User login
Rosacea Treatment Schema: An Update
When tasked with outlining updated therapy regimens for rosacea, specific patient vignettes come to mind.
A 53-year-old male golfer presents with years of central facial flushing, prominent telangiectases, erythema, and scattered pink papules. He attempted various over-the-counter topical products indicated for acne, such as salicylic acid scrub and benzoyl peroxide cream, with no improvement and much irritation. Recently, his wife has been helping him apply redness-concealing makeup in the morning and over-the-counter hydrocortisone cream in the evening, which has been slightly helpful.
This patient’s rosacea could conceivably be labeled under the papulopustular rosacea subtype; however, the conventional categories are fluid with subtype overlap and imprecise diagnostic criteria. He also seemed to display features of the erythematotelangiectatic subtype, perhaps with underlying photodamage as well as steroid rebound erythema and/or atrophy.1 Nevertheless, it is a common presentation, and certain baseline tenets should be applied. First, all steroid products and irritants (eg, benzoyl peroxide and salicylic acid ingredients, any scrub vehicle) should be discontinued. Education about avoidance of triggers (ie, sun, heat, spicy food, alcohol, stress is paramount. Because barrier inadequacy is a recent insight into rosacea pathogenesis, mild syndet- or lipid-free cleansers, daily sunscreen, and evening emollients dictate baseline skin care, as does meticulous situation-specific sun protection.2,3 The papular component and immediate erythema in and around the papules can be managed topically (prior to sunscreen or emollient application) with metronidazole gel or cream up to twice daily, ivermectin cream once daily, or azelaic acid gel or foam up to twice daily. Oral doxycycline 40 mg (delayed release) on an empty stomach or 50 mg (immediate release) with food to avert antimicrobial dosing and antibiotic resistance also could be considered if topical therapy is inadequate or irritating, though gastrointestinal comorbidities with rosacea also should be delineated before initiating oral antibiotics.4-6 (Management of this patient’s nonlesional fixed erythema, telangiectases, and flushing is discussed after the next vignette.)
What if a woman presented in a similar fashion as above, only without papules? Her family physician prescribed metronidazole gel twice daily for years with no improvement in flushing, redness, or telangiectases.
Background erythema in rosacea often is persistent with trigger-specific intensification, with or without episodic facial flushing; undoubtedly, these symptoms can be difficult to compartmentalize depending on the clarity of the patient’s history and frequency of clinic visits. The aforementioned baseline skin care and sun-protection regimen applies, and newer topical agents such as α-adrenergics (daily oxymetazoline cream or brimonidine gel) may be considered for persistent erythema; however, irritant potential and rebound erythema are common.7-9 Topical therapies such as metronidazole gel, as in this case, are inadequate for persistent background erythema or flushing. Persistent erythema and telangiectases can be reduced with pulsed dye laser or intense pulsed light modalities, particularly following conservative management of acute inflammation.5 Episodic flushing is poorly controlled with the above tactics, but anecdotally, topical or oral α-adrenergics or oral nonselective beta-blockers could be considered; the latter is also applicable to migraine therapy, which is perhaps comorbid with rosacea.5,10
A 35-year-old Hispanic woman states that the scalp, forehead, and cheeks have been flaky, pink, and pruritic for years. She saw several aestheticians for it and the admixed “acne” on the face, receiving salicylic acid chemical peels with no improvement and much dyspigmentation.
Although underreported, the commingling of rosacea with seborrheic dermatitis is common, perhaps with mutual Demodex mite overpopulation, assigning topical therapies to its management such as daily ivermectin cream or steroid-sparing pimecrolimus cream for inflammatory papules and scaly regions of the face and scalp.11-13 Further, this case exemplifies the increasing incidence and awareness of rosacea in darker skin types, along with its postinflammatory pigmentary perturbations, which necessitate repeated education about barrier control and sun protection.14
A 72-year-old male farmer presents with his wife whoinsists that his nose has been increasing in size for years; she procures a prior driver’s license photograph as proof. She also notes that he has been snoring at night and having more trouble breathing while working outdoors. The patient had not noticed.
Phymatous rosacea may exist as an additional feature of any rosacea subtype or as a singular finding, presenting as actively inflamed, fibrotic/noninflamed, or both. Management, particularly if inflamed, involves baseline gentle skin care and sun protection, avoidance of rosacea triggers, and implementation of oral therapy such as doxycycline or isotretinoin. Many cases, particularly those with a fibrotic component, warrant surgical methods such as fractionated CO2 laser or Shaw scalpel surgical sculpting. These cases frequently demonstrate varying degrees of airway compromise, validating surgery as a legitimate medical, not merely cosmetic, presentation.5,15
Final Thoughts
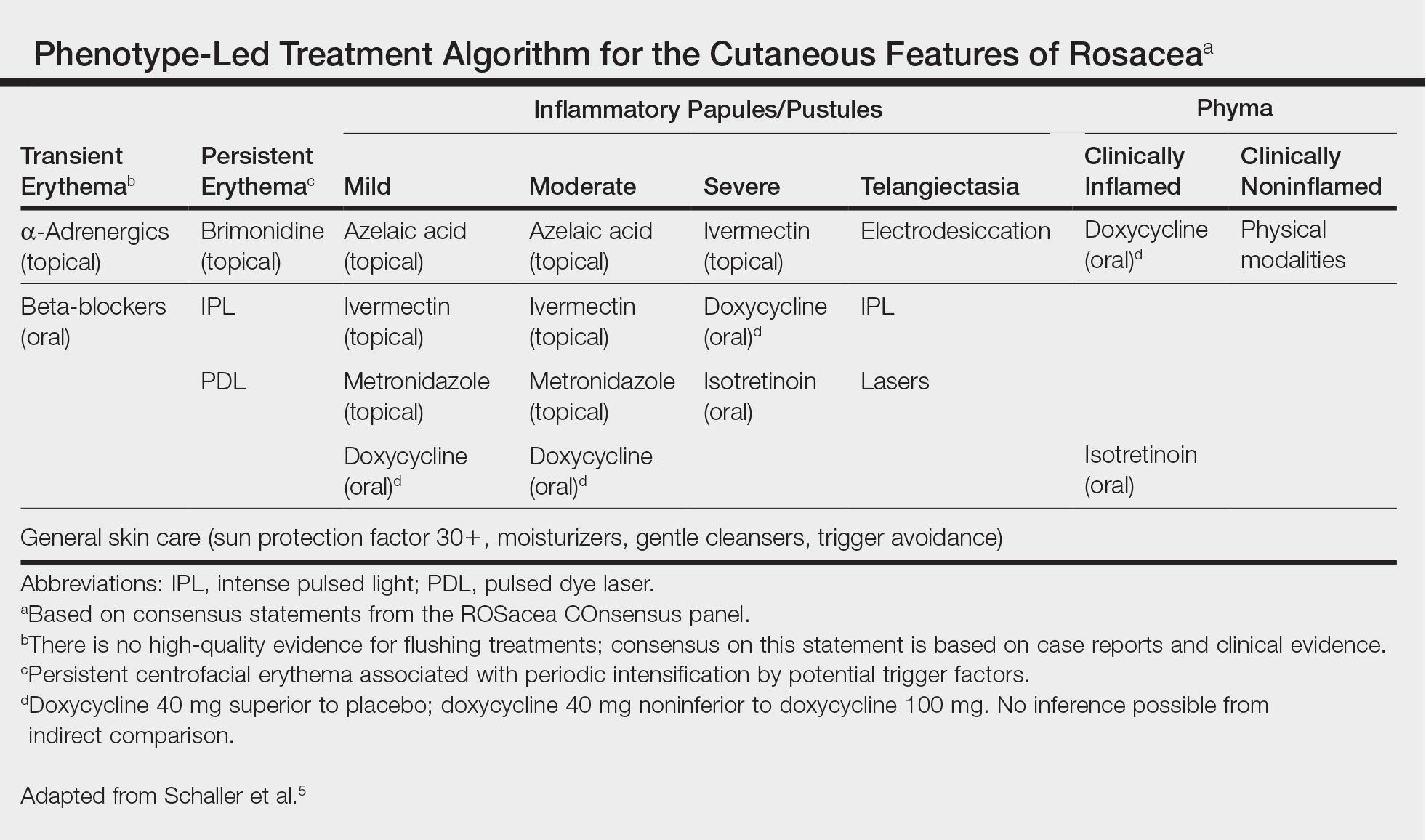
The Table, constructed as a concise therapy compendium by the ROSacea COnsensus (ROSCO) international panel of dermatologists and ophthalmologists, outlines data-driven and expert experience-based therapies for rosacea.5 This panel asserts that phenotypical features, not rigid subtypes, oblige patient-specific treatment schema. Also, as these cases outline, an evolving understanding of rosacea’s multifaceted pathogenesis, assorted presentations, and frequent pitfalls in daily skin care and initial management require individualized care.
- Tan J, Steinhoff M, Berg M, et al; Rosacea International Study Group. Shortcomings in rosacea diagnosis and classification. Br J Dermatol. 2017;176:197-199.
- Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
- Del Rosso JQ. Adjunctive skin care in the management of rosacea: cleansers, moisturizers, and photoprotectants. Cutis. 2005;75(suppl 3):17-21;discussion 33-36.
- van Zuuren EJ, Fedorowicz Z. Interventions for rosacea: abridged updated Cochrane systematic review including GRADE assessments [published online August 30, 2015]. Br J Dermatol. 2015;173:651-662.
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:465-471.
- Egeberg A, Weinstock LB, Thvssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol. 2017;176:100-106.
- Layton AM, Schaller M, Homey B, et al. Brimonidine gel 0.33% rapidly improves patient-reported outcomes by controlling facial erythema of rosacea: a randomized, double-blind, vehicle-controlled study. J Eur Acad Dermatol Venereol. 2015;29:2405-2410.
- Docherty JR, Steinhoff M, Lorton D, et al. Multidisciplinary consideration of potential pathophysiologic mechanisms of paradoxical erythema with topical brimonidine therapy [published online August 25, 2016]. Adv Ther. 2016;33:1885-1895.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Egeberg A, Ashina M, Gaist D, et al. Prevalence and risk of migraine in patients with rosacea: a population-based cohort study. J Am Acad Dermatol. 2017;76:454-458.
- Zhao YE, Peng Y, Wang XL, et al. Facial dermatosis associated with Demodex: a case-control study. J Zhejiang Univ Sci B. 2011;12:1008-1015.
- Siddiqui K, Stein Gold L, Gill J. The efficacy, safety, and tolerability of ivermectin compared with current topical treatments for the inflammatory lesions of rosacea: a network meta-analysis. Springerplus. 2016;5:1151. doi: 10.1186/s40064-016-2819-8.
- Kim MB, Kim GW, Park HJ, et al. Pimecrolimus 1% cream for the treatment of rosacea. J Dermatol. 2011;38:1135-1139.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Little SC, Stucker FJ, Compton A, et al. Nuances in the management of rhinophyma. Facial Plast Surg. 2012;28:231-237.
When tasked with outlining updated therapy regimens for rosacea, specific patient vignettes come to mind.
A 53-year-old male golfer presents with years of central facial flushing, prominent telangiectases, erythema, and scattered pink papules. He attempted various over-the-counter topical products indicated for acne, such as salicylic acid scrub and benzoyl peroxide cream, with no improvement and much irritation. Recently, his wife has been helping him apply redness-concealing makeup in the morning and over-the-counter hydrocortisone cream in the evening, which has been slightly helpful.
This patient’s rosacea could conceivably be labeled under the papulopustular rosacea subtype; however, the conventional categories are fluid with subtype overlap and imprecise diagnostic criteria. He also seemed to display features of the erythematotelangiectatic subtype, perhaps with underlying photodamage as well as steroid rebound erythema and/or atrophy.1 Nevertheless, it is a common presentation, and certain baseline tenets should be applied. First, all steroid products and irritants (eg, benzoyl peroxide and salicylic acid ingredients, any scrub vehicle) should be discontinued. Education about avoidance of triggers (ie, sun, heat, spicy food, alcohol, stress is paramount. Because barrier inadequacy is a recent insight into rosacea pathogenesis, mild syndet- or lipid-free cleansers, daily sunscreen, and evening emollients dictate baseline skin care, as does meticulous situation-specific sun protection.2,3 The papular component and immediate erythema in and around the papules can be managed topically (prior to sunscreen or emollient application) with metronidazole gel or cream up to twice daily, ivermectin cream once daily, or azelaic acid gel or foam up to twice daily. Oral doxycycline 40 mg (delayed release) on an empty stomach or 50 mg (immediate release) with food to avert antimicrobial dosing and antibiotic resistance also could be considered if topical therapy is inadequate or irritating, though gastrointestinal comorbidities with rosacea also should be delineated before initiating oral antibiotics.4-6 (Management of this patient’s nonlesional fixed erythema, telangiectases, and flushing is discussed after the next vignette.)
What if a woman presented in a similar fashion as above, only without papules? Her family physician prescribed metronidazole gel twice daily for years with no improvement in flushing, redness, or telangiectases.
Background erythema in rosacea often is persistent with trigger-specific intensification, with or without episodic facial flushing; undoubtedly, these symptoms can be difficult to compartmentalize depending on the clarity of the patient’s history and frequency of clinic visits. The aforementioned baseline skin care and sun-protection regimen applies, and newer topical agents such as α-adrenergics (daily oxymetazoline cream or brimonidine gel) may be considered for persistent erythema; however, irritant potential and rebound erythema are common.7-9 Topical therapies such as metronidazole gel, as in this case, are inadequate for persistent background erythema or flushing. Persistent erythema and telangiectases can be reduced with pulsed dye laser or intense pulsed light modalities, particularly following conservative management of acute inflammation.5 Episodic flushing is poorly controlled with the above tactics, but anecdotally, topical or oral α-adrenergics or oral nonselective beta-blockers could be considered; the latter is also applicable to migraine therapy, which is perhaps comorbid with rosacea.5,10
A 35-year-old Hispanic woman states that the scalp, forehead, and cheeks have been flaky, pink, and pruritic for years. She saw several aestheticians for it and the admixed “acne” on the face, receiving salicylic acid chemical peels with no improvement and much dyspigmentation.
Although underreported, the commingling of rosacea with seborrheic dermatitis is common, perhaps with mutual Demodex mite overpopulation, assigning topical therapies to its management such as daily ivermectin cream or steroid-sparing pimecrolimus cream for inflammatory papules and scaly regions of the face and scalp.11-13 Further, this case exemplifies the increasing incidence and awareness of rosacea in darker skin types, along with its postinflammatory pigmentary perturbations, which necessitate repeated education about barrier control and sun protection.14
A 72-year-old male farmer presents with his wife whoinsists that his nose has been increasing in size for years; she procures a prior driver’s license photograph as proof. She also notes that he has been snoring at night and having more trouble breathing while working outdoors. The patient had not noticed.
Phymatous rosacea may exist as an additional feature of any rosacea subtype or as a singular finding, presenting as actively inflamed, fibrotic/noninflamed, or both. Management, particularly if inflamed, involves baseline gentle skin care and sun protection, avoidance of rosacea triggers, and implementation of oral therapy such as doxycycline or isotretinoin. Many cases, particularly those with a fibrotic component, warrant surgical methods such as fractionated CO2 laser or Shaw scalpel surgical sculpting. These cases frequently demonstrate varying degrees of airway compromise, validating surgery as a legitimate medical, not merely cosmetic, presentation.5,15
Final Thoughts
The Table, constructed as a concise therapy compendium by the ROSacea COnsensus (ROSCO) international panel of dermatologists and ophthalmologists, outlines data-driven and expert experience-based therapies for rosacea.5 This panel asserts that phenotypical features, not rigid subtypes, oblige patient-specific treatment schema. Also, as these cases outline, an evolving understanding of rosacea’s multifaceted pathogenesis, assorted presentations, and frequent pitfalls in daily skin care and initial management require individualized care.

When tasked with outlining updated therapy regimens for rosacea, specific patient vignettes come to mind.
A 53-year-old male golfer presents with years of central facial flushing, prominent telangiectases, erythema, and scattered pink papules. He attempted various over-the-counter topical products indicated for acne, such as salicylic acid scrub and benzoyl peroxide cream, with no improvement and much irritation. Recently, his wife has been helping him apply redness-concealing makeup in the morning and over-the-counter hydrocortisone cream in the evening, which has been slightly helpful.
This patient’s rosacea could conceivably be labeled under the papulopustular rosacea subtype; however, the conventional categories are fluid with subtype overlap and imprecise diagnostic criteria. He also seemed to display features of the erythematotelangiectatic subtype, perhaps with underlying photodamage as well as steroid rebound erythema and/or atrophy.1 Nevertheless, it is a common presentation, and certain baseline tenets should be applied. First, all steroid products and irritants (eg, benzoyl peroxide and salicylic acid ingredients, any scrub vehicle) should be discontinued. Education about avoidance of triggers (ie, sun, heat, spicy food, alcohol, stress is paramount. Because barrier inadequacy is a recent insight into rosacea pathogenesis, mild syndet- or lipid-free cleansers, daily sunscreen, and evening emollients dictate baseline skin care, as does meticulous situation-specific sun protection.2,3 The papular component and immediate erythema in and around the papules can be managed topically (prior to sunscreen or emollient application) with metronidazole gel or cream up to twice daily, ivermectin cream once daily, or azelaic acid gel or foam up to twice daily. Oral doxycycline 40 mg (delayed release) on an empty stomach or 50 mg (immediate release) with food to avert antimicrobial dosing and antibiotic resistance also could be considered if topical therapy is inadequate or irritating, though gastrointestinal comorbidities with rosacea also should be delineated before initiating oral antibiotics.4-6 (Management of this patient’s nonlesional fixed erythema, telangiectases, and flushing is discussed after the next vignette.)
What if a woman presented in a similar fashion as above, only without papules? Her family physician prescribed metronidazole gel twice daily for years with no improvement in flushing, redness, or telangiectases.
Background erythema in rosacea often is persistent with trigger-specific intensification, with or without episodic facial flushing; undoubtedly, these symptoms can be difficult to compartmentalize depending on the clarity of the patient’s history and frequency of clinic visits. The aforementioned baseline skin care and sun-protection regimen applies, and newer topical agents such as α-adrenergics (daily oxymetazoline cream or brimonidine gel) may be considered for persistent erythema; however, irritant potential and rebound erythema are common.7-9 Topical therapies such as metronidazole gel, as in this case, are inadequate for persistent background erythema or flushing. Persistent erythema and telangiectases can be reduced with pulsed dye laser or intense pulsed light modalities, particularly following conservative management of acute inflammation.5 Episodic flushing is poorly controlled with the above tactics, but anecdotally, topical or oral α-adrenergics or oral nonselective beta-blockers could be considered; the latter is also applicable to migraine therapy, which is perhaps comorbid with rosacea.5,10
A 35-year-old Hispanic woman states that the scalp, forehead, and cheeks have been flaky, pink, and pruritic for years. She saw several aestheticians for it and the admixed “acne” on the face, receiving salicylic acid chemical peels with no improvement and much dyspigmentation.
Although underreported, the commingling of rosacea with seborrheic dermatitis is common, perhaps with mutual Demodex mite overpopulation, assigning topical therapies to its management such as daily ivermectin cream or steroid-sparing pimecrolimus cream for inflammatory papules and scaly regions of the face and scalp.11-13 Further, this case exemplifies the increasing incidence and awareness of rosacea in darker skin types, along with its postinflammatory pigmentary perturbations, which necessitate repeated education about barrier control and sun protection.14
A 72-year-old male farmer presents with his wife whoinsists that his nose has been increasing in size for years; she procures a prior driver’s license photograph as proof. She also notes that he has been snoring at night and having more trouble breathing while working outdoors. The patient had not noticed.
Phymatous rosacea may exist as an additional feature of any rosacea subtype or as a singular finding, presenting as actively inflamed, fibrotic/noninflamed, or both. Management, particularly if inflamed, involves baseline gentle skin care and sun protection, avoidance of rosacea triggers, and implementation of oral therapy such as doxycycline or isotretinoin. Many cases, particularly those with a fibrotic component, warrant surgical methods such as fractionated CO2 laser or Shaw scalpel surgical sculpting. These cases frequently demonstrate varying degrees of airway compromise, validating surgery as a legitimate medical, not merely cosmetic, presentation.5,15
Final Thoughts
The Table, constructed as a concise therapy compendium by the ROSacea COnsensus (ROSCO) international panel of dermatologists and ophthalmologists, outlines data-driven and expert experience-based therapies for rosacea.5 This panel asserts that phenotypical features, not rigid subtypes, oblige patient-specific treatment schema. Also, as these cases outline, an evolving understanding of rosacea’s multifaceted pathogenesis, assorted presentations, and frequent pitfalls in daily skin care and initial management require individualized care.

- Tan J, Steinhoff M, Berg M, et al; Rosacea International Study Group. Shortcomings in rosacea diagnosis and classification. Br J Dermatol. 2017;176:197-199.
- Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
- Del Rosso JQ. Adjunctive skin care in the management of rosacea: cleansers, moisturizers, and photoprotectants. Cutis. 2005;75(suppl 3):17-21;discussion 33-36.
- van Zuuren EJ, Fedorowicz Z. Interventions for rosacea: abridged updated Cochrane systematic review including GRADE assessments [published online August 30, 2015]. Br J Dermatol. 2015;173:651-662.
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:465-471.
- Egeberg A, Weinstock LB, Thvssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol. 2017;176:100-106.
- Layton AM, Schaller M, Homey B, et al. Brimonidine gel 0.33% rapidly improves patient-reported outcomes by controlling facial erythema of rosacea: a randomized, double-blind, vehicle-controlled study. J Eur Acad Dermatol Venereol. 2015;29:2405-2410.
- Docherty JR, Steinhoff M, Lorton D, et al. Multidisciplinary consideration of potential pathophysiologic mechanisms of paradoxical erythema with topical brimonidine therapy [published online August 25, 2016]. Adv Ther. 2016;33:1885-1895.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Egeberg A, Ashina M, Gaist D, et al. Prevalence and risk of migraine in patients with rosacea: a population-based cohort study. J Am Acad Dermatol. 2017;76:454-458.
- Zhao YE, Peng Y, Wang XL, et al. Facial dermatosis associated with Demodex: a case-control study. J Zhejiang Univ Sci B. 2011;12:1008-1015.
- Siddiqui K, Stein Gold L, Gill J. The efficacy, safety, and tolerability of ivermectin compared with current topical treatments for the inflammatory lesions of rosacea: a network meta-analysis. Springerplus. 2016;5:1151. doi: 10.1186/s40064-016-2819-8.
- Kim MB, Kim GW, Park HJ, et al. Pimecrolimus 1% cream for the treatment of rosacea. J Dermatol. 2011;38:1135-1139.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Little SC, Stucker FJ, Compton A, et al. Nuances in the management of rhinophyma. Facial Plast Surg. 2012;28:231-237.
- Tan J, Steinhoff M, Berg M, et al; Rosacea International Study Group. Shortcomings in rosacea diagnosis and classification. Br J Dermatol. 2017;176:197-199.
- Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
- Del Rosso JQ. Adjunctive skin care in the management of rosacea: cleansers, moisturizers, and photoprotectants. Cutis. 2005;75(suppl 3):17-21;discussion 33-36.
- van Zuuren EJ, Fedorowicz Z. Interventions for rosacea: abridged updated Cochrane systematic review including GRADE assessments [published online August 30, 2015]. Br J Dermatol. 2015;173:651-662.
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:465-471.
- Egeberg A, Weinstock LB, Thvssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol. 2017;176:100-106.
- Layton AM, Schaller M, Homey B, et al. Brimonidine gel 0.33% rapidly improves patient-reported outcomes by controlling facial erythema of rosacea: a randomized, double-blind, vehicle-controlled study. J Eur Acad Dermatol Venereol. 2015;29:2405-2410.
- Docherty JR, Steinhoff M, Lorton D, et al. Multidisciplinary consideration of potential pathophysiologic mechanisms of paradoxical erythema with topical brimonidine therapy [published online August 25, 2016]. Adv Ther. 2016;33:1885-1895.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Egeberg A, Ashina M, Gaist D, et al. Prevalence and risk of migraine in patients with rosacea: a population-based cohort study. J Am Acad Dermatol. 2017;76:454-458.
- Zhao YE, Peng Y, Wang XL, et al. Facial dermatosis associated with Demodex: a case-control study. J Zhejiang Univ Sci B. 2011;12:1008-1015.
- Siddiqui K, Stein Gold L, Gill J. The efficacy, safety, and tolerability of ivermectin compared with current topical treatments for the inflammatory lesions of rosacea: a network meta-analysis. Springerplus. 2016;5:1151. doi: 10.1186/s40064-016-2819-8.
- Kim MB, Kim GW, Park HJ, et al. Pimecrolimus 1% cream for the treatment of rosacea. J Dermatol. 2011;38:1135-1139.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Little SC, Stucker FJ, Compton A, et al. Nuances in the management of rhinophyma. Facial Plast Surg. 2012;28:231-237.
First EDition: ED Visits Increased in States That Expanded Medicaid, more
BY JEFF BAUER
There was a substantial increase in the number of ED visits in states that expanded Medicaid coverage in 2014, after the Affordable Care Act was implemented, and a decrease in the number of ED visits by uninsured patients, according to a study published in Annals of Emergency Medicine.
Researchers analyzed quarterly data on ED visits from the Agency for Healthcare Research and Quality’s Fast Stats database, which is an early-release, aggregated version of the State Emergency Department Databases and State Inpatient Databases. They compared changes in ED visits per capita and changes in share of ED visits by payer (Medicaid, uninsured, and private insurance) in states that did and did not expand Medicaid coverage in 2014.
The analysis included 25 states: 14 Medicaid expansion states (Arizona, California, Hawaii, Iowa, Illinois, Kentucky, Maryland, Minnesota, North Dakota, New Jersey, Nevada, New York, Rhode Island, and Vermont) and 11 nonexpansion states (Florida, Georgia, Indiana, Kansas, Missouri, North Carolina, Nebraska, South Carolina, South Dakota, Tennessee, and Wisconsin). Researchers defined visits that occurred during all 4 quarters of 2012 and the first 3 quarters of 2013 as the pre-expansion period, and visits from the first through fourth quarters of 2014 as the postexpansion period. Visits that occurred during the fourth quarter of 2013 were not included in the analysis because Medicaid coverage began to increase in the final quarter of 2013 for most states.
Overall, researchers found that after 2014, ED use per 1,000 people per quarter increased by 2.5 visits more in expansion states compared to nonexpansion states. Researchers estimated that 1.13 million ED visits in 2014 could be attributed to Medicaid expansion in these states. In expansion states, the share of ED visits by Medicaid patients increased by 8.8 percentage points and the share of visits by insured patients decreased by 5.3 percentage points, compared to nonexpansion states. The share of visits by insured patients did not change for expansion states but increased slightly for nonexpansion states.
An American College of Emergency Physicians press release about this study included editorial comments by Ari Friedman, MD, of Beth Israel Deaconess Medical Center in Boston, who said, “More emergency department visits by Medicaid beneficiaries is neither clearly bad nor clearly good. Insurance increases access to care, including emergency department care. We need to move beyond the value judgments that have dominated so much study of emergency department utilization towards a more rational basis for how we structure unscheduled visits in the health system. If we want to meet patients’ care needs as patients themselves define them, the emergency department has a key role to play in a flexible system.”
Nikpay S, Freedman S, Levy H, Buchmueller T. Effect of the Affordable Care Act Medicaid expansion on emergency department visits: evidence from state-level emergency department databases. Ann Emerg Med. 2017 June 26. [Epub ahead of print]. doi:http://dx.doi.org/10.1016/j.annemergmed.2017.03.023.
Child Firearm Suicide at Highest Rate in More Than a Decade
MOLLIE KALAYCIO
FRONTLINE MEDICAL NEWS
Boys, older children, and minorities are disproportionately affected when it comes to firearm injuries and deaths in US children and adolescents, and child firearm suicide rates are at the highest they have been in more than a decade, new study results revealed.
Approximately 19 children are either medically treated for a gunshot wound or killed by one every day in the United States. “The majority of these children are boys 13-17 years old, African American in the case of firearm homicide, and white and American Indian in the case of firearm suicide. Pediatric firearm injuries and deaths are an important public health problem in the United States, contributing substantially each year to premature death, illness, and disability of children,” said Katherine A. Fowler, PhD, of the National Center for Injury Prevention and Control, Atlanta, and her associates. “Finding ways to prevent such injuries and ensure that all children have safe, stable, nurturing relationships and environments remains one of our most important priorities.”
National data on fatal firearm injuries in 2011-2014 for this study were derived from death certificate data from the Centers for Disease Control and Prevention’s (CDC’s) National Vital Statistics System, obtained via the CDC’s Web-based Injury Statistics Query and Reporting System. Data on nonfatal firearm injuries for 2011-2014 were obtained from the National Electronic Injury Surveillance System.
“From 2012 to 2014, the average annual case fatality rate was 74% for firearm-related self-harm, 14% for firearm-related assaults, and 6% for unintentional firearm injuries,” the investigators reported.
Boys accounted for 82% of all child firearm deaths from 2012 to 2014. In this time period, the annual rate of firearm death for boys was 4.5 times higher than the annual rate for girls (2.8 vs. 0.6 per 100,000). This difference was even more pronounced by age, with the rate for 13- to 17-year-old boys being six times higher than the rate for same-aged girls. Similarly, boys suffer the majority of nonfatal firearm injuries treated in US EDs, accounting for 84% of all nonfatal firearm injuries medically treated each year from 2012 to 2014. The average annual rate of nonfatal firearm injuries for boys was five times the rate for girls at 13 vs. 3 per 100,000.
The annual rate of firearm homicide was 10 times higher among 13- to 17-year-olds vs. 0- to 12-year-olds (3 vs. 0.3 per 100,000). Unintentional firearm death rates were approximately twice as high when comparing these two groups (0.2 vs. 0.1 per 100,000).
Dr Fowler and her associates wrote, “Our findings indicate that most children who died of unintentional firearm injuries were shot by another child in their own age range and most often in the context of playing with a gun or showing it to others. More than one-third of the deaths of older children occurred in incidents in which the shooter thought that the gun was unloaded or thought that the safety was engaged.”
“Child firearm suicide rates showed a significant upward trend between 2007 and 2014, increasing 60% from 1.0 to 1.6 (P < .05) to the highest rate seen over the period examined,” Dr Fowler and her associates said.
Firearm suicide rates were 11 times higher among 13- to 17-year-olds vs. 10- to 12-year-olds (2 vs. 0.2 per 100,000). Older children also accounted for 88% of all nonfatal firearm injuries treated in an ED. The overall average annual rate of nonfatal firearm injuries for older children was 19 times that of younger children (24 vs. 1 per 100,000).
The annual firearm homicide rate for African American children was nearly 10 times higher than the rate for white children (4 vs. 0.4 per 100,000). However, the annual rate of firearm suicide among white children was nearly four times higher than the rate for African American children (2. vs. 0.6 per 100,000).
Awareness of the availability of firearms during times of crisis is crucial because suicides are often impulsive in young people, Dr Fowler and her associates said, “with previous findings indicating that many who attempt suicide spend 10 minutes or less deliberating. Safe storage practices (ie, unloading and locking all firearms and ammunition) can potentially be lifesaving in these instances,” as the results of previous studies in this age group attest.
Firearm deaths are the third leading cause of
Fowler KA, Dahlberg LL, Haileyesus T, Gutierrez C, Bacon S. Childhood firearm injuries in the united states. Pediatrics. 2017;140(1):e20163486.
BY JEFF BAUER
There was a substantial increase in the number of ED visits in states that expanded Medicaid coverage in 2014, after the Affordable Care Act was implemented, and a decrease in the number of ED visits by uninsured patients, according to a study published in Annals of Emergency Medicine.
Researchers analyzed quarterly data on ED visits from the Agency for Healthcare Research and Quality’s Fast Stats database, which is an early-release, aggregated version of the State Emergency Department Databases and State Inpatient Databases. They compared changes in ED visits per capita and changes in share of ED visits by payer (Medicaid, uninsured, and private insurance) in states that did and did not expand Medicaid coverage in 2014.
The analysis included 25 states: 14 Medicaid expansion states (Arizona, California, Hawaii, Iowa, Illinois, Kentucky, Maryland, Minnesota, North Dakota, New Jersey, Nevada, New York, Rhode Island, and Vermont) and 11 nonexpansion states (Florida, Georgia, Indiana, Kansas, Missouri, North Carolina, Nebraska, South Carolina, South Dakota, Tennessee, and Wisconsin). Researchers defined visits that occurred during all 4 quarters of 2012 and the first 3 quarters of 2013 as the pre-expansion period, and visits from the first through fourth quarters of 2014 as the postexpansion period. Visits that occurred during the fourth quarter of 2013 were not included in the analysis because Medicaid coverage began to increase in the final quarter of 2013 for most states.
Overall, researchers found that after 2014, ED use per 1,000 people per quarter increased by 2.5 visits more in expansion states compared to nonexpansion states. Researchers estimated that 1.13 million ED visits in 2014 could be attributed to Medicaid expansion in these states. In expansion states, the share of ED visits by Medicaid patients increased by 8.8 percentage points and the share of visits by insured patients decreased by 5.3 percentage points, compared to nonexpansion states. The share of visits by insured patients did not change for expansion states but increased slightly for nonexpansion states.
An American College of Emergency Physicians press release about this study included editorial comments by Ari Friedman, MD, of Beth Israel Deaconess Medical Center in Boston, who said, “More emergency department visits by Medicaid beneficiaries is neither clearly bad nor clearly good. Insurance increases access to care, including emergency department care. We need to move beyond the value judgments that have dominated so much study of emergency department utilization towards a more rational basis for how we structure unscheduled visits in the health system. If we want to meet patients’ care needs as patients themselves define them, the emergency department has a key role to play in a flexible system.”
Nikpay S, Freedman S, Levy H, Buchmueller T. Effect of the Affordable Care Act Medicaid expansion on emergency department visits: evidence from state-level emergency department databases. Ann Emerg Med. 2017 June 26. [Epub ahead of print]. doi:http://dx.doi.org/10.1016/j.annemergmed.2017.03.023.
Child Firearm Suicide at Highest Rate in More Than a Decade
MOLLIE KALAYCIO
FRONTLINE MEDICAL NEWS
Boys, older children, and minorities are disproportionately affected when it comes to firearm injuries and deaths in US children and adolescents, and child firearm suicide rates are at the highest they have been in more than a decade, new study results revealed.
Approximately 19 children are either medically treated for a gunshot wound or killed by one every day in the United States. “The majority of these children are boys 13-17 years old, African American in the case of firearm homicide, and white and American Indian in the case of firearm suicide. Pediatric firearm injuries and deaths are an important public health problem in the United States, contributing substantially each year to premature death, illness, and disability of children,” said Katherine A. Fowler, PhD, of the National Center for Injury Prevention and Control, Atlanta, and her associates. “Finding ways to prevent such injuries and ensure that all children have safe, stable, nurturing relationships and environments remains one of our most important priorities.”
National data on fatal firearm injuries in 2011-2014 for this study were derived from death certificate data from the Centers for Disease Control and Prevention’s (CDC’s) National Vital Statistics System, obtained via the CDC’s Web-based Injury Statistics Query and Reporting System. Data on nonfatal firearm injuries for 2011-2014 were obtained from the National Electronic Injury Surveillance System.
“From 2012 to 2014, the average annual case fatality rate was 74% for firearm-related self-harm, 14% for firearm-related assaults, and 6% for unintentional firearm injuries,” the investigators reported.
Boys accounted for 82% of all child firearm deaths from 2012 to 2014. In this time period, the annual rate of firearm death for boys was 4.5 times higher than the annual rate for girls (2.8 vs. 0.6 per 100,000). This difference was even more pronounced by age, with the rate for 13- to 17-year-old boys being six times higher than the rate for same-aged girls. Similarly, boys suffer the majority of nonfatal firearm injuries treated in US EDs, accounting for 84% of all nonfatal firearm injuries medically treated each year from 2012 to 2014. The average annual rate of nonfatal firearm injuries for boys was five times the rate for girls at 13 vs. 3 per 100,000.
The annual rate of firearm homicide was 10 times higher among 13- to 17-year-olds vs. 0- to 12-year-olds (3 vs. 0.3 per 100,000). Unintentional firearm death rates were approximately twice as high when comparing these two groups (0.2 vs. 0.1 per 100,000).
Dr Fowler and her associates wrote, “Our findings indicate that most children who died of unintentional firearm injuries were shot by another child in their own age range and most often in the context of playing with a gun or showing it to others. More than one-third of the deaths of older children occurred in incidents in which the shooter thought that the gun was unloaded or thought that the safety was engaged.”
“Child firearm suicide rates showed a significant upward trend between 2007 and 2014, increasing 60% from 1.0 to 1.6 (P < .05) to the highest rate seen over the period examined,” Dr Fowler and her associates said.
Firearm suicide rates were 11 times higher among 13- to 17-year-olds vs. 10- to 12-year-olds (2 vs. 0.2 per 100,000). Older children also accounted for 88% of all nonfatal firearm injuries treated in an ED. The overall average annual rate of nonfatal firearm injuries for older children was 19 times that of younger children (24 vs. 1 per 100,000).
The annual firearm homicide rate for African American children was nearly 10 times higher than the rate for white children (4 vs. 0.4 per 100,000). However, the annual rate of firearm suicide among white children was nearly four times higher than the rate for African American children (2. vs. 0.6 per 100,000).
Awareness of the availability of firearms during times of crisis is crucial because suicides are often impulsive in young people, Dr Fowler and her associates said, “with previous findings indicating that many who attempt suicide spend 10 minutes or less deliberating. Safe storage practices (ie, unloading and locking all firearms and ammunition) can potentially be lifesaving in these instances,” as the results of previous studies in this age group attest.
Firearm deaths are the third leading cause of
Fowler KA, Dahlberg LL, Haileyesus T, Gutierrez C, Bacon S. Childhood firearm injuries in the united states. Pediatrics. 2017;140(1):e20163486.
BY JEFF BAUER
There was a substantial increase in the number of ED visits in states that expanded Medicaid coverage in 2014, after the Affordable Care Act was implemented, and a decrease in the number of ED visits by uninsured patients, according to a study published in Annals of Emergency Medicine.
Researchers analyzed quarterly data on ED visits from the Agency for Healthcare Research and Quality’s Fast Stats database, which is an early-release, aggregated version of the State Emergency Department Databases and State Inpatient Databases. They compared changes in ED visits per capita and changes in share of ED visits by payer (Medicaid, uninsured, and private insurance) in states that did and did not expand Medicaid coverage in 2014.
The analysis included 25 states: 14 Medicaid expansion states (Arizona, California, Hawaii, Iowa, Illinois, Kentucky, Maryland, Minnesota, North Dakota, New Jersey, Nevada, New York, Rhode Island, and Vermont) and 11 nonexpansion states (Florida, Georgia, Indiana, Kansas, Missouri, North Carolina, Nebraska, South Carolina, South Dakota, Tennessee, and Wisconsin). Researchers defined visits that occurred during all 4 quarters of 2012 and the first 3 quarters of 2013 as the pre-expansion period, and visits from the first through fourth quarters of 2014 as the postexpansion period. Visits that occurred during the fourth quarter of 2013 were not included in the analysis because Medicaid coverage began to increase in the final quarter of 2013 for most states.
Overall, researchers found that after 2014, ED use per 1,000 people per quarter increased by 2.5 visits more in expansion states compared to nonexpansion states. Researchers estimated that 1.13 million ED visits in 2014 could be attributed to Medicaid expansion in these states. In expansion states, the share of ED visits by Medicaid patients increased by 8.8 percentage points and the share of visits by insured patients decreased by 5.3 percentage points, compared to nonexpansion states. The share of visits by insured patients did not change for expansion states but increased slightly for nonexpansion states.
An American College of Emergency Physicians press release about this study included editorial comments by Ari Friedman, MD, of Beth Israel Deaconess Medical Center in Boston, who said, “More emergency department visits by Medicaid beneficiaries is neither clearly bad nor clearly good. Insurance increases access to care, including emergency department care. We need to move beyond the value judgments that have dominated so much study of emergency department utilization towards a more rational basis for how we structure unscheduled visits in the health system. If we want to meet patients’ care needs as patients themselves define them, the emergency department has a key role to play in a flexible system.”
Nikpay S, Freedman S, Levy H, Buchmueller T. Effect of the Affordable Care Act Medicaid expansion on emergency department visits: evidence from state-level emergency department databases. Ann Emerg Med. 2017 June 26. [Epub ahead of print]. doi:http://dx.doi.org/10.1016/j.annemergmed.2017.03.023.
Child Firearm Suicide at Highest Rate in More Than a Decade
MOLLIE KALAYCIO
FRONTLINE MEDICAL NEWS
Boys, older children, and minorities are disproportionately affected when it comes to firearm injuries and deaths in US children and adolescents, and child firearm suicide rates are at the highest they have been in more than a decade, new study results revealed.
Approximately 19 children are either medically treated for a gunshot wound or killed by one every day in the United States. “The majority of these children are boys 13-17 years old, African American in the case of firearm homicide, and white and American Indian in the case of firearm suicide. Pediatric firearm injuries and deaths are an important public health problem in the United States, contributing substantially each year to premature death, illness, and disability of children,” said Katherine A. Fowler, PhD, of the National Center for Injury Prevention and Control, Atlanta, and her associates. “Finding ways to prevent such injuries and ensure that all children have safe, stable, nurturing relationships and environments remains one of our most important priorities.”
National data on fatal firearm injuries in 2011-2014 for this study were derived from death certificate data from the Centers for Disease Control and Prevention’s (CDC’s) National Vital Statistics System, obtained via the CDC’s Web-based Injury Statistics Query and Reporting System. Data on nonfatal firearm injuries for 2011-2014 were obtained from the National Electronic Injury Surveillance System.
“From 2012 to 2014, the average annual case fatality rate was 74% for firearm-related self-harm, 14% for firearm-related assaults, and 6% for unintentional firearm injuries,” the investigators reported.
Boys accounted for 82% of all child firearm deaths from 2012 to 2014. In this time period, the annual rate of firearm death for boys was 4.5 times higher than the annual rate for girls (2.8 vs. 0.6 per 100,000). This difference was even more pronounced by age, with the rate for 13- to 17-year-old boys being six times higher than the rate for same-aged girls. Similarly, boys suffer the majority of nonfatal firearm injuries treated in US EDs, accounting for 84% of all nonfatal firearm injuries medically treated each year from 2012 to 2014. The average annual rate of nonfatal firearm injuries for boys was five times the rate for girls at 13 vs. 3 per 100,000.
The annual rate of firearm homicide was 10 times higher among 13- to 17-year-olds vs. 0- to 12-year-olds (3 vs. 0.3 per 100,000). Unintentional firearm death rates were approximately twice as high when comparing these two groups (0.2 vs. 0.1 per 100,000).
Dr Fowler and her associates wrote, “Our findings indicate that most children who died of unintentional firearm injuries were shot by another child in their own age range and most often in the context of playing with a gun or showing it to others. More than one-third of the deaths of older children occurred in incidents in which the shooter thought that the gun was unloaded or thought that the safety was engaged.”
“Child firearm suicide rates showed a significant upward trend between 2007 and 2014, increasing 60% from 1.0 to 1.6 (P < .05) to the highest rate seen over the period examined,” Dr Fowler and her associates said.
Firearm suicide rates were 11 times higher among 13- to 17-year-olds vs. 10- to 12-year-olds (2 vs. 0.2 per 100,000). Older children also accounted for 88% of all nonfatal firearm injuries treated in an ED. The overall average annual rate of nonfatal firearm injuries for older children was 19 times that of younger children (24 vs. 1 per 100,000).
The annual firearm homicide rate for African American children was nearly 10 times higher than the rate for white children (4 vs. 0.4 per 100,000). However, the annual rate of firearm suicide among white children was nearly four times higher than the rate for African American children (2. vs. 0.6 per 100,000).
Awareness of the availability of firearms during times of crisis is crucial because suicides are often impulsive in young people, Dr Fowler and her associates said, “with previous findings indicating that many who attempt suicide spend 10 minutes or less deliberating. Safe storage practices (ie, unloading and locking all firearms and ammunition) can potentially be lifesaving in these instances,” as the results of previous studies in this age group attest.
Firearm deaths are the third leading cause of
Fowler KA, Dahlberg LL, Haileyesus T, Gutierrez C, Bacon S. Childhood firearm injuries in the united states. Pediatrics. 2017;140(1):e20163486.
Topical Cannabinoids in Dermatology
The prevalence of topical cannabinoids has risen sharply in recent years. Commercial advertisers promote their usage as a safe means to treat a multitude of skin disorders, including atopic dermatitis (AD), psoriasis, and acne. Topical compounds have garnered interest in laboratory studies, but the purchase of commercial formulations is limited to over-the-counter products from unregulated suppliers. In this article, we review the scientific evidence behind topical cannabinoids and evaluate their role in clinical dermatology.
Background
Cannabis is designated as a Schedule I drug, according to the Controlled Substances Act of 1970. This listing is given to substances with no therapeutic value and a high potential for abuse. However, as of 2017, 29 states and the District of Columbia have laws legalizing cannabis in some capacity. These regulations typically apply to medicinal use, though several states have now legalized recreational use.
Cannabinoids represent a broad class of chemical compounds derived from the cannabis plant. Originally, this class only comprised phytocannabinoids, cannabinoids produced by the cannabis plant. Tetrahydrocannabinol (THC) is the most well-known phytocannabinoid and leads to the psychoactive effects typically associated with cannabis use. Later investigation led to the discovery of endocannabinoids, cannabinoids that are naturally produced by human and animal bodies, as well as synthetic cannabinoids.1 Cannabidiol is a phytocannabinoid that has been investigated in neurologic and anti-inflammatory conditions.2-4
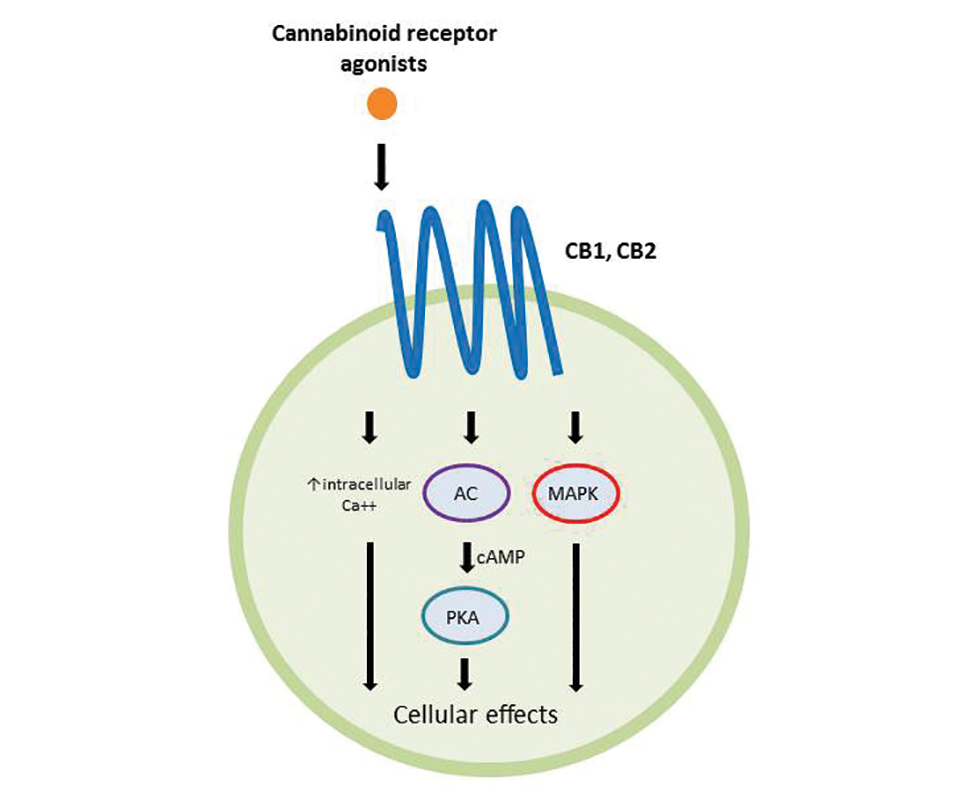
Cannabinoids act as agonists on 2 principal receptors— cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2)—which are both G protein–coupled receptors (Figure).5 Both have distinct distributions throughout different organ systems, to which cannabinoids (eg, THC, cannabidiol, endocannabinoids) show differential binding.6,7 Importantly, the expression of CB1 and CB2 has been identified on sensory nerve fibers, inflammatory cells, and adnexal structures of human skin.8 Based on these associations, topical application of cannabinoids has become a modality of interest for dermatological disorders. These formulations aim to influence cutaneous morphology without producing psychoactive effects.
Topical Cannabinoids in Inflammatory Disorders
Atopic dermatitis has emerged as an active area of investigation for cannabinoid receptors and topical agonists (Table 1). In an animal model, Kim et al9 examined the effects of CB1 agonism on skin inflammation. Mice treated with topical CB1 agonists showed greater recovery of epidermal barrier function in acutely abrogated skin relative to those treated with a vehicle preparation. In addition, agonism of CB1 led to significant (P<.001) decreases in skin fold thickness among models of acute and chronic skin inflammation.9
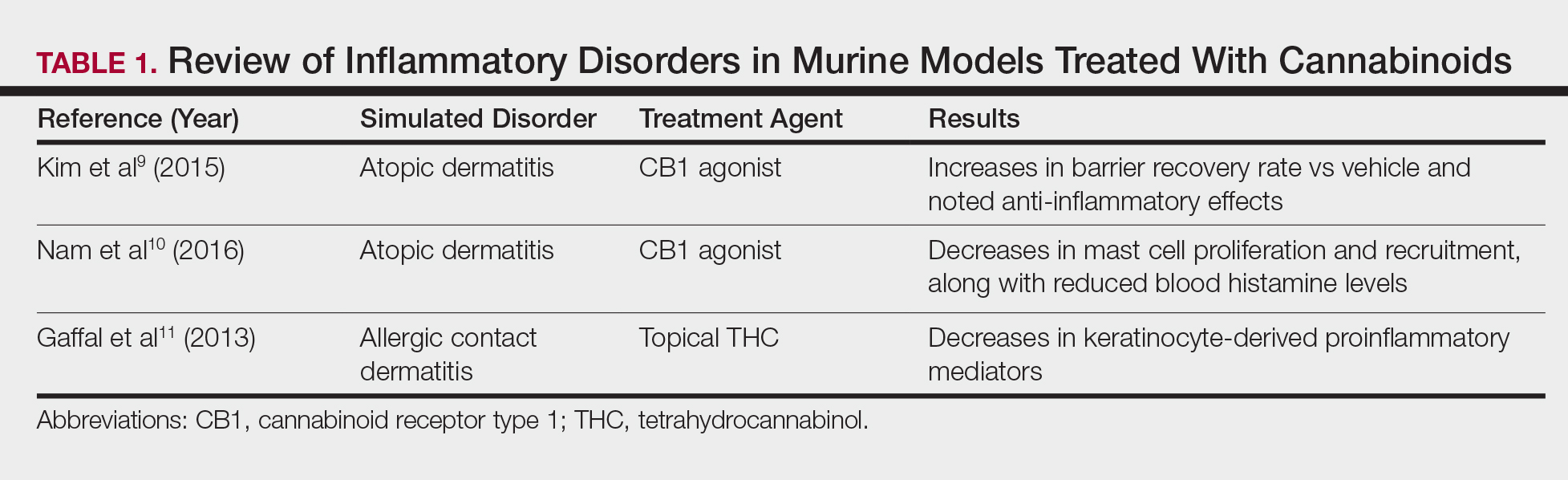
Nam et al10 also examined the role of topical CB1 agonists in mice with induced AD-like symptoms. Relative to treatment with vehicle, CB1 agonists significantly reduced the recruitment of mast cells (P<.01) and lowered the blood concentration of histamine (P<.05). Given the noted decrease in the release of inflammatory mediators, the authors speculated that topical agonsim of CB1 may prove useful in several conditions related to mast cell activation, such as AD, contact dermatitis, and psoriasis.10
The anti-inflammatory properties of topical THC were evaluated by Gaffal et al.11 In a mouse model of allergic contact dermatitis, mice treated with topical THC showed decreases in myeloid immune cell infiltration, with these beneficial effects existing even in mice with deficient CB1 and CB2 receptors. These results support a potentially wide anti-inflammatory activity of topical THC.11
Topical Cannabinoids in Pain Management
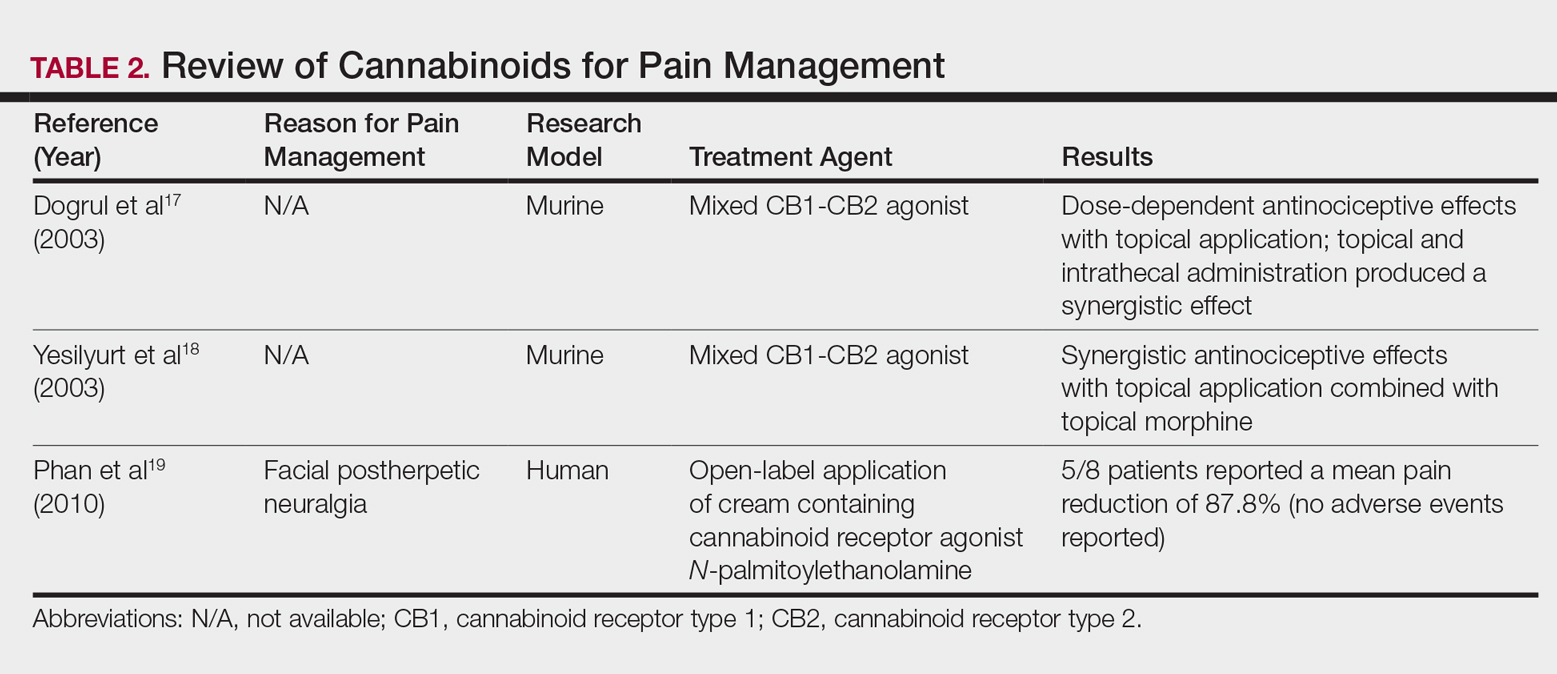
The effects of smoked cannabis in treating pain have undergone thorough investigation over recent years. Benefits have been noted in treating neuropathic pain, particularly in human immunodeficiency virus–associated sensory neuropathy.12-15 Smoked cannabis also may provide value as a synergistic therapy with opioids, thereby allowing for lower opioid doses.16
In contrast, research into the relationship between topical application of cannabinoids and nociception remains in preliminary stages (Table 2). In a mouse model, Dogrul et al17 assessed the topical antinociceptive potential of a mixed CB1-CB2 agonist. Results showed significant (P<.01) and dose-dependent antinociceptive effects relative to treatment with a vehicle.17 In a related study, Yesilyurt et al18 evaluated whether a mixed CB1-CB2 agonist could enhance the antinociceptive effects of topical opioids. Among mice treated with the combination of a cannabinoid agonist and topical morphine, a significantly (P<.05) greater analgesic effect was demonstrated relative to topical morphine alone.18
Studies in humans have been far more limited. Phan et al19 conducted a small, nonrandomized, open-label trial of a topical cannabinoid cream in patients with facial postherpetic neuralgia. Of 8 patients treated, 5 noted a mean pain reduction of 87.8%. No comparison vehicle was used. Based on this narrow study design, it is difficult to extrapolate these positive results to a broader patient population.19
Commercial Products
Although preliminary models with topical cannabinoids have shown potential, large-scale clinical trials in humans have yet to be performed. Despite this lack of investigation, commercial formulations of topical cannabinoids are available to dermatology patients. These formulations are nonstandardized, and no safety data exists regarding their use. Topical cannabinoids on the market may contain various amounts of active ingredient and may be combined with a range of other compounds.
In dermatology offices, it is not uncommon for patients to express an intention to use topical cannabinoid products following their planned treatment or procedure. Patients also have been known to use topical cannabinoid products prior to dermatologic procedures, sometimes in place of an approved topical anesthetic, without consulting the physician performing the procedure. With interventions that lead to active areas of wound healing, the application of such products may increase the risk for contamination and infection. Therefore, patients should be counseled that the use of commercial topical cannabinoids could jeopardize the success of their planned procedure, put them at risk for infection, and possibly lead to systemic absorption and/or changes in wound-healing capacities.
Conclusion
Based on the results from recent animal models, cannabinoids may have a role in future treatment algorithms for several inflammatory conditions. However, current efficacy and safety data are almost entirely limited to preliminary animal studies in rodents. In addition, the formulation of topical cannabinoid products is nonstandardized and poorly regulated. As such, the present evidence does not support the use of topical cannabinoids in dermatology practices. Dermatologists should ask patients about the use of any cannabinoid products as part of a treatment program, especially given the unsubstantiated claims often made by unscrupulous advertisers. This issue highlights the need for further research and regulation.
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389-462.
- Giacoppo S, Galuppo M, Pollastro F, et al. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. Daru. 2015;23:48.
- Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936-948.
- Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology. 2012;89:149-155.
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161-202.
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
- Svizenska I, Dubovy P, Sulcova A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav. 2008;90:501-511.
- Stander S, Schmelz M, Metze D, et al. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005;38:177-188.
- Kim HJ, Kim B, Park BM, et al. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int J Dermatol. 2015;54:E401-E408.
- Nam G, Jeong SK, Park BM, et al. Selective cannabinoid receptor-1 agonists regulate mast cell activation in an oxazolone-induced atopic dermatitis model. Ann Dermatol. 2016;28:22-29.
- Gaffal E, Cron M, Glodde N, et al. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy. 2013;68:994-1000.
- Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515-521.
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672-680.
- Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136-148.
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506-521.
- Abrams DI, Couey P, Shade SB, et al. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844-851.
- Dogrul A, Gul H, Akar A, et al. Topical cannabinoid antinociception: synergy with spinal sites. Pain. 2003;105:11-16.
- Yesilyurt O, Dogrul A, Gul H, et al. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003;105:303-308.
- Phan NQ, Siepmann D, Gralow I, et al. Adjuvant topical therapy with a cannabinoid receptor agonist in facial postherpetic neuralgia. J Dtsch Dermatol Ges. 2010;8:88-91.
The prevalence of topical cannabinoids has risen sharply in recent years. Commercial advertisers promote their usage as a safe means to treat a multitude of skin disorders, including atopic dermatitis (AD), psoriasis, and acne. Topical compounds have garnered interest in laboratory studies, but the purchase of commercial formulations is limited to over-the-counter products from unregulated suppliers. In this article, we review the scientific evidence behind topical cannabinoids and evaluate their role in clinical dermatology.
Background
Cannabis is designated as a Schedule I drug, according to the Controlled Substances Act of 1970. This listing is given to substances with no therapeutic value and a high potential for abuse. However, as of 2017, 29 states and the District of Columbia have laws legalizing cannabis in some capacity. These regulations typically apply to medicinal use, though several states have now legalized recreational use.
Cannabinoids represent a broad class of chemical compounds derived from the cannabis plant. Originally, this class only comprised phytocannabinoids, cannabinoids produced by the cannabis plant. Tetrahydrocannabinol (THC) is the most well-known phytocannabinoid and leads to the psychoactive effects typically associated with cannabis use. Later investigation led to the discovery of endocannabinoids, cannabinoids that are naturally produced by human and animal bodies, as well as synthetic cannabinoids.1 Cannabidiol is a phytocannabinoid that has been investigated in neurologic and anti-inflammatory conditions.2-4
Cannabinoids act as agonists on 2 principal receptors— cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2)—which are both G protein–coupled receptors (Figure).5 Both have distinct distributions throughout different organ systems, to which cannabinoids (eg, THC, cannabidiol, endocannabinoids) show differential binding.6,7 Importantly, the expression of CB1 and CB2 has been identified on sensory nerve fibers, inflammatory cells, and adnexal structures of human skin.8 Based on these associations, topical application of cannabinoids has become a modality of interest for dermatological disorders. These formulations aim to influence cutaneous morphology without producing psychoactive effects.

Topical Cannabinoids in Inflammatory Disorders
Atopic dermatitis has emerged as an active area of investigation for cannabinoid receptors and topical agonists (Table 1). In an animal model, Kim et al9 examined the effects of CB1 agonism on skin inflammation. Mice treated with topical CB1 agonists showed greater recovery of epidermal barrier function in acutely abrogated skin relative to those treated with a vehicle preparation. In addition, agonism of CB1 led to significant (P<.001) decreases in skin fold thickness among models of acute and chronic skin inflammation.9

Nam et al10 also examined the role of topical CB1 agonists in mice with induced AD-like symptoms. Relative to treatment with vehicle, CB1 agonists significantly reduced the recruitment of mast cells (P<.01) and lowered the blood concentration of histamine (P<.05). Given the noted decrease in the release of inflammatory mediators, the authors speculated that topical agonsim of CB1 may prove useful in several conditions related to mast cell activation, such as AD, contact dermatitis, and psoriasis.10
The anti-inflammatory properties of topical THC were evaluated by Gaffal et al.11 In a mouse model of allergic contact dermatitis, mice treated with topical THC showed decreases in myeloid immune cell infiltration, with these beneficial effects existing even in mice with deficient CB1 and CB2 receptors. These results support a potentially wide anti-inflammatory activity of topical THC.11
Topical Cannabinoids in Pain Management
The effects of smoked cannabis in treating pain have undergone thorough investigation over recent years. Benefits have been noted in treating neuropathic pain, particularly in human immunodeficiency virus–associated sensory neuropathy.12-15 Smoked cannabis also may provide value as a synergistic therapy with opioids, thereby allowing for lower opioid doses.16
In contrast, research into the relationship between topical application of cannabinoids and nociception remains in preliminary stages (Table 2). In a mouse model, Dogrul et al17 assessed the topical antinociceptive potential of a mixed CB1-CB2 agonist. Results showed significant (P<.01) and dose-dependent antinociceptive effects relative to treatment with a vehicle.17 In a related study, Yesilyurt et al18 evaluated whether a mixed CB1-CB2 agonist could enhance the antinociceptive effects of topical opioids. Among mice treated with the combination of a cannabinoid agonist and topical morphine, a significantly (P<.05) greater analgesic effect was demonstrated relative to topical morphine alone.18
Studies in humans have been far more limited. Phan et al19 conducted a small, nonrandomized, open-label trial of a topical cannabinoid cream in patients with facial postherpetic neuralgia. Of 8 patients treated, 5 noted a mean pain reduction of 87.8%. No comparison vehicle was used. Based on this narrow study design, it is difficult to extrapolate these positive results to a broader patient population.19

Commercial Products
Although preliminary models with topical cannabinoids have shown potential, large-scale clinical trials in humans have yet to be performed. Despite this lack of investigation, commercial formulations of topical cannabinoids are available to dermatology patients. These formulations are nonstandardized, and no safety data exists regarding their use. Topical cannabinoids on the market may contain various amounts of active ingredient and may be combined with a range of other compounds.
In dermatology offices, it is not uncommon for patients to express an intention to use topical cannabinoid products following their planned treatment or procedure. Patients also have been known to use topical cannabinoid products prior to dermatologic procedures, sometimes in place of an approved topical anesthetic, without consulting the physician performing the procedure. With interventions that lead to active areas of wound healing, the application of such products may increase the risk for contamination and infection. Therefore, patients should be counseled that the use of commercial topical cannabinoids could jeopardize the success of their planned procedure, put them at risk for infection, and possibly lead to systemic absorption and/or changes in wound-healing capacities.
Conclusion
Based on the results from recent animal models, cannabinoids may have a role in future treatment algorithms for several inflammatory conditions. However, current efficacy and safety data are almost entirely limited to preliminary animal studies in rodents. In addition, the formulation of topical cannabinoid products is nonstandardized and poorly regulated. As such, the present evidence does not support the use of topical cannabinoids in dermatology practices. Dermatologists should ask patients about the use of any cannabinoid products as part of a treatment program, especially given the unsubstantiated claims often made by unscrupulous advertisers. This issue highlights the need for further research and regulation.
The prevalence of topical cannabinoids has risen sharply in recent years. Commercial advertisers promote their usage as a safe means to treat a multitude of skin disorders, including atopic dermatitis (AD), psoriasis, and acne. Topical compounds have garnered interest in laboratory studies, but the purchase of commercial formulations is limited to over-the-counter products from unregulated suppliers. In this article, we review the scientific evidence behind topical cannabinoids and evaluate their role in clinical dermatology.
Background
Cannabis is designated as a Schedule I drug, according to the Controlled Substances Act of 1970. This listing is given to substances with no therapeutic value and a high potential for abuse. However, as of 2017, 29 states and the District of Columbia have laws legalizing cannabis in some capacity. These regulations typically apply to medicinal use, though several states have now legalized recreational use.
Cannabinoids represent a broad class of chemical compounds derived from the cannabis plant. Originally, this class only comprised phytocannabinoids, cannabinoids produced by the cannabis plant. Tetrahydrocannabinol (THC) is the most well-known phytocannabinoid and leads to the psychoactive effects typically associated with cannabis use. Later investigation led to the discovery of endocannabinoids, cannabinoids that are naturally produced by human and animal bodies, as well as synthetic cannabinoids.1 Cannabidiol is a phytocannabinoid that has been investigated in neurologic and anti-inflammatory conditions.2-4
Cannabinoids act as agonists on 2 principal receptors— cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2)—which are both G protein–coupled receptors (Figure).5 Both have distinct distributions throughout different organ systems, to which cannabinoids (eg, THC, cannabidiol, endocannabinoids) show differential binding.6,7 Importantly, the expression of CB1 and CB2 has been identified on sensory nerve fibers, inflammatory cells, and adnexal structures of human skin.8 Based on these associations, topical application of cannabinoids has become a modality of interest for dermatological disorders. These formulations aim to influence cutaneous morphology without producing psychoactive effects.

Topical Cannabinoids in Inflammatory Disorders
Atopic dermatitis has emerged as an active area of investigation for cannabinoid receptors and topical agonists (Table 1). In an animal model, Kim et al9 examined the effects of CB1 agonism on skin inflammation. Mice treated with topical CB1 agonists showed greater recovery of epidermal barrier function in acutely abrogated skin relative to those treated with a vehicle preparation. In addition, agonism of CB1 led to significant (P<.001) decreases in skin fold thickness among models of acute and chronic skin inflammation.9

Nam et al10 also examined the role of topical CB1 agonists in mice with induced AD-like symptoms. Relative to treatment with vehicle, CB1 agonists significantly reduced the recruitment of mast cells (P<.01) and lowered the blood concentration of histamine (P<.05). Given the noted decrease in the release of inflammatory mediators, the authors speculated that topical agonsim of CB1 may prove useful in several conditions related to mast cell activation, such as AD, contact dermatitis, and psoriasis.10
The anti-inflammatory properties of topical THC were evaluated by Gaffal et al.11 In a mouse model of allergic contact dermatitis, mice treated with topical THC showed decreases in myeloid immune cell infiltration, with these beneficial effects existing even in mice with deficient CB1 and CB2 receptors. These results support a potentially wide anti-inflammatory activity of topical THC.11
Topical Cannabinoids in Pain Management
The effects of smoked cannabis in treating pain have undergone thorough investigation over recent years. Benefits have been noted in treating neuropathic pain, particularly in human immunodeficiency virus–associated sensory neuropathy.12-15 Smoked cannabis also may provide value as a synergistic therapy with opioids, thereby allowing for lower opioid doses.16
In contrast, research into the relationship between topical application of cannabinoids and nociception remains in preliminary stages (Table 2). In a mouse model, Dogrul et al17 assessed the topical antinociceptive potential of a mixed CB1-CB2 agonist. Results showed significant (P<.01) and dose-dependent antinociceptive effects relative to treatment with a vehicle.17 In a related study, Yesilyurt et al18 evaluated whether a mixed CB1-CB2 agonist could enhance the antinociceptive effects of topical opioids. Among mice treated with the combination of a cannabinoid agonist and topical morphine, a significantly (P<.05) greater analgesic effect was demonstrated relative to topical morphine alone.18
Studies in humans have been far more limited. Phan et al19 conducted a small, nonrandomized, open-label trial of a topical cannabinoid cream in patients with facial postherpetic neuralgia. Of 8 patients treated, 5 noted a mean pain reduction of 87.8%. No comparison vehicle was used. Based on this narrow study design, it is difficult to extrapolate these positive results to a broader patient population.19

Commercial Products
Although preliminary models with topical cannabinoids have shown potential, large-scale clinical trials in humans have yet to be performed. Despite this lack of investigation, commercial formulations of topical cannabinoids are available to dermatology patients. These formulations are nonstandardized, and no safety data exists regarding their use. Topical cannabinoids on the market may contain various amounts of active ingredient and may be combined with a range of other compounds.
In dermatology offices, it is not uncommon for patients to express an intention to use topical cannabinoid products following their planned treatment or procedure. Patients also have been known to use topical cannabinoid products prior to dermatologic procedures, sometimes in place of an approved topical anesthetic, without consulting the physician performing the procedure. With interventions that lead to active areas of wound healing, the application of such products may increase the risk for contamination and infection. Therefore, patients should be counseled that the use of commercial topical cannabinoids could jeopardize the success of their planned procedure, put them at risk for infection, and possibly lead to systemic absorption and/or changes in wound-healing capacities.
Conclusion
Based on the results from recent animal models, cannabinoids may have a role in future treatment algorithms for several inflammatory conditions. However, current efficacy and safety data are almost entirely limited to preliminary animal studies in rodents. In addition, the formulation of topical cannabinoid products is nonstandardized and poorly regulated. As such, the present evidence does not support the use of topical cannabinoids in dermatology practices. Dermatologists should ask patients about the use of any cannabinoid products as part of a treatment program, especially given the unsubstantiated claims often made by unscrupulous advertisers. This issue highlights the need for further research and regulation.
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389-462.
- Giacoppo S, Galuppo M, Pollastro F, et al. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. Daru. 2015;23:48.
- Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936-948.
- Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology. 2012;89:149-155.
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161-202.
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
- Svizenska I, Dubovy P, Sulcova A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav. 2008;90:501-511.
- Stander S, Schmelz M, Metze D, et al. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005;38:177-188.
- Kim HJ, Kim B, Park BM, et al. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int J Dermatol. 2015;54:E401-E408.
- Nam G, Jeong SK, Park BM, et al. Selective cannabinoid receptor-1 agonists regulate mast cell activation in an oxazolone-induced atopic dermatitis model. Ann Dermatol. 2016;28:22-29.
- Gaffal E, Cron M, Glodde N, et al. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy. 2013;68:994-1000.
- Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515-521.
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672-680.
- Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136-148.
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506-521.
- Abrams DI, Couey P, Shade SB, et al. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844-851.
- Dogrul A, Gul H, Akar A, et al. Topical cannabinoid antinociception: synergy with spinal sites. Pain. 2003;105:11-16.
- Yesilyurt O, Dogrul A, Gul H, et al. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003;105:303-308.
- Phan NQ, Siepmann D, Gralow I, et al. Adjuvant topical therapy with a cannabinoid receptor agonist in facial postherpetic neuralgia. J Dtsch Dermatol Ges. 2010;8:88-91.
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389-462.
- Giacoppo S, Galuppo M, Pollastro F, et al. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. Daru. 2015;23:48.
- Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936-948.
- Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology. 2012;89:149-155.
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161-202.
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
- Svizenska I, Dubovy P, Sulcova A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav. 2008;90:501-511.
- Stander S, Schmelz M, Metze D, et al. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005;38:177-188.
- Kim HJ, Kim B, Park BM, et al. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int J Dermatol. 2015;54:E401-E408.
- Nam G, Jeong SK, Park BM, et al. Selective cannabinoid receptor-1 agonists regulate mast cell activation in an oxazolone-induced atopic dermatitis model. Ann Dermatol. 2016;28:22-29.
- Gaffal E, Cron M, Glodde N, et al. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy. 2013;68:994-1000.
- Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515-521.
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672-680.
- Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136-148.
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506-521.
- Abrams DI, Couey P, Shade SB, et al. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844-851.
- Dogrul A, Gul H, Akar A, et al. Topical cannabinoid antinociception: synergy with spinal sites. Pain. 2003;105:11-16.
- Yesilyurt O, Dogrul A, Gul H, et al. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003;105:303-308.
- Phan NQ, Siepmann D, Gralow I, et al. Adjuvant topical therapy with a cannabinoid receptor agonist in facial postherpetic neuralgia. J Dtsch Dermatol Ges. 2010;8:88-91.
Practice Points
- Topical cannabinoids are advertised by companies as treatment options for numerous dermatologic conditions.
- Despite promising data in rodent models, there have been no rigorous studies to date confirming efficacy or safety in humans.
- Dermatologists should therefore inquire with patients about the use of any topical cannabinoid products, especially around the time of planned procedures, as they may affect treatment outcomes.
Trauma Care: The “Golden Hour” Meets the “Golden Years”
In Emergency Medicine this month and next, Drs. Tom Scalea (See “The Golden Hourglass,” EM, April 2007), Ashley Menne, Daniel Haase, and Jay Menaker of the University of Maryland’s R Adams Cowley Shock Trauma Center paint a detailed picture of the changing landscape of trauma care over the past two decades.
In his introduction, Dr. Scalea writes “Certainly, the most important change has been the ‘graying’ of trauma patients…[whose evaluation and care] may involve a number of diagnostic tests in the ED…”, and whose care must include dealing with comorbidities, and a large number of medications that might interact with the analgesics, sedatives, and anti-seizure meds needed to treat trauma. These considerations have led many Level I trauma centers to add advanced patient age as an independent determinant for both trauma activations and subsequent ICU admissions, and to include “geriatric” consultants in the initial management.
The aging trauma patient, however, is not the only factor responsible for major changes in the management of serious trauma, as the Shock Trauma group describes the current difficulties in attempting to rapidly reverse the anticoagulation effects of the novel oral anticoagulants (NOACs) that are increasingly being prescribed instead of warfarin to manage the thromboembolic complications of atrial fibrillation, valve replacement, venous thrombosis, and pulmonary embolism in both younger and older patients. They also explain a major change in thinking regarding the optimal degree of blood pressure control in favor of “permissive hypotension” as part of “damage control resuscitation,” and in the amount and types of volume replacement, optimal blood component ratios for transfusion, monitoring, and faster and less invasive endovascular repair techniques for hemostasis. The authors also note the persistent and rising incidence of penetrating trauma from gunshot and knife wounds.
But the increasing percentages of elderly trauma victims requiring care for devastating falls and low-speed vehicular injuries in even the busiest “knife and gun club” trauma centers mandate the attention of all health care providers. In recent months, much space in this and other journals has been devoted to the health care issues of the elderly (see “Recognizing and Managing Elder Abuse in the Emergency Department,” and “Elder Abuse: A New Old Problem,” EM, May 2017) that necessitate significantly increased resources and provider time and effort now, and for at least the first half of the 21st century.
The main reason for this seismic demographic shift, dubbed by some “the silver tsunami”, is the aging post World War II “baby boomer” generation that has commanded center stage in western society throughout their development since the late 1940s. As a member of that generation, I often wonder how subsequent generations such as “Gen X” and “Millennials” view this phenomenon. Do they resent the attention, resources, and expenditures now demanded by baby boomers? If so, there is an important lesson to be learned from the changes in trauma care described in the following pages: virtually every measure now employed to enhance recovery of an elderly trauma victim will benefit younger trauma victims, as well. At most, some of the measures may not be absolutely necessary because younger adults have greater functional reserve and are more likely to survive less precise management, even if their posttraumatic courses are longer and more difficult. But younger trauma victims with comorbidities can also benefit from a more inclusive team approach from the start, as well as measures such as permissive hypotension, vascular stents and less invasive endovascular approaches, more precise blood component replacement, more accurate monitoring, and a better approach to anticoagulation and its reversal.
Faster and better quality survival of all trauma victims—including, but not limited to, the elderly—will free up needed and expensive resources for other patients and trauma victims, including those who continue to butt heads, drive two and four wheel vehicles at excessive speeds, and engage in trauma of an “interpersonal nature.”
In Emergency Medicine this month and next, Drs. Tom Scalea (See “The Golden Hourglass,” EM, April 2007), Ashley Menne, Daniel Haase, and Jay Menaker of the University of Maryland’s R Adams Cowley Shock Trauma Center paint a detailed picture of the changing landscape of trauma care over the past two decades.
In his introduction, Dr. Scalea writes “Certainly, the most important change has been the ‘graying’ of trauma patients…[whose evaluation and care] may involve a number of diagnostic tests in the ED…”, and whose care must include dealing with comorbidities, and a large number of medications that might interact with the analgesics, sedatives, and anti-seizure meds needed to treat trauma. These considerations have led many Level I trauma centers to add advanced patient age as an independent determinant for both trauma activations and subsequent ICU admissions, and to include “geriatric” consultants in the initial management.
The aging trauma patient, however, is not the only factor responsible for major changes in the management of serious trauma, as the Shock Trauma group describes the current difficulties in attempting to rapidly reverse the anticoagulation effects of the novel oral anticoagulants (NOACs) that are increasingly being prescribed instead of warfarin to manage the thromboembolic complications of atrial fibrillation, valve replacement, venous thrombosis, and pulmonary embolism in both younger and older patients. They also explain a major change in thinking regarding the optimal degree of blood pressure control in favor of “permissive hypotension” as part of “damage control resuscitation,” and in the amount and types of volume replacement, optimal blood component ratios for transfusion, monitoring, and faster and less invasive endovascular repair techniques for hemostasis. The authors also note the persistent and rising incidence of penetrating trauma from gunshot and knife wounds.
But the increasing percentages of elderly trauma victims requiring care for devastating falls and low-speed vehicular injuries in even the busiest “knife and gun club” trauma centers mandate the attention of all health care providers. In recent months, much space in this and other journals has been devoted to the health care issues of the elderly (see “Recognizing and Managing Elder Abuse in the Emergency Department,” and “Elder Abuse: A New Old Problem,” EM, May 2017) that necessitate significantly increased resources and provider time and effort now, and for at least the first half of the 21st century.
The main reason for this seismic demographic shift, dubbed by some “the silver tsunami”, is the aging post World War II “baby boomer” generation that has commanded center stage in western society throughout their development since the late 1940s. As a member of that generation, I often wonder how subsequent generations such as “Gen X” and “Millennials” view this phenomenon. Do they resent the attention, resources, and expenditures now demanded by baby boomers? If so, there is an important lesson to be learned from the changes in trauma care described in the following pages: virtually every measure now employed to enhance recovery of an elderly trauma victim will benefit younger trauma victims, as well. At most, some of the measures may not be absolutely necessary because younger adults have greater functional reserve and are more likely to survive less precise management, even if their posttraumatic courses are longer and more difficult. But younger trauma victims with comorbidities can also benefit from a more inclusive team approach from the start, as well as measures such as permissive hypotension, vascular stents and less invasive endovascular approaches, more precise blood component replacement, more accurate monitoring, and a better approach to anticoagulation and its reversal.
Faster and better quality survival of all trauma victims—including, but not limited to, the elderly—will free up needed and expensive resources for other patients and trauma victims, including those who continue to butt heads, drive two and four wheel vehicles at excessive speeds, and engage in trauma of an “interpersonal nature.”
In Emergency Medicine this month and next, Drs. Tom Scalea (See “The Golden Hourglass,” EM, April 2007), Ashley Menne, Daniel Haase, and Jay Menaker of the University of Maryland’s R Adams Cowley Shock Trauma Center paint a detailed picture of the changing landscape of trauma care over the past two decades.
In his introduction, Dr. Scalea writes “Certainly, the most important change has been the ‘graying’ of trauma patients…[whose evaluation and care] may involve a number of diagnostic tests in the ED…”, and whose care must include dealing with comorbidities, and a large number of medications that might interact with the analgesics, sedatives, and anti-seizure meds needed to treat trauma. These considerations have led many Level I trauma centers to add advanced patient age as an independent determinant for both trauma activations and subsequent ICU admissions, and to include “geriatric” consultants in the initial management.
The aging trauma patient, however, is not the only factor responsible for major changes in the management of serious trauma, as the Shock Trauma group describes the current difficulties in attempting to rapidly reverse the anticoagulation effects of the novel oral anticoagulants (NOACs) that are increasingly being prescribed instead of warfarin to manage the thromboembolic complications of atrial fibrillation, valve replacement, venous thrombosis, and pulmonary embolism in both younger and older patients. They also explain a major change in thinking regarding the optimal degree of blood pressure control in favor of “permissive hypotension” as part of “damage control resuscitation,” and in the amount and types of volume replacement, optimal blood component ratios for transfusion, monitoring, and faster and less invasive endovascular repair techniques for hemostasis. The authors also note the persistent and rising incidence of penetrating trauma from gunshot and knife wounds.
But the increasing percentages of elderly trauma victims requiring care for devastating falls and low-speed vehicular injuries in even the busiest “knife and gun club” trauma centers mandate the attention of all health care providers. In recent months, much space in this and other journals has been devoted to the health care issues of the elderly (see “Recognizing and Managing Elder Abuse in the Emergency Department,” and “Elder Abuse: A New Old Problem,” EM, May 2017) that necessitate significantly increased resources and provider time and effort now, and for at least the first half of the 21st century.
The main reason for this seismic demographic shift, dubbed by some “the silver tsunami”, is the aging post World War II “baby boomer” generation that has commanded center stage in western society throughout their development since the late 1940s. As a member of that generation, I often wonder how subsequent generations such as “Gen X” and “Millennials” view this phenomenon. Do they resent the attention, resources, and expenditures now demanded by baby boomers? If so, there is an important lesson to be learned from the changes in trauma care described in the following pages: virtually every measure now employed to enhance recovery of an elderly trauma victim will benefit younger trauma victims, as well. At most, some of the measures may not be absolutely necessary because younger adults have greater functional reserve and are more likely to survive less precise management, even if their posttraumatic courses are longer and more difficult. But younger trauma victims with comorbidities can also benefit from a more inclusive team approach from the start, as well as measures such as permissive hypotension, vascular stents and less invasive endovascular approaches, more precise blood component replacement, more accurate monitoring, and a better approach to anticoagulation and its reversal.
Faster and better quality survival of all trauma victims—including, but not limited to, the elderly—will free up needed and expensive resources for other patients and trauma victims, including those who continue to butt heads, drive two and four wheel vehicles at excessive speeds, and engage in trauma of an “interpersonal nature.”
Comparison of Salicylic Acid 30% Peel and Pneumatic Broadband Light in the Treatment of Mild to Moderately Severe Facial Acne Vulgaris
Facial acne vulgaris is a common skin disease among teenagers and adolescents that may negatively affect self-esteem, perceived facial attractiveness, and social participation.1 Treatments for acne often are multimodal and require the utmost adherence. For these reasons, acne treatments have been challenging to clinicians and patients alike, as patient compliance in maintaining the use of prescribed topical and oral medications remains essential to attain improvement in quality of life (QOL).
Salicylic acid is a popular medicament for acne treatment that frequently is used as monotherapy or as an adjuvant for other acne treatments, especially in patients with oily skin.2 Salicylic acid has a keratolytic effect, causing corneocyte discohesion in clogged pores or congested follicles,2 and it is effective in treating both inflammatory and noninflammatory acne.3,4
Light therapy, particularly with visible light, has been demonstrated to improve acne outcomes.5 Pneumatic broadband light (PBBL) is a therapeutic light treatment in the broadband range (400–1200 nm) that is combined with vacuum suction, which creates a mechanical lysis of thin-walled pustules and dislodges pore impaction. Additionally, the blue light portion of the PBBL spectrum targets endogenous porphyrins in Propionibacterium acnes, resulting in bacterial destruction.6-8
The purpose of this study was to compare the efficacy, tolerability, and safety of salicylic acid 30% peel versus PBBL in the treatment of mild to moderately severe facial acne vulgaris.
METHODS
Study Design
This single-blind, randomized, split-face pilot study was approved by the institutional review board of the University of Pennsylvania (Philadelphia, Pennsylvania). All patients provided informed consent before entering the study. The single-blind evaluation was performed by one dermatologist (C.T.) who examined the participants on every visit prior to PBBL treatment.
Before the study started, participants were randomized for which side of the face was to be treated with PBBL using a number assigned to each participant. Participants received both treatments—salicylic acid 30% peel on one side of the face and PBBL treatment on the other side of the face—once weekly for a total of 6 treatments. They were then asked to return for 2 follow-up evaluations at weeks 3 and 6 following the last treatment session and were instructed not to use any topical or oral acne medications during these follow-up periods.
Inclusion and Exclusion Criteria
Patients aged 18 years and older of any race and sex with noninflammatory papules, some inflammatory papules, and no more than 1 nodule (considered as mild to moderately severe facial acne) were included in the study. Participants had not been on any topical acne medications for at least 1 month and/or oral retinoids for at least 1 year prior to the study period. All women completed urine pregnancy tests prior to the study and were advised to utilize birth control during the study period.
Study Treatments
Salicylic Acid 30% Peel
The participant’s face was cleansed thoroughly before application of salicylic acid 30% (1.5 g/2.5 mL) to half of the face and left on for 5 minutes before being carefully rinsed off by spraying with spring water. Prior to initiating PBBL therapy, the peeled side of the participant’s face was covered with a towel.
Pneumatic Broadband Light
On the other side of the face, PBBL was performed to deliver broadband light within the spectrum range of 400 to 1200 nm at a setting approximately equivalent to a fluence of 4 to 6 J/cm2 and a vacuum setting approximately equivalent to a negative pressure of 3 lb/in2. The power setting was increased on each subsequent visit depending on each participant’s tolerability.
Participants were required to apply a moisturizer and sunscreen to the face and avoid excessive sun exposure between study visits.
Efficacy Evaluation
A comparison of the efficacy of the treatments was determined by clinical evaluation and examining the results of the outcome measurements with the modified Global Acne Grading Score (mGAGS) and Acne QOL Scale during each treatment visit. Facial photographs were taken at each visit.
Modified Global Acne Grading Score
The mGAGS is a modification of the Global Acne Grading Scale (GAGS) that has been used to evaluate acne severity in many studies.9-11 The GAGS considers 6 locations on the face with a grading factor for each location. The local score is obtained by multiplying the factor rated by location with the factor of clinical assessment: local score = factor rated by location × factor rated by clinical assessment. The total score is the sum of the individual local scores (Table 1).

Although the original GAGS incorporated the type and location of the lesions in its calculation, we felt that the number of lesions also was important to add to our grading score. Therefore, we modified the GAGS by adding a factor rated by the number of lesions to improve the accuracy of the test. Accordingly, the local mGAGS scores were calculated by multiplying the location factor by the lesion type and number of lesions factors: local score = location factor × lesion type factor × number of lesions factor.
Acne QOL Questionnaire
Acne QOL was assessed during each visit to demonstrate if the treatment results affected participants’ socialization due to appearance.12 Participants were asked to complete the questionnaire, which consisted of 9 questions with 4 rating answers (0=not affected; 1=mildly affected; 2=moderately affected; 3=markedly affected). A total score of 9 or higher (high score) indicated that acne had a substantial negative impact on the participant, while a total score below 9 (low score) meant acne scarcely impacted social aspects and daily activities of the patient.
Safety Evaluation
The safety of the treatments was evaluated by clinical inspection and by comparing the results of the Wong-Baker FACES Pain Rating Scale (WBPRS)13 after treatment. The WBPRS is used worldwide among researchers to assess pain, particularly in children.14,15 It is composed of 6 faces expressing pain with word descriptions with a corresponding number range reflecting pain severity from 0 to 5 (0=no hurt; 1=hurts little bit; 2=hurts little more; 3=hurts even more; 4=hurts whole lot; 5=hurts worst).13
Statistical Analysis
All variables were presented as the median (range). A Wilcoxon signed rank test was used to compare clinical responses between the salicylic acid 30% peel and PBBL therapies. SPSS software version 12.0 was used for all statistical analysis. A 2-tailed P value of ≤.05 was considered statistically significant.
RESULTS
Study Population
Twelve participants (2 males, 10 females) aged 17 to 36 years (median age, 22 years; mean age [SD], 23.33 [1.65] years) with both comedonal and inflammatory acne were enrolled into this study for 6 split-face treatments of salicylic acid 30% peel and PBBL at 1-week intervals for 6 weeks, with 2 subsequent follow-up sessions at weeks 3 and 6 posttreatment. Of the 12 participants, 11 were white and 1 was Asian American, with Fitzpatrick skin types II to IV. Nine participants (75%) completed the study. One participant dropped out of the study after the fourth treatment due to a scheduling conflict, and the other 2 participants did not return for follow-up. No participants withdrew from the study because of adverse therapeutic events.
Efficacy Evaluation
Comparisons between the salicylic acid 30% peel and PBBL procedures for mGAGS at each visit are shown in Table 2. There was no significant difference in treatment efficacy between the salicylic acid 30% peel and PBBL therapies during the study’s treatment and follow-up events; however, both procedures contributed to a major improvement in acne symptoms by the third treatment session and through to the last follow-up session (P≤.05). Clinical photographs at baseline, at last treatment visit (week 6), and at last follow-up (week 12) are shown in Figures 1 and 2.
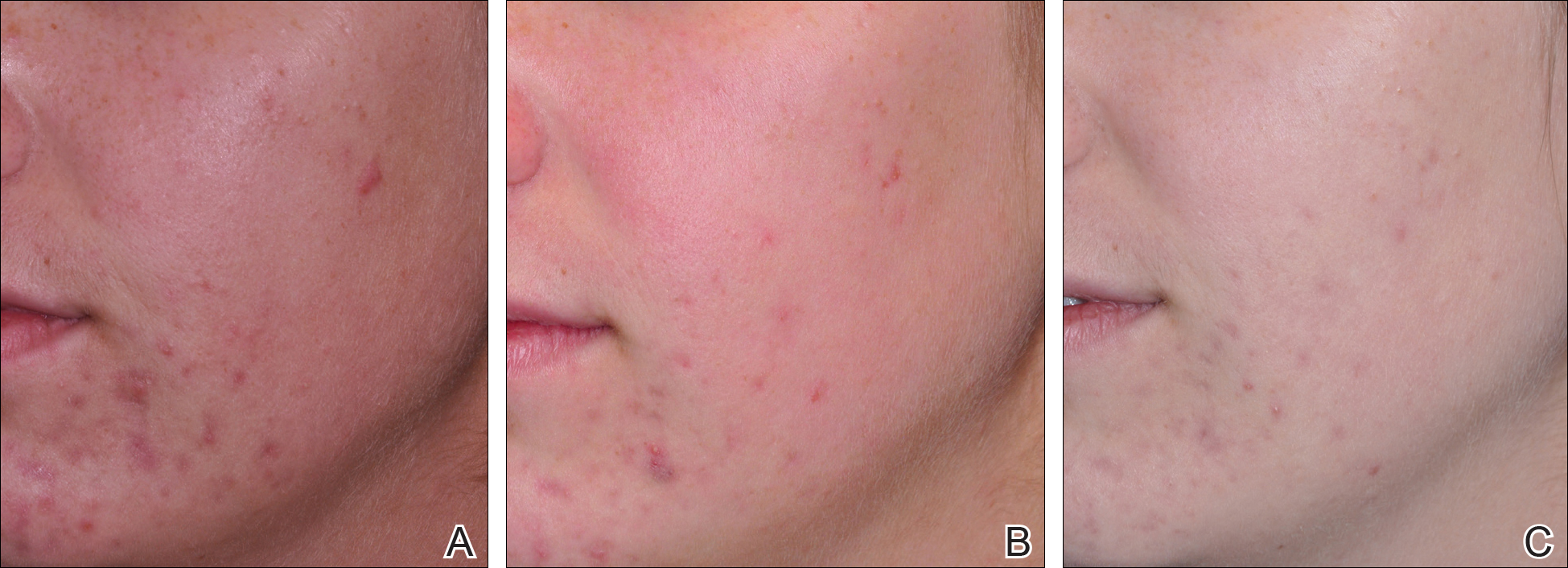
The results of the acne QOL questionnaire are shown in Table 2. Lower scores reflect a higher QOL. Median QOL scores at each visit ranged from 0.5 to 4.5. There was no significant difference found between the peel agent or PBBL based on the baseline QOL and subsequent visit assessments; however, the differences between the 2 treatments were significant at weeks 3 (P=.05) and 5 (P=.03) of treatment as well as at the last follow-up visit (P=.05).
According to the QOL scores, by the third treatment session participants were more satisfied with their improved acne condition from the PBBL procedure than the salicylic acid 30% peel as demonstrated by a positive range of the QOL assessments between PBBL and salicylic acid 30% peel (as shown in the difference in QOL in Table 2: week 3, 0–6; week 4, 0–3; week 5, 0–7). On the other hand, participants saw more improvement from the salicylic acid 30% peel than from PBBL by the last follow-up evaluation, as the differences in QOL scores between the 2 treatments resulted in a negative range (−5–0).
Safety
Pain assessment by the WBPRS at every visit showed a low pain rating associated with both salicylic acid 30% peel (range, 0–0.5) and PBBL (range, 1.0–1.5) treatments. The median pain score of the salicylic acid 30% peel appeared higher compared to the PBBL treatment, yet a significant difference between both treatments was seen only at weeks 1, 3, and 6 of treatment (P≤.05).
There were no unexpected therapeutic reactions reported in our study, and no participants withdrew from the study due to adverse events. Most participants experienced only mild adverse reactions, including redness, stinging, and a burning sensation on the salicylic acid 30% peel side, which were transient and disappeared in minutes; only redness occurred on the PBBL-treated side.
Comment
Facial acne treatment is challenging, as prolonged and/or severe acne contributes to scarring, declining self-confidence, and undesirable financial consequences. Even though salicylic acid peel is a commonly used acne treatment choice, the PBBL methodology was approved by the US Food and Drug Administration6 and has become an alternative procedure for acne treatment.
The pharmacological effects of salicylic acid are related to its corneocyte desquamation and exfoliative actions, thereby reducing corneocyte cohesion and unclogging follicular pores.16 Salicylic acid has been demonstrated to ameliorate inflammatory acne by its effects on the arachidonic acid cascade.2,4,17 In our study, salicylic acid 30% peel met participants’ satisfaction in acne improvement similar to a study showing a 50% improvement in acne scores after just 2 treatments.18 Our data support and corroborate that salicylic acid 30% peel renders an improvement in acne sequelae reported in several other studies.2,17,18
Pneumatic broadband light has been known to treat acne by the mechanism of pneumatic suction combined with photodynamic therapy using broadband-pulsed light (400–1200 nm).6-8 By applying the pneumatic device, a vacuum is created on the skin to remove sebum contents from follicles, whereas broadband light is emitted simultaneously to destroy bacteria and decrease the inflammatory process.7 During the vacuum process, the skin is stretched to reduce pain and avoid competitive chromophores (eg, hemoglobin), while the broadband light is administered.7 Broadband light encompasses 2 main light spectrums: blue light (415 nm) activates coproporphyrin III, which induces reactive free radicals and singlet oxygen species and has been reported to be the cause of bacterial cell death,19 and red light (633 nm), which renders an increase of fibroblast growth factors to work against the inflammatory processes.20 There are numerous studies showing a reduction of acne lesions after photopneumatic therapy with minimal side effects.6-8
In our study, we compared the efficacy of salicylic acid 30% peel with PBBL in the treatment of acne. Both treatments showed significant reduction of mGAGS compared to baseline starting from week 3 and lasting until week 12. Remarkably, although there were some participants who reported acne recurrence after completing all treatments at week 6, which could have happened when the treatments were ended, the final acne score at week 12 was still significantly lower than baseline. It is clear that the participants continued their acne improvement up to the 6-week follow-up period without any topical or oral medication. We do not propose that either salicylic acid peel or PBBL treatment is a solitary option but speculate that the combination of both treatments may initiate a faster resolution in the disappearance of acne.
Although there was no statistically significant difference in efficacy between salicylic acid 30% peel and PBBL procedures at each visit, QOL assessments related to treatment satisfaction did yield significant differences between baseline and the end of treatment. We noticed that participants had more positive attitudes toward the PBBL side at week 3 and week 5 but only mild satisfaction at week 4, as the differences in QOL scores between both treatments showed positive ranging values. This finding is most likely related to the immediate reduction of acne pustules by the PBBL vacuum lysis of these lesions. The differences in the QOL scores between both treatments at week 12 (the last follow-up evaluation) provided opposite findings, which meant patients had nearly even improvement in both PBBL method and salicylic acid 30% peel. Therefore, according to QOL data, acne disappeared quickly with the application of PBBL therapy but reappeared on the PBBL-treated side by the follow-up evaluations, though the acne score between both sides showed no statistically significant difference.
We reason that the PBBL therapy works better than salicylic acid 30% peel because the pneumatic system may help to unclog the pores through mechanical debridement via suctioning versus desquamation from salicylic acid 30% peel. Nonetheless, salicylic acid 30% peel sustained improvement when compared to PBBL through the follow-up periods. Both salicylic acid 30% peel and PBBL treatments are well tolerated and may initiate a faster resolution in the improvement of acne when incorporated with a medical program.
Because of the recurrence of acne after treatments were stopped, additional medical therapies are advised to be used along with this study’s clinical treatments to help mitigate the acne symptoms. These treatments should be considered in patients concerned about antibiotic resistance or those who cannot take oral antibiotics or retinoids. Salicylic acid peel is more accessible and affordable than PBBL, whereas PBBL is slightly more tolerable and less irritating than salicylic acid peel. Nevertheless, the cost of investment in PBBL is quite high—as much as $70,000—and does not include disposable, single-use tips, which cost $30 each. The machine is easy to set up, weighs about 40 lb, and requires little space to store. The average cost per visit of PBBL treatment in office is $150.00 and $75.00 for salicylic acid peel (unpublished data, Hospital of the University of Pennsylvania, 2010). Most patients may select salicylic acid peel over PBBL due to the cost and convenience of the treatment. Neither procedure should be considered as a solitary treatment option but rather as adjunctive procedures combined with oral and/or topical acne medications. After this study’s treatments were stopped and without other medications to maintain treatment effectiveness, the lesions reappeared, trending back toward baseline.
Conclusion
Both salicylic acid 30% peel and PBBL procedures are effective, safe, and well tolerated in treating acne. Although there was no significant difference in the efficacy between both treatments in this study, the small sample size and short follow-up intervals warrant further studies to support the observed outstanding outcomes and should be considered in combination with other medical treatment options. These procedures may be beneficial in holding the patient compliant until their medical therapies have an opportunity to work.
Acknowledgment
The authors would like to thank Joyce Okawa, RN (Philadelphia, Pennsylvania), for her assistance in the submission to the institutional review board of the University of Pennsylvania.
- Rapp DA, Brenes GA, Feldman SR, et al. Anger and acne: implications for quality of life, patient satisfaction and clinical care. Br J Dermatol. 2004;151:183-189.
- Zakopoulou N, Kontochristopoulos G. Superficial chemical peels. J Cosmet Dermatol. 2006;5:246-253.
- Berson DS, Cohen JL, Rendon MI, et al. Clinical role and application of superficial chemical peels in today’s practice. J Drugs Dermatol. 2009;8:803-811.
- Shalita AR. Treatment of mild and moderate acne vulgaris with salicylic acid in an alcohol-detergent vehicle. Cutis. 1981;28:556-558, 561.
- Sakamoto FH, Lopes JD, Anderson RR. Photodynamic therapy for acne vulgaris: a critical review from basics to clinical practice: part I. acne vulgaris: when and why consider photodynamic therapy? J Am Acad Dermatol. 2010;63:183-193; quiz 93-94.
- Gold MH, Biron J. Efficacy of a novel combination of pneumatic energy and broadband light for the treatment of acne. J Drugs Dermatol. 2008;7:639-642.
- Shamban AT, Enokibori M, Narurkar V, et al. Photopneumatic technology for the treatment of acne vulgaris. J Drugs Dermatol. 2008;7:139-145.
- Wanitphakdeedecha R, Tanzi EL, Alster TS. Photopneumatic therapy for the treatment of acne. J Drugs Dermatol. 2009;8:239-241.
- Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol. 1997;36:416-418.
- Weiss JW, Shavin J, Davis M. Preliminary results of a nonrandomized, multicenter, open-label study of patient satisfaction after treatment with combination benzoyl peroxide/clindamycin topical gel for mild to moderate acne. Clin Ther. 2002;24:1706-1717.
- Demircay Z, Kus S, Sur H. Predictive factors for acne flare during isotretinoin treatment. Eur J Dermatol. 2008;18:452-456.
- Gupta MA, Johnson AM, Gupta AK. The development of an Acne Quality of Life scale: reliability, validity, and relation to subjective acne severity in mild to moderate acne vulgaris. Acta Derm Venereol. 1998;78:451-456.
- Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14:9-17.
- Wong DL, Hockenberry-Eaton M, Wilson D, et al. Wong’s Essentials of Pediatric Nursing. 6th ed. St. Louis, MO: Mosby; 2001:1301.
- Zempsky WT, Robbins B, McKay K. Reduction of topical anesthetic onset time using ultrasound: a randomized controlled trial prior to venipuncture in young children. Pain Med. 2008;9:795-802.
- Imayama S, Ueda S, Isoda M. Histologic changes in the skin of hairless mice following peeling with salicylic acid. Arch Dermatol. 2000;136:1390-1395.
- Lee H, Kim I. Salicylic acid peels for the treatment of acne vulgaris in Asian patients. Dermatol Surg. 2003;29:1196-1199.
- Kessler E, Flanagan K, Chia C, et al. Comparison of alpha- and beta-hydroxy acid chemical peels in the treatment of mild to moderately severe facial acne vulgaris. Dermatol Surg. 2008;34:45-50.
- Omi T, Munavalli GS, Kawana S, et al. Ultrastructural evidencefor thermal injury to pilosebaceous units during the treatment of acne using photopneumatic (PPX) therapy. J Cosmet Laser Ther. 2008;10:7-11.
- Papageorgiou P, Katsambas A, Chu A. Phototherapy with blue (415 nm) and red (660 nm) light in the treatment of acne vulgaris. Br J Dermatol. 2000;142:973-978.
Facial acne vulgaris is a common skin disease among teenagers and adolescents that may negatively affect self-esteem, perceived facial attractiveness, and social participation.1 Treatments for acne often are multimodal and require the utmost adherence. For these reasons, acne treatments have been challenging to clinicians and patients alike, as patient compliance in maintaining the use of prescribed topical and oral medications remains essential to attain improvement in quality of life (QOL).
Salicylic acid is a popular medicament for acne treatment that frequently is used as monotherapy or as an adjuvant for other acne treatments, especially in patients with oily skin.2 Salicylic acid has a keratolytic effect, causing corneocyte discohesion in clogged pores or congested follicles,2 and it is effective in treating both inflammatory and noninflammatory acne.3,4
Light therapy, particularly with visible light, has been demonstrated to improve acne outcomes.5 Pneumatic broadband light (PBBL) is a therapeutic light treatment in the broadband range (400–1200 nm) that is combined with vacuum suction, which creates a mechanical lysis of thin-walled pustules and dislodges pore impaction. Additionally, the blue light portion of the PBBL spectrum targets endogenous porphyrins in Propionibacterium acnes, resulting in bacterial destruction.6-8
The purpose of this study was to compare the efficacy, tolerability, and safety of salicylic acid 30% peel versus PBBL in the treatment of mild to moderately severe facial acne vulgaris.
METHODS
Study Design
This single-blind, randomized, split-face pilot study was approved by the institutional review board of the University of Pennsylvania (Philadelphia, Pennsylvania). All patients provided informed consent before entering the study. The single-blind evaluation was performed by one dermatologist (C.T.) who examined the participants on every visit prior to PBBL treatment.
Before the study started, participants were randomized for which side of the face was to be treated with PBBL using a number assigned to each participant. Participants received both treatments—salicylic acid 30% peel on one side of the face and PBBL treatment on the other side of the face—once weekly for a total of 6 treatments. They were then asked to return for 2 follow-up evaluations at weeks 3 and 6 following the last treatment session and were instructed not to use any topical or oral acne medications during these follow-up periods.
Inclusion and Exclusion Criteria
Patients aged 18 years and older of any race and sex with noninflammatory papules, some inflammatory papules, and no more than 1 nodule (considered as mild to moderately severe facial acne) were included in the study. Participants had not been on any topical acne medications for at least 1 month and/or oral retinoids for at least 1 year prior to the study period. All women completed urine pregnancy tests prior to the study and were advised to utilize birth control during the study period.
Study Treatments
Salicylic Acid 30% Peel
The participant’s face was cleansed thoroughly before application of salicylic acid 30% (1.5 g/2.5 mL) to half of the face and left on for 5 minutes before being carefully rinsed off by spraying with spring water. Prior to initiating PBBL therapy, the peeled side of the participant’s face was covered with a towel.
Pneumatic Broadband Light
On the other side of the face, PBBL was performed to deliver broadband light within the spectrum range of 400 to 1200 nm at a setting approximately equivalent to a fluence of 4 to 6 J/cm2 and a vacuum setting approximately equivalent to a negative pressure of 3 lb/in2. The power setting was increased on each subsequent visit depending on each participant’s tolerability.
Participants were required to apply a moisturizer and sunscreen to the face and avoid excessive sun exposure between study visits.
Efficacy Evaluation
A comparison of the efficacy of the treatments was determined by clinical evaluation and examining the results of the outcome measurements with the modified Global Acne Grading Score (mGAGS) and Acne QOL Scale during each treatment visit. Facial photographs were taken at each visit.
Modified Global Acne Grading Score
The mGAGS is a modification of the Global Acne Grading Scale (GAGS) that has been used to evaluate acne severity in many studies.9-11 The GAGS considers 6 locations on the face with a grading factor for each location. The local score is obtained by multiplying the factor rated by location with the factor of clinical assessment: local score = factor rated by location × factor rated by clinical assessment. The total score is the sum of the individual local scores (Table 1).

Although the original GAGS incorporated the type and location of the lesions in its calculation, we felt that the number of lesions also was important to add to our grading score. Therefore, we modified the GAGS by adding a factor rated by the number of lesions to improve the accuracy of the test. Accordingly, the local mGAGS scores were calculated by multiplying the location factor by the lesion type and number of lesions factors: local score = location factor × lesion type factor × number of lesions factor.
Acne QOL Questionnaire
Acne QOL was assessed during each visit to demonstrate if the treatment results affected participants’ socialization due to appearance.12 Participants were asked to complete the questionnaire, which consisted of 9 questions with 4 rating answers (0=not affected; 1=mildly affected; 2=moderately affected; 3=markedly affected). A total score of 9 or higher (high score) indicated that acne had a substantial negative impact on the participant, while a total score below 9 (low score) meant acne scarcely impacted social aspects and daily activities of the patient.
Safety Evaluation
The safety of the treatments was evaluated by clinical inspection and by comparing the results of the Wong-Baker FACES Pain Rating Scale (WBPRS)13 after treatment. The WBPRS is used worldwide among researchers to assess pain, particularly in children.14,15 It is composed of 6 faces expressing pain with word descriptions with a corresponding number range reflecting pain severity from 0 to 5 (0=no hurt; 1=hurts little bit; 2=hurts little more; 3=hurts even more; 4=hurts whole lot; 5=hurts worst).13
Statistical Analysis
All variables were presented as the median (range). A Wilcoxon signed rank test was used to compare clinical responses between the salicylic acid 30% peel and PBBL therapies. SPSS software version 12.0 was used for all statistical analysis. A 2-tailed P value of ≤.05 was considered statistically significant.
RESULTS
Study Population
Twelve participants (2 males, 10 females) aged 17 to 36 years (median age, 22 years; mean age [SD], 23.33 [1.65] years) with both comedonal and inflammatory acne were enrolled into this study for 6 split-face treatments of salicylic acid 30% peel and PBBL at 1-week intervals for 6 weeks, with 2 subsequent follow-up sessions at weeks 3 and 6 posttreatment. Of the 12 participants, 11 were white and 1 was Asian American, with Fitzpatrick skin types II to IV. Nine participants (75%) completed the study. One participant dropped out of the study after the fourth treatment due to a scheduling conflict, and the other 2 participants did not return for follow-up. No participants withdrew from the study because of adverse therapeutic events.
Efficacy Evaluation
Comparisons between the salicylic acid 30% peel and PBBL procedures for mGAGS at each visit are shown in Table 2. There was no significant difference in treatment efficacy between the salicylic acid 30% peel and PBBL therapies during the study’s treatment and follow-up events; however, both procedures contributed to a major improvement in acne symptoms by the third treatment session and through to the last follow-up session (P≤.05). Clinical photographs at baseline, at last treatment visit (week 6), and at last follow-up (week 12) are shown in Figures 1 and 2.



The results of the acne QOL questionnaire are shown in Table 2. Lower scores reflect a higher QOL. Median QOL scores at each visit ranged from 0.5 to 4.5. There was no significant difference found between the peel agent or PBBL based on the baseline QOL and subsequent visit assessments; however, the differences between the 2 treatments were significant at weeks 3 (P=.05) and 5 (P=.03) of treatment as well as at the last follow-up visit (P=.05).
According to the QOL scores, by the third treatment session participants were more satisfied with their improved acne condition from the PBBL procedure than the salicylic acid 30% peel as demonstrated by a positive range of the QOL assessments between PBBL and salicylic acid 30% peel (as shown in the difference in QOL in Table 2: week 3, 0–6; week 4, 0–3; week 5, 0–7). On the other hand, participants saw more improvement from the salicylic acid 30% peel than from PBBL by the last follow-up evaluation, as the differences in QOL scores between the 2 treatments resulted in a negative range (−5–0).
Safety
Pain assessment by the WBPRS at every visit showed a low pain rating associated with both salicylic acid 30% peel (range, 0–0.5) and PBBL (range, 1.0–1.5) treatments. The median pain score of the salicylic acid 30% peel appeared higher compared to the PBBL treatment, yet a significant difference between both treatments was seen only at weeks 1, 3, and 6 of treatment (P≤.05).
There were no unexpected therapeutic reactions reported in our study, and no participants withdrew from the study due to adverse events. Most participants experienced only mild adverse reactions, including redness, stinging, and a burning sensation on the salicylic acid 30% peel side, which were transient and disappeared in minutes; only redness occurred on the PBBL-treated side.
Comment
Facial acne treatment is challenging, as prolonged and/or severe acne contributes to scarring, declining self-confidence, and undesirable financial consequences. Even though salicylic acid peel is a commonly used acne treatment choice, the PBBL methodology was approved by the US Food and Drug Administration6 and has become an alternative procedure for acne treatment.
The pharmacological effects of salicylic acid are related to its corneocyte desquamation and exfoliative actions, thereby reducing corneocyte cohesion and unclogging follicular pores.16 Salicylic acid has been demonstrated to ameliorate inflammatory acne by its effects on the arachidonic acid cascade.2,4,17 In our study, salicylic acid 30% peel met participants’ satisfaction in acne improvement similar to a study showing a 50% improvement in acne scores after just 2 treatments.18 Our data support and corroborate that salicylic acid 30% peel renders an improvement in acne sequelae reported in several other studies.2,17,18
Pneumatic broadband light has been known to treat acne by the mechanism of pneumatic suction combined with photodynamic therapy using broadband-pulsed light (400–1200 nm).6-8 By applying the pneumatic device, a vacuum is created on the skin to remove sebum contents from follicles, whereas broadband light is emitted simultaneously to destroy bacteria and decrease the inflammatory process.7 During the vacuum process, the skin is stretched to reduce pain and avoid competitive chromophores (eg, hemoglobin), while the broadband light is administered.7 Broadband light encompasses 2 main light spectrums: blue light (415 nm) activates coproporphyrin III, which induces reactive free radicals and singlet oxygen species and has been reported to be the cause of bacterial cell death,19 and red light (633 nm), which renders an increase of fibroblast growth factors to work against the inflammatory processes.20 There are numerous studies showing a reduction of acne lesions after photopneumatic therapy with minimal side effects.6-8
In our study, we compared the efficacy of salicylic acid 30% peel with PBBL in the treatment of acne. Both treatments showed significant reduction of mGAGS compared to baseline starting from week 3 and lasting until week 12. Remarkably, although there were some participants who reported acne recurrence after completing all treatments at week 6, which could have happened when the treatments were ended, the final acne score at week 12 was still significantly lower than baseline. It is clear that the participants continued their acne improvement up to the 6-week follow-up period without any topical or oral medication. We do not propose that either salicylic acid peel or PBBL treatment is a solitary option but speculate that the combination of both treatments may initiate a faster resolution in the disappearance of acne.
Although there was no statistically significant difference in efficacy between salicylic acid 30% peel and PBBL procedures at each visit, QOL assessments related to treatment satisfaction did yield significant differences between baseline and the end of treatment. We noticed that participants had more positive attitudes toward the PBBL side at week 3 and week 5 but only mild satisfaction at week 4, as the differences in QOL scores between both treatments showed positive ranging values. This finding is most likely related to the immediate reduction of acne pustules by the PBBL vacuum lysis of these lesions. The differences in the QOL scores between both treatments at week 12 (the last follow-up evaluation) provided opposite findings, which meant patients had nearly even improvement in both PBBL method and salicylic acid 30% peel. Therefore, according to QOL data, acne disappeared quickly with the application of PBBL therapy but reappeared on the PBBL-treated side by the follow-up evaluations, though the acne score between both sides showed no statistically significant difference.
We reason that the PBBL therapy works better than salicylic acid 30% peel because the pneumatic system may help to unclog the pores through mechanical debridement via suctioning versus desquamation from salicylic acid 30% peel. Nonetheless, salicylic acid 30% peel sustained improvement when compared to PBBL through the follow-up periods. Both salicylic acid 30% peel and PBBL treatments are well tolerated and may initiate a faster resolution in the improvement of acne when incorporated with a medical program.
Because of the recurrence of acne after treatments were stopped, additional medical therapies are advised to be used along with this study’s clinical treatments to help mitigate the acne symptoms. These treatments should be considered in patients concerned about antibiotic resistance or those who cannot take oral antibiotics or retinoids. Salicylic acid peel is more accessible and affordable than PBBL, whereas PBBL is slightly more tolerable and less irritating than salicylic acid peel. Nevertheless, the cost of investment in PBBL is quite high—as much as $70,000—and does not include disposable, single-use tips, which cost $30 each. The machine is easy to set up, weighs about 40 lb, and requires little space to store. The average cost per visit of PBBL treatment in office is $150.00 and $75.00 for salicylic acid peel (unpublished data, Hospital of the University of Pennsylvania, 2010). Most patients may select salicylic acid peel over PBBL due to the cost and convenience of the treatment. Neither procedure should be considered as a solitary treatment option but rather as adjunctive procedures combined with oral and/or topical acne medications. After this study’s treatments were stopped and without other medications to maintain treatment effectiveness, the lesions reappeared, trending back toward baseline.
Conclusion
Both salicylic acid 30% peel and PBBL procedures are effective, safe, and well tolerated in treating acne. Although there was no significant difference in the efficacy between both treatments in this study, the small sample size and short follow-up intervals warrant further studies to support the observed outstanding outcomes and should be considered in combination with other medical treatment options. These procedures may be beneficial in holding the patient compliant until their medical therapies have an opportunity to work.
Acknowledgment
The authors would like to thank Joyce Okawa, RN (Philadelphia, Pennsylvania), for her assistance in the submission to the institutional review board of the University of Pennsylvania.
Facial acne vulgaris is a common skin disease among teenagers and adolescents that may negatively affect self-esteem, perceived facial attractiveness, and social participation.1 Treatments for acne often are multimodal and require the utmost adherence. For these reasons, acne treatments have been challenging to clinicians and patients alike, as patient compliance in maintaining the use of prescribed topical and oral medications remains essential to attain improvement in quality of life (QOL).
Salicylic acid is a popular medicament for acne treatment that frequently is used as monotherapy or as an adjuvant for other acne treatments, especially in patients with oily skin.2 Salicylic acid has a keratolytic effect, causing corneocyte discohesion in clogged pores or congested follicles,2 and it is effective in treating both inflammatory and noninflammatory acne.3,4
Light therapy, particularly with visible light, has been demonstrated to improve acne outcomes.5 Pneumatic broadband light (PBBL) is a therapeutic light treatment in the broadband range (400–1200 nm) that is combined with vacuum suction, which creates a mechanical lysis of thin-walled pustules and dislodges pore impaction. Additionally, the blue light portion of the PBBL spectrum targets endogenous porphyrins in Propionibacterium acnes, resulting in bacterial destruction.6-8
The purpose of this study was to compare the efficacy, tolerability, and safety of salicylic acid 30% peel versus PBBL in the treatment of mild to moderately severe facial acne vulgaris.
METHODS
Study Design
This single-blind, randomized, split-face pilot study was approved by the institutional review board of the University of Pennsylvania (Philadelphia, Pennsylvania). All patients provided informed consent before entering the study. The single-blind evaluation was performed by one dermatologist (C.T.) who examined the participants on every visit prior to PBBL treatment.
Before the study started, participants were randomized for which side of the face was to be treated with PBBL using a number assigned to each participant. Participants received both treatments—salicylic acid 30% peel on one side of the face and PBBL treatment on the other side of the face—once weekly for a total of 6 treatments. They were then asked to return for 2 follow-up evaluations at weeks 3 and 6 following the last treatment session and were instructed not to use any topical or oral acne medications during these follow-up periods.
Inclusion and Exclusion Criteria
Patients aged 18 years and older of any race and sex with noninflammatory papules, some inflammatory papules, and no more than 1 nodule (considered as mild to moderately severe facial acne) were included in the study. Participants had not been on any topical acne medications for at least 1 month and/or oral retinoids for at least 1 year prior to the study period. All women completed urine pregnancy tests prior to the study and were advised to utilize birth control during the study period.
Study Treatments
Salicylic Acid 30% Peel
The participant’s face was cleansed thoroughly before application of salicylic acid 30% (1.5 g/2.5 mL) to half of the face and left on for 5 minutes before being carefully rinsed off by spraying with spring water. Prior to initiating PBBL therapy, the peeled side of the participant’s face was covered with a towel.
Pneumatic Broadband Light
On the other side of the face, PBBL was performed to deliver broadband light within the spectrum range of 400 to 1200 nm at a setting approximately equivalent to a fluence of 4 to 6 J/cm2 and a vacuum setting approximately equivalent to a negative pressure of 3 lb/in2. The power setting was increased on each subsequent visit depending on each participant’s tolerability.
Participants were required to apply a moisturizer and sunscreen to the face and avoid excessive sun exposure between study visits.
Efficacy Evaluation
A comparison of the efficacy of the treatments was determined by clinical evaluation and examining the results of the outcome measurements with the modified Global Acne Grading Score (mGAGS) and Acne QOL Scale during each treatment visit. Facial photographs were taken at each visit.
Modified Global Acne Grading Score
The mGAGS is a modification of the Global Acne Grading Scale (GAGS) that has been used to evaluate acne severity in many studies.9-11 The GAGS considers 6 locations on the face with a grading factor for each location. The local score is obtained by multiplying the factor rated by location with the factor of clinical assessment: local score = factor rated by location × factor rated by clinical assessment. The total score is the sum of the individual local scores (Table 1).

Although the original GAGS incorporated the type and location of the lesions in its calculation, we felt that the number of lesions also was important to add to our grading score. Therefore, we modified the GAGS by adding a factor rated by the number of lesions to improve the accuracy of the test. Accordingly, the local mGAGS scores were calculated by multiplying the location factor by the lesion type and number of lesions factors: local score = location factor × lesion type factor × number of lesions factor.
Acne QOL Questionnaire
Acne QOL was assessed during each visit to demonstrate if the treatment results affected participants’ socialization due to appearance.12 Participants were asked to complete the questionnaire, which consisted of 9 questions with 4 rating answers (0=not affected; 1=mildly affected; 2=moderately affected; 3=markedly affected). A total score of 9 or higher (high score) indicated that acne had a substantial negative impact on the participant, while a total score below 9 (low score) meant acne scarcely impacted social aspects and daily activities of the patient.
Safety Evaluation
The safety of the treatments was evaluated by clinical inspection and by comparing the results of the Wong-Baker FACES Pain Rating Scale (WBPRS)13 after treatment. The WBPRS is used worldwide among researchers to assess pain, particularly in children.14,15 It is composed of 6 faces expressing pain with word descriptions with a corresponding number range reflecting pain severity from 0 to 5 (0=no hurt; 1=hurts little bit; 2=hurts little more; 3=hurts even more; 4=hurts whole lot; 5=hurts worst).13
Statistical Analysis
All variables were presented as the median (range). A Wilcoxon signed rank test was used to compare clinical responses between the salicylic acid 30% peel and PBBL therapies. SPSS software version 12.0 was used for all statistical analysis. A 2-tailed P value of ≤.05 was considered statistically significant.
RESULTS
Study Population
Twelve participants (2 males, 10 females) aged 17 to 36 years (median age, 22 years; mean age [SD], 23.33 [1.65] years) with both comedonal and inflammatory acne were enrolled into this study for 6 split-face treatments of salicylic acid 30% peel and PBBL at 1-week intervals for 6 weeks, with 2 subsequent follow-up sessions at weeks 3 and 6 posttreatment. Of the 12 participants, 11 were white and 1 was Asian American, with Fitzpatrick skin types II to IV. Nine participants (75%) completed the study. One participant dropped out of the study after the fourth treatment due to a scheduling conflict, and the other 2 participants did not return for follow-up. No participants withdrew from the study because of adverse therapeutic events.
Efficacy Evaluation
Comparisons between the salicylic acid 30% peel and PBBL procedures for mGAGS at each visit are shown in Table 2. There was no significant difference in treatment efficacy between the salicylic acid 30% peel and PBBL therapies during the study’s treatment and follow-up events; however, both procedures contributed to a major improvement in acne symptoms by the third treatment session and through to the last follow-up session (P≤.05). Clinical photographs at baseline, at last treatment visit (week 6), and at last follow-up (week 12) are shown in Figures 1 and 2.



The results of the acne QOL questionnaire are shown in Table 2. Lower scores reflect a higher QOL. Median QOL scores at each visit ranged from 0.5 to 4.5. There was no significant difference found between the peel agent or PBBL based on the baseline QOL and subsequent visit assessments; however, the differences between the 2 treatments were significant at weeks 3 (P=.05) and 5 (P=.03) of treatment as well as at the last follow-up visit (P=.05).
According to the QOL scores, by the third treatment session participants were more satisfied with their improved acne condition from the PBBL procedure than the salicylic acid 30% peel as demonstrated by a positive range of the QOL assessments between PBBL and salicylic acid 30% peel (as shown in the difference in QOL in Table 2: week 3, 0–6; week 4, 0–3; week 5, 0–7). On the other hand, participants saw more improvement from the salicylic acid 30% peel than from PBBL by the last follow-up evaluation, as the differences in QOL scores between the 2 treatments resulted in a negative range (−5–0).
Safety
Pain assessment by the WBPRS at every visit showed a low pain rating associated with both salicylic acid 30% peel (range, 0–0.5) and PBBL (range, 1.0–1.5) treatments. The median pain score of the salicylic acid 30% peel appeared higher compared to the PBBL treatment, yet a significant difference between both treatments was seen only at weeks 1, 3, and 6 of treatment (P≤.05).
There were no unexpected therapeutic reactions reported in our study, and no participants withdrew from the study due to adverse events. Most participants experienced only mild adverse reactions, including redness, stinging, and a burning sensation on the salicylic acid 30% peel side, which were transient and disappeared in minutes; only redness occurred on the PBBL-treated side.
Comment
Facial acne treatment is challenging, as prolonged and/or severe acne contributes to scarring, declining self-confidence, and undesirable financial consequences. Even though salicylic acid peel is a commonly used acne treatment choice, the PBBL methodology was approved by the US Food and Drug Administration6 and has become an alternative procedure for acne treatment.
The pharmacological effects of salicylic acid are related to its corneocyte desquamation and exfoliative actions, thereby reducing corneocyte cohesion and unclogging follicular pores.16 Salicylic acid has been demonstrated to ameliorate inflammatory acne by its effects on the arachidonic acid cascade.2,4,17 In our study, salicylic acid 30% peel met participants’ satisfaction in acne improvement similar to a study showing a 50% improvement in acne scores after just 2 treatments.18 Our data support and corroborate that salicylic acid 30% peel renders an improvement in acne sequelae reported in several other studies.2,17,18
Pneumatic broadband light has been known to treat acne by the mechanism of pneumatic suction combined with photodynamic therapy using broadband-pulsed light (400–1200 nm).6-8 By applying the pneumatic device, a vacuum is created on the skin to remove sebum contents from follicles, whereas broadband light is emitted simultaneously to destroy bacteria and decrease the inflammatory process.7 During the vacuum process, the skin is stretched to reduce pain and avoid competitive chromophores (eg, hemoglobin), while the broadband light is administered.7 Broadband light encompasses 2 main light spectrums: blue light (415 nm) activates coproporphyrin III, which induces reactive free radicals and singlet oxygen species and has been reported to be the cause of bacterial cell death,19 and red light (633 nm), which renders an increase of fibroblast growth factors to work against the inflammatory processes.20 There are numerous studies showing a reduction of acne lesions after photopneumatic therapy with minimal side effects.6-8
In our study, we compared the efficacy of salicylic acid 30% peel with PBBL in the treatment of acne. Both treatments showed significant reduction of mGAGS compared to baseline starting from week 3 and lasting until week 12. Remarkably, although there were some participants who reported acne recurrence after completing all treatments at week 6, which could have happened when the treatments were ended, the final acne score at week 12 was still significantly lower than baseline. It is clear that the participants continued their acne improvement up to the 6-week follow-up period without any topical or oral medication. We do not propose that either salicylic acid peel or PBBL treatment is a solitary option but speculate that the combination of both treatments may initiate a faster resolution in the disappearance of acne.
Although there was no statistically significant difference in efficacy between salicylic acid 30% peel and PBBL procedures at each visit, QOL assessments related to treatment satisfaction did yield significant differences between baseline and the end of treatment. We noticed that participants had more positive attitudes toward the PBBL side at week 3 and week 5 but only mild satisfaction at week 4, as the differences in QOL scores between both treatments showed positive ranging values. This finding is most likely related to the immediate reduction of acne pustules by the PBBL vacuum lysis of these lesions. The differences in the QOL scores between both treatments at week 12 (the last follow-up evaluation) provided opposite findings, which meant patients had nearly even improvement in both PBBL method and salicylic acid 30% peel. Therefore, according to QOL data, acne disappeared quickly with the application of PBBL therapy but reappeared on the PBBL-treated side by the follow-up evaluations, though the acne score between both sides showed no statistically significant difference.
We reason that the PBBL therapy works better than salicylic acid 30% peel because the pneumatic system may help to unclog the pores through mechanical debridement via suctioning versus desquamation from salicylic acid 30% peel. Nonetheless, salicylic acid 30% peel sustained improvement when compared to PBBL through the follow-up periods. Both salicylic acid 30% peel and PBBL treatments are well tolerated and may initiate a faster resolution in the improvement of acne when incorporated with a medical program.
Because of the recurrence of acne after treatments were stopped, additional medical therapies are advised to be used along with this study’s clinical treatments to help mitigate the acne symptoms. These treatments should be considered in patients concerned about antibiotic resistance or those who cannot take oral antibiotics or retinoids. Salicylic acid peel is more accessible and affordable than PBBL, whereas PBBL is slightly more tolerable and less irritating than salicylic acid peel. Nevertheless, the cost of investment in PBBL is quite high—as much as $70,000—and does not include disposable, single-use tips, which cost $30 each. The machine is easy to set up, weighs about 40 lb, and requires little space to store. The average cost per visit of PBBL treatment in office is $150.00 and $75.00 for salicylic acid peel (unpublished data, Hospital of the University of Pennsylvania, 2010). Most patients may select salicylic acid peel over PBBL due to the cost and convenience of the treatment. Neither procedure should be considered as a solitary treatment option but rather as adjunctive procedures combined with oral and/or topical acne medications. After this study’s treatments were stopped and without other medications to maintain treatment effectiveness, the lesions reappeared, trending back toward baseline.
Conclusion
Both salicylic acid 30% peel and PBBL procedures are effective, safe, and well tolerated in treating acne. Although there was no significant difference in the efficacy between both treatments in this study, the small sample size and short follow-up intervals warrant further studies to support the observed outstanding outcomes and should be considered in combination with other medical treatment options. These procedures may be beneficial in holding the patient compliant until their medical therapies have an opportunity to work.
Acknowledgment
The authors would like to thank Joyce Okawa, RN (Philadelphia, Pennsylvania), for her assistance in the submission to the institutional review board of the University of Pennsylvania.
- Rapp DA, Brenes GA, Feldman SR, et al. Anger and acne: implications for quality of life, patient satisfaction and clinical care. Br J Dermatol. 2004;151:183-189.
- Zakopoulou N, Kontochristopoulos G. Superficial chemical peels. J Cosmet Dermatol. 2006;5:246-253.
- Berson DS, Cohen JL, Rendon MI, et al. Clinical role and application of superficial chemical peels in today’s practice. J Drugs Dermatol. 2009;8:803-811.
- Shalita AR. Treatment of mild and moderate acne vulgaris with salicylic acid in an alcohol-detergent vehicle. Cutis. 1981;28:556-558, 561.
- Sakamoto FH, Lopes JD, Anderson RR. Photodynamic therapy for acne vulgaris: a critical review from basics to clinical practice: part I. acne vulgaris: when and why consider photodynamic therapy? J Am Acad Dermatol. 2010;63:183-193; quiz 93-94.
- Gold MH, Biron J. Efficacy of a novel combination of pneumatic energy and broadband light for the treatment of acne. J Drugs Dermatol. 2008;7:639-642.
- Shamban AT, Enokibori M, Narurkar V, et al. Photopneumatic technology for the treatment of acne vulgaris. J Drugs Dermatol. 2008;7:139-145.
- Wanitphakdeedecha R, Tanzi EL, Alster TS. Photopneumatic therapy for the treatment of acne. J Drugs Dermatol. 2009;8:239-241.
- Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol. 1997;36:416-418.
- Weiss JW, Shavin J, Davis M. Preliminary results of a nonrandomized, multicenter, open-label study of patient satisfaction after treatment with combination benzoyl peroxide/clindamycin topical gel for mild to moderate acne. Clin Ther. 2002;24:1706-1717.
- Demircay Z, Kus S, Sur H. Predictive factors for acne flare during isotretinoin treatment. Eur J Dermatol. 2008;18:452-456.
- Gupta MA, Johnson AM, Gupta AK. The development of an Acne Quality of Life scale: reliability, validity, and relation to subjective acne severity in mild to moderate acne vulgaris. Acta Derm Venereol. 1998;78:451-456.
- Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14:9-17.
- Wong DL, Hockenberry-Eaton M, Wilson D, et al. Wong’s Essentials of Pediatric Nursing. 6th ed. St. Louis, MO: Mosby; 2001:1301.
- Zempsky WT, Robbins B, McKay K. Reduction of topical anesthetic onset time using ultrasound: a randomized controlled trial prior to venipuncture in young children. Pain Med. 2008;9:795-802.
- Imayama S, Ueda S, Isoda M. Histologic changes in the skin of hairless mice following peeling with salicylic acid. Arch Dermatol. 2000;136:1390-1395.
- Lee H, Kim I. Salicylic acid peels for the treatment of acne vulgaris in Asian patients. Dermatol Surg. 2003;29:1196-1199.
- Kessler E, Flanagan K, Chia C, et al. Comparison of alpha- and beta-hydroxy acid chemical peels in the treatment of mild to moderately severe facial acne vulgaris. Dermatol Surg. 2008;34:45-50.
- Omi T, Munavalli GS, Kawana S, et al. Ultrastructural evidencefor thermal injury to pilosebaceous units during the treatment of acne using photopneumatic (PPX) therapy. J Cosmet Laser Ther. 2008;10:7-11.
- Papageorgiou P, Katsambas A, Chu A. Phototherapy with blue (415 nm) and red (660 nm) light in the treatment of acne vulgaris. Br J Dermatol. 2000;142:973-978.
- Rapp DA, Brenes GA, Feldman SR, et al. Anger and acne: implications for quality of life, patient satisfaction and clinical care. Br J Dermatol. 2004;151:183-189.
- Zakopoulou N, Kontochristopoulos G. Superficial chemical peels. J Cosmet Dermatol. 2006;5:246-253.
- Berson DS, Cohen JL, Rendon MI, et al. Clinical role and application of superficial chemical peels in today’s practice. J Drugs Dermatol. 2009;8:803-811.
- Shalita AR. Treatment of mild and moderate acne vulgaris with salicylic acid in an alcohol-detergent vehicle. Cutis. 1981;28:556-558, 561.
- Sakamoto FH, Lopes JD, Anderson RR. Photodynamic therapy for acne vulgaris: a critical review from basics to clinical practice: part I. acne vulgaris: when and why consider photodynamic therapy? J Am Acad Dermatol. 2010;63:183-193; quiz 93-94.
- Gold MH, Biron J. Efficacy of a novel combination of pneumatic energy and broadband light for the treatment of acne. J Drugs Dermatol. 2008;7:639-642.
- Shamban AT, Enokibori M, Narurkar V, et al. Photopneumatic technology for the treatment of acne vulgaris. J Drugs Dermatol. 2008;7:139-145.
- Wanitphakdeedecha R, Tanzi EL, Alster TS. Photopneumatic therapy for the treatment of acne. J Drugs Dermatol. 2009;8:239-241.
- Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol. 1997;36:416-418.
- Weiss JW, Shavin J, Davis M. Preliminary results of a nonrandomized, multicenter, open-label study of patient satisfaction after treatment with combination benzoyl peroxide/clindamycin topical gel for mild to moderate acne. Clin Ther. 2002;24:1706-1717.
- Demircay Z, Kus S, Sur H. Predictive factors for acne flare during isotretinoin treatment. Eur J Dermatol. 2008;18:452-456.
- Gupta MA, Johnson AM, Gupta AK. The development of an Acne Quality of Life scale: reliability, validity, and relation to subjective acne severity in mild to moderate acne vulgaris. Acta Derm Venereol. 1998;78:451-456.
- Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14:9-17.
- Wong DL, Hockenberry-Eaton M, Wilson D, et al. Wong’s Essentials of Pediatric Nursing. 6th ed. St. Louis, MO: Mosby; 2001:1301.
- Zempsky WT, Robbins B, McKay K. Reduction of topical anesthetic onset time using ultrasound: a randomized controlled trial prior to venipuncture in young children. Pain Med. 2008;9:795-802.
- Imayama S, Ueda S, Isoda M. Histologic changes in the skin of hairless mice following peeling with salicylic acid. Arch Dermatol. 2000;136:1390-1395.
- Lee H, Kim I. Salicylic acid peels for the treatment of acne vulgaris in Asian patients. Dermatol Surg. 2003;29:1196-1199.
- Kessler E, Flanagan K, Chia C, et al. Comparison of alpha- and beta-hydroxy acid chemical peels in the treatment of mild to moderately severe facial acne vulgaris. Dermatol Surg. 2008;34:45-50.
- Omi T, Munavalli GS, Kawana S, et al. Ultrastructural evidencefor thermal injury to pilosebaceous units during the treatment of acne using photopneumatic (PPX) therapy. J Cosmet Laser Ther. 2008;10:7-11.
- Papageorgiou P, Katsambas A, Chu A. Phototherapy with blue (415 nm) and red (660 nm) light in the treatment of acne vulgaris. Br J Dermatol. 2000;142:973-978.
Practice Points
- Salicylic acid peel and pneumatic broadband light (PBBL) are good alternative options in treating acne in addition to regular oral and topical treatments.
- Both salicylic acid peel and PBBL are effective, safe, and tolerable.
U.S. goals for earlier HIV diagnosis and treatment may be out of reach
In 2015, only 66% of U.S. youth who were diagnosed with HIV in a Centers for Disease Control and Prevention program were introduced to proper care within 90 days of diagnosis, falling far short of the 2020 national goal to introduce 85% of HIV-affected youth to proper care within 30 days.
In an analysis of data from a CDC-funded program covering 61 state and local health departments and 123 community-based organizations in the United States, Puerto Rico, and the U.S. Virgin Islands, the CDC looked at HIV tests, new positive diagnoses, and linkage between patient and care within 90 days of diagnosis. Of 2,973 youths who were newly diagnosed with HIV, 1,955 (66%) were connected to care within 90 days, and 1,871 were interviewed for partner services, according to the CDC. Of 1,911 youths who had been previously diagnosed, 1,749 (92%) were not in medical care at the time of CDC testing.
“A health care provider’s testing recommendation is the most important predictor of testing among adolescents at risk for HIV infection,” the researchers said. “Increasing the number of HIV tests among youths at risk for HIV and increasing regular retesting among these youths is essential for reducing HIV infection in this vulnerable population.”
No conflicts of interest were reported by the authors.
Read more in MMWR (2017 Jun 23. doi: 10.15585/mmwr.mm6624a2).
In 2015, only 66% of U.S. youth who were diagnosed with HIV in a Centers for Disease Control and Prevention program were introduced to proper care within 90 days of diagnosis, falling far short of the 2020 national goal to introduce 85% of HIV-affected youth to proper care within 30 days.
In an analysis of data from a CDC-funded program covering 61 state and local health departments and 123 community-based organizations in the United States, Puerto Rico, and the U.S. Virgin Islands, the CDC looked at HIV tests, new positive diagnoses, and linkage between patient and care within 90 days of diagnosis. Of 2,973 youths who were newly diagnosed with HIV, 1,955 (66%) were connected to care within 90 days, and 1,871 were interviewed for partner services, according to the CDC. Of 1,911 youths who had been previously diagnosed, 1,749 (92%) were not in medical care at the time of CDC testing.
“A health care provider’s testing recommendation is the most important predictor of testing among adolescents at risk for HIV infection,” the researchers said. “Increasing the number of HIV tests among youths at risk for HIV and increasing regular retesting among these youths is essential for reducing HIV infection in this vulnerable population.”
No conflicts of interest were reported by the authors.
Read more in MMWR (2017 Jun 23. doi: 10.15585/mmwr.mm6624a2).
In 2015, only 66% of U.S. youth who were diagnosed with HIV in a Centers for Disease Control and Prevention program were introduced to proper care within 90 days of diagnosis, falling far short of the 2020 national goal to introduce 85% of HIV-affected youth to proper care within 30 days.
In an analysis of data from a CDC-funded program covering 61 state and local health departments and 123 community-based organizations in the United States, Puerto Rico, and the U.S. Virgin Islands, the CDC looked at HIV tests, new positive diagnoses, and linkage between patient and care within 90 days of diagnosis. Of 2,973 youths who were newly diagnosed with HIV, 1,955 (66%) were connected to care within 90 days, and 1,871 were interviewed for partner services, according to the CDC. Of 1,911 youths who had been previously diagnosed, 1,749 (92%) were not in medical care at the time of CDC testing.
“A health care provider’s testing recommendation is the most important predictor of testing among adolescents at risk for HIV infection,” the researchers said. “Increasing the number of HIV tests among youths at risk for HIV and increasing regular retesting among these youths is essential for reducing HIV infection in this vulnerable population.”
No conflicts of interest were reported by the authors.
Read more in MMWR (2017 Jun 23. doi: 10.15585/mmwr.mm6624a2).
FROM MMWR
Maintenance of Certification: How Physician Self-regulation Can Improve Quality of Care
QI enthusiast to QI leader: John Bulger, DO
Editor’s Note: This SHM series highlights the professional pathways of quality improvement leaders. This month features the story of John Bulger, DO, chief medical officer for Geisinger Health Plan.
As chief medical officer for Geisinger Health Plan, John Bulger, DO, MBA, is intimately acquainted with the daily challenges that intersect with the delivery of safe, quality-driven care in the hospital system.
He’s also very familiar with the intricacies of carving out a professional road map. When Dr. Bulger began practicing as an internist at Geisinger Health System in the late 1990s, there wasn’t a formal hospitalist designation. He created one and became director of the hospital medicine program. Years later, when the opportunity arose to become chief quality officer, Dr. Bulger was a natural fit for the position, having led many improvement-centered committees and projects while running the hospital medicine group.
Early in his QI immersion, Dr. Bulger sought training where available from sources such as ACP and SHM, while familiarizing himself with methodologies such as PDSA and Lean. There are far more QI training opportunities available to hospitalists today than when Dr. Bulger began his journey, but the fundamentals of success come back to finding the right mentors, team building, and implementing projects built around SMART goals.
Getting started, Dr. Bulger suggests to “pick something within your scope, like medical reconciliation for every patient, or ensuring that every patient who leaves the hospital gets an appointment with their primary physician within 7 days. Early on, we were working on issues like pneumonia core measures and providing discharge instructions.”
He cautions those starting out in QI against viewing unintended outcomes or project setbacks as failure. “If your goal is to take a (scenario) from bad to perfect, you’ll end up getting discouraged. Any effort toward making things better is helpful. If it doesn’t work you try something else.”
While Dr. Bulger is fully supportive of the impact that quality improvement projects make at the institutional level, he encourages clinicians and researchers to always keep the Institute for Healthcare Improvement Triple Aim in sight.
“We need better measures and more discussion about what is best for patients,” Dr. Bulger said. “The things we talk about in (health care) – readmission rates, glycemic control – have a minimal impact on people’s health, but the social determinants of health – the patient’s housing and economic situation – play a bigger role than anything else. As we move from provider- to patient-centric communities by fixing the Triple Aim, the experience will be better for both providers and patients.”
Claudia Stahl is content manager for the Society of Hospital Medicine.
Editor’s Note: This SHM series highlights the professional pathways of quality improvement leaders. This month features the story of John Bulger, DO, chief medical officer for Geisinger Health Plan.
As chief medical officer for Geisinger Health Plan, John Bulger, DO, MBA, is intimately acquainted with the daily challenges that intersect with the delivery of safe, quality-driven care in the hospital system.
He’s also very familiar with the intricacies of carving out a professional road map. When Dr. Bulger began practicing as an internist at Geisinger Health System in the late 1990s, there wasn’t a formal hospitalist designation. He created one and became director of the hospital medicine program. Years later, when the opportunity arose to become chief quality officer, Dr. Bulger was a natural fit for the position, having led many improvement-centered committees and projects while running the hospital medicine group.
Early in his QI immersion, Dr. Bulger sought training where available from sources such as ACP and SHM, while familiarizing himself with methodologies such as PDSA and Lean. There are far more QI training opportunities available to hospitalists today than when Dr. Bulger began his journey, but the fundamentals of success come back to finding the right mentors, team building, and implementing projects built around SMART goals.
Getting started, Dr. Bulger suggests to “pick something within your scope, like medical reconciliation for every patient, or ensuring that every patient who leaves the hospital gets an appointment with their primary physician within 7 days. Early on, we were working on issues like pneumonia core measures and providing discharge instructions.”
He cautions those starting out in QI against viewing unintended outcomes or project setbacks as failure. “If your goal is to take a (scenario) from bad to perfect, you’ll end up getting discouraged. Any effort toward making things better is helpful. If it doesn’t work you try something else.”
While Dr. Bulger is fully supportive of the impact that quality improvement projects make at the institutional level, he encourages clinicians and researchers to always keep the Institute for Healthcare Improvement Triple Aim in sight.
“We need better measures and more discussion about what is best for patients,” Dr. Bulger said. “The things we talk about in (health care) – readmission rates, glycemic control – have a minimal impact on people’s health, but the social determinants of health – the patient’s housing and economic situation – play a bigger role than anything else. As we move from provider- to patient-centric communities by fixing the Triple Aim, the experience will be better for both providers and patients.”
Claudia Stahl is content manager for the Society of Hospital Medicine.
Editor’s Note: This SHM series highlights the professional pathways of quality improvement leaders. This month features the story of John Bulger, DO, chief medical officer for Geisinger Health Plan.
As chief medical officer for Geisinger Health Plan, John Bulger, DO, MBA, is intimately acquainted with the daily challenges that intersect with the delivery of safe, quality-driven care in the hospital system.
He’s also very familiar with the intricacies of carving out a professional road map. When Dr. Bulger began practicing as an internist at Geisinger Health System in the late 1990s, there wasn’t a formal hospitalist designation. He created one and became director of the hospital medicine program. Years later, when the opportunity arose to become chief quality officer, Dr. Bulger was a natural fit for the position, having led many improvement-centered committees and projects while running the hospital medicine group.
Early in his QI immersion, Dr. Bulger sought training where available from sources such as ACP and SHM, while familiarizing himself with methodologies such as PDSA and Lean. There are far more QI training opportunities available to hospitalists today than when Dr. Bulger began his journey, but the fundamentals of success come back to finding the right mentors, team building, and implementing projects built around SMART goals.
Getting started, Dr. Bulger suggests to “pick something within your scope, like medical reconciliation for every patient, or ensuring that every patient who leaves the hospital gets an appointment with their primary physician within 7 days. Early on, we were working on issues like pneumonia core measures and providing discharge instructions.”
He cautions those starting out in QI against viewing unintended outcomes or project setbacks as failure. “If your goal is to take a (scenario) from bad to perfect, you’ll end up getting discouraged. Any effort toward making things better is helpful. If it doesn’t work you try something else.”
While Dr. Bulger is fully supportive of the impact that quality improvement projects make at the institutional level, he encourages clinicians and researchers to always keep the Institute for Healthcare Improvement Triple Aim in sight.
“We need better measures and more discussion about what is best for patients,” Dr. Bulger said. “The things we talk about in (health care) – readmission rates, glycemic control – have a minimal impact on people’s health, but the social determinants of health – the patient’s housing and economic situation – play a bigger role than anything else. As we move from provider- to patient-centric communities by fixing the Triple Aim, the experience will be better for both providers and patients.”
Claudia Stahl is content manager for the Society of Hospital Medicine.
Knotless Arthroscopic Reduction and Internal Fixation of a Displaced Anterior Cruciate Ligament Tibial Eminence Avulsion Fracture
Take-Home Points
- Technique provides optimal fixation while simultaneously protecting open growth plates.
- Self tensioning feature insures both optimal ACL tension and fracture reduction.
- No need for future hardware removal.
- 10Cross suture configuration optimizes strength of fixation for highly consistent results.
- Use fluoroscopy to avoid violation of tibial physis.
Generally occurring in the 8- to 14-year-old population, tibial eminence avulsion (TEA) fractures are a common variant of anterior cruciate ligament (ACL) ruptures and represent 2% to 5% of all knee injuries in skeletally immature individuals.1,2 Compared with adults, children likely experience this anomaly more often because of the weakness of their incompletely ossified tibial plateau relative to the strength of their native ACL.3
The open repair techniques that have been described have multiple disadvantages, including open incisions, difficult visualization of the fracture owing to the location of the fat pad, and increased risk for arthrofibrosis. Arthroscopic fixation is considered the treatment of choice for TEA fractures because it allows for direct visualization of injury, accurate reduction of fracture fragments, removal of loose fragments, and easy treatment of associated soft-tissue injuries.4-6Several fixation techniques for ACL-TEA fractures were recently described: arthroscopic reduction and internal fixation (ARIF) with Kirschner wires,7 cannulated screws,4 the Meniscus Arrow device (Bionx Implants),8 pull-out sutures,9,10 bioabsorbable nails,11 Herbert screws,12 TightRope fixation (Arthrex),13 and various other rotator cuff and meniscal repair systems.14,15 These approaches tend to have good outcomes for TEA fractures, but there are risks associated with ACL tensioning and potential tibial growth plate violation or hardware problems. Likewise, there are no studies with large numbers of patients treated with these new techniques, so the optimal method of reduction and fixation is still unknown.
In this article, we describe a new ARIF technique that involves 2 absorbable anchors with adjustable suture-tensioning technology. This technique optimizes reduction and helps surgeons avoid proximal tibial physeal damage, procedure-related morbidity, and additional surgery.
Case Report
History
The patient, an 8-year-old boy, sustained a noncontact twisting injury of the left knee during a cutting maneuver in a flag football game. He experienced immediate pain and subsequent swelling. Clinical examination revealed a moderate effusion with motion limitations secondary to swelling and irritability. The patient’s Lachman test result was 2+. Pivot shift testing was not possible because of guarding. The knee was stable to varus and valgus stress at 0° and 30° of flexion. Limited knee flexion prohibited placement of the patient in the position needed for anterior and posterior drawer testing. His patella was stable on lateral stress testing at 20° of flexion with no apprehension. Neurovascular status was intact throughout the lower extremity.
Anteroposterior and lateral radiographs showed a minimally displaced Meyers-McKeever type II TEA fracture (Figures 1A, 1B).
After discussing potential treatment options with the parents, Dr. Smith proceeded with arthroscopic surgery for definitive reduction and internal fixation of the patient’s left knee displaced ACL-TEA fracture. The new adjustable suture-tensioning fixation technique was used. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Examination Under Anesthesia
Examination with the patient under general anesthesia revealed 3+ Lachman, 2+ pivot shift with foot in internal and external rotation, and 1+ anterior drawer with foot in neutral and internal rotation. The knee was stable to varus and valgus stress testing.
Surgical Technique
Proper patient positioning and padding of bony prominences were ensured, and the limb was sterilely prepared and draped.
Given the young age of the patient, it was imperative to avoid the open proximal tibial growth plate. The surgical plan for stabilization involved use of two 3.0-mm BioComposite Knotless SutureTak anchors (Arthrex). This anchor configuration is based on a No. 2 FiberWire suture shuttled through itself to create a locking splice mechanism that allows for adjustable tensioning. The anchors were placed on each side of the tibial bony avulsion site with two No. 2 FiberWire sutures and were then crossed about the avulsion fracture fragment in an “x-type” configuration to secure the ACL back down to the bony bed.
First, a curette was used to débride fibrous tissue on the underside of the fracture fragment and on the fracture bed. Minimal amounts of cancellous bone were débrided from the tibial fracture bed to optimize fracture reduction by slightly recessing the fracture fragment to ensure optimal ACL tensioning (Figure 5).
Next, from the accessory superior medial portal, the end of the wire that had been passed through the medial aspect of the bony avulsion was retrieved through the lateral portal. This wire was used to shuttle the repair suture from the laterally positioned SutureTak anchor over and through the medial aspect of the bony fragment out of the accessory superior medial (Figure 7).
Follow-Up
Two weeks after surgery, the patient returned to clinic for suture removal. Four weeks after surgery, radiographs confirmed anatomical reduction of the TEA fracture, and outpatient physical therapy (range-of-motion exercises as tolerated) and isometric quadriceps strengthening were instituted. Twelve weeks after surgery, examination revealed full knee motion, negative Lachman and pivot shift test results, and residual quadriceps muscle atrophy, and radiographs confirmed complete fracture healing with maintenance of a normal proximal tibial growth plate (Figures 10A, 10B).
Discussion
The highlight of this case is the simplicity of an excellent reduction of a displaced ACL-TEA fracture. Minimally invasive absorbable implants did not violate the proximal tibial physis, and the unique adjustable suture-tensioning technology allowed the degree of reduction and ACL tension to be “dialed in.” SutureTak implants have strong No. 2 FiberWire suture for excellent stability with an overall small suture load, and their small size avoids the risk of violating the proximal tibial physis and avoids potential growth disturbances.
Despite the obvious risks it poses to the open proximal tibial physis, surgical reduction of Meyers-McKeever type II and type III fractures is the norm for restoring ACL stability. Screws and suture fixation are the most common and reliable methods of TEA fracture reduction.16,17 In recent systematic reviews, however, Osti and colleagues17 and Gans and colleagues18 noted there is not enough evidence to warrant a “gold standard” in pediatric tibial avulsion cases.
Other fixation methods for TEA fractures must be investigated. Anderson and colleagues19 described the biomechanics of 4 different physeal-sparing avulsion fracture reduction techniques: an ultra-high-molecular-weight polyethylene (UHMWPE) suture-suture button, a suture anchor, a polydioxanone suture-suture button, and screw fixation. Using techniques described by Kocher and colleagues,4 Berg,20 Mah and colleagues,21 Vega and colleagues,22 and Lu and colleagues,23 Anderson and colleagues19 reduced TEA fractures in skeletally immature porcine knees. Compared with suture anchors, UHMWPE suture-suture buttons provided biomechanically superior cyclic and load-to-failure results as well as more consistent fixation.
Screw fixation has shown good results but has disadvantages. Incorrect positioning of a screw can lead to impingement and articular cartilage damage, and screw removal may be needed if discomfort at the fixation site persists.24,25 Likewise, screws generally are an option only for large fracture fragments, as there is an inherent risk of fracturing small TEA fractures, which can be common in skeletally immature patients.
Brunner and colleagues26 recently found that TEA fracture repair with absorbable sutures and distal bone bridge fixation yielded 3-month radiographic and clinical healing rates similar to those obtained with nonabsorbable sutures tied around a screw. However, other authors have reported growth disturbances with use of a similar technique, owing to a disturbance of the open proximal tibial growth plate.9 In that regard, a major advantage of this new knotless suturing technique is that distal fixation is not necessary.
The minimally invasive TEA fraction reduction technique described in this article has 6 advantages: It provides excellent fixation while avoiding proximal tibial growth plate injury; the degree of tensioning is easily controlled during reduction; it uses strong suture instead of metal screws or pins; the reduction construct is low-profile; distal fixation is unnecessary; and implant removal is unnecessary, thus limiting subsequent surgical intervention. With respect to long-term outcomes, however, it is not known how this procedure will compare with other commonly used ARIF methods in physeal-sparing techniques for TEA fracture fixation.
This case report highlights a novel pediatric displaced ACL-TEA fracture reduction technique that allows for adjustable reduction and resultant ACL tensioning with excellent strong suture fixation without violating the proximal tibial physis, which could make it invaluable in the surgical treatment of this injury in skeletally immature patients.
Am J Orthop. 2017;46(4):203-208. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Eiskjaer S, Larsen ST, Schmidt MB. The significance of hemarthrosis of the knee in children. Arch Orthop Trauma Surg. 1988;107(2):96-98.
2. Luhmann SJ. Acute traumatic knee effusions in children and adolescents. J Pediatr Orthop. 2003;23(2):199-202.
3. Woo SL, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med. 1991;19(3):217-225.
4. Kocher MS, Foreman ES, Micheli LJ. Laxity and functional outcome after arthroscopic reduction and internal fixation of displaced tibial spine fractures in children. Arthroscopy. 2003;19(10):1085-1090.
5. Lubowitz JH, Elson WS, Guttmann D. Part II: arthroscopic treatment of tibial plateau fractures: intercondylar eminence avulsion fractures. Arthroscopy. 2005;21(1):86-92.
6. Vargas B, Lutz N, Dutoit M, Zambelli PY. Nonunion after fracture of the anterior tibial spine: case report and review of the literature. J Pediatr Orthop B. 2009;18(2):90-92.
7. Sommerfeldt DW. Arthroscopically assisted internal fixation of avulsion fractures of the anterior cruciate ligament during childhood and adolescence [in German]. Oper Orthop Traumatol. 2008;20(4-5):310-320.
8. Wouters DB, de Graaf JS, Hemmer PH, Burgerhof JG, Kramer WL. The arthroscopic treatment of displaced tibial spine fractures in children and adolescents using Meniscus Arrows®. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):736-739.
9. Ahn JH, Yoo JC. Clinical outcome of arthroscopic reduction and suture for displaced acute and chronic tibial spine fractures. Knee Surg Sports Traumatol Arthrosc. 2005;13(2):116-121.
10. Huang TW, Hsu KY, Cheng CY, et al. Arthroscopic suture fixation of tibial eminence avulsion fractures. Arthroscopy. 2008;24(11):1232-1238.
11. Liljeros K, Werner S, Janarv PM. Arthroscopic fixation of anterior tibial spine fractures with bioabsorbable nails in skeletally immature patients. Am J Sports Med. 2009;37(5):923-928.
12. Wiegand N, Naumov I, Vamhidy L, Not LG. Arthroscopic treatment of tibial spine fracture in children with a cannulated Herbert screw. Knee. 2014;21(2):481-485.
13. Faivre B, Benea H, Klouche S, Lespagnol F, Bauer T, Hardy P. An original arthroscopic fixation of adult’s tibial eminence fractures using the Tightrope® device: a report of 8 cases and review of literature. Knee. 2014;21(4):833-839.
14. Kluemper CT, Snyder GM, Coats AC, Johnson DL, Mair SD. Arthroscopic suture fixation of tibial eminence fractures. Orthopedics. 2013;36(11):e1401-e1406.
15. Ochiai S, Hagino T, Watanabe Y, Senga S, Haro H. One strategy for arthroscopic suture fixation of tibial intercondylar eminence fractures using the Meniscal Viper Repair System. Sports Med Arthrosc Rehabil Ther Technol. 2011;3:17.
16. Bogunovic L, Tarabichi M, Harris D, Wright R. Treatment of tibial eminence fractures: a systematic review. J Knee Surg. 2015;28(3):255-262.
17. Osti L, Buda M, Soldati F, Del Buono A, Osti R, Maffulli N. Arthroscopic treatment of tibial eminence fracture: a systematic review of different fixation methods. Br Med Bull. 2016;118(1):73-90.
18. Gans I, Baldwin KD, Ganley TJ. Treatment and management outcomes of tibial eminence fractures in pediatric patients: a systematic review. Am J Sports Med. 2014;42(7):1743-1750.
19. Anderson CN, Nyman JS, McCullough KA, et al. Biomechanical evaluation of physeal-sparing fixation methods in tibial eminence fractures. Am J Sports Med. 2013;41(7):1586-1594.
20. Berg EE. Pediatric tibial eminence fractures: arthroscopic cannulated screw fixation. Arthroscopy. 1995;11(3):328-331.
21. Mah JY, Otsuka NY, McLean J. An arthroscopic technique for the reduction and fixation of tibial-eminence fractures. J Pediatr Orthop. 1996;16(1):119-121.
22. Vega JR, Irribarra LA, Baar AK, Iniguez M, Salgado M, Gana N. Arthroscopic fixation of displaced tibial eminence fractures: a new growth plate-sparing method. Arthroscopy. 2008;24(11):1239-1243.
23. Lu XW, Hu XP, Jin C, Zhu T, Ding Y, Dai LY. Reduction and fixation of the avulsion fracture of the tibial eminence using mini-open technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1476-1480.
24. Bonin N, Jeunet L, Obert L, Dejour D. Adult tibial eminence fracture fixation: arthroscopic procedure using K-wire folded fixation. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):857-862.
25. Senekovic V, Veselko M. Anterograde arthroscopic fixation of avulsion fractures of the tibial eminence with a cannulated screw: five-year results. Arthroscopy. 2003;19(1):54-61.
26. Brunner S, Vavken P, Kilger R, et al. Absorbable and non-absorbable suture fixation results in similar outcomes for tibial eminence fractures in children and adolescents. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):723-729.
Take-Home Points
- Technique provides optimal fixation while simultaneously protecting open growth plates.
- Self tensioning feature insures both optimal ACL tension and fracture reduction.
- No need for future hardware removal.
- 10Cross suture configuration optimizes strength of fixation for highly consistent results.
- Use fluoroscopy to avoid violation of tibial physis.
Generally occurring in the 8- to 14-year-old population, tibial eminence avulsion (TEA) fractures are a common variant of anterior cruciate ligament (ACL) ruptures and represent 2% to 5% of all knee injuries in skeletally immature individuals.1,2 Compared with adults, children likely experience this anomaly more often because of the weakness of their incompletely ossified tibial plateau relative to the strength of their native ACL.3
The open repair techniques that have been described have multiple disadvantages, including open incisions, difficult visualization of the fracture owing to the location of the fat pad, and increased risk for arthrofibrosis. Arthroscopic fixation is considered the treatment of choice for TEA fractures because it allows for direct visualization of injury, accurate reduction of fracture fragments, removal of loose fragments, and easy treatment of associated soft-tissue injuries.4-6Several fixation techniques for ACL-TEA fractures were recently described: arthroscopic reduction and internal fixation (ARIF) with Kirschner wires,7 cannulated screws,4 the Meniscus Arrow device (Bionx Implants),8 pull-out sutures,9,10 bioabsorbable nails,11 Herbert screws,12 TightRope fixation (Arthrex),13 and various other rotator cuff and meniscal repair systems.14,15 These approaches tend to have good outcomes for TEA fractures, but there are risks associated with ACL tensioning and potential tibial growth plate violation or hardware problems. Likewise, there are no studies with large numbers of patients treated with these new techniques, so the optimal method of reduction and fixation is still unknown.
In this article, we describe a new ARIF technique that involves 2 absorbable anchors with adjustable suture-tensioning technology. This technique optimizes reduction and helps surgeons avoid proximal tibial physeal damage, procedure-related morbidity, and additional surgery.
Case Report
History
The patient, an 8-year-old boy, sustained a noncontact twisting injury of the left knee during a cutting maneuver in a flag football game. He experienced immediate pain and subsequent swelling. Clinical examination revealed a moderate effusion with motion limitations secondary to swelling and irritability. The patient’s Lachman test result was 2+. Pivot shift testing was not possible because of guarding. The knee was stable to varus and valgus stress at 0° and 30° of flexion. Limited knee flexion prohibited placement of the patient in the position needed for anterior and posterior drawer testing. His patella was stable on lateral stress testing at 20° of flexion with no apprehension. Neurovascular status was intact throughout the lower extremity.
Anteroposterior and lateral radiographs showed a minimally displaced Meyers-McKeever type II TEA fracture (Figures 1A, 1B).
After discussing potential treatment options with the parents, Dr. Smith proceeded with arthroscopic surgery for definitive reduction and internal fixation of the patient’s left knee displaced ACL-TEA fracture. The new adjustable suture-tensioning fixation technique was used. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Examination Under Anesthesia
Examination with the patient under general anesthesia revealed 3+ Lachman, 2+ pivot shift with foot in internal and external rotation, and 1+ anterior drawer with foot in neutral and internal rotation. The knee was stable to varus and valgus stress testing.
Surgical Technique
Proper patient positioning and padding of bony prominences were ensured, and the limb was sterilely prepared and draped.
Given the young age of the patient, it was imperative to avoid the open proximal tibial growth plate. The surgical plan for stabilization involved use of two 3.0-mm BioComposite Knotless SutureTak anchors (Arthrex). This anchor configuration is based on a No. 2 FiberWire suture shuttled through itself to create a locking splice mechanism that allows for adjustable tensioning. The anchors were placed on each side of the tibial bony avulsion site with two No. 2 FiberWire sutures and were then crossed about the avulsion fracture fragment in an “x-type” configuration to secure the ACL back down to the bony bed.
First, a curette was used to débride fibrous tissue on the underside of the fracture fragment and on the fracture bed. Minimal amounts of cancellous bone were débrided from the tibial fracture bed to optimize fracture reduction by slightly recessing the fracture fragment to ensure optimal ACL tensioning (Figure 5).
Next, from the accessory superior medial portal, the end of the wire that had been passed through the medial aspect of the bony avulsion was retrieved through the lateral portal. This wire was used to shuttle the repair suture from the laterally positioned SutureTak anchor over and through the medial aspect of the bony fragment out of the accessory superior medial (Figure 7).
Follow-Up
Two weeks after surgery, the patient returned to clinic for suture removal. Four weeks after surgery, radiographs confirmed anatomical reduction of the TEA fracture, and outpatient physical therapy (range-of-motion exercises as tolerated) and isometric quadriceps strengthening were instituted. Twelve weeks after surgery, examination revealed full knee motion, negative Lachman and pivot shift test results, and residual quadriceps muscle atrophy, and radiographs confirmed complete fracture healing with maintenance of a normal proximal tibial growth plate (Figures 10A, 10B).
Discussion
The highlight of this case is the simplicity of an excellent reduction of a displaced ACL-TEA fracture. Minimally invasive absorbable implants did not violate the proximal tibial physis, and the unique adjustable suture-tensioning technology allowed the degree of reduction and ACL tension to be “dialed in.” SutureTak implants have strong No. 2 FiberWire suture for excellent stability with an overall small suture load, and their small size avoids the risk of violating the proximal tibial physis and avoids potential growth disturbances.
Despite the obvious risks it poses to the open proximal tibial physis, surgical reduction of Meyers-McKeever type II and type III fractures is the norm for restoring ACL stability. Screws and suture fixation are the most common and reliable methods of TEA fracture reduction.16,17 In recent systematic reviews, however, Osti and colleagues17 and Gans and colleagues18 noted there is not enough evidence to warrant a “gold standard” in pediatric tibial avulsion cases.
Other fixation methods for TEA fractures must be investigated. Anderson and colleagues19 described the biomechanics of 4 different physeal-sparing avulsion fracture reduction techniques: an ultra-high-molecular-weight polyethylene (UHMWPE) suture-suture button, a suture anchor, a polydioxanone suture-suture button, and screw fixation. Using techniques described by Kocher and colleagues,4 Berg,20 Mah and colleagues,21 Vega and colleagues,22 and Lu and colleagues,23 Anderson and colleagues19 reduced TEA fractures in skeletally immature porcine knees. Compared with suture anchors, UHMWPE suture-suture buttons provided biomechanically superior cyclic and load-to-failure results as well as more consistent fixation.
Screw fixation has shown good results but has disadvantages. Incorrect positioning of a screw can lead to impingement and articular cartilage damage, and screw removal may be needed if discomfort at the fixation site persists.24,25 Likewise, screws generally are an option only for large fracture fragments, as there is an inherent risk of fracturing small TEA fractures, which can be common in skeletally immature patients.
Brunner and colleagues26 recently found that TEA fracture repair with absorbable sutures and distal bone bridge fixation yielded 3-month radiographic and clinical healing rates similar to those obtained with nonabsorbable sutures tied around a screw. However, other authors have reported growth disturbances with use of a similar technique, owing to a disturbance of the open proximal tibial growth plate.9 In that regard, a major advantage of this new knotless suturing technique is that distal fixation is not necessary.
The minimally invasive TEA fraction reduction technique described in this article has 6 advantages: It provides excellent fixation while avoiding proximal tibial growth plate injury; the degree of tensioning is easily controlled during reduction; it uses strong suture instead of metal screws or pins; the reduction construct is low-profile; distal fixation is unnecessary; and implant removal is unnecessary, thus limiting subsequent surgical intervention. With respect to long-term outcomes, however, it is not known how this procedure will compare with other commonly used ARIF methods in physeal-sparing techniques for TEA fracture fixation.
This case report highlights a novel pediatric displaced ACL-TEA fracture reduction technique that allows for adjustable reduction and resultant ACL tensioning with excellent strong suture fixation without violating the proximal tibial physis, which could make it invaluable in the surgical treatment of this injury in skeletally immature patients.
Am J Orthop. 2017;46(4):203-208. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Technique provides optimal fixation while simultaneously protecting open growth plates.
- Self tensioning feature insures both optimal ACL tension and fracture reduction.
- No need for future hardware removal.
- 10Cross suture configuration optimizes strength of fixation for highly consistent results.
- Use fluoroscopy to avoid violation of tibial physis.
Generally occurring in the 8- to 14-year-old population, tibial eminence avulsion (TEA) fractures are a common variant of anterior cruciate ligament (ACL) ruptures and represent 2% to 5% of all knee injuries in skeletally immature individuals.1,2 Compared with adults, children likely experience this anomaly more often because of the weakness of their incompletely ossified tibial plateau relative to the strength of their native ACL.3
The open repair techniques that have been described have multiple disadvantages, including open incisions, difficult visualization of the fracture owing to the location of the fat pad, and increased risk for arthrofibrosis. Arthroscopic fixation is considered the treatment of choice for TEA fractures because it allows for direct visualization of injury, accurate reduction of fracture fragments, removal of loose fragments, and easy treatment of associated soft-tissue injuries.4-6Several fixation techniques for ACL-TEA fractures were recently described: arthroscopic reduction and internal fixation (ARIF) with Kirschner wires,7 cannulated screws,4 the Meniscus Arrow device (Bionx Implants),8 pull-out sutures,9,10 bioabsorbable nails,11 Herbert screws,12 TightRope fixation (Arthrex),13 and various other rotator cuff and meniscal repair systems.14,15 These approaches tend to have good outcomes for TEA fractures, but there are risks associated with ACL tensioning and potential tibial growth plate violation or hardware problems. Likewise, there are no studies with large numbers of patients treated with these new techniques, so the optimal method of reduction and fixation is still unknown.
In this article, we describe a new ARIF technique that involves 2 absorbable anchors with adjustable suture-tensioning technology. This technique optimizes reduction and helps surgeons avoid proximal tibial physeal damage, procedure-related morbidity, and additional surgery.
Case Report
History
The patient, an 8-year-old boy, sustained a noncontact twisting injury of the left knee during a cutting maneuver in a flag football game. He experienced immediate pain and subsequent swelling. Clinical examination revealed a moderate effusion with motion limitations secondary to swelling and irritability. The patient’s Lachman test result was 2+. Pivot shift testing was not possible because of guarding. The knee was stable to varus and valgus stress at 0° and 30° of flexion. Limited knee flexion prohibited placement of the patient in the position needed for anterior and posterior drawer testing. His patella was stable on lateral stress testing at 20° of flexion with no apprehension. Neurovascular status was intact throughout the lower extremity.
Anteroposterior and lateral radiographs showed a minimally displaced Meyers-McKeever type II TEA fracture (Figures 1A, 1B).
After discussing potential treatment options with the parents, Dr. Smith proceeded with arthroscopic surgery for definitive reduction and internal fixation of the patient’s left knee displaced ACL-TEA fracture. The new adjustable suture-tensioning fixation technique was used. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Examination Under Anesthesia
Examination with the patient under general anesthesia revealed 3+ Lachman, 2+ pivot shift with foot in internal and external rotation, and 1+ anterior drawer with foot in neutral and internal rotation. The knee was stable to varus and valgus stress testing.
Surgical Technique
Proper patient positioning and padding of bony prominences were ensured, and the limb was sterilely prepared and draped.
Given the young age of the patient, it was imperative to avoid the open proximal tibial growth plate. The surgical plan for stabilization involved use of two 3.0-mm BioComposite Knotless SutureTak anchors (Arthrex). This anchor configuration is based on a No. 2 FiberWire suture shuttled through itself to create a locking splice mechanism that allows for adjustable tensioning. The anchors were placed on each side of the tibial bony avulsion site with two No. 2 FiberWire sutures and were then crossed about the avulsion fracture fragment in an “x-type” configuration to secure the ACL back down to the bony bed.
First, a curette was used to débride fibrous tissue on the underside of the fracture fragment and on the fracture bed. Minimal amounts of cancellous bone were débrided from the tibial fracture bed to optimize fracture reduction by slightly recessing the fracture fragment to ensure optimal ACL tensioning (Figure 5).
Next, from the accessory superior medial portal, the end of the wire that had been passed through the medial aspect of the bony avulsion was retrieved through the lateral portal. This wire was used to shuttle the repair suture from the laterally positioned SutureTak anchor over and through the medial aspect of the bony fragment out of the accessory superior medial (Figure 7).
Follow-Up
Two weeks after surgery, the patient returned to clinic for suture removal. Four weeks after surgery, radiographs confirmed anatomical reduction of the TEA fracture, and outpatient physical therapy (range-of-motion exercises as tolerated) and isometric quadriceps strengthening were instituted. Twelve weeks after surgery, examination revealed full knee motion, negative Lachman and pivot shift test results, and residual quadriceps muscle atrophy, and radiographs confirmed complete fracture healing with maintenance of a normal proximal tibial growth plate (Figures 10A, 10B).
Discussion
The highlight of this case is the simplicity of an excellent reduction of a displaced ACL-TEA fracture. Minimally invasive absorbable implants did not violate the proximal tibial physis, and the unique adjustable suture-tensioning technology allowed the degree of reduction and ACL tension to be “dialed in.” SutureTak implants have strong No. 2 FiberWire suture for excellent stability with an overall small suture load, and their small size avoids the risk of violating the proximal tibial physis and avoids potential growth disturbances.
Despite the obvious risks it poses to the open proximal tibial physis, surgical reduction of Meyers-McKeever type II and type III fractures is the norm for restoring ACL stability. Screws and suture fixation are the most common and reliable methods of TEA fracture reduction.16,17 In recent systematic reviews, however, Osti and colleagues17 and Gans and colleagues18 noted there is not enough evidence to warrant a “gold standard” in pediatric tibial avulsion cases.
Other fixation methods for TEA fractures must be investigated. Anderson and colleagues19 described the biomechanics of 4 different physeal-sparing avulsion fracture reduction techniques: an ultra-high-molecular-weight polyethylene (UHMWPE) suture-suture button, a suture anchor, a polydioxanone suture-suture button, and screw fixation. Using techniques described by Kocher and colleagues,4 Berg,20 Mah and colleagues,21 Vega and colleagues,22 and Lu and colleagues,23 Anderson and colleagues19 reduced TEA fractures in skeletally immature porcine knees. Compared with suture anchors, UHMWPE suture-suture buttons provided biomechanically superior cyclic and load-to-failure results as well as more consistent fixation.
Screw fixation has shown good results but has disadvantages. Incorrect positioning of a screw can lead to impingement and articular cartilage damage, and screw removal may be needed if discomfort at the fixation site persists.24,25 Likewise, screws generally are an option only for large fracture fragments, as there is an inherent risk of fracturing small TEA fractures, which can be common in skeletally immature patients.
Brunner and colleagues26 recently found that TEA fracture repair with absorbable sutures and distal bone bridge fixation yielded 3-month radiographic and clinical healing rates similar to those obtained with nonabsorbable sutures tied around a screw. However, other authors have reported growth disturbances with use of a similar technique, owing to a disturbance of the open proximal tibial growth plate.9 In that regard, a major advantage of this new knotless suturing technique is that distal fixation is not necessary.
The minimally invasive TEA fraction reduction technique described in this article has 6 advantages: It provides excellent fixation while avoiding proximal tibial growth plate injury; the degree of tensioning is easily controlled during reduction; it uses strong suture instead of metal screws or pins; the reduction construct is low-profile; distal fixation is unnecessary; and implant removal is unnecessary, thus limiting subsequent surgical intervention. With respect to long-term outcomes, however, it is not known how this procedure will compare with other commonly used ARIF methods in physeal-sparing techniques for TEA fracture fixation.
This case report highlights a novel pediatric displaced ACL-TEA fracture reduction technique that allows for adjustable reduction and resultant ACL tensioning with excellent strong suture fixation without violating the proximal tibial physis, which could make it invaluable in the surgical treatment of this injury in skeletally immature patients.
Am J Orthop. 2017;46(4):203-208. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Eiskjaer S, Larsen ST, Schmidt MB. The significance of hemarthrosis of the knee in children. Arch Orthop Trauma Surg. 1988;107(2):96-98.
2. Luhmann SJ. Acute traumatic knee effusions in children and adolescents. J Pediatr Orthop. 2003;23(2):199-202.
3. Woo SL, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med. 1991;19(3):217-225.
4. Kocher MS, Foreman ES, Micheli LJ. Laxity and functional outcome after arthroscopic reduction and internal fixation of displaced tibial spine fractures in children. Arthroscopy. 2003;19(10):1085-1090.
5. Lubowitz JH, Elson WS, Guttmann D. Part II: arthroscopic treatment of tibial plateau fractures: intercondylar eminence avulsion fractures. Arthroscopy. 2005;21(1):86-92.
6. Vargas B, Lutz N, Dutoit M, Zambelli PY. Nonunion after fracture of the anterior tibial spine: case report and review of the literature. J Pediatr Orthop B. 2009;18(2):90-92.
7. Sommerfeldt DW. Arthroscopically assisted internal fixation of avulsion fractures of the anterior cruciate ligament during childhood and adolescence [in German]. Oper Orthop Traumatol. 2008;20(4-5):310-320.
8. Wouters DB, de Graaf JS, Hemmer PH, Burgerhof JG, Kramer WL. The arthroscopic treatment of displaced tibial spine fractures in children and adolescents using Meniscus Arrows®. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):736-739.
9. Ahn JH, Yoo JC. Clinical outcome of arthroscopic reduction and suture for displaced acute and chronic tibial spine fractures. Knee Surg Sports Traumatol Arthrosc. 2005;13(2):116-121.
10. Huang TW, Hsu KY, Cheng CY, et al. Arthroscopic suture fixation of tibial eminence avulsion fractures. Arthroscopy. 2008;24(11):1232-1238.
11. Liljeros K, Werner S, Janarv PM. Arthroscopic fixation of anterior tibial spine fractures with bioabsorbable nails in skeletally immature patients. Am J Sports Med. 2009;37(5):923-928.
12. Wiegand N, Naumov I, Vamhidy L, Not LG. Arthroscopic treatment of tibial spine fracture in children with a cannulated Herbert screw. Knee. 2014;21(2):481-485.
13. Faivre B, Benea H, Klouche S, Lespagnol F, Bauer T, Hardy P. An original arthroscopic fixation of adult’s tibial eminence fractures using the Tightrope® device: a report of 8 cases and review of literature. Knee. 2014;21(4):833-839.
14. Kluemper CT, Snyder GM, Coats AC, Johnson DL, Mair SD. Arthroscopic suture fixation of tibial eminence fractures. Orthopedics. 2013;36(11):e1401-e1406.
15. Ochiai S, Hagino T, Watanabe Y, Senga S, Haro H. One strategy for arthroscopic suture fixation of tibial intercondylar eminence fractures using the Meniscal Viper Repair System. Sports Med Arthrosc Rehabil Ther Technol. 2011;3:17.
16. Bogunovic L, Tarabichi M, Harris D, Wright R. Treatment of tibial eminence fractures: a systematic review. J Knee Surg. 2015;28(3):255-262.
17. Osti L, Buda M, Soldati F, Del Buono A, Osti R, Maffulli N. Arthroscopic treatment of tibial eminence fracture: a systematic review of different fixation methods. Br Med Bull. 2016;118(1):73-90.
18. Gans I, Baldwin KD, Ganley TJ. Treatment and management outcomes of tibial eminence fractures in pediatric patients: a systematic review. Am J Sports Med. 2014;42(7):1743-1750.
19. Anderson CN, Nyman JS, McCullough KA, et al. Biomechanical evaluation of physeal-sparing fixation methods in tibial eminence fractures. Am J Sports Med. 2013;41(7):1586-1594.
20. Berg EE. Pediatric tibial eminence fractures: arthroscopic cannulated screw fixation. Arthroscopy. 1995;11(3):328-331.
21. Mah JY, Otsuka NY, McLean J. An arthroscopic technique for the reduction and fixation of tibial-eminence fractures. J Pediatr Orthop. 1996;16(1):119-121.
22. Vega JR, Irribarra LA, Baar AK, Iniguez M, Salgado M, Gana N. Arthroscopic fixation of displaced tibial eminence fractures: a new growth plate-sparing method. Arthroscopy. 2008;24(11):1239-1243.
23. Lu XW, Hu XP, Jin C, Zhu T, Ding Y, Dai LY. Reduction and fixation of the avulsion fracture of the tibial eminence using mini-open technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1476-1480.
24. Bonin N, Jeunet L, Obert L, Dejour D. Adult tibial eminence fracture fixation: arthroscopic procedure using K-wire folded fixation. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):857-862.
25. Senekovic V, Veselko M. Anterograde arthroscopic fixation of avulsion fractures of the tibial eminence with a cannulated screw: five-year results. Arthroscopy. 2003;19(1):54-61.
26. Brunner S, Vavken P, Kilger R, et al. Absorbable and non-absorbable suture fixation results in similar outcomes for tibial eminence fractures in children and adolescents. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):723-729.
1. Eiskjaer S, Larsen ST, Schmidt MB. The significance of hemarthrosis of the knee in children. Arch Orthop Trauma Surg. 1988;107(2):96-98.
2. Luhmann SJ. Acute traumatic knee effusions in children and adolescents. J Pediatr Orthop. 2003;23(2):199-202.
3. Woo SL, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med. 1991;19(3):217-225.
4. Kocher MS, Foreman ES, Micheli LJ. Laxity and functional outcome after arthroscopic reduction and internal fixation of displaced tibial spine fractures in children. Arthroscopy. 2003;19(10):1085-1090.
5. Lubowitz JH, Elson WS, Guttmann D. Part II: arthroscopic treatment of tibial plateau fractures: intercondylar eminence avulsion fractures. Arthroscopy. 2005;21(1):86-92.
6. Vargas B, Lutz N, Dutoit M, Zambelli PY. Nonunion after fracture of the anterior tibial spine: case report and review of the literature. J Pediatr Orthop B. 2009;18(2):90-92.
7. Sommerfeldt DW. Arthroscopically assisted internal fixation of avulsion fractures of the anterior cruciate ligament during childhood and adolescence [in German]. Oper Orthop Traumatol. 2008;20(4-5):310-320.
8. Wouters DB, de Graaf JS, Hemmer PH, Burgerhof JG, Kramer WL. The arthroscopic treatment of displaced tibial spine fractures in children and adolescents using Meniscus Arrows®. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):736-739.
9. Ahn JH, Yoo JC. Clinical outcome of arthroscopic reduction and suture for displaced acute and chronic tibial spine fractures. Knee Surg Sports Traumatol Arthrosc. 2005;13(2):116-121.
10. Huang TW, Hsu KY, Cheng CY, et al. Arthroscopic suture fixation of tibial eminence avulsion fractures. Arthroscopy. 2008;24(11):1232-1238.
11. Liljeros K, Werner S, Janarv PM. Arthroscopic fixation of anterior tibial spine fractures with bioabsorbable nails in skeletally immature patients. Am J Sports Med. 2009;37(5):923-928.
12. Wiegand N, Naumov I, Vamhidy L, Not LG. Arthroscopic treatment of tibial spine fracture in children with a cannulated Herbert screw. Knee. 2014;21(2):481-485.
13. Faivre B, Benea H, Klouche S, Lespagnol F, Bauer T, Hardy P. An original arthroscopic fixation of adult’s tibial eminence fractures using the Tightrope® device: a report of 8 cases and review of literature. Knee. 2014;21(4):833-839.
14. Kluemper CT, Snyder GM, Coats AC, Johnson DL, Mair SD. Arthroscopic suture fixation of tibial eminence fractures. Orthopedics. 2013;36(11):e1401-e1406.
15. Ochiai S, Hagino T, Watanabe Y, Senga S, Haro H. One strategy for arthroscopic suture fixation of tibial intercondylar eminence fractures using the Meniscal Viper Repair System. Sports Med Arthrosc Rehabil Ther Technol. 2011;3:17.
16. Bogunovic L, Tarabichi M, Harris D, Wright R. Treatment of tibial eminence fractures: a systematic review. J Knee Surg. 2015;28(3):255-262.
17. Osti L, Buda M, Soldati F, Del Buono A, Osti R, Maffulli N. Arthroscopic treatment of tibial eminence fracture: a systematic review of different fixation methods. Br Med Bull. 2016;118(1):73-90.
18. Gans I, Baldwin KD, Ganley TJ. Treatment and management outcomes of tibial eminence fractures in pediatric patients: a systematic review. Am J Sports Med. 2014;42(7):1743-1750.
19. Anderson CN, Nyman JS, McCullough KA, et al. Biomechanical evaluation of physeal-sparing fixation methods in tibial eminence fractures. Am J Sports Med. 2013;41(7):1586-1594.
20. Berg EE. Pediatric tibial eminence fractures: arthroscopic cannulated screw fixation. Arthroscopy. 1995;11(3):328-331.
21. Mah JY, Otsuka NY, McLean J. An arthroscopic technique for the reduction and fixation of tibial-eminence fractures. J Pediatr Orthop. 1996;16(1):119-121.
22. Vega JR, Irribarra LA, Baar AK, Iniguez M, Salgado M, Gana N. Arthroscopic fixation of displaced tibial eminence fractures: a new growth plate-sparing method. Arthroscopy. 2008;24(11):1239-1243.
23. Lu XW, Hu XP, Jin C, Zhu T, Ding Y, Dai LY. Reduction and fixation of the avulsion fracture of the tibial eminence using mini-open technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1476-1480.
24. Bonin N, Jeunet L, Obert L, Dejour D. Adult tibial eminence fracture fixation: arthroscopic procedure using K-wire folded fixation. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):857-862.
25. Senekovic V, Veselko M. Anterograde arthroscopic fixation of avulsion fractures of the tibial eminence with a cannulated screw: five-year results. Arthroscopy. 2003;19(1):54-61.
26. Brunner S, Vavken P, Kilger R, et al. Absorbable and non-absorbable suture fixation results in similar outcomes for tibial eminence fractures in children and adolescents. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):723-729.
A New Option for Glenoid Reconstruction in Recurrent Anterior Shoulder Instability
Take-Home Points
- Repair anterior bone defect on the glenoid related to recurrent anterior instability with preshaped, predrilled allograft.
- Avoid graft harvest complications related to coracoid (Latarjet) or iliac crest autograft.
- Simple guide system to allow for appropriate graft and screw placement.
- Soft tissues can be repaired to the allograft in predrilled suture holes either inside or outside of the graft
- Position the graft without step at the anterior glenoid.
Anteroinferior glenoid bone loss plays a significant role in recurrent glenohumeral instability. Arthroscopic capsulolabral reconstruction has been associated with a recurrence rate of 4% in the absence of significant glenoid bone loss but 67% in patients with either bone loss of more than 25% of the inferior glenoid diameter or an engaging Hill-Sachs lesion.1,2 Anteroinferior glenoid rim deficiency has been reported in up to 90% of cases of recurrent instability.3 Glenoid reconstruction is therefore recommended in patients with bone loss of more than 25% and in certain revision cases.4 Surgical strategies in these cases include coracoid transfer, iliac crest autograft, and allograft (osteochondral and iliac crest). These procedures all successfully restore stability of the glenohumeral joint. However, they carry the drawbacks of technical complexity with increased operative time or risk of neurovascular damage, or they create a nonanatomical reconstruction, which may contribute to subsequent instability arthropathy. In this article, we introduce a technique in which a preshaped allograft (Glenojet; Arthrosurface, Inc.) is used to match the contour of the glenoid defect. The graft is simple to insert and can reduce operative time.
Graft Preparation
The shaped human tissue cortical bone allograft is usually prepared from proximal or distal tibia or femur. There is no cartilage on the graft. It can be ordered in 2 sizes, 10 mm × 29 mm and 13 mm × 34 mm, for different amounts of bone loss. The more commonly used smaller graft reconstructs defects of 20% to 30% of the glenoid.
The sutures through this allograft can be prepared on the back table while the rest of the equipment is set up. Start by tying a No. 2 FiberWire (or equivalent) over a small thin object, such as a Freer elevator. Once the knot is secure, remove the Freer and trim the knot tails short. Thread another suture through the loop that has been created and pull to make the 2 tails even. Then thread these tails through one of the small holes of the graft, going from the flat side to the concave side. Pull the suture tails all the way through, including through the loop of the prior suture. The knot of the loop prevents the entire construct from pulling through. The suture tails are then able to slide as if attached to an anchor. Repeat these steps for the other 2 small holes to get a total of 3 sutures exiting the concave side of the graft (Figure 1). Alternatively, pass the suture the opposite way, if tying the capsule inside the graft is preferred.
Surgical Technique
A standard deltopectoral approach is used to expose the anterior glenoid. The subscapularis can either be split in line with its fibers or tenotomized with 1 cm to 2 cm attached to the tuberosity for later repair. In either instance, it is important to separate the muscle from the underlying capsule layer, as the capsule is what is directly repaired to the graft.
The capsule is carefully peeled off the anterior glenoid. A Fukuda or similar retractor may be used on the humerus, and a glenoid retractor is placed on the anterior glenoid, under the capsule and subscapularis, for optimal exposure. Once the anterior glenoid surface is exposed, the drill guide is placed flush against the surface of the glenoid.
The guide is removed. The cannulated reamer is introduced and advanced until the guide pin appears in the viewing window of the reamer and hits the stop—approximating the correct amount of bone to remove. This step is repeated for the second guide pin. Reaming flattens the anterior glenoid and allows for maximal stable apposition of the graft to the glenoid. The allograft is then inserted onto the pins in the correct orientation to match the surface of the native glenoid.
The length of the superior guide pin is measured with the depth gauge device. It is then removed, and the appropriate-length 3.5-mm cortical bone screw is inserted (alternatively, the guide pin is removed, and a standard depth gauge is used to measure screw length). Once the superior guide pin is secure, the process is repeated for the inferior guide pin (Figure 3).
Once the graft is secure, the capsule is attached to the graft with the use of a free needle on the suture of the graft (Figure 4).
Outcomes
Coracoid bone transfer or the Bristow-Latarjet technique has become more popular since bone loss was recognized as an important cause of failure of soft-tissue repair for anterior instability. This procedure, however, is not without complications. In a recent systematic review of 45 studies (1904 shoulders), Griesser and colleagues5 found an overall complication rate of 30% and a reoperation rate of 7%.
Given the potential complications of coracoid bone transfer, allograft reconstruction of the anteroinferior glenoid has become increasingly popular and proved successful at short- and medium-term follow-up. Allograft reconstruction avoids the drawbacks of traditional coracoid bone transfer—namely, high rates of neurovascular injury, and nonanatomical reconstruction with high rates of graft resorption and arthritis.5,6 At average 45-month follow-up after fresh distal tibia allograft reconstruction, Provencher and colleagues7 found an 89% radiographic union rate (average lysis, 3%), significantly improved patient-reported outcomes, and no recurrent instability. Similarly, in a study of iliac crest allograft reconstruction in 10 patients with an average 4-year follow-up, Mascarenhas and colleagues8 found an 80% radiographic union rate at 6 months, significantly improved patient-reported outcomes, and no recurrent shoulder instability.
The advantage of Glenojet over other allografts is that it is preshaped and predrilled and saves the surgeon the time and effort of preparing graft in the operating room. The surgical technologist can place the sutures before the patient enters the room. The 2 allograft sizes (10 mm × 29 mm, 13 mm × 34 mm) accommodate the spectrum of bone loss in glenoid deficiency, and graft contour fits the native glenoid well. So far we have implanted this allograft in 15 patients, and at short-term follow-up there are no known cases of recurrent instability.
The potential disadvantages of Glenojet are similar to those of other allografts. Care must be taken with retractor placement to avoid damaging the axillary and musculocutaneous nerves. There are concerns about graft union and subsequent resorption, but this will require long-term follow-up to determine. At 9-month follow-up, we had 1 fracture at the superior corner of the graft, which may have resulted from overtightening the screws in the graft, creating a stress concentration. After removal of this fragment arthroscopically, the patient has done very well clinically with no pain, instability and has returned to all activities. Although the graft does not have an articular surface, the capsular repair covers much of the articular side of the graft, and therefore we do not anticipate that the absence of articular cartilage will contribute to glenohumeral arthritis, though long-term follow-up is lacking. The other question many have is related to the lack of the sling effect since there is no conjoined tendon on the graft. Yamamoto and colleagues9 have reported that the conjoined tendon is the major stabilizing force at time zero in a cadaver model. However, other authors7,8 have successfully reconstructed glenoid defects in these difficult cases without the “sling effect” of the conjoined tendon with excellent clinical results. Our experience has been similar. It is likely that long-term studies will be necessary to answer this question. We have also done some cases with the tendon attached after releasing it from the coracoid, but the series is too small to make any comment about whether this is important or not.
The main limitation of this allograft technique is the lack of long-term outcome studies. However, short-term results are promising, and the ease of the procedure makes it an attractive option for either glenoid reconstruction of bony Bankart lesions or failed bone reconstruction, such as Bristow-Latarjet reconstruction.
Glenojetallograft is a new glenoid reconstruction option that is technically easy and simple to perform in cases of glenoid bone loss, while still creating an anatomical buttress with less surgical dissection than traditional coracoid bone transfer. Short-term outcomes are reassuring, though more research is needed for long-term graft follow-up and recurrent instability.
Am J Orthop. 2017;46(4):199-202. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.
2. Rowe CR, Sakellarides HT. Factors related to recurrences of anterior dislocations of the shoulder. Clin Orthop. 1961;(20):40-48.
3. Piasecki DP, Verma NN, Romeo AA, Levine WN, Bach BR Jr, Provencher MT. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17(8):482-493.
4. Sayegh ET, Mascarenhas R, Chalmers PN, Cole BJ, Verma NN, Romeo AA. Allograft reconstruction for glenoid bone loss in glenohumeral instability: a systematic review. Arthroscopy. 2014;30(12):1642-1649.
5. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operati ons after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22(2):286-292.
6. Young DC, Rockwood CA Jr. Complications of a failed Bristow procedure and their management. J Bone Joint Surg Am. 1991;73(7):969-981.
7. Provencher MT, Frank RM, Golijanin P, et al. Distal tibia allograft glenoid reconstruction in recurrent anterior shoulder instability: clinical and radiographic outcomes. Arthroscopy. 2017;33(5):891-897.
8. Mascarenhas R, Raleigh E, McRae S, Leiter J, Saltzman B, MacDonald PB. Iliac crest allograft glenoid reconstruction for recurrent anterior shoulder instability in athletes: surgical technique and results. Int J Shoulder Surg. 2014;8(4):127-132.
9. Yamamoto N, Muraki T, An KN, et al. The stabilizing mechanism of the Latarjet procedure: a cadaveric study. J Bone Joint Surg Am. 2013;95(15):1390-1397.
Take-Home Points
- Repair anterior bone defect on the glenoid related to recurrent anterior instability with preshaped, predrilled allograft.
- Avoid graft harvest complications related to coracoid (Latarjet) or iliac crest autograft.
- Simple guide system to allow for appropriate graft and screw placement.
- Soft tissues can be repaired to the allograft in predrilled suture holes either inside or outside of the graft
- Position the graft without step at the anterior glenoid.
Anteroinferior glenoid bone loss plays a significant role in recurrent glenohumeral instability. Arthroscopic capsulolabral reconstruction has been associated with a recurrence rate of 4% in the absence of significant glenoid bone loss but 67% in patients with either bone loss of more than 25% of the inferior glenoid diameter or an engaging Hill-Sachs lesion.1,2 Anteroinferior glenoid rim deficiency has been reported in up to 90% of cases of recurrent instability.3 Glenoid reconstruction is therefore recommended in patients with bone loss of more than 25% and in certain revision cases.4 Surgical strategies in these cases include coracoid transfer, iliac crest autograft, and allograft (osteochondral and iliac crest). These procedures all successfully restore stability of the glenohumeral joint. However, they carry the drawbacks of technical complexity with increased operative time or risk of neurovascular damage, or they create a nonanatomical reconstruction, which may contribute to subsequent instability arthropathy. In this article, we introduce a technique in which a preshaped allograft (Glenojet; Arthrosurface, Inc.) is used to match the contour of the glenoid defect. The graft is simple to insert and can reduce operative time.
Graft Preparation
The shaped human tissue cortical bone allograft is usually prepared from proximal or distal tibia or femur. There is no cartilage on the graft. It can be ordered in 2 sizes, 10 mm × 29 mm and 13 mm × 34 mm, for different amounts of bone loss. The more commonly used smaller graft reconstructs defects of 20% to 30% of the glenoid.
The sutures through this allograft can be prepared on the back table while the rest of the equipment is set up. Start by tying a No. 2 FiberWire (or equivalent) over a small thin object, such as a Freer elevator. Once the knot is secure, remove the Freer and trim the knot tails short. Thread another suture through the loop that has been created and pull to make the 2 tails even. Then thread these tails through one of the small holes of the graft, going from the flat side to the concave side. Pull the suture tails all the way through, including through the loop of the prior suture. The knot of the loop prevents the entire construct from pulling through. The suture tails are then able to slide as if attached to an anchor. Repeat these steps for the other 2 small holes to get a total of 3 sutures exiting the concave side of the graft (Figure 1). Alternatively, pass the suture the opposite way, if tying the capsule inside the graft is preferred.
Surgical Technique
A standard deltopectoral approach is used to expose the anterior glenoid. The subscapularis can either be split in line with its fibers or tenotomized with 1 cm to 2 cm attached to the tuberosity for later repair. In either instance, it is important to separate the muscle from the underlying capsule layer, as the capsule is what is directly repaired to the graft.
The capsule is carefully peeled off the anterior glenoid. A Fukuda or similar retractor may be used on the humerus, and a glenoid retractor is placed on the anterior glenoid, under the capsule and subscapularis, for optimal exposure. Once the anterior glenoid surface is exposed, the drill guide is placed flush against the surface of the glenoid.
The guide is removed. The cannulated reamer is introduced and advanced until the guide pin appears in the viewing window of the reamer and hits the stop—approximating the correct amount of bone to remove. This step is repeated for the second guide pin. Reaming flattens the anterior glenoid and allows for maximal stable apposition of the graft to the glenoid. The allograft is then inserted onto the pins in the correct orientation to match the surface of the native glenoid.
The length of the superior guide pin is measured with the depth gauge device. It is then removed, and the appropriate-length 3.5-mm cortical bone screw is inserted (alternatively, the guide pin is removed, and a standard depth gauge is used to measure screw length). Once the superior guide pin is secure, the process is repeated for the inferior guide pin (Figure 3).
Once the graft is secure, the capsule is attached to the graft with the use of a free needle on the suture of the graft (Figure 4).
Outcomes
Coracoid bone transfer or the Bristow-Latarjet technique has become more popular since bone loss was recognized as an important cause of failure of soft-tissue repair for anterior instability. This procedure, however, is not without complications. In a recent systematic review of 45 studies (1904 shoulders), Griesser and colleagues5 found an overall complication rate of 30% and a reoperation rate of 7%.
Given the potential complications of coracoid bone transfer, allograft reconstruction of the anteroinferior glenoid has become increasingly popular and proved successful at short- and medium-term follow-up. Allograft reconstruction avoids the drawbacks of traditional coracoid bone transfer—namely, high rates of neurovascular injury, and nonanatomical reconstruction with high rates of graft resorption and arthritis.5,6 At average 45-month follow-up after fresh distal tibia allograft reconstruction, Provencher and colleagues7 found an 89% radiographic union rate (average lysis, 3%), significantly improved patient-reported outcomes, and no recurrent instability. Similarly, in a study of iliac crest allograft reconstruction in 10 patients with an average 4-year follow-up, Mascarenhas and colleagues8 found an 80% radiographic union rate at 6 months, significantly improved patient-reported outcomes, and no recurrent shoulder instability.
The advantage of Glenojet over other allografts is that it is preshaped and predrilled and saves the surgeon the time and effort of preparing graft in the operating room. The surgical technologist can place the sutures before the patient enters the room. The 2 allograft sizes (10 mm × 29 mm, 13 mm × 34 mm) accommodate the spectrum of bone loss in glenoid deficiency, and graft contour fits the native glenoid well. So far we have implanted this allograft in 15 patients, and at short-term follow-up there are no known cases of recurrent instability.
The potential disadvantages of Glenojet are similar to those of other allografts. Care must be taken with retractor placement to avoid damaging the axillary and musculocutaneous nerves. There are concerns about graft union and subsequent resorption, but this will require long-term follow-up to determine. At 9-month follow-up, we had 1 fracture at the superior corner of the graft, which may have resulted from overtightening the screws in the graft, creating a stress concentration. After removal of this fragment arthroscopically, the patient has done very well clinically with no pain, instability and has returned to all activities. Although the graft does not have an articular surface, the capsular repair covers much of the articular side of the graft, and therefore we do not anticipate that the absence of articular cartilage will contribute to glenohumeral arthritis, though long-term follow-up is lacking. The other question many have is related to the lack of the sling effect since there is no conjoined tendon on the graft. Yamamoto and colleagues9 have reported that the conjoined tendon is the major stabilizing force at time zero in a cadaver model. However, other authors7,8 have successfully reconstructed glenoid defects in these difficult cases without the “sling effect” of the conjoined tendon with excellent clinical results. Our experience has been similar. It is likely that long-term studies will be necessary to answer this question. We have also done some cases with the tendon attached after releasing it from the coracoid, but the series is too small to make any comment about whether this is important or not.
The main limitation of this allograft technique is the lack of long-term outcome studies. However, short-term results are promising, and the ease of the procedure makes it an attractive option for either glenoid reconstruction of bony Bankart lesions or failed bone reconstruction, such as Bristow-Latarjet reconstruction.
Glenojetallograft is a new glenoid reconstruction option that is technically easy and simple to perform in cases of glenoid bone loss, while still creating an anatomical buttress with less surgical dissection than traditional coracoid bone transfer. Short-term outcomes are reassuring, though more research is needed for long-term graft follow-up and recurrent instability.
Am J Orthop. 2017;46(4):199-202. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Repair anterior bone defect on the glenoid related to recurrent anterior instability with preshaped, predrilled allograft.
- Avoid graft harvest complications related to coracoid (Latarjet) or iliac crest autograft.
- Simple guide system to allow for appropriate graft and screw placement.
- Soft tissues can be repaired to the allograft in predrilled suture holes either inside or outside of the graft
- Position the graft without step at the anterior glenoid.
Anteroinferior glenoid bone loss plays a significant role in recurrent glenohumeral instability. Arthroscopic capsulolabral reconstruction has been associated with a recurrence rate of 4% in the absence of significant glenoid bone loss but 67% in patients with either bone loss of more than 25% of the inferior glenoid diameter or an engaging Hill-Sachs lesion.1,2 Anteroinferior glenoid rim deficiency has been reported in up to 90% of cases of recurrent instability.3 Glenoid reconstruction is therefore recommended in patients with bone loss of more than 25% and in certain revision cases.4 Surgical strategies in these cases include coracoid transfer, iliac crest autograft, and allograft (osteochondral and iliac crest). These procedures all successfully restore stability of the glenohumeral joint. However, they carry the drawbacks of technical complexity with increased operative time or risk of neurovascular damage, or they create a nonanatomical reconstruction, which may contribute to subsequent instability arthropathy. In this article, we introduce a technique in which a preshaped allograft (Glenojet; Arthrosurface, Inc.) is used to match the contour of the glenoid defect. The graft is simple to insert and can reduce operative time.
Graft Preparation
The shaped human tissue cortical bone allograft is usually prepared from proximal or distal tibia or femur. There is no cartilage on the graft. It can be ordered in 2 sizes, 10 mm × 29 mm and 13 mm × 34 mm, for different amounts of bone loss. The more commonly used smaller graft reconstructs defects of 20% to 30% of the glenoid.
The sutures through this allograft can be prepared on the back table while the rest of the equipment is set up. Start by tying a No. 2 FiberWire (or equivalent) over a small thin object, such as a Freer elevator. Once the knot is secure, remove the Freer and trim the knot tails short. Thread another suture through the loop that has been created and pull to make the 2 tails even. Then thread these tails through one of the small holes of the graft, going from the flat side to the concave side. Pull the suture tails all the way through, including through the loop of the prior suture. The knot of the loop prevents the entire construct from pulling through. The suture tails are then able to slide as if attached to an anchor. Repeat these steps for the other 2 small holes to get a total of 3 sutures exiting the concave side of the graft (Figure 1). Alternatively, pass the suture the opposite way, if tying the capsule inside the graft is preferred.
Surgical Technique
A standard deltopectoral approach is used to expose the anterior glenoid. The subscapularis can either be split in line with its fibers or tenotomized with 1 cm to 2 cm attached to the tuberosity for later repair. In either instance, it is important to separate the muscle from the underlying capsule layer, as the capsule is what is directly repaired to the graft.
The capsule is carefully peeled off the anterior glenoid. A Fukuda or similar retractor may be used on the humerus, and a glenoid retractor is placed on the anterior glenoid, under the capsule and subscapularis, for optimal exposure. Once the anterior glenoid surface is exposed, the drill guide is placed flush against the surface of the glenoid.
The guide is removed. The cannulated reamer is introduced and advanced until the guide pin appears in the viewing window of the reamer and hits the stop—approximating the correct amount of bone to remove. This step is repeated for the second guide pin. Reaming flattens the anterior glenoid and allows for maximal stable apposition of the graft to the glenoid. The allograft is then inserted onto the pins in the correct orientation to match the surface of the native glenoid.
The length of the superior guide pin is measured with the depth gauge device. It is then removed, and the appropriate-length 3.5-mm cortical bone screw is inserted (alternatively, the guide pin is removed, and a standard depth gauge is used to measure screw length). Once the superior guide pin is secure, the process is repeated for the inferior guide pin (Figure 3).
Once the graft is secure, the capsule is attached to the graft with the use of a free needle on the suture of the graft (Figure 4).
Outcomes
Coracoid bone transfer or the Bristow-Latarjet technique has become more popular since bone loss was recognized as an important cause of failure of soft-tissue repair for anterior instability. This procedure, however, is not without complications. In a recent systematic review of 45 studies (1904 shoulders), Griesser and colleagues5 found an overall complication rate of 30% and a reoperation rate of 7%.
Given the potential complications of coracoid bone transfer, allograft reconstruction of the anteroinferior glenoid has become increasingly popular and proved successful at short- and medium-term follow-up. Allograft reconstruction avoids the drawbacks of traditional coracoid bone transfer—namely, high rates of neurovascular injury, and nonanatomical reconstruction with high rates of graft resorption and arthritis.5,6 At average 45-month follow-up after fresh distal tibia allograft reconstruction, Provencher and colleagues7 found an 89% radiographic union rate (average lysis, 3%), significantly improved patient-reported outcomes, and no recurrent instability. Similarly, in a study of iliac crest allograft reconstruction in 10 patients with an average 4-year follow-up, Mascarenhas and colleagues8 found an 80% radiographic union rate at 6 months, significantly improved patient-reported outcomes, and no recurrent shoulder instability.
The advantage of Glenojet over other allografts is that it is preshaped and predrilled and saves the surgeon the time and effort of preparing graft in the operating room. The surgical technologist can place the sutures before the patient enters the room. The 2 allograft sizes (10 mm × 29 mm, 13 mm × 34 mm) accommodate the spectrum of bone loss in glenoid deficiency, and graft contour fits the native glenoid well. So far we have implanted this allograft in 15 patients, and at short-term follow-up there are no known cases of recurrent instability.
The potential disadvantages of Glenojet are similar to those of other allografts. Care must be taken with retractor placement to avoid damaging the axillary and musculocutaneous nerves. There are concerns about graft union and subsequent resorption, but this will require long-term follow-up to determine. At 9-month follow-up, we had 1 fracture at the superior corner of the graft, which may have resulted from overtightening the screws in the graft, creating a stress concentration. After removal of this fragment arthroscopically, the patient has done very well clinically with no pain, instability and has returned to all activities. Although the graft does not have an articular surface, the capsular repair covers much of the articular side of the graft, and therefore we do not anticipate that the absence of articular cartilage will contribute to glenohumeral arthritis, though long-term follow-up is lacking. The other question many have is related to the lack of the sling effect since there is no conjoined tendon on the graft. Yamamoto and colleagues9 have reported that the conjoined tendon is the major stabilizing force at time zero in a cadaver model. However, other authors7,8 have successfully reconstructed glenoid defects in these difficult cases without the “sling effect” of the conjoined tendon with excellent clinical results. Our experience has been similar. It is likely that long-term studies will be necessary to answer this question. We have also done some cases with the tendon attached after releasing it from the coracoid, but the series is too small to make any comment about whether this is important or not.
The main limitation of this allograft technique is the lack of long-term outcome studies. However, short-term results are promising, and the ease of the procedure makes it an attractive option for either glenoid reconstruction of bony Bankart lesions or failed bone reconstruction, such as Bristow-Latarjet reconstruction.
Glenojetallograft is a new glenoid reconstruction option that is technically easy and simple to perform in cases of glenoid bone loss, while still creating an anatomical buttress with less surgical dissection than traditional coracoid bone transfer. Short-term outcomes are reassuring, though more research is needed for long-term graft follow-up and recurrent instability.
Am J Orthop. 2017;46(4):199-202. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.
2. Rowe CR, Sakellarides HT. Factors related to recurrences of anterior dislocations of the shoulder. Clin Orthop. 1961;(20):40-48.
3. Piasecki DP, Verma NN, Romeo AA, Levine WN, Bach BR Jr, Provencher MT. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17(8):482-493.
4. Sayegh ET, Mascarenhas R, Chalmers PN, Cole BJ, Verma NN, Romeo AA. Allograft reconstruction for glenoid bone loss in glenohumeral instability: a systematic review. Arthroscopy. 2014;30(12):1642-1649.
5. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operati ons after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22(2):286-292.
6. Young DC, Rockwood CA Jr. Complications of a failed Bristow procedure and their management. J Bone Joint Surg Am. 1991;73(7):969-981.
7. Provencher MT, Frank RM, Golijanin P, et al. Distal tibia allograft glenoid reconstruction in recurrent anterior shoulder instability: clinical and radiographic outcomes. Arthroscopy. 2017;33(5):891-897.
8. Mascarenhas R, Raleigh E, McRae S, Leiter J, Saltzman B, MacDonald PB. Iliac crest allograft glenoid reconstruction for recurrent anterior shoulder instability in athletes: surgical technique and results. Int J Shoulder Surg. 2014;8(4):127-132.
9. Yamamoto N, Muraki T, An KN, et al. The stabilizing mechanism of the Latarjet procedure: a cadaveric study. J Bone Joint Surg Am. 2013;95(15):1390-1397.
1. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.
2. Rowe CR, Sakellarides HT. Factors related to recurrences of anterior dislocations of the shoulder. Clin Orthop. 1961;(20):40-48.
3. Piasecki DP, Verma NN, Romeo AA, Levine WN, Bach BR Jr, Provencher MT. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17(8):482-493.
4. Sayegh ET, Mascarenhas R, Chalmers PN, Cole BJ, Verma NN, Romeo AA. Allograft reconstruction for glenoid bone loss in glenohumeral instability: a systematic review. Arthroscopy. 2014;30(12):1642-1649.
5. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operati ons after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22(2):286-292.
6. Young DC, Rockwood CA Jr. Complications of a failed Bristow procedure and their management. J Bone Joint Surg Am. 1991;73(7):969-981.
7. Provencher MT, Frank RM, Golijanin P, et al. Distal tibia allograft glenoid reconstruction in recurrent anterior shoulder instability: clinical and radiographic outcomes. Arthroscopy. 2017;33(5):891-897.
8. Mascarenhas R, Raleigh E, McRae S, Leiter J, Saltzman B, MacDonald PB. Iliac crest allograft glenoid reconstruction for recurrent anterior shoulder instability in athletes: surgical technique and results. Int J Shoulder Surg. 2014;8(4):127-132.
9. Yamamoto N, Muraki T, An KN, et al. The stabilizing mechanism of the Latarjet procedure: a cadaveric study. J Bone Joint Surg Am. 2013;95(15):1390-1397.