User login
Race-specific lung-function values may skew IPF testing
HONOLULU – Old habits die hard, especially when it comes to pulmonary function testing in a diverse population of patients with interstitial lung disease (ILD).
Specifically, pulmonary care clinicians may be habitually relying on outdated and inaccurate race-specific reference values when evaluating respiratory impairment in persons of African and Hispanic/Latino ancestry, which can result in underrecognition, underdiagnosis, and undertreatment, reported Ayodeji Adegunsoye, MD, from the University of Chicago, and colleagues.
“Our results make a compelling case for re-evaluating the use of race as a physiological variable, and highlight the need to offer equitable and optimal care for all patients, regardless of their race or ethnicity,” Dr. Adegunsoye said in an oral abstract session at the annual meeting of the American College of Chest Physicians (CHEST).
Flawed assumptions
In an interview, Dr. Adegunsoye noted that race-specific notions, such as the automatic assumption that Black people have less lung capacity than White people, are baked into clinical practice and passed on as clinical wisdom from one generation of clinicians to the next.
Pulmonary function reference values that are used to make a diagnosis of idiopathic pulmonary fibrosis in Black or Hispanic/Latino patients “appear flawed when we use race-specific values. And beyond the diagnosis, it also appears to impact eligibility for key interventional strategies for managing the disease itself,” he said.
The use of race-specific equations can falsely inflate percent-predicted pulmonary function values in non-White patients, and make it seem as if a patient has normal lung function when in fact he may be have impaired function.
For example, using race-based reference values a Black patient and a White patient may appear to have the same absolute forced vital capacity readings, but different FVC percent predicted (FVCpp), which can mean a missed diagnosis.
Investigators who studied the association between self-identified race and visually identified emphysema among 2,674 participants in the Coronary Artery Risk Development in Young Adults study found that using standard equations to adjust for racial differences in lung-function measures appeared to miss emphysema in a significant proportion of Black patients.
PF registry study
In the current study, to see whether the use of race-neutral equations for evaluating FVCpp could change access to health care in patients with ILD, Dr. Adegunsoye and colleagues used both race-specific and race-neutral equations to calculate FVCpp values among separate cohorts of Black, Hispanic/Latino, and White patients enrolled in the Pulmonary Fibrosis Foundation Patient Registry who had pulmonary functions test within about 90 days of enrollment.
The race-specific equations used to calculate FVCpp was that published in 1999 by Hankinson and colleagues in American Journal of Respiratory and Critical Care Medicine. The race-neutral Global Lung Function Initiative (GLI) equations by Bowerman and colleagues were developed in 2022 and published in March 2023 in the same journal.
The investigators defined access to care as enrollment in ILD clinical trials for patients with FVCpp greater than 45% but less than 90%, and US payer access to antifibrotic therapy for patients with FVCpp of greater than 55% but less than 82%.
They found that 22% of Black patients were misclassified in their eligibility for clinical trials in each of two scenarios – those who would be excluded from trials using the 1999 criteria but included using the 2022 criteria, and vice versa, that is included with 1999 criteria but excluded by the 2022 GLI criteria. In contrast, 14% of Hispanic Latino patients and 12% of White patients were misclassified.
Using the 1999 criteria to exclude patients because their values were ostensibly higher than the upper cutoff meant that 10.3% of Black patients who might benefit would be ineligible for clinical trial, compared with 0% of Hispanic/Latinos and 0.1% of Whites.
Similarly, 11.5% of Black patients but no Hispanic/Latino or White patients would be considered eligible for clinical trials using the old criteria but ineligible under the new criteria.
Regarding antifibrotic therapy eligibility, the respective misclassification rates were 21%, 17%, and 19%.
“Our study showed that use of race-specific equations may confound lung function tests, potentially leading to misclassification, delayed diagnosis, and inadequate treatment provision. While our study suggests potential disparities in access to health care for patients with interstitial lung disease facilitated by race-specific equations, further research is required to fully comprehend the implications,” the investigators wrote.
ATS statement
In an interview, Juan Wisnievsky, MD, DrPh, from Mount Sinai Medical Center, New York, who also chairs the Health Equity and Diversity Committee for the American Thoracic Society, pointed to a recent ATS statement he coauthored citing evidence for replacing race and ethnicity-specific equations with race-neutral average reference equations.
“This use of race and ethnicity may contribute to health disparities by norming differences in pulmonary function. In the United States and globally, race serves as a social construct that is based on appearance and reflects social values, structures, and practices. Classification of people into racial and ethnic groups differs geographically and temporally. These considerations challenge the notion that racial and ethnic categories have biological meaning and question the use of race in PFT interpretation,” the statement authors wrote.
“There is some agreement that race-based equations shouldn’t be used, but all the potential consequences of doing that and which equations would be the best ones to use to replace them is a bit unclear,” Dr. Wisnievsky said.
He was not involved in the study by Dr. Adegunsoye and colleagues.
Data used in the study were derived from research sponsored by F. Hoffman–La Roche and Genentech. Dr. Adegunsoye disclosed consultancy fees from AbbVie, Inogen, F. Hoffman–La Roche, Medscape, and PatientMpower; speaking/advisory fees from Boehringer Ingelheim; and grants/award from the CHEST Foundation, Pulmonary Fibrosis Foundation, and National Institutes of Health. Dr. Wisnievsky had no relevant disclosures.
HONOLULU – Old habits die hard, especially when it comes to pulmonary function testing in a diverse population of patients with interstitial lung disease (ILD).
Specifically, pulmonary care clinicians may be habitually relying on outdated and inaccurate race-specific reference values when evaluating respiratory impairment in persons of African and Hispanic/Latino ancestry, which can result in underrecognition, underdiagnosis, and undertreatment, reported Ayodeji Adegunsoye, MD, from the University of Chicago, and colleagues.
“Our results make a compelling case for re-evaluating the use of race as a physiological variable, and highlight the need to offer equitable and optimal care for all patients, regardless of their race or ethnicity,” Dr. Adegunsoye said in an oral abstract session at the annual meeting of the American College of Chest Physicians (CHEST).
Flawed assumptions
In an interview, Dr. Adegunsoye noted that race-specific notions, such as the automatic assumption that Black people have less lung capacity than White people, are baked into clinical practice and passed on as clinical wisdom from one generation of clinicians to the next.
Pulmonary function reference values that are used to make a diagnosis of idiopathic pulmonary fibrosis in Black or Hispanic/Latino patients “appear flawed when we use race-specific values. And beyond the diagnosis, it also appears to impact eligibility for key interventional strategies for managing the disease itself,” he said.
The use of race-specific equations can falsely inflate percent-predicted pulmonary function values in non-White patients, and make it seem as if a patient has normal lung function when in fact he may be have impaired function.
For example, using race-based reference values a Black patient and a White patient may appear to have the same absolute forced vital capacity readings, but different FVC percent predicted (FVCpp), which can mean a missed diagnosis.
Investigators who studied the association between self-identified race and visually identified emphysema among 2,674 participants in the Coronary Artery Risk Development in Young Adults study found that using standard equations to adjust for racial differences in lung-function measures appeared to miss emphysema in a significant proportion of Black patients.
PF registry study
In the current study, to see whether the use of race-neutral equations for evaluating FVCpp could change access to health care in patients with ILD, Dr. Adegunsoye and colleagues used both race-specific and race-neutral equations to calculate FVCpp values among separate cohorts of Black, Hispanic/Latino, and White patients enrolled in the Pulmonary Fibrosis Foundation Patient Registry who had pulmonary functions test within about 90 days of enrollment.
The race-specific equations used to calculate FVCpp was that published in 1999 by Hankinson and colleagues in American Journal of Respiratory and Critical Care Medicine. The race-neutral Global Lung Function Initiative (GLI) equations by Bowerman and colleagues were developed in 2022 and published in March 2023 in the same journal.
The investigators defined access to care as enrollment in ILD clinical trials for patients with FVCpp greater than 45% but less than 90%, and US payer access to antifibrotic therapy for patients with FVCpp of greater than 55% but less than 82%.
They found that 22% of Black patients were misclassified in their eligibility for clinical trials in each of two scenarios – those who would be excluded from trials using the 1999 criteria but included using the 2022 criteria, and vice versa, that is included with 1999 criteria but excluded by the 2022 GLI criteria. In contrast, 14% of Hispanic Latino patients and 12% of White patients were misclassified.
Using the 1999 criteria to exclude patients because their values were ostensibly higher than the upper cutoff meant that 10.3% of Black patients who might benefit would be ineligible for clinical trial, compared with 0% of Hispanic/Latinos and 0.1% of Whites.
Similarly, 11.5% of Black patients but no Hispanic/Latino or White patients would be considered eligible for clinical trials using the old criteria but ineligible under the new criteria.
Regarding antifibrotic therapy eligibility, the respective misclassification rates were 21%, 17%, and 19%.
“Our study showed that use of race-specific equations may confound lung function tests, potentially leading to misclassification, delayed diagnosis, and inadequate treatment provision. While our study suggests potential disparities in access to health care for patients with interstitial lung disease facilitated by race-specific equations, further research is required to fully comprehend the implications,” the investigators wrote.
ATS statement
In an interview, Juan Wisnievsky, MD, DrPh, from Mount Sinai Medical Center, New York, who also chairs the Health Equity and Diversity Committee for the American Thoracic Society, pointed to a recent ATS statement he coauthored citing evidence for replacing race and ethnicity-specific equations with race-neutral average reference equations.
“This use of race and ethnicity may contribute to health disparities by norming differences in pulmonary function. In the United States and globally, race serves as a social construct that is based on appearance and reflects social values, structures, and practices. Classification of people into racial and ethnic groups differs geographically and temporally. These considerations challenge the notion that racial and ethnic categories have biological meaning and question the use of race in PFT interpretation,” the statement authors wrote.
“There is some agreement that race-based equations shouldn’t be used, but all the potential consequences of doing that and which equations would be the best ones to use to replace them is a bit unclear,” Dr. Wisnievsky said.
He was not involved in the study by Dr. Adegunsoye and colleagues.
Data used in the study were derived from research sponsored by F. Hoffman–La Roche and Genentech. Dr. Adegunsoye disclosed consultancy fees from AbbVie, Inogen, F. Hoffman–La Roche, Medscape, and PatientMpower; speaking/advisory fees from Boehringer Ingelheim; and grants/award from the CHEST Foundation, Pulmonary Fibrosis Foundation, and National Institutes of Health. Dr. Wisnievsky had no relevant disclosures.
HONOLULU – Old habits die hard, especially when it comes to pulmonary function testing in a diverse population of patients with interstitial lung disease (ILD).
Specifically, pulmonary care clinicians may be habitually relying on outdated and inaccurate race-specific reference values when evaluating respiratory impairment in persons of African and Hispanic/Latino ancestry, which can result in underrecognition, underdiagnosis, and undertreatment, reported Ayodeji Adegunsoye, MD, from the University of Chicago, and colleagues.
“Our results make a compelling case for re-evaluating the use of race as a physiological variable, and highlight the need to offer equitable and optimal care for all patients, regardless of their race or ethnicity,” Dr. Adegunsoye said in an oral abstract session at the annual meeting of the American College of Chest Physicians (CHEST).
Flawed assumptions
In an interview, Dr. Adegunsoye noted that race-specific notions, such as the automatic assumption that Black people have less lung capacity than White people, are baked into clinical practice and passed on as clinical wisdom from one generation of clinicians to the next.
Pulmonary function reference values that are used to make a diagnosis of idiopathic pulmonary fibrosis in Black or Hispanic/Latino patients “appear flawed when we use race-specific values. And beyond the diagnosis, it also appears to impact eligibility for key interventional strategies for managing the disease itself,” he said.
The use of race-specific equations can falsely inflate percent-predicted pulmonary function values in non-White patients, and make it seem as if a patient has normal lung function when in fact he may be have impaired function.
For example, using race-based reference values a Black patient and a White patient may appear to have the same absolute forced vital capacity readings, but different FVC percent predicted (FVCpp), which can mean a missed diagnosis.
Investigators who studied the association between self-identified race and visually identified emphysema among 2,674 participants in the Coronary Artery Risk Development in Young Adults study found that using standard equations to adjust for racial differences in lung-function measures appeared to miss emphysema in a significant proportion of Black patients.
PF registry study
In the current study, to see whether the use of race-neutral equations for evaluating FVCpp could change access to health care in patients with ILD, Dr. Adegunsoye and colleagues used both race-specific and race-neutral equations to calculate FVCpp values among separate cohorts of Black, Hispanic/Latino, and White patients enrolled in the Pulmonary Fibrosis Foundation Patient Registry who had pulmonary functions test within about 90 days of enrollment.
The race-specific equations used to calculate FVCpp was that published in 1999 by Hankinson and colleagues in American Journal of Respiratory and Critical Care Medicine. The race-neutral Global Lung Function Initiative (GLI) equations by Bowerman and colleagues were developed in 2022 and published in March 2023 in the same journal.
The investigators defined access to care as enrollment in ILD clinical trials for patients with FVCpp greater than 45% but less than 90%, and US payer access to antifibrotic therapy for patients with FVCpp of greater than 55% but less than 82%.
They found that 22% of Black patients were misclassified in their eligibility for clinical trials in each of two scenarios – those who would be excluded from trials using the 1999 criteria but included using the 2022 criteria, and vice versa, that is included with 1999 criteria but excluded by the 2022 GLI criteria. In contrast, 14% of Hispanic Latino patients and 12% of White patients were misclassified.
Using the 1999 criteria to exclude patients because their values were ostensibly higher than the upper cutoff meant that 10.3% of Black patients who might benefit would be ineligible for clinical trial, compared with 0% of Hispanic/Latinos and 0.1% of Whites.
Similarly, 11.5% of Black patients but no Hispanic/Latino or White patients would be considered eligible for clinical trials using the old criteria but ineligible under the new criteria.
Regarding antifibrotic therapy eligibility, the respective misclassification rates were 21%, 17%, and 19%.
“Our study showed that use of race-specific equations may confound lung function tests, potentially leading to misclassification, delayed diagnosis, and inadequate treatment provision. While our study suggests potential disparities in access to health care for patients with interstitial lung disease facilitated by race-specific equations, further research is required to fully comprehend the implications,” the investigators wrote.
ATS statement
In an interview, Juan Wisnievsky, MD, DrPh, from Mount Sinai Medical Center, New York, who also chairs the Health Equity and Diversity Committee for the American Thoracic Society, pointed to a recent ATS statement he coauthored citing evidence for replacing race and ethnicity-specific equations with race-neutral average reference equations.
“This use of race and ethnicity may contribute to health disparities by norming differences in pulmonary function. In the United States and globally, race serves as a social construct that is based on appearance and reflects social values, structures, and practices. Classification of people into racial and ethnic groups differs geographically and temporally. These considerations challenge the notion that racial and ethnic categories have biological meaning and question the use of race in PFT interpretation,” the statement authors wrote.
“There is some agreement that race-based equations shouldn’t be used, but all the potential consequences of doing that and which equations would be the best ones to use to replace them is a bit unclear,” Dr. Wisnievsky said.
He was not involved in the study by Dr. Adegunsoye and colleagues.
Data used in the study were derived from research sponsored by F. Hoffman–La Roche and Genentech. Dr. Adegunsoye disclosed consultancy fees from AbbVie, Inogen, F. Hoffman–La Roche, Medscape, and PatientMpower; speaking/advisory fees from Boehringer Ingelheim; and grants/award from the CHEST Foundation, Pulmonary Fibrosis Foundation, and National Institutes of Health. Dr. Wisnievsky had no relevant disclosures.
AT CHEST 2023
PD-1 inhibitor improves outcomes in NHL subtype
, while pretreatment mutational profiles offer clues as to which patients may respond to such anti-PD-1 treatments, according to studies presented at the European Society of Medical Oncology (ESMO) Congress 2023.
“We found that toripalimab combined with radiotherapy is safe and has promising efficacy for stage I/II extranodal NK/T cell lymphoma [patients] who have poor response after previous standard chemotherapy,” said first author Ming Jiang, MD, of the department of medical oncology, Cancer Center, West China School of Medicine/West China Hospital of Sichuan University, Chengdu, China.
“This combined strategy can not only improve patient efficacy but also avoid unnecessary medication, and is worth further exploration,” she said in a presentation at ESMO on Oct. 27 in Madrid. The current standard of care for extranodal NK/T cell lymphoma, a subtype of non-Hodgkin lymphoma, is L-asparaginase or pegaspargase-based multi-agent chemotherapy combined with radiotherapy.
However, for patients who fail to respond to first-line treatment, the prognosis is poor: The median progression-free survival of those patients is approximately 4.5 months, with a median overall survival of about 6.4 months, Dr. Jiang explained.
“There is a need to establish a better first-line treatment for this group of patients,” she said.
In the prospective, single-arm, multicenter phase 2 study, Dr. Jiang and her colleagues enrolled patients with stage 1 and 2 extranodal NK/T cell lymphoma who had failed to achieve a complete response following 2-3 cycles of multi-agent chemotherapy.
Of the patients, eight (36.4%) had partial response, eight (36.2%) had stable disease, and six (27.2%) had progressive disease after the chemotherapy.
The patients were treated with toripalimab at 240 mg, once every 3 weeks, plus radiotherapy at a dose of 56 Gy, sequentially with or without two to four cycles of chemotherapy.
Patients who did not have disease progression were then continued with toripalimab for 1 year or until disease progression or intolerable toxicity.
The 22 patients had a median age of 45 (range 26-64) and 14 were male. Most were stage 1 (77.3%; 17) and the remaining were stage 2, while 81% had primary tumor invasion.
For the primary endpoint, at 3 months following radiotherapy, the overall response rate was 90.9%, with 17 patients (77.3%) having a complete response, 3 (13.6%) a partial response, and 2 (9.1%) having progressive disease.
Eight who had responded to previous chemotherapy received two additional chemotherapy cycles after completion of radiotherapy, while the others were treated with toripalimab alone.
With a median follow-up of 23 months (range 3-78), the 2-year progression-free survival was 81.6%, and overall survival was 95.0%.
Two of three patients with a partial response had a recurrence after radiotherapy at 5 and 10 months; one of the complete-response patients had a recurrence at 60 months, and two patients with progressive disease died at 9 months after radiotherapy.
In terms of safety, the most common adverse events during and after radiotherapy included oral mucositis and hypothyroidism. No adverse events of grade 3 or higher were reported.
Dr. Jiang speculated that “radiotherapy could synergize PD-1 inhibitors,” and she urged that “optimal radiotherapy and PD-1 inhibitor administration plans should be further explored.”
Genetic factors
Additional research presented in that ESMO session offered insights into the genetic factors that may play key roles in either response or resistance to anti-PD-1 therapy in peripheral T cell lymphoma (PTCL), of which extranodal NK/T cell lymphoma is a subtype.
The findings are from a genetic analysis of a phase 2 trial that demonstrated benefits the PD-1 inhibitor geptanolimab in patients with PTCL who failed initial chemotherapy.
Specifically, geptanolimab treatment was associated with an objective response rate of 40.4%, a complete response rate of 14.6%, and partial response rate of 25.8%.
Of 44 patients who had been treated with geptanolimab and had next-generation sequencing genetic data available, PD-L1 expression was found to be significantly elevated among those who had a complete or partial response, whereas PD-L1 expression was lower among those who had disease progression, which is consistent with previous research suggesting that low PD-L1 expression is linked to poorer response to anti-PD-1 therapies.
Tumor mutation burden did not exhibit significant prognostic value. However, the authors noted that this may be confounded by variation across PTCL subtypes.
Among other key findings were that JAK3 and EZH2 mutations, which are among the top genes frequently mutated in PTCL and extranodal NK/T cell lymphoma, were consistently associated with low PD-L1 expression (P < .05) and shorter progression-free survival (HR 5.97; P = .027, JAK3, and HR 4.76; P = .027 EZH2).
“Notably, we found JAK3 mutations, which are vital and prevalent in PTCL, reduced PD-L1 levels in vivo and in vitro, which are of great clinical and biological sense,” said the study’s first author, Ning Lou, MD, of the Cancer Hospital Chinese Academy of Medical Sciences & Peking Union Medical College, in Beijing.
Commenting on the study, discussant Olivier Casasnovas, MD, PhD, of the department of hematology, University Hospital Francois Mitterrand in Dijon, France, said that the findings are especially notable in relation to extranodal NK/T cell lymphoma.
“The clinical relevance of anti PD1 is mainly observed in relapsing/recurrent extranodal NK/T cell lymphoma, and much less in other T-cell lymphoma subtypes,” he told this news organization.
“So identifying molecular events associated with the chance of response to a PD1 blocker in relapsing extranodal NK/T cell lymphoma is important as PD1-blockers are recommended to treat [those] patients,” Dr. Casasnovas added.
Furthermore, “the interest of next-generation sequencing to identify JAK3 mutations associated with low level of PDL1 expression and weak response to anti PD1 blockers is important as JAK3 mutated tumors are potentially targetable by JAK inhibitors such as tofacitinib,” he said.
“Obviously this assumption has to be tested in clinical trials but it’s an interesting lead.”
The research on toripalimab additionally shows that “the combination of radiotherapy and PD1 blockers provides a high response rate in patients who are nonresponders to asparaginase-based chemotherapy on the basis of PET evaluation and could be a new option for optimizing the first line treatment of extranodal NK/T cell lymphoma patients,” Dr. Casasnovas added.
The authors and Dr. Casasnovas had no disclosures to report.
, while pretreatment mutational profiles offer clues as to which patients may respond to such anti-PD-1 treatments, according to studies presented at the European Society of Medical Oncology (ESMO) Congress 2023.
“We found that toripalimab combined with radiotherapy is safe and has promising efficacy for stage I/II extranodal NK/T cell lymphoma [patients] who have poor response after previous standard chemotherapy,” said first author Ming Jiang, MD, of the department of medical oncology, Cancer Center, West China School of Medicine/West China Hospital of Sichuan University, Chengdu, China.
“This combined strategy can not only improve patient efficacy but also avoid unnecessary medication, and is worth further exploration,” she said in a presentation at ESMO on Oct. 27 in Madrid. The current standard of care for extranodal NK/T cell lymphoma, a subtype of non-Hodgkin lymphoma, is L-asparaginase or pegaspargase-based multi-agent chemotherapy combined with radiotherapy.
However, for patients who fail to respond to first-line treatment, the prognosis is poor: The median progression-free survival of those patients is approximately 4.5 months, with a median overall survival of about 6.4 months, Dr. Jiang explained.
“There is a need to establish a better first-line treatment for this group of patients,” she said.
In the prospective, single-arm, multicenter phase 2 study, Dr. Jiang and her colleagues enrolled patients with stage 1 and 2 extranodal NK/T cell lymphoma who had failed to achieve a complete response following 2-3 cycles of multi-agent chemotherapy.
Of the patients, eight (36.4%) had partial response, eight (36.2%) had stable disease, and six (27.2%) had progressive disease after the chemotherapy.
The patients were treated with toripalimab at 240 mg, once every 3 weeks, plus radiotherapy at a dose of 56 Gy, sequentially with or without two to four cycles of chemotherapy.
Patients who did not have disease progression were then continued with toripalimab for 1 year or until disease progression or intolerable toxicity.
The 22 patients had a median age of 45 (range 26-64) and 14 were male. Most were stage 1 (77.3%; 17) and the remaining were stage 2, while 81% had primary tumor invasion.
For the primary endpoint, at 3 months following radiotherapy, the overall response rate was 90.9%, with 17 patients (77.3%) having a complete response, 3 (13.6%) a partial response, and 2 (9.1%) having progressive disease.
Eight who had responded to previous chemotherapy received two additional chemotherapy cycles after completion of radiotherapy, while the others were treated with toripalimab alone.
With a median follow-up of 23 months (range 3-78), the 2-year progression-free survival was 81.6%, and overall survival was 95.0%.
Two of three patients with a partial response had a recurrence after radiotherapy at 5 and 10 months; one of the complete-response patients had a recurrence at 60 months, and two patients with progressive disease died at 9 months after radiotherapy.
In terms of safety, the most common adverse events during and after radiotherapy included oral mucositis and hypothyroidism. No adverse events of grade 3 or higher were reported.
Dr. Jiang speculated that “radiotherapy could synergize PD-1 inhibitors,” and she urged that “optimal radiotherapy and PD-1 inhibitor administration plans should be further explored.”
Genetic factors
Additional research presented in that ESMO session offered insights into the genetic factors that may play key roles in either response or resistance to anti-PD-1 therapy in peripheral T cell lymphoma (PTCL), of which extranodal NK/T cell lymphoma is a subtype.
The findings are from a genetic analysis of a phase 2 trial that demonstrated benefits the PD-1 inhibitor geptanolimab in patients with PTCL who failed initial chemotherapy.
Specifically, geptanolimab treatment was associated with an objective response rate of 40.4%, a complete response rate of 14.6%, and partial response rate of 25.8%.
Of 44 patients who had been treated with geptanolimab and had next-generation sequencing genetic data available, PD-L1 expression was found to be significantly elevated among those who had a complete or partial response, whereas PD-L1 expression was lower among those who had disease progression, which is consistent with previous research suggesting that low PD-L1 expression is linked to poorer response to anti-PD-1 therapies.
Tumor mutation burden did not exhibit significant prognostic value. However, the authors noted that this may be confounded by variation across PTCL subtypes.
Among other key findings were that JAK3 and EZH2 mutations, which are among the top genes frequently mutated in PTCL and extranodal NK/T cell lymphoma, were consistently associated with low PD-L1 expression (P < .05) and shorter progression-free survival (HR 5.97; P = .027, JAK3, and HR 4.76; P = .027 EZH2).
“Notably, we found JAK3 mutations, which are vital and prevalent in PTCL, reduced PD-L1 levels in vivo and in vitro, which are of great clinical and biological sense,” said the study’s first author, Ning Lou, MD, of the Cancer Hospital Chinese Academy of Medical Sciences & Peking Union Medical College, in Beijing.
Commenting on the study, discussant Olivier Casasnovas, MD, PhD, of the department of hematology, University Hospital Francois Mitterrand in Dijon, France, said that the findings are especially notable in relation to extranodal NK/T cell lymphoma.
“The clinical relevance of anti PD1 is mainly observed in relapsing/recurrent extranodal NK/T cell lymphoma, and much less in other T-cell lymphoma subtypes,” he told this news organization.
“So identifying molecular events associated with the chance of response to a PD1 blocker in relapsing extranodal NK/T cell lymphoma is important as PD1-blockers are recommended to treat [those] patients,” Dr. Casasnovas added.
Furthermore, “the interest of next-generation sequencing to identify JAK3 mutations associated with low level of PDL1 expression and weak response to anti PD1 blockers is important as JAK3 mutated tumors are potentially targetable by JAK inhibitors such as tofacitinib,” he said.
“Obviously this assumption has to be tested in clinical trials but it’s an interesting lead.”
The research on toripalimab additionally shows that “the combination of radiotherapy and PD1 blockers provides a high response rate in patients who are nonresponders to asparaginase-based chemotherapy on the basis of PET evaluation and could be a new option for optimizing the first line treatment of extranodal NK/T cell lymphoma patients,” Dr. Casasnovas added.
The authors and Dr. Casasnovas had no disclosures to report.
, while pretreatment mutational profiles offer clues as to which patients may respond to such anti-PD-1 treatments, according to studies presented at the European Society of Medical Oncology (ESMO) Congress 2023.
“We found that toripalimab combined with radiotherapy is safe and has promising efficacy for stage I/II extranodal NK/T cell lymphoma [patients] who have poor response after previous standard chemotherapy,” said first author Ming Jiang, MD, of the department of medical oncology, Cancer Center, West China School of Medicine/West China Hospital of Sichuan University, Chengdu, China.
“This combined strategy can not only improve patient efficacy but also avoid unnecessary medication, and is worth further exploration,” she said in a presentation at ESMO on Oct. 27 in Madrid. The current standard of care for extranodal NK/T cell lymphoma, a subtype of non-Hodgkin lymphoma, is L-asparaginase or pegaspargase-based multi-agent chemotherapy combined with radiotherapy.
However, for patients who fail to respond to first-line treatment, the prognosis is poor: The median progression-free survival of those patients is approximately 4.5 months, with a median overall survival of about 6.4 months, Dr. Jiang explained.
“There is a need to establish a better first-line treatment for this group of patients,” she said.
In the prospective, single-arm, multicenter phase 2 study, Dr. Jiang and her colleagues enrolled patients with stage 1 and 2 extranodal NK/T cell lymphoma who had failed to achieve a complete response following 2-3 cycles of multi-agent chemotherapy.
Of the patients, eight (36.4%) had partial response, eight (36.2%) had stable disease, and six (27.2%) had progressive disease after the chemotherapy.
The patients were treated with toripalimab at 240 mg, once every 3 weeks, plus radiotherapy at a dose of 56 Gy, sequentially with or without two to four cycles of chemotherapy.
Patients who did not have disease progression were then continued with toripalimab for 1 year or until disease progression or intolerable toxicity.
The 22 patients had a median age of 45 (range 26-64) and 14 were male. Most were stage 1 (77.3%; 17) and the remaining were stage 2, while 81% had primary tumor invasion.
For the primary endpoint, at 3 months following radiotherapy, the overall response rate was 90.9%, with 17 patients (77.3%) having a complete response, 3 (13.6%) a partial response, and 2 (9.1%) having progressive disease.
Eight who had responded to previous chemotherapy received two additional chemotherapy cycles after completion of radiotherapy, while the others were treated with toripalimab alone.
With a median follow-up of 23 months (range 3-78), the 2-year progression-free survival was 81.6%, and overall survival was 95.0%.
Two of three patients with a partial response had a recurrence after radiotherapy at 5 and 10 months; one of the complete-response patients had a recurrence at 60 months, and two patients with progressive disease died at 9 months after radiotherapy.
In terms of safety, the most common adverse events during and after radiotherapy included oral mucositis and hypothyroidism. No adverse events of grade 3 or higher were reported.
Dr. Jiang speculated that “radiotherapy could synergize PD-1 inhibitors,” and she urged that “optimal radiotherapy and PD-1 inhibitor administration plans should be further explored.”
Genetic factors
Additional research presented in that ESMO session offered insights into the genetic factors that may play key roles in either response or resistance to anti-PD-1 therapy in peripheral T cell lymphoma (PTCL), of which extranodal NK/T cell lymphoma is a subtype.
The findings are from a genetic analysis of a phase 2 trial that demonstrated benefits the PD-1 inhibitor geptanolimab in patients with PTCL who failed initial chemotherapy.
Specifically, geptanolimab treatment was associated with an objective response rate of 40.4%, a complete response rate of 14.6%, and partial response rate of 25.8%.
Of 44 patients who had been treated with geptanolimab and had next-generation sequencing genetic data available, PD-L1 expression was found to be significantly elevated among those who had a complete or partial response, whereas PD-L1 expression was lower among those who had disease progression, which is consistent with previous research suggesting that low PD-L1 expression is linked to poorer response to anti-PD-1 therapies.
Tumor mutation burden did not exhibit significant prognostic value. However, the authors noted that this may be confounded by variation across PTCL subtypes.
Among other key findings were that JAK3 and EZH2 mutations, which are among the top genes frequently mutated in PTCL and extranodal NK/T cell lymphoma, were consistently associated with low PD-L1 expression (P < .05) and shorter progression-free survival (HR 5.97; P = .027, JAK3, and HR 4.76; P = .027 EZH2).
“Notably, we found JAK3 mutations, which are vital and prevalent in PTCL, reduced PD-L1 levels in vivo and in vitro, which are of great clinical and biological sense,” said the study’s first author, Ning Lou, MD, of the Cancer Hospital Chinese Academy of Medical Sciences & Peking Union Medical College, in Beijing.
Commenting on the study, discussant Olivier Casasnovas, MD, PhD, of the department of hematology, University Hospital Francois Mitterrand in Dijon, France, said that the findings are especially notable in relation to extranodal NK/T cell lymphoma.
“The clinical relevance of anti PD1 is mainly observed in relapsing/recurrent extranodal NK/T cell lymphoma, and much less in other T-cell lymphoma subtypes,” he told this news organization.
“So identifying molecular events associated with the chance of response to a PD1 blocker in relapsing extranodal NK/T cell lymphoma is important as PD1-blockers are recommended to treat [those] patients,” Dr. Casasnovas added.
Furthermore, “the interest of next-generation sequencing to identify JAK3 mutations associated with low level of PDL1 expression and weak response to anti PD1 blockers is important as JAK3 mutated tumors are potentially targetable by JAK inhibitors such as tofacitinib,” he said.
“Obviously this assumption has to be tested in clinical trials but it’s an interesting lead.”
The research on toripalimab additionally shows that “the combination of radiotherapy and PD1 blockers provides a high response rate in patients who are nonresponders to asparaginase-based chemotherapy on the basis of PET evaluation and could be a new option for optimizing the first line treatment of extranodal NK/T cell lymphoma patients,” Dr. Casasnovas added.
The authors and Dr. Casasnovas had no disclosures to report.
FROM ESMO 2023
Returning to work after a patient assault
Mr. B, age 23, is admitted to an inpatient psychiatric unit for depression. During his hospitalization, Mr. B becomes fixated on obtaining specific medications, including controlled substances. He is treated by Dr. M, a psychiatrist early in her training. In a difficult conversation, Dr. M tells Mr. B he will not be prescribed the medications he is requesting and explains why. Mr. B responds by jumping across a table and repeatedly punching Dr. M. Unit staff restrains Mr. B, and Dr. M leaves to seek medical care.
Assaults perpetrated against employees on inpatient psychiatric units are common.1 Assaults on physicians can occur at any level of training, including during residency.2 This is not a new phenomenon: concerns about patients assaulting psychiatrists and other inpatient staff have been reported for decades.3-5 Most research surrounding this topic has focused on risk factors for violence and prevention.6 Research regarding the aftermath of a patient assault and what services an employee requires have primarily centered on nurses.7,8
Practical guidance for a psychiatrist who has been assaulted and wants to return to work is difficult to find. This article provides strategies to help psychiatrists (and their colleagues) transition back to work after being the victim of a patient assault. While the recommendations we provide can be applied to trainees as well as attending physicians, there are some considerations specific to residents who have been assaulted (Box9,10).
Box
Psychiatry residents who are the targets of violence (such as Dr. M) require unique management, including evaluation of how the assault impacts their training and the role of the program director. Additionally, according to the Accreditation Council for Graduate Medical Education (ACGME) Common Program Requirements, residency programs must address residents’ wellbeing, including “evaluating workplace safety data and addressing the safety of residents and faculty members.”9 These specific considerations for residents are guided by the most recent program requirements through ACGME, as well as the policies of the specific institution overseeing the residency. Some institutions have developed resources to assist in this area, such as the WELL Toolkit from the University of Pittsburgh Medical Center.10
Having a plan for after an assault
The aftereffects of a patient assault can take a significant toll on the individual who is assaulted. A 2021 article about psychiatric mental health nurses by Dean et al8 identified multiple potential repercussions of unaddressed workplace violence, including role confusion, job dissatisfaction, decreased resiliency traits, poor coping methods, increased attrition rate, and increased expenditures related to assault injuries. Providing appropriate services and having a plan for how best to support an assaulted psychiatrist are likely to mitigate these effects. This can be grouped into 4 categories: 1) seeking immediate care, 2) removing the patient from your care, 3) easing back into the environment, and 4) finding long-term support.
1. Seeking immediate care
“Round or be rounded on” is a phrase that encapsulates many physicians’ attitude regarding their own health care and may contribute to their refusal of medical care following acute trauma such as an assault. Feelings of shock, guilt, and shame may also lead to a psychiatrist’s initial hesitation to seek treatment. However, it is important for the victim of an assault to be promptly evaluated and treated.
Elevated adrenaline in the aftermath of a physical engagement may mask the perception of injuries, and there is a risk for exposure to blood-borne pathogens. Regardless of the severity of injuries, seeking medical care establishes documentation of any injuries that can later serve as a record for workers’ compensation claims or if legal action is taken.
In addition to medical needs, immediate psychological support should be considered. Compulsory participation in crisis intervention stress debriefing, particularly when performed by untrained individuals, is not recommended due to questions about its demonstrated efficacy and potential to increase the risk of posttraumatic stress disorder (PTSD) in the long term.11,12 However, research has established the need for immediate support that does not necessarily involve a discussion of the traumatic event. One option is psychological first aid (PFA), an intervention supported by the World Health Organization. Originally developed for victims of mass crisis events, PFA easily translates to the hospital setting.12,13 PFA focuses on the immediate, basic needs of the victim to reduce distress and anxiety and encourage adaptive coping. Table 112,13 summarizes key components of PFA.

Continue to: PFA can be compared...
PFA can be compared to medical first aid in the field prior to reaching the hospital. In the case of Dr. M, other residents collaborated to transport her to the hospital, keep attendings and program directors apprised of the situation, and bring her snacks and comfort items to the hospital. Dr. M also received support from attending physicians at a neighboring hospital who helped coordinate her care. Essentially, she received a de facto version of PFA. However, given the evidence behind PFA and the unfortunate rate of violence against health care staff, institutions and organizations may offer training in PFA to ensure this level of support for all victims.
Multiple groups may take the lead to support a physician following an injury, including human resources, employee health, or other offices within the institution. The principles of PFA can be used to guide these employees in assisting the victim. Even if such employees are not trained in PFA, they can align with these principles by ensuring access to counseling and medical care, assisting with time off and accommodations, and helping the victim of an assault navigate the legal and administrative processes. Workers’ compensation can be a challenging process, and an institution’s human resources department should be available to assist the assaulted individual in navigating resources both within and outside of what they are able to offer.
2. Removing the patient from the psychiatrist’s care
During her recovery, Dr. M heard from a few peers that what happened was an occupational hazard. On some level, they were correct. While the public does not perceive a career in medicine to be physically dangerous, violence is a rampant problem in health care. Research shows that health care professionals are up to 16 times more likely to experience violence than other occupations; the odds for nurses are even higher.8
The frequency and pervasiveness of violence against health care professionals create an environment in which it can become an expected, and even accepted, phenomenon. However, violence cannot and should not be viewed as a normal part of workplace culture. A 2016 study by Moylan et al7 found that many nurses believe violence is part of their role, and therefore do not recognize the need to report such incidents or seek the necessary support. In other studies, only 30% of nurses reported violence, and the rate of reporting by physicians was 26%.14 This underreporting likely represents the role confusion surrounding whether caring for self or caring for the patient takes precedent, as well as normative expectations surrounding violence in the workplace.
It must be made clear to the victim that their safety is a priority and violence will not be tolerated. An institution’s administration can achieve this by immediately removing the patient from the victim’s care. In many cases, discharge of the patient from the clinic or facility may be warranted. A psychiatrist should not be expected to continue as the primary physician for a patient who has assaulted them; transfer to another psychiatrist is necessary if discharge is not an appropriate option. In a scenario in which a psychiatrist must maintain the treating relationship with a patient who assaulted them until the patient can be placed with another clinician (eg, as might occur on a unit with severely limited resources), staff chaperones can be considered when interacting with the patient.
Continue to: An institution's adminstration...
An institution’s administration should provide support if the psychiatrist chooses to press charges. At the core of our ethos as physicians is “do no harm,” and for some, the prospect of filing charges may be a difficult decision. However, health care professionals do not have an ethical obligation to put themselves in danger of serious bodily harm.15 While there is no one-size-fits-all answer to the question of whether or not to press charges against a patient who has committed an assault, the Occupational Safety and Health Administration considers the perception that violence is tolerated and victims are unable to report to law enforcement an organizational risk factor for workplace violence.16
As leaders in the workplace, physicians should set the precedent that violence will not be tolerated by reporting incidents to police and filing charges when appropriate. In the case of Dr. M, she received full support from her institution’s administration in filing charges against Mr. B due to the specific details of the assault.
3. Easing back into the environment
Despite assurances from her superiors that she could take time off, Dr. M wanted to return to work as soon as possible. She considered the balance between her physical injuries and desire to return to work and ultimately returned to work 5 days after the assault. She did well with supportive measures from administration and other staff, including the use of technician escorts on the unit, peer support, and frequent communication with and check-ins from management.
The decision on how quickly to return to work should always lie with the individual who was assaulted. The administration should offer time off without hesitation. Victims of an assault may feel overwhelmed by 2 diverging paths on how to return to a traumatic environment: avoid the location at all costs, or try to “face their fears” and return as quickly as possible. Research from outside medicine indicates that the timing of returning to work after a traumatic injury may not be nearly as important as the method of returning, and who makes this decision.17 Predictors of return to work after an assault include not only the severity of the trauma and amount of distress symptoms, but also any actual or perceived injustice on the part of the victim.17 Although this study was not specific to health care employees, it suggests that overall, an employee who does not feel a sense of control over their choice to return to work could perceive that as an injustice on the part of administration, leading to decreased job satisfaction.17
A study by Lamothe et al18 that was specific to health care professionals found that despite the importance of self-efficacy for the assault victim, perceived organizational support had an even greater protective effect following patient violence. Additionally, monitoring for signs of distress among victims after an episode of violence could prevent further violence by reducing the risk for subsequent victimization.18 This highlights the need for leadership of an inpatient unit to be keenly aware of how an assault on a psychiatrist or other health care professional may change the work environment and create a need to help staff navigate the new normal they may face on the unit.
Continue to: Finding long-term support
4. Finding long-term support
Longitudinal support is key in the initial transition back to work, as well as in the following weeks and months. Studies assessing the impacts of patient assault on mental health nurses indicate that while most individuals exposed to a traumatic event do not develop PTSD, many reported continued somatic symptoms, and more still reported ongoing psychological effects such as recurring thoughts of the assault, fear, generalized anger, and feeling a loss of control.8 Peer support is a common method employed by physicians and nurses alike, but administrative support is also essential.8
Regardless which form of psychotherapy, medication treatment, or peer support is utilized, access to the tools the psychiatrist finds most helpful is crucial to making them feel safe and comfortable returning to their role. Table 2 details practical steps administrators and peers can take to facilitate longitudinal support in these situations. In the case of Dr. M, administration was not only supportive in encouraging time off, but also in allowing protected time for therapy when she endorsed distress over the event. The combination of immediate responses and more long-term support greatly helped Dr. M continue her role as a psychiatrist and remain satisfied with her work.

Bottom Line
Being assaulted by a patient can make a psychiatrist reluctant to return to work. Strategies to ease this transition include seeking immediate care, removing the patient from the care of the psychiatrist who was assaulted, easing back into the environment, and finding long-term support.
Related Resources
- Lapic S, Joshi KG. What to do after a patient assaults you. Current Psychiatry. 2017;16(10):53-54.
- Joshi KG. Workplace violence: enhance your safety in outpatient settings. Current Psychiatry. 2021;20(8):37-38. doi:10.12788/cp.0163
- Su D. Harassment of health care workers: a survey. Current Psychiatry. 2021;20(6):48-50. doi:10.12788/cp.0135
- Rozel JS, Wiles C, Amin P. Too close for comfort: when the psychiatrist is stalked. Current Psychiatry. 2022;21(1): 23-28. doi:10.12788/cp.0209
1. Odes R, Chapman S, Harrison R, et al. Frequency of violence towards healthcare workers in the United States’ inpatient psychiatric hospitals: a systematic review of literature. Int J Ment Health Nurs. 2021;30(1):27-46.
2. Chaimowitz GA, Moscovitch A. Patient assaults on psychiatric residents: the Canadian experience. Can J Psychiatry. 1991;36(2):107-111.
3. Faulkner LR, Grimm NR, MacFarland BH, et al. Threats and assaults against psychiatrists. Bull Am Acad Psychiatry Law. 1990;18(1):37-46.
4. Carmel H, Hunter M. Psychiatrists injured by patient attack. Bull Am Acad Psychiatry Law. 1991;19(3):309-316.
5. Kwok S, Ostermeyer B, Coverdale J. A systematic review of the prevalence of patient assaults against residents. J Grad Med Educ. 2012;4(3):296-300.
6. Weltens I, Bak M, Verhagen S, et al. Aggression on the psychiatric ward: prevalence and risk factors. A systematic review of the literature. PLoS One. 2021;16(10):e0258346.
7. Moylan L, McManus M, Cullinan M, et al. Need for specialized support services for nurse victims of physical assault by psychiatric patients. Issues Ment Health Nurs. 2016;37(7):446-450.
8. Dean L, Butler A, Cuddigan J. The impact of workplace violence toward psychiatric mental health nurses: identifying the facilitators and barriers to supportive resources. J Am Psychiatr Nurses Assoc. 2021;27(3):189-202.
9. Accreditation Council for Graduate Medical Education. Common program requirements (Residency). July 2023. Accessed September 20, 2023. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2023v3.pdf
10. WELL Toolkit. UPMC GME Well-Being. October 3, 2022. Accessed September 20, 2023. https://gmewellness.upmc.com/
11. Rose S, Bisson J, Churchill R, et al. Psychological debriefing for preventing post traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2002;(2):CD000560.
12. Flannery RB Jr, Farley E, Rego S, et al. Characteristics of staff victims of psychiatric patient assaults: 15-year analysis of the Assaulted Staff Action Program (ASAP). Psychiatr Q. 2007;78(1):25-37.
13. Gispen F, Wu AW. Psychological first aid: CPR for mental health crises in healthcare. J Patient Saf Risk Manag. 2018:23(2):51-53.
14. Phillips JP. Workplace violence against health care workers in the United States. N Eng J Med. 2016;374(17):1661-1669.
15. Baby M, Glue P, Carlyle D. ‘Violence is not part of our job’: a thematic analysis of psychiatric mental health nurses’ experiences of patient assaults from a New Zealand perspective. Issues Ment Health Nurs. 2014;35(9):647-655.
16. Occupational Safety and Health Administration. Guidelines for Preventing Workplace Violence for Healthcare and Social Service Workers. Occupational Safety and Health Administration, US Dept of Labor; 2015.
17. Giummarra, MJ, Cameron PA, Ponsford J, et al. Return to work after traumatic injury: increased work-related disability in injured persons receiving financial compensation is mediated by perceived injustice. J Occup Rehabil. 2017;27(2):173-185.
18. Lamothe J, Boyer R, Guay S. A longitudinal analysis of psychological distress among healthcare workers following patient violence. Can J Behav Sci. 2021;53(1):48-58.
Mr. B, age 23, is admitted to an inpatient psychiatric unit for depression. During his hospitalization, Mr. B becomes fixated on obtaining specific medications, including controlled substances. He is treated by Dr. M, a psychiatrist early in her training. In a difficult conversation, Dr. M tells Mr. B he will not be prescribed the medications he is requesting and explains why. Mr. B responds by jumping across a table and repeatedly punching Dr. M. Unit staff restrains Mr. B, and Dr. M leaves to seek medical care.
Assaults perpetrated against employees on inpatient psychiatric units are common.1 Assaults on physicians can occur at any level of training, including during residency.2 This is not a new phenomenon: concerns about patients assaulting psychiatrists and other inpatient staff have been reported for decades.3-5 Most research surrounding this topic has focused on risk factors for violence and prevention.6 Research regarding the aftermath of a patient assault and what services an employee requires have primarily centered on nurses.7,8
Practical guidance for a psychiatrist who has been assaulted and wants to return to work is difficult to find. This article provides strategies to help psychiatrists (and their colleagues) transition back to work after being the victim of a patient assault. While the recommendations we provide can be applied to trainees as well as attending physicians, there are some considerations specific to residents who have been assaulted (Box9,10).
Box
Psychiatry residents who are the targets of violence (such as Dr. M) require unique management, including evaluation of how the assault impacts their training and the role of the program director. Additionally, according to the Accreditation Council for Graduate Medical Education (ACGME) Common Program Requirements, residency programs must address residents’ wellbeing, including “evaluating workplace safety data and addressing the safety of residents and faculty members.”9 These specific considerations for residents are guided by the most recent program requirements through ACGME, as well as the policies of the specific institution overseeing the residency. Some institutions have developed resources to assist in this area, such as the WELL Toolkit from the University of Pittsburgh Medical Center.10
Having a plan for after an assault
The aftereffects of a patient assault can take a significant toll on the individual who is assaulted. A 2021 article about psychiatric mental health nurses by Dean et al8 identified multiple potential repercussions of unaddressed workplace violence, including role confusion, job dissatisfaction, decreased resiliency traits, poor coping methods, increased attrition rate, and increased expenditures related to assault injuries. Providing appropriate services and having a plan for how best to support an assaulted psychiatrist are likely to mitigate these effects. This can be grouped into 4 categories: 1) seeking immediate care, 2) removing the patient from your care, 3) easing back into the environment, and 4) finding long-term support.
1. Seeking immediate care
“Round or be rounded on” is a phrase that encapsulates many physicians’ attitude regarding their own health care and may contribute to their refusal of medical care following acute trauma such as an assault. Feelings of shock, guilt, and shame may also lead to a psychiatrist’s initial hesitation to seek treatment. However, it is important for the victim of an assault to be promptly evaluated and treated.
Elevated adrenaline in the aftermath of a physical engagement may mask the perception of injuries, and there is a risk for exposure to blood-borne pathogens. Regardless of the severity of injuries, seeking medical care establishes documentation of any injuries that can later serve as a record for workers’ compensation claims or if legal action is taken.
In addition to medical needs, immediate psychological support should be considered. Compulsory participation in crisis intervention stress debriefing, particularly when performed by untrained individuals, is not recommended due to questions about its demonstrated efficacy and potential to increase the risk of posttraumatic stress disorder (PTSD) in the long term.11,12 However, research has established the need for immediate support that does not necessarily involve a discussion of the traumatic event. One option is psychological first aid (PFA), an intervention supported by the World Health Organization. Originally developed for victims of mass crisis events, PFA easily translates to the hospital setting.12,13 PFA focuses on the immediate, basic needs of the victim to reduce distress and anxiety and encourage adaptive coping. Table 112,13 summarizes key components of PFA.

Continue to: PFA can be compared...
PFA can be compared to medical first aid in the field prior to reaching the hospital. In the case of Dr. M, other residents collaborated to transport her to the hospital, keep attendings and program directors apprised of the situation, and bring her snacks and comfort items to the hospital. Dr. M also received support from attending physicians at a neighboring hospital who helped coordinate her care. Essentially, she received a de facto version of PFA. However, given the evidence behind PFA and the unfortunate rate of violence against health care staff, institutions and organizations may offer training in PFA to ensure this level of support for all victims.
Multiple groups may take the lead to support a physician following an injury, including human resources, employee health, or other offices within the institution. The principles of PFA can be used to guide these employees in assisting the victim. Even if such employees are not trained in PFA, they can align with these principles by ensuring access to counseling and medical care, assisting with time off and accommodations, and helping the victim of an assault navigate the legal and administrative processes. Workers’ compensation can be a challenging process, and an institution’s human resources department should be available to assist the assaulted individual in navigating resources both within and outside of what they are able to offer.
2. Removing the patient from the psychiatrist’s care
During her recovery, Dr. M heard from a few peers that what happened was an occupational hazard. On some level, they were correct. While the public does not perceive a career in medicine to be physically dangerous, violence is a rampant problem in health care. Research shows that health care professionals are up to 16 times more likely to experience violence than other occupations; the odds for nurses are even higher.8
The frequency and pervasiveness of violence against health care professionals create an environment in which it can become an expected, and even accepted, phenomenon. However, violence cannot and should not be viewed as a normal part of workplace culture. A 2016 study by Moylan et al7 found that many nurses believe violence is part of their role, and therefore do not recognize the need to report such incidents or seek the necessary support. In other studies, only 30% of nurses reported violence, and the rate of reporting by physicians was 26%.14 This underreporting likely represents the role confusion surrounding whether caring for self or caring for the patient takes precedent, as well as normative expectations surrounding violence in the workplace.
It must be made clear to the victim that their safety is a priority and violence will not be tolerated. An institution’s administration can achieve this by immediately removing the patient from the victim’s care. In many cases, discharge of the patient from the clinic or facility may be warranted. A psychiatrist should not be expected to continue as the primary physician for a patient who has assaulted them; transfer to another psychiatrist is necessary if discharge is not an appropriate option. In a scenario in which a psychiatrist must maintain the treating relationship with a patient who assaulted them until the patient can be placed with another clinician (eg, as might occur on a unit with severely limited resources), staff chaperones can be considered when interacting with the patient.
Continue to: An institution's adminstration...
An institution’s administration should provide support if the psychiatrist chooses to press charges. At the core of our ethos as physicians is “do no harm,” and for some, the prospect of filing charges may be a difficult decision. However, health care professionals do not have an ethical obligation to put themselves in danger of serious bodily harm.15 While there is no one-size-fits-all answer to the question of whether or not to press charges against a patient who has committed an assault, the Occupational Safety and Health Administration considers the perception that violence is tolerated and victims are unable to report to law enforcement an organizational risk factor for workplace violence.16
As leaders in the workplace, physicians should set the precedent that violence will not be tolerated by reporting incidents to police and filing charges when appropriate. In the case of Dr. M, she received full support from her institution’s administration in filing charges against Mr. B due to the specific details of the assault.
3. Easing back into the environment
Despite assurances from her superiors that she could take time off, Dr. M wanted to return to work as soon as possible. She considered the balance between her physical injuries and desire to return to work and ultimately returned to work 5 days after the assault. She did well with supportive measures from administration and other staff, including the use of technician escorts on the unit, peer support, and frequent communication with and check-ins from management.
The decision on how quickly to return to work should always lie with the individual who was assaulted. The administration should offer time off without hesitation. Victims of an assault may feel overwhelmed by 2 diverging paths on how to return to a traumatic environment: avoid the location at all costs, or try to “face their fears” and return as quickly as possible. Research from outside medicine indicates that the timing of returning to work after a traumatic injury may not be nearly as important as the method of returning, and who makes this decision.17 Predictors of return to work after an assault include not only the severity of the trauma and amount of distress symptoms, but also any actual or perceived injustice on the part of the victim.17 Although this study was not specific to health care employees, it suggests that overall, an employee who does not feel a sense of control over their choice to return to work could perceive that as an injustice on the part of administration, leading to decreased job satisfaction.17
A study by Lamothe et al18 that was specific to health care professionals found that despite the importance of self-efficacy for the assault victim, perceived organizational support had an even greater protective effect following patient violence. Additionally, monitoring for signs of distress among victims after an episode of violence could prevent further violence by reducing the risk for subsequent victimization.18 This highlights the need for leadership of an inpatient unit to be keenly aware of how an assault on a psychiatrist or other health care professional may change the work environment and create a need to help staff navigate the new normal they may face on the unit.
Continue to: Finding long-term support
4. Finding long-term support
Longitudinal support is key in the initial transition back to work, as well as in the following weeks and months. Studies assessing the impacts of patient assault on mental health nurses indicate that while most individuals exposed to a traumatic event do not develop PTSD, many reported continued somatic symptoms, and more still reported ongoing psychological effects such as recurring thoughts of the assault, fear, generalized anger, and feeling a loss of control.8 Peer support is a common method employed by physicians and nurses alike, but administrative support is also essential.8
Regardless which form of psychotherapy, medication treatment, or peer support is utilized, access to the tools the psychiatrist finds most helpful is crucial to making them feel safe and comfortable returning to their role. Table 2 details practical steps administrators and peers can take to facilitate longitudinal support in these situations. In the case of Dr. M, administration was not only supportive in encouraging time off, but also in allowing protected time for therapy when she endorsed distress over the event. The combination of immediate responses and more long-term support greatly helped Dr. M continue her role as a psychiatrist and remain satisfied with her work.

Bottom Line
Being assaulted by a patient can make a psychiatrist reluctant to return to work. Strategies to ease this transition include seeking immediate care, removing the patient from the care of the psychiatrist who was assaulted, easing back into the environment, and finding long-term support.
Related Resources
- Lapic S, Joshi KG. What to do after a patient assaults you. Current Psychiatry. 2017;16(10):53-54.
- Joshi KG. Workplace violence: enhance your safety in outpatient settings. Current Psychiatry. 2021;20(8):37-38. doi:10.12788/cp.0163
- Su D. Harassment of health care workers: a survey. Current Psychiatry. 2021;20(6):48-50. doi:10.12788/cp.0135
- Rozel JS, Wiles C, Amin P. Too close for comfort: when the psychiatrist is stalked. Current Psychiatry. 2022;21(1): 23-28. doi:10.12788/cp.0209
Mr. B, age 23, is admitted to an inpatient psychiatric unit for depression. During his hospitalization, Mr. B becomes fixated on obtaining specific medications, including controlled substances. He is treated by Dr. M, a psychiatrist early in her training. In a difficult conversation, Dr. M tells Mr. B he will not be prescribed the medications he is requesting and explains why. Mr. B responds by jumping across a table and repeatedly punching Dr. M. Unit staff restrains Mr. B, and Dr. M leaves to seek medical care.
Assaults perpetrated against employees on inpatient psychiatric units are common.1 Assaults on physicians can occur at any level of training, including during residency.2 This is not a new phenomenon: concerns about patients assaulting psychiatrists and other inpatient staff have been reported for decades.3-5 Most research surrounding this topic has focused on risk factors for violence and prevention.6 Research regarding the aftermath of a patient assault and what services an employee requires have primarily centered on nurses.7,8
Practical guidance for a psychiatrist who has been assaulted and wants to return to work is difficult to find. This article provides strategies to help psychiatrists (and their colleagues) transition back to work after being the victim of a patient assault. While the recommendations we provide can be applied to trainees as well as attending physicians, there are some considerations specific to residents who have been assaulted (Box9,10).
Box
Psychiatry residents who are the targets of violence (such as Dr. M) require unique management, including evaluation of how the assault impacts their training and the role of the program director. Additionally, according to the Accreditation Council for Graduate Medical Education (ACGME) Common Program Requirements, residency programs must address residents’ wellbeing, including “evaluating workplace safety data and addressing the safety of residents and faculty members.”9 These specific considerations for residents are guided by the most recent program requirements through ACGME, as well as the policies of the specific institution overseeing the residency. Some institutions have developed resources to assist in this area, such as the WELL Toolkit from the University of Pittsburgh Medical Center.10
Having a plan for after an assault
The aftereffects of a patient assault can take a significant toll on the individual who is assaulted. A 2021 article about psychiatric mental health nurses by Dean et al8 identified multiple potential repercussions of unaddressed workplace violence, including role confusion, job dissatisfaction, decreased resiliency traits, poor coping methods, increased attrition rate, and increased expenditures related to assault injuries. Providing appropriate services and having a plan for how best to support an assaulted psychiatrist are likely to mitigate these effects. This can be grouped into 4 categories: 1) seeking immediate care, 2) removing the patient from your care, 3) easing back into the environment, and 4) finding long-term support.
1. Seeking immediate care
“Round or be rounded on” is a phrase that encapsulates many physicians’ attitude regarding their own health care and may contribute to their refusal of medical care following acute trauma such as an assault. Feelings of shock, guilt, and shame may also lead to a psychiatrist’s initial hesitation to seek treatment. However, it is important for the victim of an assault to be promptly evaluated and treated.
Elevated adrenaline in the aftermath of a physical engagement may mask the perception of injuries, and there is a risk for exposure to blood-borne pathogens. Regardless of the severity of injuries, seeking medical care establishes documentation of any injuries that can later serve as a record for workers’ compensation claims or if legal action is taken.
In addition to medical needs, immediate psychological support should be considered. Compulsory participation in crisis intervention stress debriefing, particularly when performed by untrained individuals, is not recommended due to questions about its demonstrated efficacy and potential to increase the risk of posttraumatic stress disorder (PTSD) in the long term.11,12 However, research has established the need for immediate support that does not necessarily involve a discussion of the traumatic event. One option is psychological first aid (PFA), an intervention supported by the World Health Organization. Originally developed for victims of mass crisis events, PFA easily translates to the hospital setting.12,13 PFA focuses on the immediate, basic needs of the victim to reduce distress and anxiety and encourage adaptive coping. Table 112,13 summarizes key components of PFA.

Continue to: PFA can be compared...
PFA can be compared to medical first aid in the field prior to reaching the hospital. In the case of Dr. M, other residents collaborated to transport her to the hospital, keep attendings and program directors apprised of the situation, and bring her snacks and comfort items to the hospital. Dr. M also received support from attending physicians at a neighboring hospital who helped coordinate her care. Essentially, she received a de facto version of PFA. However, given the evidence behind PFA and the unfortunate rate of violence against health care staff, institutions and organizations may offer training in PFA to ensure this level of support for all victims.
Multiple groups may take the lead to support a physician following an injury, including human resources, employee health, or other offices within the institution. The principles of PFA can be used to guide these employees in assisting the victim. Even if such employees are not trained in PFA, they can align with these principles by ensuring access to counseling and medical care, assisting with time off and accommodations, and helping the victim of an assault navigate the legal and administrative processes. Workers’ compensation can be a challenging process, and an institution’s human resources department should be available to assist the assaulted individual in navigating resources both within and outside of what they are able to offer.
2. Removing the patient from the psychiatrist’s care
During her recovery, Dr. M heard from a few peers that what happened was an occupational hazard. On some level, they were correct. While the public does not perceive a career in medicine to be physically dangerous, violence is a rampant problem in health care. Research shows that health care professionals are up to 16 times more likely to experience violence than other occupations; the odds for nurses are even higher.8
The frequency and pervasiveness of violence against health care professionals create an environment in which it can become an expected, and even accepted, phenomenon. However, violence cannot and should not be viewed as a normal part of workplace culture. A 2016 study by Moylan et al7 found that many nurses believe violence is part of their role, and therefore do not recognize the need to report such incidents or seek the necessary support. In other studies, only 30% of nurses reported violence, and the rate of reporting by physicians was 26%.14 This underreporting likely represents the role confusion surrounding whether caring for self or caring for the patient takes precedent, as well as normative expectations surrounding violence in the workplace.
It must be made clear to the victim that their safety is a priority and violence will not be tolerated. An institution’s administration can achieve this by immediately removing the patient from the victim’s care. In many cases, discharge of the patient from the clinic or facility may be warranted. A psychiatrist should not be expected to continue as the primary physician for a patient who has assaulted them; transfer to another psychiatrist is necessary if discharge is not an appropriate option. In a scenario in which a psychiatrist must maintain the treating relationship with a patient who assaulted them until the patient can be placed with another clinician (eg, as might occur on a unit with severely limited resources), staff chaperones can be considered when interacting with the patient.
Continue to: An institution's adminstration...
An institution’s administration should provide support if the psychiatrist chooses to press charges. At the core of our ethos as physicians is “do no harm,” and for some, the prospect of filing charges may be a difficult decision. However, health care professionals do not have an ethical obligation to put themselves in danger of serious bodily harm.15 While there is no one-size-fits-all answer to the question of whether or not to press charges against a patient who has committed an assault, the Occupational Safety and Health Administration considers the perception that violence is tolerated and victims are unable to report to law enforcement an organizational risk factor for workplace violence.16
As leaders in the workplace, physicians should set the precedent that violence will not be tolerated by reporting incidents to police and filing charges when appropriate. In the case of Dr. M, she received full support from her institution’s administration in filing charges against Mr. B due to the specific details of the assault.
3. Easing back into the environment
Despite assurances from her superiors that she could take time off, Dr. M wanted to return to work as soon as possible. She considered the balance between her physical injuries and desire to return to work and ultimately returned to work 5 days after the assault. She did well with supportive measures from administration and other staff, including the use of technician escorts on the unit, peer support, and frequent communication with and check-ins from management.
The decision on how quickly to return to work should always lie with the individual who was assaulted. The administration should offer time off without hesitation. Victims of an assault may feel overwhelmed by 2 diverging paths on how to return to a traumatic environment: avoid the location at all costs, or try to “face their fears” and return as quickly as possible. Research from outside medicine indicates that the timing of returning to work after a traumatic injury may not be nearly as important as the method of returning, and who makes this decision.17 Predictors of return to work after an assault include not only the severity of the trauma and amount of distress symptoms, but also any actual or perceived injustice on the part of the victim.17 Although this study was not specific to health care employees, it suggests that overall, an employee who does not feel a sense of control over their choice to return to work could perceive that as an injustice on the part of administration, leading to decreased job satisfaction.17
A study by Lamothe et al18 that was specific to health care professionals found that despite the importance of self-efficacy for the assault victim, perceived organizational support had an even greater protective effect following patient violence. Additionally, monitoring for signs of distress among victims after an episode of violence could prevent further violence by reducing the risk for subsequent victimization.18 This highlights the need for leadership of an inpatient unit to be keenly aware of how an assault on a psychiatrist or other health care professional may change the work environment and create a need to help staff navigate the new normal they may face on the unit.
Continue to: Finding long-term support
4. Finding long-term support
Longitudinal support is key in the initial transition back to work, as well as in the following weeks and months. Studies assessing the impacts of patient assault on mental health nurses indicate that while most individuals exposed to a traumatic event do not develop PTSD, many reported continued somatic symptoms, and more still reported ongoing psychological effects such as recurring thoughts of the assault, fear, generalized anger, and feeling a loss of control.8 Peer support is a common method employed by physicians and nurses alike, but administrative support is also essential.8
Regardless which form of psychotherapy, medication treatment, or peer support is utilized, access to the tools the psychiatrist finds most helpful is crucial to making them feel safe and comfortable returning to their role. Table 2 details practical steps administrators and peers can take to facilitate longitudinal support in these situations. In the case of Dr. M, administration was not only supportive in encouraging time off, but also in allowing protected time for therapy when she endorsed distress over the event. The combination of immediate responses and more long-term support greatly helped Dr. M continue her role as a psychiatrist and remain satisfied with her work.

Bottom Line
Being assaulted by a patient can make a psychiatrist reluctant to return to work. Strategies to ease this transition include seeking immediate care, removing the patient from the care of the psychiatrist who was assaulted, easing back into the environment, and finding long-term support.
Related Resources
- Lapic S, Joshi KG. What to do after a patient assaults you. Current Psychiatry. 2017;16(10):53-54.
- Joshi KG. Workplace violence: enhance your safety in outpatient settings. Current Psychiatry. 2021;20(8):37-38. doi:10.12788/cp.0163
- Su D. Harassment of health care workers: a survey. Current Psychiatry. 2021;20(6):48-50. doi:10.12788/cp.0135
- Rozel JS, Wiles C, Amin P. Too close for comfort: when the psychiatrist is stalked. Current Psychiatry. 2022;21(1): 23-28. doi:10.12788/cp.0209
1. Odes R, Chapman S, Harrison R, et al. Frequency of violence towards healthcare workers in the United States’ inpatient psychiatric hospitals: a systematic review of literature. Int J Ment Health Nurs. 2021;30(1):27-46.
2. Chaimowitz GA, Moscovitch A. Patient assaults on psychiatric residents: the Canadian experience. Can J Psychiatry. 1991;36(2):107-111.
3. Faulkner LR, Grimm NR, MacFarland BH, et al. Threats and assaults against psychiatrists. Bull Am Acad Psychiatry Law. 1990;18(1):37-46.
4. Carmel H, Hunter M. Psychiatrists injured by patient attack. Bull Am Acad Psychiatry Law. 1991;19(3):309-316.
5. Kwok S, Ostermeyer B, Coverdale J. A systematic review of the prevalence of patient assaults against residents. J Grad Med Educ. 2012;4(3):296-300.
6. Weltens I, Bak M, Verhagen S, et al. Aggression on the psychiatric ward: prevalence and risk factors. A systematic review of the literature. PLoS One. 2021;16(10):e0258346.
7. Moylan L, McManus M, Cullinan M, et al. Need for specialized support services for nurse victims of physical assault by psychiatric patients. Issues Ment Health Nurs. 2016;37(7):446-450.
8. Dean L, Butler A, Cuddigan J. The impact of workplace violence toward psychiatric mental health nurses: identifying the facilitators and barriers to supportive resources. J Am Psychiatr Nurses Assoc. 2021;27(3):189-202.
9. Accreditation Council for Graduate Medical Education. Common program requirements (Residency). July 2023. Accessed September 20, 2023. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2023v3.pdf
10. WELL Toolkit. UPMC GME Well-Being. October 3, 2022. Accessed September 20, 2023. https://gmewellness.upmc.com/
11. Rose S, Bisson J, Churchill R, et al. Psychological debriefing for preventing post traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2002;(2):CD000560.
12. Flannery RB Jr, Farley E, Rego S, et al. Characteristics of staff victims of psychiatric patient assaults: 15-year analysis of the Assaulted Staff Action Program (ASAP). Psychiatr Q. 2007;78(1):25-37.
13. Gispen F, Wu AW. Psychological first aid: CPR for mental health crises in healthcare. J Patient Saf Risk Manag. 2018:23(2):51-53.
14. Phillips JP. Workplace violence against health care workers in the United States. N Eng J Med. 2016;374(17):1661-1669.
15. Baby M, Glue P, Carlyle D. ‘Violence is not part of our job’: a thematic analysis of psychiatric mental health nurses’ experiences of patient assaults from a New Zealand perspective. Issues Ment Health Nurs. 2014;35(9):647-655.
16. Occupational Safety and Health Administration. Guidelines for Preventing Workplace Violence for Healthcare and Social Service Workers. Occupational Safety and Health Administration, US Dept of Labor; 2015.
17. Giummarra, MJ, Cameron PA, Ponsford J, et al. Return to work after traumatic injury: increased work-related disability in injured persons receiving financial compensation is mediated by perceived injustice. J Occup Rehabil. 2017;27(2):173-185.
18. Lamothe J, Boyer R, Guay S. A longitudinal analysis of psychological distress among healthcare workers following patient violence. Can J Behav Sci. 2021;53(1):48-58.
1. Odes R, Chapman S, Harrison R, et al. Frequency of violence towards healthcare workers in the United States’ inpatient psychiatric hospitals: a systematic review of literature. Int J Ment Health Nurs. 2021;30(1):27-46.
2. Chaimowitz GA, Moscovitch A. Patient assaults on psychiatric residents: the Canadian experience. Can J Psychiatry. 1991;36(2):107-111.
3. Faulkner LR, Grimm NR, MacFarland BH, et al. Threats and assaults against psychiatrists. Bull Am Acad Psychiatry Law. 1990;18(1):37-46.
4. Carmel H, Hunter M. Psychiatrists injured by patient attack. Bull Am Acad Psychiatry Law. 1991;19(3):309-316.
5. Kwok S, Ostermeyer B, Coverdale J. A systematic review of the prevalence of patient assaults against residents. J Grad Med Educ. 2012;4(3):296-300.
6. Weltens I, Bak M, Verhagen S, et al. Aggression on the psychiatric ward: prevalence and risk factors. A systematic review of the literature. PLoS One. 2021;16(10):e0258346.
7. Moylan L, McManus M, Cullinan M, et al. Need for specialized support services for nurse victims of physical assault by psychiatric patients. Issues Ment Health Nurs. 2016;37(7):446-450.
8. Dean L, Butler A, Cuddigan J. The impact of workplace violence toward psychiatric mental health nurses: identifying the facilitators and barriers to supportive resources. J Am Psychiatr Nurses Assoc. 2021;27(3):189-202.
9. Accreditation Council for Graduate Medical Education. Common program requirements (Residency). July 2023. Accessed September 20, 2023. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2023v3.pdf
10. WELL Toolkit. UPMC GME Well-Being. October 3, 2022. Accessed September 20, 2023. https://gmewellness.upmc.com/
11. Rose S, Bisson J, Churchill R, et al. Psychological debriefing for preventing post traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2002;(2):CD000560.
12. Flannery RB Jr, Farley E, Rego S, et al. Characteristics of staff victims of psychiatric patient assaults: 15-year analysis of the Assaulted Staff Action Program (ASAP). Psychiatr Q. 2007;78(1):25-37.
13. Gispen F, Wu AW. Psychological first aid: CPR for mental health crises in healthcare. J Patient Saf Risk Manag. 2018:23(2):51-53.
14. Phillips JP. Workplace violence against health care workers in the United States. N Eng J Med. 2016;374(17):1661-1669.
15. Baby M, Glue P, Carlyle D. ‘Violence is not part of our job’: a thematic analysis of psychiatric mental health nurses’ experiences of patient assaults from a New Zealand perspective. Issues Ment Health Nurs. 2014;35(9):647-655.
16. Occupational Safety and Health Administration. Guidelines for Preventing Workplace Violence for Healthcare and Social Service Workers. Occupational Safety and Health Administration, US Dept of Labor; 2015.
17. Giummarra, MJ, Cameron PA, Ponsford J, et al. Return to work after traumatic injury: increased work-related disability in injured persons receiving financial compensation is mediated by perceived injustice. J Occup Rehabil. 2017;27(2):173-185.
18. Lamothe J, Boyer R, Guay S. A longitudinal analysis of psychological distress among healthcare workers following patient violence. Can J Behav Sci. 2021;53(1):48-58.
Adult ADHD: A sensible approach to diagnosis and treatment
Attention-deficit/hyperactivity disorder (ADHD) is common, with an estimated worldwide prevalence of 5.29% among children and adolescents and 2.5% among adults.1 DSM-5-TR classifies ADHD as a neurodevelopmental disorder, “a group of conditions with onset in the developmental period [that] typically manifest early in development, often before the child enters school.”2 Because of the expectation that ADHD symptoms emerge early in development, the diagnostic criteria specify that symptoms must have been present prior to age 12 to qualify as ADHD. However, recent years have shown a significant increase in the number of patients being diagnosed with ADHD for the first time in adulthood. One study found that the diagnosis of ADHD among adults in the United States doubled between 2007 and 2016.3
First-line treatment for ADHD is the stimulants methylphenidate and amphetamine/dextroamphetamine. In the United States, these medications are classified as Schedule II controlled substances, indicating a high risk for abuse. However, just as ADHD diagnoses among adults have increased, so have prescriptions for stimulants. For example, Olfson et al4 found that stimulant prescriptions among young adults increased by a factor of 10 between 1994 and 2009.
The increased prevalence of adult patients diagnosed with ADHD and taking stimulants frequently places clinicians in a position to consider the validity of existing diagnoses and evaluate new patients with ADHD-related concerns. In this article, we review some of the challenges associated with diagnosing ADHD in adults, discuss the risks of stimulant treatment, and present a practical approach to the diagnosis and treatment of ADHD in adults.
Challenges in diagnosis
DSM-5-TR diagnostic criteria for ADHD are summarized in Table 1. Establishing a diagnosis of adult ADHD can be challenging. As with many psychiatric conditions, symptoms of ADHD are highly subjective. Retrospectively diagnosing a developmental condition in adults is often biased by the patient’s current functioning.5 ADHD has a high heritability and adults may inquire about the diagnosis if their children are diagnosed with ADHD.6 Some experts have cautioned that clinicians must be careful in diagnosing ADHD in adults.7 Just as there are risks associated with underdiagnosing ADHD, there are risks associated with overdiagnosis. Overdiagnosis may medicalize normal variants in the population and lead to unnecessary treatment and a misappropriation of limited medical resources.8 Many false positive cases of late-onset ADHD may be attributable to nonimpairing cognitive fluctuations.9

Poor diagnostic practices can impede accuracy in establishing the presence or absence of ADHD. Unfortunately, methods of diagnosing adult ADHD have been shown to vary widely in terms of information sources, diagnostic instruments used, symptom threshold, and whether functional impairment is a requirement for diagnosis.10 A common practice in diagnosing adult ADHD involves asking patients to complete self-report questionnaires that list symptoms of ADHD, such as the Adult ADHD Self-Report Scale developed by the World Health Organization.11 However, self-reports of ADHD in adults are less reliable than informant reports, and some young adults without ADHD overreport symptoms.12,13 Symptom checklists are particularly susceptible to faking, which lessens their diagnostic value.14
The possibility of malingered symptoms of ADHD further increases the diagnostic difficulty. College students may be particularly susceptible to overreporting ADHD symptoms in order to obtain academic accommodations or stimulants in the hopes of improving school performance.15 One study found that 25% to 48% of college students self-referred for ADHD evaluations exaggerated their symptoms.16 In another study, 31% of adults failed the Word Memory Test, which suggests noncredible performance in their ADHD evaluation.17 College students can successfully feign ADHD symptoms in both self-reported symptoms and computer-based tests of attention.18 Harrison et al19 summarized many of these concerns in their 2007 study of ADHD malingering, noting the “almost perfect ability of the Faking group to choose items … that correspond to the DSM-IV symptoms, and to report these at levels even higher than persons with diagnosed ADHD.” They suggested “Clinicians should be suspicious of students or young adults presenting for a first-time diagnosis who rate themselves as being significantly symptomatic, yet have managed to achieve well in school and other life activities.”19
Another challenge in correctly diagnosing adult ADHD is identifying other conditions that may impair attention.20 Psychiatric conditions that may impair concentration include anxiety disorders, chronic stress, posttraumatic stress disorder, recent trauma, major depressive disorder (MDD), and bipolar disorder (BD). Undiagnosed learning disorders may present like ADHD. Focus can be negatively affected by sleep disorders such as sleep apnea, restless leg syndrome, or delayed sleep phase-onset disorder. Marijuana, cocaine, 3,4-methylenedioxy-methamphetamine (MDMA; “ecstasy”), caffeine, or prescription medications such as anticholinergics can also impair attention. Medical conditions that can present with attentional or executive functioning deficits include seizures, Lyme disease, HIV, encephalopathy, hypothyroidism, and “chemo brain.”21 Environmental factors such as age-related cognitive decline, sleep deprivation, inflammation, obesity, air pollution, chemical exposure, and excessive use of digital media may also produce symptoms similar to ADHD. Two studies of adult-onset ADHD concluded that 93% to 95% of cases were better explained by other conditions such as sleep disorders, substance use disorders, or another psychiatric disorder.22
Continue to: Risks associated with treatment
Risks associated with treatment
With or without an accurate ADHD diagnosis, prescribing stimulants presents certain risks (Table 223-40). One of the more well-known risks of stimulants is addiction or misuse.23 An estimated 5 million American adults misused prescription stimulants in 2016.24 Despite stimulants’ status as controlled substances, long-term concurrent use of stimulants with opioids is common among adults with ADHD.25 College students are particularly susceptible to misusing or diverting stimulants, often to improve their academic performance.26 At 1 university, 22% of students had misused stimulants in the past year.27 Prescribing short-acting stimulants (rather than extended-release formulations) increases the likelihood of misuse.28 Patients prescribed stimulants begin to receive requests to divert their medications to others as early as elementary school, and by college more than one-third of those taking stimulants have been asked to give, sell, or trade their medications.29 Diversion of stimulants by students with ADHD is prevalent, with 62% of patients engaging in diversion during their lifetime.15 Diverted stimulants can come from family members, black market sources, or deceived clinicians.30 Although students’ stimulant misuse/diversion often is academically motivated, nonmedical use of psychostimulants does not appear to have a statistically significant effect on improving grade point average.31 Despite a negligible impact on grades, most students who take stimulants identify their effect as strongly positive, producing a situation in which misusers of stimulants have little motivation to stop.32 While some patients might ask for a stimulant prescription with the rationale that liking the effects proves they have ADHD, this is inappropriate because most individuals like the effects of stimulant medications.33
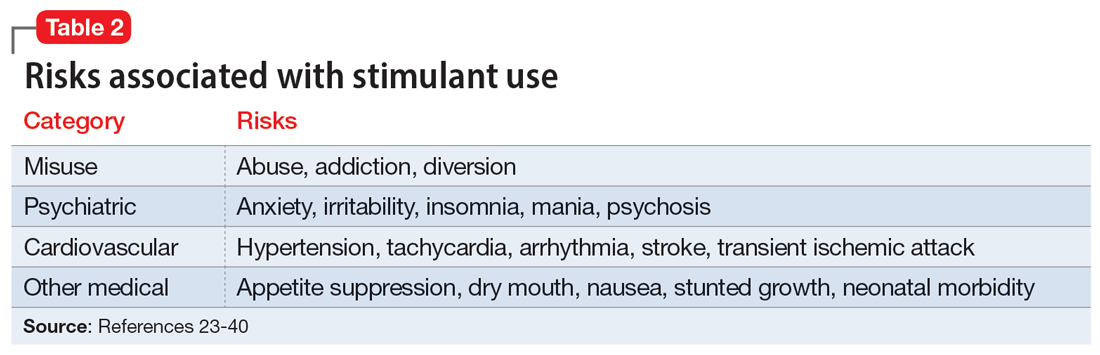
The use of stimulants increases the risk for several adverse psychiatric outcomes. Stimulants increase the risk of anxiety, so exercise caution when prescribing to patients with a comorbid anxiety disorder.34 Stimulants can also worsen irritability and insomnia, 2 issues common among patients with ADHD.32 Use of stimulant medications can trigger manic episodes. Viktorin et al35 found a >6-fold increase in manic episodes among patients with BD receiving methylphenidate monotherapy compared to those receiving a combination of methylphenidate and a mood stabilizer.35 The use of methylphenidate and amphetamine can lead to new-onset psychosis (or exacerbation of pre-existing psychotic illness); amphetamine use is associated with a higher risk of psychosis than methylphenidate.36
General medical adverse effects are also possible with stimulant use. Stimulants’ adverse effect profiles include appetite suppression, dry mouth, and nausea. Long-term use poses a risk for stunting growth in children.1 Using stimulants during pregnancy is associated with higher risk for neonatal morbidity, including preterm birth, CNS-related disorders, and seizures.37 Stimulants can raise blood pressure and increase heart rate. Serious cardiovascular events associated with stimulant use include ventricular arrhythmias, strokes, and transient ischemic attacks.38
Nonstimulant ADHD treatments are less risky than stimulants but still require monitoring for common adverse effects. Atomoxetine has been associated with sedation, growth retardation (in children), and in severe cases, liver injury or suicidal ideation.39 Bupropion (commonly used off-label for ADHD) can lower the seizure threshold and cause irritability, anorexia, and insomnia.39 Viloxazine, a newer agent, can cause hypertension, increased heart rate, nausea, drowsiness, headache, and insomnia.40
Sensible diagnosing
Given the challenges in accurately diagnosing ADHD in adults, we present a sensible approach to making the diagnosis (Table 3). The first step is to rule out other conditions that might better explain the patient’s symptoms. A thorough clinical interview (including a psychiatric review of symptoms) is the cornerstone of an initial diagnostic assessment. The use of validated screening questionnaires such as the Patient Health Questionnaire-9 and General Anxiety Disorder-7 may also provide information regarding psychiatric conditions that require additional evaluation.
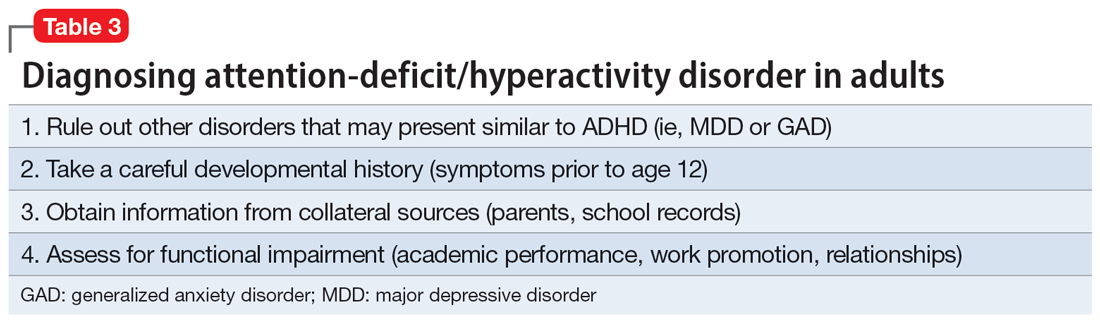
Continue to: Some of the most common conditions...
Some of the most common conditions we see mistaken for ADHD are MDD, generalized anxiety disorder (GAD), and BD. In DSM-5-TR, 1 of the diagnostic criteria for MDD is “diminished ability to think or concentrate, or indecisiveness, nearly every day (either by subjective account or as observed by others).”41 Similarly, criteria for GAD include “difficulty concentrating.”42 DSM-5-TR also includes distractibility as one of the criteria for mania/hypomania. Table 420-22,41,42 lists other psychiatric, substance-related, medical, and environmental conditions that can produce ADHD-like symptoms. Referring to some medical and environmental explanations for inattention, Aiken22 pointed out, “Patients who suffer from these problems might ask their doctor for a stimulant, but none of those syndromes require a psychopharmacologic approach.” ADHD can be comorbid with other psychiatric conditions, so the presence of another psychiatric illness does not automatically rule out ADHD. If alternative psychiatric diagnoses have been identified, these can be discussed with the patient and treatment offered that targets the specified condition.
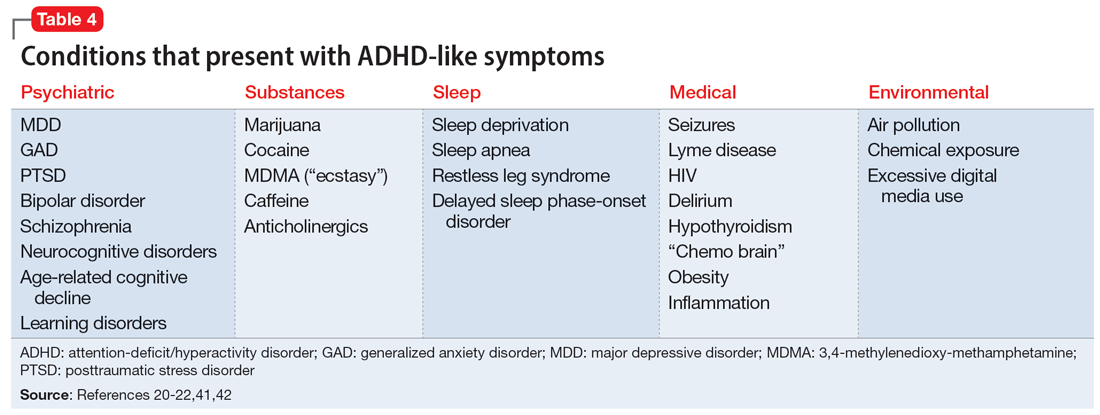
Once alternative explanations have been ruled out, focus on the patient’s developmental history. DSM-5-TR conceptualizes ADHD as a neurodevelopmental disorder, meaning it is expected to emerge early in life. Whereas previous editions of DSM specified that ADHD symptoms must be present before age 7, DSM-5 modified this age threshold to before age 12.1 This necessitates taking a careful life history in order to understand the presence or absence of symptoms at earlier developmental stages.5 ADHD should be verified by symptoms apparent in childhood and present across the lifespan.15
While this retrospective history is necessary, histories that rely on self-report alone are often unreliable. Collateral sources of information are generally more reliable when assessing for ADHD symptoms.13 Third-party sources can help confirm that any impairment is best attributed to ADHD rather than to another condition.15 Unfortunately, the difficulty of obtaining collateral information means it is often neglected, even in the literature.10 A parent is the ideal informant for gathering collateral information regarding a patient’s functioning in childhood.5 Suggested best practices also include obtaining collateral information from interviews with significant others, behavioral questionnaires completed by parents (for current and childhood symptoms), review of school records, and consideration of intellectual and achievement testing.43 If psychological testing is pursued, include validity testing to detect feigned symptoms.18,44
When evaluating for ADHD, assess not only for the presence of symptoms, but also if these symptoms produce significant functional impairment.13,15 Impairments in daily functioning can include impaired school participation, social participation, quality of relationships, family conflict, family activities, family functioning, and emotional functioning.45 Some symptoms may affect functioning in an adult’s life differently than they did during childhood, from missed work appointments to being late picking up kids from school. Research has shown that the correlation between the number of symptoms and functional impairment is weak, which means someone could experience all of the symptoms of ADHD without experiencing functional impairment.45 To make an accurate diagnosis, it is therefore important to clearly establish both the number of symptoms the patient is experiencing and whether these symptoms are clearly linked to functional impairments.10
Sensible treatment
Once a diagnosis of ADHD has been clearly established, clinicians need to consider how best to treat the condition (Table 5). Stimulants are generally considered first-line treatment for ADHD. In randomized clinical trials, they showed significant efficacy; for example, one study of 146 adults with ADHD found a 76% improvement with methylphenidate compared to 19% for the placebo group.46 Before starting a stimulant, certain comorbidities should be ruled out. If a patient has glaucoma or pheochromocytoma, they may first need treatment from or clearance by other specialists. Stimulants should likely be held in patients with hypertension, angina, or cardiovascular defects until receiving medical clearance. The risks of stimulants need to be discussed with female patients of childbearing age, weighing the benefits of treatment against the risks of medication use should the patient get pregnant. Patients with comorbid psychosis or uncontrolled bipolar illness should not receive stimulants due to the risk of exacerbation. Patients with active substance use disorders (SUDs) are generally not good candidates for stimulants because of the risk of misusing or diverting stimulants and the possibility that substance abuse may be causing their inattentive symptoms. Patients whose SUDs are in remission may cautiously be considered as candidates for stimulants. If patients misuse their prescribed stimulants, they should be switched to a nonstimulant medication such as atomoxetine, bupropion, guanfacine, or clonidine.47

Continue to: Once a patient is deemed...
Once a patient is deemed to be a candidate for stimulants, clinicians need to choose between methylphenidate or amphetamine/dextroamphetamine formulations. Table 6 lists medications that are commonly prescribed to treat ADHD; unless otherwise noted, these are FDA-approved for this indication. As a general rule, for adults, long-acting stimulant formulations are preferred over short-acting formulations.28 Immediate-release stimulants are more prone to misuse or diversion compared to extended-release medications.29 Longer-acting formulations may also provide better full-day symptom control.48
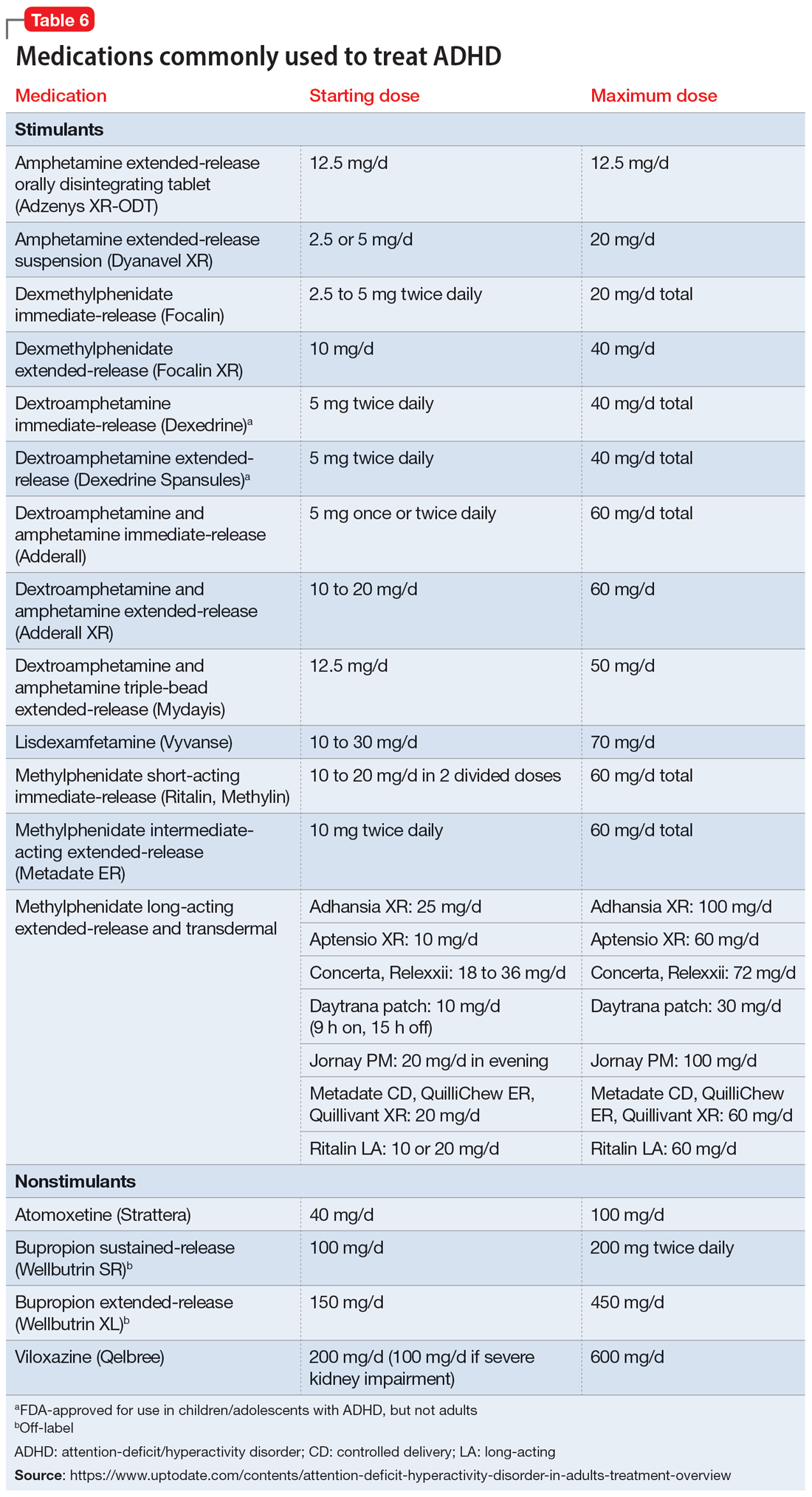
In contrast to many other psychiatric medications, it may be beneficial to encourage periodically taking breaks or “medication holidays” from stimulants. Planned medication holidays for adults can involve intentionally not taking the medication over the weekend when the patient is not involved in work or school responsibilities. Such breaks have been shown to reduce adverse effects of stimulants (such as appetite suppression and insomnia) without significantly increasing ADHD symptoms.49 Short breaks can also help prevent medication tolerance and the subsequent need to increase doses.50 Medication holidays provide an opportunity to verify the ongoing benefits of the medication. It is advisable to periodically assess whether there is a continued need for stimulant treatment.51 If patients do not tolerate stimulants or have other contraindications, nonstimulants should be considered.
Lastly, no psychiatric patient should be treated with medication alone, and nonpharmacologic approaches should be incorporated as needed. Clear instructions, visual aids, nonverbal cues, frequent breaks to stand and stretch, schedules, normalizing failure as part of growth, and identifying triggers for emotional reactivity may help patients with ADHD.52 In a study of the academic performance of 92 college students taking medication for ADHD and 146 control students, treatment with stimulants alone did not eliminate the academic achievement deficit of those individuals with ADHD.53 Good study habits (even without stimulants) appeared more important in overcoming the achievement disparity of students with ADHD.53 Providing psychoeducation and training in concrete organization and planning skills have shown benefit.54 Practice of skills on a daily basis appears to be especially beneficial.55
Bottom Line
A sensible approach to diagnosing attention-deficit/hyperactivity disorder (ADHD) in adults includes ruling out other disorders that may present similar to ADHD, taking an appropriate developmental history, obtaining collateral information, and assessing for functional impairment. Sensible treatment involves ruling out comorbidities that stimulants could worsen, selecting extended-release stimulants, incorporating medication holidays, and using nonpharmacologic interventions.
Related Resources
- National Institute for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management. https://www.nice.org.uk/guidance/ng87
- Substance Abuse and Mental Health Services Administration. Advisory: Prescription Stimulant Misuse Among Youth and Young Adults. https://store.samhsa.gov/product/prescription-stimulant-misuse-among-youth-young-adults/PEP21-06-01-003
Drug Brand Names
Amphetamine • Adzenys, Dyanavel, others
Atomoxetine • Strattera
Bupropion • Wellbutrin, Forfivo
Clonidine • Catapres, Kapvay
Dexmethylphenidate • Focalin
Dextroamphetamine • Dexedrine
Dextroamphetamine and amphetamine • Adderall, Mydayis
Guanfacine • Intuniv, Tenex
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin, others
Viloxazine • Qelbree
1. Posner J, Polanczyk GV, Sonuga-Barke E. Attention-deficit hyperactivity disorder. Lancet. 2020;395(10222):450-462.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:35.
3. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
4. Olfson M, Blanco C, Wang S, et al. Trends in office-based treatment of adults with stimulants in the United States. J Clin Psychiatry. 2013;74(1):43-50.
5. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956.
6. Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2019;24(4):562-575.
7. Solanto MV. Child vs adult onset of attention-deficit/hyperactivity disorder. JAMA Psychiatry. 2017;74(4):421.
8. Jummani RR, Hirsch E, Hirsch GS. Are we overdiagnosing and overtreating ADHD? Psychiatric Times. Published May 31, 2017. Accessed March 17, 2023. https://www.psychiatrictimes.com/view/are-we-overdiagnosing-and-overtreating-adhd
9. Sibley MH, Rohde LA, Swanson JM, et al; Multimodal Treatment Study of Children with ADHD (MTA) Cooperative Group. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149.
10. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165.
11. Ustun B, Adler LA, Rudin C, et al. The World Health Organization adult attention-deficit/hyperactivity disorder self-report screening scale for DSM-5. JAMA Psychiatry. 2017;74(5):520-527.
12. Faraone SV, Biederman J. Can attention-deficit/hyperactivity disorder onset occur in adulthood? JAMA Psychiatry. 2016;73(7):655-656.
13. Sibley MH, Pelham WE, Molina BSG, et al. When diagnosing ADHD in young adults emphasize informant reports, DSM items, and impairment. J Consult Clin Psychol. 2012;80(6):1052-1061.
14. Sollman MJ, Ranseen JD, Berry DT. Detection of feigned ADHD in college students. Psychol Assess. 2010;22(2):325-335.
15. Green AL, Rabiner DL. What do we really know about ADHD in college students? Neurotherapeutics. 2012;9(3):559-568.
16. Sullivan BK, May K, Galbally L. Symptom exaggeration by college adults in attention-deficit hyperactivity disorder and learning disorder assessments. Appl Neuropsychol. 2007;14(3):189-207.
17. Suhr J, Hammers D, Dobbins-Buckland K, et al. The relationship of malingering test failure to self-reported symptoms and neuropsychological findings in adults referred for ADHD evaluation. Arch Clin Neuropsychol. 2008;23(5):521-530.
18. Lee Booksh R, Pella RD, Singh AN, et al. Ability of college students to simulate ADHD on objective measures of attention. J Atten Disord. 2010;13(4):325-338.
19. Harrison AG, Edwards MJ, Parker KC. Identifying students faking ADHD: preliminary findings and strategies for detection. Arch Clin Neuropsychol. 2007;22(5):577-588.
20. Lopez R, Micoulaud-Franchi JA, Galeria C, et al. Is adult-onset attention deficit/hyperactivity disorder frequent in clinical practice? Psychiatry Res. 2017;257:238-241.
21. Bhatia R. Rule out these causes of inattention before diagnosing ADHD. Current Psychiatry. 2016;15(10):32-33.
22. Aiken C. Adult-onset ADHD raises questions. Psychiatric Times. 2021;38(3):24.
23. Bjorn S, Weyandt LL. Issues pertaining to misuse of ADHD prescription medications. Psychiatric Times. 2018;35(9):17-19.
24. Compton WM, Han B, Blanco C, et al. Prevalence and correlates of prescription stimulant use, misuse, use disorders, and motivations for misuse among adults in the United States. Am J Psychiatry. 2018;175(8):741-755.
25. Wei YJ, Zhu Y, Liu W, et al. Prevalence of and factors associated with long-term concurrent use of stimulants and opioids among adults with attention-deficit/hyperactivity disorder. JAMA Netw Open. 2018;1(4):e181152. doi:10.1001/jamanetworkopen.2018.1152
26. Benson K, Flory K, Humphreys KL, et al. Misuse of stimulant medication among college students: a comprehensive review and meta-analysis. Clin Child Fam Psychol Rev. 2015;18(1):50-76.
27. Benson K, Woodlief DT, Flory K, et al. Is ADHD, independent of ODD, associated with whether and why college students misuse stimulant medication? Exp Clin Psychopharmacol. 2018;26(5):476-487.
28. Froehlich TE. ADHD medication adherence in college students-- a call to action for clinicians and researchers: commentary on “transition to college and adherence to prescribed attention deficit hyperactivity disorder medication.” J Dev Behav Pediatr. 2018;39(1):77-78.
29. Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21-31.
30. Vrecko S. Everyday drug diversions: a qualitative study of the illicit exchange and non-medical use of prescription stimulants on a university campus. Soc Sci Med. 2015;131:297-304.
31. Munro BA, Weyandt LL, Marraccini ME, et al. The relationship between nonmedical use of prescription stimulants, executive functioning and academic outcomes. Addict Behav. 2017;65:250-257.
32. Rabiner DL, Anastopoulos AD, Costello EJ, et al. Motives and perceived consequences of nonmedical ADHD medication use by college students: are students treating themselves for attention problems? J Atten Disord. 2009;13(3)259-270.
33. Tayag Y. Adult ADHD is the wild west of psychiatry. The Atlantic. Published April 14, 2023. Accessed May 3, 2023. https://www.theatlantic.com/health/archive/2023/04/adult-adhd-diagnosis-treatment-adderall-shortage/673719/
34. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255-270.
35. Viktorin A, Rydén E, Thase ME, et al. The risk of treatment-emergent mania with methylphenidate in bipolar disorder. Am J Psychiatry. 2017;174(4):341-348.
36. Moran LV, Ongur D, Hsu J, et al. Psychosis with methylphenidate or amphetamine in patients with ADHD. N Engl J Med. 2019; 380(12):1128-1138.
37. Nörby U, Winbladh B, Källén K. Perinatal outcomes after treatment with ADHD medication during pregnancy. Pediatrics. 2017;140(6):e20170747. doi:10.1542/peds.2017-0747
38. Tadrous M, Shakeri A, Chu C, et al. Assessment of stimulant use and cardiovascular event risks among older adults. JAMA Netw Open. 2021;4(10):e2130795. doi:10.1001/jamanetworkopen.2021.30795
39. Daughton JM, Kratochvil CJ. Review of ADHD pharmacotherapies: advantages, disadvantages, and clinical pearls. J Am Acad Child Adolesc Psychiatry. 2009;48(3):240-248.
40. Qelbree [package insert]. Rockville, MD: Supernus Pharmaceuticals; 2021.
41. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:183.
42. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:250.
43. DuPaul GJ, Weyandt LL, O’Dell SM, et al. College students with ADHD: current status and future directions. J Atten Disord. 2009;13(3):234-250.
44. Edmundson M, Berry DTR, Combs HL, et al. The effects of symptom information coaching on the feigning of adult ADHD. Psychol Assess. 2017;29(12):1429-1436.
45. Gordon M, Antshel K, Faraone S, et al. Symptoms versus impairment: the case for respecting DSM-IV’s criterion D. J Atten Disord. 2006;9(3):465-475.
46. Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456-463.
47. Osser D, Awidi B. Treating adults with ADHD requires special considerations. Psychiatric News. Published August 30, 2018. Accessed March 17, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.pp8a1
48. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown, RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
49. Martins S, Tramontina S, Polanczyk G, et al. Weekend holidays during methylphenidate use in ADHD children: a randomized clinical trial. J Child Adolesc Psychopharmacol. 2004;14(2):195-206.
50. Ibrahim K, Donyai P. Drug holidays from ADHD medication: international experience over the past four decades. J Atten Disord. 2015;19(7):551-568.
51. Matthijssen AM, Dietrich A, Bierens M, et al. Continued benefits of methylphenidate in ADHD after 2 years in clinical practice: a randomized placebo-controlled discontinuation study. Am J Psychiatry. 2019;176(9):754-762.
52. Mason EJ, Joshi KG. Nonpharmacologic strategies for helping children with ADHD. Current Psychiatry. 2018;7(1):42,46.
53. Advokat C, Lane SM, Luo C. College students with and without ADHD: comparison of self-report of medication usage, study habits, and academic achievement. J Atten Disord. 2011;15(8):656-666.
54. Knouse LE, Cooper-Vince C, Sprich S, et al. Recent developments in the psychosocial treatment of adult ADHD. Expert Rev Neurother. 2008;8(10):1537-1548.
55. Evans SW, Owens JS, Wymbs BT, et al. Evidence-based psychosocial treatments for children and adolescents with attention deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2018;47(2):157-198.
Attention-deficit/hyperactivity disorder (ADHD) is common, with an estimated worldwide prevalence of 5.29% among children and adolescents and 2.5% among adults.1 DSM-5-TR classifies ADHD as a neurodevelopmental disorder, “a group of conditions with onset in the developmental period [that] typically manifest early in development, often before the child enters school.”2 Because of the expectation that ADHD symptoms emerge early in development, the diagnostic criteria specify that symptoms must have been present prior to age 12 to qualify as ADHD. However, recent years have shown a significant increase in the number of patients being diagnosed with ADHD for the first time in adulthood. One study found that the diagnosis of ADHD among adults in the United States doubled between 2007 and 2016.3
First-line treatment for ADHD is the stimulants methylphenidate and amphetamine/dextroamphetamine. In the United States, these medications are classified as Schedule II controlled substances, indicating a high risk for abuse. However, just as ADHD diagnoses among adults have increased, so have prescriptions for stimulants. For example, Olfson et al4 found that stimulant prescriptions among young adults increased by a factor of 10 between 1994 and 2009.
The increased prevalence of adult patients diagnosed with ADHD and taking stimulants frequently places clinicians in a position to consider the validity of existing diagnoses and evaluate new patients with ADHD-related concerns. In this article, we review some of the challenges associated with diagnosing ADHD in adults, discuss the risks of stimulant treatment, and present a practical approach to the diagnosis and treatment of ADHD in adults.
Challenges in diagnosis
DSM-5-TR diagnostic criteria for ADHD are summarized in Table 1. Establishing a diagnosis of adult ADHD can be challenging. As with many psychiatric conditions, symptoms of ADHD are highly subjective. Retrospectively diagnosing a developmental condition in adults is often biased by the patient’s current functioning.5 ADHD has a high heritability and adults may inquire about the diagnosis if their children are diagnosed with ADHD.6 Some experts have cautioned that clinicians must be careful in diagnosing ADHD in adults.7 Just as there are risks associated with underdiagnosing ADHD, there are risks associated with overdiagnosis. Overdiagnosis may medicalize normal variants in the population and lead to unnecessary treatment and a misappropriation of limited medical resources.8 Many false positive cases of late-onset ADHD may be attributable to nonimpairing cognitive fluctuations.9

Poor diagnostic practices can impede accuracy in establishing the presence or absence of ADHD. Unfortunately, methods of diagnosing adult ADHD have been shown to vary widely in terms of information sources, diagnostic instruments used, symptom threshold, and whether functional impairment is a requirement for diagnosis.10 A common practice in diagnosing adult ADHD involves asking patients to complete self-report questionnaires that list symptoms of ADHD, such as the Adult ADHD Self-Report Scale developed by the World Health Organization.11 However, self-reports of ADHD in adults are less reliable than informant reports, and some young adults without ADHD overreport symptoms.12,13 Symptom checklists are particularly susceptible to faking, which lessens their diagnostic value.14
The possibility of malingered symptoms of ADHD further increases the diagnostic difficulty. College students may be particularly susceptible to overreporting ADHD symptoms in order to obtain academic accommodations or stimulants in the hopes of improving school performance.15 One study found that 25% to 48% of college students self-referred for ADHD evaluations exaggerated their symptoms.16 In another study, 31% of adults failed the Word Memory Test, which suggests noncredible performance in their ADHD evaluation.17 College students can successfully feign ADHD symptoms in both self-reported symptoms and computer-based tests of attention.18 Harrison et al19 summarized many of these concerns in their 2007 study of ADHD malingering, noting the “almost perfect ability of the Faking group to choose items … that correspond to the DSM-IV symptoms, and to report these at levels even higher than persons with diagnosed ADHD.” They suggested “Clinicians should be suspicious of students or young adults presenting for a first-time diagnosis who rate themselves as being significantly symptomatic, yet have managed to achieve well in school and other life activities.”19
Another challenge in correctly diagnosing adult ADHD is identifying other conditions that may impair attention.20 Psychiatric conditions that may impair concentration include anxiety disorders, chronic stress, posttraumatic stress disorder, recent trauma, major depressive disorder (MDD), and bipolar disorder (BD). Undiagnosed learning disorders may present like ADHD. Focus can be negatively affected by sleep disorders such as sleep apnea, restless leg syndrome, or delayed sleep phase-onset disorder. Marijuana, cocaine, 3,4-methylenedioxy-methamphetamine (MDMA; “ecstasy”), caffeine, or prescription medications such as anticholinergics can also impair attention. Medical conditions that can present with attentional or executive functioning deficits include seizures, Lyme disease, HIV, encephalopathy, hypothyroidism, and “chemo brain.”21 Environmental factors such as age-related cognitive decline, sleep deprivation, inflammation, obesity, air pollution, chemical exposure, and excessive use of digital media may also produce symptoms similar to ADHD. Two studies of adult-onset ADHD concluded that 93% to 95% of cases were better explained by other conditions such as sleep disorders, substance use disorders, or another psychiatric disorder.22
Continue to: Risks associated with treatment
Risks associated with treatment
With or without an accurate ADHD diagnosis, prescribing stimulants presents certain risks (Table 223-40). One of the more well-known risks of stimulants is addiction or misuse.23 An estimated 5 million American adults misused prescription stimulants in 2016.24 Despite stimulants’ status as controlled substances, long-term concurrent use of stimulants with opioids is common among adults with ADHD.25 College students are particularly susceptible to misusing or diverting stimulants, often to improve their academic performance.26 At 1 university, 22% of students had misused stimulants in the past year.27 Prescribing short-acting stimulants (rather than extended-release formulations) increases the likelihood of misuse.28 Patients prescribed stimulants begin to receive requests to divert their medications to others as early as elementary school, and by college more than one-third of those taking stimulants have been asked to give, sell, or trade their medications.29 Diversion of stimulants by students with ADHD is prevalent, with 62% of patients engaging in diversion during their lifetime.15 Diverted stimulants can come from family members, black market sources, or deceived clinicians.30 Although students’ stimulant misuse/diversion often is academically motivated, nonmedical use of psychostimulants does not appear to have a statistically significant effect on improving grade point average.31 Despite a negligible impact on grades, most students who take stimulants identify their effect as strongly positive, producing a situation in which misusers of stimulants have little motivation to stop.32 While some patients might ask for a stimulant prescription with the rationale that liking the effects proves they have ADHD, this is inappropriate because most individuals like the effects of stimulant medications.33

The use of stimulants increases the risk for several adverse psychiatric outcomes. Stimulants increase the risk of anxiety, so exercise caution when prescribing to patients with a comorbid anxiety disorder.34 Stimulants can also worsen irritability and insomnia, 2 issues common among patients with ADHD.32 Use of stimulant medications can trigger manic episodes. Viktorin et al35 found a >6-fold increase in manic episodes among patients with BD receiving methylphenidate monotherapy compared to those receiving a combination of methylphenidate and a mood stabilizer.35 The use of methylphenidate and amphetamine can lead to new-onset psychosis (or exacerbation of pre-existing psychotic illness); amphetamine use is associated with a higher risk of psychosis than methylphenidate.36
General medical adverse effects are also possible with stimulant use. Stimulants’ adverse effect profiles include appetite suppression, dry mouth, and nausea. Long-term use poses a risk for stunting growth in children.1 Using stimulants during pregnancy is associated with higher risk for neonatal morbidity, including preterm birth, CNS-related disorders, and seizures.37 Stimulants can raise blood pressure and increase heart rate. Serious cardiovascular events associated with stimulant use include ventricular arrhythmias, strokes, and transient ischemic attacks.38
Nonstimulant ADHD treatments are less risky than stimulants but still require monitoring for common adverse effects. Atomoxetine has been associated with sedation, growth retardation (in children), and in severe cases, liver injury or suicidal ideation.39 Bupropion (commonly used off-label for ADHD) can lower the seizure threshold and cause irritability, anorexia, and insomnia.39 Viloxazine, a newer agent, can cause hypertension, increased heart rate, nausea, drowsiness, headache, and insomnia.40
Sensible diagnosing
Given the challenges in accurately diagnosing ADHD in adults, we present a sensible approach to making the diagnosis (Table 3). The first step is to rule out other conditions that might better explain the patient’s symptoms. A thorough clinical interview (including a psychiatric review of symptoms) is the cornerstone of an initial diagnostic assessment. The use of validated screening questionnaires such as the Patient Health Questionnaire-9 and General Anxiety Disorder-7 may also provide information regarding psychiatric conditions that require additional evaluation.

Continue to: Some of the most common conditions...
Some of the most common conditions we see mistaken for ADHD are MDD, generalized anxiety disorder (GAD), and BD. In DSM-5-TR, 1 of the diagnostic criteria for MDD is “diminished ability to think or concentrate, or indecisiveness, nearly every day (either by subjective account or as observed by others).”41 Similarly, criteria for GAD include “difficulty concentrating.”42 DSM-5-TR also includes distractibility as one of the criteria for mania/hypomania. Table 420-22,41,42 lists other psychiatric, substance-related, medical, and environmental conditions that can produce ADHD-like symptoms. Referring to some medical and environmental explanations for inattention, Aiken22 pointed out, “Patients who suffer from these problems might ask their doctor for a stimulant, but none of those syndromes require a psychopharmacologic approach.” ADHD can be comorbid with other psychiatric conditions, so the presence of another psychiatric illness does not automatically rule out ADHD. If alternative psychiatric diagnoses have been identified, these can be discussed with the patient and treatment offered that targets the specified condition.

Once alternative explanations have been ruled out, focus on the patient’s developmental history. DSM-5-TR conceptualizes ADHD as a neurodevelopmental disorder, meaning it is expected to emerge early in life. Whereas previous editions of DSM specified that ADHD symptoms must be present before age 7, DSM-5 modified this age threshold to before age 12.1 This necessitates taking a careful life history in order to understand the presence or absence of symptoms at earlier developmental stages.5 ADHD should be verified by symptoms apparent in childhood and present across the lifespan.15
While this retrospective history is necessary, histories that rely on self-report alone are often unreliable. Collateral sources of information are generally more reliable when assessing for ADHD symptoms.13 Third-party sources can help confirm that any impairment is best attributed to ADHD rather than to another condition.15 Unfortunately, the difficulty of obtaining collateral information means it is often neglected, even in the literature.10 A parent is the ideal informant for gathering collateral information regarding a patient’s functioning in childhood.5 Suggested best practices also include obtaining collateral information from interviews with significant others, behavioral questionnaires completed by parents (for current and childhood symptoms), review of school records, and consideration of intellectual and achievement testing.43 If psychological testing is pursued, include validity testing to detect feigned symptoms.18,44
When evaluating for ADHD, assess not only for the presence of symptoms, but also if these symptoms produce significant functional impairment.13,15 Impairments in daily functioning can include impaired school participation, social participation, quality of relationships, family conflict, family activities, family functioning, and emotional functioning.45 Some symptoms may affect functioning in an adult’s life differently than they did during childhood, from missed work appointments to being late picking up kids from school. Research has shown that the correlation between the number of symptoms and functional impairment is weak, which means someone could experience all of the symptoms of ADHD without experiencing functional impairment.45 To make an accurate diagnosis, it is therefore important to clearly establish both the number of symptoms the patient is experiencing and whether these symptoms are clearly linked to functional impairments.10
Sensible treatment
Once a diagnosis of ADHD has been clearly established, clinicians need to consider how best to treat the condition (Table 5). Stimulants are generally considered first-line treatment for ADHD. In randomized clinical trials, they showed significant efficacy; for example, one study of 146 adults with ADHD found a 76% improvement with methylphenidate compared to 19% for the placebo group.46 Before starting a stimulant, certain comorbidities should be ruled out. If a patient has glaucoma or pheochromocytoma, they may first need treatment from or clearance by other specialists. Stimulants should likely be held in patients with hypertension, angina, or cardiovascular defects until receiving medical clearance. The risks of stimulants need to be discussed with female patients of childbearing age, weighing the benefits of treatment against the risks of medication use should the patient get pregnant. Patients with comorbid psychosis or uncontrolled bipolar illness should not receive stimulants due to the risk of exacerbation. Patients with active substance use disorders (SUDs) are generally not good candidates for stimulants because of the risk of misusing or diverting stimulants and the possibility that substance abuse may be causing their inattentive symptoms. Patients whose SUDs are in remission may cautiously be considered as candidates for stimulants. If patients misuse their prescribed stimulants, they should be switched to a nonstimulant medication such as atomoxetine, bupropion, guanfacine, or clonidine.47

Continue to: Once a patient is deemed...
Once a patient is deemed to be a candidate for stimulants, clinicians need to choose between methylphenidate or amphetamine/dextroamphetamine formulations. Table 6 lists medications that are commonly prescribed to treat ADHD; unless otherwise noted, these are FDA-approved for this indication. As a general rule, for adults, long-acting stimulant formulations are preferred over short-acting formulations.28 Immediate-release stimulants are more prone to misuse or diversion compared to extended-release medications.29 Longer-acting formulations may also provide better full-day symptom control.48

In contrast to many other psychiatric medications, it may be beneficial to encourage periodically taking breaks or “medication holidays” from stimulants. Planned medication holidays for adults can involve intentionally not taking the medication over the weekend when the patient is not involved in work or school responsibilities. Such breaks have been shown to reduce adverse effects of stimulants (such as appetite suppression and insomnia) without significantly increasing ADHD symptoms.49 Short breaks can also help prevent medication tolerance and the subsequent need to increase doses.50 Medication holidays provide an opportunity to verify the ongoing benefits of the medication. It is advisable to periodically assess whether there is a continued need for stimulant treatment.51 If patients do not tolerate stimulants or have other contraindications, nonstimulants should be considered.
Lastly, no psychiatric patient should be treated with medication alone, and nonpharmacologic approaches should be incorporated as needed. Clear instructions, visual aids, nonverbal cues, frequent breaks to stand and stretch, schedules, normalizing failure as part of growth, and identifying triggers for emotional reactivity may help patients with ADHD.52 In a study of the academic performance of 92 college students taking medication for ADHD and 146 control students, treatment with stimulants alone did not eliminate the academic achievement deficit of those individuals with ADHD.53 Good study habits (even without stimulants) appeared more important in overcoming the achievement disparity of students with ADHD.53 Providing psychoeducation and training in concrete organization and planning skills have shown benefit.54 Practice of skills on a daily basis appears to be especially beneficial.55
Bottom Line
A sensible approach to diagnosing attention-deficit/hyperactivity disorder (ADHD) in adults includes ruling out other disorders that may present similar to ADHD, taking an appropriate developmental history, obtaining collateral information, and assessing for functional impairment. Sensible treatment involves ruling out comorbidities that stimulants could worsen, selecting extended-release stimulants, incorporating medication holidays, and using nonpharmacologic interventions.
Related Resources
- National Institute for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management. https://www.nice.org.uk/guidance/ng87
- Substance Abuse and Mental Health Services Administration. Advisory: Prescription Stimulant Misuse Among Youth and Young Adults. https://store.samhsa.gov/product/prescription-stimulant-misuse-among-youth-young-adults/PEP21-06-01-003
Drug Brand Names
Amphetamine • Adzenys, Dyanavel, others
Atomoxetine • Strattera
Bupropion • Wellbutrin, Forfivo
Clonidine • Catapres, Kapvay
Dexmethylphenidate • Focalin
Dextroamphetamine • Dexedrine
Dextroamphetamine and amphetamine • Adderall, Mydayis
Guanfacine • Intuniv, Tenex
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin, others
Viloxazine • Qelbree
Attention-deficit/hyperactivity disorder (ADHD) is common, with an estimated worldwide prevalence of 5.29% among children and adolescents and 2.5% among adults.1 DSM-5-TR classifies ADHD as a neurodevelopmental disorder, “a group of conditions with onset in the developmental period [that] typically manifest early in development, often before the child enters school.”2 Because of the expectation that ADHD symptoms emerge early in development, the diagnostic criteria specify that symptoms must have been present prior to age 12 to qualify as ADHD. However, recent years have shown a significant increase in the number of patients being diagnosed with ADHD for the first time in adulthood. One study found that the diagnosis of ADHD among adults in the United States doubled between 2007 and 2016.3
First-line treatment for ADHD is the stimulants methylphenidate and amphetamine/dextroamphetamine. In the United States, these medications are classified as Schedule II controlled substances, indicating a high risk for abuse. However, just as ADHD diagnoses among adults have increased, so have prescriptions for stimulants. For example, Olfson et al4 found that stimulant prescriptions among young adults increased by a factor of 10 between 1994 and 2009.
The increased prevalence of adult patients diagnosed with ADHD and taking stimulants frequently places clinicians in a position to consider the validity of existing diagnoses and evaluate new patients with ADHD-related concerns. In this article, we review some of the challenges associated with diagnosing ADHD in adults, discuss the risks of stimulant treatment, and present a practical approach to the diagnosis and treatment of ADHD in adults.
Challenges in diagnosis
DSM-5-TR diagnostic criteria for ADHD are summarized in Table 1. Establishing a diagnosis of adult ADHD can be challenging. As with many psychiatric conditions, symptoms of ADHD are highly subjective. Retrospectively diagnosing a developmental condition in adults is often biased by the patient’s current functioning.5 ADHD has a high heritability and adults may inquire about the diagnosis if their children are diagnosed with ADHD.6 Some experts have cautioned that clinicians must be careful in diagnosing ADHD in adults.7 Just as there are risks associated with underdiagnosing ADHD, there are risks associated with overdiagnosis. Overdiagnosis may medicalize normal variants in the population and lead to unnecessary treatment and a misappropriation of limited medical resources.8 Many false positive cases of late-onset ADHD may be attributable to nonimpairing cognitive fluctuations.9

Poor diagnostic practices can impede accuracy in establishing the presence or absence of ADHD. Unfortunately, methods of diagnosing adult ADHD have been shown to vary widely in terms of information sources, diagnostic instruments used, symptom threshold, and whether functional impairment is a requirement for diagnosis.10 A common practice in diagnosing adult ADHD involves asking patients to complete self-report questionnaires that list symptoms of ADHD, such as the Adult ADHD Self-Report Scale developed by the World Health Organization.11 However, self-reports of ADHD in adults are less reliable than informant reports, and some young adults without ADHD overreport symptoms.12,13 Symptom checklists are particularly susceptible to faking, which lessens their diagnostic value.14
The possibility of malingered symptoms of ADHD further increases the diagnostic difficulty. College students may be particularly susceptible to overreporting ADHD symptoms in order to obtain academic accommodations or stimulants in the hopes of improving school performance.15 One study found that 25% to 48% of college students self-referred for ADHD evaluations exaggerated their symptoms.16 In another study, 31% of adults failed the Word Memory Test, which suggests noncredible performance in their ADHD evaluation.17 College students can successfully feign ADHD symptoms in both self-reported symptoms and computer-based tests of attention.18 Harrison et al19 summarized many of these concerns in their 2007 study of ADHD malingering, noting the “almost perfect ability of the Faking group to choose items … that correspond to the DSM-IV symptoms, and to report these at levels even higher than persons with diagnosed ADHD.” They suggested “Clinicians should be suspicious of students or young adults presenting for a first-time diagnosis who rate themselves as being significantly symptomatic, yet have managed to achieve well in school and other life activities.”19
Another challenge in correctly diagnosing adult ADHD is identifying other conditions that may impair attention.20 Psychiatric conditions that may impair concentration include anxiety disorders, chronic stress, posttraumatic stress disorder, recent trauma, major depressive disorder (MDD), and bipolar disorder (BD). Undiagnosed learning disorders may present like ADHD. Focus can be negatively affected by sleep disorders such as sleep apnea, restless leg syndrome, or delayed sleep phase-onset disorder. Marijuana, cocaine, 3,4-methylenedioxy-methamphetamine (MDMA; “ecstasy”), caffeine, or prescription medications such as anticholinergics can also impair attention. Medical conditions that can present with attentional or executive functioning deficits include seizures, Lyme disease, HIV, encephalopathy, hypothyroidism, and “chemo brain.”21 Environmental factors such as age-related cognitive decline, sleep deprivation, inflammation, obesity, air pollution, chemical exposure, and excessive use of digital media may also produce symptoms similar to ADHD. Two studies of adult-onset ADHD concluded that 93% to 95% of cases were better explained by other conditions such as sleep disorders, substance use disorders, or another psychiatric disorder.22
Continue to: Risks associated with treatment
Risks associated with treatment
With or without an accurate ADHD diagnosis, prescribing stimulants presents certain risks (Table 223-40). One of the more well-known risks of stimulants is addiction or misuse.23 An estimated 5 million American adults misused prescription stimulants in 2016.24 Despite stimulants’ status as controlled substances, long-term concurrent use of stimulants with opioids is common among adults with ADHD.25 College students are particularly susceptible to misusing or diverting stimulants, often to improve their academic performance.26 At 1 university, 22% of students had misused stimulants in the past year.27 Prescribing short-acting stimulants (rather than extended-release formulations) increases the likelihood of misuse.28 Patients prescribed stimulants begin to receive requests to divert their medications to others as early as elementary school, and by college more than one-third of those taking stimulants have been asked to give, sell, or trade their medications.29 Diversion of stimulants by students with ADHD is prevalent, with 62% of patients engaging in diversion during their lifetime.15 Diverted stimulants can come from family members, black market sources, or deceived clinicians.30 Although students’ stimulant misuse/diversion often is academically motivated, nonmedical use of psychostimulants does not appear to have a statistically significant effect on improving grade point average.31 Despite a negligible impact on grades, most students who take stimulants identify their effect as strongly positive, producing a situation in which misusers of stimulants have little motivation to stop.32 While some patients might ask for a stimulant prescription with the rationale that liking the effects proves they have ADHD, this is inappropriate because most individuals like the effects of stimulant medications.33

The use of stimulants increases the risk for several adverse psychiatric outcomes. Stimulants increase the risk of anxiety, so exercise caution when prescribing to patients with a comorbid anxiety disorder.34 Stimulants can also worsen irritability and insomnia, 2 issues common among patients with ADHD.32 Use of stimulant medications can trigger manic episodes. Viktorin et al35 found a >6-fold increase in manic episodes among patients with BD receiving methylphenidate monotherapy compared to those receiving a combination of methylphenidate and a mood stabilizer.35 The use of methylphenidate and amphetamine can lead to new-onset psychosis (or exacerbation of pre-existing psychotic illness); amphetamine use is associated with a higher risk of psychosis than methylphenidate.36
General medical adverse effects are also possible with stimulant use. Stimulants’ adverse effect profiles include appetite suppression, dry mouth, and nausea. Long-term use poses a risk for stunting growth in children.1 Using stimulants during pregnancy is associated with higher risk for neonatal morbidity, including preterm birth, CNS-related disorders, and seizures.37 Stimulants can raise blood pressure and increase heart rate. Serious cardiovascular events associated with stimulant use include ventricular arrhythmias, strokes, and transient ischemic attacks.38
Nonstimulant ADHD treatments are less risky than stimulants but still require monitoring for common adverse effects. Atomoxetine has been associated with sedation, growth retardation (in children), and in severe cases, liver injury or suicidal ideation.39 Bupropion (commonly used off-label for ADHD) can lower the seizure threshold and cause irritability, anorexia, and insomnia.39 Viloxazine, a newer agent, can cause hypertension, increased heart rate, nausea, drowsiness, headache, and insomnia.40
Sensible diagnosing
Given the challenges in accurately diagnosing ADHD in adults, we present a sensible approach to making the diagnosis (Table 3). The first step is to rule out other conditions that might better explain the patient’s symptoms. A thorough clinical interview (including a psychiatric review of symptoms) is the cornerstone of an initial diagnostic assessment. The use of validated screening questionnaires such as the Patient Health Questionnaire-9 and General Anxiety Disorder-7 may also provide information regarding psychiatric conditions that require additional evaluation.

Continue to: Some of the most common conditions...
Some of the most common conditions we see mistaken for ADHD are MDD, generalized anxiety disorder (GAD), and BD. In DSM-5-TR, 1 of the diagnostic criteria for MDD is “diminished ability to think or concentrate, or indecisiveness, nearly every day (either by subjective account or as observed by others).”41 Similarly, criteria for GAD include “difficulty concentrating.”42 DSM-5-TR also includes distractibility as one of the criteria for mania/hypomania. Table 420-22,41,42 lists other psychiatric, substance-related, medical, and environmental conditions that can produce ADHD-like symptoms. Referring to some medical and environmental explanations for inattention, Aiken22 pointed out, “Patients who suffer from these problems might ask their doctor for a stimulant, but none of those syndromes require a psychopharmacologic approach.” ADHD can be comorbid with other psychiatric conditions, so the presence of another psychiatric illness does not automatically rule out ADHD. If alternative psychiatric diagnoses have been identified, these can be discussed with the patient and treatment offered that targets the specified condition.

Once alternative explanations have been ruled out, focus on the patient’s developmental history. DSM-5-TR conceptualizes ADHD as a neurodevelopmental disorder, meaning it is expected to emerge early in life. Whereas previous editions of DSM specified that ADHD symptoms must be present before age 7, DSM-5 modified this age threshold to before age 12.1 This necessitates taking a careful life history in order to understand the presence or absence of symptoms at earlier developmental stages.5 ADHD should be verified by symptoms apparent in childhood and present across the lifespan.15
While this retrospective history is necessary, histories that rely on self-report alone are often unreliable. Collateral sources of information are generally more reliable when assessing for ADHD symptoms.13 Third-party sources can help confirm that any impairment is best attributed to ADHD rather than to another condition.15 Unfortunately, the difficulty of obtaining collateral information means it is often neglected, even in the literature.10 A parent is the ideal informant for gathering collateral information regarding a patient’s functioning in childhood.5 Suggested best practices also include obtaining collateral information from interviews with significant others, behavioral questionnaires completed by parents (for current and childhood symptoms), review of school records, and consideration of intellectual and achievement testing.43 If psychological testing is pursued, include validity testing to detect feigned symptoms.18,44
When evaluating for ADHD, assess not only for the presence of symptoms, but also if these symptoms produce significant functional impairment.13,15 Impairments in daily functioning can include impaired school participation, social participation, quality of relationships, family conflict, family activities, family functioning, and emotional functioning.45 Some symptoms may affect functioning in an adult’s life differently than they did during childhood, from missed work appointments to being late picking up kids from school. Research has shown that the correlation between the number of symptoms and functional impairment is weak, which means someone could experience all of the symptoms of ADHD without experiencing functional impairment.45 To make an accurate diagnosis, it is therefore important to clearly establish both the number of symptoms the patient is experiencing and whether these symptoms are clearly linked to functional impairments.10
Sensible treatment
Once a diagnosis of ADHD has been clearly established, clinicians need to consider how best to treat the condition (Table 5). Stimulants are generally considered first-line treatment for ADHD. In randomized clinical trials, they showed significant efficacy; for example, one study of 146 adults with ADHD found a 76% improvement with methylphenidate compared to 19% for the placebo group.46 Before starting a stimulant, certain comorbidities should be ruled out. If a patient has glaucoma or pheochromocytoma, they may first need treatment from or clearance by other specialists. Stimulants should likely be held in patients with hypertension, angina, or cardiovascular defects until receiving medical clearance. The risks of stimulants need to be discussed with female patients of childbearing age, weighing the benefits of treatment against the risks of medication use should the patient get pregnant. Patients with comorbid psychosis or uncontrolled bipolar illness should not receive stimulants due to the risk of exacerbation. Patients with active substance use disorders (SUDs) are generally not good candidates for stimulants because of the risk of misusing or diverting stimulants and the possibility that substance abuse may be causing their inattentive symptoms. Patients whose SUDs are in remission may cautiously be considered as candidates for stimulants. If patients misuse their prescribed stimulants, they should be switched to a nonstimulant medication such as atomoxetine, bupropion, guanfacine, or clonidine.47

Continue to: Once a patient is deemed...
Once a patient is deemed to be a candidate for stimulants, clinicians need to choose between methylphenidate or amphetamine/dextroamphetamine formulations. Table 6 lists medications that are commonly prescribed to treat ADHD; unless otherwise noted, these are FDA-approved for this indication. As a general rule, for adults, long-acting stimulant formulations are preferred over short-acting formulations.28 Immediate-release stimulants are more prone to misuse or diversion compared to extended-release medications.29 Longer-acting formulations may also provide better full-day symptom control.48

In contrast to many other psychiatric medications, it may be beneficial to encourage periodically taking breaks or “medication holidays” from stimulants. Planned medication holidays for adults can involve intentionally not taking the medication over the weekend when the patient is not involved in work or school responsibilities. Such breaks have been shown to reduce adverse effects of stimulants (such as appetite suppression and insomnia) without significantly increasing ADHD symptoms.49 Short breaks can also help prevent medication tolerance and the subsequent need to increase doses.50 Medication holidays provide an opportunity to verify the ongoing benefits of the medication. It is advisable to periodically assess whether there is a continued need for stimulant treatment.51 If patients do not tolerate stimulants or have other contraindications, nonstimulants should be considered.
Lastly, no psychiatric patient should be treated with medication alone, and nonpharmacologic approaches should be incorporated as needed. Clear instructions, visual aids, nonverbal cues, frequent breaks to stand and stretch, schedules, normalizing failure as part of growth, and identifying triggers for emotional reactivity may help patients with ADHD.52 In a study of the academic performance of 92 college students taking medication for ADHD and 146 control students, treatment with stimulants alone did not eliminate the academic achievement deficit of those individuals with ADHD.53 Good study habits (even without stimulants) appeared more important in overcoming the achievement disparity of students with ADHD.53 Providing psychoeducation and training in concrete organization and planning skills have shown benefit.54 Practice of skills on a daily basis appears to be especially beneficial.55
Bottom Line
A sensible approach to diagnosing attention-deficit/hyperactivity disorder (ADHD) in adults includes ruling out other disorders that may present similar to ADHD, taking an appropriate developmental history, obtaining collateral information, and assessing for functional impairment. Sensible treatment involves ruling out comorbidities that stimulants could worsen, selecting extended-release stimulants, incorporating medication holidays, and using nonpharmacologic interventions.
Related Resources
- National Institute for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management. https://www.nice.org.uk/guidance/ng87
- Substance Abuse and Mental Health Services Administration. Advisory: Prescription Stimulant Misuse Among Youth and Young Adults. https://store.samhsa.gov/product/prescription-stimulant-misuse-among-youth-young-adults/PEP21-06-01-003
Drug Brand Names
Amphetamine • Adzenys, Dyanavel, others
Atomoxetine • Strattera
Bupropion • Wellbutrin, Forfivo
Clonidine • Catapres, Kapvay
Dexmethylphenidate • Focalin
Dextroamphetamine • Dexedrine
Dextroamphetamine and amphetamine • Adderall, Mydayis
Guanfacine • Intuniv, Tenex
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin, others
Viloxazine • Qelbree
1. Posner J, Polanczyk GV, Sonuga-Barke E. Attention-deficit hyperactivity disorder. Lancet. 2020;395(10222):450-462.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:35.
3. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
4. Olfson M, Blanco C, Wang S, et al. Trends in office-based treatment of adults with stimulants in the United States. J Clin Psychiatry. 2013;74(1):43-50.
5. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956.
6. Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2019;24(4):562-575.
7. Solanto MV. Child vs adult onset of attention-deficit/hyperactivity disorder. JAMA Psychiatry. 2017;74(4):421.
8. Jummani RR, Hirsch E, Hirsch GS. Are we overdiagnosing and overtreating ADHD? Psychiatric Times. Published May 31, 2017. Accessed March 17, 2023. https://www.psychiatrictimes.com/view/are-we-overdiagnosing-and-overtreating-adhd
9. Sibley MH, Rohde LA, Swanson JM, et al; Multimodal Treatment Study of Children with ADHD (MTA) Cooperative Group. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149.
10. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165.
11. Ustun B, Adler LA, Rudin C, et al. The World Health Organization adult attention-deficit/hyperactivity disorder self-report screening scale for DSM-5. JAMA Psychiatry. 2017;74(5):520-527.
12. Faraone SV, Biederman J. Can attention-deficit/hyperactivity disorder onset occur in adulthood? JAMA Psychiatry. 2016;73(7):655-656.
13. Sibley MH, Pelham WE, Molina BSG, et al. When diagnosing ADHD in young adults emphasize informant reports, DSM items, and impairment. J Consult Clin Psychol. 2012;80(6):1052-1061.
14. Sollman MJ, Ranseen JD, Berry DT. Detection of feigned ADHD in college students. Psychol Assess. 2010;22(2):325-335.
15. Green AL, Rabiner DL. What do we really know about ADHD in college students? Neurotherapeutics. 2012;9(3):559-568.
16. Sullivan BK, May K, Galbally L. Symptom exaggeration by college adults in attention-deficit hyperactivity disorder and learning disorder assessments. Appl Neuropsychol. 2007;14(3):189-207.
17. Suhr J, Hammers D, Dobbins-Buckland K, et al. The relationship of malingering test failure to self-reported symptoms and neuropsychological findings in adults referred for ADHD evaluation. Arch Clin Neuropsychol. 2008;23(5):521-530.
18. Lee Booksh R, Pella RD, Singh AN, et al. Ability of college students to simulate ADHD on objective measures of attention. J Atten Disord. 2010;13(4):325-338.
19. Harrison AG, Edwards MJ, Parker KC. Identifying students faking ADHD: preliminary findings and strategies for detection. Arch Clin Neuropsychol. 2007;22(5):577-588.
20. Lopez R, Micoulaud-Franchi JA, Galeria C, et al. Is adult-onset attention deficit/hyperactivity disorder frequent in clinical practice? Psychiatry Res. 2017;257:238-241.
21. Bhatia R. Rule out these causes of inattention before diagnosing ADHD. Current Psychiatry. 2016;15(10):32-33.
22. Aiken C. Adult-onset ADHD raises questions. Psychiatric Times. 2021;38(3):24.
23. Bjorn S, Weyandt LL. Issues pertaining to misuse of ADHD prescription medications. Psychiatric Times. 2018;35(9):17-19.
24. Compton WM, Han B, Blanco C, et al. Prevalence and correlates of prescription stimulant use, misuse, use disorders, and motivations for misuse among adults in the United States. Am J Psychiatry. 2018;175(8):741-755.
25. Wei YJ, Zhu Y, Liu W, et al. Prevalence of and factors associated with long-term concurrent use of stimulants and opioids among adults with attention-deficit/hyperactivity disorder. JAMA Netw Open. 2018;1(4):e181152. doi:10.1001/jamanetworkopen.2018.1152
26. Benson K, Flory K, Humphreys KL, et al. Misuse of stimulant medication among college students: a comprehensive review and meta-analysis. Clin Child Fam Psychol Rev. 2015;18(1):50-76.
27. Benson K, Woodlief DT, Flory K, et al. Is ADHD, independent of ODD, associated with whether and why college students misuse stimulant medication? Exp Clin Psychopharmacol. 2018;26(5):476-487.
28. Froehlich TE. ADHD medication adherence in college students-- a call to action for clinicians and researchers: commentary on “transition to college and adherence to prescribed attention deficit hyperactivity disorder medication.” J Dev Behav Pediatr. 2018;39(1):77-78.
29. Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21-31.
30. Vrecko S. Everyday drug diversions: a qualitative study of the illicit exchange and non-medical use of prescription stimulants on a university campus. Soc Sci Med. 2015;131:297-304.
31. Munro BA, Weyandt LL, Marraccini ME, et al. The relationship between nonmedical use of prescription stimulants, executive functioning and academic outcomes. Addict Behav. 2017;65:250-257.
32. Rabiner DL, Anastopoulos AD, Costello EJ, et al. Motives and perceived consequences of nonmedical ADHD medication use by college students: are students treating themselves for attention problems? J Atten Disord. 2009;13(3)259-270.
33. Tayag Y. Adult ADHD is the wild west of psychiatry. The Atlantic. Published April 14, 2023. Accessed May 3, 2023. https://www.theatlantic.com/health/archive/2023/04/adult-adhd-diagnosis-treatment-adderall-shortage/673719/
34. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255-270.
35. Viktorin A, Rydén E, Thase ME, et al. The risk of treatment-emergent mania with methylphenidate in bipolar disorder. Am J Psychiatry. 2017;174(4):341-348.
36. Moran LV, Ongur D, Hsu J, et al. Psychosis with methylphenidate or amphetamine in patients with ADHD. N Engl J Med. 2019; 380(12):1128-1138.
37. Nörby U, Winbladh B, Källén K. Perinatal outcomes after treatment with ADHD medication during pregnancy. Pediatrics. 2017;140(6):e20170747. doi:10.1542/peds.2017-0747
38. Tadrous M, Shakeri A, Chu C, et al. Assessment of stimulant use and cardiovascular event risks among older adults. JAMA Netw Open. 2021;4(10):e2130795. doi:10.1001/jamanetworkopen.2021.30795
39. Daughton JM, Kratochvil CJ. Review of ADHD pharmacotherapies: advantages, disadvantages, and clinical pearls. J Am Acad Child Adolesc Psychiatry. 2009;48(3):240-248.
40. Qelbree [package insert]. Rockville, MD: Supernus Pharmaceuticals; 2021.
41. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:183.
42. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:250.
43. DuPaul GJ, Weyandt LL, O’Dell SM, et al. College students with ADHD: current status and future directions. J Atten Disord. 2009;13(3):234-250.
44. Edmundson M, Berry DTR, Combs HL, et al. The effects of symptom information coaching on the feigning of adult ADHD. Psychol Assess. 2017;29(12):1429-1436.
45. Gordon M, Antshel K, Faraone S, et al. Symptoms versus impairment: the case for respecting DSM-IV’s criterion D. J Atten Disord. 2006;9(3):465-475.
46. Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456-463.
47. Osser D, Awidi B. Treating adults with ADHD requires special considerations. Psychiatric News. Published August 30, 2018. Accessed March 17, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.pp8a1
48. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown, RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
49. Martins S, Tramontina S, Polanczyk G, et al. Weekend holidays during methylphenidate use in ADHD children: a randomized clinical trial. J Child Adolesc Psychopharmacol. 2004;14(2):195-206.
50. Ibrahim K, Donyai P. Drug holidays from ADHD medication: international experience over the past four decades. J Atten Disord. 2015;19(7):551-568.
51. Matthijssen AM, Dietrich A, Bierens M, et al. Continued benefits of methylphenidate in ADHD after 2 years in clinical practice: a randomized placebo-controlled discontinuation study. Am J Psychiatry. 2019;176(9):754-762.
52. Mason EJ, Joshi KG. Nonpharmacologic strategies for helping children with ADHD. Current Psychiatry. 2018;7(1):42,46.
53. Advokat C, Lane SM, Luo C. College students with and without ADHD: comparison of self-report of medication usage, study habits, and academic achievement. J Atten Disord. 2011;15(8):656-666.
54. Knouse LE, Cooper-Vince C, Sprich S, et al. Recent developments in the psychosocial treatment of adult ADHD. Expert Rev Neurother. 2008;8(10):1537-1548.
55. Evans SW, Owens JS, Wymbs BT, et al. Evidence-based psychosocial treatments for children and adolescents with attention deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2018;47(2):157-198.
1. Posner J, Polanczyk GV, Sonuga-Barke E. Attention-deficit hyperactivity disorder. Lancet. 2020;395(10222):450-462.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:35.
3. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
4. Olfson M, Blanco C, Wang S, et al. Trends in office-based treatment of adults with stimulants in the United States. J Clin Psychiatry. 2013;74(1):43-50.
5. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956.
6. Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2019;24(4):562-575.
7. Solanto MV. Child vs adult onset of attention-deficit/hyperactivity disorder. JAMA Psychiatry. 2017;74(4):421.
8. Jummani RR, Hirsch E, Hirsch GS. Are we overdiagnosing and overtreating ADHD? Psychiatric Times. Published May 31, 2017. Accessed March 17, 2023. https://www.psychiatrictimes.com/view/are-we-overdiagnosing-and-overtreating-adhd
9. Sibley MH, Rohde LA, Swanson JM, et al; Multimodal Treatment Study of Children with ADHD (MTA) Cooperative Group. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149.
10. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165.
11. Ustun B, Adler LA, Rudin C, et al. The World Health Organization adult attention-deficit/hyperactivity disorder self-report screening scale for DSM-5. JAMA Psychiatry. 2017;74(5):520-527.
12. Faraone SV, Biederman J. Can attention-deficit/hyperactivity disorder onset occur in adulthood? JAMA Psychiatry. 2016;73(7):655-656.
13. Sibley MH, Pelham WE, Molina BSG, et al. When diagnosing ADHD in young adults emphasize informant reports, DSM items, and impairment. J Consult Clin Psychol. 2012;80(6):1052-1061.
14. Sollman MJ, Ranseen JD, Berry DT. Detection of feigned ADHD in college students. Psychol Assess. 2010;22(2):325-335.
15. Green AL, Rabiner DL. What do we really know about ADHD in college students? Neurotherapeutics. 2012;9(3):559-568.
16. Sullivan BK, May K, Galbally L. Symptom exaggeration by college adults in attention-deficit hyperactivity disorder and learning disorder assessments. Appl Neuropsychol. 2007;14(3):189-207.
17. Suhr J, Hammers D, Dobbins-Buckland K, et al. The relationship of malingering test failure to self-reported symptoms and neuropsychological findings in adults referred for ADHD evaluation. Arch Clin Neuropsychol. 2008;23(5):521-530.
18. Lee Booksh R, Pella RD, Singh AN, et al. Ability of college students to simulate ADHD on objective measures of attention. J Atten Disord. 2010;13(4):325-338.
19. Harrison AG, Edwards MJ, Parker KC. Identifying students faking ADHD: preliminary findings and strategies for detection. Arch Clin Neuropsychol. 2007;22(5):577-588.
20. Lopez R, Micoulaud-Franchi JA, Galeria C, et al. Is adult-onset attention deficit/hyperactivity disorder frequent in clinical practice? Psychiatry Res. 2017;257:238-241.
21. Bhatia R. Rule out these causes of inattention before diagnosing ADHD. Current Psychiatry. 2016;15(10):32-33.
22. Aiken C. Adult-onset ADHD raises questions. Psychiatric Times. 2021;38(3):24.
23. Bjorn S, Weyandt LL. Issues pertaining to misuse of ADHD prescription medications. Psychiatric Times. 2018;35(9):17-19.
24. Compton WM, Han B, Blanco C, et al. Prevalence and correlates of prescription stimulant use, misuse, use disorders, and motivations for misuse among adults in the United States. Am J Psychiatry. 2018;175(8):741-755.
25. Wei YJ, Zhu Y, Liu W, et al. Prevalence of and factors associated with long-term concurrent use of stimulants and opioids among adults with attention-deficit/hyperactivity disorder. JAMA Netw Open. 2018;1(4):e181152. doi:10.1001/jamanetworkopen.2018.1152
26. Benson K, Flory K, Humphreys KL, et al. Misuse of stimulant medication among college students: a comprehensive review and meta-analysis. Clin Child Fam Psychol Rev. 2015;18(1):50-76.
27. Benson K, Woodlief DT, Flory K, et al. Is ADHD, independent of ODD, associated with whether and why college students misuse stimulant medication? Exp Clin Psychopharmacol. 2018;26(5):476-487.
28. Froehlich TE. ADHD medication adherence in college students-- a call to action for clinicians and researchers: commentary on “transition to college and adherence to prescribed attention deficit hyperactivity disorder medication.” J Dev Behav Pediatr. 2018;39(1):77-78.
29. Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21-31.
30. Vrecko S. Everyday drug diversions: a qualitative study of the illicit exchange and non-medical use of prescription stimulants on a university campus. Soc Sci Med. 2015;131:297-304.
31. Munro BA, Weyandt LL, Marraccini ME, et al. The relationship between nonmedical use of prescription stimulants, executive functioning and academic outcomes. Addict Behav. 2017;65:250-257.
32. Rabiner DL, Anastopoulos AD, Costello EJ, et al. Motives and perceived consequences of nonmedical ADHD medication use by college students: are students treating themselves for attention problems? J Atten Disord. 2009;13(3)259-270.
33. Tayag Y. Adult ADHD is the wild west of psychiatry. The Atlantic. Published April 14, 2023. Accessed May 3, 2023. https://www.theatlantic.com/health/archive/2023/04/adult-adhd-diagnosis-treatment-adderall-shortage/673719/
34. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255-270.
35. Viktorin A, Rydén E, Thase ME, et al. The risk of treatment-emergent mania with methylphenidate in bipolar disorder. Am J Psychiatry. 2017;174(4):341-348.
36. Moran LV, Ongur D, Hsu J, et al. Psychosis with methylphenidate or amphetamine in patients with ADHD. N Engl J Med. 2019; 380(12):1128-1138.
37. Nörby U, Winbladh B, Källén K. Perinatal outcomes after treatment with ADHD medication during pregnancy. Pediatrics. 2017;140(6):e20170747. doi:10.1542/peds.2017-0747
38. Tadrous M, Shakeri A, Chu C, et al. Assessment of stimulant use and cardiovascular event risks among older adults. JAMA Netw Open. 2021;4(10):e2130795. doi:10.1001/jamanetworkopen.2021.30795
39. Daughton JM, Kratochvil CJ. Review of ADHD pharmacotherapies: advantages, disadvantages, and clinical pearls. J Am Acad Child Adolesc Psychiatry. 2009;48(3):240-248.
40. Qelbree [package insert]. Rockville, MD: Supernus Pharmaceuticals; 2021.
41. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:183.
42. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:250.
43. DuPaul GJ, Weyandt LL, O’Dell SM, et al. College students with ADHD: current status and future directions. J Atten Disord. 2009;13(3):234-250.
44. Edmundson M, Berry DTR, Combs HL, et al. The effects of symptom information coaching on the feigning of adult ADHD. Psychol Assess. 2017;29(12):1429-1436.
45. Gordon M, Antshel K, Faraone S, et al. Symptoms versus impairment: the case for respecting DSM-IV’s criterion D. J Atten Disord. 2006;9(3):465-475.
46. Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456-463.
47. Osser D, Awidi B. Treating adults with ADHD requires special considerations. Psychiatric News. Published August 30, 2018. Accessed March 17, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.pp8a1
48. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown, RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
49. Martins S, Tramontina S, Polanczyk G, et al. Weekend holidays during methylphenidate use in ADHD children: a randomized clinical trial. J Child Adolesc Psychopharmacol. 2004;14(2):195-206.
50. Ibrahim K, Donyai P. Drug holidays from ADHD medication: international experience over the past four decades. J Atten Disord. 2015;19(7):551-568.
51. Matthijssen AM, Dietrich A, Bierens M, et al. Continued benefits of methylphenidate in ADHD after 2 years in clinical practice: a randomized placebo-controlled discontinuation study. Am J Psychiatry. 2019;176(9):754-762.
52. Mason EJ, Joshi KG. Nonpharmacologic strategies for helping children with ADHD. Current Psychiatry. 2018;7(1):42,46.
53. Advokat C, Lane SM, Luo C. College students with and without ADHD: comparison of self-report of medication usage, study habits, and academic achievement. J Atten Disord. 2011;15(8):656-666.
54. Knouse LE, Cooper-Vince C, Sprich S, et al. Recent developments in the psychosocial treatment of adult ADHD. Expert Rev Neurother. 2008;8(10):1537-1548.
55. Evans SW, Owens JS, Wymbs BT, et al. Evidence-based psychosocial treatments for children and adolescents with attention deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2018;47(2):157-198.
Brain structural and cognitive changes during pregnancy
Pregnancy is unquestionably a major milestone in a woman’s life. During gestation, her body shape noticeably changes, but the invisible structural and cognitive changes in her brain are more striking. Some of those neurobiological changes are short-term, while others are long-lasting, well beyond delivery, and even into old age.
Physiological changes during pregnancy are extraordinary. The dramatic increases in estrogen, progesterone, and glucocorticoids help maintain pregnancy, ensure safe delivery of the baby, and trigger maternal behavior. However, other important changes also occur in the mother’s cardiac output, blood volume, renal function, respiratory output, and immune adaptations to accommodate the growth of the fetus. Gene expression also occurs to accomplish those changes, and there are lifelong repercussions from those drastic physiological changes.
During pregnancy, the brain is exposed to escalating levels of hormones released from the placenta, which the woman had never experienced. Those hormones regulate neuroplasticity, neuroinflammation, behavior, and cognition.
Structural brain changes1-6
Brain volume declines during pregnancy, reaching a nadir at the time of parturition. However, recovery occurs within 5 months after delivery. During the postpartum period, gray matter volume increases in the first 3 to 4 weeks, especially in areas involved in maternal behavior, including the amygdala, prefrontal cortex, and hypothalamus. Hippocampal gray matter decreases at 2 months postpartum compared to preconception levels, and reductions can still be observed up to 2 years following delivery. Gray matter reductions occur in multiple brain regions involved in social cognition, including the superior temporal gyrus, medial and inferior frontal cortex, fusiform areas, and hippocampus. Those changes correlate with positive maternal attachment. It is noteworthy that neural activity is highest in areas with reduced gray volume, so a decline in brain volume is associated with enhanced maternal attachment. Interestingly, those changes occur in fathers, too.
Childbearing improves stroke outcomes in middle age, but body weight will increase. The risk of Alzheimer’s disease increases with a higher number of gestations, but longevity is higher if the pregnancy occurs at an older age. Reproduction is also associated with shorter telomeres, which can elevate the risk of cancer, inflammation, diabetes, and dementia.
Cognitive changes7-10
The term “pregnancy brain” refers to cognitive changes during pregnancy and postpartum; these include decreased memory and concentration, absent-mindedness, heightened reactivity to threatening stimuli, and a decrease in motivation and executive functions. After delivery a mother has increased empathy (sometimes referred to as Theory of Mind) and greater activation in brain structures involved in empathy, including the paracingulate cortex, the posterior cingulate, and the insula. Also, the mirror neuron system becomes more activated in response to a woman’s own children compared to unfamiliar children. This incudes the ventral premotor cortex, the inferior frontal gyrus, and the posterior parietal cortex.
Certain forms of memory are impaired during pregnancy and early postpartum, including verbal free recall and working memory, as well as executive functions. Those are believed to correlate with glucocorticoids and estrogen levels.
Continue to: The following cognitive functions...
The following cognitive functions increase between the first and second trimester: verbal memory, attention, executive functions processing speed, verbal, and visuospatial abilities. Interestingly, mothers of a male fetus outperformed mothers of a female fetus on working memory and spatial ability.
Other changes11-16
- Cells from the fetus can traffic to the mother’s body and create microchimeric cells, which have short-term benefits (healing some of the other’s organs as stem cells do) but long-term downsides include future brain disorders such as Parkinson’s disease or Alzheimer’s disease, as well as autoimmune diseases and various types of cancer. The reverse also occurs with cells transferring from the mother to the fetus, persisting into infancy and childhood.
- Postpartum psychosis is associated with reductions in the volumes of the anterior cingulate, left parahippocampal gyrus, and superior temporal gyrus.
- A woman’s white matter increases during pregnancy compared to preconception. This is attributed to the high levels of prolactin, which proliferates oligodendrocytes, the glial cells that continuously manufacture myelin.
- The pituitary gland increases by 200% to 300% during pregnancy and returns to pre-pregnancy levels approximately 8 months following delivery. Prolactin also mediates the production of brain cells in the hippocampus (ie, neurogenesis).
- Sexual activity, even without pregnancy, increases neurogenesis. Plasma levels of prolactin increase significantly following an orgasm in both men and women, which indicates that sexual activity has beneficial brain effects.
- With pregnancy, the immune system shifts from proinflammatory to anti-inflammatory signaling. This protects the fetus from being attacked and rejected as foreign tissue. However, at the end of pregnancy, there is a “burst” of proinflammatory signaling, which serves as a major trigger to induce uterine contractions and initiate labor (to expel the foreign tissue).
- Brain levels of the anti-inflammatory cytokine interleukin-6 increase in the postpartum period, which represents a significant modification in the neuroimmune environment, and the maternal brain assumes an inflammatory-resistant state, which has cognitive and neuroplasticity implications. However, this neuroimmune dysregulation is implicated in postpartum depression and anxiety.
- Older females who were never pregnant or only had 1 pregnancy had better overall cognitive functioning than females who became pregnant at an young age.
- In animal studies, reproduction alleviates the negative effects of aging on several hippocampal functions, especially neurogenesis. Dendritic spine density in the CA1 region of the hippocampus is higher in pregnancy and early postpartum period compared to nulliparous females (based on animal studies).
Pregnancy is indispensable for the perpetuation of the species. Its hormonal, physiologic, neurobiological, and cognitive correlates are extensive. The cognitive changes in the postpartum period are designed by evolution to prepare a woman to care for her newborn and to ensure its survival. But the biological sequelae of pregnancy extend to the rest of a woman’s life and may predispose her to immune and brain disorders as she ages.
1. Barba-Müller E, Craddock S, Carmona S, et al. Brain plasticity in pregnancy and the postpartum period: links to maternal caregiving and mental health. Arch Womens Ment Health. 2019;22(2):289-299.
2. Pawluski JL, Hoekzema E, Leuner B, et al. Less can be more: fine tuning the maternal brain. Neurosci Biobehav Rev. 2022;133:104475. doi:10.1016/j.neubiorev.2021.11.045
3. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
4. Cárdenas EF, Kujawa A, Humphreys KL. Neurobiological changes during the peripartum period: implications for health and behavior. Soc Cogn Affect Neurosci. 2020;15(10):1097-1110.
5. Eid RS, Chaiton JA, Lieblich SE, et al. Early and late effects of maternal experience on hippocampal neurogenesis, microglia, and the circulating cytokine milieu. Neurobiol Aging. 2019;78:1-17.
6. Galea LA, Leuner B, Slattery DA. Hippocampal plasticity during the peripartum period: influence of sex steroids, stress and ageing. J Neuroendocrinol. 2014;26(10):641-648.
7. Henry JF, Sherwin BB. Hormones and cognitive functioning during late pregnancy and postpartum: a longitudinal study. Behav Neurosci. 2012;126(1):73-85.
8. Barda G, Mizrachi Y, Borokchovich I, et al. The effect of pregnancy on maternal cognition. Sci Rep. 2011;11(1)12187. doi:10.1038/s41598-021-91504-9
9. Davies SJ, Lum JA, Skouteris H, et al. Cognitive impairment during pregnancy: a meta-analysis. Med J Aust. 2018;208(1):35-40.
10. Pownall M, Hutter RRC, Rockliffe L, et al. Memory and mood changes in pregnancy: a qualitative content analysis of women’s first-hand accounts. J Reprod Infant Psychol. 2023;41(5):516-527.
11. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
12. Duarte-Guterman P, Leuner B, Galea LAM. The long and short term effects of motherhood on the brain. Front Neuroendocrinol. 2019;53:100740. doi:10.1016/j.yfrne.2019.02.004
13. Haim A, Julian D, Albin-Brooks C, et al. A survey of neuroimmune changes in pregnant and postpartum female rats. Brain Behav Immun. 2017;59:67-78.
14. Benson JC, Malyuk DF, Madhavan A, et al. Pituitary volume changes in pregnancy and the post-partum period. Neuroradiol J. 2023. doi:10.1177/19714009231196470
15. Schepanski S, Chini M, Sternemann V, et al. Pregnancy-induced maternal microchimerism shapes neurodevelopment and behavior in mice. Nat Commun. 2022;13(1):4571. doi:10.1038/s41467-022-32230-2
16. Larsen CM, Grattan DR. Prolactin, neurogenesis, and maternal behaviors. Brain Behav Immun. 2012;26(2):201-209.
Pregnancy is unquestionably a major milestone in a woman’s life. During gestation, her body shape noticeably changes, but the invisible structural and cognitive changes in her brain are more striking. Some of those neurobiological changes are short-term, while others are long-lasting, well beyond delivery, and even into old age.
Physiological changes during pregnancy are extraordinary. The dramatic increases in estrogen, progesterone, and glucocorticoids help maintain pregnancy, ensure safe delivery of the baby, and trigger maternal behavior. However, other important changes also occur in the mother’s cardiac output, blood volume, renal function, respiratory output, and immune adaptations to accommodate the growth of the fetus. Gene expression also occurs to accomplish those changes, and there are lifelong repercussions from those drastic physiological changes.
During pregnancy, the brain is exposed to escalating levels of hormones released from the placenta, which the woman had never experienced. Those hormones regulate neuroplasticity, neuroinflammation, behavior, and cognition.
Structural brain changes1-6
Brain volume declines during pregnancy, reaching a nadir at the time of parturition. However, recovery occurs within 5 months after delivery. During the postpartum period, gray matter volume increases in the first 3 to 4 weeks, especially in areas involved in maternal behavior, including the amygdala, prefrontal cortex, and hypothalamus. Hippocampal gray matter decreases at 2 months postpartum compared to preconception levels, and reductions can still be observed up to 2 years following delivery. Gray matter reductions occur in multiple brain regions involved in social cognition, including the superior temporal gyrus, medial and inferior frontal cortex, fusiform areas, and hippocampus. Those changes correlate with positive maternal attachment. It is noteworthy that neural activity is highest in areas with reduced gray volume, so a decline in brain volume is associated with enhanced maternal attachment. Interestingly, those changes occur in fathers, too.
Childbearing improves stroke outcomes in middle age, but body weight will increase. The risk of Alzheimer’s disease increases with a higher number of gestations, but longevity is higher if the pregnancy occurs at an older age. Reproduction is also associated with shorter telomeres, which can elevate the risk of cancer, inflammation, diabetes, and dementia.
Cognitive changes7-10
The term “pregnancy brain” refers to cognitive changes during pregnancy and postpartum; these include decreased memory and concentration, absent-mindedness, heightened reactivity to threatening stimuli, and a decrease in motivation and executive functions. After delivery a mother has increased empathy (sometimes referred to as Theory of Mind) and greater activation in brain structures involved in empathy, including the paracingulate cortex, the posterior cingulate, and the insula. Also, the mirror neuron system becomes more activated in response to a woman’s own children compared to unfamiliar children. This incudes the ventral premotor cortex, the inferior frontal gyrus, and the posterior parietal cortex.
Certain forms of memory are impaired during pregnancy and early postpartum, including verbal free recall and working memory, as well as executive functions. Those are believed to correlate with glucocorticoids and estrogen levels.
Continue to: The following cognitive functions...
The following cognitive functions increase between the first and second trimester: verbal memory, attention, executive functions processing speed, verbal, and visuospatial abilities. Interestingly, mothers of a male fetus outperformed mothers of a female fetus on working memory and spatial ability.
Other changes11-16
- Cells from the fetus can traffic to the mother’s body and create microchimeric cells, which have short-term benefits (healing some of the other’s organs as stem cells do) but long-term downsides include future brain disorders such as Parkinson’s disease or Alzheimer’s disease, as well as autoimmune diseases and various types of cancer. The reverse also occurs with cells transferring from the mother to the fetus, persisting into infancy and childhood.
- Postpartum psychosis is associated with reductions in the volumes of the anterior cingulate, left parahippocampal gyrus, and superior temporal gyrus.
- A woman’s white matter increases during pregnancy compared to preconception. This is attributed to the high levels of prolactin, which proliferates oligodendrocytes, the glial cells that continuously manufacture myelin.
- The pituitary gland increases by 200% to 300% during pregnancy and returns to pre-pregnancy levels approximately 8 months following delivery. Prolactin also mediates the production of brain cells in the hippocampus (ie, neurogenesis).
- Sexual activity, even without pregnancy, increases neurogenesis. Plasma levels of prolactin increase significantly following an orgasm in both men and women, which indicates that sexual activity has beneficial brain effects.
- With pregnancy, the immune system shifts from proinflammatory to anti-inflammatory signaling. This protects the fetus from being attacked and rejected as foreign tissue. However, at the end of pregnancy, there is a “burst” of proinflammatory signaling, which serves as a major trigger to induce uterine contractions and initiate labor (to expel the foreign tissue).
- Brain levels of the anti-inflammatory cytokine interleukin-6 increase in the postpartum period, which represents a significant modification in the neuroimmune environment, and the maternal brain assumes an inflammatory-resistant state, which has cognitive and neuroplasticity implications. However, this neuroimmune dysregulation is implicated in postpartum depression and anxiety.
- Older females who were never pregnant or only had 1 pregnancy had better overall cognitive functioning than females who became pregnant at an young age.
- In animal studies, reproduction alleviates the negative effects of aging on several hippocampal functions, especially neurogenesis. Dendritic spine density in the CA1 region of the hippocampus is higher in pregnancy and early postpartum period compared to nulliparous females (based on animal studies).
Pregnancy is indispensable for the perpetuation of the species. Its hormonal, physiologic, neurobiological, and cognitive correlates are extensive. The cognitive changes in the postpartum period are designed by evolution to prepare a woman to care for her newborn and to ensure its survival. But the biological sequelae of pregnancy extend to the rest of a woman’s life and may predispose her to immune and brain disorders as she ages.
Pregnancy is unquestionably a major milestone in a woman’s life. During gestation, her body shape noticeably changes, but the invisible structural and cognitive changes in her brain are more striking. Some of those neurobiological changes are short-term, while others are long-lasting, well beyond delivery, and even into old age.
Physiological changes during pregnancy are extraordinary. The dramatic increases in estrogen, progesterone, and glucocorticoids help maintain pregnancy, ensure safe delivery of the baby, and trigger maternal behavior. However, other important changes also occur in the mother’s cardiac output, blood volume, renal function, respiratory output, and immune adaptations to accommodate the growth of the fetus. Gene expression also occurs to accomplish those changes, and there are lifelong repercussions from those drastic physiological changes.
During pregnancy, the brain is exposed to escalating levels of hormones released from the placenta, which the woman had never experienced. Those hormones regulate neuroplasticity, neuroinflammation, behavior, and cognition.
Structural brain changes1-6
Brain volume declines during pregnancy, reaching a nadir at the time of parturition. However, recovery occurs within 5 months after delivery. During the postpartum period, gray matter volume increases in the first 3 to 4 weeks, especially in areas involved in maternal behavior, including the amygdala, prefrontal cortex, and hypothalamus. Hippocampal gray matter decreases at 2 months postpartum compared to preconception levels, and reductions can still be observed up to 2 years following delivery. Gray matter reductions occur in multiple brain regions involved in social cognition, including the superior temporal gyrus, medial and inferior frontal cortex, fusiform areas, and hippocampus. Those changes correlate with positive maternal attachment. It is noteworthy that neural activity is highest in areas with reduced gray volume, so a decline in brain volume is associated with enhanced maternal attachment. Interestingly, those changes occur in fathers, too.
Childbearing improves stroke outcomes in middle age, but body weight will increase. The risk of Alzheimer’s disease increases with a higher number of gestations, but longevity is higher if the pregnancy occurs at an older age. Reproduction is also associated with shorter telomeres, which can elevate the risk of cancer, inflammation, diabetes, and dementia.
Cognitive changes7-10
The term “pregnancy brain” refers to cognitive changes during pregnancy and postpartum; these include decreased memory and concentration, absent-mindedness, heightened reactivity to threatening stimuli, and a decrease in motivation and executive functions. After delivery a mother has increased empathy (sometimes referred to as Theory of Mind) and greater activation in brain structures involved in empathy, including the paracingulate cortex, the posterior cingulate, and the insula. Also, the mirror neuron system becomes more activated in response to a woman’s own children compared to unfamiliar children. This incudes the ventral premotor cortex, the inferior frontal gyrus, and the posterior parietal cortex.
Certain forms of memory are impaired during pregnancy and early postpartum, including verbal free recall and working memory, as well as executive functions. Those are believed to correlate with glucocorticoids and estrogen levels.
Continue to: The following cognitive functions...
The following cognitive functions increase between the first and second trimester: verbal memory, attention, executive functions processing speed, verbal, and visuospatial abilities. Interestingly, mothers of a male fetus outperformed mothers of a female fetus on working memory and spatial ability.
Other changes11-16
- Cells from the fetus can traffic to the mother’s body and create microchimeric cells, which have short-term benefits (healing some of the other’s organs as stem cells do) but long-term downsides include future brain disorders such as Parkinson’s disease or Alzheimer’s disease, as well as autoimmune diseases and various types of cancer. The reverse also occurs with cells transferring from the mother to the fetus, persisting into infancy and childhood.
- Postpartum psychosis is associated with reductions in the volumes of the anterior cingulate, left parahippocampal gyrus, and superior temporal gyrus.
- A woman’s white matter increases during pregnancy compared to preconception. This is attributed to the high levels of prolactin, which proliferates oligodendrocytes, the glial cells that continuously manufacture myelin.
- The pituitary gland increases by 200% to 300% during pregnancy and returns to pre-pregnancy levels approximately 8 months following delivery. Prolactin also mediates the production of brain cells in the hippocampus (ie, neurogenesis).
- Sexual activity, even without pregnancy, increases neurogenesis. Plasma levels of prolactin increase significantly following an orgasm in both men and women, which indicates that sexual activity has beneficial brain effects.
- With pregnancy, the immune system shifts from proinflammatory to anti-inflammatory signaling. This protects the fetus from being attacked and rejected as foreign tissue. However, at the end of pregnancy, there is a “burst” of proinflammatory signaling, which serves as a major trigger to induce uterine contractions and initiate labor (to expel the foreign tissue).
- Brain levels of the anti-inflammatory cytokine interleukin-6 increase in the postpartum period, which represents a significant modification in the neuroimmune environment, and the maternal brain assumes an inflammatory-resistant state, which has cognitive and neuroplasticity implications. However, this neuroimmune dysregulation is implicated in postpartum depression and anxiety.
- Older females who were never pregnant or only had 1 pregnancy had better overall cognitive functioning than females who became pregnant at an young age.
- In animal studies, reproduction alleviates the negative effects of aging on several hippocampal functions, especially neurogenesis. Dendritic spine density in the CA1 region of the hippocampus is higher in pregnancy and early postpartum period compared to nulliparous females (based on animal studies).
Pregnancy is indispensable for the perpetuation of the species. Its hormonal, physiologic, neurobiological, and cognitive correlates are extensive. The cognitive changes in the postpartum period are designed by evolution to prepare a woman to care for her newborn and to ensure its survival. But the biological sequelae of pregnancy extend to the rest of a woman’s life and may predispose her to immune and brain disorders as she ages.
1. Barba-Müller E, Craddock S, Carmona S, et al. Brain plasticity in pregnancy and the postpartum period: links to maternal caregiving and mental health. Arch Womens Ment Health. 2019;22(2):289-299.
2. Pawluski JL, Hoekzema E, Leuner B, et al. Less can be more: fine tuning the maternal brain. Neurosci Biobehav Rev. 2022;133:104475. doi:10.1016/j.neubiorev.2021.11.045
3. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
4. Cárdenas EF, Kujawa A, Humphreys KL. Neurobiological changes during the peripartum period: implications for health and behavior. Soc Cogn Affect Neurosci. 2020;15(10):1097-1110.
5. Eid RS, Chaiton JA, Lieblich SE, et al. Early and late effects of maternal experience on hippocampal neurogenesis, microglia, and the circulating cytokine milieu. Neurobiol Aging. 2019;78:1-17.
6. Galea LA, Leuner B, Slattery DA. Hippocampal plasticity during the peripartum period: influence of sex steroids, stress and ageing. J Neuroendocrinol. 2014;26(10):641-648.
7. Henry JF, Sherwin BB. Hormones and cognitive functioning during late pregnancy and postpartum: a longitudinal study. Behav Neurosci. 2012;126(1):73-85.
8. Barda G, Mizrachi Y, Borokchovich I, et al. The effect of pregnancy on maternal cognition. Sci Rep. 2011;11(1)12187. doi:10.1038/s41598-021-91504-9
9. Davies SJ, Lum JA, Skouteris H, et al. Cognitive impairment during pregnancy: a meta-analysis. Med J Aust. 2018;208(1):35-40.
10. Pownall M, Hutter RRC, Rockliffe L, et al. Memory and mood changes in pregnancy: a qualitative content analysis of women’s first-hand accounts. J Reprod Infant Psychol. 2023;41(5):516-527.
11. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
12. Duarte-Guterman P, Leuner B, Galea LAM. The long and short term effects of motherhood on the brain. Front Neuroendocrinol. 2019;53:100740. doi:10.1016/j.yfrne.2019.02.004
13. Haim A, Julian D, Albin-Brooks C, et al. A survey of neuroimmune changes in pregnant and postpartum female rats. Brain Behav Immun. 2017;59:67-78.
14. Benson JC, Malyuk DF, Madhavan A, et al. Pituitary volume changes in pregnancy and the post-partum period. Neuroradiol J. 2023. doi:10.1177/19714009231196470
15. Schepanski S, Chini M, Sternemann V, et al. Pregnancy-induced maternal microchimerism shapes neurodevelopment and behavior in mice. Nat Commun. 2022;13(1):4571. doi:10.1038/s41467-022-32230-2
16. Larsen CM, Grattan DR. Prolactin, neurogenesis, and maternal behaviors. Brain Behav Immun. 2012;26(2):201-209.
1. Barba-Müller E, Craddock S, Carmona S, et al. Brain plasticity in pregnancy and the postpartum period: links to maternal caregiving and mental health. Arch Womens Ment Health. 2019;22(2):289-299.
2. Pawluski JL, Hoekzema E, Leuner B, et al. Less can be more: fine tuning the maternal brain. Neurosci Biobehav Rev. 2022;133:104475. doi:10.1016/j.neubiorev.2021.11.045
3. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
4. Cárdenas EF, Kujawa A, Humphreys KL. Neurobiological changes during the peripartum period: implications for health and behavior. Soc Cogn Affect Neurosci. 2020;15(10):1097-1110.
5. Eid RS, Chaiton JA, Lieblich SE, et al. Early and late effects of maternal experience on hippocampal neurogenesis, microglia, and the circulating cytokine milieu. Neurobiol Aging. 2019;78:1-17.
6. Galea LA, Leuner B, Slattery DA. Hippocampal plasticity during the peripartum period: influence of sex steroids, stress and ageing. J Neuroendocrinol. 2014;26(10):641-648.
7. Henry JF, Sherwin BB. Hormones and cognitive functioning during late pregnancy and postpartum: a longitudinal study. Behav Neurosci. 2012;126(1):73-85.
8. Barda G, Mizrachi Y, Borokchovich I, et al. The effect of pregnancy on maternal cognition. Sci Rep. 2011;11(1)12187. doi:10.1038/s41598-021-91504-9
9. Davies SJ, Lum JA, Skouteris H, et al. Cognitive impairment during pregnancy: a meta-analysis. Med J Aust. 2018;208(1):35-40.
10. Pownall M, Hutter RRC, Rockliffe L, et al. Memory and mood changes in pregnancy: a qualitative content analysis of women’s first-hand accounts. J Reprod Infant Psychol. 2023;41(5):516-527.
11. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
12. Duarte-Guterman P, Leuner B, Galea LAM. The long and short term effects of motherhood on the brain. Front Neuroendocrinol. 2019;53:100740. doi:10.1016/j.yfrne.2019.02.004
13. Haim A, Julian D, Albin-Brooks C, et al. A survey of neuroimmune changes in pregnant and postpartum female rats. Brain Behav Immun. 2017;59:67-78.
14. Benson JC, Malyuk DF, Madhavan A, et al. Pituitary volume changes in pregnancy and the post-partum period. Neuroradiol J. 2023. doi:10.1177/19714009231196470
15. Schepanski S, Chini M, Sternemann V, et al. Pregnancy-induced maternal microchimerism shapes neurodevelopment and behavior in mice. Nat Commun. 2022;13(1):4571. doi:10.1038/s41467-022-32230-2
16. Larsen CM, Grattan DR. Prolactin, neurogenesis, and maternal behaviors. Brain Behav Immun. 2012;26(2):201-209.
More on climate change and mental health, burnout among surgeons
More on climate change and mental health
Your recent editorial (“A toxic and fractured political system can breed angst and PTSD”
The article suggested that psychiatrists are unequivocally tasked with managing the psychological aftermath of climate-related disasters. However, it is crucial to acknowledge that this is an assumption and lacks empirical evidence. I concur with the authors’ recognition of the grave environmental concerns posed by pollution, but it is valid to question the extent to which these concerns are fueled by mass hysteria, exacerbated by articles such as this one. Climate change undoubtedly is a multifaceted issue at times exploited for political purposes. As a result, terms such as “climate change denialism” are warped expressions that polarize the public even further, hindering constructive dialogue. Rather than denying the issue at hand, I am advocating for environmentally friendly solutions that do not come at the cost of manipulating public sentiment for political gain.
Additionally, I would argue trauma often does not arise from climate change itself, but instead from the actions of misguided radical environmentalist policy that unwittingly can cause more harm than good. The devastating destruction in Maui is a case in point. The article focuses on climate change as a cause of nihilism in this country; however, there is serious need to explore broader sociological issues that underlie this sense of nihilism and lack of life meaning, especially in the young.
It is essential to engage in a balanced and evidence-based discussion regarding climate change and its potential mental health implications. While some concerns the authors raised are valid, it is equally important to avoid fomenting hysteria and consider alternative perspectives that may help bridge gaps in understanding and unite us in effectively addressing this global challenge.
Robert Barris, MD
Flushing, New York
I want to send my appreciation for publishing in the same issue your editorial “A toxic and fractured political system can breed angst and PTSD” and the article “Climate change and mental illness: What psychiatrists can do.” I believe the issues addressed are important and belong in the mainstream of current psychiatric discussion.
Regarding the differing views of optimists and pessimists, I agree that narrative is bound for destruction. Because of that, several months ago I decided to deliberately cultivate and maintain a sense of optimism while knowing the facts! I believe that stance is the only one that strategically can lead towards progress.
I also want to comment on the “religification” of politics. While I believe secular religions exist, I also believe what we are currently seeing in the United States is not the rise of secular religions, but instead an attempt to insert extreme religious beliefs into politics while using language to create the illusion that the Constitution’s barrier against the merging of church and state is not being breached. I don’t think we are seeing secular religion, but God-based religion masking as secular religion.
Michael A. Kalm, MD
Salt Lake City, Utah
More on physician burnout
I am writing in reference to “Burnout among surgeons: Lessons for psychiatrists” (
It would behoove institutions to teach methods to mitigate burnout starting with first-year medical students instead of waiting until the increased stress, workload, and responsibility of their intern year. Knowing there is a potential negative downstream effect on patient care, in addition to the negative personal and professional impact on surgeons, is significant. By taking the time to engage all medical students in confidential, affordable, accessible mental health care, institutions would not only decrease burnout in this population of physicians but decrease the likelihood of negative outcomes in patient care.
Elina Maymind, MD
Mt. Laurel, New Jersey
More on climate change and mental health
Your recent editorial (“A toxic and fractured political system can breed angst and PTSD”
The article suggested that psychiatrists are unequivocally tasked with managing the psychological aftermath of climate-related disasters. However, it is crucial to acknowledge that this is an assumption and lacks empirical evidence. I concur with the authors’ recognition of the grave environmental concerns posed by pollution, but it is valid to question the extent to which these concerns are fueled by mass hysteria, exacerbated by articles such as this one. Climate change undoubtedly is a multifaceted issue at times exploited for political purposes. As a result, terms such as “climate change denialism” are warped expressions that polarize the public even further, hindering constructive dialogue. Rather than denying the issue at hand, I am advocating for environmentally friendly solutions that do not come at the cost of manipulating public sentiment for political gain.
Additionally, I would argue trauma often does not arise from climate change itself, but instead from the actions of misguided radical environmentalist policy that unwittingly can cause more harm than good. The devastating destruction in Maui is a case in point. The article focuses on climate change as a cause of nihilism in this country; however, there is serious need to explore broader sociological issues that underlie this sense of nihilism and lack of life meaning, especially in the young.
It is essential to engage in a balanced and evidence-based discussion regarding climate change and its potential mental health implications. While some concerns the authors raised are valid, it is equally important to avoid fomenting hysteria and consider alternative perspectives that may help bridge gaps in understanding and unite us in effectively addressing this global challenge.
Robert Barris, MD
Flushing, New York
I want to send my appreciation for publishing in the same issue your editorial “A toxic and fractured political system can breed angst and PTSD” and the article “Climate change and mental illness: What psychiatrists can do.” I believe the issues addressed are important and belong in the mainstream of current psychiatric discussion.
Regarding the differing views of optimists and pessimists, I agree that narrative is bound for destruction. Because of that, several months ago I decided to deliberately cultivate and maintain a sense of optimism while knowing the facts! I believe that stance is the only one that strategically can lead towards progress.
I also want to comment on the “religification” of politics. While I believe secular religions exist, I also believe what we are currently seeing in the United States is not the rise of secular religions, but instead an attempt to insert extreme religious beliefs into politics while using language to create the illusion that the Constitution’s barrier against the merging of church and state is not being breached. I don’t think we are seeing secular religion, but God-based religion masking as secular religion.
Michael A. Kalm, MD
Salt Lake City, Utah
More on physician burnout
I am writing in reference to “Burnout among surgeons: Lessons for psychiatrists” (
It would behoove institutions to teach methods to mitigate burnout starting with first-year medical students instead of waiting until the increased stress, workload, and responsibility of their intern year. Knowing there is a potential negative downstream effect on patient care, in addition to the negative personal and professional impact on surgeons, is significant. By taking the time to engage all medical students in confidential, affordable, accessible mental health care, institutions would not only decrease burnout in this population of physicians but decrease the likelihood of negative outcomes in patient care.
Elina Maymind, MD
Mt. Laurel, New Jersey
More on climate change and mental health
Your recent editorial (“A toxic and fractured political system can breed angst and PTSD”
The article suggested that psychiatrists are unequivocally tasked with managing the psychological aftermath of climate-related disasters. However, it is crucial to acknowledge that this is an assumption and lacks empirical evidence. I concur with the authors’ recognition of the grave environmental concerns posed by pollution, but it is valid to question the extent to which these concerns are fueled by mass hysteria, exacerbated by articles such as this one. Climate change undoubtedly is a multifaceted issue at times exploited for political purposes. As a result, terms such as “climate change denialism” are warped expressions that polarize the public even further, hindering constructive dialogue. Rather than denying the issue at hand, I am advocating for environmentally friendly solutions that do not come at the cost of manipulating public sentiment for political gain.
Additionally, I would argue trauma often does not arise from climate change itself, but instead from the actions of misguided radical environmentalist policy that unwittingly can cause more harm than good. The devastating destruction in Maui is a case in point. The article focuses on climate change as a cause of nihilism in this country; however, there is serious need to explore broader sociological issues that underlie this sense of nihilism and lack of life meaning, especially in the young.
It is essential to engage in a balanced and evidence-based discussion regarding climate change and its potential mental health implications. While some concerns the authors raised are valid, it is equally important to avoid fomenting hysteria and consider alternative perspectives that may help bridge gaps in understanding and unite us in effectively addressing this global challenge.
Robert Barris, MD
Flushing, New York
I want to send my appreciation for publishing in the same issue your editorial “A toxic and fractured political system can breed angst and PTSD” and the article “Climate change and mental illness: What psychiatrists can do.” I believe the issues addressed are important and belong in the mainstream of current psychiatric discussion.
Regarding the differing views of optimists and pessimists, I agree that narrative is bound for destruction. Because of that, several months ago I decided to deliberately cultivate and maintain a sense of optimism while knowing the facts! I believe that stance is the only one that strategically can lead towards progress.
I also want to comment on the “religification” of politics. While I believe secular religions exist, I also believe what we are currently seeing in the United States is not the rise of secular religions, but instead an attempt to insert extreme religious beliefs into politics while using language to create the illusion that the Constitution’s barrier against the merging of church and state is not being breached. I don’t think we are seeing secular religion, but God-based religion masking as secular religion.
Michael A. Kalm, MD
Salt Lake City, Utah
More on physician burnout
I am writing in reference to “Burnout among surgeons: Lessons for psychiatrists” (
It would behoove institutions to teach methods to mitigate burnout starting with first-year medical students instead of waiting until the increased stress, workload, and responsibility of their intern year. Knowing there is a potential negative downstream effect on patient care, in addition to the negative personal and professional impact on surgeons, is significant. By taking the time to engage all medical students in confidential, affordable, accessible mental health care, institutions would not only decrease burnout in this population of physicians but decrease the likelihood of negative outcomes in patient care.
Elina Maymind, MD
Mt. Laurel, New Jersey
Managing psychotropic-induced hyperhidrosis
Ms. K, age 32, presents to the psychiatric clinic for a routine follow-up. Her history includes agoraphobia, attention-deficit/hyperactivity disorder, and schizoaffective disorder. Ms. K’s current medications are oral hydroxyzine 50 mg 4 times daily as needed for anxiety and paliperidone palmitate 234 mg IM monthly. Since her last follow-up, she has been switched from oral sertraline 150 mg/d to oral paroxetine 20 mg/d. Ms. K reports having constipation (which improves by taking oral docusate 100 mg twice daily) and generalized hyperhidrosis. She wants to alleviate the hyperhidrosis without changing her paroxetine because that medication improved her symptoms.
Hyperhidrosis—excessive sweating not needed to maintain a normal body temperature—is an uncommon and uncomfortable adverse effect of many medications, including psychotropics.1 This long-term adverse effect typically is not dose-related and does not remit with continued therapy.2 Table 11-3 lists psychotropic medications associated with hyperhidrosis as well as postulated mechanisms.
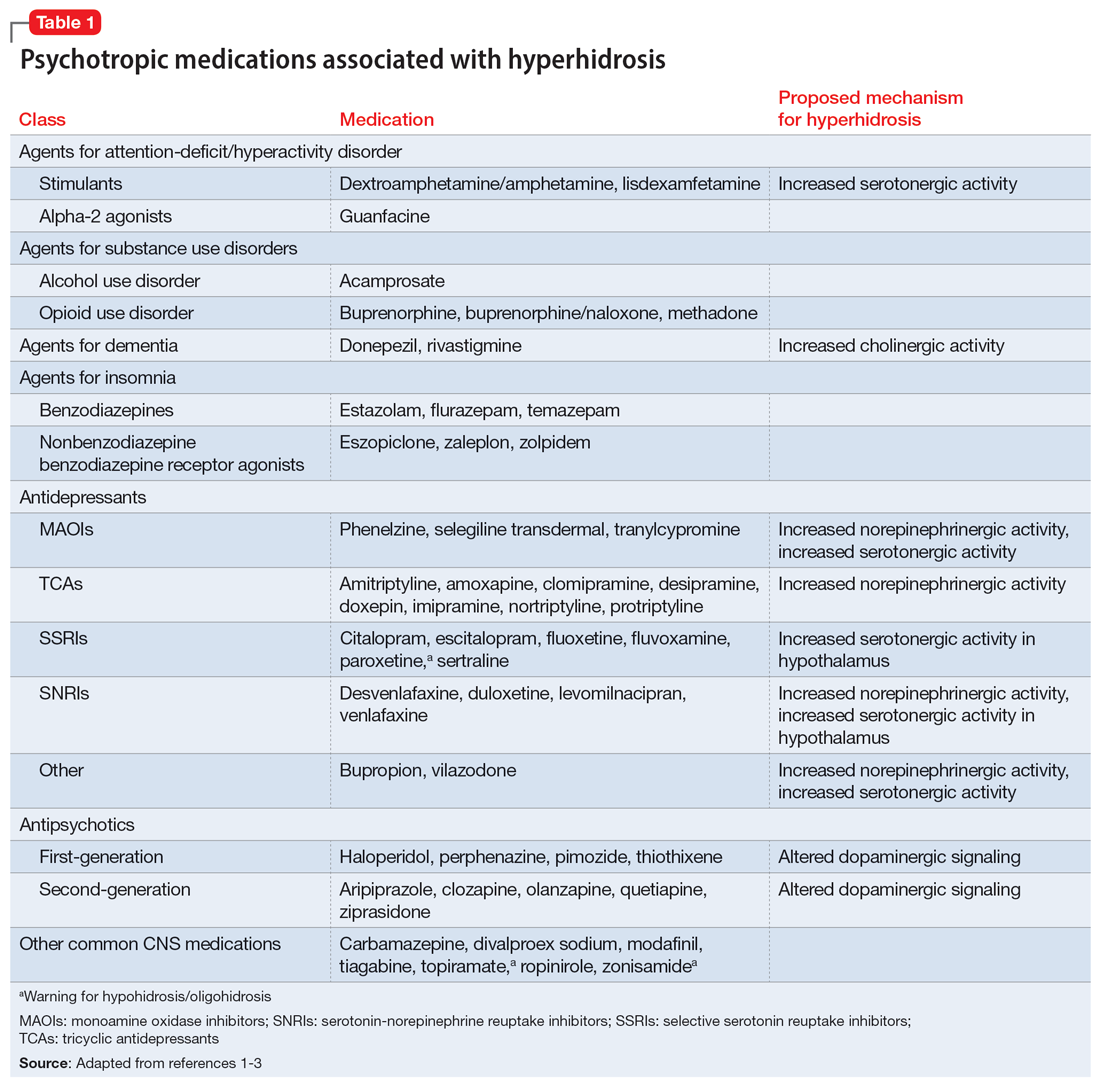
The incidence of medication-induced hyperhidrosis is unknown, but for psychotropic medications it is estimated to be 5% to 20%.3 Patients may not report hyperhidrosis due to embarrassment; in clinical trials, reporting measures may be inconsistent and, in some cases, misleading. For example, it is possible hyperhidrosis that appears to be associated with buprenorphine is actually a symptom of the withdrawal syndrome rather than a direct effect of the medication. Also, some medications, including certain psychotropics (eg, paroxetine4 and topiramate3) may cause either hyperhidrosis or hypohidrosis (decreased sweating). Few medications carry labeled warnings for hypohidrosis; the condition generally is not of clinical concern unless patients experience heat intolerance or hyperthermia.3
Psychotropic-induced hyperhidrosis is likely an idiopathic effect. There are few known predisposing factors, but some medications carry a greater risk than others. In a meta-analysis, Beyer et al2 found certain selective serotonin reuptake inhibitors (SSRIs), such as sertraline and paroxetine, had a higher risk of causing hyperhidrosis. Fluvoxamine, bupropion, and vortioxetine had the lowest risk. The class risk for SSRIs was comparable to that of serotonin-norepinephrine reuptake inhibitors (SNRIs), which all carried a comparable risk. In this analysis, neither indication nor dose were reliable indicators of risk of causing hyperhidrosis. However, the study found that for both SSRIs and SNRIs, increased affinity for the dopamine transporter was correlated with an increased risk of hyperhidrosis.2
Treatment
Treatment of hyperhidrosis depends on its cause and presentation.5 Hyperhidrosis may be categorized as primary (idiopathic) or secondary (also termed diaphoresis), and either focal or generalized.6 Many treatment recommendations focus on primary or focal hyperhidrosis and prioritize topical therapies.5 Because medication-induced hyperhidrosis most commonly presents as generalized3 and thus affects a large body surface area, the use of topical therapies is precluded. Topical therapy for psychotropic-induced hyperhidrosis should be pursued only if the patient’s sweating is localized.
Treating medication-induced hyperhidrosis becomes more complicated if it is not possible to alter the inciting medication (ie, because the medication is effective or the patient is resistant to change). In such scenarios, discontinuing the medication and initiating an alternative therapy may not be effective or feasible.2 For generalized presentations of medication-induced hyperhidrosis, if the inciting medication cannot be altered, initiating an oral systemic therapy is the preferred treatment.3,5
Oral anticholinergic medications (eg, benztropine, glycopyrrolate, and oxybutynin),4-6 act directly on muscarinic receptors within the eccrine sweat glands to decrease or stop sweating. They are considered first-line for generalized hyperhidrosis but may be inappropriate for psychotropic-induced hyperhidrosis because many psychotropics (eg, tricyclic antidepressants, paroxetine, olanzapine, quetiapine, and clozapine) have anticholinergic properties. Adding an anticholinergic medication to these patients’ regimens may increase the adverse effect burden and worsen cognitive deficits. Additionally, approximately one-third of patients discontinue anticholinergic medications due to tolerability issues (eg, dry mouth).
Continue to: However, anticholinergic medications...
However, anticholinergic medications may still have a role in treating psychotropic-induced hyperhidrosis. Benztropine3,7,8 and cyproheptadine2,3,9 may be effective options, though their role in treating psychotropic-induced hyperhidrosis should be limited and reserved for patients who have another compelling indication for these medications (eg, extrapyramidal symptoms) or when other treatment options are ineffective or intolerable.
Avoiding anticholinergic medications can also be justified based on the proposed mechanism of psychotropic-induced hyperhidrosis as an extension of the medication’s toxic effects. Conceptualizing psychotropic-induced hyperhidrosis as similar to the diaphoresis and hyperthermia observed in neuroleptic malignant syndrome and serotonin syndrome offers a clearer target for treatment. Though the specifics of the mechanisms remain unknown,2 many medications that cause hyperhidrosis do so by increasing sweat gland secretions, either directly by increasing cholinergic activity or indirectly via increased sympathetic transmission.
Considering this pathophysiology, another target for psychotropic-induced hyperhidrosis may be altered and/or excessive catecholamine activity. The use of medications such as clonidine,3-6 propranolol,4-6 or terazosin2,3,10 should be considered given their beneficial effects on the activation of the sympathetic nervous system, although clonidine also possesses anticholinergic activity. The calcium channel blocker diltiazem can improve hyperhidrosis symptoms by interfering with the calcium signaling necessary for normal sweat gland function.4,5 Comorbid cardiovascular diseases and tachycardia, an adverse effect of many psychotropic medications, may also be managed with these treatment options. Some research suggests using benzodiazepines to treat psychotropic-induced hyperhidrosis.4-6 As is the case for anticholinergic medications, the use of benzodiazepines would require another compelling indication for long-term use.
Table 23,4,6-8,10 provides recommended dosing and caveats for the use of these medications and other potentially appropriate medications.
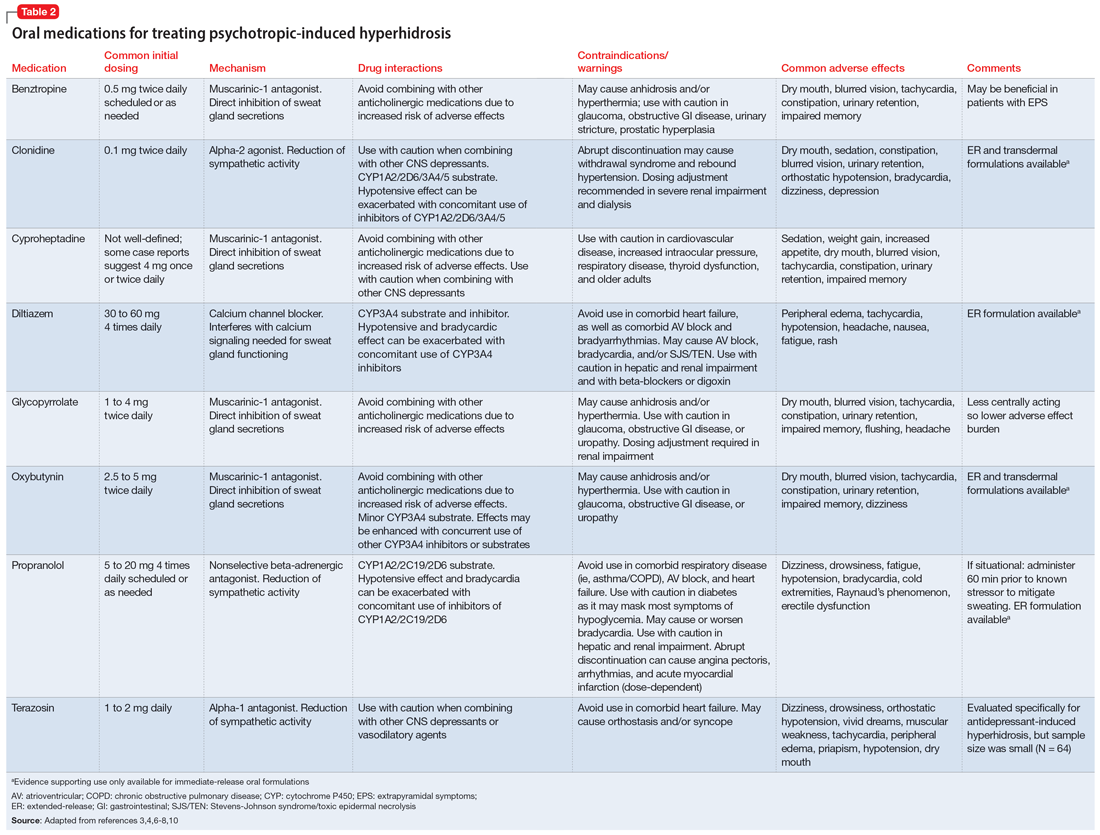
Research of investigational treatments for generalized hyperhidrosis is ongoing. It is possible some of these medications may have a future role in the treatment of psychotropic-induced hyperhidrosis, with improved efficacy and better tolerability.
Continue to: CASE CONTINUED
CASE CONTINUED
Because Ms. K’s medication-induced hyperhidrosis is generalized and therefore ineligible for topical therapies, and because the inciting medication (paroxetine) cannot be switched to an alternative, the treatment team considers adding an oral medication. Treatment with an anticholinergic medication, such as benztropine, is not preferred due to the anticholinergic activity associated with paroxetine and Ms. K’s history of constipation. After discussing other oral treatment options with Ms. K, the team ultimately decides to initiate propranolol at a low dose (5 mg twice daily) to minimize the chances of an interaction with paroxetine, and titrate based on efficacy and tolerability.
Related Resources
- International Hyperhidrosis Society. Hyperhidrosis treatment overview. www.sweathelp.org/hyperhidrosis-treatments/treatment-overview.html
Drug Brand Names
Acamprosate • Campral
Aripiprazole • Abilify
Buprenorphine • Sublocade
Buprenorphine/naloxone • Zubsolv
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clomipramine • Anafranil
Clonidine • Catapres
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextroamphetamine/amphetamine • Adderall
Diltiazem • Cardizem
Divalproex • Depakote
Donepezil • Aricept
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Guanfacine • Intuniv
Glycopyrrolate • Cuvposa
Hydroxyzine • Vistaril
Imipramine • Tofranil
Levomilnacipran • Fetzima
Lisdexamfetamine • Vyvanse
Methadone • Dolophine, Methadose
Modafinil • Provigil
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone palmitate • Invega Sustenna
Paroxetine • Paxil
Phenelzine • Nardil
Pimozide • Orap
Protriptyline • Vivactil
Quetiapine • Seroquel
Rivastigmine • Exelon
Selegiline transdermal • Emsam
Sertraline • Zoloft
Temazepam • Restoril
Thiothixene • Navane
Tiagabine • Gabitril
Topiramate • Topamax
Tranylcypromine • Parnate
Vilazodone • Viibryd
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
1. International Hyperhidrosis Society. Drugs/medications known to cause hyperhidrosis. Sweathelp.org. 2022. Accessed September 6, 2022. https://www.sweathelp.org/pdf/drugs_2009.pdf
2. Beyer C, Cappetta K, Johnson JA, et al. Meta-analysis: risk of hyperhidrosis with second-generation antidepressants. Depress Anxiety. 2017;34(12):1134-1146. doi:10.1002/da.22680
3. Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 2008;31(2):109-126. doi:10.2165/00002018-200831020-00002
4. del Boz J. Systemic treatment of hyperhidrosis. Actas Dermosifiliogr. 2015;106(4):271-277. doi:10.1016/j.ad.2014.11.012
5. Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669-680. doi:10.1016/j.jaad2018.11.066
6. Glaser DA. Oral medications. Dermatol Clin. 2014;32(4):527-532. doi:10.1016/j.det.2014.06.002
7. Garber A, Gregory RJ. Benztropine in the treatment of venlafaxine-induced sweating. J Clin Psychiatry. 1997;58(4):176-177. doi:10.4088/jcp.v58n0407e
8. Kolli V, Ramaswamy S. Improvement of antidepressant-induced sweating with as-required benztropine. Innov Clin Neurosci. 2013;10(11-12):10-11.
9. Ashton AK, Weinstein WL. Cyproheptadine for drug-induced sweating. Am J Psychiatry. 2002;159(5):875. doi:10.1176/APPI.AJP.159.5.874-A
10. Ghaleiha A, Shahidi KM, Afzali S, et al. Effect of terazosin on sweating in patients with major depressive disorder receiving sertraline: a randomized controlled trial. Int J Psychiatry Clin Pract. 2013;17(1):44-47. doi:10.3109/13651501.2012.687449
Ms. K, age 32, presents to the psychiatric clinic for a routine follow-up. Her history includes agoraphobia, attention-deficit/hyperactivity disorder, and schizoaffective disorder. Ms. K’s current medications are oral hydroxyzine 50 mg 4 times daily as needed for anxiety and paliperidone palmitate 234 mg IM monthly. Since her last follow-up, she has been switched from oral sertraline 150 mg/d to oral paroxetine 20 mg/d. Ms. K reports having constipation (which improves by taking oral docusate 100 mg twice daily) and generalized hyperhidrosis. She wants to alleviate the hyperhidrosis without changing her paroxetine because that medication improved her symptoms.
Hyperhidrosis—excessive sweating not needed to maintain a normal body temperature—is an uncommon and uncomfortable adverse effect of many medications, including psychotropics.1 This long-term adverse effect typically is not dose-related and does not remit with continued therapy.2 Table 11-3 lists psychotropic medications associated with hyperhidrosis as well as postulated mechanisms.

The incidence of medication-induced hyperhidrosis is unknown, but for psychotropic medications it is estimated to be 5% to 20%.3 Patients may not report hyperhidrosis due to embarrassment; in clinical trials, reporting measures may be inconsistent and, in some cases, misleading. For example, it is possible hyperhidrosis that appears to be associated with buprenorphine is actually a symptom of the withdrawal syndrome rather than a direct effect of the medication. Also, some medications, including certain psychotropics (eg, paroxetine4 and topiramate3) may cause either hyperhidrosis or hypohidrosis (decreased sweating). Few medications carry labeled warnings for hypohidrosis; the condition generally is not of clinical concern unless patients experience heat intolerance or hyperthermia.3
Psychotropic-induced hyperhidrosis is likely an idiopathic effect. There are few known predisposing factors, but some medications carry a greater risk than others. In a meta-analysis, Beyer et al2 found certain selective serotonin reuptake inhibitors (SSRIs), such as sertraline and paroxetine, had a higher risk of causing hyperhidrosis. Fluvoxamine, bupropion, and vortioxetine had the lowest risk. The class risk for SSRIs was comparable to that of serotonin-norepinephrine reuptake inhibitors (SNRIs), which all carried a comparable risk. In this analysis, neither indication nor dose were reliable indicators of risk of causing hyperhidrosis. However, the study found that for both SSRIs and SNRIs, increased affinity for the dopamine transporter was correlated with an increased risk of hyperhidrosis.2
Treatment
Treatment of hyperhidrosis depends on its cause and presentation.5 Hyperhidrosis may be categorized as primary (idiopathic) or secondary (also termed diaphoresis), and either focal or generalized.6 Many treatment recommendations focus on primary or focal hyperhidrosis and prioritize topical therapies.5 Because medication-induced hyperhidrosis most commonly presents as generalized3 and thus affects a large body surface area, the use of topical therapies is precluded. Topical therapy for psychotropic-induced hyperhidrosis should be pursued only if the patient’s sweating is localized.
Treating medication-induced hyperhidrosis becomes more complicated if it is not possible to alter the inciting medication (ie, because the medication is effective or the patient is resistant to change). In such scenarios, discontinuing the medication and initiating an alternative therapy may not be effective or feasible.2 For generalized presentations of medication-induced hyperhidrosis, if the inciting medication cannot be altered, initiating an oral systemic therapy is the preferred treatment.3,5
Oral anticholinergic medications (eg, benztropine, glycopyrrolate, and oxybutynin),4-6 act directly on muscarinic receptors within the eccrine sweat glands to decrease or stop sweating. They are considered first-line for generalized hyperhidrosis but may be inappropriate for psychotropic-induced hyperhidrosis because many psychotropics (eg, tricyclic antidepressants, paroxetine, olanzapine, quetiapine, and clozapine) have anticholinergic properties. Adding an anticholinergic medication to these patients’ regimens may increase the adverse effect burden and worsen cognitive deficits. Additionally, approximately one-third of patients discontinue anticholinergic medications due to tolerability issues (eg, dry mouth).
Continue to: However, anticholinergic medications...
However, anticholinergic medications may still have a role in treating psychotropic-induced hyperhidrosis. Benztropine3,7,8 and cyproheptadine2,3,9 may be effective options, though their role in treating psychotropic-induced hyperhidrosis should be limited and reserved for patients who have another compelling indication for these medications (eg, extrapyramidal symptoms) or when other treatment options are ineffective or intolerable.
Avoiding anticholinergic medications can also be justified based on the proposed mechanism of psychotropic-induced hyperhidrosis as an extension of the medication’s toxic effects. Conceptualizing psychotropic-induced hyperhidrosis as similar to the diaphoresis and hyperthermia observed in neuroleptic malignant syndrome and serotonin syndrome offers a clearer target for treatment. Though the specifics of the mechanisms remain unknown,2 many medications that cause hyperhidrosis do so by increasing sweat gland secretions, either directly by increasing cholinergic activity or indirectly via increased sympathetic transmission.
Considering this pathophysiology, another target for psychotropic-induced hyperhidrosis may be altered and/or excessive catecholamine activity. The use of medications such as clonidine,3-6 propranolol,4-6 or terazosin2,3,10 should be considered given their beneficial effects on the activation of the sympathetic nervous system, although clonidine also possesses anticholinergic activity. The calcium channel blocker diltiazem can improve hyperhidrosis symptoms by interfering with the calcium signaling necessary for normal sweat gland function.4,5 Comorbid cardiovascular diseases and tachycardia, an adverse effect of many psychotropic medications, may also be managed with these treatment options. Some research suggests using benzodiazepines to treat psychotropic-induced hyperhidrosis.4-6 As is the case for anticholinergic medications, the use of benzodiazepines would require another compelling indication for long-term use.
Table 23,4,6-8,10 provides recommended dosing and caveats for the use of these medications and other potentially appropriate medications.

Research of investigational treatments for generalized hyperhidrosis is ongoing. It is possible some of these medications may have a future role in the treatment of psychotropic-induced hyperhidrosis, with improved efficacy and better tolerability.
Continue to: CASE CONTINUED
CASE CONTINUED
Because Ms. K’s medication-induced hyperhidrosis is generalized and therefore ineligible for topical therapies, and because the inciting medication (paroxetine) cannot be switched to an alternative, the treatment team considers adding an oral medication. Treatment with an anticholinergic medication, such as benztropine, is not preferred due to the anticholinergic activity associated with paroxetine and Ms. K’s history of constipation. After discussing other oral treatment options with Ms. K, the team ultimately decides to initiate propranolol at a low dose (5 mg twice daily) to minimize the chances of an interaction with paroxetine, and titrate based on efficacy and tolerability.
Related Resources
- International Hyperhidrosis Society. Hyperhidrosis treatment overview. www.sweathelp.org/hyperhidrosis-treatments/treatment-overview.html
Drug Brand Names
Acamprosate • Campral
Aripiprazole • Abilify
Buprenorphine • Sublocade
Buprenorphine/naloxone • Zubsolv
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clomipramine • Anafranil
Clonidine • Catapres
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextroamphetamine/amphetamine • Adderall
Diltiazem • Cardizem
Divalproex • Depakote
Donepezil • Aricept
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Guanfacine • Intuniv
Glycopyrrolate • Cuvposa
Hydroxyzine • Vistaril
Imipramine • Tofranil
Levomilnacipran • Fetzima
Lisdexamfetamine • Vyvanse
Methadone • Dolophine, Methadose
Modafinil • Provigil
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone palmitate • Invega Sustenna
Paroxetine • Paxil
Phenelzine • Nardil
Pimozide • Orap
Protriptyline • Vivactil
Quetiapine • Seroquel
Rivastigmine • Exelon
Selegiline transdermal • Emsam
Sertraline • Zoloft
Temazepam • Restoril
Thiothixene • Navane
Tiagabine • Gabitril
Topiramate • Topamax
Tranylcypromine • Parnate
Vilazodone • Viibryd
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
Ms. K, age 32, presents to the psychiatric clinic for a routine follow-up. Her history includes agoraphobia, attention-deficit/hyperactivity disorder, and schizoaffective disorder. Ms. K’s current medications are oral hydroxyzine 50 mg 4 times daily as needed for anxiety and paliperidone palmitate 234 mg IM monthly. Since her last follow-up, she has been switched from oral sertraline 150 mg/d to oral paroxetine 20 mg/d. Ms. K reports having constipation (which improves by taking oral docusate 100 mg twice daily) and generalized hyperhidrosis. She wants to alleviate the hyperhidrosis without changing her paroxetine because that medication improved her symptoms.
Hyperhidrosis—excessive sweating not needed to maintain a normal body temperature—is an uncommon and uncomfortable adverse effect of many medications, including psychotropics.1 This long-term adverse effect typically is not dose-related and does not remit with continued therapy.2 Table 11-3 lists psychotropic medications associated with hyperhidrosis as well as postulated mechanisms.

The incidence of medication-induced hyperhidrosis is unknown, but for psychotropic medications it is estimated to be 5% to 20%.3 Patients may not report hyperhidrosis due to embarrassment; in clinical trials, reporting measures may be inconsistent and, in some cases, misleading. For example, it is possible hyperhidrosis that appears to be associated with buprenorphine is actually a symptom of the withdrawal syndrome rather than a direct effect of the medication. Also, some medications, including certain psychotropics (eg, paroxetine4 and topiramate3) may cause either hyperhidrosis or hypohidrosis (decreased sweating). Few medications carry labeled warnings for hypohidrosis; the condition generally is not of clinical concern unless patients experience heat intolerance or hyperthermia.3
Psychotropic-induced hyperhidrosis is likely an idiopathic effect. There are few known predisposing factors, but some medications carry a greater risk than others. In a meta-analysis, Beyer et al2 found certain selective serotonin reuptake inhibitors (SSRIs), such as sertraline and paroxetine, had a higher risk of causing hyperhidrosis. Fluvoxamine, bupropion, and vortioxetine had the lowest risk. The class risk for SSRIs was comparable to that of serotonin-norepinephrine reuptake inhibitors (SNRIs), which all carried a comparable risk. In this analysis, neither indication nor dose were reliable indicators of risk of causing hyperhidrosis. However, the study found that for both SSRIs and SNRIs, increased affinity for the dopamine transporter was correlated with an increased risk of hyperhidrosis.2
Treatment
Treatment of hyperhidrosis depends on its cause and presentation.5 Hyperhidrosis may be categorized as primary (idiopathic) or secondary (also termed diaphoresis), and either focal or generalized.6 Many treatment recommendations focus on primary or focal hyperhidrosis and prioritize topical therapies.5 Because medication-induced hyperhidrosis most commonly presents as generalized3 and thus affects a large body surface area, the use of topical therapies is precluded. Topical therapy for psychotropic-induced hyperhidrosis should be pursued only if the patient’s sweating is localized.
Treating medication-induced hyperhidrosis becomes more complicated if it is not possible to alter the inciting medication (ie, because the medication is effective or the patient is resistant to change). In such scenarios, discontinuing the medication and initiating an alternative therapy may not be effective or feasible.2 For generalized presentations of medication-induced hyperhidrosis, if the inciting medication cannot be altered, initiating an oral systemic therapy is the preferred treatment.3,5
Oral anticholinergic medications (eg, benztropine, glycopyrrolate, and oxybutynin),4-6 act directly on muscarinic receptors within the eccrine sweat glands to decrease or stop sweating. They are considered first-line for generalized hyperhidrosis but may be inappropriate for psychotropic-induced hyperhidrosis because many psychotropics (eg, tricyclic antidepressants, paroxetine, olanzapine, quetiapine, and clozapine) have anticholinergic properties. Adding an anticholinergic medication to these patients’ regimens may increase the adverse effect burden and worsen cognitive deficits. Additionally, approximately one-third of patients discontinue anticholinergic medications due to tolerability issues (eg, dry mouth).
Continue to: However, anticholinergic medications...
However, anticholinergic medications may still have a role in treating psychotropic-induced hyperhidrosis. Benztropine3,7,8 and cyproheptadine2,3,9 may be effective options, though their role in treating psychotropic-induced hyperhidrosis should be limited and reserved for patients who have another compelling indication for these medications (eg, extrapyramidal symptoms) or when other treatment options are ineffective or intolerable.
Avoiding anticholinergic medications can also be justified based on the proposed mechanism of psychotropic-induced hyperhidrosis as an extension of the medication’s toxic effects. Conceptualizing psychotropic-induced hyperhidrosis as similar to the diaphoresis and hyperthermia observed in neuroleptic malignant syndrome and serotonin syndrome offers a clearer target for treatment. Though the specifics of the mechanisms remain unknown,2 many medications that cause hyperhidrosis do so by increasing sweat gland secretions, either directly by increasing cholinergic activity or indirectly via increased sympathetic transmission.
Considering this pathophysiology, another target for psychotropic-induced hyperhidrosis may be altered and/or excessive catecholamine activity. The use of medications such as clonidine,3-6 propranolol,4-6 or terazosin2,3,10 should be considered given their beneficial effects on the activation of the sympathetic nervous system, although clonidine also possesses anticholinergic activity. The calcium channel blocker diltiazem can improve hyperhidrosis symptoms by interfering with the calcium signaling necessary for normal sweat gland function.4,5 Comorbid cardiovascular diseases and tachycardia, an adverse effect of many psychotropic medications, may also be managed with these treatment options. Some research suggests using benzodiazepines to treat psychotropic-induced hyperhidrosis.4-6 As is the case for anticholinergic medications, the use of benzodiazepines would require another compelling indication for long-term use.
Table 23,4,6-8,10 provides recommended dosing and caveats for the use of these medications and other potentially appropriate medications.

Research of investigational treatments for generalized hyperhidrosis is ongoing. It is possible some of these medications may have a future role in the treatment of psychotropic-induced hyperhidrosis, with improved efficacy and better tolerability.
Continue to: CASE CONTINUED
CASE CONTINUED
Because Ms. K’s medication-induced hyperhidrosis is generalized and therefore ineligible for topical therapies, and because the inciting medication (paroxetine) cannot be switched to an alternative, the treatment team considers adding an oral medication. Treatment with an anticholinergic medication, such as benztropine, is not preferred due to the anticholinergic activity associated with paroxetine and Ms. K’s history of constipation. After discussing other oral treatment options with Ms. K, the team ultimately decides to initiate propranolol at a low dose (5 mg twice daily) to minimize the chances of an interaction with paroxetine, and titrate based on efficacy and tolerability.
Related Resources
- International Hyperhidrosis Society. Hyperhidrosis treatment overview. www.sweathelp.org/hyperhidrosis-treatments/treatment-overview.html
Drug Brand Names
Acamprosate • Campral
Aripiprazole • Abilify
Buprenorphine • Sublocade
Buprenorphine/naloxone • Zubsolv
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clomipramine • Anafranil
Clonidine • Catapres
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextroamphetamine/amphetamine • Adderall
Diltiazem • Cardizem
Divalproex • Depakote
Donepezil • Aricept
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Guanfacine • Intuniv
Glycopyrrolate • Cuvposa
Hydroxyzine • Vistaril
Imipramine • Tofranil
Levomilnacipran • Fetzima
Lisdexamfetamine • Vyvanse
Methadone • Dolophine, Methadose
Modafinil • Provigil
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone palmitate • Invega Sustenna
Paroxetine • Paxil
Phenelzine • Nardil
Pimozide • Orap
Protriptyline • Vivactil
Quetiapine • Seroquel
Rivastigmine • Exelon
Selegiline transdermal • Emsam
Sertraline • Zoloft
Temazepam • Restoril
Thiothixene • Navane
Tiagabine • Gabitril
Topiramate • Topamax
Tranylcypromine • Parnate
Vilazodone • Viibryd
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
1. International Hyperhidrosis Society. Drugs/medications known to cause hyperhidrosis. Sweathelp.org. 2022. Accessed September 6, 2022. https://www.sweathelp.org/pdf/drugs_2009.pdf
2. Beyer C, Cappetta K, Johnson JA, et al. Meta-analysis: risk of hyperhidrosis with second-generation antidepressants. Depress Anxiety. 2017;34(12):1134-1146. doi:10.1002/da.22680
3. Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 2008;31(2):109-126. doi:10.2165/00002018-200831020-00002
4. del Boz J. Systemic treatment of hyperhidrosis. Actas Dermosifiliogr. 2015;106(4):271-277. doi:10.1016/j.ad.2014.11.012
5. Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669-680. doi:10.1016/j.jaad2018.11.066
6. Glaser DA. Oral medications. Dermatol Clin. 2014;32(4):527-532. doi:10.1016/j.det.2014.06.002
7. Garber A, Gregory RJ. Benztropine in the treatment of venlafaxine-induced sweating. J Clin Psychiatry. 1997;58(4):176-177. doi:10.4088/jcp.v58n0407e
8. Kolli V, Ramaswamy S. Improvement of antidepressant-induced sweating with as-required benztropine. Innov Clin Neurosci. 2013;10(11-12):10-11.
9. Ashton AK, Weinstein WL. Cyproheptadine for drug-induced sweating. Am J Psychiatry. 2002;159(5):875. doi:10.1176/APPI.AJP.159.5.874-A
10. Ghaleiha A, Shahidi KM, Afzali S, et al. Effect of terazosin on sweating in patients with major depressive disorder receiving sertraline: a randomized controlled trial. Int J Psychiatry Clin Pract. 2013;17(1):44-47. doi:10.3109/13651501.2012.687449
1. International Hyperhidrosis Society. Drugs/medications known to cause hyperhidrosis. Sweathelp.org. 2022. Accessed September 6, 2022. https://www.sweathelp.org/pdf/drugs_2009.pdf
2. Beyer C, Cappetta K, Johnson JA, et al. Meta-analysis: risk of hyperhidrosis with second-generation antidepressants. Depress Anxiety. 2017;34(12):1134-1146. doi:10.1002/da.22680
3. Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 2008;31(2):109-126. doi:10.2165/00002018-200831020-00002
4. del Boz J. Systemic treatment of hyperhidrosis. Actas Dermosifiliogr. 2015;106(4):271-277. doi:10.1016/j.ad.2014.11.012
5. Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669-680. doi:10.1016/j.jaad2018.11.066
6. Glaser DA. Oral medications. Dermatol Clin. 2014;32(4):527-532. doi:10.1016/j.det.2014.06.002
7. Garber A, Gregory RJ. Benztropine in the treatment of venlafaxine-induced sweating. J Clin Psychiatry. 1997;58(4):176-177. doi:10.4088/jcp.v58n0407e
8. Kolli V, Ramaswamy S. Improvement of antidepressant-induced sweating with as-required benztropine. Innov Clin Neurosci. 2013;10(11-12):10-11.
9. Ashton AK, Weinstein WL. Cyproheptadine for drug-induced sweating. Am J Psychiatry. 2002;159(5):875. doi:10.1176/APPI.AJP.159.5.874-A
10. Ghaleiha A, Shahidi KM, Afzali S, et al. Effect of terazosin on sweating in patients with major depressive disorder receiving sertraline: a randomized controlled trial. Int J Psychiatry Clin Pract. 2013;17(1):44-47. doi:10.3109/13651501.2012.687449
Supervising residents in an outpatient setting: 6 tips for success
The Accreditation Council for Graduate Medical Education (ACGME) requires supervision of residents “provides safe and effective care to patients; ensures each resident’s development of the skills, knowledge, and attitudes required to enter the unsupervised practice of medicine; and establishes a foundation for continued professional growth.”1 Beyond delineating supervision types (direct, indirect, or oversight), best practices for outpatient supervision are lacking, which perhaps contributes to challenges and discrepancies in experiences involving both trainees and supervisors.2 In this article, I provide 6 practical recommendations for supervisors to address and overcome these challenges.
1. Don’t skip orientation
Resist the pressure to jump directly into clinical care. Devote the first supervision session to learner orientation about expectations (eg, documentation and between-visit patient outreach), logistics (eg, electronic health record or absences), clinic workflow and processes (eg, no-shows or referrals), and team members. Provide this verbally and in writing; the former fosters additional discussion and prompts questions, while the latter serves as a useful reference.
2. Plan for the end at the beginning
Plan ahead for end-of-rotation issues (eg, transfers of care or clinician sign-out), particularly because handoffs are known patient safety risks.3 Provide a written sign-out template or example, set a deadline for the first draft, and ensure known verbal sign-out occurs to both you and any trainees coming into the rotation.
3. Facilitate self-identification of strengths, weaknesses, and goals
Individual learning plans (ILPs) are a fundamental component of adult learning theory, allowing for self-directed learning and ongoing assessment by trainee and supervisor. Complete the ILP together at the beginning of the rotation and regularly devote time to revisit and revise it. This process ensures targeted feedback, which will reduce the stress and potential surprises often associated with end-of-rotation evaluations.
4. Consider the homework you assign
Be intentional about assigned readings. Consider their frequency and length, highlight where you want learners to focus, provide self-reflection questions/prompts, and take time to discuss during supervision. If you use a structured curriculum, maintain flexibility so your trainees’ interests, topics arising in real-time clinical care, and relevant in-press articles can be included.
5. Use direct observation
Whenever possible, directly observe clinical care, particularly a patient’s intake. To reduce trainee (and patient) anxiety and preserve independence, state, “I’m the attending physician supervising Dr. (NAME), who will be your doctor. We provide feedback to trainees right up to graduation so I’m here to observe and will be quiet in the background.” While direct observation is associated with early learners and inpatient settings, it is also preferred by senior outpatient psychiatry residents4 and associated with positive educational and patient outcomes.5
6. Offer supplemental experiences
If feasible, offer additional interdisciplinary supervision (eg, social workers, psychologists, or peer support), scholarly opportunities (eg, case report collaboration or clinic talk), psychotherapy cases, or meeting with patients on your caseload (eg, patients with a rare diagnosis or unique presentation). These align with ACGME’s broad supervision requirements and offer much-appreciated individualized learning tailored to the trainee.
1. Accreditation Council for Graduate Medical Education. Common Program Requirements (Residency). Updated July 1, 2022. Accessed September 6, 2023. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2022v3.pdf
2. Newman M, Ravindranath D, Figueroa S, et al. Perceptions of supervision in an outpatient psychiatry clinic. Acad Psychiatry. 2016;40(1):153-156. doi:10.1007/s40596-014-0191-y
3. The Joint Commission. Inadequate hand-off communication. Sentinel Event Alert. Issue 58. September 12, 2017. Accessed September 11, 2023. https://www.jointcommission.org/-/media/tjc/documents/resources/patient-safety-topics/sentinel-event/sea_58_hand_off_comms_9_6_17_final_(1).pdf
4. Tan LL, Kam CJW. How psychiatry residents perceive clinical teaching effectiveness with or without direct supervision. The Asia-Pacific Scholar. 2020;5(2):14-21.
5. Galanter CA, Nikolov R, Green N, et al. Direct supervision in outpatient psychiatric graduate medical education. Acad Psychiatry. 2016;40(1):157-163. doi:10.1007/s40596-014-0247-z
The Accreditation Council for Graduate Medical Education (ACGME) requires supervision of residents “provides safe and effective care to patients; ensures each resident’s development of the skills, knowledge, and attitudes required to enter the unsupervised practice of medicine; and establishes a foundation for continued professional growth.”1 Beyond delineating supervision types (direct, indirect, or oversight), best practices for outpatient supervision are lacking, which perhaps contributes to challenges and discrepancies in experiences involving both trainees and supervisors.2 In this article, I provide 6 practical recommendations for supervisors to address and overcome these challenges.
1. Don’t skip orientation
Resist the pressure to jump directly into clinical care. Devote the first supervision session to learner orientation about expectations (eg, documentation and between-visit patient outreach), logistics (eg, electronic health record or absences), clinic workflow and processes (eg, no-shows or referrals), and team members. Provide this verbally and in writing; the former fosters additional discussion and prompts questions, while the latter serves as a useful reference.
2. Plan for the end at the beginning
Plan ahead for end-of-rotation issues (eg, transfers of care or clinician sign-out), particularly because handoffs are known patient safety risks.3 Provide a written sign-out template or example, set a deadline for the first draft, and ensure known verbal sign-out occurs to both you and any trainees coming into the rotation.
3. Facilitate self-identification of strengths, weaknesses, and goals
Individual learning plans (ILPs) are a fundamental component of adult learning theory, allowing for self-directed learning and ongoing assessment by trainee and supervisor. Complete the ILP together at the beginning of the rotation and regularly devote time to revisit and revise it. This process ensures targeted feedback, which will reduce the stress and potential surprises often associated with end-of-rotation evaluations.
4. Consider the homework you assign
Be intentional about assigned readings. Consider their frequency and length, highlight where you want learners to focus, provide self-reflection questions/prompts, and take time to discuss during supervision. If you use a structured curriculum, maintain flexibility so your trainees’ interests, topics arising in real-time clinical care, and relevant in-press articles can be included.
5. Use direct observation
Whenever possible, directly observe clinical care, particularly a patient’s intake. To reduce trainee (and patient) anxiety and preserve independence, state, “I’m the attending physician supervising Dr. (NAME), who will be your doctor. We provide feedback to trainees right up to graduation so I’m here to observe and will be quiet in the background.” While direct observation is associated with early learners and inpatient settings, it is also preferred by senior outpatient psychiatry residents4 and associated with positive educational and patient outcomes.5
6. Offer supplemental experiences
If feasible, offer additional interdisciplinary supervision (eg, social workers, psychologists, or peer support), scholarly opportunities (eg, case report collaboration or clinic talk), psychotherapy cases, or meeting with patients on your caseload (eg, patients with a rare diagnosis or unique presentation). These align with ACGME’s broad supervision requirements and offer much-appreciated individualized learning tailored to the trainee.
The Accreditation Council for Graduate Medical Education (ACGME) requires supervision of residents “provides safe and effective care to patients; ensures each resident’s development of the skills, knowledge, and attitudes required to enter the unsupervised practice of medicine; and establishes a foundation for continued professional growth.”1 Beyond delineating supervision types (direct, indirect, or oversight), best practices for outpatient supervision are lacking, which perhaps contributes to challenges and discrepancies in experiences involving both trainees and supervisors.2 In this article, I provide 6 practical recommendations for supervisors to address and overcome these challenges.
1. Don’t skip orientation
Resist the pressure to jump directly into clinical care. Devote the first supervision session to learner orientation about expectations (eg, documentation and between-visit patient outreach), logistics (eg, electronic health record or absences), clinic workflow and processes (eg, no-shows or referrals), and team members. Provide this verbally and in writing; the former fosters additional discussion and prompts questions, while the latter serves as a useful reference.
2. Plan for the end at the beginning
Plan ahead for end-of-rotation issues (eg, transfers of care or clinician sign-out), particularly because handoffs are known patient safety risks.3 Provide a written sign-out template or example, set a deadline for the first draft, and ensure known verbal sign-out occurs to both you and any trainees coming into the rotation.
3. Facilitate self-identification of strengths, weaknesses, and goals
Individual learning plans (ILPs) are a fundamental component of adult learning theory, allowing for self-directed learning and ongoing assessment by trainee and supervisor. Complete the ILP together at the beginning of the rotation and regularly devote time to revisit and revise it. This process ensures targeted feedback, which will reduce the stress and potential surprises often associated with end-of-rotation evaluations.
4. Consider the homework you assign
Be intentional about assigned readings. Consider their frequency and length, highlight where you want learners to focus, provide self-reflection questions/prompts, and take time to discuss during supervision. If you use a structured curriculum, maintain flexibility so your trainees’ interests, topics arising in real-time clinical care, and relevant in-press articles can be included.
5. Use direct observation
Whenever possible, directly observe clinical care, particularly a patient’s intake. To reduce trainee (and patient) anxiety and preserve independence, state, “I’m the attending physician supervising Dr. (NAME), who will be your doctor. We provide feedback to trainees right up to graduation so I’m here to observe and will be quiet in the background.” While direct observation is associated with early learners and inpatient settings, it is also preferred by senior outpatient psychiatry residents4 and associated with positive educational and patient outcomes.5
6. Offer supplemental experiences
If feasible, offer additional interdisciplinary supervision (eg, social workers, psychologists, or peer support), scholarly opportunities (eg, case report collaboration or clinic talk), psychotherapy cases, or meeting with patients on your caseload (eg, patients with a rare diagnosis or unique presentation). These align with ACGME’s broad supervision requirements and offer much-appreciated individualized learning tailored to the trainee.
1. Accreditation Council for Graduate Medical Education. Common Program Requirements (Residency). Updated July 1, 2022. Accessed September 6, 2023. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2022v3.pdf
2. Newman M, Ravindranath D, Figueroa S, et al. Perceptions of supervision in an outpatient psychiatry clinic. Acad Psychiatry. 2016;40(1):153-156. doi:10.1007/s40596-014-0191-y
3. The Joint Commission. Inadequate hand-off communication. Sentinel Event Alert. Issue 58. September 12, 2017. Accessed September 11, 2023. https://www.jointcommission.org/-/media/tjc/documents/resources/patient-safety-topics/sentinel-event/sea_58_hand_off_comms_9_6_17_final_(1).pdf
4. Tan LL, Kam CJW. How psychiatry residents perceive clinical teaching effectiveness with or without direct supervision. The Asia-Pacific Scholar. 2020;5(2):14-21.
5. Galanter CA, Nikolov R, Green N, et al. Direct supervision in outpatient psychiatric graduate medical education. Acad Psychiatry. 2016;40(1):157-163. doi:10.1007/s40596-014-0247-z
1. Accreditation Council for Graduate Medical Education. Common Program Requirements (Residency). Updated July 1, 2022. Accessed September 6, 2023. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2022v3.pdf
2. Newman M, Ravindranath D, Figueroa S, et al. Perceptions of supervision in an outpatient psychiatry clinic. Acad Psychiatry. 2016;40(1):153-156. doi:10.1007/s40596-014-0191-y
3. The Joint Commission. Inadequate hand-off communication. Sentinel Event Alert. Issue 58. September 12, 2017. Accessed September 11, 2023. https://www.jointcommission.org/-/media/tjc/documents/resources/patient-safety-topics/sentinel-event/sea_58_hand_off_comms_9_6_17_final_(1).pdf
4. Tan LL, Kam CJW. How psychiatry residents perceive clinical teaching effectiveness with or without direct supervision. The Asia-Pacific Scholar. 2020;5(2):14-21.
5. Galanter CA, Nikolov R, Green N, et al. Direct supervision in outpatient psychiatric graduate medical education. Acad Psychiatry. 2016;40(1):157-163. doi:10.1007/s40596-014-0247-z
Interviewing a patient experiencing psychosis
Clinicians of all experience levels, particularly trainees, may struggle when interviewing an individual experiencing psychosis. Many clinicians feel unsure what to say when a patient expresses fixed beliefs that are not amenable to change despite conflicting evidence, or worry about inadvertently affirming these beliefs. Supporting and empathizing with a person experiencing psychosis while avoiding reinforcing delusional beliefs is an important skillset for clinicians to have. While there is no single “correct” approach to interviewing individuals with psychosis, key principles include:
1. Do not begin by challenging delusions
People experiencing delusions often feel strongly about the validity of their beliefs and find evidence to support them. Directly challenging these beliefs from the beginning may alienate them. Instead, explore with neutral questioning: “Can you tell me more about X?” “What did you notice that made you believe Y?” Later, when rapport is established, it may be appropriate to explore discrepancies that provide insight into their delusions, a technique used in cognitive-behavioral therapy for psychosis.
2. Validate the emotion, not the psychosis
Many interviewers worry that talking about a patient’s delusions or voices will inadvertently reinforce them. Instead of agreeing with the content, listen for and empathize with the emotion (which is often fear): “That sounds frightening.” If the emotion is unclear, ask: “How did you feel when that happened?” When unsure what to say, sometimes a neutral “mmm” conveys listening without reinforcing the psychosis.
3. Explicitly state emotions and intentions
People with psychosis may have difficulty processing others’ emotions and facial expressions.1 We recommend using verbal cues to assist them in recognizing emotions and intentions: “It makes me sad to hear how alone you felt,” or “I’m here to help you.” The interviewer may mildly “amplify” their facial expressions so that the person experiencing psychosis can more clearly identify the expressed emotion, though not all individuals with psychosis respond well to this.
4. Reflect the patient’s own words
We recommend using the patient’s exact (typically nonclinical) words in referring to their experiences to build rapport and a shared understanding of their subjective experience.2 Avoid introducing clinical jargon, such as “delusion” or “hallucination.” For example, the interviewer might follow a patient’s explanation of their experiences by asking: “You heard voices in the walls—what did they say?” If the patient uses clinical jargon, the interviewer should clarify their meaning: “When you say ‘paranoid,’ what does that mean to you?”
5. Be intentional with gestures and positioning
People with schizophrenia-spectrum disorders may have difficulty interpreting gestures and are more likely to perceive gestures as self-referential.1 We recommend minimizing gestures or using simple, neutral-to-positive movements appropriate to cultural context. For example, in the United States, hands with palms up in front of the body generally convey openness, whereas arms crossed over the chest may convey anger. We recommend that to avoid appearing confrontational, interviewers do not position themselves directly in front of the patient, instead positioning themselves at an angle. Consider mirroring patients’ gestures or postures to convey empathy and build rapport.3
1. Chapellier V, Pavlidou A, Maderthaner L, et al. The impact of poor nonverbal social perception on functional capacity in schizophrenia. Front Psychol. 2022;13:804093. doi:10.3389/fpsyg.2022.804093
2. Olson M, Seikkula J, Ziedonis D. The key elements of dialogic practice in Open Dialogue: fidelity criteria. University of Massachusetts Medical School. Published September 2, 2014. Accessed August 16, 2023. https://www.umassmed.edu/globalassets/psychiatry/open-dialogue/keyelementsv1.109022014.pdf
3. Raffard S, Salesse RN, Bortolon C, et al. Using mimicry of body movements by a virtual agent to increase synchronization behavior and rapport in individuals with schizophrenia. Sci Rep. 2018;8(1):17356. doi:10.1038/s41598-018-35813-6
Clinicians of all experience levels, particularly trainees, may struggle when interviewing an individual experiencing psychosis. Many clinicians feel unsure what to say when a patient expresses fixed beliefs that are not amenable to change despite conflicting evidence, or worry about inadvertently affirming these beliefs. Supporting and empathizing with a person experiencing psychosis while avoiding reinforcing delusional beliefs is an important skillset for clinicians to have. While there is no single “correct” approach to interviewing individuals with psychosis, key principles include:
1. Do not begin by challenging delusions
People experiencing delusions often feel strongly about the validity of their beliefs and find evidence to support them. Directly challenging these beliefs from the beginning may alienate them. Instead, explore with neutral questioning: “Can you tell me more about X?” “What did you notice that made you believe Y?” Later, when rapport is established, it may be appropriate to explore discrepancies that provide insight into their delusions, a technique used in cognitive-behavioral therapy for psychosis.
2. Validate the emotion, not the psychosis
Many interviewers worry that talking about a patient’s delusions or voices will inadvertently reinforce them. Instead of agreeing with the content, listen for and empathize with the emotion (which is often fear): “That sounds frightening.” If the emotion is unclear, ask: “How did you feel when that happened?” When unsure what to say, sometimes a neutral “mmm” conveys listening without reinforcing the psychosis.
3. Explicitly state emotions and intentions
People with psychosis may have difficulty processing others’ emotions and facial expressions.1 We recommend using verbal cues to assist them in recognizing emotions and intentions: “It makes me sad to hear how alone you felt,” or “I’m here to help you.” The interviewer may mildly “amplify” their facial expressions so that the person experiencing psychosis can more clearly identify the expressed emotion, though not all individuals with psychosis respond well to this.
4. Reflect the patient’s own words
We recommend using the patient’s exact (typically nonclinical) words in referring to their experiences to build rapport and a shared understanding of their subjective experience.2 Avoid introducing clinical jargon, such as “delusion” or “hallucination.” For example, the interviewer might follow a patient’s explanation of their experiences by asking: “You heard voices in the walls—what did they say?” If the patient uses clinical jargon, the interviewer should clarify their meaning: “When you say ‘paranoid,’ what does that mean to you?”
5. Be intentional with gestures and positioning
People with schizophrenia-spectrum disorders may have difficulty interpreting gestures and are more likely to perceive gestures as self-referential.1 We recommend minimizing gestures or using simple, neutral-to-positive movements appropriate to cultural context. For example, in the United States, hands with palms up in front of the body generally convey openness, whereas arms crossed over the chest may convey anger. We recommend that to avoid appearing confrontational, interviewers do not position themselves directly in front of the patient, instead positioning themselves at an angle. Consider mirroring patients’ gestures or postures to convey empathy and build rapport.3
Clinicians of all experience levels, particularly trainees, may struggle when interviewing an individual experiencing psychosis. Many clinicians feel unsure what to say when a patient expresses fixed beliefs that are not amenable to change despite conflicting evidence, or worry about inadvertently affirming these beliefs. Supporting and empathizing with a person experiencing psychosis while avoiding reinforcing delusional beliefs is an important skillset for clinicians to have. While there is no single “correct” approach to interviewing individuals with psychosis, key principles include:
1. Do not begin by challenging delusions
People experiencing delusions often feel strongly about the validity of their beliefs and find evidence to support them. Directly challenging these beliefs from the beginning may alienate them. Instead, explore with neutral questioning: “Can you tell me more about X?” “What did you notice that made you believe Y?” Later, when rapport is established, it may be appropriate to explore discrepancies that provide insight into their delusions, a technique used in cognitive-behavioral therapy for psychosis.
2. Validate the emotion, not the psychosis
Many interviewers worry that talking about a patient’s delusions or voices will inadvertently reinforce them. Instead of agreeing with the content, listen for and empathize with the emotion (which is often fear): “That sounds frightening.” If the emotion is unclear, ask: “How did you feel when that happened?” When unsure what to say, sometimes a neutral “mmm” conveys listening without reinforcing the psychosis.
3. Explicitly state emotions and intentions
People with psychosis may have difficulty processing others’ emotions and facial expressions.1 We recommend using verbal cues to assist them in recognizing emotions and intentions: “It makes me sad to hear how alone you felt,” or “I’m here to help you.” The interviewer may mildly “amplify” their facial expressions so that the person experiencing psychosis can more clearly identify the expressed emotion, though not all individuals with psychosis respond well to this.
4. Reflect the patient’s own words
We recommend using the patient’s exact (typically nonclinical) words in referring to their experiences to build rapport and a shared understanding of their subjective experience.2 Avoid introducing clinical jargon, such as “delusion” or “hallucination.” For example, the interviewer might follow a patient’s explanation of their experiences by asking: “You heard voices in the walls—what did they say?” If the patient uses clinical jargon, the interviewer should clarify their meaning: “When you say ‘paranoid,’ what does that mean to you?”
5. Be intentional with gestures and positioning
People with schizophrenia-spectrum disorders may have difficulty interpreting gestures and are more likely to perceive gestures as self-referential.1 We recommend minimizing gestures or using simple, neutral-to-positive movements appropriate to cultural context. For example, in the United States, hands with palms up in front of the body generally convey openness, whereas arms crossed over the chest may convey anger. We recommend that to avoid appearing confrontational, interviewers do not position themselves directly in front of the patient, instead positioning themselves at an angle. Consider mirroring patients’ gestures or postures to convey empathy and build rapport.3
1. Chapellier V, Pavlidou A, Maderthaner L, et al. The impact of poor nonverbal social perception on functional capacity in schizophrenia. Front Psychol. 2022;13:804093. doi:10.3389/fpsyg.2022.804093
2. Olson M, Seikkula J, Ziedonis D. The key elements of dialogic practice in Open Dialogue: fidelity criteria. University of Massachusetts Medical School. Published September 2, 2014. Accessed August 16, 2023. https://www.umassmed.edu/globalassets/psychiatry/open-dialogue/keyelementsv1.109022014.pdf
3. Raffard S, Salesse RN, Bortolon C, et al. Using mimicry of body movements by a virtual agent to increase synchronization behavior and rapport in individuals with schizophrenia. Sci Rep. 2018;8(1):17356. doi:10.1038/s41598-018-35813-6
1. Chapellier V, Pavlidou A, Maderthaner L, et al. The impact of poor nonverbal social perception on functional capacity in schizophrenia. Front Psychol. 2022;13:804093. doi:10.3389/fpsyg.2022.804093
2. Olson M, Seikkula J, Ziedonis D. The key elements of dialogic practice in Open Dialogue: fidelity criteria. University of Massachusetts Medical School. Published September 2, 2014. Accessed August 16, 2023. https://www.umassmed.edu/globalassets/psychiatry/open-dialogue/keyelementsv1.109022014.pdf
3. Raffard S, Salesse RN, Bortolon C, et al. Using mimicry of body movements by a virtual agent to increase synchronization behavior and rapport in individuals with schizophrenia. Sci Rep. 2018;8(1):17356. doi:10.1038/s41598-018-35813-6
The ‘borderlinization’ of our society and the mental health crisis
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
We appreciated Dr. Nasrallah’s recent editorial1 that implicated smartphones, social media, and video game addiction, combined with the pandemic, in causing default mode network (DMN) dysfunction. The United States Surgeon General’s May 2023 report echoed these concerns and recommended limiting the use of these platforms.2 While devices are accelerants on a raging fire of mental illness, we observe a more insidious etiology that kindled the flame long before the proliferation of social media use during the pandemic. I (MZP) call this the “borderlinization” of society.
Imagine living somewhere in America that time had forgotten, where youth did not use smartphones and social media or play video games, and throughout the pandemic, people continued to congregate and socialize. These are the religious enclaves throughout New York and New Jersey that we (MZP and RLP) serve. Yet if devices were predominantly to blame for the contemporary mental health crisis, we would not expect the growing mental health problems we encounter. So, what is going on?
Over the past decade, mental health awareness has permeated all institutions of education, media, business, and government, which has increased compassion for marginalized groups. Consequently, people who may have previously silently suffered have become encouraged and supported in seeking help. That is good news. The bad news is that we have also come to pathologize, label, and attempt to treat nearly all of life’s struggles, and have been exporting mental disease around the world.3 We are losing the sense of “normal” when more than one-half of all Americans will receive a DSM diagnosis in their lifetime.4
Traits of borderline personality disorder (BPD)—such as abandonment fears, unstable relationships, identity disturbance, affective instability, emptiness, anger, mistrust, and dissociation5—that previously were seen less often are now more commonplace among our patients. These patients’ therapists have “validated” their “victimization” of “microaggressions” such that they now require “trigger warnings,” “safe spaces,” and psychiatric “diagnosis and treatment” to be able to function “normally.” These developments have also positioned parents, educators, employers, and psychiatrists, who may share “power and privilege,” to “walk on eggshells” so as not to offend newfound hypersensitivities. Interestingly, the DMN may be a major, reversible driver in BPD,6 a possible final common pathway that is further impaired by devices starting to creep into our communities and amplify the dysfunction.
Beyond treating individual patients, we must consider mandating time away from devices to nourish our DMN. During a 25-hour period each week, we (MZP and RLP) unplug from all forms of work and electronics, remember the past, consider the future, reflect on self and others, connect with nature, meditate, and eat mindfully—all of which are DMN functions. We call it Shabbat, which people have observed for thousands of years to process the week before and rejuvenate for the week ahead. Excluding smartphones from school premises has also been helpful7 and could be implemented as a nationwide commitment to the developing brains of our youth. Finally, we need to look to our profession to promote resilience over dependence, distress tolerance over avoidance, and empathic communication over “cancellation” to help heal a divisive society.
1. Nasrallah HA. Is the contemporary mental health crisis among youth due to DMN disruption? Current Psychiatry. 2023;22(6):10-11,21. doi:10.12788/cp.0372
2. U.S. Department of Health and Human Services. Surgeon general issues new advisory about effects social media use has on youth mental health. May 23, 2023. Accessed June 4, 2023. https://www.hhs.gov/about/news/2023/05/23/surgeon-general-issues-new-advisory-about-effects-social-media-use-has-youth-mental-health.html
3. Watters E. Crazy Like Us: The Globalization of the American Psyche. Free Press; 2011.
4. Centers for Disease Control and Prevention. About mental health. April 25, 2023. Accessed June 4, 2023. https://www.cdc.gov/mentalhealth/learn/index.htm
5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
6. Amiri S, Mirfazeli FS, Grafman J, et al. Alternation in functional connectivity within default mode network after psychodynamic psychotherapy in borderline personality disorder. Ann Gen Psychiatry. 2023;22(1):18. doi:10.1186/s12991-023-00449-y
7. Beland LP, Murphy R. Ill communication: technology, distraction & student performance. Labour Economics. 2016;41:61-76. doi:10.1016/j.labeco.2016.04.004
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
We appreciated Dr. Nasrallah’s recent editorial1 that implicated smartphones, social media, and video game addiction, combined with the pandemic, in causing default mode network (DMN) dysfunction. The United States Surgeon General’s May 2023 report echoed these concerns and recommended limiting the use of these platforms.2 While devices are accelerants on a raging fire of mental illness, we observe a more insidious etiology that kindled the flame long before the proliferation of social media use during the pandemic. I (MZP) call this the “borderlinization” of society.
Imagine living somewhere in America that time had forgotten, where youth did not use smartphones and social media or play video games, and throughout the pandemic, people continued to congregate and socialize. These are the religious enclaves throughout New York and New Jersey that we (MZP and RLP) serve. Yet if devices were predominantly to blame for the contemporary mental health crisis, we would not expect the growing mental health problems we encounter. So, what is going on?
Over the past decade, mental health awareness has permeated all institutions of education, media, business, and government, which has increased compassion for marginalized groups. Consequently, people who may have previously silently suffered have become encouraged and supported in seeking help. That is good news. The bad news is that we have also come to pathologize, label, and attempt to treat nearly all of life’s struggles, and have been exporting mental disease around the world.3 We are losing the sense of “normal” when more than one-half of all Americans will receive a DSM diagnosis in their lifetime.4
Traits of borderline personality disorder (BPD)—such as abandonment fears, unstable relationships, identity disturbance, affective instability, emptiness, anger, mistrust, and dissociation5—that previously were seen less often are now more commonplace among our patients. These patients’ therapists have “validated” their “victimization” of “microaggressions” such that they now require “trigger warnings,” “safe spaces,” and psychiatric “diagnosis and treatment” to be able to function “normally.” These developments have also positioned parents, educators, employers, and psychiatrists, who may share “power and privilege,” to “walk on eggshells” so as not to offend newfound hypersensitivities. Interestingly, the DMN may be a major, reversible driver in BPD,6 a possible final common pathway that is further impaired by devices starting to creep into our communities and amplify the dysfunction.
Beyond treating individual patients, we must consider mandating time away from devices to nourish our DMN. During a 25-hour period each week, we (MZP and RLP) unplug from all forms of work and electronics, remember the past, consider the future, reflect on self and others, connect with nature, meditate, and eat mindfully—all of which are DMN functions. We call it Shabbat, which people have observed for thousands of years to process the week before and rejuvenate for the week ahead. Excluding smartphones from school premises has also been helpful7 and could be implemented as a nationwide commitment to the developing brains of our youth. Finally, we need to look to our profession to promote resilience over dependence, distress tolerance over avoidance, and empathic communication over “cancellation” to help heal a divisive society.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
We appreciated Dr. Nasrallah’s recent editorial1 that implicated smartphones, social media, and video game addiction, combined with the pandemic, in causing default mode network (DMN) dysfunction. The United States Surgeon General’s May 2023 report echoed these concerns and recommended limiting the use of these platforms.2 While devices are accelerants on a raging fire of mental illness, we observe a more insidious etiology that kindled the flame long before the proliferation of social media use during the pandemic. I (MZP) call this the “borderlinization” of society.
Imagine living somewhere in America that time had forgotten, where youth did not use smartphones and social media or play video games, and throughout the pandemic, people continued to congregate and socialize. These are the religious enclaves throughout New York and New Jersey that we (MZP and RLP) serve. Yet if devices were predominantly to blame for the contemporary mental health crisis, we would not expect the growing mental health problems we encounter. So, what is going on?
Over the past decade, mental health awareness has permeated all institutions of education, media, business, and government, which has increased compassion for marginalized groups. Consequently, people who may have previously silently suffered have become encouraged and supported in seeking help. That is good news. The bad news is that we have also come to pathologize, label, and attempt to treat nearly all of life’s struggles, and have been exporting mental disease around the world.3 We are losing the sense of “normal” when more than one-half of all Americans will receive a DSM diagnosis in their lifetime.4
Traits of borderline personality disorder (BPD)—such as abandonment fears, unstable relationships, identity disturbance, affective instability, emptiness, anger, mistrust, and dissociation5—that previously were seen less often are now more commonplace among our patients. These patients’ therapists have “validated” their “victimization” of “microaggressions” such that they now require “trigger warnings,” “safe spaces,” and psychiatric “diagnosis and treatment” to be able to function “normally.” These developments have also positioned parents, educators, employers, and psychiatrists, who may share “power and privilege,” to “walk on eggshells” so as not to offend newfound hypersensitivities. Interestingly, the DMN may be a major, reversible driver in BPD,6 a possible final common pathway that is further impaired by devices starting to creep into our communities and amplify the dysfunction.
Beyond treating individual patients, we must consider mandating time away from devices to nourish our DMN. During a 25-hour period each week, we (MZP and RLP) unplug from all forms of work and electronics, remember the past, consider the future, reflect on self and others, connect with nature, meditate, and eat mindfully—all of which are DMN functions. We call it Shabbat, which people have observed for thousands of years to process the week before and rejuvenate for the week ahead. Excluding smartphones from school premises has also been helpful7 and could be implemented as a nationwide commitment to the developing brains of our youth. Finally, we need to look to our profession to promote resilience over dependence, distress tolerance over avoidance, and empathic communication over “cancellation” to help heal a divisive society.
1. Nasrallah HA. Is the contemporary mental health crisis among youth due to DMN disruption? Current Psychiatry. 2023;22(6):10-11,21. doi:10.12788/cp.0372
2. U.S. Department of Health and Human Services. Surgeon general issues new advisory about effects social media use has on youth mental health. May 23, 2023. Accessed June 4, 2023. https://www.hhs.gov/about/news/2023/05/23/surgeon-general-issues-new-advisory-about-effects-social-media-use-has-youth-mental-health.html
3. Watters E. Crazy Like Us: The Globalization of the American Psyche. Free Press; 2011.
4. Centers for Disease Control and Prevention. About mental health. April 25, 2023. Accessed June 4, 2023. https://www.cdc.gov/mentalhealth/learn/index.htm
5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
6. Amiri S, Mirfazeli FS, Grafman J, et al. Alternation in functional connectivity within default mode network after psychodynamic psychotherapy in borderline personality disorder. Ann Gen Psychiatry. 2023;22(1):18. doi:10.1186/s12991-023-00449-y
7. Beland LP, Murphy R. Ill communication: technology, distraction & student performance. Labour Economics. 2016;41:61-76. doi:10.1016/j.labeco.2016.04.004
1. Nasrallah HA. Is the contemporary mental health crisis among youth due to DMN disruption? Current Psychiatry. 2023;22(6):10-11,21. doi:10.12788/cp.0372
2. U.S. Department of Health and Human Services. Surgeon general issues new advisory about effects social media use has on youth mental health. May 23, 2023. Accessed June 4, 2023. https://www.hhs.gov/about/news/2023/05/23/surgeon-general-issues-new-advisory-about-effects-social-media-use-has-youth-mental-health.html
3. Watters E. Crazy Like Us: The Globalization of the American Psyche. Free Press; 2011.
4. Centers for Disease Control and Prevention. About mental health. April 25, 2023. Accessed June 4, 2023. https://www.cdc.gov/mentalhealth/learn/index.htm
5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
6. Amiri S, Mirfazeli FS, Grafman J, et al. Alternation in functional connectivity within default mode network after psychodynamic psychotherapy in borderline personality disorder. Ann Gen Psychiatry. 2023;22(1):18. doi:10.1186/s12991-023-00449-y
7. Beland LP, Murphy R. Ill communication: technology, distraction & student performance. Labour Economics. 2016;41:61-76. doi:10.1016/j.labeco.2016.04.004