User login
This tool can predict recurrence in rectal cancer watchful waiting
TOPLINE:
for both local regrowth and distant metastasis during watchful waiting.
METHODOLOGY:
- Currently, oncologists do not have a biomarker that can help select patients with rectal cancer for watchful waiting after neoadjuvant chemoradiotherapy as well as help monitor them over time. And about 25% of patients on watchful waiting will eventually relapse.
- The Immunoscore biopsy quantifies the degree of immune infiltration on pretreatment tumor biopsies, looking at the density of CD3- and CD8-positive T cells at baseline, given that greater infiltration has been associated with prolonged treatment response.
- To determine whether the Immunoscore can help select patients for watchful waiting, investigators correlated Immunoscore with time to recurrence in 249 patients with stage I-III rectal cancer who were undergoing watchful waiting after complete clinical responses to neoadjuvant chemoradiotherapy.
- CD3- and CD8-positive T-cell densities were converted into percentiles and then translated into scores of Immunoscore biopsy: low (0%-25%), intermediate (> 25%-70%), and high (> 70%-100%).
TAKEAWAY:
- The Immunoscore biopsy significantly improved predictions of recurrence: 5-year recurrence-free survival was 91.3% among patients with high scores, 62.5% among those with intermediate scores, and 53.1% among those with low scores.
- The Immunoscore was significantly associated with disease-free survival (log-rank P = .0002) and predicted both local regrowth and distant metastasis.
- On multivariate analysis, the Immunoscore’s predictive ability was independent of age, sex, tumor location, cT stage, and cN stage, and was the strongest predictor of time to recurrence (hazard ratio, high vs. low, 6.93; P = .0017).
IN PRACTICE:
This validation study confirms that the Immunoscore biopsy “is an independent parameter predicting time to recurrence” and can help physicians and patients decide whether to opt for watchful waiting, the study authors wrote.
SOURCE:
The study, led by Carine El Sissy of the Laboratory of Integrative Cancer Immunology, Paris, was published Oct. 3 in the Journal of Clinical Oncology.
LIMITATIONS:
Mismatch repair gene expression status was not assessed.
DISCLOSURES:
The work was supported by the L’Institut National de la Santé et de la Recherche Médicale and others. Investigators disclosed patents related to the work and ties to many companies, including Amgen, AstraZeneca, and Merck. One investigator is an employee of Novo Nordisk, and another is employed by Veracyte.
A version of this article first appeared on Medscape.com.
TOPLINE:
for both local regrowth and distant metastasis during watchful waiting.
METHODOLOGY:
- Currently, oncologists do not have a biomarker that can help select patients with rectal cancer for watchful waiting after neoadjuvant chemoradiotherapy as well as help monitor them over time. And about 25% of patients on watchful waiting will eventually relapse.
- The Immunoscore biopsy quantifies the degree of immune infiltration on pretreatment tumor biopsies, looking at the density of CD3- and CD8-positive T cells at baseline, given that greater infiltration has been associated with prolonged treatment response.
- To determine whether the Immunoscore can help select patients for watchful waiting, investigators correlated Immunoscore with time to recurrence in 249 patients with stage I-III rectal cancer who were undergoing watchful waiting after complete clinical responses to neoadjuvant chemoradiotherapy.
- CD3- and CD8-positive T-cell densities were converted into percentiles and then translated into scores of Immunoscore biopsy: low (0%-25%), intermediate (> 25%-70%), and high (> 70%-100%).
TAKEAWAY:
- The Immunoscore biopsy significantly improved predictions of recurrence: 5-year recurrence-free survival was 91.3% among patients with high scores, 62.5% among those with intermediate scores, and 53.1% among those with low scores.
- The Immunoscore was significantly associated with disease-free survival (log-rank P = .0002) and predicted both local regrowth and distant metastasis.
- On multivariate analysis, the Immunoscore’s predictive ability was independent of age, sex, tumor location, cT stage, and cN stage, and was the strongest predictor of time to recurrence (hazard ratio, high vs. low, 6.93; P = .0017).
IN PRACTICE:
This validation study confirms that the Immunoscore biopsy “is an independent parameter predicting time to recurrence” and can help physicians and patients decide whether to opt for watchful waiting, the study authors wrote.
SOURCE:
The study, led by Carine El Sissy of the Laboratory of Integrative Cancer Immunology, Paris, was published Oct. 3 in the Journal of Clinical Oncology.
LIMITATIONS:
Mismatch repair gene expression status was not assessed.
DISCLOSURES:
The work was supported by the L’Institut National de la Santé et de la Recherche Médicale and others. Investigators disclosed patents related to the work and ties to many companies, including Amgen, AstraZeneca, and Merck. One investigator is an employee of Novo Nordisk, and another is employed by Veracyte.
A version of this article first appeared on Medscape.com.
TOPLINE:
for both local regrowth and distant metastasis during watchful waiting.
METHODOLOGY:
- Currently, oncologists do not have a biomarker that can help select patients with rectal cancer for watchful waiting after neoadjuvant chemoradiotherapy as well as help monitor them over time. And about 25% of patients on watchful waiting will eventually relapse.
- The Immunoscore biopsy quantifies the degree of immune infiltration on pretreatment tumor biopsies, looking at the density of CD3- and CD8-positive T cells at baseline, given that greater infiltration has been associated with prolonged treatment response.
- To determine whether the Immunoscore can help select patients for watchful waiting, investigators correlated Immunoscore with time to recurrence in 249 patients with stage I-III rectal cancer who were undergoing watchful waiting after complete clinical responses to neoadjuvant chemoradiotherapy.
- CD3- and CD8-positive T-cell densities were converted into percentiles and then translated into scores of Immunoscore biopsy: low (0%-25%), intermediate (> 25%-70%), and high (> 70%-100%).
TAKEAWAY:
- The Immunoscore biopsy significantly improved predictions of recurrence: 5-year recurrence-free survival was 91.3% among patients with high scores, 62.5% among those with intermediate scores, and 53.1% among those with low scores.
- The Immunoscore was significantly associated with disease-free survival (log-rank P = .0002) and predicted both local regrowth and distant metastasis.
- On multivariate analysis, the Immunoscore’s predictive ability was independent of age, sex, tumor location, cT stage, and cN stage, and was the strongest predictor of time to recurrence (hazard ratio, high vs. low, 6.93; P = .0017).
IN PRACTICE:
This validation study confirms that the Immunoscore biopsy “is an independent parameter predicting time to recurrence” and can help physicians and patients decide whether to opt for watchful waiting, the study authors wrote.
SOURCE:
The study, led by Carine El Sissy of the Laboratory of Integrative Cancer Immunology, Paris, was published Oct. 3 in the Journal of Clinical Oncology.
LIMITATIONS:
Mismatch repair gene expression status was not assessed.
DISCLOSURES:
The work was supported by the L’Institut National de la Santé et de la Recherche Médicale and others. Investigators disclosed patents related to the work and ties to many companies, including Amgen, AstraZeneca, and Merck. One investigator is an employee of Novo Nordisk, and another is employed by Veracyte.
A version of this article first appeared on Medscape.com.
ACS expands lung cancer screening eligibility
The American Cancer Society has updated its screening guidelines for lung cancer, the leading cause of cancer-specific deaths in the United States and the largest driver of potential years of life lost from cancer.
The 2023 screening guidance, aimed principally at reducing lung cancer mortality in asymptomatic but high-risk, tobacco-exposed individuals, expands the age eligibility and lowers both the former smoking history and the years since quitting threshold for screening with low-dose CT (LDCT).
It is based on the most recent evidence on the efficacy and effectiveness of screening and lung cancer risk in persons who formerly smoked, wrote the ACS’s Guideline Development Group led by Robert A. Smith, PhD, senior vice president of early cancer detection science. The new guidelines, which replace the 2013 statement, appear in CA: A Cancer Journal for Physicians.
The primary evidence source for the update was a systematic review of LDCT lung cancer screening conducted for the U.S. Preventive Services Task Force and published in 2021.
The new guideline continues a trend of expanding eligibility for lung cancer screening, which has had low uptake, to prevent more deaths. “Recent studies have shown that extending the age for persons who smoked and formerly smoked, eliminating the ‘years since quitting’ requirement, and lowering the pack-per-year recommendation could make a real difference in saving lives,” Dr. Smith said. “The relative risk of developing lung cancer in people who have smoked most of their life compared to people who never smoked is very high – about 70 times the risk.” Although lung cancer is the third most common malignancy in the United States, it accounts for more deaths than colorectal, breast, prostate, and cervical cancers combined.
The recommendation for annual LDCT for at-risk persons remains unchanged from 2013.
Among the 2023 eligibility changes:
- Age: Expanded to 50-80 years from 55-74 years.
- Smoking status: Changed to current or previous smoker from current smoker or smoker who quit within past 15 years (number of years since quitting no longer a criterion to start or stop screening). Dr. Smith noted that both the 2013 guidelines and other groups’ updated recommendations retained the eligibility cutoff of 15 years since smoking cessation. “But had their risk declined to a level that just did not justify continuing screening?” he asked. “There wasn’t an answer to that question, so we needed to look carefully at the absolute risk of lung cancer in persons who formerly smoked compared with people who currently smoked and people who never smoked.”
- Smoking history: Reduced to 20 or more pack-years (average of 20 cigarettes a day) versus 30 or more pack-years.
- Exclusions: Expanded to health conditions that may increase harm or hinder further evaluation, surgery, or treatment; comorbidities limiting life expectancy to fewer than 5 years; unwillingness to accept treatment for screen‐detected cancer, which was changed from 2013’s life‐limiting comorbid conditions, metallic implants or devices in the chest or back, home oxygen supplementation.
In addition, decision-making should be a shared process with a health professional providing the patient with information on the benefits, limitations, and harms of LDCT screening, as well as prescreening advice on smoking cessation and the offer of assistive counseling and pharmocotherapy.
“Overall, lung cancer screening remains one of the least used early cancer detection modalities in clinical practice. The new guidance opens up lung cancer screening to all former smokers regardless of time of cessation,” said internist William E. Golden, MD, MACP, a professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock. “This may promote greater uptake in concert with greater availability of low-radiation CT scanning.”
While agreeing the expanded criteria will enfranchise nearly 5 million current and former U.S. smokers for screening and may reduce deaths, internist Aarati D. Didwania, MD, MMSCI, MACP, a professor of medicine and medical education at Northwestern University, Chicago, warned that increasing actual uptake may be an uphill battle. “The practical part of the equation is seeing that the scans get done. There is often a lag between a recommendation of a yearly test and getting insurance coverage for it, and many disadvantaged people face barriers.” Then there’s the knowledge gap. “Patients and doctors have to know what the new guidelines are and who has access,” she said.
Reaching the target population in rural areas is particularly challenging with the greater distances to imaging centers. Another barrier is that most electronic health records do not identify eligible patients based on smoking and pack‐year history.
In Dr. Didwania’s view, professional medical societies have an important role to play in educating their members, and through them, patients. “Disseminating information about the new recommendations is the first step and would be incredibly helpful.”
A brief history of lung cancer screening
1950s: By mid-20th century, the causal association between tobacco exposure and lung cancer became clear and by the late 1950s attempts were made to develop a lung cancer screening strategy for high‐risk individuals, commonly with the combination of sputum cytology and chest x-ray.
1970s: The ACS recommended annual testing for current or former smokers with chest x-ray (and sometimes sputum cytology).
1980: The ACS withdrew the above recommendation for regular radiographic screening after randomized controlled trials failed to yield convincing evidence that such screening saved lives.
2013: After the National Lung Screening Trial found three annual LDCT screenings were associated with a 20% relative mortality reduction, compared with annual chest x-ray, the ACS issued a recommendation for annual screening with LDCT: in persons 55-74 years with a pack‐year history of 30 or more who currently smoke or formerly smoked but had not exceeded 15 years since quitting and had no life-limiting morbidity.
Future mortality
Although tobacco controls are expected to reduce age‐adjusted lung cancer mortality in the United States by 79% from 2015 to 2065, 4.4 million lung cancer deaths are projected to occur in this period, the authors stated. “A large fraction of these deaths can be prevented if we embrace the urgent challenge to improve our ability to identify the population at risk and apply our knowledge to achieve high rates of participation in regular [lung cancer screening].”
The study was funded by the American Cancer Society Guideline Development Group and the National Comprehensive Cancer Network. The authors disclosed no relevant competing interests. Dr. Golden and Dr. Didwania had no relevant conflicts of interest to declare with regard to their comments.
The American Cancer Society has updated its screening guidelines for lung cancer, the leading cause of cancer-specific deaths in the United States and the largest driver of potential years of life lost from cancer.
The 2023 screening guidance, aimed principally at reducing lung cancer mortality in asymptomatic but high-risk, tobacco-exposed individuals, expands the age eligibility and lowers both the former smoking history and the years since quitting threshold for screening with low-dose CT (LDCT).
It is based on the most recent evidence on the efficacy and effectiveness of screening and lung cancer risk in persons who formerly smoked, wrote the ACS’s Guideline Development Group led by Robert A. Smith, PhD, senior vice president of early cancer detection science. The new guidelines, which replace the 2013 statement, appear in CA: A Cancer Journal for Physicians.
The primary evidence source for the update was a systematic review of LDCT lung cancer screening conducted for the U.S. Preventive Services Task Force and published in 2021.
The new guideline continues a trend of expanding eligibility for lung cancer screening, which has had low uptake, to prevent more deaths. “Recent studies have shown that extending the age for persons who smoked and formerly smoked, eliminating the ‘years since quitting’ requirement, and lowering the pack-per-year recommendation could make a real difference in saving lives,” Dr. Smith said. “The relative risk of developing lung cancer in people who have smoked most of their life compared to people who never smoked is very high – about 70 times the risk.” Although lung cancer is the third most common malignancy in the United States, it accounts for more deaths than colorectal, breast, prostate, and cervical cancers combined.
The recommendation for annual LDCT for at-risk persons remains unchanged from 2013.
Among the 2023 eligibility changes:
- Age: Expanded to 50-80 years from 55-74 years.
- Smoking status: Changed to current or previous smoker from current smoker or smoker who quit within past 15 years (number of years since quitting no longer a criterion to start or stop screening). Dr. Smith noted that both the 2013 guidelines and other groups’ updated recommendations retained the eligibility cutoff of 15 years since smoking cessation. “But had their risk declined to a level that just did not justify continuing screening?” he asked. “There wasn’t an answer to that question, so we needed to look carefully at the absolute risk of lung cancer in persons who formerly smoked compared with people who currently smoked and people who never smoked.”
- Smoking history: Reduced to 20 or more pack-years (average of 20 cigarettes a day) versus 30 or more pack-years.
- Exclusions: Expanded to health conditions that may increase harm or hinder further evaluation, surgery, or treatment; comorbidities limiting life expectancy to fewer than 5 years; unwillingness to accept treatment for screen‐detected cancer, which was changed from 2013’s life‐limiting comorbid conditions, metallic implants or devices in the chest or back, home oxygen supplementation.
In addition, decision-making should be a shared process with a health professional providing the patient with information on the benefits, limitations, and harms of LDCT screening, as well as prescreening advice on smoking cessation and the offer of assistive counseling and pharmocotherapy.
“Overall, lung cancer screening remains one of the least used early cancer detection modalities in clinical practice. The new guidance opens up lung cancer screening to all former smokers regardless of time of cessation,” said internist William E. Golden, MD, MACP, a professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock. “This may promote greater uptake in concert with greater availability of low-radiation CT scanning.”
While agreeing the expanded criteria will enfranchise nearly 5 million current and former U.S. smokers for screening and may reduce deaths, internist Aarati D. Didwania, MD, MMSCI, MACP, a professor of medicine and medical education at Northwestern University, Chicago, warned that increasing actual uptake may be an uphill battle. “The practical part of the equation is seeing that the scans get done. There is often a lag between a recommendation of a yearly test and getting insurance coverage for it, and many disadvantaged people face barriers.” Then there’s the knowledge gap. “Patients and doctors have to know what the new guidelines are and who has access,” she said.
Reaching the target population in rural areas is particularly challenging with the greater distances to imaging centers. Another barrier is that most electronic health records do not identify eligible patients based on smoking and pack‐year history.
In Dr. Didwania’s view, professional medical societies have an important role to play in educating their members, and through them, patients. “Disseminating information about the new recommendations is the first step and would be incredibly helpful.”
A brief history of lung cancer screening
1950s: By mid-20th century, the causal association between tobacco exposure and lung cancer became clear and by the late 1950s attempts were made to develop a lung cancer screening strategy for high‐risk individuals, commonly with the combination of sputum cytology and chest x-ray.
1970s: The ACS recommended annual testing for current or former smokers with chest x-ray (and sometimes sputum cytology).
1980: The ACS withdrew the above recommendation for regular radiographic screening after randomized controlled trials failed to yield convincing evidence that such screening saved lives.
2013: After the National Lung Screening Trial found three annual LDCT screenings were associated with a 20% relative mortality reduction, compared with annual chest x-ray, the ACS issued a recommendation for annual screening with LDCT: in persons 55-74 years with a pack‐year history of 30 or more who currently smoke or formerly smoked but had not exceeded 15 years since quitting and had no life-limiting morbidity.
Future mortality
Although tobacco controls are expected to reduce age‐adjusted lung cancer mortality in the United States by 79% from 2015 to 2065, 4.4 million lung cancer deaths are projected to occur in this period, the authors stated. “A large fraction of these deaths can be prevented if we embrace the urgent challenge to improve our ability to identify the population at risk and apply our knowledge to achieve high rates of participation in regular [lung cancer screening].”
The study was funded by the American Cancer Society Guideline Development Group and the National Comprehensive Cancer Network. The authors disclosed no relevant competing interests. Dr. Golden and Dr. Didwania had no relevant conflicts of interest to declare with regard to their comments.
The American Cancer Society has updated its screening guidelines for lung cancer, the leading cause of cancer-specific deaths in the United States and the largest driver of potential years of life lost from cancer.
The 2023 screening guidance, aimed principally at reducing lung cancer mortality in asymptomatic but high-risk, tobacco-exposed individuals, expands the age eligibility and lowers both the former smoking history and the years since quitting threshold for screening with low-dose CT (LDCT).
It is based on the most recent evidence on the efficacy and effectiveness of screening and lung cancer risk in persons who formerly smoked, wrote the ACS’s Guideline Development Group led by Robert A. Smith, PhD, senior vice president of early cancer detection science. The new guidelines, which replace the 2013 statement, appear in CA: A Cancer Journal for Physicians.
The primary evidence source for the update was a systematic review of LDCT lung cancer screening conducted for the U.S. Preventive Services Task Force and published in 2021.
The new guideline continues a trend of expanding eligibility for lung cancer screening, which has had low uptake, to prevent more deaths. “Recent studies have shown that extending the age for persons who smoked and formerly smoked, eliminating the ‘years since quitting’ requirement, and lowering the pack-per-year recommendation could make a real difference in saving lives,” Dr. Smith said. “The relative risk of developing lung cancer in people who have smoked most of their life compared to people who never smoked is very high – about 70 times the risk.” Although lung cancer is the third most common malignancy in the United States, it accounts for more deaths than colorectal, breast, prostate, and cervical cancers combined.
The recommendation for annual LDCT for at-risk persons remains unchanged from 2013.
Among the 2023 eligibility changes:
- Age: Expanded to 50-80 years from 55-74 years.
- Smoking status: Changed to current or previous smoker from current smoker or smoker who quit within past 15 years (number of years since quitting no longer a criterion to start or stop screening). Dr. Smith noted that both the 2013 guidelines and other groups’ updated recommendations retained the eligibility cutoff of 15 years since smoking cessation. “But had their risk declined to a level that just did not justify continuing screening?” he asked. “There wasn’t an answer to that question, so we needed to look carefully at the absolute risk of lung cancer in persons who formerly smoked compared with people who currently smoked and people who never smoked.”
- Smoking history: Reduced to 20 or more pack-years (average of 20 cigarettes a day) versus 30 or more pack-years.
- Exclusions: Expanded to health conditions that may increase harm or hinder further evaluation, surgery, or treatment; comorbidities limiting life expectancy to fewer than 5 years; unwillingness to accept treatment for screen‐detected cancer, which was changed from 2013’s life‐limiting comorbid conditions, metallic implants or devices in the chest or back, home oxygen supplementation.
In addition, decision-making should be a shared process with a health professional providing the patient with information on the benefits, limitations, and harms of LDCT screening, as well as prescreening advice on smoking cessation and the offer of assistive counseling and pharmocotherapy.
“Overall, lung cancer screening remains one of the least used early cancer detection modalities in clinical practice. The new guidance opens up lung cancer screening to all former smokers regardless of time of cessation,” said internist William E. Golden, MD, MACP, a professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock. “This may promote greater uptake in concert with greater availability of low-radiation CT scanning.”
While agreeing the expanded criteria will enfranchise nearly 5 million current and former U.S. smokers for screening and may reduce deaths, internist Aarati D. Didwania, MD, MMSCI, MACP, a professor of medicine and medical education at Northwestern University, Chicago, warned that increasing actual uptake may be an uphill battle. “The practical part of the equation is seeing that the scans get done. There is often a lag between a recommendation of a yearly test and getting insurance coverage for it, and many disadvantaged people face barriers.” Then there’s the knowledge gap. “Patients and doctors have to know what the new guidelines are and who has access,” she said.
Reaching the target population in rural areas is particularly challenging with the greater distances to imaging centers. Another barrier is that most electronic health records do not identify eligible patients based on smoking and pack‐year history.
In Dr. Didwania’s view, professional medical societies have an important role to play in educating their members, and through them, patients. “Disseminating information about the new recommendations is the first step and would be incredibly helpful.”
A brief history of lung cancer screening
1950s: By mid-20th century, the causal association between tobacco exposure and lung cancer became clear and by the late 1950s attempts were made to develop a lung cancer screening strategy for high‐risk individuals, commonly with the combination of sputum cytology and chest x-ray.
1970s: The ACS recommended annual testing for current or former smokers with chest x-ray (and sometimes sputum cytology).
1980: The ACS withdrew the above recommendation for regular radiographic screening after randomized controlled trials failed to yield convincing evidence that such screening saved lives.
2013: After the National Lung Screening Trial found three annual LDCT screenings were associated with a 20% relative mortality reduction, compared with annual chest x-ray, the ACS issued a recommendation for annual screening with LDCT: in persons 55-74 years with a pack‐year history of 30 or more who currently smoke or formerly smoked but had not exceeded 15 years since quitting and had no life-limiting morbidity.
Future mortality
Although tobacco controls are expected to reduce age‐adjusted lung cancer mortality in the United States by 79% from 2015 to 2065, 4.4 million lung cancer deaths are projected to occur in this period, the authors stated. “A large fraction of these deaths can be prevented if we embrace the urgent challenge to improve our ability to identify the population at risk and apply our knowledge to achieve high rates of participation in regular [lung cancer screening].”
The study was funded by the American Cancer Society Guideline Development Group and the National Comprehensive Cancer Network. The authors disclosed no relevant competing interests. Dr. Golden and Dr. Didwania had no relevant conflicts of interest to declare with regard to their comments.
FROM CA: A CANCER JOURNAL FOR PHYSICIANS
November 2023 - ICYMI
Gastroenterology
July
Newberry C et al. Enhancing Nutrition and Obesity Education in GI Fellowship Through Universal Curriculum Development. Gastroenterology. 2023 Jul;165(1):16-19. doi: 10.1053/j.gastro.2023.04.004. Epub 2023 Apr 13. PMID: 37061170.
Han H et al. Macrophage-derived Osteopontin (SPP1) Protects From Nonalcoholic Steatohepatitis. Gastroenterology. 2023 Jul;165(1):201-17. doi: 10.1053/j.gastro.2023.03.228. Epub 2023 Apr 5. PMID: 37028770.
Deepak P et al. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Gastroenterology. 2023 Jul;165(1):11-15. doi: 10.1053/j.gastro.2023.05.017. PMID: 37349061.
August
Guo L et al. Molecular Profiling Provides Clinical Insights Into Targeted and Immunotherapies as Well as Colorectal Cancer Prognosis. Gastroenterology. 2023 Aug;165(2):414-28.e7. doi: 10.1053/j.gastro.2023.04.029. Epub 2023 May 3. PMID: 37146911.
Huang DQ et al. Fibrosis Progression Rate in Biopsy-Proven Nonalcoholic Fatty Liver Disease Among People With Diabetes Versus People Without Diabetes: A Multicenter Study. Gastroenterology. 2023 Aug;165(2):463-72.e5. doi: 10.1053/j.gastro.2023.04.025. Epub 2023 Apr 29. PMID: 37127100.
Teoh AYB et al. EUS-Guided Choledocho-duodenostomy Using Lumen Apposing Stent Versus ERCP With Covered Metallic Stents in Patients With Unresectable Malignant Distal Biliary Obstruction: A Multicenter Randomized Controlled Trial (DRA-MBO Trial). Gastroenterology. 2023 Aug;165(2):473-82.e2. doi: 10.1053/j.gastro.2023.04.016. Epub 2023 Apr 28. PMID: 37121331.
September
Mehta RS et al. Association of Proton Pump Inhibitor Use With Incident Dementia and Cognitive Decline in Older Adults: A Prospective Cohort Study. Gastroenterology. 2023 Sep;165(3):564-72.e1. doi: 10.1053/j.gastro.2023.05.052. Epub 2023 Jun 12. PMID: 37315867; PMCID: PMC10527011.
Ballou S et al. Prevalence and Associated Factors of Bloating: Results From the Rome Foundation Global Epidemiology Study. Gastroenterology. 2023 Sep;165(3):647-55.e4. doi: 10.1053/j.gastro.2023.05.049. Epub 2023 Jun 13. PMID: 37315866; PMCID: PMC10527500.
CGH
July
Chang JW et al. Development of a Practical Guide to Implement and Monitor Diet Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023 Jul;21(7):1690-8. doi: 10.1016/j.cgh.2023.03.006. Epub 2023 Mar 16. PMID: 36933603; PMCID: PMC10293042.
Siboni S et al. Improving the Diagnostic Yield of High-Resolution Esophageal Manometry for GERD: The “Straight Leg-Raise” International Study. Clin Gastroenterol Hepatol. 2023 Jul;21(7):1761-70.e1. doi: 10.1016/j.cgh.2022.10.008. Epub 2022 Oct 19. PMID: 36270615.
August
Wechsler EV et al. Up-Front Endoscopy Maximizes Cost-Effectiveness and Cost-Satisfaction in Uninvestigated Dyspepsia. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2378-88.e28. doi: 10.1016/j.cgh.2023.01.003. Epub 2023 Jan 13. PMID: 36646234; PMCID: PMC10542651.
Frederiks CN et al. Clinical Relevance of Random Biopsies From the Esophagogastric Junction After Complete Eradication of Barrett’s Esophagus is Low. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2260-9.e9. doi: 10.1016/j.cgh.2022.11.012. Epub 2022 Nov 22. PMID: 36423874.
Rustgi SD et al. Management of Gastric Intestinal Metaplasia. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2178-82. doi: 10.1016/j.cgh.2023.03.010. Epub 2023 Apr 19. PMID: 37086748; PMCID: PMC10526696.
September
Baroud S et al. A Protocolized Management of Walled-Off Necrosis (WON) Reduces Time to WON Resolution and Improves Outcomes. Clin Gastroenterol Hepatol. 2023 Sep;21(10):2543-50.e1. doi: 10.1016/j.cgh.2023.04.029. Epub 2023 May 8. PMID: 37164115.
Arnim UV et al. Monitoring Patients With Eosinophilic Esophagitis in Routine Clinical Practice - International Expert Recommendations. Clin Gastroenterol Hepatol. 2023 Sep;21(10):2526-33. doi: 10.1016/j.cgh.2022.12.018. Epub 2022 Dec 24. PMID: 36572109.
TIGE
Kaila V et al. Does the Absence of Contrast Passage Into the Duodenum During Intraoperative Cholangiogram Truly Predict Choledocholithiasis? Techniques and Innovations in Gastrointestinal Endoscopy. 2023. https://doi.org/10.1016/j.tige.2023.05.002.
O’Keefe SJD et al. Early Enteral Feeding in Severe Acute Pancreatitis: A Randomized Clinical Trial Between Gastric vs Distal Jejunal Feeding. Techniques and Innovations in Gastrointestinal Endoscopy. 2023. https://doi.org/10.1016/j.tige.2023.06.002.
Gastro Hep Advances
Mukherjee S et al. Assessing ChatGPT’s ability to reply to queries regarding colon cancer screening based on Multi-Society Guidelines. Gastro Hep Advances. 2023. https://doi.org/10.1016/j.gastha.2023.07.008.
Lopes EW et al. Lochhead P. Improving the Consent Process with an Informed Consent Video Prior to Outpatient Colonoscopy. Gastro Hep Advances. 2023. https://doi.org/10.1016/j.gastha.2023.07.016.
Gastroenterology
July
Newberry C et al. Enhancing Nutrition and Obesity Education in GI Fellowship Through Universal Curriculum Development. Gastroenterology. 2023 Jul;165(1):16-19. doi: 10.1053/j.gastro.2023.04.004. Epub 2023 Apr 13. PMID: 37061170.
Han H et al. Macrophage-derived Osteopontin (SPP1) Protects From Nonalcoholic Steatohepatitis. Gastroenterology. 2023 Jul;165(1):201-17. doi: 10.1053/j.gastro.2023.03.228. Epub 2023 Apr 5. PMID: 37028770.
Deepak P et al. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Gastroenterology. 2023 Jul;165(1):11-15. doi: 10.1053/j.gastro.2023.05.017. PMID: 37349061.
August
Guo L et al. Molecular Profiling Provides Clinical Insights Into Targeted and Immunotherapies as Well as Colorectal Cancer Prognosis. Gastroenterology. 2023 Aug;165(2):414-28.e7. doi: 10.1053/j.gastro.2023.04.029. Epub 2023 May 3. PMID: 37146911.
Huang DQ et al. Fibrosis Progression Rate in Biopsy-Proven Nonalcoholic Fatty Liver Disease Among People With Diabetes Versus People Without Diabetes: A Multicenter Study. Gastroenterology. 2023 Aug;165(2):463-72.e5. doi: 10.1053/j.gastro.2023.04.025. Epub 2023 Apr 29. PMID: 37127100.
Teoh AYB et al. EUS-Guided Choledocho-duodenostomy Using Lumen Apposing Stent Versus ERCP With Covered Metallic Stents in Patients With Unresectable Malignant Distal Biliary Obstruction: A Multicenter Randomized Controlled Trial (DRA-MBO Trial). Gastroenterology. 2023 Aug;165(2):473-82.e2. doi: 10.1053/j.gastro.2023.04.016. Epub 2023 Apr 28. PMID: 37121331.
September
Mehta RS et al. Association of Proton Pump Inhibitor Use With Incident Dementia and Cognitive Decline in Older Adults: A Prospective Cohort Study. Gastroenterology. 2023 Sep;165(3):564-72.e1. doi: 10.1053/j.gastro.2023.05.052. Epub 2023 Jun 12. PMID: 37315867; PMCID: PMC10527011.
Ballou S et al. Prevalence and Associated Factors of Bloating: Results From the Rome Foundation Global Epidemiology Study. Gastroenterology. 2023 Sep;165(3):647-55.e4. doi: 10.1053/j.gastro.2023.05.049. Epub 2023 Jun 13. PMID: 37315866; PMCID: PMC10527500.
CGH
July
Chang JW et al. Development of a Practical Guide to Implement and Monitor Diet Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023 Jul;21(7):1690-8. doi: 10.1016/j.cgh.2023.03.006. Epub 2023 Mar 16. PMID: 36933603; PMCID: PMC10293042.
Siboni S et al. Improving the Diagnostic Yield of High-Resolution Esophageal Manometry for GERD: The “Straight Leg-Raise” International Study. Clin Gastroenterol Hepatol. 2023 Jul;21(7):1761-70.e1. doi: 10.1016/j.cgh.2022.10.008. Epub 2022 Oct 19. PMID: 36270615.
August
Wechsler EV et al. Up-Front Endoscopy Maximizes Cost-Effectiveness and Cost-Satisfaction in Uninvestigated Dyspepsia. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2378-88.e28. doi: 10.1016/j.cgh.2023.01.003. Epub 2023 Jan 13. PMID: 36646234; PMCID: PMC10542651.
Frederiks CN et al. Clinical Relevance of Random Biopsies From the Esophagogastric Junction After Complete Eradication of Barrett’s Esophagus is Low. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2260-9.e9. doi: 10.1016/j.cgh.2022.11.012. Epub 2022 Nov 22. PMID: 36423874.
Rustgi SD et al. Management of Gastric Intestinal Metaplasia. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2178-82. doi: 10.1016/j.cgh.2023.03.010. Epub 2023 Apr 19. PMID: 37086748; PMCID: PMC10526696.
September
Baroud S et al. A Protocolized Management of Walled-Off Necrosis (WON) Reduces Time to WON Resolution and Improves Outcomes. Clin Gastroenterol Hepatol. 2023 Sep;21(10):2543-50.e1. doi: 10.1016/j.cgh.2023.04.029. Epub 2023 May 8. PMID: 37164115.
Arnim UV et al. Monitoring Patients With Eosinophilic Esophagitis in Routine Clinical Practice - International Expert Recommendations. Clin Gastroenterol Hepatol. 2023 Sep;21(10):2526-33. doi: 10.1016/j.cgh.2022.12.018. Epub 2022 Dec 24. PMID: 36572109.
TIGE
Kaila V et al. Does the Absence of Contrast Passage Into the Duodenum During Intraoperative Cholangiogram Truly Predict Choledocholithiasis? Techniques and Innovations in Gastrointestinal Endoscopy. 2023. https://doi.org/10.1016/j.tige.2023.05.002.
O’Keefe SJD et al. Early Enteral Feeding in Severe Acute Pancreatitis: A Randomized Clinical Trial Between Gastric vs Distal Jejunal Feeding. Techniques and Innovations in Gastrointestinal Endoscopy. 2023. https://doi.org/10.1016/j.tige.2023.06.002.
Gastro Hep Advances
Mukherjee S et al. Assessing ChatGPT’s ability to reply to queries regarding colon cancer screening based on Multi-Society Guidelines. Gastro Hep Advances. 2023. https://doi.org/10.1016/j.gastha.2023.07.008.
Lopes EW et al. Lochhead P. Improving the Consent Process with an Informed Consent Video Prior to Outpatient Colonoscopy. Gastro Hep Advances. 2023. https://doi.org/10.1016/j.gastha.2023.07.016.
Gastroenterology
July
Newberry C et al. Enhancing Nutrition and Obesity Education in GI Fellowship Through Universal Curriculum Development. Gastroenterology. 2023 Jul;165(1):16-19. doi: 10.1053/j.gastro.2023.04.004. Epub 2023 Apr 13. PMID: 37061170.
Han H et al. Macrophage-derived Osteopontin (SPP1) Protects From Nonalcoholic Steatohepatitis. Gastroenterology. 2023 Jul;165(1):201-17. doi: 10.1053/j.gastro.2023.03.228. Epub 2023 Apr 5. PMID: 37028770.
Deepak P et al. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Gastroenterology. 2023 Jul;165(1):11-15. doi: 10.1053/j.gastro.2023.05.017. PMID: 37349061.
August
Guo L et al. Molecular Profiling Provides Clinical Insights Into Targeted and Immunotherapies as Well as Colorectal Cancer Prognosis. Gastroenterology. 2023 Aug;165(2):414-28.e7. doi: 10.1053/j.gastro.2023.04.029. Epub 2023 May 3. PMID: 37146911.
Huang DQ et al. Fibrosis Progression Rate in Biopsy-Proven Nonalcoholic Fatty Liver Disease Among People With Diabetes Versus People Without Diabetes: A Multicenter Study. Gastroenterology. 2023 Aug;165(2):463-72.e5. doi: 10.1053/j.gastro.2023.04.025. Epub 2023 Apr 29. PMID: 37127100.
Teoh AYB et al. EUS-Guided Choledocho-duodenostomy Using Lumen Apposing Stent Versus ERCP With Covered Metallic Stents in Patients With Unresectable Malignant Distal Biliary Obstruction: A Multicenter Randomized Controlled Trial (DRA-MBO Trial). Gastroenterology. 2023 Aug;165(2):473-82.e2. doi: 10.1053/j.gastro.2023.04.016. Epub 2023 Apr 28. PMID: 37121331.
September
Mehta RS et al. Association of Proton Pump Inhibitor Use With Incident Dementia and Cognitive Decline in Older Adults: A Prospective Cohort Study. Gastroenterology. 2023 Sep;165(3):564-72.e1. doi: 10.1053/j.gastro.2023.05.052. Epub 2023 Jun 12. PMID: 37315867; PMCID: PMC10527011.
Ballou S et al. Prevalence and Associated Factors of Bloating: Results From the Rome Foundation Global Epidemiology Study. Gastroenterology. 2023 Sep;165(3):647-55.e4. doi: 10.1053/j.gastro.2023.05.049. Epub 2023 Jun 13. PMID: 37315866; PMCID: PMC10527500.
CGH
July
Chang JW et al. Development of a Practical Guide to Implement and Monitor Diet Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023 Jul;21(7):1690-8. doi: 10.1016/j.cgh.2023.03.006. Epub 2023 Mar 16. PMID: 36933603; PMCID: PMC10293042.
Siboni S et al. Improving the Diagnostic Yield of High-Resolution Esophageal Manometry for GERD: The “Straight Leg-Raise” International Study. Clin Gastroenterol Hepatol. 2023 Jul;21(7):1761-70.e1. doi: 10.1016/j.cgh.2022.10.008. Epub 2022 Oct 19. PMID: 36270615.
August
Wechsler EV et al. Up-Front Endoscopy Maximizes Cost-Effectiveness and Cost-Satisfaction in Uninvestigated Dyspepsia. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2378-88.e28. doi: 10.1016/j.cgh.2023.01.003. Epub 2023 Jan 13. PMID: 36646234; PMCID: PMC10542651.
Frederiks CN et al. Clinical Relevance of Random Biopsies From the Esophagogastric Junction After Complete Eradication of Barrett’s Esophagus is Low. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2260-9.e9. doi: 10.1016/j.cgh.2022.11.012. Epub 2022 Nov 22. PMID: 36423874.
Rustgi SD et al. Management of Gastric Intestinal Metaplasia. Clin Gastroenterol Hepatol. 2023 Aug;21(9):2178-82. doi: 10.1016/j.cgh.2023.03.010. Epub 2023 Apr 19. PMID: 37086748; PMCID: PMC10526696.
September
Baroud S et al. A Protocolized Management of Walled-Off Necrosis (WON) Reduces Time to WON Resolution and Improves Outcomes. Clin Gastroenterol Hepatol. 2023 Sep;21(10):2543-50.e1. doi: 10.1016/j.cgh.2023.04.029. Epub 2023 May 8. PMID: 37164115.
Arnim UV et al. Monitoring Patients With Eosinophilic Esophagitis in Routine Clinical Practice - International Expert Recommendations. Clin Gastroenterol Hepatol. 2023 Sep;21(10):2526-33. doi: 10.1016/j.cgh.2022.12.018. Epub 2022 Dec 24. PMID: 36572109.
TIGE
Kaila V et al. Does the Absence of Contrast Passage Into the Duodenum During Intraoperative Cholangiogram Truly Predict Choledocholithiasis? Techniques and Innovations in Gastrointestinal Endoscopy. 2023. https://doi.org/10.1016/j.tige.2023.05.002.
O’Keefe SJD et al. Early Enteral Feeding in Severe Acute Pancreatitis: A Randomized Clinical Trial Between Gastric vs Distal Jejunal Feeding. Techniques and Innovations in Gastrointestinal Endoscopy. 2023. https://doi.org/10.1016/j.tige.2023.06.002.
Gastro Hep Advances
Mukherjee S et al. Assessing ChatGPT’s ability to reply to queries regarding colon cancer screening based on Multi-Society Guidelines. Gastro Hep Advances. 2023. https://doi.org/10.1016/j.gastha.2023.07.008.
Lopes EW et al. Lochhead P. Improving the Consent Process with an Informed Consent Video Prior to Outpatient Colonoscopy. Gastro Hep Advances. 2023. https://doi.org/10.1016/j.gastha.2023.07.016.
Does diabetes affect colorectal cancer outcomes?
TOPLINE:
, while those with uncomplicated diabetes had insignificantly worse cancer outcomes.
METHODOLOGY:
- This population-based retrospective cohort study used 2007-2015 data from the Taiwan Cancer Registry, which is linked to national insurance and death registry data.
- The analysis included 59,202 adults with stage I-III CRC who underwent potentially curative surgery: 44,944 without diabetes, 8,864 with uncomplicated diabetes, and 5,394 with complicated diabetes.
- The association between diabetes severity and CRC survival, overall survival (OS), disease-free survival (DFS), time to recurrence, and cancer-specific survival (CSS) was examined.
TAKEAWAY:
- Patients with uncomplicated diabetes had insignificantly worse OS (hazard ratio, 1.05), DFS (HR, 1.08), and CSS (HR, 0.98), compared with peers who did not have diabetes.
- Patients with complicated diabetes were at significantly higher risk of poor OS (HR, 1.85), DFS (HR, 1.75), and CSS (HR, 1.41), compared with those without diabetes.
- Patients with diabetes were also at higher risk for CRC recurrence than those without diabetes.
- Except for recurrence risk, the impact of complicated diabetes on CRC survival – that is, OS, DFS, and CSS – was more pronounced among women and those with early-stage cancer.
IN PRACTICE:
“These findings indicate that preventing diabetes complications may help improve survival in patients with CRC, especially [in] female patients and those in the early stages of the disease. Thus, a multidisciplinary approach is recommended for patients with CRC,” the authors conclude.
SOURCE:
The study, with first author Hsin-Yin Hsu, MD, National Taiwan University, Taipei, was published online in the journal Cancer.
LIMITATIONS:
Only patients from Taiwan were included, which limits generalizability, because CRC prognosis may vary in accordance with race or cancer treatment strategy – factors that may differ among countries. Data on glucose levels and diabetes duration were unavailable, potentially leading to misclassification of diabetes status.
DISCLOSURES:
Funding was provided by the Ministry of Science and Technology and the Ministry of Health and Welfare. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, while those with uncomplicated diabetes had insignificantly worse cancer outcomes.
METHODOLOGY:
- This population-based retrospective cohort study used 2007-2015 data from the Taiwan Cancer Registry, which is linked to national insurance and death registry data.
- The analysis included 59,202 adults with stage I-III CRC who underwent potentially curative surgery: 44,944 without diabetes, 8,864 with uncomplicated diabetes, and 5,394 with complicated diabetes.
- The association between diabetes severity and CRC survival, overall survival (OS), disease-free survival (DFS), time to recurrence, and cancer-specific survival (CSS) was examined.
TAKEAWAY:
- Patients with uncomplicated diabetes had insignificantly worse OS (hazard ratio, 1.05), DFS (HR, 1.08), and CSS (HR, 0.98), compared with peers who did not have diabetes.
- Patients with complicated diabetes were at significantly higher risk of poor OS (HR, 1.85), DFS (HR, 1.75), and CSS (HR, 1.41), compared with those without diabetes.
- Patients with diabetes were also at higher risk for CRC recurrence than those without diabetes.
- Except for recurrence risk, the impact of complicated diabetes on CRC survival – that is, OS, DFS, and CSS – was more pronounced among women and those with early-stage cancer.
IN PRACTICE:
“These findings indicate that preventing diabetes complications may help improve survival in patients with CRC, especially [in] female patients and those in the early stages of the disease. Thus, a multidisciplinary approach is recommended for patients with CRC,” the authors conclude.
SOURCE:
The study, with first author Hsin-Yin Hsu, MD, National Taiwan University, Taipei, was published online in the journal Cancer.
LIMITATIONS:
Only patients from Taiwan were included, which limits generalizability, because CRC prognosis may vary in accordance with race or cancer treatment strategy – factors that may differ among countries. Data on glucose levels and diabetes duration were unavailable, potentially leading to misclassification of diabetes status.
DISCLOSURES:
Funding was provided by the Ministry of Science and Technology and the Ministry of Health and Welfare. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, while those with uncomplicated diabetes had insignificantly worse cancer outcomes.
METHODOLOGY:
- This population-based retrospective cohort study used 2007-2015 data from the Taiwan Cancer Registry, which is linked to national insurance and death registry data.
- The analysis included 59,202 adults with stage I-III CRC who underwent potentially curative surgery: 44,944 without diabetes, 8,864 with uncomplicated diabetes, and 5,394 with complicated diabetes.
- The association between diabetes severity and CRC survival, overall survival (OS), disease-free survival (DFS), time to recurrence, and cancer-specific survival (CSS) was examined.
TAKEAWAY:
- Patients with uncomplicated diabetes had insignificantly worse OS (hazard ratio, 1.05), DFS (HR, 1.08), and CSS (HR, 0.98), compared with peers who did not have diabetes.
- Patients with complicated diabetes were at significantly higher risk of poor OS (HR, 1.85), DFS (HR, 1.75), and CSS (HR, 1.41), compared with those without diabetes.
- Patients with diabetes were also at higher risk for CRC recurrence than those without diabetes.
- Except for recurrence risk, the impact of complicated diabetes on CRC survival – that is, OS, DFS, and CSS – was more pronounced among women and those with early-stage cancer.
IN PRACTICE:
“These findings indicate that preventing diabetes complications may help improve survival in patients with CRC, especially [in] female patients and those in the early stages of the disease. Thus, a multidisciplinary approach is recommended for patients with CRC,” the authors conclude.
SOURCE:
The study, with first author Hsin-Yin Hsu, MD, National Taiwan University, Taipei, was published online in the journal Cancer.
LIMITATIONS:
Only patients from Taiwan were included, which limits generalizability, because CRC prognosis may vary in accordance with race or cancer treatment strategy – factors that may differ among countries. Data on glucose levels and diabetes duration were unavailable, potentially leading to misclassification of diabetes status.
DISCLOSURES:
Funding was provided by the Ministry of Science and Technology and the Ministry of Health and Welfare. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Five personal finance questions for the young GI
While this article will get you started, these are complex topics, and each could warrant several standalone articles. I strongly encourage you to develop some basic understanding of personal finance through books, websites, and podcasts. If you can manage Barrett’s esophagus, Crohn’s, and cirrhosis, you can understand the basics of personal finance.
1. What should I do about my student loans? Go for public service loan forgiveness or pay them off?
The first step is knowing your debt burden, knowing your options, and developing a plan to pay off student loans. Public service loan forgiveness (PSLF) can be a good option in many situations. For borrowers staying in academic or other 501(c)(3) positions, PSLF is often an obvious move. Importantly, a fall 2022 statement by the U.S. Department of Education clarified that physicians working as contractors for nonprofit hospitals in California and Texas may now qualify for PSLF.1,2
For trainees debating an academic/501(c)(3) position vs. private practice, I would generally not advise making a career choice based purely on PSLF eligibility. However, borrowers with very high federal student loan burdens (e.g., debt to income ratio of > 2:1), or who are very close to the PSLF 10-year requirement may want to consider choosing a qualifying position for a few years to receive PSLF student loan forgiveness. Please see TNG’s 2020 article3 for a deeper discussion. Consultation with a company specializing in student loan advice for physicians may be well worth the upfront cost.
2. Do I need disability insurance? What should I look for?
I would strongly advise getting disability insurance as soon as possible (including while in training). While disability insurance is not cheap, it is one of the first steps you should take and one of the most important ways to protect your financial future. It is essential to look for a specialty-specific own occupation policy. Such a policy will provide disability payments if you are no longer able to work as a gastroenterologist/hepatologist (including an injury which prevents you from doing endoscopies).
There are two major types of disability policies: group policies and individual policies. See table 1 for a detailed comparison.
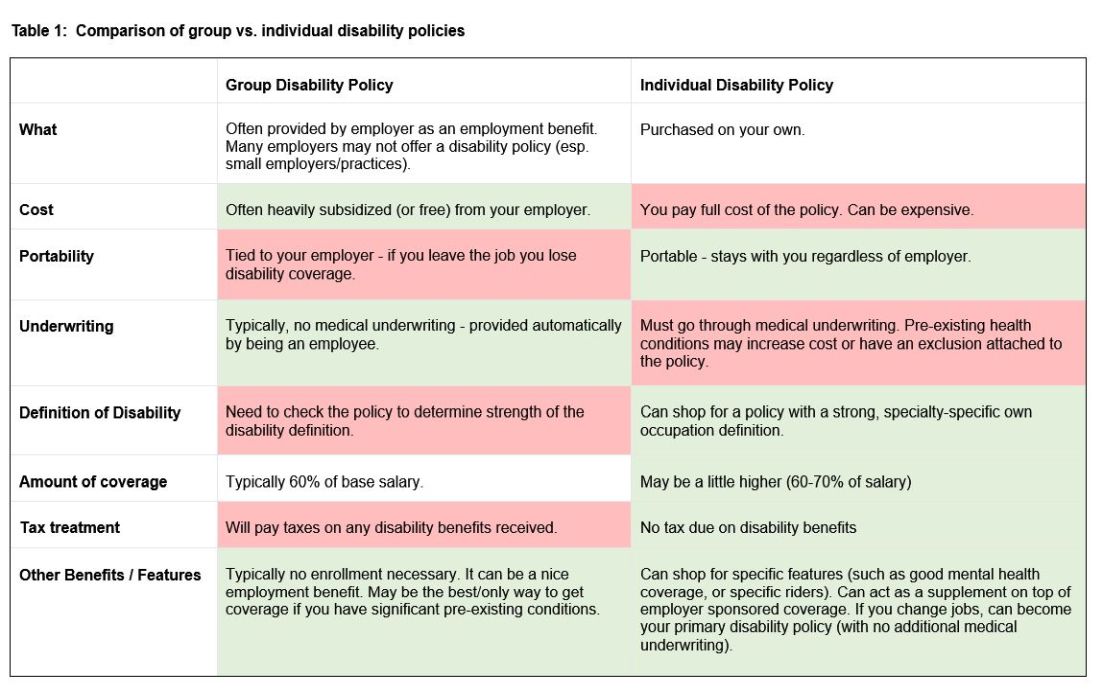
Your hospital/employer may provide a group policy at a heavily subsidized rate. Alternatively, you can purchase an individual disability policy, which is independent of your employer and will stay with you even if you change jobs. Currently, the only companies providing high quality own-occupation policies for physicians are Mass Mutual, Principal, Guardian, The Standard, and Ameritas. Because disability insurance is complicated, it is highly advisable to work with an agent experienced in physician disability policies.
Importantly, even if you have a group disability policy, you can purchase an individual policy as a supplement to provide extra coverage. If you leave employers, the individual policy can then become your primary disability policy without any additional medical underwriting.
3. Do I need life insurance? What type should I get?
If anyone is dependent on your income (partner, child, etc.), you should have life insurance. Moreover, if you expect to have dependents in the near future (e.g., children), you could consider getting life insurance now while you are younger and healthier. For a young GI with multiple financial obligations, term life insurance is generally the right product. Term life insurance is a straightforward, affordable product that can be purchased from multiple high-quality insurance carriers. There are two major considerations: The amount of coverage ($2 million, $3 million, etc.) and the length of coverage (20 years, 30 years, etc.). To estimate the appropriate amount of coverage, start with your expected annual household living expenses, and multiply by 25-30. While this is a rule of thumb, it will get you in the ballpark. For many young physicians, a $2-$5 million policy with 20- to 30-year coverage is reasonable.
Many financial advisers may suggest whole life insurance policies. These are typically not the ideal policy for young GIs who are just starting their careers. While whole life insurance may be the right choice in select cases, term life insurance will be the best product for most of TNG’s audience. As an example, a $3 million, 25-year term policy for a healthy, nonsmoking 35-year-old male would cost approximately $175 per month. A similar $3 million whole life policy could cost $2,000 per month or more.
4. What do I need to know about retirement accounts and investing?
The alphabet soup of retirement accounts can be confusing – IRA, 401k, 457. Retirement accounts provide a tax break to incentivize saving for retirement. Traditional (“non-Roth”) accounts provide a tax break today, but you will pay taxes when withdrawing the money in retirement. Roth accounts provide no tax break now but provide tax-free growth for decades, and no taxes are due when withdrawing money. See table 2 for a detailed comparison of retirement accounts.
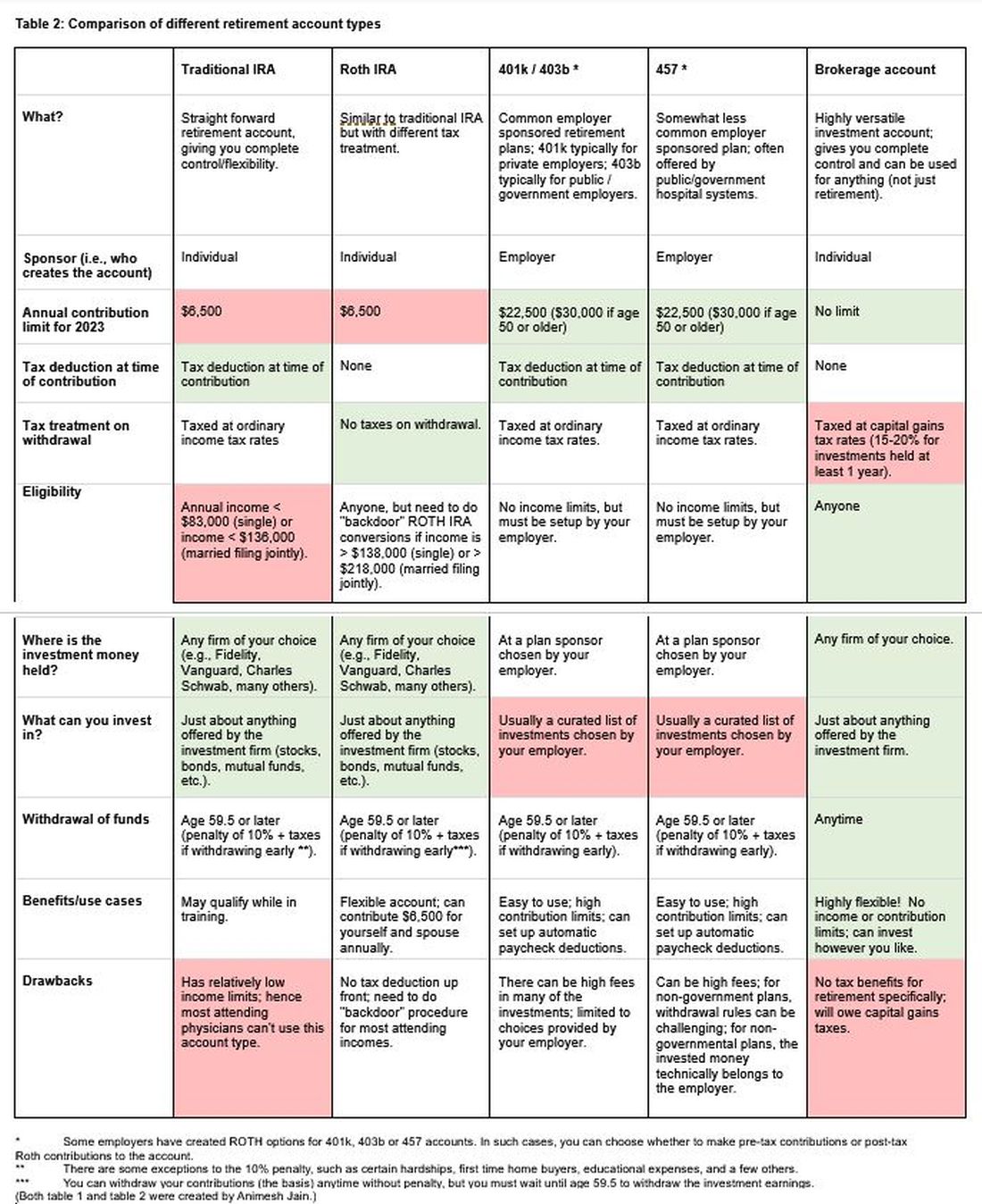
Once you place money into a retirement account, you will need to choose specific investments to grow your money. The two most common asset classes are stocks and bonds, though there are many other reasonable assets, such as real estate, commodities, and alternative currencies. It is generally recommended to have a higher proportion of stock-based investments early on (60%-90%) and then increase the ratio of bonds closer to retirement. Using low cost, passive index funds (or exchange traded funds) is a good way to get stock exposure. Target date retirement funds can be a nice tool for beginning investors since they will automatically adjust the stock/bond ratio for you.
Calculating the amount needed for retirement is beyond the scope of this article. However, saving at least 20% of your gross income specifically for retirement is a good starting point and should set you up for a reasonable retirement in about 30 years. For the average GI physician, this would mean saving $4,000 or more per month for retirement. If you aim to retire earlier, consider investing a higher percentage.
5. What do I need to know about buying a house?
The first question to ask is whether it makes sense to rent or buy a house. This is a personal and lifestyle decision, not just a financial decision. Today’s market is difficult with both high home prices and high rent costs. If there is a reasonable chance that you will be moving within 3-5 years, I would consider not buying until your long-term plans are more stable. Moreover, a high proportion of physicians change jobs.4,5,6 If you are just starting a new job, it is often wise to wait at least 6-12 months before buying a house to ensure the new job is a good fit. If you are in a stable long-term situation, it may be reasonable to buy a house. While it is commonly believed that buying a house is a “good financial move,” there are many hidden costs to home ownership, including big ticket repairs, property taxes, and real estate fees when selling a home.
First-time physician home buyers can often secure a physician mortgage with competitive interest rates and a low down payment of 0%-10% instead of the traditional 20% down payment. Moreover, a good physician mortgage should not have private mortgage insurance (PMI). Given the variation between mortgage companies, my most important piece of advice is to shop around for a good mortgage. An independent mortgage broker can be very valuable.
Dr. Jain is associate professor of medicine in the division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. He has no conflicts of interest. The information in this article is meant for general educational purposes only. For individualized personal finance advice, please seek your own financial advisor, tax accountant, insurance broker, attorney, or other financial professional. Follow Dr. Jain @AJainMD on X.
References
1. Future of PSLF Fact Sheet
2. The Loophole That Can Get Thousands of Doctors into PSLF
3. Student loan management: An introduction for the young gastroenterologist
4. Study Shows First Job after Medical Residency Often Doesn’t Last
5. More physicians want to leave their jobs as pay rates fall, survey finds
6. Physician turnover rates are climbing as they clamor for better work-life balance
While this article will get you started, these are complex topics, and each could warrant several standalone articles. I strongly encourage you to develop some basic understanding of personal finance through books, websites, and podcasts. If you can manage Barrett’s esophagus, Crohn’s, and cirrhosis, you can understand the basics of personal finance.
1. What should I do about my student loans? Go for public service loan forgiveness or pay them off?
The first step is knowing your debt burden, knowing your options, and developing a plan to pay off student loans. Public service loan forgiveness (PSLF) can be a good option in many situations. For borrowers staying in academic or other 501(c)(3) positions, PSLF is often an obvious move. Importantly, a fall 2022 statement by the U.S. Department of Education clarified that physicians working as contractors for nonprofit hospitals in California and Texas may now qualify for PSLF.1,2
For trainees debating an academic/501(c)(3) position vs. private practice, I would generally not advise making a career choice based purely on PSLF eligibility. However, borrowers with very high federal student loan burdens (e.g., debt to income ratio of > 2:1), or who are very close to the PSLF 10-year requirement may want to consider choosing a qualifying position for a few years to receive PSLF student loan forgiveness. Please see TNG’s 2020 article3 for a deeper discussion. Consultation with a company specializing in student loan advice for physicians may be well worth the upfront cost.
2. Do I need disability insurance? What should I look for?
I would strongly advise getting disability insurance as soon as possible (including while in training). While disability insurance is not cheap, it is one of the first steps you should take and one of the most important ways to protect your financial future. It is essential to look for a specialty-specific own occupation policy. Such a policy will provide disability payments if you are no longer able to work as a gastroenterologist/hepatologist (including an injury which prevents you from doing endoscopies).
There are two major types of disability policies: group policies and individual policies. See table 1 for a detailed comparison.

Your hospital/employer may provide a group policy at a heavily subsidized rate. Alternatively, you can purchase an individual disability policy, which is independent of your employer and will stay with you even if you change jobs. Currently, the only companies providing high quality own-occupation policies for physicians are Mass Mutual, Principal, Guardian, The Standard, and Ameritas. Because disability insurance is complicated, it is highly advisable to work with an agent experienced in physician disability policies.
Importantly, even if you have a group disability policy, you can purchase an individual policy as a supplement to provide extra coverage. If you leave employers, the individual policy can then become your primary disability policy without any additional medical underwriting.
3. Do I need life insurance? What type should I get?
If anyone is dependent on your income (partner, child, etc.), you should have life insurance. Moreover, if you expect to have dependents in the near future (e.g., children), you could consider getting life insurance now while you are younger and healthier. For a young GI with multiple financial obligations, term life insurance is generally the right product. Term life insurance is a straightforward, affordable product that can be purchased from multiple high-quality insurance carriers. There are two major considerations: The amount of coverage ($2 million, $3 million, etc.) and the length of coverage (20 years, 30 years, etc.). To estimate the appropriate amount of coverage, start with your expected annual household living expenses, and multiply by 25-30. While this is a rule of thumb, it will get you in the ballpark. For many young physicians, a $2-$5 million policy with 20- to 30-year coverage is reasonable.
Many financial advisers may suggest whole life insurance policies. These are typically not the ideal policy for young GIs who are just starting their careers. While whole life insurance may be the right choice in select cases, term life insurance will be the best product for most of TNG’s audience. As an example, a $3 million, 25-year term policy for a healthy, nonsmoking 35-year-old male would cost approximately $175 per month. A similar $3 million whole life policy could cost $2,000 per month or more.
4. What do I need to know about retirement accounts and investing?
The alphabet soup of retirement accounts can be confusing – IRA, 401k, 457. Retirement accounts provide a tax break to incentivize saving for retirement. Traditional (“non-Roth”) accounts provide a tax break today, but you will pay taxes when withdrawing the money in retirement. Roth accounts provide no tax break now but provide tax-free growth for decades, and no taxes are due when withdrawing money. See table 2 for a detailed comparison of retirement accounts.

Once you place money into a retirement account, you will need to choose specific investments to grow your money. The two most common asset classes are stocks and bonds, though there are many other reasonable assets, such as real estate, commodities, and alternative currencies. It is generally recommended to have a higher proportion of stock-based investments early on (60%-90%) and then increase the ratio of bonds closer to retirement. Using low cost, passive index funds (or exchange traded funds) is a good way to get stock exposure. Target date retirement funds can be a nice tool for beginning investors since they will automatically adjust the stock/bond ratio for you.
Calculating the amount needed for retirement is beyond the scope of this article. However, saving at least 20% of your gross income specifically for retirement is a good starting point and should set you up for a reasonable retirement in about 30 years. For the average GI physician, this would mean saving $4,000 or more per month for retirement. If you aim to retire earlier, consider investing a higher percentage.
5. What do I need to know about buying a house?
The first question to ask is whether it makes sense to rent or buy a house. This is a personal and lifestyle decision, not just a financial decision. Today’s market is difficult with both high home prices and high rent costs. If there is a reasonable chance that you will be moving within 3-5 years, I would consider not buying until your long-term plans are more stable. Moreover, a high proportion of physicians change jobs.4,5,6 If you are just starting a new job, it is often wise to wait at least 6-12 months before buying a house to ensure the new job is a good fit. If you are in a stable long-term situation, it may be reasonable to buy a house. While it is commonly believed that buying a house is a “good financial move,” there are many hidden costs to home ownership, including big ticket repairs, property taxes, and real estate fees when selling a home.
First-time physician home buyers can often secure a physician mortgage with competitive interest rates and a low down payment of 0%-10% instead of the traditional 20% down payment. Moreover, a good physician mortgage should not have private mortgage insurance (PMI). Given the variation between mortgage companies, my most important piece of advice is to shop around for a good mortgage. An independent mortgage broker can be very valuable.
Dr. Jain is associate professor of medicine in the division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. He has no conflicts of interest. The information in this article is meant for general educational purposes only. For individualized personal finance advice, please seek your own financial advisor, tax accountant, insurance broker, attorney, or other financial professional. Follow Dr. Jain @AJainMD on X.
References
1. Future of PSLF Fact Sheet
2. The Loophole That Can Get Thousands of Doctors into PSLF
3. Student loan management: An introduction for the young gastroenterologist
4. Study Shows First Job after Medical Residency Often Doesn’t Last
5. More physicians want to leave their jobs as pay rates fall, survey finds
6. Physician turnover rates are climbing as they clamor for better work-life balance
While this article will get you started, these are complex topics, and each could warrant several standalone articles. I strongly encourage you to develop some basic understanding of personal finance through books, websites, and podcasts. If you can manage Barrett’s esophagus, Crohn’s, and cirrhosis, you can understand the basics of personal finance.
1. What should I do about my student loans? Go for public service loan forgiveness or pay them off?
The first step is knowing your debt burden, knowing your options, and developing a plan to pay off student loans. Public service loan forgiveness (PSLF) can be a good option in many situations. For borrowers staying in academic or other 501(c)(3) positions, PSLF is often an obvious move. Importantly, a fall 2022 statement by the U.S. Department of Education clarified that physicians working as contractors for nonprofit hospitals in California and Texas may now qualify for PSLF.1,2
For trainees debating an academic/501(c)(3) position vs. private practice, I would generally not advise making a career choice based purely on PSLF eligibility. However, borrowers with very high federal student loan burdens (e.g., debt to income ratio of > 2:1), or who are very close to the PSLF 10-year requirement may want to consider choosing a qualifying position for a few years to receive PSLF student loan forgiveness. Please see TNG’s 2020 article3 for a deeper discussion. Consultation with a company specializing in student loan advice for physicians may be well worth the upfront cost.
2. Do I need disability insurance? What should I look for?
I would strongly advise getting disability insurance as soon as possible (including while in training). While disability insurance is not cheap, it is one of the first steps you should take and one of the most important ways to protect your financial future. It is essential to look for a specialty-specific own occupation policy. Such a policy will provide disability payments if you are no longer able to work as a gastroenterologist/hepatologist (including an injury which prevents you from doing endoscopies).
There are two major types of disability policies: group policies and individual policies. See table 1 for a detailed comparison.

Your hospital/employer may provide a group policy at a heavily subsidized rate. Alternatively, you can purchase an individual disability policy, which is independent of your employer and will stay with you even if you change jobs. Currently, the only companies providing high quality own-occupation policies for physicians are Mass Mutual, Principal, Guardian, The Standard, and Ameritas. Because disability insurance is complicated, it is highly advisable to work with an agent experienced in physician disability policies.
Importantly, even if you have a group disability policy, you can purchase an individual policy as a supplement to provide extra coverage. If you leave employers, the individual policy can then become your primary disability policy without any additional medical underwriting.
3. Do I need life insurance? What type should I get?
If anyone is dependent on your income (partner, child, etc.), you should have life insurance. Moreover, if you expect to have dependents in the near future (e.g., children), you could consider getting life insurance now while you are younger and healthier. For a young GI with multiple financial obligations, term life insurance is generally the right product. Term life insurance is a straightforward, affordable product that can be purchased from multiple high-quality insurance carriers. There are two major considerations: The amount of coverage ($2 million, $3 million, etc.) and the length of coverage (20 years, 30 years, etc.). To estimate the appropriate amount of coverage, start with your expected annual household living expenses, and multiply by 25-30. While this is a rule of thumb, it will get you in the ballpark. For many young physicians, a $2-$5 million policy with 20- to 30-year coverage is reasonable.
Many financial advisers may suggest whole life insurance policies. These are typically not the ideal policy for young GIs who are just starting their careers. While whole life insurance may be the right choice in select cases, term life insurance will be the best product for most of TNG’s audience. As an example, a $3 million, 25-year term policy for a healthy, nonsmoking 35-year-old male would cost approximately $175 per month. A similar $3 million whole life policy could cost $2,000 per month or more.
4. What do I need to know about retirement accounts and investing?
The alphabet soup of retirement accounts can be confusing – IRA, 401k, 457. Retirement accounts provide a tax break to incentivize saving for retirement. Traditional (“non-Roth”) accounts provide a tax break today, but you will pay taxes when withdrawing the money in retirement. Roth accounts provide no tax break now but provide tax-free growth for decades, and no taxes are due when withdrawing money. See table 2 for a detailed comparison of retirement accounts.

Once you place money into a retirement account, you will need to choose specific investments to grow your money. The two most common asset classes are stocks and bonds, though there are many other reasonable assets, such as real estate, commodities, and alternative currencies. It is generally recommended to have a higher proportion of stock-based investments early on (60%-90%) and then increase the ratio of bonds closer to retirement. Using low cost, passive index funds (or exchange traded funds) is a good way to get stock exposure. Target date retirement funds can be a nice tool for beginning investors since they will automatically adjust the stock/bond ratio for you.
Calculating the amount needed for retirement is beyond the scope of this article. However, saving at least 20% of your gross income specifically for retirement is a good starting point and should set you up for a reasonable retirement in about 30 years. For the average GI physician, this would mean saving $4,000 or more per month for retirement. If you aim to retire earlier, consider investing a higher percentage.
5. What do I need to know about buying a house?
The first question to ask is whether it makes sense to rent or buy a house. This is a personal and lifestyle decision, not just a financial decision. Today’s market is difficult with both high home prices and high rent costs. If there is a reasonable chance that you will be moving within 3-5 years, I would consider not buying until your long-term plans are more stable. Moreover, a high proportion of physicians change jobs.4,5,6 If you are just starting a new job, it is often wise to wait at least 6-12 months before buying a house to ensure the new job is a good fit. If you are in a stable long-term situation, it may be reasonable to buy a house. While it is commonly believed that buying a house is a “good financial move,” there are many hidden costs to home ownership, including big ticket repairs, property taxes, and real estate fees when selling a home.
First-time physician home buyers can often secure a physician mortgage with competitive interest rates and a low down payment of 0%-10% instead of the traditional 20% down payment. Moreover, a good physician mortgage should not have private mortgage insurance (PMI). Given the variation between mortgage companies, my most important piece of advice is to shop around for a good mortgage. An independent mortgage broker can be very valuable.
Dr. Jain is associate professor of medicine in the division of gastroenterology and hepatology, University of North Carolina School of Medicine, Chapel Hill. He has no conflicts of interest. The information in this article is meant for general educational purposes only. For individualized personal finance advice, please seek your own financial advisor, tax accountant, insurance broker, attorney, or other financial professional. Follow Dr. Jain @AJainMD on X.
References
1. Future of PSLF Fact Sheet
2. The Loophole That Can Get Thousands of Doctors into PSLF
3. Student loan management: An introduction for the young gastroenterologist
4. Study Shows First Job after Medical Residency Often Doesn’t Last
5. More physicians want to leave their jobs as pay rates fall, survey finds
6. Physician turnover rates are climbing as they clamor for better work-life balance
Advances in endoscopic therapies in inflammatory bowel disease
Introduction
Inflammatory bowel disease (IBD) is a chronic, relapsing and remitting disorder that is becoming increasingly prevalent worldwide.1 Despite major advances in this area, many patients with moderate to severe IBD do not achieve disease remission with immunosuppressive therapy.2 Dysplasia and fibrostenosis are two common consequences of uncontrolled chronic inflammation and these structural complications are often the primary reasons for surgical interventions.3 While there is certainly a time and a place for surgery in IBD, this approach is invasive and postoperative recrudescence of disease is common.4 Moreover patients with complex surgical or medical histories may not make optimal surgical candidates.
Thanks to advancements in a variety of endoscopic technologies, Over the last several years, applications of endoscopic therapies in IBD have been gaining traction and the need for these therapies is expected to continue to rise over time. As such, understanding the domains of available endoscopic options in IBD is important for the modern-day gastroenterologist. In this article, we will discuss some of the recent advancements in endoscopic therapies for IBD and how we may position these in clinical practice.
Protecting against colitis dysplasia and colon cancer
IBD is a risk factor for colorectal cancer because of the dysplasia-carcinoma sequence arising from chronic colitis. Endoscopic resection is the first-line treatment for conventional colitis-associated dysplasia (CAD).5,6 However, larger or complex lesions may not have been previously amenable to this organ-preserving approach. The application of newer techniques has extended the indication for endoscopic resection to include most CAD lesions, as an alternative to proctocolectomy. Endoscopic mucosal resection (EMR) is the most commonly used technique and its outcomes for CAD greater than 2 cm have been excellent (Figure 1).7 However, employing EMR for lesions greater than 2 cm in size may require piecemeal resection and this has been associated with a small risk of local recurrence.8 Endoscopic submucosal dissection (ESD) is an alternate method of endoscopic tissue resection that can reliably achieve en bloc (single specimen) resections even in larger lesions.9
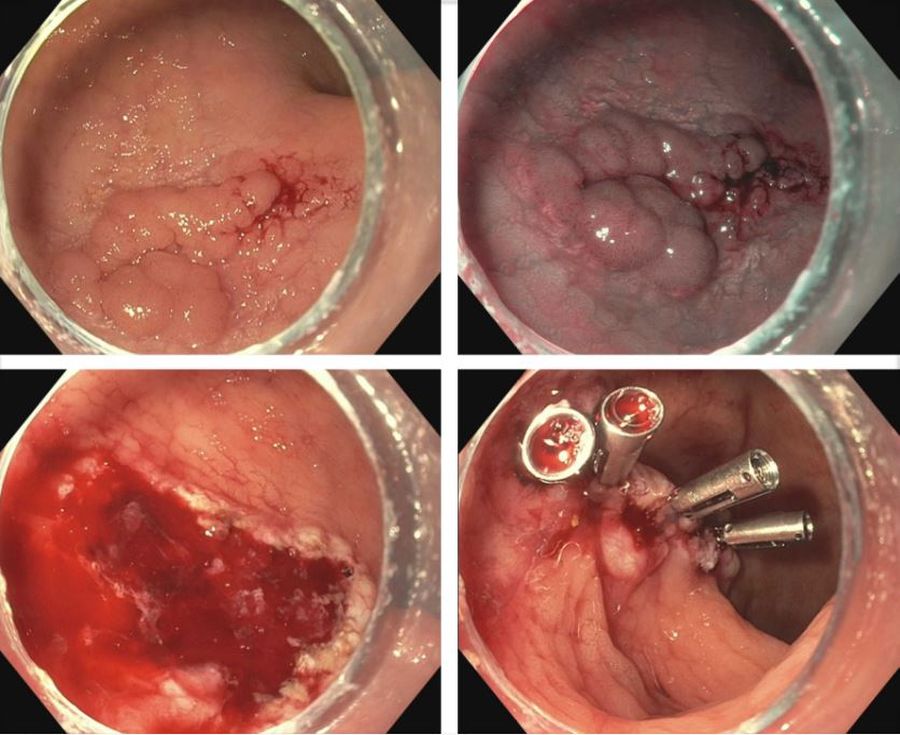
These technical advantages, however, have not been proven to result in broad clinical superiority of ESD over EMR for advanced lesions.10 The other consideration is that ESD is associated with greater risk of perforation and is more technically complex to perform.10 Yet, recent data supporting ESD in larger lesions is amounting and it may be more suitable for situations where conventional techniques fall short.11 To that end, dense submucosal fibrosis is a common characteristic of CAD and may prohibit successful EMR or ESD as a single modality. Different therapeutic methods can be incorporated in these circumstances, including combined ESD and EMR technique, tissue thermal ablation, or even full-thickness resection has been described.11-13
Taken together, we have many effective options for how we can effectively deal with CAD endoscopically and maintain our patients free of colorectal cancer. The method in which this is done may not matter as much at this juncture and may be more dependent on available local clinical expertise. Moreover, we can’t forget that metachronous lesions and neoplastic recurrence after endoscopic resection are not uncommon and a structured, vigilant endoscopic surveillance program for all patients undergoing endoscopic management of CAD is mandated.7,10
Restoring gastrointestinal tract transit
Crohn’s strictures may lead to acute intestinal obstructions or facilitate the onset of penetrating disease, such as fistula formation or abscess. These strictures are often characterized by a combination of inflammation and layered fibrosis, which requires the application of medical therapies alongside structural remodeling to successfully manage. Not all strictures may be clinically overt due to variances in visceral sensitivity, yet experts believe that treatment of all strictures should be considered to avoid occurrence of delayed complications.14 Endoscopic balloon dilation (EBD) is a well-established treatment for Crohn’s strictures up to 4-5 cm in length (Figure 2). This treatment involves inflating a balloon within the narrowed section of intestine, thereby stretching and disrupting the layered fibrotic bands to widen the stricture. EBD improves symptoms 70% of the time and successfully avoids the medium-term need for surgery in most, although it often requires repeat endoscopic procedures.15 In fact, up to 74% of patients will require repeat dilation over 2 years and 43% will require salvage surgery after EBD.16
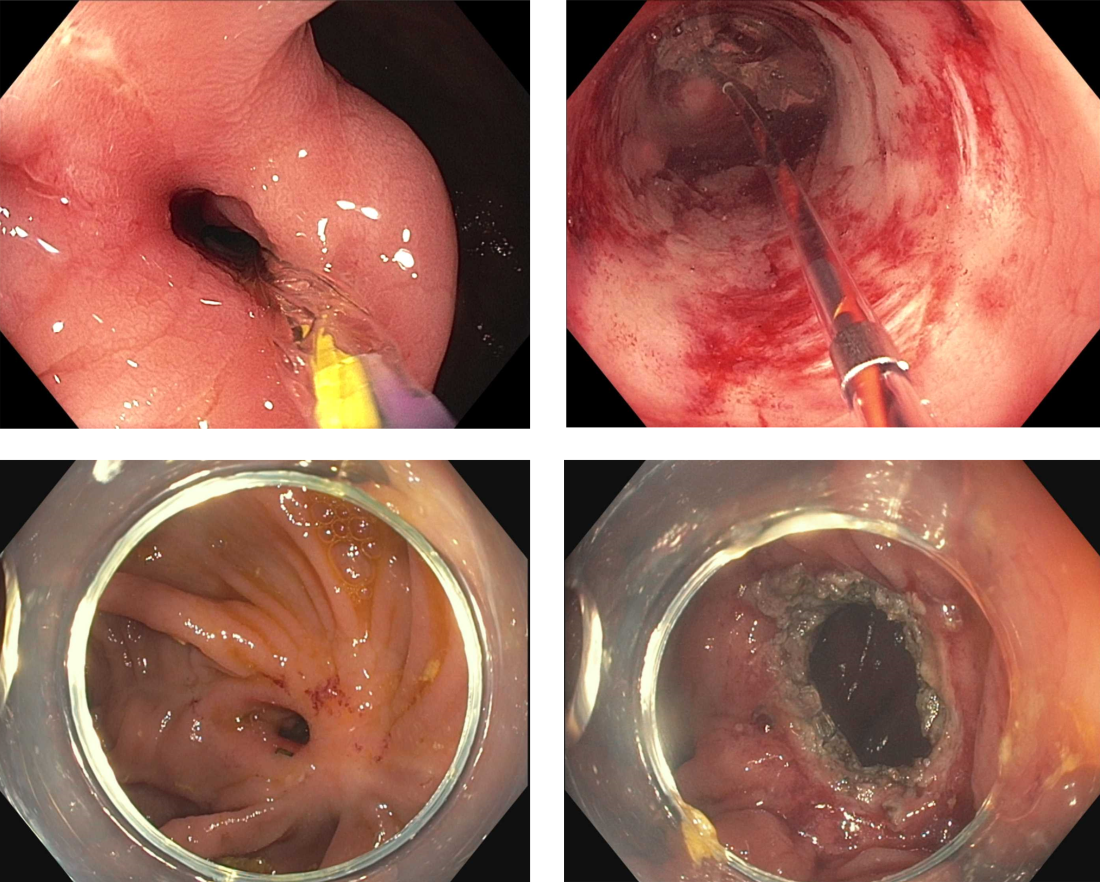
Endoscopic stricturotomy (Est) is a newer technique that involves making radial and longitudinal incisions within the stricture using an endoscopic knife (Figure 2). The ability to excise fibrotic bands allows for more advanced remodeling and thus a lower need for reintervention or surgery (9%-22.5%) in comparison with EBD, while maintaining similar technical and clinical success rates.17 Est also carries a lower risk of perforation, but a higher risk of delayed bleeding.17 Refinements in Est are ongoing as the technique continues to develop, including the application of prophylactic clips after Est or use of other hemostatic agents such as gels or powders to minimizing bleeding risk. Despite this, Est has clear benefit in durability for treating strictures especially anastomotic subtype or those refractory to balloon dilation.
Stenting is a third option for treating strictures in Crohn’s disease that is reserved for specific situations. This approach involves endoscopic implantation of a covered metallic stent within the stricture in order to promote remodeling throughout a selected dwell time (generally 2-4 weeks). Stents may be considered in nonoperative candidates with strictures longer than 5 cm, which are generally too long for EBD or Est, or in EBD-refractory strictures in which there is no clear plane for Est excision. However, given the risk of migration, stents are currently not considered a first-line treatment of IBD-related strictures.18 Perhaps with further modifications in design and availability of stent-fixation methods, their use may become more practical in the future.19
The future for endoscopic therapy is bright
Structural complications of IBD are common and can pose a significant detriment to quality of life and general well-being for patients. From mucosal resection of CAD to surgery-sparing therapies for intestinal strictures, endoscopic therapies are valuable and effective options for managing disease-related sequelae within the scope of interventional IBD practice. We can expect the availability of these options to grow as the scope of endoscopy training incorporates principles of interventional IBD, along with the concurrent development of additional therapeutic applications beyond the categories discussed here (including perianal disease, fistulas, and abscess formation). It is noteworthy to mention that while endoscopic therapies are separate treatment modalities, should not be considered mutually exclusive; endotherapies are best viewed as a complement to existing medical and surgical approaches. Thus, Interventional IBD endoscopy can serve as an integral part of the multidisciplinary IBD framework to provide comprehensive care for our patients with IBD.
Juan Reyes Genere, MD, is an assistant professor of medicine in gastroenterology at Washington University in St. Louis. He served as the corresponding author of this article. Michael Rubeiz, MD, is a physician in the internal medicine residency program at Washington University in St. Louis. Kemmian Johnson, MD, MPH, is a gastroenterologist at Washington University in St. Louis specializing in inflammatory bowel disease. Dr. Genere is a consultant for Edulis Therapeutics. Dr. Rubeiz and Dr. Johnson had no personal or financial conflicts of interest. Dr. Johnson can be reached on Instagram @KJ.1906; Dr. Rubeiz is on X @MichaelRubeiz1 and Dr. Genere can be reached via X @JPGenereMD.
References
1. Ng SC et al. Lancet. 2017;390(10114):2769-78.
2. Gordon JP et al. Eur J Gastroenterol Hepatol. 2015;27(7):804-12.
3. Sica GS and Biancone L. World J Gastroenterol. 2013;19(16):2445-8.
4. Iborra M et al. Gastroenterol Rep (Oxf). 2019;7(6):411-8.
5. Annese V et al. J Crohns Colitis. 2013;7(12):982-1018.
6. Laine L et al. Gastrointest Endosc. 2015;81(3):489-501.e426.
7. Mohan BP et al. Gastrointest Endosc. 2021;93(1):59-67.e10.
8. Briedigkeit A et al. World J Gastrointest Endosc. 2016;8(5):276-81.
9. Manta R et al. J Crohns Colitis. 2021;15(1):165-8.
10. Mohapatra S et al. Endosc Int Open. 2022;10(5):E593-601.
11. Ngamruengphong S et al. Endosc Int Open. 2022;10(4):E354-60.
12. Baker G et al. Cureus. 2022 May 3;14(5):e24688.
13. Yadav S et al. Endosc Int Open. 2019;7(8):E994-1001.
14. Schwartz DA. Gastrointestinal Endoscopy. 2023;97(5):974-6.
15. Morar PS et al. Aliment Pharmacol Ther. 2015;42(10):1137-48.
16. Bettenworth D et al. Inflamm Bowel Dis. 2017;23(1):133-42.
17. Lan N and Shen B. Inflamm Bowel Dis. 2018;24(4):897-907.
18. Loras C et al. Lancet Gastroenterol Hepatol. 2022;7(4):332-41.
19. Genere JR et al. Lancet Gastroenterol Hepatol. 2022;7(6):503-4.
Introduction
Inflammatory bowel disease (IBD) is a chronic, relapsing and remitting disorder that is becoming increasingly prevalent worldwide.1 Despite major advances in this area, many patients with moderate to severe IBD do not achieve disease remission with immunosuppressive therapy.2 Dysplasia and fibrostenosis are two common consequences of uncontrolled chronic inflammation and these structural complications are often the primary reasons for surgical interventions.3 While there is certainly a time and a place for surgery in IBD, this approach is invasive and postoperative recrudescence of disease is common.4 Moreover patients with complex surgical or medical histories may not make optimal surgical candidates.
Thanks to advancements in a variety of endoscopic technologies, Over the last several years, applications of endoscopic therapies in IBD have been gaining traction and the need for these therapies is expected to continue to rise over time. As such, understanding the domains of available endoscopic options in IBD is important for the modern-day gastroenterologist. In this article, we will discuss some of the recent advancements in endoscopic therapies for IBD and how we may position these in clinical practice.
Protecting against colitis dysplasia and colon cancer
IBD is a risk factor for colorectal cancer because of the dysplasia-carcinoma sequence arising from chronic colitis. Endoscopic resection is the first-line treatment for conventional colitis-associated dysplasia (CAD).5,6 However, larger or complex lesions may not have been previously amenable to this organ-preserving approach. The application of newer techniques has extended the indication for endoscopic resection to include most CAD lesions, as an alternative to proctocolectomy. Endoscopic mucosal resection (EMR) is the most commonly used technique and its outcomes for CAD greater than 2 cm have been excellent (Figure 1).7 However, employing EMR for lesions greater than 2 cm in size may require piecemeal resection and this has been associated with a small risk of local recurrence.8 Endoscopic submucosal dissection (ESD) is an alternate method of endoscopic tissue resection that can reliably achieve en bloc (single specimen) resections even in larger lesions.9

These technical advantages, however, have not been proven to result in broad clinical superiority of ESD over EMR for advanced lesions.10 The other consideration is that ESD is associated with greater risk of perforation and is more technically complex to perform.10 Yet, recent data supporting ESD in larger lesions is amounting and it may be more suitable for situations where conventional techniques fall short.11 To that end, dense submucosal fibrosis is a common characteristic of CAD and may prohibit successful EMR or ESD as a single modality. Different therapeutic methods can be incorporated in these circumstances, including combined ESD and EMR technique, tissue thermal ablation, or even full-thickness resection has been described.11-13
Taken together, we have many effective options for how we can effectively deal with CAD endoscopically and maintain our patients free of colorectal cancer. The method in which this is done may not matter as much at this juncture and may be more dependent on available local clinical expertise. Moreover, we can’t forget that metachronous lesions and neoplastic recurrence after endoscopic resection are not uncommon and a structured, vigilant endoscopic surveillance program for all patients undergoing endoscopic management of CAD is mandated.7,10
Restoring gastrointestinal tract transit
Crohn’s strictures may lead to acute intestinal obstructions or facilitate the onset of penetrating disease, such as fistula formation or abscess. These strictures are often characterized by a combination of inflammation and layered fibrosis, which requires the application of medical therapies alongside structural remodeling to successfully manage. Not all strictures may be clinically overt due to variances in visceral sensitivity, yet experts believe that treatment of all strictures should be considered to avoid occurrence of delayed complications.14 Endoscopic balloon dilation (EBD) is a well-established treatment for Crohn’s strictures up to 4-5 cm in length (Figure 2). This treatment involves inflating a balloon within the narrowed section of intestine, thereby stretching and disrupting the layered fibrotic bands to widen the stricture. EBD improves symptoms 70% of the time and successfully avoids the medium-term need for surgery in most, although it often requires repeat endoscopic procedures.15 In fact, up to 74% of patients will require repeat dilation over 2 years and 43% will require salvage surgery after EBD.16

Endoscopic stricturotomy (Est) is a newer technique that involves making radial and longitudinal incisions within the stricture using an endoscopic knife (Figure 2). The ability to excise fibrotic bands allows for more advanced remodeling and thus a lower need for reintervention or surgery (9%-22.5%) in comparison with EBD, while maintaining similar technical and clinical success rates.17 Est also carries a lower risk of perforation, but a higher risk of delayed bleeding.17 Refinements in Est are ongoing as the technique continues to develop, including the application of prophylactic clips after Est or use of other hemostatic agents such as gels or powders to minimizing bleeding risk. Despite this, Est has clear benefit in durability for treating strictures especially anastomotic subtype or those refractory to balloon dilation.
Stenting is a third option for treating strictures in Crohn’s disease that is reserved for specific situations. This approach involves endoscopic implantation of a covered metallic stent within the stricture in order to promote remodeling throughout a selected dwell time (generally 2-4 weeks). Stents may be considered in nonoperative candidates with strictures longer than 5 cm, which are generally too long for EBD or Est, or in EBD-refractory strictures in which there is no clear plane for Est excision. However, given the risk of migration, stents are currently not considered a first-line treatment of IBD-related strictures.18 Perhaps with further modifications in design and availability of stent-fixation methods, their use may become more practical in the future.19
The future for endoscopic therapy is bright
Structural complications of IBD are common and can pose a significant detriment to quality of life and general well-being for patients. From mucosal resection of CAD to surgery-sparing therapies for intestinal strictures, endoscopic therapies are valuable and effective options for managing disease-related sequelae within the scope of interventional IBD practice. We can expect the availability of these options to grow as the scope of endoscopy training incorporates principles of interventional IBD, along with the concurrent development of additional therapeutic applications beyond the categories discussed here (including perianal disease, fistulas, and abscess formation). It is noteworthy to mention that while endoscopic therapies are separate treatment modalities, should not be considered mutually exclusive; endotherapies are best viewed as a complement to existing medical and surgical approaches. Thus, Interventional IBD endoscopy can serve as an integral part of the multidisciplinary IBD framework to provide comprehensive care for our patients with IBD.
Juan Reyes Genere, MD, is an assistant professor of medicine in gastroenterology at Washington University in St. Louis. He served as the corresponding author of this article. Michael Rubeiz, MD, is a physician in the internal medicine residency program at Washington University in St. Louis. Kemmian Johnson, MD, MPH, is a gastroenterologist at Washington University in St. Louis specializing in inflammatory bowel disease. Dr. Genere is a consultant for Edulis Therapeutics. Dr. Rubeiz and Dr. Johnson had no personal or financial conflicts of interest. Dr. Johnson can be reached on Instagram @KJ.1906; Dr. Rubeiz is on X @MichaelRubeiz1 and Dr. Genere can be reached via X @JPGenereMD.
References
1. Ng SC et al. Lancet. 2017;390(10114):2769-78.
2. Gordon JP et al. Eur J Gastroenterol Hepatol. 2015;27(7):804-12.
3. Sica GS and Biancone L. World J Gastroenterol. 2013;19(16):2445-8.
4. Iborra M et al. Gastroenterol Rep (Oxf). 2019;7(6):411-8.
5. Annese V et al. J Crohns Colitis. 2013;7(12):982-1018.
6. Laine L et al. Gastrointest Endosc. 2015;81(3):489-501.e426.
7. Mohan BP et al. Gastrointest Endosc. 2021;93(1):59-67.e10.
8. Briedigkeit A et al. World J Gastrointest Endosc. 2016;8(5):276-81.
9. Manta R et al. J Crohns Colitis. 2021;15(1):165-8.
10. Mohapatra S et al. Endosc Int Open. 2022;10(5):E593-601.
11. Ngamruengphong S et al. Endosc Int Open. 2022;10(4):E354-60.
12. Baker G et al. Cureus. 2022 May 3;14(5):e24688.
13. Yadav S et al. Endosc Int Open. 2019;7(8):E994-1001.
14. Schwartz DA. Gastrointestinal Endoscopy. 2023;97(5):974-6.
15. Morar PS et al. Aliment Pharmacol Ther. 2015;42(10):1137-48.
16. Bettenworth D et al. Inflamm Bowel Dis. 2017;23(1):133-42.
17. Lan N and Shen B. Inflamm Bowel Dis. 2018;24(4):897-907.
18. Loras C et al. Lancet Gastroenterol Hepatol. 2022;7(4):332-41.
19. Genere JR et al. Lancet Gastroenterol Hepatol. 2022;7(6):503-4.
Introduction
Inflammatory bowel disease (IBD) is a chronic, relapsing and remitting disorder that is becoming increasingly prevalent worldwide.1 Despite major advances in this area, many patients with moderate to severe IBD do not achieve disease remission with immunosuppressive therapy.2 Dysplasia and fibrostenosis are two common consequences of uncontrolled chronic inflammation and these structural complications are often the primary reasons for surgical interventions.3 While there is certainly a time and a place for surgery in IBD, this approach is invasive and postoperative recrudescence of disease is common.4 Moreover patients with complex surgical or medical histories may not make optimal surgical candidates.
Thanks to advancements in a variety of endoscopic technologies, Over the last several years, applications of endoscopic therapies in IBD have been gaining traction and the need for these therapies is expected to continue to rise over time. As such, understanding the domains of available endoscopic options in IBD is important for the modern-day gastroenterologist. In this article, we will discuss some of the recent advancements in endoscopic therapies for IBD and how we may position these in clinical practice.
Protecting against colitis dysplasia and colon cancer
IBD is a risk factor for colorectal cancer because of the dysplasia-carcinoma sequence arising from chronic colitis. Endoscopic resection is the first-line treatment for conventional colitis-associated dysplasia (CAD).5,6 However, larger or complex lesions may not have been previously amenable to this organ-preserving approach. The application of newer techniques has extended the indication for endoscopic resection to include most CAD lesions, as an alternative to proctocolectomy. Endoscopic mucosal resection (EMR) is the most commonly used technique and its outcomes for CAD greater than 2 cm have been excellent (Figure 1).7 However, employing EMR for lesions greater than 2 cm in size may require piecemeal resection and this has been associated with a small risk of local recurrence.8 Endoscopic submucosal dissection (ESD) is an alternate method of endoscopic tissue resection that can reliably achieve en bloc (single specimen) resections even in larger lesions.9

These technical advantages, however, have not been proven to result in broad clinical superiority of ESD over EMR for advanced lesions.10 The other consideration is that ESD is associated with greater risk of perforation and is more technically complex to perform.10 Yet, recent data supporting ESD in larger lesions is amounting and it may be more suitable for situations where conventional techniques fall short.11 To that end, dense submucosal fibrosis is a common characteristic of CAD and may prohibit successful EMR or ESD as a single modality. Different therapeutic methods can be incorporated in these circumstances, including combined ESD and EMR technique, tissue thermal ablation, or even full-thickness resection has been described.11-13
Taken together, we have many effective options for how we can effectively deal with CAD endoscopically and maintain our patients free of colorectal cancer. The method in which this is done may not matter as much at this juncture and may be more dependent on available local clinical expertise. Moreover, we can’t forget that metachronous lesions and neoplastic recurrence after endoscopic resection are not uncommon and a structured, vigilant endoscopic surveillance program for all patients undergoing endoscopic management of CAD is mandated.7,10
Restoring gastrointestinal tract transit
Crohn’s strictures may lead to acute intestinal obstructions or facilitate the onset of penetrating disease, such as fistula formation or abscess. These strictures are often characterized by a combination of inflammation and layered fibrosis, which requires the application of medical therapies alongside structural remodeling to successfully manage. Not all strictures may be clinically overt due to variances in visceral sensitivity, yet experts believe that treatment of all strictures should be considered to avoid occurrence of delayed complications.14 Endoscopic balloon dilation (EBD) is a well-established treatment for Crohn’s strictures up to 4-5 cm in length (Figure 2). This treatment involves inflating a balloon within the narrowed section of intestine, thereby stretching and disrupting the layered fibrotic bands to widen the stricture. EBD improves symptoms 70% of the time and successfully avoids the medium-term need for surgery in most, although it often requires repeat endoscopic procedures.15 In fact, up to 74% of patients will require repeat dilation over 2 years and 43% will require salvage surgery after EBD.16

Endoscopic stricturotomy (Est) is a newer technique that involves making radial and longitudinal incisions within the stricture using an endoscopic knife (Figure 2). The ability to excise fibrotic bands allows for more advanced remodeling and thus a lower need for reintervention or surgery (9%-22.5%) in comparison with EBD, while maintaining similar technical and clinical success rates.17 Est also carries a lower risk of perforation, but a higher risk of delayed bleeding.17 Refinements in Est are ongoing as the technique continues to develop, including the application of prophylactic clips after Est or use of other hemostatic agents such as gels or powders to minimizing bleeding risk. Despite this, Est has clear benefit in durability for treating strictures especially anastomotic subtype or those refractory to balloon dilation.
Stenting is a third option for treating strictures in Crohn’s disease that is reserved for specific situations. This approach involves endoscopic implantation of a covered metallic stent within the stricture in order to promote remodeling throughout a selected dwell time (generally 2-4 weeks). Stents may be considered in nonoperative candidates with strictures longer than 5 cm, which are generally too long for EBD or Est, or in EBD-refractory strictures in which there is no clear plane for Est excision. However, given the risk of migration, stents are currently not considered a first-line treatment of IBD-related strictures.18 Perhaps with further modifications in design and availability of stent-fixation methods, their use may become more practical in the future.19
The future for endoscopic therapy is bright
Structural complications of IBD are common and can pose a significant detriment to quality of life and general well-being for patients. From mucosal resection of CAD to surgery-sparing therapies for intestinal strictures, endoscopic therapies are valuable and effective options for managing disease-related sequelae within the scope of interventional IBD practice. We can expect the availability of these options to grow as the scope of endoscopy training incorporates principles of interventional IBD, along with the concurrent development of additional therapeutic applications beyond the categories discussed here (including perianal disease, fistulas, and abscess formation). It is noteworthy to mention that while endoscopic therapies are separate treatment modalities, should not be considered mutually exclusive; endotherapies are best viewed as a complement to existing medical and surgical approaches. Thus, Interventional IBD endoscopy can serve as an integral part of the multidisciplinary IBD framework to provide comprehensive care for our patients with IBD.
Juan Reyes Genere, MD, is an assistant professor of medicine in gastroenterology at Washington University in St. Louis. He served as the corresponding author of this article. Michael Rubeiz, MD, is a physician in the internal medicine residency program at Washington University in St. Louis. Kemmian Johnson, MD, MPH, is a gastroenterologist at Washington University in St. Louis specializing in inflammatory bowel disease. Dr. Genere is a consultant for Edulis Therapeutics. Dr. Rubeiz and Dr. Johnson had no personal or financial conflicts of interest. Dr. Johnson can be reached on Instagram @KJ.1906; Dr. Rubeiz is on X @MichaelRubeiz1 and Dr. Genere can be reached via X @JPGenereMD.
References
1. Ng SC et al. Lancet. 2017;390(10114):2769-78.
2. Gordon JP et al. Eur J Gastroenterol Hepatol. 2015;27(7):804-12.
3. Sica GS and Biancone L. World J Gastroenterol. 2013;19(16):2445-8.
4. Iborra M et al. Gastroenterol Rep (Oxf). 2019;7(6):411-8.
5. Annese V et al. J Crohns Colitis. 2013;7(12):982-1018.
6. Laine L et al. Gastrointest Endosc. 2015;81(3):489-501.e426.
7. Mohan BP et al. Gastrointest Endosc. 2021;93(1):59-67.e10.
8. Briedigkeit A et al. World J Gastrointest Endosc. 2016;8(5):276-81.
9. Manta R et al. J Crohns Colitis. 2021;15(1):165-8.
10. Mohapatra S et al. Endosc Int Open. 2022;10(5):E593-601.
11. Ngamruengphong S et al. Endosc Int Open. 2022;10(4):E354-60.
12. Baker G et al. Cureus. 2022 May 3;14(5):e24688.
13. Yadav S et al. Endosc Int Open. 2019;7(8):E994-1001.
14. Schwartz DA. Gastrointestinal Endoscopy. 2023;97(5):974-6.
15. Morar PS et al. Aliment Pharmacol Ther. 2015;42(10):1137-48.
16. Bettenworth D et al. Inflamm Bowel Dis. 2017;23(1):133-42.
17. Lan N and Shen B. Inflamm Bowel Dis. 2018;24(4):897-907.
18. Loras C et al. Lancet Gastroenterol Hepatol. 2022;7(4):332-41.
19. Genere JR et al. Lancet Gastroenterol Hepatol. 2022;7(6):503-4.
Stripped privileges: An alarming precedent for community oncologists?
, Alliance Cancer Specialists.
The outcome, some community oncologists say, could set a new precedent in how far large health care organizations will go to take their patients or drive them out of business.
The case
On Sept. 5, Alliance sued Jefferson Health after Jefferson canceled the inpatient oncology/hematology privileges of five Alliance oncologists at three Jefferson Health-Northeast hospitals, primarily alleging that Jefferson was attempting to monopolize cancer care in the area.
Jefferson – one of the largest health care systems in the Philadelphia area that includes the NCI-designated Sidney Kimmel Cancer Center – made the move because it had entered into an exclusive agreement with its own medical group to provide inpatient and outpatient oncology/hematology services at the hospitals.
In its court filings, Jefferson said it entered into the exclusive agreement because doing so was in “the best interest of patients, as it would ensure better integration and availability of care and help ensure that Jefferson consistently provides high-quality medical care in accordance with evidence-based standards.”
Tensions had been building between Alliance and Jefferson for years, ever since, according to Alliance, the community practice declined a buyout offer from Jefferson almost a decade ago.
But the revocation of privileges ultimately tipped the scales for Alliance, sparking the lawsuit.
“For us, that crossed a line,” said Moshe Chasky, MD, one of the five Alliance oncologists and a plaintiff in the suit.
Dr. Chasky and his colleagues had provided care at the hospitals for years, with about 10-15 patients admitted at any one time. The quality of their care is not in dispute. Dr. Chasky, for instance, routinely makes Philadelphia Magazine’s Top Doc List.
Under the new arrangement, the five Alliance oncologists have to hand over care of their admitted patients to Jefferson oncologists or send their patients to another hospital farther away where they do have admitting privileges.
“Without having admitting privileges,” community oncologists “can’t look a patient in the eye and say, ‘No matter what, I’ve got you,’ ” explained Nicolas Ferreyros, managing director of policy, advocacy, and communications at the Community Oncology Alliance, a DC-based lobbying group for independent oncologists.
“A doctor doesn’t want to tell a patient that ‘once you go in the hospital, I have to hand you off.’ ” It undermines their practice, Mr. Ferreyros said.
The situation has caught the attention of other community oncologists who are worried that hospitals canceling admitting privileges might become a new tactic in what they characterize as an ongoing effort to elbow-out independent practitioners and corner the oncology market.
Dr. Chasky said he is getting “calls every day from independent oncologists throughout the country” who “are very concerned. People are watching this for sure.”
Alliance attorney Daniel Frier said that there is nothing unusual about hospitals entering into exclusive contracts with hospital-based practices.
But Mr. Frier said he’s never heard of a hospital entering into an exclusive contract and then terminating the privileges of community oncologists.
“There’s no direct precedent” for the move, he said.
Jefferson Health did not respond to requests for comment.
The ruling
U.S. District Court Judge Kai Scott, who ruled on Alliance’s motion to block the contract and preserve its oncologists’ admitting privileges, ultimately sided with Jefferson and allowed the contract to go forward.
Judge Scott wrote that, “while the court understands the plaintiffs’ concerns and desires to maintain the continuity of care for their own patients,” the court “is not persuaded that either of the two threshold elements for a temporary restraining order or preliminary injunction are met” – first, that Jefferson’s actions violate antitrust laws and second that the plaintiffs “will suffer immediate, irreparable harm” from having their admitting privileges rescinded.
Alliance argued that Jefferson’s contract violated federal antitrust laws and would allow Jefferson to monopolize the local oncology market.
However, Judge Scott called Alliance’s antitrust argument “lifeless” under the strict requirements for antitrust violations, explaining that, among other reasons, a monopoly is unlikely given that Jefferson competes with several high-profile oncology programs in the Philadelphia area, including the Fox Chase Cancer Center.
Judge Scott also expressed doubt that the Jefferson’s actions would cause irreparable harm to Alliance’s business. Alliance employs more than thirty oncologists affiliated with over a dozen hospitals in the greater Philadelphia area, and the inpatient services provided at Jefferson Health-Northeast did not represent a major part of its business.
Despite her ruling, Judge Scott did voice skepticism about some of Jefferson’s arguments.
“The court notes that the Jefferson defendants have briefly argued that Jefferson will be better able to ensure that its own patients receive fully integrated and coordinated care” under the exclusive provider agreement, but “it is unclear how the cooperation of ACS [Alliance Cancer Specialists] and JNE [Jefferson Health-Northeast] hospitalists really caused any problems for the coordinated care of” patients in the many years that they worked together.
It also “does not seem to necessarily serve the community to quickly sever the artery between the services that ACS provides and the services that JNE provides,” Judge Scott wrote.
She added that she would consider another motion from Alliance if the practice makes stronger arguments illustrating antitrust violations and demonstrating irreparable harm.
Currently, Dr. Chasky and Mr. Frier are considering their next steps in the case. The oncologists said they can appeal the judge’s decision or file a new complaint.
Meanwhile, Dr. Chasky and his four colleagues requested and were granted internal medicine privileges at Jefferson Health-Northeast, but given the considerable overlap between oncology and internal medicine, the line between what they can and cannot do remains unclear.
“It’s a mess,” he said.
A familiar story
Large health care entities have increasingly worked to push out or swallow up smaller, independent practices for years.
“What Dr. Chasky and his practice are going through is a little bit more of an aggressive version of what’s going on in the rest of the country,” said Michael Diaz, MD, a community oncologist at Florida Cancer Specialists, the largest independent medical oncology/hematology group in the United States. “The larger institutional hospitals try to make it a closed system so they can keep everything in-house and refer to their own physicians.”
The incentive, Dr. Diaz said, is the financial windfall that Section 340B of the 1992 Public Health Service Act generates for hospital-based oncology services at nonprofit hospitals, such as the Jefferson Health-Northeast facilities.
The 340B program allows nonprofit hospitals to buy primarily outpatient oncology drugs at steep discounts, sometimes 50% or more, and be reimbursed at full price.
When launched in 1992, the program was meant to help a handful of safety-net hospitals cover the cost of charity care, and now approximately more than half of U.S. hospitals participate in the program, particularly after requirements were loosened by the Affordable Care Act. But there’s little transparency on how the money is spent.
Critics say the incentives have created a feeding frenzy among 340B hospitals to either acquire outpatient oncology practices or take their business because of the particularly high margins on oncology drugs. There are similar incentives for hospital-based infusion centers.
In its lawsuit, Alliance alleged that such incentives are what motivated Jefferson’s recent actions.
“It’s all about the money at the end of the day,” said Christian Thomas, MD, a community oncologist with New England Cancer Specialists, Scarborough, Maine, who, like Dr. Diaz, said he’s seen the dynamic play out repeatedly in his career.
The American Hospital Association has been a vigorous defender of 340B in the courts and elsewhere, but the Association’s communications staff had little to say when this news organization reached out about the Jefferson-Alliance situation, except that they do not comment on “specific hospital circumstance.”
Reverberations around the country
Many community oncologists are keeping close tabs on the Jefferson-Alliance situation.
“Our group has been watching Jefferson closely because our [local] hospital is following the same playbook, but they have not yet gone after our privileges,” said Scott Herbert, MD, a community oncologist with the independent Nexus Health system, Sante Fe, N.M.
Dr. Herbert was referring to what has happened since he and his colleagues declined to renew an exclusive provider agreement early this year with St. Vincent Regional Medical Center, a nonprofit hospital in Sante Fe. The agreement allowed the hospital to take advantage of the 340B program because Nexus oncologists acted on its behalf.
St. Vincent’s owner, Christus Health, did not respond to inquiries from this news organization.
Nexus let the contract lapse because its oncologists wanted to provide services at a second, newer hospital in Santa Fe where some of their patients had begun seeking treatment.
The nonprofit hospital in Sante Fe is now building its own oncology practice. Similar to Dr. Chasky’s experience in Philadelphia, Dr. Herbert said his group has seen referrals from the hospital dry up and existing patients rechanneled to the hospital’s oncologists.
“We found over 109 patients in January and February that were referred to one of our docs that got rerouted to one of their docs,” he said.
Dr. Herbert has sent cease-and-desist letters, but “after we saw what Jefferson did, my group said, ‘You better back off of the hospital, or it’s going to take our privileges.’ ”
The Jefferson situation “is sending a message,” he said. “Frankly, we’ve been terrified” at the thought of losing privileges there. “It’s the busiest hospital in our area.”
The future of community oncology
Despite the challenges, Mr. Ferreyros at the Community Oncology Alliance remains optimistic about the future of independent oncology.
Under the competitive pressures, a lot of independent oncology practices have folded in recent years, but the ones that remain are strong. Payers are also increasingly noticing that community oncology practices are less expensive than hospital-based practices for comparable care, he said.
Relationships with hospitals aren’t always adversarial, either. “A lot of practices have collaborative agreements with local hospitals” that work out well, Mr. Ferreyros said, adding that sometimes hospitals even hand over oncology care to local independents after finding that starting and maintaining an oncology service is harder than they imagined.
“The last two decades have been difficult,” but the remaining community oncology practices “are going strong,” he said, and “we’ve never seen more engagement on our issues,” particularly around the issue of cost savings.
A version of this article first appeared on Medscape.com.
, Alliance Cancer Specialists.
The outcome, some community oncologists say, could set a new precedent in how far large health care organizations will go to take their patients or drive them out of business.
The case
On Sept. 5, Alliance sued Jefferson Health after Jefferson canceled the inpatient oncology/hematology privileges of five Alliance oncologists at three Jefferson Health-Northeast hospitals, primarily alleging that Jefferson was attempting to monopolize cancer care in the area.
Jefferson – one of the largest health care systems in the Philadelphia area that includes the NCI-designated Sidney Kimmel Cancer Center – made the move because it had entered into an exclusive agreement with its own medical group to provide inpatient and outpatient oncology/hematology services at the hospitals.
In its court filings, Jefferson said it entered into the exclusive agreement because doing so was in “the best interest of patients, as it would ensure better integration and availability of care and help ensure that Jefferson consistently provides high-quality medical care in accordance with evidence-based standards.”
Tensions had been building between Alliance and Jefferson for years, ever since, according to Alliance, the community practice declined a buyout offer from Jefferson almost a decade ago.
But the revocation of privileges ultimately tipped the scales for Alliance, sparking the lawsuit.
“For us, that crossed a line,” said Moshe Chasky, MD, one of the five Alliance oncologists and a plaintiff in the suit.
Dr. Chasky and his colleagues had provided care at the hospitals for years, with about 10-15 patients admitted at any one time. The quality of their care is not in dispute. Dr. Chasky, for instance, routinely makes Philadelphia Magazine’s Top Doc List.
Under the new arrangement, the five Alliance oncologists have to hand over care of their admitted patients to Jefferson oncologists or send their patients to another hospital farther away where they do have admitting privileges.
“Without having admitting privileges,” community oncologists “can’t look a patient in the eye and say, ‘No matter what, I’ve got you,’ ” explained Nicolas Ferreyros, managing director of policy, advocacy, and communications at the Community Oncology Alliance, a DC-based lobbying group for independent oncologists.
“A doctor doesn’t want to tell a patient that ‘once you go in the hospital, I have to hand you off.’ ” It undermines their practice, Mr. Ferreyros said.
The situation has caught the attention of other community oncologists who are worried that hospitals canceling admitting privileges might become a new tactic in what they characterize as an ongoing effort to elbow-out independent practitioners and corner the oncology market.
Dr. Chasky said he is getting “calls every day from independent oncologists throughout the country” who “are very concerned. People are watching this for sure.”
Alliance attorney Daniel Frier said that there is nothing unusual about hospitals entering into exclusive contracts with hospital-based practices.
But Mr. Frier said he’s never heard of a hospital entering into an exclusive contract and then terminating the privileges of community oncologists.
“There’s no direct precedent” for the move, he said.
Jefferson Health did not respond to requests for comment.
The ruling
U.S. District Court Judge Kai Scott, who ruled on Alliance’s motion to block the contract and preserve its oncologists’ admitting privileges, ultimately sided with Jefferson and allowed the contract to go forward.
Judge Scott wrote that, “while the court understands the plaintiffs’ concerns and desires to maintain the continuity of care for their own patients,” the court “is not persuaded that either of the two threshold elements for a temporary restraining order or preliminary injunction are met” – first, that Jefferson’s actions violate antitrust laws and second that the plaintiffs “will suffer immediate, irreparable harm” from having their admitting privileges rescinded.
Alliance argued that Jefferson’s contract violated federal antitrust laws and would allow Jefferson to monopolize the local oncology market.
However, Judge Scott called Alliance’s antitrust argument “lifeless” under the strict requirements for antitrust violations, explaining that, among other reasons, a monopoly is unlikely given that Jefferson competes with several high-profile oncology programs in the Philadelphia area, including the Fox Chase Cancer Center.
Judge Scott also expressed doubt that the Jefferson’s actions would cause irreparable harm to Alliance’s business. Alliance employs more than thirty oncologists affiliated with over a dozen hospitals in the greater Philadelphia area, and the inpatient services provided at Jefferson Health-Northeast did not represent a major part of its business.
Despite her ruling, Judge Scott did voice skepticism about some of Jefferson’s arguments.
“The court notes that the Jefferson defendants have briefly argued that Jefferson will be better able to ensure that its own patients receive fully integrated and coordinated care” under the exclusive provider agreement, but “it is unclear how the cooperation of ACS [Alliance Cancer Specialists] and JNE [Jefferson Health-Northeast] hospitalists really caused any problems for the coordinated care of” patients in the many years that they worked together.
It also “does not seem to necessarily serve the community to quickly sever the artery between the services that ACS provides and the services that JNE provides,” Judge Scott wrote.
She added that she would consider another motion from Alliance if the practice makes stronger arguments illustrating antitrust violations and demonstrating irreparable harm.
Currently, Dr. Chasky and Mr. Frier are considering their next steps in the case. The oncologists said they can appeal the judge’s decision or file a new complaint.
Meanwhile, Dr. Chasky and his four colleagues requested and were granted internal medicine privileges at Jefferson Health-Northeast, but given the considerable overlap between oncology and internal medicine, the line between what they can and cannot do remains unclear.
“It’s a mess,” he said.
A familiar story
Large health care entities have increasingly worked to push out or swallow up smaller, independent practices for years.
“What Dr. Chasky and his practice are going through is a little bit more of an aggressive version of what’s going on in the rest of the country,” said Michael Diaz, MD, a community oncologist at Florida Cancer Specialists, the largest independent medical oncology/hematology group in the United States. “The larger institutional hospitals try to make it a closed system so they can keep everything in-house and refer to their own physicians.”
The incentive, Dr. Diaz said, is the financial windfall that Section 340B of the 1992 Public Health Service Act generates for hospital-based oncology services at nonprofit hospitals, such as the Jefferson Health-Northeast facilities.
The 340B program allows nonprofit hospitals to buy primarily outpatient oncology drugs at steep discounts, sometimes 50% or more, and be reimbursed at full price.
When launched in 1992, the program was meant to help a handful of safety-net hospitals cover the cost of charity care, and now approximately more than half of U.S. hospitals participate in the program, particularly after requirements were loosened by the Affordable Care Act. But there’s little transparency on how the money is spent.
Critics say the incentives have created a feeding frenzy among 340B hospitals to either acquire outpatient oncology practices or take their business because of the particularly high margins on oncology drugs. There are similar incentives for hospital-based infusion centers.
In its lawsuit, Alliance alleged that such incentives are what motivated Jefferson’s recent actions.
“It’s all about the money at the end of the day,” said Christian Thomas, MD, a community oncologist with New England Cancer Specialists, Scarborough, Maine, who, like Dr. Diaz, said he’s seen the dynamic play out repeatedly in his career.
The American Hospital Association has been a vigorous defender of 340B in the courts and elsewhere, but the Association’s communications staff had little to say when this news organization reached out about the Jefferson-Alliance situation, except that they do not comment on “specific hospital circumstance.”
Reverberations around the country
Many community oncologists are keeping close tabs on the Jefferson-Alliance situation.
“Our group has been watching Jefferson closely because our [local] hospital is following the same playbook, but they have not yet gone after our privileges,” said Scott Herbert, MD, a community oncologist with the independent Nexus Health system, Sante Fe, N.M.
Dr. Herbert was referring to what has happened since he and his colleagues declined to renew an exclusive provider agreement early this year with St. Vincent Regional Medical Center, a nonprofit hospital in Sante Fe. The agreement allowed the hospital to take advantage of the 340B program because Nexus oncologists acted on its behalf.
St. Vincent’s owner, Christus Health, did not respond to inquiries from this news organization.
Nexus let the contract lapse because its oncologists wanted to provide services at a second, newer hospital in Santa Fe where some of their patients had begun seeking treatment.
The nonprofit hospital in Sante Fe is now building its own oncology practice. Similar to Dr. Chasky’s experience in Philadelphia, Dr. Herbert said his group has seen referrals from the hospital dry up and existing patients rechanneled to the hospital’s oncologists.
“We found over 109 patients in January and February that were referred to one of our docs that got rerouted to one of their docs,” he said.
Dr. Herbert has sent cease-and-desist letters, but “after we saw what Jefferson did, my group said, ‘You better back off of the hospital, or it’s going to take our privileges.’ ”
The Jefferson situation “is sending a message,” he said. “Frankly, we’ve been terrified” at the thought of losing privileges there. “It’s the busiest hospital in our area.”
The future of community oncology
Despite the challenges, Mr. Ferreyros at the Community Oncology Alliance remains optimistic about the future of independent oncology.
Under the competitive pressures, a lot of independent oncology practices have folded in recent years, but the ones that remain are strong. Payers are also increasingly noticing that community oncology practices are less expensive than hospital-based practices for comparable care, he said.
Relationships with hospitals aren’t always adversarial, either. “A lot of practices have collaborative agreements with local hospitals” that work out well, Mr. Ferreyros said, adding that sometimes hospitals even hand over oncology care to local independents after finding that starting and maintaining an oncology service is harder than they imagined.
“The last two decades have been difficult,” but the remaining community oncology practices “are going strong,” he said, and “we’ve never seen more engagement on our issues,” particularly around the issue of cost savings.
A version of this article first appeared on Medscape.com.
, Alliance Cancer Specialists.
The outcome, some community oncologists say, could set a new precedent in how far large health care organizations will go to take their patients or drive them out of business.
The case
On Sept. 5, Alliance sued Jefferson Health after Jefferson canceled the inpatient oncology/hematology privileges of five Alliance oncologists at three Jefferson Health-Northeast hospitals, primarily alleging that Jefferson was attempting to monopolize cancer care in the area.
Jefferson – one of the largest health care systems in the Philadelphia area that includes the NCI-designated Sidney Kimmel Cancer Center – made the move because it had entered into an exclusive agreement with its own medical group to provide inpatient and outpatient oncology/hematology services at the hospitals.
In its court filings, Jefferson said it entered into the exclusive agreement because doing so was in “the best interest of patients, as it would ensure better integration and availability of care and help ensure that Jefferson consistently provides high-quality medical care in accordance with evidence-based standards.”
Tensions had been building between Alliance and Jefferson for years, ever since, according to Alliance, the community practice declined a buyout offer from Jefferson almost a decade ago.
But the revocation of privileges ultimately tipped the scales for Alliance, sparking the lawsuit.
“For us, that crossed a line,” said Moshe Chasky, MD, one of the five Alliance oncologists and a plaintiff in the suit.
Dr. Chasky and his colleagues had provided care at the hospitals for years, with about 10-15 patients admitted at any one time. The quality of their care is not in dispute. Dr. Chasky, for instance, routinely makes Philadelphia Magazine’s Top Doc List.
Under the new arrangement, the five Alliance oncologists have to hand over care of their admitted patients to Jefferson oncologists or send their patients to another hospital farther away where they do have admitting privileges.
“Without having admitting privileges,” community oncologists “can’t look a patient in the eye and say, ‘No matter what, I’ve got you,’ ” explained Nicolas Ferreyros, managing director of policy, advocacy, and communications at the Community Oncology Alliance, a DC-based lobbying group for independent oncologists.
“A doctor doesn’t want to tell a patient that ‘once you go in the hospital, I have to hand you off.’ ” It undermines their practice, Mr. Ferreyros said.
The situation has caught the attention of other community oncologists who are worried that hospitals canceling admitting privileges might become a new tactic in what they characterize as an ongoing effort to elbow-out independent practitioners and corner the oncology market.
Dr. Chasky said he is getting “calls every day from independent oncologists throughout the country” who “are very concerned. People are watching this for sure.”
Alliance attorney Daniel Frier said that there is nothing unusual about hospitals entering into exclusive contracts with hospital-based practices.
But Mr. Frier said he’s never heard of a hospital entering into an exclusive contract and then terminating the privileges of community oncologists.
“There’s no direct precedent” for the move, he said.
Jefferson Health did not respond to requests for comment.
The ruling
U.S. District Court Judge Kai Scott, who ruled on Alliance’s motion to block the contract and preserve its oncologists’ admitting privileges, ultimately sided with Jefferson and allowed the contract to go forward.
Judge Scott wrote that, “while the court understands the plaintiffs’ concerns and desires to maintain the continuity of care for their own patients,” the court “is not persuaded that either of the two threshold elements for a temporary restraining order or preliminary injunction are met” – first, that Jefferson’s actions violate antitrust laws and second that the plaintiffs “will suffer immediate, irreparable harm” from having their admitting privileges rescinded.
Alliance argued that Jefferson’s contract violated federal antitrust laws and would allow Jefferson to monopolize the local oncology market.
However, Judge Scott called Alliance’s antitrust argument “lifeless” under the strict requirements for antitrust violations, explaining that, among other reasons, a monopoly is unlikely given that Jefferson competes with several high-profile oncology programs in the Philadelphia area, including the Fox Chase Cancer Center.
Judge Scott also expressed doubt that the Jefferson’s actions would cause irreparable harm to Alliance’s business. Alliance employs more than thirty oncologists affiliated with over a dozen hospitals in the greater Philadelphia area, and the inpatient services provided at Jefferson Health-Northeast did not represent a major part of its business.
Despite her ruling, Judge Scott did voice skepticism about some of Jefferson’s arguments.
“The court notes that the Jefferson defendants have briefly argued that Jefferson will be better able to ensure that its own patients receive fully integrated and coordinated care” under the exclusive provider agreement, but “it is unclear how the cooperation of ACS [Alliance Cancer Specialists] and JNE [Jefferson Health-Northeast] hospitalists really caused any problems for the coordinated care of” patients in the many years that they worked together.
It also “does not seem to necessarily serve the community to quickly sever the artery between the services that ACS provides and the services that JNE provides,” Judge Scott wrote.
She added that she would consider another motion from Alliance if the practice makes stronger arguments illustrating antitrust violations and demonstrating irreparable harm.
Currently, Dr. Chasky and Mr. Frier are considering their next steps in the case. The oncologists said they can appeal the judge’s decision or file a new complaint.
Meanwhile, Dr. Chasky and his four colleagues requested and were granted internal medicine privileges at Jefferson Health-Northeast, but given the considerable overlap between oncology and internal medicine, the line between what they can and cannot do remains unclear.
“It’s a mess,” he said.
A familiar story
Large health care entities have increasingly worked to push out or swallow up smaller, independent practices for years.
“What Dr. Chasky and his practice are going through is a little bit more of an aggressive version of what’s going on in the rest of the country,” said Michael Diaz, MD, a community oncologist at Florida Cancer Specialists, the largest independent medical oncology/hematology group in the United States. “The larger institutional hospitals try to make it a closed system so they can keep everything in-house and refer to their own physicians.”
The incentive, Dr. Diaz said, is the financial windfall that Section 340B of the 1992 Public Health Service Act generates for hospital-based oncology services at nonprofit hospitals, such as the Jefferson Health-Northeast facilities.
The 340B program allows nonprofit hospitals to buy primarily outpatient oncology drugs at steep discounts, sometimes 50% or more, and be reimbursed at full price.
When launched in 1992, the program was meant to help a handful of safety-net hospitals cover the cost of charity care, and now approximately more than half of U.S. hospitals participate in the program, particularly after requirements were loosened by the Affordable Care Act. But there’s little transparency on how the money is spent.
Critics say the incentives have created a feeding frenzy among 340B hospitals to either acquire outpatient oncology practices or take their business because of the particularly high margins on oncology drugs. There are similar incentives for hospital-based infusion centers.
In its lawsuit, Alliance alleged that such incentives are what motivated Jefferson’s recent actions.
“It’s all about the money at the end of the day,” said Christian Thomas, MD, a community oncologist with New England Cancer Specialists, Scarborough, Maine, who, like Dr. Diaz, said he’s seen the dynamic play out repeatedly in his career.
The American Hospital Association has been a vigorous defender of 340B in the courts and elsewhere, but the Association’s communications staff had little to say when this news organization reached out about the Jefferson-Alliance situation, except that they do not comment on “specific hospital circumstance.”
Reverberations around the country
Many community oncologists are keeping close tabs on the Jefferson-Alliance situation.
“Our group has been watching Jefferson closely because our [local] hospital is following the same playbook, but they have not yet gone after our privileges,” said Scott Herbert, MD, a community oncologist with the independent Nexus Health system, Sante Fe, N.M.
Dr. Herbert was referring to what has happened since he and his colleagues declined to renew an exclusive provider agreement early this year with St. Vincent Regional Medical Center, a nonprofit hospital in Sante Fe. The agreement allowed the hospital to take advantage of the 340B program because Nexus oncologists acted on its behalf.
St. Vincent’s owner, Christus Health, did not respond to inquiries from this news organization.
Nexus let the contract lapse because its oncologists wanted to provide services at a second, newer hospital in Santa Fe where some of their patients had begun seeking treatment.
The nonprofit hospital in Sante Fe is now building its own oncology practice. Similar to Dr. Chasky’s experience in Philadelphia, Dr. Herbert said his group has seen referrals from the hospital dry up and existing patients rechanneled to the hospital’s oncologists.
“We found over 109 patients in January and February that were referred to one of our docs that got rerouted to one of their docs,” he said.
Dr. Herbert has sent cease-and-desist letters, but “after we saw what Jefferson did, my group said, ‘You better back off of the hospital, or it’s going to take our privileges.’ ”
The Jefferson situation “is sending a message,” he said. “Frankly, we’ve been terrified” at the thought of losing privileges there. “It’s the busiest hospital in our area.”
The future of community oncology
Despite the challenges, Mr. Ferreyros at the Community Oncology Alliance remains optimistic about the future of independent oncology.
Under the competitive pressures, a lot of independent oncology practices have folded in recent years, but the ones that remain are strong. Payers are also increasingly noticing that community oncology practices are less expensive than hospital-based practices for comparable care, he said.
Relationships with hospitals aren’t always adversarial, either. “A lot of practices have collaborative agreements with local hospitals” that work out well, Mr. Ferreyros said, adding that sometimes hospitals even hand over oncology care to local independents after finding that starting and maintaining an oncology service is harder than they imagined.
“The last two decades have been difficult,” but the remaining community oncology practices “are going strong,” he said, and “we’ve never seen more engagement on our issues,” particularly around the issue of cost savings.
A version of this article first appeared on Medscape.com.
How to think about second-line therapy in NSCLC
This transcript has been edited for clarity.
I’ve been thinking lately about treatments after initial therapy for non–small cell lung cancers, what people often call second-line therapy.
I think the first thought is that, for all the regimens that are available and tested, the results are clearly not as good as seen with first-line therapy. I’ll get into some specifics in a second. That being the case, it’s really important to make the best choice for first-line therapy.
The second thing that is absolutely critical is to very carefully assess when that first-line therapy has stopped working and whether there is a need for a new systemic therapy. We very often have these situations where there is an oligoprogression, and by treating a single symptomatic lesion, you may get the patient in a very good place and may continue initial therapy. Very often, there is inconsequential growth of the cancer.
For example, if there is a 21% increase in the size of a primary tumor that is not associated with any symptoms in a person who is living their life and is not having any severe side effects, you have to think long and hard about changing that therapy. I wouldn’t even give a consolidative therapy there if they’re really doing well. Obviously, consolidative therapies are a new therapy, and they have their side effects with them as well.
With second-line therapy, sadly, none of them have a huge benefit anywhere near what we see in first line. All the rates of response are well under 50%. Just getting into it, you’re not going to shrink the cancer by more than 30% in the majority of patients, so you have to think long and hard about making that switch.
Second, our standard still remains docetaxel, and the numbers on docetaxel are really not great. It’s about a 15% rate of response and a median survival of about 5 months. Now, by adding other RET drugs to docetaxel, you can achieve better results. By adding ramucirumab, for example, the response rate just about doubles and the duration of response and progression-free survival both go up by a few months.
For patients who have KRAS G12C, in the randomized trial that has been done so far, over docetaxel, you get, again, a doubling of response. For patients where response is important, you really double that response rate, but also you get an improvement in median progression-free survival by, again, 2-3 months. There is benefit there in terms of response and progression-free survival; however, it’s not huge.
Please remember, if you’re choosing to use docetaxel, to think about using alternative dosages and schedules. When you look at the course of a person treated with docetaxel over, let’s say, a 6-month period, you often see that doses are held. When you look at the total dose, it’s very similar to an every-2-week dose of a lower amount. I routinely give a 60-mg flat dose every 2 weeks.
I urge you to look at the progress of one of your patients over a 6-month period who was given the 75-mg dose. Many of those doses end up getting held. When all is said and done, you give a lower dose over that whole time from that 75-mg dose. Giving 35 mg/m2 or a 60-mg flat dose every 2 weeks, you end up getting almost exactly the same amount of docetaxel. There’s really no convincing evidence that the higher dose is better. It’s clearly harder on the patient.
I’ve shared some thoughts about second-line therapy. We really have to do better. Please make sure that your first-line therapy is the best you can give. Make sure you’ve gotten everything out of that first-line therapy and that it will be continued as long as possible, as long as you and the patient have concluded that there’s benefit. When you do switch, try to give the most effective regimen that you have, which would be docetaxel with ramucirumab, or for patients with KRAS G12C, giving adagrasib or sotorasib at this point.
Dr. Kris is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York. He reported conflicts of interest with AstraZeneca, Roche/Genentech, Ariad Pharmaceuticals, Pfizer, and PUMA.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’ve been thinking lately about treatments after initial therapy for non–small cell lung cancers, what people often call second-line therapy.
I think the first thought is that, for all the regimens that are available and tested, the results are clearly not as good as seen with first-line therapy. I’ll get into some specifics in a second. That being the case, it’s really important to make the best choice for first-line therapy.
The second thing that is absolutely critical is to very carefully assess when that first-line therapy has stopped working and whether there is a need for a new systemic therapy. We very often have these situations where there is an oligoprogression, and by treating a single symptomatic lesion, you may get the patient in a very good place and may continue initial therapy. Very often, there is inconsequential growth of the cancer.
For example, if there is a 21% increase in the size of a primary tumor that is not associated with any symptoms in a person who is living their life and is not having any severe side effects, you have to think long and hard about changing that therapy. I wouldn’t even give a consolidative therapy there if they’re really doing well. Obviously, consolidative therapies are a new therapy, and they have their side effects with them as well.
With second-line therapy, sadly, none of them have a huge benefit anywhere near what we see in first line. All the rates of response are well under 50%. Just getting into it, you’re not going to shrink the cancer by more than 30% in the majority of patients, so you have to think long and hard about making that switch.
Second, our standard still remains docetaxel, and the numbers on docetaxel are really not great. It’s about a 15% rate of response and a median survival of about 5 months. Now, by adding other RET drugs to docetaxel, you can achieve better results. By adding ramucirumab, for example, the response rate just about doubles and the duration of response and progression-free survival both go up by a few months.
For patients who have KRAS G12C, in the randomized trial that has been done so far, over docetaxel, you get, again, a doubling of response. For patients where response is important, you really double that response rate, but also you get an improvement in median progression-free survival by, again, 2-3 months. There is benefit there in terms of response and progression-free survival; however, it’s not huge.
Please remember, if you’re choosing to use docetaxel, to think about using alternative dosages and schedules. When you look at the course of a person treated with docetaxel over, let’s say, a 6-month period, you often see that doses are held. When you look at the total dose, it’s very similar to an every-2-week dose of a lower amount. I routinely give a 60-mg flat dose every 2 weeks.
I urge you to look at the progress of one of your patients over a 6-month period who was given the 75-mg dose. Many of those doses end up getting held. When all is said and done, you give a lower dose over that whole time from that 75-mg dose. Giving 35 mg/m2 or a 60-mg flat dose every 2 weeks, you end up getting almost exactly the same amount of docetaxel. There’s really no convincing evidence that the higher dose is better. It’s clearly harder on the patient.
I’ve shared some thoughts about second-line therapy. We really have to do better. Please make sure that your first-line therapy is the best you can give. Make sure you’ve gotten everything out of that first-line therapy and that it will be continued as long as possible, as long as you and the patient have concluded that there’s benefit. When you do switch, try to give the most effective regimen that you have, which would be docetaxel with ramucirumab, or for patients with KRAS G12C, giving adagrasib or sotorasib at this point.
Dr. Kris is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York. He reported conflicts of interest with AstraZeneca, Roche/Genentech, Ariad Pharmaceuticals, Pfizer, and PUMA.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’ve been thinking lately about treatments after initial therapy for non–small cell lung cancers, what people often call second-line therapy.
I think the first thought is that, for all the regimens that are available and tested, the results are clearly not as good as seen with first-line therapy. I’ll get into some specifics in a second. That being the case, it’s really important to make the best choice for first-line therapy.
The second thing that is absolutely critical is to very carefully assess when that first-line therapy has stopped working and whether there is a need for a new systemic therapy. We very often have these situations where there is an oligoprogression, and by treating a single symptomatic lesion, you may get the patient in a very good place and may continue initial therapy. Very often, there is inconsequential growth of the cancer.
For example, if there is a 21% increase in the size of a primary tumor that is not associated with any symptoms in a person who is living their life and is not having any severe side effects, you have to think long and hard about changing that therapy. I wouldn’t even give a consolidative therapy there if they’re really doing well. Obviously, consolidative therapies are a new therapy, and they have their side effects with them as well.
With second-line therapy, sadly, none of them have a huge benefit anywhere near what we see in first line. All the rates of response are well under 50%. Just getting into it, you’re not going to shrink the cancer by more than 30% in the majority of patients, so you have to think long and hard about making that switch.
Second, our standard still remains docetaxel, and the numbers on docetaxel are really not great. It’s about a 15% rate of response and a median survival of about 5 months. Now, by adding other RET drugs to docetaxel, you can achieve better results. By adding ramucirumab, for example, the response rate just about doubles and the duration of response and progression-free survival both go up by a few months.
For patients who have KRAS G12C, in the randomized trial that has been done so far, over docetaxel, you get, again, a doubling of response. For patients where response is important, you really double that response rate, but also you get an improvement in median progression-free survival by, again, 2-3 months. There is benefit there in terms of response and progression-free survival; however, it’s not huge.
Please remember, if you’re choosing to use docetaxel, to think about using alternative dosages and schedules. When you look at the course of a person treated with docetaxel over, let’s say, a 6-month period, you often see that doses are held. When you look at the total dose, it’s very similar to an every-2-week dose of a lower amount. I routinely give a 60-mg flat dose every 2 weeks.
I urge you to look at the progress of one of your patients over a 6-month period who was given the 75-mg dose. Many of those doses end up getting held. When all is said and done, you give a lower dose over that whole time from that 75-mg dose. Giving 35 mg/m2 or a 60-mg flat dose every 2 weeks, you end up getting almost exactly the same amount of docetaxel. There’s really no convincing evidence that the higher dose is better. It’s clearly harder on the patient.
I’ve shared some thoughts about second-line therapy. We really have to do better. Please make sure that your first-line therapy is the best you can give. Make sure you’ve gotten everything out of that first-line therapy and that it will be continued as long as possible, as long as you and the patient have concluded that there’s benefit. When you do switch, try to give the most effective regimen that you have, which would be docetaxel with ramucirumab, or for patients with KRAS G12C, giving adagrasib or sotorasib at this point.
Dr. Kris is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York. He reported conflicts of interest with AstraZeneca, Roche/Genentech, Ariad Pharmaceuticals, Pfizer, and PUMA.
A version of this article first appeared on Medscape.com.
More phase 3 data support use of nemolizumab for prurigo nodularis
reported at the annual Congress of the European Academy of Dermatology and Venereology.
In the OLYMPIA 1 study, clinically significant improvements in both itch and skin lesions were seen after 16 weeks of treatment with nemolizumab compared with placebo (P < .0001).
Indeed, among the 286 patients who participated in the trial (190 on nemolizumab and 96 on placebo), 58.4% of those treated with nemolizumab and 16.7% of those who received placebo had an improvement of 4 points or more in the weekly average peak pruritus numeric rating scale (PP-NRS) at week 16 (P < .0001).
Skin lesions were assessed using an investigators general assessment (IGA) score, where IGA success was defined as a score of 0/1 indicating clear or almost clear skin or where there had been at least a 2-point change from baseline values. Over a quarter (26.3%) of nemolizumab-treated patients met these criteria versus 7.3% for those on placebo (P = .0001).
“These results confirm the results of the OLYMPIA 2 study, the other phase 3 study, and now I hope you understand why we are so excited,” lead investigator Sonja Ständer, MD, of the Center for Chronic Pruritus at University Hospital Münster, Germany, said at the meeting, where she presented the data.
The OLYMPIA 2 study included 274 patients and the results showed a weekly average PP-NRS score improvement of 56.3% vs. 20.9% for placebo and IGA success in 37.7% and 11% of patients, respectively, at 16 weeks.
First-in-class therapy
“We know how difficult it is to treat patients; they are refractory to treatment, frustrated, and this really impacts them regarding their quality of life,” said Dr. Ständer. New options are needed to help patients, and nemolizumab, a first-in-class interleukin-31 (IL-31) receptor alpha antagonist, is one treatment that may answer this call.
Prurigo nodularis is a chronic neuroimmune skin condition characterized by severe itch and multiple nodular skin lesions, Dr. Ständer explained. She added that there is evidence that IL-31 has a key role to play in the development of itch, and in differentiation of keratinocytes, type 2 and type 17 immune responses, and fibrosis associated with the condition.
The OLYMPIA 1 and 2 trials are part of a large developmental program that includes two ongoing trials. One is assessing the durability of response over 24 weeks in 40 patients and the other is a long-term extension trial involving 450 patients from the OLYMPIA 1 and 2 trials.
Inclusion criteria and additional results
For inclusion in the study, adults with prurigo nodularis for at least 6 months had to have 20 or more nodules on the body with a bilateral distribution, an IGA score of 3 or more, and an average PP-NRS of 7 or higher. The latter “was really a high bar for them to qualify for the trial,” said Dr. Ständer.
After an initial 4-week screening period, patients were randomly assigned to 24 weeks of treatment with nemolizumab or placebo given as a subcutaneous injection every 4 weeks. An 8-week “off-treatment” period followed.
The nemolizumab dose was based on the patient’s body weight, with patients weighing less than 90 kg (198 pounds) getting a loading dose of 60 mg followed by further doses of 30 mg; while patients weighing 90 kg or more receiving 50 mg of nemolizumab.
Dr. Ständer reported that nemolizumab met all of the trials’ secondary endpoints; this included at least a 4-point improvement in sleep disturbance. She noted that changes in itch and subsequent sleep disturbance occurred early, at 4 weeks of treatment – after just one injection of nemolizumab.
The response rates seen in the moderate to severe prurigo nodularis population studies are quite unique when compared with conventional therapies, Dr. Ständer maintained. “We’ve never seen something like this before.”
No safety concerns
No significant difference in tolerability was seen between the nemolizumab and placebo groups, Dr. Ständer observed. Any adverse event occurred in 71.7% and 65.3% of patients, respectively, and serious adverse events in 8.6% and 10.5%.
There was a similar rate of adverse events leading to discontinuation, respectively (4.8% vs. 4.2%).
Headache was seen more frequently among those on nemolizumab than those on placebo (7.0% vs. 2.1%), and there was a higher number of eczema cases among those on nemolizumab (5.3% vs. 1.1%). The latter is somewhat paradoxical because nemolizumab is also being studied as a treatment for atopic dermatitis, with good results seen in phase 3 trials. Asked about this finding after her presentation, Dr. Ständer said “we are following up on that to know exactly what is going on; this is a side effect of nemolizumab that is seen also with other biologics.”
JAK inhibitor trial for PN, CPUO
Nemolizumab is not the only promising new approach to treating prurigo nodularis. During a separate late-breaking news session at the meeting, Shawn Kwatra, MD, director of the Johns Hopkins Itch Center in Baltimore, presented “dramatic” data from a “proof-of-concept” phase 2 study with the Janus kinase (JAK) inhibitor abrocitinib (Cibinqo), which is approved for atopic dermatitis in the United States and Europe.
The investigator-initiated trial took a different approach from most other trials, Dr. Kwatra said. The starting point was to look at studying multiple rather than single dermatologic diseases that were perhaps being left a little by the wayside but may share some common ground. Those two diseases were prurigo nodularis and chronic pruritus of unknown origin (CPUO).
“They’re actually very analogous conditions in the way we treat, so I thought those would be a good pair,” Dr. Kwatra said, noting that there were several studies that made him think that JAK inhibition “would be an interesting concept to try.”
On that basis, 10 women with prurigo nodularis (mean age, 58 years) and two women and eight men with CPUO (mean age, 70 years) were recruited and all were treated with abrocitinib at a once-daily oral dose of 200 mg for 12 weeks.
“They all had really intense itch,” before treatment, Dr. Kwatra said. The mean baseline PP-NRS was 9.2 and 8.2 in the prurigo nodularis and CPUO groups, respectively. By the end of treatment, however, “the improvement in itch was pretty dramatic,” especially for prurigo nodularis, he said.
At 12 weeks, the PP-NRS score had fallen to 2.0 in the prurigo nodularis group, equating to a significant 78% change from baseline (P < .001). And, in the CPUO group, the 12-week PP-NRS score was 3.8, nearly a 54% drop from baseline (P = .01).
Sleep disturbance was improved for both conditions, and in the patients with prurigo nodularis, there were improvements in skin lesions. Looking at the patients who responded to treatment, Dr. Kwatra noted that “if you responded, you respond fast, and you respond almost entirely.”
Additional findings from cutaneous transcriptome analysis showed that JAK inhibition with abrocitinib was modulating Th1-, Th2-, Th17-, and Th22-mediated pathways in both groups of patients.
The overall frequency of adverse events was low, and no serious adverse events occurred.
Commenting on the potential use of abrocitinib in managing patients with PN and CPUO, Tiago dos Reis Matos, MD, PhD, MSc, Amsterdam University Medical Centers, told this news organization that JAK1 inhibitors “are showing promising results in treating several diseases.”
Dr. Matos, who was not involved in the study, added that JAK inhibition was “of special interest in prurigo nodularis and chronic pruritus, since these are some of the most difficult diseases to treat with limited therapeutic options.”
Dr. Kwatra observed: “Obviously, we need further development. But we also have clues here about how to design phase 3 trials.”
Galderma funded the OLYMPIA 1 and 2 studies. Dr. Ständer was an investigator for the trial and reported serving as a consultant, speaker, or investigator for multiple pharmaceutical companies, including Galderma.
Johns Hopkins University supported the abrocitinib study with funding from Pfizer. Dr. Kwatra is an advisory board member or consultant to several pharmaceutical companies and is an investigator for Galderma, Incyte, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
reported at the annual Congress of the European Academy of Dermatology and Venereology.
In the OLYMPIA 1 study, clinically significant improvements in both itch and skin lesions were seen after 16 weeks of treatment with nemolizumab compared with placebo (P < .0001).
Indeed, among the 286 patients who participated in the trial (190 on nemolizumab and 96 on placebo), 58.4% of those treated with nemolizumab and 16.7% of those who received placebo had an improvement of 4 points or more in the weekly average peak pruritus numeric rating scale (PP-NRS) at week 16 (P < .0001).
Skin lesions were assessed using an investigators general assessment (IGA) score, where IGA success was defined as a score of 0/1 indicating clear or almost clear skin or where there had been at least a 2-point change from baseline values. Over a quarter (26.3%) of nemolizumab-treated patients met these criteria versus 7.3% for those on placebo (P = .0001).
“These results confirm the results of the OLYMPIA 2 study, the other phase 3 study, and now I hope you understand why we are so excited,” lead investigator Sonja Ständer, MD, of the Center for Chronic Pruritus at University Hospital Münster, Germany, said at the meeting, where she presented the data.
The OLYMPIA 2 study included 274 patients and the results showed a weekly average PP-NRS score improvement of 56.3% vs. 20.9% for placebo and IGA success in 37.7% and 11% of patients, respectively, at 16 weeks.
First-in-class therapy
“We know how difficult it is to treat patients; they are refractory to treatment, frustrated, and this really impacts them regarding their quality of life,” said Dr. Ständer. New options are needed to help patients, and nemolizumab, a first-in-class interleukin-31 (IL-31) receptor alpha antagonist, is one treatment that may answer this call.
Prurigo nodularis is a chronic neuroimmune skin condition characterized by severe itch and multiple nodular skin lesions, Dr. Ständer explained. She added that there is evidence that IL-31 has a key role to play in the development of itch, and in differentiation of keratinocytes, type 2 and type 17 immune responses, and fibrosis associated with the condition.
The OLYMPIA 1 and 2 trials are part of a large developmental program that includes two ongoing trials. One is assessing the durability of response over 24 weeks in 40 patients and the other is a long-term extension trial involving 450 patients from the OLYMPIA 1 and 2 trials.
Inclusion criteria and additional results
For inclusion in the study, adults with prurigo nodularis for at least 6 months had to have 20 or more nodules on the body with a bilateral distribution, an IGA score of 3 or more, and an average PP-NRS of 7 or higher. The latter “was really a high bar for them to qualify for the trial,” said Dr. Ständer.
After an initial 4-week screening period, patients were randomly assigned to 24 weeks of treatment with nemolizumab or placebo given as a subcutaneous injection every 4 weeks. An 8-week “off-treatment” period followed.
The nemolizumab dose was based on the patient’s body weight, with patients weighing less than 90 kg (198 pounds) getting a loading dose of 60 mg followed by further doses of 30 mg; while patients weighing 90 kg or more receiving 50 mg of nemolizumab.
Dr. Ständer reported that nemolizumab met all of the trials’ secondary endpoints; this included at least a 4-point improvement in sleep disturbance. She noted that changes in itch and subsequent sleep disturbance occurred early, at 4 weeks of treatment – after just one injection of nemolizumab.
The response rates seen in the moderate to severe prurigo nodularis population studies are quite unique when compared with conventional therapies, Dr. Ständer maintained. “We’ve never seen something like this before.”
No safety concerns
No significant difference in tolerability was seen between the nemolizumab and placebo groups, Dr. Ständer observed. Any adverse event occurred in 71.7% and 65.3% of patients, respectively, and serious adverse events in 8.6% and 10.5%.
There was a similar rate of adverse events leading to discontinuation, respectively (4.8% vs. 4.2%).
Headache was seen more frequently among those on nemolizumab than those on placebo (7.0% vs. 2.1%), and there was a higher number of eczema cases among those on nemolizumab (5.3% vs. 1.1%). The latter is somewhat paradoxical because nemolizumab is also being studied as a treatment for atopic dermatitis, with good results seen in phase 3 trials. Asked about this finding after her presentation, Dr. Ständer said “we are following up on that to know exactly what is going on; this is a side effect of nemolizumab that is seen also with other biologics.”
JAK inhibitor trial for PN, CPUO
Nemolizumab is not the only promising new approach to treating prurigo nodularis. During a separate late-breaking news session at the meeting, Shawn Kwatra, MD, director of the Johns Hopkins Itch Center in Baltimore, presented “dramatic” data from a “proof-of-concept” phase 2 study with the Janus kinase (JAK) inhibitor abrocitinib (Cibinqo), which is approved for atopic dermatitis in the United States and Europe.
The investigator-initiated trial took a different approach from most other trials, Dr. Kwatra said. The starting point was to look at studying multiple rather than single dermatologic diseases that were perhaps being left a little by the wayside but may share some common ground. Those two diseases were prurigo nodularis and chronic pruritus of unknown origin (CPUO).
“They’re actually very analogous conditions in the way we treat, so I thought those would be a good pair,” Dr. Kwatra said, noting that there were several studies that made him think that JAK inhibition “would be an interesting concept to try.”
On that basis, 10 women with prurigo nodularis (mean age, 58 years) and two women and eight men with CPUO (mean age, 70 years) were recruited and all were treated with abrocitinib at a once-daily oral dose of 200 mg for 12 weeks.
“They all had really intense itch,” before treatment, Dr. Kwatra said. The mean baseline PP-NRS was 9.2 and 8.2 in the prurigo nodularis and CPUO groups, respectively. By the end of treatment, however, “the improvement in itch was pretty dramatic,” especially for prurigo nodularis, he said.
At 12 weeks, the PP-NRS score had fallen to 2.0 in the prurigo nodularis group, equating to a significant 78% change from baseline (P < .001). And, in the CPUO group, the 12-week PP-NRS score was 3.8, nearly a 54% drop from baseline (P = .01).
Sleep disturbance was improved for both conditions, and in the patients with prurigo nodularis, there were improvements in skin lesions. Looking at the patients who responded to treatment, Dr. Kwatra noted that “if you responded, you respond fast, and you respond almost entirely.”
Additional findings from cutaneous transcriptome analysis showed that JAK inhibition with abrocitinib was modulating Th1-, Th2-, Th17-, and Th22-mediated pathways in both groups of patients.
The overall frequency of adverse events was low, and no serious adverse events occurred.
Commenting on the potential use of abrocitinib in managing patients with PN and CPUO, Tiago dos Reis Matos, MD, PhD, MSc, Amsterdam University Medical Centers, told this news organization that JAK1 inhibitors “are showing promising results in treating several diseases.”
Dr. Matos, who was not involved in the study, added that JAK inhibition was “of special interest in prurigo nodularis and chronic pruritus, since these are some of the most difficult diseases to treat with limited therapeutic options.”
Dr. Kwatra observed: “Obviously, we need further development. But we also have clues here about how to design phase 3 trials.”
Galderma funded the OLYMPIA 1 and 2 studies. Dr. Ständer was an investigator for the trial and reported serving as a consultant, speaker, or investigator for multiple pharmaceutical companies, including Galderma.
Johns Hopkins University supported the abrocitinib study with funding from Pfizer. Dr. Kwatra is an advisory board member or consultant to several pharmaceutical companies and is an investigator for Galderma, Incyte, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
reported at the annual Congress of the European Academy of Dermatology and Venereology.
In the OLYMPIA 1 study, clinically significant improvements in both itch and skin lesions were seen after 16 weeks of treatment with nemolizumab compared with placebo (P < .0001).
Indeed, among the 286 patients who participated in the trial (190 on nemolizumab and 96 on placebo), 58.4% of those treated with nemolizumab and 16.7% of those who received placebo had an improvement of 4 points or more in the weekly average peak pruritus numeric rating scale (PP-NRS) at week 16 (P < .0001).
Skin lesions were assessed using an investigators general assessment (IGA) score, where IGA success was defined as a score of 0/1 indicating clear or almost clear skin or where there had been at least a 2-point change from baseline values. Over a quarter (26.3%) of nemolizumab-treated patients met these criteria versus 7.3% for those on placebo (P = .0001).
“These results confirm the results of the OLYMPIA 2 study, the other phase 3 study, and now I hope you understand why we are so excited,” lead investigator Sonja Ständer, MD, of the Center for Chronic Pruritus at University Hospital Münster, Germany, said at the meeting, where she presented the data.
The OLYMPIA 2 study included 274 patients and the results showed a weekly average PP-NRS score improvement of 56.3% vs. 20.9% for placebo and IGA success in 37.7% and 11% of patients, respectively, at 16 weeks.
First-in-class therapy
“We know how difficult it is to treat patients; they are refractory to treatment, frustrated, and this really impacts them regarding their quality of life,” said Dr. Ständer. New options are needed to help patients, and nemolizumab, a first-in-class interleukin-31 (IL-31) receptor alpha antagonist, is one treatment that may answer this call.
Prurigo nodularis is a chronic neuroimmune skin condition characterized by severe itch and multiple nodular skin lesions, Dr. Ständer explained. She added that there is evidence that IL-31 has a key role to play in the development of itch, and in differentiation of keratinocytes, type 2 and type 17 immune responses, and fibrosis associated with the condition.
The OLYMPIA 1 and 2 trials are part of a large developmental program that includes two ongoing trials. One is assessing the durability of response over 24 weeks in 40 patients and the other is a long-term extension trial involving 450 patients from the OLYMPIA 1 and 2 trials.
Inclusion criteria and additional results
For inclusion in the study, adults with prurigo nodularis for at least 6 months had to have 20 or more nodules on the body with a bilateral distribution, an IGA score of 3 or more, and an average PP-NRS of 7 or higher. The latter “was really a high bar for them to qualify for the trial,” said Dr. Ständer.
After an initial 4-week screening period, patients were randomly assigned to 24 weeks of treatment with nemolizumab or placebo given as a subcutaneous injection every 4 weeks. An 8-week “off-treatment” period followed.
The nemolizumab dose was based on the patient’s body weight, with patients weighing less than 90 kg (198 pounds) getting a loading dose of 60 mg followed by further doses of 30 mg; while patients weighing 90 kg or more receiving 50 mg of nemolizumab.
Dr. Ständer reported that nemolizumab met all of the trials’ secondary endpoints; this included at least a 4-point improvement in sleep disturbance. She noted that changes in itch and subsequent sleep disturbance occurred early, at 4 weeks of treatment – after just one injection of nemolizumab.
The response rates seen in the moderate to severe prurigo nodularis population studies are quite unique when compared with conventional therapies, Dr. Ständer maintained. “We’ve never seen something like this before.”
No safety concerns
No significant difference in tolerability was seen between the nemolizumab and placebo groups, Dr. Ständer observed. Any adverse event occurred in 71.7% and 65.3% of patients, respectively, and serious adverse events in 8.6% and 10.5%.
There was a similar rate of adverse events leading to discontinuation, respectively (4.8% vs. 4.2%).
Headache was seen more frequently among those on nemolizumab than those on placebo (7.0% vs. 2.1%), and there was a higher number of eczema cases among those on nemolizumab (5.3% vs. 1.1%). The latter is somewhat paradoxical because nemolizumab is also being studied as a treatment for atopic dermatitis, with good results seen in phase 3 trials. Asked about this finding after her presentation, Dr. Ständer said “we are following up on that to know exactly what is going on; this is a side effect of nemolizumab that is seen also with other biologics.”
JAK inhibitor trial for PN, CPUO
Nemolizumab is not the only promising new approach to treating prurigo nodularis. During a separate late-breaking news session at the meeting, Shawn Kwatra, MD, director of the Johns Hopkins Itch Center in Baltimore, presented “dramatic” data from a “proof-of-concept” phase 2 study with the Janus kinase (JAK) inhibitor abrocitinib (Cibinqo), which is approved for atopic dermatitis in the United States and Europe.
The investigator-initiated trial took a different approach from most other trials, Dr. Kwatra said. The starting point was to look at studying multiple rather than single dermatologic diseases that were perhaps being left a little by the wayside but may share some common ground. Those two diseases were prurigo nodularis and chronic pruritus of unknown origin (CPUO).
“They’re actually very analogous conditions in the way we treat, so I thought those would be a good pair,” Dr. Kwatra said, noting that there were several studies that made him think that JAK inhibition “would be an interesting concept to try.”
On that basis, 10 women with prurigo nodularis (mean age, 58 years) and two women and eight men with CPUO (mean age, 70 years) were recruited and all were treated with abrocitinib at a once-daily oral dose of 200 mg for 12 weeks.
“They all had really intense itch,” before treatment, Dr. Kwatra said. The mean baseline PP-NRS was 9.2 and 8.2 in the prurigo nodularis and CPUO groups, respectively. By the end of treatment, however, “the improvement in itch was pretty dramatic,” especially for prurigo nodularis, he said.
At 12 weeks, the PP-NRS score had fallen to 2.0 in the prurigo nodularis group, equating to a significant 78% change from baseline (P < .001). And, in the CPUO group, the 12-week PP-NRS score was 3.8, nearly a 54% drop from baseline (P = .01).
Sleep disturbance was improved for both conditions, and in the patients with prurigo nodularis, there were improvements in skin lesions. Looking at the patients who responded to treatment, Dr. Kwatra noted that “if you responded, you respond fast, and you respond almost entirely.”
Additional findings from cutaneous transcriptome analysis showed that JAK inhibition with abrocitinib was modulating Th1-, Th2-, Th17-, and Th22-mediated pathways in both groups of patients.
The overall frequency of adverse events was low, and no serious adverse events occurred.
Commenting on the potential use of abrocitinib in managing patients with PN and CPUO, Tiago dos Reis Matos, MD, PhD, MSc, Amsterdam University Medical Centers, told this news organization that JAK1 inhibitors “are showing promising results in treating several diseases.”
Dr. Matos, who was not involved in the study, added that JAK inhibition was “of special interest in prurigo nodularis and chronic pruritus, since these are some of the most difficult diseases to treat with limited therapeutic options.”
Dr. Kwatra observed: “Obviously, we need further development. But we also have clues here about how to design phase 3 trials.”
Galderma funded the OLYMPIA 1 and 2 studies. Dr. Ständer was an investigator for the trial and reported serving as a consultant, speaker, or investigator for multiple pharmaceutical companies, including Galderma.
Johns Hopkins University supported the abrocitinib study with funding from Pfizer. Dr. Kwatra is an advisory board member or consultant to several pharmaceutical companies and is an investigator for Galderma, Incyte, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Birch bark–derived treatment reduces daily dressings in patients with epidermolysis bullosa
Additional when compared with a control gel.
In a final, post hoc analysis to come from the trial, 15 of 45 (33%) patients treated with Oleogel-S10 versus 5 of 48 (10.4%) treated with the control gel were reported as no longer needing daily dressing changes at 45 days of follow-up.
Moreover, the effect was sustained, with similar percentages of patients no longer requiring daily dressing changes at 60 days (34% vs. 13%, respectively) and 90 days (36% vs. 11%) of follow-up.
The mean reduction in daily dressing changes was 1.36 for Oleogel-S10 and 0.41 for the control gel (P = .005).
“Patients who, in the beginning, had daily dressing changes had almost three fewer dressing changes every 2 weeks if they were treated with Oleogel-S10,” Dimitra Kiritsi, MD, PhD, of the department of dermatology at the University of Freiburg (Germany), reported at the annual congress of the European Academy of Dermatology and Venereology. By comparison, patients in the control group had just one fewer daily dressing change in 2 weeks.
“You might say okay, but what does this mean in terms of time?” added Dr. Kiritsi. Using historical data on the time required for whole body care (Orphanet J Rare Dis. 2020 Jan 3. doi: 10.1186/s13023-019-1279-y), it was estimated that treatment with Oleogel-S10 was associated with an overall time-saving per week of 11 hours (6.6 hours for the patient and 4.4 hours for the caregiver) and use of the control gel was associated with an overall time-saving of 4 hours (2.4 hours for the patient and 1.6 hours for the caregiver).
“This is, for our patients, important,” said Dr. Kiritsi, as “it is time that they can spend doing something nice with the family” instead, avoiding the pain and distress associated with frequent dressing changes.
Approved in Europe, not in the United States
Oleogel-S10, classified as an herbal product, contains triterpenes derived from birch bark extract, which have been formulated with sunflower oil to form a gel.
Despite being approved for use in Europe, Oleogel-S10 has not yet been approved to treat EB in the United States. The FDA did not approve Amryt Pharma’s new drug application in February 2022. The application had included data from the EASE trial.
EASE included 223 patients with dystrophic or junctional EB, including 156 children, at 58 sites in 28 countries. As such, this makes it the largest treatment study in this rare genetic disease to date.
The trial had consisted of an initial 90-day, double-blind treatment period, during which time 109 patients had used Oleogel-S10 and 114 had used a control gel. This was followed by a 24-month open-label phase, during which time all remaining patients (n = 205) had used Oleogel-S10 on top of their standard of care.
Dr. Kiritsi summarized the main results of the EASE trial as follows.
- Complete healing of target wounds (primary endpoint) in 41.3% of patients treated with Oleogel-S10 and 28.9% of patients treated with the control gel (P = .013).
- Improved total body wound burden measured by both Epidermolysis Bullosa Disease Activity and Scarring Index and Body Surface Area Percentage scores.
- Reduced frequency of dressing changes (1 less per 2 weeks for Oleogel-S10 versus 0 less per 2 weeks for control gel).
- Improved pain among participants aged 4 years and older while their dressings were being changed.
- Reduced rates of wound infection (0.9% Oleogel-S10 vs. 4.4% control gel).
- Similar rates of treatment-emergent adverse events (24.8% vs. 22.8%, respectively), which were mostly deemed to be mild or moderate.
The EASE study – an important trial for EB
EASE is an important trial for EB, the study’s principal investigator Dédée Murrell, MD, DSc, University of New South Wales, Sydney, has pointed out previously.
“This was the first EB study to meet its primary endpoint and demonstrated a statistically significant acceleration of target wound healing by day 45,” Dr. Murrell said in a press release issued by Amryt Pharma to coincide with the online publication of the trial results.
“In addition, the favorable trends we see with key secondary endpoints such as reduced wound burden, pain, and frequency of dressing changes are considered as being very meaningful for patients,” Dr. Murrell said.
The EASE study was funded by Amryt Research Limited. Dr. Kiritsi reported receiving honoraria or consultation fees from Amryt, RHEACELL GmbH, and Fibrx Derm. She also acknowledged grant or research support from DEBRA International, EB Research Partnership, Fritz-Thyssen Foundation, German Research Foundation, and RHEACELL. Dr. Murrell has ties to Amryt and Amicus and is a co-owner of the patent for topical sirolimus for EB simplex.
A version of this article appeared on Medscape.com.
Additional when compared with a control gel.
In a final, post hoc analysis to come from the trial, 15 of 45 (33%) patients treated with Oleogel-S10 versus 5 of 48 (10.4%) treated with the control gel were reported as no longer needing daily dressing changes at 45 days of follow-up.
Moreover, the effect was sustained, with similar percentages of patients no longer requiring daily dressing changes at 60 days (34% vs. 13%, respectively) and 90 days (36% vs. 11%) of follow-up.
The mean reduction in daily dressing changes was 1.36 for Oleogel-S10 and 0.41 for the control gel (P = .005).
“Patients who, in the beginning, had daily dressing changes had almost three fewer dressing changes every 2 weeks if they were treated with Oleogel-S10,” Dimitra Kiritsi, MD, PhD, of the department of dermatology at the University of Freiburg (Germany), reported at the annual congress of the European Academy of Dermatology and Venereology. By comparison, patients in the control group had just one fewer daily dressing change in 2 weeks.
“You might say okay, but what does this mean in terms of time?” added Dr. Kiritsi. Using historical data on the time required for whole body care (Orphanet J Rare Dis. 2020 Jan 3. doi: 10.1186/s13023-019-1279-y), it was estimated that treatment with Oleogel-S10 was associated with an overall time-saving per week of 11 hours (6.6 hours for the patient and 4.4 hours for the caregiver) and use of the control gel was associated with an overall time-saving of 4 hours (2.4 hours for the patient and 1.6 hours for the caregiver).
“This is, for our patients, important,” said Dr. Kiritsi, as “it is time that they can spend doing something nice with the family” instead, avoiding the pain and distress associated with frequent dressing changes.
Approved in Europe, not in the United States
Oleogel-S10, classified as an herbal product, contains triterpenes derived from birch bark extract, which have been formulated with sunflower oil to form a gel.
Despite being approved for use in Europe, Oleogel-S10 has not yet been approved to treat EB in the United States. The FDA did not approve Amryt Pharma’s new drug application in February 2022. The application had included data from the EASE trial.
EASE included 223 patients with dystrophic or junctional EB, including 156 children, at 58 sites in 28 countries. As such, this makes it the largest treatment study in this rare genetic disease to date.
The trial had consisted of an initial 90-day, double-blind treatment period, during which time 109 patients had used Oleogel-S10 and 114 had used a control gel. This was followed by a 24-month open-label phase, during which time all remaining patients (n = 205) had used Oleogel-S10 on top of their standard of care.
Dr. Kiritsi summarized the main results of the EASE trial as follows.
- Complete healing of target wounds (primary endpoint) in 41.3% of patients treated with Oleogel-S10 and 28.9% of patients treated with the control gel (P = .013).
- Improved total body wound burden measured by both Epidermolysis Bullosa Disease Activity and Scarring Index and Body Surface Area Percentage scores.
- Reduced frequency of dressing changes (1 less per 2 weeks for Oleogel-S10 versus 0 less per 2 weeks for control gel).
- Improved pain among participants aged 4 years and older while their dressings were being changed.
- Reduced rates of wound infection (0.9% Oleogel-S10 vs. 4.4% control gel).
- Similar rates of treatment-emergent adverse events (24.8% vs. 22.8%, respectively), which were mostly deemed to be mild or moderate.
The EASE study – an important trial for EB
EASE is an important trial for EB, the study’s principal investigator Dédée Murrell, MD, DSc, University of New South Wales, Sydney, has pointed out previously.
“This was the first EB study to meet its primary endpoint and demonstrated a statistically significant acceleration of target wound healing by day 45,” Dr. Murrell said in a press release issued by Amryt Pharma to coincide with the online publication of the trial results.
“In addition, the favorable trends we see with key secondary endpoints such as reduced wound burden, pain, and frequency of dressing changes are considered as being very meaningful for patients,” Dr. Murrell said.
The EASE study was funded by Amryt Research Limited. Dr. Kiritsi reported receiving honoraria or consultation fees from Amryt, RHEACELL GmbH, and Fibrx Derm. She also acknowledged grant or research support from DEBRA International, EB Research Partnership, Fritz-Thyssen Foundation, German Research Foundation, and RHEACELL. Dr. Murrell has ties to Amryt and Amicus and is a co-owner of the patent for topical sirolimus for EB simplex.
A version of this article appeared on Medscape.com.
Additional when compared with a control gel.
In a final, post hoc analysis to come from the trial, 15 of 45 (33%) patients treated with Oleogel-S10 versus 5 of 48 (10.4%) treated with the control gel were reported as no longer needing daily dressing changes at 45 days of follow-up.
Moreover, the effect was sustained, with similar percentages of patients no longer requiring daily dressing changes at 60 days (34% vs. 13%, respectively) and 90 days (36% vs. 11%) of follow-up.
The mean reduction in daily dressing changes was 1.36 for Oleogel-S10 and 0.41 for the control gel (P = .005).
“Patients who, in the beginning, had daily dressing changes had almost three fewer dressing changes every 2 weeks if they were treated with Oleogel-S10,” Dimitra Kiritsi, MD, PhD, of the department of dermatology at the University of Freiburg (Germany), reported at the annual congress of the European Academy of Dermatology and Venereology. By comparison, patients in the control group had just one fewer daily dressing change in 2 weeks.
“You might say okay, but what does this mean in terms of time?” added Dr. Kiritsi. Using historical data on the time required for whole body care (Orphanet J Rare Dis. 2020 Jan 3. doi: 10.1186/s13023-019-1279-y), it was estimated that treatment with Oleogel-S10 was associated with an overall time-saving per week of 11 hours (6.6 hours for the patient and 4.4 hours for the caregiver) and use of the control gel was associated with an overall time-saving of 4 hours (2.4 hours for the patient and 1.6 hours for the caregiver).
“This is, for our patients, important,” said Dr. Kiritsi, as “it is time that they can spend doing something nice with the family” instead, avoiding the pain and distress associated with frequent dressing changes.
Approved in Europe, not in the United States
Oleogel-S10, classified as an herbal product, contains triterpenes derived from birch bark extract, which have been formulated with sunflower oil to form a gel.
Despite being approved for use in Europe, Oleogel-S10 has not yet been approved to treat EB in the United States. The FDA did not approve Amryt Pharma’s new drug application in February 2022. The application had included data from the EASE trial.
EASE included 223 patients with dystrophic or junctional EB, including 156 children, at 58 sites in 28 countries. As such, this makes it the largest treatment study in this rare genetic disease to date.
The trial had consisted of an initial 90-day, double-blind treatment period, during which time 109 patients had used Oleogel-S10 and 114 had used a control gel. This was followed by a 24-month open-label phase, during which time all remaining patients (n = 205) had used Oleogel-S10 on top of their standard of care.
Dr. Kiritsi summarized the main results of the EASE trial as follows.
- Complete healing of target wounds (primary endpoint) in 41.3% of patients treated with Oleogel-S10 and 28.9% of patients treated with the control gel (P = .013).
- Improved total body wound burden measured by both Epidermolysis Bullosa Disease Activity and Scarring Index and Body Surface Area Percentage scores.
- Reduced frequency of dressing changes (1 less per 2 weeks for Oleogel-S10 versus 0 less per 2 weeks for control gel).
- Improved pain among participants aged 4 years and older while their dressings were being changed.
- Reduced rates of wound infection (0.9% Oleogel-S10 vs. 4.4% control gel).
- Similar rates of treatment-emergent adverse events (24.8% vs. 22.8%, respectively), which were mostly deemed to be mild or moderate.
The EASE study – an important trial for EB
EASE is an important trial for EB, the study’s principal investigator Dédée Murrell, MD, DSc, University of New South Wales, Sydney, has pointed out previously.
“This was the first EB study to meet its primary endpoint and demonstrated a statistically significant acceleration of target wound healing by day 45,” Dr. Murrell said in a press release issued by Amryt Pharma to coincide with the online publication of the trial results.
“In addition, the favorable trends we see with key secondary endpoints such as reduced wound burden, pain, and frequency of dressing changes are considered as being very meaningful for patients,” Dr. Murrell said.
The EASE study was funded by Amryt Research Limited. Dr. Kiritsi reported receiving honoraria or consultation fees from Amryt, RHEACELL GmbH, and Fibrx Derm. She also acknowledged grant or research support from DEBRA International, EB Research Partnership, Fritz-Thyssen Foundation, German Research Foundation, and RHEACELL. Dr. Murrell has ties to Amryt and Amicus and is a co-owner of the patent for topical sirolimus for EB simplex.
A version of this article appeared on Medscape.com.
FROM THE EADV CONGRESS









