User login
Addressing supply-demand mismatch in GI
Impacts of this supply-demand mismatch are felt daily in our GI practices as we strive to expand access in our clinics and endoscopy suites, particularly in rural and urban underserved communities. In gastroenterology, increased demand for care has been driven by a perfect storm of population growth, increased patient awareness of GI health, and rising incidence of digestive diseases.
Between 2019 and 2034, the U.S. population is expected to grow by 10.6%, while the population aged 65 and older expands by over 42%. Recent increases in the CRC screening–eligible population also have contributed to unprecedented demand for GI care. Furthermore, care delivery has become more complex and time-consuming with the evolution of personalized medicine and high prevalence of comorbid conditions. At the same time, we are faced with a dwindling supply of gastroenterology providers. In 2021, there were 15,678 practicing gastroenterologists in the U.S., over half of whom were 55 years or older. This translates to 1 gastroenterologist per 20,830 people captured in the U.S. Census.
Addressing this striking supply-demand mismatch in GI requires a multi-pronged approach that addresses its complex drivers. First and foremost, we must expand the number of GI fellowship training slots to boost our pipeline. There are approximately 1,840 GI fellows currently in training, a third of whom enter the workforce each year. While the number of GI fellowship slots in the GI fellowship match has slowly increased over time (from 525 available slots across 199 programs in 2019 to 657 slots across 230 programs in 2023), this incremental growth is dwarfed by overall need. Continued advocacy for increased funding to support expansion of training slots is necessary to further move the needle – such lobbying recently led to the addition of 1,000 new Medicare-supported graduate medical education positions across specialties over a 5-year period starting in 2020, illustrating that change is possible. At the same time, we must address the factors that are causing gastroenterologists to leave the workforce prematurely through early retirement or part-time work by investing in innovative solutions to address burnout, reduce administrative burdens, enhance the efficiency of care delivery, and maintain financial viability. By investing in our physician workforce and its sustainability, we can ensure that our profession is better prepared to meet the needs of our growing and increasingly complex patient population now and in the future.
We hope you enjoy the November issue of GI & Hepatology News and have a wonderful Thanksgiving.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Impacts of this supply-demand mismatch are felt daily in our GI practices as we strive to expand access in our clinics and endoscopy suites, particularly in rural and urban underserved communities. In gastroenterology, increased demand for care has been driven by a perfect storm of population growth, increased patient awareness of GI health, and rising incidence of digestive diseases.
Between 2019 and 2034, the U.S. population is expected to grow by 10.6%, while the population aged 65 and older expands by over 42%. Recent increases in the CRC screening–eligible population also have contributed to unprecedented demand for GI care. Furthermore, care delivery has become more complex and time-consuming with the evolution of personalized medicine and high prevalence of comorbid conditions. At the same time, we are faced with a dwindling supply of gastroenterology providers. In 2021, there were 15,678 practicing gastroenterologists in the U.S., over half of whom were 55 years or older. This translates to 1 gastroenterologist per 20,830 people captured in the U.S. Census.
Addressing this striking supply-demand mismatch in GI requires a multi-pronged approach that addresses its complex drivers. First and foremost, we must expand the number of GI fellowship training slots to boost our pipeline. There are approximately 1,840 GI fellows currently in training, a third of whom enter the workforce each year. While the number of GI fellowship slots in the GI fellowship match has slowly increased over time (from 525 available slots across 199 programs in 2019 to 657 slots across 230 programs in 2023), this incremental growth is dwarfed by overall need. Continued advocacy for increased funding to support expansion of training slots is necessary to further move the needle – such lobbying recently led to the addition of 1,000 new Medicare-supported graduate medical education positions across specialties over a 5-year period starting in 2020, illustrating that change is possible. At the same time, we must address the factors that are causing gastroenterologists to leave the workforce prematurely through early retirement or part-time work by investing in innovative solutions to address burnout, reduce administrative burdens, enhance the efficiency of care delivery, and maintain financial viability. By investing in our physician workforce and its sustainability, we can ensure that our profession is better prepared to meet the needs of our growing and increasingly complex patient population now and in the future.
We hope you enjoy the November issue of GI & Hepatology News and have a wonderful Thanksgiving.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Impacts of this supply-demand mismatch are felt daily in our GI practices as we strive to expand access in our clinics and endoscopy suites, particularly in rural and urban underserved communities. In gastroenterology, increased demand for care has been driven by a perfect storm of population growth, increased patient awareness of GI health, and rising incidence of digestive diseases.
Between 2019 and 2034, the U.S. population is expected to grow by 10.6%, while the population aged 65 and older expands by over 42%. Recent increases in the CRC screening–eligible population also have contributed to unprecedented demand for GI care. Furthermore, care delivery has become more complex and time-consuming with the evolution of personalized medicine and high prevalence of comorbid conditions. At the same time, we are faced with a dwindling supply of gastroenterology providers. In 2021, there were 15,678 practicing gastroenterologists in the U.S., over half of whom were 55 years or older. This translates to 1 gastroenterologist per 20,830 people captured in the U.S. Census.
Addressing this striking supply-demand mismatch in GI requires a multi-pronged approach that addresses its complex drivers. First and foremost, we must expand the number of GI fellowship training slots to boost our pipeline. There are approximately 1,840 GI fellows currently in training, a third of whom enter the workforce each year. While the number of GI fellowship slots in the GI fellowship match has slowly increased over time (from 525 available slots across 199 programs in 2019 to 657 slots across 230 programs in 2023), this incremental growth is dwarfed by overall need. Continued advocacy for increased funding to support expansion of training slots is necessary to further move the needle – such lobbying recently led to the addition of 1,000 new Medicare-supported graduate medical education positions across specialties over a 5-year period starting in 2020, illustrating that change is possible. At the same time, we must address the factors that are causing gastroenterologists to leave the workforce prematurely through early retirement or part-time work by investing in innovative solutions to address burnout, reduce administrative burdens, enhance the efficiency of care delivery, and maintain financial viability. By investing in our physician workforce and its sustainability, we can ensure that our profession is better prepared to meet the needs of our growing and increasingly complex patient population now and in the future.
We hope you enjoy the November issue of GI & Hepatology News and have a wonderful Thanksgiving.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Selecting therapies in moderate to severe inflammatory bowel disease: Key factors in decision making
Despite new advances in treatment, head to head clinical trials, which are considered the gold standard when comparing therapies, remain limited. Other comparative effectiveness studies and network meta-analyses are the currently available substitutes to guide decision making.1
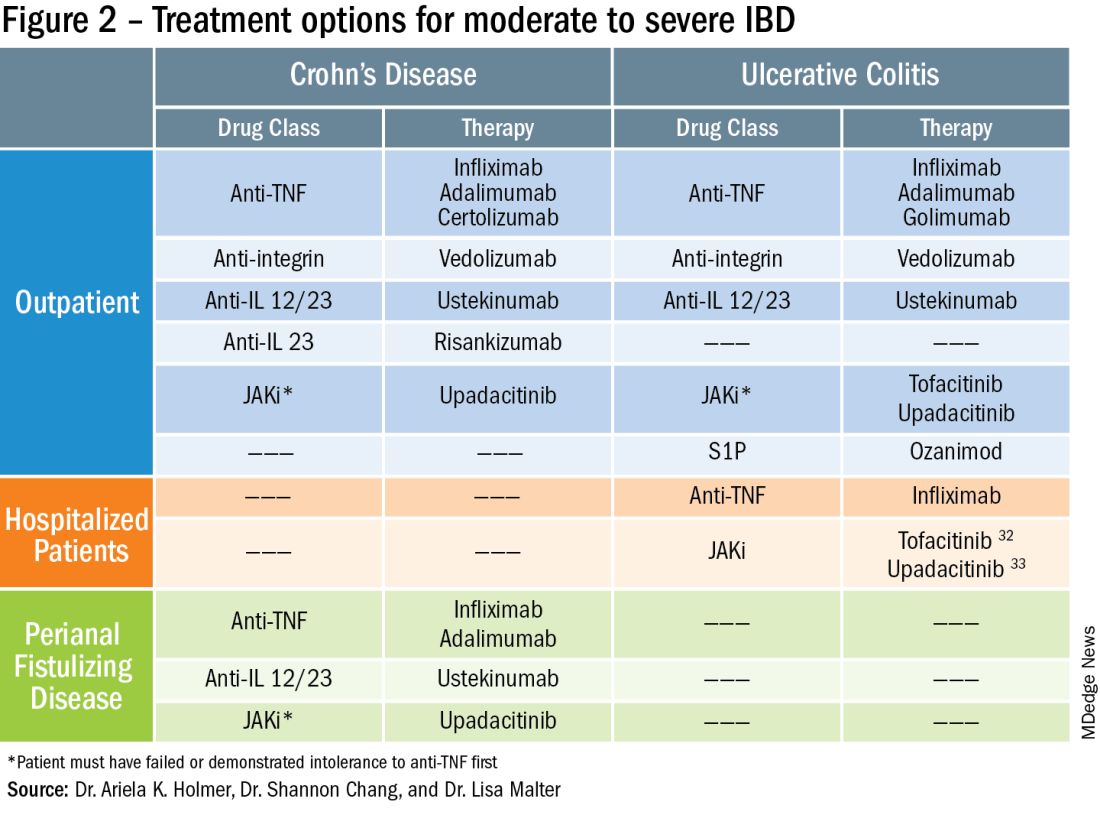
While efficacy is often considered first when choosing a drug, other critical factors play a role in tailoring a treatment plan. This article focuses on key considerations to help guide clinical decision making when treating patients with moderate to severe IBD (Figure 1).

Disease activity versus severity
Both disease activity and disease severity should be considered when evaluating a patient for treatment. Disease activity is a cross-sectional view of one’s signs and symptoms which can vary visit to visit. Standardized indices measure disease activity in both Crohn’s disease (CD) and ulcerative colitis (UC).2,3 Disease severity encompasses the overall prognosis of disease over time and includes factors such as the presence or absence of high risk features, prior medication exposure, history of surgery, hospitalizations and the impact on quality of life.4
To prevent disease complications, the goals of treatment should be aimed at both reducing active symptoms (disease activity) but also healing mucosal inflammation, preventing disease progression (disease severity) and downstream sequelae including cancer, hospitalization or surgery.5 Determining the best treatment option takes disease activity and severity into account, in addition to the other key factors listed below (Figure 2).
Extraintestinal manifestations
Inflammation of organs outside of the gastrointestinal tract is common and can occur in up to 50% of patients with IBD.6 The most prevalent extraintestinal manifestations (EIMs) involve the skin and joints, which will be the primary focus in this article. We will also focus on treatment options with the most evidence supporting their use. Peripheral arthritis is often associated with intestinal inflammation, and treatment of underlying IBD can simultaneously improve joint symptoms. Conversely, axial spondyloarthritis does not commonly parallel intestinal inflammation. Anti–tumor necrosis factor (TNF) agents including infliximab and adalimumab are effective for the treatment of both peripheral and axial disease.6
Ustekinumab, an interleukin (IL)-12/23 inhibitor, may be effective for peripheral arthritis, however is ineffective for the treatment of axial spondyloarthritis.6 Janus kinase (JAK) inhibitors which include tofacitinib and upadacitinib are oral small molecules used to treat peripheral and axial spondyloarthritis and have more recently been approved for moderate to severe IBD.6,7
Erythema nodosum (EN) and pyoderma gangrenosum (PG) are skin manifestations seen in patients with IBD. EN appears as subcutaneous nodules and parallels intestinal inflammation, while PG consists of violaceous, ulcerated plaques, and presents with more significant pain. Anti-TNFs are effective for both EN and PG, with infliximab being the only biologic studied in a randomized control trial of patients with PG.8 In addition, small case reports have described some benefit from ustekinumab and upadacitinib in the treatment of PG.9,10
Safety
The safety of IBD therapies is a key consideration and often the most important factor to patients when choosing a treatment option. It is important to note that untreated disease is associated with significant morbidity, and should be weighed when discussing risks of medications with patients. In general, anti-TNFs and JAK inhibitors may be associated with an increased risk of infection and malignancy, while ustekinumab, vedolizumab, risankizumab and ozanimod offer a more favorable safety profile.11 In large registries and observational studies, infliximab was associated with up to a two times greater risk of serious infection as compared to nonbiologic medications, with the most common infections being pneumonia, sepsis and herpes zoster.12 JAK inhibitors are associated with an increased risk of herpes zoster infection, with a dose dependent effect seen in the maintenance clinical trials with tofacitinib.7
Ozanimod may be associated with atrioventricular conduction delays and bradycardia, however long-term safety data has reported a low incidence of serious cardiac related adverse events.13 Overall, though risks of infection may vary with different therapies, other consistent risk factors associated with greater rates of serious infection include prolonged corticosteroid use, combination therapy with thiopurines, and disease severity. Anti-TNFs have also been associated with a somewhat increased risk of lymphoma, increased when used in combination with thiopurines. Reassuringly, however, in patients with a prior history of cancer, anti-TNFs and non-TNF biologics have not been found to increase the risk of new or recurrent cancer.14
Ultimately, in patients with a prior history of cancer, the choice of biologic or small molecule should be made in collaboration with a patient’s oncologist.
Anti-TNF exposure
Anti-TNFs were the first available biologics for the treatment of IBD. After the approval of vedolizumab in 2014, the first non-TNF biologic, many patients enrolled in clinical trials thereafter had already tried and failed anti-TNFs. In general, exposure to anti-TNFs may reduce the efficacy of a future biologic. In patients treated with vedolizumab, endoscopic and clinical outcomes were negatively impacted by prior anti-TNF exposure.15 However, in VARSITY, a head-to-head clinical trial where 20% of patients with UC were previously exposed to anti-TNFs other than adalimumab, vedolizumab had significantly higher rates of clinical remission and endoscopic improvement compared to adalimumab.16 Clinical remission rates with tofacitinib were not impacted by exposure to anti-TNF treatment, and similar findings were observed with ustekinumab.7,17 Risankizumab, a newly approved selective anti-IL23, also does not appear to be impacted by prior anti-TNF exposure by demonstrating similar rates of clinical remission regardless of biologic exposure status.18 Therefore, in patients with prior history of anti-TNF use, consideration of ustekinumab, risankizumab or JAK inhibitors as second line agents may be more favorable as compared to vedolizumab.
Perianal fistulizing disease
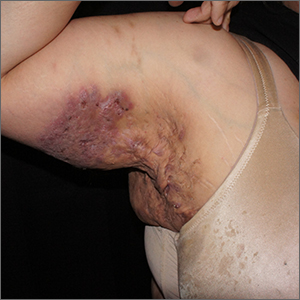
Perianal fistulizing disease can affect up to one-third of patients with CD and significantly impact a patient’s quality of life.19 The most robust data for the treatment of perianal fistulizing disease includes the use of infliximab with up to one-third of patients on maintenance therapy achieving complete resolution of fistula drainage. While no head-to-head trials compare combination therapy with infliximab plus immunomodulators versus infliximab alone for this indication specifically, one observational study demonstrated higher rates of fistula closure with combination therapy as compared to infliximab mono-therapy.19 In a post hoc analysis, higher infliximab concentrations at week 14 were associated with greater fistula response and remission rates.20 In patients with perianal disease, ustekinumab and vedolizumab may also be an effective treatment option by promoting resolution of fistula drainage.21
More recently, emerging data demonstrate that upadacitinib may be an excellent option as a second-line treatment for perianal disease in patients who have failed anti-TNF therapy. Use of upadacitinib was associated with greater rates of complete resolution of fistula drainage and higher rates of external fistula closure (Figure 2).22 Lastly, as an alternative to medical therapy, mesenchymal stem cell therapy has also shown to improve fistula drainage and improve external fistula openings in patients with CD.23 Stem cell therapy is only available through clinical trials at this time.
Patient preferences
Overall, data are lacking for evaluating patient preferences in treatment options for IBD especially with the recent increase in therapeutic options. One survey demonstrated that patient preferences were most impacted by the possibility of improving abdominal pain, with patients accepting additional risk of treatment side effects in order to reduce their abdominal pain.24 An oral route of administration and improving fatigue and bowel urgency were similarly important to patients. Patient preferences can also be highly variable with some valuing avoidance of corticosteroid use while others valuing avoidance of symptoms or risks of medication side effects and surgery. It is important to tailor the discussion on treatment strategies to each individual patient and inquire about the patient’s lifestyle, medical history, and value system, which may impact their treatment preferences utilizing shared decision making.
Access to treatment including the role of social determinants of health
The expanded therapeutic armamentarium has the potential to help patients achieve the current goals of care in IBD. However, these medications are not available to all patients due to numerous barriers including step therapy payer policies, prohibitive costs, insurance prior authorizations, and the role of social determinants of health and proximity to IBD expertise.25 While clinicians work with patients to determine the best treatment option, more often than not, the decision lies with the insurance payer. Step therapy is the protocol used by insurance companies that requires patients to try a lower-cost medication and fail to respond before they approve the originally requested treatment. This can lead to treatment delays, progression of disease, and disease complications. The option to incorporate the use of biosimilars, currently available for anti-TNFs, and other biologics in the near future, will reduce cost and potentially increase access.26 Additionally, working with a clinical pharmacist to navigate access and utilize patient assistance programs may help overcome cost related barriers to treatment and prevent delays in care.
Socioeconomic status has been shown to impact IBD disease outcomes, and compliance rates in treatment vary depending on race and ethnicity.27 Certain racial and ethnic groups remain vulnerable and may require additional support to achieve treatment goals. For example, disparities in health literacy in patients with IBD have been demonstrated with older black men at risk.28 Additionally, the patient’s proximity to their health care facility may impact treatment options. Most IBD centers are located in metropolitan areas and numerous “IBD deserts” exist, potentially limiting therapies for patients from more remote/rural settings.29 Access to treatment and the interplay of social determinants of health can have a large role in therapy selection.
Special considerations: Pregnancy and older adults
Certain patient populations warrant special consideration when approaching treatment strategies. Pregnancy in IBD will not be addressed in full depth in this article, however a key takeaway is that planning is critical and providers should emphasize the importance of steroid-free clinical remission for at least 3 months before conception.30 Additionally, biologic use during pregnancy has not been shown to increase adverse fetal outcomes, thus should be continued to minimize disease flare. Newer novel small molecules are generally avoided during pregnancy due to limited available safety data.
Older adults are the largest growing patient population with IBD. Frailty, or a state of decreased reserve, is more commonly observed in older patients and has been shown to increase adverse events including hospitalization and mortaility.31 Ultimately reducing polypharmacy, ensuring adequate nutrition, minimizing corticosteroid exposure and avoiding undertreatment of active IBD are all key in optimizing outcomes in an older patient with IBD.
Conclusion
When discussing treatment options with patients with IBD, it is important to individualize care and share the decision-making process with patients. Goals include improving symptoms and quality of life while working to achieve the goal of healing intestinal inflammation. In summary, this article can serve as a guide to clinicians for key factors in decision making when selecting therapies in moderate to severe IBD.
Dr. Holmer is a gastroenterologist with NYU Langone Health specializing in inflammatory bowel disease. Dr. Chang is director of clinical operations for the NYU Langone Health Inflammatory Bowel Disease Center. Dr. Malter is director of education for the Inflammatory Bowel Disease Center at NYU Langone Health and director of the inflammatory bowel disease program at Bellevue Hospital Center. Follow Dr. Holmer on X (formerly Twitter) at @HolmerMd and Dr. Chang @shannonchangmd. Dr. Holmer disclosed affiliations with Pfizer, Bristol Myers Squibb, and AvevoRx. Dr. Chang disclosed affiliations with Pfizer and Bristol Myers Squibb. Dr. Malter disclosed receiving educational grants form Abbvie, Janssen, Pfizer and Takeda, and serving on the advisory boards of AbbVie, Bristol Myers Squibb, Celltrion, Janssen, Merck, and Takeda.
References
1. Chang S et al. Am J Gastroenterol. 2023 Aug 24. doi: 10.14309/ajg.0000000000002485.
2. Harvey RF et al. The Lancet. 1980;1:514.
3. Lewis JD et al. Inflammatory Bowel Diseases. 2008;14:1660-1666.
4. Siegel CA et al. Gut. 2018;67(2):244-54.
5. Peyrin-Biroulet L et al. Am J Gastroenterol. 2015;110:1324-38
6. Rogler G et al. Gastroenterology. 2021;161:1118-32.
7. Sandborn WJ et al. N Engl J Med. 2017;376:1723-36.
8. Brooklyn TN et al. Gut. 2006;55:505-9.
9. Fahmy M et al. Am J Gastroenterol. 2012;107:794-5.
10. Van Eycken L et al. JAAD Case Rep. 2023;37:89-91.
11. Lasa JS et al. Lancet Gastroenterol Hepatol. 2022;7:161-70.
12. Lichtenstein GR et al. Inflamm Bowel Dis. 2018;24:490-501.
13. Long MD et al. Gastroenterology. 2022;162:S-5-S-6.
14. Holmer AK et al. Clin Gastroenterol Hepatol.2023;21:1598-1606.e5.
15. Sands BE et al. Gastroenterology. 2014;147:618-27.e3.
16. Sands BE et al. N Engl J Med. 2019;381:1215-26.
17. Sands BE et al. N Engl J Med. 2019;381:1201-14.
18. D’Haens G et al. Lancet. 2022;399:2015-30.
19. Bouguen G et al. Clin Gastroenterol Hepatol. 2013;11:975-81.e1-4.
20. Papamichael K et al. Am J Gastroenterol. 2021;116:1007-14.
21. Shehab M et al. Inflamm Bowel Dis. 2023;29:367-75.
22. Colombel JF et al. J Crohns Colitis. 2023;17:i620-i623.
23. Garcia-Olmo D et al. Dis Colon Rectum. 2022;65:713-20.
24. Louis E et al. J Crohns Colitis. 2023;17:231-9.
25. Rubin DT et al. Inflamm Bowel Dis. 2017;23:224-32.
26. Gulacsi L et al. Curr Med Chem. 2019;26:259-69.
27. Cai Q et al. BMC Gastroenterol. 2022;22:545.
28. Dos Santos Marques IC et al. Crohns Colitis 360. 2020 Oct;2(4):otaa076.
29. Deepak P et al. Gastroenterology. 2023;165:11-15.
30. Mahadevan U et al. Gastroenterology. 2019;156:1508-24.
31. Faye AS et al. Inflamm Bowel Dis. 2022;28:126-32.
32. Berinstein JA et al. Clin Gastroenterol Hepatol. 2021;19:2112-20.e1.
33. Levine J et al. Gastroenterology. 2023;164:S103-S104.
Despite new advances in treatment, head to head clinical trials, which are considered the gold standard when comparing therapies, remain limited. Other comparative effectiveness studies and network meta-analyses are the currently available substitutes to guide decision making.1
While efficacy is often considered first when choosing a drug, other critical factors play a role in tailoring a treatment plan. This article focuses on key considerations to help guide clinical decision making when treating patients with moderate to severe IBD (Figure 1).

Disease activity versus severity
Both disease activity and disease severity should be considered when evaluating a patient for treatment. Disease activity is a cross-sectional view of one’s signs and symptoms which can vary visit to visit. Standardized indices measure disease activity in both Crohn’s disease (CD) and ulcerative colitis (UC).2,3 Disease severity encompasses the overall prognosis of disease over time and includes factors such as the presence or absence of high risk features, prior medication exposure, history of surgery, hospitalizations and the impact on quality of life.4
To prevent disease complications, the goals of treatment should be aimed at both reducing active symptoms (disease activity) but also healing mucosal inflammation, preventing disease progression (disease severity) and downstream sequelae including cancer, hospitalization or surgery.5 Determining the best treatment option takes disease activity and severity into account, in addition to the other key factors listed below (Figure 2).

Extraintestinal manifestations
Inflammation of organs outside of the gastrointestinal tract is common and can occur in up to 50% of patients with IBD.6 The most prevalent extraintestinal manifestations (EIMs) involve the skin and joints, which will be the primary focus in this article. We will also focus on treatment options with the most evidence supporting their use. Peripheral arthritis is often associated with intestinal inflammation, and treatment of underlying IBD can simultaneously improve joint symptoms. Conversely, axial spondyloarthritis does not commonly parallel intestinal inflammation. Anti–tumor necrosis factor (TNF) agents including infliximab and adalimumab are effective for the treatment of both peripheral and axial disease.6
Ustekinumab, an interleukin (IL)-12/23 inhibitor, may be effective for peripheral arthritis, however is ineffective for the treatment of axial spondyloarthritis.6 Janus kinase (JAK) inhibitors which include tofacitinib and upadacitinib are oral small molecules used to treat peripheral and axial spondyloarthritis and have more recently been approved for moderate to severe IBD.6,7
Erythema nodosum (EN) and pyoderma gangrenosum (PG) are skin manifestations seen in patients with IBD. EN appears as subcutaneous nodules and parallels intestinal inflammation, while PG consists of violaceous, ulcerated plaques, and presents with more significant pain. Anti-TNFs are effective for both EN and PG, with infliximab being the only biologic studied in a randomized control trial of patients with PG.8 In addition, small case reports have described some benefit from ustekinumab and upadacitinib in the treatment of PG.9,10
Safety
The safety of IBD therapies is a key consideration and often the most important factor to patients when choosing a treatment option. It is important to note that untreated disease is associated with significant morbidity, and should be weighed when discussing risks of medications with patients. In general, anti-TNFs and JAK inhibitors may be associated with an increased risk of infection and malignancy, while ustekinumab, vedolizumab, risankizumab and ozanimod offer a more favorable safety profile.11 In large registries and observational studies, infliximab was associated with up to a two times greater risk of serious infection as compared to nonbiologic medications, with the most common infections being pneumonia, sepsis and herpes zoster.12 JAK inhibitors are associated with an increased risk of herpes zoster infection, with a dose dependent effect seen in the maintenance clinical trials with tofacitinib.7
Ozanimod may be associated with atrioventricular conduction delays and bradycardia, however long-term safety data has reported a low incidence of serious cardiac related adverse events.13 Overall, though risks of infection may vary with different therapies, other consistent risk factors associated with greater rates of serious infection include prolonged corticosteroid use, combination therapy with thiopurines, and disease severity. Anti-TNFs have also been associated with a somewhat increased risk of lymphoma, increased when used in combination with thiopurines. Reassuringly, however, in patients with a prior history of cancer, anti-TNFs and non-TNF biologics have not been found to increase the risk of new or recurrent cancer.14
Ultimately, in patients with a prior history of cancer, the choice of biologic or small molecule should be made in collaboration with a patient’s oncologist.
Anti-TNF exposure
Anti-TNFs were the first available biologics for the treatment of IBD. After the approval of vedolizumab in 2014, the first non-TNF biologic, many patients enrolled in clinical trials thereafter had already tried and failed anti-TNFs. In general, exposure to anti-TNFs may reduce the efficacy of a future biologic. In patients treated with vedolizumab, endoscopic and clinical outcomes were negatively impacted by prior anti-TNF exposure.15 However, in VARSITY, a head-to-head clinical trial where 20% of patients with UC were previously exposed to anti-TNFs other than adalimumab, vedolizumab had significantly higher rates of clinical remission and endoscopic improvement compared to adalimumab.16 Clinical remission rates with tofacitinib were not impacted by exposure to anti-TNF treatment, and similar findings were observed with ustekinumab.7,17 Risankizumab, a newly approved selective anti-IL23, also does not appear to be impacted by prior anti-TNF exposure by demonstrating similar rates of clinical remission regardless of biologic exposure status.18 Therefore, in patients with prior history of anti-TNF use, consideration of ustekinumab, risankizumab or JAK inhibitors as second line agents may be more favorable as compared to vedolizumab.
Perianal fistulizing disease
Perianal fistulizing disease can affect up to one-third of patients with CD and significantly impact a patient’s quality of life.19 The most robust data for the treatment of perianal fistulizing disease includes the use of infliximab with up to one-third of patients on maintenance therapy achieving complete resolution of fistula drainage. While no head-to-head trials compare combination therapy with infliximab plus immunomodulators versus infliximab alone for this indication specifically, one observational study demonstrated higher rates of fistula closure with combination therapy as compared to infliximab mono-therapy.19 In a post hoc analysis, higher infliximab concentrations at week 14 were associated with greater fistula response and remission rates.20 In patients with perianal disease, ustekinumab and vedolizumab may also be an effective treatment option by promoting resolution of fistula drainage.21
More recently, emerging data demonstrate that upadacitinib may be an excellent option as a second-line treatment for perianal disease in patients who have failed anti-TNF therapy. Use of upadacitinib was associated with greater rates of complete resolution of fistula drainage and higher rates of external fistula closure (Figure 2).22 Lastly, as an alternative to medical therapy, mesenchymal stem cell therapy has also shown to improve fistula drainage and improve external fistula openings in patients with CD.23 Stem cell therapy is only available through clinical trials at this time.
Patient preferences
Overall, data are lacking for evaluating patient preferences in treatment options for IBD especially with the recent increase in therapeutic options. One survey demonstrated that patient preferences were most impacted by the possibility of improving abdominal pain, with patients accepting additional risk of treatment side effects in order to reduce their abdominal pain.24 An oral route of administration and improving fatigue and bowel urgency were similarly important to patients. Patient preferences can also be highly variable with some valuing avoidance of corticosteroid use while others valuing avoidance of symptoms or risks of medication side effects and surgery. It is important to tailor the discussion on treatment strategies to each individual patient and inquire about the patient’s lifestyle, medical history, and value system, which may impact their treatment preferences utilizing shared decision making.
Access to treatment including the role of social determinants of health
The expanded therapeutic armamentarium has the potential to help patients achieve the current goals of care in IBD. However, these medications are not available to all patients due to numerous barriers including step therapy payer policies, prohibitive costs, insurance prior authorizations, and the role of social determinants of health and proximity to IBD expertise.25 While clinicians work with patients to determine the best treatment option, more often than not, the decision lies with the insurance payer. Step therapy is the protocol used by insurance companies that requires patients to try a lower-cost medication and fail to respond before they approve the originally requested treatment. This can lead to treatment delays, progression of disease, and disease complications. The option to incorporate the use of biosimilars, currently available for anti-TNFs, and other biologics in the near future, will reduce cost and potentially increase access.26 Additionally, working with a clinical pharmacist to navigate access and utilize patient assistance programs may help overcome cost related barriers to treatment and prevent delays in care.
Socioeconomic status has been shown to impact IBD disease outcomes, and compliance rates in treatment vary depending on race and ethnicity.27 Certain racial and ethnic groups remain vulnerable and may require additional support to achieve treatment goals. For example, disparities in health literacy in patients with IBD have been demonstrated with older black men at risk.28 Additionally, the patient’s proximity to their health care facility may impact treatment options. Most IBD centers are located in metropolitan areas and numerous “IBD deserts” exist, potentially limiting therapies for patients from more remote/rural settings.29 Access to treatment and the interplay of social determinants of health can have a large role in therapy selection.
Special considerations: Pregnancy and older adults
Certain patient populations warrant special consideration when approaching treatment strategies. Pregnancy in IBD will not be addressed in full depth in this article, however a key takeaway is that planning is critical and providers should emphasize the importance of steroid-free clinical remission for at least 3 months before conception.30 Additionally, biologic use during pregnancy has not been shown to increase adverse fetal outcomes, thus should be continued to minimize disease flare. Newer novel small molecules are generally avoided during pregnancy due to limited available safety data.
Older adults are the largest growing patient population with IBD. Frailty, or a state of decreased reserve, is more commonly observed in older patients and has been shown to increase adverse events including hospitalization and mortaility.31 Ultimately reducing polypharmacy, ensuring adequate nutrition, minimizing corticosteroid exposure and avoiding undertreatment of active IBD are all key in optimizing outcomes in an older patient with IBD.
Conclusion
When discussing treatment options with patients with IBD, it is important to individualize care and share the decision-making process with patients. Goals include improving symptoms and quality of life while working to achieve the goal of healing intestinal inflammation. In summary, this article can serve as a guide to clinicians for key factors in decision making when selecting therapies in moderate to severe IBD.
Dr. Holmer is a gastroenterologist with NYU Langone Health specializing in inflammatory bowel disease. Dr. Chang is director of clinical operations for the NYU Langone Health Inflammatory Bowel Disease Center. Dr. Malter is director of education for the Inflammatory Bowel Disease Center at NYU Langone Health and director of the inflammatory bowel disease program at Bellevue Hospital Center. Follow Dr. Holmer on X (formerly Twitter) at @HolmerMd and Dr. Chang @shannonchangmd. Dr. Holmer disclosed affiliations with Pfizer, Bristol Myers Squibb, and AvevoRx. Dr. Chang disclosed affiliations with Pfizer and Bristol Myers Squibb. Dr. Malter disclosed receiving educational grants form Abbvie, Janssen, Pfizer and Takeda, and serving on the advisory boards of AbbVie, Bristol Myers Squibb, Celltrion, Janssen, Merck, and Takeda.
References
1. Chang S et al. Am J Gastroenterol. 2023 Aug 24. doi: 10.14309/ajg.0000000000002485.
2. Harvey RF et al. The Lancet. 1980;1:514.
3. Lewis JD et al. Inflammatory Bowel Diseases. 2008;14:1660-1666.
4. Siegel CA et al. Gut. 2018;67(2):244-54.
5. Peyrin-Biroulet L et al. Am J Gastroenterol. 2015;110:1324-38
6. Rogler G et al. Gastroenterology. 2021;161:1118-32.
7. Sandborn WJ et al. N Engl J Med. 2017;376:1723-36.
8. Brooklyn TN et al. Gut. 2006;55:505-9.
9. Fahmy M et al. Am J Gastroenterol. 2012;107:794-5.
10. Van Eycken L et al. JAAD Case Rep. 2023;37:89-91.
11. Lasa JS et al. Lancet Gastroenterol Hepatol. 2022;7:161-70.
12. Lichtenstein GR et al. Inflamm Bowel Dis. 2018;24:490-501.
13. Long MD et al. Gastroenterology. 2022;162:S-5-S-6.
14. Holmer AK et al. Clin Gastroenterol Hepatol.2023;21:1598-1606.e5.
15. Sands BE et al. Gastroenterology. 2014;147:618-27.e3.
16. Sands BE et al. N Engl J Med. 2019;381:1215-26.
17. Sands BE et al. N Engl J Med. 2019;381:1201-14.
18. D’Haens G et al. Lancet. 2022;399:2015-30.
19. Bouguen G et al. Clin Gastroenterol Hepatol. 2013;11:975-81.e1-4.
20. Papamichael K et al. Am J Gastroenterol. 2021;116:1007-14.
21. Shehab M et al. Inflamm Bowel Dis. 2023;29:367-75.
22. Colombel JF et al. J Crohns Colitis. 2023;17:i620-i623.
23. Garcia-Olmo D et al. Dis Colon Rectum. 2022;65:713-20.
24. Louis E et al. J Crohns Colitis. 2023;17:231-9.
25. Rubin DT et al. Inflamm Bowel Dis. 2017;23:224-32.
26. Gulacsi L et al. Curr Med Chem. 2019;26:259-69.
27. Cai Q et al. BMC Gastroenterol. 2022;22:545.
28. Dos Santos Marques IC et al. Crohns Colitis 360. 2020 Oct;2(4):otaa076.
29. Deepak P et al. Gastroenterology. 2023;165:11-15.
30. Mahadevan U et al. Gastroenterology. 2019;156:1508-24.
31. Faye AS et al. Inflamm Bowel Dis. 2022;28:126-32.
32. Berinstein JA et al. Clin Gastroenterol Hepatol. 2021;19:2112-20.e1.
33. Levine J et al. Gastroenterology. 2023;164:S103-S104.
Despite new advances in treatment, head to head clinical trials, which are considered the gold standard when comparing therapies, remain limited. Other comparative effectiveness studies and network meta-analyses are the currently available substitutes to guide decision making.1
While efficacy is often considered first when choosing a drug, other critical factors play a role in tailoring a treatment plan. This article focuses on key considerations to help guide clinical decision making when treating patients with moderate to severe IBD (Figure 1).

Disease activity versus severity
Both disease activity and disease severity should be considered when evaluating a patient for treatment. Disease activity is a cross-sectional view of one’s signs and symptoms which can vary visit to visit. Standardized indices measure disease activity in both Crohn’s disease (CD) and ulcerative colitis (UC).2,3 Disease severity encompasses the overall prognosis of disease over time and includes factors such as the presence or absence of high risk features, prior medication exposure, history of surgery, hospitalizations and the impact on quality of life.4
To prevent disease complications, the goals of treatment should be aimed at both reducing active symptoms (disease activity) but also healing mucosal inflammation, preventing disease progression (disease severity) and downstream sequelae including cancer, hospitalization or surgery.5 Determining the best treatment option takes disease activity and severity into account, in addition to the other key factors listed below (Figure 2).

Extraintestinal manifestations
Inflammation of organs outside of the gastrointestinal tract is common and can occur in up to 50% of patients with IBD.6 The most prevalent extraintestinal manifestations (EIMs) involve the skin and joints, which will be the primary focus in this article. We will also focus on treatment options with the most evidence supporting their use. Peripheral arthritis is often associated with intestinal inflammation, and treatment of underlying IBD can simultaneously improve joint symptoms. Conversely, axial spondyloarthritis does not commonly parallel intestinal inflammation. Anti–tumor necrosis factor (TNF) agents including infliximab and adalimumab are effective for the treatment of both peripheral and axial disease.6
Ustekinumab, an interleukin (IL)-12/23 inhibitor, may be effective for peripheral arthritis, however is ineffective for the treatment of axial spondyloarthritis.6 Janus kinase (JAK) inhibitors which include tofacitinib and upadacitinib are oral small molecules used to treat peripheral and axial spondyloarthritis and have more recently been approved for moderate to severe IBD.6,7
Erythema nodosum (EN) and pyoderma gangrenosum (PG) are skin manifestations seen in patients with IBD. EN appears as subcutaneous nodules and parallels intestinal inflammation, while PG consists of violaceous, ulcerated plaques, and presents with more significant pain. Anti-TNFs are effective for both EN and PG, with infliximab being the only biologic studied in a randomized control trial of patients with PG.8 In addition, small case reports have described some benefit from ustekinumab and upadacitinib in the treatment of PG.9,10
Safety
The safety of IBD therapies is a key consideration and often the most important factor to patients when choosing a treatment option. It is important to note that untreated disease is associated with significant morbidity, and should be weighed when discussing risks of medications with patients. In general, anti-TNFs and JAK inhibitors may be associated with an increased risk of infection and malignancy, while ustekinumab, vedolizumab, risankizumab and ozanimod offer a more favorable safety profile.11 In large registries and observational studies, infliximab was associated with up to a two times greater risk of serious infection as compared to nonbiologic medications, with the most common infections being pneumonia, sepsis and herpes zoster.12 JAK inhibitors are associated with an increased risk of herpes zoster infection, with a dose dependent effect seen in the maintenance clinical trials with tofacitinib.7
Ozanimod may be associated with atrioventricular conduction delays and bradycardia, however long-term safety data has reported a low incidence of serious cardiac related adverse events.13 Overall, though risks of infection may vary with different therapies, other consistent risk factors associated with greater rates of serious infection include prolonged corticosteroid use, combination therapy with thiopurines, and disease severity. Anti-TNFs have also been associated with a somewhat increased risk of lymphoma, increased when used in combination with thiopurines. Reassuringly, however, in patients with a prior history of cancer, anti-TNFs and non-TNF biologics have not been found to increase the risk of new or recurrent cancer.14
Ultimately, in patients with a prior history of cancer, the choice of biologic or small molecule should be made in collaboration with a patient’s oncologist.
Anti-TNF exposure
Anti-TNFs were the first available biologics for the treatment of IBD. After the approval of vedolizumab in 2014, the first non-TNF biologic, many patients enrolled in clinical trials thereafter had already tried and failed anti-TNFs. In general, exposure to anti-TNFs may reduce the efficacy of a future biologic. In patients treated with vedolizumab, endoscopic and clinical outcomes were negatively impacted by prior anti-TNF exposure.15 However, in VARSITY, a head-to-head clinical trial where 20% of patients with UC were previously exposed to anti-TNFs other than adalimumab, vedolizumab had significantly higher rates of clinical remission and endoscopic improvement compared to adalimumab.16 Clinical remission rates with tofacitinib were not impacted by exposure to anti-TNF treatment, and similar findings were observed with ustekinumab.7,17 Risankizumab, a newly approved selective anti-IL23, also does not appear to be impacted by prior anti-TNF exposure by demonstrating similar rates of clinical remission regardless of biologic exposure status.18 Therefore, in patients with prior history of anti-TNF use, consideration of ustekinumab, risankizumab or JAK inhibitors as second line agents may be more favorable as compared to vedolizumab.
Perianal fistulizing disease
Perianal fistulizing disease can affect up to one-third of patients with CD and significantly impact a patient’s quality of life.19 The most robust data for the treatment of perianal fistulizing disease includes the use of infliximab with up to one-third of patients on maintenance therapy achieving complete resolution of fistula drainage. While no head-to-head trials compare combination therapy with infliximab plus immunomodulators versus infliximab alone for this indication specifically, one observational study demonstrated higher rates of fistula closure with combination therapy as compared to infliximab mono-therapy.19 In a post hoc analysis, higher infliximab concentrations at week 14 were associated with greater fistula response and remission rates.20 In patients with perianal disease, ustekinumab and vedolizumab may also be an effective treatment option by promoting resolution of fistula drainage.21
More recently, emerging data demonstrate that upadacitinib may be an excellent option as a second-line treatment for perianal disease in patients who have failed anti-TNF therapy. Use of upadacitinib was associated with greater rates of complete resolution of fistula drainage and higher rates of external fistula closure (Figure 2).22 Lastly, as an alternative to medical therapy, mesenchymal stem cell therapy has also shown to improve fistula drainage and improve external fistula openings in patients with CD.23 Stem cell therapy is only available through clinical trials at this time.
Patient preferences
Overall, data are lacking for evaluating patient preferences in treatment options for IBD especially with the recent increase in therapeutic options. One survey demonstrated that patient preferences were most impacted by the possibility of improving abdominal pain, with patients accepting additional risk of treatment side effects in order to reduce their abdominal pain.24 An oral route of administration and improving fatigue and bowel urgency were similarly important to patients. Patient preferences can also be highly variable with some valuing avoidance of corticosteroid use while others valuing avoidance of symptoms or risks of medication side effects and surgery. It is important to tailor the discussion on treatment strategies to each individual patient and inquire about the patient’s lifestyle, medical history, and value system, which may impact their treatment preferences utilizing shared decision making.
Access to treatment including the role of social determinants of health
The expanded therapeutic armamentarium has the potential to help patients achieve the current goals of care in IBD. However, these medications are not available to all patients due to numerous barriers including step therapy payer policies, prohibitive costs, insurance prior authorizations, and the role of social determinants of health and proximity to IBD expertise.25 While clinicians work with patients to determine the best treatment option, more often than not, the decision lies with the insurance payer. Step therapy is the protocol used by insurance companies that requires patients to try a lower-cost medication and fail to respond before they approve the originally requested treatment. This can lead to treatment delays, progression of disease, and disease complications. The option to incorporate the use of biosimilars, currently available for anti-TNFs, and other biologics in the near future, will reduce cost and potentially increase access.26 Additionally, working with a clinical pharmacist to navigate access and utilize patient assistance programs may help overcome cost related barriers to treatment and prevent delays in care.
Socioeconomic status has been shown to impact IBD disease outcomes, and compliance rates in treatment vary depending on race and ethnicity.27 Certain racial and ethnic groups remain vulnerable and may require additional support to achieve treatment goals. For example, disparities in health literacy in patients with IBD have been demonstrated with older black men at risk.28 Additionally, the patient’s proximity to their health care facility may impact treatment options. Most IBD centers are located in metropolitan areas and numerous “IBD deserts” exist, potentially limiting therapies for patients from more remote/rural settings.29 Access to treatment and the interplay of social determinants of health can have a large role in therapy selection.
Special considerations: Pregnancy and older adults
Certain patient populations warrant special consideration when approaching treatment strategies. Pregnancy in IBD will not be addressed in full depth in this article, however a key takeaway is that planning is critical and providers should emphasize the importance of steroid-free clinical remission for at least 3 months before conception.30 Additionally, biologic use during pregnancy has not been shown to increase adverse fetal outcomes, thus should be continued to minimize disease flare. Newer novel small molecules are generally avoided during pregnancy due to limited available safety data.
Older adults are the largest growing patient population with IBD. Frailty, or a state of decreased reserve, is more commonly observed in older patients and has been shown to increase adverse events including hospitalization and mortaility.31 Ultimately reducing polypharmacy, ensuring adequate nutrition, minimizing corticosteroid exposure and avoiding undertreatment of active IBD are all key in optimizing outcomes in an older patient with IBD.
Conclusion
When discussing treatment options with patients with IBD, it is important to individualize care and share the decision-making process with patients. Goals include improving symptoms and quality of life while working to achieve the goal of healing intestinal inflammation. In summary, this article can serve as a guide to clinicians for key factors in decision making when selecting therapies in moderate to severe IBD.
Dr. Holmer is a gastroenterologist with NYU Langone Health specializing in inflammatory bowel disease. Dr. Chang is director of clinical operations for the NYU Langone Health Inflammatory Bowel Disease Center. Dr. Malter is director of education for the Inflammatory Bowel Disease Center at NYU Langone Health and director of the inflammatory bowel disease program at Bellevue Hospital Center. Follow Dr. Holmer on X (formerly Twitter) at @HolmerMd and Dr. Chang @shannonchangmd. Dr. Holmer disclosed affiliations with Pfizer, Bristol Myers Squibb, and AvevoRx. Dr. Chang disclosed affiliations with Pfizer and Bristol Myers Squibb. Dr. Malter disclosed receiving educational grants form Abbvie, Janssen, Pfizer and Takeda, and serving on the advisory boards of AbbVie, Bristol Myers Squibb, Celltrion, Janssen, Merck, and Takeda.
References
1. Chang S et al. Am J Gastroenterol. 2023 Aug 24. doi: 10.14309/ajg.0000000000002485.
2. Harvey RF et al. The Lancet. 1980;1:514.
3. Lewis JD et al. Inflammatory Bowel Diseases. 2008;14:1660-1666.
4. Siegel CA et al. Gut. 2018;67(2):244-54.
5. Peyrin-Biroulet L et al. Am J Gastroenterol. 2015;110:1324-38
6. Rogler G et al. Gastroenterology. 2021;161:1118-32.
7. Sandborn WJ et al. N Engl J Med. 2017;376:1723-36.
8. Brooklyn TN et al. Gut. 2006;55:505-9.
9. Fahmy M et al. Am J Gastroenterol. 2012;107:794-5.
10. Van Eycken L et al. JAAD Case Rep. 2023;37:89-91.
11. Lasa JS et al. Lancet Gastroenterol Hepatol. 2022;7:161-70.
12. Lichtenstein GR et al. Inflamm Bowel Dis. 2018;24:490-501.
13. Long MD et al. Gastroenterology. 2022;162:S-5-S-6.
14. Holmer AK et al. Clin Gastroenterol Hepatol.2023;21:1598-1606.e5.
15. Sands BE et al. Gastroenterology. 2014;147:618-27.e3.
16. Sands BE et al. N Engl J Med. 2019;381:1215-26.
17. Sands BE et al. N Engl J Med. 2019;381:1201-14.
18. D’Haens G et al. Lancet. 2022;399:2015-30.
19. Bouguen G et al. Clin Gastroenterol Hepatol. 2013;11:975-81.e1-4.
20. Papamichael K et al. Am J Gastroenterol. 2021;116:1007-14.
21. Shehab M et al. Inflamm Bowel Dis. 2023;29:367-75.
22. Colombel JF et al. J Crohns Colitis. 2023;17:i620-i623.
23. Garcia-Olmo D et al. Dis Colon Rectum. 2022;65:713-20.
24. Louis E et al. J Crohns Colitis. 2023;17:231-9.
25. Rubin DT et al. Inflamm Bowel Dis. 2017;23:224-32.
26. Gulacsi L et al. Curr Med Chem. 2019;26:259-69.
27. Cai Q et al. BMC Gastroenterol. 2022;22:545.
28. Dos Santos Marques IC et al. Crohns Colitis 360. 2020 Oct;2(4):otaa076.
29. Deepak P et al. Gastroenterology. 2023;165:11-15.
30. Mahadevan U et al. Gastroenterology. 2019;156:1508-24.
31. Faye AS et al. Inflamm Bowel Dis. 2022;28:126-32.
32. Berinstein JA et al. Clin Gastroenterol Hepatol. 2021;19:2112-20.e1.
33. Levine J et al. Gastroenterology. 2023;164:S103-S104.
Advancing personalized medicine in IBD
Gastroenterologists have more treatments at their disposal today than ever before, particularly in the last decade. “We have had tremendous advances in many areas of understanding contributors to disease,” said Dr. Melia, an assistant professor of medicine at Johns Hopkins Medicine in Baltimore who specializes in inflammatory bowel disease (IBD). But the hurdle is in translating the science to clinical care that is individualized to each patient based on condition and stage of the condition.
“That still remains a bit of a dream,” she said. Much of her career has been devoted to chasing down a particular genetic variant that contributes to IBD, with the goal of reaching more precise treatments for patients.
In an interview, she shared how she entered this line of work, and what her research has revealed about Crohn’s disease, manganese, and a common genetic variant known as ZIP8.
Q: Your expertise is in inflammatory bowel disease and manganese deficiency. Why did you choose these two areas as your focus in GI?
Dr. Melia: In talking to many patients with IBD, I was always struck by the questions around nutritional factors related to disease. As a fellow, I was embedded in a lab that focused on genetics of IBD. A micronutrient transporter, ZIP8, has a mutation in it that increases the risk of Crohn’s disease.
I’ve dedicated the last 8 years to understanding how this mutation can increase risk. It initially started out as a project focused on zinc, because that’s what the transporter was thought to regulate. However, it’s evolved as we’ve learned more about it, underscoring the importance of manganese, another micronutrient that we derive from food.
We have established that having this mutation changes how the body handles manganese and affects downstream processes that involve manganese. What I’m doing now is trying to connect those dots on why those processes are important in Crohn’s disease and whether we can target them for treatment.
Q: How does manganese deficiency lead to chronic IBD?
Dr. Melia: In individuals with this mutation, their blood manganese levels are lower than people who don’t have this mutation. When we talk about manganese deficiency or insufficiency, what we’re really talking about is lower blood levels. But it’s more complicated than that at the tissue level.
What we and other groups are working on right now is trying to understand if the manganese levels change in the gut and what happens in inflammation. The gut is a particularly interesting area for manganese, in that much of the manganese that we eat is excreted. We only absorb a small amount of it. And so, manganese levels within the gut lumen may actually be quite high – and may be even higher in inflammation. But there are things we don’t understand about that and how it relates to mucosal levels of manganese and Crohn’s disease. The ileum, the site of the Crohn’s disease that’s specifically associated with this mutation, might be particularly sensitive to changes in the manganese levels or the downstream processes that changing manganese availability affects.
One of those processes is glycosylation. Manganese is important to properly glycosylate your proteins. Many enzymes help cells put sugars on proteins, and many of those enzymes need manganese to do it. Glycosylation of proteins is important so cells know where those proteins should go, and the sugars help them stay where they need to be. When you change protein glycosylation, you can stress the cells. We know individuals who carry this mutation have changes in the glycosylation of their proteins. What we’re working on right now is understanding which key proteins might change when that happens, and why that’s a potential problem, especially in the ileum.
Q: How might your research inform clinical practice?
Dr. Melia: We’ve seen significant progress in new medications and new pathways that have emerged. We still have this fundamental problem that our immune-targeting medicines are only helping about 50% of the patients.
It’s critical that we begin to identify new pathways. And my hope is that in studying genes like the ZIP8 (SLC39A8), which is associated with the dysregulated processing of manganese, we can understand different pathways and mechanisms to target.
As an example, if we could help correct the glycosylation problem, that would help to boost the barrier function of the gut and perhaps decrease the activation of those immune cells, because you’re just reinforcing the barrier integrity of the gut.
We want to target that glycosylation problem as we would treat patients with congenital disorders of glycosylation by giving supplemental sugars. We think this problem of glycosylation extends beyond patients with the ZIP8 mutation, but it is also really important for patients with the mutation. So, the goal would be to use ZIP8 genetics to help prioritize patients for therapy targeting this problem.
Q: You’re involved in the American Gastroenterological Association Future Leaders Program. What is your role in this program? Why is it important?
Dr. Melia: I was very grateful for the opportunity to participate in the AGA’s Future Leaders Program. I think it was exceedingly valuable for two main reasons. One, it really offered an insight into the role of the AGA and the important role that the AGA plays in the careers of gastroenterologists. Two, it was such a unique opportunity to work with colleagues nationwide and to build a network of individuals who are all at a similar stage in their careers. It was a very inspiring group to meet and to have the opportunity to work with as part of that program, and I thank the AGA for supporting such an initiative.
Q: What teacher or mentor had the greatest impact on you?
Dr. Melia: I have been blessed by many clinical and research mentors through my career. I was inspired to do science at the lab of Ramnik Xavier, MD, at Massachusetts General Hospital. At Johns Hopkins, I credit Cindy Sears, MD, and Anne Marie O’Broin Lennon, MBBCh, PhD, as two physician scientists who have really shaped how I have tried to integrate my clinical and research career.
LIGHTNING ROUND
Do you prefer texting or talking?
Texting
If you weren’t a gastroenterologist, what would you be?
Teacher
What was the last movie you watched?
Great Bear Rainforest
What is your most favorite city in the U.S.?
Surry, Maine
What song do you absolutely have to sing along with when you hear it?
Any song by Whitney Houston.
Are you an introvert or extrovert?
Introvert
How many cups of coffee do you drink per day?
One
Gastroenterologists have more treatments at their disposal today than ever before, particularly in the last decade. “We have had tremendous advances in many areas of understanding contributors to disease,” said Dr. Melia, an assistant professor of medicine at Johns Hopkins Medicine in Baltimore who specializes in inflammatory bowel disease (IBD). But the hurdle is in translating the science to clinical care that is individualized to each patient based on condition and stage of the condition.
“That still remains a bit of a dream,” she said. Much of her career has been devoted to chasing down a particular genetic variant that contributes to IBD, with the goal of reaching more precise treatments for patients.
In an interview, she shared how she entered this line of work, and what her research has revealed about Crohn’s disease, manganese, and a common genetic variant known as ZIP8.
Q: Your expertise is in inflammatory bowel disease and manganese deficiency. Why did you choose these two areas as your focus in GI?
Dr. Melia: In talking to many patients with IBD, I was always struck by the questions around nutritional factors related to disease. As a fellow, I was embedded in a lab that focused on genetics of IBD. A micronutrient transporter, ZIP8, has a mutation in it that increases the risk of Crohn’s disease.
I’ve dedicated the last 8 years to understanding how this mutation can increase risk. It initially started out as a project focused on zinc, because that’s what the transporter was thought to regulate. However, it’s evolved as we’ve learned more about it, underscoring the importance of manganese, another micronutrient that we derive from food.
We have established that having this mutation changes how the body handles manganese and affects downstream processes that involve manganese. What I’m doing now is trying to connect those dots on why those processes are important in Crohn’s disease and whether we can target them for treatment.
Q: How does manganese deficiency lead to chronic IBD?
Dr. Melia: In individuals with this mutation, their blood manganese levels are lower than people who don’t have this mutation. When we talk about manganese deficiency or insufficiency, what we’re really talking about is lower blood levels. But it’s more complicated than that at the tissue level.
What we and other groups are working on right now is trying to understand if the manganese levels change in the gut and what happens in inflammation. The gut is a particularly interesting area for manganese, in that much of the manganese that we eat is excreted. We only absorb a small amount of it. And so, manganese levels within the gut lumen may actually be quite high – and may be even higher in inflammation. But there are things we don’t understand about that and how it relates to mucosal levels of manganese and Crohn’s disease. The ileum, the site of the Crohn’s disease that’s specifically associated with this mutation, might be particularly sensitive to changes in the manganese levels or the downstream processes that changing manganese availability affects.
One of those processes is glycosylation. Manganese is important to properly glycosylate your proteins. Many enzymes help cells put sugars on proteins, and many of those enzymes need manganese to do it. Glycosylation of proteins is important so cells know where those proteins should go, and the sugars help them stay where they need to be. When you change protein glycosylation, you can stress the cells. We know individuals who carry this mutation have changes in the glycosylation of their proteins. What we’re working on right now is understanding which key proteins might change when that happens, and why that’s a potential problem, especially in the ileum.
Q: How might your research inform clinical practice?
Dr. Melia: We’ve seen significant progress in new medications and new pathways that have emerged. We still have this fundamental problem that our immune-targeting medicines are only helping about 50% of the patients.
It’s critical that we begin to identify new pathways. And my hope is that in studying genes like the ZIP8 (SLC39A8), which is associated with the dysregulated processing of manganese, we can understand different pathways and mechanisms to target.
As an example, if we could help correct the glycosylation problem, that would help to boost the barrier function of the gut and perhaps decrease the activation of those immune cells, because you’re just reinforcing the barrier integrity of the gut.
We want to target that glycosylation problem as we would treat patients with congenital disorders of glycosylation by giving supplemental sugars. We think this problem of glycosylation extends beyond patients with the ZIP8 mutation, but it is also really important for patients with the mutation. So, the goal would be to use ZIP8 genetics to help prioritize patients for therapy targeting this problem.
Q: You’re involved in the American Gastroenterological Association Future Leaders Program. What is your role in this program? Why is it important?
Dr. Melia: I was very grateful for the opportunity to participate in the AGA’s Future Leaders Program. I think it was exceedingly valuable for two main reasons. One, it really offered an insight into the role of the AGA and the important role that the AGA plays in the careers of gastroenterologists. Two, it was such a unique opportunity to work with colleagues nationwide and to build a network of individuals who are all at a similar stage in their careers. It was a very inspiring group to meet and to have the opportunity to work with as part of that program, and I thank the AGA for supporting such an initiative.
Q: What teacher or mentor had the greatest impact on you?
Dr. Melia: I have been blessed by many clinical and research mentors through my career. I was inspired to do science at the lab of Ramnik Xavier, MD, at Massachusetts General Hospital. At Johns Hopkins, I credit Cindy Sears, MD, and Anne Marie O’Broin Lennon, MBBCh, PhD, as two physician scientists who have really shaped how I have tried to integrate my clinical and research career.
LIGHTNING ROUND
Do you prefer texting or talking?
Texting
If you weren’t a gastroenterologist, what would you be?
Teacher
What was the last movie you watched?
Great Bear Rainforest
What is your most favorite city in the U.S.?
Surry, Maine
What song do you absolutely have to sing along with when you hear it?
Any song by Whitney Houston.
Are you an introvert or extrovert?
Introvert
How many cups of coffee do you drink per day?
One
Gastroenterologists have more treatments at their disposal today than ever before, particularly in the last decade. “We have had tremendous advances in many areas of understanding contributors to disease,” said Dr. Melia, an assistant professor of medicine at Johns Hopkins Medicine in Baltimore who specializes in inflammatory bowel disease (IBD). But the hurdle is in translating the science to clinical care that is individualized to each patient based on condition and stage of the condition.
“That still remains a bit of a dream,” she said. Much of her career has been devoted to chasing down a particular genetic variant that contributes to IBD, with the goal of reaching more precise treatments for patients.
In an interview, she shared how she entered this line of work, and what her research has revealed about Crohn’s disease, manganese, and a common genetic variant known as ZIP8.
Q: Your expertise is in inflammatory bowel disease and manganese deficiency. Why did you choose these two areas as your focus in GI?
Dr. Melia: In talking to many patients with IBD, I was always struck by the questions around nutritional factors related to disease. As a fellow, I was embedded in a lab that focused on genetics of IBD. A micronutrient transporter, ZIP8, has a mutation in it that increases the risk of Crohn’s disease.
I’ve dedicated the last 8 years to understanding how this mutation can increase risk. It initially started out as a project focused on zinc, because that’s what the transporter was thought to regulate. However, it’s evolved as we’ve learned more about it, underscoring the importance of manganese, another micronutrient that we derive from food.
We have established that having this mutation changes how the body handles manganese and affects downstream processes that involve manganese. What I’m doing now is trying to connect those dots on why those processes are important in Crohn’s disease and whether we can target them for treatment.
Q: How does manganese deficiency lead to chronic IBD?
Dr. Melia: In individuals with this mutation, their blood manganese levels are lower than people who don’t have this mutation. When we talk about manganese deficiency or insufficiency, what we’re really talking about is lower blood levels. But it’s more complicated than that at the tissue level.
What we and other groups are working on right now is trying to understand if the manganese levels change in the gut and what happens in inflammation. The gut is a particularly interesting area for manganese, in that much of the manganese that we eat is excreted. We only absorb a small amount of it. And so, manganese levels within the gut lumen may actually be quite high – and may be even higher in inflammation. But there are things we don’t understand about that and how it relates to mucosal levels of manganese and Crohn’s disease. The ileum, the site of the Crohn’s disease that’s specifically associated with this mutation, might be particularly sensitive to changes in the manganese levels or the downstream processes that changing manganese availability affects.
One of those processes is glycosylation. Manganese is important to properly glycosylate your proteins. Many enzymes help cells put sugars on proteins, and many of those enzymes need manganese to do it. Glycosylation of proteins is important so cells know where those proteins should go, and the sugars help them stay where they need to be. When you change protein glycosylation, you can stress the cells. We know individuals who carry this mutation have changes in the glycosylation of their proteins. What we’re working on right now is understanding which key proteins might change when that happens, and why that’s a potential problem, especially in the ileum.
Q: How might your research inform clinical practice?
Dr. Melia: We’ve seen significant progress in new medications and new pathways that have emerged. We still have this fundamental problem that our immune-targeting medicines are only helping about 50% of the patients.
It’s critical that we begin to identify new pathways. And my hope is that in studying genes like the ZIP8 (SLC39A8), which is associated with the dysregulated processing of manganese, we can understand different pathways and mechanisms to target.
As an example, if we could help correct the glycosylation problem, that would help to boost the barrier function of the gut and perhaps decrease the activation of those immune cells, because you’re just reinforcing the barrier integrity of the gut.
We want to target that glycosylation problem as we would treat patients with congenital disorders of glycosylation by giving supplemental sugars. We think this problem of glycosylation extends beyond patients with the ZIP8 mutation, but it is also really important for patients with the mutation. So, the goal would be to use ZIP8 genetics to help prioritize patients for therapy targeting this problem.
Q: You’re involved in the American Gastroenterological Association Future Leaders Program. What is your role in this program? Why is it important?
Dr. Melia: I was very grateful for the opportunity to participate in the AGA’s Future Leaders Program. I think it was exceedingly valuable for two main reasons. One, it really offered an insight into the role of the AGA and the important role that the AGA plays in the careers of gastroenterologists. Two, it was such a unique opportunity to work with colleagues nationwide and to build a network of individuals who are all at a similar stage in their careers. It was a very inspiring group to meet and to have the opportunity to work with as part of that program, and I thank the AGA for supporting such an initiative.
Q: What teacher or mentor had the greatest impact on you?
Dr. Melia: I have been blessed by many clinical and research mentors through my career. I was inspired to do science at the lab of Ramnik Xavier, MD, at Massachusetts General Hospital. At Johns Hopkins, I credit Cindy Sears, MD, and Anne Marie O’Broin Lennon, MBBCh, PhD, as two physician scientists who have really shaped how I have tried to integrate my clinical and research career.
LIGHTNING ROUND
Do you prefer texting or talking?
Texting
If you weren’t a gastroenterologist, what would you be?
Teacher
What was the last movie you watched?
Great Bear Rainforest
What is your most favorite city in the U.S.?
Surry, Maine
What song do you absolutely have to sing along with when you hear it?
Any song by Whitney Houston.
Are you an introvert or extrovert?
Introvert
How many cups of coffee do you drink per day?
One
Cysteamine and melasma
Most subjects covered in this column are botanical ingredients used for multiple conditions in topical skin care. The focus this month, though, is a natural agent garnering attention primarily for one indication. Present in many mammals and in various cells in the human body (and particularly highly concentrated in human milk), cysteamine is a stable aminothiol that acts as an antioxidant as a result of the degradation of coenzyme A and is known to play a protective function.1 Melasma, an acquired recurrent, chronic hyperpigmentary disorder, continues to be a treatment challenge and is often psychologically troublesome for those affected, approximately 90% of whom are women.2 Individuals with Fitzpatrick skin types IV and V who reside in regions where UV exposure is likely are particularly prominent among those with melasma.2 While triple combination therapy (also known as Kligman’s formula) continues to be the modern gold standard of care for melasma (over the last 30 years),3 cysteamine, a nonmelanocytotoxic molecule, is considered viable for long-term use and safer than the long-time skin-lightening gold standard over several decades, hydroquinone (HQ), which is associated with safety concerns.4 .
Recent history and the 2015 study
Prior to 2015, the quick oxidation and malodorous nature of cysteamine rendered it unsuitable for use as a topical agent. However, stabilization efforts resulted in a product that first began to show efficacy that year.5
Mansouri et al. conducted a randomized, double-blind, placebo-controlled trial to assess the efficacy of topical cysteamine 5% to treat epidermal melasma in 2015. Over 4 months, 50 volunteers (25 in each group) applied either cysteamine cream or placebo on lesions once nightly. The mean differences at baseline between pigmented and normal skin were 75.2 ± 37 in the cysteamine group and 68.9 ± 31 in the placebo group. Statistically significant differences between the groups were identified at the 2- and 4-month points. At 2 months, the mean differences were 39.7 ± 16.6 in the cysteamine group and 63.8 ± 28.6 in the placebo group; at 4 months, the respective differences were 26.2 ± 16 and 60.7 ± 27.3. Melasma area severity index (MASI) scores were significantly lower in the cysteamine group compared with the placebo group at the end of the study, and investigator global assessment scores and patient questionnaire results revealed substantial comparative efficacy of cysteamine cream.6 Topical cysteamine has also demonstrated notable efficacy in treating senile lentigines, which typically do not respond to topical depigmenting products.5
Farshi et al. used Dermacatch as a novel measurement tool to ascertain the efficacy of cysteamine cream for treating epidermal melasma in a 2018 report of a randomized, double-blind, placebo-controlled study with 40 patients. During the 4-month trial, cysteamine cream or placebo was applied nightly before sleep. Investigators measured treatment efficacy through Dermacatch, and Mexameter skin colorimetry, MASI scores, investigator global assessments, and patient questionnaires at baseline, 2 months, and 4 months. Through all measurement methods, cysteamine was found to reduce melanin content of melasma lesions, with Dermacatch performing reliably and comparably to Mexameter.7 Since then, cysteamine has been compared to several first-line melasma therapies.
Reviews
A 2019 systematic review by Austin et al. of randomized controlled trials (RCTs) on topical treatments for melasma identified 35 original RCTs evaluating a wide range of approximately 20 agents. They identified cysteamine, triple combination therapy, and tranexamic acid as the products netting the most robust recommendations. The researchers characterized cysteamine as conferring strong efficacy and reported anticancer activity while triple combination therapy poses the potential risk of ochronosis and tranexamic acid may present the risk for thrombosis. They concluded that more research is necessary, though, to establish the proper concentration and optimal formulation of cysteamine as a frontline therapy.8
More reviews have since been published to further clarify where cysteamine stands among the optimal treatments for melasma. In a May 2022 systematic PubMed review of topical agents used to treat melasma, González-Molina et al. identified 80 papers meeting inclusion criteria (double or single blinded, prospective, controlled or RCTs, reviews of literature, and meta-analysis studies), with tranexamic acid and cysteamine among the novel well-tolerated agents. Cysteamine was not associated with any severe adverse effects and is recommended as an adjuvant and maintenance therapy.3
A September 2022 review by Niazi et al. found that while the signaling mechanisms through which cysteamine suppresses melasma are not well understood, the topical application of cysteamine cream is seen as safe and effective alone or in combination with other products to treat melasma.2
A systematic review and meta-analysis reported by Gomes dos Santos-Neto et al. at the end of 2022 considered the efficacy of depigmenting formulations containing 5% cysteamine for treating melasma. The meta-analysis covered six studies, with 120 melasma patients treated. The conclusion was that 5% cysteamine was effective with adverse effects unlikely.9
Cysteamine vs. hydroquinone
In 2020, Lima et al. reported the results of a quasi-randomized, multicenter, evaluator-blinded comparative study of topical 0.56% cysteamine and 4% HQ in 40 women with facial melasma. (Note that this study originally claimed a 5% cysteamine concentration, but a letter to the editor of the International Journal of Dermatology in 2020 disputed this and proved it was 0.56%) For 120 days, volunteers applied either 0.56% cysteamine or 4% HQ nightly. Tinted sunscreen (SPF 50; PPD 19) use was required for all participants. There were no differences in colorimetric evaluations between the groups, both of which showed progressive depigmenting, or in photographic assessments. The HQ group demonstrated greater mean decreases in modified melasma area severity index (mMASI) scores (41% for HQ and 24% for cysteamine at 60 days; 53% for HQ and 38% for cysteamine at 120 days). The investigators observed that while cysteamine was safe, well tolerated, and effective, it was outperformed by HQ in terms of mMASI and melasma quality of life (MELASQoL) scores.10
Early the next year, results of a randomized, double-blind, single-center study in 20 women, conducted by Nguyen et al. comparing the efficacy of cysteamine cream with HQ for melasma treatment were published. Participants were given either treatment over 16 weeks. Ultimately, five volunteers in the cysteamine group and nine in the HQ group completed the study. There was no statistically significant difference in mMASI scores between the groups. In this notably small study, HQ was tolerated better. The researchers concluded that their findings supported the argument of comparable efficacy between cysteamine and HQ, with further studies needed to establish whether cysteamine would be an appropriate alternative to HQ.11 Notably, HQ was banned by the Food and Drug Administration in 2020 in over-the-counter products.
Cysteamine vs. Kligman’s formula
Early in 2021, Karrabi et al. published the results of a randomized, double-blind clinical trial of 50 subjects with epidermal melasma to compare cysteamine 5% with Modified Kligman’s formula. Over 4 months, participants applied once daily either cysteamine cream 5% (15 minutes exposure) or the Modified Kligman’s formula (4% hydroquinone, 0.05% retinoic acid and 0.1% betamethasone) for whole night exposure. At 2 and 4 months, a statistically significant difference in mMASI score was noted, with the percentage decline in mMASI score nearly 9% higher in the cysteamine group. The investigators concluded that cysteamine 5% demonstrated greater efficacy than the Modified Kligman’s formula and was also better tolerated.12
Cysteamine vs. tranexamic acid
Later that year, Karrabi et al. published the results of a single-blind, randomized clinical trial assessing the efficacy of tranexamic acid mesotherapy compared with cysteamine 5% cream in 54 melasma patients. For 4 consecutive months, the cysteamine 5% cream group applied the cream on lesions 30 minutes before going to sleep. Every 4 weeks until 2 months, a physician performed tranexamic acid mesotherapy (0.05 mL; 4 mg/mL) on individuals in the tranexamic acid group. The researchers concluded, after measurements using both a Dermacatch device and the mMASI, that neither treatment was significantly better than the other but fewer complications were observed in the cysteamine group.13
Safety
In 2022, Sepaskhah et al. assessed the effects of a cysteamine 5% cream and compared it with HQ 4%/ascorbic acid 3% cream for epidermal melasma in a single-blind, randomized controlled trial. Sixty-five of 80 patients completed the study. The difference in mMASI scores after 4 months was not significant between the groups nor was the improvement in quality of life, but the melanin index was significantly lower in the HQ/ascorbic acid group compared with the less substantial reduction for the cysteamine group. Nevertheless, the researchers concluded that cysteamine is a safe and suitable substitute for HQ/ascorbic acid.4
Conclusion
In the last decade, cysteamine has been established as a potent depigmenting agent. Its suitability and desirability as a top consideration for melasma treatment also appears to be compelling. More RCTs comparing cysteamine and other topline therapies are warranted, but current evidence shows that cysteamine is an effective and safe therapy for melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Konar MC et al. J Trop Pediatr. 2020 Apr 1;66(2):129-35.
2. Niazi S et al. J Cosmet Dermatol. 2022 Sep;21(9):3867-75.
3. González-Molina V et al. J Clin Aesthet Dermatol. 2022 May;15(5):19-28.
4. Sepaskhah M et al. J Cosmet Dermatol. 2022 Jul;21(7):2871-8.
5. Desai S et al. J Drugs Dermatol. 2021 Dec 1;20(12):1276-9.
6. Mansouri P et al. Br J Dermatol. 2015 Jul;173(1):209-17.
7. Farshi S et al. J Dermatolog Treat. 2018 Mar;29(2):182-9.
8. Austin E et al. J Drugs Dermatol. 2019 Nov 1;18(11):S1545961619P1156X.
9. Gomes dos Santos-Neto A et al. Dermatol Ther. 2022 Dec;35(12):e15961.
10. Lima PB et al. Int J Dermatol. 2020 Dec;59(12):1531-6.
11. Nguyen J et al. Australas J Dermatol. 2021 Feb;62(1):e41-e46.
12. Karrabi M et al. Skin Res Technol. 2021 Jan;27(1):24-31.
13. Karrabi M et al. Arch Dermatol Res. 2021 Sep;313(7):539-47.
Most subjects covered in this column are botanical ingredients used for multiple conditions in topical skin care. The focus this month, though, is a natural agent garnering attention primarily for one indication. Present in many mammals and in various cells in the human body (and particularly highly concentrated in human milk), cysteamine is a stable aminothiol that acts as an antioxidant as a result of the degradation of coenzyme A and is known to play a protective function.1 Melasma, an acquired recurrent, chronic hyperpigmentary disorder, continues to be a treatment challenge and is often psychologically troublesome for those affected, approximately 90% of whom are women.2 Individuals with Fitzpatrick skin types IV and V who reside in regions where UV exposure is likely are particularly prominent among those with melasma.2 While triple combination therapy (also known as Kligman’s formula) continues to be the modern gold standard of care for melasma (over the last 30 years),3 cysteamine, a nonmelanocytotoxic molecule, is considered viable for long-term use and safer than the long-time skin-lightening gold standard over several decades, hydroquinone (HQ), which is associated with safety concerns.4 .
Recent history and the 2015 study
Prior to 2015, the quick oxidation and malodorous nature of cysteamine rendered it unsuitable for use as a topical agent. However, stabilization efforts resulted in a product that first began to show efficacy that year.5
Mansouri et al. conducted a randomized, double-blind, placebo-controlled trial to assess the efficacy of topical cysteamine 5% to treat epidermal melasma in 2015. Over 4 months, 50 volunteers (25 in each group) applied either cysteamine cream or placebo on lesions once nightly. The mean differences at baseline between pigmented and normal skin were 75.2 ± 37 in the cysteamine group and 68.9 ± 31 in the placebo group. Statistically significant differences between the groups were identified at the 2- and 4-month points. At 2 months, the mean differences were 39.7 ± 16.6 in the cysteamine group and 63.8 ± 28.6 in the placebo group; at 4 months, the respective differences were 26.2 ± 16 and 60.7 ± 27.3. Melasma area severity index (MASI) scores were significantly lower in the cysteamine group compared with the placebo group at the end of the study, and investigator global assessment scores and patient questionnaire results revealed substantial comparative efficacy of cysteamine cream.6 Topical cysteamine has also demonstrated notable efficacy in treating senile lentigines, which typically do not respond to topical depigmenting products.5
Farshi et al. used Dermacatch as a novel measurement tool to ascertain the efficacy of cysteamine cream for treating epidermal melasma in a 2018 report of a randomized, double-blind, placebo-controlled study with 40 patients. During the 4-month trial, cysteamine cream or placebo was applied nightly before sleep. Investigators measured treatment efficacy through Dermacatch, and Mexameter skin colorimetry, MASI scores, investigator global assessments, and patient questionnaires at baseline, 2 months, and 4 months. Through all measurement methods, cysteamine was found to reduce melanin content of melasma lesions, with Dermacatch performing reliably and comparably to Mexameter.7 Since then, cysteamine has been compared to several first-line melasma therapies.
Reviews
A 2019 systematic review by Austin et al. of randomized controlled trials (RCTs) on topical treatments for melasma identified 35 original RCTs evaluating a wide range of approximately 20 agents. They identified cysteamine, triple combination therapy, and tranexamic acid as the products netting the most robust recommendations. The researchers characterized cysteamine as conferring strong efficacy and reported anticancer activity while triple combination therapy poses the potential risk of ochronosis and tranexamic acid may present the risk for thrombosis. They concluded that more research is necessary, though, to establish the proper concentration and optimal formulation of cysteamine as a frontline therapy.8
More reviews have since been published to further clarify where cysteamine stands among the optimal treatments for melasma. In a May 2022 systematic PubMed review of topical agents used to treat melasma, González-Molina et al. identified 80 papers meeting inclusion criteria (double or single blinded, prospective, controlled or RCTs, reviews of literature, and meta-analysis studies), with tranexamic acid and cysteamine among the novel well-tolerated agents. Cysteamine was not associated with any severe adverse effects and is recommended as an adjuvant and maintenance therapy.3
A September 2022 review by Niazi et al. found that while the signaling mechanisms through which cysteamine suppresses melasma are not well understood, the topical application of cysteamine cream is seen as safe and effective alone or in combination with other products to treat melasma.2
A systematic review and meta-analysis reported by Gomes dos Santos-Neto et al. at the end of 2022 considered the efficacy of depigmenting formulations containing 5% cysteamine for treating melasma. The meta-analysis covered six studies, with 120 melasma patients treated. The conclusion was that 5% cysteamine was effective with adverse effects unlikely.9
Cysteamine vs. hydroquinone
In 2020, Lima et al. reported the results of a quasi-randomized, multicenter, evaluator-blinded comparative study of topical 0.56% cysteamine and 4% HQ in 40 women with facial melasma. (Note that this study originally claimed a 5% cysteamine concentration, but a letter to the editor of the International Journal of Dermatology in 2020 disputed this and proved it was 0.56%) For 120 days, volunteers applied either 0.56% cysteamine or 4% HQ nightly. Tinted sunscreen (SPF 50; PPD 19) use was required for all participants. There were no differences in colorimetric evaluations between the groups, both of which showed progressive depigmenting, or in photographic assessments. The HQ group demonstrated greater mean decreases in modified melasma area severity index (mMASI) scores (41% for HQ and 24% for cysteamine at 60 days; 53% for HQ and 38% for cysteamine at 120 days). The investigators observed that while cysteamine was safe, well tolerated, and effective, it was outperformed by HQ in terms of mMASI and melasma quality of life (MELASQoL) scores.10
Early the next year, results of a randomized, double-blind, single-center study in 20 women, conducted by Nguyen et al. comparing the efficacy of cysteamine cream with HQ for melasma treatment were published. Participants were given either treatment over 16 weeks. Ultimately, five volunteers in the cysteamine group and nine in the HQ group completed the study. There was no statistically significant difference in mMASI scores between the groups. In this notably small study, HQ was tolerated better. The researchers concluded that their findings supported the argument of comparable efficacy between cysteamine and HQ, with further studies needed to establish whether cysteamine would be an appropriate alternative to HQ.11 Notably, HQ was banned by the Food and Drug Administration in 2020 in over-the-counter products.
Cysteamine vs. Kligman’s formula
Early in 2021, Karrabi et al. published the results of a randomized, double-blind clinical trial of 50 subjects with epidermal melasma to compare cysteamine 5% with Modified Kligman’s formula. Over 4 months, participants applied once daily either cysteamine cream 5% (15 minutes exposure) or the Modified Kligman’s formula (4% hydroquinone, 0.05% retinoic acid and 0.1% betamethasone) for whole night exposure. At 2 and 4 months, a statistically significant difference in mMASI score was noted, with the percentage decline in mMASI score nearly 9% higher in the cysteamine group. The investigators concluded that cysteamine 5% demonstrated greater efficacy than the Modified Kligman’s formula and was also better tolerated.12
Cysteamine vs. tranexamic acid
Later that year, Karrabi et al. published the results of a single-blind, randomized clinical trial assessing the efficacy of tranexamic acid mesotherapy compared with cysteamine 5% cream in 54 melasma patients. For 4 consecutive months, the cysteamine 5% cream group applied the cream on lesions 30 minutes before going to sleep. Every 4 weeks until 2 months, a physician performed tranexamic acid mesotherapy (0.05 mL; 4 mg/mL) on individuals in the tranexamic acid group. The researchers concluded, after measurements using both a Dermacatch device and the mMASI, that neither treatment was significantly better than the other but fewer complications were observed in the cysteamine group.13
Safety
In 2022, Sepaskhah et al. assessed the effects of a cysteamine 5% cream and compared it with HQ 4%/ascorbic acid 3% cream for epidermal melasma in a single-blind, randomized controlled trial. Sixty-five of 80 patients completed the study. The difference in mMASI scores after 4 months was not significant between the groups nor was the improvement in quality of life, but the melanin index was significantly lower in the HQ/ascorbic acid group compared with the less substantial reduction for the cysteamine group. Nevertheless, the researchers concluded that cysteamine is a safe and suitable substitute for HQ/ascorbic acid.4
Conclusion
In the last decade, cysteamine has been established as a potent depigmenting agent. Its suitability and desirability as a top consideration for melasma treatment also appears to be compelling. More RCTs comparing cysteamine and other topline therapies are warranted, but current evidence shows that cysteamine is an effective and safe therapy for melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Konar MC et al. J Trop Pediatr. 2020 Apr 1;66(2):129-35.
2. Niazi S et al. J Cosmet Dermatol. 2022 Sep;21(9):3867-75.
3. González-Molina V et al. J Clin Aesthet Dermatol. 2022 May;15(5):19-28.
4. Sepaskhah M et al. J Cosmet Dermatol. 2022 Jul;21(7):2871-8.
5. Desai S et al. J Drugs Dermatol. 2021 Dec 1;20(12):1276-9.
6. Mansouri P et al. Br J Dermatol. 2015 Jul;173(1):209-17.
7. Farshi S et al. J Dermatolog Treat. 2018 Mar;29(2):182-9.
8. Austin E et al. J Drugs Dermatol. 2019 Nov 1;18(11):S1545961619P1156X.
9. Gomes dos Santos-Neto A et al. Dermatol Ther. 2022 Dec;35(12):e15961.
10. Lima PB et al. Int J Dermatol. 2020 Dec;59(12):1531-6.
11. Nguyen J et al. Australas J Dermatol. 2021 Feb;62(1):e41-e46.
12. Karrabi M et al. Skin Res Technol. 2021 Jan;27(1):24-31.
13. Karrabi M et al. Arch Dermatol Res. 2021 Sep;313(7):539-47.
Most subjects covered in this column are botanical ingredients used for multiple conditions in topical skin care. The focus this month, though, is a natural agent garnering attention primarily for one indication. Present in many mammals and in various cells in the human body (and particularly highly concentrated in human milk), cysteamine is a stable aminothiol that acts as an antioxidant as a result of the degradation of coenzyme A and is known to play a protective function.1 Melasma, an acquired recurrent, chronic hyperpigmentary disorder, continues to be a treatment challenge and is often psychologically troublesome for those affected, approximately 90% of whom are women.2 Individuals with Fitzpatrick skin types IV and V who reside in regions where UV exposure is likely are particularly prominent among those with melasma.2 While triple combination therapy (also known as Kligman’s formula) continues to be the modern gold standard of care for melasma (over the last 30 years),3 cysteamine, a nonmelanocytotoxic molecule, is considered viable for long-term use and safer than the long-time skin-lightening gold standard over several decades, hydroquinone (HQ), which is associated with safety concerns.4 .
Recent history and the 2015 study
Prior to 2015, the quick oxidation and malodorous nature of cysteamine rendered it unsuitable for use as a topical agent. However, stabilization efforts resulted in a product that first began to show efficacy that year.5
Mansouri et al. conducted a randomized, double-blind, placebo-controlled trial to assess the efficacy of topical cysteamine 5% to treat epidermal melasma in 2015. Over 4 months, 50 volunteers (25 in each group) applied either cysteamine cream or placebo on lesions once nightly. The mean differences at baseline between pigmented and normal skin were 75.2 ± 37 in the cysteamine group and 68.9 ± 31 in the placebo group. Statistically significant differences between the groups were identified at the 2- and 4-month points. At 2 months, the mean differences were 39.7 ± 16.6 in the cysteamine group and 63.8 ± 28.6 in the placebo group; at 4 months, the respective differences were 26.2 ± 16 and 60.7 ± 27.3. Melasma area severity index (MASI) scores were significantly lower in the cysteamine group compared with the placebo group at the end of the study, and investigator global assessment scores and patient questionnaire results revealed substantial comparative efficacy of cysteamine cream.6 Topical cysteamine has also demonstrated notable efficacy in treating senile lentigines, which typically do not respond to topical depigmenting products.5
Farshi et al. used Dermacatch as a novel measurement tool to ascertain the efficacy of cysteamine cream for treating epidermal melasma in a 2018 report of a randomized, double-blind, placebo-controlled study with 40 patients. During the 4-month trial, cysteamine cream or placebo was applied nightly before sleep. Investigators measured treatment efficacy through Dermacatch, and Mexameter skin colorimetry, MASI scores, investigator global assessments, and patient questionnaires at baseline, 2 months, and 4 months. Through all measurement methods, cysteamine was found to reduce melanin content of melasma lesions, with Dermacatch performing reliably and comparably to Mexameter.7 Since then, cysteamine has been compared to several first-line melasma therapies.
Reviews
A 2019 systematic review by Austin et al. of randomized controlled trials (RCTs) on topical treatments for melasma identified 35 original RCTs evaluating a wide range of approximately 20 agents. They identified cysteamine, triple combination therapy, and tranexamic acid as the products netting the most robust recommendations. The researchers characterized cysteamine as conferring strong efficacy and reported anticancer activity while triple combination therapy poses the potential risk of ochronosis and tranexamic acid may present the risk for thrombosis. They concluded that more research is necessary, though, to establish the proper concentration and optimal formulation of cysteamine as a frontline therapy.8
More reviews have since been published to further clarify where cysteamine stands among the optimal treatments for melasma. In a May 2022 systematic PubMed review of topical agents used to treat melasma, González-Molina et al. identified 80 papers meeting inclusion criteria (double or single blinded, prospective, controlled or RCTs, reviews of literature, and meta-analysis studies), with tranexamic acid and cysteamine among the novel well-tolerated agents. Cysteamine was not associated with any severe adverse effects and is recommended as an adjuvant and maintenance therapy.3
A September 2022 review by Niazi et al. found that while the signaling mechanisms through which cysteamine suppresses melasma are not well understood, the topical application of cysteamine cream is seen as safe and effective alone or in combination with other products to treat melasma.2
A systematic review and meta-analysis reported by Gomes dos Santos-Neto et al. at the end of 2022 considered the efficacy of depigmenting formulations containing 5% cysteamine for treating melasma. The meta-analysis covered six studies, with 120 melasma patients treated. The conclusion was that 5% cysteamine was effective with adverse effects unlikely.9
Cysteamine vs. hydroquinone
In 2020, Lima et al. reported the results of a quasi-randomized, multicenter, evaluator-blinded comparative study of topical 0.56% cysteamine and 4% HQ in 40 women with facial melasma. (Note that this study originally claimed a 5% cysteamine concentration, but a letter to the editor of the International Journal of Dermatology in 2020 disputed this and proved it was 0.56%) For 120 days, volunteers applied either 0.56% cysteamine or 4% HQ nightly. Tinted sunscreen (SPF 50; PPD 19) use was required for all participants. There were no differences in colorimetric evaluations between the groups, both of which showed progressive depigmenting, or in photographic assessments. The HQ group demonstrated greater mean decreases in modified melasma area severity index (mMASI) scores (41% for HQ and 24% for cysteamine at 60 days; 53% for HQ and 38% for cysteamine at 120 days). The investigators observed that while cysteamine was safe, well tolerated, and effective, it was outperformed by HQ in terms of mMASI and melasma quality of life (MELASQoL) scores.10
Early the next year, results of a randomized, double-blind, single-center study in 20 women, conducted by Nguyen et al. comparing the efficacy of cysteamine cream with HQ for melasma treatment were published. Participants were given either treatment over 16 weeks. Ultimately, five volunteers in the cysteamine group and nine in the HQ group completed the study. There was no statistically significant difference in mMASI scores between the groups. In this notably small study, HQ was tolerated better. The researchers concluded that their findings supported the argument of comparable efficacy between cysteamine and HQ, with further studies needed to establish whether cysteamine would be an appropriate alternative to HQ.11 Notably, HQ was banned by the Food and Drug Administration in 2020 in over-the-counter products.
Cysteamine vs. Kligman’s formula
Early in 2021, Karrabi et al. published the results of a randomized, double-blind clinical trial of 50 subjects with epidermal melasma to compare cysteamine 5% with Modified Kligman’s formula. Over 4 months, participants applied once daily either cysteamine cream 5% (15 minutes exposure) or the Modified Kligman’s formula (4% hydroquinone, 0.05% retinoic acid and 0.1% betamethasone) for whole night exposure. At 2 and 4 months, a statistically significant difference in mMASI score was noted, with the percentage decline in mMASI score nearly 9% higher in the cysteamine group. The investigators concluded that cysteamine 5% demonstrated greater efficacy than the Modified Kligman’s formula and was also better tolerated.12
Cysteamine vs. tranexamic acid
Later that year, Karrabi et al. published the results of a single-blind, randomized clinical trial assessing the efficacy of tranexamic acid mesotherapy compared with cysteamine 5% cream in 54 melasma patients. For 4 consecutive months, the cysteamine 5% cream group applied the cream on lesions 30 minutes before going to sleep. Every 4 weeks until 2 months, a physician performed tranexamic acid mesotherapy (0.05 mL; 4 mg/mL) on individuals in the tranexamic acid group. The researchers concluded, after measurements using both a Dermacatch device and the mMASI, that neither treatment was significantly better than the other but fewer complications were observed in the cysteamine group.13
Safety
In 2022, Sepaskhah et al. assessed the effects of a cysteamine 5% cream and compared it with HQ 4%/ascorbic acid 3% cream for epidermal melasma in a single-blind, randomized controlled trial. Sixty-five of 80 patients completed the study. The difference in mMASI scores after 4 months was not significant between the groups nor was the improvement in quality of life, but the melanin index was significantly lower in the HQ/ascorbic acid group compared with the less substantial reduction for the cysteamine group. Nevertheless, the researchers concluded that cysteamine is a safe and suitable substitute for HQ/ascorbic acid.4
Conclusion
In the last decade, cysteamine has been established as a potent depigmenting agent. Its suitability and desirability as a top consideration for melasma treatment also appears to be compelling. More RCTs comparing cysteamine and other topline therapies are warranted, but current evidence shows that cysteamine is an effective and safe therapy for melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Konar MC et al. J Trop Pediatr. 2020 Apr 1;66(2):129-35.
2. Niazi S et al. J Cosmet Dermatol. 2022 Sep;21(9):3867-75.
3. González-Molina V et al. J Clin Aesthet Dermatol. 2022 May;15(5):19-28.
4. Sepaskhah M et al. J Cosmet Dermatol. 2022 Jul;21(7):2871-8.
5. Desai S et al. J Drugs Dermatol. 2021 Dec 1;20(12):1276-9.
6. Mansouri P et al. Br J Dermatol. 2015 Jul;173(1):209-17.
7. Farshi S et al. J Dermatolog Treat. 2018 Mar;29(2):182-9.
8. Austin E et al. J Drugs Dermatol. 2019 Nov 1;18(11):S1545961619P1156X.
9. Gomes dos Santos-Neto A et al. Dermatol Ther. 2022 Dec;35(12):e15961.
10. Lima PB et al. Int J Dermatol. 2020 Dec;59(12):1531-6.
11. Nguyen J et al. Australas J Dermatol. 2021 Feb;62(1):e41-e46.
12. Karrabi M et al. Skin Res Technol. 2021 Jan;27(1):24-31.
13. Karrabi M et al. Arch Dermatol Res. 2021 Sep;313(7):539-47.
Phase 3 trial supports topical JAK inhibitor for AD in young children
BERLIN – as previously shown in adolescents and adults for whom it already has an approved indication.
In this study – TRUE-AD3 – systemic exposure to ruxolitinib, which is selective for JAK1 and 2, was followed closely, and the low mean plasma concentrations “suggest systemic JAK inhibition is highly unlikely,” Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, said at the annual congress of the European Academy of Dermatology and Venereology.
For example, at a plasma concentration no greater than 27 nM in both younger and older patients at 4 weeks and again at 8 weeks, the systemic exposure was about a tenth of that (281 nM) previously associated with myelosuppression, he reported.
Given the boxed warning for oral JAK inhibitors, which was based largely on a 2022 study in adults with rheumatoid arthritis that associated tofacitinib, a nonspecific JAK inhibitor, with an increased risk of thrombotic events in adults already at risk for these events, safety was a focus of this phase 3 trial. The boxed warning is also in the labeling for topical ruxolitinib, 1.5% (Opzelura), approved for treating to mild to moderate atopic dermatitis in patients 12 years of age and older.
Dr. Eichenfield said there were no significant safety signals in the younger pediatric population. “There were no treatment-emergent adverse events suggestive of systemic JAK inhibition,” he said. This not only included the absence of serious infections, cardiac events, thromboses, or malignancies, but there was no signal of hematologic abnormalities, such as change in hemoglobin or neutrophil count.
Application site reactions
Rather, in the study of children ages 2-11, the only adverse events associated with topical ruxolitinib not observed in the control arm, which received the vehicle alone, were application site reactions, such as pain, erythema, and irritation. None of these occurred in more than 3% of those randomized to ruxolitinib regardless of dose.
Overall, in the trial, which randomized 329 patients ages from 2 to under 12 years with mild to moderate AD to ruxolitinib 1.5% cream, ruxolitinib 0.75% cream, or vehicle in a 2:2:1 fashion, there were just two (0.8%) discontinuations in the ruxolitinib groups (one in each dosing arm). There were none in the vehicle arm.
The safety supports an expansion of the AD indication for topical ruxolitinib in young children, because the rates of response were very similar to that seen in adolescents and adults in the previously published TRUE AD-1 and TRUE AD-2 trials, he said.
For the primary endpoint of Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 (almost clear) with at least a 2 grade improvement in IGA score from baseline, the response rates were 56.5%, 36.6%, and 10.8% for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively, at 8 weeks (P < .0001 for both doses relative to vehicle).
For the secondary efficacy endpoint of 75% or greater clearance on the Eczema Area and Severity Index, the rates were 67.2%, 51.5%, and 15.4%, for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively. Again, the advantage of both doses of ruxolitinib relative to vehicle was highly statistically significant (P < .0001).
Control of itch, evaluated with the Numerical Rating Scale was only evaluated in children 6-2 because of concern of the reliability of reporting in younger children. Control was defined as at least a 4-point improvement from baseline. It was achieved by 43.4%, 37.5%, and 29.7% by week 8 in the arms receiving the higher dose of ruxolitinib, the lower dose, and vehicle, respectively. The median time to achieving itch control was 11 days, 13 days, and 23 days, respectively. For all of these endpoints, the separation of the curves was readily apparent within the first 2 weeks.
The efficacy and tolerability of ruxolitinib appeared to be similar in younger children (ages 2-6) relative to older children.
Extension study in children near completion
Most of the patients who participated in TRUE AD-3 have been rolled over to the open-label extension trial, which is nearing completion. Those originally randomized to vehicle have been rerandomized to the lower or higher dose of ruxolitinib.
While this trial was focused on ruxolitinib as monotherapy, Thrasyvoulos Tzellos, MD, head of the department of dermatology, Nordland Hospital Trust, Bødo, Norway, questioned whether this is will be how it will be used in clinical practice. With the increasing array of therapies for AD, the “concept of combination therapy becomes more and more relevant,” he said after Dr. Eichenfield’s presentation.
Questioning whether an effective nonsteroidal anti-inflammatory agent like ruxolitinib should be considered a first-line treatment in mild disease or an adjunctive treatment for AD of any severity, he suggested that it might be best considered within a combination.
Dr. Eichenfield agreed. “Once we get the drug approved in a controlled trial, I think we then figure out how to use it in clinical practice.” Based on his own use of ruxolitinib in adults, he noted that he has not seen this drug replace other therapies so much as provide another option for control.
“We have an increasing armamentarium of drugs to use for involvement in different areas of the body in order to get more long-term control of disease,” he said. As an effective topical nonsteroidal drug, he believes its addition to clinical care in younger children, if approved, will be meaningful.
Dr. Eichenfield disclosed financial relationships with more multiple pharmaceutical companies, including Incyte, the manufacturer of ruxolitinib cream that provided funding for the True-AD trials. Dr. Tzellos reported financial relationships with AbbVie and UCB.
BERLIN – as previously shown in adolescents and adults for whom it already has an approved indication.
In this study – TRUE-AD3 – systemic exposure to ruxolitinib, which is selective for JAK1 and 2, was followed closely, and the low mean plasma concentrations “suggest systemic JAK inhibition is highly unlikely,” Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, said at the annual congress of the European Academy of Dermatology and Venereology.
For example, at a plasma concentration no greater than 27 nM in both younger and older patients at 4 weeks and again at 8 weeks, the systemic exposure was about a tenth of that (281 nM) previously associated with myelosuppression, he reported.
Given the boxed warning for oral JAK inhibitors, which was based largely on a 2022 study in adults with rheumatoid arthritis that associated tofacitinib, a nonspecific JAK inhibitor, with an increased risk of thrombotic events in adults already at risk for these events, safety was a focus of this phase 3 trial. The boxed warning is also in the labeling for topical ruxolitinib, 1.5% (Opzelura), approved for treating to mild to moderate atopic dermatitis in patients 12 years of age and older.
Dr. Eichenfield said there were no significant safety signals in the younger pediatric population. “There were no treatment-emergent adverse events suggestive of systemic JAK inhibition,” he said. This not only included the absence of serious infections, cardiac events, thromboses, or malignancies, but there was no signal of hematologic abnormalities, such as change in hemoglobin or neutrophil count.
Application site reactions
Rather, in the study of children ages 2-11, the only adverse events associated with topical ruxolitinib not observed in the control arm, which received the vehicle alone, were application site reactions, such as pain, erythema, and irritation. None of these occurred in more than 3% of those randomized to ruxolitinib regardless of dose.
Overall, in the trial, which randomized 329 patients ages from 2 to under 12 years with mild to moderate AD to ruxolitinib 1.5% cream, ruxolitinib 0.75% cream, or vehicle in a 2:2:1 fashion, there were just two (0.8%) discontinuations in the ruxolitinib groups (one in each dosing arm). There were none in the vehicle arm.
The safety supports an expansion of the AD indication for topical ruxolitinib in young children, because the rates of response were very similar to that seen in adolescents and adults in the previously published TRUE AD-1 and TRUE AD-2 trials, he said.
For the primary endpoint of Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 (almost clear) with at least a 2 grade improvement in IGA score from baseline, the response rates were 56.5%, 36.6%, and 10.8% for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively, at 8 weeks (P < .0001 for both doses relative to vehicle).
For the secondary efficacy endpoint of 75% or greater clearance on the Eczema Area and Severity Index, the rates were 67.2%, 51.5%, and 15.4%, for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively. Again, the advantage of both doses of ruxolitinib relative to vehicle was highly statistically significant (P < .0001).
Control of itch, evaluated with the Numerical Rating Scale was only evaluated in children 6-2 because of concern of the reliability of reporting in younger children. Control was defined as at least a 4-point improvement from baseline. It was achieved by 43.4%, 37.5%, and 29.7% by week 8 in the arms receiving the higher dose of ruxolitinib, the lower dose, and vehicle, respectively. The median time to achieving itch control was 11 days, 13 days, and 23 days, respectively. For all of these endpoints, the separation of the curves was readily apparent within the first 2 weeks.
The efficacy and tolerability of ruxolitinib appeared to be similar in younger children (ages 2-6) relative to older children.
Extension study in children near completion
Most of the patients who participated in TRUE AD-3 have been rolled over to the open-label extension trial, which is nearing completion. Those originally randomized to vehicle have been rerandomized to the lower or higher dose of ruxolitinib.
While this trial was focused on ruxolitinib as monotherapy, Thrasyvoulos Tzellos, MD, head of the department of dermatology, Nordland Hospital Trust, Bødo, Norway, questioned whether this is will be how it will be used in clinical practice. With the increasing array of therapies for AD, the “concept of combination therapy becomes more and more relevant,” he said after Dr. Eichenfield’s presentation.
Questioning whether an effective nonsteroidal anti-inflammatory agent like ruxolitinib should be considered a first-line treatment in mild disease or an adjunctive treatment for AD of any severity, he suggested that it might be best considered within a combination.
Dr. Eichenfield agreed. “Once we get the drug approved in a controlled trial, I think we then figure out how to use it in clinical practice.” Based on his own use of ruxolitinib in adults, he noted that he has not seen this drug replace other therapies so much as provide another option for control.
“We have an increasing armamentarium of drugs to use for involvement in different areas of the body in order to get more long-term control of disease,” he said. As an effective topical nonsteroidal drug, he believes its addition to clinical care in younger children, if approved, will be meaningful.
Dr. Eichenfield disclosed financial relationships with more multiple pharmaceutical companies, including Incyte, the manufacturer of ruxolitinib cream that provided funding for the True-AD trials. Dr. Tzellos reported financial relationships with AbbVie and UCB.
BERLIN – as previously shown in adolescents and adults for whom it already has an approved indication.
In this study – TRUE-AD3 – systemic exposure to ruxolitinib, which is selective for JAK1 and 2, was followed closely, and the low mean plasma concentrations “suggest systemic JAK inhibition is highly unlikely,” Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, said at the annual congress of the European Academy of Dermatology and Venereology.
For example, at a plasma concentration no greater than 27 nM in both younger and older patients at 4 weeks and again at 8 weeks, the systemic exposure was about a tenth of that (281 nM) previously associated with myelosuppression, he reported.
Given the boxed warning for oral JAK inhibitors, which was based largely on a 2022 study in adults with rheumatoid arthritis that associated tofacitinib, a nonspecific JAK inhibitor, with an increased risk of thrombotic events in adults already at risk for these events, safety was a focus of this phase 3 trial. The boxed warning is also in the labeling for topical ruxolitinib, 1.5% (Opzelura), approved for treating to mild to moderate atopic dermatitis in patients 12 years of age and older.
Dr. Eichenfield said there were no significant safety signals in the younger pediatric population. “There were no treatment-emergent adverse events suggestive of systemic JAK inhibition,” he said. This not only included the absence of serious infections, cardiac events, thromboses, or malignancies, but there was no signal of hematologic abnormalities, such as change in hemoglobin or neutrophil count.
Application site reactions
Rather, in the study of children ages 2-11, the only adverse events associated with topical ruxolitinib not observed in the control arm, which received the vehicle alone, were application site reactions, such as pain, erythema, and irritation. None of these occurred in more than 3% of those randomized to ruxolitinib regardless of dose.
Overall, in the trial, which randomized 329 patients ages from 2 to under 12 years with mild to moderate AD to ruxolitinib 1.5% cream, ruxolitinib 0.75% cream, or vehicle in a 2:2:1 fashion, there were just two (0.8%) discontinuations in the ruxolitinib groups (one in each dosing arm). There were none in the vehicle arm.
The safety supports an expansion of the AD indication for topical ruxolitinib in young children, because the rates of response were very similar to that seen in adolescents and adults in the previously published TRUE AD-1 and TRUE AD-2 trials, he said.
For the primary endpoint of Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 (almost clear) with at least a 2 grade improvement in IGA score from baseline, the response rates were 56.5%, 36.6%, and 10.8% for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively, at 8 weeks (P < .0001 for both doses relative to vehicle).
For the secondary efficacy endpoint of 75% or greater clearance on the Eczema Area and Severity Index, the rates were 67.2%, 51.5%, and 15.4%, for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively. Again, the advantage of both doses of ruxolitinib relative to vehicle was highly statistically significant (P < .0001).
Control of itch, evaluated with the Numerical Rating Scale was only evaluated in children 6-2 because of concern of the reliability of reporting in younger children. Control was defined as at least a 4-point improvement from baseline. It was achieved by 43.4%, 37.5%, and 29.7% by week 8 in the arms receiving the higher dose of ruxolitinib, the lower dose, and vehicle, respectively. The median time to achieving itch control was 11 days, 13 days, and 23 days, respectively. For all of these endpoints, the separation of the curves was readily apparent within the first 2 weeks.
The efficacy and tolerability of ruxolitinib appeared to be similar in younger children (ages 2-6) relative to older children.
Extension study in children near completion
Most of the patients who participated in TRUE AD-3 have been rolled over to the open-label extension trial, which is nearing completion. Those originally randomized to vehicle have been rerandomized to the lower or higher dose of ruxolitinib.
While this trial was focused on ruxolitinib as monotherapy, Thrasyvoulos Tzellos, MD, head of the department of dermatology, Nordland Hospital Trust, Bødo, Norway, questioned whether this is will be how it will be used in clinical practice. With the increasing array of therapies for AD, the “concept of combination therapy becomes more and more relevant,” he said after Dr. Eichenfield’s presentation.
Questioning whether an effective nonsteroidal anti-inflammatory agent like ruxolitinib should be considered a first-line treatment in mild disease or an adjunctive treatment for AD of any severity, he suggested that it might be best considered within a combination.
Dr. Eichenfield agreed. “Once we get the drug approved in a controlled trial, I think we then figure out how to use it in clinical practice.” Based on his own use of ruxolitinib in adults, he noted that he has not seen this drug replace other therapies so much as provide another option for control.
“We have an increasing armamentarium of drugs to use for involvement in different areas of the body in order to get more long-term control of disease,” he said. As an effective topical nonsteroidal drug, he believes its addition to clinical care in younger children, if approved, will be meaningful.
Dr. Eichenfield disclosed financial relationships with more multiple pharmaceutical companies, including Incyte, the manufacturer of ruxolitinib cream that provided funding for the True-AD trials. Dr. Tzellos reported financial relationships with AbbVie and UCB.
AT THE EADV CONGRESS
Painful axillary plaque
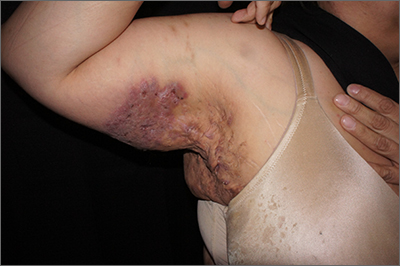
The persistent scars with recurrent abscesses and sinuses are indicative of advanced hidradenitis suppurativa. This painful and debilitating disease is characterized by the recurrent formation and inflammation of papules, cysts, sinuses, and scars in the axillae, inguinal folds, gluteal cleft, and inframammary folds. Pain, social isolation, depression, increased risk of substance abuse, and increased suicidality are all associated with hidradenitis suppurativa.
The disease may be graded based on severity, which can guide medical treatment options. The earliest stage appears similar to acne without significant sinus tract or scar formation and may be treated with topical therapies—including clindamycin 1% lotion or gel. When larger cysts associated with sinus tracts occur, systemic options with oral antibiotics (including doxycycline 100 mg bid for 3 months or combination clindamycin 300 mg and rifampin 300 mg, both bid for 3 months) are reasonable options. Intralesional triamcinolone in a concentration of 10 mg/mL injected directly into an inflamed cyst can provide acute relief. Severe disease is characterized by diffuse scars and sinus tracts. The TNF-alpha inhibitors adalimumab and infliximab are excellent options for severe disease that does not respond to antibiotics.
Surgical treatment may include either “deroofing” the sinuses or performing a wide excision of the whole area of involvement. Widely excised areas may be grafted, allowed to granulate, or closed if small enough. Although these options create significant wounds, patients experience good results; there is a 27% recurrence with deroofing and a 13% recurrence with wide excision.1
This patient underwent wide local excision of both axillae and the areas of involvement were allowed to granulate. Secondary intention healing occurred over 12 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Orenstein LAV, Nguyen TV, Damiani G, et al. Medical and surgical management of hidradenitis suppurativa: a review of international treatment guidelines and implementation in general dermatology practice. Dermatology. 2020;236:393-412. doi: 10.1159/000507323

The persistent scars with recurrent abscesses and sinuses are indicative of advanced hidradenitis suppurativa. This painful and debilitating disease is characterized by the recurrent formation and inflammation of papules, cysts, sinuses, and scars in the axillae, inguinal folds, gluteal cleft, and inframammary folds. Pain, social isolation, depression, increased risk of substance abuse, and increased suicidality are all associated with hidradenitis suppurativa.
The disease may be graded based on severity, which can guide medical treatment options. The earliest stage appears similar to acne without significant sinus tract or scar formation and may be treated with topical therapies—including clindamycin 1% lotion or gel. When larger cysts associated with sinus tracts occur, systemic options with oral antibiotics (including doxycycline 100 mg bid for 3 months or combination clindamycin 300 mg and rifampin 300 mg, both bid for 3 months) are reasonable options. Intralesional triamcinolone in a concentration of 10 mg/mL injected directly into an inflamed cyst can provide acute relief. Severe disease is characterized by diffuse scars and sinus tracts. The TNF-alpha inhibitors adalimumab and infliximab are excellent options for severe disease that does not respond to antibiotics.
Surgical treatment may include either “deroofing” the sinuses or performing a wide excision of the whole area of involvement. Widely excised areas may be grafted, allowed to granulate, or closed if small enough. Although these options create significant wounds, patients experience good results; there is a 27% recurrence with deroofing and a 13% recurrence with wide excision.1
This patient underwent wide local excision of both axillae and the areas of involvement were allowed to granulate. Secondary intention healing occurred over 12 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

The persistent scars with recurrent abscesses and sinuses are indicative of advanced hidradenitis suppurativa. This painful and debilitating disease is characterized by the recurrent formation and inflammation of papules, cysts, sinuses, and scars in the axillae, inguinal folds, gluteal cleft, and inframammary folds. Pain, social isolation, depression, increased risk of substance abuse, and increased suicidality are all associated with hidradenitis suppurativa.
The disease may be graded based on severity, which can guide medical treatment options. The earliest stage appears similar to acne without significant sinus tract or scar formation and may be treated with topical therapies—including clindamycin 1% lotion or gel. When larger cysts associated with sinus tracts occur, systemic options with oral antibiotics (including doxycycline 100 mg bid for 3 months or combination clindamycin 300 mg and rifampin 300 mg, both bid for 3 months) are reasonable options. Intralesional triamcinolone in a concentration of 10 mg/mL injected directly into an inflamed cyst can provide acute relief. Severe disease is characterized by diffuse scars and sinus tracts. The TNF-alpha inhibitors adalimumab and infliximab are excellent options for severe disease that does not respond to antibiotics.
Surgical treatment may include either “deroofing” the sinuses or performing a wide excision of the whole area of involvement. Widely excised areas may be grafted, allowed to granulate, or closed if small enough. Although these options create significant wounds, patients experience good results; there is a 27% recurrence with deroofing and a 13% recurrence with wide excision.1
This patient underwent wide local excision of both axillae and the areas of involvement were allowed to granulate. Secondary intention healing occurred over 12 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Orenstein LAV, Nguyen TV, Damiani G, et al. Medical and surgical management of hidradenitis suppurativa: a review of international treatment guidelines and implementation in general dermatology practice. Dermatology. 2020;236:393-412. doi: 10.1159/000507323
1. Orenstein LAV, Nguyen TV, Damiani G, et al. Medical and surgical management of hidradenitis suppurativa: a review of international treatment guidelines and implementation in general dermatology practice. Dermatology. 2020;236:393-412. doi: 10.1159/000507323
Ketamine no better for depression than placebo?
TOPLINE:
results of a new study suggest, contradicting prior research. Although symptoms improved in both study groups, investigators say participants’ expectations of an improvement from ketamine may be driving that result.
METHODOLOGY:
- The randomized, placebo-controlled trial included 40 patients who had previously been diagnosed with MDD and who were scheduled for elective noncardiac, nonintracranial surgery.
- Participants completed pre- and postsurgery depression screenings with the Patient Health Questionnaire–8 (inclusion score was ≥ 12) and the Montgomery-Åsberg Depression Rating Scale (MADRS).
- Patients received an infusion of 0.5 mg/kg of saline (placebo group; n = 20) or ketamine (n = 20) during surgery, along with general anesthesia.
- At the end of a 14-day follow-up, patients were asked to guess whether they had received ketamine or placebo.
TAKEAWAY:
- MADRS scores dropped by around half 1 day after treatment, indicating an improvement in depressive symptoms in both the group that received ketamine (mean decrease from 25 to 12.6 points) and the group that received placebo (mean decrease from 30 to 15.3 points). There was no significant difference between the two.
- Participants in the ketamine and placebo groups also reported high rates of clinical response (60% and 50%, respectively) and remission (50% and 35%, respectively), again with no significant difference based on treatment with ketamine or placebo.
- Only 36.8% of participants accurately guessed their treatment group. Those who guessed they had received ketamine had higher MADRS scores than those who guessed they had received placebo or said they didn’t know (10.1 vs. 19.2 vs. 23.0).
- The ketamine group had a significantly shorter hospital stay (1.9 days) than the placebo group (4 days) (P = .02).
IN PRACTICE:
“Our primary findings differ from those of previous antidepressant trials with ketamine conducted without adequate masking, which find robust effects of ketamine,” the authors wrote, adding that “regardless of the intervention being tested, participant expectations of a positive outcome – also known as hope – may drive large decreases in depression symptoms seen in antidepressant trials.”
SOURCE:
Boris D. Heifets, MD, PhD, led the study, which was published online in Nature Mental Health. The study was funded by the Society for Neuroscience in Anesthesiology and Critical Care, the National Institutes of Health, and the Stanford School of Medicine Research Office.
LIMITATIONS:
The investigators did not measure participants’ treatment expectations prior to randomization and could not determine what effect participant expectancy bias may have had on the results. In addition, there was no assessment of the blind for anesthesiologists who administered the ketamine or placebo to patients.
DISCLOSURES:
Dr. Heifets is on the scientific advisory boards of Osmind and Journey Clinical and is a consultant to Clairvoyant Therapeutics and Vine Ventures.
A version of this article first appeared on Medscape.com.
TOPLINE:
results of a new study suggest, contradicting prior research. Although symptoms improved in both study groups, investigators say participants’ expectations of an improvement from ketamine may be driving that result.
METHODOLOGY:
- The randomized, placebo-controlled trial included 40 patients who had previously been diagnosed with MDD and who were scheduled for elective noncardiac, nonintracranial surgery.
- Participants completed pre- and postsurgery depression screenings with the Patient Health Questionnaire–8 (inclusion score was ≥ 12) and the Montgomery-Åsberg Depression Rating Scale (MADRS).
- Patients received an infusion of 0.5 mg/kg of saline (placebo group; n = 20) or ketamine (n = 20) during surgery, along with general anesthesia.
- At the end of a 14-day follow-up, patients were asked to guess whether they had received ketamine or placebo.
TAKEAWAY:
- MADRS scores dropped by around half 1 day after treatment, indicating an improvement in depressive symptoms in both the group that received ketamine (mean decrease from 25 to 12.6 points) and the group that received placebo (mean decrease from 30 to 15.3 points). There was no significant difference between the two.
- Participants in the ketamine and placebo groups also reported high rates of clinical response (60% and 50%, respectively) and remission (50% and 35%, respectively), again with no significant difference based on treatment with ketamine or placebo.
- Only 36.8% of participants accurately guessed their treatment group. Those who guessed they had received ketamine had higher MADRS scores than those who guessed they had received placebo or said they didn’t know (10.1 vs. 19.2 vs. 23.0).
- The ketamine group had a significantly shorter hospital stay (1.9 days) than the placebo group (4 days) (P = .02).
IN PRACTICE:
“Our primary findings differ from those of previous antidepressant trials with ketamine conducted without adequate masking, which find robust effects of ketamine,” the authors wrote, adding that “regardless of the intervention being tested, participant expectations of a positive outcome – also known as hope – may drive large decreases in depression symptoms seen in antidepressant trials.”
SOURCE:
Boris D. Heifets, MD, PhD, led the study, which was published online in Nature Mental Health. The study was funded by the Society for Neuroscience in Anesthesiology and Critical Care, the National Institutes of Health, and the Stanford School of Medicine Research Office.
LIMITATIONS:
The investigators did not measure participants’ treatment expectations prior to randomization and could not determine what effect participant expectancy bias may have had on the results. In addition, there was no assessment of the blind for anesthesiologists who administered the ketamine or placebo to patients.
DISCLOSURES:
Dr. Heifets is on the scientific advisory boards of Osmind and Journey Clinical and is a consultant to Clairvoyant Therapeutics and Vine Ventures.
A version of this article first appeared on Medscape.com.
TOPLINE:
results of a new study suggest, contradicting prior research. Although symptoms improved in both study groups, investigators say participants’ expectations of an improvement from ketamine may be driving that result.
METHODOLOGY:
- The randomized, placebo-controlled trial included 40 patients who had previously been diagnosed with MDD and who were scheduled for elective noncardiac, nonintracranial surgery.
- Participants completed pre- and postsurgery depression screenings with the Patient Health Questionnaire–8 (inclusion score was ≥ 12) and the Montgomery-Åsberg Depression Rating Scale (MADRS).
- Patients received an infusion of 0.5 mg/kg of saline (placebo group; n = 20) or ketamine (n = 20) during surgery, along with general anesthesia.
- At the end of a 14-day follow-up, patients were asked to guess whether they had received ketamine or placebo.
TAKEAWAY:
- MADRS scores dropped by around half 1 day after treatment, indicating an improvement in depressive symptoms in both the group that received ketamine (mean decrease from 25 to 12.6 points) and the group that received placebo (mean decrease from 30 to 15.3 points). There was no significant difference between the two.
- Participants in the ketamine and placebo groups also reported high rates of clinical response (60% and 50%, respectively) and remission (50% and 35%, respectively), again with no significant difference based on treatment with ketamine or placebo.
- Only 36.8% of participants accurately guessed their treatment group. Those who guessed they had received ketamine had higher MADRS scores than those who guessed they had received placebo or said they didn’t know (10.1 vs. 19.2 vs. 23.0).
- The ketamine group had a significantly shorter hospital stay (1.9 days) than the placebo group (4 days) (P = .02).
IN PRACTICE:
“Our primary findings differ from those of previous antidepressant trials with ketamine conducted without adequate masking, which find robust effects of ketamine,” the authors wrote, adding that “regardless of the intervention being tested, participant expectations of a positive outcome – also known as hope – may drive large decreases in depression symptoms seen in antidepressant trials.”
SOURCE:
Boris D. Heifets, MD, PhD, led the study, which was published online in Nature Mental Health. The study was funded by the Society for Neuroscience in Anesthesiology and Critical Care, the National Institutes of Health, and the Stanford School of Medicine Research Office.
LIMITATIONS:
The investigators did not measure participants’ treatment expectations prior to randomization and could not determine what effect participant expectancy bias may have had on the results. In addition, there was no assessment of the blind for anesthesiologists who administered the ketamine or placebo to patients.
DISCLOSURES:
Dr. Heifets is on the scientific advisory boards of Osmind and Journey Clinical and is a consultant to Clairvoyant Therapeutics and Vine Ventures.
A version of this article first appeared on Medscape.com.
RA precision medicine using synovial biopsy ‘remains elusive’
Researchers have yet to crack how to analyze synovial tissue biopsy specimens to predict treatment responses for patients with rheumatoid arthritis.
According to new research, classifying patients by synovial fluid B-cell status was not reliable in predicting treatment response to etanercept, tocilizumab, or rituximab. More comprehensive molecular analyses may hold promise in developing models to predict treatment of a disease as heterogeneous as RA, wrote the authors, led by chief investigator Costantino Pitzalis, MD, head of the Centre for Experimental Medicine and Rheumatology at Queen Mary University of London.
The article was published in the November 2023 issue of The Lancet Rheumatology..
Trial-and-error treatment
Because clinicians do not have a way to reliably predict how patients may respond to certain medications, the current RA treatments involve trial and error.

“Both with regard to the first conventional synthetic disease-modifying antirheumatic drug (DMARD) for all patients and the first biologic DMARD for the subgroup of patients requiring these drugs, the choice of a drug is based on pragmatic arguments rather than on individual patient characteristics,” Annette H. van der Helm-van Mil, MD, PhD, professor of rheumatology at the Leiden University Medical Center, the Netherlands, wrote in an accompanying editorial.
Most research on predicting treatment response has used clinical features and blood biomarkers, but neither approach is reliably predictive. “Precision medicine, therefore, remains elusive,” she said. Newer research has focused on synovial tissue biopsy to inform research decisions.
The STRAP studies
In this newest study, researchers combined data from two clinical studies with identical design: the Stratification of Biological Therapies by Pathobiology in Biologic-Naive Patients With Rheumatoid Arthritis (STRAP) trial, taking place in the United Kingdom, and STRAP-EU, which recruited patients from the European Union. The trials are the largest biopsy-driven trials to date, according to the researchers.
In total, researchers recruited 223 biologic-naive adult patients from 26 universities in the United Kingdom and Europe. Participants underwent ultrasound-guided synovial tissue biopsy and were then randomly assigned to receive etanercept (72 patients), tocilizumab (73 patients), or rituximab (78 patients). In a histologic analysis, 121 patients were characterized as B-cell poor (BCP), 100 patients were classified as B-cell rich (BCR), and two patients were classified as undetermined.
“We hypothesized that patients with a low synovial histological or molecular B-cell score would have a lower response to rituximab than to etanercept or tocilizumab,” the authors wrote.
However, among the BCP patients, there were no significant differences between responses to treatment at week 16 in the rituximab group compared with the etanercept and tocilizumab groups put together. In both groups, around 60% of patients achieved the primary endpoint of at least 20% improvement in American College of Rheumatology response criteria.
The results suggest that “a dichotomic classification into synovial B cell poor versus rich is unable to predict treatment response in patients treated with rituximab compared with etanercept or tocilizumab,” the authors wrote.
The findings echo work from the same group of researchers in 2021. This study – the R4RA trial – enrolled 164 patients who previously had an inadequate response to tumor necrosis factor blockers. Researchers then used a similar histologic approach to compare patients’ responses to tocilizumab or rituximab.
Among patients classified as BCP, there was not a significant difference in treatment response between the tocilizumab group and the rituximab group. However, classifying patients as BCP with RNA sequencing did make a difference. In this subgroup, patients given tocilizumab demonstrated a significantly higher response rate than those treated with rituximab.
Molecular-level analyses needed
This 2021 study showed – and this most recent study further confirmed – that “histology is not the way to understand what’s going on with or be predictive with tissue,” said Harris R. Perlman, PhD, chief of rheumatology at Northwestern University in Chicago. He was not involved with the research.
“Most people now believe that you really have to understand the tissue on a single-cell basis – using the gene expression of each individual cell – to really give you an idea of what’s happening in tissue,” he noted.
“RA continues to tell us that it is more complex than just something dichotomous,” added Elena Myasoedova, MD, PhD, director of the Inflammatory Arthritis Subspecialty Group at the Mayo Clinic in Rochester, Minn. She was not involved with the work.
“Understanding more about the heterogeneity using different ‘-omics’ approaches and introducing a two- or three-dimensional approach with spatial biology can be helpful,” she said.
Spatial transcriptomics, for example, allows scientists to measure all gene activity in a tissue sample and to map where that gene activity is occurring.
“It helps us to understand and visualize molecules and their unique context within individual cells and tissues,” Dr. Myasoedova explained.
With advanced molecular analyses already available, Dr. Perlman is adamant that synovial tissue remains the key to unlocking precision medicine.
“The tissue is the golden ticket,” he said, “but it’s how you analyze it.”
And it’s clear that older analytic methods – such as histology – are not enough, he said.
A larger study of a size similar to that of STRAP that incorporates multiple sources of patient information, from gene expression to clinical symptoms, to create a predictive model would be key to understanding how to move the field of precision treatment for RA forward, he added.
“Precision medicine for RA is close,” he said. “We still have to get the numbers.”
The STRAP and STRAP-EU trials were jointly funded by the UK Medical Research Council and Versus Arthritis. Pfizer and Roche donated the study drugs through an investigator-sponsored research grant. Many authors, including Dr. Pitzalis, have multiple financial relationships with pharmaceutical companies. Dr. Van der Helm-van Mil and Dr. Myasoedova have disclosed no relevant financial relationships. Dr. Perlman consults for AbbVie, AnaptysBio, Exagen, Janssen, and Kiniksa.
A version of this article first appeared on Medscape.com.
Researchers have yet to crack how to analyze synovial tissue biopsy specimens to predict treatment responses for patients with rheumatoid arthritis.
According to new research, classifying patients by synovial fluid B-cell status was not reliable in predicting treatment response to etanercept, tocilizumab, or rituximab. More comprehensive molecular analyses may hold promise in developing models to predict treatment of a disease as heterogeneous as RA, wrote the authors, led by chief investigator Costantino Pitzalis, MD, head of the Centre for Experimental Medicine and Rheumatology at Queen Mary University of London.
The article was published in the November 2023 issue of The Lancet Rheumatology..
Trial-and-error treatment
Because clinicians do not have a way to reliably predict how patients may respond to certain medications, the current RA treatments involve trial and error.

“Both with regard to the first conventional synthetic disease-modifying antirheumatic drug (DMARD) for all patients and the first biologic DMARD for the subgroup of patients requiring these drugs, the choice of a drug is based on pragmatic arguments rather than on individual patient characteristics,” Annette H. van der Helm-van Mil, MD, PhD, professor of rheumatology at the Leiden University Medical Center, the Netherlands, wrote in an accompanying editorial.
Most research on predicting treatment response has used clinical features and blood biomarkers, but neither approach is reliably predictive. “Precision medicine, therefore, remains elusive,” she said. Newer research has focused on synovial tissue biopsy to inform research decisions.
The STRAP studies
In this newest study, researchers combined data from two clinical studies with identical design: the Stratification of Biological Therapies by Pathobiology in Biologic-Naive Patients With Rheumatoid Arthritis (STRAP) trial, taking place in the United Kingdom, and STRAP-EU, which recruited patients from the European Union. The trials are the largest biopsy-driven trials to date, according to the researchers.
In total, researchers recruited 223 biologic-naive adult patients from 26 universities in the United Kingdom and Europe. Participants underwent ultrasound-guided synovial tissue biopsy and were then randomly assigned to receive etanercept (72 patients), tocilizumab (73 patients), or rituximab (78 patients). In a histologic analysis, 121 patients were characterized as B-cell poor (BCP), 100 patients were classified as B-cell rich (BCR), and two patients were classified as undetermined.
“We hypothesized that patients with a low synovial histological or molecular B-cell score would have a lower response to rituximab than to etanercept or tocilizumab,” the authors wrote.
However, among the BCP patients, there were no significant differences between responses to treatment at week 16 in the rituximab group compared with the etanercept and tocilizumab groups put together. In both groups, around 60% of patients achieved the primary endpoint of at least 20% improvement in American College of Rheumatology response criteria.
The results suggest that “a dichotomic classification into synovial B cell poor versus rich is unable to predict treatment response in patients treated with rituximab compared with etanercept or tocilizumab,” the authors wrote.
The findings echo work from the same group of researchers in 2021. This study – the R4RA trial – enrolled 164 patients who previously had an inadequate response to tumor necrosis factor blockers. Researchers then used a similar histologic approach to compare patients’ responses to tocilizumab or rituximab.
Among patients classified as BCP, there was not a significant difference in treatment response between the tocilizumab group and the rituximab group. However, classifying patients as BCP with RNA sequencing did make a difference. In this subgroup, patients given tocilizumab demonstrated a significantly higher response rate than those treated with rituximab.
Molecular-level analyses needed
This 2021 study showed – and this most recent study further confirmed – that “histology is not the way to understand what’s going on with or be predictive with tissue,” said Harris R. Perlman, PhD, chief of rheumatology at Northwestern University in Chicago. He was not involved with the research.
“Most people now believe that you really have to understand the tissue on a single-cell basis – using the gene expression of each individual cell – to really give you an idea of what’s happening in tissue,” he noted.
“RA continues to tell us that it is more complex than just something dichotomous,” added Elena Myasoedova, MD, PhD, director of the Inflammatory Arthritis Subspecialty Group at the Mayo Clinic in Rochester, Minn. She was not involved with the work.
“Understanding more about the heterogeneity using different ‘-omics’ approaches and introducing a two- or three-dimensional approach with spatial biology can be helpful,” she said.
Spatial transcriptomics, for example, allows scientists to measure all gene activity in a tissue sample and to map where that gene activity is occurring.
“It helps us to understand and visualize molecules and their unique context within individual cells and tissues,” Dr. Myasoedova explained.
With advanced molecular analyses already available, Dr. Perlman is adamant that synovial tissue remains the key to unlocking precision medicine.
“The tissue is the golden ticket,” he said, “but it’s how you analyze it.”
And it’s clear that older analytic methods – such as histology – are not enough, he said.
A larger study of a size similar to that of STRAP that incorporates multiple sources of patient information, from gene expression to clinical symptoms, to create a predictive model would be key to understanding how to move the field of precision treatment for RA forward, he added.
“Precision medicine for RA is close,” he said. “We still have to get the numbers.”
The STRAP and STRAP-EU trials were jointly funded by the UK Medical Research Council and Versus Arthritis. Pfizer and Roche donated the study drugs through an investigator-sponsored research grant. Many authors, including Dr. Pitzalis, have multiple financial relationships with pharmaceutical companies. Dr. Van der Helm-van Mil and Dr. Myasoedova have disclosed no relevant financial relationships. Dr. Perlman consults for AbbVie, AnaptysBio, Exagen, Janssen, and Kiniksa.
A version of this article first appeared on Medscape.com.
Researchers have yet to crack how to analyze synovial tissue biopsy specimens to predict treatment responses for patients with rheumatoid arthritis.
According to new research, classifying patients by synovial fluid B-cell status was not reliable in predicting treatment response to etanercept, tocilizumab, or rituximab. More comprehensive molecular analyses may hold promise in developing models to predict treatment of a disease as heterogeneous as RA, wrote the authors, led by chief investigator Costantino Pitzalis, MD, head of the Centre for Experimental Medicine and Rheumatology at Queen Mary University of London.
The article was published in the November 2023 issue of The Lancet Rheumatology..
Trial-and-error treatment
Because clinicians do not have a way to reliably predict how patients may respond to certain medications, the current RA treatments involve trial and error.

“Both with regard to the first conventional synthetic disease-modifying antirheumatic drug (DMARD) for all patients and the first biologic DMARD for the subgroup of patients requiring these drugs, the choice of a drug is based on pragmatic arguments rather than on individual patient characteristics,” Annette H. van der Helm-van Mil, MD, PhD, professor of rheumatology at the Leiden University Medical Center, the Netherlands, wrote in an accompanying editorial.
Most research on predicting treatment response has used clinical features and blood biomarkers, but neither approach is reliably predictive. “Precision medicine, therefore, remains elusive,” she said. Newer research has focused on synovial tissue biopsy to inform research decisions.
The STRAP studies
In this newest study, researchers combined data from two clinical studies with identical design: the Stratification of Biological Therapies by Pathobiology in Biologic-Naive Patients With Rheumatoid Arthritis (STRAP) trial, taking place in the United Kingdom, and STRAP-EU, which recruited patients from the European Union. The trials are the largest biopsy-driven trials to date, according to the researchers.
In total, researchers recruited 223 biologic-naive adult patients from 26 universities in the United Kingdom and Europe. Participants underwent ultrasound-guided synovial tissue biopsy and were then randomly assigned to receive etanercept (72 patients), tocilizumab (73 patients), or rituximab (78 patients). In a histologic analysis, 121 patients were characterized as B-cell poor (BCP), 100 patients were classified as B-cell rich (BCR), and two patients were classified as undetermined.
“We hypothesized that patients with a low synovial histological or molecular B-cell score would have a lower response to rituximab than to etanercept or tocilizumab,” the authors wrote.
However, among the BCP patients, there were no significant differences between responses to treatment at week 16 in the rituximab group compared with the etanercept and tocilizumab groups put together. In both groups, around 60% of patients achieved the primary endpoint of at least 20% improvement in American College of Rheumatology response criteria.
The results suggest that “a dichotomic classification into synovial B cell poor versus rich is unable to predict treatment response in patients treated with rituximab compared with etanercept or tocilizumab,” the authors wrote.
The findings echo work from the same group of researchers in 2021. This study – the R4RA trial – enrolled 164 patients who previously had an inadequate response to tumor necrosis factor blockers. Researchers then used a similar histologic approach to compare patients’ responses to tocilizumab or rituximab.
Among patients classified as BCP, there was not a significant difference in treatment response between the tocilizumab group and the rituximab group. However, classifying patients as BCP with RNA sequencing did make a difference. In this subgroup, patients given tocilizumab demonstrated a significantly higher response rate than those treated with rituximab.
Molecular-level analyses needed
This 2021 study showed – and this most recent study further confirmed – that “histology is not the way to understand what’s going on with or be predictive with tissue,” said Harris R. Perlman, PhD, chief of rheumatology at Northwestern University in Chicago. He was not involved with the research.
“Most people now believe that you really have to understand the tissue on a single-cell basis – using the gene expression of each individual cell – to really give you an idea of what’s happening in tissue,” he noted.
“RA continues to tell us that it is more complex than just something dichotomous,” added Elena Myasoedova, MD, PhD, director of the Inflammatory Arthritis Subspecialty Group at the Mayo Clinic in Rochester, Minn. She was not involved with the work.
“Understanding more about the heterogeneity using different ‘-omics’ approaches and introducing a two- or three-dimensional approach with spatial biology can be helpful,” she said.
Spatial transcriptomics, for example, allows scientists to measure all gene activity in a tissue sample and to map where that gene activity is occurring.
“It helps us to understand and visualize molecules and their unique context within individual cells and tissues,” Dr. Myasoedova explained.
With advanced molecular analyses already available, Dr. Perlman is adamant that synovial tissue remains the key to unlocking precision medicine.
“The tissue is the golden ticket,” he said, “but it’s how you analyze it.”
And it’s clear that older analytic methods – such as histology – are not enough, he said.
A larger study of a size similar to that of STRAP that incorporates multiple sources of patient information, from gene expression to clinical symptoms, to create a predictive model would be key to understanding how to move the field of precision treatment for RA forward, he added.
“Precision medicine for RA is close,” he said. “We still have to get the numbers.”
The STRAP and STRAP-EU trials were jointly funded by the UK Medical Research Council and Versus Arthritis. Pfizer and Roche donated the study drugs through an investigator-sponsored research grant. Many authors, including Dr. Pitzalis, have multiple financial relationships with pharmaceutical companies. Dr. Van der Helm-van Mil and Dr. Myasoedova have disclosed no relevant financial relationships. Dr. Perlman consults for AbbVie, AnaptysBio, Exagen, Janssen, and Kiniksa.
A version of this article first appeared on Medscape.com.
FROM THE LANCET RHEUMATOLOGY
Drug-eluting resorbable scaffold beats angioplasty for infrapopliteal artery disease
SAN FRANCISCO – An everolimus-eluting stent with a resorbable scaffold showed superior efficacy in a randomized multicenter trial when compared with angioplasty for the treatment of patients with chronic limb threatening ischemia (CLTI) resulting from infrapopliteal artery disease.
The stent (Esprit BTK, Abbott Vascular) was also noninferior to angioplasty in terms of safety.
Presenting results of the LIFE-BTK trial at the Transcatheter Cardiovascular Therapeutics annual meeting, Ramon Varcoe, MBBS, MS, PhD, MMed, of Prince of Wales Hospital, Sydney, said that peripheral artery disease is a global epidemic, the most serious manifestation of which is CTLI.
“If not treated expeditiously, this could lead to high rates of amputation, which, as we all know, has a severe impact on patients’ quality of life and even worse impact on their life prognosis, with prognosis rates worse than most cancers.”
He said that for infrapopliteal or below-the-knee (BTK) arterial disease, treatment with angioplasty has proven superior to bypass graft surgery, but some limitations of angioplasty are elastic recoil, dissection, and restenosis, thus limiting its durability. Coronary drug-eluting stents showed promise in BTK procedures but can interfere with reintervention. Thus, LIFE-BTK compared a drug-eluting stent with resorbable scaffolding to surgery.
Esprit BTK is a drug-eluting resorbable scaffold consisting of a temporary scaffold backbone of poly(L-lactide) and a strut thickness of 99 μm. It is coated with everolimus and bioresorbable poly(D,L-lactide). Two platinum markers at each end provide radiopacity.
LIFE-BTK enrolled patients aged 18 years or older with CLTI associated with ischemic rest pain or minor tissue loss and who had infrapopliteal artery stenosis or occlusion. The trial was prospective, international multicenter, and single-blind and randomly assigned 261 patients aged 18 years or older in the ratio of 2:1 to Esprit BTK (n = 173) or to angioplasty (n = 88). Treatment of up to two target lesions was allowed with a total scaffold length less than 170 mm.
The primary efficacy endpoint was superiority of Esprit BTK over angioplasty in terms of freedom of above-ankle amputation in the index limb, binary restenosis of the target lesion, and clinically driven target lesion revascularization evaluated at 1 year.
The primary safety endpoint, evaluated at 6 months, consisted of freedom from above-ankle amputation, major reintervention at 6 months, and perioperative mortality at 30 days.
An independent committee adjudicated clinical events, and core laboratories with assessors blinded to trial group assignment adjudicated imaging results and wound assessments.
Superior efficacy, noninferior safety
Participants were about two-thirds men, largely White, with about 15% of participants being Black/African American, and more than 90% of patients in each arm had hypertension. Lesion lengths were approximately 44 mm in each group with reference vessel diameters averaging 2.82-2.94 mm before intervention. Less than 4% in each group had severe lesion calcification.
Clinical follow-up rate at 1 year in the Esprit BTK arm was 90.2% and in the angioplasty arm 90.9%. Six patients in the former arm died versus five in the latter.
At the meeting, sponsored by the Cardiovascular Research Foundation, Dr. Varcoe showed a graph of the primary efficacy results at 453 days, the extra time being allowed to achieve a diagnostic ultrasound. (P < .0001).
“As you can see, those bars start to diverge in about 5 months and continue to separate over time, showing clear superiority of the scaffold over angioplasty, absolute risk difference of 30.8% and a highly statistically significant P value,” he said. Very similar primary efficacy outcomes were seen at 393 days.
At 1 year, the secondary efficacy endpoint of binary restenosis of the target lesion occurred in 24.2% of scaffold patients versus 46.5% of the angioplasty group (P < .0001). Another secondary endpoint, freedom from above-ankle amputation, 100% total occlusion of the target vessel, or clinically driven target lesion revascularization, occurred in 82.5% of the scaffold group versus 70.4% in the angioplasty group (P = .0081).
The primary safety endpoint of freedom from a major adverse limb event plus perioperative death was 100% for angioplasty and 96.9% for Esprit BTK (P = .0019)
All subgroup analyses assessing interaction by sex, race, geographic region, or age showed Esprit BTK was superior to angioplasty, with relative risks ranging from 0.27 to 0.61.
“If this technology is approved by the FDA, it will provide a new option for our patients with very difficult-to-treat disease, which will provide them additional durability and fewer reinterventions,” Dr. Varcoe concluded. “And I think we all know deep down that’s going to translate to improved clinical outcomes and few amputations.”
David Kandzari, MD, of Peidmont Heart Institute, Atlanta, was asked to comment on the study. He said that as with other vascular interventions, the ideal technology “would be first to provide a safe and effective antiproliferative therapy that would mitigate against restenosis and for scaffolding to prevent elastic recoil and reocclusion ... and ultimately fulfill these two promises without the requisite of a permanent implant.
“Despite their common use in femoral popliteal disease, drug-coated balloons had at best demonstrated inconsistent results below the knee, and drug-coated balloons, therefore, are not approved for such indications.”
He said that drug-eluting stents have demonstrated efficacy in this indication but that these studies have been limited to fairly discrete proximal disease.
“LIFE-BTK therefore represents one of the most rigorous trials in the space of endovascular interventions as a single line and randomized trial,” Dr. Kandzari said, “showing a primary composite endpoint of both safety and effectiveness relative to conventional angioplasty.”
Dr. Kandzari pointed to strengths of the study in that it used standardization of technique with independent adjudication of both imaging and wound healing assessments. “And the study population, too, was relevant to clinical practice, representing oftentimes underrepresented groups, including those with extensive disease burden [and] clinical severity,” he said. “Importantly, in this study, nearly one-third of the population will be women.”
Panelist Jennifer Rymer, MD, of Duke University Medical Center, Durham, N.C., commented that she treats a lot of African American patients with CTLI and applauded the researchers for including those patients. “I think that this will be a groundbreaking new change in our practice,” she said.
The trial results were published simultaneously with the presentation at TCT 2023 in the New England Journal of Medicine.
Dr. Varcoe reported receiving consulting fees/honoraria from Boston Scientific Corporation, Medtronic, Abbott, BD, Intervene, Surmodics, Philips, Nectero, Alucent, W.L. Gore, Vesteck, Bard Medical, Cook Medical, and R3 Vascular. He has equity, stock, or stock options in EBR Systems and has an executive role or ownership interest in Provisio Medical and Vesteck. Dr. Kandzari received grant support/research contract from Medtronic, Teleflex, Biotronik, and CSI; consultant fees/honoraria from and is on the speaker’s bureau of CSI and Medtronic; and has equity, stock, or options in Biostar Ventures. Dr. Rymer receives grant support/research contract from Chiesi, Abbott Vascular, Abiomed, and Pfizer. The study was funded by Abbott.
A version of this article appeared on Medscape.com.
SAN FRANCISCO – An everolimus-eluting stent with a resorbable scaffold showed superior efficacy in a randomized multicenter trial when compared with angioplasty for the treatment of patients with chronic limb threatening ischemia (CLTI) resulting from infrapopliteal artery disease.
The stent (Esprit BTK, Abbott Vascular) was also noninferior to angioplasty in terms of safety.
Presenting results of the LIFE-BTK trial at the Transcatheter Cardiovascular Therapeutics annual meeting, Ramon Varcoe, MBBS, MS, PhD, MMed, of Prince of Wales Hospital, Sydney, said that peripheral artery disease is a global epidemic, the most serious manifestation of which is CTLI.
“If not treated expeditiously, this could lead to high rates of amputation, which, as we all know, has a severe impact on patients’ quality of life and even worse impact on their life prognosis, with prognosis rates worse than most cancers.”
He said that for infrapopliteal or below-the-knee (BTK) arterial disease, treatment with angioplasty has proven superior to bypass graft surgery, but some limitations of angioplasty are elastic recoil, dissection, and restenosis, thus limiting its durability. Coronary drug-eluting stents showed promise in BTK procedures but can interfere with reintervention. Thus, LIFE-BTK compared a drug-eluting stent with resorbable scaffolding to surgery.
Esprit BTK is a drug-eluting resorbable scaffold consisting of a temporary scaffold backbone of poly(L-lactide) and a strut thickness of 99 μm. It is coated with everolimus and bioresorbable poly(D,L-lactide). Two platinum markers at each end provide radiopacity.
LIFE-BTK enrolled patients aged 18 years or older with CLTI associated with ischemic rest pain or minor tissue loss and who had infrapopliteal artery stenosis or occlusion. The trial was prospective, international multicenter, and single-blind and randomly assigned 261 patients aged 18 years or older in the ratio of 2:1 to Esprit BTK (n = 173) or to angioplasty (n = 88). Treatment of up to two target lesions was allowed with a total scaffold length less than 170 mm.
The primary efficacy endpoint was superiority of Esprit BTK over angioplasty in terms of freedom of above-ankle amputation in the index limb, binary restenosis of the target lesion, and clinically driven target lesion revascularization evaluated at 1 year.
The primary safety endpoint, evaluated at 6 months, consisted of freedom from above-ankle amputation, major reintervention at 6 months, and perioperative mortality at 30 days.
An independent committee adjudicated clinical events, and core laboratories with assessors blinded to trial group assignment adjudicated imaging results and wound assessments.
Superior efficacy, noninferior safety
Participants were about two-thirds men, largely White, with about 15% of participants being Black/African American, and more than 90% of patients in each arm had hypertension. Lesion lengths were approximately 44 mm in each group with reference vessel diameters averaging 2.82-2.94 mm before intervention. Less than 4% in each group had severe lesion calcification.
Clinical follow-up rate at 1 year in the Esprit BTK arm was 90.2% and in the angioplasty arm 90.9%. Six patients in the former arm died versus five in the latter.
At the meeting, sponsored by the Cardiovascular Research Foundation, Dr. Varcoe showed a graph of the primary efficacy results at 453 days, the extra time being allowed to achieve a diagnostic ultrasound. (P < .0001).
“As you can see, those bars start to diverge in about 5 months and continue to separate over time, showing clear superiority of the scaffold over angioplasty, absolute risk difference of 30.8% and a highly statistically significant P value,” he said. Very similar primary efficacy outcomes were seen at 393 days.
At 1 year, the secondary efficacy endpoint of binary restenosis of the target lesion occurred in 24.2% of scaffold patients versus 46.5% of the angioplasty group (P < .0001). Another secondary endpoint, freedom from above-ankle amputation, 100% total occlusion of the target vessel, or clinically driven target lesion revascularization, occurred in 82.5% of the scaffold group versus 70.4% in the angioplasty group (P = .0081).
The primary safety endpoint of freedom from a major adverse limb event plus perioperative death was 100% for angioplasty and 96.9% for Esprit BTK (P = .0019)
All subgroup analyses assessing interaction by sex, race, geographic region, or age showed Esprit BTK was superior to angioplasty, with relative risks ranging from 0.27 to 0.61.
“If this technology is approved by the FDA, it will provide a new option for our patients with very difficult-to-treat disease, which will provide them additional durability and fewer reinterventions,” Dr. Varcoe concluded. “And I think we all know deep down that’s going to translate to improved clinical outcomes and few amputations.”
David Kandzari, MD, of Peidmont Heart Institute, Atlanta, was asked to comment on the study. He said that as with other vascular interventions, the ideal technology “would be first to provide a safe and effective antiproliferative therapy that would mitigate against restenosis and for scaffolding to prevent elastic recoil and reocclusion ... and ultimately fulfill these two promises without the requisite of a permanent implant.
“Despite their common use in femoral popliteal disease, drug-coated balloons had at best demonstrated inconsistent results below the knee, and drug-coated balloons, therefore, are not approved for such indications.”
He said that drug-eluting stents have demonstrated efficacy in this indication but that these studies have been limited to fairly discrete proximal disease.
“LIFE-BTK therefore represents one of the most rigorous trials in the space of endovascular interventions as a single line and randomized trial,” Dr. Kandzari said, “showing a primary composite endpoint of both safety and effectiveness relative to conventional angioplasty.”
Dr. Kandzari pointed to strengths of the study in that it used standardization of technique with independent adjudication of both imaging and wound healing assessments. “And the study population, too, was relevant to clinical practice, representing oftentimes underrepresented groups, including those with extensive disease burden [and] clinical severity,” he said. “Importantly, in this study, nearly one-third of the population will be women.”
Panelist Jennifer Rymer, MD, of Duke University Medical Center, Durham, N.C., commented that she treats a lot of African American patients with CTLI and applauded the researchers for including those patients. “I think that this will be a groundbreaking new change in our practice,” she said.
The trial results were published simultaneously with the presentation at TCT 2023 in the New England Journal of Medicine.
Dr. Varcoe reported receiving consulting fees/honoraria from Boston Scientific Corporation, Medtronic, Abbott, BD, Intervene, Surmodics, Philips, Nectero, Alucent, W.L. Gore, Vesteck, Bard Medical, Cook Medical, and R3 Vascular. He has equity, stock, or stock options in EBR Systems and has an executive role or ownership interest in Provisio Medical and Vesteck. Dr. Kandzari received grant support/research contract from Medtronic, Teleflex, Biotronik, and CSI; consultant fees/honoraria from and is on the speaker’s bureau of CSI and Medtronic; and has equity, stock, or options in Biostar Ventures. Dr. Rymer receives grant support/research contract from Chiesi, Abbott Vascular, Abiomed, and Pfizer. The study was funded by Abbott.
A version of this article appeared on Medscape.com.
SAN FRANCISCO – An everolimus-eluting stent with a resorbable scaffold showed superior efficacy in a randomized multicenter trial when compared with angioplasty for the treatment of patients with chronic limb threatening ischemia (CLTI) resulting from infrapopliteal artery disease.
The stent (Esprit BTK, Abbott Vascular) was also noninferior to angioplasty in terms of safety.
Presenting results of the LIFE-BTK trial at the Transcatheter Cardiovascular Therapeutics annual meeting, Ramon Varcoe, MBBS, MS, PhD, MMed, of Prince of Wales Hospital, Sydney, said that peripheral artery disease is a global epidemic, the most serious manifestation of which is CTLI.
“If not treated expeditiously, this could lead to high rates of amputation, which, as we all know, has a severe impact on patients’ quality of life and even worse impact on their life prognosis, with prognosis rates worse than most cancers.”
He said that for infrapopliteal or below-the-knee (BTK) arterial disease, treatment with angioplasty has proven superior to bypass graft surgery, but some limitations of angioplasty are elastic recoil, dissection, and restenosis, thus limiting its durability. Coronary drug-eluting stents showed promise in BTK procedures but can interfere with reintervention. Thus, LIFE-BTK compared a drug-eluting stent with resorbable scaffolding to surgery.
Esprit BTK is a drug-eluting resorbable scaffold consisting of a temporary scaffold backbone of poly(L-lactide) and a strut thickness of 99 μm. It is coated with everolimus and bioresorbable poly(D,L-lactide). Two platinum markers at each end provide radiopacity.
LIFE-BTK enrolled patients aged 18 years or older with CLTI associated with ischemic rest pain or minor tissue loss and who had infrapopliteal artery stenosis or occlusion. The trial was prospective, international multicenter, and single-blind and randomly assigned 261 patients aged 18 years or older in the ratio of 2:1 to Esprit BTK (n = 173) or to angioplasty (n = 88). Treatment of up to two target lesions was allowed with a total scaffold length less than 170 mm.
The primary efficacy endpoint was superiority of Esprit BTK over angioplasty in terms of freedom of above-ankle amputation in the index limb, binary restenosis of the target lesion, and clinically driven target lesion revascularization evaluated at 1 year.
The primary safety endpoint, evaluated at 6 months, consisted of freedom from above-ankle amputation, major reintervention at 6 months, and perioperative mortality at 30 days.
An independent committee adjudicated clinical events, and core laboratories with assessors blinded to trial group assignment adjudicated imaging results and wound assessments.
Superior efficacy, noninferior safety
Participants were about two-thirds men, largely White, with about 15% of participants being Black/African American, and more than 90% of patients in each arm had hypertension. Lesion lengths were approximately 44 mm in each group with reference vessel diameters averaging 2.82-2.94 mm before intervention. Less than 4% in each group had severe lesion calcification.
Clinical follow-up rate at 1 year in the Esprit BTK arm was 90.2% and in the angioplasty arm 90.9%. Six patients in the former arm died versus five in the latter.
At the meeting, sponsored by the Cardiovascular Research Foundation, Dr. Varcoe showed a graph of the primary efficacy results at 453 days, the extra time being allowed to achieve a diagnostic ultrasound. (P < .0001).
“As you can see, those bars start to diverge in about 5 months and continue to separate over time, showing clear superiority of the scaffold over angioplasty, absolute risk difference of 30.8% and a highly statistically significant P value,” he said. Very similar primary efficacy outcomes were seen at 393 days.
At 1 year, the secondary efficacy endpoint of binary restenosis of the target lesion occurred in 24.2% of scaffold patients versus 46.5% of the angioplasty group (P < .0001). Another secondary endpoint, freedom from above-ankle amputation, 100% total occlusion of the target vessel, or clinically driven target lesion revascularization, occurred in 82.5% of the scaffold group versus 70.4% in the angioplasty group (P = .0081).
The primary safety endpoint of freedom from a major adverse limb event plus perioperative death was 100% for angioplasty and 96.9% for Esprit BTK (P = .0019)
All subgroup analyses assessing interaction by sex, race, geographic region, or age showed Esprit BTK was superior to angioplasty, with relative risks ranging from 0.27 to 0.61.
“If this technology is approved by the FDA, it will provide a new option for our patients with very difficult-to-treat disease, which will provide them additional durability and fewer reinterventions,” Dr. Varcoe concluded. “And I think we all know deep down that’s going to translate to improved clinical outcomes and few amputations.”
David Kandzari, MD, of Peidmont Heart Institute, Atlanta, was asked to comment on the study. He said that as with other vascular interventions, the ideal technology “would be first to provide a safe and effective antiproliferative therapy that would mitigate against restenosis and for scaffolding to prevent elastic recoil and reocclusion ... and ultimately fulfill these two promises without the requisite of a permanent implant.
“Despite their common use in femoral popliteal disease, drug-coated balloons had at best demonstrated inconsistent results below the knee, and drug-coated balloons, therefore, are not approved for such indications.”
He said that drug-eluting stents have demonstrated efficacy in this indication but that these studies have been limited to fairly discrete proximal disease.
“LIFE-BTK therefore represents one of the most rigorous trials in the space of endovascular interventions as a single line and randomized trial,” Dr. Kandzari said, “showing a primary composite endpoint of both safety and effectiveness relative to conventional angioplasty.”
Dr. Kandzari pointed to strengths of the study in that it used standardization of technique with independent adjudication of both imaging and wound healing assessments. “And the study population, too, was relevant to clinical practice, representing oftentimes underrepresented groups, including those with extensive disease burden [and] clinical severity,” he said. “Importantly, in this study, nearly one-third of the population will be women.”
Panelist Jennifer Rymer, MD, of Duke University Medical Center, Durham, N.C., commented that she treats a lot of African American patients with CTLI and applauded the researchers for including those patients. “I think that this will be a groundbreaking new change in our practice,” she said.
The trial results were published simultaneously with the presentation at TCT 2023 in the New England Journal of Medicine.
Dr. Varcoe reported receiving consulting fees/honoraria from Boston Scientific Corporation, Medtronic, Abbott, BD, Intervene, Surmodics, Philips, Nectero, Alucent, W.L. Gore, Vesteck, Bard Medical, Cook Medical, and R3 Vascular. He has equity, stock, or stock options in EBR Systems and has an executive role or ownership interest in Provisio Medical and Vesteck. Dr. Kandzari received grant support/research contract from Medtronic, Teleflex, Biotronik, and CSI; consultant fees/honoraria from and is on the speaker’s bureau of CSI and Medtronic; and has equity, stock, or options in Biostar Ventures. Dr. Rymer receives grant support/research contract from Chiesi, Abbott Vascular, Abiomed, and Pfizer. The study was funded by Abbott.
A version of this article appeared on Medscape.com.
AT TCT 2023
Childhood trauma linked to adult headache
TOPLINE:
with more early adverse experiences raising the risk even more, a new study found.
METHODOLOGY:
- The meta-analysis included 28 observational studies with 154,739 persons in 19 countries that assessed the relationship between at least one adverse childhood experience (ACE) and primary headache (including migraine, tension-type headache, cluster headache, and chronic/severe headache) at age 21 years or older.
- From each study, researchers extracted outcome point estimates and corresponding 95% confidence intervals, number of events in each group, and covariates included in the model. They subcategorized ACEs according to those involving threat (for example, physical, emotional, or sexual abuse) and deprivation (for example, neglect, household substance misuse).
- For the primary analysis, the researchers calculated the odds ratios and hazard ratios of headache among persons with at least one ACE, compared with those with no ACEs.
- They also tested an underlying biological theory that threat and deprivation ACEs may manifest differently in neurodevelopment, with distinct impacts on primary headaches.
TAKEAWAY:
- The most commonly reported ACEs were physical abuse (77%), sexual abuse (73%), and exposure to family violence (38%).
- Compared with having experienced no ACEs, experiencing at least one was associated with primary headaches (pooled OR, 1.48; 95% confidence interval, 1.36-1.61).
- As the number of ACEs increased, the strength of the association with primary headaches increased in a dose-response relationship (P for trend < .0001).
- Both threat and deprivation were independently associated with primary headaches; the pooled main effect was consistent for threat (OR, 1.46; 95% CI, 1.32-1.60) and for deprivation (OR, 1.35; 95% CI, 1.23-1.49), suggesting possible distinct pathways of early adversity.
IN PRACTICE:
Clinicians who treat primary headaches in adults “should routinely screen for ACEs, educate patients on the connection between ACEs and health, and provide referrals for treatment strategies,” the investigators write. Strategies such as trauma-informed or attachment-based therapy may help rewire parts of the brain that have been dysregulated, they add.
SOURCE:
The study was led by Claudia Sikorski, department of health research methods, evidence, and impact, McMaster University, Hamilton, Ont. It was published online in Neurology.
LIMITATIONS:
The findings reflect a conservative estimate of the true impact of ACEs on primary headaches, because ACEs are commonly underreported. The analysis could not statistically disentangle younger adults with developing brains (age 21-26 years) from older adults. Not all included studies adjusted for age and sex, which are known risk factors for headaches. The study did not explore the relationship between ACEs and primary headache disorders in childhood and adolescence. Owing to the inherent nature of studies investigating ACEs, causation cannot be inferred.
DISCLOSURES:
The authors report no targeted funding and no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
with more early adverse experiences raising the risk even more, a new study found.
METHODOLOGY:
- The meta-analysis included 28 observational studies with 154,739 persons in 19 countries that assessed the relationship between at least one adverse childhood experience (ACE) and primary headache (including migraine, tension-type headache, cluster headache, and chronic/severe headache) at age 21 years or older.
- From each study, researchers extracted outcome point estimates and corresponding 95% confidence intervals, number of events in each group, and covariates included in the model. They subcategorized ACEs according to those involving threat (for example, physical, emotional, or sexual abuse) and deprivation (for example, neglect, household substance misuse).
- For the primary analysis, the researchers calculated the odds ratios and hazard ratios of headache among persons with at least one ACE, compared with those with no ACEs.
- They also tested an underlying biological theory that threat and deprivation ACEs may manifest differently in neurodevelopment, with distinct impacts on primary headaches.
TAKEAWAY:
- The most commonly reported ACEs were physical abuse (77%), sexual abuse (73%), and exposure to family violence (38%).
- Compared with having experienced no ACEs, experiencing at least one was associated with primary headaches (pooled OR, 1.48; 95% confidence interval, 1.36-1.61).
- As the number of ACEs increased, the strength of the association with primary headaches increased in a dose-response relationship (P for trend < .0001).
- Both threat and deprivation were independently associated with primary headaches; the pooled main effect was consistent for threat (OR, 1.46; 95% CI, 1.32-1.60) and for deprivation (OR, 1.35; 95% CI, 1.23-1.49), suggesting possible distinct pathways of early adversity.
IN PRACTICE:
Clinicians who treat primary headaches in adults “should routinely screen for ACEs, educate patients on the connection between ACEs and health, and provide referrals for treatment strategies,” the investigators write. Strategies such as trauma-informed or attachment-based therapy may help rewire parts of the brain that have been dysregulated, they add.
SOURCE:
The study was led by Claudia Sikorski, department of health research methods, evidence, and impact, McMaster University, Hamilton, Ont. It was published online in Neurology.
LIMITATIONS:
The findings reflect a conservative estimate of the true impact of ACEs on primary headaches, because ACEs are commonly underreported. The analysis could not statistically disentangle younger adults with developing brains (age 21-26 years) from older adults. Not all included studies adjusted for age and sex, which are known risk factors for headaches. The study did not explore the relationship between ACEs and primary headache disorders in childhood and adolescence. Owing to the inherent nature of studies investigating ACEs, causation cannot be inferred.
DISCLOSURES:
The authors report no targeted funding and no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
with more early adverse experiences raising the risk even more, a new study found.
METHODOLOGY:
- The meta-analysis included 28 observational studies with 154,739 persons in 19 countries that assessed the relationship between at least one adverse childhood experience (ACE) and primary headache (including migraine, tension-type headache, cluster headache, and chronic/severe headache) at age 21 years or older.
- From each study, researchers extracted outcome point estimates and corresponding 95% confidence intervals, number of events in each group, and covariates included in the model. They subcategorized ACEs according to those involving threat (for example, physical, emotional, or sexual abuse) and deprivation (for example, neglect, household substance misuse).
- For the primary analysis, the researchers calculated the odds ratios and hazard ratios of headache among persons with at least one ACE, compared with those with no ACEs.
- They also tested an underlying biological theory that threat and deprivation ACEs may manifest differently in neurodevelopment, with distinct impacts on primary headaches.
TAKEAWAY:
- The most commonly reported ACEs were physical abuse (77%), sexual abuse (73%), and exposure to family violence (38%).
- Compared with having experienced no ACEs, experiencing at least one was associated with primary headaches (pooled OR, 1.48; 95% confidence interval, 1.36-1.61).
- As the number of ACEs increased, the strength of the association with primary headaches increased in a dose-response relationship (P for trend < .0001).
- Both threat and deprivation were independently associated with primary headaches; the pooled main effect was consistent for threat (OR, 1.46; 95% CI, 1.32-1.60) and for deprivation (OR, 1.35; 95% CI, 1.23-1.49), suggesting possible distinct pathways of early adversity.
IN PRACTICE:
Clinicians who treat primary headaches in adults “should routinely screen for ACEs, educate patients on the connection between ACEs and health, and provide referrals for treatment strategies,” the investigators write. Strategies such as trauma-informed or attachment-based therapy may help rewire parts of the brain that have been dysregulated, they add.
SOURCE:
The study was led by Claudia Sikorski, department of health research methods, evidence, and impact, McMaster University, Hamilton, Ont. It was published online in Neurology.
LIMITATIONS:
The findings reflect a conservative estimate of the true impact of ACEs on primary headaches, because ACEs are commonly underreported. The analysis could not statistically disentangle younger adults with developing brains (age 21-26 years) from older adults. Not all included studies adjusted for age and sex, which are known risk factors for headaches. The study did not explore the relationship between ACEs and primary headache disorders in childhood and adolescence. Owing to the inherent nature of studies investigating ACEs, causation cannot be inferred.
DISCLOSURES:
The authors report no targeted funding and no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.