User login
GI symptoms during menopause deserve attention
This transcript has been edited for clarity.
Welcome back to another GI Common Concerns.
Today, I want to highlight some information about menopause.
Approximately 1.5 million women in the United States per year enter into menopause. Hysterectomy is also one of the most common surgeries for women worldwide, with an estimated 20%-40% undergoing this procedure by the age of 60.
Therefore, whether it’s because of biologic onset with age or surgical induction, menopause is a very common condition, and it’s important that we understand its symptoms and the latest information around it.
Impact on GI motility
One of the clearest functional symptoms to be aware of with menopause relates to alterations in hormonal balance. This has an impact on gastrointestinal (GI) motility by increasing abdominal muscle stimulation related to different patterns of secretion and can result in a number of symptomatic changes.
One such change that can occur is food intolerance. It is believed that menopause-associated food intolerance has multiple possible causes and may be related more to alterations to the microbiome, which can be contributed to by diet, activity, sleep cycle, and other factors.
When food intolerances are triggered in the perimenopausal or menopausal patient, it may lead you to recommend the well-established FODMAP diet, which is known to reduce symptoms. But the answer for every patient is not simply placing them on a FODMAP diet and telling them they have irritable bowel syndrome.
Other approaches can be considered for addressing food intolerance in these patients. The data are quite strong that adjunctive use of a dietitian is tremendously helpful in this particular population.
When it comes to menopausal patients, however, we need to consider other changes in their activity or adverse contributors to their mental health, such as stress or anxiety. These all contribute to more of a multifactorial composite in this population, for which irritable bowel syndrome serves as a similar example.
This means that we may need to expand our horizons rather than to focus on solely on antispasmodic or diet-related interventions.
Instead, we can start to consider more of a multidimensional treatment approach consisting of education, relaxation, cognitive-behavioral therapy, and physical activity. Certainly, there are now behavioral interventions using Internet-based digital formats to increase the acceptability and sustainability among patients.
Choosing such a multidisciplinary approach can be quite helpful.
The metabolic consequences of altering hormonal balance
Recent data from a rat model study investigated the metabolic impact of changing hormonal balance.
Investigators looked at ovariectomized rats and found that there was a biologic change in the diversity of the general GI biome. There were also noteworthy associations with weight fluctuations and dramatic changes in the spatial memory and cognitive performance characteristics of these rats, which was subsequently improved by supplemental estrogen.
This indicates that we may be able to remediate these effects with the similar use of supplemental hormone replacement treatments.
Another recent study looked at nonalcoholic fatty liver disease, which is very common in the general population and has a > 20% worldwide prevalence in postmenopausal women. Albeit small in numbers, this was a very interesting study.
Investigators looked at the delivery method for menopausal hormone therapy, which was transdermal for 75 patients and oral for 293 patients. Then, they looked at ultrasound definition of nonalcoholic fatty liver disease after 1 year as the endpoint. They found an approximate 7% reduction in the patients who received the transdermal administration compared with a 4% increase in the patients who received it orally.
Again, we have to remember this is a relatively small study, but the results indicate that the route of estrogen administration may be an important consideration in nonalcoholic fatty liver disease.
Sleep disturbances: fragmentation, duration, and quality
Sleep is something that’s near and dear to my heart and is the focus of a lot of our research.
Sleep disturbances are really part and parcel of menopause and are observed with hormonal imbalances and temperature intolerances. Disturbances such as sleep fragmentation, shorter sleep duration, and poorer sleep quality have a dramatic effect not only on the biome but also on sensory thresholds.
Therefore, as we start to look at mitigating strategies here, we need to focus on sleep and ask the right questions.
In my own practice, I try not to just ask, “How did you sleep last night?” That’s because sleep can be somewhat amnestic. You may have a cognitive awakening or a noncognitive awakening but still have experienced fragmentation.
As a result, my focus is on next-day function. I ask my patients, “When you get up in the morning, are you refreshed? Do you have the ability to perform daytime activities? Do you experience early fatigue or cognitive changes that occur?”
These questions can provide good insights into the sleep efficiency of the previous night.
The effect of the microbiome on osteoporosis
One final topic I found very interesting pertains to the effects of menopause on osteoporosis.
We certainly know that postmenopausal women have a very high prevalence of osteopenia, and that osteoporosis is a progression of that, as well as that increased bone-related disease affects fractures and related morbidity and mortality.
However, there’s accumulating evidence on the osteoporotic effects of biomarker changes in menopause, which shows that the biome regulates the pathophysiologic process of at least a large degree of osteoporosis.
This starts to make sense when you look at the pro-inflammatory factors that increase with changes in biome diversity, in particular tumor necrosis factor alpha (which is something we also see in inflammatory bowel disease), interleukin-1, and increased activated osteoclasts.
Therefore, when it comes to decreasing bone loss among patients who are perimenopausal or postmenopausal, we don’t yet have a clear answer. Hormone therapy, diet, activity, vitamin D supplementation, and other things may positively change the biome. They are worthy topics for patients to bring up with their ob.gyns. or primary care doctors.
Although it may be a little bit outside the scope of gastroenterology, in my opinion there are a number of new findings relating to menopause that we as a field need to be more proactive in addressing.
Ask the right questions when these people come in to you, irrespective of why they’re there. Start to ask about the quality of their sleep. What are their other functional symptoms? What are their other potential osteoporosis-related risks?
We must do a better job about individualizing care. Rather than treating patients as disease states, we must start to do specific patient-focused care.
I hope this gives you some provocative thoughts when you have your next session with a patient in the perimenopausal or menopausal state. There are lots of things that we continue to learn.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Va., and a past president of the American College of Gastroenterology. He serves as an adviser to ISOThrive and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome back to another GI Common Concerns.
Today, I want to highlight some information about menopause.
Approximately 1.5 million women in the United States per year enter into menopause. Hysterectomy is also one of the most common surgeries for women worldwide, with an estimated 20%-40% undergoing this procedure by the age of 60.
Therefore, whether it’s because of biologic onset with age or surgical induction, menopause is a very common condition, and it’s important that we understand its symptoms and the latest information around it.
Impact on GI motility
One of the clearest functional symptoms to be aware of with menopause relates to alterations in hormonal balance. This has an impact on gastrointestinal (GI) motility by increasing abdominal muscle stimulation related to different patterns of secretion and can result in a number of symptomatic changes.
One such change that can occur is food intolerance. It is believed that menopause-associated food intolerance has multiple possible causes and may be related more to alterations to the microbiome, which can be contributed to by diet, activity, sleep cycle, and other factors.
When food intolerances are triggered in the perimenopausal or menopausal patient, it may lead you to recommend the well-established FODMAP diet, which is known to reduce symptoms. But the answer for every patient is not simply placing them on a FODMAP diet and telling them they have irritable bowel syndrome.
Other approaches can be considered for addressing food intolerance in these patients. The data are quite strong that adjunctive use of a dietitian is tremendously helpful in this particular population.
When it comes to menopausal patients, however, we need to consider other changes in their activity or adverse contributors to their mental health, such as stress or anxiety. These all contribute to more of a multifactorial composite in this population, for which irritable bowel syndrome serves as a similar example.
This means that we may need to expand our horizons rather than to focus on solely on antispasmodic or diet-related interventions.
Instead, we can start to consider more of a multidimensional treatment approach consisting of education, relaxation, cognitive-behavioral therapy, and physical activity. Certainly, there are now behavioral interventions using Internet-based digital formats to increase the acceptability and sustainability among patients.
Choosing such a multidisciplinary approach can be quite helpful.
The metabolic consequences of altering hormonal balance
Recent data from a rat model study investigated the metabolic impact of changing hormonal balance.
Investigators looked at ovariectomized rats and found that there was a biologic change in the diversity of the general GI biome. There were also noteworthy associations with weight fluctuations and dramatic changes in the spatial memory and cognitive performance characteristics of these rats, which was subsequently improved by supplemental estrogen.
This indicates that we may be able to remediate these effects with the similar use of supplemental hormone replacement treatments.
Another recent study looked at nonalcoholic fatty liver disease, which is very common in the general population and has a > 20% worldwide prevalence in postmenopausal women. Albeit small in numbers, this was a very interesting study.
Investigators looked at the delivery method for menopausal hormone therapy, which was transdermal for 75 patients and oral for 293 patients. Then, they looked at ultrasound definition of nonalcoholic fatty liver disease after 1 year as the endpoint. They found an approximate 7% reduction in the patients who received the transdermal administration compared with a 4% increase in the patients who received it orally.
Again, we have to remember this is a relatively small study, but the results indicate that the route of estrogen administration may be an important consideration in nonalcoholic fatty liver disease.
Sleep disturbances: fragmentation, duration, and quality
Sleep is something that’s near and dear to my heart and is the focus of a lot of our research.
Sleep disturbances are really part and parcel of menopause and are observed with hormonal imbalances and temperature intolerances. Disturbances such as sleep fragmentation, shorter sleep duration, and poorer sleep quality have a dramatic effect not only on the biome but also on sensory thresholds.
Therefore, as we start to look at mitigating strategies here, we need to focus on sleep and ask the right questions.
In my own practice, I try not to just ask, “How did you sleep last night?” That’s because sleep can be somewhat amnestic. You may have a cognitive awakening or a noncognitive awakening but still have experienced fragmentation.
As a result, my focus is on next-day function. I ask my patients, “When you get up in the morning, are you refreshed? Do you have the ability to perform daytime activities? Do you experience early fatigue or cognitive changes that occur?”
These questions can provide good insights into the sleep efficiency of the previous night.
The effect of the microbiome on osteoporosis
One final topic I found very interesting pertains to the effects of menopause on osteoporosis.
We certainly know that postmenopausal women have a very high prevalence of osteopenia, and that osteoporosis is a progression of that, as well as that increased bone-related disease affects fractures and related morbidity and mortality.
However, there’s accumulating evidence on the osteoporotic effects of biomarker changes in menopause, which shows that the biome regulates the pathophysiologic process of at least a large degree of osteoporosis.
This starts to make sense when you look at the pro-inflammatory factors that increase with changes in biome diversity, in particular tumor necrosis factor alpha (which is something we also see in inflammatory bowel disease), interleukin-1, and increased activated osteoclasts.
Therefore, when it comes to decreasing bone loss among patients who are perimenopausal or postmenopausal, we don’t yet have a clear answer. Hormone therapy, diet, activity, vitamin D supplementation, and other things may positively change the biome. They are worthy topics for patients to bring up with their ob.gyns. or primary care doctors.
Although it may be a little bit outside the scope of gastroenterology, in my opinion there are a number of new findings relating to menopause that we as a field need to be more proactive in addressing.
Ask the right questions when these people come in to you, irrespective of why they’re there. Start to ask about the quality of their sleep. What are their other functional symptoms? What are their other potential osteoporosis-related risks?
We must do a better job about individualizing care. Rather than treating patients as disease states, we must start to do specific patient-focused care.
I hope this gives you some provocative thoughts when you have your next session with a patient in the perimenopausal or menopausal state. There are lots of things that we continue to learn.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Va., and a past president of the American College of Gastroenterology. He serves as an adviser to ISOThrive and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome back to another GI Common Concerns.
Today, I want to highlight some information about menopause.
Approximately 1.5 million women in the United States per year enter into menopause. Hysterectomy is also one of the most common surgeries for women worldwide, with an estimated 20%-40% undergoing this procedure by the age of 60.
Therefore, whether it’s because of biologic onset with age or surgical induction, menopause is a very common condition, and it’s important that we understand its symptoms and the latest information around it.
Impact on GI motility
One of the clearest functional symptoms to be aware of with menopause relates to alterations in hormonal balance. This has an impact on gastrointestinal (GI) motility by increasing abdominal muscle stimulation related to different patterns of secretion and can result in a number of symptomatic changes.
One such change that can occur is food intolerance. It is believed that menopause-associated food intolerance has multiple possible causes and may be related more to alterations to the microbiome, which can be contributed to by diet, activity, sleep cycle, and other factors.
When food intolerances are triggered in the perimenopausal or menopausal patient, it may lead you to recommend the well-established FODMAP diet, which is known to reduce symptoms. But the answer for every patient is not simply placing them on a FODMAP diet and telling them they have irritable bowel syndrome.
Other approaches can be considered for addressing food intolerance in these patients. The data are quite strong that adjunctive use of a dietitian is tremendously helpful in this particular population.
When it comes to menopausal patients, however, we need to consider other changes in their activity or adverse contributors to their mental health, such as stress or anxiety. These all contribute to more of a multifactorial composite in this population, for which irritable bowel syndrome serves as a similar example.
This means that we may need to expand our horizons rather than to focus on solely on antispasmodic or diet-related interventions.
Instead, we can start to consider more of a multidimensional treatment approach consisting of education, relaxation, cognitive-behavioral therapy, and physical activity. Certainly, there are now behavioral interventions using Internet-based digital formats to increase the acceptability and sustainability among patients.
Choosing such a multidisciplinary approach can be quite helpful.
The metabolic consequences of altering hormonal balance
Recent data from a rat model study investigated the metabolic impact of changing hormonal balance.
Investigators looked at ovariectomized rats and found that there was a biologic change in the diversity of the general GI biome. There were also noteworthy associations with weight fluctuations and dramatic changes in the spatial memory and cognitive performance characteristics of these rats, which was subsequently improved by supplemental estrogen.
This indicates that we may be able to remediate these effects with the similar use of supplemental hormone replacement treatments.
Another recent study looked at nonalcoholic fatty liver disease, which is very common in the general population and has a > 20% worldwide prevalence in postmenopausal women. Albeit small in numbers, this was a very interesting study.
Investigators looked at the delivery method for menopausal hormone therapy, which was transdermal for 75 patients and oral for 293 patients. Then, they looked at ultrasound definition of nonalcoholic fatty liver disease after 1 year as the endpoint. They found an approximate 7% reduction in the patients who received the transdermal administration compared with a 4% increase in the patients who received it orally.
Again, we have to remember this is a relatively small study, but the results indicate that the route of estrogen administration may be an important consideration in nonalcoholic fatty liver disease.
Sleep disturbances: fragmentation, duration, and quality
Sleep is something that’s near and dear to my heart and is the focus of a lot of our research.
Sleep disturbances are really part and parcel of menopause and are observed with hormonal imbalances and temperature intolerances. Disturbances such as sleep fragmentation, shorter sleep duration, and poorer sleep quality have a dramatic effect not only on the biome but also on sensory thresholds.
Therefore, as we start to look at mitigating strategies here, we need to focus on sleep and ask the right questions.
In my own practice, I try not to just ask, “How did you sleep last night?” That’s because sleep can be somewhat amnestic. You may have a cognitive awakening or a noncognitive awakening but still have experienced fragmentation.
As a result, my focus is on next-day function. I ask my patients, “When you get up in the morning, are you refreshed? Do you have the ability to perform daytime activities? Do you experience early fatigue or cognitive changes that occur?”
These questions can provide good insights into the sleep efficiency of the previous night.
The effect of the microbiome on osteoporosis
One final topic I found very interesting pertains to the effects of menopause on osteoporosis.
We certainly know that postmenopausal women have a very high prevalence of osteopenia, and that osteoporosis is a progression of that, as well as that increased bone-related disease affects fractures and related morbidity and mortality.
However, there’s accumulating evidence on the osteoporotic effects of biomarker changes in menopause, which shows that the biome regulates the pathophysiologic process of at least a large degree of osteoporosis.
This starts to make sense when you look at the pro-inflammatory factors that increase with changes in biome diversity, in particular tumor necrosis factor alpha (which is something we also see in inflammatory bowel disease), interleukin-1, and increased activated osteoclasts.
Therefore, when it comes to decreasing bone loss among patients who are perimenopausal or postmenopausal, we don’t yet have a clear answer. Hormone therapy, diet, activity, vitamin D supplementation, and other things may positively change the biome. They are worthy topics for patients to bring up with their ob.gyns. or primary care doctors.
Although it may be a little bit outside the scope of gastroenterology, in my opinion there are a number of new findings relating to menopause that we as a field need to be more proactive in addressing.
Ask the right questions when these people come in to you, irrespective of why they’re there. Start to ask about the quality of their sleep. What are their other functional symptoms? What are their other potential osteoporosis-related risks?
We must do a better job about individualizing care. Rather than treating patients as disease states, we must start to do specific patient-focused care.
I hope this gives you some provocative thoughts when you have your next session with a patient in the perimenopausal or menopausal state. There are lots of things that we continue to learn.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Va., and a past president of the American College of Gastroenterology. He serves as an adviser to ISOThrive and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
Seven metrics oncology practices can track to be successful
and see how they measure up against their peers.
“Once practices figure out what they want to measure, and obviously they want to measure things that they’re not doing so well, they can look for opportunities for improvement,” said Diana Berich Brieva, DHA, MBA, CPC-A, the CEO of Ambulatory Care Consultants, which partners with medical practices to optimize operations and increase revenue.
Benchmarking your practice against others shows you how your numbers stack up to other practices’ metrics by percentile – for instance, whether your revenue is in the 25th, 50th, or 75th percentile against similar practices.
The 2024 MIPS Value Pathways (MVP) for Advancing Cancer Care is a new CMS program with specific metric criteria. The voluntary program has a Nov. 30, 2023, deadline for practices to sign up. The purpose of the program is to help practices identify areas where they can improve. Also, oncology societies such as the American Society of Clinical Oncology (ASCO) have developed metrics for this specialty.
Still, for many practices, it’s essential to develop your own metrics according to your patient population and available resources, explained Dr. Brieva.
Here are seven popular oncology metrics that many practices track to measure success.
1. Productivity
Every practice may think about productivity differently depending on whether it focuses on new patients, revenue, business development, or a combination. You can measure physician productivity in many ways: by the number of new patients per full-time employee (FTE), work relative value units (wRVU) per FTE, which measures physician work, and established patient visits.
Some clinics measure for wRVU for chemotherapy administration and per-hospital visits as a percentage of total patients as well. “We’re a community-based oncology practice, so we don’t use RVUs, but we do use other production numbers,” said Emily Touloukian, DO, an oncologist-hematologist and president of Coastal Cancer Center with four locations around South Carolina. She is assistant professor of internal medicine at the University of South Carolina, Columbia.
“There are lots of quality programs out there that measure how well oncology practices are meeting guidelines. The one we’ve participated with since its inception is [the] Quality Oncology Practice Initiative (QOPI) through [the] American Society of Clinical Oncology (ASCO),” said Dr. Touloukian. “Basically, it’s a chart review and extraction of various indicators in accordance with quality measures.”
Pontchartrain Cancer Center, with four locations around Louisiana, tracks the number of new patients in hematology and oncology by location and provider. They also track follow-up patients. New and follow-up patient metrics are broken down by visit code.
“The E&M code tells me the level of acuity that the physician coded for,” said Kathy Oubre, MS, the CEO of Pontchartrain. Patients with complicated cases get a higher-paying code since clinics get paid differently for each code. Ms. Oubre tracks the codes by provider and says if they bill every patient with the same code, it can put your practice at risk for an audit, even when it’s the lowest billable code.
In the 2019 ASCO survey, the number of new patient visits reported by participants averaged 301 visits per FTE. Established patient visits averaged 3,334.
“When we talk about metrics and how we measure things and how successful our practice is, productivity also has to do with how satisfied the people working for you and with you are,” said Dr. Touloukian.
“If you’re not providing a supportive workplace for your physicians and employees, you’re not going to be successful,” she said. “You’ll end up with doctors coming and going every 2 years, employees quitting all the time, and a need for retraining.” Instead, if you can create a welcoming, sustainable environment where people are happy to come to work, physicians aren’t burnt out, and get to spend time away from the clinic to recharge, productivity will be more successful.
2. Revenue
When participating in their voluntary survey, practices can get a copy of revenue metric data annually from the Medical Group Management Association (MGMA). It collects the number of FTEs, gross revenue, net revenue, and collection rate and is broken down by specialties so your practice can benchmark against others. Total revenue, including oncologists’ salaries per FTE from the ASCO survey, was $7,323,900, but comparisons are difficult since practices differ in services.
Revenue metrics can consist of total revenue (cash collections), net medical excluding radiation services, drug revenue for infusion services, cash expenses including salaries, net accounts receivable, and gross accounts receivable minus contractual allowances and bad debt. Practices can differ on bad debt collection because of the emotional nature of cancer treatment. However, some use revenue cycle management companies with debt collection services; others find charity foundation funds for patients who can’t pay.
Pontchartrain also tracks when its clinic gets paid. Ms. Oubre said the best practice is that your claims receive payment within 21 days. They send claims out every 24 hours. “For example, most of that money is in drugs we’ve administered to patients we’ve likely already paid for.” Since there is a gap between paying their wholesaler for drugs and receiving reimbursement, they closely track claims and payment metrics.
Any claims that get sent back are refiled and sent out again within 24 hours. When claims hit 31 days without a response, the practice reaches out to learn the problem. “We’re proactive rather than waiting for the denial to come,” Ms. Oubre told this news organization. Dr. Brieva said for every revenue metric a practice tracks in which they’re not performing well, the practice has to find a solution. Are too many claims being denied? Do claim forms contain errors? Are most claims being paid in the 21-day window? Is the problem a user error, an issue with the clearinghouse, or an intake error on the other end? The key to successful metric use is to drill down for answers to these questions.
3. Patient satisfaction
Patient satisfaction may be one of the more straightforward metrics practices can track, though not specific to oncology. Dr. Brieva said most metric programs include patient satisfaction surveys against which you can benchmark your practice. You can also create your own emailed patient surveys. The metric can show how satisfied your patients are and how you compare against other practices.
Ms. Oubre said Pontchartrain also tracks metrics around participating in advanced care planning, survivorship care, and transitional care management. Even though most insurers require copays for these services, they’ve found patients who participate in them have an overall better experience.
“The Biden administration also has a Cancer Moon Shot initiative, which intends to reduce cancer deaths by 50% by 2047,” said Dr. Brieva. “They want to reduce deaths and improve the experience of patients. So, tracking survival rates will also be key for this program.”
4. Referrals
Oncology is typically a referral business. So, keeping track of the top referring physicians every quarter is the best way to ensure your referring clinicians are happy with your practice’s service. A best practice is that all new oncology referrals are seen within 48 hours. If your referral metric drops off, especially for top referrers, a physician from your practice should check in with the referrer.
Ms. Oubre runs reports out of the EMR and scrubs for referring providers, so she’s alerted to any issues. “It can be as simple as a front office staff who was rude on the phone,” she said. “We had an issue years ago with one of our schedulers who didn’t want to risk staying after 5:00 p.m., so she wouldn’t put anyone on the schedule after 3:00. But if I hadn’t called and identified that from the referring practitioner, I wouldn’t have known that they couldn’t get late-afternoon appointments at our clinic.”
5. No-show appointments
Practices track no-shows per week to determine which patients did not show up for their visits vs. those who rescheduled. If a patient on active treatment starts no-showing, practices must find out why. Is it a social or a transportation issue, or do they want to discontinue treatment? Often, you can help with the problem if you know what it is.
“Sometimes we can help from a social determinants of health perspective, helping to provide services like transportation, financial assistance, or other things, and patients appreciate that we would care enough to reach out to see if we can help,” said Ms. Oubre.
For recurring no-shows, practices should notify the referring provider. Letting the referring clinician know that the patient stopped coming is a professional courtesy that helps strengthen your referral relationships. You wouldn’t want the referring physician to think the patient is being treated for cancer only to find out later that they discontinued treatment.
6. Injections and infusions
By tracking the number of injections and infusions per location per week, clinics can assess how busy their chemo chairs are and how many injections they give. Benchmarks include the number of initial intravenous infusions/injections and the total number of drug administration services per patient per chair. Similar metrics in radiation oncology are helpful.
7. Pharmacy prescriptions
For practices with an in-house pharmacy, tracking how many prescriptions are written per week by each provider and whether they could fill them in-house or had to send them out to a specialty pharmacy because of an insurance issue tells you the volume of drugs your pharmacy is fulfilling. Point-of-care dispensing pharmacy revenue averaged $1,843,342 in the ASCO survey.
Dr. Brieva mentioned many other trackable metrics, such as the time to start treatment, adherence to treatment guidelines, rates of side effects and complications, patient retention rates, treatment completion rates, and coordination of care with other providers, which may be additional metrics your practice wants to track.
Dr. Touloukian said that practices must be careful how they measure some metrics because if you’re extracting data from the EMR and someone hasn’t entered it correctly, you won’t get accurate information. “I like programs like QOPI because while it’s a little labor intensive, my staff actually goes in and extracts the data from the charts and shows the proof.
“Comparing yourself to other [oncology] practices across the nation helps to ensure you’re achieving a certain level of success on some of these traditional metrics,” said Dr. Touloukian.
A version of this article first appeared on Medscape.com.
and see how they measure up against their peers.
“Once practices figure out what they want to measure, and obviously they want to measure things that they’re not doing so well, they can look for opportunities for improvement,” said Diana Berich Brieva, DHA, MBA, CPC-A, the CEO of Ambulatory Care Consultants, which partners with medical practices to optimize operations and increase revenue.
Benchmarking your practice against others shows you how your numbers stack up to other practices’ metrics by percentile – for instance, whether your revenue is in the 25th, 50th, or 75th percentile against similar practices.
The 2024 MIPS Value Pathways (MVP) for Advancing Cancer Care is a new CMS program with specific metric criteria. The voluntary program has a Nov. 30, 2023, deadline for practices to sign up. The purpose of the program is to help practices identify areas where they can improve. Also, oncology societies such as the American Society of Clinical Oncology (ASCO) have developed metrics for this specialty.
Still, for many practices, it’s essential to develop your own metrics according to your patient population and available resources, explained Dr. Brieva.
Here are seven popular oncology metrics that many practices track to measure success.
1. Productivity
Every practice may think about productivity differently depending on whether it focuses on new patients, revenue, business development, or a combination. You can measure physician productivity in many ways: by the number of new patients per full-time employee (FTE), work relative value units (wRVU) per FTE, which measures physician work, and established patient visits.
Some clinics measure for wRVU for chemotherapy administration and per-hospital visits as a percentage of total patients as well. “We’re a community-based oncology practice, so we don’t use RVUs, but we do use other production numbers,” said Emily Touloukian, DO, an oncologist-hematologist and president of Coastal Cancer Center with four locations around South Carolina. She is assistant professor of internal medicine at the University of South Carolina, Columbia.
“There are lots of quality programs out there that measure how well oncology practices are meeting guidelines. The one we’ve participated with since its inception is [the] Quality Oncology Practice Initiative (QOPI) through [the] American Society of Clinical Oncology (ASCO),” said Dr. Touloukian. “Basically, it’s a chart review and extraction of various indicators in accordance with quality measures.”
Pontchartrain Cancer Center, with four locations around Louisiana, tracks the number of new patients in hematology and oncology by location and provider. They also track follow-up patients. New and follow-up patient metrics are broken down by visit code.
“The E&M code tells me the level of acuity that the physician coded for,” said Kathy Oubre, MS, the CEO of Pontchartrain. Patients with complicated cases get a higher-paying code since clinics get paid differently for each code. Ms. Oubre tracks the codes by provider and says if they bill every patient with the same code, it can put your practice at risk for an audit, even when it’s the lowest billable code.
In the 2019 ASCO survey, the number of new patient visits reported by participants averaged 301 visits per FTE. Established patient visits averaged 3,334.
“When we talk about metrics and how we measure things and how successful our practice is, productivity also has to do with how satisfied the people working for you and with you are,” said Dr. Touloukian.
“If you’re not providing a supportive workplace for your physicians and employees, you’re not going to be successful,” she said. “You’ll end up with doctors coming and going every 2 years, employees quitting all the time, and a need for retraining.” Instead, if you can create a welcoming, sustainable environment where people are happy to come to work, physicians aren’t burnt out, and get to spend time away from the clinic to recharge, productivity will be more successful.
2. Revenue
When participating in their voluntary survey, practices can get a copy of revenue metric data annually from the Medical Group Management Association (MGMA). It collects the number of FTEs, gross revenue, net revenue, and collection rate and is broken down by specialties so your practice can benchmark against others. Total revenue, including oncologists’ salaries per FTE from the ASCO survey, was $7,323,900, but comparisons are difficult since practices differ in services.
Revenue metrics can consist of total revenue (cash collections), net medical excluding radiation services, drug revenue for infusion services, cash expenses including salaries, net accounts receivable, and gross accounts receivable minus contractual allowances and bad debt. Practices can differ on bad debt collection because of the emotional nature of cancer treatment. However, some use revenue cycle management companies with debt collection services; others find charity foundation funds for patients who can’t pay.
Pontchartrain also tracks when its clinic gets paid. Ms. Oubre said the best practice is that your claims receive payment within 21 days. They send claims out every 24 hours. “For example, most of that money is in drugs we’ve administered to patients we’ve likely already paid for.” Since there is a gap between paying their wholesaler for drugs and receiving reimbursement, they closely track claims and payment metrics.
Any claims that get sent back are refiled and sent out again within 24 hours. When claims hit 31 days without a response, the practice reaches out to learn the problem. “We’re proactive rather than waiting for the denial to come,” Ms. Oubre told this news organization. Dr. Brieva said for every revenue metric a practice tracks in which they’re not performing well, the practice has to find a solution. Are too many claims being denied? Do claim forms contain errors? Are most claims being paid in the 21-day window? Is the problem a user error, an issue with the clearinghouse, or an intake error on the other end? The key to successful metric use is to drill down for answers to these questions.
3. Patient satisfaction
Patient satisfaction may be one of the more straightforward metrics practices can track, though not specific to oncology. Dr. Brieva said most metric programs include patient satisfaction surveys against which you can benchmark your practice. You can also create your own emailed patient surveys. The metric can show how satisfied your patients are and how you compare against other practices.
Ms. Oubre said Pontchartrain also tracks metrics around participating in advanced care planning, survivorship care, and transitional care management. Even though most insurers require copays for these services, they’ve found patients who participate in them have an overall better experience.
“The Biden administration also has a Cancer Moon Shot initiative, which intends to reduce cancer deaths by 50% by 2047,” said Dr. Brieva. “They want to reduce deaths and improve the experience of patients. So, tracking survival rates will also be key for this program.”
4. Referrals
Oncology is typically a referral business. So, keeping track of the top referring physicians every quarter is the best way to ensure your referring clinicians are happy with your practice’s service. A best practice is that all new oncology referrals are seen within 48 hours. If your referral metric drops off, especially for top referrers, a physician from your practice should check in with the referrer.
Ms. Oubre runs reports out of the EMR and scrubs for referring providers, so she’s alerted to any issues. “It can be as simple as a front office staff who was rude on the phone,” she said. “We had an issue years ago with one of our schedulers who didn’t want to risk staying after 5:00 p.m., so she wouldn’t put anyone on the schedule after 3:00. But if I hadn’t called and identified that from the referring practitioner, I wouldn’t have known that they couldn’t get late-afternoon appointments at our clinic.”
5. No-show appointments
Practices track no-shows per week to determine which patients did not show up for their visits vs. those who rescheduled. If a patient on active treatment starts no-showing, practices must find out why. Is it a social or a transportation issue, or do they want to discontinue treatment? Often, you can help with the problem if you know what it is.
“Sometimes we can help from a social determinants of health perspective, helping to provide services like transportation, financial assistance, or other things, and patients appreciate that we would care enough to reach out to see if we can help,” said Ms. Oubre.
For recurring no-shows, practices should notify the referring provider. Letting the referring clinician know that the patient stopped coming is a professional courtesy that helps strengthen your referral relationships. You wouldn’t want the referring physician to think the patient is being treated for cancer only to find out later that they discontinued treatment.
6. Injections and infusions
By tracking the number of injections and infusions per location per week, clinics can assess how busy their chemo chairs are and how many injections they give. Benchmarks include the number of initial intravenous infusions/injections and the total number of drug administration services per patient per chair. Similar metrics in radiation oncology are helpful.
7. Pharmacy prescriptions
For practices with an in-house pharmacy, tracking how many prescriptions are written per week by each provider and whether they could fill them in-house or had to send them out to a specialty pharmacy because of an insurance issue tells you the volume of drugs your pharmacy is fulfilling. Point-of-care dispensing pharmacy revenue averaged $1,843,342 in the ASCO survey.
Dr. Brieva mentioned many other trackable metrics, such as the time to start treatment, adherence to treatment guidelines, rates of side effects and complications, patient retention rates, treatment completion rates, and coordination of care with other providers, which may be additional metrics your practice wants to track.
Dr. Touloukian said that practices must be careful how they measure some metrics because if you’re extracting data from the EMR and someone hasn’t entered it correctly, you won’t get accurate information. “I like programs like QOPI because while it’s a little labor intensive, my staff actually goes in and extracts the data from the charts and shows the proof.
“Comparing yourself to other [oncology] practices across the nation helps to ensure you’re achieving a certain level of success on some of these traditional metrics,” said Dr. Touloukian.
A version of this article first appeared on Medscape.com.
and see how they measure up against their peers.
“Once practices figure out what they want to measure, and obviously they want to measure things that they’re not doing so well, they can look for opportunities for improvement,” said Diana Berich Brieva, DHA, MBA, CPC-A, the CEO of Ambulatory Care Consultants, which partners with medical practices to optimize operations and increase revenue.
Benchmarking your practice against others shows you how your numbers stack up to other practices’ metrics by percentile – for instance, whether your revenue is in the 25th, 50th, or 75th percentile against similar practices.
The 2024 MIPS Value Pathways (MVP) for Advancing Cancer Care is a new CMS program with specific metric criteria. The voluntary program has a Nov. 30, 2023, deadline for practices to sign up. The purpose of the program is to help practices identify areas where they can improve. Also, oncology societies such as the American Society of Clinical Oncology (ASCO) have developed metrics for this specialty.
Still, for many practices, it’s essential to develop your own metrics according to your patient population and available resources, explained Dr. Brieva.
Here are seven popular oncology metrics that many practices track to measure success.
1. Productivity
Every practice may think about productivity differently depending on whether it focuses on new patients, revenue, business development, or a combination. You can measure physician productivity in many ways: by the number of new patients per full-time employee (FTE), work relative value units (wRVU) per FTE, which measures physician work, and established patient visits.
Some clinics measure for wRVU for chemotherapy administration and per-hospital visits as a percentage of total patients as well. “We’re a community-based oncology practice, so we don’t use RVUs, but we do use other production numbers,” said Emily Touloukian, DO, an oncologist-hematologist and president of Coastal Cancer Center with four locations around South Carolina. She is assistant professor of internal medicine at the University of South Carolina, Columbia.
“There are lots of quality programs out there that measure how well oncology practices are meeting guidelines. The one we’ve participated with since its inception is [the] Quality Oncology Practice Initiative (QOPI) through [the] American Society of Clinical Oncology (ASCO),” said Dr. Touloukian. “Basically, it’s a chart review and extraction of various indicators in accordance with quality measures.”
Pontchartrain Cancer Center, with four locations around Louisiana, tracks the number of new patients in hematology and oncology by location and provider. They also track follow-up patients. New and follow-up patient metrics are broken down by visit code.
“The E&M code tells me the level of acuity that the physician coded for,” said Kathy Oubre, MS, the CEO of Pontchartrain. Patients with complicated cases get a higher-paying code since clinics get paid differently for each code. Ms. Oubre tracks the codes by provider and says if they bill every patient with the same code, it can put your practice at risk for an audit, even when it’s the lowest billable code.
In the 2019 ASCO survey, the number of new patient visits reported by participants averaged 301 visits per FTE. Established patient visits averaged 3,334.
“When we talk about metrics and how we measure things and how successful our practice is, productivity also has to do with how satisfied the people working for you and with you are,” said Dr. Touloukian.
“If you’re not providing a supportive workplace for your physicians and employees, you’re not going to be successful,” she said. “You’ll end up with doctors coming and going every 2 years, employees quitting all the time, and a need for retraining.” Instead, if you can create a welcoming, sustainable environment where people are happy to come to work, physicians aren’t burnt out, and get to spend time away from the clinic to recharge, productivity will be more successful.
2. Revenue
When participating in their voluntary survey, practices can get a copy of revenue metric data annually from the Medical Group Management Association (MGMA). It collects the number of FTEs, gross revenue, net revenue, and collection rate and is broken down by specialties so your practice can benchmark against others. Total revenue, including oncologists’ salaries per FTE from the ASCO survey, was $7,323,900, but comparisons are difficult since practices differ in services.
Revenue metrics can consist of total revenue (cash collections), net medical excluding radiation services, drug revenue for infusion services, cash expenses including salaries, net accounts receivable, and gross accounts receivable minus contractual allowances and bad debt. Practices can differ on bad debt collection because of the emotional nature of cancer treatment. However, some use revenue cycle management companies with debt collection services; others find charity foundation funds for patients who can’t pay.
Pontchartrain also tracks when its clinic gets paid. Ms. Oubre said the best practice is that your claims receive payment within 21 days. They send claims out every 24 hours. “For example, most of that money is in drugs we’ve administered to patients we’ve likely already paid for.” Since there is a gap between paying their wholesaler for drugs and receiving reimbursement, they closely track claims and payment metrics.
Any claims that get sent back are refiled and sent out again within 24 hours. When claims hit 31 days without a response, the practice reaches out to learn the problem. “We’re proactive rather than waiting for the denial to come,” Ms. Oubre told this news organization. Dr. Brieva said for every revenue metric a practice tracks in which they’re not performing well, the practice has to find a solution. Are too many claims being denied? Do claim forms contain errors? Are most claims being paid in the 21-day window? Is the problem a user error, an issue with the clearinghouse, or an intake error on the other end? The key to successful metric use is to drill down for answers to these questions.
3. Patient satisfaction
Patient satisfaction may be one of the more straightforward metrics practices can track, though not specific to oncology. Dr. Brieva said most metric programs include patient satisfaction surveys against which you can benchmark your practice. You can also create your own emailed patient surveys. The metric can show how satisfied your patients are and how you compare against other practices.
Ms. Oubre said Pontchartrain also tracks metrics around participating in advanced care planning, survivorship care, and transitional care management. Even though most insurers require copays for these services, they’ve found patients who participate in them have an overall better experience.
“The Biden administration also has a Cancer Moon Shot initiative, which intends to reduce cancer deaths by 50% by 2047,” said Dr. Brieva. “They want to reduce deaths and improve the experience of patients. So, tracking survival rates will also be key for this program.”
4. Referrals
Oncology is typically a referral business. So, keeping track of the top referring physicians every quarter is the best way to ensure your referring clinicians are happy with your practice’s service. A best practice is that all new oncology referrals are seen within 48 hours. If your referral metric drops off, especially for top referrers, a physician from your practice should check in with the referrer.
Ms. Oubre runs reports out of the EMR and scrubs for referring providers, so she’s alerted to any issues. “It can be as simple as a front office staff who was rude on the phone,” she said. “We had an issue years ago with one of our schedulers who didn’t want to risk staying after 5:00 p.m., so she wouldn’t put anyone on the schedule after 3:00. But if I hadn’t called and identified that from the referring practitioner, I wouldn’t have known that they couldn’t get late-afternoon appointments at our clinic.”
5. No-show appointments
Practices track no-shows per week to determine which patients did not show up for their visits vs. those who rescheduled. If a patient on active treatment starts no-showing, practices must find out why. Is it a social or a transportation issue, or do they want to discontinue treatment? Often, you can help with the problem if you know what it is.
“Sometimes we can help from a social determinants of health perspective, helping to provide services like transportation, financial assistance, or other things, and patients appreciate that we would care enough to reach out to see if we can help,” said Ms. Oubre.
For recurring no-shows, practices should notify the referring provider. Letting the referring clinician know that the patient stopped coming is a professional courtesy that helps strengthen your referral relationships. You wouldn’t want the referring physician to think the patient is being treated for cancer only to find out later that they discontinued treatment.
6. Injections and infusions
By tracking the number of injections and infusions per location per week, clinics can assess how busy their chemo chairs are and how many injections they give. Benchmarks include the number of initial intravenous infusions/injections and the total number of drug administration services per patient per chair. Similar metrics in radiation oncology are helpful.
7. Pharmacy prescriptions
For practices with an in-house pharmacy, tracking how many prescriptions are written per week by each provider and whether they could fill them in-house or had to send them out to a specialty pharmacy because of an insurance issue tells you the volume of drugs your pharmacy is fulfilling. Point-of-care dispensing pharmacy revenue averaged $1,843,342 in the ASCO survey.
Dr. Brieva mentioned many other trackable metrics, such as the time to start treatment, adherence to treatment guidelines, rates of side effects and complications, patient retention rates, treatment completion rates, and coordination of care with other providers, which may be additional metrics your practice wants to track.
Dr. Touloukian said that practices must be careful how they measure some metrics because if you’re extracting data from the EMR and someone hasn’t entered it correctly, you won’t get accurate information. “I like programs like QOPI because while it’s a little labor intensive, my staff actually goes in and extracts the data from the charts and shows the proof.
“Comparing yourself to other [oncology] practices across the nation helps to ensure you’re achieving a certain level of success on some of these traditional metrics,” said Dr. Touloukian.
A version of this article first appeared on Medscape.com.
Online nicotine toothpick vendors ignore age restrictions
WASHINGTON – according to a study of 77 stores and 16 online sites.
Online nicotine toothpick sales are “the Wild West” in terms of regulation, said Abhijeet Grewal, a research assistant at Cohen Children’s Medical Center, in New Hyde Park, N.Y., who presented the findings at the annual meeting of the American Academy of Pediatrics.
Nicotine toothpicks have become popular among teenagers as a relatively inconspicuous way to access the drug, Mr. Grewal said. The nicotine content of the toothpicks varies, but many contain as much as 2-3 mg per pick compared with the 1.1-1.8–mg amount inhaled per the average cigarette, he said. The cheap price and teen-friendly flavors like cherry and mocha add to the appeal of the picks. However, data on the marketplace and accessibility of these products are lacking, Mr. Grewal said.
To find out how easily youth can buy nicotine toothpicks through in-person and online channels, Mr. Grewal and colleagues identified and called 404 brick-and-mortar retailers across the United States by phone and asked whether they required ID for purchase of nicotine toothpicks; of the 77 locations that responded, only 1 said that they would sell nicotine toothpicks without asking for proof of age.
The researchers also collected data on 16 vendor websites that sold nicotine toothpicks with shipment to the United States (identified from pixotine.com).
Overall, 11 sites (69%) prompted users to confirm that they were aged 21 years or older to either view the site or place orders, but 12 sites (75%) required no formal method of verification.
Warnings or disclaimers, such as “nicotine is an addictive chemical,” appeared on 69% of sites. Marketing statements including terms such as “discreet” and “cost-effective” to describe the toothpicks, Mr. Grewal said, and online reviews endorsed the products as “convenient” and “rich in flavor.”
The sites in the study offered a total of 32 different flavors, Mr. Grewal said, and 44% of the sites offered some type of discount on prices, which land in the range of approximately $5 for a tube of 20 toothpicks.
Nicotine toothpicks and flavored toothpicks without nicotine were originally marketed as smoking cessation aids, said Mr. Grewal, but their low price point and ability to be consumed discreetly makes them appealing to teens for nicotine use in many environments.
More research is needed to characterize youth use of nicotine toothpick products, as well as purchasing patterns, he said. However, the results highlight the need for regulation of nicotine toothpick vendors to protect youth from accessing nicotine in this form, he said.
Ask adolescents about toothpicks
“While nicotine replacement therapy [NRT] products may be an effective way for people to quit smoking, these products have the potential to introduce minors to nicotine in a seemingly innocent way resulting in dependence,” senior author Ruth Milanaik, DO, also of Cohen Children’s Medical Center, said in an interview. “Many children are intrigued by these fun flavored products, and our team was interested in examining the availability of these products to minors.”
Overall, “our team was quite pleased with brick-and-mortar stores’ spoken requirements of age verification for purchase, and quite worried about the availability of nic picks through online vendors,” she continued.
Clinicians, educators, and parents should be aware of the existence of nicotine toothpicks and the ease with which minors can attain them through online vendors, Dr. Milanaik said. “While NRT is a part of smoking cessation programs, nicotine toothpicks should not be used by minors without clinical reasons,” she said. “The innocuous and innocent nature of these toothpicks may entice minors to try and regularly use these without regard to future dependence.”
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
WASHINGTON – according to a study of 77 stores and 16 online sites.
Online nicotine toothpick sales are “the Wild West” in terms of regulation, said Abhijeet Grewal, a research assistant at Cohen Children’s Medical Center, in New Hyde Park, N.Y., who presented the findings at the annual meeting of the American Academy of Pediatrics.
Nicotine toothpicks have become popular among teenagers as a relatively inconspicuous way to access the drug, Mr. Grewal said. The nicotine content of the toothpicks varies, but many contain as much as 2-3 mg per pick compared with the 1.1-1.8–mg amount inhaled per the average cigarette, he said. The cheap price and teen-friendly flavors like cherry and mocha add to the appeal of the picks. However, data on the marketplace and accessibility of these products are lacking, Mr. Grewal said.
To find out how easily youth can buy nicotine toothpicks through in-person and online channels, Mr. Grewal and colleagues identified and called 404 brick-and-mortar retailers across the United States by phone and asked whether they required ID for purchase of nicotine toothpicks; of the 77 locations that responded, only 1 said that they would sell nicotine toothpicks without asking for proof of age.
The researchers also collected data on 16 vendor websites that sold nicotine toothpicks with shipment to the United States (identified from pixotine.com).
Overall, 11 sites (69%) prompted users to confirm that they were aged 21 years or older to either view the site or place orders, but 12 sites (75%) required no formal method of verification.
Warnings or disclaimers, such as “nicotine is an addictive chemical,” appeared on 69% of sites. Marketing statements including terms such as “discreet” and “cost-effective” to describe the toothpicks, Mr. Grewal said, and online reviews endorsed the products as “convenient” and “rich in flavor.”
The sites in the study offered a total of 32 different flavors, Mr. Grewal said, and 44% of the sites offered some type of discount on prices, which land in the range of approximately $5 for a tube of 20 toothpicks.
Nicotine toothpicks and flavored toothpicks without nicotine were originally marketed as smoking cessation aids, said Mr. Grewal, but their low price point and ability to be consumed discreetly makes them appealing to teens for nicotine use in many environments.
More research is needed to characterize youth use of nicotine toothpick products, as well as purchasing patterns, he said. However, the results highlight the need for regulation of nicotine toothpick vendors to protect youth from accessing nicotine in this form, he said.
Ask adolescents about toothpicks
“While nicotine replacement therapy [NRT] products may be an effective way for people to quit smoking, these products have the potential to introduce minors to nicotine in a seemingly innocent way resulting in dependence,” senior author Ruth Milanaik, DO, also of Cohen Children’s Medical Center, said in an interview. “Many children are intrigued by these fun flavored products, and our team was interested in examining the availability of these products to minors.”
Overall, “our team was quite pleased with brick-and-mortar stores’ spoken requirements of age verification for purchase, and quite worried about the availability of nic picks through online vendors,” she continued.
Clinicians, educators, and parents should be aware of the existence of nicotine toothpicks and the ease with which minors can attain them through online vendors, Dr. Milanaik said. “While NRT is a part of smoking cessation programs, nicotine toothpicks should not be used by minors without clinical reasons,” she said. “The innocuous and innocent nature of these toothpicks may entice minors to try and regularly use these without regard to future dependence.”
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
WASHINGTON – according to a study of 77 stores and 16 online sites.
Online nicotine toothpick sales are “the Wild West” in terms of regulation, said Abhijeet Grewal, a research assistant at Cohen Children’s Medical Center, in New Hyde Park, N.Y., who presented the findings at the annual meeting of the American Academy of Pediatrics.
Nicotine toothpicks have become popular among teenagers as a relatively inconspicuous way to access the drug, Mr. Grewal said. The nicotine content of the toothpicks varies, but many contain as much as 2-3 mg per pick compared with the 1.1-1.8–mg amount inhaled per the average cigarette, he said. The cheap price and teen-friendly flavors like cherry and mocha add to the appeal of the picks. However, data on the marketplace and accessibility of these products are lacking, Mr. Grewal said.
To find out how easily youth can buy nicotine toothpicks through in-person and online channels, Mr. Grewal and colleagues identified and called 404 brick-and-mortar retailers across the United States by phone and asked whether they required ID for purchase of nicotine toothpicks; of the 77 locations that responded, only 1 said that they would sell nicotine toothpicks without asking for proof of age.
The researchers also collected data on 16 vendor websites that sold nicotine toothpicks with shipment to the United States (identified from pixotine.com).
Overall, 11 sites (69%) prompted users to confirm that they were aged 21 years or older to either view the site or place orders, but 12 sites (75%) required no formal method of verification.
Warnings or disclaimers, such as “nicotine is an addictive chemical,” appeared on 69% of sites. Marketing statements including terms such as “discreet” and “cost-effective” to describe the toothpicks, Mr. Grewal said, and online reviews endorsed the products as “convenient” and “rich in flavor.”
The sites in the study offered a total of 32 different flavors, Mr. Grewal said, and 44% of the sites offered some type of discount on prices, which land in the range of approximately $5 for a tube of 20 toothpicks.
Nicotine toothpicks and flavored toothpicks without nicotine were originally marketed as smoking cessation aids, said Mr. Grewal, but their low price point and ability to be consumed discreetly makes them appealing to teens for nicotine use in many environments.
More research is needed to characterize youth use of nicotine toothpick products, as well as purchasing patterns, he said. However, the results highlight the need for regulation of nicotine toothpick vendors to protect youth from accessing nicotine in this form, he said.
Ask adolescents about toothpicks
“While nicotine replacement therapy [NRT] products may be an effective way for people to quit smoking, these products have the potential to introduce minors to nicotine in a seemingly innocent way resulting in dependence,” senior author Ruth Milanaik, DO, also of Cohen Children’s Medical Center, said in an interview. “Many children are intrigued by these fun flavored products, and our team was interested in examining the availability of these products to minors.”
Overall, “our team was quite pleased with brick-and-mortar stores’ spoken requirements of age verification for purchase, and quite worried about the availability of nic picks through online vendors,” she continued.
Clinicians, educators, and parents should be aware of the existence of nicotine toothpicks and the ease with which minors can attain them through online vendors, Dr. Milanaik said. “While NRT is a part of smoking cessation programs, nicotine toothpicks should not be used by minors without clinical reasons,” she said. “The innocuous and innocent nature of these toothpicks may entice minors to try and regularly use these without regard to future dependence.”
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
AT AAP 2023
More weight loss with time-restricted eating
TOPLINE:
, compared with calorie restriction, while hemoglobin A1c levels dropped with both approaches, compared with no intervention.
METHODOLOGY:
- Six-month clinical trial of 75 adult participants with type 2 diabetes and obesity, randomly assigned to either 8-hour TRE (noon to 8 p.m. only) without calorie counting, a 25% daily calorie restriction, or control.
TAKEAWAY:
- The primary outcome, change in body weight at month 6, was –3.56% (P = .004) with TRE vs. –1.78% with calorie restriction (P = .06), compared with controls.
- The mean calorie deficit over the 6 months was –313 kcal/day with TRE, –197 kcal/day with calorie restriction, and –16 kcal/day for controls.
- Self-reported adherence to the regimens was 87% of days with 8-hour TRE vs. 68% reporting adherence with calorie goals over the 6 months.
- A1c levels were reduced significantly by 0.91% in the TRE group and 0.94% in the calorie-restriction group, relative to controls, with no differences between the two intervention groups.
- No serious adverse events were reported.
- Hypoglycemia and hyperglycemia occurrences didn’t differ between groups.
IN PRACTICE:
“Our findings ... show that TRE is safe in patients who are using either diet alone or medications to control their [type 2 diabetes]. However, for people using sulfonylureas and/or insulin, adopting a TRE regimen will require medication changes and regular monitoring, particularly in the initial stages of the diet.”
SOURCE:
The study was conducted by Vasiliki Pavlou, MS, RD, of the department of kinesiology and nutrition, University of Illinois at Chicago, and colleagues. It was published online in JAMA Network Open.
LIMITATIONS:
- Relatively short trial duration.
- Lack of blinding.
- A higher proportion in the TRE group were using newer type 2 diabetes medications at baseline.
- Self-reported dietary intake.
DISCLOSURES:
The study was supported by the University of Illinois at Chicago, and by grants from the National Institutes of Health. Ms. Pavlou reports no relevant financial relationships. Several authors reported relationships with industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
, compared with calorie restriction, while hemoglobin A1c levels dropped with both approaches, compared with no intervention.
METHODOLOGY:
- Six-month clinical trial of 75 adult participants with type 2 diabetes and obesity, randomly assigned to either 8-hour TRE (noon to 8 p.m. only) without calorie counting, a 25% daily calorie restriction, or control.
TAKEAWAY:
- The primary outcome, change in body weight at month 6, was –3.56% (P = .004) with TRE vs. –1.78% with calorie restriction (P = .06), compared with controls.
- The mean calorie deficit over the 6 months was –313 kcal/day with TRE, –197 kcal/day with calorie restriction, and –16 kcal/day for controls.
- Self-reported adherence to the regimens was 87% of days with 8-hour TRE vs. 68% reporting adherence with calorie goals over the 6 months.
- A1c levels were reduced significantly by 0.91% in the TRE group and 0.94% in the calorie-restriction group, relative to controls, with no differences between the two intervention groups.
- No serious adverse events were reported.
- Hypoglycemia and hyperglycemia occurrences didn’t differ between groups.
IN PRACTICE:
“Our findings ... show that TRE is safe in patients who are using either diet alone or medications to control their [type 2 diabetes]. However, for people using sulfonylureas and/or insulin, adopting a TRE regimen will require medication changes and regular monitoring, particularly in the initial stages of the diet.”
SOURCE:
The study was conducted by Vasiliki Pavlou, MS, RD, of the department of kinesiology and nutrition, University of Illinois at Chicago, and colleagues. It was published online in JAMA Network Open.
LIMITATIONS:
- Relatively short trial duration.
- Lack of blinding.
- A higher proportion in the TRE group were using newer type 2 diabetes medications at baseline.
- Self-reported dietary intake.
DISCLOSURES:
The study was supported by the University of Illinois at Chicago, and by grants from the National Institutes of Health. Ms. Pavlou reports no relevant financial relationships. Several authors reported relationships with industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
, compared with calorie restriction, while hemoglobin A1c levels dropped with both approaches, compared with no intervention.
METHODOLOGY:
- Six-month clinical trial of 75 adult participants with type 2 diabetes and obesity, randomly assigned to either 8-hour TRE (noon to 8 p.m. only) without calorie counting, a 25% daily calorie restriction, or control.
TAKEAWAY:
- The primary outcome, change in body weight at month 6, was –3.56% (P = .004) with TRE vs. –1.78% with calorie restriction (P = .06), compared with controls.
- The mean calorie deficit over the 6 months was –313 kcal/day with TRE, –197 kcal/day with calorie restriction, and –16 kcal/day for controls.
- Self-reported adherence to the regimens was 87% of days with 8-hour TRE vs. 68% reporting adherence with calorie goals over the 6 months.
- A1c levels were reduced significantly by 0.91% in the TRE group and 0.94% in the calorie-restriction group, relative to controls, with no differences between the two intervention groups.
- No serious adverse events were reported.
- Hypoglycemia and hyperglycemia occurrences didn’t differ between groups.
IN PRACTICE:
“Our findings ... show that TRE is safe in patients who are using either diet alone or medications to control their [type 2 diabetes]. However, for people using sulfonylureas and/or insulin, adopting a TRE regimen will require medication changes and regular monitoring, particularly in the initial stages of the diet.”
SOURCE:
The study was conducted by Vasiliki Pavlou, MS, RD, of the department of kinesiology and nutrition, University of Illinois at Chicago, and colleagues. It was published online in JAMA Network Open.
LIMITATIONS:
- Relatively short trial duration.
- Lack of blinding.
- A higher proportion in the TRE group were using newer type 2 diabetes medications at baseline.
- Self-reported dietary intake.
DISCLOSURES:
The study was supported by the University of Illinois at Chicago, and by grants from the National Institutes of Health. Ms. Pavlou reports no relevant financial relationships. Several authors reported relationships with industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
Portfolio diet tied to lower risk for CVD, stroke
TOPLINE:
Close adherence to the Portfolio dietary pattern, including foods that have been shown to actively lower cholesterol (for example, plant proteins, nuts, viscous fiber, phytosterols, and plant monounsaturated fats) is associated with a 14% lower risk for total cardiovascular disease (CVD), coronary heart disease (CHD), and stroke, pooled results from three large observational studies suggest.
METHODOLOGY:
- The study included 73,924 women from the Nurses’ Health Study (NHS), 92,346 women from the Nurses’ Health Study II (NHSII), and 43,970 men from the Health Professionals Follow-Up Study (HPFS) without CVD at baseline who were followed biennially on lifestyle, medical history, and other health-related factors.
- From food-frequency questionnaires (FFQs) completed every 4 years, researchers categorized foods into the six components of the Portfolio diet:
- Plant protein such as legumes, beans, tofu, peas, and soymilk
- Nuts and seeds
- Fiber sources such as bran, oats, berries, and eggplant
- Phytosterols
- Monounsaturated fat (MUFA) sources such as olive oil and avocado
- High saturated fat and cholesterol sources such as whole-fat dairy and red and processed meats
- They scored each from 1 (least adherent) to 5 (most adherent), with a higher score indicating higher consumption.
- Researchers examined the association of this Portfolio Diet Score (PDS) with total CVD, CHD, and stroke, in the three cohorts, and associations with plasma levels of lipid and inflammatory biomarkers in a subpopulation of the cohorts.
TAKEAWAY:
- During up to 30 years of follow-up, there were 16,917 incident CVD cases, including 10,666 CHD cases and 6,473 strokes.
- In a pooled analysis of the three cohorts, the fully adjusted hazard ratio for total CVD comparing the highest with the lowest quintile of the PDS was 0.86 (95% confidence interval, 0.81-0.92; P for trend < .001).
- Also comparing extreme quintiles, the pooled HR for CHD was 0.86 (95% CI, 0.80-0.93; P for trend = .0001) and for stroke, it was 0.86 (95% CI, 0.78-0.95; P for trend = .0003).
- A higher PDS was also associated with a more favorable lipid profile and lower levels of inflammation.
IN PRACTICE:
which aligns with American Heart Association guidelines promoting consumption of whole grains, fruits and vegetables, plant-based proteins, minimally processed foods, and healthy unsaturated plant oils, the authors conclude.
SOURCE:
The study was conducted by Andrea J. Glenn, PhD, RD, department of nutrition, Harvard T.H. Chan School of Public Health, Boston, and colleagues. It was published online in the journal Circulation.
LIMITATIONS:
As the study was observational, residual confounding can’t be ruled out. Diet was self-reported, which may have resulted in measurement errors. Consumption of some recommended foods was low, even in the top quintiles, so the association with CVD risk may be underestimated. Information on a few key Portfolio diet foods, including barley and okra, was unavailable, potentially leading to underestimation of intake, which may also attenuate the findings.
DISCLOSURES:
The study was supported by the Diabetes Canada End Diabetes 100 Award. The NH and HPFS studies are supported by the National Institutes of Health. Dr. Glenn is supported by a Canadian Institutes of Health Research fellowship; she has received honoraria or travel support from the Soy Nutrition Institute Global, Vinasoy, and the Academy of Nutrition and Dietetics. See original article for disclosures of other authors.
A version of this article first appeared on Medscape.com.
TOPLINE:
Close adherence to the Portfolio dietary pattern, including foods that have been shown to actively lower cholesterol (for example, plant proteins, nuts, viscous fiber, phytosterols, and plant monounsaturated fats) is associated with a 14% lower risk for total cardiovascular disease (CVD), coronary heart disease (CHD), and stroke, pooled results from three large observational studies suggest.
METHODOLOGY:
- The study included 73,924 women from the Nurses’ Health Study (NHS), 92,346 women from the Nurses’ Health Study II (NHSII), and 43,970 men from the Health Professionals Follow-Up Study (HPFS) without CVD at baseline who were followed biennially on lifestyle, medical history, and other health-related factors.
- From food-frequency questionnaires (FFQs) completed every 4 years, researchers categorized foods into the six components of the Portfolio diet:
- Plant protein such as legumes, beans, tofu, peas, and soymilk
- Nuts and seeds
- Fiber sources such as bran, oats, berries, and eggplant
- Phytosterols
- Monounsaturated fat (MUFA) sources such as olive oil and avocado
- High saturated fat and cholesterol sources such as whole-fat dairy and red and processed meats
- They scored each from 1 (least adherent) to 5 (most adherent), with a higher score indicating higher consumption.
- Researchers examined the association of this Portfolio Diet Score (PDS) with total CVD, CHD, and stroke, in the three cohorts, and associations with plasma levels of lipid and inflammatory biomarkers in a subpopulation of the cohorts.
TAKEAWAY:
- During up to 30 years of follow-up, there were 16,917 incident CVD cases, including 10,666 CHD cases and 6,473 strokes.
- In a pooled analysis of the three cohorts, the fully adjusted hazard ratio for total CVD comparing the highest with the lowest quintile of the PDS was 0.86 (95% confidence interval, 0.81-0.92; P for trend < .001).
- Also comparing extreme quintiles, the pooled HR for CHD was 0.86 (95% CI, 0.80-0.93; P for trend = .0001) and for stroke, it was 0.86 (95% CI, 0.78-0.95; P for trend = .0003).
- A higher PDS was also associated with a more favorable lipid profile and lower levels of inflammation.
IN PRACTICE:
which aligns with American Heart Association guidelines promoting consumption of whole grains, fruits and vegetables, plant-based proteins, minimally processed foods, and healthy unsaturated plant oils, the authors conclude.
SOURCE:
The study was conducted by Andrea J. Glenn, PhD, RD, department of nutrition, Harvard T.H. Chan School of Public Health, Boston, and colleagues. It was published online in the journal Circulation.
LIMITATIONS:
As the study was observational, residual confounding can’t be ruled out. Diet was self-reported, which may have resulted in measurement errors. Consumption of some recommended foods was low, even in the top quintiles, so the association with CVD risk may be underestimated. Information on a few key Portfolio diet foods, including barley and okra, was unavailable, potentially leading to underestimation of intake, which may also attenuate the findings.
DISCLOSURES:
The study was supported by the Diabetes Canada End Diabetes 100 Award. The NH and HPFS studies are supported by the National Institutes of Health. Dr. Glenn is supported by a Canadian Institutes of Health Research fellowship; she has received honoraria or travel support from the Soy Nutrition Institute Global, Vinasoy, and the Academy of Nutrition and Dietetics. See original article for disclosures of other authors.
A version of this article first appeared on Medscape.com.
TOPLINE:
Close adherence to the Portfolio dietary pattern, including foods that have been shown to actively lower cholesterol (for example, plant proteins, nuts, viscous fiber, phytosterols, and plant monounsaturated fats) is associated with a 14% lower risk for total cardiovascular disease (CVD), coronary heart disease (CHD), and stroke, pooled results from three large observational studies suggest.
METHODOLOGY:
- The study included 73,924 women from the Nurses’ Health Study (NHS), 92,346 women from the Nurses’ Health Study II (NHSII), and 43,970 men from the Health Professionals Follow-Up Study (HPFS) without CVD at baseline who were followed biennially on lifestyle, medical history, and other health-related factors.
- From food-frequency questionnaires (FFQs) completed every 4 years, researchers categorized foods into the six components of the Portfolio diet:
- Plant protein such as legumes, beans, tofu, peas, and soymilk
- Nuts and seeds
- Fiber sources such as bran, oats, berries, and eggplant
- Phytosterols
- Monounsaturated fat (MUFA) sources such as olive oil and avocado
- High saturated fat and cholesterol sources such as whole-fat dairy and red and processed meats
- They scored each from 1 (least adherent) to 5 (most adherent), with a higher score indicating higher consumption.
- Researchers examined the association of this Portfolio Diet Score (PDS) with total CVD, CHD, and stroke, in the three cohorts, and associations with plasma levels of lipid and inflammatory biomarkers in a subpopulation of the cohorts.
TAKEAWAY:
- During up to 30 years of follow-up, there were 16,917 incident CVD cases, including 10,666 CHD cases and 6,473 strokes.
- In a pooled analysis of the three cohorts, the fully adjusted hazard ratio for total CVD comparing the highest with the lowest quintile of the PDS was 0.86 (95% confidence interval, 0.81-0.92; P for trend < .001).
- Also comparing extreme quintiles, the pooled HR for CHD was 0.86 (95% CI, 0.80-0.93; P for trend = .0001) and for stroke, it was 0.86 (95% CI, 0.78-0.95; P for trend = .0003).
- A higher PDS was also associated with a more favorable lipid profile and lower levels of inflammation.
IN PRACTICE:
which aligns with American Heart Association guidelines promoting consumption of whole grains, fruits and vegetables, plant-based proteins, minimally processed foods, and healthy unsaturated plant oils, the authors conclude.
SOURCE:
The study was conducted by Andrea J. Glenn, PhD, RD, department of nutrition, Harvard T.H. Chan School of Public Health, Boston, and colleagues. It was published online in the journal Circulation.
LIMITATIONS:
As the study was observational, residual confounding can’t be ruled out. Diet was self-reported, which may have resulted in measurement errors. Consumption of some recommended foods was low, even in the top quintiles, so the association with CVD risk may be underestimated. Information on a few key Portfolio diet foods, including barley and okra, was unavailable, potentially leading to underestimation of intake, which may also attenuate the findings.
DISCLOSURES:
The study was supported by the Diabetes Canada End Diabetes 100 Award. The NH and HPFS studies are supported by the National Institutes of Health. Dr. Glenn is supported by a Canadian Institutes of Health Research fellowship; she has received honoraria or travel support from the Soy Nutrition Institute Global, Vinasoy, and the Academy of Nutrition and Dietetics. See original article for disclosures of other authors.
A version of this article first appeared on Medscape.com.
Experts question finding that 70% cancer deaths are preventable
A new global analysis highlights the substantial burden of premature deaths from cancer around the world – a burden that could potentially be averted through prevention, early detection, and timely treatment.
According to the analysis, in 2020, over half of all cancer deaths – 5.28 million of 9.96 million – occurred prematurely (before age 70), leading to a loss of roughly 183 million life-years from the disease worldwide.
More than two-thirds of premature cancer-related deaths – 3.6 million, or 68% – were potentially preventable through lifestyle changes or early detection efforts, such as cancer screening, dietary changes, or smoking cessation, and about one-third – 1.65 million, or 31% – may have been treatable.
But two biostatisticians not involved in the study who took a deep dive into it urged caution in interpreting the data.
Nilanjan Chatterjee, PhD, Bloomberg Distinguished Professor, Bloomberg School of Public Health at Johns Hopkins University, Baltimore, said the study does a “great job in bringing a lot of diverse data together to show there is very high potential for preventing premature deaths due to cancer worldwide.”
However, for a variety of reasons, Dr. Chatterjee explained, one should not “overinterpret” the high percentage of potentially preventable cancer deaths.
Gideon Meyerowitz-Katz, PhD, an epidemiologist at the University of Wollongong, in Australia, agreed.
“It’s likely many cancer deaths are, in theory, preventable, but the numbers around just how many are necessarily vague,” said Dr. Meyerowitz-Katz. “Also, ‘in theory preventable’ doesn’t necessarily mean that we can actually do it in practice.”
Invest in cancer prevention
The study, led by researchers from the World Health Organization’s International Agency for Research on Cancer and partners, provides estimates of premature deaths from 36 cancers across 185 countries.
The findings, published in The Lancet Global Health along with a new Lancet Commission report – “Women, Power, and Cancer” also highlighted the “underrecognized” cancer burden among women around the world.
Cancer ranks in the top three causes of premature mortality among women in almost all countries worldwide, but it is often “deprioritized,” the Lancet Commission report explained.
Of the nearly 5.3 million premature cancer deaths in 2020, 2.9 million occurred in men, and 2.3 million occurred in women, the investigators found. Of the premature deaths among women, 1.5 million could have potentially been avoided through prevention or detection efforts, while the remaining 800,000 might have been averted “if all women everywhere could access optimal cancer care,” the authors said.
Lung cancer was the leading contributor to preventable premature years of life lost in countries that have medium to very high scores on the Human Development Index (HDI), whereas cervical cancer was the leading contributor in low-HDI countries. HDI rankings are based on life expectancy, education, and gross national income.
Among women, as many as 72% of cancer death were premature in low-HDI countries, vs 36% in very high-HDI countries.
Overall, across all four tiers of HDI, colorectal and breast cancers represented the major treatable cancers.
Reducing exposure to four main risk factors – tobacco smoking, alcohol consumption, high body weight, and infections – would go a long way toward reducing potentially preventable premature cancer-related deaths, the authors said.
“Globally, there are marked inequalities between countries in reaching the target of reducing premature mortality from noncommunicable diseases, including cancer,” author Isabelle Soerjomataram, MD, PhD, deputy head of cancer surveillance at the International Agency for Research on Cancer, said in a press release.
“Greater investments in cancer prevention programs can reduce the prevalence of key risk factors for cancer, and increased coverage of vaccination alongside early diagnosis and screening linked to timely treatment can and must address the current cancer inequalities that are seen worldwide,” she added.
Caveats and cautionary notes
The authors acknowledge that the study has limitations related to its methodology and underlying assumptions. For instance, some premature cancer deaths that were classified as preventable may have been averted through curative therapy as well.
The findings also represent a snapshot of premature mortality in 2020 but do not necessarily predict progress in cancer control over time.
In Dr. Chatterjee’s view, this is “an excellent descriptive study that gives a good overall picture about the potential for saving a very large fraction of premature death due to cancer by implementing what is now known about primary and secondary interventions, and treatments.”
However, estimates for the effects of various risk factors and interventions are often derived from observational nonrandomized studies, which can have various types of biases, he said.
“Additionally, availability of data, observational or randomized, are often limited from many countries in Africa, Latin America, and Asia, where the cancer burdens are increasing,” Dr. Chatterjee told this news organization. “Therefore, extrapolating evidence generated mostly from North America and European countries to other understudied settings could be problematic due to difference in background in genetics, environment, socioeconomic, and cultural differences.”
Dr. Meyerowitz-Katz said the issue with this “very complex” article is that it includes “models built upon models, all of which include layers of assumptions that aren’t always obvious and may be wrong.”
On top of that, he said, “there are questions over whether the modifiable risk factors are really modifiable. Can we really get rid of 100% of ‘lack of physical exercise’? What would that even look like?”
Overall, Dr. Meyerowitz-Katz noted, “Yes, some proportion of these cancers could be prevented, and that percentage may be large, but the exact 70% estimate is very uncertain in my opinion.”
The study had no commercial funding. The authors, Dr. Chatterjee, and Dr. Meyerowitz-Katz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new global analysis highlights the substantial burden of premature deaths from cancer around the world – a burden that could potentially be averted through prevention, early detection, and timely treatment.
According to the analysis, in 2020, over half of all cancer deaths – 5.28 million of 9.96 million – occurred prematurely (before age 70), leading to a loss of roughly 183 million life-years from the disease worldwide.
More than two-thirds of premature cancer-related deaths – 3.6 million, or 68% – were potentially preventable through lifestyle changes or early detection efforts, such as cancer screening, dietary changes, or smoking cessation, and about one-third – 1.65 million, or 31% – may have been treatable.
But two biostatisticians not involved in the study who took a deep dive into it urged caution in interpreting the data.
Nilanjan Chatterjee, PhD, Bloomberg Distinguished Professor, Bloomberg School of Public Health at Johns Hopkins University, Baltimore, said the study does a “great job in bringing a lot of diverse data together to show there is very high potential for preventing premature deaths due to cancer worldwide.”
However, for a variety of reasons, Dr. Chatterjee explained, one should not “overinterpret” the high percentage of potentially preventable cancer deaths.
Gideon Meyerowitz-Katz, PhD, an epidemiologist at the University of Wollongong, in Australia, agreed.
“It’s likely many cancer deaths are, in theory, preventable, but the numbers around just how many are necessarily vague,” said Dr. Meyerowitz-Katz. “Also, ‘in theory preventable’ doesn’t necessarily mean that we can actually do it in practice.”
Invest in cancer prevention
The study, led by researchers from the World Health Organization’s International Agency for Research on Cancer and partners, provides estimates of premature deaths from 36 cancers across 185 countries.
The findings, published in The Lancet Global Health along with a new Lancet Commission report – “Women, Power, and Cancer” also highlighted the “underrecognized” cancer burden among women around the world.
Cancer ranks in the top three causes of premature mortality among women in almost all countries worldwide, but it is often “deprioritized,” the Lancet Commission report explained.
Of the nearly 5.3 million premature cancer deaths in 2020, 2.9 million occurred in men, and 2.3 million occurred in women, the investigators found. Of the premature deaths among women, 1.5 million could have potentially been avoided through prevention or detection efforts, while the remaining 800,000 might have been averted “if all women everywhere could access optimal cancer care,” the authors said.
Lung cancer was the leading contributor to preventable premature years of life lost in countries that have medium to very high scores on the Human Development Index (HDI), whereas cervical cancer was the leading contributor in low-HDI countries. HDI rankings are based on life expectancy, education, and gross national income.
Among women, as many as 72% of cancer death were premature in low-HDI countries, vs 36% in very high-HDI countries.
Overall, across all four tiers of HDI, colorectal and breast cancers represented the major treatable cancers.
Reducing exposure to four main risk factors – tobacco smoking, alcohol consumption, high body weight, and infections – would go a long way toward reducing potentially preventable premature cancer-related deaths, the authors said.
“Globally, there are marked inequalities between countries in reaching the target of reducing premature mortality from noncommunicable diseases, including cancer,” author Isabelle Soerjomataram, MD, PhD, deputy head of cancer surveillance at the International Agency for Research on Cancer, said in a press release.
“Greater investments in cancer prevention programs can reduce the prevalence of key risk factors for cancer, and increased coverage of vaccination alongside early diagnosis and screening linked to timely treatment can and must address the current cancer inequalities that are seen worldwide,” she added.
Caveats and cautionary notes
The authors acknowledge that the study has limitations related to its methodology and underlying assumptions. For instance, some premature cancer deaths that were classified as preventable may have been averted through curative therapy as well.
The findings also represent a snapshot of premature mortality in 2020 but do not necessarily predict progress in cancer control over time.
In Dr. Chatterjee’s view, this is “an excellent descriptive study that gives a good overall picture about the potential for saving a very large fraction of premature death due to cancer by implementing what is now known about primary and secondary interventions, and treatments.”
However, estimates for the effects of various risk factors and interventions are often derived from observational nonrandomized studies, which can have various types of biases, he said.
“Additionally, availability of data, observational or randomized, are often limited from many countries in Africa, Latin America, and Asia, where the cancer burdens are increasing,” Dr. Chatterjee told this news organization. “Therefore, extrapolating evidence generated mostly from North America and European countries to other understudied settings could be problematic due to difference in background in genetics, environment, socioeconomic, and cultural differences.”
Dr. Meyerowitz-Katz said the issue with this “very complex” article is that it includes “models built upon models, all of which include layers of assumptions that aren’t always obvious and may be wrong.”
On top of that, he said, “there are questions over whether the modifiable risk factors are really modifiable. Can we really get rid of 100% of ‘lack of physical exercise’? What would that even look like?”
Overall, Dr. Meyerowitz-Katz noted, “Yes, some proportion of these cancers could be prevented, and that percentage may be large, but the exact 70% estimate is very uncertain in my opinion.”
The study had no commercial funding. The authors, Dr. Chatterjee, and Dr. Meyerowitz-Katz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new global analysis highlights the substantial burden of premature deaths from cancer around the world – a burden that could potentially be averted through prevention, early detection, and timely treatment.
According to the analysis, in 2020, over half of all cancer deaths – 5.28 million of 9.96 million – occurred prematurely (before age 70), leading to a loss of roughly 183 million life-years from the disease worldwide.
More than two-thirds of premature cancer-related deaths – 3.6 million, or 68% – were potentially preventable through lifestyle changes or early detection efforts, such as cancer screening, dietary changes, or smoking cessation, and about one-third – 1.65 million, or 31% – may have been treatable.
But two biostatisticians not involved in the study who took a deep dive into it urged caution in interpreting the data.
Nilanjan Chatterjee, PhD, Bloomberg Distinguished Professor, Bloomberg School of Public Health at Johns Hopkins University, Baltimore, said the study does a “great job in bringing a lot of diverse data together to show there is very high potential for preventing premature deaths due to cancer worldwide.”
However, for a variety of reasons, Dr. Chatterjee explained, one should not “overinterpret” the high percentage of potentially preventable cancer deaths.
Gideon Meyerowitz-Katz, PhD, an epidemiologist at the University of Wollongong, in Australia, agreed.
“It’s likely many cancer deaths are, in theory, preventable, but the numbers around just how many are necessarily vague,” said Dr. Meyerowitz-Katz. “Also, ‘in theory preventable’ doesn’t necessarily mean that we can actually do it in practice.”
Invest in cancer prevention
The study, led by researchers from the World Health Organization’s International Agency for Research on Cancer and partners, provides estimates of premature deaths from 36 cancers across 185 countries.
The findings, published in The Lancet Global Health along with a new Lancet Commission report – “Women, Power, and Cancer” also highlighted the “underrecognized” cancer burden among women around the world.
Cancer ranks in the top three causes of premature mortality among women in almost all countries worldwide, but it is often “deprioritized,” the Lancet Commission report explained.
Of the nearly 5.3 million premature cancer deaths in 2020, 2.9 million occurred in men, and 2.3 million occurred in women, the investigators found. Of the premature deaths among women, 1.5 million could have potentially been avoided through prevention or detection efforts, while the remaining 800,000 might have been averted “if all women everywhere could access optimal cancer care,” the authors said.
Lung cancer was the leading contributor to preventable premature years of life lost in countries that have medium to very high scores on the Human Development Index (HDI), whereas cervical cancer was the leading contributor in low-HDI countries. HDI rankings are based on life expectancy, education, and gross national income.
Among women, as many as 72% of cancer death were premature in low-HDI countries, vs 36% in very high-HDI countries.
Overall, across all four tiers of HDI, colorectal and breast cancers represented the major treatable cancers.
Reducing exposure to four main risk factors – tobacco smoking, alcohol consumption, high body weight, and infections – would go a long way toward reducing potentially preventable premature cancer-related deaths, the authors said.
“Globally, there are marked inequalities between countries in reaching the target of reducing premature mortality from noncommunicable diseases, including cancer,” author Isabelle Soerjomataram, MD, PhD, deputy head of cancer surveillance at the International Agency for Research on Cancer, said in a press release.
“Greater investments in cancer prevention programs can reduce the prevalence of key risk factors for cancer, and increased coverage of vaccination alongside early diagnosis and screening linked to timely treatment can and must address the current cancer inequalities that are seen worldwide,” she added.
Caveats and cautionary notes
The authors acknowledge that the study has limitations related to its methodology and underlying assumptions. For instance, some premature cancer deaths that were classified as preventable may have been averted through curative therapy as well.
The findings also represent a snapshot of premature mortality in 2020 but do not necessarily predict progress in cancer control over time.
In Dr. Chatterjee’s view, this is “an excellent descriptive study that gives a good overall picture about the potential for saving a very large fraction of premature death due to cancer by implementing what is now known about primary and secondary interventions, and treatments.”
However, estimates for the effects of various risk factors and interventions are often derived from observational nonrandomized studies, which can have various types of biases, he said.
“Additionally, availability of data, observational or randomized, are often limited from many countries in Africa, Latin America, and Asia, where the cancer burdens are increasing,” Dr. Chatterjee told this news organization. “Therefore, extrapolating evidence generated mostly from North America and European countries to other understudied settings could be problematic due to difference in background in genetics, environment, socioeconomic, and cultural differences.”
Dr. Meyerowitz-Katz said the issue with this “very complex” article is that it includes “models built upon models, all of which include layers of assumptions that aren’t always obvious and may be wrong.”
On top of that, he said, “there are questions over whether the modifiable risk factors are really modifiable. Can we really get rid of 100% of ‘lack of physical exercise’? What would that even look like?”
Overall, Dr. Meyerowitz-Katz noted, “Yes, some proportion of these cancers could be prevented, and that percentage may be large, but the exact 70% estimate is very uncertain in my opinion.”
The study had no commercial funding. The authors, Dr. Chatterjee, and Dr. Meyerowitz-Katz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET GLOBAL HEALTH
Analysis of Internal Dermatology Matches Following the COVID-19 Pandemic
Dermatology residencies continue to be among the most competitive, with only 66% of seniors in US medical schools (MD programs) successfully matching to a dermatology residency in 2023, according to the National Resident Matching Program. In 2023, there were 141 dermatology residency programs accepting applications, with a total of 499 positions offered. Of 578 medical school senior applicants, 384 of those applicants successfully matched. In contrast, of the 79 senior applicants from osteopathic medical schools, only 34 successfully matched, according to the National Resident Matching Program. A higher number of students match to either their home institution or an institution at which they completed an away (external) rotation, likely because faculty members are more comfortable matching future residents with whom they have worked because of greater familiarity with these applicants, and because applicants are more comfortable with programs familiar to them.1
Prior to the COVID-19 pandemic, the Association of Professors of Dermatology published an official statement discouraging programs from offering in-person external electives to applicants in the 2020-2021 cycle. As the pandemic progressed, this evolved: for the 2021-2022 cycle, applicants were encouraged to complete only 1 away rotation, and for the 2022-2023 cycle, applicants were encouraged to complete up to 3 away rotations.2 This most recent recommendation reflects applicant experience before the pandemic, with some students having a personal connection to up to 4 programs, including their home and away programs.
A cross-sectional study published in early 2023 analyzed internal matches prior to and until the second year of the pandemic. The prepandemic rate of internal matches—applicants who matched at their home programs—was 26.7%. This rate increased to 40.3% in the 2020-2021 cycle and was 33.5% in the 2021-2022 cycle.2,3 The increase in internal matches is likely multifactorial, including the emergence of virtual interviews, the addition of program and geographic signals, and the regulation of away rotations. Notably, the rate of internal matches inversely correlates with the number of external programs to which students have connections.
We conducted a cross-sectional study to analyze the rates of internal and regional dermatology matches in the post–COVID-19 pandemic era (2022-2023) vs prepandemic and pandemic rates.
Methods
Data were obtained from publicly available online match lists from 65 US medical schools that detailed programs where dermatology applicants matched. The data reflected the postpandemic residency application cycle (2022-2023). These data were then compared to previous match rates for the prepandemic (2020-2021) and pandemic (2021-2022) application cycles. Medical schools without corresponding dermatology residency programs were excluded from the study. Regions were determined using the Association of American Medical Colleges Residency Explorer tool. The Northeast region included schools from Vermont; Pennsylvania; New Hampshire; New Jersey; Rhode Island; Maryland; Massachusetts; New York; Connecticut; and Washington, DC. The Southern region included schools from Florida, Georgia, Kentucky, Louisiana, Arkansas, North Carolina, Alabama, South Carolina, Mississippi, Tennessee, Texas, Oklahoma, and Virginia. The Western region included schools from Oregon, New Mexico, Utah, Colorado, Arizona, Washington, and California. The Central region included schools from Illinois, Indiana, Michigan, Ohio, Wisconsin, Iowa, Kansas, Minnesota, Missouri, and Nebraska. The data collected included the number of applicants who matched into dermatology, the number of applicants who matched at their home institutions, and the regions in which applicants matched. Rates of matching were calculated as percentages, and Pearson χ2 tests were used to compare internal and regional match rates between different time periods.
Results
Results for the 2022-2023 residency cycle are summarized in the Table. Of 210 matches, 80 (38.10%) of the applicants matched at their home institution. In prepandemic cycles, 26.7% of applicants matched at their home institutions, which increased to 38.1% after the pandemic (P=.028). During the pandemic, 40.3% of applicants matched at their home institutions (P=.827).2 One hundred forty-nine of 210 (70.95%) applicants matched in the same region as their home institutions. The Western region had the highest rate of both internal matches (47.06%) and same-region matches (76.47%). However, the Central and Northeast regions were a close second (43.55% for home matches and 75.81% for same-region matches) and third (42.31% for home matches and 75.00% for same-region matches) for both rates, respectively. The Southern region had the lowest rates overall, with 29.11% for home matches and 63.29% for same-region matches.
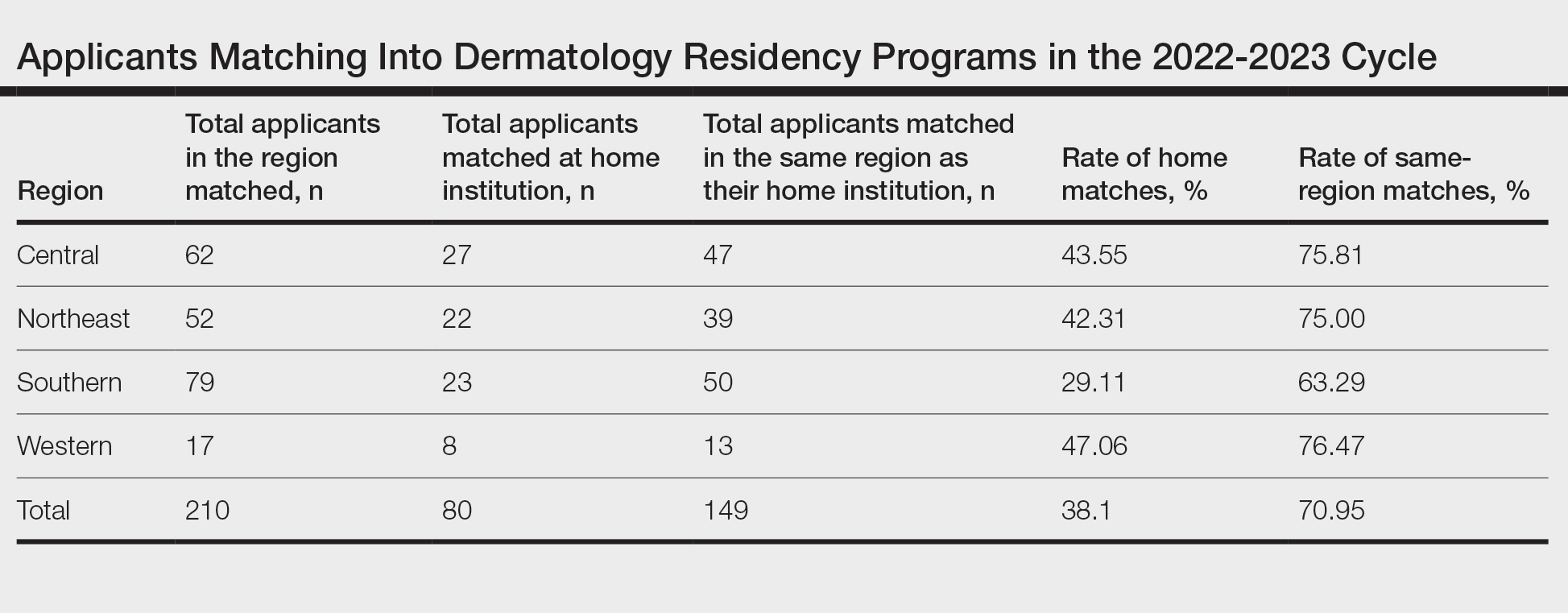
Comment
The changes to the match process resulting from the COVID-19 pandemic have had a profound impact on match outcomes since 2020. During the first year of the pandemic, internal matches increased to 40%; during the second year, the rate decreased to 33%.2 The difference between the current postpandemic internal match rate of 38.1% and the prepandemic internal match rate of 26.7% was statistically significant (P=.028). Conversely, the difference between the postpandemic internal match rate and the pandemic internal match rate was not significant (P=.827). These findings suggest that that pandemic trends have continued despite the return to multiple away rotations for students, perhaps suggesting that virtual interviews, which have been maintained at most programs despite the end of the pandemic, may be the driving force behind the increased home match rate. During the second year of the pandemic, there were greater odds (odds ratio, 2.3) of a dermatology program matching at least 1 internal applicant vs the years prior to 2020.4
The prepandemic regional match rate was 61.6% and increased to 67.5% during the pandemic.3 Following the pandemic, 70.95% of applicants matched in the same region as their home program. A study completed in 2022 using the Texas Seeking Transparency in Application to Residency database found that there was no difference in the percentage of applicants who had a geographic connection to their program when comparing the 2021 cycle to 2018-2020 cycles.5 Frequently, applicants prefer to stay within their regions due to social factors. Although applicants can again travel for external rotations, the regional match rate has stayed relatively constant before and after the pandemic, though it has trended upward throughout the latest application cycles.
During the 2022-2023 cycle, applicants were able to send preference signals to 3 programs. A survey reflecting the 2021-2022 cycle showed that 21.1% of applicants who sent a preference signal to a program were interviewed by that program, whereas only 3.7% of applicants who did not send a preference signal were interviewed. Furthermore, 19% of matched applicants sent a preference signal to the program at which they ultimately matched.6 Survey respondents included 40 accredited dermatology residency programs who reported an average of 506 applications per program. Preference signals were developed to allow applicants to connect with programs at which they were not able to rotate. It is unclear how preference signals are affecting internal or regional match rates, but similar to virtual interviewing, they may be contributing to the higher rates of internal matching.
This study is limited in the number of programs with match data publicly available for analysis. Additionally, there were no official data on how many students match at programs at which they completed external rotations. Furthermore, these data do not include reapplicants or osteopathic applicants who match within their regions. Importantly, all US medical schools were not represented in these data. Many programs, specifically in the Western region, did not have publicly available match lists. Self-reported match lists were not included in this study to avoid discrepancies. Regional rates reported here may not be representative of actual regional rates, as there were more applicants and internal matches in each region than were included in this study.
Conclusion
Although applicants were able to participate in external rotations as of the last 2 application cycles, there was still an increase in the rate of internal dermatology matches during the 2022-2023 cycle. This trend suggests an underlying disadvantage in matching for students without a home program. For the 2023-2024 cycle, applicants are recommended to complete up to 2 external rotations and may consider up to 3 if they do not have a home program. This recommended limitation in external rotations aims to allow students without a home program to develop connections with more programs.
- Luu Y, Gao W, Han J, et al. Personal connections and preference signaling: a cross-sectional analysis of the dermatology residency match during COVID-19. J Am Acad Dermatol. 2023;88:1381-1383. doi:10.1016/j.jaad.2023.01.032
- Dowdle TS, Ryan MP, Tarbox MB, et al. An analysis of internal and regional dermatology matches during the second year of the COVID-19 pandemic: a cross-sectional study. J Am Acad Dermatol. 2023;88:207-209. doi:10.1016/j.jaad.2022.04.036
- Dowdle TS, Ryan MP, Wagner RF. Internal and geographic dermatology match trends in the age of COVID-19. J Am Acad Dermatol. 2021;85:1364-1366. doi:10.1016/j.jaad.2021.08.004
- Abdelwahab R, Antezana LA, Xie KZ, et al. Cross-sectional study of dermatology residency home match incidence during the COVID-19 pandemic. J Am Acad Dermatol. 2022;87:886-888. doi:10.1016/j.jaad.2021.12.004
- Williams GE, Zimmerman JM, Wiggins CJ, et al. The indelible marks on dermatology: impacts of COVID-19 on dermatology residency Match using the Texas STAR database. Clin Dermatol. 2023;41:215-218. doi:10.1016/j.clindermatol.2022.12.001
- Dirr MA, Brownstone N, Zakria D, et al. Dermatology match preference signaling tokens: impact and implications. Dermatol Surg. 2022;48:1367-1368. doi:10.1097/DSS.0000000000003645
Dermatology residencies continue to be among the most competitive, with only 66% of seniors in US medical schools (MD programs) successfully matching to a dermatology residency in 2023, according to the National Resident Matching Program. In 2023, there were 141 dermatology residency programs accepting applications, with a total of 499 positions offered. Of 578 medical school senior applicants, 384 of those applicants successfully matched. In contrast, of the 79 senior applicants from osteopathic medical schools, only 34 successfully matched, according to the National Resident Matching Program. A higher number of students match to either their home institution or an institution at which they completed an away (external) rotation, likely because faculty members are more comfortable matching future residents with whom they have worked because of greater familiarity with these applicants, and because applicants are more comfortable with programs familiar to them.1
Prior to the COVID-19 pandemic, the Association of Professors of Dermatology published an official statement discouraging programs from offering in-person external electives to applicants in the 2020-2021 cycle. As the pandemic progressed, this evolved: for the 2021-2022 cycle, applicants were encouraged to complete only 1 away rotation, and for the 2022-2023 cycle, applicants were encouraged to complete up to 3 away rotations.2 This most recent recommendation reflects applicant experience before the pandemic, with some students having a personal connection to up to 4 programs, including their home and away programs.
A cross-sectional study published in early 2023 analyzed internal matches prior to and until the second year of the pandemic. The prepandemic rate of internal matches—applicants who matched at their home programs—was 26.7%. This rate increased to 40.3% in the 2020-2021 cycle and was 33.5% in the 2021-2022 cycle.2,3 The increase in internal matches is likely multifactorial, including the emergence of virtual interviews, the addition of program and geographic signals, and the regulation of away rotations. Notably, the rate of internal matches inversely correlates with the number of external programs to which students have connections.
We conducted a cross-sectional study to analyze the rates of internal and regional dermatology matches in the post–COVID-19 pandemic era (2022-2023) vs prepandemic and pandemic rates.
Methods
Data were obtained from publicly available online match lists from 65 US medical schools that detailed programs where dermatology applicants matched. The data reflected the postpandemic residency application cycle (2022-2023). These data were then compared to previous match rates for the prepandemic (2020-2021) and pandemic (2021-2022) application cycles. Medical schools without corresponding dermatology residency programs were excluded from the study. Regions were determined using the Association of American Medical Colleges Residency Explorer tool. The Northeast region included schools from Vermont; Pennsylvania; New Hampshire; New Jersey; Rhode Island; Maryland; Massachusetts; New York; Connecticut; and Washington, DC. The Southern region included schools from Florida, Georgia, Kentucky, Louisiana, Arkansas, North Carolina, Alabama, South Carolina, Mississippi, Tennessee, Texas, Oklahoma, and Virginia. The Western region included schools from Oregon, New Mexico, Utah, Colorado, Arizona, Washington, and California. The Central region included schools from Illinois, Indiana, Michigan, Ohio, Wisconsin, Iowa, Kansas, Minnesota, Missouri, and Nebraska. The data collected included the number of applicants who matched into dermatology, the number of applicants who matched at their home institutions, and the regions in which applicants matched. Rates of matching were calculated as percentages, and Pearson χ2 tests were used to compare internal and regional match rates between different time periods.
Results
Results for the 2022-2023 residency cycle are summarized in the Table. Of 210 matches, 80 (38.10%) of the applicants matched at their home institution. In prepandemic cycles, 26.7% of applicants matched at their home institutions, which increased to 38.1% after the pandemic (P=.028). During the pandemic, 40.3% of applicants matched at their home institutions (P=.827).2 One hundred forty-nine of 210 (70.95%) applicants matched in the same region as their home institutions. The Western region had the highest rate of both internal matches (47.06%) and same-region matches (76.47%). However, the Central and Northeast regions were a close second (43.55% for home matches and 75.81% for same-region matches) and third (42.31% for home matches and 75.00% for same-region matches) for both rates, respectively. The Southern region had the lowest rates overall, with 29.11% for home matches and 63.29% for same-region matches.

Comment
The changes to the match process resulting from the COVID-19 pandemic have had a profound impact on match outcomes since 2020. During the first year of the pandemic, internal matches increased to 40%; during the second year, the rate decreased to 33%.2 The difference between the current postpandemic internal match rate of 38.1% and the prepandemic internal match rate of 26.7% was statistically significant (P=.028). Conversely, the difference between the postpandemic internal match rate and the pandemic internal match rate was not significant (P=.827). These findings suggest that that pandemic trends have continued despite the return to multiple away rotations for students, perhaps suggesting that virtual interviews, which have been maintained at most programs despite the end of the pandemic, may be the driving force behind the increased home match rate. During the second year of the pandemic, there were greater odds (odds ratio, 2.3) of a dermatology program matching at least 1 internal applicant vs the years prior to 2020.4
The prepandemic regional match rate was 61.6% and increased to 67.5% during the pandemic.3 Following the pandemic, 70.95% of applicants matched in the same region as their home program. A study completed in 2022 using the Texas Seeking Transparency in Application to Residency database found that there was no difference in the percentage of applicants who had a geographic connection to their program when comparing the 2021 cycle to 2018-2020 cycles.5 Frequently, applicants prefer to stay within their regions due to social factors. Although applicants can again travel for external rotations, the regional match rate has stayed relatively constant before and after the pandemic, though it has trended upward throughout the latest application cycles.
During the 2022-2023 cycle, applicants were able to send preference signals to 3 programs. A survey reflecting the 2021-2022 cycle showed that 21.1% of applicants who sent a preference signal to a program were interviewed by that program, whereas only 3.7% of applicants who did not send a preference signal were interviewed. Furthermore, 19% of matched applicants sent a preference signal to the program at which they ultimately matched.6 Survey respondents included 40 accredited dermatology residency programs who reported an average of 506 applications per program. Preference signals were developed to allow applicants to connect with programs at which they were not able to rotate. It is unclear how preference signals are affecting internal or regional match rates, but similar to virtual interviewing, they may be contributing to the higher rates of internal matching.
This study is limited in the number of programs with match data publicly available for analysis. Additionally, there were no official data on how many students match at programs at which they completed external rotations. Furthermore, these data do not include reapplicants or osteopathic applicants who match within their regions. Importantly, all US medical schools were not represented in these data. Many programs, specifically in the Western region, did not have publicly available match lists. Self-reported match lists were not included in this study to avoid discrepancies. Regional rates reported here may not be representative of actual regional rates, as there were more applicants and internal matches in each region than were included in this study.
Conclusion
Although applicants were able to participate in external rotations as of the last 2 application cycles, there was still an increase in the rate of internal dermatology matches during the 2022-2023 cycle. This trend suggests an underlying disadvantage in matching for students without a home program. For the 2023-2024 cycle, applicants are recommended to complete up to 2 external rotations and may consider up to 3 if they do not have a home program. This recommended limitation in external rotations aims to allow students without a home program to develop connections with more programs.
Dermatology residencies continue to be among the most competitive, with only 66% of seniors in US medical schools (MD programs) successfully matching to a dermatology residency in 2023, according to the National Resident Matching Program. In 2023, there were 141 dermatology residency programs accepting applications, with a total of 499 positions offered. Of 578 medical school senior applicants, 384 of those applicants successfully matched. In contrast, of the 79 senior applicants from osteopathic medical schools, only 34 successfully matched, according to the National Resident Matching Program. A higher number of students match to either their home institution or an institution at which they completed an away (external) rotation, likely because faculty members are more comfortable matching future residents with whom they have worked because of greater familiarity with these applicants, and because applicants are more comfortable with programs familiar to them.1
Prior to the COVID-19 pandemic, the Association of Professors of Dermatology published an official statement discouraging programs from offering in-person external electives to applicants in the 2020-2021 cycle. As the pandemic progressed, this evolved: for the 2021-2022 cycle, applicants were encouraged to complete only 1 away rotation, and for the 2022-2023 cycle, applicants were encouraged to complete up to 3 away rotations.2 This most recent recommendation reflects applicant experience before the pandemic, with some students having a personal connection to up to 4 programs, including their home and away programs.
A cross-sectional study published in early 2023 analyzed internal matches prior to and until the second year of the pandemic. The prepandemic rate of internal matches—applicants who matched at their home programs—was 26.7%. This rate increased to 40.3% in the 2020-2021 cycle and was 33.5% in the 2021-2022 cycle.2,3 The increase in internal matches is likely multifactorial, including the emergence of virtual interviews, the addition of program and geographic signals, and the regulation of away rotations. Notably, the rate of internal matches inversely correlates with the number of external programs to which students have connections.
We conducted a cross-sectional study to analyze the rates of internal and regional dermatology matches in the post–COVID-19 pandemic era (2022-2023) vs prepandemic and pandemic rates.
Methods
Data were obtained from publicly available online match lists from 65 US medical schools that detailed programs where dermatology applicants matched. The data reflected the postpandemic residency application cycle (2022-2023). These data were then compared to previous match rates for the prepandemic (2020-2021) and pandemic (2021-2022) application cycles. Medical schools without corresponding dermatology residency programs were excluded from the study. Regions were determined using the Association of American Medical Colleges Residency Explorer tool. The Northeast region included schools from Vermont; Pennsylvania; New Hampshire; New Jersey; Rhode Island; Maryland; Massachusetts; New York; Connecticut; and Washington, DC. The Southern region included schools from Florida, Georgia, Kentucky, Louisiana, Arkansas, North Carolina, Alabama, South Carolina, Mississippi, Tennessee, Texas, Oklahoma, and Virginia. The Western region included schools from Oregon, New Mexico, Utah, Colorado, Arizona, Washington, and California. The Central region included schools from Illinois, Indiana, Michigan, Ohio, Wisconsin, Iowa, Kansas, Minnesota, Missouri, and Nebraska. The data collected included the number of applicants who matched into dermatology, the number of applicants who matched at their home institutions, and the regions in which applicants matched. Rates of matching were calculated as percentages, and Pearson χ2 tests were used to compare internal and regional match rates between different time periods.
Results
Results for the 2022-2023 residency cycle are summarized in the Table. Of 210 matches, 80 (38.10%) of the applicants matched at their home institution. In prepandemic cycles, 26.7% of applicants matched at their home institutions, which increased to 38.1% after the pandemic (P=.028). During the pandemic, 40.3% of applicants matched at their home institutions (P=.827).2 One hundred forty-nine of 210 (70.95%) applicants matched in the same region as their home institutions. The Western region had the highest rate of both internal matches (47.06%) and same-region matches (76.47%). However, the Central and Northeast regions were a close second (43.55% for home matches and 75.81% for same-region matches) and third (42.31% for home matches and 75.00% for same-region matches) for both rates, respectively. The Southern region had the lowest rates overall, with 29.11% for home matches and 63.29% for same-region matches.

Comment
The changes to the match process resulting from the COVID-19 pandemic have had a profound impact on match outcomes since 2020. During the first year of the pandemic, internal matches increased to 40%; during the second year, the rate decreased to 33%.2 The difference between the current postpandemic internal match rate of 38.1% and the prepandemic internal match rate of 26.7% was statistically significant (P=.028). Conversely, the difference between the postpandemic internal match rate and the pandemic internal match rate was not significant (P=.827). These findings suggest that that pandemic trends have continued despite the return to multiple away rotations for students, perhaps suggesting that virtual interviews, which have been maintained at most programs despite the end of the pandemic, may be the driving force behind the increased home match rate. During the second year of the pandemic, there were greater odds (odds ratio, 2.3) of a dermatology program matching at least 1 internal applicant vs the years prior to 2020.4
The prepandemic regional match rate was 61.6% and increased to 67.5% during the pandemic.3 Following the pandemic, 70.95% of applicants matched in the same region as their home program. A study completed in 2022 using the Texas Seeking Transparency in Application to Residency database found that there was no difference in the percentage of applicants who had a geographic connection to their program when comparing the 2021 cycle to 2018-2020 cycles.5 Frequently, applicants prefer to stay within their regions due to social factors. Although applicants can again travel for external rotations, the regional match rate has stayed relatively constant before and after the pandemic, though it has trended upward throughout the latest application cycles.
During the 2022-2023 cycle, applicants were able to send preference signals to 3 programs. A survey reflecting the 2021-2022 cycle showed that 21.1% of applicants who sent a preference signal to a program were interviewed by that program, whereas only 3.7% of applicants who did not send a preference signal were interviewed. Furthermore, 19% of matched applicants sent a preference signal to the program at which they ultimately matched.6 Survey respondents included 40 accredited dermatology residency programs who reported an average of 506 applications per program. Preference signals were developed to allow applicants to connect with programs at which they were not able to rotate. It is unclear how preference signals are affecting internal or regional match rates, but similar to virtual interviewing, they may be contributing to the higher rates of internal matching.
This study is limited in the number of programs with match data publicly available for analysis. Additionally, there were no official data on how many students match at programs at which they completed external rotations. Furthermore, these data do not include reapplicants or osteopathic applicants who match within their regions. Importantly, all US medical schools were not represented in these data. Many programs, specifically in the Western region, did not have publicly available match lists. Self-reported match lists were not included in this study to avoid discrepancies. Regional rates reported here may not be representative of actual regional rates, as there were more applicants and internal matches in each region than were included in this study.
Conclusion
Although applicants were able to participate in external rotations as of the last 2 application cycles, there was still an increase in the rate of internal dermatology matches during the 2022-2023 cycle. This trend suggests an underlying disadvantage in matching for students without a home program. For the 2023-2024 cycle, applicants are recommended to complete up to 2 external rotations and may consider up to 3 if they do not have a home program. This recommended limitation in external rotations aims to allow students without a home program to develop connections with more programs.
- Luu Y, Gao W, Han J, et al. Personal connections and preference signaling: a cross-sectional analysis of the dermatology residency match during COVID-19. J Am Acad Dermatol. 2023;88:1381-1383. doi:10.1016/j.jaad.2023.01.032
- Dowdle TS, Ryan MP, Tarbox MB, et al. An analysis of internal and regional dermatology matches during the second year of the COVID-19 pandemic: a cross-sectional study. J Am Acad Dermatol. 2023;88:207-209. doi:10.1016/j.jaad.2022.04.036
- Dowdle TS, Ryan MP, Wagner RF. Internal and geographic dermatology match trends in the age of COVID-19. J Am Acad Dermatol. 2021;85:1364-1366. doi:10.1016/j.jaad.2021.08.004
- Abdelwahab R, Antezana LA, Xie KZ, et al. Cross-sectional study of dermatology residency home match incidence during the COVID-19 pandemic. J Am Acad Dermatol. 2022;87:886-888. doi:10.1016/j.jaad.2021.12.004
- Williams GE, Zimmerman JM, Wiggins CJ, et al. The indelible marks on dermatology: impacts of COVID-19 on dermatology residency Match using the Texas STAR database. Clin Dermatol. 2023;41:215-218. doi:10.1016/j.clindermatol.2022.12.001
- Dirr MA, Brownstone N, Zakria D, et al. Dermatology match preference signaling tokens: impact and implications. Dermatol Surg. 2022;48:1367-1368. doi:10.1097/DSS.0000000000003645
- Luu Y, Gao W, Han J, et al. Personal connections and preference signaling: a cross-sectional analysis of the dermatology residency match during COVID-19. J Am Acad Dermatol. 2023;88:1381-1383. doi:10.1016/j.jaad.2023.01.032
- Dowdle TS, Ryan MP, Tarbox MB, et al. An analysis of internal and regional dermatology matches during the second year of the COVID-19 pandemic: a cross-sectional study. J Am Acad Dermatol. 2023;88:207-209. doi:10.1016/j.jaad.2022.04.036
- Dowdle TS, Ryan MP, Wagner RF. Internal and geographic dermatology match trends in the age of COVID-19. J Am Acad Dermatol. 2021;85:1364-1366. doi:10.1016/j.jaad.2021.08.004
- Abdelwahab R, Antezana LA, Xie KZ, et al. Cross-sectional study of dermatology residency home match incidence during the COVID-19 pandemic. J Am Acad Dermatol. 2022;87:886-888. doi:10.1016/j.jaad.2021.12.004
- Williams GE, Zimmerman JM, Wiggins CJ, et al. The indelible marks on dermatology: impacts of COVID-19 on dermatology residency Match using the Texas STAR database. Clin Dermatol. 2023;41:215-218. doi:10.1016/j.clindermatol.2022.12.001
- Dirr MA, Brownstone N, Zakria D, et al. Dermatology match preference signaling tokens: impact and implications. Dermatol Surg. 2022;48:1367-1368. doi:10.1097/DSS.0000000000003645
PRACTICE POINTS
- Following the COVID-19 pandemic, affiliation with a home program is even more impactful in successful application to dermatology residency. Applicants from institutions without dermatology programs should consider completing additional externships.
- The high rate of applicants matching to the same regions as their home programs is due to several factors. Applicants may have a larger social support system near their home institution. Additionally, programs are more comfortable matching applicants within their own regions.
Hospital Dermatology: Review of Research in 2022-2023
Dermatologists improve the diagnostic accuracy and quality of care of patients in the hospital setting. They help shorten the length of stay, improve outpatient follow-up, and reduce the rate of hospital readmission.1 Medicare beneficiaries hospitalized with skin conditions at institutions with a dermatology hospitalist—a provider with a specialty interest in inpatient dermatology—have 24% lower odds of risk-adjusted 30-day mortality and 12% lower odds of risk-adjusted 30-day readmissions.2
In the last year, research among the dermatology hospitalist community has actively contributed to our understanding of challenging inpatient skin diseases and has identified new ways in which dermatologists can contribute to the care of hospitalized patients. In this review, we highlight 4 areas of focus from the published literature in 2022-2023—severe cutaneous adverse reactions, supportive oncodermatology, cost of inpatient services, and teledermatology.
Severe Cutaneous Adverse Reactions: Old and New
Severe cutaneous adverse reactions to medications frequently are encountered in the inpatient setting. Dermatology hospitalists are well positioned to phenotype these reactions, drawing insights that aid in identifying, characterizing, risk stratifying, and managing these conditions, which have considerable morbidity and mortality.
A recent 20-year retrospective review of cases of acute generalized exanthematous pustulosis (N=340) across 10 academic systems—the largest to date—improves our understanding of the features of this rare entity.3 The authors found that acute generalized exanthematous pustulosis most often is triggered by β-lactam and other antibiotics (75.5%) and is accompanied by fever (49.7%), neutrophilia (85.1%), and eosinophilia (52.1%). Kidney and liver involvement occur in less than 10% of cases, and mortality rates are low but not zero, with an all-cause 30-day mortality rate of 3.5%.3
In a multi-institutional retrospective study of 68 patients diagnosed with DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, Sharma et al4 developed a scoring system to identify those at greatest risk for DRESS recurrence. Variables associated with recurrence including younger age, female sex, and features considered atypical for DRESS syndrome—nonmorbilliform rash; absence of facial edema; antinuclear antibody positivity; medication class other than antibiotic, antigout, or antiseizure—were used to develop a “ReDRESS” score. This predictive model had a sensitivity of 73% and specificity of 83% for predicting DRESS recurrence.4
Another case series characterized SCoRCH (sudden conjunctivitis, lymphopenia, sunburnlike rash, and hemodynamic changes), a newly described hypersensitivity reaction to trimethoprim-sulfamethoxazole.5 The onset of this reaction typically occurs 4 to 11 days after initiation of trimethoprim-sulfamethoxazole but can occur as quickly as 1 day following re-exposure. Patients are systemically ill with fever, hypotension, tachycardia, acute renal insufficiency, and transaminitis, and they have a diffuse sunburnlike erythema without scale, facial edema, and conjunctivitis. It is thought this distinct hypersensitivity reaction may be mediated by IL-6, which has a role in triggering a sepsislike physiology, with vasodilation, hypotension, and edema.5
A systematic review and meta-analysis found that sulfonamides remain the most prominent cause of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN).6 A case-control study described SJS/TEN presentations triggered by Mycoplasma, advocating for routine Mycoplasma screening, especially in patients without a clear medication culprit. Mycoplasma-induced cases carried statistically lower rates of mortality (0%) compared with medication-induced cases (22.5%).7 Another prospective open-label study evaluated SJS/TEN management by randomizing 25 patients to receive either combination therapy with methylprednisolone plus a tumor necrosis factor α inhibitor or methylprednisolone alone.8 Anti–tumor necrosis factor therapy was associated with a shorter length of initial steroid treatment and duration of the acute stage, hospitalization, and time to re-epithelialization8; however, as in a prior randomized unblinded trial,9 there was no difference in mortality between the 2 groups.
There is limited high-quality evidence to support the use of any systemic immunomodulator to decrease SJS/TEN–related mortality.10 A Cochrane systematic review highlighted the many limitations of the available data due to variations in presentation, assessment, and management.11 Because SJS/TEN is rare, powering studies based on mortality is infeasible; the authors calculated that 2872 participants were needed to detect a 50% mortality reduction among those with SCORTEN (severity-of-illness score for TEN) scores of 0 to 1.11 Therefore, collaborative efforts using appropriate outcomes measures (eg, time to re-epithelialization, length of hospital stay), standardized terminology and dosing regimens, and adaptive trial designs are needed. Consensus-derived assessment and treatment protocols could help account for variation, ensure consistency in treatment, and enable head-to-head comparisons. Members of the Society of Dermatology Hospitalists are working on efforts to standardize terminology and validate outcomes measures needed for future studies.12
Supportive Oncodermatology: A New Frontier
With the advent of immune checkpoint inhibitors (ICIs) for a growing number of cancers, dermatologists have become critical to identifying and managing cutaneous immune-related adverse events (cirAEs). Recent findings have demonstrated that dermatology input improves patient outcomes, not only regarding the treatment of dermatoses but also by augmenting cancer-related survival. One group found that patients with cirAEs who were evaluated by a dermatologist had improved progression-free (hazard ratio, 0.69; 95% CI, 0.54-0.87; P=.002) and overall survival rates (hazard ratio, 0.62; 95% CI, 0.45-0.84; P=.002), controlling for cirAE severity, age, sex, cancer type, and ICI subtype. Patients who were under the care of a dermatologist also were more likely to resume ICI therapy following an interruption (odds ratio, 10.52; 95% CI, 5.15-21.48; P<.001).13 Dermatologists help to optimize skin-directed and targeted therapies, such as dupilumab, minimizing exposure to systemic immunosuppression in these complex patients.14
Supportive oncodermatologists also have made important observations on how cirAEs relate to other adverse events and prognosis. A review of 628 patients found that almost half of those with cirAEs had co-occurring noncutaneous immune-related adverse events, most commonly pulmonary. Psoriasiform eruptions were most frequently associated with noncutaneous immune-related adverse events, and cutaneous reactions frequently preceded the development of systemic manifestations, serving as a clinical biomarker to provide prognostic information.15 A review of 95 patients found that spongiotic and lichenoid interface reactions were associated with decreased mortality rates, whereas vacuolar interface and perivascular dermatitis were associated with increased mortality.16
As with severe cutaneous adverse events, dermatology input has been critical for accurately phenotyping and risk stratifying these novel reactions. The dermatologist’s skill set is necessary for optimizing skin-directed and targeted therapies while minimizing systemic immunosuppression, thereby improving patient outcomes with respect to rash, cancer response, and survival.
The Cost of Inpatient Skin Disease
Hospitalizations account for approximately half of all health care expenditures, and hospital readmission, seen as a measure of the quality of health care delivery, can double this cost.17 Identifying and developing protocols for addressing patients with complex chronic inflammatory disorders is one strategy for improving outcomes and reducing financial burden. Inpatient dermatologists have identified hidradenitis suppurativa as one disease that can benefit from early intervention by dermatologists in the hospital, with its 30-day (17.8%) and 180-day (48.6%) readmission rates being comparable to those of heart failure.18
Following an index emergency department (ED) visit, 17.2% (3484/20,269) of patients with HS have at least 1 return ED visit within 30 days, while only 2.4% (483/20,269) have a dermatology visit within the same time frame.19 Understanding the risk factors for hospital readmission and ED utilization, including severity of illness, the presence of medical comorbidities, health coverage under Medicaid, and receipt of opioids, can allow dermatologists to anticipate those at greatest risk.19 Opportunities exist for cross-specialty interventions to anticipate and address modifiable risk factors. Shorter time to dermatology outpatient follow-up leads to improved clinic attendance and may help reduce ED utilization and hospital readmission.20
Teledermatology: Leveraging Inpatient Expertise
Although the benefit of inpatient dermatologic care is substantial, access to that care is finite. Following the COVID-19 pandemic, there is an increased acceptance of telemedicine and the long-term role it can play in leveraging dermatologic expertise, including meeting the increasing demand for inpatient dermatology care in rural and resource-poor communities.21
Recent studies conducted by dermatology hospitalists have illustrated the value of asynchronous store-and-forward technology in settings lacking access to consultative dermatology.22,23 Stephens et al22 found that expanding provider-to-provider electronic consultation (e-consultation) capacity to an inpatient rehabilitation facility resulted in completed consultations within 1.5 days compared with a 7- to 14-day wait time for patients attending an in-person urgent access dermatology clinic. In another study, the implementation of asynchronous dermatology e-consultations for immunobullous diseases, vasculitis, and herpes zoster resulted in a change in diagnosis 86% of the time, accompanied by at least 1 new systemic or topical therapy recommendation.23
Researchers also identified ways in which teledermatology can be inelegant and proposed specific supplemental data to aid in diagnosis. A review of 126 inpatient e-consultations demonstrated limitations related to the diagnosis of skin and soft-tissue infections. In two-thirds to three-quarters of cases, potentially useful descriptive information was missing, and in 70% (88/126), images were not appropriately focused. The authors developed a detailed checklist to help primary medical teams focus their differential diagnoses.24 A recent pilot study found that supplementation of clinical information with a standardized questionnaire and thermal images improved the accuracy of cellulitis diagnosis. Using this method, there was no difference in accuracy between dermatology hospitalists and other board-certified dermatologists, supporting the notion that any dermatologist can fulfill this need successfully, even without specific inpatient experience.25 Due to the high incidence and cost of cellulitis and related hospital admissions,26 such an intervention could have a considerable financial and patient safety impact.
Final Thoughts
This last year brought many changes to the health care landscape, the recession of a global pandemic, and an increasingly complex health care delivery system. Inpatient dermatologists met these challenges by providing high-quality dermatologic care and practice-modifying research in the areas of severe cutaneous adverse reactions, supportive oncodermatology, hospital readmission, telemedicine, and more, demonstrating the value of dermatologic expertise in the hospital setting.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
- Puri P, Pollock BD, Yousif M, et al. Association of Society of Dermatology hospitalist institutions with improved outcomes in Medicare beneficiaries hospitalized for skin disease. J Am Acad Dermatol. 2023;88:1372-1375.
- Creadore A, Desai S, Alloo A, et al. Clinical characteristics, disease course, and outcomes of patients with acute generalized exanthematous pustulosis in the US. JAMA Dermatol. 2022;158:176-183.
- Sharma AN, Murphy K, Shwe S, et al. Predicting DRESS syndrome recurrence—the ReDRESS score. JAMA Dermatol. 2022;158:1445-1447.
- Brian M, Rose EK, Mauskar MM, et al. Sudden conjunctivitis, lymphopenia, and rash combined with hemodynamic changes (SCoRCH) after trimethoprim-sulfamethoxazole use: a case series study of a hypersensitivity reaction. JAMA Dermatol. 2023;159:73-78.
- Lee EY, Knox C, Phillips EJ. Worldwide prevalence of antibiotic-associated Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2023;159:384-392.
- Liew YCC, Choo KJL, Oh CC, et al. Mycoplasma-induced Stevens-Johnson syndrome/toxic epidermal necrolysis: case-control analysis of a cohort managed in a specialized center. J Am Acad Dermatol. 2022;86:811-817.
- Ao S, Gao X, Zhan J, et al. Inhibition of tumor necrosis factor improves conventional steroid therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis in a cohort of patients. J Am Acad Dermatol. 2022;86:1236-1245.
- Wang CW, Yang LY, Chen CB, et al; the Taiwan Severe Cutaneous Adverse Reaction (TSCAR) Consortium. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
- Han JJ, Creadore A, Seminario-Vidal L, et al. Medical management of Stevens-Johnson syndrome/toxic epidermal necrolysis among North American dermatologists. J Am Acad Dermatol. 2022;87:429-431.
- Noe MH, Micheletti RG. Systemic interventions for treatment of Stevens-Johnson syndrome/toxic epidermal necrolysis: summary of a Cochrane review. JAMA Dermatol. 2022;158:1436-1437.
- Waters M, Dobry A, Le ST, et al. Development of a skin-directed scoring system for Stevens-Johnson syndrome and epidermal necrolysis: a Delphi consensus exercise. JAMA Dermatol. 2023;159:772-777.
- Jacoby TV, Shah N, Asdourian MS, et al. Dermatology evaluation for cutaneous immune-related adverse events is associated with improved survival in cancer patients treated with checkpoint inhibition. J Am Acad Dermatol. 2023;88:711-714.
- Said JT, Elman SA, Perez-Chada LM, et al. Treatment of immune checkpoint inhibitor-mediated psoriasis: a systematic review. J Am Acad Dermatol. 2022;87:399-400.
- Asdourian MS, Shah N, Jacoby TV, et al. Evaluating patterns of co-occurrence between cutaneous and noncutaneous immune-related adverse events after immune checkpoint inhibitor therapy. J Am Acad Dermatol. 2023;88:246-249.
- Hirotsu KE, Scott MKD, Marquez C, et al. Histologic subtype of cutaneous immune-related adverse events predicts overall survival in patients receiving immune checkpoint inhibitors. J Am Acad Dermatol. 2022;87:651-653.
- Benbassat J, Taragin M. Hospital readmissions as a measure of quality of health care: advantages and limitations. Arch Intern Med. 2000;160:1074-1081.
- Edigin E, Kaul S, Eseaton PO, et al. At 180 days hidradenitis suppurativa readmission rate is comparable to heart failure: analysis of the nationwide readmissions database. J Am Acad Dermatol. 2022;87:188-192.
- Wang CX, Buss JL, Keller M, et al. Factors associated with dermatologic follow-up vs emergency department return in patients with hidradenitis suppurativa after an initial emergency department visit. JAMA Dermatol. 2022;158:1378-1386.
- Zakaria A, Chang AY, Kim-Lim P, et al. Predictors of postdischarge follow-up attendance among hospitalized dermatology patients: disparities and potential interventions. J Am Acad Dermatol. 2022;87:186-188.
- Arnold JD, Yoon S, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2019;80:425-432. doi:10.1016/j.jaad.2018.06.070
- Stephens MR, Das S, Smith GP. Utilization and outcomes of an asynchronous teledermatology pilot for an inpatient rehabilitation hospital. J Am Acad Dermatol. 2022;87:421-423.
- Ortiz C, Khosravi H, Kettering C, et al. Concordance data for inpatient asynchronous eDermatology consultation for immunobullous disease, zoster, and vasculitis. J Am Acad Dermatol. 2022;86:918-920.
- Salle R, Hua C, Mongereau M, et al. Challenges and limitations of teledermatology for skin and soft-tissue infections: a real-world study of an expert center. J Am Acad Dermatol. 2023;88:457-459.
- Creadore A, Manjaly P, Tkachenko E, et al. The utility of augmented teledermatology to improve dermatologist diagnosis of cellulitis: a cross-sectional study. Arch Dermatol Res. 2023;315:1347-1353.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
Dermatologists improve the diagnostic accuracy and quality of care of patients in the hospital setting. They help shorten the length of stay, improve outpatient follow-up, and reduce the rate of hospital readmission.1 Medicare beneficiaries hospitalized with skin conditions at institutions with a dermatology hospitalist—a provider with a specialty interest in inpatient dermatology—have 24% lower odds of risk-adjusted 30-day mortality and 12% lower odds of risk-adjusted 30-day readmissions.2
In the last year, research among the dermatology hospitalist community has actively contributed to our understanding of challenging inpatient skin diseases and has identified new ways in which dermatologists can contribute to the care of hospitalized patients. In this review, we highlight 4 areas of focus from the published literature in 2022-2023—severe cutaneous adverse reactions, supportive oncodermatology, cost of inpatient services, and teledermatology.
Severe Cutaneous Adverse Reactions: Old and New
Severe cutaneous adverse reactions to medications frequently are encountered in the inpatient setting. Dermatology hospitalists are well positioned to phenotype these reactions, drawing insights that aid in identifying, characterizing, risk stratifying, and managing these conditions, which have considerable morbidity and mortality.
A recent 20-year retrospective review of cases of acute generalized exanthematous pustulosis (N=340) across 10 academic systems—the largest to date—improves our understanding of the features of this rare entity.3 The authors found that acute generalized exanthematous pustulosis most often is triggered by β-lactam and other antibiotics (75.5%) and is accompanied by fever (49.7%), neutrophilia (85.1%), and eosinophilia (52.1%). Kidney and liver involvement occur in less than 10% of cases, and mortality rates are low but not zero, with an all-cause 30-day mortality rate of 3.5%.3
In a multi-institutional retrospective study of 68 patients diagnosed with DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, Sharma et al4 developed a scoring system to identify those at greatest risk for DRESS recurrence. Variables associated with recurrence including younger age, female sex, and features considered atypical for DRESS syndrome—nonmorbilliform rash; absence of facial edema; antinuclear antibody positivity; medication class other than antibiotic, antigout, or antiseizure—were used to develop a “ReDRESS” score. This predictive model had a sensitivity of 73% and specificity of 83% for predicting DRESS recurrence.4
Another case series characterized SCoRCH (sudden conjunctivitis, lymphopenia, sunburnlike rash, and hemodynamic changes), a newly described hypersensitivity reaction to trimethoprim-sulfamethoxazole.5 The onset of this reaction typically occurs 4 to 11 days after initiation of trimethoprim-sulfamethoxazole but can occur as quickly as 1 day following re-exposure. Patients are systemically ill with fever, hypotension, tachycardia, acute renal insufficiency, and transaminitis, and they have a diffuse sunburnlike erythema without scale, facial edema, and conjunctivitis. It is thought this distinct hypersensitivity reaction may be mediated by IL-6, which has a role in triggering a sepsislike physiology, with vasodilation, hypotension, and edema.5
A systematic review and meta-analysis found that sulfonamides remain the most prominent cause of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN).6 A case-control study described SJS/TEN presentations triggered by Mycoplasma, advocating for routine Mycoplasma screening, especially in patients without a clear medication culprit. Mycoplasma-induced cases carried statistically lower rates of mortality (0%) compared with medication-induced cases (22.5%).7 Another prospective open-label study evaluated SJS/TEN management by randomizing 25 patients to receive either combination therapy with methylprednisolone plus a tumor necrosis factor α inhibitor or methylprednisolone alone.8 Anti–tumor necrosis factor therapy was associated with a shorter length of initial steroid treatment and duration of the acute stage, hospitalization, and time to re-epithelialization8; however, as in a prior randomized unblinded trial,9 there was no difference in mortality between the 2 groups.
There is limited high-quality evidence to support the use of any systemic immunomodulator to decrease SJS/TEN–related mortality.10 A Cochrane systematic review highlighted the many limitations of the available data due to variations in presentation, assessment, and management.11 Because SJS/TEN is rare, powering studies based on mortality is infeasible; the authors calculated that 2872 participants were needed to detect a 50% mortality reduction among those with SCORTEN (severity-of-illness score for TEN) scores of 0 to 1.11 Therefore, collaborative efforts using appropriate outcomes measures (eg, time to re-epithelialization, length of hospital stay), standardized terminology and dosing regimens, and adaptive trial designs are needed. Consensus-derived assessment and treatment protocols could help account for variation, ensure consistency in treatment, and enable head-to-head comparisons. Members of the Society of Dermatology Hospitalists are working on efforts to standardize terminology and validate outcomes measures needed for future studies.12
Supportive Oncodermatology: A New Frontier
With the advent of immune checkpoint inhibitors (ICIs) for a growing number of cancers, dermatologists have become critical to identifying and managing cutaneous immune-related adverse events (cirAEs). Recent findings have demonstrated that dermatology input improves patient outcomes, not only regarding the treatment of dermatoses but also by augmenting cancer-related survival. One group found that patients with cirAEs who were evaluated by a dermatologist had improved progression-free (hazard ratio, 0.69; 95% CI, 0.54-0.87; P=.002) and overall survival rates (hazard ratio, 0.62; 95% CI, 0.45-0.84; P=.002), controlling for cirAE severity, age, sex, cancer type, and ICI subtype. Patients who were under the care of a dermatologist also were more likely to resume ICI therapy following an interruption (odds ratio, 10.52; 95% CI, 5.15-21.48; P<.001).13 Dermatologists help to optimize skin-directed and targeted therapies, such as dupilumab, minimizing exposure to systemic immunosuppression in these complex patients.14
Supportive oncodermatologists also have made important observations on how cirAEs relate to other adverse events and prognosis. A review of 628 patients found that almost half of those with cirAEs had co-occurring noncutaneous immune-related adverse events, most commonly pulmonary. Psoriasiform eruptions were most frequently associated with noncutaneous immune-related adverse events, and cutaneous reactions frequently preceded the development of systemic manifestations, serving as a clinical biomarker to provide prognostic information.15 A review of 95 patients found that spongiotic and lichenoid interface reactions were associated with decreased mortality rates, whereas vacuolar interface and perivascular dermatitis were associated with increased mortality.16
As with severe cutaneous adverse events, dermatology input has been critical for accurately phenotyping and risk stratifying these novel reactions. The dermatologist’s skill set is necessary for optimizing skin-directed and targeted therapies while minimizing systemic immunosuppression, thereby improving patient outcomes with respect to rash, cancer response, and survival.
The Cost of Inpatient Skin Disease
Hospitalizations account for approximately half of all health care expenditures, and hospital readmission, seen as a measure of the quality of health care delivery, can double this cost.17 Identifying and developing protocols for addressing patients with complex chronic inflammatory disorders is one strategy for improving outcomes and reducing financial burden. Inpatient dermatologists have identified hidradenitis suppurativa as one disease that can benefit from early intervention by dermatologists in the hospital, with its 30-day (17.8%) and 180-day (48.6%) readmission rates being comparable to those of heart failure.18
Following an index emergency department (ED) visit, 17.2% (3484/20,269) of patients with HS have at least 1 return ED visit within 30 days, while only 2.4% (483/20,269) have a dermatology visit within the same time frame.19 Understanding the risk factors for hospital readmission and ED utilization, including severity of illness, the presence of medical comorbidities, health coverage under Medicaid, and receipt of opioids, can allow dermatologists to anticipate those at greatest risk.19 Opportunities exist for cross-specialty interventions to anticipate and address modifiable risk factors. Shorter time to dermatology outpatient follow-up leads to improved clinic attendance and may help reduce ED utilization and hospital readmission.20
Teledermatology: Leveraging Inpatient Expertise
Although the benefit of inpatient dermatologic care is substantial, access to that care is finite. Following the COVID-19 pandemic, there is an increased acceptance of telemedicine and the long-term role it can play in leveraging dermatologic expertise, including meeting the increasing demand for inpatient dermatology care in rural and resource-poor communities.21
Recent studies conducted by dermatology hospitalists have illustrated the value of asynchronous store-and-forward technology in settings lacking access to consultative dermatology.22,23 Stephens et al22 found that expanding provider-to-provider electronic consultation (e-consultation) capacity to an inpatient rehabilitation facility resulted in completed consultations within 1.5 days compared with a 7- to 14-day wait time for patients attending an in-person urgent access dermatology clinic. In another study, the implementation of asynchronous dermatology e-consultations for immunobullous diseases, vasculitis, and herpes zoster resulted in a change in diagnosis 86% of the time, accompanied by at least 1 new systemic or topical therapy recommendation.23
Researchers also identified ways in which teledermatology can be inelegant and proposed specific supplemental data to aid in diagnosis. A review of 126 inpatient e-consultations demonstrated limitations related to the diagnosis of skin and soft-tissue infections. In two-thirds to three-quarters of cases, potentially useful descriptive information was missing, and in 70% (88/126), images were not appropriately focused. The authors developed a detailed checklist to help primary medical teams focus their differential diagnoses.24 A recent pilot study found that supplementation of clinical information with a standardized questionnaire and thermal images improved the accuracy of cellulitis diagnosis. Using this method, there was no difference in accuracy between dermatology hospitalists and other board-certified dermatologists, supporting the notion that any dermatologist can fulfill this need successfully, even without specific inpatient experience.25 Due to the high incidence and cost of cellulitis and related hospital admissions,26 such an intervention could have a considerable financial and patient safety impact.
Final Thoughts
This last year brought many changes to the health care landscape, the recession of a global pandemic, and an increasingly complex health care delivery system. Inpatient dermatologists met these challenges by providing high-quality dermatologic care and practice-modifying research in the areas of severe cutaneous adverse reactions, supportive oncodermatology, hospital readmission, telemedicine, and more, demonstrating the value of dermatologic expertise in the hospital setting.
Dermatologists improve the diagnostic accuracy and quality of care of patients in the hospital setting. They help shorten the length of stay, improve outpatient follow-up, and reduce the rate of hospital readmission.1 Medicare beneficiaries hospitalized with skin conditions at institutions with a dermatology hospitalist—a provider with a specialty interest in inpatient dermatology—have 24% lower odds of risk-adjusted 30-day mortality and 12% lower odds of risk-adjusted 30-day readmissions.2
In the last year, research among the dermatology hospitalist community has actively contributed to our understanding of challenging inpatient skin diseases and has identified new ways in which dermatologists can contribute to the care of hospitalized patients. In this review, we highlight 4 areas of focus from the published literature in 2022-2023—severe cutaneous adverse reactions, supportive oncodermatology, cost of inpatient services, and teledermatology.
Severe Cutaneous Adverse Reactions: Old and New
Severe cutaneous adverse reactions to medications frequently are encountered in the inpatient setting. Dermatology hospitalists are well positioned to phenotype these reactions, drawing insights that aid in identifying, characterizing, risk stratifying, and managing these conditions, which have considerable morbidity and mortality.
A recent 20-year retrospective review of cases of acute generalized exanthematous pustulosis (N=340) across 10 academic systems—the largest to date—improves our understanding of the features of this rare entity.3 The authors found that acute generalized exanthematous pustulosis most often is triggered by β-lactam and other antibiotics (75.5%) and is accompanied by fever (49.7%), neutrophilia (85.1%), and eosinophilia (52.1%). Kidney and liver involvement occur in less than 10% of cases, and mortality rates are low but not zero, with an all-cause 30-day mortality rate of 3.5%.3
In a multi-institutional retrospective study of 68 patients diagnosed with DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, Sharma et al4 developed a scoring system to identify those at greatest risk for DRESS recurrence. Variables associated with recurrence including younger age, female sex, and features considered atypical for DRESS syndrome—nonmorbilliform rash; absence of facial edema; antinuclear antibody positivity; medication class other than antibiotic, antigout, or antiseizure—were used to develop a “ReDRESS” score. This predictive model had a sensitivity of 73% and specificity of 83% for predicting DRESS recurrence.4
Another case series characterized SCoRCH (sudden conjunctivitis, lymphopenia, sunburnlike rash, and hemodynamic changes), a newly described hypersensitivity reaction to trimethoprim-sulfamethoxazole.5 The onset of this reaction typically occurs 4 to 11 days after initiation of trimethoprim-sulfamethoxazole but can occur as quickly as 1 day following re-exposure. Patients are systemically ill with fever, hypotension, tachycardia, acute renal insufficiency, and transaminitis, and they have a diffuse sunburnlike erythema without scale, facial edema, and conjunctivitis. It is thought this distinct hypersensitivity reaction may be mediated by IL-6, which has a role in triggering a sepsislike physiology, with vasodilation, hypotension, and edema.5
A systematic review and meta-analysis found that sulfonamides remain the most prominent cause of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN).6 A case-control study described SJS/TEN presentations triggered by Mycoplasma, advocating for routine Mycoplasma screening, especially in patients without a clear medication culprit. Mycoplasma-induced cases carried statistically lower rates of mortality (0%) compared with medication-induced cases (22.5%).7 Another prospective open-label study evaluated SJS/TEN management by randomizing 25 patients to receive either combination therapy with methylprednisolone plus a tumor necrosis factor α inhibitor or methylprednisolone alone.8 Anti–tumor necrosis factor therapy was associated with a shorter length of initial steroid treatment and duration of the acute stage, hospitalization, and time to re-epithelialization8; however, as in a prior randomized unblinded trial,9 there was no difference in mortality between the 2 groups.
There is limited high-quality evidence to support the use of any systemic immunomodulator to decrease SJS/TEN–related mortality.10 A Cochrane systematic review highlighted the many limitations of the available data due to variations in presentation, assessment, and management.11 Because SJS/TEN is rare, powering studies based on mortality is infeasible; the authors calculated that 2872 participants were needed to detect a 50% mortality reduction among those with SCORTEN (severity-of-illness score for TEN) scores of 0 to 1.11 Therefore, collaborative efforts using appropriate outcomes measures (eg, time to re-epithelialization, length of hospital stay), standardized terminology and dosing regimens, and adaptive trial designs are needed. Consensus-derived assessment and treatment protocols could help account for variation, ensure consistency in treatment, and enable head-to-head comparisons. Members of the Society of Dermatology Hospitalists are working on efforts to standardize terminology and validate outcomes measures needed for future studies.12
Supportive Oncodermatology: A New Frontier
With the advent of immune checkpoint inhibitors (ICIs) for a growing number of cancers, dermatologists have become critical to identifying and managing cutaneous immune-related adverse events (cirAEs). Recent findings have demonstrated that dermatology input improves patient outcomes, not only regarding the treatment of dermatoses but also by augmenting cancer-related survival. One group found that patients with cirAEs who were evaluated by a dermatologist had improved progression-free (hazard ratio, 0.69; 95% CI, 0.54-0.87; P=.002) and overall survival rates (hazard ratio, 0.62; 95% CI, 0.45-0.84; P=.002), controlling for cirAE severity, age, sex, cancer type, and ICI subtype. Patients who were under the care of a dermatologist also were more likely to resume ICI therapy following an interruption (odds ratio, 10.52; 95% CI, 5.15-21.48; P<.001).13 Dermatologists help to optimize skin-directed and targeted therapies, such as dupilumab, minimizing exposure to systemic immunosuppression in these complex patients.14
Supportive oncodermatologists also have made important observations on how cirAEs relate to other adverse events and prognosis. A review of 628 patients found that almost half of those with cirAEs had co-occurring noncutaneous immune-related adverse events, most commonly pulmonary. Psoriasiform eruptions were most frequently associated with noncutaneous immune-related adverse events, and cutaneous reactions frequently preceded the development of systemic manifestations, serving as a clinical biomarker to provide prognostic information.15 A review of 95 patients found that spongiotic and lichenoid interface reactions were associated with decreased mortality rates, whereas vacuolar interface and perivascular dermatitis were associated with increased mortality.16
As with severe cutaneous adverse events, dermatology input has been critical for accurately phenotyping and risk stratifying these novel reactions. The dermatologist’s skill set is necessary for optimizing skin-directed and targeted therapies while minimizing systemic immunosuppression, thereby improving patient outcomes with respect to rash, cancer response, and survival.
The Cost of Inpatient Skin Disease
Hospitalizations account for approximately half of all health care expenditures, and hospital readmission, seen as a measure of the quality of health care delivery, can double this cost.17 Identifying and developing protocols for addressing patients with complex chronic inflammatory disorders is one strategy for improving outcomes and reducing financial burden. Inpatient dermatologists have identified hidradenitis suppurativa as one disease that can benefit from early intervention by dermatologists in the hospital, with its 30-day (17.8%) and 180-day (48.6%) readmission rates being comparable to those of heart failure.18
Following an index emergency department (ED) visit, 17.2% (3484/20,269) of patients with HS have at least 1 return ED visit within 30 days, while only 2.4% (483/20,269) have a dermatology visit within the same time frame.19 Understanding the risk factors for hospital readmission and ED utilization, including severity of illness, the presence of medical comorbidities, health coverage under Medicaid, and receipt of opioids, can allow dermatologists to anticipate those at greatest risk.19 Opportunities exist for cross-specialty interventions to anticipate and address modifiable risk factors. Shorter time to dermatology outpatient follow-up leads to improved clinic attendance and may help reduce ED utilization and hospital readmission.20
Teledermatology: Leveraging Inpatient Expertise
Although the benefit of inpatient dermatologic care is substantial, access to that care is finite. Following the COVID-19 pandemic, there is an increased acceptance of telemedicine and the long-term role it can play in leveraging dermatologic expertise, including meeting the increasing demand for inpatient dermatology care in rural and resource-poor communities.21
Recent studies conducted by dermatology hospitalists have illustrated the value of asynchronous store-and-forward technology in settings lacking access to consultative dermatology.22,23 Stephens et al22 found that expanding provider-to-provider electronic consultation (e-consultation) capacity to an inpatient rehabilitation facility resulted in completed consultations within 1.5 days compared with a 7- to 14-day wait time for patients attending an in-person urgent access dermatology clinic. In another study, the implementation of asynchronous dermatology e-consultations for immunobullous diseases, vasculitis, and herpes zoster resulted in a change in diagnosis 86% of the time, accompanied by at least 1 new systemic or topical therapy recommendation.23
Researchers also identified ways in which teledermatology can be inelegant and proposed specific supplemental data to aid in diagnosis. A review of 126 inpatient e-consultations demonstrated limitations related to the diagnosis of skin and soft-tissue infections. In two-thirds to three-quarters of cases, potentially useful descriptive information was missing, and in 70% (88/126), images were not appropriately focused. The authors developed a detailed checklist to help primary medical teams focus their differential diagnoses.24 A recent pilot study found that supplementation of clinical information with a standardized questionnaire and thermal images improved the accuracy of cellulitis diagnosis. Using this method, there was no difference in accuracy between dermatology hospitalists and other board-certified dermatologists, supporting the notion that any dermatologist can fulfill this need successfully, even without specific inpatient experience.25 Due to the high incidence and cost of cellulitis and related hospital admissions,26 such an intervention could have a considerable financial and patient safety impact.
Final Thoughts
This last year brought many changes to the health care landscape, the recession of a global pandemic, and an increasingly complex health care delivery system. Inpatient dermatologists met these challenges by providing high-quality dermatologic care and practice-modifying research in the areas of severe cutaneous adverse reactions, supportive oncodermatology, hospital readmission, telemedicine, and more, demonstrating the value of dermatologic expertise in the hospital setting.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
- Puri P, Pollock BD, Yousif M, et al. Association of Society of Dermatology hospitalist institutions with improved outcomes in Medicare beneficiaries hospitalized for skin disease. J Am Acad Dermatol. 2023;88:1372-1375.
- Creadore A, Desai S, Alloo A, et al. Clinical characteristics, disease course, and outcomes of patients with acute generalized exanthematous pustulosis in the US. JAMA Dermatol. 2022;158:176-183.
- Sharma AN, Murphy K, Shwe S, et al. Predicting DRESS syndrome recurrence—the ReDRESS score. JAMA Dermatol. 2022;158:1445-1447.
- Brian M, Rose EK, Mauskar MM, et al. Sudden conjunctivitis, lymphopenia, and rash combined with hemodynamic changes (SCoRCH) after trimethoprim-sulfamethoxazole use: a case series study of a hypersensitivity reaction. JAMA Dermatol. 2023;159:73-78.
- Lee EY, Knox C, Phillips EJ. Worldwide prevalence of antibiotic-associated Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2023;159:384-392.
- Liew YCC, Choo KJL, Oh CC, et al. Mycoplasma-induced Stevens-Johnson syndrome/toxic epidermal necrolysis: case-control analysis of a cohort managed in a specialized center. J Am Acad Dermatol. 2022;86:811-817.
- Ao S, Gao X, Zhan J, et al. Inhibition of tumor necrosis factor improves conventional steroid therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis in a cohort of patients. J Am Acad Dermatol. 2022;86:1236-1245.
- Wang CW, Yang LY, Chen CB, et al; the Taiwan Severe Cutaneous Adverse Reaction (TSCAR) Consortium. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
- Han JJ, Creadore A, Seminario-Vidal L, et al. Medical management of Stevens-Johnson syndrome/toxic epidermal necrolysis among North American dermatologists. J Am Acad Dermatol. 2022;87:429-431.
- Noe MH, Micheletti RG. Systemic interventions for treatment of Stevens-Johnson syndrome/toxic epidermal necrolysis: summary of a Cochrane review. JAMA Dermatol. 2022;158:1436-1437.
- Waters M, Dobry A, Le ST, et al. Development of a skin-directed scoring system for Stevens-Johnson syndrome and epidermal necrolysis: a Delphi consensus exercise. JAMA Dermatol. 2023;159:772-777.
- Jacoby TV, Shah N, Asdourian MS, et al. Dermatology evaluation for cutaneous immune-related adverse events is associated with improved survival in cancer patients treated with checkpoint inhibition. J Am Acad Dermatol. 2023;88:711-714.
- Said JT, Elman SA, Perez-Chada LM, et al. Treatment of immune checkpoint inhibitor-mediated psoriasis: a systematic review. J Am Acad Dermatol. 2022;87:399-400.
- Asdourian MS, Shah N, Jacoby TV, et al. Evaluating patterns of co-occurrence between cutaneous and noncutaneous immune-related adverse events after immune checkpoint inhibitor therapy. J Am Acad Dermatol. 2023;88:246-249.
- Hirotsu KE, Scott MKD, Marquez C, et al. Histologic subtype of cutaneous immune-related adverse events predicts overall survival in patients receiving immune checkpoint inhibitors. J Am Acad Dermatol. 2022;87:651-653.
- Benbassat J, Taragin M. Hospital readmissions as a measure of quality of health care: advantages and limitations. Arch Intern Med. 2000;160:1074-1081.
- Edigin E, Kaul S, Eseaton PO, et al. At 180 days hidradenitis suppurativa readmission rate is comparable to heart failure: analysis of the nationwide readmissions database. J Am Acad Dermatol. 2022;87:188-192.
- Wang CX, Buss JL, Keller M, et al. Factors associated with dermatologic follow-up vs emergency department return in patients with hidradenitis suppurativa after an initial emergency department visit. JAMA Dermatol. 2022;158:1378-1386.
- Zakaria A, Chang AY, Kim-Lim P, et al. Predictors of postdischarge follow-up attendance among hospitalized dermatology patients: disparities and potential interventions. J Am Acad Dermatol. 2022;87:186-188.
- Arnold JD, Yoon S, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2019;80:425-432. doi:10.1016/j.jaad.2018.06.070
- Stephens MR, Das S, Smith GP. Utilization and outcomes of an asynchronous teledermatology pilot for an inpatient rehabilitation hospital. J Am Acad Dermatol. 2022;87:421-423.
- Ortiz C, Khosravi H, Kettering C, et al. Concordance data for inpatient asynchronous eDermatology consultation for immunobullous disease, zoster, and vasculitis. J Am Acad Dermatol. 2022;86:918-920.
- Salle R, Hua C, Mongereau M, et al. Challenges and limitations of teledermatology for skin and soft-tissue infections: a real-world study of an expert center. J Am Acad Dermatol. 2023;88:457-459.
- Creadore A, Manjaly P, Tkachenko E, et al. The utility of augmented teledermatology to improve dermatologist diagnosis of cellulitis: a cross-sectional study. Arch Dermatol Res. 2023;315:1347-1353.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
- Puri P, Pollock BD, Yousif M, et al. Association of Society of Dermatology hospitalist institutions with improved outcomes in Medicare beneficiaries hospitalized for skin disease. J Am Acad Dermatol. 2023;88:1372-1375.
- Creadore A, Desai S, Alloo A, et al. Clinical characteristics, disease course, and outcomes of patients with acute generalized exanthematous pustulosis in the US. JAMA Dermatol. 2022;158:176-183.
- Sharma AN, Murphy K, Shwe S, et al. Predicting DRESS syndrome recurrence—the ReDRESS score. JAMA Dermatol. 2022;158:1445-1447.
- Brian M, Rose EK, Mauskar MM, et al. Sudden conjunctivitis, lymphopenia, and rash combined with hemodynamic changes (SCoRCH) after trimethoprim-sulfamethoxazole use: a case series study of a hypersensitivity reaction. JAMA Dermatol. 2023;159:73-78.
- Lee EY, Knox C, Phillips EJ. Worldwide prevalence of antibiotic-associated Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2023;159:384-392.
- Liew YCC, Choo KJL, Oh CC, et al. Mycoplasma-induced Stevens-Johnson syndrome/toxic epidermal necrolysis: case-control analysis of a cohort managed in a specialized center. J Am Acad Dermatol. 2022;86:811-817.
- Ao S, Gao X, Zhan J, et al. Inhibition of tumor necrosis factor improves conventional steroid therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis in a cohort of patients. J Am Acad Dermatol. 2022;86:1236-1245.
- Wang CW, Yang LY, Chen CB, et al; the Taiwan Severe Cutaneous Adverse Reaction (TSCAR) Consortium. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
- Han JJ, Creadore A, Seminario-Vidal L, et al. Medical management of Stevens-Johnson syndrome/toxic epidermal necrolysis among North American dermatologists. J Am Acad Dermatol. 2022;87:429-431.
- Noe MH, Micheletti RG. Systemic interventions for treatment of Stevens-Johnson syndrome/toxic epidermal necrolysis: summary of a Cochrane review. JAMA Dermatol. 2022;158:1436-1437.
- Waters M, Dobry A, Le ST, et al. Development of a skin-directed scoring system for Stevens-Johnson syndrome and epidermal necrolysis: a Delphi consensus exercise. JAMA Dermatol. 2023;159:772-777.
- Jacoby TV, Shah N, Asdourian MS, et al. Dermatology evaluation for cutaneous immune-related adverse events is associated with improved survival in cancer patients treated with checkpoint inhibition. J Am Acad Dermatol. 2023;88:711-714.
- Said JT, Elman SA, Perez-Chada LM, et al. Treatment of immune checkpoint inhibitor-mediated psoriasis: a systematic review. J Am Acad Dermatol. 2022;87:399-400.
- Asdourian MS, Shah N, Jacoby TV, et al. Evaluating patterns of co-occurrence between cutaneous and noncutaneous immune-related adverse events after immune checkpoint inhibitor therapy. J Am Acad Dermatol. 2023;88:246-249.
- Hirotsu KE, Scott MKD, Marquez C, et al. Histologic subtype of cutaneous immune-related adverse events predicts overall survival in patients receiving immune checkpoint inhibitors. J Am Acad Dermatol. 2022;87:651-653.
- Benbassat J, Taragin M. Hospital readmissions as a measure of quality of health care: advantages and limitations. Arch Intern Med. 2000;160:1074-1081.
- Edigin E, Kaul S, Eseaton PO, et al. At 180 days hidradenitis suppurativa readmission rate is comparable to heart failure: analysis of the nationwide readmissions database. J Am Acad Dermatol. 2022;87:188-192.
- Wang CX, Buss JL, Keller M, et al. Factors associated with dermatologic follow-up vs emergency department return in patients with hidradenitis suppurativa after an initial emergency department visit. JAMA Dermatol. 2022;158:1378-1386.
- Zakaria A, Chang AY, Kim-Lim P, et al. Predictors of postdischarge follow-up attendance among hospitalized dermatology patients: disparities and potential interventions. J Am Acad Dermatol. 2022;87:186-188.
- Arnold JD, Yoon S, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2019;80:425-432. doi:10.1016/j.jaad.2018.06.070
- Stephens MR, Das S, Smith GP. Utilization and outcomes of an asynchronous teledermatology pilot for an inpatient rehabilitation hospital. J Am Acad Dermatol. 2022;87:421-423.
- Ortiz C, Khosravi H, Kettering C, et al. Concordance data for inpatient asynchronous eDermatology consultation for immunobullous disease, zoster, and vasculitis. J Am Acad Dermatol. 2022;86:918-920.
- Salle R, Hua C, Mongereau M, et al. Challenges and limitations of teledermatology for skin and soft-tissue infections: a real-world study of an expert center. J Am Acad Dermatol. 2023;88:457-459.
- Creadore A, Manjaly P, Tkachenko E, et al. The utility of augmented teledermatology to improve dermatologist diagnosis of cellulitis: a cross-sectional study. Arch Dermatol Res. 2023;315:1347-1353.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
Practice Points
- A severe hypersensitivity reaction to trimethoprim-sulfamethoxazole—sudden conjunctivitis, lymphopenia, sunburnlike rash, and hemodynamic changes (SCoRCH)—has been described.
- Patients experiencing cutaneous reactions to immune checkpoint inhibitors have improved progression-free and overall survival rates if evaluated by a dermatologist who can optimize skin-directed and targeted therapies.
- Interventions, including shorter time to dermatology outpatient follow-up, are needed to reduce emergency department utilization by patients with hidradenitis suppurativa.
- Asynchronous store-and-forward dermatology e-consultation is effective for immunobullous diseases, vasculitis, herpes zoster, and cellulitis, demonstrating the utility of teledermatology in the inpatient setting, particularly when standardized data capture tools are used.
Botanical Briefs: Australian Stinging Tree (Dendrocnide moroides)
Clinical Importance
Dendrocnide moroides is arguably the most brutal of stinging plants, even leading to death in dogs, horses, and humans in rare cases.1-3 Commonly called gympie-gympie (based on its discovery by gold miners near the town of Gympie in Queensland, Australia), D moroides also has been referred to as the mulberrylike stinging tree or stinger.2,4-6
Family and Nomenclature
The Australian stinging tree belongs to the family Urticaceae (known as the nettle family) within the order Rosales.1,2,3,5 Urticaceae is derived from the Latin term urere (to burn)—an apt description of the clinical experience of patients with D moroides–induced urticaria.
Urticaceae includes 54 genera, comprising herbs, shrubs, small trees, and vines found predominantly in tropical regions. Dendrocnide comprises approximately 40 species, all commonly known in Australia as stinging trees.2,7,8
Distribution
Dendrocnide moroides is found in the rainforests of Australia and Southeast Asia.2 Because the plant has a strong need for sunlight and wind protection, it typically is found in light-filled gaps within the rainforest, in moist ravines, along the edges of creeks, and on land bordering the rainforest.3,6
Appearance
Although D moroides is referred to as a tree, it is an understory shrub that typically grows to 3 m, with heart-shaped, serrated, dark green leaves that are 50-cm wide (Figure 1).6 The leaves are produced consistently through the year, with variable growth depending on the season.9

The plant is covered in what appears to be soft downy fur made up of trichomes (or plant hairs).1,6 The density of the hairs on leaves decreases as they age.2,9 The fruit, which is actually edible (if one is careful to avoid hairs), appears similar to red to dark purple raspberries growing on long stems.5,6
Cutaneous Manifestations
Symptoms of contact with the stems and leaves of D moroides range from slight irritation to serious neurologic disorders, including neuropathy. The severity of the reaction depends on the person, how much skin was contacted, and how one came into contact with the plant.1,5 Upon touch, there is an immediate reaction, with burning, urticaria, and edema. Pain increases, peaking 30 minutes later; then the pain slowly subsides.1 Tachycardia and throbbing regional lymphadenopathy can occur for 1 to 4 hours.1,6
Cutaneous Findings—Examination reveals immediate piloerection, erythema due to arteriolar dilation, and local swelling.2 These findings may disappear after 1 hour or last as long as 24 hours.1 Although objective signs may fade, subjective pain, pruritus, and burning can persist for months.3
Dermatitis-Inducing Plant Parts
After contact with the stems or leaves, the sharp trichomes become embedded in the skin, making them difficult to remove.1 The toxins are contained in siliceous hairs that the human body cannot break down.3 Symptoms can be experienced for as long as 1 year after contact, especially when the skin is pressed firmly or washed with hot or cold water.3,6 Because the plant’s hairs are shed continuously, being in close proximity to D moroides for longer than 20 minutes can lead to extreme sneezing, nosebleeds, and major respiratory damage from inhaling hairs.1,6,9
The stinging hairs of D moroides differ from irritant hairs on other plants because they contain physiologically active substances. Stinging hairs are classified as either a hypodermic syringe, which expels liquid only, or as a tragia-type syringe, in which liquid and sharp crystals are injected.
The Australian stinging tree falls into the first of these 2 groups (Figure 2)1; the sharp tip of the hair breaks on contact, leading to expulsion of the toxin into skin.1,4 The hairs function as a defense against mammalian herbivores but typically have no impact on pests.1 Nocturnal beetles and on occasion possums and red-legged pademelons dare to eat D moroides.3,6
The Irritant
Initially, formic acid was proposed as the irritant chemical in D moroides1; other candidates have included neurotransmitters, such as histamine, acetylcholine, and serotonin, as well as inorganic ions, such as potassium. These compounds may play a role but none explain the persistent sensory effects and years-long stable nature of the toxin.1,4
The most likely culprit irritant is a member of a newly discovered family of neurotoxins, the gympietides. These knot-shaped chemicals, found in D moroides and some spider venoms, have the ability to activate voltage-gated sodium channels of cutaneous neurons and cause local cutaneous vasodilation by stimulating neurotransmitter release.4 These neurotoxins not only generate pain but also suppress the mechanism used to interrupt those pain signals.10 Synthesized gympietides can replicate the effects of natural contact, indicating that they are the primary active toxins. These toxins are ultrastable, thus producing lasting effects.1
Although much is understood about the evolution and distribution of D moroides and the ecological role that it plays, there is still more to learn about the plant’s toxicology.
Prevention and Treatment
Prevention—Dendrocnide moroides dermatitis is best prevented by avoiding contact with the plant and related species, as well as wearing upper body clothing with long sleeves, pants, and boots, though plant hairs can still penetrate garments and sting.2,3
Therapy—There is no reversal therapy of D moroides dermatitis but symptoms can be managed.4 For pain, analgesics, such as opioids, have been used; on occasion, however, pain is so intense that even morphine does not help.4,10
Systemic or topical corticosteroids are the main therapy for many forms of plant-induced dermatitis because they are able to decrease cytokine production and stop lymphocyte production. Adding an oral antihistamine can alleviate histamine-mediated pruritus but not pruritus that is mediated by other chemicals.11
Other methods of relieving symptoms of D moroides dermatitis have been proposed or reported anecdotally. Diluted hydrochloric acid can be applied to the skin to denature remaining toxin.4 The sap of Alocasia brisbanensis (the cunjevoi plant) can be rubbed on affected areas to provide a cooling effect, but do not allow A brisbanensis sap to enter the mouth, as it contains calcium oxalate, a toxic irritant found in dumb cane (Dieffenbachia species). The roots of the Australian stinging tree also can be ground and made into a paste, which is applied to the skin.3 However, given the stability of the toxin, we do not recommend these remedies.
Instead, heavy-duty masking tape or hot wax can be applied to remove plant hairs from the skin. The most successful method of removing plant hair is hair removal wax strips, which are considered an essential component of a first aid kit where D moroides is found.3
- Ensikat H-J, Wessely H, Engeser M, et al. Distribution, ecology, chemistry and toxicology of plant stinging hairs. Toxins (Basel). 2021;13:141. doi:10.3390/toxins13020141
- Schmitt C, Parola P, de Haro L. Painful sting after exposure to Dendrocnide sp: two case reports. Wilderness Environ Med. 2013;24:471-473. doi:10.1016/j.wem.2013.03.021
- Hurley M. Selective stingers. ECOS. 2000;105:18-23. Accessed October 13, 2023. https://www.writingclearscience.com.au/wp-content/uploads/2015/06/stingers.pdf
- Gilding EK, Jami S, Deuis JR, et al. Neurotoxic peptides from the venom of the giant Australian stinging tree. Sci Adv. 2020;6:eabb8828. doi:10.1126/sciadv.abb8828
- Dendrocnide moroides. James Cook University Australia website. Accessed Accessed October 13, 2023. https://www.jcu.edu.au/discover-nature-at-jcu/plants/plants-by-scientific-name2/dendrocnide-moroides
- Hurley M. ‘The worst kind of pain you can imagine’—what it’s like to be stung by a stinging tree. The Conversation. September 28, 2018. Accessed October 13, 2023. https://theconversation.com/the-worst-kind-of-pain-you-can-imagine-what-its-like-to-be-stung-by-a-stinging-tree-103220
- Urticaceae: plant family. Britannica [Internet]. Accessed October 13, 2023. https://www.britannica.com/plant/Urticaceae
- Stinging trees (genus Dendrocnide). iNaturalist.ca [Internet]. Accessed October 13, 2023. https://inaturalist.ca/taxa/129502-Dendrocnide
- Hurley M. Growth dynamics and leaf quality of the stinging trees Dendrocnide moroides and Dendrocnide cordifolia (family Urticaceae) in Australian tropical rainforest: implications for herbivores. Aust J Bot. 2000;48:191-201. doi:10.1071/BT98006
- How the giant stinging tree of Australia can inflict months of agony. Nature. September 17, 2020. Accessed October 13, 2023. https://www.nature.com/articles/d41586-020-02668-9
- Chang Y-T, Shen J-J, Wong W-R, et al. Alternative therapy for autosensitization dermatitis. Chang Gung Med J. 2009;32:668-673.
Clinical Importance
Dendrocnide moroides is arguably the most brutal of stinging plants, even leading to death in dogs, horses, and humans in rare cases.1-3 Commonly called gympie-gympie (based on its discovery by gold miners near the town of Gympie in Queensland, Australia), D moroides also has been referred to as the mulberrylike stinging tree or stinger.2,4-6
Family and Nomenclature
The Australian stinging tree belongs to the family Urticaceae (known as the nettle family) within the order Rosales.1,2,3,5 Urticaceae is derived from the Latin term urere (to burn)—an apt description of the clinical experience of patients with D moroides–induced urticaria.
Urticaceae includes 54 genera, comprising herbs, shrubs, small trees, and vines found predominantly in tropical regions. Dendrocnide comprises approximately 40 species, all commonly known in Australia as stinging trees.2,7,8
Distribution
Dendrocnide moroides is found in the rainforests of Australia and Southeast Asia.2 Because the plant has a strong need for sunlight and wind protection, it typically is found in light-filled gaps within the rainforest, in moist ravines, along the edges of creeks, and on land bordering the rainforest.3,6
Appearance
Although D moroides is referred to as a tree, it is an understory shrub that typically grows to 3 m, with heart-shaped, serrated, dark green leaves that are 50-cm wide (Figure 1).6 The leaves are produced consistently through the year, with variable growth depending on the season.9

The plant is covered in what appears to be soft downy fur made up of trichomes (or plant hairs).1,6 The density of the hairs on leaves decreases as they age.2,9 The fruit, which is actually edible (if one is careful to avoid hairs), appears similar to red to dark purple raspberries growing on long stems.5,6
Cutaneous Manifestations
Symptoms of contact with the stems and leaves of D moroides range from slight irritation to serious neurologic disorders, including neuropathy. The severity of the reaction depends on the person, how much skin was contacted, and how one came into contact with the plant.1,5 Upon touch, there is an immediate reaction, with burning, urticaria, and edema. Pain increases, peaking 30 minutes later; then the pain slowly subsides.1 Tachycardia and throbbing regional lymphadenopathy can occur for 1 to 4 hours.1,6
Cutaneous Findings—Examination reveals immediate piloerection, erythema due to arteriolar dilation, and local swelling.2 These findings may disappear after 1 hour or last as long as 24 hours.1 Although objective signs may fade, subjective pain, pruritus, and burning can persist for months.3
Dermatitis-Inducing Plant Parts
After contact with the stems or leaves, the sharp trichomes become embedded in the skin, making them difficult to remove.1 The toxins are contained in siliceous hairs that the human body cannot break down.3 Symptoms can be experienced for as long as 1 year after contact, especially when the skin is pressed firmly or washed with hot or cold water.3,6 Because the plant’s hairs are shed continuously, being in close proximity to D moroides for longer than 20 minutes can lead to extreme sneezing, nosebleeds, and major respiratory damage from inhaling hairs.1,6,9
The stinging hairs of D moroides differ from irritant hairs on other plants because they contain physiologically active substances. Stinging hairs are classified as either a hypodermic syringe, which expels liquid only, or as a tragia-type syringe, in which liquid and sharp crystals are injected.
The Australian stinging tree falls into the first of these 2 groups (Figure 2)1; the sharp tip of the hair breaks on contact, leading to expulsion of the toxin into skin.1,4 The hairs function as a defense against mammalian herbivores but typically have no impact on pests.1 Nocturnal beetles and on occasion possums and red-legged pademelons dare to eat D moroides.3,6

The Irritant
Initially, formic acid was proposed as the irritant chemical in D moroides1; other candidates have included neurotransmitters, such as histamine, acetylcholine, and serotonin, as well as inorganic ions, such as potassium. These compounds may play a role but none explain the persistent sensory effects and years-long stable nature of the toxin.1,4
The most likely culprit irritant is a member of a newly discovered family of neurotoxins, the gympietides. These knot-shaped chemicals, found in D moroides and some spider venoms, have the ability to activate voltage-gated sodium channels of cutaneous neurons and cause local cutaneous vasodilation by stimulating neurotransmitter release.4 These neurotoxins not only generate pain but also suppress the mechanism used to interrupt those pain signals.10 Synthesized gympietides can replicate the effects of natural contact, indicating that they are the primary active toxins. These toxins are ultrastable, thus producing lasting effects.1
Although much is understood about the evolution and distribution of D moroides and the ecological role that it plays, there is still more to learn about the plant’s toxicology.
Prevention and Treatment
Prevention—Dendrocnide moroides dermatitis is best prevented by avoiding contact with the plant and related species, as well as wearing upper body clothing with long sleeves, pants, and boots, though plant hairs can still penetrate garments and sting.2,3
Therapy—There is no reversal therapy of D moroides dermatitis but symptoms can be managed.4 For pain, analgesics, such as opioids, have been used; on occasion, however, pain is so intense that even morphine does not help.4,10
Systemic or topical corticosteroids are the main therapy for many forms of plant-induced dermatitis because they are able to decrease cytokine production and stop lymphocyte production. Adding an oral antihistamine can alleviate histamine-mediated pruritus but not pruritus that is mediated by other chemicals.11
Other methods of relieving symptoms of D moroides dermatitis have been proposed or reported anecdotally. Diluted hydrochloric acid can be applied to the skin to denature remaining toxin.4 The sap of Alocasia brisbanensis (the cunjevoi plant) can be rubbed on affected areas to provide a cooling effect, but do not allow A brisbanensis sap to enter the mouth, as it contains calcium oxalate, a toxic irritant found in dumb cane (Dieffenbachia species). The roots of the Australian stinging tree also can be ground and made into a paste, which is applied to the skin.3 However, given the stability of the toxin, we do not recommend these remedies.
Instead, heavy-duty masking tape or hot wax can be applied to remove plant hairs from the skin. The most successful method of removing plant hair is hair removal wax strips, which are considered an essential component of a first aid kit where D moroides is found.3
Clinical Importance
Dendrocnide moroides is arguably the most brutal of stinging plants, even leading to death in dogs, horses, and humans in rare cases.1-3 Commonly called gympie-gympie (based on its discovery by gold miners near the town of Gympie in Queensland, Australia), D moroides also has been referred to as the mulberrylike stinging tree or stinger.2,4-6
Family and Nomenclature
The Australian stinging tree belongs to the family Urticaceae (known as the nettle family) within the order Rosales.1,2,3,5 Urticaceae is derived from the Latin term urere (to burn)—an apt description of the clinical experience of patients with D moroides–induced urticaria.
Urticaceae includes 54 genera, comprising herbs, shrubs, small trees, and vines found predominantly in tropical regions. Dendrocnide comprises approximately 40 species, all commonly known in Australia as stinging trees.2,7,8
Distribution
Dendrocnide moroides is found in the rainforests of Australia and Southeast Asia.2 Because the plant has a strong need for sunlight and wind protection, it typically is found in light-filled gaps within the rainforest, in moist ravines, along the edges of creeks, and on land bordering the rainforest.3,6
Appearance
Although D moroides is referred to as a tree, it is an understory shrub that typically grows to 3 m, with heart-shaped, serrated, dark green leaves that are 50-cm wide (Figure 1).6 The leaves are produced consistently through the year, with variable growth depending on the season.9

The plant is covered in what appears to be soft downy fur made up of trichomes (or plant hairs).1,6 The density of the hairs on leaves decreases as they age.2,9 The fruit, which is actually edible (if one is careful to avoid hairs), appears similar to red to dark purple raspberries growing on long stems.5,6
Cutaneous Manifestations
Symptoms of contact with the stems and leaves of D moroides range from slight irritation to serious neurologic disorders, including neuropathy. The severity of the reaction depends on the person, how much skin was contacted, and how one came into contact with the plant.1,5 Upon touch, there is an immediate reaction, with burning, urticaria, and edema. Pain increases, peaking 30 minutes later; then the pain slowly subsides.1 Tachycardia and throbbing regional lymphadenopathy can occur for 1 to 4 hours.1,6
Cutaneous Findings—Examination reveals immediate piloerection, erythema due to arteriolar dilation, and local swelling.2 These findings may disappear after 1 hour or last as long as 24 hours.1 Although objective signs may fade, subjective pain, pruritus, and burning can persist for months.3
Dermatitis-Inducing Plant Parts
After contact with the stems or leaves, the sharp trichomes become embedded in the skin, making them difficult to remove.1 The toxins are contained in siliceous hairs that the human body cannot break down.3 Symptoms can be experienced for as long as 1 year after contact, especially when the skin is pressed firmly or washed with hot or cold water.3,6 Because the plant’s hairs are shed continuously, being in close proximity to D moroides for longer than 20 minutes can lead to extreme sneezing, nosebleeds, and major respiratory damage from inhaling hairs.1,6,9
The stinging hairs of D moroides differ from irritant hairs on other plants because they contain physiologically active substances. Stinging hairs are classified as either a hypodermic syringe, which expels liquid only, or as a tragia-type syringe, in which liquid and sharp crystals are injected.
The Australian stinging tree falls into the first of these 2 groups (Figure 2)1; the sharp tip of the hair breaks on contact, leading to expulsion of the toxin into skin.1,4 The hairs function as a defense against mammalian herbivores but typically have no impact on pests.1 Nocturnal beetles and on occasion possums and red-legged pademelons dare to eat D moroides.3,6

The Irritant
Initially, formic acid was proposed as the irritant chemical in D moroides1; other candidates have included neurotransmitters, such as histamine, acetylcholine, and serotonin, as well as inorganic ions, such as potassium. These compounds may play a role but none explain the persistent sensory effects and years-long stable nature of the toxin.1,4
The most likely culprit irritant is a member of a newly discovered family of neurotoxins, the gympietides. These knot-shaped chemicals, found in D moroides and some spider venoms, have the ability to activate voltage-gated sodium channels of cutaneous neurons and cause local cutaneous vasodilation by stimulating neurotransmitter release.4 These neurotoxins not only generate pain but also suppress the mechanism used to interrupt those pain signals.10 Synthesized gympietides can replicate the effects of natural contact, indicating that they are the primary active toxins. These toxins are ultrastable, thus producing lasting effects.1
Although much is understood about the evolution and distribution of D moroides and the ecological role that it plays, there is still more to learn about the plant’s toxicology.
Prevention and Treatment
Prevention—Dendrocnide moroides dermatitis is best prevented by avoiding contact with the plant and related species, as well as wearing upper body clothing with long sleeves, pants, and boots, though plant hairs can still penetrate garments and sting.2,3
Therapy—There is no reversal therapy of D moroides dermatitis but symptoms can be managed.4 For pain, analgesics, such as opioids, have been used; on occasion, however, pain is so intense that even morphine does not help.4,10
Systemic or topical corticosteroids are the main therapy for many forms of plant-induced dermatitis because they are able to decrease cytokine production and stop lymphocyte production. Adding an oral antihistamine can alleviate histamine-mediated pruritus but not pruritus that is mediated by other chemicals.11
Other methods of relieving symptoms of D moroides dermatitis have been proposed or reported anecdotally. Diluted hydrochloric acid can be applied to the skin to denature remaining toxin.4 The sap of Alocasia brisbanensis (the cunjevoi plant) can be rubbed on affected areas to provide a cooling effect, but do not allow A brisbanensis sap to enter the mouth, as it contains calcium oxalate, a toxic irritant found in dumb cane (Dieffenbachia species). The roots of the Australian stinging tree also can be ground and made into a paste, which is applied to the skin.3 However, given the stability of the toxin, we do not recommend these remedies.
Instead, heavy-duty masking tape or hot wax can be applied to remove plant hairs from the skin. The most successful method of removing plant hair is hair removal wax strips, which are considered an essential component of a first aid kit where D moroides is found.3
- Ensikat H-J, Wessely H, Engeser M, et al. Distribution, ecology, chemistry and toxicology of plant stinging hairs. Toxins (Basel). 2021;13:141. doi:10.3390/toxins13020141
- Schmitt C, Parola P, de Haro L. Painful sting after exposure to Dendrocnide sp: two case reports. Wilderness Environ Med. 2013;24:471-473. doi:10.1016/j.wem.2013.03.021
- Hurley M. Selective stingers. ECOS. 2000;105:18-23. Accessed October 13, 2023. https://www.writingclearscience.com.au/wp-content/uploads/2015/06/stingers.pdf
- Gilding EK, Jami S, Deuis JR, et al. Neurotoxic peptides from the venom of the giant Australian stinging tree. Sci Adv. 2020;6:eabb8828. doi:10.1126/sciadv.abb8828
- Dendrocnide moroides. James Cook University Australia website. Accessed Accessed October 13, 2023. https://www.jcu.edu.au/discover-nature-at-jcu/plants/plants-by-scientific-name2/dendrocnide-moroides
- Hurley M. ‘The worst kind of pain you can imagine’—what it’s like to be stung by a stinging tree. The Conversation. September 28, 2018. Accessed October 13, 2023. https://theconversation.com/the-worst-kind-of-pain-you-can-imagine-what-its-like-to-be-stung-by-a-stinging-tree-103220
- Urticaceae: plant family. Britannica [Internet]. Accessed October 13, 2023. https://www.britannica.com/plant/Urticaceae
- Stinging trees (genus Dendrocnide). iNaturalist.ca [Internet]. Accessed October 13, 2023. https://inaturalist.ca/taxa/129502-Dendrocnide
- Hurley M. Growth dynamics and leaf quality of the stinging trees Dendrocnide moroides and Dendrocnide cordifolia (family Urticaceae) in Australian tropical rainforest: implications for herbivores. Aust J Bot. 2000;48:191-201. doi:10.1071/BT98006
- How the giant stinging tree of Australia can inflict months of agony. Nature. September 17, 2020. Accessed October 13, 2023. https://www.nature.com/articles/d41586-020-02668-9
- Chang Y-T, Shen J-J, Wong W-R, et al. Alternative therapy for autosensitization dermatitis. Chang Gung Med J. 2009;32:668-673.
- Ensikat H-J, Wessely H, Engeser M, et al. Distribution, ecology, chemistry and toxicology of plant stinging hairs. Toxins (Basel). 2021;13:141. doi:10.3390/toxins13020141
- Schmitt C, Parola P, de Haro L. Painful sting after exposure to Dendrocnide sp: two case reports. Wilderness Environ Med. 2013;24:471-473. doi:10.1016/j.wem.2013.03.021
- Hurley M. Selective stingers. ECOS. 2000;105:18-23. Accessed October 13, 2023. https://www.writingclearscience.com.au/wp-content/uploads/2015/06/stingers.pdf
- Gilding EK, Jami S, Deuis JR, et al. Neurotoxic peptides from the venom of the giant Australian stinging tree. Sci Adv. 2020;6:eabb8828. doi:10.1126/sciadv.abb8828
- Dendrocnide moroides. James Cook University Australia website. Accessed Accessed October 13, 2023. https://www.jcu.edu.au/discover-nature-at-jcu/plants/plants-by-scientific-name2/dendrocnide-moroides
- Hurley M. ‘The worst kind of pain you can imagine’—what it’s like to be stung by a stinging tree. The Conversation. September 28, 2018. Accessed October 13, 2023. https://theconversation.com/the-worst-kind-of-pain-you-can-imagine-what-its-like-to-be-stung-by-a-stinging-tree-103220
- Urticaceae: plant family. Britannica [Internet]. Accessed October 13, 2023. https://www.britannica.com/plant/Urticaceae
- Stinging trees (genus Dendrocnide). iNaturalist.ca [Internet]. Accessed October 13, 2023. https://inaturalist.ca/taxa/129502-Dendrocnide
- Hurley M. Growth dynamics and leaf quality of the stinging trees Dendrocnide moroides and Dendrocnide cordifolia (family Urticaceae) in Australian tropical rainforest: implications for herbivores. Aust J Bot. 2000;48:191-201. doi:10.1071/BT98006
- How the giant stinging tree of Australia can inflict months of agony. Nature. September 17, 2020. Accessed October 13, 2023. https://www.nature.com/articles/d41586-020-02668-9
- Chang Y-T, Shen J-J, Wong W-R, et al. Alternative therapy for autosensitization dermatitis. Chang Gung Med J. 2009;32:668-673.
Practice Points
- Dendrocnide moroides is arguably the most brutal of stinging plants, even leading to death in dogs, horses, and humans in rare cases.
- Clinical observations after contact reveal immediate piloerection and local swelling, which may disappear after 1 hour or last as long as 24 hours, but subjective pain, pruritus, and burning can persist for months.
- The most successful method of removing plant hair is hair removal wax strips, which are considered an essential component of a first aid kit where D moroides is found.
Suture Selection to Minimize Postoperative Postinflammatory Hyperpigmentation in Patients With Skin of Color During Mohs Micrographic Surgery
Practice Gap
Proper suture selection is imperative for appropriate wound healing to minimize the risk for infection and inflammation and to reduce scarring. In Mohs micrographic surgery (MMS), suture selection should be given high consideration in patients with skin of color.1 Using the right type of suture and wound closure technique can lead to favorable aesthetic outcomes by preventing postoperative postinflammatory hyperpigmentation (PIH) and keloids. Data on the choice of suture material in patients with skin of color are limited.
Suture selection depends on a variety of factors including but not limited to the location of the wound on the body, risk for infection, cost, availability, and the personal preference and experience of the MMS surgeon. During the COVID-19 pandemic, suturepreference among dermatologic surgeons shifted to fast-absorbing gut sutures,2 offering alternatives to synthetic monofilament polypropylene and nylon sutures. Absorbable sutures reduced the need for in-person follow-up visits without increasing the incidence of postoperative complications.
Despite these benefits, research suggests that natural absorbable gut sutures induce cutaneous inflammation and should be avoided in patients with skin of color.1,3,4 Nonabsorbable sutures are less reactive, reducing PIH after MMS in patients with skin of color.
Tools and Technique
Use of nonabsorbable stitches is a practical solution to reduce the risk for inflammation in patients with skin of color. Increased inflammation can lead to PIH and increase the risk for keloids in this patient population. Some patients will experience PIH after a surgical procedure regardless of the sutures used to repair the closure; however, one of our goals with patients with skin of color undergoing MMS is to reduce the inflammatory risk that could lead to PIH to ensure optimal aesthetic outcomes.
A middle-aged African woman with darker skin and a history of developing PIH after trauma to the skin presented to our clinic for MMS of a dermatofibrosarcoma protuberans on the upper abdomen. We used a simple running suture with 4-0 nylon to close the surgical wound. We avoided fast-absorbing gut sutures because they have high tissue reactivity1,4; use of sutures with low tissue reactivity, such as nylon and polypropylene, decreases the risk for inflammation without compromising alignment of wound edges and overall cosmesis of the repair. Prolene also is cost-effective and presents a decreased risk for wound dehiscence.5 After cauterizing the wound, we placed multiple synthetic absorbable sutures first to close the wound. We then did a double-running suture of nonabsorbable monofilament suture to reapproximate the epidermal edges with minimal tension. We placed 2 sets of running stitches to minimize the risk for dehiscence along the scar.
The patient was required to return for removal of the nonabsorbable sutures; this postoperative visit was covered by health insurance at no additional cost to the patient. In comparison, long-term repeat visits to treat PIH with a laser or chemical peel would have been more costly. Given that treatment of PIH is considered cosmetic, laser treatment would have been priced at several hundred dollars per session at our institution, and the patient would likely have had a copay for a pretreatment lightening cream such as hydroquinone. Our patient had a favorable cosmetic outcome and reported no or minimal evidence of PIH months after the procedure.
Patients should be instructed to apply petrolatum twice daily, use sun-protective clothing, and cover sutures to minimize exposure to the sun and prevent crusting of the wound. Postinflammatory hyperpigmentation can be proactively treated postoperatively with topical hydroquinone, which was not needed in our patient.
Practice Implications
Although some studies suggest that there are no cosmetic differences between absorbable and nonabsorbable sutures, the effect of suture type in patients with skin of color undergoing MMS often is unreported or is not studied.6,7 The high reactivity and cutaneous inflammation associated with absorbable gut sutures are important considerations in this patient population.
In patients with skin of color undergoing MMS, we use nonabsorbable epidermal sutures such as nylon and Prolene because of their low reactivity and association with favorable aesthetic outcomes. Nonabsorbable sutures can be safely used in patients of all ages who are undergoing MMS under local anesthesia.
An exception would be the use of the absorbable suture Monocryl (J&J MedTech) in patients with skin of color who need a running subcuticular wound closure because it has low tissue reactivity and maintains high tensile strength. Monocryl has been shown to create less-reactive scars, which decreases the risk for keloids.8,9
More clinical studies are needed to assess the increased susceptibility to PIH in patients with skin of color when using absorbable gut sutures.
- Williams R, Ciocon D. Mohs micrographic surgery in skin of color. J Drugs Dermatol. 2022;21:536-541. doi:10.36849/JDD.6469
- Gallop J, Andrasik W, Lucas J. Successful use of percutaneous dissolvable sutures during COVID-19 pandemic: a retrospective review. J Cutan Med Surg. 2023;27:34-38. doi:10.1177/12034754221143083
- Byrne M, Aly A. The surgical suture. Aesthet Surg J. 2019;39(suppl 2):S67-S72. doi:10.1093/asj/sjz036
- Koppa M, House R, Tobin V, et al. Suture material choice can increase risk of hypersensitivity in hand trauma patients. Eur J Plast Surg. 2023;46:239-243. doi:10.1007/s00238-022-01986-7
- Pandey S, Singh M, Singh K, et al. A prospective randomized study comparing non-absorbable polypropylene (Prolene®) and delayed absorbable polyglactin 910 (Vicryl®) suture material in mass closure of vertical laparotomy wounds. Indian J Surg. 2013;75:306-310. doi:10.1007/s12262-012-0492-x
- Parell GJ, Becker GD. Comparison of absorbable with nonabsorbable sutures in closure of facial skin wounds. Arch Facial Plast Surg. 2003;5:488-490. doi:10.1001/archfaci.5.6.488
- Kim J, Singh Maan H, Cool AJ, et al. Fast absorbing gut suture versus cyanoacrylate tissue adhesive in the epidermal closure of linear repairs following Mohs micrographic surgery. J Clin Aesthet Dermatol. 2015;8:24-29.
- Niessen FB, Spauwen PH, Kon M. The role of suture material in hypertrophic scar formation: Monocryl vs. Vicryl-Rapide. Ann Plast Surg. 1997;39:254-260. doi:10.1097/00000637-199709000-00006
- Fosko SW, Heap D. Surgical pearl: an economical means of skin closure with absorbable suture. J Am Acad Dermatol. 1998;39(2 pt 1):248-250. doi:10.1016/s0190-9622(98)70084-2
Practice Gap
Proper suture selection is imperative for appropriate wound healing to minimize the risk for infection and inflammation and to reduce scarring. In Mohs micrographic surgery (MMS), suture selection should be given high consideration in patients with skin of color.1 Using the right type of suture and wound closure technique can lead to favorable aesthetic outcomes by preventing postoperative postinflammatory hyperpigmentation (PIH) and keloids. Data on the choice of suture material in patients with skin of color are limited.
Suture selection depends on a variety of factors including but not limited to the location of the wound on the body, risk for infection, cost, availability, and the personal preference and experience of the MMS surgeon. During the COVID-19 pandemic, suturepreference among dermatologic surgeons shifted to fast-absorbing gut sutures,2 offering alternatives to synthetic monofilament polypropylene and nylon sutures. Absorbable sutures reduced the need for in-person follow-up visits without increasing the incidence of postoperative complications.
Despite these benefits, research suggests that natural absorbable gut sutures induce cutaneous inflammation and should be avoided in patients with skin of color.1,3,4 Nonabsorbable sutures are less reactive, reducing PIH after MMS in patients with skin of color.
Tools and Technique
Use of nonabsorbable stitches is a practical solution to reduce the risk for inflammation in patients with skin of color. Increased inflammation can lead to PIH and increase the risk for keloids in this patient population. Some patients will experience PIH after a surgical procedure regardless of the sutures used to repair the closure; however, one of our goals with patients with skin of color undergoing MMS is to reduce the inflammatory risk that could lead to PIH to ensure optimal aesthetic outcomes.
A middle-aged African woman with darker skin and a history of developing PIH after trauma to the skin presented to our clinic for MMS of a dermatofibrosarcoma protuberans on the upper abdomen. We used a simple running suture with 4-0 nylon to close the surgical wound. We avoided fast-absorbing gut sutures because they have high tissue reactivity1,4; use of sutures with low tissue reactivity, such as nylon and polypropylene, decreases the risk for inflammation without compromising alignment of wound edges and overall cosmesis of the repair. Prolene also is cost-effective and presents a decreased risk for wound dehiscence.5 After cauterizing the wound, we placed multiple synthetic absorbable sutures first to close the wound. We then did a double-running suture of nonabsorbable monofilament suture to reapproximate the epidermal edges with minimal tension. We placed 2 sets of running stitches to minimize the risk for dehiscence along the scar.
The patient was required to return for removal of the nonabsorbable sutures; this postoperative visit was covered by health insurance at no additional cost to the patient. In comparison, long-term repeat visits to treat PIH with a laser or chemical peel would have been more costly. Given that treatment of PIH is considered cosmetic, laser treatment would have been priced at several hundred dollars per session at our institution, and the patient would likely have had a copay for a pretreatment lightening cream such as hydroquinone. Our patient had a favorable cosmetic outcome and reported no or minimal evidence of PIH months after the procedure.
Patients should be instructed to apply petrolatum twice daily, use sun-protective clothing, and cover sutures to minimize exposure to the sun and prevent crusting of the wound. Postinflammatory hyperpigmentation can be proactively treated postoperatively with topical hydroquinone, which was not needed in our patient.
Practice Implications
Although some studies suggest that there are no cosmetic differences between absorbable and nonabsorbable sutures, the effect of suture type in patients with skin of color undergoing MMS often is unreported or is not studied.6,7 The high reactivity and cutaneous inflammation associated with absorbable gut sutures are important considerations in this patient population.
In patients with skin of color undergoing MMS, we use nonabsorbable epidermal sutures such as nylon and Prolene because of their low reactivity and association with favorable aesthetic outcomes. Nonabsorbable sutures can be safely used in patients of all ages who are undergoing MMS under local anesthesia.
An exception would be the use of the absorbable suture Monocryl (J&J MedTech) in patients with skin of color who need a running subcuticular wound closure because it has low tissue reactivity and maintains high tensile strength. Monocryl has been shown to create less-reactive scars, which decreases the risk for keloids.8,9
More clinical studies are needed to assess the increased susceptibility to PIH in patients with skin of color when using absorbable gut sutures.
Practice Gap
Proper suture selection is imperative for appropriate wound healing to minimize the risk for infection and inflammation and to reduce scarring. In Mohs micrographic surgery (MMS), suture selection should be given high consideration in patients with skin of color.1 Using the right type of suture and wound closure technique can lead to favorable aesthetic outcomes by preventing postoperative postinflammatory hyperpigmentation (PIH) and keloids. Data on the choice of suture material in patients with skin of color are limited.
Suture selection depends on a variety of factors including but not limited to the location of the wound on the body, risk for infection, cost, availability, and the personal preference and experience of the MMS surgeon. During the COVID-19 pandemic, suturepreference among dermatologic surgeons shifted to fast-absorbing gut sutures,2 offering alternatives to synthetic monofilament polypropylene and nylon sutures. Absorbable sutures reduced the need for in-person follow-up visits without increasing the incidence of postoperative complications.
Despite these benefits, research suggests that natural absorbable gut sutures induce cutaneous inflammation and should be avoided in patients with skin of color.1,3,4 Nonabsorbable sutures are less reactive, reducing PIH after MMS in patients with skin of color.
Tools and Technique
Use of nonabsorbable stitches is a practical solution to reduce the risk for inflammation in patients with skin of color. Increased inflammation can lead to PIH and increase the risk for keloids in this patient population. Some patients will experience PIH after a surgical procedure regardless of the sutures used to repair the closure; however, one of our goals with patients with skin of color undergoing MMS is to reduce the inflammatory risk that could lead to PIH to ensure optimal aesthetic outcomes.
A middle-aged African woman with darker skin and a history of developing PIH after trauma to the skin presented to our clinic for MMS of a dermatofibrosarcoma protuberans on the upper abdomen. We used a simple running suture with 4-0 nylon to close the surgical wound. We avoided fast-absorbing gut sutures because they have high tissue reactivity1,4; use of sutures with low tissue reactivity, such as nylon and polypropylene, decreases the risk for inflammation without compromising alignment of wound edges and overall cosmesis of the repair. Prolene also is cost-effective and presents a decreased risk for wound dehiscence.5 After cauterizing the wound, we placed multiple synthetic absorbable sutures first to close the wound. We then did a double-running suture of nonabsorbable monofilament suture to reapproximate the epidermal edges with minimal tension. We placed 2 sets of running stitches to minimize the risk for dehiscence along the scar.
The patient was required to return for removal of the nonabsorbable sutures; this postoperative visit was covered by health insurance at no additional cost to the patient. In comparison, long-term repeat visits to treat PIH with a laser or chemical peel would have been more costly. Given that treatment of PIH is considered cosmetic, laser treatment would have been priced at several hundred dollars per session at our institution, and the patient would likely have had a copay for a pretreatment lightening cream such as hydroquinone. Our patient had a favorable cosmetic outcome and reported no or minimal evidence of PIH months after the procedure.
Patients should be instructed to apply petrolatum twice daily, use sun-protective clothing, and cover sutures to minimize exposure to the sun and prevent crusting of the wound. Postinflammatory hyperpigmentation can be proactively treated postoperatively with topical hydroquinone, which was not needed in our patient.
Practice Implications
Although some studies suggest that there are no cosmetic differences between absorbable and nonabsorbable sutures, the effect of suture type in patients with skin of color undergoing MMS often is unreported or is not studied.6,7 The high reactivity and cutaneous inflammation associated with absorbable gut sutures are important considerations in this patient population.
In patients with skin of color undergoing MMS, we use nonabsorbable epidermal sutures such as nylon and Prolene because of their low reactivity and association with favorable aesthetic outcomes. Nonabsorbable sutures can be safely used in patients of all ages who are undergoing MMS under local anesthesia.
An exception would be the use of the absorbable suture Monocryl (J&J MedTech) in patients with skin of color who need a running subcuticular wound closure because it has low tissue reactivity and maintains high tensile strength. Monocryl has been shown to create less-reactive scars, which decreases the risk for keloids.8,9
More clinical studies are needed to assess the increased susceptibility to PIH in patients with skin of color when using absorbable gut sutures.
- Williams R, Ciocon D. Mohs micrographic surgery in skin of color. J Drugs Dermatol. 2022;21:536-541. doi:10.36849/JDD.6469
- Gallop J, Andrasik W, Lucas J. Successful use of percutaneous dissolvable sutures during COVID-19 pandemic: a retrospective review. J Cutan Med Surg. 2023;27:34-38. doi:10.1177/12034754221143083
- Byrne M, Aly A. The surgical suture. Aesthet Surg J. 2019;39(suppl 2):S67-S72. doi:10.1093/asj/sjz036
- Koppa M, House R, Tobin V, et al. Suture material choice can increase risk of hypersensitivity in hand trauma patients. Eur J Plast Surg. 2023;46:239-243. doi:10.1007/s00238-022-01986-7
- Pandey S, Singh M, Singh K, et al. A prospective randomized study comparing non-absorbable polypropylene (Prolene®) and delayed absorbable polyglactin 910 (Vicryl®) suture material in mass closure of vertical laparotomy wounds. Indian J Surg. 2013;75:306-310. doi:10.1007/s12262-012-0492-x
- Parell GJ, Becker GD. Comparison of absorbable with nonabsorbable sutures in closure of facial skin wounds. Arch Facial Plast Surg. 2003;5:488-490. doi:10.1001/archfaci.5.6.488
- Kim J, Singh Maan H, Cool AJ, et al. Fast absorbing gut suture versus cyanoacrylate tissue adhesive in the epidermal closure of linear repairs following Mohs micrographic surgery. J Clin Aesthet Dermatol. 2015;8:24-29.
- Niessen FB, Spauwen PH, Kon M. The role of suture material in hypertrophic scar formation: Monocryl vs. Vicryl-Rapide. Ann Plast Surg. 1997;39:254-260. doi:10.1097/00000637-199709000-00006
- Fosko SW, Heap D. Surgical pearl: an economical means of skin closure with absorbable suture. J Am Acad Dermatol. 1998;39(2 pt 1):248-250. doi:10.1016/s0190-9622(98)70084-2
- Williams R, Ciocon D. Mohs micrographic surgery in skin of color. J Drugs Dermatol. 2022;21:536-541. doi:10.36849/JDD.6469
- Gallop J, Andrasik W, Lucas J. Successful use of percutaneous dissolvable sutures during COVID-19 pandemic: a retrospective review. J Cutan Med Surg. 2023;27:34-38. doi:10.1177/12034754221143083
- Byrne M, Aly A. The surgical suture. Aesthet Surg J. 2019;39(suppl 2):S67-S72. doi:10.1093/asj/sjz036
- Koppa M, House R, Tobin V, et al. Suture material choice can increase risk of hypersensitivity in hand trauma patients. Eur J Plast Surg. 2023;46:239-243. doi:10.1007/s00238-022-01986-7
- Pandey S, Singh M, Singh K, et al. A prospective randomized study comparing non-absorbable polypropylene (Prolene®) and delayed absorbable polyglactin 910 (Vicryl®) suture material in mass closure of vertical laparotomy wounds. Indian J Surg. 2013;75:306-310. doi:10.1007/s12262-012-0492-x
- Parell GJ, Becker GD. Comparison of absorbable with nonabsorbable sutures in closure of facial skin wounds. Arch Facial Plast Surg. 2003;5:488-490. doi:10.1001/archfaci.5.6.488
- Kim J, Singh Maan H, Cool AJ, et al. Fast absorbing gut suture versus cyanoacrylate tissue adhesive in the epidermal closure of linear repairs following Mohs micrographic surgery. J Clin Aesthet Dermatol. 2015;8:24-29.
- Niessen FB, Spauwen PH, Kon M. The role of suture material in hypertrophic scar formation: Monocryl vs. Vicryl-Rapide. Ann Plast Surg. 1997;39:254-260. doi:10.1097/00000637-199709000-00006
- Fosko SW, Heap D. Surgical pearl: an economical means of skin closure with absorbable suture. J Am Acad Dermatol. 1998;39(2 pt 1):248-250. doi:10.1016/s0190-9622(98)70084-2
