User login
Congratulations!
Dear Colleagues,
Congratulations to the new gastroenterology fellows who have just begun their fellowships and also to those who have just finished and are starting their careers. It is certainly an exciting time of year for so many! A letter from AGA President Sheila Crowe, included in this issue, details the benefits and opportunities our organization offers GIs entering practice and academia.
This issue also contains an informative perspective about pursuing a career in medical education by Suzanne Rose (University of Connecticut), an incredibly passionate educator who has dedicated her career to this endeavor. Additionally, Katherine Garman (Duke University) and Latha Alaparthi (Gastroenterology Center of Connecticut/Yale University) provide a recap of this year’s AGA Women’s Leadership conference, which brought together a large group of early-career and experienced women from many different career pathways within the field of gastroenterology.
As student loans are an issue for many, Common Bond, the AGA’s official student loan partner, highlights an early-career gastroenterologist’s experience with student loans, as well as important factors in refinancing and paying off student loans. Finally, in the first of a two-part series on medical malpractice, an experienced group of attorneys from Eckert Seamans Cherin & Mellott, LLC (Philadelphia) provide a concise overview of the basics of malpractice as well as tips to help minimize your risk of being sued.
I hope that you enjoy this issue of The New Gastroenterologist. For those in the early-career group on the AGA Community (http://community.gastro.org/), these articles will be posted to the library to further enhance access. You can also find The New Gastroenterologist online and via the free app. If you have ideas for future issues or would be interested in contributing, please e-mail either me at [email protected] or Managing Editor Ryan Farrell at [email protected].
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Dr. Bryson W. Katona is an instructor of medicine in the division of gastroenterology at the University of Pennsylvania.
Dear Colleagues,
Congratulations to the new gastroenterology fellows who have just begun their fellowships and also to those who have just finished and are starting their careers. It is certainly an exciting time of year for so many! A letter from AGA President Sheila Crowe, included in this issue, details the benefits and opportunities our organization offers GIs entering practice and academia.
This issue also contains an informative perspective about pursuing a career in medical education by Suzanne Rose (University of Connecticut), an incredibly passionate educator who has dedicated her career to this endeavor. Additionally, Katherine Garman (Duke University) and Latha Alaparthi (Gastroenterology Center of Connecticut/Yale University) provide a recap of this year’s AGA Women’s Leadership conference, which brought together a large group of early-career and experienced women from many different career pathways within the field of gastroenterology.
As student loans are an issue for many, Common Bond, the AGA’s official student loan partner, highlights an early-career gastroenterologist’s experience with student loans, as well as important factors in refinancing and paying off student loans. Finally, in the first of a two-part series on medical malpractice, an experienced group of attorneys from Eckert Seamans Cherin & Mellott, LLC (Philadelphia) provide a concise overview of the basics of malpractice as well as tips to help minimize your risk of being sued.
I hope that you enjoy this issue of The New Gastroenterologist. For those in the early-career group on the AGA Community (http://community.gastro.org/), these articles will be posted to the library to further enhance access. You can also find The New Gastroenterologist online and via the free app. If you have ideas for future issues or would be interested in contributing, please e-mail either me at [email protected] or Managing Editor Ryan Farrell at [email protected].
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Dr. Bryson W. Katona is an instructor of medicine in the division of gastroenterology at the University of Pennsylvania.
Dear Colleagues,
Congratulations to the new gastroenterology fellows who have just begun their fellowships and also to those who have just finished and are starting their careers. It is certainly an exciting time of year for so many! A letter from AGA President Sheila Crowe, included in this issue, details the benefits and opportunities our organization offers GIs entering practice and academia.
This issue also contains an informative perspective about pursuing a career in medical education by Suzanne Rose (University of Connecticut), an incredibly passionate educator who has dedicated her career to this endeavor. Additionally, Katherine Garman (Duke University) and Latha Alaparthi (Gastroenterology Center of Connecticut/Yale University) provide a recap of this year’s AGA Women’s Leadership conference, which brought together a large group of early-career and experienced women from many different career pathways within the field of gastroenterology.
As student loans are an issue for many, Common Bond, the AGA’s official student loan partner, highlights an early-career gastroenterologist’s experience with student loans, as well as important factors in refinancing and paying off student loans. Finally, in the first of a two-part series on medical malpractice, an experienced group of attorneys from Eckert Seamans Cherin & Mellott, LLC (Philadelphia) provide a concise overview of the basics of malpractice as well as tips to help minimize your risk of being sued.
I hope that you enjoy this issue of The New Gastroenterologist. For those in the early-career group on the AGA Community (http://community.gastro.org/), these articles will be posted to the library to further enhance access. You can also find The New Gastroenterologist online and via the free app. If you have ideas for future issues or would be interested in contributing, please e-mail either me at [email protected] or Managing Editor Ryan Farrell at [email protected].
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Dr. Bryson W. Katona is an instructor of medicine in the division of gastroenterology at the University of Pennsylvania.
Minocycline may delay conversion to MS
Minocycline, an antibiotic that has immune-modulating properties and crosses the blood-brain barrier, appears to delay conversion to multiple sclerosis in patients who have an initial focal demyelinating event, according to a report published online June 1 in the New England Journal of Medicine.
Two small clinical trials involving patients with relapsing-remitting multiple sclerosis (MS) recently showed that minocycline reduces the number of lesions detected on MRI with gadolinium enhancement. So researchers led by Luanne M. Metz, MD, of the Cumming School of Medicine and the Hotchkiss Brain Institute, Calgary, Alta., conducted a randomized, double-blind, placebo-controlled trial at 12 Canadian MS clinics to determine whether the drug might delay conversion to MS after a first, clinically isolated demyelinating event, such as optic neuritis or a brainstem, cerebral, cerebellar, or myelopathy syndrome.
The primary outcome of conversion to MS within 6 months of randomization occurred in 23 (32%) patients taking minocycline, compared with 41 (59%) taking placebo – a difference that exceeded the prespecified clinically meaningful difference between the two groups. After the data were adjusted to account for the number of brain lesions at baseline, the difference in risk at 6 months was 18.5 percentage points, a magnitude of effect that is similar to what has been reported for other therapies such as interferon beta-1b, interferon beta-1a, teriflunomide, and oral cladribine.
The findings were similar in every sensitivity and subgroup analysis. All secondary outcomes, such as the decrease in mean lesion volume and the mean number of new lesions after 6 months of treatment, also favored minocycline over placebo, the investigators said (N Engl J Med. 2017 June 1. doi: 10.1056/NEJMoa1608889).
Minocycline’s neuroprotective effect persisted through 12 months of follow-up, according to a post hoc analysis, but was no longer sustained at 24 months of follow-up, they noted. In addition, post hoc analyses showed that minocycline held no significant benefit over placebo with respect to relapse or disability outcomes at either 6 months or 24 months.
This study was supported by the Multiple Sclerosis Society of Canada. Dr. Metz reported receiving grant support from Hoffmann–La Roche outside of this work; her associates reported ties to numerous industry sources.
The intriguing findings of Metz et al., together with the established safety profile and low cost of minocycline, make a compelling case for more research into the drug’s use in early MS.
However, it would be premature to begin using minocycline for MS until its benefits can be confirmed in larger and longer-term clinical trials.
Zongqi Xia, MD, PhD, is in the Program in Translational Neurology and Neuroinflammation at the Pittsburgh Institute of Neurodegenerative Diseases and at the Institute of Multiple Sclerosis Care and Research at the University of Pittsburgh. Robert M. Friedlander, MD, is in the Neuroapoptosis Laboratory and the department of neurosurgery at the University of Pittsburgh. They reported having no relevant financial disclosures. Dr. Xia and Dr. Friedlander made these remarks in an editorial accompanying Dr. Metz and colleagues’ report (N Engl J Med. 2017 June 1. doi: 10.1056/NEJMe1703230).
The intriguing findings of Metz et al., together with the established safety profile and low cost of minocycline, make a compelling case for more research into the drug’s use in early MS.
However, it would be premature to begin using minocycline for MS until its benefits can be confirmed in larger and longer-term clinical trials.
Zongqi Xia, MD, PhD, is in the Program in Translational Neurology and Neuroinflammation at the Pittsburgh Institute of Neurodegenerative Diseases and at the Institute of Multiple Sclerosis Care and Research at the University of Pittsburgh. Robert M. Friedlander, MD, is in the Neuroapoptosis Laboratory and the department of neurosurgery at the University of Pittsburgh. They reported having no relevant financial disclosures. Dr. Xia and Dr. Friedlander made these remarks in an editorial accompanying Dr. Metz and colleagues’ report (N Engl J Med. 2017 June 1. doi: 10.1056/NEJMe1703230).
The intriguing findings of Metz et al., together with the established safety profile and low cost of minocycline, make a compelling case for more research into the drug’s use in early MS.
However, it would be premature to begin using minocycline for MS until its benefits can be confirmed in larger and longer-term clinical trials.
Zongqi Xia, MD, PhD, is in the Program in Translational Neurology and Neuroinflammation at the Pittsburgh Institute of Neurodegenerative Diseases and at the Institute of Multiple Sclerosis Care and Research at the University of Pittsburgh. Robert M. Friedlander, MD, is in the Neuroapoptosis Laboratory and the department of neurosurgery at the University of Pittsburgh. They reported having no relevant financial disclosures. Dr. Xia and Dr. Friedlander made these remarks in an editorial accompanying Dr. Metz and colleagues’ report (N Engl J Med. 2017 June 1. doi: 10.1056/NEJMe1703230).
Minocycline, an antibiotic that has immune-modulating properties and crosses the blood-brain barrier, appears to delay conversion to multiple sclerosis in patients who have an initial focal demyelinating event, according to a report published online June 1 in the New England Journal of Medicine.
Two small clinical trials involving patients with relapsing-remitting multiple sclerosis (MS) recently showed that minocycline reduces the number of lesions detected on MRI with gadolinium enhancement. So researchers led by Luanne M. Metz, MD, of the Cumming School of Medicine and the Hotchkiss Brain Institute, Calgary, Alta., conducted a randomized, double-blind, placebo-controlled trial at 12 Canadian MS clinics to determine whether the drug might delay conversion to MS after a first, clinically isolated demyelinating event, such as optic neuritis or a brainstem, cerebral, cerebellar, or myelopathy syndrome.
The primary outcome of conversion to MS within 6 months of randomization occurred in 23 (32%) patients taking minocycline, compared with 41 (59%) taking placebo – a difference that exceeded the prespecified clinically meaningful difference between the two groups. After the data were adjusted to account for the number of brain lesions at baseline, the difference in risk at 6 months was 18.5 percentage points, a magnitude of effect that is similar to what has been reported for other therapies such as interferon beta-1b, interferon beta-1a, teriflunomide, and oral cladribine.
The findings were similar in every sensitivity and subgroup analysis. All secondary outcomes, such as the decrease in mean lesion volume and the mean number of new lesions after 6 months of treatment, also favored minocycline over placebo, the investigators said (N Engl J Med. 2017 June 1. doi: 10.1056/NEJMoa1608889).
Minocycline’s neuroprotective effect persisted through 12 months of follow-up, according to a post hoc analysis, but was no longer sustained at 24 months of follow-up, they noted. In addition, post hoc analyses showed that minocycline held no significant benefit over placebo with respect to relapse or disability outcomes at either 6 months or 24 months.
This study was supported by the Multiple Sclerosis Society of Canada. Dr. Metz reported receiving grant support from Hoffmann–La Roche outside of this work; her associates reported ties to numerous industry sources.
Minocycline, an antibiotic that has immune-modulating properties and crosses the blood-brain barrier, appears to delay conversion to multiple sclerosis in patients who have an initial focal demyelinating event, according to a report published online June 1 in the New England Journal of Medicine.
Two small clinical trials involving patients with relapsing-remitting multiple sclerosis (MS) recently showed that minocycline reduces the number of lesions detected on MRI with gadolinium enhancement. So researchers led by Luanne M. Metz, MD, of the Cumming School of Medicine and the Hotchkiss Brain Institute, Calgary, Alta., conducted a randomized, double-blind, placebo-controlled trial at 12 Canadian MS clinics to determine whether the drug might delay conversion to MS after a first, clinically isolated demyelinating event, such as optic neuritis or a brainstem, cerebral, cerebellar, or myelopathy syndrome.
The primary outcome of conversion to MS within 6 months of randomization occurred in 23 (32%) patients taking minocycline, compared with 41 (59%) taking placebo – a difference that exceeded the prespecified clinically meaningful difference between the two groups. After the data were adjusted to account for the number of brain lesions at baseline, the difference in risk at 6 months was 18.5 percentage points, a magnitude of effect that is similar to what has been reported for other therapies such as interferon beta-1b, interferon beta-1a, teriflunomide, and oral cladribine.
The findings were similar in every sensitivity and subgroup analysis. All secondary outcomes, such as the decrease in mean lesion volume and the mean number of new lesions after 6 months of treatment, also favored minocycline over placebo, the investigators said (N Engl J Med. 2017 June 1. doi: 10.1056/NEJMoa1608889).
Minocycline’s neuroprotective effect persisted through 12 months of follow-up, according to a post hoc analysis, but was no longer sustained at 24 months of follow-up, they noted. In addition, post hoc analyses showed that minocycline held no significant benefit over placebo with respect to relapse or disability outcomes at either 6 months or 24 months.
This study was supported by the Multiple Sclerosis Society of Canada. Dr. Metz reported receiving grant support from Hoffmann–La Roche outside of this work; her associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: The primary outcome, conversion to MS within 6 months of randomization, occurred in 23 (32%) patients taking minocycline, compared with 41 (59%) taking placebo.
Data source: A multicenter, randomized, double-blind, placebo-controlled trial involving 142 adults treated for up to 24 months.
Disclosures: This study was supported by the Multiple Sclerosis Society of Canada. Dr. Metz reported receiving grant support from Hoffmann–La Roche outside of this work; her associates reported ties to numerous industry sources.
Practical Considerations for Moderate to Severe Asthma, Part 1: Management, Biomarkers, and When to Refer
This newsletter, Practical Considerations for Moderate to Severe Asthma, Part 1: Management, Biomarkers, and When to Refer, provides detailed guidance for nurse practitioners and physician assistants on how to identify, evaluate, and treat patients with poorly controlled, moderate to severe asthma.
Click here to read the supplement
Clinical Research Coordinator
Boys Town National Research Hospital
Boys Town, Nebraska
Kevin R. Murphy, MD
Director of Allergy, Asthma, and Pulmonary Research
Boys Town National Research Hospital
Boys Town, Nebraska
Department of Pediatrics
University of Nebraska Medical Center
Creighton University School of Medicine
Omaha, Nebraska
This newsletter, Practical Considerations for Moderate to Severe Asthma, Part 1: Management, Biomarkers, and When to Refer, provides detailed guidance for nurse practitioners and physician assistants on how to identify, evaluate, and treat patients with poorly controlled, moderate to severe asthma.
Click here to read the supplement
Clinical Research Coordinator
Boys Town National Research Hospital
Boys Town, Nebraska
Kevin R. Murphy, MD
Director of Allergy, Asthma, and Pulmonary Research
Boys Town National Research Hospital
Boys Town, Nebraska
Department of Pediatrics
University of Nebraska Medical Center
Creighton University School of Medicine
Omaha, Nebraska
This newsletter, Practical Considerations for Moderate to Severe Asthma, Part 1: Management, Biomarkers, and When to Refer, provides detailed guidance for nurse practitioners and physician assistants on how to identify, evaluate, and treat patients with poorly controlled, moderate to severe asthma.
Click here to read the supplement
Clinical Research Coordinator
Boys Town National Research Hospital
Boys Town, Nebraska
Kevin R. Murphy, MD
Director of Allergy, Asthma, and Pulmonary Research
Boys Town National Research Hospital
Boys Town, Nebraska
Department of Pediatrics
University of Nebraska Medical Center
Creighton University School of Medicine
Omaha, Nebraska
Highlights from The 2017 Society of Gynecologic Surgeons Scientific Meeting
PART 1

Robert E. Gutman, MD
FPMRS Program Director
MedStar Washington Hospital Center
Associate Professor
Departments of Urology and Obstetrics/Gynecology
Georgetown University
Washington, DC

Elizabeth R. Mueller, MD, MSME
Professor, Departments of Urology and Obstetrics/Gynecology
Loyola University Chicago Stritch School of Medicine
Loyola University Medical Center
Maywood, Illinois

Janet Bickel, MA
Leadership and Career Development Coach
Falls Church, Virginia

Kristin M. Jacobs, MD
Steering Committee Chair, AUGS-SGS Group of FPRN®
FPMRS Fellow, Division of Urogynecology and Reconstructive Pelvic Surgery
Brown University
Providence, Rhode Island

Lior Lowenstein, MD, MS, MHA
Clinical Associate Professor, Department of Obstetrics and Gynecology
Rambam Health Center Campus, Ruth and Bruce Rappaport Faculty of Medicine
Technion Israel Institute of Technology
Haifa, Israel
Drs. Gutman, Jacobs, and Lowenstein and Ms. Bickel report no financial relationships relevant to their articles. Dr. Mueller reports that she is an investigator for and is on the advisory board of Astellas Medical and Scientific Affairs.
PART 2

Geoffrey W. Cundiff, MD
Dr. Victor Gomel Professor and Head
Department of Obstetrics and Gynaecology
University of Bristish Columbia
Vancouver, British Columbia

Kimberly Kenton, MD, MS
Professor, Obstetrics and Gynaecology and Urology
Divison Chief and Fellowship Program Director
Female Pelvis Medicine and Reconstructive Surgery
Medicial Director, Women's Integrated Pelvic Health Program
Northwestern Medicine/Northwestern University Feinberg School of Medicince
Chicago, Illinois

Denise M. Elser, MD
Urogynecologist
Women's Health Institute of Illinois
Oak Lawn, Illinois
Drs. Cundiff and Elser report no financial relationships relevant to their articles. Dr. Kenton reports that she receives grant or research support from Boston Scientific and the National Institutes of Health, and that she serves as an expert witness for the Butler Snow Law Firm/Ethicon.

PART 1

Robert E. Gutman, MD
FPMRS Program Director
MedStar Washington Hospital Center
Associate Professor
Departments of Urology and Obstetrics/Gynecology
Georgetown University
Washington, DC

Elizabeth R. Mueller, MD, MSME
Professor, Departments of Urology and Obstetrics/Gynecology
Loyola University Chicago Stritch School of Medicine
Loyola University Medical Center
Maywood, Illinois

Janet Bickel, MA
Leadership and Career Development Coach
Falls Church, Virginia

Kristin M. Jacobs, MD
Steering Committee Chair, AUGS-SGS Group of FPRN®
FPMRS Fellow, Division of Urogynecology and Reconstructive Pelvic Surgery
Brown University
Providence, Rhode Island

Lior Lowenstein, MD, MS, MHA
Clinical Associate Professor, Department of Obstetrics and Gynecology
Rambam Health Center Campus, Ruth and Bruce Rappaport Faculty of Medicine
Technion Israel Institute of Technology
Haifa, Israel
Drs. Gutman, Jacobs, and Lowenstein and Ms. Bickel report no financial relationships relevant to their articles. Dr. Mueller reports that she is an investigator for and is on the advisory board of Astellas Medical and Scientific Affairs.

PART 2

Geoffrey W. Cundiff, MD
Dr. Victor Gomel Professor and Head
Department of Obstetrics and Gynaecology
University of Bristish Columbia
Vancouver, British Columbia

Kimberly Kenton, MD, MS
Professor, Obstetrics and Gynaecology and Urology
Divison Chief and Fellowship Program Director
Female Pelvis Medicine and Reconstructive Surgery
Medicial Director, Women's Integrated Pelvic Health Program
Northwestern Medicine/Northwestern University Feinberg School of Medicince
Chicago, Illinois

Denise M. Elser, MD
Urogynecologist
Women's Health Institute of Illinois
Oak Lawn, Illinois
Drs. Cundiff and Elser report no financial relationships relevant to their articles. Dr. Kenton reports that she receives grant or research support from Boston Scientific and the National Institutes of Health, and that she serves as an expert witness for the Butler Snow Law Firm/Ethicon.

PART 1

Robert E. Gutman, MD
FPMRS Program Director
MedStar Washington Hospital Center
Associate Professor
Departments of Urology and Obstetrics/Gynecology
Georgetown University
Washington, DC

Elizabeth R. Mueller, MD, MSME
Professor, Departments of Urology and Obstetrics/Gynecology
Loyola University Chicago Stritch School of Medicine
Loyola University Medical Center
Maywood, Illinois

Janet Bickel, MA
Leadership and Career Development Coach
Falls Church, Virginia

Kristin M. Jacobs, MD
Steering Committee Chair, AUGS-SGS Group of FPRN®
FPMRS Fellow, Division of Urogynecology and Reconstructive Pelvic Surgery
Brown University
Providence, Rhode Island

Lior Lowenstein, MD, MS, MHA
Clinical Associate Professor, Department of Obstetrics and Gynecology
Rambam Health Center Campus, Ruth and Bruce Rappaport Faculty of Medicine
Technion Israel Institute of Technology
Haifa, Israel
Drs. Gutman, Jacobs, and Lowenstein and Ms. Bickel report no financial relationships relevant to their articles. Dr. Mueller reports that she is an investigator for and is on the advisory board of Astellas Medical and Scientific Affairs.

PART 2

Geoffrey W. Cundiff, MD
Dr. Victor Gomel Professor and Head
Department of Obstetrics and Gynaecology
University of Bristish Columbia
Vancouver, British Columbia

Kimberly Kenton, MD, MS
Professor, Obstetrics and Gynaecology and Urology
Divison Chief and Fellowship Program Director
Female Pelvis Medicine and Reconstructive Surgery
Medicial Director, Women's Integrated Pelvic Health Program
Northwestern Medicine/Northwestern University Feinberg School of Medicince
Chicago, Illinois

Denise M. Elser, MD
Urogynecologist
Women's Health Institute of Illinois
Oak Lawn, Illinois
Drs. Cundiff and Elser report no financial relationships relevant to their articles. Dr. Kenton reports that she receives grant or research support from Boston Scientific and the National Institutes of Health, and that she serves as an expert witness for the Butler Snow Law Firm/Ethicon.


ENDEAR Study Demonstrates Efficacy of Nusinersen in Infants With Spinal Muscular Atrophy
BOSTON—Infants with spinal muscular atrophy (SMA) type 1 who were treated with nusinersen demonstrated clinically and statistically significant gains across multiple efficacy end points, according to a report presented at the 69th Annual Meeting of the American Academy of Neurology. Nancy L. Kuntz, MD, an attending physician at the Ann and Robert H. Lurie Children’s Hospital of Chicago, on behalf of the ENDEAR Study Group, reported the final results of the phase III ENDEAR study assessing efficacy and safety of nusinersen in infants with SMA.
SMA is a rare, debilitating, autosomal recessive neuromuscular disorder causing varying degrees of weakness. The disease is caused by insufficient levels of SMN protein. Nusinersen is an antisense oligonucleotide that promotes the production of full-length SMN protein.
The ENDEAR study was a phase III, randomized, double-blind, sham-procedure controlled 13-month study to assess the efficacy and safety of nusinersen in infants with SMA. The ENDEAR study had an interim efficacy analysis in September of 2016. This analysis showed that the primary end point—motor milestone response—was positive in 41% of nusinersen-treated infants, and information was submitted to the FDA. Under priority review, Spinraza (nusinersen) was approved for the treatment of SMA in pediatric and adult patients by the FDA on December 23, 2016.
Study Design
Symptomatic infants diagnosed with SMA (with clinical features consistent with type 1 SMA) were randomized (2:1) to receive intrathecal nusinersen (12-mg scaled equivalent dose) or sham procedure. For both groups, four doses were given over two months, on days 1, 15, 29, and 64. This was followed by a maintenance phase, with dosing every four months.
Key eligibility criteria included 5q SMN1 homozygous gene deletion or mutation, two SMN2 gene copies, onset of SMA symptoms at younger than 6 months, and no hypoxemia at baseline screening at age 7 months or younger. A total of 122 infants were enrolled.
Primary end points included proportion of modified section 2 Hammersmith Infant Neurological Examination (HINE) motor milestone responders (ie, more categories improving [≥ 2-point increase or maximal score in kicking ability, or ≥ 1-point increase in head control, rolling, sitting, crawling, standing, or walking] than worsening) and event-free survival (time to death or permanent ventilation). Secondary end points included percentage of Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND) responders (≥ 4-point increase), overall survival, and percentage of peroneal nerve compound muscle action potential (CMAP) responders (amplitude ≥ 1 mV).
The preplanned interim efficacy analysis was triggered when two-thirds of the infants reached day 183 involvement in the study. Because the primary end point—motor milestone response—was positive, the study was ended, and all of the infants were transferred into the open-label extension study, which is called SHINE. Event-free survival and all of the secondary end points were not assessed at the ENDEAR interim analysis. With further analysis now complete, Dr. Kuntz presented the end-of-study data set.
ENDEAR Final Results
At the end of the study, there was a significantly greater proportion of nusinersen-treated motor milestone responders versus sham-control responders (51% vs 0%), demonstrating continued improvement over the previous interim analysis (41% vs 0%). In the nusinersen-treated group, 22% of infants developed full head control, 10% of the infants developed the ability to independently roll from supine to prone positions, 8% developed independent sitting, with half of those being able to sit and pivot, and one infant was able to stand with minimal to moderate support.
Looking at change over time, the improvement in HINE motor milestone scores seen in ENDEAR matches the trajectory seen in a previous open-label trial. Patients in the previous trial have now been followed for another year or so, and they slowly continue to attain their motor milestones. Additionally, infants with presymptomatic SMA who were identified and treated within the first six weeks of life showed improvements in the rate and the range of their motor skills that were much greater than those in the other groups, suggesting that early treatment makes a difference.
Additional analyses included event-free survival, overall survival, CHOP INTEND score, peroneal nerve CMAP response, and need for mechanical ventilation. A significant nusinersen treatment benefit was seen with regard to event-free survival (hazard ratio = 0.530) and overall survival (hazard ratio = 0.372). Dr. Kuntz reported that 61% of the nusinersen-treated infants were alive at the end of the study, compared with 32% of controls. For nusinersen versus sham-control infants, 71% versus 3% were CHOP INTEND responders, and 36% versus 5% were CMAP responders. The risk of permanent ventilation was 34% lower in the nusinersen-treated group. Over the course of the study, 31% of the nusinersen-treated infants required permanent ventilation, defined as at least 16 hours per day, compared with 48% of the control infants.
The ENDEAR study was supported by Ionis Pharmaceuticals and Biogen.
Good News, Bad News
Following Dr. Kuntz’s plenary presentation of the ENDEAR study results, Charlotte J. Sumner, MD, Associate Professor of Neurology at Johns Hopkins University in Baltimore, served as the discussant. While Dr. Sumner praised the study findings and the breakthrough they represent, she did point out the staggering cost of the drug. At about $120,000 per dose, the price “has raised issues about insurance approval and reimbursement and raises concerns about delays
to treatment initiation and institutional risk,” she said. “But I would say that despite these challenges, well over 100 patients have already been dosed commercially at very different ages, and this is very promising that we will be able to deliver this drug in a widespread way.”
—Glenn S. Williams
Suggested Reading
Finkel RS, Chiriboga CA, Vajsar J, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet. 2016;388(10063):3017-3026.
BOSTON—Infants with spinal muscular atrophy (SMA) type 1 who were treated with nusinersen demonstrated clinically and statistically significant gains across multiple efficacy end points, according to a report presented at the 69th Annual Meeting of the American Academy of Neurology. Nancy L. Kuntz, MD, an attending physician at the Ann and Robert H. Lurie Children’s Hospital of Chicago, on behalf of the ENDEAR Study Group, reported the final results of the phase III ENDEAR study assessing efficacy and safety of nusinersen in infants with SMA.
SMA is a rare, debilitating, autosomal recessive neuromuscular disorder causing varying degrees of weakness. The disease is caused by insufficient levels of SMN protein. Nusinersen is an antisense oligonucleotide that promotes the production of full-length SMN protein.
The ENDEAR study was a phase III, randomized, double-blind, sham-procedure controlled 13-month study to assess the efficacy and safety of nusinersen in infants with SMA. The ENDEAR study had an interim efficacy analysis in September of 2016. This analysis showed that the primary end point—motor milestone response—was positive in 41% of nusinersen-treated infants, and information was submitted to the FDA. Under priority review, Spinraza (nusinersen) was approved for the treatment of SMA in pediatric and adult patients by the FDA on December 23, 2016.
Study Design
Symptomatic infants diagnosed with SMA (with clinical features consistent with type 1 SMA) were randomized (2:1) to receive intrathecal nusinersen (12-mg scaled equivalent dose) or sham procedure. For both groups, four doses were given over two months, on days 1, 15, 29, and 64. This was followed by a maintenance phase, with dosing every four months.
Key eligibility criteria included 5q SMN1 homozygous gene deletion or mutation, two SMN2 gene copies, onset of SMA symptoms at younger than 6 months, and no hypoxemia at baseline screening at age 7 months or younger. A total of 122 infants were enrolled.
Primary end points included proportion of modified section 2 Hammersmith Infant Neurological Examination (HINE) motor milestone responders (ie, more categories improving [≥ 2-point increase or maximal score in kicking ability, or ≥ 1-point increase in head control, rolling, sitting, crawling, standing, or walking] than worsening) and event-free survival (time to death or permanent ventilation). Secondary end points included percentage of Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND) responders (≥ 4-point increase), overall survival, and percentage of peroneal nerve compound muscle action potential (CMAP) responders (amplitude ≥ 1 mV).
The preplanned interim efficacy analysis was triggered when two-thirds of the infants reached day 183 involvement in the study. Because the primary end point—motor milestone response—was positive, the study was ended, and all of the infants were transferred into the open-label extension study, which is called SHINE. Event-free survival and all of the secondary end points were not assessed at the ENDEAR interim analysis. With further analysis now complete, Dr. Kuntz presented the end-of-study data set.
ENDEAR Final Results
At the end of the study, there was a significantly greater proportion of nusinersen-treated motor milestone responders versus sham-control responders (51% vs 0%), demonstrating continued improvement over the previous interim analysis (41% vs 0%). In the nusinersen-treated group, 22% of infants developed full head control, 10% of the infants developed the ability to independently roll from supine to prone positions, 8% developed independent sitting, with half of those being able to sit and pivot, and one infant was able to stand with minimal to moderate support.
Looking at change over time, the improvement in HINE motor milestone scores seen in ENDEAR matches the trajectory seen in a previous open-label trial. Patients in the previous trial have now been followed for another year or so, and they slowly continue to attain their motor milestones. Additionally, infants with presymptomatic SMA who were identified and treated within the first six weeks of life showed improvements in the rate and the range of their motor skills that were much greater than those in the other groups, suggesting that early treatment makes a difference.
Additional analyses included event-free survival, overall survival, CHOP INTEND score, peroneal nerve CMAP response, and need for mechanical ventilation. A significant nusinersen treatment benefit was seen with regard to event-free survival (hazard ratio = 0.530) and overall survival (hazard ratio = 0.372). Dr. Kuntz reported that 61% of the nusinersen-treated infants were alive at the end of the study, compared with 32% of controls. For nusinersen versus sham-control infants, 71% versus 3% were CHOP INTEND responders, and 36% versus 5% were CMAP responders. The risk of permanent ventilation was 34% lower in the nusinersen-treated group. Over the course of the study, 31% of the nusinersen-treated infants required permanent ventilation, defined as at least 16 hours per day, compared with 48% of the control infants.
The ENDEAR study was supported by Ionis Pharmaceuticals and Biogen.
Good News, Bad News
Following Dr. Kuntz’s plenary presentation of the ENDEAR study results, Charlotte J. Sumner, MD, Associate Professor of Neurology at Johns Hopkins University in Baltimore, served as the discussant. While Dr. Sumner praised the study findings and the breakthrough they represent, she did point out the staggering cost of the drug. At about $120,000 per dose, the price “has raised issues about insurance approval and reimbursement and raises concerns about delays
to treatment initiation and institutional risk,” she said. “But I would say that despite these challenges, well over 100 patients have already been dosed commercially at very different ages, and this is very promising that we will be able to deliver this drug in a widespread way.”
—Glenn S. Williams
Suggested Reading
Finkel RS, Chiriboga CA, Vajsar J, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet. 2016;388(10063):3017-3026.
BOSTON—Infants with spinal muscular atrophy (SMA) type 1 who were treated with nusinersen demonstrated clinically and statistically significant gains across multiple efficacy end points, according to a report presented at the 69th Annual Meeting of the American Academy of Neurology. Nancy L. Kuntz, MD, an attending physician at the Ann and Robert H. Lurie Children’s Hospital of Chicago, on behalf of the ENDEAR Study Group, reported the final results of the phase III ENDEAR study assessing efficacy and safety of nusinersen in infants with SMA.
SMA is a rare, debilitating, autosomal recessive neuromuscular disorder causing varying degrees of weakness. The disease is caused by insufficient levels of SMN protein. Nusinersen is an antisense oligonucleotide that promotes the production of full-length SMN protein.
The ENDEAR study was a phase III, randomized, double-blind, sham-procedure controlled 13-month study to assess the efficacy and safety of nusinersen in infants with SMA. The ENDEAR study had an interim efficacy analysis in September of 2016. This analysis showed that the primary end point—motor milestone response—was positive in 41% of nusinersen-treated infants, and information was submitted to the FDA. Under priority review, Spinraza (nusinersen) was approved for the treatment of SMA in pediatric and adult patients by the FDA on December 23, 2016.
Study Design
Symptomatic infants diagnosed with SMA (with clinical features consistent with type 1 SMA) were randomized (2:1) to receive intrathecal nusinersen (12-mg scaled equivalent dose) or sham procedure. For both groups, four doses were given over two months, on days 1, 15, 29, and 64. This was followed by a maintenance phase, with dosing every four months.
Key eligibility criteria included 5q SMN1 homozygous gene deletion or mutation, two SMN2 gene copies, onset of SMA symptoms at younger than 6 months, and no hypoxemia at baseline screening at age 7 months or younger. A total of 122 infants were enrolled.
Primary end points included proportion of modified section 2 Hammersmith Infant Neurological Examination (HINE) motor milestone responders (ie, more categories improving [≥ 2-point increase or maximal score in kicking ability, or ≥ 1-point increase in head control, rolling, sitting, crawling, standing, or walking] than worsening) and event-free survival (time to death or permanent ventilation). Secondary end points included percentage of Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND) responders (≥ 4-point increase), overall survival, and percentage of peroneal nerve compound muscle action potential (CMAP) responders (amplitude ≥ 1 mV).
The preplanned interim efficacy analysis was triggered when two-thirds of the infants reached day 183 involvement in the study. Because the primary end point—motor milestone response—was positive, the study was ended, and all of the infants were transferred into the open-label extension study, which is called SHINE. Event-free survival and all of the secondary end points were not assessed at the ENDEAR interim analysis. With further analysis now complete, Dr. Kuntz presented the end-of-study data set.
ENDEAR Final Results
At the end of the study, there was a significantly greater proportion of nusinersen-treated motor milestone responders versus sham-control responders (51% vs 0%), demonstrating continued improvement over the previous interim analysis (41% vs 0%). In the nusinersen-treated group, 22% of infants developed full head control, 10% of the infants developed the ability to independently roll from supine to prone positions, 8% developed independent sitting, with half of those being able to sit and pivot, and one infant was able to stand with minimal to moderate support.
Looking at change over time, the improvement in HINE motor milestone scores seen in ENDEAR matches the trajectory seen in a previous open-label trial. Patients in the previous trial have now been followed for another year or so, and they slowly continue to attain their motor milestones. Additionally, infants with presymptomatic SMA who were identified and treated within the first six weeks of life showed improvements in the rate and the range of their motor skills that were much greater than those in the other groups, suggesting that early treatment makes a difference.
Additional analyses included event-free survival, overall survival, CHOP INTEND score, peroneal nerve CMAP response, and need for mechanical ventilation. A significant nusinersen treatment benefit was seen with regard to event-free survival (hazard ratio = 0.530) and overall survival (hazard ratio = 0.372). Dr. Kuntz reported that 61% of the nusinersen-treated infants were alive at the end of the study, compared with 32% of controls. For nusinersen versus sham-control infants, 71% versus 3% were CHOP INTEND responders, and 36% versus 5% were CMAP responders. The risk of permanent ventilation was 34% lower in the nusinersen-treated group. Over the course of the study, 31% of the nusinersen-treated infants required permanent ventilation, defined as at least 16 hours per day, compared with 48% of the control infants.
The ENDEAR study was supported by Ionis Pharmaceuticals and Biogen.
Good News, Bad News
Following Dr. Kuntz’s plenary presentation of the ENDEAR study results, Charlotte J. Sumner, MD, Associate Professor of Neurology at Johns Hopkins University in Baltimore, served as the discussant. While Dr. Sumner praised the study findings and the breakthrough they represent, she did point out the staggering cost of the drug. At about $120,000 per dose, the price “has raised issues about insurance approval and reimbursement and raises concerns about delays
to treatment initiation and institutional risk,” she said. “But I would say that despite these challenges, well over 100 patients have already been dosed commercially at very different ages, and this is very promising that we will be able to deliver this drug in a widespread way.”
—Glenn S. Williams
Suggested Reading
Finkel RS, Chiriboga CA, Vajsar J, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet. 2016;388(10063):3017-3026.
Management of Poorly Controlled Indolent Systemic Mastocytosis Using Narrowband UVB Phototherapy
Systemic mastocytosis is a heterogeneous disorder of stem cell origin defined by abnormal hyperplasia and accumulation of mast cells (MCs) in one or more tissues.1,2 The most commonly affected tissues are the bone marrow, gastrointestinal tract, and skin. Based on a number of major and minor criteria defined by the World Health Organization (WHO), the mastocytoses are subdivided into 7 variants that range from isolated cutaneous involvement to widespread systemic disease.1-4 The most frequently diagnosed subtype is indolent systemic mastocytosis (ISM), a chronic disorder characterized by diffuse cutaneous macules and papules as well as bone marrow involvement in the form of multifocal dense infiltrates of MCs that frequently are phenotypically positive for c-KIT and tryptase. Serum tryptase levels are nearly invariably elevated in patients with this condition.1,2
Symptoms of ISM are determined by the intermittent release of histamine and leukotrienes from hyperproliferating MCs as well as IL-6 and eosinophil chemotactic factors. As the burden of MC secretory products increases, patients experience worsening pruritus, flushing, palpitations, vomiting, and anaphylaxis in severe instances.1,2,5 The mainstay of treatment of this condition involves symptom control through the inhibition of MC mediators.1 The majority of patients respond well to antihistamines, antileukotriene agents, and oral corticosteroids during severe episodes of MC degranulation.1,2,5
Unfortunately, some patients are unable to achieve adequate symptom control through the use of mediator-targeting treatments alone. In these cases, physicians often are faced with the following treatment dilemma: Either attempt to use therapies such as interferon alfa, which is cytoreductive to MCs, or 2-chlorodeoxyadenosine to reduce the overall MC burden, or turn to newer nonimmunosuppressive second-line options. We present the case of a patient with chronic ISM with progressive cutaneous lesions and poorly controlled pruritus that was previously managed with topical corticosteroids and antihistamines who responded favorably to treatment with narrowband UVB (NB-UVB) phototherapy.
Case Report
A 57-year-old woman presented with a 10-year history of widespread red-brown macules and papules on the trunk and upper and lower extremities. The lesions were intermittently pruritic, a symptom that was exacerbated on sun and heat exposure. A skin biopsy performed by an outside dermatologist 9 years prior confirmed the presence of mastocytosis. The patient was originally treated with triamcinolone cream and oral antihistamines, which controlled her symptoms successfully for nearly a decade.
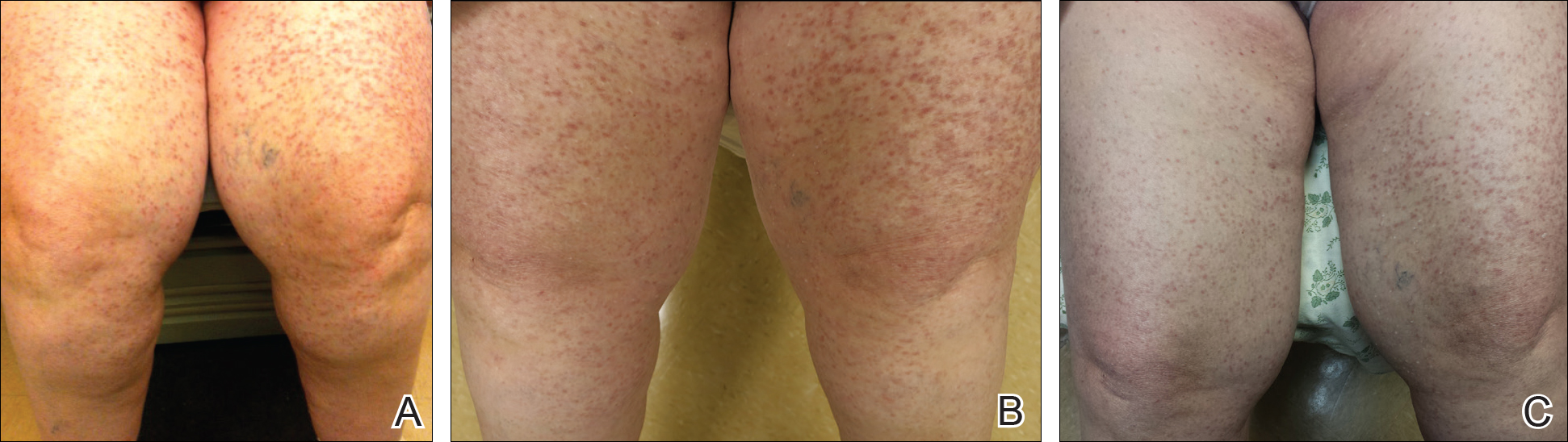
At the current presentation, the patient reported increasingly severe pruritus and lesional spread to the neck and face of 15 months’ duration. She denied any symptoms of flushing, diarrhea, syncopal episodes, or lightheadedness. Physical examination revealed a well-appearing middle-aged woman with multiple 3- to 8-mm, red-brown, blanchable macules and papules with areas coalescing into plaques that primarily involved the legs (Figure 1A); arms; back; and to a lesser extent the abdomen, neck, and face. There was no palpable lymphadenopathy.
Laboratory results revealed a complete blood cell count and basic metabolic profile within reference range; however, the serum tryptase level was elevated at 65 ng/mL (reference range, <11.4 ng/mL). A positron emission tomography–computed tomography scan was negative, as well as a c-KIT mutation analysis. A review of the skin biopsy from 9 years prior demonstrated slight acanthosis with dermal proliferation of mononuclear cells (Figure 2A), some of which had abundant cytoplasm and oval-shaped nuclei. There were few eosinophils and marked dermal telangiectasias. Giemsa stain revealed increased numbers of MCs in the upper dermis (Figure 2B). A bone marrow biopsy performed 9 years later showed multifocal lesions composed of MCs with associated lymphoid aggregates without notable myelodyspoiesis (or myeloproliferative neoplasm). These features were all consistent with WHO criteria for ISM. Based on the most current clinical, laboratory, and histopathologic findings, the patient was diagnosed with category IB ISM.

The patient’s symptoms had remained stable for 9 years with a regimen of triamcinolone cream 0.1% twice daily, doxepin cream 5% daily as needed, and oral fexofenadine 180 mg once daily. The patient continues to use topical steroids and oral antihistamines. Due to inadequate symptom control, breakthrough pruritus, and the development of new skin lesions on the head and neck, she was started on NB-UVB treatment 2 months after presentation. The patient’s symptoms and the extent of cutaneous maculopapular lesions improved after 20 light treatments (Figure 1B), with even more dramatic results after 40 cycles of therapy (Figure 1C). Overall, the lower legs have proved most recalcitrant to this treatment modality. She is currently continuing to receive NB-UVB treatment twice weekly.
Comment
Systemic mastocytosis is a heterogeneous disorder characterized by the proliferation and accumulation of atypical MCs in tissues, principally in the bone marrow and skin, though involvement of the gastrointestinal tract, liver, spleen, and lymphatic system also have been reported.1,2,6 The WHO classification of mastocytosis divides this condition into 7 subtypes.4 Indolent systemic mastocytosis is the most common variant.2,6 The etiology of ISM is not fully understood, but there is evidence suggesting that an activating mutation of KIT proto-oncogene receptor tyrosine kinase, KIT (usually D816V), present in the MCs of nearly 80% of patients with ISM may be involved.1,3-5,7 Patients occasionally present with predominantly cutaneous findings but typically seek medical attention due to the recurrent systemic symptoms of the disease (eg, pruritus, flushing, syncope, palpitations, headache, dyspepsia, vomiting, diarrhea), which are related to the release of MC mediators.1,2
The management of ISM is complex and based primarily on symptom reduction without alteration of disease course.1,2,5,7 Patients should avoid symptom triggers such as heat, humidity, emotional and physical stress, alcohol, and certain medications (ie, aspirin, opioids, radiocontrast agents).7 Patients are initially treated with histamine H1- and H2-receptor antagonists to alleviate MC mediator release symptoms.1,2,8 Although H1 blockers are most effective in mitigating cutaneous symptoms and limiting pruritus, H2 blockers are used to control gastric hypersecretion and dyspepsia.2 Proton pump inhibitors are useful in patients with peptic ulcer disease who are unresponsive to H2-receptor antagonist therapy.2,7 Cromolyn sodium and ketotifen fumarate are MC stabilizers that help prevent degranulation, which is helpful in relieving most major ISM symptoms. Leukotriene antagonists, such as zafirlukast, montelukast sodium, or zileuton, also may be employed to target the proinflammatory and pruritogenic leukotrienes, also products of the MC protein.2,7 Imatinib mesylate and masitinib mesylate, both tyrosine kinase inhibitors, have been shown to improve symptoms and reduce MC mediator levels in ISM; however, most patients harbor the resistant KIT D816V mutation, which limits the utility of this medication.Patients with sensitive KIT mutations or those who have the wild-type KIT D816 mutation may be more appropriate candidates for imatinib or masitinib therapy, which can ameliorate symptoms of flushing, pruritus, and depression.7-10 Treatment with omalizumab, a humanized murine anti-IgE monoclonal antibody, can be effective in treating recurrent, treatment-refractory anaphylaxis in ISM patients.5,7
Symptoms unresponsive to these therapies can be effectively treated with a short course of oral corticosteroids,6,7 while MC cytoreductive therapies such as interferon alfa or 2-chlorodeoxyadenosine (cladribine/2-CdA) are reserved for refractory cases.2,7 Alternative therapies such as NB-UVB2 or psoralen plus UVA phototherapy11 also have demonstrated success in treating ISM symptoms. In the past, NB-UVB has shown efficacy in controlling pruriginous conditions ranging from chronic urticaria12,13 to atopic dermatitis14 to psoriasis.15 This evidence has spurred studies to evaluate if NB-UVB has a role in the management of uncontrolled cases of cutaneous and ISM.2,13,16,17 To date, the evidence has been promising. The majority of patients treated with this regimen report subjective reduction in pruritus in addition to clinical cutaneous disease burden.2,11 Also, laboratory analysis demonstrates decreased levels of tryptase in patients utilizing NB-UVB phototherapy.2 Thus far, the use of NB-UVB phototherapy in the treatment of pruriginous disorders such as ISM has not been associated with any severe side effects such as increased rates of anaphylaxis, though some research has suggested that this therapy may lower the threshold for patients to develop symptomatic dermographism.12 Overall, patients treated with NB-UVB phototherapy report improved quality of life related to more effective symptom control.16
Although ISM is currently considered an incurable chronic condition,6 this case illustrates that symptomatic management is possible, even in cases of long-standing, severe disease. Patients should still be encouraged to avoid triggering factors and be vigilant in preventing potential anaphylaxis. However, NB-UVB phototherapy provides a supplemental or alternative treatment choice when other therapies have failed. We hope that the success of NB-UVB demonstrated in this case provides further evidence that this light-based therapy is a valuable treatment option in mastocytosis patients with unremitting or poorly controlled symptoms.
- Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2012.
- Brazzelli V, Grasso V, Manna G, et al. Indolent systemic mastocytosis treated with narrow-band UVB phototherapy: study of five cases [published online May 13, 2011]. J Eur Acad Dermatol Venereol. 2012;26:465-469.
- Pardanani A, Lim KH, Lasho TL, et al. WHO subvariants of indolent mastocytosis: clinical details and prognostic evaluation in 159 consecutive adults. Blood. 2010;115:150-151.
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes [published online April 8, 2009]. Blood. 2009;114:937-951.
- Wolff K, Komar M, Petzelbauer P. Clinical and histopathological aspects of cutaneous mastocytosis. Leuk Res. 2001;25:519-528.
- Marone G, Spadaro G, Granata F, et al. Treatment of mastocytosis: pharmacologic basis and current concepts. Leuk Res. 2001;25:583-594.
- Pardanani A. How I treat patients with indolent and smoldering mastocytosis (rare conditions but difficult to manage)[published online February 20, 2013]. Blood. 2013;121:3085-3094.
- Hartmann K, Henz BM. Mastocytosis: recent advances in defining the disease. Br J Dermatol. 2001;144:682-695.
- Vega-Ruiz A, Cortes JE, Sever M, et al. Phase II study of imatinib mesylate as therapy for patients with systemic mastocytosis. Leuk Res. 2009;33:1481-1484.
- Lortholary O, Chandesris MO, Bulai Livideanu C, et al. Masitinib for treatment of severely symptomatic indolent systemic mastocytosis: a randomised, placebo-controlled, phase 3 study. Lancet. 2017;389:612-620.
- Godt O, Proksch E, Streit V, et al. Short-and long-term effectiveness of oral and bath PUVA therapy in urticaria pigmentosa and systemic mastocytosis. Dermatology. 1997;1:35-39.
- Berroeta L, Clark C, Ibbotson SH, et al. Narrow-band (TL-01) ultraviolet B phototherapy for chronic urticaria. Clin Exp Dermatol. 2004;29:91-99.
- Engin B, Ozdemir M, Balevi A, et al. Treatment of chronic urticaria with narrowband ultraviolet B phototherapy: a randomized controlled trial. Acta Derm Venereol. 2008;3:247-251.
- Meduri NB, Vandergriff T, Rasmussen H, et al. Phototherapy in the management of atopic dermatitis: a systemic review. Photodermatol Photoimmunol Photomed. 2007;23:106-112.
- Nguyen T, Gattu S, Pugashetti R, et al. Practice of phototherapy in the treatment of moderate-to severe psoriasis. Curr Probl Dermatol. 2009;38:59-78.
- Brazzelli V, Grassi S, Merante S, et al. Narrow-band UVB phototherapy and psoralen-ultraviolet A photochemotherapy in the treatment of cutaneous mastocytosis: a study in 20 patients. Photodermatol Photoimmunol Photomed. 2016;32:238-246.
- Prignano F, Troiano M, Lotti T. Cutaneous mastocytosis: successful treatment with narrowband ultraviolet B phototherapy. Clin Exp Dermatol. 2010;35:914-915.
Systemic mastocytosis is a heterogeneous disorder of stem cell origin defined by abnormal hyperplasia and accumulation of mast cells (MCs) in one or more tissues.1,2 The most commonly affected tissues are the bone marrow, gastrointestinal tract, and skin. Based on a number of major and minor criteria defined by the World Health Organization (WHO), the mastocytoses are subdivided into 7 variants that range from isolated cutaneous involvement to widespread systemic disease.1-4 The most frequently diagnosed subtype is indolent systemic mastocytosis (ISM), a chronic disorder characterized by diffuse cutaneous macules and papules as well as bone marrow involvement in the form of multifocal dense infiltrates of MCs that frequently are phenotypically positive for c-KIT and tryptase. Serum tryptase levels are nearly invariably elevated in patients with this condition.1,2
Symptoms of ISM are determined by the intermittent release of histamine and leukotrienes from hyperproliferating MCs as well as IL-6 and eosinophil chemotactic factors. As the burden of MC secretory products increases, patients experience worsening pruritus, flushing, palpitations, vomiting, and anaphylaxis in severe instances.1,2,5 The mainstay of treatment of this condition involves symptom control through the inhibition of MC mediators.1 The majority of patients respond well to antihistamines, antileukotriene agents, and oral corticosteroids during severe episodes of MC degranulation.1,2,5
Unfortunately, some patients are unable to achieve adequate symptom control through the use of mediator-targeting treatments alone. In these cases, physicians often are faced with the following treatment dilemma: Either attempt to use therapies such as interferon alfa, which is cytoreductive to MCs, or 2-chlorodeoxyadenosine to reduce the overall MC burden, or turn to newer nonimmunosuppressive second-line options. We present the case of a patient with chronic ISM with progressive cutaneous lesions and poorly controlled pruritus that was previously managed with topical corticosteroids and antihistamines who responded favorably to treatment with narrowband UVB (NB-UVB) phototherapy.
Case Report
A 57-year-old woman presented with a 10-year history of widespread red-brown macules and papules on the trunk and upper and lower extremities. The lesions were intermittently pruritic, a symptom that was exacerbated on sun and heat exposure. A skin biopsy performed by an outside dermatologist 9 years prior confirmed the presence of mastocytosis. The patient was originally treated with triamcinolone cream and oral antihistamines, which controlled her symptoms successfully for nearly a decade.
At the current presentation, the patient reported increasingly severe pruritus and lesional spread to the neck and face of 15 months’ duration. She denied any symptoms of flushing, diarrhea, syncopal episodes, or lightheadedness. Physical examination revealed a well-appearing middle-aged woman with multiple 3- to 8-mm, red-brown, blanchable macules and papules with areas coalescing into plaques that primarily involved the legs (Figure 1A); arms; back; and to a lesser extent the abdomen, neck, and face. There was no palpable lymphadenopathy.

Laboratory results revealed a complete blood cell count and basic metabolic profile within reference range; however, the serum tryptase level was elevated at 65 ng/mL (reference range, <11.4 ng/mL). A positron emission tomography–computed tomography scan was negative, as well as a c-KIT mutation analysis. A review of the skin biopsy from 9 years prior demonstrated slight acanthosis with dermal proliferation of mononuclear cells (Figure 2A), some of which had abundant cytoplasm and oval-shaped nuclei. There were few eosinophils and marked dermal telangiectasias. Giemsa stain revealed increased numbers of MCs in the upper dermis (Figure 2B). A bone marrow biopsy performed 9 years later showed multifocal lesions composed of MCs with associated lymphoid aggregates without notable myelodyspoiesis (or myeloproliferative neoplasm). These features were all consistent with WHO criteria for ISM. Based on the most current clinical, laboratory, and histopathologic findings, the patient was diagnosed with category IB ISM.

The patient’s symptoms had remained stable for 9 years with a regimen of triamcinolone cream 0.1% twice daily, doxepin cream 5% daily as needed, and oral fexofenadine 180 mg once daily. The patient continues to use topical steroids and oral antihistamines. Due to inadequate symptom control, breakthrough pruritus, and the development of new skin lesions on the head and neck, she was started on NB-UVB treatment 2 months after presentation. The patient’s symptoms and the extent of cutaneous maculopapular lesions improved after 20 light treatments (Figure 1B), with even more dramatic results after 40 cycles of therapy (Figure 1C). Overall, the lower legs have proved most recalcitrant to this treatment modality. She is currently continuing to receive NB-UVB treatment twice weekly.
Comment
Systemic mastocytosis is a heterogeneous disorder characterized by the proliferation and accumulation of atypical MCs in tissues, principally in the bone marrow and skin, though involvement of the gastrointestinal tract, liver, spleen, and lymphatic system also have been reported.1,2,6 The WHO classification of mastocytosis divides this condition into 7 subtypes.4 Indolent systemic mastocytosis is the most common variant.2,6 The etiology of ISM is not fully understood, but there is evidence suggesting that an activating mutation of KIT proto-oncogene receptor tyrosine kinase, KIT (usually D816V), present in the MCs of nearly 80% of patients with ISM may be involved.1,3-5,7 Patients occasionally present with predominantly cutaneous findings but typically seek medical attention due to the recurrent systemic symptoms of the disease (eg, pruritus, flushing, syncope, palpitations, headache, dyspepsia, vomiting, diarrhea), which are related to the release of MC mediators.1,2
The management of ISM is complex and based primarily on symptom reduction without alteration of disease course.1,2,5,7 Patients should avoid symptom triggers such as heat, humidity, emotional and physical stress, alcohol, and certain medications (ie, aspirin, opioids, radiocontrast agents).7 Patients are initially treated with histamine H1- and H2-receptor antagonists to alleviate MC mediator release symptoms.1,2,8 Although H1 blockers are most effective in mitigating cutaneous symptoms and limiting pruritus, H2 blockers are used to control gastric hypersecretion and dyspepsia.2 Proton pump inhibitors are useful in patients with peptic ulcer disease who are unresponsive to H2-receptor antagonist therapy.2,7 Cromolyn sodium and ketotifen fumarate are MC stabilizers that help prevent degranulation, which is helpful in relieving most major ISM symptoms. Leukotriene antagonists, such as zafirlukast, montelukast sodium, or zileuton, also may be employed to target the proinflammatory and pruritogenic leukotrienes, also products of the MC protein.2,7 Imatinib mesylate and masitinib mesylate, both tyrosine kinase inhibitors, have been shown to improve symptoms and reduce MC mediator levels in ISM; however, most patients harbor the resistant KIT D816V mutation, which limits the utility of this medication.Patients with sensitive KIT mutations or those who have the wild-type KIT D816 mutation may be more appropriate candidates for imatinib or masitinib therapy, which can ameliorate symptoms of flushing, pruritus, and depression.7-10 Treatment with omalizumab, a humanized murine anti-IgE monoclonal antibody, can be effective in treating recurrent, treatment-refractory anaphylaxis in ISM patients.5,7
Symptoms unresponsive to these therapies can be effectively treated with a short course of oral corticosteroids,6,7 while MC cytoreductive therapies such as interferon alfa or 2-chlorodeoxyadenosine (cladribine/2-CdA) are reserved for refractory cases.2,7 Alternative therapies such as NB-UVB2 or psoralen plus UVA phototherapy11 also have demonstrated success in treating ISM symptoms. In the past, NB-UVB has shown efficacy in controlling pruriginous conditions ranging from chronic urticaria12,13 to atopic dermatitis14 to psoriasis.15 This evidence has spurred studies to evaluate if NB-UVB has a role in the management of uncontrolled cases of cutaneous and ISM.2,13,16,17 To date, the evidence has been promising. The majority of patients treated with this regimen report subjective reduction in pruritus in addition to clinical cutaneous disease burden.2,11 Also, laboratory analysis demonstrates decreased levels of tryptase in patients utilizing NB-UVB phototherapy.2 Thus far, the use of NB-UVB phototherapy in the treatment of pruriginous disorders such as ISM has not been associated with any severe side effects such as increased rates of anaphylaxis, though some research has suggested that this therapy may lower the threshold for patients to develop symptomatic dermographism.12 Overall, patients treated with NB-UVB phototherapy report improved quality of life related to more effective symptom control.16
Although ISM is currently considered an incurable chronic condition,6 this case illustrates that symptomatic management is possible, even in cases of long-standing, severe disease. Patients should still be encouraged to avoid triggering factors and be vigilant in preventing potential anaphylaxis. However, NB-UVB phototherapy provides a supplemental or alternative treatment choice when other therapies have failed. We hope that the success of NB-UVB demonstrated in this case provides further evidence that this light-based therapy is a valuable treatment option in mastocytosis patients with unremitting or poorly controlled symptoms.
Systemic mastocytosis is a heterogeneous disorder of stem cell origin defined by abnormal hyperplasia and accumulation of mast cells (MCs) in one or more tissues.1,2 The most commonly affected tissues are the bone marrow, gastrointestinal tract, and skin. Based on a number of major and minor criteria defined by the World Health Organization (WHO), the mastocytoses are subdivided into 7 variants that range from isolated cutaneous involvement to widespread systemic disease.1-4 The most frequently diagnosed subtype is indolent systemic mastocytosis (ISM), a chronic disorder characterized by diffuse cutaneous macules and papules as well as bone marrow involvement in the form of multifocal dense infiltrates of MCs that frequently are phenotypically positive for c-KIT and tryptase. Serum tryptase levels are nearly invariably elevated in patients with this condition.1,2
Symptoms of ISM are determined by the intermittent release of histamine and leukotrienes from hyperproliferating MCs as well as IL-6 and eosinophil chemotactic factors. As the burden of MC secretory products increases, patients experience worsening pruritus, flushing, palpitations, vomiting, and anaphylaxis in severe instances.1,2,5 The mainstay of treatment of this condition involves symptom control through the inhibition of MC mediators.1 The majority of patients respond well to antihistamines, antileukotriene agents, and oral corticosteroids during severe episodes of MC degranulation.1,2,5
Unfortunately, some patients are unable to achieve adequate symptom control through the use of mediator-targeting treatments alone. In these cases, physicians often are faced with the following treatment dilemma: Either attempt to use therapies such as interferon alfa, which is cytoreductive to MCs, or 2-chlorodeoxyadenosine to reduce the overall MC burden, or turn to newer nonimmunosuppressive second-line options. We present the case of a patient with chronic ISM with progressive cutaneous lesions and poorly controlled pruritus that was previously managed with topical corticosteroids and antihistamines who responded favorably to treatment with narrowband UVB (NB-UVB) phototherapy.
Case Report
A 57-year-old woman presented with a 10-year history of widespread red-brown macules and papules on the trunk and upper and lower extremities. The lesions were intermittently pruritic, a symptom that was exacerbated on sun and heat exposure. A skin biopsy performed by an outside dermatologist 9 years prior confirmed the presence of mastocytosis. The patient was originally treated with triamcinolone cream and oral antihistamines, which controlled her symptoms successfully for nearly a decade.
At the current presentation, the patient reported increasingly severe pruritus and lesional spread to the neck and face of 15 months’ duration. She denied any symptoms of flushing, diarrhea, syncopal episodes, or lightheadedness. Physical examination revealed a well-appearing middle-aged woman with multiple 3- to 8-mm, red-brown, blanchable macules and papules with areas coalescing into plaques that primarily involved the legs (Figure 1A); arms; back; and to a lesser extent the abdomen, neck, and face. There was no palpable lymphadenopathy.

Laboratory results revealed a complete blood cell count and basic metabolic profile within reference range; however, the serum tryptase level was elevated at 65 ng/mL (reference range, <11.4 ng/mL). A positron emission tomography–computed tomography scan was negative, as well as a c-KIT mutation analysis. A review of the skin biopsy from 9 years prior demonstrated slight acanthosis with dermal proliferation of mononuclear cells (Figure 2A), some of which had abundant cytoplasm and oval-shaped nuclei. There were few eosinophils and marked dermal telangiectasias. Giemsa stain revealed increased numbers of MCs in the upper dermis (Figure 2B). A bone marrow biopsy performed 9 years later showed multifocal lesions composed of MCs with associated lymphoid aggregates without notable myelodyspoiesis (or myeloproliferative neoplasm). These features were all consistent with WHO criteria for ISM. Based on the most current clinical, laboratory, and histopathologic findings, the patient was diagnosed with category IB ISM.

The patient’s symptoms had remained stable for 9 years with a regimen of triamcinolone cream 0.1% twice daily, doxepin cream 5% daily as needed, and oral fexofenadine 180 mg once daily. The patient continues to use topical steroids and oral antihistamines. Due to inadequate symptom control, breakthrough pruritus, and the development of new skin lesions on the head and neck, she was started on NB-UVB treatment 2 months after presentation. The patient’s symptoms and the extent of cutaneous maculopapular lesions improved after 20 light treatments (Figure 1B), with even more dramatic results after 40 cycles of therapy (Figure 1C). Overall, the lower legs have proved most recalcitrant to this treatment modality. She is currently continuing to receive NB-UVB treatment twice weekly.
Comment
Systemic mastocytosis is a heterogeneous disorder characterized by the proliferation and accumulation of atypical MCs in tissues, principally in the bone marrow and skin, though involvement of the gastrointestinal tract, liver, spleen, and lymphatic system also have been reported.1,2,6 The WHO classification of mastocytosis divides this condition into 7 subtypes.4 Indolent systemic mastocytosis is the most common variant.2,6 The etiology of ISM is not fully understood, but there is evidence suggesting that an activating mutation of KIT proto-oncogene receptor tyrosine kinase, KIT (usually D816V), present in the MCs of nearly 80% of patients with ISM may be involved.1,3-5,7 Patients occasionally present with predominantly cutaneous findings but typically seek medical attention due to the recurrent systemic symptoms of the disease (eg, pruritus, flushing, syncope, palpitations, headache, dyspepsia, vomiting, diarrhea), which are related to the release of MC mediators.1,2
The management of ISM is complex and based primarily on symptom reduction without alteration of disease course.1,2,5,7 Patients should avoid symptom triggers such as heat, humidity, emotional and physical stress, alcohol, and certain medications (ie, aspirin, opioids, radiocontrast agents).7 Patients are initially treated with histamine H1- and H2-receptor antagonists to alleviate MC mediator release symptoms.1,2,8 Although H1 blockers are most effective in mitigating cutaneous symptoms and limiting pruritus, H2 blockers are used to control gastric hypersecretion and dyspepsia.2 Proton pump inhibitors are useful in patients with peptic ulcer disease who are unresponsive to H2-receptor antagonist therapy.2,7 Cromolyn sodium and ketotifen fumarate are MC stabilizers that help prevent degranulation, which is helpful in relieving most major ISM symptoms. Leukotriene antagonists, such as zafirlukast, montelukast sodium, or zileuton, also may be employed to target the proinflammatory and pruritogenic leukotrienes, also products of the MC protein.2,7 Imatinib mesylate and masitinib mesylate, both tyrosine kinase inhibitors, have been shown to improve symptoms and reduce MC mediator levels in ISM; however, most patients harbor the resistant KIT D816V mutation, which limits the utility of this medication.Patients with sensitive KIT mutations or those who have the wild-type KIT D816 mutation may be more appropriate candidates for imatinib or masitinib therapy, which can ameliorate symptoms of flushing, pruritus, and depression.7-10 Treatment with omalizumab, a humanized murine anti-IgE monoclonal antibody, can be effective in treating recurrent, treatment-refractory anaphylaxis in ISM patients.5,7
Symptoms unresponsive to these therapies can be effectively treated with a short course of oral corticosteroids,6,7 while MC cytoreductive therapies such as interferon alfa or 2-chlorodeoxyadenosine (cladribine/2-CdA) are reserved for refractory cases.2,7 Alternative therapies such as NB-UVB2 or psoralen plus UVA phototherapy11 also have demonstrated success in treating ISM symptoms. In the past, NB-UVB has shown efficacy in controlling pruriginous conditions ranging from chronic urticaria12,13 to atopic dermatitis14 to psoriasis.15 This evidence has spurred studies to evaluate if NB-UVB has a role in the management of uncontrolled cases of cutaneous and ISM.2,13,16,17 To date, the evidence has been promising. The majority of patients treated with this regimen report subjective reduction in pruritus in addition to clinical cutaneous disease burden.2,11 Also, laboratory analysis demonstrates decreased levels of tryptase in patients utilizing NB-UVB phototherapy.2 Thus far, the use of NB-UVB phototherapy in the treatment of pruriginous disorders such as ISM has not been associated with any severe side effects such as increased rates of anaphylaxis, though some research has suggested that this therapy may lower the threshold for patients to develop symptomatic dermographism.12 Overall, patients treated with NB-UVB phototherapy report improved quality of life related to more effective symptom control.16
Although ISM is currently considered an incurable chronic condition,6 this case illustrates that symptomatic management is possible, even in cases of long-standing, severe disease. Patients should still be encouraged to avoid triggering factors and be vigilant in preventing potential anaphylaxis. However, NB-UVB phototherapy provides a supplemental or alternative treatment choice when other therapies have failed. We hope that the success of NB-UVB demonstrated in this case provides further evidence that this light-based therapy is a valuable treatment option in mastocytosis patients with unremitting or poorly controlled symptoms.
- Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2012.
- Brazzelli V, Grasso V, Manna G, et al. Indolent systemic mastocytosis treated with narrow-band UVB phototherapy: study of five cases [published online May 13, 2011]. J Eur Acad Dermatol Venereol. 2012;26:465-469.
- Pardanani A, Lim KH, Lasho TL, et al. WHO subvariants of indolent mastocytosis: clinical details and prognostic evaluation in 159 consecutive adults. Blood. 2010;115:150-151.
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes [published online April 8, 2009]. Blood. 2009;114:937-951.
- Wolff K, Komar M, Petzelbauer P. Clinical and histopathological aspects of cutaneous mastocytosis. Leuk Res. 2001;25:519-528.
- Marone G, Spadaro G, Granata F, et al. Treatment of mastocytosis: pharmacologic basis and current concepts. Leuk Res. 2001;25:583-594.
- Pardanani A. How I treat patients with indolent and smoldering mastocytosis (rare conditions but difficult to manage)[published online February 20, 2013]. Blood. 2013;121:3085-3094.
- Hartmann K, Henz BM. Mastocytosis: recent advances in defining the disease. Br J Dermatol. 2001;144:682-695.
- Vega-Ruiz A, Cortes JE, Sever M, et al. Phase II study of imatinib mesylate as therapy for patients with systemic mastocytosis. Leuk Res. 2009;33:1481-1484.
- Lortholary O, Chandesris MO, Bulai Livideanu C, et al. Masitinib for treatment of severely symptomatic indolent systemic mastocytosis: a randomised, placebo-controlled, phase 3 study. Lancet. 2017;389:612-620.
- Godt O, Proksch E, Streit V, et al. Short-and long-term effectiveness of oral and bath PUVA therapy in urticaria pigmentosa and systemic mastocytosis. Dermatology. 1997;1:35-39.
- Berroeta L, Clark C, Ibbotson SH, et al. Narrow-band (TL-01) ultraviolet B phototherapy for chronic urticaria. Clin Exp Dermatol. 2004;29:91-99.
- Engin B, Ozdemir M, Balevi A, et al. Treatment of chronic urticaria with narrowband ultraviolet B phototherapy: a randomized controlled trial. Acta Derm Venereol. 2008;3:247-251.
- Meduri NB, Vandergriff T, Rasmussen H, et al. Phototherapy in the management of atopic dermatitis: a systemic review. Photodermatol Photoimmunol Photomed. 2007;23:106-112.
- Nguyen T, Gattu S, Pugashetti R, et al. Practice of phototherapy in the treatment of moderate-to severe psoriasis. Curr Probl Dermatol. 2009;38:59-78.
- Brazzelli V, Grassi S, Merante S, et al. Narrow-band UVB phototherapy and psoralen-ultraviolet A photochemotherapy in the treatment of cutaneous mastocytosis: a study in 20 patients. Photodermatol Photoimmunol Photomed. 2016;32:238-246.
- Prignano F, Troiano M, Lotti T. Cutaneous mastocytosis: successful treatment with narrowband ultraviolet B phototherapy. Clin Exp Dermatol. 2010;35:914-915.
- Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2012.
- Brazzelli V, Grasso V, Manna G, et al. Indolent systemic mastocytosis treated with narrow-band UVB phototherapy: study of five cases [published online May 13, 2011]. J Eur Acad Dermatol Venereol. 2012;26:465-469.
- Pardanani A, Lim KH, Lasho TL, et al. WHO subvariants of indolent mastocytosis: clinical details and prognostic evaluation in 159 consecutive adults. Blood. 2010;115:150-151.
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes [published online April 8, 2009]. Blood. 2009;114:937-951.
- Wolff K, Komar M, Petzelbauer P. Clinical and histopathological aspects of cutaneous mastocytosis. Leuk Res. 2001;25:519-528.
- Marone G, Spadaro G, Granata F, et al. Treatment of mastocytosis: pharmacologic basis and current concepts. Leuk Res. 2001;25:583-594.
- Pardanani A. How I treat patients with indolent and smoldering mastocytosis (rare conditions but difficult to manage)[published online February 20, 2013]. Blood. 2013;121:3085-3094.
- Hartmann K, Henz BM. Mastocytosis: recent advances in defining the disease. Br J Dermatol. 2001;144:682-695.
- Vega-Ruiz A, Cortes JE, Sever M, et al. Phase II study of imatinib mesylate as therapy for patients with systemic mastocytosis. Leuk Res. 2009;33:1481-1484.
- Lortholary O, Chandesris MO, Bulai Livideanu C, et al. Masitinib for treatment of severely symptomatic indolent systemic mastocytosis: a randomised, placebo-controlled, phase 3 study. Lancet. 2017;389:612-620.
- Godt O, Proksch E, Streit V, et al. Short-and long-term effectiveness of oral and bath PUVA therapy in urticaria pigmentosa and systemic mastocytosis. Dermatology. 1997;1:35-39.
- Berroeta L, Clark C, Ibbotson SH, et al. Narrow-band (TL-01) ultraviolet B phototherapy for chronic urticaria. Clin Exp Dermatol. 2004;29:91-99.
- Engin B, Ozdemir M, Balevi A, et al. Treatment of chronic urticaria with narrowband ultraviolet B phototherapy: a randomized controlled trial. Acta Derm Venereol. 2008;3:247-251.
- Meduri NB, Vandergriff T, Rasmussen H, et al. Phototherapy in the management of atopic dermatitis: a systemic review. Photodermatol Photoimmunol Photomed. 2007;23:106-112.
- Nguyen T, Gattu S, Pugashetti R, et al. Practice of phototherapy in the treatment of moderate-to severe psoriasis. Curr Probl Dermatol. 2009;38:59-78.
- Brazzelli V, Grassi S, Merante S, et al. Narrow-band UVB phototherapy and psoralen-ultraviolet A photochemotherapy in the treatment of cutaneous mastocytosis: a study in 20 patients. Photodermatol Photoimmunol Photomed. 2016;32:238-246.
- Prignano F, Troiano M, Lotti T. Cutaneous mastocytosis: successful treatment with narrowband ultraviolet B phototherapy. Clin Exp Dermatol. 2010;35:914-915.
Practice Points
- Patients with cutaneous lesions and symptoms consistent with mastocytosis should be worked up for potential systemic involvement.
- Symptoms of indolent systemic mastocytosis (ISM) include pruritus, flushing, palpitations, vomiting, and anaphylaxis in severe instances.
- Most patients respond well to antihistamines, antileukotriene agents, and oral corticosteroids during severe episodes of mast cell degranulation.
- Narrowband UVB is a safe, effective, and well-tolerated treatment option for symptom control in refractory ISM cases.
Session to Spotlight Key Research Papers
VAM attendees have the opportunity to learn about several medical journal articles of relevance to their vascular surgery practice during a session on Saturday.
Entitled “Beyond the Journal of Vascular Surgery: ‘Top Ten’ Papers Relevant to Vascular Surgery,” the session “serves to inform VAM attendees on important literature outside of our core vascular surgery journals that might be missed in a busy practice,” said session co-moderator Ellen Dillavou, MD, a vascular surgeon in the department of surgery at Duke University Medical Center, Durham, N.C. “Additionally, the articles are discussed by experts in our field, providing a clinical context for interpretation.”
The session’s other co-moderator is Jon S. Matsumura, MD, professor and chairman of the division of vascular surgery at the University of Wisconsin School of Medicine and Public Health, Madison, Wis.
The moderators reported having no financial disclosures.
Saturday, June 3
10:30 a.m. - 12:00 p.m.
SDCC, Room 6 A/B
F2: Beyond the Journal of Vascular Surgery: “Top Ten” Papers Relevant to Vascular Surgery
VAM attendees have the opportunity to learn about several medical journal articles of relevance to their vascular surgery practice during a session on Saturday.
Entitled “Beyond the Journal of Vascular Surgery: ‘Top Ten’ Papers Relevant to Vascular Surgery,” the session “serves to inform VAM attendees on important literature outside of our core vascular surgery journals that might be missed in a busy practice,” said session co-moderator Ellen Dillavou, MD, a vascular surgeon in the department of surgery at Duke University Medical Center, Durham, N.C. “Additionally, the articles are discussed by experts in our field, providing a clinical context for interpretation.”
The session’s other co-moderator is Jon S. Matsumura, MD, professor and chairman of the division of vascular surgery at the University of Wisconsin School of Medicine and Public Health, Madison, Wis.
The moderators reported having no financial disclosures.
Saturday, June 3
10:30 a.m. - 12:00 p.m.
SDCC, Room 6 A/B
F2: Beyond the Journal of Vascular Surgery: “Top Ten” Papers Relevant to Vascular Surgery
VAM attendees have the opportunity to learn about several medical journal articles of relevance to their vascular surgery practice during a session on Saturday.
Entitled “Beyond the Journal of Vascular Surgery: ‘Top Ten’ Papers Relevant to Vascular Surgery,” the session “serves to inform VAM attendees on important literature outside of our core vascular surgery journals that might be missed in a busy practice,” said session co-moderator Ellen Dillavou, MD, a vascular surgeon in the department of surgery at Duke University Medical Center, Durham, N.C. “Additionally, the articles are discussed by experts in our field, providing a clinical context for interpretation.”
The session’s other co-moderator is Jon S. Matsumura, MD, professor and chairman of the division of vascular surgery at the University of Wisconsin School of Medicine and Public Health, Madison, Wis.
The moderators reported having no financial disclosures.
Saturday, June 3
10:30 a.m. - 12:00 p.m.
SDCC, Room 6 A/B
F2: Beyond the Journal of Vascular Surgery: “Top Ten” Papers Relevant to Vascular Surgery
How the Team Approach Can Drive a High-Performance Vascular Practice
On Saturday, June 3, at 10:30 a.m., vascular surgeons and other members of the vascular care team will come together for a session entitled “Building the Vascular Team – Evolving Collaboration of Surgeons and Nurses.”
This session, run jointly by the Society for Vascular Surgery and the Society for Vascular Nursing, will offer attendees “advice and information about how best to value your team, form your team, educate your team, and utilize your team,” said co-moderator Kellie R. Brown, MD, of the Medical College of Wisconsin, Milwaukee.
Yaron Sternbach, MD, and Marie Rossi, RN, BS, both of The Vascular Group, PLLC, in Albany, N. Y., will give the physician and the nursing perspective on “Defining the High-Performing Vascular Practice,” with an emphasis on forming and maintaining a high-performing team, said Dr. Brown.
In turn, Dr. Brown and co-moderator Tiffany K. Street, MSN, ACNP-BC, of Vanderbilt University School of Nursing will give the physician and the advanced practice provider’s perspective on integrating advanced practice providers into a vascular practice.
Dr. Brown said that a vascular surgeon can really benefit from understanding “how best to have the nursing and advanced practice members of your team really work to the top of their licenses.” This, she said, is when a high-performance vascular team really starts working well: “You have a whole team contributing to the care of the patient. The surgeon’s doing surgery, the nurse is doing nursing, and the advanced practice providers are complementing the surgeon’s work in pre- and post-op care.”
Ms. Street agreed. “From a physician’s perspective, we all have unique roles and all have something to contribute toward the care of the patient. How do we get the right people in the right positions, doing the right type of work?” This, she said, will be a key take-away from the session.
Ms. Rossi, also a co-moderator, will speak in more detail about vascular specialty nursing, in a session entitled, “The Case for Vascular Specialty Nursing: What We Know and What Remains to be Clarified.” In another session, attendees will hear from Kathy Rich, PhD, a vascular nurse in Chesterton, Ind. Dr. Rich sits on the editorial board of the Journal of Vascular Nursing; her presentation is entitled “Expanding the Knowledge Base: Training Paradigms in Vascular Nursing.”
The morning’s presentations will be rounded out by Ms. Street, who will give a nuts-and-bolts presentation entitled, “Integrating Nursing Roles into Daily Practice.” Here, she plans to touch on some of the realities of creating and maintaining a high-performing vascular team. These include scope of practice, competencies, compliance, and billing perspectives, all of which can be perceived hurdles to integrating nurse specialists and advanced practice providers into a vascular practice.
Ms. Street will also provide information about potential career paths within vascular nursing in a presentation entitled “Careers in Vascular Nursing: Opportunities for Growth.”
The joint session, said Dr. Brown, represents “an exciting direction” for the Society for Vascular Surgery. “There will be more opportunities for collaboration between individuals in our two career paths,” as the use of advanced practice providers and specialty nurses grows within vascular surgery.
Ms. Street agreed, adding that the team approach doesn’t negate the surgeon’s role. “Surgery can’t be surgery without surgeons. It’s just about using people in the right positions to take the best care of patients,” she said.
Dr. Brown said that the goal of the team approach in vascular surgery is to achieve both high-quality and efficient care; reaching this mark is an especially relevant goal today, since “the need is going to keep rising, and we can’t train vascular surgeons fast enough,” she said. The team approach is a way to have the vascular surgeon care for more patients as demand soars. “In this era of declining reimbursement, we all need to be more efficient,” said Dr. Brown.
Dr. Brown reported being an investigator for the LEOPARD trial. Ms. Street reported no relevant financial relationships.
Saturday, June 3
10:30 a.m. – 12:00 p.m. SDCC, Room 3
C13: Building the Vascular Team
On Saturday, June 3, at 10:30 a.m., vascular surgeons and other members of the vascular care team will come together for a session entitled “Building the Vascular Team – Evolving Collaboration of Surgeons and Nurses.”
This session, run jointly by the Society for Vascular Surgery and the Society for Vascular Nursing, will offer attendees “advice and information about how best to value your team, form your team, educate your team, and utilize your team,” said co-moderator Kellie R. Brown, MD, of the Medical College of Wisconsin, Milwaukee.
Yaron Sternbach, MD, and Marie Rossi, RN, BS, both of The Vascular Group, PLLC, in Albany, N. Y., will give the physician and the nursing perspective on “Defining the High-Performing Vascular Practice,” with an emphasis on forming and maintaining a high-performing team, said Dr. Brown.
In turn, Dr. Brown and co-moderator Tiffany K. Street, MSN, ACNP-BC, of Vanderbilt University School of Nursing will give the physician and the advanced practice provider’s perspective on integrating advanced practice providers into a vascular practice.
Dr. Brown said that a vascular surgeon can really benefit from understanding “how best to have the nursing and advanced practice members of your team really work to the top of their licenses.” This, she said, is when a high-performance vascular team really starts working well: “You have a whole team contributing to the care of the patient. The surgeon’s doing surgery, the nurse is doing nursing, and the advanced practice providers are complementing the surgeon’s work in pre- and post-op care.”
Ms. Street agreed. “From a physician’s perspective, we all have unique roles and all have something to contribute toward the care of the patient. How do we get the right people in the right positions, doing the right type of work?” This, she said, will be a key take-away from the session.
Ms. Rossi, also a co-moderator, will speak in more detail about vascular specialty nursing, in a session entitled, “The Case for Vascular Specialty Nursing: What We Know and What Remains to be Clarified.” In another session, attendees will hear from Kathy Rich, PhD, a vascular nurse in Chesterton, Ind. Dr. Rich sits on the editorial board of the Journal of Vascular Nursing; her presentation is entitled “Expanding the Knowledge Base: Training Paradigms in Vascular Nursing.”
The morning’s presentations will be rounded out by Ms. Street, who will give a nuts-and-bolts presentation entitled, “Integrating Nursing Roles into Daily Practice.” Here, she plans to touch on some of the realities of creating and maintaining a high-performing vascular team. These include scope of practice, competencies, compliance, and billing perspectives, all of which can be perceived hurdles to integrating nurse specialists and advanced practice providers into a vascular practice.
Ms. Street will also provide information about potential career paths within vascular nursing in a presentation entitled “Careers in Vascular Nursing: Opportunities for Growth.”
The joint session, said Dr. Brown, represents “an exciting direction” for the Society for Vascular Surgery. “There will be more opportunities for collaboration between individuals in our two career paths,” as the use of advanced practice providers and specialty nurses grows within vascular surgery.
Ms. Street agreed, adding that the team approach doesn’t negate the surgeon’s role. “Surgery can’t be surgery without surgeons. It’s just about using people in the right positions to take the best care of patients,” she said.
Dr. Brown said that the goal of the team approach in vascular surgery is to achieve both high-quality and efficient care; reaching this mark is an especially relevant goal today, since “the need is going to keep rising, and we can’t train vascular surgeons fast enough,” she said. The team approach is a way to have the vascular surgeon care for more patients as demand soars. “In this era of declining reimbursement, we all need to be more efficient,” said Dr. Brown.
Dr. Brown reported being an investigator for the LEOPARD trial. Ms. Street reported no relevant financial relationships.
Saturday, June 3
10:30 a.m. – 12:00 p.m. SDCC, Room 3
C13: Building the Vascular Team
On Saturday, June 3, at 10:30 a.m., vascular surgeons and other members of the vascular care team will come together for a session entitled “Building the Vascular Team – Evolving Collaboration of Surgeons and Nurses.”
This session, run jointly by the Society for Vascular Surgery and the Society for Vascular Nursing, will offer attendees “advice and information about how best to value your team, form your team, educate your team, and utilize your team,” said co-moderator Kellie R. Brown, MD, of the Medical College of Wisconsin, Milwaukee.
Yaron Sternbach, MD, and Marie Rossi, RN, BS, both of The Vascular Group, PLLC, in Albany, N. Y., will give the physician and the nursing perspective on “Defining the High-Performing Vascular Practice,” with an emphasis on forming and maintaining a high-performing team, said Dr. Brown.
In turn, Dr. Brown and co-moderator Tiffany K. Street, MSN, ACNP-BC, of Vanderbilt University School of Nursing will give the physician and the advanced practice provider’s perspective on integrating advanced practice providers into a vascular practice.
Dr. Brown said that a vascular surgeon can really benefit from understanding “how best to have the nursing and advanced practice members of your team really work to the top of their licenses.” This, she said, is when a high-performance vascular team really starts working well: “You have a whole team contributing to the care of the patient. The surgeon’s doing surgery, the nurse is doing nursing, and the advanced practice providers are complementing the surgeon’s work in pre- and post-op care.”
Ms. Street agreed. “From a physician’s perspective, we all have unique roles and all have something to contribute toward the care of the patient. How do we get the right people in the right positions, doing the right type of work?” This, she said, will be a key take-away from the session.
Ms. Rossi, also a co-moderator, will speak in more detail about vascular specialty nursing, in a session entitled, “The Case for Vascular Specialty Nursing: What We Know and What Remains to be Clarified.” In another session, attendees will hear from Kathy Rich, PhD, a vascular nurse in Chesterton, Ind. Dr. Rich sits on the editorial board of the Journal of Vascular Nursing; her presentation is entitled “Expanding the Knowledge Base: Training Paradigms in Vascular Nursing.”
The morning’s presentations will be rounded out by Ms. Street, who will give a nuts-and-bolts presentation entitled, “Integrating Nursing Roles into Daily Practice.” Here, she plans to touch on some of the realities of creating and maintaining a high-performing vascular team. These include scope of practice, competencies, compliance, and billing perspectives, all of which can be perceived hurdles to integrating nurse specialists and advanced practice providers into a vascular practice.
Ms. Street will also provide information about potential career paths within vascular nursing in a presentation entitled “Careers in Vascular Nursing: Opportunities for Growth.”
The joint session, said Dr. Brown, represents “an exciting direction” for the Society for Vascular Surgery. “There will be more opportunities for collaboration between individuals in our two career paths,” as the use of advanced practice providers and specialty nurses grows within vascular surgery.
Ms. Street agreed, adding that the team approach doesn’t negate the surgeon’s role. “Surgery can’t be surgery without surgeons. It’s just about using people in the right positions to take the best care of patients,” she said.
Dr. Brown said that the goal of the team approach in vascular surgery is to achieve both high-quality and efficient care; reaching this mark is an especially relevant goal today, since “the need is going to keep rising, and we can’t train vascular surgeons fast enough,” she said. The team approach is a way to have the vascular surgeon care for more patients as demand soars. “In this era of declining reimbursement, we all need to be more efficient,” said Dr. Brown.
Dr. Brown reported being an investigator for the LEOPARD trial. Ms. Street reported no relevant financial relationships.
Saturday, June 3
10:30 a.m. – 12:00 p.m. SDCC, Room 3
C13: Building the Vascular Team
Erenumab May Reduce Headache Days in Episodic Migraine
BOSTON—Erenumab reduces the number of migraine days per month in patients with episodic migraine, according to a study presented at the 69th Annual Meeting of the American Academy of Neurology. The treatment also reduces the use of acute migraine medication and improves function.
Erenumab is a fully human monoclonal antibody developed to block the pathway of calcitonin gene-related peptide (CGRP). The treatment showed promise in a phase II study in patients with episodic migraine and in a phase II study of patients with chronic migraine, according to Peter Goadsby, MD, PhD, Professor of Neurology at the University of California, San Francisco School of Medicine.
A Test of Two Doses
Dr. Goadsby and colleagues conducted a phase III study of 955 participants to examine whether erenumab would be an effective preventive treatment for episodic migraine. Eligible patients had between four and 14 migraine days per month and were allowed to take one concomitant migraine medication. Patients who previously had failed two preventive medications were excluded. After screening, patients underwent a four-week baseline period during which they kept electronic migraine diaries. The investigators then randomized patients in groups of equal size to placebo, 70 mg of subcutaneous erenumab, or 140 mg of subcutaneous erenumab. The treatment period lasted for six months.
As has been the case in many studies of migraine during the past 25 years, the typical patient in this study was a 41-year-old Caucasian woman, said Dr. Goadsby. Slightly more than half of participants had no previous exposure to a preventive therapy. The mean number of migraine days in all treatment groups was eight, and the mean number of headache days was nine. The treatment groups were well balanced.
Treatment Reduced Medication Use
During the last three months of treatment, the mean number of monthly migraine days decreased by 3.2 for patients receiving 70 mg of erenumab and by 3.7 for patients receiving 140 mg of erenumab, compared with 1.8 for patients receiving placebo. The difference between the active and control arms was statistically significant. The rate of participants who had a 50% reduction in migraine attacks was 26.6% for controls, 43% for patients receiving the 70-mg dose, and 50% for patients receiving the 140-mg dose.
The use of acute medicines decreased by 1.1 days for patients receiving 70 mg of erenumab and by 1.6 days for patients receiving 140 mg, compared with a decrease of 0.2 days for patients receiving placebo. Using the Migraine Physical Function Impact Diary, participants in the 70-mg group reported that the impact of migraine on everyday activities decreased by 5.5 points. Participants in the 140-mg group reported a 5.9-point reduction in this end point. Participants in the placebo group reported a 3.3-point reduction. Physical impairment decreased by 4.2 points for the 70-mg group and by 4.8 points for the 140-mg group versus 2.4 points for the placebo group.
Erenumab was well tolerated, and none of the serious adverse events appeared to be related to treatment. The most common adverse event was injection-site reactions. About 6% of the entire cohort developed antibodies to erenumab, including 8% in the 70-mg group and 3.2% in the 140-mg group. One patient receiving 70 mg had a neutralizing antibody, and no patients in the 140-mg group had neutralizing antibodies.
The study results are exciting, said Dr. Goadsby. In the future, treatments that target the CGRP pathway could be provided to migraineurs who do not respond to or cannot tolerate the current therapies, he added.
The trial was sponsored by Amgen, and Dr. Goadsby previously has consulted for the company.
—Erik Greb
BOSTON—Erenumab reduces the number of migraine days per month in patients with episodic migraine, according to a study presented at the 69th Annual Meeting of the American Academy of Neurology. The treatment also reduces the use of acute migraine medication and improves function.
Erenumab is a fully human monoclonal antibody developed to block the pathway of calcitonin gene-related peptide (CGRP). The treatment showed promise in a phase II study in patients with episodic migraine and in a phase II study of patients with chronic migraine, according to Peter Goadsby, MD, PhD, Professor of Neurology at the University of California, San Francisco School of Medicine.
A Test of Two Doses
Dr. Goadsby and colleagues conducted a phase III study of 955 participants to examine whether erenumab would be an effective preventive treatment for episodic migraine. Eligible patients had between four and 14 migraine days per month and were allowed to take one concomitant migraine medication. Patients who previously had failed two preventive medications were excluded. After screening, patients underwent a four-week baseline period during which they kept electronic migraine diaries. The investigators then randomized patients in groups of equal size to placebo, 70 mg of subcutaneous erenumab, or 140 mg of subcutaneous erenumab. The treatment period lasted for six months.
As has been the case in many studies of migraine during the past 25 years, the typical patient in this study was a 41-year-old Caucasian woman, said Dr. Goadsby. Slightly more than half of participants had no previous exposure to a preventive therapy. The mean number of migraine days in all treatment groups was eight, and the mean number of headache days was nine. The treatment groups were well balanced.
Treatment Reduced Medication Use
During the last three months of treatment, the mean number of monthly migraine days decreased by 3.2 for patients receiving 70 mg of erenumab and by 3.7 for patients receiving 140 mg of erenumab, compared with 1.8 for patients receiving placebo. The difference between the active and control arms was statistically significant. The rate of participants who had a 50% reduction in migraine attacks was 26.6% for controls, 43% for patients receiving the 70-mg dose, and 50% for patients receiving the 140-mg dose.
The use of acute medicines decreased by 1.1 days for patients receiving 70 mg of erenumab and by 1.6 days for patients receiving 140 mg, compared with a decrease of 0.2 days for patients receiving placebo. Using the Migraine Physical Function Impact Diary, participants in the 70-mg group reported that the impact of migraine on everyday activities decreased by 5.5 points. Participants in the 140-mg group reported a 5.9-point reduction in this end point. Participants in the placebo group reported a 3.3-point reduction. Physical impairment decreased by 4.2 points for the 70-mg group and by 4.8 points for the 140-mg group versus 2.4 points for the placebo group.
Erenumab was well tolerated, and none of the serious adverse events appeared to be related to treatment. The most common adverse event was injection-site reactions. About 6% of the entire cohort developed antibodies to erenumab, including 8% in the 70-mg group and 3.2% in the 140-mg group. One patient receiving 70 mg had a neutralizing antibody, and no patients in the 140-mg group had neutralizing antibodies.
The study results are exciting, said Dr. Goadsby. In the future, treatments that target the CGRP pathway could be provided to migraineurs who do not respond to or cannot tolerate the current therapies, he added.
The trial was sponsored by Amgen, and Dr. Goadsby previously has consulted for the company.
—Erik Greb
BOSTON—Erenumab reduces the number of migraine days per month in patients with episodic migraine, according to a study presented at the 69th Annual Meeting of the American Academy of Neurology. The treatment also reduces the use of acute migraine medication and improves function.
Erenumab is a fully human monoclonal antibody developed to block the pathway of calcitonin gene-related peptide (CGRP). The treatment showed promise in a phase II study in patients with episodic migraine and in a phase II study of patients with chronic migraine, according to Peter Goadsby, MD, PhD, Professor of Neurology at the University of California, San Francisco School of Medicine.
A Test of Two Doses
Dr. Goadsby and colleagues conducted a phase III study of 955 participants to examine whether erenumab would be an effective preventive treatment for episodic migraine. Eligible patients had between four and 14 migraine days per month and were allowed to take one concomitant migraine medication. Patients who previously had failed two preventive medications were excluded. After screening, patients underwent a four-week baseline period during which they kept electronic migraine diaries. The investigators then randomized patients in groups of equal size to placebo, 70 mg of subcutaneous erenumab, or 140 mg of subcutaneous erenumab. The treatment period lasted for six months.
As has been the case in many studies of migraine during the past 25 years, the typical patient in this study was a 41-year-old Caucasian woman, said Dr. Goadsby. Slightly more than half of participants had no previous exposure to a preventive therapy. The mean number of migraine days in all treatment groups was eight, and the mean number of headache days was nine. The treatment groups were well balanced.
Treatment Reduced Medication Use
During the last three months of treatment, the mean number of monthly migraine days decreased by 3.2 for patients receiving 70 mg of erenumab and by 3.7 for patients receiving 140 mg of erenumab, compared with 1.8 for patients receiving placebo. The difference between the active and control arms was statistically significant. The rate of participants who had a 50% reduction in migraine attacks was 26.6% for controls, 43% for patients receiving the 70-mg dose, and 50% for patients receiving the 140-mg dose.
The use of acute medicines decreased by 1.1 days for patients receiving 70 mg of erenumab and by 1.6 days for patients receiving 140 mg, compared with a decrease of 0.2 days for patients receiving placebo. Using the Migraine Physical Function Impact Diary, participants in the 70-mg group reported that the impact of migraine on everyday activities decreased by 5.5 points. Participants in the 140-mg group reported a 5.9-point reduction in this end point. Participants in the placebo group reported a 3.3-point reduction. Physical impairment decreased by 4.2 points for the 70-mg group and by 4.8 points for the 140-mg group versus 2.4 points for the placebo group.
Erenumab was well tolerated, and none of the serious adverse events appeared to be related to treatment. The most common adverse event was injection-site reactions. About 6% of the entire cohort developed antibodies to erenumab, including 8% in the 70-mg group and 3.2% in the 140-mg group. One patient receiving 70 mg had a neutralizing antibody, and no patients in the 140-mg group had neutralizing antibodies.
The study results are exciting, said Dr. Goadsby. In the future, treatments that target the CGRP pathway could be provided to migraineurs who do not respond to or cannot tolerate the current therapies, he added.
The trial was sponsored by Amgen, and Dr. Goadsby previously has consulted for the company.
—Erik Greb
Tips for Recovering From Concussion
Click here to download the PDF.
Click here to download the PDF.
Click here to download the PDF.