User login
Access new surgeon and resident well-being resources
Personal and professional well-being are vital to the success of members of the American College of Surgeons (ACS) and your patients. Many health care professionals experience periods of distress, yet few physicians seek help. In an effort to provide relief to interested surgeons, the ACS has compiled several resources to support surgeons and residents as they confront the challenges associated with surgical care.
Visit the ACS Surgeon Well-Being page at facs.org/burnout to learn more about the tool and how to access it, as well as to review other helpful resources.
Personal and professional well-being are vital to the success of members of the American College of Surgeons (ACS) and your patients. Many health care professionals experience periods of distress, yet few physicians seek help. In an effort to provide relief to interested surgeons, the ACS has compiled several resources to support surgeons and residents as they confront the challenges associated with surgical care.
Visit the ACS Surgeon Well-Being page at facs.org/burnout to learn more about the tool and how to access it, as well as to review other helpful resources.
Personal and professional well-being are vital to the success of members of the American College of Surgeons (ACS) and your patients. Many health care professionals experience periods of distress, yet few physicians seek help. In an effort to provide relief to interested surgeons, the ACS has compiled several resources to support surgeons and residents as they confront the challenges associated with surgical care.
Visit the ACS Surgeon Well-Being page at facs.org/burnout to learn more about the tool and how to access it, as well as to review other helpful resources.
Three days in the life of a surgeon
By sheer happenstance, I was visiting a surgery program on the day after “the Match.” As all of you know, four days before the official release of the placement of every new surgical trainee, both the medical students involved and the programs affected are informed as to whether they have been matched. Students don’t know where they are going, just that the last rung of training is now in place. They have a job and a relatively secure future. Those who have not been matched and those programs that did not fill all their slots now enter into a scramble (officially called SOAP) to find students for the remaining slots. This year, the scramble occurred on a Wednesday and was orchestrated by a set of rules I’d never been privy to before.
On Tuesday night, all the programs in need of students for their open slots, whether categorical or preliminary, looked over the list of candidates remaining and made their choices. So did the students now hoping to find a place. At 10 a.m., the offers went out to students in the first round. Next, in precisely timed order, the programs found out who had accepted the offers. And, if slots were left over, the programs had a short time to put up another set of offers – and so on throughout the day until all the slots were gone. Like a game of musical chairs, the music finally stopped and the Match was over for the entering class of residents for 2017.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, and coeditor of ACS Surgery News.
By sheer happenstance, I was visiting a surgery program on the day after “the Match.” As all of you know, four days before the official release of the placement of every new surgical trainee, both the medical students involved and the programs affected are informed as to whether they have been matched. Students don’t know where they are going, just that the last rung of training is now in place. They have a job and a relatively secure future. Those who have not been matched and those programs that did not fill all their slots now enter into a scramble (officially called SOAP) to find students for the remaining slots. This year, the scramble occurred on a Wednesday and was orchestrated by a set of rules I’d never been privy to before.
On Tuesday night, all the programs in need of students for their open slots, whether categorical or preliminary, looked over the list of candidates remaining and made their choices. So did the students now hoping to find a place. At 10 a.m., the offers went out to students in the first round. Next, in precisely timed order, the programs found out who had accepted the offers. And, if slots were left over, the programs had a short time to put up another set of offers – and so on throughout the day until all the slots were gone. Like a game of musical chairs, the music finally stopped and the Match was over for the entering class of residents for 2017.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, and coeditor of ACS Surgery News.
By sheer happenstance, I was visiting a surgery program on the day after “the Match.” As all of you know, four days before the official release of the placement of every new surgical trainee, both the medical students involved and the programs affected are informed as to whether they have been matched. Students don’t know where they are going, just that the last rung of training is now in place. They have a job and a relatively secure future. Those who have not been matched and those programs that did not fill all their slots now enter into a scramble (officially called SOAP) to find students for the remaining slots. This year, the scramble occurred on a Wednesday and was orchestrated by a set of rules I’d never been privy to before.
On Tuesday night, all the programs in need of students for their open slots, whether categorical or preliminary, looked over the list of candidates remaining and made their choices. So did the students now hoping to find a place. At 10 a.m., the offers went out to students in the first round. Next, in precisely timed order, the programs found out who had accepted the offers. And, if slots were left over, the programs had a short time to put up another set of offers – and so on throughout the day until all the slots were gone. Like a game of musical chairs, the music finally stopped and the Match was over for the entering class of residents for 2017.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, and coeditor of ACS Surgery News.
Legends Come to Lunch
A highlight of the Postgraduate Symposia at the AATS Centennial will be the Legends Luncheons. This year, renowned cardiothoracic surgeons, William I. Norwood, MD, Valerie W. Rusch, MD, and Magdi H. Yacoub, MD, will share their experiences at the interactive luncheons on Sunday at each of the Postgraduate Symposia. You must be registered for a Sunday Postgraduate Symposia to attend the Legend Luncheons, but you may select any luncheon to attend.
William I. Norwood, MD (Congenital Heart Surgery)
Dr. Norwood trained in cardiac surgery at the University of Minnesota as well as at Brigham and Women’s Hospital. He took a staff position at Boston Children’s Hospital in 1976. In 1981, he and his colleageues first reported the hypoplastic left heart syndrome (HLHS) procedure that bears his name.
HLHS is congenital heart defect in which one or more of the left-sided cardiac structures are underdeveloped and unable to support the systemic circulation. Dr. Norwood’s operation, improved over the years with subsequent stages and refinements, alleviated the universally fatal condition, allowing survivors to live functional lives, even if restricted to varying degreess.
Dr. Norwood became chief of cardiac surgery at Children’s Hospital of Philadelphia, after which, he and Aldo Castaneda, MD, established a private clinic in Geneva, Switzerland in 1994. Dr. Norwood later moved to Nemours/Alfred I. duPont Hospital for Children, from which he retired from clinical practice in 2003.
Valerie W. Rusch, MD (General Thoraic Surgery)
Dr. Rusch is the Vice Chair for Clinical Research, Department of Surgery, and Miner Family Chair in Intrathoracic Cancers at Memorial Sloan Kettering Cancer Center. Dr. Rusch was a pioneer among women in cardiothoracic surgery, and was among the first women in the country to be board certified. In 2015, Dr. Rusch was elected Chair of the Board of Regents of the American College of Surgeons, one of the most prestigious positions a surgeon can attain.
Dr. Rusch specializes in the diagnosis and treatment of patients with a variety of thoracic cancers, including lung, esophageal, mediastinaal, chest wall, and pleural.
She is a national leader in clinical trials for thoracic malignancies, and has received the Thoracic Surgery Foundation for Research and Education Socrates Award. Dr. Rusch is co-author on more than 260 papers and numerous book chapters and invited texts and has received numerous teaching and patient-care excellence awards.
Magdi H. Yacoub, MD (Adult Cardiac Surgery and Transplantation)
Dr. Yacoub is Professor of Cardiothoracic Surgery at the National Heart and Lung Institute, Imperial College London, and Founder and Director of Research at the Magdi Yacoub Institute, Harefield Heart Science Centre, which focuses on tissue engineering, myocardial regeneration, stem cell biology, end stage heart failure, and transplant immunology. He is also Founder and Director of Magdi Yacoub Research Network which has created the Qatar Cardiovascular Research Center in collaboration with Qatar Foundation and Hamad Medical Corporation.
Dr. Yacoub was born in Egypt and held an assistant professorship at the University of Chicago. He was a British Heart Foundation Professor of Cardiothoracic Surgery for over 20 years, and a consultant cardiothoracic surgeon at Harefield Hospital from 1969-2001 and Royal Brompton Hospital from 1986-2001.
Dr. Yacoub established the largest heart and lung transplantation program in the world (in the United Kingdom) where more than 2,500 transplant operations have been performed. Among his many other accomplishments, he pioneered a live-saving surgical technique for treating infants born with transposition of the great arteries.
Dr. Yacoub was knighted for his services to medicine and surgery in 1991, awarded Fellowship of the Academy of Medical Sciences in 1998 and became a Fellow of The Royal Society in 1999.
Dr. Yacoub is author or co-author on more than 1,000 articles
A highlight of the Postgraduate Symposia at the AATS Centennial will be the Legends Luncheons. This year, renowned cardiothoracic surgeons, William I. Norwood, MD, Valerie W. Rusch, MD, and Magdi H. Yacoub, MD, will share their experiences at the interactive luncheons on Sunday at each of the Postgraduate Symposia. You must be registered for a Sunday Postgraduate Symposia to attend the Legend Luncheons, but you may select any luncheon to attend.
William I. Norwood, MD (Congenital Heart Surgery)
Dr. Norwood trained in cardiac surgery at the University of Minnesota as well as at Brigham and Women’s Hospital. He took a staff position at Boston Children’s Hospital in 1976. In 1981, he and his colleageues first reported the hypoplastic left heart syndrome (HLHS) procedure that bears his name.
HLHS is congenital heart defect in which one or more of the left-sided cardiac structures are underdeveloped and unable to support the systemic circulation. Dr. Norwood’s operation, improved over the years with subsequent stages and refinements, alleviated the universally fatal condition, allowing survivors to live functional lives, even if restricted to varying degreess.
Dr. Norwood became chief of cardiac surgery at Children’s Hospital of Philadelphia, after which, he and Aldo Castaneda, MD, established a private clinic in Geneva, Switzerland in 1994. Dr. Norwood later moved to Nemours/Alfred I. duPont Hospital for Children, from which he retired from clinical practice in 2003.
Valerie W. Rusch, MD (General Thoraic Surgery)
Dr. Rusch is the Vice Chair for Clinical Research, Department of Surgery, and Miner Family Chair in Intrathoracic Cancers at Memorial Sloan Kettering Cancer Center. Dr. Rusch was a pioneer among women in cardiothoracic surgery, and was among the first women in the country to be board certified. In 2015, Dr. Rusch was elected Chair of the Board of Regents of the American College of Surgeons, one of the most prestigious positions a surgeon can attain.
Dr. Rusch specializes in the diagnosis and treatment of patients with a variety of thoracic cancers, including lung, esophageal, mediastinaal, chest wall, and pleural.
She is a national leader in clinical trials for thoracic malignancies, and has received the Thoracic Surgery Foundation for Research and Education Socrates Award. Dr. Rusch is co-author on more than 260 papers and numerous book chapters and invited texts and has received numerous teaching and patient-care excellence awards.
Magdi H. Yacoub, MD (Adult Cardiac Surgery and Transplantation)
Dr. Yacoub is Professor of Cardiothoracic Surgery at the National Heart and Lung Institute, Imperial College London, and Founder and Director of Research at the Magdi Yacoub Institute, Harefield Heart Science Centre, which focuses on tissue engineering, myocardial regeneration, stem cell biology, end stage heart failure, and transplant immunology. He is also Founder and Director of Magdi Yacoub Research Network which has created the Qatar Cardiovascular Research Center in collaboration with Qatar Foundation and Hamad Medical Corporation.
Dr. Yacoub was born in Egypt and held an assistant professorship at the University of Chicago. He was a British Heart Foundation Professor of Cardiothoracic Surgery for over 20 years, and a consultant cardiothoracic surgeon at Harefield Hospital from 1969-2001 and Royal Brompton Hospital from 1986-2001.
Dr. Yacoub established the largest heart and lung transplantation program in the world (in the United Kingdom) where more than 2,500 transplant operations have been performed. Among his many other accomplishments, he pioneered a live-saving surgical technique for treating infants born with transposition of the great arteries.
Dr. Yacoub was knighted for his services to medicine and surgery in 1991, awarded Fellowship of the Academy of Medical Sciences in 1998 and became a Fellow of The Royal Society in 1999.
Dr. Yacoub is author or co-author on more than 1,000 articles
A highlight of the Postgraduate Symposia at the AATS Centennial will be the Legends Luncheons. This year, renowned cardiothoracic surgeons, William I. Norwood, MD, Valerie W. Rusch, MD, and Magdi H. Yacoub, MD, will share their experiences at the interactive luncheons on Sunday at each of the Postgraduate Symposia. You must be registered for a Sunday Postgraduate Symposia to attend the Legend Luncheons, but you may select any luncheon to attend.
William I. Norwood, MD (Congenital Heart Surgery)
Dr. Norwood trained in cardiac surgery at the University of Minnesota as well as at Brigham and Women’s Hospital. He took a staff position at Boston Children’s Hospital in 1976. In 1981, he and his colleageues first reported the hypoplastic left heart syndrome (HLHS) procedure that bears his name.
HLHS is congenital heart defect in which one or more of the left-sided cardiac structures are underdeveloped and unable to support the systemic circulation. Dr. Norwood’s operation, improved over the years with subsequent stages and refinements, alleviated the universally fatal condition, allowing survivors to live functional lives, even if restricted to varying degreess.
Dr. Norwood became chief of cardiac surgery at Children’s Hospital of Philadelphia, after which, he and Aldo Castaneda, MD, established a private clinic in Geneva, Switzerland in 1994. Dr. Norwood later moved to Nemours/Alfred I. duPont Hospital for Children, from which he retired from clinical practice in 2003.
Valerie W. Rusch, MD (General Thoraic Surgery)
Dr. Rusch is the Vice Chair for Clinical Research, Department of Surgery, and Miner Family Chair in Intrathoracic Cancers at Memorial Sloan Kettering Cancer Center. Dr. Rusch was a pioneer among women in cardiothoracic surgery, and was among the first women in the country to be board certified. In 2015, Dr. Rusch was elected Chair of the Board of Regents of the American College of Surgeons, one of the most prestigious positions a surgeon can attain.
Dr. Rusch specializes in the diagnosis and treatment of patients with a variety of thoracic cancers, including lung, esophageal, mediastinaal, chest wall, and pleural.
She is a national leader in clinical trials for thoracic malignancies, and has received the Thoracic Surgery Foundation for Research and Education Socrates Award. Dr. Rusch is co-author on more than 260 papers and numerous book chapters and invited texts and has received numerous teaching and patient-care excellence awards.
Magdi H. Yacoub, MD (Adult Cardiac Surgery and Transplantation)
Dr. Yacoub is Professor of Cardiothoracic Surgery at the National Heart and Lung Institute, Imperial College London, and Founder and Director of Research at the Magdi Yacoub Institute, Harefield Heart Science Centre, which focuses on tissue engineering, myocardial regeneration, stem cell biology, end stage heart failure, and transplant immunology. He is also Founder and Director of Magdi Yacoub Research Network which has created the Qatar Cardiovascular Research Center in collaboration with Qatar Foundation and Hamad Medical Corporation.
Dr. Yacoub was born in Egypt and held an assistant professorship at the University of Chicago. He was a British Heart Foundation Professor of Cardiothoracic Surgery for over 20 years, and a consultant cardiothoracic surgeon at Harefield Hospital from 1969-2001 and Royal Brompton Hospital from 1986-2001.
Dr. Yacoub established the largest heart and lung transplantation program in the world (in the United Kingdom) where more than 2,500 transplant operations have been performed. Among his many other accomplishments, he pioneered a live-saving surgical technique for treating infants born with transposition of the great arteries.
Dr. Yacoub was knighted for his services to medicine and surgery in 1991, awarded Fellowship of the Academy of Medical Sciences in 1998 and became a Fellow of The Royal Society in 1999.
Dr. Yacoub is author or co-author on more than 1,000 articles
Teens’ marijuana use higher during pregnancy
The prevalence of marijuana use among pregnant teenagers is more than double that among teens who are not pregnant, according to a study involving 410,000 females aged 12-44 years.
For pregnant teens aged 12-17 years, the past-month prevalence of marijuana use was 14% between 2002 and 2015, compared with 6.5% for their nonpregnant peers, Nora D. Volkow, MD, director of the National Institute on Drug Abuse in Bethesda, Md., and her associates reported in a letter on April 17 (Ann Intern Med. 2017 Apr 17. doi: 10.7326/L17-0067).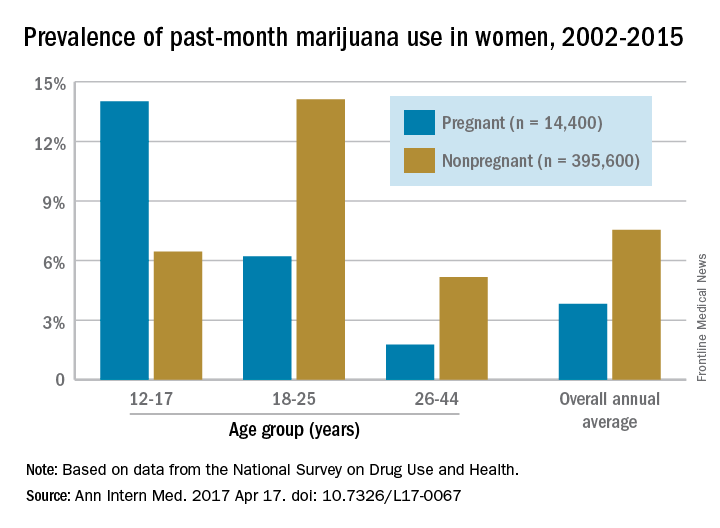
The pattern of use in the youngest age group “may reflect underlying risky behavior common to both teen pregnancy and early substance use and suggests the importance of intervention for teenagers,” the researchers wrote. “Because of consistent overlap between use of marijuana and other substances, identification of marijuana use during pregnancy warrants evaluation for comorbid substance abuse.”
The overall annual average prevalence of marijuana use was 3.8% among the 14,400 pregnant women and 7.5% for the 395,600 nonpregnant women who responded to the survey from 2002 to 2015. The investigators also found that marijuana use was higher in the first trimester (6.4%) than in the second (3.3%) or third (1.8%) trimesters and that use was higher in pregnant black women (6.5%) than in white (3.8%) or Hispanic women(2.9%) or women of other races/ethnicities (1.4%).
Although evidence on the effects of marijuana on prenatal development is limited, “pregnant females and those considering becoming pregnant should be advised not to use marijuana or other cannabinoids recreationally or to treat nausea,” Dr. Volkow and her associates wrote.
The study was sponsored by the National Institute on Drug Abuse and the Substance Abuse and Mental Health Services Administration. Dr. Volkow reported having no financial disclosures. One coauthor reported stock ownership of Pfizer, and another reported stock ownership of Sanofi and Eli Lilly.
The prevalence of marijuana use among pregnant teenagers is more than double that among teens who are not pregnant, according to a study involving 410,000 females aged 12-44 years.
For pregnant teens aged 12-17 years, the past-month prevalence of marijuana use was 14% between 2002 and 2015, compared with 6.5% for their nonpregnant peers, Nora D. Volkow, MD, director of the National Institute on Drug Abuse in Bethesda, Md., and her associates reported in a letter on April 17 (Ann Intern Med. 2017 Apr 17. doi: 10.7326/L17-0067).
The pattern of use in the youngest age group “may reflect underlying risky behavior common to both teen pregnancy and early substance use and suggests the importance of intervention for teenagers,” the researchers wrote. “Because of consistent overlap between use of marijuana and other substances, identification of marijuana use during pregnancy warrants evaluation for comorbid substance abuse.”
The overall annual average prevalence of marijuana use was 3.8% among the 14,400 pregnant women and 7.5% for the 395,600 nonpregnant women who responded to the survey from 2002 to 2015. The investigators also found that marijuana use was higher in the first trimester (6.4%) than in the second (3.3%) or third (1.8%) trimesters and that use was higher in pregnant black women (6.5%) than in white (3.8%) or Hispanic women(2.9%) or women of other races/ethnicities (1.4%).
Although evidence on the effects of marijuana on prenatal development is limited, “pregnant females and those considering becoming pregnant should be advised not to use marijuana or other cannabinoids recreationally or to treat nausea,” Dr. Volkow and her associates wrote.
The study was sponsored by the National Institute on Drug Abuse and the Substance Abuse and Mental Health Services Administration. Dr. Volkow reported having no financial disclosures. One coauthor reported stock ownership of Pfizer, and another reported stock ownership of Sanofi and Eli Lilly.
The prevalence of marijuana use among pregnant teenagers is more than double that among teens who are not pregnant, according to a study involving 410,000 females aged 12-44 years.
For pregnant teens aged 12-17 years, the past-month prevalence of marijuana use was 14% between 2002 and 2015, compared with 6.5% for their nonpregnant peers, Nora D. Volkow, MD, director of the National Institute on Drug Abuse in Bethesda, Md., and her associates reported in a letter on April 17 (Ann Intern Med. 2017 Apr 17. doi: 10.7326/L17-0067).
The pattern of use in the youngest age group “may reflect underlying risky behavior common to both teen pregnancy and early substance use and suggests the importance of intervention for teenagers,” the researchers wrote. “Because of consistent overlap between use of marijuana and other substances, identification of marijuana use during pregnancy warrants evaluation for comorbid substance abuse.”
The overall annual average prevalence of marijuana use was 3.8% among the 14,400 pregnant women and 7.5% for the 395,600 nonpregnant women who responded to the survey from 2002 to 2015. The investigators also found that marijuana use was higher in the first trimester (6.4%) than in the second (3.3%) or third (1.8%) trimesters and that use was higher in pregnant black women (6.5%) than in white (3.8%) or Hispanic women(2.9%) or women of other races/ethnicities (1.4%).
Although evidence on the effects of marijuana on prenatal development is limited, “pregnant females and those considering becoming pregnant should be advised not to use marijuana or other cannabinoids recreationally or to treat nausea,” Dr. Volkow and her associates wrote.
The study was sponsored by the National Institute on Drug Abuse and the Substance Abuse and Mental Health Services Administration. Dr. Volkow reported having no financial disclosures. One coauthor reported stock ownership of Pfizer, and another reported stock ownership of Sanofi and Eli Lilly.
FROM ANNALS OF INTERNAL MEDICINE
Proceed cautiously with liver cancer resection in elderly patients
MIAMI BEACH – A decision to proceed with major hepatectomy in patients 75 and older with perihilar cholangiocarcinoma should be made on a case-by-case basis following strict selection, Thuy Tran, MD, said, based on a study of 210 patients.
“As the U.S. population ages, an increasing number of elderly patients are being evaluated for resection of GI malignancies,” Dr. Tran said at the annual meeting of the Americas Hepato-Pancreato-Biliary Association.
“Advanced age has been regarded as contraindication to resection for complex hepatobiliary malignancies,” she explained, with concerns that “it may be too risky, and the survival benefit is limited in elderly populations. However, the oncologic benefit of aggressive surgical strategies in perihilar cholangiocarcinoma remains a subject of debate.”
Dr. Tran and her colleagues identified patients who underwent curative resection for perihilar cholangiocarcinoma (CCA) in the U.S. Extrahepatic Biliary Malignancy Consortium database. They compared outcomes of those younger than 75 years versus patients 75 years and older. A total of 59% of the cohort were men, 20% were 75 years and older, and the median age was 66 years.
CCA is a rare and aggressive malignancy, often presenting with obstructive jaundice, said Dr. Tran, a postdoctoral research fellow in general surgery at Stanford (Calif.) University.
Preoperative characteristics were similar in the groups, except that cardiac morbidity was higher in the older cohort. In addition, pathology characteristics did not differ significantly between age groups, including tumor stage, nodal status, grade, size, and margin status.
The in-hospital mortality was double for the older patients, 15% versus 8%, despite the similarities, Dr. Tran said. “This supports the notion that it is more difficult to salvage older patients when they run into a complication,” she added.
Postoperative morbidity was also higher in older patients, 78% versus 68%, but did not reach statistical significance (P = .34).
The 90-day mortality rate was 22% in patients 75 years and older, compared with 10% in younger patients, a nonsignificant difference (P = .09).
Five-year survival was 15% in the older cohort and 22% for the younger patients (P = .003). There was a “more significant drop in the survival curves in the older age group, but then they get closer,” Dr. Tran said. The disease-specific survival did not differ significantly at 46 months versus 37 months, respectively.
Advanced-stage cancer and elevated CA 19-9 tumor marker levels were independent predictors of survival in a multivariate analysis, but age was not. Higher body mass index was associated with a higher perioperative mortality in older patients, but sex, cardiac morbidity, and ASA status were not. Dr. Tran said, “Lower BMI may be a useful tool to select elderly patients,” she noted.
“Elderly patients have double the mortality following major hepatectomy for perihilar cholangiocarcinoma,” Dr. Tran said. “However, the long-term, cancer-specific outcome appears similar to that of younger patients.” Physiologically robust older patients may be better candidates for surgery, she suggested.
Of the six patients who died in the 75 and older group, two patients died from liver failure, one from sepsis, one intraoperatively, one from unknown causes, and one died who required reoperation for postop bleeding, Dr. Tran said. “We did not find patients dying from MI or pneumonia, probably due to the small number of patients.”
Dr. Tran said that 2.5% of the older group and 5% of the younger group received neoadjuvant chemotherapy, which was not a statistically significant difference.
A meeting attendee asked if left versus right hepatectomy made a difference, and why the researchers chose 75 years as the cutoff age between younger and older groups.
“Left vs. right laterality does not seem to make an impact in terms of survival,” Dr. Tran said. “The median age was 66 years, and we used the upper limit of standard deviation, which was 75.”
Dr. Tran had no relevant financial disclosures.
MIAMI BEACH – A decision to proceed with major hepatectomy in patients 75 and older with perihilar cholangiocarcinoma should be made on a case-by-case basis following strict selection, Thuy Tran, MD, said, based on a study of 210 patients.
“As the U.S. population ages, an increasing number of elderly patients are being evaluated for resection of GI malignancies,” Dr. Tran said at the annual meeting of the Americas Hepato-Pancreato-Biliary Association.
“Advanced age has been regarded as contraindication to resection for complex hepatobiliary malignancies,” she explained, with concerns that “it may be too risky, and the survival benefit is limited in elderly populations. However, the oncologic benefit of aggressive surgical strategies in perihilar cholangiocarcinoma remains a subject of debate.”
Dr. Tran and her colleagues identified patients who underwent curative resection for perihilar cholangiocarcinoma (CCA) in the U.S. Extrahepatic Biliary Malignancy Consortium database. They compared outcomes of those younger than 75 years versus patients 75 years and older. A total of 59% of the cohort were men, 20% were 75 years and older, and the median age was 66 years.
CCA is a rare and aggressive malignancy, often presenting with obstructive jaundice, said Dr. Tran, a postdoctoral research fellow in general surgery at Stanford (Calif.) University.
Preoperative characteristics were similar in the groups, except that cardiac morbidity was higher in the older cohort. In addition, pathology characteristics did not differ significantly between age groups, including tumor stage, nodal status, grade, size, and margin status.
The in-hospital mortality was double for the older patients, 15% versus 8%, despite the similarities, Dr. Tran said. “This supports the notion that it is more difficult to salvage older patients when they run into a complication,” she added.
Postoperative morbidity was also higher in older patients, 78% versus 68%, but did not reach statistical significance (P = .34).
The 90-day mortality rate was 22% in patients 75 years and older, compared with 10% in younger patients, a nonsignificant difference (P = .09).
Five-year survival was 15% in the older cohort and 22% for the younger patients (P = .003). There was a “more significant drop in the survival curves in the older age group, but then they get closer,” Dr. Tran said. The disease-specific survival did not differ significantly at 46 months versus 37 months, respectively.
Advanced-stage cancer and elevated CA 19-9 tumor marker levels were independent predictors of survival in a multivariate analysis, but age was not. Higher body mass index was associated with a higher perioperative mortality in older patients, but sex, cardiac morbidity, and ASA status were not. Dr. Tran said, “Lower BMI may be a useful tool to select elderly patients,” she noted.
“Elderly patients have double the mortality following major hepatectomy for perihilar cholangiocarcinoma,” Dr. Tran said. “However, the long-term, cancer-specific outcome appears similar to that of younger patients.” Physiologically robust older patients may be better candidates for surgery, she suggested.
Of the six patients who died in the 75 and older group, two patients died from liver failure, one from sepsis, one intraoperatively, one from unknown causes, and one died who required reoperation for postop bleeding, Dr. Tran said. “We did not find patients dying from MI or pneumonia, probably due to the small number of patients.”
Dr. Tran said that 2.5% of the older group and 5% of the younger group received neoadjuvant chemotherapy, which was not a statistically significant difference.
A meeting attendee asked if left versus right hepatectomy made a difference, and why the researchers chose 75 years as the cutoff age between younger and older groups.
“Left vs. right laterality does not seem to make an impact in terms of survival,” Dr. Tran said. “The median age was 66 years, and we used the upper limit of standard deviation, which was 75.”
Dr. Tran had no relevant financial disclosures.
MIAMI BEACH – A decision to proceed with major hepatectomy in patients 75 and older with perihilar cholangiocarcinoma should be made on a case-by-case basis following strict selection, Thuy Tran, MD, said, based on a study of 210 patients.
“As the U.S. population ages, an increasing number of elderly patients are being evaluated for resection of GI malignancies,” Dr. Tran said at the annual meeting of the Americas Hepato-Pancreato-Biliary Association.
“Advanced age has been regarded as contraindication to resection for complex hepatobiliary malignancies,” she explained, with concerns that “it may be too risky, and the survival benefit is limited in elderly populations. However, the oncologic benefit of aggressive surgical strategies in perihilar cholangiocarcinoma remains a subject of debate.”
Dr. Tran and her colleagues identified patients who underwent curative resection for perihilar cholangiocarcinoma (CCA) in the U.S. Extrahepatic Biliary Malignancy Consortium database. They compared outcomes of those younger than 75 years versus patients 75 years and older. A total of 59% of the cohort were men, 20% were 75 years and older, and the median age was 66 years.
CCA is a rare and aggressive malignancy, often presenting with obstructive jaundice, said Dr. Tran, a postdoctoral research fellow in general surgery at Stanford (Calif.) University.
Preoperative characteristics were similar in the groups, except that cardiac morbidity was higher in the older cohort. In addition, pathology characteristics did not differ significantly between age groups, including tumor stage, nodal status, grade, size, and margin status.
The in-hospital mortality was double for the older patients, 15% versus 8%, despite the similarities, Dr. Tran said. “This supports the notion that it is more difficult to salvage older patients when they run into a complication,” she added.
Postoperative morbidity was also higher in older patients, 78% versus 68%, but did not reach statistical significance (P = .34).
The 90-day mortality rate was 22% in patients 75 years and older, compared with 10% in younger patients, a nonsignificant difference (P = .09).
Five-year survival was 15% in the older cohort and 22% for the younger patients (P = .003). There was a “more significant drop in the survival curves in the older age group, but then they get closer,” Dr. Tran said. The disease-specific survival did not differ significantly at 46 months versus 37 months, respectively.
Advanced-stage cancer and elevated CA 19-9 tumor marker levels were independent predictors of survival in a multivariate analysis, but age was not. Higher body mass index was associated with a higher perioperative mortality in older patients, but sex, cardiac morbidity, and ASA status were not. Dr. Tran said, “Lower BMI may be a useful tool to select elderly patients,” she noted.
“Elderly patients have double the mortality following major hepatectomy for perihilar cholangiocarcinoma,” Dr. Tran said. “However, the long-term, cancer-specific outcome appears similar to that of younger patients.” Physiologically robust older patients may be better candidates for surgery, she suggested.
Of the six patients who died in the 75 and older group, two patients died from liver failure, one from sepsis, one intraoperatively, one from unknown causes, and one died who required reoperation for postop bleeding, Dr. Tran said. “We did not find patients dying from MI or pneumonia, probably due to the small number of patients.”
Dr. Tran said that 2.5% of the older group and 5% of the younger group received neoadjuvant chemotherapy, which was not a statistically significant difference.
A meeting attendee asked if left versus right hepatectomy made a difference, and why the researchers chose 75 years as the cutoff age between younger and older groups.
“Left vs. right laterality does not seem to make an impact in terms of survival,” Dr. Tran said. “The median age was 66 years, and we used the upper limit of standard deviation, which was 75.”
Dr. Tran had no relevant financial disclosures.
Key clinical point: Patients 75 years and older undergoing hepatectomy for perihilar cholangiocarcinoma trended toward higher mortality but experienced cancer-specific outcomes similar to younger patients.
Major finding: 90-day mortality was 22% in patients 75 years and older versus 10% in younger patients (nonsignificant, P = .09).
Data source: Retrospective database study of 210 people who had curative intent resection for perihilar cholangiocarcinoma.
Disclosures: Dr. Tran had no relevant financial disclosures.
FDA: REMS no longer necessary for epoetin, darbepoetin
The Food and Drug Administration no longer requires certification of doctors and hospitals to prescribe epoetin alfa (Procrit, Epogen) or darbepoetin alfa (Aranesp) for chemotherapy anemia.
A Risk Evaluation and Mitigation Strategy (REMS) program was put in place in 2011 to make sure that the benefits of erythropoiesis-stimulating agents (ESAs) outweighed the risks when prescribed. Under the program, providers were required to become certified in the ESA REMS and to demonstrate that each patient had received counseling on the benefits and risks of the therapies prior to use.
Amgen’s prescriber surveys demonstrated “acceptable knowledge” of the need to counsel patients about the risks. Utilization data indicated “appropriate prescribing” as an alternative to transfusion.
In addition, in an evaluation of the impact of multiple regulatory actions, the FDA determined that full implementation of the ESA REMS in 2011 had minimal impact on trends in ESA utilization metrics, the FDA wrote.
The FDA concluded that regulatory actions and label changes – and the cut in payments for nonrenal indications from the Center for Medicare & Medicaid Services – were enough to reduce overuse in chemotherapy.
However, while the REMS is no longer necessary, the FDA says serious risks of shortened overall survival and/or increased risk of tumor progression or recurrence associated with these drugs remain and health care providers should continue to discuss the risks and benefits of using ESAs with each patient before initiating use.
The Food and Drug Administration no longer requires certification of doctors and hospitals to prescribe epoetin alfa (Procrit, Epogen) or darbepoetin alfa (Aranesp) for chemotherapy anemia.
A Risk Evaluation and Mitigation Strategy (REMS) program was put in place in 2011 to make sure that the benefits of erythropoiesis-stimulating agents (ESAs) outweighed the risks when prescribed. Under the program, providers were required to become certified in the ESA REMS and to demonstrate that each patient had received counseling on the benefits and risks of the therapies prior to use.
Amgen’s prescriber surveys demonstrated “acceptable knowledge” of the need to counsel patients about the risks. Utilization data indicated “appropriate prescribing” as an alternative to transfusion.
In addition, in an evaluation of the impact of multiple regulatory actions, the FDA determined that full implementation of the ESA REMS in 2011 had minimal impact on trends in ESA utilization metrics, the FDA wrote.
The FDA concluded that regulatory actions and label changes – and the cut in payments for nonrenal indications from the Center for Medicare & Medicaid Services – were enough to reduce overuse in chemotherapy.
However, while the REMS is no longer necessary, the FDA says serious risks of shortened overall survival and/or increased risk of tumor progression or recurrence associated with these drugs remain and health care providers should continue to discuss the risks and benefits of using ESAs with each patient before initiating use.
The Food and Drug Administration no longer requires certification of doctors and hospitals to prescribe epoetin alfa (Procrit, Epogen) or darbepoetin alfa (Aranesp) for chemotherapy anemia.
A Risk Evaluation and Mitigation Strategy (REMS) program was put in place in 2011 to make sure that the benefits of erythropoiesis-stimulating agents (ESAs) outweighed the risks when prescribed. Under the program, providers were required to become certified in the ESA REMS and to demonstrate that each patient had received counseling on the benefits and risks of the therapies prior to use.
Amgen’s prescriber surveys demonstrated “acceptable knowledge” of the need to counsel patients about the risks. Utilization data indicated “appropriate prescribing” as an alternative to transfusion.
In addition, in an evaluation of the impact of multiple regulatory actions, the FDA determined that full implementation of the ESA REMS in 2011 had minimal impact on trends in ESA utilization metrics, the FDA wrote.
The FDA concluded that regulatory actions and label changes – and the cut in payments for nonrenal indications from the Center for Medicare & Medicaid Services – were enough to reduce overuse in chemotherapy.
However, while the REMS is no longer necessary, the FDA says serious risks of shortened overall survival and/or increased risk of tumor progression or recurrence associated with these drugs remain and health care providers should continue to discuss the risks and benefits of using ESAs with each patient before initiating use.
Reduced-intensity conditioning may not preserve fertility in young girls after bone marrow transplant
ORLANDO – Girls who undergo reduced-intensity conditioning for a bone marrow transplant may face fertility problems in the future, even if they experience an outwardly normal puberty.
In the first-ever study to compare high- and low-intensity chemotherapeutic conditioning regimens among young girls, significantly more who underwent the reduced-intensity regimen had normal estradiol, luteinizing hormone, and follicle-stimulating hormone compared with those who had high-intensity conditioning. But anti-Müllerian hormone was low or absent in almost all the girls, no matter which conditioning regimen they had, Jonathan C. Howell, MD, PhD, said at the annual meeting of the Endocrine Society.
While not a perfect predictor of future fertility, anti-Müllerian hormone is a good indicator of ovarian follicular reserve, said Dr. Howell, a pediatric endocrinologist at Cincinnati Children’s Hospital Medical Center.
Dr. Howell and his colleagues, Holly R. Hoefgen, MD, Kasiani C. Myers, MD, and Helen Oquendo-Del Toro, MD, all of Cincinnati Children’s Hospital Medical Center, are following 49 females aged 1-40 years who had preconditioning chemotherapy in advance of hematopoietic stem cell transplantation.
At the meeting, Dr. Howell reported data on 23 girls who were in puberty during their treatment (mean age 12 years). The mean follow-up was 4 years, but this varied widely, from 1 to 13 years. Most (16) had high-intensity myeloablation; the remainder had reduced-intensity conditioning. Diagnoses varied between the groups. Among those with high-intensity conditioning, malignancy and bone marrow failure were the most common indications (seven patients each); one patient had an immunodeficiency, and the cause was unknown for another.
Among those who had the reduced-intensity regimen, five had an immunodeficiency and two had bone marrow failure.
The discrepancy in diagnoses between the groups isn’t surprising, Dr. Howell said. “Diagnosis can dictate which treatment patients receive. People with malignancies or a prior history of leukemia or lymphoma often receive the high-intensity conditioning. You want to wipe out every single malignant cell.”
Reduced-intensity conditioning may be an option for patients with other problems such as bone marrow failure, immunodeficiencies, or genetic or metabolic problems. The less-intense regimen does confer some benefits, Dr. Howell noted. “The short-term need for intensive medical therapy while getting the stem cells is less. The medical benefit of these less-intense regimens is certainly there, but the long-term endocrine impact has yet to be defined.”
Most of the girls in the high-intensity regimen group (64%) had high follicle stimulating hormone and luteinizing hormone, suggesting primary ovarian failure; 71% of them also had low estradiol levels. However, all of these hormones were normal in the reduced-intensity group. But regardless of conditioning treatment, anti-Müllerian hormone was abnormally low in nearly all of the patients (87%). Only one girl with myeloablative conditioning and two girls with reduced intensity condition had normal anti-Müllerian levels. “This tells us that fertility potential may not be preserved, despite [their] getting the reduced-intensity conditioning,” Dr. Howell said.
The story here is only beginning to unfold, he said. “Fertility is defined as the ability to conceive a child, and that’s not something we have looked at yet. We would like to know the long-term outcomes of fertility in these patients, and whether they can conceive when they’re ready to start a family. Our goal is to follow these young women into their 20s and 30s, and to see if that’s an opportunity they are able to experience.”
The study is a cooperative project involving the hospital’s divisions of Pediatric and Adolescent Gynecology, Bone Marrow Transplantation and Immunology, and Endocrinology.
Neither Dr. Howell nor any of his colleagues had any financial disclosures.
Fertility preservation talks: The earlier, the better
A talk about fertility preservation can be the first step into a new future for families of children with a cancer diagnosis.
“Talking about your baby having a baby can be the farthest thing from your mind,” when you’re the parent of a child about to undergo cancer treatment, said Dr. Hoefgen. “But we know from survivors that this can be a very important issue in the future. We simply start by telling parents, ‘This will be important to your child at some point, and we want to talk about it now, while there is still something we may be able to do about it.’ ”
Dr. Hoefgen, a staff member at the hospital’s Comprehensive Fertility Care and Preservation Program, said parents “sometimes find it weird” to be talking about unborn grandchildren when they’re consumed with making critical decisions for their own child. But by asking them to consider that child’s long-term future, the discussion offers its own message of hope.
The talks always begin with a basic discussion of how cancer treatments can affect the reproductive organs. The hospital has a series of short animated videos that are very helpful in relaying the information. Another video in that series describes the different methods of fertility preservation: mature oocyte or sperm harvesting, or, for younger patients, removing and freezing ovarian and testicular tissue. Parents and children can watch them together, get grounding in the basics, and be prepared for a productive conversation.
Talks always include the team oncologist, who creates a specialized risk assessment for each patient. The group discusses each preservation method, the risks and benefits, and the cost. But the talks are exploratory, too, helping both clinicians and families understand what’s most important to them, she said.
“Common things that we typically talk about are genetics, religion, and ethics – which may mean different things to different families.”
Dr. Hoefgen and her team reach out to more than 95% of families that face a pediatric cancer diagnosis. After the in-depth discussions, she said, about 20% decide to investigate some form of fertility preservation.
“The most important thing is having the conversation early, while we still have options,” she said.
Dr. Hoefgen had no financial disclosures.
ORLANDO – Girls who undergo reduced-intensity conditioning for a bone marrow transplant may face fertility problems in the future, even if they experience an outwardly normal puberty.
In the first-ever study to compare high- and low-intensity chemotherapeutic conditioning regimens among young girls, significantly more who underwent the reduced-intensity regimen had normal estradiol, luteinizing hormone, and follicle-stimulating hormone compared with those who had high-intensity conditioning. But anti-Müllerian hormone was low or absent in almost all the girls, no matter which conditioning regimen they had, Jonathan C. Howell, MD, PhD, said at the annual meeting of the Endocrine Society.
While not a perfect predictor of future fertility, anti-Müllerian hormone is a good indicator of ovarian follicular reserve, said Dr. Howell, a pediatric endocrinologist at Cincinnati Children’s Hospital Medical Center.
Dr. Howell and his colleagues, Holly R. Hoefgen, MD, Kasiani C. Myers, MD, and Helen Oquendo-Del Toro, MD, all of Cincinnati Children’s Hospital Medical Center, are following 49 females aged 1-40 years who had preconditioning chemotherapy in advance of hematopoietic stem cell transplantation.
At the meeting, Dr. Howell reported data on 23 girls who were in puberty during their treatment (mean age 12 years). The mean follow-up was 4 years, but this varied widely, from 1 to 13 years. Most (16) had high-intensity myeloablation; the remainder had reduced-intensity conditioning. Diagnoses varied between the groups. Among those with high-intensity conditioning, malignancy and bone marrow failure were the most common indications (seven patients each); one patient had an immunodeficiency, and the cause was unknown for another.
Among those who had the reduced-intensity regimen, five had an immunodeficiency and two had bone marrow failure.
The discrepancy in diagnoses between the groups isn’t surprising, Dr. Howell said. “Diagnosis can dictate which treatment patients receive. People with malignancies or a prior history of leukemia or lymphoma often receive the high-intensity conditioning. You want to wipe out every single malignant cell.”
Reduced-intensity conditioning may be an option for patients with other problems such as bone marrow failure, immunodeficiencies, or genetic or metabolic problems. The less-intense regimen does confer some benefits, Dr. Howell noted. “The short-term need for intensive medical therapy while getting the stem cells is less. The medical benefit of these less-intense regimens is certainly there, but the long-term endocrine impact has yet to be defined.”
Most of the girls in the high-intensity regimen group (64%) had high follicle stimulating hormone and luteinizing hormone, suggesting primary ovarian failure; 71% of them also had low estradiol levels. However, all of these hormones were normal in the reduced-intensity group. But regardless of conditioning treatment, anti-Müllerian hormone was abnormally low in nearly all of the patients (87%). Only one girl with myeloablative conditioning and two girls with reduced intensity condition had normal anti-Müllerian levels. “This tells us that fertility potential may not be preserved, despite [their] getting the reduced-intensity conditioning,” Dr. Howell said.
The story here is only beginning to unfold, he said. “Fertility is defined as the ability to conceive a child, and that’s not something we have looked at yet. We would like to know the long-term outcomes of fertility in these patients, and whether they can conceive when they’re ready to start a family. Our goal is to follow these young women into their 20s and 30s, and to see if that’s an opportunity they are able to experience.”
The study is a cooperative project involving the hospital’s divisions of Pediatric and Adolescent Gynecology, Bone Marrow Transplantation and Immunology, and Endocrinology.
Neither Dr. Howell nor any of his colleagues had any financial disclosures.
Fertility preservation talks: The earlier, the better
A talk about fertility preservation can be the first step into a new future for families of children with a cancer diagnosis.
“Talking about your baby having a baby can be the farthest thing from your mind,” when you’re the parent of a child about to undergo cancer treatment, said Dr. Hoefgen. “But we know from survivors that this can be a very important issue in the future. We simply start by telling parents, ‘This will be important to your child at some point, and we want to talk about it now, while there is still something we may be able to do about it.’ ”
Dr. Hoefgen, a staff member at the hospital’s Comprehensive Fertility Care and Preservation Program, said parents “sometimes find it weird” to be talking about unborn grandchildren when they’re consumed with making critical decisions for their own child. But by asking them to consider that child’s long-term future, the discussion offers its own message of hope.
The talks always begin with a basic discussion of how cancer treatments can affect the reproductive organs. The hospital has a series of short animated videos that are very helpful in relaying the information. Another video in that series describes the different methods of fertility preservation: mature oocyte or sperm harvesting, or, for younger patients, removing and freezing ovarian and testicular tissue. Parents and children can watch them together, get grounding in the basics, and be prepared for a productive conversation.
Talks always include the team oncologist, who creates a specialized risk assessment for each patient. The group discusses each preservation method, the risks and benefits, and the cost. But the talks are exploratory, too, helping both clinicians and families understand what’s most important to them, she said.
“Common things that we typically talk about are genetics, religion, and ethics – which may mean different things to different families.”
Dr. Hoefgen and her team reach out to more than 95% of families that face a pediatric cancer diagnosis. After the in-depth discussions, she said, about 20% decide to investigate some form of fertility preservation.
“The most important thing is having the conversation early, while we still have options,” she said.
Dr. Hoefgen had no financial disclosures.
ORLANDO – Girls who undergo reduced-intensity conditioning for a bone marrow transplant may face fertility problems in the future, even if they experience an outwardly normal puberty.
In the first-ever study to compare high- and low-intensity chemotherapeutic conditioning regimens among young girls, significantly more who underwent the reduced-intensity regimen had normal estradiol, luteinizing hormone, and follicle-stimulating hormone compared with those who had high-intensity conditioning. But anti-Müllerian hormone was low or absent in almost all the girls, no matter which conditioning regimen they had, Jonathan C. Howell, MD, PhD, said at the annual meeting of the Endocrine Society.
While not a perfect predictor of future fertility, anti-Müllerian hormone is a good indicator of ovarian follicular reserve, said Dr. Howell, a pediatric endocrinologist at Cincinnati Children’s Hospital Medical Center.
Dr. Howell and his colleagues, Holly R. Hoefgen, MD, Kasiani C. Myers, MD, and Helen Oquendo-Del Toro, MD, all of Cincinnati Children’s Hospital Medical Center, are following 49 females aged 1-40 years who had preconditioning chemotherapy in advance of hematopoietic stem cell transplantation.
At the meeting, Dr. Howell reported data on 23 girls who were in puberty during their treatment (mean age 12 years). The mean follow-up was 4 years, but this varied widely, from 1 to 13 years. Most (16) had high-intensity myeloablation; the remainder had reduced-intensity conditioning. Diagnoses varied between the groups. Among those with high-intensity conditioning, malignancy and bone marrow failure were the most common indications (seven patients each); one patient had an immunodeficiency, and the cause was unknown for another.
Among those who had the reduced-intensity regimen, five had an immunodeficiency and two had bone marrow failure.
The discrepancy in diagnoses between the groups isn’t surprising, Dr. Howell said. “Diagnosis can dictate which treatment patients receive. People with malignancies or a prior history of leukemia or lymphoma often receive the high-intensity conditioning. You want to wipe out every single malignant cell.”
Reduced-intensity conditioning may be an option for patients with other problems such as bone marrow failure, immunodeficiencies, or genetic or metabolic problems. The less-intense regimen does confer some benefits, Dr. Howell noted. “The short-term need for intensive medical therapy while getting the stem cells is less. The medical benefit of these less-intense regimens is certainly there, but the long-term endocrine impact has yet to be defined.”
Most of the girls in the high-intensity regimen group (64%) had high follicle stimulating hormone and luteinizing hormone, suggesting primary ovarian failure; 71% of them also had low estradiol levels. However, all of these hormones were normal in the reduced-intensity group. But regardless of conditioning treatment, anti-Müllerian hormone was abnormally low in nearly all of the patients (87%). Only one girl with myeloablative conditioning and two girls with reduced intensity condition had normal anti-Müllerian levels. “This tells us that fertility potential may not be preserved, despite [their] getting the reduced-intensity conditioning,” Dr. Howell said.
The story here is only beginning to unfold, he said. “Fertility is defined as the ability to conceive a child, and that’s not something we have looked at yet. We would like to know the long-term outcomes of fertility in these patients, and whether they can conceive when they’re ready to start a family. Our goal is to follow these young women into their 20s and 30s, and to see if that’s an opportunity they are able to experience.”
The study is a cooperative project involving the hospital’s divisions of Pediatric and Adolescent Gynecology, Bone Marrow Transplantation and Immunology, and Endocrinology.
Neither Dr. Howell nor any of his colleagues had any financial disclosures.
Fertility preservation talks: The earlier, the better
A talk about fertility preservation can be the first step into a new future for families of children with a cancer diagnosis.
“Talking about your baby having a baby can be the farthest thing from your mind,” when you’re the parent of a child about to undergo cancer treatment, said Dr. Hoefgen. “But we know from survivors that this can be a very important issue in the future. We simply start by telling parents, ‘This will be important to your child at some point, and we want to talk about it now, while there is still something we may be able to do about it.’ ”
Dr. Hoefgen, a staff member at the hospital’s Comprehensive Fertility Care and Preservation Program, said parents “sometimes find it weird” to be talking about unborn grandchildren when they’re consumed with making critical decisions for their own child. But by asking them to consider that child’s long-term future, the discussion offers its own message of hope.
The talks always begin with a basic discussion of how cancer treatments can affect the reproductive organs. The hospital has a series of short animated videos that are very helpful in relaying the information. Another video in that series describes the different methods of fertility preservation: mature oocyte or sperm harvesting, or, for younger patients, removing and freezing ovarian and testicular tissue. Parents and children can watch them together, get grounding in the basics, and be prepared for a productive conversation.
Talks always include the team oncologist, who creates a specialized risk assessment for each patient. The group discusses each preservation method, the risks and benefits, and the cost. But the talks are exploratory, too, helping both clinicians and families understand what’s most important to them, she said.
“Common things that we typically talk about are genetics, religion, and ethics – which may mean different things to different families.”
Dr. Hoefgen and her team reach out to more than 95% of families that face a pediatric cancer diagnosis. After the in-depth discussions, she said, about 20% decide to investigate some form of fertility preservation.
“The most important thing is having the conversation early, while we still have options,” she said.
Dr. Hoefgen had no financial disclosures.
AT ENDO 2017
Key clinical point:
Major finding: Anti-Müllerian hormone was abnormally low or absent in all treated girls, whether they had reduced-intensity or high-intensity conditioning.
Data source: The prospective study is following 49 females aged 1-40 years.
Disclosures: Neither Dr. Howell nor any of his colleagues had any financial disclosures.
Serotonergic dysfunction documented in hoarding disorder
SAN FRANCISCO – Hoarding disorder is characterized by a distinct pattern of serotonergic dysfunction different from that seen in obsessive-compulsive disorder (OCD) or major depression, Sanjaya Saxena, MD, reported at the annual conference of the Anxiety and Depression Association of America.
He presented the findings of the first-ever study to examine neurotransmitter function in patients with hoarding disorder. In contrast to hoarding disorder, the neurobiology of OCD is among the most extensively studied and best understood of any psychiatric disorder, with Dr. Saxena having made important contributions to this body of knowledge.
The findings underscore the prescience of the authors of DSM-5 in removing hoarding disorder from under the umbrella of OCD and giving it its own separate diagnostic category.
“Our study provides more evidence suggesting that OCD and hoarding disorder are two separate disorders that do sometimes occur together in patients, but not necessarily more often than other disorders do. OCD is found in only about 15% of patients with hoarding disorder, and vice versa. Depression is much more commonly comorbid, and [attention-deficit/hyperactivity disorder] is present in about one-quarter of hoarding disorder patients,” according to the psychiatrist.
His study included 8 patients with hoarding disorder and 14 age- and sex-matched normal controls who underwent positron emission tomography with carbon-11-labeled DASP, a ligand that binds with high selectivity to the brain serotonin transporter. The serotonin transporter is a neuronal membrane protein whose main function is to recycle serotonin from synapse back into the neuronal cell body. The serotonin transporter also is the primary site to which serotonin reuptake inhibitor medications bind. Serotonin transporter binding is associated with fear conditioning, amygdala reactivity, the cortisol response, and state anxiety.
Subjects with hoarding disorder proved to have significantly greater serotonin transporter binding than healthy controls in the bilateral amygdala, nucleus accumbens, putamen, and right caudate nucleus. In contrast, their serotonin transporter binding was significantly diminished, compared with controls, in the bilateral retrosplenial posterior cingulate cortex. Severity of hoarding symptoms was correlated with serotonin transporter binding in the right anterior cingulate cortex and right orbitofrontal cortex, but negatively correlated with serotonin transporter binding in the bilateral posterior thalamus, Dr. Saxena reported.
“The binding pattern is almost opposite that seen in OCD,” he observed.
The new study of serotonergic function in hoarding disorder, taken together with earlier brain imaging studies of regional glucose metabolism and functional MRI studies conducted by Dr. Saxena and other research groups, point to a couple of areas of the brain as being strongly implicated in the pathophysiology of hoarding disorder – most notably, the anterior and posterior cingulate cortex.
Particularly intriguing, in Dr. Saxena’s view, is the retrosplenial posterior cingulate cortex, located in Brodmann areas 29 and 30. The retrosplenial posterior cingulate cortex, part of the default mode network, is involved in emotional functioning, learning, planning, navigation, as well as autobiographical, episodic, and visuospatial memory.
“Abnormalities of all those functions are found in hoarding disorder patients, so the retrosplenial posterior cingulate cortex is a candidate for further study,” according to Dr. Saxena.
His neurotransmitter study was funded by the university’s department of psychiatry. He reported having no financial conflicts.
SAN FRANCISCO – Hoarding disorder is characterized by a distinct pattern of serotonergic dysfunction different from that seen in obsessive-compulsive disorder (OCD) or major depression, Sanjaya Saxena, MD, reported at the annual conference of the Anxiety and Depression Association of America.
He presented the findings of the first-ever study to examine neurotransmitter function in patients with hoarding disorder. In contrast to hoarding disorder, the neurobiology of OCD is among the most extensively studied and best understood of any psychiatric disorder, with Dr. Saxena having made important contributions to this body of knowledge.
The findings underscore the prescience of the authors of DSM-5 in removing hoarding disorder from under the umbrella of OCD and giving it its own separate diagnostic category.
“Our study provides more evidence suggesting that OCD and hoarding disorder are two separate disorders that do sometimes occur together in patients, but not necessarily more often than other disorders do. OCD is found in only about 15% of patients with hoarding disorder, and vice versa. Depression is much more commonly comorbid, and [attention-deficit/hyperactivity disorder] is present in about one-quarter of hoarding disorder patients,” according to the psychiatrist.
His study included 8 patients with hoarding disorder and 14 age- and sex-matched normal controls who underwent positron emission tomography with carbon-11-labeled DASP, a ligand that binds with high selectivity to the brain serotonin transporter. The serotonin transporter is a neuronal membrane protein whose main function is to recycle serotonin from synapse back into the neuronal cell body. The serotonin transporter also is the primary site to which serotonin reuptake inhibitor medications bind. Serotonin transporter binding is associated with fear conditioning, amygdala reactivity, the cortisol response, and state anxiety.
Subjects with hoarding disorder proved to have significantly greater serotonin transporter binding than healthy controls in the bilateral amygdala, nucleus accumbens, putamen, and right caudate nucleus. In contrast, their serotonin transporter binding was significantly diminished, compared with controls, in the bilateral retrosplenial posterior cingulate cortex. Severity of hoarding symptoms was correlated with serotonin transporter binding in the right anterior cingulate cortex and right orbitofrontal cortex, but negatively correlated with serotonin transporter binding in the bilateral posterior thalamus, Dr. Saxena reported.
“The binding pattern is almost opposite that seen in OCD,” he observed.
The new study of serotonergic function in hoarding disorder, taken together with earlier brain imaging studies of regional glucose metabolism and functional MRI studies conducted by Dr. Saxena and other research groups, point to a couple of areas of the brain as being strongly implicated in the pathophysiology of hoarding disorder – most notably, the anterior and posterior cingulate cortex.
Particularly intriguing, in Dr. Saxena’s view, is the retrosplenial posterior cingulate cortex, located in Brodmann areas 29 and 30. The retrosplenial posterior cingulate cortex, part of the default mode network, is involved in emotional functioning, learning, planning, navigation, as well as autobiographical, episodic, and visuospatial memory.
“Abnormalities of all those functions are found in hoarding disorder patients, so the retrosplenial posterior cingulate cortex is a candidate for further study,” according to Dr. Saxena.
His neurotransmitter study was funded by the university’s department of psychiatry. He reported having no financial conflicts.
SAN FRANCISCO – Hoarding disorder is characterized by a distinct pattern of serotonergic dysfunction different from that seen in obsessive-compulsive disorder (OCD) or major depression, Sanjaya Saxena, MD, reported at the annual conference of the Anxiety and Depression Association of America.
He presented the findings of the first-ever study to examine neurotransmitter function in patients with hoarding disorder. In contrast to hoarding disorder, the neurobiology of OCD is among the most extensively studied and best understood of any psychiatric disorder, with Dr. Saxena having made important contributions to this body of knowledge.
The findings underscore the prescience of the authors of DSM-5 in removing hoarding disorder from under the umbrella of OCD and giving it its own separate diagnostic category.
“Our study provides more evidence suggesting that OCD and hoarding disorder are two separate disorders that do sometimes occur together in patients, but not necessarily more often than other disorders do. OCD is found in only about 15% of patients with hoarding disorder, and vice versa. Depression is much more commonly comorbid, and [attention-deficit/hyperactivity disorder] is present in about one-quarter of hoarding disorder patients,” according to the psychiatrist.
His study included 8 patients with hoarding disorder and 14 age- and sex-matched normal controls who underwent positron emission tomography with carbon-11-labeled DASP, a ligand that binds with high selectivity to the brain serotonin transporter. The serotonin transporter is a neuronal membrane protein whose main function is to recycle serotonin from synapse back into the neuronal cell body. The serotonin transporter also is the primary site to which serotonin reuptake inhibitor medications bind. Serotonin transporter binding is associated with fear conditioning, amygdala reactivity, the cortisol response, and state anxiety.
Subjects with hoarding disorder proved to have significantly greater serotonin transporter binding than healthy controls in the bilateral amygdala, nucleus accumbens, putamen, and right caudate nucleus. In contrast, their serotonin transporter binding was significantly diminished, compared with controls, in the bilateral retrosplenial posterior cingulate cortex. Severity of hoarding symptoms was correlated with serotonin transporter binding in the right anterior cingulate cortex and right orbitofrontal cortex, but negatively correlated with serotonin transporter binding in the bilateral posterior thalamus, Dr. Saxena reported.
“The binding pattern is almost opposite that seen in OCD,” he observed.
The new study of serotonergic function in hoarding disorder, taken together with earlier brain imaging studies of regional glucose metabolism and functional MRI studies conducted by Dr. Saxena and other research groups, point to a couple of areas of the brain as being strongly implicated in the pathophysiology of hoarding disorder – most notably, the anterior and posterior cingulate cortex.
Particularly intriguing, in Dr. Saxena’s view, is the retrosplenial posterior cingulate cortex, located in Brodmann areas 29 and 30. The retrosplenial posterior cingulate cortex, part of the default mode network, is involved in emotional functioning, learning, planning, navigation, as well as autobiographical, episodic, and visuospatial memory.
“Abnormalities of all those functions are found in hoarding disorder patients, so the retrosplenial posterior cingulate cortex is a candidate for further study,” according to Dr. Saxena.
His neurotransmitter study was funded by the university’s department of psychiatry. He reported having no financial conflicts.
Key clinical point:
Major finding: Serotonin transporter binding is markedly reduced in the retrosplenial posterior cingulate cortex of hoarding disorder patients, compared with normal controls.
Data source: This positron emission tomography study, the first ever to examine brain serotonin transporter binding patterns in hoarding disorder, included 8 affected patients and 14 matched normal controls.
Disclosures: The study was funded by the University of California, San Diego, department of psychiatry. The presenter reported having no financial conflicts.
Maternal Tdap vaccination highly protective against infant pertussis
, results of a study show.
From 2010 to 2015, researchers collected data from a cohort study of full-term infants born at Kaiser Permanente of Northern California. “We estimated the effectiveness of maternal pertussis vaccination for protecting newborns against pertussis in the first 2 months of life and in the first year of life, accounting for each infant DTaP dose,” said Roger Baxter, MD, and his associates at Kaiser Permanente Vaccine Study Center, Oakland, Calif.
The vaccine effectiveness was an estimated 88% before infants had any DTaP vaccine doses, 81% between doses 1 and 2, 6% between doses 2 and 3, and 66% after infants had 3 DTaP doses.
“This result is consistent with 2 earlier studies in the United Kingdom” showing protection in infants less than 3 months of age, and a U.S. study showing maternal Tdap vaccination conferring protection to infants in the first 8 weeks of life, the researchers said.
In conclusion, even after infant DTaP dosing, there was evidence of additional protection from maternal Tdap vaccination for the first year of life (Pediatrics. 2017 Apr 3. doi: 10.1542/peds.2016-4091).
, results of a study show.
From 2010 to 2015, researchers collected data from a cohort study of full-term infants born at Kaiser Permanente of Northern California. “We estimated the effectiveness of maternal pertussis vaccination for protecting newborns against pertussis in the first 2 months of life and in the first year of life, accounting for each infant DTaP dose,” said Roger Baxter, MD, and his associates at Kaiser Permanente Vaccine Study Center, Oakland, Calif.
The vaccine effectiveness was an estimated 88% before infants had any DTaP vaccine doses, 81% between doses 1 and 2, 6% between doses 2 and 3, and 66% after infants had 3 DTaP doses.
“This result is consistent with 2 earlier studies in the United Kingdom” showing protection in infants less than 3 months of age, and a U.S. study showing maternal Tdap vaccination conferring protection to infants in the first 8 weeks of life, the researchers said.
In conclusion, even after infant DTaP dosing, there was evidence of additional protection from maternal Tdap vaccination for the first year of life (Pediatrics. 2017 Apr 3. doi: 10.1542/peds.2016-4091).
, results of a study show.
From 2010 to 2015, researchers collected data from a cohort study of full-term infants born at Kaiser Permanente of Northern California. “We estimated the effectiveness of maternal pertussis vaccination for protecting newborns against pertussis in the first 2 months of life and in the first year of life, accounting for each infant DTaP dose,” said Roger Baxter, MD, and his associates at Kaiser Permanente Vaccine Study Center, Oakland, Calif.
The vaccine effectiveness was an estimated 88% before infants had any DTaP vaccine doses, 81% between doses 1 and 2, 6% between doses 2 and 3, and 66% after infants had 3 DTaP doses.
“This result is consistent with 2 earlier studies in the United Kingdom” showing protection in infants less than 3 months of age, and a U.S. study showing maternal Tdap vaccination conferring protection to infants in the first 8 weeks of life, the researchers said.
In conclusion, even after infant DTaP dosing, there was evidence of additional protection from maternal Tdap vaccination for the first year of life (Pediatrics. 2017 Apr 3. doi: 10.1542/peds.2016-4091).
App may improve CPAP adherence
Use of a mobile app may help sleep apnea patients adhere to continuous positive airway pressure (CPAP) therapy, a small study suggests.
The app – SleepMapper (SM) – has interactive algorithms that are modeled on the same theories of behavior change that have improved adherence to CPAP when delivered in person or through telephone-linked communication, wrote Jordanna M. Hostler of Walter Reed National Military Medical Center, Bethesda, Md., and her coinvestigators (J Sleep Res. 2017;26:139-46).
“Despite our small sample size, patients in the SM group were more than three times as likely to meet Medicare criteria for [CPAP] adherence (greater than 4 hours per night for 70% of nights), a trend that just missed statistical significance (P = .06),” the researchers noted.
“The magnitude of the increase [in CPAP use] indicates likely clinical benefit,” they added.
SleepMapper allows patients to self-monitor the outcomes of positive airway pressure (PAP) therapy by providing information on their adherence, Apnea-Hypopnea Index, and mask leak. The app, which can be downloaded on a smartphone or personal computer, also includes training modules on how to use PAP. The system, owned by Phillips Respironics, will sync with Philips Respironics’ Encore Anywhere software program.
The study comprised 61 patients who had been diagnosed with obstructive sleep apnea (OSA) via overnight, in-lab polysomnography. The patients were initiating PAP for the first time at Walter Reed National Military Medical Center’s Sleep Disorders Center in Bethesda, Md., as participants in the center’s program. Such a program included group sessions with instruction on sleep hygiene and training in the use of PAP, an initial one-on-one meeting with a physician, and a follow-up appointment with a physician 4 weeks after initiating the therapy.
Thirty of the program participants included in the study used SleepMapper in addition to the center’s standard education and follow-up. The researchers analyzed 11 weeks of data for all 61 study participants.
Patients in the SleepMapper group used their PAP machines for a greater percentage of days and achieved more than 4 hours of use on more days of participation in the program, compared with patients who did not use the app. The patients using the app also showed a trend toward using PAP for more hours per night overall. Specifically, nine of the patients in the app group used their PAP machines greater than 4 hours per night for 70% of nights, versus three of the patients in the control group (P = .06). SleepMapper usage remained significantly associated with percentage of nights including greater than 4 hours of PAP use, in a multivariate linear regression analysis.
The researchers observed many similarities between patients using the app and patients in the control group, including Apnea-Hypopnea Index scores, central apnea index scores, and percentages of time spent in periodic breathing.
Some additional advantages to use of SleepMapper over simply participating in the center’s educational program are that the app provides ongoing coaching and immediate access to Apnea-Hypopnea Index and PAP use data, according to the researchers. They touted the app’s educational videos about OSA and PAP therapy and “structured motivational enhancement techniques such as feedback and goal setting, which have shown benefit when delivered by health care professionals in other studies.”
The researchers called for an additional study of the app’s usefulness in a larger group of patients.
The authors did not receive any funding or support for this study.
Feedback to patients regarding their clinical status is rarely a bad thing. I’m a big fan of tools that give patients information on their CPAP adherence and outcomes, of which several now exist, including some that are not affiliated with any particular respiratory device company, and are able to read a number of different device downloads.
In the end, the use of technology for monitoring CPAP adherence is here to stay, and the incremental cost of making it available to our patients is low. Taking the time to educate patients who are newly prescribed CPAP about interpreting their outcome data is likely to be time well spent.
Feedback to patients regarding their clinical status is rarely a bad thing. I’m a big fan of tools that give patients information on their CPAP adherence and outcomes, of which several now exist, including some that are not affiliated with any particular respiratory device company, and are able to read a number of different device downloads.
In the end, the use of technology for monitoring CPAP adherence is here to stay, and the incremental cost of making it available to our patients is low. Taking the time to educate patients who are newly prescribed CPAP about interpreting their outcome data is likely to be time well spent.
Feedback to patients regarding their clinical status is rarely a bad thing. I’m a big fan of tools that give patients information on their CPAP adherence and outcomes, of which several now exist, including some that are not affiliated with any particular respiratory device company, and are able to read a number of different device downloads.
In the end, the use of technology for monitoring CPAP adherence is here to stay, and the incremental cost of making it available to our patients is low. Taking the time to educate patients who are newly prescribed CPAP about interpreting their outcome data is likely to be time well spent.
Use of a mobile app may help sleep apnea patients adhere to continuous positive airway pressure (CPAP) therapy, a small study suggests.
The app – SleepMapper (SM) – has interactive algorithms that are modeled on the same theories of behavior change that have improved adherence to CPAP when delivered in person or through telephone-linked communication, wrote Jordanna M. Hostler of Walter Reed National Military Medical Center, Bethesda, Md., and her coinvestigators (J Sleep Res. 2017;26:139-46).
“Despite our small sample size, patients in the SM group were more than three times as likely to meet Medicare criteria for [CPAP] adherence (greater than 4 hours per night for 70% of nights), a trend that just missed statistical significance (P = .06),” the researchers noted.
“The magnitude of the increase [in CPAP use] indicates likely clinical benefit,” they added.
SleepMapper allows patients to self-monitor the outcomes of positive airway pressure (PAP) therapy by providing information on their adherence, Apnea-Hypopnea Index, and mask leak. The app, which can be downloaded on a smartphone or personal computer, also includes training modules on how to use PAP. The system, owned by Phillips Respironics, will sync with Philips Respironics’ Encore Anywhere software program.
The study comprised 61 patients who had been diagnosed with obstructive sleep apnea (OSA) via overnight, in-lab polysomnography. The patients were initiating PAP for the first time at Walter Reed National Military Medical Center’s Sleep Disorders Center in Bethesda, Md., as participants in the center’s program. Such a program included group sessions with instruction on sleep hygiene and training in the use of PAP, an initial one-on-one meeting with a physician, and a follow-up appointment with a physician 4 weeks after initiating the therapy.
Thirty of the program participants included in the study used SleepMapper in addition to the center’s standard education and follow-up. The researchers analyzed 11 weeks of data for all 61 study participants.
Patients in the SleepMapper group used their PAP machines for a greater percentage of days and achieved more than 4 hours of use on more days of participation in the program, compared with patients who did not use the app. The patients using the app also showed a trend toward using PAP for more hours per night overall. Specifically, nine of the patients in the app group used their PAP machines greater than 4 hours per night for 70% of nights, versus three of the patients in the control group (P = .06). SleepMapper usage remained significantly associated with percentage of nights including greater than 4 hours of PAP use, in a multivariate linear regression analysis.
The researchers observed many similarities between patients using the app and patients in the control group, including Apnea-Hypopnea Index scores, central apnea index scores, and percentages of time spent in periodic breathing.
Some additional advantages to use of SleepMapper over simply participating in the center’s educational program are that the app provides ongoing coaching and immediate access to Apnea-Hypopnea Index and PAP use data, according to the researchers. They touted the app’s educational videos about OSA and PAP therapy and “structured motivational enhancement techniques such as feedback and goal setting, which have shown benefit when delivered by health care professionals in other studies.”
The researchers called for an additional study of the app’s usefulness in a larger group of patients.
The authors did not receive any funding or support for this study.
Use of a mobile app may help sleep apnea patients adhere to continuous positive airway pressure (CPAP) therapy, a small study suggests.
The app – SleepMapper (SM) – has interactive algorithms that are modeled on the same theories of behavior change that have improved adherence to CPAP when delivered in person or through telephone-linked communication, wrote Jordanna M. Hostler of Walter Reed National Military Medical Center, Bethesda, Md., and her coinvestigators (J Sleep Res. 2017;26:139-46).
“Despite our small sample size, patients in the SM group were more than three times as likely to meet Medicare criteria for [CPAP] adherence (greater than 4 hours per night for 70% of nights), a trend that just missed statistical significance (P = .06),” the researchers noted.
“The magnitude of the increase [in CPAP use] indicates likely clinical benefit,” they added.
SleepMapper allows patients to self-monitor the outcomes of positive airway pressure (PAP) therapy by providing information on their adherence, Apnea-Hypopnea Index, and mask leak. The app, which can be downloaded on a smartphone or personal computer, also includes training modules on how to use PAP. The system, owned by Phillips Respironics, will sync with Philips Respironics’ Encore Anywhere software program.
The study comprised 61 patients who had been diagnosed with obstructive sleep apnea (OSA) via overnight, in-lab polysomnography. The patients were initiating PAP for the first time at Walter Reed National Military Medical Center’s Sleep Disorders Center in Bethesda, Md., as participants in the center’s program. Such a program included group sessions with instruction on sleep hygiene and training in the use of PAP, an initial one-on-one meeting with a physician, and a follow-up appointment with a physician 4 weeks after initiating the therapy.
Thirty of the program participants included in the study used SleepMapper in addition to the center’s standard education and follow-up. The researchers analyzed 11 weeks of data for all 61 study participants.
Patients in the SleepMapper group used their PAP machines for a greater percentage of days and achieved more than 4 hours of use on more days of participation in the program, compared with patients who did not use the app. The patients using the app also showed a trend toward using PAP for more hours per night overall. Specifically, nine of the patients in the app group used their PAP machines greater than 4 hours per night for 70% of nights, versus three of the patients in the control group (P = .06). SleepMapper usage remained significantly associated with percentage of nights including greater than 4 hours of PAP use, in a multivariate linear regression analysis.
The researchers observed many similarities between patients using the app and patients in the control group, including Apnea-Hypopnea Index scores, central apnea index scores, and percentages of time spent in periodic breathing.
Some additional advantages to use of SleepMapper over simply participating in the center’s educational program are that the app provides ongoing coaching and immediate access to Apnea-Hypopnea Index and PAP use data, according to the researchers. They touted the app’s educational videos about OSA and PAP therapy and “structured motivational enhancement techniques such as feedback and goal setting, which have shown benefit when delivered by health care professionals in other studies.”
The researchers called for an additional study of the app’s usefulness in a larger group of patients.
The authors did not receive any funding or support for this study.