User login
Conjoint Sessions With Clinical Pharmacy and Health Psychology for Chronic Pain
Providing comprehensive, integrated, behavioral intervention services to address the prevalent condition of chronic, noncancer pain is a growing concern. Although the biopsychosocial model (BPS) and stepped-care approaches have been understood and discussed for some time, clinician and patient understanding and investment in these approaches continue to face challenges. Moreover, even when resources (eg, staffing, referral options, space) are available, clinicians and patients must engage in meaningful communication to achieve this type of care.
Importantly, engagement means moving beyond diagnosis and assessment and offering interventions that provide psychoeducation related to the chronic pain cycle. These interventions address maladaptive cognitions and beliefs about movement and pain; promote paced, daily physical activity and engagement in life; and help increase coping skills to improve low mood or distress, all fundamental components of the BPS understanding of chronic pain.
Background
Chronic, noncancer pain is a prevalent presentation in primary care settings in the U.S. and even more so for veterans.1 Fifty percent of male veterans and 75% of female veterans report chronic pain as an important condition that impacts their health.2 An important aspect of this prevalence is the focus on opioid pain medication and medical procedures, both of which draw more narrowly on the biomedical model. Additional information on the longer term use of pain procedures and opioid medications is now available,and given some risks and limitations (eg, tolerance, decreasing efficacy, opioid-induced medical complications), the need to study and offer other options is gaining attention.3 Behavioral chronic pain management has a clear historic role that draws on the BPS modeland Gate Control Theory.3-6
More recently, the National Strategy of Chronic Pain collaborative and stepped-care models extended this literature, outlining collaboration and levels of care depending on the chronicity of the pain experience as well as co-occurring conditions and patient presentations.7,8 The Commission on Accreditation of Rehabilitation Facilities (CARF), the gold standard in interdisciplinary pain management programs, calls for further resources and coordination of these efforts, including a tertiary level of care representing the highest step in the stepped-care model.8
These interdisciplinary, integrative pain management programs, which include functional restoration and cognitive behavioral therapy (CBT) interventions, have been effective for the treatment of chronic pain.9-12However, the staffing, resources, clinical access, and coordination of this complex care may not be feasible in many health care settings. For example, a 2005 survey reported that there were only 200 multidisciplinary pain programs in the U.S., and only 84 of them were CARF accredited.13 By 2011 the number of CARF-accredited programs had decreased to 64 (the number of nonaccredited programs was not reported for 2011).13
Furthermore, engagement in behavioral pain management services is a challenge: Studies show that psychosocial interventions are underused, and a majority of studies may not report quantitatively or qualitatively on patient adherence or engagement in these services.14 These realities introduce the idea that coordinated appointments between 2 or 3 different disciplines available in primary care may be a feasible step toward implementing more comprehensive, optimal care models.
Behavioral pain management interventions that uphold the BPS also call on the idea of active self-management. Therefore, effective communication is fundamental at both the provider-patient and interprofessional levels to enhance engagement in health care, receptiveness to interventions, and to self-management of chronic pain.11,15 How clinicians conceptualize, hold assumptions about, and communicate with patients about chronic pain management has received more attention.15,16
Clinician Considerations for Pain Management
On theclinicians’ side, monitoring assumptions about patients and awareness of their beliefs as well as the care itself are foundational in patient interactions, impacting the success of patient engagement. Awareness of the language used in these interactions and how clinicians collaborate with other professionals become salient. Coupled with the reality of high attrition, this discussion lends itself in important ways to the motivational interviewing (MI) approach that aims to meet patients “where they are” by use of open-ended questions and reflective listening to guide the conversation in the direction of contemplating or actual behavior change.17 For example, “What do you think are the best ways to manage your pain?” and “It sounds like sometimes the medicine helps, but you also want more options to feel in control of your pain.”
Given the historic focus on the biomedical approach to chronic pain, including the use of opioid medications and medical procedures as well as traditional challenges to engagement in CBT, researchers have explored whether alternative methods may increase participation and improve outcomes for behavioral self-management.3 Drawing on a history of assessing readiness for change in pain management, Kerns and colleagues offered tailored cognitive strategies or behavioral skills training depending on patient preferences.18,19 These researchers also incorporated motivational enhancement strategies in the tailored interventions and compared engagement with standard CBT for chronic pain protocol. Although they did not find significant differences in engagement between the 2 groups, participation and treatment adherence were associated with posttreatment improvements in both groups.19 Taking a step back from enhancing intervention engagement, first assessing readiness to self-manage becomes another salient exploration and step in the process.
Another element of engagement in services is referral to other clinicians. Dorflinger and colleagues made this point in a conceptual paper that broadly outlined interdisciplinary, integrative, and more comprehensive models of care for chronic pain.15 We know from integrated models that referral-based care may decrease the likelihood of participation in health care services. That is, if a patient needs to make a separate appointment and meet with a new clinician, they are more likely to decline, cancel, or not show, particularly if they are not “ready” for change. Co-located or embedded care and conjoint sessions that include a warm handoff or another clinician who joins the first appointment may reduce stigma and other relevant barriers for introducing a patient to new ideas.20
Using a conjoint session that involves a clinical pharmacy pain specialist and a health psychologist is one way in which veterans can be exposed to more chronic pain-related BPS concepts and behavioral health services than they might be exposed to otherwise. The purpose of this project was to bring awareness to a practical and clinically relevant integrated approach to the dissemination of BPS information for chronic pain management.
In providing this information through effective communication at the patient-provider and interprofessional levels, the clinicians’ intention was to increase patient engagement and use of BPS strategies in the self-management of chronic pain. This project also aimed to enhance engagement and improve the quality of services before acquiring additional positions and funding for a specialized pain management team. These sessions were offered at the VA Ann Arbor Healthcare System (VAAAHS) in Michigan. Quantitative and qualitative information was examined from the conjoint and subsequent sessions that occurred in this setting.
Methods
With the above concepts in mind, VAAAHS offered veterans conjoint sessions involving a health psychologist and clinical pharmacy specialist during a 3-month period while this resource was available. The conjoint sessions were part of a preexisting pharmacist-run pain medication clinic embedded in primary care. The conjoint session was presented to patients as part of general clinic flow to reduce stigma of engagement in psychological services and allow for the dissemination of BPS information.
Participants
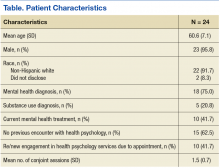
The electronic health records (EHR) of 24 veteran patients with chronic pain, who participated in a conjoint health psychology/pain pharmacy session, were reviewed for the current study. Most of the patients were male (95.8%) and non-Hispanic white (91.7%); the remaining participants did not disclose their ethnicity. The mean age was 60.6 years (SD 7.1; range 50-80). A total of 75% had a mental health diagnosis, and 41.7% were in mental health treatment at the time of the conjoint appointment. Among the sample, 20.8% had a current diagnosis of a substance use disorder (SUD), and no individuals were in treatment for a SUD at the time of the conjoint appointment. Patients received an average of 1.5 conjoint sessions (SD 0.7; range 1-3).
Procedure
The veterans for this project were chosen from a panel of patients followed by the pain medication clinical pharmacy specialist in the primary care pain medication clinic. The selected veterans were offered a joint session with their clinical pharmacy provider and the health psychology resident during their scheduled visit in the pain medication clinic. Each veteran was informed that the goal of the joint visit was to enhance self-directed nonpharmacologic chronic pain management skills as an additional set of tools in the tool kit for particularly difficult pain days. Veterans were assured that their usual care would not be compromised if they declined the session.
During the encounter(s), the health psychologist contributed to the veteran’s care by using MI and CBT for chronic pain skills. The health psychologist further assessed concerns and needs and guided the discussion as appropriate. With veteran readiness, these discussions explored the degree of knowledge and cognitive and behavioral coping skills the patient used. These conjoint sessions also documented the types of discussions and degree of engagement in the encounter(s) as well additional referrals, complementary services, and/or offered follow-up services for either additional conjoint sessions or further health psychology-related services.
A total of 24 EHRs from these conjoint and subsequent encounters were reviewed for evidence of the procedures by a psychology intern involved in chronic pain management services. Of these 24 records, 6 also were reviewed by a board-certified health psychologist for consensus building and agreement on coding (Sidebar, Record Coding).
Using the coding system and SPSS Version 2.1 (IBM, Armonk NY), descriptive statistics were used to examine conjoint session content and new- or re-engagement in health psychology services following the conjoint sessions. For those patients who followed up with additional services, the content, type, and outcome of these services were explored. Next, linear regression was used to determine whether number of conjoint sessions was associated with a qualitative treatment outcome, and 2 logistic regressions were used to determine whether the number of sessions was associated with the likelihood of accepting services and follow-through with services after accepting them. An additional logistic regression examined whether having a mental health diagnosis (yes/no) was associated with whether the individual accepted additional health psychology services. Finally, independent sample t tests examined differences between those who accepted services vs those who declined follow-up services in substance use diagnosis, mental health diagnosis, and previous health psychology services engagement. Of note, given the small sample size, the Levene’s test for equality of variances was conducted and unequal variances were assumed.
Results
All 24 patients agreed to have the conjoint session with the clinical pharmacy specialist and health psychologist. Of the participants, 62.5% had no previous interaction with health psychology services. Among those who had previous encounters with health psychology services, 12.5% had participated in 1 or more group sessions, another 12.5% had participated in 1 or more individual sessions, and an additional 12.5% had been referred for health psychology services but had not followed through. A total of 10 participants represented a new- or re-engagement in health psychology services following the conjoint appointment. Two patients were referred for additional services as a result of their conjoint appointment (1 to specialty mental health and another to Primary Care-Mental Health Integration [PC-MHI]), and 1 of the participants followed through with the referral. Finally, with regard to the content of the initial session, 37.5% of the sessions contained some form of psychoeducation, 54.2% contained a functional assessment, and 41.7% contained an introduction of skills.
Half of the veterans participated in health psychology services beyond the initial conjoint session. Four of these veterans participated in additional conjoint sessions, and the remaining 8 engaged in health psychology services, which took the form of telephone sessions (3), in-person sessions (3), or a combination of both telephone and in-person sessions (2). Twelve veterans participated in an average of 3.4 (SD 3.7) follow-up sessions. In terms of the content of these follow-up sessions, across all formats and types, 3 included some introduction to coping skills, with no documented evidence of follow-through. For 2 of the veterans engaging in some type of follow-up, there was documented use of coping skills, and 2 used the coping skills with self-reported success and benefit. Finally, documentation revealed evidence that 3 of these veterans were not only using the coping skills with benefit, but also reported an improvement in pain management overall. One also was connected with a different service.
Regarding reasons for completion of services, 2 veterans were terminated due to completing treatment/meeting goals, 2 were terminated because they did not follow up after a session, 7 were terminated due to patient declining additional sessions, and 1 veteran was still receiving services at the time of the review. Linear regression indicated that the number of conjoint sessions was not associated with qualitative treatment outcome. Two logistic regressions indicated that number of conjoint sessions was not related to whether the veteran accepted follow-up services or whether the veteran followed through with services after accepting. Of note, logistic regression indicated that having a mental health diagnosiswas associated with a decreased likelihood of accepting health psychology services (P = .03). Regarding the independent samples t tests, veterans who did not accept follow-up services were more likely to have a mental health diagnosis (P = .03). The groups did not differ significantly with regard to substance use diagnosis or previous engagement in health psychology services.
Discussion
Results showed that all 24 veterans who were offered a conjoint session with a clinical pharmacy specialist and health psychologist engaged in at least 1 session. Half the veterans participated in further services as well. Both the initial conjoint and follow-up sessions offered a greater degree of communication related to the cognitive-behavioral and functional restoration components of behavioral pain management. Given that a majority of the sample had not participated in behavioral or mental health services previously, this may represent a greater penetration rate of exposure to mental health service for veterans than would have been available otherwise.
More specifically, qualitative results suggest that in these conjoint sessions, the veterans were exposed to behavioral psychotherapeutic approaches to chronic pain management (eg, health behavior change, motivational enhancement, health-related psychoeducation, and CBT for chronic pain) that again may not have been provided otherwise (ie, via referral and separately scheduled sessions). These findings are supported by theories consistent with the Transtheoretical Model, which indicates that individuals fall in varying degrees of readiness for behavioral change (ie, precontemplative, contemplative, planning, action, maintenance).21,22 Thus, behavioral intervention approaches must be adaptive and adjust format and communication, including the amount and type of psychoeducation offered. Moreover, the integrated theory of health behavior change in the context of chronic pain management calls for fostering awareness, knowledge, and beliefs through effective communication and education for a wide range of individuals who are at varying stages of change.23 In addition to the conjoint session and subsequent service(s) content that were reviewed and coded in this current project, future projects might draw on these theoretical models and code sessions for patients’ stages of change and assess whether a patient made progress across phases of change (eg, the patient shifted from contemplative to the planning stage of change).
Within this project’s conjoint sessions and consistent with MI principles, veterans were offered discussions related to the bidirectional and BPS aspects of their own chronic pain experience. That is, while discussing responses and adjustment to pain medication(s), veterans received reflections with MI and heard feedback related to their current coping strategies, methods to enhance coping, as well as potential psychosocial impacts of their chronic pain experience. With permission, veterans also were introduced to themes that comprised evidence-based CBT for chronic pain (CBT-CP) intervention. Understanding what change means in the context of chronic pain management is critical. That is, tipping the conversation toward consideration of alternative modalities (eg, relaxation, stress management, cognitions, and pain) in conjunction with or in place of the traditional modalities (eg, medication, pain procedures) is paramount.
Clinicians must listen for patient ambivalence related to procedures, interventions, medication changes, and/or the behavioral self-management of chronic pain. This type of active listening and exploration may be more likely when there is collaboration and effective team functioning among clinicians than when clinicians provide care independently. Future quality improvement (QI) or research projects could extend the EHR review and evaluate clinician-patient transcripts for fidelity to the CBT-CP and MI models. Such efforts could assess for associations between clinician MI consistent behaviors and change talk on the part of the patient. Furthermore, clinician communication and patient change talk from transcripts could be evaluated in relation to evidence from the EHR regarding patient use of coping skills and behavior change.
Consistent with behavioral health literature, having a mental health diagnosis was associated with declining additional behavioral health psychology services in this project. Research has shown that individuals with a mental health diagnosis tend to engage less in behavioral health self-management programs, such as chronic headache and weight management.24-26 This phenomenon lends support for the importance of health care professionals (HCPs) to increase access and exposure to mental and behavioral health services, such as the PC-MHI model.20 In fact, chronic pain management program development efforts within the VA system nationwide include collaboration with the PC-MHI services. One of the initial goals for PC-MHI services is to increase penetration rates into the general outpatient medical clinics and enhance engagement in mental health services.
Using conjoint sessions as was offered in the current project is one step in the development of more comprehensive interdisciplinary teams through interprofessional collaboration and the use of effective clinical communication. In turn, it will be important to directly explore the communication skills and attitudes of these HCPs with regard to interdisciplinary program development and collaboration as teams continue to integrate more broadly into the medical system and enhance chronic pain management services.11 Similarly, measuring the perceptions of clinical pharmacy specialists, physicians, health psychologists, or other clinical disciplines involved in chronic pain management could be another area to explore. More specific to MI, clinician confidence in the use of effective communication and MI skills represents still another area for future study.16
Limitations
Some limitations and suggested future directions found as part of this QI project have been outlined earlier. Other limitations include the used of a retrospective review of information available in patient medical charts. More developed measurement-based care or research could collect self-reports of patient satisfaction with care, functioning, knowledge, readiness for change, and mood in addition to what is noted and documented in clinical observations. Second, the sample was small and did not include any female and few younger veterans, even though these are important subpopulations when examining pain management services. When resources are available for a larger sample size, some exploratory analyses could be conducted for differences in engagement among subgroups. Third, this project may have further confounding variables as this was not an experimental or a controlled study, which could directly compare conjoint sessions with referral-based care and/or those not offered conjoint sessions.
Conclusion
The optimal method of behavioral pain management suggests the need for an interdisciplinary, coordinated team approach, in which the gold standard programs meet requirements set by CARF. However, on a practical level, optimal behavioral pain management may not be feasible at all health care facilities. Furthermore, in an effort to provide best practices to individuals with chronic pain, clinicians must be adaptive and skilled in using effective communication and specialized interventions, such as CBT and MI.
Approaching the more optimal behavioral self-management of chronic pain from a multimodal interdisciplinary perspective and further engaging veterans in this care is paramount. This project is merely one step in this effort that can shed light on the function and logistic outcomes of using a practical, integrated approach to chronic pain. It demonstrates that implementing best practices founded in sound theoretical models despite staffing and resource constraints is possible. Thus, continuing to explore the utility of alternate modalities may offer important applied and translational information to help disseminate and improve chronic pain management services.
Future research could focus on important subpopulations and enhance experimental design with pre- and postmeasures, controlling for possible confounding variables and if possible a controlled design.
Acknowledgments
This quality improvement project was unfunded, and approval was confirmed with the VA Ann Arbor Healthcare System Institutional Review Board and Research & Development committees. The authors also thank Associate Chief of Staff, Ambulatory Care, Clinton Greenstone, MD, and Chief of Primary Care Adam Tremblay, MD, for their leadership and support of these integrative services and quality improvement efforts. The authors especially recognize the veterans for whom they aim to provide the highest quality of services possible.
1. Brooks PM. The burden of musculoskeletal disease—a global perspective. Clin Rheumatol. 2006;25(6):778-781.
2. Haskell SG, Heapy A, Reid MC, Papas RK, Kerns RD. The prevalence and age-related characteristics of pain in a sample of women veterans receiving primary care. J Womens Health (Larchmt). 2006;15(7):862-869.
3. Roth RS, Geisser ME, Williams DA. Interventional pain medicine: retreat from the biopsychosocial model of pain. Transl Behav Med. 2012;2(1):106-116.
4. Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129-136.
5. Borrell-Carrió F, Suchman AL, Epstein RM. The biopsychosocial model 25 years later: principles, practice, and scientific inquiry. Ann Fam Med. 2004;2(6):576-582.
6. Wall PD. The gate control theory of pain mechanisms: a re-examination and re-statement. Brain. 1978;101(1):1-18.
7. Dobscha SK, Corson K, Perrin NA, et al. Collaborative care for chronic pain in primary care: a cluster randomized trial. JAMA. 2009;301(12):1242-1252.
8. Von Korff, Moore JC. Stepped care for back pain: activating approaches for primary care. Ann Intern Med. 2001;134(9, pt 2):911-917.
9. Oslund S, Robinson RC, Clark TC, et al. Long-term effectiveness of a comprehensive pain management program: strengthening the case for interdisciplinary care. Proc (Bayl Univ Med Cent). 2009;22(3):211-214.
10. Scascighini L, Toma V, Dober-Spielmann S, Sprott H. Multidisciplinary treatment for chronic pain: a systematic review of interventions and outcomes. Rheumatology (Oxford). 2008;47(5):670-678.
11. Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119-130.
12. McCracken LM, Turk DC. Behavioral and cognitive-behavioral treatment for chronic pain: outcome, predictors of outcome, and treatment process. Spine (Phila Pa 1976). 2002;27(22): 2564-2573.
13. Jeffery MM, Butler M, Stark A, Kane RL. Multidisciplinary Pain Programs for Chronic Noncancer Pain. Comparative Effectiveness Technical Briefs, No 8. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
14. Ehde DM, Dilworth TM, Turner JA. Cognitive-behavioral therapy for individuals with chronic pain: efficacy, innovations, and directions for research. Am Psychol. 2014;69(2):153-166.
15. Dorflinger L, Kerns RD, Auerbach SM. Providers’ roles in enhancing patients’ adherence to pain self management. Transl Behav Med. 2013;3(1):39-46.
16. Pellico LH, Gilliam WP, Lee AW, Kerns RD. Hearing new voices: registered nurses and health technicians experience caring for chronic pain patients in primary care clinics. Open Nurs J. 2014;8:25-33.
17. Rollnick S, Miller WR, Butler CC, eds. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, NY: The Guilford Press; 2008.
18. Kerns RD, Habib S. A critical review of the pain readiness to change model. J Pain. 2004;5(7):357-367.19. Kerns RD, Burns JW, Shulman M, et al. Can we improve cognitive-behavior therapy for chronic back pain treatment engagement and adherence? A controlled trial of tailored versus standard therapy. Health Psychol. 2014;33(9):938-947.
20. Kearney LK, Post EP, Zeiss A, Goldstein MG, Dundon M. The role of mental and behavioral health in the application of the patient-centered medical home in the Department of Veterans Affairs. Transl Behav Med. 2011;1(4):624-628.
21. Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51(3):390-395.
22. Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. Am Psychol. 1992;47(9):1102-1114.
23. Ryan P. Integrated theory of health behavior change: background and intervention development. Clin Nurse Spec. 2009;23(3):161-172.
24. Evans DD , Blanchard EB. Prediction of early termination from the self-regulatory treatment of chronic headache. Biofeedback Self Regul. 1988;13(3):245-256.
25. Maguen S, Hoerster KD, Littman AJ, et al. Iraq and Afghanistan veterans with PTSD participate less in VA’s weight loss program than those without PTSD. J Affect Disord. 2016;193:289-294.
26. Bloor LE. Improving weight management services for female veterans: design and participation factors with a women only program, and comparisons with gender neutral services. Med Res Arch. 2015;2.
Providing comprehensive, integrated, behavioral intervention services to address the prevalent condition of chronic, noncancer pain is a growing concern. Although the biopsychosocial model (BPS) and stepped-care approaches have been understood and discussed for some time, clinician and patient understanding and investment in these approaches continue to face challenges. Moreover, even when resources (eg, staffing, referral options, space) are available, clinicians and patients must engage in meaningful communication to achieve this type of care.
Importantly, engagement means moving beyond diagnosis and assessment and offering interventions that provide psychoeducation related to the chronic pain cycle. These interventions address maladaptive cognitions and beliefs about movement and pain; promote paced, daily physical activity and engagement in life; and help increase coping skills to improve low mood or distress, all fundamental components of the BPS understanding of chronic pain.
Background
Chronic, noncancer pain is a prevalent presentation in primary care settings in the U.S. and even more so for veterans.1 Fifty percent of male veterans and 75% of female veterans report chronic pain as an important condition that impacts their health.2 An important aspect of this prevalence is the focus on opioid pain medication and medical procedures, both of which draw more narrowly on the biomedical model. Additional information on the longer term use of pain procedures and opioid medications is now available,and given some risks and limitations (eg, tolerance, decreasing efficacy, opioid-induced medical complications), the need to study and offer other options is gaining attention.3 Behavioral chronic pain management has a clear historic role that draws on the BPS modeland Gate Control Theory.3-6
More recently, the National Strategy of Chronic Pain collaborative and stepped-care models extended this literature, outlining collaboration and levels of care depending on the chronicity of the pain experience as well as co-occurring conditions and patient presentations.7,8 The Commission on Accreditation of Rehabilitation Facilities (CARF), the gold standard in interdisciplinary pain management programs, calls for further resources and coordination of these efforts, including a tertiary level of care representing the highest step in the stepped-care model.8
These interdisciplinary, integrative pain management programs, which include functional restoration and cognitive behavioral therapy (CBT) interventions, have been effective for the treatment of chronic pain.9-12However, the staffing, resources, clinical access, and coordination of this complex care may not be feasible in many health care settings. For example, a 2005 survey reported that there were only 200 multidisciplinary pain programs in the U.S., and only 84 of them were CARF accredited.13 By 2011 the number of CARF-accredited programs had decreased to 64 (the number of nonaccredited programs was not reported for 2011).13
Furthermore, engagement in behavioral pain management services is a challenge: Studies show that psychosocial interventions are underused, and a majority of studies may not report quantitatively or qualitatively on patient adherence or engagement in these services.14 These realities introduce the idea that coordinated appointments between 2 or 3 different disciplines available in primary care may be a feasible step toward implementing more comprehensive, optimal care models.
Behavioral pain management interventions that uphold the BPS also call on the idea of active self-management. Therefore, effective communication is fundamental at both the provider-patient and interprofessional levels to enhance engagement in health care, receptiveness to interventions, and to self-management of chronic pain.11,15 How clinicians conceptualize, hold assumptions about, and communicate with patients about chronic pain management has received more attention.15,16
Clinician Considerations for Pain Management
On theclinicians’ side, monitoring assumptions about patients and awareness of their beliefs as well as the care itself are foundational in patient interactions, impacting the success of patient engagement. Awareness of the language used in these interactions and how clinicians collaborate with other professionals become salient. Coupled with the reality of high attrition, this discussion lends itself in important ways to the motivational interviewing (MI) approach that aims to meet patients “where they are” by use of open-ended questions and reflective listening to guide the conversation in the direction of contemplating or actual behavior change.17 For example, “What do you think are the best ways to manage your pain?” and “It sounds like sometimes the medicine helps, but you also want more options to feel in control of your pain.”
Given the historic focus on the biomedical approach to chronic pain, including the use of opioid medications and medical procedures as well as traditional challenges to engagement in CBT, researchers have explored whether alternative methods may increase participation and improve outcomes for behavioral self-management.3 Drawing on a history of assessing readiness for change in pain management, Kerns and colleagues offered tailored cognitive strategies or behavioral skills training depending on patient preferences.18,19 These researchers also incorporated motivational enhancement strategies in the tailored interventions and compared engagement with standard CBT for chronic pain protocol. Although they did not find significant differences in engagement between the 2 groups, participation and treatment adherence were associated with posttreatment improvements in both groups.19 Taking a step back from enhancing intervention engagement, first assessing readiness to self-manage becomes another salient exploration and step in the process.
Another element of engagement in services is referral to other clinicians. Dorflinger and colleagues made this point in a conceptual paper that broadly outlined interdisciplinary, integrative, and more comprehensive models of care for chronic pain.15 We know from integrated models that referral-based care may decrease the likelihood of participation in health care services. That is, if a patient needs to make a separate appointment and meet with a new clinician, they are more likely to decline, cancel, or not show, particularly if they are not “ready” for change. Co-located or embedded care and conjoint sessions that include a warm handoff or another clinician who joins the first appointment may reduce stigma and other relevant barriers for introducing a patient to new ideas.20
Using a conjoint session that involves a clinical pharmacy pain specialist and a health psychologist is one way in which veterans can be exposed to more chronic pain-related BPS concepts and behavioral health services than they might be exposed to otherwise. The purpose of this project was to bring awareness to a practical and clinically relevant integrated approach to the dissemination of BPS information for chronic pain management.
In providing this information through effective communication at the patient-provider and interprofessional levels, the clinicians’ intention was to increase patient engagement and use of BPS strategies in the self-management of chronic pain. This project also aimed to enhance engagement and improve the quality of services before acquiring additional positions and funding for a specialized pain management team. These sessions were offered at the VA Ann Arbor Healthcare System (VAAAHS) in Michigan. Quantitative and qualitative information was examined from the conjoint and subsequent sessions that occurred in this setting.
Methods
With the above concepts in mind, VAAAHS offered veterans conjoint sessions involving a health psychologist and clinical pharmacy specialist during a 3-month period while this resource was available. The conjoint sessions were part of a preexisting pharmacist-run pain medication clinic embedded in primary care. The conjoint session was presented to patients as part of general clinic flow to reduce stigma of engagement in psychological services and allow for the dissemination of BPS information.
Participants
The electronic health records (EHR) of 24 veteran patients with chronic pain, who participated in a conjoint health psychology/pain pharmacy session, were reviewed for the current study. Most of the patients were male (95.8%) and non-Hispanic white (91.7%); the remaining participants did not disclose their ethnicity. The mean age was 60.6 years (SD 7.1; range 50-80). A total of 75% had a mental health diagnosis, and 41.7% were in mental health treatment at the time of the conjoint appointment. Among the sample, 20.8% had a current diagnosis of a substance use disorder (SUD), and no individuals were in treatment for a SUD at the time of the conjoint appointment. Patients received an average of 1.5 conjoint sessions (SD 0.7; range 1-3).
Procedure
The veterans for this project were chosen from a panel of patients followed by the pain medication clinical pharmacy specialist in the primary care pain medication clinic. The selected veterans were offered a joint session with their clinical pharmacy provider and the health psychology resident during their scheduled visit in the pain medication clinic. Each veteran was informed that the goal of the joint visit was to enhance self-directed nonpharmacologic chronic pain management skills as an additional set of tools in the tool kit for particularly difficult pain days. Veterans were assured that their usual care would not be compromised if they declined the session.
During the encounter(s), the health psychologist contributed to the veteran’s care by using MI and CBT for chronic pain skills. The health psychologist further assessed concerns and needs and guided the discussion as appropriate. With veteran readiness, these discussions explored the degree of knowledge and cognitive and behavioral coping skills the patient used. These conjoint sessions also documented the types of discussions and degree of engagement in the encounter(s) as well additional referrals, complementary services, and/or offered follow-up services for either additional conjoint sessions or further health psychology-related services.
A total of 24 EHRs from these conjoint and subsequent encounters were reviewed for evidence of the procedures by a psychology intern involved in chronic pain management services. Of these 24 records, 6 also were reviewed by a board-certified health psychologist for consensus building and agreement on coding (Sidebar, Record Coding).
Using the coding system and SPSS Version 2.1 (IBM, Armonk NY), descriptive statistics were used to examine conjoint session content and new- or re-engagement in health psychology services following the conjoint sessions. For those patients who followed up with additional services, the content, type, and outcome of these services were explored. Next, linear regression was used to determine whether number of conjoint sessions was associated with a qualitative treatment outcome, and 2 logistic regressions were used to determine whether the number of sessions was associated with the likelihood of accepting services and follow-through with services after accepting them. An additional logistic regression examined whether having a mental health diagnosis (yes/no) was associated with whether the individual accepted additional health psychology services. Finally, independent sample t tests examined differences between those who accepted services vs those who declined follow-up services in substance use diagnosis, mental health diagnosis, and previous health psychology services engagement. Of note, given the small sample size, the Levene’s test for equality of variances was conducted and unequal variances were assumed.
Results
All 24 patients agreed to have the conjoint session with the clinical pharmacy specialist and health psychologist. Of the participants, 62.5% had no previous interaction with health psychology services. Among those who had previous encounters with health psychology services, 12.5% had participated in 1 or more group sessions, another 12.5% had participated in 1 or more individual sessions, and an additional 12.5% had been referred for health psychology services but had not followed through. A total of 10 participants represented a new- or re-engagement in health psychology services following the conjoint appointment. Two patients were referred for additional services as a result of their conjoint appointment (1 to specialty mental health and another to Primary Care-Mental Health Integration [PC-MHI]), and 1 of the participants followed through with the referral. Finally, with regard to the content of the initial session, 37.5% of the sessions contained some form of psychoeducation, 54.2% contained a functional assessment, and 41.7% contained an introduction of skills.
Half of the veterans participated in health psychology services beyond the initial conjoint session. Four of these veterans participated in additional conjoint sessions, and the remaining 8 engaged in health psychology services, which took the form of telephone sessions (3), in-person sessions (3), or a combination of both telephone and in-person sessions (2). Twelve veterans participated in an average of 3.4 (SD 3.7) follow-up sessions. In terms of the content of these follow-up sessions, across all formats and types, 3 included some introduction to coping skills, with no documented evidence of follow-through. For 2 of the veterans engaging in some type of follow-up, there was documented use of coping skills, and 2 used the coping skills with self-reported success and benefit. Finally, documentation revealed evidence that 3 of these veterans were not only using the coping skills with benefit, but also reported an improvement in pain management overall. One also was connected with a different service.
Regarding reasons for completion of services, 2 veterans were terminated due to completing treatment/meeting goals, 2 were terminated because they did not follow up after a session, 7 were terminated due to patient declining additional sessions, and 1 veteran was still receiving services at the time of the review. Linear regression indicated that the number of conjoint sessions was not associated with qualitative treatment outcome. Two logistic regressions indicated that number of conjoint sessions was not related to whether the veteran accepted follow-up services or whether the veteran followed through with services after accepting. Of note, logistic regression indicated that having a mental health diagnosiswas associated with a decreased likelihood of accepting health psychology services (P = .03). Regarding the independent samples t tests, veterans who did not accept follow-up services were more likely to have a mental health diagnosis (P = .03). The groups did not differ significantly with regard to substance use diagnosis or previous engagement in health psychology services.
Discussion
Results showed that all 24 veterans who were offered a conjoint session with a clinical pharmacy specialist and health psychologist engaged in at least 1 session. Half the veterans participated in further services as well. Both the initial conjoint and follow-up sessions offered a greater degree of communication related to the cognitive-behavioral and functional restoration components of behavioral pain management. Given that a majority of the sample had not participated in behavioral or mental health services previously, this may represent a greater penetration rate of exposure to mental health service for veterans than would have been available otherwise.
More specifically, qualitative results suggest that in these conjoint sessions, the veterans were exposed to behavioral psychotherapeutic approaches to chronic pain management (eg, health behavior change, motivational enhancement, health-related psychoeducation, and CBT for chronic pain) that again may not have been provided otherwise (ie, via referral and separately scheduled sessions). These findings are supported by theories consistent with the Transtheoretical Model, which indicates that individuals fall in varying degrees of readiness for behavioral change (ie, precontemplative, contemplative, planning, action, maintenance).21,22 Thus, behavioral intervention approaches must be adaptive and adjust format and communication, including the amount and type of psychoeducation offered. Moreover, the integrated theory of health behavior change in the context of chronic pain management calls for fostering awareness, knowledge, and beliefs through effective communication and education for a wide range of individuals who are at varying stages of change.23 In addition to the conjoint session and subsequent service(s) content that were reviewed and coded in this current project, future projects might draw on these theoretical models and code sessions for patients’ stages of change and assess whether a patient made progress across phases of change (eg, the patient shifted from contemplative to the planning stage of change).
Within this project’s conjoint sessions and consistent with MI principles, veterans were offered discussions related to the bidirectional and BPS aspects of their own chronic pain experience. That is, while discussing responses and adjustment to pain medication(s), veterans received reflections with MI and heard feedback related to their current coping strategies, methods to enhance coping, as well as potential psychosocial impacts of their chronic pain experience. With permission, veterans also were introduced to themes that comprised evidence-based CBT for chronic pain (CBT-CP) intervention. Understanding what change means in the context of chronic pain management is critical. That is, tipping the conversation toward consideration of alternative modalities (eg, relaxation, stress management, cognitions, and pain) in conjunction with or in place of the traditional modalities (eg, medication, pain procedures) is paramount.
Clinicians must listen for patient ambivalence related to procedures, interventions, medication changes, and/or the behavioral self-management of chronic pain. This type of active listening and exploration may be more likely when there is collaboration and effective team functioning among clinicians than when clinicians provide care independently. Future quality improvement (QI) or research projects could extend the EHR review and evaluate clinician-patient transcripts for fidelity to the CBT-CP and MI models. Such efforts could assess for associations between clinician MI consistent behaviors and change talk on the part of the patient. Furthermore, clinician communication and patient change talk from transcripts could be evaluated in relation to evidence from the EHR regarding patient use of coping skills and behavior change.
Consistent with behavioral health literature, having a mental health diagnosis was associated with declining additional behavioral health psychology services in this project. Research has shown that individuals with a mental health diagnosis tend to engage less in behavioral health self-management programs, such as chronic headache and weight management.24-26 This phenomenon lends support for the importance of health care professionals (HCPs) to increase access and exposure to mental and behavioral health services, such as the PC-MHI model.20 In fact, chronic pain management program development efforts within the VA system nationwide include collaboration with the PC-MHI services. One of the initial goals for PC-MHI services is to increase penetration rates into the general outpatient medical clinics and enhance engagement in mental health services.
Using conjoint sessions as was offered in the current project is one step in the development of more comprehensive interdisciplinary teams through interprofessional collaboration and the use of effective clinical communication. In turn, it will be important to directly explore the communication skills and attitudes of these HCPs with regard to interdisciplinary program development and collaboration as teams continue to integrate more broadly into the medical system and enhance chronic pain management services.11 Similarly, measuring the perceptions of clinical pharmacy specialists, physicians, health psychologists, or other clinical disciplines involved in chronic pain management could be another area to explore. More specific to MI, clinician confidence in the use of effective communication and MI skills represents still another area for future study.16
Limitations
Some limitations and suggested future directions found as part of this QI project have been outlined earlier. Other limitations include the used of a retrospective review of information available in patient medical charts. More developed measurement-based care or research could collect self-reports of patient satisfaction with care, functioning, knowledge, readiness for change, and mood in addition to what is noted and documented in clinical observations. Second, the sample was small and did not include any female and few younger veterans, even though these are important subpopulations when examining pain management services. When resources are available for a larger sample size, some exploratory analyses could be conducted for differences in engagement among subgroups. Third, this project may have further confounding variables as this was not an experimental or a controlled study, which could directly compare conjoint sessions with referral-based care and/or those not offered conjoint sessions.
Conclusion
The optimal method of behavioral pain management suggests the need for an interdisciplinary, coordinated team approach, in which the gold standard programs meet requirements set by CARF. However, on a practical level, optimal behavioral pain management may not be feasible at all health care facilities. Furthermore, in an effort to provide best practices to individuals with chronic pain, clinicians must be adaptive and skilled in using effective communication and specialized interventions, such as CBT and MI.
Approaching the more optimal behavioral self-management of chronic pain from a multimodal interdisciplinary perspective and further engaging veterans in this care is paramount. This project is merely one step in this effort that can shed light on the function and logistic outcomes of using a practical, integrated approach to chronic pain. It demonstrates that implementing best practices founded in sound theoretical models despite staffing and resource constraints is possible. Thus, continuing to explore the utility of alternate modalities may offer important applied and translational information to help disseminate and improve chronic pain management services.
Future research could focus on important subpopulations and enhance experimental design with pre- and postmeasures, controlling for possible confounding variables and if possible a controlled design.
Acknowledgments
This quality improvement project was unfunded, and approval was confirmed with the VA Ann Arbor Healthcare System Institutional Review Board and Research & Development committees. The authors also thank Associate Chief of Staff, Ambulatory Care, Clinton Greenstone, MD, and Chief of Primary Care Adam Tremblay, MD, for their leadership and support of these integrative services and quality improvement efforts. The authors especially recognize the veterans for whom they aim to provide the highest quality of services possible.
Providing comprehensive, integrated, behavioral intervention services to address the prevalent condition of chronic, noncancer pain is a growing concern. Although the biopsychosocial model (BPS) and stepped-care approaches have been understood and discussed for some time, clinician and patient understanding and investment in these approaches continue to face challenges. Moreover, even when resources (eg, staffing, referral options, space) are available, clinicians and patients must engage in meaningful communication to achieve this type of care.
Importantly, engagement means moving beyond diagnosis and assessment and offering interventions that provide psychoeducation related to the chronic pain cycle. These interventions address maladaptive cognitions and beliefs about movement and pain; promote paced, daily physical activity and engagement in life; and help increase coping skills to improve low mood or distress, all fundamental components of the BPS understanding of chronic pain.
Background
Chronic, noncancer pain is a prevalent presentation in primary care settings in the U.S. and even more so for veterans.1 Fifty percent of male veterans and 75% of female veterans report chronic pain as an important condition that impacts their health.2 An important aspect of this prevalence is the focus on opioid pain medication and medical procedures, both of which draw more narrowly on the biomedical model. Additional information on the longer term use of pain procedures and opioid medications is now available,and given some risks and limitations (eg, tolerance, decreasing efficacy, opioid-induced medical complications), the need to study and offer other options is gaining attention.3 Behavioral chronic pain management has a clear historic role that draws on the BPS modeland Gate Control Theory.3-6
More recently, the National Strategy of Chronic Pain collaborative and stepped-care models extended this literature, outlining collaboration and levels of care depending on the chronicity of the pain experience as well as co-occurring conditions and patient presentations.7,8 The Commission on Accreditation of Rehabilitation Facilities (CARF), the gold standard in interdisciplinary pain management programs, calls for further resources and coordination of these efforts, including a tertiary level of care representing the highest step in the stepped-care model.8
These interdisciplinary, integrative pain management programs, which include functional restoration and cognitive behavioral therapy (CBT) interventions, have been effective for the treatment of chronic pain.9-12However, the staffing, resources, clinical access, and coordination of this complex care may not be feasible in many health care settings. For example, a 2005 survey reported that there were only 200 multidisciplinary pain programs in the U.S., and only 84 of them were CARF accredited.13 By 2011 the number of CARF-accredited programs had decreased to 64 (the number of nonaccredited programs was not reported for 2011).13
Furthermore, engagement in behavioral pain management services is a challenge: Studies show that psychosocial interventions are underused, and a majority of studies may not report quantitatively or qualitatively on patient adherence or engagement in these services.14 These realities introduce the idea that coordinated appointments between 2 or 3 different disciplines available in primary care may be a feasible step toward implementing more comprehensive, optimal care models.
Behavioral pain management interventions that uphold the BPS also call on the idea of active self-management. Therefore, effective communication is fundamental at both the provider-patient and interprofessional levels to enhance engagement in health care, receptiveness to interventions, and to self-management of chronic pain.11,15 How clinicians conceptualize, hold assumptions about, and communicate with patients about chronic pain management has received more attention.15,16
Clinician Considerations for Pain Management
On theclinicians’ side, monitoring assumptions about patients and awareness of their beliefs as well as the care itself are foundational in patient interactions, impacting the success of patient engagement. Awareness of the language used in these interactions and how clinicians collaborate with other professionals become salient. Coupled with the reality of high attrition, this discussion lends itself in important ways to the motivational interviewing (MI) approach that aims to meet patients “where they are” by use of open-ended questions and reflective listening to guide the conversation in the direction of contemplating or actual behavior change.17 For example, “What do you think are the best ways to manage your pain?” and “It sounds like sometimes the medicine helps, but you also want more options to feel in control of your pain.”
Given the historic focus on the biomedical approach to chronic pain, including the use of opioid medications and medical procedures as well as traditional challenges to engagement in CBT, researchers have explored whether alternative methods may increase participation and improve outcomes for behavioral self-management.3 Drawing on a history of assessing readiness for change in pain management, Kerns and colleagues offered tailored cognitive strategies or behavioral skills training depending on patient preferences.18,19 These researchers also incorporated motivational enhancement strategies in the tailored interventions and compared engagement with standard CBT for chronic pain protocol. Although they did not find significant differences in engagement between the 2 groups, participation and treatment adherence were associated with posttreatment improvements in both groups.19 Taking a step back from enhancing intervention engagement, first assessing readiness to self-manage becomes another salient exploration and step in the process.
Another element of engagement in services is referral to other clinicians. Dorflinger and colleagues made this point in a conceptual paper that broadly outlined interdisciplinary, integrative, and more comprehensive models of care for chronic pain.15 We know from integrated models that referral-based care may decrease the likelihood of participation in health care services. That is, if a patient needs to make a separate appointment and meet with a new clinician, they are more likely to decline, cancel, or not show, particularly if they are not “ready” for change. Co-located or embedded care and conjoint sessions that include a warm handoff or another clinician who joins the first appointment may reduce stigma and other relevant barriers for introducing a patient to new ideas.20
Using a conjoint session that involves a clinical pharmacy pain specialist and a health psychologist is one way in which veterans can be exposed to more chronic pain-related BPS concepts and behavioral health services than they might be exposed to otherwise. The purpose of this project was to bring awareness to a practical and clinically relevant integrated approach to the dissemination of BPS information for chronic pain management.
In providing this information through effective communication at the patient-provider and interprofessional levels, the clinicians’ intention was to increase patient engagement and use of BPS strategies in the self-management of chronic pain. This project also aimed to enhance engagement and improve the quality of services before acquiring additional positions and funding for a specialized pain management team. These sessions were offered at the VA Ann Arbor Healthcare System (VAAAHS) in Michigan. Quantitative and qualitative information was examined from the conjoint and subsequent sessions that occurred in this setting.
Methods
With the above concepts in mind, VAAAHS offered veterans conjoint sessions involving a health psychologist and clinical pharmacy specialist during a 3-month period while this resource was available. The conjoint sessions were part of a preexisting pharmacist-run pain medication clinic embedded in primary care. The conjoint session was presented to patients as part of general clinic flow to reduce stigma of engagement in psychological services and allow for the dissemination of BPS information.
Participants
The electronic health records (EHR) of 24 veteran patients with chronic pain, who participated in a conjoint health psychology/pain pharmacy session, were reviewed for the current study. Most of the patients were male (95.8%) and non-Hispanic white (91.7%); the remaining participants did not disclose their ethnicity. The mean age was 60.6 years (SD 7.1; range 50-80). A total of 75% had a mental health diagnosis, and 41.7% were in mental health treatment at the time of the conjoint appointment. Among the sample, 20.8% had a current diagnosis of a substance use disorder (SUD), and no individuals were in treatment for a SUD at the time of the conjoint appointment. Patients received an average of 1.5 conjoint sessions (SD 0.7; range 1-3).
Procedure
The veterans for this project were chosen from a panel of patients followed by the pain medication clinical pharmacy specialist in the primary care pain medication clinic. The selected veterans were offered a joint session with their clinical pharmacy provider and the health psychology resident during their scheduled visit in the pain medication clinic. Each veteran was informed that the goal of the joint visit was to enhance self-directed nonpharmacologic chronic pain management skills as an additional set of tools in the tool kit for particularly difficult pain days. Veterans were assured that their usual care would not be compromised if they declined the session.
During the encounter(s), the health psychologist contributed to the veteran’s care by using MI and CBT for chronic pain skills. The health psychologist further assessed concerns and needs and guided the discussion as appropriate. With veteran readiness, these discussions explored the degree of knowledge and cognitive and behavioral coping skills the patient used. These conjoint sessions also documented the types of discussions and degree of engagement in the encounter(s) as well additional referrals, complementary services, and/or offered follow-up services for either additional conjoint sessions or further health psychology-related services.
A total of 24 EHRs from these conjoint and subsequent encounters were reviewed for evidence of the procedures by a psychology intern involved in chronic pain management services. Of these 24 records, 6 also were reviewed by a board-certified health psychologist for consensus building and agreement on coding (Sidebar, Record Coding).
Using the coding system and SPSS Version 2.1 (IBM, Armonk NY), descriptive statistics were used to examine conjoint session content and new- or re-engagement in health psychology services following the conjoint sessions. For those patients who followed up with additional services, the content, type, and outcome of these services were explored. Next, linear regression was used to determine whether number of conjoint sessions was associated with a qualitative treatment outcome, and 2 logistic regressions were used to determine whether the number of sessions was associated with the likelihood of accepting services and follow-through with services after accepting them. An additional logistic regression examined whether having a mental health diagnosis (yes/no) was associated with whether the individual accepted additional health psychology services. Finally, independent sample t tests examined differences between those who accepted services vs those who declined follow-up services in substance use diagnosis, mental health diagnosis, and previous health psychology services engagement. Of note, given the small sample size, the Levene’s test for equality of variances was conducted and unequal variances were assumed.
Results
All 24 patients agreed to have the conjoint session with the clinical pharmacy specialist and health psychologist. Of the participants, 62.5% had no previous interaction with health psychology services. Among those who had previous encounters with health psychology services, 12.5% had participated in 1 or more group sessions, another 12.5% had participated in 1 or more individual sessions, and an additional 12.5% had been referred for health psychology services but had not followed through. A total of 10 participants represented a new- or re-engagement in health psychology services following the conjoint appointment. Two patients were referred for additional services as a result of their conjoint appointment (1 to specialty mental health and another to Primary Care-Mental Health Integration [PC-MHI]), and 1 of the participants followed through with the referral. Finally, with regard to the content of the initial session, 37.5% of the sessions contained some form of psychoeducation, 54.2% contained a functional assessment, and 41.7% contained an introduction of skills.
Half of the veterans participated in health psychology services beyond the initial conjoint session. Four of these veterans participated in additional conjoint sessions, and the remaining 8 engaged in health psychology services, which took the form of telephone sessions (3), in-person sessions (3), or a combination of both telephone and in-person sessions (2). Twelve veterans participated in an average of 3.4 (SD 3.7) follow-up sessions. In terms of the content of these follow-up sessions, across all formats and types, 3 included some introduction to coping skills, with no documented evidence of follow-through. For 2 of the veterans engaging in some type of follow-up, there was documented use of coping skills, and 2 used the coping skills with self-reported success and benefit. Finally, documentation revealed evidence that 3 of these veterans were not only using the coping skills with benefit, but also reported an improvement in pain management overall. One also was connected with a different service.
Regarding reasons for completion of services, 2 veterans were terminated due to completing treatment/meeting goals, 2 were terminated because they did not follow up after a session, 7 were terminated due to patient declining additional sessions, and 1 veteran was still receiving services at the time of the review. Linear regression indicated that the number of conjoint sessions was not associated with qualitative treatment outcome. Two logistic regressions indicated that number of conjoint sessions was not related to whether the veteran accepted follow-up services or whether the veteran followed through with services after accepting. Of note, logistic regression indicated that having a mental health diagnosiswas associated with a decreased likelihood of accepting health psychology services (P = .03). Regarding the independent samples t tests, veterans who did not accept follow-up services were more likely to have a mental health diagnosis (P = .03). The groups did not differ significantly with regard to substance use diagnosis or previous engagement in health psychology services.
Discussion
Results showed that all 24 veterans who were offered a conjoint session with a clinical pharmacy specialist and health psychologist engaged in at least 1 session. Half the veterans participated in further services as well. Both the initial conjoint and follow-up sessions offered a greater degree of communication related to the cognitive-behavioral and functional restoration components of behavioral pain management. Given that a majority of the sample had not participated in behavioral or mental health services previously, this may represent a greater penetration rate of exposure to mental health service for veterans than would have been available otherwise.
More specifically, qualitative results suggest that in these conjoint sessions, the veterans were exposed to behavioral psychotherapeutic approaches to chronic pain management (eg, health behavior change, motivational enhancement, health-related psychoeducation, and CBT for chronic pain) that again may not have been provided otherwise (ie, via referral and separately scheduled sessions). These findings are supported by theories consistent with the Transtheoretical Model, which indicates that individuals fall in varying degrees of readiness for behavioral change (ie, precontemplative, contemplative, planning, action, maintenance).21,22 Thus, behavioral intervention approaches must be adaptive and adjust format and communication, including the amount and type of psychoeducation offered. Moreover, the integrated theory of health behavior change in the context of chronic pain management calls for fostering awareness, knowledge, and beliefs through effective communication and education for a wide range of individuals who are at varying stages of change.23 In addition to the conjoint session and subsequent service(s) content that were reviewed and coded in this current project, future projects might draw on these theoretical models and code sessions for patients’ stages of change and assess whether a patient made progress across phases of change (eg, the patient shifted from contemplative to the planning stage of change).
Within this project’s conjoint sessions and consistent with MI principles, veterans were offered discussions related to the bidirectional and BPS aspects of their own chronic pain experience. That is, while discussing responses and adjustment to pain medication(s), veterans received reflections with MI and heard feedback related to their current coping strategies, methods to enhance coping, as well as potential psychosocial impacts of their chronic pain experience. With permission, veterans also were introduced to themes that comprised evidence-based CBT for chronic pain (CBT-CP) intervention. Understanding what change means in the context of chronic pain management is critical. That is, tipping the conversation toward consideration of alternative modalities (eg, relaxation, stress management, cognitions, and pain) in conjunction with or in place of the traditional modalities (eg, medication, pain procedures) is paramount.
Clinicians must listen for patient ambivalence related to procedures, interventions, medication changes, and/or the behavioral self-management of chronic pain. This type of active listening and exploration may be more likely when there is collaboration and effective team functioning among clinicians than when clinicians provide care independently. Future quality improvement (QI) or research projects could extend the EHR review and evaluate clinician-patient transcripts for fidelity to the CBT-CP and MI models. Such efforts could assess for associations between clinician MI consistent behaviors and change talk on the part of the patient. Furthermore, clinician communication and patient change talk from transcripts could be evaluated in relation to evidence from the EHR regarding patient use of coping skills and behavior change.
Consistent with behavioral health literature, having a mental health diagnosis was associated with declining additional behavioral health psychology services in this project. Research has shown that individuals with a mental health diagnosis tend to engage less in behavioral health self-management programs, such as chronic headache and weight management.24-26 This phenomenon lends support for the importance of health care professionals (HCPs) to increase access and exposure to mental and behavioral health services, such as the PC-MHI model.20 In fact, chronic pain management program development efforts within the VA system nationwide include collaboration with the PC-MHI services. One of the initial goals for PC-MHI services is to increase penetration rates into the general outpatient medical clinics and enhance engagement in mental health services.
Using conjoint sessions as was offered in the current project is one step in the development of more comprehensive interdisciplinary teams through interprofessional collaboration and the use of effective clinical communication. In turn, it will be important to directly explore the communication skills and attitudes of these HCPs with regard to interdisciplinary program development and collaboration as teams continue to integrate more broadly into the medical system and enhance chronic pain management services.11 Similarly, measuring the perceptions of clinical pharmacy specialists, physicians, health psychologists, or other clinical disciplines involved in chronic pain management could be another area to explore. More specific to MI, clinician confidence in the use of effective communication and MI skills represents still another area for future study.16
Limitations
Some limitations and suggested future directions found as part of this QI project have been outlined earlier. Other limitations include the used of a retrospective review of information available in patient medical charts. More developed measurement-based care or research could collect self-reports of patient satisfaction with care, functioning, knowledge, readiness for change, and mood in addition to what is noted and documented in clinical observations. Second, the sample was small and did not include any female and few younger veterans, even though these are important subpopulations when examining pain management services. When resources are available for a larger sample size, some exploratory analyses could be conducted for differences in engagement among subgroups. Third, this project may have further confounding variables as this was not an experimental or a controlled study, which could directly compare conjoint sessions with referral-based care and/or those not offered conjoint sessions.
Conclusion
The optimal method of behavioral pain management suggests the need for an interdisciplinary, coordinated team approach, in which the gold standard programs meet requirements set by CARF. However, on a practical level, optimal behavioral pain management may not be feasible at all health care facilities. Furthermore, in an effort to provide best practices to individuals with chronic pain, clinicians must be adaptive and skilled in using effective communication and specialized interventions, such as CBT and MI.
Approaching the more optimal behavioral self-management of chronic pain from a multimodal interdisciplinary perspective and further engaging veterans in this care is paramount. This project is merely one step in this effort that can shed light on the function and logistic outcomes of using a practical, integrated approach to chronic pain. It demonstrates that implementing best practices founded in sound theoretical models despite staffing and resource constraints is possible. Thus, continuing to explore the utility of alternate modalities may offer important applied and translational information to help disseminate and improve chronic pain management services.
Future research could focus on important subpopulations and enhance experimental design with pre- and postmeasures, controlling for possible confounding variables and if possible a controlled design.
Acknowledgments
This quality improvement project was unfunded, and approval was confirmed with the VA Ann Arbor Healthcare System Institutional Review Board and Research & Development committees. The authors also thank Associate Chief of Staff, Ambulatory Care, Clinton Greenstone, MD, and Chief of Primary Care Adam Tremblay, MD, for their leadership and support of these integrative services and quality improvement efforts. The authors especially recognize the veterans for whom they aim to provide the highest quality of services possible.
1. Brooks PM. The burden of musculoskeletal disease—a global perspective. Clin Rheumatol. 2006;25(6):778-781.
2. Haskell SG, Heapy A, Reid MC, Papas RK, Kerns RD. The prevalence and age-related characteristics of pain in a sample of women veterans receiving primary care. J Womens Health (Larchmt). 2006;15(7):862-869.
3. Roth RS, Geisser ME, Williams DA. Interventional pain medicine: retreat from the biopsychosocial model of pain. Transl Behav Med. 2012;2(1):106-116.
4. Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129-136.
5. Borrell-Carrió F, Suchman AL, Epstein RM. The biopsychosocial model 25 years later: principles, practice, and scientific inquiry. Ann Fam Med. 2004;2(6):576-582.
6. Wall PD. The gate control theory of pain mechanisms: a re-examination and re-statement. Brain. 1978;101(1):1-18.
7. Dobscha SK, Corson K, Perrin NA, et al. Collaborative care for chronic pain in primary care: a cluster randomized trial. JAMA. 2009;301(12):1242-1252.
8. Von Korff, Moore JC. Stepped care for back pain: activating approaches for primary care. Ann Intern Med. 2001;134(9, pt 2):911-917.
9. Oslund S, Robinson RC, Clark TC, et al. Long-term effectiveness of a comprehensive pain management program: strengthening the case for interdisciplinary care. Proc (Bayl Univ Med Cent). 2009;22(3):211-214.
10. Scascighini L, Toma V, Dober-Spielmann S, Sprott H. Multidisciplinary treatment for chronic pain: a systematic review of interventions and outcomes. Rheumatology (Oxford). 2008;47(5):670-678.
11. Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119-130.
12. McCracken LM, Turk DC. Behavioral and cognitive-behavioral treatment for chronic pain: outcome, predictors of outcome, and treatment process. Spine (Phila Pa 1976). 2002;27(22): 2564-2573.
13. Jeffery MM, Butler M, Stark A, Kane RL. Multidisciplinary Pain Programs for Chronic Noncancer Pain. Comparative Effectiveness Technical Briefs, No 8. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
14. Ehde DM, Dilworth TM, Turner JA. Cognitive-behavioral therapy for individuals with chronic pain: efficacy, innovations, and directions for research. Am Psychol. 2014;69(2):153-166.
15. Dorflinger L, Kerns RD, Auerbach SM. Providers’ roles in enhancing patients’ adherence to pain self management. Transl Behav Med. 2013;3(1):39-46.
16. Pellico LH, Gilliam WP, Lee AW, Kerns RD. Hearing new voices: registered nurses and health technicians experience caring for chronic pain patients in primary care clinics. Open Nurs J. 2014;8:25-33.
17. Rollnick S, Miller WR, Butler CC, eds. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, NY: The Guilford Press; 2008.
18. Kerns RD, Habib S. A critical review of the pain readiness to change model. J Pain. 2004;5(7):357-367.19. Kerns RD, Burns JW, Shulman M, et al. Can we improve cognitive-behavior therapy for chronic back pain treatment engagement and adherence? A controlled trial of tailored versus standard therapy. Health Psychol. 2014;33(9):938-947.
20. Kearney LK, Post EP, Zeiss A, Goldstein MG, Dundon M. The role of mental and behavioral health in the application of the patient-centered medical home in the Department of Veterans Affairs. Transl Behav Med. 2011;1(4):624-628.
21. Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51(3):390-395.
22. Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. Am Psychol. 1992;47(9):1102-1114.
23. Ryan P. Integrated theory of health behavior change: background and intervention development. Clin Nurse Spec. 2009;23(3):161-172.
24. Evans DD , Blanchard EB. Prediction of early termination from the self-regulatory treatment of chronic headache. Biofeedback Self Regul. 1988;13(3):245-256.
25. Maguen S, Hoerster KD, Littman AJ, et al. Iraq and Afghanistan veterans with PTSD participate less in VA’s weight loss program than those without PTSD. J Affect Disord. 2016;193:289-294.
26. Bloor LE. Improving weight management services for female veterans: design and participation factors with a women only program, and comparisons with gender neutral services. Med Res Arch. 2015;2.
1. Brooks PM. The burden of musculoskeletal disease—a global perspective. Clin Rheumatol. 2006;25(6):778-781.
2. Haskell SG, Heapy A, Reid MC, Papas RK, Kerns RD. The prevalence and age-related characteristics of pain in a sample of women veterans receiving primary care. J Womens Health (Larchmt). 2006;15(7):862-869.
3. Roth RS, Geisser ME, Williams DA. Interventional pain medicine: retreat from the biopsychosocial model of pain. Transl Behav Med. 2012;2(1):106-116.
4. Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129-136.
5. Borrell-Carrió F, Suchman AL, Epstein RM. The biopsychosocial model 25 years later: principles, practice, and scientific inquiry. Ann Fam Med. 2004;2(6):576-582.
6. Wall PD. The gate control theory of pain mechanisms: a re-examination and re-statement. Brain. 1978;101(1):1-18.
7. Dobscha SK, Corson K, Perrin NA, et al. Collaborative care for chronic pain in primary care: a cluster randomized trial. JAMA. 2009;301(12):1242-1252.
8. Von Korff, Moore JC. Stepped care for back pain: activating approaches for primary care. Ann Intern Med. 2001;134(9, pt 2):911-917.
9. Oslund S, Robinson RC, Clark TC, et al. Long-term effectiveness of a comprehensive pain management program: strengthening the case for interdisciplinary care. Proc (Bayl Univ Med Cent). 2009;22(3):211-214.
10. Scascighini L, Toma V, Dober-Spielmann S, Sprott H. Multidisciplinary treatment for chronic pain: a systematic review of interventions and outcomes. Rheumatology (Oxford). 2008;47(5):670-678.
11. Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119-130.
12. McCracken LM, Turk DC. Behavioral and cognitive-behavioral treatment for chronic pain: outcome, predictors of outcome, and treatment process. Spine (Phila Pa 1976). 2002;27(22): 2564-2573.
13. Jeffery MM, Butler M, Stark A, Kane RL. Multidisciplinary Pain Programs for Chronic Noncancer Pain. Comparative Effectiveness Technical Briefs, No 8. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
14. Ehde DM, Dilworth TM, Turner JA. Cognitive-behavioral therapy for individuals with chronic pain: efficacy, innovations, and directions for research. Am Psychol. 2014;69(2):153-166.
15. Dorflinger L, Kerns RD, Auerbach SM. Providers’ roles in enhancing patients’ adherence to pain self management. Transl Behav Med. 2013;3(1):39-46.
16. Pellico LH, Gilliam WP, Lee AW, Kerns RD. Hearing new voices: registered nurses and health technicians experience caring for chronic pain patients in primary care clinics. Open Nurs J. 2014;8:25-33.
17. Rollnick S, Miller WR, Butler CC, eds. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, NY: The Guilford Press; 2008.
18. Kerns RD, Habib S. A critical review of the pain readiness to change model. J Pain. 2004;5(7):357-367.19. Kerns RD, Burns JW, Shulman M, et al. Can we improve cognitive-behavior therapy for chronic back pain treatment engagement and adherence? A controlled trial of tailored versus standard therapy. Health Psychol. 2014;33(9):938-947.
20. Kearney LK, Post EP, Zeiss A, Goldstein MG, Dundon M. The role of mental and behavioral health in the application of the patient-centered medical home in the Department of Veterans Affairs. Transl Behav Med. 2011;1(4):624-628.
21. Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51(3):390-395.
22. Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. Am Psychol. 1992;47(9):1102-1114.
23. Ryan P. Integrated theory of health behavior change: background and intervention development. Clin Nurse Spec. 2009;23(3):161-172.
24. Evans DD , Blanchard EB. Prediction of early termination from the self-regulatory treatment of chronic headache. Biofeedback Self Regul. 1988;13(3):245-256.
25. Maguen S, Hoerster KD, Littman AJ, et al. Iraq and Afghanistan veterans with PTSD participate less in VA’s weight loss program than those without PTSD. J Affect Disord. 2016;193:289-294.
26. Bloor LE. Improving weight management services for female veterans: design and participation factors with a women only program, and comparisons with gender neutral services. Med Res Arch. 2015;2.
Early prophylaxis may preserve joint health in hemophilia patients
New research suggests that starting prophylactic factor VIII (FVIII) therapy early in life can preserve joint health in patients with hemophilia A.
In studying data on more than 6000 patients, researchers found the use of FVIII prophylaxis increased from 1999 to 2010.
And the rate of bleeding events, including joint bleeds, decreased over this time period.
The study also indicated that starting FVIII prophylaxis before age 4 decreases a patient’s risk of losing normal range of motion (ROM) in his joints.
Marilyn J. Manco-Johnson, MD, of the University of Colorado Denver in Aurora, Colorado, and her colleagues reported these findings in Blood.
The team studied information collected from 6196 males between 2 and 69 years of age with severe hemophilia A. Altogether, these patients had 26,614 visits to US hemophilia treatment centers between 1999 and 2010.
The data collected from the clinic visits were used to examine trends in FVIII prophylaxis use and changes in the patients’ health over the study period.
Prophylaxis and bleeds
Overall, prophylaxis use increased from 31% in 1999 to 59% in 2010. Three-quarters of patients younger than 20 years of age were on prophylaxis by 2010.
The rate of total bleeds fell 17% from 1999 to 2010 for patients receiving prophylaxis—from a mean of 4.91 bleeds every 6 months to a mean of 4.07 bleeds every 6 months. For patients not on prophylaxis, the rate of total bleeds fell 30%, from 14.2 to 9.87 bleeds.
For patients on prophylaxis, the rate of joint bleeding fell 22%, from a mean of 3.03 bleeds every 6 months in 1999 to a mean of 2.36 bleeds every 6 months in 2010. Among patients not on prophylaxis, the rate of joint bleeding fell 23%, from 9.42 to 7.25 bleeds.
The researchers noted that rates of joint bleeding and total bleeding events in patients not on prophylaxis were roughly twice the rates for patients who were using prophylaxis.
Joint ROM
The researchers also conducted longitudinal analyses to assess joint ROM on 3078 patients who had 14,130 visits to hemophilia treatment centers during the study period.
The team found a few factors that were significantly associated with a decrease in overall joint ROM at the patients’ initial visit. This included advancing age (P<0.001), non-white race (P<0.001), and obesity (P=0.003).
Obesity was associated with a significant increase in loss of joint ROM over time as well (P<0.001).
On the other hand, starting prophylaxis before age 4 was associated with a significant decrease in loss of joint ROM over time (P=0.03).
Questions and next steps
The researchers said it isn’t clear why prophylaxis is best able to protect joint ROM when treatment is started at a very young age. And it’s not clear why hemophilia patients who might benefit from prophylaxis aren’t using it.
The team said studies are needed to understand why some hemophilia patients don’t use prophylaxis and to develop strategies that are successful in changing this behavior.
In addition, more work is needed to understand the factors that may lead to joint bleeds and to develop treatment strategies for hemophilia patients who have a higher risk for joint disease. ![]()
New research suggests that starting prophylactic factor VIII (FVIII) therapy early in life can preserve joint health in patients with hemophilia A.
In studying data on more than 6000 patients, researchers found the use of FVIII prophylaxis increased from 1999 to 2010.
And the rate of bleeding events, including joint bleeds, decreased over this time period.
The study also indicated that starting FVIII prophylaxis before age 4 decreases a patient’s risk of losing normal range of motion (ROM) in his joints.
Marilyn J. Manco-Johnson, MD, of the University of Colorado Denver in Aurora, Colorado, and her colleagues reported these findings in Blood.
The team studied information collected from 6196 males between 2 and 69 years of age with severe hemophilia A. Altogether, these patients had 26,614 visits to US hemophilia treatment centers between 1999 and 2010.
The data collected from the clinic visits were used to examine trends in FVIII prophylaxis use and changes in the patients’ health over the study period.
Prophylaxis and bleeds
Overall, prophylaxis use increased from 31% in 1999 to 59% in 2010. Three-quarters of patients younger than 20 years of age were on prophylaxis by 2010.
The rate of total bleeds fell 17% from 1999 to 2010 for patients receiving prophylaxis—from a mean of 4.91 bleeds every 6 months to a mean of 4.07 bleeds every 6 months. For patients not on prophylaxis, the rate of total bleeds fell 30%, from 14.2 to 9.87 bleeds.
For patients on prophylaxis, the rate of joint bleeding fell 22%, from a mean of 3.03 bleeds every 6 months in 1999 to a mean of 2.36 bleeds every 6 months in 2010. Among patients not on prophylaxis, the rate of joint bleeding fell 23%, from 9.42 to 7.25 bleeds.
The researchers noted that rates of joint bleeding and total bleeding events in patients not on prophylaxis were roughly twice the rates for patients who were using prophylaxis.
Joint ROM
The researchers also conducted longitudinal analyses to assess joint ROM on 3078 patients who had 14,130 visits to hemophilia treatment centers during the study period.
The team found a few factors that were significantly associated with a decrease in overall joint ROM at the patients’ initial visit. This included advancing age (P<0.001), non-white race (P<0.001), and obesity (P=0.003).
Obesity was associated with a significant increase in loss of joint ROM over time as well (P<0.001).
On the other hand, starting prophylaxis before age 4 was associated with a significant decrease in loss of joint ROM over time (P=0.03).
Questions and next steps
The researchers said it isn’t clear why prophylaxis is best able to protect joint ROM when treatment is started at a very young age. And it’s not clear why hemophilia patients who might benefit from prophylaxis aren’t using it.
The team said studies are needed to understand why some hemophilia patients don’t use prophylaxis and to develop strategies that are successful in changing this behavior.
In addition, more work is needed to understand the factors that may lead to joint bleeds and to develop treatment strategies for hemophilia patients who have a higher risk for joint disease. ![]()
New research suggests that starting prophylactic factor VIII (FVIII) therapy early in life can preserve joint health in patients with hemophilia A.
In studying data on more than 6000 patients, researchers found the use of FVIII prophylaxis increased from 1999 to 2010.
And the rate of bleeding events, including joint bleeds, decreased over this time period.
The study also indicated that starting FVIII prophylaxis before age 4 decreases a patient’s risk of losing normal range of motion (ROM) in his joints.
Marilyn J. Manco-Johnson, MD, of the University of Colorado Denver in Aurora, Colorado, and her colleagues reported these findings in Blood.
The team studied information collected from 6196 males between 2 and 69 years of age with severe hemophilia A. Altogether, these patients had 26,614 visits to US hemophilia treatment centers between 1999 and 2010.
The data collected from the clinic visits were used to examine trends in FVIII prophylaxis use and changes in the patients’ health over the study period.
Prophylaxis and bleeds
Overall, prophylaxis use increased from 31% in 1999 to 59% in 2010. Three-quarters of patients younger than 20 years of age were on prophylaxis by 2010.
The rate of total bleeds fell 17% from 1999 to 2010 for patients receiving prophylaxis—from a mean of 4.91 bleeds every 6 months to a mean of 4.07 bleeds every 6 months. For patients not on prophylaxis, the rate of total bleeds fell 30%, from 14.2 to 9.87 bleeds.
For patients on prophylaxis, the rate of joint bleeding fell 22%, from a mean of 3.03 bleeds every 6 months in 1999 to a mean of 2.36 bleeds every 6 months in 2010. Among patients not on prophylaxis, the rate of joint bleeding fell 23%, from 9.42 to 7.25 bleeds.
The researchers noted that rates of joint bleeding and total bleeding events in patients not on prophylaxis were roughly twice the rates for patients who were using prophylaxis.
Joint ROM
The researchers also conducted longitudinal analyses to assess joint ROM on 3078 patients who had 14,130 visits to hemophilia treatment centers during the study period.
The team found a few factors that were significantly associated with a decrease in overall joint ROM at the patients’ initial visit. This included advancing age (P<0.001), non-white race (P<0.001), and obesity (P=0.003).
Obesity was associated with a significant increase in loss of joint ROM over time as well (P<0.001).
On the other hand, starting prophylaxis before age 4 was associated with a significant decrease in loss of joint ROM over time (P=0.03).
Questions and next steps
The researchers said it isn’t clear why prophylaxis is best able to protect joint ROM when treatment is started at a very young age. And it’s not clear why hemophilia patients who might benefit from prophylaxis aren’t using it.
The team said studies are needed to understand why some hemophilia patients don’t use prophylaxis and to develop strategies that are successful in changing this behavior.
In addition, more work is needed to understand the factors that may lead to joint bleeds and to develop treatment strategies for hemophilia patients who have a higher risk for joint disease. ![]()
Clinical Comestibles?
1. This 45-year-old woman’s skin has multiple small, soft, compressible papules, one of which is apt to bleed when scratched. She is concerned that these “cherry red” lesions are precancerous.
Diagnosis: Cherry angiomas, also known as de Morgan spots, are extremely common lesions; though usually asymptomatic, they may bleed with trauma. They occur most commonly as multiple asymptomatic lesions on the trunk and arms. These capillary hemangiomas are dome-shaped, small (0.1 to 0.5 cm in diameter), and bright red to violaceous; they can be flat, raised, or nodular.
Cherry angiomas form as a result of the development of multiple capillaries with narrow lumens and prominent endothelial cells arranged in a lobular pattern in the papillary dermis. Effective treatment options include curettage, laser ablation, and electrodesiccation.
For more information, see Kim, J-H, Park H-Y, Ahn SK. Cherry Angiomas on the Scalp. Case Rep Dermatol. 2009;1(1):82–86.
2. A 60-year-old African-American woman presents with painful swelling of two years’ duration. She delayed care due to lack of insurance. The patient is hypertensive, and her left breast and nipple are retracted, with darkened skin and a peau d’orange texture.
Diagnosis: Palpation of the firm, matted nodes in the left axilla elucidated the diagnosis of breast cancer with lymphedema. Lymphedema causes the skin of the breast to resemble that of an orange.
The patient was referred to the local university’s breast center. Although the prognosis was poor, it was important to make every effort to have the disease staged to determine the most appropriate therapy.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ. Breast cancer. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:551-556.
For more information, see “Swollen breast and arm.” The Journal of Family Practice. February 6, 2015.
3. This patient presents with her cheeks quite red and covered with hyperkeratotic, rough, pinpoint papules. No other blemishes or lesions are seen on her face, but there are hundreds of hyperkeratotic papules on her bilateral triceps, giving a “chicken skin” appearance.
Diagnosis: Keratosis pilaris (KP) is an extremely common and harmless problem that affects up to 70% of newborns, though it may not fully express until age 1 or 2. Caused by an overproduction of perifollicular keratin, KP is inherited in an autosomal dominant pattern and is often seen in conjunction with atopic dermatitis and related conditions (eg, eczema, xerosis, asthma, icthyosis). KP can manifest anywhere on the body except glabrous skin (palms and soles).
Variants of KP are also common; the one affecting this patient is keratosis pilaris rubra facei. This condition is often confused with acne, but treating it as such worsens irritation—especially in the wintertime, when humidity levels are low.
For more information, see “Acne: Maybe She's Born With It?” Clinician Reviews. 2016 July;26(7):W1.
4. A 5-year-old girl has a fever of 102.4°F and “strawberry tongue.” The posterior pharynx is erythematous with slight exudate visible. The anterior cervical lymph nodes are mildly tender and somewhat enlarged. No rashes are noted.
Diagnosis: The child’s strawberry tongue and scarlet fever were caused by strep pharyngitis. Strawberry tongue, identified by prominent papillae along with erythema (resembling a strawberry), is most commonly seen in children with scarlet fever or Kawasaki disease and usually develops within the first two to three days of illness. A white or yellowish coating typically precedes the distinctive red tongue with white papillae.
In this case, oral penicillin VK was prescribed, along with ibuprofen for the fever and sore throat. Improvement was noted within 24 hours, but the full 10-day course of pencillin was completed to prevent rheumatic fever.
For more information, see “Papillae on tongue.” Journal of Family Practice. January 24, 2014.
1. This 45-year-old woman’s skin has multiple small, soft, compressible papules, one of which is apt to bleed when scratched. She is concerned that these “cherry red” lesions are precancerous.

Diagnosis: Cherry angiomas, also known as de Morgan spots, are extremely common lesions; though usually asymptomatic, they may bleed with trauma. They occur most commonly as multiple asymptomatic lesions on the trunk and arms. These capillary hemangiomas are dome-shaped, small (0.1 to 0.5 cm in diameter), and bright red to violaceous; they can be flat, raised, or nodular.
Cherry angiomas form as a result of the development of multiple capillaries with narrow lumens and prominent endothelial cells arranged in a lobular pattern in the papillary dermis. Effective treatment options include curettage, laser ablation, and electrodesiccation.
For more information, see Kim, J-H, Park H-Y, Ahn SK. Cherry Angiomas on the Scalp. Case Rep Dermatol. 2009;1(1):82–86.
2. A 60-year-old African-American woman presents with painful swelling of two years’ duration. She delayed care due to lack of insurance. The patient is hypertensive, and her left breast and nipple are retracted, with darkened skin and a peau d’orange texture.

Diagnosis: Palpation of the firm, matted nodes in the left axilla elucidated the diagnosis of breast cancer with lymphedema. Lymphedema causes the skin of the breast to resemble that of an orange.
The patient was referred to the local university’s breast center. Although the prognosis was poor, it was important to make every effort to have the disease staged to determine the most appropriate therapy.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ. Breast cancer. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:551-556.
For more information, see “Swollen breast and arm.” The Journal of Family Practice. February 6, 2015.
3. This patient presents with her cheeks quite red and covered with hyperkeratotic, rough, pinpoint papules. No other blemishes or lesions are seen on her face, but there are hundreds of hyperkeratotic papules on her bilateral triceps, giving a “chicken skin” appearance.

Diagnosis: Keratosis pilaris (KP) is an extremely common and harmless problem that affects up to 70% of newborns, though it may not fully express until age 1 or 2. Caused by an overproduction of perifollicular keratin, KP is inherited in an autosomal dominant pattern and is often seen in conjunction with atopic dermatitis and related conditions (eg, eczema, xerosis, asthma, icthyosis). KP can manifest anywhere on the body except glabrous skin (palms and soles).
Variants of KP are also common; the one affecting this patient is keratosis pilaris rubra facei. This condition is often confused with acne, but treating it as such worsens irritation—especially in the wintertime, when humidity levels are low.
For more information, see “Acne: Maybe She's Born With It?” Clinician Reviews. 2016 July;26(7):W1.
4. A 5-year-old girl has a fever of 102.4°F and “strawberry tongue.” The posterior pharynx is erythematous with slight exudate visible. The anterior cervical lymph nodes are mildly tender and somewhat enlarged. No rashes are noted.

Diagnosis: The child’s strawberry tongue and scarlet fever were caused by strep pharyngitis. Strawberry tongue, identified by prominent papillae along with erythema (resembling a strawberry), is most commonly seen in children with scarlet fever or Kawasaki disease and usually develops within the first two to three days of illness. A white or yellowish coating typically precedes the distinctive red tongue with white papillae.
In this case, oral penicillin VK was prescribed, along with ibuprofen for the fever and sore throat. Improvement was noted within 24 hours, but the full 10-day course of pencillin was completed to prevent rheumatic fever.
For more information, see “Papillae on tongue.” Journal of Family Practice. January 24, 2014.
1. This 45-year-old woman’s skin has multiple small, soft, compressible papules, one of which is apt to bleed when scratched. She is concerned that these “cherry red” lesions are precancerous.

Diagnosis: Cherry angiomas, also known as de Morgan spots, are extremely common lesions; though usually asymptomatic, they may bleed with trauma. They occur most commonly as multiple asymptomatic lesions on the trunk and arms. These capillary hemangiomas are dome-shaped, small (0.1 to 0.5 cm in diameter), and bright red to violaceous; they can be flat, raised, or nodular.
Cherry angiomas form as a result of the development of multiple capillaries with narrow lumens and prominent endothelial cells arranged in a lobular pattern in the papillary dermis. Effective treatment options include curettage, laser ablation, and electrodesiccation.
For more information, see Kim, J-H, Park H-Y, Ahn SK. Cherry Angiomas on the Scalp. Case Rep Dermatol. 2009;1(1):82–86.
2. A 60-year-old African-American woman presents with painful swelling of two years’ duration. She delayed care due to lack of insurance. The patient is hypertensive, and her left breast and nipple are retracted, with darkened skin and a peau d’orange texture.

Diagnosis: Palpation of the firm, matted nodes in the left axilla elucidated the diagnosis of breast cancer with lymphedema. Lymphedema causes the skin of the breast to resemble that of an orange.
The patient was referred to the local university’s breast center. Although the prognosis was poor, it was important to make every effort to have the disease staged to determine the most appropriate therapy.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ. Breast cancer. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:551-556.
For more information, see “Swollen breast and arm.” The Journal of Family Practice. February 6, 2015.
3. This patient presents with her cheeks quite red and covered with hyperkeratotic, rough, pinpoint papules. No other blemishes or lesions are seen on her face, but there are hundreds of hyperkeratotic papules on her bilateral triceps, giving a “chicken skin” appearance.

Diagnosis: Keratosis pilaris (KP) is an extremely common and harmless problem that affects up to 70% of newborns, though it may not fully express until age 1 or 2. Caused by an overproduction of perifollicular keratin, KP is inherited in an autosomal dominant pattern and is often seen in conjunction with atopic dermatitis and related conditions (eg, eczema, xerosis, asthma, icthyosis). KP can manifest anywhere on the body except glabrous skin (palms and soles).
Variants of KP are also common; the one affecting this patient is keratosis pilaris rubra facei. This condition is often confused with acne, but treating it as such worsens irritation—especially in the wintertime, when humidity levels are low.
For more information, see “Acne: Maybe She's Born With It?” Clinician Reviews. 2016 July;26(7):W1.
4. A 5-year-old girl has a fever of 102.4°F and “strawberry tongue.” The posterior pharynx is erythematous with slight exudate visible. The anterior cervical lymph nodes are mildly tender and somewhat enlarged. No rashes are noted.

Diagnosis: The child’s strawberry tongue and scarlet fever were caused by strep pharyngitis. Strawberry tongue, identified by prominent papillae along with erythema (resembling a strawberry), is most commonly seen in children with scarlet fever or Kawasaki disease and usually develops within the first two to three days of illness. A white or yellowish coating typically precedes the distinctive red tongue with white papillae.
In this case, oral penicillin VK was prescribed, along with ibuprofen for the fever and sore throat. Improvement was noted within 24 hours, but the full 10-day course of pencillin was completed to prevent rheumatic fever.
For more information, see “Papillae on tongue.” Journal of Family Practice. January 24, 2014.
Best Practices: In the Management of Hemophilia
The treatment of patients with hemophilia has rapidly evolved from one-size-fits-all factor replacement strategies to highly individualized, patient-specific care.
Faculty:
Erik Berntorp, MD, PhD
Malmö Centre for Thrombosis and Haemostasis
Lund University
Malmö, Sweden
Faculty Disclosures:
This sponsored content was prepared by Dr. Berntorp and reviewed by Shire. Dr. Berntorp discloses that he is a consultant and on the advisory boards and speakers’ bureaus for Bayer, CSL Behring, Octapharma, Shire, and Sobi. The production of this section did not involve the news or editorial staff of Frontline Medical Communications.
S28001
2/17
Click here to read the supplement
The treatment of patients with hemophilia has rapidly evolved from one-size-fits-all factor replacement strategies to highly individualized, patient-specific care.
Faculty:
Erik Berntorp, MD, PhD
Malmö Centre for Thrombosis and Haemostasis
Lund University
Malmö, Sweden
Faculty Disclosures:
This sponsored content was prepared by Dr. Berntorp and reviewed by Shire. Dr. Berntorp discloses that he is a consultant and on the advisory boards and speakers’ bureaus for Bayer, CSL Behring, Octapharma, Shire, and Sobi. The production of this section did not involve the news or editorial staff of Frontline Medical Communications.
S28001
2/17
Click here to read the supplement
The treatment of patients with hemophilia has rapidly evolved from one-size-fits-all factor replacement strategies to highly individualized, patient-specific care.
Faculty:
Erik Berntorp, MD, PhD
Malmö Centre for Thrombosis and Haemostasis
Lund University
Malmö, Sweden
Faculty Disclosures:
This sponsored content was prepared by Dr. Berntorp and reviewed by Shire. Dr. Berntorp discloses that he is a consultant and on the advisory boards and speakers’ bureaus for Bayer, CSL Behring, Octapharma, Shire, and Sobi. The production of this section did not involve the news or editorial staff of Frontline Medical Communications.
S28001
2/17
Click here to read the supplement
Imbalance drives development of B-ALL, team says
Researchers say they have discovered an imbalance that drives the development of B-cell acute lymphoblastic leukemia (B-ALL).
The group’s study suggests that activation of STAT5 causes competition among other transcription factors that leads to B-ALL.
Therefore, the researchers believe that inhibiting the activation of STAT5 and restoring the natural balance of proteins could mean more effective treatment for B-ALL.
Seth Frietze, PhD, of the University of Vermont in Burlington, Vermont, and his colleagues conducted this research and reported the results in Nature Immunology.
The researchers first studied the role of STAT5 in B-ALL using mouse models.
The experiments revealed that STAT5 activation and defects in a signaling pathway worked together to promote B-ALL. The defects were in signaling components of the B-cell antigen receptor precursor—IKAROS, NF-κB, BLNK, BTK, and PKCβ.
With further investigation, the researchers found that STAT5 “antagonized NF-κB and IKAROS by opposing the regulation of shared target genes.”
The team also studied samples from patients with B-ALL and found that patients with a high ratio of active STAT5 to NF-κB or IKAROS had more aggressive disease.
Specifically, the ratio of active STAT5 to IKAROS was negatively correlated with patient survival and the duration of remission. The ratio of active STAT5 to the NF-κB subunit RELA correlated with remission duration but not survival.
“The major outcome of this story is that a signature emerged from looking at the level of activated proteins compared to other proteins that’s very predictive of how a patient will respond to therapy,” Dr Frietze said.
“That’s a novel finding. If we could find drugs to target that activation, that could be an incredibly effective way to treat leukemia.” ![]()
Researchers say they have discovered an imbalance that drives the development of B-cell acute lymphoblastic leukemia (B-ALL).
The group’s study suggests that activation of STAT5 causes competition among other transcription factors that leads to B-ALL.
Therefore, the researchers believe that inhibiting the activation of STAT5 and restoring the natural balance of proteins could mean more effective treatment for B-ALL.
Seth Frietze, PhD, of the University of Vermont in Burlington, Vermont, and his colleagues conducted this research and reported the results in Nature Immunology.
The researchers first studied the role of STAT5 in B-ALL using mouse models.
The experiments revealed that STAT5 activation and defects in a signaling pathway worked together to promote B-ALL. The defects were in signaling components of the B-cell antigen receptor precursor—IKAROS, NF-κB, BLNK, BTK, and PKCβ.
With further investigation, the researchers found that STAT5 “antagonized NF-κB and IKAROS by opposing the regulation of shared target genes.”
The team also studied samples from patients with B-ALL and found that patients with a high ratio of active STAT5 to NF-κB or IKAROS had more aggressive disease.
Specifically, the ratio of active STAT5 to IKAROS was negatively correlated with patient survival and the duration of remission. The ratio of active STAT5 to the NF-κB subunit RELA correlated with remission duration but not survival.
“The major outcome of this story is that a signature emerged from looking at the level of activated proteins compared to other proteins that’s very predictive of how a patient will respond to therapy,” Dr Frietze said.
“That’s a novel finding. If we could find drugs to target that activation, that could be an incredibly effective way to treat leukemia.” ![]()
Researchers say they have discovered an imbalance that drives the development of B-cell acute lymphoblastic leukemia (B-ALL).
The group’s study suggests that activation of STAT5 causes competition among other transcription factors that leads to B-ALL.
Therefore, the researchers believe that inhibiting the activation of STAT5 and restoring the natural balance of proteins could mean more effective treatment for B-ALL.
Seth Frietze, PhD, of the University of Vermont in Burlington, Vermont, and his colleagues conducted this research and reported the results in Nature Immunology.
The researchers first studied the role of STAT5 in B-ALL using mouse models.
The experiments revealed that STAT5 activation and defects in a signaling pathway worked together to promote B-ALL. The defects were in signaling components of the B-cell antigen receptor precursor—IKAROS, NF-κB, BLNK, BTK, and PKCβ.
With further investigation, the researchers found that STAT5 “antagonized NF-κB and IKAROS by opposing the regulation of shared target genes.”
The team also studied samples from patients with B-ALL and found that patients with a high ratio of active STAT5 to NF-κB or IKAROS had more aggressive disease.
Specifically, the ratio of active STAT5 to IKAROS was negatively correlated with patient survival and the duration of remission. The ratio of active STAT5 to the NF-κB subunit RELA correlated with remission duration but not survival.
“The major outcome of this story is that a signature emerged from looking at the level of activated proteins compared to other proteins that’s very predictive of how a patient will respond to therapy,” Dr Frietze said.
“That’s a novel finding. If we could find drugs to target that activation, that could be an incredibly effective way to treat leukemia.” ![]()
TKI shows promise in preclinical study of AML
WASHINGTON, DC—Preclinical data suggest a novel tyrosine kinase inhibitor (TKI) may be an effective treatment for patients with NTRK-rearranged acute myeloid leukemia (AML).
Entrectinib is a TKI targeting tumors that harbor TRK, ROS1, or ALK fusions.
Researchers found that entrectinib inhibited cell proliferation in NTRK-rearranged AML cell lines.
In mouse models of NTRK-rearranged AML, entrectinib induced tumor regression and eliminated residual AML cells from the bone marrow.
These results were presented at the AACR Annual Meeting 2017 (abstract 5158). The research was conducted by employees of Ignyta, Inc., the company developing entrectinib.
The researchers first tested entrectinib in AML cell lines. They observed “potent” anti-proliferative activity in a pair of NTRK-fusion-positive AML cell lines, IMS-M2 and M0-91.
Entrectinib inhibited TRK signaling and induced cell-cycle arrest in these cell lines. The TKI also induced both caspase 3-dependent apoptosis and PARP cleavage in a dose- and time-dependent manner.
However, entrectinib showed minimal activity against an NTRK-fusion-negative AML cell line, Kasumi-1.
The researchers also tested entrectinib in mouse models of NTRK-fusion-driven AML.
The TKI induced tumor regression in both IMS-M2 and M0-91 models, and the drug eliminated leukemic cells in the bone marrow.
The researchers said these results provide rationale for the clinical development of entrectinib in molecularly defined hematologic malignancies.
Entrectinib is currently being studied in a phase 2 trial of solid tumor malignancies. ![]()
WASHINGTON, DC—Preclinical data suggest a novel tyrosine kinase inhibitor (TKI) may be an effective treatment for patients with NTRK-rearranged acute myeloid leukemia (AML).
Entrectinib is a TKI targeting tumors that harbor TRK, ROS1, or ALK fusions.
Researchers found that entrectinib inhibited cell proliferation in NTRK-rearranged AML cell lines.
In mouse models of NTRK-rearranged AML, entrectinib induced tumor regression and eliminated residual AML cells from the bone marrow.
These results were presented at the AACR Annual Meeting 2017 (abstract 5158). The research was conducted by employees of Ignyta, Inc., the company developing entrectinib.
The researchers first tested entrectinib in AML cell lines. They observed “potent” anti-proliferative activity in a pair of NTRK-fusion-positive AML cell lines, IMS-M2 and M0-91.
Entrectinib inhibited TRK signaling and induced cell-cycle arrest in these cell lines. The TKI also induced both caspase 3-dependent apoptosis and PARP cleavage in a dose- and time-dependent manner.
However, entrectinib showed minimal activity against an NTRK-fusion-negative AML cell line, Kasumi-1.
The researchers also tested entrectinib in mouse models of NTRK-fusion-driven AML.
The TKI induced tumor regression in both IMS-M2 and M0-91 models, and the drug eliminated leukemic cells in the bone marrow.
The researchers said these results provide rationale for the clinical development of entrectinib in molecularly defined hematologic malignancies.
Entrectinib is currently being studied in a phase 2 trial of solid tumor malignancies. ![]()
WASHINGTON, DC—Preclinical data suggest a novel tyrosine kinase inhibitor (TKI) may be an effective treatment for patients with NTRK-rearranged acute myeloid leukemia (AML).
Entrectinib is a TKI targeting tumors that harbor TRK, ROS1, or ALK fusions.
Researchers found that entrectinib inhibited cell proliferation in NTRK-rearranged AML cell lines.
In mouse models of NTRK-rearranged AML, entrectinib induced tumor regression and eliminated residual AML cells from the bone marrow.
These results were presented at the AACR Annual Meeting 2017 (abstract 5158). The research was conducted by employees of Ignyta, Inc., the company developing entrectinib.
The researchers first tested entrectinib in AML cell lines. They observed “potent” anti-proliferative activity in a pair of NTRK-fusion-positive AML cell lines, IMS-M2 and M0-91.
Entrectinib inhibited TRK signaling and induced cell-cycle arrest in these cell lines. The TKI also induced both caspase 3-dependent apoptosis and PARP cleavage in a dose- and time-dependent manner.
However, entrectinib showed minimal activity against an NTRK-fusion-negative AML cell line, Kasumi-1.
The researchers also tested entrectinib in mouse models of NTRK-fusion-driven AML.
The TKI induced tumor regression in both IMS-M2 and M0-91 models, and the drug eliminated leukemic cells in the bone marrow.
The researchers said these results provide rationale for the clinical development of entrectinib in molecularly defined hematologic malignancies.
Entrectinib is currently being studied in a phase 2 trial of solid tumor malignancies. ![]()
Minimal residual disease eyed for myeloma management
NEW YORK – Testing for minimal residual disease is clearly the state-of-the-art way to gauge the status of treated patients with multiple myeloma, agreed experts. The issue now is whether reliance on MRD to guide management remains investigational or ready for routine practice.
Paul G. Richardson, MD, gave MRD full endorsement as the wave of the future, but cautioned against routine use right now. “MRD is an exciting new tool [but] is not yet the standard of care for routine practice,” he said at the conference held by Imedex.
He cited results from a recent meta-analysis that included data from 14 studies of multiple myeloma patients that examined the correlation of MRD status with progression-free survival and 12 studies that addressed how MRD related to overall survival. The meta-analysis results showed that, among patients in complete remission, the average duration of progression-free survival was 56 months in patients who began follow-up without detectable MRD and 34 months in patients with detectable MRD (JAMA Oncology. 2017 Jan;3[1]:28-35). The average duration of overall survival was 112 months in patients with undetectable MRD and 82 months in those with detectable MRD.
But MRD assessment still has several limitations. Evaluations of its strength for prognosis have largely been based on flow cytometry measures of MRD, an approach recently eclipsed by next-generation sequencing and polymerase chain reaction–based assays. Lack of standardization across the multiple tests now available is another hindrance. In addition, undetectable MRD in a myeloma patient in no way means that the malignancy is gone and that treatment can stop. Patients with undetectable MRD may have better medium-term outcomes, compared with patients with detectable MRD, but even patients with undetectable multiple myeloma still succumb to disease progression several years later.
“Patients negative for MRD do better than patients with detectable MRD, but they still relapse and die. You must maintain treatment in patients negative for MRD,” Dr. Richardson said. The clinical experience “supports the idea that, going forward, MRD has real potential, but MRD is not yet standard of care for routine practice,” he said.
In late 2015, Memorial Sloan-Kettering Cancer Center began routinely using MRD to assess multiple myeloma patients, said C. Ola Landgren, MD, PhD, professor of medicine at Weill Cornell Medical College and chief of the Myeloma Service at Memorial Sloan-Kettering.
He and his associates focus on newly diagnosed multiple myeloma patients who have undergone induction therapy. Patients who come out of the regimen with an undetectable level of MRD at a sensitivity of one cell in a million have the option of receiving high-dose melphalan (Alkeran) followed by autologous stem cell transplantation or maintenance therapy with lenalidomide (Revlimid). Patients who finish induction with detectable MRD can either receive immediate treatment with high-dose melphalan followed by autologous stem cell transplantation or further combination treatment with lenalidomide, carfilzomib (Kyprolis), and dexamethasone, then followed by melphalan and stem cell transplantation (Bone Marrow Transplantation. 2016 July;51[7]:913-4).
“The data show that patients who become MRD negative have longer progression-free survival and overall survival, and we find that more and more patients achieve a MRD-negative state,” said Dr. Landgren. “In our practice, it’s about half of newly diagnosed patients. I think that achieving MRD negativity is more important than the specific treatment a patient receives” for predicting prognosis, he said.
Dr. Richardson has been a consultant to Celgene, Genmab, Janssen, Novartis, Oncopeptides, and Takeda and has received research funding from Celgene and Takeda. Dr. Landgren has been a consultant to Amgen and Takeda and a speaker on behalf of Plexus.
[email protected]
On Twitter @mitchelzoler
NEW YORK – Testing for minimal residual disease is clearly the state-of-the-art way to gauge the status of treated patients with multiple myeloma, agreed experts. The issue now is whether reliance on MRD to guide management remains investigational or ready for routine practice.
Paul G. Richardson, MD, gave MRD full endorsement as the wave of the future, but cautioned against routine use right now. “MRD is an exciting new tool [but] is not yet the standard of care for routine practice,” he said at the conference held by Imedex.
He cited results from a recent meta-analysis that included data from 14 studies of multiple myeloma patients that examined the correlation of MRD status with progression-free survival and 12 studies that addressed how MRD related to overall survival. The meta-analysis results showed that, among patients in complete remission, the average duration of progression-free survival was 56 months in patients who began follow-up without detectable MRD and 34 months in patients with detectable MRD (JAMA Oncology. 2017 Jan;3[1]:28-35). The average duration of overall survival was 112 months in patients with undetectable MRD and 82 months in those with detectable MRD.
But MRD assessment still has several limitations. Evaluations of its strength for prognosis have largely been based on flow cytometry measures of MRD, an approach recently eclipsed by next-generation sequencing and polymerase chain reaction–based assays. Lack of standardization across the multiple tests now available is another hindrance. In addition, undetectable MRD in a myeloma patient in no way means that the malignancy is gone and that treatment can stop. Patients with undetectable MRD may have better medium-term outcomes, compared with patients with detectable MRD, but even patients with undetectable multiple myeloma still succumb to disease progression several years later.
“Patients negative for MRD do better than patients with detectable MRD, but they still relapse and die. You must maintain treatment in patients negative for MRD,” Dr. Richardson said. The clinical experience “supports the idea that, going forward, MRD has real potential, but MRD is not yet standard of care for routine practice,” he said.
In late 2015, Memorial Sloan-Kettering Cancer Center began routinely using MRD to assess multiple myeloma patients, said C. Ola Landgren, MD, PhD, professor of medicine at Weill Cornell Medical College and chief of the Myeloma Service at Memorial Sloan-Kettering.
He and his associates focus on newly diagnosed multiple myeloma patients who have undergone induction therapy. Patients who come out of the regimen with an undetectable level of MRD at a sensitivity of one cell in a million have the option of receiving high-dose melphalan (Alkeran) followed by autologous stem cell transplantation or maintenance therapy with lenalidomide (Revlimid). Patients who finish induction with detectable MRD can either receive immediate treatment with high-dose melphalan followed by autologous stem cell transplantation or further combination treatment with lenalidomide, carfilzomib (Kyprolis), and dexamethasone, then followed by melphalan and stem cell transplantation (Bone Marrow Transplantation. 2016 July;51[7]:913-4).
“The data show that patients who become MRD negative have longer progression-free survival and overall survival, and we find that more and more patients achieve a MRD-negative state,” said Dr. Landgren. “In our practice, it’s about half of newly diagnosed patients. I think that achieving MRD negativity is more important than the specific treatment a patient receives” for predicting prognosis, he said.
Dr. Richardson has been a consultant to Celgene, Genmab, Janssen, Novartis, Oncopeptides, and Takeda and has received research funding from Celgene and Takeda. Dr. Landgren has been a consultant to Amgen and Takeda and a speaker on behalf of Plexus.
[email protected]
On Twitter @mitchelzoler
NEW YORK – Testing for minimal residual disease is clearly the state-of-the-art way to gauge the status of treated patients with multiple myeloma, agreed experts. The issue now is whether reliance on MRD to guide management remains investigational or ready for routine practice.
Paul G. Richardson, MD, gave MRD full endorsement as the wave of the future, but cautioned against routine use right now. “MRD is an exciting new tool [but] is not yet the standard of care for routine practice,” he said at the conference held by Imedex.
He cited results from a recent meta-analysis that included data from 14 studies of multiple myeloma patients that examined the correlation of MRD status with progression-free survival and 12 studies that addressed how MRD related to overall survival. The meta-analysis results showed that, among patients in complete remission, the average duration of progression-free survival was 56 months in patients who began follow-up without detectable MRD and 34 months in patients with detectable MRD (JAMA Oncology. 2017 Jan;3[1]:28-35). The average duration of overall survival was 112 months in patients with undetectable MRD and 82 months in those with detectable MRD.
But MRD assessment still has several limitations. Evaluations of its strength for prognosis have largely been based on flow cytometry measures of MRD, an approach recently eclipsed by next-generation sequencing and polymerase chain reaction–based assays. Lack of standardization across the multiple tests now available is another hindrance. In addition, undetectable MRD in a myeloma patient in no way means that the malignancy is gone and that treatment can stop. Patients with undetectable MRD may have better medium-term outcomes, compared with patients with detectable MRD, but even patients with undetectable multiple myeloma still succumb to disease progression several years later.
“Patients negative for MRD do better than patients with detectable MRD, but they still relapse and die. You must maintain treatment in patients negative for MRD,” Dr. Richardson said. The clinical experience “supports the idea that, going forward, MRD has real potential, but MRD is not yet standard of care for routine practice,” he said.
In late 2015, Memorial Sloan-Kettering Cancer Center began routinely using MRD to assess multiple myeloma patients, said C. Ola Landgren, MD, PhD, professor of medicine at Weill Cornell Medical College and chief of the Myeloma Service at Memorial Sloan-Kettering.
He and his associates focus on newly diagnosed multiple myeloma patients who have undergone induction therapy. Patients who come out of the regimen with an undetectable level of MRD at a sensitivity of one cell in a million have the option of receiving high-dose melphalan (Alkeran) followed by autologous stem cell transplantation or maintenance therapy with lenalidomide (Revlimid). Patients who finish induction with detectable MRD can either receive immediate treatment with high-dose melphalan followed by autologous stem cell transplantation or further combination treatment with lenalidomide, carfilzomib (Kyprolis), and dexamethasone, then followed by melphalan and stem cell transplantation (Bone Marrow Transplantation. 2016 July;51[7]:913-4).
“The data show that patients who become MRD negative have longer progression-free survival and overall survival, and we find that more and more patients achieve a MRD-negative state,” said Dr. Landgren. “In our practice, it’s about half of newly diagnosed patients. I think that achieving MRD negativity is more important than the specific treatment a patient receives” for predicting prognosis, he said.
Dr. Richardson has been a consultant to Celgene, Genmab, Janssen, Novartis, Oncopeptides, and Takeda and has received research funding from Celgene and Takeda. Dr. Landgren has been a consultant to Amgen and Takeda and a speaker on behalf of Plexus.
[email protected]
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM A MEETING ON HEMATOLOGIC MALIGNANCIES
Order entry tool halves prescription drug costs
SAN DIEGO – What if you could cut patient drug costs, improve adherence, and cut hospitalization by using an online tool?
“Sounds like science fiction, right? I’m here to tell you it’s reality,” Alan A. Kubey, MD, said at the annual meeting of the American College of Physicians.
During his medical internship 3 years ago, Dr. Kubey met an 83-year-old woman on Medicare Part A and B, but not D, coverage. She was on a cardiology service, came in with a non-ST segment elevation myocardial infarction, and was treated conservatively. “She confided in me that it was hard for her, on a fixed income, to afford her medications and to do the things she loved to do,” said Dr. Kubey, who is now a hospitalist at Mayo Clinic, Rochester, Minn., and at Thomas Jefferson University Hospital, Philadelphia. After researching pharmacy options online, Dr. Kubey told the patient that he could get her medication cost down from $465 per day to $65 per day. “She was blown away, and I was blown away,” he said. “It seemed too good to be true. I then did a long retrospective analysis of patients in similar situations.”
During the ACP’s “Dragon’s Lair” competition at the meeting, Dr. Kubey earned the top prize of $7,500 to study the idea further. To date, he and his fellow researchers have used the tool for 25 patients and have saved each one about $5,600 per year in medication costs. Specifically, average costs have dropped from $6,282.54 per year to $598.84 per year, a savings of 88%. “Our plan now is to prospectively validate this in a small, single-site trial to show that this works,” he said. “If it does, we want to study it and develop it further and provide it more broadly to empower clinicians to improve the lives of patients.”
Each year, patients in the United States spend $325 billion on outpatient medications that hold the power to improve health and to save lives. “Yet, we know that up to 45% of patients don’t fill those scripts because of cost,” Dr. Kubey said. “Medication nonadherence leads to an additional $100 billion to $300 billion per year in excess care and an incalculable human cost in lost health. Providers are responsible for guiding patients in their medication decision making, yet study after study shows that physicians are woefully ill informed on the cost of medications. Yet even the astute clinician faces a Byzantine landscape that makes no sense, where a medication may cost up to 69-fold from one pharmacy to the next. What are we to do?
“We believe that you have to do it at the point of entry. There are innovative tools for patients like GoodRx, but, in my opinion, that puts too much on the patients’ shoulders. We want to bring this information directly to the provider – to build upon the progress that the likes of GoodRx have accomplished and add our advanced algorithm to rapidly empower the physician to send the patient to the most cost-effective pharmacy for the most cost-effective regimen on day 1.”
Dr. Kubey reported having no financial disclosures.
SAN DIEGO – What if you could cut patient drug costs, improve adherence, and cut hospitalization by using an online tool?
“Sounds like science fiction, right? I’m here to tell you it’s reality,” Alan A. Kubey, MD, said at the annual meeting of the American College of Physicians.
During his medical internship 3 years ago, Dr. Kubey met an 83-year-old woman on Medicare Part A and B, but not D, coverage. She was on a cardiology service, came in with a non-ST segment elevation myocardial infarction, and was treated conservatively. “She confided in me that it was hard for her, on a fixed income, to afford her medications and to do the things she loved to do,” said Dr. Kubey, who is now a hospitalist at Mayo Clinic, Rochester, Minn., and at Thomas Jefferson University Hospital, Philadelphia. After researching pharmacy options online, Dr. Kubey told the patient that he could get her medication cost down from $465 per day to $65 per day. “She was blown away, and I was blown away,” he said. “It seemed too good to be true. I then did a long retrospective analysis of patients in similar situations.”
During the ACP’s “Dragon’s Lair” competition at the meeting, Dr. Kubey earned the top prize of $7,500 to study the idea further. To date, he and his fellow researchers have used the tool for 25 patients and have saved each one about $5,600 per year in medication costs. Specifically, average costs have dropped from $6,282.54 per year to $598.84 per year, a savings of 88%. “Our plan now is to prospectively validate this in a small, single-site trial to show that this works,” he said. “If it does, we want to study it and develop it further and provide it more broadly to empower clinicians to improve the lives of patients.”
Each year, patients in the United States spend $325 billion on outpatient medications that hold the power to improve health and to save lives. “Yet, we know that up to 45% of patients don’t fill those scripts because of cost,” Dr. Kubey said. “Medication nonadherence leads to an additional $100 billion to $300 billion per year in excess care and an incalculable human cost in lost health. Providers are responsible for guiding patients in their medication decision making, yet study after study shows that physicians are woefully ill informed on the cost of medications. Yet even the astute clinician faces a Byzantine landscape that makes no sense, where a medication may cost up to 69-fold from one pharmacy to the next. What are we to do?
“We believe that you have to do it at the point of entry. There are innovative tools for patients like GoodRx, but, in my opinion, that puts too much on the patients’ shoulders. We want to bring this information directly to the provider – to build upon the progress that the likes of GoodRx have accomplished and add our advanced algorithm to rapidly empower the physician to send the patient to the most cost-effective pharmacy for the most cost-effective regimen on day 1.”
Dr. Kubey reported having no financial disclosures.
SAN DIEGO – What if you could cut patient drug costs, improve adherence, and cut hospitalization by using an online tool?
“Sounds like science fiction, right? I’m here to tell you it’s reality,” Alan A. Kubey, MD, said at the annual meeting of the American College of Physicians.
During his medical internship 3 years ago, Dr. Kubey met an 83-year-old woman on Medicare Part A and B, but not D, coverage. She was on a cardiology service, came in with a non-ST segment elevation myocardial infarction, and was treated conservatively. “She confided in me that it was hard for her, on a fixed income, to afford her medications and to do the things she loved to do,” said Dr. Kubey, who is now a hospitalist at Mayo Clinic, Rochester, Minn., and at Thomas Jefferson University Hospital, Philadelphia. After researching pharmacy options online, Dr. Kubey told the patient that he could get her medication cost down from $465 per day to $65 per day. “She was blown away, and I was blown away,” he said. “It seemed too good to be true. I then did a long retrospective analysis of patients in similar situations.”
During the ACP’s “Dragon’s Lair” competition at the meeting, Dr. Kubey earned the top prize of $7,500 to study the idea further. To date, he and his fellow researchers have used the tool for 25 patients and have saved each one about $5,600 per year in medication costs. Specifically, average costs have dropped from $6,282.54 per year to $598.84 per year, a savings of 88%. “Our plan now is to prospectively validate this in a small, single-site trial to show that this works,” he said. “If it does, we want to study it and develop it further and provide it more broadly to empower clinicians to improve the lives of patients.”
Each year, patients in the United States spend $325 billion on outpatient medications that hold the power to improve health and to save lives. “Yet, we know that up to 45% of patients don’t fill those scripts because of cost,” Dr. Kubey said. “Medication nonadherence leads to an additional $100 billion to $300 billion per year in excess care and an incalculable human cost in lost health. Providers are responsible for guiding patients in their medication decision making, yet study after study shows that physicians are woefully ill informed on the cost of medications. Yet even the astute clinician faces a Byzantine landscape that makes no sense, where a medication may cost up to 69-fold from one pharmacy to the next. What are we to do?
“We believe that you have to do it at the point of entry. There are innovative tools for patients like GoodRx, but, in my opinion, that puts too much on the patients’ shoulders. We want to bring this information directly to the provider – to build upon the progress that the likes of GoodRx have accomplished and add our advanced algorithm to rapidly empower the physician to send the patient to the most cost-effective pharmacy for the most cost-effective regimen on day 1.”
Dr. Kubey reported having no financial disclosures.
EXPERT ANALYSIS AT ACP INTERNAL MEDICINE
VIDEO: GI innovators dive into the Shark Tank
BOSTON – The innovators who presented their novel technologies to a “shark tank” panel during the 2017 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology proved that innovation is alive and well in the field of gastroenterology.
“Shark Tank this year was fabulous,” Dr. Michael L. Kochman, MD, AGAF, executive committee chair of the AGA Center for GI Innovation and Technology, said in a video interview. “It was great to see some really novel ideas and some great, innovative applications.”
Presenters received feedback on their proposals from representatives of the physician, medtech, and regulatory communities and were uniformly positive about the experience, citing the value of such information to decide whether or how to move their projects forward.
“Diving into meet with the “sharks” was “a great opportunity,” says Susan Hutfless, PhD., an epidemiologist at Johns Hopkins University School of Medicine, Baltimore. “I’m still swimming, still alive ... I would do it again.”
BOSTON – The innovators who presented their novel technologies to a “shark tank” panel during the 2017 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology proved that innovation is alive and well in the field of gastroenterology.
“Shark Tank this year was fabulous,” Dr. Michael L. Kochman, MD, AGAF, executive committee chair of the AGA Center for GI Innovation and Technology, said in a video interview. “It was great to see some really novel ideas and some great, innovative applications.”
Presenters received feedback on their proposals from representatives of the physician, medtech, and regulatory communities and were uniformly positive about the experience, citing the value of such information to decide whether or how to move their projects forward.
“Diving into meet with the “sharks” was “a great opportunity,” says Susan Hutfless, PhD., an epidemiologist at Johns Hopkins University School of Medicine, Baltimore. “I’m still swimming, still alive ... I would do it again.”
BOSTON – The innovators who presented their novel technologies to a “shark tank” panel during the 2017 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology proved that innovation is alive and well in the field of gastroenterology.
“Shark Tank this year was fabulous,” Dr. Michael L. Kochman, MD, AGAF, executive committee chair of the AGA Center for GI Innovation and Technology, said in a video interview. “It was great to see some really novel ideas and some great, innovative applications.”
Presenters received feedback on their proposals from representatives of the physician, medtech, and regulatory communities and were uniformly positive about the experience, citing the value of such information to decide whether or how to move their projects forward.
“Diving into meet with the “sharks” was “a great opportunity,” says Susan Hutfless, PhD., an epidemiologist at Johns Hopkins University School of Medicine, Baltimore. “I’m still swimming, still alive ... I would do it again.”
FROM THE AGA 2017 TECH SUMMIT
Three strategies target high quality care with new technologies
BOSTON – Unforeseen complications and steep learning curves represent the downside of new devices and new procedures, but there are some strategies to minimize these risks, according to experts outlining three such approaches during a session on quality outcomes at the 2017 AGA Tech Summit.
One approach involves a reorientation in skill acquisition in health care delivery. Another involves novel strategies to identify problems with new devices more rapidly. The third involves an ongoing evolution in mentoring clinicians through telecommunication.
Mastering new skills
The strategy described for equipping clinicians with the skills to produce uniform, reproducible outcomes is a technique referred to as simulation-based mastery learning, explained E. Matthew Ritter, MD, vice chairman for education and program director of the general surgery residency program at Walter Reed National Military Medical Center’s Uniformed Services University, Bethesda, Md. The technique is based on the goal of eliciting a “specific level of performance without regard to a time-based approach that is the typical standard in learning processes.”
“More and more evidence is available that performing something a lot of times does not necessarily correlate with performing something well,” Dr. Ritter said. He proposed that direct measurements of skill acquisition address this problem.
“My work has been primarily in laparoscopic surgery, but this approach is not specialty-specific,” Dr. Ritter said in an interview, explaining that the approach focuses on breaking down clinical procedures into tasks for which mastery can be objectively defined. The mastery learning for any one task is complete when performance criteria are met rather than after any specific number of repetitions.
“There is substantial evidence that this approach improves skill acquisition and improves outcome,” Dr. Ritter reported.
Identifying problems more quickly
To reduce the risks and complications of new devices, various strategies for postmarketing surveillance are being pursued simultaneously, according to Dana Telem, MD, MPH, director of the Michigan Comprehensive Hernia Program and an associate professor of surgery at the University of Michigan, Ann Arbor. As has been demonstrated repeatedly, the premarket testing and regulatory approval process does not rule out risk of unforeseen complications, including serious risks that may ultimately lead to the technology being discarded.
One approach has been the increasing use of registries so that constant capture of patient data allows isolated but recurring events to become rapidly identifiable, but Dr. Telem said that the value of these registries can be increased “by encouraging and empowering patients to report issues when they occur” to improve capture of adverse events.
As an example of a strategy to more rapidly drill down to the cause of complications, Dr. Telem reported on an effort to encourage creation of unique device identifiers for a given device. As devices are commonly updated and modified, the serial number may be useful when trying to determine whether the issue can be linked to a specific design change. More importantly, these numbers would be identifiable in billing data and potentially linked to registries to improve real time identification of adverse event patterns.
“The problem with adding new layers of premarketing regulation is the risk of stifling innovation, but we have not done a great job of postmarketing surveillance, and I think the focus now being placed on better strategies will greatly improve patient safety,” Dr. Telem reported.
Adding more layers of review and testing prior to approval of a new technology does not necessarily identify all risks, because not all complications are foreseeable, Dr. Telem explained.
“The long-term monitoring of new devices and technologies is mission critical if we really hope to safely innovate in the future.” Dr. Telem said.
Improving learning through telementoring
Employing avenues of telecommunication to better mentor clinicians acquiring new skills was the third example of an innovative area of improving quality assurance. A large focus of the presentation by Christopher Schlachta, MD, professor of surgery, Schulich School of Medicine and Dentistry, Western University, London, Ont., was on telementoring. He explained that this concept is not new, but it is becoming increasingly sophisticated, and there is a growing body of objective evidence that it is effective.
The Internet expands the possibilities. Remote screens can accommodate multiple images, including, in the case of surgical procedures, the surgical field and situational cameras that provide one or more views of the surgical team as they proceed. For laparoscopy, the information being fed to the mentor is often even more comprehensive.
“So much of what we do now is computer assisted, so the chip on the end of scope, for example, is capturing images digitally. These data can be transmitted and translated into images essentially simultaneously for the operator and a mentor who could be hundreds of miles away,” Dr. Schlachta reported.
There are now “plenty of studies that telementoring is effective in a variety of different surgical procedures,” Dr. Schlachta added. While the data confirming the value of telementoring for teaching skills in endoscopic procedures are fewer, Dr. Schlachta predicted this will soon change. He noted that the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) recently created a telementoring task force.
Reviewing several do-it-yourself and proprietary systems that have been developed for telemonitoring, Dr. Schlachta acknowledged that is it not always clear who should pay for this form of learning. He noted that there is potential value for mentees, hospitals wishing to expand services, and health systems attempting to improve quality outcomes. So far, industry has been the primary source of funds.
“For a company introducing a new technology, there is a risk that the technology will fail rapidly if there are bad results in the early going, making it valuable for industry to invest in these telementoring programs,” Dr. Schlacta explained.
“There are related technologies, such as those involving the use of tablets, that are also likely to contribute to the ongoing evolution in how procedural skills are taught and validated. I think these will accelerate and improve learning with the very important potential of better patient outcomes,” Dr. Schlachta reported.
BOSTON – Unforeseen complications and steep learning curves represent the downside of new devices and new procedures, but there are some strategies to minimize these risks, according to experts outlining three such approaches during a session on quality outcomes at the 2017 AGA Tech Summit.
One approach involves a reorientation in skill acquisition in health care delivery. Another involves novel strategies to identify problems with new devices more rapidly. The third involves an ongoing evolution in mentoring clinicians through telecommunication.
Mastering new skills
The strategy described for equipping clinicians with the skills to produce uniform, reproducible outcomes is a technique referred to as simulation-based mastery learning, explained E. Matthew Ritter, MD, vice chairman for education and program director of the general surgery residency program at Walter Reed National Military Medical Center’s Uniformed Services University, Bethesda, Md. The technique is based on the goal of eliciting a “specific level of performance without regard to a time-based approach that is the typical standard in learning processes.”
“More and more evidence is available that performing something a lot of times does not necessarily correlate with performing something well,” Dr. Ritter said. He proposed that direct measurements of skill acquisition address this problem.
“My work has been primarily in laparoscopic surgery, but this approach is not specialty-specific,” Dr. Ritter said in an interview, explaining that the approach focuses on breaking down clinical procedures into tasks for which mastery can be objectively defined. The mastery learning for any one task is complete when performance criteria are met rather than after any specific number of repetitions.
“There is substantial evidence that this approach improves skill acquisition and improves outcome,” Dr. Ritter reported.
Identifying problems more quickly
To reduce the risks and complications of new devices, various strategies for postmarketing surveillance are being pursued simultaneously, according to Dana Telem, MD, MPH, director of the Michigan Comprehensive Hernia Program and an associate professor of surgery at the University of Michigan, Ann Arbor. As has been demonstrated repeatedly, the premarket testing and regulatory approval process does not rule out risk of unforeseen complications, including serious risks that may ultimately lead to the technology being discarded.
One approach has been the increasing use of registries so that constant capture of patient data allows isolated but recurring events to become rapidly identifiable, but Dr. Telem said that the value of these registries can be increased “by encouraging and empowering patients to report issues when they occur” to improve capture of adverse events.
As an example of a strategy to more rapidly drill down to the cause of complications, Dr. Telem reported on an effort to encourage creation of unique device identifiers for a given device. As devices are commonly updated and modified, the serial number may be useful when trying to determine whether the issue can be linked to a specific design change. More importantly, these numbers would be identifiable in billing data and potentially linked to registries to improve real time identification of adverse event patterns.
“The problem with adding new layers of premarketing regulation is the risk of stifling innovation, but we have not done a great job of postmarketing surveillance, and I think the focus now being placed on better strategies will greatly improve patient safety,” Dr. Telem reported.
Adding more layers of review and testing prior to approval of a new technology does not necessarily identify all risks, because not all complications are foreseeable, Dr. Telem explained.
“The long-term monitoring of new devices and technologies is mission critical if we really hope to safely innovate in the future.” Dr. Telem said.
Improving learning through telementoring
Employing avenues of telecommunication to better mentor clinicians acquiring new skills was the third example of an innovative area of improving quality assurance. A large focus of the presentation by Christopher Schlachta, MD, professor of surgery, Schulich School of Medicine and Dentistry, Western University, London, Ont., was on telementoring. He explained that this concept is not new, but it is becoming increasingly sophisticated, and there is a growing body of objective evidence that it is effective.
The Internet expands the possibilities. Remote screens can accommodate multiple images, including, in the case of surgical procedures, the surgical field and situational cameras that provide one or more views of the surgical team as they proceed. For laparoscopy, the information being fed to the mentor is often even more comprehensive.
“So much of what we do now is computer assisted, so the chip on the end of scope, for example, is capturing images digitally. These data can be transmitted and translated into images essentially simultaneously for the operator and a mentor who could be hundreds of miles away,” Dr. Schlachta reported.
There are now “plenty of studies that telementoring is effective in a variety of different surgical procedures,” Dr. Schlachta added. While the data confirming the value of telementoring for teaching skills in endoscopic procedures are fewer, Dr. Schlachta predicted this will soon change. He noted that the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) recently created a telementoring task force.
Reviewing several do-it-yourself and proprietary systems that have been developed for telemonitoring, Dr. Schlachta acknowledged that is it not always clear who should pay for this form of learning. He noted that there is potential value for mentees, hospitals wishing to expand services, and health systems attempting to improve quality outcomes. So far, industry has been the primary source of funds.
“For a company introducing a new technology, there is a risk that the technology will fail rapidly if there are bad results in the early going, making it valuable for industry to invest in these telementoring programs,” Dr. Schlacta explained.
“There are related technologies, such as those involving the use of tablets, that are also likely to contribute to the ongoing evolution in how procedural skills are taught and validated. I think these will accelerate and improve learning with the very important potential of better patient outcomes,” Dr. Schlachta reported.
BOSTON – Unforeseen complications and steep learning curves represent the downside of new devices and new procedures, but there are some strategies to minimize these risks, according to experts outlining three such approaches during a session on quality outcomes at the 2017 AGA Tech Summit.
One approach involves a reorientation in skill acquisition in health care delivery. Another involves novel strategies to identify problems with new devices more rapidly. The third involves an ongoing evolution in mentoring clinicians through telecommunication.
Mastering new skills
The strategy described for equipping clinicians with the skills to produce uniform, reproducible outcomes is a technique referred to as simulation-based mastery learning, explained E. Matthew Ritter, MD, vice chairman for education and program director of the general surgery residency program at Walter Reed National Military Medical Center’s Uniformed Services University, Bethesda, Md. The technique is based on the goal of eliciting a “specific level of performance without regard to a time-based approach that is the typical standard in learning processes.”
“More and more evidence is available that performing something a lot of times does not necessarily correlate with performing something well,” Dr. Ritter said. He proposed that direct measurements of skill acquisition address this problem.
“My work has been primarily in laparoscopic surgery, but this approach is not specialty-specific,” Dr. Ritter said in an interview, explaining that the approach focuses on breaking down clinical procedures into tasks for which mastery can be objectively defined. The mastery learning for any one task is complete when performance criteria are met rather than after any specific number of repetitions.
“There is substantial evidence that this approach improves skill acquisition and improves outcome,” Dr. Ritter reported.
Identifying problems more quickly
To reduce the risks and complications of new devices, various strategies for postmarketing surveillance are being pursued simultaneously, according to Dana Telem, MD, MPH, director of the Michigan Comprehensive Hernia Program and an associate professor of surgery at the University of Michigan, Ann Arbor. As has been demonstrated repeatedly, the premarket testing and regulatory approval process does not rule out risk of unforeseen complications, including serious risks that may ultimately lead to the technology being discarded.
One approach has been the increasing use of registries so that constant capture of patient data allows isolated but recurring events to become rapidly identifiable, but Dr. Telem said that the value of these registries can be increased “by encouraging and empowering patients to report issues when they occur” to improve capture of adverse events.
As an example of a strategy to more rapidly drill down to the cause of complications, Dr. Telem reported on an effort to encourage creation of unique device identifiers for a given device. As devices are commonly updated and modified, the serial number may be useful when trying to determine whether the issue can be linked to a specific design change. More importantly, these numbers would be identifiable in billing data and potentially linked to registries to improve real time identification of adverse event patterns.
“The problem with adding new layers of premarketing regulation is the risk of stifling innovation, but we have not done a great job of postmarketing surveillance, and I think the focus now being placed on better strategies will greatly improve patient safety,” Dr. Telem reported.
Adding more layers of review and testing prior to approval of a new technology does not necessarily identify all risks, because not all complications are foreseeable, Dr. Telem explained.
“The long-term monitoring of new devices and technologies is mission critical if we really hope to safely innovate in the future.” Dr. Telem said.
Improving learning through telementoring
Employing avenues of telecommunication to better mentor clinicians acquiring new skills was the third example of an innovative area of improving quality assurance. A large focus of the presentation by Christopher Schlachta, MD, professor of surgery, Schulich School of Medicine and Dentistry, Western University, London, Ont., was on telementoring. He explained that this concept is not new, but it is becoming increasingly sophisticated, and there is a growing body of objective evidence that it is effective.
The Internet expands the possibilities. Remote screens can accommodate multiple images, including, in the case of surgical procedures, the surgical field and situational cameras that provide one or more views of the surgical team as they proceed. For laparoscopy, the information being fed to the mentor is often even more comprehensive.
“So much of what we do now is computer assisted, so the chip on the end of scope, for example, is capturing images digitally. These data can be transmitted and translated into images essentially simultaneously for the operator and a mentor who could be hundreds of miles away,” Dr. Schlachta reported.
There are now “plenty of studies that telementoring is effective in a variety of different surgical procedures,” Dr. Schlachta added. While the data confirming the value of telementoring for teaching skills in endoscopic procedures are fewer, Dr. Schlachta predicted this will soon change. He noted that the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) recently created a telementoring task force.
Reviewing several do-it-yourself and proprietary systems that have been developed for telemonitoring, Dr. Schlachta acknowledged that is it not always clear who should pay for this form of learning. He noted that there is potential value for mentees, hospitals wishing to expand services, and health systems attempting to improve quality outcomes. So far, industry has been the primary source of funds.
“For a company introducing a new technology, there is a risk that the technology will fail rapidly if there are bad results in the early going, making it valuable for industry to invest in these telementoring programs,” Dr. Schlacta explained.
“There are related technologies, such as those involving the use of tablets, that are also likely to contribute to the ongoing evolution in how procedural skills are taught and validated. I think these will accelerate and improve learning with the very important potential of better patient outcomes,” Dr. Schlachta reported.
FROM THE 2017 AGA TECH SUMMIT