User login
Paraneoplastic Palmoplantar Keratoderma Secondary to Metastatic Uterine Adenocarcinoma
Paraneoplastic palmoplantar keratoderma (PPK) is an acquired dermatosis that presents with hyperkeratosis of the palms and soles in association with visceral malignancies, such as esophageal, gastric, pulmonary, and bladder carcinomas. This condition may either be acquired or inherited.1
Case Report
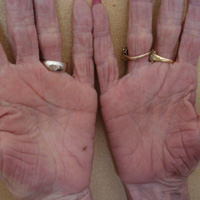
A 72-year-old woman was referred to our dermatology clinic for evaluation of a nonpruritic hyperkeratotic eruption predominantly on the palms and soles of 2 to 3 months’ duration (Figure 1A). Review of systems was remarkable for chronic anxiety, unintentional weight loss of 10 lb over the last 6 months, and a mild cough of 10 days’ duration. The differential diagnosis included eczematous dermatitis, tinea manuum, new-onset palmoplantar psoriasis, and PPK.
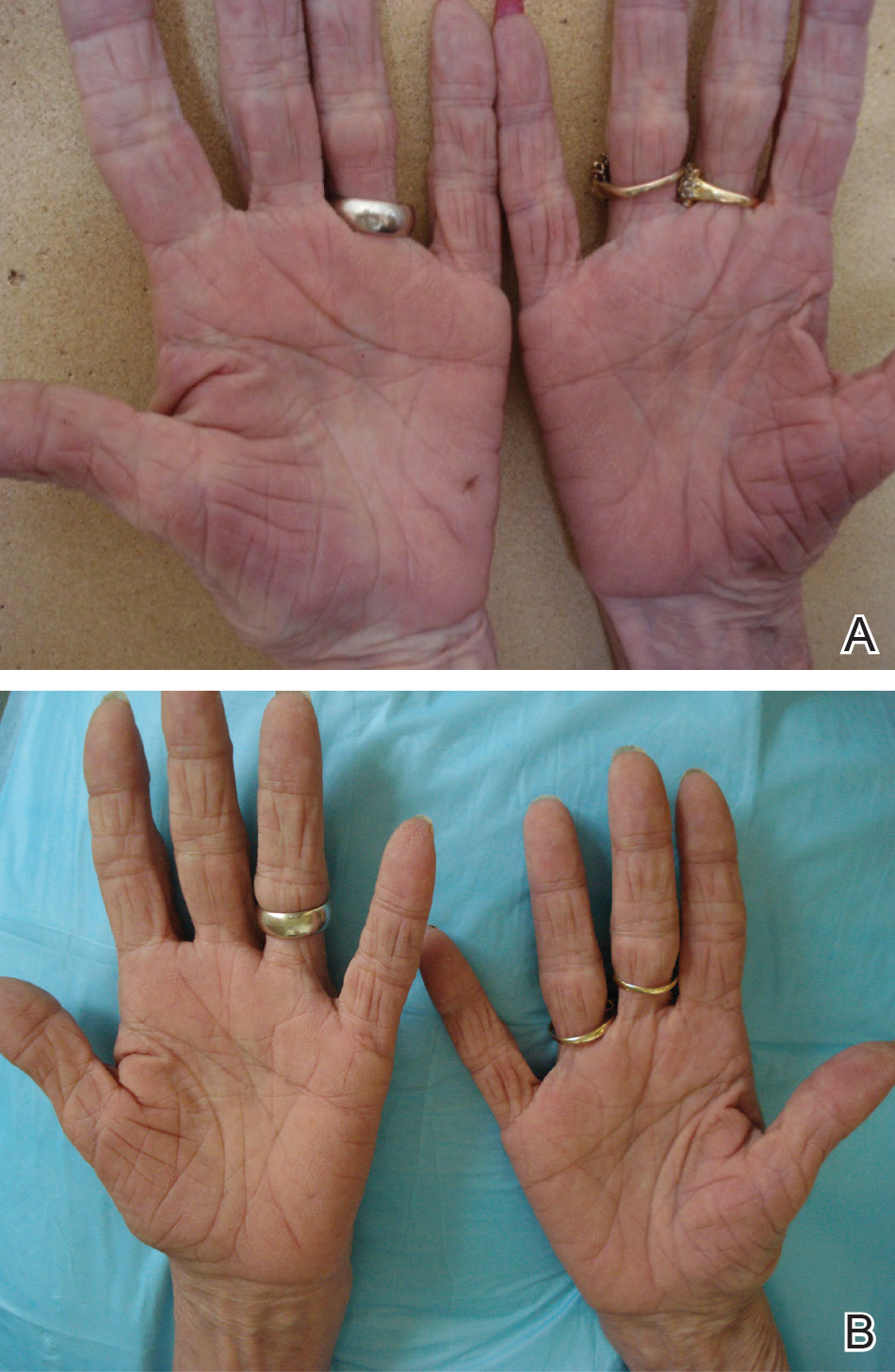
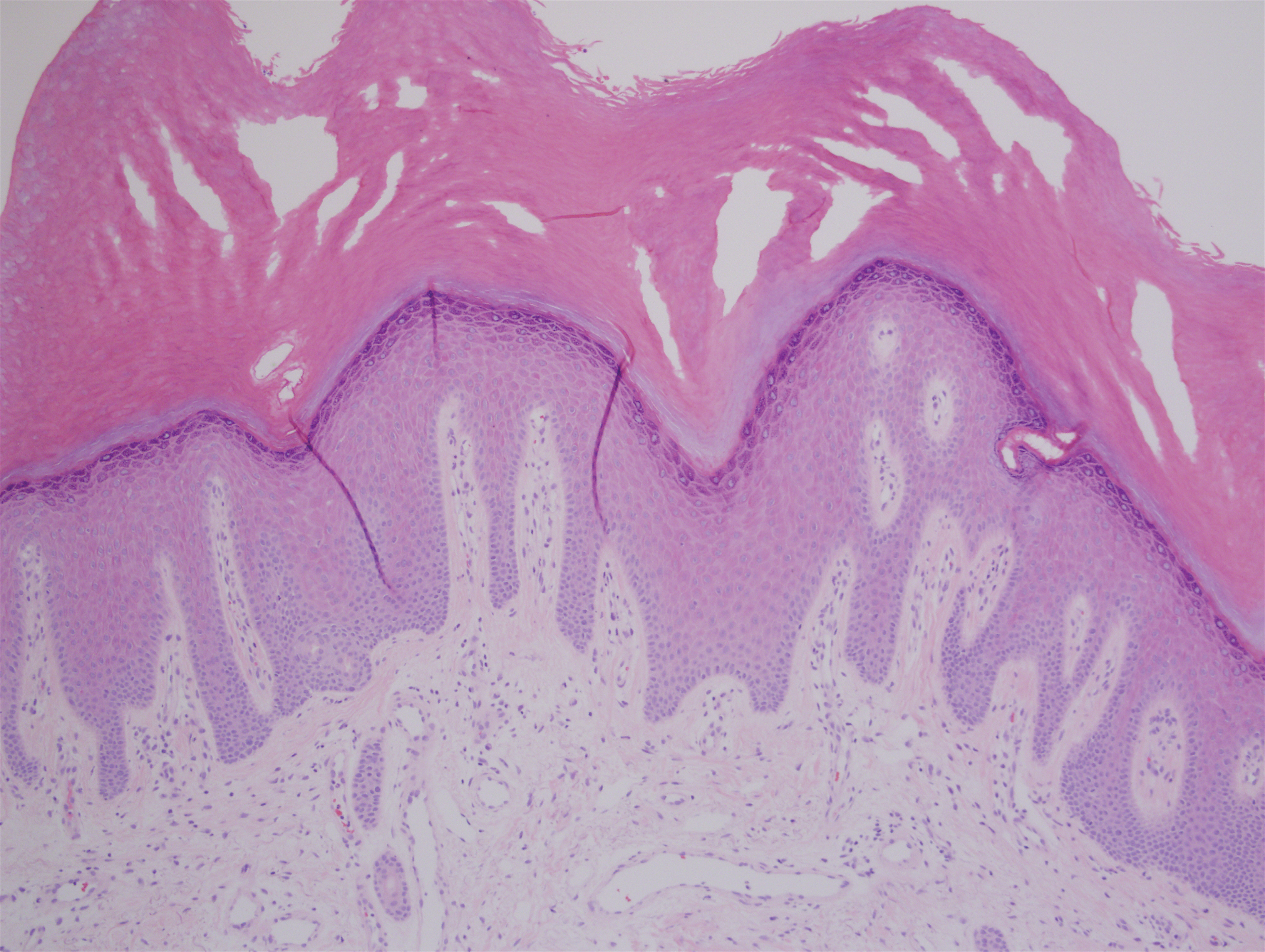
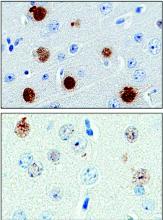
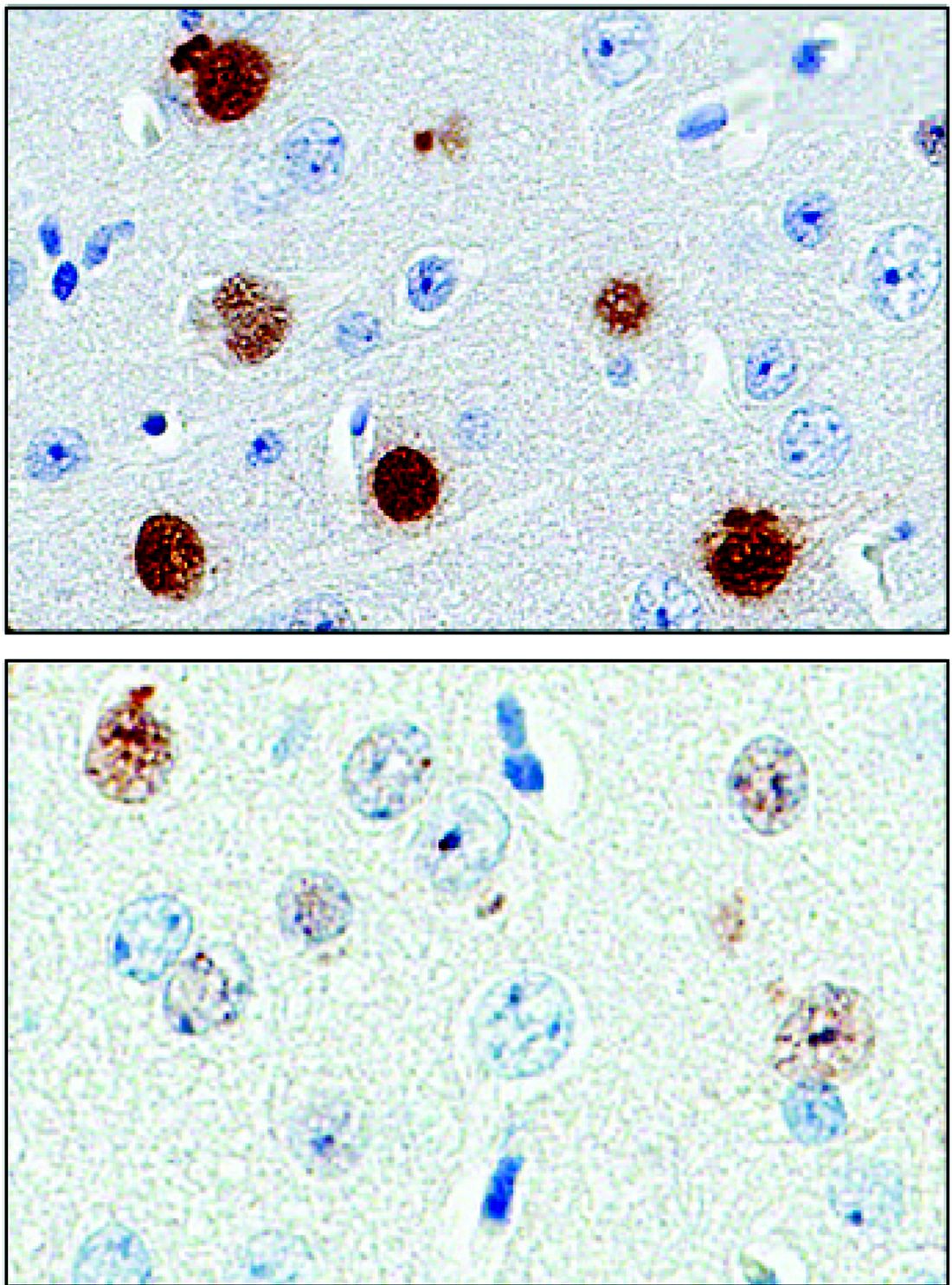
A punch biopsy of the medial hypothenar eminence of the left hand was performed, revealing notable lichenified hyperkeratosis with vascular ectasia (Figure 2). Periodic acid–Schiff staining was negative for fungal elements. Given the suspicion of PPK, multiple carcinoma markers were ordered. Cancer antigen 125 measured at 68 U/mL (reference range upper limit, 21 U/mL). Cancer antigen 27-29 was 50 U/mL (reference range, <38 U/mL) and cancer antigen 19-9 was 24 U/mL (reference range, <37 U/mL). Computed tomography of the chest revealed a large mass in the left lower lung associated with hilar lymphadenopathy. The patient was referred to oncology for further evaluation. Computed tomography–guided biopsy revealed metastatic uterine adenocarcinoma, which prompted subsequent chemotherapy. The combination of visceral malignancy with PPK led to the diagnosis of acquired PPK secondary to uterine cancer. After the completion of chemotherapy, the palmar dermatosis notably decreased (Figure 1B).
Comment
Paraneoplastic PPK is not uncommon. Ninety percent of acquired diffuse PPK is secondary to cancer,2 which occurs more frequently in male patients. Associated visceral malignancies include localized esophageal,3 myeloma,4 pulmonary, urinary/bladder,5 and gastric carcinoma.6 Paraneoplastic PPK in women is rare but has been linked to ovarian and breast carcinoma.7
The findings under light microscopy include thickening of any or all of the cell layers of the epidermis, which can include hyperkeratosis, acanthosis, and papillomatosis (Figure 2). A moderate amount of mononuclear cell infiltrates also can be visualized.
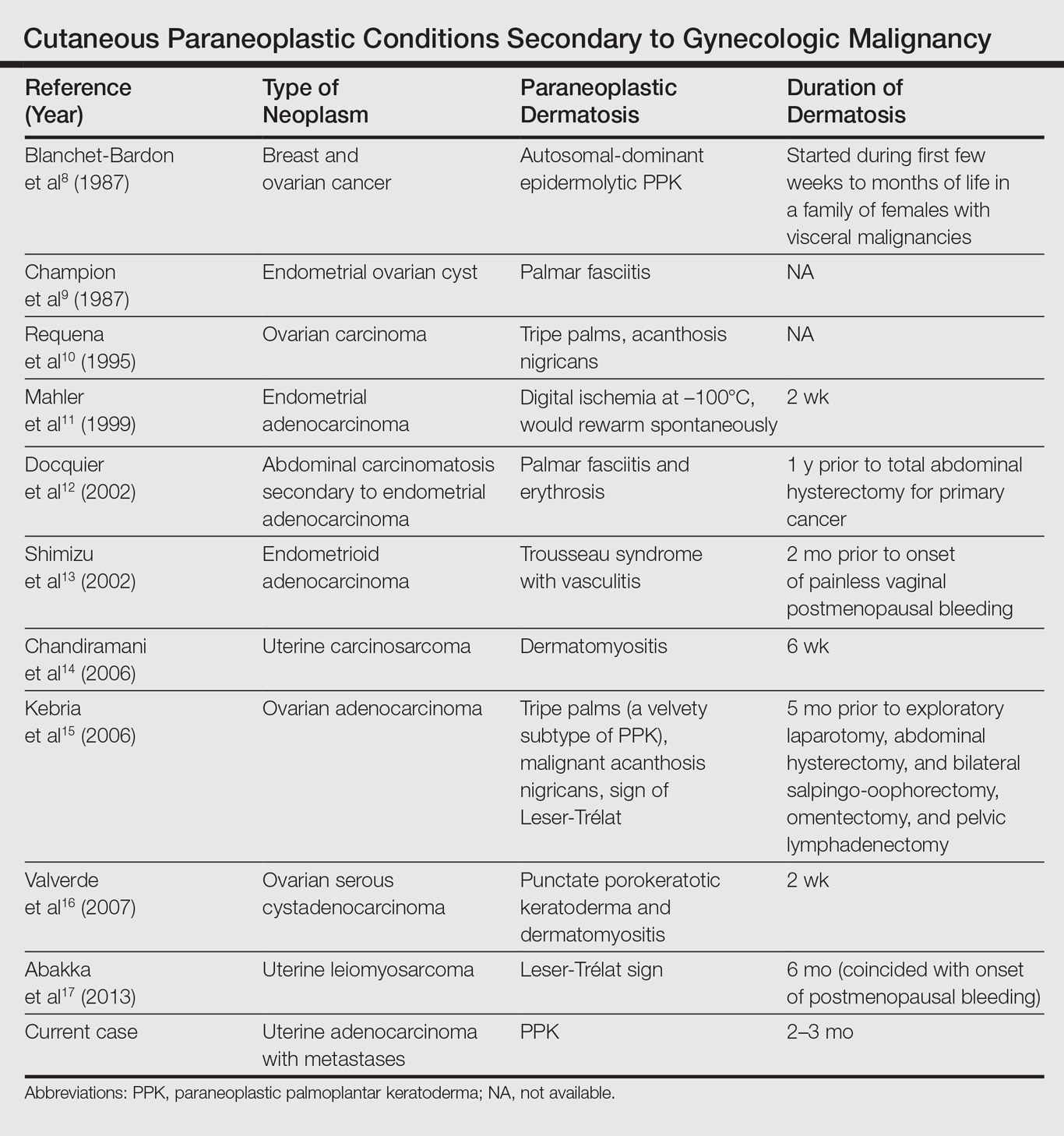
Palmoplantar keratoderma associated with uterine malignancy is rare. However, many other paraneoplastic dermatoses resulting from uterine cancer have been described as well as nonuterine gynecological malignancies (Table).8-17
The first step in managing acquired PPK is to determine its etiology via a complete history and a total-body skin examination. If findings are consistent with a hereditary PPK, then genetic workup is advised. Other suspected etiologies should be investigated via imaging and laboratory analysis.18
The first approach in managing acquired PPK is to treat the underlying cause. In prior cases, complete resolution of skin findings resulted once the malignancy or associated dermatosis had been treated.8-17 Adjunctive medication includes topical keratolytics (eg, urea, salicylic acid, lactic acid), topical retinoids, topical psoralen plus UVA, and topical corticosteroids.18 Vitamin A analogues have been found to be an effective treatment of many hyperkeratotic dermatoses.19 Isotretinoin and etretinate have been used to treat the cutaneous findings and prevent the onset and progression of esophageal malignancy of the inherited forms of PPK. The oral retinoid acitretin has been shown to rapidly resolve lesions, have persistent effects after 5 months of cessation, and have minimal side effects. Thus, it has been suggested as the first-line treatment of chronic PPK.19 One study found no response to topical keratolytics (urea cream and salicylic acid ointment) and a 2-week course of oral prednisone; however, low-dose oral acitretin 10 mg once daily resulted in notable improvement over several weeks.7 Physical debridement also may be necessary.18
Conclusion
Palmoplantar keratoderma is a condition that presents with hyperkeratosis of the palms and soles. Acquired PPK often occurs as a paraneoplastic response as well as a stigma of other dermatoses. It occurs more frequently in male patients. Reports of PPK secondary to uterine cancer are not common in the literature. Management of PPK includes a complete history and total-body skin examination. After appropriate imaging and laboratory analysis, treatment of the underlying cause is the best approach. Adjunctive medications include topical keratolytics, topical retinoids, topical psoralen plus UVA, and topical corticosteroids. Oral isotretinoin and etretinate have demonstrated promising results.
- Zamiri M, van Steensel MA, Munro CS. Inherited palmoplantar keratodermas. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012:538-548.
- Cohen PR, Grossman ME, Silvers DN. Tripe palms and cancer. Clin Dermatol. 1993;11:165-173.
- Belmar P, Marquet A, Martín-Sáez E. Symmetric palmar hyperkeratosis and esophageal carcinoma [in Spanish]. Actas Dermosifiliogr. 2008;99:149-150.
- Smith CH, Barker JN, Hay RJ. Diffuse plane xanthomatosis and acquired palmoplantar keratoderma in association with myeloma. Br J Dermatol. 1995;132:286-289.
- Küchmeister B, Rasokat H. Acquired disseminated papulous palmar keratoses—a paraneoplastic syndrome in cancers of the urinary bladder and lung? [in German]. Z Hautkr. 1984;59:1123-1124.
- Stieler K, Blume-Peytavi U, Vogel A, et al. Hyperkeratoses as paraneoplastic syndrome [published online June 1, 2012]. J Dtsch Dermatol Ges. 2012;10:593-595.
- Vignale RA, Espasandín J, Paciel J, et al. Diagnostic value of keratosis palmaris as indicative sign of visceral cancer [in Spanish]. Med Cutan Ibero Lat Am. 1983;11:287-292.
- Blanchet-Bardon C, Nazzaro V, Chevrant-Breton J, et al. Hereditary epidermolytic palmoplantar keratoderma associated with breast and ovarian cancer in a large kindred. Br J Dermatol. 1987;117:363-370.
- Champion GD, Saxon JA, Kossard S. The syndrome of palmar fibromatosis (fasciitis) and polyarthritis. J Rheumatol. 1987;14:1196-1198.
- Requena L, Aguilar A, Renedo G, et al. Tripe palms: a cutaneous marker of internal malignancy. J Dermatol. 1995;22:492-495.
- Mahler V, Neureiter D, Kirchner T, et al. Digital ischemia as paraneoplastic marker of metastatic endometrial carcinoma [in German]. Hautarzt. 1999;50:748-752.
- Docquier Ch, Majois F, Mitine C. Palmar fasciitis and arthritis: association with endometrial adenocarcinoma. Clin Rheumatol. 2002;21:63-65.
- Shimizu Y, Uchiyama S, Mori G, et al. A young patient with endometrioid adenocarcinoma who suffered Trousseau’s syndrome associated with vasculitis [in Japanese]. Rinsho Shinkeigaku. 2002;42:227-232.
- Chandiramani M, Joynson C, Panchal R, et al. Dermatomyositis as a paraneoplastic syndrome in carcinosarcoma of uterine origin. Clin Oncol (R Coll Radiol). 2006;18:641-648.
- Kebria MM, Belinson J, Kim R, et al. Malignant acanthosis nigricans, tripe palms and the sign of Leser-Trélat, a hint to the diagnosis of early stage ovarian cancer: a case report and review of the literature [published online January 27, 2006]. Gynecol Oncol. 2006;101:353-355.
- Valverde R, Sánchez-Caminero MP, Calzado L, et al. Dermatomyositis and punctate porokeratotic keratoderma as paraneoplastic syndrome of ovarian carcinoma [in Spanish]. Actas Dermosifiliogr. 2007;98:358-360.
- Abakka S, Elhalouat H, Khoummane N, et al. Uterine leiomyosarcoma and Leser-Trélat sign. Lancet. 2013;381:88.
- Patel S, Zirwas M, English JC 3rd. Acquired palmoplantar keratoderma. Am J Clin Dermatol. 2007;8:1-11.
- Capella GL, Fracchiolla C, Frigerio E, et al. A controlled study of comparative efficacy of oral retinoids and topical betamethasone/salicylic acid for chronic hyperkeratotic palmoplantar dermatitis. J Dermatolog Treat. 2004;15:88-93.
Paraneoplastic palmoplantar keratoderma (PPK) is an acquired dermatosis that presents with hyperkeratosis of the palms and soles in association with visceral malignancies, such as esophageal, gastric, pulmonary, and bladder carcinomas. This condition may either be acquired or inherited.1
Case Report
A 72-year-old woman was referred to our dermatology clinic for evaluation of a nonpruritic hyperkeratotic eruption predominantly on the palms and soles of 2 to 3 months’ duration (Figure 1A). Review of systems was remarkable for chronic anxiety, unintentional weight loss of 10 lb over the last 6 months, and a mild cough of 10 days’ duration. The differential diagnosis included eczematous dermatitis, tinea manuum, new-onset palmoplantar psoriasis, and PPK.


A punch biopsy of the medial hypothenar eminence of the left hand was performed, revealing notable lichenified hyperkeratosis with vascular ectasia (Figure 2). Periodic acid–Schiff staining was negative for fungal elements. Given the suspicion of PPK, multiple carcinoma markers were ordered. Cancer antigen 125 measured at 68 U/mL (reference range upper limit, 21 U/mL). Cancer antigen 27-29 was 50 U/mL (reference range, <38 U/mL) and cancer antigen 19-9 was 24 U/mL (reference range, <37 U/mL). Computed tomography of the chest revealed a large mass in the left lower lung associated with hilar lymphadenopathy. The patient was referred to oncology for further evaluation. Computed tomography–guided biopsy revealed metastatic uterine adenocarcinoma, which prompted subsequent chemotherapy. The combination of visceral malignancy with PPK led to the diagnosis of acquired PPK secondary to uterine cancer. After the completion of chemotherapy, the palmar dermatosis notably decreased (Figure 1B).
Comment
Paraneoplastic PPK is not uncommon. Ninety percent of acquired diffuse PPK is secondary to cancer,2 which occurs more frequently in male patients. Associated visceral malignancies include localized esophageal,3 myeloma,4 pulmonary, urinary/bladder,5 and gastric carcinoma.6 Paraneoplastic PPK in women is rare but has been linked to ovarian and breast carcinoma.7
The findings under light microscopy include thickening of any or all of the cell layers of the epidermis, which can include hyperkeratosis, acanthosis, and papillomatosis (Figure 2). A moderate amount of mononuclear cell infiltrates also can be visualized.
Palmoplantar keratoderma associated with uterine malignancy is rare. However, many other paraneoplastic dermatoses resulting from uterine cancer have been described as well as nonuterine gynecological malignancies (Table).8-17

The first step in managing acquired PPK is to determine its etiology via a complete history and a total-body skin examination. If findings are consistent with a hereditary PPK, then genetic workup is advised. Other suspected etiologies should be investigated via imaging and laboratory analysis.18
The first approach in managing acquired PPK is to treat the underlying cause. In prior cases, complete resolution of skin findings resulted once the malignancy or associated dermatosis had been treated.8-17 Adjunctive medication includes topical keratolytics (eg, urea, salicylic acid, lactic acid), topical retinoids, topical psoralen plus UVA, and topical corticosteroids.18 Vitamin A analogues have been found to be an effective treatment of many hyperkeratotic dermatoses.19 Isotretinoin and etretinate have been used to treat the cutaneous findings and prevent the onset and progression of esophageal malignancy of the inherited forms of PPK. The oral retinoid acitretin has been shown to rapidly resolve lesions, have persistent effects after 5 months of cessation, and have minimal side effects. Thus, it has been suggested as the first-line treatment of chronic PPK.19 One study found no response to topical keratolytics (urea cream and salicylic acid ointment) and a 2-week course of oral prednisone; however, low-dose oral acitretin 10 mg once daily resulted in notable improvement over several weeks.7 Physical debridement also may be necessary.18
Conclusion
Palmoplantar keratoderma is a condition that presents with hyperkeratosis of the palms and soles. Acquired PPK often occurs as a paraneoplastic response as well as a stigma of other dermatoses. It occurs more frequently in male patients. Reports of PPK secondary to uterine cancer are not common in the literature. Management of PPK includes a complete history and total-body skin examination. After appropriate imaging and laboratory analysis, treatment of the underlying cause is the best approach. Adjunctive medications include topical keratolytics, topical retinoids, topical psoralen plus UVA, and topical corticosteroids. Oral isotretinoin and etretinate have demonstrated promising results.
Paraneoplastic palmoplantar keratoderma (PPK) is an acquired dermatosis that presents with hyperkeratosis of the palms and soles in association with visceral malignancies, such as esophageal, gastric, pulmonary, and bladder carcinomas. This condition may either be acquired or inherited.1
Case Report
A 72-year-old woman was referred to our dermatology clinic for evaluation of a nonpruritic hyperkeratotic eruption predominantly on the palms and soles of 2 to 3 months’ duration (Figure 1A). Review of systems was remarkable for chronic anxiety, unintentional weight loss of 10 lb over the last 6 months, and a mild cough of 10 days’ duration. The differential diagnosis included eczematous dermatitis, tinea manuum, new-onset palmoplantar psoriasis, and PPK.


A punch biopsy of the medial hypothenar eminence of the left hand was performed, revealing notable lichenified hyperkeratosis with vascular ectasia (Figure 2). Periodic acid–Schiff staining was negative for fungal elements. Given the suspicion of PPK, multiple carcinoma markers were ordered. Cancer antigen 125 measured at 68 U/mL (reference range upper limit, 21 U/mL). Cancer antigen 27-29 was 50 U/mL (reference range, <38 U/mL) and cancer antigen 19-9 was 24 U/mL (reference range, <37 U/mL). Computed tomography of the chest revealed a large mass in the left lower lung associated with hilar lymphadenopathy. The patient was referred to oncology for further evaluation. Computed tomography–guided biopsy revealed metastatic uterine adenocarcinoma, which prompted subsequent chemotherapy. The combination of visceral malignancy with PPK led to the diagnosis of acquired PPK secondary to uterine cancer. After the completion of chemotherapy, the palmar dermatosis notably decreased (Figure 1B).
Comment
Paraneoplastic PPK is not uncommon. Ninety percent of acquired diffuse PPK is secondary to cancer,2 which occurs more frequently in male patients. Associated visceral malignancies include localized esophageal,3 myeloma,4 pulmonary, urinary/bladder,5 and gastric carcinoma.6 Paraneoplastic PPK in women is rare but has been linked to ovarian and breast carcinoma.7
The findings under light microscopy include thickening of any or all of the cell layers of the epidermis, which can include hyperkeratosis, acanthosis, and papillomatosis (Figure 2). A moderate amount of mononuclear cell infiltrates also can be visualized.
Palmoplantar keratoderma associated with uterine malignancy is rare. However, many other paraneoplastic dermatoses resulting from uterine cancer have been described as well as nonuterine gynecological malignancies (Table).8-17

The first step in managing acquired PPK is to determine its etiology via a complete history and a total-body skin examination. If findings are consistent with a hereditary PPK, then genetic workup is advised. Other suspected etiologies should be investigated via imaging and laboratory analysis.18
The first approach in managing acquired PPK is to treat the underlying cause. In prior cases, complete resolution of skin findings resulted once the malignancy or associated dermatosis had been treated.8-17 Adjunctive medication includes topical keratolytics (eg, urea, salicylic acid, lactic acid), topical retinoids, topical psoralen plus UVA, and topical corticosteroids.18 Vitamin A analogues have been found to be an effective treatment of many hyperkeratotic dermatoses.19 Isotretinoin and etretinate have been used to treat the cutaneous findings and prevent the onset and progression of esophageal malignancy of the inherited forms of PPK. The oral retinoid acitretin has been shown to rapidly resolve lesions, have persistent effects after 5 months of cessation, and have minimal side effects. Thus, it has been suggested as the first-line treatment of chronic PPK.19 One study found no response to topical keratolytics (urea cream and salicylic acid ointment) and a 2-week course of oral prednisone; however, low-dose oral acitretin 10 mg once daily resulted in notable improvement over several weeks.7 Physical debridement also may be necessary.18
Conclusion
Palmoplantar keratoderma is a condition that presents with hyperkeratosis of the palms and soles. Acquired PPK often occurs as a paraneoplastic response as well as a stigma of other dermatoses. It occurs more frequently in male patients. Reports of PPK secondary to uterine cancer are not common in the literature. Management of PPK includes a complete history and total-body skin examination. After appropriate imaging and laboratory analysis, treatment of the underlying cause is the best approach. Adjunctive medications include topical keratolytics, topical retinoids, topical psoralen plus UVA, and topical corticosteroids. Oral isotretinoin and etretinate have demonstrated promising results.
- Zamiri M, van Steensel MA, Munro CS. Inherited palmoplantar keratodermas. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012:538-548.
- Cohen PR, Grossman ME, Silvers DN. Tripe palms and cancer. Clin Dermatol. 1993;11:165-173.
- Belmar P, Marquet A, Martín-Sáez E. Symmetric palmar hyperkeratosis and esophageal carcinoma [in Spanish]. Actas Dermosifiliogr. 2008;99:149-150.
- Smith CH, Barker JN, Hay RJ. Diffuse plane xanthomatosis and acquired palmoplantar keratoderma in association with myeloma. Br J Dermatol. 1995;132:286-289.
- Küchmeister B, Rasokat H. Acquired disseminated papulous palmar keratoses—a paraneoplastic syndrome in cancers of the urinary bladder and lung? [in German]. Z Hautkr. 1984;59:1123-1124.
- Stieler K, Blume-Peytavi U, Vogel A, et al. Hyperkeratoses as paraneoplastic syndrome [published online June 1, 2012]. J Dtsch Dermatol Ges. 2012;10:593-595.
- Vignale RA, Espasandín J, Paciel J, et al. Diagnostic value of keratosis palmaris as indicative sign of visceral cancer [in Spanish]. Med Cutan Ibero Lat Am. 1983;11:287-292.
- Blanchet-Bardon C, Nazzaro V, Chevrant-Breton J, et al. Hereditary epidermolytic palmoplantar keratoderma associated with breast and ovarian cancer in a large kindred. Br J Dermatol. 1987;117:363-370.
- Champion GD, Saxon JA, Kossard S. The syndrome of palmar fibromatosis (fasciitis) and polyarthritis. J Rheumatol. 1987;14:1196-1198.
- Requena L, Aguilar A, Renedo G, et al. Tripe palms: a cutaneous marker of internal malignancy. J Dermatol. 1995;22:492-495.
- Mahler V, Neureiter D, Kirchner T, et al. Digital ischemia as paraneoplastic marker of metastatic endometrial carcinoma [in German]. Hautarzt. 1999;50:748-752.
- Docquier Ch, Majois F, Mitine C. Palmar fasciitis and arthritis: association with endometrial adenocarcinoma. Clin Rheumatol. 2002;21:63-65.
- Shimizu Y, Uchiyama S, Mori G, et al. A young patient with endometrioid adenocarcinoma who suffered Trousseau’s syndrome associated with vasculitis [in Japanese]. Rinsho Shinkeigaku. 2002;42:227-232.
- Chandiramani M, Joynson C, Panchal R, et al. Dermatomyositis as a paraneoplastic syndrome in carcinosarcoma of uterine origin. Clin Oncol (R Coll Radiol). 2006;18:641-648.
- Kebria MM, Belinson J, Kim R, et al. Malignant acanthosis nigricans, tripe palms and the sign of Leser-Trélat, a hint to the diagnosis of early stage ovarian cancer: a case report and review of the literature [published online January 27, 2006]. Gynecol Oncol. 2006;101:353-355.
- Valverde R, Sánchez-Caminero MP, Calzado L, et al. Dermatomyositis and punctate porokeratotic keratoderma as paraneoplastic syndrome of ovarian carcinoma [in Spanish]. Actas Dermosifiliogr. 2007;98:358-360.
- Abakka S, Elhalouat H, Khoummane N, et al. Uterine leiomyosarcoma and Leser-Trélat sign. Lancet. 2013;381:88.
- Patel S, Zirwas M, English JC 3rd. Acquired palmoplantar keratoderma. Am J Clin Dermatol. 2007;8:1-11.
- Capella GL, Fracchiolla C, Frigerio E, et al. A controlled study of comparative efficacy of oral retinoids and topical betamethasone/salicylic acid for chronic hyperkeratotic palmoplantar dermatitis. J Dermatolog Treat. 2004;15:88-93.
- Zamiri M, van Steensel MA, Munro CS. Inherited palmoplantar keratodermas. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012:538-548.
- Cohen PR, Grossman ME, Silvers DN. Tripe palms and cancer. Clin Dermatol. 1993;11:165-173.
- Belmar P, Marquet A, Martín-Sáez E. Symmetric palmar hyperkeratosis and esophageal carcinoma [in Spanish]. Actas Dermosifiliogr. 2008;99:149-150.
- Smith CH, Barker JN, Hay RJ. Diffuse plane xanthomatosis and acquired palmoplantar keratoderma in association with myeloma. Br J Dermatol. 1995;132:286-289.
- Küchmeister B, Rasokat H. Acquired disseminated papulous palmar keratoses—a paraneoplastic syndrome in cancers of the urinary bladder and lung? [in German]. Z Hautkr. 1984;59:1123-1124.
- Stieler K, Blume-Peytavi U, Vogel A, et al. Hyperkeratoses as paraneoplastic syndrome [published online June 1, 2012]. J Dtsch Dermatol Ges. 2012;10:593-595.
- Vignale RA, Espasandín J, Paciel J, et al. Diagnostic value of keratosis palmaris as indicative sign of visceral cancer [in Spanish]. Med Cutan Ibero Lat Am. 1983;11:287-292.
- Blanchet-Bardon C, Nazzaro V, Chevrant-Breton J, et al. Hereditary epidermolytic palmoplantar keratoderma associated with breast and ovarian cancer in a large kindred. Br J Dermatol. 1987;117:363-370.
- Champion GD, Saxon JA, Kossard S. The syndrome of palmar fibromatosis (fasciitis) and polyarthritis. J Rheumatol. 1987;14:1196-1198.
- Requena L, Aguilar A, Renedo G, et al. Tripe palms: a cutaneous marker of internal malignancy. J Dermatol. 1995;22:492-495.
- Mahler V, Neureiter D, Kirchner T, et al. Digital ischemia as paraneoplastic marker of metastatic endometrial carcinoma [in German]. Hautarzt. 1999;50:748-752.
- Docquier Ch, Majois F, Mitine C. Palmar fasciitis and arthritis: association with endometrial adenocarcinoma. Clin Rheumatol. 2002;21:63-65.
- Shimizu Y, Uchiyama S, Mori G, et al. A young patient with endometrioid adenocarcinoma who suffered Trousseau’s syndrome associated with vasculitis [in Japanese]. Rinsho Shinkeigaku. 2002;42:227-232.
- Chandiramani M, Joynson C, Panchal R, et al. Dermatomyositis as a paraneoplastic syndrome in carcinosarcoma of uterine origin. Clin Oncol (R Coll Radiol). 2006;18:641-648.
- Kebria MM, Belinson J, Kim R, et al. Malignant acanthosis nigricans, tripe palms and the sign of Leser-Trélat, a hint to the diagnosis of early stage ovarian cancer: a case report and review of the literature [published online January 27, 2006]. Gynecol Oncol. 2006;101:353-355.
- Valverde R, Sánchez-Caminero MP, Calzado L, et al. Dermatomyositis and punctate porokeratotic keratoderma as paraneoplastic syndrome of ovarian carcinoma [in Spanish]. Actas Dermosifiliogr. 2007;98:358-360.
- Abakka S, Elhalouat H, Khoummane N, et al. Uterine leiomyosarcoma and Leser-Trélat sign. Lancet. 2013;381:88.
- Patel S, Zirwas M, English JC 3rd. Acquired palmoplantar keratoderma. Am J Clin Dermatol. 2007;8:1-11.
- Capella GL, Fracchiolla C, Frigerio E, et al. A controlled study of comparative efficacy of oral retinoids and topical betamethasone/salicylic acid for chronic hyperkeratotic palmoplantar dermatitis. J Dermatolog Treat. 2004;15:88-93.
Practice Points
- Paraneoplastic palmoplantar keratoderma (PPK) is an acquired dermatosis that presents with hyperkeratosis of the palms and soles in association with visceral malignancies (eg, esophageal, gastric, pulmonary, and urinary/bladder carcinomas).
- Palmoplantar keratoderma secondary to uterine cancer is rare.
- Light microscopy shows thickening of any or all of the cell layers of the epidermis (hyperkeratosis, acanthosis, and papillomatosis) and mononuclear cells.
- Management of acquired PPK includes treatment of the underlying malignancy. Adjunctive vitamin A analogues may be of additional utility.
Heat-stable rotavirus vaccine shows promising results
A new low-cost rotavirus gastroenteritis vaccine that does not require cold storage showed a vaccine efficacy of 67% in a per-protocol test of 3,508 Nigerien infants, according to a study by Sheila Isanaka of the department of research at Epicentre, Paris, and her associates.
In a double blind, placebo-controlled test of the oral bovine rotavirus pentavalent vaccine (BRV-PV), 31 severe cases of rotavirus were found in the vaccinated group of 1,780, while 87 cases were found in the placebo group of 1,728 (2.14 vs. 6.44 cases per 100 person-years, respectively), according to Ms. Isanaka and her colleagues (N Engl J Med. 2017 Mar 23;376[12]:1121-30).
Researchers gathered infants with severe symptoms and administered three injections of either BRV-PV or the placebo from August 2014 through November 2015. The BRV-PV vaccine, manufactured by Serum Institute of India, contains the rotavirus serotypes G1, G2, G3, G4, and G9.
With regard to adverse events, there was no distinction between the two groups (P greater than .15). According to medical investigators, the most common cause of deaths – 27 and 22 in the vaccine and placebo groups, respectively – was infections and infestations (in 37 infants) and metabolism and nutrition disorders (in 6).
Part of what makes this vaccine unique is its shelf life of 2 years when kept at 37° C, or 6 months at 40° C, a key point for Ms. Isanaka and her colleagues.
“The global supply of the vaccines is constrained, and unreliable transportation and storage systems make delivery of vaccines that require refrigeration difficult,” Ms. Isanaka and her colleagues reported. “The introduction of BRV-PV may help to minimize the burden on already strained immunization programs.” The vaccine is also “for sale at or below the current price of the two WHO prequalified vaccines that are supported by the Gavi alliance,” making it possibly more financially accessible as well.
While the per-protocol test found efficacy at 67%, the intention-to-treat population reported a higher efficacy of 73%. Ms. Isanaka and her colleagues believe this number “may more closely represent the efficacy under real-world conditions,” due to the more flexible vaccination schedule.
This study was limited by a short time frame, which did not allow researchers to gather genotyping data pertaining to the efficacy against changing serotypes. BRV-PV also was not given consistently with the oral polio vaccine.
The study was supported by Médecins sans Frontières Operational Center in Geneva and the Kavli Foundation. Ms. Isanaka’s institution, Epicentre, receives core funding from Médecins sans Frontières. Ms. McNeal reports grant support from Epicentre for the study, and other support from Merck and GlaxoSmithKline outside the submitted work. None of the other researchers had relevant financial disclosures.
[email protected]
On Twitter @EAZTweets
Rotavirus is the leading cause of diarrhea-associated death in children aged under 5 years, and with 85% of all deaths occurring in Africa and Asia, proper channels of vaccine dissemination are critical. There is no question to the positive influence of rotavirus vaccines, as research suggests they could save 2.46 million children’s lives and prevent another 83 million from living with a disability between 2011 and 2030.
While two vaccines, Rotarix and RotaTeq, are already being introduced to patients in 48 countries, diminishing vaccine uptake is standing in the way of these interventions reaching their full potential. Despite subsidies from Gavi (the vaccine alliance) and the World Health Organization, costs are still too high and the necessity for cold storage poses a financial and logistical problem for a majority of high risk countries. The BRV-PV vaccine developed by Serum Institute of India seems to be a step in the right direction in helping to ease this burden.
While its efficacy is modest, BRV-PV has shown promise through its heat stability as well as its cost, which falls between the prices of the two already available vaccines.
It is not perfect; as a freeze-dried vaccine, it may pose problems in areas where oral liquid, all-in-one vaccinations are preferred. Yet, there is no doubt that an increase in affordable, programmatically suitable options will help achieve the goal of ending rotavirus related deaths globally.
Mathuram Santosham, MD, is professor of pediatrics and pediatric infectious diseases at Johns Hopkins University, Baltimore. Duncan Steele, PhD, is a microbiologist and deputy director and strategic lead for enteric vaccines and enteric and diarrheal diseases for the Bill & Melinda Gates Foundation, Seattle. They coauthored the editorial regarding the article by Isanaka et al. (N Engl J Med. 2017 March 23;376[12]:1170-2). Dr. Santosham reported no relevant financial disclosures. Dr. Steele reports that he is employed at the Bill & Melinda Gates Foundation, which has supported through funding, the development of multiple rotavirus vaccine candidates including the lyophilized rotavirus vaccine produced by Serum Institute.
Rotavirus is the leading cause of diarrhea-associated death in children aged under 5 years, and with 85% of all deaths occurring in Africa and Asia, proper channels of vaccine dissemination are critical. There is no question to the positive influence of rotavirus vaccines, as research suggests they could save 2.46 million children’s lives and prevent another 83 million from living with a disability between 2011 and 2030.
While two vaccines, Rotarix and RotaTeq, are already being introduced to patients in 48 countries, diminishing vaccine uptake is standing in the way of these interventions reaching their full potential. Despite subsidies from Gavi (the vaccine alliance) and the World Health Organization, costs are still too high and the necessity for cold storage poses a financial and logistical problem for a majority of high risk countries. The BRV-PV vaccine developed by Serum Institute of India seems to be a step in the right direction in helping to ease this burden.
While its efficacy is modest, BRV-PV has shown promise through its heat stability as well as its cost, which falls between the prices of the two already available vaccines.
It is not perfect; as a freeze-dried vaccine, it may pose problems in areas where oral liquid, all-in-one vaccinations are preferred. Yet, there is no doubt that an increase in affordable, programmatically suitable options will help achieve the goal of ending rotavirus related deaths globally.
Mathuram Santosham, MD, is professor of pediatrics and pediatric infectious diseases at Johns Hopkins University, Baltimore. Duncan Steele, PhD, is a microbiologist and deputy director and strategic lead for enteric vaccines and enteric and diarrheal diseases for the Bill & Melinda Gates Foundation, Seattle. They coauthored the editorial regarding the article by Isanaka et al. (N Engl J Med. 2017 March 23;376[12]:1170-2). Dr. Santosham reported no relevant financial disclosures. Dr. Steele reports that he is employed at the Bill & Melinda Gates Foundation, which has supported through funding, the development of multiple rotavirus vaccine candidates including the lyophilized rotavirus vaccine produced by Serum Institute.
Rotavirus is the leading cause of diarrhea-associated death in children aged under 5 years, and with 85% of all deaths occurring in Africa and Asia, proper channels of vaccine dissemination are critical. There is no question to the positive influence of rotavirus vaccines, as research suggests they could save 2.46 million children’s lives and prevent another 83 million from living with a disability between 2011 and 2030.
While two vaccines, Rotarix and RotaTeq, are already being introduced to patients in 48 countries, diminishing vaccine uptake is standing in the way of these interventions reaching their full potential. Despite subsidies from Gavi (the vaccine alliance) and the World Health Organization, costs are still too high and the necessity for cold storage poses a financial and logistical problem for a majority of high risk countries. The BRV-PV vaccine developed by Serum Institute of India seems to be a step in the right direction in helping to ease this burden.
While its efficacy is modest, BRV-PV has shown promise through its heat stability as well as its cost, which falls between the prices of the two already available vaccines.
It is not perfect; as a freeze-dried vaccine, it may pose problems in areas where oral liquid, all-in-one vaccinations are preferred. Yet, there is no doubt that an increase in affordable, programmatically suitable options will help achieve the goal of ending rotavirus related deaths globally.
Mathuram Santosham, MD, is professor of pediatrics and pediatric infectious diseases at Johns Hopkins University, Baltimore. Duncan Steele, PhD, is a microbiologist and deputy director and strategic lead for enteric vaccines and enteric and diarrheal diseases for the Bill & Melinda Gates Foundation, Seattle. They coauthored the editorial regarding the article by Isanaka et al. (N Engl J Med. 2017 March 23;376[12]:1170-2). Dr. Santosham reported no relevant financial disclosures. Dr. Steele reports that he is employed at the Bill & Melinda Gates Foundation, which has supported through funding, the development of multiple rotavirus vaccine candidates including the lyophilized rotavirus vaccine produced by Serum Institute.
A new low-cost rotavirus gastroenteritis vaccine that does not require cold storage showed a vaccine efficacy of 67% in a per-protocol test of 3,508 Nigerien infants, according to a study by Sheila Isanaka of the department of research at Epicentre, Paris, and her associates.
In a double blind, placebo-controlled test of the oral bovine rotavirus pentavalent vaccine (BRV-PV), 31 severe cases of rotavirus were found in the vaccinated group of 1,780, while 87 cases were found in the placebo group of 1,728 (2.14 vs. 6.44 cases per 100 person-years, respectively), according to Ms. Isanaka and her colleagues (N Engl J Med. 2017 Mar 23;376[12]:1121-30).
Researchers gathered infants with severe symptoms and administered three injections of either BRV-PV or the placebo from August 2014 through November 2015. The BRV-PV vaccine, manufactured by Serum Institute of India, contains the rotavirus serotypes G1, G2, G3, G4, and G9.
With regard to adverse events, there was no distinction between the two groups (P greater than .15). According to medical investigators, the most common cause of deaths – 27 and 22 in the vaccine and placebo groups, respectively – was infections and infestations (in 37 infants) and metabolism and nutrition disorders (in 6).
Part of what makes this vaccine unique is its shelf life of 2 years when kept at 37° C, or 6 months at 40° C, a key point for Ms. Isanaka and her colleagues.
“The global supply of the vaccines is constrained, and unreliable transportation and storage systems make delivery of vaccines that require refrigeration difficult,” Ms. Isanaka and her colleagues reported. “The introduction of BRV-PV may help to minimize the burden on already strained immunization programs.” The vaccine is also “for sale at or below the current price of the two WHO prequalified vaccines that are supported by the Gavi alliance,” making it possibly more financially accessible as well.
While the per-protocol test found efficacy at 67%, the intention-to-treat population reported a higher efficacy of 73%. Ms. Isanaka and her colleagues believe this number “may more closely represent the efficacy under real-world conditions,” due to the more flexible vaccination schedule.
This study was limited by a short time frame, which did not allow researchers to gather genotyping data pertaining to the efficacy against changing serotypes. BRV-PV also was not given consistently with the oral polio vaccine.
The study was supported by Médecins sans Frontières Operational Center in Geneva and the Kavli Foundation. Ms. Isanaka’s institution, Epicentre, receives core funding from Médecins sans Frontières. Ms. McNeal reports grant support from Epicentre for the study, and other support from Merck and GlaxoSmithKline outside the submitted work. None of the other researchers had relevant financial disclosures.
[email protected]
On Twitter @EAZTweets
A new low-cost rotavirus gastroenteritis vaccine that does not require cold storage showed a vaccine efficacy of 67% in a per-protocol test of 3,508 Nigerien infants, according to a study by Sheila Isanaka of the department of research at Epicentre, Paris, and her associates.
In a double blind, placebo-controlled test of the oral bovine rotavirus pentavalent vaccine (BRV-PV), 31 severe cases of rotavirus were found in the vaccinated group of 1,780, while 87 cases were found in the placebo group of 1,728 (2.14 vs. 6.44 cases per 100 person-years, respectively), according to Ms. Isanaka and her colleagues (N Engl J Med. 2017 Mar 23;376[12]:1121-30).
Researchers gathered infants with severe symptoms and administered three injections of either BRV-PV or the placebo from August 2014 through November 2015. The BRV-PV vaccine, manufactured by Serum Institute of India, contains the rotavirus serotypes G1, G2, G3, G4, and G9.
With regard to adverse events, there was no distinction between the two groups (P greater than .15). According to medical investigators, the most common cause of deaths – 27 and 22 in the vaccine and placebo groups, respectively – was infections and infestations (in 37 infants) and metabolism and nutrition disorders (in 6).
Part of what makes this vaccine unique is its shelf life of 2 years when kept at 37° C, or 6 months at 40° C, a key point for Ms. Isanaka and her colleagues.
“The global supply of the vaccines is constrained, and unreliable transportation and storage systems make delivery of vaccines that require refrigeration difficult,” Ms. Isanaka and her colleagues reported. “The introduction of BRV-PV may help to minimize the burden on already strained immunization programs.” The vaccine is also “for sale at or below the current price of the two WHO prequalified vaccines that are supported by the Gavi alliance,” making it possibly more financially accessible as well.
While the per-protocol test found efficacy at 67%, the intention-to-treat population reported a higher efficacy of 73%. Ms. Isanaka and her colleagues believe this number “may more closely represent the efficacy under real-world conditions,” due to the more flexible vaccination schedule.
This study was limited by a short time frame, which did not allow researchers to gather genotyping data pertaining to the efficacy against changing serotypes. BRV-PV also was not given consistently with the oral polio vaccine.
The study was supported by Médecins sans Frontières Operational Center in Geneva and the Kavli Foundation. Ms. Isanaka’s institution, Epicentre, receives core funding from Médecins sans Frontières. Ms. McNeal reports grant support from Epicentre for the study, and other support from Merck and GlaxoSmithKline outside the submitted work. None of the other researchers had relevant financial disclosures.
[email protected]
On Twitter @EAZTweets
Key clinical point:
Major finding: Of 3,508 infants studied, 31 cases of severe rotavirus gastroenteritis were found in the vaccine group, and 87 cases in the placebo group, putting efficacy at 67%.
Data source: Double blind, placebo-controlled test of 3,508 Nigerian infants whose symptoms were measured via 20-point Vesikari scoring.
Disclosures: The study was supported by Médecins sans Frontières Operational Center in Geneva and the Kavli Foundation. Ms. Isanaka’s institution, Epicentre, receives core funding from Médecins sans Frontières. Ms. McNeal reports grant support from Epicentre for the study, and other support from Merck and GlaxoSmithKline outside the submitted work. None of the other researchers had relevant financial disclosures.
Milk: Friend to bones, foe to faces?
ORLANDO – A greasy hamburger and fries and a chocolate milkshake may all earn the finger of blame when teens fret over acne. But which of these foods is the real culprit?
A growing body of data suggests it may be the milk – especially if it’s fat-free milk, according to Andrea Zaenglein, MD, who spoke at the annual meeting of the American Academy of Dermatology. Skim milk has been at the center of a long-simmering acne controversy, said Dr. Zaenglein, professor of dermatology and pediatric dermatology at Pennsylvania State University, Hershey.
The same association was seen in a subsequent study of 4,273 teen boys, published in 2008. There was a 10% increased risk for acne associated with intake of whole or 2% milk, a 17% increased risk for 1% milk, and a 19% increased risk for skim milk (J Am Acad Dermatol. 2008 May;58[5]: 787-93).
A prospective study of about 6,000 girls found similar risks associated with all types of milk: a 19% increased risk for whole milk, 17% for low-fat milk, and 19% for skim milk (Dermatol Online J. 2006 May 30;12[4]:1).
They found positive associations with total dairy and with nonfat dairy, but not with whole-fat or low-fat dairy. “The association was driven by the nonfat dairy,” Dr. Zaenglein said. “When we took nonfat [dairy] out of the total dairy, the association there was no longer significant.” They also found no significant association with body mass index, glycemic index, or glycemic load, she added.
“You have to wonder, what could this association between dairy – and skim milk in particular – be? Could dairy actually be involved in the pathogenesis of acne?” There are a number of proposed mechanisms, none of which have ever been confirmed, she said. “Could it be related to steroids? Milk is a very bioactive substance with estrogens and other hormones, but these are fat soluble and would be removed in skim milk.”
Another theory suggests that insulinlike growth factor-1, either in milk or endogenously stimulated by its consumption, may make a contribution. “People who are passionate about this have published prolifically about the activation of this pathway,” Dr. Zaenglein said, “but it remains speculative.”
She added, there are a plethora of studies showing milk’s benefits in many other areas, including the benefits exerted by milk’s medium-chain fatty acids on cardiovascular health, glycemic control, insulin regulation, and even obesity.
Finally, dairy’s importance to bone health in the United States can’t be ignored. In fact, dairy products are the most commonly recommended foods for ensuring adequate calcium intake in children, teens, and young adults.
“It’s really hard to make a firm recommendation to eliminate dairy, because, in this country, it makes up a good portion of the calcium teens need during their bone-building years, and kids are already at high risk for not meeting these requirements.”
National nutritional guidelines recommend 1,300 mg of calcium every day, which can be accomplished in three to five servings of dairy. “An 8-ounce glass of milk has 300 mg. Yogurt, cheese, and calcium-fortified juice are all highly accepted by teens. But, to get that same amount from vegetables, for example, you’d have to eat 3 cups of cooked kale. That’s a lot of kale,” Dr. Zaenglein said.
She had no relevant financial disclosures.
[email protected]
On Twitter @alz_gal
ORLANDO – A greasy hamburger and fries and a chocolate milkshake may all earn the finger of blame when teens fret over acne. But which of these foods is the real culprit?
A growing body of data suggests it may be the milk – especially if it’s fat-free milk, according to Andrea Zaenglein, MD, who spoke at the annual meeting of the American Academy of Dermatology. Skim milk has been at the center of a long-simmering acne controversy, said Dr. Zaenglein, professor of dermatology and pediatric dermatology at Pennsylvania State University, Hershey.
The same association was seen in a subsequent study of 4,273 teen boys, published in 2008. There was a 10% increased risk for acne associated with intake of whole or 2% milk, a 17% increased risk for 1% milk, and a 19% increased risk for skim milk (J Am Acad Dermatol. 2008 May;58[5]: 787-93).
A prospective study of about 6,000 girls found similar risks associated with all types of milk: a 19% increased risk for whole milk, 17% for low-fat milk, and 19% for skim milk (Dermatol Online J. 2006 May 30;12[4]:1).
They found positive associations with total dairy and with nonfat dairy, but not with whole-fat or low-fat dairy. “The association was driven by the nonfat dairy,” Dr. Zaenglein said. “When we took nonfat [dairy] out of the total dairy, the association there was no longer significant.” They also found no significant association with body mass index, glycemic index, or glycemic load, she added.
“You have to wonder, what could this association between dairy – and skim milk in particular – be? Could dairy actually be involved in the pathogenesis of acne?” There are a number of proposed mechanisms, none of which have ever been confirmed, she said. “Could it be related to steroids? Milk is a very bioactive substance with estrogens and other hormones, but these are fat soluble and would be removed in skim milk.”
Another theory suggests that insulinlike growth factor-1, either in milk or endogenously stimulated by its consumption, may make a contribution. “People who are passionate about this have published prolifically about the activation of this pathway,” Dr. Zaenglein said, “but it remains speculative.”
She added, there are a plethora of studies showing milk’s benefits in many other areas, including the benefits exerted by milk’s medium-chain fatty acids on cardiovascular health, glycemic control, insulin regulation, and even obesity.
Finally, dairy’s importance to bone health in the United States can’t be ignored. In fact, dairy products are the most commonly recommended foods for ensuring adequate calcium intake in children, teens, and young adults.
“It’s really hard to make a firm recommendation to eliminate dairy, because, in this country, it makes up a good portion of the calcium teens need during their bone-building years, and kids are already at high risk for not meeting these requirements.”
National nutritional guidelines recommend 1,300 mg of calcium every day, which can be accomplished in three to five servings of dairy. “An 8-ounce glass of milk has 300 mg. Yogurt, cheese, and calcium-fortified juice are all highly accepted by teens. But, to get that same amount from vegetables, for example, you’d have to eat 3 cups of cooked kale. That’s a lot of kale,” Dr. Zaenglein said.
She had no relevant financial disclosures.
[email protected]
On Twitter @alz_gal
ORLANDO – A greasy hamburger and fries and a chocolate milkshake may all earn the finger of blame when teens fret over acne. But which of these foods is the real culprit?
A growing body of data suggests it may be the milk – especially if it’s fat-free milk, according to Andrea Zaenglein, MD, who spoke at the annual meeting of the American Academy of Dermatology. Skim milk has been at the center of a long-simmering acne controversy, said Dr. Zaenglein, professor of dermatology and pediatric dermatology at Pennsylvania State University, Hershey.
The same association was seen in a subsequent study of 4,273 teen boys, published in 2008. There was a 10% increased risk for acne associated with intake of whole or 2% milk, a 17% increased risk for 1% milk, and a 19% increased risk for skim milk (J Am Acad Dermatol. 2008 May;58[5]: 787-93).
A prospective study of about 6,000 girls found similar risks associated with all types of milk: a 19% increased risk for whole milk, 17% for low-fat milk, and 19% for skim milk (Dermatol Online J. 2006 May 30;12[4]:1).
They found positive associations with total dairy and with nonfat dairy, but not with whole-fat or low-fat dairy. “The association was driven by the nonfat dairy,” Dr. Zaenglein said. “When we took nonfat [dairy] out of the total dairy, the association there was no longer significant.” They also found no significant association with body mass index, glycemic index, or glycemic load, she added.
“You have to wonder, what could this association between dairy – and skim milk in particular – be? Could dairy actually be involved in the pathogenesis of acne?” There are a number of proposed mechanisms, none of which have ever been confirmed, she said. “Could it be related to steroids? Milk is a very bioactive substance with estrogens and other hormones, but these are fat soluble and would be removed in skim milk.”
Another theory suggests that insulinlike growth factor-1, either in milk or endogenously stimulated by its consumption, may make a contribution. “People who are passionate about this have published prolifically about the activation of this pathway,” Dr. Zaenglein said, “but it remains speculative.”
She added, there are a plethora of studies showing milk’s benefits in many other areas, including the benefits exerted by milk’s medium-chain fatty acids on cardiovascular health, glycemic control, insulin regulation, and even obesity.
Finally, dairy’s importance to bone health in the United States can’t be ignored. In fact, dairy products are the most commonly recommended foods for ensuring adequate calcium intake in children, teens, and young adults.
“It’s really hard to make a firm recommendation to eliminate dairy, because, in this country, it makes up a good portion of the calcium teens need during their bone-building years, and kids are already at high risk for not meeting these requirements.”
National nutritional guidelines recommend 1,300 mg of calcium every day, which can be accomplished in three to five servings of dairy. “An 8-ounce glass of milk has 300 mg. Yogurt, cheese, and calcium-fortified juice are all highly accepted by teens. But, to get that same amount from vegetables, for example, you’d have to eat 3 cups of cooked kale. That’s a lot of kale,” Dr. Zaenglein said.
She had no relevant financial disclosures.
[email protected]
On Twitter @alz_gal
EXPERT ANALYSIS FROM AAD 17
Of six milestones, only two left on route to hep B cure
PHILADELPHIA – The road to absolute cure of hepatitis B virus (HBV) is so well understood that the strategies now being actively pursued may conceivably eliminate this infection from the human population, according to a summary of progress presented at Digestive Diseases: New Advances.
“As in HIV and hepatitis C infections, combination therapies of drugs with different mechanisms of action will be the solution. Which combination will deliver the ultimate cure is yet to be determined, but within 5 years we should have a cure,” reported Vinod K. Rustgi, MD, chief of hepatology at Robert Wood Johnson Medical School, New Brunswick, N.J.
The biggest hurdle to cure may be clearing covalently closed circular DNA (cccDNA) from the hepatocytes of HBV-infected individuals. This structure is derived from cytoplasmic capsid–relaxed circular DNA (rcDNA) and creates a transcriptional template in the nucleus of hepatocytes. This HBV reservoir has been thus far resistant to therapy. According to Dr. Rustgi, eliminating cccDNA along with integrated HBV RNA sequences represents the final two milestones in the road to cure. There are at least theoretical approaches to eliminating both obstacles.
For many patients, available therapies already provide a functional cure of HBV, defined as viral loads below detection. Long-term data show that such patients can anticipate a lifespan equivalent to those without HBV infection, according to Dr. Rustgi, who listed this as one of the three milestones on the road to cure that have already been achieved. The first milestone was identified as a simple reduction in viral load. The second was anti-HBe seroconversion, which can be achieved in most with available treatments. Following functional cure, the fourth milestone, clearance of HBsAg antigen, is not yet reliably achieved, although this has been documented in a proportion of patients on the most effective long-term regimens.
Understanding these milestones is important, because each has prognostic relevance, according to Dr. Rustgi. Noting the direct correlation between increasing levels of serum HBV DNA and risk of hepatocellular carcinoma (HCC) over time, he indicated that reducing HBV DNA replication already reduces risk of complications. It is possible that virologic cure, which he defined as a stable off-drug suppression of HBV viremia and normalization of liver function tests, may be achieved in a larger proportion of patients with more potent versions of existing therapies. However, absolute cure, which includes complete clearance of cccDNA and other integrated HBV DNA, is the ultimate goal. It is expected that complete clearance will eliminate the threat of HCC or other liver complications.
Based on what is understood about HBV infection, increasing the potency of nucleoside analogues, which are the mainstay of current HBV therapy, will only go so far toward absolute cure. These drugs reduce viral replication to prevent liver damage. Although new drugs to prevent cccDNA formation and HBV life cycle events such as capsid assembly and HBeAg expression may further improve outcomes, they still may not be curative without better host defenses.
“One of the ways for trying to get rid of the virus is restoration of antiviral immunity, and there are several strategies to accomplish this,” reported Dr. Rustgi, citing active efforts to mobilize T cells that attack the virus and create toll-like receptor agonists, which play a key role in the innate immune response. He suggested that several strategies now demonstrating benefit in cancer, such as blocking proteins involved in the inhibition of the immune response, are being explored in the treatment of HBV. All of these strategies may be needed.
“No single approach will be sufficient to deliver a cure,” maintained Dr. Rustgi, outlining the many mechanisms HBV employs to defend against eradication. However, he provided a long list of HBV-targeted drugs and immunotherapies, including vaccines, which are now in clinical trials, enabling combinations to attack this infection from multiple fronts.
“A curative regimen might consist of an HBV antigen inhibitor, an immune activator, a cccDNA inhibitor, and a potent nucleoside analogue,” Dr. Rustgi said. Despite the multiple mechanisms that HBV now employs to evade eradication, there are now strategies being pursued to address most of the known vulnerabilities, he said. “I hope I have given you an idea where we are going.”
Digestive Diseases: New Advances was held by Rutgers, the State University of New Jersey, and Global Academy for Medical Education. Global Academy and this news organization are owned by the same company. Dr. Rustgi reported financial relationships with AbbVie, Gilead, Innovimmune, Intercept, and Merck.
PHILADELPHIA – The road to absolute cure of hepatitis B virus (HBV) is so well understood that the strategies now being actively pursued may conceivably eliminate this infection from the human population, according to a summary of progress presented at Digestive Diseases: New Advances.
“As in HIV and hepatitis C infections, combination therapies of drugs with different mechanisms of action will be the solution. Which combination will deliver the ultimate cure is yet to be determined, but within 5 years we should have a cure,” reported Vinod K. Rustgi, MD, chief of hepatology at Robert Wood Johnson Medical School, New Brunswick, N.J.
The biggest hurdle to cure may be clearing covalently closed circular DNA (cccDNA) from the hepatocytes of HBV-infected individuals. This structure is derived from cytoplasmic capsid–relaxed circular DNA (rcDNA) and creates a transcriptional template in the nucleus of hepatocytes. This HBV reservoir has been thus far resistant to therapy. According to Dr. Rustgi, eliminating cccDNA along with integrated HBV RNA sequences represents the final two milestones in the road to cure. There are at least theoretical approaches to eliminating both obstacles.
For many patients, available therapies already provide a functional cure of HBV, defined as viral loads below detection. Long-term data show that such patients can anticipate a lifespan equivalent to those without HBV infection, according to Dr. Rustgi, who listed this as one of the three milestones on the road to cure that have already been achieved. The first milestone was identified as a simple reduction in viral load. The second was anti-HBe seroconversion, which can be achieved in most with available treatments. Following functional cure, the fourth milestone, clearance of HBsAg antigen, is not yet reliably achieved, although this has been documented in a proportion of patients on the most effective long-term regimens.
Understanding these milestones is important, because each has prognostic relevance, according to Dr. Rustgi. Noting the direct correlation between increasing levels of serum HBV DNA and risk of hepatocellular carcinoma (HCC) over time, he indicated that reducing HBV DNA replication already reduces risk of complications. It is possible that virologic cure, which he defined as a stable off-drug suppression of HBV viremia and normalization of liver function tests, may be achieved in a larger proportion of patients with more potent versions of existing therapies. However, absolute cure, which includes complete clearance of cccDNA and other integrated HBV DNA, is the ultimate goal. It is expected that complete clearance will eliminate the threat of HCC or other liver complications.
Based on what is understood about HBV infection, increasing the potency of nucleoside analogues, which are the mainstay of current HBV therapy, will only go so far toward absolute cure. These drugs reduce viral replication to prevent liver damage. Although new drugs to prevent cccDNA formation and HBV life cycle events such as capsid assembly and HBeAg expression may further improve outcomes, they still may not be curative without better host defenses.
“One of the ways for trying to get rid of the virus is restoration of antiviral immunity, and there are several strategies to accomplish this,” reported Dr. Rustgi, citing active efforts to mobilize T cells that attack the virus and create toll-like receptor agonists, which play a key role in the innate immune response. He suggested that several strategies now demonstrating benefit in cancer, such as blocking proteins involved in the inhibition of the immune response, are being explored in the treatment of HBV. All of these strategies may be needed.
“No single approach will be sufficient to deliver a cure,” maintained Dr. Rustgi, outlining the many mechanisms HBV employs to defend against eradication. However, he provided a long list of HBV-targeted drugs and immunotherapies, including vaccines, which are now in clinical trials, enabling combinations to attack this infection from multiple fronts.
“A curative regimen might consist of an HBV antigen inhibitor, an immune activator, a cccDNA inhibitor, and a potent nucleoside analogue,” Dr. Rustgi said. Despite the multiple mechanisms that HBV now employs to evade eradication, there are now strategies being pursued to address most of the known vulnerabilities, he said. “I hope I have given you an idea where we are going.”
Digestive Diseases: New Advances was held by Rutgers, the State University of New Jersey, and Global Academy for Medical Education. Global Academy and this news organization are owned by the same company. Dr. Rustgi reported financial relationships with AbbVie, Gilead, Innovimmune, Intercept, and Merck.
PHILADELPHIA – The road to absolute cure of hepatitis B virus (HBV) is so well understood that the strategies now being actively pursued may conceivably eliminate this infection from the human population, according to a summary of progress presented at Digestive Diseases: New Advances.
“As in HIV and hepatitis C infections, combination therapies of drugs with different mechanisms of action will be the solution. Which combination will deliver the ultimate cure is yet to be determined, but within 5 years we should have a cure,” reported Vinod K. Rustgi, MD, chief of hepatology at Robert Wood Johnson Medical School, New Brunswick, N.J.
The biggest hurdle to cure may be clearing covalently closed circular DNA (cccDNA) from the hepatocytes of HBV-infected individuals. This structure is derived from cytoplasmic capsid–relaxed circular DNA (rcDNA) and creates a transcriptional template in the nucleus of hepatocytes. This HBV reservoir has been thus far resistant to therapy. According to Dr. Rustgi, eliminating cccDNA along with integrated HBV RNA sequences represents the final two milestones in the road to cure. There are at least theoretical approaches to eliminating both obstacles.
For many patients, available therapies already provide a functional cure of HBV, defined as viral loads below detection. Long-term data show that such patients can anticipate a lifespan equivalent to those without HBV infection, according to Dr. Rustgi, who listed this as one of the three milestones on the road to cure that have already been achieved. The first milestone was identified as a simple reduction in viral load. The second was anti-HBe seroconversion, which can be achieved in most with available treatments. Following functional cure, the fourth milestone, clearance of HBsAg antigen, is not yet reliably achieved, although this has been documented in a proportion of patients on the most effective long-term regimens.
Understanding these milestones is important, because each has prognostic relevance, according to Dr. Rustgi. Noting the direct correlation between increasing levels of serum HBV DNA and risk of hepatocellular carcinoma (HCC) over time, he indicated that reducing HBV DNA replication already reduces risk of complications. It is possible that virologic cure, which he defined as a stable off-drug suppression of HBV viremia and normalization of liver function tests, may be achieved in a larger proportion of patients with more potent versions of existing therapies. However, absolute cure, which includes complete clearance of cccDNA and other integrated HBV DNA, is the ultimate goal. It is expected that complete clearance will eliminate the threat of HCC or other liver complications.
Based on what is understood about HBV infection, increasing the potency of nucleoside analogues, which are the mainstay of current HBV therapy, will only go so far toward absolute cure. These drugs reduce viral replication to prevent liver damage. Although new drugs to prevent cccDNA formation and HBV life cycle events such as capsid assembly and HBeAg expression may further improve outcomes, they still may not be curative without better host defenses.
“One of the ways for trying to get rid of the virus is restoration of antiviral immunity, and there are several strategies to accomplish this,” reported Dr. Rustgi, citing active efforts to mobilize T cells that attack the virus and create toll-like receptor agonists, which play a key role in the innate immune response. He suggested that several strategies now demonstrating benefit in cancer, such as blocking proteins involved in the inhibition of the immune response, are being explored in the treatment of HBV. All of these strategies may be needed.
“No single approach will be sufficient to deliver a cure,” maintained Dr. Rustgi, outlining the many mechanisms HBV employs to defend against eradication. However, he provided a long list of HBV-targeted drugs and immunotherapies, including vaccines, which are now in clinical trials, enabling combinations to attack this infection from multiple fronts.
“A curative regimen might consist of an HBV antigen inhibitor, an immune activator, a cccDNA inhibitor, and a potent nucleoside analogue,” Dr. Rustgi said. Despite the multiple mechanisms that HBV now employs to evade eradication, there are now strategies being pursued to address most of the known vulnerabilities, he said. “I hope I have given you an idea where we are going.”
Digestive Diseases: New Advances was held by Rutgers, the State University of New Jersey, and Global Academy for Medical Education. Global Academy and this news organization are owned by the same company. Dr. Rustgi reported financial relationships with AbbVie, Gilead, Innovimmune, Intercept, and Merck.
EXPERT ANALYSIS FROM DIGESTIVE DISEASES: NEW ADVANCES
Robot-assisted surgery can be a pain
It’s early days for research on the physical impact of robot-assisted surgery on operators. But a study of surgeons who regularly do this kind of work suggests that surgical robots can be the cause of workplace injuries, despite their reputation for good ergonomic design and low stress on surgeon hands, wrists, backs, and necks
More than half (236) of 432 surveyed surgeons with at least 10 robotic surgeries annually reported physical discomfort associated with robotics consoles, according to a study out of Johns Hopkins University, Baltimore.
Most participants were male (71%) and averaged 48 years of age; their specialties comprised gynecology (68%), urology (20%), general surgery (8%), and others (3%).
Of the 432 participants, they reported physical discomfort in the following areas: fingers, 78%; necks, 74%; upper backs, 53%; and 43%, 34%, and 33% in the lower backs, eyes, and wrists, respectively.
Most of those who responded to the survey (80.8%) performed surgery with the da Vinci Si as their primary robotic system, with the rest using a different iteration of the da Vinci system.
Dr. Lee and his colleagues estimate the high rates of reported discomfort in fingers and necks are because of the structure of the robotics console.
“Due to the absence of tactile feedback at the master controller of the surgeon console, some robotic surgeons might close their fingers excessively when holding objects with instruments,” researchers said. “During the performance of suturing and knot-tying tasks, surgeons must squeeze their grip to hold a needle in place because there is no locking mechanism, which is present with open and laparoscopic needle holders.”
Researchers credit high rates of neck pain to the console as well, which “requires [surgeons] to maintain their neck position in a fixed place for extended period of time.”
While the rate of physical discomfort was 56%, participants rated the ergonomic functions of the console an average of 4 out of 5, with 5 being the highest score.
In contrast, surgeons gave low ratings to the communications systems used by the surgeon and the in-room supporting OR staff – an average of 2.87 out of 5 – noting an urgency for system updates.
Overall, researchers found that surgeons with high confidence in their ergonomic console settings were more likely to feel confident in the use of robotics in their surgical procedures and less likely to report physical discomfort. This finding led researchers to conclude the importance of surgeons new to robot-assisted surgery to receive education in ergonomic settings.
“Formal robotic surgery training programs should include this crucially important knowledge about optimal ergonomic guidelines so that any surgeon starting their training in robotic surgery would have the knowledge to maintain sound body posture and to minimize any physical strains while acquiring the best skill set,” according to Dr. Lee and his associates.
This study was limited by the self-reported data, which could create possible reporting bias, as well as by a small sample size. Since surgeons conducted more than one type of surgery annually, researchers found it difficult to identify what had caused the physical symptoms with complete confidence.
Researchers declared no relevant financial disclosures.
[email protected]
On Twitter @EAZTweets
It’s early days for research on the physical impact of robot-assisted surgery on operators. But a study of surgeons who regularly do this kind of work suggests that surgical robots can be the cause of workplace injuries, despite their reputation for good ergonomic design and low stress on surgeon hands, wrists, backs, and necks
More than half (236) of 432 surveyed surgeons with at least 10 robotic surgeries annually reported physical discomfort associated with robotics consoles, according to a study out of Johns Hopkins University, Baltimore.
Most participants were male (71%) and averaged 48 years of age; their specialties comprised gynecology (68%), urology (20%), general surgery (8%), and others (3%).
Of the 432 participants, they reported physical discomfort in the following areas: fingers, 78%; necks, 74%; upper backs, 53%; and 43%, 34%, and 33% in the lower backs, eyes, and wrists, respectively.
Most of those who responded to the survey (80.8%) performed surgery with the da Vinci Si as their primary robotic system, with the rest using a different iteration of the da Vinci system.
Dr. Lee and his colleagues estimate the high rates of reported discomfort in fingers and necks are because of the structure of the robotics console.
“Due to the absence of tactile feedback at the master controller of the surgeon console, some robotic surgeons might close their fingers excessively when holding objects with instruments,” researchers said. “During the performance of suturing and knot-tying tasks, surgeons must squeeze their grip to hold a needle in place because there is no locking mechanism, which is present with open and laparoscopic needle holders.”
Researchers credit high rates of neck pain to the console as well, which “requires [surgeons] to maintain their neck position in a fixed place for extended period of time.”
While the rate of physical discomfort was 56%, participants rated the ergonomic functions of the console an average of 4 out of 5, with 5 being the highest score.
In contrast, surgeons gave low ratings to the communications systems used by the surgeon and the in-room supporting OR staff – an average of 2.87 out of 5 – noting an urgency for system updates.
Overall, researchers found that surgeons with high confidence in their ergonomic console settings were more likely to feel confident in the use of robotics in their surgical procedures and less likely to report physical discomfort. This finding led researchers to conclude the importance of surgeons new to robot-assisted surgery to receive education in ergonomic settings.
“Formal robotic surgery training programs should include this crucially important knowledge about optimal ergonomic guidelines so that any surgeon starting their training in robotic surgery would have the knowledge to maintain sound body posture and to minimize any physical strains while acquiring the best skill set,” according to Dr. Lee and his associates.
This study was limited by the self-reported data, which could create possible reporting bias, as well as by a small sample size. Since surgeons conducted more than one type of surgery annually, researchers found it difficult to identify what had caused the physical symptoms with complete confidence.
Researchers declared no relevant financial disclosures.
[email protected]
On Twitter @EAZTweets
It’s early days for research on the physical impact of robot-assisted surgery on operators. But a study of surgeons who regularly do this kind of work suggests that surgical robots can be the cause of workplace injuries, despite their reputation for good ergonomic design and low stress on surgeon hands, wrists, backs, and necks
More than half (236) of 432 surveyed surgeons with at least 10 robotic surgeries annually reported physical discomfort associated with robotics consoles, according to a study out of Johns Hopkins University, Baltimore.
Most participants were male (71%) and averaged 48 years of age; their specialties comprised gynecology (68%), urology (20%), general surgery (8%), and others (3%).
Of the 432 participants, they reported physical discomfort in the following areas: fingers, 78%; necks, 74%; upper backs, 53%; and 43%, 34%, and 33% in the lower backs, eyes, and wrists, respectively.
Most of those who responded to the survey (80.8%) performed surgery with the da Vinci Si as their primary robotic system, with the rest using a different iteration of the da Vinci system.
Dr. Lee and his colleagues estimate the high rates of reported discomfort in fingers and necks are because of the structure of the robotics console.
“Due to the absence of tactile feedback at the master controller of the surgeon console, some robotic surgeons might close their fingers excessively when holding objects with instruments,” researchers said. “During the performance of suturing and knot-tying tasks, surgeons must squeeze their grip to hold a needle in place because there is no locking mechanism, which is present with open and laparoscopic needle holders.”
Researchers credit high rates of neck pain to the console as well, which “requires [surgeons] to maintain their neck position in a fixed place for extended period of time.”
While the rate of physical discomfort was 56%, participants rated the ergonomic functions of the console an average of 4 out of 5, with 5 being the highest score.
In contrast, surgeons gave low ratings to the communications systems used by the surgeon and the in-room supporting OR staff – an average of 2.87 out of 5 – noting an urgency for system updates.
Overall, researchers found that surgeons with high confidence in their ergonomic console settings were more likely to feel confident in the use of robotics in their surgical procedures and less likely to report physical discomfort. This finding led researchers to conclude the importance of surgeons new to robot-assisted surgery to receive education in ergonomic settings.
“Formal robotic surgery training programs should include this crucially important knowledge about optimal ergonomic guidelines so that any surgeon starting their training in robotic surgery would have the knowledge to maintain sound body posture and to minimize any physical strains while acquiring the best skill set,” according to Dr. Lee and his associates.
This study was limited by the self-reported data, which could create possible reporting bias, as well as by a small sample size. Since surgeons conducted more than one type of surgery annually, researchers found it difficult to identify what had caused the physical symptoms with complete confidence.
Researchers declared no relevant financial disclosures.
[email protected]
On Twitter @EAZTweets
Key clinical point:
Major finding: Of 432 participating surgeons, 236 (56.1%) reported having physical discomfort during or after using the surgical robot.
Data source: A 20-question, self-reporting survey disseminated to surgeons via email, analyzed using logistic regression.
Disclosures: Researchers declared no relevant financial disclosures.
VIDEO: How to start an oncology sexual health clinic
NATIONAL HARBOR, MD – Start small, but anticipate growth. Engage your administration from the start. Be smart about resources, and consider using advanced practice providers to keep costs down. Above all, keep lines of communication open with physicians and other members of the care team.
In a video interview, Joanne Rash, PA-C, a certified physician assistant at the University of Wisconsin–Madison offers these and other tips. She explains her collaborative work with David Kushner, MD, director of the gynecologic oncology program and professor at the University of Wisconsin School of Medicine and Public Health, and a colleague to develop the Women’s Integrative Sexual Health (WISH) program.
WISH is modeled on the University of Chicago’s Program in Integrative Sex and Medicine for Women and Girls with Cancer (PRISM) and participates in the PRISM registry, which studies ways to prevent and treat sexual problems for women and girls with cancer.
“I think what makes the WISH program unique is that we carve time out,” said Ms. Rash. “Of course, we address some of these issues in my gynecologic oncology practice, but, when we do it in WISH, the format is different,” and there’s just more time for discussion.
Communication is key to the model’s success in safe integration of sexual health into cancer care, she said. “We certainly don’t want to do something that compromises cancer care, and so, it’s important that we have those conversations with that woman’s team. And now we get to be a part of that team, which is a real privilege.”
Ms. Rash reported no conflicts of interest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
NATIONAL HARBOR, MD – Start small, but anticipate growth. Engage your administration from the start. Be smart about resources, and consider using advanced practice providers to keep costs down. Above all, keep lines of communication open with physicians and other members of the care team.
In a video interview, Joanne Rash, PA-C, a certified physician assistant at the University of Wisconsin–Madison offers these and other tips. She explains her collaborative work with David Kushner, MD, director of the gynecologic oncology program and professor at the University of Wisconsin School of Medicine and Public Health, and a colleague to develop the Women’s Integrative Sexual Health (WISH) program.
WISH is modeled on the University of Chicago’s Program in Integrative Sex and Medicine for Women and Girls with Cancer (PRISM) and participates in the PRISM registry, which studies ways to prevent and treat sexual problems for women and girls with cancer.
“I think what makes the WISH program unique is that we carve time out,” said Ms. Rash. “Of course, we address some of these issues in my gynecologic oncology practice, but, when we do it in WISH, the format is different,” and there’s just more time for discussion.
Communication is key to the model’s success in safe integration of sexual health into cancer care, she said. “We certainly don’t want to do something that compromises cancer care, and so, it’s important that we have those conversations with that woman’s team. And now we get to be a part of that team, which is a real privilege.”
Ms. Rash reported no conflicts of interest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
NATIONAL HARBOR, MD – Start small, but anticipate growth. Engage your administration from the start. Be smart about resources, and consider using advanced practice providers to keep costs down. Above all, keep lines of communication open with physicians and other members of the care team.
In a video interview, Joanne Rash, PA-C, a certified physician assistant at the University of Wisconsin–Madison offers these and other tips. She explains her collaborative work with David Kushner, MD, director of the gynecologic oncology program and professor at the University of Wisconsin School of Medicine and Public Health, and a colleague to develop the Women’s Integrative Sexual Health (WISH) program.
WISH is modeled on the University of Chicago’s Program in Integrative Sex and Medicine for Women and Girls with Cancer (PRISM) and participates in the PRISM registry, which studies ways to prevent and treat sexual problems for women and girls with cancer.
“I think what makes the WISH program unique is that we carve time out,” said Ms. Rash. “Of course, we address some of these issues in my gynecologic oncology practice, but, when we do it in WISH, the format is different,” and there’s just more time for discussion.
Communication is key to the model’s success in safe integration of sexual health into cancer care, she said. “We certainly don’t want to do something that compromises cancer care, and so, it’s important that we have those conversations with that woman’s team. And now we get to be a part of that team, which is a real privilege.”
Ms. Rash reported no conflicts of interest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Do you attend a patient’s funeral?
I’ve never been to a patient’s funeral, though I know plenty of other doctors who have.
I suppose this is a highly personal decision. Some feel they should go out of respect to the patient, or if they had a particularly strong or longstanding relationship with them.
Part of it is feeling like an outsider. To me, funerals are a chance for loved ones and close friends to say their goodbyes. I generally try to keep a professional distance. It makes the job easier.
Another is simply a reluctance to take time off from the office. Even though someone I cared for is gone, that person is not the only one that I see. I have to continue caring for the patients who still need me.
There’s also an aspect of fear. Family members who don’t know you well may see your presence as a sign of guilt that you did something wrong. Or, in the irrational nature of grief and anger, become belligerent, accusing you of incompetence. These sorts of confrontations can never end well for either side.
All of us are facing death sooner or later. As physicians, our job is to prolong and improve quality of life as best we can, knowing that inevitably we’ll lose. When that happens, the most we can ever ask is that we did our best. And that we continue to care for those who still depend on us.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
[polldaddy:9711658]
I’ve never been to a patient’s funeral, though I know plenty of other doctors who have.
I suppose this is a highly personal decision. Some feel they should go out of respect to the patient, or if they had a particularly strong or longstanding relationship with them.
Part of it is feeling like an outsider. To me, funerals are a chance for loved ones and close friends to say their goodbyes. I generally try to keep a professional distance. It makes the job easier.
Another is simply a reluctance to take time off from the office. Even though someone I cared for is gone, that person is not the only one that I see. I have to continue caring for the patients who still need me.
There’s also an aspect of fear. Family members who don’t know you well may see your presence as a sign of guilt that you did something wrong. Or, in the irrational nature of grief and anger, become belligerent, accusing you of incompetence. These sorts of confrontations can never end well for either side.
All of us are facing death sooner or later. As physicians, our job is to prolong and improve quality of life as best we can, knowing that inevitably we’ll lose. When that happens, the most we can ever ask is that we did our best. And that we continue to care for those who still depend on us.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
[polldaddy:9711658]
I’ve never been to a patient’s funeral, though I know plenty of other doctors who have.
I suppose this is a highly personal decision. Some feel they should go out of respect to the patient, or if they had a particularly strong or longstanding relationship with them.
Part of it is feeling like an outsider. To me, funerals are a chance for loved ones and close friends to say their goodbyes. I generally try to keep a professional distance. It makes the job easier.
Another is simply a reluctance to take time off from the office. Even though someone I cared for is gone, that person is not the only one that I see. I have to continue caring for the patients who still need me.
There’s also an aspect of fear. Family members who don’t know you well may see your presence as a sign of guilt that you did something wrong. Or, in the irrational nature of grief and anger, become belligerent, accusing you of incompetence. These sorts of confrontations can never end well for either side.
All of us are facing death sooner or later. As physicians, our job is to prolong and improve quality of life as best we can, knowing that inevitably we’ll lose. When that happens, the most we can ever ask is that we did our best. And that we continue to care for those who still depend on us.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
[polldaddy:9711658]
Novel protein biomarker could accelerate ALS research
A protein found in the cerebrospinal fluid and peripheral blood cells of patients with a common type of amyotrophic lateral sclerosis may serve as a “pharmacodynamic marker,” providing a mechanism to assess RNA-based therapies that are now in clinical trials.
The protein, poly(GP), is expressed in patients who have a mutation in the gene chromosome 9 open reading frame 72 (C9ORF72), which causes one type of amyotrophic lateral sclerosis (ALS). This means that detecting poly(GP) also, eventually, may help to identify “presymptomatic individuals who are expected to benefit from early therapeutic interventions,” wrote the authors of a newly published paper (Sci Transl Med. 2017;9[383]:eaai7866).
Tania Gendron, PhD, of the department of neuroscience at the Mayo Clinic, Jacksonville, Fla., and her colleagues noted that poly(GP) was found in the cerebrospinal fluid (CSF) of both symptomatic and asymptomatic patients who carried the C9ORF72 mutation. The mutation can cause ALS, and is also associated with frontotemporal dementia (FTD) in a pattern with incomplete overlap with ALS.
However, “A limitation in moving such treatments from bench to bedside is a lack of pharmacodynamics markers for use in clinical trials,” Dr. Gendron and her collaborators wrote. The discovery that poly(GP) tracks well with C9ORF72 means that it has the potential to serve as the kind of biomarker that’s been missing in drug development for this family of neurodegenerative diseases, they said.
“To prepare for upcoming clinical trials for c9ALS, the present study used patient CSF and several preclinical models to investigate the hypothesis that poly(GP) proteins could serve as an urgently needed pharmacodynamics marker for developing and testing therapies for treating c9ALS,” Dr. Gendron and her colleagues wrote.
They looked at CSF samples from 83 patients with c9ALS and 27 patients who were asymptomatic C9ORF72 repeat expansion carriers, as well as 24 carriers who had a neurologic disease besides ALS or FTD (total n = 134). They also examined CSF samples from 120 study participants who lacked the mutation, 48 of whom were healthy controls; the remainder had ALS (n = 57) or another neurological disease.
The investigators, who were blinded to individuals’ disease status in each study arm, found that CSF poly(GP) levels were significantly higher in patients who had the C9ORF72 mutation (P less than .0001 in unadjusted and adjusted analyses). Poly(GP) was present in both asymptomatic and symptomatic carriers of the mutation, and not significantly different between these groups when data were adjusted for multiple comparisons, age, and gender.
When Dr. Gendron and her colleagues looked at poly(GP) levels over time for patients whose longitudinal data were available, they found that levels for an individual study participant were “relatively constant,” without any significant change over the median 12.9 months that these levels were tracked (P = .84).
However, “poly(GP) is not a prognostic marker,” wrote Dr. Gendron and her colleagues. They found no consistent association between levels of the protein and disease severity of progression, age at onset, or the development of FTD. Women were more likely to have lower levels, but the significance of that finding is not known, they said. There was a trend, which lost significance after statistical adjustment, for patients with cognitive impairment to have higher poly(GP) levels (adjusted P = .12).
Treatments under investigation for ALS and FTD include the use of an antisense oligonucleotide (ASO) to bind to the repeated RNA sequences and negate their ill effects. The investigators wrote that in vitro investigations using patient-derived cell models showed that poly(GP) levels dropped when cells were exposed to an ASO for 10 days. “The data indicate that poly(GP) production mirrors expression of repeat-containing C9ORF72 transcripts in lymphoblastoid cell lines,” they wrote.
The authors reported multiple governmental and private foundation sources of support for the research. Dr. Gendron and several of her coauthors are investigators in clinical trials for an ASO to target C9ORF72. Several authors reported paid and unpaid relationships and stock positions with pharmaceutical companies, including ones developing treatments for ALS and FTD.
[email protected]
On Twitter @karioakes
A protein found in the cerebrospinal fluid and peripheral blood cells of patients with a common type of amyotrophic lateral sclerosis may serve as a “pharmacodynamic marker,” providing a mechanism to assess RNA-based therapies that are now in clinical trials.
The protein, poly(GP), is expressed in patients who have a mutation in the gene chromosome 9 open reading frame 72 (C9ORF72), which causes one type of amyotrophic lateral sclerosis (ALS). This means that detecting poly(GP) also, eventually, may help to identify “presymptomatic individuals who are expected to benefit from early therapeutic interventions,” wrote the authors of a newly published paper (Sci Transl Med. 2017;9[383]:eaai7866).
Tania Gendron, PhD, of the department of neuroscience at the Mayo Clinic, Jacksonville, Fla., and her colleagues noted that poly(GP) was found in the cerebrospinal fluid (CSF) of both symptomatic and asymptomatic patients who carried the C9ORF72 mutation. The mutation can cause ALS, and is also associated with frontotemporal dementia (FTD) in a pattern with incomplete overlap with ALS.
However, “A limitation in moving such treatments from bench to bedside is a lack of pharmacodynamics markers for use in clinical trials,” Dr. Gendron and her collaborators wrote. The discovery that poly(GP) tracks well with C9ORF72 means that it has the potential to serve as the kind of biomarker that’s been missing in drug development for this family of neurodegenerative diseases, they said.
“To prepare for upcoming clinical trials for c9ALS, the present study used patient CSF and several preclinical models to investigate the hypothesis that poly(GP) proteins could serve as an urgently needed pharmacodynamics marker for developing and testing therapies for treating c9ALS,” Dr. Gendron and her colleagues wrote.
They looked at CSF samples from 83 patients with c9ALS and 27 patients who were asymptomatic C9ORF72 repeat expansion carriers, as well as 24 carriers who had a neurologic disease besides ALS or FTD (total n = 134). They also examined CSF samples from 120 study participants who lacked the mutation, 48 of whom were healthy controls; the remainder had ALS (n = 57) or another neurological disease.
The investigators, who were blinded to individuals’ disease status in each study arm, found that CSF poly(GP) levels were significantly higher in patients who had the C9ORF72 mutation (P less than .0001 in unadjusted and adjusted analyses). Poly(GP) was present in both asymptomatic and symptomatic carriers of the mutation, and not significantly different between these groups when data were adjusted for multiple comparisons, age, and gender.
When Dr. Gendron and her colleagues looked at poly(GP) levels over time for patients whose longitudinal data were available, they found that levels for an individual study participant were “relatively constant,” without any significant change over the median 12.9 months that these levels were tracked (P = .84).
However, “poly(GP) is not a prognostic marker,” wrote Dr. Gendron and her colleagues. They found no consistent association between levels of the protein and disease severity of progression, age at onset, or the development of FTD. Women were more likely to have lower levels, but the significance of that finding is not known, they said. There was a trend, which lost significance after statistical adjustment, for patients with cognitive impairment to have higher poly(GP) levels (adjusted P = .12).
Treatments under investigation for ALS and FTD include the use of an antisense oligonucleotide (ASO) to bind to the repeated RNA sequences and negate their ill effects. The investigators wrote that in vitro investigations using patient-derived cell models showed that poly(GP) levels dropped when cells were exposed to an ASO for 10 days. “The data indicate that poly(GP) production mirrors expression of repeat-containing C9ORF72 transcripts in lymphoblastoid cell lines,” they wrote.
The authors reported multiple governmental and private foundation sources of support for the research. Dr. Gendron and several of her coauthors are investigators in clinical trials for an ASO to target C9ORF72. Several authors reported paid and unpaid relationships and stock positions with pharmaceutical companies, including ones developing treatments for ALS and FTD.
[email protected]
On Twitter @karioakes
A protein found in the cerebrospinal fluid and peripheral blood cells of patients with a common type of amyotrophic lateral sclerosis may serve as a “pharmacodynamic marker,” providing a mechanism to assess RNA-based therapies that are now in clinical trials.
The protein, poly(GP), is expressed in patients who have a mutation in the gene chromosome 9 open reading frame 72 (C9ORF72), which causes one type of amyotrophic lateral sclerosis (ALS). This means that detecting poly(GP) also, eventually, may help to identify “presymptomatic individuals who are expected to benefit from early therapeutic interventions,” wrote the authors of a newly published paper (Sci Transl Med. 2017;9[383]:eaai7866).
Tania Gendron, PhD, of the department of neuroscience at the Mayo Clinic, Jacksonville, Fla., and her colleagues noted that poly(GP) was found in the cerebrospinal fluid (CSF) of both symptomatic and asymptomatic patients who carried the C9ORF72 mutation. The mutation can cause ALS, and is also associated with frontotemporal dementia (FTD) in a pattern with incomplete overlap with ALS.
However, “A limitation in moving such treatments from bench to bedside is a lack of pharmacodynamics markers for use in clinical trials,” Dr. Gendron and her collaborators wrote. The discovery that poly(GP) tracks well with C9ORF72 means that it has the potential to serve as the kind of biomarker that’s been missing in drug development for this family of neurodegenerative diseases, they said.
“To prepare for upcoming clinical trials for c9ALS, the present study used patient CSF and several preclinical models to investigate the hypothesis that poly(GP) proteins could serve as an urgently needed pharmacodynamics marker for developing and testing therapies for treating c9ALS,” Dr. Gendron and her colleagues wrote.
They looked at CSF samples from 83 patients with c9ALS and 27 patients who were asymptomatic C9ORF72 repeat expansion carriers, as well as 24 carriers who had a neurologic disease besides ALS or FTD (total n = 134). They also examined CSF samples from 120 study participants who lacked the mutation, 48 of whom were healthy controls; the remainder had ALS (n = 57) or another neurological disease.
The investigators, who were blinded to individuals’ disease status in each study arm, found that CSF poly(GP) levels were significantly higher in patients who had the C9ORF72 mutation (P less than .0001 in unadjusted and adjusted analyses). Poly(GP) was present in both asymptomatic and symptomatic carriers of the mutation, and not significantly different between these groups when data were adjusted for multiple comparisons, age, and gender.
When Dr. Gendron and her colleagues looked at poly(GP) levels over time for patients whose longitudinal data were available, they found that levels for an individual study participant were “relatively constant,” without any significant change over the median 12.9 months that these levels were tracked (P = .84).
However, “poly(GP) is not a prognostic marker,” wrote Dr. Gendron and her colleagues. They found no consistent association between levels of the protein and disease severity of progression, age at onset, or the development of FTD. Women were more likely to have lower levels, but the significance of that finding is not known, they said. There was a trend, which lost significance after statistical adjustment, for patients with cognitive impairment to have higher poly(GP) levels (adjusted P = .12).
Treatments under investigation for ALS and FTD include the use of an antisense oligonucleotide (ASO) to bind to the repeated RNA sequences and negate their ill effects. The investigators wrote that in vitro investigations using patient-derived cell models showed that poly(GP) levels dropped when cells were exposed to an ASO for 10 days. “The data indicate that poly(GP) production mirrors expression of repeat-containing C9ORF72 transcripts in lymphoblastoid cell lines,” they wrote.
The authors reported multiple governmental and private foundation sources of support for the research. Dr. Gendron and several of her coauthors are investigators in clinical trials for an ASO to target C9ORF72. Several authors reported paid and unpaid relationships and stock positions with pharmaceutical companies, including ones developing treatments for ALS and FTD.
[email protected]
On Twitter @karioakes
Key clinical point:
Major finding: Patients with a mutation predisposing them to ALS had significantly higher levels of the protein poly(GP) (P less than .0001 in unadjusted and adjusted analyses).
Data source: Prospective blinded study of CSF samples from 83 patients with c9ALS, 27 patients who were asymptomatic C9ORF72 repeat expansion carriers, 24 carriers who had a neurologic disease besides ALS or FTD, and 120 study participants who lacked the C9ORF72 mutation.
Disclosures: The authors reported multiple governmental and private foundation sources of support for their research. Dr. Gendron and several of her coauthors are investigators in clinical trials for an ASO to target C9ORF72. Several authors reported paid and unpaid relationships and stock positions with pharmaceutical companies, including ones developing treatments for ALS and FTD.
Safe to avoid sentinel node biopsy in some breast cancer patients
SEATTLE – Sentinel lymph node biopsy is widely used in patients with early-stage breast cancer for staging the axilla, but it can be safely omitted in some patients, according to new research presented at the annual Society of Surgical Oncology Cancer Symposium.
In women aged 70 years and older with hormone receptor (HR)–positive invasive breast cancer, the risk of nodal involvement is 14%-15%, which adds support to the premise that sentinel lymph node surgery could be avoided in many of the women deemed to be low risk.
The Choosing Wisely campaign was initiated to reduce excess cost and expenditures in health care. The Society of Surgical Oncology recently released five Choosing Wisely guidelines that included specific tests or procedures commonly ordered but not always necessary in surgical oncology, explained study author Jessemae Welsh, MD, of the Mayo Clinic, Rochester, Minn. One of the recommendations was to avoid routine sentinel node biopsy in clinically node-negative women over age 70 years with hormone receptor–positive invasive breast cancer.
“Their rationale is that hormone therapy is the standard of care in these women and sentinel node surgery has shown no impact on local regional recurrence or breast cancer mortality,” said Dr. Welsh. “Therefore it would be safe to treat this population without any axillary node staging.”
She noted that the average 70-year-old woman may live another 14-16 years. “So the question is, how should we be applying the Choosing Wisely guidelines?”
Dr. Welsh and her colleagues evaluated the factors that might be impacting nodal positivity in this population, and in particular, they looked at T stage and tumor grade.
They used two large databases to identify all women over the age of 70 years with HR+ cN0 invasive disease in the institutional breast surgery database (IBSD, 2008-2016) from the Mayo Clinic and the National Cancer Database (NCDB, 2004-2013).
The rates of patients who were node positive (pN+) were based on those who had undergone axillary surgery.
The researchers then stratified patients by clinical T stage and tumor grade to compare risk of pN+ across strata.
Of 705 selected patients in the IBSD, 191 or 14.3% were pN+ and a similar rate was observed in the NCDB; 15.2% (19,607/129,216). Tumor grade and clinical T stage were associated with pN+.
“The overall rates were about 14% for both databases, and when we stratified this by T stage, we could see increasing node positivity with increasing T stage,” said Dr. Welsh.
In similar fashion, the researchers observed comparable increases when they stratified it by grade. “Increasing grades were associated with increasing rates, especially for grade 2 and higher,” said Dr. Welsh.
When the two factors were combined, the researchers were able to define low-risk criteria as clinical T1a-b, grade 1-2 or clinical T1c, grade 1. The low-risk group accounted for 54.3% (IBSD) and 43.2% (NCDB) of patients, and pN+ rates within this group were 7.6% (IBSD) and 7.4% (NCDB).
Patients outside of this subcohort had pN+ rates of 22.4% (IBSD) and 23.0% (NCDB), which extrapolated to a relative risk of 2.95 (95% CI: 1.97-4.42) and 3.11 (95% CI: 2.99-3.23), respectively (each P less than .001).
“Women in the high-risk group had three times the risk of node positivity as the low-risk group,” she said. “Based on our data, we can say that for grade 1 T1a-c we can omit sentinel node surgery, and also for grade 2 T1 a-b.”
But for grade 3, T2 or higher, or any grade 2 Tc tumors, clinicians should continue to consider sentinel node surgery, taking into account individual patient factors.
The investigator had no disclosures.
Are there patients older than 70 years of age who have a low risk of nodal metastasis and/or even if they had nodal metastasis, could be adequately treated with anti-hormones?
If the answer is “yes,” then sentinel node sampling can be avoided in these patients. This study identified a group with a low risk for nodal metastasis. Even though it may be difficult to estimate tumor size preoperatively for lobular cancers, this is less of a problem for ductal cancers. Another approach is to use molecular profiling to determine which patients may “skip” sentinel node biopsy. Molecular profiling can identify patients who will have an excellent outcome with adjuvant anti-hormones even in the presence of nodal metastases.
Maureen Chung, MD, FACS, is medical director of the breast care program at Southcoast Health, North Dartmouth, Mass.
Are there patients older than 70 years of age who have a low risk of nodal metastasis and/or even if they had nodal metastasis, could be adequately treated with anti-hormones?
If the answer is “yes,” then sentinel node sampling can be avoided in these patients. This study identified a group with a low risk for nodal metastasis. Even though it may be difficult to estimate tumor size preoperatively for lobular cancers, this is less of a problem for ductal cancers. Another approach is to use molecular profiling to determine which patients may “skip” sentinel node biopsy. Molecular profiling can identify patients who will have an excellent outcome with adjuvant anti-hormones even in the presence of nodal metastases.
Maureen Chung, MD, FACS, is medical director of the breast care program at Southcoast Health, North Dartmouth, Mass.
Are there patients older than 70 years of age who have a low risk of nodal metastasis and/or even if they had nodal metastasis, could be adequately treated with anti-hormones?
If the answer is “yes,” then sentinel node sampling can be avoided in these patients. This study identified a group with a low risk for nodal metastasis. Even though it may be difficult to estimate tumor size preoperatively for lobular cancers, this is less of a problem for ductal cancers. Another approach is to use molecular profiling to determine which patients may “skip” sentinel node biopsy. Molecular profiling can identify patients who will have an excellent outcome with adjuvant anti-hormones even in the presence of nodal metastases.
Maureen Chung, MD, FACS, is medical director of the breast care program at Southcoast Health, North Dartmouth, Mass.
SEATTLE – Sentinel lymph node biopsy is widely used in patients with early-stage breast cancer for staging the axilla, but it can be safely omitted in some patients, according to new research presented at the annual Society of Surgical Oncology Cancer Symposium.
In women aged 70 years and older with hormone receptor (HR)–positive invasive breast cancer, the risk of nodal involvement is 14%-15%, which adds support to the premise that sentinel lymph node surgery could be avoided in many of the women deemed to be low risk.
The Choosing Wisely campaign was initiated to reduce excess cost and expenditures in health care. The Society of Surgical Oncology recently released five Choosing Wisely guidelines that included specific tests or procedures commonly ordered but not always necessary in surgical oncology, explained study author Jessemae Welsh, MD, of the Mayo Clinic, Rochester, Minn. One of the recommendations was to avoid routine sentinel node biopsy in clinically node-negative women over age 70 years with hormone receptor–positive invasive breast cancer.
“Their rationale is that hormone therapy is the standard of care in these women and sentinel node surgery has shown no impact on local regional recurrence or breast cancer mortality,” said Dr. Welsh. “Therefore it would be safe to treat this population without any axillary node staging.”
She noted that the average 70-year-old woman may live another 14-16 years. “So the question is, how should we be applying the Choosing Wisely guidelines?”
Dr. Welsh and her colleagues evaluated the factors that might be impacting nodal positivity in this population, and in particular, they looked at T stage and tumor grade.
They used two large databases to identify all women over the age of 70 years with HR+ cN0 invasive disease in the institutional breast surgery database (IBSD, 2008-2016) from the Mayo Clinic and the National Cancer Database (NCDB, 2004-2013).
The rates of patients who were node positive (pN+) were based on those who had undergone axillary surgery.
The researchers then stratified patients by clinical T stage and tumor grade to compare risk of pN+ across strata.
Of 705 selected patients in the IBSD, 191 or 14.3% were pN+ and a similar rate was observed in the NCDB; 15.2% (19,607/129,216). Tumor grade and clinical T stage were associated with pN+.
“The overall rates were about 14% for both databases, and when we stratified this by T stage, we could see increasing node positivity with increasing T stage,” said Dr. Welsh.
In similar fashion, the researchers observed comparable increases when they stratified it by grade. “Increasing grades were associated with increasing rates, especially for grade 2 and higher,” said Dr. Welsh.
When the two factors were combined, the researchers were able to define low-risk criteria as clinical T1a-b, grade 1-2 or clinical T1c, grade 1. The low-risk group accounted for 54.3% (IBSD) and 43.2% (NCDB) of patients, and pN+ rates within this group were 7.6% (IBSD) and 7.4% (NCDB).
Patients outside of this subcohort had pN+ rates of 22.4% (IBSD) and 23.0% (NCDB), which extrapolated to a relative risk of 2.95 (95% CI: 1.97-4.42) and 3.11 (95% CI: 2.99-3.23), respectively (each P less than .001).
“Women in the high-risk group had three times the risk of node positivity as the low-risk group,” she said. “Based on our data, we can say that for grade 1 T1a-c we can omit sentinel node surgery, and also for grade 2 T1 a-b.”
But for grade 3, T2 or higher, or any grade 2 Tc tumors, clinicians should continue to consider sentinel node surgery, taking into account individual patient factors.
The investigator had no disclosures.
SEATTLE – Sentinel lymph node biopsy is widely used in patients with early-stage breast cancer for staging the axilla, but it can be safely omitted in some patients, according to new research presented at the annual Society of Surgical Oncology Cancer Symposium.
In women aged 70 years and older with hormone receptor (HR)–positive invasive breast cancer, the risk of nodal involvement is 14%-15%, which adds support to the premise that sentinel lymph node surgery could be avoided in many of the women deemed to be low risk.
The Choosing Wisely campaign was initiated to reduce excess cost and expenditures in health care. The Society of Surgical Oncology recently released five Choosing Wisely guidelines that included specific tests or procedures commonly ordered but not always necessary in surgical oncology, explained study author Jessemae Welsh, MD, of the Mayo Clinic, Rochester, Minn. One of the recommendations was to avoid routine sentinel node biopsy in clinically node-negative women over age 70 years with hormone receptor–positive invasive breast cancer.
“Their rationale is that hormone therapy is the standard of care in these women and sentinel node surgery has shown no impact on local regional recurrence or breast cancer mortality,” said Dr. Welsh. “Therefore it would be safe to treat this population without any axillary node staging.”
She noted that the average 70-year-old woman may live another 14-16 years. “So the question is, how should we be applying the Choosing Wisely guidelines?”
Dr. Welsh and her colleagues evaluated the factors that might be impacting nodal positivity in this population, and in particular, they looked at T stage and tumor grade.
They used two large databases to identify all women over the age of 70 years with HR+ cN0 invasive disease in the institutional breast surgery database (IBSD, 2008-2016) from the Mayo Clinic and the National Cancer Database (NCDB, 2004-2013).
The rates of patients who were node positive (pN+) were based on those who had undergone axillary surgery.
The researchers then stratified patients by clinical T stage and tumor grade to compare risk of pN+ across strata.
Of 705 selected patients in the IBSD, 191 or 14.3% were pN+ and a similar rate was observed in the NCDB; 15.2% (19,607/129,216). Tumor grade and clinical T stage were associated with pN+.
“The overall rates were about 14% for both databases, and when we stratified this by T stage, we could see increasing node positivity with increasing T stage,” said Dr. Welsh.
In similar fashion, the researchers observed comparable increases when they stratified it by grade. “Increasing grades were associated with increasing rates, especially for grade 2 and higher,” said Dr. Welsh.
When the two factors were combined, the researchers were able to define low-risk criteria as clinical T1a-b, grade 1-2 or clinical T1c, grade 1. The low-risk group accounted for 54.3% (IBSD) and 43.2% (NCDB) of patients, and pN+ rates within this group were 7.6% (IBSD) and 7.4% (NCDB).
Patients outside of this subcohort had pN+ rates of 22.4% (IBSD) and 23.0% (NCDB), which extrapolated to a relative risk of 2.95 (95% CI: 1.97-4.42) and 3.11 (95% CI: 2.99-3.23), respectively (each P less than .001).
“Women in the high-risk group had three times the risk of node positivity as the low-risk group,” she said. “Based on our data, we can say that for grade 1 T1a-c we can omit sentinel node surgery, and also for grade 2 T1 a-b.”
But for grade 3, T2 or higher, or any grade 2 Tc tumors, clinicians should continue to consider sentinel node surgery, taking into account individual patient factors.
The investigator had no disclosures.
Key clinical point: Sentinel node biopsy can be safely avoided in certain populations of breast cancer patients.
Major finding: In women 70 years and older with hormone receptor (HR)–positive invasive breast cancer who are at low risk, sentinel node surgery can safely be avoided.
Data source: Two large databases of more than 150,000 women, from the Mayo Clinic and the National Cancer Database.
Disclosures: There was no funding source disclosed. The author had no disclosures.
VIDEO: Sex, intimacy part of history in gynecologic oncology care
NATIONAL HARBOR, MD. – Cancer care can feel like a sequence of crises, large and small; a comprehensive cancer care approach can keep the patient at the center of this flurry of events. But understanding a patient’s sexual history and assessing how a gynecologic malignancy has affected that patient’s sexual health can, too often, get lost in the shuffle, said Don Dizon, MD, of Massachusetts General Hospital, Boston.
“I think there are multiple reasons for that, but it has to be the job of the clinician – not necessarily the oncologist-physician – but somebody has to be able to say, ‘What is your sexual history? Can I ask about your sexual history?’ ” he said in a video interview at the annual meeting of the Society of Gynecologic Oncology.
Dr. Dizon, director of the Oncology Sexual Health Clinic at Massachusetts General Hospital*, emphasized the importance of providing sensitive sexual health care while staying true to one’s own framework of care. “I teach clinicians not to run away from their schema,” he said. “Having said that, the job of a clinician is to normalize the history, and to normalize that sex, intimacy, relationships are a part of someone’s history.”
Dr. Dizon sits on the board of the Patty Brisben Foundation and the Young Survival Coalition.
[email protected]
On Twitter @karioakes
*A previous version of this article misstated Dr. Dizon's affiliation.
NATIONAL HARBOR, MD. – Cancer care can feel like a sequence of crises, large and small; a comprehensive cancer care approach can keep the patient at the center of this flurry of events. But understanding a patient’s sexual history and assessing how a gynecologic malignancy has affected that patient’s sexual health can, too often, get lost in the shuffle, said Don Dizon, MD, of Massachusetts General Hospital, Boston.
“I think there are multiple reasons for that, but it has to be the job of the clinician – not necessarily the oncologist-physician – but somebody has to be able to say, ‘What is your sexual history? Can I ask about your sexual history?’ ” he said in a video interview at the annual meeting of the Society of Gynecologic Oncology.
Dr. Dizon, director of the Oncology Sexual Health Clinic at Massachusetts General Hospital*, emphasized the importance of providing sensitive sexual health care while staying true to one’s own framework of care. “I teach clinicians not to run away from their schema,” he said. “Having said that, the job of a clinician is to normalize the history, and to normalize that sex, intimacy, relationships are a part of someone’s history.”
Dr. Dizon sits on the board of the Patty Brisben Foundation and the Young Survival Coalition.
[email protected]
On Twitter @karioakes
*A previous version of this article misstated Dr. Dizon's affiliation.
NATIONAL HARBOR, MD. – Cancer care can feel like a sequence of crises, large and small; a comprehensive cancer care approach can keep the patient at the center of this flurry of events. But understanding a patient’s sexual history and assessing how a gynecologic malignancy has affected that patient’s sexual health can, too often, get lost in the shuffle, said Don Dizon, MD, of Massachusetts General Hospital, Boston.
“I think there are multiple reasons for that, but it has to be the job of the clinician – not necessarily the oncologist-physician – but somebody has to be able to say, ‘What is your sexual history? Can I ask about your sexual history?’ ” he said in a video interview at the annual meeting of the Society of Gynecologic Oncology.
Dr. Dizon, director of the Oncology Sexual Health Clinic at Massachusetts General Hospital*, emphasized the importance of providing sensitive sexual health care while staying true to one’s own framework of care. “I teach clinicians not to run away from their schema,” he said. “Having said that, the job of a clinician is to normalize the history, and to normalize that sex, intimacy, relationships are a part of someone’s history.”
Dr. Dizon sits on the board of the Patty Brisben Foundation and the Young Survival Coalition.
[email protected]
On Twitter @karioakes
*A previous version of this article misstated Dr. Dizon's affiliation.
AT THE ANNUAL MEETING ON WOMEN'S CANCER