User login
Health law changes under new administration
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
A veteran who is suicidal while sleeping
CASE Suicidal while asleep
Mr. R, age 28, an Iraq and Afghanistan veteran with major depressive disorder and posttraumatic stress disorder (PTSD), is awoken by his wife to check on their daughter approximately 30 minutes after he takes his nightly regimen of zolpidem, 10 mg, melatonin, 6 mg, and hydroxyzine, 20 mg. When Mr. R returns to the bedroom, he appears to be confused. Mr. R grabs an unloaded gun from under the mattress, puts it in his mouth, and pulls the trigger. Then Mr. R holds the gun to his head and pulls the trigger while saying that his wife and children will be better off without him. His wife takes the gun away, but he grabs another gun from his gun box and loads it. His wife convinces him to remove the ammunition; however, Mr. R gets the other unloaded gun and pulls the trigger on himself again. After his wife takes this gun away, he tries cutting himself with a pocketknife, causing superficial cuts. Eventually, Mr. R goes back to bed. He does not remember these events in the morning.
What increased the likelihood of parasomnia in Mr. R?
a) high zolpidem dosage
b) concomitant use of other sedating agents
c) sleep deprivation
d) dehydration
[polldaddy:9712545]
The authors’ observations
Parasomnias are sleep-wake transition disorders classified by the sleep stage from which they arise, either NREM or rapid eye movement (REM). NREM parasomnias could result from incomplete awakening from NREM sleep, typically in Stage N3 (slow-wave) sleep.1 DSM-5 describes NREM parasomnias as arousal disorders in which the disturbance is not attributable to the physiological effects of substance; substance/medication-induced sleep disorder, parasomnia type, is when the disturbance can be attributed to a substance.2 The latter also can occur during REM sleep.
NREM parasomnias are characterized by abnormal behaviors during sleep with significant harm potential.3 Somnambulism or sleepwalking and sleep terrors are the 2 types of NREM parasomnias in DSM-5. Sleepwalking could involve complex behaviors, including:
- eating
- talking
- cooking
- shopping
- driving
- sexual activity.
Zolpidem, a benzodiazepine receptor agonist, is a preferred hypnotic agent for insomnia because of its low risk for abuse and daytime sedation.4 However, the drug has been associated with NREM parasomnias, namely somnambulism or sleepwalking, and its variants including sleep-driving, sleep-related eating disorder, and rarely sexsomnia (sleep-sex), with anterograde amnesia for the event.5 Suicidal behavior that occurs while the patient is asleep with next-day amnesia is another variant of somnambulism. There are several reports of suicidal behavior during sleep,6,7 but to our knowledge, there are only 2 previous cases implicating zolpidem as the cause:
- Gibson et al8 described a 49-year-old man who sustained a self-inflicted gunshot wound to his head while asleep. He just had started taking zolpidem, and in the weeks before the incident he had several episodes of sleepwalking and sleep-eating. He had consumed alcohol the night of the self-inflicted gunshot wound, but had no other psychiatric history.
- Chopra et al4 described a 37-year-old man, with no prior episodes of sleepwalking or associated complex behaviors, who was taking zolpidem, 10 mg/d, for chronic insomnia. He shot a gun in the basement of his home, and then held the loaded gun to his neck while asleep. The authors attributed the event to zolpidem in combination with other predisposing factors, including dehydration after intense exercise and alcohol use. The authors categorized this type of event as “para-suicidal amnestic behavior,” although “sleep-related pseudo-suicidal behavior” might be a better term for this type of parasomnia because of its occurrence during sleep and non-deliberate nature.
In another case report, a 27-year-old man took additional zolpidem after he did not experience desired sedative effects from an initial 20 mg.9 Because the patient remembered the suicidal thoughts, the authors believed that the patient attempted suicide while under the influence of zolpidem. The authors did not believe the incident to be sleep-related suicidal behavior, because it was uncertain if he attempted suicide while asleep.
Mr. R does not remember the events his wife witnessed while he was asleep. To our knowledge, Mr. R’s case is the first sleep-related pseudo-suicidal behavior case resulting from zolpidem, 10 mg/d, without concurrent alcohol use in an adult male veteran with PTSD and no suicidal ideation while awake.
HISTORY Further details revealed
Mr. R says that in the days leading to the incident he was not sleep-deprived and was getting at least 6 hours of restful sleep every night. He had been taking zolpidem every night. He has no childhood or family history of NREM parasomnias. He says he did not engage in intense exercise that evening or have a fever the night of the incident and has abstained from alcohol for 2 years.
His wife says that after he took zolpidem, when he was woken up, “He was not there; his eyes were glazed and glossy, and it’s like he was in another world,” and his speech and behavior were bizarre. She also reports that his eyes were open when he engaged in this behavior that appeared suicidal.
Three months before the incident, Mr. R had reported nightmares with dream enactment behaviors, hypervigilance on awakening and during the daytime, irritability, and anxious and depressed mood with neurovegetative symptoms, and was referred to our clinic for medication management. He also reported no prior or current manic or psychotic symptoms, denied suicidal thoughts, and had no history of suicide attempts. Mr. R’s medication regimen included tramadol, 400 mg/d, for chronic knee pain; fluoxetine, 60 mg/d, for depression and PTSD; and propranolol ER, 60 mg/d, and propranolol, 10 mg/d as needed, for anxiety. He was started on prazosin, 2 mg/d, titrated to 4 mg/d, for medication management of nightmares.
Mr. R also was referred to the sleep laboratory for a polysomnogram (PSG) because of reported loud snoring and witnessed apneas, especially because sleep apnea can cause nightmares and dream enactment behaviors. The PSG was negative for sleep apnea or excessive periodic limb movements of sleep, but showed increased electromyographic (EMG) activity during REM sleep, which was consistent with his report of dream enactment behaviors. Two months later, he reported improvement in nightmares and depression, but not in dream enactment behaviors. Because of prominent anxiety and irritability, he was started on gabapentin, 300 mg, 3 times a day.
What factor increases the risk of NREM parasomnias with zolpidem compared with benzodiazepines?
a) greater preservation of Stage N3 sleep
b) lesser degree of muscle relaxation
c) both a and b
d) none of the above
[polldaddy:9712556]
The authors’ observations
Factors that increase the likelihood of parasomnias include:
- zolpidem >10 mg at bedtime
- concomitant use of other CNS depressants, including sedative hypnotic agents and alcohol
- female sex
- not falling asleep immediately after taking zolpidem
- personal or family history of parasomnias
- living alone
- poor pill management
- presence of sleep disruptors such as sleep apnea and periodic limb movements of sleep.1,4,5,10
Higher dosages of zolpidem (>10 mg/d) have been identified as the predictive risk factor.5 In the Chopra et al4 case report on sleep-related suicidal behavior related to zolpidem, 10 mg at bedtime, concomitant dehydration and alcohol use were implicated as facilitating factors. Dehydration could increase serum levels of zolpidem resulting in greater CNS effects. Alcohol use was implicated in the Gibson et al8 case report as well, and the patient had multiple episodes of sleepwalking and sleep-related eating.However, Mr. R was not dehydrated or using alcohol.
An interesting feature of Mr. R’s case is that he was taking fluoxetine. Cytochrome P450 (CYP) 3A4 is involved in metabolizing zolpidem, and norfluoxetine, a metabolite of fluoxetine, inhibits CYP3A4. Although studies have not found pharmacokinetic interactions between fluoxetine and zolpidem, these studies did not investigate fluoxetine dosages >20 mg/d.11 The inhibition of CYP enzymes by fluoxetine likely is dose-dependent,12 and therefore concomitant administration of high-dosage fluoxetine (>20 mg/d) with zolpidem might result in higher serum levels of zolpidem.
Mr. R also was taking several sedating agents (gabapentin, hydroxyzine, melatonin, and tramadol). The concomitant use of these sedative-hypnotic agents could have increased his risk of parasomnia. A review of the literature did not reveal any reports of gabapentin, hydroxyzine, melatonin, or tramadol causing parasomnias. This observation, as well as the well-known role of zolpidem5 in etiopathogenesis of parasomnias, indicates that the pseudo-suicidal behavior Mr. R displayed while asleep likely was a direct result of zolpidem use in presence of other facilitating factors. Gabapentin, which is known to increase the depth of sleep, was added to his regimen 1 month before his parasomnia episode. Therefore, gabapentin could have triggered parasomnia with zolpidem therapy.1,13
Conditions that provoke repeated cortical arousals (eg, periodic limb movement disorder [PLMD] and sleep apnea) or increase depth or pressure of sleep (intense exercise in the evening, fever, sleep deprivation) are thought to be associated with NREM parasomnias.1-4 However, Mr. R underwent in-laboratory PSG and tested negative for major cortical arousal-inducing conditions, such as PLMD and sleep apnea.
Some other sleep disruptors likely were involved in Mr. R’s case. Auditory and tactile stimuli are known to cause cortical arousals, with additive effect seen when these 2 stimuli are combined.3,14 Additionally, these exogenous stimuli are known to trigger sleep-related violent parasomnias.15 Mr. R displayed this behavior after his wife woke him up. The auditory stimulus of his wife’s voice and/or tactile stimulus involved in the act of waking Mr. R likely played a role in the suicidal and violent nature of his NREM parasomnia.
[polldaddy:9712581]
The authors’ observations
In general, the mechanisms by which zolpidem causes NREM parasomnias are not completely understood. The sedation-related amnestic properties of zolpidem might explain some of these behaviors. Patients could perform these behaviors after waking and have subsequent amnesia.4 There is greater preservation of Stage N3 sleep with zolpidem compared with benzodiazepines. Benzodiazepines also cause muscle relaxation while the motor system remains relatively more active during sleep with zolpidem because of its selectivity for α-1 subunit of gamma-aminobutyric acid A receptor. These factors might increase the likelihood of NREM parasomnias with zolpidem compared with benzodiazepines.4
Types of parasomnias
According to DSM-5, there are 2 categories of parasomnias based on the sleep stage from which a parasomnia emerges.2 REM sleep behavior disorder (RBD) refers to complex motor and/or vocalizations during REM sleep, accompanied by increased EMG activity during REM sleep (Table).2,3
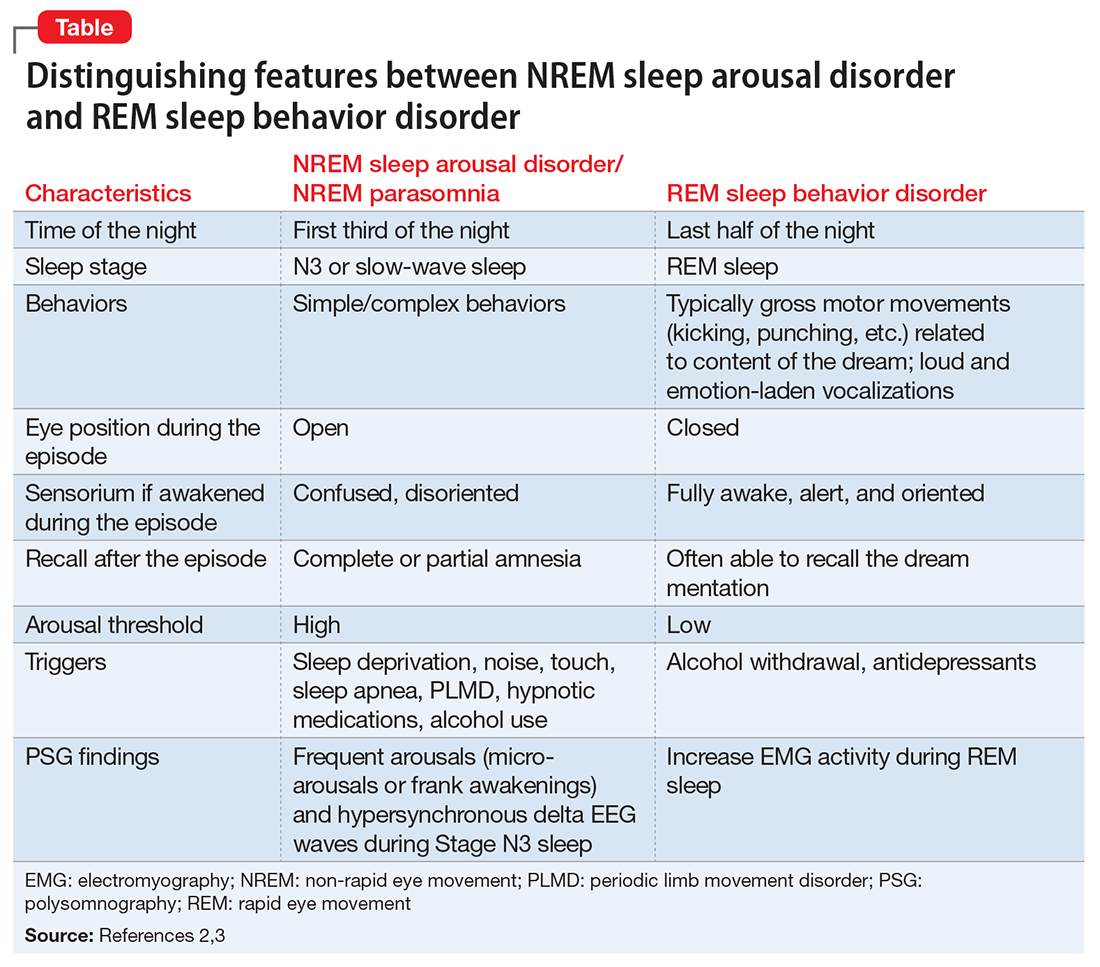
The pseudo-suicidal behavior Mr. R displayed likely was NREM parasomnia because it occurred in the first third of the night with his eyes open and impaired recall after the event. Interestingly, Mr. R had RBD in addition to the NREM parasomnia likely caused by zolpidem. This is evident from Mr. R’s frequent dream enactment behaviors, such as kicking, thrashing, and punching during sleep, along with increased EMG activity during REM sleep as recorded on the PSG.10 The presence of RBD could be explained by selective serotonin reuptake inhibitor (fluoxetine) use, and comorbidity with PTSD.2,16
Management of parasomnias
Initial management of parasomnias involves decreasing the risk of parasomnia-related injury. Suggested safety measures include:
- sleeping away from windows
- sleeping in a sleeping bag
- sleeping on a lower floor
- locking windows and doors
- removing potentially dangerous objects from the bedroom
- putting gates across stairwells
- installing bells or alarms on door knobs.15
Removing access to firearms or other weapons such as knives is of utmost importance especially with patients who have easy access during wakefulness. If removing weapons is not feasible, consider disarming, securing, or locking them.15 These considerations are relevant to veterans with PTSD because of the high prevalence of symptoms, including depression, insomnia, and pain, which require sedating medications.17 A review of parasomnias among a large sample of psychiatric outpatients revealed that a variety of sedating medications, including antidepressants, can lead to NREM parasomnias.18 Therefore, exercise caution when prescribing sedating medications, especially in patients vulnerable to developing dangerous parasomnias, such as a veteran with PTSD and easy access to guns.19
TREATMENT Zolpidem stopped
Mr. R immediately stops taking zolpidem because he is aware of its association with abnormal behaviors during sleep, and his wife removes his access to firearms and knives at night. Because of his history of clinical benefit and no history of parasomnias with mirtazapine, Mr. R is started on mirtazapine for insomnia that previously was treated with zolpidem, and residual depression. Six months after discontinuing zolpidem, he does not experience NREM parasomnias, and there are no changes in his dream enactment behaviors.
Summing up
Zolpidem therapy could be associated with unusual variants of NREM parasomnia, sleepwalking type; sleep-related pseudo-suicidal behavior is one such variant. Several factors could play a role in increasing the likelihood of NREM parasomnia with zolpidem therapy. In Mr. R’s case, the pharmacokinetic drug interactions between fluoxetine and zolpidem, as well as concomitant use of several sedating agents could have played a role in increasing the likelihood of NREM parasomnia, with audio-tactile stimuli contributing to the violent and suicidal nature of the parasomnia. Exercise caution when using CYP enzyme inhibitors, such as fluoxetine and paroxetine, in combination with zolpidem. Knowledge of the potential interaction between zolpidem and fluoxetine is important because antidepressants and hypnotics are commonly co-prescribed because insomnia often is comorbid with other psychiatric disorders.
In veterans with PTSD who do not have suicidal ideations while awake, life-threatening non-intentional behavior is a risk because of easy access to guns or other weapons. Sedative-hypnotic medications commonly are prescribed to patients with PTSD. Exercise caution when using hypnotic agents such as zolpidem, and consider sleep aids with a lower risk of parasomnias (based on the author’s experience, trazodone, mirtazapine, melatonin, and gabapentin) when possible. Non-pharmacologic treatments of insomnia, such as sleep hygiene education and, more importantly, cognitive-behavioral therapy for insomnia, are preferred. If a patient is already taking zolpidem, nightly dosage should not be >10 mg. Polypharmacy with other sedating medications should be avoided when possible and both exogenous (noise, pets) and endogenous sleep disruptors (sleep apnea, PLMD) should be addressed. Advise the patient to avoid alcohol and remove firearms and other potential weapons. Discontinue zolpidem if the patient develops sleep-related abnormal behavior because of its potential to take on violent forms.
1. Howell MJ. Parasomnias: an updated review. Neurotherapeutics. 2012;9(4):753-775.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Zadra A, Desautels A, Petit D, et al. Somnambulism: clinical aspects and pathophysiological hypotheses. Lancet Neurol. 2013;12(3):285-294.
4. Chopra A, Selim B, Silber MH, et al. Para-suicidal amnestic behavior associated with chronic zolpidem use: implications for patient safety. Psychosomatics. 2013;54(5):498-501.
5. Hwang TJ, Ni HC, Chen HC, et al. Risk predictors for hypnosedative-related complex sleep behaviors: a retrospective, cross-sectional pilot study. J Clin Psychiatry. 2010;71(10):1331-1335.
6. Shatkin JP, Feinfield K, Strober M. The misinterpretation of a non-REM sleep parasomnia as suicidal behavior in an adolescent. Sleep Breath. 2002;6(4):175-179.
7. Mahowald MW, Schenck CH, Goldner M, et al. Parasomnia pseudo-suicide. J Forensic Sci. 2003;48(5):1158-1162.
8. Gibson CE, Caplan JP. Zolpidem-associated parasomnia with serious self-injury: a shot in the dark. Psychosomatics. 2011;52(1):88-91.
9. Mortaz Hejri S, Faizi M, Babaeian M. Zolpidem-induced suicide attempt: a case report. Daru. 2013;20;21(1):77.
10. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
11. Hesse LM, von Moltke LL, Greenblatt DJ. Clinically important drug interactions with zopiclone, zolpidem and zaleplon. CNS Drugs. 2003;17(7):513-532.
12. Catterson ML, Preskorn SH. Pharmacokinetics of selective serotonin reuptake inhibitors: clinical relevance. Pharmacol Toxicol. 1996;78(4):203-208.
13. Rosenberg RP, Hull SG, Lankford DA, et al. A randomized, double-blind, single-dose, placebo-controlled, multicenter, polysomnographic study of gabapentin in transient insomnia induced by sleep phase advance. J Clin Sleep Med. 2014;10(10):1093-1100.
14. Kato T, Montplaisir JY, Lavigne GJ. Experimentally induced arousals during sleep: a cross-modality matching paradigm. J Sleep Res. 2004;13(3):229-238.
15. Siclari F, Khatami R, Urbaniok F, et al. Violence in sleep. Brain. 2010;133(pt 12):3494-3509.
16. Husain AM, Miller PP, Carwile ST. Rem sleep behavior disorder: potential relationship to post-traumatic stress disorder. J Clin Neurophysiol. 2001;18(2):148-157.
17. Bernardy NC, Lund BC, Alexander B, et al. Increased polysedative use in veterans with posttraumatic stress disorder. Pain Med. 2014;15(7):1083-1090.
18. Lam SP, Fong SY, Ho CK, et al. Parasomnia among psychiatric outpatients: a clinical, epidemiologic, cross-sectional study. J Clin Psychiatry. 2008;69(9):1374-1382.
19. Freeman TW, Roca V, Kimbrell T. A survey of gun collection and use among three groups of veteran patients admitted to veterans affairs hospital treatment programs. South Med J. 2003;96(3):240-243.
CASE Suicidal while asleep
Mr. R, age 28, an Iraq and Afghanistan veteran with major depressive disorder and posttraumatic stress disorder (PTSD), is awoken by his wife to check on their daughter approximately 30 minutes after he takes his nightly regimen of zolpidem, 10 mg, melatonin, 6 mg, and hydroxyzine, 20 mg. When Mr. R returns to the bedroom, he appears to be confused. Mr. R grabs an unloaded gun from under the mattress, puts it in his mouth, and pulls the trigger. Then Mr. R holds the gun to his head and pulls the trigger while saying that his wife and children will be better off without him. His wife takes the gun away, but he grabs another gun from his gun box and loads it. His wife convinces him to remove the ammunition; however, Mr. R gets the other unloaded gun and pulls the trigger on himself again. After his wife takes this gun away, he tries cutting himself with a pocketknife, causing superficial cuts. Eventually, Mr. R goes back to bed. He does not remember these events in the morning.
What increased the likelihood of parasomnia in Mr. R?
a) high zolpidem dosage
b) concomitant use of other sedating agents
c) sleep deprivation
d) dehydration
[polldaddy:9712545]
The authors’ observations
Parasomnias are sleep-wake transition disorders classified by the sleep stage from which they arise, either NREM or rapid eye movement (REM). NREM parasomnias could result from incomplete awakening from NREM sleep, typically in Stage N3 (slow-wave) sleep.1 DSM-5 describes NREM parasomnias as arousal disorders in which the disturbance is not attributable to the physiological effects of substance; substance/medication-induced sleep disorder, parasomnia type, is when the disturbance can be attributed to a substance.2 The latter also can occur during REM sleep.
NREM parasomnias are characterized by abnormal behaviors during sleep with significant harm potential.3 Somnambulism or sleepwalking and sleep terrors are the 2 types of NREM parasomnias in DSM-5. Sleepwalking could involve complex behaviors, including:
- eating
- talking
- cooking
- shopping
- driving
- sexual activity.
Zolpidem, a benzodiazepine receptor agonist, is a preferred hypnotic agent for insomnia because of its low risk for abuse and daytime sedation.4 However, the drug has been associated with NREM parasomnias, namely somnambulism or sleepwalking, and its variants including sleep-driving, sleep-related eating disorder, and rarely sexsomnia (sleep-sex), with anterograde amnesia for the event.5 Suicidal behavior that occurs while the patient is asleep with next-day amnesia is another variant of somnambulism. There are several reports of suicidal behavior during sleep,6,7 but to our knowledge, there are only 2 previous cases implicating zolpidem as the cause:
- Gibson et al8 described a 49-year-old man who sustained a self-inflicted gunshot wound to his head while asleep. He just had started taking zolpidem, and in the weeks before the incident he had several episodes of sleepwalking and sleep-eating. He had consumed alcohol the night of the self-inflicted gunshot wound, but had no other psychiatric history.
- Chopra et al4 described a 37-year-old man, with no prior episodes of sleepwalking or associated complex behaviors, who was taking zolpidem, 10 mg/d, for chronic insomnia. He shot a gun in the basement of his home, and then held the loaded gun to his neck while asleep. The authors attributed the event to zolpidem in combination with other predisposing factors, including dehydration after intense exercise and alcohol use. The authors categorized this type of event as “para-suicidal amnestic behavior,” although “sleep-related pseudo-suicidal behavior” might be a better term for this type of parasomnia because of its occurrence during sleep and non-deliberate nature.
In another case report, a 27-year-old man took additional zolpidem after he did not experience desired sedative effects from an initial 20 mg.9 Because the patient remembered the suicidal thoughts, the authors believed that the patient attempted suicide while under the influence of zolpidem. The authors did not believe the incident to be sleep-related suicidal behavior, because it was uncertain if he attempted suicide while asleep.
Mr. R does not remember the events his wife witnessed while he was asleep. To our knowledge, Mr. R’s case is the first sleep-related pseudo-suicidal behavior case resulting from zolpidem, 10 mg/d, without concurrent alcohol use in an adult male veteran with PTSD and no suicidal ideation while awake.
HISTORY Further details revealed
Mr. R says that in the days leading to the incident he was not sleep-deprived and was getting at least 6 hours of restful sleep every night. He had been taking zolpidem every night. He has no childhood or family history of NREM parasomnias. He says he did not engage in intense exercise that evening or have a fever the night of the incident and has abstained from alcohol for 2 years.
His wife says that after he took zolpidem, when he was woken up, “He was not there; his eyes were glazed and glossy, and it’s like he was in another world,” and his speech and behavior were bizarre. She also reports that his eyes were open when he engaged in this behavior that appeared suicidal.
Three months before the incident, Mr. R had reported nightmares with dream enactment behaviors, hypervigilance on awakening and during the daytime, irritability, and anxious and depressed mood with neurovegetative symptoms, and was referred to our clinic for medication management. He also reported no prior or current manic or psychotic symptoms, denied suicidal thoughts, and had no history of suicide attempts. Mr. R’s medication regimen included tramadol, 400 mg/d, for chronic knee pain; fluoxetine, 60 mg/d, for depression and PTSD; and propranolol ER, 60 mg/d, and propranolol, 10 mg/d as needed, for anxiety. He was started on prazosin, 2 mg/d, titrated to 4 mg/d, for medication management of nightmares.
Mr. R also was referred to the sleep laboratory for a polysomnogram (PSG) because of reported loud snoring and witnessed apneas, especially because sleep apnea can cause nightmares and dream enactment behaviors. The PSG was negative for sleep apnea or excessive periodic limb movements of sleep, but showed increased electromyographic (EMG) activity during REM sleep, which was consistent with his report of dream enactment behaviors. Two months later, he reported improvement in nightmares and depression, but not in dream enactment behaviors. Because of prominent anxiety and irritability, he was started on gabapentin, 300 mg, 3 times a day.
What factor increases the risk of NREM parasomnias with zolpidem compared with benzodiazepines?
a) greater preservation of Stage N3 sleep
b) lesser degree of muscle relaxation
c) both a and b
d) none of the above
[polldaddy:9712556]
The authors’ observations
Factors that increase the likelihood of parasomnias include:
- zolpidem >10 mg at bedtime
- concomitant use of other CNS depressants, including sedative hypnotic agents and alcohol
- female sex
- not falling asleep immediately after taking zolpidem
- personal or family history of parasomnias
- living alone
- poor pill management
- presence of sleep disruptors such as sleep apnea and periodic limb movements of sleep.1,4,5,10
Higher dosages of zolpidem (>10 mg/d) have been identified as the predictive risk factor.5 In the Chopra et al4 case report on sleep-related suicidal behavior related to zolpidem, 10 mg at bedtime, concomitant dehydration and alcohol use were implicated as facilitating factors. Dehydration could increase serum levels of zolpidem resulting in greater CNS effects. Alcohol use was implicated in the Gibson et al8 case report as well, and the patient had multiple episodes of sleepwalking and sleep-related eating.However, Mr. R was not dehydrated or using alcohol.
An interesting feature of Mr. R’s case is that he was taking fluoxetine. Cytochrome P450 (CYP) 3A4 is involved in metabolizing zolpidem, and norfluoxetine, a metabolite of fluoxetine, inhibits CYP3A4. Although studies have not found pharmacokinetic interactions between fluoxetine and zolpidem, these studies did not investigate fluoxetine dosages >20 mg/d.11 The inhibition of CYP enzymes by fluoxetine likely is dose-dependent,12 and therefore concomitant administration of high-dosage fluoxetine (>20 mg/d) with zolpidem might result in higher serum levels of zolpidem.
Mr. R also was taking several sedating agents (gabapentin, hydroxyzine, melatonin, and tramadol). The concomitant use of these sedative-hypnotic agents could have increased his risk of parasomnia. A review of the literature did not reveal any reports of gabapentin, hydroxyzine, melatonin, or tramadol causing parasomnias. This observation, as well as the well-known role of zolpidem5 in etiopathogenesis of parasomnias, indicates that the pseudo-suicidal behavior Mr. R displayed while asleep likely was a direct result of zolpidem use in presence of other facilitating factors. Gabapentin, which is known to increase the depth of sleep, was added to his regimen 1 month before his parasomnia episode. Therefore, gabapentin could have triggered parasomnia with zolpidem therapy.1,13
Conditions that provoke repeated cortical arousals (eg, periodic limb movement disorder [PLMD] and sleep apnea) or increase depth or pressure of sleep (intense exercise in the evening, fever, sleep deprivation) are thought to be associated with NREM parasomnias.1-4 However, Mr. R underwent in-laboratory PSG and tested negative for major cortical arousal-inducing conditions, such as PLMD and sleep apnea.
Some other sleep disruptors likely were involved in Mr. R’s case. Auditory and tactile stimuli are known to cause cortical arousals, with additive effect seen when these 2 stimuli are combined.3,14 Additionally, these exogenous stimuli are known to trigger sleep-related violent parasomnias.15 Mr. R displayed this behavior after his wife woke him up. The auditory stimulus of his wife’s voice and/or tactile stimulus involved in the act of waking Mr. R likely played a role in the suicidal and violent nature of his NREM parasomnia.
[polldaddy:9712581]
The authors’ observations
In general, the mechanisms by which zolpidem causes NREM parasomnias are not completely understood. The sedation-related amnestic properties of zolpidem might explain some of these behaviors. Patients could perform these behaviors after waking and have subsequent amnesia.4 There is greater preservation of Stage N3 sleep with zolpidem compared with benzodiazepines. Benzodiazepines also cause muscle relaxation while the motor system remains relatively more active during sleep with zolpidem because of its selectivity for α-1 subunit of gamma-aminobutyric acid A receptor. These factors might increase the likelihood of NREM parasomnias with zolpidem compared with benzodiazepines.4
Types of parasomnias
According to DSM-5, there are 2 categories of parasomnias based on the sleep stage from which a parasomnia emerges.2 REM sleep behavior disorder (RBD) refers to complex motor and/or vocalizations during REM sleep, accompanied by increased EMG activity during REM sleep (Table).2,3

The pseudo-suicidal behavior Mr. R displayed likely was NREM parasomnia because it occurred in the first third of the night with his eyes open and impaired recall after the event. Interestingly, Mr. R had RBD in addition to the NREM parasomnia likely caused by zolpidem. This is evident from Mr. R’s frequent dream enactment behaviors, such as kicking, thrashing, and punching during sleep, along with increased EMG activity during REM sleep as recorded on the PSG.10 The presence of RBD could be explained by selective serotonin reuptake inhibitor (fluoxetine) use, and comorbidity with PTSD.2,16
Management of parasomnias
Initial management of parasomnias involves decreasing the risk of parasomnia-related injury. Suggested safety measures include:
- sleeping away from windows
- sleeping in a sleeping bag
- sleeping on a lower floor
- locking windows and doors
- removing potentially dangerous objects from the bedroom
- putting gates across stairwells
- installing bells or alarms on door knobs.15
Removing access to firearms or other weapons such as knives is of utmost importance especially with patients who have easy access during wakefulness. If removing weapons is not feasible, consider disarming, securing, or locking them.15 These considerations are relevant to veterans with PTSD because of the high prevalence of symptoms, including depression, insomnia, and pain, which require sedating medications.17 A review of parasomnias among a large sample of psychiatric outpatients revealed that a variety of sedating medications, including antidepressants, can lead to NREM parasomnias.18 Therefore, exercise caution when prescribing sedating medications, especially in patients vulnerable to developing dangerous parasomnias, such as a veteran with PTSD and easy access to guns.19
TREATMENT Zolpidem stopped
Mr. R immediately stops taking zolpidem because he is aware of its association with abnormal behaviors during sleep, and his wife removes his access to firearms and knives at night. Because of his history of clinical benefit and no history of parasomnias with mirtazapine, Mr. R is started on mirtazapine for insomnia that previously was treated with zolpidem, and residual depression. Six months after discontinuing zolpidem, he does not experience NREM parasomnias, and there are no changes in his dream enactment behaviors.
Summing up
Zolpidem therapy could be associated with unusual variants of NREM parasomnia, sleepwalking type; sleep-related pseudo-suicidal behavior is one such variant. Several factors could play a role in increasing the likelihood of NREM parasomnia with zolpidem therapy. In Mr. R’s case, the pharmacokinetic drug interactions between fluoxetine and zolpidem, as well as concomitant use of several sedating agents could have played a role in increasing the likelihood of NREM parasomnia, with audio-tactile stimuli contributing to the violent and suicidal nature of the parasomnia. Exercise caution when using CYP enzyme inhibitors, such as fluoxetine and paroxetine, in combination with zolpidem. Knowledge of the potential interaction between zolpidem and fluoxetine is important because antidepressants and hypnotics are commonly co-prescribed because insomnia often is comorbid with other psychiatric disorders.
In veterans with PTSD who do not have suicidal ideations while awake, life-threatening non-intentional behavior is a risk because of easy access to guns or other weapons. Sedative-hypnotic medications commonly are prescribed to patients with PTSD. Exercise caution when using hypnotic agents such as zolpidem, and consider sleep aids with a lower risk of parasomnias (based on the author’s experience, trazodone, mirtazapine, melatonin, and gabapentin) when possible. Non-pharmacologic treatments of insomnia, such as sleep hygiene education and, more importantly, cognitive-behavioral therapy for insomnia, are preferred. If a patient is already taking zolpidem, nightly dosage should not be >10 mg. Polypharmacy with other sedating medications should be avoided when possible and both exogenous (noise, pets) and endogenous sleep disruptors (sleep apnea, PLMD) should be addressed. Advise the patient to avoid alcohol and remove firearms and other potential weapons. Discontinue zolpidem if the patient develops sleep-related abnormal behavior because of its potential to take on violent forms.
CASE Suicidal while asleep
Mr. R, age 28, an Iraq and Afghanistan veteran with major depressive disorder and posttraumatic stress disorder (PTSD), is awoken by his wife to check on their daughter approximately 30 minutes after he takes his nightly regimen of zolpidem, 10 mg, melatonin, 6 mg, and hydroxyzine, 20 mg. When Mr. R returns to the bedroom, he appears to be confused. Mr. R grabs an unloaded gun from under the mattress, puts it in his mouth, and pulls the trigger. Then Mr. R holds the gun to his head and pulls the trigger while saying that his wife and children will be better off without him. His wife takes the gun away, but he grabs another gun from his gun box and loads it. His wife convinces him to remove the ammunition; however, Mr. R gets the other unloaded gun and pulls the trigger on himself again. After his wife takes this gun away, he tries cutting himself with a pocketknife, causing superficial cuts. Eventually, Mr. R goes back to bed. He does not remember these events in the morning.
What increased the likelihood of parasomnia in Mr. R?
a) high zolpidem dosage
b) concomitant use of other sedating agents
c) sleep deprivation
d) dehydration
[polldaddy:9712545]
The authors’ observations
Parasomnias are sleep-wake transition disorders classified by the sleep stage from which they arise, either NREM or rapid eye movement (REM). NREM parasomnias could result from incomplete awakening from NREM sleep, typically in Stage N3 (slow-wave) sleep.1 DSM-5 describes NREM parasomnias as arousal disorders in which the disturbance is not attributable to the physiological effects of substance; substance/medication-induced sleep disorder, parasomnia type, is when the disturbance can be attributed to a substance.2 The latter also can occur during REM sleep.
NREM parasomnias are characterized by abnormal behaviors during sleep with significant harm potential.3 Somnambulism or sleepwalking and sleep terrors are the 2 types of NREM parasomnias in DSM-5. Sleepwalking could involve complex behaviors, including:
- eating
- talking
- cooking
- shopping
- driving
- sexual activity.
Zolpidem, a benzodiazepine receptor agonist, is a preferred hypnotic agent for insomnia because of its low risk for abuse and daytime sedation.4 However, the drug has been associated with NREM parasomnias, namely somnambulism or sleepwalking, and its variants including sleep-driving, sleep-related eating disorder, and rarely sexsomnia (sleep-sex), with anterograde amnesia for the event.5 Suicidal behavior that occurs while the patient is asleep with next-day amnesia is another variant of somnambulism. There are several reports of suicidal behavior during sleep,6,7 but to our knowledge, there are only 2 previous cases implicating zolpidem as the cause:
- Gibson et al8 described a 49-year-old man who sustained a self-inflicted gunshot wound to his head while asleep. He just had started taking zolpidem, and in the weeks before the incident he had several episodes of sleepwalking and sleep-eating. He had consumed alcohol the night of the self-inflicted gunshot wound, but had no other psychiatric history.
- Chopra et al4 described a 37-year-old man, with no prior episodes of sleepwalking or associated complex behaviors, who was taking zolpidem, 10 mg/d, for chronic insomnia. He shot a gun in the basement of his home, and then held the loaded gun to his neck while asleep. The authors attributed the event to zolpidem in combination with other predisposing factors, including dehydration after intense exercise and alcohol use. The authors categorized this type of event as “para-suicidal amnestic behavior,” although “sleep-related pseudo-suicidal behavior” might be a better term for this type of parasomnia because of its occurrence during sleep and non-deliberate nature.
In another case report, a 27-year-old man took additional zolpidem after he did not experience desired sedative effects from an initial 20 mg.9 Because the patient remembered the suicidal thoughts, the authors believed that the patient attempted suicide while under the influence of zolpidem. The authors did not believe the incident to be sleep-related suicidal behavior, because it was uncertain if he attempted suicide while asleep.
Mr. R does not remember the events his wife witnessed while he was asleep. To our knowledge, Mr. R’s case is the first sleep-related pseudo-suicidal behavior case resulting from zolpidem, 10 mg/d, without concurrent alcohol use in an adult male veteran with PTSD and no suicidal ideation while awake.
HISTORY Further details revealed
Mr. R says that in the days leading to the incident he was not sleep-deprived and was getting at least 6 hours of restful sleep every night. He had been taking zolpidem every night. He has no childhood or family history of NREM parasomnias. He says he did not engage in intense exercise that evening or have a fever the night of the incident and has abstained from alcohol for 2 years.
His wife says that after he took zolpidem, when he was woken up, “He was not there; his eyes were glazed and glossy, and it’s like he was in another world,” and his speech and behavior were bizarre. She also reports that his eyes were open when he engaged in this behavior that appeared suicidal.
Three months before the incident, Mr. R had reported nightmares with dream enactment behaviors, hypervigilance on awakening and during the daytime, irritability, and anxious and depressed mood with neurovegetative symptoms, and was referred to our clinic for medication management. He also reported no prior or current manic or psychotic symptoms, denied suicidal thoughts, and had no history of suicide attempts. Mr. R’s medication regimen included tramadol, 400 mg/d, for chronic knee pain; fluoxetine, 60 mg/d, for depression and PTSD; and propranolol ER, 60 mg/d, and propranolol, 10 mg/d as needed, for anxiety. He was started on prazosin, 2 mg/d, titrated to 4 mg/d, for medication management of nightmares.
Mr. R also was referred to the sleep laboratory for a polysomnogram (PSG) because of reported loud snoring and witnessed apneas, especially because sleep apnea can cause nightmares and dream enactment behaviors. The PSG was negative for sleep apnea or excessive periodic limb movements of sleep, but showed increased electromyographic (EMG) activity during REM sleep, which was consistent with his report of dream enactment behaviors. Two months later, he reported improvement in nightmares and depression, but not in dream enactment behaviors. Because of prominent anxiety and irritability, he was started on gabapentin, 300 mg, 3 times a day.
What factor increases the risk of NREM parasomnias with zolpidem compared with benzodiazepines?
a) greater preservation of Stage N3 sleep
b) lesser degree of muscle relaxation
c) both a and b
d) none of the above
[polldaddy:9712556]
The authors’ observations
Factors that increase the likelihood of parasomnias include:
- zolpidem >10 mg at bedtime
- concomitant use of other CNS depressants, including sedative hypnotic agents and alcohol
- female sex
- not falling asleep immediately after taking zolpidem
- personal or family history of parasomnias
- living alone
- poor pill management
- presence of sleep disruptors such as sleep apnea and periodic limb movements of sleep.1,4,5,10
Higher dosages of zolpidem (>10 mg/d) have been identified as the predictive risk factor.5 In the Chopra et al4 case report on sleep-related suicidal behavior related to zolpidem, 10 mg at bedtime, concomitant dehydration and alcohol use were implicated as facilitating factors. Dehydration could increase serum levels of zolpidem resulting in greater CNS effects. Alcohol use was implicated in the Gibson et al8 case report as well, and the patient had multiple episodes of sleepwalking and sleep-related eating.However, Mr. R was not dehydrated or using alcohol.
An interesting feature of Mr. R’s case is that he was taking fluoxetine. Cytochrome P450 (CYP) 3A4 is involved in metabolizing zolpidem, and norfluoxetine, a metabolite of fluoxetine, inhibits CYP3A4. Although studies have not found pharmacokinetic interactions between fluoxetine and zolpidem, these studies did not investigate fluoxetine dosages >20 mg/d.11 The inhibition of CYP enzymes by fluoxetine likely is dose-dependent,12 and therefore concomitant administration of high-dosage fluoxetine (>20 mg/d) with zolpidem might result in higher serum levels of zolpidem.
Mr. R also was taking several sedating agents (gabapentin, hydroxyzine, melatonin, and tramadol). The concomitant use of these sedative-hypnotic agents could have increased his risk of parasomnia. A review of the literature did not reveal any reports of gabapentin, hydroxyzine, melatonin, or tramadol causing parasomnias. This observation, as well as the well-known role of zolpidem5 in etiopathogenesis of parasomnias, indicates that the pseudo-suicidal behavior Mr. R displayed while asleep likely was a direct result of zolpidem use in presence of other facilitating factors. Gabapentin, which is known to increase the depth of sleep, was added to his regimen 1 month before his parasomnia episode. Therefore, gabapentin could have triggered parasomnia with zolpidem therapy.1,13
Conditions that provoke repeated cortical arousals (eg, periodic limb movement disorder [PLMD] and sleep apnea) or increase depth or pressure of sleep (intense exercise in the evening, fever, sleep deprivation) are thought to be associated with NREM parasomnias.1-4 However, Mr. R underwent in-laboratory PSG and tested negative for major cortical arousal-inducing conditions, such as PLMD and sleep apnea.
Some other sleep disruptors likely were involved in Mr. R’s case. Auditory and tactile stimuli are known to cause cortical arousals, with additive effect seen when these 2 stimuli are combined.3,14 Additionally, these exogenous stimuli are known to trigger sleep-related violent parasomnias.15 Mr. R displayed this behavior after his wife woke him up. The auditory stimulus of his wife’s voice and/or tactile stimulus involved in the act of waking Mr. R likely played a role in the suicidal and violent nature of his NREM parasomnia.
[polldaddy:9712581]
The authors’ observations
In general, the mechanisms by which zolpidem causes NREM parasomnias are not completely understood. The sedation-related amnestic properties of zolpidem might explain some of these behaviors. Patients could perform these behaviors after waking and have subsequent amnesia.4 There is greater preservation of Stage N3 sleep with zolpidem compared with benzodiazepines. Benzodiazepines also cause muscle relaxation while the motor system remains relatively more active during sleep with zolpidem because of its selectivity for α-1 subunit of gamma-aminobutyric acid A receptor. These factors might increase the likelihood of NREM parasomnias with zolpidem compared with benzodiazepines.4
Types of parasomnias
According to DSM-5, there are 2 categories of parasomnias based on the sleep stage from which a parasomnia emerges.2 REM sleep behavior disorder (RBD) refers to complex motor and/or vocalizations during REM sleep, accompanied by increased EMG activity during REM sleep (Table).2,3

The pseudo-suicidal behavior Mr. R displayed likely was NREM parasomnia because it occurred in the first third of the night with his eyes open and impaired recall after the event. Interestingly, Mr. R had RBD in addition to the NREM parasomnia likely caused by zolpidem. This is evident from Mr. R’s frequent dream enactment behaviors, such as kicking, thrashing, and punching during sleep, along with increased EMG activity during REM sleep as recorded on the PSG.10 The presence of RBD could be explained by selective serotonin reuptake inhibitor (fluoxetine) use, and comorbidity with PTSD.2,16
Management of parasomnias
Initial management of parasomnias involves decreasing the risk of parasomnia-related injury. Suggested safety measures include:
- sleeping away from windows
- sleeping in a sleeping bag
- sleeping on a lower floor
- locking windows and doors
- removing potentially dangerous objects from the bedroom
- putting gates across stairwells
- installing bells or alarms on door knobs.15
Removing access to firearms or other weapons such as knives is of utmost importance especially with patients who have easy access during wakefulness. If removing weapons is not feasible, consider disarming, securing, or locking them.15 These considerations are relevant to veterans with PTSD because of the high prevalence of symptoms, including depression, insomnia, and pain, which require sedating medications.17 A review of parasomnias among a large sample of psychiatric outpatients revealed that a variety of sedating medications, including antidepressants, can lead to NREM parasomnias.18 Therefore, exercise caution when prescribing sedating medications, especially in patients vulnerable to developing dangerous parasomnias, such as a veteran with PTSD and easy access to guns.19
TREATMENT Zolpidem stopped
Mr. R immediately stops taking zolpidem because he is aware of its association with abnormal behaviors during sleep, and his wife removes his access to firearms and knives at night. Because of his history of clinical benefit and no history of parasomnias with mirtazapine, Mr. R is started on mirtazapine for insomnia that previously was treated with zolpidem, and residual depression. Six months after discontinuing zolpidem, he does not experience NREM parasomnias, and there are no changes in his dream enactment behaviors.
Summing up
Zolpidem therapy could be associated with unusual variants of NREM parasomnia, sleepwalking type; sleep-related pseudo-suicidal behavior is one such variant. Several factors could play a role in increasing the likelihood of NREM parasomnia with zolpidem therapy. In Mr. R’s case, the pharmacokinetic drug interactions between fluoxetine and zolpidem, as well as concomitant use of several sedating agents could have played a role in increasing the likelihood of NREM parasomnia, with audio-tactile stimuli contributing to the violent and suicidal nature of the parasomnia. Exercise caution when using CYP enzyme inhibitors, such as fluoxetine and paroxetine, in combination with zolpidem. Knowledge of the potential interaction between zolpidem and fluoxetine is important because antidepressants and hypnotics are commonly co-prescribed because insomnia often is comorbid with other psychiatric disorders.
In veterans with PTSD who do not have suicidal ideations while awake, life-threatening non-intentional behavior is a risk because of easy access to guns or other weapons. Sedative-hypnotic medications commonly are prescribed to patients with PTSD. Exercise caution when using hypnotic agents such as zolpidem, and consider sleep aids with a lower risk of parasomnias (based on the author’s experience, trazodone, mirtazapine, melatonin, and gabapentin) when possible. Non-pharmacologic treatments of insomnia, such as sleep hygiene education and, more importantly, cognitive-behavioral therapy for insomnia, are preferred. If a patient is already taking zolpidem, nightly dosage should not be >10 mg. Polypharmacy with other sedating medications should be avoided when possible and both exogenous (noise, pets) and endogenous sleep disruptors (sleep apnea, PLMD) should be addressed. Advise the patient to avoid alcohol and remove firearms and other potential weapons. Discontinue zolpidem if the patient develops sleep-related abnormal behavior because of its potential to take on violent forms.
1. Howell MJ. Parasomnias: an updated review. Neurotherapeutics. 2012;9(4):753-775.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Zadra A, Desautels A, Petit D, et al. Somnambulism: clinical aspects and pathophysiological hypotheses. Lancet Neurol. 2013;12(3):285-294.
4. Chopra A, Selim B, Silber MH, et al. Para-suicidal amnestic behavior associated with chronic zolpidem use: implications for patient safety. Psychosomatics. 2013;54(5):498-501.
5. Hwang TJ, Ni HC, Chen HC, et al. Risk predictors for hypnosedative-related complex sleep behaviors: a retrospective, cross-sectional pilot study. J Clin Psychiatry. 2010;71(10):1331-1335.
6. Shatkin JP, Feinfield K, Strober M. The misinterpretation of a non-REM sleep parasomnia as suicidal behavior in an adolescent. Sleep Breath. 2002;6(4):175-179.
7. Mahowald MW, Schenck CH, Goldner M, et al. Parasomnia pseudo-suicide. J Forensic Sci. 2003;48(5):1158-1162.
8. Gibson CE, Caplan JP. Zolpidem-associated parasomnia with serious self-injury: a shot in the dark. Psychosomatics. 2011;52(1):88-91.
9. Mortaz Hejri S, Faizi M, Babaeian M. Zolpidem-induced suicide attempt: a case report. Daru. 2013;20;21(1):77.
10. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
11. Hesse LM, von Moltke LL, Greenblatt DJ. Clinically important drug interactions with zopiclone, zolpidem and zaleplon. CNS Drugs. 2003;17(7):513-532.
12. Catterson ML, Preskorn SH. Pharmacokinetics of selective serotonin reuptake inhibitors: clinical relevance. Pharmacol Toxicol. 1996;78(4):203-208.
13. Rosenberg RP, Hull SG, Lankford DA, et al. A randomized, double-blind, single-dose, placebo-controlled, multicenter, polysomnographic study of gabapentin in transient insomnia induced by sleep phase advance. J Clin Sleep Med. 2014;10(10):1093-1100.
14. Kato T, Montplaisir JY, Lavigne GJ. Experimentally induced arousals during sleep: a cross-modality matching paradigm. J Sleep Res. 2004;13(3):229-238.
15. Siclari F, Khatami R, Urbaniok F, et al. Violence in sleep. Brain. 2010;133(pt 12):3494-3509.
16. Husain AM, Miller PP, Carwile ST. Rem sleep behavior disorder: potential relationship to post-traumatic stress disorder. J Clin Neurophysiol. 2001;18(2):148-157.
17. Bernardy NC, Lund BC, Alexander B, et al. Increased polysedative use in veterans with posttraumatic stress disorder. Pain Med. 2014;15(7):1083-1090.
18. Lam SP, Fong SY, Ho CK, et al. Parasomnia among psychiatric outpatients: a clinical, epidemiologic, cross-sectional study. J Clin Psychiatry. 2008;69(9):1374-1382.
19. Freeman TW, Roca V, Kimbrell T. A survey of gun collection and use among three groups of veteran patients admitted to veterans affairs hospital treatment programs. South Med J. 2003;96(3):240-243.
1. Howell MJ. Parasomnias: an updated review. Neurotherapeutics. 2012;9(4):753-775.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Zadra A, Desautels A, Petit D, et al. Somnambulism: clinical aspects and pathophysiological hypotheses. Lancet Neurol. 2013;12(3):285-294.
4. Chopra A, Selim B, Silber MH, et al. Para-suicidal amnestic behavior associated with chronic zolpidem use: implications for patient safety. Psychosomatics. 2013;54(5):498-501.
5. Hwang TJ, Ni HC, Chen HC, et al. Risk predictors for hypnosedative-related complex sleep behaviors: a retrospective, cross-sectional pilot study. J Clin Psychiatry. 2010;71(10):1331-1335.
6. Shatkin JP, Feinfield K, Strober M. The misinterpretation of a non-REM sleep parasomnia as suicidal behavior in an adolescent. Sleep Breath. 2002;6(4):175-179.
7. Mahowald MW, Schenck CH, Goldner M, et al. Parasomnia pseudo-suicide. J Forensic Sci. 2003;48(5):1158-1162.
8. Gibson CE, Caplan JP. Zolpidem-associated parasomnia with serious self-injury: a shot in the dark. Psychosomatics. 2011;52(1):88-91.
9. Mortaz Hejri S, Faizi M, Babaeian M. Zolpidem-induced suicide attempt: a case report. Daru. 2013;20;21(1):77.
10. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
11. Hesse LM, von Moltke LL, Greenblatt DJ. Clinically important drug interactions with zopiclone, zolpidem and zaleplon. CNS Drugs. 2003;17(7):513-532.
12. Catterson ML, Preskorn SH. Pharmacokinetics of selective serotonin reuptake inhibitors: clinical relevance. Pharmacol Toxicol. 1996;78(4):203-208.
13. Rosenberg RP, Hull SG, Lankford DA, et al. A randomized, double-blind, single-dose, placebo-controlled, multicenter, polysomnographic study of gabapentin in transient insomnia induced by sleep phase advance. J Clin Sleep Med. 2014;10(10):1093-1100.
14. Kato T, Montplaisir JY, Lavigne GJ. Experimentally induced arousals during sleep: a cross-modality matching paradigm. J Sleep Res. 2004;13(3):229-238.
15. Siclari F, Khatami R, Urbaniok F, et al. Violence in sleep. Brain. 2010;133(pt 12):3494-3509.
16. Husain AM, Miller PP, Carwile ST. Rem sleep behavior disorder: potential relationship to post-traumatic stress disorder. J Clin Neurophysiol. 2001;18(2):148-157.
17. Bernardy NC, Lund BC, Alexander B, et al. Increased polysedative use in veterans with posttraumatic stress disorder. Pain Med. 2014;15(7):1083-1090.
18. Lam SP, Fong SY, Ho CK, et al. Parasomnia among psychiatric outpatients: a clinical, epidemiologic, cross-sectional study. J Clin Psychiatry. 2008;69(9):1374-1382.
19. Freeman TW, Roca V, Kimbrell T. A survey of gun collection and use among three groups of veteran patients admitted to veterans affairs hospital treatment programs. South Med J. 2003;96(3):240-243.
Evaluating and monitoring drug and alcohol use during child custody disputes
Alcohol or drug use is frequently reported as a factor in divorce; 10.6% of divorcing couples list it as a precipitant for the marriage dissolution, surpassed by infidelity (21.6%) and incompatibility (19.2%).1 An effective drug and alcohol evaluation and monitoring plan during a child custody dispute safeguards the well-being of the minor children and protects—as much as possible—the parenting time of drug- or alcohol-involved parents. The evaluation maneuvers discussed in this article most likely will produce a complete, fair, and transparent evaluation and monitoring plan.
An evaluator—usually a clinician trained in diagnosing and treating a substance use disorder (SUD) and other psychiatric illnesses—performs a comprehensive alcohol/drug evaluation, prepares a monitoring program, or both. The evaluation and monitoring plan should be fair and transparent to all parties. Specific evaluation maneuvers, such as forensic-quality testing, detailed interviews with collateral informants, and ongoing collaboration with attorneys, are likely to yield a thorough evaluation and an effective and fair monitoring program. The evaluating clinician should strive for objectivity, accuracy, and practical workability when constructing these reports and monitoring plans. However, the evaluator should—in most cases—not provide treatment because this likely would represent a boundary violation between clinical treatment and forensic evaluation.
Addiction-specific evaluation maneuvers
As in all forensic matters, the evaluator’s report must answer the court’s “psycho-legal question as objectively as possible”2 rather than benefit the subject of that report. (Describing the individual being examined as the “subject” rather than “patient” emphasizes the forensic rather than clinical nature of the evaluation and the absence of a doctor–patient relationship.) Similarly, a monitoring program for drug/alcohol use should be designed to flag use of banned substances and protect the well-being of the minor child, not the parents.
Acting more as a detective than a clinician, the evaluator should maintain a skeptical—although not cynical or disrespectful—attitude when interviewing individuals who might have knowledge of the subject’s drug or alcohol use, including friends, co-workers, therapists, physicians, and even the soon-to-be-ex spouse. These collateral informants will have their own preferences or loyalties, and the examining clinician must consider these biases in the final report. A spouse often is biased and could exaggerate, emphasize, or invent addictive behaviors committed by the subject.
Collaboration among attorneys and evaluators/monitors
A strong collaboration between the judge and the attorney requesting a drug/alcohol evaluation or monitoring plan likely will result in a better outcome. This collaboration must begin with a clear delineation of the report’s purpose:
- Is the court appointing the evaluator to help gauge a drug/alcohol-involved parent’s ongoing ability to care for a child?
- Is an attorney looking for advice on how to best present the matter to the court?
- Is the evaluator expected to present and maintain a position in a court proceeding against another evaluator in a “battle of the experts?”
- Is the evaluator to consider only drug use? Only illicit drug use?
- Is the subject banned from using the substance at all times or just when she (he) is caring for the child?
A clear understanding of the evaluator’s mission is important, in part because the subject must fully comprehend the plan to consent to having the results disseminated.
To foster an effective collaboration with legal personnel the evaluator should frame the final report, testimony, and monitoring plan using clinical rather than colloquial language. To best describe the subject’s situation, diagnosis, and likely prognosis, these clinical terms often will require explanation or clarification. For example, urine drug screens (UDS) should be described as “positive for the cocaine metabolite benzoylecgonine” rather than “dirty,” and the subject might be described as “meeting criteria for alcohol use disorder” rather than an “alcoholic” or “abuser.” Using DSM-5 terminology allows for a respectful, reasonably reproducible diagnostic assessment that promotes civil discussion about disagreements, rather than name-calling in the courtroom. Professional third-party evaluation and monitoring programs in custody dispute proceedings can de-escalate the tension between the parents around issues of substance use. The conversation becomes professional, dispassionate, and focused on the best interests of the child.
Use of appropriate language allows the evaluator to expand the parameters of the report or recommend an expansion of it. If a drug/alcohol evaluation finds a relevant mental illness—in addition to a SUD—or finds another caregiver who seems incompetent, the evaluator might be professionally obligated to bring up these points, even if they are outside the purview of the requested report and monitoring plan.
Planning a monitoring program
If the evaluation determines a monitoring plan is indicated and the court orders a testing program, the evaluator must design a program that accomplishes the specific goals established by the court order. The evaluator might help the court draft that plan, but the evaluator must accommodate the final court order. Table 2 lists vital aspects of a monitoring program in a child custody dispute.
Describe goals. A court-ordered monitoring program includes:
- a clear description of goals
- what specific substances are being tested for
- how and when they are being tested for
- who pays for the testing
- what will happen after a positive or missed test.
The situation will determine whether random, scheduled, or for-cause testing is indicated.
A frequent sticking point is the decision as to whether an individual can use alcohol or other substances while he (she) is not caring for the child. A person who does not meet criteria for a SUD could argue that abstinence from alcohol or any sort of testing is unwarranted when another person is taking care of the child. The evaluator should provide input, but the court will determine the outcome.
Develop a testing program. The evaluator should develop a testing program that accomplishes the goals set out by the court, usually to protect the child from possible harm caused by a parent who uses alcohol or drugs. However, this narrow goal often is expanded to allow testing for drugs/alcohol at all times, because the parent’s slip or relapse could harm the child in the long or short term.
Describe consequences. A carefully structured definition of the consequences of a positive or missed test is an important aspect of the monitoring program. In protecting the best interests of the child, the consequences usually include the immediate transfer of the child to a safe environment. This often involves the person who receives the positive test result—usually with a physician monitoring the testing—notifying the other parent or the other parent’s attorney of the positive test result.
Testing
Although an important part of evaluation and monitoring, drug and alcohol testing alone does not diagnose a SUD or even misuse.3 Adults often use alcohol with no consequence to their children, and illicit drug use is not a prima facie bar to parenthood or taking care of a child. Also, the results of a thorough alcohol or drug evaluation cannot determine the ideal custody arrangement. The court’s final decision is based on a more wide-ranging evaluation of the family system as a whole, with the drug/alcohol issue as 1 component. In addition, the court could use the results of a forensic examiner’s assessment to advocate or mandate the appropriate treatment.
With that caveat, the specific tests used and the timing of those tests are important in the context of a child custody dispute. Once the parties have agreed on the time frame of the testing (ongoing or only during visits with the child), the specific substances that are tested for must be listed. Forensic quality testing—often called “employment testing” in clinical laboratories—decreases but does not eliminate the possibility of evasion of the test. Although addiction clinicians usually request a full screen for drugs of abuse for their patients, in the legal sphere, often only the problematic substances are tested for, which are listed in the court order.
UDS, the most common test, is non-invasive, although awkward and intrusive for the subject when done with the strictest “observed” protocol. Most testing protocols do not require a “directly observed” urine collection unless there is a suspicion that the testee has substituted her (his) urine for a sample from someone else. Breath testing, although similarly non-invasive, is only useful for alcohol testing and can detect use only several hours before the test.
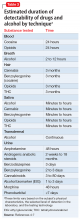
The urine test for the alcohol metabolites ethyl glucuronide (EtG) and ethyl sulfate (EtS) points toward alcohol use in the previous 3 days, but the test is plagued with false-positives at the lower cutoff values.8 EtG can be accurately assayed in human hair.9
Other tests. Dried blood spot testing for phosphatidylethanol is accurate in finding moderate to heavy alcohol use up to 3 weeks before the test.10 Saliva tests also can be useful for point-of-service testing, but the dearth of studies for this methodology makes it less useful in a courtroom setting. Newer technologies using handheld breathalyzers connected to a device with facial recognition software11,12 allow for random and “for-cause” alcohol testing, and can be useful in child custody negotiations. Hair sample testing, which can detect drug use over the 3 months before the test, is becoming more acceptable in the legal setting. However, hair testing cannot identify drug use 7 to 10 days before the test and does not test for alcohol13; and some questions remain regarding its reliability for different ethnic groups.14
Table 4 summarizes some of the most productive testing methods for child custody disputes. Selecting the best tissue, method, and timing for testing will depend on the clinical scenario, as well as the court’s requirements. For example, negotiations between parties could result in a testing protocol that uses both random and for-cause testing of urine, breath, and hair to prove that the individual does not use any illicit substances. In a less serious clinical circumstance—or less contentious legal situation—the testing protocol may necessitate only occasional UDS to make sure that the subject is not using prohibited substances.
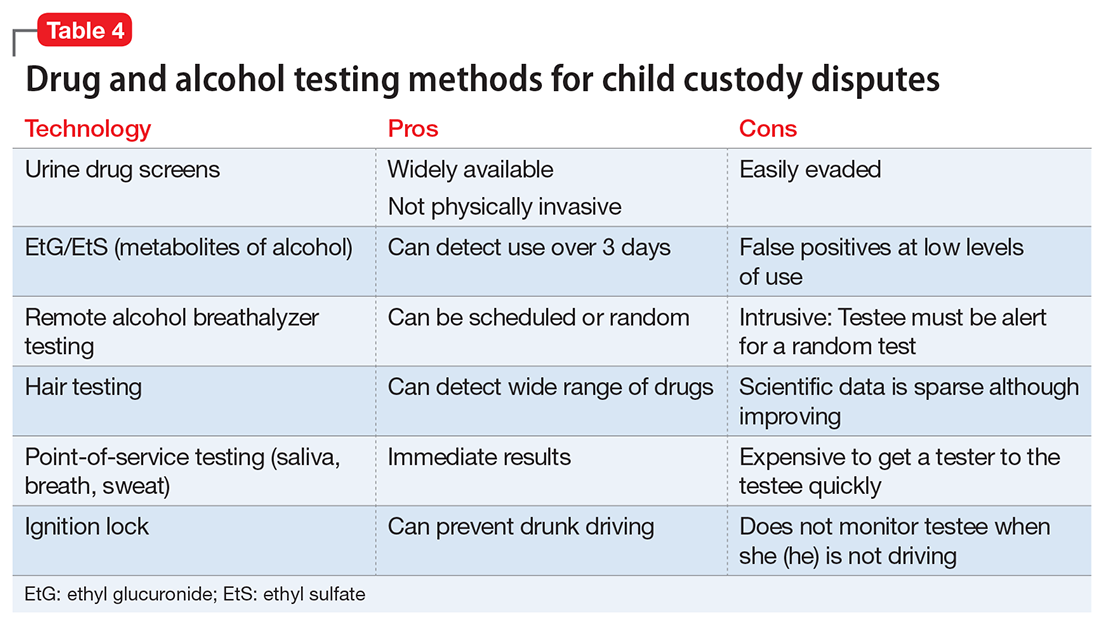
Practical considerations
It is important to remember that drug/alcohol evaluation and testing does not provide a clear-cut answer in child custody proceedings. Any drug or alcohol use must be evaluated under the standard used in child custody disputes: “the best interests of the child.” However, what is in the child’s best interests can be disputed in a courtroom. One California judge understood this as a process to identify the parent who can best provide the child with “… the ethical, emotional, and intellectual guidance the parent gives the child throughout his formative years, and beyond ….”15 However, in determining child custody the need for assuring the child’s physical and emotional safety overrules these long-term goals, and the parents’ emotional needs are disregarded. In a custody dispute, the conflict between parents vying for custody of their child is matched by a corresponding tension between the state’s interest in protecting a minor child while preserving an adult’s right to parent her child.
The Montana custody dispute described in Stout v Stout16 demonstrates some aspects typical of these cases. In deciding to grant custody of a then 3-year-old girl to the father, the presiding judge noted that, although the mother had completed an inpatient alcohol treatment program, her apparent unwillingness or inability to stop drinking or enroll in outpatient treatment, combined with a driving under the influence arrest while the child was in her care, were too worrisome to allow her to have physical custody of the child. The judge mentioned other factors that supported granting custody to the father, but a deciding factor was that “the evidence shows that her drinking adversely affects her parenting ability.” The judge’s ruling demonstrates his judgment in balancing the mother’s legal but harmful alcohol use with potential catastrophic effects for the child.
Although a thorough drug/alcohol evaluation, an evidence-based set of treatment recommendations, and a well-planned monitoring program all promote progress in a child custody dispute, the reality is that the clinical situation could change and all 3 aspects would have to be modified.
Manualized diagnostic rubrics and formal psychological testing, although often used in general forensic assessments, usually are not central to the drug/alcohol evaluation in a child custody dispute,17 because confirming a SUD diagnosis might not be relevant to the task of attending to the child’s best interest. Rather, the danger—or potential danger—of the subject’s substance use to the minor child is paramount, regardless of the diagnosis. Of course, an established diagnosis of a SUD might be relevant to the parent being examined, and might necessitate modifications in the testing protocol, the tissues examined, and the monitor’s overall level of skepticism about testing results.
The evaluator and monitor should be prepared to respond quickly to a slip or relapse, while remaining vigilant for exaggerated, inaccurate, or even deceitful accusations about the subject from the co-parent or others. The evaluator should assess all the relevant sources of information when performing an evaluation and use careful interviewing and testing techniques during the monitoring process. Even with this sort of deliberate evaluation and monitoring the evaluator should never assert that any testing regimen is incapable of error, and always keep in mind that the primary goal—and presumably the interest of all parties involved—is to protect the child’s well-being.
1. Amato PT, Previti D. People’s reasons for divorcing: gender, social class, the life course, and adjustment. J Fam Issues. 2003;24(5):602-606.
2. Glancy GD, Ash P, Bath EP, et al. AAPL practice guideline for the forensic assessment. J Am Acad Psychiatry Law. 2015;43(suppl 2):S3-S53.
3. Center for Substance Abuse Treatment. Drug testing in child welfare: practice and policy considerations. HHS Pub. No. (SMA) 10-4556. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2010.
4. Macdonald DI, DuPont RL. The role of the medical review officer. In: Graham AW, Schultz TK, eds. Principles of addiction medicine, 2nd ed. Chevy Chase, MD: American Society of Addiction Medicine; 1998:1259.
5. Swotinsky RB. The medical review officer’s manual: MROCC’s guide to drug testing. 5th ed. Beverly Farms, MA: OEM Health Information; 2015.
6. Marques PR, McKnight AS. Evaluating transdermal alcohol measuring devices. Calverton, MD: Pacific Institute for Research and Evaluation; 2007.
7. Steroidal.com. How steroid drug testing works. https://www.steroidal.com/steroid-detection-times. Accessed March 8, 2017.
8. Substance Abuse and Mental Health Services Administration. The role of biomarkers in the treatment of alcohol use disorders, 2012 revision. SAMHSA Advisory. 2012;11(2):1-8.
9. United States Drug Testing Laboratories, Inc. Detection of the direct alcohol biomarker ethyl glucuronide (EtG) in hair: an annotated bibliography. http://www.usdtl.com/media/white-papers/ETG_hair_annotated_bibliography_032014.pdf. Accessed March 8, 2017.
10. Viel G, Boscolo-Berto R, Cecchetto G, et al. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13(11):14788-14812.
11. SoberLink. https://www.soberlink.com. Accessed March 8, 2017.
12. Scram Systems. https://www.scramsystems.com/products/scram-continuous-alcohol-monitoring/?gclid=CIqUr8Kqx9ICFZmCswodI0QKPA. Accessed March 8, 2017.
13. Swotinsky RB. The medical review officer’s manual: MROCC’s guide to drug testing. 5th ed. Beverly Farms, MA: OEM Health Information; 2015:208.
14. Chamberlain RT. Legal review for testing of drugs in hair. Forensic Sci Rev. 2007;19(1-2):85-94.
15. Marriage of Carney, 24 Cal 3d725,157 Cal Rptr 383 (1979).
16. Marriage of Stout, 216 Mont 342 (Mont 1985).
17. Hynan DJ. Child custody evaluation, new theoretical applications and research. In: Hynan DJ. Difficult evaluation challenges: domestic violence, child abuse, substance abuse, and relocations. Springfield, IL: Charles C. Thomas Publisher; 2014:178-195.
Alcohol or drug use is frequently reported as a factor in divorce; 10.6% of divorcing couples list it as a precipitant for the marriage dissolution, surpassed by infidelity (21.6%) and incompatibility (19.2%).1 An effective drug and alcohol evaluation and monitoring plan during a child custody dispute safeguards the well-being of the minor children and protects—as much as possible—the parenting time of drug- or alcohol-involved parents. The evaluation maneuvers discussed in this article most likely will produce a complete, fair, and transparent evaluation and monitoring plan.
An evaluator—usually a clinician trained in diagnosing and treating a substance use disorder (SUD) and other psychiatric illnesses—performs a comprehensive alcohol/drug evaluation, prepares a monitoring program, or both. The evaluation and monitoring plan should be fair and transparent to all parties. Specific evaluation maneuvers, such as forensic-quality testing, detailed interviews with collateral informants, and ongoing collaboration with attorneys, are likely to yield a thorough evaluation and an effective and fair monitoring program. The evaluating clinician should strive for objectivity, accuracy, and practical workability when constructing these reports and monitoring plans. However, the evaluator should—in most cases—not provide treatment because this likely would represent a boundary violation between clinical treatment and forensic evaluation.
Addiction-specific evaluation maneuvers
As in all forensic matters, the evaluator’s report must answer the court’s “psycho-legal question as objectively as possible”2 rather than benefit the subject of that report. (Describing the individual being examined as the “subject” rather than “patient” emphasizes the forensic rather than clinical nature of the evaluation and the absence of a doctor–patient relationship.) Similarly, a monitoring program for drug/alcohol use should be designed to flag use of banned substances and protect the well-being of the minor child, not the parents.
Acting more as a detective than a clinician, the evaluator should maintain a skeptical—although not cynical or disrespectful—attitude when interviewing individuals who might have knowledge of the subject’s drug or alcohol use, including friends, co-workers, therapists, physicians, and even the soon-to-be-ex spouse. These collateral informants will have their own preferences or loyalties, and the examining clinician must consider these biases in the final report. A spouse often is biased and could exaggerate, emphasize, or invent addictive behaviors committed by the subject.
Collaboration among attorneys and evaluators/monitors
A strong collaboration between the judge and the attorney requesting a drug/alcohol evaluation or monitoring plan likely will result in a better outcome. This collaboration must begin with a clear delineation of the report’s purpose:
- Is the court appointing the evaluator to help gauge a drug/alcohol-involved parent’s ongoing ability to care for a child?
- Is an attorney looking for advice on how to best present the matter to the court?
- Is the evaluator expected to present and maintain a position in a court proceeding against another evaluator in a “battle of the experts?”
- Is the evaluator to consider only drug use? Only illicit drug use?
- Is the subject banned from using the substance at all times or just when she (he) is caring for the child?
A clear understanding of the evaluator’s mission is important, in part because the subject must fully comprehend the plan to consent to having the results disseminated.
To foster an effective collaboration with legal personnel the evaluator should frame the final report, testimony, and monitoring plan using clinical rather than colloquial language. To best describe the subject’s situation, diagnosis, and likely prognosis, these clinical terms often will require explanation or clarification. For example, urine drug screens (UDS) should be described as “positive for the cocaine metabolite benzoylecgonine” rather than “dirty,” and the subject might be described as “meeting criteria for alcohol use disorder” rather than an “alcoholic” or “abuser.” Using DSM-5 terminology allows for a respectful, reasonably reproducible diagnostic assessment that promotes civil discussion about disagreements, rather than name-calling in the courtroom. Professional third-party evaluation and monitoring programs in custody dispute proceedings can de-escalate the tension between the parents around issues of substance use. The conversation becomes professional, dispassionate, and focused on the best interests of the child.
Use of appropriate language allows the evaluator to expand the parameters of the report or recommend an expansion of it. If a drug/alcohol evaluation finds a relevant mental illness—in addition to a SUD—or finds another caregiver who seems incompetent, the evaluator might be professionally obligated to bring up these points, even if they are outside the purview of the requested report and monitoring plan.
Planning a monitoring program
If the evaluation determines a monitoring plan is indicated and the court orders a testing program, the evaluator must design a program that accomplishes the specific goals established by the court order. The evaluator might help the court draft that plan, but the evaluator must accommodate the final court order. Table 2 lists vital aspects of a monitoring program in a child custody dispute.
Describe goals. A court-ordered monitoring program includes:
- a clear description of goals
- what specific substances are being tested for
- how and when they are being tested for
- who pays for the testing
- what will happen after a positive or missed test.
The situation will determine whether random, scheduled, or for-cause testing is indicated.
A frequent sticking point is the decision as to whether an individual can use alcohol or other substances while he (she) is not caring for the child. A person who does not meet criteria for a SUD could argue that abstinence from alcohol or any sort of testing is unwarranted when another person is taking care of the child. The evaluator should provide input, but the court will determine the outcome.
Develop a testing program. The evaluator should develop a testing program that accomplishes the goals set out by the court, usually to protect the child from possible harm caused by a parent who uses alcohol or drugs. However, this narrow goal often is expanded to allow testing for drugs/alcohol at all times, because the parent’s slip or relapse could harm the child in the long or short term.
Describe consequences. A carefully structured definition of the consequences of a positive or missed test is an important aspect of the monitoring program. In protecting the best interests of the child, the consequences usually include the immediate transfer of the child to a safe environment. This often involves the person who receives the positive test result—usually with a physician monitoring the testing—notifying the other parent or the other parent’s attorney of the positive test result.
Testing
Although an important part of evaluation and monitoring, drug and alcohol testing alone does not diagnose a SUD or even misuse.3 Adults often use alcohol with no consequence to their children, and illicit drug use is not a prima facie bar to parenthood or taking care of a child. Also, the results of a thorough alcohol or drug evaluation cannot determine the ideal custody arrangement. The court’s final decision is based on a more wide-ranging evaluation of the family system as a whole, with the drug/alcohol issue as 1 component. In addition, the court could use the results of a forensic examiner’s assessment to advocate or mandate the appropriate treatment.
With that caveat, the specific tests used and the timing of those tests are important in the context of a child custody dispute. Once the parties have agreed on the time frame of the testing (ongoing or only during visits with the child), the specific substances that are tested for must be listed. Forensic quality testing—often called “employment testing” in clinical laboratories—decreases but does not eliminate the possibility of evasion of the test. Although addiction clinicians usually request a full screen for drugs of abuse for their patients, in the legal sphere, often only the problematic substances are tested for, which are listed in the court order.
UDS, the most common test, is non-invasive, although awkward and intrusive for the subject when done with the strictest “observed” protocol. Most testing protocols do not require a “directly observed” urine collection unless there is a suspicion that the testee has substituted her (his) urine for a sample from someone else. Breath testing, although similarly non-invasive, is only useful for alcohol testing and can detect use only several hours before the test.
The urine test for the alcohol metabolites ethyl glucuronide (EtG) and ethyl sulfate (EtS) points toward alcohol use in the previous 3 days, but the test is plagued with false-positives at the lower cutoff values.8 EtG can be accurately assayed in human hair.9
Other tests. Dried blood spot testing for phosphatidylethanol is accurate in finding moderate to heavy alcohol use up to 3 weeks before the test.10 Saliva tests also can be useful for point-of-service testing, but the dearth of studies for this methodology makes it less useful in a courtroom setting. Newer technologies using handheld breathalyzers connected to a device with facial recognition software11,12 allow for random and “for-cause” alcohol testing, and can be useful in child custody negotiations. Hair sample testing, which can detect drug use over the 3 months before the test, is becoming more acceptable in the legal setting. However, hair testing cannot identify drug use 7 to 10 days before the test and does not test for alcohol13; and some questions remain regarding its reliability for different ethnic groups.14
Table 4 summarizes some of the most productive testing methods for child custody disputes. Selecting the best tissue, method, and timing for testing will depend on the clinical scenario, as well as the court’s requirements. For example, negotiations between parties could result in a testing protocol that uses both random and for-cause testing of urine, breath, and hair to prove that the individual does not use any illicit substances. In a less serious clinical circumstance—or less contentious legal situation—the testing protocol may necessitate only occasional UDS to make sure that the subject is not using prohibited substances.

Practical considerations
It is important to remember that drug/alcohol evaluation and testing does not provide a clear-cut answer in child custody proceedings. Any drug or alcohol use must be evaluated under the standard used in child custody disputes: “the best interests of the child.” However, what is in the child’s best interests can be disputed in a courtroom. One California judge understood this as a process to identify the parent who can best provide the child with “… the ethical, emotional, and intellectual guidance the parent gives the child throughout his formative years, and beyond ….”15 However, in determining child custody the need for assuring the child’s physical and emotional safety overrules these long-term goals, and the parents’ emotional needs are disregarded. In a custody dispute, the conflict between parents vying for custody of their child is matched by a corresponding tension between the state’s interest in protecting a minor child while preserving an adult’s right to parent her child.
The Montana custody dispute described in Stout v Stout16 demonstrates some aspects typical of these cases. In deciding to grant custody of a then 3-year-old girl to the father, the presiding judge noted that, although the mother had completed an inpatient alcohol treatment program, her apparent unwillingness or inability to stop drinking or enroll in outpatient treatment, combined with a driving under the influence arrest while the child was in her care, were too worrisome to allow her to have physical custody of the child. The judge mentioned other factors that supported granting custody to the father, but a deciding factor was that “the evidence shows that her drinking adversely affects her parenting ability.” The judge’s ruling demonstrates his judgment in balancing the mother’s legal but harmful alcohol use with potential catastrophic effects for the child.
Although a thorough drug/alcohol evaluation, an evidence-based set of treatment recommendations, and a well-planned monitoring program all promote progress in a child custody dispute, the reality is that the clinical situation could change and all 3 aspects would have to be modified.
Manualized diagnostic rubrics and formal psychological testing, although often used in general forensic assessments, usually are not central to the drug/alcohol evaluation in a child custody dispute,17 because confirming a SUD diagnosis might not be relevant to the task of attending to the child’s best interest. Rather, the danger—or potential danger—of the subject’s substance use to the minor child is paramount, regardless of the diagnosis. Of course, an established diagnosis of a SUD might be relevant to the parent being examined, and might necessitate modifications in the testing protocol, the tissues examined, and the monitor’s overall level of skepticism about testing results.
The evaluator and monitor should be prepared to respond quickly to a slip or relapse, while remaining vigilant for exaggerated, inaccurate, or even deceitful accusations about the subject from the co-parent or others. The evaluator should assess all the relevant sources of information when performing an evaluation and use careful interviewing and testing techniques during the monitoring process. Even with this sort of deliberate evaluation and monitoring the evaluator should never assert that any testing regimen is incapable of error, and always keep in mind that the primary goal—and presumably the interest of all parties involved—is to protect the child’s well-being.
Alcohol or drug use is frequently reported as a factor in divorce; 10.6% of divorcing couples list it as a precipitant for the marriage dissolution, surpassed by infidelity (21.6%) and incompatibility (19.2%).1 An effective drug and alcohol evaluation and monitoring plan during a child custody dispute safeguards the well-being of the minor children and protects—as much as possible—the parenting time of drug- or alcohol-involved parents. The evaluation maneuvers discussed in this article most likely will produce a complete, fair, and transparent evaluation and monitoring plan.
An evaluator—usually a clinician trained in diagnosing and treating a substance use disorder (SUD) and other psychiatric illnesses—performs a comprehensive alcohol/drug evaluation, prepares a monitoring program, or both. The evaluation and monitoring plan should be fair and transparent to all parties. Specific evaluation maneuvers, such as forensic-quality testing, detailed interviews with collateral informants, and ongoing collaboration with attorneys, are likely to yield a thorough evaluation and an effective and fair monitoring program. The evaluating clinician should strive for objectivity, accuracy, and practical workability when constructing these reports and monitoring plans. However, the evaluator should—in most cases—not provide treatment because this likely would represent a boundary violation between clinical treatment and forensic evaluation.
Addiction-specific evaluation maneuvers
As in all forensic matters, the evaluator’s report must answer the court’s “psycho-legal question as objectively as possible”2 rather than benefit the subject of that report. (Describing the individual being examined as the “subject” rather than “patient” emphasizes the forensic rather than clinical nature of the evaluation and the absence of a doctor–patient relationship.) Similarly, a monitoring program for drug/alcohol use should be designed to flag use of banned substances and protect the well-being of the minor child, not the parents.
Acting more as a detective than a clinician, the evaluator should maintain a skeptical—although not cynical or disrespectful—attitude when interviewing individuals who might have knowledge of the subject’s drug or alcohol use, including friends, co-workers, therapists, physicians, and even the soon-to-be-ex spouse. These collateral informants will have their own preferences or loyalties, and the examining clinician must consider these biases in the final report. A spouse often is biased and could exaggerate, emphasize, or invent addictive behaviors committed by the subject.
Collaboration among attorneys and evaluators/monitors
A strong collaboration between the judge and the attorney requesting a drug/alcohol evaluation or monitoring plan likely will result in a better outcome. This collaboration must begin with a clear delineation of the report’s purpose:
- Is the court appointing the evaluator to help gauge a drug/alcohol-involved parent’s ongoing ability to care for a child?
- Is an attorney looking for advice on how to best present the matter to the court?
- Is the evaluator expected to present and maintain a position in a court proceeding against another evaluator in a “battle of the experts?”
- Is the evaluator to consider only drug use? Only illicit drug use?
- Is the subject banned from using the substance at all times or just when she (he) is caring for the child?
A clear understanding of the evaluator’s mission is important, in part because the subject must fully comprehend the plan to consent to having the results disseminated.
To foster an effective collaboration with legal personnel the evaluator should frame the final report, testimony, and monitoring plan using clinical rather than colloquial language. To best describe the subject’s situation, diagnosis, and likely prognosis, these clinical terms often will require explanation or clarification. For example, urine drug screens (UDS) should be described as “positive for the cocaine metabolite benzoylecgonine” rather than “dirty,” and the subject might be described as “meeting criteria for alcohol use disorder” rather than an “alcoholic” or “abuser.” Using DSM-5 terminology allows for a respectful, reasonably reproducible diagnostic assessment that promotes civil discussion about disagreements, rather than name-calling in the courtroom. Professional third-party evaluation and monitoring programs in custody dispute proceedings can de-escalate the tension between the parents around issues of substance use. The conversation becomes professional, dispassionate, and focused on the best interests of the child.
Use of appropriate language allows the evaluator to expand the parameters of the report or recommend an expansion of it. If a drug/alcohol evaluation finds a relevant mental illness—in addition to a SUD—or finds another caregiver who seems incompetent, the evaluator might be professionally obligated to bring up these points, even if they are outside the purview of the requested report and monitoring plan.
Planning a monitoring program
If the evaluation determines a monitoring plan is indicated and the court orders a testing program, the evaluator must design a program that accomplishes the specific goals established by the court order. The evaluator might help the court draft that plan, but the evaluator must accommodate the final court order. Table 2 lists vital aspects of a monitoring program in a child custody dispute.
Describe goals. A court-ordered monitoring program includes:
- a clear description of goals
- what specific substances are being tested for
- how and when they are being tested for
- who pays for the testing
- what will happen after a positive or missed test.
The situation will determine whether random, scheduled, or for-cause testing is indicated.
A frequent sticking point is the decision as to whether an individual can use alcohol or other substances while he (she) is not caring for the child. A person who does not meet criteria for a SUD could argue that abstinence from alcohol or any sort of testing is unwarranted when another person is taking care of the child. The evaluator should provide input, but the court will determine the outcome.
Develop a testing program. The evaluator should develop a testing program that accomplishes the goals set out by the court, usually to protect the child from possible harm caused by a parent who uses alcohol or drugs. However, this narrow goal often is expanded to allow testing for drugs/alcohol at all times, because the parent’s slip or relapse could harm the child in the long or short term.
Describe consequences. A carefully structured definition of the consequences of a positive or missed test is an important aspect of the monitoring program. In protecting the best interests of the child, the consequences usually include the immediate transfer of the child to a safe environment. This often involves the person who receives the positive test result—usually with a physician monitoring the testing—notifying the other parent or the other parent’s attorney of the positive test result.
Testing
Although an important part of evaluation and monitoring, drug and alcohol testing alone does not diagnose a SUD or even misuse.3 Adults often use alcohol with no consequence to their children, and illicit drug use is not a prima facie bar to parenthood or taking care of a child. Also, the results of a thorough alcohol or drug evaluation cannot determine the ideal custody arrangement. The court’s final decision is based on a more wide-ranging evaluation of the family system as a whole, with the drug/alcohol issue as 1 component. In addition, the court could use the results of a forensic examiner’s assessment to advocate or mandate the appropriate treatment.
With that caveat, the specific tests used and the timing of those tests are important in the context of a child custody dispute. Once the parties have agreed on the time frame of the testing (ongoing or only during visits with the child), the specific substances that are tested for must be listed. Forensic quality testing—often called “employment testing” in clinical laboratories—decreases but does not eliminate the possibility of evasion of the test. Although addiction clinicians usually request a full screen for drugs of abuse for their patients, in the legal sphere, often only the problematic substances are tested for, which are listed in the court order.
UDS, the most common test, is non-invasive, although awkward and intrusive for the subject when done with the strictest “observed” protocol. Most testing protocols do not require a “directly observed” urine collection unless there is a suspicion that the testee has substituted her (his) urine for a sample from someone else. Breath testing, although similarly non-invasive, is only useful for alcohol testing and can detect use only several hours before the test.
The urine test for the alcohol metabolites ethyl glucuronide (EtG) and ethyl sulfate (EtS) points toward alcohol use in the previous 3 days, but the test is plagued with false-positives at the lower cutoff values.8 EtG can be accurately assayed in human hair.9
Other tests. Dried blood spot testing for phosphatidylethanol is accurate in finding moderate to heavy alcohol use up to 3 weeks before the test.10 Saliva tests also can be useful for point-of-service testing, but the dearth of studies for this methodology makes it less useful in a courtroom setting. Newer technologies using handheld breathalyzers connected to a device with facial recognition software11,12 allow for random and “for-cause” alcohol testing, and can be useful in child custody negotiations. Hair sample testing, which can detect drug use over the 3 months before the test, is becoming more acceptable in the legal setting. However, hair testing cannot identify drug use 7 to 10 days before the test and does not test for alcohol13; and some questions remain regarding its reliability for different ethnic groups.14
Table 4 summarizes some of the most productive testing methods for child custody disputes. Selecting the best tissue, method, and timing for testing will depend on the clinical scenario, as well as the court’s requirements. For example, negotiations between parties could result in a testing protocol that uses both random and for-cause testing of urine, breath, and hair to prove that the individual does not use any illicit substances. In a less serious clinical circumstance—or less contentious legal situation—the testing protocol may necessitate only occasional UDS to make sure that the subject is not using prohibited substances.

Practical considerations
It is important to remember that drug/alcohol evaluation and testing does not provide a clear-cut answer in child custody proceedings. Any drug or alcohol use must be evaluated under the standard used in child custody disputes: “the best interests of the child.” However, what is in the child’s best interests can be disputed in a courtroom. One California judge understood this as a process to identify the parent who can best provide the child with “… the ethical, emotional, and intellectual guidance the parent gives the child throughout his formative years, and beyond ….”15 However, in determining child custody the need for assuring the child’s physical and emotional safety overrules these long-term goals, and the parents’ emotional needs are disregarded. In a custody dispute, the conflict between parents vying for custody of their child is matched by a corresponding tension between the state’s interest in protecting a minor child while preserving an adult’s right to parent her child.
The Montana custody dispute described in Stout v Stout16 demonstrates some aspects typical of these cases. In deciding to grant custody of a then 3-year-old girl to the father, the presiding judge noted that, although the mother had completed an inpatient alcohol treatment program, her apparent unwillingness or inability to stop drinking or enroll in outpatient treatment, combined with a driving under the influence arrest while the child was in her care, were too worrisome to allow her to have physical custody of the child. The judge mentioned other factors that supported granting custody to the father, but a deciding factor was that “the evidence shows that her drinking adversely affects her parenting ability.” The judge’s ruling demonstrates his judgment in balancing the mother’s legal but harmful alcohol use with potential catastrophic effects for the child.
Although a thorough drug/alcohol evaluation, an evidence-based set of treatment recommendations, and a well-planned monitoring program all promote progress in a child custody dispute, the reality is that the clinical situation could change and all 3 aspects would have to be modified.
Manualized diagnostic rubrics and formal psychological testing, although often used in general forensic assessments, usually are not central to the drug/alcohol evaluation in a child custody dispute,17 because confirming a SUD diagnosis might not be relevant to the task of attending to the child’s best interest. Rather, the danger—or potential danger—of the subject’s substance use to the minor child is paramount, regardless of the diagnosis. Of course, an established diagnosis of a SUD might be relevant to the parent being examined, and might necessitate modifications in the testing protocol, the tissues examined, and the monitor’s overall level of skepticism about testing results.
The evaluator and monitor should be prepared to respond quickly to a slip or relapse, while remaining vigilant for exaggerated, inaccurate, or even deceitful accusations about the subject from the co-parent or others. The evaluator should assess all the relevant sources of information when performing an evaluation and use careful interviewing and testing techniques during the monitoring process. Even with this sort of deliberate evaluation and monitoring the evaluator should never assert that any testing regimen is incapable of error, and always keep in mind that the primary goal—and presumably the interest of all parties involved—is to protect the child’s well-being.
1. Amato PT, Previti D. People’s reasons for divorcing: gender, social class, the life course, and adjustment. J Fam Issues. 2003;24(5):602-606.
2. Glancy GD, Ash P, Bath EP, et al. AAPL practice guideline for the forensic assessment. J Am Acad Psychiatry Law. 2015;43(suppl 2):S3-S53.
3. Center for Substance Abuse Treatment. Drug testing in child welfare: practice and policy considerations. HHS Pub. No. (SMA) 10-4556. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2010.
4. Macdonald DI, DuPont RL. The role of the medical review officer. In: Graham AW, Schultz TK, eds. Principles of addiction medicine, 2nd ed. Chevy Chase, MD: American Society of Addiction Medicine; 1998:1259.
5. Swotinsky RB. The medical review officer’s manual: MROCC’s guide to drug testing. 5th ed. Beverly Farms, MA: OEM Health Information; 2015.
6. Marques PR, McKnight AS. Evaluating transdermal alcohol measuring devices. Calverton, MD: Pacific Institute for Research and Evaluation; 2007.
7. Steroidal.com. How steroid drug testing works. https://www.steroidal.com/steroid-detection-times. Accessed March 8, 2017.
8. Substance Abuse and Mental Health Services Administration. The role of biomarkers in the treatment of alcohol use disorders, 2012 revision. SAMHSA Advisory. 2012;11(2):1-8.
9. United States Drug Testing Laboratories, Inc. Detection of the direct alcohol biomarker ethyl glucuronide (EtG) in hair: an annotated bibliography. http://www.usdtl.com/media/white-papers/ETG_hair_annotated_bibliography_032014.pdf. Accessed March 8, 2017.
10. Viel G, Boscolo-Berto R, Cecchetto G, et al. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13(11):14788-14812.
11. SoberLink. https://www.soberlink.com. Accessed March 8, 2017.
12. Scram Systems. https://www.scramsystems.com/products/scram-continuous-alcohol-monitoring/?gclid=CIqUr8Kqx9ICFZmCswodI0QKPA. Accessed March 8, 2017.
13. Swotinsky RB. The medical review officer’s manual: MROCC’s guide to drug testing. 5th ed. Beverly Farms, MA: OEM Health Information; 2015:208.
14. Chamberlain RT. Legal review for testing of drugs in hair. Forensic Sci Rev. 2007;19(1-2):85-94.
15. Marriage of Carney, 24 Cal 3d725,157 Cal Rptr 383 (1979).
16. Marriage of Stout, 216 Mont 342 (Mont 1985).
17. Hynan DJ. Child custody evaluation, new theoretical applications and research. In: Hynan DJ. Difficult evaluation challenges: domestic violence, child abuse, substance abuse, and relocations. Springfield, IL: Charles C. Thomas Publisher; 2014:178-195.
1. Amato PT, Previti D. People’s reasons for divorcing: gender, social class, the life course, and adjustment. J Fam Issues. 2003;24(5):602-606.
2. Glancy GD, Ash P, Bath EP, et al. AAPL practice guideline for the forensic assessment. J Am Acad Psychiatry Law. 2015;43(suppl 2):S3-S53.
3. Center for Substance Abuse Treatment. Drug testing in child welfare: practice and policy considerations. HHS Pub. No. (SMA) 10-4556. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2010.
4. Macdonald DI, DuPont RL. The role of the medical review officer. In: Graham AW, Schultz TK, eds. Principles of addiction medicine, 2nd ed. Chevy Chase, MD: American Society of Addiction Medicine; 1998:1259.
5. Swotinsky RB. The medical review officer’s manual: MROCC’s guide to drug testing. 5th ed. Beverly Farms, MA: OEM Health Information; 2015.
6. Marques PR, McKnight AS. Evaluating transdermal alcohol measuring devices. Calverton, MD: Pacific Institute for Research and Evaluation; 2007.
7. Steroidal.com. How steroid drug testing works. https://www.steroidal.com/steroid-detection-times. Accessed March 8, 2017.
8. Substance Abuse and Mental Health Services Administration. The role of biomarkers in the treatment of alcohol use disorders, 2012 revision. SAMHSA Advisory. 2012;11(2):1-8.
9. United States Drug Testing Laboratories, Inc. Detection of the direct alcohol biomarker ethyl glucuronide (EtG) in hair: an annotated bibliography. http://www.usdtl.com/media/white-papers/ETG_hair_annotated_bibliography_032014.pdf. Accessed March 8, 2017.
10. Viel G, Boscolo-Berto R, Cecchetto G, et al. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13(11):14788-14812.
11. SoberLink. https://www.soberlink.com. Accessed March 8, 2017.
12. Scram Systems. https://www.scramsystems.com/products/scram-continuous-alcohol-monitoring/?gclid=CIqUr8Kqx9ICFZmCswodI0QKPA. Accessed March 8, 2017.
13. Swotinsky RB. The medical review officer’s manual: MROCC’s guide to drug testing. 5th ed. Beverly Farms, MA: OEM Health Information; 2015:208.
14. Chamberlain RT. Legal review for testing of drugs in hair. Forensic Sci Rev. 2007;19(1-2):85-94.
15. Marriage of Carney, 24 Cal 3d725,157 Cal Rptr 383 (1979).
16. Marriage of Stout, 216 Mont 342 (Mont 1985).
17. Hynan DJ. Child custody evaluation, new theoretical applications and research. In: Hynan DJ. Difficult evaluation challenges: domestic violence, child abuse, substance abuse, and relocations. Springfield, IL: Charles C. Thomas Publisher; 2014:178-195.
How would repeal of the Affordable Care Act affect mental health care?
With the changing political landscape in Washington, there has been much talk about health care in the United States. The Affordable Care Act (ACA) is at risk for repeal or, at least, substantial change. As the debate heats up, many psychiatric clinicians wonder what repeal could mean for mental health care and treatment of substance use disorders.
To examine this issue, we need to understand what the ACA has accomplished so far. The Patient Protection and Affordable Care Act—known as “Obamacare”—was enacted on March 23, 2010. From 2010 to 2014, various provisions were implemented; more provisions are slated for completion by 2017 if the law remains in place. These provisions are at the heart of how those with mental illness or substance use disorders could be affected by repeal of the ACA.
Since the ACA’s implementation, an estimated 20 million Americans have gained health insurance.1 The ACA includes several provisions that made this number possible, such as the expansion of Medicaid in some states. In addition to plans offered through the Health Insurance Marketplace, private insurers are required to provide insurance to some who previously fell into non-coverage gaps.1 Young adults can remain on a parent’s plan until age 26, which is significant to mental health care because many psychiatric disorders emerge in young adulthood, and this age group is vulnerable to developing substance use disorders.
The ACA also requires private insurance plans to cover those with preexisting health conditions. This has been crucial for persons with mental illness because before the ACA, mental health disorders were the second most common preexisting condition that precipitated either an increase in the cost of a plan or coverage denial.2
These provisions have helped ensure coverage for the approximately 20% of adults in the United States who have a mental illness.3 Before the ACA, 18% of individuals who purchased their own insurance did not have mental health coverage, and more than one-third of insurers did not cover substance use disorders.4 According to the CDC, the uninsured rate for those with serious mental health disorders fell from 28.1% in 2012 to 19.5% in 2015.5 Likewise, the number of adults with mental illness who could not afford needed care decreased during the same years.5 A University of Minnesota study found that persons with mental illness are disproportionately represented among the uninsured.6 Before the ACA, 18% of individual health plans did not cover prescriptions, including those indicated for psychiatric illness.7 Simply put, the ACA has allowed people to seek assessment and treatment for mental health, whereas it would not have been as accessible before the legislation.
What does the ACA cover?
The ACA required health plans to cover Essential Health Benefits starting January 1, 2014. These include:
- medical services such as doctor visits
- emergency and urgent care services
- hospital physician and facility services
- prenatal, delivery, and postnatal care
- evaluation and treatment of mental health conditions
- services to address substance use including behavioral health treatment
- coverage of prescription medications
- rehabilitation services
- diagnostic tests and imaging
- preventive and wellness care and management of chronic diseases
- pediatric care.
As of March 2013, only 2% of existing health plans in the United States provided all of these benefits required by the ACA.7
Required coverage of mental health care and substance use disorders increases patient access to those services. Including preventive care extends the reach of mental health screening to primary care providers, who can screen for mood disorders and substance use in adults and adolescents and for autism and behavioral issues in children.8
The ACA provides further expansion and enforcement of mental health parity. In 2008, the Mental Health Parity and Addiction Equity Act was passed with the intent of providing behavioral health benefits at the same level as medical care. Although this law was beneficial in theory, it did not require insurers to cover behavioral health treatment. Rather, it only required parity if large group plans already provided behavioral health coverage; parity laws did not apply to individual or small group plans. The Essential Health Benefits of the ACA specify that insurers must provide mental health and substance use treatment. Essentially, the ACA gave the parity law teeth. The law would matter very little if low-income patients, who often suffer from mental health symptoms, have no insurance coverage.
Perhaps more concerning are the implications for those battling substance use disorders. If the ACA is repealed without appropriate replacement measures, it is unclear how those with limited income or preexisting substance use disorders would access evidence-based treatment.
Opioid use disorder affects >2 million individuals in the United States and caused 33,000 overdose deaths in 2015.9 The Opioid Initiative, established in 2015 by the U.S. Department of Health and Human Services (HHS), has worked to improve prescribing practices, increase use of naloxone to treat overdose, and expand access to medication-assisted treatment and psychosocial support. The success of this initiative relies on accessible health insurance coverage. Medication-assisted treatment and psychosocial support services would be threatened most by repeal of the ACA.9 In 2016, the HHS provided $94 million in grants, through the ACA, for free clinics to screen and treat patients for substance use disorders.10 Continued funding for these programs would be jeopardized if the ACA was repealed without replacement.
Repair rather than replace
The ACA is not without its flaws, but perhaps the best approach is to build on its successes while repairing its weaknesses. Researcher Peter Phalen, MA, looked at changes in rates, usage, affordability, and satisfaction with services for those with moderate and severe mental illness after implementation of the ACA.11 Using a nationally representative sample (N = 35,602), he discovered that those with moderate mental illness, as measured by psychological distress scales, experienced greater gains in finding affordable coverage than those without mental illness.11 However, individuals with severe mental illness showed no improvement on these measures, with the exception of increased satisfaction with current coverage and care. There were no reported increases in health care use or affordability for either group.11
Although the ACA requires prescription coverage, there is no regulation of what insurers choose to include in their formularies, and often brand name drugs, particularly antipsychotics, are not covered. The National Alliance on Mental Illness released a report in 2015 noting that, even with the ACA, individuals continue to experience difficulty accessing behavioral health providers in a timely manner, especially in rural areas. The report also described a lack of parity enforcement for behavioral health coverage.12
What if?
If the ACA is repealed, other legislative acts could continue, in some way, to address the needs of those with mental illness. The 21st Century Cures Act, which has bipartisan support, was passed in 2016 in the hope of reforming national mental health care. The American Psychiatric Association (APA) president, Maria Oquendo, MD, PhD, indicated that the bill enhances parity laws and provides better coordination for national agencies involved in treating psychiatric illness.13 The APA applauded this effort and highlighted these provisions:
- reauthorizing grants to support integrated care models
- reauthorizing grants to train school staff to identify students who need mental health care
- requiring the HHS to develop a plan to enforce parity laws
- providing $1 billion in state grants to address the opioid epidemic.13
The APA has voiced its concern about repealing the ACA without replacement. The APA issued a letter to Congressional leadership stating the organization’s concerns, emphasizing that current law has eased the burden for Americans to access “appropriate and evidence-based mental health care.”14 The APA requested that, in considering reforms to health care law, Congress does not “undo the gains which have been made over the past several years for individuals with mental illness.”14 The APA noted that the proposed ACA replacement bill, released on March 3, 2017, would “negatively impact care for people with mental illness and substance use disorders.”15
Since the ACA was implemented, we have taken for granted many provisions as permanent fixtures of our nation’s health care system. Who now can imagine a denial of coverage for a preexisting condition? How many young adults are ready to purchase their own insurance plans immediately after high school or college if employment is not readily available? Is it reasonable that an insurance plan does not provide prescription coverage or behavioral health services? How will those with mental illness or substance use disorders have reliable access to assessment and treatment?
Repealing, replacing, or enhancing the ACA is a complicated balancing act. We must be vigilant and vocal in asking Congress to continue considering the needs of those with mental illness and substance use disorders.
1. U.S. Department of Health and Human Services. 20 million people have gained health insurance coverage because of the Affordable Care Act, new estimates show. http://wayback.archive-it.org/3926/20170127190440/https://www.hhs.gov/about/news/2016/03/03/20-million-people-have-gained-health-insurance-coverage-because-affordable-care-act-new-estimates. Published March 16, 2016. Accessed February 15, 2017.
2. U.S. Department of Health and Human Services. Health insurance coverage for Americans with pre-existing conditions: the impact of the Affordable Care Act. https://aspe.hhs.gov/pdf-report/health-insurance-coverage-americans-pre-existing-conditions-impact-affordable-care-act. Published January 5, 2017. Accessed February 20, 2017.
3. National Alliance on Mental Illness. Mental health by the numbers. http://www.nami.org/Learn-More/Mental-Health-By-the-Numbers. Accessed February 20, 2017.
4. U.S. Department of Health and Human Services. Essential health benefits: individual market place. https://aspe.hhs.gov/pdf-report/essential-health-benefits-individual-market-coverage. Published December 16, 2011. Accessed February 18, 2017.
5. Cohen R, Zammitti EP. Access to care among adults aged 18-64 with serious psychological distress: early release of estimates from the National Health Interview Survey, 2012-September 2015. https://www.cdc.gov/nchs/data/nhis/earlyrelease/er_spd_access_2015_f_auer.pdf. Published May 2016. Accessed February 1, 2017.
6. Rowan K, McAlpine DD, Blewett LA. Access and cost barriers to mental health care by insurance status, 1999-2010. Health Aff (Millwood). 2013;32(10):1723-1730.
7. Health Pocket. Almost no existing health plans meet new ACA essential health benefit standards. https://www.healthpocket.com/healthcare-research/infostat/few-existing-health-plans-meet-new-aca-essential-health-benefit-standards/#.WLSSdqPMxmC. Published March 7, 2013. Accessed February 20, 2017.
8. U.S. Department of Health and Human Services. Health benefits and coverage: preventive health services. https://www.healthcare.gov/coverage/preventive-care-benefits. Accessed February 26, 2017.
9. U.S. Department of Health and Human Services. Continuing progress on the opioid epidemic: the role of the Affordable Care Act. https://aspe.hhs.gov/pdf-report/continuing-progress-opioid-epidemic-role-affordable-care-act. Published January 11, 2017. Accessed February 15, 2017.
10. U.S. Department of Health and Human Services. HHS awards 94 million to health centers help treat the prescription opioid abuse and heroin epidemic in America. http://wayback.archive-it.org/3926/20170127185615/https://www.hhs.gov/about/news/2016/03/11/hhs-awards-94-million-to-health-centers.html. Published March 11, 2016. Accessed February 1, 2017.
11. Phalen P. Psychological distress and rates of health insurance coverage and use and affordability of mental health services, 2013-2014 [published online December 15, 2016]. Psychiatr Serv. http://dx.doi.org/10.1176/appi.ps.201500544.
12. National Alliance on Mental Illness. A long road ahead: achieving true parity in mental health and substance use care. https://www.nami.org/About-NAMI/Publications-Reports/Public-Policy-Reports/A-Long-Road-Ahead/2015-ALongRoadAhead.pdf. Published April 2015. Accessed February 15, 2017.
13. American Psychiatric Association. APA commends house for approving mental health reform bill. https://www.psychiatry.org/newsroom/news-releases/apa-commends-house-for-approving-mental-health-reform-bill?_ga=1.239819267.1833283241.1466442827. Published November 30, 2016. Accessed February 26, 2017.
14. American Psychiatric Association. APA calls on Congress to protect patient access to healthcare. https://www.psychiatry.org/newsroom/news-releases/apa-calls-on-congress-to-protect-patient-access-to-health-care?_ga=1.240843011.1833283241.1466442827. Published January 5, 2017. Accessed February 15, 2017.
15. American Psychiatric Association. APA concerned about proposed ACA replacement bill. https://www.psychiatry.org/newsroom/news-releases/apa-concerned-about-proposed-aca-replacement-bill. Published March 7, 2017. Accessed March 17, 2017.
With the changing political landscape in Washington, there has been much talk about health care in the United States. The Affordable Care Act (ACA) is at risk for repeal or, at least, substantial change. As the debate heats up, many psychiatric clinicians wonder what repeal could mean for mental health care and treatment of substance use disorders.
To examine this issue, we need to understand what the ACA has accomplished so far. The Patient Protection and Affordable Care Act—known as “Obamacare”—was enacted on March 23, 2010. From 2010 to 2014, various provisions were implemented; more provisions are slated for completion by 2017 if the law remains in place. These provisions are at the heart of how those with mental illness or substance use disorders could be affected by repeal of the ACA.
Since the ACA’s implementation, an estimated 20 million Americans have gained health insurance.1 The ACA includes several provisions that made this number possible, such as the expansion of Medicaid in some states. In addition to plans offered through the Health Insurance Marketplace, private insurers are required to provide insurance to some who previously fell into non-coverage gaps.1 Young adults can remain on a parent’s plan until age 26, which is significant to mental health care because many psychiatric disorders emerge in young adulthood, and this age group is vulnerable to developing substance use disorders.
The ACA also requires private insurance plans to cover those with preexisting health conditions. This has been crucial for persons with mental illness because before the ACA, mental health disorders were the second most common preexisting condition that precipitated either an increase in the cost of a plan or coverage denial.2
These provisions have helped ensure coverage for the approximately 20% of adults in the United States who have a mental illness.3 Before the ACA, 18% of individuals who purchased their own insurance did not have mental health coverage, and more than one-third of insurers did not cover substance use disorders.4 According to the CDC, the uninsured rate for those with serious mental health disorders fell from 28.1% in 2012 to 19.5% in 2015.5 Likewise, the number of adults with mental illness who could not afford needed care decreased during the same years.5 A University of Minnesota study found that persons with mental illness are disproportionately represented among the uninsured.6 Before the ACA, 18% of individual health plans did not cover prescriptions, including those indicated for psychiatric illness.7 Simply put, the ACA has allowed people to seek assessment and treatment for mental health, whereas it would not have been as accessible before the legislation.
What does the ACA cover?
The ACA required health plans to cover Essential Health Benefits starting January 1, 2014. These include:
- medical services such as doctor visits
- emergency and urgent care services
- hospital physician and facility services
- prenatal, delivery, and postnatal care
- evaluation and treatment of mental health conditions
- services to address substance use including behavioral health treatment
- coverage of prescription medications
- rehabilitation services
- diagnostic tests and imaging
- preventive and wellness care and management of chronic diseases
- pediatric care.
As of March 2013, only 2% of existing health plans in the United States provided all of these benefits required by the ACA.7
Required coverage of mental health care and substance use disorders increases patient access to those services. Including preventive care extends the reach of mental health screening to primary care providers, who can screen for mood disorders and substance use in adults and adolescents and for autism and behavioral issues in children.8
The ACA provides further expansion and enforcement of mental health parity. In 2008, the Mental Health Parity and Addiction Equity Act was passed with the intent of providing behavioral health benefits at the same level as medical care. Although this law was beneficial in theory, it did not require insurers to cover behavioral health treatment. Rather, it only required parity if large group plans already provided behavioral health coverage; parity laws did not apply to individual or small group plans. The Essential Health Benefits of the ACA specify that insurers must provide mental health and substance use treatment. Essentially, the ACA gave the parity law teeth. The law would matter very little if low-income patients, who often suffer from mental health symptoms, have no insurance coverage.
Perhaps more concerning are the implications for those battling substance use disorders. If the ACA is repealed without appropriate replacement measures, it is unclear how those with limited income or preexisting substance use disorders would access evidence-based treatment.
Opioid use disorder affects >2 million individuals in the United States and caused 33,000 overdose deaths in 2015.9 The Opioid Initiative, established in 2015 by the U.S. Department of Health and Human Services (HHS), has worked to improve prescribing practices, increase use of naloxone to treat overdose, and expand access to medication-assisted treatment and psychosocial support. The success of this initiative relies on accessible health insurance coverage. Medication-assisted treatment and psychosocial support services would be threatened most by repeal of the ACA.9 In 2016, the HHS provided $94 million in grants, through the ACA, for free clinics to screen and treat patients for substance use disorders.10 Continued funding for these programs would be jeopardized if the ACA was repealed without replacement.
Repair rather than replace
The ACA is not without its flaws, but perhaps the best approach is to build on its successes while repairing its weaknesses. Researcher Peter Phalen, MA, looked at changes in rates, usage, affordability, and satisfaction with services for those with moderate and severe mental illness after implementation of the ACA.11 Using a nationally representative sample (N = 35,602), he discovered that those with moderate mental illness, as measured by psychological distress scales, experienced greater gains in finding affordable coverage than those without mental illness.11 However, individuals with severe mental illness showed no improvement on these measures, with the exception of increased satisfaction with current coverage and care. There were no reported increases in health care use or affordability for either group.11
Although the ACA requires prescription coverage, there is no regulation of what insurers choose to include in their formularies, and often brand name drugs, particularly antipsychotics, are not covered. The National Alliance on Mental Illness released a report in 2015 noting that, even with the ACA, individuals continue to experience difficulty accessing behavioral health providers in a timely manner, especially in rural areas. The report also described a lack of parity enforcement for behavioral health coverage.12
What if?
If the ACA is repealed, other legislative acts could continue, in some way, to address the needs of those with mental illness. The 21st Century Cures Act, which has bipartisan support, was passed in 2016 in the hope of reforming national mental health care. The American Psychiatric Association (APA) president, Maria Oquendo, MD, PhD, indicated that the bill enhances parity laws and provides better coordination for national agencies involved in treating psychiatric illness.13 The APA applauded this effort and highlighted these provisions:
- reauthorizing grants to support integrated care models
- reauthorizing grants to train school staff to identify students who need mental health care
- requiring the HHS to develop a plan to enforce parity laws
- providing $1 billion in state grants to address the opioid epidemic.13
The APA has voiced its concern about repealing the ACA without replacement. The APA issued a letter to Congressional leadership stating the organization’s concerns, emphasizing that current law has eased the burden for Americans to access “appropriate and evidence-based mental health care.”14 The APA requested that, in considering reforms to health care law, Congress does not “undo the gains which have been made over the past several years for individuals with mental illness.”14 The APA noted that the proposed ACA replacement bill, released on March 3, 2017, would “negatively impact care for people with mental illness and substance use disorders.”15
Since the ACA was implemented, we have taken for granted many provisions as permanent fixtures of our nation’s health care system. Who now can imagine a denial of coverage for a preexisting condition? How many young adults are ready to purchase their own insurance plans immediately after high school or college if employment is not readily available? Is it reasonable that an insurance plan does not provide prescription coverage or behavioral health services? How will those with mental illness or substance use disorders have reliable access to assessment and treatment?
Repealing, replacing, or enhancing the ACA is a complicated balancing act. We must be vigilant and vocal in asking Congress to continue considering the needs of those with mental illness and substance use disorders.
With the changing political landscape in Washington, there has been much talk about health care in the United States. The Affordable Care Act (ACA) is at risk for repeal or, at least, substantial change. As the debate heats up, many psychiatric clinicians wonder what repeal could mean for mental health care and treatment of substance use disorders.
To examine this issue, we need to understand what the ACA has accomplished so far. The Patient Protection and Affordable Care Act—known as “Obamacare”—was enacted on March 23, 2010. From 2010 to 2014, various provisions were implemented; more provisions are slated for completion by 2017 if the law remains in place. These provisions are at the heart of how those with mental illness or substance use disorders could be affected by repeal of the ACA.
Since the ACA’s implementation, an estimated 20 million Americans have gained health insurance.1 The ACA includes several provisions that made this number possible, such as the expansion of Medicaid in some states. In addition to plans offered through the Health Insurance Marketplace, private insurers are required to provide insurance to some who previously fell into non-coverage gaps.1 Young adults can remain on a parent’s plan until age 26, which is significant to mental health care because many psychiatric disorders emerge in young adulthood, and this age group is vulnerable to developing substance use disorders.
The ACA also requires private insurance plans to cover those with preexisting health conditions. This has been crucial for persons with mental illness because before the ACA, mental health disorders were the second most common preexisting condition that precipitated either an increase in the cost of a plan or coverage denial.2
These provisions have helped ensure coverage for the approximately 20% of adults in the United States who have a mental illness.3 Before the ACA, 18% of individuals who purchased their own insurance did not have mental health coverage, and more than one-third of insurers did not cover substance use disorders.4 According to the CDC, the uninsured rate for those with serious mental health disorders fell from 28.1% in 2012 to 19.5% in 2015.5 Likewise, the number of adults with mental illness who could not afford needed care decreased during the same years.5 A University of Minnesota study found that persons with mental illness are disproportionately represented among the uninsured.6 Before the ACA, 18% of individual health plans did not cover prescriptions, including those indicated for psychiatric illness.7 Simply put, the ACA has allowed people to seek assessment and treatment for mental health, whereas it would not have been as accessible before the legislation.
What does the ACA cover?
The ACA required health plans to cover Essential Health Benefits starting January 1, 2014. These include:
- medical services such as doctor visits
- emergency and urgent care services
- hospital physician and facility services
- prenatal, delivery, and postnatal care
- evaluation and treatment of mental health conditions
- services to address substance use including behavioral health treatment
- coverage of prescription medications
- rehabilitation services
- diagnostic tests and imaging
- preventive and wellness care and management of chronic diseases
- pediatric care.
As of March 2013, only 2% of existing health plans in the United States provided all of these benefits required by the ACA.7
Required coverage of mental health care and substance use disorders increases patient access to those services. Including preventive care extends the reach of mental health screening to primary care providers, who can screen for mood disorders and substance use in adults and adolescents and for autism and behavioral issues in children.8
The ACA provides further expansion and enforcement of mental health parity. In 2008, the Mental Health Parity and Addiction Equity Act was passed with the intent of providing behavioral health benefits at the same level as medical care. Although this law was beneficial in theory, it did not require insurers to cover behavioral health treatment. Rather, it only required parity if large group plans already provided behavioral health coverage; parity laws did not apply to individual or small group plans. The Essential Health Benefits of the ACA specify that insurers must provide mental health and substance use treatment. Essentially, the ACA gave the parity law teeth. The law would matter very little if low-income patients, who often suffer from mental health symptoms, have no insurance coverage.
Perhaps more concerning are the implications for those battling substance use disorders. If the ACA is repealed without appropriate replacement measures, it is unclear how those with limited income or preexisting substance use disorders would access evidence-based treatment.
Opioid use disorder affects >2 million individuals in the United States and caused 33,000 overdose deaths in 2015.9 The Opioid Initiative, established in 2015 by the U.S. Department of Health and Human Services (HHS), has worked to improve prescribing practices, increase use of naloxone to treat overdose, and expand access to medication-assisted treatment and psychosocial support. The success of this initiative relies on accessible health insurance coverage. Medication-assisted treatment and psychosocial support services would be threatened most by repeal of the ACA.9 In 2016, the HHS provided $94 million in grants, through the ACA, for free clinics to screen and treat patients for substance use disorders.10 Continued funding for these programs would be jeopardized if the ACA was repealed without replacement.
Repair rather than replace
The ACA is not without its flaws, but perhaps the best approach is to build on its successes while repairing its weaknesses. Researcher Peter Phalen, MA, looked at changes in rates, usage, affordability, and satisfaction with services for those with moderate and severe mental illness after implementation of the ACA.11 Using a nationally representative sample (N = 35,602), he discovered that those with moderate mental illness, as measured by psychological distress scales, experienced greater gains in finding affordable coverage than those without mental illness.11 However, individuals with severe mental illness showed no improvement on these measures, with the exception of increased satisfaction with current coverage and care. There were no reported increases in health care use or affordability for either group.11
Although the ACA requires prescription coverage, there is no regulation of what insurers choose to include in their formularies, and often brand name drugs, particularly antipsychotics, are not covered. The National Alliance on Mental Illness released a report in 2015 noting that, even with the ACA, individuals continue to experience difficulty accessing behavioral health providers in a timely manner, especially in rural areas. The report also described a lack of parity enforcement for behavioral health coverage.12
What if?
If the ACA is repealed, other legislative acts could continue, in some way, to address the needs of those with mental illness. The 21st Century Cures Act, which has bipartisan support, was passed in 2016 in the hope of reforming national mental health care. The American Psychiatric Association (APA) president, Maria Oquendo, MD, PhD, indicated that the bill enhances parity laws and provides better coordination for national agencies involved in treating psychiatric illness.13 The APA applauded this effort and highlighted these provisions:
- reauthorizing grants to support integrated care models
- reauthorizing grants to train school staff to identify students who need mental health care
- requiring the HHS to develop a plan to enforce parity laws
- providing $1 billion in state grants to address the opioid epidemic.13
The APA has voiced its concern about repealing the ACA without replacement. The APA issued a letter to Congressional leadership stating the organization’s concerns, emphasizing that current law has eased the burden for Americans to access “appropriate and evidence-based mental health care.”14 The APA requested that, in considering reforms to health care law, Congress does not “undo the gains which have been made over the past several years for individuals with mental illness.”14 The APA noted that the proposed ACA replacement bill, released on March 3, 2017, would “negatively impact care for people with mental illness and substance use disorders.”15
Since the ACA was implemented, we have taken for granted many provisions as permanent fixtures of our nation’s health care system. Who now can imagine a denial of coverage for a preexisting condition? How many young adults are ready to purchase their own insurance plans immediately after high school or college if employment is not readily available? Is it reasonable that an insurance plan does not provide prescription coverage or behavioral health services? How will those with mental illness or substance use disorders have reliable access to assessment and treatment?
Repealing, replacing, or enhancing the ACA is a complicated balancing act. We must be vigilant and vocal in asking Congress to continue considering the needs of those with mental illness and substance use disorders.
1. U.S. Department of Health and Human Services. 20 million people have gained health insurance coverage because of the Affordable Care Act, new estimates show. http://wayback.archive-it.org/3926/20170127190440/https://www.hhs.gov/about/news/2016/03/03/20-million-people-have-gained-health-insurance-coverage-because-affordable-care-act-new-estimates. Published March 16, 2016. Accessed February 15, 2017.
2. U.S. Department of Health and Human Services. Health insurance coverage for Americans with pre-existing conditions: the impact of the Affordable Care Act. https://aspe.hhs.gov/pdf-report/health-insurance-coverage-americans-pre-existing-conditions-impact-affordable-care-act. Published January 5, 2017. Accessed February 20, 2017.
3. National Alliance on Mental Illness. Mental health by the numbers. http://www.nami.org/Learn-More/Mental-Health-By-the-Numbers. Accessed February 20, 2017.
4. U.S. Department of Health and Human Services. Essential health benefits: individual market place. https://aspe.hhs.gov/pdf-report/essential-health-benefits-individual-market-coverage. Published December 16, 2011. Accessed February 18, 2017.
5. Cohen R, Zammitti EP. Access to care among adults aged 18-64 with serious psychological distress: early release of estimates from the National Health Interview Survey, 2012-September 2015. https://www.cdc.gov/nchs/data/nhis/earlyrelease/er_spd_access_2015_f_auer.pdf. Published May 2016. Accessed February 1, 2017.
6. Rowan K, McAlpine DD, Blewett LA. Access and cost barriers to mental health care by insurance status, 1999-2010. Health Aff (Millwood). 2013;32(10):1723-1730.
7. Health Pocket. Almost no existing health plans meet new ACA essential health benefit standards. https://www.healthpocket.com/healthcare-research/infostat/few-existing-health-plans-meet-new-aca-essential-health-benefit-standards/#.WLSSdqPMxmC. Published March 7, 2013. Accessed February 20, 2017.
8. U.S. Department of Health and Human Services. Health benefits and coverage: preventive health services. https://www.healthcare.gov/coverage/preventive-care-benefits. Accessed February 26, 2017.
9. U.S. Department of Health and Human Services. Continuing progress on the opioid epidemic: the role of the Affordable Care Act. https://aspe.hhs.gov/pdf-report/continuing-progress-opioid-epidemic-role-affordable-care-act. Published January 11, 2017. Accessed February 15, 2017.
10. U.S. Department of Health and Human Services. HHS awards 94 million to health centers help treat the prescription opioid abuse and heroin epidemic in America. http://wayback.archive-it.org/3926/20170127185615/https://www.hhs.gov/about/news/2016/03/11/hhs-awards-94-million-to-health-centers.html. Published March 11, 2016. Accessed February 1, 2017.
11. Phalen P. Psychological distress and rates of health insurance coverage and use and affordability of mental health services, 2013-2014 [published online December 15, 2016]. Psychiatr Serv. http://dx.doi.org/10.1176/appi.ps.201500544.
12. National Alliance on Mental Illness. A long road ahead: achieving true parity in mental health and substance use care. https://www.nami.org/About-NAMI/Publications-Reports/Public-Policy-Reports/A-Long-Road-Ahead/2015-ALongRoadAhead.pdf. Published April 2015. Accessed February 15, 2017.
13. American Psychiatric Association. APA commends house for approving mental health reform bill. https://www.psychiatry.org/newsroom/news-releases/apa-commends-house-for-approving-mental-health-reform-bill?_ga=1.239819267.1833283241.1466442827. Published November 30, 2016. Accessed February 26, 2017.
14. American Psychiatric Association. APA calls on Congress to protect patient access to healthcare. https://www.psychiatry.org/newsroom/news-releases/apa-calls-on-congress-to-protect-patient-access-to-health-care?_ga=1.240843011.1833283241.1466442827. Published January 5, 2017. Accessed February 15, 2017.
15. American Psychiatric Association. APA concerned about proposed ACA replacement bill. https://www.psychiatry.org/newsroom/news-releases/apa-concerned-about-proposed-aca-replacement-bill. Published March 7, 2017. Accessed March 17, 2017.
1. U.S. Department of Health and Human Services. 20 million people have gained health insurance coverage because of the Affordable Care Act, new estimates show. http://wayback.archive-it.org/3926/20170127190440/https://www.hhs.gov/about/news/2016/03/03/20-million-people-have-gained-health-insurance-coverage-because-affordable-care-act-new-estimates. Published March 16, 2016. Accessed February 15, 2017.
2. U.S. Department of Health and Human Services. Health insurance coverage for Americans with pre-existing conditions: the impact of the Affordable Care Act. https://aspe.hhs.gov/pdf-report/health-insurance-coverage-americans-pre-existing-conditions-impact-affordable-care-act. Published January 5, 2017. Accessed February 20, 2017.
3. National Alliance on Mental Illness. Mental health by the numbers. http://www.nami.org/Learn-More/Mental-Health-By-the-Numbers. Accessed February 20, 2017.
4. U.S. Department of Health and Human Services. Essential health benefits: individual market place. https://aspe.hhs.gov/pdf-report/essential-health-benefits-individual-market-coverage. Published December 16, 2011. Accessed February 18, 2017.
5. Cohen R, Zammitti EP. Access to care among adults aged 18-64 with serious psychological distress: early release of estimates from the National Health Interview Survey, 2012-September 2015. https://www.cdc.gov/nchs/data/nhis/earlyrelease/er_spd_access_2015_f_auer.pdf. Published May 2016. Accessed February 1, 2017.
6. Rowan K, McAlpine DD, Blewett LA. Access and cost barriers to mental health care by insurance status, 1999-2010. Health Aff (Millwood). 2013;32(10):1723-1730.
7. Health Pocket. Almost no existing health plans meet new ACA essential health benefit standards. https://www.healthpocket.com/healthcare-research/infostat/few-existing-health-plans-meet-new-aca-essential-health-benefit-standards/#.WLSSdqPMxmC. Published March 7, 2013. Accessed February 20, 2017.
8. U.S. Department of Health and Human Services. Health benefits and coverage: preventive health services. https://www.healthcare.gov/coverage/preventive-care-benefits. Accessed February 26, 2017.
9. U.S. Department of Health and Human Services. Continuing progress on the opioid epidemic: the role of the Affordable Care Act. https://aspe.hhs.gov/pdf-report/continuing-progress-opioid-epidemic-role-affordable-care-act. Published January 11, 2017. Accessed February 15, 2017.
10. U.S. Department of Health and Human Services. HHS awards 94 million to health centers help treat the prescription opioid abuse and heroin epidemic in America. http://wayback.archive-it.org/3926/20170127185615/https://www.hhs.gov/about/news/2016/03/11/hhs-awards-94-million-to-health-centers.html. Published March 11, 2016. Accessed February 1, 2017.
11. Phalen P. Psychological distress and rates of health insurance coverage and use and affordability of mental health services, 2013-2014 [published online December 15, 2016]. Psychiatr Serv. http://dx.doi.org/10.1176/appi.ps.201500544.
12. National Alliance on Mental Illness. A long road ahead: achieving true parity in mental health and substance use care. https://www.nami.org/About-NAMI/Publications-Reports/Public-Policy-Reports/A-Long-Road-Ahead/2015-ALongRoadAhead.pdf. Published April 2015. Accessed February 15, 2017.
13. American Psychiatric Association. APA commends house for approving mental health reform bill. https://www.psychiatry.org/newsroom/news-releases/apa-commends-house-for-approving-mental-health-reform-bill?_ga=1.239819267.1833283241.1466442827. Published November 30, 2016. Accessed February 26, 2017.
14. American Psychiatric Association. APA calls on Congress to protect patient access to healthcare. https://www.psychiatry.org/newsroom/news-releases/apa-calls-on-congress-to-protect-patient-access-to-health-care?_ga=1.240843011.1833283241.1466442827. Published January 5, 2017. Accessed February 15, 2017.
15. American Psychiatric Association. APA concerned about proposed ACA replacement bill. https://www.psychiatry.org/newsroom/news-releases/apa-concerned-about-proposed-aca-replacement-bill. Published March 7, 2017. Accessed March 17, 2017.
Advancing the role of advanced practice psychiatric nurses in today’s psychiatric workforce
The number of psychiatric prescribers per capita is at one of the lowest levels in history.1 Approximately 43.4 million persons (17.9%) in the United States have a diagnosable mental illness2; 9.8 million (4%) are diagnosed with a serious and persistent mental illness, such as schizophrenia, bipolar disorder, and major depressive disorder (these figures do not include substance use disorders).3
Of the 45,000 licensed psychiatrists, approximately 25,000 are in active practice.4 By comparison, there are approximately 19,000 practicing licensed psychiatric advanced practice registered nurses (APRNs).5 Annually, approximately 1,300 physicians graduate from psychiatric residency programs6 and 700 APRNs from master’s or Doctor of Nursing Practice programs.7 Combining the 2 prescribing workforces (44,000) yields a ratio of 986 patients per licensed prescriber. Seeing each patient only once every 2 months would equate to 25 patients daily considering a 5-day work week. Recognizing that some patients need much more frequent follow-up, this is an impossible task even if these providers and patients were dispersed uniformly across the United States. Currently, ratios are calculated based on the number of psychiatrists per 100,000 individuals, which in the United States is 16.8 Most psychiatrists practice in urban areas,9 whereas psychiatric nurse practitioners are found primarily in rural and less populated urban areas.10
Who can provide care?
Although the growing number of psychiatric APRNs is encouraging for the mental health workforce, their limited role and function remain a battle in the 27 states that do not grant full practice authority. This dispute has become so contentious that the Federal Trade Commission (FTC) has stated that the debate over scope of practice represents federal restraint of trade,11 while patients and their families suffer from lack of access to care.
Recognizing that 9 million patients age <65 who were enrolled in Medicaid in 2011 and treated for a mental health disorder (20% of enrollees) accounted for 50% of all Medicaid expenditures prompts the question, “Who is treating these patients?” According to the American Academy of Nurse Practitioners, 75% of nurse practitioners accept and treat both Medicaid and Medicare patients compared with 43% of psychiatrists who accepted Medicaid and 54% who accepted Medicare in 2011 (these numbers do not include potential overlap).12
Who are APRNs?
The first master’s degree in nursing was created by Hildegard Peplau, EdD, at Rutgers University in 1954, using the title Clinical Specialist in Psychiatric Mental Health Nursing (PMH-CNS). As a master’s prepared clinician, the PMH-CNS could function independently, and many chose to open private practices. Other universities began to create clinical specialty programs in a variety of disciplines. In 1996, 41 states granted prescriptive authority to the PMH-CNS. Psychiatric nurse practitioners were first certified in 2000 to meet the statutory requirements for prescriptive authority of the other 9 states. However this created 4 PMH-APRN roles: Adult and Child/Adolescent CNS and Adult and Family PMHNPs.
Clinical specialists in most areas of health care—except for psychiatry—were primarily working in institutional settings, whereas nurse practitioners were hired principally in primary care community-based settings. The public grew familiar with the term “nurse practitioner,” but these professionals functioned primarily under institutional protocols, while the PMH-CNS had the ability to practice independently. In the mid-1990s, the 4 advanced practice nursing roles of nurse midwife, nurse anesthetist, nurse practitioner, and clinical nurse specialist were encompassed under 1 title: APRN. In 2010 the American Psychiatric Nurses Association endorsed one title for the psychiatric mental health advanced practice registered nurse (PMH-APRN), the psychiatric nurse practitioner, to be educated across the lifespan.
Today, the title PMH-APRN encompasses both the PMHNP and PMH-CNS; the majority specialize in the adult population.
Licensure, accreditation, certification, and education
In 2008, after several years of heated debate among members of >70 nursing organizations, a consensus model governing advanced practice nursing was ratified. This document outlined requirements for licensure, accreditation, certification, and education of the 4 primary advanced practice nursing roles.13 According to the model, the 4 nursing roles would address 1 of 6 major patient populations: neonatal, pediatric, adult-geriatric, family, women’s health/gender-related, and psychiatric. Licensure in each state would be converted to APRN from the existing 26 titles. Each student would have to graduate from a nationally accredited program. In addition to health promotion and advanced roles, educational programs would be required to include advanced courses in pathophysiology, pharmacotherapeutics, and physicalassessment as well as population-specific courses in these same categories. In addition, supervised clinical hour minimums were established for the various population-specific programs.
Concomitantly, graduate educational programs were wrestling with the 2005 statement from the American Association of Colleges of Nursing (AACN) that all advanced practice nursing education should be at the doctoral level by 2015. Because of the knowledge explosion, nurses needed more than what could be achieved in a master’s program to meet practice requirements as well as leadership, systems evaluation, quality improvement, research, and program development. Currently, there are 264 Doctor of Nursing Practice programs in the United States with less than one-half having a PMHNP program.14
Nursing education at the collegiate level has been evolving, which is fostered and supported by the 2010 Institute of Medicine (IOM) Report on the Future of Nursing that identified 4 key recommendations to promote a workforce at capacity to help care for our nation’s growing population:
- Remove scope of practice barriers
- Expand opportunities for nurses to lead and diffuse collaborative improvement efforts
- Implement nurse residency programs
- Increase the proportion of nurses with a baccalaureate degree to 80% by 2020.
The current status of advanced practice nursing
Each of the 50 states is in varying levels of compliance with the 2015 mandates from the consensus model and the AACN. From the psychiatric workforce perspective, many state boards of nursing are concerned because titles often are linked to legislative statute or rules. Despite the 2010 IOM recommendations and the FTC, the American Medical Association (AMA) has stationed AMA lobbyists in the legislatures that are poised to open the nurse practice act to comply with the consensus model. The sole purpose of these lobbyists is to block independent practice for APRNs in the 26 states that are seeking this status and to remove independent practice from the states where it already exists. For example, in Washington the title is ARNP but to change it to APRN will require opening the state’s legislative action. The AMA is eager to remove the autonomy that has existed in that state since 1978. One of the reasons is because where the APRN is required to be in a collaborative or supervisory relationship with a physician, the physician can charge the APRN to be compliant with state regulations. (In some states, the APRN cannot see patients or be on call if the collaborator is on vacation).
This has turned into a cottage industry for many physicians. However, there are many who do not charge because they are able to add additional patients to the practice by adding an APRN and generate more revenue. Others do not charge because they are supportive and committed to the APRN role.
Some thoughts about our mutual field
Can we move past the guild issue and come together to respect our given scopes of practice? I see psychiatry far ahead of the curve compared with APRNs in other specialties. The PMH-APRN is a highly educated nurse with a specific scope of practice that provides skilled psychiatric care (assessment, diagnosis, prescribing, psychotherapy) from a nursing perspective. Independent practice certainly does not imply that we do not collaborate with one another in a professional manner.
Mental Health Professional Shortage Areas
As of January 1, 2017, there are 4,627 Mental Health Professional Shortage Areas (MHPSA) in the United States and Territories (Table), which translates to only 44.2% of the need for psychiatric practitioners being met.15 To eliminate the designation of a MHSPA there must be a population to psychiatric provider ratio of at least 30,000 to 1 (20,000 to 1 if there are unusually high needs in the community). Currently 3,397 practitioners are needed to remove the designation across the United States. The state in most need of providers is Texas with 271 clinicians required to meet the need.
Considering that approximately 700 PMH-APRNs graduate each year16 and 1,317 psychiatry residents17 entered PGY-1 residency in 2016, it will be decades—or longer—before there are enough new providers to eliminate MHPSAs, particularly because the current workforce is aging (average age of the PMH-APRN is 55).
Because there are more than enough patients to go around, I encourage the APA to take a stand against the AMA and unite with the psychiatric APRNs to remove unnecessary barriers to practice and promote a unified and collegial workforce. This will transmit a strong message to the most underserved of our communities that psychiatrists and psychiatric nurse practitioners can emulate the therapeutic relationship by virtue of presenting a unified force. Imagine psychiatrists and psychiatric nurse practitioners going arm in arm to lobby county commissioners, state legislators, and Congressional Representatives and Senators. Together we could be a true force to be reckoned with.
1. Heisler EJ, Bagalman E. The mental health workforce: a primer. http://digitalcommons.ilr.cornell.edu/key_workplace/1410. Published April 16, 2015. Accessed March 13, 2017.
2. National Institute of Mental Health. Any mental illness (AMI) among U.S. adults. https://www.nimh.nih.gov/health/statistics/prevalence/any-mental-illness-ami-among-us-adults.shtml. Accessed March 13, 2017.
3. National Institute of Mental Health. Serious mental illness (SMI) among U.S. adults. https://www.nimh.nih.gov/health/statistics/prevalence/serious-mental-illness-smi-among-us-adults.shtml. Accessed March 13, 2017.
4. Bureau of Labor Statistics. Occupational employment and wages, May 2015. https://www.bls.gov/oes/current/oes291066.htm. Updated March 30, 2016. Accessed March 13, 2017.
5. American Association of Colleges of Nursing. Program directory. http://www.aacn.nche.edu/dnp/program-directory. Accessed March 13, 2017.
7. Fang D, Li Y, Stauffer DC, et al. 2015-2016 Enrollment and graduations in baccalaureate and graduate programs in nursing. http://www.nonpf.org/resource/resmgr/docs/NPTables15-16.pdf. Published 2016. Accessed March 13, 2017.
8. Tasman A. Too few psychiatrists for too many. Psychiatric Times. http://www.psychiatrictimes.com/cultural-psychiatry/too-few-psychiatrists-too-many. Published April 16, 2015. Accessed March 15, 2017.
9. U.S. Department of Health and Human Services. National projections of supply and demand for selected behavioral health practitioners: 2013-2025. https://bhw.hrsa.gov/sites/default/files/bhw/health-workforce-analysis/research/projections/behavioral-health2013-2025.pdf. Published November 2016. Accessed March 13, 2017.
10. Hanrahan NP, Hartley D. Employment of advanced-practice psychiatric nurses to stem rural mental health workforce shortages. Psychiatr Serv. 2008;59(1):109-111.
11. Koslov T. The doctor (or nurse practitioner) will see you now: competition and the regulation of advanced practice nurses. https://www.ftc.gov/news-events/blogs/competition-matters/2014/03/doctor-or-nurse-practitioner-will-see-you-now. Published March 7, 2014. Accessed March 14, 2017.
12. Bishop TF, Press MJ, Keyhani S, et al. Acceptance of insurance by psychiatrists and the implications for access to mental health care. JAMA Psychiatry. 2014;71(2):176-181.
13. National Council of State Boards of Nursing. APRN consensus model. The consensus model for APRN regulation, licensure, accreditation, certification and education. https://www.ncsbn.org/736.htm. Accessed March 13, 2017.
14. National Council of State Boards of Nursing. APRN title map. NCSBN’s APRN campaign for consensus: State progress toward uniformity. https://www.ncsbn.org/5398.htm. Accessed March 13, 2017.
15. Kaiser Family Foundation. Mental health care health professional shortage areas (HPSAs). http://kff.org/other/state-indicator/mental-health-care-health-professional-shortage-areas-hpsas/?activeTab=map¤tTimeframe=0&selectedDistributions=total-mental-health-care-hpsa-designations&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed March 13, 2017.
16. Commission on Collegiate Nursing Education. CCNE-Accredited Doctor of Nursing Practice (DNP) Programs. http://directory.ccnecommunity.org/reports/rptAccreditedPrograms_New.asp?sort=state&sProgramType=3. Accessed March 15, 2017.
17. National Residency Match Program. 2016 match results by state, specialty, and applicant type. http://www.nrmp.org/wp-content/uploads/2016/04/Main-Match-Results-by-State-and-Specialty-2016.pdf. Accessed March 13, 2017.
The number of psychiatric prescribers per capita is at one of the lowest levels in history.1 Approximately 43.4 million persons (17.9%) in the United States have a diagnosable mental illness2; 9.8 million (4%) are diagnosed with a serious and persistent mental illness, such as schizophrenia, bipolar disorder, and major depressive disorder (these figures do not include substance use disorders).3
Of the 45,000 licensed psychiatrists, approximately 25,000 are in active practice.4 By comparison, there are approximately 19,000 practicing licensed psychiatric advanced practice registered nurses (APRNs).5 Annually, approximately 1,300 physicians graduate from psychiatric residency programs6 and 700 APRNs from master’s or Doctor of Nursing Practice programs.7 Combining the 2 prescribing workforces (44,000) yields a ratio of 986 patients per licensed prescriber. Seeing each patient only once every 2 months would equate to 25 patients daily considering a 5-day work week. Recognizing that some patients need much more frequent follow-up, this is an impossible task even if these providers and patients were dispersed uniformly across the United States. Currently, ratios are calculated based on the number of psychiatrists per 100,000 individuals, which in the United States is 16.8 Most psychiatrists practice in urban areas,9 whereas psychiatric nurse practitioners are found primarily in rural and less populated urban areas.10
Who can provide care?
Although the growing number of psychiatric APRNs is encouraging for the mental health workforce, their limited role and function remain a battle in the 27 states that do not grant full practice authority. This dispute has become so contentious that the Federal Trade Commission (FTC) has stated that the debate over scope of practice represents federal restraint of trade,11 while patients and their families suffer from lack of access to care.
Recognizing that 9 million patients age <65 who were enrolled in Medicaid in 2011 and treated for a mental health disorder (20% of enrollees) accounted for 50% of all Medicaid expenditures prompts the question, “Who is treating these patients?” According to the American Academy of Nurse Practitioners, 75% of nurse practitioners accept and treat both Medicaid and Medicare patients compared with 43% of psychiatrists who accepted Medicaid and 54% who accepted Medicare in 2011 (these numbers do not include potential overlap).12
Who are APRNs?
The first master’s degree in nursing was created by Hildegard Peplau, EdD, at Rutgers University in 1954, using the title Clinical Specialist in Psychiatric Mental Health Nursing (PMH-CNS). As a master’s prepared clinician, the PMH-CNS could function independently, and many chose to open private practices. Other universities began to create clinical specialty programs in a variety of disciplines. In 1996, 41 states granted prescriptive authority to the PMH-CNS. Psychiatric nurse practitioners were first certified in 2000 to meet the statutory requirements for prescriptive authority of the other 9 states. However this created 4 PMH-APRN roles: Adult and Child/Adolescent CNS and Adult and Family PMHNPs.
Clinical specialists in most areas of health care—except for psychiatry—were primarily working in institutional settings, whereas nurse practitioners were hired principally in primary care community-based settings. The public grew familiar with the term “nurse practitioner,” but these professionals functioned primarily under institutional protocols, while the PMH-CNS had the ability to practice independently. In the mid-1990s, the 4 advanced practice nursing roles of nurse midwife, nurse anesthetist, nurse practitioner, and clinical nurse specialist were encompassed under 1 title: APRN. In 2010 the American Psychiatric Nurses Association endorsed one title for the psychiatric mental health advanced practice registered nurse (PMH-APRN), the psychiatric nurse practitioner, to be educated across the lifespan.
Today, the title PMH-APRN encompasses both the PMHNP and PMH-CNS; the majority specialize in the adult population.
Licensure, accreditation, certification, and education
In 2008, after several years of heated debate among members of >70 nursing organizations, a consensus model governing advanced practice nursing was ratified. This document outlined requirements for licensure, accreditation, certification, and education of the 4 primary advanced practice nursing roles.13 According to the model, the 4 nursing roles would address 1 of 6 major patient populations: neonatal, pediatric, adult-geriatric, family, women’s health/gender-related, and psychiatric. Licensure in each state would be converted to APRN from the existing 26 titles. Each student would have to graduate from a nationally accredited program. In addition to health promotion and advanced roles, educational programs would be required to include advanced courses in pathophysiology, pharmacotherapeutics, and physicalassessment as well as population-specific courses in these same categories. In addition, supervised clinical hour minimums were established for the various population-specific programs.
Concomitantly, graduate educational programs were wrestling with the 2005 statement from the American Association of Colleges of Nursing (AACN) that all advanced practice nursing education should be at the doctoral level by 2015. Because of the knowledge explosion, nurses needed more than what could be achieved in a master’s program to meet practice requirements as well as leadership, systems evaluation, quality improvement, research, and program development. Currently, there are 264 Doctor of Nursing Practice programs in the United States with less than one-half having a PMHNP program.14
Nursing education at the collegiate level has been evolving, which is fostered and supported by the 2010 Institute of Medicine (IOM) Report on the Future of Nursing that identified 4 key recommendations to promote a workforce at capacity to help care for our nation’s growing population:
- Remove scope of practice barriers
- Expand opportunities for nurses to lead and diffuse collaborative improvement efforts
- Implement nurse residency programs
- Increase the proportion of nurses with a baccalaureate degree to 80% by 2020.
The current status of advanced practice nursing
Each of the 50 states is in varying levels of compliance with the 2015 mandates from the consensus model and the AACN. From the psychiatric workforce perspective, many state boards of nursing are concerned because titles often are linked to legislative statute or rules. Despite the 2010 IOM recommendations and the FTC, the American Medical Association (AMA) has stationed AMA lobbyists in the legislatures that are poised to open the nurse practice act to comply with the consensus model. The sole purpose of these lobbyists is to block independent practice for APRNs in the 26 states that are seeking this status and to remove independent practice from the states where it already exists. For example, in Washington the title is ARNP but to change it to APRN will require opening the state’s legislative action. The AMA is eager to remove the autonomy that has existed in that state since 1978. One of the reasons is because where the APRN is required to be in a collaborative or supervisory relationship with a physician, the physician can charge the APRN to be compliant with state regulations. (In some states, the APRN cannot see patients or be on call if the collaborator is on vacation).
This has turned into a cottage industry for many physicians. However, there are many who do not charge because they are able to add additional patients to the practice by adding an APRN and generate more revenue. Others do not charge because they are supportive and committed to the APRN role.
Some thoughts about our mutual field
Can we move past the guild issue and come together to respect our given scopes of practice? I see psychiatry far ahead of the curve compared with APRNs in other specialties. The PMH-APRN is a highly educated nurse with a specific scope of practice that provides skilled psychiatric care (assessment, diagnosis, prescribing, psychotherapy) from a nursing perspective. Independent practice certainly does not imply that we do not collaborate with one another in a professional manner.
Mental Health Professional Shortage Areas
As of January 1, 2017, there are 4,627 Mental Health Professional Shortage Areas (MHPSA) in the United States and Territories (Table), which translates to only 44.2% of the need for psychiatric practitioners being met.15 To eliminate the designation of a MHSPA there must be a population to psychiatric provider ratio of at least 30,000 to 1 (20,000 to 1 if there are unusually high needs in the community). Currently 3,397 practitioners are needed to remove the designation across the United States. The state in most need of providers is Texas with 271 clinicians required to meet the need.

Considering that approximately 700 PMH-APRNs graduate each year16 and 1,317 psychiatry residents17 entered PGY-1 residency in 2016, it will be decades—or longer—before there are enough new providers to eliminate MHPSAs, particularly because the current workforce is aging (average age of the PMH-APRN is 55).
Because there are more than enough patients to go around, I encourage the APA to take a stand against the AMA and unite with the psychiatric APRNs to remove unnecessary barriers to practice and promote a unified and collegial workforce. This will transmit a strong message to the most underserved of our communities that psychiatrists and psychiatric nurse practitioners can emulate the therapeutic relationship by virtue of presenting a unified force. Imagine psychiatrists and psychiatric nurse practitioners going arm in arm to lobby county commissioners, state legislators, and Congressional Representatives and Senators. Together we could be a true force to be reckoned with.
The number of psychiatric prescribers per capita is at one of the lowest levels in history.1 Approximately 43.4 million persons (17.9%) in the United States have a diagnosable mental illness2; 9.8 million (4%) are diagnosed with a serious and persistent mental illness, such as schizophrenia, bipolar disorder, and major depressive disorder (these figures do not include substance use disorders).3
Of the 45,000 licensed psychiatrists, approximately 25,000 are in active practice.4 By comparison, there are approximately 19,000 practicing licensed psychiatric advanced practice registered nurses (APRNs).5 Annually, approximately 1,300 physicians graduate from psychiatric residency programs6 and 700 APRNs from master’s or Doctor of Nursing Practice programs.7 Combining the 2 prescribing workforces (44,000) yields a ratio of 986 patients per licensed prescriber. Seeing each patient only once every 2 months would equate to 25 patients daily considering a 5-day work week. Recognizing that some patients need much more frequent follow-up, this is an impossible task even if these providers and patients were dispersed uniformly across the United States. Currently, ratios are calculated based on the number of psychiatrists per 100,000 individuals, which in the United States is 16.8 Most psychiatrists practice in urban areas,9 whereas psychiatric nurse practitioners are found primarily in rural and less populated urban areas.10
Who can provide care?
Although the growing number of psychiatric APRNs is encouraging for the mental health workforce, their limited role and function remain a battle in the 27 states that do not grant full practice authority. This dispute has become so contentious that the Federal Trade Commission (FTC) has stated that the debate over scope of practice represents federal restraint of trade,11 while patients and their families suffer from lack of access to care.
Recognizing that 9 million patients age <65 who were enrolled in Medicaid in 2011 and treated for a mental health disorder (20% of enrollees) accounted for 50% of all Medicaid expenditures prompts the question, “Who is treating these patients?” According to the American Academy of Nurse Practitioners, 75% of nurse practitioners accept and treat both Medicaid and Medicare patients compared with 43% of psychiatrists who accepted Medicaid and 54% who accepted Medicare in 2011 (these numbers do not include potential overlap).12
Who are APRNs?
The first master’s degree in nursing was created by Hildegard Peplau, EdD, at Rutgers University in 1954, using the title Clinical Specialist in Psychiatric Mental Health Nursing (PMH-CNS). As a master’s prepared clinician, the PMH-CNS could function independently, and many chose to open private practices. Other universities began to create clinical specialty programs in a variety of disciplines. In 1996, 41 states granted prescriptive authority to the PMH-CNS. Psychiatric nurse practitioners were first certified in 2000 to meet the statutory requirements for prescriptive authority of the other 9 states. However this created 4 PMH-APRN roles: Adult and Child/Adolescent CNS and Adult and Family PMHNPs.
Clinical specialists in most areas of health care—except for psychiatry—were primarily working in institutional settings, whereas nurse practitioners were hired principally in primary care community-based settings. The public grew familiar with the term “nurse practitioner,” but these professionals functioned primarily under institutional protocols, while the PMH-CNS had the ability to practice independently. In the mid-1990s, the 4 advanced practice nursing roles of nurse midwife, nurse anesthetist, nurse practitioner, and clinical nurse specialist were encompassed under 1 title: APRN. In 2010 the American Psychiatric Nurses Association endorsed one title for the psychiatric mental health advanced practice registered nurse (PMH-APRN), the psychiatric nurse practitioner, to be educated across the lifespan.
Today, the title PMH-APRN encompasses both the PMHNP and PMH-CNS; the majority specialize in the adult population.
Licensure, accreditation, certification, and education
In 2008, after several years of heated debate among members of >70 nursing organizations, a consensus model governing advanced practice nursing was ratified. This document outlined requirements for licensure, accreditation, certification, and education of the 4 primary advanced practice nursing roles.13 According to the model, the 4 nursing roles would address 1 of 6 major patient populations: neonatal, pediatric, adult-geriatric, family, women’s health/gender-related, and psychiatric. Licensure in each state would be converted to APRN from the existing 26 titles. Each student would have to graduate from a nationally accredited program. In addition to health promotion and advanced roles, educational programs would be required to include advanced courses in pathophysiology, pharmacotherapeutics, and physicalassessment as well as population-specific courses in these same categories. In addition, supervised clinical hour minimums were established for the various population-specific programs.
Concomitantly, graduate educational programs were wrestling with the 2005 statement from the American Association of Colleges of Nursing (AACN) that all advanced practice nursing education should be at the doctoral level by 2015. Because of the knowledge explosion, nurses needed more than what could be achieved in a master’s program to meet practice requirements as well as leadership, systems evaluation, quality improvement, research, and program development. Currently, there are 264 Doctor of Nursing Practice programs in the United States with less than one-half having a PMHNP program.14
Nursing education at the collegiate level has been evolving, which is fostered and supported by the 2010 Institute of Medicine (IOM) Report on the Future of Nursing that identified 4 key recommendations to promote a workforce at capacity to help care for our nation’s growing population:
- Remove scope of practice barriers
- Expand opportunities for nurses to lead and diffuse collaborative improvement efforts
- Implement nurse residency programs
- Increase the proportion of nurses with a baccalaureate degree to 80% by 2020.
The current status of advanced practice nursing
Each of the 50 states is in varying levels of compliance with the 2015 mandates from the consensus model and the AACN. From the psychiatric workforce perspective, many state boards of nursing are concerned because titles often are linked to legislative statute or rules. Despite the 2010 IOM recommendations and the FTC, the American Medical Association (AMA) has stationed AMA lobbyists in the legislatures that are poised to open the nurse practice act to comply with the consensus model. The sole purpose of these lobbyists is to block independent practice for APRNs in the 26 states that are seeking this status and to remove independent practice from the states where it already exists. For example, in Washington the title is ARNP but to change it to APRN will require opening the state’s legislative action. The AMA is eager to remove the autonomy that has existed in that state since 1978. One of the reasons is because where the APRN is required to be in a collaborative or supervisory relationship with a physician, the physician can charge the APRN to be compliant with state regulations. (In some states, the APRN cannot see patients or be on call if the collaborator is on vacation).
This has turned into a cottage industry for many physicians. However, there are many who do not charge because they are able to add additional patients to the practice by adding an APRN and generate more revenue. Others do not charge because they are supportive and committed to the APRN role.
Some thoughts about our mutual field
Can we move past the guild issue and come together to respect our given scopes of practice? I see psychiatry far ahead of the curve compared with APRNs in other specialties. The PMH-APRN is a highly educated nurse with a specific scope of practice that provides skilled psychiatric care (assessment, diagnosis, prescribing, psychotherapy) from a nursing perspective. Independent practice certainly does not imply that we do not collaborate with one another in a professional manner.
Mental Health Professional Shortage Areas
As of January 1, 2017, there are 4,627 Mental Health Professional Shortage Areas (MHPSA) in the United States and Territories (Table), which translates to only 44.2% of the need for psychiatric practitioners being met.15 To eliminate the designation of a MHSPA there must be a population to psychiatric provider ratio of at least 30,000 to 1 (20,000 to 1 if there are unusually high needs in the community). Currently 3,397 practitioners are needed to remove the designation across the United States. The state in most need of providers is Texas with 271 clinicians required to meet the need.

Considering that approximately 700 PMH-APRNs graduate each year16 and 1,317 psychiatry residents17 entered PGY-1 residency in 2016, it will be decades—or longer—before there are enough new providers to eliminate MHPSAs, particularly because the current workforce is aging (average age of the PMH-APRN is 55).
Because there are more than enough patients to go around, I encourage the APA to take a stand against the AMA and unite with the psychiatric APRNs to remove unnecessary barriers to practice and promote a unified and collegial workforce. This will transmit a strong message to the most underserved of our communities that psychiatrists and psychiatric nurse practitioners can emulate the therapeutic relationship by virtue of presenting a unified force. Imagine psychiatrists and psychiatric nurse practitioners going arm in arm to lobby county commissioners, state legislators, and Congressional Representatives and Senators. Together we could be a true force to be reckoned with.
1. Heisler EJ, Bagalman E. The mental health workforce: a primer. http://digitalcommons.ilr.cornell.edu/key_workplace/1410. Published April 16, 2015. Accessed March 13, 2017.
2. National Institute of Mental Health. Any mental illness (AMI) among U.S. adults. https://www.nimh.nih.gov/health/statistics/prevalence/any-mental-illness-ami-among-us-adults.shtml. Accessed March 13, 2017.
3. National Institute of Mental Health. Serious mental illness (SMI) among U.S. adults. https://www.nimh.nih.gov/health/statistics/prevalence/serious-mental-illness-smi-among-us-adults.shtml. Accessed March 13, 2017.
4. Bureau of Labor Statistics. Occupational employment and wages, May 2015. https://www.bls.gov/oes/current/oes291066.htm. Updated March 30, 2016. Accessed March 13, 2017.
5. American Association of Colleges of Nursing. Program directory. http://www.aacn.nche.edu/dnp/program-directory. Accessed March 13, 2017.
7. Fang D, Li Y, Stauffer DC, et al. 2015-2016 Enrollment and graduations in baccalaureate and graduate programs in nursing. http://www.nonpf.org/resource/resmgr/docs/NPTables15-16.pdf. Published 2016. Accessed March 13, 2017.
8. Tasman A. Too few psychiatrists for too many. Psychiatric Times. http://www.psychiatrictimes.com/cultural-psychiatry/too-few-psychiatrists-too-many. Published April 16, 2015. Accessed March 15, 2017.
9. U.S. Department of Health and Human Services. National projections of supply and demand for selected behavioral health practitioners: 2013-2025. https://bhw.hrsa.gov/sites/default/files/bhw/health-workforce-analysis/research/projections/behavioral-health2013-2025.pdf. Published November 2016. Accessed March 13, 2017.
10. Hanrahan NP, Hartley D. Employment of advanced-practice psychiatric nurses to stem rural mental health workforce shortages. Psychiatr Serv. 2008;59(1):109-111.
11. Koslov T. The doctor (or nurse practitioner) will see you now: competition and the regulation of advanced practice nurses. https://www.ftc.gov/news-events/blogs/competition-matters/2014/03/doctor-or-nurse-practitioner-will-see-you-now. Published March 7, 2014. Accessed March 14, 2017.
12. Bishop TF, Press MJ, Keyhani S, et al. Acceptance of insurance by psychiatrists and the implications for access to mental health care. JAMA Psychiatry. 2014;71(2):176-181.
13. National Council of State Boards of Nursing. APRN consensus model. The consensus model for APRN regulation, licensure, accreditation, certification and education. https://www.ncsbn.org/736.htm. Accessed March 13, 2017.
14. National Council of State Boards of Nursing. APRN title map. NCSBN’s APRN campaign for consensus: State progress toward uniformity. https://www.ncsbn.org/5398.htm. Accessed March 13, 2017.
15. Kaiser Family Foundation. Mental health care health professional shortage areas (HPSAs). http://kff.org/other/state-indicator/mental-health-care-health-professional-shortage-areas-hpsas/?activeTab=map¤tTimeframe=0&selectedDistributions=total-mental-health-care-hpsa-designations&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed March 13, 2017.
16. Commission on Collegiate Nursing Education. CCNE-Accredited Doctor of Nursing Practice (DNP) Programs. http://directory.ccnecommunity.org/reports/rptAccreditedPrograms_New.asp?sort=state&sProgramType=3. Accessed March 15, 2017.
17. National Residency Match Program. 2016 match results by state, specialty, and applicant type. http://www.nrmp.org/wp-content/uploads/2016/04/Main-Match-Results-by-State-and-Specialty-2016.pdf. Accessed March 13, 2017.
1. Heisler EJ, Bagalman E. The mental health workforce: a primer. http://digitalcommons.ilr.cornell.edu/key_workplace/1410. Published April 16, 2015. Accessed March 13, 2017.
2. National Institute of Mental Health. Any mental illness (AMI) among U.S. adults. https://www.nimh.nih.gov/health/statistics/prevalence/any-mental-illness-ami-among-us-adults.shtml. Accessed March 13, 2017.
3. National Institute of Mental Health. Serious mental illness (SMI) among U.S. adults. https://www.nimh.nih.gov/health/statistics/prevalence/serious-mental-illness-smi-among-us-adults.shtml. Accessed March 13, 2017.
4. Bureau of Labor Statistics. Occupational employment and wages, May 2015. https://www.bls.gov/oes/current/oes291066.htm. Updated March 30, 2016. Accessed March 13, 2017.
5. American Association of Colleges of Nursing. Program directory. http://www.aacn.nche.edu/dnp/program-directory. Accessed March 13, 2017.
7. Fang D, Li Y, Stauffer DC, et al. 2015-2016 Enrollment and graduations in baccalaureate and graduate programs in nursing. http://www.nonpf.org/resource/resmgr/docs/NPTables15-16.pdf. Published 2016. Accessed March 13, 2017.
8. Tasman A. Too few psychiatrists for too many. Psychiatric Times. http://www.psychiatrictimes.com/cultural-psychiatry/too-few-psychiatrists-too-many. Published April 16, 2015. Accessed March 15, 2017.
9. U.S. Department of Health and Human Services. National projections of supply and demand for selected behavioral health practitioners: 2013-2025. https://bhw.hrsa.gov/sites/default/files/bhw/health-workforce-analysis/research/projections/behavioral-health2013-2025.pdf. Published November 2016. Accessed March 13, 2017.
10. Hanrahan NP, Hartley D. Employment of advanced-practice psychiatric nurses to stem rural mental health workforce shortages. Psychiatr Serv. 2008;59(1):109-111.
11. Koslov T. The doctor (or nurse practitioner) will see you now: competition and the regulation of advanced practice nurses. https://www.ftc.gov/news-events/blogs/competition-matters/2014/03/doctor-or-nurse-practitioner-will-see-you-now. Published March 7, 2014. Accessed March 14, 2017.
12. Bishop TF, Press MJ, Keyhani S, et al. Acceptance of insurance by psychiatrists and the implications for access to mental health care. JAMA Psychiatry. 2014;71(2):176-181.
13. National Council of State Boards of Nursing. APRN consensus model. The consensus model for APRN regulation, licensure, accreditation, certification and education. https://www.ncsbn.org/736.htm. Accessed March 13, 2017.
14. National Council of State Boards of Nursing. APRN title map. NCSBN’s APRN campaign for consensus: State progress toward uniformity. https://www.ncsbn.org/5398.htm. Accessed March 13, 2017.
15. Kaiser Family Foundation. Mental health care health professional shortage areas (HPSAs). http://kff.org/other/state-indicator/mental-health-care-health-professional-shortage-areas-hpsas/?activeTab=map¤tTimeframe=0&selectedDistributions=total-mental-health-care-hpsa-designations&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed March 13, 2017.
16. Commission on Collegiate Nursing Education. CCNE-Accredited Doctor of Nursing Practice (DNP) Programs. http://directory.ccnecommunity.org/reports/rptAccreditedPrograms_New.asp?sort=state&sProgramType=3. Accessed March 15, 2017.
17. National Residency Match Program. 2016 match results by state, specialty, and applicant type. http://www.nrmp.org/wp-content/uploads/2016/04/Main-Match-Results-by-State-and-Specialty-2016.pdf. Accessed March 13, 2017.
Tips for significant others
Heather Howell has gotten pretty good at making the most out of SHM’s annual meeting. It’s not that she has a system for wending through scores of educational offerings, a knack for interpersonal networking or award-winning research.
It’s that she’s the spouse of former SHM President Eric Howell, MD, MHM, and a long-time annual meeting attendee with her husband.
Welcome to HM17, family style. While thousands of hospitalists, nonphysician practitioners, and other attendees swarm the Mandalay Bay Resort and Casino for a four-day crash course on all things hospital medicine, thousands more family members tag along. Husbands and wives, like Mrs. Howell, and, in years past, children like the Howells’ 14-year-old son Mason and 12-year-old daughter Anna. The kids aren’t traveling this year, which is tip No. 1.
“It gets harder as they’re older to drag them to San Diego or Vegas in the middle of a school year, which is when [the annual meeting] is usually held,” said Mrs. Howell, whose day job is as a real estate agent.
Tip No. 2? Make friends the first time around. Maybe it’s with spouses of other physicians from your significant other’s practice. Or maybe it’s with your spouse’s old friends from past jobs. For Mrs. Howell, it’s SHM staff and the families of board members her husband has worked with for years.
“I’ve been doing it for so long that I’ve met a lot of the other [spouses] that do go,” she said. “Usually, if Eric is in meetings all day, I will connect with some of the other spouses and we will go on excursions that are in that town. There is usually so much going on.”
Las Vegas is certainly no exception. In fact, SHM has a dedicated web page recommending family activities. Recommendations include hanging out at the 11-acre Mandalay Beach, which encompasses 2,700 tons of sand, three pools and a lazy river. There’s also the popular Shark Reef Aquarium, a 1.6 million-gallon saltwater habitat with some 2,000 creatures.
Mrs. Howell says excursions further afield could include Red Rock Canyon National Conservation Area, which lies a 25-minute drive from the convention, or the Grand Canyon, which is about two hours east. But planning too much, especially with children, can become a challenge.
“When I arrive, there always seems to be a group of people that are going to do things,” Mrs. Howell said. “It’s very easy to hook up with the other spouses that aren’t involved in the meeting. We always tend to find each other.”
Heather Howell has gotten pretty good at making the most out of SHM’s annual meeting. It’s not that she has a system for wending through scores of educational offerings, a knack for interpersonal networking or award-winning research.
It’s that she’s the spouse of former SHM President Eric Howell, MD, MHM, and a long-time annual meeting attendee with her husband.
Welcome to HM17, family style. While thousands of hospitalists, nonphysician practitioners, and other attendees swarm the Mandalay Bay Resort and Casino for a four-day crash course on all things hospital medicine, thousands more family members tag along. Husbands and wives, like Mrs. Howell, and, in years past, children like the Howells’ 14-year-old son Mason and 12-year-old daughter Anna. The kids aren’t traveling this year, which is tip No. 1.
“It gets harder as they’re older to drag them to San Diego or Vegas in the middle of a school year, which is when [the annual meeting] is usually held,” said Mrs. Howell, whose day job is as a real estate agent.
Tip No. 2? Make friends the first time around. Maybe it’s with spouses of other physicians from your significant other’s practice. Or maybe it’s with your spouse’s old friends from past jobs. For Mrs. Howell, it’s SHM staff and the families of board members her husband has worked with for years.
“I’ve been doing it for so long that I’ve met a lot of the other [spouses] that do go,” she said. “Usually, if Eric is in meetings all day, I will connect with some of the other spouses and we will go on excursions that are in that town. There is usually so much going on.”
Las Vegas is certainly no exception. In fact, SHM has a dedicated web page recommending family activities. Recommendations include hanging out at the 11-acre Mandalay Beach, which encompasses 2,700 tons of sand, three pools and a lazy river. There’s also the popular Shark Reef Aquarium, a 1.6 million-gallon saltwater habitat with some 2,000 creatures.
Mrs. Howell says excursions further afield could include Red Rock Canyon National Conservation Area, which lies a 25-minute drive from the convention, or the Grand Canyon, which is about two hours east. But planning too much, especially with children, can become a challenge.
“When I arrive, there always seems to be a group of people that are going to do things,” Mrs. Howell said. “It’s very easy to hook up with the other spouses that aren’t involved in the meeting. We always tend to find each other.”
Heather Howell has gotten pretty good at making the most out of SHM’s annual meeting. It’s not that she has a system for wending through scores of educational offerings, a knack for interpersonal networking or award-winning research.
It’s that she’s the spouse of former SHM President Eric Howell, MD, MHM, and a long-time annual meeting attendee with her husband.
Welcome to HM17, family style. While thousands of hospitalists, nonphysician practitioners, and other attendees swarm the Mandalay Bay Resort and Casino for a four-day crash course on all things hospital medicine, thousands more family members tag along. Husbands and wives, like Mrs. Howell, and, in years past, children like the Howells’ 14-year-old son Mason and 12-year-old daughter Anna. The kids aren’t traveling this year, which is tip No. 1.
“It gets harder as they’re older to drag them to San Diego or Vegas in the middle of a school year, which is when [the annual meeting] is usually held,” said Mrs. Howell, whose day job is as a real estate agent.
Tip No. 2? Make friends the first time around. Maybe it’s with spouses of other physicians from your significant other’s practice. Or maybe it’s with your spouse’s old friends from past jobs. For Mrs. Howell, it’s SHM staff and the families of board members her husband has worked with for years.
“I’ve been doing it for so long that I’ve met a lot of the other [spouses] that do go,” she said. “Usually, if Eric is in meetings all day, I will connect with some of the other spouses and we will go on excursions that are in that town. There is usually so much going on.”
Las Vegas is certainly no exception. In fact, SHM has a dedicated web page recommending family activities. Recommendations include hanging out at the 11-acre Mandalay Beach, which encompasses 2,700 tons of sand, three pools and a lazy river. There’s also the popular Shark Reef Aquarium, a 1.6 million-gallon saltwater habitat with some 2,000 creatures.
Mrs. Howell says excursions further afield could include Red Rock Canyon National Conservation Area, which lies a 25-minute drive from the convention, or the Grand Canyon, which is about two hours east. But planning too much, especially with children, can become a challenge.
“When I arrive, there always seems to be a group of people that are going to do things,” Mrs. Howell said. “It’s very easy to hook up with the other spouses that aren’t involved in the meeting. We always tend to find each other.”
Hot Topics in Primary Care
This supplement includes 2 CME credits. (scroll down)
Topics include:
- Biologics, Biosimilars, and Generics
- Community-Acquired Bacterial Pneumonia
- Cardiovascular Safety of Medications for Type 2 Diabetes Mellitus
- Dual therapy for Type 2 Diabetes Mellitus
- GLP-1R Agonists
- Medication Adherence in Type 2 Diabetes Mellitus
- NSAIDs
- Sublingual Immunotherapy
This supplement offers the opportunity to earn a total of 2 CME credits.
Credit is awarded for successful completion of the online evaluations at the links below; these links may also be found within the supplement on the first page of each article.
- Diagnosis of Cirrhosis and Evaluation of Hepatic Encephalopathy: Common Errors and Their Significance for the PCP
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go to http://www.pceconsortium.org/liver.
- Role of the Microbiome in Disease: Implications for Treatment of Irritable Bowel Syndrome
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go to http://www.pceconsortium.org/microbiome.
This supplement includes 2 CME credits. (scroll down)
Topics include:
- Biologics, Biosimilars, and Generics
- Community-Acquired Bacterial Pneumonia
- Cardiovascular Safety of Medications for Type 2 Diabetes Mellitus
- Dual therapy for Type 2 Diabetes Mellitus
- GLP-1R Agonists
- Medication Adherence in Type 2 Diabetes Mellitus
- NSAIDs
- Sublingual Immunotherapy
This supplement offers the opportunity to earn a total of 2 CME credits.
Credit is awarded for successful completion of the online evaluations at the links below; these links may also be found within the supplement on the first page of each article.
- Diagnosis of Cirrhosis and Evaluation of Hepatic Encephalopathy: Common Errors and Their Significance for the PCP
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go to http://www.pceconsortium.org/liver.
- Role of the Microbiome in Disease: Implications for Treatment of Irritable Bowel Syndrome
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go to http://www.pceconsortium.org/microbiome.
This supplement includes 2 CME credits. (scroll down)
Topics include:
- Biologics, Biosimilars, and Generics
- Community-Acquired Bacterial Pneumonia
- Cardiovascular Safety of Medications for Type 2 Diabetes Mellitus
- Dual therapy for Type 2 Diabetes Mellitus
- GLP-1R Agonists
- Medication Adherence in Type 2 Diabetes Mellitus
- NSAIDs
- Sublingual Immunotherapy
This supplement offers the opportunity to earn a total of 2 CME credits.
Credit is awarded for successful completion of the online evaluations at the links below; these links may also be found within the supplement on the first page of each article.
- Diagnosis of Cirrhosis and Evaluation of Hepatic Encephalopathy: Common Errors and Their Significance for the PCP
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go to http://www.pceconsortium.org/liver.
- Role of the Microbiome in Disease: Implications for Treatment of Irritable Bowel Syndrome
- To complete the online evaluation and receive 1 CME credit for this article: please click on the link at the end of the article or go to http://www.pceconsortium.org/microbiome.
VIDEO: When early-stage vulvar cancer recurs, the prognosis is poor
NATIONAL HARBOR, MD – Cancer of the vulva is becoming more common, and as incidence is rising, so are death rates from the relatively rare malignancy. A new examination of women with early-stage vulvar cancer showed that recurrence was common, and carried a poor prognosis.
Cancer in “nearly 25% of women with stage IB squamous cell carcinoma of the vulva will recur within 3 years of initial diagnosis,” said the study’s lead author, Rebecca Stone, MD, at the annual meeting of the Society of Gynecologic Oncology. Although most (85%) of the cancers that recur do so locally, salvage rates for patients with recurrences are poor, she said in a video interview.
Dr. Stone of Johns Hopkins University, Baltimore, said that the study looked at the subset of 59 patients with recurrence of their stage IB vulvar cancer, drawing from a total of 244 stage IB patients for whom complete data were available.
Current National Comprehensive Cancer Network (NCCN) guidelines for the treatment of early-stage vulvar squamous cell carcinoma (meaning T1 or 4 cm or smaller T2 lesions) call for adjuvant radiotherapy for patients with positive margins or unresectable lesions. For patients with negative margins after the primary procedure or re-excision, the guidelines say that radiotherapy decisions should be guided by risk factors including margin width, tumor size, the presence of lymphovascular invasion, and the pattern and depth of invasion.
However, Dr. Stone said, “There’s a knowledge gap in our current management of early-stage disease.” Some unknowns include what the threshold should be for tumor recurrence in terms of depth of invasion and size of tumor, whether perineural invasion is a risk factor, and whether adverse clinical factors are important. Also, she said, it’s not known how risk factors combine to increase recurrence risk.
Tumor size, invasion depth over 6 mm, and patient age were all risk factors identified by the study; perineural and lymphovascular invasion were also potential risk factors for recurrence.
The retrospective study, said Dr. Stone, is the largest multi-institutional cohort study examining stage IB vulvar cancer that has been completed, and it accomplished a detailed examination of which patients have recurrences, factors associated with recurrence, and postrecurrence salvage rates.
Dr. Stone reported no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
NATIONAL HARBOR, MD – Cancer of the vulva is becoming more common, and as incidence is rising, so are death rates from the relatively rare malignancy. A new examination of women with early-stage vulvar cancer showed that recurrence was common, and carried a poor prognosis.
Cancer in “nearly 25% of women with stage IB squamous cell carcinoma of the vulva will recur within 3 years of initial diagnosis,” said the study’s lead author, Rebecca Stone, MD, at the annual meeting of the Society of Gynecologic Oncology. Although most (85%) of the cancers that recur do so locally, salvage rates for patients with recurrences are poor, she said in a video interview.
Dr. Stone of Johns Hopkins University, Baltimore, said that the study looked at the subset of 59 patients with recurrence of their stage IB vulvar cancer, drawing from a total of 244 stage IB patients for whom complete data were available.
Current National Comprehensive Cancer Network (NCCN) guidelines for the treatment of early-stage vulvar squamous cell carcinoma (meaning T1 or 4 cm or smaller T2 lesions) call for adjuvant radiotherapy for patients with positive margins or unresectable lesions. For patients with negative margins after the primary procedure or re-excision, the guidelines say that radiotherapy decisions should be guided by risk factors including margin width, tumor size, the presence of lymphovascular invasion, and the pattern and depth of invasion.
However, Dr. Stone said, “There’s a knowledge gap in our current management of early-stage disease.” Some unknowns include what the threshold should be for tumor recurrence in terms of depth of invasion and size of tumor, whether perineural invasion is a risk factor, and whether adverse clinical factors are important. Also, she said, it’s not known how risk factors combine to increase recurrence risk.
Tumor size, invasion depth over 6 mm, and patient age were all risk factors identified by the study; perineural and lymphovascular invasion were also potential risk factors for recurrence.
The retrospective study, said Dr. Stone, is the largest multi-institutional cohort study examining stage IB vulvar cancer that has been completed, and it accomplished a detailed examination of which patients have recurrences, factors associated with recurrence, and postrecurrence salvage rates.
Dr. Stone reported no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
NATIONAL HARBOR, MD – Cancer of the vulva is becoming more common, and as incidence is rising, so are death rates from the relatively rare malignancy. A new examination of women with early-stage vulvar cancer showed that recurrence was common, and carried a poor prognosis.
Cancer in “nearly 25% of women with stage IB squamous cell carcinoma of the vulva will recur within 3 years of initial diagnosis,” said the study’s lead author, Rebecca Stone, MD, at the annual meeting of the Society of Gynecologic Oncology. Although most (85%) of the cancers that recur do so locally, salvage rates for patients with recurrences are poor, she said in a video interview.
Dr. Stone of Johns Hopkins University, Baltimore, said that the study looked at the subset of 59 patients with recurrence of their stage IB vulvar cancer, drawing from a total of 244 stage IB patients for whom complete data were available.
Current National Comprehensive Cancer Network (NCCN) guidelines for the treatment of early-stage vulvar squamous cell carcinoma (meaning T1 or 4 cm or smaller T2 lesions) call for adjuvant radiotherapy for patients with positive margins or unresectable lesions. For patients with negative margins after the primary procedure or re-excision, the guidelines say that radiotherapy decisions should be guided by risk factors including margin width, tumor size, the presence of lymphovascular invasion, and the pattern and depth of invasion.
However, Dr. Stone said, “There’s a knowledge gap in our current management of early-stage disease.” Some unknowns include what the threshold should be for tumor recurrence in terms of depth of invasion and size of tumor, whether perineural invasion is a risk factor, and whether adverse clinical factors are important. Also, she said, it’s not known how risk factors combine to increase recurrence risk.
Tumor size, invasion depth over 6 mm, and patient age were all risk factors identified by the study; perineural and lymphovascular invasion were also potential risk factors for recurrence.
The retrospective study, said Dr. Stone, is the largest multi-institutional cohort study examining stage IB vulvar cancer that has been completed, and it accomplished a detailed examination of which patients have recurrences, factors associated with recurrence, and postrecurrence salvage rates.
Dr. Stone reported no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Subscapularis Tenotomy Versus Lesser Tuberosity Osteotomy for Total Shoulder Arthroplasty: A Systematic Review
Take-Home Points
- According to the orthopedic literature, ST and LTO for a TSA produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
- Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions.
- ST and LTO approaches for a TSA result in similar Constant scores, pain scores, radiographic outcomes, and complication rates.
During total shoulder arthroplasty (TSA) exposure, the subscapularis muscle must be mobilized; its repair is crucial to the stability of the arthroplasty. The subscapularis is the largest rotator cuff muscle and has a contractile force equal to that of the other 3 muscles combined.1,2 Traditionally it is mobilized with a tenotomy just medial to the tendon’s insertion onto the lesser tuberosity. Over the past 15 years, however, numerous authors have reported dysfunction after subscapularis tenotomy (ST). In 2003, Miller and colleagues3 reported that, at 2-year follow-up, almost 70% of patients had abnormal belly-press and liftoff tests, surrogate markers of subscapularis function. Other authors have found increased rates of anterior instability after subscapularis rupture.4,5
In 2005, Gerber and colleagues6 introduced a technique for circumventing surgical division of the subscapularis. They described a lesser tuberosity osteotomy (LTO), in which the subscapularis tendon is detached with a bone fragment 5 mm to 10 mm in thickness and 3 cm to 4 cm in length. This approach was based on the premise that bone-to-bone healing is more reliable than tendon-to-tendon healing. Initial studies reported successful osteotomy healing, improved clinical outcome scores, and fewer abnormalities with belly-press and liftoff tests.2,6 More recent literature, however, has questioned the necessity of LTO.2,4,7-9We performed a systematic review to evaluate the literature, describe ST and LTO, and summarize the radiographic and clinical outcomes of both techniques. We hypothesized there would be no significant clinical differences between these approaches.
Methods
Search Strategy and Study Selection
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we systematically reviewed the literature.10 Searches were completed in September 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Two reviewers (Dr. Louie, Dr. Levy) independently performed the search and assessed eligibility of all relevant studies based on predetermined inclusion criteria. Disagreements between reviewers were resolved by discussion. Key word selection was designed to capture all English-language studies with clinical and/or radiographic outcomes and level I to IV evidence. We used an electronic search algorithm with key words and a series of NOT phrases to match certain exclusion criteria:
(((((((((((((((((((((((((((((((((((((total[Text Word]) AND shoulder[Title]) AND arthroplasty[Title] AND (English[lang]))) NOT reverse[Title/Abstract]) NOT hemiarthroplasty[Title]) NOT nonoperative[Title]) NOT nonsurgical[Title] AND (English[lang]))) NOT rheumatoid[Title/Abstract]) NOT inflammatory[Title/Abstract]) NOT elbow[Title/Abstract]) NOT wrist[Title/Abstract]) NOT hip[Title/Abstract]) NOT knee[Title/Abstract]) NOT ankle[Title/Abstract] AND (English[lang]))) NOT biomechanic[Title/Abstract]) NOT biomechanics[Title/Abstract]) NOT biomechanical [Title/Abstract]) NOT cadaveric[Title/Abstract]) NOT revision[Title]) NOT resurfacing[Title/Abstract]) NOT surface[Title/Abstract]) NOT interphalangeal[Title/Abstract] AND (English[lang]))) NOT radiostereometric[Title/Abstract] AND (English[lang]))) NOT cmc[Title/Abstract]) NOT carpometacarpal[Title/Abstract]) NOT cervical[Title/Abstract]) NOT histology[Title/Abstract]) NOT histological[Title/Abstract]) NOT collagen[Title/Abstract] AND (English[lang]))) NOT kinematic[Title/Abstract]) NOT kinematics[Title/Abstract] AND (English[lang]))) NOT vitro[Title/Abstract] AND (English[lang]))) NOT inverted[Title/Abstract]) NOT grammont[Title/Abstract]) NOT arthrodesis[Title/Abstract]) NOT fusion[Title/Abstract]) NOT reverse[Title/Abstract] AND (English[lang]))
Study exclusion criteria consisted of cadaveric, biomechanical, histologic, and kinematic results as well as analyses of nonoperative management, hemiarthroplasty, or reverse TSA. Studies were excluded if they did not report clinical and/or radiographic data. Minimum mean follow-up was 2 years. To discount the effect of other TSA technical innovations, we evaluated the same period for the 2 surgical approaches. The first study with clinical outcomes after LTO was published in early 2005,6 so all studies published before 2005 were excluded.
We reviewed all references within the studies included by the initial search algorithm: randomized control trials, retrospective and prospective cohort designs, case series, and treatment studies. Technical notes, review papers, letters to the editor, and level V evidence reviews were excluded. To avoid counting patients twice, we compared each study’s authors and data collection period with those of the other studies. If there was overlap in authorship, period, and place, only the study with the longer follow-up or more comprehensive data was included. All trials comparing ST and LTO were included. If the authors of a TSA study did not describe the approach used, that study was excluded from our review.
Data Extraction
We collected details of study design, sample size, and patient demographics (sex, age, hand dominance, primary diagnosis). We also abstracted surgical factors about the glenoid component (cemented vs uncemented; pegged vs keeled; all-polyethylene vs metal-backed) and the humeral component (cemented vs press-fit; stemmed vs stemless). Clinical outcomes included pain scores, functional scores, number of revisions, range of motion (ROM), and subscapularis-specific tests (eg, belly-press, liftoff). As pain scales varied between studies, all values were converted to a 10-point scoring scale (0 = no pain; 10 = maximum pain) for comparisons. Numerous functional outcome scores were reported, but the Constant score was the only one consistently used across studies, making it a good choice for comparisons. One study used Penn Shoulder Scores (PSSs) and directly compared ST and LTO groups, so its data were included. In addition, radiographic data were compiled: radiolucencies around the humeral stem and glenoid component, humeral head subluxation/migration, and osteotomy healing. The only consistent radiographic parameter available for comparisons between groups was the presence of radiolucencies.
The Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues,11 was used to evaluate the methodologic quality of each study. The MCMS is a 15-item instrument that has been used to assess both randomized and nonrandomized trials.12,13 It has a scaled score ranging from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor). Study quality was not factored into the data synthesis analysis.
Statistical Analysis
Data are reported as weighted means and standard deviations. A mean was calculated for each study reporting on a respective data point and was then weighed according to the study sample size. The result was that the nonweighted means from studies with smaller samples did not carry as much weight as those from studies with larger samples. Student t tests and 2-way analysis of variance were used to compare the ST and LTO groups and assess differences over time (SPSS Version 18; IBM). An α of 0.05 was set as statistically significant.
Results
Twenty studies (1420 shoulders, 1392 patients) were included in the final dataset (Figure).2,6,8,14-30
The youngest patients in the ST and LTO groups were 22 years and 19 years of age, respectively.
Table 2 lists the details regarding the surgical components. For glenoid components, the ST and LTO groups’ fixation types and material used were not significantly different.
Table 3 lists the clinical and radiographic outcomes most consistently reported in the literature. Physical examination data were reported in 18 ST populations8,14-16,21-30 and 11 LTO populations.2,6,14-20
Constant scores were reported in 4 ST studies14,22,24,27 and 3 LTO studies14,17,18 (Table 3). There was no significant difference (P = .37) in post-TSA Constant score improvement between the 2 groups. In the one study that performed direct comparisons, PSS improved on average from 29 to 81 in the ST group and from 29 to 92 in the LTO group.15 Several ST studies reported improved scores on various indices: WOOS (Western Ontario Osteoarthritis of the Shoulder), ASES (American Shoulder and Elbow Surgeons), SST (Simple Shoulder Test), DASH (Disabilities of the Arm, Shoulder, and Hand), SF-12 (Short Form 12-Item Health Survey), MACTAR (McMaster Toronto Arthritis Patient Preference Disability Questionnaire), and Neer shoulder impingement test.8,14,15,21,23-25,27-30 However, these outcomes were not reported in LTO cohorts for comparison. Similarly, 2 LTO cohorts reported improvements in SSV (subjective shoulder value) scores, but this measure was not used in the ST cohorts.6,17 Five ST studies recorded patients’ subjective satisfaction: 58% of patients indicated an excellent outcome, 35% a satisfactory outcome, and 7% a less than satisfactory outcome.21,23,25,26,29 Only 1 LTO study reported patient satisfaction: 69% excellent, 31% satisfactory, 0% dissatisfied.17
Complications were reported in 16 ST studies8,15,21-30 and 6 LTO studies.15,17-19 There were 117 complications (17.8%) and 58 revisions (10.0%) in the ST group and 52 complications (17.2%) and 49 revisions (16.2%) in the LTO group. In the ST group, aseptic loosening (6.2%) was the most common complication, followed by subscapularis tear or attenuation (5.2%), dislocation (2.1%), and deep infection (0.5%). In the LTO group, aseptic loosening was again the most common (9.0%), followed by dislocation (4.0%), subscapularis tear or attenuation (2.2%), and deep infection (0.7%). There were no significant differences in the incidence of individual complications between groups. The difference in revision rates was not statistically significant (P = .31).
Radiolucency data were reported in 12 ST studies19,21-26,28,30 and 2 LTO studies.17,18 There were no discussions of humeral component radiolucencies in the LTO studies. At final follow-up, radiolucencies of the glenoid component were detected in 42.3% of patients in the ST group and 40.7% of patients in the LTO group (P = .76).
Discussion
Our goal in this systematic review was to analyze outcomes associated with ST and LTO in a heterogenous TSA population. We hypothesized TSA with ST or LTO would produce similar clinical and radiographic outcomes. There were no significant differences in Constant scores, pain scores, radiolucencies, or complications between the 2 groups. The ST group showed trends toward wider ROM improvements and fewer revisions, but only the change in forward elevation was significant. The components used in the 2 groups were similar with the exception of a lack of keeled glenoids and cemented humeral stems in the LTO group; data stratification controlling for these differences revealed no change in outcomes.
The optimal method of subscapularis mobilization for TSA remains a source of debate. Jackson and colleagues23 found significant improvements in Neer and DASH scores after ST. However, 7 of 15 patients ruptured the subscapularis after 6 months and had significantly lower DASH scores. In 2005, Gerber and colleagues6 first described the LTO technique as an alternative to ST. After a mean of 39 months, 89% of their patients had a negative belly-press test, and 75% had a normal liftoff test. Radiographic evaluation revealed that the osteotomized fragment had healed in an anatomical position in all shoulders. In a large case series, Small and colleagues20 used radiographs and computed tomography to further investigate LTO healing rates and found that 89% of patients had bony union by 6 months and that smoking was a significant risk factor for nonunion.
Biomechanical studies comparing ST and LTO approaches have shown mixed results. Ponce and colleagues2 found decreased cyclic displacement and increased maximum load to failure with LTO, but Giuseffi and colleagues32 showed less cyclic displacement with ST and no difference in load to failure. Others authors have found no significant differences in stiffness or maximum load to failure.33 Van den Berghe and colleagues7 reported a higher failure rate in bone-to-bone repairs compared with tendon-to-tendon constructs. Moreover, they found that suture cut-out through bone tunnels is the primary mode of LTO failure, so many LTO surgeons now pass sutures around the humeral stem instead.
Three TSA studies directly compared ST and LTO approaches. Buckley and colleagues14 analyzed 60 TSAs and found no significant differences in WOOS, DASH, or Constant scores between groups. The authors described an ST subgroup with subscapularis attenuation on ultrasound but did not report the group as having any inferior functional outcome. Scalise and colleagues15 showed improved strength and PSSs in both groups after 2 years. However, the LTO group had a lower rate of subscapularis tears and significantly higher PSSs. Finally, Jandhyala and colleagues16 reported more favorable outcomes with LTO, which trended toward wider ROM and significantly higher belly-press test grades. Lapner and colleagues34 conducted a randomized, controlled trial (often referenced) and found no significant differences between the 2 groups in terms of strength or functional outcome at 2-year follow-up. Their study, however, included hemiarthroplasties and did not substratify the TSA population, so we did not include it in our review.
Our systematic review found significantly more forward elevation improvement for the ST group than the LTO group, which may suggest improved ROM with a soft-tissue approach than a bony approach. At the same time, the ST group trended toward better passive external rotation relative to the LTO group. This trend indicates fewer constraints to external rotation in the ST group, possibly attributable to a more attenuated subscapularis after tenotomy. Subscapularis tear or attenuation was more commonly reported in the ST group than in the LTO group, though not significantly so. This may indicate that more ST studies than LTO studies specially emphasized postoperative subscapularis function, but these data also highlight some authors’ concerns regarding subscapularis dysfunction after tenotomy.6,15,16The study populations’ complication rates were similar, just over 17%. The LTO group trended toward a higher revision rate, but it was not statistically significant. The LTO group also had significantly fewer patients with osteoarthritis and more patients with posttraumatic arthritis, so this group may have had more complex patients predisposed to a higher likelihood of revision surgery. Revisions were most commonly performed for aseptic loosening; theoretically, if osteotomies heal less effectively than tenotomies, the LTO approach could produce component instability and aseptic loosening. However, no prior studies or other clinical findings from this review suggest LTO predisposes to aseptic loosening. Overall, the uneven revision rates represent a clinical concern that should be monitored as larger samples of patients undergo ST and LTO procedures.
Glenoid radiolucencies were the only radiographic parameter consistently reported in the included studies. Twelve ST studies had radiolucency data—compared with only 2 LTO studies. Thus, our ability to compare radiographic outcomes was limited. Our data revealed similar rates of glenoid radiolucencies between the 2 approaches. The clinical relevance of radiolucencies is questioned by some authors, and, indeed, Razmjou and colleagues25 found no correlation of radiolucencies with patient satisfaction. Nevertheless, early presence of radiolucencies may raise concerns about progressive loss of fixation,35,36 so this should be monitored.
Limitations of this systematic review reflect the studies analyzed. We minimized selection bias by including level I to IV evidence, but most studies were level IV, and only 1 was level I. As such, there was a relative paucity of consistent clinical and radiographic data. For instance, although many ST studies reported patient satisfaction as an outcomes measure, only 1 LTO study commented on it. Perhaps the relative novelty of the LTO approach has prompted some authors to focus more on technical details and less on reporting a variety of outcome measures. As mentioned earlier, the significance of radiolucency data is controversial, and determination of their presence or absence depends on the observer. A radiolucency found in one study may not qualify as one in a study that uses different criteria. However, lucency data were the most frequently and reliably reported radiographic parameter, so we deemed it the most appropriate method for comparing radiographic outcomes. Finally, the baseline differences in diagnosis between the ST and LTO groups complicated comparisons. We stratified the groups by component design because use of keeled or pegged implants or humeral cemented or press-fit stems was usually a uniform feature of each study—enabling removal of certain studies for data stratification. However, we were unable to stratify by original diagnosis because these groups were not stratified within the individual studies.
Conclusion
Our systematic review found similar Constant scores, pain scores, radiographic outcomes, and complication rates for the ST and LTO approaches. Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions. Although not definitive, these data suggest the ST approach may provide more stability over the long term, but additional comprehensive studies are needed to increase the sample size and the power of the trends elucidated in this review. According to the orthopedic literature, both techniques produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
Am J Orthop. 2017;46(2):E131-E138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Keating JF, Waterworth P, Shaw-Dunn J, Crossan J. The relative strengths of the rotator cuff muscles. A cadaver study. J Bone Joint Surg Br. 1993;75(1):137-140.
2. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity repair technique in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87(suppl 2):1-8.
3. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34.
4. Gerber A, Ghalambor N, Warner JJ. Instability of shoulder arthroplasty: balancing mobility and stability. Orthop Clin North Am. 2001;32(4):661-670, ix.
5. Moeckel BH, Altchek DW, Warren RF, Wickiewicz TL, Dines DM. Instability of the shoulder after arthroplasty. J Bone Joint Surg Am. 1993;75(4):492-497.
6. Gerber C, Yian EH, Pfirrmann CA, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745.
7. Van den Berghe GR, Nguyen B, Patil S, et al. A biomechanical evaluation of three surgical techniques for subscapularis repair. J Shoulder Elbow Surg. 2008;17(1):156-161.
8. Caplan JL, Whitfield B, Neviaser RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196.
9. Armstrong A, Lashgari C, Teefey S, Menendez J, Yamaguchi K, Galatz LM. Ultrasound evaluation and clinical correlation of subscapularis repair after total shoulder arthroplasty. J Shoulder Elbow Surg. 2006;15(5):541-548.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
11. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
12. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
13. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
14. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317.
15. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic, and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634.
16. Jandhyala S, Unnithan A, Hughes S, Hong T. Subscapularis tenotomy versus lesser tuberosity osteotomy during total shoulder replacement: a comparison of patient outcomes. J Shoulder Elbow Surg. 2011;20(7):1102-1107.
17. Fucentese SF, Costouros JG, Kühnel SP, Gerber C. Total shoulder arthroplasty with an uncemented soft-metal-backed glenoid component. J Shoulder Elbow Surg. 2010;19(4):624-631.
18. Clement ND, Duckworth AD, Colling RC, Stirrat AN. An uncemented metal-backed glenoid component in total shoulder arthroplasty for osteoarthritis: factors affecting survival and outcome. J Orthop Sci. 2013;18(1):22-28.
19. Rosenberg N, Neumann L, Modi A, Mersich IJ, Wallace AW. Improvements in survival of the uncemented Nottingham Total Shoulder prosthesis: a prospective comparative study. BMC Musculoskelet Disord. 2007;8(1):76.
20. Small KM, Siegel EJ, Miller LR, Higgins LD. Imaging characteristics of lesser tuberosity osteotomy after total shoulder replacement: a study of 220 patients. J Shoulder Elbow Surg. 2014;23(9):1318-1326.
21. Mileti J, Sperling JW, Cofield RH, Harrington JR, Hoskin TL. Monoblock and modular total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Br. 2005;87(4):496-500.
22. Merolla G, Paladini P, Campi F, Porcellini G. Efficacy of anatomical prostheses in primary glenohumeral osteoarthritis. Chir Organi Mov. 2008;91(2):109-115.
23. Jackson JD, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090.
24. Jost PW, Dines JS, Griffith MH, Angel M, Altchek DW, Dines DM. Total shoulder arthroplasty utilizing mini-stem humeral components: technique and short-term results. HSS J. 2011;7(3):213-217.
25. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
26. Raiss P, Schmitt M, Bruckner T, et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94(23):e1711-1710.
27. Litchfied RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthritis of the shoulder: a prospective, randomized, double-blind clinical trial—a JOINTs Canada Project. J Shoulder Elbow Surg. 2011;20(4):529-536.
28. Martin SD, Zurakowski D, Thornhill TS. Uncemented glenoid component in total shoulder arthroplasty. Survivorship and outcomes. J Bone Joint Surg Am. 2005;87(6):1284-1292.
29. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188.
30. Budge MD, Nolan EM, Heisey MH, Baker K, Wiater JM. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Shoulder Elbow Surg. 2013;22(4):535-541.
31. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510.
32. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095.
33. Van Thiel GS, Wang VM, Wang FC, et al. Biomechanical similarities among subscapularis repairs after shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(5):657-663.
34. Lapner PL, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of lesser tuberosity osteotomy to subscapularis peel in shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(24):2239-2246.
35. Cofield RH. Total shoulder arthroplasty with the Neer prosthesis. J Bone Joint Surg Am. 1984;66(6):899-906.
36. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
Take-Home Points
- According to the orthopedic literature, ST and LTO for a TSA produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
- Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions.
- ST and LTO approaches for a TSA result in similar Constant scores, pain scores, radiographic outcomes, and complication rates.
During total shoulder arthroplasty (TSA) exposure, the subscapularis muscle must be mobilized; its repair is crucial to the stability of the arthroplasty. The subscapularis is the largest rotator cuff muscle and has a contractile force equal to that of the other 3 muscles combined.1,2 Traditionally it is mobilized with a tenotomy just medial to the tendon’s insertion onto the lesser tuberosity. Over the past 15 years, however, numerous authors have reported dysfunction after subscapularis tenotomy (ST). In 2003, Miller and colleagues3 reported that, at 2-year follow-up, almost 70% of patients had abnormal belly-press and liftoff tests, surrogate markers of subscapularis function. Other authors have found increased rates of anterior instability after subscapularis rupture.4,5
In 2005, Gerber and colleagues6 introduced a technique for circumventing surgical division of the subscapularis. They described a lesser tuberosity osteotomy (LTO), in which the subscapularis tendon is detached with a bone fragment 5 mm to 10 mm in thickness and 3 cm to 4 cm in length. This approach was based on the premise that bone-to-bone healing is more reliable than tendon-to-tendon healing. Initial studies reported successful osteotomy healing, improved clinical outcome scores, and fewer abnormalities with belly-press and liftoff tests.2,6 More recent literature, however, has questioned the necessity of LTO.2,4,7-9We performed a systematic review to evaluate the literature, describe ST and LTO, and summarize the radiographic and clinical outcomes of both techniques. We hypothesized there would be no significant clinical differences between these approaches.
Methods
Search Strategy and Study Selection
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we systematically reviewed the literature.10 Searches were completed in September 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Two reviewers (Dr. Louie, Dr. Levy) independently performed the search and assessed eligibility of all relevant studies based on predetermined inclusion criteria. Disagreements between reviewers were resolved by discussion. Key word selection was designed to capture all English-language studies with clinical and/or radiographic outcomes and level I to IV evidence. We used an electronic search algorithm with key words and a series of NOT phrases to match certain exclusion criteria:
(((((((((((((((((((((((((((((((((((((total[Text Word]) AND shoulder[Title]) AND arthroplasty[Title] AND (English[lang]))) NOT reverse[Title/Abstract]) NOT hemiarthroplasty[Title]) NOT nonoperative[Title]) NOT nonsurgical[Title] AND (English[lang]))) NOT rheumatoid[Title/Abstract]) NOT inflammatory[Title/Abstract]) NOT elbow[Title/Abstract]) NOT wrist[Title/Abstract]) NOT hip[Title/Abstract]) NOT knee[Title/Abstract]) NOT ankle[Title/Abstract] AND (English[lang]))) NOT biomechanic[Title/Abstract]) NOT biomechanics[Title/Abstract]) NOT biomechanical [Title/Abstract]) NOT cadaveric[Title/Abstract]) NOT revision[Title]) NOT resurfacing[Title/Abstract]) NOT surface[Title/Abstract]) NOT interphalangeal[Title/Abstract] AND (English[lang]))) NOT radiostereometric[Title/Abstract] AND (English[lang]))) NOT cmc[Title/Abstract]) NOT carpometacarpal[Title/Abstract]) NOT cervical[Title/Abstract]) NOT histology[Title/Abstract]) NOT histological[Title/Abstract]) NOT collagen[Title/Abstract] AND (English[lang]))) NOT kinematic[Title/Abstract]) NOT kinematics[Title/Abstract] AND (English[lang]))) NOT vitro[Title/Abstract] AND (English[lang]))) NOT inverted[Title/Abstract]) NOT grammont[Title/Abstract]) NOT arthrodesis[Title/Abstract]) NOT fusion[Title/Abstract]) NOT reverse[Title/Abstract] AND (English[lang]))
Study exclusion criteria consisted of cadaveric, biomechanical, histologic, and kinematic results as well as analyses of nonoperative management, hemiarthroplasty, or reverse TSA. Studies were excluded if they did not report clinical and/or radiographic data. Minimum mean follow-up was 2 years. To discount the effect of other TSA technical innovations, we evaluated the same period for the 2 surgical approaches. The first study with clinical outcomes after LTO was published in early 2005,6 so all studies published before 2005 were excluded.
We reviewed all references within the studies included by the initial search algorithm: randomized control trials, retrospective and prospective cohort designs, case series, and treatment studies. Technical notes, review papers, letters to the editor, and level V evidence reviews were excluded. To avoid counting patients twice, we compared each study’s authors and data collection period with those of the other studies. If there was overlap in authorship, period, and place, only the study with the longer follow-up or more comprehensive data was included. All trials comparing ST and LTO were included. If the authors of a TSA study did not describe the approach used, that study was excluded from our review.
Data Extraction
We collected details of study design, sample size, and patient demographics (sex, age, hand dominance, primary diagnosis). We also abstracted surgical factors about the glenoid component (cemented vs uncemented; pegged vs keeled; all-polyethylene vs metal-backed) and the humeral component (cemented vs press-fit; stemmed vs stemless). Clinical outcomes included pain scores, functional scores, number of revisions, range of motion (ROM), and subscapularis-specific tests (eg, belly-press, liftoff). As pain scales varied between studies, all values were converted to a 10-point scoring scale (0 = no pain; 10 = maximum pain) for comparisons. Numerous functional outcome scores were reported, but the Constant score was the only one consistently used across studies, making it a good choice for comparisons. One study used Penn Shoulder Scores (PSSs) and directly compared ST and LTO groups, so its data were included. In addition, radiographic data were compiled: radiolucencies around the humeral stem and glenoid component, humeral head subluxation/migration, and osteotomy healing. The only consistent radiographic parameter available for comparisons between groups was the presence of radiolucencies.
The Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues,11 was used to evaluate the methodologic quality of each study. The MCMS is a 15-item instrument that has been used to assess both randomized and nonrandomized trials.12,13 It has a scaled score ranging from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor). Study quality was not factored into the data synthesis analysis.
Statistical Analysis
Data are reported as weighted means and standard deviations. A mean was calculated for each study reporting on a respective data point and was then weighed according to the study sample size. The result was that the nonweighted means from studies with smaller samples did not carry as much weight as those from studies with larger samples. Student t tests and 2-way analysis of variance were used to compare the ST and LTO groups and assess differences over time (SPSS Version 18; IBM). An α of 0.05 was set as statistically significant.
Results
Twenty studies (1420 shoulders, 1392 patients) were included in the final dataset (Figure).2,6,8,14-30
The youngest patients in the ST and LTO groups were 22 years and 19 years of age, respectively.
Table 2 lists the details regarding the surgical components. For glenoid components, the ST and LTO groups’ fixation types and material used were not significantly different.
Table 3 lists the clinical and radiographic outcomes most consistently reported in the literature. Physical examination data were reported in 18 ST populations8,14-16,21-30 and 11 LTO populations.2,6,14-20
Constant scores were reported in 4 ST studies14,22,24,27 and 3 LTO studies14,17,18 (Table 3). There was no significant difference (P = .37) in post-TSA Constant score improvement between the 2 groups. In the one study that performed direct comparisons, PSS improved on average from 29 to 81 in the ST group and from 29 to 92 in the LTO group.15 Several ST studies reported improved scores on various indices: WOOS (Western Ontario Osteoarthritis of the Shoulder), ASES (American Shoulder and Elbow Surgeons), SST (Simple Shoulder Test), DASH (Disabilities of the Arm, Shoulder, and Hand), SF-12 (Short Form 12-Item Health Survey), MACTAR (McMaster Toronto Arthritis Patient Preference Disability Questionnaire), and Neer shoulder impingement test.8,14,15,21,23-25,27-30 However, these outcomes were not reported in LTO cohorts for comparison. Similarly, 2 LTO cohorts reported improvements in SSV (subjective shoulder value) scores, but this measure was not used in the ST cohorts.6,17 Five ST studies recorded patients’ subjective satisfaction: 58% of patients indicated an excellent outcome, 35% a satisfactory outcome, and 7% a less than satisfactory outcome.21,23,25,26,29 Only 1 LTO study reported patient satisfaction: 69% excellent, 31% satisfactory, 0% dissatisfied.17
Complications were reported in 16 ST studies8,15,21-30 and 6 LTO studies.15,17-19 There were 117 complications (17.8%) and 58 revisions (10.0%) in the ST group and 52 complications (17.2%) and 49 revisions (16.2%) in the LTO group. In the ST group, aseptic loosening (6.2%) was the most common complication, followed by subscapularis tear or attenuation (5.2%), dislocation (2.1%), and deep infection (0.5%). In the LTO group, aseptic loosening was again the most common (9.0%), followed by dislocation (4.0%), subscapularis tear or attenuation (2.2%), and deep infection (0.7%). There were no significant differences in the incidence of individual complications between groups. The difference in revision rates was not statistically significant (P = .31).
Radiolucency data were reported in 12 ST studies19,21-26,28,30 and 2 LTO studies.17,18 There were no discussions of humeral component radiolucencies in the LTO studies. At final follow-up, radiolucencies of the glenoid component were detected in 42.3% of patients in the ST group and 40.7% of patients in the LTO group (P = .76).
Discussion
Our goal in this systematic review was to analyze outcomes associated with ST and LTO in a heterogenous TSA population. We hypothesized TSA with ST or LTO would produce similar clinical and radiographic outcomes. There were no significant differences in Constant scores, pain scores, radiolucencies, or complications between the 2 groups. The ST group showed trends toward wider ROM improvements and fewer revisions, but only the change in forward elevation was significant. The components used in the 2 groups were similar with the exception of a lack of keeled glenoids and cemented humeral stems in the LTO group; data stratification controlling for these differences revealed no change in outcomes.
The optimal method of subscapularis mobilization for TSA remains a source of debate. Jackson and colleagues23 found significant improvements in Neer and DASH scores after ST. However, 7 of 15 patients ruptured the subscapularis after 6 months and had significantly lower DASH scores. In 2005, Gerber and colleagues6 first described the LTO technique as an alternative to ST. After a mean of 39 months, 89% of their patients had a negative belly-press test, and 75% had a normal liftoff test. Radiographic evaluation revealed that the osteotomized fragment had healed in an anatomical position in all shoulders. In a large case series, Small and colleagues20 used radiographs and computed tomography to further investigate LTO healing rates and found that 89% of patients had bony union by 6 months and that smoking was a significant risk factor for nonunion.
Biomechanical studies comparing ST and LTO approaches have shown mixed results. Ponce and colleagues2 found decreased cyclic displacement and increased maximum load to failure with LTO, but Giuseffi and colleagues32 showed less cyclic displacement with ST and no difference in load to failure. Others authors have found no significant differences in stiffness or maximum load to failure.33 Van den Berghe and colleagues7 reported a higher failure rate in bone-to-bone repairs compared with tendon-to-tendon constructs. Moreover, they found that suture cut-out through bone tunnels is the primary mode of LTO failure, so many LTO surgeons now pass sutures around the humeral stem instead.
Three TSA studies directly compared ST and LTO approaches. Buckley and colleagues14 analyzed 60 TSAs and found no significant differences in WOOS, DASH, or Constant scores between groups. The authors described an ST subgroup with subscapularis attenuation on ultrasound but did not report the group as having any inferior functional outcome. Scalise and colleagues15 showed improved strength and PSSs in both groups after 2 years. However, the LTO group had a lower rate of subscapularis tears and significantly higher PSSs. Finally, Jandhyala and colleagues16 reported more favorable outcomes with LTO, which trended toward wider ROM and significantly higher belly-press test grades. Lapner and colleagues34 conducted a randomized, controlled trial (often referenced) and found no significant differences between the 2 groups in terms of strength or functional outcome at 2-year follow-up. Their study, however, included hemiarthroplasties and did not substratify the TSA population, so we did not include it in our review.
Our systematic review found significantly more forward elevation improvement for the ST group than the LTO group, which may suggest improved ROM with a soft-tissue approach than a bony approach. At the same time, the ST group trended toward better passive external rotation relative to the LTO group. This trend indicates fewer constraints to external rotation in the ST group, possibly attributable to a more attenuated subscapularis after tenotomy. Subscapularis tear or attenuation was more commonly reported in the ST group than in the LTO group, though not significantly so. This may indicate that more ST studies than LTO studies specially emphasized postoperative subscapularis function, but these data also highlight some authors’ concerns regarding subscapularis dysfunction after tenotomy.6,15,16The study populations’ complication rates were similar, just over 17%. The LTO group trended toward a higher revision rate, but it was not statistically significant. The LTO group also had significantly fewer patients with osteoarthritis and more patients with posttraumatic arthritis, so this group may have had more complex patients predisposed to a higher likelihood of revision surgery. Revisions were most commonly performed for aseptic loosening; theoretically, if osteotomies heal less effectively than tenotomies, the LTO approach could produce component instability and aseptic loosening. However, no prior studies or other clinical findings from this review suggest LTO predisposes to aseptic loosening. Overall, the uneven revision rates represent a clinical concern that should be monitored as larger samples of patients undergo ST and LTO procedures.
Glenoid radiolucencies were the only radiographic parameter consistently reported in the included studies. Twelve ST studies had radiolucency data—compared with only 2 LTO studies. Thus, our ability to compare radiographic outcomes was limited. Our data revealed similar rates of glenoid radiolucencies between the 2 approaches. The clinical relevance of radiolucencies is questioned by some authors, and, indeed, Razmjou and colleagues25 found no correlation of radiolucencies with patient satisfaction. Nevertheless, early presence of radiolucencies may raise concerns about progressive loss of fixation,35,36 so this should be monitored.
Limitations of this systematic review reflect the studies analyzed. We minimized selection bias by including level I to IV evidence, but most studies were level IV, and only 1 was level I. As such, there was a relative paucity of consistent clinical and radiographic data. For instance, although many ST studies reported patient satisfaction as an outcomes measure, only 1 LTO study commented on it. Perhaps the relative novelty of the LTO approach has prompted some authors to focus more on technical details and less on reporting a variety of outcome measures. As mentioned earlier, the significance of radiolucency data is controversial, and determination of their presence or absence depends on the observer. A radiolucency found in one study may not qualify as one in a study that uses different criteria. However, lucency data were the most frequently and reliably reported radiographic parameter, so we deemed it the most appropriate method for comparing radiographic outcomes. Finally, the baseline differences in diagnosis between the ST and LTO groups complicated comparisons. We stratified the groups by component design because use of keeled or pegged implants or humeral cemented or press-fit stems was usually a uniform feature of each study—enabling removal of certain studies for data stratification. However, we were unable to stratify by original diagnosis because these groups were not stratified within the individual studies.
Conclusion
Our systematic review found similar Constant scores, pain scores, radiographic outcomes, and complication rates for the ST and LTO approaches. Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions. Although not definitive, these data suggest the ST approach may provide more stability over the long term, but additional comprehensive studies are needed to increase the sample size and the power of the trends elucidated in this review. According to the orthopedic literature, both techniques produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
Am J Orthop. 2017;46(2):E131-E138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- According to the orthopedic literature, ST and LTO for a TSA produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
- Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions.
- ST and LTO approaches for a TSA result in similar Constant scores, pain scores, radiographic outcomes, and complication rates.
During total shoulder arthroplasty (TSA) exposure, the subscapularis muscle must be mobilized; its repair is crucial to the stability of the arthroplasty. The subscapularis is the largest rotator cuff muscle and has a contractile force equal to that of the other 3 muscles combined.1,2 Traditionally it is mobilized with a tenotomy just medial to the tendon’s insertion onto the lesser tuberosity. Over the past 15 years, however, numerous authors have reported dysfunction after subscapularis tenotomy (ST). In 2003, Miller and colleagues3 reported that, at 2-year follow-up, almost 70% of patients had abnormal belly-press and liftoff tests, surrogate markers of subscapularis function. Other authors have found increased rates of anterior instability after subscapularis rupture.4,5
In 2005, Gerber and colleagues6 introduced a technique for circumventing surgical division of the subscapularis. They described a lesser tuberosity osteotomy (LTO), in which the subscapularis tendon is detached with a bone fragment 5 mm to 10 mm in thickness and 3 cm to 4 cm in length. This approach was based on the premise that bone-to-bone healing is more reliable than tendon-to-tendon healing. Initial studies reported successful osteotomy healing, improved clinical outcome scores, and fewer abnormalities with belly-press and liftoff tests.2,6 More recent literature, however, has questioned the necessity of LTO.2,4,7-9We performed a systematic review to evaluate the literature, describe ST and LTO, and summarize the radiographic and clinical outcomes of both techniques. We hypothesized there would be no significant clinical differences between these approaches.
Methods
Search Strategy and Study Selection
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we systematically reviewed the literature.10 Searches were completed in September 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Two reviewers (Dr. Louie, Dr. Levy) independently performed the search and assessed eligibility of all relevant studies based on predetermined inclusion criteria. Disagreements between reviewers were resolved by discussion. Key word selection was designed to capture all English-language studies with clinical and/or radiographic outcomes and level I to IV evidence. We used an electronic search algorithm with key words and a series of NOT phrases to match certain exclusion criteria:
(((((((((((((((((((((((((((((((((((((total[Text Word]) AND shoulder[Title]) AND arthroplasty[Title] AND (English[lang]))) NOT reverse[Title/Abstract]) NOT hemiarthroplasty[Title]) NOT nonoperative[Title]) NOT nonsurgical[Title] AND (English[lang]))) NOT rheumatoid[Title/Abstract]) NOT inflammatory[Title/Abstract]) NOT elbow[Title/Abstract]) NOT wrist[Title/Abstract]) NOT hip[Title/Abstract]) NOT knee[Title/Abstract]) NOT ankle[Title/Abstract] AND (English[lang]))) NOT biomechanic[Title/Abstract]) NOT biomechanics[Title/Abstract]) NOT biomechanical [Title/Abstract]) NOT cadaveric[Title/Abstract]) NOT revision[Title]) NOT resurfacing[Title/Abstract]) NOT surface[Title/Abstract]) NOT interphalangeal[Title/Abstract] AND (English[lang]))) NOT radiostereometric[Title/Abstract] AND (English[lang]))) NOT cmc[Title/Abstract]) NOT carpometacarpal[Title/Abstract]) NOT cervical[Title/Abstract]) NOT histology[Title/Abstract]) NOT histological[Title/Abstract]) NOT collagen[Title/Abstract] AND (English[lang]))) NOT kinematic[Title/Abstract]) NOT kinematics[Title/Abstract] AND (English[lang]))) NOT vitro[Title/Abstract] AND (English[lang]))) NOT inverted[Title/Abstract]) NOT grammont[Title/Abstract]) NOT arthrodesis[Title/Abstract]) NOT fusion[Title/Abstract]) NOT reverse[Title/Abstract] AND (English[lang]))
Study exclusion criteria consisted of cadaveric, biomechanical, histologic, and kinematic results as well as analyses of nonoperative management, hemiarthroplasty, or reverse TSA. Studies were excluded if they did not report clinical and/or radiographic data. Minimum mean follow-up was 2 years. To discount the effect of other TSA technical innovations, we evaluated the same period for the 2 surgical approaches. The first study with clinical outcomes after LTO was published in early 2005,6 so all studies published before 2005 were excluded.
We reviewed all references within the studies included by the initial search algorithm: randomized control trials, retrospective and prospective cohort designs, case series, and treatment studies. Technical notes, review papers, letters to the editor, and level V evidence reviews were excluded. To avoid counting patients twice, we compared each study’s authors and data collection period with those of the other studies. If there was overlap in authorship, period, and place, only the study with the longer follow-up or more comprehensive data was included. All trials comparing ST and LTO were included. If the authors of a TSA study did not describe the approach used, that study was excluded from our review.
Data Extraction
We collected details of study design, sample size, and patient demographics (sex, age, hand dominance, primary diagnosis). We also abstracted surgical factors about the glenoid component (cemented vs uncemented; pegged vs keeled; all-polyethylene vs metal-backed) and the humeral component (cemented vs press-fit; stemmed vs stemless). Clinical outcomes included pain scores, functional scores, number of revisions, range of motion (ROM), and subscapularis-specific tests (eg, belly-press, liftoff). As pain scales varied between studies, all values were converted to a 10-point scoring scale (0 = no pain; 10 = maximum pain) for comparisons. Numerous functional outcome scores were reported, but the Constant score was the only one consistently used across studies, making it a good choice for comparisons. One study used Penn Shoulder Scores (PSSs) and directly compared ST and LTO groups, so its data were included. In addition, radiographic data were compiled: radiolucencies around the humeral stem and glenoid component, humeral head subluxation/migration, and osteotomy healing. The only consistent radiographic parameter available for comparisons between groups was the presence of radiolucencies.
The Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues,11 was used to evaluate the methodologic quality of each study. The MCMS is a 15-item instrument that has been used to assess both randomized and nonrandomized trials.12,13 It has a scaled score ranging from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor). Study quality was not factored into the data synthesis analysis.
Statistical Analysis
Data are reported as weighted means and standard deviations. A mean was calculated for each study reporting on a respective data point and was then weighed according to the study sample size. The result was that the nonweighted means from studies with smaller samples did not carry as much weight as those from studies with larger samples. Student t tests and 2-way analysis of variance were used to compare the ST and LTO groups and assess differences over time (SPSS Version 18; IBM). An α of 0.05 was set as statistically significant.
Results
Twenty studies (1420 shoulders, 1392 patients) were included in the final dataset (Figure).2,6,8,14-30
The youngest patients in the ST and LTO groups were 22 years and 19 years of age, respectively.
Table 2 lists the details regarding the surgical components. For glenoid components, the ST and LTO groups’ fixation types and material used were not significantly different.
Table 3 lists the clinical and radiographic outcomes most consistently reported in the literature. Physical examination data were reported in 18 ST populations8,14-16,21-30 and 11 LTO populations.2,6,14-20
Constant scores were reported in 4 ST studies14,22,24,27 and 3 LTO studies14,17,18 (Table 3). There was no significant difference (P = .37) in post-TSA Constant score improvement between the 2 groups. In the one study that performed direct comparisons, PSS improved on average from 29 to 81 in the ST group and from 29 to 92 in the LTO group.15 Several ST studies reported improved scores on various indices: WOOS (Western Ontario Osteoarthritis of the Shoulder), ASES (American Shoulder and Elbow Surgeons), SST (Simple Shoulder Test), DASH (Disabilities of the Arm, Shoulder, and Hand), SF-12 (Short Form 12-Item Health Survey), MACTAR (McMaster Toronto Arthritis Patient Preference Disability Questionnaire), and Neer shoulder impingement test.8,14,15,21,23-25,27-30 However, these outcomes were not reported in LTO cohorts for comparison. Similarly, 2 LTO cohorts reported improvements in SSV (subjective shoulder value) scores, but this measure was not used in the ST cohorts.6,17 Five ST studies recorded patients’ subjective satisfaction: 58% of patients indicated an excellent outcome, 35% a satisfactory outcome, and 7% a less than satisfactory outcome.21,23,25,26,29 Only 1 LTO study reported patient satisfaction: 69% excellent, 31% satisfactory, 0% dissatisfied.17
Complications were reported in 16 ST studies8,15,21-30 and 6 LTO studies.15,17-19 There were 117 complications (17.8%) and 58 revisions (10.0%) in the ST group and 52 complications (17.2%) and 49 revisions (16.2%) in the LTO group. In the ST group, aseptic loosening (6.2%) was the most common complication, followed by subscapularis tear or attenuation (5.2%), dislocation (2.1%), and deep infection (0.5%). In the LTO group, aseptic loosening was again the most common (9.0%), followed by dislocation (4.0%), subscapularis tear or attenuation (2.2%), and deep infection (0.7%). There were no significant differences in the incidence of individual complications between groups. The difference in revision rates was not statistically significant (P = .31).
Radiolucency data were reported in 12 ST studies19,21-26,28,30 and 2 LTO studies.17,18 There were no discussions of humeral component radiolucencies in the LTO studies. At final follow-up, radiolucencies of the glenoid component were detected in 42.3% of patients in the ST group and 40.7% of patients in the LTO group (P = .76).
Discussion
Our goal in this systematic review was to analyze outcomes associated with ST and LTO in a heterogenous TSA population. We hypothesized TSA with ST or LTO would produce similar clinical and radiographic outcomes. There were no significant differences in Constant scores, pain scores, radiolucencies, or complications between the 2 groups. The ST group showed trends toward wider ROM improvements and fewer revisions, but only the change in forward elevation was significant. The components used in the 2 groups were similar with the exception of a lack of keeled glenoids and cemented humeral stems in the LTO group; data stratification controlling for these differences revealed no change in outcomes.
The optimal method of subscapularis mobilization for TSA remains a source of debate. Jackson and colleagues23 found significant improvements in Neer and DASH scores after ST. However, 7 of 15 patients ruptured the subscapularis after 6 months and had significantly lower DASH scores. In 2005, Gerber and colleagues6 first described the LTO technique as an alternative to ST. After a mean of 39 months, 89% of their patients had a negative belly-press test, and 75% had a normal liftoff test. Radiographic evaluation revealed that the osteotomized fragment had healed in an anatomical position in all shoulders. In a large case series, Small and colleagues20 used radiographs and computed tomography to further investigate LTO healing rates and found that 89% of patients had bony union by 6 months and that smoking was a significant risk factor for nonunion.
Biomechanical studies comparing ST and LTO approaches have shown mixed results. Ponce and colleagues2 found decreased cyclic displacement and increased maximum load to failure with LTO, but Giuseffi and colleagues32 showed less cyclic displacement with ST and no difference in load to failure. Others authors have found no significant differences in stiffness or maximum load to failure.33 Van den Berghe and colleagues7 reported a higher failure rate in bone-to-bone repairs compared with tendon-to-tendon constructs. Moreover, they found that suture cut-out through bone tunnels is the primary mode of LTO failure, so many LTO surgeons now pass sutures around the humeral stem instead.
Three TSA studies directly compared ST and LTO approaches. Buckley and colleagues14 analyzed 60 TSAs and found no significant differences in WOOS, DASH, or Constant scores between groups. The authors described an ST subgroup with subscapularis attenuation on ultrasound but did not report the group as having any inferior functional outcome. Scalise and colleagues15 showed improved strength and PSSs in both groups after 2 years. However, the LTO group had a lower rate of subscapularis tears and significantly higher PSSs. Finally, Jandhyala and colleagues16 reported more favorable outcomes with LTO, which trended toward wider ROM and significantly higher belly-press test grades. Lapner and colleagues34 conducted a randomized, controlled trial (often referenced) and found no significant differences between the 2 groups in terms of strength or functional outcome at 2-year follow-up. Their study, however, included hemiarthroplasties and did not substratify the TSA population, so we did not include it in our review.
Our systematic review found significantly more forward elevation improvement for the ST group than the LTO group, which may suggest improved ROM with a soft-tissue approach than a bony approach. At the same time, the ST group trended toward better passive external rotation relative to the LTO group. This trend indicates fewer constraints to external rotation in the ST group, possibly attributable to a more attenuated subscapularis after tenotomy. Subscapularis tear or attenuation was more commonly reported in the ST group than in the LTO group, though not significantly so. This may indicate that more ST studies than LTO studies specially emphasized postoperative subscapularis function, but these data also highlight some authors’ concerns regarding subscapularis dysfunction after tenotomy.6,15,16The study populations’ complication rates were similar, just over 17%. The LTO group trended toward a higher revision rate, but it was not statistically significant. The LTO group also had significantly fewer patients with osteoarthritis and more patients with posttraumatic arthritis, so this group may have had more complex patients predisposed to a higher likelihood of revision surgery. Revisions were most commonly performed for aseptic loosening; theoretically, if osteotomies heal less effectively than tenotomies, the LTO approach could produce component instability and aseptic loosening. However, no prior studies or other clinical findings from this review suggest LTO predisposes to aseptic loosening. Overall, the uneven revision rates represent a clinical concern that should be monitored as larger samples of patients undergo ST and LTO procedures.
Glenoid radiolucencies were the only radiographic parameter consistently reported in the included studies. Twelve ST studies had radiolucency data—compared with only 2 LTO studies. Thus, our ability to compare radiographic outcomes was limited. Our data revealed similar rates of glenoid radiolucencies between the 2 approaches. The clinical relevance of radiolucencies is questioned by some authors, and, indeed, Razmjou and colleagues25 found no correlation of radiolucencies with patient satisfaction. Nevertheless, early presence of radiolucencies may raise concerns about progressive loss of fixation,35,36 so this should be monitored.
Limitations of this systematic review reflect the studies analyzed. We minimized selection bias by including level I to IV evidence, but most studies were level IV, and only 1 was level I. As such, there was a relative paucity of consistent clinical and radiographic data. For instance, although many ST studies reported patient satisfaction as an outcomes measure, only 1 LTO study commented on it. Perhaps the relative novelty of the LTO approach has prompted some authors to focus more on technical details and less on reporting a variety of outcome measures. As mentioned earlier, the significance of radiolucency data is controversial, and determination of their presence or absence depends on the observer. A radiolucency found in one study may not qualify as one in a study that uses different criteria. However, lucency data were the most frequently and reliably reported radiographic parameter, so we deemed it the most appropriate method for comparing radiographic outcomes. Finally, the baseline differences in diagnosis between the ST and LTO groups complicated comparisons. We stratified the groups by component design because use of keeled or pegged implants or humeral cemented or press-fit stems was usually a uniform feature of each study—enabling removal of certain studies for data stratification. However, we were unable to stratify by original diagnosis because these groups were not stratified within the individual studies.
Conclusion
Our systematic review found similar Constant scores, pain scores, radiographic outcomes, and complication rates for the ST and LTO approaches. Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions. Although not definitive, these data suggest the ST approach may provide more stability over the long term, but additional comprehensive studies are needed to increase the sample size and the power of the trends elucidated in this review. According to the orthopedic literature, both techniques produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
Am J Orthop. 2017;46(2):E131-E138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Keating JF, Waterworth P, Shaw-Dunn J, Crossan J. The relative strengths of the rotator cuff muscles. A cadaver study. J Bone Joint Surg Br. 1993;75(1):137-140.
2. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity repair technique in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87(suppl 2):1-8.
3. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34.
4. Gerber A, Ghalambor N, Warner JJ. Instability of shoulder arthroplasty: balancing mobility and stability. Orthop Clin North Am. 2001;32(4):661-670, ix.
5. Moeckel BH, Altchek DW, Warren RF, Wickiewicz TL, Dines DM. Instability of the shoulder after arthroplasty. J Bone Joint Surg Am. 1993;75(4):492-497.
6. Gerber C, Yian EH, Pfirrmann CA, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745.
7. Van den Berghe GR, Nguyen B, Patil S, et al. A biomechanical evaluation of three surgical techniques for subscapularis repair. J Shoulder Elbow Surg. 2008;17(1):156-161.
8. Caplan JL, Whitfield B, Neviaser RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196.
9. Armstrong A, Lashgari C, Teefey S, Menendez J, Yamaguchi K, Galatz LM. Ultrasound evaluation and clinical correlation of subscapularis repair after total shoulder arthroplasty. J Shoulder Elbow Surg. 2006;15(5):541-548.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
11. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
12. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
13. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
14. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317.
15. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic, and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634.
16. Jandhyala S, Unnithan A, Hughes S, Hong T. Subscapularis tenotomy versus lesser tuberosity osteotomy during total shoulder replacement: a comparison of patient outcomes. J Shoulder Elbow Surg. 2011;20(7):1102-1107.
17. Fucentese SF, Costouros JG, Kühnel SP, Gerber C. Total shoulder arthroplasty with an uncemented soft-metal-backed glenoid component. J Shoulder Elbow Surg. 2010;19(4):624-631.
18. Clement ND, Duckworth AD, Colling RC, Stirrat AN. An uncemented metal-backed glenoid component in total shoulder arthroplasty for osteoarthritis: factors affecting survival and outcome. J Orthop Sci. 2013;18(1):22-28.
19. Rosenberg N, Neumann L, Modi A, Mersich IJ, Wallace AW. Improvements in survival of the uncemented Nottingham Total Shoulder prosthesis: a prospective comparative study. BMC Musculoskelet Disord. 2007;8(1):76.
20. Small KM, Siegel EJ, Miller LR, Higgins LD. Imaging characteristics of lesser tuberosity osteotomy after total shoulder replacement: a study of 220 patients. J Shoulder Elbow Surg. 2014;23(9):1318-1326.
21. Mileti J, Sperling JW, Cofield RH, Harrington JR, Hoskin TL. Monoblock and modular total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Br. 2005;87(4):496-500.
22. Merolla G, Paladini P, Campi F, Porcellini G. Efficacy of anatomical prostheses in primary glenohumeral osteoarthritis. Chir Organi Mov. 2008;91(2):109-115.
23. Jackson JD, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090.
24. Jost PW, Dines JS, Griffith MH, Angel M, Altchek DW, Dines DM. Total shoulder arthroplasty utilizing mini-stem humeral components: technique and short-term results. HSS J. 2011;7(3):213-217.
25. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
26. Raiss P, Schmitt M, Bruckner T, et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94(23):e1711-1710.
27. Litchfied RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthritis of the shoulder: a prospective, randomized, double-blind clinical trial—a JOINTs Canada Project. J Shoulder Elbow Surg. 2011;20(4):529-536.
28. Martin SD, Zurakowski D, Thornhill TS. Uncemented glenoid component in total shoulder arthroplasty. Survivorship and outcomes. J Bone Joint Surg Am. 2005;87(6):1284-1292.
29. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188.
30. Budge MD, Nolan EM, Heisey MH, Baker K, Wiater JM. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Shoulder Elbow Surg. 2013;22(4):535-541.
31. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510.
32. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095.
33. Van Thiel GS, Wang VM, Wang FC, et al. Biomechanical similarities among subscapularis repairs after shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(5):657-663.
34. Lapner PL, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of lesser tuberosity osteotomy to subscapularis peel in shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(24):2239-2246.
35. Cofield RH. Total shoulder arthroplasty with the Neer prosthesis. J Bone Joint Surg Am. 1984;66(6):899-906.
36. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
1. Keating JF, Waterworth P, Shaw-Dunn J, Crossan J. The relative strengths of the rotator cuff muscles. A cadaver study. J Bone Joint Surg Br. 1993;75(1):137-140.
2. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity repair technique in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87(suppl 2):1-8.
3. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34.
4. Gerber A, Ghalambor N, Warner JJ. Instability of shoulder arthroplasty: balancing mobility and stability. Orthop Clin North Am. 2001;32(4):661-670, ix.
5. Moeckel BH, Altchek DW, Warren RF, Wickiewicz TL, Dines DM. Instability of the shoulder after arthroplasty. J Bone Joint Surg Am. 1993;75(4):492-497.
6. Gerber C, Yian EH, Pfirrmann CA, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745.
7. Van den Berghe GR, Nguyen B, Patil S, et al. A biomechanical evaluation of three surgical techniques for subscapularis repair. J Shoulder Elbow Surg. 2008;17(1):156-161.
8. Caplan JL, Whitfield B, Neviaser RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196.
9. Armstrong A, Lashgari C, Teefey S, Menendez J, Yamaguchi K, Galatz LM. Ultrasound evaluation and clinical correlation of subscapularis repair after total shoulder arthroplasty. J Shoulder Elbow Surg. 2006;15(5):541-548.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
11. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
12. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
13. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
14. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317.
15. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic, and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634.
16. Jandhyala S, Unnithan A, Hughes S, Hong T. Subscapularis tenotomy versus lesser tuberosity osteotomy during total shoulder replacement: a comparison of patient outcomes. J Shoulder Elbow Surg. 2011;20(7):1102-1107.
17. Fucentese SF, Costouros JG, Kühnel SP, Gerber C. Total shoulder arthroplasty with an uncemented soft-metal-backed glenoid component. J Shoulder Elbow Surg. 2010;19(4):624-631.
18. Clement ND, Duckworth AD, Colling RC, Stirrat AN. An uncemented metal-backed glenoid component in total shoulder arthroplasty for osteoarthritis: factors affecting survival and outcome. J Orthop Sci. 2013;18(1):22-28.
19. Rosenberg N, Neumann L, Modi A, Mersich IJ, Wallace AW. Improvements in survival of the uncemented Nottingham Total Shoulder prosthesis: a prospective comparative study. BMC Musculoskelet Disord. 2007;8(1):76.
20. Small KM, Siegel EJ, Miller LR, Higgins LD. Imaging characteristics of lesser tuberosity osteotomy after total shoulder replacement: a study of 220 patients. J Shoulder Elbow Surg. 2014;23(9):1318-1326.
21. Mileti J, Sperling JW, Cofield RH, Harrington JR, Hoskin TL. Monoblock and modular total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Br. 2005;87(4):496-500.
22. Merolla G, Paladini P, Campi F, Porcellini G. Efficacy of anatomical prostheses in primary glenohumeral osteoarthritis. Chir Organi Mov. 2008;91(2):109-115.
23. Jackson JD, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090.
24. Jost PW, Dines JS, Griffith MH, Angel M, Altchek DW, Dines DM. Total shoulder arthroplasty utilizing mini-stem humeral components: technique and short-term results. HSS J. 2011;7(3):213-217.
25. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
26. Raiss P, Schmitt M, Bruckner T, et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94(23):e1711-1710.
27. Litchfied RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthritis of the shoulder: a prospective, randomized, double-blind clinical trial—a JOINTs Canada Project. J Shoulder Elbow Surg. 2011;20(4):529-536.
28. Martin SD, Zurakowski D, Thornhill TS. Uncemented glenoid component in total shoulder arthroplasty. Survivorship and outcomes. J Bone Joint Surg Am. 2005;87(6):1284-1292.
29. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188.
30. Budge MD, Nolan EM, Heisey MH, Baker K, Wiater JM. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Shoulder Elbow Surg. 2013;22(4):535-541.
31. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510.
32. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095.
33. Van Thiel GS, Wang VM, Wang FC, et al. Biomechanical similarities among subscapularis repairs after shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(5):657-663.
34. Lapner PL, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of lesser tuberosity osteotomy to subscapularis peel in shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(24):2239-2246.
35. Cofield RH. Total shoulder arthroplasty with the Neer prosthesis. J Bone Joint Surg Am. 1984;66(6):899-906.
36. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
Doubt expressed about potential of any single regimen to treat all hep C
PHILADELPHIA – Despite combination therapies for hepatitis C virus (HCV) that are now showing high rates of sustained viral remission (SVR) across all genotypes, a one-size-fits-all treatment will not be practical in the near future, according to a review of current and coming HCV therapies at Digestive Diseases: New Advances meeting held by Rutgers, the State University of New Jersey, and Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
The focus for improving HCV care is “now shifting, I think, from this one-size-fits-all treatment to identifying, really, the one-size-fits all provider,” reported Kimberly A. Brown, MD, division head of gastroenterology/hepatology at Henry Ford Health System, Detroit.
The presence of cirrhosis in some patients demonstrates the need for this care, for example. Many of the most effective single-pill DAA combinations are demonstrating high SVR rates for HCV patients with cirrhosis, but Dr. Brown said that identification of cirrhosis prior to HCV treatment “remains imperative.” Some pangenotypic therapies require a longer duration of treatment when cirrhosis is present, and patients with cirrhosis require posttreatment monitoring for decompensation and hepatocellular carcinoma (HCC). Patients who have failed a prior anti-HCV regimen or who are in renal failure also require more individualized care.
“There is no current therapy in my opinion that allows for one combination, for one length of treatment, without consideration of any patient characteristics,” Dr. Brown said at the meeting. Although several newer combination drugs with pangenotypic properties are likely to be approved for HCV in 2017, Dr. Brown believes that the one-size-fits-all ideal is not going to be fulfilled “anytime soon.”
However, Dr. Brown does believe that HCV care can and should be shifted to trained primary care providers in order to increase the proportion of infected patients who are treated. She indicated that the pangenotypic drugs are making this easier to accomplish and cited a study from the most recent annual meeting of the American Society for the Study of Liver Diseases that showed comparable SVR rates for primary care physicians, nurse practitioners, and specialists when the primary care clinicians underwent a uniform 3-hour training program (Emmanuel B. et al. AASLD 2016;Abstract 22).
“There is an evidence basis for shifting care to primary care providers in order to expand treatment, but, certainly, these providers must have an interest,” Dr. Brown said. She also said that treatment from a primary care provider must be accompanied by follow-up care, which, for example, might include clinics specializing in alcohol or drug dependency.
In treatment-naive patients with uncomplicated HCV, nearly 100% of patients will achieve an SVR on 8-12 weeks of therapy, regardless of genotype, with the newest and most potent DAA regimens, according to data cited by Dr. Brown. However, she cautioned that, even in these patients, it would be inaccurate to conclude that one-size-fits-all therapy is sufficient.
One relatively recent concern is HBV activation. “The reactivation of HBV appears to be temporally related to use of DAAs, and this seems to be independent of HCV genotype, type of DAA received, or [the patient’s] HCV parameters,” Dr. Brown reported, citing data from the Food and Drug Administration. “The clinical implications for this are that HBV DNA must now be monitored, so this is another level of complexity for our care providers.”
Other considerations for care of HCV despite achieving SVR with current treatments include monitoring for HCC and preventing reinfection. Dr. Brown cautioned that the risk of HCC, although greatly reduced after SVR, is not eliminated, and specific monitoring strategies are particularly important for those with fibrosis or cirrhosis prior to SVR.
In addition, the same risks for primary HCV are relevant for reinfection, according to Dr. Brown. She pointed out that these reinfection rates can be substantial in populations that persist in behaviors that result in HCV exposure.
“We are getting very close to the ideal of a one-size-fits-all treatment regimen for HCV, which would include no need to check genotype, no contraindications, no need for close monitoring, and no need to document cirrhosis, but we are not there yet,” Dr. Brown said. Even if such a regimen does emerge, she indicated that clinicians are not likely to ever be absolved from important management decisions that ensure an optimal long-term outcome.
PHILADELPHIA – Despite combination therapies for hepatitis C virus (HCV) that are now showing high rates of sustained viral remission (SVR) across all genotypes, a one-size-fits-all treatment will not be practical in the near future, according to a review of current and coming HCV therapies at Digestive Diseases: New Advances meeting held by Rutgers, the State University of New Jersey, and Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
The focus for improving HCV care is “now shifting, I think, from this one-size-fits-all treatment to identifying, really, the one-size-fits all provider,” reported Kimberly A. Brown, MD, division head of gastroenterology/hepatology at Henry Ford Health System, Detroit.
The presence of cirrhosis in some patients demonstrates the need for this care, for example. Many of the most effective single-pill DAA combinations are demonstrating high SVR rates for HCV patients with cirrhosis, but Dr. Brown said that identification of cirrhosis prior to HCV treatment “remains imperative.” Some pangenotypic therapies require a longer duration of treatment when cirrhosis is present, and patients with cirrhosis require posttreatment monitoring for decompensation and hepatocellular carcinoma (HCC). Patients who have failed a prior anti-HCV regimen or who are in renal failure also require more individualized care.
“There is no current therapy in my opinion that allows for one combination, for one length of treatment, without consideration of any patient characteristics,” Dr. Brown said at the meeting. Although several newer combination drugs with pangenotypic properties are likely to be approved for HCV in 2017, Dr. Brown believes that the one-size-fits-all ideal is not going to be fulfilled “anytime soon.”
However, Dr. Brown does believe that HCV care can and should be shifted to trained primary care providers in order to increase the proportion of infected patients who are treated. She indicated that the pangenotypic drugs are making this easier to accomplish and cited a study from the most recent annual meeting of the American Society for the Study of Liver Diseases that showed comparable SVR rates for primary care physicians, nurse practitioners, and specialists when the primary care clinicians underwent a uniform 3-hour training program (Emmanuel B. et al. AASLD 2016;Abstract 22).
“There is an evidence basis for shifting care to primary care providers in order to expand treatment, but, certainly, these providers must have an interest,” Dr. Brown said. She also said that treatment from a primary care provider must be accompanied by follow-up care, which, for example, might include clinics specializing in alcohol or drug dependency.
In treatment-naive patients with uncomplicated HCV, nearly 100% of patients will achieve an SVR on 8-12 weeks of therapy, regardless of genotype, with the newest and most potent DAA regimens, according to data cited by Dr. Brown. However, she cautioned that, even in these patients, it would be inaccurate to conclude that one-size-fits-all therapy is sufficient.
One relatively recent concern is HBV activation. “The reactivation of HBV appears to be temporally related to use of DAAs, and this seems to be independent of HCV genotype, type of DAA received, or [the patient’s] HCV parameters,” Dr. Brown reported, citing data from the Food and Drug Administration. “The clinical implications for this are that HBV DNA must now be monitored, so this is another level of complexity for our care providers.”
Other considerations for care of HCV despite achieving SVR with current treatments include monitoring for HCC and preventing reinfection. Dr. Brown cautioned that the risk of HCC, although greatly reduced after SVR, is not eliminated, and specific monitoring strategies are particularly important for those with fibrosis or cirrhosis prior to SVR.
In addition, the same risks for primary HCV are relevant for reinfection, according to Dr. Brown. She pointed out that these reinfection rates can be substantial in populations that persist in behaviors that result in HCV exposure.
“We are getting very close to the ideal of a one-size-fits-all treatment regimen for HCV, which would include no need to check genotype, no contraindications, no need for close monitoring, and no need to document cirrhosis, but we are not there yet,” Dr. Brown said. Even if such a regimen does emerge, she indicated that clinicians are not likely to ever be absolved from important management decisions that ensure an optimal long-term outcome.
PHILADELPHIA – Despite combination therapies for hepatitis C virus (HCV) that are now showing high rates of sustained viral remission (SVR) across all genotypes, a one-size-fits-all treatment will not be practical in the near future, according to a review of current and coming HCV therapies at Digestive Diseases: New Advances meeting held by Rutgers, the State University of New Jersey, and Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
The focus for improving HCV care is “now shifting, I think, from this one-size-fits-all treatment to identifying, really, the one-size-fits all provider,” reported Kimberly A. Brown, MD, division head of gastroenterology/hepatology at Henry Ford Health System, Detroit.
The presence of cirrhosis in some patients demonstrates the need for this care, for example. Many of the most effective single-pill DAA combinations are demonstrating high SVR rates for HCV patients with cirrhosis, but Dr. Brown said that identification of cirrhosis prior to HCV treatment “remains imperative.” Some pangenotypic therapies require a longer duration of treatment when cirrhosis is present, and patients with cirrhosis require posttreatment monitoring for decompensation and hepatocellular carcinoma (HCC). Patients who have failed a prior anti-HCV regimen or who are in renal failure also require more individualized care.
“There is no current therapy in my opinion that allows for one combination, for one length of treatment, without consideration of any patient characteristics,” Dr. Brown said at the meeting. Although several newer combination drugs with pangenotypic properties are likely to be approved for HCV in 2017, Dr. Brown believes that the one-size-fits-all ideal is not going to be fulfilled “anytime soon.”
However, Dr. Brown does believe that HCV care can and should be shifted to trained primary care providers in order to increase the proportion of infected patients who are treated. She indicated that the pangenotypic drugs are making this easier to accomplish and cited a study from the most recent annual meeting of the American Society for the Study of Liver Diseases that showed comparable SVR rates for primary care physicians, nurse practitioners, and specialists when the primary care clinicians underwent a uniform 3-hour training program (Emmanuel B. et al. AASLD 2016;Abstract 22).
“There is an evidence basis for shifting care to primary care providers in order to expand treatment, but, certainly, these providers must have an interest,” Dr. Brown said. She also said that treatment from a primary care provider must be accompanied by follow-up care, which, for example, might include clinics specializing in alcohol or drug dependency.
In treatment-naive patients with uncomplicated HCV, nearly 100% of patients will achieve an SVR on 8-12 weeks of therapy, regardless of genotype, with the newest and most potent DAA regimens, according to data cited by Dr. Brown. However, she cautioned that, even in these patients, it would be inaccurate to conclude that one-size-fits-all therapy is sufficient.
One relatively recent concern is HBV activation. “The reactivation of HBV appears to be temporally related to use of DAAs, and this seems to be independent of HCV genotype, type of DAA received, or [the patient’s] HCV parameters,” Dr. Brown reported, citing data from the Food and Drug Administration. “The clinical implications for this are that HBV DNA must now be monitored, so this is another level of complexity for our care providers.”
Other considerations for care of HCV despite achieving SVR with current treatments include monitoring for HCC and preventing reinfection. Dr. Brown cautioned that the risk of HCC, although greatly reduced after SVR, is not eliminated, and specific monitoring strategies are particularly important for those with fibrosis or cirrhosis prior to SVR.
In addition, the same risks for primary HCV are relevant for reinfection, according to Dr. Brown. She pointed out that these reinfection rates can be substantial in populations that persist in behaviors that result in HCV exposure.
“We are getting very close to the ideal of a one-size-fits-all treatment regimen for HCV, which would include no need to check genotype, no contraindications, no need for close monitoring, and no need to document cirrhosis, but we are not there yet,” Dr. Brown said. Even if such a regimen does emerge, she indicated that clinicians are not likely to ever be absolved from important management decisions that ensure an optimal long-term outcome.
AT DIGESTIVE DISEASES: NEW ADVANCES MEETING
Key clinical point: Pangenotypic efficacy is not sufficient to create a one-size-fits-all regimen for HCV treatment, according to an assessment of evolving options.
Major finding: More-effective pills have moved the focus for progress in hepatitis C to more effective delivery of care.
Data source: A research review of current and coming HCV therapies.
Disclosures: Dr. Brown reported financial relationships with AbbVie, Bayer, Bristol-Myers Squibb, Gilead, Merck, and Novartis.