User login
For Latinos, misperceptions and lack of medical care make preventing melanoma risky business
ORLANDO – Ignorance and exposure are teaming up to put Latinos in the bull’s-eye of skin cancer.
Many believe that they are not at risk for either melanoma or nonmelanoma skin cancers – and too often, their physicians believe the same, Maritza Perez, MD, said at the annual meeting of the American Academy of Dermatology. Because of such incorrect perceptions, Latino patients get little counseling about risky behaviors, and so their exposure to those dangers continues unabated.
“The behavior of many Hispanic patients is very risky,” said Dr. Perez, a clinical professor of dermatology, at Mount Sinai Medical Center, New York. “They don’t wear sunscreen. They don’t do skin self-exams. They use tanning beds. And because of these beliefs, they don’t educate their children about sun safety.”
A research letter published in the Journal of the American Academy of Dermatology in 2011 broke down levels of skin cancer awareness by race and ethnicity among 165 whites, Hispanics, blacks, and Asians surveyed in New York City (64[1]:198-200). Compared with whites, Hispanics were significantly less likely to have ever had a doctor perform a full body skin exam (21% vs. 61%) or to have performed a self-exam (37% vs. 54%). Significantly fewer believed that skin cancer could occur in darker skin (78% vs. 91%). Only 8% had heard of the ABCDs of early melanoma detection, compared with 27% of whites. And about half as many Hispanics said they wore sunscreen (55% vs. 96%).
Unfortunately, Dr. Perez said, doctors aren’t correcting these misperceptions. Many physicians display a similar lack of understanding. They may correctly believe that the risk for skin cancer is less among Hispanics than it is among whites overall, but fail to communicate individual risk.
What these physicians may not understand, Dr. Perez said, is that the Hispanic population comprises an incredible variety of ethnic backgrounds. The population’s centuries-long genetic mixing bowl means there is no “typical” Hispanic skin. Instead, it includes every Fitzpatrick skin type, from fair-skinned redheads to the darkest brown and black skins.
Inadequate healthcare access exerts yet another damaging force. Like other ethnic minorities, many Hispanic patients lack insurance or adequate access to medical care. Instead of seeking regular primary care that would include skin cancer screenings, they tend to rely on urgent care or emergency departments to address emergent health issues, Dr. Perez said. When primary and preventive care falls by the wayside, melanomas that could be diagnosed at a curable stage invariably progress.
“We know that the only way of curing melanoma is with a scalpel. And the only way to remove it is by treating early disease. We’re not doing that. Our melanoma patients are diagnosed at younger ages with more advanced disease with more lymph node involvement than Caucasians, so there is also more mortality. We achieve early-stage diagnosis in 91% of Caucasians, but only 74% of Hispanics.”
A 2011 paper on racial and ethnic variations in the incidence and survival of melanoma, based on national cancer registry data covering almost 70% of the U.S. population, from 1999-2006, provided more information on the differences between the white and Hispanic populations (J Am Acad Dermatol. 2011 Nov;65[5 Suppl 1]:S26-37). Compared with non-Hispanic whites, Hispanics presented with thicker tumors (more than 1 mm, 35% vs. 25%), more regional involvement (12% vs. 8%), and more distant metastasis (7% vs. 4%).
Because adult Hispanic patients lack knowledge about their melanoma risk, they aren’t improving the outlook for their children, Dr. Perez said. The Hispanic demographic in the United States is already a young one. According on 2014 data cited by the Pew Research Center, 58% of Hispanics in the United States are aged 33 years or younger; 32% are younger than 18 years.
These young people are already endangering their health with unsafe sun behavior, Dr. Perez said. A 2007 study surveyed 369 white Hispanic and white non-Hispanic high school students in Miami about sun protection behaviors and skin cancer risk. The Hispanic teens were 2.5 times more likely to have used a tanning bed in the previous year; they were also less likely to wear sunscreen and protective clothing. The Hispanic students generally believed they were less likely to get skin cancer than the Caucasian students. They were 60% less likely to have heard of a skin self-exam and 70% less likely to have been told how to do one (Arch Dermatol. 2007;143[8]:983-8).
The oil to calm these troubled waters is education, Dr. Perez said. She takes this commitment very seriously, and said a simple conversation is the first step.
“I tell all my patients, no matter what ethnicity you are or what skin type you have, you can get skin cancer and you need regular, complete skin exams. And I teach them to do this for themselves.”
A senior vice-president for the Skin Cancer Foundation, Dr. Perez is coauthor of “Understanding Melanoma: What You Need to Know,” which is now in its fifth edition.
The book, originally published in 1996, is aimed at melanoma patients and their families. It covers the four types of melanoma and their causes and risk factors. Information on melanoma diagnosis, staging, treatment options, prognosis, and hereditary and genetic factors is also included, as well as guidelines for prevention.
The updated edition contains information on the latest immunotherapy and genetically targeted treatments, including ipilimumab (Yervoy), pembrolizumab (Keytruda), nivolumab (Opdivo), vemurafenib (Zelboraf), dabrafenib (Tafinlar) and trametinib (Mekinist). The book is available for download for a nominal fee.
She has also committed to educating physicians about the issue.
“If we want to decrease the incidence of melanoma in Latinos, decrease the tumor depth at diagnosis and bring down the higher mortality, we have to first educate the doctors who are taking care of these patients and correct the message delivered to Latinos by telling them that they are as prone to skin cancer as Caucasians. We simply have to get the message across that, just like everyone else, they need protection from the sun by applying sunblocks, using sunglasses, and covering their bodies with sun-protective clothing and large-rim hats. And we have to make medical care more accessible so that these people can be diagnosed and saved. This is what we need to do now. But I don’t know how many decades it will take to turn the tables.”
Dr. Perez had no disclosures relevant to her lecture.
[email protected]
On Twitter @Alz_Gal
ORLANDO – Ignorance and exposure are teaming up to put Latinos in the bull’s-eye of skin cancer.
Many believe that they are not at risk for either melanoma or nonmelanoma skin cancers – and too often, their physicians believe the same, Maritza Perez, MD, said at the annual meeting of the American Academy of Dermatology. Because of such incorrect perceptions, Latino patients get little counseling about risky behaviors, and so their exposure to those dangers continues unabated.
“The behavior of many Hispanic patients is very risky,” said Dr. Perez, a clinical professor of dermatology, at Mount Sinai Medical Center, New York. “They don’t wear sunscreen. They don’t do skin self-exams. They use tanning beds. And because of these beliefs, they don’t educate their children about sun safety.”
A research letter published in the Journal of the American Academy of Dermatology in 2011 broke down levels of skin cancer awareness by race and ethnicity among 165 whites, Hispanics, blacks, and Asians surveyed in New York City (64[1]:198-200). Compared with whites, Hispanics were significantly less likely to have ever had a doctor perform a full body skin exam (21% vs. 61%) or to have performed a self-exam (37% vs. 54%). Significantly fewer believed that skin cancer could occur in darker skin (78% vs. 91%). Only 8% had heard of the ABCDs of early melanoma detection, compared with 27% of whites. And about half as many Hispanics said they wore sunscreen (55% vs. 96%).
Unfortunately, Dr. Perez said, doctors aren’t correcting these misperceptions. Many physicians display a similar lack of understanding. They may correctly believe that the risk for skin cancer is less among Hispanics than it is among whites overall, but fail to communicate individual risk.
What these physicians may not understand, Dr. Perez said, is that the Hispanic population comprises an incredible variety of ethnic backgrounds. The population’s centuries-long genetic mixing bowl means there is no “typical” Hispanic skin. Instead, it includes every Fitzpatrick skin type, from fair-skinned redheads to the darkest brown and black skins.
Inadequate healthcare access exerts yet another damaging force. Like other ethnic minorities, many Hispanic patients lack insurance or adequate access to medical care. Instead of seeking regular primary care that would include skin cancer screenings, they tend to rely on urgent care or emergency departments to address emergent health issues, Dr. Perez said. When primary and preventive care falls by the wayside, melanomas that could be diagnosed at a curable stage invariably progress.
“We know that the only way of curing melanoma is with a scalpel. And the only way to remove it is by treating early disease. We’re not doing that. Our melanoma patients are diagnosed at younger ages with more advanced disease with more lymph node involvement than Caucasians, so there is also more mortality. We achieve early-stage diagnosis in 91% of Caucasians, but only 74% of Hispanics.”
A 2011 paper on racial and ethnic variations in the incidence and survival of melanoma, based on national cancer registry data covering almost 70% of the U.S. population, from 1999-2006, provided more information on the differences between the white and Hispanic populations (J Am Acad Dermatol. 2011 Nov;65[5 Suppl 1]:S26-37). Compared with non-Hispanic whites, Hispanics presented with thicker tumors (more than 1 mm, 35% vs. 25%), more regional involvement (12% vs. 8%), and more distant metastasis (7% vs. 4%).
Because adult Hispanic patients lack knowledge about their melanoma risk, they aren’t improving the outlook for their children, Dr. Perez said. The Hispanic demographic in the United States is already a young one. According on 2014 data cited by the Pew Research Center, 58% of Hispanics in the United States are aged 33 years or younger; 32% are younger than 18 years.
These young people are already endangering their health with unsafe sun behavior, Dr. Perez said. A 2007 study surveyed 369 white Hispanic and white non-Hispanic high school students in Miami about sun protection behaviors and skin cancer risk. The Hispanic teens were 2.5 times more likely to have used a tanning bed in the previous year; they were also less likely to wear sunscreen and protective clothing. The Hispanic students generally believed they were less likely to get skin cancer than the Caucasian students. They were 60% less likely to have heard of a skin self-exam and 70% less likely to have been told how to do one (Arch Dermatol. 2007;143[8]:983-8).
The oil to calm these troubled waters is education, Dr. Perez said. She takes this commitment very seriously, and said a simple conversation is the first step.
“I tell all my patients, no matter what ethnicity you are or what skin type you have, you can get skin cancer and you need regular, complete skin exams. And I teach them to do this for themselves.”
A senior vice-president for the Skin Cancer Foundation, Dr. Perez is coauthor of “Understanding Melanoma: What You Need to Know,” which is now in its fifth edition.
The book, originally published in 1996, is aimed at melanoma patients and their families. It covers the four types of melanoma and their causes and risk factors. Information on melanoma diagnosis, staging, treatment options, prognosis, and hereditary and genetic factors is also included, as well as guidelines for prevention.
The updated edition contains information on the latest immunotherapy and genetically targeted treatments, including ipilimumab (Yervoy), pembrolizumab (Keytruda), nivolumab (Opdivo), vemurafenib (Zelboraf), dabrafenib (Tafinlar) and trametinib (Mekinist). The book is available for download for a nominal fee.
She has also committed to educating physicians about the issue.
“If we want to decrease the incidence of melanoma in Latinos, decrease the tumor depth at diagnosis and bring down the higher mortality, we have to first educate the doctors who are taking care of these patients and correct the message delivered to Latinos by telling them that they are as prone to skin cancer as Caucasians. We simply have to get the message across that, just like everyone else, they need protection from the sun by applying sunblocks, using sunglasses, and covering their bodies with sun-protective clothing and large-rim hats. And we have to make medical care more accessible so that these people can be diagnosed and saved. This is what we need to do now. But I don’t know how many decades it will take to turn the tables.”
Dr. Perez had no disclosures relevant to her lecture.
[email protected]
On Twitter @Alz_Gal
ORLANDO – Ignorance and exposure are teaming up to put Latinos in the bull’s-eye of skin cancer.
Many believe that they are not at risk for either melanoma or nonmelanoma skin cancers – and too often, their physicians believe the same, Maritza Perez, MD, said at the annual meeting of the American Academy of Dermatology. Because of such incorrect perceptions, Latino patients get little counseling about risky behaviors, and so their exposure to those dangers continues unabated.
“The behavior of many Hispanic patients is very risky,” said Dr. Perez, a clinical professor of dermatology, at Mount Sinai Medical Center, New York. “They don’t wear sunscreen. They don’t do skin self-exams. They use tanning beds. And because of these beliefs, they don’t educate their children about sun safety.”
A research letter published in the Journal of the American Academy of Dermatology in 2011 broke down levels of skin cancer awareness by race and ethnicity among 165 whites, Hispanics, blacks, and Asians surveyed in New York City (64[1]:198-200). Compared with whites, Hispanics were significantly less likely to have ever had a doctor perform a full body skin exam (21% vs. 61%) or to have performed a self-exam (37% vs. 54%). Significantly fewer believed that skin cancer could occur in darker skin (78% vs. 91%). Only 8% had heard of the ABCDs of early melanoma detection, compared with 27% of whites. And about half as many Hispanics said they wore sunscreen (55% vs. 96%).
Unfortunately, Dr. Perez said, doctors aren’t correcting these misperceptions. Many physicians display a similar lack of understanding. They may correctly believe that the risk for skin cancer is less among Hispanics than it is among whites overall, but fail to communicate individual risk.
What these physicians may not understand, Dr. Perez said, is that the Hispanic population comprises an incredible variety of ethnic backgrounds. The population’s centuries-long genetic mixing bowl means there is no “typical” Hispanic skin. Instead, it includes every Fitzpatrick skin type, from fair-skinned redheads to the darkest brown and black skins.
Inadequate healthcare access exerts yet another damaging force. Like other ethnic minorities, many Hispanic patients lack insurance or adequate access to medical care. Instead of seeking regular primary care that would include skin cancer screenings, they tend to rely on urgent care or emergency departments to address emergent health issues, Dr. Perez said. When primary and preventive care falls by the wayside, melanomas that could be diagnosed at a curable stage invariably progress.
“We know that the only way of curing melanoma is with a scalpel. And the only way to remove it is by treating early disease. We’re not doing that. Our melanoma patients are diagnosed at younger ages with more advanced disease with more lymph node involvement than Caucasians, so there is also more mortality. We achieve early-stage diagnosis in 91% of Caucasians, but only 74% of Hispanics.”
A 2011 paper on racial and ethnic variations in the incidence and survival of melanoma, based on national cancer registry data covering almost 70% of the U.S. population, from 1999-2006, provided more information on the differences between the white and Hispanic populations (J Am Acad Dermatol. 2011 Nov;65[5 Suppl 1]:S26-37). Compared with non-Hispanic whites, Hispanics presented with thicker tumors (more than 1 mm, 35% vs. 25%), more regional involvement (12% vs. 8%), and more distant metastasis (7% vs. 4%).
Because adult Hispanic patients lack knowledge about their melanoma risk, they aren’t improving the outlook for their children, Dr. Perez said. The Hispanic demographic in the United States is already a young one. According on 2014 data cited by the Pew Research Center, 58% of Hispanics in the United States are aged 33 years or younger; 32% are younger than 18 years.
These young people are already endangering their health with unsafe sun behavior, Dr. Perez said. A 2007 study surveyed 369 white Hispanic and white non-Hispanic high school students in Miami about sun protection behaviors and skin cancer risk. The Hispanic teens were 2.5 times more likely to have used a tanning bed in the previous year; they were also less likely to wear sunscreen and protective clothing. The Hispanic students generally believed they were less likely to get skin cancer than the Caucasian students. They were 60% less likely to have heard of a skin self-exam and 70% less likely to have been told how to do one (Arch Dermatol. 2007;143[8]:983-8).
The oil to calm these troubled waters is education, Dr. Perez said. She takes this commitment very seriously, and said a simple conversation is the first step.
“I tell all my patients, no matter what ethnicity you are or what skin type you have, you can get skin cancer and you need regular, complete skin exams. And I teach them to do this for themselves.”
A senior vice-president for the Skin Cancer Foundation, Dr. Perez is coauthor of “Understanding Melanoma: What You Need to Know,” which is now in its fifth edition.
The book, originally published in 1996, is aimed at melanoma patients and their families. It covers the four types of melanoma and their causes and risk factors. Information on melanoma diagnosis, staging, treatment options, prognosis, and hereditary and genetic factors is also included, as well as guidelines for prevention.
The updated edition contains information on the latest immunotherapy and genetically targeted treatments, including ipilimumab (Yervoy), pembrolizumab (Keytruda), nivolumab (Opdivo), vemurafenib (Zelboraf), dabrafenib (Tafinlar) and trametinib (Mekinist). The book is available for download for a nominal fee.
She has also committed to educating physicians about the issue.
“If we want to decrease the incidence of melanoma in Latinos, decrease the tumor depth at diagnosis and bring down the higher mortality, we have to first educate the doctors who are taking care of these patients and correct the message delivered to Latinos by telling them that they are as prone to skin cancer as Caucasians. We simply have to get the message across that, just like everyone else, they need protection from the sun by applying sunblocks, using sunglasses, and covering their bodies with sun-protective clothing and large-rim hats. And we have to make medical care more accessible so that these people can be diagnosed and saved. This is what we need to do now. But I don’t know how many decades it will take to turn the tables.”
Dr. Perez had no disclosures relevant to her lecture.
[email protected]
On Twitter @Alz_Gal
EXPERT ANALYSIS FROM AAD 17
Clinicians still seek the best uses for apremilast
WAILEA, HAWAII – Oral apremilast is a drug in search of a compelling indication, Craig L. Leonardi, MD, declared at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Research Foundation.
“Apremilast is the least effective systemic agent for the treatment of psoriasis. That’s a statement of fact. I think the drug is mismatched for the treatment of moderate to severe plaque psoriasis. In fact, many of us gave the company that advice early on, but it was a program that was set in stone at that point,” according to Dr. Leonardi, a dermatologist at Saint Louis University and a prominent psoriasis clinical trialist.
“You have a drug that’s modest with regard to clearing psoriasis and it’s modest in controlling psoriatic arthritis. So you have to ask yourself what’s the patient population for this drug. I would just say that in my hands – and this is off-label – I use it only in patients with mild to moderate psoriasis,” Dr. Leonardi said.
“We all have psoriasis patients who come in with 3% or 4% of their skin involved, they’re actually using the class I topical steroids that I prescribe for them, yet they’re still not clear or almost clear and they want more. At that point, that’s when I might take them down this pathway and put them on apremilast along with a topical steroid. And I think that’s the appropriate place for this drug. I don’t use it in patients with 10% or more body surface area involvement,” he said.
He added that he would like to be able to prescribe apremilast in conjunction with a biologic agent in patients with more severe psoriasis to obtain synergistic efficacy, but payers balk at that because, at close to $3,000 per month, apremilast costs far more than methotrexate and other generic conventional disease-modifying antirheumatic drugs.
“The insurance industry is all over this drug. They require preauthorization, and they tell me, ‘No way, have a nice day,’ ” according to the dermatologist.
Dr. Leonardi described a couple of other practical caveats regarding apremilast. In patients with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a dose reduction to 30 mg once daily in the morning is necessary. And apremilast is not recommended for use in patients who are on a strong inducer of the CYP 450 enzyme, such as rifampin or phenytoin.
An intriguing side effect of apremilast is that it causes weight loss: A 5%-10% weight loss was seen in the major psoriasis clinical trials. But it’s been established that there is no correlation at all between the weight loss and clinical efficacy for skin clearance.
What efficacy can be anticipated?
In the pivotal phase III ESTEEM I trial conducted in 844 patients with moderate to severe psoriasis (J Am Acad Dermatol. 2015 Jul;73[1]:37-49), the overall PASI-75 response rate at week 16 in patients assigned to apremilast at 30 mg twice daily was 33%, significantly better than the 5% placebo response rate, but substantially less than what’s achieved with the tumor necrosis factor inhibitors and other injectable biologic agents.
Moreover, in the pivotal phase III PALACE 1 study of apremilast for the treatment of psoriatic arthritis, the primary outcome of at least a 20% improvement in the modified American College of Rheumatology response criteria at week 16 was achieved in 40% of patients randomized to apremilast at 30 mg twice daily, compared with 19% on placebo (Ann Rheum Dis. 2014 Jun;73[6]:1020-6). Again, that doesn’t approach the efficacy of a TNF inhibitor, Dr. Leonardi noted.
On the plus side, an oral agent such as apremilast is an attractive option for treatment-adherent patients. Plus, the drug has a favorable safety profile and is well tolerated, with mild to moderate side effects that appear early and are self-limited. In ESTEEM I, for example, more than 96% of patients had either no or only mild to moderate adverse events. The incidence of the two most common adverse events, diarrhea and nausea, at 19% and 16%, respectively, was more than double that in placebo-treated controls, but rates of other adverse events were similar in the two treatment arms.
Symposium codirector Linda Stein Gold, MD, director of dermatology research at the Henry Ford Health System in Detroit, said a clinical trial of apremilast conducted specifically in patients with moderate psoriasis has recently been completed. When those results become available they should shore up the drug’s use in that population.
Finding potential in off-label use
“This drug may have been brought to market for psoriasis, but I think its utility is so much more in other diseases where inflammation is an important mechanism,” said Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego. “Most of my use of apremilast is off-label for atopic dermatitis, for lichen planus, and I’ve tried it in a lot of patients where it has worked for discoid lupus. There’s so much potential for apremilast,” said Dr. Bhatia.
Dr. Leonardi remained unpersuaded.
“Your glass of water is half full, mine is half empty in this case,” he replied. “This drug has been approved now for at least 3 years, and we are still looking at the occasional favorable case report that flies up. I’ve got to say this drug is having a hard time finding a place outside of psoriasis, but we’ll all see.”
Dr. Leonardi reported having financial relationships with more than a dozen pharmaceutical companies, including Celgene, which markets apremilast. Dr. Stein Gold, too, has received research grants from and serves as a consultant to numerous drug companies, including Celgene. Dr. Bhatia declared having financial relationships with more than two dozen.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – Oral apremilast is a drug in search of a compelling indication, Craig L. Leonardi, MD, declared at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Research Foundation.
“Apremilast is the least effective systemic agent for the treatment of psoriasis. That’s a statement of fact. I think the drug is mismatched for the treatment of moderate to severe plaque psoriasis. In fact, many of us gave the company that advice early on, but it was a program that was set in stone at that point,” according to Dr. Leonardi, a dermatologist at Saint Louis University and a prominent psoriasis clinical trialist.
“You have a drug that’s modest with regard to clearing psoriasis and it’s modest in controlling psoriatic arthritis. So you have to ask yourself what’s the patient population for this drug. I would just say that in my hands – and this is off-label – I use it only in patients with mild to moderate psoriasis,” Dr. Leonardi said.
“We all have psoriasis patients who come in with 3% or 4% of their skin involved, they’re actually using the class I topical steroids that I prescribe for them, yet they’re still not clear or almost clear and they want more. At that point, that’s when I might take them down this pathway and put them on apremilast along with a topical steroid. And I think that’s the appropriate place for this drug. I don’t use it in patients with 10% or more body surface area involvement,” he said.
He added that he would like to be able to prescribe apremilast in conjunction with a biologic agent in patients with more severe psoriasis to obtain synergistic efficacy, but payers balk at that because, at close to $3,000 per month, apremilast costs far more than methotrexate and other generic conventional disease-modifying antirheumatic drugs.
“The insurance industry is all over this drug. They require preauthorization, and they tell me, ‘No way, have a nice day,’ ” according to the dermatologist.
Dr. Leonardi described a couple of other practical caveats regarding apremilast. In patients with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a dose reduction to 30 mg once daily in the morning is necessary. And apremilast is not recommended for use in patients who are on a strong inducer of the CYP 450 enzyme, such as rifampin or phenytoin.
An intriguing side effect of apremilast is that it causes weight loss: A 5%-10% weight loss was seen in the major psoriasis clinical trials. But it’s been established that there is no correlation at all between the weight loss and clinical efficacy for skin clearance.
What efficacy can be anticipated?
In the pivotal phase III ESTEEM I trial conducted in 844 patients with moderate to severe psoriasis (J Am Acad Dermatol. 2015 Jul;73[1]:37-49), the overall PASI-75 response rate at week 16 in patients assigned to apremilast at 30 mg twice daily was 33%, significantly better than the 5% placebo response rate, but substantially less than what’s achieved with the tumor necrosis factor inhibitors and other injectable biologic agents.
Moreover, in the pivotal phase III PALACE 1 study of apremilast for the treatment of psoriatic arthritis, the primary outcome of at least a 20% improvement in the modified American College of Rheumatology response criteria at week 16 was achieved in 40% of patients randomized to apremilast at 30 mg twice daily, compared with 19% on placebo (Ann Rheum Dis. 2014 Jun;73[6]:1020-6). Again, that doesn’t approach the efficacy of a TNF inhibitor, Dr. Leonardi noted.
On the plus side, an oral agent such as apremilast is an attractive option for treatment-adherent patients. Plus, the drug has a favorable safety profile and is well tolerated, with mild to moderate side effects that appear early and are self-limited. In ESTEEM I, for example, more than 96% of patients had either no or only mild to moderate adverse events. The incidence of the two most common adverse events, diarrhea and nausea, at 19% and 16%, respectively, was more than double that in placebo-treated controls, but rates of other adverse events were similar in the two treatment arms.
Symposium codirector Linda Stein Gold, MD, director of dermatology research at the Henry Ford Health System in Detroit, said a clinical trial of apremilast conducted specifically in patients with moderate psoriasis has recently been completed. When those results become available they should shore up the drug’s use in that population.
Finding potential in off-label use
“This drug may have been brought to market for psoriasis, but I think its utility is so much more in other diseases where inflammation is an important mechanism,” said Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego. “Most of my use of apremilast is off-label for atopic dermatitis, for lichen planus, and I’ve tried it in a lot of patients where it has worked for discoid lupus. There’s so much potential for apremilast,” said Dr. Bhatia.
Dr. Leonardi remained unpersuaded.
“Your glass of water is half full, mine is half empty in this case,” he replied. “This drug has been approved now for at least 3 years, and we are still looking at the occasional favorable case report that flies up. I’ve got to say this drug is having a hard time finding a place outside of psoriasis, but we’ll all see.”
Dr. Leonardi reported having financial relationships with more than a dozen pharmaceutical companies, including Celgene, which markets apremilast. Dr. Stein Gold, too, has received research grants from and serves as a consultant to numerous drug companies, including Celgene. Dr. Bhatia declared having financial relationships with more than two dozen.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – Oral apremilast is a drug in search of a compelling indication, Craig L. Leonardi, MD, declared at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Research Foundation.
“Apremilast is the least effective systemic agent for the treatment of psoriasis. That’s a statement of fact. I think the drug is mismatched for the treatment of moderate to severe plaque psoriasis. In fact, many of us gave the company that advice early on, but it was a program that was set in stone at that point,” according to Dr. Leonardi, a dermatologist at Saint Louis University and a prominent psoriasis clinical trialist.
“You have a drug that’s modest with regard to clearing psoriasis and it’s modest in controlling psoriatic arthritis. So you have to ask yourself what’s the patient population for this drug. I would just say that in my hands – and this is off-label – I use it only in patients with mild to moderate psoriasis,” Dr. Leonardi said.
“We all have psoriasis patients who come in with 3% or 4% of their skin involved, they’re actually using the class I topical steroids that I prescribe for them, yet they’re still not clear or almost clear and they want more. At that point, that’s when I might take them down this pathway and put them on apremilast along with a topical steroid. And I think that’s the appropriate place for this drug. I don’t use it in patients with 10% or more body surface area involvement,” he said.
He added that he would like to be able to prescribe apremilast in conjunction with a biologic agent in patients with more severe psoriasis to obtain synergistic efficacy, but payers balk at that because, at close to $3,000 per month, apremilast costs far more than methotrexate and other generic conventional disease-modifying antirheumatic drugs.
“The insurance industry is all over this drug. They require preauthorization, and they tell me, ‘No way, have a nice day,’ ” according to the dermatologist.
Dr. Leonardi described a couple of other practical caveats regarding apremilast. In patients with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a dose reduction to 30 mg once daily in the morning is necessary. And apremilast is not recommended for use in patients who are on a strong inducer of the CYP 450 enzyme, such as rifampin or phenytoin.
An intriguing side effect of apremilast is that it causes weight loss: A 5%-10% weight loss was seen in the major psoriasis clinical trials. But it’s been established that there is no correlation at all between the weight loss and clinical efficacy for skin clearance.
What efficacy can be anticipated?
In the pivotal phase III ESTEEM I trial conducted in 844 patients with moderate to severe psoriasis (J Am Acad Dermatol. 2015 Jul;73[1]:37-49), the overall PASI-75 response rate at week 16 in patients assigned to apremilast at 30 mg twice daily was 33%, significantly better than the 5% placebo response rate, but substantially less than what’s achieved with the tumor necrosis factor inhibitors and other injectable biologic agents.
Moreover, in the pivotal phase III PALACE 1 study of apremilast for the treatment of psoriatic arthritis, the primary outcome of at least a 20% improvement in the modified American College of Rheumatology response criteria at week 16 was achieved in 40% of patients randomized to apremilast at 30 mg twice daily, compared with 19% on placebo (Ann Rheum Dis. 2014 Jun;73[6]:1020-6). Again, that doesn’t approach the efficacy of a TNF inhibitor, Dr. Leonardi noted.
On the plus side, an oral agent such as apremilast is an attractive option for treatment-adherent patients. Plus, the drug has a favorable safety profile and is well tolerated, with mild to moderate side effects that appear early and are self-limited. In ESTEEM I, for example, more than 96% of patients had either no or only mild to moderate adverse events. The incidence of the two most common adverse events, diarrhea and nausea, at 19% and 16%, respectively, was more than double that in placebo-treated controls, but rates of other adverse events were similar in the two treatment arms.
Symposium codirector Linda Stein Gold, MD, director of dermatology research at the Henry Ford Health System in Detroit, said a clinical trial of apremilast conducted specifically in patients with moderate psoriasis has recently been completed. When those results become available they should shore up the drug’s use in that population.
Finding potential in off-label use
“This drug may have been brought to market for psoriasis, but I think its utility is so much more in other diseases where inflammation is an important mechanism,” said Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego. “Most of my use of apremilast is off-label for atopic dermatitis, for lichen planus, and I’ve tried it in a lot of patients where it has worked for discoid lupus. There’s so much potential for apremilast,” said Dr. Bhatia.
Dr. Leonardi remained unpersuaded.
“Your glass of water is half full, mine is half empty in this case,” he replied. “This drug has been approved now for at least 3 years, and we are still looking at the occasional favorable case report that flies up. I’ve got to say this drug is having a hard time finding a place outside of psoriasis, but we’ll all see.”
Dr. Leonardi reported having financial relationships with more than a dozen pharmaceutical companies, including Celgene, which markets apremilast. Dr. Stein Gold, too, has received research grants from and serves as a consultant to numerous drug companies, including Celgene. Dr. Bhatia declared having financial relationships with more than two dozen.
SDEF and this news organization are owned by the same parent company.
Adolescent opioid use common but decreasing
About a quarter of high school seniors have taken prescription opioids, medically or nonmedically, but exposures have declined over the past 2 years, according to a study.
“The recent declines in medical use of prescription opioids and nonmedical use of prescribed opioids from 2013 through 2015 found in the current study coincide with similar recent declines in U.S. opioid analgesic prescribing,” reported Sean Esteban McCabe, PhD, of the University of Michigan, Ann Arbor, and his associates (Pediatrics 2017 March 20. doi: 10.1542/peds.2016-2387). “Among adolescents who report both medical use of prescription opioids and nonmedical use of prescribed opioids, medical use before initiating nonmedical use of prescribed opioids tended to be most prevalent, and this pattern may be driven by the one-third of adolescents who report nonmedical use of prescribed opioids involving leftover opioid medications from their own previous prescriptions.”
The researchers analyzed data from the Monitoring the Future study from 1976 through 2015, an annual cross-sectional survey of high school seniors from 135 public and private high schools in the United States. Cohorts were nationally representative and varied in size from 2,181 to 3,791 students.
Students were asked whether they had ever been prescribed opioids by a doctor and how often they had ever taken opioids nonmedically. The list of opioids included all the ones available that year, including Vicodin, OxyContin, Percodan, Percocet, Demerol, Ultram, methadone, morphine, opium, and codeine.
Use of opioids for any reason ranged from 16.5% to 24% over the 4 decades, with medical use ranging from a low of 8.5% in 2000 to a peak of 14.4% in 1989. Though 16% of teens had used medical opioids in 1976, prevalence rose to 20% in 1989, then dropped to 13% in 1997 and remained stable. Rates sharply increased to 20% in 2002 – the year Vicodin, OxyContin, and Percocet were included in the questions – and then began declining again in 2013.
Nonmedical use correlated with medical use though remained less prevalent throughout the study period. This correlation was stronger for males, who are more likely to use opioids recreationally, than for females, who are more likely to use them to self-treat pain, the authors wrote.
Both medical and nonmedical use were more prevalent among white students than black students, in line with previous research showing “health disparities for receiving prescription opioids among racial minority patients,” the authors wrote.
Black teens are more often motivated to use opioids for pain relief, suggesting that their lower exposure rates “could result from a lack of adequate treatment, insufficient availability, overprescribing among white populations or underprescribing among nonwhite populations,” they wrote.
One limitation of the study was that absent students or those who had dropped out have a higher risk of opioid use, thereby possibly contributing to underreporting.
The National Institute on Drug Abuse and the National Institutes of Health funded the research. The researchers had no disclosures.
This research is important because nonmedical use of prescribed opiates is believed to be a major cause of morbidity and mortality associated with opiates. The consistency of the Monitoring the Future survey provides a high-level view of opiate use across the country from 1975 to 2015. Although helpful, this perspective can obscure critically important changes in local areas or within specific populations.
It is important to recognize the strong relationship between opioid prescription and nonmedical opioid use. These findings support the policy recommendations to prescribe opioids only when patients have strong indications for opioids and no better treatment options are available.
We view the recent decrease in the medical use of prescription opioids and nonmedical opioid use as an important finding, but there are significant small-area variations that would not appear in a national study. The epidemic of opioid use disproportionately affects some urban and more rural areas. Nonmedical use of prescribed opiates in general has become more common in rural areas. West Virginia, a predominantly rural state, has the highest rate of opioid overdose fatality in the country at 41.5 deaths per 100,000 in 2015. The state also has the second highest rate of opioid prescriptions per capita.
We are heartened to see a recent decrease, but we see it as a measured improvement. We understand that the appropriate use of opioids to manage pain can be helpful for our patients, but we must continue to search for solutions to the current crisis. Possible interventions include better education of our patients and families when we prescribe these drugs, better drug regulation, development of new affordable approaches to pain management that have lower potential for abuse, and accessible and affordable treatment programs for those already afflicted.
These comments were taken from an editorial published in Pediatrics by David A. Rosen, MD, FAAP, and Pamela J. Murray, MD, MHP, FAAP, both of West Virginia University in Morgantown. They reported having no disclosures or external funding.
This research is important because nonmedical use of prescribed opiates is believed to be a major cause of morbidity and mortality associated with opiates. The consistency of the Monitoring the Future survey provides a high-level view of opiate use across the country from 1975 to 2015. Although helpful, this perspective can obscure critically important changes in local areas or within specific populations.
It is important to recognize the strong relationship between opioid prescription and nonmedical opioid use. These findings support the policy recommendations to prescribe opioids only when patients have strong indications for opioids and no better treatment options are available.
We view the recent decrease in the medical use of prescription opioids and nonmedical opioid use as an important finding, but there are significant small-area variations that would not appear in a national study. The epidemic of opioid use disproportionately affects some urban and more rural areas. Nonmedical use of prescribed opiates in general has become more common in rural areas. West Virginia, a predominantly rural state, has the highest rate of opioid overdose fatality in the country at 41.5 deaths per 100,000 in 2015. The state also has the second highest rate of opioid prescriptions per capita.
We are heartened to see a recent decrease, but we see it as a measured improvement. We understand that the appropriate use of opioids to manage pain can be helpful for our patients, but we must continue to search for solutions to the current crisis. Possible interventions include better education of our patients and families when we prescribe these drugs, better drug regulation, development of new affordable approaches to pain management that have lower potential for abuse, and accessible and affordable treatment programs for those already afflicted.
These comments were taken from an editorial published in Pediatrics by David A. Rosen, MD, FAAP, and Pamela J. Murray, MD, MHP, FAAP, both of West Virginia University in Morgantown. They reported having no disclosures or external funding.
This research is important because nonmedical use of prescribed opiates is believed to be a major cause of morbidity and mortality associated with opiates. The consistency of the Monitoring the Future survey provides a high-level view of opiate use across the country from 1975 to 2015. Although helpful, this perspective can obscure critically important changes in local areas or within specific populations.
It is important to recognize the strong relationship between opioid prescription and nonmedical opioid use. These findings support the policy recommendations to prescribe opioids only when patients have strong indications for opioids and no better treatment options are available.
We view the recent decrease in the medical use of prescription opioids and nonmedical opioid use as an important finding, but there are significant small-area variations that would not appear in a national study. The epidemic of opioid use disproportionately affects some urban and more rural areas. Nonmedical use of prescribed opiates in general has become more common in rural areas. West Virginia, a predominantly rural state, has the highest rate of opioid overdose fatality in the country at 41.5 deaths per 100,000 in 2015. The state also has the second highest rate of opioid prescriptions per capita.
We are heartened to see a recent decrease, but we see it as a measured improvement. We understand that the appropriate use of opioids to manage pain can be helpful for our patients, but we must continue to search for solutions to the current crisis. Possible interventions include better education of our patients and families when we prescribe these drugs, better drug regulation, development of new affordable approaches to pain management that have lower potential for abuse, and accessible and affordable treatment programs for those already afflicted.
These comments were taken from an editorial published in Pediatrics by David A. Rosen, MD, FAAP, and Pamela J. Murray, MD, MHP, FAAP, both of West Virginia University in Morgantown. They reported having no disclosures or external funding.
About a quarter of high school seniors have taken prescription opioids, medically or nonmedically, but exposures have declined over the past 2 years, according to a study.
“The recent declines in medical use of prescription opioids and nonmedical use of prescribed opioids from 2013 through 2015 found in the current study coincide with similar recent declines in U.S. opioid analgesic prescribing,” reported Sean Esteban McCabe, PhD, of the University of Michigan, Ann Arbor, and his associates (Pediatrics 2017 March 20. doi: 10.1542/peds.2016-2387). “Among adolescents who report both medical use of prescription opioids and nonmedical use of prescribed opioids, medical use before initiating nonmedical use of prescribed opioids tended to be most prevalent, and this pattern may be driven by the one-third of adolescents who report nonmedical use of prescribed opioids involving leftover opioid medications from their own previous prescriptions.”
The researchers analyzed data from the Monitoring the Future study from 1976 through 2015, an annual cross-sectional survey of high school seniors from 135 public and private high schools in the United States. Cohorts were nationally representative and varied in size from 2,181 to 3,791 students.
Students were asked whether they had ever been prescribed opioids by a doctor and how often they had ever taken opioids nonmedically. The list of opioids included all the ones available that year, including Vicodin, OxyContin, Percodan, Percocet, Demerol, Ultram, methadone, morphine, opium, and codeine.
Use of opioids for any reason ranged from 16.5% to 24% over the 4 decades, with medical use ranging from a low of 8.5% in 2000 to a peak of 14.4% in 1989. Though 16% of teens had used medical opioids in 1976, prevalence rose to 20% in 1989, then dropped to 13% in 1997 and remained stable. Rates sharply increased to 20% in 2002 – the year Vicodin, OxyContin, and Percocet were included in the questions – and then began declining again in 2013.
Nonmedical use correlated with medical use though remained less prevalent throughout the study period. This correlation was stronger for males, who are more likely to use opioids recreationally, than for females, who are more likely to use them to self-treat pain, the authors wrote.
Both medical and nonmedical use were more prevalent among white students than black students, in line with previous research showing “health disparities for receiving prescription opioids among racial minority patients,” the authors wrote.
Black teens are more often motivated to use opioids for pain relief, suggesting that their lower exposure rates “could result from a lack of adequate treatment, insufficient availability, overprescribing among white populations or underprescribing among nonwhite populations,” they wrote.
One limitation of the study was that absent students or those who had dropped out have a higher risk of opioid use, thereby possibly contributing to underreporting.
The National Institute on Drug Abuse and the National Institutes of Health funded the research. The researchers had no disclosures.
About a quarter of high school seniors have taken prescription opioids, medically or nonmedically, but exposures have declined over the past 2 years, according to a study.
“The recent declines in medical use of prescription opioids and nonmedical use of prescribed opioids from 2013 through 2015 found in the current study coincide with similar recent declines in U.S. opioid analgesic prescribing,” reported Sean Esteban McCabe, PhD, of the University of Michigan, Ann Arbor, and his associates (Pediatrics 2017 March 20. doi: 10.1542/peds.2016-2387). “Among adolescents who report both medical use of prescription opioids and nonmedical use of prescribed opioids, medical use before initiating nonmedical use of prescribed opioids tended to be most prevalent, and this pattern may be driven by the one-third of adolescents who report nonmedical use of prescribed opioids involving leftover opioid medications from their own previous prescriptions.”
The researchers analyzed data from the Monitoring the Future study from 1976 through 2015, an annual cross-sectional survey of high school seniors from 135 public and private high schools in the United States. Cohorts were nationally representative and varied in size from 2,181 to 3,791 students.
Students were asked whether they had ever been prescribed opioids by a doctor and how often they had ever taken opioids nonmedically. The list of opioids included all the ones available that year, including Vicodin, OxyContin, Percodan, Percocet, Demerol, Ultram, methadone, morphine, opium, and codeine.
Use of opioids for any reason ranged from 16.5% to 24% over the 4 decades, with medical use ranging from a low of 8.5% in 2000 to a peak of 14.4% in 1989. Though 16% of teens had used medical opioids in 1976, prevalence rose to 20% in 1989, then dropped to 13% in 1997 and remained stable. Rates sharply increased to 20% in 2002 – the year Vicodin, OxyContin, and Percocet were included in the questions – and then began declining again in 2013.
Nonmedical use correlated with medical use though remained less prevalent throughout the study period. This correlation was stronger for males, who are more likely to use opioids recreationally, than for females, who are more likely to use them to self-treat pain, the authors wrote.
Both medical and nonmedical use were more prevalent among white students than black students, in line with previous research showing “health disparities for receiving prescription opioids among racial minority patients,” the authors wrote.
Black teens are more often motivated to use opioids for pain relief, suggesting that their lower exposure rates “could result from a lack of adequate treatment, insufficient availability, overprescribing among white populations or underprescribing among nonwhite populations,” they wrote.
One limitation of the study was that absent students or those who had dropped out have a higher risk of opioid use, thereby possibly contributing to underreporting.
The National Institute on Drug Abuse and the National Institutes of Health funded the research. The researchers had no disclosures.
FROM PEDIATRICS
Key clinical point: Teen opioid use began to decline from 2013 through 2015 but remains common.
Major finding: Prevalence of teens’ medical or nonmedical use of prescription opioids ranged from 16.5% to 24% between 1976 and 2015.
Data source: Analysis of 40 annual surveys of U.S. high school seniors from 1976 through 2015.
Disclosures: The National Institute on Drug Abuse and the National Institutes of Health funded the research. The researchers had no disclosures.
Antioxidants plus sunscreens may be two-punch knockout for melasma
ORLANDO – A two-step regimen of high-potency topical antioxidants followed by a mineral-based sunscreen may help repair light-induced skin damage and protect against new damage in patients with melasma.
Recent studies suggest that the antioxidants tamp down inflammatory cytokines and damage of oxidative stress, Maria Ivonne Arellano-Mendoza, MD, said during a special focus session on Latino skin held at the annual meeting of the American Academy of Dermatology.
“Many compounds are being studied for this purpose,” said Dr. Arellano-Mendoza, head of the dermatology department of the General Hospital of Mexico, Mexico City. “One combination is vitamin C, vitamin E, ubiquinone, and grape-seed extract. This effectively prevented infrared-A radiation–induced matrix metalloproteinase-1 messenger RNA expression in human skin. This combination can be found now in some sunscreens and daily care products.”
Another effective combination seems to be a mixture of ferulic acid, tocopherol, and vitamin C, she said.
The two-step process of regularly using a topical product with antioxidants before applying a sunscreen is all there is for now, she added, because so far it’s been impossible to combine the agents in a single product.
“The challenge will be how to create a product that stays on the surface of the skin to protect it from light, while liberating the antioxidants to penetrate the skin,” she commented.
The antioxidants’ benefits, however, will be obliterated by the continued effects of some light wavelengths that aggravate melasma unless they are used in sequence with a light-scattering sunscreen.
Sunscreens are critical components of a melasma treatment regimen, Dr. Arellano-Mendoza said. “Sunscreens are a cornerstone of treatment. We clearly tell our patients that sunscreens have to be used every day, forever, and if they are not used properly, they will have no improvement.”
Patients with hyperpigmentation disorders are susceptible to longer wavelengths that aren’t covered by chemically derived sunscreens. Longer wavelengths, including infrared light and visible light, have been shown to increase expression of matrix metalloproteinase (MMP) -1 and -9, decrease expression of type 1 procollagen, and can induce macrophage infiltration. These wavelengths also increase reactive oxygen species and proinflammatory cytokines in vitro, Dr. Arellano-Mendoza noted.
Visible light can cause erythema, transient and long-lasting hyperpigmentation, thermal damage, free radical production, and premature photoaging. It also can stimulate the production of reactive oxygen species that can damage DNA.
Mineral-based, inorganic sunscreens, however – like those with titanium dioxide, zinc oxide, and iron oxide – scatter all wavelengths.
“These micronized forms of metal oxides not only scatter and reflect light, they also absorb ultraviolet radiation. The compounds aren’t new,” she said. In 1991, Dr. Elaine Kaye of Harvard University, Boston, and associates described (Arch Dermatol. 1991;127:351-5) opaque physical sunscreens that were useful blockers of visible light and found that transmittance of light can be lowered by adding iron oxide, Dr. Arellano-Mendoza pointed out.
The inorganic sunscreens have never been widely adopted because they are highly pigmented with white or, in the case of iron oxide, with red. “Not many people accepted [the iron-containing compounds] because of the redness, but now different shades are going to be hitting the market soon,” and the hope is that consumers will find them more appealing, she said.
This is good news, as the data emerging around iron oxide are intriguingly positive. A 2015 study showed that a sunscreen with iron oxides prevented melasma relapse during the summer months. Patients were randomized to the same ultraviolet filter topical sunscreen, but for one group, micronized iron oxide was added to it. After 6 months, the median melasma area severity index score was significantly better in the group using the iron oxide compound (J Am Acad Dermatol. 2015 Jan;72[1]:189-90.e1).
Support for the two-step regimen appeared in 2014, when a small study randomized 30 healthy volunteers to an SPF 30 sunscreen or the same sunscreen supplemented with an antioxidant cocktail of grape seed extract, vitamin E, ubiquinone, and vitamin C. The endpoint was MMP-1 upregulation after exposure to infrared-A light. Skin treated with the combination regimen showed significantly lower MMP-1 activation, leading the authors to conclude that the combination of topical antioxidants conferred protection against the irradiation (Photochem Photobiol. 2015 Jan-Feb;91[1]:248-50).
Those same authors published a companion article (Photodermatol Photoimmunol Photomed. 2014;30:167-74) suggesting that another antioxidant mixture (ferulic acid, tocopherol, and vitamin C) was similarly effective.
Using a combination of antioxidants is important, Dr. Arellano-Mendoza said, because different antioxidants work differently. Some (catalase, glutathione peroxidase, and superoxide dismutase) are enzymatic, catalyzing reactions that convert free radicals to oxygen and water. Others terminate free radicals by preventing the propagation of oxidative chain reactions (vitamins A and C, flavonoids, uric acid, bilirubin, albumin, and members of the thiol group). A third group consists of metal-binding proteins that sequester free iron or copper to prevent free radical production (ferritin, transferrin, lactoferrin, and ceruloplasmin).
“I think we can now consider antioxidants a part of the tools we use in treating some pigmentary disorders as melasma,” she said. “We need to choose the compounds carefully, and we definitely need more in vivo research, but the findings are very encouraging.”
Dr. Arellano-Mendoza had no relevant financial disclosures.
This article was updated 3/20/17.
[email protected]
On Twitter @Alz_Gal
ORLANDO – A two-step regimen of high-potency topical antioxidants followed by a mineral-based sunscreen may help repair light-induced skin damage and protect against new damage in patients with melasma.
Recent studies suggest that the antioxidants tamp down inflammatory cytokines and damage of oxidative stress, Maria Ivonne Arellano-Mendoza, MD, said during a special focus session on Latino skin held at the annual meeting of the American Academy of Dermatology.
“Many compounds are being studied for this purpose,” said Dr. Arellano-Mendoza, head of the dermatology department of the General Hospital of Mexico, Mexico City. “One combination is vitamin C, vitamin E, ubiquinone, and grape-seed extract. This effectively prevented infrared-A radiation–induced matrix metalloproteinase-1 messenger RNA expression in human skin. This combination can be found now in some sunscreens and daily care products.”
Another effective combination seems to be a mixture of ferulic acid, tocopherol, and vitamin C, she said.
The two-step process of regularly using a topical product with antioxidants before applying a sunscreen is all there is for now, she added, because so far it’s been impossible to combine the agents in a single product.
“The challenge will be how to create a product that stays on the surface of the skin to protect it from light, while liberating the antioxidants to penetrate the skin,” she commented.
The antioxidants’ benefits, however, will be obliterated by the continued effects of some light wavelengths that aggravate melasma unless they are used in sequence with a light-scattering sunscreen.
Sunscreens are critical components of a melasma treatment regimen, Dr. Arellano-Mendoza said. “Sunscreens are a cornerstone of treatment. We clearly tell our patients that sunscreens have to be used every day, forever, and if they are not used properly, they will have no improvement.”
Patients with hyperpigmentation disorders are susceptible to longer wavelengths that aren’t covered by chemically derived sunscreens. Longer wavelengths, including infrared light and visible light, have been shown to increase expression of matrix metalloproteinase (MMP) -1 and -9, decrease expression of type 1 procollagen, and can induce macrophage infiltration. These wavelengths also increase reactive oxygen species and proinflammatory cytokines in vitro, Dr. Arellano-Mendoza noted.
Visible light can cause erythema, transient and long-lasting hyperpigmentation, thermal damage, free radical production, and premature photoaging. It also can stimulate the production of reactive oxygen species that can damage DNA.
Mineral-based, inorganic sunscreens, however – like those with titanium dioxide, zinc oxide, and iron oxide – scatter all wavelengths.
“These micronized forms of metal oxides not only scatter and reflect light, they also absorb ultraviolet radiation. The compounds aren’t new,” she said. In 1991, Dr. Elaine Kaye of Harvard University, Boston, and associates described (Arch Dermatol. 1991;127:351-5) opaque physical sunscreens that were useful blockers of visible light and found that transmittance of light can be lowered by adding iron oxide, Dr. Arellano-Mendoza pointed out.
The inorganic sunscreens have never been widely adopted because they are highly pigmented with white or, in the case of iron oxide, with red. “Not many people accepted [the iron-containing compounds] because of the redness, but now different shades are going to be hitting the market soon,” and the hope is that consumers will find them more appealing, she said.
This is good news, as the data emerging around iron oxide are intriguingly positive. A 2015 study showed that a sunscreen with iron oxides prevented melasma relapse during the summer months. Patients were randomized to the same ultraviolet filter topical sunscreen, but for one group, micronized iron oxide was added to it. After 6 months, the median melasma area severity index score was significantly better in the group using the iron oxide compound (J Am Acad Dermatol. 2015 Jan;72[1]:189-90.e1).
Support for the two-step regimen appeared in 2014, when a small study randomized 30 healthy volunteers to an SPF 30 sunscreen or the same sunscreen supplemented with an antioxidant cocktail of grape seed extract, vitamin E, ubiquinone, and vitamin C. The endpoint was MMP-1 upregulation after exposure to infrared-A light. Skin treated with the combination regimen showed significantly lower MMP-1 activation, leading the authors to conclude that the combination of topical antioxidants conferred protection against the irradiation (Photochem Photobiol. 2015 Jan-Feb;91[1]:248-50).
Those same authors published a companion article (Photodermatol Photoimmunol Photomed. 2014;30:167-74) suggesting that another antioxidant mixture (ferulic acid, tocopherol, and vitamin C) was similarly effective.
Using a combination of antioxidants is important, Dr. Arellano-Mendoza said, because different antioxidants work differently. Some (catalase, glutathione peroxidase, and superoxide dismutase) are enzymatic, catalyzing reactions that convert free radicals to oxygen and water. Others terminate free radicals by preventing the propagation of oxidative chain reactions (vitamins A and C, flavonoids, uric acid, bilirubin, albumin, and members of the thiol group). A third group consists of metal-binding proteins that sequester free iron or copper to prevent free radical production (ferritin, transferrin, lactoferrin, and ceruloplasmin).
“I think we can now consider antioxidants a part of the tools we use in treating some pigmentary disorders as melasma,” she said. “We need to choose the compounds carefully, and we definitely need more in vivo research, but the findings are very encouraging.”
Dr. Arellano-Mendoza had no relevant financial disclosures.
This article was updated 3/20/17.
[email protected]
On Twitter @Alz_Gal
ORLANDO – A two-step regimen of high-potency topical antioxidants followed by a mineral-based sunscreen may help repair light-induced skin damage and protect against new damage in patients with melasma.
Recent studies suggest that the antioxidants tamp down inflammatory cytokines and damage of oxidative stress, Maria Ivonne Arellano-Mendoza, MD, said during a special focus session on Latino skin held at the annual meeting of the American Academy of Dermatology.
“Many compounds are being studied for this purpose,” said Dr. Arellano-Mendoza, head of the dermatology department of the General Hospital of Mexico, Mexico City. “One combination is vitamin C, vitamin E, ubiquinone, and grape-seed extract. This effectively prevented infrared-A radiation–induced matrix metalloproteinase-1 messenger RNA expression in human skin. This combination can be found now in some sunscreens and daily care products.”
Another effective combination seems to be a mixture of ferulic acid, tocopherol, and vitamin C, she said.
The two-step process of regularly using a topical product with antioxidants before applying a sunscreen is all there is for now, she added, because so far it’s been impossible to combine the agents in a single product.
“The challenge will be how to create a product that stays on the surface of the skin to protect it from light, while liberating the antioxidants to penetrate the skin,” she commented.
The antioxidants’ benefits, however, will be obliterated by the continued effects of some light wavelengths that aggravate melasma unless they are used in sequence with a light-scattering sunscreen.
Sunscreens are critical components of a melasma treatment regimen, Dr. Arellano-Mendoza said. “Sunscreens are a cornerstone of treatment. We clearly tell our patients that sunscreens have to be used every day, forever, and if they are not used properly, they will have no improvement.”
Patients with hyperpigmentation disorders are susceptible to longer wavelengths that aren’t covered by chemically derived sunscreens. Longer wavelengths, including infrared light and visible light, have been shown to increase expression of matrix metalloproteinase (MMP) -1 and -9, decrease expression of type 1 procollagen, and can induce macrophage infiltration. These wavelengths also increase reactive oxygen species and proinflammatory cytokines in vitro, Dr. Arellano-Mendoza noted.
Visible light can cause erythema, transient and long-lasting hyperpigmentation, thermal damage, free radical production, and premature photoaging. It also can stimulate the production of reactive oxygen species that can damage DNA.
Mineral-based, inorganic sunscreens, however – like those with titanium dioxide, zinc oxide, and iron oxide – scatter all wavelengths.
“These micronized forms of metal oxides not only scatter and reflect light, they also absorb ultraviolet radiation. The compounds aren’t new,” she said. In 1991, Dr. Elaine Kaye of Harvard University, Boston, and associates described (Arch Dermatol. 1991;127:351-5) opaque physical sunscreens that were useful blockers of visible light and found that transmittance of light can be lowered by adding iron oxide, Dr. Arellano-Mendoza pointed out.
The inorganic sunscreens have never been widely adopted because they are highly pigmented with white or, in the case of iron oxide, with red. “Not many people accepted [the iron-containing compounds] because of the redness, but now different shades are going to be hitting the market soon,” and the hope is that consumers will find them more appealing, she said.
This is good news, as the data emerging around iron oxide are intriguingly positive. A 2015 study showed that a sunscreen with iron oxides prevented melasma relapse during the summer months. Patients were randomized to the same ultraviolet filter topical sunscreen, but for one group, micronized iron oxide was added to it. After 6 months, the median melasma area severity index score was significantly better in the group using the iron oxide compound (J Am Acad Dermatol. 2015 Jan;72[1]:189-90.e1).
Support for the two-step regimen appeared in 2014, when a small study randomized 30 healthy volunteers to an SPF 30 sunscreen or the same sunscreen supplemented with an antioxidant cocktail of grape seed extract, vitamin E, ubiquinone, and vitamin C. The endpoint was MMP-1 upregulation after exposure to infrared-A light. Skin treated with the combination regimen showed significantly lower MMP-1 activation, leading the authors to conclude that the combination of topical antioxidants conferred protection against the irradiation (Photochem Photobiol. 2015 Jan-Feb;91[1]:248-50).
Those same authors published a companion article (Photodermatol Photoimmunol Photomed. 2014;30:167-74) suggesting that another antioxidant mixture (ferulic acid, tocopherol, and vitamin C) was similarly effective.
Using a combination of antioxidants is important, Dr. Arellano-Mendoza said, because different antioxidants work differently. Some (catalase, glutathione peroxidase, and superoxide dismutase) are enzymatic, catalyzing reactions that convert free radicals to oxygen and water. Others terminate free radicals by preventing the propagation of oxidative chain reactions (vitamins A and C, flavonoids, uric acid, bilirubin, albumin, and members of the thiol group). A third group consists of metal-binding proteins that sequester free iron or copper to prevent free radical production (ferritin, transferrin, lactoferrin, and ceruloplasmin).
“I think we can now consider antioxidants a part of the tools we use in treating some pigmentary disorders as melasma,” she said. “We need to choose the compounds carefully, and we definitely need more in vivo research, but the findings are very encouraging.”
Dr. Arellano-Mendoza had no relevant financial disclosures.
This article was updated 3/20/17.
[email protected]
On Twitter @Alz_Gal
EXPERT ANALYSIS FROM AAD 2017
EXPERT ANALYSIS FROM AAD 2017
Get With the Guidelines propels stroke thrombolytic therapy
HOUSTON – Thrombolytic therapy for U.S. patients experiencing an acute ischemic stroke is no longer the poster child for proven, but neglected, therapies.
Long bemoaned since its introduction in the mid-1990s as an effective but woefully underused treatment, thrombolytic therapy with tissue plasminogen activator (tPA) has seen robust growth in U.S. practice recently, largely due to the promotion and quality-improvement efforts of a voluntary, non-profit program, Get With the Guidelines (GWTG)-Stroke.
“We’ve seen a dramatic increase in tPA treatment in eligible patients,” said Dr. Smith, a neurologist and medical director of the Cognitive Neurosciences Clinic at the University of Calgary (Alta.).
“This represents remarkable and clinically meaningful improvements in stroke care, overcoming substantial barriers using a systems of care approach,” Gregg C. Fonarow, MD, professor of medicine and cochief of clinical cardiology at the University of California, Los Angeles and one of the long-time leaders of GWTG-Stroke, said in an interview. “This represents one of the most transformative improvements and success stories ever observed in stroke care.”
“These data show dramatic changes in the GWTG-Stroke hospitals,” agreed Lee H. Schwamm, MD, professor of neurology at Harvard Medical School and chief of stroke services at Massachusetts General Hospital in Boston and another leader of the GWTG-Stroke program. However, “we still have work to do,” cautioned Dr. Schwamm, who notes that 68% remains significantly short of the ideal 100%. The positive is that several GWTG-Stroke participating hospitals have surged to a better than 90% rate of administering tPA to eligible patients within a 60-minute door-to-needle window.
What is GWTG-Stroke?
GWTG was launched in 2000 by the American Heart Association as a hospital-based–quality improvement initiative focused on U.S. management of acute MI. In 2003, acute stroke became another focus of the program and inspired added participation of the American Stroke Association.
“Improvements in tPA use in eligible patients were seen soon after the introduction of GWTG-Stroke in 2003, but there was minimal improvement in the percentage of patients with door-to-needle times within 60 minutes despite national guidelines,” recalled Dr. Fonarow. This slow rate of advance led him, Dr. Schwamm, and others to create a more activist program within GWTG-Stroke, Target Stroke, that not only provided resources and collected data from participating hospitals but also set acute-treatment goals that challenged participating hospitals to up their game. Target Stroke phase I asked them to try to achieve a tPA door-to-needle time within 60 minutes in at least 50% of eligible patients. By mid-2013, the program reached this goal with 53% of patients treated in the allotted time frame (JAMA. 2014 Apr 23;311[16]:1632-40).
These time-based goals lead directly to meaningful improvements in patient outcomes. “We know that, for every 15 minute reduction in tPA door-to-needle time, there is a 5% reduction of in-hospital mortality and a substantial increase in the percentage of patients who are discharged home instead of to a rehabilitation hospital or nursing home,” Dr. Schwamm said.
Dr. Smith highlighted the 11 best practices steps that Target Stroke promotes to GWTG-Stroke hospitals as their road map to achieving the performance goals. These include things such as notification of a hospital by emergency medical services that a patient showing signs of an acute ischemic stroke is on the way, transfer of the patient after hospital arrival directly to a CT or MRI scanner to get the imaging that can confirm tPA eligibility, rapid reading of the scan, premixing of the tPA, a team-based approach to acute stroke management, and quick feedback to the team regarding their performance on each stroke patient they treat.
Dr. Schwamm noted that he, Dr. Fonarow, and other program leaders are already planning a phase III for Target Stroke that will branch out to even more facets of acute stroke care, such as incorporation of goals for using endovascular thrombectomy in appropriate patients and for using telemedicine to quicken and broaden the availability of expert neurologic consults for identifying tPA- and thrombectomy-eligible patients.
Growing the program
The success of GWTG-Stroke in transforming U.S. acute stroke care has not only relied on setting aggressive performance goals but also on advancing by boosting the number of U.S. hospitals participating of more U.S. hospitals. During the Target Stroke years since the start of 2010, the number of hospitals active in GWTG-Stroke jumped from 1427 to 1,950 by mid-2016, including a 12% year-over-year jump in 2016, compared with 2015. During October 2015-October 2016, participating hospitals treated more than 570,000 acute stroke patients, or roughly 71% of the estimated 800,000 U.S. patients who have an acute stroke each year.
These numbers seem on track to continue expanding. “With the recent evidence showing that participating in GWTG-Stroke improves care and outcomes there has been even greater interest by hospitals, recently, in joining,” said Dr. Fonarow. “While many hospitals currently participate, ideally all would join and be active.”
“Hospitals now feel that they can’t afford not to focus on stroke as a quality improvement program, so they join,” said Dr. Schwamm. He believes that essentially all of the roughly 200 certified U.S. Comprehensive Stroke Centers and of the roughly 1,000 certified U.S. Primary Stroke Centers are already members of GWTG-Stroke. “We’re now in all the high-value hospitals, based on their higher numbers of patients. It’s the Acute Stroke Ready hospitals where we now have the greatest potential to penetrate, hospitals that generally treat about 20-50 stroke patients a year,” he said.
Joining GWTG-Stroke holds major attraction for hospitals because the program gives them a tool for measuring their performance, data that hospitals often now need to prove the value of the care they deliver to insurers and to avoid penalization for readmissions. However, the barrier to hospitals, especially smaller hospitals, is that data collection can be expensive. It’s something that larger hospitals now routinely do, but smaller hospitals have often balked because of the expense. To address this, the GWTG-Stroke program is trying to develop a “lighter” version of their data collection tool that involves a smaller financial burden.
“I think we can sell a trimmed down version of GWTG-Stroke to smaller hospitals that is affordable and use it to recruit another 1,000 hospitals. That’s a realistic goal,” Dr. Schwamm said.
What is also notable about GWTG-Stroke is that hospitals sign up despite the somewhat ambiguous payback they receive.
“In the past, stroke was the third-leading U.S. cause of death, but now it’s fifth,” noted Steven R. Messe, MD, a stroke neurologist at the University of Pennsylvania in Philadelphia and a member of the GWTG-Stroke steering committee. “That’s an amazing accomplishment – to drop stroke from third place to fifth – and it’s due to advances in thrombolytic use and to improved systems of care and quality improvement measures.”
Get With the Guidelines-Stroke is a program of the American Heart Association and American Stroke Association and uses funding provided by several drug companies. Dr. Smith, Dr. Fonarow, Dr. Schwamm and Dr. Messe had no relevant commercial disclosures.
[email protected]
On Twitter @mitchelzoler
HOUSTON – Thrombolytic therapy for U.S. patients experiencing an acute ischemic stroke is no longer the poster child for proven, but neglected, therapies.
Long bemoaned since its introduction in the mid-1990s as an effective but woefully underused treatment, thrombolytic therapy with tissue plasminogen activator (tPA) has seen robust growth in U.S. practice recently, largely due to the promotion and quality-improvement efforts of a voluntary, non-profit program, Get With the Guidelines (GWTG)-Stroke.
“We’ve seen a dramatic increase in tPA treatment in eligible patients,” said Dr. Smith, a neurologist and medical director of the Cognitive Neurosciences Clinic at the University of Calgary (Alta.).
“This represents remarkable and clinically meaningful improvements in stroke care, overcoming substantial barriers using a systems of care approach,” Gregg C. Fonarow, MD, professor of medicine and cochief of clinical cardiology at the University of California, Los Angeles and one of the long-time leaders of GWTG-Stroke, said in an interview. “This represents one of the most transformative improvements and success stories ever observed in stroke care.”
“These data show dramatic changes in the GWTG-Stroke hospitals,” agreed Lee H. Schwamm, MD, professor of neurology at Harvard Medical School and chief of stroke services at Massachusetts General Hospital in Boston and another leader of the GWTG-Stroke program. However, “we still have work to do,” cautioned Dr. Schwamm, who notes that 68% remains significantly short of the ideal 100%. The positive is that several GWTG-Stroke participating hospitals have surged to a better than 90% rate of administering tPA to eligible patients within a 60-minute door-to-needle window.
What is GWTG-Stroke?
GWTG was launched in 2000 by the American Heart Association as a hospital-based–quality improvement initiative focused on U.S. management of acute MI. In 2003, acute stroke became another focus of the program and inspired added participation of the American Stroke Association.
“Improvements in tPA use in eligible patients were seen soon after the introduction of GWTG-Stroke in 2003, but there was minimal improvement in the percentage of patients with door-to-needle times within 60 minutes despite national guidelines,” recalled Dr. Fonarow. This slow rate of advance led him, Dr. Schwamm, and others to create a more activist program within GWTG-Stroke, Target Stroke, that not only provided resources and collected data from participating hospitals but also set acute-treatment goals that challenged participating hospitals to up their game. Target Stroke phase I asked them to try to achieve a tPA door-to-needle time within 60 minutes in at least 50% of eligible patients. By mid-2013, the program reached this goal with 53% of patients treated in the allotted time frame (JAMA. 2014 Apr 23;311[16]:1632-40).
These time-based goals lead directly to meaningful improvements in patient outcomes. “We know that, for every 15 minute reduction in tPA door-to-needle time, there is a 5% reduction of in-hospital mortality and a substantial increase in the percentage of patients who are discharged home instead of to a rehabilitation hospital or nursing home,” Dr. Schwamm said.
Dr. Smith highlighted the 11 best practices steps that Target Stroke promotes to GWTG-Stroke hospitals as their road map to achieving the performance goals. These include things such as notification of a hospital by emergency medical services that a patient showing signs of an acute ischemic stroke is on the way, transfer of the patient after hospital arrival directly to a CT or MRI scanner to get the imaging that can confirm tPA eligibility, rapid reading of the scan, premixing of the tPA, a team-based approach to acute stroke management, and quick feedback to the team regarding their performance on each stroke patient they treat.
Dr. Schwamm noted that he, Dr. Fonarow, and other program leaders are already planning a phase III for Target Stroke that will branch out to even more facets of acute stroke care, such as incorporation of goals for using endovascular thrombectomy in appropriate patients and for using telemedicine to quicken and broaden the availability of expert neurologic consults for identifying tPA- and thrombectomy-eligible patients.
Growing the program
The success of GWTG-Stroke in transforming U.S. acute stroke care has not only relied on setting aggressive performance goals but also on advancing by boosting the number of U.S. hospitals participating of more U.S. hospitals. During the Target Stroke years since the start of 2010, the number of hospitals active in GWTG-Stroke jumped from 1427 to 1,950 by mid-2016, including a 12% year-over-year jump in 2016, compared with 2015. During October 2015-October 2016, participating hospitals treated more than 570,000 acute stroke patients, or roughly 71% of the estimated 800,000 U.S. patients who have an acute stroke each year.
These numbers seem on track to continue expanding. “With the recent evidence showing that participating in GWTG-Stroke improves care and outcomes there has been even greater interest by hospitals, recently, in joining,” said Dr. Fonarow. “While many hospitals currently participate, ideally all would join and be active.”
“Hospitals now feel that they can’t afford not to focus on stroke as a quality improvement program, so they join,” said Dr. Schwamm. He believes that essentially all of the roughly 200 certified U.S. Comprehensive Stroke Centers and of the roughly 1,000 certified U.S. Primary Stroke Centers are already members of GWTG-Stroke. “We’re now in all the high-value hospitals, based on their higher numbers of patients. It’s the Acute Stroke Ready hospitals where we now have the greatest potential to penetrate, hospitals that generally treat about 20-50 stroke patients a year,” he said.
Joining GWTG-Stroke holds major attraction for hospitals because the program gives them a tool for measuring their performance, data that hospitals often now need to prove the value of the care they deliver to insurers and to avoid penalization for readmissions. However, the barrier to hospitals, especially smaller hospitals, is that data collection can be expensive. It’s something that larger hospitals now routinely do, but smaller hospitals have often balked because of the expense. To address this, the GWTG-Stroke program is trying to develop a “lighter” version of their data collection tool that involves a smaller financial burden.
“I think we can sell a trimmed down version of GWTG-Stroke to smaller hospitals that is affordable and use it to recruit another 1,000 hospitals. That’s a realistic goal,” Dr. Schwamm said.
What is also notable about GWTG-Stroke is that hospitals sign up despite the somewhat ambiguous payback they receive.
“In the past, stroke was the third-leading U.S. cause of death, but now it’s fifth,” noted Steven R. Messe, MD, a stroke neurologist at the University of Pennsylvania in Philadelphia and a member of the GWTG-Stroke steering committee. “That’s an amazing accomplishment – to drop stroke from third place to fifth – and it’s due to advances in thrombolytic use and to improved systems of care and quality improvement measures.”
Get With the Guidelines-Stroke is a program of the American Heart Association and American Stroke Association and uses funding provided by several drug companies. Dr. Smith, Dr. Fonarow, Dr. Schwamm and Dr. Messe had no relevant commercial disclosures.
[email protected]
On Twitter @mitchelzoler
HOUSTON – Thrombolytic therapy for U.S. patients experiencing an acute ischemic stroke is no longer the poster child for proven, but neglected, therapies.
Long bemoaned since its introduction in the mid-1990s as an effective but woefully underused treatment, thrombolytic therapy with tissue plasminogen activator (tPA) has seen robust growth in U.S. practice recently, largely due to the promotion and quality-improvement efforts of a voluntary, non-profit program, Get With the Guidelines (GWTG)-Stroke.
“We’ve seen a dramatic increase in tPA treatment in eligible patients,” said Dr. Smith, a neurologist and medical director of the Cognitive Neurosciences Clinic at the University of Calgary (Alta.).
“This represents remarkable and clinically meaningful improvements in stroke care, overcoming substantial barriers using a systems of care approach,” Gregg C. Fonarow, MD, professor of medicine and cochief of clinical cardiology at the University of California, Los Angeles and one of the long-time leaders of GWTG-Stroke, said in an interview. “This represents one of the most transformative improvements and success stories ever observed in stroke care.”
“These data show dramatic changes in the GWTG-Stroke hospitals,” agreed Lee H. Schwamm, MD, professor of neurology at Harvard Medical School and chief of stroke services at Massachusetts General Hospital in Boston and another leader of the GWTG-Stroke program. However, “we still have work to do,” cautioned Dr. Schwamm, who notes that 68% remains significantly short of the ideal 100%. The positive is that several GWTG-Stroke participating hospitals have surged to a better than 90% rate of administering tPA to eligible patients within a 60-minute door-to-needle window.
What is GWTG-Stroke?
GWTG was launched in 2000 by the American Heart Association as a hospital-based–quality improvement initiative focused on U.S. management of acute MI. In 2003, acute stroke became another focus of the program and inspired added participation of the American Stroke Association.
“Improvements in tPA use in eligible patients were seen soon after the introduction of GWTG-Stroke in 2003, but there was minimal improvement in the percentage of patients with door-to-needle times within 60 minutes despite national guidelines,” recalled Dr. Fonarow. This slow rate of advance led him, Dr. Schwamm, and others to create a more activist program within GWTG-Stroke, Target Stroke, that not only provided resources and collected data from participating hospitals but also set acute-treatment goals that challenged participating hospitals to up their game. Target Stroke phase I asked them to try to achieve a tPA door-to-needle time within 60 minutes in at least 50% of eligible patients. By mid-2013, the program reached this goal with 53% of patients treated in the allotted time frame (JAMA. 2014 Apr 23;311[16]:1632-40).
These time-based goals lead directly to meaningful improvements in patient outcomes. “We know that, for every 15 minute reduction in tPA door-to-needle time, there is a 5% reduction of in-hospital mortality and a substantial increase in the percentage of patients who are discharged home instead of to a rehabilitation hospital or nursing home,” Dr. Schwamm said.
Dr. Smith highlighted the 11 best practices steps that Target Stroke promotes to GWTG-Stroke hospitals as their road map to achieving the performance goals. These include things such as notification of a hospital by emergency medical services that a patient showing signs of an acute ischemic stroke is on the way, transfer of the patient after hospital arrival directly to a CT or MRI scanner to get the imaging that can confirm tPA eligibility, rapid reading of the scan, premixing of the tPA, a team-based approach to acute stroke management, and quick feedback to the team regarding their performance on each stroke patient they treat.
Dr. Schwamm noted that he, Dr. Fonarow, and other program leaders are already planning a phase III for Target Stroke that will branch out to even more facets of acute stroke care, such as incorporation of goals for using endovascular thrombectomy in appropriate patients and for using telemedicine to quicken and broaden the availability of expert neurologic consults for identifying tPA- and thrombectomy-eligible patients.
Growing the program
The success of GWTG-Stroke in transforming U.S. acute stroke care has not only relied on setting aggressive performance goals but also on advancing by boosting the number of U.S. hospitals participating of more U.S. hospitals. During the Target Stroke years since the start of 2010, the number of hospitals active in GWTG-Stroke jumped from 1427 to 1,950 by mid-2016, including a 12% year-over-year jump in 2016, compared with 2015. During October 2015-October 2016, participating hospitals treated more than 570,000 acute stroke patients, or roughly 71% of the estimated 800,000 U.S. patients who have an acute stroke each year.
These numbers seem on track to continue expanding. “With the recent evidence showing that participating in GWTG-Stroke improves care and outcomes there has been even greater interest by hospitals, recently, in joining,” said Dr. Fonarow. “While many hospitals currently participate, ideally all would join and be active.”
“Hospitals now feel that they can’t afford not to focus on stroke as a quality improvement program, so they join,” said Dr. Schwamm. He believes that essentially all of the roughly 200 certified U.S. Comprehensive Stroke Centers and of the roughly 1,000 certified U.S. Primary Stroke Centers are already members of GWTG-Stroke. “We’re now in all the high-value hospitals, based on their higher numbers of patients. It’s the Acute Stroke Ready hospitals where we now have the greatest potential to penetrate, hospitals that generally treat about 20-50 stroke patients a year,” he said.
Joining GWTG-Stroke holds major attraction for hospitals because the program gives them a tool for measuring their performance, data that hospitals often now need to prove the value of the care they deliver to insurers and to avoid penalization for readmissions. However, the barrier to hospitals, especially smaller hospitals, is that data collection can be expensive. It’s something that larger hospitals now routinely do, but smaller hospitals have often balked because of the expense. To address this, the GWTG-Stroke program is trying to develop a “lighter” version of their data collection tool that involves a smaller financial burden.
“I think we can sell a trimmed down version of GWTG-Stroke to smaller hospitals that is affordable and use it to recruit another 1,000 hospitals. That’s a realistic goal,” Dr. Schwamm said.
What is also notable about GWTG-Stroke is that hospitals sign up despite the somewhat ambiguous payback they receive.
“In the past, stroke was the third-leading U.S. cause of death, but now it’s fifth,” noted Steven R. Messe, MD, a stroke neurologist at the University of Pennsylvania in Philadelphia and a member of the GWTG-Stroke steering committee. “That’s an amazing accomplishment – to drop stroke from third place to fifth – and it’s due to advances in thrombolytic use and to improved systems of care and quality improvement measures.”
Get With the Guidelines-Stroke is a program of the American Heart Association and American Stroke Association and uses funding provided by several drug companies. Dr. Smith, Dr. Fonarow, Dr. Schwamm and Dr. Messe had no relevant commercial disclosures.
[email protected]
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE INTERNATIONAL STROKE CONFERENCE
Hold your breath
“Exercising my ‘reasoned judgment,’ I have no doubt that the right to a climate system capable of sustaining human life is fundamental to a free and ordered society.”
– U.S. District Judge Ann Aiken in Kelsey Cascadia Rose Juliana vs. United States of America, et al.
In many areas of the world, the simple act of breathing has become hazardous to people’s health.
According to the World Health Organization, more people die every day from air pollution than from HIV/AIDS, tuberculosis, and road injuries combined. In China, more than 1 million deaths annually are linked to polluted air (76/100,000); in India the number of deaths is more than 600,000 annually (49/100,000); and in the United States, that figure comes to more than 38,000 (12/100,000).
And yet, nonpolluting, alternative options – such as sun and wind power – are readily available.
Dirty air is visible on a hot summer day – when, mixed with other substances, it forms smog. Higher temperatures can then speed up the chemical reactions that form smog. We breathe in that polluted air, especially on days when the air is stagnant or there is temperature inversion.
The health effects of climate change
Black carbon found in air pollution leads to drug-resistant bacteria and alters antibiotic tolerance.1 The pollution also is associated with multiple cancers: lung, liver, ovarian, and, possibly, breast.2,3,4,5 It causes inflammation linked to the development of coronary artery disease (seen even in children!) and plaque formation leading to heart attacks and cardiac arrhythmias – including atrial fibrillation. Air pollution causes, triggers, or worsens respiratory illnesses – chronic obstructive pulmonary disease, emphysema, asthma, infections – and is responsible for lifelong diminished lung volume in children (a reason families are leaving Beijing.) Exponentially increased rates of autism are linked to bad air quality, as are autoimmune diseases, which also are on the rise.6,7 Polluted air causes brain inflammation – living near sources of air pollution increases the risk of dementia – and other neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s, and amyotrophic lateral sclerosis.8 The blood brain barrier protects the brain from most foreign matter, but particulate matter, especially ultrafine particulate matter of less than 1 mcm such as magnetite, can cross directly into the brain via the olfactory nerve. (Magnetite has been identified in the brain tissue of residents living in areas where the substance is produced as a result of industrial waste.) While particulate matter of 2.5 mcmis measured in the United States, ultrafine particulate matter is not.
Psychiatric symptoms and chronic psychiatric disorders also are associated with polluted air: On days with poor air quality, a statistically significant increase is seen in suicide threats and visits to emergency departments for panic attacks.9,10
A rise in aggression occurs when there are abnormally high temperatures and significant changes in rainfall. More assaults, murders, suicides, domestic violence, and child abuse can be expected, and a rise in unrest around the world should come as no surprise.
As a consequence of increased CO2 in the atmosphere, temperatures have already risen by 2° F: Sixteen of the hottest years on record have occurred in the last 17 years, with 2016 as the hottest year ever recorded. In Iraq and Kuwait, the temperature last summer reached 129.2° F.
We are experiencing more frequent and extreme weather events, chronic climate conditions, and the cascading disruption of ecosystems. Drought and sea level rise are leading to physical and psychological impacts – both direct and indirect. Some regions of the world have become destabilized, triggering migrations and the refugee crisis.
Along with these psychological impacts, CO2 affects cognition: A recent study by the Harvard School of Public Health, Boston, shows that the indoor levels of CO2 to which American workers typically are exposed impair cognitive functioning, particularly in the areas of strategic thinking, information processing, and crisis management.11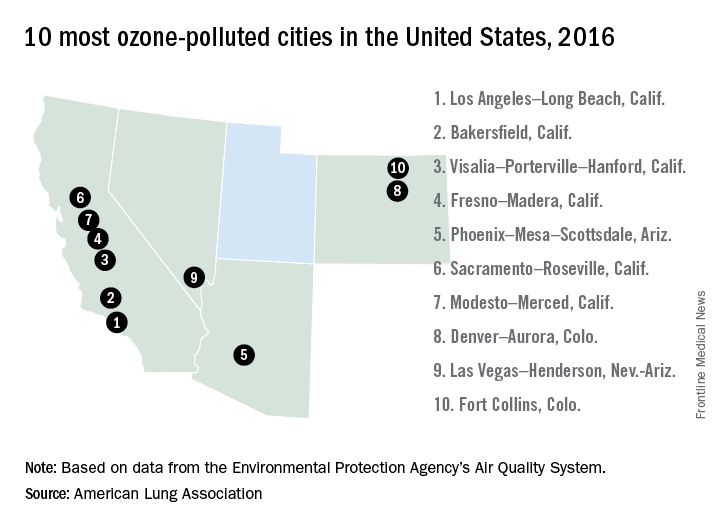
What do we do about it?
As mental health professionals, we know that aggression can be overt or passive (from inaction). Overwhelming evidence shows harm to public health from burning fossil fuels, and yet, though we are making progress, resistance still exists in the transition to clean, renewable energy critical for the health of our families and communities. When political will is what stands between us and getting back on a path to breathing clean air, how can inaction be understood as anything but an act of aggression?
This issue has reached U.S. courts: In a landmark case, 21 youths aged 9-20 years represented by “Our Children’s Trust” are suing the U.S. government in the Oregon U.S. District Court for failure to act on climate. The case, heard by Judge Ann Aiken, is now headed to trial.
All of us have a duty to collectively, repeatedly, and forcefully call on policy makers to take action.
That leads me to what we can do as doctors. In this effort to quickly transition to safe, clean renewable energy, we all have a role to play. The notion that we can’t do anything as individuals is no more credible than saying “my vote doesn’t matter.” Just as our actions as voters in a democracy demonstrate the collective civic responsibility we owe one another, so too do our actions on climate. As global citizens, all actions that we take to help us live within the planet’s means are opportunities to restore balance.
What we do collectively drives markets and determines the social norms that powerfully influence the decisions of others – sometimes even unconsciously.
As doctors, we have a unique role to play in the places we work – urging hospitals, clinics, academic centers, and other organizations and facilities to lead by example, become role models for energy efficiency, and choose clean renewable energy sources over the ones harming our health. We can start by choosing wind and solar to power our homes and influencing others to do the same.
We are the voices because this is a health message.
Dr. Van Susteren is a practicing general and forensic psychiatrist in Washington. She serves on the advisory board of the Center for Health and the Global Environment at Harvard T.H. Chan School of Public Health, Boston. Dr. Van Susteren is a former member of the board of directors of the National Wildlife Federation and coauthor of group’s report, “The Psychological Effects of Global Warming on the United States – Why the U.S. Mental Health System is Not Prepared.” In 2006, Dr. Van Susteren sought the Democratic nomination for a U.S. Senate seat in Maryland. She also founded Lucky Planet Foods, a company that provides plant-based, low carbon foods.
References
1. Environ Microbiol. 2017 Feb 14. doi: 10.1111/1462-2920.13686.
2. Environ Health Perspect. 2017 Mar;125[3]:378-84.
3. J Hepatol. 2015;63[6]:1397-1404.
4. J Toxicol Environ Health A. 2012;75[3]:174-82.
5. Environ Health Perspect. 2012 Nov; 118[11]:1578-83.
6. J Child Psychol Psychiatry. 2016; 57[3]:271-92.
7. Curr Opin Pediatr. 2010;22[2]219-25.
8. Inhal Toxicol. 2008;20[5]:499-506.
9. J Psychiatr Res. 2015 Mar;62:130-5.
10. Schizophr Res. 2016 Oct 5. doi: 10.1016/j.schres.2016.10.003.
11. Environ Health Perspect. 2016 Jun;124[6]:805-12.
“Exercising my ‘reasoned judgment,’ I have no doubt that the right to a climate system capable of sustaining human life is fundamental to a free and ordered society.”
– U.S. District Judge Ann Aiken in Kelsey Cascadia Rose Juliana vs. United States of America, et al.
In many areas of the world, the simple act of breathing has become hazardous to people’s health.
According to the World Health Organization, more people die every day from air pollution than from HIV/AIDS, tuberculosis, and road injuries combined. In China, more than 1 million deaths annually are linked to polluted air (76/100,000); in India the number of deaths is more than 600,000 annually (49/100,000); and in the United States, that figure comes to more than 38,000 (12/100,000).
And yet, nonpolluting, alternative options – such as sun and wind power – are readily available.
Dirty air is visible on a hot summer day – when, mixed with other substances, it forms smog. Higher temperatures can then speed up the chemical reactions that form smog. We breathe in that polluted air, especially on days when the air is stagnant or there is temperature inversion.
The health effects of climate change
Black carbon found in air pollution leads to drug-resistant bacteria and alters antibiotic tolerance.1 The pollution also is associated with multiple cancers: lung, liver, ovarian, and, possibly, breast.2,3,4,5 It causes inflammation linked to the development of coronary artery disease (seen even in children!) and plaque formation leading to heart attacks and cardiac arrhythmias – including atrial fibrillation. Air pollution causes, triggers, or worsens respiratory illnesses – chronic obstructive pulmonary disease, emphysema, asthma, infections – and is responsible for lifelong diminished lung volume in children (a reason families are leaving Beijing.) Exponentially increased rates of autism are linked to bad air quality, as are autoimmune diseases, which also are on the rise.6,7 Polluted air causes brain inflammation – living near sources of air pollution increases the risk of dementia – and other neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s, and amyotrophic lateral sclerosis.8 The blood brain barrier protects the brain from most foreign matter, but particulate matter, especially ultrafine particulate matter of less than 1 mcm such as magnetite, can cross directly into the brain via the olfactory nerve. (Magnetite has been identified in the brain tissue of residents living in areas where the substance is produced as a result of industrial waste.) While particulate matter of 2.5 mcmis measured in the United States, ultrafine particulate matter is not.
Psychiatric symptoms and chronic psychiatric disorders also are associated with polluted air: On days with poor air quality, a statistically significant increase is seen in suicide threats and visits to emergency departments for panic attacks.9,10
A rise in aggression occurs when there are abnormally high temperatures and significant changes in rainfall. More assaults, murders, suicides, domestic violence, and child abuse can be expected, and a rise in unrest around the world should come as no surprise.
As a consequence of increased CO2 in the atmosphere, temperatures have already risen by 2° F: Sixteen of the hottest years on record have occurred in the last 17 years, with 2016 as the hottest year ever recorded. In Iraq and Kuwait, the temperature last summer reached 129.2° F.
We are experiencing more frequent and extreme weather events, chronic climate conditions, and the cascading disruption of ecosystems. Drought and sea level rise are leading to physical and psychological impacts – both direct and indirect. Some regions of the world have become destabilized, triggering migrations and the refugee crisis.
Along with these psychological impacts, CO2 affects cognition: A recent study by the Harvard School of Public Health, Boston, shows that the indoor levels of CO2 to which American workers typically are exposed impair cognitive functioning, particularly in the areas of strategic thinking, information processing, and crisis management.11
What do we do about it?
As mental health professionals, we know that aggression can be overt or passive (from inaction). Overwhelming evidence shows harm to public health from burning fossil fuels, and yet, though we are making progress, resistance still exists in the transition to clean, renewable energy critical for the health of our families and communities. When political will is what stands between us and getting back on a path to breathing clean air, how can inaction be understood as anything but an act of aggression?
This issue has reached U.S. courts: In a landmark case, 21 youths aged 9-20 years represented by “Our Children’s Trust” are suing the U.S. government in the Oregon U.S. District Court for failure to act on climate. The case, heard by Judge Ann Aiken, is now headed to trial.
All of us have a duty to collectively, repeatedly, and forcefully call on policy makers to take action.
That leads me to what we can do as doctors. In this effort to quickly transition to safe, clean renewable energy, we all have a role to play. The notion that we can’t do anything as individuals is no more credible than saying “my vote doesn’t matter.” Just as our actions as voters in a democracy demonstrate the collective civic responsibility we owe one another, so too do our actions on climate. As global citizens, all actions that we take to help us live within the planet’s means are opportunities to restore balance.
What we do collectively drives markets and determines the social norms that powerfully influence the decisions of others – sometimes even unconsciously.
As doctors, we have a unique role to play in the places we work – urging hospitals, clinics, academic centers, and other organizations and facilities to lead by example, become role models for energy efficiency, and choose clean renewable energy sources over the ones harming our health. We can start by choosing wind and solar to power our homes and influencing others to do the same.
We are the voices because this is a health message.
Dr. Van Susteren is a practicing general and forensic psychiatrist in Washington. She serves on the advisory board of the Center for Health and the Global Environment at Harvard T.H. Chan School of Public Health, Boston. Dr. Van Susteren is a former member of the board of directors of the National Wildlife Federation and coauthor of group’s report, “The Psychological Effects of Global Warming on the United States – Why the U.S. Mental Health System is Not Prepared.” In 2006, Dr. Van Susteren sought the Democratic nomination for a U.S. Senate seat in Maryland. She also founded Lucky Planet Foods, a company that provides plant-based, low carbon foods.
References
1. Environ Microbiol. 2017 Feb 14. doi: 10.1111/1462-2920.13686.
2. Environ Health Perspect. 2017 Mar;125[3]:378-84.
3. J Hepatol. 2015;63[6]:1397-1404.
4. J Toxicol Environ Health A. 2012;75[3]:174-82.
5. Environ Health Perspect. 2012 Nov; 118[11]:1578-83.
6. J Child Psychol Psychiatry. 2016; 57[3]:271-92.
7. Curr Opin Pediatr. 2010;22[2]219-25.
8. Inhal Toxicol. 2008;20[5]:499-506.
9. J Psychiatr Res. 2015 Mar;62:130-5.
10. Schizophr Res. 2016 Oct 5. doi: 10.1016/j.schres.2016.10.003.
11. Environ Health Perspect. 2016 Jun;124[6]:805-12.
“Exercising my ‘reasoned judgment,’ I have no doubt that the right to a climate system capable of sustaining human life is fundamental to a free and ordered society.”
– U.S. District Judge Ann Aiken in Kelsey Cascadia Rose Juliana vs. United States of America, et al.
In many areas of the world, the simple act of breathing has become hazardous to people’s health.
According to the World Health Organization, more people die every day from air pollution than from HIV/AIDS, tuberculosis, and road injuries combined. In China, more than 1 million deaths annually are linked to polluted air (76/100,000); in India the number of deaths is more than 600,000 annually (49/100,000); and in the United States, that figure comes to more than 38,000 (12/100,000).
And yet, nonpolluting, alternative options – such as sun and wind power – are readily available.
Dirty air is visible on a hot summer day – when, mixed with other substances, it forms smog. Higher temperatures can then speed up the chemical reactions that form smog. We breathe in that polluted air, especially on days when the air is stagnant or there is temperature inversion.
The health effects of climate change
Black carbon found in air pollution leads to drug-resistant bacteria and alters antibiotic tolerance.1 The pollution also is associated with multiple cancers: lung, liver, ovarian, and, possibly, breast.2,3,4,5 It causes inflammation linked to the development of coronary artery disease (seen even in children!) and plaque formation leading to heart attacks and cardiac arrhythmias – including atrial fibrillation. Air pollution causes, triggers, or worsens respiratory illnesses – chronic obstructive pulmonary disease, emphysema, asthma, infections – and is responsible for lifelong diminished lung volume in children (a reason families are leaving Beijing.) Exponentially increased rates of autism are linked to bad air quality, as are autoimmune diseases, which also are on the rise.6,7 Polluted air causes brain inflammation – living near sources of air pollution increases the risk of dementia – and other neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s, and amyotrophic lateral sclerosis.8 The blood brain barrier protects the brain from most foreign matter, but particulate matter, especially ultrafine particulate matter of less than 1 mcm such as magnetite, can cross directly into the brain via the olfactory nerve. (Magnetite has been identified in the brain tissue of residents living in areas where the substance is produced as a result of industrial waste.) While particulate matter of 2.5 mcmis measured in the United States, ultrafine particulate matter is not.
Psychiatric symptoms and chronic psychiatric disorders also are associated with polluted air: On days with poor air quality, a statistically significant increase is seen in suicide threats and visits to emergency departments for panic attacks.9,10
A rise in aggression occurs when there are abnormally high temperatures and significant changes in rainfall. More assaults, murders, suicides, domestic violence, and child abuse can be expected, and a rise in unrest around the world should come as no surprise.
As a consequence of increased CO2 in the atmosphere, temperatures have already risen by 2° F: Sixteen of the hottest years on record have occurred in the last 17 years, with 2016 as the hottest year ever recorded. In Iraq and Kuwait, the temperature last summer reached 129.2° F.
We are experiencing more frequent and extreme weather events, chronic climate conditions, and the cascading disruption of ecosystems. Drought and sea level rise are leading to physical and psychological impacts – both direct and indirect. Some regions of the world have become destabilized, triggering migrations and the refugee crisis.
Along with these psychological impacts, CO2 affects cognition: A recent study by the Harvard School of Public Health, Boston, shows that the indoor levels of CO2 to which American workers typically are exposed impair cognitive functioning, particularly in the areas of strategic thinking, information processing, and crisis management.11
What do we do about it?
As mental health professionals, we know that aggression can be overt or passive (from inaction). Overwhelming evidence shows harm to public health from burning fossil fuels, and yet, though we are making progress, resistance still exists in the transition to clean, renewable energy critical for the health of our families and communities. When political will is what stands between us and getting back on a path to breathing clean air, how can inaction be understood as anything but an act of aggression?
This issue has reached U.S. courts: In a landmark case, 21 youths aged 9-20 years represented by “Our Children’s Trust” are suing the U.S. government in the Oregon U.S. District Court for failure to act on climate. The case, heard by Judge Ann Aiken, is now headed to trial.
All of us have a duty to collectively, repeatedly, and forcefully call on policy makers to take action.
That leads me to what we can do as doctors. In this effort to quickly transition to safe, clean renewable energy, we all have a role to play. The notion that we can’t do anything as individuals is no more credible than saying “my vote doesn’t matter.” Just as our actions as voters in a democracy demonstrate the collective civic responsibility we owe one another, so too do our actions on climate. As global citizens, all actions that we take to help us live within the planet’s means are opportunities to restore balance.
What we do collectively drives markets and determines the social norms that powerfully influence the decisions of others – sometimes even unconsciously.
As doctors, we have a unique role to play in the places we work – urging hospitals, clinics, academic centers, and other organizations and facilities to lead by example, become role models for energy efficiency, and choose clean renewable energy sources over the ones harming our health. We can start by choosing wind and solar to power our homes and influencing others to do the same.
We are the voices because this is a health message.
Dr. Van Susteren is a practicing general and forensic psychiatrist in Washington. She serves on the advisory board of the Center for Health and the Global Environment at Harvard T.H. Chan School of Public Health, Boston. Dr. Van Susteren is a former member of the board of directors of the National Wildlife Federation and coauthor of group’s report, “The Psychological Effects of Global Warming on the United States – Why the U.S. Mental Health System is Not Prepared.” In 2006, Dr. Van Susteren sought the Democratic nomination for a U.S. Senate seat in Maryland. She also founded Lucky Planet Foods, a company that provides plant-based, low carbon foods.
References
1. Environ Microbiol. 2017 Feb 14. doi: 10.1111/1462-2920.13686.
2. Environ Health Perspect. 2017 Mar;125[3]:378-84.
3. J Hepatol. 2015;63[6]:1397-1404.
4. J Toxicol Environ Health A. 2012;75[3]:174-82.
5. Environ Health Perspect. 2012 Nov; 118[11]:1578-83.
6. J Child Psychol Psychiatry. 2016; 57[3]:271-92.
7. Curr Opin Pediatr. 2010;22[2]219-25.
8. Inhal Toxicol. 2008;20[5]:499-506.
9. J Psychiatr Res. 2015 Mar;62:130-5.
10. Schizophr Res. 2016 Oct 5. doi: 10.1016/j.schres.2016.10.003.
11. Environ Health Perspect. 2016 Jun;124[6]:805-12.
Pronator Teres Myotendinous Tear
Take-Home Points
- Pronator teres muscle injuries are rare.
- Injury can be mistaken for MUCL injury in athletes.
- Tenderness and weak/painful forearm pronation are common findings.
- MRI confirms the diagnosis and helps grade the muscle strain injury.
- Conservative treatment is recommended and prognosis is excellent even for high-grade strains.
Pronator teres muscle strain is a rare sporting injury reported only in cricket players, and now in a golfer whose forearm experienced an eccentric force during resisted elbow flexion and pronation.1,2 The injury occurs when the sporting club or racket strikes the ground during a swing, impeding forward progress and subjecting the pronator teres muscle to eccentric forces in excess of what it can withstand. The pronator teres, one of several muscles that comprise the flexor wad of the forearm, consists of 2 heads, originating proximally from the medical epicondyle and attaching distally to the shaft of the radius on its lateral surface and just distal to the supinator. The oblique orientation of the muscle belly allows it to serve in its primary rotatory role as the main pronator of the forearm. Injuries to the soft tissue of the medial forearm are common in both elite and recreational athletes, especially in racket and club sports.3 Often, these injuries are related to overuse and chronic fatigue of the surrounding soft tissue—caused by repetitive flexing, gripping, or swinging. Even when identified early, these injuries can result in a significant loss of training time.4 In this article, we report a case of pronator teres muscle tear at the myotendinous junction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A right-hand–dominant 36-year-old man presented to the clinic with pain on the medial side of his right elbow after sustaining an injury to the elbow while playing golf several days earlier. The patient, an advertising executive, was playing recreational golf several times a month and had no significant medical history or previous symptoms related to the elbow. Initial pain symptoms began during a second round of play, immediately after the patient miss-hit an iron shot, making contact mostly with the ground and causing the club to forcefully stop. The pain was on the medial side of the elbow and forearm. The patient noted progressive swelling and bruising at the pain site and development of forearm weakness. Physical examination during the clinic presentation revealed ecchymosis on the anterior medial forearm, medial elbow, and medial triceps (Figure 1).
Noncontrast magnetic resonance imaging (MRI) showed a high-grade partial tear of the pronator teres myotendinous junction (Figures 2A-2C).
The patient was instructed to rest the elbow from strenuous activity, golf in particular, for 4 weeks. Physical therapy for ROM and forearm strengthening of the surrounding flexor wad was initiated at 2 weeks and continued for 4 weeks. The patient was advised to take over-the-counter nonsteroidal anti-inflammatory drugs as needed for comfort. On repeat examination at 4 weeks, with tenderness or weakness with pronation absent and full ROM regained, the patient was released back to full activity. He was able to return to golf and reported being symptom-free and having no sense of weakness or loss of control.
Discussion
A tear of the pronator teres is an exceedingly rare injury. Our results with conservative treatment and a full return to previous activity level are consistent with the only other case reported in the literature.5 In contrast to our patient, the previous patient sustained a tear of the pronator teres after a prolonged period of batting during a recreational cricket match.
Our patient’s pronator teres injury occurred at the myotendinous junction, a muscle-tendon transition zone often susceptible to injury. What is unusual for this athletic medial elbow injury is that the patient reported no previous symptoms, and it appears that, though the surrounding muscle may have been fatigued by overuse from the round of golf earlier that day, the pathology was caused by an acute eccentric force. During a golf swing, tremendous forces are put on the entire body, from the lower extremities to the forearm and the fingers. Successful completion of the transfer of energy from the golf club to the ball requires both proper technique and proper functioning of key muscles. Specifically, parameters such as ball positioning, club angle, and wrist control play a major role.6 Altered forearm positioning or swing arc can significantly affect club head velocity and energy transfer without putting more stress on the golfer.7 Therefore, it is easy to understand how prolonged or extended play may fatigue the surrounding elbow muscles, leading to altered technique and increased susceptibility to acute injury. Biomechanical analysis of shoulder motion can provide a helpful baseline for assessing injury-related changes in golf swing and developing specific exercise and rehabilitation programs.8,9Although injury to the pronator teres is rare, sport physicians should be aware that, after a valgus stress or force, bruising and swelling along the medial elbow do not always indicate a medial ulnar collateral ligament (MUCL) tear or medial epicondylitis. The key examination findings that differentiate this injury from a MUCL injury are the exact location of pain, the milking maneuver for MUCL incompetence, and the extensive bruising over the muscle course of the pronator teres. MRI plays a pivotal role in proper diagnosis.4 In addition, MRI allows for evaluation of any concomitant injuries that may be obscuring the clinical presentation.
Successful treatment of such injuries is important for both elite and recreational athletes. With rest and physical therapy, our patient recovered from this rare isolated injury to the pronator teres with complete resolution of symptoms and full ROM. In the literature, we found no other reports of isolated full-thickness myotendinous rupture of the pronator teres or avulsion from the medial epicondyle. Therefore, it is unclear whether the same outcome can be expected with conservative therapy. However, because of the good outcomes for partial-thickness injuries treated conservatively and the lack of robust tendinous tissue to repair at the myotendinous junction, we recommend an initial course of conservative treatment. Sports physicians should be aware of this exceedingly rare injury to the elbow and understand the large forces experienced by the soft tissues of the forearm during the golf swing.9,10
Conclusion
Pronator teres muscle strain is a rare sporting injury reported in cricket and golf players. The elbow experiences a large eccentric force during resisted elbow flexion and pronation. The injury appears to occur when the sporting club or racket strikes the ground during a forceful swing impeding forward progress of the arm. The injury can be confused with a MUCL injury, or exacerbation of medial epicondylitis. Physical examination reveals bruising and tenderness over the course of the pronator teres, often distal to the elbow. Advanced imaging confirms the diagnosis and helps grade the severity of muscle strain. Treatment is often conservative, with return to function and sport after 4 to 6 weeks of rest and restricted activities. The patient in this case report had complete return to sporting function, with no residual weakness or pain.
Am J Orthop. 2017;46(2):E105-E107. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Field LD, Savoie FH. Common elbow injuries in sport. Sports Med. 1998;26(3):193-205.
2. Loomer RL. Elbow injuries in athletes. Can J Appl Sport Sci. 1982;7(3):164-166.
3. Dines JS, Bedi A, Williams PN, et al. Tennis injuries: epidemiology, pathophysiology, and treatment. J Am Acad Orthop Surg. 2015;23(3):181-189.
4. Banks KP, Ly JQ, Beall DP, Grayson DE, Bancroft LW, Tall MA. Overuse injuries of the upper extremity in the competitive athlete: magnetic resonance imaging findings associated with repetitive trauma. Curr Probl Diagn Radiol. 2005;34(4):127-142.
5. Niebulski HZ, Richardson ML. High-grade pronator teres tear in a cricket batsman. Radiol Case Rep. 2015;6(3):540.
6. Zhang X, Shan G. Where do golf drive swings go wrong? Factors influencing driver swing consistency. Scand J Med Sci Sports. 2014;24(5):749-757.
7. Nesbit SM, McGinnis RS. Kinetic constrained optimization of the golf swing hub path. J Sports Sci Med. 2014;13(4):859-873.
8. Helton MS. Conservative treatment of a proximal full-thickness biceps brachii muscle tear in a special operations soldier. Phys Ther. 2014;94(4):571-577.
9. Mitchell K, Banks S, Morgan D, Sugaya H. Shoulder motions during the golf swing in male amateur golfers. J Orthop Sports Phys Ther. 2003;33(4):196-203.
10. Grimshaw P, Giles A, Tong R, Grimmer K. Lower back and elbow injuries in golf. Sports Med. 2002;32(10):655-666.
Take-Home Points
- Pronator teres muscle injuries are rare.
- Injury can be mistaken for MUCL injury in athletes.
- Tenderness and weak/painful forearm pronation are common findings.
- MRI confirms the diagnosis and helps grade the muscle strain injury.
- Conservative treatment is recommended and prognosis is excellent even for high-grade strains.
Pronator teres muscle strain is a rare sporting injury reported only in cricket players, and now in a golfer whose forearm experienced an eccentric force during resisted elbow flexion and pronation.1,2 The injury occurs when the sporting club or racket strikes the ground during a swing, impeding forward progress and subjecting the pronator teres muscle to eccentric forces in excess of what it can withstand. The pronator teres, one of several muscles that comprise the flexor wad of the forearm, consists of 2 heads, originating proximally from the medical epicondyle and attaching distally to the shaft of the radius on its lateral surface and just distal to the supinator. The oblique orientation of the muscle belly allows it to serve in its primary rotatory role as the main pronator of the forearm. Injuries to the soft tissue of the medial forearm are common in both elite and recreational athletes, especially in racket and club sports.3 Often, these injuries are related to overuse and chronic fatigue of the surrounding soft tissue—caused by repetitive flexing, gripping, or swinging. Even when identified early, these injuries can result in a significant loss of training time.4 In this article, we report a case of pronator teres muscle tear at the myotendinous junction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A right-hand–dominant 36-year-old man presented to the clinic with pain on the medial side of his right elbow after sustaining an injury to the elbow while playing golf several days earlier. The patient, an advertising executive, was playing recreational golf several times a month and had no significant medical history or previous symptoms related to the elbow. Initial pain symptoms began during a second round of play, immediately after the patient miss-hit an iron shot, making contact mostly with the ground and causing the club to forcefully stop. The pain was on the medial side of the elbow and forearm. The patient noted progressive swelling and bruising at the pain site and development of forearm weakness. Physical examination during the clinic presentation revealed ecchymosis on the anterior medial forearm, medial elbow, and medial triceps (Figure 1).
Noncontrast magnetic resonance imaging (MRI) showed a high-grade partial tear of the pronator teres myotendinous junction (Figures 2A-2C).
The patient was instructed to rest the elbow from strenuous activity, golf in particular, for 4 weeks. Physical therapy for ROM and forearm strengthening of the surrounding flexor wad was initiated at 2 weeks and continued for 4 weeks. The patient was advised to take over-the-counter nonsteroidal anti-inflammatory drugs as needed for comfort. On repeat examination at 4 weeks, with tenderness or weakness with pronation absent and full ROM regained, the patient was released back to full activity. He was able to return to golf and reported being symptom-free and having no sense of weakness or loss of control.
Discussion
A tear of the pronator teres is an exceedingly rare injury. Our results with conservative treatment and a full return to previous activity level are consistent with the only other case reported in the literature.5 In contrast to our patient, the previous patient sustained a tear of the pronator teres after a prolonged period of batting during a recreational cricket match.
Our patient’s pronator teres injury occurred at the myotendinous junction, a muscle-tendon transition zone often susceptible to injury. What is unusual for this athletic medial elbow injury is that the patient reported no previous symptoms, and it appears that, though the surrounding muscle may have been fatigued by overuse from the round of golf earlier that day, the pathology was caused by an acute eccentric force. During a golf swing, tremendous forces are put on the entire body, from the lower extremities to the forearm and the fingers. Successful completion of the transfer of energy from the golf club to the ball requires both proper technique and proper functioning of key muscles. Specifically, parameters such as ball positioning, club angle, and wrist control play a major role.6 Altered forearm positioning or swing arc can significantly affect club head velocity and energy transfer without putting more stress on the golfer.7 Therefore, it is easy to understand how prolonged or extended play may fatigue the surrounding elbow muscles, leading to altered technique and increased susceptibility to acute injury. Biomechanical analysis of shoulder motion can provide a helpful baseline for assessing injury-related changes in golf swing and developing specific exercise and rehabilitation programs.8,9Although injury to the pronator teres is rare, sport physicians should be aware that, after a valgus stress or force, bruising and swelling along the medial elbow do not always indicate a medial ulnar collateral ligament (MUCL) tear or medial epicondylitis. The key examination findings that differentiate this injury from a MUCL injury are the exact location of pain, the milking maneuver for MUCL incompetence, and the extensive bruising over the muscle course of the pronator teres. MRI plays a pivotal role in proper diagnosis.4 In addition, MRI allows for evaluation of any concomitant injuries that may be obscuring the clinical presentation.
Successful treatment of such injuries is important for both elite and recreational athletes. With rest and physical therapy, our patient recovered from this rare isolated injury to the pronator teres with complete resolution of symptoms and full ROM. In the literature, we found no other reports of isolated full-thickness myotendinous rupture of the pronator teres or avulsion from the medial epicondyle. Therefore, it is unclear whether the same outcome can be expected with conservative therapy. However, because of the good outcomes for partial-thickness injuries treated conservatively and the lack of robust tendinous tissue to repair at the myotendinous junction, we recommend an initial course of conservative treatment. Sports physicians should be aware of this exceedingly rare injury to the elbow and understand the large forces experienced by the soft tissues of the forearm during the golf swing.9,10
Conclusion
Pronator teres muscle strain is a rare sporting injury reported in cricket and golf players. The elbow experiences a large eccentric force during resisted elbow flexion and pronation. The injury appears to occur when the sporting club or racket strikes the ground during a forceful swing impeding forward progress of the arm. The injury can be confused with a MUCL injury, or exacerbation of medial epicondylitis. Physical examination reveals bruising and tenderness over the course of the pronator teres, often distal to the elbow. Advanced imaging confirms the diagnosis and helps grade the severity of muscle strain. Treatment is often conservative, with return to function and sport after 4 to 6 weeks of rest and restricted activities. The patient in this case report had complete return to sporting function, with no residual weakness or pain.
Am J Orthop. 2017;46(2):E105-E107. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Pronator teres muscle injuries are rare.
- Injury can be mistaken for MUCL injury in athletes.
- Tenderness and weak/painful forearm pronation are common findings.
- MRI confirms the diagnosis and helps grade the muscle strain injury.
- Conservative treatment is recommended and prognosis is excellent even for high-grade strains.
Pronator teres muscle strain is a rare sporting injury reported only in cricket players, and now in a golfer whose forearm experienced an eccentric force during resisted elbow flexion and pronation.1,2 The injury occurs when the sporting club or racket strikes the ground during a swing, impeding forward progress and subjecting the pronator teres muscle to eccentric forces in excess of what it can withstand. The pronator teres, one of several muscles that comprise the flexor wad of the forearm, consists of 2 heads, originating proximally from the medical epicondyle and attaching distally to the shaft of the radius on its lateral surface and just distal to the supinator. The oblique orientation of the muscle belly allows it to serve in its primary rotatory role as the main pronator of the forearm. Injuries to the soft tissue of the medial forearm are common in both elite and recreational athletes, especially in racket and club sports.3 Often, these injuries are related to overuse and chronic fatigue of the surrounding soft tissue—caused by repetitive flexing, gripping, or swinging. Even when identified early, these injuries can result in a significant loss of training time.4 In this article, we report a case of pronator teres muscle tear at the myotendinous junction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A right-hand–dominant 36-year-old man presented to the clinic with pain on the medial side of his right elbow after sustaining an injury to the elbow while playing golf several days earlier. The patient, an advertising executive, was playing recreational golf several times a month and had no significant medical history or previous symptoms related to the elbow. Initial pain symptoms began during a second round of play, immediately after the patient miss-hit an iron shot, making contact mostly with the ground and causing the club to forcefully stop. The pain was on the medial side of the elbow and forearm. The patient noted progressive swelling and bruising at the pain site and development of forearm weakness. Physical examination during the clinic presentation revealed ecchymosis on the anterior medial forearm, medial elbow, and medial triceps (Figure 1).
Noncontrast magnetic resonance imaging (MRI) showed a high-grade partial tear of the pronator teres myotendinous junction (Figures 2A-2C).
The patient was instructed to rest the elbow from strenuous activity, golf in particular, for 4 weeks. Physical therapy for ROM and forearm strengthening of the surrounding flexor wad was initiated at 2 weeks and continued for 4 weeks. The patient was advised to take over-the-counter nonsteroidal anti-inflammatory drugs as needed for comfort. On repeat examination at 4 weeks, with tenderness or weakness with pronation absent and full ROM regained, the patient was released back to full activity. He was able to return to golf and reported being symptom-free and having no sense of weakness or loss of control.
Discussion
A tear of the pronator teres is an exceedingly rare injury. Our results with conservative treatment and a full return to previous activity level are consistent with the only other case reported in the literature.5 In contrast to our patient, the previous patient sustained a tear of the pronator teres after a prolonged period of batting during a recreational cricket match.
Our patient’s pronator teres injury occurred at the myotendinous junction, a muscle-tendon transition zone often susceptible to injury. What is unusual for this athletic medial elbow injury is that the patient reported no previous symptoms, and it appears that, though the surrounding muscle may have been fatigued by overuse from the round of golf earlier that day, the pathology was caused by an acute eccentric force. During a golf swing, tremendous forces are put on the entire body, from the lower extremities to the forearm and the fingers. Successful completion of the transfer of energy from the golf club to the ball requires both proper technique and proper functioning of key muscles. Specifically, parameters such as ball positioning, club angle, and wrist control play a major role.6 Altered forearm positioning or swing arc can significantly affect club head velocity and energy transfer without putting more stress on the golfer.7 Therefore, it is easy to understand how prolonged or extended play may fatigue the surrounding elbow muscles, leading to altered technique and increased susceptibility to acute injury. Biomechanical analysis of shoulder motion can provide a helpful baseline for assessing injury-related changes in golf swing and developing specific exercise and rehabilitation programs.8,9Although injury to the pronator teres is rare, sport physicians should be aware that, after a valgus stress or force, bruising and swelling along the medial elbow do not always indicate a medial ulnar collateral ligament (MUCL) tear or medial epicondylitis. The key examination findings that differentiate this injury from a MUCL injury are the exact location of pain, the milking maneuver for MUCL incompetence, and the extensive bruising over the muscle course of the pronator teres. MRI plays a pivotal role in proper diagnosis.4 In addition, MRI allows for evaluation of any concomitant injuries that may be obscuring the clinical presentation.
Successful treatment of such injuries is important for both elite and recreational athletes. With rest and physical therapy, our patient recovered from this rare isolated injury to the pronator teres with complete resolution of symptoms and full ROM. In the literature, we found no other reports of isolated full-thickness myotendinous rupture of the pronator teres or avulsion from the medial epicondyle. Therefore, it is unclear whether the same outcome can be expected with conservative therapy. However, because of the good outcomes for partial-thickness injuries treated conservatively and the lack of robust tendinous tissue to repair at the myotendinous junction, we recommend an initial course of conservative treatment. Sports physicians should be aware of this exceedingly rare injury to the elbow and understand the large forces experienced by the soft tissues of the forearm during the golf swing.9,10
Conclusion
Pronator teres muscle strain is a rare sporting injury reported in cricket and golf players. The elbow experiences a large eccentric force during resisted elbow flexion and pronation. The injury appears to occur when the sporting club or racket strikes the ground during a forceful swing impeding forward progress of the arm. The injury can be confused with a MUCL injury, or exacerbation of medial epicondylitis. Physical examination reveals bruising and tenderness over the course of the pronator teres, often distal to the elbow. Advanced imaging confirms the diagnosis and helps grade the severity of muscle strain. Treatment is often conservative, with return to function and sport after 4 to 6 weeks of rest and restricted activities. The patient in this case report had complete return to sporting function, with no residual weakness or pain.
Am J Orthop. 2017;46(2):E105-E107. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Field LD, Savoie FH. Common elbow injuries in sport. Sports Med. 1998;26(3):193-205.
2. Loomer RL. Elbow injuries in athletes. Can J Appl Sport Sci. 1982;7(3):164-166.
3. Dines JS, Bedi A, Williams PN, et al. Tennis injuries: epidemiology, pathophysiology, and treatment. J Am Acad Orthop Surg. 2015;23(3):181-189.
4. Banks KP, Ly JQ, Beall DP, Grayson DE, Bancroft LW, Tall MA. Overuse injuries of the upper extremity in the competitive athlete: magnetic resonance imaging findings associated with repetitive trauma. Curr Probl Diagn Radiol. 2005;34(4):127-142.
5. Niebulski HZ, Richardson ML. High-grade pronator teres tear in a cricket batsman. Radiol Case Rep. 2015;6(3):540.
6. Zhang X, Shan G. Where do golf drive swings go wrong? Factors influencing driver swing consistency. Scand J Med Sci Sports. 2014;24(5):749-757.
7. Nesbit SM, McGinnis RS. Kinetic constrained optimization of the golf swing hub path. J Sports Sci Med. 2014;13(4):859-873.
8. Helton MS. Conservative treatment of a proximal full-thickness biceps brachii muscle tear in a special operations soldier. Phys Ther. 2014;94(4):571-577.
9. Mitchell K, Banks S, Morgan D, Sugaya H. Shoulder motions during the golf swing in male amateur golfers. J Orthop Sports Phys Ther. 2003;33(4):196-203.
10. Grimshaw P, Giles A, Tong R, Grimmer K. Lower back and elbow injuries in golf. Sports Med. 2002;32(10):655-666.
1. Field LD, Savoie FH. Common elbow injuries in sport. Sports Med. 1998;26(3):193-205.
2. Loomer RL. Elbow injuries in athletes. Can J Appl Sport Sci. 1982;7(3):164-166.
3. Dines JS, Bedi A, Williams PN, et al. Tennis injuries: epidemiology, pathophysiology, and treatment. J Am Acad Orthop Surg. 2015;23(3):181-189.
4. Banks KP, Ly JQ, Beall DP, Grayson DE, Bancroft LW, Tall MA. Overuse injuries of the upper extremity in the competitive athlete: magnetic resonance imaging findings associated with repetitive trauma. Curr Probl Diagn Radiol. 2005;34(4):127-142.
5. Niebulski HZ, Richardson ML. High-grade pronator teres tear in a cricket batsman. Radiol Case Rep. 2015;6(3):540.
6. Zhang X, Shan G. Where do golf drive swings go wrong? Factors influencing driver swing consistency. Scand J Med Sci Sports. 2014;24(5):749-757.
7. Nesbit SM, McGinnis RS. Kinetic constrained optimization of the golf swing hub path. J Sports Sci Med. 2014;13(4):859-873.
8. Helton MS. Conservative treatment of a proximal full-thickness biceps brachii muscle tear in a special operations soldier. Phys Ther. 2014;94(4):571-577.
9. Mitchell K, Banks S, Morgan D, Sugaya H. Shoulder motions during the golf swing in male amateur golfers. J Orthop Sports Phys Ther. 2003;33(4):196-203.
10. Grimshaw P, Giles A, Tong R, Grimmer K. Lower back and elbow injuries in golf. Sports Med. 2002;32(10):655-666.
Dupilumab improved eczema scores in children in open label trial
ORLANDO – Treatment with dupilumab in children and adolescents with moderate to severe atopic dermatitis (AD) reduced severity and pruritus scores from baseline and was well tolerated, in a multicenter, open-label trial of 78 children and adolescents.
Dupilumab’s “powerful ability” to block interleukin-4 and interleukin-13 pathways of inflammation is “especially exciting because AD is even more Th-2 cell driven in children,” said Michael J. Cork, MD, PhD, head of dermatologic research at the University of Sheffield (England), who presented the findings during a late-breaking research session at the annual meeting of the American Academy of Dermatology.
The study assessed the pharmacokinetics, safety, and efficacy of dupilumab and was conducted with 38 children, aged 6-11 years, and 40 adolescents, aged 12-17 years, with moderate to severe AD. All had failed topical corticosteroid therapy. Some of the children (16%) and adolescents (22.5%) had failed at least one systemic therapy.
Both age groups were given either a 2 mg/kg or a 4 mg/kg dose of dupilumab (administered subcutaneously), nothing for 8 weeks, followed by 4 weekly doses of their respective regimens. Mean Eczema Area and Severity Index (EASI) scores at baseline were 31.7 among the adolescents and 35.9 among the children.
At week 12, mean scores in the younger cohort given either 2 mg/kg or 4 mg/kg had improved by 76.2% and 63.4%, respectively, from baseline. In the adolescents, EASI scores at week 12 had improved by a mean of 66.4% in the 2-mg/kg group and 69.7% in the 4-mg/kg group.
Itch also improved “dramatically,” according to Dr. Cork. In the younger children, peak pruritus Numerical Rating Scale scores improved from baseline by a mean of 41.6% in the lower-dose group and 39.6% in the higher-dose group. In the older cohort, pruritus scores improved from baseline by a mean of 30.8% in the lower-dose group and 37.6% in the higher-dose group.
Treatment was well tolerated across the study, and adverse events were “mild, transient, and unrelated,” Dr. Cork said. “I would like to emphasize that these were not related to dupilumab, as they occurred during the period of time after the first dose, in weeks 6 and 7,” he commented. He attributed AD flares experienced in the study to the quick clearance of the drug in the first few weeks.
Dr. Cork reported numerous disclosures, including serving as an adviser, consultant, and investigator for Regeneron, the sponsor of the trial, and Sanofi; the companies developing dupilumab.
[email protected]
On Twitter @whitneymcknight
ORLANDO – Treatment with dupilumab in children and adolescents with moderate to severe atopic dermatitis (AD) reduced severity and pruritus scores from baseline and was well tolerated, in a multicenter, open-label trial of 78 children and adolescents.
Dupilumab’s “powerful ability” to block interleukin-4 and interleukin-13 pathways of inflammation is “especially exciting because AD is even more Th-2 cell driven in children,” said Michael J. Cork, MD, PhD, head of dermatologic research at the University of Sheffield (England), who presented the findings during a late-breaking research session at the annual meeting of the American Academy of Dermatology.
The study assessed the pharmacokinetics, safety, and efficacy of dupilumab and was conducted with 38 children, aged 6-11 years, and 40 adolescents, aged 12-17 years, with moderate to severe AD. All had failed topical corticosteroid therapy. Some of the children (16%) and adolescents (22.5%) had failed at least one systemic therapy.
Both age groups were given either a 2 mg/kg or a 4 mg/kg dose of dupilumab (administered subcutaneously), nothing for 8 weeks, followed by 4 weekly doses of their respective regimens. Mean Eczema Area and Severity Index (EASI) scores at baseline were 31.7 among the adolescents and 35.9 among the children.
At week 12, mean scores in the younger cohort given either 2 mg/kg or 4 mg/kg had improved by 76.2% and 63.4%, respectively, from baseline. In the adolescents, EASI scores at week 12 had improved by a mean of 66.4% in the 2-mg/kg group and 69.7% in the 4-mg/kg group.
Itch also improved “dramatically,” according to Dr. Cork. In the younger children, peak pruritus Numerical Rating Scale scores improved from baseline by a mean of 41.6% in the lower-dose group and 39.6% in the higher-dose group. In the older cohort, pruritus scores improved from baseline by a mean of 30.8% in the lower-dose group and 37.6% in the higher-dose group.
Treatment was well tolerated across the study, and adverse events were “mild, transient, and unrelated,” Dr. Cork said. “I would like to emphasize that these were not related to dupilumab, as they occurred during the period of time after the first dose, in weeks 6 and 7,” he commented. He attributed AD flares experienced in the study to the quick clearance of the drug in the first few weeks.
Dr. Cork reported numerous disclosures, including serving as an adviser, consultant, and investigator for Regeneron, the sponsor of the trial, and Sanofi; the companies developing dupilumab.
[email protected]
On Twitter @whitneymcknight
ORLANDO – Treatment with dupilumab in children and adolescents with moderate to severe atopic dermatitis (AD) reduced severity and pruritus scores from baseline and was well tolerated, in a multicenter, open-label trial of 78 children and adolescents.
Dupilumab’s “powerful ability” to block interleukin-4 and interleukin-13 pathways of inflammation is “especially exciting because AD is even more Th-2 cell driven in children,” said Michael J. Cork, MD, PhD, head of dermatologic research at the University of Sheffield (England), who presented the findings during a late-breaking research session at the annual meeting of the American Academy of Dermatology.
The study assessed the pharmacokinetics, safety, and efficacy of dupilumab and was conducted with 38 children, aged 6-11 years, and 40 adolescents, aged 12-17 years, with moderate to severe AD. All had failed topical corticosteroid therapy. Some of the children (16%) and adolescents (22.5%) had failed at least one systemic therapy.
Both age groups were given either a 2 mg/kg or a 4 mg/kg dose of dupilumab (administered subcutaneously), nothing for 8 weeks, followed by 4 weekly doses of their respective regimens. Mean Eczema Area and Severity Index (EASI) scores at baseline were 31.7 among the adolescents and 35.9 among the children.
At week 12, mean scores in the younger cohort given either 2 mg/kg or 4 mg/kg had improved by 76.2% and 63.4%, respectively, from baseline. In the adolescents, EASI scores at week 12 had improved by a mean of 66.4% in the 2-mg/kg group and 69.7% in the 4-mg/kg group.
Itch also improved “dramatically,” according to Dr. Cork. In the younger children, peak pruritus Numerical Rating Scale scores improved from baseline by a mean of 41.6% in the lower-dose group and 39.6% in the higher-dose group. In the older cohort, pruritus scores improved from baseline by a mean of 30.8% in the lower-dose group and 37.6% in the higher-dose group.
Treatment was well tolerated across the study, and adverse events were “mild, transient, and unrelated,” Dr. Cork said. “I would like to emphasize that these were not related to dupilumab, as they occurred during the period of time after the first dose, in weeks 6 and 7,” he commented. He attributed AD flares experienced in the study to the quick clearance of the drug in the first few weeks.
Dr. Cork reported numerous disclosures, including serving as an adviser, consultant, and investigator for Regeneron, the sponsor of the trial, and Sanofi; the companies developing dupilumab.
[email protected]
On Twitter @whitneymcknight
AT AAD 17
Key clinical point: Data on dupilumab for treating moderate to severe atopic dermatitis in children and adolescents are promising.
Major finding: At week 12, treatment with dupilumab at 2 mg/kg and 4 mg/kg doses, across 8 weeks in two pediatric cohorts improved baseline EASI scores by 69.7% and pruritus scores by a third and was well tolerated.
Data source: A phase IIa, multicenter, open label pharmacokinetics, safety, and efficacy trial evaluating two dosing regimens of dupilumab in 78 children with moderate to severe AD.
Disclosures: Dr. Cork reported numerous disclosures, including serving as an adviser, consultant, and investigator for Regeneron, the sponsor of the trial, and Sanofi – the two companies developing dupilumab.
For optimal care, consider all acne in Latinos inflammatory
ORLANDO – Postinflammatory hyperpigmentation is a common consequence of acne in Latino skin, and aggressive medical treatments or procedures can aggravate the problem.
In fact, the hyperpigmentation that remains after acne is treated can be even more upsetting than the acne itself, according to Mercedes Florez-White, MD.
“Most of the time, the postinflammatory hyperpigmentation [PIH] has much more impact on the patient’s quality of life even than the disease itself, so you need to be very careful when treating these patients,” said Dr. Florez-White of Florida International University, Miami.
Every Fitzpatrick phototype can be observed in the Latino population, but one feature seems to link them: inflammation linked with all types of acne, even comedonal. “Even patients with very pale skin can show, at the periphery of the comedone, a little darkness signaling inflammation. The skin of a mestizo [a person of mixed Spanish/Native American ancestry] is especially prone to this,” she said at the annual meeting of the American Academy of Dermatology.
There is a paucity of research on the treatment of acne in people with skin of color, Dr. Florez-White said. Even the most recent acne treatment guidelines, published in 2016 by the AAD, acknowledge the area as a “research gap” that needs to be addressed.
In the meantime, she offered what she called “pearls of acne wisdom” gleaned from 35 years of treating Latino patients in South America and the United States.
Overview and diagnosis
Nodulocystic acne is the most common type in this population, followed by comedonal acne. Although the comedonal type is not generally considered inflammatory acne, it should be in Latino patients, given their sensitivity to skin inflammation and its outcome. “There is inflammation there from the beginning – treat it as an inflammatory disease for the best results.”
• Acne in Latino patients occurs most commonly, but not exclusively, on the lower face. “We have also seen this on the neck and chest,” she noted.
• A thorough history – including a rundown of hair and skin products the patient is using – is critical. “You never know when something you think is acne is really something else,” like an allergic reaction, she said.
Treatment
There are no AAD-generated treatment guidelines for acne in Latino patients. Dr. Florez-White manages her patients according to a treatment algorithm published by the Ibero-Latin American College of Dermatology and a paper published in the Journal of Dermatologic Treatment (2010 May;21[3]:206-11), as well as her own clinical experience.
Topical therapy
• Start topical retinoids at a lower concentration, two to three times a week, and gradually increase the dose and frequency until the desired effect is reached. Also, start benzoyl peroxide at lower doses.
• For a patient with very proinflammatory cystic acne, initiate a short course of corticosteroids to decrease inflammation – this will reduce the risk of PIH.
• Add azelaic acid in a 20% cream or 15% gel. “It’s an anti-inflammatory and antibacterial, and it really does help reduce the chance of PIH. This is used all over Latin American and Asia, but it is an off-label use in the U.S.” she said.
• For adult women, consider 5% dapsone gel, but not in combination with benzoyl peroxide.
Oral therapy
• Doxycycline is first-line treatment for moderate to severe papulopustular or nodulocystic acne. Other primary agents could be minocycline or tetracycline. Second-line antibiotic choices include erythromycin, azithromycin, and sulfamethoxazole/trimethoprim. Antibiotics should be given with benzoyl peroxide to reduce the chance of bacterial resistance.
• For nonresponsive cases, use oral isotretinoin, started at half the recommended dose. Increase gradually to treatment response.
For women with hormonally mediated acne, give a trial of oral contraceptives with antiandrogen properties, or spironolactone. “I like to combine oral contraceptive pills with isotretinoin. You can give this because the absorption of isotretinoin is very superficial. You won’t have any problems with that,” she noted.
Skin care
Good skin care will help promote healing and generally increase patient compliance with medication, Dr. Florez-White said.
• A noncomedogenic broad-spectrum sunscreen should be used daily. Consider one with mineral oxides, especially iron oxide, as only these preparations scatter both visible light and infrared radiation, which both increase the risk of PIH.
• Cleanse with a synthetic detergent or lipid-free cleanser.
• Recommend daily use of a salicylic, lactic, or glycolic acid.
Camouflage is very important for some patients, especially teens. “I always recommend a noncomedogenic fluid makeup,” she said.
Procedures
• Comedo extraction is an essential part of a comprehensive acne treatment program, but hold off until after a month of retinoid treatment. Removing the lesions significantly decreases the chance of PIH.
• Very superficial chemical peels with salicylic acid alone, or in combination with mandelic acid, may improve outcome faster and decrease PIH that already exists. These can be used in conjunction with oral agents, including isotretinoin.
Dr. Florez-White had no financial disclosures.
[email protected]
On Twitter @alz_gal
ORLANDO – Postinflammatory hyperpigmentation is a common consequence of acne in Latino skin, and aggressive medical treatments or procedures can aggravate the problem.
In fact, the hyperpigmentation that remains after acne is treated can be even more upsetting than the acne itself, according to Mercedes Florez-White, MD.
“Most of the time, the postinflammatory hyperpigmentation [PIH] has much more impact on the patient’s quality of life even than the disease itself, so you need to be very careful when treating these patients,” said Dr. Florez-White of Florida International University, Miami.
Every Fitzpatrick phototype can be observed in the Latino population, but one feature seems to link them: inflammation linked with all types of acne, even comedonal. “Even patients with very pale skin can show, at the periphery of the comedone, a little darkness signaling inflammation. The skin of a mestizo [a person of mixed Spanish/Native American ancestry] is especially prone to this,” she said at the annual meeting of the American Academy of Dermatology.
There is a paucity of research on the treatment of acne in people with skin of color, Dr. Florez-White said. Even the most recent acne treatment guidelines, published in 2016 by the AAD, acknowledge the area as a “research gap” that needs to be addressed.
In the meantime, she offered what she called “pearls of acne wisdom” gleaned from 35 years of treating Latino patients in South America and the United States.
Overview and diagnosis
Nodulocystic acne is the most common type in this population, followed by comedonal acne. Although the comedonal type is not generally considered inflammatory acne, it should be in Latino patients, given their sensitivity to skin inflammation and its outcome. “There is inflammation there from the beginning – treat it as an inflammatory disease for the best results.”
• Acne in Latino patients occurs most commonly, but not exclusively, on the lower face. “We have also seen this on the neck and chest,” she noted.
• A thorough history – including a rundown of hair and skin products the patient is using – is critical. “You never know when something you think is acne is really something else,” like an allergic reaction, she said.
Treatment
There are no AAD-generated treatment guidelines for acne in Latino patients. Dr. Florez-White manages her patients according to a treatment algorithm published by the Ibero-Latin American College of Dermatology and a paper published in the Journal of Dermatologic Treatment (2010 May;21[3]:206-11), as well as her own clinical experience.
Topical therapy
• Start topical retinoids at a lower concentration, two to three times a week, and gradually increase the dose and frequency until the desired effect is reached. Also, start benzoyl peroxide at lower doses.
• For a patient with very proinflammatory cystic acne, initiate a short course of corticosteroids to decrease inflammation – this will reduce the risk of PIH.
• Add azelaic acid in a 20% cream or 15% gel. “It’s an anti-inflammatory and antibacterial, and it really does help reduce the chance of PIH. This is used all over Latin American and Asia, but it is an off-label use in the U.S.” she said.
• For adult women, consider 5% dapsone gel, but not in combination with benzoyl peroxide.
Oral therapy
• Doxycycline is first-line treatment for moderate to severe papulopustular or nodulocystic acne. Other primary agents could be minocycline or tetracycline. Second-line antibiotic choices include erythromycin, azithromycin, and sulfamethoxazole/trimethoprim. Antibiotics should be given with benzoyl peroxide to reduce the chance of bacterial resistance.
• For nonresponsive cases, use oral isotretinoin, started at half the recommended dose. Increase gradually to treatment response.
For women with hormonally mediated acne, give a trial of oral contraceptives with antiandrogen properties, or spironolactone. “I like to combine oral contraceptive pills with isotretinoin. You can give this because the absorption of isotretinoin is very superficial. You won’t have any problems with that,” she noted.
Skin care
Good skin care will help promote healing and generally increase patient compliance with medication, Dr. Florez-White said.
• A noncomedogenic broad-spectrum sunscreen should be used daily. Consider one with mineral oxides, especially iron oxide, as only these preparations scatter both visible light and infrared radiation, which both increase the risk of PIH.
• Cleanse with a synthetic detergent or lipid-free cleanser.
• Recommend daily use of a salicylic, lactic, or glycolic acid.
Camouflage is very important for some patients, especially teens. “I always recommend a noncomedogenic fluid makeup,” she said.
Procedures
• Comedo extraction is an essential part of a comprehensive acne treatment program, but hold off until after a month of retinoid treatment. Removing the lesions significantly decreases the chance of PIH.
• Very superficial chemical peels with salicylic acid alone, or in combination with mandelic acid, may improve outcome faster and decrease PIH that already exists. These can be used in conjunction with oral agents, including isotretinoin.
Dr. Florez-White had no financial disclosures.
[email protected]
On Twitter @alz_gal
ORLANDO – Postinflammatory hyperpigmentation is a common consequence of acne in Latino skin, and aggressive medical treatments or procedures can aggravate the problem.
In fact, the hyperpigmentation that remains after acne is treated can be even more upsetting than the acne itself, according to Mercedes Florez-White, MD.
“Most of the time, the postinflammatory hyperpigmentation [PIH] has much more impact on the patient’s quality of life even than the disease itself, so you need to be very careful when treating these patients,” said Dr. Florez-White of Florida International University, Miami.
Every Fitzpatrick phototype can be observed in the Latino population, but one feature seems to link them: inflammation linked with all types of acne, even comedonal. “Even patients with very pale skin can show, at the periphery of the comedone, a little darkness signaling inflammation. The skin of a mestizo [a person of mixed Spanish/Native American ancestry] is especially prone to this,” she said at the annual meeting of the American Academy of Dermatology.
There is a paucity of research on the treatment of acne in people with skin of color, Dr. Florez-White said. Even the most recent acne treatment guidelines, published in 2016 by the AAD, acknowledge the area as a “research gap” that needs to be addressed.
In the meantime, she offered what she called “pearls of acne wisdom” gleaned from 35 years of treating Latino patients in South America and the United States.
Overview and diagnosis
Nodulocystic acne is the most common type in this population, followed by comedonal acne. Although the comedonal type is not generally considered inflammatory acne, it should be in Latino patients, given their sensitivity to skin inflammation and its outcome. “There is inflammation there from the beginning – treat it as an inflammatory disease for the best results.”
• Acne in Latino patients occurs most commonly, but not exclusively, on the lower face. “We have also seen this on the neck and chest,” she noted.
• A thorough history – including a rundown of hair and skin products the patient is using – is critical. “You never know when something you think is acne is really something else,” like an allergic reaction, she said.
Treatment
There are no AAD-generated treatment guidelines for acne in Latino patients. Dr. Florez-White manages her patients according to a treatment algorithm published by the Ibero-Latin American College of Dermatology and a paper published in the Journal of Dermatologic Treatment (2010 May;21[3]:206-11), as well as her own clinical experience.
Topical therapy
• Start topical retinoids at a lower concentration, two to three times a week, and gradually increase the dose and frequency until the desired effect is reached. Also, start benzoyl peroxide at lower doses.
• For a patient with very proinflammatory cystic acne, initiate a short course of corticosteroids to decrease inflammation – this will reduce the risk of PIH.
• Add azelaic acid in a 20% cream or 15% gel. “It’s an anti-inflammatory and antibacterial, and it really does help reduce the chance of PIH. This is used all over Latin American and Asia, but it is an off-label use in the U.S.” she said.
• For adult women, consider 5% dapsone gel, but not in combination with benzoyl peroxide.
Oral therapy
• Doxycycline is first-line treatment for moderate to severe papulopustular or nodulocystic acne. Other primary agents could be minocycline or tetracycline. Second-line antibiotic choices include erythromycin, azithromycin, and sulfamethoxazole/trimethoprim. Antibiotics should be given with benzoyl peroxide to reduce the chance of bacterial resistance.
• For nonresponsive cases, use oral isotretinoin, started at half the recommended dose. Increase gradually to treatment response.
For women with hormonally mediated acne, give a trial of oral contraceptives with antiandrogen properties, or spironolactone. “I like to combine oral contraceptive pills with isotretinoin. You can give this because the absorption of isotretinoin is very superficial. You won’t have any problems with that,” she noted.
Skin care
Good skin care will help promote healing and generally increase patient compliance with medication, Dr. Florez-White said.
• A noncomedogenic broad-spectrum sunscreen should be used daily. Consider one with mineral oxides, especially iron oxide, as only these preparations scatter both visible light and infrared radiation, which both increase the risk of PIH.
• Cleanse with a synthetic detergent or lipid-free cleanser.
• Recommend daily use of a salicylic, lactic, or glycolic acid.
Camouflage is very important for some patients, especially teens. “I always recommend a noncomedogenic fluid makeup,” she said.
Procedures
• Comedo extraction is an essential part of a comprehensive acne treatment program, but hold off until after a month of retinoid treatment. Removing the lesions significantly decreases the chance of PIH.
• Very superficial chemical peels with salicylic acid alone, or in combination with mandelic acid, may improve outcome faster and decrease PIH that already exists. These can be used in conjunction with oral agents, including isotretinoin.
Dr. Florez-White had no financial disclosures.
[email protected]
On Twitter @alz_gal
EXPERT ANALYSIS FROM AAD 17