User login
Education: Register Now for the 2017 VAM
The topic is vascular care. The subjects to be covered are nearly too numerous to count. And the clock is ticking for signing up.
Registration and housing will open in early March for the 2017 Vascular Annual Meeting, set for May 31 to June 3 in San Diego, Calif., with plenaries and exhibits from June 1 to 3. This premier meeting of vascular specialists will be held at the San Diego Convention Center. The headquarters hotel, the Marriott Marquis San Diego Marina Hotel, adjoins the center.
There is plenty going on.
Additional programming: Expanded programming begins Wednesday and continues through Saturday. Be sure to plan your travel to attend these informative sessions. This year’s meeting includes more concurrent sessions, and the number of joint society programs has doubled from last year.
Free postgraduate courses for SVS members: Once again, all SVS member-registrants receive free admittance to the six Wednesday postgraduate courses, a savings of $300. Non-members plus anyone registering only for the postgraduate courses must pay the appropriate registration fee. Self-assessment credit is available to all physician attendees.
SVS/STS Aortic Summit: New this year, and a highlight for Saturday, is the SVS/STS Summit: Advances and Controversies in the Management of Complex Thoracoabdominal Aneurysmal Diseases and Type B Aortic Dissection. This program will take place from 1 to 5 p.m. and is co-sponsored by the Society of Thoracic Surgeons. An additional fee is required. 
Revamped workshops: Wednesday’s workshops will feature a new format, with four separate two-hour timeslots, and the opportunity to rotate between sessions in each time period. Cost is $100 for each timeslot and registration is required. Attendance is limited to 25 registrants per workshop; register early for the best selection.
Expanded international programming: A new session has been added to Wednesday’s International program: “International Consortium of Vascular Registries: Quality Improvement in Vascular Surgery Goes Global.” This presentation will be held from 8 to 10 a.m. and kicks off a full day of international events.
VQI @ VAM – VQI’s Second Annual Meeting: Vascular Quality Initiative’s second Annual Meeting has expanded to 1 ½ days, beginning Tuesday afternoon, May 30, and continuing all day Wednesday. Also new this year are a poster session and networking reception. Registration and a separate $200 fee are required.
Exhibit Hall: Learn first-hand from our industry partners about the latest devices, products and services. The Exhibit Hall also hosts the Opening Reception, box lunches and coffee breaks Thursday through Saturday. Industry participation in the exhibits underwrites a signification portion of VAM – allowing us to keep registration fees at lower rates than other industry meetings – so please support our industry partners.
And more: VAM also will include: breakfast sessions; a discount for the On-Demand Library; texting of questions; Interactive Poster Session, programming for fellows, residents and students; the Physician Vascular Interpretation Examination Review course; SVS Member Business Luncheon, with the election of officers plus the presentation of SVS and SVS Foundation awards; Vascular Live sessions highlighting the latest products and developments; plus socializing, with alumni receptions and other events.
For information and to register, visit vsweb.org/VAM17.
The topic is vascular care. The subjects to be covered are nearly too numerous to count. And the clock is ticking for signing up.
Registration and housing will open in early March for the 2017 Vascular Annual Meeting, set for May 31 to June 3 in San Diego, Calif., with plenaries and exhibits from June 1 to 3. This premier meeting of vascular specialists will be held at the San Diego Convention Center. The headquarters hotel, the Marriott Marquis San Diego Marina Hotel, adjoins the center.
There is plenty going on.
Additional programming: Expanded programming begins Wednesday and continues through Saturday. Be sure to plan your travel to attend these informative sessions. This year’s meeting includes more concurrent sessions, and the number of joint society programs has doubled from last year.
Free postgraduate courses for SVS members: Once again, all SVS member-registrants receive free admittance to the six Wednesday postgraduate courses, a savings of $300. Non-members plus anyone registering only for the postgraduate courses must pay the appropriate registration fee. Self-assessment credit is available to all physician attendees.
SVS/STS Aortic Summit: New this year, and a highlight for Saturday, is the SVS/STS Summit: Advances and Controversies in the Management of Complex Thoracoabdominal Aneurysmal Diseases and Type B Aortic Dissection. This program will take place from 1 to 5 p.m. and is co-sponsored by the Society of Thoracic Surgeons. An additional fee is required. 
Revamped workshops: Wednesday’s workshops will feature a new format, with four separate two-hour timeslots, and the opportunity to rotate between sessions in each time period. Cost is $100 for each timeslot and registration is required. Attendance is limited to 25 registrants per workshop; register early for the best selection.
Expanded international programming: A new session has been added to Wednesday’s International program: “International Consortium of Vascular Registries: Quality Improvement in Vascular Surgery Goes Global.” This presentation will be held from 8 to 10 a.m. and kicks off a full day of international events.
VQI @ VAM – VQI’s Second Annual Meeting: Vascular Quality Initiative’s second Annual Meeting has expanded to 1 ½ days, beginning Tuesday afternoon, May 30, and continuing all day Wednesday. Also new this year are a poster session and networking reception. Registration and a separate $200 fee are required.
Exhibit Hall: Learn first-hand from our industry partners about the latest devices, products and services. The Exhibit Hall also hosts the Opening Reception, box lunches and coffee breaks Thursday through Saturday. Industry participation in the exhibits underwrites a signification portion of VAM – allowing us to keep registration fees at lower rates than other industry meetings – so please support our industry partners.
And more: VAM also will include: breakfast sessions; a discount for the On-Demand Library; texting of questions; Interactive Poster Session, programming for fellows, residents and students; the Physician Vascular Interpretation Examination Review course; SVS Member Business Luncheon, with the election of officers plus the presentation of SVS and SVS Foundation awards; Vascular Live sessions highlighting the latest products and developments; plus socializing, with alumni receptions and other events.
For information and to register, visit vsweb.org/VAM17.
The topic is vascular care. The subjects to be covered are nearly too numerous to count. And the clock is ticking for signing up.
Registration and housing will open in early March for the 2017 Vascular Annual Meeting, set for May 31 to June 3 in San Diego, Calif., with plenaries and exhibits from June 1 to 3. This premier meeting of vascular specialists will be held at the San Diego Convention Center. The headquarters hotel, the Marriott Marquis San Diego Marina Hotel, adjoins the center.
There is plenty going on.
Additional programming: Expanded programming begins Wednesday and continues through Saturday. Be sure to plan your travel to attend these informative sessions. This year’s meeting includes more concurrent sessions, and the number of joint society programs has doubled from last year.
Free postgraduate courses for SVS members: Once again, all SVS member-registrants receive free admittance to the six Wednesday postgraduate courses, a savings of $300. Non-members plus anyone registering only for the postgraduate courses must pay the appropriate registration fee. Self-assessment credit is available to all physician attendees.
SVS/STS Aortic Summit: New this year, and a highlight for Saturday, is the SVS/STS Summit: Advances and Controversies in the Management of Complex Thoracoabdominal Aneurysmal Diseases and Type B Aortic Dissection. This program will take place from 1 to 5 p.m. and is co-sponsored by the Society of Thoracic Surgeons. An additional fee is required. 
Revamped workshops: Wednesday’s workshops will feature a new format, with four separate two-hour timeslots, and the opportunity to rotate between sessions in each time period. Cost is $100 for each timeslot and registration is required. Attendance is limited to 25 registrants per workshop; register early for the best selection.
Expanded international programming: A new session has been added to Wednesday’s International program: “International Consortium of Vascular Registries: Quality Improvement in Vascular Surgery Goes Global.” This presentation will be held from 8 to 10 a.m. and kicks off a full day of international events.
VQI @ VAM – VQI’s Second Annual Meeting: Vascular Quality Initiative’s second Annual Meeting has expanded to 1 ½ days, beginning Tuesday afternoon, May 30, and continuing all day Wednesday. Also new this year are a poster session and networking reception. Registration and a separate $200 fee are required.
Exhibit Hall: Learn first-hand from our industry partners about the latest devices, products and services. The Exhibit Hall also hosts the Opening Reception, box lunches and coffee breaks Thursday through Saturday. Industry participation in the exhibits underwrites a signification portion of VAM – allowing us to keep registration fees at lower rates than other industry meetings – so please support our industry partners.
And more: VAM also will include: breakfast sessions; a discount for the On-Demand Library; texting of questions; Interactive Poster Session, programming for fellows, residents and students; the Physician Vascular Interpretation Examination Review course; SVS Member Business Luncheon, with the election of officers plus the presentation of SVS and SVS Foundation awards; Vascular Live sessions highlighting the latest products and developments; plus socializing, with alumni receptions and other events.
For information and to register, visit vsweb.org/VAM17.
Preoperative variables can predict prolonged air leak
Prolonged air leak is a well-known complication after lung cancer surgery that can worsen patient outcomes and drive up costs, and while international authors have developed tools to calculate the risk of PAL, their use has been limited in the United States for various reasons. Researchers at the University of Pittsburgh have reported on a predictive model that uses easy-to-obtain patient factors, such as forced expiratory volume and smoking history, to help surgeons identify patients at greatest risk for complications and implement preventative measures.
Adam Attaar and his coauthors reported that their nomogram had an accuracy rate of 76%, with a 95% confidence interval, for predicting PAL after surgery (J Thorac Cardiovasc Surg. 2017 March;153[3]:690-9). “Using readily available candidate variables, our nomogram predicts increasing risk of prolonged air leak with good discriminatory ability,” noted Mr. Attaar, a student at University of Pittsburgh, and his coauthors.
Previously published reports put the incidence of PAL complications at 6%-18%, they noted. In the University of Pittsburgh series of 2,317 patients who had pulmonary resection for lung cancer or nodules from January 2009 to June 2014, the incidence was 8.6%.
In this series, patients with PAL were more likely to be older, men, and smokers, and to have a lower body mass index, peripheral vascular disease, chronic obstructive pulmonary disease, a history of steroid use, a high Zubrod score and lower forced expiratory volume.“They were less likely to have diabetes or to be hospitalized before surgery,” the researchers said. Surgical factors that characterized patients with PAL were resection for primary lung cancer rather than benign or metastatic tumors; lobectomy/segmentectomy or bilobectomy rather than wedge resection; a right-sided resection; thoracotomy; and a surgeon with higher annual caseloads.
Not all those factors made it into the nomogram, however. The nomogram scores each of these 10 variables to calculate the risk of PAL, in order of their weighting: lower forced expiratory volume, procedure type, BMI, right-sided thoracotomy, preoperative hospitalization, annual surgeon caseload, wedge resection by thoracotomy, reoperation, smoking history, and Zubrod score. A second nomogram drops out surgeon volume to make it more generalizable to other institutions.
In explaining higher surgeon volume as a risk factor for PAL, the researchers said that high-volume surgeons may be operating on patients with variables not accounted for in the Society of Thoracic Surgeons General Thoracic Surgery Database. “These unmeasured variables … could reveal modifiable technical factors to reduce the incidence of PAL and require further study,” the researchers said.
Fast-track discharge has gained acceptance in recent years as a way to spare patients a prolonged hospital stay and cut costs, but in this series the median hospital stay for patients with PAL was 10 days vs. 4 days for non-PAL patients (P less than 0.001).
“An accurate and generalizable PAL risk stratification tool could facilitate surgical decision making and patient-specific care” and aid in the design of trials to evaluate air-leak reduction methods such as sealants, buttressed staple lines, and pneumoperitoneum the researchers wrote.
Going forward, further development of the model would involve a multicenter study and inclusion of risk factors not accounted for in the thoracic surgery database, they noted.
The researchers had no relevant financial relationships to disclose.
The authors of this study “have performed a rigorous set of analyses to create this model,” Chi-Fu Jeffrey Yang, MD, of Duke University, Durham, N.C., noted in his invited commentary (J Thorac Cardiovasc Surg. 2017 March;53[3]:700-1). “The strengths of this study include its sound statistical analysis and study design,” Dr. Yang wrote. He gave the authors credit for using bootstrapping to internally validate the model.
However, Dr. Yang said that the database used by the researchers did not account for “numerous important variables,” including presence of pleural adhesions and emphysema status. The analysis also grouped lobectomy and segmentectomy together, and did not consider intraoperative variables such as sealant use, or postoperative management.
While Dr. Yang commended the study authors for developing a “reliable nomogram,” getting it implemented in the clinic is another hurdle. “It is commonly cited that it takes approximately 17 years for research evidence to translate into daily practice,” he said. To shorten that time line, he suggested the authors take a cue from various tech groups: Develop an app that surgeons can use.
Dr. Yang had no relevant financial relationships to disclose.
The authors of this study “have performed a rigorous set of analyses to create this model,” Chi-Fu Jeffrey Yang, MD, of Duke University, Durham, N.C., noted in his invited commentary (J Thorac Cardiovasc Surg. 2017 March;53[3]:700-1). “The strengths of this study include its sound statistical analysis and study design,” Dr. Yang wrote. He gave the authors credit for using bootstrapping to internally validate the model.
However, Dr. Yang said that the database used by the researchers did not account for “numerous important variables,” including presence of pleural adhesions and emphysema status. The analysis also grouped lobectomy and segmentectomy together, and did not consider intraoperative variables such as sealant use, or postoperative management.
While Dr. Yang commended the study authors for developing a “reliable nomogram,” getting it implemented in the clinic is another hurdle. “It is commonly cited that it takes approximately 17 years for research evidence to translate into daily practice,” he said. To shorten that time line, he suggested the authors take a cue from various tech groups: Develop an app that surgeons can use.
Dr. Yang had no relevant financial relationships to disclose.
The authors of this study “have performed a rigorous set of analyses to create this model,” Chi-Fu Jeffrey Yang, MD, of Duke University, Durham, N.C., noted in his invited commentary (J Thorac Cardiovasc Surg. 2017 March;53[3]:700-1). “The strengths of this study include its sound statistical analysis and study design,” Dr. Yang wrote. He gave the authors credit for using bootstrapping to internally validate the model.
However, Dr. Yang said that the database used by the researchers did not account for “numerous important variables,” including presence of pleural adhesions and emphysema status. The analysis also grouped lobectomy and segmentectomy together, and did not consider intraoperative variables such as sealant use, or postoperative management.
While Dr. Yang commended the study authors for developing a “reliable nomogram,” getting it implemented in the clinic is another hurdle. “It is commonly cited that it takes approximately 17 years for research evidence to translate into daily practice,” he said. To shorten that time line, he suggested the authors take a cue from various tech groups: Develop an app that surgeons can use.
Dr. Yang had no relevant financial relationships to disclose.
Prolonged air leak is a well-known complication after lung cancer surgery that can worsen patient outcomes and drive up costs, and while international authors have developed tools to calculate the risk of PAL, their use has been limited in the United States for various reasons. Researchers at the University of Pittsburgh have reported on a predictive model that uses easy-to-obtain patient factors, such as forced expiratory volume and smoking history, to help surgeons identify patients at greatest risk for complications and implement preventative measures.
Adam Attaar and his coauthors reported that their nomogram had an accuracy rate of 76%, with a 95% confidence interval, for predicting PAL after surgery (J Thorac Cardiovasc Surg. 2017 March;153[3]:690-9). “Using readily available candidate variables, our nomogram predicts increasing risk of prolonged air leak with good discriminatory ability,” noted Mr. Attaar, a student at University of Pittsburgh, and his coauthors.
Previously published reports put the incidence of PAL complications at 6%-18%, they noted. In the University of Pittsburgh series of 2,317 patients who had pulmonary resection for lung cancer or nodules from January 2009 to June 2014, the incidence was 8.6%.
In this series, patients with PAL were more likely to be older, men, and smokers, and to have a lower body mass index, peripheral vascular disease, chronic obstructive pulmonary disease, a history of steroid use, a high Zubrod score and lower forced expiratory volume.“They were less likely to have diabetes or to be hospitalized before surgery,” the researchers said. Surgical factors that characterized patients with PAL were resection for primary lung cancer rather than benign or metastatic tumors; lobectomy/segmentectomy or bilobectomy rather than wedge resection; a right-sided resection; thoracotomy; and a surgeon with higher annual caseloads.
Not all those factors made it into the nomogram, however. The nomogram scores each of these 10 variables to calculate the risk of PAL, in order of their weighting: lower forced expiratory volume, procedure type, BMI, right-sided thoracotomy, preoperative hospitalization, annual surgeon caseload, wedge resection by thoracotomy, reoperation, smoking history, and Zubrod score. A second nomogram drops out surgeon volume to make it more generalizable to other institutions.
In explaining higher surgeon volume as a risk factor for PAL, the researchers said that high-volume surgeons may be operating on patients with variables not accounted for in the Society of Thoracic Surgeons General Thoracic Surgery Database. “These unmeasured variables … could reveal modifiable technical factors to reduce the incidence of PAL and require further study,” the researchers said.
Fast-track discharge has gained acceptance in recent years as a way to spare patients a prolonged hospital stay and cut costs, but in this series the median hospital stay for patients with PAL was 10 days vs. 4 days for non-PAL patients (P less than 0.001).
“An accurate and generalizable PAL risk stratification tool could facilitate surgical decision making and patient-specific care” and aid in the design of trials to evaluate air-leak reduction methods such as sealants, buttressed staple lines, and pneumoperitoneum the researchers wrote.
Going forward, further development of the model would involve a multicenter study and inclusion of risk factors not accounted for in the thoracic surgery database, they noted.
The researchers had no relevant financial relationships to disclose.
Prolonged air leak is a well-known complication after lung cancer surgery that can worsen patient outcomes and drive up costs, and while international authors have developed tools to calculate the risk of PAL, their use has been limited in the United States for various reasons. Researchers at the University of Pittsburgh have reported on a predictive model that uses easy-to-obtain patient factors, such as forced expiratory volume and smoking history, to help surgeons identify patients at greatest risk for complications and implement preventative measures.
Adam Attaar and his coauthors reported that their nomogram had an accuracy rate of 76%, with a 95% confidence interval, for predicting PAL after surgery (J Thorac Cardiovasc Surg. 2017 March;153[3]:690-9). “Using readily available candidate variables, our nomogram predicts increasing risk of prolonged air leak with good discriminatory ability,” noted Mr. Attaar, a student at University of Pittsburgh, and his coauthors.
Previously published reports put the incidence of PAL complications at 6%-18%, they noted. In the University of Pittsburgh series of 2,317 patients who had pulmonary resection for lung cancer or nodules from January 2009 to June 2014, the incidence was 8.6%.
In this series, patients with PAL were more likely to be older, men, and smokers, and to have a lower body mass index, peripheral vascular disease, chronic obstructive pulmonary disease, a history of steroid use, a high Zubrod score and lower forced expiratory volume.“They were less likely to have diabetes or to be hospitalized before surgery,” the researchers said. Surgical factors that characterized patients with PAL were resection for primary lung cancer rather than benign or metastatic tumors; lobectomy/segmentectomy or bilobectomy rather than wedge resection; a right-sided resection; thoracotomy; and a surgeon with higher annual caseloads.
Not all those factors made it into the nomogram, however. The nomogram scores each of these 10 variables to calculate the risk of PAL, in order of their weighting: lower forced expiratory volume, procedure type, BMI, right-sided thoracotomy, preoperative hospitalization, annual surgeon caseload, wedge resection by thoracotomy, reoperation, smoking history, and Zubrod score. A second nomogram drops out surgeon volume to make it more generalizable to other institutions.
In explaining higher surgeon volume as a risk factor for PAL, the researchers said that high-volume surgeons may be operating on patients with variables not accounted for in the Society of Thoracic Surgeons General Thoracic Surgery Database. “These unmeasured variables … could reveal modifiable technical factors to reduce the incidence of PAL and require further study,” the researchers said.
Fast-track discharge has gained acceptance in recent years as a way to spare patients a prolonged hospital stay and cut costs, but in this series the median hospital stay for patients with PAL was 10 days vs. 4 days for non-PAL patients (P less than 0.001).
“An accurate and generalizable PAL risk stratification tool could facilitate surgical decision making and patient-specific care” and aid in the design of trials to evaluate air-leak reduction methods such as sealants, buttressed staple lines, and pneumoperitoneum the researchers wrote.
Going forward, further development of the model would involve a multicenter study and inclusion of risk factors not accounted for in the thoracic surgery database, they noted.
The researchers had no relevant financial relationships to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Preoperative variables can be evaluated to determine patient risk for prolonged air leak (PAL) in lung resection for cancer.
Major finding: A nomogram demonstrated 76% discriminatory accuracy in predicting PAL after lung resection.
Data source: Analysis of 2,522 pulmonary resections performed at eight hospitals within the University of Pittsburgh health system from January 2009 to June 2014.
Disclosures: The researchers had no conflicts of interest to disclose.
Colonic Diaphragm Disease: An Important NSAID Complication to Know
Colonic diaphragm disease (CDD) is a rare but serious complication of nonsteroidal anti‐inflammatory drug (NSAID) use in which diaphragm‐like strictures develop in the large and/or small intestines. There have been about 100 reported cases of CDD since this finding was first reported in the medical literature in 1989.1,2 However, given the frequency of NSAID use, providers should consider this diagnosis.
Case Report
An 85-year‐old woman presented to the emergency department (ED) with generalized weakness, occult positive stool, and severe anemia requiring blood transfusions. Eight months prior, she had presented to her primary care physician (PCP) for a routine visit and was found to have anemia. She was referred to gastroenterology by her PCP for an upper endoscopy but refused the referral despite clear warnings of the potential for serious complications. She was started on ferrous sulfate 325 mg po tid at that time. The patient’s hemoglobin (Hbg) levels ranged from 10.8 to 11.2 g/dL prior to her ED presentation, and random fecal occult blood testing had been negative.
In the ED, the patient reported no abdominal pain, nausea emesis or constipation. Her past medical history was significant for compensated diastolic heart failure, paroxysmal atrial fibrillation controlled by medication, and chronic lumbago. The patient had been prescribed NSAIDs for 10 years for the chronic lumbago diagnosis; however, she was inconsistent in taking this medication until more recent years. Surgical history included a hysterectomy and back surgeries in the distant past. She had no symptoms or history of inflammatory bowel disease. The patient’s medications included both the oral and topical forms of diclofenac, conjugated estrogen, nitroglycerin, amlodipine, hydrocodone bitartrate and acetaminophen 5 mg once daily, amiodarone, diazepam, valsartan, furosemide, oxycodone 5 mg nightly prn for severe pain, and ferrous sulfate 325 mg.
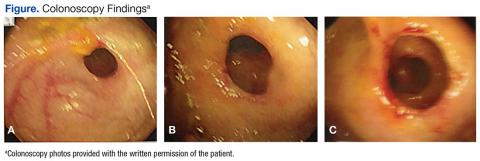
Significant laboratory results in the ED included a Hbg level of 8.3 g/dL; a ferritin level of 11.9 ng/mL, and a serum iron level of 12 mg/dL. A computerized tomography scan of the abdomen and pelvis was normal. The patient was admitted, received a blood transfusion, and a gastroenterology consult was obtained for an upper and lower endoscopy. Although copy was normal, the colonoscopy revealed evidence of CDD of the ascending colon.
Colonic Diaphragm Disease
The gross findings seen on this patient’s colonoscopy showed evidence of the development of an internal colonic stricture from the fibrous overgrowth of the diaphragm (Figures A, B, & C). The narrow lumen is exhibited particularly well in Figure A in the upper left image. The external surface of the intestine appears normal.
Presentation and Evaluation
The presenting symptoms of CDD can vary and include abdominal pain, vomiting, lower gastrointestinal bleeding, anemia, and intestinal obstruction/perforation. Evidence seems to suggest a greater association with use of the oxicams (meloxicam, piroxicam) or diclofenac.1,2 Some researchers have suggested an association with long‐acting NSAID formulations.1 The use of misoprostol or proton pump inhibitors does not seem to lessen the chance of stricture formulation.2 Cox‐2 inhibitors are less likely players in the development of CDD.2 Most diaphragms in the large colon have been noted in the cecum, ascending and transverse colon, though descending colonic lesions have been documented as well.1
The incidence of CDD is higher among women and occurs most often in the seventh decade of life. Correlation with the duration of NSAID use prior to disease onset varies widely, with studies documenting disease onset from 3 months to 5 years after initiating consistent NSAID use.1 The patient in this case study was prescribed NSAIDs for almost 10 years, although she had poor adherence. One study also notes a possible association of CYP2C9*3 genetic polymorphism with the propensity to develop CDD. This finding is particularly interesting since NSAIDs are principally metabolized by CYP2C9 in the liver. CYP2C9 polymorphisms are thought to induce higher plasma concentrations of NSAIDs over time, which may lead to the formation of colonic diaphragms.2
Endoscopy with biopsy is the diagnostic modality of choice. Use of capsule endoscopy is limited because the capsule may not be able to pass through narrow stricture walls.
Pathology and Histology
The mechanism by which NSAIDs induce the formation of colonic diaphragms may be related to these medications’ effects on mucosal integrity, vasoconstriction, and vascular spasm. These effects may lead to “erosions, ulceration, and diaphragm-like strictures.”3
The primary histologic feature of these strictures is submucosal fibrosis with an intact muscularis propria. Prominent features also include areas of ulceration and granulation.1 The widened submucosa results in annular constriction of the intestinal lumen with disorganized bundles of smooth muscle, unmyelinated nerve bundles, scattered ganglion cells, and blood vessels. This morphology can resemble a node or mass.4 The mucosa is typically normal.5 Conditions such as vascular and neuromuscular hamartoma can resemble CDD. Interestingly, the fibrous changes in CDD have been noted apart from NSAID use, leading to speculation that diaphragm formation may be a result of injury and not exclusively a reaction to NSAID use.5
Treatment
Recommendations include NSAID withdrawal, endoscopic/fluoroscopic dilatation, and surgical resection. Outcomes with these treatment approaches have been highly variable. For some patients, NSAID withdrawal alone or following resection has resulted in success. However, even with withdrawal of NSAIDs, symptoms may improve, although the histologic findings of CDD may not be reversed.3,6 In one case report, a colonic stricture persisted endoscopically after NSAID withdrawal but resolved after a 20-week course of prednisone.7
Conclusion
Despite NSAID cessation, the patient in this case study presented a month later with an acute bowel obstruction. The bowel obstruction responded to conservative treatment, and the patient declined surgical intervention. The patient was readmitted again 4 months later with an acute bowel obstruction, and underwent an emergent colonic resection. The pathology report was consistent with CDD. In the 3 years since the resection, the patient has had no recurrences and continues to avoid all NSAIDs. She has refused a follow-up postresection colonoscopy.
1. Munipalle PC, Garud T, Light D. Diaphragmatic disease of the colon: systematic review. Colorectal Dis. 2013;15(9):1063-1069.
2. Ishihara M, Ohmiya N, Nakamura M, et al. Risk factors of symptomatic NSAID-induced small intestinal injury and diaphragm disease. Aliment Pharmacol Ther. 2014;40(5):538-547.
3. Keeling AN, Davis JL, Williams A, Sabharwal T, Adam A. Fluoroscopically guided balloon dilation of NSAID-induced colonic diaphragm. J Vasc Interv Radiol. 2007;18(8):1060-1062.
4. Yousfi MM, De Petris G, Leighton JA, et al. Diaphragm disease after use of nonsteroidal anti-inflammatory agents: first report of diagnosis with capsule endoscopy. J Clin Gastroenterol. 2004;38(8):686-691.
5. Pilgrim S, Velchuru V, Waters G, Tsiamis A, Lal R. Diaphragm disease and small bowel enteropathy due to nonsteroidal anti-inflammatory drugs: a surgical perspective. Colorectal Dis. 2011;13(4):463-466.
6. Munipalle PC, Little M, Garud T, Henderson D. NSAID-induced diaphragmatic disease of the colon. BMJ Case Rep. 2013;2013:pii:bcr2012008448.
7. Penner RM, Williams CN. Resolution of multiple severe nonsteroidal anti-inflammatory drug-induced colonic strictures with prednisone therapy: a case report and review of the literature. Can J Gastroenterol. 2003;17(8):497-500.
Colonic diaphragm disease (CDD) is a rare but serious complication of nonsteroidal anti‐inflammatory drug (NSAID) use in which diaphragm‐like strictures develop in the large and/or small intestines. There have been about 100 reported cases of CDD since this finding was first reported in the medical literature in 1989.1,2 However, given the frequency of NSAID use, providers should consider this diagnosis.
Case Report
An 85-year‐old woman presented to the emergency department (ED) with generalized weakness, occult positive stool, and severe anemia requiring blood transfusions. Eight months prior, she had presented to her primary care physician (PCP) for a routine visit and was found to have anemia. She was referred to gastroenterology by her PCP for an upper endoscopy but refused the referral despite clear warnings of the potential for serious complications. She was started on ferrous sulfate 325 mg po tid at that time. The patient’s hemoglobin (Hbg) levels ranged from 10.8 to 11.2 g/dL prior to her ED presentation, and random fecal occult blood testing had been negative.
In the ED, the patient reported no abdominal pain, nausea emesis or constipation. Her past medical history was significant for compensated diastolic heart failure, paroxysmal atrial fibrillation controlled by medication, and chronic lumbago. The patient had been prescribed NSAIDs for 10 years for the chronic lumbago diagnosis; however, she was inconsistent in taking this medication until more recent years. Surgical history included a hysterectomy and back surgeries in the distant past. She had no symptoms or history of inflammatory bowel disease. The patient’s medications included both the oral and topical forms of diclofenac, conjugated estrogen, nitroglycerin, amlodipine, hydrocodone bitartrate and acetaminophen 5 mg once daily, amiodarone, diazepam, valsartan, furosemide, oxycodone 5 mg nightly prn for severe pain, and ferrous sulfate 325 mg.
Significant laboratory results in the ED included a Hbg level of 8.3 g/dL; a ferritin level of 11.9 ng/mL, and a serum iron level of 12 mg/dL. A computerized tomography scan of the abdomen and pelvis was normal. The patient was admitted, received a blood transfusion, and a gastroenterology consult was obtained for an upper and lower endoscopy. Although copy was normal, the colonoscopy revealed evidence of CDD of the ascending colon.
Colonic Diaphragm Disease
The gross findings seen on this patient’s colonoscopy showed evidence of the development of an internal colonic stricture from the fibrous overgrowth of the diaphragm (Figures A, B, & C). The narrow lumen is exhibited particularly well in Figure A in the upper left image. The external surface of the intestine appears normal.
Presentation and Evaluation
The presenting symptoms of CDD can vary and include abdominal pain, vomiting, lower gastrointestinal bleeding, anemia, and intestinal obstruction/perforation. Evidence seems to suggest a greater association with use of the oxicams (meloxicam, piroxicam) or diclofenac.1,2 Some researchers have suggested an association with long‐acting NSAID formulations.1 The use of misoprostol or proton pump inhibitors does not seem to lessen the chance of stricture formulation.2 Cox‐2 inhibitors are less likely players in the development of CDD.2 Most diaphragms in the large colon have been noted in the cecum, ascending and transverse colon, though descending colonic lesions have been documented as well.1
The incidence of CDD is higher among women and occurs most often in the seventh decade of life. Correlation with the duration of NSAID use prior to disease onset varies widely, with studies documenting disease onset from 3 months to 5 years after initiating consistent NSAID use.1 The patient in this case study was prescribed NSAIDs for almost 10 years, although she had poor adherence. One study also notes a possible association of CYP2C9*3 genetic polymorphism with the propensity to develop CDD. This finding is particularly interesting since NSAIDs are principally metabolized by CYP2C9 in the liver. CYP2C9 polymorphisms are thought to induce higher plasma concentrations of NSAIDs over time, which may lead to the formation of colonic diaphragms.2
Endoscopy with biopsy is the diagnostic modality of choice. Use of capsule endoscopy is limited because the capsule may not be able to pass through narrow stricture walls.
Pathology and Histology
The mechanism by which NSAIDs induce the formation of colonic diaphragms may be related to these medications’ effects on mucosal integrity, vasoconstriction, and vascular spasm. These effects may lead to “erosions, ulceration, and diaphragm-like strictures.”3
The primary histologic feature of these strictures is submucosal fibrosis with an intact muscularis propria. Prominent features also include areas of ulceration and granulation.1 The widened submucosa results in annular constriction of the intestinal lumen with disorganized bundles of smooth muscle, unmyelinated nerve bundles, scattered ganglion cells, and blood vessels. This morphology can resemble a node or mass.4 The mucosa is typically normal.5 Conditions such as vascular and neuromuscular hamartoma can resemble CDD. Interestingly, the fibrous changes in CDD have been noted apart from NSAID use, leading to speculation that diaphragm formation may be a result of injury and not exclusively a reaction to NSAID use.5
Treatment
Recommendations include NSAID withdrawal, endoscopic/fluoroscopic dilatation, and surgical resection. Outcomes with these treatment approaches have been highly variable. For some patients, NSAID withdrawal alone or following resection has resulted in success. However, even with withdrawal of NSAIDs, symptoms may improve, although the histologic findings of CDD may not be reversed.3,6 In one case report, a colonic stricture persisted endoscopically after NSAID withdrawal but resolved after a 20-week course of prednisone.7
Conclusion
Despite NSAID cessation, the patient in this case study presented a month later with an acute bowel obstruction. The bowel obstruction responded to conservative treatment, and the patient declined surgical intervention. The patient was readmitted again 4 months later with an acute bowel obstruction, and underwent an emergent colonic resection. The pathology report was consistent with CDD. In the 3 years since the resection, the patient has had no recurrences and continues to avoid all NSAIDs. She has refused a follow-up postresection colonoscopy.
Colonic diaphragm disease (CDD) is a rare but serious complication of nonsteroidal anti‐inflammatory drug (NSAID) use in which diaphragm‐like strictures develop in the large and/or small intestines. There have been about 100 reported cases of CDD since this finding was first reported in the medical literature in 1989.1,2 However, given the frequency of NSAID use, providers should consider this diagnosis.
Case Report
An 85-year‐old woman presented to the emergency department (ED) with generalized weakness, occult positive stool, and severe anemia requiring blood transfusions. Eight months prior, she had presented to her primary care physician (PCP) for a routine visit and was found to have anemia. She was referred to gastroenterology by her PCP for an upper endoscopy but refused the referral despite clear warnings of the potential for serious complications. She was started on ferrous sulfate 325 mg po tid at that time. The patient’s hemoglobin (Hbg) levels ranged from 10.8 to 11.2 g/dL prior to her ED presentation, and random fecal occult blood testing had been negative.
In the ED, the patient reported no abdominal pain, nausea emesis or constipation. Her past medical history was significant for compensated diastolic heart failure, paroxysmal atrial fibrillation controlled by medication, and chronic lumbago. The patient had been prescribed NSAIDs for 10 years for the chronic lumbago diagnosis; however, she was inconsistent in taking this medication until more recent years. Surgical history included a hysterectomy and back surgeries in the distant past. She had no symptoms or history of inflammatory bowel disease. The patient’s medications included both the oral and topical forms of diclofenac, conjugated estrogen, nitroglycerin, amlodipine, hydrocodone bitartrate and acetaminophen 5 mg once daily, amiodarone, diazepam, valsartan, furosemide, oxycodone 5 mg nightly prn for severe pain, and ferrous sulfate 325 mg.
Significant laboratory results in the ED included a Hbg level of 8.3 g/dL; a ferritin level of 11.9 ng/mL, and a serum iron level of 12 mg/dL. A computerized tomography scan of the abdomen and pelvis was normal. The patient was admitted, received a blood transfusion, and a gastroenterology consult was obtained for an upper and lower endoscopy. Although copy was normal, the colonoscopy revealed evidence of CDD of the ascending colon.
Colonic Diaphragm Disease
The gross findings seen on this patient’s colonoscopy showed evidence of the development of an internal colonic stricture from the fibrous overgrowth of the diaphragm (Figures A, B, & C). The narrow lumen is exhibited particularly well in Figure A in the upper left image. The external surface of the intestine appears normal.
Presentation and Evaluation
The presenting symptoms of CDD can vary and include abdominal pain, vomiting, lower gastrointestinal bleeding, anemia, and intestinal obstruction/perforation. Evidence seems to suggest a greater association with use of the oxicams (meloxicam, piroxicam) or diclofenac.1,2 Some researchers have suggested an association with long‐acting NSAID formulations.1 The use of misoprostol or proton pump inhibitors does not seem to lessen the chance of stricture formulation.2 Cox‐2 inhibitors are less likely players in the development of CDD.2 Most diaphragms in the large colon have been noted in the cecum, ascending and transverse colon, though descending colonic lesions have been documented as well.1
The incidence of CDD is higher among women and occurs most often in the seventh decade of life. Correlation with the duration of NSAID use prior to disease onset varies widely, with studies documenting disease onset from 3 months to 5 years after initiating consistent NSAID use.1 The patient in this case study was prescribed NSAIDs for almost 10 years, although she had poor adherence. One study also notes a possible association of CYP2C9*3 genetic polymorphism with the propensity to develop CDD. This finding is particularly interesting since NSAIDs are principally metabolized by CYP2C9 in the liver. CYP2C9 polymorphisms are thought to induce higher plasma concentrations of NSAIDs over time, which may lead to the formation of colonic diaphragms.2
Endoscopy with biopsy is the diagnostic modality of choice. Use of capsule endoscopy is limited because the capsule may not be able to pass through narrow stricture walls.
Pathology and Histology
The mechanism by which NSAIDs induce the formation of colonic diaphragms may be related to these medications’ effects on mucosal integrity, vasoconstriction, and vascular spasm. These effects may lead to “erosions, ulceration, and diaphragm-like strictures.”3
The primary histologic feature of these strictures is submucosal fibrosis with an intact muscularis propria. Prominent features also include areas of ulceration and granulation.1 The widened submucosa results in annular constriction of the intestinal lumen with disorganized bundles of smooth muscle, unmyelinated nerve bundles, scattered ganglion cells, and blood vessels. This morphology can resemble a node or mass.4 The mucosa is typically normal.5 Conditions such as vascular and neuromuscular hamartoma can resemble CDD. Interestingly, the fibrous changes in CDD have been noted apart from NSAID use, leading to speculation that diaphragm formation may be a result of injury and not exclusively a reaction to NSAID use.5
Treatment
Recommendations include NSAID withdrawal, endoscopic/fluoroscopic dilatation, and surgical resection. Outcomes with these treatment approaches have been highly variable. For some patients, NSAID withdrawal alone or following resection has resulted in success. However, even with withdrawal of NSAIDs, symptoms may improve, although the histologic findings of CDD may not be reversed.3,6 In one case report, a colonic stricture persisted endoscopically after NSAID withdrawal but resolved after a 20-week course of prednisone.7
Conclusion
Despite NSAID cessation, the patient in this case study presented a month later with an acute bowel obstruction. The bowel obstruction responded to conservative treatment, and the patient declined surgical intervention. The patient was readmitted again 4 months later with an acute bowel obstruction, and underwent an emergent colonic resection. The pathology report was consistent with CDD. In the 3 years since the resection, the patient has had no recurrences and continues to avoid all NSAIDs. She has refused a follow-up postresection colonoscopy.
1. Munipalle PC, Garud T, Light D. Diaphragmatic disease of the colon: systematic review. Colorectal Dis. 2013;15(9):1063-1069.
2. Ishihara M, Ohmiya N, Nakamura M, et al. Risk factors of symptomatic NSAID-induced small intestinal injury and diaphragm disease. Aliment Pharmacol Ther. 2014;40(5):538-547.
3. Keeling AN, Davis JL, Williams A, Sabharwal T, Adam A. Fluoroscopically guided balloon dilation of NSAID-induced colonic diaphragm. J Vasc Interv Radiol. 2007;18(8):1060-1062.
4. Yousfi MM, De Petris G, Leighton JA, et al. Diaphragm disease after use of nonsteroidal anti-inflammatory agents: first report of diagnosis with capsule endoscopy. J Clin Gastroenterol. 2004;38(8):686-691.
5. Pilgrim S, Velchuru V, Waters G, Tsiamis A, Lal R. Diaphragm disease and small bowel enteropathy due to nonsteroidal anti-inflammatory drugs: a surgical perspective. Colorectal Dis. 2011;13(4):463-466.
6. Munipalle PC, Little M, Garud T, Henderson D. NSAID-induced diaphragmatic disease of the colon. BMJ Case Rep. 2013;2013:pii:bcr2012008448.
7. Penner RM, Williams CN. Resolution of multiple severe nonsteroidal anti-inflammatory drug-induced colonic strictures with prednisone therapy: a case report and review of the literature. Can J Gastroenterol. 2003;17(8):497-500.
1. Munipalle PC, Garud T, Light D. Diaphragmatic disease of the colon: systematic review. Colorectal Dis. 2013;15(9):1063-1069.
2. Ishihara M, Ohmiya N, Nakamura M, et al. Risk factors of symptomatic NSAID-induced small intestinal injury and diaphragm disease. Aliment Pharmacol Ther. 2014;40(5):538-547.
3. Keeling AN, Davis JL, Williams A, Sabharwal T, Adam A. Fluoroscopically guided balloon dilation of NSAID-induced colonic diaphragm. J Vasc Interv Radiol. 2007;18(8):1060-1062.
4. Yousfi MM, De Petris G, Leighton JA, et al. Diaphragm disease after use of nonsteroidal anti-inflammatory agents: first report of diagnosis with capsule endoscopy. J Clin Gastroenterol. 2004;38(8):686-691.
5. Pilgrim S, Velchuru V, Waters G, Tsiamis A, Lal R. Diaphragm disease and small bowel enteropathy due to nonsteroidal anti-inflammatory drugs: a surgical perspective. Colorectal Dis. 2011;13(4):463-466.
6. Munipalle PC, Little M, Garud T, Henderson D. NSAID-induced diaphragmatic disease of the colon. BMJ Case Rep. 2013;2013:pii:bcr2012008448.
7. Penner RM, Williams CN. Resolution of multiple severe nonsteroidal anti-inflammatory drug-induced colonic strictures with prednisone therapy: a case report and review of the literature. Can J Gastroenterol. 2003;17(8):497-500.
Apixaban bests warfarin in real-world analysis
WASHINGTON, DC—An analysis of real-world data suggests elderly patients with non-valvular atrial fibrillation (NVAF) have a lower risk of stroke or systemic embolism and a lower risk of major bleeding if they receive apixaban rather than warfarin.
The study also indicates that elderly NVAF patients who receive dabigatran have a similar risk of stroke/systemic embolism as those on warfarin, but the risk of major bleeding is lower with dabigatran.
And rivaroxaban poses a lower risk of stroke/systemic embolism than warfarin but a higher risk of major bleeding.
However, the researchers who conducted this analysis stressed that it cannot be used as stand-alone evidence to validate the efficacy and/or safety of any of the drugs studied.
The results of the analysis were presented at the American College of Cardiology’s 66th Annual Scientific Session (abstract 1134M-13).
The researchers also presented data on the financial costs of major bleeding associated with apixaban, dabigatran, rivaroxaban, and warfarin (abstract 1189-085/085).
The research was conducted by employees from Bristol-Myers Squibb Company and Pfizer Inc., the companies marketing apixaban, as well as other researchers who have relationships with the companies.
The team evaluated medical and pharmacy claims from the US Medicare fee-for-service database. They looked at NVAF patients age 65 and older who were newly prescribed apixaban, dabigatran, rivaroxaban, or warfarin between January 1, 2013, and December 31, 2014.
The researchers compared each of the direct oral anticoagulants to warfarin using 1:1 propensity score matching methodology to balance select demographic and clinical characteristics between the groups.
The team used Cox proportional hazards models to estimate the hazard ratio (HR) of stroke/systemic embolism and major bleeding.
Results
In a comparison of apixaban and warfarin (20,803 patients in each treatment group), apixaban was associated with a significantly lower risk of stroke or systemic embolism than warfarin (HR: 0.40, 95% CI: 0.31-0.53; P<0.0001).
Apixaban was also associated with a significantly lower risk of major bleeding than warfarin (HR: 0.51, 95% CI: 0.44-0.58; P<0.0001). The cost of major bleeding per patient per month was $286 with apixaban and $537 with warfarin (P<0.0001).
In a comparison of dabigatran and warfarin (16,731 patients in each treatment group), the risk of stroke or systemic embolism was similar between the cohorts (HR: 0.94, 95% CI: 0.74-1.21; P=0.647).
However, the risk of major bleeding was significantly lower with dabigatran than with warfarin (HR: 0.79, 95% CI: 0.69-0.91; P=0.001). The cost of major bleeding per patient per month was $367 with dabigatran and $452 with warfarin (P=0.032).
In a comparison of rivaroxaban and warfarin (52,476 patients in each group), rivaroxaban was associated with a significantly lower risk of stroke or systemic embolism than warfarin (HR: 0.72, 95% CI: 0.63-0.83; P<0.0001).
However, the risk of major bleeding was significantly higher with rivaroxaban than with warfarin (HR: 1.17, 95% CI: 1.10-1.26; P<0.0001). The cost of major bleeding per patient per month was $524 with rivaroxaban and $500 with warfarin (P=0.154).
Limitations
The researchers noted that this analysis has several limitations. For instance, information on laboratory results and time in therapeutic range were not available. Diagnoses were identified through ICD-9 codes, and drug prescriptions were identified through prescription claims.
Propensity score matching was used to mimic randomization by balancing pre-defined demographic and clinical characteristics at baseline for both treatment cohorts. However, unobserved confounders (such as laboratory values and patient preferences) may exist.
As with any real-world data analysis, missing values, coding errors, and a lack of clinical accuracy may have introduced bias.
The researchers stressed that, due to such limitations, real-world data analyses cannot be used as stand-alone evidence to validate the efficacy and/or safety of a treatment. However, these analyses add to information provided by randomized clinical trials.
“Studies such as this large US Medicare database analysis supplement pivotal trials by broadening and deepening our scientific knowledge of how patients respond to direct oral anticoagulants in everyday clinical practice,” said Alpesh Amin, MD, principal investigator and professor of medicine at the University of California, Irvine.
“Given the diversity of patients with non-valvular atrial fibrillation, analyses of real-world data provide further information that adds to data generated in randomized clinical trials.” ![]()
WASHINGTON, DC—An analysis of real-world data suggests elderly patients with non-valvular atrial fibrillation (NVAF) have a lower risk of stroke or systemic embolism and a lower risk of major bleeding if they receive apixaban rather than warfarin.
The study also indicates that elderly NVAF patients who receive dabigatran have a similar risk of stroke/systemic embolism as those on warfarin, but the risk of major bleeding is lower with dabigatran.
And rivaroxaban poses a lower risk of stroke/systemic embolism than warfarin but a higher risk of major bleeding.
However, the researchers who conducted this analysis stressed that it cannot be used as stand-alone evidence to validate the efficacy and/or safety of any of the drugs studied.
The results of the analysis were presented at the American College of Cardiology’s 66th Annual Scientific Session (abstract 1134M-13).
The researchers also presented data on the financial costs of major bleeding associated with apixaban, dabigatran, rivaroxaban, and warfarin (abstract 1189-085/085).
The research was conducted by employees from Bristol-Myers Squibb Company and Pfizer Inc., the companies marketing apixaban, as well as other researchers who have relationships with the companies.
The team evaluated medical and pharmacy claims from the US Medicare fee-for-service database. They looked at NVAF patients age 65 and older who were newly prescribed apixaban, dabigatran, rivaroxaban, or warfarin between January 1, 2013, and December 31, 2014.
The researchers compared each of the direct oral anticoagulants to warfarin using 1:1 propensity score matching methodology to balance select demographic and clinical characteristics between the groups.
The team used Cox proportional hazards models to estimate the hazard ratio (HR) of stroke/systemic embolism and major bleeding.
Results
In a comparison of apixaban and warfarin (20,803 patients in each treatment group), apixaban was associated with a significantly lower risk of stroke or systemic embolism than warfarin (HR: 0.40, 95% CI: 0.31-0.53; P<0.0001).
Apixaban was also associated with a significantly lower risk of major bleeding than warfarin (HR: 0.51, 95% CI: 0.44-0.58; P<0.0001). The cost of major bleeding per patient per month was $286 with apixaban and $537 with warfarin (P<0.0001).
In a comparison of dabigatran and warfarin (16,731 patients in each treatment group), the risk of stroke or systemic embolism was similar between the cohorts (HR: 0.94, 95% CI: 0.74-1.21; P=0.647).
However, the risk of major bleeding was significantly lower with dabigatran than with warfarin (HR: 0.79, 95% CI: 0.69-0.91; P=0.001). The cost of major bleeding per patient per month was $367 with dabigatran and $452 with warfarin (P=0.032).
In a comparison of rivaroxaban and warfarin (52,476 patients in each group), rivaroxaban was associated with a significantly lower risk of stroke or systemic embolism than warfarin (HR: 0.72, 95% CI: 0.63-0.83; P<0.0001).
However, the risk of major bleeding was significantly higher with rivaroxaban than with warfarin (HR: 1.17, 95% CI: 1.10-1.26; P<0.0001). The cost of major bleeding per patient per month was $524 with rivaroxaban and $500 with warfarin (P=0.154).
Limitations
The researchers noted that this analysis has several limitations. For instance, information on laboratory results and time in therapeutic range were not available. Diagnoses were identified through ICD-9 codes, and drug prescriptions were identified through prescription claims.
Propensity score matching was used to mimic randomization by balancing pre-defined demographic and clinical characteristics at baseline for both treatment cohorts. However, unobserved confounders (such as laboratory values and patient preferences) may exist.
As with any real-world data analysis, missing values, coding errors, and a lack of clinical accuracy may have introduced bias.
The researchers stressed that, due to such limitations, real-world data analyses cannot be used as stand-alone evidence to validate the efficacy and/or safety of a treatment. However, these analyses add to information provided by randomized clinical trials.
“Studies such as this large US Medicare database analysis supplement pivotal trials by broadening and deepening our scientific knowledge of how patients respond to direct oral anticoagulants in everyday clinical practice,” said Alpesh Amin, MD, principal investigator and professor of medicine at the University of California, Irvine.
“Given the diversity of patients with non-valvular atrial fibrillation, analyses of real-world data provide further information that adds to data generated in randomized clinical trials.” ![]()
WASHINGTON, DC—An analysis of real-world data suggests elderly patients with non-valvular atrial fibrillation (NVAF) have a lower risk of stroke or systemic embolism and a lower risk of major bleeding if they receive apixaban rather than warfarin.
The study also indicates that elderly NVAF patients who receive dabigatran have a similar risk of stroke/systemic embolism as those on warfarin, but the risk of major bleeding is lower with dabigatran.
And rivaroxaban poses a lower risk of stroke/systemic embolism than warfarin but a higher risk of major bleeding.
However, the researchers who conducted this analysis stressed that it cannot be used as stand-alone evidence to validate the efficacy and/or safety of any of the drugs studied.
The results of the analysis were presented at the American College of Cardiology’s 66th Annual Scientific Session (abstract 1134M-13).
The researchers also presented data on the financial costs of major bleeding associated with apixaban, dabigatran, rivaroxaban, and warfarin (abstract 1189-085/085).
The research was conducted by employees from Bristol-Myers Squibb Company and Pfizer Inc., the companies marketing apixaban, as well as other researchers who have relationships with the companies.
The team evaluated medical and pharmacy claims from the US Medicare fee-for-service database. They looked at NVAF patients age 65 and older who were newly prescribed apixaban, dabigatran, rivaroxaban, or warfarin between January 1, 2013, and December 31, 2014.
The researchers compared each of the direct oral anticoagulants to warfarin using 1:1 propensity score matching methodology to balance select demographic and clinical characteristics between the groups.
The team used Cox proportional hazards models to estimate the hazard ratio (HR) of stroke/systemic embolism and major bleeding.
Results
In a comparison of apixaban and warfarin (20,803 patients in each treatment group), apixaban was associated with a significantly lower risk of stroke or systemic embolism than warfarin (HR: 0.40, 95% CI: 0.31-0.53; P<0.0001).
Apixaban was also associated with a significantly lower risk of major bleeding than warfarin (HR: 0.51, 95% CI: 0.44-0.58; P<0.0001). The cost of major bleeding per patient per month was $286 with apixaban and $537 with warfarin (P<0.0001).
In a comparison of dabigatran and warfarin (16,731 patients in each treatment group), the risk of stroke or systemic embolism was similar between the cohorts (HR: 0.94, 95% CI: 0.74-1.21; P=0.647).
However, the risk of major bleeding was significantly lower with dabigatran than with warfarin (HR: 0.79, 95% CI: 0.69-0.91; P=0.001). The cost of major bleeding per patient per month was $367 with dabigatran and $452 with warfarin (P=0.032).
In a comparison of rivaroxaban and warfarin (52,476 patients in each group), rivaroxaban was associated with a significantly lower risk of stroke or systemic embolism than warfarin (HR: 0.72, 95% CI: 0.63-0.83; P<0.0001).
However, the risk of major bleeding was significantly higher with rivaroxaban than with warfarin (HR: 1.17, 95% CI: 1.10-1.26; P<0.0001). The cost of major bleeding per patient per month was $524 with rivaroxaban and $500 with warfarin (P=0.154).
Limitations
The researchers noted that this analysis has several limitations. For instance, information on laboratory results and time in therapeutic range were not available. Diagnoses were identified through ICD-9 codes, and drug prescriptions were identified through prescription claims.
Propensity score matching was used to mimic randomization by balancing pre-defined demographic and clinical characteristics at baseline for both treatment cohorts. However, unobserved confounders (such as laboratory values and patient preferences) may exist.
As with any real-world data analysis, missing values, coding errors, and a lack of clinical accuracy may have introduced bias.
The researchers stressed that, due to such limitations, real-world data analyses cannot be used as stand-alone evidence to validate the efficacy and/or safety of a treatment. However, these analyses add to information provided by randomized clinical trials.
“Studies such as this large US Medicare database analysis supplement pivotal trials by broadening and deepening our scientific knowledge of how patients respond to direct oral anticoagulants in everyday clinical practice,” said Alpesh Amin, MD, principal investigator and professor of medicine at the University of California, Irvine.
“Given the diversity of patients with non-valvular atrial fibrillation, analyses of real-world data provide further information that adds to data generated in randomized clinical trials.” ![]()
Major bleeding lower with dabigatran than warfarin
WASHINGTON, DC—Results from the RE-CIRCUIT trial suggest that uninterrupted dabigatran poses a lower risk of major bleeding than uninterrupted warfarin in patients with non-valvular atrial fibrillation (NVAF) who are undergoing catheter ablation.
Patients who received dabigatran also had fewer serious and severe adverse events (AEs).
The incidence of minor bleeding was similar between the treatment groups, and the incidence of AEs leading to treatment discontinuation was higher with dabigatran.
These results were presented at the American College of Cardiology’s 66th Annual Scientific Session and simultaneously published in NEJM.
This study was sponsored by Boehringer Ingelheim Pharmaceuticals, Inc.
The RE-CIRCUIT trial enrolled 704 patients with paroxysmal or persistent NVAF, and 635 of them ultimately underwent catheter ablation with uninterrupted anticoagulation.
The patients were randomized to receive dabigatran at 150 mg twice daily or warfarin (with a target international normalized ratio [INR] of 2.0–3.0) in a 1:1 ratio and remained on this treatment for the duration of the trial.
The mean adherence to dabigatran was 97.6%, and patients receiving warfarin were within the guideline-defined target INR range 66% of the time. The majority of patients in both groups (86.1% in the dabigatran group and 84.3% in the warfarin group) received study treatment for at least 8 weeks after ablation.
Results
The study’s primary endpoint was the incidence of major bleeding events, as defined by the International Society on Thrombosis and Hemostasis, during the ablation procedure and up to 2 months after.
There was a significantly lower incidence of major bleeding with uninterrupted dabigatran than with uninterrupted warfarin—1.6% (n=5) and 6.9% (n=22), respectively (P<0.001).
Major bleeding events (in the dabigatran and warfarin groups, respectively) included pericardial tamponade (1 and 6), pericardial effusion (1 and 0), groin bleeds (2 in both), groin hematomas (0 and 8), gastrointestinal bleeds (1 and 2), intracranial bleeds (0 and 2), pseudoaneurysm (0 and 1), and hematomas (0 and 2).
The incidence of minor bleeding was similar between the treatment groups—18.6% (n=59) in the dabigatran group and 17.0% (n=54) in the warfarin group.
The incidence of serious AEs was 18.6% in the dabigatran group and 22.2% in the warfarin group. The most frequent serious AEs were atrial flutter (5.9% and 5.6%, respectively), atrial fibrillation (1.8% and 3.8%, respectively), and cardiac tamponade (0.3% and 1.2%, respectively).
The incidence of severe AEs was 3.3% in the dabigatran group and 6.2% in the warfarin group. The incidence of AEs leading to treatment discontinuation was 5.6% and 2.4%, respectively.
There were no cases of stroke, systemic embolism, or transient ischemic attack in the dabigatran group. However, there was a transient ischemic attack in the warfarin group.
“These results are exciting news for the medical community,” said study investigator Hugh Calkins, MD, of Johns Hopkins Hospital in Baltimore, Maryland.
“During an ablation procedure, patients are at risk of potential major complications, including stroke and bleeding. Therefore, anticoagulation management at the time of AFib ablation is critically important. In RE-CIRCUIT, we have seen that uninterrupted anticoagulation with dabigatran showed significantly lower major bleeding complications than warfarin in atrial fibrillation patients undergoing cardiac ablation.” ![]()
WASHINGTON, DC—Results from the RE-CIRCUIT trial suggest that uninterrupted dabigatran poses a lower risk of major bleeding than uninterrupted warfarin in patients with non-valvular atrial fibrillation (NVAF) who are undergoing catheter ablation.
Patients who received dabigatran also had fewer serious and severe adverse events (AEs).
The incidence of minor bleeding was similar between the treatment groups, and the incidence of AEs leading to treatment discontinuation was higher with dabigatran.
These results were presented at the American College of Cardiology’s 66th Annual Scientific Session and simultaneously published in NEJM.
This study was sponsored by Boehringer Ingelheim Pharmaceuticals, Inc.
The RE-CIRCUIT trial enrolled 704 patients with paroxysmal or persistent NVAF, and 635 of them ultimately underwent catheter ablation with uninterrupted anticoagulation.
The patients were randomized to receive dabigatran at 150 mg twice daily or warfarin (with a target international normalized ratio [INR] of 2.0–3.0) in a 1:1 ratio and remained on this treatment for the duration of the trial.
The mean adherence to dabigatran was 97.6%, and patients receiving warfarin were within the guideline-defined target INR range 66% of the time. The majority of patients in both groups (86.1% in the dabigatran group and 84.3% in the warfarin group) received study treatment for at least 8 weeks after ablation.
Results
The study’s primary endpoint was the incidence of major bleeding events, as defined by the International Society on Thrombosis and Hemostasis, during the ablation procedure and up to 2 months after.
There was a significantly lower incidence of major bleeding with uninterrupted dabigatran than with uninterrupted warfarin—1.6% (n=5) and 6.9% (n=22), respectively (P<0.001).
Major bleeding events (in the dabigatran and warfarin groups, respectively) included pericardial tamponade (1 and 6), pericardial effusion (1 and 0), groin bleeds (2 in both), groin hematomas (0 and 8), gastrointestinal bleeds (1 and 2), intracranial bleeds (0 and 2), pseudoaneurysm (0 and 1), and hematomas (0 and 2).
The incidence of minor bleeding was similar between the treatment groups—18.6% (n=59) in the dabigatran group and 17.0% (n=54) in the warfarin group.
The incidence of serious AEs was 18.6% in the dabigatran group and 22.2% in the warfarin group. The most frequent serious AEs were atrial flutter (5.9% and 5.6%, respectively), atrial fibrillation (1.8% and 3.8%, respectively), and cardiac tamponade (0.3% and 1.2%, respectively).
The incidence of severe AEs was 3.3% in the dabigatran group and 6.2% in the warfarin group. The incidence of AEs leading to treatment discontinuation was 5.6% and 2.4%, respectively.
There were no cases of stroke, systemic embolism, or transient ischemic attack in the dabigatran group. However, there was a transient ischemic attack in the warfarin group.
“These results are exciting news for the medical community,” said study investigator Hugh Calkins, MD, of Johns Hopkins Hospital in Baltimore, Maryland.
“During an ablation procedure, patients are at risk of potential major complications, including stroke and bleeding. Therefore, anticoagulation management at the time of AFib ablation is critically important. In RE-CIRCUIT, we have seen that uninterrupted anticoagulation with dabigatran showed significantly lower major bleeding complications than warfarin in atrial fibrillation patients undergoing cardiac ablation.” ![]()
WASHINGTON, DC—Results from the RE-CIRCUIT trial suggest that uninterrupted dabigatran poses a lower risk of major bleeding than uninterrupted warfarin in patients with non-valvular atrial fibrillation (NVAF) who are undergoing catheter ablation.
Patients who received dabigatran also had fewer serious and severe adverse events (AEs).
The incidence of minor bleeding was similar between the treatment groups, and the incidence of AEs leading to treatment discontinuation was higher with dabigatran.
These results were presented at the American College of Cardiology’s 66th Annual Scientific Session and simultaneously published in NEJM.
This study was sponsored by Boehringer Ingelheim Pharmaceuticals, Inc.
The RE-CIRCUIT trial enrolled 704 patients with paroxysmal or persistent NVAF, and 635 of them ultimately underwent catheter ablation with uninterrupted anticoagulation.
The patients were randomized to receive dabigatran at 150 mg twice daily or warfarin (with a target international normalized ratio [INR] of 2.0–3.0) in a 1:1 ratio and remained on this treatment for the duration of the trial.
The mean adherence to dabigatran was 97.6%, and patients receiving warfarin were within the guideline-defined target INR range 66% of the time. The majority of patients in both groups (86.1% in the dabigatran group and 84.3% in the warfarin group) received study treatment for at least 8 weeks after ablation.
Results
The study’s primary endpoint was the incidence of major bleeding events, as defined by the International Society on Thrombosis and Hemostasis, during the ablation procedure and up to 2 months after.
There was a significantly lower incidence of major bleeding with uninterrupted dabigatran than with uninterrupted warfarin—1.6% (n=5) and 6.9% (n=22), respectively (P<0.001).
Major bleeding events (in the dabigatran and warfarin groups, respectively) included pericardial tamponade (1 and 6), pericardial effusion (1 and 0), groin bleeds (2 in both), groin hematomas (0 and 8), gastrointestinal bleeds (1 and 2), intracranial bleeds (0 and 2), pseudoaneurysm (0 and 1), and hematomas (0 and 2).
The incidence of minor bleeding was similar between the treatment groups—18.6% (n=59) in the dabigatran group and 17.0% (n=54) in the warfarin group.
The incidence of serious AEs was 18.6% in the dabigatran group and 22.2% in the warfarin group. The most frequent serious AEs were atrial flutter (5.9% and 5.6%, respectively), atrial fibrillation (1.8% and 3.8%, respectively), and cardiac tamponade (0.3% and 1.2%, respectively).
The incidence of severe AEs was 3.3% in the dabigatran group and 6.2% in the warfarin group. The incidence of AEs leading to treatment discontinuation was 5.6% and 2.4%, respectively.
There were no cases of stroke, systemic embolism, or transient ischemic attack in the dabigatran group. However, there was a transient ischemic attack in the warfarin group.
“These results are exciting news for the medical community,” said study investigator Hugh Calkins, MD, of Johns Hopkins Hospital in Baltimore, Maryland.
“During an ablation procedure, patients are at risk of potential major complications, including stroke and bleeding. Therefore, anticoagulation management at the time of AFib ablation is critically important. In RE-CIRCUIT, we have seen that uninterrupted anticoagulation with dabigatran showed significantly lower major bleeding complications than warfarin in atrial fibrillation patients undergoing cardiac ablation.” ![]()
ACS launches AHRQ Safety Program for ERAS
The American College of Surgeons (ACS), in collaboration with the Johns Hopkins Medicine Armstrong Institute for Patient Safety and Quality, Baltimore, MD, has launched the AHRQ (Agency for Healthcare Research and Quality) Safety Program for Enhanced Recovery after Surgery (ERAS). This new surgical quality improvement program is funded and guided by AHRQ.
The AHRQ Safety Program for ERAS will support hospitals in implementing perioperative evidence-based protocols to meaningfully improve clinical outcomes, reduce health care utilization, and improve the patient experience. This program aims to enroll at least 750 hospitals throughout the five-year contract. Hospitals within the U.S., Puerto Rico, and the District of Columbia are eligible to participate across five service lines: colorectal, orthopaedic, bariatric, gynecology, and emergency general surgery.
Participating hospitals will have access to the international leaders in ERAS, including representatives of surgery, anesthesiology, and nursing; prototype ERAS protocols developed for five procedures based on up-to-date evidence review; literature to support protocols; tools and educational materials to facilitate implementation; quality improvement specialist support; and coaching calls to support hospital work.
Program enrollment will begin in spring 2017. For more information, contact [email protected].
The American College of Surgeons (ACS), in collaboration with the Johns Hopkins Medicine Armstrong Institute for Patient Safety and Quality, Baltimore, MD, has launched the AHRQ (Agency for Healthcare Research and Quality) Safety Program for Enhanced Recovery after Surgery (ERAS). This new surgical quality improvement program is funded and guided by AHRQ.
The AHRQ Safety Program for ERAS will support hospitals in implementing perioperative evidence-based protocols to meaningfully improve clinical outcomes, reduce health care utilization, and improve the patient experience. This program aims to enroll at least 750 hospitals throughout the five-year contract. Hospitals within the U.S., Puerto Rico, and the District of Columbia are eligible to participate across five service lines: colorectal, orthopaedic, bariatric, gynecology, and emergency general surgery.
Participating hospitals will have access to the international leaders in ERAS, including representatives of surgery, anesthesiology, and nursing; prototype ERAS protocols developed for five procedures based on up-to-date evidence review; literature to support protocols; tools and educational materials to facilitate implementation; quality improvement specialist support; and coaching calls to support hospital work.
Program enrollment will begin in spring 2017. For more information, contact [email protected].
The American College of Surgeons (ACS), in collaboration with the Johns Hopkins Medicine Armstrong Institute for Patient Safety and Quality, Baltimore, MD, has launched the AHRQ (Agency for Healthcare Research and Quality) Safety Program for Enhanced Recovery after Surgery (ERAS). This new surgical quality improvement program is funded and guided by AHRQ.
The AHRQ Safety Program for ERAS will support hospitals in implementing perioperative evidence-based protocols to meaningfully improve clinical outcomes, reduce health care utilization, and improve the patient experience. This program aims to enroll at least 750 hospitals throughout the five-year contract. Hospitals within the U.S., Puerto Rico, and the District of Columbia are eligible to participate across five service lines: colorectal, orthopaedic, bariatric, gynecology, and emergency general surgery.
Participating hospitals will have access to the international leaders in ERAS, including representatives of surgery, anesthesiology, and nursing; prototype ERAS protocols developed for five procedures based on up-to-date evidence review; literature to support protocols; tools and educational materials to facilitate implementation; quality improvement specialist support; and coaching calls to support hospital work.
Program enrollment will begin in spring 2017. For more information, contact [email protected].
Surgical History Group student and resident papers now available online
The Surgical History Group (SHG) of the American College of Surgeons (ACS) has unveiled the inaugural issue of the Bulletin of the Surgical History Group: Papers from the 2016 Poster Competition. This publication is a compendium of articles based on abstracts that medical students and residents submitted for the SHG’s poster competition at Clinical Congress 2016 in Washington, DC.
The SHG sponsors a poster competition at the annual Clinical Congress, featuring the scholarly work of students and residents. Submissions cover a range of surgical history topics.
More than 40 abstracts were submitted for presentation at Clinical Congress 2016 in Washington, DC. A panel of judges led by J. Patrick O’Leary, MD, FACS, and Patrick Greiffenstein, MD, FACS, Chair and Co-Chair, respectively, of the Poster Competition Committee, selected 21 posters for the program and chose two posters among the high-quality entries for top prizes. The SHG decided that the students’ and residents’ scholarship deserved wider distribution in a more permanent format than posters. All participants were invited to submit their work in written form for this collection of articles available for the study and enjoyment of both Fellows and the public interested in the history of surgery.
The e-bulletin can be accessed on the SHG website at facs.org/about-acs/archives/shg.
The Surgical History Group (SHG) of the American College of Surgeons (ACS) has unveiled the inaugural issue of the Bulletin of the Surgical History Group: Papers from the 2016 Poster Competition. This publication is a compendium of articles based on abstracts that medical students and residents submitted for the SHG’s poster competition at Clinical Congress 2016 in Washington, DC.
The SHG sponsors a poster competition at the annual Clinical Congress, featuring the scholarly work of students and residents. Submissions cover a range of surgical history topics.
More than 40 abstracts were submitted for presentation at Clinical Congress 2016 in Washington, DC. A panel of judges led by J. Patrick O’Leary, MD, FACS, and Patrick Greiffenstein, MD, FACS, Chair and Co-Chair, respectively, of the Poster Competition Committee, selected 21 posters for the program and chose two posters among the high-quality entries for top prizes. The SHG decided that the students’ and residents’ scholarship deserved wider distribution in a more permanent format than posters. All participants were invited to submit their work in written form for this collection of articles available for the study and enjoyment of both Fellows and the public interested in the history of surgery.
The e-bulletin can be accessed on the SHG website at facs.org/about-acs/archives/shg.
The Surgical History Group (SHG) of the American College of Surgeons (ACS) has unveiled the inaugural issue of the Bulletin of the Surgical History Group: Papers from the 2016 Poster Competition. This publication is a compendium of articles based on abstracts that medical students and residents submitted for the SHG’s poster competition at Clinical Congress 2016 in Washington, DC.
The SHG sponsors a poster competition at the annual Clinical Congress, featuring the scholarly work of students and residents. Submissions cover a range of surgical history topics.
More than 40 abstracts were submitted for presentation at Clinical Congress 2016 in Washington, DC. A panel of judges led by J. Patrick O’Leary, MD, FACS, and Patrick Greiffenstein, MD, FACS, Chair and Co-Chair, respectively, of the Poster Competition Committee, selected 21 posters for the program and chose two posters among the high-quality entries for top prizes. The SHG decided that the students’ and residents’ scholarship deserved wider distribution in a more permanent format than posters. All participants were invited to submit their work in written form for this collection of articles available for the study and enjoyment of both Fellows and the public interested in the history of surgery.
The e-bulletin can be accessed on the SHG website at facs.org/about-acs/archives/shg.
ACS: Ensuring the integrity of the profession and the quality of patient care
With a new administration and Congress in place, as well as many exciting internal changes, the American College of Surgeons (ACS) is looking forward to an exciting year ahead.
Quality improvement
Making certain that surgeons have the tools they need to measure and evaluate their performance is a key mission of the College. To this end, we have initiated the database integration system, which will bring together under a single platform the ACS National Surgical Quality Improvement Program (ACS NSQIP®), National Cancer Database, National Trauma Data Bank, and Surgeon Specific Registry (SSRTM). This project, which is being implemented incrementally, will make it easier for surgeons to meet American Board of Surgery (ABS) Maintenance of Certification requirements and Medicare payment mandates.
Furthermore, the College intends to publish an ACS quality manual this year. This comprehensive guidebook will outline strategies and resources needed to ensure the delivery of optimal surgical care.
Advocacy
The Medicare Access and CHIP Reauthorization Act (MACRA), enacted in 2015, repealed the flawed sustainable growth rate (SGR) methodology that was used for many years to calculate Medicare physician reimbursement. Replacing the SGR is the Quality Payment Program (QPP), which advances a longstanding policy goal of basing payment on value rather than on volume.
Surgeons can participate in the QPP through one of two pathways—the Merit-Based Incentive Payment System (MIPS) or the Advanced Alternative Payment Models (APMs). MIPS is the default QPP pathway most physicians will use initially. The College has taken a number of steps to ensure that surgeons are able to comply with MIPS’ reporting requirements and performance measures. For example, we have created an online Resource Center (https://www.facs.org/advocacy/qpp) for surgeons seeking information about the QPP. In addition, we are working with health policy experts at Brandeis University, Waltham, MA, and Brigham and Women’s Hospital, Boston, to propose surgical APMs.
We anticipate that the new presidential administration and Republican-controlled Congress will leave QPP untouched—at least for a while. However, we also speculate that they will attempt to repeal at least some provisions in the Affordable Care Act (ACA). The ACS intends to play an active role in what is certain to be a highly charged debate—just as we did when the ACA was under consideration. As we enter this discussion, we will advocate for the policies that we believe will have the greatest benefit toward ensuring that all surgical patients have access to necessary services. We will not be swayed by politics but rather, will promote our enduring principles for health care reform: quality improvement and patient safety, patient access to surgical care, reduction of health care costs, and medical liability reform.
Education
Surgical education and training have been at the heart of the College’s mission since the organization’s inception. We believe the ACS’ education and training programs are the cornerstones of excellence, transform possibilities into realities, and instill the joy of lifelong learning.
Of particular concern in recent years have been reports that a significant percentage of general surgeon residency graduates leave training feeling insecure about their ability to perform advanced procedures and to manage a practice. In response, the College launched the Transition to Practice in General Surgery program, which provides opportunities for recently graduated residents to engage in a period of mentored practice.
In addition, the College has been working with other stakeholders, including the ABS, the Accreditation Council for Graduate Medical Education, the Association of Program Directors in Surgery, and the Residency Review Committee for Surgery, to develop a road map to secure the future of general surgery. Concepts discussed in these meetings include the following:
- Medical student boot camps
- Further training after five years of general surgery residency
- Modification to duty-hour requirements
- Competency-based education and skills assessment
- Guidelines for self-assessment during residency
- A faculty development requirement
- Career-long record keeping, starting in medical school
Today’s residents are tomorrow’s surgeons. Given the aging population that will be seeking their services, it is imperative that the House of Surgery take responsibility for ensuring that graduates of general surgery training programs have the full range of skills and the confidence necessary to care for these vulnerable patients.
Member services and communication
Our Member Services area continues to develop programs that are designed to encourage surgeon engagement and well-being.
The 2017 Leadership & Advocacy Summit, May 6-9 in Washington, DC, will address such topics as team building, managing critical situations, burnout, common mistakes in leadership, and domestic volunteerism. During the advocacy portion of the meeting, members will have opportunities to flex their leadership muscle and advocate on their patients’ behalf.
The College recognizes that surgeons need to be mentally, emotionally, and physically healthy to provide optimal care to their patients. With this thought in mind, the ACS invites all active, dues-paying members to use the Physician Well-Being Index. This validated screening tool provides an opportunity for surgeons to better understand their overall well-being and identify areas of risk compared to physicians across the nation. Access to local and national resources will also be targeted to surgeons based on their results. Access the ACS Surgeon Well-Being page to learn more about the tool.
Furthermore, the College has continued to make its communications vehicles more interactive and user-friendly. The ACS Communities are thriving, allowing members to share their common concerns and interests in a protected environment. We also are working to have all of our major publications, including the Bulletin and the Journal of the American College of Surgeons, moved to fully digital platforms.
I am proud of the strides the College is making and look forward to an exciting and productive year. As always, please let us know how we can better serve you and your patients.
Dr. Hoyt is the Executive Director of the American College of Surgeons.
With a new administration and Congress in place, as well as many exciting internal changes, the American College of Surgeons (ACS) is looking forward to an exciting year ahead.
Quality improvement
Making certain that surgeons have the tools they need to measure and evaluate their performance is a key mission of the College. To this end, we have initiated the database integration system, which will bring together under a single platform the ACS National Surgical Quality Improvement Program (ACS NSQIP®), National Cancer Database, National Trauma Data Bank, and Surgeon Specific Registry (SSRTM). This project, which is being implemented incrementally, will make it easier for surgeons to meet American Board of Surgery (ABS) Maintenance of Certification requirements and Medicare payment mandates.
Furthermore, the College intends to publish an ACS quality manual this year. This comprehensive guidebook will outline strategies and resources needed to ensure the delivery of optimal surgical care.
Advocacy
The Medicare Access and CHIP Reauthorization Act (MACRA), enacted in 2015, repealed the flawed sustainable growth rate (SGR) methodology that was used for many years to calculate Medicare physician reimbursement. Replacing the SGR is the Quality Payment Program (QPP), which advances a longstanding policy goal of basing payment on value rather than on volume.
Surgeons can participate in the QPP through one of two pathways—the Merit-Based Incentive Payment System (MIPS) or the Advanced Alternative Payment Models (APMs). MIPS is the default QPP pathway most physicians will use initially. The College has taken a number of steps to ensure that surgeons are able to comply with MIPS’ reporting requirements and performance measures. For example, we have created an online Resource Center (https://www.facs.org/advocacy/qpp) for surgeons seeking information about the QPP. In addition, we are working with health policy experts at Brandeis University, Waltham, MA, and Brigham and Women’s Hospital, Boston, to propose surgical APMs.
We anticipate that the new presidential administration and Republican-controlled Congress will leave QPP untouched—at least for a while. However, we also speculate that they will attempt to repeal at least some provisions in the Affordable Care Act (ACA). The ACS intends to play an active role in what is certain to be a highly charged debate—just as we did when the ACA was under consideration. As we enter this discussion, we will advocate for the policies that we believe will have the greatest benefit toward ensuring that all surgical patients have access to necessary services. We will not be swayed by politics but rather, will promote our enduring principles for health care reform: quality improvement and patient safety, patient access to surgical care, reduction of health care costs, and medical liability reform.
Education
Surgical education and training have been at the heart of the College’s mission since the organization’s inception. We believe the ACS’ education and training programs are the cornerstones of excellence, transform possibilities into realities, and instill the joy of lifelong learning.
Of particular concern in recent years have been reports that a significant percentage of general surgeon residency graduates leave training feeling insecure about their ability to perform advanced procedures and to manage a practice. In response, the College launched the Transition to Practice in General Surgery program, which provides opportunities for recently graduated residents to engage in a period of mentored practice.
In addition, the College has been working with other stakeholders, including the ABS, the Accreditation Council for Graduate Medical Education, the Association of Program Directors in Surgery, and the Residency Review Committee for Surgery, to develop a road map to secure the future of general surgery. Concepts discussed in these meetings include the following:
- Medical student boot camps
- Further training after five years of general surgery residency
- Modification to duty-hour requirements
- Competency-based education and skills assessment
- Guidelines for self-assessment during residency
- A faculty development requirement
- Career-long record keeping, starting in medical school
Today’s residents are tomorrow’s surgeons. Given the aging population that will be seeking their services, it is imperative that the House of Surgery take responsibility for ensuring that graduates of general surgery training programs have the full range of skills and the confidence necessary to care for these vulnerable patients.
Member services and communication
Our Member Services area continues to develop programs that are designed to encourage surgeon engagement and well-being.
The 2017 Leadership & Advocacy Summit, May 6-9 in Washington, DC, will address such topics as team building, managing critical situations, burnout, common mistakes in leadership, and domestic volunteerism. During the advocacy portion of the meeting, members will have opportunities to flex their leadership muscle and advocate on their patients’ behalf.
The College recognizes that surgeons need to be mentally, emotionally, and physically healthy to provide optimal care to their patients. With this thought in mind, the ACS invites all active, dues-paying members to use the Physician Well-Being Index. This validated screening tool provides an opportunity for surgeons to better understand their overall well-being and identify areas of risk compared to physicians across the nation. Access to local and national resources will also be targeted to surgeons based on their results. Access the ACS Surgeon Well-Being page to learn more about the tool.
Furthermore, the College has continued to make its communications vehicles more interactive and user-friendly. The ACS Communities are thriving, allowing members to share their common concerns and interests in a protected environment. We also are working to have all of our major publications, including the Bulletin and the Journal of the American College of Surgeons, moved to fully digital platforms.
I am proud of the strides the College is making and look forward to an exciting and productive year. As always, please let us know how we can better serve you and your patients.
Dr. Hoyt is the Executive Director of the American College of Surgeons.
With a new administration and Congress in place, as well as many exciting internal changes, the American College of Surgeons (ACS) is looking forward to an exciting year ahead.
Quality improvement
Making certain that surgeons have the tools they need to measure and evaluate their performance is a key mission of the College. To this end, we have initiated the database integration system, which will bring together under a single platform the ACS National Surgical Quality Improvement Program (ACS NSQIP®), National Cancer Database, National Trauma Data Bank, and Surgeon Specific Registry (SSRTM). This project, which is being implemented incrementally, will make it easier for surgeons to meet American Board of Surgery (ABS) Maintenance of Certification requirements and Medicare payment mandates.
Furthermore, the College intends to publish an ACS quality manual this year. This comprehensive guidebook will outline strategies and resources needed to ensure the delivery of optimal surgical care.
Advocacy
The Medicare Access and CHIP Reauthorization Act (MACRA), enacted in 2015, repealed the flawed sustainable growth rate (SGR) methodology that was used for many years to calculate Medicare physician reimbursement. Replacing the SGR is the Quality Payment Program (QPP), which advances a longstanding policy goal of basing payment on value rather than on volume.
Surgeons can participate in the QPP through one of two pathways—the Merit-Based Incentive Payment System (MIPS) or the Advanced Alternative Payment Models (APMs). MIPS is the default QPP pathway most physicians will use initially. The College has taken a number of steps to ensure that surgeons are able to comply with MIPS’ reporting requirements and performance measures. For example, we have created an online Resource Center (https://www.facs.org/advocacy/qpp) for surgeons seeking information about the QPP. In addition, we are working with health policy experts at Brandeis University, Waltham, MA, and Brigham and Women’s Hospital, Boston, to propose surgical APMs.
We anticipate that the new presidential administration and Republican-controlled Congress will leave QPP untouched—at least for a while. However, we also speculate that they will attempt to repeal at least some provisions in the Affordable Care Act (ACA). The ACS intends to play an active role in what is certain to be a highly charged debate—just as we did when the ACA was under consideration. As we enter this discussion, we will advocate for the policies that we believe will have the greatest benefit toward ensuring that all surgical patients have access to necessary services. We will not be swayed by politics but rather, will promote our enduring principles for health care reform: quality improvement and patient safety, patient access to surgical care, reduction of health care costs, and medical liability reform.
Education
Surgical education and training have been at the heart of the College’s mission since the organization’s inception. We believe the ACS’ education and training programs are the cornerstones of excellence, transform possibilities into realities, and instill the joy of lifelong learning.
Of particular concern in recent years have been reports that a significant percentage of general surgeon residency graduates leave training feeling insecure about their ability to perform advanced procedures and to manage a practice. In response, the College launched the Transition to Practice in General Surgery program, which provides opportunities for recently graduated residents to engage in a period of mentored practice.
In addition, the College has been working with other stakeholders, including the ABS, the Accreditation Council for Graduate Medical Education, the Association of Program Directors in Surgery, and the Residency Review Committee for Surgery, to develop a road map to secure the future of general surgery. Concepts discussed in these meetings include the following:
- Medical student boot camps
- Further training after five years of general surgery residency
- Modification to duty-hour requirements
- Competency-based education and skills assessment
- Guidelines for self-assessment during residency
- A faculty development requirement
- Career-long record keeping, starting in medical school
Today’s residents are tomorrow’s surgeons. Given the aging population that will be seeking their services, it is imperative that the House of Surgery take responsibility for ensuring that graduates of general surgery training programs have the full range of skills and the confidence necessary to care for these vulnerable patients.
Member services and communication
Our Member Services area continues to develop programs that are designed to encourage surgeon engagement and well-being.
The 2017 Leadership & Advocacy Summit, May 6-9 in Washington, DC, will address such topics as team building, managing critical situations, burnout, common mistakes in leadership, and domestic volunteerism. During the advocacy portion of the meeting, members will have opportunities to flex their leadership muscle and advocate on their patients’ behalf.
The College recognizes that surgeons need to be mentally, emotionally, and physically healthy to provide optimal care to their patients. With this thought in mind, the ACS invites all active, dues-paying members to use the Physician Well-Being Index. This validated screening tool provides an opportunity for surgeons to better understand their overall well-being and identify areas of risk compared to physicians across the nation. Access to local and national resources will also be targeted to surgeons based on their results. Access the ACS Surgeon Well-Being page to learn more about the tool.
Furthermore, the College has continued to make its communications vehicles more interactive and user-friendly. The ACS Communities are thriving, allowing members to share their common concerns and interests in a protected environment. We also are working to have all of our major publications, including the Bulletin and the Journal of the American College of Surgeons, moved to fully digital platforms.
I am proud of the strides the College is making and look forward to an exciting and productive year. As always, please let us know how we can better serve you and your patients.
Dr. Hoyt is the Executive Director of the American College of Surgeons.
ACS NSQIP conference gets new name, expanded focus
To provide a more comprehensive look at the American College of Surgeons (ACS) quality improvement efforts, the College has announced that the ACS National Surgical Quality Improvement Program (ACS NSQIP®) Annual Conference will now be the 2017 ACS Quality and Safety Conference. The meeting will take place July 21-24 at the New York Hilton Midtown, NY.
The annual ACS NSQIP conference has grown rapidly in recent years – the 2016 conference in San Diego, CA, drew nearly 1,500 surgeon champions, surgical clinical reviewers (SCRs), and other quality improvement professionals. The ACS Quality and Safety Conference will build on that success, featuring leaders in surgery as speakers and various presentations focused on ACS NSQIP, while offering expanded content on the following ACS Quality Programs:
• ACS NSQIP Pediatric
• Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program
• Children’s Surgery Verification™ Quality Improvement Program
• Surgeon Specific Registry
Achieving quality
The theme of the expanded conference, Achieving Quality: Present and Future, will serve as the basis of the meeting’s proceedings. To achieve the goal of improving quality, presenters and organizers are striving to accomplish the following objectives:
Provide a forum to share the most up-to-date knowledge pertaining to local, national, and international quality improvement initiatives in surgery.
Present methods used to analyze clinical registry data and demonstrate practical ways to use the data.
Assist hospitals in analyzing, managing, and interpreting data by providing education on proven methods that will empower hospitals to make a positive impact on patient care.
Enhance the learning experience by offering breakout sessions that educate attendees on topic areas of interest to them, with consideration of their level of experience in ACS quality improvement programs.
Conference highlights
In addition to talks from surgical leaders, the 2017 ACS Quality and Safety Conference will offer other notable events.
Keynote speaker Blake Haxton, a member of the 2016 U.S. Paralympic Team in rowing, will share his unique insight with attendees. Mr. Haxton contracted necrotizing fasciitis in March 2009, in his senior year of high school. The infection led to heart, lung, kidney, and liver failure, as well as the loss of both legs; however, after an intensive rehabilitation regimen, he was able to attend and graduate from college and law school.
Another conference highlight will be abstract competitions in four categories: Medical Student and Surgical Resident Abstract Competition, SCR Abstract Competition, Clinical Abstract Competition, and Abstract Poster Competition.
Clifford Y. Ko, MD, MS, MSHS, FACS, FASCRS, Director, ACS Division of Research and Optimal Patient Care, which oversees all ACS Quality Programs, is enthusiastic about the newly expanded ACS Quality and Safety Conference. “We are excited to have multiple quality programs of the American College of Surgeons coming together for this conference so that we can all learn how to get better, become more efficient, and provide high-value care in all types of settings,” Dr. Ko said. “This is the first time we’ve put together a conference like this, which we hope will be the first of many.”
More information about the 2017 ACS Quality and Safety Conference can be found at facs.org/quality-programs/quality-safety-conference.
To provide a more comprehensive look at the American College of Surgeons (ACS) quality improvement efforts, the College has announced that the ACS National Surgical Quality Improvement Program (ACS NSQIP®) Annual Conference will now be the 2017 ACS Quality and Safety Conference. The meeting will take place July 21-24 at the New York Hilton Midtown, NY.
The annual ACS NSQIP conference has grown rapidly in recent years – the 2016 conference in San Diego, CA, drew nearly 1,500 surgeon champions, surgical clinical reviewers (SCRs), and other quality improvement professionals. The ACS Quality and Safety Conference will build on that success, featuring leaders in surgery as speakers and various presentations focused on ACS NSQIP, while offering expanded content on the following ACS Quality Programs:
• ACS NSQIP Pediatric
• Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program
• Children’s Surgery Verification™ Quality Improvement Program
• Surgeon Specific Registry
Achieving quality
The theme of the expanded conference, Achieving Quality: Present and Future, will serve as the basis of the meeting’s proceedings. To achieve the goal of improving quality, presenters and organizers are striving to accomplish the following objectives:
Provide a forum to share the most up-to-date knowledge pertaining to local, national, and international quality improvement initiatives in surgery.
Present methods used to analyze clinical registry data and demonstrate practical ways to use the data.
Assist hospitals in analyzing, managing, and interpreting data by providing education on proven methods that will empower hospitals to make a positive impact on patient care.
Enhance the learning experience by offering breakout sessions that educate attendees on topic areas of interest to them, with consideration of their level of experience in ACS quality improvement programs.
Conference highlights
In addition to talks from surgical leaders, the 2017 ACS Quality and Safety Conference will offer other notable events.
Keynote speaker Blake Haxton, a member of the 2016 U.S. Paralympic Team in rowing, will share his unique insight with attendees. Mr. Haxton contracted necrotizing fasciitis in March 2009, in his senior year of high school. The infection led to heart, lung, kidney, and liver failure, as well as the loss of both legs; however, after an intensive rehabilitation regimen, he was able to attend and graduate from college and law school.
Another conference highlight will be abstract competitions in four categories: Medical Student and Surgical Resident Abstract Competition, SCR Abstract Competition, Clinical Abstract Competition, and Abstract Poster Competition.
Clifford Y. Ko, MD, MS, MSHS, FACS, FASCRS, Director, ACS Division of Research and Optimal Patient Care, which oversees all ACS Quality Programs, is enthusiastic about the newly expanded ACS Quality and Safety Conference. “We are excited to have multiple quality programs of the American College of Surgeons coming together for this conference so that we can all learn how to get better, become more efficient, and provide high-value care in all types of settings,” Dr. Ko said. “This is the first time we’ve put together a conference like this, which we hope will be the first of many.”
More information about the 2017 ACS Quality and Safety Conference can be found at facs.org/quality-programs/quality-safety-conference.
To provide a more comprehensive look at the American College of Surgeons (ACS) quality improvement efforts, the College has announced that the ACS National Surgical Quality Improvement Program (ACS NSQIP®) Annual Conference will now be the 2017 ACS Quality and Safety Conference. The meeting will take place July 21-24 at the New York Hilton Midtown, NY.
The annual ACS NSQIP conference has grown rapidly in recent years – the 2016 conference in San Diego, CA, drew nearly 1,500 surgeon champions, surgical clinical reviewers (SCRs), and other quality improvement professionals. The ACS Quality and Safety Conference will build on that success, featuring leaders in surgery as speakers and various presentations focused on ACS NSQIP, while offering expanded content on the following ACS Quality Programs:
• ACS NSQIP Pediatric
• Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program
• Children’s Surgery Verification™ Quality Improvement Program
• Surgeon Specific Registry
Achieving quality
The theme of the expanded conference, Achieving Quality: Present and Future, will serve as the basis of the meeting’s proceedings. To achieve the goal of improving quality, presenters and organizers are striving to accomplish the following objectives:
Provide a forum to share the most up-to-date knowledge pertaining to local, national, and international quality improvement initiatives in surgery.
Present methods used to analyze clinical registry data and demonstrate practical ways to use the data.
Assist hospitals in analyzing, managing, and interpreting data by providing education on proven methods that will empower hospitals to make a positive impact on patient care.
Enhance the learning experience by offering breakout sessions that educate attendees on topic areas of interest to them, with consideration of their level of experience in ACS quality improvement programs.
Conference highlights
In addition to talks from surgical leaders, the 2017 ACS Quality and Safety Conference will offer other notable events.
Keynote speaker Blake Haxton, a member of the 2016 U.S. Paralympic Team in rowing, will share his unique insight with attendees. Mr. Haxton contracted necrotizing fasciitis in March 2009, in his senior year of high school. The infection led to heart, lung, kidney, and liver failure, as well as the loss of both legs; however, after an intensive rehabilitation regimen, he was able to attend and graduate from college and law school.
Another conference highlight will be abstract competitions in four categories: Medical Student and Surgical Resident Abstract Competition, SCR Abstract Competition, Clinical Abstract Competition, and Abstract Poster Competition.
Clifford Y. Ko, MD, MS, MSHS, FACS, FASCRS, Director, ACS Division of Research and Optimal Patient Care, which oversees all ACS Quality Programs, is enthusiastic about the newly expanded ACS Quality and Safety Conference. “We are excited to have multiple quality programs of the American College of Surgeons coming together for this conference so that we can all learn how to get better, become more efficient, and provide high-value care in all types of settings,” Dr. Ko said. “This is the first time we’ve put together a conference like this, which we hope will be the first of many.”
More information about the 2017 ACS Quality and Safety Conference can be found at facs.org/quality-programs/quality-safety-conference.
Post-election health policy takes center stage at AMA HOD meeting
The American Medical Association (AMA) Interim House of Delegates (HOD) meeting took place November 12–15, 2016, in Orlando, FL. A total of 530 state medical society and specialty society delegates, including the six members of the American College of Surgeons’ (ACS) delegation, debated the policy implications of 32 reports and 101 resolutions. Occurring within a week of the national elections, a central focus of the meeting was the uncertainty about the future of the Affordable Care Act (ACA). On the other hand, the Stop the Bleed® program received an enthusiastic reception.
ACS delegation sponsors Stop the Bleed skills course
AMA meetings provide an opportunity for the ACS delegates to share College initiatives with physician leaders from a breadth of geographic locations, specialties, and career stages. In this spirit, ACS delegates, all of whom are Stop the Bleed instructors, along with Leonard J. Weireter, MD, FACS, Vice-Chair, ACS Committee on Trauma, presented the skills course to 125 practicing physicians, residents, and medical student delegates. Through four half-hour sessions, participants refreshed their hands-on skills in bleeding control and became advocates for bringing the course back to their communities. Course success was recognized before the entire HOD.
Surgical Caucus focuses on mass casualty readiness
The Surgical Caucus sponsored a one-hour educational session, The Hartford Consensus: Strategies to Enhance Survival in Active Shooter and Intentional Mass Casualty Events. Dr. Weireter shared an overview of the Hartford Consensus recommendations for effective response to active shooter and mass casualty events and highlighted the value of the Stop the Bleed course in improving survival of casualties from these events.
Orlando trauma surgeon Michael Cheatham, MD, FACS, gave a synopsis of the Orlando Regional Medical Center response to the Pulse nightclub shooting in June 2016. In addition to conducting relevant readiness drills, he emphasized the importance of including casualty family assistance and post-event hospital staff counseling in mass casualty plans.
At this meeting, the AMA endorsed recommendations from a 2015 call to action by eight health professional organizations and the American Bar Association to reduce the public health consequences of firearm-related injury.
U.S. elections put health care system in spotlight
Five resolutions covering a spectrum of opinions about AMA engagement in ACA reform were discussed. The five proposals were consolidated into one adopted resolution, which calls for the AMA, in collaboration with state and specialty medical societies, to actively discuss the future of health care reform with the new presidential administration and Congress. AMA executive vice-president James Madera, MD, sent a letter to congressional leaders on January 3 emphasizing the AMA’s interest in proposals that “make coverage more affordable, provide greater choice, and increase the number of those insured.”
The ACS delegation focused on the ACS Health Care Reform General Principles, which promote a systems-based approach to health care quality and safety, patient access to surgical care, reduction of health care costs, and medical liability reform.
Maintenance of Certification
General disaffection with Maintenance of Certification (MOC) requirements persists in multiple specialties, with particular concerns related to its use in credentialing and privileging decisions. The HOD adopted a policy that directs the AMA to increase efforts to ensure that MOC does not become a requirement for insurance panel participation, state medical licensure, and medical staff membership (initial and ongoing).
Medical student and resident training
Delegates agreed with a need for formal leadership training during medical school. The AMA now advocates for the creation of leadership programs that emphasize experiential learning of skills necessary to lead inter-professional teams. Delegates also recognized the importance of having training program policies that support residents who are breastfeeding. As a result, the AMA will now work with appropriate professional regulatory organizations to put policies for protected times and locations for breastfeeding into program requirements.
Surgeon management of patients with perioperative pain
A resolution intended to reduce perioperative opioid consumption was introduced, calling for hospitals to adopt practices for perioperative pain management, which include services dedicated to acute pain management. This proposal generated a great deal of concern among surgical and anesthesiology delegates. The HOD appreciated that surgeons are trained to manage the perioperative pain of their patients and may consult for additional services as needed. Thus, existing AMA efforts to promote appropriate clinical use of opioid analgesics were reaffirmed in lieu of the resolution.
Next meeting
The next meeting of the AMA HOD is scheduled for June 10–14 in Chicago. This meeting will be the first since the inauguration of President Donald Trump, and the ACS delegates anticipate that national health care policy will again dominate the discussion. ACS members with suggestions for potential resolutions should forward them to Jon Sutton at [email protected].
ACS Delegation at the AMA HOD
• John H. Armstrong, MD, FACS (Delegation Chair), acute care surgery, Ocala, FL
• Brian J. Gavitt, MD, MPH (also Young Physicians Section delegate), general surgery, Cincinnati, OH
• Jacob Moalem, MD, FACS (also Young Physicians Section delegate), general surgery, Rochester, NY
• Leigh A. Neumayer, MD, FACS, general surgery, Tucson, AZ; Vice-Chair, ACS Board of Regents
• Naveen F. Sangji, MD, general surgery resident, Boston, MA
• Patricia L. Turner, MD, FACS, general surgery, Chicago, IL; Director, ACS Division of Member Services; Chair, AMA Council on Medical Education
Dr. Armstrong is a member of the American College of Surgeons (ACS) Health Policy and Advocacy Group, and Past-Chair, ACS Professional Association political action committee (ACSPA-SurgeonsPAC).
Mr. Sutton is Manager, State Affairs, ACS Division of Advocacy and Health Policy, Washington, DC.
The American Medical Association (AMA) Interim House of Delegates (HOD) meeting took place November 12–15, 2016, in Orlando, FL. A total of 530 state medical society and specialty society delegates, including the six members of the American College of Surgeons’ (ACS) delegation, debated the policy implications of 32 reports and 101 resolutions. Occurring within a week of the national elections, a central focus of the meeting was the uncertainty about the future of the Affordable Care Act (ACA). On the other hand, the Stop the Bleed® program received an enthusiastic reception.
ACS delegation sponsors Stop the Bleed skills course
AMA meetings provide an opportunity for the ACS delegates to share College initiatives with physician leaders from a breadth of geographic locations, specialties, and career stages. In this spirit, ACS delegates, all of whom are Stop the Bleed instructors, along with Leonard J. Weireter, MD, FACS, Vice-Chair, ACS Committee on Trauma, presented the skills course to 125 practicing physicians, residents, and medical student delegates. Through four half-hour sessions, participants refreshed their hands-on skills in bleeding control and became advocates for bringing the course back to their communities. Course success was recognized before the entire HOD.
Surgical Caucus focuses on mass casualty readiness
The Surgical Caucus sponsored a one-hour educational session, The Hartford Consensus: Strategies to Enhance Survival in Active Shooter and Intentional Mass Casualty Events. Dr. Weireter shared an overview of the Hartford Consensus recommendations for effective response to active shooter and mass casualty events and highlighted the value of the Stop the Bleed course in improving survival of casualties from these events.
Orlando trauma surgeon Michael Cheatham, MD, FACS, gave a synopsis of the Orlando Regional Medical Center response to the Pulse nightclub shooting in June 2016. In addition to conducting relevant readiness drills, he emphasized the importance of including casualty family assistance and post-event hospital staff counseling in mass casualty plans.
At this meeting, the AMA endorsed recommendations from a 2015 call to action by eight health professional organizations and the American Bar Association to reduce the public health consequences of firearm-related injury.
U.S. elections put health care system in spotlight
Five resolutions covering a spectrum of opinions about AMA engagement in ACA reform were discussed. The five proposals were consolidated into one adopted resolution, which calls for the AMA, in collaboration with state and specialty medical societies, to actively discuss the future of health care reform with the new presidential administration and Congress. AMA executive vice-president James Madera, MD, sent a letter to congressional leaders on January 3 emphasizing the AMA’s interest in proposals that “make coverage more affordable, provide greater choice, and increase the number of those insured.”
The ACS delegation focused on the ACS Health Care Reform General Principles, which promote a systems-based approach to health care quality and safety, patient access to surgical care, reduction of health care costs, and medical liability reform.
Maintenance of Certification
General disaffection with Maintenance of Certification (MOC) requirements persists in multiple specialties, with particular concerns related to its use in credentialing and privileging decisions. The HOD adopted a policy that directs the AMA to increase efforts to ensure that MOC does not become a requirement for insurance panel participation, state medical licensure, and medical staff membership (initial and ongoing).
Medical student and resident training
Delegates agreed with a need for formal leadership training during medical school. The AMA now advocates for the creation of leadership programs that emphasize experiential learning of skills necessary to lead inter-professional teams. Delegates also recognized the importance of having training program policies that support residents who are breastfeeding. As a result, the AMA will now work with appropriate professional regulatory organizations to put policies for protected times and locations for breastfeeding into program requirements.
Surgeon management of patients with perioperative pain
A resolution intended to reduce perioperative opioid consumption was introduced, calling for hospitals to adopt practices for perioperative pain management, which include services dedicated to acute pain management. This proposal generated a great deal of concern among surgical and anesthesiology delegates. The HOD appreciated that surgeons are trained to manage the perioperative pain of their patients and may consult for additional services as needed. Thus, existing AMA efforts to promote appropriate clinical use of opioid analgesics were reaffirmed in lieu of the resolution.
Next meeting
The next meeting of the AMA HOD is scheduled for June 10–14 in Chicago. This meeting will be the first since the inauguration of President Donald Trump, and the ACS delegates anticipate that national health care policy will again dominate the discussion. ACS members with suggestions for potential resolutions should forward them to Jon Sutton at [email protected].
ACS Delegation at the AMA HOD
• John H. Armstrong, MD, FACS (Delegation Chair), acute care surgery, Ocala, FL
• Brian J. Gavitt, MD, MPH (also Young Physicians Section delegate), general surgery, Cincinnati, OH
• Jacob Moalem, MD, FACS (also Young Physicians Section delegate), general surgery, Rochester, NY
• Leigh A. Neumayer, MD, FACS, general surgery, Tucson, AZ; Vice-Chair, ACS Board of Regents
• Naveen F. Sangji, MD, general surgery resident, Boston, MA
• Patricia L. Turner, MD, FACS, general surgery, Chicago, IL; Director, ACS Division of Member Services; Chair, AMA Council on Medical Education
Dr. Armstrong is a member of the American College of Surgeons (ACS) Health Policy and Advocacy Group, and Past-Chair, ACS Professional Association political action committee (ACSPA-SurgeonsPAC).
Mr. Sutton is Manager, State Affairs, ACS Division of Advocacy and Health Policy, Washington, DC.
The American Medical Association (AMA) Interim House of Delegates (HOD) meeting took place November 12–15, 2016, in Orlando, FL. A total of 530 state medical society and specialty society delegates, including the six members of the American College of Surgeons’ (ACS) delegation, debated the policy implications of 32 reports and 101 resolutions. Occurring within a week of the national elections, a central focus of the meeting was the uncertainty about the future of the Affordable Care Act (ACA). On the other hand, the Stop the Bleed® program received an enthusiastic reception.
ACS delegation sponsors Stop the Bleed skills course
AMA meetings provide an opportunity for the ACS delegates to share College initiatives with physician leaders from a breadth of geographic locations, specialties, and career stages. In this spirit, ACS delegates, all of whom are Stop the Bleed instructors, along with Leonard J. Weireter, MD, FACS, Vice-Chair, ACS Committee on Trauma, presented the skills course to 125 practicing physicians, residents, and medical student delegates. Through four half-hour sessions, participants refreshed their hands-on skills in bleeding control and became advocates for bringing the course back to their communities. Course success was recognized before the entire HOD.
Surgical Caucus focuses on mass casualty readiness
The Surgical Caucus sponsored a one-hour educational session, The Hartford Consensus: Strategies to Enhance Survival in Active Shooter and Intentional Mass Casualty Events. Dr. Weireter shared an overview of the Hartford Consensus recommendations for effective response to active shooter and mass casualty events and highlighted the value of the Stop the Bleed course in improving survival of casualties from these events.
Orlando trauma surgeon Michael Cheatham, MD, FACS, gave a synopsis of the Orlando Regional Medical Center response to the Pulse nightclub shooting in June 2016. In addition to conducting relevant readiness drills, he emphasized the importance of including casualty family assistance and post-event hospital staff counseling in mass casualty plans.
At this meeting, the AMA endorsed recommendations from a 2015 call to action by eight health professional organizations and the American Bar Association to reduce the public health consequences of firearm-related injury.
U.S. elections put health care system in spotlight
Five resolutions covering a spectrum of opinions about AMA engagement in ACA reform were discussed. The five proposals were consolidated into one adopted resolution, which calls for the AMA, in collaboration with state and specialty medical societies, to actively discuss the future of health care reform with the new presidential administration and Congress. AMA executive vice-president James Madera, MD, sent a letter to congressional leaders on January 3 emphasizing the AMA’s interest in proposals that “make coverage more affordable, provide greater choice, and increase the number of those insured.”
The ACS delegation focused on the ACS Health Care Reform General Principles, which promote a systems-based approach to health care quality and safety, patient access to surgical care, reduction of health care costs, and medical liability reform.
Maintenance of Certification
General disaffection with Maintenance of Certification (MOC) requirements persists in multiple specialties, with particular concerns related to its use in credentialing and privileging decisions. The HOD adopted a policy that directs the AMA to increase efforts to ensure that MOC does not become a requirement for insurance panel participation, state medical licensure, and medical staff membership (initial and ongoing).
Medical student and resident training
Delegates agreed with a need for formal leadership training during medical school. The AMA now advocates for the creation of leadership programs that emphasize experiential learning of skills necessary to lead inter-professional teams. Delegates also recognized the importance of having training program policies that support residents who are breastfeeding. As a result, the AMA will now work with appropriate professional regulatory organizations to put policies for protected times and locations for breastfeeding into program requirements.
Surgeon management of patients with perioperative pain
A resolution intended to reduce perioperative opioid consumption was introduced, calling for hospitals to adopt practices for perioperative pain management, which include services dedicated to acute pain management. This proposal generated a great deal of concern among surgical and anesthesiology delegates. The HOD appreciated that surgeons are trained to manage the perioperative pain of their patients and may consult for additional services as needed. Thus, existing AMA efforts to promote appropriate clinical use of opioid analgesics were reaffirmed in lieu of the resolution.
Next meeting
The next meeting of the AMA HOD is scheduled for June 10–14 in Chicago. This meeting will be the first since the inauguration of President Donald Trump, and the ACS delegates anticipate that national health care policy will again dominate the discussion. ACS members with suggestions for potential resolutions should forward them to Jon Sutton at [email protected].
ACS Delegation at the AMA HOD
• John H. Armstrong, MD, FACS (Delegation Chair), acute care surgery, Ocala, FL
• Brian J. Gavitt, MD, MPH (also Young Physicians Section delegate), general surgery, Cincinnati, OH
• Jacob Moalem, MD, FACS (also Young Physicians Section delegate), general surgery, Rochester, NY
• Leigh A. Neumayer, MD, FACS, general surgery, Tucson, AZ; Vice-Chair, ACS Board of Regents
• Naveen F. Sangji, MD, general surgery resident, Boston, MA
• Patricia L. Turner, MD, FACS, general surgery, Chicago, IL; Director, ACS Division of Member Services; Chair, AMA Council on Medical Education
Dr. Armstrong is a member of the American College of Surgeons (ACS) Health Policy and Advocacy Group, and Past-Chair, ACS Professional Association political action committee (ACSPA-SurgeonsPAC).
Mr. Sutton is Manager, State Affairs, ACS Division of Advocacy and Health Policy, Washington, DC.