User login
Endometriosis survey findings show doctors aren’t asking key questions
, according to the findings of a recent survey.
The survey, conducted by HealthyWomen with support from drug-maker AbbVie, highlights some of the knowledge gaps surrounding endometriosis. They surveyed 1,211 adult women in the United States and 352 health care providers, including ob.gyns, primary care physicians, reproductive endocrinologists, gynecologic specialists, and nurse practitioners.
Another symptom that may be going unrecognized, according to the survey, is pelvic pain. Just one-third of the providers surveyed asked patients at each visit if pelvic pain interferes with their daily activities. However, among 260 women who said they had been diagnosed with endometriosis, 86% reported that their condition interferes with daily activities at least some of the time.
The survey also reinforced findings from previous research about delayed diagnosis of endometriosis. Among 260 respondents who identified themselves as diagnosed with endometriosis, 72% reported seeing two or more providers before receiving a diagnosis, and nearly a quarter saw four or more providers.
The survey was conducted online from Dec. 7, 2016, to Feb. 6, 2017.
[email protected]
On Twitter @maryellenny
, according to the findings of a recent survey.
The survey, conducted by HealthyWomen with support from drug-maker AbbVie, highlights some of the knowledge gaps surrounding endometriosis. They surveyed 1,211 adult women in the United States and 352 health care providers, including ob.gyns, primary care physicians, reproductive endocrinologists, gynecologic specialists, and nurse practitioners.
Another symptom that may be going unrecognized, according to the survey, is pelvic pain. Just one-third of the providers surveyed asked patients at each visit if pelvic pain interferes with their daily activities. However, among 260 women who said they had been diagnosed with endometriosis, 86% reported that their condition interferes with daily activities at least some of the time.
The survey also reinforced findings from previous research about delayed diagnosis of endometriosis. Among 260 respondents who identified themselves as diagnosed with endometriosis, 72% reported seeing two or more providers before receiving a diagnosis, and nearly a quarter saw four or more providers.
The survey was conducted online from Dec. 7, 2016, to Feb. 6, 2017.
[email protected]
On Twitter @maryellenny
, according to the findings of a recent survey.
The survey, conducted by HealthyWomen with support from drug-maker AbbVie, highlights some of the knowledge gaps surrounding endometriosis. They surveyed 1,211 adult women in the United States and 352 health care providers, including ob.gyns, primary care physicians, reproductive endocrinologists, gynecologic specialists, and nurse practitioners.
Another symptom that may be going unrecognized, according to the survey, is pelvic pain. Just one-third of the providers surveyed asked patients at each visit if pelvic pain interferes with their daily activities. However, among 260 women who said they had been diagnosed with endometriosis, 86% reported that their condition interferes with daily activities at least some of the time.
The survey also reinforced findings from previous research about delayed diagnosis of endometriosis. Among 260 respondents who identified themselves as diagnosed with endometriosis, 72% reported seeing two or more providers before receiving a diagnosis, and nearly a quarter saw four or more providers.
The survey was conducted online from Dec. 7, 2016, to Feb. 6, 2017.
[email protected]
On Twitter @maryellenny
Genetic variant linked to more antibiotics, corticosteroid use in COPD
A genetic variant associated with a poorer therapeutic response in patients with asthma may also be linked to more severe chronic obstructive pulmonary disease, researchers have found.
The polymorphisms at codons 16 and 27 of the beta-2-adrenoreceptor (ADRB2) gene are responsible for enhanced down-regulation of the beta-2-adrenoreceptor, and research suggests that Arg/Arg homozygosity at position 16 is associated with worse control of disease in patients with bronchial asthma.
However, the results of studies exploring the impact of this variant “on the clinical response to the administration of the beta-2-adrenoreceptor agonists in COPD patients are “parse and inconclusive,” according to Justyna Emeryk-Maksymiuk and colleagues at the Medical University of Lublin in Poland.
In a study published in the April issue of Pulmonary Pharmacology & Therapeutics, the researchers looked for variants of the ADRB2 gene in blood samples taken from 92 patients with stable grade COPD.
They collected data on each patient’s disease course during the previous 12 months, including the frequency of exacerbations requiring hospitalization, and antibiotic and systemic corticosteroid use.
They found significant differences between patients with either the Arg/Arg (n = 18), Arg/Gly (n = 61) and Gly/Gly (n = 13) polymorphism at codon 16 of the ADRB2 gene (Pulm Pharmacol Ther. 2017. doi: 10.1016/j.pupt.2017.01.005).
Those who were Arg/Arg homozygotes were significantly more likely to require two or more courses of antibiotic therapy: 33% of this group required two courses of antibiotics compared to 16.4% of those with the Arg/Gly polymorphism and none of those with the Gly/Gly polymorphism.
Those with the Arg/Arg polymorphism also required significantly more corticosteroid therapy; 16.7% needed three or more courses of systemic corticosteroid therapy, compared to none of the patients with the other polymorphisms.
However there were no significant differences between the three groups in the number of hospitalizations over the prior 12 months.
The researchers did not see any significant effects on hospitalizations, courses of corticosteroids or antibiotics from polymorphisms at codon 27 of the ADRB2 gene.
“The majority of researchers focus on the bronchodilator effect brought by the activation of the beta-2-adrenoreceptors, with less emphasis on the facts that these receptors are also involved in the inhibition of mast cell degranulation, chemotaxis, adhesion and activation of leukocytes, as well as in the improvement of mucociliary clearance of respiratory epithelium,” the authors wrote.
“The results of these studies confirmed that the Arg/Arg genotype at codon 16 predisposes patients to clinically more severe manifestation of obstructive respiratory disorders.”
The authors noted that the differences in the effect of genetic polymorphisms in the ADRB2 gene could also be the result of differences in the use of inhaled glucocorticoids, as these can prevent the desensitization of the beta-2-adrenoreceptor.
Previous research has found that nonusage of inhaled glucocorticoids in asthma patients with the Arg/Arg phenotype is associated with a twofold greater odds of uncontrolled asthma, when compared with patients with the Gly/Gly phenotype.
While patients with asthma are recommended to have inhaled glucocorticoids in conjunction with beta-2-mimetics, a considerable fraction of patients with COPD would not be administered glucocorticoids.
“Therefore, it cannot be excluded that a more severe course of asthma and COPD in patients with [the] Arg/Arg genotype of [the] ADRB2 gene at codon 16 does not result solely from the polymorphism itself, but also from the lack of [inhaled glucocorticoids].”
The Ministry of Science and Education supported the study. No conflicts of interest were declared.
A genetic variant associated with a poorer therapeutic response in patients with asthma may also be linked to more severe chronic obstructive pulmonary disease, researchers have found.
The polymorphisms at codons 16 and 27 of the beta-2-adrenoreceptor (ADRB2) gene are responsible for enhanced down-regulation of the beta-2-adrenoreceptor, and research suggests that Arg/Arg homozygosity at position 16 is associated with worse control of disease in patients with bronchial asthma.
However, the results of studies exploring the impact of this variant “on the clinical response to the administration of the beta-2-adrenoreceptor agonists in COPD patients are “parse and inconclusive,” according to Justyna Emeryk-Maksymiuk and colleagues at the Medical University of Lublin in Poland.
In a study published in the April issue of Pulmonary Pharmacology & Therapeutics, the researchers looked for variants of the ADRB2 gene in blood samples taken from 92 patients with stable grade COPD.
They collected data on each patient’s disease course during the previous 12 months, including the frequency of exacerbations requiring hospitalization, and antibiotic and systemic corticosteroid use.
They found significant differences between patients with either the Arg/Arg (n = 18), Arg/Gly (n = 61) and Gly/Gly (n = 13) polymorphism at codon 16 of the ADRB2 gene (Pulm Pharmacol Ther. 2017. doi: 10.1016/j.pupt.2017.01.005).
Those who were Arg/Arg homozygotes were significantly more likely to require two or more courses of antibiotic therapy: 33% of this group required two courses of antibiotics compared to 16.4% of those with the Arg/Gly polymorphism and none of those with the Gly/Gly polymorphism.
Those with the Arg/Arg polymorphism also required significantly more corticosteroid therapy; 16.7% needed three or more courses of systemic corticosteroid therapy, compared to none of the patients with the other polymorphisms.
However there were no significant differences between the three groups in the number of hospitalizations over the prior 12 months.
The researchers did not see any significant effects on hospitalizations, courses of corticosteroids or antibiotics from polymorphisms at codon 27 of the ADRB2 gene.
“The majority of researchers focus on the bronchodilator effect brought by the activation of the beta-2-adrenoreceptors, with less emphasis on the facts that these receptors are also involved in the inhibition of mast cell degranulation, chemotaxis, adhesion and activation of leukocytes, as well as in the improvement of mucociliary clearance of respiratory epithelium,” the authors wrote.
“The results of these studies confirmed that the Arg/Arg genotype at codon 16 predisposes patients to clinically more severe manifestation of obstructive respiratory disorders.”
The authors noted that the differences in the effect of genetic polymorphisms in the ADRB2 gene could also be the result of differences in the use of inhaled glucocorticoids, as these can prevent the desensitization of the beta-2-adrenoreceptor.
Previous research has found that nonusage of inhaled glucocorticoids in asthma patients with the Arg/Arg phenotype is associated with a twofold greater odds of uncontrolled asthma, when compared with patients with the Gly/Gly phenotype.
While patients with asthma are recommended to have inhaled glucocorticoids in conjunction with beta-2-mimetics, a considerable fraction of patients with COPD would not be administered glucocorticoids.
“Therefore, it cannot be excluded that a more severe course of asthma and COPD in patients with [the] Arg/Arg genotype of [the] ADRB2 gene at codon 16 does not result solely from the polymorphism itself, but also from the lack of [inhaled glucocorticoids].”
The Ministry of Science and Education supported the study. No conflicts of interest were declared.
A genetic variant associated with a poorer therapeutic response in patients with asthma may also be linked to more severe chronic obstructive pulmonary disease, researchers have found.
The polymorphisms at codons 16 and 27 of the beta-2-adrenoreceptor (ADRB2) gene are responsible for enhanced down-regulation of the beta-2-adrenoreceptor, and research suggests that Arg/Arg homozygosity at position 16 is associated with worse control of disease in patients with bronchial asthma.
However, the results of studies exploring the impact of this variant “on the clinical response to the administration of the beta-2-adrenoreceptor agonists in COPD patients are “parse and inconclusive,” according to Justyna Emeryk-Maksymiuk and colleagues at the Medical University of Lublin in Poland.
In a study published in the April issue of Pulmonary Pharmacology & Therapeutics, the researchers looked for variants of the ADRB2 gene in blood samples taken from 92 patients with stable grade COPD.
They collected data on each patient’s disease course during the previous 12 months, including the frequency of exacerbations requiring hospitalization, and antibiotic and systemic corticosteroid use.
They found significant differences between patients with either the Arg/Arg (n = 18), Arg/Gly (n = 61) and Gly/Gly (n = 13) polymorphism at codon 16 of the ADRB2 gene (Pulm Pharmacol Ther. 2017. doi: 10.1016/j.pupt.2017.01.005).
Those who were Arg/Arg homozygotes were significantly more likely to require two or more courses of antibiotic therapy: 33% of this group required two courses of antibiotics compared to 16.4% of those with the Arg/Gly polymorphism and none of those with the Gly/Gly polymorphism.
Those with the Arg/Arg polymorphism also required significantly more corticosteroid therapy; 16.7% needed three or more courses of systemic corticosteroid therapy, compared to none of the patients with the other polymorphisms.
However there were no significant differences between the three groups in the number of hospitalizations over the prior 12 months.
The researchers did not see any significant effects on hospitalizations, courses of corticosteroids or antibiotics from polymorphisms at codon 27 of the ADRB2 gene.
“The majority of researchers focus on the bronchodilator effect brought by the activation of the beta-2-adrenoreceptors, with less emphasis on the facts that these receptors are also involved in the inhibition of mast cell degranulation, chemotaxis, adhesion and activation of leukocytes, as well as in the improvement of mucociliary clearance of respiratory epithelium,” the authors wrote.
“The results of these studies confirmed that the Arg/Arg genotype at codon 16 predisposes patients to clinically more severe manifestation of obstructive respiratory disorders.”
The authors noted that the differences in the effect of genetic polymorphisms in the ADRB2 gene could also be the result of differences in the use of inhaled glucocorticoids, as these can prevent the desensitization of the beta-2-adrenoreceptor.
Previous research has found that nonusage of inhaled glucocorticoids in asthma patients with the Arg/Arg phenotype is associated with a twofold greater odds of uncontrolled asthma, when compared with patients with the Gly/Gly phenotype.
While patients with asthma are recommended to have inhaled glucocorticoids in conjunction with beta-2-mimetics, a considerable fraction of patients with COPD would not be administered glucocorticoids.
“Therefore, it cannot be excluded that a more severe course of asthma and COPD in patients with [the] Arg/Arg genotype of [the] ADRB2 gene at codon 16 does not result solely from the polymorphism itself, but also from the lack of [inhaled glucocorticoids].”
The Ministry of Science and Education supported the study. No conflicts of interest were declared.
FROM PULMONARY PHARMACOLOGY & THERAPEUTICS
Key clinical point: A genetic variation linked to poorer disease control in asthma is associated with a similar effect in patients with chronic obstructive pulmonary disease.
Major finding: Patients with COPD who are Arg/Arg homozygotes at codon 16 of the beta-2-adrenoreceptor gene were significantly more likely to require two or more courses of antibiotic therapy and more systemic corticosteroid therapy than were patients with other polymorphisms.
Data source: A retrospective cohort study of 92 patients with stable grade COPD.
Disclosures: The Ministry of Science and Education supported the study. No conflicts of interest were declared.
Contract negotiations
As more and more physicians, both young and experienced, choose to merge with larger practices or join multispecialty groups, I am getting numerous questions about the contracts they are being asked to sign. Obviously, every circumstance will be unique; but some common issues and avoidable mistakes are worth mentioning.
The most common error I see is failing to retain an attorney in a timely manner. Incredibly, many physicians try to do their own negotiating, and call a lawyer only when unpleasant discoveries are made after the contract has been signed. You will need counsel from the very beginning – and not your brother-in-law, or a family friend. Get referrals from colleagues who have gone through the process and are happy with their contracts.
Ambiguous provisions that could later become the subjects of dispute should be kept to a minimum. One example I have seen more than once is, “physician shall share call duties.” Don’t rely on the employer to be fair and reasonable with the call schedule. Get specific language that does not hinge on factors outside your control, such as the health or diligence of other physicians in the practice.
The conditions of your employment may also be inadequately defined. Office hours, administrative duties, medical record responsibilities, and access to specialized equipment and support staff are all negotiable, and should be clearly delineated, preferably prior to any discussion of compensation.
Other provisions may be defined, but not the way you might define them. When a contract puts a specific definition on a specific term, it will highlight the term in italics or boldface, then define it in the “Definitions” section. Read that section carefully! In court, the term will mean what the contract says it means, not what you may think it means. For example, if you can be terminated for “professional misconduct,” make sure you know how the agreement defines that transgression. Look carefully at any other termination provisions as well; make sure they are fair, reasonable, and well defined. Vague conditions such as “conduct detrimental to the practice” should be clarified.
When you discuss compensation, pay close attention to fringe benefits, such as vacation and sick leave, dues, allowances, profit sharing and retirement plans, and various insurances. Most are open to negotiation, even if the employers do not volunteer that they are. An experienced contract lawyer may also propose additional benefits that aren’t listed, and that you may not have thought of.
Incentive provisions require particularly close scrutiny. Beware of bonus triggers that an unscrupulous employer could manipulate against your interests. I’ve seen contracts that award a percentage of net income as a bonus; net income is subjective, and easy to manipulate. Owners can pay themselves a higher salary and drive down the practice’s net income. Such bonuses should be based on gross income numbers, which are more objective and easier to pin down. Incentive plans should protect you as well as your employer.
Be sure to include specific language protecting your rights to outside or additional income, such as lecture honoraria, writing royalties, expert witness testimony, and patent royalties. And carefully consider all of the implications of signing a noncompetition clause. Negotiate the clause cautiously; you won’t want to spend time and money litigating this issue if you leave.
Finally, don’t neglect researching your prospective employer, and colleagues already employed there. A friend of 30 years recently told me that merging his practice with a large conglomerate was “the worst mistake I’ve ever made,” largely because of important promises that were not kept. Due diligence, he now admits, would have revealed that the organization has a long history of promising the world, but failing to deliver. He also discovered – too late – a series of pending government sanctions, malpractice claims, and other litigation that diminish his own previously impeccable reputation, and may well affect his compensation and profit sharing for years.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
As more and more physicians, both young and experienced, choose to merge with larger practices or join multispecialty groups, I am getting numerous questions about the contracts they are being asked to sign. Obviously, every circumstance will be unique; but some common issues and avoidable mistakes are worth mentioning.
The most common error I see is failing to retain an attorney in a timely manner. Incredibly, many physicians try to do their own negotiating, and call a lawyer only when unpleasant discoveries are made after the contract has been signed. You will need counsel from the very beginning – and not your brother-in-law, or a family friend. Get referrals from colleagues who have gone through the process and are happy with their contracts.
Ambiguous provisions that could later become the subjects of dispute should be kept to a minimum. One example I have seen more than once is, “physician shall share call duties.” Don’t rely on the employer to be fair and reasonable with the call schedule. Get specific language that does not hinge on factors outside your control, such as the health or diligence of other physicians in the practice.
The conditions of your employment may also be inadequately defined. Office hours, administrative duties, medical record responsibilities, and access to specialized equipment and support staff are all negotiable, and should be clearly delineated, preferably prior to any discussion of compensation.
Other provisions may be defined, but not the way you might define them. When a contract puts a specific definition on a specific term, it will highlight the term in italics or boldface, then define it in the “Definitions” section. Read that section carefully! In court, the term will mean what the contract says it means, not what you may think it means. For example, if you can be terminated for “professional misconduct,” make sure you know how the agreement defines that transgression. Look carefully at any other termination provisions as well; make sure they are fair, reasonable, and well defined. Vague conditions such as “conduct detrimental to the practice” should be clarified.
When you discuss compensation, pay close attention to fringe benefits, such as vacation and sick leave, dues, allowances, profit sharing and retirement plans, and various insurances. Most are open to negotiation, even if the employers do not volunteer that they are. An experienced contract lawyer may also propose additional benefits that aren’t listed, and that you may not have thought of.
Incentive provisions require particularly close scrutiny. Beware of bonus triggers that an unscrupulous employer could manipulate against your interests. I’ve seen contracts that award a percentage of net income as a bonus; net income is subjective, and easy to manipulate. Owners can pay themselves a higher salary and drive down the practice’s net income. Such bonuses should be based on gross income numbers, which are more objective and easier to pin down. Incentive plans should protect you as well as your employer.
Be sure to include specific language protecting your rights to outside or additional income, such as lecture honoraria, writing royalties, expert witness testimony, and patent royalties. And carefully consider all of the implications of signing a noncompetition clause. Negotiate the clause cautiously; you won’t want to spend time and money litigating this issue if you leave.
Finally, don’t neglect researching your prospective employer, and colleagues already employed there. A friend of 30 years recently told me that merging his practice with a large conglomerate was “the worst mistake I’ve ever made,” largely because of important promises that were not kept. Due diligence, he now admits, would have revealed that the organization has a long history of promising the world, but failing to deliver. He also discovered – too late – a series of pending government sanctions, malpractice claims, and other litigation that diminish his own previously impeccable reputation, and may well affect his compensation and profit sharing for years.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
As more and more physicians, both young and experienced, choose to merge with larger practices or join multispecialty groups, I am getting numerous questions about the contracts they are being asked to sign. Obviously, every circumstance will be unique; but some common issues and avoidable mistakes are worth mentioning.
The most common error I see is failing to retain an attorney in a timely manner. Incredibly, many physicians try to do their own negotiating, and call a lawyer only when unpleasant discoveries are made after the contract has been signed. You will need counsel from the very beginning – and not your brother-in-law, or a family friend. Get referrals from colleagues who have gone through the process and are happy with their contracts.
Ambiguous provisions that could later become the subjects of dispute should be kept to a minimum. One example I have seen more than once is, “physician shall share call duties.” Don’t rely on the employer to be fair and reasonable with the call schedule. Get specific language that does not hinge on factors outside your control, such as the health or diligence of other physicians in the practice.
The conditions of your employment may also be inadequately defined. Office hours, administrative duties, medical record responsibilities, and access to specialized equipment and support staff are all negotiable, and should be clearly delineated, preferably prior to any discussion of compensation.
Other provisions may be defined, but not the way you might define them. When a contract puts a specific definition on a specific term, it will highlight the term in italics or boldface, then define it in the “Definitions” section. Read that section carefully! In court, the term will mean what the contract says it means, not what you may think it means. For example, if you can be terminated for “professional misconduct,” make sure you know how the agreement defines that transgression. Look carefully at any other termination provisions as well; make sure they are fair, reasonable, and well defined. Vague conditions such as “conduct detrimental to the practice” should be clarified.
When you discuss compensation, pay close attention to fringe benefits, such as vacation and sick leave, dues, allowances, profit sharing and retirement plans, and various insurances. Most are open to negotiation, even if the employers do not volunteer that they are. An experienced contract lawyer may also propose additional benefits that aren’t listed, and that you may not have thought of.
Incentive provisions require particularly close scrutiny. Beware of bonus triggers that an unscrupulous employer could manipulate against your interests. I’ve seen contracts that award a percentage of net income as a bonus; net income is subjective, and easy to manipulate. Owners can pay themselves a higher salary and drive down the practice’s net income. Such bonuses should be based on gross income numbers, which are more objective and easier to pin down. Incentive plans should protect you as well as your employer.
Be sure to include specific language protecting your rights to outside or additional income, such as lecture honoraria, writing royalties, expert witness testimony, and patent royalties. And carefully consider all of the implications of signing a noncompetition clause. Negotiate the clause cautiously; you won’t want to spend time and money litigating this issue if you leave.
Finally, don’t neglect researching your prospective employer, and colleagues already employed there. A friend of 30 years recently told me that merging his practice with a large conglomerate was “the worst mistake I’ve ever made,” largely because of important promises that were not kept. Due diligence, he now admits, would have revealed that the organization has a long history of promising the world, but failing to deliver. He also discovered – too late – a series of pending government sanctions, malpractice claims, and other litigation that diminish his own previously impeccable reputation, and may well affect his compensation and profit sharing for years.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Generalized Vaccinia After Smallpox Vaccination With Concomitant Primary Epstein Barr Virus Infection
Generalized vaccinia (GV) is a rare, self-limiting complication of the smallpox vaccination that is caused by the systemic spread of the virus from the inoculation site. The incidence of GV became rare after routine vaccination was discontinued in the U.S. in 1971 and globally in the 1980s after the disease was eradicated.1,2 However in 2002, heightened concerns for the deliberate release of the smallpox virus as a bioweapon led the U.S. military to restart its smallpox vaccination program for soldiers and public health workers.3,4 Here, the authors describe a patient with concomitant GV and mononucleosis.
Case Report
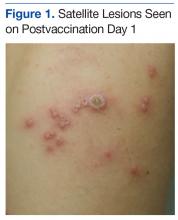
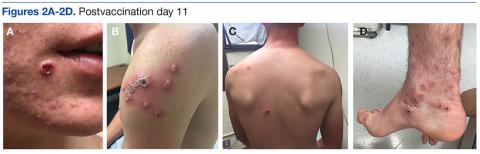
A 19-year-old active-duty marine presented to his battalion aid station with concern for a spreading vesicular rash 9 days after a primary inoculation with the smallpox vaccine. The rash was limited to the inoculation site on his left shoulder (Figure 1). He had no medical history of eczema, atopic dermatitis, or other rashes and reported no systemic symptoms. His vitals also were within normal limits. A clinical diagnosis of inadvertent inoculation (also termed accidental infection) with satellite lesions was made, and he was discharged with counseling on wound care and close follow-up. Two days later, on postvaccination day 11, he presented with new symptoms of a headache, fever, chills, diffuse myalgia, sore throat, and spreading erythematous macules, papules, and vesicles on his arms, chest, abdomen, back, legs, and face (Figures 2A-2D). His vital signs were remarkable for tachycardia with heart rate of 100 bpm and a fever of 103º F (39.4º C). He was sent to the emergency department with a presumed GV diagnosis.
A complete blood count, liver function tests, and basic metabolic panel were unremarkable. Given his symptom of pharyngitis, a rapid strep test was performed. The test was negative, and a throat culture showed no growth. A mononucleosis screen also was performed and was positive. The patient was diagnosed with mononucleosis and GV. His condition improved, and his vital signs stabilized with conservative treatment without the need for vaccine immune globulin (VIG). He convalesced for 72 hours and was referred to dermatology on the following day. Quarantining him in a single occupancy barracks room until all lesions crusted over addressed the concern for spread of the virus to nonimmunized marines or family members.
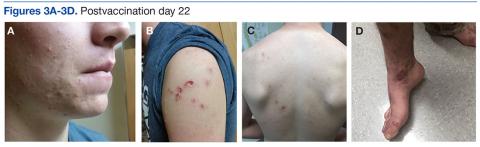
On postvaccination day 12, the patient continued to be clinically well, and he remained afebrile. The dermatologist obtained a skin biopsy from a lesion on the patient’s right shin. The biopsy demonstrated marked epidermal necrosis with peripheral keratinocytes showing ballooning degeneration and viral cytopathic changes consistent with GV. Antibody titers showing high levels of Epstein-Barr virus (EBV) capsid IgM and IgG present confirmed mononucleosis infection within the past 6 months. The patient remained clinically well and was released from quarantine on postvaccination day 22 when all lesions crusted over (Figures 3A-3D).
Discussion
The CDC current definition for GV is “the spread of lesions to other parts of the body that are benign in appearance and occur as a result of viremia.”5 Although the exact mechanisms of viral spread are unknown, it may be due to a subtle immunologic defect, specifically in the B-cell line.6,7 Epstein-Barr virus affects the B-cell line, and concurrent infection may depress humoral immunity and allow for systemic spread of the virus.8,9
This case illustrates the potential for a severe reaction after smallpox vaccination in a patient with a concomitant EBV infection. Service members primarily receive the smallpox vaccination early in their career when the risk of mononucleosis is at its highest incidence among young adults, 11 to 48 per 1,000.10-13 Although the potential for disseminated vaccinia following vaccination is rare, clinicians need to remain cognizant of the risk, which may be enhanced by recent or subsequent infection with EBV. However, regular screening for EBV would be of questionable value given the large number of tests needed to prevent a single case of GV.
Generalized vaccinia is a rare complication after smallpox vaccination. Despite its dire appearance, GV typically resolves spontaneously with limited adverse effects (AEs).14 The pre-eradication reported incidence was 17.7 per 1,000,000 recipients in a national survey.15 Posteradication the incidence of GV was 3 times as high with 2 reported cases in 2003 after administration of 38,440 vaccinations.16 Inflammatory reactions can be common; however, these reactions are not due to systemic viral spread.5 When dealing with a vaccinia-specific AE, it is important to distinguish the benign inadvertent inoculations and GV from the more serious reactions of eczema vaccinatum (EV) or progressive vaccinia (PV). 5
Inadvertent inoculations and GV are usually benign and self-limited—requiring only prevention of secondary transmission and nosocomial infection. Eczema vaccinatum occurs among persons with atopic dermatitis or eczema.5 The
Conclusion
The smallpox vaccination is unique among vaccinations. It is the only vaccine that is administered via inoculation with a bifurcated needle, requires regular follow-up care, and can be spread to casual contacts.5
It is important for any practitioner administering the smallpox vaccine to be aware of associated AEs. A greater knowledge of the unique challenges with the smallpox vaccine allows for better patient selection that eliminates those with conditions that impair their immune system and improves patient education.
1. Centers for Disease Control and prevention. Public Health Service recommendation on smallpox vaccination. MMWR Recomm Rep. 1971;20:339
2. The global eradication of smallpox. World Health Organization Web site. http://apps.who.int/iris/bitstream/10665/39253/1/a41438.pdf. Accessed February 8, 2017.
3. Belongia EA, Naleway A. Smallpox vaccine: the good, the bad and the ugly. Clin Med Res. 2003;1(2):87-92.
4. Wharton M, Strikas RA, Harpaz R, et al. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52(RR-7):1-16.
5. Cono J, Casey CG, Bell DM. Smallpox vaccination and adverse reactions. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Updated February 10, 2003. Accessed February 2, 2017.
6. Chahroudi A, Chavan R, Kozyr N, Waller EK, Silvestri G, Feinberg MB. Vaccinia virus tropism for primary hematolymphoid cells is determined by restricted expression of a unique virus receptor. J Virol. 2005;79(16):10397-10407.
7. , , Blasco R. of different leukocyte cell types to Vaccinia virus infection. Virol J. 2004;1(1):10.
8. Küppers R. B cells under influence: transformation of B cells by Epstein-Barr virus. Nat Rev Immunol. 2003;3(10):801-812.
9. Nemerow G, Cooper N. Infection of B lymphocytes by a human herpesvirus, Epstein-Barr virus, is blocked by calmodulin antagonists. Proc Natl Acad Sci U S A. 1984;81(15):4955-4959.
10. Hallee TJ, Evans AS, Niederman JC, Brooks CM, Voegtly JH. Infectious Mononucleosis at the United States Military Academy. A prospective study of a single class over four years. Yale J Biol Med. 1974;47(3):182-195.
11. Evans AS, Robinton ED. An epidemiological study of infectious mononucleosis. N Engl J Med. 1950;242:492-496.
12. Niederman JC, Evans AS, Subrahmanyan L, McCollum RW. Prevalence, incidence and persistence of EB virus antibody in young adults. N Engl J Med. 1970;282(7):361-365.
13. Sawyer RN, Evans AS, Niederman JC, McCollum RW. Prospective studies of a group of Yale University freshmen. I. Occurrence of infectious mononucleosis. J Infect Dis. 1971;123(3):263-270.
14. Henderson DA, Borio LL, Lane MJ. Smallpox and vaccinia. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 4th ed. Philadelphia, PA: Elsevier; 2004:123-153.
15. Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968—national surveillance in the United States. N Engl J Med. 1969;281(22):1201-1208.
16. Vellozzi C, Lane JM, Averhoff F, et al. Generalized vaccinia, progressive vaccinia and eczema vaccinatum are rare following smallpox (vaccinia) vaccination: United States surveillance, 2003. Clin Infect Dis. 2005;41(5):689-697.
17. Reed J, Scott D. Bray M. Eczema Vaccinatum. Clin Infect Dis. 2012;54(6):832-840.
18. Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36(6):766-774.
19. Fulginiti V, Kempe C, Hathaway W, et al. Progressive vaccinia in immunologically deficient individuals. Birth Defects Orig Artic Ser. 1968;4:129-145.
Generalized vaccinia (GV) is a rare, self-limiting complication of the smallpox vaccination that is caused by the systemic spread of the virus from the inoculation site. The incidence of GV became rare after routine vaccination was discontinued in the U.S. in 1971 and globally in the 1980s after the disease was eradicated.1,2 However in 2002, heightened concerns for the deliberate release of the smallpox virus as a bioweapon led the U.S. military to restart its smallpox vaccination program for soldiers and public health workers.3,4 Here, the authors describe a patient with concomitant GV and mononucleosis.
Case Report
A 19-year-old active-duty marine presented to his battalion aid station with concern for a spreading vesicular rash 9 days after a primary inoculation with the smallpox vaccine. The rash was limited to the inoculation site on his left shoulder (Figure 1). He had no medical history of eczema, atopic dermatitis, or other rashes and reported no systemic symptoms. His vitals also were within normal limits. A clinical diagnosis of inadvertent inoculation (also termed accidental infection) with satellite lesions was made, and he was discharged with counseling on wound care and close follow-up. Two days later, on postvaccination day 11, he presented with new symptoms of a headache, fever, chills, diffuse myalgia, sore throat, and spreading erythematous macules, papules, and vesicles on his arms, chest, abdomen, back, legs, and face (Figures 2A-2D). His vital signs were remarkable for tachycardia with heart rate of 100 bpm and a fever of 103º F (39.4º C). He was sent to the emergency department with a presumed GV diagnosis.
A complete blood count, liver function tests, and basic metabolic panel were unremarkable. Given his symptom of pharyngitis, a rapid strep test was performed. The test was negative, and a throat culture showed no growth. A mononucleosis screen also was performed and was positive. The patient was diagnosed with mononucleosis and GV. His condition improved, and his vital signs stabilized with conservative treatment without the need for vaccine immune globulin (VIG). He convalesced for 72 hours and was referred to dermatology on the following day. Quarantining him in a single occupancy barracks room until all lesions crusted over addressed the concern for spread of the virus to nonimmunized marines or family members.
On postvaccination day 12, the patient continued to be clinically well, and he remained afebrile. The dermatologist obtained a skin biopsy from a lesion on the patient’s right shin. The biopsy demonstrated marked epidermal necrosis with peripheral keratinocytes showing ballooning degeneration and viral cytopathic changes consistent with GV. Antibody titers showing high levels of Epstein-Barr virus (EBV) capsid IgM and IgG present confirmed mononucleosis infection within the past 6 months. The patient remained clinically well and was released from quarantine on postvaccination day 22 when all lesions crusted over (Figures 3A-3D).
Discussion
The CDC current definition for GV is “the spread of lesions to other parts of the body that are benign in appearance and occur as a result of viremia.”5 Although the exact mechanisms of viral spread are unknown, it may be due to a subtle immunologic defect, specifically in the B-cell line.6,7 Epstein-Barr virus affects the B-cell line, and concurrent infection may depress humoral immunity and allow for systemic spread of the virus.8,9
This case illustrates the potential for a severe reaction after smallpox vaccination in a patient with a concomitant EBV infection. Service members primarily receive the smallpox vaccination early in their career when the risk of mononucleosis is at its highest incidence among young adults, 11 to 48 per 1,000.10-13 Although the potential for disseminated vaccinia following vaccination is rare, clinicians need to remain cognizant of the risk, which may be enhanced by recent or subsequent infection with EBV. However, regular screening for EBV would be of questionable value given the large number of tests needed to prevent a single case of GV.
Generalized vaccinia is a rare complication after smallpox vaccination. Despite its dire appearance, GV typically resolves spontaneously with limited adverse effects (AEs).14 The pre-eradication reported incidence was 17.7 per 1,000,000 recipients in a national survey.15 Posteradication the incidence of GV was 3 times as high with 2 reported cases in 2003 after administration of 38,440 vaccinations.16 Inflammatory reactions can be common; however, these reactions are not due to systemic viral spread.5 When dealing with a vaccinia-specific AE, it is important to distinguish the benign inadvertent inoculations and GV from the more serious reactions of eczema vaccinatum (EV) or progressive vaccinia (PV). 5
Inadvertent inoculations and GV are usually benign and self-limited—requiring only prevention of secondary transmission and nosocomial infection. Eczema vaccinatum occurs among persons with atopic dermatitis or eczema.5 The
Conclusion
The smallpox vaccination is unique among vaccinations. It is the only vaccine that is administered via inoculation with a bifurcated needle, requires regular follow-up care, and can be spread to casual contacts.5
It is important for any practitioner administering the smallpox vaccine to be aware of associated AEs. A greater knowledge of the unique challenges with the smallpox vaccine allows for better patient selection that eliminates those with conditions that impair their immune system and improves patient education.
Generalized vaccinia (GV) is a rare, self-limiting complication of the smallpox vaccination that is caused by the systemic spread of the virus from the inoculation site. The incidence of GV became rare after routine vaccination was discontinued in the U.S. in 1971 and globally in the 1980s after the disease was eradicated.1,2 However in 2002, heightened concerns for the deliberate release of the smallpox virus as a bioweapon led the U.S. military to restart its smallpox vaccination program for soldiers and public health workers.3,4 Here, the authors describe a patient with concomitant GV and mononucleosis.
Case Report
A 19-year-old active-duty marine presented to his battalion aid station with concern for a spreading vesicular rash 9 days after a primary inoculation with the smallpox vaccine. The rash was limited to the inoculation site on his left shoulder (Figure 1). He had no medical history of eczema, atopic dermatitis, or other rashes and reported no systemic symptoms. His vitals also were within normal limits. A clinical diagnosis of inadvertent inoculation (also termed accidental infection) with satellite lesions was made, and he was discharged with counseling on wound care and close follow-up. Two days later, on postvaccination day 11, he presented with new symptoms of a headache, fever, chills, diffuse myalgia, sore throat, and spreading erythematous macules, papules, and vesicles on his arms, chest, abdomen, back, legs, and face (Figures 2A-2D). His vital signs were remarkable for tachycardia with heart rate of 100 bpm and a fever of 103º F (39.4º C). He was sent to the emergency department with a presumed GV diagnosis.
A complete blood count, liver function tests, and basic metabolic panel were unremarkable. Given his symptom of pharyngitis, a rapid strep test was performed. The test was negative, and a throat culture showed no growth. A mononucleosis screen also was performed and was positive. The patient was diagnosed with mononucleosis and GV. His condition improved, and his vital signs stabilized with conservative treatment without the need for vaccine immune globulin (VIG). He convalesced for 72 hours and was referred to dermatology on the following day. Quarantining him in a single occupancy barracks room until all lesions crusted over addressed the concern for spread of the virus to nonimmunized marines or family members.
On postvaccination day 12, the patient continued to be clinically well, and he remained afebrile. The dermatologist obtained a skin biopsy from a lesion on the patient’s right shin. The biopsy demonstrated marked epidermal necrosis with peripheral keratinocytes showing ballooning degeneration and viral cytopathic changes consistent with GV. Antibody titers showing high levels of Epstein-Barr virus (EBV) capsid IgM and IgG present confirmed mononucleosis infection within the past 6 months. The patient remained clinically well and was released from quarantine on postvaccination day 22 when all lesions crusted over (Figures 3A-3D).
Discussion
The CDC current definition for GV is “the spread of lesions to other parts of the body that are benign in appearance and occur as a result of viremia.”5 Although the exact mechanisms of viral spread are unknown, it may be due to a subtle immunologic defect, specifically in the B-cell line.6,7 Epstein-Barr virus affects the B-cell line, and concurrent infection may depress humoral immunity and allow for systemic spread of the virus.8,9
This case illustrates the potential for a severe reaction after smallpox vaccination in a patient with a concomitant EBV infection. Service members primarily receive the smallpox vaccination early in their career when the risk of mononucleosis is at its highest incidence among young adults, 11 to 48 per 1,000.10-13 Although the potential for disseminated vaccinia following vaccination is rare, clinicians need to remain cognizant of the risk, which may be enhanced by recent or subsequent infection with EBV. However, regular screening for EBV would be of questionable value given the large number of tests needed to prevent a single case of GV.
Generalized vaccinia is a rare complication after smallpox vaccination. Despite its dire appearance, GV typically resolves spontaneously with limited adverse effects (AEs).14 The pre-eradication reported incidence was 17.7 per 1,000,000 recipients in a national survey.15 Posteradication the incidence of GV was 3 times as high with 2 reported cases in 2003 after administration of 38,440 vaccinations.16 Inflammatory reactions can be common; however, these reactions are not due to systemic viral spread.5 When dealing with a vaccinia-specific AE, it is important to distinguish the benign inadvertent inoculations and GV from the more serious reactions of eczema vaccinatum (EV) or progressive vaccinia (PV). 5
Inadvertent inoculations and GV are usually benign and self-limited—requiring only prevention of secondary transmission and nosocomial infection. Eczema vaccinatum occurs among persons with atopic dermatitis or eczema.5 The
Conclusion
The smallpox vaccination is unique among vaccinations. It is the only vaccine that is administered via inoculation with a bifurcated needle, requires regular follow-up care, and can be spread to casual contacts.5
It is important for any practitioner administering the smallpox vaccine to be aware of associated AEs. A greater knowledge of the unique challenges with the smallpox vaccine allows for better patient selection that eliminates those with conditions that impair their immune system and improves patient education.
1. Centers for Disease Control and prevention. Public Health Service recommendation on smallpox vaccination. MMWR Recomm Rep. 1971;20:339
2. The global eradication of smallpox. World Health Organization Web site. http://apps.who.int/iris/bitstream/10665/39253/1/a41438.pdf. Accessed February 8, 2017.
3. Belongia EA, Naleway A. Smallpox vaccine: the good, the bad and the ugly. Clin Med Res. 2003;1(2):87-92.
4. Wharton M, Strikas RA, Harpaz R, et al. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52(RR-7):1-16.
5. Cono J, Casey CG, Bell DM. Smallpox vaccination and adverse reactions. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Updated February 10, 2003. Accessed February 2, 2017.
6. Chahroudi A, Chavan R, Kozyr N, Waller EK, Silvestri G, Feinberg MB. Vaccinia virus tropism for primary hematolymphoid cells is determined by restricted expression of a unique virus receptor. J Virol. 2005;79(16):10397-10407.
7. , , Blasco R. of different leukocyte cell types to Vaccinia virus infection. Virol J. 2004;1(1):10.
8. Küppers R. B cells under influence: transformation of B cells by Epstein-Barr virus. Nat Rev Immunol. 2003;3(10):801-812.
9. Nemerow G, Cooper N. Infection of B lymphocytes by a human herpesvirus, Epstein-Barr virus, is blocked by calmodulin antagonists. Proc Natl Acad Sci U S A. 1984;81(15):4955-4959.
10. Hallee TJ, Evans AS, Niederman JC, Brooks CM, Voegtly JH. Infectious Mononucleosis at the United States Military Academy. A prospective study of a single class over four years. Yale J Biol Med. 1974;47(3):182-195.
11. Evans AS, Robinton ED. An epidemiological study of infectious mononucleosis. N Engl J Med. 1950;242:492-496.
12. Niederman JC, Evans AS, Subrahmanyan L, McCollum RW. Prevalence, incidence and persistence of EB virus antibody in young adults. N Engl J Med. 1970;282(7):361-365.
13. Sawyer RN, Evans AS, Niederman JC, McCollum RW. Prospective studies of a group of Yale University freshmen. I. Occurrence of infectious mononucleosis. J Infect Dis. 1971;123(3):263-270.
14. Henderson DA, Borio LL, Lane MJ. Smallpox and vaccinia. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 4th ed. Philadelphia, PA: Elsevier; 2004:123-153.
15. Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968—national surveillance in the United States. N Engl J Med. 1969;281(22):1201-1208.
16. Vellozzi C, Lane JM, Averhoff F, et al. Generalized vaccinia, progressive vaccinia and eczema vaccinatum are rare following smallpox (vaccinia) vaccination: United States surveillance, 2003. Clin Infect Dis. 2005;41(5):689-697.
17. Reed J, Scott D. Bray M. Eczema Vaccinatum. Clin Infect Dis. 2012;54(6):832-840.
18. Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36(6):766-774.
19. Fulginiti V, Kempe C, Hathaway W, et al. Progressive vaccinia in immunologically deficient individuals. Birth Defects Orig Artic Ser. 1968;4:129-145.
1. Centers for Disease Control and prevention. Public Health Service recommendation on smallpox vaccination. MMWR Recomm Rep. 1971;20:339
2. The global eradication of smallpox. World Health Organization Web site. http://apps.who.int/iris/bitstream/10665/39253/1/a41438.pdf. Accessed February 8, 2017.
3. Belongia EA, Naleway A. Smallpox vaccine: the good, the bad and the ugly. Clin Med Res. 2003;1(2):87-92.
4. Wharton M, Strikas RA, Harpaz R, et al. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52(RR-7):1-16.
5. Cono J, Casey CG, Bell DM. Smallpox vaccination and adverse reactions. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Updated February 10, 2003. Accessed February 2, 2017.
6. Chahroudi A, Chavan R, Kozyr N, Waller EK, Silvestri G, Feinberg MB. Vaccinia virus tropism for primary hematolymphoid cells is determined by restricted expression of a unique virus receptor. J Virol. 2005;79(16):10397-10407.
7. , , Blasco R. of different leukocyte cell types to Vaccinia virus infection. Virol J. 2004;1(1):10.
8. Küppers R. B cells under influence: transformation of B cells by Epstein-Barr virus. Nat Rev Immunol. 2003;3(10):801-812.
9. Nemerow G, Cooper N. Infection of B lymphocytes by a human herpesvirus, Epstein-Barr virus, is blocked by calmodulin antagonists. Proc Natl Acad Sci U S A. 1984;81(15):4955-4959.
10. Hallee TJ, Evans AS, Niederman JC, Brooks CM, Voegtly JH. Infectious Mononucleosis at the United States Military Academy. A prospective study of a single class over four years. Yale J Biol Med. 1974;47(3):182-195.
11. Evans AS, Robinton ED. An epidemiological study of infectious mononucleosis. N Engl J Med. 1950;242:492-496.
12. Niederman JC, Evans AS, Subrahmanyan L, McCollum RW. Prevalence, incidence and persistence of EB virus antibody in young adults. N Engl J Med. 1970;282(7):361-365.
13. Sawyer RN, Evans AS, Niederman JC, McCollum RW. Prospective studies of a group of Yale University freshmen. I. Occurrence of infectious mononucleosis. J Infect Dis. 1971;123(3):263-270.
14. Henderson DA, Borio LL, Lane MJ. Smallpox and vaccinia. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 4th ed. Philadelphia, PA: Elsevier; 2004:123-153.
15. Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968—national surveillance in the United States. N Engl J Med. 1969;281(22):1201-1208.
16. Vellozzi C, Lane JM, Averhoff F, et al. Generalized vaccinia, progressive vaccinia and eczema vaccinatum are rare following smallpox (vaccinia) vaccination: United States surveillance, 2003. Clin Infect Dis. 2005;41(5):689-697.
17. Reed J, Scott D. Bray M. Eczema Vaccinatum. Clin Infect Dis. 2012;54(6):832-840.
18. Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36(6):766-774.
19. Fulginiti V, Kempe C, Hathaway W, et al. Progressive vaccinia in immunologically deficient individuals. Birth Defects Orig Artic Ser. 1968;4:129-145.
Therapy can produce durable CRs in NHL
When given after low-dose chemotherapy, an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy can produce durable complete responses (CRs) in patients with relapsed/refractory non-Hodgkin lymphoma (NHL), according to research published in the Journal of Clinical Oncology.
In this phase 1 study, the overall response rate was 73%, and 50% of patients had an ongoing CR at last follow-up.
Fifty-five percent of patients experienced grade 3/4 neurologic toxicities, though these events eventually resolved.
This research was conducted under a cooperative research and development agreement between the National Cancer Institute and Kite Pharma, Inc.
Kite is developing the CAR T-cell therapy axicabtagene ciloleucel (formerly known as KTE-C19), and the therapy tested in this trial has the same CAR construct as axicabtagene ciloleucel.
Results from this study (NCT00924326) were previously published in the Journal of Clinical Oncology in 2014.
The current report included 22 patients with relapsed/refractory NHL. Seventeen patients had diffuse large B-cell lymphoma (DLBCL), 2 had primary mediastinal B-cell lymphoma (PMBCL), 2 had follicular lymphoma (FL), and 1 had mantle cell lymphoma (MCL).
Patients received a single dose of CAR T cells 2 days after a low-dose chemotherapy conditioning regimen consisting of cyclophosphamide and fludarabine.
Response
The overall response rate was 73% (16/22), with a CR rate of 55% (n=12) and a partial response (PR) rate of 18% (n=4).
Among patients with DLBCL, there were 9 CRs, 4 PRs, 1 patient with stable disease, and 3 patients with progressive disease.
Both FL patients achieved a CR, as did the patient with MCL. One patient with PMBCL had stable disease, and the other progressed.
Eleven of the 12 CRs are ongoing, with durations ranging from more than 7 months to more than 24 months. The median duration of CR is 12.5 months.
The researchers found that serum IL-15 levels and CAR T-cell expansion correlated with treatment response (CR or PR).
The median peak blood CAR+ cell level was 98/μL in patients who achieved a response and 15/μL in those who did not (P=0.027).
High serum IL-15 levels were significantly associated with high peak blood CAR+ cell levels (P=0.001) and response (P<0.001).
Toxicity
Fifty-five percent of patients had grade 3 or 4 neurologic toxicities, the most common of which were dysphasia (n=9) and confusion (n=8).
The researchers said all acute toxicities resolved completely, and none of the patients died as a result of toxicity.
One patient experienced vision loss 3 months after receiving CAR T-cell therapy. The researchers said they could not confirm the cause of the vision loss, but it is consistent with fludarabine toxicity.
One patient developed myelodysplastic syndrome, which was thought to be related to prior therapy.
The researchers noted that patients who experienced grade 3/4 neurologic toxicity had significantly higher levels of blood CAR+ cells than patients who had neurologic toxicities of a lower grade (P=0.003).
In addition, peak levels of serum IL-10 and IL-15 were higher in patients with grade 3/4 neurologic toxicities (P=0.006 and 0.014, respectively). ![]()
When given after low-dose chemotherapy, an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy can produce durable complete responses (CRs) in patients with relapsed/refractory non-Hodgkin lymphoma (NHL), according to research published in the Journal of Clinical Oncology.
In this phase 1 study, the overall response rate was 73%, and 50% of patients had an ongoing CR at last follow-up.
Fifty-five percent of patients experienced grade 3/4 neurologic toxicities, though these events eventually resolved.
This research was conducted under a cooperative research and development agreement between the National Cancer Institute and Kite Pharma, Inc.
Kite is developing the CAR T-cell therapy axicabtagene ciloleucel (formerly known as KTE-C19), and the therapy tested in this trial has the same CAR construct as axicabtagene ciloleucel.
Results from this study (NCT00924326) were previously published in the Journal of Clinical Oncology in 2014.
The current report included 22 patients with relapsed/refractory NHL. Seventeen patients had diffuse large B-cell lymphoma (DLBCL), 2 had primary mediastinal B-cell lymphoma (PMBCL), 2 had follicular lymphoma (FL), and 1 had mantle cell lymphoma (MCL).
Patients received a single dose of CAR T cells 2 days after a low-dose chemotherapy conditioning regimen consisting of cyclophosphamide and fludarabine.
Response
The overall response rate was 73% (16/22), with a CR rate of 55% (n=12) and a partial response (PR) rate of 18% (n=4).
Among patients with DLBCL, there were 9 CRs, 4 PRs, 1 patient with stable disease, and 3 patients with progressive disease.
Both FL patients achieved a CR, as did the patient with MCL. One patient with PMBCL had stable disease, and the other progressed.
Eleven of the 12 CRs are ongoing, with durations ranging from more than 7 months to more than 24 months. The median duration of CR is 12.5 months.
The researchers found that serum IL-15 levels and CAR T-cell expansion correlated with treatment response (CR or PR).
The median peak blood CAR+ cell level was 98/μL in patients who achieved a response and 15/μL in those who did not (P=0.027).
High serum IL-15 levels were significantly associated with high peak blood CAR+ cell levels (P=0.001) and response (P<0.001).
Toxicity
Fifty-five percent of patients had grade 3 or 4 neurologic toxicities, the most common of which were dysphasia (n=9) and confusion (n=8).
The researchers said all acute toxicities resolved completely, and none of the patients died as a result of toxicity.
One patient experienced vision loss 3 months after receiving CAR T-cell therapy. The researchers said they could not confirm the cause of the vision loss, but it is consistent with fludarabine toxicity.
One patient developed myelodysplastic syndrome, which was thought to be related to prior therapy.
The researchers noted that patients who experienced grade 3/4 neurologic toxicity had significantly higher levels of blood CAR+ cells than patients who had neurologic toxicities of a lower grade (P=0.003).
In addition, peak levels of serum IL-10 and IL-15 were higher in patients with grade 3/4 neurologic toxicities (P=0.006 and 0.014, respectively). ![]()
When given after low-dose chemotherapy, an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy can produce durable complete responses (CRs) in patients with relapsed/refractory non-Hodgkin lymphoma (NHL), according to research published in the Journal of Clinical Oncology.
In this phase 1 study, the overall response rate was 73%, and 50% of patients had an ongoing CR at last follow-up.
Fifty-five percent of patients experienced grade 3/4 neurologic toxicities, though these events eventually resolved.
This research was conducted under a cooperative research and development agreement between the National Cancer Institute and Kite Pharma, Inc.
Kite is developing the CAR T-cell therapy axicabtagene ciloleucel (formerly known as KTE-C19), and the therapy tested in this trial has the same CAR construct as axicabtagene ciloleucel.
Results from this study (NCT00924326) were previously published in the Journal of Clinical Oncology in 2014.
The current report included 22 patients with relapsed/refractory NHL. Seventeen patients had diffuse large B-cell lymphoma (DLBCL), 2 had primary mediastinal B-cell lymphoma (PMBCL), 2 had follicular lymphoma (FL), and 1 had mantle cell lymphoma (MCL).
Patients received a single dose of CAR T cells 2 days after a low-dose chemotherapy conditioning regimen consisting of cyclophosphamide and fludarabine.
Response
The overall response rate was 73% (16/22), with a CR rate of 55% (n=12) and a partial response (PR) rate of 18% (n=4).
Among patients with DLBCL, there were 9 CRs, 4 PRs, 1 patient with stable disease, and 3 patients with progressive disease.
Both FL patients achieved a CR, as did the patient with MCL. One patient with PMBCL had stable disease, and the other progressed.
Eleven of the 12 CRs are ongoing, with durations ranging from more than 7 months to more than 24 months. The median duration of CR is 12.5 months.
The researchers found that serum IL-15 levels and CAR T-cell expansion correlated with treatment response (CR or PR).
The median peak blood CAR+ cell level was 98/μL in patients who achieved a response and 15/μL in those who did not (P=0.027).
High serum IL-15 levels were significantly associated with high peak blood CAR+ cell levels (P=0.001) and response (P<0.001).
Toxicity
Fifty-five percent of patients had grade 3 or 4 neurologic toxicities, the most common of which were dysphasia (n=9) and confusion (n=8).
The researchers said all acute toxicities resolved completely, and none of the patients died as a result of toxicity.
One patient experienced vision loss 3 months after receiving CAR T-cell therapy. The researchers said they could not confirm the cause of the vision loss, but it is consistent with fludarabine toxicity.
One patient developed myelodysplastic syndrome, which was thought to be related to prior therapy.
The researchers noted that patients who experienced grade 3/4 neurologic toxicity had significantly higher levels of blood CAR+ cells than patients who had neurologic toxicities of a lower grade (P=0.003).
In addition, peak levels of serum IL-10 and IL-15 were higher in patients with grade 3/4 neurologic toxicities (P=0.006 and 0.014, respectively). ![]()
ADCs could treat myeloma, other malignancies
A class of antibody-drug conjugates (ADCs) have shown promise for treating hematologic and solid tumor malignancies, according to research published in Cell Chemical Biology.
The ADCs, known as selenomab-drug conjugates, demonstrated in vitro activity against breast cancer and multiple myeloma (MM).
The ADCs also inhibited tumor growth and prolonged survival in mouse models of both malignancies.
“We’ve been working on this technology for some time,” said study author Christoph Rader, PhD, of The Scripps Research Institute (TSRI) in Jupiter, Florida.
“It’s based on the rarely used natural amino acid selenocysteine, which we insert into our antibodies. We refer to these engineered antibodies as selenomabs.”
He then explained that selenomab-drug conjugates are ADCs that “utilize the unique reactivity of selenocysteine for drug attachment.”
For this study, Dr Rader and his colleagues generated selective selenomab-drug conjugates and tested them in vitro and in vivo.
The team found that CD138-targeting selenomab-drug conjugates were effective against MM cell lines (U266 and H929), and HER2-targeting selenomab-drug conjugates were effective against breast cancer cell lines.
Both types of ADCs demonstrated efficacy in mouse models as well.
One of the CD138-targeting selenomab-drug conjugates, known as CN29, was tested in a mouse model of MM.
One group of mice received CN29 at 3 mg/kg every 4 days for a total of 4 cycles, another group received unconjugated selenomab, and a third received vehicle control.
CN29 significantly inhibited tumor growth (P=0.000085) and extended survival time (P=0.0083) in the mice.
Based on these results, Dr Rader said selenomab-drug conjugates “promise broad utility for cancer therapy.” ![]()
A class of antibody-drug conjugates (ADCs) have shown promise for treating hematologic and solid tumor malignancies, according to research published in Cell Chemical Biology.
The ADCs, known as selenomab-drug conjugates, demonstrated in vitro activity against breast cancer and multiple myeloma (MM).
The ADCs also inhibited tumor growth and prolonged survival in mouse models of both malignancies.
“We’ve been working on this technology for some time,” said study author Christoph Rader, PhD, of The Scripps Research Institute (TSRI) in Jupiter, Florida.
“It’s based on the rarely used natural amino acid selenocysteine, which we insert into our antibodies. We refer to these engineered antibodies as selenomabs.”
He then explained that selenomab-drug conjugates are ADCs that “utilize the unique reactivity of selenocysteine for drug attachment.”
For this study, Dr Rader and his colleagues generated selective selenomab-drug conjugates and tested them in vitro and in vivo.
The team found that CD138-targeting selenomab-drug conjugates were effective against MM cell lines (U266 and H929), and HER2-targeting selenomab-drug conjugates were effective against breast cancer cell lines.
Both types of ADCs demonstrated efficacy in mouse models as well.
One of the CD138-targeting selenomab-drug conjugates, known as CN29, was tested in a mouse model of MM.
One group of mice received CN29 at 3 mg/kg every 4 days for a total of 4 cycles, another group received unconjugated selenomab, and a third received vehicle control.
CN29 significantly inhibited tumor growth (P=0.000085) and extended survival time (P=0.0083) in the mice.
Based on these results, Dr Rader said selenomab-drug conjugates “promise broad utility for cancer therapy.” ![]()
A class of antibody-drug conjugates (ADCs) have shown promise for treating hematologic and solid tumor malignancies, according to research published in Cell Chemical Biology.
The ADCs, known as selenomab-drug conjugates, demonstrated in vitro activity against breast cancer and multiple myeloma (MM).
The ADCs also inhibited tumor growth and prolonged survival in mouse models of both malignancies.
“We’ve been working on this technology for some time,” said study author Christoph Rader, PhD, of The Scripps Research Institute (TSRI) in Jupiter, Florida.
“It’s based on the rarely used natural amino acid selenocysteine, which we insert into our antibodies. We refer to these engineered antibodies as selenomabs.”
He then explained that selenomab-drug conjugates are ADCs that “utilize the unique reactivity of selenocysteine for drug attachment.”
For this study, Dr Rader and his colleagues generated selective selenomab-drug conjugates and tested them in vitro and in vivo.
The team found that CD138-targeting selenomab-drug conjugates were effective against MM cell lines (U266 and H929), and HER2-targeting selenomab-drug conjugates were effective against breast cancer cell lines.
Both types of ADCs demonstrated efficacy in mouse models as well.
One of the CD138-targeting selenomab-drug conjugates, known as CN29, was tested in a mouse model of MM.
One group of mice received CN29 at 3 mg/kg every 4 days for a total of 4 cycles, another group received unconjugated selenomab, and a third received vehicle control.
CN29 significantly inhibited tumor growth (P=0.000085) and extended survival time (P=0.0083) in the mice.
Based on these results, Dr Rader said selenomab-drug conjugates “promise broad utility for cancer therapy.” ![]()
Funders could do more to reduce research waste, team says
A study published in The Lancet suggests agencies that distribute public funds for research could do more to reduce waste.
Investigators evaluated 11 agencies from various countries and found the agencies aren’t always transparent about what they are doing to prevent waste in research.
The investigators also found evidence to suggest that some agencies are not taking certain steps that could reduce waste, and the governments responsible for the public money these agencies distribute are not holding them to account.
“Our investigation has shown that, on the whole, information about the policies and processes used by national funding agencies across the funding landscape are not transparent or readily available,” said Mona Nasser, DDS, of Plymouth University in the UK.
“It would appear that governments around the world often do not hold these agencies accountable for adding value to research and reducing research waste. This is not a call for governments to reduce spending on medical research, but, rather, as public funds become increasingly squeezed, there is no better time for funding agencies and governments to work together to ensure that we will all get the best ‘bang for the buck.’”
For this study, Dr Nasser and her colleagues investigated how research funders monitor and take steps to reduce waste in the research they support. The team also examined how funders support methodology research and the development of research infrastructures to reduce waste.
The investigators looked through the websites of 11 national research funders that distribute public funds in the US, UK, Australia, Canada, Germany, France, The Netherlands, Denmark, and Norway.
The team looked for information on how the agencies decide what to fund and how they ensure what they fund is not wasteful. The investigators also contacted these agencies to verify their findings.
The team found that approaches vary among the funders, but there are weaknesses that are applicable across all funding bodies.
One weakness is that grant committees tend to be dominated by academics and clinicians, which is a problem because patients’ interests may be overlooked.
The funders with the “most extensive” involvement of the general public are the National Institute of Health Research (NIHR) in the UK and ZonMW in The Netherlands.
Another weakness is the fact that practice and policy decisions are often made without the systematic assessment of existing research evidence.
The only funder to require reference to relevant systematic reviews in all funding applications is NIHR.
Yet another weakness is that only 6 of the 11 funding agencies require the publication of full reports of the research they have funded. And none of the funders have a comprehensive strategy to make data from all research projects freely available.
Based on these results, Dr Nasser and her colleagues concluded that more should be done to ensure transparency and accountability.
“In simple terms, there is a 2-pronged requirement for medical research funding bodies which distribute public funds,” Dr Nasser said. “The first is that they need to be fully responsible for how and why those funds are distributed because they are ultimately answerable to every tax payer in their home countries.”
“The second is that they need to ensure that public funds are not only invested wisely in research projects which represent both good value and waste-limited practice, but also to ensure that the results of these studies are made available in a usable format to the people who need them.” ![]()
A study published in The Lancet suggests agencies that distribute public funds for research could do more to reduce waste.
Investigators evaluated 11 agencies from various countries and found the agencies aren’t always transparent about what they are doing to prevent waste in research.
The investigators also found evidence to suggest that some agencies are not taking certain steps that could reduce waste, and the governments responsible for the public money these agencies distribute are not holding them to account.
“Our investigation has shown that, on the whole, information about the policies and processes used by national funding agencies across the funding landscape are not transparent or readily available,” said Mona Nasser, DDS, of Plymouth University in the UK.
“It would appear that governments around the world often do not hold these agencies accountable for adding value to research and reducing research waste. This is not a call for governments to reduce spending on medical research, but, rather, as public funds become increasingly squeezed, there is no better time for funding agencies and governments to work together to ensure that we will all get the best ‘bang for the buck.’”
For this study, Dr Nasser and her colleagues investigated how research funders monitor and take steps to reduce waste in the research they support. The team also examined how funders support methodology research and the development of research infrastructures to reduce waste.
The investigators looked through the websites of 11 national research funders that distribute public funds in the US, UK, Australia, Canada, Germany, France, The Netherlands, Denmark, and Norway.
The team looked for information on how the agencies decide what to fund and how they ensure what they fund is not wasteful. The investigators also contacted these agencies to verify their findings.
The team found that approaches vary among the funders, but there are weaknesses that are applicable across all funding bodies.
One weakness is that grant committees tend to be dominated by academics and clinicians, which is a problem because patients’ interests may be overlooked.
The funders with the “most extensive” involvement of the general public are the National Institute of Health Research (NIHR) in the UK and ZonMW in The Netherlands.
Another weakness is the fact that practice and policy decisions are often made without the systematic assessment of existing research evidence.
The only funder to require reference to relevant systematic reviews in all funding applications is NIHR.
Yet another weakness is that only 6 of the 11 funding agencies require the publication of full reports of the research they have funded. And none of the funders have a comprehensive strategy to make data from all research projects freely available.
Based on these results, Dr Nasser and her colleagues concluded that more should be done to ensure transparency and accountability.
“In simple terms, there is a 2-pronged requirement for medical research funding bodies which distribute public funds,” Dr Nasser said. “The first is that they need to be fully responsible for how and why those funds are distributed because they are ultimately answerable to every tax payer in their home countries.”
“The second is that they need to ensure that public funds are not only invested wisely in research projects which represent both good value and waste-limited practice, but also to ensure that the results of these studies are made available in a usable format to the people who need them.” ![]()
A study published in The Lancet suggests agencies that distribute public funds for research could do more to reduce waste.
Investigators evaluated 11 agencies from various countries and found the agencies aren’t always transparent about what they are doing to prevent waste in research.
The investigators also found evidence to suggest that some agencies are not taking certain steps that could reduce waste, and the governments responsible for the public money these agencies distribute are not holding them to account.
“Our investigation has shown that, on the whole, information about the policies and processes used by national funding agencies across the funding landscape are not transparent or readily available,” said Mona Nasser, DDS, of Plymouth University in the UK.
“It would appear that governments around the world often do not hold these agencies accountable for adding value to research and reducing research waste. This is not a call for governments to reduce spending on medical research, but, rather, as public funds become increasingly squeezed, there is no better time for funding agencies and governments to work together to ensure that we will all get the best ‘bang for the buck.’”
For this study, Dr Nasser and her colleagues investigated how research funders monitor and take steps to reduce waste in the research they support. The team also examined how funders support methodology research and the development of research infrastructures to reduce waste.
The investigators looked through the websites of 11 national research funders that distribute public funds in the US, UK, Australia, Canada, Germany, France, The Netherlands, Denmark, and Norway.
The team looked for information on how the agencies decide what to fund and how they ensure what they fund is not wasteful. The investigators also contacted these agencies to verify their findings.
The team found that approaches vary among the funders, but there are weaknesses that are applicable across all funding bodies.
One weakness is that grant committees tend to be dominated by academics and clinicians, which is a problem because patients’ interests may be overlooked.
The funders with the “most extensive” involvement of the general public are the National Institute of Health Research (NIHR) in the UK and ZonMW in The Netherlands.
Another weakness is the fact that practice and policy decisions are often made without the systematic assessment of existing research evidence.
The only funder to require reference to relevant systematic reviews in all funding applications is NIHR.
Yet another weakness is that only 6 of the 11 funding agencies require the publication of full reports of the research they have funded. And none of the funders have a comprehensive strategy to make data from all research projects freely available.
Based on these results, Dr Nasser and her colleagues concluded that more should be done to ensure transparency and accountability.
“In simple terms, there is a 2-pronged requirement for medical research funding bodies which distribute public funds,” Dr Nasser said. “The first is that they need to be fully responsible for how and why those funds are distributed because they are ultimately answerable to every tax payer in their home countries.”
“The second is that they need to ensure that public funds are not only invested wisely in research projects which represent both good value and waste-limited practice, but also to ensure that the results of these studies are made available in a usable format to the people who need them.” ![]()
What are indications, complications of acute blood transfusions in sickle cell anemia? Key Points Additional Reading
Case
A 19-year-old female with a history of sickle cell anemia and hemoglobin SS, presents with a 2-day history of worsening lower back pain and dyspnea. Physical exam reveals oxygen saturation of 87% on room air, a temperature of 39.2° C, respiratory rate of 24 breaths per minute, and right-sided rales. Her hemoglobin is 5.3 g/dL (baseline hemoglobin of 7.8 g/dL). Chest radiograph reveals a right upper lobe pneumonia, and she is diagnosed with acute chest syndrome.
What are indications and complications of acute transfusion in sickle cell anemia?
Background
Chronic hemolytic anemia is a trademark of sickle cell anemia (SCA) or hemoglobin (Hb) SS as is acute anemia during illness or vaso-occlusive crises. Blood transfusions were the first therapy used in sickle cell disease, long before the pathophysiology was understood. Transfusion of red blood cells (RBC) increases the percentage of circulating normal Hb A, thereby decreasing the percentage of abnormal, sickled cells. This increases the oxygen-carrying capacity of the patient’s RBCs, improves organ perfusion, prevents organ damage, and can be life saving. SCA patients are the largest users of the United States rare donor blood bank registry.1
Unfortunately, transfusion comes with many risks including infection, transfusion reactions, alloimmunization, iron overload, hyperviscosity, and volume overload.
As SCA is a low-prevalence disease in a minority population, very few studies have been performed. Currently, the guidance available regarding blood transfusion is primarily based on expert opinion.
What to transfuse
Leukoreduced and intensive phenotypically matched RBC are not possible in many medical centers. Previous studies have noted decreased incidence of febrile nonhemolytic anemia transfusion reactions, cytomegalovirus transmission, and human leukocyte antigen alloimmunization in leukoreduced blood transfusions, however, these studies did not include SCA patients.2
Complications from transfusion
Complications from blood transfusions include febrile nonhemolytic transfusion reaction, acute hemolytic transfusion reaction (ABO incompatibility), transfusion-associated lung injury (TRALI), transfusion-associated circulatory overload (TACO), infections, and anaphylaxis. The National Heart, Lung, and Blood Institute guidelines specifically highlight the complications of delayed hemolytic transfusion reaction, iron overload, and hyperviscosity in SCA.Approximately 30% of SCA patients have alloantibodies.2 SCA patients may also develop autoimmunization, an immune response to their own RBC, particularly if the patient has multiple autoantibodies.
Infection is a risk for all individuals receiving transfusion. Screening for hepatitis B, hepatitis C, HIV, human T-cell lymphotropic virus, syphilis, West Nile virus, Trympanosoma, and bacteria are routinely performed but not 100% conclusive. Other diseases not routinely screened for include Creutzfeldt-Jakob disease, Babesia, human herpesvirus-8, dengue fever, malaria, and newer concerns such as Zika virus. 2,3
Febrile nonhemolytic transfusion reactions present as an increase in body temperature of more than 1° C during or shortly after receiving a blood transfusion in the absence of other pyrexic stimulus. Febrile nonhemolytic transfusion reaction occurs more frequently in patients with a previous history of transfusions. The use of leukoreduced RBCs reduces the occurrence to less than 1%.2
TRALI presents with the acute onset of hypoxemia and noncardiogenic pulmonary edema within 6 hours of a blood transfusion in the absence of other etiologies. The mechanism of TRALI is caused by an inflammatory response causing injury to the alveolar capillary membrane and the development of pulmonary edema.1
TACO presents with cardiogenic pulmonary edema not from another etiology. This is usually seen after transfusion of excessive volumes of blood or after excessively rapid rates of transfusion.1
Delayed hemolytic transfusion reaction (DHTR) may be a life-threatening immune response to donor cell antigens. The reaction is identified by a drop in the patient’s hemoglobin below the pretransfusion level, reticulocytopenia, a positive direct Coombs test, and occasionally jaundice on physical exam.2 Patients may have an unexpectedly high hemoglobin S% after transfusion from the hemolysis of donor cells. The pathognomonic feature is development of a new alloantibody. DHTR occurs more often in individuals who have received recurrent transfusions and has been reported in 4%-11% of transfused SCA patients.3 Donor and native cells hemolyze intra- and extravascularly 5-20 days after receiving a transfusion.2 DHTR is likely underestimated in SCA as it may be confused for a vaso-occlusive crisis.
Iron overload from recurrent transfusions is a slow, chronic process resulting in end organ damage of the heart, liver, and pancreas. It is associated with more frequent hospitalizations and higher mortality in SCA.3 The average person has 4-5 g of iron with no process to remove the excess. One unit of packed red blood cells adds 250-300 mg of iron.2 Ferritin somewhat correlates to iron overload but is not a reliable method because it is an acute-phase reactant. Liver biopsy is the current diagnostic gold standard, however, noninvasive MRI is gaining diagnostic credibility.
Hb SS blood has up to 10 times higher viscosity than does non–sickle cell blood at the same hemoglobin level. RBC transfusion increases the already hyperviscous state of SCA resulting in slow blood flow through vessels. The slow flow through small vessels from hyperviscosity may result in additional sickling and trigger or worsen a vaso-occlusive crisis. Avascular necrosis is theorized to be a result of hyperviscosity as it occurs more commonly in sickle cell patients with higher hemoglobin. It is important not to transfuse to baseline or above a hemoglobin of 10 g/dL to avoid worsening hyperviscosity.2
When to consider transfusion
Unfortunately, there are no strong randomized controlled trials to definitively dictate when simple transfusions or exchange transfusions are indicated. Acute simple transfusions should be considered in certain circumstances including acute chest syndrome, acute stroke, aplastic anemia, preoperative transfusion, splenic sequestration plus severe anemia, acute hepatic sequestration, and severe acute intrahepatic cholestasis.2
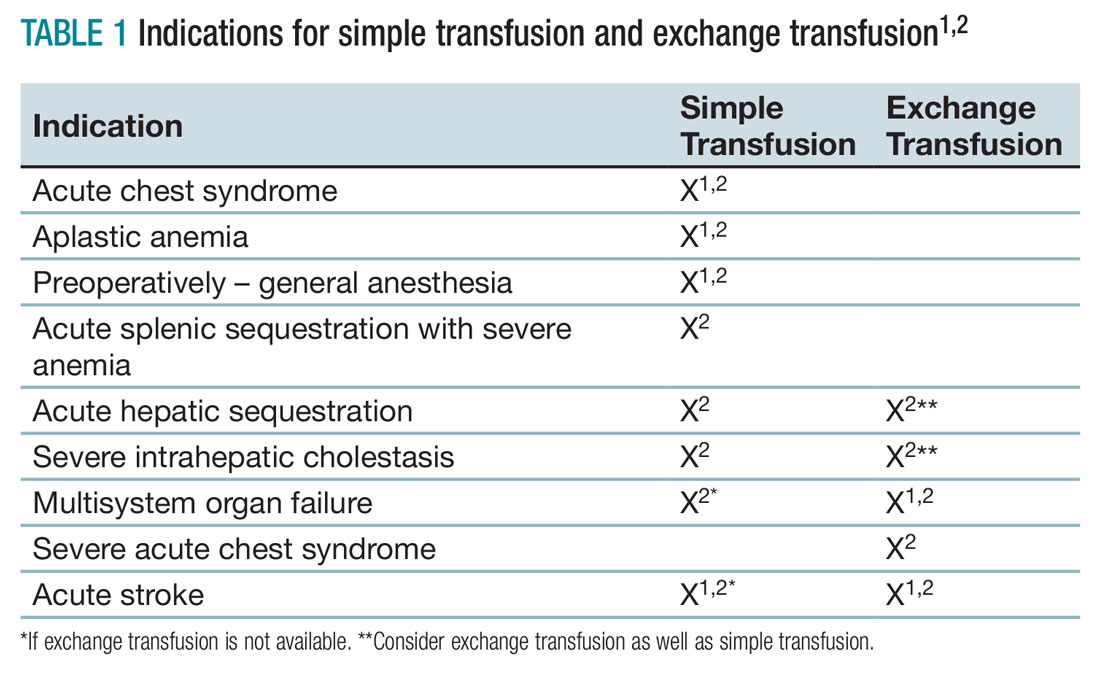
Few studies compare simple transfusion and exchange transfusion.2 The decision to use exchange transfusion over simple transfusion often is based on availability of exchange transfusion, ability of simple transfusion to decrease the percentage of hemoglobin S, and/or the patient’s current hemoglobin to avoid hyperviscosity from simple transfusion.3 Exchange transfusion should be considered for hemoglobin greater than 8-9 g/dL.2
Acute hepatic sequestration (AHS) occurs with the sequestration of RBCs in the liver and is marked by greater than 2 g/dL decrease in hemoglobin and hepatic enlargement, compared with baseline. The stretching of the hepatic capsule results in right upper quadrant pain. AHS often develops over a few hours to a few days with only mild elevation of liver function tests. AHS may be underestimated as two-thirds of SCA patients have hepatomegaly. Unless the hepatomegaly is radiographically monitored it may not be possible to determine an acute increase in liver size.2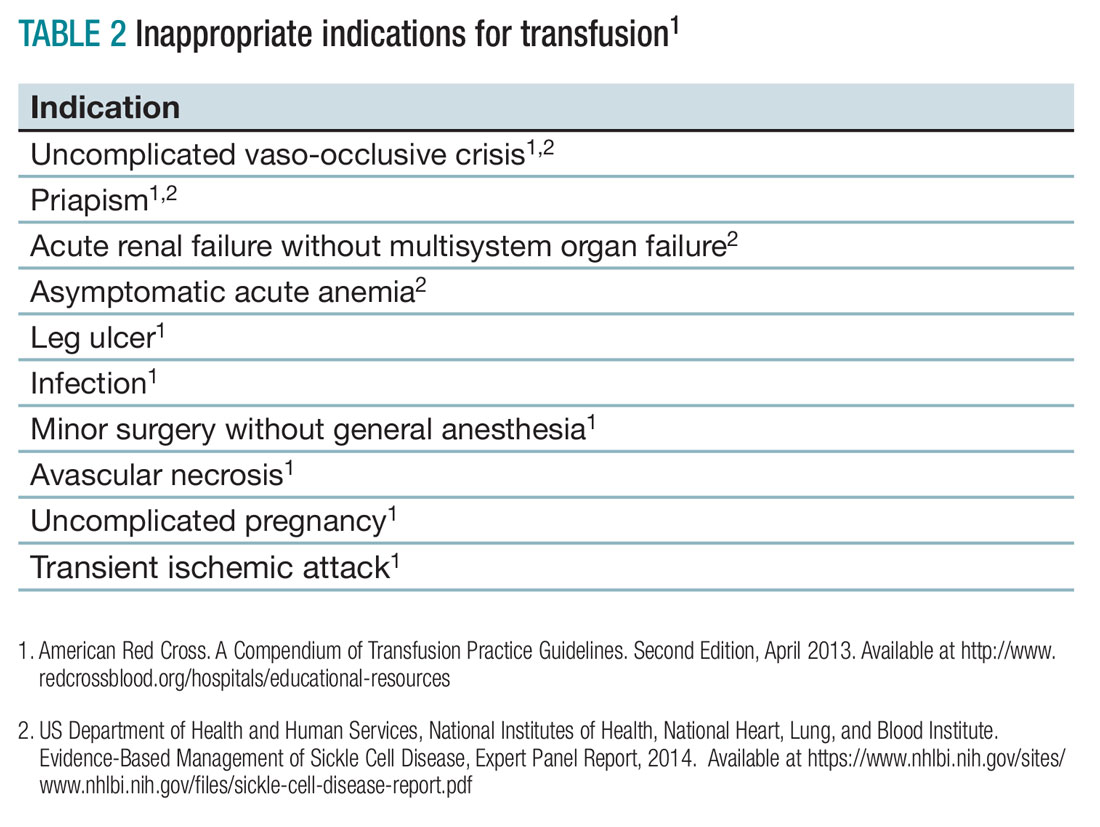
Aplastic crisis presents as a gradual onset of fatigue, shortness of breath, and sometimes syncope or fever. Physical examination may reveal tachycardia and occasionally frank heart failure. The hemoglobin is usually far below the patient’s baseline level with an inappropriate, severely low reticulocyte count. Aplastic crisis should be transfused immediately because of the markedly short life expectancy of hemoglobin S RBCs, but does not need to be transfused to baseline.2
Acute splenic sequestration presents as a decrease in hemoglobin by greater than 2 g/dL, elevated reticulocyte count and circulating nucleated red blood cells, thrombocytopenia, and sudden splenomegaly.2 The goal of transfusion is for partial correction because of the risk of hyperviscosity when the spleen releases the sequestered RBCs.
Acute chest syndrome (ACS) presents as a pneumonia radiographically consistent with a respiratory tract infection caused by cough, shortness of breath, retractions, and/or rales. ACS is the most common cause of death in SCA. ACS is usually from infection but may be because of fat embolism, intrapulmonary aggregates of sickled cells, atelectasis, or pulmonary edema.2 If ACS has a hemoglobin decrease of greater than 1g/dL, consider transfusion.1,2
Severe acute chest syndrome is distinguished by radiographic evidence of multilobe pneumonia, increased work of breathing, pleural effusions, and oxygen saturation below 95% with supplemental oxygen. Severe ACS may have a decrease in hemoglobin despite receiving transfusion. Exchange transfusion is recommended because of the high mortality in severe ACS.2
Preoperative transfusion is used to decrease the incidence of postoperative vaso-occlusive crisis, acute stroke, or ACS for patients receiving general anesthesia. The goal for transfusion hemoglobin is 10g/dL. In SCA patients with a hemoglobin greater than 9g/dL, exchange transfusion may be considered to avoid hyperviscosity.1,2
Multisystem organ failure (MSOF) is severe and life-threatening lung, liver, and/or kidney failure. MSOF may occur after several days of hospitalization. It is often unanticipated and swift, frequently presenting with fever, a rapid increase in anemia, thrombocytopenia, and altered mental status. Lung failure often presents as ACS. Liver failure is marked by hyperbilirubinemia, elevated transaminases, and coagulopathy. Kidney failure is marked by elevated creatinine, with or without change in urine output and hyperkalemia. Rapid treatment with transfusion or exchange transfusion reduces mortality.
The incidence of acute ischemic stroke in SCA decreases with prophylactic transfusion of patients with elevated transcranial Dopplers. Acute stroke is usually secondary to stenosis or an occlusion of the internal carotid or middle cerebral artery. Acute hemorrhagic stroke may present as severe headache and loss of consciousness. Acute stroke should be confirmed radiographically, then exchange transfusion instituted rapidly.2
When not to transfuse
- Do not transfuse for simple vaso-occlusive crisis in the absence of symptoms attributable to acute anemia.1-3
- Do not transfuse for priapism.2
- Do not transfuse for acute renal failure unless there is MSOF.2
Back to the case
The patient was admitted for vaso-occlusive crisis and was started on patient-controlled analgesia with hydromorphone and IV fluids. Azithromycin and ceftriaxone were initiated empirically for community-acquired pneumonia. She was given one unit of phenotypically matched, leukoreduced RBCs for acute chest syndrome. Her hemoglobin increased to 6.1 g/dL. Her fever resolved on day 2, and her dyspnea improved on day 3 of hospitalization. She was weaned off of her patient-controlled analgesia on day 4 and discharged home on day 5 with moxifloxacin to complete 7 days of antibiotics.
Bottom line
Acute simple transfusions and exchange transfusions are indicated for multiple serious and life-threatening complications in SCA. However, transfusion has many serious and life-threatening potential adverse effects. It is essential to conduct a thorough risk-benefit analysis for each individual SCA patient. Whenever possible, intensive phenotypically matched and leukoreduced RBCs should be used. TH
References
1. American Red Cross. A Compendium of Transfusion Practice Guidelines. Second Edition, April 2013.
2. US Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute. Evidence-Based Management of Sickle Cell Disease, Expert Panel Report, 2014.
3. Smith-Whitely, K and Thompson, AA. Indications and complications of transfusions in sickle cell disease. Pediatr Blood Cancer. 2012;59(2):358-64.
- SCA patients are at risk for serious transfusion complications including iron overload, delayed hemolytic transfusion reaction, and hyperviscosity in addition to the usual transfusion risks.
- Do not transfuse an uncomplicated vaso-occlusive crisis without symptomatic anemia.1-3
- Repeated transfusions create alloimmunization in SCA patients increasing risk for life-threatening transfusion reactions and difficulty locating phenotypically matched RBCs.
- Transfusion should be considered in SCA patients experiencing acute chest syndrome, aplastic anemia, splenic sequestration with acute anemia, acute hepatic sequestration, and severe intrahepatic cholestasis.1,2
- If available, exchange transfusion should be considered for SCA patients experiencing multisystem organ failure, acute stroke, and severe acute chest syndrome.1,2
- American Red Cross. A Compendium of Transfusion Practice Guidelines. Second Edition, April 2013.
Case
A 19-year-old female with a history of sickle cell anemia and hemoglobin SS, presents with a 2-day history of worsening lower back pain and dyspnea. Physical exam reveals oxygen saturation of 87% on room air, a temperature of 39.2° C, respiratory rate of 24 breaths per minute, and right-sided rales. Her hemoglobin is 5.3 g/dL (baseline hemoglobin of 7.8 g/dL). Chest radiograph reveals a right upper lobe pneumonia, and she is diagnosed with acute chest syndrome.
What are indications and complications of acute transfusion in sickle cell anemia?
Background
Chronic hemolytic anemia is a trademark of sickle cell anemia (SCA) or hemoglobin (Hb) SS as is acute anemia during illness or vaso-occlusive crises. Blood transfusions were the first therapy used in sickle cell disease, long before the pathophysiology was understood. Transfusion of red blood cells (RBC) increases the percentage of circulating normal Hb A, thereby decreasing the percentage of abnormal, sickled cells. This increases the oxygen-carrying capacity of the patient’s RBCs, improves organ perfusion, prevents organ damage, and can be life saving. SCA patients are the largest users of the United States rare donor blood bank registry.1
Unfortunately, transfusion comes with many risks including infection, transfusion reactions, alloimmunization, iron overload, hyperviscosity, and volume overload.
As SCA is a low-prevalence disease in a minority population, very few studies have been performed. Currently, the guidance available regarding blood transfusion is primarily based on expert opinion.
What to transfuse
Leukoreduced and intensive phenotypically matched RBC are not possible in many medical centers. Previous studies have noted decreased incidence of febrile nonhemolytic anemia transfusion reactions, cytomegalovirus transmission, and human leukocyte antigen alloimmunization in leukoreduced blood transfusions, however, these studies did not include SCA patients.2
Complications from transfusion
Complications from blood transfusions include febrile nonhemolytic transfusion reaction, acute hemolytic transfusion reaction (ABO incompatibility), transfusion-associated lung injury (TRALI), transfusion-associated circulatory overload (TACO), infections, and anaphylaxis. The National Heart, Lung, and Blood Institute guidelines specifically highlight the complications of delayed hemolytic transfusion reaction, iron overload, and hyperviscosity in SCA.Approximately 30% of SCA patients have alloantibodies.2 SCA patients may also develop autoimmunization, an immune response to their own RBC, particularly if the patient has multiple autoantibodies.
Infection is a risk for all individuals receiving transfusion. Screening for hepatitis B, hepatitis C, HIV, human T-cell lymphotropic virus, syphilis, West Nile virus, Trympanosoma, and bacteria are routinely performed but not 100% conclusive. Other diseases not routinely screened for include Creutzfeldt-Jakob disease, Babesia, human herpesvirus-8, dengue fever, malaria, and newer concerns such as Zika virus. 2,3
Febrile nonhemolytic transfusion reactions present as an increase in body temperature of more than 1° C during or shortly after receiving a blood transfusion in the absence of other pyrexic stimulus. Febrile nonhemolytic transfusion reaction occurs more frequently in patients with a previous history of transfusions. The use of leukoreduced RBCs reduces the occurrence to less than 1%.2
TRALI presents with the acute onset of hypoxemia and noncardiogenic pulmonary edema within 6 hours of a blood transfusion in the absence of other etiologies. The mechanism of TRALI is caused by an inflammatory response causing injury to the alveolar capillary membrane and the development of pulmonary edema.1
TACO presents with cardiogenic pulmonary edema not from another etiology. This is usually seen after transfusion of excessive volumes of blood or after excessively rapid rates of transfusion.1
Delayed hemolytic transfusion reaction (DHTR) may be a life-threatening immune response to donor cell antigens. The reaction is identified by a drop in the patient’s hemoglobin below the pretransfusion level, reticulocytopenia, a positive direct Coombs test, and occasionally jaundice on physical exam.2 Patients may have an unexpectedly high hemoglobin S% after transfusion from the hemolysis of donor cells. The pathognomonic feature is development of a new alloantibody. DHTR occurs more often in individuals who have received recurrent transfusions and has been reported in 4%-11% of transfused SCA patients.3 Donor and native cells hemolyze intra- and extravascularly 5-20 days after receiving a transfusion.2 DHTR is likely underestimated in SCA as it may be confused for a vaso-occlusive crisis.
Iron overload from recurrent transfusions is a slow, chronic process resulting in end organ damage of the heart, liver, and pancreas. It is associated with more frequent hospitalizations and higher mortality in SCA.3 The average person has 4-5 g of iron with no process to remove the excess. One unit of packed red blood cells adds 250-300 mg of iron.2 Ferritin somewhat correlates to iron overload but is not a reliable method because it is an acute-phase reactant. Liver biopsy is the current diagnostic gold standard, however, noninvasive MRI is gaining diagnostic credibility.
Hb SS blood has up to 10 times higher viscosity than does non–sickle cell blood at the same hemoglobin level. RBC transfusion increases the already hyperviscous state of SCA resulting in slow blood flow through vessels. The slow flow through small vessels from hyperviscosity may result in additional sickling and trigger or worsen a vaso-occlusive crisis. Avascular necrosis is theorized to be a result of hyperviscosity as it occurs more commonly in sickle cell patients with higher hemoglobin. It is important not to transfuse to baseline or above a hemoglobin of 10 g/dL to avoid worsening hyperviscosity.2
When to consider transfusion
Unfortunately, there are no strong randomized controlled trials to definitively dictate when simple transfusions or exchange transfusions are indicated. Acute simple transfusions should be considered in certain circumstances including acute chest syndrome, acute stroke, aplastic anemia, preoperative transfusion, splenic sequestration plus severe anemia, acute hepatic sequestration, and severe acute intrahepatic cholestasis.2

Few studies compare simple transfusion and exchange transfusion.2 The decision to use exchange transfusion over simple transfusion often is based on availability of exchange transfusion, ability of simple transfusion to decrease the percentage of hemoglobin S, and/or the patient’s current hemoglobin to avoid hyperviscosity from simple transfusion.3 Exchange transfusion should be considered for hemoglobin greater than 8-9 g/dL.2
Acute hepatic sequestration (AHS) occurs with the sequestration of RBCs in the liver and is marked by greater than 2 g/dL decrease in hemoglobin and hepatic enlargement, compared with baseline. The stretching of the hepatic capsule results in right upper quadrant pain. AHS often develops over a few hours to a few days with only mild elevation of liver function tests. AHS may be underestimated as two-thirds of SCA patients have hepatomegaly. Unless the hepatomegaly is radiographically monitored it may not be possible to determine an acute increase in liver size.2
Aplastic crisis presents as a gradual onset of fatigue, shortness of breath, and sometimes syncope or fever. Physical examination may reveal tachycardia and occasionally frank heart failure. The hemoglobin is usually far below the patient’s baseline level with an inappropriate, severely low reticulocyte count. Aplastic crisis should be transfused immediately because of the markedly short life expectancy of hemoglobin S RBCs, but does not need to be transfused to baseline.2
Acute splenic sequestration presents as a decrease in hemoglobin by greater than 2 g/dL, elevated reticulocyte count and circulating nucleated red blood cells, thrombocytopenia, and sudden splenomegaly.2 The goal of transfusion is for partial correction because of the risk of hyperviscosity when the spleen releases the sequestered RBCs.
Acute chest syndrome (ACS) presents as a pneumonia radiographically consistent with a respiratory tract infection caused by cough, shortness of breath, retractions, and/or rales. ACS is the most common cause of death in SCA. ACS is usually from infection but may be because of fat embolism, intrapulmonary aggregates of sickled cells, atelectasis, or pulmonary edema.2 If ACS has a hemoglobin decrease of greater than 1g/dL, consider transfusion.1,2
Severe acute chest syndrome is distinguished by radiographic evidence of multilobe pneumonia, increased work of breathing, pleural effusions, and oxygen saturation below 95% with supplemental oxygen. Severe ACS may have a decrease in hemoglobin despite receiving transfusion. Exchange transfusion is recommended because of the high mortality in severe ACS.2
Preoperative transfusion is used to decrease the incidence of postoperative vaso-occlusive crisis, acute stroke, or ACS for patients receiving general anesthesia. The goal for transfusion hemoglobin is 10g/dL. In SCA patients with a hemoglobin greater than 9g/dL, exchange transfusion may be considered to avoid hyperviscosity.1,2
Multisystem organ failure (MSOF) is severe and life-threatening lung, liver, and/or kidney failure. MSOF may occur after several days of hospitalization. It is often unanticipated and swift, frequently presenting with fever, a rapid increase in anemia, thrombocytopenia, and altered mental status. Lung failure often presents as ACS. Liver failure is marked by hyperbilirubinemia, elevated transaminases, and coagulopathy. Kidney failure is marked by elevated creatinine, with or without change in urine output and hyperkalemia. Rapid treatment with transfusion or exchange transfusion reduces mortality.
The incidence of acute ischemic stroke in SCA decreases with prophylactic transfusion of patients with elevated transcranial Dopplers. Acute stroke is usually secondary to stenosis or an occlusion of the internal carotid or middle cerebral artery. Acute hemorrhagic stroke may present as severe headache and loss of consciousness. Acute stroke should be confirmed radiographically, then exchange transfusion instituted rapidly.2
When not to transfuse
- Do not transfuse for simple vaso-occlusive crisis in the absence of symptoms attributable to acute anemia.1-3
- Do not transfuse for priapism.2
- Do not transfuse for acute renal failure unless there is MSOF.2
Back to the case
The patient was admitted for vaso-occlusive crisis and was started on patient-controlled analgesia with hydromorphone and IV fluids. Azithromycin and ceftriaxone were initiated empirically for community-acquired pneumonia. She was given one unit of phenotypically matched, leukoreduced RBCs for acute chest syndrome. Her hemoglobin increased to 6.1 g/dL. Her fever resolved on day 2, and her dyspnea improved on day 3 of hospitalization. She was weaned off of her patient-controlled analgesia on day 4 and discharged home on day 5 with moxifloxacin to complete 7 days of antibiotics.
Bottom line
Acute simple transfusions and exchange transfusions are indicated for multiple serious and life-threatening complications in SCA. However, transfusion has many serious and life-threatening potential adverse effects. It is essential to conduct a thorough risk-benefit analysis for each individual SCA patient. Whenever possible, intensive phenotypically matched and leukoreduced RBCs should be used. TH
References
1. American Red Cross. A Compendium of Transfusion Practice Guidelines. Second Edition, April 2013.
2. US Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute. Evidence-Based Management of Sickle Cell Disease, Expert Panel Report, 2014.
3. Smith-Whitely, K and Thompson, AA. Indications and complications of transfusions in sickle cell disease. Pediatr Blood Cancer. 2012;59(2):358-64.
- SCA patients are at risk for serious transfusion complications including iron overload, delayed hemolytic transfusion reaction, and hyperviscosity in addition to the usual transfusion risks.
- Do not transfuse an uncomplicated vaso-occlusive crisis without symptomatic anemia.1-3
- Repeated transfusions create alloimmunization in SCA patients increasing risk for life-threatening transfusion reactions and difficulty locating phenotypically matched RBCs.
- Transfusion should be considered in SCA patients experiencing acute chest syndrome, aplastic anemia, splenic sequestration with acute anemia, acute hepatic sequestration, and severe intrahepatic cholestasis.1,2
- If available, exchange transfusion should be considered for SCA patients experiencing multisystem organ failure, acute stroke, and severe acute chest syndrome.1,2
- American Red Cross. A Compendium of Transfusion Practice Guidelines. Second Edition, April 2013.
Case
A 19-year-old female with a history of sickle cell anemia and hemoglobin SS, presents with a 2-day history of worsening lower back pain and dyspnea. Physical exam reveals oxygen saturation of 87% on room air, a temperature of 39.2° C, respiratory rate of 24 breaths per minute, and right-sided rales. Her hemoglobin is 5.3 g/dL (baseline hemoglobin of 7.8 g/dL). Chest radiograph reveals a right upper lobe pneumonia, and she is diagnosed with acute chest syndrome.
What are indications and complications of acute transfusion in sickle cell anemia?
Background
Chronic hemolytic anemia is a trademark of sickle cell anemia (SCA) or hemoglobin (Hb) SS as is acute anemia during illness or vaso-occlusive crises. Blood transfusions were the first therapy used in sickle cell disease, long before the pathophysiology was understood. Transfusion of red blood cells (RBC) increases the percentage of circulating normal Hb A, thereby decreasing the percentage of abnormal, sickled cells. This increases the oxygen-carrying capacity of the patient’s RBCs, improves organ perfusion, prevents organ damage, and can be life saving. SCA patients are the largest users of the United States rare donor blood bank registry.1
Unfortunately, transfusion comes with many risks including infection, transfusion reactions, alloimmunization, iron overload, hyperviscosity, and volume overload.
As SCA is a low-prevalence disease in a minority population, very few studies have been performed. Currently, the guidance available regarding blood transfusion is primarily based on expert opinion.
What to transfuse
Leukoreduced and intensive phenotypically matched RBC are not possible in many medical centers. Previous studies have noted decreased incidence of febrile nonhemolytic anemia transfusion reactions, cytomegalovirus transmission, and human leukocyte antigen alloimmunization in leukoreduced blood transfusions, however, these studies did not include SCA patients.2
Complications from transfusion
Complications from blood transfusions include febrile nonhemolytic transfusion reaction, acute hemolytic transfusion reaction (ABO incompatibility), transfusion-associated lung injury (TRALI), transfusion-associated circulatory overload (TACO), infections, and anaphylaxis. The National Heart, Lung, and Blood Institute guidelines specifically highlight the complications of delayed hemolytic transfusion reaction, iron overload, and hyperviscosity in SCA.Approximately 30% of SCA patients have alloantibodies.2 SCA patients may also develop autoimmunization, an immune response to their own RBC, particularly if the patient has multiple autoantibodies.
Infection is a risk for all individuals receiving transfusion. Screening for hepatitis B, hepatitis C, HIV, human T-cell lymphotropic virus, syphilis, West Nile virus, Trympanosoma, and bacteria are routinely performed but not 100% conclusive. Other diseases not routinely screened for include Creutzfeldt-Jakob disease, Babesia, human herpesvirus-8, dengue fever, malaria, and newer concerns such as Zika virus. 2,3
Febrile nonhemolytic transfusion reactions present as an increase in body temperature of more than 1° C during or shortly after receiving a blood transfusion in the absence of other pyrexic stimulus. Febrile nonhemolytic transfusion reaction occurs more frequently in patients with a previous history of transfusions. The use of leukoreduced RBCs reduces the occurrence to less than 1%.2
TRALI presents with the acute onset of hypoxemia and noncardiogenic pulmonary edema within 6 hours of a blood transfusion in the absence of other etiologies. The mechanism of TRALI is caused by an inflammatory response causing injury to the alveolar capillary membrane and the development of pulmonary edema.1
TACO presents with cardiogenic pulmonary edema not from another etiology. This is usually seen after transfusion of excessive volumes of blood or after excessively rapid rates of transfusion.1
Delayed hemolytic transfusion reaction (DHTR) may be a life-threatening immune response to donor cell antigens. The reaction is identified by a drop in the patient’s hemoglobin below the pretransfusion level, reticulocytopenia, a positive direct Coombs test, and occasionally jaundice on physical exam.2 Patients may have an unexpectedly high hemoglobin S% after transfusion from the hemolysis of donor cells. The pathognomonic feature is development of a new alloantibody. DHTR occurs more often in individuals who have received recurrent transfusions and has been reported in 4%-11% of transfused SCA patients.3 Donor and native cells hemolyze intra- and extravascularly 5-20 days after receiving a transfusion.2 DHTR is likely underestimated in SCA as it may be confused for a vaso-occlusive crisis.
Iron overload from recurrent transfusions is a slow, chronic process resulting in end organ damage of the heart, liver, and pancreas. It is associated with more frequent hospitalizations and higher mortality in SCA.3 The average person has 4-5 g of iron with no process to remove the excess. One unit of packed red blood cells adds 250-300 mg of iron.2 Ferritin somewhat correlates to iron overload but is not a reliable method because it is an acute-phase reactant. Liver biopsy is the current diagnostic gold standard, however, noninvasive MRI is gaining diagnostic credibility.
Hb SS blood has up to 10 times higher viscosity than does non–sickle cell blood at the same hemoglobin level. RBC transfusion increases the already hyperviscous state of SCA resulting in slow blood flow through vessels. The slow flow through small vessels from hyperviscosity may result in additional sickling and trigger or worsen a vaso-occlusive crisis. Avascular necrosis is theorized to be a result of hyperviscosity as it occurs more commonly in sickle cell patients with higher hemoglobin. It is important not to transfuse to baseline or above a hemoglobin of 10 g/dL to avoid worsening hyperviscosity.2
When to consider transfusion
Unfortunately, there are no strong randomized controlled trials to definitively dictate when simple transfusions or exchange transfusions are indicated. Acute simple transfusions should be considered in certain circumstances including acute chest syndrome, acute stroke, aplastic anemia, preoperative transfusion, splenic sequestration plus severe anemia, acute hepatic sequestration, and severe acute intrahepatic cholestasis.2

Few studies compare simple transfusion and exchange transfusion.2 The decision to use exchange transfusion over simple transfusion often is based on availability of exchange transfusion, ability of simple transfusion to decrease the percentage of hemoglobin S, and/or the patient’s current hemoglobin to avoid hyperviscosity from simple transfusion.3 Exchange transfusion should be considered for hemoglobin greater than 8-9 g/dL.2
Acute hepatic sequestration (AHS) occurs with the sequestration of RBCs in the liver and is marked by greater than 2 g/dL decrease in hemoglobin and hepatic enlargement, compared with baseline. The stretching of the hepatic capsule results in right upper quadrant pain. AHS often develops over a few hours to a few days with only mild elevation of liver function tests. AHS may be underestimated as two-thirds of SCA patients have hepatomegaly. Unless the hepatomegaly is radiographically monitored it may not be possible to determine an acute increase in liver size.2
Aplastic crisis presents as a gradual onset of fatigue, shortness of breath, and sometimes syncope or fever. Physical examination may reveal tachycardia and occasionally frank heart failure. The hemoglobin is usually far below the patient’s baseline level with an inappropriate, severely low reticulocyte count. Aplastic crisis should be transfused immediately because of the markedly short life expectancy of hemoglobin S RBCs, but does not need to be transfused to baseline.2
Acute splenic sequestration presents as a decrease in hemoglobin by greater than 2 g/dL, elevated reticulocyte count and circulating nucleated red blood cells, thrombocytopenia, and sudden splenomegaly.2 The goal of transfusion is for partial correction because of the risk of hyperviscosity when the spleen releases the sequestered RBCs.
Acute chest syndrome (ACS) presents as a pneumonia radiographically consistent with a respiratory tract infection caused by cough, shortness of breath, retractions, and/or rales. ACS is the most common cause of death in SCA. ACS is usually from infection but may be because of fat embolism, intrapulmonary aggregates of sickled cells, atelectasis, or pulmonary edema.2 If ACS has a hemoglobin decrease of greater than 1g/dL, consider transfusion.1,2
Severe acute chest syndrome is distinguished by radiographic evidence of multilobe pneumonia, increased work of breathing, pleural effusions, and oxygen saturation below 95% with supplemental oxygen. Severe ACS may have a decrease in hemoglobin despite receiving transfusion. Exchange transfusion is recommended because of the high mortality in severe ACS.2
Preoperative transfusion is used to decrease the incidence of postoperative vaso-occlusive crisis, acute stroke, or ACS for patients receiving general anesthesia. The goal for transfusion hemoglobin is 10g/dL. In SCA patients with a hemoglobin greater than 9g/dL, exchange transfusion may be considered to avoid hyperviscosity.1,2
Multisystem organ failure (MSOF) is severe and life-threatening lung, liver, and/or kidney failure. MSOF may occur after several days of hospitalization. It is often unanticipated and swift, frequently presenting with fever, a rapid increase in anemia, thrombocytopenia, and altered mental status. Lung failure often presents as ACS. Liver failure is marked by hyperbilirubinemia, elevated transaminases, and coagulopathy. Kidney failure is marked by elevated creatinine, with or without change in urine output and hyperkalemia. Rapid treatment with transfusion or exchange transfusion reduces mortality.
The incidence of acute ischemic stroke in SCA decreases with prophylactic transfusion of patients with elevated transcranial Dopplers. Acute stroke is usually secondary to stenosis or an occlusion of the internal carotid or middle cerebral artery. Acute hemorrhagic stroke may present as severe headache and loss of consciousness. Acute stroke should be confirmed radiographically, then exchange transfusion instituted rapidly.2
When not to transfuse
- Do not transfuse for simple vaso-occlusive crisis in the absence of symptoms attributable to acute anemia.1-3
- Do not transfuse for priapism.2
- Do not transfuse for acute renal failure unless there is MSOF.2
Back to the case
The patient was admitted for vaso-occlusive crisis and was started on patient-controlled analgesia with hydromorphone and IV fluids. Azithromycin and ceftriaxone were initiated empirically for community-acquired pneumonia. She was given one unit of phenotypically matched, leukoreduced RBCs for acute chest syndrome. Her hemoglobin increased to 6.1 g/dL. Her fever resolved on day 2, and her dyspnea improved on day 3 of hospitalization. She was weaned off of her patient-controlled analgesia on day 4 and discharged home on day 5 with moxifloxacin to complete 7 days of antibiotics.
Bottom line
Acute simple transfusions and exchange transfusions are indicated for multiple serious and life-threatening complications in SCA. However, transfusion has many serious and life-threatening potential adverse effects. It is essential to conduct a thorough risk-benefit analysis for each individual SCA patient. Whenever possible, intensive phenotypically matched and leukoreduced RBCs should be used. TH
References
1. American Red Cross. A Compendium of Transfusion Practice Guidelines. Second Edition, April 2013.
2. US Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute. Evidence-Based Management of Sickle Cell Disease, Expert Panel Report, 2014.
3. Smith-Whitely, K and Thompson, AA. Indications and complications of transfusions in sickle cell disease. Pediatr Blood Cancer. 2012;59(2):358-64.
- SCA patients are at risk for serious transfusion complications including iron overload, delayed hemolytic transfusion reaction, and hyperviscosity in addition to the usual transfusion risks.
- Do not transfuse an uncomplicated vaso-occlusive crisis without symptomatic anemia.1-3
- Repeated transfusions create alloimmunization in SCA patients increasing risk for life-threatening transfusion reactions and difficulty locating phenotypically matched RBCs.
- Transfusion should be considered in SCA patients experiencing acute chest syndrome, aplastic anemia, splenic sequestration with acute anemia, acute hepatic sequestration, and severe intrahepatic cholestasis.1,2
- If available, exchange transfusion should be considered for SCA patients experiencing multisystem organ failure, acute stroke, and severe acute chest syndrome.1,2
- American Red Cross. A Compendium of Transfusion Practice Guidelines. Second Edition, April 2013.
EBUS scope, EUS-FNA similarly effective
When assessing a patient for lung cancer, a procedure involving the insertion of an EBUS scope in the esophagus – EUS-B-FNA – can achieve similarly accurate results as endoscopic ultrasound guided–fine-needle aspiration (EUS-FNA), according to a new study.
This finding could lead patients to choose EUS-B-FNA over EUS-FNA – the standard of care for analyzing potential metastasis of the left adrenal glands (LAGs) – resulting in both time and cost savings for patients.
“A recent report showed that LAG visualization using the EBUS scope was possible in 85% of patients,” according to the authors of this study, including Prof. Jouke T. Annema, MD of the University of Amsterdam. Prior to this new research, it was unknown to what extent a single EBUS scope adequately assess and sample the LAGs and how its performance related to the use of a conventional endoscopic ultrasound–guided scope (Lung Cancer. 2017. doi: org/10.1016/j.lungcan.2017.02.011).
Dr. Annema and his coauthors recruited patients from four centers – three in the Netherlands, one in Poland – and followed them prospectively. Patients with “(suspected) lung cancer [who] had an indication for both mediastinal lymph node and LAG sampling” were recruited for the study. The researchers followed 44 patients through final diagnosis to determine if they ultimately had lung cancer.
Subjects first received complete mediastinal and hilar staging of lung cancer and any present tumors via an EBUS and EUS-B procedure. Following an EBUS examination of the mediastinum, the EBUS scope was retracted from the trachea and positioned into the esophagus for an examination of the mediastinal nodes. Then, the EBUS scope was advanced into the stomach for identification of the LAG. Afterward, the routine EUS-FNA was performed. LAG analysis across both methods involved visualizing the LAG and collecting an adequate tissue sample for testing.
“In short, in order to locate the LAG, a structured three step approach was used according to the EUS assessment tool (EUS-AT): identification of the liver, abdominal aorta, coeliac trunk, left kidney, and LAG,” the authors noted. “By turning the EBUS scope clockwise from the liver, the abdominal aorta and coeliac trun[k] are identified. By subsequently turning the EBUS scope gently in caudal direction, the left kidney and LAG are identified.”
Endoscopists then evaluated both procedures in each subject according to feasibility and practicability to determine if the findings of the experimental procedure were usable. Finally, a cytologic exam was conducted, using Giemsa or Papanicolaou staining to determine if any present cancer had metastasized, and a final diagnosis was made.
LAG analysis had a success rate of 89% (39/44; 95% confidence interval, 76-95%) for EUS-B-FNA, compared with 93% (41/44; 95% CI, 82-98%) for EUS-FNA. Similarly, when looking at the rate of sensitivity for LAG metastases, EUS-B had a rate of sensitivity for LAG metastases of at least 87% (95% CI, 65-97%), while EUS-FNA was found to be at least 83% (95% CI, 62-95%. Endoscopists were equally satisfied with both procedures in the “majority” of cases in this study.
“In [five] cases (11%), the EUS-B-FNA procedure was unsuccessful, due to the inability to make good contact of the ultrasound transducer and the stomach wall,” the authors explained. “The conventional EUS scope is more stable as a result of the increased tube diameter. Another advantage of the conventional echo-endoscope is its wider scanning angle. ... The conventional EUS scope is also longer than the EBUS scope, [but that] does not seem to be the limiting factor.”
No funding source was disclosed for this study. The authors reported no relevant financial disclosures.
When assessing a patient for lung cancer, a procedure involving the insertion of an EBUS scope in the esophagus – EUS-B-FNA – can achieve similarly accurate results as endoscopic ultrasound guided–fine-needle aspiration (EUS-FNA), according to a new study.
This finding could lead patients to choose EUS-B-FNA over EUS-FNA – the standard of care for analyzing potential metastasis of the left adrenal glands (LAGs) – resulting in both time and cost savings for patients.
“A recent report showed that LAG visualization using the EBUS scope was possible in 85% of patients,” according to the authors of this study, including Prof. Jouke T. Annema, MD of the University of Amsterdam. Prior to this new research, it was unknown to what extent a single EBUS scope adequately assess and sample the LAGs and how its performance related to the use of a conventional endoscopic ultrasound–guided scope (Lung Cancer. 2017. doi: org/10.1016/j.lungcan.2017.02.011).
Dr. Annema and his coauthors recruited patients from four centers – three in the Netherlands, one in Poland – and followed them prospectively. Patients with “(suspected) lung cancer [who] had an indication for both mediastinal lymph node and LAG sampling” were recruited for the study. The researchers followed 44 patients through final diagnosis to determine if they ultimately had lung cancer.
Subjects first received complete mediastinal and hilar staging of lung cancer and any present tumors via an EBUS and EUS-B procedure. Following an EBUS examination of the mediastinum, the EBUS scope was retracted from the trachea and positioned into the esophagus for an examination of the mediastinal nodes. Then, the EBUS scope was advanced into the stomach for identification of the LAG. Afterward, the routine EUS-FNA was performed. LAG analysis across both methods involved visualizing the LAG and collecting an adequate tissue sample for testing.
“In short, in order to locate the LAG, a structured three step approach was used according to the EUS assessment tool (EUS-AT): identification of the liver, abdominal aorta, coeliac trunk, left kidney, and LAG,” the authors noted. “By turning the EBUS scope clockwise from the liver, the abdominal aorta and coeliac trun[k] are identified. By subsequently turning the EBUS scope gently in caudal direction, the left kidney and LAG are identified.”
Endoscopists then evaluated both procedures in each subject according to feasibility and practicability to determine if the findings of the experimental procedure were usable. Finally, a cytologic exam was conducted, using Giemsa or Papanicolaou staining to determine if any present cancer had metastasized, and a final diagnosis was made.
LAG analysis had a success rate of 89% (39/44; 95% confidence interval, 76-95%) for EUS-B-FNA, compared with 93% (41/44; 95% CI, 82-98%) for EUS-FNA. Similarly, when looking at the rate of sensitivity for LAG metastases, EUS-B had a rate of sensitivity for LAG metastases of at least 87% (95% CI, 65-97%), while EUS-FNA was found to be at least 83% (95% CI, 62-95%. Endoscopists were equally satisfied with both procedures in the “majority” of cases in this study.
“In [five] cases (11%), the EUS-B-FNA procedure was unsuccessful, due to the inability to make good contact of the ultrasound transducer and the stomach wall,” the authors explained. “The conventional EUS scope is more stable as a result of the increased tube diameter. Another advantage of the conventional echo-endoscope is its wider scanning angle. ... The conventional EUS scope is also longer than the EBUS scope, [but that] does not seem to be the limiting factor.”
No funding source was disclosed for this study. The authors reported no relevant financial disclosures.
When assessing a patient for lung cancer, a procedure involving the insertion of an EBUS scope in the esophagus – EUS-B-FNA – can achieve similarly accurate results as endoscopic ultrasound guided–fine-needle aspiration (EUS-FNA), according to a new study.
This finding could lead patients to choose EUS-B-FNA over EUS-FNA – the standard of care for analyzing potential metastasis of the left adrenal glands (LAGs) – resulting in both time and cost savings for patients.
“A recent report showed that LAG visualization using the EBUS scope was possible in 85% of patients,” according to the authors of this study, including Prof. Jouke T. Annema, MD of the University of Amsterdam. Prior to this new research, it was unknown to what extent a single EBUS scope adequately assess and sample the LAGs and how its performance related to the use of a conventional endoscopic ultrasound–guided scope (Lung Cancer. 2017. doi: org/10.1016/j.lungcan.2017.02.011).
Dr. Annema and his coauthors recruited patients from four centers – three in the Netherlands, one in Poland – and followed them prospectively. Patients with “(suspected) lung cancer [who] had an indication for both mediastinal lymph node and LAG sampling” were recruited for the study. The researchers followed 44 patients through final diagnosis to determine if they ultimately had lung cancer.
Subjects first received complete mediastinal and hilar staging of lung cancer and any present tumors via an EBUS and EUS-B procedure. Following an EBUS examination of the mediastinum, the EBUS scope was retracted from the trachea and positioned into the esophagus for an examination of the mediastinal nodes. Then, the EBUS scope was advanced into the stomach for identification of the LAG. Afterward, the routine EUS-FNA was performed. LAG analysis across both methods involved visualizing the LAG and collecting an adequate tissue sample for testing.
“In short, in order to locate the LAG, a structured three step approach was used according to the EUS assessment tool (EUS-AT): identification of the liver, abdominal aorta, coeliac trunk, left kidney, and LAG,” the authors noted. “By turning the EBUS scope clockwise from the liver, the abdominal aorta and coeliac trun[k] are identified. By subsequently turning the EBUS scope gently in caudal direction, the left kidney and LAG are identified.”
Endoscopists then evaluated both procedures in each subject according to feasibility and practicability to determine if the findings of the experimental procedure were usable. Finally, a cytologic exam was conducted, using Giemsa or Papanicolaou staining to determine if any present cancer had metastasized, and a final diagnosis was made.
LAG analysis had a success rate of 89% (39/44; 95% confidence interval, 76-95%) for EUS-B-FNA, compared with 93% (41/44; 95% CI, 82-98%) for EUS-FNA. Similarly, when looking at the rate of sensitivity for LAG metastases, EUS-B had a rate of sensitivity for LAG metastases of at least 87% (95% CI, 65-97%), while EUS-FNA was found to be at least 83% (95% CI, 62-95%. Endoscopists were equally satisfied with both procedures in the “majority” of cases in this study.
“In [five] cases (11%), the EUS-B-FNA procedure was unsuccessful, due to the inability to make good contact of the ultrasound transducer and the stomach wall,” the authors explained. “The conventional EUS scope is more stable as a result of the increased tube diameter. Another advantage of the conventional echo-endoscope is its wider scanning angle. ... The conventional EUS scope is also longer than the EBUS scope, [but that] does not seem to be the limiting factor.”
No funding source was disclosed for this study. The authors reported no relevant financial disclosures.
FROM LUNG CANCER
Key clinical point:
Major finding: EUS-B-FNA had a success rate of 89%, versus 93% for EUS-FNA, while sensitivity for LAG metastases were 87% and 83%, respectively.
Data source: Multicenter, prospective study of 44 consecutive suspected lung cancer patients.
Disclosures: No funding source disclosed. Authors reported no relevant financial disclosures.
Antiviral medication successful for treating HCV in hepatocellular carcinoma
Direct-acting antiviral (DAA) medication was successful in treating hepatitis C in 74.5% of patients with hepatocellular carcinoma, and 93.4% of patients with HCC who underwent liver transplants, according to a study funded by Veterans Affairs.
In order to study the effectiveness of DAAs in this setting, Lauren A. Beste, MD, and her colleagues studied a cohort of 17,487 veterans; 624 patients reported having HCC, including 142 with HCC and liver transplantation (J Hepatol. 2017. doi. org/10.1016/j.jhep.2017.02.027).
Effects of the DAAs were also studied based on the genotype of patients’ HCV. According to analysis, patients with the genotype 1 HCV virus were most susceptible to the medication, with sustained virologic response (SVR) rates calculated at 79.1% for patients with HCC, 96.4% for HCC and transplant, and 93.1% for non-HCC.
For patients with genotype 2 virus, the SVR rate was 68.9% for those with HCC, and 86.5% for patients without HCC; for genotype 3, the rate of SVR was 68.9% and 86.5% for patients with and without HCC, respectively; and for genotype 4, the SVR rate was 50% and 90.2% for patients with and without HCC, respectively.
Unlike the genotype 1 population, which had 111 patients with HCC and liver transplantation, genotypes 2, 3, and 4 had only 4, 18, and 0 patients, respectively.
Dr. Beste and her colleagues attribute this to how common genotype 1 is, which made up 11,761 of 11,871 patients (99%) with known genotypes treated with either of the two medications.
An LDV/SOF-based regimen was given to more of those with genotype 1 who had HCC (88.1%) or HCC and liver transplantation (99.1%) than to those without HCC.
When comparing fibrosis and cirrhosis (FIB-4) scores among patients with an LDV/SOF-based and PrOD p/m ribavirin regimens, patients given PrOD regimens were less likely to have a higher FIB-4 score (47.7% vs. 73.1%), thrombocytopenia (23.1% vs. 40.2%), or elevated bilirubin (21.6% vs. 35.9%).
Patients with genotype 4 showed similar results in favor of PrOD treatment and genotype 2 patients only received LDV/SOF-based treatment; however genotype 3 showed the most positive results with LDV/SOF-based regimens, reporting a 100% success rate for the seven patients treated in the specific sample.
Overall, treatment was less successful for patients with HCC, compared with those without or who underwent transplantation. While Dr. Beste and her colleagues could not definitively explain this, the researchers suggested that it might be from the HCC itself. “The association between HCC and treatment failure persisted after adjustment for cirrhosis, markers of liver dysfunction, and genotype,” said Dr. Beste. “Therefore, these factors cannot explain the lower SVR in patients with HCC, and lead us to suspect that HCC itself could be causally linked to antiviral treatment failure.”
The researcher’s presented the hypothesis that “altered hepatic immune processes may predispose both to HCC and to poorer antiviral treatment outcomes.”
While the study was strengthened by the size and scope of the cohort, researchers were limited by a lack of data, including SVR data for 11.6% of HCC patients and 6.3% of HCC patients with transplantations. Researchers were also unable to attain HCC treatment data for nearly 24% of nontransplanted cases. Finally, the sample size was “overwhelmingly” male, which may give the study “limited generalizability to women.”
[email protected]
On Twitter @EAZweets
Direct-acting antiviral (DAA) medication was successful in treating hepatitis C in 74.5% of patients with hepatocellular carcinoma, and 93.4% of patients with HCC who underwent liver transplants, according to a study funded by Veterans Affairs.
In order to study the effectiveness of DAAs in this setting, Lauren A. Beste, MD, and her colleagues studied a cohort of 17,487 veterans; 624 patients reported having HCC, including 142 with HCC and liver transplantation (J Hepatol. 2017. doi. org/10.1016/j.jhep.2017.02.027).
Effects of the DAAs were also studied based on the genotype of patients’ HCV. According to analysis, patients with the genotype 1 HCV virus were most susceptible to the medication, with sustained virologic response (SVR) rates calculated at 79.1% for patients with HCC, 96.4% for HCC and transplant, and 93.1% for non-HCC.
For patients with genotype 2 virus, the SVR rate was 68.9% for those with HCC, and 86.5% for patients without HCC; for genotype 3, the rate of SVR was 68.9% and 86.5% for patients with and without HCC, respectively; and for genotype 4, the SVR rate was 50% and 90.2% for patients with and without HCC, respectively.
Unlike the genotype 1 population, which had 111 patients with HCC and liver transplantation, genotypes 2, 3, and 4 had only 4, 18, and 0 patients, respectively.
Dr. Beste and her colleagues attribute this to how common genotype 1 is, which made up 11,761 of 11,871 patients (99%) with known genotypes treated with either of the two medications.
An LDV/SOF-based regimen was given to more of those with genotype 1 who had HCC (88.1%) or HCC and liver transplantation (99.1%) than to those without HCC.
When comparing fibrosis and cirrhosis (FIB-4) scores among patients with an LDV/SOF-based and PrOD p/m ribavirin regimens, patients given PrOD regimens were less likely to have a higher FIB-4 score (47.7% vs. 73.1%), thrombocytopenia (23.1% vs. 40.2%), or elevated bilirubin (21.6% vs. 35.9%).
Patients with genotype 4 showed similar results in favor of PrOD treatment and genotype 2 patients only received LDV/SOF-based treatment; however genotype 3 showed the most positive results with LDV/SOF-based regimens, reporting a 100% success rate for the seven patients treated in the specific sample.
Overall, treatment was less successful for patients with HCC, compared with those without or who underwent transplantation. While Dr. Beste and her colleagues could not definitively explain this, the researchers suggested that it might be from the HCC itself. “The association between HCC and treatment failure persisted after adjustment for cirrhosis, markers of liver dysfunction, and genotype,” said Dr. Beste. “Therefore, these factors cannot explain the lower SVR in patients with HCC, and lead us to suspect that HCC itself could be causally linked to antiviral treatment failure.”
The researcher’s presented the hypothesis that “altered hepatic immune processes may predispose both to HCC and to poorer antiviral treatment outcomes.”
While the study was strengthened by the size and scope of the cohort, researchers were limited by a lack of data, including SVR data for 11.6% of HCC patients and 6.3% of HCC patients with transplantations. Researchers were also unable to attain HCC treatment data for nearly 24% of nontransplanted cases. Finally, the sample size was “overwhelmingly” male, which may give the study “limited generalizability to women.”
[email protected]
On Twitter @EAZweets
Direct-acting antiviral (DAA) medication was successful in treating hepatitis C in 74.5% of patients with hepatocellular carcinoma, and 93.4% of patients with HCC who underwent liver transplants, according to a study funded by Veterans Affairs.
In order to study the effectiveness of DAAs in this setting, Lauren A. Beste, MD, and her colleagues studied a cohort of 17,487 veterans; 624 patients reported having HCC, including 142 with HCC and liver transplantation (J Hepatol. 2017. doi. org/10.1016/j.jhep.2017.02.027).
Effects of the DAAs were also studied based on the genotype of patients’ HCV. According to analysis, patients with the genotype 1 HCV virus were most susceptible to the medication, with sustained virologic response (SVR) rates calculated at 79.1% for patients with HCC, 96.4% for HCC and transplant, and 93.1% for non-HCC.
For patients with genotype 2 virus, the SVR rate was 68.9% for those with HCC, and 86.5% for patients without HCC; for genotype 3, the rate of SVR was 68.9% and 86.5% for patients with and without HCC, respectively; and for genotype 4, the SVR rate was 50% and 90.2% for patients with and without HCC, respectively.
Unlike the genotype 1 population, which had 111 patients with HCC and liver transplantation, genotypes 2, 3, and 4 had only 4, 18, and 0 patients, respectively.
Dr. Beste and her colleagues attribute this to how common genotype 1 is, which made up 11,761 of 11,871 patients (99%) with known genotypes treated with either of the two medications.
An LDV/SOF-based regimen was given to more of those with genotype 1 who had HCC (88.1%) or HCC and liver transplantation (99.1%) than to those without HCC.
When comparing fibrosis and cirrhosis (FIB-4) scores among patients with an LDV/SOF-based and PrOD p/m ribavirin regimens, patients given PrOD regimens were less likely to have a higher FIB-4 score (47.7% vs. 73.1%), thrombocytopenia (23.1% vs. 40.2%), or elevated bilirubin (21.6% vs. 35.9%).
Patients with genotype 4 showed similar results in favor of PrOD treatment and genotype 2 patients only received LDV/SOF-based treatment; however genotype 3 showed the most positive results with LDV/SOF-based regimens, reporting a 100% success rate for the seven patients treated in the specific sample.
Overall, treatment was less successful for patients with HCC, compared with those without or who underwent transplantation. While Dr. Beste and her colleagues could not definitively explain this, the researchers suggested that it might be from the HCC itself. “The association between HCC and treatment failure persisted after adjustment for cirrhosis, markers of liver dysfunction, and genotype,” said Dr. Beste. “Therefore, these factors cannot explain the lower SVR in patients with HCC, and lead us to suspect that HCC itself could be causally linked to antiviral treatment failure.”
The researcher’s presented the hypothesis that “altered hepatic immune processes may predispose both to HCC and to poorer antiviral treatment outcomes.”
While the study was strengthened by the size and scope of the cohort, researchers were limited by a lack of data, including SVR data for 11.6% of HCC patients and 6.3% of HCC patients with transplantations. Researchers were also unable to attain HCC treatment data for nearly 24% of nontransplanted cases. Finally, the sample size was “overwhelmingly” male, which may give the study “limited generalizability to women.”
[email protected]
On Twitter @EAZweets
FROM JOURNAL OF HEPATOLOGY
Key clinical point:
Major finding: Of the 17,487 patients given HCV treatment, sustained virologic response was found in 91.9% of patients without HCC, 74.5% with HCC, and 93.4% of patients with HCC and liver transplantation.
Data source: 17,487 patient records from 2014-2015 obtained through the Veterans Affairs Corporate Data Warehouse. Tests were approved by the VA Puget Sound Institutional Review Board.
Disclosures: The study was funded in part by Clinical Science Research and Development, Office of Research and Development, Veterans Affairs. Researchers reported no conflicts of interest.