User login
Chemoradiation standard of care in muscle-invasive bladder cancer
Orlando – The updated results of a large phase III trial support the use of chemoradiation with 5-fluorouracil (5-FU) and mitomycin C (MMC) and confirm that this treatment regimen should be a standard of care for muscle-invasive bladder cancer (MIBC).
When comparing patients who received radiation therapy with those who received chemoradiation, there was a robust improvement in bladder cancer specific survival for the latter when adjusted for known prognostic factors (hazard ratio, 0.73; P = .043).
There was also a borderline significant improvement in metastasis-free survival (HR, 0.78) and a significant reduction in the need for salvage cystectomy in the patients treated with chemoradiation (2-year rate, chemoradiotherapy11% vs. radiation therapy:17%, HR, 0.54; P = .03).
There were no statistically significant differences between groups when it came to overall survival, but, even though overall survival did not reach significance, at 2 years, there was a hint of separation of the curves, explained study author Emma Hall, MD, from the Institute of Cancer Research, London at the 2017 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, American Society for Radiation Oncology, and the Society of Urologic Oncology.
One of the treatment arms received a reduced rate of radiation therapy to see if that would decrease toxicity. “The radiation therapy volume modification that we used did not reduce toxicity, but there is no evidence of an increase in local failure rate, suggesting it is safe to pursue clinical trials of volume sparing radiation therapy using newer technology adaptive delivery techniques,” said Dr. Hall.
The initial findings of the BC2001 study showed that adding chemotherapy (5-FU + MMC) to radiotherapy significantly improved rates of MIBC locoregional control but that reduced high-dose volume versus standard radiotherapy did not significantly reduce late side effects.
This study was a clinical trial set up to test two different questions in the treatment of MIBC, as an alternative to cystectomy. “We wanted to see if adding synchronous chemotherapy to radiotherapy would improve locoregional recurrence control and if reducing the radiation dose to uninvolved bladder would reduce toxicity and not impact local regional recurrence control,” according to Dr. Hall.
Under the 2 x 2 partial factorial design, 458 patients were randomized to radiation therapy (n = 178) or chemoradiation (n = 182) and/or to standard radiation therapy (n = 108) or reduced high-dose volume radiation therapy (n = 111).
The primary endpoint was locoregional control, and secondary endpoints included overall survival, bladder-cancer specific survival, metastasis-free survival, and salvage cystectomy rates.
The initial patients received radiation therapy instead of chemoradiation, and there was a robust improvement in bladder cancer–specific survival when adjusted for known prognostic factors (HR, 0.73; P = .043).
The analysis, presented in 2012, showed a reduction of about one-third of locoregional recurrence. The local control rates were 54% in the radiotherapy-alone arm and 67% in the chemoradiotherapy arm.
There was no significant difference in overall survival at that time.
For the radiotherapy comparison, the rate of late toxicity was low, and much lower than was anticipated, at the outset of the trial, and there was no difference in treatment groups, said Dr. Hall.
In an updated analysis, with a median of 10 years of follow-up, 70% of the patients were now deceased. “These represent robust data, and it is unlikely we will see any changes to the data,” she noted.
The findings presented now had an additional 4 years of follow-up, and while there were additional late events, the results were basically the same.
The rate of local control now showed a 40% reduction in the risk of recurrence and 5-year local control rates of 49% in the radiotherapy arm and 63% in the chemoradiotherapy arm.
“With 10 years follow up, an improvement in locoregional control and a reduced salvage cystectomy rate is confirmed with chemoradiotherapy,” Dr. Hall concluded, “and, taken together with the good quality of life data we have, this is important for this group.”
In a discussion of the paper, Dr. Jonathan Rosenberg, MD, from Memorial Sloan Kettering Cancer Center in New York, agrees with the conclusion that the data continue to support the use of chemoradiotherapy and that 5-FU + MMC is a good option.
He noted that 5-FU + MMC is a standard of care regardless of cisplatin eligibility, but he cannot draw conclusions on dose volume. “There are also other options for chemosensitization,” he said, but it is also import to determine the best way to select patients who will derive the most benefit from chemoradiation.
“There is a high need for robust predictive biomarkers, and we need novel approaches to move beyond chemotherapy,” he said.
The study was supported by Cancer Research UK. Dr Hall has received research funding from Accuray, AstraZeneca, Aventis, and Bayer. Several co-authors also have disclosed relationships with industry. Dr. Rosenberg has disclosed multiple relationships with industry.
Orlando – The updated results of a large phase III trial support the use of chemoradiation with 5-fluorouracil (5-FU) and mitomycin C (MMC) and confirm that this treatment regimen should be a standard of care for muscle-invasive bladder cancer (MIBC).
When comparing patients who received radiation therapy with those who received chemoradiation, there was a robust improvement in bladder cancer specific survival for the latter when adjusted for known prognostic factors (hazard ratio, 0.73; P = .043).
There was also a borderline significant improvement in metastasis-free survival (HR, 0.78) and a significant reduction in the need for salvage cystectomy in the patients treated with chemoradiation (2-year rate, chemoradiotherapy11% vs. radiation therapy:17%, HR, 0.54; P = .03).
There were no statistically significant differences between groups when it came to overall survival, but, even though overall survival did not reach significance, at 2 years, there was a hint of separation of the curves, explained study author Emma Hall, MD, from the Institute of Cancer Research, London at the 2017 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, American Society for Radiation Oncology, and the Society of Urologic Oncology.
One of the treatment arms received a reduced rate of radiation therapy to see if that would decrease toxicity. “The radiation therapy volume modification that we used did not reduce toxicity, but there is no evidence of an increase in local failure rate, suggesting it is safe to pursue clinical trials of volume sparing radiation therapy using newer technology adaptive delivery techniques,” said Dr. Hall.
The initial findings of the BC2001 study showed that adding chemotherapy (5-FU + MMC) to radiotherapy significantly improved rates of MIBC locoregional control but that reduced high-dose volume versus standard radiotherapy did not significantly reduce late side effects.
This study was a clinical trial set up to test two different questions in the treatment of MIBC, as an alternative to cystectomy. “We wanted to see if adding synchronous chemotherapy to radiotherapy would improve locoregional recurrence control and if reducing the radiation dose to uninvolved bladder would reduce toxicity and not impact local regional recurrence control,” according to Dr. Hall.
Under the 2 x 2 partial factorial design, 458 patients were randomized to radiation therapy (n = 178) or chemoradiation (n = 182) and/or to standard radiation therapy (n = 108) or reduced high-dose volume radiation therapy (n = 111).
The primary endpoint was locoregional control, and secondary endpoints included overall survival, bladder-cancer specific survival, metastasis-free survival, and salvage cystectomy rates.
The initial patients received radiation therapy instead of chemoradiation, and there was a robust improvement in bladder cancer–specific survival when adjusted for known prognostic factors (HR, 0.73; P = .043).
The analysis, presented in 2012, showed a reduction of about one-third of locoregional recurrence. The local control rates were 54% in the radiotherapy-alone arm and 67% in the chemoradiotherapy arm.
There was no significant difference in overall survival at that time.
For the radiotherapy comparison, the rate of late toxicity was low, and much lower than was anticipated, at the outset of the trial, and there was no difference in treatment groups, said Dr. Hall.
In an updated analysis, with a median of 10 years of follow-up, 70% of the patients were now deceased. “These represent robust data, and it is unlikely we will see any changes to the data,” she noted.
The findings presented now had an additional 4 years of follow-up, and while there were additional late events, the results were basically the same.
The rate of local control now showed a 40% reduction in the risk of recurrence and 5-year local control rates of 49% in the radiotherapy arm and 63% in the chemoradiotherapy arm.
“With 10 years follow up, an improvement in locoregional control and a reduced salvage cystectomy rate is confirmed with chemoradiotherapy,” Dr. Hall concluded, “and, taken together with the good quality of life data we have, this is important for this group.”
In a discussion of the paper, Dr. Jonathan Rosenberg, MD, from Memorial Sloan Kettering Cancer Center in New York, agrees with the conclusion that the data continue to support the use of chemoradiotherapy and that 5-FU + MMC is a good option.
He noted that 5-FU + MMC is a standard of care regardless of cisplatin eligibility, but he cannot draw conclusions on dose volume. “There are also other options for chemosensitization,” he said, but it is also import to determine the best way to select patients who will derive the most benefit from chemoradiation.
“There is a high need for robust predictive biomarkers, and we need novel approaches to move beyond chemotherapy,” he said.
The study was supported by Cancer Research UK. Dr Hall has received research funding from Accuray, AstraZeneca, Aventis, and Bayer. Several co-authors also have disclosed relationships with industry. Dr. Rosenberg has disclosed multiple relationships with industry.
Orlando – The updated results of a large phase III trial support the use of chemoradiation with 5-fluorouracil (5-FU) and mitomycin C (MMC) and confirm that this treatment regimen should be a standard of care for muscle-invasive bladder cancer (MIBC).
When comparing patients who received radiation therapy with those who received chemoradiation, there was a robust improvement in bladder cancer specific survival for the latter when adjusted for known prognostic factors (hazard ratio, 0.73; P = .043).
There was also a borderline significant improvement in metastasis-free survival (HR, 0.78) and a significant reduction in the need for salvage cystectomy in the patients treated with chemoradiation (2-year rate, chemoradiotherapy11% vs. radiation therapy:17%, HR, 0.54; P = .03).
There were no statistically significant differences between groups when it came to overall survival, but, even though overall survival did not reach significance, at 2 years, there was a hint of separation of the curves, explained study author Emma Hall, MD, from the Institute of Cancer Research, London at the 2017 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, American Society for Radiation Oncology, and the Society of Urologic Oncology.
One of the treatment arms received a reduced rate of radiation therapy to see if that would decrease toxicity. “The radiation therapy volume modification that we used did not reduce toxicity, but there is no evidence of an increase in local failure rate, suggesting it is safe to pursue clinical trials of volume sparing radiation therapy using newer technology adaptive delivery techniques,” said Dr. Hall.
The initial findings of the BC2001 study showed that adding chemotherapy (5-FU + MMC) to radiotherapy significantly improved rates of MIBC locoregional control but that reduced high-dose volume versus standard radiotherapy did not significantly reduce late side effects.
This study was a clinical trial set up to test two different questions in the treatment of MIBC, as an alternative to cystectomy. “We wanted to see if adding synchronous chemotherapy to radiotherapy would improve locoregional recurrence control and if reducing the radiation dose to uninvolved bladder would reduce toxicity and not impact local regional recurrence control,” according to Dr. Hall.
Under the 2 x 2 partial factorial design, 458 patients were randomized to radiation therapy (n = 178) or chemoradiation (n = 182) and/or to standard radiation therapy (n = 108) or reduced high-dose volume radiation therapy (n = 111).
The primary endpoint was locoregional control, and secondary endpoints included overall survival, bladder-cancer specific survival, metastasis-free survival, and salvage cystectomy rates.
The initial patients received radiation therapy instead of chemoradiation, and there was a robust improvement in bladder cancer–specific survival when adjusted for known prognostic factors (HR, 0.73; P = .043).
The analysis, presented in 2012, showed a reduction of about one-third of locoregional recurrence. The local control rates were 54% in the radiotherapy-alone arm and 67% in the chemoradiotherapy arm.
There was no significant difference in overall survival at that time.
For the radiotherapy comparison, the rate of late toxicity was low, and much lower than was anticipated, at the outset of the trial, and there was no difference in treatment groups, said Dr. Hall.
In an updated analysis, with a median of 10 years of follow-up, 70% of the patients were now deceased. “These represent robust data, and it is unlikely we will see any changes to the data,” she noted.
The findings presented now had an additional 4 years of follow-up, and while there were additional late events, the results were basically the same.
The rate of local control now showed a 40% reduction in the risk of recurrence and 5-year local control rates of 49% in the radiotherapy arm and 63% in the chemoradiotherapy arm.
“With 10 years follow up, an improvement in locoregional control and a reduced salvage cystectomy rate is confirmed with chemoradiotherapy,” Dr. Hall concluded, “and, taken together with the good quality of life data we have, this is important for this group.”
In a discussion of the paper, Dr. Jonathan Rosenberg, MD, from Memorial Sloan Kettering Cancer Center in New York, agrees with the conclusion that the data continue to support the use of chemoradiotherapy and that 5-FU + MMC is a good option.
He noted that 5-FU + MMC is a standard of care regardless of cisplatin eligibility, but he cannot draw conclusions on dose volume. “There are also other options for chemosensitization,” he said, but it is also import to determine the best way to select patients who will derive the most benefit from chemoradiation.
“There is a high need for robust predictive biomarkers, and we need novel approaches to move beyond chemotherapy,” he said.
The study was supported by Cancer Research UK. Dr Hall has received research funding from Accuray, AstraZeneca, Aventis, and Bayer. Several co-authors also have disclosed relationships with industry. Dr. Rosenberg has disclosed multiple relationships with industry.
AT THE GENITOURINARY CANCERS SYMPOSIUM
Key clinical point: Chemoradiation with 5-FU + MMC should be a standard of care in muscle-invasive bladder cancer.
Major finding: When comparing radiation therapy versus chemoradiation, there was a robust improvement in bladder cancer specific survival when adjusted for known prognostic factors (HR, 0.73; P = .043).
Data source: A long-term phase III randomized trial that included 458 patients with MIBC.
Disclosures: The study was supported by Cancer Research UK. Dr. Hall has received research funding from Accuray, AstraZeneca, Aventis , and Bayer. Several coauthors also have disclosed relationships with industry. Dr. Rosenberg has disclosed multiple relationships with industry.
Hyperkalemia in Adults: Review of a Common Electrolyte Imbalance
CE/CME No: CR-1703
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the pathophysiology and causes of hyperkalemia.
• Identify patients who are susceptible to hyperkalemia.
• Recognize the clinical sequelae of hyperkalemia.
• Formulate assessment and treatment plans for patients with hyperkalemia.
FACULTY
Melanie Douglas is a Physician Assistant in the Medicine Department at NYU Langone Medical Center in New York, New York. Denise Rizzolo is a Clinical Assistant Professor in the PA Program at Pace University in New York, New York, and Research Director in the Program of PA Studies at Kean University in Union, New Jersey. Danielle Kruger is an Academic Coordinator and Associate Professor in the PA Program at St. John’s University in Queens, New York. The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of March 2017.
Article begins on next page >>
Hyperkalemia is a common electrolyte disorder associated with life-threatening cardiac arrhythmias. Prompt recognition and appropriate treatment are essential in preventing serious cardiac complications. Although clinical manifestations of hyperkalemia are usually nonspecific or absent, laboratory testing and electrocardiography performed by the astute clinician aware of predisposing risk factors can help direct management.
Potassium is contained mostly in intracellular fluid; only about 2% is found in the extracellular space.1 The average total body potassium is about 50 mEq per kg of body weight (eg, a 70-kg individual has a total body potassium of approximately 3,500 mEq).2 Levels are tightly regulated by alterations in excretion in the distal renal tubule in response to potassium load and balance, and potassium distribution is influenced by insulin, aldosterone, catecholamines, and acid-base status.2 Movement of potassium across cell membranes is driven by the sodium-potassium adenosine triphosphatase (Na-K-ATPase) pump.3 In this article, we use the common serum potassium reference range of 3.5 to 5.0 mEq/L and define hyperkalemia as a serum potassium concentration greater than 5.5 mEq/L.4
Hyperkalemia can lead to life-threatening complications of cardiac arrhythmias, asystole, hypotension, flaccid paralysis, tetany, dyspnea, and altered mental status.5 Among patients with end-stage renal disease (ESRD), hyperkalemia is thought to contribute to 2% to 5% of deaths.6 A retrospective study found that patients with serum potassium levels exceeding 6.0 mEq/L on ICU admission had a significantly higher death rate within 30 days than patients who were normokalemic on presentation.7
RISK FACTORS
It is estimated that more than 35% of patients age 70 and older have chronic kidney disease (CKD) stage 3 or higher.8 Hyperkalemia is closely associated with CKD, increasing linearly in relation to the degree of renal impairment.8 As such, the prevalence of hyperkalemia in older adults is high, and it will increase overall as the US population ages. In a retrospective analysis of veterans older than 65 with CKD stage 3 or higher, the prevalence of hyperkalemia was 2.5%.9 Use of certain medications is also associated with hyperkalemia. Another retrospective study analyzed records obtained from 70,873 patients with CKD (estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73 m2) hospitalized in the Veterans Health Administration system. It found that patients treated with renin-angiotensin-aldosterone system (RAAS) blockers, such as ACE inhibitors (ACEis) or angiotensin-receptor blockers (ARBs), had a higher incidence of hyperkalemia (potassium level ≥ 5.5 mEq/L) than patients not treated with these medications (8.22 vs 1.77 events per 100 patient-months).9,10
POTASSIUM HOMEOSTASIS
Tight control over extracellular potassium is maintained in part by the Na-K-ATPase pump, which uses adenosine triphosphatase to move potassium and sodium ions in opposite directions across cell membranes.3 Specifically, three sodium ions are pumped out of the cell for every two potassium ions pumped in, resulting in a potassium gradient that is partially responsible for maintaining a resting membrane potential. This resting membrane potential, which determines myocardial, skeletal muscle, and nerve cell excitability and signaling, is highly sensitive to changes in the extracellular potassium level.4 Even small extracellular imbalances can induce cell depolarization and evoke an action potential. Increased extracellular potassium concentration decreases the resting membrane potential of the myocardium, shortens repolarization time, and decreases the rate of myocardial cell conduction, and also slows down neuromuscular conduction.11,12
Renal tubular function plays a significant role in potassium homeostasis, with approximately 90% of dietary potassium intake ex
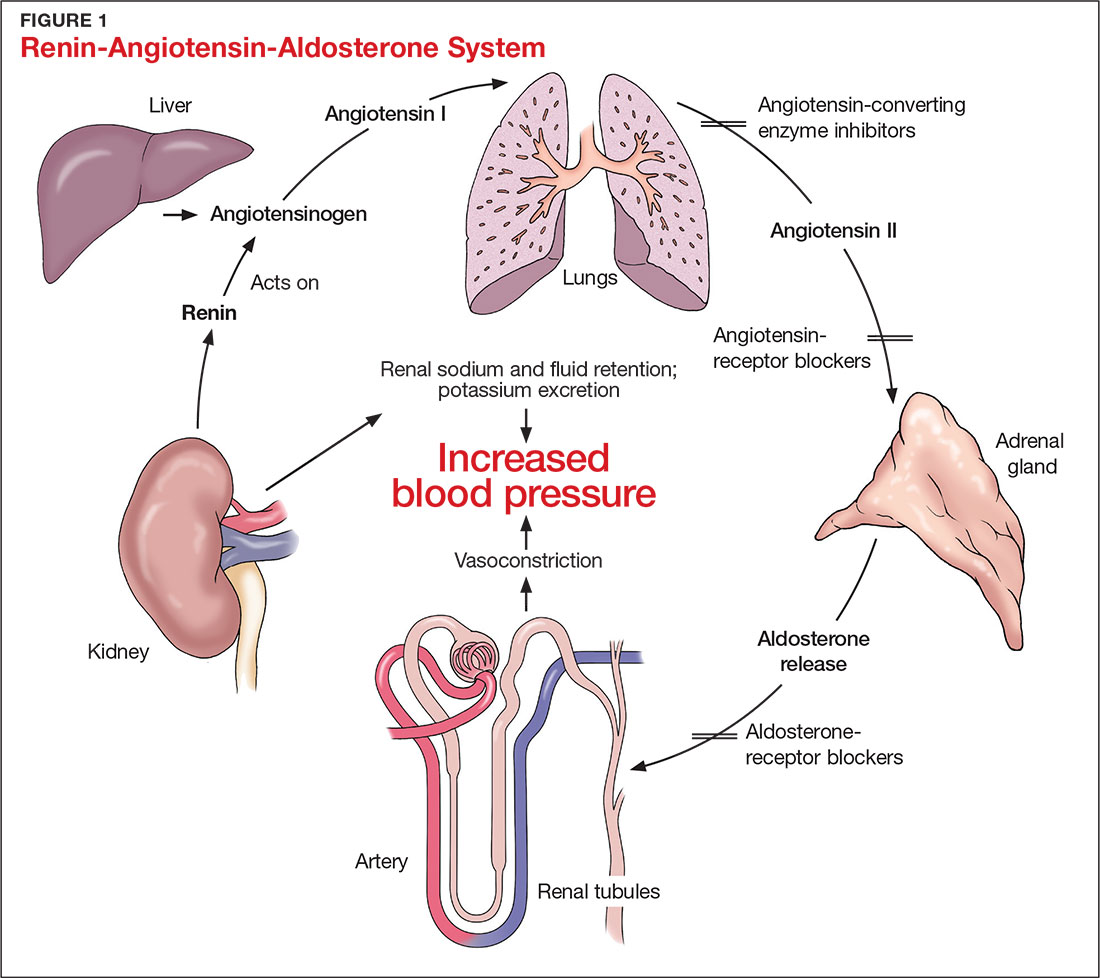
The RAAS is a signal transduction pathway that regulates potassium excretion by the kidneys. Renin is secreted by the kidney in response to low renal perfusion, catecholamines, ß-adrenergic stimulation, potassium and sodium levels, and other factors. Secretion of renin triggers a signaling cascade that eventually results in the release of aldosterone from the adrenal cortex.5 Aldosterone binds to a receptor in the kidney’s collecting ducts where it increases potassium excretion by stimulating sodium reabsorption and fluid retention (see Figure 1).5
CAUSES OF HYPERKALEMIA
The pathophysiology of hyperkalemia generally involves either decreased renal excretion or shifts in extracellular potassium. Causes of hyperkalemia are listed in the Table. Potassium excretion can be disrupted in acute kidney injury (AKI), sepsis, cardiac ischemia, heart failure, diabetic ketoacidosis (DKA), insulin deficiency, tumor lysis syndrome (TLS), sickle cell disease, systemic lupus erythematosus, renal transplant, hepatorenal syndrome, adrenal insufficiency, and obstructive uropathy.15 In addition, certain medications can impair potassium excretion (eg, RAAS blockers, potassium-sparing diuretics in patients with CKD, digoxin toxicity).16 The following sections highlight the pathophysiology and manifestations of more common causes of hyperkalemia.

Renal impairment
Hyperkalemia may be a manifestation of worsening renal function. Potassium excretion is reduced in CKD, and CKD is the most common cause of hyperkalemia due to lower GFR.8,17 Patients with lower GFR tend to be older and male, and frequently have comorbid conditions such as type 2 diabetes, chronic liver disease, and heart failure.17
In CKD, decreased delivery of sodium to the distal tubules and reduced filtration capacity of the kidney diminishes the collecting duct’s ability to excrete potassium in exchange for sodium.2 Metabolic acidosis, which often contributes to AKI or CKD, causes potassium to shift from the intracellular to the extracellular compartment.4 Renal impairment may present clinically with dehydration, oliguria, nausea, vomiting, constipation, altered mental status, or weakness.
Hyperglycemia
Insulin and catecholamines (eg, epinephrine and norepinephrine) drive potassium into cells. Insulin increases potassium uptake into liver and muscle cells.13 A decrease in insulin levels, as may occur in type 2 diabetes or DKA, can cause a buildup of extracellular potassium.4 Also, serum hypertonicity from hyperglycemia results in water movement from the intracellular to the extracellular compartment; this raises the intracellular concentration of potassium, further promoting its movement to the extracellular space.4,14 Patients with hyperglycemia may present with dizziness, polyuria, polydipsia, nausea, vomiting, altered mental status, or fatigue.
Rhabdomyolysis
Rhabdomyolysis is a rapid breakdown of skeletal muscle that results in leakage of cellular contents into the extracellular space.4,18 Causes of rhabdomyolysis include use of medications such as statins, illicit drugs (eg, cocaine), or alcohol; rigorous exercise; and trauma.19
Muscle cell contents that are released into the circulation include potassium and other electrolytes, enzymes (eg, lactate dehydrogenase, aspartate transaminase, aldolase), and myoglobin.19 In rhabdomyolysis, myoglobin accumulation and hypovolemia lead to AKI and hyperkalemia.19 Patients may present with myalgias, extremity paresthesias, generalized weakness, nausea, altered mental status, fever, or darkened urine.18,19
Adrenal insufficiency
During critical illness such as sepsis, adrenal insufficiency can result from destruction of the adrenal glands, leading to hypoaldosteronism.20 Reduced aldosterone in adrenal insufficiency enables sodium and water to be eliminated from the body more easily, but as a result, less potassium gets excreted through the renal system and more is driven into the plasma.15
Acute adrenal insufficiency may manifest with hypotension, nausea, vomiting, or altered mental status, and labwork may reveal hyperkalemia as well as hypoglycemia or hyponatremia. Additionally, long-term glucocorticoid therapy can suppress the hypothalamic-pituitary axis and cause adrenal atrophy; rapid discontinuation of steroids can lead to adrenal insufficiency and hyperkalemia.21
Medications
RAAS blockers reduce CKD progression in patients with an eGFR of 29 mL/min/1.73 m2 or greater.22 Nonetheless, prescribing two or more drugs from the ACEi or ARB classes is not recommended. The Veterans Administration Nephron-Diabetes Trial (VA-NEPHRON-D) was terminated early because patients with stage 3 CKD due to diabetes who received dual ACEi/ARB therapy had higher rates of hyperkalemia but no slowing of CKD.22
Within the RAAS cascade, ACEis block the formation of angiotensin II and ARBs prevent angiotensin II from binding to the adrenal receptor. This impairs renal excretion of potassium and potentially contributes to hyperkalemia.5 Nonetheless, when patients on ACEis or ARBs develop hyperkalemia, aldosterone concentrations usually decrease due to preexisting illnesses (eg, diabetes, heart failure, CKD, AKI) or drug effects (eg, potassium-sparing diuretics, ß-blockers, digoxin).5 Ultimately, a combination of factors resulting from ACEi or ARB therapy causes reductions in renal perfusion and predisposes patients to hyperkalemia.5
NSAIDs may lead to hyperkalemia, as they interfere with prostaglandin release, decrease renal perfusion, and reduce renin and aldosterone levels.22 ß-blockers and tacrolimus inhibit renin release, leading to decreased aldosterone levels.5 Potassium-sparing diuretics block the interaction of aldosterone with the aldosterone receptor in the nephron.5 Digoxin decreases the activity of Na-K-ATPase, diminishing potassium uptake by cells.9 Potassium supplements, often prescribed for patients on diuretics, may contribute to hyperkalemia in patients with CKD. In the hospital setting, potassium tablets or IV formulations are utilized to correct hypokalemia. Especially in patients with CKD, clinicians should prescribe these agents with caution to avoid inducing hyperkalemia. Salt substitutes, which commonly contain potassium chloride, may be appealing to patients concerned about their sodium intake. However, consumption of these substitutes may contribute to hyperkalemia, especially in patients with CKD, heart failure, or type 2 diabetes.23
Tumor lysis syndrome
TLS involves rapid release of electrolytes and other intracellular contents into the extracellular space during the lysis of tumor cells.24 Nucleic acids within DNA strands break down and build up extracellularly, leading to hyperuricemia and often AKI. Potassium and other electrolytes released into the plasma during cell lysis can usually be removed by a healthy renal system. In TLS, however, AKI due to uric acid nephropathy prevents kidneys from removing the excess electrolytes from the bloodstream.24 Patients with rapidly growing hematologic tumors undergoing chemotherapy are especially at risk.
Pseudohyperkalemia
Pseudohyperkalemia is a transiently elevated serum potassium level that erroneously represents the true serum potassium level. It results from hemolysis due to mechanical trauma during the blood draw (eg, a tourniquet tied too tightly or use of a small-bore needle) or during specimen handling afterwards.25 Furthermore, leukocytosis, thrombocytosis, and polycythemia make red blood cells more fragile, increasing the chance of hemolysis and potassium leakage.26 Blood transfusion also can lead to pseudohyperkalemia. When blood is stored, potassium leakage from the cells and cell lysis, along with diminished Na-K-ATPase activity, lead to a buildup of potassium in the medium surrounding the stored red blood cells.27,28 The rise in serum potassium levels post-transfusion is usually transient, as the blood cells redistribute the potassium load once they become metabolically active.27,29
CLINICAL MANIFESTATIONS
Clinical manifestations of mild to moderate hyperkalemia (serum potassium > 5.5 mEq/L but < 6.5 mEq/L) include fatigue, generalized weakness, nausea, vomiting, constipation, and diarrhea.15 In many patients, mild to moderate hyperkalemia may not be associated with any acute symptoms and vital signs may be normal.13 Severe hyperkalemia (serum potassium > 6.5 mEq/L) may present clinically with acute extremity paresthesias, muscle weakness and paralysis, heart palpitations, dyspnea, altered mental status, cardiac arrhythmias, and cardiac arrest.30,31 Irregular heart rhythm, decreased deep tendon reflexes, or decreased strength may be revealed on physical exam.3 Individuals with ESRD on hemodialysis seem to tolerate higher levels of potassium than the general population without displaying clinical symptoms. However, these individuals are still susceptible to the cardiac effects of hyperkalemia.32
INITIAL ASSESSMENT
In assessing hyperkalemia, the clinician must perform a focused history and physical exam and review the patient’s medication list, including supplements and dietary habits that impact potassium intake. Potassium-rich foods include meat, fish, milk, almonds, spinach, cantaloupe, bananas, oranges, mushrooms, and potatoes.33 Hyperkalemia may present in association with various medical emergencies. The clinician should have an index of suspicion, depending on the patient’s overall medical profile and presentation, for emergencies such as cardiac ischemia, sepsis, adrenal crisis, DKA, TLS, and digoxin overdose.
The clinician must identify whether an elevated potassium level requires emergent therapy; assessment of vital signs is paramount in determining this. Orthostatic hypotension and tachycardia may hint that the patient is volume depleted. The patient should be examined for signs of hemodynamic shock with the CAB sequence: circulation, airway, breathing.34 Symptoms such as chest pain, shortness of breath, muscle weakness, paralysis, and altered mental status suggest that an expedited evaluation is warranted.
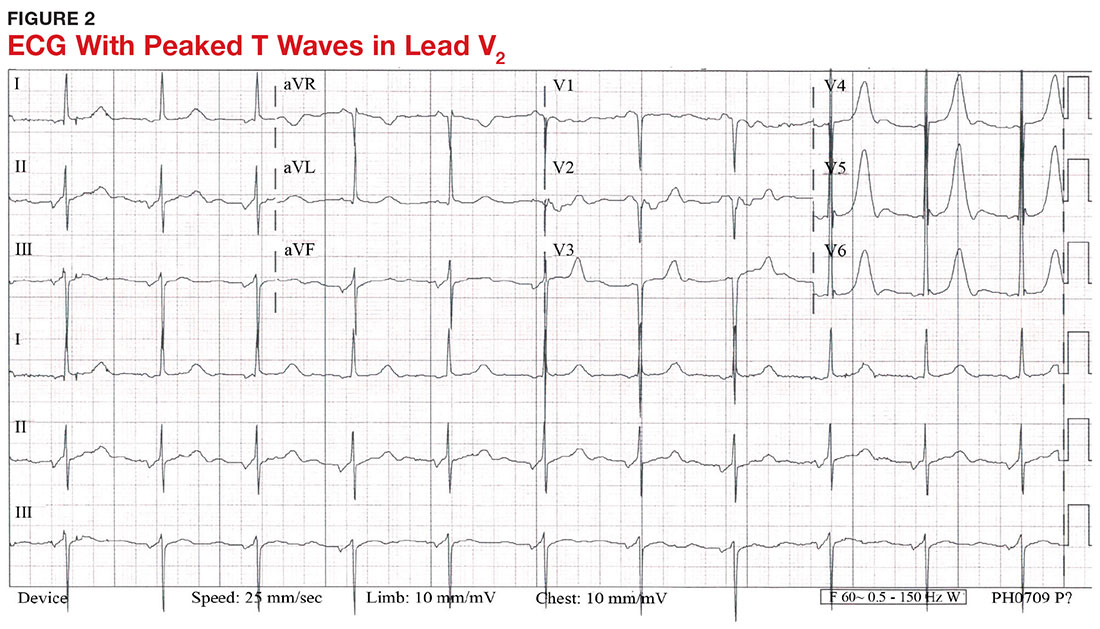
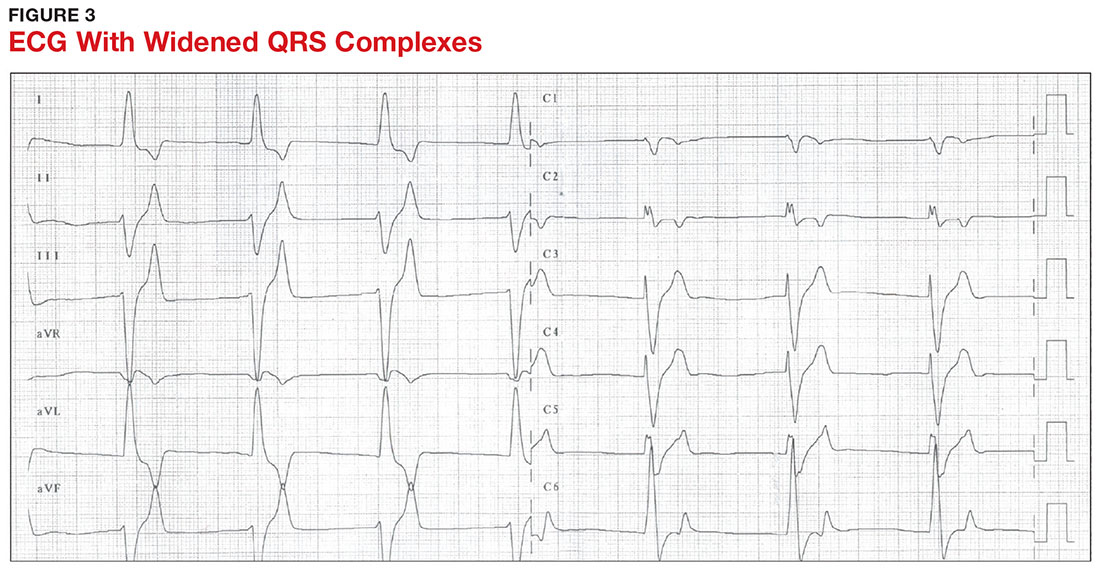
With a serum potassium level > 5.5 mEq/L, urgent electrocardiography should be performed.26 ECG findings observed with serum potassium levels of 5.5-6.5 mEq/L usually include peaked T-waves and prolonged PR intervals (see Figure 2). With potassium levels > 6.5 mEq/L consistent with further cardiac destabilization, the P-wave flattens then disappears, the QRS complex broadens, and sinus bradycardia or ectopic beats may occur.12,26 ST depression, T-wave inversion, or ST elevation also may be seen.12 With serum potassium levels > 7.5 mEq/L, progressive widening of the QRS complex to a sine-wave with bundle branch blocks or fascicular blocks may occur (see Figure 3).26 Without prompt intervention, ventricular fibrillation may ensue.26
An extensive laboratory workup may be necessary to investigate the etiology; this includes a complete blood count, metabolic panel, liver function tests, cardiac enzymes, blood gas analysis, serum/urine osmolality, urinalysis, urine electrolytes, and toxicology screen.13,26 Arterial blood gas (ABG) analysis may show metabolic acidosis with AKI or DKA, or an elevated lactate may occur with sepsis. In patients with hyperglycemia, besides checking for acidosis, obtaining blood/urine ketone levels and a metabolic panel with anion gap to evaluate for DKA is useful.35
When assessing a patient with an elevated creatinine, the GFR at the time of evaluation should be compared with the patient’s baseline GFR to determine chronicity and duration of his/her kidney disease.36 Obtaining a urinalysis and urine electrolytes in addition to the basic metabolic panel can help narrow the etiology.36 A Foley catheter should be placed in cases of urinary retention because without intervention, urinary obstruction may lead to AKI and hyperkalemia. Myoglobinuria on urinalysis and an elevated creatine kinase are diagnostic markers of rhabdomyolysis.18
TLS should be considered in patients who recently received chemotherapy, especially those with proliferative hematologic malignancies, such as acute lymphoblastic leukemia, acute myeloid leukemia, and Burkitt lymphoma.24 In TLS, bloodwork often reveals hyperkalemia along with AKI, an elevated uric acid level, hyperphosphatemia, and hypocalcemia.24
Patients presenting with hyperkalemia, hypotension, hypoglycemia, and hyponatremia may have adrenal insufficiency.20 If insufficiency is suspected, a cortisol level may be checked during morning hours; a low level is often suggestive of this diagnosis.37 Treatment includes daily doses of steroids, and consultation with an endocrinologist is recommended.37
If an elevated potassium level is not accompanied by renal dysfunction, electrolyte imbalances, ECG changes, or inciting medications, pseudohyperkalemia should be considered.38 A repeat lab sample should be checked. Consider obtaining an ABG analysis, as the shorter time interval between drawing the blood sample and the sample analysis reportedly increases the reliability of the resulting potassium level.38
THERAPY
Emergent
Emergent treatment is needed for severe hyperkalemia (see Figure 4). Any hyperkalemia-inciting medications or potassium supplements should be immediately discontinued.39 IV access and cardiac telemetry monitoring should be promptly applied.26
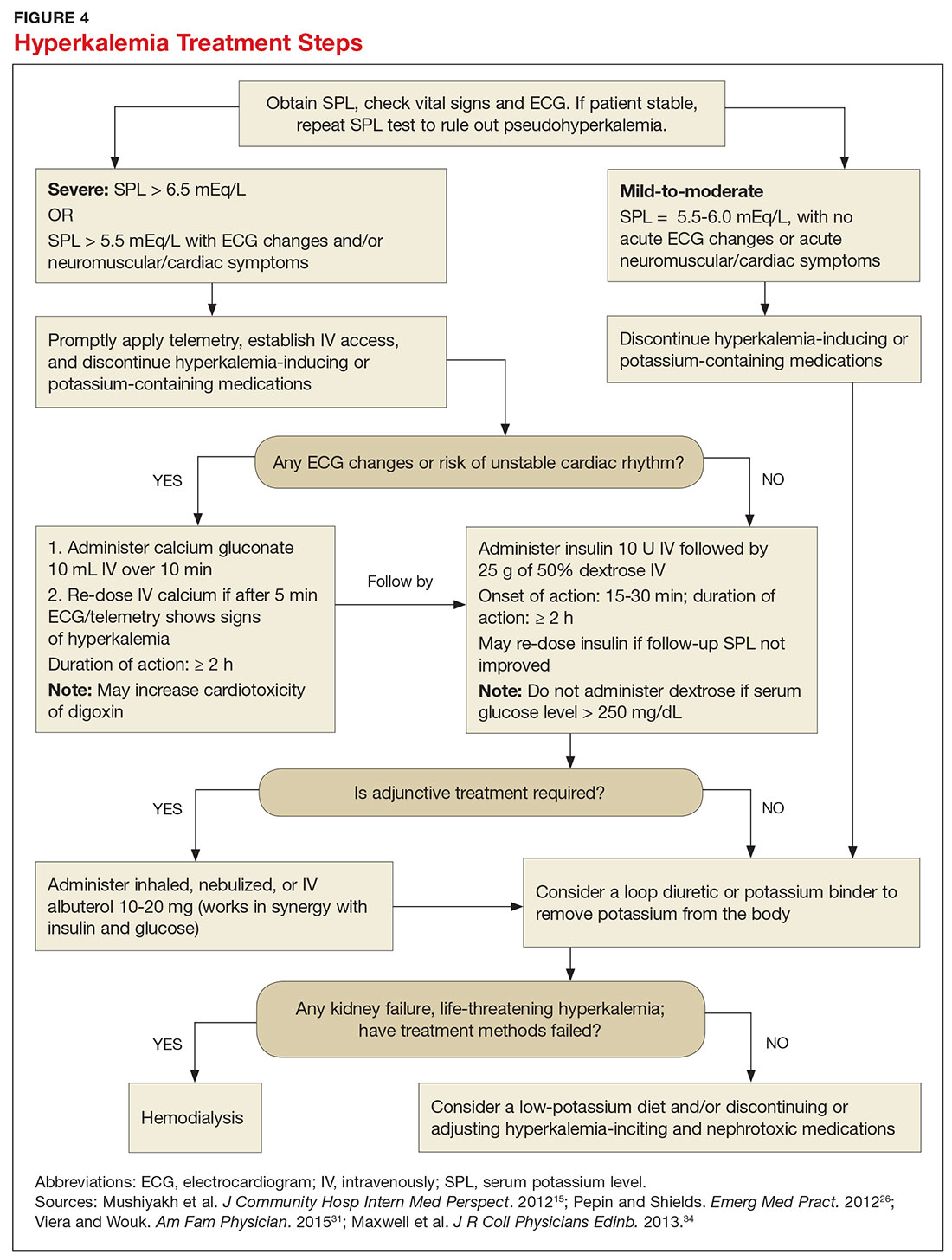
In cases of severe hyperkalemia that involve cardiac arrhythmias, manifestations on ECG, or risk for arrhythmias, calcium gluconate (10 mL IV over 10 min) should be urgently administered, followed by IV insulin in conjunction with dextrose.26 Calcium chloride should be utilized for hyperkalemia in the context of the advanced cardiac life support (ACLS) protocol for cardiac arrest.26 The patient should remain on cardiac telemetry during this treatment to monitor for ventricular fibrillation or other arrhythmias.15 IV calcium does not lower serum potassium but rather antagonizes the effects of potassium on the cardiac cell membranes, helping to prevent or terminate arrhythmias.15,34 It should be noted, however, that firstline treatment for patients who develop hyperkalemia in the setting of digoxin toxicity involves administration of digoxin-specific antibody, while calcium infusion may be utilized later.34 Alternatively, if the patient is dialysis-dependent with ESRD, dialysis may be considered as a prompt initial treatment, with nephrologist consultation.
Administration of 10 U of regular insulin plus 25 g of 50% dextrose via IV will shift potassium intracellularly (see Figure 4). The dextrose will offset the resultant hypoglycemia.31,34 Of note, this treatment is often firstline for moderate to severe hyperkalemia in patients with a stable cardiac rhythm and ECG. Blood glucose should be monitored with a fingerstick within 30 to 60 minutes of infusion and every hour thereafter for up to six hours following insulin administration.34 Potassium levels should be checked every one to two hours after this treatment step until the serum potassium level stabilizes. Thereafter, recheck the levels every four to six hours to gauge whether further treatment is needed.34
Adjunctive
After performing firstline treatment strategies for severe hyperkalemia, there are alternate therapies to consider that can help lower total body potassium. Nebulized albuterol may be used, which pushes potassium into cells; this works in synergy with insulin and glucose.26,33 Sodium bicarbonate may be effective in cases in which the ABG analysis or labs show metabolic acidosis, as this infusion shifts potassium into cells by increasing the blood pH.33
In patients with dehydration, sepsis, TLS, or rhabdomyolysis, administration of IV fluids to maintain appropriate vascular volume is important. However, excessive fluid resuscitation can result in fluid overload, inducing complications such as respiratory failure and worsened renal function.40 A Foley catheter may be placed for strict intake and output monitoring.
The patient’s volume status must be carefully assessed. Hyperkalemia may present in association with heart failure exacerbation or ascites, which are usually hypervolemic states. Loop diuretics may be used to compensate for volume overload and to help remove potassium from the body, but these medications are contraindicated in anuric patients.13,41
Removing total body potassium
After emergent therapy is carried out, potassium may need to be removed from the body through diuresis, hemodialysis, or potassium binders. Loop diuretics or potassium binders may be used to treat mild to moderate hyperkalemia or to continue to stabilize the potassium level after emergent therapy is carried out. If severe hyperkalemia persists with kidney injury or with absence of urine output, hemodialysis is the therapy of choice.13
The potassium binder sodium polystyrene sulfonate (SPS) exchanges sodium for potassium in the intestine.42 This agent is contraindicated if the patient has intestinal obstruction. SPS’s slow onset of action (two to six hours) makes it ineffective as firstline therapy for severe hyperkalemia.3 In addition, SPS has serious but rare adverse effects, more commonly seen in patients who have uremia after kidney transplant or who have had recent abdominal surgery, bowel injury, or intestinal perforation.41 Adverse effects of SPS include aspiration pneumonitis, upper gastrointestinal injury, colonic necrosis, and rectal stenosis.41 However, there have been documented events of colonic necrosis due to SPS in patients without ESRD who have not had abdominal surgery.43,44 In 2009, the FDA advised against concomitant administration of sorbitol with SPS. However, this drug preparation continues to be the only one stocked by many hospital pharmacies.44 Because SPS has potentially harmful adverse effects and generally is not effective in promptly lowering serum potassium, it is prudent for clinicians to implement other management strategies first.44
MONITORING AT-RISK PATIENTS
Patients with a GFR < 45 mL/min/1.73 m2 and a baseline serum potassium level > 4.5 mEq/L are at risk for hyperkalemia while taking an ACEi or an ARB and should be advised to adhere to a potassium-restrictive diet with frequent laboratory checkups.22 Depending on the serum potassium and GFR levels at checkups, these medication doses may need to be reduced or discontinued altogether.
NEW DRUG DEVELOPMENTS
A potassium binder approved for daily use would benefit patients on aggressive heart failure medication regimens, as hyperkalemia commonly occurs with these regimens. As discussed, the widely available potassium binder SPS has been associated with severe gastrointestinal adverse effects, limiting its potential for routine use.44,45 In clinical trials, new potassium binders patiromer and zirconium cyclosilicate (ZS-9) have demonstrated an ability to maintain normokalemia over weeks of therapy with acceptable adverse effect profiles.45 In 2015, patiromer was approved by the FDA as therapy for hyperkalemia.46 An in-depth discussion, which is outside the scope of this article, will be presented by experts in the April 2017 edition of Renal Consult.
CONCLUSION
The best treatment for hyperkalemia is prevention through close surveillance of at-risk patients. Clinicians should be aware of predisposing risk factors for hyperkalemia, as it can have an insidious onset, with symptoms manifesting only when this electrolyte imbalance becomes life-threatening. It is particularly important to recognize when this condition mandates emergent treatment so that critical cardiac arrhythmias can be prevented.26
1. An JN, Lee JP, Jeon HJ, et al. Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care. 2012; 16(6):R225.
2. Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ. 2016;40(4):480-490.
3. Medford-Davis L, Rafique Z. Derangements of potassium. Emerg Med Clin North Am. 2014;32(2):329-347.
4. Eleftheriadis T, Leivaditis K, Antoniadi G, Liakopoulos V. Differential diagnosis of hyperkalemia: an update to a complex problem. Hippokratia. 2012;16(4):294-302.
5. Raebel M. Hyperkalemia associated with use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Cardiovasc Ther. 2012;30(3):156-166.
6. Korgaonkar S, Tilea A, Gillespie BW, et al. Serum potassium and outcomes in CKD: insights from the RRI-CKD Cohort Study. Clin J Am Soc Nephrol. 2010;5(5):762-769.
7. McMahon GM, Mendu ML, Gibbons FK, Christopher KB. Association between hyperkalemia at critical care initiation and mortality. Intensive Care Med. 2012;38(11):1834-1842.
8. Drawz PE, Babineau DC, Rahman M. Metabolic complications in elderly adults with chronic kidney disease. J Am Geriatr Soc. 2012;60(2):310-315.
9. Sarafidis PA, Georgianos PI, Bakris GL. Advances in treatment of hyperkalemia in chronic kidney disease. Expert Opin Pharmacother. 2015;16(14):2205-2215.
10. Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156-1162.
11. Khanagavi J, Gupta T, Aronow WS, et al. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10(2):251-257.
12. Berkova M, Berka Z, Topinkova E. Arrhythmias and ECG changes in life threatening hyperkalemia in older patients treated by potassium sparing drugs. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158(1):84-91.
13. Lehnhardt A, Kemper MJ. Pathogenesis, diagnosis and management of hyperkalemia. Pediatr Nephrol. 2011;26(3):377-384.
14. Palmer BF. A physiologic-based approach to the evaluation of a patient with hyperkalemia. Am J Kidney Dis. 2010;56(2):387-393.
15. Mushiyakh Y, Dangaria H, Qavi S, et al. Treatment and pathogenesis of acute hyperkalemia. J Community Hosp Intern Med Perspect. 2012;1(4):7372.
16. Elliott MJ, Ronksley PE, Clase CM, et al. Management of patients with acute hyperkalemia. CMAJ. 2010;182(15):1631-1635.
17. Wiebe N, Klarenbach SW, Allan GM, et al. Potentially preventable hospitalization as a complication of CKD: a cohort study. Am J Kidney Dis. 2014;64(2):230-238.
18. Zutt R, van der Kooi AJ, Linthorst GE, et al. Rhabdomyolysis: review of the literature. Neuromuscul Disord. 2014;24(8):651-659.
19. Zimmerman JL, Shen MC. Rhabdomyolysis. Chest. 2013; 144(3):1058-1065.
20. Khardori R, Castillo D. Endocrine and metabolic changes during sepsis: an update. Med Clin North Am. 2012;96(6):1095-1105.
21. Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2014;4(20):739-769.
22. Lazich I, Bakris GL. Prediction and management of hyperkalemia across the spectrum of chronic kidney disease. Semin Nephrol. 2014;34(3):333-339.
23. Ayach T, Nappo R, Paugh-Miller J, Ross E. Life-threatening hyperkalemia in a patient with normal renal function. Clin Kidney J. 2014;7(1):49-52.
24. Wilson FP, Berns JS. Tumor lysis syndrome: new challenges and recent advances. Adv Chronic Kidney Dis. 2014;21(1):18-26.
25. Asiryatham JR, Moses V, Bjornson L. Errors in potassium measurement: a laboratory perspective for the clinician. N Am J Med Sci. 2013;5(4):255-259.
26. Pepin J, Shields C. Advances in diagnosis and management of hypokalemic and hyperkalemic emergencies. Emerg Med Pract. 2012;14(2):1-17.
27. Vraets A, Lin Y, Callum JL. Transfusion-associated hyperkalemia. Transfus Med Rev. 2011;25(3):184-196.
28. Aboudara MC, Hurst FP, Abbott KC, Perkins RM. Hyperkalemia after packed red blood cell transfusion in trauma patients. J Trauma. 2008;64(2 suppl):S86-S91.
29. Olson J, Talekar M, Sachdev M, et al. Potassium changes associated with blood transfusion in pediatric patients. Am J Clin Pathol. 2013;139(6):800-805.
30. Chon S, Kwak YH, Hwang SS, et al. Severe hyperkalemia can be detected immediately by quantitative electrocardiography and clinical history in patients with symptomatic or extreme bradycardia: a retrospective cross-sectional study. J Crit Care. 2013;28(6):1112.e7-1112.e13.
31. Viera AJ, Wouk N. Potassium disorders: hypokalemia and hyperkalemia. Am Fam Physician. 2015;92(6):487-495.
32. Sanghavi S, Whitling S, Uribarri J. Potassium balance in dialysis patients. Semin Dial. 2013;26(5):597-603.
33. Crawford AH. Hyperkalemia: Recognition and management of a critical electrolyte disturbance. J Infus Nurs. 2014;37(3):167-175.
34. Maxwell AP, Linden K, O’Donnell S, et al. Management of hyperkalemia. J R Coll Physicians Edinb. 2013;43(3):246-251.
35. Seth P, Kaur H, Kaur M. Clinical profile of diabetic ketoacidosis: a prospective study in a tertiary care hospital. J Clin Diagn Res. 2015;9(6):OC01-OC04.
36. Rahman M, Shad F, Smith M. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician. 2012;86(7): 631-639.
37. Puar TH, Stikkelbroeck NM, Smans LC, et al. Adrenal crisis: still a deadly event in the 21st century. Am J Med. 2016;129(3):339.e1-9.
38. Liamis G, Liberopoulos E, Barkas F, Elisaf M. Spurious electrolyte disorders: a diagnostic challenge for clinicians. Am J Nephrol. 2013;38(1):50-57.
39. Kovesdy CP. Management of hyperkalemia: an update for the internist. Am J Med. 2015;128(12):1281-1287.
40. Labib M, Khalid R, Khan A, Khan S. Volume management in the critically ill patient with acute kidney injury. Crit Care Res Pract. 2013;2013:792830.
41. Watson M, Abbott KC, Yuan CM. Damned if you do, damned if you don’t: potassium binding resins in hyperkalemia. Clin J Am Soc Nephrol. 2010;5(10):1723-1726.
42. Nguyen T, Ondrik D, Zhufyak O, et al. Hyperkalemia and potential pitfalls of sodium polystyrene sulfonate. JAAPA. 2015; 28(3):41-45.
43. McGowan CE, Saha S, Resnick MB, Moss SF. Intestinal necrosis due to sodium polystyrene sulfonate (Kayexalate) in sorbitol. South Med J. 2009;102(5):493-497.
44. Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21:733-735.
45. Pitt B, Bakris GL. New potassium binders for the treatment of hyperkalemia: current data and opportunities for the future. Hypertension. 2015;66(4):731-738.
46. Epstein M, Pitt B. Recent advances in pharmacological treatments of hyperkalemia: focus on patiromer. Expert Opin Pharmacother. 2016;17(10):1435-1448.
CE/CME No: CR-1703
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the pathophysiology and causes of hyperkalemia.
• Identify patients who are susceptible to hyperkalemia.
• Recognize the clinical sequelae of hyperkalemia.
• Formulate assessment and treatment plans for patients with hyperkalemia.
FACULTY
Melanie Douglas is a Physician Assistant in the Medicine Department at NYU Langone Medical Center in New York, New York. Denise Rizzolo is a Clinical Assistant Professor in the PA Program at Pace University in New York, New York, and Research Director in the Program of PA Studies at Kean University in Union, New Jersey. Danielle Kruger is an Academic Coordinator and Associate Professor in the PA Program at St. John’s University in Queens, New York. The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of March 2017.
Article begins on next page >>
Hyperkalemia is a common electrolyte disorder associated with life-threatening cardiac arrhythmias. Prompt recognition and appropriate treatment are essential in preventing serious cardiac complications. Although clinical manifestations of hyperkalemia are usually nonspecific or absent, laboratory testing and electrocardiography performed by the astute clinician aware of predisposing risk factors can help direct management.
Potassium is contained mostly in intracellular fluid; only about 2% is found in the extracellular space.1 The average total body potassium is about 50 mEq per kg of body weight (eg, a 70-kg individual has a total body potassium of approximately 3,500 mEq).2 Levels are tightly regulated by alterations in excretion in the distal renal tubule in response to potassium load and balance, and potassium distribution is influenced by insulin, aldosterone, catecholamines, and acid-base status.2 Movement of potassium across cell membranes is driven by the sodium-potassium adenosine triphosphatase (Na-K-ATPase) pump.3 In this article, we use the common serum potassium reference range of 3.5 to 5.0 mEq/L and define hyperkalemia as a serum potassium concentration greater than 5.5 mEq/L.4
Hyperkalemia can lead to life-threatening complications of cardiac arrhythmias, asystole, hypotension, flaccid paralysis, tetany, dyspnea, and altered mental status.5 Among patients with end-stage renal disease (ESRD), hyperkalemia is thought to contribute to 2% to 5% of deaths.6 A retrospective study found that patients with serum potassium levels exceeding 6.0 mEq/L on ICU admission had a significantly higher death rate within 30 days than patients who were normokalemic on presentation.7
RISK FACTORS
It is estimated that more than 35% of patients age 70 and older have chronic kidney disease (CKD) stage 3 or higher.8 Hyperkalemia is closely associated with CKD, increasing linearly in relation to the degree of renal impairment.8 As such, the prevalence of hyperkalemia in older adults is high, and it will increase overall as the US population ages. In a retrospective analysis of veterans older than 65 with CKD stage 3 or higher, the prevalence of hyperkalemia was 2.5%.9 Use of certain medications is also associated with hyperkalemia. Another retrospective study analyzed records obtained from 70,873 patients with CKD (estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73 m2) hospitalized in the Veterans Health Administration system. It found that patients treated with renin-angiotensin-aldosterone system (RAAS) blockers, such as ACE inhibitors (ACEis) or angiotensin-receptor blockers (ARBs), had a higher incidence of hyperkalemia (potassium level ≥ 5.5 mEq/L) than patients not treated with these medications (8.22 vs 1.77 events per 100 patient-months).9,10
POTASSIUM HOMEOSTASIS
Tight control over extracellular potassium is maintained in part by the Na-K-ATPase pump, which uses adenosine triphosphatase to move potassium and sodium ions in opposite directions across cell membranes.3 Specifically, three sodium ions are pumped out of the cell for every two potassium ions pumped in, resulting in a potassium gradient that is partially responsible for maintaining a resting membrane potential. This resting membrane potential, which determines myocardial, skeletal muscle, and nerve cell excitability and signaling, is highly sensitive to changes in the extracellular potassium level.4 Even small extracellular imbalances can induce cell depolarization and evoke an action potential. Increased extracellular potassium concentration decreases the resting membrane potential of the myocardium, shortens repolarization time, and decreases the rate of myocardial cell conduction, and also slows down neuromuscular conduction.11,12
Renal tubular function plays a significant role in potassium homeostasis, with approximately 90% of dietary potassium intake ex
The RAAS is a signal transduction pathway that regulates potassium excretion by the kidneys. Renin is secreted by the kidney in response to low renal perfusion, catecholamines, ß-adrenergic stimulation, potassium and sodium levels, and other factors. Secretion of renin triggers a signaling cascade that eventually results in the release of aldosterone from the adrenal cortex.5 Aldosterone binds to a receptor in the kidney’s collecting ducts where it increases potassium excretion by stimulating sodium reabsorption and fluid retention (see Figure 1).5

CAUSES OF HYPERKALEMIA
The pathophysiology of hyperkalemia generally involves either decreased renal excretion or shifts in extracellular potassium. Causes of hyperkalemia are listed in the Table. Potassium excretion can be disrupted in acute kidney injury (AKI), sepsis, cardiac ischemia, heart failure, diabetic ketoacidosis (DKA), insulin deficiency, tumor lysis syndrome (TLS), sickle cell disease, systemic lupus erythematosus, renal transplant, hepatorenal syndrome, adrenal insufficiency, and obstructive uropathy.15 In addition, certain medications can impair potassium excretion (eg, RAAS blockers, potassium-sparing diuretics in patients with CKD, digoxin toxicity).16 The following sections highlight the pathophysiology and manifestations of more common causes of hyperkalemia.

Renal impairment
Hyperkalemia may be a manifestation of worsening renal function. Potassium excretion is reduced in CKD, and CKD is the most common cause of hyperkalemia due to lower GFR.8,17 Patients with lower GFR tend to be older and male, and frequently have comorbid conditions such as type 2 diabetes, chronic liver disease, and heart failure.17
In CKD, decreased delivery of sodium to the distal tubules and reduced filtration capacity of the kidney diminishes the collecting duct’s ability to excrete potassium in exchange for sodium.2 Metabolic acidosis, which often contributes to AKI or CKD, causes potassium to shift from the intracellular to the extracellular compartment.4 Renal impairment may present clinically with dehydration, oliguria, nausea, vomiting, constipation, altered mental status, or weakness.
Hyperglycemia
Insulin and catecholamines (eg, epinephrine and norepinephrine) drive potassium into cells. Insulin increases potassium uptake into liver and muscle cells.13 A decrease in insulin levels, as may occur in type 2 diabetes or DKA, can cause a buildup of extracellular potassium.4 Also, serum hypertonicity from hyperglycemia results in water movement from the intracellular to the extracellular compartment; this raises the intracellular concentration of potassium, further promoting its movement to the extracellular space.4,14 Patients with hyperglycemia may present with dizziness, polyuria, polydipsia, nausea, vomiting, altered mental status, or fatigue.
Rhabdomyolysis
Rhabdomyolysis is a rapid breakdown of skeletal muscle that results in leakage of cellular contents into the extracellular space.4,18 Causes of rhabdomyolysis include use of medications such as statins, illicit drugs (eg, cocaine), or alcohol; rigorous exercise; and trauma.19
Muscle cell contents that are released into the circulation include potassium and other electrolytes, enzymes (eg, lactate dehydrogenase, aspartate transaminase, aldolase), and myoglobin.19 In rhabdomyolysis, myoglobin accumulation and hypovolemia lead to AKI and hyperkalemia.19 Patients may present with myalgias, extremity paresthesias, generalized weakness, nausea, altered mental status, fever, or darkened urine.18,19
Adrenal insufficiency
During critical illness such as sepsis, adrenal insufficiency can result from destruction of the adrenal glands, leading to hypoaldosteronism.20 Reduced aldosterone in adrenal insufficiency enables sodium and water to be eliminated from the body more easily, but as a result, less potassium gets excreted through the renal system and more is driven into the plasma.15
Acute adrenal insufficiency may manifest with hypotension, nausea, vomiting, or altered mental status, and labwork may reveal hyperkalemia as well as hypoglycemia or hyponatremia. Additionally, long-term glucocorticoid therapy can suppress the hypothalamic-pituitary axis and cause adrenal atrophy; rapid discontinuation of steroids can lead to adrenal insufficiency and hyperkalemia.21
Medications
RAAS blockers reduce CKD progression in patients with an eGFR of 29 mL/min/1.73 m2 or greater.22 Nonetheless, prescribing two or more drugs from the ACEi or ARB classes is not recommended. The Veterans Administration Nephron-Diabetes Trial (VA-NEPHRON-D) was terminated early because patients with stage 3 CKD due to diabetes who received dual ACEi/ARB therapy had higher rates of hyperkalemia but no slowing of CKD.22
Within the RAAS cascade, ACEis block the formation of angiotensin II and ARBs prevent angiotensin II from binding to the adrenal receptor. This impairs renal excretion of potassium and potentially contributes to hyperkalemia.5 Nonetheless, when patients on ACEis or ARBs develop hyperkalemia, aldosterone concentrations usually decrease due to preexisting illnesses (eg, diabetes, heart failure, CKD, AKI) or drug effects (eg, potassium-sparing diuretics, ß-blockers, digoxin).5 Ultimately, a combination of factors resulting from ACEi or ARB therapy causes reductions in renal perfusion and predisposes patients to hyperkalemia.5
NSAIDs may lead to hyperkalemia, as they interfere with prostaglandin release, decrease renal perfusion, and reduce renin and aldosterone levels.22 ß-blockers and tacrolimus inhibit renin release, leading to decreased aldosterone levels.5 Potassium-sparing diuretics block the interaction of aldosterone with the aldosterone receptor in the nephron.5 Digoxin decreases the activity of Na-K-ATPase, diminishing potassium uptake by cells.9 Potassium supplements, often prescribed for patients on diuretics, may contribute to hyperkalemia in patients with CKD. In the hospital setting, potassium tablets or IV formulations are utilized to correct hypokalemia. Especially in patients with CKD, clinicians should prescribe these agents with caution to avoid inducing hyperkalemia. Salt substitutes, which commonly contain potassium chloride, may be appealing to patients concerned about their sodium intake. However, consumption of these substitutes may contribute to hyperkalemia, especially in patients with CKD, heart failure, or type 2 diabetes.23
Tumor lysis syndrome
TLS involves rapid release of electrolytes and other intracellular contents into the extracellular space during the lysis of tumor cells.24 Nucleic acids within DNA strands break down and build up extracellularly, leading to hyperuricemia and often AKI. Potassium and other electrolytes released into the plasma during cell lysis can usually be removed by a healthy renal system. In TLS, however, AKI due to uric acid nephropathy prevents kidneys from removing the excess electrolytes from the bloodstream.24 Patients with rapidly growing hematologic tumors undergoing chemotherapy are especially at risk.
Pseudohyperkalemia
Pseudohyperkalemia is a transiently elevated serum potassium level that erroneously represents the true serum potassium level. It results from hemolysis due to mechanical trauma during the blood draw (eg, a tourniquet tied too tightly or use of a small-bore needle) or during specimen handling afterwards.25 Furthermore, leukocytosis, thrombocytosis, and polycythemia make red blood cells more fragile, increasing the chance of hemolysis and potassium leakage.26 Blood transfusion also can lead to pseudohyperkalemia. When blood is stored, potassium leakage from the cells and cell lysis, along with diminished Na-K-ATPase activity, lead to a buildup of potassium in the medium surrounding the stored red blood cells.27,28 The rise in serum potassium levels post-transfusion is usually transient, as the blood cells redistribute the potassium load once they become metabolically active.27,29
CLINICAL MANIFESTATIONS
Clinical manifestations of mild to moderate hyperkalemia (serum potassium > 5.5 mEq/L but < 6.5 mEq/L) include fatigue, generalized weakness, nausea, vomiting, constipation, and diarrhea.15 In many patients, mild to moderate hyperkalemia may not be associated with any acute symptoms and vital signs may be normal.13 Severe hyperkalemia (serum potassium > 6.5 mEq/L) may present clinically with acute extremity paresthesias, muscle weakness and paralysis, heart palpitations, dyspnea, altered mental status, cardiac arrhythmias, and cardiac arrest.30,31 Irregular heart rhythm, decreased deep tendon reflexes, or decreased strength may be revealed on physical exam.3 Individuals with ESRD on hemodialysis seem to tolerate higher levels of potassium than the general population without displaying clinical symptoms. However, these individuals are still susceptible to the cardiac effects of hyperkalemia.32
INITIAL ASSESSMENT
In assessing hyperkalemia, the clinician must perform a focused history and physical exam and review the patient’s medication list, including supplements and dietary habits that impact potassium intake. Potassium-rich foods include meat, fish, milk, almonds, spinach, cantaloupe, bananas, oranges, mushrooms, and potatoes.33 Hyperkalemia may present in association with various medical emergencies. The clinician should have an index of suspicion, depending on the patient’s overall medical profile and presentation, for emergencies such as cardiac ischemia, sepsis, adrenal crisis, DKA, TLS, and digoxin overdose.
The clinician must identify whether an elevated potassium level requires emergent therapy; assessment of vital signs is paramount in determining this. Orthostatic hypotension and tachycardia may hint that the patient is volume depleted. The patient should be examined for signs of hemodynamic shock with the CAB sequence: circulation, airway, breathing.34 Symptoms such as chest pain, shortness of breath, muscle weakness, paralysis, and altered mental status suggest that an expedited evaluation is warranted.

With a serum potassium level > 5.5 mEq/L, urgent electrocardiography should be performed.26 ECG findings observed with serum potassium levels of 5.5-6.5 mEq/L usually include peaked T-waves and prolonged PR intervals (see Figure 2). With potassium levels > 6.5 mEq/L consistent with further cardiac destabilization, the P-wave flattens then disappears, the QRS complex broadens, and sinus bradycardia or ectopic beats may occur.12,26 ST depression, T-wave inversion, or ST elevation also may be seen.12 With serum potassium levels > 7.5 mEq/L, progressive widening of the QRS complex to a sine-wave with bundle branch blocks or fascicular blocks may occur (see Figure 3).26 Without prompt intervention, ventricular fibrillation may ensue.26

An extensive laboratory workup may be necessary to investigate the etiology; this includes a complete blood count, metabolic panel, liver function tests, cardiac enzymes, blood gas analysis, serum/urine osmolality, urinalysis, urine electrolytes, and toxicology screen.13,26 Arterial blood gas (ABG) analysis may show metabolic acidosis with AKI or DKA, or an elevated lactate may occur with sepsis. In patients with hyperglycemia, besides checking for acidosis, obtaining blood/urine ketone levels and a metabolic panel with anion gap to evaluate for DKA is useful.35
When assessing a patient with an elevated creatinine, the GFR at the time of evaluation should be compared with the patient’s baseline GFR to determine chronicity and duration of his/her kidney disease.36 Obtaining a urinalysis and urine electrolytes in addition to the basic metabolic panel can help narrow the etiology.36 A Foley catheter should be placed in cases of urinary retention because without intervention, urinary obstruction may lead to AKI and hyperkalemia. Myoglobinuria on urinalysis and an elevated creatine kinase are diagnostic markers of rhabdomyolysis.18
TLS should be considered in patients who recently received chemotherapy, especially those with proliferative hematologic malignancies, such as acute lymphoblastic leukemia, acute myeloid leukemia, and Burkitt lymphoma.24 In TLS, bloodwork often reveals hyperkalemia along with AKI, an elevated uric acid level, hyperphosphatemia, and hypocalcemia.24
Patients presenting with hyperkalemia, hypotension, hypoglycemia, and hyponatremia may have adrenal insufficiency.20 If insufficiency is suspected, a cortisol level may be checked during morning hours; a low level is often suggestive of this diagnosis.37 Treatment includes daily doses of steroids, and consultation with an endocrinologist is recommended.37
If an elevated potassium level is not accompanied by renal dysfunction, electrolyte imbalances, ECG changes, or inciting medications, pseudohyperkalemia should be considered.38 A repeat lab sample should be checked. Consider obtaining an ABG analysis, as the shorter time interval between drawing the blood sample and the sample analysis reportedly increases the reliability of the resulting potassium level.38
THERAPY
Emergent
Emergent treatment is needed for severe hyperkalemia (see Figure 4). Any hyperkalemia-inciting medications or potassium supplements should be immediately discontinued.39 IV access and cardiac telemetry monitoring should be promptly applied.26

In cases of severe hyperkalemia that involve cardiac arrhythmias, manifestations on ECG, or risk for arrhythmias, calcium gluconate (10 mL IV over 10 min) should be urgently administered, followed by IV insulin in conjunction with dextrose.26 Calcium chloride should be utilized for hyperkalemia in the context of the advanced cardiac life support (ACLS) protocol for cardiac arrest.26 The patient should remain on cardiac telemetry during this treatment to monitor for ventricular fibrillation or other arrhythmias.15 IV calcium does not lower serum potassium but rather antagonizes the effects of potassium on the cardiac cell membranes, helping to prevent or terminate arrhythmias.15,34 It should be noted, however, that firstline treatment for patients who develop hyperkalemia in the setting of digoxin toxicity involves administration of digoxin-specific antibody, while calcium infusion may be utilized later.34 Alternatively, if the patient is dialysis-dependent with ESRD, dialysis may be considered as a prompt initial treatment, with nephrologist consultation.
Administration of 10 U of regular insulin plus 25 g of 50% dextrose via IV will shift potassium intracellularly (see Figure 4). The dextrose will offset the resultant hypoglycemia.31,34 Of note, this treatment is often firstline for moderate to severe hyperkalemia in patients with a stable cardiac rhythm and ECG. Blood glucose should be monitored with a fingerstick within 30 to 60 minutes of infusion and every hour thereafter for up to six hours following insulin administration.34 Potassium levels should be checked every one to two hours after this treatment step until the serum potassium level stabilizes. Thereafter, recheck the levels every four to six hours to gauge whether further treatment is needed.34
Adjunctive
After performing firstline treatment strategies for severe hyperkalemia, there are alternate therapies to consider that can help lower total body potassium. Nebulized albuterol may be used, which pushes potassium into cells; this works in synergy with insulin and glucose.26,33 Sodium bicarbonate may be effective in cases in which the ABG analysis or labs show metabolic acidosis, as this infusion shifts potassium into cells by increasing the blood pH.33
In patients with dehydration, sepsis, TLS, or rhabdomyolysis, administration of IV fluids to maintain appropriate vascular volume is important. However, excessive fluid resuscitation can result in fluid overload, inducing complications such as respiratory failure and worsened renal function.40 A Foley catheter may be placed for strict intake and output monitoring.
The patient’s volume status must be carefully assessed. Hyperkalemia may present in association with heart failure exacerbation or ascites, which are usually hypervolemic states. Loop diuretics may be used to compensate for volume overload and to help remove potassium from the body, but these medications are contraindicated in anuric patients.13,41
Removing total body potassium
After emergent therapy is carried out, potassium may need to be removed from the body through diuresis, hemodialysis, or potassium binders. Loop diuretics or potassium binders may be used to treat mild to moderate hyperkalemia or to continue to stabilize the potassium level after emergent therapy is carried out. If severe hyperkalemia persists with kidney injury or with absence of urine output, hemodialysis is the therapy of choice.13
The potassium binder sodium polystyrene sulfonate (SPS) exchanges sodium for potassium in the intestine.42 This agent is contraindicated if the patient has intestinal obstruction. SPS’s slow onset of action (two to six hours) makes it ineffective as firstline therapy for severe hyperkalemia.3 In addition, SPS has serious but rare adverse effects, more commonly seen in patients who have uremia after kidney transplant or who have had recent abdominal surgery, bowel injury, or intestinal perforation.41 Adverse effects of SPS include aspiration pneumonitis, upper gastrointestinal injury, colonic necrosis, and rectal stenosis.41 However, there have been documented events of colonic necrosis due to SPS in patients without ESRD who have not had abdominal surgery.43,44 In 2009, the FDA advised against concomitant administration of sorbitol with SPS. However, this drug preparation continues to be the only one stocked by many hospital pharmacies.44 Because SPS has potentially harmful adverse effects and generally is not effective in promptly lowering serum potassium, it is prudent for clinicians to implement other management strategies first.44
MONITORING AT-RISK PATIENTS
Patients with a GFR < 45 mL/min/1.73 m2 and a baseline serum potassium level > 4.5 mEq/L are at risk for hyperkalemia while taking an ACEi or an ARB and should be advised to adhere to a potassium-restrictive diet with frequent laboratory checkups.22 Depending on the serum potassium and GFR levels at checkups, these medication doses may need to be reduced or discontinued altogether.
NEW DRUG DEVELOPMENTS
A potassium binder approved for daily use would benefit patients on aggressive heart failure medication regimens, as hyperkalemia commonly occurs with these regimens. As discussed, the widely available potassium binder SPS has been associated with severe gastrointestinal adverse effects, limiting its potential for routine use.44,45 In clinical trials, new potassium binders patiromer and zirconium cyclosilicate (ZS-9) have demonstrated an ability to maintain normokalemia over weeks of therapy with acceptable adverse effect profiles.45 In 2015, patiromer was approved by the FDA as therapy for hyperkalemia.46 An in-depth discussion, which is outside the scope of this article, will be presented by experts in the April 2017 edition of Renal Consult.
CONCLUSION
The best treatment for hyperkalemia is prevention through close surveillance of at-risk patients. Clinicians should be aware of predisposing risk factors for hyperkalemia, as it can have an insidious onset, with symptoms manifesting only when this electrolyte imbalance becomes life-threatening. It is particularly important to recognize when this condition mandates emergent treatment so that critical cardiac arrhythmias can be prevented.26
CE/CME No: CR-1703
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the pathophysiology and causes of hyperkalemia.
• Identify patients who are susceptible to hyperkalemia.
• Recognize the clinical sequelae of hyperkalemia.
• Formulate assessment and treatment plans for patients with hyperkalemia.
FACULTY
Melanie Douglas is a Physician Assistant in the Medicine Department at NYU Langone Medical Center in New York, New York. Denise Rizzolo is a Clinical Assistant Professor in the PA Program at Pace University in New York, New York, and Research Director in the Program of PA Studies at Kean University in Union, New Jersey. Danielle Kruger is an Academic Coordinator and Associate Professor in the PA Program at St. John’s University in Queens, New York. The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of March 2017.
Article begins on next page >>
Hyperkalemia is a common electrolyte disorder associated with life-threatening cardiac arrhythmias. Prompt recognition and appropriate treatment are essential in preventing serious cardiac complications. Although clinical manifestations of hyperkalemia are usually nonspecific or absent, laboratory testing and electrocardiography performed by the astute clinician aware of predisposing risk factors can help direct management.
Potassium is contained mostly in intracellular fluid; only about 2% is found in the extracellular space.1 The average total body potassium is about 50 mEq per kg of body weight (eg, a 70-kg individual has a total body potassium of approximately 3,500 mEq).2 Levels are tightly regulated by alterations in excretion in the distal renal tubule in response to potassium load and balance, and potassium distribution is influenced by insulin, aldosterone, catecholamines, and acid-base status.2 Movement of potassium across cell membranes is driven by the sodium-potassium adenosine triphosphatase (Na-K-ATPase) pump.3 In this article, we use the common serum potassium reference range of 3.5 to 5.0 mEq/L and define hyperkalemia as a serum potassium concentration greater than 5.5 mEq/L.4
Hyperkalemia can lead to life-threatening complications of cardiac arrhythmias, asystole, hypotension, flaccid paralysis, tetany, dyspnea, and altered mental status.5 Among patients with end-stage renal disease (ESRD), hyperkalemia is thought to contribute to 2% to 5% of deaths.6 A retrospective study found that patients with serum potassium levels exceeding 6.0 mEq/L on ICU admission had a significantly higher death rate within 30 days than patients who were normokalemic on presentation.7
RISK FACTORS
It is estimated that more than 35% of patients age 70 and older have chronic kidney disease (CKD) stage 3 or higher.8 Hyperkalemia is closely associated with CKD, increasing linearly in relation to the degree of renal impairment.8 As such, the prevalence of hyperkalemia in older adults is high, and it will increase overall as the US population ages. In a retrospective analysis of veterans older than 65 with CKD stage 3 or higher, the prevalence of hyperkalemia was 2.5%.9 Use of certain medications is also associated with hyperkalemia. Another retrospective study analyzed records obtained from 70,873 patients with CKD (estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73 m2) hospitalized in the Veterans Health Administration system. It found that patients treated with renin-angiotensin-aldosterone system (RAAS) blockers, such as ACE inhibitors (ACEis) or angiotensin-receptor blockers (ARBs), had a higher incidence of hyperkalemia (potassium level ≥ 5.5 mEq/L) than patients not treated with these medications (8.22 vs 1.77 events per 100 patient-months).9,10
POTASSIUM HOMEOSTASIS
Tight control over extracellular potassium is maintained in part by the Na-K-ATPase pump, which uses adenosine triphosphatase to move potassium and sodium ions in opposite directions across cell membranes.3 Specifically, three sodium ions are pumped out of the cell for every two potassium ions pumped in, resulting in a potassium gradient that is partially responsible for maintaining a resting membrane potential. This resting membrane potential, which determines myocardial, skeletal muscle, and nerve cell excitability and signaling, is highly sensitive to changes in the extracellular potassium level.4 Even small extracellular imbalances can induce cell depolarization and evoke an action potential. Increased extracellular potassium concentration decreases the resting membrane potential of the myocardium, shortens repolarization time, and decreases the rate of myocardial cell conduction, and also slows down neuromuscular conduction.11,12
Renal tubular function plays a significant role in potassium homeostasis, with approximately 90% of dietary potassium intake ex
The RAAS is a signal transduction pathway that regulates potassium excretion by the kidneys. Renin is secreted by the kidney in response to low renal perfusion, catecholamines, ß-adrenergic stimulation, potassium and sodium levels, and other factors. Secretion of renin triggers a signaling cascade that eventually results in the release of aldosterone from the adrenal cortex.5 Aldosterone binds to a receptor in the kidney’s collecting ducts where it increases potassium excretion by stimulating sodium reabsorption and fluid retention (see Figure 1).5

CAUSES OF HYPERKALEMIA
The pathophysiology of hyperkalemia generally involves either decreased renal excretion or shifts in extracellular potassium. Causes of hyperkalemia are listed in the Table. Potassium excretion can be disrupted in acute kidney injury (AKI), sepsis, cardiac ischemia, heart failure, diabetic ketoacidosis (DKA), insulin deficiency, tumor lysis syndrome (TLS), sickle cell disease, systemic lupus erythematosus, renal transplant, hepatorenal syndrome, adrenal insufficiency, and obstructive uropathy.15 In addition, certain medications can impair potassium excretion (eg, RAAS blockers, potassium-sparing diuretics in patients with CKD, digoxin toxicity).16 The following sections highlight the pathophysiology and manifestations of more common causes of hyperkalemia.

Renal impairment
Hyperkalemia may be a manifestation of worsening renal function. Potassium excretion is reduced in CKD, and CKD is the most common cause of hyperkalemia due to lower GFR.8,17 Patients with lower GFR tend to be older and male, and frequently have comorbid conditions such as type 2 diabetes, chronic liver disease, and heart failure.17
In CKD, decreased delivery of sodium to the distal tubules and reduced filtration capacity of the kidney diminishes the collecting duct’s ability to excrete potassium in exchange for sodium.2 Metabolic acidosis, which often contributes to AKI or CKD, causes potassium to shift from the intracellular to the extracellular compartment.4 Renal impairment may present clinically with dehydration, oliguria, nausea, vomiting, constipation, altered mental status, or weakness.
Hyperglycemia
Insulin and catecholamines (eg, epinephrine and norepinephrine) drive potassium into cells. Insulin increases potassium uptake into liver and muscle cells.13 A decrease in insulin levels, as may occur in type 2 diabetes or DKA, can cause a buildup of extracellular potassium.4 Also, serum hypertonicity from hyperglycemia results in water movement from the intracellular to the extracellular compartment; this raises the intracellular concentration of potassium, further promoting its movement to the extracellular space.4,14 Patients with hyperglycemia may present with dizziness, polyuria, polydipsia, nausea, vomiting, altered mental status, or fatigue.
Rhabdomyolysis
Rhabdomyolysis is a rapid breakdown of skeletal muscle that results in leakage of cellular contents into the extracellular space.4,18 Causes of rhabdomyolysis include use of medications such as statins, illicit drugs (eg, cocaine), or alcohol; rigorous exercise; and trauma.19
Muscle cell contents that are released into the circulation include potassium and other electrolytes, enzymes (eg, lactate dehydrogenase, aspartate transaminase, aldolase), and myoglobin.19 In rhabdomyolysis, myoglobin accumulation and hypovolemia lead to AKI and hyperkalemia.19 Patients may present with myalgias, extremity paresthesias, generalized weakness, nausea, altered mental status, fever, or darkened urine.18,19
Adrenal insufficiency
During critical illness such as sepsis, adrenal insufficiency can result from destruction of the adrenal glands, leading to hypoaldosteronism.20 Reduced aldosterone in adrenal insufficiency enables sodium and water to be eliminated from the body more easily, but as a result, less potassium gets excreted through the renal system and more is driven into the plasma.15
Acute adrenal insufficiency may manifest with hypotension, nausea, vomiting, or altered mental status, and labwork may reveal hyperkalemia as well as hypoglycemia or hyponatremia. Additionally, long-term glucocorticoid therapy can suppress the hypothalamic-pituitary axis and cause adrenal atrophy; rapid discontinuation of steroids can lead to adrenal insufficiency and hyperkalemia.21
Medications
RAAS blockers reduce CKD progression in patients with an eGFR of 29 mL/min/1.73 m2 or greater.22 Nonetheless, prescribing two or more drugs from the ACEi or ARB classes is not recommended. The Veterans Administration Nephron-Diabetes Trial (VA-NEPHRON-D) was terminated early because patients with stage 3 CKD due to diabetes who received dual ACEi/ARB therapy had higher rates of hyperkalemia but no slowing of CKD.22
Within the RAAS cascade, ACEis block the formation of angiotensin II and ARBs prevent angiotensin II from binding to the adrenal receptor. This impairs renal excretion of potassium and potentially contributes to hyperkalemia.5 Nonetheless, when patients on ACEis or ARBs develop hyperkalemia, aldosterone concentrations usually decrease due to preexisting illnesses (eg, diabetes, heart failure, CKD, AKI) or drug effects (eg, potassium-sparing diuretics, ß-blockers, digoxin).5 Ultimately, a combination of factors resulting from ACEi or ARB therapy causes reductions in renal perfusion and predisposes patients to hyperkalemia.5
NSAIDs may lead to hyperkalemia, as they interfere with prostaglandin release, decrease renal perfusion, and reduce renin and aldosterone levels.22 ß-blockers and tacrolimus inhibit renin release, leading to decreased aldosterone levels.5 Potassium-sparing diuretics block the interaction of aldosterone with the aldosterone receptor in the nephron.5 Digoxin decreases the activity of Na-K-ATPase, diminishing potassium uptake by cells.9 Potassium supplements, often prescribed for patients on diuretics, may contribute to hyperkalemia in patients with CKD. In the hospital setting, potassium tablets or IV formulations are utilized to correct hypokalemia. Especially in patients with CKD, clinicians should prescribe these agents with caution to avoid inducing hyperkalemia. Salt substitutes, which commonly contain potassium chloride, may be appealing to patients concerned about their sodium intake. However, consumption of these substitutes may contribute to hyperkalemia, especially in patients with CKD, heart failure, or type 2 diabetes.23
Tumor lysis syndrome
TLS involves rapid release of electrolytes and other intracellular contents into the extracellular space during the lysis of tumor cells.24 Nucleic acids within DNA strands break down and build up extracellularly, leading to hyperuricemia and often AKI. Potassium and other electrolytes released into the plasma during cell lysis can usually be removed by a healthy renal system. In TLS, however, AKI due to uric acid nephropathy prevents kidneys from removing the excess electrolytes from the bloodstream.24 Patients with rapidly growing hematologic tumors undergoing chemotherapy are especially at risk.
Pseudohyperkalemia
Pseudohyperkalemia is a transiently elevated serum potassium level that erroneously represents the true serum potassium level. It results from hemolysis due to mechanical trauma during the blood draw (eg, a tourniquet tied too tightly or use of a small-bore needle) or during specimen handling afterwards.25 Furthermore, leukocytosis, thrombocytosis, and polycythemia make red blood cells more fragile, increasing the chance of hemolysis and potassium leakage.26 Blood transfusion also can lead to pseudohyperkalemia. When blood is stored, potassium leakage from the cells and cell lysis, along with diminished Na-K-ATPase activity, lead to a buildup of potassium in the medium surrounding the stored red blood cells.27,28 The rise in serum potassium levels post-transfusion is usually transient, as the blood cells redistribute the potassium load once they become metabolically active.27,29
CLINICAL MANIFESTATIONS
Clinical manifestations of mild to moderate hyperkalemia (serum potassium > 5.5 mEq/L but < 6.5 mEq/L) include fatigue, generalized weakness, nausea, vomiting, constipation, and diarrhea.15 In many patients, mild to moderate hyperkalemia may not be associated with any acute symptoms and vital signs may be normal.13 Severe hyperkalemia (serum potassium > 6.5 mEq/L) may present clinically with acute extremity paresthesias, muscle weakness and paralysis, heart palpitations, dyspnea, altered mental status, cardiac arrhythmias, and cardiac arrest.30,31 Irregular heart rhythm, decreased deep tendon reflexes, or decreased strength may be revealed on physical exam.3 Individuals with ESRD on hemodialysis seem to tolerate higher levels of potassium than the general population without displaying clinical symptoms. However, these individuals are still susceptible to the cardiac effects of hyperkalemia.32
INITIAL ASSESSMENT
In assessing hyperkalemia, the clinician must perform a focused history and physical exam and review the patient’s medication list, including supplements and dietary habits that impact potassium intake. Potassium-rich foods include meat, fish, milk, almonds, spinach, cantaloupe, bananas, oranges, mushrooms, and potatoes.33 Hyperkalemia may present in association with various medical emergencies. The clinician should have an index of suspicion, depending on the patient’s overall medical profile and presentation, for emergencies such as cardiac ischemia, sepsis, adrenal crisis, DKA, TLS, and digoxin overdose.
The clinician must identify whether an elevated potassium level requires emergent therapy; assessment of vital signs is paramount in determining this. Orthostatic hypotension and tachycardia may hint that the patient is volume depleted. The patient should be examined for signs of hemodynamic shock with the CAB sequence: circulation, airway, breathing.34 Symptoms such as chest pain, shortness of breath, muscle weakness, paralysis, and altered mental status suggest that an expedited evaluation is warranted.

With a serum potassium level > 5.5 mEq/L, urgent electrocardiography should be performed.26 ECG findings observed with serum potassium levels of 5.5-6.5 mEq/L usually include peaked T-waves and prolonged PR intervals (see Figure 2). With potassium levels > 6.5 mEq/L consistent with further cardiac destabilization, the P-wave flattens then disappears, the QRS complex broadens, and sinus bradycardia or ectopic beats may occur.12,26 ST depression, T-wave inversion, or ST elevation also may be seen.12 With serum potassium levels > 7.5 mEq/L, progressive widening of the QRS complex to a sine-wave with bundle branch blocks or fascicular blocks may occur (see Figure 3).26 Without prompt intervention, ventricular fibrillation may ensue.26

An extensive laboratory workup may be necessary to investigate the etiology; this includes a complete blood count, metabolic panel, liver function tests, cardiac enzymes, blood gas analysis, serum/urine osmolality, urinalysis, urine electrolytes, and toxicology screen.13,26 Arterial blood gas (ABG) analysis may show metabolic acidosis with AKI or DKA, or an elevated lactate may occur with sepsis. In patients with hyperglycemia, besides checking for acidosis, obtaining blood/urine ketone levels and a metabolic panel with anion gap to evaluate for DKA is useful.35
When assessing a patient with an elevated creatinine, the GFR at the time of evaluation should be compared with the patient’s baseline GFR to determine chronicity and duration of his/her kidney disease.36 Obtaining a urinalysis and urine electrolytes in addition to the basic metabolic panel can help narrow the etiology.36 A Foley catheter should be placed in cases of urinary retention because without intervention, urinary obstruction may lead to AKI and hyperkalemia. Myoglobinuria on urinalysis and an elevated creatine kinase are diagnostic markers of rhabdomyolysis.18
TLS should be considered in patients who recently received chemotherapy, especially those with proliferative hematologic malignancies, such as acute lymphoblastic leukemia, acute myeloid leukemia, and Burkitt lymphoma.24 In TLS, bloodwork often reveals hyperkalemia along with AKI, an elevated uric acid level, hyperphosphatemia, and hypocalcemia.24
Patients presenting with hyperkalemia, hypotension, hypoglycemia, and hyponatremia may have adrenal insufficiency.20 If insufficiency is suspected, a cortisol level may be checked during morning hours; a low level is often suggestive of this diagnosis.37 Treatment includes daily doses of steroids, and consultation with an endocrinologist is recommended.37
If an elevated potassium level is not accompanied by renal dysfunction, electrolyte imbalances, ECG changes, or inciting medications, pseudohyperkalemia should be considered.38 A repeat lab sample should be checked. Consider obtaining an ABG analysis, as the shorter time interval between drawing the blood sample and the sample analysis reportedly increases the reliability of the resulting potassium level.38
THERAPY
Emergent
Emergent treatment is needed for severe hyperkalemia (see Figure 4). Any hyperkalemia-inciting medications or potassium supplements should be immediately discontinued.39 IV access and cardiac telemetry monitoring should be promptly applied.26

In cases of severe hyperkalemia that involve cardiac arrhythmias, manifestations on ECG, or risk for arrhythmias, calcium gluconate (10 mL IV over 10 min) should be urgently administered, followed by IV insulin in conjunction with dextrose.26 Calcium chloride should be utilized for hyperkalemia in the context of the advanced cardiac life support (ACLS) protocol for cardiac arrest.26 The patient should remain on cardiac telemetry during this treatment to monitor for ventricular fibrillation or other arrhythmias.15 IV calcium does not lower serum potassium but rather antagonizes the effects of potassium on the cardiac cell membranes, helping to prevent or terminate arrhythmias.15,34 It should be noted, however, that firstline treatment for patients who develop hyperkalemia in the setting of digoxin toxicity involves administration of digoxin-specific antibody, while calcium infusion may be utilized later.34 Alternatively, if the patient is dialysis-dependent with ESRD, dialysis may be considered as a prompt initial treatment, with nephrologist consultation.
Administration of 10 U of regular insulin plus 25 g of 50% dextrose via IV will shift potassium intracellularly (see Figure 4). The dextrose will offset the resultant hypoglycemia.31,34 Of note, this treatment is often firstline for moderate to severe hyperkalemia in patients with a stable cardiac rhythm and ECG. Blood glucose should be monitored with a fingerstick within 30 to 60 minutes of infusion and every hour thereafter for up to six hours following insulin administration.34 Potassium levels should be checked every one to two hours after this treatment step until the serum potassium level stabilizes. Thereafter, recheck the levels every four to six hours to gauge whether further treatment is needed.34
Adjunctive
After performing firstline treatment strategies for severe hyperkalemia, there are alternate therapies to consider that can help lower total body potassium. Nebulized albuterol may be used, which pushes potassium into cells; this works in synergy with insulin and glucose.26,33 Sodium bicarbonate may be effective in cases in which the ABG analysis or labs show metabolic acidosis, as this infusion shifts potassium into cells by increasing the blood pH.33
In patients with dehydration, sepsis, TLS, or rhabdomyolysis, administration of IV fluids to maintain appropriate vascular volume is important. However, excessive fluid resuscitation can result in fluid overload, inducing complications such as respiratory failure and worsened renal function.40 A Foley catheter may be placed for strict intake and output monitoring.
The patient’s volume status must be carefully assessed. Hyperkalemia may present in association with heart failure exacerbation or ascites, which are usually hypervolemic states. Loop diuretics may be used to compensate for volume overload and to help remove potassium from the body, but these medications are contraindicated in anuric patients.13,41
Removing total body potassium
After emergent therapy is carried out, potassium may need to be removed from the body through diuresis, hemodialysis, or potassium binders. Loop diuretics or potassium binders may be used to treat mild to moderate hyperkalemia or to continue to stabilize the potassium level after emergent therapy is carried out. If severe hyperkalemia persists with kidney injury or with absence of urine output, hemodialysis is the therapy of choice.13
The potassium binder sodium polystyrene sulfonate (SPS) exchanges sodium for potassium in the intestine.42 This agent is contraindicated if the patient has intestinal obstruction. SPS’s slow onset of action (two to six hours) makes it ineffective as firstline therapy for severe hyperkalemia.3 In addition, SPS has serious but rare adverse effects, more commonly seen in patients who have uremia after kidney transplant or who have had recent abdominal surgery, bowel injury, or intestinal perforation.41 Adverse effects of SPS include aspiration pneumonitis, upper gastrointestinal injury, colonic necrosis, and rectal stenosis.41 However, there have been documented events of colonic necrosis due to SPS in patients without ESRD who have not had abdominal surgery.43,44 In 2009, the FDA advised against concomitant administration of sorbitol with SPS. However, this drug preparation continues to be the only one stocked by many hospital pharmacies.44 Because SPS has potentially harmful adverse effects and generally is not effective in promptly lowering serum potassium, it is prudent for clinicians to implement other management strategies first.44
MONITORING AT-RISK PATIENTS
Patients with a GFR < 45 mL/min/1.73 m2 and a baseline serum potassium level > 4.5 mEq/L are at risk for hyperkalemia while taking an ACEi or an ARB and should be advised to adhere to a potassium-restrictive diet with frequent laboratory checkups.22 Depending on the serum potassium and GFR levels at checkups, these medication doses may need to be reduced or discontinued altogether.
NEW DRUG DEVELOPMENTS
A potassium binder approved for daily use would benefit patients on aggressive heart failure medication regimens, as hyperkalemia commonly occurs with these regimens. As discussed, the widely available potassium binder SPS has been associated with severe gastrointestinal adverse effects, limiting its potential for routine use.44,45 In clinical trials, new potassium binders patiromer and zirconium cyclosilicate (ZS-9) have demonstrated an ability to maintain normokalemia over weeks of therapy with acceptable adverse effect profiles.45 In 2015, patiromer was approved by the FDA as therapy for hyperkalemia.46 An in-depth discussion, which is outside the scope of this article, will be presented by experts in the April 2017 edition of Renal Consult.
CONCLUSION
The best treatment for hyperkalemia is prevention through close surveillance of at-risk patients. Clinicians should be aware of predisposing risk factors for hyperkalemia, as it can have an insidious onset, with symptoms manifesting only when this electrolyte imbalance becomes life-threatening. It is particularly important to recognize when this condition mandates emergent treatment so that critical cardiac arrhythmias can be prevented.26
1. An JN, Lee JP, Jeon HJ, et al. Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care. 2012; 16(6):R225.
2. Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ. 2016;40(4):480-490.
3. Medford-Davis L, Rafique Z. Derangements of potassium. Emerg Med Clin North Am. 2014;32(2):329-347.
4. Eleftheriadis T, Leivaditis K, Antoniadi G, Liakopoulos V. Differential diagnosis of hyperkalemia: an update to a complex problem. Hippokratia. 2012;16(4):294-302.
5. Raebel M. Hyperkalemia associated with use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Cardiovasc Ther. 2012;30(3):156-166.
6. Korgaonkar S, Tilea A, Gillespie BW, et al. Serum potassium and outcomes in CKD: insights from the RRI-CKD Cohort Study. Clin J Am Soc Nephrol. 2010;5(5):762-769.
7. McMahon GM, Mendu ML, Gibbons FK, Christopher KB. Association between hyperkalemia at critical care initiation and mortality. Intensive Care Med. 2012;38(11):1834-1842.
8. Drawz PE, Babineau DC, Rahman M. Metabolic complications in elderly adults with chronic kidney disease. J Am Geriatr Soc. 2012;60(2):310-315.
9. Sarafidis PA, Georgianos PI, Bakris GL. Advances in treatment of hyperkalemia in chronic kidney disease. Expert Opin Pharmacother. 2015;16(14):2205-2215.
10. Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156-1162.
11. Khanagavi J, Gupta T, Aronow WS, et al. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10(2):251-257.
12. Berkova M, Berka Z, Topinkova E. Arrhythmias and ECG changes in life threatening hyperkalemia in older patients treated by potassium sparing drugs. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158(1):84-91.
13. Lehnhardt A, Kemper MJ. Pathogenesis, diagnosis and management of hyperkalemia. Pediatr Nephrol. 2011;26(3):377-384.
14. Palmer BF. A physiologic-based approach to the evaluation of a patient with hyperkalemia. Am J Kidney Dis. 2010;56(2):387-393.
15. Mushiyakh Y, Dangaria H, Qavi S, et al. Treatment and pathogenesis of acute hyperkalemia. J Community Hosp Intern Med Perspect. 2012;1(4):7372.
16. Elliott MJ, Ronksley PE, Clase CM, et al. Management of patients with acute hyperkalemia. CMAJ. 2010;182(15):1631-1635.
17. Wiebe N, Klarenbach SW, Allan GM, et al. Potentially preventable hospitalization as a complication of CKD: a cohort study. Am J Kidney Dis. 2014;64(2):230-238.
18. Zutt R, van der Kooi AJ, Linthorst GE, et al. Rhabdomyolysis: review of the literature. Neuromuscul Disord. 2014;24(8):651-659.
19. Zimmerman JL, Shen MC. Rhabdomyolysis. Chest. 2013; 144(3):1058-1065.
20. Khardori R, Castillo D. Endocrine and metabolic changes during sepsis: an update. Med Clin North Am. 2012;96(6):1095-1105.
21. Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2014;4(20):739-769.
22. Lazich I, Bakris GL. Prediction and management of hyperkalemia across the spectrum of chronic kidney disease. Semin Nephrol. 2014;34(3):333-339.
23. Ayach T, Nappo R, Paugh-Miller J, Ross E. Life-threatening hyperkalemia in a patient with normal renal function. Clin Kidney J. 2014;7(1):49-52.
24. Wilson FP, Berns JS. Tumor lysis syndrome: new challenges and recent advances. Adv Chronic Kidney Dis. 2014;21(1):18-26.
25. Asiryatham JR, Moses V, Bjornson L. Errors in potassium measurement: a laboratory perspective for the clinician. N Am J Med Sci. 2013;5(4):255-259.
26. Pepin J, Shields C. Advances in diagnosis and management of hypokalemic and hyperkalemic emergencies. Emerg Med Pract. 2012;14(2):1-17.
27. Vraets A, Lin Y, Callum JL. Transfusion-associated hyperkalemia. Transfus Med Rev. 2011;25(3):184-196.
28. Aboudara MC, Hurst FP, Abbott KC, Perkins RM. Hyperkalemia after packed red blood cell transfusion in trauma patients. J Trauma. 2008;64(2 suppl):S86-S91.
29. Olson J, Talekar M, Sachdev M, et al. Potassium changes associated with blood transfusion in pediatric patients. Am J Clin Pathol. 2013;139(6):800-805.
30. Chon S, Kwak YH, Hwang SS, et al. Severe hyperkalemia can be detected immediately by quantitative electrocardiography and clinical history in patients with symptomatic or extreme bradycardia: a retrospective cross-sectional study. J Crit Care. 2013;28(6):1112.e7-1112.e13.
31. Viera AJ, Wouk N. Potassium disorders: hypokalemia and hyperkalemia. Am Fam Physician. 2015;92(6):487-495.
32. Sanghavi S, Whitling S, Uribarri J. Potassium balance in dialysis patients. Semin Dial. 2013;26(5):597-603.
33. Crawford AH. Hyperkalemia: Recognition and management of a critical electrolyte disturbance. J Infus Nurs. 2014;37(3):167-175.
34. Maxwell AP, Linden K, O’Donnell S, et al. Management of hyperkalemia. J R Coll Physicians Edinb. 2013;43(3):246-251.
35. Seth P, Kaur H, Kaur M. Clinical profile of diabetic ketoacidosis: a prospective study in a tertiary care hospital. J Clin Diagn Res. 2015;9(6):OC01-OC04.
36. Rahman M, Shad F, Smith M. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician. 2012;86(7): 631-639.
37. Puar TH, Stikkelbroeck NM, Smans LC, et al. Adrenal crisis: still a deadly event in the 21st century. Am J Med. 2016;129(3):339.e1-9.
38. Liamis G, Liberopoulos E, Barkas F, Elisaf M. Spurious electrolyte disorders: a diagnostic challenge for clinicians. Am J Nephrol. 2013;38(1):50-57.
39. Kovesdy CP. Management of hyperkalemia: an update for the internist. Am J Med. 2015;128(12):1281-1287.
40. Labib M, Khalid R, Khan A, Khan S. Volume management in the critically ill patient with acute kidney injury. Crit Care Res Pract. 2013;2013:792830.
41. Watson M, Abbott KC, Yuan CM. Damned if you do, damned if you don’t: potassium binding resins in hyperkalemia. Clin J Am Soc Nephrol. 2010;5(10):1723-1726.
42. Nguyen T, Ondrik D, Zhufyak O, et al. Hyperkalemia and potential pitfalls of sodium polystyrene sulfonate. JAAPA. 2015; 28(3):41-45.
43. McGowan CE, Saha S, Resnick MB, Moss SF. Intestinal necrosis due to sodium polystyrene sulfonate (Kayexalate) in sorbitol. South Med J. 2009;102(5):493-497.
44. Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21:733-735.
45. Pitt B, Bakris GL. New potassium binders for the treatment of hyperkalemia: current data and opportunities for the future. Hypertension. 2015;66(4):731-738.
46. Epstein M, Pitt B. Recent advances in pharmacological treatments of hyperkalemia: focus on patiromer. Expert Opin Pharmacother. 2016;17(10):1435-1448.
1. An JN, Lee JP, Jeon HJ, et al. Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care. 2012; 16(6):R225.
2. Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ. 2016;40(4):480-490.
3. Medford-Davis L, Rafique Z. Derangements of potassium. Emerg Med Clin North Am. 2014;32(2):329-347.
4. Eleftheriadis T, Leivaditis K, Antoniadi G, Liakopoulos V. Differential diagnosis of hyperkalemia: an update to a complex problem. Hippokratia. 2012;16(4):294-302.
5. Raebel M. Hyperkalemia associated with use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Cardiovasc Ther. 2012;30(3):156-166.
6. Korgaonkar S, Tilea A, Gillespie BW, et al. Serum potassium and outcomes in CKD: insights from the RRI-CKD Cohort Study. Clin J Am Soc Nephrol. 2010;5(5):762-769.
7. McMahon GM, Mendu ML, Gibbons FK, Christopher KB. Association between hyperkalemia at critical care initiation and mortality. Intensive Care Med. 2012;38(11):1834-1842.
8. Drawz PE, Babineau DC, Rahman M. Metabolic complications in elderly adults with chronic kidney disease. J Am Geriatr Soc. 2012;60(2):310-315.
9. Sarafidis PA, Georgianos PI, Bakris GL. Advances in treatment of hyperkalemia in chronic kidney disease. Expert Opin Pharmacother. 2015;16(14):2205-2215.
10. Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156-1162.
11. Khanagavi J, Gupta T, Aronow WS, et al. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10(2):251-257.
12. Berkova M, Berka Z, Topinkova E. Arrhythmias and ECG changes in life threatening hyperkalemia in older patients treated by potassium sparing drugs. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158(1):84-91.
13. Lehnhardt A, Kemper MJ. Pathogenesis, diagnosis and management of hyperkalemia. Pediatr Nephrol. 2011;26(3):377-384.
14. Palmer BF. A physiologic-based approach to the evaluation of a patient with hyperkalemia. Am J Kidney Dis. 2010;56(2):387-393.
15. Mushiyakh Y, Dangaria H, Qavi S, et al. Treatment and pathogenesis of acute hyperkalemia. J Community Hosp Intern Med Perspect. 2012;1(4):7372.
16. Elliott MJ, Ronksley PE, Clase CM, et al. Management of patients with acute hyperkalemia. CMAJ. 2010;182(15):1631-1635.
17. Wiebe N, Klarenbach SW, Allan GM, et al. Potentially preventable hospitalization as a complication of CKD: a cohort study. Am J Kidney Dis. 2014;64(2):230-238.
18. Zutt R, van der Kooi AJ, Linthorst GE, et al. Rhabdomyolysis: review of the literature. Neuromuscul Disord. 2014;24(8):651-659.
19. Zimmerman JL, Shen MC. Rhabdomyolysis. Chest. 2013; 144(3):1058-1065.
20. Khardori R, Castillo D. Endocrine and metabolic changes during sepsis: an update. Med Clin North Am. 2012;96(6):1095-1105.
21. Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2014;4(20):739-769.
22. Lazich I, Bakris GL. Prediction and management of hyperkalemia across the spectrum of chronic kidney disease. Semin Nephrol. 2014;34(3):333-339.
23. Ayach T, Nappo R, Paugh-Miller J, Ross E. Life-threatening hyperkalemia in a patient with normal renal function. Clin Kidney J. 2014;7(1):49-52.
24. Wilson FP, Berns JS. Tumor lysis syndrome: new challenges and recent advances. Adv Chronic Kidney Dis. 2014;21(1):18-26.
25. Asiryatham JR, Moses V, Bjornson L. Errors in potassium measurement: a laboratory perspective for the clinician. N Am J Med Sci. 2013;5(4):255-259.
26. Pepin J, Shields C. Advances in diagnosis and management of hypokalemic and hyperkalemic emergencies. Emerg Med Pract. 2012;14(2):1-17.
27. Vraets A, Lin Y, Callum JL. Transfusion-associated hyperkalemia. Transfus Med Rev. 2011;25(3):184-196.
28. Aboudara MC, Hurst FP, Abbott KC, Perkins RM. Hyperkalemia after packed red blood cell transfusion in trauma patients. J Trauma. 2008;64(2 suppl):S86-S91.
29. Olson J, Talekar M, Sachdev M, et al. Potassium changes associated with blood transfusion in pediatric patients. Am J Clin Pathol. 2013;139(6):800-805.
30. Chon S, Kwak YH, Hwang SS, et al. Severe hyperkalemia can be detected immediately by quantitative electrocardiography and clinical history in patients with symptomatic or extreme bradycardia: a retrospective cross-sectional study. J Crit Care. 2013;28(6):1112.e7-1112.e13.
31. Viera AJ, Wouk N. Potassium disorders: hypokalemia and hyperkalemia. Am Fam Physician. 2015;92(6):487-495.
32. Sanghavi S, Whitling S, Uribarri J. Potassium balance in dialysis patients. Semin Dial. 2013;26(5):597-603.
33. Crawford AH. Hyperkalemia: Recognition and management of a critical electrolyte disturbance. J Infus Nurs. 2014;37(3):167-175.
34. Maxwell AP, Linden K, O’Donnell S, et al. Management of hyperkalemia. J R Coll Physicians Edinb. 2013;43(3):246-251.
35. Seth P, Kaur H, Kaur M. Clinical profile of diabetic ketoacidosis: a prospective study in a tertiary care hospital. J Clin Diagn Res. 2015;9(6):OC01-OC04.
36. Rahman M, Shad F, Smith M. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician. 2012;86(7): 631-639.
37. Puar TH, Stikkelbroeck NM, Smans LC, et al. Adrenal crisis: still a deadly event in the 21st century. Am J Med. 2016;129(3):339.e1-9.
38. Liamis G, Liberopoulos E, Barkas F, Elisaf M. Spurious electrolyte disorders: a diagnostic challenge for clinicians. Am J Nephrol. 2013;38(1):50-57.
39. Kovesdy CP. Management of hyperkalemia: an update for the internist. Am J Med. 2015;128(12):1281-1287.
40. Labib M, Khalid R, Khan A, Khan S. Volume management in the critically ill patient with acute kidney injury. Crit Care Res Pract. 2013;2013:792830.
41. Watson M, Abbott KC, Yuan CM. Damned if you do, damned if you don’t: potassium binding resins in hyperkalemia. Clin J Am Soc Nephrol. 2010;5(10):1723-1726.
42. Nguyen T, Ondrik D, Zhufyak O, et al. Hyperkalemia and potential pitfalls of sodium polystyrene sulfonate. JAAPA. 2015; 28(3):41-45.
43. McGowan CE, Saha S, Resnick MB, Moss SF. Intestinal necrosis due to sodium polystyrene sulfonate (Kayexalate) in sorbitol. South Med J. 2009;102(5):493-497.
44. Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21:733-735.
45. Pitt B, Bakris GL. New potassium binders for the treatment of hyperkalemia: current data and opportunities for the future. Hypertension. 2015;66(4):731-738.
46. Epstein M, Pitt B. Recent advances in pharmacological treatments of hyperkalemia: focus on patiromer. Expert Opin Pharmacother. 2016;17(10):1435-1448.
Vitiligo linked to moles, tanning ability, blistering sunburn
Upper extremity moles were associated with a 37% increase in the likelihood of vitiligo among white women, according to an analysis of the prospective Nurses’ Health Study.
“Women with a higher tanning ability and women who had a history of blistering sunburns in childhood were also found to have a higher risk of developing vitiligo,” Rachel Dunlap, MD, of the department of dermatology, Brown University in Providence, R.I., and her associates wrote in the Journal of Investigative Dermatology.
Vitiligo is the most common cutaneous depigmentation disorder, but associated risk factors are poorly understood, the investigators noted. They examined ties between skin pigmentation, reactions to sun exposure, and new onset vitiligo in the Nurses’ Health Study, a population-based prospective cohort study. Study participants were asked to report the number of moles on their left arms measuring at least 3 mm in diameter, their reactions to sunburn and ability to tan during childhood, and whether they had vitiligo diagnosed by a physician. A total of 51,337 women answered the question about moles, and 68,590 women answered the question about vitiligo, the investigators said (J Invest Dermatol. 2017 Feb 14. doi: 10.1016/j.jid.2017.02.004).
A total of 271 cases of vitiligo developed over 835,594 person-years. Women who reported at least one left arm mole larger than 3 mm were significantly more likely to report incident vitiligo, compared with women without moles (hazard ratio, 1.37; 95% confidence interval, 1.02-1.83), even after controlling for age, hair color, history of exposure to direct sunlight, skin tanning ability, and severity of reaction to sunburn. Developing an “average” tan or a “deep” tan after prolonged sun exposure also were significantly associated with vitiligo with hazard ratios of 2.28 (95% CI, 1.12-4.65) and 2.59 (95% CI, 1.21-5.54), respectively, “when compared to those who had minimal skin reactions or less severe burns when exposed to the sun,” the authors wrote.
A history of at least one blistering sunburn after 2 hours of sun exposure also predicted vitiligo (HR, 2.17; 95% CI, 1.15-4.10), while hair color did not.
“The benefits of good sun protection can be expanded to include potential vitiligo prevention, which may be particularly applicable to adult patients with vitiligo who are concerned about their children developing the condition,” the investigators commented. “Future studies will examine the incidence of other influencing factors, such as melanoma and melanoma associated leukoderma in this population.”
External funding sources included the National Institutes of Health and Dermatology Foundation. The investigators reported having no conflicts of interest.
Upper extremity moles were associated with a 37% increase in the likelihood of vitiligo among white women, according to an analysis of the prospective Nurses’ Health Study.
“Women with a higher tanning ability and women who had a history of blistering sunburns in childhood were also found to have a higher risk of developing vitiligo,” Rachel Dunlap, MD, of the department of dermatology, Brown University in Providence, R.I., and her associates wrote in the Journal of Investigative Dermatology.
Vitiligo is the most common cutaneous depigmentation disorder, but associated risk factors are poorly understood, the investigators noted. They examined ties between skin pigmentation, reactions to sun exposure, and new onset vitiligo in the Nurses’ Health Study, a population-based prospective cohort study. Study participants were asked to report the number of moles on their left arms measuring at least 3 mm in diameter, their reactions to sunburn and ability to tan during childhood, and whether they had vitiligo diagnosed by a physician. A total of 51,337 women answered the question about moles, and 68,590 women answered the question about vitiligo, the investigators said (J Invest Dermatol. 2017 Feb 14. doi: 10.1016/j.jid.2017.02.004).
A total of 271 cases of vitiligo developed over 835,594 person-years. Women who reported at least one left arm mole larger than 3 mm were significantly more likely to report incident vitiligo, compared with women without moles (hazard ratio, 1.37; 95% confidence interval, 1.02-1.83), even after controlling for age, hair color, history of exposure to direct sunlight, skin tanning ability, and severity of reaction to sunburn. Developing an “average” tan or a “deep” tan after prolonged sun exposure also were significantly associated with vitiligo with hazard ratios of 2.28 (95% CI, 1.12-4.65) and 2.59 (95% CI, 1.21-5.54), respectively, “when compared to those who had minimal skin reactions or less severe burns when exposed to the sun,” the authors wrote.
A history of at least one blistering sunburn after 2 hours of sun exposure also predicted vitiligo (HR, 2.17; 95% CI, 1.15-4.10), while hair color did not.
“The benefits of good sun protection can be expanded to include potential vitiligo prevention, which may be particularly applicable to adult patients with vitiligo who are concerned about their children developing the condition,” the investigators commented. “Future studies will examine the incidence of other influencing factors, such as melanoma and melanoma associated leukoderma in this population.”
External funding sources included the National Institutes of Health and Dermatology Foundation. The investigators reported having no conflicts of interest.
Upper extremity moles were associated with a 37% increase in the likelihood of vitiligo among white women, according to an analysis of the prospective Nurses’ Health Study.
“Women with a higher tanning ability and women who had a history of blistering sunburns in childhood were also found to have a higher risk of developing vitiligo,” Rachel Dunlap, MD, of the department of dermatology, Brown University in Providence, R.I., and her associates wrote in the Journal of Investigative Dermatology.
Vitiligo is the most common cutaneous depigmentation disorder, but associated risk factors are poorly understood, the investigators noted. They examined ties between skin pigmentation, reactions to sun exposure, and new onset vitiligo in the Nurses’ Health Study, a population-based prospective cohort study. Study participants were asked to report the number of moles on their left arms measuring at least 3 mm in diameter, their reactions to sunburn and ability to tan during childhood, and whether they had vitiligo diagnosed by a physician. A total of 51,337 women answered the question about moles, and 68,590 women answered the question about vitiligo, the investigators said (J Invest Dermatol. 2017 Feb 14. doi: 10.1016/j.jid.2017.02.004).
A total of 271 cases of vitiligo developed over 835,594 person-years. Women who reported at least one left arm mole larger than 3 mm were significantly more likely to report incident vitiligo, compared with women without moles (hazard ratio, 1.37; 95% confidence interval, 1.02-1.83), even after controlling for age, hair color, history of exposure to direct sunlight, skin tanning ability, and severity of reaction to sunburn. Developing an “average” tan or a “deep” tan after prolonged sun exposure also were significantly associated with vitiligo with hazard ratios of 2.28 (95% CI, 1.12-4.65) and 2.59 (95% CI, 1.21-5.54), respectively, “when compared to those who had minimal skin reactions or less severe burns when exposed to the sun,” the authors wrote.
A history of at least one blistering sunburn after 2 hours of sun exposure also predicted vitiligo (HR, 2.17; 95% CI, 1.15-4.10), while hair color did not.
“The benefits of good sun protection can be expanded to include potential vitiligo prevention, which may be particularly applicable to adult patients with vitiligo who are concerned about their children developing the condition,” the investigators commented. “Future studies will examine the incidence of other influencing factors, such as melanoma and melanoma associated leukoderma in this population.”
External funding sources included the National Institutes of Health and Dermatology Foundation. The investigators reported having no conflicts of interest.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Key clinical point: Upper extremity moles, tanning ability, and a history of blistering sunburn were significant risk factors for vitiligo among white women.
Major finding: In the multivariate analysis, hazard ratios were 1.37 (95% confidence interval, 1.02-1.83), 2.28 (95% CI, 1.12-4.65), 2.59 (95% CI, 1.21-5.54), and 2.17 (95% CI, 1.15-4.10), respectively.
Data source: An analysis of 51,337 white women from the Nurses’ Health Study.
Disclosures: Funding sources included the National Institutes of Health and Dermatology Foundation. The investigators reported having no conflicts of interest.
Spotlight shifts to active treatment for concussions
The prevailing notion that concussions should be managed solely or primarily with prescribed cognitive and physical rest is shifting.
Experts in concussion management are increasingly in agreement that concussion is a much more heterogeneous injury than previously believed, and that “active” approaches targeting specific symptoms and impairments can be initiated early and may improve recovery for patients who have concussions from sports as well as from falls, motor vehicle accidents, or other accidents.
Evidence for such active approaches to rehabilitation – including vestibular, vision, and behavioral therapies, and submaximal aerobic training – is deemed “preliminary” by experts and comes from small, mostly single-center studies.
“There’s emerging evidence that strict or prolonged rest is not good, and there’s emerging consensus that we need to start looking at concussion subtypes” and then target treatments to those clinical profiles, he said. “We’re at the cusp of dramatic changes [in management].”
An article published recently in Neurosurgery and coauthored by several dozen concussion experts details 16 “statements of agreement” on targeted evaluation and active management. The experts – from neuropsychology, sports medicine, neurology and neurosurgery, athletic training, and other fields – convened in Pittsburgh in late 2015 at the invitation of Michael W. Collins, PhD, and his colleagues at the University of Pittsburgh Medical Center (UPMC), where the Sports Medicine Concussion Program was established in 2000 (Neurosurgery. 2016 Dec;79[6]:912-29).
Thus far, guidelines and statements on concussion in sport have advised a rest-based approach to management that’s dependent on the spontaneous resolution of symptoms. In some documents, rest is recommended until patients are asymptomatic. Other statements mention the possibility of symptom-based approaches after initial rest, but offer little if any guidance.
The theory of prescribed rest has been twofold, driven both by concern about re-injury in sport and by the belief that cognitive and physical activity can exacerbate symptoms and concussion-associated impairments, thus prolonging recovery, the Pittsburgh paper says.
However, “avoiding contact during the vulnerable period after concussion and prescribed rest represent two separate strategies,” the experts wrote. Avoiding contact “is always recommended to avoid further head impacts,” they say, but monitored activity does not appear to worsen injury.
Recent research suggests, moreover, that prolonged physical and cognitive rest – not activity – is associated with increased symptoms and delayed recovery. And strict rest – avoidance of nearly all brain stimulation – is “not empirically supported ... and may have unintended adverse effects,” the experts wrote.
Physicians and others “have to avoid treating concussion as a punishment,” said Dr. Halstead of St. Louis Children’s Hospital in Chesterfield, Mo., who coauthored a recent editorial entitled “Rethinking Cognitive Rest” (Br J Sports Med. 2017;51:147).
“It’s been taken to the extreme. When we talk about not texting or not going out or not doing anything physically active until you’re without symptoms ... students can develop symptoms of depression and anxiety that then just complicate the injury altogether,” he said.
Physicians also need to dig beneath symptom checklists and perform multidomain assessments to better understand root contributors of symptoms that, without active treatment, can persist for weeks upon weeks in some patients. “There could be neck injuries, sleep issues, vestibular issues, oculomotor issues” and different types of headache, Dr. Halstead said. “If we can identify these things, we can actually be doing rehabilitation to fix these injuries.”
At UPMC’s Sports Medicine Concussion Program, concussions are generally categorized into six clinical profiles – vestibular, ocular-motor, cognitive/fatigue, posttraumatic migraine, cervical, and anxiety/mood. The profiles are not mutually exclusive, but each drives particular rehabilitation recommendations. Clinicians at other concussion programs and centers are similarly attempting to classify concussions.
“We still need to come to agreement as to what exactly the clinical profiles are,” said UPMC’s Dr. Collins, who directs the concussion program and is the lead author of the Neurosurgery paper. “But I think the big concept to come out of our meeting is that we now agree there are different profiles and that we have to match treatment.”
Additional research is needed to determine and validate concussion clinical profiles, to identify biomarkers to assess recovery and determine the effectiveness of treatments, and to determine the best timing of specific active treatments, he and his coauthors said.
Applying individualized and active treatments after concussion is consistent with approaches taken for other injuries and conditions, noted Dr. Hainline, who attended both the UPMC conference and the 5th International Conference on Concussion in Sport.
“It’s rare that prolonged rest is the answer. Look at stroke – you don’t have patients resting indefinitely. You have to get their nervous systems re-engaged,” he said.
“If you keep [concussion] patients with predominantly vestibular symptoms at rest [for example], the vestibular symptoms can become more centralized, and that then becomes the behavior of the nervous system,” he said. “Another example is convergence insufficiencies – if you keep resting [the vision system] and don’t rehabilitate it,” symptoms perpetuate.
Prolonged rest also may lead to deconditioning that lowers tolerance for physical activity. Randomized clinical trials are needed to compare the benefits of physical rest with those of more physically active treatments, but “emerging clinical research” suggests that exposing patients to supervised low-level physical activity (for example, submaximal aerobic training) after an initial period of rest is “not only safe but effective,” the Pittsburgh conference paper says.
The Pittsburgh conference paper “rang a bell with me,” Dr. Wergin said. “Rest is still important, but prolonged rest may not be best for all patients, and maybe it’s possible to do some interventions. We need to stay tuned as we get more of an evidence base.”
The conference – coined the TEAM (for targeted evaluation and active management) Approaches to Treating Concussion Meeting – was attended by staff from the National Institutes of Health, the Centers for Disease Control and Prevention, the Department of Defense, and the National Football League (NFL) and other sporting organizations, Dr. Collins said. It was sponsored by UPMC and the NFL. Coauthors reported numerous disclosures, including various advising and consulting roles with the NFL.
The prevailing notion that concussions should be managed solely or primarily with prescribed cognitive and physical rest is shifting.
Experts in concussion management are increasingly in agreement that concussion is a much more heterogeneous injury than previously believed, and that “active” approaches targeting specific symptoms and impairments can be initiated early and may improve recovery for patients who have concussions from sports as well as from falls, motor vehicle accidents, or other accidents.
Evidence for such active approaches to rehabilitation – including vestibular, vision, and behavioral therapies, and submaximal aerobic training – is deemed “preliminary” by experts and comes from small, mostly single-center studies.
“There’s emerging evidence that strict or prolonged rest is not good, and there’s emerging consensus that we need to start looking at concussion subtypes” and then target treatments to those clinical profiles, he said. “We’re at the cusp of dramatic changes [in management].”
An article published recently in Neurosurgery and coauthored by several dozen concussion experts details 16 “statements of agreement” on targeted evaluation and active management. The experts – from neuropsychology, sports medicine, neurology and neurosurgery, athletic training, and other fields – convened in Pittsburgh in late 2015 at the invitation of Michael W. Collins, PhD, and his colleagues at the University of Pittsburgh Medical Center (UPMC), where the Sports Medicine Concussion Program was established in 2000 (Neurosurgery. 2016 Dec;79[6]:912-29).
Thus far, guidelines and statements on concussion in sport have advised a rest-based approach to management that’s dependent on the spontaneous resolution of symptoms. In some documents, rest is recommended until patients are asymptomatic. Other statements mention the possibility of symptom-based approaches after initial rest, but offer little if any guidance.
The theory of prescribed rest has been twofold, driven both by concern about re-injury in sport and by the belief that cognitive and physical activity can exacerbate symptoms and concussion-associated impairments, thus prolonging recovery, the Pittsburgh paper says.
However, “avoiding contact during the vulnerable period after concussion and prescribed rest represent two separate strategies,” the experts wrote. Avoiding contact “is always recommended to avoid further head impacts,” they say, but monitored activity does not appear to worsen injury.
Recent research suggests, moreover, that prolonged physical and cognitive rest – not activity – is associated with increased symptoms and delayed recovery. And strict rest – avoidance of nearly all brain stimulation – is “not empirically supported ... and may have unintended adverse effects,” the experts wrote.
Physicians and others “have to avoid treating concussion as a punishment,” said Dr. Halstead of St. Louis Children’s Hospital in Chesterfield, Mo., who coauthored a recent editorial entitled “Rethinking Cognitive Rest” (Br J Sports Med. 2017;51:147).
“It’s been taken to the extreme. When we talk about not texting or not going out or not doing anything physically active until you’re without symptoms ... students can develop symptoms of depression and anxiety that then just complicate the injury altogether,” he said.
Physicians also need to dig beneath symptom checklists and perform multidomain assessments to better understand root contributors of symptoms that, without active treatment, can persist for weeks upon weeks in some patients. “There could be neck injuries, sleep issues, vestibular issues, oculomotor issues” and different types of headache, Dr. Halstead said. “If we can identify these things, we can actually be doing rehabilitation to fix these injuries.”
At UPMC’s Sports Medicine Concussion Program, concussions are generally categorized into six clinical profiles – vestibular, ocular-motor, cognitive/fatigue, posttraumatic migraine, cervical, and anxiety/mood. The profiles are not mutually exclusive, but each drives particular rehabilitation recommendations. Clinicians at other concussion programs and centers are similarly attempting to classify concussions.
“We still need to come to agreement as to what exactly the clinical profiles are,” said UPMC’s Dr. Collins, who directs the concussion program and is the lead author of the Neurosurgery paper. “But I think the big concept to come out of our meeting is that we now agree there are different profiles and that we have to match treatment.”
Additional research is needed to determine and validate concussion clinical profiles, to identify biomarkers to assess recovery and determine the effectiveness of treatments, and to determine the best timing of specific active treatments, he and his coauthors said.
Applying individualized and active treatments after concussion is consistent with approaches taken for other injuries and conditions, noted Dr. Hainline, who attended both the UPMC conference and the 5th International Conference on Concussion in Sport.
“It’s rare that prolonged rest is the answer. Look at stroke – you don’t have patients resting indefinitely. You have to get their nervous systems re-engaged,” he said.
“If you keep [concussion] patients with predominantly vestibular symptoms at rest [for example], the vestibular symptoms can become more centralized, and that then becomes the behavior of the nervous system,” he said. “Another example is convergence insufficiencies – if you keep resting [the vision system] and don’t rehabilitate it,” symptoms perpetuate.
Prolonged rest also may lead to deconditioning that lowers tolerance for physical activity. Randomized clinical trials are needed to compare the benefits of physical rest with those of more physically active treatments, but “emerging clinical research” suggests that exposing patients to supervised low-level physical activity (for example, submaximal aerobic training) after an initial period of rest is “not only safe but effective,” the Pittsburgh conference paper says.
The Pittsburgh conference paper “rang a bell with me,” Dr. Wergin said. “Rest is still important, but prolonged rest may not be best for all patients, and maybe it’s possible to do some interventions. We need to stay tuned as we get more of an evidence base.”
The conference – coined the TEAM (for targeted evaluation and active management) Approaches to Treating Concussion Meeting – was attended by staff from the National Institutes of Health, the Centers for Disease Control and Prevention, the Department of Defense, and the National Football League (NFL) and other sporting organizations, Dr. Collins said. It was sponsored by UPMC and the NFL. Coauthors reported numerous disclosures, including various advising and consulting roles with the NFL.
The prevailing notion that concussions should be managed solely or primarily with prescribed cognitive and physical rest is shifting.
Experts in concussion management are increasingly in agreement that concussion is a much more heterogeneous injury than previously believed, and that “active” approaches targeting specific symptoms and impairments can be initiated early and may improve recovery for patients who have concussions from sports as well as from falls, motor vehicle accidents, or other accidents.
Evidence for such active approaches to rehabilitation – including vestibular, vision, and behavioral therapies, and submaximal aerobic training – is deemed “preliminary” by experts and comes from small, mostly single-center studies.
“There’s emerging evidence that strict or prolonged rest is not good, and there’s emerging consensus that we need to start looking at concussion subtypes” and then target treatments to those clinical profiles, he said. “We’re at the cusp of dramatic changes [in management].”
An article published recently in Neurosurgery and coauthored by several dozen concussion experts details 16 “statements of agreement” on targeted evaluation and active management. The experts – from neuropsychology, sports medicine, neurology and neurosurgery, athletic training, and other fields – convened in Pittsburgh in late 2015 at the invitation of Michael W. Collins, PhD, and his colleagues at the University of Pittsburgh Medical Center (UPMC), where the Sports Medicine Concussion Program was established in 2000 (Neurosurgery. 2016 Dec;79[6]:912-29).
Thus far, guidelines and statements on concussion in sport have advised a rest-based approach to management that’s dependent on the spontaneous resolution of symptoms. In some documents, rest is recommended until patients are asymptomatic. Other statements mention the possibility of symptom-based approaches after initial rest, but offer little if any guidance.
The theory of prescribed rest has been twofold, driven both by concern about re-injury in sport and by the belief that cognitive and physical activity can exacerbate symptoms and concussion-associated impairments, thus prolonging recovery, the Pittsburgh paper says.
However, “avoiding contact during the vulnerable period after concussion and prescribed rest represent two separate strategies,” the experts wrote. Avoiding contact “is always recommended to avoid further head impacts,” they say, but monitored activity does not appear to worsen injury.
Recent research suggests, moreover, that prolonged physical and cognitive rest – not activity – is associated with increased symptoms and delayed recovery. And strict rest – avoidance of nearly all brain stimulation – is “not empirically supported ... and may have unintended adverse effects,” the experts wrote.
Physicians and others “have to avoid treating concussion as a punishment,” said Dr. Halstead of St. Louis Children’s Hospital in Chesterfield, Mo., who coauthored a recent editorial entitled “Rethinking Cognitive Rest” (Br J Sports Med. 2017;51:147).
“It’s been taken to the extreme. When we talk about not texting or not going out or not doing anything physically active until you’re without symptoms ... students can develop symptoms of depression and anxiety that then just complicate the injury altogether,” he said.
Physicians also need to dig beneath symptom checklists and perform multidomain assessments to better understand root contributors of symptoms that, without active treatment, can persist for weeks upon weeks in some patients. “There could be neck injuries, sleep issues, vestibular issues, oculomotor issues” and different types of headache, Dr. Halstead said. “If we can identify these things, we can actually be doing rehabilitation to fix these injuries.”
At UPMC’s Sports Medicine Concussion Program, concussions are generally categorized into six clinical profiles – vestibular, ocular-motor, cognitive/fatigue, posttraumatic migraine, cervical, and anxiety/mood. The profiles are not mutually exclusive, but each drives particular rehabilitation recommendations. Clinicians at other concussion programs and centers are similarly attempting to classify concussions.
“We still need to come to agreement as to what exactly the clinical profiles are,” said UPMC’s Dr. Collins, who directs the concussion program and is the lead author of the Neurosurgery paper. “But I think the big concept to come out of our meeting is that we now agree there are different profiles and that we have to match treatment.”
Additional research is needed to determine and validate concussion clinical profiles, to identify biomarkers to assess recovery and determine the effectiveness of treatments, and to determine the best timing of specific active treatments, he and his coauthors said.
Applying individualized and active treatments after concussion is consistent with approaches taken for other injuries and conditions, noted Dr. Hainline, who attended both the UPMC conference and the 5th International Conference on Concussion in Sport.
“It’s rare that prolonged rest is the answer. Look at stroke – you don’t have patients resting indefinitely. You have to get their nervous systems re-engaged,” he said.
“If you keep [concussion] patients with predominantly vestibular symptoms at rest [for example], the vestibular symptoms can become more centralized, and that then becomes the behavior of the nervous system,” he said. “Another example is convergence insufficiencies – if you keep resting [the vision system] and don’t rehabilitate it,” symptoms perpetuate.
Prolonged rest also may lead to deconditioning that lowers tolerance for physical activity. Randomized clinical trials are needed to compare the benefits of physical rest with those of more physically active treatments, but “emerging clinical research” suggests that exposing patients to supervised low-level physical activity (for example, submaximal aerobic training) after an initial period of rest is “not only safe but effective,” the Pittsburgh conference paper says.
The Pittsburgh conference paper “rang a bell with me,” Dr. Wergin said. “Rest is still important, but prolonged rest may not be best for all patients, and maybe it’s possible to do some interventions. We need to stay tuned as we get more of an evidence base.”
The conference – coined the TEAM (for targeted evaluation and active management) Approaches to Treating Concussion Meeting – was attended by staff from the National Institutes of Health, the Centers for Disease Control and Prevention, the Department of Defense, and the National Football League (NFL) and other sporting organizations, Dr. Collins said. It was sponsored by UPMC and the NFL. Coauthors reported numerous disclosures, including various advising and consulting roles with the NFL.
Thrombotic microangiopathy may signal IV drug abuse
Extended-release oxymorphone hydrochloride (Opana ER) tablets contain an inert ingredient that can trigger acute microangiopathic hemolytic anemia and thrombocytopenia in those who abuse the drug via intravenous injection, Ryan Hunt, of the Food and Drug Administration, and his colleagues reported.
High-molecular-weight polyethylene oxide appeared to be the cause of acute cases of thrombotic microangiopathy in three patients who were treated in the emergency department of a single hospital in Tennessee. All had chest pain, dyspnea, visual impairment, microangiopathic hemolytic anemia and thrombocytopenia, increased lactate dehydrogenase, and undetectable haptoglobin serum levels. Two patients also had acute renal failure. Plasma exchange therapy was initiated in all three; one required additional hemodialysis.
ADAMTS13 activity was normal in blood samples obtained in two patients before they started plasma exchange. Histologic evidence of thrombotic microangiopathy with endothelial swelling of arterioles and acute tubular injury without deposition of immune complexes was seen in kidney biopsies performed in two patients. In addition, gelatinous material occluded the dialysis catheter and apheresis tubings during the initial plasma exchange sessions, the researchers reported (Blood. 2017;129[7]:896-905).
To test the association of polyethylene oxide with thrombotic microangiopathy, the researchers heated a 40-mg Opana ER tablet cut in several pieces in a spoon with 2 mL water until boiling. They injected guinea pigs with 0.1 or 0.3 mg/kg of the extracted polyethylene oxide, either as a single dose or as five doses given at 1.5-hour intervals. A dose-dependent increase of polyethylene oxide in plasma peaked at 8 hours after the first dose was measured and paralleled with intravascular hemolysis with increased plasma concentration of free hemoglobin, schistocytes in the peripheral blood smear, and thrombocytopenia. Spiking control blood samples in vitro with polyethylene oxide did not result in hemolysis.
“Although injection abuse of prescription opioids is highly concentrated in certain regions of the United States, particularly in rural Appalachia, all physicians should be highly inquisitive of IV drug abuse when presented with cases of [thrombotic microangiopathy],” the researchers concluded.
The researchers had no relevant conflicts of interest. One of the researchers is employed by Quest Diagnostics.
[email protected]
On Twitter @maryjodales
Extended-release oxymorphone hydrochloride (Opana ER) tablets contain an inert ingredient that can trigger acute microangiopathic hemolytic anemia and thrombocytopenia in those who abuse the drug via intravenous injection, Ryan Hunt, of the Food and Drug Administration, and his colleagues reported.
High-molecular-weight polyethylene oxide appeared to be the cause of acute cases of thrombotic microangiopathy in three patients who were treated in the emergency department of a single hospital in Tennessee. All had chest pain, dyspnea, visual impairment, microangiopathic hemolytic anemia and thrombocytopenia, increased lactate dehydrogenase, and undetectable haptoglobin serum levels. Two patients also had acute renal failure. Plasma exchange therapy was initiated in all three; one required additional hemodialysis.
ADAMTS13 activity was normal in blood samples obtained in two patients before they started plasma exchange. Histologic evidence of thrombotic microangiopathy with endothelial swelling of arterioles and acute tubular injury without deposition of immune complexes was seen in kidney biopsies performed in two patients. In addition, gelatinous material occluded the dialysis catheter and apheresis tubings during the initial plasma exchange sessions, the researchers reported (Blood. 2017;129[7]:896-905).
To test the association of polyethylene oxide with thrombotic microangiopathy, the researchers heated a 40-mg Opana ER tablet cut in several pieces in a spoon with 2 mL water until boiling. They injected guinea pigs with 0.1 or 0.3 mg/kg of the extracted polyethylene oxide, either as a single dose or as five doses given at 1.5-hour intervals. A dose-dependent increase of polyethylene oxide in plasma peaked at 8 hours after the first dose was measured and paralleled with intravascular hemolysis with increased plasma concentration of free hemoglobin, schistocytes in the peripheral blood smear, and thrombocytopenia. Spiking control blood samples in vitro with polyethylene oxide did not result in hemolysis.
“Although injection abuse of prescription opioids is highly concentrated in certain regions of the United States, particularly in rural Appalachia, all physicians should be highly inquisitive of IV drug abuse when presented with cases of [thrombotic microangiopathy],” the researchers concluded.
The researchers had no relevant conflicts of interest. One of the researchers is employed by Quest Diagnostics.
[email protected]
On Twitter @maryjodales
Extended-release oxymorphone hydrochloride (Opana ER) tablets contain an inert ingredient that can trigger acute microangiopathic hemolytic anemia and thrombocytopenia in those who abuse the drug via intravenous injection, Ryan Hunt, of the Food and Drug Administration, and his colleagues reported.
High-molecular-weight polyethylene oxide appeared to be the cause of acute cases of thrombotic microangiopathy in three patients who were treated in the emergency department of a single hospital in Tennessee. All had chest pain, dyspnea, visual impairment, microangiopathic hemolytic anemia and thrombocytopenia, increased lactate dehydrogenase, and undetectable haptoglobin serum levels. Two patients also had acute renal failure. Plasma exchange therapy was initiated in all three; one required additional hemodialysis.
ADAMTS13 activity was normal in blood samples obtained in two patients before they started plasma exchange. Histologic evidence of thrombotic microangiopathy with endothelial swelling of arterioles and acute tubular injury without deposition of immune complexes was seen in kidney biopsies performed in two patients. In addition, gelatinous material occluded the dialysis catheter and apheresis tubings during the initial plasma exchange sessions, the researchers reported (Blood. 2017;129[7]:896-905).
To test the association of polyethylene oxide with thrombotic microangiopathy, the researchers heated a 40-mg Opana ER tablet cut in several pieces in a spoon with 2 mL water until boiling. They injected guinea pigs with 0.1 or 0.3 mg/kg of the extracted polyethylene oxide, either as a single dose or as five doses given at 1.5-hour intervals. A dose-dependent increase of polyethylene oxide in plasma peaked at 8 hours after the first dose was measured and paralleled with intravascular hemolysis with increased plasma concentration of free hemoglobin, schistocytes in the peripheral blood smear, and thrombocytopenia. Spiking control blood samples in vitro with polyethylene oxide did not result in hemolysis.
“Although injection abuse of prescription opioids is highly concentrated in certain regions of the United States, particularly in rural Appalachia, all physicians should be highly inquisitive of IV drug abuse when presented with cases of [thrombotic microangiopathy],” the researchers concluded.
The researchers had no relevant conflicts of interest. One of the researchers is employed by Quest Diagnostics.
[email protected]
On Twitter @maryjodales
Key clinical point:
Major finding: Extended-release oxymorphone hydrochloride (Opana ER) tablets contain an inert ingredient that can trigger acute microangiopathic hemolytic anemia and thrombocytopenia in those who abuse the drug via intravenous injection.
Data source: Observational study of three patients and translational research study of inert drug components in guinea pigs.
Disclosures: The researchers had no conflicts of interest. One of the researchers is employed by Quest Diagnostics.
Factors linked to B-NHL in Palestinians, Israelis
New research has revealed factors that may increase the risk of B-cell non-Hodgkin lymphoma (B-NHL) in Israelis and Palestinians.
This large-scale, epidemiological study indicated that each group had its own unique risk factors.
However, in both groups, recreational sun exposure, black hair-dye use, a history of hospitalization for infection, and having a first-degree relative with a hematopoietic malignancy were all associated with B-NHL.
A team of Palestinian and Israeli researchers reported these findings in PLOS ONE.
The researchers noted that Israelis and Palestinians share the same ecosystem but differ in terms of lifestyle, health behaviors, and medical systems. Yet both populations report high incidences of NHL.
To gain some insight into this phenomenon, the team conducted a study examining risk factors for B-NHL and its subtypes in these two populations.
The researchers looked at medical history, environmental factors, and lifestyle factors in 823 B-NHL patients and 808 healthy controls.
There were 516 Israeli Jews with B-NHL and 307 Palestinian Arabs with B-NHL. The mean age at diagnosis was 60 and 51, respectively.
The proportion of patients with diffuse large B-cell lymphoma was 71% of Palestinian Arabs and 41% of Israeli Jews. The proportion of patients with follicular lymphoma was 14% and 28%, respectively. And the proportion of patients with marginal zone lymphoma was 2% and 14%, respectively.
Using data from questionnaires, pathology review, serology, and genotyping, the researchers uncovered potential risk factors for B-NHL common to both populations and other factors unique to each population.
Results
The data showed that, in both Palestinian Arabs and Israeli Jews, B-NHL was associated with:
- Recreational sun exposure (odds ratio [OR]=1.4)
- Black hair-dye use (OR=1.70)
- A history of hospitalization for infection (OR=1.68)
- Having a first-degree relative with a hematopoietic malignancy (OR=1.69).
Smoking was associated with follicular lymphoma in both populations (OR=1.46). And greater-than-monthly indoor pesticide use was associated with diffuse large B-cell lymphoma in both populations (OR=2.01).
There was an inverse association between alcohol use and B-NHL for both populations (OR=0.46).
Among Palestinian Arabs only, risk factors for B-NHL included gardening (OR=1.93) and a history of herpes (OR=3.73), mononucleosis (OR=6.34), rubella (OR=2.86), and blood transfusion (OR=2.53).
Risk factors that applied to Israeli Jews only included growing fruits and vegetables (OR=1.87) and self-reported autoimmune diseases (OR=1.99).
The researchers said differences in risk factors by ethnicity could reflect differences in lifestyle, medical systems, and reporting patterns, while variations by B-NHL subtypes suggest specific causal factors for different types of disease. However, these findings require further investigation to reveal their mechanisms.
“Apart from the scientific contribution that this research provides in terms of understanding risk factors for NHL, the study entails an important research cooperation among many institutions,” said study author Ora Paltiel, of Hadassah-Hebrew University Medical Organization in Jerusalem, Israel.
“The study provided opportunities for training Palestinian and Israeli researchers and will provide for intellectual interaction for years to come. The data collected will also provide a research platform for the future study of lymphoma. Epidemiologic research has the potential to improve and preserve human health, and it can also serve as a bridge to dialogue among nations.” ![]()
New research has revealed factors that may increase the risk of B-cell non-Hodgkin lymphoma (B-NHL) in Israelis and Palestinians.
This large-scale, epidemiological study indicated that each group had its own unique risk factors.
However, in both groups, recreational sun exposure, black hair-dye use, a history of hospitalization for infection, and having a first-degree relative with a hematopoietic malignancy were all associated with B-NHL.
A team of Palestinian and Israeli researchers reported these findings in PLOS ONE.
The researchers noted that Israelis and Palestinians share the same ecosystem but differ in terms of lifestyle, health behaviors, and medical systems. Yet both populations report high incidences of NHL.
To gain some insight into this phenomenon, the team conducted a study examining risk factors for B-NHL and its subtypes in these two populations.
The researchers looked at medical history, environmental factors, and lifestyle factors in 823 B-NHL patients and 808 healthy controls.
There were 516 Israeli Jews with B-NHL and 307 Palestinian Arabs with B-NHL. The mean age at diagnosis was 60 and 51, respectively.
The proportion of patients with diffuse large B-cell lymphoma was 71% of Palestinian Arabs and 41% of Israeli Jews. The proportion of patients with follicular lymphoma was 14% and 28%, respectively. And the proportion of patients with marginal zone lymphoma was 2% and 14%, respectively.
Using data from questionnaires, pathology review, serology, and genotyping, the researchers uncovered potential risk factors for B-NHL common to both populations and other factors unique to each population.
Results
The data showed that, in both Palestinian Arabs and Israeli Jews, B-NHL was associated with:
- Recreational sun exposure (odds ratio [OR]=1.4)
- Black hair-dye use (OR=1.70)
- A history of hospitalization for infection (OR=1.68)
- Having a first-degree relative with a hematopoietic malignancy (OR=1.69).
Smoking was associated with follicular lymphoma in both populations (OR=1.46). And greater-than-monthly indoor pesticide use was associated with diffuse large B-cell lymphoma in both populations (OR=2.01).
There was an inverse association between alcohol use and B-NHL for both populations (OR=0.46).
Among Palestinian Arabs only, risk factors for B-NHL included gardening (OR=1.93) and a history of herpes (OR=3.73), mononucleosis (OR=6.34), rubella (OR=2.86), and blood transfusion (OR=2.53).
Risk factors that applied to Israeli Jews only included growing fruits and vegetables (OR=1.87) and self-reported autoimmune diseases (OR=1.99).
The researchers said differences in risk factors by ethnicity could reflect differences in lifestyle, medical systems, and reporting patterns, while variations by B-NHL subtypes suggest specific causal factors for different types of disease. However, these findings require further investigation to reveal their mechanisms.
“Apart from the scientific contribution that this research provides in terms of understanding risk factors for NHL, the study entails an important research cooperation among many institutions,” said study author Ora Paltiel, of Hadassah-Hebrew University Medical Organization in Jerusalem, Israel.
“The study provided opportunities for training Palestinian and Israeli researchers and will provide for intellectual interaction for years to come. The data collected will also provide a research platform for the future study of lymphoma. Epidemiologic research has the potential to improve and preserve human health, and it can also serve as a bridge to dialogue among nations.” ![]()
New research has revealed factors that may increase the risk of B-cell non-Hodgkin lymphoma (B-NHL) in Israelis and Palestinians.
This large-scale, epidemiological study indicated that each group had its own unique risk factors.
However, in both groups, recreational sun exposure, black hair-dye use, a history of hospitalization for infection, and having a first-degree relative with a hematopoietic malignancy were all associated with B-NHL.
A team of Palestinian and Israeli researchers reported these findings in PLOS ONE.
The researchers noted that Israelis and Palestinians share the same ecosystem but differ in terms of lifestyle, health behaviors, and medical systems. Yet both populations report high incidences of NHL.
To gain some insight into this phenomenon, the team conducted a study examining risk factors for B-NHL and its subtypes in these two populations.
The researchers looked at medical history, environmental factors, and lifestyle factors in 823 B-NHL patients and 808 healthy controls.
There were 516 Israeli Jews with B-NHL and 307 Palestinian Arabs with B-NHL. The mean age at diagnosis was 60 and 51, respectively.
The proportion of patients with diffuse large B-cell lymphoma was 71% of Palestinian Arabs and 41% of Israeli Jews. The proportion of patients with follicular lymphoma was 14% and 28%, respectively. And the proportion of patients with marginal zone lymphoma was 2% and 14%, respectively.
Using data from questionnaires, pathology review, serology, and genotyping, the researchers uncovered potential risk factors for B-NHL common to both populations and other factors unique to each population.
Results
The data showed that, in both Palestinian Arabs and Israeli Jews, B-NHL was associated with:
- Recreational sun exposure (odds ratio [OR]=1.4)
- Black hair-dye use (OR=1.70)
- A history of hospitalization for infection (OR=1.68)
- Having a first-degree relative with a hematopoietic malignancy (OR=1.69).
Smoking was associated with follicular lymphoma in both populations (OR=1.46). And greater-than-monthly indoor pesticide use was associated with diffuse large B-cell lymphoma in both populations (OR=2.01).
There was an inverse association between alcohol use and B-NHL for both populations (OR=0.46).
Among Palestinian Arabs only, risk factors for B-NHL included gardening (OR=1.93) and a history of herpes (OR=3.73), mononucleosis (OR=6.34), rubella (OR=2.86), and blood transfusion (OR=2.53).
Risk factors that applied to Israeli Jews only included growing fruits and vegetables (OR=1.87) and self-reported autoimmune diseases (OR=1.99).
The researchers said differences in risk factors by ethnicity could reflect differences in lifestyle, medical systems, and reporting patterns, while variations by B-NHL subtypes suggest specific causal factors for different types of disease. However, these findings require further investigation to reveal their mechanisms.
“Apart from the scientific contribution that this research provides in terms of understanding risk factors for NHL, the study entails an important research cooperation among many institutions,” said study author Ora Paltiel, of Hadassah-Hebrew University Medical Organization in Jerusalem, Israel.
“The study provided opportunities for training Palestinian and Israeli researchers and will provide for intellectual interaction for years to come. The data collected will also provide a research platform for the future study of lymphoma. Epidemiologic research has the potential to improve and preserve human health, and it can also serve as a bridge to dialogue among nations.” ![]()
Software predicts HSPC differentiation
Deep learning can be used to determine how murine hematopoietic stem and progenitor cells (HSPCs) will differentiate, according to research published in Nature Methods.
Deep learning algorithms simulate the learning processes in people using artificial neural networks.
Researchers have reported the development of software that uses deep learning to predict which type of cell murine HSPCs will differentiate into, based on microscopy images.
“A hematopoietic stem cell’s decision to become a certain cell type cannot be observed,” said study author Carsten Marr, PhD, of Helmholtz Zentrum München–German Research Center for Environmental Health in Neuherberg, Germany.
“At this time, it is only possible to verify the decision retrospectively with cell surface markers.”
Therefore, Dr Marr and his team set out to develop an algorithm that can predict the decision in advance, and deep learning was key.
“Deep neural networks play a major role in our method,” Dr Marr said. “Our algorithm classifies light microscopic images and videos of individual cells by comparing these data with past experience from the development of such cells. In this way, the algorithm ‘learns’ how certain cells behave.”
Specifically, the researchers examined murine HSPCs filmed under a microscope in the lab. Using information on the cells’ appearance and speed, the software was able to “memorize” the corresponding behavior patterns and then make its prediction.
“Compared to conventional methods, such as fluorescent antibodies against certain surface proteins, we know how the cells will decide 3 cell generations earlier,” said Felix Buggenthin, PhD, of Helmholtz Zentrum München–German Research Center for Environmental Health.
“Since we now know which cells will develop in which way, we can isolate them earlier than before and examine how they differ at a molecular level,” Dr Marr added. “We want to use this information to understand how the choices are made for particular developmental traits.” ![]()
Deep learning can be used to determine how murine hematopoietic stem and progenitor cells (HSPCs) will differentiate, according to research published in Nature Methods.
Deep learning algorithms simulate the learning processes in people using artificial neural networks.
Researchers have reported the development of software that uses deep learning to predict which type of cell murine HSPCs will differentiate into, based on microscopy images.
“A hematopoietic stem cell’s decision to become a certain cell type cannot be observed,” said study author Carsten Marr, PhD, of Helmholtz Zentrum München–German Research Center for Environmental Health in Neuherberg, Germany.
“At this time, it is only possible to verify the decision retrospectively with cell surface markers.”
Therefore, Dr Marr and his team set out to develop an algorithm that can predict the decision in advance, and deep learning was key.
“Deep neural networks play a major role in our method,” Dr Marr said. “Our algorithm classifies light microscopic images and videos of individual cells by comparing these data with past experience from the development of such cells. In this way, the algorithm ‘learns’ how certain cells behave.”
Specifically, the researchers examined murine HSPCs filmed under a microscope in the lab. Using information on the cells’ appearance and speed, the software was able to “memorize” the corresponding behavior patterns and then make its prediction.
“Compared to conventional methods, such as fluorescent antibodies against certain surface proteins, we know how the cells will decide 3 cell generations earlier,” said Felix Buggenthin, PhD, of Helmholtz Zentrum München–German Research Center for Environmental Health.
“Since we now know which cells will develop in which way, we can isolate them earlier than before and examine how they differ at a molecular level,” Dr Marr added. “We want to use this information to understand how the choices are made for particular developmental traits.” ![]()
Deep learning can be used to determine how murine hematopoietic stem and progenitor cells (HSPCs) will differentiate, according to research published in Nature Methods.
Deep learning algorithms simulate the learning processes in people using artificial neural networks.
Researchers have reported the development of software that uses deep learning to predict which type of cell murine HSPCs will differentiate into, based on microscopy images.
“A hematopoietic stem cell’s decision to become a certain cell type cannot be observed,” said study author Carsten Marr, PhD, of Helmholtz Zentrum München–German Research Center for Environmental Health in Neuherberg, Germany.
“At this time, it is only possible to verify the decision retrospectively with cell surface markers.”
Therefore, Dr Marr and his team set out to develop an algorithm that can predict the decision in advance, and deep learning was key.
“Deep neural networks play a major role in our method,” Dr Marr said. “Our algorithm classifies light microscopic images and videos of individual cells by comparing these data with past experience from the development of such cells. In this way, the algorithm ‘learns’ how certain cells behave.”
Specifically, the researchers examined murine HSPCs filmed under a microscope in the lab. Using information on the cells’ appearance and speed, the software was able to “memorize” the corresponding behavior patterns and then make its prediction.
“Compared to conventional methods, such as fluorescent antibodies against certain surface proteins, we know how the cells will decide 3 cell generations earlier,” said Felix Buggenthin, PhD, of Helmholtz Zentrum München–German Research Center for Environmental Health.
“Since we now know which cells will develop in which way, we can isolate them earlier than before and examine how they differ at a molecular level,” Dr Marr added. “We want to use this information to understand how the choices are made for particular developmental traits.” ![]()
European Commission approves rituximab biosimilar
The European Commission has approved a biosimilar rituximab product, Truxima™, for all the same indications as the reference product, MabThera.
This means Truxima (formerly called CT-P10) is approved for use in the European Union to treat patients with non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis (RA), granulomatosis with polyangiitis (GPA), and microscopic polyangiitis (MPA).
Truxima, a product of Celltrion Healthcare Hungary Kft, is the first biosimilar monoclonal antibody approved in an oncology indication worldwide.
The approval is based on data submitted to the European Medicines Agency.
The agency’s Committee for Medicinal Products for Human Use (CHMP) said the evidence suggests Truxima and MabThera are similar in terms of efficacy, safety, immunogenicity, pharmacodynamics, and pharmacokinetics in patients with RA and advanced follicular lymphoma (FL).
Therefore, the European Commission approved Truxima for the following indications.
Non-Hodgkin lymphoma
Truxima is indicated for use in combination with chemotherapy to treat previously untreated patients with stage III-IV FL.
Truxima maintenance therapy is indicated for the treatment of FL patients responding to induction therapy.
Truxima monotherapy is indicated for the treatment of patients with stage III-IV FL who are chemo-resistant or are in their second or subsequent relapse after chemotherapy.
Truxima is indicated for use in combination with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) for the treatment of patients with CD20-positive diffuse large B-cell lymphoma.
Chronic lymphocytic leukemia
Truxima in combination with chemotherapy is indicated for the treatment of patients with previously untreated and relapsed/refractory chronic lymphocytic leukemia.
The CHMP noted that limited efficacy and safety data are available for patients previously treated with monoclonal antibodies, including rituximab, or patients who are refractory to previous rituximab plus chemotherapy.
RA, GPA, and MPA
Truxima in combination with methotrexate is indicated for the treatment of adults with severe, active RA who have had an inadequate response to or cannot tolerate other disease-modifying anti-rheumatic drugs, including one or more tumor necrosis factor inhibitor therapies.
Truxima in combination with glucocorticoids is indicated for the induction of remission in adults with severe, active GPA or MPA.
Truxima studies
There are 3 ongoing, phase 3 trials of Truxima in patients with RA (NCT02149121), advanced FL (NCT02162771), and low-tumor-burden FL (NCT02260804).
Results from the phase 1/3 trial in patients with newly diagnosed, advanced FL suggest that Truxima and the reference rituximab are similar with regard to pharmacokinetics, immunogenicity, and safety (B Coiffier et al. ASH 2016, abstract 1807).
Results from the phase 3 study of RA patients indicate that Truxima is similar to reference products (EU and US-sourced rituximab) with regard to pharmacodynamics, safety, and efficacy for up to 24 weeks (DH Yoo et al. 2016 ACR/ARHP Annual Meeting, abstract 1635). ![]()
The European Commission has approved a biosimilar rituximab product, Truxima™, for all the same indications as the reference product, MabThera.
This means Truxima (formerly called CT-P10) is approved for use in the European Union to treat patients with non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis (RA), granulomatosis with polyangiitis (GPA), and microscopic polyangiitis (MPA).
Truxima, a product of Celltrion Healthcare Hungary Kft, is the first biosimilar monoclonal antibody approved in an oncology indication worldwide.
The approval is based on data submitted to the European Medicines Agency.
The agency’s Committee for Medicinal Products for Human Use (CHMP) said the evidence suggests Truxima and MabThera are similar in terms of efficacy, safety, immunogenicity, pharmacodynamics, and pharmacokinetics in patients with RA and advanced follicular lymphoma (FL).
Therefore, the European Commission approved Truxima for the following indications.
Non-Hodgkin lymphoma
Truxima is indicated for use in combination with chemotherapy to treat previously untreated patients with stage III-IV FL.
Truxima maintenance therapy is indicated for the treatment of FL patients responding to induction therapy.
Truxima monotherapy is indicated for the treatment of patients with stage III-IV FL who are chemo-resistant or are in their second or subsequent relapse after chemotherapy.
Truxima is indicated for use in combination with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) for the treatment of patients with CD20-positive diffuse large B-cell lymphoma.
Chronic lymphocytic leukemia
Truxima in combination with chemotherapy is indicated for the treatment of patients with previously untreated and relapsed/refractory chronic lymphocytic leukemia.
The CHMP noted that limited efficacy and safety data are available for patients previously treated with monoclonal antibodies, including rituximab, or patients who are refractory to previous rituximab plus chemotherapy.
RA, GPA, and MPA
Truxima in combination with methotrexate is indicated for the treatment of adults with severe, active RA who have had an inadequate response to or cannot tolerate other disease-modifying anti-rheumatic drugs, including one or more tumor necrosis factor inhibitor therapies.
Truxima in combination with glucocorticoids is indicated for the induction of remission in adults with severe, active GPA or MPA.
Truxima studies
There are 3 ongoing, phase 3 trials of Truxima in patients with RA (NCT02149121), advanced FL (NCT02162771), and low-tumor-burden FL (NCT02260804).
Results from the phase 1/3 trial in patients with newly diagnosed, advanced FL suggest that Truxima and the reference rituximab are similar with regard to pharmacokinetics, immunogenicity, and safety (B Coiffier et al. ASH 2016, abstract 1807).
Results from the phase 3 study of RA patients indicate that Truxima is similar to reference products (EU and US-sourced rituximab) with regard to pharmacodynamics, safety, and efficacy for up to 24 weeks (DH Yoo et al. 2016 ACR/ARHP Annual Meeting, abstract 1635). ![]()
The European Commission has approved a biosimilar rituximab product, Truxima™, for all the same indications as the reference product, MabThera.
This means Truxima (formerly called CT-P10) is approved for use in the European Union to treat patients with non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis (RA), granulomatosis with polyangiitis (GPA), and microscopic polyangiitis (MPA).
Truxima, a product of Celltrion Healthcare Hungary Kft, is the first biosimilar monoclonal antibody approved in an oncology indication worldwide.
The approval is based on data submitted to the European Medicines Agency.
The agency’s Committee for Medicinal Products for Human Use (CHMP) said the evidence suggests Truxima and MabThera are similar in terms of efficacy, safety, immunogenicity, pharmacodynamics, and pharmacokinetics in patients with RA and advanced follicular lymphoma (FL).
Therefore, the European Commission approved Truxima for the following indications.
Non-Hodgkin lymphoma
Truxima is indicated for use in combination with chemotherapy to treat previously untreated patients with stage III-IV FL.
Truxima maintenance therapy is indicated for the treatment of FL patients responding to induction therapy.
Truxima monotherapy is indicated for the treatment of patients with stage III-IV FL who are chemo-resistant or are in their second or subsequent relapse after chemotherapy.
Truxima is indicated for use in combination with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) for the treatment of patients with CD20-positive diffuse large B-cell lymphoma.
Chronic lymphocytic leukemia
Truxima in combination with chemotherapy is indicated for the treatment of patients with previously untreated and relapsed/refractory chronic lymphocytic leukemia.
The CHMP noted that limited efficacy and safety data are available for patients previously treated with monoclonal antibodies, including rituximab, or patients who are refractory to previous rituximab plus chemotherapy.
RA, GPA, and MPA
Truxima in combination with methotrexate is indicated for the treatment of adults with severe, active RA who have had an inadequate response to or cannot tolerate other disease-modifying anti-rheumatic drugs, including one or more tumor necrosis factor inhibitor therapies.
Truxima in combination with glucocorticoids is indicated for the induction of remission in adults with severe, active GPA or MPA.
Truxima studies
There are 3 ongoing, phase 3 trials of Truxima in patients with RA (NCT02149121), advanced FL (NCT02162771), and low-tumor-burden FL (NCT02260804).
Results from the phase 1/3 trial in patients with newly diagnosed, advanced FL suggest that Truxima and the reference rituximab are similar with regard to pharmacokinetics, immunogenicity, and safety (B Coiffier et al. ASH 2016, abstract 1807).
Results from the phase 3 study of RA patients indicate that Truxima is similar to reference products (EU and US-sourced rituximab) with regard to pharmacodynamics, safety, and efficacy for up to 24 weeks (DH Yoo et al. 2016 ACR/ARHP Annual Meeting, abstract 1635). ![]()
Tips for taming atopic dermatitis and managing expectations
MIAMI – Tactics for managing patients with atopic dermatitis can go a long way to educate patients, set realistic expectations, and devise strategies for existing therapies, even as clinicians await some promising agents expected on the market soon.
“The good news is this is the Age of Eczema. In the last couple of years we’ve seen an explosion in the literature,” Adam Friedman, MD, of the department of dermatology, George Washington University, Washington, D.C., said at the Orlando Dermatology Aesthetic and Clinical Conference. Some of this research is spurring new therapeutics. a phosphodiesterase 4 inhibitor.
Crisaborole ointment, 2% (Eucrisa), a phosphodiesterase 4 inhibitor, was approved by the Food and Drug Administration in December 2016 for treating patients aged 2 years and older with mild to moderate AD, for example. It is a novel, nonsteroidal anti-inflammatory and the first prescription agent approved in the United States for atopic dermatitis in more than 10 years.
Dr. Friedman has no personal experience with crisaborole, which just became available. “But the data look encouraging. From what I’ve seen this may be a nonburning alternative to calcineurin inhibitors. It will be interesting to see how this will fit in our practices.”
Systemic management of pruritus
There’s also promise for patients troubled by one of the top manifestations of AD – the itch. “We have new targeted therapies coming down the pike, some hopefully [gaining approval] in the next few months. We have biologics going after the cytokines of itch. It’s a very, very exciting time right now,” Dr. Friedman said.
Current clinical trials are not only focusing on AD but also specifically on pruritus, he added.
In the meantime, itch can be managed with prescription and over-the-counter topical agents, as well as systemic therapies such as gabapentin, some antidepressants, and the antiemetic aprepitant. Aprepitant is a substance P antagonist (through blocking neurokinin 1 receptor) and can be effective for some patients when taken three times a week, but it is not indicated for itch, Dr. Friedman said. Because of its off label indication “it’s a little tricky getting [insurance] coverage.”
Back to basics
“Even with all the excitement, even with the new therapeutics, you have to stick with the basics,” he said. “Put the lotion on, put the cream on. You have to put moisturizer on wet skin and be cautious with soaps.” He added, “don’t be afraid to ask for help. The National Eczema Association has a wonderful website with research, education, tools – you name it.”
Keeping it real
For regional eczemas like hand dermatitis, what are the options? “Tell patients they can glove up, there are various latex alternatives … but it probably won’t fly in the real world,” Dr. Friedman said. Zinc oxide “works like armor, and patients will probably do well,” but the aesthetics are unacceptable for most, he added. “Newer alternatives, such as those with aluminum magnesium hydroxide stearate, have similar protecting power, but are not opaque and rub on easier.”
A goal of topical therapy is to get rid of the inflammation, and steroids have a long history of evidence supporting their use, but “topical steroid phobia in parents” is a problem, he said. To counter the reluctance or refusal to use topical steroids, he suggested exploring reasons for noncompliance, dispelling any myths, and working with parent to make it easier to apply the steroids to their child.
Interestingly, there is some evidence that a simpler regimen may work well for some patients. “We always say ‘apply twice a day.’ Why? Because all the clinical trials had participants apply steroids twice a day. But there is no evidence to show twice a day is better than once a day, and in fact, a meta-analysis suggests once a day works just as well” (Br J Dermatol. 2005 Jan;152[1]:130-41).
Topical calcineurin inhibitors are another option. In general, Dr. Friedman prescribes these agents for delicate areas, for patients with thin skin, or for patients who use a topical steroid “on and on and on and can’t seem to get off it.” Calcineurin inhibitors can also be used on in-between days during steroid maintenance therapy, he added. When prescribing, warn patients about the initial burn (due to substance P release) that commonly occurs so that they have realistic expectations.
Education remains essential
“I encourage you to educate your patients and empathize with them,” he said. “Show them how to apply a moisturizer. Also, use your nurses and assistants to help with education – really empower them to be part of the process.”
“Explain, explain, explain, so they have realistic expectations,” and know that there is no cure, so that when they experience a flare, they understand that “it’s not that the steroid didn’t work – this is a chronic disease,” added Dr. Friedman, who recommends providing patients with handouts that answer many of their questions.
Maximize moisturizing
When it comes to moisturizing, more is usually better. Effective products contain all the key ingredients: emollients to soften the skin, an occlusive to keep the water there, and a humectant to bond the water. “Just one or two is not going to cut it,” he said.
“Something we now know is that starting early is key,” he pointed out, referring to recent studies that have shown that in babies at high risk for AD, starting moisturizers early can decrease their risk for developing AD later (J Allergy Clin Immunol. 2014 Oct; 134[4]: 818-23).
“Another study that received a ton of press was in JAMA Pediatrics recently,” Dr. Friedman said. The study concluded that the use of different moisturizers to prevent AD in high risk babies was likely to be cost-effective (JAMA Pediatr. 2017 Feb 6;171[2]:e163909. doi: 10.1001/jamapediatrics.2016.3909). Although some news reports claimed starting babies with Vaseline as a moisturizer will prevent AD, “that’s actually not what the study showed. All the over-the-counter moisturizers they used worked, but Vaseline was the least expensive,” Dr. Friedman noted
Help patients select the right soap
Educate patients to avoid “true soaps” such as Dial, Ivory, Irish Spring, or Lever 2000. “Soaps can be a real enemy here. You want lower pH types of soaps. Depending on skin type, our skin is somewhere between 5.5 and 6.5 pH,” Dr. Friedman explained. “The paradigm shift for your patients is to hydrate, not to clean. Showers are okay if they’re not blaring hot. Baths are okay ... but you should not be sitting in a sudsy bath.”
Also, instruct patients to avoid irritating fabrics, dryer sheets, or harsh laundry detergents that could exacerbate AD.
‘You’re not alone’
Sometimes it’s helpful to assure patients with AD that they’re not alone, and that many researchers and clinicians are working on effective treatment strategies. “We’re all familiar with atopic dermatitis because there’s so much of it. The numbers are surprisingly high,” Dr. Friedman said. Compared with the estimated 2.2 million Americans with psoriasis, AD eclipses their numbers substantially, affecting about 17 million people.
Dr. Friedman disclosed that he is a speaker for Amgen, Janssen, and Promius; receives research grants from Valeant; and is a consultant and/or advisory board member for Amgen, Aveeno, Biogen, Encore, Exeltis, Ferndale, Galderma, G&W Laboratories, Intraderm, La Roche-Posay, Loreal, Microcures, Nano Bio-Med, Novartis, Oakstone Institute, Occulus, Onset, Pfizer, Promius, Sanova Works, and Valeant. Dr. Friedman is also an editorial advisory board member for Dermatology News.
MIAMI – Tactics for managing patients with atopic dermatitis can go a long way to educate patients, set realistic expectations, and devise strategies for existing therapies, even as clinicians await some promising agents expected on the market soon.
“The good news is this is the Age of Eczema. In the last couple of years we’ve seen an explosion in the literature,” Adam Friedman, MD, of the department of dermatology, George Washington University, Washington, D.C., said at the Orlando Dermatology Aesthetic and Clinical Conference. Some of this research is spurring new therapeutics. a phosphodiesterase 4 inhibitor.
Crisaborole ointment, 2% (Eucrisa), a phosphodiesterase 4 inhibitor, was approved by the Food and Drug Administration in December 2016 for treating patients aged 2 years and older with mild to moderate AD, for example. It is a novel, nonsteroidal anti-inflammatory and the first prescription agent approved in the United States for atopic dermatitis in more than 10 years.
Dr. Friedman has no personal experience with crisaborole, which just became available. “But the data look encouraging. From what I’ve seen this may be a nonburning alternative to calcineurin inhibitors. It will be interesting to see how this will fit in our practices.”
Systemic management of pruritus
There’s also promise for patients troubled by one of the top manifestations of AD – the itch. “We have new targeted therapies coming down the pike, some hopefully [gaining approval] in the next few months. We have biologics going after the cytokines of itch. It’s a very, very exciting time right now,” Dr. Friedman said.
Current clinical trials are not only focusing on AD but also specifically on pruritus, he added.
In the meantime, itch can be managed with prescription and over-the-counter topical agents, as well as systemic therapies such as gabapentin, some antidepressants, and the antiemetic aprepitant. Aprepitant is a substance P antagonist (through blocking neurokinin 1 receptor) and can be effective for some patients when taken three times a week, but it is not indicated for itch, Dr. Friedman said. Because of its off label indication “it’s a little tricky getting [insurance] coverage.”
Back to basics
“Even with all the excitement, even with the new therapeutics, you have to stick with the basics,” he said. “Put the lotion on, put the cream on. You have to put moisturizer on wet skin and be cautious with soaps.” He added, “don’t be afraid to ask for help. The National Eczema Association has a wonderful website with research, education, tools – you name it.”
Keeping it real
For regional eczemas like hand dermatitis, what are the options? “Tell patients they can glove up, there are various latex alternatives … but it probably won’t fly in the real world,” Dr. Friedman said. Zinc oxide “works like armor, and patients will probably do well,” but the aesthetics are unacceptable for most, he added. “Newer alternatives, such as those with aluminum magnesium hydroxide stearate, have similar protecting power, but are not opaque and rub on easier.”
A goal of topical therapy is to get rid of the inflammation, and steroids have a long history of evidence supporting their use, but “topical steroid phobia in parents” is a problem, he said. To counter the reluctance or refusal to use topical steroids, he suggested exploring reasons for noncompliance, dispelling any myths, and working with parent to make it easier to apply the steroids to their child.
Interestingly, there is some evidence that a simpler regimen may work well for some patients. “We always say ‘apply twice a day.’ Why? Because all the clinical trials had participants apply steroids twice a day. But there is no evidence to show twice a day is better than once a day, and in fact, a meta-analysis suggests once a day works just as well” (Br J Dermatol. 2005 Jan;152[1]:130-41).
Topical calcineurin inhibitors are another option. In general, Dr. Friedman prescribes these agents for delicate areas, for patients with thin skin, or for patients who use a topical steroid “on and on and on and can’t seem to get off it.” Calcineurin inhibitors can also be used on in-between days during steroid maintenance therapy, he added. When prescribing, warn patients about the initial burn (due to substance P release) that commonly occurs so that they have realistic expectations.
Education remains essential
“I encourage you to educate your patients and empathize with them,” he said. “Show them how to apply a moisturizer. Also, use your nurses and assistants to help with education – really empower them to be part of the process.”
“Explain, explain, explain, so they have realistic expectations,” and know that there is no cure, so that when they experience a flare, they understand that “it’s not that the steroid didn’t work – this is a chronic disease,” added Dr. Friedman, who recommends providing patients with handouts that answer many of their questions.
Maximize moisturizing
When it comes to moisturizing, more is usually better. Effective products contain all the key ingredients: emollients to soften the skin, an occlusive to keep the water there, and a humectant to bond the water. “Just one or two is not going to cut it,” he said.
“Something we now know is that starting early is key,” he pointed out, referring to recent studies that have shown that in babies at high risk for AD, starting moisturizers early can decrease their risk for developing AD later (J Allergy Clin Immunol. 2014 Oct; 134[4]: 818-23).
“Another study that received a ton of press was in JAMA Pediatrics recently,” Dr. Friedman said. The study concluded that the use of different moisturizers to prevent AD in high risk babies was likely to be cost-effective (JAMA Pediatr. 2017 Feb 6;171[2]:e163909. doi: 10.1001/jamapediatrics.2016.3909). Although some news reports claimed starting babies with Vaseline as a moisturizer will prevent AD, “that’s actually not what the study showed. All the over-the-counter moisturizers they used worked, but Vaseline was the least expensive,” Dr. Friedman noted
Help patients select the right soap
Educate patients to avoid “true soaps” such as Dial, Ivory, Irish Spring, or Lever 2000. “Soaps can be a real enemy here. You want lower pH types of soaps. Depending on skin type, our skin is somewhere between 5.5 and 6.5 pH,” Dr. Friedman explained. “The paradigm shift for your patients is to hydrate, not to clean. Showers are okay if they’re not blaring hot. Baths are okay ... but you should not be sitting in a sudsy bath.”
Also, instruct patients to avoid irritating fabrics, dryer sheets, or harsh laundry detergents that could exacerbate AD.
‘You’re not alone’
Sometimes it’s helpful to assure patients with AD that they’re not alone, and that many researchers and clinicians are working on effective treatment strategies. “We’re all familiar with atopic dermatitis because there’s so much of it. The numbers are surprisingly high,” Dr. Friedman said. Compared with the estimated 2.2 million Americans with psoriasis, AD eclipses their numbers substantially, affecting about 17 million people.
Dr. Friedman disclosed that he is a speaker for Amgen, Janssen, and Promius; receives research grants from Valeant; and is a consultant and/or advisory board member for Amgen, Aveeno, Biogen, Encore, Exeltis, Ferndale, Galderma, G&W Laboratories, Intraderm, La Roche-Posay, Loreal, Microcures, Nano Bio-Med, Novartis, Oakstone Institute, Occulus, Onset, Pfizer, Promius, Sanova Works, and Valeant. Dr. Friedman is also an editorial advisory board member for Dermatology News.
MIAMI – Tactics for managing patients with atopic dermatitis can go a long way to educate patients, set realistic expectations, and devise strategies for existing therapies, even as clinicians await some promising agents expected on the market soon.
“The good news is this is the Age of Eczema. In the last couple of years we’ve seen an explosion in the literature,” Adam Friedman, MD, of the department of dermatology, George Washington University, Washington, D.C., said at the Orlando Dermatology Aesthetic and Clinical Conference. Some of this research is spurring new therapeutics. a phosphodiesterase 4 inhibitor.
Crisaborole ointment, 2% (Eucrisa), a phosphodiesterase 4 inhibitor, was approved by the Food and Drug Administration in December 2016 for treating patients aged 2 years and older with mild to moderate AD, for example. It is a novel, nonsteroidal anti-inflammatory and the first prescription agent approved in the United States for atopic dermatitis in more than 10 years.
Dr. Friedman has no personal experience with crisaborole, which just became available. “But the data look encouraging. From what I’ve seen this may be a nonburning alternative to calcineurin inhibitors. It will be interesting to see how this will fit in our practices.”
Systemic management of pruritus
There’s also promise for patients troubled by one of the top manifestations of AD – the itch. “We have new targeted therapies coming down the pike, some hopefully [gaining approval] in the next few months. We have biologics going after the cytokines of itch. It’s a very, very exciting time right now,” Dr. Friedman said.
Current clinical trials are not only focusing on AD but also specifically on pruritus, he added.
In the meantime, itch can be managed with prescription and over-the-counter topical agents, as well as systemic therapies such as gabapentin, some antidepressants, and the antiemetic aprepitant. Aprepitant is a substance P antagonist (through blocking neurokinin 1 receptor) and can be effective for some patients when taken three times a week, but it is not indicated for itch, Dr. Friedman said. Because of its off label indication “it’s a little tricky getting [insurance] coverage.”
Back to basics
“Even with all the excitement, even with the new therapeutics, you have to stick with the basics,” he said. “Put the lotion on, put the cream on. You have to put moisturizer on wet skin and be cautious with soaps.” He added, “don’t be afraid to ask for help. The National Eczema Association has a wonderful website with research, education, tools – you name it.”
Keeping it real
For regional eczemas like hand dermatitis, what are the options? “Tell patients they can glove up, there are various latex alternatives … but it probably won’t fly in the real world,” Dr. Friedman said. Zinc oxide “works like armor, and patients will probably do well,” but the aesthetics are unacceptable for most, he added. “Newer alternatives, such as those with aluminum magnesium hydroxide stearate, have similar protecting power, but are not opaque and rub on easier.”
A goal of topical therapy is to get rid of the inflammation, and steroids have a long history of evidence supporting their use, but “topical steroid phobia in parents” is a problem, he said. To counter the reluctance or refusal to use topical steroids, he suggested exploring reasons for noncompliance, dispelling any myths, and working with parent to make it easier to apply the steroids to their child.
Interestingly, there is some evidence that a simpler regimen may work well for some patients. “We always say ‘apply twice a day.’ Why? Because all the clinical trials had participants apply steroids twice a day. But there is no evidence to show twice a day is better than once a day, and in fact, a meta-analysis suggests once a day works just as well” (Br J Dermatol. 2005 Jan;152[1]:130-41).
Topical calcineurin inhibitors are another option. In general, Dr. Friedman prescribes these agents for delicate areas, for patients with thin skin, or for patients who use a topical steroid “on and on and on and can’t seem to get off it.” Calcineurin inhibitors can also be used on in-between days during steroid maintenance therapy, he added. When prescribing, warn patients about the initial burn (due to substance P release) that commonly occurs so that they have realistic expectations.
Education remains essential
“I encourage you to educate your patients and empathize with them,” he said. “Show them how to apply a moisturizer. Also, use your nurses and assistants to help with education – really empower them to be part of the process.”
“Explain, explain, explain, so they have realistic expectations,” and know that there is no cure, so that when they experience a flare, they understand that “it’s not that the steroid didn’t work – this is a chronic disease,” added Dr. Friedman, who recommends providing patients with handouts that answer many of their questions.
Maximize moisturizing
When it comes to moisturizing, more is usually better. Effective products contain all the key ingredients: emollients to soften the skin, an occlusive to keep the water there, and a humectant to bond the water. “Just one or two is not going to cut it,” he said.
“Something we now know is that starting early is key,” he pointed out, referring to recent studies that have shown that in babies at high risk for AD, starting moisturizers early can decrease their risk for developing AD later (J Allergy Clin Immunol. 2014 Oct; 134[4]: 818-23).
“Another study that received a ton of press was in JAMA Pediatrics recently,” Dr. Friedman said. The study concluded that the use of different moisturizers to prevent AD in high risk babies was likely to be cost-effective (JAMA Pediatr. 2017 Feb 6;171[2]:e163909. doi: 10.1001/jamapediatrics.2016.3909). Although some news reports claimed starting babies with Vaseline as a moisturizer will prevent AD, “that’s actually not what the study showed. All the over-the-counter moisturizers they used worked, but Vaseline was the least expensive,” Dr. Friedman noted
Help patients select the right soap
Educate patients to avoid “true soaps” such as Dial, Ivory, Irish Spring, or Lever 2000. “Soaps can be a real enemy here. You want lower pH types of soaps. Depending on skin type, our skin is somewhere between 5.5 and 6.5 pH,” Dr. Friedman explained. “The paradigm shift for your patients is to hydrate, not to clean. Showers are okay if they’re not blaring hot. Baths are okay ... but you should not be sitting in a sudsy bath.”
Also, instruct patients to avoid irritating fabrics, dryer sheets, or harsh laundry detergents that could exacerbate AD.
‘You’re not alone’
Sometimes it’s helpful to assure patients with AD that they’re not alone, and that many researchers and clinicians are working on effective treatment strategies. “We’re all familiar with atopic dermatitis because there’s so much of it. The numbers are surprisingly high,” Dr. Friedman said. Compared with the estimated 2.2 million Americans with psoriasis, AD eclipses their numbers substantially, affecting about 17 million people.
Dr. Friedman disclosed that he is a speaker for Amgen, Janssen, and Promius; receives research grants from Valeant; and is a consultant and/or advisory board member for Amgen, Aveeno, Biogen, Encore, Exeltis, Ferndale, Galderma, G&W Laboratories, Intraderm, La Roche-Posay, Loreal, Microcures, Nano Bio-Med, Novartis, Oakstone Institute, Occulus, Onset, Pfizer, Promius, Sanova Works, and Valeant. Dr. Friedman is also an editorial advisory board member for Dermatology News.
AT ODAC 2017
Antibiotic prophylaxis for artificial joints
A 66-year-old woman 3 years status post hip replacement is seen for dental work. The dentist contacts the clinic for an antibiotic prescription. The patient has a penicillin allergy (rash). What do you recommend?
A. Clindamycin one dose before dental work.
B. Amoxicillin one dose before dental work.
C. Amoxicillin one dose before, one dose 4 hours after dental work.
D. Clindamycin one dose before dental work, one dose 4 hours after dental work.
E. No antibiotics.
Many patients with prosthetic joints will request antibiotics to take prior to dental procedures. Sometimes this request comes from the dental office.
When I ask patients why they feel they need antibiotics, they often reply that they were told by their orthopedic surgeons or their dentist that they would need to take antibiotics before dental procedures.
In an era when Clostridium difficile infection is a common and dangerous complication in the elderly, avoidance of unnecessary antibiotics is critical. In the United States, it is estimated that there are 240,000 patients infected with C. difficile annually, with 24,000 deaths at a cost of $6 billion.1
Is there compelling evidence to justify giving antibiotic prophylaxis for dental procedures to patients with prosthetic joints?
This information has called into question the wisdom of giving antibiotic prophylaxis for dental procedures when the same patients have transient bacteremias as a regular part of day-to-day life, and mouth organisms were infrequent causes of prosthetic joint infections.
The American Academy of Orthopaedic Surgeons (AAOS) and the American Dental Association (ADA) released an advisory statement 20 years ago on antibiotic prophylaxis for patients with dental replacements, which concluded: “Antibiotic prophylaxis is not indicated for dental patients with pins, plates, and screws, nor is it routinely indicated for most dental patients with total joint replacements.”4
In 2003, the AAOS and the ADA released updated guidelines that stated: “Presently, no scientific evidence supports the position that antibiotic prophylaxis to prevent hematogenous infections is required prior to dental treatment in patients with total joint prostheses. The risk/benefit and cost/effectiveness ratios fail to justify the administration of routine antibiotics.”5
Great confusion arose in 2009 when the AAOS published a position paper on its website that reversed this position.6 Interestingly, the statement was done by the AAOS alone, and not done in conjunction with the ADA.
In this position paper, the AAOS recommended that health care providers consider antibiotic prophylaxis prior to invasive procedures on all patients who had prosthetic joints, regardless of how long those joints have been in place. This major change in recommendations was not based on any new evidence that had been reviewed since the 2003 guidelines.
There are two studies that address outcome of patients with prosthetic joints who have and have not received prophylactic antibiotics.
Elie Berbari, MD, and colleagues reported on the results of a prospective case-control study comparing patients with prosthetic joints hospitalized with hip or knee infections with patients who had prosthetic joints hospitalized at the same time who did not have hip or knee infections.7
There was no increased risk of prosthetic hip or knee infection for patients undergoing a dental procedure who were not receiving antibiotic prophylaxis (odds ratio, 0.8; 95% confidence interval, 0.4-1.6), compared with the risk for patients not undergoing a dental procedure (OR, 0.6; 95% CI, 0.4-1.1). Antibiotic prophylaxis in patients undergoing high and low risk dental procedures did not decrease the risk of prosthetic joint infections.
In 2012, the AAOS and the ADA published updated guidelines with the following summary recommendation: “The practitioner might consider discontinuing the practice of routinely prescribing prophylactic antibiotics for patients with hip and knee prosthetic joint implants undergoing dental procedures.”8 They referenced the Berbari study as the best available evidence.
Feng-Chen Kao, MD, and colleagues published a study this year with a design very similar to the Berbari study, with similar results.9 All Taiwanese residents who had received hip or knee replacements over a 12-year period were screened. Those who had received dental procedures were matched with individuals who had not had dental procedures. The dental procedure group was subdivided into a group that received antibiotics and one that didn’t.
There was no difference in infection rates between the group that had received dental procedures and the group that did not, and no difference in infection rates between those who received prophylactic antibiotics and those who didn’t.
I think this myth can be put to rest. There is no evidence to give patients with joint prostheses prophylactic antibiotics before dental procedures.
References
1. Steckelberg J.M., Osmon D.R. Prosthetic joint infections. In: Bisno A.L., Waldvogel F.A., eds. Infections associated with indwelling medical devices. Third ed., Washington, D.C.: American Society of Microbiology Press, 2000:173-209.
2. J Dent Res. 2004 Feb;83(2):170-4.
3. J Clin Periodontol. 2006 Feb;33(6):401-7.
4. J Am Dent Assoc. 1997 Jul;128(7):1004-8.
5. J Am Dent Assoc. 2003 Jul;134(7):895-9.
6. Spec Care Dentist. 2009 Nov-Dec;29(6):229-31.
7. Clin Infect Dis. 2010 Jan 1;50(1):8-16.
8. J Dent (Shiraz). 2013 Mar;14(1):49-52.
9. Infect Control Hosp Epidemiol. 2017 Feb;38(2):154-61.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A 66-year-old woman 3 years status post hip replacement is seen for dental work. The dentist contacts the clinic for an antibiotic prescription. The patient has a penicillin allergy (rash). What do you recommend?
A. Clindamycin one dose before dental work.
B. Amoxicillin one dose before dental work.
C. Amoxicillin one dose before, one dose 4 hours after dental work.
D. Clindamycin one dose before dental work, one dose 4 hours after dental work.
E. No antibiotics.
Many patients with prosthetic joints will request antibiotics to take prior to dental procedures. Sometimes this request comes from the dental office.
When I ask patients why they feel they need antibiotics, they often reply that they were told by their orthopedic surgeons or their dentist that they would need to take antibiotics before dental procedures.
In an era when Clostridium difficile infection is a common and dangerous complication in the elderly, avoidance of unnecessary antibiotics is critical. In the United States, it is estimated that there are 240,000 patients infected with C. difficile annually, with 24,000 deaths at a cost of $6 billion.1
Is there compelling evidence to justify giving antibiotic prophylaxis for dental procedures to patients with prosthetic joints?
This information has called into question the wisdom of giving antibiotic prophylaxis for dental procedures when the same patients have transient bacteremias as a regular part of day-to-day life, and mouth organisms were infrequent causes of prosthetic joint infections.
The American Academy of Orthopaedic Surgeons (AAOS) and the American Dental Association (ADA) released an advisory statement 20 years ago on antibiotic prophylaxis for patients with dental replacements, which concluded: “Antibiotic prophylaxis is not indicated for dental patients with pins, plates, and screws, nor is it routinely indicated for most dental patients with total joint replacements.”4
In 2003, the AAOS and the ADA released updated guidelines that stated: “Presently, no scientific evidence supports the position that antibiotic prophylaxis to prevent hematogenous infections is required prior to dental treatment in patients with total joint prostheses. The risk/benefit and cost/effectiveness ratios fail to justify the administration of routine antibiotics.”5
Great confusion arose in 2009 when the AAOS published a position paper on its website that reversed this position.6 Interestingly, the statement was done by the AAOS alone, and not done in conjunction with the ADA.
In this position paper, the AAOS recommended that health care providers consider antibiotic prophylaxis prior to invasive procedures on all patients who had prosthetic joints, regardless of how long those joints have been in place. This major change in recommendations was not based on any new evidence that had been reviewed since the 2003 guidelines.
There are two studies that address outcome of patients with prosthetic joints who have and have not received prophylactic antibiotics.
Elie Berbari, MD, and colleagues reported on the results of a prospective case-control study comparing patients with prosthetic joints hospitalized with hip or knee infections with patients who had prosthetic joints hospitalized at the same time who did not have hip or knee infections.7
There was no increased risk of prosthetic hip or knee infection for patients undergoing a dental procedure who were not receiving antibiotic prophylaxis (odds ratio, 0.8; 95% confidence interval, 0.4-1.6), compared with the risk for patients not undergoing a dental procedure (OR, 0.6; 95% CI, 0.4-1.1). Antibiotic prophylaxis in patients undergoing high and low risk dental procedures did not decrease the risk of prosthetic joint infections.
In 2012, the AAOS and the ADA published updated guidelines with the following summary recommendation: “The practitioner might consider discontinuing the practice of routinely prescribing prophylactic antibiotics for patients with hip and knee prosthetic joint implants undergoing dental procedures.”8 They referenced the Berbari study as the best available evidence.
Feng-Chen Kao, MD, and colleagues published a study this year with a design very similar to the Berbari study, with similar results.9 All Taiwanese residents who had received hip or knee replacements over a 12-year period were screened. Those who had received dental procedures were matched with individuals who had not had dental procedures. The dental procedure group was subdivided into a group that received antibiotics and one that didn’t.
There was no difference in infection rates between the group that had received dental procedures and the group that did not, and no difference in infection rates between those who received prophylactic antibiotics and those who didn’t.
I think this myth can be put to rest. There is no evidence to give patients with joint prostheses prophylactic antibiotics before dental procedures.
References
1. Steckelberg J.M., Osmon D.R. Prosthetic joint infections. In: Bisno A.L., Waldvogel F.A., eds. Infections associated with indwelling medical devices. Third ed., Washington, D.C.: American Society of Microbiology Press, 2000:173-209.
2. J Dent Res. 2004 Feb;83(2):170-4.
3. J Clin Periodontol. 2006 Feb;33(6):401-7.
4. J Am Dent Assoc. 1997 Jul;128(7):1004-8.
5. J Am Dent Assoc. 2003 Jul;134(7):895-9.
6. Spec Care Dentist. 2009 Nov-Dec;29(6):229-31.
7. Clin Infect Dis. 2010 Jan 1;50(1):8-16.
8. J Dent (Shiraz). 2013 Mar;14(1):49-52.
9. Infect Control Hosp Epidemiol. 2017 Feb;38(2):154-61.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A 66-year-old woman 3 years status post hip replacement is seen for dental work. The dentist contacts the clinic for an antibiotic prescription. The patient has a penicillin allergy (rash). What do you recommend?
A. Clindamycin one dose before dental work.
B. Amoxicillin one dose before dental work.
C. Amoxicillin one dose before, one dose 4 hours after dental work.
D. Clindamycin one dose before dental work, one dose 4 hours after dental work.
E. No antibiotics.
Many patients with prosthetic joints will request antibiotics to take prior to dental procedures. Sometimes this request comes from the dental office.
When I ask patients why they feel they need antibiotics, they often reply that they were told by their orthopedic surgeons or their dentist that they would need to take antibiotics before dental procedures.
In an era when Clostridium difficile infection is a common and dangerous complication in the elderly, avoidance of unnecessary antibiotics is critical. In the United States, it is estimated that there are 240,000 patients infected with C. difficile annually, with 24,000 deaths at a cost of $6 billion.1
Is there compelling evidence to justify giving antibiotic prophylaxis for dental procedures to patients with prosthetic joints?
This information has called into question the wisdom of giving antibiotic prophylaxis for dental procedures when the same patients have transient bacteremias as a regular part of day-to-day life, and mouth organisms were infrequent causes of prosthetic joint infections.
The American Academy of Orthopaedic Surgeons (AAOS) and the American Dental Association (ADA) released an advisory statement 20 years ago on antibiotic prophylaxis for patients with dental replacements, which concluded: “Antibiotic prophylaxis is not indicated for dental patients with pins, plates, and screws, nor is it routinely indicated for most dental patients with total joint replacements.”4
In 2003, the AAOS and the ADA released updated guidelines that stated: “Presently, no scientific evidence supports the position that antibiotic prophylaxis to prevent hematogenous infections is required prior to dental treatment in patients with total joint prostheses. The risk/benefit and cost/effectiveness ratios fail to justify the administration of routine antibiotics.”5
Great confusion arose in 2009 when the AAOS published a position paper on its website that reversed this position.6 Interestingly, the statement was done by the AAOS alone, and not done in conjunction with the ADA.
In this position paper, the AAOS recommended that health care providers consider antibiotic prophylaxis prior to invasive procedures on all patients who had prosthetic joints, regardless of how long those joints have been in place. This major change in recommendations was not based on any new evidence that had been reviewed since the 2003 guidelines.
There are two studies that address outcome of patients with prosthetic joints who have and have not received prophylactic antibiotics.
Elie Berbari, MD, and colleagues reported on the results of a prospective case-control study comparing patients with prosthetic joints hospitalized with hip or knee infections with patients who had prosthetic joints hospitalized at the same time who did not have hip or knee infections.7
There was no increased risk of prosthetic hip or knee infection for patients undergoing a dental procedure who were not receiving antibiotic prophylaxis (odds ratio, 0.8; 95% confidence interval, 0.4-1.6), compared with the risk for patients not undergoing a dental procedure (OR, 0.6; 95% CI, 0.4-1.1). Antibiotic prophylaxis in patients undergoing high and low risk dental procedures did not decrease the risk of prosthetic joint infections.
In 2012, the AAOS and the ADA published updated guidelines with the following summary recommendation: “The practitioner might consider discontinuing the practice of routinely prescribing prophylactic antibiotics for patients with hip and knee prosthetic joint implants undergoing dental procedures.”8 They referenced the Berbari study as the best available evidence.
Feng-Chen Kao, MD, and colleagues published a study this year with a design very similar to the Berbari study, with similar results.9 All Taiwanese residents who had received hip or knee replacements over a 12-year period were screened. Those who had received dental procedures were matched with individuals who had not had dental procedures. The dental procedure group was subdivided into a group that received antibiotics and one that didn’t.
There was no difference in infection rates between the group that had received dental procedures and the group that did not, and no difference in infection rates between those who received prophylactic antibiotics and those who didn’t.
I think this myth can be put to rest. There is no evidence to give patients with joint prostheses prophylactic antibiotics before dental procedures.
References
1. Steckelberg J.M., Osmon D.R. Prosthetic joint infections. In: Bisno A.L., Waldvogel F.A., eds. Infections associated with indwelling medical devices. Third ed., Washington, D.C.: American Society of Microbiology Press, 2000:173-209.
2. J Dent Res. 2004 Feb;83(2):170-4.
3. J Clin Periodontol. 2006 Feb;33(6):401-7.
4. J Am Dent Assoc. 1997 Jul;128(7):1004-8.
5. J Am Dent Assoc. 2003 Jul;134(7):895-9.
6. Spec Care Dentist. 2009 Nov-Dec;29(6):229-31.
7. Clin Infect Dis. 2010 Jan 1;50(1):8-16.
8. J Dent (Shiraz). 2013 Mar;14(1):49-52.
9. Infect Control Hosp Epidemiol. 2017 Feb;38(2):154-61.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].