User login
Anticoagulant resumption after ICH aids patients
HOUSTON – Even when patients on an oral anticoagulant have the dreaded complication of an intracerebral hemorrhage, resumption of their oral anticoagulation regimen appears to produce the best midterm outcomes, based on a meta-analysis of data from more than 1,000 patients collected in three observational studies.
Resumption of oral anticoagulation therapy (OAT) is a “major dilemma” when managing patients who developed an intracerebral hemorrhage (ICH) while on OAT, said Alessandro Biffi, MD, explaining why he performed this meta-analysis that he presented at the International Stroke Conference sponsored by the American Heart Association.
He used individual patient data collected from a total of 1,027 patients enrolled in any of three different observational studies: the German-wide Multicenter Analysis of Oral Anticoagulation Associated Intracerebral Hemorrhage (RETRACE) study, the MGH longitudinal ICH study, or the Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study. Overall 26% of the patients resumed OAT following their ICH, although the rate ranged from a low of 20% in one study to a high of 42% in another. The vast majority of patients received a vitamin K antagonist as their anticoagulant; very few received a new oral anticoagulant.
Using propensity score matching to compare similar patients who resumed or stayed off OAT, Dr. Biffi found that, during the year following the index ICH, mortality was 71%-74% lower among patients who resumed OAT. Recurrent all-cause stroke was 49%-55% lower with resumed OAT, and favorable functional outcomes (a score of 0-3 on the modified Rankin scale) were more than fourfold higher with OAT resumption, he reported.
Dr. Biffi calculated these relative rates, both for patients with a lumbar location of their ICH and for those with a nonlumbar location, and found that location had no influence on responsiveness to OAT. Patients with an index ICH in a lumbar location had a trend toward more recurrent ICH on OAT, a 26% higher rate relative to patients not resumed on OAT, but this difference fell short of statistical significance.
The only factor he found that linked with whether or not patients resumed OAT was the severity of their index ICH. The more severe their bleed, the less likely were patients to resume. Aside from that, “there is a lot of variation in practice,” he said. “We are gathering additional data” to try to further address this question.
Dr. Biffi had no disclosures.
[email protected]
On Twitter @mitchelzoler
Resuming oral anticoagulation following an intracerebral hemorrhage is one of the most vexing problems today in vascular neurology. It’s a situation that often happens, and it will grow increasingly more common as the number of patients with atrial fibrillation escalates and even more people start oral anticoagulation.
It’s also very important to remember that patients like these who need oral anticoagulation but now have a history of ICH must have all their other cardiovascular disease risk factors very well controlled: their blood pressure, their diabetes, their smoking, etc. Oral anticoagulation may be important for these patients, but tight risk factor control is even more important.
I agree with Dr. Biffi that a prospective, randomized trial is the best way to get more information to help guide resuming oral anticoagulation. Observational studies are significantly limited by ascertainment bias, and for these patients there are also many variables – at least a dozen – that can influence whether or not a patient resumes oral anticoagulation. Dr. Biffi’s findings are interesting, but the limitations of his data prevent the results from being truly compelling.
It would be very helpful to have data from a trial that randomized ICH patients who required anticoagulation to a full-dose NOAC, a reduced-dose NOAC, or aspirin and see which group had the best long-term outcome. Whatever the results, it would change practice. It’s intriguing to speculate that a reduced-dose NOAC might provide adequate ischemic protection with a reduced risk for more bleeding.
Mark J. Alberts, MD , is chief of neurology at Hartford (Conn.) Hospital. He had no disclosures. He made these comments in an interview and during a press conference.
Resuming oral anticoagulation following an intracerebral hemorrhage is one of the most vexing problems today in vascular neurology. It’s a situation that often happens, and it will grow increasingly more common as the number of patients with atrial fibrillation escalates and even more people start oral anticoagulation.
It’s also very important to remember that patients like these who need oral anticoagulation but now have a history of ICH must have all their other cardiovascular disease risk factors very well controlled: their blood pressure, their diabetes, their smoking, etc. Oral anticoagulation may be important for these patients, but tight risk factor control is even more important.
I agree with Dr. Biffi that a prospective, randomized trial is the best way to get more information to help guide resuming oral anticoagulation. Observational studies are significantly limited by ascertainment bias, and for these patients there are also many variables – at least a dozen – that can influence whether or not a patient resumes oral anticoagulation. Dr. Biffi’s findings are interesting, but the limitations of his data prevent the results from being truly compelling.
It would be very helpful to have data from a trial that randomized ICH patients who required anticoagulation to a full-dose NOAC, a reduced-dose NOAC, or aspirin and see which group had the best long-term outcome. Whatever the results, it would change practice. It’s intriguing to speculate that a reduced-dose NOAC might provide adequate ischemic protection with a reduced risk for more bleeding.
Mark J. Alberts, MD , is chief of neurology at Hartford (Conn.) Hospital. He had no disclosures. He made these comments in an interview and during a press conference.
Resuming oral anticoagulation following an intracerebral hemorrhage is one of the most vexing problems today in vascular neurology. It’s a situation that often happens, and it will grow increasingly more common as the number of patients with atrial fibrillation escalates and even more people start oral anticoagulation.
It’s also very important to remember that patients like these who need oral anticoagulation but now have a history of ICH must have all their other cardiovascular disease risk factors very well controlled: their blood pressure, their diabetes, their smoking, etc. Oral anticoagulation may be important for these patients, but tight risk factor control is even more important.
I agree with Dr. Biffi that a prospective, randomized trial is the best way to get more information to help guide resuming oral anticoagulation. Observational studies are significantly limited by ascertainment bias, and for these patients there are also many variables – at least a dozen – that can influence whether or not a patient resumes oral anticoagulation. Dr. Biffi’s findings are interesting, but the limitations of his data prevent the results from being truly compelling.
It would be very helpful to have data from a trial that randomized ICH patients who required anticoagulation to a full-dose NOAC, a reduced-dose NOAC, or aspirin and see which group had the best long-term outcome. Whatever the results, it would change practice. It’s intriguing to speculate that a reduced-dose NOAC might provide adequate ischemic protection with a reduced risk for more bleeding.
Mark J. Alberts, MD , is chief of neurology at Hartford (Conn.) Hospital. He had no disclosures. He made these comments in an interview and during a press conference.
HOUSTON – Even when patients on an oral anticoagulant have the dreaded complication of an intracerebral hemorrhage, resumption of their oral anticoagulation regimen appears to produce the best midterm outcomes, based on a meta-analysis of data from more than 1,000 patients collected in three observational studies.
Resumption of oral anticoagulation therapy (OAT) is a “major dilemma” when managing patients who developed an intracerebral hemorrhage (ICH) while on OAT, said Alessandro Biffi, MD, explaining why he performed this meta-analysis that he presented at the International Stroke Conference sponsored by the American Heart Association.
He used individual patient data collected from a total of 1,027 patients enrolled in any of three different observational studies: the German-wide Multicenter Analysis of Oral Anticoagulation Associated Intracerebral Hemorrhage (RETRACE) study, the MGH longitudinal ICH study, or the Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study. Overall 26% of the patients resumed OAT following their ICH, although the rate ranged from a low of 20% in one study to a high of 42% in another. The vast majority of patients received a vitamin K antagonist as their anticoagulant; very few received a new oral anticoagulant.
Using propensity score matching to compare similar patients who resumed or stayed off OAT, Dr. Biffi found that, during the year following the index ICH, mortality was 71%-74% lower among patients who resumed OAT. Recurrent all-cause stroke was 49%-55% lower with resumed OAT, and favorable functional outcomes (a score of 0-3 on the modified Rankin scale) were more than fourfold higher with OAT resumption, he reported.
Dr. Biffi calculated these relative rates, both for patients with a lumbar location of their ICH and for those with a nonlumbar location, and found that location had no influence on responsiveness to OAT. Patients with an index ICH in a lumbar location had a trend toward more recurrent ICH on OAT, a 26% higher rate relative to patients not resumed on OAT, but this difference fell short of statistical significance.
The only factor he found that linked with whether or not patients resumed OAT was the severity of their index ICH. The more severe their bleed, the less likely were patients to resume. Aside from that, “there is a lot of variation in practice,” he said. “We are gathering additional data” to try to further address this question.
Dr. Biffi had no disclosures.
[email protected]
On Twitter @mitchelzoler
HOUSTON – Even when patients on an oral anticoagulant have the dreaded complication of an intracerebral hemorrhage, resumption of their oral anticoagulation regimen appears to produce the best midterm outcomes, based on a meta-analysis of data from more than 1,000 patients collected in three observational studies.
Resumption of oral anticoagulation therapy (OAT) is a “major dilemma” when managing patients who developed an intracerebral hemorrhage (ICH) while on OAT, said Alessandro Biffi, MD, explaining why he performed this meta-analysis that he presented at the International Stroke Conference sponsored by the American Heart Association.
He used individual patient data collected from a total of 1,027 patients enrolled in any of three different observational studies: the German-wide Multicenter Analysis of Oral Anticoagulation Associated Intracerebral Hemorrhage (RETRACE) study, the MGH longitudinal ICH study, or the Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study. Overall 26% of the patients resumed OAT following their ICH, although the rate ranged from a low of 20% in one study to a high of 42% in another. The vast majority of patients received a vitamin K antagonist as their anticoagulant; very few received a new oral anticoagulant.
Using propensity score matching to compare similar patients who resumed or stayed off OAT, Dr. Biffi found that, during the year following the index ICH, mortality was 71%-74% lower among patients who resumed OAT. Recurrent all-cause stroke was 49%-55% lower with resumed OAT, and favorable functional outcomes (a score of 0-3 on the modified Rankin scale) were more than fourfold higher with OAT resumption, he reported.
Dr. Biffi calculated these relative rates, both for patients with a lumbar location of their ICH and for those with a nonlumbar location, and found that location had no influence on responsiveness to OAT. Patients with an index ICH in a lumbar location had a trend toward more recurrent ICH on OAT, a 26% higher rate relative to patients not resumed on OAT, but this difference fell short of statistical significance.
The only factor he found that linked with whether or not patients resumed OAT was the severity of their index ICH. The more severe their bleed, the less likely were patients to resume. Aside from that, “there is a lot of variation in practice,” he said. “We are gathering additional data” to try to further address this question.
Dr. Biffi had no disclosures.
[email protected]
On Twitter @mitchelzoler
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point:
Major finding: One-year mortality was 71%-74% lower among patients who resumed oral anticoagulation relative to those who did not.
Data source: Meta-analysis of data from 1,027 patients collected in three observational studies.
Disclosures: Dr. Biffi had no disclosures.
Prenatal surveillance vital in monochorionic twin pregnancies
Prenatal care is important for every pregnancy. It ensures the health and safety of the mother and baby throughout gestation, and alerts the ob.gyn. to any possible complications that may arise. Today, more than ever before, we have a wide array of powerful tools to augment the care we provide for our patients – from imaging technologies, to genomic screens, to advances in fetal surgery. However, every pregnancy can present its own set of challenges, and successful delivery of a healthy newborn cannot be taken for granted.
The importance of proper prenatal care is most essential to women with higher-risk pregnancies, which includes those involving multiple fetuses. From the babies’ perspective, complications of multiple fetuses can include intrauterine growth restriction, cerebral palsy, and stillbirth; from the mother’s perspective, complications of multiple fetuses can include preterm labor, gestational diabetes mellitus, preeclampsia, and placental abruption.
This month, we focus on the range of tools and approaches used to treat complications that can occur in monochorionic twin pregnancies, including twin-to-twin transfusion syndrome, selective fetal growth restriction, twin anemia polycythemia sequence, and twin reversed arterial perfusion. Despite the challenges these conditions pose, increased ultrasonographic and echocardiographic surveillance allow for earlier detection and possible intervention to slow progression or, in some cases, correct defects that would have terminated the pregnancy not too long ago. Additionally, fetal therapy programs utilizing in-utero surgical techniques, such as fetoscopic laser coagulation, have significantly broadened the management and treatment options we can now offer these patients.
Dr. M. Ozhan Turan, an associate professor and director of fetal therapy and complex obstetric surgery in the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, provides an overview of these techniques and technologies which, when applied appropriately, can significantly improve pregnancy outcomes.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Prenatal care is important for every pregnancy. It ensures the health and safety of the mother and baby throughout gestation, and alerts the ob.gyn. to any possible complications that may arise. Today, more than ever before, we have a wide array of powerful tools to augment the care we provide for our patients – from imaging technologies, to genomic screens, to advances in fetal surgery. However, every pregnancy can present its own set of challenges, and successful delivery of a healthy newborn cannot be taken for granted.
The importance of proper prenatal care is most essential to women with higher-risk pregnancies, which includes those involving multiple fetuses. From the babies’ perspective, complications of multiple fetuses can include intrauterine growth restriction, cerebral palsy, and stillbirth; from the mother’s perspective, complications of multiple fetuses can include preterm labor, gestational diabetes mellitus, preeclampsia, and placental abruption.
This month, we focus on the range of tools and approaches used to treat complications that can occur in monochorionic twin pregnancies, including twin-to-twin transfusion syndrome, selective fetal growth restriction, twin anemia polycythemia sequence, and twin reversed arterial perfusion. Despite the challenges these conditions pose, increased ultrasonographic and echocardiographic surveillance allow for earlier detection and possible intervention to slow progression or, in some cases, correct defects that would have terminated the pregnancy not too long ago. Additionally, fetal therapy programs utilizing in-utero surgical techniques, such as fetoscopic laser coagulation, have significantly broadened the management and treatment options we can now offer these patients.
Dr. M. Ozhan Turan, an associate professor and director of fetal therapy and complex obstetric surgery in the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, provides an overview of these techniques and technologies which, when applied appropriately, can significantly improve pregnancy outcomes.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Prenatal care is important for every pregnancy. It ensures the health and safety of the mother and baby throughout gestation, and alerts the ob.gyn. to any possible complications that may arise. Today, more than ever before, we have a wide array of powerful tools to augment the care we provide for our patients – from imaging technologies, to genomic screens, to advances in fetal surgery. However, every pregnancy can present its own set of challenges, and successful delivery of a healthy newborn cannot be taken for granted.
The importance of proper prenatal care is most essential to women with higher-risk pregnancies, which includes those involving multiple fetuses. From the babies’ perspective, complications of multiple fetuses can include intrauterine growth restriction, cerebral palsy, and stillbirth; from the mother’s perspective, complications of multiple fetuses can include preterm labor, gestational diabetes mellitus, preeclampsia, and placental abruption.
This month, we focus on the range of tools and approaches used to treat complications that can occur in monochorionic twin pregnancies, including twin-to-twin transfusion syndrome, selective fetal growth restriction, twin anemia polycythemia sequence, and twin reversed arterial perfusion. Despite the challenges these conditions pose, increased ultrasonographic and echocardiographic surveillance allow for earlier detection and possible intervention to slow progression or, in some cases, correct defects that would have terminated the pregnancy not too long ago. Additionally, fetal therapy programs utilizing in-utero surgical techniques, such as fetoscopic laser coagulation, have significantly broadened the management and treatment options we can now offer these patients.
Dr. M. Ozhan Turan, an associate professor and director of fetal therapy and complex obstetric surgery in the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, provides an overview of these techniques and technologies which, when applied appropriately, can significantly improve pregnancy outcomes.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
For mantle cell lymphoma, VR-CAP beat R-CHOP
For patients with newly diagnosed mantle cell lymphoma, duration and quality of response were superior with a regimen of bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) when compared with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), based on a post hoc analysis of the randomized, phase III LYM-3002 trial.
The difference was especially evident among patients who had a low- or medium-risk mantle cell lymphoma international prognostic index, Gregor Verhoef, MD, of University Hospital Leuven (Belgium) and his associates wrote in Haematologica.
In LYM-3002, 487 patients with newly diagnosed stage II-IV mantle cell lymphoma received six to eight 21-day cycles of intravenous VR-CAP or R-CHOP. Although overall response rates were similar for both groups, VR-CAP was associated with better duration of response and progression-free survival (PFS) and extended time to next treatment. To further explore these differences, the post hoc analysis stratified outcomes by response categories and analyzed depth of response based on computed tomography (CT) scans. Patients had a median age of about 65 years, and most were white males with stage-IV disease at diagnosis and an Eastern Cooperative Oncology Group performance status of 1 (Haematologica. 2017 Feb 9. doi: 10.3324/haematol.2016.152496).The superiority of VR-CAP held up across response categories. Complete responders to VR-CAP had more than twice the median PFS as did complete responders to R-CHOP (40.9 vs. 19.8 months; hazard ratio, 0.58; 95% confidence interval, 0.39-0.84; P = .004). Among partial responders, median PFS was 17.1 vs. 11.7 months, respectively (HR, 0.62; 95% CI, 0.43-0.89; P = .01). Respective median duration of overall response was 42.1 months for VR-CAP vs. 18.5 months among complete responders (HR, 0.42; P less than .001), and 20.2 vs. 9.6 months among partial responders (HR, 0.57; P = .006).
Median time to next treatment also favored VR-CAP over R-CHOP among both complete responders (not evaluable vs. 26.6 months; HR, 0.42; P less than .001) and partial responders (35.3 vs. 24.3 months; HR, 0.57; P = .006), the researchers said. Further, CT scans showed that proportionally more patients in each response category became lesion-negative on VR-CAP than on R-CHOP. Among complete responders, rates of lesion negativity were 72% and 59%, respectively. Among partial responders, rates were 48% and 28%.
The effects of VR-CAP were most evident among patients with a low or medium-risk mantle cell lymphoma international prognostic index. Perhaps high-risk status signifies more rapidly proliferative disease, which negates the deeper responses with VR-CAP, compared with R-CHOP, they added.
The LYM-3002 study was supported by Janssen Research & Development and Millennium Pharmaceuticals. Dr. Verhoef had no disclosures. Nine coinvestigators disclosed ties to Janssen, Roche, GlaxoSmithKline, and several other pharmaceutical companies.
For patients with newly diagnosed mantle cell lymphoma, duration and quality of response were superior with a regimen of bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) when compared with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), based on a post hoc analysis of the randomized, phase III LYM-3002 trial.
The difference was especially evident among patients who had a low- or medium-risk mantle cell lymphoma international prognostic index, Gregor Verhoef, MD, of University Hospital Leuven (Belgium) and his associates wrote in Haematologica.
In LYM-3002, 487 patients with newly diagnosed stage II-IV mantle cell lymphoma received six to eight 21-day cycles of intravenous VR-CAP or R-CHOP. Although overall response rates were similar for both groups, VR-CAP was associated with better duration of response and progression-free survival (PFS) and extended time to next treatment. To further explore these differences, the post hoc analysis stratified outcomes by response categories and analyzed depth of response based on computed tomography (CT) scans. Patients had a median age of about 65 years, and most were white males with stage-IV disease at diagnosis and an Eastern Cooperative Oncology Group performance status of 1 (Haematologica. 2017 Feb 9. doi: 10.3324/haematol.2016.152496).The superiority of VR-CAP held up across response categories. Complete responders to VR-CAP had more than twice the median PFS as did complete responders to R-CHOP (40.9 vs. 19.8 months; hazard ratio, 0.58; 95% confidence interval, 0.39-0.84; P = .004). Among partial responders, median PFS was 17.1 vs. 11.7 months, respectively (HR, 0.62; 95% CI, 0.43-0.89; P = .01). Respective median duration of overall response was 42.1 months for VR-CAP vs. 18.5 months among complete responders (HR, 0.42; P less than .001), and 20.2 vs. 9.6 months among partial responders (HR, 0.57; P = .006).
Median time to next treatment also favored VR-CAP over R-CHOP among both complete responders (not evaluable vs. 26.6 months; HR, 0.42; P less than .001) and partial responders (35.3 vs. 24.3 months; HR, 0.57; P = .006), the researchers said. Further, CT scans showed that proportionally more patients in each response category became lesion-negative on VR-CAP than on R-CHOP. Among complete responders, rates of lesion negativity were 72% and 59%, respectively. Among partial responders, rates were 48% and 28%.
The effects of VR-CAP were most evident among patients with a low or medium-risk mantle cell lymphoma international prognostic index. Perhaps high-risk status signifies more rapidly proliferative disease, which negates the deeper responses with VR-CAP, compared with R-CHOP, they added.
The LYM-3002 study was supported by Janssen Research & Development and Millennium Pharmaceuticals. Dr. Verhoef had no disclosures. Nine coinvestigators disclosed ties to Janssen, Roche, GlaxoSmithKline, and several other pharmaceutical companies.
For patients with newly diagnosed mantle cell lymphoma, duration and quality of response were superior with a regimen of bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) when compared with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), based on a post hoc analysis of the randomized, phase III LYM-3002 trial.
The difference was especially evident among patients who had a low- or medium-risk mantle cell lymphoma international prognostic index, Gregor Verhoef, MD, of University Hospital Leuven (Belgium) and his associates wrote in Haematologica.
In LYM-3002, 487 patients with newly diagnosed stage II-IV mantle cell lymphoma received six to eight 21-day cycles of intravenous VR-CAP or R-CHOP. Although overall response rates were similar for both groups, VR-CAP was associated with better duration of response and progression-free survival (PFS) and extended time to next treatment. To further explore these differences, the post hoc analysis stratified outcomes by response categories and analyzed depth of response based on computed tomography (CT) scans. Patients had a median age of about 65 years, and most were white males with stage-IV disease at diagnosis and an Eastern Cooperative Oncology Group performance status of 1 (Haematologica. 2017 Feb 9. doi: 10.3324/haematol.2016.152496).The superiority of VR-CAP held up across response categories. Complete responders to VR-CAP had more than twice the median PFS as did complete responders to R-CHOP (40.9 vs. 19.8 months; hazard ratio, 0.58; 95% confidence interval, 0.39-0.84; P = .004). Among partial responders, median PFS was 17.1 vs. 11.7 months, respectively (HR, 0.62; 95% CI, 0.43-0.89; P = .01). Respective median duration of overall response was 42.1 months for VR-CAP vs. 18.5 months among complete responders (HR, 0.42; P less than .001), and 20.2 vs. 9.6 months among partial responders (HR, 0.57; P = .006).
Median time to next treatment also favored VR-CAP over R-CHOP among both complete responders (not evaluable vs. 26.6 months; HR, 0.42; P less than .001) and partial responders (35.3 vs. 24.3 months; HR, 0.57; P = .006), the researchers said. Further, CT scans showed that proportionally more patients in each response category became lesion-negative on VR-CAP than on R-CHOP. Among complete responders, rates of lesion negativity were 72% and 59%, respectively. Among partial responders, rates were 48% and 28%.
The effects of VR-CAP were most evident among patients with a low or medium-risk mantle cell lymphoma international prognostic index. Perhaps high-risk status signifies more rapidly proliferative disease, which negates the deeper responses with VR-CAP, compared with R-CHOP, they added.
The LYM-3002 study was supported by Janssen Research & Development and Millennium Pharmaceuticals. Dr. Verhoef had no disclosures. Nine coinvestigators disclosed ties to Janssen, Roche, GlaxoSmithKline, and several other pharmaceutical companies.
FROM HAEMATOLOGICA
Key clinical point: A regimen of bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) led to superior duration and quality of response when compared with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in patients with newly diagnosed mantle cell lymphoma.
Major finding: Among complete responders, median progression-free survival on VR-CAP was nearly twice that of R-CHOP (40.9 vs. 19.8 months; hazard ratio, 0.58; 95% confidence interval, 0.39-0.84; P = .004).
Data source: A post hoc analysis of a phase III trial comparing VR-CAP with R-CHOP in 487 patients with newly diagnosed, measurable stage II-IV mantle cell lymphoma (LYM-3002).
Disclosures: The LYM-3002 study was supported by Janssen Research & Development and Millennium Pharmaceuticals. Dr. Verhoef had no disclosures. Nine coinvestigators disclosed ties to Janssen, Roche, GlaxoSmithKline, and several other pharmaceutical companies.
Anticoagulation Management Outcomes in Veterans: Office vs Telephone Visits
Oral anticoagulation with warfarin is used for the treatment and prevention of a variety of thrombotic disorders, including deep venous thrombosis (DVT), pulmonary embolism (PE), stroke prevention in atrial fibrillation (AF) and atrial flutter, and other hypercoagulable conditions. Although a mainstay in the treatment for these conditions, warfarin requires close monitoring due to its narrow therapeutic range, extensive drug and dietary interactions, and dosage variability among patients.1 Patients outside the therapeutic range are at risk of having a thrombotic or bleeding event that could lead to hospitalization or fatality.1 To reduce the risk of these events, patients on warfarin are managed by dose adjustment based on the international normalized ratio (INR). Research has shown that patients on warfarin in pharmacist-managed specialty anticoagulation clinics have more consistent monitoring and lower rates of adverse events (AEs) compared with traditional physician or nurse clinics.2-6 Management through these clinics can be achieved through office visits or telephone visits.
There are advantages and disadvantages to each model of anticoagulation management for patients.Telephone clinics provide time and cost savings, increased access to care, and convenience. However, disadvantages include missed phone calls or inability to contact the patient, difficulty for the patient to hear the provider’s instructions over the phone, and patient unavailability when a critical INR is of concern. Office visits are beneficial in that providers can provide both written and verbal instruction to patients, perform visual or physical patient assessments, and provide timely care if needed. Disadvantages of office visits may include long wait times and inconvenience for patients who live far away.
Telephone anticoagulation clinics have been evaluated for their efficacy and cost-effectiveness in several studies.5,7,8 However, few studies are available that compare patient outcomes between office visits and telephone visits. Two prior studies comparing groups of anticoagulation patients managed by telephone or by office visit concluded that there is no difference in outcomes between the 2 management models.9,10 However, a retrospective study by Stoudenmire and colleagues examined extreme INR values (≤ 1.5 or ≥ 4.5) in each management model and found that telephone clinic patients have a significant increase in extreme INR values but no difference in AEs between the 2 management models.11
The VA North Texas Health Care System (VANTHCS) includes a major medical center, 3 outlying medical facilities, and 5 community-based outpatient clinics (CBOCs). A centralized pharmacist-managed anticoagulation clinic is used to manage more than 2,500 VANTHCS anticoagulation patients. To meet the National Patient Safety Goal measures and provide consistent management across the system, all anticoagulation patients from CBOCs and medical facilities are enrolled in the clinic.12 To facilitate access to care, many patients transitioned from office visits to telephone visits. It was essential to evaluate the transition of patients from office to telephone visits to ensure continued stability and continuity of care across both models. The objective of this study was to determine whether a difference in anticoagulation outcomes exists when patients are transitioned from office to telephone visits.
Methods
The VANTHCS anticoagulation clinic policy for office visits requires that patients arrive at the Dallas VAMC 2 hours before their appointment for INR lab draw. During the office visit, the anticoagulation pharmacist evaluates the INR and pertinent changes since the previous visit. The patient is provided verbal instructions and a written dosage adjustment card. Telephone clinic protocol is similar to office visits with a few exceptions. Any patient, regardless of INR stability, may be enrolled in the telephone clinic as long as the patient provides consent and has a working telephone with voice mail. Patients enrolled in the telephone clinic access blood draws at the nearest VA facility and are given a questionnaire that includes pertinent questions asked during an office visit. Anticoagulation pharmacists evaluate the questionnaire and INR then contact the patient within 1 business day to provide the patient with instructions. If a patient fails to answer the telephone, the anticoagulation pharmacist leaves a voicemail message.
Study Design
This retrospective study was conducted by chart review using Computerized Patient Record System (CPRS) at VANTHCS on patients who met inclusion criteria between January 1, 2011 and May 31, 2014, and it was approved by the institutional review board and research and development committee. The study included patients aged ≥ 18 years on warfarin therapy managed by the VANTHCS anticoagulation clinic who were previously managed in office visits for ≥ 180 days before the telephone transition, then in telephone visits for another ≥ 180 days. Only INR values obtained through the VANTHCS anticoagulation clinic were assessed.
Patients were excluded from the study if they were not managed by the VANTHCS anticoagulation clinic or received direct oral anticoagulants (DOACs). The INR values were excluded if they were nonclinic related INR values (ie, results reported that do not reflect management by the anticoagulation clinic), the first INR after hospitalization, or INRs obtained during the first month of initial warfarin treatment for a patient.
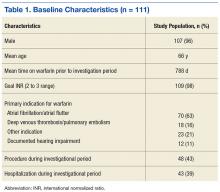
For all patients included in the study, demographic information, goal INR range (2 to 3 or 2.5 to 3.5), indication for warfarin therapy, and duration of warfarin therapy (defined as the first prescription filled for warfarin at the VA) were obtained. Individual INR values were obtained for each patient during the period of investigation and type of visit (office or telephone) for each INR drawn was specified. Any major bleeding or thrombotic events (bleed requiring an emergency department [ED] visit, hospitalization, vitamin K administration, blood transfusion, and/or warfarin therapy hold/discontinuation) were documented. Procedures and number of hospitalizations also during the investigation were recorded.
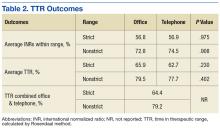
The primary outcomes measures evaluated INRs for time in therapeutic range (TTR) using the Rosendaal method and percentage of INRs within range.13 The therapeutic range was either 2 to 3 or 2.5 to 3.5 (the “strict range” for INR management). Because many patients fluctuate around the strict range and it is common to avoid therapy adjustment based on slightly elevated or lower values, a “nonstrict” range (1.8 to 3.2 or 2.3 to 3.7) also was evaluated.14 The secondary outcomes examined differences between the 2 management models in rates of major AEs, including thrombosis and major bleeding events as defined earlier.Frequencies, percentages, and other descriptive statistics were used to describe nominal data. A paired t test was used to compare TTR of patients transitioned from office to telephone visits. A P value of < .05 was used for statistical significance.
Results
A total of 111 patients met inclusion criteria (Table 1). Most patients were elderly males with AF or atrial flutter as their primary indication for warfarin therapy. No statistically significant difference was found for percentage INRs in strict range (56.8% in office vs 56.9% in telephone, P = .98) or TTR (65.9% in office vs 62.72% in telephone, P = .23) for patients who transitioned from office to telephone visits (Table 2). Similar results were found within the nonstrict range.
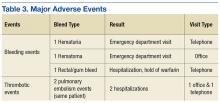
In examining safety, 5 major AEs occurred. One patient had 2 thrombotic pulmonary embolism events. This patient had a history of nonadherence with warfarin therapy. Three major bleeding events occurred (2 in the telephone group and 1 in the office group). Two bleeding events led to ED visits, and 1 event led to hospitalization. Although 43% of patients had a procedure during the study period, only a portion of patients received bridging with low-molecular-weight heparin (LMWH). None of the 3 reported bleeding events discovered during the study were associated with recent LMWH use. No events were fatal (Table 3).
Discussion
This study demonstrates that patients transitioned from office to telephone visits for warfarin management will have no significant change in their TTR. Additionally, patients had similar rates of major AEs before and after transition, although there were few events overall.
Previous research comparing anticoagulation outcomes in telephone vs office visits also has described outcomes to be similar between these 2 management models. Wittkowsky and colleagues examined 2 university-affiliated clinics to evaluate warfarin outcomes and AEs in patients in each management model (office vs telephone) and found no difference in outcomes between the 2 management models.9
Staresinic and colleagues designed a prospective study of 192 patients to evaluate TTR and AEs of the 2 management models at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin.10 This study found no difference between the 2 groups in percentage of time maintained within INR range or AEs and concluded that the telephone model was effective for anticoagulant management.
A retrospective study by Stoudenmire and colleagues evaluated office vs telephone management effects on extreme INR values (≤ 1.5 or ≥ 4.5), TTR, and AEs.11 This study found overall TTR and AEs to be similar between groups, but the telephone clinic had a 2-fold increase in extreme INR values compared with the office clinic.11
The current study differs from the previously discussed studies in that it evaluated outcomes for the same patients before and after the transition to telephone. This study did not exclude specific patients from telephone clinic. In the Wittkowsky study, patients were enrolled in the telephone clinic based on criteria such as patient disability or living long distances from the clinic.9 Additionally, in the current study, patients transitioned to telephone visits did not have scheduled office visits for anticoagulation management. In contrast, patients in the Staresinic study had routine anticoagulation office visits every 3 months, thus it was not a true telephone-only clinic.10
This study’s findings support prior studies’ findings that telephone clinics are acceptable for anticoagulation management. Furthermore, safety does not seem to be affected when transitioning patients, although there were few AEs to review. Providers can use telephone clinics to potentially decrease cost and facilitate access to care for patients.
Limitations
Patients were required to be in office and telephone for a sequential 6 months, and this may have produced selection biases toward patients who adhered to appointments and who were on long-term warfarin therapy. Many patients that were excluded from the study transitioned back and forth between the 2 management models. Due to the retrospective nature of this study, the authors were unable to control for all confounding variables. Patients also were not randomly assigned to be transitioned from office to telephone. Although a strength of this study was the limited telephone clinic selection criteria, there may be a few individual situations in which the pharmacist’s clinical judgment influenced the transition to the telephone clinic, creating selection bias.
There may be time bias present as clinical guidelines, providers, and clinic population size differed over the study period and might have influenced management. The population of VA patients was mainly elderly males; therefore, the study results may not be applicable to other populations. Last, the results of the study are reflective of the VANTHCS clinic structure and may not be applicable to other clinic designs.
Conclusion
Veterans in a pharmacist-managed anticoagulation clinic experienced the same outcomes in terms of TTR and major AEs when transitioned from the traditional face-to-face office visits to telephone visits. The study supports the safety and efficacy of transitioning patients from a pharmacist-managed anticoagulation office clinic to telephone clinic.
1. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(suppl 6):160S-198S.
2. Rudd KM, Dier JG. Comparison of two different models of anticoagulation management services with usual medical care. Pharmacotherapy. 2010;30(4):330-338.
3. Bungard TJ, Gardner L, Archer SL, et al. Evaluation of a pharmacist-managed anticoagulation clinic: improving patient care. Open Med. 2009;3(1):e16-e21.
4. Chiquette E, Amato MG, Bussey HI. Comparison of an anticoagulation clinic with usual medical care: anticoagulation control, patient outcomes, and health care costs. Arch Intern Med. 1998;158(15):1641-1647.
5. Waterman AD, Banet G, Milligan PE, et al. Patient and physician satisfaction with a telephone-based anticoagulation service. J Gen Intern Med. 2001;16(7):460-463.
6. Hasan SS, Shamala R, Syed IA, et al. Factors affecting warfarin-related knowledge and INR control of patients attending physician- and pharmacist-managed anticoagulation clinics. J Pharm Pract. 2011;24(5):485-493.
7. Hassan S, Naboush A, Radbel J, et al. Telephone-based anticoagulation management in the homebound setting: a retrospective observational study. Int J Gen Med. 2013;6:869-875.
8. Moherman LJ, Kolar MM. Complication rates for a telephone-based anticoagulation service. Am J Health Syst Pharm. 1999;56(15):1540-1542.
9. Wittkowsky AK, Nutescu EA, Blackburn J, et al. Outcomes of oral anticoagulant therapy managed by telephone vs in-office visits in an anticoagulation clinic setting. Chest. 2006;130(5):1385-1389.
10. Staresinic AG, Sorkness CA, Goodman BM, Pigarelli DW. Comparison of outcomes using 2 delivery models of anticoagulation care. Arch Intern Med. 2006;166(9):997-1002.
11. Stoudenmire LG, DeRemer CE, Elewa H. Telephone versus office-based management of warfarin: impact on international normalized ratios and outcomes. Int J Hematol. 2014;100(2):119-124.
12. The Joint Commission. National Patient Safety Goals Effective January 1, 2015. http://www.jointcommission.org/assets/1/6/2015_NPSG_AHC1.PDF. Published 2014. Accessed November 23, 2016.
13. Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236-239.
14. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(suppl 2):7S-47S.
Oral anticoagulation with warfarin is used for the treatment and prevention of a variety of thrombotic disorders, including deep venous thrombosis (DVT), pulmonary embolism (PE), stroke prevention in atrial fibrillation (AF) and atrial flutter, and other hypercoagulable conditions. Although a mainstay in the treatment for these conditions, warfarin requires close monitoring due to its narrow therapeutic range, extensive drug and dietary interactions, and dosage variability among patients.1 Patients outside the therapeutic range are at risk of having a thrombotic or bleeding event that could lead to hospitalization or fatality.1 To reduce the risk of these events, patients on warfarin are managed by dose adjustment based on the international normalized ratio (INR). Research has shown that patients on warfarin in pharmacist-managed specialty anticoagulation clinics have more consistent monitoring and lower rates of adverse events (AEs) compared with traditional physician or nurse clinics.2-6 Management through these clinics can be achieved through office visits or telephone visits.
There are advantages and disadvantages to each model of anticoagulation management for patients.Telephone clinics provide time and cost savings, increased access to care, and convenience. However, disadvantages include missed phone calls or inability to contact the patient, difficulty for the patient to hear the provider’s instructions over the phone, and patient unavailability when a critical INR is of concern. Office visits are beneficial in that providers can provide both written and verbal instruction to patients, perform visual or physical patient assessments, and provide timely care if needed. Disadvantages of office visits may include long wait times and inconvenience for patients who live far away.
Telephone anticoagulation clinics have been evaluated for their efficacy and cost-effectiveness in several studies.5,7,8 However, few studies are available that compare patient outcomes between office visits and telephone visits. Two prior studies comparing groups of anticoagulation patients managed by telephone or by office visit concluded that there is no difference in outcomes between the 2 management models.9,10 However, a retrospective study by Stoudenmire and colleagues examined extreme INR values (≤ 1.5 or ≥ 4.5) in each management model and found that telephone clinic patients have a significant increase in extreme INR values but no difference in AEs between the 2 management models.11
The VA North Texas Health Care System (VANTHCS) includes a major medical center, 3 outlying medical facilities, and 5 community-based outpatient clinics (CBOCs). A centralized pharmacist-managed anticoagulation clinic is used to manage more than 2,500 VANTHCS anticoagulation patients. To meet the National Patient Safety Goal measures and provide consistent management across the system, all anticoagulation patients from CBOCs and medical facilities are enrolled in the clinic.12 To facilitate access to care, many patients transitioned from office visits to telephone visits. It was essential to evaluate the transition of patients from office to telephone visits to ensure continued stability and continuity of care across both models. The objective of this study was to determine whether a difference in anticoagulation outcomes exists when patients are transitioned from office to telephone visits.
Methods
The VANTHCS anticoagulation clinic policy for office visits requires that patients arrive at the Dallas VAMC 2 hours before their appointment for INR lab draw. During the office visit, the anticoagulation pharmacist evaluates the INR and pertinent changes since the previous visit. The patient is provided verbal instructions and a written dosage adjustment card. Telephone clinic protocol is similar to office visits with a few exceptions. Any patient, regardless of INR stability, may be enrolled in the telephone clinic as long as the patient provides consent and has a working telephone with voice mail. Patients enrolled in the telephone clinic access blood draws at the nearest VA facility and are given a questionnaire that includes pertinent questions asked during an office visit. Anticoagulation pharmacists evaluate the questionnaire and INR then contact the patient within 1 business day to provide the patient with instructions. If a patient fails to answer the telephone, the anticoagulation pharmacist leaves a voicemail message.
Study Design
This retrospective study was conducted by chart review using Computerized Patient Record System (CPRS) at VANTHCS on patients who met inclusion criteria between January 1, 2011 and May 31, 2014, and it was approved by the institutional review board and research and development committee. The study included patients aged ≥ 18 years on warfarin therapy managed by the VANTHCS anticoagulation clinic who were previously managed in office visits for ≥ 180 days before the telephone transition, then in telephone visits for another ≥ 180 days. Only INR values obtained through the VANTHCS anticoagulation clinic were assessed.
Patients were excluded from the study if they were not managed by the VANTHCS anticoagulation clinic or received direct oral anticoagulants (DOACs). The INR values were excluded if they were nonclinic related INR values (ie, results reported that do not reflect management by the anticoagulation clinic), the first INR after hospitalization, or INRs obtained during the first month of initial warfarin treatment for a patient.
For all patients included in the study, demographic information, goal INR range (2 to 3 or 2.5 to 3.5), indication for warfarin therapy, and duration of warfarin therapy (defined as the first prescription filled for warfarin at the VA) were obtained. Individual INR values were obtained for each patient during the period of investigation and type of visit (office or telephone) for each INR drawn was specified. Any major bleeding or thrombotic events (bleed requiring an emergency department [ED] visit, hospitalization, vitamin K administration, blood transfusion, and/or warfarin therapy hold/discontinuation) were documented. Procedures and number of hospitalizations also during the investigation were recorded.
The primary outcomes measures evaluated INRs for time in therapeutic range (TTR) using the Rosendaal method and percentage of INRs within range.13 The therapeutic range was either 2 to 3 or 2.5 to 3.5 (the “strict range” for INR management). Because many patients fluctuate around the strict range and it is common to avoid therapy adjustment based on slightly elevated or lower values, a “nonstrict” range (1.8 to 3.2 or 2.3 to 3.7) also was evaluated.14 The secondary outcomes examined differences between the 2 management models in rates of major AEs, including thrombosis and major bleeding events as defined earlier.Frequencies, percentages, and other descriptive statistics were used to describe nominal data. A paired t test was used to compare TTR of patients transitioned from office to telephone visits. A P value of < .05 was used for statistical significance.
Results
A total of 111 patients met inclusion criteria (Table 1). Most patients were elderly males with AF or atrial flutter as their primary indication for warfarin therapy. No statistically significant difference was found for percentage INRs in strict range (56.8% in office vs 56.9% in telephone, P = .98) or TTR (65.9% in office vs 62.72% in telephone, P = .23) for patients who transitioned from office to telephone visits (Table 2). Similar results were found within the nonstrict range.
In examining safety, 5 major AEs occurred. One patient had 2 thrombotic pulmonary embolism events. This patient had a history of nonadherence with warfarin therapy. Three major bleeding events occurred (2 in the telephone group and 1 in the office group). Two bleeding events led to ED visits, and 1 event led to hospitalization. Although 43% of patients had a procedure during the study period, only a portion of patients received bridging with low-molecular-weight heparin (LMWH). None of the 3 reported bleeding events discovered during the study were associated with recent LMWH use. No events were fatal (Table 3).
Discussion
This study demonstrates that patients transitioned from office to telephone visits for warfarin management will have no significant change in their TTR. Additionally, patients had similar rates of major AEs before and after transition, although there were few events overall.
Previous research comparing anticoagulation outcomes in telephone vs office visits also has described outcomes to be similar between these 2 management models. Wittkowsky and colleagues examined 2 university-affiliated clinics to evaluate warfarin outcomes and AEs in patients in each management model (office vs telephone) and found no difference in outcomes between the 2 management models.9
Staresinic and colleagues designed a prospective study of 192 patients to evaluate TTR and AEs of the 2 management models at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin.10 This study found no difference between the 2 groups in percentage of time maintained within INR range or AEs and concluded that the telephone model was effective for anticoagulant management.
A retrospective study by Stoudenmire and colleagues evaluated office vs telephone management effects on extreme INR values (≤ 1.5 or ≥ 4.5), TTR, and AEs.11 This study found overall TTR and AEs to be similar between groups, but the telephone clinic had a 2-fold increase in extreme INR values compared with the office clinic.11
The current study differs from the previously discussed studies in that it evaluated outcomes for the same patients before and after the transition to telephone. This study did not exclude specific patients from telephone clinic. In the Wittkowsky study, patients were enrolled in the telephone clinic based on criteria such as patient disability or living long distances from the clinic.9 Additionally, in the current study, patients transitioned to telephone visits did not have scheduled office visits for anticoagulation management. In contrast, patients in the Staresinic study had routine anticoagulation office visits every 3 months, thus it was not a true telephone-only clinic.10
This study’s findings support prior studies’ findings that telephone clinics are acceptable for anticoagulation management. Furthermore, safety does not seem to be affected when transitioning patients, although there were few AEs to review. Providers can use telephone clinics to potentially decrease cost and facilitate access to care for patients.
Limitations
Patients were required to be in office and telephone for a sequential 6 months, and this may have produced selection biases toward patients who adhered to appointments and who were on long-term warfarin therapy. Many patients that were excluded from the study transitioned back and forth between the 2 management models. Due to the retrospective nature of this study, the authors were unable to control for all confounding variables. Patients also were not randomly assigned to be transitioned from office to telephone. Although a strength of this study was the limited telephone clinic selection criteria, there may be a few individual situations in which the pharmacist’s clinical judgment influenced the transition to the telephone clinic, creating selection bias.
There may be time bias present as clinical guidelines, providers, and clinic population size differed over the study period and might have influenced management. The population of VA patients was mainly elderly males; therefore, the study results may not be applicable to other populations. Last, the results of the study are reflective of the VANTHCS clinic structure and may not be applicable to other clinic designs.
Conclusion
Veterans in a pharmacist-managed anticoagulation clinic experienced the same outcomes in terms of TTR and major AEs when transitioned from the traditional face-to-face office visits to telephone visits. The study supports the safety and efficacy of transitioning patients from a pharmacist-managed anticoagulation office clinic to telephone clinic.
Oral anticoagulation with warfarin is used for the treatment and prevention of a variety of thrombotic disorders, including deep venous thrombosis (DVT), pulmonary embolism (PE), stroke prevention in atrial fibrillation (AF) and atrial flutter, and other hypercoagulable conditions. Although a mainstay in the treatment for these conditions, warfarin requires close monitoring due to its narrow therapeutic range, extensive drug and dietary interactions, and dosage variability among patients.1 Patients outside the therapeutic range are at risk of having a thrombotic or bleeding event that could lead to hospitalization or fatality.1 To reduce the risk of these events, patients on warfarin are managed by dose adjustment based on the international normalized ratio (INR). Research has shown that patients on warfarin in pharmacist-managed specialty anticoagulation clinics have more consistent monitoring and lower rates of adverse events (AEs) compared with traditional physician or nurse clinics.2-6 Management through these clinics can be achieved through office visits or telephone visits.
There are advantages and disadvantages to each model of anticoagulation management for patients.Telephone clinics provide time and cost savings, increased access to care, and convenience. However, disadvantages include missed phone calls or inability to contact the patient, difficulty for the patient to hear the provider’s instructions over the phone, and patient unavailability when a critical INR is of concern. Office visits are beneficial in that providers can provide both written and verbal instruction to patients, perform visual or physical patient assessments, and provide timely care if needed. Disadvantages of office visits may include long wait times and inconvenience for patients who live far away.
Telephone anticoagulation clinics have been evaluated for their efficacy and cost-effectiveness in several studies.5,7,8 However, few studies are available that compare patient outcomes between office visits and telephone visits. Two prior studies comparing groups of anticoagulation patients managed by telephone or by office visit concluded that there is no difference in outcomes between the 2 management models.9,10 However, a retrospective study by Stoudenmire and colleagues examined extreme INR values (≤ 1.5 or ≥ 4.5) in each management model and found that telephone clinic patients have a significant increase in extreme INR values but no difference in AEs between the 2 management models.11
The VA North Texas Health Care System (VANTHCS) includes a major medical center, 3 outlying medical facilities, and 5 community-based outpatient clinics (CBOCs). A centralized pharmacist-managed anticoagulation clinic is used to manage more than 2,500 VANTHCS anticoagulation patients. To meet the National Patient Safety Goal measures and provide consistent management across the system, all anticoagulation patients from CBOCs and medical facilities are enrolled in the clinic.12 To facilitate access to care, many patients transitioned from office visits to telephone visits. It was essential to evaluate the transition of patients from office to telephone visits to ensure continued stability and continuity of care across both models. The objective of this study was to determine whether a difference in anticoagulation outcomes exists when patients are transitioned from office to telephone visits.
Methods
The VANTHCS anticoagulation clinic policy for office visits requires that patients arrive at the Dallas VAMC 2 hours before their appointment for INR lab draw. During the office visit, the anticoagulation pharmacist evaluates the INR and pertinent changes since the previous visit. The patient is provided verbal instructions and a written dosage adjustment card. Telephone clinic protocol is similar to office visits with a few exceptions. Any patient, regardless of INR stability, may be enrolled in the telephone clinic as long as the patient provides consent and has a working telephone with voice mail. Patients enrolled in the telephone clinic access blood draws at the nearest VA facility and are given a questionnaire that includes pertinent questions asked during an office visit. Anticoagulation pharmacists evaluate the questionnaire and INR then contact the patient within 1 business day to provide the patient with instructions. If a patient fails to answer the telephone, the anticoagulation pharmacist leaves a voicemail message.
Study Design
This retrospective study was conducted by chart review using Computerized Patient Record System (CPRS) at VANTHCS on patients who met inclusion criteria between January 1, 2011 and May 31, 2014, and it was approved by the institutional review board and research and development committee. The study included patients aged ≥ 18 years on warfarin therapy managed by the VANTHCS anticoagulation clinic who were previously managed in office visits for ≥ 180 days before the telephone transition, then in telephone visits for another ≥ 180 days. Only INR values obtained through the VANTHCS anticoagulation clinic were assessed.
Patients were excluded from the study if they were not managed by the VANTHCS anticoagulation clinic or received direct oral anticoagulants (DOACs). The INR values were excluded if they were nonclinic related INR values (ie, results reported that do not reflect management by the anticoagulation clinic), the first INR after hospitalization, or INRs obtained during the first month of initial warfarin treatment for a patient.
For all patients included in the study, demographic information, goal INR range (2 to 3 or 2.5 to 3.5), indication for warfarin therapy, and duration of warfarin therapy (defined as the first prescription filled for warfarin at the VA) were obtained. Individual INR values were obtained for each patient during the period of investigation and type of visit (office or telephone) for each INR drawn was specified. Any major bleeding or thrombotic events (bleed requiring an emergency department [ED] visit, hospitalization, vitamin K administration, blood transfusion, and/or warfarin therapy hold/discontinuation) were documented. Procedures and number of hospitalizations also during the investigation were recorded.
The primary outcomes measures evaluated INRs for time in therapeutic range (TTR) using the Rosendaal method and percentage of INRs within range.13 The therapeutic range was either 2 to 3 or 2.5 to 3.5 (the “strict range” for INR management). Because many patients fluctuate around the strict range and it is common to avoid therapy adjustment based on slightly elevated or lower values, a “nonstrict” range (1.8 to 3.2 or 2.3 to 3.7) also was evaluated.14 The secondary outcomes examined differences between the 2 management models in rates of major AEs, including thrombosis and major bleeding events as defined earlier.Frequencies, percentages, and other descriptive statistics were used to describe nominal data. A paired t test was used to compare TTR of patients transitioned from office to telephone visits. A P value of < .05 was used for statistical significance.
Results
A total of 111 patients met inclusion criteria (Table 1). Most patients were elderly males with AF or atrial flutter as their primary indication for warfarin therapy. No statistically significant difference was found for percentage INRs in strict range (56.8% in office vs 56.9% in telephone, P = .98) or TTR (65.9% in office vs 62.72% in telephone, P = .23) for patients who transitioned from office to telephone visits (Table 2). Similar results were found within the nonstrict range.
In examining safety, 5 major AEs occurred. One patient had 2 thrombotic pulmonary embolism events. This patient had a history of nonadherence with warfarin therapy. Three major bleeding events occurred (2 in the telephone group and 1 in the office group). Two bleeding events led to ED visits, and 1 event led to hospitalization. Although 43% of patients had a procedure during the study period, only a portion of patients received bridging with low-molecular-weight heparin (LMWH). None of the 3 reported bleeding events discovered during the study were associated with recent LMWH use. No events were fatal (Table 3).
Discussion
This study demonstrates that patients transitioned from office to telephone visits for warfarin management will have no significant change in their TTR. Additionally, patients had similar rates of major AEs before and after transition, although there were few events overall.
Previous research comparing anticoagulation outcomes in telephone vs office visits also has described outcomes to be similar between these 2 management models. Wittkowsky and colleagues examined 2 university-affiliated clinics to evaluate warfarin outcomes and AEs in patients in each management model (office vs telephone) and found no difference in outcomes between the 2 management models.9
Staresinic and colleagues designed a prospective study of 192 patients to evaluate TTR and AEs of the 2 management models at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin.10 This study found no difference between the 2 groups in percentage of time maintained within INR range or AEs and concluded that the telephone model was effective for anticoagulant management.
A retrospective study by Stoudenmire and colleagues evaluated office vs telephone management effects on extreme INR values (≤ 1.5 or ≥ 4.5), TTR, and AEs.11 This study found overall TTR and AEs to be similar between groups, but the telephone clinic had a 2-fold increase in extreme INR values compared with the office clinic.11
The current study differs from the previously discussed studies in that it evaluated outcomes for the same patients before and after the transition to telephone. This study did not exclude specific patients from telephone clinic. In the Wittkowsky study, patients were enrolled in the telephone clinic based on criteria such as patient disability or living long distances from the clinic.9 Additionally, in the current study, patients transitioned to telephone visits did not have scheduled office visits for anticoagulation management. In contrast, patients in the Staresinic study had routine anticoagulation office visits every 3 months, thus it was not a true telephone-only clinic.10
This study’s findings support prior studies’ findings that telephone clinics are acceptable for anticoagulation management. Furthermore, safety does not seem to be affected when transitioning patients, although there were few AEs to review. Providers can use telephone clinics to potentially decrease cost and facilitate access to care for patients.
Limitations
Patients were required to be in office and telephone for a sequential 6 months, and this may have produced selection biases toward patients who adhered to appointments and who were on long-term warfarin therapy. Many patients that were excluded from the study transitioned back and forth between the 2 management models. Due to the retrospective nature of this study, the authors were unable to control for all confounding variables. Patients also were not randomly assigned to be transitioned from office to telephone. Although a strength of this study was the limited telephone clinic selection criteria, there may be a few individual situations in which the pharmacist’s clinical judgment influenced the transition to the telephone clinic, creating selection bias.
There may be time bias present as clinical guidelines, providers, and clinic population size differed over the study period and might have influenced management. The population of VA patients was mainly elderly males; therefore, the study results may not be applicable to other populations. Last, the results of the study are reflective of the VANTHCS clinic structure and may not be applicable to other clinic designs.
Conclusion
Veterans in a pharmacist-managed anticoagulation clinic experienced the same outcomes in terms of TTR and major AEs when transitioned from the traditional face-to-face office visits to telephone visits. The study supports the safety and efficacy of transitioning patients from a pharmacist-managed anticoagulation office clinic to telephone clinic.
1. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(suppl 6):160S-198S.
2. Rudd KM, Dier JG. Comparison of two different models of anticoagulation management services with usual medical care. Pharmacotherapy. 2010;30(4):330-338.
3. Bungard TJ, Gardner L, Archer SL, et al. Evaluation of a pharmacist-managed anticoagulation clinic: improving patient care. Open Med. 2009;3(1):e16-e21.
4. Chiquette E, Amato MG, Bussey HI. Comparison of an anticoagulation clinic with usual medical care: anticoagulation control, patient outcomes, and health care costs. Arch Intern Med. 1998;158(15):1641-1647.
5. Waterman AD, Banet G, Milligan PE, et al. Patient and physician satisfaction with a telephone-based anticoagulation service. J Gen Intern Med. 2001;16(7):460-463.
6. Hasan SS, Shamala R, Syed IA, et al. Factors affecting warfarin-related knowledge and INR control of patients attending physician- and pharmacist-managed anticoagulation clinics. J Pharm Pract. 2011;24(5):485-493.
7. Hassan S, Naboush A, Radbel J, et al. Telephone-based anticoagulation management in the homebound setting: a retrospective observational study. Int J Gen Med. 2013;6:869-875.
8. Moherman LJ, Kolar MM. Complication rates for a telephone-based anticoagulation service. Am J Health Syst Pharm. 1999;56(15):1540-1542.
9. Wittkowsky AK, Nutescu EA, Blackburn J, et al. Outcomes of oral anticoagulant therapy managed by telephone vs in-office visits in an anticoagulation clinic setting. Chest. 2006;130(5):1385-1389.
10. Staresinic AG, Sorkness CA, Goodman BM, Pigarelli DW. Comparison of outcomes using 2 delivery models of anticoagulation care. Arch Intern Med. 2006;166(9):997-1002.
11. Stoudenmire LG, DeRemer CE, Elewa H. Telephone versus office-based management of warfarin: impact on international normalized ratios and outcomes. Int J Hematol. 2014;100(2):119-124.
12. The Joint Commission. National Patient Safety Goals Effective January 1, 2015. http://www.jointcommission.org/assets/1/6/2015_NPSG_AHC1.PDF. Published 2014. Accessed November 23, 2016.
13. Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236-239.
14. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(suppl 2):7S-47S.
1. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(suppl 6):160S-198S.
2. Rudd KM, Dier JG. Comparison of two different models of anticoagulation management services with usual medical care. Pharmacotherapy. 2010;30(4):330-338.
3. Bungard TJ, Gardner L, Archer SL, et al. Evaluation of a pharmacist-managed anticoagulation clinic: improving patient care. Open Med. 2009;3(1):e16-e21.
4. Chiquette E, Amato MG, Bussey HI. Comparison of an anticoagulation clinic with usual medical care: anticoagulation control, patient outcomes, and health care costs. Arch Intern Med. 1998;158(15):1641-1647.
5. Waterman AD, Banet G, Milligan PE, et al. Patient and physician satisfaction with a telephone-based anticoagulation service. J Gen Intern Med. 2001;16(7):460-463.
6. Hasan SS, Shamala R, Syed IA, et al. Factors affecting warfarin-related knowledge and INR control of patients attending physician- and pharmacist-managed anticoagulation clinics. J Pharm Pract. 2011;24(5):485-493.
7. Hassan S, Naboush A, Radbel J, et al. Telephone-based anticoagulation management in the homebound setting: a retrospective observational study. Int J Gen Med. 2013;6:869-875.
8. Moherman LJ, Kolar MM. Complication rates for a telephone-based anticoagulation service. Am J Health Syst Pharm. 1999;56(15):1540-1542.
9. Wittkowsky AK, Nutescu EA, Blackburn J, et al. Outcomes of oral anticoagulant therapy managed by telephone vs in-office visits in an anticoagulation clinic setting. Chest. 2006;130(5):1385-1389.
10. Staresinic AG, Sorkness CA, Goodman BM, Pigarelli DW. Comparison of outcomes using 2 delivery models of anticoagulation care. Arch Intern Med. 2006;166(9):997-1002.
11. Stoudenmire LG, DeRemer CE, Elewa H. Telephone versus office-based management of warfarin: impact on international normalized ratios and outcomes. Int J Hematol. 2014;100(2):119-124.
12. The Joint Commission. National Patient Safety Goals Effective January 1, 2015. http://www.jointcommission.org/assets/1/6/2015_NPSG_AHC1.PDF. Published 2014. Accessed November 23, 2016.
13. Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236-239.
14. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(suppl 2):7S-47S.
Sprint to find Zika vaccine could hinge on summer outbreaks
As warmer temperatures herald the arrival of pesky mosquitoes, researchers are feverishly working on several promising vaccines against Zika, a virus notorious for infecting humans through this insect’s bite.
The speed and debilitating effects of last year’s Zika outbreak in the Western Hemisphere prompted a sprint to develop a vaccine. Just a little more than a year after the pandemic was declared a global health emergency, a handful of candidates are undergoing preliminary testing in humans.
“On one hand, you don’t want to see outbreaks of infection,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases. “But on the other hand, [without that testing] you might have to wait a long time to make sure that the vaccine works.”
All the vaccines currently being tested are in phase I clinical trials, which means they are being tested for safety in a small number of people. According to a review paper published Tuesday in the journal Immunity, the vaccines represent a variety of scientific techniques to thwart the disease, ranging from inactivating the virus to manipulating its DNA.
The NIAID announced Tuesday it is launching yet another phase I trial for a vaccine made out of proteins found in mosquito saliva. The product is intended to trigger a human immune system response to the mosquito’s saliva and any viruses mixed with it. If successful, the product could protect humans against a spectrum of mosquito-transmitted diseases, including Zika.
Col. Nelson Michael, MD, PhD, director of the U.S. Military HIV Research Program at the Walter Reed Army Institute of Research in Silver Spring, Md., and coauthor of the paper, said he expects preliminary reports on the safety of some of the older vaccines in April. As of now, he said, it is impossible to guess which vaccine will prove most effective in providing immunity.
“Sometimes it’s difficult to predict which horse will win the race,” Michael said.
Zika – which is spread from infected people to others by mosquito bites or sexual contact, often infects people without showing symptoms. In some cases, it causes flu-like symptoms, such as fever, muscle aches and joint pain in adults – and, in rare cases, Guillain-Barré syndrome, which can cause temporary paralysis. But it is most notorious for causing some children to be born with microcephaly – a birth defect in which a child’s head is smaller than the average size – if their mothers were exposed to Zika.
The virus garnered international attention after hundreds of cases of disabled babies surfaced in Brazil. It quickly swept through South America and the Caribbean before stopping on the southern coast of the U.S.
The World Health Organization declared the outbreak a “public health emergency of international concern” on Feb. 1, 2016, then ended the alert on Nov. 18.
Vaccines that meet the safety standard in phase I clinical trials undergo subsequent rounds of testing to gauge effectiveness. To measure this, researchers rely on the gold standard of administering the vaccine to large number of individuals already exposed to the virus. However, Zika’s recent arrival to the Western Hemisphere means researchers don’t know whether the virus will become a perennial threat or a one-time explosion.
The uncertainty poses several implications for the surge in Zika vaccine development. A lull in the outbreak could cause significant delays in testing, pushing back the timetable for a commercially available product, Dr. Fauci said.
While researchers can use alternative methods to measure efficacy without large-scale testing, a decline in the circulation of the Zika virus could set progress back by years because the vaccine testing would be ineffective.
“If we don’t get a lot of infections this season in South America and Puerto Rico, it may take years to make sure the vaccine works,” he said.
Dr. Fauci expects to launch the next round of human trials for a DNA vaccine developed by the NIAID next month.
Dr. Michael also worries that a lag in the number of Zika cases could lead the private sector to pull funds from vaccine development. It takes millions of dollars to develop a drug or vaccine, and pharmaceutical companies play a critical role in making and manufacturing them, he said. But those companies have many competing interests, he noted, and if it is hard to test a vaccine this year, the public and private Zika prevention efforts may turn attention elsewhere.
“This is a constant issue where you put your resources,” Dr. Michael said.
So far, signs suggest that the climate could be ripe for Zika again this year. Warmer-than-usual temperatures are affecting areas across the Western Hemisphere, CBS reported, including hotbeds of the Zika outbreaks in Brazil. The higher temperatures increase the voracity of Zika’s main transmitter, the Aedes aegypti mosquito.
In the United States, areas with populations of the Aedes aegypti are closely monitoring their numbers. Last year, Texas and Florida dealt with locally acquired cases of Zika infection.
In Texas, public health officials have monitored mosquito populations throughout the winter to track their numbers and any presence of the virus. Despite unseasonably warm weather, said Chris Van Deusen, spokesman for the Texas Department of State Health Services, they have seen lower numbers of the Aedes aegypti and no cases of Zika.
Mr. Van Deusen said the state is also monitoring the outbreak in Mexico, since heavy traffic across the border increases the possibility of transmission. Officials are expecting another outbreak of locally transmitted cases of disease, Mr. Van Deusen said.
“There’s so many factors that go into it, it’s really impossible to make an ironclad prediction,” he said.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
As warmer temperatures herald the arrival of pesky mosquitoes, researchers are feverishly working on several promising vaccines against Zika, a virus notorious for infecting humans through this insect’s bite.
The speed and debilitating effects of last year’s Zika outbreak in the Western Hemisphere prompted a sprint to develop a vaccine. Just a little more than a year after the pandemic was declared a global health emergency, a handful of candidates are undergoing preliminary testing in humans.
“On one hand, you don’t want to see outbreaks of infection,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases. “But on the other hand, [without that testing] you might have to wait a long time to make sure that the vaccine works.”
All the vaccines currently being tested are in phase I clinical trials, which means they are being tested for safety in a small number of people. According to a review paper published Tuesday in the journal Immunity, the vaccines represent a variety of scientific techniques to thwart the disease, ranging from inactivating the virus to manipulating its DNA.
The NIAID announced Tuesday it is launching yet another phase I trial for a vaccine made out of proteins found in mosquito saliva. The product is intended to trigger a human immune system response to the mosquito’s saliva and any viruses mixed with it. If successful, the product could protect humans against a spectrum of mosquito-transmitted diseases, including Zika.
Col. Nelson Michael, MD, PhD, director of the U.S. Military HIV Research Program at the Walter Reed Army Institute of Research in Silver Spring, Md., and coauthor of the paper, said he expects preliminary reports on the safety of some of the older vaccines in April. As of now, he said, it is impossible to guess which vaccine will prove most effective in providing immunity.
“Sometimes it’s difficult to predict which horse will win the race,” Michael said.
Zika – which is spread from infected people to others by mosquito bites or sexual contact, often infects people without showing symptoms. In some cases, it causes flu-like symptoms, such as fever, muscle aches and joint pain in adults – and, in rare cases, Guillain-Barré syndrome, which can cause temporary paralysis. But it is most notorious for causing some children to be born with microcephaly – a birth defect in which a child’s head is smaller than the average size – if their mothers were exposed to Zika.
The virus garnered international attention after hundreds of cases of disabled babies surfaced in Brazil. It quickly swept through South America and the Caribbean before stopping on the southern coast of the U.S.
The World Health Organization declared the outbreak a “public health emergency of international concern” on Feb. 1, 2016, then ended the alert on Nov. 18.
Vaccines that meet the safety standard in phase I clinical trials undergo subsequent rounds of testing to gauge effectiveness. To measure this, researchers rely on the gold standard of administering the vaccine to large number of individuals already exposed to the virus. However, Zika’s recent arrival to the Western Hemisphere means researchers don’t know whether the virus will become a perennial threat or a one-time explosion.
The uncertainty poses several implications for the surge in Zika vaccine development. A lull in the outbreak could cause significant delays in testing, pushing back the timetable for a commercially available product, Dr. Fauci said.
While researchers can use alternative methods to measure efficacy without large-scale testing, a decline in the circulation of the Zika virus could set progress back by years because the vaccine testing would be ineffective.
“If we don’t get a lot of infections this season in South America and Puerto Rico, it may take years to make sure the vaccine works,” he said.
Dr. Fauci expects to launch the next round of human trials for a DNA vaccine developed by the NIAID next month.
Dr. Michael also worries that a lag in the number of Zika cases could lead the private sector to pull funds from vaccine development. It takes millions of dollars to develop a drug or vaccine, and pharmaceutical companies play a critical role in making and manufacturing them, he said. But those companies have many competing interests, he noted, and if it is hard to test a vaccine this year, the public and private Zika prevention efforts may turn attention elsewhere.
“This is a constant issue where you put your resources,” Dr. Michael said.
So far, signs suggest that the climate could be ripe for Zika again this year. Warmer-than-usual temperatures are affecting areas across the Western Hemisphere, CBS reported, including hotbeds of the Zika outbreaks in Brazil. The higher temperatures increase the voracity of Zika’s main transmitter, the Aedes aegypti mosquito.
In the United States, areas with populations of the Aedes aegypti are closely monitoring their numbers. Last year, Texas and Florida dealt with locally acquired cases of Zika infection.
In Texas, public health officials have monitored mosquito populations throughout the winter to track their numbers and any presence of the virus. Despite unseasonably warm weather, said Chris Van Deusen, spokesman for the Texas Department of State Health Services, they have seen lower numbers of the Aedes aegypti and no cases of Zika.
Mr. Van Deusen said the state is also monitoring the outbreak in Mexico, since heavy traffic across the border increases the possibility of transmission. Officials are expecting another outbreak of locally transmitted cases of disease, Mr. Van Deusen said.
“There’s so many factors that go into it, it’s really impossible to make an ironclad prediction,” he said.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
As warmer temperatures herald the arrival of pesky mosquitoes, researchers are feverishly working on several promising vaccines against Zika, a virus notorious for infecting humans through this insect’s bite.
The speed and debilitating effects of last year’s Zika outbreak in the Western Hemisphere prompted a sprint to develop a vaccine. Just a little more than a year after the pandemic was declared a global health emergency, a handful of candidates are undergoing preliminary testing in humans.
“On one hand, you don’t want to see outbreaks of infection,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases. “But on the other hand, [without that testing] you might have to wait a long time to make sure that the vaccine works.”
All the vaccines currently being tested are in phase I clinical trials, which means they are being tested for safety in a small number of people. According to a review paper published Tuesday in the journal Immunity, the vaccines represent a variety of scientific techniques to thwart the disease, ranging from inactivating the virus to manipulating its DNA.
The NIAID announced Tuesday it is launching yet another phase I trial for a vaccine made out of proteins found in mosquito saliva. The product is intended to trigger a human immune system response to the mosquito’s saliva and any viruses mixed with it. If successful, the product could protect humans against a spectrum of mosquito-transmitted diseases, including Zika.
Col. Nelson Michael, MD, PhD, director of the U.S. Military HIV Research Program at the Walter Reed Army Institute of Research in Silver Spring, Md., and coauthor of the paper, said he expects preliminary reports on the safety of some of the older vaccines in April. As of now, he said, it is impossible to guess which vaccine will prove most effective in providing immunity.
“Sometimes it’s difficult to predict which horse will win the race,” Michael said.
Zika – which is spread from infected people to others by mosquito bites or sexual contact, often infects people without showing symptoms. In some cases, it causes flu-like symptoms, such as fever, muscle aches and joint pain in adults – and, in rare cases, Guillain-Barré syndrome, which can cause temporary paralysis. But it is most notorious for causing some children to be born with microcephaly – a birth defect in which a child’s head is smaller than the average size – if their mothers were exposed to Zika.
The virus garnered international attention after hundreds of cases of disabled babies surfaced in Brazil. It quickly swept through South America and the Caribbean before stopping on the southern coast of the U.S.
The World Health Organization declared the outbreak a “public health emergency of international concern” on Feb. 1, 2016, then ended the alert on Nov. 18.
Vaccines that meet the safety standard in phase I clinical trials undergo subsequent rounds of testing to gauge effectiveness. To measure this, researchers rely on the gold standard of administering the vaccine to large number of individuals already exposed to the virus. However, Zika’s recent arrival to the Western Hemisphere means researchers don’t know whether the virus will become a perennial threat or a one-time explosion.
The uncertainty poses several implications for the surge in Zika vaccine development. A lull in the outbreak could cause significant delays in testing, pushing back the timetable for a commercially available product, Dr. Fauci said.
While researchers can use alternative methods to measure efficacy without large-scale testing, a decline in the circulation of the Zika virus could set progress back by years because the vaccine testing would be ineffective.
“If we don’t get a lot of infections this season in South America and Puerto Rico, it may take years to make sure the vaccine works,” he said.
Dr. Fauci expects to launch the next round of human trials for a DNA vaccine developed by the NIAID next month.
Dr. Michael also worries that a lag in the number of Zika cases could lead the private sector to pull funds from vaccine development. It takes millions of dollars to develop a drug or vaccine, and pharmaceutical companies play a critical role in making and manufacturing them, he said. But those companies have many competing interests, he noted, and if it is hard to test a vaccine this year, the public and private Zika prevention efforts may turn attention elsewhere.
“This is a constant issue where you put your resources,” Dr. Michael said.
So far, signs suggest that the climate could be ripe for Zika again this year. Warmer-than-usual temperatures are affecting areas across the Western Hemisphere, CBS reported, including hotbeds of the Zika outbreaks in Brazil. The higher temperatures increase the voracity of Zika’s main transmitter, the Aedes aegypti mosquito.
In the United States, areas with populations of the Aedes aegypti are closely monitoring their numbers. Last year, Texas and Florida dealt with locally acquired cases of Zika infection.
In Texas, public health officials have monitored mosquito populations throughout the winter to track their numbers and any presence of the virus. Despite unseasonably warm weather, said Chris Van Deusen, spokesman for the Texas Department of State Health Services, they have seen lower numbers of the Aedes aegypti and no cases of Zika.
Mr. Van Deusen said the state is also monitoring the outbreak in Mexico, since heavy traffic across the border increases the possibility of transmission. Officials are expecting another outbreak of locally transmitted cases of disease, Mr. Van Deusen said.
“There’s so many factors that go into it, it’s really impossible to make an ironclad prediction,” he said.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Survey highlights interest in diet’s effects on RA
Nearly one-quarter of patients with long-standing rheumatoid arthritis who participated in a recent survey reported that their diets affect their RA symptoms.
Of 217 participants with a median disease duration of 17 years, 52 (24%) reported that certain foods either improve or worsen symptoms. Foods most commonly associated with improved symptoms were blueberries (11.1%), fish (10.9%), and spinach; foods most commonly associated with exacerbated symptoms were desserts (12.7%) and soda with sugar (12.4%, ), Sara K. Tedeschi, MD, of Brigham and Women’s Hospital, Boston, and her colleagues reported online in Arthritis Care & Research.
Participants came from a single-center RA registry (the Brigham RA Sequential Study, or BRASS) at a large academic center and were surveyed between May 2015 and December 2015. They were asked about the effects of 20 different foods that have been popularized as “inflammatory” or “anti-inflammatory” and about the effects of four lifestyle/environment factors. Most (83%) were women, and 58% were using a biologic disease-modifying antirheumatic drug.
The findings indicate that there is substantial patient interest in the effects of diet on RA symptoms and highlight the need for prospective studies on the topic, the investigators concluded. While strong conclusions cannot be drawn based on this survey, further study regarding a potential link between sugar consumption and inflammation is warranted.
Dr. Tedeschi’s work on this project was supported by the National Institutes of Health. The Brigham RA Sequential Study received funding from UCB, Crescendo Biosciences, Bristol-Myers Squibb, Amgen, and DxTerity.
Nearly one-quarter of patients with long-standing rheumatoid arthritis who participated in a recent survey reported that their diets affect their RA symptoms.
Of 217 participants with a median disease duration of 17 years, 52 (24%) reported that certain foods either improve or worsen symptoms. Foods most commonly associated with improved symptoms were blueberries (11.1%), fish (10.9%), and spinach; foods most commonly associated with exacerbated symptoms were desserts (12.7%) and soda with sugar (12.4%, ), Sara K. Tedeschi, MD, of Brigham and Women’s Hospital, Boston, and her colleagues reported online in Arthritis Care & Research.
Participants came from a single-center RA registry (the Brigham RA Sequential Study, or BRASS) at a large academic center and were surveyed between May 2015 and December 2015. They were asked about the effects of 20 different foods that have been popularized as “inflammatory” or “anti-inflammatory” and about the effects of four lifestyle/environment factors. Most (83%) were women, and 58% were using a biologic disease-modifying antirheumatic drug.
The findings indicate that there is substantial patient interest in the effects of diet on RA symptoms and highlight the need for prospective studies on the topic, the investigators concluded. While strong conclusions cannot be drawn based on this survey, further study regarding a potential link between sugar consumption and inflammation is warranted.
Dr. Tedeschi’s work on this project was supported by the National Institutes of Health. The Brigham RA Sequential Study received funding from UCB, Crescendo Biosciences, Bristol-Myers Squibb, Amgen, and DxTerity.
Nearly one-quarter of patients with long-standing rheumatoid arthritis who participated in a recent survey reported that their diets affect their RA symptoms.
Of 217 participants with a median disease duration of 17 years, 52 (24%) reported that certain foods either improve or worsen symptoms. Foods most commonly associated with improved symptoms were blueberries (11.1%), fish (10.9%), and spinach; foods most commonly associated with exacerbated symptoms were desserts (12.7%) and soda with sugar (12.4%, ), Sara K. Tedeschi, MD, of Brigham and Women’s Hospital, Boston, and her colleagues reported online in Arthritis Care & Research.
Participants came from a single-center RA registry (the Brigham RA Sequential Study, or BRASS) at a large academic center and were surveyed between May 2015 and December 2015. They were asked about the effects of 20 different foods that have been popularized as “inflammatory” or “anti-inflammatory” and about the effects of four lifestyle/environment factors. Most (83%) were women, and 58% were using a biologic disease-modifying antirheumatic drug.
The findings indicate that there is substantial patient interest in the effects of diet on RA symptoms and highlight the need for prospective studies on the topic, the investigators concluded. While strong conclusions cannot be drawn based on this survey, further study regarding a potential link between sugar consumption and inflammation is warranted.
Dr. Tedeschi’s work on this project was supported by the National Institutes of Health. The Brigham RA Sequential Study received funding from UCB, Crescendo Biosciences, Bristol-Myers Squibb, Amgen, and DxTerity.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point:
Major finding: 24% of respondents reported that diet affects RA symptoms.
Data source: A survey of 217 participants in the Brigham RA Sequential Study.
Disclosures: Dr. Tedeschi’s work on this project was supported by the National Institutes of Health. The Brigham RA Sequential Study received funding from UCB, Crescendo Biosciences, Bristol-Myers Squibb, Amgen, and DxTerity.
A familiar face
A friend of mine recently fell and sustained a complex wrist fracture. She is more than a month post injury, and her forearm, with all its external hardware, looks like an 11-year-old’s science project gone horribly wrong. As she related the story of her fall, the surgery, and her recovery, she mentioned that, since the surgery, she has had four follow-up visits, none of them with the same provider.
Two visits were with nurse practitioners and two with physicians’ assistants. Each of the folks that she saw was pleasant and courteous and appeared genuinely concerned about how she was doing. From a purely economic standpoint, I can understand why a surgeon feels he can be more productive in the operating room than when he is doing follow-ups in the office. Personally, I would have preferred to have at least a quick look at my handiwork. What I found most troubling, however, was the fact that my friend’s injury hadn’t received even the smallest dose of continuity during her recovery.
Does not seeing the same provider at each visit make a difference? In my friend’s case it may have been important because it wasn’t until the last visit that she discovered that she was supposed to be wiggling her fingers. Continuity may not have prevented this oversight, but the discontinuity didn’t help.
People feel more comfortable in situations in which they see a familiar face, whether it’s a bank teller, a barber, or the person at the check-out counter in the grocery store. This calming effect of familiarity can be even more important when it comes to transmitting bad news or supporting a patient through a challenging illness.
If you find that argument for continuity a little too touchy-feely, consider it instead as an effective efficiency booster. Does it take you longer to see one of your colleague’s patients whom you may not have seen before or a 5-year-old patient you have seen several times a year since she was born? The time-saving advantage of continuity increases exponentially with the complexity of the patient’s presenting problem.
When you are seeing patients with whom you aren’t familiar, there are always those extra minutes with your eyes on the computer screen trying to get some sense of context. There are those time-gobbling ventures down therapeutic paths that are going to blind ends, simply because the patient doesn’t know you well enough to trust your advice.
These are just some of the reasons that make continuity important and why it should be one of the driving principles behind scheduling in every physician’s office. Where does continuity sit on the priority list in the practice where you work? Do providers leave enough time in their schedules to allow for same day visits and follow-ups? Are the providers flexible enough to allow their patients to see them for almost every visit?
You may agree with me on the importance of continuity, but you may also be struggling with that quality of life/professional responsibility thing. If, like an increasing number of pediatricians, you would like to work part time, but you realize that cutting back your hours also will mean that maintaining continuity with your patients will be more difficult, careful use of a mid-level provider might help soften the transition. Would 2 full days and 2 half-days a week be more continuity-friendly than 3 full days? You’d be working the same number of hours, but the first option may create the illusion that your familiar face is in the office more often than it is. Regardless of where your practice trajectory is going, don’t discount the value of continuity.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
A friend of mine recently fell and sustained a complex wrist fracture. She is more than a month post injury, and her forearm, with all its external hardware, looks like an 11-year-old’s science project gone horribly wrong. As she related the story of her fall, the surgery, and her recovery, she mentioned that, since the surgery, she has had four follow-up visits, none of them with the same provider.
Two visits were with nurse practitioners and two with physicians’ assistants. Each of the folks that she saw was pleasant and courteous and appeared genuinely concerned about how she was doing. From a purely economic standpoint, I can understand why a surgeon feels he can be more productive in the operating room than when he is doing follow-ups in the office. Personally, I would have preferred to have at least a quick look at my handiwork. What I found most troubling, however, was the fact that my friend’s injury hadn’t received even the smallest dose of continuity during her recovery.
Does not seeing the same provider at each visit make a difference? In my friend’s case it may have been important because it wasn’t until the last visit that she discovered that she was supposed to be wiggling her fingers. Continuity may not have prevented this oversight, but the discontinuity didn’t help.
People feel more comfortable in situations in which they see a familiar face, whether it’s a bank teller, a barber, or the person at the check-out counter in the grocery store. This calming effect of familiarity can be even more important when it comes to transmitting bad news or supporting a patient through a challenging illness.
If you find that argument for continuity a little too touchy-feely, consider it instead as an effective efficiency booster. Does it take you longer to see one of your colleague’s patients whom you may not have seen before or a 5-year-old patient you have seen several times a year since she was born? The time-saving advantage of continuity increases exponentially with the complexity of the patient’s presenting problem.
When you are seeing patients with whom you aren’t familiar, there are always those extra minutes with your eyes on the computer screen trying to get some sense of context. There are those time-gobbling ventures down therapeutic paths that are going to blind ends, simply because the patient doesn’t know you well enough to trust your advice.
These are just some of the reasons that make continuity important and why it should be one of the driving principles behind scheduling in every physician’s office. Where does continuity sit on the priority list in the practice where you work? Do providers leave enough time in their schedules to allow for same day visits and follow-ups? Are the providers flexible enough to allow their patients to see them for almost every visit?
You may agree with me on the importance of continuity, but you may also be struggling with that quality of life/professional responsibility thing. If, like an increasing number of pediatricians, you would like to work part time, but you realize that cutting back your hours also will mean that maintaining continuity with your patients will be more difficult, careful use of a mid-level provider might help soften the transition. Would 2 full days and 2 half-days a week be more continuity-friendly than 3 full days? You’d be working the same number of hours, but the first option may create the illusion that your familiar face is in the office more often than it is. Regardless of where your practice trajectory is going, don’t discount the value of continuity.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
A friend of mine recently fell and sustained a complex wrist fracture. She is more than a month post injury, and her forearm, with all its external hardware, looks like an 11-year-old’s science project gone horribly wrong. As she related the story of her fall, the surgery, and her recovery, she mentioned that, since the surgery, she has had four follow-up visits, none of them with the same provider.
Two visits were with nurse practitioners and two with physicians’ assistants. Each of the folks that she saw was pleasant and courteous and appeared genuinely concerned about how she was doing. From a purely economic standpoint, I can understand why a surgeon feels he can be more productive in the operating room than when he is doing follow-ups in the office. Personally, I would have preferred to have at least a quick look at my handiwork. What I found most troubling, however, was the fact that my friend’s injury hadn’t received even the smallest dose of continuity during her recovery.
Does not seeing the same provider at each visit make a difference? In my friend’s case it may have been important because it wasn’t until the last visit that she discovered that she was supposed to be wiggling her fingers. Continuity may not have prevented this oversight, but the discontinuity didn’t help.
People feel more comfortable in situations in which they see a familiar face, whether it’s a bank teller, a barber, or the person at the check-out counter in the grocery store. This calming effect of familiarity can be even more important when it comes to transmitting bad news or supporting a patient through a challenging illness.
If you find that argument for continuity a little too touchy-feely, consider it instead as an effective efficiency booster. Does it take you longer to see one of your colleague’s patients whom you may not have seen before or a 5-year-old patient you have seen several times a year since she was born? The time-saving advantage of continuity increases exponentially with the complexity of the patient’s presenting problem.
When you are seeing patients with whom you aren’t familiar, there are always those extra minutes with your eyes on the computer screen trying to get some sense of context. There are those time-gobbling ventures down therapeutic paths that are going to blind ends, simply because the patient doesn’t know you well enough to trust your advice.
These are just some of the reasons that make continuity important and why it should be one of the driving principles behind scheduling in every physician’s office. Where does continuity sit on the priority list in the practice where you work? Do providers leave enough time in their schedules to allow for same day visits and follow-ups? Are the providers flexible enough to allow their patients to see them for almost every visit?
You may agree with me on the importance of continuity, but you may also be struggling with that quality of life/professional responsibility thing. If, like an increasing number of pediatricians, you would like to work part time, but you realize that cutting back your hours also will mean that maintaining continuity with your patients will be more difficult, careful use of a mid-level provider might help soften the transition. Would 2 full days and 2 half-days a week be more continuity-friendly than 3 full days? You’d be working the same number of hours, but the first option may create the illusion that your familiar face is in the office more often than it is. Regardless of where your practice trajectory is going, don’t discount the value of continuity.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Pilot trial of first in kind biologic shows RA treatment potential
The novel biologic mavrilimumab that targets the GM-CSF pathway has shown therapeutic potential in the treatment of rheumatoid arthritis in a proof-of-concept trial, particularly in patients who have failed to respond to biologics that target other pathways.
Writing in the Annals of the Rheumatic Diseases, researchers led by Gerd R. Burmester, MD, director and professor of medicine in the department of rheumatology and clinical immunology at Charité University Hospital and the Free University and Humboldt University of Berlin, noted that despite the success of the currently available biologics to treat rheumatoid arthritis (RA), a considerable number of patients do not achieve long-term responses (Ann Rheum Dis. 2017 Feb 17. doi: 10.1136/annrheumdis-2016-210624).
Mavrilimumab is a fully human monoclonal antibody that blocks GM-CSFR and is the first biologic of its kind to target the GM-CSF pathway, they noted.
The multicenter, phase IIb, randomized trial EARTH EXPLORER 1 involved 305 patients with moderate to severe RA who were randomized in a ratio of 1:1:1:1 to subcutaneous mavrilimumab 150 mg, 100 mg, 30 mg, or placebo every other week plus methotrexate for 24 weeks.
The results showed that the GM-CSFR blocker met one of its primary endpoints by significantly reducing the 28-joint Disease Activity Score based on C-reactive protein (DAS28–CRP) from baseline to week 12 when compared with placebo. The amount of reduction increased with the dose, from –1.37 with 30 mg, to –1.64 with 100 mg, to –1.90 with 150 mg, compared with –0.68 with placebo (P less than .001 for all vs. placebo).
The biologic also met its other primary endpoint of achieving ACR20 level of response by 24 weeks in significantly more patients than with placebo: 73.4% for 150 mg, 61.2% for 100 mg, and 50.6% for 30 mg vs. 24.7% for placebo (P less than .001).
Adverse events were reported in all treatment dose groups (42%-54% of patients in each group), and no treatment-related safety signals were observed. Only one case each of pneumonia and angioedema were considered to be related to treatment by the investigators.
The research team suggested that the blocking of GM-CSF signaling could be applicable to patients who have failed treatment with biologics that target other pathways or for people with other inflammatory or autoimmune diseases.
“This proof-of-concept study confirms that inhibition of GM-CSF activity is a promising and novel therapeutic approach for patients with RA, including those who do not adequately respond to currently available therapies,” they concluded.
The study was funded by AstraZeneca/MedImmune. Several of the authors are employees of MedImmune and several reported financial ties to other pharmaceutical companies.
The novel biologic mavrilimumab that targets the GM-CSF pathway has shown therapeutic potential in the treatment of rheumatoid arthritis in a proof-of-concept trial, particularly in patients who have failed to respond to biologics that target other pathways.
Writing in the Annals of the Rheumatic Diseases, researchers led by Gerd R. Burmester, MD, director and professor of medicine in the department of rheumatology and clinical immunology at Charité University Hospital and the Free University and Humboldt University of Berlin, noted that despite the success of the currently available biologics to treat rheumatoid arthritis (RA), a considerable number of patients do not achieve long-term responses (Ann Rheum Dis. 2017 Feb 17. doi: 10.1136/annrheumdis-2016-210624).
Mavrilimumab is a fully human monoclonal antibody that blocks GM-CSFR and is the first biologic of its kind to target the GM-CSF pathway, they noted.
The multicenter, phase IIb, randomized trial EARTH EXPLORER 1 involved 305 patients with moderate to severe RA who were randomized in a ratio of 1:1:1:1 to subcutaneous mavrilimumab 150 mg, 100 mg, 30 mg, or placebo every other week plus methotrexate for 24 weeks.
The results showed that the GM-CSFR blocker met one of its primary endpoints by significantly reducing the 28-joint Disease Activity Score based on C-reactive protein (DAS28–CRP) from baseline to week 12 when compared with placebo. The amount of reduction increased with the dose, from –1.37 with 30 mg, to –1.64 with 100 mg, to –1.90 with 150 mg, compared with –0.68 with placebo (P less than .001 for all vs. placebo).
The biologic also met its other primary endpoint of achieving ACR20 level of response by 24 weeks in significantly more patients than with placebo: 73.4% for 150 mg, 61.2% for 100 mg, and 50.6% for 30 mg vs. 24.7% for placebo (P less than .001).
Adverse events were reported in all treatment dose groups (42%-54% of patients in each group), and no treatment-related safety signals were observed. Only one case each of pneumonia and angioedema were considered to be related to treatment by the investigators.
The research team suggested that the blocking of GM-CSF signaling could be applicable to patients who have failed treatment with biologics that target other pathways or for people with other inflammatory or autoimmune diseases.
“This proof-of-concept study confirms that inhibition of GM-CSF activity is a promising and novel therapeutic approach for patients with RA, including those who do not adequately respond to currently available therapies,” they concluded.
The study was funded by AstraZeneca/MedImmune. Several of the authors are employees of MedImmune and several reported financial ties to other pharmaceutical companies.
The novel biologic mavrilimumab that targets the GM-CSF pathway has shown therapeutic potential in the treatment of rheumatoid arthritis in a proof-of-concept trial, particularly in patients who have failed to respond to biologics that target other pathways.
Writing in the Annals of the Rheumatic Diseases, researchers led by Gerd R. Burmester, MD, director and professor of medicine in the department of rheumatology and clinical immunology at Charité University Hospital and the Free University and Humboldt University of Berlin, noted that despite the success of the currently available biologics to treat rheumatoid arthritis (RA), a considerable number of patients do not achieve long-term responses (Ann Rheum Dis. 2017 Feb 17. doi: 10.1136/annrheumdis-2016-210624).
Mavrilimumab is a fully human monoclonal antibody that blocks GM-CSFR and is the first biologic of its kind to target the GM-CSF pathway, they noted.
The multicenter, phase IIb, randomized trial EARTH EXPLORER 1 involved 305 patients with moderate to severe RA who were randomized in a ratio of 1:1:1:1 to subcutaneous mavrilimumab 150 mg, 100 mg, 30 mg, or placebo every other week plus methotrexate for 24 weeks.
The results showed that the GM-CSFR blocker met one of its primary endpoints by significantly reducing the 28-joint Disease Activity Score based on C-reactive protein (DAS28–CRP) from baseline to week 12 when compared with placebo. The amount of reduction increased with the dose, from –1.37 with 30 mg, to –1.64 with 100 mg, to –1.90 with 150 mg, compared with –0.68 with placebo (P less than .001 for all vs. placebo).
The biologic also met its other primary endpoint of achieving ACR20 level of response by 24 weeks in significantly more patients than with placebo: 73.4% for 150 mg, 61.2% for 100 mg, and 50.6% for 30 mg vs. 24.7% for placebo (P less than .001).
Adverse events were reported in all treatment dose groups (42%-54% of patients in each group), and no treatment-related safety signals were observed. Only one case each of pneumonia and angioedema were considered to be related to treatment by the investigators.
The research team suggested that the blocking of GM-CSF signaling could be applicable to patients who have failed treatment with biologics that target other pathways or for people with other inflammatory or autoimmune diseases.
“This proof-of-concept study confirms that inhibition of GM-CSF activity is a promising and novel therapeutic approach for patients with RA, including those who do not adequately respond to currently available therapies,” they concluded.
The study was funded by AstraZeneca/MedImmune. Several of the authors are employees of MedImmune and several reported financial ties to other pharmaceutical companies.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: Mavrilimumab significantly reduced DAS28–CRP scores from baseline to week 12 when compared with placebo, and the mount of reduction increased with the dose, from –1.37 with 30 mg, to –1.64 with 100 mg, to –1.90 with 150 mg, compared with –0.68 with placebo (P less than .001 for all vs. placebo).
Data source: A multicenter, randomized, double-blind, placebo-controlled, phase IIb trial of 305 RA patients with moderate to severe RA.
Disclosures: The study was funded by AstraZeneca/MedImmune. Several of the authors are employees of MedImmune and several reported financial ties to other pharmaceutical companies.
Prolonged dual-antiplatelet therapy after PCI challenged
WASHINGTON – Guidelines were recently modified to permit shorter duration of dual-antiplatelet therapy (DAPT) after percutaneous coronary intervention, but a series of ongoing trials are evaluating whether DAPT can be abandoned altogether in many if not most percutaneous coronary intervention (PCI) patients, according to a review of this major potential change in direction presented at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
“The 1-year duration of dual-antiplatelet therapy post PCI with a drug eluting stent is based on anecdotal historical data,” asserted Patrick W. Serruys, MD, PhD, professor of cardiology, Imperial College, London. Citing several sets of data consistent with the conclusion that single agents provide adequate protection against thrombus formation but reduced risk of bleeding relative to DAPT, he suggested that it is now critical to challenge the old standard.
It has long been understood that greater protection against thrombus formation with more aggressive antiplatelet therapy is purchased with a higher risk of bleeding, but there appears to be a fundamental change in orientation. Several new pieces of evidence, including data showing that shorter duration of DAPT is as good as longer duration, has placed this trade-off in doubt at least over the longer term.
To some degree, the current standard was based on the premise that thrombotic events are more important than bleeding events, according to Usman Baber, MD, assistant professor of cardiology, Icahn School of Medicine at Mount Sinai, New York. He said, “That thought process really dominated thinking for many years, but this is completely unsupported by the data.” Instead, he noted that hazard ratios after thrombotic and bleeding events are almost identical, but the risk of death after bleeding is more persistent, while risk of ischemic events typically diminishes after an initial peak.
There is no shortage of studies that have attempted to determine the ideal combination and duration of antiplatelet therapies after PCI, but the heterogeneity of study design has prohibited definitive conclusions. In particular, Dr. Serruys suggested that there is no level 1 evidence confirming the value of adding aspirin, which he emphasized has a relatively nonspecific effect, over that of P2Y12 inhibitor alone.
In the design phase of the GLOBAL LEADERS trial, Dr. Serruys recounted, he first argued for a design in which aspirin was eliminated altogether and then for a protocol with only a single week of aspirin, but was met with strong objections each time. In the end, the experimental protocol calls for 1 month of aspirin plus ticagrelor before patients are continued on ticagrelor alone. This is being compared with the current standard, which is aspirin plus ticagrelor or clopidogrel for 12 months followed by another 12 months of aspirin alone.
GLOBAL LEADERS is an all-comers trial in which patients are randomized before PCI. All patients at the 131 participating centers in 18 countries are receiving the same stent (BioMatrix Flex). The primary endpoint is all-cause mortality, and enrollment is completed. The results are expected in November of this year.
There are numerous other studies addressing the same question. Like GLOBAL LEADERS, the TWILIGHT trial is also investigator-initiated and is near the halfway mark for a 9,000-patient enrollment. In this study, patients are being randomized to aspirin plus ticagrelor or ticagrelor alone after they have achieved a successful placement of a drug-eluting stent. This trial, however, is restricted to those with diabetes, chronic kidney disease, or other high-risk features. The primary endpoint is major bleeding. Completion is expected in 2019.
The SMART-CHOICE trial is enrolling roughly 5,000 PCI patients receiving a drug-eluting stent. Patients are being randomized to a P2Y12 antagonist monotherapy plus aspirin or the P2Y12 antagonist alone. The primary endpoint is a composite of major adverse cardiovascular events as well as major bleeding events.
After the STOP DAPT trial showed that 3 months of DAPT after PCI was as safe as prolonged DAPT in patients receiving a everolimus-eluting chromium-cobalt stent (Cardiovasc Interv Ther. 2016;31:196-209), the same group of Japanese investigators conceived the STOP-DAPT2 trial. In this trial, 3,000 patients are being randomized a standard DAPT or clopidogrel monotherapy beginning 1 month after PCI. The primary outcome is similar to that of SMART-CHOICE.
In yet another trial cited by Dr. Serruys, patients will receive DAPT only if the PCI outcome is considered suboptimal. For those judged to have a good result, patients will receive ticagrelor alone. Outcomes at the end of 1 year will be monitored.
The movement toward antiplatelet monotherapy is driven by recognition that “the need to mitigate the risk of bleeding is an important as the need to mitigate thrombosis,” Dr. Baber explained. Like Dr. Serruys, he believes it is important to challenge the standard.
“By testing single, specific, and potent antiplatelet therapy and getting rid of the old and nonspecific platelet drug called acetylsalicylic acid, we may be able to simplify risk management after PCI,” agreed Dr. Serruys. If, as expected, the GLOBAL LEADERS and other monotherapy antiplatelet trials meet their endpoints, it will mean a major evolution in postprocedural risk management.
Dr. Serruys reported no financial relationships to disclose.
WASHINGTON – Guidelines were recently modified to permit shorter duration of dual-antiplatelet therapy (DAPT) after percutaneous coronary intervention, but a series of ongoing trials are evaluating whether DAPT can be abandoned altogether in many if not most percutaneous coronary intervention (PCI) patients, according to a review of this major potential change in direction presented at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
“The 1-year duration of dual-antiplatelet therapy post PCI with a drug eluting stent is based on anecdotal historical data,” asserted Patrick W. Serruys, MD, PhD, professor of cardiology, Imperial College, London. Citing several sets of data consistent with the conclusion that single agents provide adequate protection against thrombus formation but reduced risk of bleeding relative to DAPT, he suggested that it is now critical to challenge the old standard.
It has long been understood that greater protection against thrombus formation with more aggressive antiplatelet therapy is purchased with a higher risk of bleeding, but there appears to be a fundamental change in orientation. Several new pieces of evidence, including data showing that shorter duration of DAPT is as good as longer duration, has placed this trade-off in doubt at least over the longer term.
To some degree, the current standard was based on the premise that thrombotic events are more important than bleeding events, according to Usman Baber, MD, assistant professor of cardiology, Icahn School of Medicine at Mount Sinai, New York. He said, “That thought process really dominated thinking for many years, but this is completely unsupported by the data.” Instead, he noted that hazard ratios after thrombotic and bleeding events are almost identical, but the risk of death after bleeding is more persistent, while risk of ischemic events typically diminishes after an initial peak.
There is no shortage of studies that have attempted to determine the ideal combination and duration of antiplatelet therapies after PCI, but the heterogeneity of study design has prohibited definitive conclusions. In particular, Dr. Serruys suggested that there is no level 1 evidence confirming the value of adding aspirin, which he emphasized has a relatively nonspecific effect, over that of P2Y12 inhibitor alone.
In the design phase of the GLOBAL LEADERS trial, Dr. Serruys recounted, he first argued for a design in which aspirin was eliminated altogether and then for a protocol with only a single week of aspirin, but was met with strong objections each time. In the end, the experimental protocol calls for 1 month of aspirin plus ticagrelor before patients are continued on ticagrelor alone. This is being compared with the current standard, which is aspirin plus ticagrelor or clopidogrel for 12 months followed by another 12 months of aspirin alone.
GLOBAL LEADERS is an all-comers trial in which patients are randomized before PCI. All patients at the 131 participating centers in 18 countries are receiving the same stent (BioMatrix Flex). The primary endpoint is all-cause mortality, and enrollment is completed. The results are expected in November of this year.
There are numerous other studies addressing the same question. Like GLOBAL LEADERS, the TWILIGHT trial is also investigator-initiated and is near the halfway mark for a 9,000-patient enrollment. In this study, patients are being randomized to aspirin plus ticagrelor or ticagrelor alone after they have achieved a successful placement of a drug-eluting stent. This trial, however, is restricted to those with diabetes, chronic kidney disease, or other high-risk features. The primary endpoint is major bleeding. Completion is expected in 2019.
The SMART-CHOICE trial is enrolling roughly 5,000 PCI patients receiving a drug-eluting stent. Patients are being randomized to a P2Y12 antagonist monotherapy plus aspirin or the P2Y12 antagonist alone. The primary endpoint is a composite of major adverse cardiovascular events as well as major bleeding events.
After the STOP DAPT trial showed that 3 months of DAPT after PCI was as safe as prolonged DAPT in patients receiving a everolimus-eluting chromium-cobalt stent (Cardiovasc Interv Ther. 2016;31:196-209), the same group of Japanese investigators conceived the STOP-DAPT2 trial. In this trial, 3,000 patients are being randomized a standard DAPT or clopidogrel monotherapy beginning 1 month after PCI. The primary outcome is similar to that of SMART-CHOICE.
In yet another trial cited by Dr. Serruys, patients will receive DAPT only if the PCI outcome is considered suboptimal. For those judged to have a good result, patients will receive ticagrelor alone. Outcomes at the end of 1 year will be monitored.
The movement toward antiplatelet monotherapy is driven by recognition that “the need to mitigate the risk of bleeding is an important as the need to mitigate thrombosis,” Dr. Baber explained. Like Dr. Serruys, he believes it is important to challenge the standard.
“By testing single, specific, and potent antiplatelet therapy and getting rid of the old and nonspecific platelet drug called acetylsalicylic acid, we may be able to simplify risk management after PCI,” agreed Dr. Serruys. If, as expected, the GLOBAL LEADERS and other monotherapy antiplatelet trials meet their endpoints, it will mean a major evolution in postprocedural risk management.
Dr. Serruys reported no financial relationships to disclose.
WASHINGTON – Guidelines were recently modified to permit shorter duration of dual-antiplatelet therapy (DAPT) after percutaneous coronary intervention, but a series of ongoing trials are evaluating whether DAPT can be abandoned altogether in many if not most percutaneous coronary intervention (PCI) patients, according to a review of this major potential change in direction presented at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
“The 1-year duration of dual-antiplatelet therapy post PCI with a drug eluting stent is based on anecdotal historical data,” asserted Patrick W. Serruys, MD, PhD, professor of cardiology, Imperial College, London. Citing several sets of data consistent with the conclusion that single agents provide adequate protection against thrombus formation but reduced risk of bleeding relative to DAPT, he suggested that it is now critical to challenge the old standard.
It has long been understood that greater protection against thrombus formation with more aggressive antiplatelet therapy is purchased with a higher risk of bleeding, but there appears to be a fundamental change in orientation. Several new pieces of evidence, including data showing that shorter duration of DAPT is as good as longer duration, has placed this trade-off in doubt at least over the longer term.
To some degree, the current standard was based on the premise that thrombotic events are more important than bleeding events, according to Usman Baber, MD, assistant professor of cardiology, Icahn School of Medicine at Mount Sinai, New York. He said, “That thought process really dominated thinking for many years, but this is completely unsupported by the data.” Instead, he noted that hazard ratios after thrombotic and bleeding events are almost identical, but the risk of death after bleeding is more persistent, while risk of ischemic events typically diminishes after an initial peak.
There is no shortage of studies that have attempted to determine the ideal combination and duration of antiplatelet therapies after PCI, but the heterogeneity of study design has prohibited definitive conclusions. In particular, Dr. Serruys suggested that there is no level 1 evidence confirming the value of adding aspirin, which he emphasized has a relatively nonspecific effect, over that of P2Y12 inhibitor alone.
In the design phase of the GLOBAL LEADERS trial, Dr. Serruys recounted, he first argued for a design in which aspirin was eliminated altogether and then for a protocol with only a single week of aspirin, but was met with strong objections each time. In the end, the experimental protocol calls for 1 month of aspirin plus ticagrelor before patients are continued on ticagrelor alone. This is being compared with the current standard, which is aspirin plus ticagrelor or clopidogrel for 12 months followed by another 12 months of aspirin alone.
GLOBAL LEADERS is an all-comers trial in which patients are randomized before PCI. All patients at the 131 participating centers in 18 countries are receiving the same stent (BioMatrix Flex). The primary endpoint is all-cause mortality, and enrollment is completed. The results are expected in November of this year.
There are numerous other studies addressing the same question. Like GLOBAL LEADERS, the TWILIGHT trial is also investigator-initiated and is near the halfway mark for a 9,000-patient enrollment. In this study, patients are being randomized to aspirin plus ticagrelor or ticagrelor alone after they have achieved a successful placement of a drug-eluting stent. This trial, however, is restricted to those with diabetes, chronic kidney disease, or other high-risk features. The primary endpoint is major bleeding. Completion is expected in 2019.
The SMART-CHOICE trial is enrolling roughly 5,000 PCI patients receiving a drug-eluting stent. Patients are being randomized to a P2Y12 antagonist monotherapy plus aspirin or the P2Y12 antagonist alone. The primary endpoint is a composite of major adverse cardiovascular events as well as major bleeding events.
After the STOP DAPT trial showed that 3 months of DAPT after PCI was as safe as prolonged DAPT in patients receiving a everolimus-eluting chromium-cobalt stent (Cardiovasc Interv Ther. 2016;31:196-209), the same group of Japanese investigators conceived the STOP-DAPT2 trial. In this trial, 3,000 patients are being randomized a standard DAPT or clopidogrel monotherapy beginning 1 month after PCI. The primary outcome is similar to that of SMART-CHOICE.
In yet another trial cited by Dr. Serruys, patients will receive DAPT only if the PCI outcome is considered suboptimal. For those judged to have a good result, patients will receive ticagrelor alone. Outcomes at the end of 1 year will be monitored.
The movement toward antiplatelet monotherapy is driven by recognition that “the need to mitigate the risk of bleeding is an important as the need to mitigate thrombosis,” Dr. Baber explained. Like Dr. Serruys, he believes it is important to challenge the standard.
“By testing single, specific, and potent antiplatelet therapy and getting rid of the old and nonspecific platelet drug called acetylsalicylic acid, we may be able to simplify risk management after PCI,” agreed Dr. Serruys. If, as expected, the GLOBAL LEADERS and other monotherapy antiplatelet trials meet their endpoints, it will mean a major evolution in postprocedural risk management.
Dr. Serruys reported no financial relationships to disclose.
EXPERT ANALYSIS FROM CRT 2017
Single BEP adjuvant chemotherapy cycle ‘sufficient’ in testicular cancer
ORLANDO – Although two cycles of bleomycin-etoposide-cisplatin (BEP) chemotherapy typically comprise adjuvant treatment for nonseminomatous or combined germ cell testicular cancer, investigators suggest one cycle may be sufficient.
“Over recent years, evidence has begun to accumulate that one single cycle of BEP may be sufficient to reduce the recurrence rate to below 5%,” said Robert Anthony Huddart, MBBS, MRCP, FRCR, PhD, of the Institute of Cancer Research in Sutton, England. “If this is confirmed, it means we could have a similar cure rate to two cycles of lower-dose BEP and that would reduce the overall burden of chemotherapy and health care resource usage.”
Dr. Huddart and his coauthors assessed 246 people from 33 centers in the United Kingdom. The patients had stage I nonseminomatous or combined germ cell testicular cancer. Instead of the standard two cycles of lower-dose etoposide regimen, BE360P post orchidectomy, the investigators administered one dose of BE500P and then followed patients for a mean 39 months.
The primary endpoint of the single-arm study was malignant recurrence at 2 years. Some patients will recur with acute, undifferentiated disease at multiple sites, often with rising markers; the treatment for these patients is further chemotherapy, Dr. Huddart explained. In contrast, other patients present with differentiated teratomas, usually in the peritoneum; they tend to be marker negative and candidates for surgical resection.
Malignant recurrence rate
“The headline result for the study is … we had three malignant recurrences, for a 1.3% rate, with an upper confidence limit of 4% – below our 5% target,” Dr. Huddart said at the 2017 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology. “We also had three patients – or 1.3% – who presented with differential teratoma[s], so the overall event rate was 2.6% at 2 years.”
“This adjuvant chemotherapy is highly successful in reducing recurrence, with a 2-year recurrence-free rate of 98%. And it does avoid the need for intensive surveillance,” Dr. Huddart said.
“This paper demonstrates that single-cycle treatment is safe and effective and two cycles are unnecessary,” said study discussant Noel W. Clarke, MBBS, FRCS, of the Christie Hospital NHS Foundation Trust in Manchester, England. “So why don’t we give all patients this kind of treatment? It doesn’t come without toxicity,” he said.
Adverse events
Myelosuppression comprised the majority of grade 3 and 4 acute toxicities in the study, which “is what you would expect with BEP chemotherapy,” Dr. Huddart said. The most common adverse events were neutropenia, leukopenia, and febrile neutropenia. He pointed out that the febrile neutropenia rate “was a low 6.4%.” A total of 104 patients experienced any toxicity.
A meeting attendee asked why the investigators did not consider a lower dose of etoposide in the study. Dr. Huddart replied, “We used a standard dose BEP because etoposide reduction might reduce the toxicity, but it might also reduce the efficacy.”
In terms of overall survival, three participants died during the study. One presented 6 months after adjuvant chemotherapy with a large, intra-abdominal recurrence; another patient died from a secondary primary lung cancer; and the third from a drug overdose.
In addition to the 2-year primary outcomes, by 4 years, another malignancy recurrence occurred, for a cumulative rate of 1.8%; including teratomas, the all-recurrence rate increased to 3.1%. “Therefore, we can successfully demonstrate the rate of malignancy after one cycle of BEP is less than 5%,” Dr. Huddart said.
Participants were aged 16 years and older with clinical stage I disease, including both mixed or pure nonseminomatous germ cell tumors. Patients also received two prophylactic agents. “We were keen to reduce the incidence of neutropenic sepsis and gave propped up G-CSF [granulocyte-colony stimulating factor] and propped up antibiotics,” Dr. Huddart said.
“This is a new treatment approach for an uncommon cancer,” Dr. Huddart said. Adoption of the protocol would reduce the overall exposure to chemotherapy in a young patient population, he added. “Just 20% of patients were [older than] age 40.”
ORLANDO – Although two cycles of bleomycin-etoposide-cisplatin (BEP) chemotherapy typically comprise adjuvant treatment for nonseminomatous or combined germ cell testicular cancer, investigators suggest one cycle may be sufficient.
“Over recent years, evidence has begun to accumulate that one single cycle of BEP may be sufficient to reduce the recurrence rate to below 5%,” said Robert Anthony Huddart, MBBS, MRCP, FRCR, PhD, of the Institute of Cancer Research in Sutton, England. “If this is confirmed, it means we could have a similar cure rate to two cycles of lower-dose BEP and that would reduce the overall burden of chemotherapy and health care resource usage.”
Dr. Huddart and his coauthors assessed 246 people from 33 centers in the United Kingdom. The patients had stage I nonseminomatous or combined germ cell testicular cancer. Instead of the standard two cycles of lower-dose etoposide regimen, BE360P post orchidectomy, the investigators administered one dose of BE500P and then followed patients for a mean 39 months.
The primary endpoint of the single-arm study was malignant recurrence at 2 years. Some patients will recur with acute, undifferentiated disease at multiple sites, often with rising markers; the treatment for these patients is further chemotherapy, Dr. Huddart explained. In contrast, other patients present with differentiated teratomas, usually in the peritoneum; they tend to be marker negative and candidates for surgical resection.
Malignant recurrence rate
“The headline result for the study is … we had three malignant recurrences, for a 1.3% rate, with an upper confidence limit of 4% – below our 5% target,” Dr. Huddart said at the 2017 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology. “We also had three patients – or 1.3% – who presented with differential teratoma[s], so the overall event rate was 2.6% at 2 years.”
“This adjuvant chemotherapy is highly successful in reducing recurrence, with a 2-year recurrence-free rate of 98%. And it does avoid the need for intensive surveillance,” Dr. Huddart said.
“This paper demonstrates that single-cycle treatment is safe and effective and two cycles are unnecessary,” said study discussant Noel W. Clarke, MBBS, FRCS, of the Christie Hospital NHS Foundation Trust in Manchester, England. “So why don’t we give all patients this kind of treatment? It doesn’t come without toxicity,” he said.
Adverse events
Myelosuppression comprised the majority of grade 3 and 4 acute toxicities in the study, which “is what you would expect with BEP chemotherapy,” Dr. Huddart said. The most common adverse events were neutropenia, leukopenia, and febrile neutropenia. He pointed out that the febrile neutropenia rate “was a low 6.4%.” A total of 104 patients experienced any toxicity.
A meeting attendee asked why the investigators did not consider a lower dose of etoposide in the study. Dr. Huddart replied, “We used a standard dose BEP because etoposide reduction might reduce the toxicity, but it might also reduce the efficacy.”
In terms of overall survival, three participants died during the study. One presented 6 months after adjuvant chemotherapy with a large, intra-abdominal recurrence; another patient died from a secondary primary lung cancer; and the third from a drug overdose.
In addition to the 2-year primary outcomes, by 4 years, another malignancy recurrence occurred, for a cumulative rate of 1.8%; including teratomas, the all-recurrence rate increased to 3.1%. “Therefore, we can successfully demonstrate the rate of malignancy after one cycle of BEP is less than 5%,” Dr. Huddart said.
Participants were aged 16 years and older with clinical stage I disease, including both mixed or pure nonseminomatous germ cell tumors. Patients also received two prophylactic agents. “We were keen to reduce the incidence of neutropenic sepsis and gave propped up G-CSF [granulocyte-colony stimulating factor] and propped up antibiotics,” Dr. Huddart said.
“This is a new treatment approach for an uncommon cancer,” Dr. Huddart said. Adoption of the protocol would reduce the overall exposure to chemotherapy in a young patient population, he added. “Just 20% of patients were [older than] age 40.”
ORLANDO – Although two cycles of bleomycin-etoposide-cisplatin (BEP) chemotherapy typically comprise adjuvant treatment for nonseminomatous or combined germ cell testicular cancer, investigators suggest one cycle may be sufficient.
“Over recent years, evidence has begun to accumulate that one single cycle of BEP may be sufficient to reduce the recurrence rate to below 5%,” said Robert Anthony Huddart, MBBS, MRCP, FRCR, PhD, of the Institute of Cancer Research in Sutton, England. “If this is confirmed, it means we could have a similar cure rate to two cycles of lower-dose BEP and that would reduce the overall burden of chemotherapy and health care resource usage.”
Dr. Huddart and his coauthors assessed 246 people from 33 centers in the United Kingdom. The patients had stage I nonseminomatous or combined germ cell testicular cancer. Instead of the standard two cycles of lower-dose etoposide regimen, BE360P post orchidectomy, the investigators administered one dose of BE500P and then followed patients for a mean 39 months.
The primary endpoint of the single-arm study was malignant recurrence at 2 years. Some patients will recur with acute, undifferentiated disease at multiple sites, often with rising markers; the treatment for these patients is further chemotherapy, Dr. Huddart explained. In contrast, other patients present with differentiated teratomas, usually in the peritoneum; they tend to be marker negative and candidates for surgical resection.
Malignant recurrence rate
“The headline result for the study is … we had three malignant recurrences, for a 1.3% rate, with an upper confidence limit of 4% – below our 5% target,” Dr. Huddart said at the 2017 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology. “We also had three patients – or 1.3% – who presented with differential teratoma[s], so the overall event rate was 2.6% at 2 years.”
“This adjuvant chemotherapy is highly successful in reducing recurrence, with a 2-year recurrence-free rate of 98%. And it does avoid the need for intensive surveillance,” Dr. Huddart said.
“This paper demonstrates that single-cycle treatment is safe and effective and two cycles are unnecessary,” said study discussant Noel W. Clarke, MBBS, FRCS, of the Christie Hospital NHS Foundation Trust in Manchester, England. “So why don’t we give all patients this kind of treatment? It doesn’t come without toxicity,” he said.
Adverse events
Myelosuppression comprised the majority of grade 3 and 4 acute toxicities in the study, which “is what you would expect with BEP chemotherapy,” Dr. Huddart said. The most common adverse events were neutropenia, leukopenia, and febrile neutropenia. He pointed out that the febrile neutropenia rate “was a low 6.4%.” A total of 104 patients experienced any toxicity.
A meeting attendee asked why the investigators did not consider a lower dose of etoposide in the study. Dr. Huddart replied, “We used a standard dose BEP because etoposide reduction might reduce the toxicity, but it might also reduce the efficacy.”
In terms of overall survival, three participants died during the study. One presented 6 months after adjuvant chemotherapy with a large, intra-abdominal recurrence; another patient died from a secondary primary lung cancer; and the third from a drug overdose.
In addition to the 2-year primary outcomes, by 4 years, another malignancy recurrence occurred, for a cumulative rate of 1.8%; including teratomas, the all-recurrence rate increased to 3.1%. “Therefore, we can successfully demonstrate the rate of malignancy after one cycle of BEP is less than 5%,” Dr. Huddart said.
Participants were aged 16 years and older with clinical stage I disease, including both mixed or pure nonseminomatous germ cell tumors. Patients also received two prophylactic agents. “We were keen to reduce the incidence of neutropenic sepsis and gave propped up G-CSF [granulocyte-colony stimulating factor] and propped up antibiotics,” Dr. Huddart said.
“This is a new treatment approach for an uncommon cancer,” Dr. Huddart said. Adoption of the protocol would reduce the overall exposure to chemotherapy in a young patient population, he added. “Just 20% of patients were [older than] age 40.”
AT THE GENITOURINARY CANCERS SYMPOSIUM
Key clinical point: A single cycle of adjuvant BEP looks promising in testicular cancer.
Major finding: A malignant recurrence rate of 1.3% at 2 years following a single cycle of BEP.
Data source: Multicenter study of 246 men with stage I nonseminomatous or combined germ cell testicular cancer.
Disclosures: The Institute of Cancer Research UK and the Queen Elizabeth Hospital Birmingham funded the study. Dr. Huddart is a consultant/adviser for Astellas Pharma and Merck Sharp & Dohme. He also receives research funding from Janssen, Lilly, and Roche.