User login
Precision and Accuracy of Identification of Anatomical Surface Landmarks by 30 Expert Hip Arthroscopists
Take-Home Points
- Surface landmarks are routinely used for physical examination and surgical technique.
- Common surface landmarks used in establishing arthroscopic portals may be more difficult to accurately identify than previously thought.
- The greater trochanter was the surface landmark most precisely identified by expert examiners.
- Ultrasound examination identified landmarks varied from landmarks identified by palpation alone.
Anatomical surface landmarks about the hip and lower abdomen are often referenced when placing arthroscopic portals and office-based injections.1-3 However, the degree to which these landmarks can be reproducibly identified using only visual inspection and palpation is unknown.
Safe access to the hip joint and surrounding structures during hip arthroscopy has been a focus in the orthopedic literature. Authors have described anatomical relationships of recommended portals to neurovascular and other anatomical structures.4-6 This information has been reported in millimeters to centimeters of safety based on cadaver dissection studies.4-7We conducted a study to assess expert hip arthroscopists’ ability to identify, using only physical examination techniques, the anatomical structures used for reference when creating safe starting points for arthroscopic access. We hypothesized that variance in examiner-identified points would exceed safe distances from neurovascular structures for the most commonly used hip arthroscopic portals. The volunteer in this study provided written informed consent for print and electronic publication of this article.
Methods
In this study, we prospectively assessed 30 expert hip arthroscopic surgeons’ ability to identify commonly referenced surface landmarks on the adult male hip, using only inspection and manual palpation. Surgeons were defined as experts on the basis of their status as hip arthroscopy instructors at the Orthopaedic Learning Center (Rosemont, IL) for the Arthroscopy Association of North America and industry-sponsored hip arthroscopy education faculty (Arthrex). Five surface landmarks were selected for their relevance to publications on safe portal placement2-5: anterior superior iliac spine (ASIS), tip of greater trochanter (GT), rectus origin (RO), superficial inguinal ring (SIR), and psoas tendon (PT).
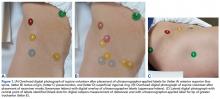
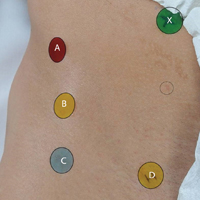
A healthy adult male volunteer was placed supine on an examination table and exposed distally from the mid abdomen, with the perineum and the genital area covered bikini-style. An expert musculoskeletal ultrasonographer used a handheld musculoskeletal ultrasound transducer (Sonosite) to identify the 5 landmarks. Short- and long-axis images of each structure were obtained. The examiner applied a round (1 cm in diameter), uniquely colored adhesive label to the skin over each location. A professional photographer using a Canon digital camera and fixed mounts made precise overhead and lateral images. The positional integrity and scale of these images were confirmed with referral to constant anatomical skin features. Images were archived for analysis (Figure 1A).
After the ultrasonographer’s labels were removed, each of the 30 expert hip arthroscopic surgeons identified the structures by static physical examination (inspection and palpation only) and applied the same colored labels to the skin.
Imaging software (Adobe Photoshop Creative Suite 5.1) was used to superimpose the digital images of the examiner labels on those of the ultrasound-verified anatomical labels (Figure 1C). Measurements were then taken with digital calipers to determine average distance from ultrasound label; accuracy within 10 mm of verified ultrasound label; true average location (TAL) determined by 95% confidence interval (CI); and interobserver variability calculated by 95% prediction interval, which determined the probability of where an additional examiner data point would lie.
In the second arm of the study, examiner data were compared with previously published data on arthroscopic portal safety.
Results
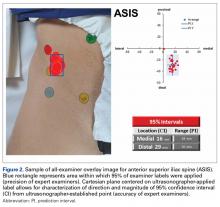
Average absolute distance from examiner labels to ultrasonographer labels was 31 mm for ASIS, 24 mm for GT, 26 mm for RO, 19 mm for SIR, and 35 mm for PT (Figure 2).
Of the 30 surgeons, 1 (3%) came within 10 mm of the ultrasound for ASIS, 1 (3%) for GT, 4 (13%) for RO, 5 (17%) for SIR, and 1 (3%) for PT (Table 1).
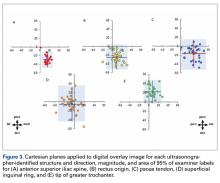
TAL as determined by CI was 16 mm medial and 29 mm inferior for ASIS; 8 mm anterior and 22 mm superior for GT; 10 mm medial and 25 mm inferior for RO; 5 mm lateral and 5 mm inferior for SIR; and 28 mm medial and 16 mm inferior for PT (Figure 3, Table 2). Interobserver variability determined by prediction interval had a range of 18 mm medial to lateral × 36 mm proximal to distal for ASIS; 33 mm anterior to posterior × 48 mm superior to inferior for GT; 41 mm medial to distal × 54 mm proximal to distal for RO; 51 mm medial to lateral × 74 mm proximal to distal for SIR; and 49 mm medial to distal × 61 mm proximal to distal for PT.
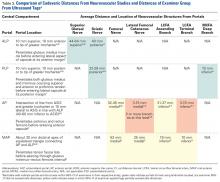
Given the difference between examiner data (direction and distance from ultrasound labels) and published data (distance to significant neurovascular structures), inaccurate identification of surface landmarks has the potential to lead to AP and MAP damage (Table 3). The examiner GT and ASIS surface landmarks used for AP overlapped directly with the safe distances for the lateral femoral cutaneous nerve and the terminal branch of the lateral circumflex femoral artery.
Discussion
Others have investigated examiners’ use of palpation, compared with ultrasound, to identify common shoulder and knee structures.8-10 In a 2011 systematic review, Gilliland and colleagues11 confirmed that accuracy was improved with use of ultrasound (vs palpation) for injections in the shoulder, hip, knee, wrist, and ankle. Given the scarcity of data in this setting, we conducted the present study to assess the precision and accuracy of expert arthroscopists in identifying common surface landmarks. We hypothesized that physical examination and ultrasound examination would differ significantly in precisely and accurately identifying these landmarks.
Working with a standard awake volunteer, our test group of examiners was consistently inaccurate when they accepted ultrasonographer-placed labels as the ideal. Precision within the group, however, trended toward close agreement; examiners consistently placed labels in the same direction and approximate magnitude away from ultrasonographer labels. This suggests that a discrepancy between the ultrasonographic surface structure definitions taught to ultrasonographers and the manually identified definitions taught to surgeons for arthroscopy (training bias) can generate differences in landmark identification.
Given reported low rates of complications in the creation of standard surface anatomy portals, more data is needed to correlate whether safe distance guidelines best apply to the points identified by hip experts or the points identified by ultrasonographers. In a 2013 systematic review, Harris and colleagues8 found a 7.5% overall complication rate, with temporary neuropraxia 1 of the 2 most common complications. Whether adding ultrasound to physical examination for the creation of some or all portals will reduce the incidence of these problems is unknown. Regardless of the anatomical area referenced by experts for portal creation, the tight grouping of examiner marks in our study supports a consensus regarding the location of the landmarks studied.
In our study of the use of surface anatomical landmarks for the creation of portals, we analyzed 4 previously described locations: ALP, AP, PLP, and MAP. ALP, AP, and PLP directly reference at least 1 surface anatomical structure; AP references 2 anatomical structures (ASIS, GT); and MAP indirectly references ASIS and GT and directly references ALP and AP. In cadaveric and radiographic studies, 7 neurovascular structures have been described in proximity to ALP, AP, MAP, and PLP: superior gluteal nerve, sciatic nerve, femoral nerve, lateral femoral cutaneous nerve, lateral circumflex femoral artery, and medial circumflex femoral artery.5,6 Our results showed that use of surface anatomy in AP and MAP creation most likely places structures at risk, given the overlap of examiner CIs and the previously published cadaveric5,6 and radiographic7 data.
Hua and colleagues12 confirmed the feasibility of using ultrasound for the creation of hip arthroscopy portals. More data is needed to assess how the standard palpation-and-fluoroscopy method described by Byrd3 compares with an ultrasound-guided technique in safety and cost. However, data from our study should not be used to justify a demand for ultrasound during arthroscopy portal establishment, as limitations do not permit such a recommendation.
With diagnostic injection remaining a mainstay of differential diagnosis and treatment about the hip,1 the data presented here suggest a potential for ultrasound in enhancing outcomes. There is evidence supporting the role of image guidance in improving palpation accuracy in the area of the biceps tendon in the forearm.10 Potentially, identification and treatment of specific extra-articular structures surrounding the hip could be made safer with more routine use of ultrasound.
Limitations
This study had several limitations. The surgeons were limited to palpation and static examination of a body in its natural state. Hip arthroscopic portals typically are created under traction and after a standard perineal post is placed for hip arthroscopy. In addition, in an awake injection setting, the clinician may receive patient feedback in the form of limb movement or speech. To what degree palpation or ultrasound will be affected in these scenarios is unknown.
Another limitation is the lack of serial examination by each examiner—intrarater variability could not be gauged. In addition, with only 1 ultrasonographic examination performed, there is the potential that adding ultrasonographic examinations, or having an examiner perform serial physical examinations, could better define the precision of each component. Given the practical limitations of our volunteer’s time and the schedules of 30 expert arthroscopists, we kept the chosen study design for its single setting.
Conclusion
Visual inspection and manual palpation are standard means of identifying common surface anatomical landmarks for the creation of arthroscopy portals and the placement of injections. Our study results showed variance in landmark identification between expert examiners and an ultrasonographer. The degree of variance exceeded established neurovascular safe zones, particularly for AP and MAP. This new evidence calls for further investigation into the best, safest means of performing hip arthroscopic techniques and injection-based interventions.
Am J Orthop. 2017;46(1):E65-E70. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Byrd JW, Potts EA, Allison RK, Jones KS. Ultrasound-guided hip injections: a comparative study with fluoroscopy-guided injections. Arthroscopy. 2014;30(1):42-46.
2. Dienst M, Seil R, Kohn DM. Safe arthroscopic access to the central compartment of the hip. Arthroscopy. 2005;21(12):1510-1514.
3. Byrd JW. Hip arthroscopy, the supine approach: technique and anatomy of the intraarticular and peripheral compartments. Tech Orthop. 2005;20(1):17-31.
4. Bond JL, Knutson ZA, Ebert A, Guanche CA. The 23-point arthroscopic examination of the hip: basic setup, portal placement, and surgical technique. Arthroscopy. 2009;25(4):416-429.
5. Roberson WJ, Kelly BT. The safe zone for hip arthroscopy: a cadaveric assessment of central, peripheral, and lateral compartment portal placement. Arthroscopy. 2008;24(9):1019-1026.
6. Byrd JW, Pappas JN, Pedley MJ. Hip arthroscopy: an anatomic study of portal placement and relationship to the extra-articular structures. Arthroscopy. 1995;11(4):418-423.
7. Watson JN, Bohnenkamp F, El-Bitar Y, Moretti V, Domb BG. Variability in locations of hip neurovascular structures and their proximity to hip arthroscopic portals. Arthroscopy. 2014;30(4):462-467.
8. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
9. Jacobson JA, Bedi A, Sekiya JK, Blankenbaker DG. Evaluation of the painful athletic hip: imaging options and imaging-guided injections. AJR Am J Roentgenol. 2012;199(3):516-524.
10. Gazzillo GP, Finnoff JT, Hall MM, Sayeed YA, Smith J. Accuracy of palpating the long head of the biceps tendon: an ultrasonographic study. PM R. 2011;3(11):1035-1040.
11. Gilliland CA, Salazar LD, Borchers JR. Ultrasound versus anatomic guidance for intra-articular and periarticular injection: a systematic review. Phys Sportsmed. 2011;39(3):121-131.
12. Hua Y, Yang Y, Chen S, et al. Ultrasound-guided establishment of hip arthroscopy portals. Arthroscopy. 2009;25(12):1491-1495.
Take-Home Points
- Surface landmarks are routinely used for physical examination and surgical technique.
- Common surface landmarks used in establishing arthroscopic portals may be more difficult to accurately identify than previously thought.
- The greater trochanter was the surface landmark most precisely identified by expert examiners.
- Ultrasound examination identified landmarks varied from landmarks identified by palpation alone.
Anatomical surface landmarks about the hip and lower abdomen are often referenced when placing arthroscopic portals and office-based injections.1-3 However, the degree to which these landmarks can be reproducibly identified using only visual inspection and palpation is unknown.
Safe access to the hip joint and surrounding structures during hip arthroscopy has been a focus in the orthopedic literature. Authors have described anatomical relationships of recommended portals to neurovascular and other anatomical structures.4-6 This information has been reported in millimeters to centimeters of safety based on cadaver dissection studies.4-7We conducted a study to assess expert hip arthroscopists’ ability to identify, using only physical examination techniques, the anatomical structures used for reference when creating safe starting points for arthroscopic access. We hypothesized that variance in examiner-identified points would exceed safe distances from neurovascular structures for the most commonly used hip arthroscopic portals. The volunteer in this study provided written informed consent for print and electronic publication of this article.
Methods
In this study, we prospectively assessed 30 expert hip arthroscopic surgeons’ ability to identify commonly referenced surface landmarks on the adult male hip, using only inspection and manual palpation. Surgeons were defined as experts on the basis of their status as hip arthroscopy instructors at the Orthopaedic Learning Center (Rosemont, IL) for the Arthroscopy Association of North America and industry-sponsored hip arthroscopy education faculty (Arthrex). Five surface landmarks were selected for their relevance to publications on safe portal placement2-5: anterior superior iliac spine (ASIS), tip of greater trochanter (GT), rectus origin (RO), superficial inguinal ring (SIR), and psoas tendon (PT).
A healthy adult male volunteer was placed supine on an examination table and exposed distally from the mid abdomen, with the perineum and the genital area covered bikini-style. An expert musculoskeletal ultrasonographer used a handheld musculoskeletal ultrasound transducer (Sonosite) to identify the 5 landmarks. Short- and long-axis images of each structure were obtained. The examiner applied a round (1 cm in diameter), uniquely colored adhesive label to the skin over each location. A professional photographer using a Canon digital camera and fixed mounts made precise overhead and lateral images. The positional integrity and scale of these images were confirmed with referral to constant anatomical skin features. Images were archived for analysis (Figure 1A).
After the ultrasonographer’s labels were removed, each of the 30 expert hip arthroscopic surgeons identified the structures by static physical examination (inspection and palpation only) and applied the same colored labels to the skin.
Imaging software (Adobe Photoshop Creative Suite 5.1) was used to superimpose the digital images of the examiner labels on those of the ultrasound-verified anatomical labels (Figure 1C). Measurements were then taken with digital calipers to determine average distance from ultrasound label; accuracy within 10 mm of verified ultrasound label; true average location (TAL) determined by 95% confidence interval (CI); and interobserver variability calculated by 95% prediction interval, which determined the probability of where an additional examiner data point would lie.
In the second arm of the study, examiner data were compared with previously published data on arthroscopic portal safety.
Results
Average absolute distance from examiner labels to ultrasonographer labels was 31 mm for ASIS, 24 mm for GT, 26 mm for RO, 19 mm for SIR, and 35 mm for PT (Figure 2).
Of the 30 surgeons, 1 (3%) came within 10 mm of the ultrasound for ASIS, 1 (3%) for GT, 4 (13%) for RO, 5 (17%) for SIR, and 1 (3%) for PT (Table 1).
TAL as determined by CI was 16 mm medial and 29 mm inferior for ASIS; 8 mm anterior and 22 mm superior for GT; 10 mm medial and 25 mm inferior for RO; 5 mm lateral and 5 mm inferior for SIR; and 28 mm medial and 16 mm inferior for PT (Figure 3, Table 2). Interobserver variability determined by prediction interval had a range of 18 mm medial to lateral × 36 mm proximal to distal for ASIS; 33 mm anterior to posterior × 48 mm superior to inferior for GT; 41 mm medial to distal × 54 mm proximal to distal for RO; 51 mm medial to lateral × 74 mm proximal to distal for SIR; and 49 mm medial to distal × 61 mm proximal to distal for PT.
Given the difference between examiner data (direction and distance from ultrasound labels) and published data (distance to significant neurovascular structures), inaccurate identification of surface landmarks has the potential to lead to AP and MAP damage (Table 3). The examiner GT and ASIS surface landmarks used for AP overlapped directly with the safe distances for the lateral femoral cutaneous nerve and the terminal branch of the lateral circumflex femoral artery.
Discussion
Others have investigated examiners’ use of palpation, compared with ultrasound, to identify common shoulder and knee structures.8-10 In a 2011 systematic review, Gilliland and colleagues11 confirmed that accuracy was improved with use of ultrasound (vs palpation) for injections in the shoulder, hip, knee, wrist, and ankle. Given the scarcity of data in this setting, we conducted the present study to assess the precision and accuracy of expert arthroscopists in identifying common surface landmarks. We hypothesized that physical examination and ultrasound examination would differ significantly in precisely and accurately identifying these landmarks.
Working with a standard awake volunteer, our test group of examiners was consistently inaccurate when they accepted ultrasonographer-placed labels as the ideal. Precision within the group, however, trended toward close agreement; examiners consistently placed labels in the same direction and approximate magnitude away from ultrasonographer labels. This suggests that a discrepancy between the ultrasonographic surface structure definitions taught to ultrasonographers and the manually identified definitions taught to surgeons for arthroscopy (training bias) can generate differences in landmark identification.
Given reported low rates of complications in the creation of standard surface anatomy portals, more data is needed to correlate whether safe distance guidelines best apply to the points identified by hip experts or the points identified by ultrasonographers. In a 2013 systematic review, Harris and colleagues8 found a 7.5% overall complication rate, with temporary neuropraxia 1 of the 2 most common complications. Whether adding ultrasound to physical examination for the creation of some or all portals will reduce the incidence of these problems is unknown. Regardless of the anatomical area referenced by experts for portal creation, the tight grouping of examiner marks in our study supports a consensus regarding the location of the landmarks studied.
In our study of the use of surface anatomical landmarks for the creation of portals, we analyzed 4 previously described locations: ALP, AP, PLP, and MAP. ALP, AP, and PLP directly reference at least 1 surface anatomical structure; AP references 2 anatomical structures (ASIS, GT); and MAP indirectly references ASIS and GT and directly references ALP and AP. In cadaveric and radiographic studies, 7 neurovascular structures have been described in proximity to ALP, AP, MAP, and PLP: superior gluteal nerve, sciatic nerve, femoral nerve, lateral femoral cutaneous nerve, lateral circumflex femoral artery, and medial circumflex femoral artery.5,6 Our results showed that use of surface anatomy in AP and MAP creation most likely places structures at risk, given the overlap of examiner CIs and the previously published cadaveric5,6 and radiographic7 data.
Hua and colleagues12 confirmed the feasibility of using ultrasound for the creation of hip arthroscopy portals. More data is needed to assess how the standard palpation-and-fluoroscopy method described by Byrd3 compares with an ultrasound-guided technique in safety and cost. However, data from our study should not be used to justify a demand for ultrasound during arthroscopy portal establishment, as limitations do not permit such a recommendation.
With diagnostic injection remaining a mainstay of differential diagnosis and treatment about the hip,1 the data presented here suggest a potential for ultrasound in enhancing outcomes. There is evidence supporting the role of image guidance in improving palpation accuracy in the area of the biceps tendon in the forearm.10 Potentially, identification and treatment of specific extra-articular structures surrounding the hip could be made safer with more routine use of ultrasound.
Limitations
This study had several limitations. The surgeons were limited to palpation and static examination of a body in its natural state. Hip arthroscopic portals typically are created under traction and after a standard perineal post is placed for hip arthroscopy. In addition, in an awake injection setting, the clinician may receive patient feedback in the form of limb movement or speech. To what degree palpation or ultrasound will be affected in these scenarios is unknown.
Another limitation is the lack of serial examination by each examiner—intrarater variability could not be gauged. In addition, with only 1 ultrasonographic examination performed, there is the potential that adding ultrasonographic examinations, or having an examiner perform serial physical examinations, could better define the precision of each component. Given the practical limitations of our volunteer’s time and the schedules of 30 expert arthroscopists, we kept the chosen study design for its single setting.
Conclusion
Visual inspection and manual palpation are standard means of identifying common surface anatomical landmarks for the creation of arthroscopy portals and the placement of injections. Our study results showed variance in landmark identification between expert examiners and an ultrasonographer. The degree of variance exceeded established neurovascular safe zones, particularly for AP and MAP. This new evidence calls for further investigation into the best, safest means of performing hip arthroscopic techniques and injection-based interventions.
Am J Orthop. 2017;46(1):E65-E70. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Surface landmarks are routinely used for physical examination and surgical technique.
- Common surface landmarks used in establishing arthroscopic portals may be more difficult to accurately identify than previously thought.
- The greater trochanter was the surface landmark most precisely identified by expert examiners.
- Ultrasound examination identified landmarks varied from landmarks identified by palpation alone.
Anatomical surface landmarks about the hip and lower abdomen are often referenced when placing arthroscopic portals and office-based injections.1-3 However, the degree to which these landmarks can be reproducibly identified using only visual inspection and palpation is unknown.
Safe access to the hip joint and surrounding structures during hip arthroscopy has been a focus in the orthopedic literature. Authors have described anatomical relationships of recommended portals to neurovascular and other anatomical structures.4-6 This information has been reported in millimeters to centimeters of safety based on cadaver dissection studies.4-7We conducted a study to assess expert hip arthroscopists’ ability to identify, using only physical examination techniques, the anatomical structures used for reference when creating safe starting points for arthroscopic access. We hypothesized that variance in examiner-identified points would exceed safe distances from neurovascular structures for the most commonly used hip arthroscopic portals. The volunteer in this study provided written informed consent for print and electronic publication of this article.
Methods
In this study, we prospectively assessed 30 expert hip arthroscopic surgeons’ ability to identify commonly referenced surface landmarks on the adult male hip, using only inspection and manual palpation. Surgeons were defined as experts on the basis of their status as hip arthroscopy instructors at the Orthopaedic Learning Center (Rosemont, IL) for the Arthroscopy Association of North America and industry-sponsored hip arthroscopy education faculty (Arthrex). Five surface landmarks were selected for their relevance to publications on safe portal placement2-5: anterior superior iliac spine (ASIS), tip of greater trochanter (GT), rectus origin (RO), superficial inguinal ring (SIR), and psoas tendon (PT).
A healthy adult male volunteer was placed supine on an examination table and exposed distally from the mid abdomen, with the perineum and the genital area covered bikini-style. An expert musculoskeletal ultrasonographer used a handheld musculoskeletal ultrasound transducer (Sonosite) to identify the 5 landmarks. Short- and long-axis images of each structure were obtained. The examiner applied a round (1 cm in diameter), uniquely colored adhesive label to the skin over each location. A professional photographer using a Canon digital camera and fixed mounts made precise overhead and lateral images. The positional integrity and scale of these images were confirmed with referral to constant anatomical skin features. Images were archived for analysis (Figure 1A).
After the ultrasonographer’s labels were removed, each of the 30 expert hip arthroscopic surgeons identified the structures by static physical examination (inspection and palpation only) and applied the same colored labels to the skin.
Imaging software (Adobe Photoshop Creative Suite 5.1) was used to superimpose the digital images of the examiner labels on those of the ultrasound-verified anatomical labels (Figure 1C). Measurements were then taken with digital calipers to determine average distance from ultrasound label; accuracy within 10 mm of verified ultrasound label; true average location (TAL) determined by 95% confidence interval (CI); and interobserver variability calculated by 95% prediction interval, which determined the probability of where an additional examiner data point would lie.
In the second arm of the study, examiner data were compared with previously published data on arthroscopic portal safety.
Results
Average absolute distance from examiner labels to ultrasonographer labels was 31 mm for ASIS, 24 mm for GT, 26 mm for RO, 19 mm for SIR, and 35 mm for PT (Figure 2).
Of the 30 surgeons, 1 (3%) came within 10 mm of the ultrasound for ASIS, 1 (3%) for GT, 4 (13%) for RO, 5 (17%) for SIR, and 1 (3%) for PT (Table 1).
TAL as determined by CI was 16 mm medial and 29 mm inferior for ASIS; 8 mm anterior and 22 mm superior for GT; 10 mm medial and 25 mm inferior for RO; 5 mm lateral and 5 mm inferior for SIR; and 28 mm medial and 16 mm inferior for PT (Figure 3, Table 2). Interobserver variability determined by prediction interval had a range of 18 mm medial to lateral × 36 mm proximal to distal for ASIS; 33 mm anterior to posterior × 48 mm superior to inferior for GT; 41 mm medial to distal × 54 mm proximal to distal for RO; 51 mm medial to lateral × 74 mm proximal to distal for SIR; and 49 mm medial to distal × 61 mm proximal to distal for PT.
Given the difference between examiner data (direction and distance from ultrasound labels) and published data (distance to significant neurovascular structures), inaccurate identification of surface landmarks has the potential to lead to AP and MAP damage (Table 3). The examiner GT and ASIS surface landmarks used for AP overlapped directly with the safe distances for the lateral femoral cutaneous nerve and the terminal branch of the lateral circumflex femoral artery.
Discussion
Others have investigated examiners’ use of palpation, compared with ultrasound, to identify common shoulder and knee structures.8-10 In a 2011 systematic review, Gilliland and colleagues11 confirmed that accuracy was improved with use of ultrasound (vs palpation) for injections in the shoulder, hip, knee, wrist, and ankle. Given the scarcity of data in this setting, we conducted the present study to assess the precision and accuracy of expert arthroscopists in identifying common surface landmarks. We hypothesized that physical examination and ultrasound examination would differ significantly in precisely and accurately identifying these landmarks.
Working with a standard awake volunteer, our test group of examiners was consistently inaccurate when they accepted ultrasonographer-placed labels as the ideal. Precision within the group, however, trended toward close agreement; examiners consistently placed labels in the same direction and approximate magnitude away from ultrasonographer labels. This suggests that a discrepancy between the ultrasonographic surface structure definitions taught to ultrasonographers and the manually identified definitions taught to surgeons for arthroscopy (training bias) can generate differences in landmark identification.
Given reported low rates of complications in the creation of standard surface anatomy portals, more data is needed to correlate whether safe distance guidelines best apply to the points identified by hip experts or the points identified by ultrasonographers. In a 2013 systematic review, Harris and colleagues8 found a 7.5% overall complication rate, with temporary neuropraxia 1 of the 2 most common complications. Whether adding ultrasound to physical examination for the creation of some or all portals will reduce the incidence of these problems is unknown. Regardless of the anatomical area referenced by experts for portal creation, the tight grouping of examiner marks in our study supports a consensus regarding the location of the landmarks studied.
In our study of the use of surface anatomical landmarks for the creation of portals, we analyzed 4 previously described locations: ALP, AP, PLP, and MAP. ALP, AP, and PLP directly reference at least 1 surface anatomical structure; AP references 2 anatomical structures (ASIS, GT); and MAP indirectly references ASIS and GT and directly references ALP and AP. In cadaveric and radiographic studies, 7 neurovascular structures have been described in proximity to ALP, AP, MAP, and PLP: superior gluteal nerve, sciatic nerve, femoral nerve, lateral femoral cutaneous nerve, lateral circumflex femoral artery, and medial circumflex femoral artery.5,6 Our results showed that use of surface anatomy in AP and MAP creation most likely places structures at risk, given the overlap of examiner CIs and the previously published cadaveric5,6 and radiographic7 data.
Hua and colleagues12 confirmed the feasibility of using ultrasound for the creation of hip arthroscopy portals. More data is needed to assess how the standard palpation-and-fluoroscopy method described by Byrd3 compares with an ultrasound-guided technique in safety and cost. However, data from our study should not be used to justify a demand for ultrasound during arthroscopy portal establishment, as limitations do not permit such a recommendation.
With diagnostic injection remaining a mainstay of differential diagnosis and treatment about the hip,1 the data presented here suggest a potential for ultrasound in enhancing outcomes. There is evidence supporting the role of image guidance in improving palpation accuracy in the area of the biceps tendon in the forearm.10 Potentially, identification and treatment of specific extra-articular structures surrounding the hip could be made safer with more routine use of ultrasound.
Limitations
This study had several limitations. The surgeons were limited to palpation and static examination of a body in its natural state. Hip arthroscopic portals typically are created under traction and after a standard perineal post is placed for hip arthroscopy. In addition, in an awake injection setting, the clinician may receive patient feedback in the form of limb movement or speech. To what degree palpation or ultrasound will be affected in these scenarios is unknown.
Another limitation is the lack of serial examination by each examiner—intrarater variability could not be gauged. In addition, with only 1 ultrasonographic examination performed, there is the potential that adding ultrasonographic examinations, or having an examiner perform serial physical examinations, could better define the precision of each component. Given the practical limitations of our volunteer’s time and the schedules of 30 expert arthroscopists, we kept the chosen study design for its single setting.
Conclusion
Visual inspection and manual palpation are standard means of identifying common surface anatomical landmarks for the creation of arthroscopy portals and the placement of injections. Our study results showed variance in landmark identification between expert examiners and an ultrasonographer. The degree of variance exceeded established neurovascular safe zones, particularly for AP and MAP. This new evidence calls for further investigation into the best, safest means of performing hip arthroscopic techniques and injection-based interventions.
Am J Orthop. 2017;46(1):E65-E70. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Byrd JW, Potts EA, Allison RK, Jones KS. Ultrasound-guided hip injections: a comparative study with fluoroscopy-guided injections. Arthroscopy. 2014;30(1):42-46.
2. Dienst M, Seil R, Kohn DM. Safe arthroscopic access to the central compartment of the hip. Arthroscopy. 2005;21(12):1510-1514.
3. Byrd JW. Hip arthroscopy, the supine approach: technique and anatomy of the intraarticular and peripheral compartments. Tech Orthop. 2005;20(1):17-31.
4. Bond JL, Knutson ZA, Ebert A, Guanche CA. The 23-point arthroscopic examination of the hip: basic setup, portal placement, and surgical technique. Arthroscopy. 2009;25(4):416-429.
5. Roberson WJ, Kelly BT. The safe zone for hip arthroscopy: a cadaveric assessment of central, peripheral, and lateral compartment portal placement. Arthroscopy. 2008;24(9):1019-1026.
6. Byrd JW, Pappas JN, Pedley MJ. Hip arthroscopy: an anatomic study of portal placement and relationship to the extra-articular structures. Arthroscopy. 1995;11(4):418-423.
7. Watson JN, Bohnenkamp F, El-Bitar Y, Moretti V, Domb BG. Variability in locations of hip neurovascular structures and their proximity to hip arthroscopic portals. Arthroscopy. 2014;30(4):462-467.
8. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
9. Jacobson JA, Bedi A, Sekiya JK, Blankenbaker DG. Evaluation of the painful athletic hip: imaging options and imaging-guided injections. AJR Am J Roentgenol. 2012;199(3):516-524.
10. Gazzillo GP, Finnoff JT, Hall MM, Sayeed YA, Smith J. Accuracy of palpating the long head of the biceps tendon: an ultrasonographic study. PM R. 2011;3(11):1035-1040.
11. Gilliland CA, Salazar LD, Borchers JR. Ultrasound versus anatomic guidance for intra-articular and periarticular injection: a systematic review. Phys Sportsmed. 2011;39(3):121-131.
12. Hua Y, Yang Y, Chen S, et al. Ultrasound-guided establishment of hip arthroscopy portals. Arthroscopy. 2009;25(12):1491-1495.
1. Byrd JW, Potts EA, Allison RK, Jones KS. Ultrasound-guided hip injections: a comparative study with fluoroscopy-guided injections. Arthroscopy. 2014;30(1):42-46.
2. Dienst M, Seil R, Kohn DM. Safe arthroscopic access to the central compartment of the hip. Arthroscopy. 2005;21(12):1510-1514.
3. Byrd JW. Hip arthroscopy, the supine approach: technique and anatomy of the intraarticular and peripheral compartments. Tech Orthop. 2005;20(1):17-31.
4. Bond JL, Knutson ZA, Ebert A, Guanche CA. The 23-point arthroscopic examination of the hip: basic setup, portal placement, and surgical technique. Arthroscopy. 2009;25(4):416-429.
5. Roberson WJ, Kelly BT. The safe zone for hip arthroscopy: a cadaveric assessment of central, peripheral, and lateral compartment portal placement. Arthroscopy. 2008;24(9):1019-1026.
6. Byrd JW, Pappas JN, Pedley MJ. Hip arthroscopy: an anatomic study of portal placement and relationship to the extra-articular structures. Arthroscopy. 1995;11(4):418-423.
7. Watson JN, Bohnenkamp F, El-Bitar Y, Moretti V, Domb BG. Variability in locations of hip neurovascular structures and their proximity to hip arthroscopic portals. Arthroscopy. 2014;30(4):462-467.
8. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
9. Jacobson JA, Bedi A, Sekiya JK, Blankenbaker DG. Evaluation of the painful athletic hip: imaging options and imaging-guided injections. AJR Am J Roentgenol. 2012;199(3):516-524.
10. Gazzillo GP, Finnoff JT, Hall MM, Sayeed YA, Smith J. Accuracy of palpating the long head of the biceps tendon: an ultrasonographic study. PM R. 2011;3(11):1035-1040.
11. Gilliland CA, Salazar LD, Borchers JR. Ultrasound versus anatomic guidance for intra-articular and periarticular injection: a systematic review. Phys Sportsmed. 2011;39(3):121-131.
12. Hua Y, Yang Y, Chen S, et al. Ultrasound-guided establishment of hip arthroscopy portals. Arthroscopy. 2009;25(12):1491-1495.
Gluten-free diets related to high levels of arsenic, mercury
Individuals who adopt a gluten-free diet are putting themselves at risk for uncommonly high levels of arsenic and mercury, according to the findings of a recent study published in Epidemiology.
“Despite [less than] 1% of Americans having diagnosed celiac disease, an estimated 25% of American consumers reported consuming gluten-free food in 2015, a 67% increase from 2013,” wrote the authors of the study, led by Maria Argos, PhD, of the University of Illinois at Chicago. “Despite such a dramatic shift in the diet of many Americans, little is known about how gluten-free diets might affect exposure to toxic metals found in certain foods,” they noted.
Dr. Argos and her colleagues analyzed data collected from the National Health and Nutrition Examination Survey (NHANES), which included self-reported questionnaires in which subjects indicated what type of diet they were on, if any. Data on those who indicated that they followed a gluten-free diet were analyzed to determine if their urinary and blood biomarkers indicated any exposure to toxic metals. A total of 7,471 subjects from the NHANES were included in the analysis.
“We accounted for the complex sampling design of NHANES [by] using Taylor series linearization and sampling weights, per the NHANES analytic guidelines, to ensure unbiased and nationally representative estimates,” the authors explained.
A total of 73 subjects identified themselves as following a gluten-free diet. Within this group, the mean total arsenic level in urine was found to be 12.1 mcg/L, compared to 7.8 mcg/L for the other 7,398 subjects. Levels of dimethylarsinic acid averaged 5.3 mcg/L for those who were gluten-free, but only 3.7 for everyone else, while cadmium and lead levels were also slightly higher for gluten-free individuals: 0.18 mcg/L vs. 0.16 mcg/L, and 0.40 mcg/L vs. 0.37 mcg/L, respectively.
Blood analyses showed that total mercury levels were also substantially higher in the gluten-free group, at a mean of 1.3 mcg/L compared to 0.8 mcg/L. While cadmium levels were the same between the two – both showed a mean level of 0.29 mcg/L – lead measured 1.1 mcg/dL and inorganic mercury measured 0.30 mcg/L, compared to 0.96 mcg/L and 0.28 mcg/L in everyone else, respectively.
Geometric mean ratios showed that total arsenic, total arsenic 1, and total mercury levels had the largest disparity between the two groups. Total arsenic registered a 1.5 (95% CI, 1.2-2.0), total mercury a 1.7 (95% CI, 1.1-2.4), and total arsenic 1 a 1.9 (95% CI 1.3-2.6), meaning the gluten-free group had nearly double the risk for higher levels than those on other diets.
“These findings may have important health implications since the health effects of low-level arsenic and mercury exposure from food sources are uncertain but may increase the risk for cancer and other chronic diseases,” Dr. Argos and her coauthors concluded, adding that “future studies are needed to more fully examine exposure to toxic metals from consuming gluten-free foods.”
The study was funded by a grant from the National Institutes of Health. Dr. Argos and her coauthors did not report any relevant financial disclosures.
Individuals who adopt a gluten-free diet are putting themselves at risk for uncommonly high levels of arsenic and mercury, according to the findings of a recent study published in Epidemiology.
“Despite [less than] 1% of Americans having diagnosed celiac disease, an estimated 25% of American consumers reported consuming gluten-free food in 2015, a 67% increase from 2013,” wrote the authors of the study, led by Maria Argos, PhD, of the University of Illinois at Chicago. “Despite such a dramatic shift in the diet of many Americans, little is known about how gluten-free diets might affect exposure to toxic metals found in certain foods,” they noted.
Dr. Argos and her colleagues analyzed data collected from the National Health and Nutrition Examination Survey (NHANES), which included self-reported questionnaires in which subjects indicated what type of diet they were on, if any. Data on those who indicated that they followed a gluten-free diet were analyzed to determine if their urinary and blood biomarkers indicated any exposure to toxic metals. A total of 7,471 subjects from the NHANES were included in the analysis.
“We accounted for the complex sampling design of NHANES [by] using Taylor series linearization and sampling weights, per the NHANES analytic guidelines, to ensure unbiased and nationally representative estimates,” the authors explained.
A total of 73 subjects identified themselves as following a gluten-free diet. Within this group, the mean total arsenic level in urine was found to be 12.1 mcg/L, compared to 7.8 mcg/L for the other 7,398 subjects. Levels of dimethylarsinic acid averaged 5.3 mcg/L for those who were gluten-free, but only 3.7 for everyone else, while cadmium and lead levels were also slightly higher for gluten-free individuals: 0.18 mcg/L vs. 0.16 mcg/L, and 0.40 mcg/L vs. 0.37 mcg/L, respectively.
Blood analyses showed that total mercury levels were also substantially higher in the gluten-free group, at a mean of 1.3 mcg/L compared to 0.8 mcg/L. While cadmium levels were the same between the two – both showed a mean level of 0.29 mcg/L – lead measured 1.1 mcg/dL and inorganic mercury measured 0.30 mcg/L, compared to 0.96 mcg/L and 0.28 mcg/L in everyone else, respectively.
Geometric mean ratios showed that total arsenic, total arsenic 1, and total mercury levels had the largest disparity between the two groups. Total arsenic registered a 1.5 (95% CI, 1.2-2.0), total mercury a 1.7 (95% CI, 1.1-2.4), and total arsenic 1 a 1.9 (95% CI 1.3-2.6), meaning the gluten-free group had nearly double the risk for higher levels than those on other diets.
“These findings may have important health implications since the health effects of low-level arsenic and mercury exposure from food sources are uncertain but may increase the risk for cancer and other chronic diseases,” Dr. Argos and her coauthors concluded, adding that “future studies are needed to more fully examine exposure to toxic metals from consuming gluten-free foods.”
The study was funded by a grant from the National Institutes of Health. Dr. Argos and her coauthors did not report any relevant financial disclosures.
Individuals who adopt a gluten-free diet are putting themselves at risk for uncommonly high levels of arsenic and mercury, according to the findings of a recent study published in Epidemiology.
“Despite [less than] 1% of Americans having diagnosed celiac disease, an estimated 25% of American consumers reported consuming gluten-free food in 2015, a 67% increase from 2013,” wrote the authors of the study, led by Maria Argos, PhD, of the University of Illinois at Chicago. “Despite such a dramatic shift in the diet of many Americans, little is known about how gluten-free diets might affect exposure to toxic metals found in certain foods,” they noted.
Dr. Argos and her colleagues analyzed data collected from the National Health and Nutrition Examination Survey (NHANES), which included self-reported questionnaires in which subjects indicated what type of diet they were on, if any. Data on those who indicated that they followed a gluten-free diet were analyzed to determine if their urinary and blood biomarkers indicated any exposure to toxic metals. A total of 7,471 subjects from the NHANES were included in the analysis.
“We accounted for the complex sampling design of NHANES [by] using Taylor series linearization and sampling weights, per the NHANES analytic guidelines, to ensure unbiased and nationally representative estimates,” the authors explained.
A total of 73 subjects identified themselves as following a gluten-free diet. Within this group, the mean total arsenic level in urine was found to be 12.1 mcg/L, compared to 7.8 mcg/L for the other 7,398 subjects. Levels of dimethylarsinic acid averaged 5.3 mcg/L for those who were gluten-free, but only 3.7 for everyone else, while cadmium and lead levels were also slightly higher for gluten-free individuals: 0.18 mcg/L vs. 0.16 mcg/L, and 0.40 mcg/L vs. 0.37 mcg/L, respectively.
Blood analyses showed that total mercury levels were also substantially higher in the gluten-free group, at a mean of 1.3 mcg/L compared to 0.8 mcg/L. While cadmium levels were the same between the two – both showed a mean level of 0.29 mcg/L – lead measured 1.1 mcg/dL and inorganic mercury measured 0.30 mcg/L, compared to 0.96 mcg/L and 0.28 mcg/L in everyone else, respectively.
Geometric mean ratios showed that total arsenic, total arsenic 1, and total mercury levels had the largest disparity between the two groups. Total arsenic registered a 1.5 (95% CI, 1.2-2.0), total mercury a 1.7 (95% CI, 1.1-2.4), and total arsenic 1 a 1.9 (95% CI 1.3-2.6), meaning the gluten-free group had nearly double the risk for higher levels than those on other diets.
“These findings may have important health implications since the health effects of low-level arsenic and mercury exposure from food sources are uncertain but may increase the risk for cancer and other chronic diseases,” Dr. Argos and her coauthors concluded, adding that “future studies are needed to more fully examine exposure to toxic metals from consuming gluten-free foods.”
The study was funded by a grant from the National Institutes of Health. Dr. Argos and her coauthors did not report any relevant financial disclosures.
FROM EPIDEMIOLOGY
Key clinical point:
Major finding: Total arsenic and mercury levels were higher in those that self-reported being on gluten-free diet than in those who weren’t: 12.1 mcg/L vs. 7.8 mcg/L for arsenic and 1.3 mcg/L vs. 0.8 mcg/L for mercury.
Data source: Retrospective analysis of data from the National Health and Nutrition Examination Survey during 2009-2014.
Disclosures: The National Institutes of Health funded the study. The authors had no relevant financial disclosures.
Cooperation must overcome polarization
Each profession has its own core set of knowledge, skills, and values. For physicians, the core set of knowledge is anatomy, pathophysiology, and pharmacology. Skills include taking a history, the physical exam, and surgical procedures. The core values traditionally have been compassion and altruism. For modern medical practice, I urge adding cooperation as a core value. Medical school and the apprenticeship of residency are designed to teach, role model, foster, develop, and groom these core competencies.
Getting into medical school is highly competitive. Medical training uses methods that are very different than those used to train elite Olympic and professional athletes. Some competitiveness persists in medical school, but in general the faculty emphasizes cooperation rather than competition. The metric is not whether one student or resident is better than another. Gold and silver medals are not awarded. It is about whether each physician-to-be has passed the milestones needed to practice medicine.
But American health care is threatened by the continued polarization of our government and our society. For years both Cleveland Clinic and Dana Farber have held annual fundraising events at Mar-a-Lago. In February 2017, because of that location’s association with President Trump, some people associated with the organizations advocated boycotting those important fund-raisers. These past few months, it seems every action and every purchase has become a political statement. One restaurant mentioned immigrants on its receipt. The action went viral and caused other people to advocate boycotting the restaurant or not tipping the wait staff (“The new political battleground: Your restaurant receipt,” The Washington Post, by Maura Judkis, Feb. 14, 2017).
Secondary boycotts are an ethical quandary. In labor disputes, organized unions can go on strike. In the 1970s, Japanese cars were not welcome in the employee parking lot of a Ford assembly plant. People do vote with their pocketbook. But in labor disputes, there are legal restrictions on secondary boycotts against other companies. People do need to get along with their neighbors and so do businesses. Politics is the art of encouraging cooperation on one project amongst people who disagree about the goals of many other proposed projects. The Preamble to the United States Constitution enumerates the benefits of cooperation.
In any large-scale human endeavor, conflicts arise that may limit cooperation. Accommodating conscientious objection is the safety valve that permits cooperation when dealing with contested government endeavors such as war, abortion, and physician-assisted suicide. It is meant as a last ditch effort to maintain cohesion of both societal and individual moral integrity. But if every proposed action is met with votes divided along party lines, conscientious objection loses its moral high ground.
Judge Neil Gorsuch, the nominee for the U.S. Supreme Court, has a record of supporting religious freedom in Yellowbear v. Lambert (10th Cir. 2014). He would likely support conscientious objection in relation to assisted dying by physicians, contrary to the arguments made recently by bioethicists Julian Savulescu and Udo Schuklenk. In related news, the liberty of physicians to address gun safety was affirmed when a Florida appeals court upheld the overturning of the state’s Privacy of Firearm Owners Act.
Summing up a tumultuous month of medical ethics, I leave you with the words of Voltaire: “Cherish those who seek the truth but beware of those who find it.”
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
Each profession has its own core set of knowledge, skills, and values. For physicians, the core set of knowledge is anatomy, pathophysiology, and pharmacology. Skills include taking a history, the physical exam, and surgical procedures. The core values traditionally have been compassion and altruism. For modern medical practice, I urge adding cooperation as a core value. Medical school and the apprenticeship of residency are designed to teach, role model, foster, develop, and groom these core competencies.
Getting into medical school is highly competitive. Medical training uses methods that are very different than those used to train elite Olympic and professional athletes. Some competitiveness persists in medical school, but in general the faculty emphasizes cooperation rather than competition. The metric is not whether one student or resident is better than another. Gold and silver medals are not awarded. It is about whether each physician-to-be has passed the milestones needed to practice medicine.
But American health care is threatened by the continued polarization of our government and our society. For years both Cleveland Clinic and Dana Farber have held annual fundraising events at Mar-a-Lago. In February 2017, because of that location’s association with President Trump, some people associated with the organizations advocated boycotting those important fund-raisers. These past few months, it seems every action and every purchase has become a political statement. One restaurant mentioned immigrants on its receipt. The action went viral and caused other people to advocate boycotting the restaurant or not tipping the wait staff (“The new political battleground: Your restaurant receipt,” The Washington Post, by Maura Judkis, Feb. 14, 2017).
Secondary boycotts are an ethical quandary. In labor disputes, organized unions can go on strike. In the 1970s, Japanese cars were not welcome in the employee parking lot of a Ford assembly plant. People do vote with their pocketbook. But in labor disputes, there are legal restrictions on secondary boycotts against other companies. People do need to get along with their neighbors and so do businesses. Politics is the art of encouraging cooperation on one project amongst people who disagree about the goals of many other proposed projects. The Preamble to the United States Constitution enumerates the benefits of cooperation.
In any large-scale human endeavor, conflicts arise that may limit cooperation. Accommodating conscientious objection is the safety valve that permits cooperation when dealing with contested government endeavors such as war, abortion, and physician-assisted suicide. It is meant as a last ditch effort to maintain cohesion of both societal and individual moral integrity. But if every proposed action is met with votes divided along party lines, conscientious objection loses its moral high ground.
Judge Neil Gorsuch, the nominee for the U.S. Supreme Court, has a record of supporting religious freedom in Yellowbear v. Lambert (10th Cir. 2014). He would likely support conscientious objection in relation to assisted dying by physicians, contrary to the arguments made recently by bioethicists Julian Savulescu and Udo Schuklenk. In related news, the liberty of physicians to address gun safety was affirmed when a Florida appeals court upheld the overturning of the state’s Privacy of Firearm Owners Act.
Summing up a tumultuous month of medical ethics, I leave you with the words of Voltaire: “Cherish those who seek the truth but beware of those who find it.”
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
Each profession has its own core set of knowledge, skills, and values. For physicians, the core set of knowledge is anatomy, pathophysiology, and pharmacology. Skills include taking a history, the physical exam, and surgical procedures. The core values traditionally have been compassion and altruism. For modern medical practice, I urge adding cooperation as a core value. Medical school and the apprenticeship of residency are designed to teach, role model, foster, develop, and groom these core competencies.
Getting into medical school is highly competitive. Medical training uses methods that are very different than those used to train elite Olympic and professional athletes. Some competitiveness persists in medical school, but in general the faculty emphasizes cooperation rather than competition. The metric is not whether one student or resident is better than another. Gold and silver medals are not awarded. It is about whether each physician-to-be has passed the milestones needed to practice medicine.
But American health care is threatened by the continued polarization of our government and our society. For years both Cleveland Clinic and Dana Farber have held annual fundraising events at Mar-a-Lago. In February 2017, because of that location’s association with President Trump, some people associated with the organizations advocated boycotting those important fund-raisers. These past few months, it seems every action and every purchase has become a political statement. One restaurant mentioned immigrants on its receipt. The action went viral and caused other people to advocate boycotting the restaurant or not tipping the wait staff (“The new political battleground: Your restaurant receipt,” The Washington Post, by Maura Judkis, Feb. 14, 2017).
Secondary boycotts are an ethical quandary. In labor disputes, organized unions can go on strike. In the 1970s, Japanese cars were not welcome in the employee parking lot of a Ford assembly plant. People do vote with their pocketbook. But in labor disputes, there are legal restrictions on secondary boycotts against other companies. People do need to get along with their neighbors and so do businesses. Politics is the art of encouraging cooperation on one project amongst people who disagree about the goals of many other proposed projects. The Preamble to the United States Constitution enumerates the benefits of cooperation.
In any large-scale human endeavor, conflicts arise that may limit cooperation. Accommodating conscientious objection is the safety valve that permits cooperation when dealing with contested government endeavors such as war, abortion, and physician-assisted suicide. It is meant as a last ditch effort to maintain cohesion of both societal and individual moral integrity. But if every proposed action is met with votes divided along party lines, conscientious objection loses its moral high ground.
Judge Neil Gorsuch, the nominee for the U.S. Supreme Court, has a record of supporting religious freedom in Yellowbear v. Lambert (10th Cir. 2014). He would likely support conscientious objection in relation to assisted dying by physicians, contrary to the arguments made recently by bioethicists Julian Savulescu and Udo Schuklenk. In related news, the liberty of physicians to address gun safety was affirmed when a Florida appeals court upheld the overturning of the state’s Privacy of Firearm Owners Act.
Summing up a tumultuous month of medical ethics, I leave you with the words of Voltaire: “Cherish those who seek the truth but beware of those who find it.”
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
DoD Rolls Out EHR Genesis at Fairchild Air Force Base
After years of preparation, a new electronic health record (EHR) system is now live at Fairchild Air Force Base in Washington. According to DoD officials, the system remains on schedule for the next step in the rollout later this year and the eventual completion in 2022. “This is just the first step in implementing what will be the largest integrated inpatient and outpatient electronic health record in the United States,” said VADM Raquel Bono, director of the Defense Health Agency.
Home to the 92nd Medical Group, Fairchild has a number of outpatient clinics and a pharmacy. According to Col Margaret Carey, the 92nd Medical Group commander, Genesis is being used by all the medical personnel at Fairchild.
For many years, the VA and DoD discussed possibly developing a single EHR across both systems before rejecting the idea for being too costly and complicated. Instead the 2 agencies looked to make their systems interoperable and to improve data sharing across the systems. The 2 agencies developed the Joint Legacy Viewer system to improve data sharing. The Joint Legacy Viewer is a clinical application that provides an integrated, read-only display of health data from DoD, VA, private sector partners, and the current military medical record system in a common data viewer.
The DoD originally awarded the $4.3 billion contract to Leidos in 2014 to develop Genesis, but the system was delayed to address cyber-security concerns. The concerns also caused DoD to reduce a larger rollout to focus initially on Fairchild.
“I can report firsthand from the command center that everything is going as expected,” reported Stacy Cummings, program executive officer for Defense Healthcare Management Systems. “Initial feedback from [health care] providers is positive.”
According to Dr. Paul Cordts, director, functional champion for the MHS at the Defense Health Agency, the biggest change that Genesis will bring is offering open medical notes to patients to “empower our patients to know what medical data are in the medical records and to use that data to improve their health and their health care over time.” Patients will be able to access data through a patient portal.
“This EHR is built to enable a team approach to providing health services to patients,” said U.S. Air Force Surgeon General Lt Gen Mark Ediger, MD. “In medicine today we really leverage a number of different skill sets on a health care team… That’s why in the Air Force we are piloting the addition of a health coaching capability here at Fairchild to leverage capabilities that are in MHS, so that health coaches can interact with patients between visits to work on things like tobacco cessation and weight loss, exercise plans and things of that nature.”
After years of preparation, a new electronic health record (EHR) system is now live at Fairchild Air Force Base in Washington. According to DoD officials, the system remains on schedule for the next step in the rollout later this year and the eventual completion in 2022. “This is just the first step in implementing what will be the largest integrated inpatient and outpatient electronic health record in the United States,” said VADM Raquel Bono, director of the Defense Health Agency.
Home to the 92nd Medical Group, Fairchild has a number of outpatient clinics and a pharmacy. According to Col Margaret Carey, the 92nd Medical Group commander, Genesis is being used by all the medical personnel at Fairchild.
For many years, the VA and DoD discussed possibly developing a single EHR across both systems before rejecting the idea for being too costly and complicated. Instead the 2 agencies looked to make their systems interoperable and to improve data sharing across the systems. The 2 agencies developed the Joint Legacy Viewer system to improve data sharing. The Joint Legacy Viewer is a clinical application that provides an integrated, read-only display of health data from DoD, VA, private sector partners, and the current military medical record system in a common data viewer.
The DoD originally awarded the $4.3 billion contract to Leidos in 2014 to develop Genesis, but the system was delayed to address cyber-security concerns. The concerns also caused DoD to reduce a larger rollout to focus initially on Fairchild.
“I can report firsthand from the command center that everything is going as expected,” reported Stacy Cummings, program executive officer for Defense Healthcare Management Systems. “Initial feedback from [health care] providers is positive.”
According to Dr. Paul Cordts, director, functional champion for the MHS at the Defense Health Agency, the biggest change that Genesis will bring is offering open medical notes to patients to “empower our patients to know what medical data are in the medical records and to use that data to improve their health and their health care over time.” Patients will be able to access data through a patient portal.
“This EHR is built to enable a team approach to providing health services to patients,” said U.S. Air Force Surgeon General Lt Gen Mark Ediger, MD. “In medicine today we really leverage a number of different skill sets on a health care team… That’s why in the Air Force we are piloting the addition of a health coaching capability here at Fairchild to leverage capabilities that are in MHS, so that health coaches can interact with patients between visits to work on things like tobacco cessation and weight loss, exercise plans and things of that nature.”
After years of preparation, a new electronic health record (EHR) system is now live at Fairchild Air Force Base in Washington. According to DoD officials, the system remains on schedule for the next step in the rollout later this year and the eventual completion in 2022. “This is just the first step in implementing what will be the largest integrated inpatient and outpatient electronic health record in the United States,” said VADM Raquel Bono, director of the Defense Health Agency.
Home to the 92nd Medical Group, Fairchild has a number of outpatient clinics and a pharmacy. According to Col Margaret Carey, the 92nd Medical Group commander, Genesis is being used by all the medical personnel at Fairchild.
For many years, the VA and DoD discussed possibly developing a single EHR across both systems before rejecting the idea for being too costly and complicated. Instead the 2 agencies looked to make their systems interoperable and to improve data sharing across the systems. The 2 agencies developed the Joint Legacy Viewer system to improve data sharing. The Joint Legacy Viewer is a clinical application that provides an integrated, read-only display of health data from DoD, VA, private sector partners, and the current military medical record system in a common data viewer.
The DoD originally awarded the $4.3 billion contract to Leidos in 2014 to develop Genesis, but the system was delayed to address cyber-security concerns. The concerns also caused DoD to reduce a larger rollout to focus initially on Fairchild.
“I can report firsthand from the command center that everything is going as expected,” reported Stacy Cummings, program executive officer for Defense Healthcare Management Systems. “Initial feedback from [health care] providers is positive.”
According to Dr. Paul Cordts, director, functional champion for the MHS at the Defense Health Agency, the biggest change that Genesis will bring is offering open medical notes to patients to “empower our patients to know what medical data are in the medical records and to use that data to improve their health and their health care over time.” Patients will be able to access data through a patient portal.
“This EHR is built to enable a team approach to providing health services to patients,” said U.S. Air Force Surgeon General Lt Gen Mark Ediger, MD. “In medicine today we really leverage a number of different skill sets on a health care team… That’s why in the Air Force we are piloting the addition of a health coaching capability here at Fairchild to leverage capabilities that are in MHS, so that health coaches can interact with patients between visits to work on things like tobacco cessation and weight loss, exercise plans and things of that nature.”
NIOSH Guide Promotes Holistic View of Worker Health
Stress levels, access to sick leave (or lack thereof), hazardous conditions, and interactions with coworkers have a ripple effect on the lives of workers, their families, and their communities. A safe workplace that supports the well-being of workers can have far-reaching benefits. That’s the premise and promise of Fundamentals of Total Worker Health Approaches: Essential Elements for Advancing Worker Safety, Health, and Well-Being, created by the National Institute for Occupational Safety and Health (NIOSH).
The workbook provides a “user-friendly entry point” into total worker health with examples and tips, such as, “design programs with a long-term outlook to ensure sustainability. Short-term approaches have short-term value.” It also includes a self-assessment tool and resources to develop an action plan and measure progress specific to the organization. A new conceptual model—a “hierarchy of controls”—lists ways to minimize or eliminate exposure to hazards in the workplace, from least effective (eg, providing personal protective equipment) to most effective (eg, physically removing the hazard).
Each workplace is unique, NIOSH says, and because the experiences of people who manage and work in them differ, the workbook is not intended as a one-size-fits-all tool. But it can be used to provide a “snapshot” of where the organization is on the path to total worker health.
Stress levels, access to sick leave (or lack thereof), hazardous conditions, and interactions with coworkers have a ripple effect on the lives of workers, their families, and their communities. A safe workplace that supports the well-being of workers can have far-reaching benefits. That’s the premise and promise of Fundamentals of Total Worker Health Approaches: Essential Elements for Advancing Worker Safety, Health, and Well-Being, created by the National Institute for Occupational Safety and Health (NIOSH).
The workbook provides a “user-friendly entry point” into total worker health with examples and tips, such as, “design programs with a long-term outlook to ensure sustainability. Short-term approaches have short-term value.” It also includes a self-assessment tool and resources to develop an action plan and measure progress specific to the organization. A new conceptual model—a “hierarchy of controls”—lists ways to minimize or eliminate exposure to hazards in the workplace, from least effective (eg, providing personal protective equipment) to most effective (eg, physically removing the hazard).
Each workplace is unique, NIOSH says, and because the experiences of people who manage and work in them differ, the workbook is not intended as a one-size-fits-all tool. But it can be used to provide a “snapshot” of where the organization is on the path to total worker health.
Stress levels, access to sick leave (or lack thereof), hazardous conditions, and interactions with coworkers have a ripple effect on the lives of workers, their families, and their communities. A safe workplace that supports the well-being of workers can have far-reaching benefits. That’s the premise and promise of Fundamentals of Total Worker Health Approaches: Essential Elements for Advancing Worker Safety, Health, and Well-Being, created by the National Institute for Occupational Safety and Health (NIOSH).
The workbook provides a “user-friendly entry point” into total worker health with examples and tips, such as, “design programs with a long-term outlook to ensure sustainability. Short-term approaches have short-term value.” It also includes a self-assessment tool and resources to develop an action plan and measure progress specific to the organization. A new conceptual model—a “hierarchy of controls”—lists ways to minimize or eliminate exposure to hazards in the workplace, from least effective (eg, providing personal protective equipment) to most effective (eg, physically removing the hazard).
Each workplace is unique, NIOSH says, and because the experiences of people who manage and work in them differ, the workbook is not intended as a one-size-fits-all tool. But it can be used to provide a “snapshot” of where the organization is on the path to total worker health.
Adrenal “incidentalomas”
Appeals court strikes down doctor gun gag law
An appeals court has struck down a Florida law that banned physicians from asking patients about firearms, ruling that the so-called gun gag law violates doctors’ First Amendment rights.
The decision by the 11th U.S. Circuit Court of Appeals enables Florida physicians to once again query patients and families about gun ownership and record firearm information in their records. The court, however, left intact a provision that allows patients to refuse answering such questions and upheld another rule that physicians cannot discriminate against patients based on firearm status.
”While the chapter does not wish to impinge on the rights of gun owners, it has fought this legislation in the legislature and in the courts because it is essential that physicians and patients have the right to an open dialogue, free from government restrictions,” Dr. Goldman said in a statement. “[The] ruling is a victory for patients and the profession.”
Florida Gov. Rick Scott (R) did not respond to a request for comment.
The Florida Firearms Owners’ Privacy Act (FOPA) was enacted in 2011 after complaints from patients that physicians were inquiring about firearms in the home and in some cases, refusing to provide care if patients declined to answer. The law barred physicians from asking about firearms and from recording information about firearms in patient records. The statute also precluded doctors from unnecessarily harassing patients about firearm ownership during an examination and prevented physicians from discriminating against patients based solely on firearm ownership. Violations of the law could result in a $10,000 fine per offense, a letter of reprimand, probation, suspension, compulsory remedial education, or permanent license revocation.
Shortly after the law passed, the ACP Florida Chapter sued the state; the suit was joined by the American Academy of Family Physicians Florida Chapter, the American Academy of Pediatrics Florida chapter, and a group of physicians. The plaintiffs argued that doctors routinely ask patients about potential health and safety risks, including firearms, to assess safety risks, educate patients and parents, and encourage gun safety. The gag law violated doctors’ free speech protections, according to the plaintiffs. The state argued that FOPA ensures patient privacy and protects gun owners’ right to own and bear arms from private encumbrances. A district court ruled in favor of the plaintiffs.
In the ruling, judges for the 11th U.S. Circuit Court of Appeals wrote that the right to own and possess firearms does not preclude questions about, commentary on, or criticism for the exercise of that right.
“As the district court aptly noted, there is no actual conflict between the First Amendment rights of doctors and medical professionals and the Second Amendment rights of patients that justifies FOPA’s speaker-focused and content-based restrictions on speech,” judges wrote. “Even if there were some possible conflict between the First Amendment rights of doctors and medical professionals and the Second Amendment rights of patients, the record-keeping, inquiry, and anti-harassment provisions do not advance [the legislative goals] in a permissible way.”
“The legislature has every right to regulate any profession to protect the public from discrimination and abuse,” Ms. Hammer said in an interview. “Doctors are businessmen, not gods. This activist decision attempts to use the First Amendment as a sword to terrorize the Second Amendment and completely disregards the rights and the will of the elected representatives of the people of Florida.”
The American Medical Association called the court ruling a clear victory against censorship of private medical discussions between patients and physicians.
“The court agreed that in the fields of medicine and public health, information saves lives,” according to an AMA statement. “Studies show that patients who received physician counseling on firearm safety are more likely to adopt one or more safe gun-storage practices. … Counseling patients we care for makes a difference in preventing gun-related injuries and deaths.”
[email protected]
On Twitter @legal_med
An appeals court has struck down a Florida law that banned physicians from asking patients about firearms, ruling that the so-called gun gag law violates doctors’ First Amendment rights.
The decision by the 11th U.S. Circuit Court of Appeals enables Florida physicians to once again query patients and families about gun ownership and record firearm information in their records. The court, however, left intact a provision that allows patients to refuse answering such questions and upheld another rule that physicians cannot discriminate against patients based on firearm status.
”While the chapter does not wish to impinge on the rights of gun owners, it has fought this legislation in the legislature and in the courts because it is essential that physicians and patients have the right to an open dialogue, free from government restrictions,” Dr. Goldman said in a statement. “[The] ruling is a victory for patients and the profession.”
Florida Gov. Rick Scott (R) did not respond to a request for comment.
The Florida Firearms Owners’ Privacy Act (FOPA) was enacted in 2011 after complaints from patients that physicians were inquiring about firearms in the home and in some cases, refusing to provide care if patients declined to answer. The law barred physicians from asking about firearms and from recording information about firearms in patient records. The statute also precluded doctors from unnecessarily harassing patients about firearm ownership during an examination and prevented physicians from discriminating against patients based solely on firearm ownership. Violations of the law could result in a $10,000 fine per offense, a letter of reprimand, probation, suspension, compulsory remedial education, or permanent license revocation.
Shortly after the law passed, the ACP Florida Chapter sued the state; the suit was joined by the American Academy of Family Physicians Florida Chapter, the American Academy of Pediatrics Florida chapter, and a group of physicians. The plaintiffs argued that doctors routinely ask patients about potential health and safety risks, including firearms, to assess safety risks, educate patients and parents, and encourage gun safety. The gag law violated doctors’ free speech protections, according to the plaintiffs. The state argued that FOPA ensures patient privacy and protects gun owners’ right to own and bear arms from private encumbrances. A district court ruled in favor of the plaintiffs.
In the ruling, judges for the 11th U.S. Circuit Court of Appeals wrote that the right to own and possess firearms does not preclude questions about, commentary on, or criticism for the exercise of that right.
“As the district court aptly noted, there is no actual conflict between the First Amendment rights of doctors and medical professionals and the Second Amendment rights of patients that justifies FOPA’s speaker-focused and content-based restrictions on speech,” judges wrote. “Even if there were some possible conflict between the First Amendment rights of doctors and medical professionals and the Second Amendment rights of patients, the record-keeping, inquiry, and anti-harassment provisions do not advance [the legislative goals] in a permissible way.”
“The legislature has every right to regulate any profession to protect the public from discrimination and abuse,” Ms. Hammer said in an interview. “Doctors are businessmen, not gods. This activist decision attempts to use the First Amendment as a sword to terrorize the Second Amendment and completely disregards the rights and the will of the elected representatives of the people of Florida.”
The American Medical Association called the court ruling a clear victory against censorship of private medical discussions between patients and physicians.
“The court agreed that in the fields of medicine and public health, information saves lives,” according to an AMA statement. “Studies show that patients who received physician counseling on firearm safety are more likely to adopt one or more safe gun-storage practices. … Counseling patients we care for makes a difference in preventing gun-related injuries and deaths.”
[email protected]
On Twitter @legal_med
An appeals court has struck down a Florida law that banned physicians from asking patients about firearms, ruling that the so-called gun gag law violates doctors’ First Amendment rights.
The decision by the 11th U.S. Circuit Court of Appeals enables Florida physicians to once again query patients and families about gun ownership and record firearm information in their records. The court, however, left intact a provision that allows patients to refuse answering such questions and upheld another rule that physicians cannot discriminate against patients based on firearm status.
”While the chapter does not wish to impinge on the rights of gun owners, it has fought this legislation in the legislature and in the courts because it is essential that physicians and patients have the right to an open dialogue, free from government restrictions,” Dr. Goldman said in a statement. “[The] ruling is a victory for patients and the profession.”
Florida Gov. Rick Scott (R) did not respond to a request for comment.
The Florida Firearms Owners’ Privacy Act (FOPA) was enacted in 2011 after complaints from patients that physicians were inquiring about firearms in the home and in some cases, refusing to provide care if patients declined to answer. The law barred physicians from asking about firearms and from recording information about firearms in patient records. The statute also precluded doctors from unnecessarily harassing patients about firearm ownership during an examination and prevented physicians from discriminating against patients based solely on firearm ownership. Violations of the law could result in a $10,000 fine per offense, a letter of reprimand, probation, suspension, compulsory remedial education, or permanent license revocation.
Shortly after the law passed, the ACP Florida Chapter sued the state; the suit was joined by the American Academy of Family Physicians Florida Chapter, the American Academy of Pediatrics Florida chapter, and a group of physicians. The plaintiffs argued that doctors routinely ask patients about potential health and safety risks, including firearms, to assess safety risks, educate patients and parents, and encourage gun safety. The gag law violated doctors’ free speech protections, according to the plaintiffs. The state argued that FOPA ensures patient privacy and protects gun owners’ right to own and bear arms from private encumbrances. A district court ruled in favor of the plaintiffs.
In the ruling, judges for the 11th U.S. Circuit Court of Appeals wrote that the right to own and possess firearms does not preclude questions about, commentary on, or criticism for the exercise of that right.
“As the district court aptly noted, there is no actual conflict between the First Amendment rights of doctors and medical professionals and the Second Amendment rights of patients that justifies FOPA’s speaker-focused and content-based restrictions on speech,” judges wrote. “Even if there were some possible conflict between the First Amendment rights of doctors and medical professionals and the Second Amendment rights of patients, the record-keeping, inquiry, and anti-harassment provisions do not advance [the legislative goals] in a permissible way.”
“The legislature has every right to regulate any profession to protect the public from discrimination and abuse,” Ms. Hammer said in an interview. “Doctors are businessmen, not gods. This activist decision attempts to use the First Amendment as a sword to terrorize the Second Amendment and completely disregards the rights and the will of the elected representatives of the people of Florida.”
The American Medical Association called the court ruling a clear victory against censorship of private medical discussions between patients and physicians.
“The court agreed that in the fields of medicine and public health, information saves lives,” according to an AMA statement. “Studies show that patients who received physician counseling on firearm safety are more likely to adopt one or more safe gun-storage practices. … Counseling patients we care for makes a difference in preventing gun-related injuries and deaths.”
[email protected]
On Twitter @legal_med
How long Zika remains in body fluids
A study published in NEJM provides evidence that Zika virus RNA remain longer in blood and semen than in other body fluids, which suggests these may be superior diagnostic specimens.
This is the first study in which researchers examined multiple body fluids for the presence of Zika virus over a length of time.
The team sought to determine the frequency and duration of detectable Zika virus RNA in serum, saliva, urine, semen, and vaginal secretions.
They collected such specimens from 150 men and women in Puerto Rico who initially tested positive for Zika virus in urine or blood. The specimens were collected weekly for the first month and then at 2 months, 4 months, and 6 months.
The researchers tested all specimens using the Trioplex RT-PCR assay, a test that can be used to detect dengue, chikungunya, and Zika virus RNA. There have been allegations that this assay is less effective than a test used to detect Zika virus alone.
The researchers also performed validation analyses for the use of the Trioplex RT-PCR assay in semen. And they tested serum using the Zika IgM Antibody Capture Enzyme-Linked Immunosorbent Assay (Zika MAC-ELISA).
The US Centers for Disease Control and Prevention (CDC) developed both Zika MAC-ELISA and the Trioplex RT-PCR assay. This research was supported by the CDC.
After sample testing was complete, the researchers used parametric Weibull regression models to estimate the time to the loss of Zika virus RNA, which they reported in medians and 95th percentiles.
Results
The researchers said 88% of subjects (132/150) had detectable Zika virus RNA in at least 1 serum specimen. The median time to the loss of RNA detection in serum was 14 days (95% confidence interval [CI], 11 to 17), and the 95th percentile of time was 54 days (95% CI, 43 to 64).
The team also found that 61.7% of eligible subjects (92/149) had detectable Zika virus RNA in at least 1 urine specimen. The median time to the loss of RNA detection in urine was 8 days (95% CI, 6 to 10), and the 95th percentile of time was 39 days (95% CI, 31 to 47).
Fifteen subjects (10.1%) had detectable Zika virus RNA in urine but not serum, and 55 (36.7%) had RNA in serum but not urine.
Fifty-six percent of eligible male subjects (31/55) had Zika virus RNA in at least 1 semen specimen. The median time to loss of RNA detection in semen was 34 days (95% CI, 28 to 41), and the 95th percentile of time was 81 days (95% CI, 64 to 98).
The researchers noted that 11 of the 55 subjects had Zika virus RNA in their semen at their last visit and were still being followed at the time the NEJM article was written. The maximum duration of RNA detection was 125 days after the onset of symptoms.
Zika virus RNA levels were detectable in few saliva samples, with 10.2% of eligible subjects (15/147) having detectable levels in at least 1 saliva specimen.
Only 1 of 50 women (2%) had detectable Zika virus RNA in vaginal secretions.
“The findings of this study are important for both diagnostic and prevention purposes,” said study author Eli Rosenberg, PhD, of Emory University in Atlanta, Georgia.
“The results fully support current CDC sexual transmission recommendations but also provide critical information to help in understanding how often and how long evidence of Zika virus can be found in different body fluids. This knowledge is key to improving accuracy and effectiveness of testing methods while providing important baseline information for future research.” ![]()
A study published in NEJM provides evidence that Zika virus RNA remain longer in blood and semen than in other body fluids, which suggests these may be superior diagnostic specimens.
This is the first study in which researchers examined multiple body fluids for the presence of Zika virus over a length of time.
The team sought to determine the frequency and duration of detectable Zika virus RNA in serum, saliva, urine, semen, and vaginal secretions.
They collected such specimens from 150 men and women in Puerto Rico who initially tested positive for Zika virus in urine or blood. The specimens were collected weekly for the first month and then at 2 months, 4 months, and 6 months.
The researchers tested all specimens using the Trioplex RT-PCR assay, a test that can be used to detect dengue, chikungunya, and Zika virus RNA. There have been allegations that this assay is less effective than a test used to detect Zika virus alone.
The researchers also performed validation analyses for the use of the Trioplex RT-PCR assay in semen. And they tested serum using the Zika IgM Antibody Capture Enzyme-Linked Immunosorbent Assay (Zika MAC-ELISA).
The US Centers for Disease Control and Prevention (CDC) developed both Zika MAC-ELISA and the Trioplex RT-PCR assay. This research was supported by the CDC.
After sample testing was complete, the researchers used parametric Weibull regression models to estimate the time to the loss of Zika virus RNA, which they reported in medians and 95th percentiles.
Results
The researchers said 88% of subjects (132/150) had detectable Zika virus RNA in at least 1 serum specimen. The median time to the loss of RNA detection in serum was 14 days (95% confidence interval [CI], 11 to 17), and the 95th percentile of time was 54 days (95% CI, 43 to 64).
The team also found that 61.7% of eligible subjects (92/149) had detectable Zika virus RNA in at least 1 urine specimen. The median time to the loss of RNA detection in urine was 8 days (95% CI, 6 to 10), and the 95th percentile of time was 39 days (95% CI, 31 to 47).
Fifteen subjects (10.1%) had detectable Zika virus RNA in urine but not serum, and 55 (36.7%) had RNA in serum but not urine.
Fifty-six percent of eligible male subjects (31/55) had Zika virus RNA in at least 1 semen specimen. The median time to loss of RNA detection in semen was 34 days (95% CI, 28 to 41), and the 95th percentile of time was 81 days (95% CI, 64 to 98).
The researchers noted that 11 of the 55 subjects had Zika virus RNA in their semen at their last visit and were still being followed at the time the NEJM article was written. The maximum duration of RNA detection was 125 days after the onset of symptoms.
Zika virus RNA levels were detectable in few saliva samples, with 10.2% of eligible subjects (15/147) having detectable levels in at least 1 saliva specimen.
Only 1 of 50 women (2%) had detectable Zika virus RNA in vaginal secretions.
“The findings of this study are important for both diagnostic and prevention purposes,” said study author Eli Rosenberg, PhD, of Emory University in Atlanta, Georgia.
“The results fully support current CDC sexual transmission recommendations but also provide critical information to help in understanding how often and how long evidence of Zika virus can be found in different body fluids. This knowledge is key to improving accuracy and effectiveness of testing methods while providing important baseline information for future research.” ![]()
A study published in NEJM provides evidence that Zika virus RNA remain longer in blood and semen than in other body fluids, which suggests these may be superior diagnostic specimens.
This is the first study in which researchers examined multiple body fluids for the presence of Zika virus over a length of time.
The team sought to determine the frequency and duration of detectable Zika virus RNA in serum, saliva, urine, semen, and vaginal secretions.
They collected such specimens from 150 men and women in Puerto Rico who initially tested positive for Zika virus in urine or blood. The specimens were collected weekly for the first month and then at 2 months, 4 months, and 6 months.
The researchers tested all specimens using the Trioplex RT-PCR assay, a test that can be used to detect dengue, chikungunya, and Zika virus RNA. There have been allegations that this assay is less effective than a test used to detect Zika virus alone.
The researchers also performed validation analyses for the use of the Trioplex RT-PCR assay in semen. And they tested serum using the Zika IgM Antibody Capture Enzyme-Linked Immunosorbent Assay (Zika MAC-ELISA).
The US Centers for Disease Control and Prevention (CDC) developed both Zika MAC-ELISA and the Trioplex RT-PCR assay. This research was supported by the CDC.
After sample testing was complete, the researchers used parametric Weibull regression models to estimate the time to the loss of Zika virus RNA, which they reported in medians and 95th percentiles.
Results
The researchers said 88% of subjects (132/150) had detectable Zika virus RNA in at least 1 serum specimen. The median time to the loss of RNA detection in serum was 14 days (95% confidence interval [CI], 11 to 17), and the 95th percentile of time was 54 days (95% CI, 43 to 64).
The team also found that 61.7% of eligible subjects (92/149) had detectable Zika virus RNA in at least 1 urine specimen. The median time to the loss of RNA detection in urine was 8 days (95% CI, 6 to 10), and the 95th percentile of time was 39 days (95% CI, 31 to 47).
Fifteen subjects (10.1%) had detectable Zika virus RNA in urine but not serum, and 55 (36.7%) had RNA in serum but not urine.
Fifty-six percent of eligible male subjects (31/55) had Zika virus RNA in at least 1 semen specimen. The median time to loss of RNA detection in semen was 34 days (95% CI, 28 to 41), and the 95th percentile of time was 81 days (95% CI, 64 to 98).
The researchers noted that 11 of the 55 subjects had Zika virus RNA in their semen at their last visit and were still being followed at the time the NEJM article was written. The maximum duration of RNA detection was 125 days after the onset of symptoms.
Zika virus RNA levels were detectable in few saliva samples, with 10.2% of eligible subjects (15/147) having detectable levels in at least 1 saliva specimen.
Only 1 of 50 women (2%) had detectable Zika virus RNA in vaginal secretions.
“The findings of this study are important for both diagnostic and prevention purposes,” said study author Eli Rosenberg, PhD, of Emory University in Atlanta, Georgia.
“The results fully support current CDC sexual transmission recommendations but also provide critical information to help in understanding how often and how long evidence of Zika virus can be found in different body fluids. This knowledge is key to improving accuracy and effectiveness of testing methods while providing important baseline information for future research.” ![]()
LEEP tops cryotherapy for cervical dysplasia in women with HIV
SEATTLE – HIV-positive women treated with cryotherapy for cervical intraepithelial neoplasia (CIN) 2/3 were 52% more likely than women treated with loop excision to have a recurrence within 2 years, according to a randomized trial in Kenya.
The investigators called on the World Health Organization and other groups to support loop excision as the first-line option for HIV-positive women in sub-Saharan Africa and other low-resource settings, but the findings also support its use, when possible, in Western countries.
For the study, 200 HIV-positive women were randomized to cryotherapy and 200 to LEEP for treatment of CIN 2/3 at the Coptic Hope Center for Infectious Diseases in Nairobi, with follow-up Pap smears every 6 months afterwards for 2 years.
At 12 months, 36 women in the LEEP group (18%) had recurrent high-grade squamous intraepithelial lesions (HSIL), versus 54 women (27%) in the cryotherapy arm. At 24 months, HSIL was detected in 52 women who had LEEP (26%), versus 74 who had cryotherapy (37%).
Overall, the rate of recurrence of HSIL was 21.1 per 100 woman-years after cryotherapy and 14.0 per 100 woman-years after LEEP.
It’s unclear how those results would have played out in the United States, Ms. Green said, noting that LEEP failure rates are far lower among women who do not have HIV, but the success of LEEP in HIV-positive women in the United States has not been well studied.
The World Health Organization “recommends posttreatment follow-up at 12 months, regardless of HIV status. Our findings indicate that women should be screened at more frequent intervals, particularly following cryotherapy,” she said at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
Women were excluded from the study if they were pregnant. The median age was 37 years, and almost all the subjects were on antiretroviral therapy at the time of intervention, with a median CD4 count of 380 cells/mcL. Median follow-up was 2.1 years in both arms; most of the women completed all four follow-up visits. The majority in both groups had CIN 3; about 10 in each arm had carcinoma in situ.
“Cervical screening and treatment using visual inspection with acetic acid and cryotherapy is often implemented in resource-limited settings with high HIV-1 endemicity; however … HIV testing and referral for LEEP may be more effective” and cost effective if it prevents later hysterectomy, Ms. Greene said.
Ms. Greene had no disclosures. The work was funded by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) and the Centers for Disease Control and Prevention.
SEATTLE – HIV-positive women treated with cryotherapy for cervical intraepithelial neoplasia (CIN) 2/3 were 52% more likely than women treated with loop excision to have a recurrence within 2 years, according to a randomized trial in Kenya.
The investigators called on the World Health Organization and other groups to support loop excision as the first-line option for HIV-positive women in sub-Saharan Africa and other low-resource settings, but the findings also support its use, when possible, in Western countries.
For the study, 200 HIV-positive women were randomized to cryotherapy and 200 to LEEP for treatment of CIN 2/3 at the Coptic Hope Center for Infectious Diseases in Nairobi, with follow-up Pap smears every 6 months afterwards for 2 years.
At 12 months, 36 women in the LEEP group (18%) had recurrent high-grade squamous intraepithelial lesions (HSIL), versus 54 women (27%) in the cryotherapy arm. At 24 months, HSIL was detected in 52 women who had LEEP (26%), versus 74 who had cryotherapy (37%).
Overall, the rate of recurrence of HSIL was 21.1 per 100 woman-years after cryotherapy and 14.0 per 100 woman-years after LEEP.
It’s unclear how those results would have played out in the United States, Ms. Green said, noting that LEEP failure rates are far lower among women who do not have HIV, but the success of LEEP in HIV-positive women in the United States has not been well studied.
The World Health Organization “recommends posttreatment follow-up at 12 months, regardless of HIV status. Our findings indicate that women should be screened at more frequent intervals, particularly following cryotherapy,” she said at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
Women were excluded from the study if they were pregnant. The median age was 37 years, and almost all the subjects were on antiretroviral therapy at the time of intervention, with a median CD4 count of 380 cells/mcL. Median follow-up was 2.1 years in both arms; most of the women completed all four follow-up visits. The majority in both groups had CIN 3; about 10 in each arm had carcinoma in situ.
“Cervical screening and treatment using visual inspection with acetic acid and cryotherapy is often implemented in resource-limited settings with high HIV-1 endemicity; however … HIV testing and referral for LEEP may be more effective” and cost effective if it prevents later hysterectomy, Ms. Greene said.
Ms. Greene had no disclosures. The work was funded by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) and the Centers for Disease Control and Prevention.
SEATTLE – HIV-positive women treated with cryotherapy for cervical intraepithelial neoplasia (CIN) 2/3 were 52% more likely than women treated with loop excision to have a recurrence within 2 years, according to a randomized trial in Kenya.
The investigators called on the World Health Organization and other groups to support loop excision as the first-line option for HIV-positive women in sub-Saharan Africa and other low-resource settings, but the findings also support its use, when possible, in Western countries.
For the study, 200 HIV-positive women were randomized to cryotherapy and 200 to LEEP for treatment of CIN 2/3 at the Coptic Hope Center for Infectious Diseases in Nairobi, with follow-up Pap smears every 6 months afterwards for 2 years.
At 12 months, 36 women in the LEEP group (18%) had recurrent high-grade squamous intraepithelial lesions (HSIL), versus 54 women (27%) in the cryotherapy arm. At 24 months, HSIL was detected in 52 women who had LEEP (26%), versus 74 who had cryotherapy (37%).
Overall, the rate of recurrence of HSIL was 21.1 per 100 woman-years after cryotherapy and 14.0 per 100 woman-years after LEEP.
It’s unclear how those results would have played out in the United States, Ms. Green said, noting that LEEP failure rates are far lower among women who do not have HIV, but the success of LEEP in HIV-positive women in the United States has not been well studied.
The World Health Organization “recommends posttreatment follow-up at 12 months, regardless of HIV status. Our findings indicate that women should be screened at more frequent intervals, particularly following cryotherapy,” she said at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
Women were excluded from the study if they were pregnant. The median age was 37 years, and almost all the subjects were on antiretroviral therapy at the time of intervention, with a median CD4 count of 380 cells/mcL. Median follow-up was 2.1 years in both arms; most of the women completed all four follow-up visits. The majority in both groups had CIN 3; about 10 in each arm had carcinoma in situ.
“Cervical screening and treatment using visual inspection with acetic acid and cryotherapy is often implemented in resource-limited settings with high HIV-1 endemicity; however … HIV testing and referral for LEEP may be more effective” and cost effective if it prevents later hysterectomy, Ms. Greene said.
Ms. Greene had no disclosures. The work was funded by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) and the Centers for Disease Control and Prevention.
AT CROI
Key clinical point:
Major finding: At 12 months, 36 women in the LEEP group (18%) had recurrent high-grade squamous intraepithelial lesions, versus 54 women (27%) in the cryotherapy group. At 24 months, HSIL was detected in 52 women in the LEEP arm (26%) versus 74 who had cryotherapy (37%).
Data source: Randomized trial of 400 women in Kenya.
Disclosures: The lead investigator had no disclosures. The work was funded by the U.S. President’s Emergency Plan for AIDS Relief and the Centers for Disease Control and Prevention.
How Should the Treatment Costs of Distal Radius Fractures Be Measured?
Take-Home Points
- Physician fees, operating room costs, therapy costs, and missed work account for most (92%) of the costs in distal radius fractures.
- Indirect costs (especially missed work) contribute a significant amount to the total cost of injury.
- Patients continue to accrue costs up to 3-6 months post-injury.
- Implant costs make up only 6% of the total costs of operatively treated distal radius fractures.
Distal radius fractures (DRFs) account for 20% of all fractures seen in the emergency department, and are the most common fractures in all patients under age 75 years.1,2 Apart from causing pain and disability, DRFs have a large associated economic burden.3-6 In addition, over the past decade, the fixation technology used for DRF treatment has expanded rapidly and revolutionized operative management. With this expansion has come a growing body of high-level evidence guiding treatment decisions regarding patient outcomes.7-11 As operative treatment of these injuries has evolved, researchers have begun to critically evaluate both health outcomes and the cost-effectiveness of treatment choices.12,13
Determining the cost-effectiveness of any medical intervention requires an accurate and standardized method for measuring the total cost of a course of treatment. Although several studies have attempted to evaluate the treatment costs of DRFs,14-18 none has rigorously examined exactly what needs to be measured, and for how long, to accurately describe the overall cost. Many studies have examined only direct costs (treatment-related costs incurred in the hospital or clinic itself) and neglected indirect costs (eg, missed work, time in treatment, additional care requirements). As patient-reported disability from these injuries can be high,19-22 it is likely that the additional indirect costs, often borne by the patient, are correspondingly high. This relationship has been suggested by indirect data from large retrospective epidemiologic studies3-6 but has never been evaluated with primary data obtained in a prospective study.
Given these questions, we conducted an in-depth study of the treatment costs of these injuries to identify which factors should be captured, and for how long, to accurately describe the overall cost without missing any of the major cost-drivers. We hypothesized that indirect costs (particularly missed work) would be significant and variable cost-drivers in the overall economic impact of these injuries, and that direct prospective measurement of these costs would be the most reliable method for accurately assessing them. In short, this was a prospective, observational study of all the direct and indirect costs associated with treating DRFs. Its 2 main goals were to determine how much of the overall cost was attributable to indirect costs, and which cost factors should be measured, and for how long, to capture the true economic cost of these injuries.
Patients and Methods
Study Design
This prospective, observational study was approved by our hospital’s Institutional Review Board, and patients gave informed consent to participate. Patients with an isolated DRF that was treated either operatively or nonoperatively and followed at our hospital were eligible for the study. Treatment decisions for each patient were made by the treating surgeon and were based on injury characteristics. Patients with multiple concomitant injuries (polytrauma) were excluded. The AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification system was used to grade all fractures.23
Patients were seen 2 weeks, 1 month, 3 months, 6 months, and 1 year after injury. Each time, clinical data (strength, range of motion, patient-rated outcome forms) and economic data were collected. A patient’s economic data were considered complete if the patient had full follow-up in our clinic up to 1 year after injury or, if applicable, the patient returned to work and had all recurring direct and indirect costs resolved. Costs were measured and calculated from the broadest possible perspective (overall societal costs) rather than from payer-specific perspectives (eg, institution costs, insurance costs).
Treatment and Rehabilitation Protocol
Each patient who underwent nonoperative treatment was placed in a molded sugar-tong splint with hand motion encouraged and followed in clinic. At 4 to 6 weeks, the splint was removed, and the patient was placed in a removable cock-up wrist splint for another 2 to 4 weeks. Throughout this period, the patient worked on elbow and finger motion with an occupational therapist (OT). On discontinuation of the wrist splint, the patient returned to the OT for gentle wrist motion and continuation of elbow and finger motion.
For each patient who underwent operative treatment, implant and approach were based on fracture pattern. Implants used included isolated Kirschner wires (K-wires), volar locked plates, dorsal plates, radial column plates, and ulnar plates. After fixation, the patient was placed in a well-padded volar splint and encouraged to start immediate finger motion. Ten to 14 days after surgery, the splint was removed, and the patient was referred to an OT for gentle wrist, finger, and elbow motion. Therapy was continued until wrist, finger, and elbow motion was full.
Direct Costs
Direct costs were obtained from hospital billing and collections records. Cost items measured included physician fees, imaging fees, inpatient bed fees (when applicable), operating room (OR) facility fees, implant costs, and OT costs. Whenever possible, the final amount collected (vs charged) was used for the cost, as this was thought to be the most reliable indicator of the real cost of an item. Total cost was obtained from ultimate collection/reimbursement for all physician, imaging, and OT fees.
In a few cases, ultimate amount collected was not in our system and instead was calculated by normalizing the charges based on internal departmental cost-to-charge ratios. Cost-to-charge ratios were used for OR/emergency department facility fees, inpatient bed fees, and implant costs.
Indirect Costs
Indirect costs were calculated from questionnaires completed by patients at initial enrollment and at each follow-up visit. The initial enrollment form captured basic demographic information, employment status and work type, and annual income. The follow-up form included questions about current work status, physical/occupational therapy frequency, and extra recurring expenses related to transportation, household chores, and personal care, among other items. Total recurring expenses from transportation, chores, and personal care were calculated by multiplying the weekly expenses listed at a given visit by the time since the previous visit.
Costs for missed work were estimated as a function of preinjury wages multiplied by decreased level of productivity and period of work missed. For a patient who indicated part-time work status, decreased level of productivity was calculated by dividing the patient’s weekly hours by 40 (assumes 40-hour week is full-time), which yielded a percentage of full-time capacity. The patient was also asked to indicate any change in work status, which allowed for an accurate accounting of how long the patient was away from work and how much the patient’s capacity was decreased, ultimately providing an estimate of total amount of work missed. Multiplying that period by annual preinjury wages gave the value used for total cost of missed work.
Results
Of the 82 patients enrolled in the study, 36 were treated operatively and 46 nonoperatively. Table 1 lists additional demographic information about the study population.
Table 2 provides a full breakdown of costs. OT costs were similar between groups but proportionally made up 27% of the costs for the nonoperative group and 4.9% for the operative group.
Indirect costs accounted for 28% of the total cost for the operative group and 36% for the nonoperative group. Missed work was the major contributor to overall indirect cost, accounting for 93% of all indirect costs. Additional transportation, household chores, and personal care costs accounted for 4.7%, 1.7%, and 0.8% of total indirect costs, respectively.
Of the nonoperatively treated patients who had been working before being injured, 25% missed at least some work. Except for 1 patient, all were back working full-time within 3 months after injury. Of the operatively treated patients who had been working before injury, 48% missed at least some work, and 24% were still missing at least some work between 3 and 6 months after injury. All patients in both groups were back working within 1 year after injury.
Indirect costs largely paralleled work status, with 50% of patients still incurring some costs up to 6 months after injury (Figure).
Discussion
The drive to use evidence-based treatments in medicine has led to increased scrutiny of the benefits of novel treatments and technologies. However, in addition to carefully measuring clinical benefits, we must monitor costs. Implementation of new treatments based on small clinical advantages, without consideration of economic impact, will not be sustainable over the long term.
This study was not intended to report the “true” cost of treating these injuries, or to make direct comparisons between operative and nonoperative groups (regional and institutional costs and practices vary so much that no single-site study can report a meaningful number for cost). Furthermore, the observational (nonrandomized) nature of this study makes direct comparison of operative and nonoperative groups too confounded to draw conclusions. Simply, this study was conducted to help determine what needs to be measured, with the ultimate goal being to obtain a relatively reliable estimate of the total cost to society of a given injury and its treatment.
In this study, physician fees and facility fees were major direct expenses—not surprising given the value of physician time and OR time. In addition, OT was a fairly large direct-cost driver, particularly for nonoperative patients, for whom other costs were relatively low. This finding supports what has been reported in studies of the frequency and duration of therapy as potential targets for cost containment.24 Surprisingly, OT costs were lower for operatively (vs nonoperatively) treated patients. This finding may be attributable to earlier wrist motion in operatively treated patients (10-14 days) relative to nonoperatively treated patients (6-8 weeks), as earlier wrist motion may reduce stiffness and total need for therapy. Alternatively, the finding may be attributable to sampling error caused by difficulty in obtaining accurate OT costs, as some patients received therapy at multiple private offices, with records unavailable.
Although significant attention is often focused on implant costs, these actually comprised a relatively small portion (6%) of the total treatment costs for these injuries. However, implant costs vary significantly between institutions.
Indirect costs were a major factor, accounting for about one-third of total cost. Missed work was the single largest cost item in this study, comprising 93% of the indirect cost and 27% of the total cost. These findings suggest that the cost of missed work is crucial and should be measured in any study that compares the cost-effectiveness of different treatment modalities.
In orthopedic trauma, earlier return to work is often cited as a potential benefit of surgical intervention. However, without defining the exact economic impact of missed work, it is difficult to decide if earlier return to work justifies the added cost of surgery. The situation is further muddled by conflicting priorities, as the entities that bear the cost of missed work (patient, disability insurance) are often different from the entity that bears the cost of surgery (medical insurance). In the light of this complex decision-making with multiple and sometimes conflicting stakeholders, accurate understanding of the economic impact of missed work is paramount. Our data showed return to work took slightly longer for operatively (vs nonoperatively) treated patients, though we think this is more likely a result of higher injury severity than treatment choice.
Patients in both groups were still not back working up to 6 months after injury, indicating that return of function after these injuries is not as rapid as we might hope or expect, and may play a role in setting expectations during initial discussions with patients.
The major strength of this study is that it was the first of its kind to prospectively measure these costs at a single institution in order to make direct comparisons of different cost factors. Whenever possible, rather than relying on cost-to-charge ratio estimates, we analyzed costs obtained directly from collections reports, which improved the validity of the results generated. Missed work was captured by directly asking patients about work capacity, not by retrospectively reviewing disability applications, which for a variety of reasons often inaccurately reflects true work productivity. In addition, our final follow-up rate was relatively high (91%), which helped minimize bias. Although this study focused on DRFs, the hope is that these data can serve as a template for the kinds of factors that need to be measured to accurately describe the cost of many different upper extremity injuries. This idea, however, needs to be formally tested.
This study had several limitations. First, some costs (OR time, facility fees) still had to be estimated with cost-to-charge ratios—a less precise method. Second, measuring the societal cost of missed work is controversial. We calculated this cost by using standard economic techniques, valuing the decreased productivity period according to baseline salary, though the true “loss” to society is less clear. Third, our data represent the costs at one hospital in one city and might be very different at other institutions with different cost structures. Fourth, this study was observational (vs randomized) and subject to the usual bias of such studies, so conclusions between treatment choices and cost or clinical outcomes could not be drawn (which was not our intent in this study). Although these issues limited our ability to calculate the exact “cost” of these injuries, the relative impact of the different cost factors could be measured (which was our intent).
DRFs are common injuries that can have significant associated expenses, many of which were not captured in previous cost analyses. In the present study, we found that measuring physician, OR, therapy, and missed work costs for at least 6 months after injury was generally sufficient for accurate capture of major costs. We hope these data can help in planning studies of the treatment costs of upper extremity injuries. Only through accurate and conscientious data gathering can we evaluate the clinical and economic effects of novel technologies and ensure delivery of high-quality care while containing costs and improving efficiency.
Am J Orthop. 2017;46(1):E54-E59. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Simic PM, Weiland AJ. Fractures of the distal aspect of the radius: changes in treatment over the past two decades. Instr Course Lect. 2003;52:185-195.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Trybus M, Guzik P. The economic impact of hand injury [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2003;68(4):269-273.
4. Dias JJ, Garcia-Elias M. Hand injury costs. Injury. 2006;37(11):1071-1077.
5. Wüthrich P. Epidemiology and socioeconomic significance of hand injuries [in German]. Z Unfallchir Versicherungsmed Berufskr. 1986;79(1):5-14.
6. de Putter CE, Selles RW, Polinder S, Panneman MJ, Hovius SE, van Beeck EF. Economic impact of hand and wrist injuries: health-care costs and productivity costs in a population-based study. J Bone Joint Surg Am. 2012;94(9):e56.
7. Wong TC, Chiu Y, Tsang WL, Leung WY, Yam SK, Yeung SH. Casting versus percutaneous pinning for extra-articular fractures of the distal radius in an elderly Chinese population: a prospective randomised controlled trial. J Hand Surg Eur Vol. 2010;35(3):202-208.
8. Krukhaug Y, Ugland S, Lie SA, Hove LM. External fixation of fractures of the distal radius: a randomized comparison of the Hoffman Compact II non-bridging fixator and the Dynawrist fixator in 75 patients followed for 1 year. Acta Orthop. 2009;80(1):104-108.
9. Xu GG, Chan SP, Puhaindran ME, Chew WY. Prospective randomised study of intra-articular fractures of the distal radius: comparison between external fixation and plate fixation. Ann Acad Med Singapore. 2009;38(7):600-606.
10. Egol K, Walsh M, Tejwani N, McLaurin T, Wynn C, Paksima N. Bridging external fixation and supplementary Kirschner-wire fixation versus volar locked plating for unstable fractures of the distal radius: a randomised, prospective trial. J Bone Joint Surg Br. 2008;90(9):1214-1221.
11. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
12. Shauver MJ, Clapham PJ, Chung KC. An economic analysis of outcomes and complications of treating distal radius fractures in the elderly. J Hand Surg Am. 2011;36(12):1912-1918.e1-e3.
13. Espinosa Gutiérrez A, Moreno Velázquez A. Cost–benefit of various treatments for patients with distal radius fracture [in Spanish]. Acta Ortop Mex. 2010;24(2):61-65.
14. Shyamalan G, Theokli C, Pearse Y, Tennent D. Volar locking plates versus Kirschner wires for distal radial fractures—a cost analysis study. Injury. 2009;40(12):1279-1281.
15. Kakarlapudi TK, Santini A, Shahane SA, Douglas D. The cost of treatment of distal radial fractures. Injury. 2000;31(4):229-232.
16. Do TT, Strub WM, Foad SL, Mehlman CT, Crawford AH. Reduction versus remodeling in pediatric distal forearm fractures: a preliminary cost analysis. J Pediatr Orthop B. 2003;12(2):109-115.
17. Miller BS, Taylor B, Widmann RF, Bae DS, Snyder BD, Waters PM. Cast immobilization versus percutaneous pin fixation of displaced distal radius fractures in children: a prospective, randomized study. J Pediatr Orthop. 2005;25(4):490-494.
18. Shauver MJ, Yin H, Banerjee M, Chung KC. Current and future national costs to Medicare for the treatment of distal radius fracture in the elderly. J Hand Surg Am. 2011;36(8):1282-1287.
19. Handoll HH, Madhok R, Howe TE. Rehabilitation for distal radial fractures in adults. Cochrane Database Syst Rev. 2006;(3):CD003324.
20. Handoll HH, Huntley JS, Madhok R. External fixation versus conservative treatment for distal radial fractures in adults. Cochrane Database Syst Rev. 2007;(3):CD006194.
21. Handoll HH, Vaghela MV, Madhok R. Percutaneous pinning for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2007;(3):CD006080.
22. Handoll HH, Huntley JS, Madhok R. Different methods of external fixation for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2008;(1):CD006522.
23. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
24. Souer JS, Buijze G, Ring D. A prospective randomized controlled trial comparing occupational therapy with independent exercises after volar plate fixation of a fracture of the distal part of the radius. J Bone Joint Surg Am. 2011;93(19):1761-1766.
Take-Home Points
- Physician fees, operating room costs, therapy costs, and missed work account for most (92%) of the costs in distal radius fractures.
- Indirect costs (especially missed work) contribute a significant amount to the total cost of injury.
- Patients continue to accrue costs up to 3-6 months post-injury.
- Implant costs make up only 6% of the total costs of operatively treated distal radius fractures.
Distal radius fractures (DRFs) account for 20% of all fractures seen in the emergency department, and are the most common fractures in all patients under age 75 years.1,2 Apart from causing pain and disability, DRFs have a large associated economic burden.3-6 In addition, over the past decade, the fixation technology used for DRF treatment has expanded rapidly and revolutionized operative management. With this expansion has come a growing body of high-level evidence guiding treatment decisions regarding patient outcomes.7-11 As operative treatment of these injuries has evolved, researchers have begun to critically evaluate both health outcomes and the cost-effectiveness of treatment choices.12,13
Determining the cost-effectiveness of any medical intervention requires an accurate and standardized method for measuring the total cost of a course of treatment. Although several studies have attempted to evaluate the treatment costs of DRFs,14-18 none has rigorously examined exactly what needs to be measured, and for how long, to accurately describe the overall cost. Many studies have examined only direct costs (treatment-related costs incurred in the hospital or clinic itself) and neglected indirect costs (eg, missed work, time in treatment, additional care requirements). As patient-reported disability from these injuries can be high,19-22 it is likely that the additional indirect costs, often borne by the patient, are correspondingly high. This relationship has been suggested by indirect data from large retrospective epidemiologic studies3-6 but has never been evaluated with primary data obtained in a prospective study.
Given these questions, we conducted an in-depth study of the treatment costs of these injuries to identify which factors should be captured, and for how long, to accurately describe the overall cost without missing any of the major cost-drivers. We hypothesized that indirect costs (particularly missed work) would be significant and variable cost-drivers in the overall economic impact of these injuries, and that direct prospective measurement of these costs would be the most reliable method for accurately assessing them. In short, this was a prospective, observational study of all the direct and indirect costs associated with treating DRFs. Its 2 main goals were to determine how much of the overall cost was attributable to indirect costs, and which cost factors should be measured, and for how long, to capture the true economic cost of these injuries.
Patients and Methods
Study Design
This prospective, observational study was approved by our hospital’s Institutional Review Board, and patients gave informed consent to participate. Patients with an isolated DRF that was treated either operatively or nonoperatively and followed at our hospital were eligible for the study. Treatment decisions for each patient were made by the treating surgeon and were based on injury characteristics. Patients with multiple concomitant injuries (polytrauma) were excluded. The AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification system was used to grade all fractures.23
Patients were seen 2 weeks, 1 month, 3 months, 6 months, and 1 year after injury. Each time, clinical data (strength, range of motion, patient-rated outcome forms) and economic data were collected. A patient’s economic data were considered complete if the patient had full follow-up in our clinic up to 1 year after injury or, if applicable, the patient returned to work and had all recurring direct and indirect costs resolved. Costs were measured and calculated from the broadest possible perspective (overall societal costs) rather than from payer-specific perspectives (eg, institution costs, insurance costs).
Treatment and Rehabilitation Protocol
Each patient who underwent nonoperative treatment was placed in a molded sugar-tong splint with hand motion encouraged and followed in clinic. At 4 to 6 weeks, the splint was removed, and the patient was placed in a removable cock-up wrist splint for another 2 to 4 weeks. Throughout this period, the patient worked on elbow and finger motion with an occupational therapist (OT). On discontinuation of the wrist splint, the patient returned to the OT for gentle wrist motion and continuation of elbow and finger motion.
For each patient who underwent operative treatment, implant and approach were based on fracture pattern. Implants used included isolated Kirschner wires (K-wires), volar locked plates, dorsal plates, radial column plates, and ulnar plates. After fixation, the patient was placed in a well-padded volar splint and encouraged to start immediate finger motion. Ten to 14 days after surgery, the splint was removed, and the patient was referred to an OT for gentle wrist, finger, and elbow motion. Therapy was continued until wrist, finger, and elbow motion was full.
Direct Costs
Direct costs were obtained from hospital billing and collections records. Cost items measured included physician fees, imaging fees, inpatient bed fees (when applicable), operating room (OR) facility fees, implant costs, and OT costs. Whenever possible, the final amount collected (vs charged) was used for the cost, as this was thought to be the most reliable indicator of the real cost of an item. Total cost was obtained from ultimate collection/reimbursement for all physician, imaging, and OT fees.
In a few cases, ultimate amount collected was not in our system and instead was calculated by normalizing the charges based on internal departmental cost-to-charge ratios. Cost-to-charge ratios were used for OR/emergency department facility fees, inpatient bed fees, and implant costs.
Indirect Costs
Indirect costs were calculated from questionnaires completed by patients at initial enrollment and at each follow-up visit. The initial enrollment form captured basic demographic information, employment status and work type, and annual income. The follow-up form included questions about current work status, physical/occupational therapy frequency, and extra recurring expenses related to transportation, household chores, and personal care, among other items. Total recurring expenses from transportation, chores, and personal care were calculated by multiplying the weekly expenses listed at a given visit by the time since the previous visit.
Costs for missed work were estimated as a function of preinjury wages multiplied by decreased level of productivity and period of work missed. For a patient who indicated part-time work status, decreased level of productivity was calculated by dividing the patient’s weekly hours by 40 (assumes 40-hour week is full-time), which yielded a percentage of full-time capacity. The patient was also asked to indicate any change in work status, which allowed for an accurate accounting of how long the patient was away from work and how much the patient’s capacity was decreased, ultimately providing an estimate of total amount of work missed. Multiplying that period by annual preinjury wages gave the value used for total cost of missed work.
Results
Of the 82 patients enrolled in the study, 36 were treated operatively and 46 nonoperatively. Table 1 lists additional demographic information about the study population.
Table 2 provides a full breakdown of costs. OT costs were similar between groups but proportionally made up 27% of the costs for the nonoperative group and 4.9% for the operative group.
Indirect costs accounted for 28% of the total cost for the operative group and 36% for the nonoperative group. Missed work was the major contributor to overall indirect cost, accounting for 93% of all indirect costs. Additional transportation, household chores, and personal care costs accounted for 4.7%, 1.7%, and 0.8% of total indirect costs, respectively.
Of the nonoperatively treated patients who had been working before being injured, 25% missed at least some work. Except for 1 patient, all were back working full-time within 3 months after injury. Of the operatively treated patients who had been working before injury, 48% missed at least some work, and 24% were still missing at least some work between 3 and 6 months after injury. All patients in both groups were back working within 1 year after injury.
Indirect costs largely paralleled work status, with 50% of patients still incurring some costs up to 6 months after injury (Figure).
Discussion
The drive to use evidence-based treatments in medicine has led to increased scrutiny of the benefits of novel treatments and technologies. However, in addition to carefully measuring clinical benefits, we must monitor costs. Implementation of new treatments based on small clinical advantages, without consideration of economic impact, will not be sustainable over the long term.
This study was not intended to report the “true” cost of treating these injuries, or to make direct comparisons between operative and nonoperative groups (regional and institutional costs and practices vary so much that no single-site study can report a meaningful number for cost). Furthermore, the observational (nonrandomized) nature of this study makes direct comparison of operative and nonoperative groups too confounded to draw conclusions. Simply, this study was conducted to help determine what needs to be measured, with the ultimate goal being to obtain a relatively reliable estimate of the total cost to society of a given injury and its treatment.
In this study, physician fees and facility fees were major direct expenses—not surprising given the value of physician time and OR time. In addition, OT was a fairly large direct-cost driver, particularly for nonoperative patients, for whom other costs were relatively low. This finding supports what has been reported in studies of the frequency and duration of therapy as potential targets for cost containment.24 Surprisingly, OT costs were lower for operatively (vs nonoperatively) treated patients. This finding may be attributable to earlier wrist motion in operatively treated patients (10-14 days) relative to nonoperatively treated patients (6-8 weeks), as earlier wrist motion may reduce stiffness and total need for therapy. Alternatively, the finding may be attributable to sampling error caused by difficulty in obtaining accurate OT costs, as some patients received therapy at multiple private offices, with records unavailable.
Although significant attention is often focused on implant costs, these actually comprised a relatively small portion (6%) of the total treatment costs for these injuries. However, implant costs vary significantly between institutions.
Indirect costs were a major factor, accounting for about one-third of total cost. Missed work was the single largest cost item in this study, comprising 93% of the indirect cost and 27% of the total cost. These findings suggest that the cost of missed work is crucial and should be measured in any study that compares the cost-effectiveness of different treatment modalities.
In orthopedic trauma, earlier return to work is often cited as a potential benefit of surgical intervention. However, without defining the exact economic impact of missed work, it is difficult to decide if earlier return to work justifies the added cost of surgery. The situation is further muddled by conflicting priorities, as the entities that bear the cost of missed work (patient, disability insurance) are often different from the entity that bears the cost of surgery (medical insurance). In the light of this complex decision-making with multiple and sometimes conflicting stakeholders, accurate understanding of the economic impact of missed work is paramount. Our data showed return to work took slightly longer for operatively (vs nonoperatively) treated patients, though we think this is more likely a result of higher injury severity than treatment choice.
Patients in both groups were still not back working up to 6 months after injury, indicating that return of function after these injuries is not as rapid as we might hope or expect, and may play a role in setting expectations during initial discussions with patients.
The major strength of this study is that it was the first of its kind to prospectively measure these costs at a single institution in order to make direct comparisons of different cost factors. Whenever possible, rather than relying on cost-to-charge ratio estimates, we analyzed costs obtained directly from collections reports, which improved the validity of the results generated. Missed work was captured by directly asking patients about work capacity, not by retrospectively reviewing disability applications, which for a variety of reasons often inaccurately reflects true work productivity. In addition, our final follow-up rate was relatively high (91%), which helped minimize bias. Although this study focused on DRFs, the hope is that these data can serve as a template for the kinds of factors that need to be measured to accurately describe the cost of many different upper extremity injuries. This idea, however, needs to be formally tested.
This study had several limitations. First, some costs (OR time, facility fees) still had to be estimated with cost-to-charge ratios—a less precise method. Second, measuring the societal cost of missed work is controversial. We calculated this cost by using standard economic techniques, valuing the decreased productivity period according to baseline salary, though the true “loss” to society is less clear. Third, our data represent the costs at one hospital in one city and might be very different at other institutions with different cost structures. Fourth, this study was observational (vs randomized) and subject to the usual bias of such studies, so conclusions between treatment choices and cost or clinical outcomes could not be drawn (which was not our intent in this study). Although these issues limited our ability to calculate the exact “cost” of these injuries, the relative impact of the different cost factors could be measured (which was our intent).
DRFs are common injuries that can have significant associated expenses, many of which were not captured in previous cost analyses. In the present study, we found that measuring physician, OR, therapy, and missed work costs for at least 6 months after injury was generally sufficient for accurate capture of major costs. We hope these data can help in planning studies of the treatment costs of upper extremity injuries. Only through accurate and conscientious data gathering can we evaluate the clinical and economic effects of novel technologies and ensure delivery of high-quality care while containing costs and improving efficiency.
Am J Orthop. 2017;46(1):E54-E59. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Physician fees, operating room costs, therapy costs, and missed work account for most (92%) of the costs in distal radius fractures.
- Indirect costs (especially missed work) contribute a significant amount to the total cost of injury.
- Patients continue to accrue costs up to 3-6 months post-injury.
- Implant costs make up only 6% of the total costs of operatively treated distal radius fractures.
Distal radius fractures (DRFs) account for 20% of all fractures seen in the emergency department, and are the most common fractures in all patients under age 75 years.1,2 Apart from causing pain and disability, DRFs have a large associated economic burden.3-6 In addition, over the past decade, the fixation technology used for DRF treatment has expanded rapidly and revolutionized operative management. With this expansion has come a growing body of high-level evidence guiding treatment decisions regarding patient outcomes.7-11 As operative treatment of these injuries has evolved, researchers have begun to critically evaluate both health outcomes and the cost-effectiveness of treatment choices.12,13
Determining the cost-effectiveness of any medical intervention requires an accurate and standardized method for measuring the total cost of a course of treatment. Although several studies have attempted to evaluate the treatment costs of DRFs,14-18 none has rigorously examined exactly what needs to be measured, and for how long, to accurately describe the overall cost. Many studies have examined only direct costs (treatment-related costs incurred in the hospital or clinic itself) and neglected indirect costs (eg, missed work, time in treatment, additional care requirements). As patient-reported disability from these injuries can be high,19-22 it is likely that the additional indirect costs, often borne by the patient, are correspondingly high. This relationship has been suggested by indirect data from large retrospective epidemiologic studies3-6 but has never been evaluated with primary data obtained in a prospective study.
Given these questions, we conducted an in-depth study of the treatment costs of these injuries to identify which factors should be captured, and for how long, to accurately describe the overall cost without missing any of the major cost-drivers. We hypothesized that indirect costs (particularly missed work) would be significant and variable cost-drivers in the overall economic impact of these injuries, and that direct prospective measurement of these costs would be the most reliable method for accurately assessing them. In short, this was a prospective, observational study of all the direct and indirect costs associated with treating DRFs. Its 2 main goals were to determine how much of the overall cost was attributable to indirect costs, and which cost factors should be measured, and for how long, to capture the true economic cost of these injuries.
Patients and Methods
Study Design
This prospective, observational study was approved by our hospital’s Institutional Review Board, and patients gave informed consent to participate. Patients with an isolated DRF that was treated either operatively or nonoperatively and followed at our hospital were eligible for the study. Treatment decisions for each patient were made by the treating surgeon and were based on injury characteristics. Patients with multiple concomitant injuries (polytrauma) were excluded. The AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification system was used to grade all fractures.23
Patients were seen 2 weeks, 1 month, 3 months, 6 months, and 1 year after injury. Each time, clinical data (strength, range of motion, patient-rated outcome forms) and economic data were collected. A patient’s economic data were considered complete if the patient had full follow-up in our clinic up to 1 year after injury or, if applicable, the patient returned to work and had all recurring direct and indirect costs resolved. Costs were measured and calculated from the broadest possible perspective (overall societal costs) rather than from payer-specific perspectives (eg, institution costs, insurance costs).
Treatment and Rehabilitation Protocol
Each patient who underwent nonoperative treatment was placed in a molded sugar-tong splint with hand motion encouraged and followed in clinic. At 4 to 6 weeks, the splint was removed, and the patient was placed in a removable cock-up wrist splint for another 2 to 4 weeks. Throughout this period, the patient worked on elbow and finger motion with an occupational therapist (OT). On discontinuation of the wrist splint, the patient returned to the OT for gentle wrist motion and continuation of elbow and finger motion.
For each patient who underwent operative treatment, implant and approach were based on fracture pattern. Implants used included isolated Kirschner wires (K-wires), volar locked plates, dorsal plates, radial column plates, and ulnar plates. After fixation, the patient was placed in a well-padded volar splint and encouraged to start immediate finger motion. Ten to 14 days after surgery, the splint was removed, and the patient was referred to an OT for gentle wrist, finger, and elbow motion. Therapy was continued until wrist, finger, and elbow motion was full.
Direct Costs
Direct costs were obtained from hospital billing and collections records. Cost items measured included physician fees, imaging fees, inpatient bed fees (when applicable), operating room (OR) facility fees, implant costs, and OT costs. Whenever possible, the final amount collected (vs charged) was used for the cost, as this was thought to be the most reliable indicator of the real cost of an item. Total cost was obtained from ultimate collection/reimbursement for all physician, imaging, and OT fees.
In a few cases, ultimate amount collected was not in our system and instead was calculated by normalizing the charges based on internal departmental cost-to-charge ratios. Cost-to-charge ratios were used for OR/emergency department facility fees, inpatient bed fees, and implant costs.
Indirect Costs
Indirect costs were calculated from questionnaires completed by patients at initial enrollment and at each follow-up visit. The initial enrollment form captured basic demographic information, employment status and work type, and annual income. The follow-up form included questions about current work status, physical/occupational therapy frequency, and extra recurring expenses related to transportation, household chores, and personal care, among other items. Total recurring expenses from transportation, chores, and personal care were calculated by multiplying the weekly expenses listed at a given visit by the time since the previous visit.
Costs for missed work were estimated as a function of preinjury wages multiplied by decreased level of productivity and period of work missed. For a patient who indicated part-time work status, decreased level of productivity was calculated by dividing the patient’s weekly hours by 40 (assumes 40-hour week is full-time), which yielded a percentage of full-time capacity. The patient was also asked to indicate any change in work status, which allowed for an accurate accounting of how long the patient was away from work and how much the patient’s capacity was decreased, ultimately providing an estimate of total amount of work missed. Multiplying that period by annual preinjury wages gave the value used for total cost of missed work.
Results
Of the 82 patients enrolled in the study, 36 were treated operatively and 46 nonoperatively. Table 1 lists additional demographic information about the study population.
Table 2 provides a full breakdown of costs. OT costs were similar between groups but proportionally made up 27% of the costs for the nonoperative group and 4.9% for the operative group.
Indirect costs accounted for 28% of the total cost for the operative group and 36% for the nonoperative group. Missed work was the major contributor to overall indirect cost, accounting for 93% of all indirect costs. Additional transportation, household chores, and personal care costs accounted for 4.7%, 1.7%, and 0.8% of total indirect costs, respectively.
Of the nonoperatively treated patients who had been working before being injured, 25% missed at least some work. Except for 1 patient, all were back working full-time within 3 months after injury. Of the operatively treated patients who had been working before injury, 48% missed at least some work, and 24% were still missing at least some work between 3 and 6 months after injury. All patients in both groups were back working within 1 year after injury.
Indirect costs largely paralleled work status, with 50% of patients still incurring some costs up to 6 months after injury (Figure).
Discussion
The drive to use evidence-based treatments in medicine has led to increased scrutiny of the benefits of novel treatments and technologies. However, in addition to carefully measuring clinical benefits, we must monitor costs. Implementation of new treatments based on small clinical advantages, without consideration of economic impact, will not be sustainable over the long term.
This study was not intended to report the “true” cost of treating these injuries, or to make direct comparisons between operative and nonoperative groups (regional and institutional costs and practices vary so much that no single-site study can report a meaningful number for cost). Furthermore, the observational (nonrandomized) nature of this study makes direct comparison of operative and nonoperative groups too confounded to draw conclusions. Simply, this study was conducted to help determine what needs to be measured, with the ultimate goal being to obtain a relatively reliable estimate of the total cost to society of a given injury and its treatment.
In this study, physician fees and facility fees were major direct expenses—not surprising given the value of physician time and OR time. In addition, OT was a fairly large direct-cost driver, particularly for nonoperative patients, for whom other costs were relatively low. This finding supports what has been reported in studies of the frequency and duration of therapy as potential targets for cost containment.24 Surprisingly, OT costs were lower for operatively (vs nonoperatively) treated patients. This finding may be attributable to earlier wrist motion in operatively treated patients (10-14 days) relative to nonoperatively treated patients (6-8 weeks), as earlier wrist motion may reduce stiffness and total need for therapy. Alternatively, the finding may be attributable to sampling error caused by difficulty in obtaining accurate OT costs, as some patients received therapy at multiple private offices, with records unavailable.
Although significant attention is often focused on implant costs, these actually comprised a relatively small portion (6%) of the total treatment costs for these injuries. However, implant costs vary significantly between institutions.
Indirect costs were a major factor, accounting for about one-third of total cost. Missed work was the single largest cost item in this study, comprising 93% of the indirect cost and 27% of the total cost. These findings suggest that the cost of missed work is crucial and should be measured in any study that compares the cost-effectiveness of different treatment modalities.
In orthopedic trauma, earlier return to work is often cited as a potential benefit of surgical intervention. However, without defining the exact economic impact of missed work, it is difficult to decide if earlier return to work justifies the added cost of surgery. The situation is further muddled by conflicting priorities, as the entities that bear the cost of missed work (patient, disability insurance) are often different from the entity that bears the cost of surgery (medical insurance). In the light of this complex decision-making with multiple and sometimes conflicting stakeholders, accurate understanding of the economic impact of missed work is paramount. Our data showed return to work took slightly longer for operatively (vs nonoperatively) treated patients, though we think this is more likely a result of higher injury severity than treatment choice.
Patients in both groups were still not back working up to 6 months after injury, indicating that return of function after these injuries is not as rapid as we might hope or expect, and may play a role in setting expectations during initial discussions with patients.
The major strength of this study is that it was the first of its kind to prospectively measure these costs at a single institution in order to make direct comparisons of different cost factors. Whenever possible, rather than relying on cost-to-charge ratio estimates, we analyzed costs obtained directly from collections reports, which improved the validity of the results generated. Missed work was captured by directly asking patients about work capacity, not by retrospectively reviewing disability applications, which for a variety of reasons often inaccurately reflects true work productivity. In addition, our final follow-up rate was relatively high (91%), which helped minimize bias. Although this study focused on DRFs, the hope is that these data can serve as a template for the kinds of factors that need to be measured to accurately describe the cost of many different upper extremity injuries. This idea, however, needs to be formally tested.
This study had several limitations. First, some costs (OR time, facility fees) still had to be estimated with cost-to-charge ratios—a less precise method. Second, measuring the societal cost of missed work is controversial. We calculated this cost by using standard economic techniques, valuing the decreased productivity period according to baseline salary, though the true “loss” to society is less clear. Third, our data represent the costs at one hospital in one city and might be very different at other institutions with different cost structures. Fourth, this study was observational (vs randomized) and subject to the usual bias of such studies, so conclusions between treatment choices and cost or clinical outcomes could not be drawn (which was not our intent in this study). Although these issues limited our ability to calculate the exact “cost” of these injuries, the relative impact of the different cost factors could be measured (which was our intent).
DRFs are common injuries that can have significant associated expenses, many of which were not captured in previous cost analyses. In the present study, we found that measuring physician, OR, therapy, and missed work costs for at least 6 months after injury was generally sufficient for accurate capture of major costs. We hope these data can help in planning studies of the treatment costs of upper extremity injuries. Only through accurate and conscientious data gathering can we evaluate the clinical and economic effects of novel technologies and ensure delivery of high-quality care while containing costs and improving efficiency.
Am J Orthop. 2017;46(1):E54-E59. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Simic PM, Weiland AJ. Fractures of the distal aspect of the radius: changes in treatment over the past two decades. Instr Course Lect. 2003;52:185-195.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Trybus M, Guzik P. The economic impact of hand injury [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2003;68(4):269-273.
4. Dias JJ, Garcia-Elias M. Hand injury costs. Injury. 2006;37(11):1071-1077.
5. Wüthrich P. Epidemiology and socioeconomic significance of hand injuries [in German]. Z Unfallchir Versicherungsmed Berufskr. 1986;79(1):5-14.
6. de Putter CE, Selles RW, Polinder S, Panneman MJ, Hovius SE, van Beeck EF. Economic impact of hand and wrist injuries: health-care costs and productivity costs in a population-based study. J Bone Joint Surg Am. 2012;94(9):e56.
7. Wong TC, Chiu Y, Tsang WL, Leung WY, Yam SK, Yeung SH. Casting versus percutaneous pinning for extra-articular fractures of the distal radius in an elderly Chinese population: a prospective randomised controlled trial. J Hand Surg Eur Vol. 2010;35(3):202-208.
8. Krukhaug Y, Ugland S, Lie SA, Hove LM. External fixation of fractures of the distal radius: a randomized comparison of the Hoffman Compact II non-bridging fixator and the Dynawrist fixator in 75 patients followed for 1 year. Acta Orthop. 2009;80(1):104-108.
9. Xu GG, Chan SP, Puhaindran ME, Chew WY. Prospective randomised study of intra-articular fractures of the distal radius: comparison between external fixation and plate fixation. Ann Acad Med Singapore. 2009;38(7):600-606.
10. Egol K, Walsh M, Tejwani N, McLaurin T, Wynn C, Paksima N. Bridging external fixation and supplementary Kirschner-wire fixation versus volar locked plating for unstable fractures of the distal radius: a randomised, prospective trial. J Bone Joint Surg Br. 2008;90(9):1214-1221.
11. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
12. Shauver MJ, Clapham PJ, Chung KC. An economic analysis of outcomes and complications of treating distal radius fractures in the elderly. J Hand Surg Am. 2011;36(12):1912-1918.e1-e3.
13. Espinosa Gutiérrez A, Moreno Velázquez A. Cost–benefit of various treatments for patients with distal radius fracture [in Spanish]. Acta Ortop Mex. 2010;24(2):61-65.
14. Shyamalan G, Theokli C, Pearse Y, Tennent D. Volar locking plates versus Kirschner wires for distal radial fractures—a cost analysis study. Injury. 2009;40(12):1279-1281.
15. Kakarlapudi TK, Santini A, Shahane SA, Douglas D. The cost of treatment of distal radial fractures. Injury. 2000;31(4):229-232.
16. Do TT, Strub WM, Foad SL, Mehlman CT, Crawford AH. Reduction versus remodeling in pediatric distal forearm fractures: a preliminary cost analysis. J Pediatr Orthop B. 2003;12(2):109-115.
17. Miller BS, Taylor B, Widmann RF, Bae DS, Snyder BD, Waters PM. Cast immobilization versus percutaneous pin fixation of displaced distal radius fractures in children: a prospective, randomized study. J Pediatr Orthop. 2005;25(4):490-494.
18. Shauver MJ, Yin H, Banerjee M, Chung KC. Current and future national costs to Medicare for the treatment of distal radius fracture in the elderly. J Hand Surg Am. 2011;36(8):1282-1287.
19. Handoll HH, Madhok R, Howe TE. Rehabilitation for distal radial fractures in adults. Cochrane Database Syst Rev. 2006;(3):CD003324.
20. Handoll HH, Huntley JS, Madhok R. External fixation versus conservative treatment for distal radial fractures in adults. Cochrane Database Syst Rev. 2007;(3):CD006194.
21. Handoll HH, Vaghela MV, Madhok R. Percutaneous pinning for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2007;(3):CD006080.
22. Handoll HH, Huntley JS, Madhok R. Different methods of external fixation for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2008;(1):CD006522.
23. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
24. Souer JS, Buijze G, Ring D. A prospective randomized controlled trial comparing occupational therapy with independent exercises after volar plate fixation of a fracture of the distal part of the radius. J Bone Joint Surg Am. 2011;93(19):1761-1766.
1. Simic PM, Weiland AJ. Fractures of the distal aspect of the radius: changes in treatment over the past two decades. Instr Course Lect. 2003;52:185-195.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Trybus M, Guzik P. The economic impact of hand injury [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2003;68(4):269-273.
4. Dias JJ, Garcia-Elias M. Hand injury costs. Injury. 2006;37(11):1071-1077.
5. Wüthrich P. Epidemiology and socioeconomic significance of hand injuries [in German]. Z Unfallchir Versicherungsmed Berufskr. 1986;79(1):5-14.
6. de Putter CE, Selles RW, Polinder S, Panneman MJ, Hovius SE, van Beeck EF. Economic impact of hand and wrist injuries: health-care costs and productivity costs in a population-based study. J Bone Joint Surg Am. 2012;94(9):e56.
7. Wong TC, Chiu Y, Tsang WL, Leung WY, Yam SK, Yeung SH. Casting versus percutaneous pinning for extra-articular fractures of the distal radius in an elderly Chinese population: a prospective randomised controlled trial. J Hand Surg Eur Vol. 2010;35(3):202-208.
8. Krukhaug Y, Ugland S, Lie SA, Hove LM. External fixation of fractures of the distal radius: a randomized comparison of the Hoffman Compact II non-bridging fixator and the Dynawrist fixator in 75 patients followed for 1 year. Acta Orthop. 2009;80(1):104-108.
9. Xu GG, Chan SP, Puhaindran ME, Chew WY. Prospective randomised study of intra-articular fractures of the distal radius: comparison between external fixation and plate fixation. Ann Acad Med Singapore. 2009;38(7):600-606.
10. Egol K, Walsh M, Tejwani N, McLaurin T, Wynn C, Paksima N. Bridging external fixation and supplementary Kirschner-wire fixation versus volar locked plating for unstable fractures of the distal radius: a randomised, prospective trial. J Bone Joint Surg Br. 2008;90(9):1214-1221.
11. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
12. Shauver MJ, Clapham PJ, Chung KC. An economic analysis of outcomes and complications of treating distal radius fractures in the elderly. J Hand Surg Am. 2011;36(12):1912-1918.e1-e3.
13. Espinosa Gutiérrez A, Moreno Velázquez A. Cost–benefit of various treatments for patients with distal radius fracture [in Spanish]. Acta Ortop Mex. 2010;24(2):61-65.
14. Shyamalan G, Theokli C, Pearse Y, Tennent D. Volar locking plates versus Kirschner wires for distal radial fractures—a cost analysis study. Injury. 2009;40(12):1279-1281.
15. Kakarlapudi TK, Santini A, Shahane SA, Douglas D. The cost of treatment of distal radial fractures. Injury. 2000;31(4):229-232.
16. Do TT, Strub WM, Foad SL, Mehlman CT, Crawford AH. Reduction versus remodeling in pediatric distal forearm fractures: a preliminary cost analysis. J Pediatr Orthop B. 2003;12(2):109-115.
17. Miller BS, Taylor B, Widmann RF, Bae DS, Snyder BD, Waters PM. Cast immobilization versus percutaneous pin fixation of displaced distal radius fractures in children: a prospective, randomized study. J Pediatr Orthop. 2005;25(4):490-494.
18. Shauver MJ, Yin H, Banerjee M, Chung KC. Current and future national costs to Medicare for the treatment of distal radius fracture in the elderly. J Hand Surg Am. 2011;36(8):1282-1287.
19. Handoll HH, Madhok R, Howe TE. Rehabilitation for distal radial fractures in adults. Cochrane Database Syst Rev. 2006;(3):CD003324.
20. Handoll HH, Huntley JS, Madhok R. External fixation versus conservative treatment for distal radial fractures in adults. Cochrane Database Syst Rev. 2007;(3):CD006194.
21. Handoll HH, Vaghela MV, Madhok R. Percutaneous pinning for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2007;(3):CD006080.
22. Handoll HH, Huntley JS, Madhok R. Different methods of external fixation for treating distal radial fractures in adults. Cochrane Database Syst Rev. 2008;(1):CD006522.
23. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
24. Souer JS, Buijze G, Ring D. A prospective randomized controlled trial comparing occupational therapy with independent exercises after volar plate fixation of a fracture of the distal part of the radius. J Bone Joint Surg Am. 2011;93(19):1761-1766.