User login
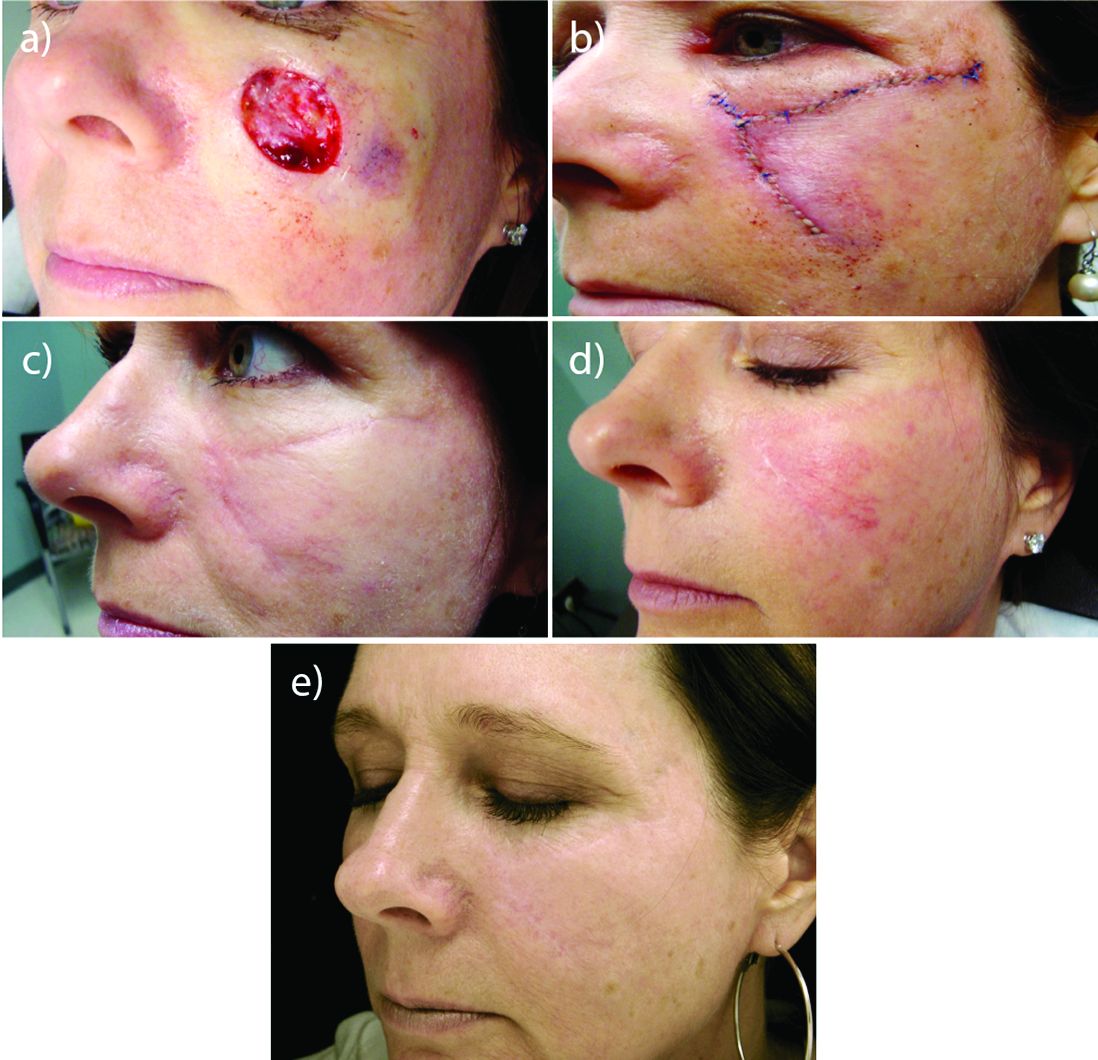
Laser resurfacing can effectively minimize post surgery scars
MIAMI – In his practice, Joel L. Cohen, MD, spends a good part of his day doing Mohs surgery, “with the goal of cancer removal, and after surgery, having the patient look good,” he said at the Orlando Dermatology Aesthetic and Clinical Conference.
“Having resurfacing in my practice has allowed me to treat not only wrinkles and etched lines, but also help skin cancer patients by blending and minimizing their skin cancer scars,” said Dr. Cohen, an aesthetic dermatologist and Mohs surgeon in private practice in Denver.
Resurfacing in his practice using a variety of lasers is very helpful, Dr. Cohen said. He published a study in November that compared pulse dye laser, CO2 ablative fractional lasers, or a combination of both for modification of scars following Mohs surgery (J Drugs Dermatol. 2016 Nov 1;15[11]:1315-9).
The prospective, multicenter study revealed that although both monotherapy approaches were safe and effective, the combination of pulse dye laser and fractional ablative laser offered some synergy that was preferred by patients.
Perioral resurfacing possible
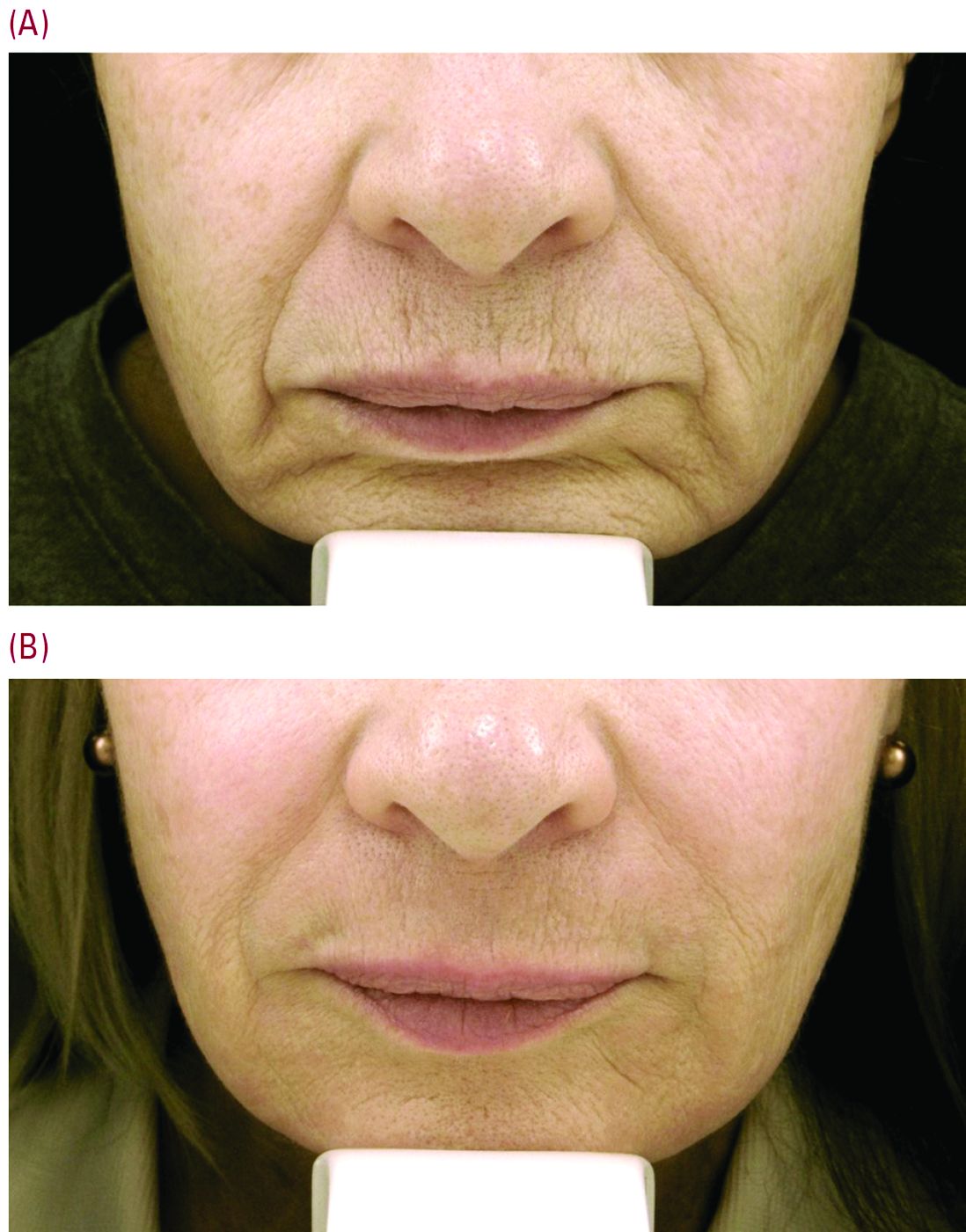
Beyond the world of treating scars, a typical cosmetic patient in Dr. Cohen’s practice presents with numerous lines around the perioral area. “When people think about rejuvenation of the lips, they only think of fillers. But fillers are not the only way to rejuvenate this area, and it is really about choosing the right tool for the right job – where resurfacing lasers are needed.”
Set realistic expectations
Setting the right expectations for people is extremely important, Dr. Cohen said. “You can educate the patient that if you’re putting the needle into the lines, you’re only treating the larger lines that you can get a 30-g needle into, but there are often a host of other lines in that area – many of which are too small to get a needle into.”
As a starting point, neuromodulators can have a role in trying to prevent or delay etched-in lines from forming around the mouth in the first place. “These are the lines between the musculature, the ones you see when you ask the patient to purse their lips,” Dr. Cohen said. He typically injects a medium dose of one of three neuromodulators – such as 6-10 U of onabotulinumtoxinA (Botox), 6-10 U of incobotulinumtoxinA (Xeomin) or 14-18 U of abobotulinumtoxinA (Dysport). “Then somewhere between week 8 and 10, there is an attenuation of the effect, and I often will see patients back then for additional treatment with a neuromodulator,” he added.
“For our every day patient complaining of lots of etched perioral lines, we have laser resurfacing,” Dr. Cohen noted. He is a bigger proponent of full-field erbium treatment versus fractional ablative laser resurfacing for these prominent upper cutaneous lip lines because the results are much more impressive with a single treatment. He added that dermatologists could do fractional treatment around the rest of the face, and reserve the erbium resurfacing to improve the appearance of lines around the mouth and prominent creping skin around the eyes.
Realistic postprocedure expectations are especially essential in the days after erbium laser resurfacing – as it is a tough downtime procedure for patients, often taking 7-9 days to re-epithelialize. “Having photos to show patients what they will look like is really helpful,” Dr. Cohen said. He suggested showing patients a chronologic set of photos of the downtime period as well as the results – so they realize improvement occurs slowly over time. “Getting people to understand they are gong to look terrible for 1.5-2 weeks is superimportant.”
“I like to have them back in the office for a postprocedure check a few days after the bigger laser resurfacing procedures are done, just to check on them,” Dr. Cohen said. “A lot of hand holding is often needed, as there is significantly more healing time with the full-field ablative resurfacing than there is with fractional. Full-field resurfacing patients will experience postprocedure erythema for a few weeks or even months,” Dr. Cohen said. A prescription of topical steroids, and sometimes some brimonidine topical gel (Mirvaso) as well can help reduce the redness.
Toxin injection then laser resurfacing
For some patients, injection of a neuromodulator a week or 2 before laser resurfacing treatment can decrease some of the movement and contraction of the muscle, “and hopefully give them better results,” Dr. Cohen said.
Timing is important. “You don’t want to use neuromodulators on the same day of treatment,” he advised. “The thinking is swelling could potentially cause the neuromodulators to spread to unwanted adjacent muscles.”
Safety first
Another tip for the postprocedure period is to supply patients with very specific written instructions. “I wish they would follow them. Patients don’t always listen to what we advise, demonstrate, and also have written down for them,” he commented. For example, one patient had resurfacing several weeks before leaving on an undisclosed kayaking trip. Despite instructions to use sunscreen, she said she wore a hat for sun protection and developed postinflammatory hyperpigmentation around the mouth that lasted for several months, Dr. Cohen said.*
With heavy resurfacing and ablative resurfacing in general, it is advised to always give patients an antiviral prophylaxis course such as valacyclovir, but it is unfortunate that not all patients will adhere to the recommended regimen, he added.
Another patient had an adverse reaction after resurfacing because she did not follow instructions to apply white petrolatum to her chest following laser resurfacing, Dr. Cohen said. She used Neosporin, “even though in all our paperwork we say never use Neosporin and just use the petrolatum. She had a big contact dermatitis reaction to the Neosporin.”
“So you really need to caution people about the importance of following instructions very carefully,” he emphasized.
Dr. Cohen is a consultant for Sciton and for companies that manufacture injectables, including Allergan, Galderma, and Merz.
Correction 2/24/17: An earlier version of this article mischaracterized the type of pigmentation disorder that the patient developed.
MIAMI – In his practice, Joel L. Cohen, MD, spends a good part of his day doing Mohs surgery, “with the goal of cancer removal, and after surgery, having the patient look good,” he said at the Orlando Dermatology Aesthetic and Clinical Conference.
“Having resurfacing in my practice has allowed me to treat not only wrinkles and etched lines, but also help skin cancer patients by blending and minimizing their skin cancer scars,” said Dr. Cohen, an aesthetic dermatologist and Mohs surgeon in private practice in Denver.
Resurfacing in his practice using a variety of lasers is very helpful, Dr. Cohen said. He published a study in November that compared pulse dye laser, CO2 ablative fractional lasers, or a combination of both for modification of scars following Mohs surgery (J Drugs Dermatol. 2016 Nov 1;15[11]:1315-9).
The prospective, multicenter study revealed that although both monotherapy approaches were safe and effective, the combination of pulse dye laser and fractional ablative laser offered some synergy that was preferred by patients.

Perioral resurfacing possible
Beyond the world of treating scars, a typical cosmetic patient in Dr. Cohen’s practice presents with numerous lines around the perioral area. “When people think about rejuvenation of the lips, they only think of fillers. But fillers are not the only way to rejuvenate this area, and it is really about choosing the right tool for the right job – where resurfacing lasers are needed.”

Set realistic expectations
Setting the right expectations for people is extremely important, Dr. Cohen said. “You can educate the patient that if you’re putting the needle into the lines, you’re only treating the larger lines that you can get a 30-g needle into, but there are often a host of other lines in that area – many of which are too small to get a needle into.”
As a starting point, neuromodulators can have a role in trying to prevent or delay etched-in lines from forming around the mouth in the first place. “These are the lines between the musculature, the ones you see when you ask the patient to purse their lips,” Dr. Cohen said. He typically injects a medium dose of one of three neuromodulators – such as 6-10 U of onabotulinumtoxinA (Botox), 6-10 U of incobotulinumtoxinA (Xeomin) or 14-18 U of abobotulinumtoxinA (Dysport). “Then somewhere between week 8 and 10, there is an attenuation of the effect, and I often will see patients back then for additional treatment with a neuromodulator,” he added.
“For our every day patient complaining of lots of etched perioral lines, we have laser resurfacing,” Dr. Cohen noted. He is a bigger proponent of full-field erbium treatment versus fractional ablative laser resurfacing for these prominent upper cutaneous lip lines because the results are much more impressive with a single treatment. He added that dermatologists could do fractional treatment around the rest of the face, and reserve the erbium resurfacing to improve the appearance of lines around the mouth and prominent creping skin around the eyes.
Realistic postprocedure expectations are especially essential in the days after erbium laser resurfacing – as it is a tough downtime procedure for patients, often taking 7-9 days to re-epithelialize. “Having photos to show patients what they will look like is really helpful,” Dr. Cohen said. He suggested showing patients a chronologic set of photos of the downtime period as well as the results – so they realize improvement occurs slowly over time. “Getting people to understand they are gong to look terrible for 1.5-2 weeks is superimportant.”
“I like to have them back in the office for a postprocedure check a few days after the bigger laser resurfacing procedures are done, just to check on them,” Dr. Cohen said. “A lot of hand holding is often needed, as there is significantly more healing time with the full-field ablative resurfacing than there is with fractional. Full-field resurfacing patients will experience postprocedure erythema for a few weeks or even months,” Dr. Cohen said. A prescription of topical steroids, and sometimes some brimonidine topical gel (Mirvaso) as well can help reduce the redness.
Toxin injection then laser resurfacing
For some patients, injection of a neuromodulator a week or 2 before laser resurfacing treatment can decrease some of the movement and contraction of the muscle, “and hopefully give them better results,” Dr. Cohen said.
Timing is important. “You don’t want to use neuromodulators on the same day of treatment,” he advised. “The thinking is swelling could potentially cause the neuromodulators to spread to unwanted adjacent muscles.”
Safety first
Another tip for the postprocedure period is to supply patients with very specific written instructions. “I wish they would follow them. Patients don’t always listen to what we advise, demonstrate, and also have written down for them,” he commented. For example, one patient had resurfacing several weeks before leaving on an undisclosed kayaking trip. Despite instructions to use sunscreen, she said she wore a hat for sun protection and developed postinflammatory hyperpigmentation around the mouth that lasted for several months, Dr. Cohen said.*
With heavy resurfacing and ablative resurfacing in general, it is advised to always give patients an antiviral prophylaxis course such as valacyclovir, but it is unfortunate that not all patients will adhere to the recommended regimen, he added.
Another patient had an adverse reaction after resurfacing because she did not follow instructions to apply white petrolatum to her chest following laser resurfacing, Dr. Cohen said. She used Neosporin, “even though in all our paperwork we say never use Neosporin and just use the petrolatum. She had a big contact dermatitis reaction to the Neosporin.”
“So you really need to caution people about the importance of following instructions very carefully,” he emphasized.
Dr. Cohen is a consultant for Sciton and for companies that manufacture injectables, including Allergan, Galderma, and Merz.
Correction 2/24/17: An earlier version of this article mischaracterized the type of pigmentation disorder that the patient developed.
MIAMI – In his practice, Joel L. Cohen, MD, spends a good part of his day doing Mohs surgery, “with the goal of cancer removal, and after surgery, having the patient look good,” he said at the Orlando Dermatology Aesthetic and Clinical Conference.
“Having resurfacing in my practice has allowed me to treat not only wrinkles and etched lines, but also help skin cancer patients by blending and minimizing their skin cancer scars,” said Dr. Cohen, an aesthetic dermatologist and Mohs surgeon in private practice in Denver.
Resurfacing in his practice using a variety of lasers is very helpful, Dr. Cohen said. He published a study in November that compared pulse dye laser, CO2 ablative fractional lasers, or a combination of both for modification of scars following Mohs surgery (J Drugs Dermatol. 2016 Nov 1;15[11]:1315-9).
The prospective, multicenter study revealed that although both monotherapy approaches were safe and effective, the combination of pulse dye laser and fractional ablative laser offered some synergy that was preferred by patients.

Perioral resurfacing possible
Beyond the world of treating scars, a typical cosmetic patient in Dr. Cohen’s practice presents with numerous lines around the perioral area. “When people think about rejuvenation of the lips, they only think of fillers. But fillers are not the only way to rejuvenate this area, and it is really about choosing the right tool for the right job – where resurfacing lasers are needed.”

Set realistic expectations
Setting the right expectations for people is extremely important, Dr. Cohen said. “You can educate the patient that if you’re putting the needle into the lines, you’re only treating the larger lines that you can get a 30-g needle into, but there are often a host of other lines in that area – many of which are too small to get a needle into.”
As a starting point, neuromodulators can have a role in trying to prevent or delay etched-in lines from forming around the mouth in the first place. “These are the lines between the musculature, the ones you see when you ask the patient to purse their lips,” Dr. Cohen said. He typically injects a medium dose of one of three neuromodulators – such as 6-10 U of onabotulinumtoxinA (Botox), 6-10 U of incobotulinumtoxinA (Xeomin) or 14-18 U of abobotulinumtoxinA (Dysport). “Then somewhere between week 8 and 10, there is an attenuation of the effect, and I often will see patients back then for additional treatment with a neuromodulator,” he added.
“For our every day patient complaining of lots of etched perioral lines, we have laser resurfacing,” Dr. Cohen noted. He is a bigger proponent of full-field erbium treatment versus fractional ablative laser resurfacing for these prominent upper cutaneous lip lines because the results are much more impressive with a single treatment. He added that dermatologists could do fractional treatment around the rest of the face, and reserve the erbium resurfacing to improve the appearance of lines around the mouth and prominent creping skin around the eyes.
Realistic postprocedure expectations are especially essential in the days after erbium laser resurfacing – as it is a tough downtime procedure for patients, often taking 7-9 days to re-epithelialize. “Having photos to show patients what they will look like is really helpful,” Dr. Cohen said. He suggested showing patients a chronologic set of photos of the downtime period as well as the results – so they realize improvement occurs slowly over time. “Getting people to understand they are gong to look terrible for 1.5-2 weeks is superimportant.”
“I like to have them back in the office for a postprocedure check a few days after the bigger laser resurfacing procedures are done, just to check on them,” Dr. Cohen said. “A lot of hand holding is often needed, as there is significantly more healing time with the full-field ablative resurfacing than there is with fractional. Full-field resurfacing patients will experience postprocedure erythema for a few weeks or even months,” Dr. Cohen said. A prescription of topical steroids, and sometimes some brimonidine topical gel (Mirvaso) as well can help reduce the redness.
Toxin injection then laser resurfacing
For some patients, injection of a neuromodulator a week or 2 before laser resurfacing treatment can decrease some of the movement and contraction of the muscle, “and hopefully give them better results,” Dr. Cohen said.
Timing is important. “You don’t want to use neuromodulators on the same day of treatment,” he advised. “The thinking is swelling could potentially cause the neuromodulators to spread to unwanted adjacent muscles.”
Safety first
Another tip for the postprocedure period is to supply patients with very specific written instructions. “I wish they would follow them. Patients don’t always listen to what we advise, demonstrate, and also have written down for them,” he commented. For example, one patient had resurfacing several weeks before leaving on an undisclosed kayaking trip. Despite instructions to use sunscreen, she said she wore a hat for sun protection and developed postinflammatory hyperpigmentation around the mouth that lasted for several months, Dr. Cohen said.*
With heavy resurfacing and ablative resurfacing in general, it is advised to always give patients an antiviral prophylaxis course such as valacyclovir, but it is unfortunate that not all patients will adhere to the recommended regimen, he added.
Another patient had an adverse reaction after resurfacing because she did not follow instructions to apply white petrolatum to her chest following laser resurfacing, Dr. Cohen said. She used Neosporin, “even though in all our paperwork we say never use Neosporin and just use the petrolatum. She had a big contact dermatitis reaction to the Neosporin.”
“So you really need to caution people about the importance of following instructions very carefully,” he emphasized.
Dr. Cohen is a consultant for Sciton and for companies that manufacture injectables, including Allergan, Galderma, and Merz.
Correction 2/24/17: An earlier version of this article mischaracterized the type of pigmentation disorder that the patient developed.
EXPERT ANALYSIS FROM THE ODAC CONFERENCE
Can a nomogram foretell invasive pulmonary adenocarcinoma?
The diagnosis of solitary peripheral subsolid nodule carries with it an undefined risk of invasive pulmonary carcinoma, but clinicians have not had a tool that can help guide their planning for surgery. However, researchers in China have developed a nomogram that they said may aid clinicians to predict the risk of invasive pulmonary adenocarcinoma in these patients.
“Validation by the use of bootstrap resampling revealed optimal discrimination and calibration, indicating that the nomogram may have clinical utility,” said Chenghua Jin, MD, and Jinlin Cao, MD, of Zhejiang University, Hangzhou, China, and coauthors. They reported their findings in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:42-9).
The nomogram accounts for the following factors: computed tomography attenuation; nodule size; spiculation; signs of vascular convergence; pleural tags; and solid proportion. “The nomogram showed a robust discrimination with an area under the receiver operating characteristic curve of 0.894,” Dr. Jin and coauthors reported. An area under the curve of 1 is equivalent to 100%, so the area under the curve this study reported shows close to 90% accuracy.
The study involved a retrospective analysis of 273 consecutive patients who had resection of a solitary peripheral subsolid nodule at Zhejiang University School of Medicine from January 2013 to December 2014. Subsolid pulmonary nodules include pure ground-glass nodules and part-solid nodules that feature both solid and ground-glass components. “The optimal management of patients with a subsolid nodule is of growing clinical concern, because the most common diagnosis for resected subsolid nodules is lung adenocarcinoma,” Dr. Jin and colleagues indicated.
Of the study population, 58% were diagnosed with invasive pulmonary adenocarcinoma. Other diagnoses within the group were benign (13%), atypical adenomatous hyperplasia (1%), adenocarcinoma in situ (6.5%) and minimally invasive adenocarcinoma (21%).
Results of the multivariable analyses showed that invasive pulmonary adenocarcinoma correlated with the following characteristics: lesion size; spiculation; vascular convergence; and pleural tag. Factors that were not significant included age, family history of lung cancer, CT attenuation, and solid proportion. However, the researchers did include CT attenuation, along with solid proportion, in the final regression analysis based on their contributions to the statistical analysis.
For the model, CT attenuation of –500 to –200 Hounsfield units carried an odds ratio of 1.690 (P = .228) while CT attenuation greater than –200 HU had an OR of 1.791 (P = .645). Positive spiculation had an OR of 3.312 (no P value given) and negative vascular convergence an OR of 0.300 (no P value given).
While a number of prediction models have been devised and validated to evaluate the likelihood of malignancy in pulmonary nodules, they have not given subsolid nodules “specific or detailed consideration,” Dr. Jin and and coauthors said. “To our knowledge, this study was the first to construct a quantitative nomogram to predict the probability of invasive pulmonary adenocarcinoma in patients with subsolid nodules,” the researchers wrote.
One limitation of the study is its selection bias toward patients with a greater probability of having a malignancy. Also, validation of the nomogram requires external analysis with additional databases from other countries and with more diverse ethnic groups. Another shortcoming is the retrospective nature of the study and a small number of patients who had positron emission tomography. “Further data collection, wider geographic recruitment, and incorporation of positron emission tomography results and some molecular factors could improve this model for future use,” Dr. Jin and coauthors concluded.
Dr. Jin and Dr. Cao had no relevant financial disclosures. The study received funding from the Zhejiang Province Science and Technology Plan.
The nomogram Dr. Jin and coauthors present can be a valuable tool for determining the extent of resection of subsolid pulmonary nodules and to distinguish invasive from preinvasive disease where preoperative needle biopsy and intraopertiave frozen section typically cannot, Bryan Burt, MD, of Baylor College of Medicine, Houston, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:460-1).
“However,” Dr. Burt added, “as the accuracy of frozen section for this disease improves, as it has in select centers, the clinical utility of such a nomogram will diminish.”
Use of the nomogram relies on experienced chest radiologists to aid in scoring variables and a validation methodology that a retrospective trial cannot meet, Dr. Burt said. “Of note, this nomogram was constructed from a dataset composed of only surgically resected lesions, and it will be imperative to validate these methods among a larger cohort of individuals with subsolid pulmonary nodules treated both surgically and nonsurgically, ideally in a prospective trial,” Dr. Burt concluded.
Dr. Burt had no relevant financial disclosures.
The nomogram Dr. Jin and coauthors present can be a valuable tool for determining the extent of resection of subsolid pulmonary nodules and to distinguish invasive from preinvasive disease where preoperative needle biopsy and intraopertiave frozen section typically cannot, Bryan Burt, MD, of Baylor College of Medicine, Houston, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:460-1).
“However,” Dr. Burt added, “as the accuracy of frozen section for this disease improves, as it has in select centers, the clinical utility of such a nomogram will diminish.”
Use of the nomogram relies on experienced chest radiologists to aid in scoring variables and a validation methodology that a retrospective trial cannot meet, Dr. Burt said. “Of note, this nomogram was constructed from a dataset composed of only surgically resected lesions, and it will be imperative to validate these methods among a larger cohort of individuals with subsolid pulmonary nodules treated both surgically and nonsurgically, ideally in a prospective trial,” Dr. Burt concluded.
Dr. Burt had no relevant financial disclosures.
The nomogram Dr. Jin and coauthors present can be a valuable tool for determining the extent of resection of subsolid pulmonary nodules and to distinguish invasive from preinvasive disease where preoperative needle biopsy and intraopertiave frozen section typically cannot, Bryan Burt, MD, of Baylor College of Medicine, Houston, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:460-1).
“However,” Dr. Burt added, “as the accuracy of frozen section for this disease improves, as it has in select centers, the clinical utility of such a nomogram will diminish.”
Use of the nomogram relies on experienced chest radiologists to aid in scoring variables and a validation methodology that a retrospective trial cannot meet, Dr. Burt said. “Of note, this nomogram was constructed from a dataset composed of only surgically resected lesions, and it will be imperative to validate these methods among a larger cohort of individuals with subsolid pulmonary nodules treated both surgically and nonsurgically, ideally in a prospective trial,” Dr. Burt concluded.
Dr. Burt had no relevant financial disclosures.
The diagnosis of solitary peripheral subsolid nodule carries with it an undefined risk of invasive pulmonary carcinoma, but clinicians have not had a tool that can help guide their planning for surgery. However, researchers in China have developed a nomogram that they said may aid clinicians to predict the risk of invasive pulmonary adenocarcinoma in these patients.
“Validation by the use of bootstrap resampling revealed optimal discrimination and calibration, indicating that the nomogram may have clinical utility,” said Chenghua Jin, MD, and Jinlin Cao, MD, of Zhejiang University, Hangzhou, China, and coauthors. They reported their findings in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:42-9).
The nomogram accounts for the following factors: computed tomography attenuation; nodule size; spiculation; signs of vascular convergence; pleural tags; and solid proportion. “The nomogram showed a robust discrimination with an area under the receiver operating characteristic curve of 0.894,” Dr. Jin and coauthors reported. An area under the curve of 1 is equivalent to 100%, so the area under the curve this study reported shows close to 90% accuracy.
The study involved a retrospective analysis of 273 consecutive patients who had resection of a solitary peripheral subsolid nodule at Zhejiang University School of Medicine from January 2013 to December 2014. Subsolid pulmonary nodules include pure ground-glass nodules and part-solid nodules that feature both solid and ground-glass components. “The optimal management of patients with a subsolid nodule is of growing clinical concern, because the most common diagnosis for resected subsolid nodules is lung adenocarcinoma,” Dr. Jin and colleagues indicated.
Of the study population, 58% were diagnosed with invasive pulmonary adenocarcinoma. Other diagnoses within the group were benign (13%), atypical adenomatous hyperplasia (1%), adenocarcinoma in situ (6.5%) and minimally invasive adenocarcinoma (21%).
Results of the multivariable analyses showed that invasive pulmonary adenocarcinoma correlated with the following characteristics: lesion size; spiculation; vascular convergence; and pleural tag. Factors that were not significant included age, family history of lung cancer, CT attenuation, and solid proportion. However, the researchers did include CT attenuation, along with solid proportion, in the final regression analysis based on their contributions to the statistical analysis.
For the model, CT attenuation of –500 to –200 Hounsfield units carried an odds ratio of 1.690 (P = .228) while CT attenuation greater than –200 HU had an OR of 1.791 (P = .645). Positive spiculation had an OR of 3.312 (no P value given) and negative vascular convergence an OR of 0.300 (no P value given).
While a number of prediction models have been devised and validated to evaluate the likelihood of malignancy in pulmonary nodules, they have not given subsolid nodules “specific or detailed consideration,” Dr. Jin and and coauthors said. “To our knowledge, this study was the first to construct a quantitative nomogram to predict the probability of invasive pulmonary adenocarcinoma in patients with subsolid nodules,” the researchers wrote.
One limitation of the study is its selection bias toward patients with a greater probability of having a malignancy. Also, validation of the nomogram requires external analysis with additional databases from other countries and with more diverse ethnic groups. Another shortcoming is the retrospective nature of the study and a small number of patients who had positron emission tomography. “Further data collection, wider geographic recruitment, and incorporation of positron emission tomography results and some molecular factors could improve this model for future use,” Dr. Jin and coauthors concluded.
Dr. Jin and Dr. Cao had no relevant financial disclosures. The study received funding from the Zhejiang Province Science and Technology Plan.
The diagnosis of solitary peripheral subsolid nodule carries with it an undefined risk of invasive pulmonary carcinoma, but clinicians have not had a tool that can help guide their planning for surgery. However, researchers in China have developed a nomogram that they said may aid clinicians to predict the risk of invasive pulmonary adenocarcinoma in these patients.
“Validation by the use of bootstrap resampling revealed optimal discrimination and calibration, indicating that the nomogram may have clinical utility,” said Chenghua Jin, MD, and Jinlin Cao, MD, of Zhejiang University, Hangzhou, China, and coauthors. They reported their findings in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:42-9).
The nomogram accounts for the following factors: computed tomography attenuation; nodule size; spiculation; signs of vascular convergence; pleural tags; and solid proportion. “The nomogram showed a robust discrimination with an area under the receiver operating characteristic curve of 0.894,” Dr. Jin and coauthors reported. An area under the curve of 1 is equivalent to 100%, so the area under the curve this study reported shows close to 90% accuracy.
The study involved a retrospective analysis of 273 consecutive patients who had resection of a solitary peripheral subsolid nodule at Zhejiang University School of Medicine from January 2013 to December 2014. Subsolid pulmonary nodules include pure ground-glass nodules and part-solid nodules that feature both solid and ground-glass components. “The optimal management of patients with a subsolid nodule is of growing clinical concern, because the most common diagnosis for resected subsolid nodules is lung adenocarcinoma,” Dr. Jin and colleagues indicated.
Of the study population, 58% were diagnosed with invasive pulmonary adenocarcinoma. Other diagnoses within the group were benign (13%), atypical adenomatous hyperplasia (1%), adenocarcinoma in situ (6.5%) and minimally invasive adenocarcinoma (21%).
Results of the multivariable analyses showed that invasive pulmonary adenocarcinoma correlated with the following characteristics: lesion size; spiculation; vascular convergence; and pleural tag. Factors that were not significant included age, family history of lung cancer, CT attenuation, and solid proportion. However, the researchers did include CT attenuation, along with solid proportion, in the final regression analysis based on their contributions to the statistical analysis.
For the model, CT attenuation of –500 to –200 Hounsfield units carried an odds ratio of 1.690 (P = .228) while CT attenuation greater than –200 HU had an OR of 1.791 (P = .645). Positive spiculation had an OR of 3.312 (no P value given) and negative vascular convergence an OR of 0.300 (no P value given).
While a number of prediction models have been devised and validated to evaluate the likelihood of malignancy in pulmonary nodules, they have not given subsolid nodules “specific or detailed consideration,” Dr. Jin and and coauthors said. “To our knowledge, this study was the first to construct a quantitative nomogram to predict the probability of invasive pulmonary adenocarcinoma in patients with subsolid nodules,” the researchers wrote.
One limitation of the study is its selection bias toward patients with a greater probability of having a malignancy. Also, validation of the nomogram requires external analysis with additional databases from other countries and with more diverse ethnic groups. Another shortcoming is the retrospective nature of the study and a small number of patients who had positron emission tomography. “Further data collection, wider geographic recruitment, and incorporation of positron emission tomography results and some molecular factors could improve this model for future use,” Dr. Jin and coauthors concluded.
Dr. Jin and Dr. Cao had no relevant financial disclosures. The study received funding from the Zhejiang Province Science and Technology Plan.
EXPERT ANALYSIS FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Investigators developed a nomogram that may help predict the risk of invasive pulmonary adenocarcinoma for patients with a solitary peripheral subsolid nodule.
Major finding: This nomogram may help clinicians individualize each patient’s prognosis for invasive pulmonary adenocarcinoma and develop treatment plans accordingly.
Data source: Retrospective analysis of 273 consecutive patients who had surgery to remove a solitary peripheral subsolid nodule at a single center.
Disclosure: The investigators received support from the Zhejiang Province Science and Technology Plan. Dr. Jin and Dr. Cao reported having no relevant financial disclosures.
Site of care may impact survival for AYAs with ALL, AML
Receiving treatment at specialized cancer centers may improve survival for adolescents and young adults (AYAs) with acute leukemia, according to a study published in Cancer Epidemiology, Biomarkers & Prevention.
The study showed that, when patients were treated at specialized cancer centers, survival rates were similar for younger AYAs and children with acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML).
However, older AYAs did not appear to reap the same survival benefit from receiving treatment at a specialized cancer center.
And AYAs of all ages had significantly worse survival than children if they were not treated at specialized cancer centers.
AYAs (patients ages 15 to 39) with ALL and AML have significantly worse survival outcomes than children ages 14 and under, according to study author Julie Wolfson, MD, of the University of Alabama at Birmingham.
“A much smaller percentage of AYAs with cancer are treated at specialized cancer centers than children with cancer,” she added. “We wanted to understand whether this difference in the site of cancer care is associated with the difference in survival outcomes.”
Dr Wolfson and her colleagues used data from the Los Angeles County Cancer Surveillance Program to identify patients diagnosed with ALL or AML from ages 1 to 39.
Patients were said to have been treated at a specialized cancer center if, at any age, they were cared for at any of the National Cancer Institute-designated Comprehensive Cancer Centers (CCC) in Los Angeles County or if, at the age of 21 or younger, they were cared for at any of the Children’s Oncology Group (COG) sites not designated a Comprehensive Cancer Center.
Included in the analysis were 978 patients diagnosed with ALL as a child (ages 1 to 14), 402 patients diagnosed with ALL as an AYA (ages 15 to 39), 131 patients diagnosed with AML as a child, and 359 patients diagnosed with AML as an AYA.
Seventy percent of the children with ALL and 30% of the AYAs with ALL were treated at CCC/COG sites. Seventy-four percent of the children with AML and 22% of the AYAs with AML were treated at CCC/COG sites.
Results in ALL
AYAs diagnosed with ALL at ages 15 to 21 and 22 to 29 who were treated at CCC/COG sites had comparable survival to children who were diagnosed with ALL from ages 10 to 14 and treated at CCC/COG sites. The hazard ratios (HRs) were 1.3 for the 15-21 age group (P=0.3) and 1.2 for the 22-29 age group (P=0.8).
The reference group for this analysis was 10- to 14-year-olds treated at CCC/COG sites because the researchers excluded data from children with ALL ages 1 to 9. These patients were excluded because they have significantly better survival than the other groups, potentially as a result of different disease biology.
The researchers also found that treatment at CCC/COG sites did not improve survival for 30- to 39-year-olds with ALL. The HR for them was 3.4 (P<0.001).
Likewise, all AYAs with ALL who were not treated at CCC/COG sites had worse survival than 10- to 14-year-olds treated at CCC/COG sites. The HRs were 1.9 for the 15-21 age group (P=0.005), 2.6 for the 22-29 age group (P<0.001), and 3.0 for the 30-39 age group (P<0.001).
Results in AML
For AML patients, the reference group was 1- to 14-year-olds treated at CCC/COG sites. Compared to these patients, 15- to 21-year-olds not treated at CCC/COG sites had an increased hazard of death (HR=1.7, P=0.02), whereas 15- to 21-year-olds treated at CCC/COG sites did not (HR=1.3; P=0.4).
All 22- to 39-year-olds, regardless of the site of care, had an increased hazard of death compared to the children treated at CCC/COG sites. The HR was 3.4 (P<0.001) for 22- to 39-year-olds treated at CCC/COG sites, and the HR was 3.0 (P<0.001) for 22- to 39-year-olds not treated at CCC/COG sites.
“The fact that the older AYAs did not appear to benefit from treatment at a specialized cancer center suggests to us that the biology of the disease in these patients differs from that in younger individuals,” Dr Wolfson said.
“The most important thing we can do to help these patients is to enroll them on clinical trials so that we can better understand the biology of the disease and its response to therapy. ![]()
Receiving treatment at specialized cancer centers may improve survival for adolescents and young adults (AYAs) with acute leukemia, according to a study published in Cancer Epidemiology, Biomarkers & Prevention.
The study showed that, when patients were treated at specialized cancer centers, survival rates were similar for younger AYAs and children with acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML).
However, older AYAs did not appear to reap the same survival benefit from receiving treatment at a specialized cancer center.
And AYAs of all ages had significantly worse survival than children if they were not treated at specialized cancer centers.
AYAs (patients ages 15 to 39) with ALL and AML have significantly worse survival outcomes than children ages 14 and under, according to study author Julie Wolfson, MD, of the University of Alabama at Birmingham.
“A much smaller percentage of AYAs with cancer are treated at specialized cancer centers than children with cancer,” she added. “We wanted to understand whether this difference in the site of cancer care is associated with the difference in survival outcomes.”
Dr Wolfson and her colleagues used data from the Los Angeles County Cancer Surveillance Program to identify patients diagnosed with ALL or AML from ages 1 to 39.
Patients were said to have been treated at a specialized cancer center if, at any age, they were cared for at any of the National Cancer Institute-designated Comprehensive Cancer Centers (CCC) in Los Angeles County or if, at the age of 21 or younger, they were cared for at any of the Children’s Oncology Group (COG) sites not designated a Comprehensive Cancer Center.
Included in the analysis were 978 patients diagnosed with ALL as a child (ages 1 to 14), 402 patients diagnosed with ALL as an AYA (ages 15 to 39), 131 patients diagnosed with AML as a child, and 359 patients diagnosed with AML as an AYA.
Seventy percent of the children with ALL and 30% of the AYAs with ALL were treated at CCC/COG sites. Seventy-four percent of the children with AML and 22% of the AYAs with AML were treated at CCC/COG sites.
Results in ALL
AYAs diagnosed with ALL at ages 15 to 21 and 22 to 29 who were treated at CCC/COG sites had comparable survival to children who were diagnosed with ALL from ages 10 to 14 and treated at CCC/COG sites. The hazard ratios (HRs) were 1.3 for the 15-21 age group (P=0.3) and 1.2 for the 22-29 age group (P=0.8).
The reference group for this analysis was 10- to 14-year-olds treated at CCC/COG sites because the researchers excluded data from children with ALL ages 1 to 9. These patients were excluded because they have significantly better survival than the other groups, potentially as a result of different disease biology.
The researchers also found that treatment at CCC/COG sites did not improve survival for 30- to 39-year-olds with ALL. The HR for them was 3.4 (P<0.001).
Likewise, all AYAs with ALL who were not treated at CCC/COG sites had worse survival than 10- to 14-year-olds treated at CCC/COG sites. The HRs were 1.9 for the 15-21 age group (P=0.005), 2.6 for the 22-29 age group (P<0.001), and 3.0 for the 30-39 age group (P<0.001).
Results in AML
For AML patients, the reference group was 1- to 14-year-olds treated at CCC/COG sites. Compared to these patients, 15- to 21-year-olds not treated at CCC/COG sites had an increased hazard of death (HR=1.7, P=0.02), whereas 15- to 21-year-olds treated at CCC/COG sites did not (HR=1.3; P=0.4).
All 22- to 39-year-olds, regardless of the site of care, had an increased hazard of death compared to the children treated at CCC/COG sites. The HR was 3.4 (P<0.001) for 22- to 39-year-olds treated at CCC/COG sites, and the HR was 3.0 (P<0.001) for 22- to 39-year-olds not treated at CCC/COG sites.
“The fact that the older AYAs did not appear to benefit from treatment at a specialized cancer center suggests to us that the biology of the disease in these patients differs from that in younger individuals,” Dr Wolfson said.
“The most important thing we can do to help these patients is to enroll them on clinical trials so that we can better understand the biology of the disease and its response to therapy. ![]()
Receiving treatment at specialized cancer centers may improve survival for adolescents and young adults (AYAs) with acute leukemia, according to a study published in Cancer Epidemiology, Biomarkers & Prevention.
The study showed that, when patients were treated at specialized cancer centers, survival rates were similar for younger AYAs and children with acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML).
However, older AYAs did not appear to reap the same survival benefit from receiving treatment at a specialized cancer center.
And AYAs of all ages had significantly worse survival than children if they were not treated at specialized cancer centers.
AYAs (patients ages 15 to 39) with ALL and AML have significantly worse survival outcomes than children ages 14 and under, according to study author Julie Wolfson, MD, of the University of Alabama at Birmingham.
“A much smaller percentage of AYAs with cancer are treated at specialized cancer centers than children with cancer,” she added. “We wanted to understand whether this difference in the site of cancer care is associated with the difference in survival outcomes.”
Dr Wolfson and her colleagues used data from the Los Angeles County Cancer Surveillance Program to identify patients diagnosed with ALL or AML from ages 1 to 39.
Patients were said to have been treated at a specialized cancer center if, at any age, they were cared for at any of the National Cancer Institute-designated Comprehensive Cancer Centers (CCC) in Los Angeles County or if, at the age of 21 or younger, they were cared for at any of the Children’s Oncology Group (COG) sites not designated a Comprehensive Cancer Center.
Included in the analysis were 978 patients diagnosed with ALL as a child (ages 1 to 14), 402 patients diagnosed with ALL as an AYA (ages 15 to 39), 131 patients diagnosed with AML as a child, and 359 patients diagnosed with AML as an AYA.
Seventy percent of the children with ALL and 30% of the AYAs with ALL were treated at CCC/COG sites. Seventy-four percent of the children with AML and 22% of the AYAs with AML were treated at CCC/COG sites.
Results in ALL
AYAs diagnosed with ALL at ages 15 to 21 and 22 to 29 who were treated at CCC/COG sites had comparable survival to children who were diagnosed with ALL from ages 10 to 14 and treated at CCC/COG sites. The hazard ratios (HRs) were 1.3 for the 15-21 age group (P=0.3) and 1.2 for the 22-29 age group (P=0.8).
The reference group for this analysis was 10- to 14-year-olds treated at CCC/COG sites because the researchers excluded data from children with ALL ages 1 to 9. These patients were excluded because they have significantly better survival than the other groups, potentially as a result of different disease biology.
The researchers also found that treatment at CCC/COG sites did not improve survival for 30- to 39-year-olds with ALL. The HR for them was 3.4 (P<0.001).
Likewise, all AYAs with ALL who were not treated at CCC/COG sites had worse survival than 10- to 14-year-olds treated at CCC/COG sites. The HRs were 1.9 for the 15-21 age group (P=0.005), 2.6 for the 22-29 age group (P<0.001), and 3.0 for the 30-39 age group (P<0.001).
Results in AML
For AML patients, the reference group was 1- to 14-year-olds treated at CCC/COG sites. Compared to these patients, 15- to 21-year-olds not treated at CCC/COG sites had an increased hazard of death (HR=1.7, P=0.02), whereas 15- to 21-year-olds treated at CCC/COG sites did not (HR=1.3; P=0.4).
All 22- to 39-year-olds, regardless of the site of care, had an increased hazard of death compared to the children treated at CCC/COG sites. The HR was 3.4 (P<0.001) for 22- to 39-year-olds treated at CCC/COG sites, and the HR was 3.0 (P<0.001) for 22- to 39-year-olds not treated at CCC/COG sites.
“The fact that the older AYAs did not appear to benefit from treatment at a specialized cancer center suggests to us that the biology of the disease in these patients differs from that in younger individuals,” Dr Wolfson said.
“The most important thing we can do to help these patients is to enroll them on clinical trials so that we can better understand the biology of the disease and its response to therapy. ![]()
Using mRNA to treat hemophilia B
Researchers have reported the successful treatment of hemophilia B in mice using messenger RNA (mRNA).
In experiments, protein-replacement therapy using mRNA was effective longer than recombinant factor IX (FIX) therapy.
The mRNA therapy also appeared to be safe and produced mild, transient immune responses.
In addition, the researchers noted that the mRNA therapy can be produced more cheaply than recombinant FIX therapy.
They said this research, published in PNAS, is proof-of-concept that mRNA therapy could be used to treat hemophilia B and other genetic diseases.
“We are really excited about this work because, short of correcting a faulty gene, protein-replacement therapy using mRNA is one of the most promising techniques we have at our disposal,” said study author Inder Verma, PhD, of the Salk Institute for Biological Studies in La Jolla, California.
“Now, we have proof that we can successfully treat a disease—with virtually no side effects—at a lower cost than manufacturing the needed protein.”
This work is a collaboration between the Verma lab and Arcturus Therapeutics, a biotech company that developed a system of encapsulating mRNA within lipid nanoparticles.
The researchers created an mRNA blueprint for human FIX nanoparticles and delivered them, via injection, to mice with a faulty FIX gene.
Once in the bloodstream, the nanoparticles traveled to the liver, where their fatty casing helped them ease into cells and deliver to the protein-making machinery the mRNA instructions to assemble FIX.
The hemophilic mice were given 3 injections over a 5-month period, during which their coagulation and immune responses were carefully monitored.
Normal clotting occurred in the mice within 4 hours of receiving the therapy, and the results lasted for up to 6 days.
The mice had a weak immune response to the treatment, which quickly returned to baseline.
“One of the issues with both nanoparticles and mRNA treatment is toxicity, and, in our study, we did not see much evidence of that,” said study author Suvasini Ramaswamy, PhD, of the Salk Institute for Biological Studies.
“We gave the treatment over a long span to give the immune system time to see it and react to it, but the immune response looked more like a mild allergic response and quickly returned to normal, so the technology seems pretty reliable and safe in our mouse model.”
The researchers also gave mice recombinant FIX, directly comparing it to the mRNA therapy. The mRNA therapy proved more effective, maintaining 20% more clotting activity 4 days after injection.
“Conceptually, in vivo mRNA delivery has been around for a long time, but its therapeutic use has been limited by poor stability, immune-reactivity, and problems with reproducible systemic delivery,” said Pad Chivukula, chief scientific officer at Arcturus Therapeutics.
“The results suggest that nanoparticle delivery technology overcomes these challenges and might allow for the development of novel, cost-effective mRNA therapeutics.”
This work was funded by the National Institutes of Health, the Ipsen Foundation, the H.N. and Frances C. Berger Foundation, the Glenn Center for Research on Aging, The Leona M. and Harry B. Helmsley Charitable Trust, and the California Institute for Regenerative Medicine. The lipid nanoparticles were developed by Arcturus Therapeutics. ![]()
Researchers have reported the successful treatment of hemophilia B in mice using messenger RNA (mRNA).
In experiments, protein-replacement therapy using mRNA was effective longer than recombinant factor IX (FIX) therapy.
The mRNA therapy also appeared to be safe and produced mild, transient immune responses.
In addition, the researchers noted that the mRNA therapy can be produced more cheaply than recombinant FIX therapy.
They said this research, published in PNAS, is proof-of-concept that mRNA therapy could be used to treat hemophilia B and other genetic diseases.
“We are really excited about this work because, short of correcting a faulty gene, protein-replacement therapy using mRNA is one of the most promising techniques we have at our disposal,” said study author Inder Verma, PhD, of the Salk Institute for Biological Studies in La Jolla, California.
“Now, we have proof that we can successfully treat a disease—with virtually no side effects—at a lower cost than manufacturing the needed protein.”
This work is a collaboration between the Verma lab and Arcturus Therapeutics, a biotech company that developed a system of encapsulating mRNA within lipid nanoparticles.
The researchers created an mRNA blueprint for human FIX nanoparticles and delivered them, via injection, to mice with a faulty FIX gene.
Once in the bloodstream, the nanoparticles traveled to the liver, where their fatty casing helped them ease into cells and deliver to the protein-making machinery the mRNA instructions to assemble FIX.
The hemophilic mice were given 3 injections over a 5-month period, during which their coagulation and immune responses were carefully monitored.
Normal clotting occurred in the mice within 4 hours of receiving the therapy, and the results lasted for up to 6 days.
The mice had a weak immune response to the treatment, which quickly returned to baseline.
“One of the issues with both nanoparticles and mRNA treatment is toxicity, and, in our study, we did not see much evidence of that,” said study author Suvasini Ramaswamy, PhD, of the Salk Institute for Biological Studies.
“We gave the treatment over a long span to give the immune system time to see it and react to it, but the immune response looked more like a mild allergic response and quickly returned to normal, so the technology seems pretty reliable and safe in our mouse model.”
The researchers also gave mice recombinant FIX, directly comparing it to the mRNA therapy. The mRNA therapy proved more effective, maintaining 20% more clotting activity 4 days after injection.
“Conceptually, in vivo mRNA delivery has been around for a long time, but its therapeutic use has been limited by poor stability, immune-reactivity, and problems with reproducible systemic delivery,” said Pad Chivukula, chief scientific officer at Arcturus Therapeutics.
“The results suggest that nanoparticle delivery technology overcomes these challenges and might allow for the development of novel, cost-effective mRNA therapeutics.”
This work was funded by the National Institutes of Health, the Ipsen Foundation, the H.N. and Frances C. Berger Foundation, the Glenn Center for Research on Aging, The Leona M. and Harry B. Helmsley Charitable Trust, and the California Institute for Regenerative Medicine. The lipid nanoparticles were developed by Arcturus Therapeutics. ![]()
Researchers have reported the successful treatment of hemophilia B in mice using messenger RNA (mRNA).
In experiments, protein-replacement therapy using mRNA was effective longer than recombinant factor IX (FIX) therapy.
The mRNA therapy also appeared to be safe and produced mild, transient immune responses.
In addition, the researchers noted that the mRNA therapy can be produced more cheaply than recombinant FIX therapy.
They said this research, published in PNAS, is proof-of-concept that mRNA therapy could be used to treat hemophilia B and other genetic diseases.
“We are really excited about this work because, short of correcting a faulty gene, protein-replacement therapy using mRNA is one of the most promising techniques we have at our disposal,” said study author Inder Verma, PhD, of the Salk Institute for Biological Studies in La Jolla, California.
“Now, we have proof that we can successfully treat a disease—with virtually no side effects—at a lower cost than manufacturing the needed protein.”
This work is a collaboration between the Verma lab and Arcturus Therapeutics, a biotech company that developed a system of encapsulating mRNA within lipid nanoparticles.
The researchers created an mRNA blueprint for human FIX nanoparticles and delivered them, via injection, to mice with a faulty FIX gene.
Once in the bloodstream, the nanoparticles traveled to the liver, where their fatty casing helped them ease into cells and deliver to the protein-making machinery the mRNA instructions to assemble FIX.
The hemophilic mice were given 3 injections over a 5-month period, during which their coagulation and immune responses were carefully monitored.
Normal clotting occurred in the mice within 4 hours of receiving the therapy, and the results lasted for up to 6 days.
The mice had a weak immune response to the treatment, which quickly returned to baseline.
“One of the issues with both nanoparticles and mRNA treatment is toxicity, and, in our study, we did not see much evidence of that,” said study author Suvasini Ramaswamy, PhD, of the Salk Institute for Biological Studies.
“We gave the treatment over a long span to give the immune system time to see it and react to it, but the immune response looked more like a mild allergic response and quickly returned to normal, so the technology seems pretty reliable and safe in our mouse model.”
The researchers also gave mice recombinant FIX, directly comparing it to the mRNA therapy. The mRNA therapy proved more effective, maintaining 20% more clotting activity 4 days after injection.
“Conceptually, in vivo mRNA delivery has been around for a long time, but its therapeutic use has been limited by poor stability, immune-reactivity, and problems with reproducible systemic delivery,” said Pad Chivukula, chief scientific officer at Arcturus Therapeutics.
“The results suggest that nanoparticle delivery technology overcomes these challenges and might allow for the development of novel, cost-effective mRNA therapeutics.”
This work was funded by the National Institutes of Health, the Ipsen Foundation, the H.N. and Frances C. Berger Foundation, the Glenn Center for Research on Aging, The Leona M. and Harry B. Helmsley Charitable Trust, and the California Institute for Regenerative Medicine. The lipid nanoparticles were developed by Arcturus Therapeutics. ![]()
Vaccine offers some protection during malaria season
In a placebo-controlled trial, an investigational vaccine protected a small but statistically significant proportion of healthy volunteers against naturally acquired malaria infection for the duration of the malaria season in Mali.
The vaccine, known as the PfSPZ Vaccine, contains live but weakened Plasmodium falciparum sporozoites and was developed by scientists at Sanaria Inc.
Results of the phase 1 trial were published in The Lancet Infectious Diseases.
Sanaria was the regulatory sponsor of the trial. The National Institute of Allergy and Infectious Diseases (NIAID) supported the development of the vaccine.
Earlier research suggested the PfSPZ Vaccine was safe and could protect against malaria infection for up to a year in healthy US adults who had not been previously exposed to malaria.
The Mali study was launched in January 2014 to provide additional safety data for the PfSPZ Vaccine and determine if it could protect adults living in a malaria-endemic area against naturally occurring malaria infection.
The study enrolled 109 healthy African men and non-pregnant women ages 18 to 35. Subjects received either 5 doses of the intravenous PfSPZ Vaccine (2.7 × 10⁵) or 5 doses of placebo (saline) over 5 months of the dry season at the study’s clinical site in the Donéguébougou village in rural Mali.
The subjects also received combined artemether and lumefantrine (4 tablets twice per day over 3 days) to eliminate P falciparum before the first and last vaccinations.
Clinical staff monitored the subjects during the 6-month malaria-transmission season for the presence of malaria parasites in the blood. The team collected blood smears every 2 weeks and during any illness for 24 weeks after the fifth vaccination.
The investigators said they detected no significant differences in local or systemic adverse events or laboratory abnormalities between the PfSPZ Vaccine group and the placebo group.
The most common solicited systemic adverse events were headache (7% in the vaccine group and 9% in the placebo group), fatigue (2% in the placebo group), fever (2% in the placebo group), and myalgia (2% in each group).
Efficacy
Ninety-three percent of evaluable subjects in the placebo group (37/40) and 66% of evaluable subjects in the PfSPZ Vaccine group (27/41) developed P falciparum malaria infections.
The investigators said the PfSPZ Vaccine demonstrated a 48% protective efficacy by time to first positive malaria blood smear and 29% efficacy by the proportion of participants with at least 1 positive malaria blood smear during a full 20-week malaria transmission season.
By both measures of protective efficacy, there was statistically significant protection in the vaccine group compared to the placebo group.
“This level of sustained efficacy against malaria infection in a region with an intense transmission season has not been seen in previous malaria vaccine studies in Africa,” said Sara Healy, MD, of NIAID in Bethesda, Maryland.
“It is a very encouraging finding that we can, hopefully, build upon.”
The investigators noted that the vaccine-induced antibody response was considerably lower in the Mali study than in the US trial, even though subjects received the same vaccine regimen.
“The poor antibody response to PfSPZ Vaccine among Malians could have been because of the participants’ lifelong exposure to P falciparum,” said Patrick E. Duffy, MD, of NIAID.
Feasibility
The investigators reported that there was no problem administering the PfSPZ Vaccine in a rural, malaria-endemic area—an initial concern about the vaccine’s design.
“Direct venous inoculation is not currently used for any licensed vaccines to prevent an infectious disease,” said Ogobara Doumbo, MD, PhD, of the University of Science, Techniques and Technologies of Bamako in Bamako, Mali.
“In this study, we administered 491 inoculations in a rural setting without a problem, and the dosages were delivered in a matter of seconds. It shows that this approach is feasible from both a logistical and public health standpoint.”
According to the investigators, a malaria vaccine employed in mass vaccination programs to eliminate P falciparum from geographically defined areas would need to prevent malaria infection or transmission in at least 80% of recipients throughout the malaria transmission season.
Clinical trials now underway in Africa, Europe, and the US have been designed to boost the PfSPZ Vaccine’s efficacy by increasing dosage levels and varying the timing and number of doses.
The experimental vaccine is also being examined in demographic groups other than healthy adults, including adolescents, children, and infants. ![]()
In a placebo-controlled trial, an investigational vaccine protected a small but statistically significant proportion of healthy volunteers against naturally acquired malaria infection for the duration of the malaria season in Mali.
The vaccine, known as the PfSPZ Vaccine, contains live but weakened Plasmodium falciparum sporozoites and was developed by scientists at Sanaria Inc.
Results of the phase 1 trial were published in The Lancet Infectious Diseases.
Sanaria was the regulatory sponsor of the trial. The National Institute of Allergy and Infectious Diseases (NIAID) supported the development of the vaccine.
Earlier research suggested the PfSPZ Vaccine was safe and could protect against malaria infection for up to a year in healthy US adults who had not been previously exposed to malaria.
The Mali study was launched in January 2014 to provide additional safety data for the PfSPZ Vaccine and determine if it could protect adults living in a malaria-endemic area against naturally occurring malaria infection.
The study enrolled 109 healthy African men and non-pregnant women ages 18 to 35. Subjects received either 5 doses of the intravenous PfSPZ Vaccine (2.7 × 10⁵) or 5 doses of placebo (saline) over 5 months of the dry season at the study’s clinical site in the Donéguébougou village in rural Mali.
The subjects also received combined artemether and lumefantrine (4 tablets twice per day over 3 days) to eliminate P falciparum before the first and last vaccinations.
Clinical staff monitored the subjects during the 6-month malaria-transmission season for the presence of malaria parasites in the blood. The team collected blood smears every 2 weeks and during any illness for 24 weeks after the fifth vaccination.
The investigators said they detected no significant differences in local or systemic adverse events or laboratory abnormalities between the PfSPZ Vaccine group and the placebo group.
The most common solicited systemic adverse events were headache (7% in the vaccine group and 9% in the placebo group), fatigue (2% in the placebo group), fever (2% in the placebo group), and myalgia (2% in each group).
Efficacy
Ninety-three percent of evaluable subjects in the placebo group (37/40) and 66% of evaluable subjects in the PfSPZ Vaccine group (27/41) developed P falciparum malaria infections.
The investigators said the PfSPZ Vaccine demonstrated a 48% protective efficacy by time to first positive malaria blood smear and 29% efficacy by the proportion of participants with at least 1 positive malaria blood smear during a full 20-week malaria transmission season.
By both measures of protective efficacy, there was statistically significant protection in the vaccine group compared to the placebo group.
“This level of sustained efficacy against malaria infection in a region with an intense transmission season has not been seen in previous malaria vaccine studies in Africa,” said Sara Healy, MD, of NIAID in Bethesda, Maryland.
“It is a very encouraging finding that we can, hopefully, build upon.”
The investigators noted that the vaccine-induced antibody response was considerably lower in the Mali study than in the US trial, even though subjects received the same vaccine regimen.
“The poor antibody response to PfSPZ Vaccine among Malians could have been because of the participants’ lifelong exposure to P falciparum,” said Patrick E. Duffy, MD, of NIAID.
Feasibility
The investigators reported that there was no problem administering the PfSPZ Vaccine in a rural, malaria-endemic area—an initial concern about the vaccine’s design.
“Direct venous inoculation is not currently used for any licensed vaccines to prevent an infectious disease,” said Ogobara Doumbo, MD, PhD, of the University of Science, Techniques and Technologies of Bamako in Bamako, Mali.
“In this study, we administered 491 inoculations in a rural setting without a problem, and the dosages were delivered in a matter of seconds. It shows that this approach is feasible from both a logistical and public health standpoint.”
According to the investigators, a malaria vaccine employed in mass vaccination programs to eliminate P falciparum from geographically defined areas would need to prevent malaria infection or transmission in at least 80% of recipients throughout the malaria transmission season.
Clinical trials now underway in Africa, Europe, and the US have been designed to boost the PfSPZ Vaccine’s efficacy by increasing dosage levels and varying the timing and number of doses.
The experimental vaccine is also being examined in demographic groups other than healthy adults, including adolescents, children, and infants. ![]()
In a placebo-controlled trial, an investigational vaccine protected a small but statistically significant proportion of healthy volunteers against naturally acquired malaria infection for the duration of the malaria season in Mali.
The vaccine, known as the PfSPZ Vaccine, contains live but weakened Plasmodium falciparum sporozoites and was developed by scientists at Sanaria Inc.
Results of the phase 1 trial were published in The Lancet Infectious Diseases.
Sanaria was the regulatory sponsor of the trial. The National Institute of Allergy and Infectious Diseases (NIAID) supported the development of the vaccine.
Earlier research suggested the PfSPZ Vaccine was safe and could protect against malaria infection for up to a year in healthy US adults who had not been previously exposed to malaria.
The Mali study was launched in January 2014 to provide additional safety data for the PfSPZ Vaccine and determine if it could protect adults living in a malaria-endemic area against naturally occurring malaria infection.
The study enrolled 109 healthy African men and non-pregnant women ages 18 to 35. Subjects received either 5 doses of the intravenous PfSPZ Vaccine (2.7 × 10⁵) or 5 doses of placebo (saline) over 5 months of the dry season at the study’s clinical site in the Donéguébougou village in rural Mali.
The subjects also received combined artemether and lumefantrine (4 tablets twice per day over 3 days) to eliminate P falciparum before the first and last vaccinations.
Clinical staff monitored the subjects during the 6-month malaria-transmission season for the presence of malaria parasites in the blood. The team collected blood smears every 2 weeks and during any illness for 24 weeks after the fifth vaccination.
The investigators said they detected no significant differences in local or systemic adverse events or laboratory abnormalities between the PfSPZ Vaccine group and the placebo group.
The most common solicited systemic adverse events were headache (7% in the vaccine group and 9% in the placebo group), fatigue (2% in the placebo group), fever (2% in the placebo group), and myalgia (2% in each group).
Efficacy
Ninety-three percent of evaluable subjects in the placebo group (37/40) and 66% of evaluable subjects in the PfSPZ Vaccine group (27/41) developed P falciparum malaria infections.
The investigators said the PfSPZ Vaccine demonstrated a 48% protective efficacy by time to first positive malaria blood smear and 29% efficacy by the proportion of participants with at least 1 positive malaria blood smear during a full 20-week malaria transmission season.
By both measures of protective efficacy, there was statistically significant protection in the vaccine group compared to the placebo group.
“This level of sustained efficacy against malaria infection in a region with an intense transmission season has not been seen in previous malaria vaccine studies in Africa,” said Sara Healy, MD, of NIAID in Bethesda, Maryland.
“It is a very encouraging finding that we can, hopefully, build upon.”
The investigators noted that the vaccine-induced antibody response was considerably lower in the Mali study than in the US trial, even though subjects received the same vaccine regimen.
“The poor antibody response to PfSPZ Vaccine among Malians could have been because of the participants’ lifelong exposure to P falciparum,” said Patrick E. Duffy, MD, of NIAID.
Feasibility
The investigators reported that there was no problem administering the PfSPZ Vaccine in a rural, malaria-endemic area—an initial concern about the vaccine’s design.
“Direct venous inoculation is not currently used for any licensed vaccines to prevent an infectious disease,” said Ogobara Doumbo, MD, PhD, of the University of Science, Techniques and Technologies of Bamako in Bamako, Mali.
“In this study, we administered 491 inoculations in a rural setting without a problem, and the dosages were delivered in a matter of seconds. It shows that this approach is feasible from both a logistical and public health standpoint.”
According to the investigators, a malaria vaccine employed in mass vaccination programs to eliminate P falciparum from geographically defined areas would need to prevent malaria infection or transmission in at least 80% of recipients throughout the malaria transmission season.
Clinical trials now underway in Africa, Europe, and the US have been designed to boost the PfSPZ Vaccine’s efficacy by increasing dosage levels and varying the timing and number of doses.
The experimental vaccine is also being examined in demographic groups other than healthy adults, including adolescents, children, and infants. ![]()
Zika slowing, but not going away
New cases of pregnant women with laboratory evidence of Zika virus infection were down for the 2 weeks ending Feb. 7, with a big drop in the U.S. territories offsetting an increase among the 50 states and the District of Columbia, according to the Centers for Disease Control and Prevention.
There were 146 new cases of Zika infection reported in pregnant women over that period, compared with 233 over the previous 2 weeks. Of the new cases, 85 were reported in the U.S. territories (down from 186) and 61 were reported in the 50 states and D.C. (up from 47), the CDC reported. These are not real-time estimates, the CDC noted. They reflect only the number of reports received, so the cases may have occurred during earlier reporting periods.
Of the cases in the states/D.C., 1,047 pregnancies have been completed with or without birth defects. There have been 43 liveborn infants with Zika-related birth defects and five pregnancy losses with Zika-related defects, the CDC said. The number of liveborn infants was up from 38 for the previous 2-week span, which was the largest increase since mid-November.
The Zika caseload for all Americans is 42,063 (from Jan. 1, 2015 to Feb. 15, 2017), of which 5,040 cases were reported in the 50 states/D.C. and 37,023 were reported in the territories (98% in Puerto Rico), the CDC reported.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
New cases of pregnant women with laboratory evidence of Zika virus infection were down for the 2 weeks ending Feb. 7, with a big drop in the U.S. territories offsetting an increase among the 50 states and the District of Columbia, according to the Centers for Disease Control and Prevention.
There were 146 new cases of Zika infection reported in pregnant women over that period, compared with 233 over the previous 2 weeks. Of the new cases, 85 were reported in the U.S. territories (down from 186) and 61 were reported in the 50 states and D.C. (up from 47), the CDC reported. These are not real-time estimates, the CDC noted. They reflect only the number of reports received, so the cases may have occurred during earlier reporting periods.
Of the cases in the states/D.C., 1,047 pregnancies have been completed with or without birth defects. There have been 43 liveborn infants with Zika-related birth defects and five pregnancy losses with Zika-related defects, the CDC said. The number of liveborn infants was up from 38 for the previous 2-week span, which was the largest increase since mid-November.
The Zika caseload for all Americans is 42,063 (from Jan. 1, 2015 to Feb. 15, 2017), of which 5,040 cases were reported in the 50 states/D.C. and 37,023 were reported in the territories (98% in Puerto Rico), the CDC reported.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
New cases of pregnant women with laboratory evidence of Zika virus infection were down for the 2 weeks ending Feb. 7, with a big drop in the U.S. territories offsetting an increase among the 50 states and the District of Columbia, according to the Centers for Disease Control and Prevention.
There were 146 new cases of Zika infection reported in pregnant women over that period, compared with 233 over the previous 2 weeks. Of the new cases, 85 were reported in the U.S. territories (down from 186) and 61 were reported in the 50 states and D.C. (up from 47), the CDC reported. These are not real-time estimates, the CDC noted. They reflect only the number of reports received, so the cases may have occurred during earlier reporting periods.
Of the cases in the states/D.C., 1,047 pregnancies have been completed with or without birth defects. There have been 43 liveborn infants with Zika-related birth defects and five pregnancy losses with Zika-related defects, the CDC said. The number of liveborn infants was up from 38 for the previous 2-week span, which was the largest increase since mid-November.
The Zika caseload for all Americans is 42,063 (from Jan. 1, 2015 to Feb. 15, 2017), of which 5,040 cases were reported in the 50 states/D.C. and 37,023 were reported in the territories (98% in Puerto Rico), the CDC reported.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
CMS spending projections come with a caveat
The Centers for Medicare & Medicaid Services is predicting an average annual growth rate of 5.6% in health care expenditures over the next 10 years – but with a big asterisk.
The projections are based on current law and make no assumptions about potential repair, repeal, or replacement of the Affordable Care Act, leaving the projections to serve as a benchmark rather than a forecast.
In the short term, growth rates in 2016 and 2017 are the slowest in the forecast period, at 4.8% and 5.4%, respectively. For 2018 and beyond, the growth rate picks up, with both Medicare and Medicaid projected to grow faster and more rapidly, compared with private health insurance spending.
The CMS actuaries attribute this to the increase in Medicare spending over recent historic lows, a Medicaid population that is expected to become older and sicker, additional Baby Boomers entering the Medicare program, and decreased demand for health services as prices increase.
The percentage of population with either public or private health insurance coverage is expected to grow under current law from 90.9% in 2015 to 91.5% in 2025, “mainly a result of continued growth in enrollment in private health insurance – in particular, employer-sponsored health insurance – in the first year of the projection period, as well as enrollment growth in public programs throughout the period,” Mr. Keegan and colleagues wrote.
The Centers for Medicare & Medicaid Services is predicting an average annual growth rate of 5.6% in health care expenditures over the next 10 years – but with a big asterisk.
The projections are based on current law and make no assumptions about potential repair, repeal, or replacement of the Affordable Care Act, leaving the projections to serve as a benchmark rather than a forecast.
In the short term, growth rates in 2016 and 2017 are the slowest in the forecast period, at 4.8% and 5.4%, respectively. For 2018 and beyond, the growth rate picks up, with both Medicare and Medicaid projected to grow faster and more rapidly, compared with private health insurance spending.
The CMS actuaries attribute this to the increase in Medicare spending over recent historic lows, a Medicaid population that is expected to become older and sicker, additional Baby Boomers entering the Medicare program, and decreased demand for health services as prices increase.
The percentage of population with either public or private health insurance coverage is expected to grow under current law from 90.9% in 2015 to 91.5% in 2025, “mainly a result of continued growth in enrollment in private health insurance – in particular, employer-sponsored health insurance – in the first year of the projection period, as well as enrollment growth in public programs throughout the period,” Mr. Keegan and colleagues wrote.
The Centers for Medicare & Medicaid Services is predicting an average annual growth rate of 5.6% in health care expenditures over the next 10 years – but with a big asterisk.
The projections are based on current law and make no assumptions about potential repair, repeal, or replacement of the Affordable Care Act, leaving the projections to serve as a benchmark rather than a forecast.
In the short term, growth rates in 2016 and 2017 are the slowest in the forecast period, at 4.8% and 5.4%, respectively. For 2018 and beyond, the growth rate picks up, with both Medicare and Medicaid projected to grow faster and more rapidly, compared with private health insurance spending.
The CMS actuaries attribute this to the increase in Medicare spending over recent historic lows, a Medicaid population that is expected to become older and sicker, additional Baby Boomers entering the Medicare program, and decreased demand for health services as prices increase.
The percentage of population with either public or private health insurance coverage is expected to grow under current law from 90.9% in 2015 to 91.5% in 2025, “mainly a result of continued growth in enrollment in private health insurance – in particular, employer-sponsored health insurance – in the first year of the projection period, as well as enrollment growth in public programs throughout the period,” Mr. Keegan and colleagues wrote.
Survival gains with pembrolizumab in urothelial cancer
Treatment with checkpoint inhibitor pembrolizumab is associated with significant gains in overall survival among patients with treatment-refractory advanced urothelial carcinoma, according to new research.
Data from the KEYNOTE-045 trial was presented simultaneously at the Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology, and published in the New England Journal of Medicine.
The open-label, international phase III trial involved 542 patients with advanced urothelial carcinoma that had recurred or progressed after platinum-based chemotherapy. Participants were randomized either to 200 mg pembrolizumab every 3 weeks, or the investigator’s choice of paclitaxel, docetaxel, or vinflunine chemotherapy.
After a median follow-up of 14.1 months, researchers saw a 27% lower hazard ratio for death among the pembrolizumab group, compared with the chemotherapy group (P = .002), with a median overall survival of 10.3 months with pembrolizumab, compared with 7.4 months with chemotherapy (N Engl J Med. 2017 Feb 17. doi: 10.1056/NEJMoa1613683).
The estimated overall survival rate at 1 year was 43.9% among those treated with pembrolizumab and 30.7% in the chemotherapy group. The authors also saw no significant differences when each chemotherapy regimen was examined separately.
Patients treated with pembrolizumab also showed a significant higher objective response rate, compared with those treated with chemotherapy (21.1% vs. 11.4%, P = .001). At the time of data cut-off, 72% of patients in the pembrolizumab group showed a continued response, compared with 35% of the chemotherapy group.
“Most responses in patients in the pembrolizumab group occurred quickly and were reported at the first scheduled imaging assessment,” wrote Joaquim Bellmunt, MD, PhD, from the Dana-Farber Cancer Institute, Boston and his coauthors. “Continued disease regression over time in some patients resulted in radiologically confirmed responses that were reported as long as 6.3 months after the start of therapy.”
They also looked at how the level of programmed death 1 ligand (PD-L1) expression in the tumor influenced response by examining outcomes in a subgroup of patients whose tumor PD-L1 combined positive score – the percentage of PD-L1 expressing tumor and infiltrating immune cells relative to the total number of tumor cells – exceeded 10%.
In this group, median overall survival was 8 months with pembrolizumab, compared with 5.2 months with chemotherapy, representing a significant 43% reduction in mortality with immunotherapy.
There was an interaction between smoking status and response, with current smokers showing a significantly greater response that favored pembrolizumab. The authors pointed out that this effect has also been observed in other advanced cancers, and may reflect the impact of a greater mutational load in the cancers of smokers.
The study did not find any difference in the duration of progression-free survival between the two groups, nor among participants with a PD-L1 combined positive score of 10% of more. Median progression-free survival was 2.1 months in the pembrolizumab group and 3.3 months in the chemotherapy group.
Researchers also noted a lower rate of adverse events among patients treated with pembrolizumab, compared with chemotherapy. Treatment-related adverse events of grade 3-5 occurred in 49.4% of patients on chemotherapy, compared with 15% of those on pembrolizumab.
There was one death from treatment-related pneumonitis in the pembrolizumab group, and three deaths attributed to the study treatment; one urinary tract obstruction, one malignant neoplasm, and one unspecified cause. In the chemotherapy group, there were two treatment-related deaths from sepsis, one from septic shock, and one unspecified.
The most common adverse events with chemotherapy were alopecia, fatigue, and anemia, while in the pembrolizumab group the most common side effects were pruritis, fatigue, and nausea.
The study was supported by Merck. Sixteen authors declared personal fees, grants, honoraria, consultancies, and advisory board positions with pharmaceutical companies including Merck, three authors were employees of Merck with stock options, and one had nothing to declare.
In the context of cancer therapy, PD-L1 and PD-1 inhibitors may be aptly described as the metaphorical rising tide that lifts all boats. These monoclonal antibodies have yielded major advances across multiple malignant conditions by unleashing the antitumor activity of T-lymphocytes by targeting this T-cell inhibitory pathway.
The KEYNOTE-045 trial will have a practice-changing effect. The longer survival and lower rate of toxic adverse effects with pembrolizumab than with chemotherapy confer an improved therapeutic index in these generally elderly patients with coexisting conditions. As we celebrate the major advance that is provided by pembrolizumab, it is important to remember that this remains an incremental advance overall, although the responses were remarkably durable.
Guru Sonpavde, MD, is from the University of Alabama at Birmingham Comprehensive Cancer Center. These comments are taken from an accompanying editorial (N Engl J Med. 2017 Feb 17. doi: 10.1056/NEJMe1701182). Dr. Sonpavde reported a range of grants and personal fees from the pharmaceutical industry, including from Merck.
In the context of cancer therapy, PD-L1 and PD-1 inhibitors may be aptly described as the metaphorical rising tide that lifts all boats. These monoclonal antibodies have yielded major advances across multiple malignant conditions by unleashing the antitumor activity of T-lymphocytes by targeting this T-cell inhibitory pathway.
The KEYNOTE-045 trial will have a practice-changing effect. The longer survival and lower rate of toxic adverse effects with pembrolizumab than with chemotherapy confer an improved therapeutic index in these generally elderly patients with coexisting conditions. As we celebrate the major advance that is provided by pembrolizumab, it is important to remember that this remains an incremental advance overall, although the responses were remarkably durable.
Guru Sonpavde, MD, is from the University of Alabama at Birmingham Comprehensive Cancer Center. These comments are taken from an accompanying editorial (N Engl J Med. 2017 Feb 17. doi: 10.1056/NEJMe1701182). Dr. Sonpavde reported a range of grants and personal fees from the pharmaceutical industry, including from Merck.
In the context of cancer therapy, PD-L1 and PD-1 inhibitors may be aptly described as the metaphorical rising tide that lifts all boats. These monoclonal antibodies have yielded major advances across multiple malignant conditions by unleashing the antitumor activity of T-lymphocytes by targeting this T-cell inhibitory pathway.
The KEYNOTE-045 trial will have a practice-changing effect. The longer survival and lower rate of toxic adverse effects with pembrolizumab than with chemotherapy confer an improved therapeutic index in these generally elderly patients with coexisting conditions. As we celebrate the major advance that is provided by pembrolizumab, it is important to remember that this remains an incremental advance overall, although the responses were remarkably durable.
Guru Sonpavde, MD, is from the University of Alabama at Birmingham Comprehensive Cancer Center. These comments are taken from an accompanying editorial (N Engl J Med. 2017 Feb 17. doi: 10.1056/NEJMe1701182). Dr. Sonpavde reported a range of grants and personal fees from the pharmaceutical industry, including from Merck.
Treatment with checkpoint inhibitor pembrolizumab is associated with significant gains in overall survival among patients with treatment-refractory advanced urothelial carcinoma, according to new research.
Data from the KEYNOTE-045 trial was presented simultaneously at the Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology, and published in the New England Journal of Medicine.
The open-label, international phase III trial involved 542 patients with advanced urothelial carcinoma that had recurred or progressed after platinum-based chemotherapy. Participants were randomized either to 200 mg pembrolizumab every 3 weeks, or the investigator’s choice of paclitaxel, docetaxel, or vinflunine chemotherapy.
After a median follow-up of 14.1 months, researchers saw a 27% lower hazard ratio for death among the pembrolizumab group, compared with the chemotherapy group (P = .002), with a median overall survival of 10.3 months with pembrolizumab, compared with 7.4 months with chemotherapy (N Engl J Med. 2017 Feb 17. doi: 10.1056/NEJMoa1613683).
The estimated overall survival rate at 1 year was 43.9% among those treated with pembrolizumab and 30.7% in the chemotherapy group. The authors also saw no significant differences when each chemotherapy regimen was examined separately.
Patients treated with pembrolizumab also showed a significant higher objective response rate, compared with those treated with chemotherapy (21.1% vs. 11.4%, P = .001). At the time of data cut-off, 72% of patients in the pembrolizumab group showed a continued response, compared with 35% of the chemotherapy group.
“Most responses in patients in the pembrolizumab group occurred quickly and were reported at the first scheduled imaging assessment,” wrote Joaquim Bellmunt, MD, PhD, from the Dana-Farber Cancer Institute, Boston and his coauthors. “Continued disease regression over time in some patients resulted in radiologically confirmed responses that were reported as long as 6.3 months after the start of therapy.”
They also looked at how the level of programmed death 1 ligand (PD-L1) expression in the tumor influenced response by examining outcomes in a subgroup of patients whose tumor PD-L1 combined positive score – the percentage of PD-L1 expressing tumor and infiltrating immune cells relative to the total number of tumor cells – exceeded 10%.
In this group, median overall survival was 8 months with pembrolizumab, compared with 5.2 months with chemotherapy, representing a significant 43% reduction in mortality with immunotherapy.
There was an interaction between smoking status and response, with current smokers showing a significantly greater response that favored pembrolizumab. The authors pointed out that this effect has also been observed in other advanced cancers, and may reflect the impact of a greater mutational load in the cancers of smokers.
The study did not find any difference in the duration of progression-free survival between the two groups, nor among participants with a PD-L1 combined positive score of 10% of more. Median progression-free survival was 2.1 months in the pembrolizumab group and 3.3 months in the chemotherapy group.
Researchers also noted a lower rate of adverse events among patients treated with pembrolizumab, compared with chemotherapy. Treatment-related adverse events of grade 3-5 occurred in 49.4% of patients on chemotherapy, compared with 15% of those on pembrolizumab.
There was one death from treatment-related pneumonitis in the pembrolizumab group, and three deaths attributed to the study treatment; one urinary tract obstruction, one malignant neoplasm, and one unspecified cause. In the chemotherapy group, there were two treatment-related deaths from sepsis, one from septic shock, and one unspecified.
The most common adverse events with chemotherapy were alopecia, fatigue, and anemia, while in the pembrolizumab group the most common side effects were pruritis, fatigue, and nausea.
The study was supported by Merck. Sixteen authors declared personal fees, grants, honoraria, consultancies, and advisory board positions with pharmaceutical companies including Merck, three authors were employees of Merck with stock options, and one had nothing to declare.
Treatment with checkpoint inhibitor pembrolizumab is associated with significant gains in overall survival among patients with treatment-refractory advanced urothelial carcinoma, according to new research.
Data from the KEYNOTE-045 trial was presented simultaneously at the Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology, and published in the New England Journal of Medicine.
The open-label, international phase III trial involved 542 patients with advanced urothelial carcinoma that had recurred or progressed after platinum-based chemotherapy. Participants were randomized either to 200 mg pembrolizumab every 3 weeks, or the investigator’s choice of paclitaxel, docetaxel, or vinflunine chemotherapy.
After a median follow-up of 14.1 months, researchers saw a 27% lower hazard ratio for death among the pembrolizumab group, compared with the chemotherapy group (P = .002), with a median overall survival of 10.3 months with pembrolizumab, compared with 7.4 months with chemotherapy (N Engl J Med. 2017 Feb 17. doi: 10.1056/NEJMoa1613683).
The estimated overall survival rate at 1 year was 43.9% among those treated with pembrolizumab and 30.7% in the chemotherapy group. The authors also saw no significant differences when each chemotherapy regimen was examined separately.
Patients treated with pembrolizumab also showed a significant higher objective response rate, compared with those treated with chemotherapy (21.1% vs. 11.4%, P = .001). At the time of data cut-off, 72% of patients in the pembrolizumab group showed a continued response, compared with 35% of the chemotherapy group.
“Most responses in patients in the pembrolizumab group occurred quickly and were reported at the first scheduled imaging assessment,” wrote Joaquim Bellmunt, MD, PhD, from the Dana-Farber Cancer Institute, Boston and his coauthors. “Continued disease regression over time in some patients resulted in radiologically confirmed responses that were reported as long as 6.3 months after the start of therapy.”
They also looked at how the level of programmed death 1 ligand (PD-L1) expression in the tumor influenced response by examining outcomes in a subgroup of patients whose tumor PD-L1 combined positive score – the percentage of PD-L1 expressing tumor and infiltrating immune cells relative to the total number of tumor cells – exceeded 10%.
In this group, median overall survival was 8 months with pembrolizumab, compared with 5.2 months with chemotherapy, representing a significant 43% reduction in mortality with immunotherapy.
There was an interaction between smoking status and response, with current smokers showing a significantly greater response that favored pembrolizumab. The authors pointed out that this effect has also been observed in other advanced cancers, and may reflect the impact of a greater mutational load in the cancers of smokers.
The study did not find any difference in the duration of progression-free survival between the two groups, nor among participants with a PD-L1 combined positive score of 10% of more. Median progression-free survival was 2.1 months in the pembrolizumab group and 3.3 months in the chemotherapy group.
Researchers also noted a lower rate of adverse events among patients treated with pembrolizumab, compared with chemotherapy. Treatment-related adverse events of grade 3-5 occurred in 49.4% of patients on chemotherapy, compared with 15% of those on pembrolizumab.
There was one death from treatment-related pneumonitis in the pembrolizumab group, and three deaths attributed to the study treatment; one urinary tract obstruction, one malignant neoplasm, and one unspecified cause. In the chemotherapy group, there were two treatment-related deaths from sepsis, one from septic shock, and one unspecified.
The most common adverse events with chemotherapy were alopecia, fatigue, and anemia, while in the pembrolizumab group the most common side effects were pruritis, fatigue, and nausea.
The study was supported by Merck. Sixteen authors declared personal fees, grants, honoraria, consultancies, and advisory board positions with pharmaceutical companies including Merck, three authors were employees of Merck with stock options, and one had nothing to declare.
FROM THE GENITOURINARY CANCERS SYMPOSIUM
Key clinical point: Pembrolizumab is associated with significant gains in overall survival among patients with treatment-refractory advanced urothelial carcinoma.
Major finding: Patients treated with pembrolizumab had a 27% lower mortality, compared with those treated with chemotherapy.
Data source: The KEYNOTE-045 open-label, international phase III trial in 542 patients with advanced urothelial carcinoma.
Disclosures: The study was supported by Merck. Sixteen authors declared personal fees, grants, honoraria, consultancies, and advisory board positions with pharmaceutical companies including Merck, three authors were employees of Merck with stock options, and one had nothing to declare.
Overweight, obesity linked to pelvic organ prolapse risk
Older age and parity are established risk factors for pelvic organ prolapse, but the role of obesity has not been precisely determined. Results from a new meta-analysis show that overweight and obese women are significantly more likely to experience pelvic organ prolapse, compared with women of normal weight.
Researchers identified 22 published observational studies of varying design that evaluated pelvic organ prolapse (POP) and obesity. The studies enrolled 96,875 participants and logged 17,249 POP cases, of which 3,043 were considered clinically significant, and 2,359 cases that were classified as self-reported/symptomatic POP (Am J Obstet Gynecol. 2017 Feb 7. doi: 10.1016/j.ajog.2017.01.039)
Ayush Giri, PhD, of Vanderbilt University in Nashville, and his colleagues, found that overweight women (BMI of 25-30 kg/m2) had a risk ratio ranging from 1.36 to 1.40, compared with normal-weight women. For obese women (BMI of 30 kg/m2 or greater) there was a risk ratio ranging from 1.47 to 1.61, compared with normal-weight women. The association between overweight and obesity and POP was stronger when the POP was clinically significant rather than self-reported and symptomatic.
The researchers called for more studies to prospectively evaluate the the association between obesity and POP.
In the meantime, they wrote, more can be done to educate women. “From a policy perspective, educating parous women about the association between obesity and POP, a common, yet less often talked about condition with debilitating effects on quality of life, may not only help reduce future burden of POP but may also help reduce obesity and related comorbidities in the population.”
The researchers reported having no conflicts of interest.
Older age and parity are established risk factors for pelvic organ prolapse, but the role of obesity has not been precisely determined. Results from a new meta-analysis show that overweight and obese women are significantly more likely to experience pelvic organ prolapse, compared with women of normal weight.
Researchers identified 22 published observational studies of varying design that evaluated pelvic organ prolapse (POP) and obesity. The studies enrolled 96,875 participants and logged 17,249 POP cases, of which 3,043 were considered clinically significant, and 2,359 cases that were classified as self-reported/symptomatic POP (Am J Obstet Gynecol. 2017 Feb 7. doi: 10.1016/j.ajog.2017.01.039)
Ayush Giri, PhD, of Vanderbilt University in Nashville, and his colleagues, found that overweight women (BMI of 25-30 kg/m2) had a risk ratio ranging from 1.36 to 1.40, compared with normal-weight women. For obese women (BMI of 30 kg/m2 or greater) there was a risk ratio ranging from 1.47 to 1.61, compared with normal-weight women. The association between overweight and obesity and POP was stronger when the POP was clinically significant rather than self-reported and symptomatic.
The researchers called for more studies to prospectively evaluate the the association between obesity and POP.
In the meantime, they wrote, more can be done to educate women. “From a policy perspective, educating parous women about the association between obesity and POP, a common, yet less often talked about condition with debilitating effects on quality of life, may not only help reduce future burden of POP but may also help reduce obesity and related comorbidities in the population.”
The researchers reported having no conflicts of interest.
Older age and parity are established risk factors for pelvic organ prolapse, but the role of obesity has not been precisely determined. Results from a new meta-analysis show that overweight and obese women are significantly more likely to experience pelvic organ prolapse, compared with women of normal weight.
Researchers identified 22 published observational studies of varying design that evaluated pelvic organ prolapse (POP) and obesity. The studies enrolled 96,875 participants and logged 17,249 POP cases, of which 3,043 were considered clinically significant, and 2,359 cases that were classified as self-reported/symptomatic POP (Am J Obstet Gynecol. 2017 Feb 7. doi: 10.1016/j.ajog.2017.01.039)
Ayush Giri, PhD, of Vanderbilt University in Nashville, and his colleagues, found that overweight women (BMI of 25-30 kg/m2) had a risk ratio ranging from 1.36 to 1.40, compared with normal-weight women. For obese women (BMI of 30 kg/m2 or greater) there was a risk ratio ranging from 1.47 to 1.61, compared with normal-weight women. The association between overweight and obesity and POP was stronger when the POP was clinically significant rather than self-reported and symptomatic.
The researchers called for more studies to prospectively evaluate the the association between obesity and POP.
In the meantime, they wrote, more can be done to educate women. “From a policy perspective, educating parous women about the association between obesity and POP, a common, yet less often talked about condition with debilitating effects on quality of life, may not only help reduce future burden of POP but may also help reduce obesity and related comorbidities in the population.”
The researchers reported having no conflicts of interest.
FROM THE AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
Key clinical point:
Major finding: Compared with women of normal weight, overweight women had a risk ratio of 1.36-1.40 for pelvic organ prolapse, while obese women had a risk ratio of 1.47-1.61.
Data source: A meta-analysis of 22 observational studies, enrolling nearly 100,000 women, including about 17,000 POP cases, 3,000 of which were considered clinically significant.
Disclosures: The researchers reported having no conflicts of interest.
Will genome editing advance animal-to-human transplantation?
Advances in gene editing are pushing the possibility of raising pigs for organs that may be transplanted into humans with immunosuppression regimens comparable to those now used in human-to-human transplants, coauthors James Butler, MD, and A. Joseph Tector, MD, PhD, stated in an expert opinion in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:488-92).
Developments in genome editing could bring new approaches to management of cardiopulmonary diseases, Dr. Butler and Dr. Tector noted. “Recently, cardiac-specific and lung-specific applications have been described, which will allow for the rapid creation of new models of heart and lung disease,” they said. Specifically, they noted gene targeting might eventually offer a way to treat challenging genetic problems “like the heterogeneous nature of nonsquamous cell lung cancer.”
Dr. Butler is with the department of surgery at Indiana University, Indianapolis, and Dr. Tector is with the department of surgery at the University of Alabama at Birmingham.
CRISPR technology has been used in developing multiple gene knockout pigs and neutralizing three separate porcine genes that encode human xenoantigens in a single reaction, leading to efficient methods for creating pigs with multiple genetic modifications.
According to the website of the Broad Institute of MIT and Harvard, Cambridge, Mass., where researchers perfected the system to work in eukaryotes, CRISPR works by using short RNA sequences designed by researchers to guide the system to matching sequences of DNA. When the target DNA is found, Cas9 – one of the enzymes produced by the CRISPR system – binds to the DNA and cuts it, shutting the targeted gene off.
“By facilitating high-throughput model creation, CRISPR has elucidated which modifications are necessary and which are not; despite the ability to alter many loci concurrently, recent evidence has implicated three porcine genes that are responsible for the majority of human-antiporcine humoral immunity,” Dr. Butler and Dr. Tector wrote.
Those genes are the Gal[alpha]1-3 epitope (Gal-alpha), CMAH and B4GaINT2 genes. “Each of these three genes is expressed in pigs but has been evolutionarily silenced in humans,” the coauthors added.
While CRISPR genome editing has yet to reach its full potential, researchers and clinicians should pay attention, according to Dr. Butler and Dr. Tector.
More recent modifications of CRISPR technology have shown promise in not just knocking out or turning off specific genes, but rather guiding directed replacement of genes with researcher-designed substitutes. This can enable permanent transformation of functional genes with altered behavior, according to the Broad Institute website.
Dr. Tector disclosed he has received funding from United Therapeutics and founded Xenobridge with patents for xenotransplantation. Dr. Butler has no relevant financial relationships to disclose.
CRISPR and CRISPR-associated proteins have emerged as effective genome editing techniques that may lead to cardiac and lung models and possibly xenotransplantation, Ari A. Mennander, MD, PhD, of the Tampere (Finland) University Heart Hospital, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:492).
The concept Dr. Butler and Dr. Tector discuss involves not using antibodies to ameliorate porcine antibodies that cause rejection in humans, but rather reengineering the genetic composition of pigs to eliminate those antibodies. “According to the wildest of dreams, these genes affecting porcine glycan expression may be silenced, and the human–antiporcine humoral immunity is controlled down to the level comparable with human allograft rejection,” Dr. Mennander said.
However, such a breakthrough carries with it consequences, Dr. Mennander said. “Should one worry about the induction of zoonosis, as well as the ethical aspects of transplanting the patient a whole organ of a pig? Would even a successful xenotransplant program seriously compete with artificial hearts or allografts?” Embracing the method too early would open its advocates to ridicule, he said.
“We are to applaud the researchers for ever-lasting and exemplary enthusiasm for a futuristic new surgical solution; the future may lie as much in current clinical solutions as in innovative discoveries based on persistent scientific experiments,” Dr. Mennander said.
Dr. Mennander had no relevant financial relationships to disclose.
CRISPR and CRISPR-associated proteins have emerged as effective genome editing techniques that may lead to cardiac and lung models and possibly xenotransplantation, Ari A. Mennander, MD, PhD, of the Tampere (Finland) University Heart Hospital, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:492).
The concept Dr. Butler and Dr. Tector discuss involves not using antibodies to ameliorate porcine antibodies that cause rejection in humans, but rather reengineering the genetic composition of pigs to eliminate those antibodies. “According to the wildest of dreams, these genes affecting porcine glycan expression may be silenced, and the human–antiporcine humoral immunity is controlled down to the level comparable with human allograft rejection,” Dr. Mennander said.
However, such a breakthrough carries with it consequences, Dr. Mennander said. “Should one worry about the induction of zoonosis, as well as the ethical aspects of transplanting the patient a whole organ of a pig? Would even a successful xenotransplant program seriously compete with artificial hearts or allografts?” Embracing the method too early would open its advocates to ridicule, he said.
“We are to applaud the researchers for ever-lasting and exemplary enthusiasm for a futuristic new surgical solution; the future may lie as much in current clinical solutions as in innovative discoveries based on persistent scientific experiments,” Dr. Mennander said.
Dr. Mennander had no relevant financial relationships to disclose.
CRISPR and CRISPR-associated proteins have emerged as effective genome editing techniques that may lead to cardiac and lung models and possibly xenotransplantation, Ari A. Mennander, MD, PhD, of the Tampere (Finland) University Heart Hospital, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:492).
The concept Dr. Butler and Dr. Tector discuss involves not using antibodies to ameliorate porcine antibodies that cause rejection in humans, but rather reengineering the genetic composition of pigs to eliminate those antibodies. “According to the wildest of dreams, these genes affecting porcine glycan expression may be silenced, and the human–antiporcine humoral immunity is controlled down to the level comparable with human allograft rejection,” Dr. Mennander said.
However, such a breakthrough carries with it consequences, Dr. Mennander said. “Should one worry about the induction of zoonosis, as well as the ethical aspects of transplanting the patient a whole organ of a pig? Would even a successful xenotransplant program seriously compete with artificial hearts or allografts?” Embracing the method too early would open its advocates to ridicule, he said.
“We are to applaud the researchers for ever-lasting and exemplary enthusiasm for a futuristic new surgical solution; the future may lie as much in current clinical solutions as in innovative discoveries based on persistent scientific experiments,” Dr. Mennander said.
Dr. Mennander had no relevant financial relationships to disclose.
Advances in gene editing are pushing the possibility of raising pigs for organs that may be transplanted into humans with immunosuppression regimens comparable to those now used in human-to-human transplants, coauthors James Butler, MD, and A. Joseph Tector, MD, PhD, stated in an expert opinion in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:488-92).
Developments in genome editing could bring new approaches to management of cardiopulmonary diseases, Dr. Butler and Dr. Tector noted. “Recently, cardiac-specific and lung-specific applications have been described, which will allow for the rapid creation of new models of heart and lung disease,” they said. Specifically, they noted gene targeting might eventually offer a way to treat challenging genetic problems “like the heterogeneous nature of nonsquamous cell lung cancer.”
Dr. Butler is with the department of surgery at Indiana University, Indianapolis, and Dr. Tector is with the department of surgery at the University of Alabama at Birmingham.
CRISPR technology has been used in developing multiple gene knockout pigs and neutralizing three separate porcine genes that encode human xenoantigens in a single reaction, leading to efficient methods for creating pigs with multiple genetic modifications.
According to the website of the Broad Institute of MIT and Harvard, Cambridge, Mass., where researchers perfected the system to work in eukaryotes, CRISPR works by using short RNA sequences designed by researchers to guide the system to matching sequences of DNA. When the target DNA is found, Cas9 – one of the enzymes produced by the CRISPR system – binds to the DNA and cuts it, shutting the targeted gene off.
“By facilitating high-throughput model creation, CRISPR has elucidated which modifications are necessary and which are not; despite the ability to alter many loci concurrently, recent evidence has implicated three porcine genes that are responsible for the majority of human-antiporcine humoral immunity,” Dr. Butler and Dr. Tector wrote.
Those genes are the Gal[alpha]1-3 epitope (Gal-alpha), CMAH and B4GaINT2 genes. “Each of these three genes is expressed in pigs but has been evolutionarily silenced in humans,” the coauthors added.
While CRISPR genome editing has yet to reach its full potential, researchers and clinicians should pay attention, according to Dr. Butler and Dr. Tector.
More recent modifications of CRISPR technology have shown promise in not just knocking out or turning off specific genes, but rather guiding directed replacement of genes with researcher-designed substitutes. This can enable permanent transformation of functional genes with altered behavior, according to the Broad Institute website.
Dr. Tector disclosed he has received funding from United Therapeutics and founded Xenobridge with patents for xenotransplantation. Dr. Butler has no relevant financial relationships to disclose.
Advances in gene editing are pushing the possibility of raising pigs for organs that may be transplanted into humans with immunosuppression regimens comparable to those now used in human-to-human transplants, coauthors James Butler, MD, and A. Joseph Tector, MD, PhD, stated in an expert opinion in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:488-92).
Developments in genome editing could bring new approaches to management of cardiopulmonary diseases, Dr. Butler and Dr. Tector noted. “Recently, cardiac-specific and lung-specific applications have been described, which will allow for the rapid creation of new models of heart and lung disease,” they said. Specifically, they noted gene targeting might eventually offer a way to treat challenging genetic problems “like the heterogeneous nature of nonsquamous cell lung cancer.”
Dr. Butler is with the department of surgery at Indiana University, Indianapolis, and Dr. Tector is with the department of surgery at the University of Alabama at Birmingham.
CRISPR technology has been used in developing multiple gene knockout pigs and neutralizing three separate porcine genes that encode human xenoantigens in a single reaction, leading to efficient methods for creating pigs with multiple genetic modifications.
According to the website of the Broad Institute of MIT and Harvard, Cambridge, Mass., where researchers perfected the system to work in eukaryotes, CRISPR works by using short RNA sequences designed by researchers to guide the system to matching sequences of DNA. When the target DNA is found, Cas9 – one of the enzymes produced by the CRISPR system – binds to the DNA and cuts it, shutting the targeted gene off.
“By facilitating high-throughput model creation, CRISPR has elucidated which modifications are necessary and which are not; despite the ability to alter many loci concurrently, recent evidence has implicated three porcine genes that are responsible for the majority of human-antiporcine humoral immunity,” Dr. Butler and Dr. Tector wrote.
Those genes are the Gal[alpha]1-3 epitope (Gal-alpha), CMAH and B4GaINT2 genes. “Each of these three genes is expressed in pigs but has been evolutionarily silenced in humans,” the coauthors added.
While CRISPR genome editing has yet to reach its full potential, researchers and clinicians should pay attention, according to Dr. Butler and Dr. Tector.
More recent modifications of CRISPR technology have shown promise in not just knocking out or turning off specific genes, but rather guiding directed replacement of genes with researcher-designed substitutes. This can enable permanent transformation of functional genes with altered behavior, according to the Broad Institute website.
Dr. Tector disclosed he has received funding from United Therapeutics and founded Xenobridge with patents for xenotransplantation. Dr. Butler has no relevant financial relationships to disclose.
Key clinical point: CRISPR/Cas9 genome editing is advancing the creation of animal models for xenotransplantation into humans.
Major finding: Genome editing tools are moving xenotransplantation models quickly toward potential treatments for cardiopulmonary disease.
Data source: Expert opinion with literature review.
Disclosures: Dr. Tector disclosed he has received funding from United Therapeutics and founded Xenobridge with patents for xenotransplantation. Dr. Butler reported having no relevant financial disclosures.