User login
Impact of patient-centered discharge tools: A systematic review
Patient-centered care, defined by the Institute of Medicine as “health care that establishes a partnership among practitioners, patients, and their families to ensure that decisions respect patients’ wants, needs and preferences and that patients have the education and support they need to make decisions and participate in their own care,” has been recognized as an important factor in improving care transitions after discharge from the hospital.1 Previous efforts to improve the discharge process for hospitalized patients and reduce avoidable readmissions have focused on improving systems surrounding the patient, such as by increasing the availability of outpatient follow-up or standardizing communication between the inpatient and outpatient care teams.1,2 In fact, successful programs such as Project BOOST and the Care Transitions Interventions™ provide healthcare institutions with a “bundle” of evidence-based transitional care guidelines for discharge: they provide postdischarge transition coaches, assistance with medication self-management, timely follow-up tips, and improved patient records in order to improve postdischarge outcomes.3,4 Successful interventions, however, may not provide more services, but also engage the patient in their own care.5,6 The impact of engaging the patient in his or her own care by providing patient-friendly discharge instructions alone, however, is unknown.
A patient-centered discharge may use tools that were designed with patients, or may involve engaging patients in an interactive process of reviewing discharge instructions and empowering them to manage aspects of their own care after leaving the hospital. This endeavour may lead to more effective use of discharge instructions and reduce the need for additional or more intensive (and costly) interventions. For example, a patient-centered discharge tool could include an educational intervention that uses the “teach-back” method, in which patients are asked to restate in their own words what they thought they heard, or in which staff use additional media or a visual design tool meant to enhance comprehension of discharge instructions.6,7 Visual aids and the use of larger fonts are particularly useful design elements for improving comprehension among non-English speakers and patients with low health literacy, who tend to have poorer recall of instructions.8-10 What may constitute essential design elements to include in a discharge instruction tool, however, is not clear.
Moreover, whether the use of discharge tools with a specific focus on patient engagement may improve postdischarge outcomes is not known. Particularly, the ability of patient-centered discharge tools to improve outcomes beyond comprehension such as self-management, adherence to discharge instructions, a reduction in unplanned visits, and a reduction in mortality has not been studied systematically. The objective of this systematic review was to review the literature on discharge instruction tools with a focus on patient engagement and their impact among hospitalized patients.
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement was followed as a guideline for reporting throughout this review.11
Data Sources
A literature search was undertaken using the following databases from January 1994 or their inception date to May 2014: Medline, Embase, SIGLE, HTA, Bioethics, ASSIA, Psych Lit, CINAHL, Cochrane Library, EconLit, ERIC, and BioMed Central. We also searched relevant design-focused journals such as Design Issues, Journal of Design Research, Information Design Journal, Innovation, Design Studies, and International Journal of Design, as well as reference lists from studies obtained by electronic searching. The following key words and combination of key words were used with the assistance of a medical librarian: patient discharge, patient-centered discharge, patient-centered design, design thinking, user based design, patient education, discharge summary, education. Additional search terms were added when identified from relevant articles (Appendix).
Inclusion Criteria
We included all English-language studies with patients admitted to the hospital irrespective of age, sex, or medical condition, which included a control group or time period and which measured patient outcomes within 3 months of discharge. The 3-month period after discharge is often cited as a time when outcomes could reasonably be associated with an intervention at discharge.2
Exclusion Criteria
Studies that did not have clear implementation of a patient-centered tool, a control group, or those whose tool was used in the emergency department or as an outpatient were excluded. Studies that included postdischarge tools such as home visits or telephone calls were excluded unless independent effects of the predischarge interventions were measured. Studies with outcomes reported after 3 months were excluded unless outcomes before 3 months were also clearly noted.
All searches were entered into Endnote and duplicates were removed. A 2-stage inclusion process was used. Titles and abstracts of articles were first screened for meeting inclusion and exclusion criteria by 1 reviewer. A second reviewer independently checked a 10% random sample of all the abstracts that met the initial screening criteria. If the agreement to exclude studies was less than 95%, criteria were reviewed before checking the rest of the 90% sample. In the second stage, 2 independent reviewers examined paper copies of the full articles selected in the first stage. Disagreement between reviewers was resolved by discussion or a third reviewer if no agreement could be reached.
Data Analysis and Synthesis
The following information was extracted from the full reference: type of study, population studied, control group or time period, tool used, and outcomes measured. Based on the National Health Care Quality report’s priorities and goals on patient and/or family engagement during transitions of care, educational tools were further described based on method of teaching, involvement of the care team, involvement of the patient in the design or delivery of the tool, and/or the use of visual aids.12 All primary outcomes were classified according to 3 categories: improved knowledge/comprehension, patient experience (patient satisfaction, self-management/efficacy such as functional status, both physical and mental), and health outcomes (unscheduled visits or readmissions, adherence with medications, diet, exercise, or follow-up, and mortality).
No quantitative pooling of results or meta-analysis was done given the variability and heterogeneity of studies reviewed. However, following guidelines for Effect Practice and Organisation of Care (EPOC) Risk of Bias criteria,13 studies that had a higher risk of bias such as uncontrolled before-after studies or studies with only 1 intervention or control site (historical controls, eg) were excluded from the final review because of the difficulties in attributing causation. Only primary outcomes were reported in order to minimize type II errors.
RESULTS
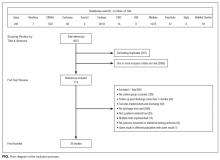
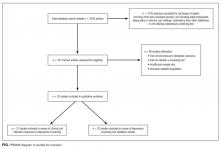
Our search revealed a total of 3699 studies after duplicates had been removed (Figure). A total of 714 references were included after initial review by title and abstract and 30 studies after full-text review. Agreement on a 10% random sample of all abstracts and full text was 79% (k=0.58) and 86% (k=0.72), respectively. Discussion was needed for fewer than 100 references, and agreement was subsequently reached for 100%.
There were 22 randomized controlled trials and 8 nonrandomized studies (5 nonrandomized controlled trials and 3 controlled before-after studies). Most of these studies were conducted in the United States (13/30 studies), followed by other European countries (5 studies), and the United Kingdom (4 studies). A large number of studies were conducted among patients with cardiovascular disease or risk factors (10 studies), followed by postsurgical patients such as coronary artery bypass graft surgery or orthopaedic surgery (5 studies). Five of 30 studies were conducted among individuals older than 65 years. Most studies excluded patients who did not speak English or the country’s official language; only 3 studies included patients with limited literacy, patients who spoke other languages, or caregivers if the patients could not communicate.
Most studies tested the impact of educational discharge interventions (28 of 30 studies) (Table 1). Quite often, it was a member of the research team who carried out the patient education. Only 3 studies involved multiple members of the care team in designing or reviewing the discharge tool with the patient. Almost half (12 studies) targeted multiple aspects of postdischarge care, including medications and side effects, signs and symptoms to consider, plans for follow-up, dietary restrictions, and/or exercise modifications. Many (19 studies) provided education using one-on-one teaching in association with a discharge tool, accompanied by a written handout (13 studies), audiotape (2 studies), or video (3 studies). While 13 studies had patients involved in creating what content was discussed and 14 studies had patients involved in the delivery of the tool, only 6 studies had patients involved in both design and delivery of the tool. Nine studies also used visual aids such as pictures, larger font, or use of a tool enhanced for patients with language barriers or limited health literacy.
Among all 30 studies included, 16 studies tested the impact of their tool on comprehension postdischarge, with 10 studies demonstrating an improvement among patients who had received the tool (Table 2). Five studies evaluated healthcare utilization outcomes such as readmission, length of stay, or physician visits after discharge and 2 studies found improvements. Twelve studies also studied the impact on adherence with medications, diet, exercise, or follow-up instructions postdischarge. However, only 4 of these 12 studies showed a positive impact. Only 2 studies tested the impact on a patient’s ability to self-manage once at home, and both studies reported positive statistical outcomes. Few studies measured patient experience (such as patient satisfaction or improvement in self-efficacy) or mortality postdischarge.
DISCUSSION/CONCLUSION
Our systematic review found 30 studies that engaged patients during the design or the delivery of a discharge instruction tool and that tested the effect of the tool on postdischarge outcomes.6-10,14–38 Our review suggests that there is sufficient evidence that patient-centered discharge tools improve comprehension. However, evidence is currently insufficient to determine if patient-centered tools improve adherence with discharge instructions. Moreover, though limited studies show promising results, more studies are needed to determine if patient engagement improves self-efficacy and healthcare utilization after discharge.
A major limitation of current studies is the variability in the level of patient engagement in tool design or delivery. Patients were involved in the design mostly through targeted development of a discharge management plan and the delivery by encouraging them to ask questions. Few studies involved patients in the design of the tool such that patients were responsible for coming up with content that was of interest to them. The few that did, often with the additional use of video media, demonstrated significant outcomes. Only a minority of studies used an interactive process to assess understanding such as “teach-back” or maximize patient comprehension such as visual aids. Even fewer studies engaged patients in both developing the discharge tool and providing discharge instructions.
Several previous studies have demonstrated that most complications after discharge are the result of ineffective communication, which can be exacerbated by lack of fluency in English or by limited health literacy.2,39-43 As a result, poor understanding of discharge instructions by patients and their caregivers can create an important care gap.44 Therefore, the use of patient-centered tools to engage patients at discharge in their own care is needed. How to engage patients consistently and effectively is perhaps less evident, as demonstrated in this review of the literature in which different levels of patient engagement were found. Many of the tools tested placed attention on patient education, sometimes in the context of bundled care along with home visits or follow-up, all of which can require extensive resources and time. Providing patients with information that the patients themselves state is of value may be the easiest refinement to a discharge educational tool, although this was surprisingly uncommon.6,9,10,17,23,33,37 Only 2 studies were found that engaged patients in the initial stage of design of the discharge tool, by incorporating information of interest to them.23,32 For example, a study testing the impact of a computer-generated written education package on poststroke outcomes designed the information by asking patients to identify which topics they would like to receive information about (along with the amount of information and font size).23 Secondly, although most of the discharge tools reviewed included the use of one-on-one teaching and the use of media such as patient handouts, these tools were often used in such a way that patients were passive recipients. In fact, studies that used additional video media that incorporated personalized content were the most likely to demonstrate positive outcomes.17,34 The next level of patient engagement may therefore be to involve the patient as an interactive partner when delivering the tool in order to empower patients to self-care. For example, 1 study designed a structured education program by first assessing lifestyle risk factors related to hypertension that were modifiable along with preconceived notions through open-ended questions during a one-on-one interview.37 Patients were subsequently educated on any knowledge deficits regarding the management of their lifestyle. Another level of patient engagement may be to use visual aids during discussions, as a well-known complement to verbal instructions.45,46 For example, in a controlled study that randomized a ward of elderly patients with 4 or more prescriptions to predischarge counseling, the counseling session aimed to review reasons for their prescriptions along with corresponding side effects, doses, and dosage times with the help of a medicine reminder card. Other uses of visual aid tools identified in our review included the use of pictograms or illustrations or, at minimum, attention to font size.7,8,16,29,33,35 In the absence of a visual aid, asking the patient to repeat or demonstrate what was just communicated can be used to assess the amount of information retained.18,33
An important result discovered in our review of the literature was also the lack of studies that tested the impact of discharge tools on usability of discharge information once at home. Conducting an evaluation of the benefits to patients after discharge can help objectify vague outcomes like health gains or qualify benefits in patient’s views. This might also explain why many studies with documented patient engagement at the time of discharge were able to demonstrate improvements in comprehension but not adherence to instructions. Although patients and caregivers may understand the information, this comprehension does not necessarily mean they will find the information useful or adhere to it once at home. For example, in 1 study, patients discharged with at least 1 medication were randomized to a structured discharge interview during which the treatment plan was reviewed verbally and questions clarified along with a visually enhanced treatment card.26 Although knowledge of medications increased, no effect was found on adherence at 1 week postdischarge. However, use of the treatment card at home was not assessed. Similarly, another study tested the effect of an individualized video of exercises and failed to find a difference in patient adherence at 4 weeks.28 The authors suggested that the lack of benefit may have been because patients were not using the video once at home. This is in contrast to 2 studies that involved patients in their own care by requiring them to request their medication as part of a self-medication tool predischarge.16,30 Patients were engaged in the process such that increasing independence was given to patients based on their demonstration of understanding and adherence to their treatment while still in the hospital, a learning tool that can be applied once at home. Feeling knowledgeable and involved, as others have suggested, may be the intermediary outcomes that led to improved adherence.47 It is also possible that adherence to discharge instructions may vary based on complexity of the information provided, such that instructions focusing solely on medication use may require less patient engagement than discharge instructions that include information on medications, diet, exercise modifications, and follow-up.48
Our review has a few limitations. Previous systematic reviews have demonstrated that bundled discharge interventions that include patient-centered education have a positive effect on outcomes postdischarge.2,5 However, we sought to describe and study the individual and distinct impact of patient engagement in the creation and delivery of discharge tools on outcomes postdischarge. We hoped that this may provide others with key information regarding elements of patient engagement that were particularly useful when designing a new discharge tool. The variability of the studies we identified, however, made it difficult to ascertain what level of patient engagement is required to observe improvements in health outcomes. It is also possible that a higher level of patient engagement may have been used but not described in the studies we reviewed. As only primary outcomes were included, we may have underestimated the effect of patient-centered discharge tools on outcomes that were reported as secondary outcomes. As we were interested in reviewing as many studies of patient-centered discharge tools as possible, we did not assess the quality of the studies and cannot comment on the role of bias in these studies. However, we excluded studies with study designs known to have the highest risk of bias. Lastly, we also cannot comment on whether patient-centered tools may have an effect on outcomes more than 3 months after a hospital discharge. However, several studies included in this review suggest a sustained effect beyond this time period.8,25,32,37
Patient-centered discharge tools in which patients were engaged in the design or the delivery were found to improve comprehension of but not adherence with discharge instructions. The perceived lack of improved adherence may be due to a lack of studies that measured the usefulness and utilization of information for patients once at home. There was also substantial variability in the extent of patient involvement in designing the style and content of information provided to patients at discharge, as well as the extent of patient engagement when receiving discharge instructions. Future studies would benefit from detailing the level of patient engagement needed in designing and delivery of discharge tools. This information may lead to the discovery of barriers and facilitators to utilization of discharge information once at home and lead to a better understanding of the patient’s journey from hospital to home and onwards.
C.M.B. and this work were funded by a CIHR Canadian Patient Safety Institute Chair in Patient Safety and Continuity of Care. Funding was provided to cover fees to obtain articles from the Donald J. Matthews Complex Care Fund of the University Health Network in Toronto, Canada. The Toronto Central Local Health Integration Network provided funding for the design and implementation of a patient-oriented discharge summary. None of the funding or supportive agencies were involved in the design or conduct of the present study, analysis, or interpretation of the data, or approval of the manuscript.
Disclosures
The authors report no conflicts of interest.
1. Hurtad
2. Mistiaen P, Francke AL, Poot E. Interventions aimed at reducing problems in adult patients discharged from hospital to home: a systematic meta-review. BMC Health Serv Res. 2007;7:47. PubMed
3. Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822-1828. PubMed
4. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. PubMed
5. Hansen LO, Young RS, Hinami K, et al. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. PubMed
6. Osman LM, Calder C, Godden DJ, et al. A randomised trial of self-management planning for adult patients admitted to hospital with acute asthma. Thorax. 2002;57(10):869-874. PubMed
7. Cordasco KM, Asch SM, Bell DS, et al. A low-literacy medication education tool for safety-net hospital patients. Am J Prev Med. 2009;37(6 suppl 1):S209-S216. PubMed
8. Morice AH, Wrench C. The role of the asthma nurse in treatment compliance and self-management following hospital admission. Resp Med. 2001;95(11):851-856. PubMed
9. Haerem JW, Ronning EJ, Leidal R. Home access to hospital discharge information on audiotape reduces sick leave and readmissions in patients with first-time myocardial infarction. Scand Cardiovasc J. 2000;34(2):219-222. PubMed
10. Legrain S, Tubach F, Bonnet-Zamponi D, et al. A new multimodal geriatric discharge-planning intervention to prevent emergency visits and rehospitalizations of older adults: the optimization of medication in AGEd multicenter randomized controlled trial. J Am Geriatr Soc. 2011;59(11):2017-2028. PubMed
11. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269. PubMed
12. Partnership NP. National Priorities and Goals: Aligning Our Efforts to Transform America’s Healthcare. Washington, DC: National Quality Forum; 2008.
13. Effective Practice and Organisation of Care (EPOC). EPOC-specific resources for review authors. Oslo, Norway: Norwegian Knowledge Centre for the Health Services; 2013. http://epoc.cochrane.org/epoc-specific-resources-review-authors. Accessed December 21, 2016.
14. Manning DM, O’Meara JG, Williams AR, et al. 3D: a tool for medication discharge education. Qual Saf Health Care. 2007;16(1):71-76. PubMed
15. Perera KY, Ranasinghe P, Adikari AM, et al. Medium of language in discharge summaries: would the use of native language improve patients’ knowledge of their illness and medications? J Health Commun. 2012;17(2):141-148. PubMed
16. Lowe CJ, Raynor DK, Courtney EA, et al. Effects of self medication programme on knowledge of drugs and compliance with treatment in elderly patients. BMJ. 1995;310(6989):1229-1231. PubMed
17. Mahler HI, Kulik JA, Tarazi RY. Effects of a videotape information intervention at discharge on diet and exercise compliance after coronary bypass surgery. J Cardiopulm Rehabil. 1999;19(3):170-177. PubMed
18. Al-Rashed SA, Wright DJ, Roebuck N, et al. The value of inpatient pharmaceutical counseling to elderly patients prior to discharge. Br J Clin Pharmacol. 2002;54(6):657-664. PubMed
19. Drenth-van Maanen AC, Wilting I, Jansen PA, et al. Effect of a discharge medication intervention on the incidence and nature of medication discrepancies in older adults. J Am Geriatr Soc. 2013;61(3):456-458. PubMed
20. Eshah NF. Predischarge education improves adherence to a healthy lifestyle among Jordanian patients with acute coronary syndrome. Nurs Health Sci. 2013;15(3):273-279. PubMed
21. Gwadry-Sridhar FH, Arnold JM, Zhang Y,et al. Pilot study to determine the impact of a multidisciplinary educational intervention in patients hospitalized with heart failure. Am Heart J. 2005;150(5):982. PubMed
22. Ho SM, Heh SS, Jevitt CM, et al. Effectiveness of a discharge education program in reducing the severity of postpartum depression: a randomized controlled evaluation study. Patient Educ Couns. 2009;77(1):68-71. PubMed
23. Hoffmann T, McKenna K, Worrall L, et al. Randomised trial of a computer-generated tailored written education package for patients following stroke. Age Ageing. 2007;36(3):280-286. PubMed
24. Jenkins HM, Blank V, Miller K, et al. A randomized single-blind evaluation of a discharge teaching book for pediatric patients with burns. J Burn Care Rehabil. 1996;17(1):49-61. PubMed
25. Kommuri NV, Johnson ML, Koelling TM. Relationship between improvements in heart failure patient disease specific knowledge and clinical events as part of a randomized controlled trial. Patient Educ Couns. 2012;86(2):233-238. PubMed
26. Louis-Simonet M, Kossovsky MP, Sarasin FP, et al. Effects of a structured patient-centered discharge interview on patients’ knowledge about their medications. Am J Med. 2004;117(8):563-568. PubMed
27. Lucas KS. Outcomes evaluation of a pharmacist discharge medication teaching service. Am J Health Syst Pharm. 1998;55(24 suppl 4):S32-S35. PubMed
28. Lysack C, Dama M, Neufeld S, et al. A compliance and satisfaction with home exercise: a comparison of computer-assisted video instruction and routine rehabilitation practice. J Allied Health. 2005;34(2):76-82. PubMed
29. Moore SM. The effects of a discharge information intervention on recovery outcomes following coronary artery bypass surgery. Int J Nurs Stud. 1996;33(2):181-189. PubMed
30. Pereles L, Romonko L, Murzyn T, et al. Evaluation of a self-medication program. J Am Geriatr Soc. 1996;44(2):161-165. PubMed
31. Reynolds MA. Postoperative pain management discharge teaching in a rural population. Pain Manag Nurs. 2009;10(2):76-84. PubMed
32. Sabariego C, Barrera AE, Neubert S, et al. Evaluation of an ICF-based patient education programme for stroke patients: a randomized, single-blinded, controlled, multicentre trial of the effects on self-efficacy, life satisfaction and functioning. Br J Health Psychol. 2013;18(4):707-728. PubMed
33. Shieh SJ, Chen HL, Liu FC, et al. The effectiveness of structured discharge education on maternal confidence, caring knowledge and growth of premature newborns. J Clin Nurs. 2010;19(23-24):3307-3313. PubMed
34. Steinberg TG, Diercks MJ, Millspaugh J. An evaluation of the effectiveness of a videotape for discharge teaching of organ transplant recipients. J Transpl Coord. 1996;6(2):59-63. PubMed
35. Whitby M, McLaws ML, Doidge S, et al. Post-discharge surgical site surveillance: does patient education improve reliability of diagnosis? J Hosp Infect. 2007;66(3):237-242. PubMed
36. Williford SL, Johnson DF. Impact of pharmacist counseling on medication knowledge and compliance. Mil Med. 1995;160(11):561–564. PubMed
37. Zernike W, Henderson A. Evaluating the effectiveness of two teaching strategies for patients diagnosed with hypertension. J Clin Nurs. 1998;7(1):37–44. PubMed
38. Press VG, Arora V, Constantine KL, et al. Forget me not: a randomized trial of the durability of hospital-based education on inhalers for patients with COPD or asthma [abstract]. J Gen Intern Med. 2014;29(1 suppl):S102.
39. Davis TC, Wolf MS, Bass PF, et al. Literacy and misunderstanding prescription drug labels. Ann Intern Med. 2006;145(12):887–894. PubMed
40. McCarthy DM, Waite KR, Curtis LM, et al. What did the doctor say? Health literacy and recall of medical instructions. Med Care. 2012;50(4):277–282. PubMed
41. Tarn DM, Heritage J, Paterniti DA, et al. Physician communication when prescribing new medications. Arch Intern Med. 2006;166(17):1855–1862. PubMed
42. Cawthon C, Walia S, Osborn CY, et al. Improving care transitions: the patient perspective. J Health Commun. 2012;17(suppl 3):312–324. PubMed
43. Karliner LS, Auerbach A, Nápoles A, et al. Language barriers and understanding of hospital discharge instructions. Med Care. 2012;50(4):283–289. PubMed
44. Enhancing the Continuum of Care. Report of the Avoidable Hospitalization Advisory Panel. http://www.health.gov.on.ca/en/common/ministry/publications/reports/baker_2011/baker_2011.pdf. Published November 2011. Accessed December 22, 2016.
45. Chugh A, Williams MV, Grigsby J, et al. Better transitions: improving comprehension of discharge instructions. Front Health Serv Manage. 2009;25(3):11–32. PubMed
46. Schillinger D, Machtinger EL, Wang F, et al. Language, literacy, and communication regarding medication in an anticoagulation clinic: a comparison of verbal vs. visual assessment. J Health Commun. 2006;11(7):651–664. PubMed
47. Epstein RM, Street RL, Jr. The values and value of patient-centered care. Ann Fam Med. 2011;9(2):100–103. PubMed
48. Albrecht JS, Gruber-Baldini AL, Hirshon JM, et al. Hospital discharge instructions: comprehension and compliance among older adults. J Gen Intern Med. 2014;29(11):1491–1498. PubMed
Patient-centered care, defined by the Institute of Medicine as “health care that establishes a partnership among practitioners, patients, and their families to ensure that decisions respect patients’ wants, needs and preferences and that patients have the education and support they need to make decisions and participate in their own care,” has been recognized as an important factor in improving care transitions after discharge from the hospital.1 Previous efforts to improve the discharge process for hospitalized patients and reduce avoidable readmissions have focused on improving systems surrounding the patient, such as by increasing the availability of outpatient follow-up or standardizing communication between the inpatient and outpatient care teams.1,2 In fact, successful programs such as Project BOOST and the Care Transitions Interventions™ provide healthcare institutions with a “bundle” of evidence-based transitional care guidelines for discharge: they provide postdischarge transition coaches, assistance with medication self-management, timely follow-up tips, and improved patient records in order to improve postdischarge outcomes.3,4 Successful interventions, however, may not provide more services, but also engage the patient in their own care.5,6 The impact of engaging the patient in his or her own care by providing patient-friendly discharge instructions alone, however, is unknown.
A patient-centered discharge may use tools that were designed with patients, or may involve engaging patients in an interactive process of reviewing discharge instructions and empowering them to manage aspects of their own care after leaving the hospital. This endeavour may lead to more effective use of discharge instructions and reduce the need for additional or more intensive (and costly) interventions. For example, a patient-centered discharge tool could include an educational intervention that uses the “teach-back” method, in which patients are asked to restate in their own words what they thought they heard, or in which staff use additional media or a visual design tool meant to enhance comprehension of discharge instructions.6,7 Visual aids and the use of larger fonts are particularly useful design elements for improving comprehension among non-English speakers and patients with low health literacy, who tend to have poorer recall of instructions.8-10 What may constitute essential design elements to include in a discharge instruction tool, however, is not clear.
Moreover, whether the use of discharge tools with a specific focus on patient engagement may improve postdischarge outcomes is not known. Particularly, the ability of patient-centered discharge tools to improve outcomes beyond comprehension such as self-management, adherence to discharge instructions, a reduction in unplanned visits, and a reduction in mortality has not been studied systematically. The objective of this systematic review was to review the literature on discharge instruction tools with a focus on patient engagement and their impact among hospitalized patients.
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement was followed as a guideline for reporting throughout this review.11
Data Sources
A literature search was undertaken using the following databases from January 1994 or their inception date to May 2014: Medline, Embase, SIGLE, HTA, Bioethics, ASSIA, Psych Lit, CINAHL, Cochrane Library, EconLit, ERIC, and BioMed Central. We also searched relevant design-focused journals such as Design Issues, Journal of Design Research, Information Design Journal, Innovation, Design Studies, and International Journal of Design, as well as reference lists from studies obtained by electronic searching. The following key words and combination of key words were used with the assistance of a medical librarian: patient discharge, patient-centered discharge, patient-centered design, design thinking, user based design, patient education, discharge summary, education. Additional search terms were added when identified from relevant articles (Appendix).
Inclusion Criteria
We included all English-language studies with patients admitted to the hospital irrespective of age, sex, or medical condition, which included a control group or time period and which measured patient outcomes within 3 months of discharge. The 3-month period after discharge is often cited as a time when outcomes could reasonably be associated with an intervention at discharge.2
Exclusion Criteria
Studies that did not have clear implementation of a patient-centered tool, a control group, or those whose tool was used in the emergency department or as an outpatient were excluded. Studies that included postdischarge tools such as home visits or telephone calls were excluded unless independent effects of the predischarge interventions were measured. Studies with outcomes reported after 3 months were excluded unless outcomes before 3 months were also clearly noted.
All searches were entered into Endnote and duplicates were removed. A 2-stage inclusion process was used. Titles and abstracts of articles were first screened for meeting inclusion and exclusion criteria by 1 reviewer. A second reviewer independently checked a 10% random sample of all the abstracts that met the initial screening criteria. If the agreement to exclude studies was less than 95%, criteria were reviewed before checking the rest of the 90% sample. In the second stage, 2 independent reviewers examined paper copies of the full articles selected in the first stage. Disagreement between reviewers was resolved by discussion or a third reviewer if no agreement could be reached.
Data Analysis and Synthesis
The following information was extracted from the full reference: type of study, population studied, control group or time period, tool used, and outcomes measured. Based on the National Health Care Quality report’s priorities and goals on patient and/or family engagement during transitions of care, educational tools were further described based on method of teaching, involvement of the care team, involvement of the patient in the design or delivery of the tool, and/or the use of visual aids.12 All primary outcomes were classified according to 3 categories: improved knowledge/comprehension, patient experience (patient satisfaction, self-management/efficacy such as functional status, both physical and mental), and health outcomes (unscheduled visits or readmissions, adherence with medications, diet, exercise, or follow-up, and mortality).
No quantitative pooling of results or meta-analysis was done given the variability and heterogeneity of studies reviewed. However, following guidelines for Effect Practice and Organisation of Care (EPOC) Risk of Bias criteria,13 studies that had a higher risk of bias such as uncontrolled before-after studies or studies with only 1 intervention or control site (historical controls, eg) were excluded from the final review because of the difficulties in attributing causation. Only primary outcomes were reported in order to minimize type II errors.
RESULTS
Our search revealed a total of 3699 studies after duplicates had been removed (Figure). A total of 714 references were included after initial review by title and abstract and 30 studies after full-text review. Agreement on a 10% random sample of all abstracts and full text was 79% (k=0.58) and 86% (k=0.72), respectively. Discussion was needed for fewer than 100 references, and agreement was subsequently reached for 100%.
There were 22 randomized controlled trials and 8 nonrandomized studies (5 nonrandomized controlled trials and 3 controlled before-after studies). Most of these studies were conducted in the United States (13/30 studies), followed by other European countries (5 studies), and the United Kingdom (4 studies). A large number of studies were conducted among patients with cardiovascular disease or risk factors (10 studies), followed by postsurgical patients such as coronary artery bypass graft surgery or orthopaedic surgery (5 studies). Five of 30 studies were conducted among individuals older than 65 years. Most studies excluded patients who did not speak English or the country’s official language; only 3 studies included patients with limited literacy, patients who spoke other languages, or caregivers if the patients could not communicate.
Most studies tested the impact of educational discharge interventions (28 of 30 studies) (Table 1). Quite often, it was a member of the research team who carried out the patient education. Only 3 studies involved multiple members of the care team in designing or reviewing the discharge tool with the patient. Almost half (12 studies) targeted multiple aspects of postdischarge care, including medications and side effects, signs and symptoms to consider, plans for follow-up, dietary restrictions, and/or exercise modifications. Many (19 studies) provided education using one-on-one teaching in association with a discharge tool, accompanied by a written handout (13 studies), audiotape (2 studies), or video (3 studies). While 13 studies had patients involved in creating what content was discussed and 14 studies had patients involved in the delivery of the tool, only 6 studies had patients involved in both design and delivery of the tool. Nine studies also used visual aids such as pictures, larger font, or use of a tool enhanced for patients with language barriers or limited health literacy.
Among all 30 studies included, 16 studies tested the impact of their tool on comprehension postdischarge, with 10 studies demonstrating an improvement among patients who had received the tool (Table 2). Five studies evaluated healthcare utilization outcomes such as readmission, length of stay, or physician visits after discharge and 2 studies found improvements. Twelve studies also studied the impact on adherence with medications, diet, exercise, or follow-up instructions postdischarge. However, only 4 of these 12 studies showed a positive impact. Only 2 studies tested the impact on a patient’s ability to self-manage once at home, and both studies reported positive statistical outcomes. Few studies measured patient experience (such as patient satisfaction or improvement in self-efficacy) or mortality postdischarge.
DISCUSSION/CONCLUSION
Our systematic review found 30 studies that engaged patients during the design or the delivery of a discharge instruction tool and that tested the effect of the tool on postdischarge outcomes.6-10,14–38 Our review suggests that there is sufficient evidence that patient-centered discharge tools improve comprehension. However, evidence is currently insufficient to determine if patient-centered tools improve adherence with discharge instructions. Moreover, though limited studies show promising results, more studies are needed to determine if patient engagement improves self-efficacy and healthcare utilization after discharge.
A major limitation of current studies is the variability in the level of patient engagement in tool design or delivery. Patients were involved in the design mostly through targeted development of a discharge management plan and the delivery by encouraging them to ask questions. Few studies involved patients in the design of the tool such that patients were responsible for coming up with content that was of interest to them. The few that did, often with the additional use of video media, demonstrated significant outcomes. Only a minority of studies used an interactive process to assess understanding such as “teach-back” or maximize patient comprehension such as visual aids. Even fewer studies engaged patients in both developing the discharge tool and providing discharge instructions.
Several previous studies have demonstrated that most complications after discharge are the result of ineffective communication, which can be exacerbated by lack of fluency in English or by limited health literacy.2,39-43 As a result, poor understanding of discharge instructions by patients and their caregivers can create an important care gap.44 Therefore, the use of patient-centered tools to engage patients at discharge in their own care is needed. How to engage patients consistently and effectively is perhaps less evident, as demonstrated in this review of the literature in which different levels of patient engagement were found. Many of the tools tested placed attention on patient education, sometimes in the context of bundled care along with home visits or follow-up, all of which can require extensive resources and time. Providing patients with information that the patients themselves state is of value may be the easiest refinement to a discharge educational tool, although this was surprisingly uncommon.6,9,10,17,23,33,37 Only 2 studies were found that engaged patients in the initial stage of design of the discharge tool, by incorporating information of interest to them.23,32 For example, a study testing the impact of a computer-generated written education package on poststroke outcomes designed the information by asking patients to identify which topics they would like to receive information about (along with the amount of information and font size).23 Secondly, although most of the discharge tools reviewed included the use of one-on-one teaching and the use of media such as patient handouts, these tools were often used in such a way that patients were passive recipients. In fact, studies that used additional video media that incorporated personalized content were the most likely to demonstrate positive outcomes.17,34 The next level of patient engagement may therefore be to involve the patient as an interactive partner when delivering the tool in order to empower patients to self-care. For example, 1 study designed a structured education program by first assessing lifestyle risk factors related to hypertension that were modifiable along with preconceived notions through open-ended questions during a one-on-one interview.37 Patients were subsequently educated on any knowledge deficits regarding the management of their lifestyle. Another level of patient engagement may be to use visual aids during discussions, as a well-known complement to verbal instructions.45,46 For example, in a controlled study that randomized a ward of elderly patients with 4 or more prescriptions to predischarge counseling, the counseling session aimed to review reasons for their prescriptions along with corresponding side effects, doses, and dosage times with the help of a medicine reminder card. Other uses of visual aid tools identified in our review included the use of pictograms or illustrations or, at minimum, attention to font size.7,8,16,29,33,35 In the absence of a visual aid, asking the patient to repeat or demonstrate what was just communicated can be used to assess the amount of information retained.18,33
An important result discovered in our review of the literature was also the lack of studies that tested the impact of discharge tools on usability of discharge information once at home. Conducting an evaluation of the benefits to patients after discharge can help objectify vague outcomes like health gains or qualify benefits in patient’s views. This might also explain why many studies with documented patient engagement at the time of discharge were able to demonstrate improvements in comprehension but not adherence to instructions. Although patients and caregivers may understand the information, this comprehension does not necessarily mean they will find the information useful or adhere to it once at home. For example, in 1 study, patients discharged with at least 1 medication were randomized to a structured discharge interview during which the treatment plan was reviewed verbally and questions clarified along with a visually enhanced treatment card.26 Although knowledge of medications increased, no effect was found on adherence at 1 week postdischarge. However, use of the treatment card at home was not assessed. Similarly, another study tested the effect of an individualized video of exercises and failed to find a difference in patient adherence at 4 weeks.28 The authors suggested that the lack of benefit may have been because patients were not using the video once at home. This is in contrast to 2 studies that involved patients in their own care by requiring them to request their medication as part of a self-medication tool predischarge.16,30 Patients were engaged in the process such that increasing independence was given to patients based on their demonstration of understanding and adherence to their treatment while still in the hospital, a learning tool that can be applied once at home. Feeling knowledgeable and involved, as others have suggested, may be the intermediary outcomes that led to improved adherence.47 It is also possible that adherence to discharge instructions may vary based on complexity of the information provided, such that instructions focusing solely on medication use may require less patient engagement than discharge instructions that include information on medications, diet, exercise modifications, and follow-up.48
Our review has a few limitations. Previous systematic reviews have demonstrated that bundled discharge interventions that include patient-centered education have a positive effect on outcomes postdischarge.2,5 However, we sought to describe and study the individual and distinct impact of patient engagement in the creation and delivery of discharge tools on outcomes postdischarge. We hoped that this may provide others with key information regarding elements of patient engagement that were particularly useful when designing a new discharge tool. The variability of the studies we identified, however, made it difficult to ascertain what level of patient engagement is required to observe improvements in health outcomes. It is also possible that a higher level of patient engagement may have been used but not described in the studies we reviewed. As only primary outcomes were included, we may have underestimated the effect of patient-centered discharge tools on outcomes that were reported as secondary outcomes. As we were interested in reviewing as many studies of patient-centered discharge tools as possible, we did not assess the quality of the studies and cannot comment on the role of bias in these studies. However, we excluded studies with study designs known to have the highest risk of bias. Lastly, we also cannot comment on whether patient-centered tools may have an effect on outcomes more than 3 months after a hospital discharge. However, several studies included in this review suggest a sustained effect beyond this time period.8,25,32,37
Patient-centered discharge tools in which patients were engaged in the design or the delivery were found to improve comprehension of but not adherence with discharge instructions. The perceived lack of improved adherence may be due to a lack of studies that measured the usefulness and utilization of information for patients once at home. There was also substantial variability in the extent of patient involvement in designing the style and content of information provided to patients at discharge, as well as the extent of patient engagement when receiving discharge instructions. Future studies would benefit from detailing the level of patient engagement needed in designing and delivery of discharge tools. This information may lead to the discovery of barriers and facilitators to utilization of discharge information once at home and lead to a better understanding of the patient’s journey from hospital to home and onwards.
C.M.B. and this work were funded by a CIHR Canadian Patient Safety Institute Chair in Patient Safety and Continuity of Care. Funding was provided to cover fees to obtain articles from the Donald J. Matthews Complex Care Fund of the University Health Network in Toronto, Canada. The Toronto Central Local Health Integration Network provided funding for the design and implementation of a patient-oriented discharge summary. None of the funding or supportive agencies were involved in the design or conduct of the present study, analysis, or interpretation of the data, or approval of the manuscript.
Disclosures
The authors report no conflicts of interest.
Patient-centered care, defined by the Institute of Medicine as “health care that establishes a partnership among practitioners, patients, and their families to ensure that decisions respect patients’ wants, needs and preferences and that patients have the education and support they need to make decisions and participate in their own care,” has been recognized as an important factor in improving care transitions after discharge from the hospital.1 Previous efforts to improve the discharge process for hospitalized patients and reduce avoidable readmissions have focused on improving systems surrounding the patient, such as by increasing the availability of outpatient follow-up or standardizing communication between the inpatient and outpatient care teams.1,2 In fact, successful programs such as Project BOOST and the Care Transitions Interventions™ provide healthcare institutions with a “bundle” of evidence-based transitional care guidelines for discharge: they provide postdischarge transition coaches, assistance with medication self-management, timely follow-up tips, and improved patient records in order to improve postdischarge outcomes.3,4 Successful interventions, however, may not provide more services, but also engage the patient in their own care.5,6 The impact of engaging the patient in his or her own care by providing patient-friendly discharge instructions alone, however, is unknown.
A patient-centered discharge may use tools that were designed with patients, or may involve engaging patients in an interactive process of reviewing discharge instructions and empowering them to manage aspects of their own care after leaving the hospital. This endeavour may lead to more effective use of discharge instructions and reduce the need for additional or more intensive (and costly) interventions. For example, a patient-centered discharge tool could include an educational intervention that uses the “teach-back” method, in which patients are asked to restate in their own words what they thought they heard, or in which staff use additional media or a visual design tool meant to enhance comprehension of discharge instructions.6,7 Visual aids and the use of larger fonts are particularly useful design elements for improving comprehension among non-English speakers and patients with low health literacy, who tend to have poorer recall of instructions.8-10 What may constitute essential design elements to include in a discharge instruction tool, however, is not clear.
Moreover, whether the use of discharge tools with a specific focus on patient engagement may improve postdischarge outcomes is not known. Particularly, the ability of patient-centered discharge tools to improve outcomes beyond comprehension such as self-management, adherence to discharge instructions, a reduction in unplanned visits, and a reduction in mortality has not been studied systematically. The objective of this systematic review was to review the literature on discharge instruction tools with a focus on patient engagement and their impact among hospitalized patients.
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement was followed as a guideline for reporting throughout this review.11
Data Sources
A literature search was undertaken using the following databases from January 1994 or their inception date to May 2014: Medline, Embase, SIGLE, HTA, Bioethics, ASSIA, Psych Lit, CINAHL, Cochrane Library, EconLit, ERIC, and BioMed Central. We also searched relevant design-focused journals such as Design Issues, Journal of Design Research, Information Design Journal, Innovation, Design Studies, and International Journal of Design, as well as reference lists from studies obtained by electronic searching. The following key words and combination of key words were used with the assistance of a medical librarian: patient discharge, patient-centered discharge, patient-centered design, design thinking, user based design, patient education, discharge summary, education. Additional search terms were added when identified from relevant articles (Appendix).
Inclusion Criteria
We included all English-language studies with patients admitted to the hospital irrespective of age, sex, or medical condition, which included a control group or time period and which measured patient outcomes within 3 months of discharge. The 3-month period after discharge is often cited as a time when outcomes could reasonably be associated with an intervention at discharge.2
Exclusion Criteria
Studies that did not have clear implementation of a patient-centered tool, a control group, or those whose tool was used in the emergency department or as an outpatient were excluded. Studies that included postdischarge tools such as home visits or telephone calls were excluded unless independent effects of the predischarge interventions were measured. Studies with outcomes reported after 3 months were excluded unless outcomes before 3 months were also clearly noted.
All searches were entered into Endnote and duplicates were removed. A 2-stage inclusion process was used. Titles and abstracts of articles were first screened for meeting inclusion and exclusion criteria by 1 reviewer. A second reviewer independently checked a 10% random sample of all the abstracts that met the initial screening criteria. If the agreement to exclude studies was less than 95%, criteria were reviewed before checking the rest of the 90% sample. In the second stage, 2 independent reviewers examined paper copies of the full articles selected in the first stage. Disagreement between reviewers was resolved by discussion or a third reviewer if no agreement could be reached.
Data Analysis and Synthesis
The following information was extracted from the full reference: type of study, population studied, control group or time period, tool used, and outcomes measured. Based on the National Health Care Quality report’s priorities and goals on patient and/or family engagement during transitions of care, educational tools were further described based on method of teaching, involvement of the care team, involvement of the patient in the design or delivery of the tool, and/or the use of visual aids.12 All primary outcomes were classified according to 3 categories: improved knowledge/comprehension, patient experience (patient satisfaction, self-management/efficacy such as functional status, both physical and mental), and health outcomes (unscheduled visits or readmissions, adherence with medications, diet, exercise, or follow-up, and mortality).
No quantitative pooling of results or meta-analysis was done given the variability and heterogeneity of studies reviewed. However, following guidelines for Effect Practice and Organisation of Care (EPOC) Risk of Bias criteria,13 studies that had a higher risk of bias such as uncontrolled before-after studies or studies with only 1 intervention or control site (historical controls, eg) were excluded from the final review because of the difficulties in attributing causation. Only primary outcomes were reported in order to minimize type II errors.
RESULTS
Our search revealed a total of 3699 studies after duplicates had been removed (Figure). A total of 714 references were included after initial review by title and abstract and 30 studies after full-text review. Agreement on a 10% random sample of all abstracts and full text was 79% (k=0.58) and 86% (k=0.72), respectively. Discussion was needed for fewer than 100 references, and agreement was subsequently reached for 100%.
There were 22 randomized controlled trials and 8 nonrandomized studies (5 nonrandomized controlled trials and 3 controlled before-after studies). Most of these studies were conducted in the United States (13/30 studies), followed by other European countries (5 studies), and the United Kingdom (4 studies). A large number of studies were conducted among patients with cardiovascular disease or risk factors (10 studies), followed by postsurgical patients such as coronary artery bypass graft surgery or orthopaedic surgery (5 studies). Five of 30 studies were conducted among individuals older than 65 years. Most studies excluded patients who did not speak English or the country’s official language; only 3 studies included patients with limited literacy, patients who spoke other languages, or caregivers if the patients could not communicate.
Most studies tested the impact of educational discharge interventions (28 of 30 studies) (Table 1). Quite often, it was a member of the research team who carried out the patient education. Only 3 studies involved multiple members of the care team in designing or reviewing the discharge tool with the patient. Almost half (12 studies) targeted multiple aspects of postdischarge care, including medications and side effects, signs and symptoms to consider, plans for follow-up, dietary restrictions, and/or exercise modifications. Many (19 studies) provided education using one-on-one teaching in association with a discharge tool, accompanied by a written handout (13 studies), audiotape (2 studies), or video (3 studies). While 13 studies had patients involved in creating what content was discussed and 14 studies had patients involved in the delivery of the tool, only 6 studies had patients involved in both design and delivery of the tool. Nine studies also used visual aids such as pictures, larger font, or use of a tool enhanced for patients with language barriers or limited health literacy.
Among all 30 studies included, 16 studies tested the impact of their tool on comprehension postdischarge, with 10 studies demonstrating an improvement among patients who had received the tool (Table 2). Five studies evaluated healthcare utilization outcomes such as readmission, length of stay, or physician visits after discharge and 2 studies found improvements. Twelve studies also studied the impact on adherence with medications, diet, exercise, or follow-up instructions postdischarge. However, only 4 of these 12 studies showed a positive impact. Only 2 studies tested the impact on a patient’s ability to self-manage once at home, and both studies reported positive statistical outcomes. Few studies measured patient experience (such as patient satisfaction or improvement in self-efficacy) or mortality postdischarge.
DISCUSSION/CONCLUSION
Our systematic review found 30 studies that engaged patients during the design or the delivery of a discharge instruction tool and that tested the effect of the tool on postdischarge outcomes.6-10,14–38 Our review suggests that there is sufficient evidence that patient-centered discharge tools improve comprehension. However, evidence is currently insufficient to determine if patient-centered tools improve adherence with discharge instructions. Moreover, though limited studies show promising results, more studies are needed to determine if patient engagement improves self-efficacy and healthcare utilization after discharge.
A major limitation of current studies is the variability in the level of patient engagement in tool design or delivery. Patients were involved in the design mostly through targeted development of a discharge management plan and the delivery by encouraging them to ask questions. Few studies involved patients in the design of the tool such that patients were responsible for coming up with content that was of interest to them. The few that did, often with the additional use of video media, demonstrated significant outcomes. Only a minority of studies used an interactive process to assess understanding such as “teach-back” or maximize patient comprehension such as visual aids. Even fewer studies engaged patients in both developing the discharge tool and providing discharge instructions.
Several previous studies have demonstrated that most complications after discharge are the result of ineffective communication, which can be exacerbated by lack of fluency in English or by limited health literacy.2,39-43 As a result, poor understanding of discharge instructions by patients and their caregivers can create an important care gap.44 Therefore, the use of patient-centered tools to engage patients at discharge in their own care is needed. How to engage patients consistently and effectively is perhaps less evident, as demonstrated in this review of the literature in which different levels of patient engagement were found. Many of the tools tested placed attention on patient education, sometimes in the context of bundled care along with home visits or follow-up, all of which can require extensive resources and time. Providing patients with information that the patients themselves state is of value may be the easiest refinement to a discharge educational tool, although this was surprisingly uncommon.6,9,10,17,23,33,37 Only 2 studies were found that engaged patients in the initial stage of design of the discharge tool, by incorporating information of interest to them.23,32 For example, a study testing the impact of a computer-generated written education package on poststroke outcomes designed the information by asking patients to identify which topics they would like to receive information about (along with the amount of information and font size).23 Secondly, although most of the discharge tools reviewed included the use of one-on-one teaching and the use of media such as patient handouts, these tools were often used in such a way that patients were passive recipients. In fact, studies that used additional video media that incorporated personalized content were the most likely to demonstrate positive outcomes.17,34 The next level of patient engagement may therefore be to involve the patient as an interactive partner when delivering the tool in order to empower patients to self-care. For example, 1 study designed a structured education program by first assessing lifestyle risk factors related to hypertension that were modifiable along with preconceived notions through open-ended questions during a one-on-one interview.37 Patients were subsequently educated on any knowledge deficits regarding the management of their lifestyle. Another level of patient engagement may be to use visual aids during discussions, as a well-known complement to verbal instructions.45,46 For example, in a controlled study that randomized a ward of elderly patients with 4 or more prescriptions to predischarge counseling, the counseling session aimed to review reasons for their prescriptions along with corresponding side effects, doses, and dosage times with the help of a medicine reminder card. Other uses of visual aid tools identified in our review included the use of pictograms or illustrations or, at minimum, attention to font size.7,8,16,29,33,35 In the absence of a visual aid, asking the patient to repeat or demonstrate what was just communicated can be used to assess the amount of information retained.18,33
An important result discovered in our review of the literature was also the lack of studies that tested the impact of discharge tools on usability of discharge information once at home. Conducting an evaluation of the benefits to patients after discharge can help objectify vague outcomes like health gains or qualify benefits in patient’s views. This might also explain why many studies with documented patient engagement at the time of discharge were able to demonstrate improvements in comprehension but not adherence to instructions. Although patients and caregivers may understand the information, this comprehension does not necessarily mean they will find the information useful or adhere to it once at home. For example, in 1 study, patients discharged with at least 1 medication were randomized to a structured discharge interview during which the treatment plan was reviewed verbally and questions clarified along with a visually enhanced treatment card.26 Although knowledge of medications increased, no effect was found on adherence at 1 week postdischarge. However, use of the treatment card at home was not assessed. Similarly, another study tested the effect of an individualized video of exercises and failed to find a difference in patient adherence at 4 weeks.28 The authors suggested that the lack of benefit may have been because patients were not using the video once at home. This is in contrast to 2 studies that involved patients in their own care by requiring them to request their medication as part of a self-medication tool predischarge.16,30 Patients were engaged in the process such that increasing independence was given to patients based on their demonstration of understanding and adherence to their treatment while still in the hospital, a learning tool that can be applied once at home. Feeling knowledgeable and involved, as others have suggested, may be the intermediary outcomes that led to improved adherence.47 It is also possible that adherence to discharge instructions may vary based on complexity of the information provided, such that instructions focusing solely on medication use may require less patient engagement than discharge instructions that include information on medications, diet, exercise modifications, and follow-up.48
Our review has a few limitations. Previous systematic reviews have demonstrated that bundled discharge interventions that include patient-centered education have a positive effect on outcomes postdischarge.2,5 However, we sought to describe and study the individual and distinct impact of patient engagement in the creation and delivery of discharge tools on outcomes postdischarge. We hoped that this may provide others with key information regarding elements of patient engagement that were particularly useful when designing a new discharge tool. The variability of the studies we identified, however, made it difficult to ascertain what level of patient engagement is required to observe improvements in health outcomes. It is also possible that a higher level of patient engagement may have been used but not described in the studies we reviewed. As only primary outcomes were included, we may have underestimated the effect of patient-centered discharge tools on outcomes that were reported as secondary outcomes. As we were interested in reviewing as many studies of patient-centered discharge tools as possible, we did not assess the quality of the studies and cannot comment on the role of bias in these studies. However, we excluded studies with study designs known to have the highest risk of bias. Lastly, we also cannot comment on whether patient-centered tools may have an effect on outcomes more than 3 months after a hospital discharge. However, several studies included in this review suggest a sustained effect beyond this time period.8,25,32,37
Patient-centered discharge tools in which patients were engaged in the design or the delivery were found to improve comprehension of but not adherence with discharge instructions. The perceived lack of improved adherence may be due to a lack of studies that measured the usefulness and utilization of information for patients once at home. There was also substantial variability in the extent of patient involvement in designing the style and content of information provided to patients at discharge, as well as the extent of patient engagement when receiving discharge instructions. Future studies would benefit from detailing the level of patient engagement needed in designing and delivery of discharge tools. This information may lead to the discovery of barriers and facilitators to utilization of discharge information once at home and lead to a better understanding of the patient’s journey from hospital to home and onwards.
C.M.B. and this work were funded by a CIHR Canadian Patient Safety Institute Chair in Patient Safety and Continuity of Care. Funding was provided to cover fees to obtain articles from the Donald J. Matthews Complex Care Fund of the University Health Network in Toronto, Canada. The Toronto Central Local Health Integration Network provided funding for the design and implementation of a patient-oriented discharge summary. None of the funding or supportive agencies were involved in the design or conduct of the present study, analysis, or interpretation of the data, or approval of the manuscript.
Disclosures
The authors report no conflicts of interest.
1. Hurtad
2. Mistiaen P, Francke AL, Poot E. Interventions aimed at reducing problems in adult patients discharged from hospital to home: a systematic meta-review. BMC Health Serv Res. 2007;7:47. PubMed
3. Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822-1828. PubMed
4. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. PubMed
5. Hansen LO, Young RS, Hinami K, et al. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. PubMed
6. Osman LM, Calder C, Godden DJ, et al. A randomised trial of self-management planning for adult patients admitted to hospital with acute asthma. Thorax. 2002;57(10):869-874. PubMed
7. Cordasco KM, Asch SM, Bell DS, et al. A low-literacy medication education tool for safety-net hospital patients. Am J Prev Med. 2009;37(6 suppl 1):S209-S216. PubMed
8. Morice AH, Wrench C. The role of the asthma nurse in treatment compliance and self-management following hospital admission. Resp Med. 2001;95(11):851-856. PubMed
9. Haerem JW, Ronning EJ, Leidal R. Home access to hospital discharge information on audiotape reduces sick leave and readmissions in patients with first-time myocardial infarction. Scand Cardiovasc J. 2000;34(2):219-222. PubMed
10. Legrain S, Tubach F, Bonnet-Zamponi D, et al. A new multimodal geriatric discharge-planning intervention to prevent emergency visits and rehospitalizations of older adults: the optimization of medication in AGEd multicenter randomized controlled trial. J Am Geriatr Soc. 2011;59(11):2017-2028. PubMed
11. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269. PubMed
12. Partnership NP. National Priorities and Goals: Aligning Our Efforts to Transform America’s Healthcare. Washington, DC: National Quality Forum; 2008.
13. Effective Practice and Organisation of Care (EPOC). EPOC-specific resources for review authors. Oslo, Norway: Norwegian Knowledge Centre for the Health Services; 2013. http://epoc.cochrane.org/epoc-specific-resources-review-authors. Accessed December 21, 2016.
14. Manning DM, O’Meara JG, Williams AR, et al. 3D: a tool for medication discharge education. Qual Saf Health Care. 2007;16(1):71-76. PubMed
15. Perera KY, Ranasinghe P, Adikari AM, et al. Medium of language in discharge summaries: would the use of native language improve patients’ knowledge of their illness and medications? J Health Commun. 2012;17(2):141-148. PubMed
16. Lowe CJ, Raynor DK, Courtney EA, et al. Effects of self medication programme on knowledge of drugs and compliance with treatment in elderly patients. BMJ. 1995;310(6989):1229-1231. PubMed
17. Mahler HI, Kulik JA, Tarazi RY. Effects of a videotape information intervention at discharge on diet and exercise compliance after coronary bypass surgery. J Cardiopulm Rehabil. 1999;19(3):170-177. PubMed
18. Al-Rashed SA, Wright DJ, Roebuck N, et al. The value of inpatient pharmaceutical counseling to elderly patients prior to discharge. Br J Clin Pharmacol. 2002;54(6):657-664. PubMed
19. Drenth-van Maanen AC, Wilting I, Jansen PA, et al. Effect of a discharge medication intervention on the incidence and nature of medication discrepancies in older adults. J Am Geriatr Soc. 2013;61(3):456-458. PubMed
20. Eshah NF. Predischarge education improves adherence to a healthy lifestyle among Jordanian patients with acute coronary syndrome. Nurs Health Sci. 2013;15(3):273-279. PubMed
21. Gwadry-Sridhar FH, Arnold JM, Zhang Y,et al. Pilot study to determine the impact of a multidisciplinary educational intervention in patients hospitalized with heart failure. Am Heart J. 2005;150(5):982. PubMed
22. Ho SM, Heh SS, Jevitt CM, et al. Effectiveness of a discharge education program in reducing the severity of postpartum depression: a randomized controlled evaluation study. Patient Educ Couns. 2009;77(1):68-71. PubMed
23. Hoffmann T, McKenna K, Worrall L, et al. Randomised trial of a computer-generated tailored written education package for patients following stroke. Age Ageing. 2007;36(3):280-286. PubMed
24. Jenkins HM, Blank V, Miller K, et al. A randomized single-blind evaluation of a discharge teaching book for pediatric patients with burns. J Burn Care Rehabil. 1996;17(1):49-61. PubMed
25. Kommuri NV, Johnson ML, Koelling TM. Relationship between improvements in heart failure patient disease specific knowledge and clinical events as part of a randomized controlled trial. Patient Educ Couns. 2012;86(2):233-238. PubMed
26. Louis-Simonet M, Kossovsky MP, Sarasin FP, et al. Effects of a structured patient-centered discharge interview on patients’ knowledge about their medications. Am J Med. 2004;117(8):563-568. PubMed
27. Lucas KS. Outcomes evaluation of a pharmacist discharge medication teaching service. Am J Health Syst Pharm. 1998;55(24 suppl 4):S32-S35. PubMed
28. Lysack C, Dama M, Neufeld S, et al. A compliance and satisfaction with home exercise: a comparison of computer-assisted video instruction and routine rehabilitation practice. J Allied Health. 2005;34(2):76-82. PubMed
29. Moore SM. The effects of a discharge information intervention on recovery outcomes following coronary artery bypass surgery. Int J Nurs Stud. 1996;33(2):181-189. PubMed
30. Pereles L, Romonko L, Murzyn T, et al. Evaluation of a self-medication program. J Am Geriatr Soc. 1996;44(2):161-165. PubMed
31. Reynolds MA. Postoperative pain management discharge teaching in a rural population. Pain Manag Nurs. 2009;10(2):76-84. PubMed
32. Sabariego C, Barrera AE, Neubert S, et al. Evaluation of an ICF-based patient education programme for stroke patients: a randomized, single-blinded, controlled, multicentre trial of the effects on self-efficacy, life satisfaction and functioning. Br J Health Psychol. 2013;18(4):707-728. PubMed
33. Shieh SJ, Chen HL, Liu FC, et al. The effectiveness of structured discharge education on maternal confidence, caring knowledge and growth of premature newborns. J Clin Nurs. 2010;19(23-24):3307-3313. PubMed
34. Steinberg TG, Diercks MJ, Millspaugh J. An evaluation of the effectiveness of a videotape for discharge teaching of organ transplant recipients. J Transpl Coord. 1996;6(2):59-63. PubMed
35. Whitby M, McLaws ML, Doidge S, et al. Post-discharge surgical site surveillance: does patient education improve reliability of diagnosis? J Hosp Infect. 2007;66(3):237-242. PubMed
36. Williford SL, Johnson DF. Impact of pharmacist counseling on medication knowledge and compliance. Mil Med. 1995;160(11):561–564. PubMed
37. Zernike W, Henderson A. Evaluating the effectiveness of two teaching strategies for patients diagnosed with hypertension. J Clin Nurs. 1998;7(1):37–44. PubMed
38. Press VG, Arora V, Constantine KL, et al. Forget me not: a randomized trial of the durability of hospital-based education on inhalers for patients with COPD or asthma [abstract]. J Gen Intern Med. 2014;29(1 suppl):S102.
39. Davis TC, Wolf MS, Bass PF, et al. Literacy and misunderstanding prescription drug labels. Ann Intern Med. 2006;145(12):887–894. PubMed
40. McCarthy DM, Waite KR, Curtis LM, et al. What did the doctor say? Health literacy and recall of medical instructions. Med Care. 2012;50(4):277–282. PubMed
41. Tarn DM, Heritage J, Paterniti DA, et al. Physician communication when prescribing new medications. Arch Intern Med. 2006;166(17):1855–1862. PubMed
42. Cawthon C, Walia S, Osborn CY, et al. Improving care transitions: the patient perspective. J Health Commun. 2012;17(suppl 3):312–324. PubMed
43. Karliner LS, Auerbach A, Nápoles A, et al. Language barriers and understanding of hospital discharge instructions. Med Care. 2012;50(4):283–289. PubMed
44. Enhancing the Continuum of Care. Report of the Avoidable Hospitalization Advisory Panel. http://www.health.gov.on.ca/en/common/ministry/publications/reports/baker_2011/baker_2011.pdf. Published November 2011. Accessed December 22, 2016.
45. Chugh A, Williams MV, Grigsby J, et al. Better transitions: improving comprehension of discharge instructions. Front Health Serv Manage. 2009;25(3):11–32. PubMed
46. Schillinger D, Machtinger EL, Wang F, et al. Language, literacy, and communication regarding medication in an anticoagulation clinic: a comparison of verbal vs. visual assessment. J Health Commun. 2006;11(7):651–664. PubMed
47. Epstein RM, Street RL, Jr. The values and value of patient-centered care. Ann Fam Med. 2011;9(2):100–103. PubMed
48. Albrecht JS, Gruber-Baldini AL, Hirshon JM, et al. Hospital discharge instructions: comprehension and compliance among older adults. J Gen Intern Med. 2014;29(11):1491–1498. PubMed
1. Hurtad
2. Mistiaen P, Francke AL, Poot E. Interventions aimed at reducing problems in adult patients discharged from hospital to home: a systematic meta-review. BMC Health Serv Res. 2007;7:47. PubMed
3. Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822-1828. PubMed
4. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. PubMed
5. Hansen LO, Young RS, Hinami K, et al. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. PubMed
6. Osman LM, Calder C, Godden DJ, et al. A randomised trial of self-management planning for adult patients admitted to hospital with acute asthma. Thorax. 2002;57(10):869-874. PubMed
7. Cordasco KM, Asch SM, Bell DS, et al. A low-literacy medication education tool for safety-net hospital patients. Am J Prev Med. 2009;37(6 suppl 1):S209-S216. PubMed
8. Morice AH, Wrench C. The role of the asthma nurse in treatment compliance and self-management following hospital admission. Resp Med. 2001;95(11):851-856. PubMed
9. Haerem JW, Ronning EJ, Leidal R. Home access to hospital discharge information on audiotape reduces sick leave and readmissions in patients with first-time myocardial infarction. Scand Cardiovasc J. 2000;34(2):219-222. PubMed
10. Legrain S, Tubach F, Bonnet-Zamponi D, et al. A new multimodal geriatric discharge-planning intervention to prevent emergency visits and rehospitalizations of older adults: the optimization of medication in AGEd multicenter randomized controlled trial. J Am Geriatr Soc. 2011;59(11):2017-2028. PubMed
11. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269. PubMed
12. Partnership NP. National Priorities and Goals: Aligning Our Efforts to Transform America’s Healthcare. Washington, DC: National Quality Forum; 2008.
13. Effective Practice and Organisation of Care (EPOC). EPOC-specific resources for review authors. Oslo, Norway: Norwegian Knowledge Centre for the Health Services; 2013. http://epoc.cochrane.org/epoc-specific-resources-review-authors. Accessed December 21, 2016.
14. Manning DM, O’Meara JG, Williams AR, et al. 3D: a tool for medication discharge education. Qual Saf Health Care. 2007;16(1):71-76. PubMed
15. Perera KY, Ranasinghe P, Adikari AM, et al. Medium of language in discharge summaries: would the use of native language improve patients’ knowledge of their illness and medications? J Health Commun. 2012;17(2):141-148. PubMed
16. Lowe CJ, Raynor DK, Courtney EA, et al. Effects of self medication programme on knowledge of drugs and compliance with treatment in elderly patients. BMJ. 1995;310(6989):1229-1231. PubMed
17. Mahler HI, Kulik JA, Tarazi RY. Effects of a videotape information intervention at discharge on diet and exercise compliance after coronary bypass surgery. J Cardiopulm Rehabil. 1999;19(3):170-177. PubMed
18. Al-Rashed SA, Wright DJ, Roebuck N, et al. The value of inpatient pharmaceutical counseling to elderly patients prior to discharge. Br J Clin Pharmacol. 2002;54(6):657-664. PubMed
19. Drenth-van Maanen AC, Wilting I, Jansen PA, et al. Effect of a discharge medication intervention on the incidence and nature of medication discrepancies in older adults. J Am Geriatr Soc. 2013;61(3):456-458. PubMed
20. Eshah NF. Predischarge education improves adherence to a healthy lifestyle among Jordanian patients with acute coronary syndrome. Nurs Health Sci. 2013;15(3):273-279. PubMed
21. Gwadry-Sridhar FH, Arnold JM, Zhang Y,et al. Pilot study to determine the impact of a multidisciplinary educational intervention in patients hospitalized with heart failure. Am Heart J. 2005;150(5):982. PubMed
22. Ho SM, Heh SS, Jevitt CM, et al. Effectiveness of a discharge education program in reducing the severity of postpartum depression: a randomized controlled evaluation study. Patient Educ Couns. 2009;77(1):68-71. PubMed
23. Hoffmann T, McKenna K, Worrall L, et al. Randomised trial of a computer-generated tailored written education package for patients following stroke. Age Ageing. 2007;36(3):280-286. PubMed
24. Jenkins HM, Blank V, Miller K, et al. A randomized single-blind evaluation of a discharge teaching book for pediatric patients with burns. J Burn Care Rehabil. 1996;17(1):49-61. PubMed
25. Kommuri NV, Johnson ML, Koelling TM. Relationship between improvements in heart failure patient disease specific knowledge and clinical events as part of a randomized controlled trial. Patient Educ Couns. 2012;86(2):233-238. PubMed
26. Louis-Simonet M, Kossovsky MP, Sarasin FP, et al. Effects of a structured patient-centered discharge interview on patients’ knowledge about their medications. Am J Med. 2004;117(8):563-568. PubMed
27. Lucas KS. Outcomes evaluation of a pharmacist discharge medication teaching service. Am J Health Syst Pharm. 1998;55(24 suppl 4):S32-S35. PubMed
28. Lysack C, Dama M, Neufeld S, et al. A compliance and satisfaction with home exercise: a comparison of computer-assisted video instruction and routine rehabilitation practice. J Allied Health. 2005;34(2):76-82. PubMed
29. Moore SM. The effects of a discharge information intervention on recovery outcomes following coronary artery bypass surgery. Int J Nurs Stud. 1996;33(2):181-189. PubMed
30. Pereles L, Romonko L, Murzyn T, et al. Evaluation of a self-medication program. J Am Geriatr Soc. 1996;44(2):161-165. PubMed
31. Reynolds MA. Postoperative pain management discharge teaching in a rural population. Pain Manag Nurs. 2009;10(2):76-84. PubMed
32. Sabariego C, Barrera AE, Neubert S, et al. Evaluation of an ICF-based patient education programme for stroke patients: a randomized, single-blinded, controlled, multicentre trial of the effects on self-efficacy, life satisfaction and functioning. Br J Health Psychol. 2013;18(4):707-728. PubMed
33. Shieh SJ, Chen HL, Liu FC, et al. The effectiveness of structured discharge education on maternal confidence, caring knowledge and growth of premature newborns. J Clin Nurs. 2010;19(23-24):3307-3313. PubMed
34. Steinberg TG, Diercks MJ, Millspaugh J. An evaluation of the effectiveness of a videotape for discharge teaching of organ transplant recipients. J Transpl Coord. 1996;6(2):59-63. PubMed
35. Whitby M, McLaws ML, Doidge S, et al. Post-discharge surgical site surveillance: does patient education improve reliability of diagnosis? J Hosp Infect. 2007;66(3):237-242. PubMed
36. Williford SL, Johnson DF. Impact of pharmacist counseling on medication knowledge and compliance. Mil Med. 1995;160(11):561–564. PubMed
37. Zernike W, Henderson A. Evaluating the effectiveness of two teaching strategies for patients diagnosed with hypertension. J Clin Nurs. 1998;7(1):37–44. PubMed
38. Press VG, Arora V, Constantine KL, et al. Forget me not: a randomized trial of the durability of hospital-based education on inhalers for patients with COPD or asthma [abstract]. J Gen Intern Med. 2014;29(1 suppl):S102.
39. Davis TC, Wolf MS, Bass PF, et al. Literacy and misunderstanding prescription drug labels. Ann Intern Med. 2006;145(12):887–894. PubMed
40. McCarthy DM, Waite KR, Curtis LM, et al. What did the doctor say? Health literacy and recall of medical instructions. Med Care. 2012;50(4):277–282. PubMed
41. Tarn DM, Heritage J, Paterniti DA, et al. Physician communication when prescribing new medications. Arch Intern Med. 2006;166(17):1855–1862. PubMed
42. Cawthon C, Walia S, Osborn CY, et al. Improving care transitions: the patient perspective. J Health Commun. 2012;17(suppl 3):312–324. PubMed
43. Karliner LS, Auerbach A, Nápoles A, et al. Language barriers and understanding of hospital discharge instructions. Med Care. 2012;50(4):283–289. PubMed
44. Enhancing the Continuum of Care. Report of the Avoidable Hospitalization Advisory Panel. http://www.health.gov.on.ca/en/common/ministry/publications/reports/baker_2011/baker_2011.pdf. Published November 2011. Accessed December 22, 2016.
45. Chugh A, Williams MV, Grigsby J, et al. Better transitions: improving comprehension of discharge instructions. Front Health Serv Manage. 2009;25(3):11–32. PubMed
46. Schillinger D, Machtinger EL, Wang F, et al. Language, literacy, and communication regarding medication in an anticoagulation clinic: a comparison of verbal vs. visual assessment. J Health Commun. 2006;11(7):651–664. PubMed
47. Epstein RM, Street RL, Jr. The values and value of patient-centered care. Ann Fam Med. 2011;9(2):100–103. PubMed
48. Albrecht JS, Gruber-Baldini AL, Hirshon JM, et al. Hospital discharge instructions: comprehension and compliance among older adults. J Gen Intern Med. 2014;29(11):1491–1498. PubMed
Screening for depression in hospitalized medical patients
In our current healthcare system, pressure to provide cost- and time-efficient care is immense. Inpatient care often focuses on assessing the patient’s presenting illness or injury and treating that condition in a manner that gets the patient on their feet and out of the hospital quickly. Because depression is not an indication for hospitalization so long as active suicidality is absent, inpatient physicians may view it as a problem best managed in the outpatient setting. Yet both psychosocial and physical factors associated with depression put patients at risk for rehospitalization.1 Furthermore, hospitalization represents an unrecognized opportunity to optimize both mental and physical health outcomes.2
Indeed, poor physical and mental health often occur together. Depressed inpatients have poorer outcomes, increased length of stay, and greater vulnerability to hospital readmission.3,4 Among elderly hospitalized patients, depression is particularly common, especially in those with poor physical health, alcoholism,5 hip fracture, and stroke.6 Yet little is known about how often depression goes unrecognized, undiagnosed, and, therefore, untreated.
The US Preventive Services Task Force (USPSTF) recommends screening for depression in the general adult population, including pregnant and postpartum women, and further suggests that screening should be implemented “with adequate systems in place to ensure accurate diagnosis, effective treatment, and appropriate follow-up.”2 The USPSTF guidelines do not distinguish between inpatient and outpatient settings. However, the preponderance of evidence for screening comes from outpatient care settings, and little is known about screening among inpatient populations.7
This study had 2 objectives. First, we sought to examine the performance of depression screening tools in inpatient settings. If depression screening were to become routine in hospital settings, screening tools would need to be sensitive and specific as well as brief and suitable for self-administration by patients or for administration by nurses, resident physicians, or hospitalists. It is also important to consider administration by mental health professionals, who may be best trained to administer such tests. We, therefore, examined 3 types of studies: (1) studies that tested a self-administered screening instrument, (2) studies that tested screening by individuals without formal training, and (3) studies that compared screening tools administered by mental health professionals. Second, we sought to describe associations between depression and clinical or utilization outcomes among hospitalized patients.
METHODS
We adhered to recommendations in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement,8,9 including designing the analysis before performing the review. However, we did not post a protocol in an online registry, formally assess study quality, or perform a meta-analysis.
Data Sources and Searches
We searched PsycINFO and PubMed databases for articles published between 1990 and 2016 (as of July 31, 2016). In PubMed, 2 search term strings were used to capture studies of depression screening tools in inpatient settings. The first used the advanced search option to exclude studies related to primary care settings or children and adolescents, and the second used MeSH terms to ensure that a wide variety of studies were included. Specific search terms are included in the Appendix. A similar search was conducted in the PsycINFO database and these search terms are also included in the Appendix.
Study Selection
Articles were eligible if they were published in English in peer-reviewed journals, included at least 20 adults hospitalized for nonpsychiatric reasons, and described the use of at least 1 measure of depression. The studies must have either tested the validity of a depression screening tool or examined the association between depression screening and clinical or utilization outcomes. Two investigators reviewed each title, abstract, and full-text article to determine eligibility, then reached a consensus on which studies to include in this review.
Data Extraction
Two investigators reviewed each full-text article to extract information related to study design, population, and outcomes regarding screening tool analysis or clinical results. From articles that assessed the performance of depression screening tools, we extracted information related to the nature and application of the index test, the nature and application of the reference test, the prevalence of depression, and the sensitivity and specificity of the index test compared with the reference test. For articles that focused on the association between depression screening and clinical or utilization outcomes, the data on relevant clinical outcomes included symptom severity, quality of life, and daily functioning, whereas the data on utilization outcomes included length of stay, readmission, and the cost of care.
RESULTS
Altogether, the search identified 3226 records. After eliminating duplicates and abstracts not suitable for inclusion (Figure), 101 articles underwent full-text review and 32 were found to be eligible. Of these, 12 focused on the association between depression and clinical or utilization outcomes, while 20 assessed the performance of depression screening tools.
Depression Screening Tools
Table 1 describes the index and reference instruments as well as methods of administration, the prevalence of depression, and the sensitivity and specificity of the index instruments relative to the reference instruments. Across the 20 studies, the prevalence of depression ranged from 15% to 60%, with a median of 34%.10–29 This finding may reflect different methods of screening or variation among diverse hospitalized populations. Many of the studies excluded patients with cognitive impairment or communication barriers.
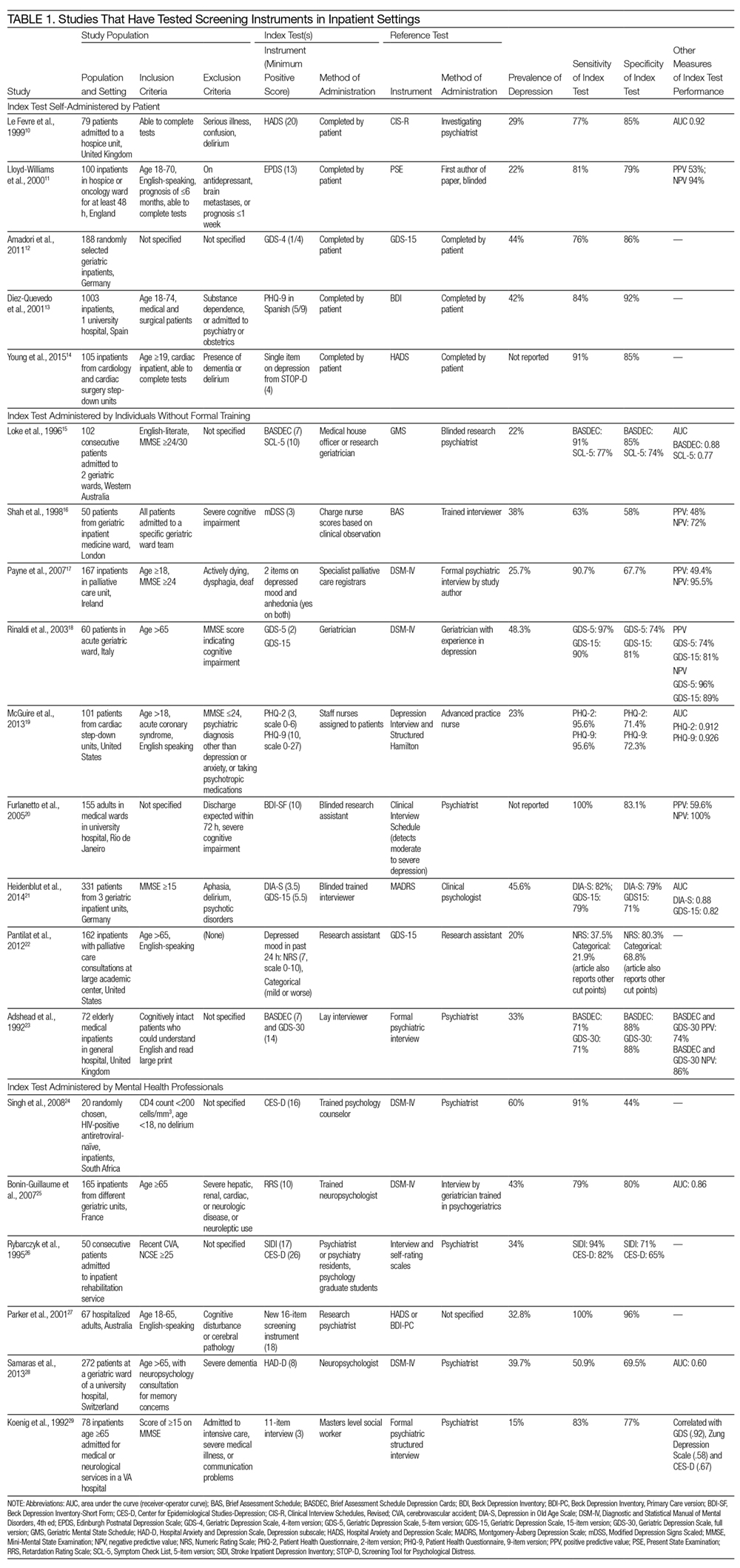
The included studies tested a wide range of unique instruments, and compared them with diverse reference standards. Five studies examined instruments that were self-administered by patients10–14; 9 studies assessed instruments administered by nurses, physicians, or research staff members without formal psychiatric training15–23; and 6 studies evaluated instruments administered by mental health professionals.24–29 Four studies compared different instruments that were administered in the same manner (eg, both self-administered by patients).12–14,22 In the remaining studies, both instruments and methods of administration differed between the index and reference conditions.
Eight studies tested brief instruments with 5 or fewer items, most of which exhibited good sensitivity (range 38%–91%) and specificity (range 68%–86%) relative to longer instruments.12,14–19,22 In 2 of these studies, instruments were self-administered. In 1 case, a single self-administered item from the STOP-D instrument (“Over the past 2 weeks, how much have you been bothered by feeling sad, down, or uninterested in life?”) performed nearly as well as the 14-item Hospital Anxiety and Depression Scale.14 In the other 6 studies testing brief instruments, the instruments were administered by individuals without formal training.15–19,22 In 1 such study, geriatricians asking 2 questions about depressed mood and anhedonia performed well compared with a formal psychiatric interview.17
Four studies tested variations of the Geriatric Depression Scale (GDS).12,18,21,23 In 3 of these studies, abbreviated versions of the GDS exhibited relatively high sensitivity and specificity.12,18,21 However, a study comparing the 15-item GDS (GDS-15) with the GDS-4 found that GDS-15 correctly classified 10% more patients with suspected depression.12 Two studies examined variations of the Patient Health Questionnaire (PHQ). One study found that both the PHQ-2 and PHQ-9 obtained by staff nurses performed well relative to a comprehensive assessment by a trained advanced practice nurse.13,19
When reported, positive predictive value, negative predictive value, and area under the receiver-operator curve were generally high.
Depression and Clinical or Utilization Outcomes
Of the 12 studies that reported either clinical or utilization outcomes for depression screening in an inpatient setting,4,30–40 3 measured rates of rehospitalization.4,31,39 The other 9 studies tested for associations between symptoms of depression and either health or treatment outcomes. Table 2 provides a more detailed description of the study designs and results.
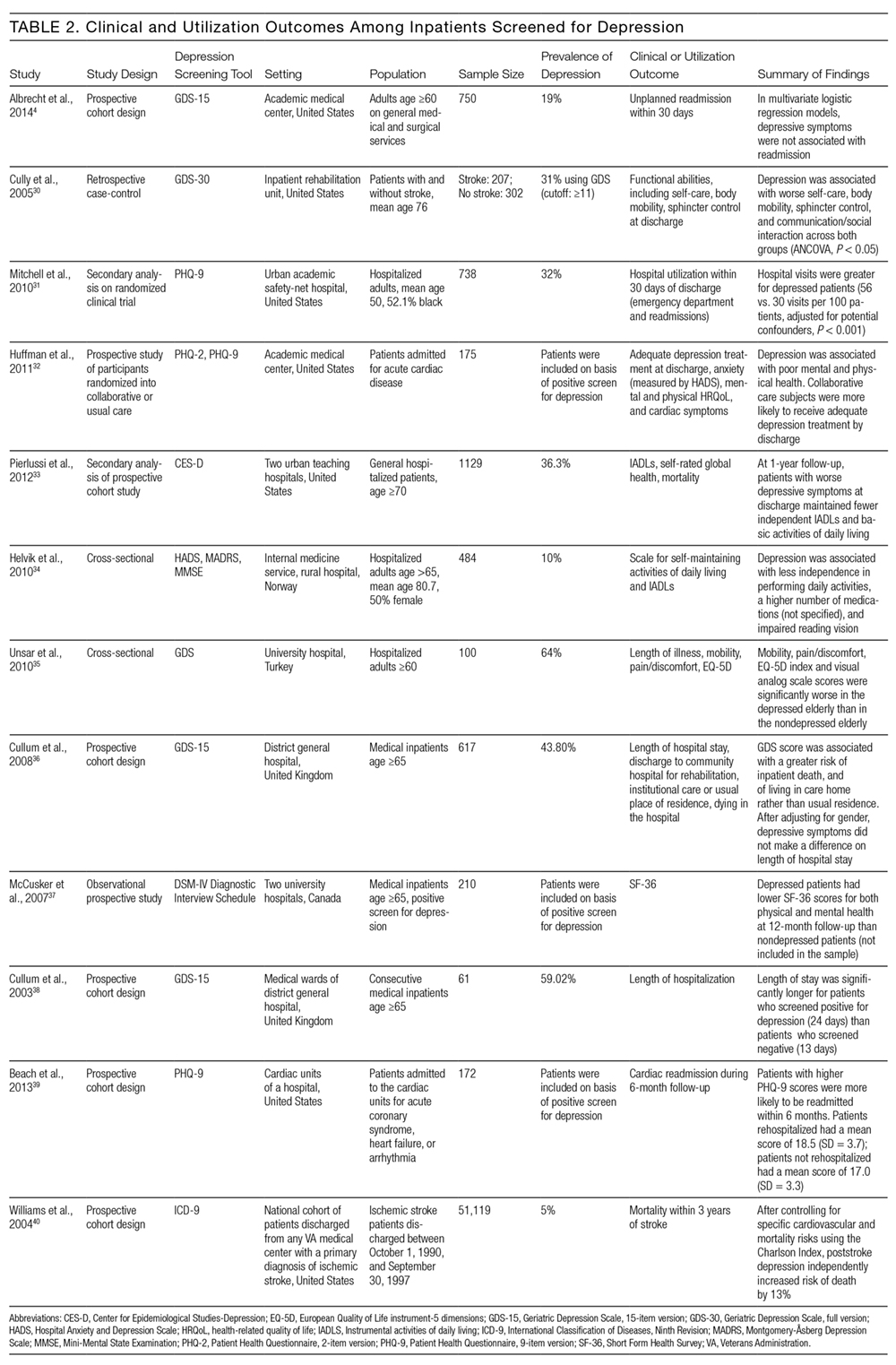
Other studies found that depression was associated with reduced functional abilities such as mobility and self-care,30,32–34 and increased hospital readmission31 as well as physical and mental health deficits.37 Interestingly, although 1 study did not find that depression and hospital readmission were closely linked (frequency at 19%), it found that comorbid illness and previous hospitalizations predicted readmission.4
We also evaluated the associations between depression diagnosed in the inpatient studies and 2 types of outcomes. The first type includes clinical outcomes including symptom severity, quality of life, and daily functioning. Most studies we identified assessed clinical outcomes, and all detected an association between depression and worse clinical outcomes. The second type includes healthcare utilization, which can be measured with the patients’ length of hospital stay, readmission and cost of care. In 1 such study, Mitchell aet al.31 reported a 54% increase in readmission within 30 days of discharge among patients who screened positive for depression.31 Additionally, Cully et al.30 found that depression may impinge on the recovery process of acute rehabilitation patients.
DISCUSSION
The purpose of this study was to describe the feasibility and performance of depression screening tools in inpatient medical settings, as well as associations between depression diagnosed in the inpatient setting and clinical and utilization outcomes. The median rate at which depression was detected among inpatients was 33%, ranging from 5% to 60%. Studies from several individual hospitals indicated that depression can be associated with higher healthcare utilization, including return to the hospital after discharge, as well as worse clinical outcomes. To detect undiagnosed depression among inpatients, screening appears feasible. Depression screening instruments generally exhibited good sensitivity and specificity relative to comprehensive clinical evaluations by mental health professionals. Furthermore, several self-administered and brief instruments had good performance. Prior authors have reported that screening for depression among inpatients may not be particularly burdensome to patients or staff members.41
The studies we reviewed used diverse screening instruments. Further research is needed to determine which tools are preferable in which patient populations, and to confirm that brief instruments are adequate for screening. The GDS is widely used, and many patients hospitalized in the United States fall into the geriatric group. The PHQ has been validated for self-administration and is widely used among outpatients42; it may be more suitable for younger populations. We found that several abbreviated versions of these and other screening instruments have exhibited good sensitivity and specificity among inpatients. However, many of the studies excluded patients with cognitive impairment or communication barriers. For individuals with auditory impairment, the Brief Assessment Schedule Depression Cards (BASDEC) might be an option. Used in 2 studies, the BASDEC involves showing patients a deck of 19 easy-to-read cards. The time required to administer the BASDEC is modest.15,23 Sets of smiley face diagrams might also be suitable for some patients with communication barriers or cognitive impairment. An ineligible study among stroke survivors found that selecting a sad face had a sensitivity of 76% and specificity of 77% relative to a formal diagnostic evaluation for depression.43
In considering the instruments that may be most suitable for inpatients, the role of somatic symptoms is also important because these can overlap between depression and the medical conditions that lead to hospitalization.44–46 Prior investigators found, for example, that 47% of Beck Depression Inventory (BDI) scores were attributable to somatic symptoms among patients hospitalized after myocardial infarction, whereas 37% of BDI scores were attributable to somatic symptoms among depressed outpatients.47 Future research is needed to determine the significance of somatic symptoms among inpatients, including whether they should be considered during screening, add prognostic value, or warrant specific treatment. In addition, although positive and negative predictive values were generally high among the screening instruments we evaluated, confirming the diagnosis of depression with a thorough clinical assessment is likely to be necessary.44,45
Despite the high prevalence of depression, associations with suboptimal outcomes, and the good performance of screening tools to date, screening for depression in the inpatient setting has received little attention. Prior authors have questioned whether hospital-based screening is an efficient and effective way to detect depression, and have raised valid concerns regarding false-positive diagnoses and unnecessary treatment, as well as a lack of randomized controlled trials.7,48,49 Whereas some studies suggest that depression is associated with greater healthcare utilization,3,4 little information exists regarding whether screening during hospitalization and treating previously undiagnosed depression improves clinical outcomes or reduces healthcare utilization.
Several important questions remain. What is the pathophysiology of depressed mood during hospitalization? How often does depressed mood during hospitalization reflect longstanding undiagnosed depression, longstanding undertreated depression, an acute stress disorder, or a normal if unpleasant short-term reaction to the stress of acute illnesses? Do the manifestations and effects of depressed mood differ among these situations? What is the prognosis of depressed mood occurring during hospitalization, and how many patients continue to have depression after recovery from acute illness; what factors affect prognosis? In a small sample of hospitalized patients, nearly 50% of those who had been depressed at intake remained depressed 1 month after discharge.50 Given that most antidepressant medications have to be taken for several weeks before effects can be detected, what, if any, approach to treatment should be taken? More research is needed on the effectiveness and cost-effectiveness of diagnosing and treating depression in the inpatient setting.
This work has several limitations. We found relatively few studies meeting eligibility criteria, particularly studies assessing clinical and utilization outcomes among depressed inpatients. Among the screening tools that were studied in the hospital setting, the highly diverse instruments and modes of administration precluded a quantitative synthesis such as meta-analysis. Prior meta-analyses on specific screening tools have focused on outpatient populations.51–53 Furthermore, we did not evaluate study quality or risk of bias.
In conclusion, screening for depression in the inpatient setting via patient self-assessment or assessment by hospital staff appears feasible. Several brief screening tools are available that have good sensitivity and specificity relative to diagnoses made by mental health professionals. Limited evidence suggests that screening tools for depression may be ready to integrate into inpatient care.41 Yet, although depression appears to be common and associated with worse clinical outcomes and higher healthcare utilization, more research is needed on the benefits, risks, and potential costs of adding depression screening in the inpatient healthcare setting.
Disclosures
The authors report no conflicts of interest.
1. Kahn KL, Keeler EB, Sherwood MJ, et al. Comparing outcomes of care before and after implementation of the DRG-based prospective payment system. JAMA. 1990;264(15):1984-1988. PubMed
2. U.S. Preventive Services Task Force (USPSTF). Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(4):380-387. PubMed
3. Dennis M, Kadri A, Coffey J. Depression in older people in the general hospital: a systematic review of screening instruments. Age Ageing. 2012;41(2):148-154. PubMed
4. Albrecht JS, Gruber-Baldini AL, Hirshon JM, et al. Depressive symptoms and hospital readmission in older adults. J Am Geriatr Soc. 2014;62(3):495-499. PubMed
5. Grant BF, Hasin DS, Harford TC. Screening for major depression among alcoholics: an application of receiver operating characteristic analysis. Drug Alcohol Depend. 1989;23(2):123-131. PubMed
6. Lieberman D, Galinsky D, Fried V, et al. Geriatric Depression Screening Scale (GDS) in patients hospitalized for physical rehabilitation. Int J Geriatr Psychiatry. 1999;14(7):549-555. PubMed
7. Canadian Task Force on Preventive Health Care. Recommendations on screening for depression in adults. CMAJ. 2013;185(9):775-782.
8. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. PubMed
9. Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013-1020. PubMed
10. Le Fevre P, Devereux J, Smith S, Lawrie SM, Cornbleet M. Screening for psychiatric illness in the palliative care inpatient setting: a comparison between the Hospital Anxiety and Depression Scale and the General Health Questionnaire-12. Palliat Med. 1999;13(5):399-407. PubMed
11. Lloyd-Williams M, Friedman T, Rudd N. Criterion validation of the Edinburgh Postnatal Depression Scale as a screening tool for depression in patients with advanced metastatic cancer. J Pain Symptom Manag. 2000;20(4):259-265. PubMed
12. Amadori K, Herrmann E, Püllen RK. Comparison of the 15-item Geriatric Depression Scale (GDS-15) and the GDS-4 during screening for depression in an in-patient geriatric patient group. J Am Geriatr Soc. 2011;59(1):171-172. PubMed
13. Diez-Quevedo C, Rangil T, Sanchez-Planell L, Kroenke K, Spitzer RL. Validation and utility of the Patient Health Questionnaire in diagnosing mental disorders in 1003 general hospital Spanish inpatients. Psychosom Med. 2001;63(4):679-686. PubMed
14. Young Q-R, Nguyen M, Roth S, Broadberry A, Mackay MH. Single-item measures for depression and anxiety: validation of the screening tool for psychological distress in an inpatient cardiology setting. Eur J Cardiovasc Nursing. 2015;14(6):544-551. PubMed
15. Loke B, Nicklason F, Burvill P. Screening for depression: clinical validation of geriatricians’ diagnosis, the Brief Assessment Schedule Depression Cards and the 5-item version of the Symptom Check List among non-demented geriatric inpatients. Int J Geriatr Psychiatry. 1996;11(5):461-465.
16. Shah A, Karasu M, De T. Nursing staff and screening for depression among acutely ill geriatric inpatients: a pilot study. Aging Ment Health. 1998;2(1):71-74.
17. Payne A, Barry S, Creedon B, et al. Sensitivity and specificity of a two-question screening tool for depression in a specialist palliative care unit. Palliat Med. 2007;21(3):193-198. PubMed
18. Rinaldi P, Mecocci P, Benedetti C, et al. Validation of the five-item geriatric depression scale in elderly subjects in three different settings. J Am Geriatr Soc. 2003;51(5):694-698. PubMed
19. McGuire AW, Eastwood J, Macabasco-O’Connell A, Hays RD, Doering LV. Depression screening: utility of the Patient Health Questionnaire in patients with acute coronary syndrome. Am J Crit Care. 2013;22(1):12-19. PubMed
20. Furlanetto LM, Mendlowicz MV, Bueno JR. The validity of the Beck Depression Inventory-Short Form as a screening and diagnostic instrument for moderate and severe depression in medical inpatients. J Affect Disord. 2005;86(1):87-91. PubMed
21. Heidenblut S, Zank S. Screening for depression with the Depression in Old Age Scale (DIA-S) and the Geriatric Depression Scale (GDS15): diagnostic accuracy in a geriatric inpatient setting. GeroPsych (Bern). 2014;27(1):41. PubMed
22. Pantilat SZ, O’Riordan DL, Dibble SL, Landefeld CS. An assessment of the screening performance of a single-item measure of depression from the Edmonton Symptom Assessment Scale among chronically ill hospitalized patients. J Pain Symptom Manage. 2012;43(5):866-873. PubMed
23. Adshead F, Cody DD, Pitt B. BASDEC: a novel screening instrument for depression in elderly medical inpatients. BMJ. 1992;305(6850):397. PubMed
24. Singh D, Sunpath H, John S, Eastham L, Gouden R. The utility of a rapid screening tool for depression and HIV dementia amongst patients with low CD4 counts – a preliminary report. Afr J Psychiatry (Johannesbg). 2008;11(4):282-286. PubMed
25. Bonin-Guillaume S, Sautel L, Demattei C, Jouve E, Blin O. Validation of the Retardation Rating Scale for detecting in geriatric inpatients. Int J Geriatr Psychiatry. 2007;22(1):68-76. PubMed
26. Rybarczyk B, Winemiller DR, Lazarus LW, Haut A, Hartman C. Validation of a depression screening measure for stroke inpatients. Am J Geriatr Psychiatry. 1996;4(2):131-139.
27. Parker G, Hilton T, Hadzi-Pavlovic D, Bains J. Screening for depression in the medically ill: the suggested utility of a cognitive-based approach. Aust N Z J Psychiatry. 2001;35(4):474-480. PubMed
28. Samaras N, Herrmann FR, Samaras D, et al. The Hospital Anxiety and Depression Scale: low sensitivity for depression screening in demented and non-demented hospitalized elderly. Int Psychogeriatr. 2013;25(1):82-87. PubMed
29. Koenig HG, Cohen HJ, Blazer DG, Meador KG, Westlund R. A brief depression scale for use in the medically ill. Int J Psychiatry Med. 1992;22(2):183-195. PubMed
30. Cully JA, Gfeller JD, Heise RA, Ross MJ, Teal CR, Kunik ME. Geriatric depression, medical diagnosis, and functional recovery during acute rehabilitation. Arch Phys Med Rehabil. 2005;86(12):2256-2260. PubMed
31. Mitchell SE, Paasche-Orlow MK, Forsythe SR, et al. Post-discharge hospital utilization among adult medical inpatients with depressive symptoms. J Hosp Med. 2010;5(7):378-384. PubMed
32. Huffman JC, Mastromauro CA, Sowden GL, Wittmann C, Rodman R, Januzzi JL. A collaborative care depression management program for cardiac inpatients: depression characteristics and in-hospital outcomes. Psychosomatics. 2011;52(1):26-3. 2007;22(11):1596-1602.J Gen Intern Med PubMed
53. Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. 2010;126(3):335-348.J Affect Disord. PubMed
52. Mitchell AJ, Meader N, Symonds P. Diagnostic validity of the Hospital Anxiety and Depression Scale (HADS) in cancer and palliative settings: a meta-analysis. 2010;69(4):371-378.J Psychosom Res. PubMed
51. Brennan C, Worrall-Davis A, McMillan D, Gilbody S, House A. The Hospital Anxiety and Depression Scale: a diagnostic meta-analysis of case-finding ability. . 1992;22(3):281-289.Int J Psychiatry Med PubMed
50. Pomerantz AS, de-Nesnera A, West AN. Resolution of depressive symptoms in medical inpatients after discharge. 2014;12(1):13.BMC Med PubMed
49. Thombs BD, Ziegelstein RC, Roseman M, Kloda LA, Ioannidis JPA. There are no randomized controlled trials that support the United States Preventive Services Task Force guideline on screening for depression in primary care: a systematic review. 2013;1(4):E159-E167.CMAJ Open PubMed
48. Keshavarz H, Fitzpatrick-Lewis D, Streiner DL, et al. Screening for depression: a systematic review and meta-analysis. 2012;73(3):157-162.J Psychosom Res. PubMed
47. Delisle VC, Beck AT, Ziegelstein RC, Thombs BD. Symptoms of heart disease or its treatment may increase Beck Depression Inventory Scores in hospitalized post-myocardial infarction patients. 2014;23(9):1079.Psychooncology PubMed
46. Palmer SC. Study provides little insight into routine screening for depression.
2005;20(3):289.Int J Geriatr Psychiatry. PubMed
45. Baldwin RC. Validation of short screening tests for depression, response to Seymour [letter to the editor]. 2005;20(3):289.Int J Geriatr Psychiatry.
44. Seymour J. Validation of short screening tests for depression: comment on Goring et al. (2004) [letter to the editor]. 2008;45(7):1081-1089.Int J Nurs Stud. PubMed
43. Lee ACK, Tang SW, Yu GKK, Cheung RTF. The smiley as a simple screening tool for depression after stroke: a preliminary study. 1999;282(18):1737-1744.JAMA. PubMed
42. Spitzer RL, Kroenke K, Williams JW. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. . 2011;14(3):275-279.J Palliat Med PubMed
41. Rao S, Ferris FD, Irwin SA. Ease of screening for depression and delirium in patients enrolled in inpatient hospice care. . 2004;161(6):1090-1095.Am J Psychiatry PubMed
40. Williams LS, Ghose SS, Swindle RW. Depression and other mental health diagnoses increase mortality risk after ischemic stroke. 2013;75(5):409-413.J Psychosom Res. PubMed
39. Beach SR, Januzzi JL, Mastromauro CA, et al. Patient Health Questionnaire-9 score and adverse cardiac outcomes in patients hospitalized for acute cardiac disease. 2003;18(4):358-359.Int J Geriatr Psychiatry PubMed
38. Cullum S, Nandhra H, Darley J, Todd C. Screening for depression in older people on medical wards: which cut-point should we use? 2007;29(4):340-348.Gen Hosp Psychiatry. PubMed
37. McCusker J, Cole M, Ciampi A, Latimer E, Windholz S, Belzile E. Major depression in older medical inpatients predicts poor physical and mental health status over 12 months. 2008;37(6):690-695.Age Ageing PubMed
36. Cullum S, Metcalfe C, Todd C, Brayne C. Does depression predict adverse outcomes for older medical inpatients? A prospective cohort study of individuals screened for a trial.
. 2010;50(1):6-10.Arch Gerontol Geriatr PubMed
35. Unsar S, Sut N. Depression and health status in elderly hospitalized patients with chronic illness. 150-159.:2010;25(2)Int J Geriatr Psychiatry. PubMed
34. Helvik A-S, Skancke RH, Selbæk G. Screening for depression in elderly medical inpatients from rural area of Norway: prevalence and associated factors. . 2012;60(12):2254-2262.J Am Geriatr Soc PubMed
33. Pierlussi E, Mehta KM, Kirby KA, et al. Depressive symptoms after hospitalization in older adults: function and mortality outcomes. PubMed
In our current healthcare system, pressure to provide cost- and time-efficient care is immense. Inpatient care often focuses on assessing the patient’s presenting illness or injury and treating that condition in a manner that gets the patient on their feet and out of the hospital quickly. Because depression is not an indication for hospitalization so long as active suicidality is absent, inpatient physicians may view it as a problem best managed in the outpatient setting. Yet both psychosocial and physical factors associated with depression put patients at risk for rehospitalization.1 Furthermore, hospitalization represents an unrecognized opportunity to optimize both mental and physical health outcomes.2
Indeed, poor physical and mental health often occur together. Depressed inpatients have poorer outcomes, increased length of stay, and greater vulnerability to hospital readmission.3,4 Among elderly hospitalized patients, depression is particularly common, especially in those with poor physical health, alcoholism,5 hip fracture, and stroke.6 Yet little is known about how often depression goes unrecognized, undiagnosed, and, therefore, untreated.
The US Preventive Services Task Force (USPSTF) recommends screening for depression in the general adult population, including pregnant and postpartum women, and further suggests that screening should be implemented “with adequate systems in place to ensure accurate diagnosis, effective treatment, and appropriate follow-up.”2 The USPSTF guidelines do not distinguish between inpatient and outpatient settings. However, the preponderance of evidence for screening comes from outpatient care settings, and little is known about screening among inpatient populations.7
This study had 2 objectives. First, we sought to examine the performance of depression screening tools in inpatient settings. If depression screening were to become routine in hospital settings, screening tools would need to be sensitive and specific as well as brief and suitable for self-administration by patients or for administration by nurses, resident physicians, or hospitalists. It is also important to consider administration by mental health professionals, who may be best trained to administer such tests. We, therefore, examined 3 types of studies: (1) studies that tested a self-administered screening instrument, (2) studies that tested screening by individuals without formal training, and (3) studies that compared screening tools administered by mental health professionals. Second, we sought to describe associations between depression and clinical or utilization outcomes among hospitalized patients.
METHODS
We adhered to recommendations in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement,8,9 including designing the analysis before performing the review. However, we did not post a protocol in an online registry, formally assess study quality, or perform a meta-analysis.
Data Sources and Searches
We searched PsycINFO and PubMed databases for articles published between 1990 and 2016 (as of July 31, 2016). In PubMed, 2 search term strings were used to capture studies of depression screening tools in inpatient settings. The first used the advanced search option to exclude studies related to primary care settings or children and adolescents, and the second used MeSH terms to ensure that a wide variety of studies were included. Specific search terms are included in the Appendix. A similar search was conducted in the PsycINFO database and these search terms are also included in the Appendix.
Study Selection
Articles were eligible if they were published in English in peer-reviewed journals, included at least 20 adults hospitalized for nonpsychiatric reasons, and described the use of at least 1 measure of depression. The studies must have either tested the validity of a depression screening tool or examined the association between depression screening and clinical or utilization outcomes. Two investigators reviewed each title, abstract, and full-text article to determine eligibility, then reached a consensus on which studies to include in this review.
Data Extraction
Two investigators reviewed each full-text article to extract information related to study design, population, and outcomes regarding screening tool analysis or clinical results. From articles that assessed the performance of depression screening tools, we extracted information related to the nature and application of the index test, the nature and application of the reference test, the prevalence of depression, and the sensitivity and specificity of the index test compared with the reference test. For articles that focused on the association between depression screening and clinical or utilization outcomes, the data on relevant clinical outcomes included symptom severity, quality of life, and daily functioning, whereas the data on utilization outcomes included length of stay, readmission, and the cost of care.
RESULTS
Altogether, the search identified 3226 records. After eliminating duplicates and abstracts not suitable for inclusion (Figure), 101 articles underwent full-text review and 32 were found to be eligible. Of these, 12 focused on the association between depression and clinical or utilization outcomes, while 20 assessed the performance of depression screening tools.
Depression Screening Tools
Table 1 describes the index and reference instruments as well as methods of administration, the prevalence of depression, and the sensitivity and specificity of the index instruments relative to the reference instruments. Across the 20 studies, the prevalence of depression ranged from 15% to 60%, with a median of 34%.10–29 This finding may reflect different methods of screening or variation among diverse hospitalized populations. Many of the studies excluded patients with cognitive impairment or communication barriers.

The included studies tested a wide range of unique instruments, and compared them with diverse reference standards. Five studies examined instruments that were self-administered by patients10–14; 9 studies assessed instruments administered by nurses, physicians, or research staff members without formal psychiatric training15–23; and 6 studies evaluated instruments administered by mental health professionals.24–29 Four studies compared different instruments that were administered in the same manner (eg, both self-administered by patients).12–14,22 In the remaining studies, both instruments and methods of administration differed between the index and reference conditions.
Eight studies tested brief instruments with 5 or fewer items, most of which exhibited good sensitivity (range 38%–91%) and specificity (range 68%–86%) relative to longer instruments.12,14–19,22 In 2 of these studies, instruments were self-administered. In 1 case, a single self-administered item from the STOP-D instrument (“Over the past 2 weeks, how much have you been bothered by feeling sad, down, or uninterested in life?”) performed nearly as well as the 14-item Hospital Anxiety and Depression Scale.14 In the other 6 studies testing brief instruments, the instruments were administered by individuals without formal training.15–19,22 In 1 such study, geriatricians asking 2 questions about depressed mood and anhedonia performed well compared with a formal psychiatric interview.17
Four studies tested variations of the Geriatric Depression Scale (GDS).12,18,21,23 In 3 of these studies, abbreviated versions of the GDS exhibited relatively high sensitivity and specificity.12,18,21 However, a study comparing the 15-item GDS (GDS-15) with the GDS-4 found that GDS-15 correctly classified 10% more patients with suspected depression.12 Two studies examined variations of the Patient Health Questionnaire (PHQ). One study found that both the PHQ-2 and PHQ-9 obtained by staff nurses performed well relative to a comprehensive assessment by a trained advanced practice nurse.13,19
When reported, positive predictive value, negative predictive value, and area under the receiver-operator curve were generally high.
Depression and Clinical or Utilization Outcomes
Of the 12 studies that reported either clinical or utilization outcomes for depression screening in an inpatient setting,4,30–40 3 measured rates of rehospitalization.4,31,39 The other 9 studies tested for associations between symptoms of depression and either health or treatment outcomes. Table 2 provides a more detailed description of the study designs and results.

Other studies found that depression was associated with reduced functional abilities such as mobility and self-care,30,32–34 and increased hospital readmission31 as well as physical and mental health deficits.37 Interestingly, although 1 study did not find that depression and hospital readmission were closely linked (frequency at 19%), it found that comorbid illness and previous hospitalizations predicted readmission.4
We also evaluated the associations between depression diagnosed in the inpatient studies and 2 types of outcomes. The first type includes clinical outcomes including symptom severity, quality of life, and daily functioning. Most studies we identified assessed clinical outcomes, and all detected an association between depression and worse clinical outcomes. The second type includes healthcare utilization, which can be measured with the patients’ length of hospital stay, readmission and cost of care. In 1 such study, Mitchell aet al.31 reported a 54% increase in readmission within 30 days of discharge among patients who screened positive for depression.31 Additionally, Cully et al.30 found that depression may impinge on the recovery process of acute rehabilitation patients.
DISCUSSION
The purpose of this study was to describe the feasibility and performance of depression screening tools in inpatient medical settings, as well as associations between depression diagnosed in the inpatient setting and clinical and utilization outcomes. The median rate at which depression was detected among inpatients was 33%, ranging from 5% to 60%. Studies from several individual hospitals indicated that depression can be associated with higher healthcare utilization, including return to the hospital after discharge, as well as worse clinical outcomes. To detect undiagnosed depression among inpatients, screening appears feasible. Depression screening instruments generally exhibited good sensitivity and specificity relative to comprehensive clinical evaluations by mental health professionals. Furthermore, several self-administered and brief instruments had good performance. Prior authors have reported that screening for depression among inpatients may not be particularly burdensome to patients or staff members.41
The studies we reviewed used diverse screening instruments. Further research is needed to determine which tools are preferable in which patient populations, and to confirm that brief instruments are adequate for screening. The GDS is widely used, and many patients hospitalized in the United States fall into the geriatric group. The PHQ has been validated for self-administration and is widely used among outpatients42; it may be more suitable for younger populations. We found that several abbreviated versions of these and other screening instruments have exhibited good sensitivity and specificity among inpatients. However, many of the studies excluded patients with cognitive impairment or communication barriers. For individuals with auditory impairment, the Brief Assessment Schedule Depression Cards (BASDEC) might be an option. Used in 2 studies, the BASDEC involves showing patients a deck of 19 easy-to-read cards. The time required to administer the BASDEC is modest.15,23 Sets of smiley face diagrams might also be suitable for some patients with communication barriers or cognitive impairment. An ineligible study among stroke survivors found that selecting a sad face had a sensitivity of 76% and specificity of 77% relative to a formal diagnostic evaluation for depression.43
In considering the instruments that may be most suitable for inpatients, the role of somatic symptoms is also important because these can overlap between depression and the medical conditions that lead to hospitalization.44–46 Prior investigators found, for example, that 47% of Beck Depression Inventory (BDI) scores were attributable to somatic symptoms among patients hospitalized after myocardial infarction, whereas 37% of BDI scores were attributable to somatic symptoms among depressed outpatients.47 Future research is needed to determine the significance of somatic symptoms among inpatients, including whether they should be considered during screening, add prognostic value, or warrant specific treatment. In addition, although positive and negative predictive values were generally high among the screening instruments we evaluated, confirming the diagnosis of depression with a thorough clinical assessment is likely to be necessary.44,45
Despite the high prevalence of depression, associations with suboptimal outcomes, and the good performance of screening tools to date, screening for depression in the inpatient setting has received little attention. Prior authors have questioned whether hospital-based screening is an efficient and effective way to detect depression, and have raised valid concerns regarding false-positive diagnoses and unnecessary treatment, as well as a lack of randomized controlled trials.7,48,49 Whereas some studies suggest that depression is associated with greater healthcare utilization,3,4 little information exists regarding whether screening during hospitalization and treating previously undiagnosed depression improves clinical outcomes or reduces healthcare utilization.
Several important questions remain. What is the pathophysiology of depressed mood during hospitalization? How often does depressed mood during hospitalization reflect longstanding undiagnosed depression, longstanding undertreated depression, an acute stress disorder, or a normal if unpleasant short-term reaction to the stress of acute illnesses? Do the manifestations and effects of depressed mood differ among these situations? What is the prognosis of depressed mood occurring during hospitalization, and how many patients continue to have depression after recovery from acute illness; what factors affect prognosis? In a small sample of hospitalized patients, nearly 50% of those who had been depressed at intake remained depressed 1 month after discharge.50 Given that most antidepressant medications have to be taken for several weeks before effects can be detected, what, if any, approach to treatment should be taken? More research is needed on the effectiveness and cost-effectiveness of diagnosing and treating depression in the inpatient setting.
This work has several limitations. We found relatively few studies meeting eligibility criteria, particularly studies assessing clinical and utilization outcomes among depressed inpatients. Among the screening tools that were studied in the hospital setting, the highly diverse instruments and modes of administration precluded a quantitative synthesis such as meta-analysis. Prior meta-analyses on specific screening tools have focused on outpatient populations.51–53 Furthermore, we did not evaluate study quality or risk of bias.
In conclusion, screening for depression in the inpatient setting via patient self-assessment or assessment by hospital staff appears feasible. Several brief screening tools are available that have good sensitivity and specificity relative to diagnoses made by mental health professionals. Limited evidence suggests that screening tools for depression may be ready to integrate into inpatient care.41 Yet, although depression appears to be common and associated with worse clinical outcomes and higher healthcare utilization, more research is needed on the benefits, risks, and potential costs of adding depression screening in the inpatient healthcare setting.
Disclosures
The authors report no conflicts of interest.
In our current healthcare system, pressure to provide cost- and time-efficient care is immense. Inpatient care often focuses on assessing the patient’s presenting illness or injury and treating that condition in a manner that gets the patient on their feet and out of the hospital quickly. Because depression is not an indication for hospitalization so long as active suicidality is absent, inpatient physicians may view it as a problem best managed in the outpatient setting. Yet both psychosocial and physical factors associated with depression put patients at risk for rehospitalization.1 Furthermore, hospitalization represents an unrecognized opportunity to optimize both mental and physical health outcomes.2
Indeed, poor physical and mental health often occur together. Depressed inpatients have poorer outcomes, increased length of stay, and greater vulnerability to hospital readmission.3,4 Among elderly hospitalized patients, depression is particularly common, especially in those with poor physical health, alcoholism,5 hip fracture, and stroke.6 Yet little is known about how often depression goes unrecognized, undiagnosed, and, therefore, untreated.
The US Preventive Services Task Force (USPSTF) recommends screening for depression in the general adult population, including pregnant and postpartum women, and further suggests that screening should be implemented “with adequate systems in place to ensure accurate diagnosis, effective treatment, and appropriate follow-up.”2 The USPSTF guidelines do not distinguish between inpatient and outpatient settings. However, the preponderance of evidence for screening comes from outpatient care settings, and little is known about screening among inpatient populations.7
This study had 2 objectives. First, we sought to examine the performance of depression screening tools in inpatient settings. If depression screening were to become routine in hospital settings, screening tools would need to be sensitive and specific as well as brief and suitable for self-administration by patients or for administration by nurses, resident physicians, or hospitalists. It is also important to consider administration by mental health professionals, who may be best trained to administer such tests. We, therefore, examined 3 types of studies: (1) studies that tested a self-administered screening instrument, (2) studies that tested screening by individuals without formal training, and (3) studies that compared screening tools administered by mental health professionals. Second, we sought to describe associations between depression and clinical or utilization outcomes among hospitalized patients.
METHODS
We adhered to recommendations in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement,8,9 including designing the analysis before performing the review. However, we did not post a protocol in an online registry, formally assess study quality, or perform a meta-analysis.
Data Sources and Searches
We searched PsycINFO and PubMed databases for articles published between 1990 and 2016 (as of July 31, 2016). In PubMed, 2 search term strings were used to capture studies of depression screening tools in inpatient settings. The first used the advanced search option to exclude studies related to primary care settings or children and adolescents, and the second used MeSH terms to ensure that a wide variety of studies were included. Specific search terms are included in the Appendix. A similar search was conducted in the PsycINFO database and these search terms are also included in the Appendix.
Study Selection
Articles were eligible if they were published in English in peer-reviewed journals, included at least 20 adults hospitalized for nonpsychiatric reasons, and described the use of at least 1 measure of depression. The studies must have either tested the validity of a depression screening tool or examined the association between depression screening and clinical or utilization outcomes. Two investigators reviewed each title, abstract, and full-text article to determine eligibility, then reached a consensus on which studies to include in this review.
Data Extraction
Two investigators reviewed each full-text article to extract information related to study design, population, and outcomes regarding screening tool analysis or clinical results. From articles that assessed the performance of depression screening tools, we extracted information related to the nature and application of the index test, the nature and application of the reference test, the prevalence of depression, and the sensitivity and specificity of the index test compared with the reference test. For articles that focused on the association between depression screening and clinical or utilization outcomes, the data on relevant clinical outcomes included symptom severity, quality of life, and daily functioning, whereas the data on utilization outcomes included length of stay, readmission, and the cost of care.
RESULTS
Altogether, the search identified 3226 records. After eliminating duplicates and abstracts not suitable for inclusion (Figure), 101 articles underwent full-text review and 32 were found to be eligible. Of these, 12 focused on the association between depression and clinical or utilization outcomes, while 20 assessed the performance of depression screening tools.
Depression Screening Tools
Table 1 describes the index and reference instruments as well as methods of administration, the prevalence of depression, and the sensitivity and specificity of the index instruments relative to the reference instruments. Across the 20 studies, the prevalence of depression ranged from 15% to 60%, with a median of 34%.10–29 This finding may reflect different methods of screening or variation among diverse hospitalized populations. Many of the studies excluded patients with cognitive impairment or communication barriers.

The included studies tested a wide range of unique instruments, and compared them with diverse reference standards. Five studies examined instruments that were self-administered by patients10–14; 9 studies assessed instruments administered by nurses, physicians, or research staff members without formal psychiatric training15–23; and 6 studies evaluated instruments administered by mental health professionals.24–29 Four studies compared different instruments that were administered in the same manner (eg, both self-administered by patients).12–14,22 In the remaining studies, both instruments and methods of administration differed between the index and reference conditions.
Eight studies tested brief instruments with 5 or fewer items, most of which exhibited good sensitivity (range 38%–91%) and specificity (range 68%–86%) relative to longer instruments.12,14–19,22 In 2 of these studies, instruments were self-administered. In 1 case, a single self-administered item from the STOP-D instrument (“Over the past 2 weeks, how much have you been bothered by feeling sad, down, or uninterested in life?”) performed nearly as well as the 14-item Hospital Anxiety and Depression Scale.14 In the other 6 studies testing brief instruments, the instruments were administered by individuals without formal training.15–19,22 In 1 such study, geriatricians asking 2 questions about depressed mood and anhedonia performed well compared with a formal psychiatric interview.17
Four studies tested variations of the Geriatric Depression Scale (GDS).12,18,21,23 In 3 of these studies, abbreviated versions of the GDS exhibited relatively high sensitivity and specificity.12,18,21 However, a study comparing the 15-item GDS (GDS-15) with the GDS-4 found that GDS-15 correctly classified 10% more patients with suspected depression.12 Two studies examined variations of the Patient Health Questionnaire (PHQ). One study found that both the PHQ-2 and PHQ-9 obtained by staff nurses performed well relative to a comprehensive assessment by a trained advanced practice nurse.13,19
When reported, positive predictive value, negative predictive value, and area under the receiver-operator curve were generally high.
Depression and Clinical or Utilization Outcomes
Of the 12 studies that reported either clinical or utilization outcomes for depression screening in an inpatient setting,4,30–40 3 measured rates of rehospitalization.4,31,39 The other 9 studies tested for associations between symptoms of depression and either health or treatment outcomes. Table 2 provides a more detailed description of the study designs and results.

Other studies found that depression was associated with reduced functional abilities such as mobility and self-care,30,32–34 and increased hospital readmission31 as well as physical and mental health deficits.37 Interestingly, although 1 study did not find that depression and hospital readmission were closely linked (frequency at 19%), it found that comorbid illness and previous hospitalizations predicted readmission.4
We also evaluated the associations between depression diagnosed in the inpatient studies and 2 types of outcomes. The first type includes clinical outcomes including symptom severity, quality of life, and daily functioning. Most studies we identified assessed clinical outcomes, and all detected an association between depression and worse clinical outcomes. The second type includes healthcare utilization, which can be measured with the patients’ length of hospital stay, readmission and cost of care. In 1 such study, Mitchell aet al.31 reported a 54% increase in readmission within 30 days of discharge among patients who screened positive for depression.31 Additionally, Cully et al.30 found that depression may impinge on the recovery process of acute rehabilitation patients.
DISCUSSION
The purpose of this study was to describe the feasibility and performance of depression screening tools in inpatient medical settings, as well as associations between depression diagnosed in the inpatient setting and clinical and utilization outcomes. The median rate at which depression was detected among inpatients was 33%, ranging from 5% to 60%. Studies from several individual hospitals indicated that depression can be associated with higher healthcare utilization, including return to the hospital after discharge, as well as worse clinical outcomes. To detect undiagnosed depression among inpatients, screening appears feasible. Depression screening instruments generally exhibited good sensitivity and specificity relative to comprehensive clinical evaluations by mental health professionals. Furthermore, several self-administered and brief instruments had good performance. Prior authors have reported that screening for depression among inpatients may not be particularly burdensome to patients or staff members.41
The studies we reviewed used diverse screening instruments. Further research is needed to determine which tools are preferable in which patient populations, and to confirm that brief instruments are adequate for screening. The GDS is widely used, and many patients hospitalized in the United States fall into the geriatric group. The PHQ has been validated for self-administration and is widely used among outpatients42; it may be more suitable for younger populations. We found that several abbreviated versions of these and other screening instruments have exhibited good sensitivity and specificity among inpatients. However, many of the studies excluded patients with cognitive impairment or communication barriers. For individuals with auditory impairment, the Brief Assessment Schedule Depression Cards (BASDEC) might be an option. Used in 2 studies, the BASDEC involves showing patients a deck of 19 easy-to-read cards. The time required to administer the BASDEC is modest.15,23 Sets of smiley face diagrams might also be suitable for some patients with communication barriers or cognitive impairment. An ineligible study among stroke survivors found that selecting a sad face had a sensitivity of 76% and specificity of 77% relative to a formal diagnostic evaluation for depression.43
In considering the instruments that may be most suitable for inpatients, the role of somatic symptoms is also important because these can overlap between depression and the medical conditions that lead to hospitalization.44–46 Prior investigators found, for example, that 47% of Beck Depression Inventory (BDI) scores were attributable to somatic symptoms among patients hospitalized after myocardial infarction, whereas 37% of BDI scores were attributable to somatic symptoms among depressed outpatients.47 Future research is needed to determine the significance of somatic symptoms among inpatients, including whether they should be considered during screening, add prognostic value, or warrant specific treatment. In addition, although positive and negative predictive values were generally high among the screening instruments we evaluated, confirming the diagnosis of depression with a thorough clinical assessment is likely to be necessary.44,45
Despite the high prevalence of depression, associations with suboptimal outcomes, and the good performance of screening tools to date, screening for depression in the inpatient setting has received little attention. Prior authors have questioned whether hospital-based screening is an efficient and effective way to detect depression, and have raised valid concerns regarding false-positive diagnoses and unnecessary treatment, as well as a lack of randomized controlled trials.7,48,49 Whereas some studies suggest that depression is associated with greater healthcare utilization,3,4 little information exists regarding whether screening during hospitalization and treating previously undiagnosed depression improves clinical outcomes or reduces healthcare utilization.
Several important questions remain. What is the pathophysiology of depressed mood during hospitalization? How often does depressed mood during hospitalization reflect longstanding undiagnosed depression, longstanding undertreated depression, an acute stress disorder, or a normal if unpleasant short-term reaction to the stress of acute illnesses? Do the manifestations and effects of depressed mood differ among these situations? What is the prognosis of depressed mood occurring during hospitalization, and how many patients continue to have depression after recovery from acute illness; what factors affect prognosis? In a small sample of hospitalized patients, nearly 50% of those who had been depressed at intake remained depressed 1 month after discharge.50 Given that most antidepressant medications have to be taken for several weeks before effects can be detected, what, if any, approach to treatment should be taken? More research is needed on the effectiveness and cost-effectiveness of diagnosing and treating depression in the inpatient setting.
This work has several limitations. We found relatively few studies meeting eligibility criteria, particularly studies assessing clinical and utilization outcomes among depressed inpatients. Among the screening tools that were studied in the hospital setting, the highly diverse instruments and modes of administration precluded a quantitative synthesis such as meta-analysis. Prior meta-analyses on specific screening tools have focused on outpatient populations.51–53 Furthermore, we did not evaluate study quality or risk of bias.
In conclusion, screening for depression in the inpatient setting via patient self-assessment or assessment by hospital staff appears feasible. Several brief screening tools are available that have good sensitivity and specificity relative to diagnoses made by mental health professionals. Limited evidence suggests that screening tools for depression may be ready to integrate into inpatient care.41 Yet, although depression appears to be common and associated with worse clinical outcomes and higher healthcare utilization, more research is needed on the benefits, risks, and potential costs of adding depression screening in the inpatient healthcare setting.
Disclosures
The authors report no conflicts of interest.
1. Kahn KL, Keeler EB, Sherwood MJ, et al. Comparing outcomes of care before and after implementation of the DRG-based prospective payment system. JAMA. 1990;264(15):1984-1988. PubMed
2. U.S. Preventive Services Task Force (USPSTF). Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(4):380-387. PubMed
3. Dennis M, Kadri A, Coffey J. Depression in older people in the general hospital: a systematic review of screening instruments. Age Ageing. 2012;41(2):148-154. PubMed
4. Albrecht JS, Gruber-Baldini AL, Hirshon JM, et al. Depressive symptoms and hospital readmission in older adults. J Am Geriatr Soc. 2014;62(3):495-499. PubMed
5. Grant BF, Hasin DS, Harford TC. Screening for major depression among alcoholics: an application of receiver operating characteristic analysis. Drug Alcohol Depend. 1989;23(2):123-131. PubMed
6. Lieberman D, Galinsky D, Fried V, et al. Geriatric Depression Screening Scale (GDS) in patients hospitalized for physical rehabilitation. Int J Geriatr Psychiatry. 1999;14(7):549-555. PubMed
7. Canadian Task Force on Preventive Health Care. Recommendations on screening for depression in adults. CMAJ. 2013;185(9):775-782.
8. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. PubMed
9. Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013-1020. PubMed
10. Le Fevre P, Devereux J, Smith S, Lawrie SM, Cornbleet M. Screening for psychiatric illness in the palliative care inpatient setting: a comparison between the Hospital Anxiety and Depression Scale and the General Health Questionnaire-12. Palliat Med. 1999;13(5):399-407. PubMed
11. Lloyd-Williams M, Friedman T, Rudd N. Criterion validation of the Edinburgh Postnatal Depression Scale as a screening tool for depression in patients with advanced metastatic cancer. J Pain Symptom Manag. 2000;20(4):259-265. PubMed
12. Amadori K, Herrmann E, Püllen RK. Comparison of the 15-item Geriatric Depression Scale (GDS-15) and the GDS-4 during screening for depression in an in-patient geriatric patient group. J Am Geriatr Soc. 2011;59(1):171-172. PubMed
13. Diez-Quevedo C, Rangil T, Sanchez-Planell L, Kroenke K, Spitzer RL. Validation and utility of the Patient Health Questionnaire in diagnosing mental disorders in 1003 general hospital Spanish inpatients. Psychosom Med. 2001;63(4):679-686. PubMed
14. Young Q-R, Nguyen M, Roth S, Broadberry A, Mackay MH. Single-item measures for depression and anxiety: validation of the screening tool for psychological distress in an inpatient cardiology setting. Eur J Cardiovasc Nursing. 2015;14(6):544-551. PubMed
15. Loke B, Nicklason F, Burvill P. Screening for depression: clinical validation of geriatricians’ diagnosis, the Brief Assessment Schedule Depression Cards and the 5-item version of the Symptom Check List among non-demented geriatric inpatients. Int J Geriatr Psychiatry. 1996;11(5):461-465.
16. Shah A, Karasu M, De T. Nursing staff and screening for depression among acutely ill geriatric inpatients: a pilot study. Aging Ment Health. 1998;2(1):71-74.
17. Payne A, Barry S, Creedon B, et al. Sensitivity and specificity of a two-question screening tool for depression in a specialist palliative care unit. Palliat Med. 2007;21(3):193-198. PubMed
18. Rinaldi P, Mecocci P, Benedetti C, et al. Validation of the five-item geriatric depression scale in elderly subjects in three different settings. J Am Geriatr Soc. 2003;51(5):694-698. PubMed
19. McGuire AW, Eastwood J, Macabasco-O’Connell A, Hays RD, Doering LV. Depression screening: utility of the Patient Health Questionnaire in patients with acute coronary syndrome. Am J Crit Care. 2013;22(1):12-19. PubMed
20. Furlanetto LM, Mendlowicz MV, Bueno JR. The validity of the Beck Depression Inventory-Short Form as a screening and diagnostic instrument for moderate and severe depression in medical inpatients. J Affect Disord. 2005;86(1):87-91. PubMed
21. Heidenblut S, Zank S. Screening for depression with the Depression in Old Age Scale (DIA-S) and the Geriatric Depression Scale (GDS15): diagnostic accuracy in a geriatric inpatient setting. GeroPsych (Bern). 2014;27(1):41. PubMed
22. Pantilat SZ, O’Riordan DL, Dibble SL, Landefeld CS. An assessment of the screening performance of a single-item measure of depression from the Edmonton Symptom Assessment Scale among chronically ill hospitalized patients. J Pain Symptom Manage. 2012;43(5):866-873. PubMed
23. Adshead F, Cody DD, Pitt B. BASDEC: a novel screening instrument for depression in elderly medical inpatients. BMJ. 1992;305(6850):397. PubMed
24. Singh D, Sunpath H, John S, Eastham L, Gouden R. The utility of a rapid screening tool for depression and HIV dementia amongst patients with low CD4 counts – a preliminary report. Afr J Psychiatry (Johannesbg). 2008;11(4):282-286. PubMed
25. Bonin-Guillaume S, Sautel L, Demattei C, Jouve E, Blin O. Validation of the Retardation Rating Scale for detecting in geriatric inpatients. Int J Geriatr Psychiatry. 2007;22(1):68-76. PubMed
26. Rybarczyk B, Winemiller DR, Lazarus LW, Haut A, Hartman C. Validation of a depression screening measure for stroke inpatients. Am J Geriatr Psychiatry. 1996;4(2):131-139.
27. Parker G, Hilton T, Hadzi-Pavlovic D, Bains J. Screening for depression in the medically ill: the suggested utility of a cognitive-based approach. Aust N Z J Psychiatry. 2001;35(4):474-480. PubMed
28. Samaras N, Herrmann FR, Samaras D, et al. The Hospital Anxiety and Depression Scale: low sensitivity for depression screening in demented and non-demented hospitalized elderly. Int Psychogeriatr. 2013;25(1):82-87. PubMed
29. Koenig HG, Cohen HJ, Blazer DG, Meador KG, Westlund R. A brief depression scale for use in the medically ill. Int J Psychiatry Med. 1992;22(2):183-195. PubMed
30. Cully JA, Gfeller JD, Heise RA, Ross MJ, Teal CR, Kunik ME. Geriatric depression, medical diagnosis, and functional recovery during acute rehabilitation. Arch Phys Med Rehabil. 2005;86(12):2256-2260. PubMed
31. Mitchell SE, Paasche-Orlow MK, Forsythe SR, et al. Post-discharge hospital utilization among adult medical inpatients with depressive symptoms. J Hosp Med. 2010;5(7):378-384. PubMed
32. Huffman JC, Mastromauro CA, Sowden GL, Wittmann C, Rodman R, Januzzi JL. A collaborative care depression management program for cardiac inpatients: depression characteristics and in-hospital outcomes. Psychosomatics. 2011;52(1):26-3. 2007;22(11):1596-1602.J Gen Intern Med PubMed
53. Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. 2010;126(3):335-348.J Affect Disord. PubMed
52. Mitchell AJ, Meader N, Symonds P. Diagnostic validity of the Hospital Anxiety and Depression Scale (HADS) in cancer and palliative settings: a meta-analysis. 2010;69(4):371-378.J Psychosom Res. PubMed
51. Brennan C, Worrall-Davis A, McMillan D, Gilbody S, House A. The Hospital Anxiety and Depression Scale: a diagnostic meta-analysis of case-finding ability. . 1992;22(3):281-289.Int J Psychiatry Med PubMed
50. Pomerantz AS, de-Nesnera A, West AN. Resolution of depressive symptoms in medical inpatients after discharge. 2014;12(1):13.BMC Med PubMed
49. Thombs BD, Ziegelstein RC, Roseman M, Kloda LA, Ioannidis JPA. There are no randomized controlled trials that support the United States Preventive Services Task Force guideline on screening for depression in primary care: a systematic review. 2013;1(4):E159-E167.CMAJ Open PubMed
48. Keshavarz H, Fitzpatrick-Lewis D, Streiner DL, et al. Screening for depression: a systematic review and meta-analysis. 2012;73(3):157-162.J Psychosom Res. PubMed
47. Delisle VC, Beck AT, Ziegelstein RC, Thombs BD. Symptoms of heart disease or its treatment may increase Beck Depression Inventory Scores in hospitalized post-myocardial infarction patients. 2014;23(9):1079.Psychooncology PubMed
46. Palmer SC. Study provides little insight into routine screening for depression.
2005;20(3):289.Int J Geriatr Psychiatry. PubMed
45. Baldwin RC. Validation of short screening tests for depression, response to Seymour [letter to the editor]. 2005;20(3):289.Int J Geriatr Psychiatry.
44. Seymour J. Validation of short screening tests for depression: comment on Goring et al. (2004) [letter to the editor]. 2008;45(7):1081-1089.Int J Nurs Stud. PubMed
43. Lee ACK, Tang SW, Yu GKK, Cheung RTF. The smiley as a simple screening tool for depression after stroke: a preliminary study. 1999;282(18):1737-1744.JAMA. PubMed
42. Spitzer RL, Kroenke K, Williams JW. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. . 2011;14(3):275-279.J Palliat Med PubMed
41. Rao S, Ferris FD, Irwin SA. Ease of screening for depression and delirium in patients enrolled in inpatient hospice care. . 2004;161(6):1090-1095.Am J Psychiatry PubMed
40. Williams LS, Ghose SS, Swindle RW. Depression and other mental health diagnoses increase mortality risk after ischemic stroke. 2013;75(5):409-413.J Psychosom Res. PubMed
39. Beach SR, Januzzi JL, Mastromauro CA, et al. Patient Health Questionnaire-9 score and adverse cardiac outcomes in patients hospitalized for acute cardiac disease. 2003;18(4):358-359.Int J Geriatr Psychiatry PubMed
38. Cullum S, Nandhra H, Darley J, Todd C. Screening for depression in older people on medical wards: which cut-point should we use? 2007;29(4):340-348.Gen Hosp Psychiatry. PubMed
37. McCusker J, Cole M, Ciampi A, Latimer E, Windholz S, Belzile E. Major depression in older medical inpatients predicts poor physical and mental health status over 12 months. 2008;37(6):690-695.Age Ageing PubMed
36. Cullum S, Metcalfe C, Todd C, Brayne C. Does depression predict adverse outcomes for older medical inpatients? A prospective cohort study of individuals screened for a trial.
. 2010;50(1):6-10.Arch Gerontol Geriatr PubMed
35. Unsar S, Sut N. Depression and health status in elderly hospitalized patients with chronic illness. 150-159.:2010;25(2)Int J Geriatr Psychiatry. PubMed
34. Helvik A-S, Skancke RH, Selbæk G. Screening for depression in elderly medical inpatients from rural area of Norway: prevalence and associated factors. . 2012;60(12):2254-2262.J Am Geriatr Soc PubMed
33. Pierlussi E, Mehta KM, Kirby KA, et al. Depressive symptoms after hospitalization in older adults: function and mortality outcomes. PubMed
1. Kahn KL, Keeler EB, Sherwood MJ, et al. Comparing outcomes of care before and after implementation of the DRG-based prospective payment system. JAMA. 1990;264(15):1984-1988. PubMed
2. U.S. Preventive Services Task Force (USPSTF). Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(4):380-387. PubMed
3. Dennis M, Kadri A, Coffey J. Depression in older people in the general hospital: a systematic review of screening instruments. Age Ageing. 2012;41(2):148-154. PubMed
4. Albrecht JS, Gruber-Baldini AL, Hirshon JM, et al. Depressive symptoms and hospital readmission in older adults. J Am Geriatr Soc. 2014;62(3):495-499. PubMed
5. Grant BF, Hasin DS, Harford TC. Screening for major depression among alcoholics: an application of receiver operating characteristic analysis. Drug Alcohol Depend. 1989;23(2):123-131. PubMed
6. Lieberman D, Galinsky D, Fried V, et al. Geriatric Depression Screening Scale (GDS) in patients hospitalized for physical rehabilitation. Int J Geriatr Psychiatry. 1999;14(7):549-555. PubMed
7. Canadian Task Force on Preventive Health Care. Recommendations on screening for depression in adults. CMAJ. 2013;185(9):775-782.
8. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. PubMed
9. Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013-1020. PubMed
10. Le Fevre P, Devereux J, Smith S, Lawrie SM, Cornbleet M. Screening for psychiatric illness in the palliative care inpatient setting: a comparison between the Hospital Anxiety and Depression Scale and the General Health Questionnaire-12. Palliat Med. 1999;13(5):399-407. PubMed
11. Lloyd-Williams M, Friedman T, Rudd N. Criterion validation of the Edinburgh Postnatal Depression Scale as a screening tool for depression in patients with advanced metastatic cancer. J Pain Symptom Manag. 2000;20(4):259-265. PubMed
12. Amadori K, Herrmann E, Püllen RK. Comparison of the 15-item Geriatric Depression Scale (GDS-15) and the GDS-4 during screening for depression in an in-patient geriatric patient group. J Am Geriatr Soc. 2011;59(1):171-172. PubMed
13. Diez-Quevedo C, Rangil T, Sanchez-Planell L, Kroenke K, Spitzer RL. Validation and utility of the Patient Health Questionnaire in diagnosing mental disorders in 1003 general hospital Spanish inpatients. Psychosom Med. 2001;63(4):679-686. PubMed
14. Young Q-R, Nguyen M, Roth S, Broadberry A, Mackay MH. Single-item measures for depression and anxiety: validation of the screening tool for psychological distress in an inpatient cardiology setting. Eur J Cardiovasc Nursing. 2015;14(6):544-551. PubMed
15. Loke B, Nicklason F, Burvill P. Screening for depression: clinical validation of geriatricians’ diagnosis, the Brief Assessment Schedule Depression Cards and the 5-item version of the Symptom Check List among non-demented geriatric inpatients. Int J Geriatr Psychiatry. 1996;11(5):461-465.
16. Shah A, Karasu M, De T. Nursing staff and screening for depression among acutely ill geriatric inpatients: a pilot study. Aging Ment Health. 1998;2(1):71-74.
17. Payne A, Barry S, Creedon B, et al. Sensitivity and specificity of a two-question screening tool for depression in a specialist palliative care unit. Palliat Med. 2007;21(3):193-198. PubMed
18. Rinaldi P, Mecocci P, Benedetti C, et al. Validation of the five-item geriatric depression scale in elderly subjects in three different settings. J Am Geriatr Soc. 2003;51(5):694-698. PubMed
19. McGuire AW, Eastwood J, Macabasco-O’Connell A, Hays RD, Doering LV. Depression screening: utility of the Patient Health Questionnaire in patients with acute coronary syndrome. Am J Crit Care. 2013;22(1):12-19. PubMed
20. Furlanetto LM, Mendlowicz MV, Bueno JR. The validity of the Beck Depression Inventory-Short Form as a screening and diagnostic instrument for moderate and severe depression in medical inpatients. J Affect Disord. 2005;86(1):87-91. PubMed
21. Heidenblut S, Zank S. Screening for depression with the Depression in Old Age Scale (DIA-S) and the Geriatric Depression Scale (GDS15): diagnostic accuracy in a geriatric inpatient setting. GeroPsych (Bern). 2014;27(1):41. PubMed
22. Pantilat SZ, O’Riordan DL, Dibble SL, Landefeld CS. An assessment of the screening performance of a single-item measure of depression from the Edmonton Symptom Assessment Scale among chronically ill hospitalized patients. J Pain Symptom Manage. 2012;43(5):866-873. PubMed
23. Adshead F, Cody DD, Pitt B. BASDEC: a novel screening instrument for depression in elderly medical inpatients. BMJ. 1992;305(6850):397. PubMed
24. Singh D, Sunpath H, John S, Eastham L, Gouden R. The utility of a rapid screening tool for depression and HIV dementia amongst patients with low CD4 counts – a preliminary report. Afr J Psychiatry (Johannesbg). 2008;11(4):282-286. PubMed
25. Bonin-Guillaume S, Sautel L, Demattei C, Jouve E, Blin O. Validation of the Retardation Rating Scale for detecting in geriatric inpatients. Int J Geriatr Psychiatry. 2007;22(1):68-76. PubMed
26. Rybarczyk B, Winemiller DR, Lazarus LW, Haut A, Hartman C. Validation of a depression screening measure for stroke inpatients. Am J Geriatr Psychiatry. 1996;4(2):131-139.
27. Parker G, Hilton T, Hadzi-Pavlovic D, Bains J. Screening for depression in the medically ill: the suggested utility of a cognitive-based approach. Aust N Z J Psychiatry. 2001;35(4):474-480. PubMed
28. Samaras N, Herrmann FR, Samaras D, et al. The Hospital Anxiety and Depression Scale: low sensitivity for depression screening in demented and non-demented hospitalized elderly. Int Psychogeriatr. 2013;25(1):82-87. PubMed
29. Koenig HG, Cohen HJ, Blazer DG, Meador KG, Westlund R. A brief depression scale for use in the medically ill. Int J Psychiatry Med. 1992;22(2):183-195. PubMed
30. Cully JA, Gfeller JD, Heise RA, Ross MJ, Teal CR, Kunik ME. Geriatric depression, medical diagnosis, and functional recovery during acute rehabilitation. Arch Phys Med Rehabil. 2005;86(12):2256-2260. PubMed
31. Mitchell SE, Paasche-Orlow MK, Forsythe SR, et al. Post-discharge hospital utilization among adult medical inpatients with depressive symptoms. J Hosp Med. 2010;5(7):378-384. PubMed
32. Huffman JC, Mastromauro CA, Sowden GL, Wittmann C, Rodman R, Januzzi JL. A collaborative care depression management program for cardiac inpatients: depression characteristics and in-hospital outcomes. Psychosomatics. 2011;52(1):26-3. 2007;22(11):1596-1602.J Gen Intern Med PubMed
53. Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. 2010;126(3):335-348.J Affect Disord. PubMed
52. Mitchell AJ, Meader N, Symonds P. Diagnostic validity of the Hospital Anxiety and Depression Scale (HADS) in cancer and palliative settings: a meta-analysis. 2010;69(4):371-378.J Psychosom Res. PubMed
51. Brennan C, Worrall-Davis A, McMillan D, Gilbody S, House A. The Hospital Anxiety and Depression Scale: a diagnostic meta-analysis of case-finding ability. . 1992;22(3):281-289.Int J Psychiatry Med PubMed
50. Pomerantz AS, de-Nesnera A, West AN. Resolution of depressive symptoms in medical inpatients after discharge. 2014;12(1):13.BMC Med PubMed
49. Thombs BD, Ziegelstein RC, Roseman M, Kloda LA, Ioannidis JPA. There are no randomized controlled trials that support the United States Preventive Services Task Force guideline on screening for depression in primary care: a systematic review. 2013;1(4):E159-E167.CMAJ Open PubMed
48. Keshavarz H, Fitzpatrick-Lewis D, Streiner DL, et al. Screening for depression: a systematic review and meta-analysis. 2012;73(3):157-162.J Psychosom Res. PubMed
47. Delisle VC, Beck AT, Ziegelstein RC, Thombs BD. Symptoms of heart disease or its treatment may increase Beck Depression Inventory Scores in hospitalized post-myocardial infarction patients. 2014;23(9):1079.Psychooncology PubMed
46. Palmer SC. Study provides little insight into routine screening for depression.
2005;20(3):289.Int J Geriatr Psychiatry. PubMed
45. Baldwin RC. Validation of short screening tests for depression, response to Seymour [letter to the editor]. 2005;20(3):289.Int J Geriatr Psychiatry.
44. Seymour J. Validation of short screening tests for depression: comment on Goring et al. (2004) [letter to the editor]. 2008;45(7):1081-1089.Int J Nurs Stud. PubMed
43. Lee ACK, Tang SW, Yu GKK, Cheung RTF. The smiley as a simple screening tool for depression after stroke: a preliminary study. 1999;282(18):1737-1744.JAMA. PubMed
42. Spitzer RL, Kroenke K, Williams JW. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. . 2011;14(3):275-279.J Palliat Med PubMed
41. Rao S, Ferris FD, Irwin SA. Ease of screening for depression and delirium in patients enrolled in inpatient hospice care. . 2004;161(6):1090-1095.Am J Psychiatry PubMed
40. Williams LS, Ghose SS, Swindle RW. Depression and other mental health diagnoses increase mortality risk after ischemic stroke. 2013;75(5):409-413.J Psychosom Res. PubMed
39. Beach SR, Januzzi JL, Mastromauro CA, et al. Patient Health Questionnaire-9 score and adverse cardiac outcomes in patients hospitalized for acute cardiac disease. 2003;18(4):358-359.Int J Geriatr Psychiatry PubMed
38. Cullum S, Nandhra H, Darley J, Todd C. Screening for depression in older people on medical wards: which cut-point should we use? 2007;29(4):340-348.Gen Hosp Psychiatry. PubMed
37. McCusker J, Cole M, Ciampi A, Latimer E, Windholz S, Belzile E. Major depression in older medical inpatients predicts poor physical and mental health status over 12 months. 2008;37(6):690-695.Age Ageing PubMed
36. Cullum S, Metcalfe C, Todd C, Brayne C. Does depression predict adverse outcomes for older medical inpatients? A prospective cohort study of individuals screened for a trial.
. 2010;50(1):6-10.Arch Gerontol Geriatr PubMed
35. Unsar S, Sut N. Depression and health status in elderly hospitalized patients with chronic illness. 150-159.:2010;25(2)Int J Geriatr Psychiatry. PubMed
34. Helvik A-S, Skancke RH, Selbæk G. Screening for depression in elderly medical inpatients from rural area of Norway: prevalence and associated factors. . 2012;60(12):2254-2262.J Am Geriatr Soc PubMed
33. Pierlussi E, Mehta KM, Kirby KA, et al. Depressive symptoms after hospitalization in older adults: function and mortality outcomes. PubMed
© 2017 Society of Hospital Medicine
Acute kidney injury is important in the hospital and afterward
Acute kidney injury (AKI) is a major contributor to morbidity and mortality in hospitalized patients across the world.1 Affecting up to 20% of all admissions (depending on which definition of AKI is used),2 AKI is the most common reason for new-inpatient nephrology consultation. Recent data suggest that AKI incidence has risen rapidly, by up to 10% per year.3,4
AKI is associated with a variety of serious short- and long-term complications. Approximately 33% to 60% of critically ill patients who develop dialysis-requiring AKI do not survive to hospital discharge, and mortality associated with dialysis-requiring AKI is greater than that associated with other serious conditions such as myocardial infarction or acute respiratory distress syndrome.5 Even relatively mild AKI in the acute inpatient setting appears to be an independent risk factor for mortality.6
For several decades, many physicians believed that AKI was a self-limited process followed by complete recovery of renal function to pre-AKI levels among survivors. (Numerous trainees have been taught some variant of the old adage: “If the patients survive, so will their kidneys.”) But studies linking AKI with the development of new-onset chronic kidney disease (CKD) or the accelerated progression of pre-existing CKD have changed this view.7 One important reason the long-term impact of AKI hasn’t been appreciated is that, traditionally, clinical studies of AKI examined inhospital outcomes such as short-term mortality and resource usage and did not consider what transpired months to years after discharge. More recently, epidemiologic studies linking inpatient events with outpatient outcomes have filled this knowledge gap.8 Contemporary animal models of AKI have shed light on potential mechanisms of maladaptive repair after AKI, characterized by fibrosis, vascular rarefaction, tubular loss, glomerulosclerosis, and chronic interstitial inflammation, all of which result in renal function decline. So over the last decade there has been a paradigm shift in how we think about AKI and CKD. Rather than distinct entities, AKI and CKD are now viewed as interconnected syndromes since AKI is a risk factor for CKD progression and CKD is a risk factor for new episodes of AKI.9
Two studies published in this issue of the Journal of Hospital Medicine augment our understanding of AKI and its clinical impact in hospitalized patients. Analyzing data from the National Inpatient Sample, Silver et al.10 found that hospitalizations that include AKI are substantially costlier and associated with longer lengths of stay than hospitalizations without AKI. The authors also highlight that the additional economic costs of AKI exceeded those of many other higher-profile yet less-common acute medical conditions, such as myocardial infarction and gastrointestinal bleeding. These results re-emphasize the important economic burden of AKI at a national level and expand on prior literature by confirming findings previously limited to single-center and regional studies. Better defining the impact AKI has on our healthcare system could help ensure that adequate resources are invested to combat AKI.
The second study, by Rutter et al.,11 found that among hospitalized patients with normal baseline renal function, use of vancomycin in combination with piperacillin-tazobactam is associated with a higher incidence of AKI after antibiotic exposure than use of either agent as monotherapy. This association persisted even after adjusting for potential confounders such as underlying comorbidities, exposure to nephrotoxic agents, documented hypotension, and baseline renal impairment. This study adds to a growing body of literature that suggests synergistic nephrotoxicity between vancomycin and piperacillin-tazobactam. It underscores that any medical intervention—even treatments typically envisioned as non-hazardous and frequently life-saving—involve inherent risks and should prompt the medical community to promote proper antimicrobial stewardship. Whether such exposures to vancomycin or beta-lactam derivatives cause AKI via direct tubular damage, interstitial nephritis, or some other novel mechanism remains to be elucidated. Better delineation of the contemporary causes of AKI, including increased antibiotic exposure, is the first step toward identifying ways to reduce AKI incidence.
Both of these papers serve to highlight the clinical importance of AKI among hospitalized patients. Their findings re-emphasize the need for vigilance in detecting AKI and intervening early to achieve the best clinical outcomes.
Given recent understanding that survivors of AKI are at greater risk for more rapid loss of renal function long after hospital discharge, one goal the US Department of Health and Human Services put forth for Healthy People 2020 is to “increase the proportion of hospital patients who incurred AKI who have follow-up renal evaluation in 6 months post-discharge” (10% improvement targeted).12 Transitions of care after hospitalizations complicated by AKI require special attention to ensure that patients’ needs are optimally monitored and managed during the critical post-discharge period. One recent study analyzing discharge documentation for hospitalizations including AKI found that fewer than half of the discharge summaries and patient instructions commented on the presence, cause, or course of AKI, indicating clear room for improvement.13 And currently, it appears that only a minority of patients with AKI—even AKI severe enough to require dialysis—are seen by a nephrologist within 90 days of discharge.14
Hospitalists play a crucial role in coordinating care as vulnerable patients transition from the inpatient to outpatient setting. We suggest that AKI should be properly documented in the discharge summary. In addition, patients should be informed that they experienced AKI so they can discuss with future caregivers potential strategies to avoid additional renal insults. Discharge referrals to nephrology should be arranged for high-risk patients, including those whose renal function remains decreased at discharge or those who had recurrent AKI episodes during prior hospitalizations. For patients with pre-hospitalization baseline CKD, nephrology should be consulted before indefinitely discontinuing medications like angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. These medications are indispensable in retarding the progression of proteinuric CKD, even though they may predispose patients to AKI under certain circumstances (eg, in states of decreased renal perfusion). Adopting these simple steps may substantially improve the long-term outcomes of patients who experience AKI during hospitalization.
Acknowledgments
The authors are supported by NIH-NIDDK Grants T32DK007219 (BJL) and K24DK92291 (CYH).
Disclosure
Nothing to report.
1. Lameire NH, Bagga A, Cruz D, et al. Acute kidney injury: an increasing global concern. Lancet. 2013;382(9887):170-179. PubMed
2. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12-20. PubMed
3. Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2013;24(1):37-42. PubMed
4. Siew ED, Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int. 2015;87(1):46-61. PubMed
5. Cerdá J, Liu KD, Cruz DN, et al. Promoting kidney function recovery in patients with AKI requiring RRT. Clin J Am Soc Nephrol. 2015;10(10):1859-1867. PubMed
6. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365-3370. PubMed
7. Hsu CY. Yes, AKI truly leads to CKD. J Am Soc Nephrol. 2012;23(6):967-969. PubMed
8. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442-448. PubMed
9. Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. New Engl J Med. 2014;371(1):58-66. PubMed
10. Silver SA, Long J, Zheng Y, Chertow GM. Cost of acute kidney injury in hospitalized patients. J Hosp Med. 2017;12(2):70-76. Full Text
11. Rutter WC, Burgess DR, Talbert JC, Burgess DS. Acute kidney injury in patients treated with vancomycin and piperacillin-tazobactam: a retrospective cohort analysis. J Hosp Med. 2017;12(2):77-82. Full Text
12. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2020. Available at: https://www.healthypeople.gov/node/4093/data_details. Accessed September 2, 2016.
13. Greer RC, Liu Y, Crews DC, Jaar BG, Rabb H, Boulware LE. Hospital discharge communications during care transitions for patients with acute kidney injury: a cross-sectional study. BMC Health Serv Res. 2016;16:449. PubMed
14. Siew ED, Peterson JF, Eden SK, et al. Outpatient nephrology referral rates after acute kidney injury. J Am Soc Nephrol. 2012;23(2):305-312. PubMed
Acute kidney injury (AKI) is a major contributor to morbidity and mortality in hospitalized patients across the world.1 Affecting up to 20% of all admissions (depending on which definition of AKI is used),2 AKI is the most common reason for new-inpatient nephrology consultation. Recent data suggest that AKI incidence has risen rapidly, by up to 10% per year.3,4
AKI is associated with a variety of serious short- and long-term complications. Approximately 33% to 60% of critically ill patients who develop dialysis-requiring AKI do not survive to hospital discharge, and mortality associated with dialysis-requiring AKI is greater than that associated with other serious conditions such as myocardial infarction or acute respiratory distress syndrome.5 Even relatively mild AKI in the acute inpatient setting appears to be an independent risk factor for mortality.6
For several decades, many physicians believed that AKI was a self-limited process followed by complete recovery of renal function to pre-AKI levels among survivors. (Numerous trainees have been taught some variant of the old adage: “If the patients survive, so will their kidneys.”) But studies linking AKI with the development of new-onset chronic kidney disease (CKD) or the accelerated progression of pre-existing CKD have changed this view.7 One important reason the long-term impact of AKI hasn’t been appreciated is that, traditionally, clinical studies of AKI examined inhospital outcomes such as short-term mortality and resource usage and did not consider what transpired months to years after discharge. More recently, epidemiologic studies linking inpatient events with outpatient outcomes have filled this knowledge gap.8 Contemporary animal models of AKI have shed light on potential mechanisms of maladaptive repair after AKI, characterized by fibrosis, vascular rarefaction, tubular loss, glomerulosclerosis, and chronic interstitial inflammation, all of which result in renal function decline. So over the last decade there has been a paradigm shift in how we think about AKI and CKD. Rather than distinct entities, AKI and CKD are now viewed as interconnected syndromes since AKI is a risk factor for CKD progression and CKD is a risk factor for new episodes of AKI.9
Two studies published in this issue of the Journal of Hospital Medicine augment our understanding of AKI and its clinical impact in hospitalized patients. Analyzing data from the National Inpatient Sample, Silver et al.10 found that hospitalizations that include AKI are substantially costlier and associated with longer lengths of stay than hospitalizations without AKI. The authors also highlight that the additional economic costs of AKI exceeded those of many other higher-profile yet less-common acute medical conditions, such as myocardial infarction and gastrointestinal bleeding. These results re-emphasize the important economic burden of AKI at a national level and expand on prior literature by confirming findings previously limited to single-center and regional studies. Better defining the impact AKI has on our healthcare system could help ensure that adequate resources are invested to combat AKI.
The second study, by Rutter et al.,11 found that among hospitalized patients with normal baseline renal function, use of vancomycin in combination with piperacillin-tazobactam is associated with a higher incidence of AKI after antibiotic exposure than use of either agent as monotherapy. This association persisted even after adjusting for potential confounders such as underlying comorbidities, exposure to nephrotoxic agents, documented hypotension, and baseline renal impairment. This study adds to a growing body of literature that suggests synergistic nephrotoxicity between vancomycin and piperacillin-tazobactam. It underscores that any medical intervention—even treatments typically envisioned as non-hazardous and frequently life-saving—involve inherent risks and should prompt the medical community to promote proper antimicrobial stewardship. Whether such exposures to vancomycin or beta-lactam derivatives cause AKI via direct tubular damage, interstitial nephritis, or some other novel mechanism remains to be elucidated. Better delineation of the contemporary causes of AKI, including increased antibiotic exposure, is the first step toward identifying ways to reduce AKI incidence.
Both of these papers serve to highlight the clinical importance of AKI among hospitalized patients. Their findings re-emphasize the need for vigilance in detecting AKI and intervening early to achieve the best clinical outcomes.
Given recent understanding that survivors of AKI are at greater risk for more rapid loss of renal function long after hospital discharge, one goal the US Department of Health and Human Services put forth for Healthy People 2020 is to “increase the proportion of hospital patients who incurred AKI who have follow-up renal evaluation in 6 months post-discharge” (10% improvement targeted).12 Transitions of care after hospitalizations complicated by AKI require special attention to ensure that patients’ needs are optimally monitored and managed during the critical post-discharge period. One recent study analyzing discharge documentation for hospitalizations including AKI found that fewer than half of the discharge summaries and patient instructions commented on the presence, cause, or course of AKI, indicating clear room for improvement.13 And currently, it appears that only a minority of patients with AKI—even AKI severe enough to require dialysis—are seen by a nephrologist within 90 days of discharge.14
Hospitalists play a crucial role in coordinating care as vulnerable patients transition from the inpatient to outpatient setting. We suggest that AKI should be properly documented in the discharge summary. In addition, patients should be informed that they experienced AKI so they can discuss with future caregivers potential strategies to avoid additional renal insults. Discharge referrals to nephrology should be arranged for high-risk patients, including those whose renal function remains decreased at discharge or those who had recurrent AKI episodes during prior hospitalizations. For patients with pre-hospitalization baseline CKD, nephrology should be consulted before indefinitely discontinuing medications like angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. These medications are indispensable in retarding the progression of proteinuric CKD, even though they may predispose patients to AKI under certain circumstances (eg, in states of decreased renal perfusion). Adopting these simple steps may substantially improve the long-term outcomes of patients who experience AKI during hospitalization.
Acknowledgments
The authors are supported by NIH-NIDDK Grants T32DK007219 (BJL) and K24DK92291 (CYH).
Disclosure
Nothing to report.
Acute kidney injury (AKI) is a major contributor to morbidity and mortality in hospitalized patients across the world.1 Affecting up to 20% of all admissions (depending on which definition of AKI is used),2 AKI is the most common reason for new-inpatient nephrology consultation. Recent data suggest that AKI incidence has risen rapidly, by up to 10% per year.3,4
AKI is associated with a variety of serious short- and long-term complications. Approximately 33% to 60% of critically ill patients who develop dialysis-requiring AKI do not survive to hospital discharge, and mortality associated with dialysis-requiring AKI is greater than that associated with other serious conditions such as myocardial infarction or acute respiratory distress syndrome.5 Even relatively mild AKI in the acute inpatient setting appears to be an independent risk factor for mortality.6
For several decades, many physicians believed that AKI was a self-limited process followed by complete recovery of renal function to pre-AKI levels among survivors. (Numerous trainees have been taught some variant of the old adage: “If the patients survive, so will their kidneys.”) But studies linking AKI with the development of new-onset chronic kidney disease (CKD) or the accelerated progression of pre-existing CKD have changed this view.7 One important reason the long-term impact of AKI hasn’t been appreciated is that, traditionally, clinical studies of AKI examined inhospital outcomes such as short-term mortality and resource usage and did not consider what transpired months to years after discharge. More recently, epidemiologic studies linking inpatient events with outpatient outcomes have filled this knowledge gap.8 Contemporary animal models of AKI have shed light on potential mechanisms of maladaptive repair after AKI, characterized by fibrosis, vascular rarefaction, tubular loss, glomerulosclerosis, and chronic interstitial inflammation, all of which result in renal function decline. So over the last decade there has been a paradigm shift in how we think about AKI and CKD. Rather than distinct entities, AKI and CKD are now viewed as interconnected syndromes since AKI is a risk factor for CKD progression and CKD is a risk factor for new episodes of AKI.9
Two studies published in this issue of the Journal of Hospital Medicine augment our understanding of AKI and its clinical impact in hospitalized patients. Analyzing data from the National Inpatient Sample, Silver et al.10 found that hospitalizations that include AKI are substantially costlier and associated with longer lengths of stay than hospitalizations without AKI. The authors also highlight that the additional economic costs of AKI exceeded those of many other higher-profile yet less-common acute medical conditions, such as myocardial infarction and gastrointestinal bleeding. These results re-emphasize the important economic burden of AKI at a national level and expand on prior literature by confirming findings previously limited to single-center and regional studies. Better defining the impact AKI has on our healthcare system could help ensure that adequate resources are invested to combat AKI.
The second study, by Rutter et al.,11 found that among hospitalized patients with normal baseline renal function, use of vancomycin in combination with piperacillin-tazobactam is associated with a higher incidence of AKI after antibiotic exposure than use of either agent as monotherapy. This association persisted even after adjusting for potential confounders such as underlying comorbidities, exposure to nephrotoxic agents, documented hypotension, and baseline renal impairment. This study adds to a growing body of literature that suggests synergistic nephrotoxicity between vancomycin and piperacillin-tazobactam. It underscores that any medical intervention—even treatments typically envisioned as non-hazardous and frequently life-saving—involve inherent risks and should prompt the medical community to promote proper antimicrobial stewardship. Whether such exposures to vancomycin or beta-lactam derivatives cause AKI via direct tubular damage, interstitial nephritis, or some other novel mechanism remains to be elucidated. Better delineation of the contemporary causes of AKI, including increased antibiotic exposure, is the first step toward identifying ways to reduce AKI incidence.
Both of these papers serve to highlight the clinical importance of AKI among hospitalized patients. Their findings re-emphasize the need for vigilance in detecting AKI and intervening early to achieve the best clinical outcomes.
Given recent understanding that survivors of AKI are at greater risk for more rapid loss of renal function long after hospital discharge, one goal the US Department of Health and Human Services put forth for Healthy People 2020 is to “increase the proportion of hospital patients who incurred AKI who have follow-up renal evaluation in 6 months post-discharge” (10% improvement targeted).12 Transitions of care after hospitalizations complicated by AKI require special attention to ensure that patients’ needs are optimally monitored and managed during the critical post-discharge period. One recent study analyzing discharge documentation for hospitalizations including AKI found that fewer than half of the discharge summaries and patient instructions commented on the presence, cause, or course of AKI, indicating clear room for improvement.13 And currently, it appears that only a minority of patients with AKI—even AKI severe enough to require dialysis—are seen by a nephrologist within 90 days of discharge.14
Hospitalists play a crucial role in coordinating care as vulnerable patients transition from the inpatient to outpatient setting. We suggest that AKI should be properly documented in the discharge summary. In addition, patients should be informed that they experienced AKI so they can discuss with future caregivers potential strategies to avoid additional renal insults. Discharge referrals to nephrology should be arranged for high-risk patients, including those whose renal function remains decreased at discharge or those who had recurrent AKI episodes during prior hospitalizations. For patients with pre-hospitalization baseline CKD, nephrology should be consulted before indefinitely discontinuing medications like angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. These medications are indispensable in retarding the progression of proteinuric CKD, even though they may predispose patients to AKI under certain circumstances (eg, in states of decreased renal perfusion). Adopting these simple steps may substantially improve the long-term outcomes of patients who experience AKI during hospitalization.
Acknowledgments
The authors are supported by NIH-NIDDK Grants T32DK007219 (BJL) and K24DK92291 (CYH).
Disclosure
Nothing to report.
1. Lameire NH, Bagga A, Cruz D, et al. Acute kidney injury: an increasing global concern. Lancet. 2013;382(9887):170-179. PubMed
2. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12-20. PubMed
3. Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2013;24(1):37-42. PubMed
4. Siew ED, Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int. 2015;87(1):46-61. PubMed
5. Cerdá J, Liu KD, Cruz DN, et al. Promoting kidney function recovery in patients with AKI requiring RRT. Clin J Am Soc Nephrol. 2015;10(10):1859-1867. PubMed
6. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365-3370. PubMed
7. Hsu CY. Yes, AKI truly leads to CKD. J Am Soc Nephrol. 2012;23(6):967-969. PubMed
8. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442-448. PubMed
9. Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. New Engl J Med. 2014;371(1):58-66. PubMed
10. Silver SA, Long J, Zheng Y, Chertow GM. Cost of acute kidney injury in hospitalized patients. J Hosp Med. 2017;12(2):70-76. Full Text
11. Rutter WC, Burgess DR, Talbert JC, Burgess DS. Acute kidney injury in patients treated with vancomycin and piperacillin-tazobactam: a retrospective cohort analysis. J Hosp Med. 2017;12(2):77-82. Full Text
12. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2020. Available at: https://www.healthypeople.gov/node/4093/data_details. Accessed September 2, 2016.
13. Greer RC, Liu Y, Crews DC, Jaar BG, Rabb H, Boulware LE. Hospital discharge communications during care transitions for patients with acute kidney injury: a cross-sectional study. BMC Health Serv Res. 2016;16:449. PubMed
14. Siew ED, Peterson JF, Eden SK, et al. Outpatient nephrology referral rates after acute kidney injury. J Am Soc Nephrol. 2012;23(2):305-312. PubMed
1. Lameire NH, Bagga A, Cruz D, et al. Acute kidney injury: an increasing global concern. Lancet. 2013;382(9887):170-179. PubMed
2. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12-20. PubMed
3. Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2013;24(1):37-42. PubMed
4. Siew ED, Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int. 2015;87(1):46-61. PubMed
5. Cerdá J, Liu KD, Cruz DN, et al. Promoting kidney function recovery in patients with AKI requiring RRT. Clin J Am Soc Nephrol. 2015;10(10):1859-1867. PubMed
6. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365-3370. PubMed
7. Hsu CY. Yes, AKI truly leads to CKD. J Am Soc Nephrol. 2012;23(6):967-969. PubMed
8. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442-448. PubMed
9. Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. New Engl J Med. 2014;371(1):58-66. PubMed
10. Silver SA, Long J, Zheng Y, Chertow GM. Cost of acute kidney injury in hospitalized patients. J Hosp Med. 2017;12(2):70-76. Full Text
11. Rutter WC, Burgess DR, Talbert JC, Burgess DS. Acute kidney injury in patients treated with vancomycin and piperacillin-tazobactam: a retrospective cohort analysis. J Hosp Med. 2017;12(2):77-82. Full Text
12. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2020. Available at: https://www.healthypeople.gov/node/4093/data_details. Accessed September 2, 2016.
13. Greer RC, Liu Y, Crews DC, Jaar BG, Rabb H, Boulware LE. Hospital discharge communications during care transitions for patients with acute kidney injury: a cross-sectional study. BMC Health Serv Res. 2016;16:449. PubMed
14. Siew ED, Peterson JF, Eden SK, et al. Outpatient nephrology referral rates after acute kidney injury. J Am Soc Nephrol. 2012;23(2):305-312. PubMed
© 2017 Society of Hospital Medicine
SAVR an option for elderly with aortic stenosis
HOUSTON – Surgical aortic valve replacement can be performed in intermediate-risk elderly patients with an operative mortality rate of 4.1%, which is better than expected, according to results from a large multicenter analysis. However, the rate of in-hospital stroke was 5.4% – twice what was expected.
“This is most likely secondary to neurologic assessment [that was conducted] for all patients postoperatively,” Vinod H. Thourani, MD, said at the annual meeting of the Society of Thoracic Surgeons.
The findings come from an in-depth analysis of SAVR outcomes in patients who participated in the Placement of Aortic Transcatheter Valves trial, known as PARTNER 2A. Conducted from December 2011 to November 2013, PARTNER 2A evaluated 2,032 medium-risk patients with aortic stenosis who were randomized to SAVR or transcatheter aortic valve replacement (TAVR) in 57 North American centers and found no significant difference in the 2-year rate of death or disabling stroke (N Engl J Med. 2016 Apr 28;3749[17]:1609-20).
Dr. Thourani’s analysis focused on the 937 patients who underwent SAVR. The main objectives were to describe operative mortality and hospital morbidities compared with STS benchmarks, describe time-related mortality and stroke including preoperative predictors for these outcomes, evaluate the effect of concomitant procedures on mortality and hospital morbidities, and evaluate longitudinal valve performance after SAVR.
The average age of these patients was 82 years, 45% were female, and their mean STS risk score was 5.8. In addition, 26% had prior coronary artery bypass surgery, 10% had a previous stroke, and 12% had previous pacemaker placement. Of the 30% of patients with chronic obstructive pulmonary disease, 9.6% were oxygen dependent going into the operating room, reported Dr. Thourani, one of the PARTNER 2A investigators, and a cardiothoracic surgeon at Emory University, Atlanta.
Most of the patients (85%) had a full sternotomy, while 15% had a mini sternotomy. Isolated AVR was done in 79% of patients, 15% of patients had AVR plus CABG, and 6% had AVR and other concomitant procedures. The mean coronary bypass time for isolated AVR was 98 minutes, and rose to a mean of 129 minutes when a concomitant procedure was added. The mean cross-clamp time was 69 minutes, and rose to a mean of 95 minutes when a concomitant procedure was added.
The investigators observed that all-cause operative mortality was 4.1%, which is lower than STS predicted-risk models. At the same time, mortality for AVR plus a concomitant procedure was 5%, followed by isolated AVR (4.2%) and AVR plus CABG plus a concomitant procedure (2.9%). The rate of in-hospital stroke was 5.4% and the rate of in-hospital deep sternal wound infection was 0.8%. At 2 years postoperatively, mortality was 17% among those who underwent isolated AVR, 18% among those who underwent AVR plus CABG, and 21% among those who underwent AVR plus a concomitant procedure, differences that did not reach statistical significance. The rate of stroke at 2 years also was similar between groups: 12% among those who underwent isolated AVR, 11% in those who underwent AVR plus a concomitant procedure, and 8.2% in those who underwent AVR plus CABG.
The main risk factor for early death after SAVR was longer procedure time (P less than .0001), while risk factors for later deaths included cachexia (P = .02), lower ejection fraction (P = .01), higher creatinine (P = .03), coronary artery disease (P = .03), and smaller prostheses (P = .01)
Dr. Thourani and his associates also found that 33% of patients had severe prosthesis-patient mismatch, yet they had survival rates similar to the rates of those without severe prosthesis-patient mismatch.
“From this adjudicated, prospectively collected data in the contemporary era, SAVR can be performed in intermediate-risk elderly patients with mortality commensurate with national benchmarks,” he concluded. “Continued surveillance of these patients remains extremely important.”
Dr. Thourani disclosed that he is a consultant for and has received research support from Edwards Lifesciences. Other authors of the study reported having numerous relevant financial disclosures.
This analysis of the surgical arm of the PARTNER 2A trial reveals respectable outcome for those so-called intermediaterisk patients with severe symptomatic aortic stenosis. The fact that mortality at 2 years was similar between the surgical and the catheter arm of the trial (upward of 17%), speaks of the multiple comorbidities present in these patients (N Engl J Med.
This analysis of the surgical arm of the PARTNER 2A trial reveals respectable outcome for those so-called intermediaterisk patients with severe symptomatic aortic stenosis. The fact that mortality at 2 years was similar between the surgical and the catheter arm of the trial (upward of 17%), speaks of the multiple comorbidities present in these patients (N Engl J Med.
This analysis of the surgical arm of the PARTNER 2A trial reveals respectable outcome for those so-called intermediaterisk patients with severe symptomatic aortic stenosis. The fact that mortality at 2 years was similar between the surgical and the catheter arm of the trial (upward of 17%), speaks of the multiple comorbidities present in these patients (N Engl J Med.
HOUSTON – Surgical aortic valve replacement can be performed in intermediate-risk elderly patients with an operative mortality rate of 4.1%, which is better than expected, according to results from a large multicenter analysis. However, the rate of in-hospital stroke was 5.4% – twice what was expected.
“This is most likely secondary to neurologic assessment [that was conducted] for all patients postoperatively,” Vinod H. Thourani, MD, said at the annual meeting of the Society of Thoracic Surgeons.
The findings come from an in-depth analysis of SAVR outcomes in patients who participated in the Placement of Aortic Transcatheter Valves trial, known as PARTNER 2A. Conducted from December 2011 to November 2013, PARTNER 2A evaluated 2,032 medium-risk patients with aortic stenosis who were randomized to SAVR or transcatheter aortic valve replacement (TAVR) in 57 North American centers and found no significant difference in the 2-year rate of death or disabling stroke (N Engl J Med. 2016 Apr 28;3749[17]:1609-20).
Dr. Thourani’s analysis focused on the 937 patients who underwent SAVR. The main objectives were to describe operative mortality and hospital morbidities compared with STS benchmarks, describe time-related mortality and stroke including preoperative predictors for these outcomes, evaluate the effect of concomitant procedures on mortality and hospital morbidities, and evaluate longitudinal valve performance after SAVR.
The average age of these patients was 82 years, 45% were female, and their mean STS risk score was 5.8. In addition, 26% had prior coronary artery bypass surgery, 10% had a previous stroke, and 12% had previous pacemaker placement. Of the 30% of patients with chronic obstructive pulmonary disease, 9.6% were oxygen dependent going into the operating room, reported Dr. Thourani, one of the PARTNER 2A investigators, and a cardiothoracic surgeon at Emory University, Atlanta.
Most of the patients (85%) had a full sternotomy, while 15% had a mini sternotomy. Isolated AVR was done in 79% of patients, 15% of patients had AVR plus CABG, and 6% had AVR and other concomitant procedures. The mean coronary bypass time for isolated AVR was 98 minutes, and rose to a mean of 129 minutes when a concomitant procedure was added. The mean cross-clamp time was 69 minutes, and rose to a mean of 95 minutes when a concomitant procedure was added.
The investigators observed that all-cause operative mortality was 4.1%, which is lower than STS predicted-risk models. At the same time, mortality for AVR plus a concomitant procedure was 5%, followed by isolated AVR (4.2%) and AVR plus CABG plus a concomitant procedure (2.9%). The rate of in-hospital stroke was 5.4% and the rate of in-hospital deep sternal wound infection was 0.8%. At 2 years postoperatively, mortality was 17% among those who underwent isolated AVR, 18% among those who underwent AVR plus CABG, and 21% among those who underwent AVR plus a concomitant procedure, differences that did not reach statistical significance. The rate of stroke at 2 years also was similar between groups: 12% among those who underwent isolated AVR, 11% in those who underwent AVR plus a concomitant procedure, and 8.2% in those who underwent AVR plus CABG.
The main risk factor for early death after SAVR was longer procedure time (P less than .0001), while risk factors for later deaths included cachexia (P = .02), lower ejection fraction (P = .01), higher creatinine (P = .03), coronary artery disease (P = .03), and smaller prostheses (P = .01)
Dr. Thourani and his associates also found that 33% of patients had severe prosthesis-patient mismatch, yet they had survival rates similar to the rates of those without severe prosthesis-patient mismatch.
“From this adjudicated, prospectively collected data in the contemporary era, SAVR can be performed in intermediate-risk elderly patients with mortality commensurate with national benchmarks,” he concluded. “Continued surveillance of these patients remains extremely important.”
Dr. Thourani disclosed that he is a consultant for and has received research support from Edwards Lifesciences. Other authors of the study reported having numerous relevant financial disclosures.
HOUSTON – Surgical aortic valve replacement can be performed in intermediate-risk elderly patients with an operative mortality rate of 4.1%, which is better than expected, according to results from a large multicenter analysis. However, the rate of in-hospital stroke was 5.4% – twice what was expected.
“This is most likely secondary to neurologic assessment [that was conducted] for all patients postoperatively,” Vinod H. Thourani, MD, said at the annual meeting of the Society of Thoracic Surgeons.
The findings come from an in-depth analysis of SAVR outcomes in patients who participated in the Placement of Aortic Transcatheter Valves trial, known as PARTNER 2A. Conducted from December 2011 to November 2013, PARTNER 2A evaluated 2,032 medium-risk patients with aortic stenosis who were randomized to SAVR or transcatheter aortic valve replacement (TAVR) in 57 North American centers and found no significant difference in the 2-year rate of death or disabling stroke (N Engl J Med. 2016 Apr 28;3749[17]:1609-20).
Dr. Thourani’s analysis focused on the 937 patients who underwent SAVR. The main objectives were to describe operative mortality and hospital morbidities compared with STS benchmarks, describe time-related mortality and stroke including preoperative predictors for these outcomes, evaluate the effect of concomitant procedures on mortality and hospital morbidities, and evaluate longitudinal valve performance after SAVR.
The average age of these patients was 82 years, 45% were female, and their mean STS risk score was 5.8. In addition, 26% had prior coronary artery bypass surgery, 10% had a previous stroke, and 12% had previous pacemaker placement. Of the 30% of patients with chronic obstructive pulmonary disease, 9.6% were oxygen dependent going into the operating room, reported Dr. Thourani, one of the PARTNER 2A investigators, and a cardiothoracic surgeon at Emory University, Atlanta.
Most of the patients (85%) had a full sternotomy, while 15% had a mini sternotomy. Isolated AVR was done in 79% of patients, 15% of patients had AVR plus CABG, and 6% had AVR and other concomitant procedures. The mean coronary bypass time for isolated AVR was 98 minutes, and rose to a mean of 129 minutes when a concomitant procedure was added. The mean cross-clamp time was 69 minutes, and rose to a mean of 95 minutes when a concomitant procedure was added.
The investigators observed that all-cause operative mortality was 4.1%, which is lower than STS predicted-risk models. At the same time, mortality for AVR plus a concomitant procedure was 5%, followed by isolated AVR (4.2%) and AVR plus CABG plus a concomitant procedure (2.9%). The rate of in-hospital stroke was 5.4% and the rate of in-hospital deep sternal wound infection was 0.8%. At 2 years postoperatively, mortality was 17% among those who underwent isolated AVR, 18% among those who underwent AVR plus CABG, and 21% among those who underwent AVR plus a concomitant procedure, differences that did not reach statistical significance. The rate of stroke at 2 years also was similar between groups: 12% among those who underwent isolated AVR, 11% in those who underwent AVR plus a concomitant procedure, and 8.2% in those who underwent AVR plus CABG.
The main risk factor for early death after SAVR was longer procedure time (P less than .0001), while risk factors for later deaths included cachexia (P = .02), lower ejection fraction (P = .01), higher creatinine (P = .03), coronary artery disease (P = .03), and smaller prostheses (P = .01)
Dr. Thourani and his associates also found that 33% of patients had severe prosthesis-patient mismatch, yet they had survival rates similar to the rates of those without severe prosthesis-patient mismatch.
“From this adjudicated, prospectively collected data in the contemporary era, SAVR can be performed in intermediate-risk elderly patients with mortality commensurate with national benchmarks,” he concluded. “Continued surveillance of these patients remains extremely important.”
Dr. Thourani disclosed that he is a consultant for and has received research support from Edwards Lifesciences. Other authors of the study reported having numerous relevant financial disclosures.
AT THE STS ANNUAL MEETING
Key clinical point:
Major finding: All-cause operative mortality was 4.1%, which is lower than STS predicted risk models.
Data source: A study of 937 medium-risk patients with aortic stenosis who were randomized to SAVR in the PARTNER 2A trial.
Disclosures: Dr. Thourani is a consultant for and has received research support from Edwards Lifesciences. Other authors of the study reported having numerous relevant financial disclosures.
Clinical Challenges - February 2017: So-called carcinosarcoma of the esophagus
What's Your Diagnosis?
The Diagnosis
Carcinosarcoma is a rare malignant entity, representing less than 2% of all esophageal neoplasms. It usually shows a bulky appearance of an intraluminal polypoid lesion owing to predominant sarcomatous development with little stromal proliferation.
References
1. Hung J.J., Li A.F., Liu J.S., et al. Esophageal carcinosarcoma with basaloid squamous cell carcinoma and osteosarcoma. Ann Thorac Surg. 2008;85[3]:1102-4.
2. Madan A.K., Long A.E., Weldon C.B., et al. Esophageal carcinosarcoma. J Gastrointest Surg. 2001;5[4]:414-7.
The Diagnosis
Carcinosarcoma is a rare malignant entity, representing less than 2% of all esophageal neoplasms. It usually shows a bulky appearance of an intraluminal polypoid lesion owing to predominant sarcomatous development with little stromal proliferation.
References
1. Hung J.J., Li A.F., Liu J.S., et al. Esophageal carcinosarcoma with basaloid squamous cell carcinoma and osteosarcoma. Ann Thorac Surg. 2008;85[3]:1102-4.
2. Madan A.K., Long A.E., Weldon C.B., et al. Esophageal carcinosarcoma. J Gastrointest Surg. 2001;5[4]:414-7.
The Diagnosis
Carcinosarcoma is a rare malignant entity, representing less than 2% of all esophageal neoplasms. It usually shows a bulky appearance of an intraluminal polypoid lesion owing to predominant sarcomatous development with little stromal proliferation.
References
1. Hung J.J., Li A.F., Liu J.S., et al. Esophageal carcinosarcoma with basaloid squamous cell carcinoma and osteosarcoma. Ann Thorac Surg. 2008;85[3]:1102-4.
2. Madan A.K., Long A.E., Weldon C.B., et al. Esophageal carcinosarcoma. J Gastrointest Surg. 2001;5[4]:414-7.
What's Your Diagnosis?
What's Your Diagnosis?
By Kensuke Adachi, MD, PhD, and Kazuaki Enatsu, MD.
Published previously in Gastroenterology (2013;144[1]:32, 251).
Researchers find ‘feedback loop’ key to reducing high blood pressure
Research with mice and rats in Germany may have found a new way to treat high blood pressure.
Using epifluorescence intravital video microscopy imaging, researchers examined mice to whom they had given angiotensin II hormones to induce arterial hypertension.
They determined that the mice with low levels of thrombin-driven factor XI (FXI) – either naturally or inhibited by antisense oligonucleotides – had healthier endothelium.
“Specificity of the effects of FXI depletion was confirmed by continuous in vivo supplementation with human FXI,” wrote Sabine Kossmann, PhD, of the Center for Thrombosis and Hemostasis, University Medical Center, Mainz (Germany), and her coauthors (Sci Transl Med. 2017 Feb 1. doi: 10.1126/scitranslmed.aah4923).
Targeting the “feedback loop” between the FXI and a receptor that helps thrombin propagate on platelets reduced both vascular inflammation and blood pressure.
“Our findings suggest that inhibiting the ... thrombin-FXI–amplifying loop may provide added cardiovascular benefits that are synergistic with those of established platelet inhibitors,” the authors wrote.
This work was supported by grants from the Stiftung Pathobiochemie und Molekulare Diagnostik and the Federal Ministry of Education and Research. The authors disclosed funding and grants from the German Research Society, the European Research Council, NIH, and other sources. One of the researchers is inventor of five patents related to the FXI inhibitor and equity holder in Aronora, and may have financial interest in the findings of the research.
Research with mice and rats in Germany may have found a new way to treat high blood pressure.
Using epifluorescence intravital video microscopy imaging, researchers examined mice to whom they had given angiotensin II hormones to induce arterial hypertension.
They determined that the mice with low levels of thrombin-driven factor XI (FXI) – either naturally or inhibited by antisense oligonucleotides – had healthier endothelium.
“Specificity of the effects of FXI depletion was confirmed by continuous in vivo supplementation with human FXI,” wrote Sabine Kossmann, PhD, of the Center for Thrombosis and Hemostasis, University Medical Center, Mainz (Germany), and her coauthors (Sci Transl Med. 2017 Feb 1. doi: 10.1126/scitranslmed.aah4923).
Targeting the “feedback loop” between the FXI and a receptor that helps thrombin propagate on platelets reduced both vascular inflammation and blood pressure.
“Our findings suggest that inhibiting the ... thrombin-FXI–amplifying loop may provide added cardiovascular benefits that are synergistic with those of established platelet inhibitors,” the authors wrote.
This work was supported by grants from the Stiftung Pathobiochemie und Molekulare Diagnostik and the Federal Ministry of Education and Research. The authors disclosed funding and grants from the German Research Society, the European Research Council, NIH, and other sources. One of the researchers is inventor of five patents related to the FXI inhibitor and equity holder in Aronora, and may have financial interest in the findings of the research.
Research with mice and rats in Germany may have found a new way to treat high blood pressure.
Using epifluorescence intravital video microscopy imaging, researchers examined mice to whom they had given angiotensin II hormones to induce arterial hypertension.
They determined that the mice with low levels of thrombin-driven factor XI (FXI) – either naturally or inhibited by antisense oligonucleotides – had healthier endothelium.
“Specificity of the effects of FXI depletion was confirmed by continuous in vivo supplementation with human FXI,” wrote Sabine Kossmann, PhD, of the Center for Thrombosis and Hemostasis, University Medical Center, Mainz (Germany), and her coauthors (Sci Transl Med. 2017 Feb 1. doi: 10.1126/scitranslmed.aah4923).
Targeting the “feedback loop” between the FXI and a receptor that helps thrombin propagate on platelets reduced both vascular inflammation and blood pressure.
“Our findings suggest that inhibiting the ... thrombin-FXI–amplifying loop may provide added cardiovascular benefits that are synergistic with those of established platelet inhibitors,” the authors wrote.
This work was supported by grants from the Stiftung Pathobiochemie und Molekulare Diagnostik and the Federal Ministry of Education and Research. The authors disclosed funding and grants from the German Research Society, the European Research Council, NIH, and other sources. One of the researchers is inventor of five patents related to the FXI inhibitor and equity holder in Aronora, and may have financial interest in the findings of the research.
FROM SCIENCE TRANSLATIONAL MEDICINE
Unpublished study on Bendectin prompts questions on hidden data
Doubt is being cast on the efficacy of Diclegis – the only prescription drug approved in the United States for treating nausea and vomiting in pregnancy – after researchers exposed flaws in previously unpublished data that served as the basis for the drug’s approval.
But the larger point, according to the researcher who brought the unpublished study to light, is the danger of relying too heavily on hidden data.
“It’s not like there’s some special concern over the safety of Diclegis. It’s that there is this commonly prescribed medication that hasn’t been proven to be effective,” Navindra Persaud, MD, a family physician and researcher at St. Michael’s Hospital in Toronto, said in an interview.
The 8-Way Bendectin Study was a double-blind, multicentered, randomized, placebo-controlled study of 2,359 women with morning sickness in the first trimester, conducted in the United States across multiple sites in 1976 by the now-defunct Wm. S. Merrell Co. The aim was to find a replacement formulation of a three-agent formula (Bendectin) for morning sickness, after one of the ingredients – dicyclomine hydrochloride – was determined ineffective for pregnancy-related nausea and vomiting.
Participants in the study, which had seven treatment arms and one control group, were asked to keep diaries for a week, detailing their bouts of nausea and vomiting. Clinicians then evaluated and rated the diary entries. In all, data for 1,599 of the women were analyzed, with all seven treatment arms besting placebo. Doxylamine-pyridoxine was rated “moderate or excellent” with a 21% absolute difference, compared with placebo (95% confidence interval, 11-30). The most commonly reported side effect across the study was drowsiness.
Dr. Persaud said he thinks the study was never published because of multiple flaws. For instance, there was not a clear baseline for symptoms, or clear parameters for how the clinicians rated those symptoms; outcome data for more than a third of controls were missing, as were completed reports about potential adverse outcomes; and P values were one sided and not adjusted to account for all eight study arms, he said.
“While the analyzed data indicate differences from placebo for several combinations, the questionable data integrity, high dropout rate, and other methodological concerns mean that the prescribing of this medication should not be based on this trial,” wrote Dr. Persaud and Dr. Zhang in their analysis.
The newly published analysis brings back the rocky history of morning sickness treatments in the United States, notably the withdrawal of Bendectin in 1983 following a barrage of teratogenicity claims against the drug maker that made it unprofitable to continue marketing.
This is all beside the point, according to Dr. Persaud. “For every medication, you’re going to find some of these associations. They might be real; they might be not. So you have to weigh potential harm against the benefit. The real problem here is that there is no demonstrated benefit even though the claim seems to be that there is,” he said.
Duchesnay defended the efficacy of the drug.
“The conclusions expressed in the report published in PLOS ONE are highly inconsistent with the large and comprehensive body of evidence regarding this combination drug,” Michael Gallo, Duchesnay vice president for regulatory and medical affairs, said in a statement posted on the company’s website. In its response to Dr. Persaud and Dr. Zhang’s analysis, the company also said that doxylamine succinate and pyridoxine hydrochloride – the two agents in the treatment – are “ the most studied drug combination used in pregnancy. The safety and efficacy of [Diclegis] have been proven in 16 cohort studies, two meta-analyses, an ecological study, a neurological development study, and numerous others.”
“It’s unclear if the [unpublished] study was carefully reassessed in the lead-up to the recent approval of Diclegis,” he said. “The available FDA review documents for the recent approval of Diclectin [pyridoxine/doxylamine] do not mention the problems with the study.”
One factor in the treatment’s place in standard of care might be anecdotal influences from some of the more than 35 million women around the world thought to have used the treatment, according to Dr. Persaud. “Lots of women have taken this medication and felt better shortly after, so they feel strongly that the medication is effective,” he said, but because nausea and vomiting in pregnancy is common in more than three-quarters of women, and typically does not last more than several weeks, most likely the patients would have gotten better over time anyway.
“Some women suffer greatly and do seem to get relief from medication,” Dr. Chambers said, but noted that Diclegis is not the only option available for women.
When Bendectin was pulled from the U.S. market, for example, Dr. Chambers said women turned to combinations of vitamin B6 and over-the-counter medications that contain the antihistamine doxylamine.
Dr. Persaud said his interest in the review started after a patient expressed her concerns over the medication. “She was reluctant to take it, and asked me if I was sure about it. I reassured her, but then after she left, I did wonder if I was correct,” he recalled. He said he checked all the guidelines, but could not find anything to justify its use other than the manufacturer’s monograph.
He said he suspects this is not the only prescription medication that would not withstand such scrutiny, but that uncovering the necessary data would be very difficult. “I was shocked it was very difficult to get access to this information as a clinician,” he said, adding that it also is impractical to expect physicians to spend 5 years to track the information down.
In their analysis of the study, Dr. Persaud and Dr. Zhang stated that their objective is to contribute to a movement across all of medicine to end the risks of data secrecy, and instead “restore invisible and abandoned trials” (RIAT). The U.S. Department of Health & Human Services has been pushing to make more clinical trials data public through ClinicalTrials.gov, including issuing federal regulations requiring information to be made public for certain trials involving drugs and devices regulated by the FDA.
As for how his own practice has been impacted by this research, Dr. Persaud said he no longer prescribes Diclegis.
Dr. Persaud, Dr. Zhang, and Dr. Chambers had no relevant financial disclosures.
[email protected]
On Twitter @whitneymcknight
Between 1956 and 1983, the primary treatment for nausea/vomiting of pregnancy (NVP) was Bendectin, a combination of doxylamine and pyridoxine. In 1983, it was removed from the market by the manufacturer because of litigation expense. Following this, there was a marked increase in the incidence of hyperemesis gravidarum, the most severe form of NVP, which was probably due to ineffective treatment of the condition.
Several organizations have stated that the combination of doxylamine/pyridoxine is safe and effective for use in pregnancy. In 2002, the Society of Obstetricians and Gynaecologists of Canada concluded that the doxylamine/pyridoxine combination should be the standard of care because it had the greatest evidence to support its efficacy and safety. In 2004, the American College of Obstetricians and Gynecologists stated that the combination was safe and effective and was the first-line treatment for NVP.
If NVP is not controlled with 2 tablets at bedtime, the Diclegis dose can be increased up to 4 tablets per day – 1 in the morning, 1 in midafternoon, and 2 at bedtime.
Gerald G. Briggs, BPharm, FCCP, is a clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
Between 1956 and 1983, the primary treatment for nausea/vomiting of pregnancy (NVP) was Bendectin, a combination of doxylamine and pyridoxine. In 1983, it was removed from the market by the manufacturer because of litigation expense. Following this, there was a marked increase in the incidence of hyperemesis gravidarum, the most severe form of NVP, which was probably due to ineffective treatment of the condition.
Several organizations have stated that the combination of doxylamine/pyridoxine is safe and effective for use in pregnancy. In 2002, the Society of Obstetricians and Gynaecologists of Canada concluded that the doxylamine/pyridoxine combination should be the standard of care because it had the greatest evidence to support its efficacy and safety. In 2004, the American College of Obstetricians and Gynecologists stated that the combination was safe and effective and was the first-line treatment for NVP.
If NVP is not controlled with 2 tablets at bedtime, the Diclegis dose can be increased up to 4 tablets per day – 1 in the morning, 1 in midafternoon, and 2 at bedtime.
Gerald G. Briggs, BPharm, FCCP, is a clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
Between 1956 and 1983, the primary treatment for nausea/vomiting of pregnancy (NVP) was Bendectin, a combination of doxylamine and pyridoxine. In 1983, it was removed from the market by the manufacturer because of litigation expense. Following this, there was a marked increase in the incidence of hyperemesis gravidarum, the most severe form of NVP, which was probably due to ineffective treatment of the condition.
Several organizations have stated that the combination of doxylamine/pyridoxine is safe and effective for use in pregnancy. In 2002, the Society of Obstetricians and Gynaecologists of Canada concluded that the doxylamine/pyridoxine combination should be the standard of care because it had the greatest evidence to support its efficacy and safety. In 2004, the American College of Obstetricians and Gynecologists stated that the combination was safe and effective and was the first-line treatment for NVP.
If NVP is not controlled with 2 tablets at bedtime, the Diclegis dose can be increased up to 4 tablets per day – 1 in the morning, 1 in midafternoon, and 2 at bedtime.
Gerald G. Briggs, BPharm, FCCP, is a clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
Doubt is being cast on the efficacy of Diclegis – the only prescription drug approved in the United States for treating nausea and vomiting in pregnancy – after researchers exposed flaws in previously unpublished data that served as the basis for the drug’s approval.
But the larger point, according to the researcher who brought the unpublished study to light, is the danger of relying too heavily on hidden data.
“It’s not like there’s some special concern over the safety of Diclegis. It’s that there is this commonly prescribed medication that hasn’t been proven to be effective,” Navindra Persaud, MD, a family physician and researcher at St. Michael’s Hospital in Toronto, said in an interview.
The 8-Way Bendectin Study was a double-blind, multicentered, randomized, placebo-controlled study of 2,359 women with morning sickness in the first trimester, conducted in the United States across multiple sites in 1976 by the now-defunct Wm. S. Merrell Co. The aim was to find a replacement formulation of a three-agent formula (Bendectin) for morning sickness, after one of the ingredients – dicyclomine hydrochloride – was determined ineffective for pregnancy-related nausea and vomiting.
Participants in the study, which had seven treatment arms and one control group, were asked to keep diaries for a week, detailing their bouts of nausea and vomiting. Clinicians then evaluated and rated the diary entries. In all, data for 1,599 of the women were analyzed, with all seven treatment arms besting placebo. Doxylamine-pyridoxine was rated “moderate or excellent” with a 21% absolute difference, compared with placebo (95% confidence interval, 11-30). The most commonly reported side effect across the study was drowsiness.
Dr. Persaud said he thinks the study was never published because of multiple flaws. For instance, there was not a clear baseline for symptoms, or clear parameters for how the clinicians rated those symptoms; outcome data for more than a third of controls were missing, as were completed reports about potential adverse outcomes; and P values were one sided and not adjusted to account for all eight study arms, he said.
“While the analyzed data indicate differences from placebo for several combinations, the questionable data integrity, high dropout rate, and other methodological concerns mean that the prescribing of this medication should not be based on this trial,” wrote Dr. Persaud and Dr. Zhang in their analysis.
The newly published analysis brings back the rocky history of morning sickness treatments in the United States, notably the withdrawal of Bendectin in 1983 following a barrage of teratogenicity claims against the drug maker that made it unprofitable to continue marketing.
This is all beside the point, according to Dr. Persaud. “For every medication, you’re going to find some of these associations. They might be real; they might be not. So you have to weigh potential harm against the benefit. The real problem here is that there is no demonstrated benefit even though the claim seems to be that there is,” he said.
Duchesnay defended the efficacy of the drug.
“The conclusions expressed in the report published in PLOS ONE are highly inconsistent with the large and comprehensive body of evidence regarding this combination drug,” Michael Gallo, Duchesnay vice president for regulatory and medical affairs, said in a statement posted on the company’s website. In its response to Dr. Persaud and Dr. Zhang’s analysis, the company also said that doxylamine succinate and pyridoxine hydrochloride – the two agents in the treatment – are “ the most studied drug combination used in pregnancy. The safety and efficacy of [Diclegis] have been proven in 16 cohort studies, two meta-analyses, an ecological study, a neurological development study, and numerous others.”
“It’s unclear if the [unpublished] study was carefully reassessed in the lead-up to the recent approval of Diclegis,” he said. “The available FDA review documents for the recent approval of Diclectin [pyridoxine/doxylamine] do not mention the problems with the study.”
One factor in the treatment’s place in standard of care might be anecdotal influences from some of the more than 35 million women around the world thought to have used the treatment, according to Dr. Persaud. “Lots of women have taken this medication and felt better shortly after, so they feel strongly that the medication is effective,” he said, but because nausea and vomiting in pregnancy is common in more than three-quarters of women, and typically does not last more than several weeks, most likely the patients would have gotten better over time anyway.
“Some women suffer greatly and do seem to get relief from medication,” Dr. Chambers said, but noted that Diclegis is not the only option available for women.
When Bendectin was pulled from the U.S. market, for example, Dr. Chambers said women turned to combinations of vitamin B6 and over-the-counter medications that contain the antihistamine doxylamine.
Dr. Persaud said his interest in the review started after a patient expressed her concerns over the medication. “She was reluctant to take it, and asked me if I was sure about it. I reassured her, but then after she left, I did wonder if I was correct,” he recalled. He said he checked all the guidelines, but could not find anything to justify its use other than the manufacturer’s monograph.
He said he suspects this is not the only prescription medication that would not withstand such scrutiny, but that uncovering the necessary data would be very difficult. “I was shocked it was very difficult to get access to this information as a clinician,” he said, adding that it also is impractical to expect physicians to spend 5 years to track the information down.
In their analysis of the study, Dr. Persaud and Dr. Zhang stated that their objective is to contribute to a movement across all of medicine to end the risks of data secrecy, and instead “restore invisible and abandoned trials” (RIAT). The U.S. Department of Health & Human Services has been pushing to make more clinical trials data public through ClinicalTrials.gov, including issuing federal regulations requiring information to be made public for certain trials involving drugs and devices regulated by the FDA.
As for how his own practice has been impacted by this research, Dr. Persaud said he no longer prescribes Diclegis.
Dr. Persaud, Dr. Zhang, and Dr. Chambers had no relevant financial disclosures.
[email protected]
On Twitter @whitneymcknight
Doubt is being cast on the efficacy of Diclegis – the only prescription drug approved in the United States for treating nausea and vomiting in pregnancy – after researchers exposed flaws in previously unpublished data that served as the basis for the drug’s approval.
But the larger point, according to the researcher who brought the unpublished study to light, is the danger of relying too heavily on hidden data.
“It’s not like there’s some special concern over the safety of Diclegis. It’s that there is this commonly prescribed medication that hasn’t been proven to be effective,” Navindra Persaud, MD, a family physician and researcher at St. Michael’s Hospital in Toronto, said in an interview.
The 8-Way Bendectin Study was a double-blind, multicentered, randomized, placebo-controlled study of 2,359 women with morning sickness in the first trimester, conducted in the United States across multiple sites in 1976 by the now-defunct Wm. S. Merrell Co. The aim was to find a replacement formulation of a three-agent formula (Bendectin) for morning sickness, after one of the ingredients – dicyclomine hydrochloride – was determined ineffective for pregnancy-related nausea and vomiting.
Participants in the study, which had seven treatment arms and one control group, were asked to keep diaries for a week, detailing their bouts of nausea and vomiting. Clinicians then evaluated and rated the diary entries. In all, data for 1,599 of the women were analyzed, with all seven treatment arms besting placebo. Doxylamine-pyridoxine was rated “moderate or excellent” with a 21% absolute difference, compared with placebo (95% confidence interval, 11-30). The most commonly reported side effect across the study was drowsiness.
Dr. Persaud said he thinks the study was never published because of multiple flaws. For instance, there was not a clear baseline for symptoms, or clear parameters for how the clinicians rated those symptoms; outcome data for more than a third of controls were missing, as were completed reports about potential adverse outcomes; and P values were one sided and not adjusted to account for all eight study arms, he said.
“While the analyzed data indicate differences from placebo for several combinations, the questionable data integrity, high dropout rate, and other methodological concerns mean that the prescribing of this medication should not be based on this trial,” wrote Dr. Persaud and Dr. Zhang in their analysis.
The newly published analysis brings back the rocky history of morning sickness treatments in the United States, notably the withdrawal of Bendectin in 1983 following a barrage of teratogenicity claims against the drug maker that made it unprofitable to continue marketing.
This is all beside the point, according to Dr. Persaud. “For every medication, you’re going to find some of these associations. They might be real; they might be not. So you have to weigh potential harm against the benefit. The real problem here is that there is no demonstrated benefit even though the claim seems to be that there is,” he said.
Duchesnay defended the efficacy of the drug.
“The conclusions expressed in the report published in PLOS ONE are highly inconsistent with the large and comprehensive body of evidence regarding this combination drug,” Michael Gallo, Duchesnay vice president for regulatory and medical affairs, said in a statement posted on the company’s website. In its response to Dr. Persaud and Dr. Zhang’s analysis, the company also said that doxylamine succinate and pyridoxine hydrochloride – the two agents in the treatment – are “ the most studied drug combination used in pregnancy. The safety and efficacy of [Diclegis] have been proven in 16 cohort studies, two meta-analyses, an ecological study, a neurological development study, and numerous others.”
“It’s unclear if the [unpublished] study was carefully reassessed in the lead-up to the recent approval of Diclegis,” he said. “The available FDA review documents for the recent approval of Diclectin [pyridoxine/doxylamine] do not mention the problems with the study.”
One factor in the treatment’s place in standard of care might be anecdotal influences from some of the more than 35 million women around the world thought to have used the treatment, according to Dr. Persaud. “Lots of women have taken this medication and felt better shortly after, so they feel strongly that the medication is effective,” he said, but because nausea and vomiting in pregnancy is common in more than three-quarters of women, and typically does not last more than several weeks, most likely the patients would have gotten better over time anyway.
“Some women suffer greatly and do seem to get relief from medication,” Dr. Chambers said, but noted that Diclegis is not the only option available for women.
When Bendectin was pulled from the U.S. market, for example, Dr. Chambers said women turned to combinations of vitamin B6 and over-the-counter medications that contain the antihistamine doxylamine.
Dr. Persaud said his interest in the review started after a patient expressed her concerns over the medication. “She was reluctant to take it, and asked me if I was sure about it. I reassured her, but then after she left, I did wonder if I was correct,” he recalled. He said he checked all the guidelines, but could not find anything to justify its use other than the manufacturer’s monograph.
He said he suspects this is not the only prescription medication that would not withstand such scrutiny, but that uncovering the necessary data would be very difficult. “I was shocked it was very difficult to get access to this information as a clinician,” he said, adding that it also is impractical to expect physicians to spend 5 years to track the information down.
In their analysis of the study, Dr. Persaud and Dr. Zhang stated that their objective is to contribute to a movement across all of medicine to end the risks of data secrecy, and instead “restore invisible and abandoned trials” (RIAT). The U.S. Department of Health & Human Services has been pushing to make more clinical trials data public through ClinicalTrials.gov, including issuing federal regulations requiring information to be made public for certain trials involving drugs and devices regulated by the FDA.
As for how his own practice has been impacted by this research, Dr. Persaud said he no longer prescribes Diclegis.
Dr. Persaud, Dr. Zhang, and Dr. Chambers had no relevant financial disclosures.
[email protected]
On Twitter @whitneymcknight
Pairing vascular reconstruction, pancreatic cancer resection
CHICAGO – More than 53,000 people will develop pancreatic ductal adenocarcinoma in the United States this year, and upwards of 41,000 will die from the disease, many of them with tumors considered unresectable because they involve adjacent vessels. However, researchers at the University of California, Irvine, have found that careful removal of the tumor around involved veins and arteries, even in borderline cases, can improve outcomes for these patients.
Roy M. Fujitani, MD, updated previously published data on a single-center study he coauthored in 2015 of 270 patients who had undergone a Whipple operation, 183 for pancreatic adenocarcinoma (J Vasc Surg. 2015;61:475-80) at a symposium on vascular surgery sponsored by Northwestern University.
Resection of pancreatic tumors without vascular involvement is fairly straightforward for surgical oncologists to perform, Dr. Fujitani said, but pancreatic tumors enter the borderline resectable category when preoperative CT scan shows portal vein abutment, for which vascular surgery should provide counsel and assist. However, even in some cases when preoperative CT scan shows unresectable, locally advanced pancreatic tumor with celiac artery encasement, neoadjuvant therapy may downstage the disease into the borderline category, he said.
“Patients with borderline resectable or stage II disease are those one should consider for reconstruction,” Dr. Fujitani said. Resectable findings of borderline disease include encasement of the portal vein, superior mesenteric vein and the confluence of the portal venous system (with suitable proximal and distal targets for reconstruction); and less-than-circumferential involvement of the common hepatic artery or right hepatic artery – but without involvement of the superior mesenteric artery or the celiac axis and “certainly not” the aorta. “This would account for about one-fourth of patients in high-volume centers as being able to receive concomitant vascular reconstruction,” Dr. Fujitani said.
In the UCI series, 60 patients with borderline lesions underwent vascular reconstruction. “As it turned out, there was no significant difference in survival between the reconstruction group and the nonreconstruction group,” Dr. Fujitani said, “but it’s important to note that these patients who had the reconstruction would never have been operated on if we were not able to do the reconstruction.” Thirty-day mortality was around 5% and 1-year survival around 70% in both groups, he said. However, at about 1.5 years the Kaplan-Meier survival curves between the two groups diverged, which Dr. Fujitani attributed to more advanced disease in the reconstruction group.
“We found lymph node status and tumor margins were most important in determining survival of these patients,” he said. “Gaining an R0 resection is the most important thing that determines favorable survivability.”
Dr. Fujitani also reviewed different techniques for vascular reconstruction, and while differences in complication rates or 1-, 2-, or 3-year survival were not statistically significant, he did note that mean survival with lateral venorrhaphy exceeded that of primary anastomosis and interposition graft – 21 months vs. 13 months vs. 4 months, suggesting the merits of a more aggressive approach to vascular resection and reconstruction.
“Improvement of survival outcomes may be achieved with concomitant advanced vascular reconstruction in carefully selected patients,” Dr. Fujitani said. “There are multiple options for vascular reconstruction for mesenteric portal venous and visceral arterial involvement using standard vascular surgical techniques.” He added that a dedicated team of experienced surgical oncologists and vascular surgeons for these reconstructions “is essential for successful outcomes.”
Dr. Fujitani had no relevant financial relationships to disclose.
CHICAGO – More than 53,000 people will develop pancreatic ductal adenocarcinoma in the United States this year, and upwards of 41,000 will die from the disease, many of them with tumors considered unresectable because they involve adjacent vessels. However, researchers at the University of California, Irvine, have found that careful removal of the tumor around involved veins and arteries, even in borderline cases, can improve outcomes for these patients.
Roy M. Fujitani, MD, updated previously published data on a single-center study he coauthored in 2015 of 270 patients who had undergone a Whipple operation, 183 for pancreatic adenocarcinoma (J Vasc Surg. 2015;61:475-80) at a symposium on vascular surgery sponsored by Northwestern University.
Resection of pancreatic tumors without vascular involvement is fairly straightforward for surgical oncologists to perform, Dr. Fujitani said, but pancreatic tumors enter the borderline resectable category when preoperative CT scan shows portal vein abutment, for which vascular surgery should provide counsel and assist. However, even in some cases when preoperative CT scan shows unresectable, locally advanced pancreatic tumor with celiac artery encasement, neoadjuvant therapy may downstage the disease into the borderline category, he said.
“Patients with borderline resectable or stage II disease are those one should consider for reconstruction,” Dr. Fujitani said. Resectable findings of borderline disease include encasement of the portal vein, superior mesenteric vein and the confluence of the portal venous system (with suitable proximal and distal targets for reconstruction); and less-than-circumferential involvement of the common hepatic artery or right hepatic artery – but without involvement of the superior mesenteric artery or the celiac axis and “certainly not” the aorta. “This would account for about one-fourth of patients in high-volume centers as being able to receive concomitant vascular reconstruction,” Dr. Fujitani said.
In the UCI series, 60 patients with borderline lesions underwent vascular reconstruction. “As it turned out, there was no significant difference in survival between the reconstruction group and the nonreconstruction group,” Dr. Fujitani said, “but it’s important to note that these patients who had the reconstruction would never have been operated on if we were not able to do the reconstruction.” Thirty-day mortality was around 5% and 1-year survival around 70% in both groups, he said. However, at about 1.5 years the Kaplan-Meier survival curves between the two groups diverged, which Dr. Fujitani attributed to more advanced disease in the reconstruction group.
“We found lymph node status and tumor margins were most important in determining survival of these patients,” he said. “Gaining an R0 resection is the most important thing that determines favorable survivability.”
Dr. Fujitani also reviewed different techniques for vascular reconstruction, and while differences in complication rates or 1-, 2-, or 3-year survival were not statistically significant, he did note that mean survival with lateral venorrhaphy exceeded that of primary anastomosis and interposition graft – 21 months vs. 13 months vs. 4 months, suggesting the merits of a more aggressive approach to vascular resection and reconstruction.
“Improvement of survival outcomes may be achieved with concomitant advanced vascular reconstruction in carefully selected patients,” Dr. Fujitani said. “There are multiple options for vascular reconstruction for mesenteric portal venous and visceral arterial involvement using standard vascular surgical techniques.” He added that a dedicated team of experienced surgical oncologists and vascular surgeons for these reconstructions “is essential for successful outcomes.”
Dr. Fujitani had no relevant financial relationships to disclose.
CHICAGO – More than 53,000 people will develop pancreatic ductal adenocarcinoma in the United States this year, and upwards of 41,000 will die from the disease, many of them with tumors considered unresectable because they involve adjacent vessels. However, researchers at the University of California, Irvine, have found that careful removal of the tumor around involved veins and arteries, even in borderline cases, can improve outcomes for these patients.
Roy M. Fujitani, MD, updated previously published data on a single-center study he coauthored in 2015 of 270 patients who had undergone a Whipple operation, 183 for pancreatic adenocarcinoma (J Vasc Surg. 2015;61:475-80) at a symposium on vascular surgery sponsored by Northwestern University.
Resection of pancreatic tumors without vascular involvement is fairly straightforward for surgical oncologists to perform, Dr. Fujitani said, but pancreatic tumors enter the borderline resectable category when preoperative CT scan shows portal vein abutment, for which vascular surgery should provide counsel and assist. However, even in some cases when preoperative CT scan shows unresectable, locally advanced pancreatic tumor with celiac artery encasement, neoadjuvant therapy may downstage the disease into the borderline category, he said.
“Patients with borderline resectable or stage II disease are those one should consider for reconstruction,” Dr. Fujitani said. Resectable findings of borderline disease include encasement of the portal vein, superior mesenteric vein and the confluence of the portal venous system (with suitable proximal and distal targets for reconstruction); and less-than-circumferential involvement of the common hepatic artery or right hepatic artery – but without involvement of the superior mesenteric artery or the celiac axis and “certainly not” the aorta. “This would account for about one-fourth of patients in high-volume centers as being able to receive concomitant vascular reconstruction,” Dr. Fujitani said.
In the UCI series, 60 patients with borderline lesions underwent vascular reconstruction. “As it turned out, there was no significant difference in survival between the reconstruction group and the nonreconstruction group,” Dr. Fujitani said, “but it’s important to note that these patients who had the reconstruction would never have been operated on if we were not able to do the reconstruction.” Thirty-day mortality was around 5% and 1-year survival around 70% in both groups, he said. However, at about 1.5 years the Kaplan-Meier survival curves between the two groups diverged, which Dr. Fujitani attributed to more advanced disease in the reconstruction group.
“We found lymph node status and tumor margins were most important in determining survival of these patients,” he said. “Gaining an R0 resection is the most important thing that determines favorable survivability.”
Dr. Fujitani also reviewed different techniques for vascular reconstruction, and while differences in complication rates or 1-, 2-, or 3-year survival were not statistically significant, he did note that mean survival with lateral venorrhaphy exceeded that of primary anastomosis and interposition graft – 21 months vs. 13 months vs. 4 months, suggesting the merits of a more aggressive approach to vascular resection and reconstruction.
“Improvement of survival outcomes may be achieved with concomitant advanced vascular reconstruction in carefully selected patients,” Dr. Fujitani said. “There are multiple options for vascular reconstruction for mesenteric portal venous and visceral arterial involvement using standard vascular surgical techniques.” He added that a dedicated team of experienced surgical oncologists and vascular surgeons for these reconstructions “is essential for successful outcomes.”
Dr. Fujitani had no relevant financial relationships to disclose.
AT THE NORTHWESTERN VASCULAR SYMPOSIUM
Key clinical point: A more aggressive vascular resection and reconstruction in pancreatic cancer may improve outcomes and palliation in these patients.
Major finding: Mean survival with lateral venorrhaphy exceeded primary anastomosis and interposition graft (21 months vs. 13 months vs. 4 months).
Data source: Updated data of previously published single-center retrospective review of 183 patients who had Whipple procedure for pancreatic adenocarcinoma.
Disclosures: Dr. Fujitani reported having no financial disclosures.
Effect of PCSK9 Inhibitors on Coronary Artery Disease Progression
Study Overview
Objective. To determine if evolocumab, a PCSK9 inhibitor, affects the progression of coronary artery disease in patients treated with statins.
Design. Multicenter, international, double-blind, placebo-controlled, randomized clinical trial.
Setting and participants. 197 community and academic hospitals worldwide enrolled 978 participants who underwent serial intravascular ultrasounds (IVUS) to measure their burden of coronary atherosclerosis. A total of 2628 patients were screened. Patients were considered for inclusion if they were 18 years of age or older and had at least 1 coronary artery stenosis of at least 20% on a clinically indicated catheterization. Additionally, the target vessel had to meet IVUS imaging quality and visibility standards. Participants were required to have been on stable statin therapy for at least 4 weeks with an LDL level of > 80 mg/dL or between 60–80 mg/dL with either 1 major or 3 minor cardiovascular risk factors. Major risk factors were noncoronary atherosclerotic disease, myocardial infarction (MI) or hospitalization for unstable angina within the past 2 years, or type 2 diabetes. Minor risk factors included current tobacco use, hypertension, low HDL-C levels, family history of early coronary disease, hsCRP level of 2 mg/L or greater, and age older than 50 years for men and 55 years for women. Patients with uncontrolled hypertension, uncontrolled diabetes, heart failure, renal insufficiency, or liver disease were excluded.
Intervention. Patients were randomized to either treatment with monthly subcutaneous injections of 420 mg evolocumab or placebo injections for 76 weeks. Participants attended 7 follow-up visits during the study period and then underwent repeat IVUS imaging at the 78th week. Research staff, who were blinded to both treatment status and imaging sequence, collected and assessed target vessel measurements, including the vessel lumen and external elastic membrane dimensions. IVUS imaging has been used in numerous clinical studies and has been shown to be accurate and reliable [1].
Main outcome measures. The primary outcome was the target artery change in percent atheroma volume (PAV) from baseline to week 78. PAV was calculated from IVUS measurements. Nominal change in PAV was then determined by calculating the difference of the PAV at baseline and at week 78.
The secondary measure was the normalized total atheroma volume (TAV). TAV addresses variability in the length of vessel segments and the number of images collected during IVUS catheter pullback. The nominal change in TAV was then determined by the difference at baseline and at week 78.
Additional secondary efficacy endpoints included number of patients with regression of plaque and change in lipid parameters. Safety outcomes were investigated through evaluation of the incidence of adjudicated clinical events, including all-cause mortality, cardiovascular death, MI, unstable angina requiring hospitalization, coronary revascularization, stroke, transient ischemic attack, and heart failure requiring hospitalization. Post-hoc analysis compared baseline LDL-C level and change in PAV and regression of PAV. The association between LDL lowering and plaque progression was also assessed post hoc.
IVUS measurements were evaluated as least squares means. Comparison of treatment groups was conducted using analysis of covariance on rank transformed data that accounted for baseline value and geographic location. Investigators used a step-down statistical procedure to evaluate primary and secondary endpoints. The statistical model accounted for confounders such as baseline LDL-C, baseline PAV, intensity of statin therapy, geographic region, age, and sex.
Main results. 484 participants were randomized to the evolocumab group and 484 to the placebo group, and 423 participants in both groups completed both baseline and follow-up IVUS imaging. Treatment and control groups contained participants matched for age, gender, ethnicity, cardiovascular risk factors, and baseline medication use, including lipid-lowering agents, ACE inhibitors, ARBs, beta-blockers, and antiplatelet therapies. Both groups consisted of a majority of white (93.4% in placebo and 94.2% in treatment) males (72.3% in placebo and 72.1% in treatment). Approximately 80% of participants had hypertension (83.7% in placebo and 82.2% in treatment), about 35% had prior MIs (35.3% in placebo and 34.9% in treatment), and roughly a fifth of participants had diabetes (21.5% in placebo and 20.2% in treatment). At baseline 98.6% of participants were treated with statins, with 58.9% on high-intensity therapy and 39.4% on moderate-intensity. Mean LDL-C level at baseline was 92.5 (SD, 27.2) mg/dL.
After 76 weeks of treatment, mean LDL-C level in the placebo group was 93.0 mg/dL and 36.6 mg/dL in the treatment group, which corresponds to a 0.2 mg/dL increase in the placebo group and a 56.3 mg/dL reduction in the treatment group. The change in LDL-C level was statistically significant (P < 0.001).
Placebo group participants had no significant change in PAV (0.05%, P = 0.78), but the evolocumab group experienced a 0.95% decrease from baseline (P < 0.001). Similarly, the placebo group had no change in TAV from baseline (–0.9 mm3, P = 0.45), but the treatment group had a 5.8 mm3 reduction in TAV from baseline (P < 0.001). The treatment group had a greater proportion of patients who experienced PAV regression (64.3% vs. 47.3%, P < 0.001) and TAV regression (61.5% vs. 48.9%, P < 0.001).
Subgroup analysis did not demonstrate a significant association between change in PAV and specific study participant characteristics (eg, age, gender, ethnicity).
Post-hoc analysis using local regression (LOESS) curve revealed a linear relationship between achieved LDL-C level and change in PAV for LDL-C levels from 110 mg/dL to 20 mg/dL.
The treatment group did not exhibit a significant increase in adverse drug events, which included injection site reactions, myalgias, neurocognitive events, and incidence of diabetes. There was no significant difference in adverse cardiovascular outcomes between groups; however, there were numerically fewer nonfatal MIs and coronary revascularizations in the treatment group.
Conclusion. The use of evolocumab in statin-treated patients resulted in greater reduction of PAV than use of statins alone.
Commentary
Evolocumab is a monoclonal antibody that inhibits pro-protein convertase subtilisin-kexin type 9 (PCSK9), which is involved in LDL-C receptor recycling. By reducing removal of LDL-C receptors, evolocumab amplifies LDL-C clearance and has been shown to reduce LDL-C levels by approximately 61% from baseline with 12 weeks oftreatment [2]. Studies have shown that the lipid-lowering potential of evolocumab is superior to statins alone and to combination therapy with statins and ezetimibe [2]. Furthermore, PCSK9 inhibitors have been effective at LDL-lowering in patients who failed or could not tolerate standard of care therapy with statins and ezetimibe [3,4]. PCSK9 inhibitors hold great promise for reducing morbidity and mortality of cardiovascular disease; however, LDL-lowering is not equivalent to improved clinical outcomes.
The GLAGOV study moves toward demonstration of the clinical benefit of evolocumab. The study shows that combined therapy with statins and evolocumab, versus statins alone, not only achieves better stability of atherosclerotic plaque dimensions but actually results in regression of plaque size. In the study, plaque burden is extrapolated from vessel measurements obtained through IVUS, and nominal changes in PAV and TAV serve as markers for atherosclerosis, but these surrogates cannot be equated to a reduction in cardiovascular events. The GLAGOV trial does explore clinical outcomes such as MI, stroke, unstable angina, coronary revascularization, and death; however, the study is not powered to evaluate the statistical significance of these events. We await sufficiently powered phase 3 clinical trials to determine the clinical benefits of PCSK9 inhibitors on cardiovascular disease.
The GLAGOV trial has several strengths, including its design as an international, double-blind, placebo-controlled, randomized clinical trial. The intervention is simple and the outcomes are clearly defined. The statistical assessment yields significant results. Nonetheless, there are multiple limitations to the study. The lead author has received research support from Amgen, the maker of evolocumab. Amgen also participated in study design and maintenance of trial databases; however, data analysis was conducted by an independent statistician. Additionally, the majority of study participants were white males with very few minority patients despite inclusion of study sites around the globe. The homogeneity of the study cohort makes the data difficult to generalize to a larger population. Similarly, patients who lacked a clinical indication for coronary catheterization and those with uncontrolled diabetes, hypertension, and heart failure were excluded, which further limits application of this study to many patients with atherosclerosis. Another limitation is study attrition; only 87% of participants completed the 78-week IVUS and were included in the data analysis, and results may have differed if those lost to follow-up had completed the trial. Furthermore, study duration was limited to 76 weeks and the magnitude and durability of study outcomes after this time point remain unknown.
Applications for Clinical Practice
Reduction in PAV and TAV are surrogate endpoints and are not indicative of a clinical benefit. Nonetheless, the GLAGOV study demonstrates that evolocumab, when used in conjunction with statins, can promote regression of atherosclerosis greater than treatment with statins alone. More studies are needed to evaluate a clinical benefit of adding evolocumab to the regularly used arsenal of lipid-lowering therapies for the treatment of atherosclerosis. Furthermore, cost-effectiveness of evolocumab has not been shown. In 2015 the yearly wholesale price of evolcumab was $14,350. A cost-effectiveness analysis based on this price estimates that treatment of atherosclerotic coronary vascular disease with evolocumab has a cost of $414,000 per quality-adjusted life year [5]. Evolocumab is well tolerated, but additional studies for cardiovascular and mortality outcomes are needed before it can be considered part of the standard of treatment for coronary artery disease.
—Lauren Brooks, MD, University of Maryland School of Medicine, Baltimore, MD
1. Nicholls SJ, Hsu A, Wolski K, et al. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol 2010;55:2399–407.
2. Sabatine MS, Giugliano RP, Wiviolt SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1500–9.
3. Giugliano RP, Sabatine MS. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field. J Am Coll Cardiol 2015;65:2639–51.
4. Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol 2014;63:2541–8.
5. Dhruv KS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic coronary artery disease. JAMA 2016;316:743–53.
Study Overview
Objective. To determine if evolocumab, a PCSK9 inhibitor, affects the progression of coronary artery disease in patients treated with statins.
Design. Multicenter, international, double-blind, placebo-controlled, randomized clinical trial.
Setting and participants. 197 community and academic hospitals worldwide enrolled 978 participants who underwent serial intravascular ultrasounds (IVUS) to measure their burden of coronary atherosclerosis. A total of 2628 patients were screened. Patients were considered for inclusion if they were 18 years of age or older and had at least 1 coronary artery stenosis of at least 20% on a clinically indicated catheterization. Additionally, the target vessel had to meet IVUS imaging quality and visibility standards. Participants were required to have been on stable statin therapy for at least 4 weeks with an LDL level of > 80 mg/dL or between 60–80 mg/dL with either 1 major or 3 minor cardiovascular risk factors. Major risk factors were noncoronary atherosclerotic disease, myocardial infarction (MI) or hospitalization for unstable angina within the past 2 years, or type 2 diabetes. Minor risk factors included current tobacco use, hypertension, low HDL-C levels, family history of early coronary disease, hsCRP level of 2 mg/L or greater, and age older than 50 years for men and 55 years for women. Patients with uncontrolled hypertension, uncontrolled diabetes, heart failure, renal insufficiency, or liver disease were excluded.
Intervention. Patients were randomized to either treatment with monthly subcutaneous injections of 420 mg evolocumab or placebo injections for 76 weeks. Participants attended 7 follow-up visits during the study period and then underwent repeat IVUS imaging at the 78th week. Research staff, who were blinded to both treatment status and imaging sequence, collected and assessed target vessel measurements, including the vessel lumen and external elastic membrane dimensions. IVUS imaging has been used in numerous clinical studies and has been shown to be accurate and reliable [1].
Main outcome measures. The primary outcome was the target artery change in percent atheroma volume (PAV) from baseline to week 78. PAV was calculated from IVUS measurements. Nominal change in PAV was then determined by calculating the difference of the PAV at baseline and at week 78.
The secondary measure was the normalized total atheroma volume (TAV). TAV addresses variability in the length of vessel segments and the number of images collected during IVUS catheter pullback. The nominal change in TAV was then determined by the difference at baseline and at week 78.
Additional secondary efficacy endpoints included number of patients with regression of plaque and change in lipid parameters. Safety outcomes were investigated through evaluation of the incidence of adjudicated clinical events, including all-cause mortality, cardiovascular death, MI, unstable angina requiring hospitalization, coronary revascularization, stroke, transient ischemic attack, and heart failure requiring hospitalization. Post-hoc analysis compared baseline LDL-C level and change in PAV and regression of PAV. The association between LDL lowering and plaque progression was also assessed post hoc.
IVUS measurements were evaluated as least squares means. Comparison of treatment groups was conducted using analysis of covariance on rank transformed data that accounted for baseline value and geographic location. Investigators used a step-down statistical procedure to evaluate primary and secondary endpoints. The statistical model accounted for confounders such as baseline LDL-C, baseline PAV, intensity of statin therapy, geographic region, age, and sex.
Main results. 484 participants were randomized to the evolocumab group and 484 to the placebo group, and 423 participants in both groups completed both baseline and follow-up IVUS imaging. Treatment and control groups contained participants matched for age, gender, ethnicity, cardiovascular risk factors, and baseline medication use, including lipid-lowering agents, ACE inhibitors, ARBs, beta-blockers, and antiplatelet therapies. Both groups consisted of a majority of white (93.4% in placebo and 94.2% in treatment) males (72.3% in placebo and 72.1% in treatment). Approximately 80% of participants had hypertension (83.7% in placebo and 82.2% in treatment), about 35% had prior MIs (35.3% in placebo and 34.9% in treatment), and roughly a fifth of participants had diabetes (21.5% in placebo and 20.2% in treatment). At baseline 98.6% of participants were treated with statins, with 58.9% on high-intensity therapy and 39.4% on moderate-intensity. Mean LDL-C level at baseline was 92.5 (SD, 27.2) mg/dL.
After 76 weeks of treatment, mean LDL-C level in the placebo group was 93.0 mg/dL and 36.6 mg/dL in the treatment group, which corresponds to a 0.2 mg/dL increase in the placebo group and a 56.3 mg/dL reduction in the treatment group. The change in LDL-C level was statistically significant (P < 0.001).
Placebo group participants had no significant change in PAV (0.05%, P = 0.78), but the evolocumab group experienced a 0.95% decrease from baseline (P < 0.001). Similarly, the placebo group had no change in TAV from baseline (–0.9 mm3, P = 0.45), but the treatment group had a 5.8 mm3 reduction in TAV from baseline (P < 0.001). The treatment group had a greater proportion of patients who experienced PAV regression (64.3% vs. 47.3%, P < 0.001) and TAV regression (61.5% vs. 48.9%, P < 0.001).
Subgroup analysis did not demonstrate a significant association between change in PAV and specific study participant characteristics (eg, age, gender, ethnicity).
Post-hoc analysis using local regression (LOESS) curve revealed a linear relationship between achieved LDL-C level and change in PAV for LDL-C levels from 110 mg/dL to 20 mg/dL.
The treatment group did not exhibit a significant increase in adverse drug events, which included injection site reactions, myalgias, neurocognitive events, and incidence of diabetes. There was no significant difference in adverse cardiovascular outcomes between groups; however, there were numerically fewer nonfatal MIs and coronary revascularizations in the treatment group.
Conclusion. The use of evolocumab in statin-treated patients resulted in greater reduction of PAV than use of statins alone.
Commentary
Evolocumab is a monoclonal antibody that inhibits pro-protein convertase subtilisin-kexin type 9 (PCSK9), which is involved in LDL-C receptor recycling. By reducing removal of LDL-C receptors, evolocumab amplifies LDL-C clearance and has been shown to reduce LDL-C levels by approximately 61% from baseline with 12 weeks oftreatment [2]. Studies have shown that the lipid-lowering potential of evolocumab is superior to statins alone and to combination therapy with statins and ezetimibe [2]. Furthermore, PCSK9 inhibitors have been effective at LDL-lowering in patients who failed or could not tolerate standard of care therapy with statins and ezetimibe [3,4]. PCSK9 inhibitors hold great promise for reducing morbidity and mortality of cardiovascular disease; however, LDL-lowering is not equivalent to improved clinical outcomes.
The GLAGOV study moves toward demonstration of the clinical benefit of evolocumab. The study shows that combined therapy with statins and evolocumab, versus statins alone, not only achieves better stability of atherosclerotic plaque dimensions but actually results in regression of plaque size. In the study, plaque burden is extrapolated from vessel measurements obtained through IVUS, and nominal changes in PAV and TAV serve as markers for atherosclerosis, but these surrogates cannot be equated to a reduction in cardiovascular events. The GLAGOV trial does explore clinical outcomes such as MI, stroke, unstable angina, coronary revascularization, and death; however, the study is not powered to evaluate the statistical significance of these events. We await sufficiently powered phase 3 clinical trials to determine the clinical benefits of PCSK9 inhibitors on cardiovascular disease.
The GLAGOV trial has several strengths, including its design as an international, double-blind, placebo-controlled, randomized clinical trial. The intervention is simple and the outcomes are clearly defined. The statistical assessment yields significant results. Nonetheless, there are multiple limitations to the study. The lead author has received research support from Amgen, the maker of evolocumab. Amgen also participated in study design and maintenance of trial databases; however, data analysis was conducted by an independent statistician. Additionally, the majority of study participants were white males with very few minority patients despite inclusion of study sites around the globe. The homogeneity of the study cohort makes the data difficult to generalize to a larger population. Similarly, patients who lacked a clinical indication for coronary catheterization and those with uncontrolled diabetes, hypertension, and heart failure were excluded, which further limits application of this study to many patients with atherosclerosis. Another limitation is study attrition; only 87% of participants completed the 78-week IVUS and were included in the data analysis, and results may have differed if those lost to follow-up had completed the trial. Furthermore, study duration was limited to 76 weeks and the magnitude and durability of study outcomes after this time point remain unknown.
Applications for Clinical Practice
Reduction in PAV and TAV are surrogate endpoints and are not indicative of a clinical benefit. Nonetheless, the GLAGOV study demonstrates that evolocumab, when used in conjunction with statins, can promote regression of atherosclerosis greater than treatment with statins alone. More studies are needed to evaluate a clinical benefit of adding evolocumab to the regularly used arsenal of lipid-lowering therapies for the treatment of atherosclerosis. Furthermore, cost-effectiveness of evolocumab has not been shown. In 2015 the yearly wholesale price of evolcumab was $14,350. A cost-effectiveness analysis based on this price estimates that treatment of atherosclerotic coronary vascular disease with evolocumab has a cost of $414,000 per quality-adjusted life year [5]. Evolocumab is well tolerated, but additional studies for cardiovascular and mortality outcomes are needed before it can be considered part of the standard of treatment for coronary artery disease.
—Lauren Brooks, MD, University of Maryland School of Medicine, Baltimore, MD
Study Overview
Objective. To determine if evolocumab, a PCSK9 inhibitor, affects the progression of coronary artery disease in patients treated with statins.
Design. Multicenter, international, double-blind, placebo-controlled, randomized clinical trial.
Setting and participants. 197 community and academic hospitals worldwide enrolled 978 participants who underwent serial intravascular ultrasounds (IVUS) to measure their burden of coronary atherosclerosis. A total of 2628 patients were screened. Patients were considered for inclusion if they were 18 years of age or older and had at least 1 coronary artery stenosis of at least 20% on a clinically indicated catheterization. Additionally, the target vessel had to meet IVUS imaging quality and visibility standards. Participants were required to have been on stable statin therapy for at least 4 weeks with an LDL level of > 80 mg/dL or between 60–80 mg/dL with either 1 major or 3 minor cardiovascular risk factors. Major risk factors were noncoronary atherosclerotic disease, myocardial infarction (MI) or hospitalization for unstable angina within the past 2 years, or type 2 diabetes. Minor risk factors included current tobacco use, hypertension, low HDL-C levels, family history of early coronary disease, hsCRP level of 2 mg/L or greater, and age older than 50 years for men and 55 years for women. Patients with uncontrolled hypertension, uncontrolled diabetes, heart failure, renal insufficiency, or liver disease were excluded.
Intervention. Patients were randomized to either treatment with monthly subcutaneous injections of 420 mg evolocumab or placebo injections for 76 weeks. Participants attended 7 follow-up visits during the study period and then underwent repeat IVUS imaging at the 78th week. Research staff, who were blinded to both treatment status and imaging sequence, collected and assessed target vessel measurements, including the vessel lumen and external elastic membrane dimensions. IVUS imaging has been used in numerous clinical studies and has been shown to be accurate and reliable [1].
Main outcome measures. The primary outcome was the target artery change in percent atheroma volume (PAV) from baseline to week 78. PAV was calculated from IVUS measurements. Nominal change in PAV was then determined by calculating the difference of the PAV at baseline and at week 78.
The secondary measure was the normalized total atheroma volume (TAV). TAV addresses variability in the length of vessel segments and the number of images collected during IVUS catheter pullback. The nominal change in TAV was then determined by the difference at baseline and at week 78.
Additional secondary efficacy endpoints included number of patients with regression of plaque and change in lipid parameters. Safety outcomes were investigated through evaluation of the incidence of adjudicated clinical events, including all-cause mortality, cardiovascular death, MI, unstable angina requiring hospitalization, coronary revascularization, stroke, transient ischemic attack, and heart failure requiring hospitalization. Post-hoc analysis compared baseline LDL-C level and change in PAV and regression of PAV. The association between LDL lowering and plaque progression was also assessed post hoc.
IVUS measurements were evaluated as least squares means. Comparison of treatment groups was conducted using analysis of covariance on rank transformed data that accounted for baseline value and geographic location. Investigators used a step-down statistical procedure to evaluate primary and secondary endpoints. The statistical model accounted for confounders such as baseline LDL-C, baseline PAV, intensity of statin therapy, geographic region, age, and sex.
Main results. 484 participants were randomized to the evolocumab group and 484 to the placebo group, and 423 participants in both groups completed both baseline and follow-up IVUS imaging. Treatment and control groups contained participants matched for age, gender, ethnicity, cardiovascular risk factors, and baseline medication use, including lipid-lowering agents, ACE inhibitors, ARBs, beta-blockers, and antiplatelet therapies. Both groups consisted of a majority of white (93.4% in placebo and 94.2% in treatment) males (72.3% in placebo and 72.1% in treatment). Approximately 80% of participants had hypertension (83.7% in placebo and 82.2% in treatment), about 35% had prior MIs (35.3% in placebo and 34.9% in treatment), and roughly a fifth of participants had diabetes (21.5% in placebo and 20.2% in treatment). At baseline 98.6% of participants were treated with statins, with 58.9% on high-intensity therapy and 39.4% on moderate-intensity. Mean LDL-C level at baseline was 92.5 (SD, 27.2) mg/dL.
After 76 weeks of treatment, mean LDL-C level in the placebo group was 93.0 mg/dL and 36.6 mg/dL in the treatment group, which corresponds to a 0.2 mg/dL increase in the placebo group and a 56.3 mg/dL reduction in the treatment group. The change in LDL-C level was statistically significant (P < 0.001).
Placebo group participants had no significant change in PAV (0.05%, P = 0.78), but the evolocumab group experienced a 0.95% decrease from baseline (P < 0.001). Similarly, the placebo group had no change in TAV from baseline (–0.9 mm3, P = 0.45), but the treatment group had a 5.8 mm3 reduction in TAV from baseline (P < 0.001). The treatment group had a greater proportion of patients who experienced PAV regression (64.3% vs. 47.3%, P < 0.001) and TAV regression (61.5% vs. 48.9%, P < 0.001).
Subgroup analysis did not demonstrate a significant association between change in PAV and specific study participant characteristics (eg, age, gender, ethnicity).
Post-hoc analysis using local regression (LOESS) curve revealed a linear relationship between achieved LDL-C level and change in PAV for LDL-C levels from 110 mg/dL to 20 mg/dL.
The treatment group did not exhibit a significant increase in adverse drug events, which included injection site reactions, myalgias, neurocognitive events, and incidence of diabetes. There was no significant difference in adverse cardiovascular outcomes between groups; however, there were numerically fewer nonfatal MIs and coronary revascularizations in the treatment group.
Conclusion. The use of evolocumab in statin-treated patients resulted in greater reduction of PAV than use of statins alone.
Commentary
Evolocumab is a monoclonal antibody that inhibits pro-protein convertase subtilisin-kexin type 9 (PCSK9), which is involved in LDL-C receptor recycling. By reducing removal of LDL-C receptors, evolocumab amplifies LDL-C clearance and has been shown to reduce LDL-C levels by approximately 61% from baseline with 12 weeks oftreatment [2]. Studies have shown that the lipid-lowering potential of evolocumab is superior to statins alone and to combination therapy with statins and ezetimibe [2]. Furthermore, PCSK9 inhibitors have been effective at LDL-lowering in patients who failed or could not tolerate standard of care therapy with statins and ezetimibe [3,4]. PCSK9 inhibitors hold great promise for reducing morbidity and mortality of cardiovascular disease; however, LDL-lowering is not equivalent to improved clinical outcomes.
The GLAGOV study moves toward demonstration of the clinical benefit of evolocumab. The study shows that combined therapy with statins and evolocumab, versus statins alone, not only achieves better stability of atherosclerotic plaque dimensions but actually results in regression of plaque size. In the study, plaque burden is extrapolated from vessel measurements obtained through IVUS, and nominal changes in PAV and TAV serve as markers for atherosclerosis, but these surrogates cannot be equated to a reduction in cardiovascular events. The GLAGOV trial does explore clinical outcomes such as MI, stroke, unstable angina, coronary revascularization, and death; however, the study is not powered to evaluate the statistical significance of these events. We await sufficiently powered phase 3 clinical trials to determine the clinical benefits of PCSK9 inhibitors on cardiovascular disease.
The GLAGOV trial has several strengths, including its design as an international, double-blind, placebo-controlled, randomized clinical trial. The intervention is simple and the outcomes are clearly defined. The statistical assessment yields significant results. Nonetheless, there are multiple limitations to the study. The lead author has received research support from Amgen, the maker of evolocumab. Amgen also participated in study design and maintenance of trial databases; however, data analysis was conducted by an independent statistician. Additionally, the majority of study participants were white males with very few minority patients despite inclusion of study sites around the globe. The homogeneity of the study cohort makes the data difficult to generalize to a larger population. Similarly, patients who lacked a clinical indication for coronary catheterization and those with uncontrolled diabetes, hypertension, and heart failure were excluded, which further limits application of this study to many patients with atherosclerosis. Another limitation is study attrition; only 87% of participants completed the 78-week IVUS and were included in the data analysis, and results may have differed if those lost to follow-up had completed the trial. Furthermore, study duration was limited to 76 weeks and the magnitude and durability of study outcomes after this time point remain unknown.
Applications for Clinical Practice
Reduction in PAV and TAV are surrogate endpoints and are not indicative of a clinical benefit. Nonetheless, the GLAGOV study demonstrates that evolocumab, when used in conjunction with statins, can promote regression of atherosclerosis greater than treatment with statins alone. More studies are needed to evaluate a clinical benefit of adding evolocumab to the regularly used arsenal of lipid-lowering therapies for the treatment of atherosclerosis. Furthermore, cost-effectiveness of evolocumab has not been shown. In 2015 the yearly wholesale price of evolcumab was $14,350. A cost-effectiveness analysis based on this price estimates that treatment of atherosclerotic coronary vascular disease with evolocumab has a cost of $414,000 per quality-adjusted life year [5]. Evolocumab is well tolerated, but additional studies for cardiovascular and mortality outcomes are needed before it can be considered part of the standard of treatment for coronary artery disease.
—Lauren Brooks, MD, University of Maryland School of Medicine, Baltimore, MD
1. Nicholls SJ, Hsu A, Wolski K, et al. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol 2010;55:2399–407.
2. Sabatine MS, Giugliano RP, Wiviolt SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1500–9.
3. Giugliano RP, Sabatine MS. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field. J Am Coll Cardiol 2015;65:2639–51.
4. Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol 2014;63:2541–8.
5. Dhruv KS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic coronary artery disease. JAMA 2016;316:743–53.
1. Nicholls SJ, Hsu A, Wolski K, et al. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol 2010;55:2399–407.
2. Sabatine MS, Giugliano RP, Wiviolt SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1500–9.
3. Giugliano RP, Sabatine MS. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field. J Am Coll Cardiol 2015;65:2639–51.
4. Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol 2014;63:2541–8.
5. Dhruv KS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic coronary artery disease. JAMA 2016;316:743–53.
Adjuvant GEMOX disappoints for localized biliary tract cancer
SAN FRANCISCO – Post-surgery adjuvant treatment with gemcitabine and oxaliplatin (GEMOX) was feasible, but failed to significantly improve relapse-free survival when compared with surveillance among patients with localized biliary tract cancer in the randomized phase III PRODIGE 12-ACCORD 18 (UNICANCER GI) trial.
Relapse-free survival at a median of 44.3 months in 196 patients who were randomized within 3 months of R0 or R1 resection of a localized biliary tract cancer to receive either GEMOX for 12 cycles or surveillance was 30.4 months vs. 22.0 months in the groups, respectively. At 4 years, relapse-free survival was 39.3% and 33.2%, respectively, Julien Edeline, MD, of Oncology Medical Eugene Marquis Comprehensive Cancer Center, Rennes, France, reported at the symposium, sponsored by ASCO, ASTRO, the American Gastroenterological Association, and Society of Surgical Oncology.
No difference was seen between the groups with respect to the co-primary endpoint of 12- and 24-month global health-related quality of life scores (70.8 vs. 83.3, and 75.0 vs. 83.3, respectively), he said.
Study subjects had localized intra-hepatic, perihilar, or extra-hepatic cholangiocarcinoma, or gallbladder cancer and were enrolled from 33 centers between July 2009 and February 2014. They had ECOG performance status of 0-2, and adequate liver function. The treatment and surveillance arms were well balanced, with similar primary disease sites, Dr. Edeline said.
Those in the GEMOX arm received 12 cycles (6 months) of gemcitabine 1,000 mg/m2 on day 1 and oxaliplatin 85 mg/m2 on day 2. Those in the surveillance arm underwent ACE, CA19.9 testing, and CT scans every 3 months for 2 years then every 6 months for 3 years.
In the treatment and surveillance arms, respectively, R0 resection rates were 86.2% and 87.9%, and lymph node invasion was present in 37.2% and 36.4%.
The maximal grade of adverse events was 3 in 57.5% vs. 22.2% of patients in the groups, respectively, and grade 4 in 17.0% vs. 9.1%. One patient in each arm died during treatment.
The main grade 3 or greater adverse events were peripheral neuropathy in 50.0% vs. 1.1% and neutropenia in 22.3% vs. 0% for GEMOX vs. surveillance group patients.
“As you know, there is a high risk of relapse following surgery for localized biliary tract cancer. There is currently no proven adjuvant or neoadjuvant treatment,” Dr. Edeline said. “In the palliative setting, we know that the combination of gemcitabine and cisplatin improves overall survival. GEMOX is considered an active regimen based on data from phase II trials. At the time of the design of our study, GEMOX was considered the standard first line treatment for biliary tract cancer.”
Based on this background, the aim of the current phase III trial was to assess whether GEMOX would increase relapse-free survival while maintaining health-related quality of life in patients with localized disease.
“We showed that adjuvant GEMOX was feasible. Toxicities were as expected and manageable, and we didn’t see detrimental effects on quality of life. However, adjuvant GEMOX was not associated in the PRODIGE 12 trial with an improvement in relapse-free survival,” he said, noting that this was also true in subgroup analyses, which showed no benefit of GEMOX with respect to relapse-free survival in any predefined subgroups.
“Clearly, further research through international collaboration is required to improve outcomes in these patients,” he concluded.
Dr. Edeline reported having no disclosures.
SAN FRANCISCO – Post-surgery adjuvant treatment with gemcitabine and oxaliplatin (GEMOX) was feasible, but failed to significantly improve relapse-free survival when compared with surveillance among patients with localized biliary tract cancer in the randomized phase III PRODIGE 12-ACCORD 18 (UNICANCER GI) trial.
Relapse-free survival at a median of 44.3 months in 196 patients who were randomized within 3 months of R0 or R1 resection of a localized biliary tract cancer to receive either GEMOX for 12 cycles or surveillance was 30.4 months vs. 22.0 months in the groups, respectively. At 4 years, relapse-free survival was 39.3% and 33.2%, respectively, Julien Edeline, MD, of Oncology Medical Eugene Marquis Comprehensive Cancer Center, Rennes, France, reported at the symposium, sponsored by ASCO, ASTRO, the American Gastroenterological Association, and Society of Surgical Oncology.
No difference was seen between the groups with respect to the co-primary endpoint of 12- and 24-month global health-related quality of life scores (70.8 vs. 83.3, and 75.0 vs. 83.3, respectively), he said.
Study subjects had localized intra-hepatic, perihilar, or extra-hepatic cholangiocarcinoma, or gallbladder cancer and were enrolled from 33 centers between July 2009 and February 2014. They had ECOG performance status of 0-2, and adequate liver function. The treatment and surveillance arms were well balanced, with similar primary disease sites, Dr. Edeline said.
Those in the GEMOX arm received 12 cycles (6 months) of gemcitabine 1,000 mg/m2 on day 1 and oxaliplatin 85 mg/m2 on day 2. Those in the surveillance arm underwent ACE, CA19.9 testing, and CT scans every 3 months for 2 years then every 6 months for 3 years.
In the treatment and surveillance arms, respectively, R0 resection rates were 86.2% and 87.9%, and lymph node invasion was present in 37.2% and 36.4%.
The maximal grade of adverse events was 3 in 57.5% vs. 22.2% of patients in the groups, respectively, and grade 4 in 17.0% vs. 9.1%. One patient in each arm died during treatment.
The main grade 3 or greater adverse events were peripheral neuropathy in 50.0% vs. 1.1% and neutropenia in 22.3% vs. 0% for GEMOX vs. surveillance group patients.
“As you know, there is a high risk of relapse following surgery for localized biliary tract cancer. There is currently no proven adjuvant or neoadjuvant treatment,” Dr. Edeline said. “In the palliative setting, we know that the combination of gemcitabine and cisplatin improves overall survival. GEMOX is considered an active regimen based on data from phase II trials. At the time of the design of our study, GEMOX was considered the standard first line treatment for biliary tract cancer.”
Based on this background, the aim of the current phase III trial was to assess whether GEMOX would increase relapse-free survival while maintaining health-related quality of life in patients with localized disease.
“We showed that adjuvant GEMOX was feasible. Toxicities were as expected and manageable, and we didn’t see detrimental effects on quality of life. However, adjuvant GEMOX was not associated in the PRODIGE 12 trial with an improvement in relapse-free survival,” he said, noting that this was also true in subgroup analyses, which showed no benefit of GEMOX with respect to relapse-free survival in any predefined subgroups.
“Clearly, further research through international collaboration is required to improve outcomes in these patients,” he concluded.
Dr. Edeline reported having no disclosures.
SAN FRANCISCO – Post-surgery adjuvant treatment with gemcitabine and oxaliplatin (GEMOX) was feasible, but failed to significantly improve relapse-free survival when compared with surveillance among patients with localized biliary tract cancer in the randomized phase III PRODIGE 12-ACCORD 18 (UNICANCER GI) trial.
Relapse-free survival at a median of 44.3 months in 196 patients who were randomized within 3 months of R0 or R1 resection of a localized biliary tract cancer to receive either GEMOX for 12 cycles or surveillance was 30.4 months vs. 22.0 months in the groups, respectively. At 4 years, relapse-free survival was 39.3% and 33.2%, respectively, Julien Edeline, MD, of Oncology Medical Eugene Marquis Comprehensive Cancer Center, Rennes, France, reported at the symposium, sponsored by ASCO, ASTRO, the American Gastroenterological Association, and Society of Surgical Oncology.
No difference was seen between the groups with respect to the co-primary endpoint of 12- and 24-month global health-related quality of life scores (70.8 vs. 83.3, and 75.0 vs. 83.3, respectively), he said.
Study subjects had localized intra-hepatic, perihilar, or extra-hepatic cholangiocarcinoma, or gallbladder cancer and were enrolled from 33 centers between July 2009 and February 2014. They had ECOG performance status of 0-2, and adequate liver function. The treatment and surveillance arms were well balanced, with similar primary disease sites, Dr. Edeline said.
Those in the GEMOX arm received 12 cycles (6 months) of gemcitabine 1,000 mg/m2 on day 1 and oxaliplatin 85 mg/m2 on day 2. Those in the surveillance arm underwent ACE, CA19.9 testing, and CT scans every 3 months for 2 years then every 6 months for 3 years.
In the treatment and surveillance arms, respectively, R0 resection rates were 86.2% and 87.9%, and lymph node invasion was present in 37.2% and 36.4%.
The maximal grade of adverse events was 3 in 57.5% vs. 22.2% of patients in the groups, respectively, and grade 4 in 17.0% vs. 9.1%. One patient in each arm died during treatment.
The main grade 3 or greater adverse events were peripheral neuropathy in 50.0% vs. 1.1% and neutropenia in 22.3% vs. 0% for GEMOX vs. surveillance group patients.
“As you know, there is a high risk of relapse following surgery for localized biliary tract cancer. There is currently no proven adjuvant or neoadjuvant treatment,” Dr. Edeline said. “In the palliative setting, we know that the combination of gemcitabine and cisplatin improves overall survival. GEMOX is considered an active regimen based on data from phase II trials. At the time of the design of our study, GEMOX was considered the standard first line treatment for biliary tract cancer.”
Based on this background, the aim of the current phase III trial was to assess whether GEMOX would increase relapse-free survival while maintaining health-related quality of life in patients with localized disease.
“We showed that adjuvant GEMOX was feasible. Toxicities were as expected and manageable, and we didn’t see detrimental effects on quality of life. However, adjuvant GEMOX was not associated in the PRODIGE 12 trial with an improvement in relapse-free survival,” he said, noting that this was also true in subgroup analyses, which showed no benefit of GEMOX with respect to relapse-free survival in any predefined subgroups.
“Clearly, further research through international collaboration is required to improve outcomes in these patients,” he concluded.
Dr. Edeline reported having no disclosures.
AT THE 2017 GASTROINTESTINAL CANCERS SYMPOSIUM
Key clinical point:
Major finding: Relapse-free survival at 4 years was 39.3% and 33.2% with GEMOX and surveillance, respectively.
Data source: The randomized, phase III PRODIGE 12-ACCORD 18 trial.
Disclosures: Dr. Edeline reported having no disclosures.