User login
Group proposes new prognostic model for PTCL-NOS

T-cell Lymphoma Forum
Photo by Larry Young
SAN FRANCISCO—Researchers have used data from the T-Cell Project (TCP) to create a prognostic model for peripheral T-cell lymphoma not otherwise specified (PTCL-NOS).
Analyses have suggested the TCP model is more accurate for PTCL-NOS than 4 other prognostic models—the International Prognostic Index (IPI), the Prognostic Index for T-cell Lymphoma (PIT), the International Peripheral T-cell Lymphoma Project score (IPTCLP), and the modified PIT (mPIT).
Massimo Federico, MD, of the University of Modena and Reggio Emilia in Italy, described the TCP model at the 9th Annual T-cell Lymphoma Forum.
Creating the model
TCP is a prospective registry that includes data from T-cell lymphoma patients in 15 countries located in 5 different regions of the world. As of December 31, 2016, 1523 cases of T-cell lymphoma have been registered with TCP.
Dr Federico and his colleagues used these data to create their prognostic model. There were 311 patients with PTCL-NOS who had adequate data for analysis. The 5-year overall survival (OS) for these patients was 36%.
The researchers chose 13 variables from the literature that have been reported to have a prognostic impact on survival in PTCL-NOS:
- Age > 60
- Lactate dehydrogenase > upper limit of normal
- Albumin < 3.5 g/dL
- Hemoglobin < 12 g/dL
- Platelets < 150/mm3
- Lymphocyte to monocyte ratio ≤ 2.1
- Neutrophil to lymphocyte ratio > 6.5
- Absolute neutrophil count (ANC) > 6.5/mm3
- ECOG performance status > 1
- Stage III-IV disease
- B symptoms
- Extra nodal sites > 1
- Male gender.
In univariate analysis, nearly all of these factors were significantly associated with OS in the cohort of TCP patients. (The 2 exceptions were age older than 60 and having more than 1 extranodal site.)
However, Dr Federico and his colleagues said the factors with the greatest prognostic impact were:
- ECOG performance status > 1, with a hazard ratio (HR) of 2.12 (P<0.001)
- Albumin < 3.5 g/dL, with an HR of 2.03 (P<0.001)
- ANC > 6.5/mm3, with an HR of 1.85 (P<0.001)
- Stage III-IV disease, with an HR of 1.74 (P=0.010).
So the researchers used these factors in their model, which has 3 risk categories.
Risk categories
Patients were considered low-risk if they had 0 of the 4 risk factors. These patients had a 3-year OS of 76% and a 5-year OS of 69%.
Patients were considered intermediate-risk if they had 1 to 2 risk factors. These patients had a 3-year OS of 43% and a 5-year OS of 31%. Compared to low-risk patients, the HR was 3.08 (P<0.001).
Patients were considered high-risk if they had 3 to 4 risk factors. The 3-year OS was 11% for these patients, and the 5-year OS was 8%.
The HR was 8.88 (P<0.001) for high-risk compared to low-risk patients and 2.88 (P<0.001) for high-risk compared to intermediate-risk patients.
Validation
The researchers tested the TCP model in a validation cohort of 98 patients from the COMPLETE registry. As with the training cohort of TCP patients, the model revealed 3 different risk groups (in terms of OS) in the validation cohort.
Dr Federico noted that there were no significant differences between the training and validation cohorts, except when it came to follow-up. The median follow-up was 46 months in the TCP group and 18 months in the COMPLETE group.
The researchers also found the TCP could classify patients into 3 different risk groups according to progression-free survival.
Comparison
Finally, Dr Federico and his colleagues compared the TCP model to the IPI, PIT, IPTCLP, and mPIT models using 208 patients.
“The discriminant power of the proposed model is superior to the others in terms of all of the statistical tests we adopted,” Dr Federico said.
| Model | c-Harrell*
(95% CI) |
D-Royston
(SE) |
R2 | AIC (95% CI) | AUC,
3-year OS |
| TCP | 0.666 (0.618-0.713) | 1.152 (0.191) | 0.31 (0.14-0.46) | 983 | 0.714 |
| PIT | 0.614 (0.563-0.664) | 0.750 (0.195) | 0.15 (0.06-0.31) | 1004 | 0.696 |
| IPI | 0.645 (0.594-0.696) | 0.883 (0.191) | 0.22 (0.08-0.38) | 987 | 0.704 |
| IPITCLP | 0.606 (0.549-0.663) | 0.631 (0.188) | 0.12 (0.03-0.28) | 1006 | 0.704 |
| mPIT | 0.640 (0.586-0.694) | 0.762 (0.170) | 0.16 (0.05-0.33) | 999 | 0.681 |
In closing, Dr Federico said the TCP model clearly defines risk groups in PTCL-NOS and identifies patients with relatively good prognosis.
However, there is a need for emerging biologic variables to be tested for prognostic value and included in prognostic tools to allow for better risk stratification. ![]()
*c-Harrel: Harrell’s concordance index, 95% CI: confidence interval, D-Royston: Royston/Sauerbrei’s D statistic (Stat Med 2004 Mar 15, 23[5]:723-48), SE: standard error, R2: explained randomness, AIC: Akaike information criterion, AUC: area under the curve (according to Heagerty et al, Biometrics, 2000 Jun, 56[2]:337-44).

T-cell Lymphoma Forum
Photo by Larry Young
SAN FRANCISCO—Researchers have used data from the T-Cell Project (TCP) to create a prognostic model for peripheral T-cell lymphoma not otherwise specified (PTCL-NOS).
Analyses have suggested the TCP model is more accurate for PTCL-NOS than 4 other prognostic models—the International Prognostic Index (IPI), the Prognostic Index for T-cell Lymphoma (PIT), the International Peripheral T-cell Lymphoma Project score (IPTCLP), and the modified PIT (mPIT).
Massimo Federico, MD, of the University of Modena and Reggio Emilia in Italy, described the TCP model at the 9th Annual T-cell Lymphoma Forum.
Creating the model
TCP is a prospective registry that includes data from T-cell lymphoma patients in 15 countries located in 5 different regions of the world. As of December 31, 2016, 1523 cases of T-cell lymphoma have been registered with TCP.
Dr Federico and his colleagues used these data to create their prognostic model. There were 311 patients with PTCL-NOS who had adequate data for analysis. The 5-year overall survival (OS) for these patients was 36%.
The researchers chose 13 variables from the literature that have been reported to have a prognostic impact on survival in PTCL-NOS:
- Age > 60
- Lactate dehydrogenase > upper limit of normal
- Albumin < 3.5 g/dL
- Hemoglobin < 12 g/dL
- Platelets < 150/mm3
- Lymphocyte to monocyte ratio ≤ 2.1
- Neutrophil to lymphocyte ratio > 6.5
- Absolute neutrophil count (ANC) > 6.5/mm3
- ECOG performance status > 1
- Stage III-IV disease
- B symptoms
- Extra nodal sites > 1
- Male gender.
In univariate analysis, nearly all of these factors were significantly associated with OS in the cohort of TCP patients. (The 2 exceptions were age older than 60 and having more than 1 extranodal site.)
However, Dr Federico and his colleagues said the factors with the greatest prognostic impact were:
- ECOG performance status > 1, with a hazard ratio (HR) of 2.12 (P<0.001)
- Albumin < 3.5 g/dL, with an HR of 2.03 (P<0.001)
- ANC > 6.5/mm3, with an HR of 1.85 (P<0.001)
- Stage III-IV disease, with an HR of 1.74 (P=0.010).
So the researchers used these factors in their model, which has 3 risk categories.
Risk categories
Patients were considered low-risk if they had 0 of the 4 risk factors. These patients had a 3-year OS of 76% and a 5-year OS of 69%.
Patients were considered intermediate-risk if they had 1 to 2 risk factors. These patients had a 3-year OS of 43% and a 5-year OS of 31%. Compared to low-risk patients, the HR was 3.08 (P<0.001).
Patients were considered high-risk if they had 3 to 4 risk factors. The 3-year OS was 11% for these patients, and the 5-year OS was 8%.
The HR was 8.88 (P<0.001) for high-risk compared to low-risk patients and 2.88 (P<0.001) for high-risk compared to intermediate-risk patients.
Validation
The researchers tested the TCP model in a validation cohort of 98 patients from the COMPLETE registry. As with the training cohort of TCP patients, the model revealed 3 different risk groups (in terms of OS) in the validation cohort.
Dr Federico noted that there were no significant differences between the training and validation cohorts, except when it came to follow-up. The median follow-up was 46 months in the TCP group and 18 months in the COMPLETE group.
The researchers also found the TCP could classify patients into 3 different risk groups according to progression-free survival.
Comparison
Finally, Dr Federico and his colleagues compared the TCP model to the IPI, PIT, IPTCLP, and mPIT models using 208 patients.
“The discriminant power of the proposed model is superior to the others in terms of all of the statistical tests we adopted,” Dr Federico said.
| Model | c-Harrell*
(95% CI) |
D-Royston
(SE) |
R2 | AIC (95% CI) | AUC,
3-year OS |
| TCP | 0.666 (0.618-0.713) | 1.152 (0.191) | 0.31 (0.14-0.46) | 983 | 0.714 |
| PIT | 0.614 (0.563-0.664) | 0.750 (0.195) | 0.15 (0.06-0.31) | 1004 | 0.696 |
| IPI | 0.645 (0.594-0.696) | 0.883 (0.191) | 0.22 (0.08-0.38) | 987 | 0.704 |
| IPITCLP | 0.606 (0.549-0.663) | 0.631 (0.188) | 0.12 (0.03-0.28) | 1006 | 0.704 |
| mPIT | 0.640 (0.586-0.694) | 0.762 (0.170) | 0.16 (0.05-0.33) | 999 | 0.681 |
In closing, Dr Federico said the TCP model clearly defines risk groups in PTCL-NOS and identifies patients with relatively good prognosis.
However, there is a need for emerging biologic variables to be tested for prognostic value and included in prognostic tools to allow for better risk stratification. ![]()
*c-Harrel: Harrell’s concordance index, 95% CI: confidence interval, D-Royston: Royston/Sauerbrei’s D statistic (Stat Med 2004 Mar 15, 23[5]:723-48), SE: standard error, R2: explained randomness, AIC: Akaike information criterion, AUC: area under the curve (according to Heagerty et al, Biometrics, 2000 Jun, 56[2]:337-44).

T-cell Lymphoma Forum
Photo by Larry Young
SAN FRANCISCO—Researchers have used data from the T-Cell Project (TCP) to create a prognostic model for peripheral T-cell lymphoma not otherwise specified (PTCL-NOS).
Analyses have suggested the TCP model is more accurate for PTCL-NOS than 4 other prognostic models—the International Prognostic Index (IPI), the Prognostic Index for T-cell Lymphoma (PIT), the International Peripheral T-cell Lymphoma Project score (IPTCLP), and the modified PIT (mPIT).
Massimo Federico, MD, of the University of Modena and Reggio Emilia in Italy, described the TCP model at the 9th Annual T-cell Lymphoma Forum.
Creating the model
TCP is a prospective registry that includes data from T-cell lymphoma patients in 15 countries located in 5 different regions of the world. As of December 31, 2016, 1523 cases of T-cell lymphoma have been registered with TCP.
Dr Federico and his colleagues used these data to create their prognostic model. There were 311 patients with PTCL-NOS who had adequate data for analysis. The 5-year overall survival (OS) for these patients was 36%.
The researchers chose 13 variables from the literature that have been reported to have a prognostic impact on survival in PTCL-NOS:
- Age > 60
- Lactate dehydrogenase > upper limit of normal
- Albumin < 3.5 g/dL
- Hemoglobin < 12 g/dL
- Platelets < 150/mm3
- Lymphocyte to monocyte ratio ≤ 2.1
- Neutrophil to lymphocyte ratio > 6.5
- Absolute neutrophil count (ANC) > 6.5/mm3
- ECOG performance status > 1
- Stage III-IV disease
- B symptoms
- Extra nodal sites > 1
- Male gender.
In univariate analysis, nearly all of these factors were significantly associated with OS in the cohort of TCP patients. (The 2 exceptions were age older than 60 and having more than 1 extranodal site.)
However, Dr Federico and his colleagues said the factors with the greatest prognostic impact were:
- ECOG performance status > 1, with a hazard ratio (HR) of 2.12 (P<0.001)
- Albumin < 3.5 g/dL, with an HR of 2.03 (P<0.001)
- ANC > 6.5/mm3, with an HR of 1.85 (P<0.001)
- Stage III-IV disease, with an HR of 1.74 (P=0.010).
So the researchers used these factors in their model, which has 3 risk categories.
Risk categories
Patients were considered low-risk if they had 0 of the 4 risk factors. These patients had a 3-year OS of 76% and a 5-year OS of 69%.
Patients were considered intermediate-risk if they had 1 to 2 risk factors. These patients had a 3-year OS of 43% and a 5-year OS of 31%. Compared to low-risk patients, the HR was 3.08 (P<0.001).
Patients were considered high-risk if they had 3 to 4 risk factors. The 3-year OS was 11% for these patients, and the 5-year OS was 8%.
The HR was 8.88 (P<0.001) for high-risk compared to low-risk patients and 2.88 (P<0.001) for high-risk compared to intermediate-risk patients.
Validation
The researchers tested the TCP model in a validation cohort of 98 patients from the COMPLETE registry. As with the training cohort of TCP patients, the model revealed 3 different risk groups (in terms of OS) in the validation cohort.
Dr Federico noted that there were no significant differences between the training and validation cohorts, except when it came to follow-up. The median follow-up was 46 months in the TCP group and 18 months in the COMPLETE group.
The researchers also found the TCP could classify patients into 3 different risk groups according to progression-free survival.
Comparison
Finally, Dr Federico and his colleagues compared the TCP model to the IPI, PIT, IPTCLP, and mPIT models using 208 patients.
“The discriminant power of the proposed model is superior to the others in terms of all of the statistical tests we adopted,” Dr Federico said.
| Model | c-Harrell*
(95% CI) |
D-Royston
(SE) |
R2 | AIC (95% CI) | AUC,
3-year OS |
| TCP | 0.666 (0.618-0.713) | 1.152 (0.191) | 0.31 (0.14-0.46) | 983 | 0.714 |
| PIT | 0.614 (0.563-0.664) | 0.750 (0.195) | 0.15 (0.06-0.31) | 1004 | 0.696 |
| IPI | 0.645 (0.594-0.696) | 0.883 (0.191) | 0.22 (0.08-0.38) | 987 | 0.704 |
| IPITCLP | 0.606 (0.549-0.663) | 0.631 (0.188) | 0.12 (0.03-0.28) | 1006 | 0.704 |
| mPIT | 0.640 (0.586-0.694) | 0.762 (0.170) | 0.16 (0.05-0.33) | 999 | 0.681 |
In closing, Dr Federico said the TCP model clearly defines risk groups in PTCL-NOS and identifies patients with relatively good prognosis.
However, there is a need for emerging biologic variables to be tested for prognostic value and included in prognostic tools to allow for better risk stratification. ![]()
*c-Harrel: Harrell’s concordance index, 95% CI: confidence interval, D-Royston: Royston/Sauerbrei’s D statistic (Stat Med 2004 Mar 15, 23[5]:723-48), SE: standard error, R2: explained randomness, AIC: Akaike information criterion, AUC: area under the curve (according to Heagerty et al, Biometrics, 2000 Jun, 56[2]:337-44).
Project provides insight into T-cell lymphoma

Photo by Larry Young
SAN FRANCISCO—The T-Cell Project has provided information that can enhance our understanding of T-cell lymphomas, according to a presentation at the 9th Annual T-cell Lymphoma Forum.
The project is a prospective registry that includes data from T-cell lymphoma patients in 15 countries located in 5 different regions of the world.
The data showed that peripheral T-cell lymphoma not otherwise specified (PTCL-NOS) is the most common subtype of T-cell lymphoma in all 5 regions, although the distribution of other subtypes varies.
A majority of patients in the registry received chemotherapy as induction, and anthracycline-containing regimens were the most popular treatment choice.
Although 60% of patients in the registry had low-risk or low/intermediate-risk disease, progression-free survival (PFS) and overall survival (OS) rates were low. The 5-year PFS was 32%, and the 5-year OS was 42%.
Massimo Federico, MD, of the University of Modena and Reggio Emilia in Italy, presented these data at the meeting.
About the project
Dr Federico said the goals of the T-Cell Project are to determine if prospective data collection provides more accurate information to better define prognosis of the most frequent subtypes of T-cell lymphoma and to improve our knowledge of clinical and biological characteristics, as well as outcomes, of the more uncommon subtypes.
“Why did we choose to propose a prospective registry for the collection of information in T-cell lymphoma?” Dr Federico asked. “Because it is, by far, less expensive than a clinical trial, but also because it can offer excellent data for generating new research programs and is a great opportunity for academic cooperation.”
As of December 31, 2016, the registry included 1523 patients. There were 75 sites (with at least 1 patient) active in the registry.
Fifteen countries in 5 geographic regions were represented. Europe was the greatest contributor (44%), followed by North America (US only, 25%), South America (20%), the Far East (9%), the Middle East (2%), and Oceania (<1%).
Subtypes
Overall, the distribution of the different T-cell lymphoma subtypes is as follows:
PTCL-NOS—36%
Angioimmunoblastic T-cell lymphoma (AITL)—17%
ALK- anaplastic large-cell lymphoma (ALCL)—16%
NK/T-cell lymphoma (NKTCL)—11%
ALK+ ALCL—8%
Enteropathy-associated T-cell lymphoma—4%
Unclassifiable T-cell lymphoma—3%
Hepatosplenic T-cell lymphoma—2%
Subcutaneous panniculitis-like T-cell lymphoma—2%
Peripheral gamma delta T-cell lymphoma—1%
Geographic distribution
The most common T-cell lymphoma subtypes in Europe were PTCL-NOS (37%), AITL (21%), and ALK- ALCL (14%). Likewise, the most common subtypes in the US were PTCL-NOS (35%), AITL (21%), and ALK- ALCL (13%).
In the Middle East, the most common subtypes were PTCL-NOS (40%), AITL (16%), and ALK+ ALCL (13%). In South America, they were PTCL-NOS (41%), ALK- ALCL (26%), and NKTCL (10%). And in Asia, they were PTCL-NOS (29%), NKTCL (29%), and AITL (17%).
Patient characteristics
Dr Federico presented data on patient characteristics for 1391 individuals, validated as of April 30, 2016.
The patients’ median age was 56 (range, 18-89). Forty-four percent were 60 or older, and 60% were male. Twenty-six percent had ECOG performance status > 1, 50% had B symptoms, and 72% had disease-related discomfort.
Sixty percent had low-risk or low/intermediate-risk disease according to the International Prognostic Index (IPI) and the Prognostic Index for T-cell Lymphoma (PIT).
Treatment
Treatment details are available for 1022 patients. Ninety-two percent received therapy with curative intent.
For induction, 76% of patients received chemotherapy alone, 14% received chemotherapy and radiotherapy, 8% received best supportive care, and 2% received radiotherapy alone.
Seventy-one percent of patients who received chemotherapy had an anthracycline-containing regimen, 13% received etoposide-containing chemotherapy, 9% received chemotherapy containing an anthracycline and etoposide, and 7% of patients received other therapy.
Thirteen percent of patients received a transplant as salvage treatment, and 7% received a transplant as consolidation.
Outcomes
Data on patient responses to initial treatment were available for 888 individuals. The 84 patients who received best supportive care were not included, and 50 patients were not evaluable for response.
The complete response/unconfirmed complete response rate was 53%, and the partial response rate was 19%. Twenty-eight percent of patients had no response or progressed.
The median PFS was 16 months. The 5-year PFS rate was 32% overall, 23% for PTCL-NOS, 28% for AITL, 39% for ALK- ALCL, and 57% for ALK+ ALCL.
The median OS was 36 months. The 5-year OS was 42% overall, 34% for PTCL-NOS, 42% for AITL, 46% for ALK- ALCL, and 76% for ALK+ ALCL.
Dr Federico and his colleagues have used these data to develop a prognostic model for PTCL-NOS that, they say, is more accurate than current models. ![]()

Photo by Larry Young
SAN FRANCISCO—The T-Cell Project has provided information that can enhance our understanding of T-cell lymphomas, according to a presentation at the 9th Annual T-cell Lymphoma Forum.
The project is a prospective registry that includes data from T-cell lymphoma patients in 15 countries located in 5 different regions of the world.
The data showed that peripheral T-cell lymphoma not otherwise specified (PTCL-NOS) is the most common subtype of T-cell lymphoma in all 5 regions, although the distribution of other subtypes varies.
A majority of patients in the registry received chemotherapy as induction, and anthracycline-containing regimens were the most popular treatment choice.
Although 60% of patients in the registry had low-risk or low/intermediate-risk disease, progression-free survival (PFS) and overall survival (OS) rates were low. The 5-year PFS was 32%, and the 5-year OS was 42%.
Massimo Federico, MD, of the University of Modena and Reggio Emilia in Italy, presented these data at the meeting.
About the project
Dr Federico said the goals of the T-Cell Project are to determine if prospective data collection provides more accurate information to better define prognosis of the most frequent subtypes of T-cell lymphoma and to improve our knowledge of clinical and biological characteristics, as well as outcomes, of the more uncommon subtypes.
“Why did we choose to propose a prospective registry for the collection of information in T-cell lymphoma?” Dr Federico asked. “Because it is, by far, less expensive than a clinical trial, but also because it can offer excellent data for generating new research programs and is a great opportunity for academic cooperation.”
As of December 31, 2016, the registry included 1523 patients. There were 75 sites (with at least 1 patient) active in the registry.
Fifteen countries in 5 geographic regions were represented. Europe was the greatest contributor (44%), followed by North America (US only, 25%), South America (20%), the Far East (9%), the Middle East (2%), and Oceania (<1%).
Subtypes
Overall, the distribution of the different T-cell lymphoma subtypes is as follows:
PTCL-NOS—36%
Angioimmunoblastic T-cell lymphoma (AITL)—17%
ALK- anaplastic large-cell lymphoma (ALCL)—16%
NK/T-cell lymphoma (NKTCL)—11%
ALK+ ALCL—8%
Enteropathy-associated T-cell lymphoma—4%
Unclassifiable T-cell lymphoma—3%
Hepatosplenic T-cell lymphoma—2%
Subcutaneous panniculitis-like T-cell lymphoma—2%
Peripheral gamma delta T-cell lymphoma—1%
Geographic distribution
The most common T-cell lymphoma subtypes in Europe were PTCL-NOS (37%), AITL (21%), and ALK- ALCL (14%). Likewise, the most common subtypes in the US were PTCL-NOS (35%), AITL (21%), and ALK- ALCL (13%).
In the Middle East, the most common subtypes were PTCL-NOS (40%), AITL (16%), and ALK+ ALCL (13%). In South America, they were PTCL-NOS (41%), ALK- ALCL (26%), and NKTCL (10%). And in Asia, they were PTCL-NOS (29%), NKTCL (29%), and AITL (17%).
Patient characteristics
Dr Federico presented data on patient characteristics for 1391 individuals, validated as of April 30, 2016.
The patients’ median age was 56 (range, 18-89). Forty-four percent were 60 or older, and 60% were male. Twenty-six percent had ECOG performance status > 1, 50% had B symptoms, and 72% had disease-related discomfort.
Sixty percent had low-risk or low/intermediate-risk disease according to the International Prognostic Index (IPI) and the Prognostic Index for T-cell Lymphoma (PIT).
Treatment
Treatment details are available for 1022 patients. Ninety-two percent received therapy with curative intent.
For induction, 76% of patients received chemotherapy alone, 14% received chemotherapy and radiotherapy, 8% received best supportive care, and 2% received radiotherapy alone.
Seventy-one percent of patients who received chemotherapy had an anthracycline-containing regimen, 13% received etoposide-containing chemotherapy, 9% received chemotherapy containing an anthracycline and etoposide, and 7% of patients received other therapy.
Thirteen percent of patients received a transplant as salvage treatment, and 7% received a transplant as consolidation.
Outcomes
Data on patient responses to initial treatment were available for 888 individuals. The 84 patients who received best supportive care were not included, and 50 patients were not evaluable for response.
The complete response/unconfirmed complete response rate was 53%, and the partial response rate was 19%. Twenty-eight percent of patients had no response or progressed.
The median PFS was 16 months. The 5-year PFS rate was 32% overall, 23% for PTCL-NOS, 28% for AITL, 39% for ALK- ALCL, and 57% for ALK+ ALCL.
The median OS was 36 months. The 5-year OS was 42% overall, 34% for PTCL-NOS, 42% for AITL, 46% for ALK- ALCL, and 76% for ALK+ ALCL.
Dr Federico and his colleagues have used these data to develop a prognostic model for PTCL-NOS that, they say, is more accurate than current models. ![]()

Photo by Larry Young
SAN FRANCISCO—The T-Cell Project has provided information that can enhance our understanding of T-cell lymphomas, according to a presentation at the 9th Annual T-cell Lymphoma Forum.
The project is a prospective registry that includes data from T-cell lymphoma patients in 15 countries located in 5 different regions of the world.
The data showed that peripheral T-cell lymphoma not otherwise specified (PTCL-NOS) is the most common subtype of T-cell lymphoma in all 5 regions, although the distribution of other subtypes varies.
A majority of patients in the registry received chemotherapy as induction, and anthracycline-containing regimens were the most popular treatment choice.
Although 60% of patients in the registry had low-risk or low/intermediate-risk disease, progression-free survival (PFS) and overall survival (OS) rates were low. The 5-year PFS was 32%, and the 5-year OS was 42%.
Massimo Federico, MD, of the University of Modena and Reggio Emilia in Italy, presented these data at the meeting.
About the project
Dr Federico said the goals of the T-Cell Project are to determine if prospective data collection provides more accurate information to better define prognosis of the most frequent subtypes of T-cell lymphoma and to improve our knowledge of clinical and biological characteristics, as well as outcomes, of the more uncommon subtypes.
“Why did we choose to propose a prospective registry for the collection of information in T-cell lymphoma?” Dr Federico asked. “Because it is, by far, less expensive than a clinical trial, but also because it can offer excellent data for generating new research programs and is a great opportunity for academic cooperation.”
As of December 31, 2016, the registry included 1523 patients. There were 75 sites (with at least 1 patient) active in the registry.
Fifteen countries in 5 geographic regions were represented. Europe was the greatest contributor (44%), followed by North America (US only, 25%), South America (20%), the Far East (9%), the Middle East (2%), and Oceania (<1%).
Subtypes
Overall, the distribution of the different T-cell lymphoma subtypes is as follows:
PTCL-NOS—36%
Angioimmunoblastic T-cell lymphoma (AITL)—17%
ALK- anaplastic large-cell lymphoma (ALCL)—16%
NK/T-cell lymphoma (NKTCL)—11%
ALK+ ALCL—8%
Enteropathy-associated T-cell lymphoma—4%
Unclassifiable T-cell lymphoma—3%
Hepatosplenic T-cell lymphoma—2%
Subcutaneous panniculitis-like T-cell lymphoma—2%
Peripheral gamma delta T-cell lymphoma—1%
Geographic distribution
The most common T-cell lymphoma subtypes in Europe were PTCL-NOS (37%), AITL (21%), and ALK- ALCL (14%). Likewise, the most common subtypes in the US were PTCL-NOS (35%), AITL (21%), and ALK- ALCL (13%).
In the Middle East, the most common subtypes were PTCL-NOS (40%), AITL (16%), and ALK+ ALCL (13%). In South America, they were PTCL-NOS (41%), ALK- ALCL (26%), and NKTCL (10%). And in Asia, they were PTCL-NOS (29%), NKTCL (29%), and AITL (17%).
Patient characteristics
Dr Federico presented data on patient characteristics for 1391 individuals, validated as of April 30, 2016.
The patients’ median age was 56 (range, 18-89). Forty-four percent were 60 or older, and 60% were male. Twenty-six percent had ECOG performance status > 1, 50% had B symptoms, and 72% had disease-related discomfort.
Sixty percent had low-risk or low/intermediate-risk disease according to the International Prognostic Index (IPI) and the Prognostic Index for T-cell Lymphoma (PIT).
Treatment
Treatment details are available for 1022 patients. Ninety-two percent received therapy with curative intent.
For induction, 76% of patients received chemotherapy alone, 14% received chemotherapy and radiotherapy, 8% received best supportive care, and 2% received radiotherapy alone.
Seventy-one percent of patients who received chemotherapy had an anthracycline-containing regimen, 13% received etoposide-containing chemotherapy, 9% received chemotherapy containing an anthracycline and etoposide, and 7% of patients received other therapy.
Thirteen percent of patients received a transplant as salvage treatment, and 7% received a transplant as consolidation.
Outcomes
Data on patient responses to initial treatment were available for 888 individuals. The 84 patients who received best supportive care were not included, and 50 patients were not evaluable for response.
The complete response/unconfirmed complete response rate was 53%, and the partial response rate was 19%. Twenty-eight percent of patients had no response or progressed.
The median PFS was 16 months. The 5-year PFS rate was 32% overall, 23% for PTCL-NOS, 28% for AITL, 39% for ALK- ALCL, and 57% for ALK+ ALCL.
The median OS was 36 months. The 5-year OS was 42% overall, 34% for PTCL-NOS, 42% for AITL, 46% for ALK- ALCL, and 76% for ALK+ ALCL.
Dr Federico and his colleagues have used these data to develop a prognostic model for PTCL-NOS that, they say, is more accurate than current models. ![]()
Recent price hikes for generic cancer meds exceed 100%

Photo by Steven Harbour
AMSTERDAM—The UK has seen substantial price increases for some generic cancer drugs over the last few years, according to a study presented at ECCO 2017: European Cancer Congress (abstract 966).
Of the 89 drugs analyzed in this study, 21 of them—including 17 generics—had price increases from 2011 to 2016.
Fourteen of the generic cancer drugs had price increases over 100%, and 2 of the drugs had increases exceeding 1000%.
“We were surprised to find several companies consistently raising the prices of cancer treatment,” said study investigator Andrew Hill, PhD, of the University of Liverpool in the UK.
“Twenty treatments have shown rises of over 100% in the last 5 years, and in 2—busulfan (used to treat leukemia) and tamoxifen (breast cancer)—prices have increased by over 1000%. We have found that some companies take over the supply of some generic cancer medicines and then raise the price progressively.”
Dr Hill and his co-investigator Melissa Barber, of the London School of Hygiene and Tropical Medicine in the UK, analyzed prices for 190 formulations of 89 cancer drugs.
Twenty-eight formulations of 21 drugs had price increases from 2011 to 2016. Seventeen of these 21 drugs were generic in 2016.
Twenty formulations of 14 generic cancer drugs had price increases exceeding 100%.
For example, the cost per tablet or injection increased for:
- Ifosfamide (2 g vial)—from £89 to £180, or 103%.
- Melphalan (50 mg vial)—from £33 to £137, or 315%.
- Chlorambucil (2 mg)—from £0.33 to £1.62, or 390%.
- Cyclophosphamide (50 mg)—from £0.20 to £1.39, or 695%.
- Busulfan (2 mg)—from £0.21 to £2.61, or 1227%.
Dr Hill said the UK’s Department of Health is aware of this issue and has introduced the Health Services Medical Supplies (Costs) Bill to enable price regulation in the future.
Companies found to be raising prices with no clear justification will be referred to the Competition and Markets Authority, and they could face fines.
However, Dr Hill and Barber said they found large price increases for generic cancer drugs in other European countries as well.
In Spain and Italy, failure to accept the high prices demanded for some generic drugs has led to warnings from companies that they could stop the supply of these drugs.
For instance, Italy fined the generic company Aspen €5 million after a 1500% increase in the price of cancer drugs, including melphalan and chlorambucil. Aspen then threatened Italy with drug shortages unless higher prices were accepted.
In Spain, Aspen demanded a 4000% increase in melphalan prices.
“We hope that, by explaining what we have found in the UK, other European countries will take note and protect themselves against these kinds of price rises,” Dr Hill said. “At a time when cancer patients are living longer and better lives due to effective treatments, this situation is particularly worrying.” ![]()

Photo by Steven Harbour
AMSTERDAM—The UK has seen substantial price increases for some generic cancer drugs over the last few years, according to a study presented at ECCO 2017: European Cancer Congress (abstract 966).
Of the 89 drugs analyzed in this study, 21 of them—including 17 generics—had price increases from 2011 to 2016.
Fourteen of the generic cancer drugs had price increases over 100%, and 2 of the drugs had increases exceeding 1000%.
“We were surprised to find several companies consistently raising the prices of cancer treatment,” said study investigator Andrew Hill, PhD, of the University of Liverpool in the UK.
“Twenty treatments have shown rises of over 100% in the last 5 years, and in 2—busulfan (used to treat leukemia) and tamoxifen (breast cancer)—prices have increased by over 1000%. We have found that some companies take over the supply of some generic cancer medicines and then raise the price progressively.”
Dr Hill and his co-investigator Melissa Barber, of the London School of Hygiene and Tropical Medicine in the UK, analyzed prices for 190 formulations of 89 cancer drugs.
Twenty-eight formulations of 21 drugs had price increases from 2011 to 2016. Seventeen of these 21 drugs were generic in 2016.
Twenty formulations of 14 generic cancer drugs had price increases exceeding 100%.
For example, the cost per tablet or injection increased for:
- Ifosfamide (2 g vial)—from £89 to £180, or 103%.
- Melphalan (50 mg vial)—from £33 to £137, or 315%.
- Chlorambucil (2 mg)—from £0.33 to £1.62, or 390%.
- Cyclophosphamide (50 mg)—from £0.20 to £1.39, or 695%.
- Busulfan (2 mg)—from £0.21 to £2.61, or 1227%.
Dr Hill said the UK’s Department of Health is aware of this issue and has introduced the Health Services Medical Supplies (Costs) Bill to enable price regulation in the future.
Companies found to be raising prices with no clear justification will be referred to the Competition and Markets Authority, and they could face fines.
However, Dr Hill and Barber said they found large price increases for generic cancer drugs in other European countries as well.
In Spain and Italy, failure to accept the high prices demanded for some generic drugs has led to warnings from companies that they could stop the supply of these drugs.
For instance, Italy fined the generic company Aspen €5 million after a 1500% increase in the price of cancer drugs, including melphalan and chlorambucil. Aspen then threatened Italy with drug shortages unless higher prices were accepted.
In Spain, Aspen demanded a 4000% increase in melphalan prices.
“We hope that, by explaining what we have found in the UK, other European countries will take note and protect themselves against these kinds of price rises,” Dr Hill said. “At a time when cancer patients are living longer and better lives due to effective treatments, this situation is particularly worrying.” ![]()

Photo by Steven Harbour
AMSTERDAM—The UK has seen substantial price increases for some generic cancer drugs over the last few years, according to a study presented at ECCO 2017: European Cancer Congress (abstract 966).
Of the 89 drugs analyzed in this study, 21 of them—including 17 generics—had price increases from 2011 to 2016.
Fourteen of the generic cancer drugs had price increases over 100%, and 2 of the drugs had increases exceeding 1000%.
“We were surprised to find several companies consistently raising the prices of cancer treatment,” said study investigator Andrew Hill, PhD, of the University of Liverpool in the UK.
“Twenty treatments have shown rises of over 100% in the last 5 years, and in 2—busulfan (used to treat leukemia) and tamoxifen (breast cancer)—prices have increased by over 1000%. We have found that some companies take over the supply of some generic cancer medicines and then raise the price progressively.”
Dr Hill and his co-investigator Melissa Barber, of the London School of Hygiene and Tropical Medicine in the UK, analyzed prices for 190 formulations of 89 cancer drugs.
Twenty-eight formulations of 21 drugs had price increases from 2011 to 2016. Seventeen of these 21 drugs were generic in 2016.
Twenty formulations of 14 generic cancer drugs had price increases exceeding 100%.
For example, the cost per tablet or injection increased for:
- Ifosfamide (2 g vial)—from £89 to £180, or 103%.
- Melphalan (50 mg vial)—from £33 to £137, or 315%.
- Chlorambucil (2 mg)—from £0.33 to £1.62, or 390%.
- Cyclophosphamide (50 mg)—from £0.20 to £1.39, or 695%.
- Busulfan (2 mg)—from £0.21 to £2.61, or 1227%.
Dr Hill said the UK’s Department of Health is aware of this issue and has introduced the Health Services Medical Supplies (Costs) Bill to enable price regulation in the future.
Companies found to be raising prices with no clear justification will be referred to the Competition and Markets Authority, and they could face fines.
However, Dr Hill and Barber said they found large price increases for generic cancer drugs in other European countries as well.
In Spain and Italy, failure to accept the high prices demanded for some generic drugs has led to warnings from companies that they could stop the supply of these drugs.
For instance, Italy fined the generic company Aspen €5 million after a 1500% increase in the price of cancer drugs, including melphalan and chlorambucil. Aspen then threatened Italy with drug shortages unless higher prices were accepted.
In Spain, Aspen demanded a 4000% increase in melphalan prices.
“We hope that, by explaining what we have found in the UK, other European countries will take note and protect themselves against these kinds of price rises,” Dr Hill said. “At a time when cancer patients are living longer and better lives due to effective treatments, this situation is particularly worrying.” ![]()
Switzerland to lift lifetime ban on MSM blood donors

The Swiss Agency for Therapeutic Products (Swissmedic) has agreed to lift the ban on blood donations from men who have sex with men (MSM).
Instead, MSMs will be allowed to donate blood if it has been at least 12 months since their last sexual contact with another man.
Swiss Transfusion SRC Inc. expects to implement the modified donation criteria for MSMs in regional blood transfusion services starting in mid-2017.
However, Swissmedic’s decision is subject to certain conditions.
Specifically, blood transfusion services will have to record additional data on the effects of the modified donation criteria and donors’ compliance with them, as well as closely monitor the risk trend.
Swissmedic said that, since January 2016, the tests for specific pathogens in donated blood in Switzerland have been further refined, resulting in a higher level of sensitivity.
The diagnostic window—the period in which any infections carried by blood donors cannot yet be discovered—for the relevant pathogens has been further narrowed. Depending on the virus, the diagnostic window is 3 days to 15 days after infection.
Therefore, Swissmedic believes that a 12-month deferral period for MSM blood donors would not expose recipients of blood transfusions to an increased risk of contracting a blood-borne infection.
Swissmedic noted that approximately half of all new HIV infections in Switzerland are attributable to MSMs. This is one of the reasons MSMs have been permanently excluded from giving blood since 1977.
The new 12-month deferral period for MSMs is in line with the precautionary measures applicable to various other behaviors that have been shown to increase the risk of HIV transmission, such as changing sexual partners, staying in countries with a high AIDS rate, and sexual contact with partners who have stayed in countries with a high AIDS rate for a lengthy period.
The change to a 1-year deferral period for MSM blood donors brings Switzerland into line with other nations that have adopted similar policies, such as Ireland, Canada, the US, and the UK. ![]()

The Swiss Agency for Therapeutic Products (Swissmedic) has agreed to lift the ban on blood donations from men who have sex with men (MSM).
Instead, MSMs will be allowed to donate blood if it has been at least 12 months since their last sexual contact with another man.
Swiss Transfusion SRC Inc. expects to implement the modified donation criteria for MSMs in regional blood transfusion services starting in mid-2017.
However, Swissmedic’s decision is subject to certain conditions.
Specifically, blood transfusion services will have to record additional data on the effects of the modified donation criteria and donors’ compliance with them, as well as closely monitor the risk trend.
Swissmedic said that, since January 2016, the tests for specific pathogens in donated blood in Switzerland have been further refined, resulting in a higher level of sensitivity.
The diagnostic window—the period in which any infections carried by blood donors cannot yet be discovered—for the relevant pathogens has been further narrowed. Depending on the virus, the diagnostic window is 3 days to 15 days after infection.
Therefore, Swissmedic believes that a 12-month deferral period for MSM blood donors would not expose recipients of blood transfusions to an increased risk of contracting a blood-borne infection.
Swissmedic noted that approximately half of all new HIV infections in Switzerland are attributable to MSMs. This is one of the reasons MSMs have been permanently excluded from giving blood since 1977.
The new 12-month deferral period for MSMs is in line with the precautionary measures applicable to various other behaviors that have been shown to increase the risk of HIV transmission, such as changing sexual partners, staying in countries with a high AIDS rate, and sexual contact with partners who have stayed in countries with a high AIDS rate for a lengthy period.
The change to a 1-year deferral period for MSM blood donors brings Switzerland into line with other nations that have adopted similar policies, such as Ireland, Canada, the US, and the UK. ![]()

The Swiss Agency for Therapeutic Products (Swissmedic) has agreed to lift the ban on blood donations from men who have sex with men (MSM).
Instead, MSMs will be allowed to donate blood if it has been at least 12 months since their last sexual contact with another man.
Swiss Transfusion SRC Inc. expects to implement the modified donation criteria for MSMs in regional blood transfusion services starting in mid-2017.
However, Swissmedic’s decision is subject to certain conditions.
Specifically, blood transfusion services will have to record additional data on the effects of the modified donation criteria and donors’ compliance with them, as well as closely monitor the risk trend.
Swissmedic said that, since January 2016, the tests for specific pathogens in donated blood in Switzerland have been further refined, resulting in a higher level of sensitivity.
The diagnostic window—the period in which any infections carried by blood donors cannot yet be discovered—for the relevant pathogens has been further narrowed. Depending on the virus, the diagnostic window is 3 days to 15 days after infection.
Therefore, Swissmedic believes that a 12-month deferral period for MSM blood donors would not expose recipients of blood transfusions to an increased risk of contracting a blood-borne infection.
Swissmedic noted that approximately half of all new HIV infections in Switzerland are attributable to MSMs. This is one of the reasons MSMs have been permanently excluded from giving blood since 1977.
The new 12-month deferral period for MSMs is in line with the precautionary measures applicable to various other behaviors that have been shown to increase the risk of HIV transmission, such as changing sexual partners, staying in countries with a high AIDS rate, and sexual contact with partners who have stayed in countries with a high AIDS rate for a lengthy period.
The change to a 1-year deferral period for MSM blood donors brings Switzerland into line with other nations that have adopted similar policies, such as Ireland, Canada, the US, and the UK. ![]()
The VA Is Not Just a Hospital, It Is a Community
The residency applicant walking with me through the lobby of the Albuquerque VA hospital on the way to an interview in my office asked me, “Are all VAs like this?” She was referring to the mariachi band that was entertaining veterans, families, and staff and the volunteer who was serving popcorn—for many years a regular feature at our VA. I responded, “No, they are all a little different, but yes, every VA is more than a hospital.” If she had asked a follow-up question, I would have added, “It is a community.”
Merriam-Webster’s Collegiate Dictionary has multiple definitions of community, and it is remarkable that most of them in one way or another describe the VA from the perspective of many veterans and even career employees:
- 1: a unified body of individuals: as
a: state, commonwealth
b: the people with common interests living in a particular area; broadly: the area itself (eg, the problems of a large community)
c: an interacting population of various kinds of individuals (as species) in a common location
d: a group of people with a common characteristic or interest in living together within a larger society (eg, a community of retired persons)
e: a group linked by a common policy
f: a body of persons or nations having a common history or common social, economic, and political interests (eg, the international community)
g: a body of persons of common and especially professional interests scattered through a larger society (eg, the academic community) - 2: society at large
- 3a: joint ownership or participation (community of goods)
b: common character: likeness (community of interests)
c: social activity: fellowship d: a social state or condition
d: a social state or condition
There is much talk in the media about the privatization of the VA. There are zealous critics who argue that privatization would improve access and quality of care. I won’t debate that here.
What I want to consider is what the VA represents and provides in addition to health care. Each VA hospital and clinic serves “a body of persons or nations having a common history or common social, economic, and political interests.” Sit in the waiting area of any VA emergency department or pharmacy and you will hear bonding conversations between veterans. Even when the conversation is critical of the VA, it is because it is their hospital. That “joint ownership or participation” means that every VA employee, including the nearly 30% who wore a uniform, is there for a single purpose: to help veterans. That is our sole mission and advocacy.
Back to my VA. We are “a group of people with a common characteristic or interest living together within a larger society.” Similar to most other large medical centers, this VA is like the army base where I was born and raised—a small village. The single most popular service at my VA is the barber shop where veterans can get a haircut and shave. We also have an extensive clothing closet where eligible veterans experiencing tough times can get decent clothes.
Our VA, like almost any military base, has a post exchange that sells a little bit of everything from snacks to small appliances. When I was an intern, I treated an elderly patient who was in a deep psychotic depression and was transferred with only the clothes he was wearing. After several electroconvulsive treatments, I could tell he was feeling better when he asked me to buy him underwear from the post exchange downstairs. What this patient needed, the community provided.
A VA medical center is “a group of people with a common characteristic or interest living together within a larger society.” Like any American small town, there is a chapel where twice a year chaplains hold a memorial service for families and staff of patients who recently passed away in our hospital. At other times, we gather as a family of various and no faiths to grieve over the loss of a beloved fellow employee who, all too often, died too soon under tragic circumstances.
Much of this interaction naturally takes place around food. In the morning, there is a line at the coffee shop in the lobby that matches any Starbucks in town. Our VA also has an award-winning canteen that knows the favorite dishes of veterans and employees. If you go for breakfast or lunch, you will almost always run in to someone you have not seen in a while and have a quick visit.
At our VA, you also can browse kiosks of handcrafted items and military memorabilia and support small veteran-owned businesses. In good weather you can buy fruits and vegetables at the veteran farmers market and hear the stories of backyard gardeners and small farmers.
There are special events for every season. In the summer, concerts are held in the gazebo and veteran and guest musicians play all types of music. We even have a VA all-star band made up of current and former employees. The band is a big hit with patients and staff alike.
Although many of these community resources are unique to my VA, the effort to provide a welcoming atmosphere for veterans and health care providers to come together as a community is not unusual. Most VA medical centers have developed cultural responses to the needs of the veterans who return often over the course of years to their VA community.
One definition that does not apply to the large, diverse veteran population or to their health care providers is “a unified body of individuals.” There are many veterans who never have and never will set foot inside a VA hospital for many complex reasons. But for those who do call it home and want to receive care under VA auspices, a private VA would result in a deep and abiding loss of community. This loss is especially true for the most disadvantaged and vulnerable for whom the VA provides a broad and compassionate safety net. Under that protective tent, unbefriended veterans may grow closer to employees who have cared for them for years than to their family. Patients with complex medical and psychiatric needs, such as spinal cord injuries, polytrauma, substance use disorders, and posttraumatic stress disorder find specialized services dedicated to them that would be difficult to rival anywhere in the private sector.What also is not appreciated amid the fierce and too often well-deserved criticisms of VA business processes is that all VA health care practitioners are “a group linked by a common policy.” Even if we do not always live up to them, the VA has higher regulatory and ethical standards than almost any civilian health care organization. Ensuring those standards are followed in a myriad of health care entities not under VA policy and federal regulation seems a shibboleth.
The residency applicant walking with me through the lobby of the Albuquerque VA hospital on the way to an interview in my office asked me, “Are all VAs like this?” She was referring to the mariachi band that was entertaining veterans, families, and staff and the volunteer who was serving popcorn—for many years a regular feature at our VA. I responded, “No, they are all a little different, but yes, every VA is more than a hospital.” If she had asked a follow-up question, I would have added, “It is a community.”
Merriam-Webster’s Collegiate Dictionary has multiple definitions of community, and it is remarkable that most of them in one way or another describe the VA from the perspective of many veterans and even career employees:
- 1: a unified body of individuals: as
a: state, commonwealth
b: the people with common interests living in a particular area; broadly: the area itself (eg, the problems of a large community)
c: an interacting population of various kinds of individuals (as species) in a common location
d: a group of people with a common characteristic or interest in living together within a larger society (eg, a community of retired persons)
e: a group linked by a common policy
f: a body of persons or nations having a common history or common social, economic, and political interests (eg, the international community)
g: a body of persons of common and especially professional interests scattered through a larger society (eg, the academic community) - 2: society at large
- 3a: joint ownership or participation (community of goods)
b: common character: likeness (community of interests)
c: social activity: fellowship d: a social state or condition
d: a social state or condition
There is much talk in the media about the privatization of the VA. There are zealous critics who argue that privatization would improve access and quality of care. I won’t debate that here.
What I want to consider is what the VA represents and provides in addition to health care. Each VA hospital and clinic serves “a body of persons or nations having a common history or common social, economic, and political interests.” Sit in the waiting area of any VA emergency department or pharmacy and you will hear bonding conversations between veterans. Even when the conversation is critical of the VA, it is because it is their hospital. That “joint ownership or participation” means that every VA employee, including the nearly 30% who wore a uniform, is there for a single purpose: to help veterans. That is our sole mission and advocacy.
Back to my VA. We are “a group of people with a common characteristic or interest living together within a larger society.” Similar to most other large medical centers, this VA is like the army base where I was born and raised—a small village. The single most popular service at my VA is the barber shop where veterans can get a haircut and shave. We also have an extensive clothing closet where eligible veterans experiencing tough times can get decent clothes.
Our VA, like almost any military base, has a post exchange that sells a little bit of everything from snacks to small appliances. When I was an intern, I treated an elderly patient who was in a deep psychotic depression and was transferred with only the clothes he was wearing. After several electroconvulsive treatments, I could tell he was feeling better when he asked me to buy him underwear from the post exchange downstairs. What this patient needed, the community provided.
A VA medical center is “a group of people with a common characteristic or interest living together within a larger society.” Like any American small town, there is a chapel where twice a year chaplains hold a memorial service for families and staff of patients who recently passed away in our hospital. At other times, we gather as a family of various and no faiths to grieve over the loss of a beloved fellow employee who, all too often, died too soon under tragic circumstances.
Much of this interaction naturally takes place around food. In the morning, there is a line at the coffee shop in the lobby that matches any Starbucks in town. Our VA also has an award-winning canteen that knows the favorite dishes of veterans and employees. If you go for breakfast or lunch, you will almost always run in to someone you have not seen in a while and have a quick visit.
At our VA, you also can browse kiosks of handcrafted items and military memorabilia and support small veteran-owned businesses. In good weather you can buy fruits and vegetables at the veteran farmers market and hear the stories of backyard gardeners and small farmers.
There are special events for every season. In the summer, concerts are held in the gazebo and veteran and guest musicians play all types of music. We even have a VA all-star band made up of current and former employees. The band is a big hit with patients and staff alike.
Although many of these community resources are unique to my VA, the effort to provide a welcoming atmosphere for veterans and health care providers to come together as a community is not unusual. Most VA medical centers have developed cultural responses to the needs of the veterans who return often over the course of years to their VA community.
One definition that does not apply to the large, diverse veteran population or to their health care providers is “a unified body of individuals.” There are many veterans who never have and never will set foot inside a VA hospital for many complex reasons. But for those who do call it home and want to receive care under VA auspices, a private VA would result in a deep and abiding loss of community. This loss is especially true for the most disadvantaged and vulnerable for whom the VA provides a broad and compassionate safety net. Under that protective tent, unbefriended veterans may grow closer to employees who have cared for them for years than to their family. Patients with complex medical and psychiatric needs, such as spinal cord injuries, polytrauma, substance use disorders, and posttraumatic stress disorder find specialized services dedicated to them that would be difficult to rival anywhere in the private sector.What also is not appreciated amid the fierce and too often well-deserved criticisms of VA business processes is that all VA health care practitioners are “a group linked by a common policy.” Even if we do not always live up to them, the VA has higher regulatory and ethical standards than almost any civilian health care organization. Ensuring those standards are followed in a myriad of health care entities not under VA policy and federal regulation seems a shibboleth.
The residency applicant walking with me through the lobby of the Albuquerque VA hospital on the way to an interview in my office asked me, “Are all VAs like this?” She was referring to the mariachi band that was entertaining veterans, families, and staff and the volunteer who was serving popcorn—for many years a regular feature at our VA. I responded, “No, they are all a little different, but yes, every VA is more than a hospital.” If she had asked a follow-up question, I would have added, “It is a community.”
Merriam-Webster’s Collegiate Dictionary has multiple definitions of community, and it is remarkable that most of them in one way or another describe the VA from the perspective of many veterans and even career employees:
- 1: a unified body of individuals: as
a: state, commonwealth
b: the people with common interests living in a particular area; broadly: the area itself (eg, the problems of a large community)
c: an interacting population of various kinds of individuals (as species) in a common location
d: a group of people with a common characteristic or interest in living together within a larger society (eg, a community of retired persons)
e: a group linked by a common policy
f: a body of persons or nations having a common history or common social, economic, and political interests (eg, the international community)
g: a body of persons of common and especially professional interests scattered through a larger society (eg, the academic community) - 2: society at large
- 3a: joint ownership or participation (community of goods)
b: common character: likeness (community of interests)
c: social activity: fellowship d: a social state or condition
d: a social state or condition
There is much talk in the media about the privatization of the VA. There are zealous critics who argue that privatization would improve access and quality of care. I won’t debate that here.
What I want to consider is what the VA represents and provides in addition to health care. Each VA hospital and clinic serves “a body of persons or nations having a common history or common social, economic, and political interests.” Sit in the waiting area of any VA emergency department or pharmacy and you will hear bonding conversations between veterans. Even when the conversation is critical of the VA, it is because it is their hospital. That “joint ownership or participation” means that every VA employee, including the nearly 30% who wore a uniform, is there for a single purpose: to help veterans. That is our sole mission and advocacy.
Back to my VA. We are “a group of people with a common characteristic or interest living together within a larger society.” Similar to most other large medical centers, this VA is like the army base where I was born and raised—a small village. The single most popular service at my VA is the barber shop where veterans can get a haircut and shave. We also have an extensive clothing closet where eligible veterans experiencing tough times can get decent clothes.
Our VA, like almost any military base, has a post exchange that sells a little bit of everything from snacks to small appliances. When I was an intern, I treated an elderly patient who was in a deep psychotic depression and was transferred with only the clothes he was wearing. After several electroconvulsive treatments, I could tell he was feeling better when he asked me to buy him underwear from the post exchange downstairs. What this patient needed, the community provided.
A VA medical center is “a group of people with a common characteristic or interest living together within a larger society.” Like any American small town, there is a chapel where twice a year chaplains hold a memorial service for families and staff of patients who recently passed away in our hospital. At other times, we gather as a family of various and no faiths to grieve over the loss of a beloved fellow employee who, all too often, died too soon under tragic circumstances.
Much of this interaction naturally takes place around food. In the morning, there is a line at the coffee shop in the lobby that matches any Starbucks in town. Our VA also has an award-winning canteen that knows the favorite dishes of veterans and employees. If you go for breakfast or lunch, you will almost always run in to someone you have not seen in a while and have a quick visit.
At our VA, you also can browse kiosks of handcrafted items and military memorabilia and support small veteran-owned businesses. In good weather you can buy fruits and vegetables at the veteran farmers market and hear the stories of backyard gardeners and small farmers.
There are special events for every season. In the summer, concerts are held in the gazebo and veteran and guest musicians play all types of music. We even have a VA all-star band made up of current and former employees. The band is a big hit with patients and staff alike.
Although many of these community resources are unique to my VA, the effort to provide a welcoming atmosphere for veterans and health care providers to come together as a community is not unusual. Most VA medical centers have developed cultural responses to the needs of the veterans who return often over the course of years to their VA community.
One definition that does not apply to the large, diverse veteran population or to their health care providers is “a unified body of individuals.” There are many veterans who never have and never will set foot inside a VA hospital for many complex reasons. But for those who do call it home and want to receive care under VA auspices, a private VA would result in a deep and abiding loss of community. This loss is especially true for the most disadvantaged and vulnerable for whom the VA provides a broad and compassionate safety net. Under that protective tent, unbefriended veterans may grow closer to employees who have cared for them for years than to their family. Patients with complex medical and psychiatric needs, such as spinal cord injuries, polytrauma, substance use disorders, and posttraumatic stress disorder find specialized services dedicated to them that would be difficult to rival anywhere in the private sector.What also is not appreciated amid the fierce and too often well-deserved criticisms of VA business processes is that all VA health care practitioners are “a group linked by a common policy.” Even if we do not always live up to them, the VA has higher regulatory and ethical standards than almost any civilian health care organization. Ensuring those standards are followed in a myriad of health care entities not under VA policy and federal regulation seems a shibboleth.
Expanding APRN practice and more
Medical psychiatry: The skill of integrating medical and psychiatric care
Although the meaning of these terms varied from department to department, biologically oriented programs—influenced by Eli Robins and Samuel Guze and DSM-III—were focused on descriptive psychiatry: reliable, observable, and symptom-based elements of psychiatric illness. Related and important elements were a focus on psychopharmacologic treatments, genetics, epidemiology, and putative mechanisms for both diseases and treatments. Psychodynamic programs had a primary focus on psychodynamic theory, with extensive training in long-term, depth-oriented psychotherapy. Many of these are programs employed charismatic and brilliant teachers whose supervisory and interviewing skills were legendary. And, of course, all the programs claimed they did everything and did it well.
However, none of these programs were exactly what I was looking for. Although I had a long-standing interest in psychodynamics and was fascinated by the implications of—what was then a far more nascent—neurobiology, I was looking for a program that had all of these elements, but also had a focus on, what I thought of as, “medical psychiatry.” Although this may have meant different things to others, and was known as “psychosomatic medicine” or “consultation-liaison psychiatry,” to me, it was about the psychiatric manifestations of medical and neurologic disorders.
My years training in internal medicine were full of patients with neuropsychiatric illness due to a host of general medical and neurologic disorders. When I was an intern, the most common admitting diagnosis was what we called “Delta MS”—change in mental status. As I advanced in my residency and focused on a subspecialty of internal medicine, it became clear that whichever illnesses I studied, conditions such as anxiety disorders in Grave’s disease or the psychotic symptoms in lupus held my interest. Finally, the only specialty left was psychiatry.
The only program I found that seemed to understand medical psychiatry at the time was at Massachusetts General Hospital (MGH). MGH not only had eminent psychiatrists in every area of the field, it seemed, but also a special focus on training psychiatrists in medical settings and as medical experts. My first Chief of Psychiatry was Thomas P. Hackett, MD—a brilliant clinician, raconteur, and polymath—who had written a cri de coeur on the importance of medical skills and training in psychiatry.1 At last, I had found a place where I could remain a physician and think and learn about every aspect of psychiatry, especially medical psychiatry.
What is medical psychiatry, and why is it relevant now?
There has been substantial and increasing interest in the integration of medical and psychiatric care. Whether it is collaborative care or co-location models, the recognition of the high rate of combined medical and psychiatric illnesses and associated increased mortality and total health care costs of these patients requires psychiatrists to be deeply familiar with the interactions among medical and psychiatric conditions.
Building on long-developed expertise in consultation-liaison psychiatry and other forms of medical psychiatric training, such as double-board medicine–psychiatry programs, medical psychiatry includes several specific areas of knowledge and skill sets, including understanding the impact that psychiatric illnesses and the medications used to treat them can have on medical illnesses and the ways in which the presence of medical disorders can change the presentation of psychiatric illnesses. Similarly, the psychiatric impact of the general medical pharmacopeia and the ways in which psychiatric illness can affect the presentation of medical illness are important for all psychiatrists to know. Most importantly, medical psychiatry should focus on the medical and neurologic causes of psychiatric illnesses. Many general medical conditions produce symptoms, which, in whole or in part, mimic psychiatric illnesses and, in some cases, could lead to psychiatric disorders, which makes identification of the underlying cause difficult.
Whether due to infectious, autoimmune, metabolic, or endocrinologic disorders, being aware of these conditions and, where clinical circumstances warrant, be able to diagnose them, with other specialists as needed, and ensure they are appropriately treated should be an essential skill for psychiatrists.
An illustrative case
I remember a case from early in my training of a woman with a late-onset mood disorder with abulia, wide-based gait, and urinary incontinence, in addition to withdrawal and loss of pleasure. Despite the skepticism of the neurology team, at autopsy she was found to have arteriosclerosis of the deep, penetrating arterioles causing white matter hyperintensities—Binswanger’s disease. There was no question that despite the neurologic cause of her symptoms treating her depression with antidepressants was needed and helpful. It also was important that her family was aware of her underlying medical condition and its implications for her care.2
Medicine is our calling
Many of these illnesses, even when identified, require expert psychiatric management of psychiatric symptoms. This should not be surprising to psychiatrists or other clinicians. No one expects a cardiologist to beg off the care of a patient with heart failure caused by alcohol abuse or a virus rather than vascular heart disease, and psychiatrists likewise need to manage psychosis due to steroid use or N-methyl-
Medical psychiatry has a broader and more inclusive perspective than what we generally mean by “biological psychiatry,” if by the latter, we mean a focus on the neurobiology and psychopharmacology of “primary” psychiatric conditions that are not secondary to other medical or neurologic disorders. As important and fundamental as deep understanding of neurobiology, genetics, and psychopharmacology are, medical psychiatry embeds our work more broadly in all of human biology and requires the full breadth of our medical training.
At a time when political battles over prescriptive privileges by non-medically trained mental health clinicians engage legislatures and professional organizations, medical psychiatry is a powerful reminder that prescribing or not prescribing medications is the final step in, what should be, an extensive, clinical evaluation including a thorough medical work up and consideration of the medical–psychiatric interactions and the differential diagnosis of these illnesses. It is, after all, what physicians do and is essential to our calling as psychiatric physicians. If psychiatrists are not at home in medicine, as Tom Hackett reminded us in 19771—at a time when psychiatry had temporarily eliminated the requirement for medical internships—then, indeed, psychiatry would be “homeless.”
2. Summergrad P. Depression in Binswanger’s encephalopathy responsive to tranylcypromine: case report. J Clin Psychiatry. 1985;46(2):69-70.
Although the meaning of these terms varied from department to department, biologically oriented programs—influenced by Eli Robins and Samuel Guze and DSM-III—were focused on descriptive psychiatry: reliable, observable, and symptom-based elements of psychiatric illness. Related and important elements were a focus on psychopharmacologic treatments, genetics, epidemiology, and putative mechanisms for both diseases and treatments. Psychodynamic programs had a primary focus on psychodynamic theory, with extensive training in long-term, depth-oriented psychotherapy. Many of these are programs employed charismatic and brilliant teachers whose supervisory and interviewing skills were legendary. And, of course, all the programs claimed they did everything and did it well.
However, none of these programs were exactly what I was looking for. Although I had a long-standing interest in psychodynamics and was fascinated by the implications of—what was then a far more nascent—neurobiology, I was looking for a program that had all of these elements, but also had a focus on, what I thought of as, “medical psychiatry.” Although this may have meant different things to others, and was known as “psychosomatic medicine” or “consultation-liaison psychiatry,” to me, it was about the psychiatric manifestations of medical and neurologic disorders.
My years training in internal medicine were full of patients with neuropsychiatric illness due to a host of general medical and neurologic disorders. When I was an intern, the most common admitting diagnosis was what we called “Delta MS”—change in mental status. As I advanced in my residency and focused on a subspecialty of internal medicine, it became clear that whichever illnesses I studied, conditions such as anxiety disorders in Grave’s disease or the psychotic symptoms in lupus held my interest. Finally, the only specialty left was psychiatry.
The only program I found that seemed to understand medical psychiatry at the time was at Massachusetts General Hospital (MGH). MGH not only had eminent psychiatrists in every area of the field, it seemed, but also a special focus on training psychiatrists in medical settings and as medical experts. My first Chief of Psychiatry was Thomas P. Hackett, MD—a brilliant clinician, raconteur, and polymath—who had written a cri de coeur on the importance of medical skills and training in psychiatry.1 At last, I had found a place where I could remain a physician and think and learn about every aspect of psychiatry, especially medical psychiatry.
What is medical psychiatry, and why is it relevant now?
There has been substantial and increasing interest in the integration of medical and psychiatric care. Whether it is collaborative care or co-location models, the recognition of the high rate of combined medical and psychiatric illnesses and associated increased mortality and total health care costs of these patients requires psychiatrists to be deeply familiar with the interactions among medical and psychiatric conditions.
Building on long-developed expertise in consultation-liaison psychiatry and other forms of medical psychiatric training, such as double-board medicine–psychiatry programs, medical psychiatry includes several specific areas of knowledge and skill sets, including understanding the impact that psychiatric illnesses and the medications used to treat them can have on medical illnesses and the ways in which the presence of medical disorders can change the presentation of psychiatric illnesses. Similarly, the psychiatric impact of the general medical pharmacopeia and the ways in which psychiatric illness can affect the presentation of medical illness are important for all psychiatrists to know. Most importantly, medical psychiatry should focus on the medical and neurologic causes of psychiatric illnesses. Many general medical conditions produce symptoms, which, in whole or in part, mimic psychiatric illnesses and, in some cases, could lead to psychiatric disorders, which makes identification of the underlying cause difficult.
Whether due to infectious, autoimmune, metabolic, or endocrinologic disorders, being aware of these conditions and, where clinical circumstances warrant, be able to diagnose them, with other specialists as needed, and ensure they are appropriately treated should be an essential skill for psychiatrists.
An illustrative case
I remember a case from early in my training of a woman with a late-onset mood disorder with abulia, wide-based gait, and urinary incontinence, in addition to withdrawal and loss of pleasure. Despite the skepticism of the neurology team, at autopsy she was found to have arteriosclerosis of the deep, penetrating arterioles causing white matter hyperintensities—Binswanger’s disease. There was no question that despite the neurologic cause of her symptoms treating her depression with antidepressants was needed and helpful. It also was important that her family was aware of her underlying medical condition and its implications for her care.2
Medicine is our calling
Many of these illnesses, even when identified, require expert psychiatric management of psychiatric symptoms. This should not be surprising to psychiatrists or other clinicians. No one expects a cardiologist to beg off the care of a patient with heart failure caused by alcohol abuse or a virus rather than vascular heart disease, and psychiatrists likewise need to manage psychosis due to steroid use or N-methyl-
Medical psychiatry has a broader and more inclusive perspective than what we generally mean by “biological psychiatry,” if by the latter, we mean a focus on the neurobiology and psychopharmacology of “primary” psychiatric conditions that are not secondary to other medical or neurologic disorders. As important and fundamental as deep understanding of neurobiology, genetics, and psychopharmacology are, medical psychiatry embeds our work more broadly in all of human biology and requires the full breadth of our medical training.
At a time when political battles over prescriptive privileges by non-medically trained mental health clinicians engage legislatures and professional organizations, medical psychiatry is a powerful reminder that prescribing or not prescribing medications is the final step in, what should be, an extensive, clinical evaluation including a thorough medical work up and consideration of the medical–psychiatric interactions and the differential diagnosis of these illnesses. It is, after all, what physicians do and is essential to our calling as psychiatric physicians. If psychiatrists are not at home in medicine, as Tom Hackett reminded us in 19771—at a time when psychiatry had temporarily eliminated the requirement for medical internships—then, indeed, psychiatry would be “homeless.”
Although the meaning of these terms varied from department to department, biologically oriented programs—influenced by Eli Robins and Samuel Guze and DSM-III—were focused on descriptive psychiatry: reliable, observable, and symptom-based elements of psychiatric illness. Related and important elements were a focus on psychopharmacologic treatments, genetics, epidemiology, and putative mechanisms for both diseases and treatments. Psychodynamic programs had a primary focus on psychodynamic theory, with extensive training in long-term, depth-oriented psychotherapy. Many of these are programs employed charismatic and brilliant teachers whose supervisory and interviewing skills were legendary. And, of course, all the programs claimed they did everything and did it well.
However, none of these programs were exactly what I was looking for. Although I had a long-standing interest in psychodynamics and was fascinated by the implications of—what was then a far more nascent—neurobiology, I was looking for a program that had all of these elements, but also had a focus on, what I thought of as, “medical psychiatry.” Although this may have meant different things to others, and was known as “psychosomatic medicine” or “consultation-liaison psychiatry,” to me, it was about the psychiatric manifestations of medical and neurologic disorders.
My years training in internal medicine were full of patients with neuropsychiatric illness due to a host of general medical and neurologic disorders. When I was an intern, the most common admitting diagnosis was what we called “Delta MS”—change in mental status. As I advanced in my residency and focused on a subspecialty of internal medicine, it became clear that whichever illnesses I studied, conditions such as anxiety disorders in Grave’s disease or the psychotic symptoms in lupus held my interest. Finally, the only specialty left was psychiatry.
The only program I found that seemed to understand medical psychiatry at the time was at Massachusetts General Hospital (MGH). MGH not only had eminent psychiatrists in every area of the field, it seemed, but also a special focus on training psychiatrists in medical settings and as medical experts. My first Chief of Psychiatry was Thomas P. Hackett, MD—a brilliant clinician, raconteur, and polymath—who had written a cri de coeur on the importance of medical skills and training in psychiatry.1 At last, I had found a place where I could remain a physician and think and learn about every aspect of psychiatry, especially medical psychiatry.
What is medical psychiatry, and why is it relevant now?
There has been substantial and increasing interest in the integration of medical and psychiatric care. Whether it is collaborative care or co-location models, the recognition of the high rate of combined medical and psychiatric illnesses and associated increased mortality and total health care costs of these patients requires psychiatrists to be deeply familiar with the interactions among medical and psychiatric conditions.
Building on long-developed expertise in consultation-liaison psychiatry and other forms of medical psychiatric training, such as double-board medicine–psychiatry programs, medical psychiatry includes several specific areas of knowledge and skill sets, including understanding the impact that psychiatric illnesses and the medications used to treat them can have on medical illnesses and the ways in which the presence of medical disorders can change the presentation of psychiatric illnesses. Similarly, the psychiatric impact of the general medical pharmacopeia and the ways in which psychiatric illness can affect the presentation of medical illness are important for all psychiatrists to know. Most importantly, medical psychiatry should focus on the medical and neurologic causes of psychiatric illnesses. Many general medical conditions produce symptoms, which, in whole or in part, mimic psychiatric illnesses and, in some cases, could lead to psychiatric disorders, which makes identification of the underlying cause difficult.
Whether due to infectious, autoimmune, metabolic, or endocrinologic disorders, being aware of these conditions and, where clinical circumstances warrant, be able to diagnose them, with other specialists as needed, and ensure they are appropriately treated should be an essential skill for psychiatrists.
An illustrative case
I remember a case from early in my training of a woman with a late-onset mood disorder with abulia, wide-based gait, and urinary incontinence, in addition to withdrawal and loss of pleasure. Despite the skepticism of the neurology team, at autopsy she was found to have arteriosclerosis of the deep, penetrating arterioles causing white matter hyperintensities—Binswanger’s disease. There was no question that despite the neurologic cause of her symptoms treating her depression with antidepressants was needed and helpful. It also was important that her family was aware of her underlying medical condition and its implications for her care.2
Medicine is our calling
Many of these illnesses, even when identified, require expert psychiatric management of psychiatric symptoms. This should not be surprising to psychiatrists or other clinicians. No one expects a cardiologist to beg off the care of a patient with heart failure caused by alcohol abuse or a virus rather than vascular heart disease, and psychiatrists likewise need to manage psychosis due to steroid use or N-methyl-
Medical psychiatry has a broader and more inclusive perspective than what we generally mean by “biological psychiatry,” if by the latter, we mean a focus on the neurobiology and psychopharmacology of “primary” psychiatric conditions that are not secondary to other medical or neurologic disorders. As important and fundamental as deep understanding of neurobiology, genetics, and psychopharmacology are, medical psychiatry embeds our work more broadly in all of human biology and requires the full breadth of our medical training.
At a time when political battles over prescriptive privileges by non-medically trained mental health clinicians engage legislatures and professional organizations, medical psychiatry is a powerful reminder that prescribing or not prescribing medications is the final step in, what should be, an extensive, clinical evaluation including a thorough medical work up and consideration of the medical–psychiatric interactions and the differential diagnosis of these illnesses. It is, after all, what physicians do and is essential to our calling as psychiatric physicians. If psychiatrists are not at home in medicine, as Tom Hackett reminded us in 19771—at a time when psychiatry had temporarily eliminated the requirement for medical internships—then, indeed, psychiatry would be “homeless.”
2. Summergrad P. Depression in Binswanger’s encephalopathy responsive to tranylcypromine: case report. J Clin Psychiatry. 1985;46(2):69-70.
2. Summergrad P. Depression in Binswanger’s encephalopathy responsive to tranylcypromine: case report. J Clin Psychiatry. 1985;46(2):69-70.
Don’t balk at using medical therapy to manage alcohol use disorder
There is ample evidence in the medical literature, as well as clinical experience, that patients seeking help for chemical dependency benefit from pharmacotherapy. It is common, however, for physicians, patients, and family to balk at the idea. Even within the psychiatry community, where there should be better understanding of substance use disorders, many practitioners hesitate to employ medications, especially for alcohol use disorder (AUD).
Efficacy for such FDA-approved medications has been demonstrated in well-designed, randomized controlled trials, but many trainees, and even experienced professionals, have never seen these medications used effectively and appropriately. Medication-assisted treatment (MAT) is not an alternative to biopsychosocial approaches but is an augmentation that can (1) help stabilize the patient until he (she) can be educated in relapse prevention skills and (2) allow the brain to rewire and heal until he regains impulse control.
Diverse presentations
Do you remember that patient who often arrived for appointments intoxicated, promising that he plans to cut down? How about the man you saw in the emergency department with an elevated blood alcohol level, who was constantly endorsing suicidal thoughts that subsided when he reached clinical sobriety? What about the college student who often was treated for alcohol poisoning after binge drinking on weekends, but who never considered this behavior problematic? And, how about the elderly woman who was evaluated for anxiety, but had been drinking 4 beers nightly for the past 30 years?
Despite the diverse presentations, these patients all have a chronic disease and we fail them when we do not apply evidence-based medicine to their treatment.
As psychiatrists, we encounter many patients with AUD as a primary or comorbid diagnosis. This is a global problem associated with significant human and financial cost. With 80% of American adolescents having reported using alcohol in the past year, the problem will continue to grow.1 Furthermore, a greater prevalence of AUD is noted in clinical populations undergoing psychiatric treatment.2 Ongoing alcohol abuse complicates the course of medical and psychiatric conditions and incites significant societal exclusion.
Pharmacotherapy is underutilized
Despite an increase in the use of psychotropic medications for treating psychiatric illness, pharmacotherapy for AUD is underutilized: only 3% of patients have received an FDA-approved treatment.2,3 Nearly one-third of adults are affected by AUD during their lifetime, yet only 20% seek help.3 Management today remains limited to episodic, brief inpatient detoxification and psychosocial therapy.
Recovery rates are highest when addiction treatment that monitors abstinence is continuous; yet, for the most part, alcohol addiction is treated in discrete episodes upon relapse. Although MAT is recommended by experts for “moderate” and “severe” substance use disorders, practitioners, in general, have demonstrated considerable resistance to using this modality as part of routine practice.4,5 This is regrettable: Regardless of terminology used to describe their condition, these people suffer a potentially fatal disease characterized by high post-treatment recidivism.
Neuroscience supports the brain disease model of addiction, with neuroplasticity changes being made during phases of drug use. Medications are shown to assist in preventing relapse while the brain is healing and normal emotional and decision-making capacities are being restored.6
Why hesitate to use pharmacotherapeutics?
There are diverse pharmacotherapeutic options that can be pursued for treating AUD with minimal disruption to home and work life. Alarmingly, many trainees have never prescribed or even considered such medications. Despite modest effect sizes in randomized controlled trials, efficacy has been demonstrated in reducing relapse rates and overall severity of drinking days.4,5 So, from where does the ambivalence of patients and providers about using these treatments to achieve lasting recovery stem?
Starting MAT certainly requires both parties to be in agreement. A patient might decline medication because of a fear of dependence or because he overestimates his ability to achieve remission on his own. There also may be financial barriers in a current alcohol treatment system that is traditionally non-medically oriented. Prescribers also fail to offer medications because of:
- lack of familiarity with available agents
- absence of guidelines for use
- disbelief that the condition is treatable.
Given that treatment often is based on a 12-step approach, such as Alcoholics Anonymous (AA), providers might hesitate to prescribe medication for an illness that is thought to be managed through psychosocial interventions, such as group and motivational therapy.
Therapeutic options
Choice of medication depends on the prescriber’s comfort level, reputation of the medication, potential side-effect profile, medical contraindications, and affordability; the most important consideration, however, should be the overall goals and expectations of the patient.
There are 4 FDA-approved medications for AUD (Table); many others are off-label. It is advisable to start with an FDA-approved medication such as disulfiram for the motivated patient who has a collaborator and desires complete abstinence; naltrexone for a patient who wants to cut down on intake (a long-acting formulation can be used for poorly adherent patients); and acamprosate for a patient with at least some established sobriety who needs help with post-withdrawal sleep disturbances.
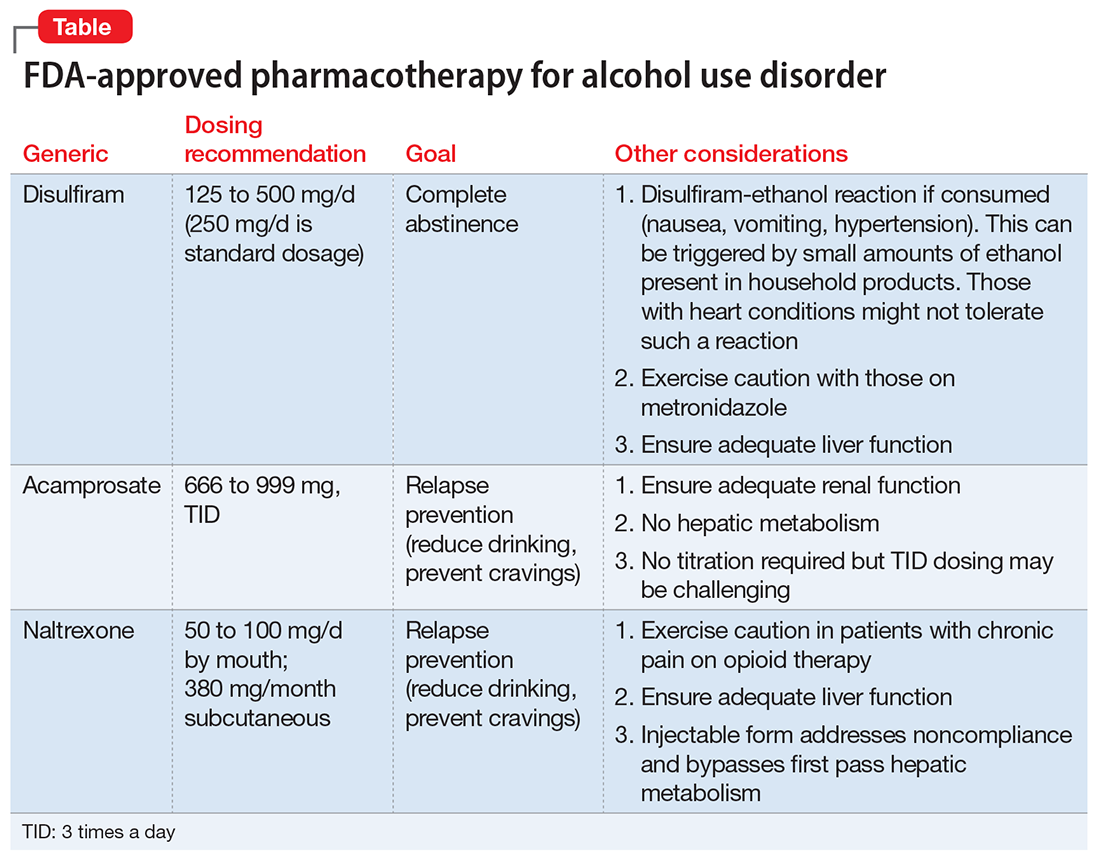
With regard to off-label medications, topiramate has the highest evidence for efficacy. Gabapentin can augment naltrexone and also helps with sleep, anxiety, withdrawal, and cravings.4,5
Psychosocial interventions
Medications are just 1 tool in recovery; patients should be engaged in a program of counseling. Encourage attendance at AA meetings. An up-and-coming concept is the use of smartphone applications to prevent relapse (or even induce remission); apps that provide an accurate blood alcohol tracking systems and integrated psychosocial therapies are in the pipeline. The novel Reddit online forum r/StopDrinking is a 24-hour peer-support community that relies on fellowship, accountability, monitoring, and anonymity; the forum can compete with motivational interviewing for efficacy in increasing abstinence and preventing relapse.
The authors would like to thank Thomas M. Penders, MS, MD, Medical Director for Consultation-Liaison Psychiatry at Cape Cod Healthcare, Hyannis, Massachusetts, and Affiliate Professor at East Carolina University, Greenville, North Carolina, for all his guidance, support, and mentorship.
In July 2017, Dr. Stanciu will be entering PGY-5 Addiction Psychiatry Fellowship, Geisel School of Medicine, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, and Dr. Gnanasegaram has accepted a Clinical Instructor position, Department of Psychiatric Medicine, Dartmouth-Hitchcock, New Hampshire.
1. Johnson L, O’Malley P, Miech RA, et al. Monitoring the Future national survey results on drug use, 1975-2015: overview, key findings on adolescent drug use. http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf. Published February 2016. Accessed January 20, 2016.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: mental health findings, NSDUH Series H-49, HHS Publication No. (SMA) 14-4887. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
3. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiological Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
4. Robinson S, Meeks TW, Geniza C. Medication for alcohol use disorder: which agents work best. Current Psychiatry. 2014;13(1):22-29.
5. Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism. Medication for the treatment of alcohol use disorder: a brief guide. HHS Publication No. (SMA) 15-4907. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
6. Volkow ND, Koob GF, McLellan AT. Neurobiological advances from the brain disease model of addiction. N Engl J Med. 2016;374(4):363-371.
There is ample evidence in the medical literature, as well as clinical experience, that patients seeking help for chemical dependency benefit from pharmacotherapy. It is common, however, for physicians, patients, and family to balk at the idea. Even within the psychiatry community, where there should be better understanding of substance use disorders, many practitioners hesitate to employ medications, especially for alcohol use disorder (AUD).
Efficacy for such FDA-approved medications has been demonstrated in well-designed, randomized controlled trials, but many trainees, and even experienced professionals, have never seen these medications used effectively and appropriately. Medication-assisted treatment (MAT) is not an alternative to biopsychosocial approaches but is an augmentation that can (1) help stabilize the patient until he (she) can be educated in relapse prevention skills and (2) allow the brain to rewire and heal until he regains impulse control.
Diverse presentations
Do you remember that patient who often arrived for appointments intoxicated, promising that he plans to cut down? How about the man you saw in the emergency department with an elevated blood alcohol level, who was constantly endorsing suicidal thoughts that subsided when he reached clinical sobriety? What about the college student who often was treated for alcohol poisoning after binge drinking on weekends, but who never considered this behavior problematic? And, how about the elderly woman who was evaluated for anxiety, but had been drinking 4 beers nightly for the past 30 years?
Despite the diverse presentations, these patients all have a chronic disease and we fail them when we do not apply evidence-based medicine to their treatment.
As psychiatrists, we encounter many patients with AUD as a primary or comorbid diagnosis. This is a global problem associated with significant human and financial cost. With 80% of American adolescents having reported using alcohol in the past year, the problem will continue to grow.1 Furthermore, a greater prevalence of AUD is noted in clinical populations undergoing psychiatric treatment.2 Ongoing alcohol abuse complicates the course of medical and psychiatric conditions and incites significant societal exclusion.
Pharmacotherapy is underutilized
Despite an increase in the use of psychotropic medications for treating psychiatric illness, pharmacotherapy for AUD is underutilized: only 3% of patients have received an FDA-approved treatment.2,3 Nearly one-third of adults are affected by AUD during their lifetime, yet only 20% seek help.3 Management today remains limited to episodic, brief inpatient detoxification and psychosocial therapy.
Recovery rates are highest when addiction treatment that monitors abstinence is continuous; yet, for the most part, alcohol addiction is treated in discrete episodes upon relapse. Although MAT is recommended by experts for “moderate” and “severe” substance use disorders, practitioners, in general, have demonstrated considerable resistance to using this modality as part of routine practice.4,5 This is regrettable: Regardless of terminology used to describe their condition, these people suffer a potentially fatal disease characterized by high post-treatment recidivism.
Neuroscience supports the brain disease model of addiction, with neuroplasticity changes being made during phases of drug use. Medications are shown to assist in preventing relapse while the brain is healing and normal emotional and decision-making capacities are being restored.6
Why hesitate to use pharmacotherapeutics?
There are diverse pharmacotherapeutic options that can be pursued for treating AUD with minimal disruption to home and work life. Alarmingly, many trainees have never prescribed or even considered such medications. Despite modest effect sizes in randomized controlled trials, efficacy has been demonstrated in reducing relapse rates and overall severity of drinking days.4,5 So, from where does the ambivalence of patients and providers about using these treatments to achieve lasting recovery stem?
Starting MAT certainly requires both parties to be in agreement. A patient might decline medication because of a fear of dependence or because he overestimates his ability to achieve remission on his own. There also may be financial barriers in a current alcohol treatment system that is traditionally non-medically oriented. Prescribers also fail to offer medications because of:
- lack of familiarity with available agents
- absence of guidelines for use
- disbelief that the condition is treatable.
Given that treatment often is based on a 12-step approach, such as Alcoholics Anonymous (AA), providers might hesitate to prescribe medication for an illness that is thought to be managed through psychosocial interventions, such as group and motivational therapy.
Therapeutic options
Choice of medication depends on the prescriber’s comfort level, reputation of the medication, potential side-effect profile, medical contraindications, and affordability; the most important consideration, however, should be the overall goals and expectations of the patient.
There are 4 FDA-approved medications for AUD (Table); many others are off-label. It is advisable to start with an FDA-approved medication such as disulfiram for the motivated patient who has a collaborator and desires complete abstinence; naltrexone for a patient who wants to cut down on intake (a long-acting formulation can be used for poorly adherent patients); and acamprosate for a patient with at least some established sobriety who needs help with post-withdrawal sleep disturbances.

With regard to off-label medications, topiramate has the highest evidence for efficacy. Gabapentin can augment naltrexone and also helps with sleep, anxiety, withdrawal, and cravings.4,5
Psychosocial interventions
Medications are just 1 tool in recovery; patients should be engaged in a program of counseling. Encourage attendance at AA meetings. An up-and-coming concept is the use of smartphone applications to prevent relapse (or even induce remission); apps that provide an accurate blood alcohol tracking systems and integrated psychosocial therapies are in the pipeline. The novel Reddit online forum r/StopDrinking is a 24-hour peer-support community that relies on fellowship, accountability, monitoring, and anonymity; the forum can compete with motivational interviewing for efficacy in increasing abstinence and preventing relapse.
The authors would like to thank Thomas M. Penders, MS, MD, Medical Director for Consultation-Liaison Psychiatry at Cape Cod Healthcare, Hyannis, Massachusetts, and Affiliate Professor at East Carolina University, Greenville, North Carolina, for all his guidance, support, and mentorship.
In July 2017, Dr. Stanciu will be entering PGY-5 Addiction Psychiatry Fellowship, Geisel School of Medicine, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, and Dr. Gnanasegaram has accepted a Clinical Instructor position, Department of Psychiatric Medicine, Dartmouth-Hitchcock, New Hampshire.
There is ample evidence in the medical literature, as well as clinical experience, that patients seeking help for chemical dependency benefit from pharmacotherapy. It is common, however, for physicians, patients, and family to balk at the idea. Even within the psychiatry community, where there should be better understanding of substance use disorders, many practitioners hesitate to employ medications, especially for alcohol use disorder (AUD).
Efficacy for such FDA-approved medications has been demonstrated in well-designed, randomized controlled trials, but many trainees, and even experienced professionals, have never seen these medications used effectively and appropriately. Medication-assisted treatment (MAT) is not an alternative to biopsychosocial approaches but is an augmentation that can (1) help stabilize the patient until he (she) can be educated in relapse prevention skills and (2) allow the brain to rewire and heal until he regains impulse control.
Diverse presentations
Do you remember that patient who often arrived for appointments intoxicated, promising that he plans to cut down? How about the man you saw in the emergency department with an elevated blood alcohol level, who was constantly endorsing suicidal thoughts that subsided when he reached clinical sobriety? What about the college student who often was treated for alcohol poisoning after binge drinking on weekends, but who never considered this behavior problematic? And, how about the elderly woman who was evaluated for anxiety, but had been drinking 4 beers nightly for the past 30 years?
Despite the diverse presentations, these patients all have a chronic disease and we fail them when we do not apply evidence-based medicine to their treatment.
As psychiatrists, we encounter many patients with AUD as a primary or comorbid diagnosis. This is a global problem associated with significant human and financial cost. With 80% of American adolescents having reported using alcohol in the past year, the problem will continue to grow.1 Furthermore, a greater prevalence of AUD is noted in clinical populations undergoing psychiatric treatment.2 Ongoing alcohol abuse complicates the course of medical and psychiatric conditions and incites significant societal exclusion.
Pharmacotherapy is underutilized
Despite an increase in the use of psychotropic medications for treating psychiatric illness, pharmacotherapy for AUD is underutilized: only 3% of patients have received an FDA-approved treatment.2,3 Nearly one-third of adults are affected by AUD during their lifetime, yet only 20% seek help.3 Management today remains limited to episodic, brief inpatient detoxification and psychosocial therapy.
Recovery rates are highest when addiction treatment that monitors abstinence is continuous; yet, for the most part, alcohol addiction is treated in discrete episodes upon relapse. Although MAT is recommended by experts for “moderate” and “severe” substance use disorders, practitioners, in general, have demonstrated considerable resistance to using this modality as part of routine practice.4,5 This is regrettable: Regardless of terminology used to describe their condition, these people suffer a potentially fatal disease characterized by high post-treatment recidivism.
Neuroscience supports the brain disease model of addiction, with neuroplasticity changes being made during phases of drug use. Medications are shown to assist in preventing relapse while the brain is healing and normal emotional and decision-making capacities are being restored.6
Why hesitate to use pharmacotherapeutics?
There are diverse pharmacotherapeutic options that can be pursued for treating AUD with minimal disruption to home and work life. Alarmingly, many trainees have never prescribed or even considered such medications. Despite modest effect sizes in randomized controlled trials, efficacy has been demonstrated in reducing relapse rates and overall severity of drinking days.4,5 So, from where does the ambivalence of patients and providers about using these treatments to achieve lasting recovery stem?
Starting MAT certainly requires both parties to be in agreement. A patient might decline medication because of a fear of dependence or because he overestimates his ability to achieve remission on his own. There also may be financial barriers in a current alcohol treatment system that is traditionally non-medically oriented. Prescribers also fail to offer medications because of:
- lack of familiarity with available agents
- absence of guidelines for use
- disbelief that the condition is treatable.
Given that treatment often is based on a 12-step approach, such as Alcoholics Anonymous (AA), providers might hesitate to prescribe medication for an illness that is thought to be managed through psychosocial interventions, such as group and motivational therapy.
Therapeutic options
Choice of medication depends on the prescriber’s comfort level, reputation of the medication, potential side-effect profile, medical contraindications, and affordability; the most important consideration, however, should be the overall goals and expectations of the patient.
There are 4 FDA-approved medications for AUD (Table); many others are off-label. It is advisable to start with an FDA-approved medication such as disulfiram for the motivated patient who has a collaborator and desires complete abstinence; naltrexone for a patient who wants to cut down on intake (a long-acting formulation can be used for poorly adherent patients); and acamprosate for a patient with at least some established sobriety who needs help with post-withdrawal sleep disturbances.

With regard to off-label medications, topiramate has the highest evidence for efficacy. Gabapentin can augment naltrexone and also helps with sleep, anxiety, withdrawal, and cravings.4,5
Psychosocial interventions
Medications are just 1 tool in recovery; patients should be engaged in a program of counseling. Encourage attendance at AA meetings. An up-and-coming concept is the use of smartphone applications to prevent relapse (or even induce remission); apps that provide an accurate blood alcohol tracking systems and integrated psychosocial therapies are in the pipeline. The novel Reddit online forum r/StopDrinking is a 24-hour peer-support community that relies on fellowship, accountability, monitoring, and anonymity; the forum can compete with motivational interviewing for efficacy in increasing abstinence and preventing relapse.
The authors would like to thank Thomas M. Penders, MS, MD, Medical Director for Consultation-Liaison Psychiatry at Cape Cod Healthcare, Hyannis, Massachusetts, and Affiliate Professor at East Carolina University, Greenville, North Carolina, for all his guidance, support, and mentorship.
In July 2017, Dr. Stanciu will be entering PGY-5 Addiction Psychiatry Fellowship, Geisel School of Medicine, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, and Dr. Gnanasegaram has accepted a Clinical Instructor position, Department of Psychiatric Medicine, Dartmouth-Hitchcock, New Hampshire.
1. Johnson L, O’Malley P, Miech RA, et al. Monitoring the Future national survey results on drug use, 1975-2015: overview, key findings on adolescent drug use. http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf. Published February 2016. Accessed January 20, 2016.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: mental health findings, NSDUH Series H-49, HHS Publication No. (SMA) 14-4887. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
3. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiological Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
4. Robinson S, Meeks TW, Geniza C. Medication for alcohol use disorder: which agents work best. Current Psychiatry. 2014;13(1):22-29.
5. Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism. Medication for the treatment of alcohol use disorder: a brief guide. HHS Publication No. (SMA) 15-4907. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
6. Volkow ND, Koob GF, McLellan AT. Neurobiological advances from the brain disease model of addiction. N Engl J Med. 2016;374(4):363-371.
1. Johnson L, O’Malley P, Miech RA, et al. Monitoring the Future national survey results on drug use, 1975-2015: overview, key findings on adolescent drug use. http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf. Published February 2016. Accessed January 20, 2016.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: mental health findings, NSDUH Series H-49, HHS Publication No. (SMA) 14-4887. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
3. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiological Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
4. Robinson S, Meeks TW, Geniza C. Medication for alcohol use disorder: which agents work best. Current Psychiatry. 2014;13(1):22-29.
5. Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism. Medication for the treatment of alcohol use disorder: a brief guide. HHS Publication No. (SMA) 15-4907. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
6. Volkow ND, Koob GF, McLellan AT. Neurobiological advances from the brain disease model of addiction. N Engl J Med. 2016;374(4):363-371.
When to consider cranial electrotherapy stimulation for patients with PTSD
Individuals with posttraumatic stress disorder (PTSD) often report cognitive and sleep disturbances, such as insomnia and poor concentration. Although many patients report improvement with traditional evidence-based treatments, such as pharmacotherapy and psychotherapy, it might be valuable to consider complementary or alternative therapies. Many patients seek treatments that they can self-administer as needed, at their convenience, particularly during symptom exacerbation. One treatment option is cranial electrotherapy stimulation (CES).
As a medical device, CES has been cleared—rather than approved, as is the case for medications—by the FDA to treat depression, insomnia, and anxiety.1 In the United States, CES devices require a prescription from a licensed health care practitioner, but they are available without a prescription in other countries. Cost for devices range from $600 to $1,200 and $10 to $20 for electrodes and contact solution. However, insurance companies that provide coverage for durable medical equipment might cover some or all of this expense.
How CES works
After applying contact solution, depending on the device used, the user attaches electrodes to the earlobes, mastoid processes, or other parts of the head that deliver a pulsed current, usually from AA batteries for 20 to 60 minutes.1 The current causes cortical deactivation and could affect emotional regulation by influencing neurotransmission in the thalamus, hypothalamus, and limbic system.1,2 CES increases cerebrospinal fluid levels of beta-endorphin, adrenocorticotropic hormone, and serotonin, which play a role in depression and anxiety.3
There are no known contraindications for CES. Adverse effects are rare, temporary, and mild; skin irritation, vertigo, or headache are the most common.1
Evidence of efficacy
There are no double-blind placebo-controlled trials evaluating the efficacy of CES for PTSD. However, there is a case series and a large survey of patients supporting its use.
- In a case series, 2 patients reported improved occupational functioning and reduced PTSD symptoms after using CES, 100 to 500 mA, 20 to 60 minutes a day, 3 to 5 days per week.4
- In an online survey of 145 veterans and active-duty military personnel, 60% of individuals used CES for PTSD, and 20% of those individuals were not receiving pharmacotherapy.5 Participants reported at least a 25% reduction in symptoms using CES for at least 20 minutes, once or twice daily, with a current of 100 to 600 mA.5
- In an expert opinion, patients noted improved sleep quality and reduced alcohol and drug withdrawal symptoms after 20-minute treatments, twice a day, with a current of 2 mA. Currents could be increased to 4 mA, if there was no improvement after 2 weeks.6
Some patients experiencing exacerbation of PTSD symptoms could benefit from using the device for 1 hour several times a day until symptoms subside.5
Optimal strength, frequency, and duration of treatment vary among patients, and further studies are needed to assess these parameters as well as efficacy because definitive studies are currently lacking. CES has not always shown efficacy, such as in some patients with depression.7 Despite the limited evidence base, it is reasonable to consider CES for patients with PTSD. This modality might be helpful for patients who have comorbid pain, anxiety, and insomnia, or for those who seek a complementary, convenient, safe, self-administered treatment.
1. Kirsch DL, Nichols F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013;36(1):169-176.
2. Feusner JD, Madsen S, Moody TD, et al. Effects of cranial electrotherapy stimulation on resting state brain activity. Brain Behav. 2012;2(3):211-220.
3. Shealy CN, Cady RK, Culver-Veehoff D, et al. Cerebrospinal fluid and plasma neurochemicals: response to cranial electrical stimulation. J Neuro Orthop Med Surg. 1998;18(2):94-97.
4. Bracciano AG, Chang WP, Kokesh S, et al. Cranial electrotherapy stimulation in the treatment of posttraumatic stress disorder: a pilot study of two military veterans. J Neurother. 2012;16(1):60-69.
5. Kirsch DL, Price LR, Nichols F, et al. Military service member and veteran self reports of efficacy of cranial electrotherapy stimulation for anxiety, posttraumatic stress disorder, insomnia, and depression. US Army Med Dep J. 2014:46-54.
6. Xenakis SN. The rise of cranial electrotherapy. Psychiatric Times. http://www.psychiatrictimes.com/electroconvulsive-therapy/rise-cranial-electrotherapy. Published July 24, 2014. Accessed December 20, 2016.
7. Mischoulon D, De Jong MF, Vitolo OV, et al. Efficacy and safety of a form of cranial electrical stimulation (CES) as an add-on intervention for treatment-resistant major depressive disorder: a three week double blind pilot study. J Psychiatr Res. 2015;70:98-105.
Individuals with posttraumatic stress disorder (PTSD) often report cognitive and sleep disturbances, such as insomnia and poor concentration. Although many patients report improvement with traditional evidence-based treatments, such as pharmacotherapy and psychotherapy, it might be valuable to consider complementary or alternative therapies. Many patients seek treatments that they can self-administer as needed, at their convenience, particularly during symptom exacerbation. One treatment option is cranial electrotherapy stimulation (CES).
As a medical device, CES has been cleared—rather than approved, as is the case for medications—by the FDA to treat depression, insomnia, and anxiety.1 In the United States, CES devices require a prescription from a licensed health care practitioner, but they are available without a prescription in other countries. Cost for devices range from $600 to $1,200 and $10 to $20 for electrodes and contact solution. However, insurance companies that provide coverage for durable medical equipment might cover some or all of this expense.
How CES works
After applying contact solution, depending on the device used, the user attaches electrodes to the earlobes, mastoid processes, or other parts of the head that deliver a pulsed current, usually from AA batteries for 20 to 60 minutes.1 The current causes cortical deactivation and could affect emotional regulation by influencing neurotransmission in the thalamus, hypothalamus, and limbic system.1,2 CES increases cerebrospinal fluid levels of beta-endorphin, adrenocorticotropic hormone, and serotonin, which play a role in depression and anxiety.3
There are no known contraindications for CES. Adverse effects are rare, temporary, and mild; skin irritation, vertigo, or headache are the most common.1
Evidence of efficacy
There are no double-blind placebo-controlled trials evaluating the efficacy of CES for PTSD. However, there is a case series and a large survey of patients supporting its use.
- In a case series, 2 patients reported improved occupational functioning and reduced PTSD symptoms after using CES, 100 to 500 mA, 20 to 60 minutes a day, 3 to 5 days per week.4
- In an online survey of 145 veterans and active-duty military personnel, 60% of individuals used CES for PTSD, and 20% of those individuals were not receiving pharmacotherapy.5 Participants reported at least a 25% reduction in symptoms using CES for at least 20 minutes, once or twice daily, with a current of 100 to 600 mA.5
- In an expert opinion, patients noted improved sleep quality and reduced alcohol and drug withdrawal symptoms after 20-minute treatments, twice a day, with a current of 2 mA. Currents could be increased to 4 mA, if there was no improvement after 2 weeks.6
Some patients experiencing exacerbation of PTSD symptoms could benefit from using the device for 1 hour several times a day until symptoms subside.5
Optimal strength, frequency, and duration of treatment vary among patients, and further studies are needed to assess these parameters as well as efficacy because definitive studies are currently lacking. CES has not always shown efficacy, such as in some patients with depression.7 Despite the limited evidence base, it is reasonable to consider CES for patients with PTSD. This modality might be helpful for patients who have comorbid pain, anxiety, and insomnia, or for those who seek a complementary, convenient, safe, self-administered treatment.
Individuals with posttraumatic stress disorder (PTSD) often report cognitive and sleep disturbances, such as insomnia and poor concentration. Although many patients report improvement with traditional evidence-based treatments, such as pharmacotherapy and psychotherapy, it might be valuable to consider complementary or alternative therapies. Many patients seek treatments that they can self-administer as needed, at their convenience, particularly during symptom exacerbation. One treatment option is cranial electrotherapy stimulation (CES).
As a medical device, CES has been cleared—rather than approved, as is the case for medications—by the FDA to treat depression, insomnia, and anxiety.1 In the United States, CES devices require a prescription from a licensed health care practitioner, but they are available without a prescription in other countries. Cost for devices range from $600 to $1,200 and $10 to $20 for electrodes and contact solution. However, insurance companies that provide coverage for durable medical equipment might cover some or all of this expense.
How CES works
After applying contact solution, depending on the device used, the user attaches electrodes to the earlobes, mastoid processes, or other parts of the head that deliver a pulsed current, usually from AA batteries for 20 to 60 minutes.1 The current causes cortical deactivation and could affect emotional regulation by influencing neurotransmission in the thalamus, hypothalamus, and limbic system.1,2 CES increases cerebrospinal fluid levels of beta-endorphin, adrenocorticotropic hormone, and serotonin, which play a role in depression and anxiety.3
There are no known contraindications for CES. Adverse effects are rare, temporary, and mild; skin irritation, vertigo, or headache are the most common.1
Evidence of efficacy
There are no double-blind placebo-controlled trials evaluating the efficacy of CES for PTSD. However, there is a case series and a large survey of patients supporting its use.
- In a case series, 2 patients reported improved occupational functioning and reduced PTSD symptoms after using CES, 100 to 500 mA, 20 to 60 minutes a day, 3 to 5 days per week.4
- In an online survey of 145 veterans and active-duty military personnel, 60% of individuals used CES for PTSD, and 20% of those individuals were not receiving pharmacotherapy.5 Participants reported at least a 25% reduction in symptoms using CES for at least 20 minutes, once or twice daily, with a current of 100 to 600 mA.5
- In an expert opinion, patients noted improved sleep quality and reduced alcohol and drug withdrawal symptoms after 20-minute treatments, twice a day, with a current of 2 mA. Currents could be increased to 4 mA, if there was no improvement after 2 weeks.6
Some patients experiencing exacerbation of PTSD symptoms could benefit from using the device for 1 hour several times a day until symptoms subside.5
Optimal strength, frequency, and duration of treatment vary among patients, and further studies are needed to assess these parameters as well as efficacy because definitive studies are currently lacking. CES has not always shown efficacy, such as in some patients with depression.7 Despite the limited evidence base, it is reasonable to consider CES for patients with PTSD. This modality might be helpful for patients who have comorbid pain, anxiety, and insomnia, or for those who seek a complementary, convenient, safe, self-administered treatment.
1. Kirsch DL, Nichols F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013;36(1):169-176.
2. Feusner JD, Madsen S, Moody TD, et al. Effects of cranial electrotherapy stimulation on resting state brain activity. Brain Behav. 2012;2(3):211-220.
3. Shealy CN, Cady RK, Culver-Veehoff D, et al. Cerebrospinal fluid and plasma neurochemicals: response to cranial electrical stimulation. J Neuro Orthop Med Surg. 1998;18(2):94-97.
4. Bracciano AG, Chang WP, Kokesh S, et al. Cranial electrotherapy stimulation in the treatment of posttraumatic stress disorder: a pilot study of two military veterans. J Neurother. 2012;16(1):60-69.
5. Kirsch DL, Price LR, Nichols F, et al. Military service member and veteran self reports of efficacy of cranial electrotherapy stimulation for anxiety, posttraumatic stress disorder, insomnia, and depression. US Army Med Dep J. 2014:46-54.
6. Xenakis SN. The rise of cranial electrotherapy. Psychiatric Times. http://www.psychiatrictimes.com/electroconvulsive-therapy/rise-cranial-electrotherapy. Published July 24, 2014. Accessed December 20, 2016.
7. Mischoulon D, De Jong MF, Vitolo OV, et al. Efficacy and safety of a form of cranial electrical stimulation (CES) as an add-on intervention for treatment-resistant major depressive disorder: a three week double blind pilot study. J Psychiatr Res. 2015;70:98-105.
1. Kirsch DL, Nichols F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013;36(1):169-176.
2. Feusner JD, Madsen S, Moody TD, et al. Effects of cranial electrotherapy stimulation on resting state brain activity. Brain Behav. 2012;2(3):211-220.
3. Shealy CN, Cady RK, Culver-Veehoff D, et al. Cerebrospinal fluid and plasma neurochemicals: response to cranial electrical stimulation. J Neuro Orthop Med Surg. 1998;18(2):94-97.
4. Bracciano AG, Chang WP, Kokesh S, et al. Cranial electrotherapy stimulation in the treatment of posttraumatic stress disorder: a pilot study of two military veterans. J Neurother. 2012;16(1):60-69.
5. Kirsch DL, Price LR, Nichols F, et al. Military service member and veteran self reports of efficacy of cranial electrotherapy stimulation for anxiety, posttraumatic stress disorder, insomnia, and depression. US Army Med Dep J. 2014:46-54.
6. Xenakis SN. The rise of cranial electrotherapy. Psychiatric Times. http://www.psychiatrictimes.com/electroconvulsive-therapy/rise-cranial-electrotherapy. Published July 24, 2014. Accessed December 20, 2016.
7. Mischoulon D, De Jong MF, Vitolo OV, et al. Efficacy and safety of a form of cranial electrical stimulation (CES) as an add-on intervention for treatment-resistant major depressive disorder: a three week double blind pilot study. J Psychiatr Res. 2015;70:98-105.
Risks of increasingly potent Cannabis: The joint effects of potency and frequency
In the United States, the average potency of Cannabis has increased significantly over the past few decades in response to consumer demand and policies in some states that have legalized marijuana for medicinal and recreational purposes.1 Whereas the delta-9-tetrahydrocannabinol (THC) content of “street” marijuana was <1% in the 1970s and 4% in the 1990s, by 2012, analyses of Cannabis samples seized by law enforcement agencies documented a rise in average THC potency to >12%.1-3
Although this increase in potency has been overstated in the media because studies did not control for the effects of changes in sampling methods on freshness, it is estimated that Cannabis potency increased 7-fold from 1970 to 2010.3 Also, Cannabis preparations such as hashish and hash oil extracts containing THC well above average—from 35% to 90% THC—are now more widely available. In states where marijuana has been legalized, high-potency Cannabis (HPC) in the form of “edibles” (eg, marijuana added to baked goods, candy, or drinks) and hash oil extracts (Table 1)4-13 can be readily obtained from dispensaries or even at local farmers’ markets.
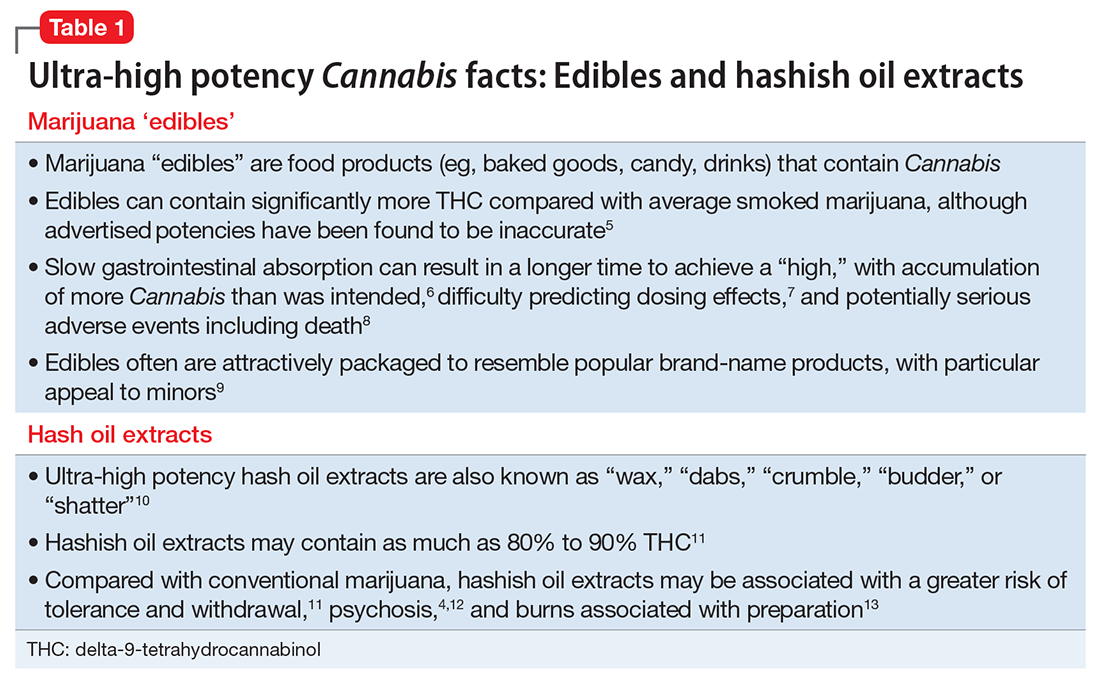
The potency of Cannabis, typically defined as the percentage of THC, its chief psychoactive component, varies depending on the genetic strain of the plant, cultivation techniques, and methods of processing and storage. For example, relative to “average marijuana,” hemp (Cannabis bred for industrial purposes) has very little THC, while sinsemilla (flowering buds from unpollinated female plants), hashish (Cannabis resin), and extracted hash oil contain increasing amounts of THC (Table 2).1,2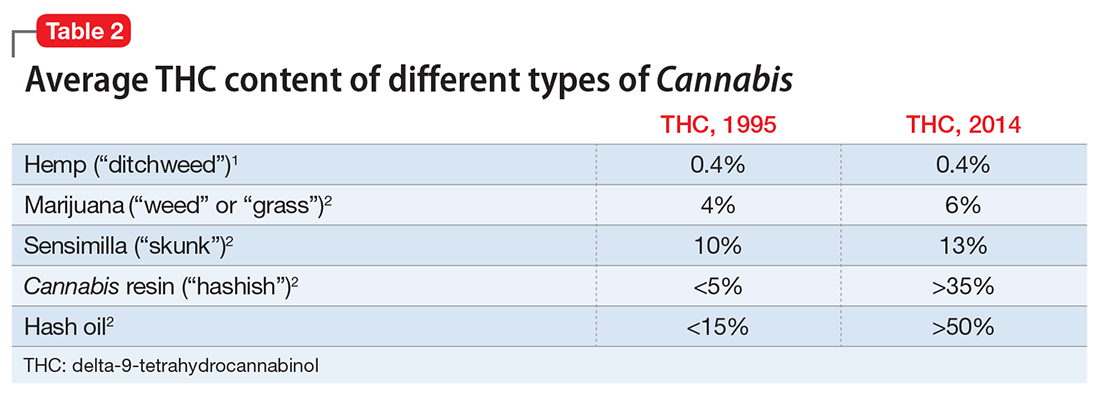
As THC levels in Cannabis have risen over time, cannabidiol (CBD) levels have dropped to <0.2%.2 Although THC appears to be largely responsible for the psychiatric morbidity associated with Cannabis, CBD may have neuroprotective and antipsychotic properties.14,15 The sharp spike in the THC:CBD ratio in recent years therefore raises the possibility that Cannabis use today might carry a much greater risk of psychiatric sequelae than it did in previous generations.
This article reviews the evidence for an increased risk of psychiatric morbidity with increasing Cannabis potency.
Cannabis use disorder
Recent data indicate that the prevalence of Cannabis use disorders (eg, abuse and dependence) in the United States is approximately 3% among the general population and >30% among Cannabis users.16 The availability of increasingly potent forms of Cannabis has been cited as a possible explanation for this rise, despite no change in the prevalence of overall marijuana use between 1991 to 1992 and 2001 to 2002.17 However, while the prevalence of marijuana use disorders has continued to rise—nearly doubling from 2001 to 2002 to 2012 to 2013—this latest increase occurred with a significant increase in overall marijuana use, such that the actual rate of Cannabis use disorders among users seems to have plateaued, despite the continued rise in marijuana potency.16 This discrepancy could be explained if Cannabis users cut back past a specific threshold of increasing potency. However, 2 studies have called into question how effective such titration efforts might be in practice. In one study, Cannabis users who preferred more potent Cannabis inhaled lower volumes of smoke, but did not fully compensate for the increased potency, such that use of HPC still resulted in greater THC exposure.18 Another study found that HPC users rolled less marijuana into their joints but not enough to mitigate the impact of greater potency.19 Therefore, it appears that HPC users typically expose themselves to greater amounts of THC, which could place them at higher risk of addiction.
Although a causal association between increasing Cannabis potency and the rate of substance use disorders among users remains unclear based on epidemiologic studies from the United States, a recent study from the United Kingdom examined the impact of Cannabis potency on dependence.20 This cross-sectional survey found that, although HPC was preferred by users and was rated as offering the “best high,” its use was associated with increasing severity of dependence, especially among young people. The limited available evidence supports a greater risk of Cannabis use disorders with increasing potency.
Psychosis
Based on longitudinal studies published over the past 30 years, it is clear that using Cannabis at a young age (age <15 to 18) increases the risk of developing a psychotic disorder.21 This association appears to be dose-dependent, with studies consistently demonstrating that psychosis risk increases with greater frequency of Cannabis use.22 The accumulated evidence to date is strong enough to view the psychotic potential of Cannabis as a significant public health concern.21
If risk of psychosis is proportional to the amount of Cannabis used as measured by frequency, it follows that this risk might be affected similarly by Cannabis potency. In another paper, I discussed the potential for greater risk of psychosis in the context of medical marijuana and synthetic cannabinoids.23 My colleagues and I also have published case reports describing emerging psychosis among regular Cannabis users after escalating to higher potency medical marijuana24 and a hyperconcentrated form of hash oil known as Cannabis “wax” or “dabs” that contains as much as 90% THC.4 Preliminary anecdotal evidence supports the plausibility of HPC being more psychotoxic than less potent forms.
Several studies from a research group in the United Kingdom, where sinsemilla has increasingly dominated the drug market, likewise have reported that the use of HPC is associated with a greater risk of psychosis. The first of these studies, published in 2009, found that adults hospitalized for first-episode psychosis were more likely to have used HPC than healthy controls.25 Among Cannabis users, HPC use was associated with a 7-fold increased risk of psychosis, with daily HPC use associated with a 12-fold increased risk.
Based on a larger dataset, a second study reported that high-potency, but not low-potency, Cannabis increased the risk of first-episode psychosis with increasing frequency of use.26 Daily users of HPC had a 5-fold higher risk of psychosis compared with those that had never used Cannabis. A third study reported that HPC use and daily Cannabis use were independently associated with an earlier onset of first-episode psychosis, with daily HPC users developing first-episode psychosis an average of 6 years earlier than non-Cannabis users.27 Finally, a prospective study following patients with first-episode psychosis over 2 years found that the greatest risk of relapse—defined by hospital admission caused by exacerbation of psychotic symptoms—was found among self-reported daily users of HPC, while the lowest risk was among those who stopped using Cannabis after their initial psychotic episode.28
The findings from these 4 studies suggest that the increased risk of psychosis with Cannabis is proportional to overall exposure, determined by both frequency of use and Cannabis potency.
Cognition
There is little doubt that using Cannabis can impair cognition acutely, “after all, this is the basic reason for its recreational use,” as one author wrote.29 As with psychosis, the available evidence indicates that the degree of cognitive impairment is related to the frequency and duration of Cannabis use as well as age of onset of use.30,31
Few studies have assessed cognitive functioning in relation to Cannabis potency with most only examining the effects of relatively low-potency Cannabis with inconsistent results. For example, 2 studies compared cognitive performance in individuals smoking Cannabis with 1.8% and 3.9% THC. One study found that using higher potency Cannabis resulted in prolonged time needed to complete certain cognitive tasks,32 whereas the other found greater impairment in performance on a decision-making task at both potencies compared with non-users but no differences between the 2 dosages.33 Detecting significant differences may be difficult within the narrow range of low Cannabis potency studied where any findings have limited applicability in the context of today’s Cannabis with much higher THC content.
To date, only 1 study has assessed cognition at higher Cannabis potencies, comparing Cannabis with 4% THC to 13% THC.34 Cognitive impairments increased with higher potency, especially in tasks that measured motor control and executive functioning. Therefore it appears that higher potency Cannabis use is associated with greater acute cognitive impairment.
The longer-term effects on cognition are less clear, with conflicting evidence about whether Cannabis use can result in residual cognitive impairment despite abstinence.30,35 A recent review concluded that “the magnitude of neuropsychological impairment and the extent to which it persists after abstinence may depend on the frequency and the duration of Cannabis use, length of abstinence, and age at onset of use.”31 The effects of HPC on long-term cognitive deficits have not been studied.
Structural brain changes
A number of studies have determined an association between Cannabis use and brain changes involving structures governing memory and emotional processing, including reduced volume of the hippocampus,36 temporal cortex, insula, and orbitofrontal cortex.37 Although many of these changes appear to be dose-related, some morphologic changes have been reported among young recreational users without Cannabis dependence.38 This has resulted in an understandable concern about the effects of Cannabis on the brains of young people with limited exposure; however, it is not yet clear to what extent detected brain changes are pathological and reflect functional deficits.
Recent research using newer neuroimaging modalities provides preliminary support of Cannabis use associated with white matter changes that, in turn, are correlated with cognitive impairment.39 One study comparing low-potency Cannabis and HPC users with and without first-episode psychosis found a significant effect of Cannabis potency on disturbances in white matter microstructural organization in the corpus callosum.40 These findings provide sufficient cause for concern that structural brain changes associated with cognitive impairment are more likely to occur with HPC use.
Recommendations for clinicians
Similar to any drug, the effects of THC and its psychiatric sequelae can be expected to increase with dosage. To date, much of the information about psychiatric risks has been based on studies of low- and moderate-potency Cannabis rather than the much higher potency Cannabis products, such as hyper-concentrated “wax dabs,” that are available today. Data from social media suggest that these products may be associated with novel patterns of use, such as with the intention of “passing out.”41 It is likely that clinicians will encounter greater psychiatric morbidity associated with HPC use.
Although clinicians may be accustomed to asking about the frequency and duration of Cannabis use, it is now prudent also to ask patients about Cannabis potency to better assess the potential risks of use. The potency of different marijuana products is openly advertised within some “medical marijuana” dispensaries, although the accuracy of information in products such as “edibles” has been called into question.5
Physicians are increasingly asked to provide recommendations on “medical marijuana” use. A recent paper outlined characteristics of appropriate candidates for “medical marijuana” including:
- having a debilitating condition that might benefit from Cannabis
- multiple failed trials of conventional pharmacotherapies including FDA-approved cannabinoids
- lack of substance use disorders, psychosis, or unstable mood or anxiety disorders
- residence in a state where “medical marijuana” is legal.42
As part of the informed consent process, physicians providing recommendations for “medical marijuana” now must consider the effects of HPC when weighing potential risks against any benefits of Cannabis use. Those monitoring patients using Cannabis should be aware of the potential for greater psychiatric morbidity with HPC and should educate patients about that risk. Failure to adequately warn patients about such morbidity or to screen for risk factors such as psychosis could leave physicians vulnerable to malpractice litigation.
In the United States, the average potency of Cannabis has increased significantly over the past few decades in response to consumer demand and policies in some states that have legalized marijuana for medicinal and recreational purposes.1 Whereas the delta-9-tetrahydrocannabinol (THC) content of “street” marijuana was <1% in the 1970s and 4% in the 1990s, by 2012, analyses of Cannabis samples seized by law enforcement agencies documented a rise in average THC potency to >12%.1-3
Although this increase in potency has been overstated in the media because studies did not control for the effects of changes in sampling methods on freshness, it is estimated that Cannabis potency increased 7-fold from 1970 to 2010.3 Also, Cannabis preparations such as hashish and hash oil extracts containing THC well above average—from 35% to 90% THC—are now more widely available. In states where marijuana has been legalized, high-potency Cannabis (HPC) in the form of “edibles” (eg, marijuana added to baked goods, candy, or drinks) and hash oil extracts (Table 1)4-13 can be readily obtained from dispensaries or even at local farmers’ markets.

The potency of Cannabis, typically defined as the percentage of THC, its chief psychoactive component, varies depending on the genetic strain of the plant, cultivation techniques, and methods of processing and storage. For example, relative to “average marijuana,” hemp (Cannabis bred for industrial purposes) has very little THC, while sinsemilla (flowering buds from unpollinated female plants), hashish (Cannabis resin), and extracted hash oil contain increasing amounts of THC (Table 2).1,2
As THC levels in Cannabis have risen over time, cannabidiol (CBD) levels have dropped to <0.2%.2 Although THC appears to be largely responsible for the psychiatric morbidity associated with Cannabis, CBD may have neuroprotective and antipsychotic properties.14,15 The sharp spike in the THC:CBD ratio in recent years therefore raises the possibility that Cannabis use today might carry a much greater risk of psychiatric sequelae than it did in previous generations.
This article reviews the evidence for an increased risk of psychiatric morbidity with increasing Cannabis potency.
Cannabis use disorder
Recent data indicate that the prevalence of Cannabis use disorders (eg, abuse and dependence) in the United States is approximately 3% among the general population and >30% among Cannabis users.16 The availability of increasingly potent forms of Cannabis has been cited as a possible explanation for this rise, despite no change in the prevalence of overall marijuana use between 1991 to 1992 and 2001 to 2002.17 However, while the prevalence of marijuana use disorders has continued to rise—nearly doubling from 2001 to 2002 to 2012 to 2013—this latest increase occurred with a significant increase in overall marijuana use, such that the actual rate of Cannabis use disorders among users seems to have plateaued, despite the continued rise in marijuana potency.16 This discrepancy could be explained if Cannabis users cut back past a specific threshold of increasing potency. However, 2 studies have called into question how effective such titration efforts might be in practice. In one study, Cannabis users who preferred more potent Cannabis inhaled lower volumes of smoke, but did not fully compensate for the increased potency, such that use of HPC still resulted in greater THC exposure.18 Another study found that HPC users rolled less marijuana into their joints but not enough to mitigate the impact of greater potency.19 Therefore, it appears that HPC users typically expose themselves to greater amounts of THC, which could place them at higher risk of addiction.
Although a causal association between increasing Cannabis potency and the rate of substance use disorders among users remains unclear based on epidemiologic studies from the United States, a recent study from the United Kingdom examined the impact of Cannabis potency on dependence.20 This cross-sectional survey found that, although HPC was preferred by users and was rated as offering the “best high,” its use was associated with increasing severity of dependence, especially among young people. The limited available evidence supports a greater risk of Cannabis use disorders with increasing potency.
Psychosis
Based on longitudinal studies published over the past 30 years, it is clear that using Cannabis at a young age (age <15 to 18) increases the risk of developing a psychotic disorder.21 This association appears to be dose-dependent, with studies consistently demonstrating that psychosis risk increases with greater frequency of Cannabis use.22 The accumulated evidence to date is strong enough to view the psychotic potential of Cannabis as a significant public health concern.21
If risk of psychosis is proportional to the amount of Cannabis used as measured by frequency, it follows that this risk might be affected similarly by Cannabis potency. In another paper, I discussed the potential for greater risk of psychosis in the context of medical marijuana and synthetic cannabinoids.23 My colleagues and I also have published case reports describing emerging psychosis among regular Cannabis users after escalating to higher potency medical marijuana24 and a hyperconcentrated form of hash oil known as Cannabis “wax” or “dabs” that contains as much as 90% THC.4 Preliminary anecdotal evidence supports the plausibility of HPC being more psychotoxic than less potent forms.
Several studies from a research group in the United Kingdom, where sinsemilla has increasingly dominated the drug market, likewise have reported that the use of HPC is associated with a greater risk of psychosis. The first of these studies, published in 2009, found that adults hospitalized for first-episode psychosis were more likely to have used HPC than healthy controls.25 Among Cannabis users, HPC use was associated with a 7-fold increased risk of psychosis, with daily HPC use associated with a 12-fold increased risk.
Based on a larger dataset, a second study reported that high-potency, but not low-potency, Cannabis increased the risk of first-episode psychosis with increasing frequency of use.26 Daily users of HPC had a 5-fold higher risk of psychosis compared with those that had never used Cannabis. A third study reported that HPC use and daily Cannabis use were independently associated with an earlier onset of first-episode psychosis, with daily HPC users developing first-episode psychosis an average of 6 years earlier than non-Cannabis users.27 Finally, a prospective study following patients with first-episode psychosis over 2 years found that the greatest risk of relapse—defined by hospital admission caused by exacerbation of psychotic symptoms—was found among self-reported daily users of HPC, while the lowest risk was among those who stopped using Cannabis after their initial psychotic episode.28
The findings from these 4 studies suggest that the increased risk of psychosis with Cannabis is proportional to overall exposure, determined by both frequency of use and Cannabis potency.
Cognition
There is little doubt that using Cannabis can impair cognition acutely, “after all, this is the basic reason for its recreational use,” as one author wrote.29 As with psychosis, the available evidence indicates that the degree of cognitive impairment is related to the frequency and duration of Cannabis use as well as age of onset of use.30,31
Few studies have assessed cognitive functioning in relation to Cannabis potency with most only examining the effects of relatively low-potency Cannabis with inconsistent results. For example, 2 studies compared cognitive performance in individuals smoking Cannabis with 1.8% and 3.9% THC. One study found that using higher potency Cannabis resulted in prolonged time needed to complete certain cognitive tasks,32 whereas the other found greater impairment in performance on a decision-making task at both potencies compared with non-users but no differences between the 2 dosages.33 Detecting significant differences may be difficult within the narrow range of low Cannabis potency studied where any findings have limited applicability in the context of today’s Cannabis with much higher THC content.
To date, only 1 study has assessed cognition at higher Cannabis potencies, comparing Cannabis with 4% THC to 13% THC.34 Cognitive impairments increased with higher potency, especially in tasks that measured motor control and executive functioning. Therefore it appears that higher potency Cannabis use is associated with greater acute cognitive impairment.
The longer-term effects on cognition are less clear, with conflicting evidence about whether Cannabis use can result in residual cognitive impairment despite abstinence.30,35 A recent review concluded that “the magnitude of neuropsychological impairment and the extent to which it persists after abstinence may depend on the frequency and the duration of Cannabis use, length of abstinence, and age at onset of use.”31 The effects of HPC on long-term cognitive deficits have not been studied.
Structural brain changes
A number of studies have determined an association between Cannabis use and brain changes involving structures governing memory and emotional processing, including reduced volume of the hippocampus,36 temporal cortex, insula, and orbitofrontal cortex.37 Although many of these changes appear to be dose-related, some morphologic changes have been reported among young recreational users without Cannabis dependence.38 This has resulted in an understandable concern about the effects of Cannabis on the brains of young people with limited exposure; however, it is not yet clear to what extent detected brain changes are pathological and reflect functional deficits.
Recent research using newer neuroimaging modalities provides preliminary support of Cannabis use associated with white matter changes that, in turn, are correlated with cognitive impairment.39 One study comparing low-potency Cannabis and HPC users with and without first-episode psychosis found a significant effect of Cannabis potency on disturbances in white matter microstructural organization in the corpus callosum.40 These findings provide sufficient cause for concern that structural brain changes associated with cognitive impairment are more likely to occur with HPC use.
Recommendations for clinicians
Similar to any drug, the effects of THC and its psychiatric sequelae can be expected to increase with dosage. To date, much of the information about psychiatric risks has been based on studies of low- and moderate-potency Cannabis rather than the much higher potency Cannabis products, such as hyper-concentrated “wax dabs,” that are available today. Data from social media suggest that these products may be associated with novel patterns of use, such as with the intention of “passing out.”41 It is likely that clinicians will encounter greater psychiatric morbidity associated with HPC use.
Although clinicians may be accustomed to asking about the frequency and duration of Cannabis use, it is now prudent also to ask patients about Cannabis potency to better assess the potential risks of use. The potency of different marijuana products is openly advertised within some “medical marijuana” dispensaries, although the accuracy of information in products such as “edibles” has been called into question.5
Physicians are increasingly asked to provide recommendations on “medical marijuana” use. A recent paper outlined characteristics of appropriate candidates for “medical marijuana” including:
- having a debilitating condition that might benefit from Cannabis
- multiple failed trials of conventional pharmacotherapies including FDA-approved cannabinoids
- lack of substance use disorders, psychosis, or unstable mood or anxiety disorders
- residence in a state where “medical marijuana” is legal.42
As part of the informed consent process, physicians providing recommendations for “medical marijuana” now must consider the effects of HPC when weighing potential risks against any benefits of Cannabis use. Those monitoring patients using Cannabis should be aware of the potential for greater psychiatric morbidity with HPC and should educate patients about that risk. Failure to adequately warn patients about such morbidity or to screen for risk factors such as psychosis could leave physicians vulnerable to malpractice litigation.
In the United States, the average potency of Cannabis has increased significantly over the past few decades in response to consumer demand and policies in some states that have legalized marijuana for medicinal and recreational purposes.1 Whereas the delta-9-tetrahydrocannabinol (THC) content of “street” marijuana was <1% in the 1970s and 4% in the 1990s, by 2012, analyses of Cannabis samples seized by law enforcement agencies documented a rise in average THC potency to >12%.1-3
Although this increase in potency has been overstated in the media because studies did not control for the effects of changes in sampling methods on freshness, it is estimated that Cannabis potency increased 7-fold from 1970 to 2010.3 Also, Cannabis preparations such as hashish and hash oil extracts containing THC well above average—from 35% to 90% THC—are now more widely available. In states where marijuana has been legalized, high-potency Cannabis (HPC) in the form of “edibles” (eg, marijuana added to baked goods, candy, or drinks) and hash oil extracts (Table 1)4-13 can be readily obtained from dispensaries or even at local farmers’ markets.

The potency of Cannabis, typically defined as the percentage of THC, its chief psychoactive component, varies depending on the genetic strain of the plant, cultivation techniques, and methods of processing and storage. For example, relative to “average marijuana,” hemp (Cannabis bred for industrial purposes) has very little THC, while sinsemilla (flowering buds from unpollinated female plants), hashish (Cannabis resin), and extracted hash oil contain increasing amounts of THC (Table 2).1,2
As THC levels in Cannabis have risen over time, cannabidiol (CBD) levels have dropped to <0.2%.2 Although THC appears to be largely responsible for the psychiatric morbidity associated with Cannabis, CBD may have neuroprotective and antipsychotic properties.14,15 The sharp spike in the THC:CBD ratio in recent years therefore raises the possibility that Cannabis use today might carry a much greater risk of psychiatric sequelae than it did in previous generations.
This article reviews the evidence for an increased risk of psychiatric morbidity with increasing Cannabis potency.
Cannabis use disorder
Recent data indicate that the prevalence of Cannabis use disorders (eg, abuse and dependence) in the United States is approximately 3% among the general population and >30% among Cannabis users.16 The availability of increasingly potent forms of Cannabis has been cited as a possible explanation for this rise, despite no change in the prevalence of overall marijuana use between 1991 to 1992 and 2001 to 2002.17 However, while the prevalence of marijuana use disorders has continued to rise—nearly doubling from 2001 to 2002 to 2012 to 2013—this latest increase occurred with a significant increase in overall marijuana use, such that the actual rate of Cannabis use disorders among users seems to have plateaued, despite the continued rise in marijuana potency.16 This discrepancy could be explained if Cannabis users cut back past a specific threshold of increasing potency. However, 2 studies have called into question how effective such titration efforts might be in practice. In one study, Cannabis users who preferred more potent Cannabis inhaled lower volumes of smoke, but did not fully compensate for the increased potency, such that use of HPC still resulted in greater THC exposure.18 Another study found that HPC users rolled less marijuana into their joints but not enough to mitigate the impact of greater potency.19 Therefore, it appears that HPC users typically expose themselves to greater amounts of THC, which could place them at higher risk of addiction.
Although a causal association between increasing Cannabis potency and the rate of substance use disorders among users remains unclear based on epidemiologic studies from the United States, a recent study from the United Kingdom examined the impact of Cannabis potency on dependence.20 This cross-sectional survey found that, although HPC was preferred by users and was rated as offering the “best high,” its use was associated with increasing severity of dependence, especially among young people. The limited available evidence supports a greater risk of Cannabis use disorders with increasing potency.
Psychosis
Based on longitudinal studies published over the past 30 years, it is clear that using Cannabis at a young age (age <15 to 18) increases the risk of developing a psychotic disorder.21 This association appears to be dose-dependent, with studies consistently demonstrating that psychosis risk increases with greater frequency of Cannabis use.22 The accumulated evidence to date is strong enough to view the psychotic potential of Cannabis as a significant public health concern.21
If risk of psychosis is proportional to the amount of Cannabis used as measured by frequency, it follows that this risk might be affected similarly by Cannabis potency. In another paper, I discussed the potential for greater risk of psychosis in the context of medical marijuana and synthetic cannabinoids.23 My colleagues and I also have published case reports describing emerging psychosis among regular Cannabis users after escalating to higher potency medical marijuana24 and a hyperconcentrated form of hash oil known as Cannabis “wax” or “dabs” that contains as much as 90% THC.4 Preliminary anecdotal evidence supports the plausibility of HPC being more psychotoxic than less potent forms.
Several studies from a research group in the United Kingdom, where sinsemilla has increasingly dominated the drug market, likewise have reported that the use of HPC is associated with a greater risk of psychosis. The first of these studies, published in 2009, found that adults hospitalized for first-episode psychosis were more likely to have used HPC than healthy controls.25 Among Cannabis users, HPC use was associated with a 7-fold increased risk of psychosis, with daily HPC use associated with a 12-fold increased risk.
Based on a larger dataset, a second study reported that high-potency, but not low-potency, Cannabis increased the risk of first-episode psychosis with increasing frequency of use.26 Daily users of HPC had a 5-fold higher risk of psychosis compared with those that had never used Cannabis. A third study reported that HPC use and daily Cannabis use were independently associated with an earlier onset of first-episode psychosis, with daily HPC users developing first-episode psychosis an average of 6 years earlier than non-Cannabis users.27 Finally, a prospective study following patients with first-episode psychosis over 2 years found that the greatest risk of relapse—defined by hospital admission caused by exacerbation of psychotic symptoms—was found among self-reported daily users of HPC, while the lowest risk was among those who stopped using Cannabis after their initial psychotic episode.28
The findings from these 4 studies suggest that the increased risk of psychosis with Cannabis is proportional to overall exposure, determined by both frequency of use and Cannabis potency.
Cognition
There is little doubt that using Cannabis can impair cognition acutely, “after all, this is the basic reason for its recreational use,” as one author wrote.29 As with psychosis, the available evidence indicates that the degree of cognitive impairment is related to the frequency and duration of Cannabis use as well as age of onset of use.30,31
Few studies have assessed cognitive functioning in relation to Cannabis potency with most only examining the effects of relatively low-potency Cannabis with inconsistent results. For example, 2 studies compared cognitive performance in individuals smoking Cannabis with 1.8% and 3.9% THC. One study found that using higher potency Cannabis resulted in prolonged time needed to complete certain cognitive tasks,32 whereas the other found greater impairment in performance on a decision-making task at both potencies compared with non-users but no differences between the 2 dosages.33 Detecting significant differences may be difficult within the narrow range of low Cannabis potency studied where any findings have limited applicability in the context of today’s Cannabis with much higher THC content.
To date, only 1 study has assessed cognition at higher Cannabis potencies, comparing Cannabis with 4% THC to 13% THC.34 Cognitive impairments increased with higher potency, especially in tasks that measured motor control and executive functioning. Therefore it appears that higher potency Cannabis use is associated with greater acute cognitive impairment.
The longer-term effects on cognition are less clear, with conflicting evidence about whether Cannabis use can result in residual cognitive impairment despite abstinence.30,35 A recent review concluded that “the magnitude of neuropsychological impairment and the extent to which it persists after abstinence may depend on the frequency and the duration of Cannabis use, length of abstinence, and age at onset of use.”31 The effects of HPC on long-term cognitive deficits have not been studied.
Structural brain changes
A number of studies have determined an association between Cannabis use and brain changes involving structures governing memory and emotional processing, including reduced volume of the hippocampus,36 temporal cortex, insula, and orbitofrontal cortex.37 Although many of these changes appear to be dose-related, some morphologic changes have been reported among young recreational users without Cannabis dependence.38 This has resulted in an understandable concern about the effects of Cannabis on the brains of young people with limited exposure; however, it is not yet clear to what extent detected brain changes are pathological and reflect functional deficits.
Recent research using newer neuroimaging modalities provides preliminary support of Cannabis use associated with white matter changes that, in turn, are correlated with cognitive impairment.39 One study comparing low-potency Cannabis and HPC users with and without first-episode psychosis found a significant effect of Cannabis potency on disturbances in white matter microstructural organization in the corpus callosum.40 These findings provide sufficient cause for concern that structural brain changes associated with cognitive impairment are more likely to occur with HPC use.
Recommendations for clinicians
Similar to any drug, the effects of THC and its psychiatric sequelae can be expected to increase with dosage. To date, much of the information about psychiatric risks has been based on studies of low- and moderate-potency Cannabis rather than the much higher potency Cannabis products, such as hyper-concentrated “wax dabs,” that are available today. Data from social media suggest that these products may be associated with novel patterns of use, such as with the intention of “passing out.”41 It is likely that clinicians will encounter greater psychiatric morbidity associated with HPC use.
Although clinicians may be accustomed to asking about the frequency and duration of Cannabis use, it is now prudent also to ask patients about Cannabis potency to better assess the potential risks of use. The potency of different marijuana products is openly advertised within some “medical marijuana” dispensaries, although the accuracy of information in products such as “edibles” has been called into question.5
Physicians are increasingly asked to provide recommendations on “medical marijuana” use. A recent paper outlined characteristics of appropriate candidates for “medical marijuana” including:
- having a debilitating condition that might benefit from Cannabis
- multiple failed trials of conventional pharmacotherapies including FDA-approved cannabinoids
- lack of substance use disorders, psychosis, or unstable mood or anxiety disorders
- residence in a state where “medical marijuana” is legal.42
As part of the informed consent process, physicians providing recommendations for “medical marijuana” now must consider the effects of HPC when weighing potential risks against any benefits of Cannabis use. Those monitoring patients using Cannabis should be aware of the potential for greater psychiatric morbidity with HPC and should educate patients about that risk. Failure to adequately warn patients about such morbidity or to screen for risk factors such as psychosis could leave physicians vulnerable to malpractice litigation.




