User login
Pornography warps children’s concept of sex, sexual identity
SAN FRANCISCO – The pornography industry has taken over children’s sense of self and sexuality and warped their concept of what sex and a sexual identity is, said Gail Dines, PhD.
She challenged pediatricians to shape policy and help parents in wrangling back that control in a presentation at the annual meeting of the American Academy of Pediatrics.
The culprit, Dr. Dines charged, is the multibillion-dollar porn industry that exploded around the year 2000 with the Internet. Then, in 2011, the business model shifted to free pornography to hook young boys in their adolescence and hopefully maintain them as customers after age 18 when they could get their own credit cards.
The average age of a boy’s first encounter with pornography is age 11, explained Dr. Dines, a professor of sociology and women’s studies at Wheelock College in Chestnut Hill, Mass.
Instead of a father’s Playboy featuring a naked woman in a cornfield, as many male pediatricians in the room might have been introduced to pornography or sexuality, today’s youth are introduced via the brutalization and dehumanization of women, she said. Such experiences traumatize the children viewing them, who become confused about who they are if they are masturbating to images and video of sexual violence, and then they enter a cycle of retraumatization that engenders shame while bringing children back to those sites again and again.
“Hence, in the business model of free porn, you are building in trauma, which is building in addiction,” Dr. Dines said. The effects of this exposure and addiction, based on decades of research, include limited capacity for intimacy, a greater likelihood of using coercive tactics for sex, decreased empathy for rape victims, increased depression and anxiety, and, most recently, rates of erectile dysfunction in males aged 15-27 that mirror the rates in those aged 27-35.
“We have never brought up boys with access to hard core pornography 24-7,” Dr. Dines said. The best way to tackle hard-core pornography is a public health model that educates parents and pediatricians who can band together to raise awareness. Her organization, Culture Reframed, is attempting to do precisely that.
Dr. Dines founded the nonprofit Culture Reframed, which attempts to counter the effects of the pornography industry and media sexuality. Her presentation used no external funding.
SAN FRANCISCO – The pornography industry has taken over children’s sense of self and sexuality and warped their concept of what sex and a sexual identity is, said Gail Dines, PhD.
She challenged pediatricians to shape policy and help parents in wrangling back that control in a presentation at the annual meeting of the American Academy of Pediatrics.
The culprit, Dr. Dines charged, is the multibillion-dollar porn industry that exploded around the year 2000 with the Internet. Then, in 2011, the business model shifted to free pornography to hook young boys in their adolescence and hopefully maintain them as customers after age 18 when they could get their own credit cards.
The average age of a boy’s first encounter with pornography is age 11, explained Dr. Dines, a professor of sociology and women’s studies at Wheelock College in Chestnut Hill, Mass.
Instead of a father’s Playboy featuring a naked woman in a cornfield, as many male pediatricians in the room might have been introduced to pornography or sexuality, today’s youth are introduced via the brutalization and dehumanization of women, she said. Such experiences traumatize the children viewing them, who become confused about who they are if they are masturbating to images and video of sexual violence, and then they enter a cycle of retraumatization that engenders shame while bringing children back to those sites again and again.
“Hence, in the business model of free porn, you are building in trauma, which is building in addiction,” Dr. Dines said. The effects of this exposure and addiction, based on decades of research, include limited capacity for intimacy, a greater likelihood of using coercive tactics for sex, decreased empathy for rape victims, increased depression and anxiety, and, most recently, rates of erectile dysfunction in males aged 15-27 that mirror the rates in those aged 27-35.
“We have never brought up boys with access to hard core pornography 24-7,” Dr. Dines said. The best way to tackle hard-core pornography is a public health model that educates parents and pediatricians who can band together to raise awareness. Her organization, Culture Reframed, is attempting to do precisely that.
Dr. Dines founded the nonprofit Culture Reframed, which attempts to counter the effects of the pornography industry and media sexuality. Her presentation used no external funding.
SAN FRANCISCO – The pornography industry has taken over children’s sense of self and sexuality and warped their concept of what sex and a sexual identity is, said Gail Dines, PhD.
She challenged pediatricians to shape policy and help parents in wrangling back that control in a presentation at the annual meeting of the American Academy of Pediatrics.
The culprit, Dr. Dines charged, is the multibillion-dollar porn industry that exploded around the year 2000 with the Internet. Then, in 2011, the business model shifted to free pornography to hook young boys in their adolescence and hopefully maintain them as customers after age 18 when they could get their own credit cards.
The average age of a boy’s first encounter with pornography is age 11, explained Dr. Dines, a professor of sociology and women’s studies at Wheelock College in Chestnut Hill, Mass.
Instead of a father’s Playboy featuring a naked woman in a cornfield, as many male pediatricians in the room might have been introduced to pornography or sexuality, today’s youth are introduced via the brutalization and dehumanization of women, she said. Such experiences traumatize the children viewing them, who become confused about who they are if they are masturbating to images and video of sexual violence, and then they enter a cycle of retraumatization that engenders shame while bringing children back to those sites again and again.
“Hence, in the business model of free porn, you are building in trauma, which is building in addiction,” Dr. Dines said. The effects of this exposure and addiction, based on decades of research, include limited capacity for intimacy, a greater likelihood of using coercive tactics for sex, decreased empathy for rape victims, increased depression and anxiety, and, most recently, rates of erectile dysfunction in males aged 15-27 that mirror the rates in those aged 27-35.
“We have never brought up boys with access to hard core pornography 24-7,” Dr. Dines said. The best way to tackle hard-core pornography is a public health model that educates parents and pediatricians who can band together to raise awareness. Her organization, Culture Reframed, is attempting to do precisely that.
Dr. Dines founded the nonprofit Culture Reframed, which attempts to counter the effects of the pornography industry and media sexuality. Her presentation used no external funding.
EXPERT ANALYSIS FROM AAP 16
New criteria estimate systemic sclerosis to be more prevalent in primary biliary cholangitis patients
The use of outdated criteria for the identification of systemic sclerosis in primary biliary cholangitis likely led to an underestimation of the comorbidity’s prevalence.
Furthermore, more recent criteria estimate the prevalence of systemic sclerosis in primary biliary cholangitis to be around 23%.
[[{"fid":"172520","view_mode":"medstat_image_flush_right","fields":{"format":"medstat_image_flush_right","field_file_image_alt_text[und][0][value]":"Clinical appearance of acrosclerotic piece-meal necrosis of the first digit in a patient with systemic sclerosis.","field_file_image_credit[und][0][value]":"BMC Dermatology 2004, 4:11. doi:10.1186/1471-5945-4-11 ","field_file_image_caption[und][0][value]":""},"type":"media","attributes":{"class":"media-element file-medstat_image_flush_right"}}]]In 1980, the American College of Rheumatology defined “highly specific but not sensitive” criteria for the identification of primary biliary cholangitis patients who also had systemic sclerosis, reported the study’s lead investigator Dr. Boyang Zheng of the University of Montreal Hospital Center and associates (J Rheumatol. 2016 Oct 28. doi: 10.3899/jrheum.160243).
In 2001, LeRoy and Medsger proposed and validated a new set of criteria that centrally required the observation of Raynaud phenomenon and “allowed for greater sensitivity and diagnosis of earlier disease by incorporating advances in nailfold capillary microscopy and [systemic sclerosis]–specific antibodies,” the investigators wrote.
Most recently, the ACR and the European League Against Rheumatism jointly developed new “weighted-point criteria endorsed for use in systemic sclerosis inclusion studies.” These new criteria, which were published in 2013, “the addition of at least a clinical or radiological feature to be positive.”
The purpose of this study, the first of its kind, according to investigators, was to compare the prevalence estimates of systemic sclerosis in primary biliary cholangitis patients as predicted by each of the three criteria sets.
A total of 100 patients who had previously been diagnosed with primary biliary cholangitis but not systemic sclerosis were recruited into the study. The majority of the patients were female (91%), the mean age at first visit was 57 years, and the mean primary biliary cholangitis Mayo score of disease severity and survival was 4.14.
At time of study enrollment, medical histories were obtained. All patients also underwent nailfold capillary microscopy, and serum samples were collected and analyzed for the presence of primary biliary cholangitis antibodies and the following systemic sclerosis–specific antibodies: anti–CENP-B, anti–topo I, anti–RNAP III, anti-Th/To.
Clinical data, presence of antibodies, and capillarascopic patterns were analyzed, and patients were retroactively evaluated for the fulfillment of each of the three systemic sclerosis criteria sets.
“A total of 23 patients satisfied at least one set of criteria, with 22 being positive for LeRoy and Medsger criteria, 17 for ACR/EULAR criteria, and only 1 for the ACR 1980 criteria,” Dr. Zheng and his associates reported.
The most frequent systemic sclerosis–associated features in the study population were Raynaud phenomenon (39%), systemic sclerosis antibodies (26%), abnormal nailfold capillary microscopy (20%), and capillary telangiectases (17%), while clinically evident skin changes were the most rare, investigators explained.
The 1980 ACR criteria likely led to an underestimation of systemic sclerosis in primary biliary cirrhosis, and given the benefit of early diagnosis and treatment of systemic sclerosis, patients with primary biliary cholangitis should be screened for Raynaud phenomenon and systemic sclerosis antibodies and undergo nailfold capillaroscopic microscopy, the investigators recommended.
“Clinicians need to remain alert for this sometimes insidious comorbidity,” the researchers added.
Dr. Zheng had no relevant financial disclosures.
[email protected]
On Twitter @jessnicolecraig
The use of outdated criteria for the identification of systemic sclerosis in primary biliary cholangitis likely led to an underestimation of the comorbidity’s prevalence.
Furthermore, more recent criteria estimate the prevalence of systemic sclerosis in primary biliary cholangitis to be around 23%.
[[{"fid":"172520","view_mode":"medstat_image_flush_right","fields":{"format":"medstat_image_flush_right","field_file_image_alt_text[und][0][value]":"Clinical appearance of acrosclerotic piece-meal necrosis of the first digit in a patient with systemic sclerosis.","field_file_image_credit[und][0][value]":"BMC Dermatology 2004, 4:11. doi:10.1186/1471-5945-4-11 ","field_file_image_caption[und][0][value]":""},"type":"media","attributes":{"class":"media-element file-medstat_image_flush_right"}}]]In 1980, the American College of Rheumatology defined “highly specific but not sensitive” criteria for the identification of primary biliary cholangitis patients who also had systemic sclerosis, reported the study’s lead investigator Dr. Boyang Zheng of the University of Montreal Hospital Center and associates (J Rheumatol. 2016 Oct 28. doi: 10.3899/jrheum.160243).
In 2001, LeRoy and Medsger proposed and validated a new set of criteria that centrally required the observation of Raynaud phenomenon and “allowed for greater sensitivity and diagnosis of earlier disease by incorporating advances in nailfold capillary microscopy and [systemic sclerosis]–specific antibodies,” the investigators wrote.
Most recently, the ACR and the European League Against Rheumatism jointly developed new “weighted-point criteria endorsed for use in systemic sclerosis inclusion studies.” These new criteria, which were published in 2013, “the addition of at least a clinical or radiological feature to be positive.”
The purpose of this study, the first of its kind, according to investigators, was to compare the prevalence estimates of systemic sclerosis in primary biliary cholangitis patients as predicted by each of the three criteria sets.
A total of 100 patients who had previously been diagnosed with primary biliary cholangitis but not systemic sclerosis were recruited into the study. The majority of the patients were female (91%), the mean age at first visit was 57 years, and the mean primary biliary cholangitis Mayo score of disease severity and survival was 4.14.
At time of study enrollment, medical histories were obtained. All patients also underwent nailfold capillary microscopy, and serum samples were collected and analyzed for the presence of primary biliary cholangitis antibodies and the following systemic sclerosis–specific antibodies: anti–CENP-B, anti–topo I, anti–RNAP III, anti-Th/To.
Clinical data, presence of antibodies, and capillarascopic patterns were analyzed, and patients were retroactively evaluated for the fulfillment of each of the three systemic sclerosis criteria sets.
“A total of 23 patients satisfied at least one set of criteria, with 22 being positive for LeRoy and Medsger criteria, 17 for ACR/EULAR criteria, and only 1 for the ACR 1980 criteria,” Dr. Zheng and his associates reported.
The most frequent systemic sclerosis–associated features in the study population were Raynaud phenomenon (39%), systemic sclerosis antibodies (26%), abnormal nailfold capillary microscopy (20%), and capillary telangiectases (17%), while clinically evident skin changes were the most rare, investigators explained.
The 1980 ACR criteria likely led to an underestimation of systemic sclerosis in primary biliary cirrhosis, and given the benefit of early diagnosis and treatment of systemic sclerosis, patients with primary biliary cholangitis should be screened for Raynaud phenomenon and systemic sclerosis antibodies and undergo nailfold capillaroscopic microscopy, the investigators recommended.
“Clinicians need to remain alert for this sometimes insidious comorbidity,” the researchers added.
Dr. Zheng had no relevant financial disclosures.
[email protected]
On Twitter @jessnicolecraig
The use of outdated criteria for the identification of systemic sclerosis in primary biliary cholangitis likely led to an underestimation of the comorbidity’s prevalence.
Furthermore, more recent criteria estimate the prevalence of systemic sclerosis in primary biliary cholangitis to be around 23%.
[[{"fid":"172520","view_mode":"medstat_image_flush_right","fields":{"format":"medstat_image_flush_right","field_file_image_alt_text[und][0][value]":"Clinical appearance of acrosclerotic piece-meal necrosis of the first digit in a patient with systemic sclerosis.","field_file_image_credit[und][0][value]":"BMC Dermatology 2004, 4:11. doi:10.1186/1471-5945-4-11 ","field_file_image_caption[und][0][value]":""},"type":"media","attributes":{"class":"media-element file-medstat_image_flush_right"}}]]In 1980, the American College of Rheumatology defined “highly specific but not sensitive” criteria for the identification of primary biliary cholangitis patients who also had systemic sclerosis, reported the study’s lead investigator Dr. Boyang Zheng of the University of Montreal Hospital Center and associates (J Rheumatol. 2016 Oct 28. doi: 10.3899/jrheum.160243).
In 2001, LeRoy and Medsger proposed and validated a new set of criteria that centrally required the observation of Raynaud phenomenon and “allowed for greater sensitivity and diagnosis of earlier disease by incorporating advances in nailfold capillary microscopy and [systemic sclerosis]–specific antibodies,” the investigators wrote.
Most recently, the ACR and the European League Against Rheumatism jointly developed new “weighted-point criteria endorsed for use in systemic sclerosis inclusion studies.” These new criteria, which were published in 2013, “the addition of at least a clinical or radiological feature to be positive.”
The purpose of this study, the first of its kind, according to investigators, was to compare the prevalence estimates of systemic sclerosis in primary biliary cholangitis patients as predicted by each of the three criteria sets.
A total of 100 patients who had previously been diagnosed with primary biliary cholangitis but not systemic sclerosis were recruited into the study. The majority of the patients were female (91%), the mean age at first visit was 57 years, and the mean primary biliary cholangitis Mayo score of disease severity and survival was 4.14.
At time of study enrollment, medical histories were obtained. All patients also underwent nailfold capillary microscopy, and serum samples were collected and analyzed for the presence of primary biliary cholangitis antibodies and the following systemic sclerosis–specific antibodies: anti–CENP-B, anti–topo I, anti–RNAP III, anti-Th/To.
Clinical data, presence of antibodies, and capillarascopic patterns were analyzed, and patients were retroactively evaluated for the fulfillment of each of the three systemic sclerosis criteria sets.
“A total of 23 patients satisfied at least one set of criteria, with 22 being positive for LeRoy and Medsger criteria, 17 for ACR/EULAR criteria, and only 1 for the ACR 1980 criteria,” Dr. Zheng and his associates reported.
The most frequent systemic sclerosis–associated features in the study population were Raynaud phenomenon (39%), systemic sclerosis antibodies (26%), abnormal nailfold capillary microscopy (20%), and capillary telangiectases (17%), while clinically evident skin changes were the most rare, investigators explained.
The 1980 ACR criteria likely led to an underestimation of systemic sclerosis in primary biliary cirrhosis, and given the benefit of early diagnosis and treatment of systemic sclerosis, patients with primary biliary cholangitis should be screened for Raynaud phenomenon and systemic sclerosis antibodies and undergo nailfold capillaroscopic microscopy, the investigators recommended.
“Clinicians need to remain alert for this sometimes insidious comorbidity,” the researchers added.
Dr. Zheng had no relevant financial disclosures.
[email protected]
On Twitter @jessnicolecraig
FROM THE JOURNAL OF RHEUMATOLOGY
Key clinical point:
Major finding: The prevalence of systemic sclerosis in primary biliary cholangitis, according to new criteria, is around 23%.
Data source: Evaluation of systemic sclerosis in 100 patients previously diagnosed with primary biliary cholangitis.
Disclosures: Dr. Zheng had no relevant financial disclosures.
In era of infliximab, ulcerative colitis surgical outcomes worsen
WASHINGTON – The era of powerful biologics has led to unforeseen surgical outcomes in patients with ulcerative colitis.
Patients undergoing surgery for ulcerative colitis now are 38% more likely to die in the hospital than they were 15 years ago, before infliximab and other biologics were adopted as medical therapy for the disease. A database review covering 18 years found that other surgical outcomes are worse, too, Jonathan Abelson, MD, said at the annual clinical congress of the American College of Surgeons.
“These very powerful agents could be completely eliminating the need for surgery in patients with mild disease, leaving surgery for those who have very advanced disease and didn’t respond well to medical therapy,” said Dr. Abelson, a clinical research fellow at New York–Presbyterian Hospital, N.Y. “We are operating now only on patients with very severe disease, not the wider range of patients we had 15 years ago, when there weren’t as effective medical options.”
He and his colleagues used the New York Statewide Planning and Research Cooperative System (SPARCS) database to identify 7,070 patients who had undergone bowel resection for ulcerative colitis during two epochs: prebiologics (1995-2005) and postbiologics (2006-2013). The cohorts were about evenly split in numbers.
There were some statistically significant differences in baseline characteristics. Patients in epoch 2 were about a year older (51 vs. 50 years). Significantly more of them had at least two major comorbidities (28% vs. 18%). Minimally invasive surgery was significantly more common in epoch 2 (28% vs. 3%).
Significantly more surgeries in epoch 2 were staged into three or more procedures (14% vs. 9%). This finding probably reflects the level of disease severity in those presenting for surgery or the fact that they underwent surgery after recently receiving biologics, Dr. Abelson said.
“One of the limits of this study is that we don’t know exactly the reasons for these one-, two-, or three-stage surgeries. The theory is that patients who were more ill at presentation are more likely to have a multistaged surgery. Another reason could be that if they are on these powerful immunosuppressive regimens, the surgeon might be concerned about not healing well from a definitive one- or two-stage surgery.”
He then conducted a multivariate analysis that controlled for baseline factors, including a variety of individual comorbid conditions. In this analysis, patients in epoch 2 were 38% more likely to die in the hospital and 51% more likely to experience a major postoperative event, like shock, pulmonary embolism, stroke, or heart attack. The chance of a surgical complication was increased by 39%, and these patients were 25% more likely to need a transfusion during surgery than those from epoch 1.
The poorer outcomes held for an at least an entire year after surgery, Dr. Abelson said. At 1 year, patients in epoch 2 were 36% more likely to have a readmission than those in epoch 1. Major events and procedural complications were both 46% more likely. Patients were also 36% more likely to require an additional procedure.
“These are not the outcomes we want to see, especially in this era when our surgical techniques have improved so much,” Dr. Abelson said. “If what this represents, though, is that we are now operating on a higher-risk population, we can’t just say, ‘Well, that’s how it’s going to be.’ We need to figure out how to minimize morbidity and mortality in this high-risk patient population.”
One goal, he suggested, would be to assess response to a biologic regimen earlier in the hopes of determining who will respond well, and moving ahead with surgery in those who don’t.
This is a tough sell for patients, he said.
“There is a big fear of this surgery. It usually requires a temporary ileostomy and a stoma bag, and patients are terrified of that. There have been a few studies demonstrating that earlier referral to surgery improves quality of life; living with advanced ulcerative colitis can be extremely difficult and patients often feel a lot better after we remove their diseased colon. But getting there is a challenge.”
Dr. Abelson had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
WASHINGTON – The era of powerful biologics has led to unforeseen surgical outcomes in patients with ulcerative colitis.
Patients undergoing surgery for ulcerative colitis now are 38% more likely to die in the hospital than they were 15 years ago, before infliximab and other biologics were adopted as medical therapy for the disease. A database review covering 18 years found that other surgical outcomes are worse, too, Jonathan Abelson, MD, said at the annual clinical congress of the American College of Surgeons.
“These very powerful agents could be completely eliminating the need for surgery in patients with mild disease, leaving surgery for those who have very advanced disease and didn’t respond well to medical therapy,” said Dr. Abelson, a clinical research fellow at New York–Presbyterian Hospital, N.Y. “We are operating now only on patients with very severe disease, not the wider range of patients we had 15 years ago, when there weren’t as effective medical options.”
He and his colleagues used the New York Statewide Planning and Research Cooperative System (SPARCS) database to identify 7,070 patients who had undergone bowel resection for ulcerative colitis during two epochs: prebiologics (1995-2005) and postbiologics (2006-2013). The cohorts were about evenly split in numbers.
There were some statistically significant differences in baseline characteristics. Patients in epoch 2 were about a year older (51 vs. 50 years). Significantly more of them had at least two major comorbidities (28% vs. 18%). Minimally invasive surgery was significantly more common in epoch 2 (28% vs. 3%).
Significantly more surgeries in epoch 2 were staged into three or more procedures (14% vs. 9%). This finding probably reflects the level of disease severity in those presenting for surgery or the fact that they underwent surgery after recently receiving biologics, Dr. Abelson said.
“One of the limits of this study is that we don’t know exactly the reasons for these one-, two-, or three-stage surgeries. The theory is that patients who were more ill at presentation are more likely to have a multistaged surgery. Another reason could be that if they are on these powerful immunosuppressive regimens, the surgeon might be concerned about not healing well from a definitive one- or two-stage surgery.”
He then conducted a multivariate analysis that controlled for baseline factors, including a variety of individual comorbid conditions. In this analysis, patients in epoch 2 were 38% more likely to die in the hospital and 51% more likely to experience a major postoperative event, like shock, pulmonary embolism, stroke, or heart attack. The chance of a surgical complication was increased by 39%, and these patients were 25% more likely to need a transfusion during surgery than those from epoch 1.
The poorer outcomes held for an at least an entire year after surgery, Dr. Abelson said. At 1 year, patients in epoch 2 were 36% more likely to have a readmission than those in epoch 1. Major events and procedural complications were both 46% more likely. Patients were also 36% more likely to require an additional procedure.
“These are not the outcomes we want to see, especially in this era when our surgical techniques have improved so much,” Dr. Abelson said. “If what this represents, though, is that we are now operating on a higher-risk population, we can’t just say, ‘Well, that’s how it’s going to be.’ We need to figure out how to minimize morbidity and mortality in this high-risk patient population.”
One goal, he suggested, would be to assess response to a biologic regimen earlier in the hopes of determining who will respond well, and moving ahead with surgery in those who don’t.
This is a tough sell for patients, he said.
“There is a big fear of this surgery. It usually requires a temporary ileostomy and a stoma bag, and patients are terrified of that. There have been a few studies demonstrating that earlier referral to surgery improves quality of life; living with advanced ulcerative colitis can be extremely difficult and patients often feel a lot better after we remove their diseased colon. But getting there is a challenge.”
Dr. Abelson had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
WASHINGTON – The era of powerful biologics has led to unforeseen surgical outcomes in patients with ulcerative colitis.
Patients undergoing surgery for ulcerative colitis now are 38% more likely to die in the hospital than they were 15 years ago, before infliximab and other biologics were adopted as medical therapy for the disease. A database review covering 18 years found that other surgical outcomes are worse, too, Jonathan Abelson, MD, said at the annual clinical congress of the American College of Surgeons.
“These very powerful agents could be completely eliminating the need for surgery in patients with mild disease, leaving surgery for those who have very advanced disease and didn’t respond well to medical therapy,” said Dr. Abelson, a clinical research fellow at New York–Presbyterian Hospital, N.Y. “We are operating now only on patients with very severe disease, not the wider range of patients we had 15 years ago, when there weren’t as effective medical options.”
He and his colleagues used the New York Statewide Planning and Research Cooperative System (SPARCS) database to identify 7,070 patients who had undergone bowel resection for ulcerative colitis during two epochs: prebiologics (1995-2005) and postbiologics (2006-2013). The cohorts were about evenly split in numbers.
There were some statistically significant differences in baseline characteristics. Patients in epoch 2 were about a year older (51 vs. 50 years). Significantly more of them had at least two major comorbidities (28% vs. 18%). Minimally invasive surgery was significantly more common in epoch 2 (28% vs. 3%).
Significantly more surgeries in epoch 2 were staged into three or more procedures (14% vs. 9%). This finding probably reflects the level of disease severity in those presenting for surgery or the fact that they underwent surgery after recently receiving biologics, Dr. Abelson said.
“One of the limits of this study is that we don’t know exactly the reasons for these one-, two-, or three-stage surgeries. The theory is that patients who were more ill at presentation are more likely to have a multistaged surgery. Another reason could be that if they are on these powerful immunosuppressive regimens, the surgeon might be concerned about not healing well from a definitive one- or two-stage surgery.”
He then conducted a multivariate analysis that controlled for baseline factors, including a variety of individual comorbid conditions. In this analysis, patients in epoch 2 were 38% more likely to die in the hospital and 51% more likely to experience a major postoperative event, like shock, pulmonary embolism, stroke, or heart attack. The chance of a surgical complication was increased by 39%, and these patients were 25% more likely to need a transfusion during surgery than those from epoch 1.
The poorer outcomes held for an at least an entire year after surgery, Dr. Abelson said. At 1 year, patients in epoch 2 were 36% more likely to have a readmission than those in epoch 1. Major events and procedural complications were both 46% more likely. Patients were also 36% more likely to require an additional procedure.
“These are not the outcomes we want to see, especially in this era when our surgical techniques have improved so much,” Dr. Abelson said. “If what this represents, though, is that we are now operating on a higher-risk population, we can’t just say, ‘Well, that’s how it’s going to be.’ We need to figure out how to minimize morbidity and mortality in this high-risk patient population.”
One goal, he suggested, would be to assess response to a biologic regimen earlier in the hopes of determining who will respond well, and moving ahead with surgery in those who don’t.
This is a tough sell for patients, he said.
“There is a big fear of this surgery. It usually requires a temporary ileostomy and a stoma bag, and patients are terrified of that. There have been a few studies demonstrating that earlier referral to surgery improves quality of life; living with advanced ulcerative colitis can be extremely difficult and patients often feel a lot better after we remove their diseased colon. But getting there is a challenge.”
Dr. Abelson had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
AT THE ACS CLINICAL CONGRESS
Key clinical point:
Major finding: Patients are 38% more likely to die in the hospital than they were 15 years ago.
Data source: The 18-year database review comprised more than 7,000 surgeries.
Disclosures: Dr. Abelson had no financial disclosures.
School-located influenza vaccination programs can be effective
School-located influenza vaccination (SLIV) increased seasonal influenza vaccination rates countywide and in both suburban and urban settings, a study found.
“Schools have a stake in influenza vaccination because immunization of schoolchildren can reduce absenteeism throughout the community. Nevertheless, only 6% of childhood influenza vaccinations occur at school. SLIV poses logistical challenges: obtaining parental consent, ordering and administering vaccine, and billing,” said Peter G. Szilagyi, MD, of Mattel Children’s Hospital, Los Angeles, and his associates.
From 2014 to 2015, 44 elementary schools were randomized in upstate New York in an organized cluster-randomized trial in which 19,776 children were eligible candidates. Seven percent of SLIV school students, 5% of suburban SLIV school students, and 9% of urban SLIV students were vaccinated at SLIV clinics. Children in SLIV schools had higher flu vaccination rates than did children in control schools countywide (54% vs. 47%, P less than .001) and in suburban (62% vs. 54%, P less than .001) and urban schools (44% vs. 39%; P less than .001).
SLIV did substitute for vaccination for urban settings serving more Vaccines for Children–covered students, but did not substitute for practice-based vaccination in the suburbs, where pediatricians often preorder influenza vaccine.
“SLIV, using Web-based consent, is a potential strategy to improve influenza vaccination coverage among large populations of children,” the researchers concluded.
Read the full story here: Pediatrics. 2016. doi: 10.1542/peds.2016-1746.
School-located influenza vaccination (SLIV) increased seasonal influenza vaccination rates countywide and in both suburban and urban settings, a study found.
“Schools have a stake in influenza vaccination because immunization of schoolchildren can reduce absenteeism throughout the community. Nevertheless, only 6% of childhood influenza vaccinations occur at school. SLIV poses logistical challenges: obtaining parental consent, ordering and administering vaccine, and billing,” said Peter G. Szilagyi, MD, of Mattel Children’s Hospital, Los Angeles, and his associates.
From 2014 to 2015, 44 elementary schools were randomized in upstate New York in an organized cluster-randomized trial in which 19,776 children were eligible candidates. Seven percent of SLIV school students, 5% of suburban SLIV school students, and 9% of urban SLIV students were vaccinated at SLIV clinics. Children in SLIV schools had higher flu vaccination rates than did children in control schools countywide (54% vs. 47%, P less than .001) and in suburban (62% vs. 54%, P less than .001) and urban schools (44% vs. 39%; P less than .001).
SLIV did substitute for vaccination for urban settings serving more Vaccines for Children–covered students, but did not substitute for practice-based vaccination in the suburbs, where pediatricians often preorder influenza vaccine.
“SLIV, using Web-based consent, is a potential strategy to improve influenza vaccination coverage among large populations of children,” the researchers concluded.
Read the full story here: Pediatrics. 2016. doi: 10.1542/peds.2016-1746.
School-located influenza vaccination (SLIV) increased seasonal influenza vaccination rates countywide and in both suburban and urban settings, a study found.
“Schools have a stake in influenza vaccination because immunization of schoolchildren can reduce absenteeism throughout the community. Nevertheless, only 6% of childhood influenza vaccinations occur at school. SLIV poses logistical challenges: obtaining parental consent, ordering and administering vaccine, and billing,” said Peter G. Szilagyi, MD, of Mattel Children’s Hospital, Los Angeles, and his associates.
From 2014 to 2015, 44 elementary schools were randomized in upstate New York in an organized cluster-randomized trial in which 19,776 children were eligible candidates. Seven percent of SLIV school students, 5% of suburban SLIV school students, and 9% of urban SLIV students were vaccinated at SLIV clinics. Children in SLIV schools had higher flu vaccination rates than did children in control schools countywide (54% vs. 47%, P less than .001) and in suburban (62% vs. 54%, P less than .001) and urban schools (44% vs. 39%; P less than .001).
SLIV did substitute for vaccination for urban settings serving more Vaccines for Children–covered students, but did not substitute for practice-based vaccination in the suburbs, where pediatricians often preorder influenza vaccine.
“SLIV, using Web-based consent, is a potential strategy to improve influenza vaccination coverage among large populations of children,” the researchers concluded.
Read the full story here: Pediatrics. 2016. doi: 10.1542/peds.2016-1746.
FROM PEDIATRICS
Cardiorespiratory fitness improves survival after depression
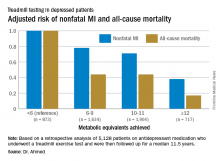
ROME – Cardiorespiratory fitness provided strong and graded protection against all-cause mortality and nonfatal MI in a study of more than 5,000 patients treated for depression, Amjad M. Ahmed, MD, reported at the annual congress of the European Society of Cardiology.
“These results highlight the importance of assessing fitness to identify risk as well as promoting an active lifestyle in patients with depression,” said Dr. Ahmed of Abdulaziz University for Health Sciences in Riyadh, Saudi Arabia.
This analysis focused on the 5,128 subjects who were on antidepressant medication at the time of their treadmill test. Their baseline cardiorespiratory fitness, as estimated by achieved peak metabolic equivalents (METs) on the treadmill, varied inversely with their risks of acute MI and all-cause mortality in the years to come. However, the less fit a patient was, the greater the burden of traditional cardiovascular risk factors. For example, the prevalence of hypertension was 86% in patients who achieved fewer than 6 METs, 75% in those who achieved 6-9 METs, 62% in depressed patients who reached 10-11 METs, and 51% in those who achieved 12 METs or more.
For this reason, Dr. Ahmed and coinvestigators performed a Cox multivariate regression analysis adjusted extensively for potential confounders, including age, sex, race, cardiovascular risk factors, known coronary artery disease, the use of cardiovascular medications, and the reason for the referral for stress testing.
When an achieved MET below 6 was used as the reference standard, for every 1 MET above 6 that patients achieved, their adjusted risk of all-cause mortality decreased by 18%, and the risk of nonfatal MI fell by 8%.
Session cochair Martin Halle, MD, pointed out what he viewed as a major limitation of the study.
“You didn’t follow their physical fitness over time, so you can’t say that increasing their METs would bring a better prognosis,” said Dr. Halle, professor and chairman of the department of preventive and rehabilitative sports medicine at the Technical University of Munich.
Dr. Ahmed reported having no financial conflicts of interest related to the Henry Ford FIT Project.
ROME – Cardiorespiratory fitness provided strong and graded protection against all-cause mortality and nonfatal MI in a study of more than 5,000 patients treated for depression, Amjad M. Ahmed, MD, reported at the annual congress of the European Society of Cardiology.
“These results highlight the importance of assessing fitness to identify risk as well as promoting an active lifestyle in patients with depression,” said Dr. Ahmed of Abdulaziz University for Health Sciences in Riyadh, Saudi Arabia.
This analysis focused on the 5,128 subjects who were on antidepressant medication at the time of their treadmill test. Their baseline cardiorespiratory fitness, as estimated by achieved peak metabolic equivalents (METs) on the treadmill, varied inversely with their risks of acute MI and all-cause mortality in the years to come. However, the less fit a patient was, the greater the burden of traditional cardiovascular risk factors. For example, the prevalence of hypertension was 86% in patients who achieved fewer than 6 METs, 75% in those who achieved 6-9 METs, 62% in depressed patients who reached 10-11 METs, and 51% in those who achieved 12 METs or more.
For this reason, Dr. Ahmed and coinvestigators performed a Cox multivariate regression analysis adjusted extensively for potential confounders, including age, sex, race, cardiovascular risk factors, known coronary artery disease, the use of cardiovascular medications, and the reason for the referral for stress testing.
When an achieved MET below 6 was used as the reference standard, for every 1 MET above 6 that patients achieved, their adjusted risk of all-cause mortality decreased by 18%, and the risk of nonfatal MI fell by 8%.
Session cochair Martin Halle, MD, pointed out what he viewed as a major limitation of the study.
“You didn’t follow their physical fitness over time, so you can’t say that increasing their METs would bring a better prognosis,” said Dr. Halle, professor and chairman of the department of preventive and rehabilitative sports medicine at the Technical University of Munich.
Dr. Ahmed reported having no financial conflicts of interest related to the Henry Ford FIT Project.
ROME – Cardiorespiratory fitness provided strong and graded protection against all-cause mortality and nonfatal MI in a study of more than 5,000 patients treated for depression, Amjad M. Ahmed, MD, reported at the annual congress of the European Society of Cardiology.
“These results highlight the importance of assessing fitness to identify risk as well as promoting an active lifestyle in patients with depression,” said Dr. Ahmed of Abdulaziz University for Health Sciences in Riyadh, Saudi Arabia.
This analysis focused on the 5,128 subjects who were on antidepressant medication at the time of their treadmill test. Their baseline cardiorespiratory fitness, as estimated by achieved peak metabolic equivalents (METs) on the treadmill, varied inversely with their risks of acute MI and all-cause mortality in the years to come. However, the less fit a patient was, the greater the burden of traditional cardiovascular risk factors. For example, the prevalence of hypertension was 86% in patients who achieved fewer than 6 METs, 75% in those who achieved 6-9 METs, 62% in depressed patients who reached 10-11 METs, and 51% in those who achieved 12 METs or more.
For this reason, Dr. Ahmed and coinvestigators performed a Cox multivariate regression analysis adjusted extensively for potential confounders, including age, sex, race, cardiovascular risk factors, known coronary artery disease, the use of cardiovascular medications, and the reason for the referral for stress testing.
When an achieved MET below 6 was used as the reference standard, for every 1 MET above 6 that patients achieved, their adjusted risk of all-cause mortality decreased by 18%, and the risk of nonfatal MI fell by 8%.
Session cochair Martin Halle, MD, pointed out what he viewed as a major limitation of the study.
“You didn’t follow their physical fitness over time, so you can’t say that increasing their METs would bring a better prognosis,” said Dr. Halle, professor and chairman of the department of preventive and rehabilitative sports medicine at the Technical University of Munich.
Dr. Ahmed reported having no financial conflicts of interest related to the Henry Ford FIT Project.
AT THE ESC CONGRESS 2016
Key clinical point:
Major finding: For every 1-MET increase a patient on antidepressant medication achieved above 6 METs during a Bruce protocol treadmill exercise test, the risk of all-cause mortality during the subsequent 11.5 years decreased by an adjusted 18%.
Data source: A retrospective analysis of 5,128 patients on antidepressant medication who underwent a treadmill exercise test as part of the Henry Ford Exercise Testing Project and were then followed up for a median of 11.5 years.
Disclosures: The study presenter reported having no relevant financial conflicts.
A Better Way to Predict Colorectal Cancer Relapse?
Carcinoembryonic antigen (CEA) is often used as a marker for relapse in colorectal cancer. But in as many as 40% of recurrences, the serum CEA shows unmeasurable elevations. And some patients with resected colorectal cancer (CRC) have transient elevations of CEA levels; the false-positive rate during follow-up has been as high as 16%, say researchers from Kaohsiung Medical University, Taiwan. They propose “a more powerful tool”: a membrane array-based multigene biomarker assay, or biomarker chip, which detects circulating tumor cells in the peripheral blood.
Related: Colorectal Carcinoma and Emerging Targeted Therapies
The researchers conducted a study in 298 patients with CRC to test that alternative. The patients were enrolled after radical curative resection for primary CRC tumor; 82 were stage I, 102 were stage II, and 114 were stage III. Patients were followed for a median of 28.4 months, every 3 months for 3 years, then every 6 months. At each follow-up visit, laboratory studies included serum CEA levels. Elevated CEA levels were defined as 2 consecutive measurements of >5 ng/mL at a 3-month interval.
During the study period, 48 patients (16.1%) had postoperative relapse, and 26 (8.7%) died. Of all 298 patients, 62 (20.8%) had a total biomarker chip score higher than the cutoff value. Of the 48 who relapsed, 42 (87.5%) showed positive biochip results prior to relapse.
The positive biochip results were significantly associated with postoperative relapse. In fact, the biomarker chip was better for predicting relapse than were the postoperative serum CEA levels with higher sensitivity (87.5% vs 60.4%), specificity (92.0% vs 83.2%), positive predictive value (67.7% vs 40.8%), negative predictive value (97.5% vs 91.6%), and accuracy (91.3% vs 79.5%).
Moreover, the biochip predicted relapse “considerably earlier” than did CEA levels (10.7 vs 2.8 months). The researchers note that CRC-related deaths are largely attributable to clinical relapse. The sooner a relapse is diagnosed, the more amenable the tumor may be to resection, increasing the likelihood of long-term survival.
In sum, the biomarker chip would be a more accurate tool for predicting relapse, the researchers say. They also suggest that, in clinical practice, combining the 2 tests could enhance confidence in the diagnosis.
Source:
Chang YT, Huang MY, Huang CW, et al. PLoS One. 2016;11(10):e0163264.
doi: 10.1371/journal.pone.0163264.
Carcinoembryonic antigen (CEA) is often used as a marker for relapse in colorectal cancer. But in as many as 40% of recurrences, the serum CEA shows unmeasurable elevations. And some patients with resected colorectal cancer (CRC) have transient elevations of CEA levels; the false-positive rate during follow-up has been as high as 16%, say researchers from Kaohsiung Medical University, Taiwan. They propose “a more powerful tool”: a membrane array-based multigene biomarker assay, or biomarker chip, which detects circulating tumor cells in the peripheral blood.
Related: Colorectal Carcinoma and Emerging Targeted Therapies
The researchers conducted a study in 298 patients with CRC to test that alternative. The patients were enrolled after radical curative resection for primary CRC tumor; 82 were stage I, 102 were stage II, and 114 were stage III. Patients were followed for a median of 28.4 months, every 3 months for 3 years, then every 6 months. At each follow-up visit, laboratory studies included serum CEA levels. Elevated CEA levels were defined as 2 consecutive measurements of >5 ng/mL at a 3-month interval.
During the study period, 48 patients (16.1%) had postoperative relapse, and 26 (8.7%) died. Of all 298 patients, 62 (20.8%) had a total biomarker chip score higher than the cutoff value. Of the 48 who relapsed, 42 (87.5%) showed positive biochip results prior to relapse.
The positive biochip results were significantly associated with postoperative relapse. In fact, the biomarker chip was better for predicting relapse than were the postoperative serum CEA levels with higher sensitivity (87.5% vs 60.4%), specificity (92.0% vs 83.2%), positive predictive value (67.7% vs 40.8%), negative predictive value (97.5% vs 91.6%), and accuracy (91.3% vs 79.5%).
Moreover, the biochip predicted relapse “considerably earlier” than did CEA levels (10.7 vs 2.8 months). The researchers note that CRC-related deaths are largely attributable to clinical relapse. The sooner a relapse is diagnosed, the more amenable the tumor may be to resection, increasing the likelihood of long-term survival.
In sum, the biomarker chip would be a more accurate tool for predicting relapse, the researchers say. They also suggest that, in clinical practice, combining the 2 tests could enhance confidence in the diagnosis.
Source:
Chang YT, Huang MY, Huang CW, et al. PLoS One. 2016;11(10):e0163264.
doi: 10.1371/journal.pone.0163264.
Carcinoembryonic antigen (CEA) is often used as a marker for relapse in colorectal cancer. But in as many as 40% of recurrences, the serum CEA shows unmeasurable elevations. And some patients with resected colorectal cancer (CRC) have transient elevations of CEA levels; the false-positive rate during follow-up has been as high as 16%, say researchers from Kaohsiung Medical University, Taiwan. They propose “a more powerful tool”: a membrane array-based multigene biomarker assay, or biomarker chip, which detects circulating tumor cells in the peripheral blood.
Related: Colorectal Carcinoma and Emerging Targeted Therapies
The researchers conducted a study in 298 patients with CRC to test that alternative. The patients were enrolled after radical curative resection for primary CRC tumor; 82 were stage I, 102 were stage II, and 114 were stage III. Patients were followed for a median of 28.4 months, every 3 months for 3 years, then every 6 months. At each follow-up visit, laboratory studies included serum CEA levels. Elevated CEA levels were defined as 2 consecutive measurements of >5 ng/mL at a 3-month interval.
During the study period, 48 patients (16.1%) had postoperative relapse, and 26 (8.7%) died. Of all 298 patients, 62 (20.8%) had a total biomarker chip score higher than the cutoff value. Of the 48 who relapsed, 42 (87.5%) showed positive biochip results prior to relapse.
The positive biochip results were significantly associated with postoperative relapse. In fact, the biomarker chip was better for predicting relapse than were the postoperative serum CEA levels with higher sensitivity (87.5% vs 60.4%), specificity (92.0% vs 83.2%), positive predictive value (67.7% vs 40.8%), negative predictive value (97.5% vs 91.6%), and accuracy (91.3% vs 79.5%).
Moreover, the biochip predicted relapse “considerably earlier” than did CEA levels (10.7 vs 2.8 months). The researchers note that CRC-related deaths are largely attributable to clinical relapse. The sooner a relapse is diagnosed, the more amenable the tumor may be to resection, increasing the likelihood of long-term survival.
In sum, the biomarker chip would be a more accurate tool for predicting relapse, the researchers say. They also suggest that, in clinical practice, combining the 2 tests could enhance confidence in the diagnosis.
Source:
Chang YT, Huang MY, Huang CW, et al. PLoS One. 2016;11(10):e0163264.
doi: 10.1371/journal.pone.0163264.
Scoring formula consolidates stroke, bleeding risk in atrial fib patients
ROME – A new risk-stratification formula for atrial fibrillation patients starting oral anticoagulant therapy helps sort out their potential net benefit on edoxaban, compared with warfarin.
This risk score “could help guide selection of treatment” with a vitamin K antagonist such as warfarin or a new oral anticoagulant (NOAC) such as edoxaban, Christina L. Fanola, MD, said at the annual congress of the European Society of Cardiology.
“It’s a great time to think about this type of score, because so many more patients are being diagnosed with atrial fibrillation and there is a lot of clinical equipoise” over which anticoagulant to start patients on, said Dr. Fanola, a cardiologist at Brigham and Women’s Hospital in Boston. She said she and her associates hope to externally validate the score and test it in cohorts that received other NOACs, such as apixaban (Eliquis), dabigatran (Pradaxa), or rivaroxaban (Xarelto), but it is very possible that scoring might differ from one NOAC to the next. “Each NOAC may need its own scoring formula,” Dr. Fanola said in an interview.
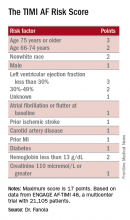
A Cox proportional hazards model identified 10 demographic, clinical, and laboratory features that had significant, independent correlations to a primary outcome of disabling stroke, life-threatening bleeding, or death. After weighing the point allocation for each item by the strength of its association, the researchers developed a scoring formula in a model that could account for about 69% of the three combined adverse outcomes.
An analysis that applied the scoring formula back to the ENGAGE AF-TIMI 48 database showed that a low-risk score of 0-6 correlated with a 4% per year rate of disabling stroke, life-threatening bleed, or death; an intermediate-risk score of 7-9 correlated with a 10% per year incidence of this combined outcome, and a high-risk score of 10 or greater linked with a 21% annual event rate.
Dr. Fanola and her associates ran a further analysis that evaluated the efficacy of edoxaban, compared with warfarin, among the patients in each of these risk strata. The high-risk patients received a major benefit from edoxaban, with a 30% overall incidence of the combined endpoint during 3 years of follow-up, compared with a 51% rate among patients on warfarin, a 21-percentage-point reduction in adverse events. Intermediate-risk patients also received a significant benefit, with a 26% event rate on warfarin and an 18% rate on edoxaban. But low-risk patients had identical 10% event rates with either treatment.
These findings suggest that atrial fibrillation patients with a TIMI AF score that is high or intermediate would have a better chance for a good outcome on edoxaban, or perhaps a different NOAC, than on warfarin. Low-risk patients seem to have similar outcomes on edoxaban or warfarin, so other considerations can come into play for choosing between these drug options, such as the cost of treatment and the inconvenience of regular warfarin monitoring, Dr. Fanola said.
ENGAGE AF-TIMI 48 was sponsored by Daiichi Sankyo, the company that markets edoxaban. Dr. Fanola had no relevant financial disclosures.
[email protected]
On Twitter @mitchelzoler
ROME – A new risk-stratification formula for atrial fibrillation patients starting oral anticoagulant therapy helps sort out their potential net benefit on edoxaban, compared with warfarin.
This risk score “could help guide selection of treatment” with a vitamin K antagonist such as warfarin or a new oral anticoagulant (NOAC) such as edoxaban, Christina L. Fanola, MD, said at the annual congress of the European Society of Cardiology.
“It’s a great time to think about this type of score, because so many more patients are being diagnosed with atrial fibrillation and there is a lot of clinical equipoise” over which anticoagulant to start patients on, said Dr. Fanola, a cardiologist at Brigham and Women’s Hospital in Boston. She said she and her associates hope to externally validate the score and test it in cohorts that received other NOACs, such as apixaban (Eliquis), dabigatran (Pradaxa), or rivaroxaban (Xarelto), but it is very possible that scoring might differ from one NOAC to the next. “Each NOAC may need its own scoring formula,” Dr. Fanola said in an interview.
A Cox proportional hazards model identified 10 demographic, clinical, and laboratory features that had significant, independent correlations to a primary outcome of disabling stroke, life-threatening bleeding, or death. After weighing the point allocation for each item by the strength of its association, the researchers developed a scoring formula in a model that could account for about 69% of the three combined adverse outcomes.
An analysis that applied the scoring formula back to the ENGAGE AF-TIMI 48 database showed that a low-risk score of 0-6 correlated with a 4% per year rate of disabling stroke, life-threatening bleed, or death; an intermediate-risk score of 7-9 correlated with a 10% per year incidence of this combined outcome, and a high-risk score of 10 or greater linked with a 21% annual event rate.
Dr. Fanola and her associates ran a further analysis that evaluated the efficacy of edoxaban, compared with warfarin, among the patients in each of these risk strata. The high-risk patients received a major benefit from edoxaban, with a 30% overall incidence of the combined endpoint during 3 years of follow-up, compared with a 51% rate among patients on warfarin, a 21-percentage-point reduction in adverse events. Intermediate-risk patients also received a significant benefit, with a 26% event rate on warfarin and an 18% rate on edoxaban. But low-risk patients had identical 10% event rates with either treatment.
These findings suggest that atrial fibrillation patients with a TIMI AF score that is high or intermediate would have a better chance for a good outcome on edoxaban, or perhaps a different NOAC, than on warfarin. Low-risk patients seem to have similar outcomes on edoxaban or warfarin, so other considerations can come into play for choosing between these drug options, such as the cost of treatment and the inconvenience of regular warfarin monitoring, Dr. Fanola said.
ENGAGE AF-TIMI 48 was sponsored by Daiichi Sankyo, the company that markets edoxaban. Dr. Fanola had no relevant financial disclosures.
[email protected]
On Twitter @mitchelzoler
ROME – A new risk-stratification formula for atrial fibrillation patients starting oral anticoagulant therapy helps sort out their potential net benefit on edoxaban, compared with warfarin.
This risk score “could help guide selection of treatment” with a vitamin K antagonist such as warfarin or a new oral anticoagulant (NOAC) such as edoxaban, Christina L. Fanola, MD, said at the annual congress of the European Society of Cardiology.
“It’s a great time to think about this type of score, because so many more patients are being diagnosed with atrial fibrillation and there is a lot of clinical equipoise” over which anticoagulant to start patients on, said Dr. Fanola, a cardiologist at Brigham and Women’s Hospital in Boston. She said she and her associates hope to externally validate the score and test it in cohorts that received other NOACs, such as apixaban (Eliquis), dabigatran (Pradaxa), or rivaroxaban (Xarelto), but it is very possible that scoring might differ from one NOAC to the next. “Each NOAC may need its own scoring formula,” Dr. Fanola said in an interview.
A Cox proportional hazards model identified 10 demographic, clinical, and laboratory features that had significant, independent correlations to a primary outcome of disabling stroke, life-threatening bleeding, or death. After weighing the point allocation for each item by the strength of its association, the researchers developed a scoring formula in a model that could account for about 69% of the three combined adverse outcomes.
An analysis that applied the scoring formula back to the ENGAGE AF-TIMI 48 database showed that a low-risk score of 0-6 correlated with a 4% per year rate of disabling stroke, life-threatening bleed, or death; an intermediate-risk score of 7-9 correlated with a 10% per year incidence of this combined outcome, and a high-risk score of 10 or greater linked with a 21% annual event rate.
Dr. Fanola and her associates ran a further analysis that evaluated the efficacy of edoxaban, compared with warfarin, among the patients in each of these risk strata. The high-risk patients received a major benefit from edoxaban, with a 30% overall incidence of the combined endpoint during 3 years of follow-up, compared with a 51% rate among patients on warfarin, a 21-percentage-point reduction in adverse events. Intermediate-risk patients also received a significant benefit, with a 26% event rate on warfarin and an 18% rate on edoxaban. But low-risk patients had identical 10% event rates with either treatment.
These findings suggest that atrial fibrillation patients with a TIMI AF score that is high or intermediate would have a better chance for a good outcome on edoxaban, or perhaps a different NOAC, than on warfarin. Low-risk patients seem to have similar outcomes on edoxaban or warfarin, so other considerations can come into play for choosing between these drug options, such as the cost of treatment and the inconvenience of regular warfarin monitoring, Dr. Fanola said.
ENGAGE AF-TIMI 48 was sponsored by Daiichi Sankyo, the company that markets edoxaban. Dr. Fanola had no relevant financial disclosures.
[email protected]
On Twitter @mitchelzoler
AT THE ESC CONGRESS 2016
Key clinical point:
Major finding: Among high-risk patients, edoxaban cut adverse events by 21 percentage points, compared with warfarin.
Data source: ENGAGE AF-TIMI 48, a multicenter trial with 21,105 patients.
Disclosures: ENGAGE AF-TIMI 48 was sponsored by Daiichi Sankyo, the company that markets edoxaban (Savaysa). Dr. Fanola had no relevant financial disclosures.
Giving women HIV self-tests increases male partner testing
Providing Kenyan women attending prenatal or postpartum health care visits with HIV self-testing kits raised the rates of partner and couples testing to more than 90%, according to a report published online in PLOS Medicine.
HIV testing remains underused in many parts of sub-Saharan Africa, particularly among men, for reasons including social stigma, fear of poor prognosis, lack of awareness of HIV risk, fear that their results would be disclosed, inconvenience, and transportation costs. To assess one strategy for improving male testing rates, researchers performed a study at a hospital and two clinics in urban and suburban Kisumu, Kenya, where the HIV prevalence is approximately 20% among adult residents.
The trial involved 600 women aged 18-39 years (mean age, 24 years) who were seeking either prenatal or postpartum health care and agreed to participate. They were randomly assigned to receive either a few HIV self-test kits plus counseling regarding HIV testing (intervention group) or counseling alone (control group) and followed for 3 months. A total of 95% of the women – 284 in the intervention group and 286 in the control group – completed the study, said Harsha Thirumurthy, PhD, of the department of health policy and management, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, and his associates.
At follow-up, 258 (90.8%) of the women in the intervention group reported that their partners had been tested for HIV, compared with 148 (51.7%) of the control group. This significant difference persisted across all subgroups of patients, regardless of study site and whether or not partners said they had been tested during the preceding year. “This result is encouraging since it suggests that the strategy of giving multiple self-tests to women can effectively increase access to HIV testing in hard-to-reach populations such as men who do not test regularly,” the investigators said (PLOS Med. 2016 Nov 8. doi: 10.1371/journal.pmed.1002166).
In three-fourths of the cases where male partners were tested for HIV, both members of the couple were tested together. This is beneficial because it helps women learn their partners’ HIV status, and because couples who test together are “more likely to adopt a range of HIV prevention and care behaviors,” Dr. Thirumurthy and his associates wrote.
Approximately one-third of the women who were eligible for this study declined to participate, often because they feared that their partners would become violent if offered an HIV self-test. Even women who did participate reported a high rate (27%) of partner violence at baseline. It is encouraging that none of the study participants reported any such incidents in response to the HIV testing, the investigators added.
The International Initiative for Impact Evaluation funded the study. Dr. Thirumurthy and his associates reported having no relevant financial disclosures.
Providing Kenyan women attending prenatal or postpartum health care visits with HIV self-testing kits raised the rates of partner and couples testing to more than 90%, according to a report published online in PLOS Medicine.
HIV testing remains underused in many parts of sub-Saharan Africa, particularly among men, for reasons including social stigma, fear of poor prognosis, lack of awareness of HIV risk, fear that their results would be disclosed, inconvenience, and transportation costs. To assess one strategy for improving male testing rates, researchers performed a study at a hospital and two clinics in urban and suburban Kisumu, Kenya, where the HIV prevalence is approximately 20% among adult residents.
The trial involved 600 women aged 18-39 years (mean age, 24 years) who were seeking either prenatal or postpartum health care and agreed to participate. They were randomly assigned to receive either a few HIV self-test kits plus counseling regarding HIV testing (intervention group) or counseling alone (control group) and followed for 3 months. A total of 95% of the women – 284 in the intervention group and 286 in the control group – completed the study, said Harsha Thirumurthy, PhD, of the department of health policy and management, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, and his associates.
At follow-up, 258 (90.8%) of the women in the intervention group reported that their partners had been tested for HIV, compared with 148 (51.7%) of the control group. This significant difference persisted across all subgroups of patients, regardless of study site and whether or not partners said they had been tested during the preceding year. “This result is encouraging since it suggests that the strategy of giving multiple self-tests to women can effectively increase access to HIV testing in hard-to-reach populations such as men who do not test regularly,” the investigators said (PLOS Med. 2016 Nov 8. doi: 10.1371/journal.pmed.1002166).
In three-fourths of the cases where male partners were tested for HIV, both members of the couple were tested together. This is beneficial because it helps women learn their partners’ HIV status, and because couples who test together are “more likely to adopt a range of HIV prevention and care behaviors,” Dr. Thirumurthy and his associates wrote.
Approximately one-third of the women who were eligible for this study declined to participate, often because they feared that their partners would become violent if offered an HIV self-test. Even women who did participate reported a high rate (27%) of partner violence at baseline. It is encouraging that none of the study participants reported any such incidents in response to the HIV testing, the investigators added.
The International Initiative for Impact Evaluation funded the study. Dr. Thirumurthy and his associates reported having no relevant financial disclosures.
Providing Kenyan women attending prenatal or postpartum health care visits with HIV self-testing kits raised the rates of partner and couples testing to more than 90%, according to a report published online in PLOS Medicine.
HIV testing remains underused in many parts of sub-Saharan Africa, particularly among men, for reasons including social stigma, fear of poor prognosis, lack of awareness of HIV risk, fear that their results would be disclosed, inconvenience, and transportation costs. To assess one strategy for improving male testing rates, researchers performed a study at a hospital and two clinics in urban and suburban Kisumu, Kenya, where the HIV prevalence is approximately 20% among adult residents.
The trial involved 600 women aged 18-39 years (mean age, 24 years) who were seeking either prenatal or postpartum health care and agreed to participate. They were randomly assigned to receive either a few HIV self-test kits plus counseling regarding HIV testing (intervention group) or counseling alone (control group) and followed for 3 months. A total of 95% of the women – 284 in the intervention group and 286 in the control group – completed the study, said Harsha Thirumurthy, PhD, of the department of health policy and management, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, and his associates.
At follow-up, 258 (90.8%) of the women in the intervention group reported that their partners had been tested for HIV, compared with 148 (51.7%) of the control group. This significant difference persisted across all subgroups of patients, regardless of study site and whether or not partners said they had been tested during the preceding year. “This result is encouraging since it suggests that the strategy of giving multiple self-tests to women can effectively increase access to HIV testing in hard-to-reach populations such as men who do not test regularly,” the investigators said (PLOS Med. 2016 Nov 8. doi: 10.1371/journal.pmed.1002166).
In three-fourths of the cases where male partners were tested for HIV, both members of the couple were tested together. This is beneficial because it helps women learn their partners’ HIV status, and because couples who test together are “more likely to adopt a range of HIV prevention and care behaviors,” Dr. Thirumurthy and his associates wrote.
Approximately one-third of the women who were eligible for this study declined to participate, often because they feared that their partners would become violent if offered an HIV self-test. Even women who did participate reported a high rate (27%) of partner violence at baseline. It is encouraging that none of the study participants reported any such incidents in response to the HIV testing, the investigators added.
The International Initiative for Impact Evaluation funded the study. Dr. Thirumurthy and his associates reported having no relevant financial disclosures.
FROM PLOS MEDICINE
Key clinical point: Providing Kenyan women attending prenatal or postpartum health care visits with HIV self-testing kits raised the rates of partner and couples testing to more than 90%.
Major finding: After 3 months, 258 women (90.8%) in the intervention group reported that their partners had been tested for HIV, compared with 148 (51.7%) of the control group.
Data source: A multicenter randomized trial involving 600 women in Kenya receiving prenatal or postpartum care, and their sexual partners.
Disclosures: The International Initiative for Impact Evaluation funded the study. Dr. Thirumurthy and his associates reported having no relevant financial disclosures.
FDA addresses unmet need in drug development guidance for female sexual dysfunction
On Oct. 25, 2016, the Food and Drug Administration issued a draft guidance document titled “Low Sexual Interest, Desire, and/or Arousal in Women: Developing Drugs for Treatment.” The document outlines FDA’s current thinking about how best to design phase III trials for drugs to treat these problems. The purpose of this guidance is to provide pharmaceutical companies with a road map of what the FDA recommends should be addressed in clinical trials designed for new drug approval.
The FDA makes it clear that this guidance is only a “recommendation,” reflecting the agency’s current thinking on this important issue, and is not a requirement (unless specific regulatory or statutory requirements are cited). That may well be, but this puts me in mind of my graduate school days when my advisor used to tell me that his suggestions for my dissertation were only “recommendations.” I knew, of course, that to ignore these recommendations was folly.
Specifically, I would like to highlight several remarkable advances in this document. First, the guidance document acknowledges that there is a medical need for drugs to treat women with sexual dysfunction. Second, the guidance supports retaining the disorders of Hypoactive Sexual Desire Disorder (HSDD) and Female Sexual Arousal Disorder (FSAD) from the DSM-IV as treatment indications. Further, the guidance recognizes that the DSM-5 revisions of female sexual disorders, in this case, Female Sexual Interest and/or Arousal Disorder (FSIAD) “have not been universally accepted the scientific community.”
With regard to clinical trial design, I commend the FDA for removing Satisfying Sexual Events (SSEs) as a “required” primary endpoint and for allowing sponsors more flexibility to choose primary endpoints that will better align regulatory standards with the definition and key symptoms of HSDD (as well as FSAD and FSIAD). These endpoints include validated patient-reported outcome instruments that assess the symptoms of low desire (or arousal) and sexually related distress.
On the negative side, the guidance also reflects some carryover myths that are not in keeping with evidence or expert opinion. Specifically, the FDA cautions against the use of the Female Sexual Function Index desire (FSFI-D) subdomain as a primary endpoint in its current form. The FSFI-D has excellent construct and content validity. Furthermore, the guidance cautions against using a long recall period – 28 days/4 weeks – reflecting the FDA’s concern that women’s recall over that period of time will be inaccurate. They strongly recommend a 24-hour recall period.
Although it is certainly appropriate to evaluate on-demand treatments using more immediate and specific time frames, longer recall of desire is still appropriate as well. Longer recall periods provide a more accurate assessment of desire, which is best understood as state as of mind. A 24-hour recall (e.g., daily log of desire) is more akin to assessing one’s current hunger, whereas a 28-day look-back reflects one’s overall appetite, a concept more similar to desire.
And finally, although the FDA supports inclusion of postmenopausal women as a target population, it would be best if they not divide women into “groups” based on menopausal status unless hormonal status is relevant. This could further marginalize postmenopausal women and delay access to treatments for this population.
Once finalized, this draft guidance should serve its stated purpose to enhance discussion among FDA, pharmaceutical companies, academics, and the public.
Dr. Kingsberg is chief of the division of behavioral medicine in the department of obstetrics and gynecology at MacDonald Women’s Hospital, part of the University Hospitals Cleveland Medical Center, Ohio. She is also a professor in the departments of reproductive biology and psychiatry at Case Western Reserve University, Cleveland. Dr. Kingsberg reported being a consultant or member of the scientific advisory board for Acerus, AMAG, Bayer, Emotional Brain, Endoceutics, NovoNordisk, Palatin, Pfizer, Shionogi, Sprout, TherapeuticsMD, Sermonix, Strategic Science & Technologies, and Valeant. Valeant is the manufacturer of the HSDD drug flibanserin.
On Oct. 25, 2016, the Food and Drug Administration issued a draft guidance document titled “Low Sexual Interest, Desire, and/or Arousal in Women: Developing Drugs for Treatment.” The document outlines FDA’s current thinking about how best to design phase III trials for drugs to treat these problems. The purpose of this guidance is to provide pharmaceutical companies with a road map of what the FDA recommends should be addressed in clinical trials designed for new drug approval.
The FDA makes it clear that this guidance is only a “recommendation,” reflecting the agency’s current thinking on this important issue, and is not a requirement (unless specific regulatory or statutory requirements are cited). That may well be, but this puts me in mind of my graduate school days when my advisor used to tell me that his suggestions for my dissertation were only “recommendations.” I knew, of course, that to ignore these recommendations was folly.
Specifically, I would like to highlight several remarkable advances in this document. First, the guidance document acknowledges that there is a medical need for drugs to treat women with sexual dysfunction. Second, the guidance supports retaining the disorders of Hypoactive Sexual Desire Disorder (HSDD) and Female Sexual Arousal Disorder (FSAD) from the DSM-IV as treatment indications. Further, the guidance recognizes that the DSM-5 revisions of female sexual disorders, in this case, Female Sexual Interest and/or Arousal Disorder (FSIAD) “have not been universally accepted the scientific community.”
With regard to clinical trial design, I commend the FDA for removing Satisfying Sexual Events (SSEs) as a “required” primary endpoint and for allowing sponsors more flexibility to choose primary endpoints that will better align regulatory standards with the definition and key symptoms of HSDD (as well as FSAD and FSIAD). These endpoints include validated patient-reported outcome instruments that assess the symptoms of low desire (or arousal) and sexually related distress.
On the negative side, the guidance also reflects some carryover myths that are not in keeping with evidence or expert opinion. Specifically, the FDA cautions against the use of the Female Sexual Function Index desire (FSFI-D) subdomain as a primary endpoint in its current form. The FSFI-D has excellent construct and content validity. Furthermore, the guidance cautions against using a long recall period – 28 days/4 weeks – reflecting the FDA’s concern that women’s recall over that period of time will be inaccurate. They strongly recommend a 24-hour recall period.
Although it is certainly appropriate to evaluate on-demand treatments using more immediate and specific time frames, longer recall of desire is still appropriate as well. Longer recall periods provide a more accurate assessment of desire, which is best understood as state as of mind. A 24-hour recall (e.g., daily log of desire) is more akin to assessing one’s current hunger, whereas a 28-day look-back reflects one’s overall appetite, a concept more similar to desire.
And finally, although the FDA supports inclusion of postmenopausal women as a target population, it would be best if they not divide women into “groups” based on menopausal status unless hormonal status is relevant. This could further marginalize postmenopausal women and delay access to treatments for this population.
Once finalized, this draft guidance should serve its stated purpose to enhance discussion among FDA, pharmaceutical companies, academics, and the public.
Dr. Kingsberg is chief of the division of behavioral medicine in the department of obstetrics and gynecology at MacDonald Women’s Hospital, part of the University Hospitals Cleveland Medical Center, Ohio. She is also a professor in the departments of reproductive biology and psychiatry at Case Western Reserve University, Cleveland. Dr. Kingsberg reported being a consultant or member of the scientific advisory board for Acerus, AMAG, Bayer, Emotional Brain, Endoceutics, NovoNordisk, Palatin, Pfizer, Shionogi, Sprout, TherapeuticsMD, Sermonix, Strategic Science & Technologies, and Valeant. Valeant is the manufacturer of the HSDD drug flibanserin.
On Oct. 25, 2016, the Food and Drug Administration issued a draft guidance document titled “Low Sexual Interest, Desire, and/or Arousal in Women: Developing Drugs for Treatment.” The document outlines FDA’s current thinking about how best to design phase III trials for drugs to treat these problems. The purpose of this guidance is to provide pharmaceutical companies with a road map of what the FDA recommends should be addressed in clinical trials designed for new drug approval.
The FDA makes it clear that this guidance is only a “recommendation,” reflecting the agency’s current thinking on this important issue, and is not a requirement (unless specific regulatory or statutory requirements are cited). That may well be, but this puts me in mind of my graduate school days when my advisor used to tell me that his suggestions for my dissertation were only “recommendations.” I knew, of course, that to ignore these recommendations was folly.
Specifically, I would like to highlight several remarkable advances in this document. First, the guidance document acknowledges that there is a medical need for drugs to treat women with sexual dysfunction. Second, the guidance supports retaining the disorders of Hypoactive Sexual Desire Disorder (HSDD) and Female Sexual Arousal Disorder (FSAD) from the DSM-IV as treatment indications. Further, the guidance recognizes that the DSM-5 revisions of female sexual disorders, in this case, Female Sexual Interest and/or Arousal Disorder (FSIAD) “have not been universally accepted the scientific community.”
With regard to clinical trial design, I commend the FDA for removing Satisfying Sexual Events (SSEs) as a “required” primary endpoint and for allowing sponsors more flexibility to choose primary endpoints that will better align regulatory standards with the definition and key symptoms of HSDD (as well as FSAD and FSIAD). These endpoints include validated patient-reported outcome instruments that assess the symptoms of low desire (or arousal) and sexually related distress.
On the negative side, the guidance also reflects some carryover myths that are not in keeping with evidence or expert opinion. Specifically, the FDA cautions against the use of the Female Sexual Function Index desire (FSFI-D) subdomain as a primary endpoint in its current form. The FSFI-D has excellent construct and content validity. Furthermore, the guidance cautions against using a long recall period – 28 days/4 weeks – reflecting the FDA’s concern that women’s recall over that period of time will be inaccurate. They strongly recommend a 24-hour recall period.
Although it is certainly appropriate to evaluate on-demand treatments using more immediate and specific time frames, longer recall of desire is still appropriate as well. Longer recall periods provide a more accurate assessment of desire, which is best understood as state as of mind. A 24-hour recall (e.g., daily log of desire) is more akin to assessing one’s current hunger, whereas a 28-day look-back reflects one’s overall appetite, a concept more similar to desire.
And finally, although the FDA supports inclusion of postmenopausal women as a target population, it would be best if they not divide women into “groups” based on menopausal status unless hormonal status is relevant. This could further marginalize postmenopausal women and delay access to treatments for this population.
Once finalized, this draft guidance should serve its stated purpose to enhance discussion among FDA, pharmaceutical companies, academics, and the public.
Dr. Kingsberg is chief of the division of behavioral medicine in the department of obstetrics and gynecology at MacDonald Women’s Hospital, part of the University Hospitals Cleveland Medical Center, Ohio. She is also a professor in the departments of reproductive biology and psychiatry at Case Western Reserve University, Cleveland. Dr. Kingsberg reported being a consultant or member of the scientific advisory board for Acerus, AMAG, Bayer, Emotional Brain, Endoceutics, NovoNordisk, Palatin, Pfizer, Shionogi, Sprout, TherapeuticsMD, Sermonix, Strategic Science & Technologies, and Valeant. Valeant is the manufacturer of the HSDD drug flibanserin.
Mental health integration into pediatric primary care offers multiple benefits
SAN FRANCISCO – Integrating pediatric mental health care into your primary care office can be an effective way to ensure your patients get the care they really need – and it’s easier than you think.
That’s the message Jay Rabinowitz, MD, MPH, a clinical professor of pediatrics at the University of Colorado at Denver, Aurora, delivered to a packed room at the annual meeting of the American Academy of Pediatrics.
He noted that depression and anxiety are among the top five conditions driving overall health costs in the United States, and a 1999 Surgeon General’s Report found that one in five children have a diagnosable mental disorder – but only a fifth to a quarter of these children receive treatment. The rub is that treatment is highly successful; it’s just difficult to access for many families, so making it a part of a child’s medical home just makes sense, Dr. Rabinowitz said.
To drive home his point, he described a case of a depressed adolescent with cutting and suicidal ideation, and the steps he would need to take without integration: find out their insurance, get a list of covered mental health professionals, refer to someone he may or may not heard of, and then rarely receive follow-up reports, much less confirmation the patient had gone to the appointment. With integrated care, parents can make appointments on their way out, he can read the psychologist’s report immediately after the visit, and he can drop in to say hello during the child’s mental health appointment.
“Sometimes there’s a question abut a medication or something, and sometimes it’s an inopportune moment if the child is sad or crying, but generally it seems to be pretty popular,” he said.
Taking steps toward integration
If providers are interesting in exploring the possibility of integration, they need to consider and decide on several issues before taking any concrete steps, Dr. Rabinowitz said. One is the type of arrangement that would work best for your practice: hiring on mental health professionals as employees of the practice, hiring independent contractors, coordinating a space share agreement or creating an out-of-office agreement.
“In our practice, psychologists are employees of the practice, but there are other arrangements,” he said, and some may depend on what is easiest based on state law or billing procedures.
The next question is what kind of provider(s) you would hire. His office has child psychologists with a PhD and postdoctorate fellowships working with children, but other possibilities include social workers, licensed counselors, psychiatric nurse practitioners, or psychiatrists.
Another consideration is what diagnoses your office will handle because it’s not possible to see everything. His practice sees patients in-house for attention deficit/hyperactivity disorder (ADHD), depression, anxiety, drug counseling, and behavioral and adjustment disorders. They choose to refer out educational testing, autism, difficult divorce cases, and complex cases that require more than 20 sessions. They refer out divorce cases because they frequently require specialized knowledge and a lot of court time and phone calls. Aside from ADHD evaluations, his office does not see the staff’s children.
Providers also should consider options for adapting their physical space to accommodate integration. His practice converted an exam room into a consultation room, making it homier with a throw rug, soft chairs, a painted wall, and office decor.
Establishing effective protocols with integration
The next step after providers decide to integrate is to determine the office protocols that govern what forms get used, who can schedule appointments and how long they last, billing, and similar procedures.
“You need to have certain protocols, and some of these things you don’t think about it until you start doing it,” Dr. Rabinowitz said. Should mental health appointments be 50 minutes, for example, or 20 to 25 minutes? His office has gradually shrunk these appointments from 50 to 30 minutes, but they give psychologists an hour of time each day for follow-up phone calls.
Forms to consider developing include a disclosure form, notice of privacy practices, late cancel/no show policy, financial policy, and a summary of parent concerns. His office’s charting includes an extensive intake form with medical, treatment, family, and social history, an intake summary, and a progress note.
It’s with reimbursement, of course, that providers will need to do the most research, particularly with regard to their state’s laws and in looking for grants to provide funding – which is more available than many realize.
“Money is often out there if you look for it,” Dr. Rabinowitz said.” Mental health is an area where no one is really against it: You get together the NRA (National Rifle Association) and the anti-gun movement, and they are both for it.”
Planning for reimbursement challenges
Reimbursement barriers can include lack of payment if mental health codes are used instead of pediatrics ones (depending on the practice arrangement), lack of “incident to” payments, same day billing of physical and mental health appointments, reimbursement for screening, and lack of payment for non–face-to-face services. Although a concierge or fee-for-service option solves many of these, it excludes Medicaid patients and is an economic barrier for many families.
Mental health networks offer a different route, but they can involve poor reimbursement and an additional layer of administration, which makes financial integration more viable as long as providers investigate their options.
“It’s going to be a regional variation, and you need to look at state rules and regulations,” Dr. Rabinowitz said, explaining that his office then sought insurance contracts to include mental health care reimbursement through their office and then sought the same from Medicaid.
“We weren’t about to see Medicaid patients for fear of an audit unless we got written permission, but we got that,” he said. His office simply asked for it and received in writing a letter starting as follows: “Under Department policy, they (our psychologists) may submit E&M claims to Medicaid under a supervising physician’s billing ID. It is not mandatory they be credentialed into a BHO (Behavioral Healthcare Options) network…”
He also noted that his state allows inclusion of psychologists on medical malpractice insurance policies, which is far less expensive for mental health professionals, compared with medical doctors.
Ultimately, the result of mental health integration into primary care practices is greater satisfaction among patients and pediatricians as well as potentially better health outcomes, Dr. Rabinowitz said. An in-house patient satisfaction survey his office conducted found that 91% of parents felt it was convenient for their child to receive mental health services at the same location as medical care, and 90% were satisfied with their care. Only 9% cited barriers to their child seeing a psychologist at their office, and 89% found the services beneficial for their child. Similarly, providers find integration more convenient, easier for follow-up, less stressful, and more efficient while improving communication, confidence, and follow-up.
Dr. Rabinowitz reported no disclosures. No external funding was used for the presentation.
SAN FRANCISCO – Integrating pediatric mental health care into your primary care office can be an effective way to ensure your patients get the care they really need – and it’s easier than you think.
That’s the message Jay Rabinowitz, MD, MPH, a clinical professor of pediatrics at the University of Colorado at Denver, Aurora, delivered to a packed room at the annual meeting of the American Academy of Pediatrics.
He noted that depression and anxiety are among the top five conditions driving overall health costs in the United States, and a 1999 Surgeon General’s Report found that one in five children have a diagnosable mental disorder – but only a fifth to a quarter of these children receive treatment. The rub is that treatment is highly successful; it’s just difficult to access for many families, so making it a part of a child’s medical home just makes sense, Dr. Rabinowitz said.
To drive home his point, he described a case of a depressed adolescent with cutting and suicidal ideation, and the steps he would need to take without integration: find out their insurance, get a list of covered mental health professionals, refer to someone he may or may not heard of, and then rarely receive follow-up reports, much less confirmation the patient had gone to the appointment. With integrated care, parents can make appointments on their way out, he can read the psychologist’s report immediately after the visit, and he can drop in to say hello during the child’s mental health appointment.
“Sometimes there’s a question abut a medication or something, and sometimes it’s an inopportune moment if the child is sad or crying, but generally it seems to be pretty popular,” he said.
Taking steps toward integration
If providers are interesting in exploring the possibility of integration, they need to consider and decide on several issues before taking any concrete steps, Dr. Rabinowitz said. One is the type of arrangement that would work best for your practice: hiring on mental health professionals as employees of the practice, hiring independent contractors, coordinating a space share agreement or creating an out-of-office agreement.
“In our practice, psychologists are employees of the practice, but there are other arrangements,” he said, and some may depend on what is easiest based on state law or billing procedures.
The next question is what kind of provider(s) you would hire. His office has child psychologists with a PhD and postdoctorate fellowships working with children, but other possibilities include social workers, licensed counselors, psychiatric nurse practitioners, or psychiatrists.
Another consideration is what diagnoses your office will handle because it’s not possible to see everything. His practice sees patients in-house for attention deficit/hyperactivity disorder (ADHD), depression, anxiety, drug counseling, and behavioral and adjustment disorders. They choose to refer out educational testing, autism, difficult divorce cases, and complex cases that require more than 20 sessions. They refer out divorce cases because they frequently require specialized knowledge and a lot of court time and phone calls. Aside from ADHD evaluations, his office does not see the staff’s children.
Providers also should consider options for adapting their physical space to accommodate integration. His practice converted an exam room into a consultation room, making it homier with a throw rug, soft chairs, a painted wall, and office decor.
Establishing effective protocols with integration
The next step after providers decide to integrate is to determine the office protocols that govern what forms get used, who can schedule appointments and how long they last, billing, and similar procedures.
“You need to have certain protocols, and some of these things you don’t think about it until you start doing it,” Dr. Rabinowitz said. Should mental health appointments be 50 minutes, for example, or 20 to 25 minutes? His office has gradually shrunk these appointments from 50 to 30 minutes, but they give psychologists an hour of time each day for follow-up phone calls.
Forms to consider developing include a disclosure form, notice of privacy practices, late cancel/no show policy, financial policy, and a summary of parent concerns. His office’s charting includes an extensive intake form with medical, treatment, family, and social history, an intake summary, and a progress note.
It’s with reimbursement, of course, that providers will need to do the most research, particularly with regard to their state’s laws and in looking for grants to provide funding – which is more available than many realize.
“Money is often out there if you look for it,” Dr. Rabinowitz said.” Mental health is an area where no one is really against it: You get together the NRA (National Rifle Association) and the anti-gun movement, and they are both for it.”
Planning for reimbursement challenges
Reimbursement barriers can include lack of payment if mental health codes are used instead of pediatrics ones (depending on the practice arrangement), lack of “incident to” payments, same day billing of physical and mental health appointments, reimbursement for screening, and lack of payment for non–face-to-face services. Although a concierge or fee-for-service option solves many of these, it excludes Medicaid patients and is an economic barrier for many families.
Mental health networks offer a different route, but they can involve poor reimbursement and an additional layer of administration, which makes financial integration more viable as long as providers investigate their options.
“It’s going to be a regional variation, and you need to look at state rules and regulations,” Dr. Rabinowitz said, explaining that his office then sought insurance contracts to include mental health care reimbursement through their office and then sought the same from Medicaid.
“We weren’t about to see Medicaid patients for fear of an audit unless we got written permission, but we got that,” he said. His office simply asked for it and received in writing a letter starting as follows: “Under Department policy, they (our psychologists) may submit E&M claims to Medicaid under a supervising physician’s billing ID. It is not mandatory they be credentialed into a BHO (Behavioral Healthcare Options) network…”
He also noted that his state allows inclusion of psychologists on medical malpractice insurance policies, which is far less expensive for mental health professionals, compared with medical doctors.
Ultimately, the result of mental health integration into primary care practices is greater satisfaction among patients and pediatricians as well as potentially better health outcomes, Dr. Rabinowitz said. An in-house patient satisfaction survey his office conducted found that 91% of parents felt it was convenient for their child to receive mental health services at the same location as medical care, and 90% were satisfied with their care. Only 9% cited barriers to their child seeing a psychologist at their office, and 89% found the services beneficial for their child. Similarly, providers find integration more convenient, easier for follow-up, less stressful, and more efficient while improving communication, confidence, and follow-up.
Dr. Rabinowitz reported no disclosures. No external funding was used for the presentation.
SAN FRANCISCO – Integrating pediatric mental health care into your primary care office can be an effective way to ensure your patients get the care they really need – and it’s easier than you think.
That’s the message Jay Rabinowitz, MD, MPH, a clinical professor of pediatrics at the University of Colorado at Denver, Aurora, delivered to a packed room at the annual meeting of the American Academy of Pediatrics.
He noted that depression and anxiety are among the top five conditions driving overall health costs in the United States, and a 1999 Surgeon General’s Report found that one in five children have a diagnosable mental disorder – but only a fifth to a quarter of these children receive treatment. The rub is that treatment is highly successful; it’s just difficult to access for many families, so making it a part of a child’s medical home just makes sense, Dr. Rabinowitz said.
To drive home his point, he described a case of a depressed adolescent with cutting and suicidal ideation, and the steps he would need to take without integration: find out their insurance, get a list of covered mental health professionals, refer to someone he may or may not heard of, and then rarely receive follow-up reports, much less confirmation the patient had gone to the appointment. With integrated care, parents can make appointments on their way out, he can read the psychologist’s report immediately after the visit, and he can drop in to say hello during the child’s mental health appointment.
“Sometimes there’s a question abut a medication or something, and sometimes it’s an inopportune moment if the child is sad or crying, but generally it seems to be pretty popular,” he said.
Taking steps toward integration
If providers are interesting in exploring the possibility of integration, they need to consider and decide on several issues before taking any concrete steps, Dr. Rabinowitz said. One is the type of arrangement that would work best for your practice: hiring on mental health professionals as employees of the practice, hiring independent contractors, coordinating a space share agreement or creating an out-of-office agreement.
“In our practice, psychologists are employees of the practice, but there are other arrangements,” he said, and some may depend on what is easiest based on state law or billing procedures.
The next question is what kind of provider(s) you would hire. His office has child psychologists with a PhD and postdoctorate fellowships working with children, but other possibilities include social workers, licensed counselors, psychiatric nurse practitioners, or psychiatrists.
Another consideration is what diagnoses your office will handle because it’s not possible to see everything. His practice sees patients in-house for attention deficit/hyperactivity disorder (ADHD), depression, anxiety, drug counseling, and behavioral and adjustment disorders. They choose to refer out educational testing, autism, difficult divorce cases, and complex cases that require more than 20 sessions. They refer out divorce cases because they frequently require specialized knowledge and a lot of court time and phone calls. Aside from ADHD evaluations, his office does not see the staff’s children.
Providers also should consider options for adapting their physical space to accommodate integration. His practice converted an exam room into a consultation room, making it homier with a throw rug, soft chairs, a painted wall, and office decor.
Establishing effective protocols with integration
The next step after providers decide to integrate is to determine the office protocols that govern what forms get used, who can schedule appointments and how long they last, billing, and similar procedures.
“You need to have certain protocols, and some of these things you don’t think about it until you start doing it,” Dr. Rabinowitz said. Should mental health appointments be 50 minutes, for example, or 20 to 25 minutes? His office has gradually shrunk these appointments from 50 to 30 minutes, but they give psychologists an hour of time each day for follow-up phone calls.
Forms to consider developing include a disclosure form, notice of privacy practices, late cancel/no show policy, financial policy, and a summary of parent concerns. His office’s charting includes an extensive intake form with medical, treatment, family, and social history, an intake summary, and a progress note.
It’s with reimbursement, of course, that providers will need to do the most research, particularly with regard to their state’s laws and in looking for grants to provide funding – which is more available than many realize.
“Money is often out there if you look for it,” Dr. Rabinowitz said.” Mental health is an area where no one is really against it: You get together the NRA (National Rifle Association) and the anti-gun movement, and they are both for it.”
Planning for reimbursement challenges
Reimbursement barriers can include lack of payment if mental health codes are used instead of pediatrics ones (depending on the practice arrangement), lack of “incident to” payments, same day billing of physical and mental health appointments, reimbursement for screening, and lack of payment for non–face-to-face services. Although a concierge or fee-for-service option solves many of these, it excludes Medicaid patients and is an economic barrier for many families.
Mental health networks offer a different route, but they can involve poor reimbursement and an additional layer of administration, which makes financial integration more viable as long as providers investigate their options.
“It’s going to be a regional variation, and you need to look at state rules and regulations,” Dr. Rabinowitz said, explaining that his office then sought insurance contracts to include mental health care reimbursement through their office and then sought the same from Medicaid.
“We weren’t about to see Medicaid patients for fear of an audit unless we got written permission, but we got that,” he said. His office simply asked for it and received in writing a letter starting as follows: “Under Department policy, they (our psychologists) may submit E&M claims to Medicaid under a supervising physician’s billing ID. It is not mandatory they be credentialed into a BHO (Behavioral Healthcare Options) network…”
He also noted that his state allows inclusion of psychologists on medical malpractice insurance policies, which is far less expensive for mental health professionals, compared with medical doctors.
Ultimately, the result of mental health integration into primary care practices is greater satisfaction among patients and pediatricians as well as potentially better health outcomes, Dr. Rabinowitz said. An in-house patient satisfaction survey his office conducted found that 91% of parents felt it was convenient for their child to receive mental health services at the same location as medical care, and 90% were satisfied with their care. Only 9% cited barriers to their child seeing a psychologist at their office, and 89% found the services beneficial for their child. Similarly, providers find integration more convenient, easier for follow-up, less stressful, and more efficient while improving communication, confidence, and follow-up.
Dr. Rabinowitz reported no disclosures. No external funding was used for the presentation.
EXPERT ANALYSIS FROM AAP 16