User login
Docs may be uninformed about biosimilars
(filgrastim-sndz), the first
biosimilar approved in the US
© Sandoz Inc. 2015
WASHINGTON, DC—A survey of roughly 1200 US physicians has revealed gaps in their knowledge about biosimilars.
The results showed that some physicians had difficulty defining biosimilars, did not fully understand how the US Food and Drug Administration (FDA) approves biosimilars, and may not know how to prescribe biosimilars appropriately.
The results were published in Advances in Therapy and presented at the DIA Biosimilars 2016 conference.
The 19-question survey was created by the Biosimilars Forum and conducted by SERMO, a global social network organization for physicians, from November 20, 2015, to January 4, 2016.
Responses were obtained from 1201 US physicians across specialties that are high prescribers of biologics, including hematologist-oncologists, dermatologists, gastroenterologists, medical oncologists, nephrologists, and rheumatologists.
Knowledge gaps
The survey revealed 5 major knowledge gaps regarding biosimilars:
- Defining biologics, biosimilars, and biosimilarity1
- Understanding the approval process and the FDA’s use of “totality of evidence” to evaluate biosimilars
- Understanding that the safety profile of a biosimilar is expected to be the same as that of the originator biologic
- Understanding how decisions are made by the FDA for extrapolation2 of indications
- Defining interchangeability3 and the related rules regarding pharmacy-level substitution.
“With 4 biosimilars approved by the FDA and more than 60 in development, the survey highlights the need for greater biosimilars education for physicians and healthcare professionals,” said study author Hillel Cohen, PhD, executive director of scientific affairs at Sandoz Inc.
“Education will help physicians and healthcare professionals have a better understanding and knowledge of biosimilars so that they feel comfortable about administering biosimilars to patients when appropriate.”
FDA approval
Most of the physicians surveyed did not clearly understand the concept of extrapolation as applied to biosimilars. Only 12% of survey respondents said they trust extrapolation of the studied biosimilar indication(s) as the basis to obtain approval of other licensed indications of the originator product.
Almost 60% of respondents correctly understood that, to be approved as “interchangeable” with the originator, a biosimilar must be shown to be safe and effective for back-and-forth switching, with no negative impacts to safety or efficacy.
However, almost 80% of respondents did not agree, or potentially did not realize, that an FDA designation of “interchangeable” may enable a pharmacist to switch between the originator biologic and biosimilar and vice versa.
A little more than half of physicians surveyed knew that, for a biosimilar to be approved, the FDA must find the biosimilar to be equally effective (62.3%) and safe (57.2%) when compared to the originator biologic.
Most physicians surveyed (74.5%) said they trust the FDA’s biosimilar approval decisions.
Biosimilar use
Fewer than half of the survey respondents (44.8%) believed that biosimilars will be safe and appropriate for use in existing patients as well as naïve patients.
Overall, respondents expressed a general agreement that switching an existing patient to a biosimilar may be appropriate, with 9 in 10 (91%) saying they would consider switching a patient from an originator biologic to a biosimilar as an effective alternative to the originator if it would help the patient have better access to his/her medications.
In addition, 82.2% of respondents believed biosimilars will expand treatment options and provide savings to patients and the healthcare system.
“The Biosimilars Forum launched the Partnership for Biosimilar Education and Access to provide evidence-based education for healthcare professionals, patients, and the public,” Dr Cohen said. “The forum will use the survey results to provide education on the key concepts of biosimilars as we advance our mission to encourage awareness, access, and adoption of these important medicines.”
The Biosimilars Forum intends to conduct a similar survey in 2 to 3 years with the same design in order to monitor trends in the awareness, knowledge, and perceptions of biosimilars over time. ![]()
1. A biosimilar medicine is a biologic that is highly similar to an FDA-approved biological medicine, known as an originator biologic. A biosimilar must (1) be highly similar to the originator biologic, notwithstanding minor differences in clinically inactive components, and (2) have no clinically meaningful differences in safety or effectiveness compared to the originator biologic. Biosimilars Forum. (2016, March 28). Frequently Asked Questions. Retrieved from: http://www.biosimilarsforum.org/sites/default/files/uploads/biosimilars_faqs_032816opt_0.pdf
2. Extrapolation is the process by which a biosimilar may be approved for one or more indications for which its reference biological product is licensed but for which there was no head-to-head clinical comparison. This approval is based on the extrapolation of the totality of data obtained with the biosimilar molecule in direct comparison to the originator biologic. Every indication for which extrapolation is sought must be scientifically justified. Biosimilars Forum. (2016, March 28). Frequently Asked Questions. Retrieved from: http://www.biosimilarsforum.org/sites/default/files/uploads/biosimilars_faqs_032816opt_0.pdf
3. An interchangeable biologic is a biosimilar that has been demonstrated to produce the same clinical result as its originator biologic in any given patient. In addition, for a product that is administered multiple times to an individual, the risk in terms of safety or diminished efficacy of alternating between use of the interchangeable biologic and its originator biologic is not greater than the risk of using the originator biologic alone. Designation of interchangeability requires additional supporting evidence, which will be further defined by the FDA. An interchangeable biologic may be substituted by a pharmacist for the originator biologic or vice-versa without the intervention of the healthcare professional who wrote the prescription. State laws govern the substitution process, and communication to the healthcare provider who wrote the prescription may be required after the product is dispensed. Biosimilars Forum. (2016, March 28). Frequently Asked Questions. Retrieved from: http://www.biosimilarsforum.org/sites/default/files/uploads/biosimilars_faqs_032816opt_0.pdf

(filgrastim-sndz), the first
biosimilar approved in the US
© Sandoz Inc. 2015
WASHINGTON, DC—A survey of roughly 1200 US physicians has revealed gaps in their knowledge about biosimilars.
The results showed that some physicians had difficulty defining biosimilars, did not fully understand how the US Food and Drug Administration (FDA) approves biosimilars, and may not know how to prescribe biosimilars appropriately.
The results were published in Advances in Therapy and presented at the DIA Biosimilars 2016 conference.
The 19-question survey was created by the Biosimilars Forum and conducted by SERMO, a global social network organization for physicians, from November 20, 2015, to January 4, 2016.
Responses were obtained from 1201 US physicians across specialties that are high prescribers of biologics, including hematologist-oncologists, dermatologists, gastroenterologists, medical oncologists, nephrologists, and rheumatologists.
Knowledge gaps
The survey revealed 5 major knowledge gaps regarding biosimilars:
- Defining biologics, biosimilars, and biosimilarity1
- Understanding the approval process and the FDA’s use of “totality of evidence” to evaluate biosimilars
- Understanding that the safety profile of a biosimilar is expected to be the same as that of the originator biologic
- Understanding how decisions are made by the FDA for extrapolation2 of indications
- Defining interchangeability3 and the related rules regarding pharmacy-level substitution.
“With 4 biosimilars approved by the FDA and more than 60 in development, the survey highlights the need for greater biosimilars education for physicians and healthcare professionals,” said study author Hillel Cohen, PhD, executive director of scientific affairs at Sandoz Inc.
“Education will help physicians and healthcare professionals have a better understanding and knowledge of biosimilars so that they feel comfortable about administering biosimilars to patients when appropriate.”
FDA approval
Most of the physicians surveyed did not clearly understand the concept of extrapolation as applied to biosimilars. Only 12% of survey respondents said they trust extrapolation of the studied biosimilar indication(s) as the basis to obtain approval of other licensed indications of the originator product.
Almost 60% of respondents correctly understood that, to be approved as “interchangeable” with the originator, a biosimilar must be shown to be safe and effective for back-and-forth switching, with no negative impacts to safety or efficacy.
However, almost 80% of respondents did not agree, or potentially did not realize, that an FDA designation of “interchangeable” may enable a pharmacist to switch between the originator biologic and biosimilar and vice versa.
A little more than half of physicians surveyed knew that, for a biosimilar to be approved, the FDA must find the biosimilar to be equally effective (62.3%) and safe (57.2%) when compared to the originator biologic.
Most physicians surveyed (74.5%) said they trust the FDA’s biosimilar approval decisions.
Biosimilar use
Fewer than half of the survey respondents (44.8%) believed that biosimilars will be safe and appropriate for use in existing patients as well as naïve patients.
Overall, respondents expressed a general agreement that switching an existing patient to a biosimilar may be appropriate, with 9 in 10 (91%) saying they would consider switching a patient from an originator biologic to a biosimilar as an effective alternative to the originator if it would help the patient have better access to his/her medications.
In addition, 82.2% of respondents believed biosimilars will expand treatment options and provide savings to patients and the healthcare system.
“The Biosimilars Forum launched the Partnership for Biosimilar Education and Access to provide evidence-based education for healthcare professionals, patients, and the public,” Dr Cohen said. “The forum will use the survey results to provide education on the key concepts of biosimilars as we advance our mission to encourage awareness, access, and adoption of these important medicines.”
The Biosimilars Forum intends to conduct a similar survey in 2 to 3 years with the same design in order to monitor trends in the awareness, knowledge, and perceptions of biosimilars over time. ![]()
1. A biosimilar medicine is a biologic that is highly similar to an FDA-approved biological medicine, known as an originator biologic. A biosimilar must (1) be highly similar to the originator biologic, notwithstanding minor differences in clinically inactive components, and (2) have no clinically meaningful differences in safety or effectiveness compared to the originator biologic. Biosimilars Forum. (2016, March 28). Frequently Asked Questions. Retrieved from: http://www.biosimilarsforum.org/sites/default/files/uploads/biosimilars_faqs_032816opt_0.pdf
2. Extrapolation is the process by which a biosimilar may be approved for one or more indications for which its reference biological product is licensed but for which there was no head-to-head clinical comparison. This approval is based on the extrapolation of the totality of data obtained with the biosimilar molecule in direct comparison to the originator biologic. Every indication for which extrapolation is sought must be scientifically justified. Biosimilars Forum. (2016, March 28). Frequently Asked Questions. Retrieved from: http://www.biosimilarsforum.org/sites/default/files/uploads/biosimilars_faqs_032816opt_0.pdf
3. An interchangeable biologic is a biosimilar that has been demonstrated to produce the same clinical result as its originator biologic in any given patient. In addition, for a product that is administered multiple times to an individual, the risk in terms of safety or diminished efficacy of alternating between use of the interchangeable biologic and its originator biologic is not greater than the risk of using the originator biologic alone. Designation of interchangeability requires additional supporting evidence, which will be further defined by the FDA. An interchangeable biologic may be substituted by a pharmacist for the originator biologic or vice-versa without the intervention of the healthcare professional who wrote the prescription. State laws govern the substitution process, and communication to the healthcare provider who wrote the prescription may be required after the product is dispensed. Biosimilars Forum. (2016, March 28). Frequently Asked Questions. Retrieved from: http://www.biosimilarsforum.org/sites/default/files/uploads/biosimilars_faqs_032816opt_0.pdf

(filgrastim-sndz), the first
biosimilar approved in the US
© Sandoz Inc. 2015
WASHINGTON, DC—A survey of roughly 1200 US physicians has revealed gaps in their knowledge about biosimilars.
The results showed that some physicians had difficulty defining biosimilars, did not fully understand how the US Food and Drug Administration (FDA) approves biosimilars, and may not know how to prescribe biosimilars appropriately.
The results were published in Advances in Therapy and presented at the DIA Biosimilars 2016 conference.
The 19-question survey was created by the Biosimilars Forum and conducted by SERMO, a global social network organization for physicians, from November 20, 2015, to January 4, 2016.
Responses were obtained from 1201 US physicians across specialties that are high prescribers of biologics, including hematologist-oncologists, dermatologists, gastroenterologists, medical oncologists, nephrologists, and rheumatologists.
Knowledge gaps
The survey revealed 5 major knowledge gaps regarding biosimilars:
- Defining biologics, biosimilars, and biosimilarity1
- Understanding the approval process and the FDA’s use of “totality of evidence” to evaluate biosimilars
- Understanding that the safety profile of a biosimilar is expected to be the same as that of the originator biologic
- Understanding how decisions are made by the FDA for extrapolation2 of indications
- Defining interchangeability3 and the related rules regarding pharmacy-level substitution.
“With 4 biosimilars approved by the FDA and more than 60 in development, the survey highlights the need for greater biosimilars education for physicians and healthcare professionals,” said study author Hillel Cohen, PhD, executive director of scientific affairs at Sandoz Inc.
“Education will help physicians and healthcare professionals have a better understanding and knowledge of biosimilars so that they feel comfortable about administering biosimilars to patients when appropriate.”
FDA approval
Most of the physicians surveyed did not clearly understand the concept of extrapolation as applied to biosimilars. Only 12% of survey respondents said they trust extrapolation of the studied biosimilar indication(s) as the basis to obtain approval of other licensed indications of the originator product.
Almost 60% of respondents correctly understood that, to be approved as “interchangeable” with the originator, a biosimilar must be shown to be safe and effective for back-and-forth switching, with no negative impacts to safety or efficacy.
However, almost 80% of respondents did not agree, or potentially did not realize, that an FDA designation of “interchangeable” may enable a pharmacist to switch between the originator biologic and biosimilar and vice versa.
A little more than half of physicians surveyed knew that, for a biosimilar to be approved, the FDA must find the biosimilar to be equally effective (62.3%) and safe (57.2%) when compared to the originator biologic.
Most physicians surveyed (74.5%) said they trust the FDA’s biosimilar approval decisions.
Biosimilar use
Fewer than half of the survey respondents (44.8%) believed that biosimilars will be safe and appropriate for use in existing patients as well as naïve patients.
Overall, respondents expressed a general agreement that switching an existing patient to a biosimilar may be appropriate, with 9 in 10 (91%) saying they would consider switching a patient from an originator biologic to a biosimilar as an effective alternative to the originator if it would help the patient have better access to his/her medications.
In addition, 82.2% of respondents believed biosimilars will expand treatment options and provide savings to patients and the healthcare system.
“The Biosimilars Forum launched the Partnership for Biosimilar Education and Access to provide evidence-based education for healthcare professionals, patients, and the public,” Dr Cohen said. “The forum will use the survey results to provide education on the key concepts of biosimilars as we advance our mission to encourage awareness, access, and adoption of these important medicines.”
The Biosimilars Forum intends to conduct a similar survey in 2 to 3 years with the same design in order to monitor trends in the awareness, knowledge, and perceptions of biosimilars over time. ![]()
1. A biosimilar medicine is a biologic that is highly similar to an FDA-approved biological medicine, known as an originator biologic. A biosimilar must (1) be highly similar to the originator biologic, notwithstanding minor differences in clinically inactive components, and (2) have no clinically meaningful differences in safety or effectiveness compared to the originator biologic. Biosimilars Forum. (2016, March 28). Frequently Asked Questions. Retrieved from: http://www.biosimilarsforum.org/sites/default/files/uploads/biosimilars_faqs_032816opt_0.pdf
2. Extrapolation is the process by which a biosimilar may be approved for one or more indications for which its reference biological product is licensed but for which there was no head-to-head clinical comparison. This approval is based on the extrapolation of the totality of data obtained with the biosimilar molecule in direct comparison to the originator biologic. Every indication for which extrapolation is sought must be scientifically justified. Biosimilars Forum. (2016, March 28). Frequently Asked Questions. Retrieved from: http://www.biosimilarsforum.org/sites/default/files/uploads/biosimilars_faqs_032816opt_0.pdf
3. An interchangeable biologic is a biosimilar that has been demonstrated to produce the same clinical result as its originator biologic in any given patient. In addition, for a product that is administered multiple times to an individual, the risk in terms of safety or diminished efficacy of alternating between use of the interchangeable biologic and its originator biologic is not greater than the risk of using the originator biologic alone. Designation of interchangeability requires additional supporting evidence, which will be further defined by the FDA. An interchangeable biologic may be substituted by a pharmacist for the originator biologic or vice-versa without the intervention of the healthcare professional who wrote the prescription. State laws govern the substitution process, and communication to the healthcare provider who wrote the prescription may be required after the product is dispensed. Biosimilars Forum. (2016, March 28). Frequently Asked Questions. Retrieved from: http://www.biosimilarsforum.org/sites/default/files/uploads/biosimilars_faqs_032816opt_0.pdf
Study provides new insight into RBC production

Andes who suffers from
chronic mountain sickness
Photo courtesy of
UC San Diego Health
Findings from a study of people living at high altitude have implications for the treatment of red blood cell (RBC) disorders such as anemia and polycythemia, according to researchers.
To better understand why some people adapt well to life at high altitude while others don’t, the researchers studied RBCs derived from representatives of both groups who were living in the Andes Mountains.
The study revealed that high-altitude dwellers prone to chronic mountain sickness produce massive amounts of RBCs thanks to overproduction of the enzyme SENP1.
The researchers reported these findings in the Journal of Experimental Medicine.
“In addition to improving the health of millions of people around the world who live above 8000 feet, information on how Andeans have adapted—or not adapted—to high-altitude life might teach us how to speed up red blood cell production at lower altitudes, such as in anemia or when blood transfusions are needed rapidly,” said study author Gabriel Haddad, MD, of the University of California San Diego School of Medicine.
Dr Haddad and his colleagues noted that chronic mountain sickness affects approximately 20% of people who live at high altitudes, and a critical aspect of the condition is polycythemia.
Some extra RBCs can be beneficial in high-altitude, low-oxygen environments by helping to keep blood oxygenated. However, too many RBCs can increase the risk of heart attack and stroke, even in young adults.
For this study, the researchers collected skin cells from people living in the Andes Mountains—4 who were healthy and 5 who suffer from chronic mountain sickness—plus an additional 3 healthy people who live at sea level and served as controls.
To produce enough RBCs from each participant to study them in the lab, the researchers converted the skin cells into induced pluripotent stem cells (iPSCs).
Then, adding a cocktail of growth factors and other molecules, the team coaxed the iPSCs to differentiate into RBCs. Multiple samples were tested for each person, for a total of at least 24 iPSC lines.
The researchers exposed the RBCs to low-oxygen conditions that mimic high altitude—5% oxygen—for 3 weeks.
RBCs from healthy sea-level or high-altitude-dwelling donors increased a little or not at all. In contrast, RBC counts from high-altitude dwellers with chronic mountain sickness increased 60-fold.
This result led the researchers to question why people with chronic mountain sickness produce so many extra RBCs in response to low oxygen.
In a previous study, the team had compared the genomes of high-altitude dwellers with and without chronic mountain sickness. This revealed a gene that varied between the 2 groups—SENP1, which is increased in low-oxygen situations in people with chronic mountain sickness but not in healthy individuals.
In the current study, the researchers set out to determine if SENP1 plays a role in high-altitude adaptation.
The team inhibited the SENP1 gene in iPSCs from patients with chronic mountain sickness. As a result, excessive RBC production was reduced by more than 90%.
When the researchers added extra SENP1 to healthy, adapted highlander iPSCs, RBC production increased 30-fold, nearly recapitulating that seen in patients with chronic mountains sickness.
Further experiments revealed how SENP1 affects RBC production. Elevated levels of the enzyme in chronic mountain sickness boost levels of several other proteins that promote cell division and survival, including VEGF, GATA1, and Bcl-xL.
“We’re interested in determining the early steps in this process—how low oxygen triggers SENP1 in the first place,” said study author Priti Azad, PhD, of the University of California San Diego School of Medicine.
“We are also investigating how existing altitude sickness medications, such as Diamox, work and whether or not it’s through this same mechanism.” ![]()

Andes who suffers from
chronic mountain sickness
Photo courtesy of
UC San Diego Health
Findings from a study of people living at high altitude have implications for the treatment of red blood cell (RBC) disorders such as anemia and polycythemia, according to researchers.
To better understand why some people adapt well to life at high altitude while others don’t, the researchers studied RBCs derived from representatives of both groups who were living in the Andes Mountains.
The study revealed that high-altitude dwellers prone to chronic mountain sickness produce massive amounts of RBCs thanks to overproduction of the enzyme SENP1.
The researchers reported these findings in the Journal of Experimental Medicine.
“In addition to improving the health of millions of people around the world who live above 8000 feet, information on how Andeans have adapted—or not adapted—to high-altitude life might teach us how to speed up red blood cell production at lower altitudes, such as in anemia or when blood transfusions are needed rapidly,” said study author Gabriel Haddad, MD, of the University of California San Diego School of Medicine.
Dr Haddad and his colleagues noted that chronic mountain sickness affects approximately 20% of people who live at high altitudes, and a critical aspect of the condition is polycythemia.
Some extra RBCs can be beneficial in high-altitude, low-oxygen environments by helping to keep blood oxygenated. However, too many RBCs can increase the risk of heart attack and stroke, even in young adults.
For this study, the researchers collected skin cells from people living in the Andes Mountains—4 who were healthy and 5 who suffer from chronic mountain sickness—plus an additional 3 healthy people who live at sea level and served as controls.
To produce enough RBCs from each participant to study them in the lab, the researchers converted the skin cells into induced pluripotent stem cells (iPSCs).
Then, adding a cocktail of growth factors and other molecules, the team coaxed the iPSCs to differentiate into RBCs. Multiple samples were tested for each person, for a total of at least 24 iPSC lines.
The researchers exposed the RBCs to low-oxygen conditions that mimic high altitude—5% oxygen—for 3 weeks.
RBCs from healthy sea-level or high-altitude-dwelling donors increased a little or not at all. In contrast, RBC counts from high-altitude dwellers with chronic mountain sickness increased 60-fold.
This result led the researchers to question why people with chronic mountain sickness produce so many extra RBCs in response to low oxygen.
In a previous study, the team had compared the genomes of high-altitude dwellers with and without chronic mountain sickness. This revealed a gene that varied between the 2 groups—SENP1, which is increased in low-oxygen situations in people with chronic mountain sickness but not in healthy individuals.
In the current study, the researchers set out to determine if SENP1 plays a role in high-altitude adaptation.
The team inhibited the SENP1 gene in iPSCs from patients with chronic mountain sickness. As a result, excessive RBC production was reduced by more than 90%.
When the researchers added extra SENP1 to healthy, adapted highlander iPSCs, RBC production increased 30-fold, nearly recapitulating that seen in patients with chronic mountains sickness.
Further experiments revealed how SENP1 affects RBC production. Elevated levels of the enzyme in chronic mountain sickness boost levels of several other proteins that promote cell division and survival, including VEGF, GATA1, and Bcl-xL.
“We’re interested in determining the early steps in this process—how low oxygen triggers SENP1 in the first place,” said study author Priti Azad, PhD, of the University of California San Diego School of Medicine.
“We are also investigating how existing altitude sickness medications, such as Diamox, work and whether or not it’s through this same mechanism.” ![]()

Andes who suffers from
chronic mountain sickness
Photo courtesy of
UC San Diego Health
Findings from a study of people living at high altitude have implications for the treatment of red blood cell (RBC) disorders such as anemia and polycythemia, according to researchers.
To better understand why some people adapt well to life at high altitude while others don’t, the researchers studied RBCs derived from representatives of both groups who were living in the Andes Mountains.
The study revealed that high-altitude dwellers prone to chronic mountain sickness produce massive amounts of RBCs thanks to overproduction of the enzyme SENP1.
The researchers reported these findings in the Journal of Experimental Medicine.
“In addition to improving the health of millions of people around the world who live above 8000 feet, information on how Andeans have adapted—or not adapted—to high-altitude life might teach us how to speed up red blood cell production at lower altitudes, such as in anemia or when blood transfusions are needed rapidly,” said study author Gabriel Haddad, MD, of the University of California San Diego School of Medicine.
Dr Haddad and his colleagues noted that chronic mountain sickness affects approximately 20% of people who live at high altitudes, and a critical aspect of the condition is polycythemia.
Some extra RBCs can be beneficial in high-altitude, low-oxygen environments by helping to keep blood oxygenated. However, too many RBCs can increase the risk of heart attack and stroke, even in young adults.
For this study, the researchers collected skin cells from people living in the Andes Mountains—4 who were healthy and 5 who suffer from chronic mountain sickness—plus an additional 3 healthy people who live at sea level and served as controls.
To produce enough RBCs from each participant to study them in the lab, the researchers converted the skin cells into induced pluripotent stem cells (iPSCs).
Then, adding a cocktail of growth factors and other molecules, the team coaxed the iPSCs to differentiate into RBCs. Multiple samples were tested for each person, for a total of at least 24 iPSC lines.
The researchers exposed the RBCs to low-oxygen conditions that mimic high altitude—5% oxygen—for 3 weeks.
RBCs from healthy sea-level or high-altitude-dwelling donors increased a little or not at all. In contrast, RBC counts from high-altitude dwellers with chronic mountain sickness increased 60-fold.
This result led the researchers to question why people with chronic mountain sickness produce so many extra RBCs in response to low oxygen.
In a previous study, the team had compared the genomes of high-altitude dwellers with and without chronic mountain sickness. This revealed a gene that varied between the 2 groups—SENP1, which is increased in low-oxygen situations in people with chronic mountain sickness but not in healthy individuals.
In the current study, the researchers set out to determine if SENP1 plays a role in high-altitude adaptation.
The team inhibited the SENP1 gene in iPSCs from patients with chronic mountain sickness. As a result, excessive RBC production was reduced by more than 90%.
When the researchers added extra SENP1 to healthy, adapted highlander iPSCs, RBC production increased 30-fold, nearly recapitulating that seen in patients with chronic mountains sickness.
Further experiments revealed how SENP1 affects RBC production. Elevated levels of the enzyme in chronic mountain sickness boost levels of several other proteins that promote cell division and survival, including VEGF, GATA1, and Bcl-xL.
“We’re interested in determining the early steps in this process—how low oxygen triggers SENP1 in the first place,” said study author Priti Azad, PhD, of the University of California San Diego School of Medicine.
“We are also investigating how existing altitude sickness medications, such as Diamox, work and whether or not it’s through this same mechanism.” ![]()
Antiviral appears active against tough-to-treat CMV
NEW ORLEANS—The antiviral agent maribavir has shown activity against cytomegalovirus (CMV) in a phase 2 trial.
The trial included transplant recipients with a CMV infection that was resistant or refractory to prior treatment with (val)ganciclovir or foscarnet.
Sixty-seven percent of patients treated with varying doses of maribavir for up to 24 weeks had no detectable levels of CMV in their plasma within 6 weeks of starting treatment.
However, CMV infection did recur in some of these patients.
Dysgeusia was the most commonly reported treatment-emergent adverse event (AE), and one patient died of an AE that was considered possibly related to maribavir.
These results were presented at IDWeek 2016 (abstract 78). The study was sponsored by Shire.
“Cytomegalovirus infection that is resistant or refractory to standard therapy in transplant patients is associated with significant complications and high mortality,” said study investigator Genovefa Papanicolaou, MD, of Memorial Sloan Kettering Cancer Center in New York, New York.
“The phase 2 findings support further research to confirm these results among patients who have limited options to combat the infection.”
Maribavir is a member of a class of drugs called benzimidazole ribosides. Maribavir is thought to inhibit viral DNA assembly and egress of viral capsids from the nucleus of infected cells. The drug has not been shown to affect the maturation of viral DNA or affect the viral DNA polymerase.
This phase 2 study of maribavir included 120 patients ages 12 and older with CMV infection (≥1000 DNA copies/mL of blood plasma) that was resistant or refractory to (val)ganciclovir or foscarnet.
Forty-seven of the patients had received a hematopoietic stem cell transplant, and 73 had a solid organ transplant. The patients’ median age was 55 (range, 18 to 74).
The patients were randomized to 1 of 3 twice-daily oral doses of maribavir—400 mg, 800 mg, or 1200 mg—for up to 24 weeks of treatment.
Efficacy
The study’s primary efficacy endpoint was the proportion of patients with confirmed undetectable plasma CMV DNA within 6 weeks of treatment.
Overall, 67% (80/120) of patients met the primary efficacy endpoint. This included 70% (n=28) of patients in the 400 mg group, 63% (n=25) in the 800 mg group, and 67% (n=27) in the 1200 mg group.
CMV infection recurred in 30 patients, including 7 in the 400 mg group, 11 in the 800 mg group, and 12 in the 1200 mg group.
Safety
The primary safety analysis was focused on the incidence of treatment-emergent AEs. The incidence was 78% (n=93) overall, including 78% (n=31) in the 400 mg group, 80% (n=32) in the 800 mg group, and 75% (n=30) in the 1200 mg group.
Twenty-seven percent of patients died due to any AE, 1 of which (multi-organ failure) was considered possibly related to maribavir.
Forty-one patients (34%) discontinued treatment with maribavir due to an AE, including 17 patients who discontinued due to CMV infection.
Dysgeusia was the most common treatment-emergent AE and led to treatment discontinuation in 1 patient. Dysgeusia occurred in 65% (n=78) of all patients, including 60% (n=24) in the 400 mg group, 63% (n=25) in the 800 mg group, and 73% (n=29) in the 1200 mg group.
Other treatment-emergent AEs (occurring in at least 20% of patients) for all doses included nausea, vomiting, CMV infection, diarrhea, fatigue, and anemia. Immunosuppressant drug level increases were reported as an AE in 10% of all patients. ![]()

NEW ORLEANS—The antiviral agent maribavir has shown activity against cytomegalovirus (CMV) in a phase 2 trial.
The trial included transplant recipients with a CMV infection that was resistant or refractory to prior treatment with (val)ganciclovir or foscarnet.
Sixty-seven percent of patients treated with varying doses of maribavir for up to 24 weeks had no detectable levels of CMV in their plasma within 6 weeks of starting treatment.
However, CMV infection did recur in some of these patients.
Dysgeusia was the most commonly reported treatment-emergent adverse event (AE), and one patient died of an AE that was considered possibly related to maribavir.
These results were presented at IDWeek 2016 (abstract 78). The study was sponsored by Shire.
“Cytomegalovirus infection that is resistant or refractory to standard therapy in transplant patients is associated with significant complications and high mortality,” said study investigator Genovefa Papanicolaou, MD, of Memorial Sloan Kettering Cancer Center in New York, New York.
“The phase 2 findings support further research to confirm these results among patients who have limited options to combat the infection.”
Maribavir is a member of a class of drugs called benzimidazole ribosides. Maribavir is thought to inhibit viral DNA assembly and egress of viral capsids from the nucleus of infected cells. The drug has not been shown to affect the maturation of viral DNA or affect the viral DNA polymerase.
This phase 2 study of maribavir included 120 patients ages 12 and older with CMV infection (≥1000 DNA copies/mL of blood plasma) that was resistant or refractory to (val)ganciclovir or foscarnet.
Forty-seven of the patients had received a hematopoietic stem cell transplant, and 73 had a solid organ transplant. The patients’ median age was 55 (range, 18 to 74).
The patients were randomized to 1 of 3 twice-daily oral doses of maribavir—400 mg, 800 mg, or 1200 mg—for up to 24 weeks of treatment.
Efficacy
The study’s primary efficacy endpoint was the proportion of patients with confirmed undetectable plasma CMV DNA within 6 weeks of treatment.
Overall, 67% (80/120) of patients met the primary efficacy endpoint. This included 70% (n=28) of patients in the 400 mg group, 63% (n=25) in the 800 mg group, and 67% (n=27) in the 1200 mg group.
CMV infection recurred in 30 patients, including 7 in the 400 mg group, 11 in the 800 mg group, and 12 in the 1200 mg group.
Safety
The primary safety analysis was focused on the incidence of treatment-emergent AEs. The incidence was 78% (n=93) overall, including 78% (n=31) in the 400 mg group, 80% (n=32) in the 800 mg group, and 75% (n=30) in the 1200 mg group.
Twenty-seven percent of patients died due to any AE, 1 of which (multi-organ failure) was considered possibly related to maribavir.
Forty-one patients (34%) discontinued treatment with maribavir due to an AE, including 17 patients who discontinued due to CMV infection.
Dysgeusia was the most common treatment-emergent AE and led to treatment discontinuation in 1 patient. Dysgeusia occurred in 65% (n=78) of all patients, including 60% (n=24) in the 400 mg group, 63% (n=25) in the 800 mg group, and 73% (n=29) in the 1200 mg group.
Other treatment-emergent AEs (occurring in at least 20% of patients) for all doses included nausea, vomiting, CMV infection, diarrhea, fatigue, and anemia. Immunosuppressant drug level increases were reported as an AE in 10% of all patients. ![]()

NEW ORLEANS—The antiviral agent maribavir has shown activity against cytomegalovirus (CMV) in a phase 2 trial.
The trial included transplant recipients with a CMV infection that was resistant or refractory to prior treatment with (val)ganciclovir or foscarnet.
Sixty-seven percent of patients treated with varying doses of maribavir for up to 24 weeks had no detectable levels of CMV in their plasma within 6 weeks of starting treatment.
However, CMV infection did recur in some of these patients.
Dysgeusia was the most commonly reported treatment-emergent adverse event (AE), and one patient died of an AE that was considered possibly related to maribavir.
These results were presented at IDWeek 2016 (abstract 78). The study was sponsored by Shire.
“Cytomegalovirus infection that is resistant or refractory to standard therapy in transplant patients is associated with significant complications and high mortality,” said study investigator Genovefa Papanicolaou, MD, of Memorial Sloan Kettering Cancer Center in New York, New York.
“The phase 2 findings support further research to confirm these results among patients who have limited options to combat the infection.”
Maribavir is a member of a class of drugs called benzimidazole ribosides. Maribavir is thought to inhibit viral DNA assembly and egress of viral capsids from the nucleus of infected cells. The drug has not been shown to affect the maturation of viral DNA or affect the viral DNA polymerase.
This phase 2 study of maribavir included 120 patients ages 12 and older with CMV infection (≥1000 DNA copies/mL of blood plasma) that was resistant or refractory to (val)ganciclovir or foscarnet.
Forty-seven of the patients had received a hematopoietic stem cell transplant, and 73 had a solid organ transplant. The patients’ median age was 55 (range, 18 to 74).
The patients were randomized to 1 of 3 twice-daily oral doses of maribavir—400 mg, 800 mg, or 1200 mg—for up to 24 weeks of treatment.
Efficacy
The study’s primary efficacy endpoint was the proportion of patients with confirmed undetectable plasma CMV DNA within 6 weeks of treatment.
Overall, 67% (80/120) of patients met the primary efficacy endpoint. This included 70% (n=28) of patients in the 400 mg group, 63% (n=25) in the 800 mg group, and 67% (n=27) in the 1200 mg group.
CMV infection recurred in 30 patients, including 7 in the 400 mg group, 11 in the 800 mg group, and 12 in the 1200 mg group.
Safety
The primary safety analysis was focused on the incidence of treatment-emergent AEs. The incidence was 78% (n=93) overall, including 78% (n=31) in the 400 mg group, 80% (n=32) in the 800 mg group, and 75% (n=30) in the 1200 mg group.
Twenty-seven percent of patients died due to any AE, 1 of which (multi-organ failure) was considered possibly related to maribavir.
Forty-one patients (34%) discontinued treatment with maribavir due to an AE, including 17 patients who discontinued due to CMV infection.
Dysgeusia was the most common treatment-emergent AE and led to treatment discontinuation in 1 patient. Dysgeusia occurred in 65% (n=78) of all patients, including 60% (n=24) in the 400 mg group, 63% (n=25) in the 800 mg group, and 73% (n=29) in the 1200 mg group.
Other treatment-emergent AEs (occurring in at least 20% of patients) for all doses included nausea, vomiting, CMV infection, diarrhea, fatigue, and anemia. Immunosuppressant drug level increases were reported as an AE in 10% of all patients. ![]()
Drug elicits responses in heavily pretreated cHL

Photo from Business Wire
COLOGNE—Results from the CheckMate -205 study suggest nivolumab can produce a high objective response rate (ORR) in patients with heavily pretreated classical Hodgkin lymphoma (cHL).
Investigators recently presented results from one of the cohorts in this phase 2 trial—cohort C—which included cHL patients who received nivolumab after undergoing autologous hematopoietic stem cell transplant (auto-HSCT) and receiving treatment with brentuximab vedotin (BV).
At a median follow-up of 8.8 months, the ORR, as assessed by an independent radiologic review committee (IRRC), was 73%.
The median progression-free survival (PFS) was 11.2 months, and the 6-month overall survival (OS) was 94%.
Investigators said the safety profile of nivolumab in this patient population was consistent with previously reported data in patients with cHL, and no new clinically meaningful safety signals were identified.
“These data from cohort C build on existing evidence supporting the benefit of Opdivo [nivolumab] in classical Hodgkin lymphoma patients who have relapsed or progressed after autologous hematopoietic stem cell transplantation and post-transplantation brentuximab vedotin,” said investigator Andreas Engert, MD, of the University Hospital of Cologne in Germany.
“Results from cohort C indicated a benefit with Opdivo regardless of the order of prior treatment with autologous hematopoietic stem cell transplantation and brentuximab vedotin, providing important insights as we continue researching the potential role Opdivo could provide for heavily pretreated classical Hodgkin lymphoma patients.”
The results were presented at the 10th International Symposium on Hodgkin Lymphoma (abstract T022). Abstracts from this meeting have been published in haematologica.
The CheckMate -205 trial is sponsored by Bristol-Myers Squibb.
About the trial
CheckMate -205 is a multi-cohort study in which investigators are evaluating the safety and efficacy of nivolumab in adults with cHL.
Cohort A included cHL patients who had received auto-HSCT and were BV-naïve (n=63). Cohort B included cHL patients who had received auto-HSCT followed by BV (n=80).
Cohort C included cHL patients who had received BV before and/or after auto-HSCT (n=100).
The study also includes a cohort D, which is currently enrolling and evaluating nivolumab in combination with chemotherapy in newly diagnosed, advanced-stage cHL patients who are treatment-naïve (n=50).
Patients enrolled in this trial have received nivolumab at 3 mg/kg intravenously every 2 weeks until disease progression or unacceptable toxicity. In cohort C, patients were also treated until investigator-assessed complete response (CR) lasting 1 year.
The study’s primary endpoint was ORR by IRRC assessment. Secondary and other exploratory endpoints included duration of response by IRRC assessment for CR rate and partial response rate, PFS by IRRC assessment, OS, and safety.
Response
The investigators presented results from cohort C (n=100), which included patients with cHL who had received BV before and/or after auto-HSCT.
At a median follow-up of 8.8 months, ORR per the IRRC was 73% (n=73) overall. The investigators said ORR was consistent across patient subgroups, regardless of the timing of prior BV relative to auto-HSCT.
The ORR was 70% (n=23) in patients who received BV only before auto-HSCT, 72% (n=41) in patients who received BV only after auto-HSCT, and 88% (n=7) in patients who received BV before and after auto-HSCT.
The CR rate was 17% (n=17) overall, 18.2% (n=6) in patients who received BV only before auto-HSCT, 12.3% (n=7) in patients who received BV only after auto-HSCT, and 38% (n=3) in patients who received BV before and after auto-HSCT.
Survival
The median PFS was 11.2 months (range, 8.5 months to not achieved) overall, 11.2 months (range, 8.5 months to not achieved) in patients who received BV only before auto-HSCT, 8.9 months (range, 8.3 months to not achieved) in patients who received BV only after auto-HSCT, and not achieved (range, 5.6 months to not achieved) in patients who received BV before and after auto-HSCT.
The 6-month OS was 93.9% overall, 97% in patients who received BV only before auto-HSCT, 91% in patients who received BV only after auto-HSCT, and 100% in patients who received BV before and after auto-HSCT.
Safety
Treatment-related adverse events (AEs) occurred in 68% of patients between the first dose and 30 days after the last dose of nivolumab. The most common treatment-related AEs were diarrhea, infusion-related reaction, and fatigue (11% each).
Grade 3/4 AEs occurred in 19% of patients. Serious treatment-related AEs were reported in 17% of patients, and treatment-related AEs leading to discontinuation occurred in 6% of patients.
At present, no treatment-related deaths have been reported. ![]()

Photo from Business Wire
COLOGNE—Results from the CheckMate -205 study suggest nivolumab can produce a high objective response rate (ORR) in patients with heavily pretreated classical Hodgkin lymphoma (cHL).
Investigators recently presented results from one of the cohorts in this phase 2 trial—cohort C—which included cHL patients who received nivolumab after undergoing autologous hematopoietic stem cell transplant (auto-HSCT) and receiving treatment with brentuximab vedotin (BV).
At a median follow-up of 8.8 months, the ORR, as assessed by an independent radiologic review committee (IRRC), was 73%.
The median progression-free survival (PFS) was 11.2 months, and the 6-month overall survival (OS) was 94%.
Investigators said the safety profile of nivolumab in this patient population was consistent with previously reported data in patients with cHL, and no new clinically meaningful safety signals were identified.
“These data from cohort C build on existing evidence supporting the benefit of Opdivo [nivolumab] in classical Hodgkin lymphoma patients who have relapsed or progressed after autologous hematopoietic stem cell transplantation and post-transplantation brentuximab vedotin,” said investigator Andreas Engert, MD, of the University Hospital of Cologne in Germany.
“Results from cohort C indicated a benefit with Opdivo regardless of the order of prior treatment with autologous hematopoietic stem cell transplantation and brentuximab vedotin, providing important insights as we continue researching the potential role Opdivo could provide for heavily pretreated classical Hodgkin lymphoma patients.”
The results were presented at the 10th International Symposium on Hodgkin Lymphoma (abstract T022). Abstracts from this meeting have been published in haematologica.
The CheckMate -205 trial is sponsored by Bristol-Myers Squibb.
About the trial
CheckMate -205 is a multi-cohort study in which investigators are evaluating the safety and efficacy of nivolumab in adults with cHL.
Cohort A included cHL patients who had received auto-HSCT and were BV-naïve (n=63). Cohort B included cHL patients who had received auto-HSCT followed by BV (n=80).
Cohort C included cHL patients who had received BV before and/or after auto-HSCT (n=100).
The study also includes a cohort D, which is currently enrolling and evaluating nivolumab in combination with chemotherapy in newly diagnosed, advanced-stage cHL patients who are treatment-naïve (n=50).
Patients enrolled in this trial have received nivolumab at 3 mg/kg intravenously every 2 weeks until disease progression or unacceptable toxicity. In cohort C, patients were also treated until investigator-assessed complete response (CR) lasting 1 year.
The study’s primary endpoint was ORR by IRRC assessment. Secondary and other exploratory endpoints included duration of response by IRRC assessment for CR rate and partial response rate, PFS by IRRC assessment, OS, and safety.
Response
The investigators presented results from cohort C (n=100), which included patients with cHL who had received BV before and/or after auto-HSCT.
At a median follow-up of 8.8 months, ORR per the IRRC was 73% (n=73) overall. The investigators said ORR was consistent across patient subgroups, regardless of the timing of prior BV relative to auto-HSCT.
The ORR was 70% (n=23) in patients who received BV only before auto-HSCT, 72% (n=41) in patients who received BV only after auto-HSCT, and 88% (n=7) in patients who received BV before and after auto-HSCT.
The CR rate was 17% (n=17) overall, 18.2% (n=6) in patients who received BV only before auto-HSCT, 12.3% (n=7) in patients who received BV only after auto-HSCT, and 38% (n=3) in patients who received BV before and after auto-HSCT.
Survival
The median PFS was 11.2 months (range, 8.5 months to not achieved) overall, 11.2 months (range, 8.5 months to not achieved) in patients who received BV only before auto-HSCT, 8.9 months (range, 8.3 months to not achieved) in patients who received BV only after auto-HSCT, and not achieved (range, 5.6 months to not achieved) in patients who received BV before and after auto-HSCT.
The 6-month OS was 93.9% overall, 97% in patients who received BV only before auto-HSCT, 91% in patients who received BV only after auto-HSCT, and 100% in patients who received BV before and after auto-HSCT.
Safety
Treatment-related adverse events (AEs) occurred in 68% of patients between the first dose and 30 days after the last dose of nivolumab. The most common treatment-related AEs were diarrhea, infusion-related reaction, and fatigue (11% each).
Grade 3/4 AEs occurred in 19% of patients. Serious treatment-related AEs were reported in 17% of patients, and treatment-related AEs leading to discontinuation occurred in 6% of patients.
At present, no treatment-related deaths have been reported. ![]()

Photo from Business Wire
COLOGNE—Results from the CheckMate -205 study suggest nivolumab can produce a high objective response rate (ORR) in patients with heavily pretreated classical Hodgkin lymphoma (cHL).
Investigators recently presented results from one of the cohorts in this phase 2 trial—cohort C—which included cHL patients who received nivolumab after undergoing autologous hematopoietic stem cell transplant (auto-HSCT) and receiving treatment with brentuximab vedotin (BV).
At a median follow-up of 8.8 months, the ORR, as assessed by an independent radiologic review committee (IRRC), was 73%.
The median progression-free survival (PFS) was 11.2 months, and the 6-month overall survival (OS) was 94%.
Investigators said the safety profile of nivolumab in this patient population was consistent with previously reported data in patients with cHL, and no new clinically meaningful safety signals were identified.
“These data from cohort C build on existing evidence supporting the benefit of Opdivo [nivolumab] in classical Hodgkin lymphoma patients who have relapsed or progressed after autologous hematopoietic stem cell transplantation and post-transplantation brentuximab vedotin,” said investigator Andreas Engert, MD, of the University Hospital of Cologne in Germany.
“Results from cohort C indicated a benefit with Opdivo regardless of the order of prior treatment with autologous hematopoietic stem cell transplantation and brentuximab vedotin, providing important insights as we continue researching the potential role Opdivo could provide for heavily pretreated classical Hodgkin lymphoma patients.”
The results were presented at the 10th International Symposium on Hodgkin Lymphoma (abstract T022). Abstracts from this meeting have been published in haematologica.
The CheckMate -205 trial is sponsored by Bristol-Myers Squibb.
About the trial
CheckMate -205 is a multi-cohort study in which investigators are evaluating the safety and efficacy of nivolumab in adults with cHL.
Cohort A included cHL patients who had received auto-HSCT and were BV-naïve (n=63). Cohort B included cHL patients who had received auto-HSCT followed by BV (n=80).
Cohort C included cHL patients who had received BV before and/or after auto-HSCT (n=100).
The study also includes a cohort D, which is currently enrolling and evaluating nivolumab in combination with chemotherapy in newly diagnosed, advanced-stage cHL patients who are treatment-naïve (n=50).
Patients enrolled in this trial have received nivolumab at 3 mg/kg intravenously every 2 weeks until disease progression or unacceptable toxicity. In cohort C, patients were also treated until investigator-assessed complete response (CR) lasting 1 year.
The study’s primary endpoint was ORR by IRRC assessment. Secondary and other exploratory endpoints included duration of response by IRRC assessment for CR rate and partial response rate, PFS by IRRC assessment, OS, and safety.
Response
The investigators presented results from cohort C (n=100), which included patients with cHL who had received BV before and/or after auto-HSCT.
At a median follow-up of 8.8 months, ORR per the IRRC was 73% (n=73) overall. The investigators said ORR was consistent across patient subgroups, regardless of the timing of prior BV relative to auto-HSCT.
The ORR was 70% (n=23) in patients who received BV only before auto-HSCT, 72% (n=41) in patients who received BV only after auto-HSCT, and 88% (n=7) in patients who received BV before and after auto-HSCT.
The CR rate was 17% (n=17) overall, 18.2% (n=6) in patients who received BV only before auto-HSCT, 12.3% (n=7) in patients who received BV only after auto-HSCT, and 38% (n=3) in patients who received BV before and after auto-HSCT.
Survival
The median PFS was 11.2 months (range, 8.5 months to not achieved) overall, 11.2 months (range, 8.5 months to not achieved) in patients who received BV only before auto-HSCT, 8.9 months (range, 8.3 months to not achieved) in patients who received BV only after auto-HSCT, and not achieved (range, 5.6 months to not achieved) in patients who received BV before and after auto-HSCT.
The 6-month OS was 93.9% overall, 97% in patients who received BV only before auto-HSCT, 91% in patients who received BV only after auto-HSCT, and 100% in patients who received BV before and after auto-HSCT.
Safety
Treatment-related adverse events (AEs) occurred in 68% of patients between the first dose and 30 days after the last dose of nivolumab. The most common treatment-related AEs were diarrhea, infusion-related reaction, and fatigue (11% each).
Grade 3/4 AEs occurred in 19% of patients. Serious treatment-related AEs were reported in 17% of patients, and treatment-related AEs leading to discontinuation occurred in 6% of patients.
At present, no treatment-related deaths have been reported. ![]()
Cutting Down on Dialysis-Related Infections
Each year about 37,000 people get potentially deadly bloodstream infections related to dialysis. But those infections could be cut dramatically by implementing evidence-based recommendations, the CDC says. For several years, facilities that have followed CDC recommendations have successfully reduced bloodstream infections in dialysis patients. "Making evidence-based safety steps a routine part of patient care is a proven strategy to keep dialysis patients safe from bloodstream infections," said CDC Director Tom Frieden, MD, MPH.
Related: Needlesticks and Infections: Still Not Enough Information
The CDC is teaming up with a coalition of health care and patient advocacy organizations and other public health partners in the Making Dialysis Safer for Patients Coalition, an initiative to expand the use of the recommendations and tools to improve dialysis patient safety.
The coalition will promote the use of the CDC’s Core Interventions and provide facilities with resources, including patient and staff education materials with tips to prevent infection; protocols for dialysis facilities; dialysis audit tools and checklists on, for example, catheter care; and videos and DVDs on best practices.
Related: Hospital-Acquired Infections on the Decline
“Dialysis patients are particularly vulnerable to infections,” said Dr. Priti Patel, medical director of the coalition. “We want to get lifesaving tools into the right hands to make a real impact on patients’ lives.”
Each year about 37,000 people get potentially deadly bloodstream infections related to dialysis. But those infections could be cut dramatically by implementing evidence-based recommendations, the CDC says. For several years, facilities that have followed CDC recommendations have successfully reduced bloodstream infections in dialysis patients. "Making evidence-based safety steps a routine part of patient care is a proven strategy to keep dialysis patients safe from bloodstream infections," said CDC Director Tom Frieden, MD, MPH.
Related: Needlesticks and Infections: Still Not Enough Information
The CDC is teaming up with a coalition of health care and patient advocacy organizations and other public health partners in the Making Dialysis Safer for Patients Coalition, an initiative to expand the use of the recommendations and tools to improve dialysis patient safety.
The coalition will promote the use of the CDC’s Core Interventions and provide facilities with resources, including patient and staff education materials with tips to prevent infection; protocols for dialysis facilities; dialysis audit tools and checklists on, for example, catheter care; and videos and DVDs on best practices.
Related: Hospital-Acquired Infections on the Decline
“Dialysis patients are particularly vulnerable to infections,” said Dr. Priti Patel, medical director of the coalition. “We want to get lifesaving tools into the right hands to make a real impact on patients’ lives.”
Each year about 37,000 people get potentially deadly bloodstream infections related to dialysis. But those infections could be cut dramatically by implementing evidence-based recommendations, the CDC says. For several years, facilities that have followed CDC recommendations have successfully reduced bloodstream infections in dialysis patients. "Making evidence-based safety steps a routine part of patient care is a proven strategy to keep dialysis patients safe from bloodstream infections," said CDC Director Tom Frieden, MD, MPH.
Related: Needlesticks and Infections: Still Not Enough Information
The CDC is teaming up with a coalition of health care and patient advocacy organizations and other public health partners in the Making Dialysis Safer for Patients Coalition, an initiative to expand the use of the recommendations and tools to improve dialysis patient safety.
The coalition will promote the use of the CDC’s Core Interventions and provide facilities with resources, including patient and staff education materials with tips to prevent infection; protocols for dialysis facilities; dialysis audit tools and checklists on, for example, catheter care; and videos and DVDs on best practices.
Related: Hospital-Acquired Infections on the Decline
“Dialysis patients are particularly vulnerable to infections,” said Dr. Priti Patel, medical director of the coalition. “We want to get lifesaving tools into the right hands to make a real impact on patients’ lives.”
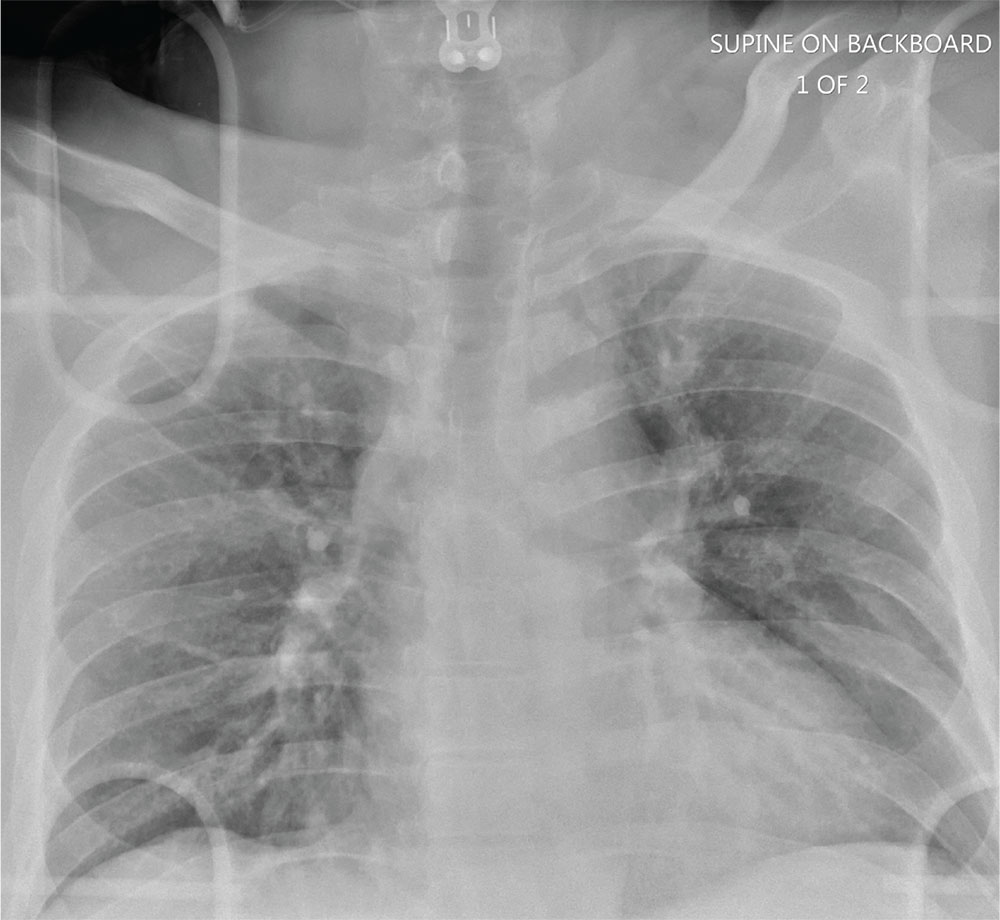
Man Thrown From Balky Bike

ANSWER
The radiograph does not demonstrate any evidence of acute chest or intrathoracic injury. Of note, it appears that the left humerus is dislocated anteriorly and inferiorly.
Dedicated shoulder radiographs were obtained, which confirmed the dislocation with no evidence of fracture. Orthopedics was consulted for evaluation and subsequent reduction.

ANSWER
The radiograph does not demonstrate any evidence of acute chest or intrathoracic injury. Of note, it appears that the left humerus is dislocated anteriorly and inferiorly.
Dedicated shoulder radiographs were obtained, which confirmed the dislocation with no evidence of fracture. Orthopedics was consulted for evaluation and subsequent reduction.

ANSWER
The radiograph does not demonstrate any evidence of acute chest or intrathoracic injury. Of note, it appears that the left humerus is dislocated anteriorly and inferiorly.
Dedicated shoulder radiographs were obtained, which confirmed the dislocation with no evidence of fracture. Orthopedics was consulted for evaluation and subsequent reduction.
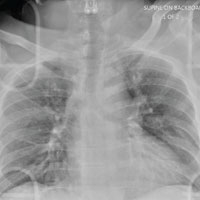
A 55-year-old man is brought to your facility following a motorcycle accident. He was a helmeted rider who was inadvertently thrown from the motorcycle when the accelerator got stuck. Bystanders reported he had brief loss of consciousness, but upon arrival to your facility, he is awake.
History is limited as the patient is confused and repetitive in his speech, indicating he is post concussive. He is complaining of head, face, and chest wall pain. His vital signs are all within normal limits; he is hemodynamically stable. His O2 saturation is 98% on room air. Breath sounds are clear.
As you are completing your primary survey, portable chest and pelvic radiographs are obtained. The chest radiograph is shown. What is your impression?
Most infective endocarditis calls for early surgery
CHICAGO - Turning to surgery earlier in infective endocarditis may hold the key to a cure for some patients. Upcoming guidelines for surgical treatment of infective endocarditis lend evidence-based support to early surgical intervention in this high-mortality condition.
“Infective endocarditis is the most severe and potentially devastating complication for heart valve disease,” said Joseph Coselli, MD, in a presentation that reviewed current trends in incidence of infective endocarditis (IE) and laid out a rationale and strategy for early surgical intervention in some patients.
“Untreated infective endocarditis is universally fatal,” said Dr. Coselli. Even with current treatments, however, overall mortality for infective endocarditis is 20%-25%, he said.
Speaking at the joint AATS-ACC Heart Valve Summit, Dr. Coselli, chief of the division of cardiothoracic surgery at Baylor College of Medicine, Houston, reviewed the key points in the upcoming guideline and the evidence that backs up the guidelines.
Dr. Coselli served on the writing committee for the 2016 AATS consensus guidelines for the surgical treatment of infective endocarditis; the guidelines are currently in press.
The guidelines propose that “at the time of surgery, the patient should be on an effective antimicrobial regimen to which the causative agent is sensitive,” he said. This is a level I recommendation, as is the recommendation that the surgeon should understand the pathology as well as possible before the procedure. Usually, say the guidelines, this is obtained by means of a transesophageal echocardiogram (TEE), assigning level I status to this recommendation as well.
According to the guidelines, patients with IE who may be surgical candidates during their hospitalization, regardless of whether their antimicrobial course is complete, include those who present with valve dysfunction that results in symptoms of heart failure. Surgery should also be considered in patients with left-sided IE with S. aureus, fungi, or other highly resistant organisms as the causative pathogen. If heart block, an aortic or annular abscess, or destructive penetrating lesions are present, surgery is also indicated. Finally, the guidelines recommend considering surgery if patients have persistent bacteremia or fevers at 5 to 7 days after initiation of appropriate antimicrobial therapy. All of these are class I indications in the upcoming guidelines, he said.
The patient who has relapsing infection, defined by the guidelines as recurrent bacteremia “after a complete course of appropriate antibiotics and subsequently negative blood culture,” who has no other identifiable source of infection, may also be a candidate.
Given the dearth of randomized trials in the area, no recommendation for intervention is backed by a level of evidence greater than B, said Dr. Coselli. And knowledge gaps persist in many areas, such as the appropriate timing of surgery in IE when there are neurological complications. Also, he said, “embolism risk needs to be better understood.” Imaging improvements would help guide decision-making, as would better data about contemporary rates of IE relapse and recurrence, said Dr. Coselli.
Though these surgeries should be done at centers that can field a complete team, and by experience valve surgeons, early intervention may be a key to success: “Operate before a devastating complication occurs,” said Dr. Coselli. “Understand what you see; don’t be afraid of radical debridement, and master alternative options to reconstruction” depending on the heart’s appearance in the OR, he said.
Surgeons can run into trouble in IE cases if they wait too long. “A patient who’s already had an embolic stroke may be too sick,” said Dr. Coselli. Insensitive organisms and ineffective antimicrobial therapy set the patient up for recurrent IE or treatment failure as well.
Having guidance for surgical intervention is important because cardiologists and surgeons will be seeing more infective endocarditis patients as heroin and other illicit intravenous drug use continues to rise, said Dr. Coselli. IE in intravenous drug users now accounts for up to 30% of all patients who seek treatment for IE, he said, citing a study that tracked characteristics of endocarditis patients undergoing surgery at a single institution from 2002-2014 (J Thorac Cardiovasc Surg. 2016 Sep;152:832-41). Incidence in intravenous drug users can range to 2,000 cases per 100,000 patient-years, he said.
The study, conducted by Joon Bum Kim, MD, PhD, and his colleagues at Massachusetts General and Brigham and Women’s hospitals, both in Boston, followed 436 patients with IE, 78 of whom were intravenous drug users (IVDUs) at the time of diagnosis. Overall, the IVDUs were younger (mean age, 36 plus or minus 10 years) when compared with the non-IVDU group (mean age, 58 plus or minus 14 years; P less than 0.001). The non-IVDU cohort were also significantly more likely to have hypertension and diabetes, but less likely to smoke. However, IVDUs were more likely to have embolic events, and to have right-sided valve involvement.
Though early mortality was better in the IVDU group post-surgically, late complications, including reinfection and reoperation, were significantly more likely to occur in the IVDUs, with reinfection more than four times as frequent in IVDUs (aggregate valve-related complications, 41% in IVDUs vs. 10% in non-IVDUs; P = 0.001).
Despite the additional morbidity seen in IVDU-associated endocarditis, the 10-year survival rate was virtually identical between the two groups.
For many IE patients, said Dr. Coselli, “the arguments against surgery have lost strength.” Active systemic infections are treatable, sicker patients can be operated on earlier, and surgeons will gain experience with this sometimes technically challenging surgery, he said. Finally, Dr. Coselli said, even though the best available data support early surgical intervention in select IE patients, “final cure of IE is always the result of antimicrobial treatment and the patient’s own defense.”
[email protected]
On Twitter @karioakes
CHICAGO - Turning to surgery earlier in infective endocarditis may hold the key to a cure for some patients. Upcoming guidelines for surgical treatment of infective endocarditis lend evidence-based support to early surgical intervention in this high-mortality condition.
“Infective endocarditis is the most severe and potentially devastating complication for heart valve disease,” said Joseph Coselli, MD, in a presentation that reviewed current trends in incidence of infective endocarditis (IE) and laid out a rationale and strategy for early surgical intervention in some patients.
“Untreated infective endocarditis is universally fatal,” said Dr. Coselli. Even with current treatments, however, overall mortality for infective endocarditis is 20%-25%, he said.
Speaking at the joint AATS-ACC Heart Valve Summit, Dr. Coselli, chief of the division of cardiothoracic surgery at Baylor College of Medicine, Houston, reviewed the key points in the upcoming guideline and the evidence that backs up the guidelines.
Dr. Coselli served on the writing committee for the 2016 AATS consensus guidelines for the surgical treatment of infective endocarditis; the guidelines are currently in press.
The guidelines propose that “at the time of surgery, the patient should be on an effective antimicrobial regimen to which the causative agent is sensitive,” he said. This is a level I recommendation, as is the recommendation that the surgeon should understand the pathology as well as possible before the procedure. Usually, say the guidelines, this is obtained by means of a transesophageal echocardiogram (TEE), assigning level I status to this recommendation as well.
According to the guidelines, patients with IE who may be surgical candidates during their hospitalization, regardless of whether their antimicrobial course is complete, include those who present with valve dysfunction that results in symptoms of heart failure. Surgery should also be considered in patients with left-sided IE with S. aureus, fungi, or other highly resistant organisms as the causative pathogen. If heart block, an aortic or annular abscess, or destructive penetrating lesions are present, surgery is also indicated. Finally, the guidelines recommend considering surgery if patients have persistent bacteremia or fevers at 5 to 7 days after initiation of appropriate antimicrobial therapy. All of these are class I indications in the upcoming guidelines, he said.
The patient who has relapsing infection, defined by the guidelines as recurrent bacteremia “after a complete course of appropriate antibiotics and subsequently negative blood culture,” who has no other identifiable source of infection, may also be a candidate.
Given the dearth of randomized trials in the area, no recommendation for intervention is backed by a level of evidence greater than B, said Dr. Coselli. And knowledge gaps persist in many areas, such as the appropriate timing of surgery in IE when there are neurological complications. Also, he said, “embolism risk needs to be better understood.” Imaging improvements would help guide decision-making, as would better data about contemporary rates of IE relapse and recurrence, said Dr. Coselli.
Though these surgeries should be done at centers that can field a complete team, and by experience valve surgeons, early intervention may be a key to success: “Operate before a devastating complication occurs,” said Dr. Coselli. “Understand what you see; don’t be afraid of radical debridement, and master alternative options to reconstruction” depending on the heart’s appearance in the OR, he said.
Surgeons can run into trouble in IE cases if they wait too long. “A patient who’s already had an embolic stroke may be too sick,” said Dr. Coselli. Insensitive organisms and ineffective antimicrobial therapy set the patient up for recurrent IE or treatment failure as well.
Having guidance for surgical intervention is important because cardiologists and surgeons will be seeing more infective endocarditis patients as heroin and other illicit intravenous drug use continues to rise, said Dr. Coselli. IE in intravenous drug users now accounts for up to 30% of all patients who seek treatment for IE, he said, citing a study that tracked characteristics of endocarditis patients undergoing surgery at a single institution from 2002-2014 (J Thorac Cardiovasc Surg. 2016 Sep;152:832-41). Incidence in intravenous drug users can range to 2,000 cases per 100,000 patient-years, he said.
The study, conducted by Joon Bum Kim, MD, PhD, and his colleagues at Massachusetts General and Brigham and Women’s hospitals, both in Boston, followed 436 patients with IE, 78 of whom were intravenous drug users (IVDUs) at the time of diagnosis. Overall, the IVDUs were younger (mean age, 36 plus or minus 10 years) when compared with the non-IVDU group (mean age, 58 plus or minus 14 years; P less than 0.001). The non-IVDU cohort were also significantly more likely to have hypertension and diabetes, but less likely to smoke. However, IVDUs were more likely to have embolic events, and to have right-sided valve involvement.
Though early mortality was better in the IVDU group post-surgically, late complications, including reinfection and reoperation, were significantly more likely to occur in the IVDUs, with reinfection more than four times as frequent in IVDUs (aggregate valve-related complications, 41% in IVDUs vs. 10% in non-IVDUs; P = 0.001).
Despite the additional morbidity seen in IVDU-associated endocarditis, the 10-year survival rate was virtually identical between the two groups.
For many IE patients, said Dr. Coselli, “the arguments against surgery have lost strength.” Active systemic infections are treatable, sicker patients can be operated on earlier, and surgeons will gain experience with this sometimes technically challenging surgery, he said. Finally, Dr. Coselli said, even though the best available data support early surgical intervention in select IE patients, “final cure of IE is always the result of antimicrobial treatment and the patient’s own defense.”
[email protected]
On Twitter @karioakes
CHICAGO - Turning to surgery earlier in infective endocarditis may hold the key to a cure for some patients. Upcoming guidelines for surgical treatment of infective endocarditis lend evidence-based support to early surgical intervention in this high-mortality condition.
“Infective endocarditis is the most severe and potentially devastating complication for heart valve disease,” said Joseph Coselli, MD, in a presentation that reviewed current trends in incidence of infective endocarditis (IE) and laid out a rationale and strategy for early surgical intervention in some patients.
“Untreated infective endocarditis is universally fatal,” said Dr. Coselli. Even with current treatments, however, overall mortality for infective endocarditis is 20%-25%, he said.
Speaking at the joint AATS-ACC Heart Valve Summit, Dr. Coselli, chief of the division of cardiothoracic surgery at Baylor College of Medicine, Houston, reviewed the key points in the upcoming guideline and the evidence that backs up the guidelines.
Dr. Coselli served on the writing committee for the 2016 AATS consensus guidelines for the surgical treatment of infective endocarditis; the guidelines are currently in press.
The guidelines propose that “at the time of surgery, the patient should be on an effective antimicrobial regimen to which the causative agent is sensitive,” he said. This is a level I recommendation, as is the recommendation that the surgeon should understand the pathology as well as possible before the procedure. Usually, say the guidelines, this is obtained by means of a transesophageal echocardiogram (TEE), assigning level I status to this recommendation as well.
According to the guidelines, patients with IE who may be surgical candidates during their hospitalization, regardless of whether their antimicrobial course is complete, include those who present with valve dysfunction that results in symptoms of heart failure. Surgery should also be considered in patients with left-sided IE with S. aureus, fungi, or other highly resistant organisms as the causative pathogen. If heart block, an aortic or annular abscess, or destructive penetrating lesions are present, surgery is also indicated. Finally, the guidelines recommend considering surgery if patients have persistent bacteremia or fevers at 5 to 7 days after initiation of appropriate antimicrobial therapy. All of these are class I indications in the upcoming guidelines, he said.
The patient who has relapsing infection, defined by the guidelines as recurrent bacteremia “after a complete course of appropriate antibiotics and subsequently negative blood culture,” who has no other identifiable source of infection, may also be a candidate.
Given the dearth of randomized trials in the area, no recommendation for intervention is backed by a level of evidence greater than B, said Dr. Coselli. And knowledge gaps persist in many areas, such as the appropriate timing of surgery in IE when there are neurological complications. Also, he said, “embolism risk needs to be better understood.” Imaging improvements would help guide decision-making, as would better data about contemporary rates of IE relapse and recurrence, said Dr. Coselli.
Though these surgeries should be done at centers that can field a complete team, and by experience valve surgeons, early intervention may be a key to success: “Operate before a devastating complication occurs,” said Dr. Coselli. “Understand what you see; don’t be afraid of radical debridement, and master alternative options to reconstruction” depending on the heart’s appearance in the OR, he said.
Surgeons can run into trouble in IE cases if they wait too long. “A patient who’s already had an embolic stroke may be too sick,” said Dr. Coselli. Insensitive organisms and ineffective antimicrobial therapy set the patient up for recurrent IE or treatment failure as well.
Having guidance for surgical intervention is important because cardiologists and surgeons will be seeing more infective endocarditis patients as heroin and other illicit intravenous drug use continues to rise, said Dr. Coselli. IE in intravenous drug users now accounts for up to 30% of all patients who seek treatment for IE, he said, citing a study that tracked characteristics of endocarditis patients undergoing surgery at a single institution from 2002-2014 (J Thorac Cardiovasc Surg. 2016 Sep;152:832-41). Incidence in intravenous drug users can range to 2,000 cases per 100,000 patient-years, he said.
The study, conducted by Joon Bum Kim, MD, PhD, and his colleagues at Massachusetts General and Brigham and Women’s hospitals, both in Boston, followed 436 patients with IE, 78 of whom were intravenous drug users (IVDUs) at the time of diagnosis. Overall, the IVDUs were younger (mean age, 36 plus or minus 10 years) when compared with the non-IVDU group (mean age, 58 plus or minus 14 years; P less than 0.001). The non-IVDU cohort were also significantly more likely to have hypertension and diabetes, but less likely to smoke. However, IVDUs were more likely to have embolic events, and to have right-sided valve involvement.
Though early mortality was better in the IVDU group post-surgically, late complications, including reinfection and reoperation, were significantly more likely to occur in the IVDUs, with reinfection more than four times as frequent in IVDUs (aggregate valve-related complications, 41% in IVDUs vs. 10% in non-IVDUs; P = 0.001).
Despite the additional morbidity seen in IVDU-associated endocarditis, the 10-year survival rate was virtually identical between the two groups.
For many IE patients, said Dr. Coselli, “the arguments against surgery have lost strength.” Active systemic infections are treatable, sicker patients can be operated on earlier, and surgeons will gain experience with this sometimes technically challenging surgery, he said. Finally, Dr. Coselli said, even though the best available data support early surgical intervention in select IE patients, “final cure of IE is always the result of antimicrobial treatment and the patient’s own defense.”
[email protected]
On Twitter @karioakes
EXPERT ANALYSIS FROM THE HEART VALVE SUMMIT 2016
Roommates
The American Academy of Pediatrics has recently released a new policy for parents on safe sleep practices that in addition to the previous warnings about bed sharing and positioning includes the recommendation that an infant sleep in the same room as her parent for at least the first 6 months (Pediatrics. 2016 Oct;138[5]:e20162938). Apparently what prompted this new set of recommendations is the observation that deaths from sudden unexpected infant deaths (SUIDS) and sudden infant deaths (SIDS) has plateaued since the dramatic decline we witnessed in the 1990s following the Back-to-Sleep campaign.
Although the policy statement refers to “new research” that has become available since the last policy statement was released in 2011, I have had trouble finding convincing evidence in the references I reviewed to support the room sharing recommendation. In some studies, room sharing was the cultural norm, making it difficult to establish a control group. In one of the most frequently cited papers from New Zealand, the authors could not sort out the effects of prone sleeping and sleeping alone, and wonder whether both factors may be affecting risk “through a common mechanism” (Lancet. 1996 Jan 6;347[8993]:7-12).
For some, parents attempting to follow this recommendation may not be without its negative consequences. Sleeping like a baby is not the same as sleeping quietly. Infants often breathe in a pattern that includes long, anxiety-provoking pauses. The implication of this policy recommendation is that parents can prevent crib death by being more vigilant at night. Do we have enough evidence that this is indeed the case?
Most parents are already anxious, and none of them are getting enough sleep. I can envision that trying to follow this recommendation could aggravate both conditions for some parents. Sleep-deprived parents often are not as capable parents as they could be. And they certainly aren’t as happy as they could be. Postpartum depression compounded by sleep deprivation continues to be an underreported and inadequately managed condition that can have negative effects for the health of the child.
For some parents, room sharing is something they gravitate toward naturally, and it can help them deal with the anxiety of new parenthood. They may sleep better with their infant close by. But for others, the better solution to their own sleep deprivation lies in sleep training, a strategy that is very difficult, if not impossible, for parents who are sharing their bedroom with their infant.
As the authors of one of the most frequently quoted papers that supports room sharing have written, “the traditional habit of labeling one sleep arrangement as being superior to another without awareness of the family context is not only wrong but potentially harmful” (Paediatric Resp Review. 2005, Jun;6[2]:134-52).
I think the academy has gone too far or at least moved prematurely with its room sharing recommendation. For some families, room sharing is a better arrangement, for others it is not. It may well be that the plateau in crib deaths is telling us that we have reached the limits of our abilities to effect any further decline with our recommendations about sleep environments. But more research needs to be done.
On a more positive note, the new recommendation may force parents to reevaluate their habit of having a television in their bedroom. Will it be baby or TV in the bedroom? Unfortunately, I fear too many will opt to have both.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.” Email him at [email protected].
The American Academy of Pediatrics has recently released a new policy for parents on safe sleep practices that in addition to the previous warnings about bed sharing and positioning includes the recommendation that an infant sleep in the same room as her parent for at least the first 6 months (Pediatrics. 2016 Oct;138[5]:e20162938). Apparently what prompted this new set of recommendations is the observation that deaths from sudden unexpected infant deaths (SUIDS) and sudden infant deaths (SIDS) has plateaued since the dramatic decline we witnessed in the 1990s following the Back-to-Sleep campaign.
Although the policy statement refers to “new research” that has become available since the last policy statement was released in 2011, I have had trouble finding convincing evidence in the references I reviewed to support the room sharing recommendation. In some studies, room sharing was the cultural norm, making it difficult to establish a control group. In one of the most frequently cited papers from New Zealand, the authors could not sort out the effects of prone sleeping and sleeping alone, and wonder whether both factors may be affecting risk “through a common mechanism” (Lancet. 1996 Jan 6;347[8993]:7-12).
For some, parents attempting to follow this recommendation may not be without its negative consequences. Sleeping like a baby is not the same as sleeping quietly. Infants often breathe in a pattern that includes long, anxiety-provoking pauses. The implication of this policy recommendation is that parents can prevent crib death by being more vigilant at night. Do we have enough evidence that this is indeed the case?
Most parents are already anxious, and none of them are getting enough sleep. I can envision that trying to follow this recommendation could aggravate both conditions for some parents. Sleep-deprived parents often are not as capable parents as they could be. And they certainly aren’t as happy as they could be. Postpartum depression compounded by sleep deprivation continues to be an underreported and inadequately managed condition that can have negative effects for the health of the child.
For some parents, room sharing is something they gravitate toward naturally, and it can help them deal with the anxiety of new parenthood. They may sleep better with their infant close by. But for others, the better solution to their own sleep deprivation lies in sleep training, a strategy that is very difficult, if not impossible, for parents who are sharing their bedroom with their infant.
As the authors of one of the most frequently quoted papers that supports room sharing have written, “the traditional habit of labeling one sleep arrangement as being superior to another without awareness of the family context is not only wrong but potentially harmful” (Paediatric Resp Review. 2005, Jun;6[2]:134-52).
I think the academy has gone too far or at least moved prematurely with its room sharing recommendation. For some families, room sharing is a better arrangement, for others it is not. It may well be that the plateau in crib deaths is telling us that we have reached the limits of our abilities to effect any further decline with our recommendations about sleep environments. But more research needs to be done.
On a more positive note, the new recommendation may force parents to reevaluate their habit of having a television in their bedroom. Will it be baby or TV in the bedroom? Unfortunately, I fear too many will opt to have both.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.” Email him at [email protected].
The American Academy of Pediatrics has recently released a new policy for parents on safe sleep practices that in addition to the previous warnings about bed sharing and positioning includes the recommendation that an infant sleep in the same room as her parent for at least the first 6 months (Pediatrics. 2016 Oct;138[5]:e20162938). Apparently what prompted this new set of recommendations is the observation that deaths from sudden unexpected infant deaths (SUIDS) and sudden infant deaths (SIDS) has plateaued since the dramatic decline we witnessed in the 1990s following the Back-to-Sleep campaign.
Although the policy statement refers to “new research” that has become available since the last policy statement was released in 2011, I have had trouble finding convincing evidence in the references I reviewed to support the room sharing recommendation. In some studies, room sharing was the cultural norm, making it difficult to establish a control group. In one of the most frequently cited papers from New Zealand, the authors could not sort out the effects of prone sleeping and sleeping alone, and wonder whether both factors may be affecting risk “through a common mechanism” (Lancet. 1996 Jan 6;347[8993]:7-12).
For some, parents attempting to follow this recommendation may not be without its negative consequences. Sleeping like a baby is not the same as sleeping quietly. Infants often breathe in a pattern that includes long, anxiety-provoking pauses. The implication of this policy recommendation is that parents can prevent crib death by being more vigilant at night. Do we have enough evidence that this is indeed the case?
Most parents are already anxious, and none of them are getting enough sleep. I can envision that trying to follow this recommendation could aggravate both conditions for some parents. Sleep-deprived parents often are not as capable parents as they could be. And they certainly aren’t as happy as they could be. Postpartum depression compounded by sleep deprivation continues to be an underreported and inadequately managed condition that can have negative effects for the health of the child.
For some parents, room sharing is something they gravitate toward naturally, and it can help them deal with the anxiety of new parenthood. They may sleep better with their infant close by. But for others, the better solution to their own sleep deprivation lies in sleep training, a strategy that is very difficult, if not impossible, for parents who are sharing their bedroom with their infant.
As the authors of one of the most frequently quoted papers that supports room sharing have written, “the traditional habit of labeling one sleep arrangement as being superior to another without awareness of the family context is not only wrong but potentially harmful” (Paediatric Resp Review. 2005, Jun;6[2]:134-52).
I think the academy has gone too far or at least moved prematurely with its room sharing recommendation. For some families, room sharing is a better arrangement, for others it is not. It may well be that the plateau in crib deaths is telling us that we have reached the limits of our abilities to effect any further decline with our recommendations about sleep environments. But more research needs to be done.
On a more positive note, the new recommendation may force parents to reevaluate their habit of having a television in their bedroom. Will it be baby or TV in the bedroom? Unfortunately, I fear too many will opt to have both.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.” Email him at [email protected].
Cancer type, age at time of diagnosis implicated in risk of CVD-related deaths
Survivorship data derived from a U.K. cancer registry make it possible to more closely pinpoint the risk of cardiovascular disease in patients treated for cancer as adolescents and young adults.
Researchers report that 6% of the 2,016 deaths occurring in 200,945 cancer survivors diagnosed between the ages of 15 and 39 years were directly related to cardiovascular disease. A multivariable Poisson regression analysis of data from the Teenage and Young Adult Cancer Survivor Study also showed that survivors who were diagnosed between the ages of 15 and 19 years had 4.2 times the risk (95% confidence interval, 3.4-5.2) of death from cardiovascular disease, compared with their peers in the general population. But for survivors who were aged 35-39 years when diagnosed, that risk decreased to 1.2 times (95% CI, 1.1-1.3) that of their general population peers (P less than .0001). The standardized mortality ratios and absolute excess risks for ischemic heart disease, valvular heart disease, and cardiomyopathy were similar (Circulation. 2016;134:1521-33).
The findings should help clinicians craft more effective after-cancer care, according to Mike Hawkins, DPhil. “It helps them focus the most intensive follow-up care on those most at risk,” Dr. Hawkins, an epidemiology professor and director of the Centre for Childhood Cancer Survivor Studies at the University of Birmingham (England), said in a statement. “It is important for survivors because it empowers them by providing them with their long-term chances of a specific side effect of cancer treatment.”
The most significant relationship between cardiovascular disease and cancer occurred in those diagnosed with Hodgkin lymphoma, and at an earlier age. Overall, Hodgkin lymphoma survivors had a 3.8 times higher risk of cardiovascular disease–related death than their peers not diagnosed with any cancer. In those diagnosed at age 15-19 years, 6.9% had died from cardiovascular disease by age 55 years, compared with 2% of those who’d been diagnosed at age 35-39 years. Among these two age groups in the general population, fewer than 1% typically die from cardiovascular disease–related deaths. In Hodgkin lymphoma survivors aged 60 years or older, 27.5% of excess deaths were from cardiovascular disease.
Although not stratified by treatment, the study includes risk estimates for other cancers diagnosed in the teen and young adult years, stratified by the age at diagnosis, something the authors of the study noted is “a considerable advance on previous knowledge.”
Survivors of all age groups in the cohort diagnosed with a variety of cancers experienced a greater risk of death from heart disease, compared with their peers in the general population.
[email protected]
On Twitter @whitneymcknight
Survivorship data derived from a U.K. cancer registry make it possible to more closely pinpoint the risk of cardiovascular disease in patients treated for cancer as adolescents and young adults.
Researchers report that 6% of the 2,016 deaths occurring in 200,945 cancer survivors diagnosed between the ages of 15 and 39 years were directly related to cardiovascular disease. A multivariable Poisson regression analysis of data from the Teenage and Young Adult Cancer Survivor Study also showed that survivors who were diagnosed between the ages of 15 and 19 years had 4.2 times the risk (95% confidence interval, 3.4-5.2) of death from cardiovascular disease, compared with their peers in the general population. But for survivors who were aged 35-39 years when diagnosed, that risk decreased to 1.2 times (95% CI, 1.1-1.3) that of their general population peers (P less than .0001). The standardized mortality ratios and absolute excess risks for ischemic heart disease, valvular heart disease, and cardiomyopathy were similar (Circulation. 2016;134:1521-33).
The findings should help clinicians craft more effective after-cancer care, according to Mike Hawkins, DPhil. “It helps them focus the most intensive follow-up care on those most at risk,” Dr. Hawkins, an epidemiology professor and director of the Centre for Childhood Cancer Survivor Studies at the University of Birmingham (England), said in a statement. “It is important for survivors because it empowers them by providing them with their long-term chances of a specific side effect of cancer treatment.”
The most significant relationship between cardiovascular disease and cancer occurred in those diagnosed with Hodgkin lymphoma, and at an earlier age. Overall, Hodgkin lymphoma survivors had a 3.8 times higher risk of cardiovascular disease–related death than their peers not diagnosed with any cancer. In those diagnosed at age 15-19 years, 6.9% had died from cardiovascular disease by age 55 years, compared with 2% of those who’d been diagnosed at age 35-39 years. Among these two age groups in the general population, fewer than 1% typically die from cardiovascular disease–related deaths. In Hodgkin lymphoma survivors aged 60 years or older, 27.5% of excess deaths were from cardiovascular disease.
Although not stratified by treatment, the study includes risk estimates for other cancers diagnosed in the teen and young adult years, stratified by the age at diagnosis, something the authors of the study noted is “a considerable advance on previous knowledge.”
Survivors of all age groups in the cohort diagnosed with a variety of cancers experienced a greater risk of death from heart disease, compared with their peers in the general population.
[email protected]
On Twitter @whitneymcknight
Survivorship data derived from a U.K. cancer registry make it possible to more closely pinpoint the risk of cardiovascular disease in patients treated for cancer as adolescents and young adults.
Researchers report that 6% of the 2,016 deaths occurring in 200,945 cancer survivors diagnosed between the ages of 15 and 39 years were directly related to cardiovascular disease. A multivariable Poisson regression analysis of data from the Teenage and Young Adult Cancer Survivor Study also showed that survivors who were diagnosed between the ages of 15 and 19 years had 4.2 times the risk (95% confidence interval, 3.4-5.2) of death from cardiovascular disease, compared with their peers in the general population. But for survivors who were aged 35-39 years when diagnosed, that risk decreased to 1.2 times (95% CI, 1.1-1.3) that of their general population peers (P less than .0001). The standardized mortality ratios and absolute excess risks for ischemic heart disease, valvular heart disease, and cardiomyopathy were similar (Circulation. 2016;134:1521-33).
The findings should help clinicians craft more effective after-cancer care, according to Mike Hawkins, DPhil. “It helps them focus the most intensive follow-up care on those most at risk,” Dr. Hawkins, an epidemiology professor and director of the Centre for Childhood Cancer Survivor Studies at the University of Birmingham (England), said in a statement. “It is important for survivors because it empowers them by providing them with their long-term chances of a specific side effect of cancer treatment.”
The most significant relationship between cardiovascular disease and cancer occurred in those diagnosed with Hodgkin lymphoma, and at an earlier age. Overall, Hodgkin lymphoma survivors had a 3.8 times higher risk of cardiovascular disease–related death than their peers not diagnosed with any cancer. In those diagnosed at age 15-19 years, 6.9% had died from cardiovascular disease by age 55 years, compared with 2% of those who’d been diagnosed at age 35-39 years. Among these two age groups in the general population, fewer than 1% typically die from cardiovascular disease–related deaths. In Hodgkin lymphoma survivors aged 60 years or older, 27.5% of excess deaths were from cardiovascular disease.
Although not stratified by treatment, the study includes risk estimates for other cancers diagnosed in the teen and young adult years, stratified by the age at diagnosis, something the authors of the study noted is “a considerable advance on previous knowledge.”
Survivors of all age groups in the cohort diagnosed with a variety of cancers experienced a greater risk of death from heart disease, compared with their peers in the general population.
[email protected]
On Twitter @whitneymcknight
FROM CIRCULATION
Key clinical point:
Major finding: Cancer survivors who were diagnosed at age 15-19 years had 4.2 times the risk of death from cardiovascular disease than did their peers in the general population.
Data source: A U.K. cancer registry of 200,945 persons between 15 and 39 years at time of diagnosis.
Disclosures: This study was supported by the National Institute for Health Research in the United Kingdom. The authors had no relevant disclosures.
Abatacept may benefit ACPA-negative undifferentiated arthritis
Abatacept may benefit ACPA-negative undifferentiated arthritis, which generally has a poor prognosis and for which there is no proven therapy currently available, according to a report published in Rheumatology.
A 1-year course of abatacept (Orencia) reduced disease activity and an ultrasound measure of synovial inflammation in a manufacturer-funded, open-label, proof-of-concept study. However, it did not achieve the primary composite endpoint of remission on the DAS44 (44-joint Disease Activity Score), a maximum of one swollen joint for at least 3 consecutive months, and no radiographic progression after 6 months of treatment.
They assessed 20 adults with undifferentiated arthritis that had lasted 12 weeks to 18 months who showed definite, active synovitis in at least 1 of 20 scanned joints and had no previous disease-modifying antirheumatic drug (DMARD) therapy; these study subjects were considered likely to progress to rheumatoid arthritis. They received 14 doses of IV abatacept for 1 year.
Two patients withdrew from the study at 6 months and 12 months because of adverse events, and another three were lost to follow-up. Only 2 of the 20 patients (10%) achieved the composite primary endpoint. The majority of patients met two of the individual components – 15 patients showed no radiographic progression and 12 had a maximum of one swollen joint – but only 6 achieved DAS44 remission, the investigators said (Rheumatology [Oxford]. 2016 Oct 22. doi: 10.1093/rheumatology/kew357).
The treatment appeared to suppress C-reactive protein levels immediately, from a median of 9 mg/L at baseline to 0 mg/L at 3 and 6 months. This implies a clear biologic effect. The median number of swollen joints also decreased from a median of 2 at baseline to 0 at 6 and 12 months. There were small reductions in median scores on a disability index and a small decrease in patient-reported visual analog scale disease activity from a median of 52 at baseline to 28 at 6 months and 24 at 12 months. Median ultrasound scores of synovitis decreased from 10 at baseline to 3 at both 6 and 12 months.
Most of these benefits persisted for 1 year after abatacept therapy was stopped, but approximately half of patients required a synthetic DMARD to maintain the benefits. “Overall, these data suggest that in the vast majority of patients (18 of 20), abatacept therapy prevented further progression of disease, but on cessation, additional therapy was indicated to maintain this,” Dr. Buch and her associates said.
There were no serious adverse events during treatment, including no infections and no abnormal liver function tests. A total of 131 nonserious adverse events were reported.
This study was supported by a research grant from Bristol-Myers Squibb and by institution-level grants from Arthritis UK and the U.K. National Institute for Health Research. Dr. Buch reported ties to Bristol-Myers Squibb, AbbVie, AstraZeneca, Pfizer, and Roche-Chugai; her associates reported ties to numerous industry sources.
Abatacept may benefit ACPA-negative undifferentiated arthritis, which generally has a poor prognosis and for which there is no proven therapy currently available, according to a report published in Rheumatology.
A 1-year course of abatacept (Orencia) reduced disease activity and an ultrasound measure of synovial inflammation in a manufacturer-funded, open-label, proof-of-concept study. However, it did not achieve the primary composite endpoint of remission on the DAS44 (44-joint Disease Activity Score), a maximum of one swollen joint for at least 3 consecutive months, and no radiographic progression after 6 months of treatment.
They assessed 20 adults with undifferentiated arthritis that had lasted 12 weeks to 18 months who showed definite, active synovitis in at least 1 of 20 scanned joints and had no previous disease-modifying antirheumatic drug (DMARD) therapy; these study subjects were considered likely to progress to rheumatoid arthritis. They received 14 doses of IV abatacept for 1 year.
Two patients withdrew from the study at 6 months and 12 months because of adverse events, and another three were lost to follow-up. Only 2 of the 20 patients (10%) achieved the composite primary endpoint. The majority of patients met two of the individual components – 15 patients showed no radiographic progression and 12 had a maximum of one swollen joint – but only 6 achieved DAS44 remission, the investigators said (Rheumatology [Oxford]. 2016 Oct 22. doi: 10.1093/rheumatology/kew357).
The treatment appeared to suppress C-reactive protein levels immediately, from a median of 9 mg/L at baseline to 0 mg/L at 3 and 6 months. This implies a clear biologic effect. The median number of swollen joints also decreased from a median of 2 at baseline to 0 at 6 and 12 months. There were small reductions in median scores on a disability index and a small decrease in patient-reported visual analog scale disease activity from a median of 52 at baseline to 28 at 6 months and 24 at 12 months. Median ultrasound scores of synovitis decreased from 10 at baseline to 3 at both 6 and 12 months.
Most of these benefits persisted for 1 year after abatacept therapy was stopped, but approximately half of patients required a synthetic DMARD to maintain the benefits. “Overall, these data suggest that in the vast majority of patients (18 of 20), abatacept therapy prevented further progression of disease, but on cessation, additional therapy was indicated to maintain this,” Dr. Buch and her associates said.
There were no serious adverse events during treatment, including no infections and no abnormal liver function tests. A total of 131 nonserious adverse events were reported.
This study was supported by a research grant from Bristol-Myers Squibb and by institution-level grants from Arthritis UK and the U.K. National Institute for Health Research. Dr. Buch reported ties to Bristol-Myers Squibb, AbbVie, AstraZeneca, Pfizer, and Roche-Chugai; her associates reported ties to numerous industry sources.
Abatacept may benefit ACPA-negative undifferentiated arthritis, which generally has a poor prognosis and for which there is no proven therapy currently available, according to a report published in Rheumatology.
A 1-year course of abatacept (Orencia) reduced disease activity and an ultrasound measure of synovial inflammation in a manufacturer-funded, open-label, proof-of-concept study. However, it did not achieve the primary composite endpoint of remission on the DAS44 (44-joint Disease Activity Score), a maximum of one swollen joint for at least 3 consecutive months, and no radiographic progression after 6 months of treatment.
They assessed 20 adults with undifferentiated arthritis that had lasted 12 weeks to 18 months who showed definite, active synovitis in at least 1 of 20 scanned joints and had no previous disease-modifying antirheumatic drug (DMARD) therapy; these study subjects were considered likely to progress to rheumatoid arthritis. They received 14 doses of IV abatacept for 1 year.
Two patients withdrew from the study at 6 months and 12 months because of adverse events, and another three were lost to follow-up. Only 2 of the 20 patients (10%) achieved the composite primary endpoint. The majority of patients met two of the individual components – 15 patients showed no radiographic progression and 12 had a maximum of one swollen joint – but only 6 achieved DAS44 remission, the investigators said (Rheumatology [Oxford]. 2016 Oct 22. doi: 10.1093/rheumatology/kew357).
The treatment appeared to suppress C-reactive protein levels immediately, from a median of 9 mg/L at baseline to 0 mg/L at 3 and 6 months. This implies a clear biologic effect. The median number of swollen joints also decreased from a median of 2 at baseline to 0 at 6 and 12 months. There were small reductions in median scores on a disability index and a small decrease in patient-reported visual analog scale disease activity from a median of 52 at baseline to 28 at 6 months and 24 at 12 months. Median ultrasound scores of synovitis decreased from 10 at baseline to 3 at both 6 and 12 months.
Most of these benefits persisted for 1 year after abatacept therapy was stopped, but approximately half of patients required a synthetic DMARD to maintain the benefits. “Overall, these data suggest that in the vast majority of patients (18 of 20), abatacept therapy prevented further progression of disease, but on cessation, additional therapy was indicated to maintain this,” Dr. Buch and her associates said.
There were no serious adverse events during treatment, including no infections and no abnormal liver function tests. A total of 131 nonserious adverse events were reported.
This study was supported by a research grant from Bristol-Myers Squibb and by institution-level grants from Arthritis UK and the U.K. National Institute for Health Research. Dr. Buch reported ties to Bristol-Myers Squibb, AbbVie, AstraZeneca, Pfizer, and Roche-Chugai; her associates reported ties to numerous industry sources.
Key clinical point:
Major finding: Only 2 of the 20 patients (10%) achieved the composite primary endpoint of DAS44 remission, a maximum of 1 swollen joint for at least 3 consecutive months, and no radiographic progression at 6-month follow-up.
Data source: A manufacturer-supported, open-label, proof-of-concept study involving 20 adults treated for 1 year and followed for 1 further year.
Disclosures: This study was supported by a research grant from Bristol-Myers Squibb and by institution-level grants from Arthritis UK and the U.K. National Institute for Health Research. Dr. Buch reported ties to Bristol-Myers Squibb, AbbVie, AstraZeneca, Pfizer, and Roche-Chugai; her associates reported ties to numerous industry sources.