User login
Novel antibiotic hits skin and soft tissue infections with one-two punch
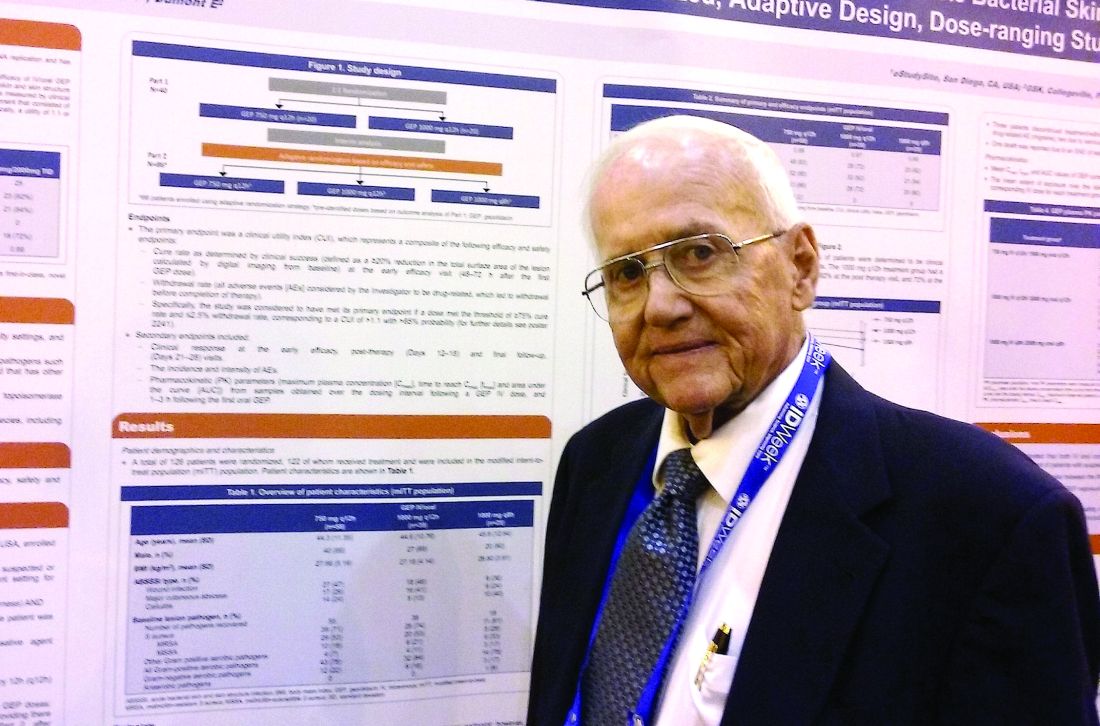
NEW ORLEANS – A novel antibiotic in development fared well in terms of efficacy and safety for patients hospitalized for suspected or confirmed Gram-positive acute skin and soft tissue infections, reveals the first reported findings of a phase II, randomized study.
Investigators randomized 122 patients over 18 years of age with wound infections, major cutaneous abscesses, or cellulitis to three different dosing intravenous/oral regimens of gepotidacin (GlaxoSmithKline). Patients in the 750-mg/1,500-mg q12h and 1,000-mg/2,000-mg q8h groups met the primary efficacy endpoint of an 80% or greater clinical success (83% and 92%, respectively) within 2-3 days. A third group, randomized to 1,000-mg/2,000-mg q12h, had a 72% early success rate.
All three groups of patients achieved the primary safety outcome, defined as less than a 2.5% withdrawal rate due to drug-related adverse events during gepotidacin treatment. One patient in the 750-mg q12h group withdrew because of a migraine related to the study drug.
Gepotidacin cleaves bacterial DNA in two places to block replication. “Because of its dual mechanism, there are a lot of potential applications,” Dr. O’Riordan said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Gepotidacin is also being assessed in ongoing gonorrhea, complicated intra-abdominal infections, and urinary tract infection studies.
The researchers in the current study also measured clinical success at post therapy days 12-18. They found 90% of the 750-mg/1,500-mg q12h group, 82% of the 1,000-mg/2,000-mg q8h, and 84% of the 1,000-mg/2,000-mg q12h group achieved the composite efficacy endpoint.
Overall, 84 or 69% of study participants experienced an adverse event. Nausea, diarrhea, and vomiting were the most common mild-to-moderate adverse events associated with the 10 days of gepotidacin treatment. Two serious adverse events not related to treatment also occurred during the study.
The “low adverse events and reproducible resolution of skin infections” in this phase II study support further development of gepotidacin, Dr. O’Riordan said.
Dr. O’Riordan had no relevant disclosures. Some study coauthors are GlaxoSmithKline employees.
NEW ORLEANS – A novel antibiotic in development fared well in terms of efficacy and safety for patients hospitalized for suspected or confirmed Gram-positive acute skin and soft tissue infections, reveals the first reported findings of a phase II, randomized study.
Investigators randomized 122 patients over 18 years of age with wound infections, major cutaneous abscesses, or cellulitis to three different dosing intravenous/oral regimens of gepotidacin (GlaxoSmithKline). Patients in the 750-mg/1,500-mg q12h and 1,000-mg/2,000-mg q8h groups met the primary efficacy endpoint of an 80% or greater clinical success (83% and 92%, respectively) within 2-3 days. A third group, randomized to 1,000-mg/2,000-mg q12h, had a 72% early success rate.
All three groups of patients achieved the primary safety outcome, defined as less than a 2.5% withdrawal rate due to drug-related adverse events during gepotidacin treatment. One patient in the 750-mg q12h group withdrew because of a migraine related to the study drug.
Gepotidacin cleaves bacterial DNA in two places to block replication. “Because of its dual mechanism, there are a lot of potential applications,” Dr. O’Riordan said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Gepotidacin is also being assessed in ongoing gonorrhea, complicated intra-abdominal infections, and urinary tract infection studies.
The researchers in the current study also measured clinical success at post therapy days 12-18. They found 90% of the 750-mg/1,500-mg q12h group, 82% of the 1,000-mg/2,000-mg q8h, and 84% of the 1,000-mg/2,000-mg q12h group achieved the composite efficacy endpoint.
Overall, 84 or 69% of study participants experienced an adverse event. Nausea, diarrhea, and vomiting were the most common mild-to-moderate adverse events associated with the 10 days of gepotidacin treatment. Two serious adverse events not related to treatment also occurred during the study.
The “low adverse events and reproducible resolution of skin infections” in this phase II study support further development of gepotidacin, Dr. O’Riordan said.
Dr. O’Riordan had no relevant disclosures. Some study coauthors are GlaxoSmithKline employees.
NEW ORLEANS – A novel antibiotic in development fared well in terms of efficacy and safety for patients hospitalized for suspected or confirmed Gram-positive acute skin and soft tissue infections, reveals the first reported findings of a phase II, randomized study.
Investigators randomized 122 patients over 18 years of age with wound infections, major cutaneous abscesses, or cellulitis to three different dosing intravenous/oral regimens of gepotidacin (GlaxoSmithKline). Patients in the 750-mg/1,500-mg q12h and 1,000-mg/2,000-mg q8h groups met the primary efficacy endpoint of an 80% or greater clinical success (83% and 92%, respectively) within 2-3 days. A third group, randomized to 1,000-mg/2,000-mg q12h, had a 72% early success rate.
All three groups of patients achieved the primary safety outcome, defined as less than a 2.5% withdrawal rate due to drug-related adverse events during gepotidacin treatment. One patient in the 750-mg q12h group withdrew because of a migraine related to the study drug.
Gepotidacin cleaves bacterial DNA in two places to block replication. “Because of its dual mechanism, there are a lot of potential applications,” Dr. O’Riordan said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Gepotidacin is also being assessed in ongoing gonorrhea, complicated intra-abdominal infections, and urinary tract infection studies.
The researchers in the current study also measured clinical success at post therapy days 12-18. They found 90% of the 750-mg/1,500-mg q12h group, 82% of the 1,000-mg/2,000-mg q8h, and 84% of the 1,000-mg/2,000-mg q12h group achieved the composite efficacy endpoint.
Overall, 84 or 69% of study participants experienced an adverse event. Nausea, diarrhea, and vomiting were the most common mild-to-moderate adverse events associated with the 10 days of gepotidacin treatment. Two serious adverse events not related to treatment also occurred during the study.
The “low adverse events and reproducible resolution of skin infections” in this phase II study support further development of gepotidacin, Dr. O’Riordan said.
Dr. O’Riordan had no relevant disclosures. Some study coauthors are GlaxoSmithKline employees.
AT IDWEEK 2016
Key clinical point: A dual-mechanism-of-action antibiotic in development shows good efficacy and a low adverse event rate in a phase II study.
Major finding: A total 71 of 122 adult patients achieved clinical success within 48 to 72 hours with gepotidacin treatment.
Data source: 122 patients over 18 years of age with wound infections, major cutaneous abscesses, or cellulitis.
Disclosures: Dr. O’Riordan had no relevant disclosures. Some study coauthors are GlaxoSmithKline employees.
Investigational AML drugs boosted remission rates
Two investigational drugs appear to be improving outcomes for patients with acute myeloid leukemia (AML), based on results reported in separate abstracts of studies that will be featured at press conferences to be held during the annual meeting of the American Society of Hematology.
In the first study, induction therapy with the investigational drug CPX-351 (Vyxeos), a liposomal formulation of cytarabine and daunorubicin, allowed a higher proportion of patients over age 60 with secondary AML to qualify for allogeneic hematopoietic cell transplants. Those patients went on to have improved survival, compared with patients who received standard 7+3 cytarabine and daunorubicin, Jeffrey E. Lancet, MD, of the H. Lee Moffitt Cancer Center and Research Institute, Tampa, and his colleagues reported in abstract 906.
The finding that CPX-351 may be an effective bridge to successful transplant for older patients with newly diagnosed secondary AML comes from an exploratory analysis of a phase III study comparing induction therapy with CPX-351 and standard cytarabine and daunorubicin. Initial data from the randomized open-label study, reported last June at the annual meeting of the American Society of Clinical Oncology, indicated CPX-351 significantly improved overall survival, event-free survival, and treatment response without an increase in 60-day mortality or in the frequency and severity of adverse events, compared with the standard 7+3 regimen of cytarabine and daunorubicin.
The data to be presented at ASH 2016 will examine the outcomes of 52 patients in the CPX-351 arm and 39 patients in the standard cytarabine and daunorubicin arm who underwent allogeneic hematopoietic cell transplantation (HCT) after induction. Data reported in the abstract indicate that 18 of the 52 patients in the CPX-351 arm and 26 of the 39 patients in the standard cytarabine and daunorubicin arm have died. The median survival time was 10.25 months with standard therapy; median survival has not yet been reached in the CPX-351 arm. The results indicate 53% fewer deaths occurred within 100 days of transplant in the CPX-351 group.
Newly diagnosed secondary AML was defined as having a history of prior cytotoxic treatment, antecedent myelodysplastic syndrome (MDS) with or without prior treatment with hypomethylating agents, or AML with World Health Organization–defined MDS-related cytogenetic abnormalities.
For the trial, conducted over 2 years at 39 U.S. and Canadian sites, 153 patients were randomized to the CPX-351 arm and 156 randomized to the standard therapy arm. Of 125 patients who had a complete response (CR) or a CR with incomplete (CRi) platelet or neutrophil recovery, 91 underwent allogeneic HCT: 52 (34%) from the CPX-351 arm and 39 (25%) from the standard therapy arm. Each arm had a similar percentage of patients who underwent transplant in CR/CRi status; however, the CPX-351 arm contained a higher percentage of patients age 70 and older (31% vs. 15%). Mortality at 100 days after transplant was 9.6% for patients in the CPX-351 arm and 20.5% for patients in the standard therapy arm. Deaths that occurred within 100 days after allogeneic HCT were due to refractory AML (CPX-351, 3.8%; standard therapy, 7.7%); graft vs. host disease (CPX-351, 3.8%; standard therapy, 2.6%); or renal, respiratory, or multiorgan failure, or septic shock (CPX-351, 0 for each; standard therapy, 2.6% for each), or the cause of death was unknown (CPX-351, 1.9%; standard therapy, 0).
For the 91 patients who had transplants, those in the CPX-351 arm had markedly better overall survival (hazard ratio, 0.46; P = .0046). The time-dependent Cox hazard ratio for overall survival in the CPX-351 arm vs. the 7+3 arm was 0.51 (95% confidence interval, 0.35–0.75; P = .0007).
In the phase Ib trial of vadastuximab talirine, 42 patients received the drug on days 1 and 4 of standard 7+3 cytarabine and daunorubicin induction therapy. Most patients had intermediate (40%) or adverse (43%) cytogenetic risk by Medical Research Council criteria, and 17% of patients had secondary AML. Response was assessed on days 15 and 28; MRD was assessed centrally by bone marrow examination using a multiparametric flow cytometric assay. The investigator chose whether to do a second induction regimen and any postremission therapies, which did not include additional administration of vadastuximab talirine.
Of the 40 patients who could be evaluated for efficacy, 24 (60%) had a CR and 7 (18%) had a CRi, and 4 (10%) reached a morphologic leukemia-free state. Nearly all (94%) of CR and CRi responses occurred after one cycle of induction therapy, and 23 of the 31 patients who reached CR or CRi achieved MRD-negative status.
Extramedullary adverse events, including hepatic toxicity, and induction mortality rates were similar to reported rates for 7+3 cytarabine and daunorubicin alone. All patients had grade 4 myelosuppression. In patients who achieved CR or CRi, the estimated median time to count recovery from day 1 of therapy was 33 days for neutrophils and 35 days for platelets. The 30- and 60-day mortality rates were 0% and 7%, respectively.
An alternative schedule of single-day dosing on day 1 is under investigation, and enrollment continues.
The CPX-351 (Vyxeos) study was supported by the drug’s maker, Celator Pharmaceuticals, which is a subsidiary of Jazz Pharmaceuticals. Dr. Lancet is a consultant to Celator as well as numerous other drug companies. Several of his colleagues disclosed a wide variety of relationships with drug companies, including Celator. Two of the study investigators disclosed employment by and equity ownership in Celator.
The vadastuximab talirine study was sponsored by the drug’s maker, Seattle Genetics. Dr. Erba disclosed a wide variety of relationships with drug companies, including research funding from Seattle Genetics. His colleagues had a similar wide variety of relationships, and two disclosed employment by and equity ownership in Seattle Genetics.
Abstract 906 Survival Following Allogeneic Hematopoietic Cell Transplantation in Older High-Risk Acute Myeloid Leukemia Patients Initially Treated With CPX-351 Liposome Injection Versus Standard Cytarabine and Daunorubicin: Subgroup Analysis of a Large Phase III Trial, will be presented in session 616 at 4:00 p.m. on Monday, Dec. 5.
Abstract 211 A Phase Ib Study of Vadastuximab Talirine in Combination With 7+3 Induction Therapy for Patients With Newly Diagnosed Acute Myeloid Leukemia (AML) will be presented in session 613 at 4:00 p.m. on Saturday, Dec. 3.
[email protected]
On Twitter @maryjodales
Two investigational drugs appear to be improving outcomes for patients with acute myeloid leukemia (AML), based on results reported in separate abstracts of studies that will be featured at press conferences to be held during the annual meeting of the American Society of Hematology.
In the first study, induction therapy with the investigational drug CPX-351 (Vyxeos), a liposomal formulation of cytarabine and daunorubicin, allowed a higher proportion of patients over age 60 with secondary AML to qualify for allogeneic hematopoietic cell transplants. Those patients went on to have improved survival, compared with patients who received standard 7+3 cytarabine and daunorubicin, Jeffrey E. Lancet, MD, of the H. Lee Moffitt Cancer Center and Research Institute, Tampa, and his colleagues reported in abstract 906.
The finding that CPX-351 may be an effective bridge to successful transplant for older patients with newly diagnosed secondary AML comes from an exploratory analysis of a phase III study comparing induction therapy with CPX-351 and standard cytarabine and daunorubicin. Initial data from the randomized open-label study, reported last June at the annual meeting of the American Society of Clinical Oncology, indicated CPX-351 significantly improved overall survival, event-free survival, and treatment response without an increase in 60-day mortality or in the frequency and severity of adverse events, compared with the standard 7+3 regimen of cytarabine and daunorubicin.
The data to be presented at ASH 2016 will examine the outcomes of 52 patients in the CPX-351 arm and 39 patients in the standard cytarabine and daunorubicin arm who underwent allogeneic hematopoietic cell transplantation (HCT) after induction. Data reported in the abstract indicate that 18 of the 52 patients in the CPX-351 arm and 26 of the 39 patients in the standard cytarabine and daunorubicin arm have died. The median survival time was 10.25 months with standard therapy; median survival has not yet been reached in the CPX-351 arm. The results indicate 53% fewer deaths occurred within 100 days of transplant in the CPX-351 group.
Newly diagnosed secondary AML was defined as having a history of prior cytotoxic treatment, antecedent myelodysplastic syndrome (MDS) with or without prior treatment with hypomethylating agents, or AML with World Health Organization–defined MDS-related cytogenetic abnormalities.
For the trial, conducted over 2 years at 39 U.S. and Canadian sites, 153 patients were randomized to the CPX-351 arm and 156 randomized to the standard therapy arm. Of 125 patients who had a complete response (CR) or a CR with incomplete (CRi) platelet or neutrophil recovery, 91 underwent allogeneic HCT: 52 (34%) from the CPX-351 arm and 39 (25%) from the standard therapy arm. Each arm had a similar percentage of patients who underwent transplant in CR/CRi status; however, the CPX-351 arm contained a higher percentage of patients age 70 and older (31% vs. 15%). Mortality at 100 days after transplant was 9.6% for patients in the CPX-351 arm and 20.5% for patients in the standard therapy arm. Deaths that occurred within 100 days after allogeneic HCT were due to refractory AML (CPX-351, 3.8%; standard therapy, 7.7%); graft vs. host disease (CPX-351, 3.8%; standard therapy, 2.6%); or renal, respiratory, or multiorgan failure, or septic shock (CPX-351, 0 for each; standard therapy, 2.6% for each), or the cause of death was unknown (CPX-351, 1.9%; standard therapy, 0).
For the 91 patients who had transplants, those in the CPX-351 arm had markedly better overall survival (hazard ratio, 0.46; P = .0046). The time-dependent Cox hazard ratio for overall survival in the CPX-351 arm vs. the 7+3 arm was 0.51 (95% confidence interval, 0.35–0.75; P = .0007).
In the phase Ib trial of vadastuximab talirine, 42 patients received the drug on days 1 and 4 of standard 7+3 cytarabine and daunorubicin induction therapy. Most patients had intermediate (40%) or adverse (43%) cytogenetic risk by Medical Research Council criteria, and 17% of patients had secondary AML. Response was assessed on days 15 and 28; MRD was assessed centrally by bone marrow examination using a multiparametric flow cytometric assay. The investigator chose whether to do a second induction regimen and any postremission therapies, which did not include additional administration of vadastuximab talirine.
Of the 40 patients who could be evaluated for efficacy, 24 (60%) had a CR and 7 (18%) had a CRi, and 4 (10%) reached a morphologic leukemia-free state. Nearly all (94%) of CR and CRi responses occurred after one cycle of induction therapy, and 23 of the 31 patients who reached CR or CRi achieved MRD-negative status.
Extramedullary adverse events, including hepatic toxicity, and induction mortality rates were similar to reported rates for 7+3 cytarabine and daunorubicin alone. All patients had grade 4 myelosuppression. In patients who achieved CR or CRi, the estimated median time to count recovery from day 1 of therapy was 33 days for neutrophils and 35 days for platelets. The 30- and 60-day mortality rates were 0% and 7%, respectively.
An alternative schedule of single-day dosing on day 1 is under investigation, and enrollment continues.
The CPX-351 (Vyxeos) study was supported by the drug’s maker, Celator Pharmaceuticals, which is a subsidiary of Jazz Pharmaceuticals. Dr. Lancet is a consultant to Celator as well as numerous other drug companies. Several of his colleagues disclosed a wide variety of relationships with drug companies, including Celator. Two of the study investigators disclosed employment by and equity ownership in Celator.
The vadastuximab talirine study was sponsored by the drug’s maker, Seattle Genetics. Dr. Erba disclosed a wide variety of relationships with drug companies, including research funding from Seattle Genetics. His colleagues had a similar wide variety of relationships, and two disclosed employment by and equity ownership in Seattle Genetics.
Abstract 906 Survival Following Allogeneic Hematopoietic Cell Transplantation in Older High-Risk Acute Myeloid Leukemia Patients Initially Treated With CPX-351 Liposome Injection Versus Standard Cytarabine and Daunorubicin: Subgroup Analysis of a Large Phase III Trial, will be presented in session 616 at 4:00 p.m. on Monday, Dec. 5.
Abstract 211 A Phase Ib Study of Vadastuximab Talirine in Combination With 7+3 Induction Therapy for Patients With Newly Diagnosed Acute Myeloid Leukemia (AML) will be presented in session 613 at 4:00 p.m. on Saturday, Dec. 3.
[email protected]
On Twitter @maryjodales
Two investigational drugs appear to be improving outcomes for patients with acute myeloid leukemia (AML), based on results reported in separate abstracts of studies that will be featured at press conferences to be held during the annual meeting of the American Society of Hematology.
In the first study, induction therapy with the investigational drug CPX-351 (Vyxeos), a liposomal formulation of cytarabine and daunorubicin, allowed a higher proportion of patients over age 60 with secondary AML to qualify for allogeneic hematopoietic cell transplants. Those patients went on to have improved survival, compared with patients who received standard 7+3 cytarabine and daunorubicin, Jeffrey E. Lancet, MD, of the H. Lee Moffitt Cancer Center and Research Institute, Tampa, and his colleagues reported in abstract 906.
The finding that CPX-351 may be an effective bridge to successful transplant for older patients with newly diagnosed secondary AML comes from an exploratory analysis of a phase III study comparing induction therapy with CPX-351 and standard cytarabine and daunorubicin. Initial data from the randomized open-label study, reported last June at the annual meeting of the American Society of Clinical Oncology, indicated CPX-351 significantly improved overall survival, event-free survival, and treatment response without an increase in 60-day mortality or in the frequency and severity of adverse events, compared with the standard 7+3 regimen of cytarabine and daunorubicin.
The data to be presented at ASH 2016 will examine the outcomes of 52 patients in the CPX-351 arm and 39 patients in the standard cytarabine and daunorubicin arm who underwent allogeneic hematopoietic cell transplantation (HCT) after induction. Data reported in the abstract indicate that 18 of the 52 patients in the CPX-351 arm and 26 of the 39 patients in the standard cytarabine and daunorubicin arm have died. The median survival time was 10.25 months with standard therapy; median survival has not yet been reached in the CPX-351 arm. The results indicate 53% fewer deaths occurred within 100 days of transplant in the CPX-351 group.
Newly diagnosed secondary AML was defined as having a history of prior cytotoxic treatment, antecedent myelodysplastic syndrome (MDS) with or without prior treatment with hypomethylating agents, or AML with World Health Organization–defined MDS-related cytogenetic abnormalities.
For the trial, conducted over 2 years at 39 U.S. and Canadian sites, 153 patients were randomized to the CPX-351 arm and 156 randomized to the standard therapy arm. Of 125 patients who had a complete response (CR) or a CR with incomplete (CRi) platelet or neutrophil recovery, 91 underwent allogeneic HCT: 52 (34%) from the CPX-351 arm and 39 (25%) from the standard therapy arm. Each arm had a similar percentage of patients who underwent transplant in CR/CRi status; however, the CPX-351 arm contained a higher percentage of patients age 70 and older (31% vs. 15%). Mortality at 100 days after transplant was 9.6% for patients in the CPX-351 arm and 20.5% for patients in the standard therapy arm. Deaths that occurred within 100 days after allogeneic HCT were due to refractory AML (CPX-351, 3.8%; standard therapy, 7.7%); graft vs. host disease (CPX-351, 3.8%; standard therapy, 2.6%); or renal, respiratory, or multiorgan failure, or septic shock (CPX-351, 0 for each; standard therapy, 2.6% for each), or the cause of death was unknown (CPX-351, 1.9%; standard therapy, 0).
For the 91 patients who had transplants, those in the CPX-351 arm had markedly better overall survival (hazard ratio, 0.46; P = .0046). The time-dependent Cox hazard ratio for overall survival in the CPX-351 arm vs. the 7+3 arm was 0.51 (95% confidence interval, 0.35–0.75; P = .0007).
In the phase Ib trial of vadastuximab talirine, 42 patients received the drug on days 1 and 4 of standard 7+3 cytarabine and daunorubicin induction therapy. Most patients had intermediate (40%) or adverse (43%) cytogenetic risk by Medical Research Council criteria, and 17% of patients had secondary AML. Response was assessed on days 15 and 28; MRD was assessed centrally by bone marrow examination using a multiparametric flow cytometric assay. The investigator chose whether to do a second induction regimen and any postremission therapies, which did not include additional administration of vadastuximab talirine.
Of the 40 patients who could be evaluated for efficacy, 24 (60%) had a CR and 7 (18%) had a CRi, and 4 (10%) reached a morphologic leukemia-free state. Nearly all (94%) of CR and CRi responses occurred after one cycle of induction therapy, and 23 of the 31 patients who reached CR or CRi achieved MRD-negative status.
Extramedullary adverse events, including hepatic toxicity, and induction mortality rates were similar to reported rates for 7+3 cytarabine and daunorubicin alone. All patients had grade 4 myelosuppression. In patients who achieved CR or CRi, the estimated median time to count recovery from day 1 of therapy was 33 days for neutrophils and 35 days for platelets. The 30- and 60-day mortality rates were 0% and 7%, respectively.
An alternative schedule of single-day dosing on day 1 is under investigation, and enrollment continues.
The CPX-351 (Vyxeos) study was supported by the drug’s maker, Celator Pharmaceuticals, which is a subsidiary of Jazz Pharmaceuticals. Dr. Lancet is a consultant to Celator as well as numerous other drug companies. Several of his colleagues disclosed a wide variety of relationships with drug companies, including Celator. Two of the study investigators disclosed employment by and equity ownership in Celator.
The vadastuximab talirine study was sponsored by the drug’s maker, Seattle Genetics. Dr. Erba disclosed a wide variety of relationships with drug companies, including research funding from Seattle Genetics. His colleagues had a similar wide variety of relationships, and two disclosed employment by and equity ownership in Seattle Genetics.
Abstract 906 Survival Following Allogeneic Hematopoietic Cell Transplantation in Older High-Risk Acute Myeloid Leukemia Patients Initially Treated With CPX-351 Liposome Injection Versus Standard Cytarabine and Daunorubicin: Subgroup Analysis of a Large Phase III Trial, will be presented in session 616 at 4:00 p.m. on Monday, Dec. 5.
Abstract 211 A Phase Ib Study of Vadastuximab Talirine in Combination With 7+3 Induction Therapy for Patients With Newly Diagnosed Acute Myeloid Leukemia (AML) will be presented in session 613 at 4:00 p.m. on Saturday, Dec. 3.
[email protected]
On Twitter @maryjodales
FROM ASH 2016
What will the Trump administration mean for medicine?
The Affordable Care Act is in the crosshairs as the transition to the Trump administration begins Nov. 9.
The primary tenet of Donald J. Trump’s health care platform calls for Congress to repeal the ACA.
In fact, Mr. Trump has called for ACA repeal efforts to begin on his administration’s first day.
The Trump administration is likely to find plentiful allies in Congress as both the House and the Senate were projected at press time to have Republican majorities, albeit slim ones. Since the ACA’s passage in 2010, House Republicans have put forward repeal legislation scores of times.
While many medical specialty societies have supported the ACA and other major health care reforms enacted over the last 8 years – Meaningful Use from the HITECH ACT and value-based payment from MACRA among them – large numbers of physicians have chafed under the myriad reporting requirements and administrative hassles.
A recent survey commissioned by the Physicians Foundation and conducted by Merritt Hawkins found that nearly half (48%) of physicians are considering a change of practice – including leaving medicine – in the next 1-3 years. Reasons cited by survey respondents included the MACRA (Medicare Access and CHIP Reauthorization Act of 2015) transition to value-based care, the increased coding required by ICD-10, the growth of physician employment, the continued sale of private practices to hospitals and health systems, the increased number of patients in the system because of the ACA coupled with a shortage of physicians, and the “businessification” of heath care.
“If any of these [changes] occurred in a period of time, it would be impactful,” Walker Ray, MD, president of the Physicians Foundation, said in an interview. “But to have all occur simultaneously, we say now that to be a physician is to feel the ground shaking under your feet. This is the landscape in which the survey was taken.”
Mr. Trump supports several free market reforms to replace repealed provisions of the ACA, as well as address other issues in the health care system. The proposals include the following:
• Foster interstate insurance sales.
• Reinstate the tax deductibility of health insurance premiums.
• Promote the more widespread use of health savings accounts.
• Require price transparency so that patients can shop for medical procedures, exams, and tests.
• Block grant Medicaid to the states.
• Allow patients to import drugs from outside of the United States.
The Trump platform also promises to reduce fraud and waste, as well as save approximately $11 billion annually by not providing health care to illegal immigrants.
Speculation has also begun regarding who might lead health care agencies and policy for the Trump administration. Among the names that have been floated for secretary of Health and Human Services are Ben Carson, MD, the former presidential candidate and retired neurosurgeon; former House Speaker Newt Gingrich (who also has been suggested as a potential secretary of State); as well as Florida Gov. Rick Scott, former chief executive of Columbia/HCA, according to Politico.com.
[email protected]
On Twitter @denisefulton
Gregory Twachtman contributed to this story.
The Affordable Care Act is in the crosshairs as the transition to the Trump administration begins Nov. 9.
The primary tenet of Donald J. Trump’s health care platform calls for Congress to repeal the ACA.
In fact, Mr. Trump has called for ACA repeal efforts to begin on his administration’s first day.
The Trump administration is likely to find plentiful allies in Congress as both the House and the Senate were projected at press time to have Republican majorities, albeit slim ones. Since the ACA’s passage in 2010, House Republicans have put forward repeal legislation scores of times.
While many medical specialty societies have supported the ACA and other major health care reforms enacted over the last 8 years – Meaningful Use from the HITECH ACT and value-based payment from MACRA among them – large numbers of physicians have chafed under the myriad reporting requirements and administrative hassles.
A recent survey commissioned by the Physicians Foundation and conducted by Merritt Hawkins found that nearly half (48%) of physicians are considering a change of practice – including leaving medicine – in the next 1-3 years. Reasons cited by survey respondents included the MACRA (Medicare Access and CHIP Reauthorization Act of 2015) transition to value-based care, the increased coding required by ICD-10, the growth of physician employment, the continued sale of private practices to hospitals and health systems, the increased number of patients in the system because of the ACA coupled with a shortage of physicians, and the “businessification” of heath care.
“If any of these [changes] occurred in a period of time, it would be impactful,” Walker Ray, MD, president of the Physicians Foundation, said in an interview. “But to have all occur simultaneously, we say now that to be a physician is to feel the ground shaking under your feet. This is the landscape in which the survey was taken.”
Mr. Trump supports several free market reforms to replace repealed provisions of the ACA, as well as address other issues in the health care system. The proposals include the following:
• Foster interstate insurance sales.
• Reinstate the tax deductibility of health insurance premiums.
• Promote the more widespread use of health savings accounts.
• Require price transparency so that patients can shop for medical procedures, exams, and tests.
• Block grant Medicaid to the states.
• Allow patients to import drugs from outside of the United States.
The Trump platform also promises to reduce fraud and waste, as well as save approximately $11 billion annually by not providing health care to illegal immigrants.
Speculation has also begun regarding who might lead health care agencies and policy for the Trump administration. Among the names that have been floated for secretary of Health and Human Services are Ben Carson, MD, the former presidential candidate and retired neurosurgeon; former House Speaker Newt Gingrich (who also has been suggested as a potential secretary of State); as well as Florida Gov. Rick Scott, former chief executive of Columbia/HCA, according to Politico.com.
[email protected]
On Twitter @denisefulton
Gregory Twachtman contributed to this story.
The Affordable Care Act is in the crosshairs as the transition to the Trump administration begins Nov. 9.
The primary tenet of Donald J. Trump’s health care platform calls for Congress to repeal the ACA.
In fact, Mr. Trump has called for ACA repeal efforts to begin on his administration’s first day.
The Trump administration is likely to find plentiful allies in Congress as both the House and the Senate were projected at press time to have Republican majorities, albeit slim ones. Since the ACA’s passage in 2010, House Republicans have put forward repeal legislation scores of times.
While many medical specialty societies have supported the ACA and other major health care reforms enacted over the last 8 years – Meaningful Use from the HITECH ACT and value-based payment from MACRA among them – large numbers of physicians have chafed under the myriad reporting requirements and administrative hassles.
A recent survey commissioned by the Physicians Foundation and conducted by Merritt Hawkins found that nearly half (48%) of physicians are considering a change of practice – including leaving medicine – in the next 1-3 years. Reasons cited by survey respondents included the MACRA (Medicare Access and CHIP Reauthorization Act of 2015) transition to value-based care, the increased coding required by ICD-10, the growth of physician employment, the continued sale of private practices to hospitals and health systems, the increased number of patients in the system because of the ACA coupled with a shortage of physicians, and the “businessification” of heath care.
“If any of these [changes] occurred in a period of time, it would be impactful,” Walker Ray, MD, president of the Physicians Foundation, said in an interview. “But to have all occur simultaneously, we say now that to be a physician is to feel the ground shaking under your feet. This is the landscape in which the survey was taken.”
Mr. Trump supports several free market reforms to replace repealed provisions of the ACA, as well as address other issues in the health care system. The proposals include the following:
• Foster interstate insurance sales.
• Reinstate the tax deductibility of health insurance premiums.
• Promote the more widespread use of health savings accounts.
• Require price transparency so that patients can shop for medical procedures, exams, and tests.
• Block grant Medicaid to the states.
• Allow patients to import drugs from outside of the United States.
The Trump platform also promises to reduce fraud and waste, as well as save approximately $11 billion annually by not providing health care to illegal immigrants.
Speculation has also begun regarding who might lead health care agencies and policy for the Trump administration. Among the names that have been floated for secretary of Health and Human Services are Ben Carson, MD, the former presidential candidate and retired neurosurgeon; former House Speaker Newt Gingrich (who also has been suggested as a potential secretary of State); as well as Florida Gov. Rick Scott, former chief executive of Columbia/HCA, according to Politico.com.
[email protected]
On Twitter @denisefulton
Gregory Twachtman contributed to this story.
Everything You Need to Know About the Bundled Payments for Care Improvement Initiative
As far back as 1983 —13 years before the birth of HM—Medicare created what was then called an “inpatient prospective payment system,” which essentially let Medicare pay a fixed amount for the entirety of a hospital stay, based on diagnosis. Then in 1991, the Centers for Medicare & Medicaid Services (CMS) introduced one payment for coronary artery bypass graft surgery, and even included 90-day readmission in the check.
Fast forward to the past 10 years when accountable care organizations (ACOs) and value-based purchasing (VBP) have been the focus of HM executives looking to take the lead in how to make bundled payments work for them.
The Bundled Payments for Care Improvement (BPCI) initiative was introduced by CMS’s Center for Medicare & Medicaid Innovation (CMMI) in 2011 and is now compiling its first data sets for the next frontier of payments for episodic care.
For rank-and-file hospitalists who have felt inundated by the regulations and promised payment reforms from ACOs and VBPs, why is this program so important?
“The reason this is so special is that it is one of the few CMS programs that allows providers to be in the driver’s seat,” says Kerry Weiner, MD, chief medical officer of acute and post-acute services at TeamHealth-IPC The Hospitalist Company. “They have the opportunity to be accountable and to actually be the designers of reengineering care. The other programs that you just mentioned, like value-based purchasing, largely originate from health systems or the federal government and dictate the principles and the metrics that as a provider you’re going to be evaluated upon.
“This model, the bundled model, gives us the flexibility, scale and brackets of risk that we want to accept and thereby gives us a lot more control over what physicians and physician groups can manage successfully.”
BPCI might be a game-changer for HM because it’s the first of the bundled-payment initiatives that truly falls direct to the care provided by hospitalists. In short, the plan covers 48 defined episodes of care and would parse out payments for those episodes in a holistic—and some say more appropriate—way. Currently, a hospitalist would get paid for a patient’s stay in the hospital and a primary-care physician (PCP) could be paid for some follow-up. If the patient ends up back in the hospital quickly, the hospitalist could get paid again and, upon discharge, a PCP could, too.
But under BPCI, pay would be determined based on the episode of care. The details of who gets paid what and the rules that apply are all likely to evolve, of course, but it’s hoped the basic premise of bundled payments would lower the overall cost of healthcare.
How It Works
Under the Patient Protection and Affordable Care Act (ACA) of 2009, it was mandated that the government establish a five-year pilot program by 2013 that bundled payments for inpatient care, according to the American Hospital Association.
The program has now ramped up to include more than 650 participating organization, not including thousands of physicians that then partner with those groups, over four models. The initiative covers defined episodes of care, both medical and surgical, that begin at the time of inpatient admission and stretch 30, 60 or 90 days post-discharge.
And hospitalists are poised to take the lead on how payment models, especially bundled payments, are shaped over the next few years, says John Nelson, MD, MHM, a co-founder and past president of SHM and and principal in Nelson Flores Hospital Medicine Consultants in Bellevue, Wash. Nelson says his consulting firm has seen an uptick in calls over the past two years dealing with alternative payment models (APMs).
“Hospitalists find themselves at a vitally important nexus of performance and success on new payment models,” he adds.
Win Whitcomb, MD, MHM, chief medical officer of Remedy Partners in Darien, Conn., agrees that BPCI and future iterations of bundled payment programs “are likely to be a potent driver of an evolving hospitalist specialty.” His hypothesis is that APMs such as BPCI are an important way for Medicare to reach its stated goal of having 50% of its fee-for-service payments running through APMs by the end of 2018. To further entice that process, physicians who document at least 25% of their revenue as coming through APMs will get a 5% bonus.
“The stakes are high now,” says Dr. Whitcomb, a past SHM president whose employer is an Awardee Convener in the BPCI initiative, meaning it administers the program. “Medicare [has] laid out the
course for the next two and a half, three years and beyond… It will be crucial for hospitalists to have a path to participate broadly in APMs..”
Dr. Whitcomb says BPCI is the program that should excite hospitalists most because it is more applicable to them moving forward than ACOs, heralded by many healthcare executives several years ago as the future of payment reform.
“With a focus on ambulatory care, ACOs have not broadly involved hospitalists,” he says. “If you look at the State of Hospital Medicine surveys, you look at how many hospitalists are meaningfully working at a system level on ACOs and committees and so forth to improve the performance of the ACO, and it’s very low.”
In fact, just 13.9% of HM groups serving adults only had formed or were participating in a functioning ACO, according to SHM’s 2014 State of Hospital Medicine report. Another 6% were in the process of forming or participating, the paper reported.
“ACOs have not yet widely worked alongside hospitalist teams to optimize where patients go after hospitalization, which is arguably the most important way to deal with post-acute-care utilization” Dr. Whitcomb adds. “whereas nearly all hospitalists working in bundle payments are focusing on a ‘high-value’ transition out of the hospital.”
Improving Care
While BPCI is focused on payment structure, the program could breed process improvements as well as improve care, says hospitalist Patrick Conway, MD, MHM, MSc, CMS’s chief medical officer and deputy administrator for innovation and quality.
“In addition to assessing the quality of patient outcomes and patient experience, CMS is also monitoring for unintended consequences, including whether there is an increase in the number of specific clinical episodes [such as specific elective surgeries] that would not have been expected in the absence of BPCI,” Dr. Conway says. “CMS can audit and intervene if it detects unintended negative consequences for beneficiaries.”
Dr. Whitcomb says two main ways that hospitalists can use BPCI to calculate value is by having better metrics on post-acute facility utilization and reduced readmission.
Immediate past SHM President Robert Harrington Jr., MD, SFHM, says that BPCI is a major stepping stone to merging quality and payment, along the lines of using Physician Quality Reporting System (PQRS) data in the value-based payment modifier.
“CMS is saying to all of us in the provider world, ‘We want to get out of the business of unit economics, and we want to start paying for episodes of care and providers should be at risk for quality outcomes,” he says. “BPCI, to me, is one of the rungs in the ladder.”
Dr. Harrington, chief medical officer at Reliant Post-Acute Care Solutions in Atlanta, says that the program’s inclusion of acute-care hospitals, skilled nursing facilities (SNFs), physician group practices, long-term care hospitals, inpatient rehabilitation facilities, and home health agencies working together is what differentiates it from past attempts at payment reform.
“Population health is sort of where this is headed,” he adds. “You sit in a CFO seat at a hospital or healthcare system right now, and five years ago, they’d buy an MRI machine and they wanted throughput through that MRI machine and they wanted as many people run through that MRI machine in the fee-for-service world as they could get to go through that machine. Nowadays, you start to look at it from a population health standpoint and the CFO is going to say to you, ‘I don’t want anybody going through that MRI machine unless they have to.’
“So it’s a total reversal of perspective when hospitals either become joined at the hip with the payors or become the payors and they start taking risk on population health and I think BPCI is one way that Medicare has allowed all of us to test the waters and get comfortable with that.”
Getting Involved
Dr. Weiner is aware that some hospitalists are nervous about bundled payments because their reimbursement is, in part based on care provided outside of their control. Take a surgical procedure where a hospitalist managing the post-surgery care is left to deal with any potential mistakes made. Or the process works fine until there is poor management by ambulatory care once the patient is discharged.
“That is the reason this program exists,” he says. “It poses the question, who is going to be accountable for the care outside of the traditional site of care that providers have been practicing in, your traditional boundaries? I would argue that physicians are more or are just as valuable as any other segment of the healthcare system in managing the transitions of care and in managing the gaps in the system.”
Given how HM has moved into post-discharge care via SNFs and other post-acute care facilities in recent years, Dr. Weiner says that while hospitalists can’t actually deliver all of the care in an “episode,” they can shepherd that process.
Hospitalists “have control over where the patient goes after they leave the acute-care facility, for example,” he says. “They write the orders on what level of care is needed, and they should have the intimate knowledge about what’s available in their community to ensure the patient gets the best care possible. As long as they have the accountability and the power to direct care, then they have the ability to negotiate and recommend care that is best for the patient, so they can select the better facilities in the community, the better agencies in the community, the better resources in the community to ensure that there is better care once the patient leaves the hospital.”
Dr. Conway suggests HM practitioners view BPCI as a model based on “quality and value.” He says early participants helped define clinical episodes, length of episode, and risk track, making the program better suited to address the actual needs of hospitalists.
“I would encourage hospital medicine physicians and care teams to view bundled payment models as an opportunity for them and their patients for better care and smarter spending,” he adds. “CMS continues to explore ways to pay for value and not just volume. Many of the organizations that are participating in BPCI have partnered with their physician communities and established gainsharing agreement. …Most importantly, this model focuses on care coordination for patients across episodes of care.
And that’s the key for Dr. Weiner.
Hospitalists who embrace BPCI can shape it as the predominant inpatient funding model for hospitals over the next five or 10 years. HM administrators and practitioners who don’t seize the opportunity to flesh out the program tacitly cede control to people outside the hospital who may not tailor the program nearly as well, he says.
“Those who have accountability in the end, the systems, the people, the entities, the providers that have the ability, the accountability for it will ultimately design it,” Dr. Weiner adds. “I think physicians, especially hospitalists, should be at that table. We should play an active role in designing the system.” TH
Richard Quinn is a freelance writer in New Jersey.
As far back as 1983 —13 years before the birth of HM—Medicare created what was then called an “inpatient prospective payment system,” which essentially let Medicare pay a fixed amount for the entirety of a hospital stay, based on diagnosis. Then in 1991, the Centers for Medicare & Medicaid Services (CMS) introduced one payment for coronary artery bypass graft surgery, and even included 90-day readmission in the check.
Fast forward to the past 10 years when accountable care organizations (ACOs) and value-based purchasing (VBP) have been the focus of HM executives looking to take the lead in how to make bundled payments work for them.
The Bundled Payments for Care Improvement (BPCI) initiative was introduced by CMS’s Center for Medicare & Medicaid Innovation (CMMI) in 2011 and is now compiling its first data sets for the next frontier of payments for episodic care.
For rank-and-file hospitalists who have felt inundated by the regulations and promised payment reforms from ACOs and VBPs, why is this program so important?
“The reason this is so special is that it is one of the few CMS programs that allows providers to be in the driver’s seat,” says Kerry Weiner, MD, chief medical officer of acute and post-acute services at TeamHealth-IPC The Hospitalist Company. “They have the opportunity to be accountable and to actually be the designers of reengineering care. The other programs that you just mentioned, like value-based purchasing, largely originate from health systems or the federal government and dictate the principles and the metrics that as a provider you’re going to be evaluated upon.
“This model, the bundled model, gives us the flexibility, scale and brackets of risk that we want to accept and thereby gives us a lot more control over what physicians and physician groups can manage successfully.”
BPCI might be a game-changer for HM because it’s the first of the bundled-payment initiatives that truly falls direct to the care provided by hospitalists. In short, the plan covers 48 defined episodes of care and would parse out payments for those episodes in a holistic—and some say more appropriate—way. Currently, a hospitalist would get paid for a patient’s stay in the hospital and a primary-care physician (PCP) could be paid for some follow-up. If the patient ends up back in the hospital quickly, the hospitalist could get paid again and, upon discharge, a PCP could, too.
But under BPCI, pay would be determined based on the episode of care. The details of who gets paid what and the rules that apply are all likely to evolve, of course, but it’s hoped the basic premise of bundled payments would lower the overall cost of healthcare.
How It Works
Under the Patient Protection and Affordable Care Act (ACA) of 2009, it was mandated that the government establish a five-year pilot program by 2013 that bundled payments for inpatient care, according to the American Hospital Association.
The program has now ramped up to include more than 650 participating organization, not including thousands of physicians that then partner with those groups, over four models. The initiative covers defined episodes of care, both medical and surgical, that begin at the time of inpatient admission and stretch 30, 60 or 90 days post-discharge.
And hospitalists are poised to take the lead on how payment models, especially bundled payments, are shaped over the next few years, says John Nelson, MD, MHM, a co-founder and past president of SHM and and principal in Nelson Flores Hospital Medicine Consultants in Bellevue, Wash. Nelson says his consulting firm has seen an uptick in calls over the past two years dealing with alternative payment models (APMs).
“Hospitalists find themselves at a vitally important nexus of performance and success on new payment models,” he adds.
Win Whitcomb, MD, MHM, chief medical officer of Remedy Partners in Darien, Conn., agrees that BPCI and future iterations of bundled payment programs “are likely to be a potent driver of an evolving hospitalist specialty.” His hypothesis is that APMs such as BPCI are an important way for Medicare to reach its stated goal of having 50% of its fee-for-service payments running through APMs by the end of 2018. To further entice that process, physicians who document at least 25% of their revenue as coming through APMs will get a 5% bonus.
“The stakes are high now,” says Dr. Whitcomb, a past SHM president whose employer is an Awardee Convener in the BPCI initiative, meaning it administers the program. “Medicare [has] laid out the
course for the next two and a half, three years and beyond… It will be crucial for hospitalists to have a path to participate broadly in APMs..”
Dr. Whitcomb says BPCI is the program that should excite hospitalists most because it is more applicable to them moving forward than ACOs, heralded by many healthcare executives several years ago as the future of payment reform.
“With a focus on ambulatory care, ACOs have not broadly involved hospitalists,” he says. “If you look at the State of Hospital Medicine surveys, you look at how many hospitalists are meaningfully working at a system level on ACOs and committees and so forth to improve the performance of the ACO, and it’s very low.”
In fact, just 13.9% of HM groups serving adults only had formed or were participating in a functioning ACO, according to SHM’s 2014 State of Hospital Medicine report. Another 6% were in the process of forming or participating, the paper reported.
“ACOs have not yet widely worked alongside hospitalist teams to optimize where patients go after hospitalization, which is arguably the most important way to deal with post-acute-care utilization” Dr. Whitcomb adds. “whereas nearly all hospitalists working in bundle payments are focusing on a ‘high-value’ transition out of the hospital.”
Improving Care
While BPCI is focused on payment structure, the program could breed process improvements as well as improve care, says hospitalist Patrick Conway, MD, MHM, MSc, CMS’s chief medical officer and deputy administrator for innovation and quality.
“In addition to assessing the quality of patient outcomes and patient experience, CMS is also monitoring for unintended consequences, including whether there is an increase in the number of specific clinical episodes [such as specific elective surgeries] that would not have been expected in the absence of BPCI,” Dr. Conway says. “CMS can audit and intervene if it detects unintended negative consequences for beneficiaries.”
Dr. Whitcomb says two main ways that hospitalists can use BPCI to calculate value is by having better metrics on post-acute facility utilization and reduced readmission.
Immediate past SHM President Robert Harrington Jr., MD, SFHM, says that BPCI is a major stepping stone to merging quality and payment, along the lines of using Physician Quality Reporting System (PQRS) data in the value-based payment modifier.
“CMS is saying to all of us in the provider world, ‘We want to get out of the business of unit economics, and we want to start paying for episodes of care and providers should be at risk for quality outcomes,” he says. “BPCI, to me, is one of the rungs in the ladder.”
Dr. Harrington, chief medical officer at Reliant Post-Acute Care Solutions in Atlanta, says that the program’s inclusion of acute-care hospitals, skilled nursing facilities (SNFs), physician group practices, long-term care hospitals, inpatient rehabilitation facilities, and home health agencies working together is what differentiates it from past attempts at payment reform.
“Population health is sort of where this is headed,” he adds. “You sit in a CFO seat at a hospital or healthcare system right now, and five years ago, they’d buy an MRI machine and they wanted throughput through that MRI machine and they wanted as many people run through that MRI machine in the fee-for-service world as they could get to go through that machine. Nowadays, you start to look at it from a population health standpoint and the CFO is going to say to you, ‘I don’t want anybody going through that MRI machine unless they have to.’
“So it’s a total reversal of perspective when hospitals either become joined at the hip with the payors or become the payors and they start taking risk on population health and I think BPCI is one way that Medicare has allowed all of us to test the waters and get comfortable with that.”
Getting Involved
Dr. Weiner is aware that some hospitalists are nervous about bundled payments because their reimbursement is, in part based on care provided outside of their control. Take a surgical procedure where a hospitalist managing the post-surgery care is left to deal with any potential mistakes made. Or the process works fine until there is poor management by ambulatory care once the patient is discharged.
“That is the reason this program exists,” he says. “It poses the question, who is going to be accountable for the care outside of the traditional site of care that providers have been practicing in, your traditional boundaries? I would argue that physicians are more or are just as valuable as any other segment of the healthcare system in managing the transitions of care and in managing the gaps in the system.”
Given how HM has moved into post-discharge care via SNFs and other post-acute care facilities in recent years, Dr. Weiner says that while hospitalists can’t actually deliver all of the care in an “episode,” they can shepherd that process.
Hospitalists “have control over where the patient goes after they leave the acute-care facility, for example,” he says. “They write the orders on what level of care is needed, and they should have the intimate knowledge about what’s available in their community to ensure the patient gets the best care possible. As long as they have the accountability and the power to direct care, then they have the ability to negotiate and recommend care that is best for the patient, so they can select the better facilities in the community, the better agencies in the community, the better resources in the community to ensure that there is better care once the patient leaves the hospital.”
Dr. Conway suggests HM practitioners view BPCI as a model based on “quality and value.” He says early participants helped define clinical episodes, length of episode, and risk track, making the program better suited to address the actual needs of hospitalists.
“I would encourage hospital medicine physicians and care teams to view bundled payment models as an opportunity for them and their patients for better care and smarter spending,” he adds. “CMS continues to explore ways to pay for value and not just volume. Many of the organizations that are participating in BPCI have partnered with their physician communities and established gainsharing agreement. …Most importantly, this model focuses on care coordination for patients across episodes of care.
And that’s the key for Dr. Weiner.
Hospitalists who embrace BPCI can shape it as the predominant inpatient funding model for hospitals over the next five or 10 years. HM administrators and practitioners who don’t seize the opportunity to flesh out the program tacitly cede control to people outside the hospital who may not tailor the program nearly as well, he says.
“Those who have accountability in the end, the systems, the people, the entities, the providers that have the ability, the accountability for it will ultimately design it,” Dr. Weiner adds. “I think physicians, especially hospitalists, should be at that table. We should play an active role in designing the system.” TH
Richard Quinn is a freelance writer in New Jersey.
As far back as 1983 —13 years before the birth of HM—Medicare created what was then called an “inpatient prospective payment system,” which essentially let Medicare pay a fixed amount for the entirety of a hospital stay, based on diagnosis. Then in 1991, the Centers for Medicare & Medicaid Services (CMS) introduced one payment for coronary artery bypass graft surgery, and even included 90-day readmission in the check.
Fast forward to the past 10 years when accountable care organizations (ACOs) and value-based purchasing (VBP) have been the focus of HM executives looking to take the lead in how to make bundled payments work for them.
The Bundled Payments for Care Improvement (BPCI) initiative was introduced by CMS’s Center for Medicare & Medicaid Innovation (CMMI) in 2011 and is now compiling its first data sets for the next frontier of payments for episodic care.
For rank-and-file hospitalists who have felt inundated by the regulations and promised payment reforms from ACOs and VBPs, why is this program so important?
“The reason this is so special is that it is one of the few CMS programs that allows providers to be in the driver’s seat,” says Kerry Weiner, MD, chief medical officer of acute and post-acute services at TeamHealth-IPC The Hospitalist Company. “They have the opportunity to be accountable and to actually be the designers of reengineering care. The other programs that you just mentioned, like value-based purchasing, largely originate from health systems or the federal government and dictate the principles and the metrics that as a provider you’re going to be evaluated upon.
“This model, the bundled model, gives us the flexibility, scale and brackets of risk that we want to accept and thereby gives us a lot more control over what physicians and physician groups can manage successfully.”
BPCI might be a game-changer for HM because it’s the first of the bundled-payment initiatives that truly falls direct to the care provided by hospitalists. In short, the plan covers 48 defined episodes of care and would parse out payments for those episodes in a holistic—and some say more appropriate—way. Currently, a hospitalist would get paid for a patient’s stay in the hospital and a primary-care physician (PCP) could be paid for some follow-up. If the patient ends up back in the hospital quickly, the hospitalist could get paid again and, upon discharge, a PCP could, too.
But under BPCI, pay would be determined based on the episode of care. The details of who gets paid what and the rules that apply are all likely to evolve, of course, but it’s hoped the basic premise of bundled payments would lower the overall cost of healthcare.
How It Works
Under the Patient Protection and Affordable Care Act (ACA) of 2009, it was mandated that the government establish a five-year pilot program by 2013 that bundled payments for inpatient care, according to the American Hospital Association.
The program has now ramped up to include more than 650 participating organization, not including thousands of physicians that then partner with those groups, over four models. The initiative covers defined episodes of care, both medical and surgical, that begin at the time of inpatient admission and stretch 30, 60 or 90 days post-discharge.
And hospitalists are poised to take the lead on how payment models, especially bundled payments, are shaped over the next few years, says John Nelson, MD, MHM, a co-founder and past president of SHM and and principal in Nelson Flores Hospital Medicine Consultants in Bellevue, Wash. Nelson says his consulting firm has seen an uptick in calls over the past two years dealing with alternative payment models (APMs).
“Hospitalists find themselves at a vitally important nexus of performance and success on new payment models,” he adds.
Win Whitcomb, MD, MHM, chief medical officer of Remedy Partners in Darien, Conn., agrees that BPCI and future iterations of bundled payment programs “are likely to be a potent driver of an evolving hospitalist specialty.” His hypothesis is that APMs such as BPCI are an important way for Medicare to reach its stated goal of having 50% of its fee-for-service payments running through APMs by the end of 2018. To further entice that process, physicians who document at least 25% of their revenue as coming through APMs will get a 5% bonus.
“The stakes are high now,” says Dr. Whitcomb, a past SHM president whose employer is an Awardee Convener in the BPCI initiative, meaning it administers the program. “Medicare [has] laid out the
course for the next two and a half, three years and beyond… It will be crucial for hospitalists to have a path to participate broadly in APMs..”
Dr. Whitcomb says BPCI is the program that should excite hospitalists most because it is more applicable to them moving forward than ACOs, heralded by many healthcare executives several years ago as the future of payment reform.
“With a focus on ambulatory care, ACOs have not broadly involved hospitalists,” he says. “If you look at the State of Hospital Medicine surveys, you look at how many hospitalists are meaningfully working at a system level on ACOs and committees and so forth to improve the performance of the ACO, and it’s very low.”
In fact, just 13.9% of HM groups serving adults only had formed or were participating in a functioning ACO, according to SHM’s 2014 State of Hospital Medicine report. Another 6% were in the process of forming or participating, the paper reported.
“ACOs have not yet widely worked alongside hospitalist teams to optimize where patients go after hospitalization, which is arguably the most important way to deal with post-acute-care utilization” Dr. Whitcomb adds. “whereas nearly all hospitalists working in bundle payments are focusing on a ‘high-value’ transition out of the hospital.”
Improving Care
While BPCI is focused on payment structure, the program could breed process improvements as well as improve care, says hospitalist Patrick Conway, MD, MHM, MSc, CMS’s chief medical officer and deputy administrator for innovation and quality.
“In addition to assessing the quality of patient outcomes and patient experience, CMS is also monitoring for unintended consequences, including whether there is an increase in the number of specific clinical episodes [such as specific elective surgeries] that would not have been expected in the absence of BPCI,” Dr. Conway says. “CMS can audit and intervene if it detects unintended negative consequences for beneficiaries.”
Dr. Whitcomb says two main ways that hospitalists can use BPCI to calculate value is by having better metrics on post-acute facility utilization and reduced readmission.
Immediate past SHM President Robert Harrington Jr., MD, SFHM, says that BPCI is a major stepping stone to merging quality and payment, along the lines of using Physician Quality Reporting System (PQRS) data in the value-based payment modifier.
“CMS is saying to all of us in the provider world, ‘We want to get out of the business of unit economics, and we want to start paying for episodes of care and providers should be at risk for quality outcomes,” he says. “BPCI, to me, is one of the rungs in the ladder.”
Dr. Harrington, chief medical officer at Reliant Post-Acute Care Solutions in Atlanta, says that the program’s inclusion of acute-care hospitals, skilled nursing facilities (SNFs), physician group practices, long-term care hospitals, inpatient rehabilitation facilities, and home health agencies working together is what differentiates it from past attempts at payment reform.
“Population health is sort of where this is headed,” he adds. “You sit in a CFO seat at a hospital or healthcare system right now, and five years ago, they’d buy an MRI machine and they wanted throughput through that MRI machine and they wanted as many people run through that MRI machine in the fee-for-service world as they could get to go through that machine. Nowadays, you start to look at it from a population health standpoint and the CFO is going to say to you, ‘I don’t want anybody going through that MRI machine unless they have to.’
“So it’s a total reversal of perspective when hospitals either become joined at the hip with the payors or become the payors and they start taking risk on population health and I think BPCI is one way that Medicare has allowed all of us to test the waters and get comfortable with that.”
Getting Involved
Dr. Weiner is aware that some hospitalists are nervous about bundled payments because their reimbursement is, in part based on care provided outside of their control. Take a surgical procedure where a hospitalist managing the post-surgery care is left to deal with any potential mistakes made. Or the process works fine until there is poor management by ambulatory care once the patient is discharged.
“That is the reason this program exists,” he says. “It poses the question, who is going to be accountable for the care outside of the traditional site of care that providers have been practicing in, your traditional boundaries? I would argue that physicians are more or are just as valuable as any other segment of the healthcare system in managing the transitions of care and in managing the gaps in the system.”
Given how HM has moved into post-discharge care via SNFs and other post-acute care facilities in recent years, Dr. Weiner says that while hospitalists can’t actually deliver all of the care in an “episode,” they can shepherd that process.
Hospitalists “have control over where the patient goes after they leave the acute-care facility, for example,” he says. “They write the orders on what level of care is needed, and they should have the intimate knowledge about what’s available in their community to ensure the patient gets the best care possible. As long as they have the accountability and the power to direct care, then they have the ability to negotiate and recommend care that is best for the patient, so they can select the better facilities in the community, the better agencies in the community, the better resources in the community to ensure that there is better care once the patient leaves the hospital.”
Dr. Conway suggests HM practitioners view BPCI as a model based on “quality and value.” He says early participants helped define clinical episodes, length of episode, and risk track, making the program better suited to address the actual needs of hospitalists.
“I would encourage hospital medicine physicians and care teams to view bundled payment models as an opportunity for them and their patients for better care and smarter spending,” he adds. “CMS continues to explore ways to pay for value and not just volume. Many of the organizations that are participating in BPCI have partnered with their physician communities and established gainsharing agreement. …Most importantly, this model focuses on care coordination for patients across episodes of care.
And that’s the key for Dr. Weiner.
Hospitalists who embrace BPCI can shape it as the predominant inpatient funding model for hospitals over the next five or 10 years. HM administrators and practitioners who don’t seize the opportunity to flesh out the program tacitly cede control to people outside the hospital who may not tailor the program nearly as well, he says.
“Those who have accountability in the end, the systems, the people, the entities, the providers that have the ability, the accountability for it will ultimately design it,” Dr. Weiner adds. “I think physicians, especially hospitalists, should be at that table. We should play an active role in designing the system.” TH
Richard Quinn is a freelance writer in New Jersey.
Link between hemolysis and infection explained

plate showing a positive
streptococcus infection
Photo by Bill Branson
Patients who suffer from hemolysis have an increased risk of developing bacterial infections, and new research provides an explanation for this phenomenon.
The study refutes the idea that excess circulating iron is to blame.
Instead, it suggests that heme prevents macrophages from engulfing bacteria. And targeting this activity might reduce the risk of bacterial infection in patients with hemolytic disorders.
Sylvia Knapp, MD, PhD, of the Medical University of Vienna in Austria, and her colleagues reported these findings in Nature Immunology.
For decades, iron has been considered the prime suspect responsible for the high rate of bacterial infections in patients with hemolysis. Iron has long been established as an essential nutrient for bacteria.
Since hemolysis leads to the release of iron-containing heme, the threat of serious bacterial infections in these patients was attributed to the excess availability of circulating iron (heme).
However, Dr Knapp and her colleagues found that heme does not act as a willing nutrient to bacteria.
“Using in vitro and preclinical models, we could clearly demonstrate that heme-derived iron is not at all vital for bacterial growth,” said Rui Martins, a PhD student at the Medical University of Vienna.
“In contrast, we found that heme acts on macrophages, the most significant immune cells that are required for mounting an antibacterial response, and it furthermore prevented these cells from eliminating bacteria.”
Heme interfered with the cytoskeleton of macrophages, thereby immobilizing them.
“Heme causes cells to form numerous spikes—like hair standing on end—and then ‘stuns’ the cells within minutes,” Martins explained. “It is reminiscent of a cartoon character sticking his finger in an electrical outlet.”
The cytoskeleton is crucial for the basic functions of macrophages. It consists of long, branching filaments that act as the cell’s internal, highly flexible, and mobile framework.
Through targeted build-up and breakdown of these filaments, phagocytes can move in any direction and engulf invading bacteria. However, this requires a finely tuned signaling program in which the protein DOCK8 plays a central role.
“Through chemical proteomics and biochemical experiments, we discovered that heme interacted with DOCK8, which led to the permanent activation of its downstream target, Cdc42, with deleterious effects,” Dr Knapp said.
In the presence of heme, the cytoskeletal resilience was lost, as filaments grew rampant in all directions, resulting in macrophage paralysis. The cells lost their ability to shape-shift and could no longer chase down and engulf the invading bacteria, allowing the bacteria to multiply virtually unrestricted.
However, Dr Knapp and her colleagues found that an antimalarial drug can restore the functionality of these paralyzed macrophages.
“Quinine, which is clinically used to treat malaria and is suspected to bind heme, blocks the interaction of heme with DOCK8 and thereby improves the outcome from sepsis,” Dr Knapp said.
“This is very promising. We conclusively demonstrate that it is indeed feasible to therapeutically ‘protect’ immune cells and to restore the body’s immune defense against bacteria in hemolytic conditions.” ![]()

plate showing a positive
streptococcus infection
Photo by Bill Branson
Patients who suffer from hemolysis have an increased risk of developing bacterial infections, and new research provides an explanation for this phenomenon.
The study refutes the idea that excess circulating iron is to blame.
Instead, it suggests that heme prevents macrophages from engulfing bacteria. And targeting this activity might reduce the risk of bacterial infection in patients with hemolytic disorders.
Sylvia Knapp, MD, PhD, of the Medical University of Vienna in Austria, and her colleagues reported these findings in Nature Immunology.
For decades, iron has been considered the prime suspect responsible for the high rate of bacterial infections in patients with hemolysis. Iron has long been established as an essential nutrient for bacteria.
Since hemolysis leads to the release of iron-containing heme, the threat of serious bacterial infections in these patients was attributed to the excess availability of circulating iron (heme).
However, Dr Knapp and her colleagues found that heme does not act as a willing nutrient to bacteria.
“Using in vitro and preclinical models, we could clearly demonstrate that heme-derived iron is not at all vital for bacterial growth,” said Rui Martins, a PhD student at the Medical University of Vienna.
“In contrast, we found that heme acts on macrophages, the most significant immune cells that are required for mounting an antibacterial response, and it furthermore prevented these cells from eliminating bacteria.”
Heme interfered with the cytoskeleton of macrophages, thereby immobilizing them.
“Heme causes cells to form numerous spikes—like hair standing on end—and then ‘stuns’ the cells within minutes,” Martins explained. “It is reminiscent of a cartoon character sticking his finger in an electrical outlet.”
The cytoskeleton is crucial for the basic functions of macrophages. It consists of long, branching filaments that act as the cell’s internal, highly flexible, and mobile framework.
Through targeted build-up and breakdown of these filaments, phagocytes can move in any direction and engulf invading bacteria. However, this requires a finely tuned signaling program in which the protein DOCK8 plays a central role.
“Through chemical proteomics and biochemical experiments, we discovered that heme interacted with DOCK8, which led to the permanent activation of its downstream target, Cdc42, with deleterious effects,” Dr Knapp said.
In the presence of heme, the cytoskeletal resilience was lost, as filaments grew rampant in all directions, resulting in macrophage paralysis. The cells lost their ability to shape-shift and could no longer chase down and engulf the invading bacteria, allowing the bacteria to multiply virtually unrestricted.
However, Dr Knapp and her colleagues found that an antimalarial drug can restore the functionality of these paralyzed macrophages.
“Quinine, which is clinically used to treat malaria and is suspected to bind heme, blocks the interaction of heme with DOCK8 and thereby improves the outcome from sepsis,” Dr Knapp said.
“This is very promising. We conclusively demonstrate that it is indeed feasible to therapeutically ‘protect’ immune cells and to restore the body’s immune defense against bacteria in hemolytic conditions.” ![]()

plate showing a positive
streptococcus infection
Photo by Bill Branson
Patients who suffer from hemolysis have an increased risk of developing bacterial infections, and new research provides an explanation for this phenomenon.
The study refutes the idea that excess circulating iron is to blame.
Instead, it suggests that heme prevents macrophages from engulfing bacteria. And targeting this activity might reduce the risk of bacterial infection in patients with hemolytic disorders.
Sylvia Knapp, MD, PhD, of the Medical University of Vienna in Austria, and her colleagues reported these findings in Nature Immunology.
For decades, iron has been considered the prime suspect responsible for the high rate of bacterial infections in patients with hemolysis. Iron has long been established as an essential nutrient for bacteria.
Since hemolysis leads to the release of iron-containing heme, the threat of serious bacterial infections in these patients was attributed to the excess availability of circulating iron (heme).
However, Dr Knapp and her colleagues found that heme does not act as a willing nutrient to bacteria.
“Using in vitro and preclinical models, we could clearly demonstrate that heme-derived iron is not at all vital for bacterial growth,” said Rui Martins, a PhD student at the Medical University of Vienna.
“In contrast, we found that heme acts on macrophages, the most significant immune cells that are required for mounting an antibacterial response, and it furthermore prevented these cells from eliminating bacteria.”
Heme interfered with the cytoskeleton of macrophages, thereby immobilizing them.
“Heme causes cells to form numerous spikes—like hair standing on end—and then ‘stuns’ the cells within minutes,” Martins explained. “It is reminiscent of a cartoon character sticking his finger in an electrical outlet.”
The cytoskeleton is crucial for the basic functions of macrophages. It consists of long, branching filaments that act as the cell’s internal, highly flexible, and mobile framework.
Through targeted build-up and breakdown of these filaments, phagocytes can move in any direction and engulf invading bacteria. However, this requires a finely tuned signaling program in which the protein DOCK8 plays a central role.
“Through chemical proteomics and biochemical experiments, we discovered that heme interacted with DOCK8, which led to the permanent activation of its downstream target, Cdc42, with deleterious effects,” Dr Knapp said.
In the presence of heme, the cytoskeletal resilience was lost, as filaments grew rampant in all directions, resulting in macrophage paralysis. The cells lost their ability to shape-shift and could no longer chase down and engulf the invading bacteria, allowing the bacteria to multiply virtually unrestricted.
However, Dr Knapp and her colleagues found that an antimalarial drug can restore the functionality of these paralyzed macrophages.
“Quinine, which is clinically used to treat malaria and is suspected to bind heme, blocks the interaction of heme with DOCK8 and thereby improves the outcome from sepsis,” Dr Knapp said.
“This is very promising. We conclusively demonstrate that it is indeed feasible to therapeutically ‘protect’ immune cells and to restore the body’s immune defense against bacteria in hemolytic conditions.” ![]()
Generic bivalirudin available in US
Photo from Business Wire
Fresenius Kabi’s Bivalirudin for Injection, a generic alternative to The Medicines Company’s Angiomax, is now available in the US.
Bivalirudin is a direct thrombin inhibitor indicated for use as an anticoagulant.
Fresenius Kabi’s Bivalirudin for Injection was approved by the US Food and Drug Administration in October.
Bivalirudin for Injection is now available in single-dose vials, each containing 250 mg of bivalirudin.
Bivalirudin for Injection was developed and is manufactured in the US.
Bivalirudin for Injection is indicated for use in:
- Patients with unstable angina who are undergoing percutaneous transluminal coronary angioplasty (PTCA)
- Patients undergoing percutaneous coronary intervention (PCI) with provisional use of glycoprotein IIb/IIIa inhibitors, as in the REPLACE-2 study
- Patients with, or at risk of, heparin-induced thrombocytopenia or heparin-induced thrombocytopenia and thrombosis syndrome who are undergoing PCI.
Bivalirudin for Injection is intended for use with aspirin.
The safety and effectiveness of Bivalirudin for Injection has not been established in patients with acute coronary syndromes who are not undergoing PTCA or PCI. ![]()

Photo from Business Wire
Fresenius Kabi’s Bivalirudin for Injection, a generic alternative to The Medicines Company’s Angiomax, is now available in the US.
Bivalirudin is a direct thrombin inhibitor indicated for use as an anticoagulant.
Fresenius Kabi’s Bivalirudin for Injection was approved by the US Food and Drug Administration in October.
Bivalirudin for Injection is now available in single-dose vials, each containing 250 mg of bivalirudin.
Bivalirudin for Injection was developed and is manufactured in the US.
Bivalirudin for Injection is indicated for use in:
- Patients with unstable angina who are undergoing percutaneous transluminal coronary angioplasty (PTCA)
- Patients undergoing percutaneous coronary intervention (PCI) with provisional use of glycoprotein IIb/IIIa inhibitors, as in the REPLACE-2 study
- Patients with, or at risk of, heparin-induced thrombocytopenia or heparin-induced thrombocytopenia and thrombosis syndrome who are undergoing PCI.
Bivalirudin for Injection is intended for use with aspirin.
The safety and effectiveness of Bivalirudin for Injection has not been established in patients with acute coronary syndromes who are not undergoing PTCA or PCI. ![]()

Photo from Business Wire
Fresenius Kabi’s Bivalirudin for Injection, a generic alternative to The Medicines Company’s Angiomax, is now available in the US.
Bivalirudin is a direct thrombin inhibitor indicated for use as an anticoagulant.
Fresenius Kabi’s Bivalirudin for Injection was approved by the US Food and Drug Administration in October.
Bivalirudin for Injection is now available in single-dose vials, each containing 250 mg of bivalirudin.
Bivalirudin for Injection was developed and is manufactured in the US.
Bivalirudin for Injection is indicated for use in:
- Patients with unstable angina who are undergoing percutaneous transluminal coronary angioplasty (PTCA)
- Patients undergoing percutaneous coronary intervention (PCI) with provisional use of glycoprotein IIb/IIIa inhibitors, as in the REPLACE-2 study
- Patients with, or at risk of, heparin-induced thrombocytopenia or heparin-induced thrombocytopenia and thrombosis syndrome who are undergoing PCI.
Bivalirudin for Injection is intended for use with aspirin.
The safety and effectiveness of Bivalirudin for Injection has not been established in patients with acute coronary syndromes who are not undergoing PTCA or PCI. ![]()
Phase 2 trial of MM drug placed on clinical hold

A phase 2 study of the antibody BI-505 in patients with multiple myeloma (MM) has been placed on full clinical hold.
BioInvent International, the company developing BI-505, said it has received verbal notice of the clinical hold from the US Food and Drug Administration (FDA).
The clinical hold means no new subjects can be enrolled on the trial, and there can be no further dosing of subjects who are already enrolled.
BioInvent International said it has yet to receive written notice of the clinical hold from the FDA. However, based on verbal communications, the clinical hold is due to an adverse cardiopulmonary event.
The study (NCT02756728) is being conducted by BioInvent International in collaboration with investigators at the University of Pennsylvania.
The goal of the study is to determine if BI-505 can deepen therapeutic response and thereby prevent or delay relapse in MM patients undergoing autologous stem cell transplant with high-dose melphalan.
BioInvent International said it will analyze the possibility to obtain release of the clinical hold and will provide updates when there is further information to report.
About BI-505
BI-505 is a human antibody targeting ICAM-1, a protein that is elevated in MM cells. BI-505 has been shown to attack MM in 2 ways—by inducing apoptosis in MM cells and by engaging macrophages to attack and kill MM cells.
According to BioInvent International, the development strategy for BI-505 is focused on eliminating residual disease by combining the antibody with modern standard-of-care drugs used to treat MM.
The company said BI-505 proved safe in a phase 1 trial of patients with relapsed/refractory MM, as well as demonstrating “signs of a positive effect against the disease.” This study was published in Clinical Cancer Research in June 2015.
BI-505 has received orphan drug designation as a treatment for MM from both the FDA and the European Medicines Agency. ![]()

A phase 2 study of the antibody BI-505 in patients with multiple myeloma (MM) has been placed on full clinical hold.
BioInvent International, the company developing BI-505, said it has received verbal notice of the clinical hold from the US Food and Drug Administration (FDA).
The clinical hold means no new subjects can be enrolled on the trial, and there can be no further dosing of subjects who are already enrolled.
BioInvent International said it has yet to receive written notice of the clinical hold from the FDA. However, based on verbal communications, the clinical hold is due to an adverse cardiopulmonary event.
The study (NCT02756728) is being conducted by BioInvent International in collaboration with investigators at the University of Pennsylvania.
The goal of the study is to determine if BI-505 can deepen therapeutic response and thereby prevent or delay relapse in MM patients undergoing autologous stem cell transplant with high-dose melphalan.
BioInvent International said it will analyze the possibility to obtain release of the clinical hold and will provide updates when there is further information to report.
About BI-505
BI-505 is a human antibody targeting ICAM-1, a protein that is elevated in MM cells. BI-505 has been shown to attack MM in 2 ways—by inducing apoptosis in MM cells and by engaging macrophages to attack and kill MM cells.
According to BioInvent International, the development strategy for BI-505 is focused on eliminating residual disease by combining the antibody with modern standard-of-care drugs used to treat MM.
The company said BI-505 proved safe in a phase 1 trial of patients with relapsed/refractory MM, as well as demonstrating “signs of a positive effect against the disease.” This study was published in Clinical Cancer Research in June 2015.
BI-505 has received orphan drug designation as a treatment for MM from both the FDA and the European Medicines Agency. ![]()

A phase 2 study of the antibody BI-505 in patients with multiple myeloma (MM) has been placed on full clinical hold.
BioInvent International, the company developing BI-505, said it has received verbal notice of the clinical hold from the US Food and Drug Administration (FDA).
The clinical hold means no new subjects can be enrolled on the trial, and there can be no further dosing of subjects who are already enrolled.
BioInvent International said it has yet to receive written notice of the clinical hold from the FDA. However, based on verbal communications, the clinical hold is due to an adverse cardiopulmonary event.
The study (NCT02756728) is being conducted by BioInvent International in collaboration with investigators at the University of Pennsylvania.
The goal of the study is to determine if BI-505 can deepen therapeutic response and thereby prevent or delay relapse in MM patients undergoing autologous stem cell transplant with high-dose melphalan.
BioInvent International said it will analyze the possibility to obtain release of the clinical hold and will provide updates when there is further information to report.
About BI-505
BI-505 is a human antibody targeting ICAM-1, a protein that is elevated in MM cells. BI-505 has been shown to attack MM in 2 ways—by inducing apoptosis in MM cells and by engaging macrophages to attack and kill MM cells.
According to BioInvent International, the development strategy for BI-505 is focused on eliminating residual disease by combining the antibody with modern standard-of-care drugs used to treat MM.
The company said BI-505 proved safe in a phase 1 trial of patients with relapsed/refractory MM, as well as demonstrating “signs of a positive effect against the disease.” This study was published in Clinical Cancer Research in June 2015.
BI-505 has received orphan drug designation as a treatment for MM from both the FDA and the European Medicines Agency. ![]()
Panobinostat might treat high-risk ALL subtype

Photo courtesy of Novartis
Researchers say they have identified a high-risk subtype of acute lymphoblastic leukemia (ALL) that may respond to treatment with the histone deacetylase (HDAC) inhibitor panobinostat.
The ALL subtype is characterized by chromosomal rearrangements that involve the MEF2D gene and 1 of 6 partner genes, most often BCL9.
The researchers described this subtype, known as MEF2D-rearranged ALL, in Nature Communications.
“MEF2D is a transcription factor that switches on expression of other genes during normal development,” said study author Charles Mullighan, MD, MBBS, of the St. Jude Children’s Research Hospital in Memphis, Tennessee.
“We found that MEF2D chromosomal rearrangements disrupt expression of those genes and create a vulnerability to at least one targeted therapy, the drug panobinostat.”
Dr Mullighan and his colleagues performed genomic analyses on samples from a total of 1724 children, adolescents, and adults with ALL. This revealed 52 patients with MEF2D rearrangements.
MEF2D-rearranged ALL
The researchers calculated that MEF2D-rearranged ALL accounted for 5.3% of the ALL cases whose genetic basis was unknown.
The team also noted that MEF2D-rearranged ALL occurred most frequently in adolescents. Although, overall, ALL occurs most often in children between 3 and 5 years old, the average patient with MEF2D-rearranged ALL was 14.
In addition, MEF2D-rearranged ALL was associated with reduced survival when compared to some other ALL subtypes. The 5-year cancer-free survival for MEF2D-rearranged ALL patients was 71.6%.
The researchers also found that a fusion protein resulting from the MEF2D rearrangement led to sustained growth of mouse cells when compared to wild-type MEF2D or other proteins.
“That indicates the MEF2D fusion is a key step in transforming a normal white blood cell with a finite lifespan into a leukemic cell that is immortal,” Dr Mullighan said.
Role for panobinostat
MEF2D-rearranged leukemic cells produced high levels of HDAC9, which is targeted by panobinostat.
The researchers tested panobinostat in the lab and found the drug stopped proliferation of human MEF2D-rearranged leukemic cells.
Dr Mullighan said MEF2D-rearranged leukemic cells were exquisitely sensitive to panobinostat, which suggested the drug might function in a more targeted manner against cells with the rearrangement.
“If further testing of panobinostat, either alone or in combination therapy, confirms the anti-proliferative activity, that would lay the foundation for a clinical trial in patients, particularly patients with high-risk disease or those who have relapsed,” he said. ![]()

Photo courtesy of Novartis
Researchers say they have identified a high-risk subtype of acute lymphoblastic leukemia (ALL) that may respond to treatment with the histone deacetylase (HDAC) inhibitor panobinostat.
The ALL subtype is characterized by chromosomal rearrangements that involve the MEF2D gene and 1 of 6 partner genes, most often BCL9.
The researchers described this subtype, known as MEF2D-rearranged ALL, in Nature Communications.
“MEF2D is a transcription factor that switches on expression of other genes during normal development,” said study author Charles Mullighan, MD, MBBS, of the St. Jude Children’s Research Hospital in Memphis, Tennessee.
“We found that MEF2D chromosomal rearrangements disrupt expression of those genes and create a vulnerability to at least one targeted therapy, the drug panobinostat.”
Dr Mullighan and his colleagues performed genomic analyses on samples from a total of 1724 children, adolescents, and adults with ALL. This revealed 52 patients with MEF2D rearrangements.
MEF2D-rearranged ALL
The researchers calculated that MEF2D-rearranged ALL accounted for 5.3% of the ALL cases whose genetic basis was unknown.
The team also noted that MEF2D-rearranged ALL occurred most frequently in adolescents. Although, overall, ALL occurs most often in children between 3 and 5 years old, the average patient with MEF2D-rearranged ALL was 14.
In addition, MEF2D-rearranged ALL was associated with reduced survival when compared to some other ALL subtypes. The 5-year cancer-free survival for MEF2D-rearranged ALL patients was 71.6%.
The researchers also found that a fusion protein resulting from the MEF2D rearrangement led to sustained growth of mouse cells when compared to wild-type MEF2D or other proteins.
“That indicates the MEF2D fusion is a key step in transforming a normal white blood cell with a finite lifespan into a leukemic cell that is immortal,” Dr Mullighan said.
Role for panobinostat
MEF2D-rearranged leukemic cells produced high levels of HDAC9, which is targeted by panobinostat.
The researchers tested panobinostat in the lab and found the drug stopped proliferation of human MEF2D-rearranged leukemic cells.
Dr Mullighan said MEF2D-rearranged leukemic cells were exquisitely sensitive to panobinostat, which suggested the drug might function in a more targeted manner against cells with the rearrangement.
“If further testing of panobinostat, either alone or in combination therapy, confirms the anti-proliferative activity, that would lay the foundation for a clinical trial in patients, particularly patients with high-risk disease or those who have relapsed,” he said. ![]()

Photo courtesy of Novartis
Researchers say they have identified a high-risk subtype of acute lymphoblastic leukemia (ALL) that may respond to treatment with the histone deacetylase (HDAC) inhibitor panobinostat.
The ALL subtype is characterized by chromosomal rearrangements that involve the MEF2D gene and 1 of 6 partner genes, most often BCL9.
The researchers described this subtype, known as MEF2D-rearranged ALL, in Nature Communications.
“MEF2D is a transcription factor that switches on expression of other genes during normal development,” said study author Charles Mullighan, MD, MBBS, of the St. Jude Children’s Research Hospital in Memphis, Tennessee.
“We found that MEF2D chromosomal rearrangements disrupt expression of those genes and create a vulnerability to at least one targeted therapy, the drug panobinostat.”
Dr Mullighan and his colleagues performed genomic analyses on samples from a total of 1724 children, adolescents, and adults with ALL. This revealed 52 patients with MEF2D rearrangements.
MEF2D-rearranged ALL
The researchers calculated that MEF2D-rearranged ALL accounted for 5.3% of the ALL cases whose genetic basis was unknown.
The team also noted that MEF2D-rearranged ALL occurred most frequently in adolescents. Although, overall, ALL occurs most often in children between 3 and 5 years old, the average patient with MEF2D-rearranged ALL was 14.
In addition, MEF2D-rearranged ALL was associated with reduced survival when compared to some other ALL subtypes. The 5-year cancer-free survival for MEF2D-rearranged ALL patients was 71.6%.
The researchers also found that a fusion protein resulting from the MEF2D rearrangement led to sustained growth of mouse cells when compared to wild-type MEF2D or other proteins.
“That indicates the MEF2D fusion is a key step in transforming a normal white blood cell with a finite lifespan into a leukemic cell that is immortal,” Dr Mullighan said.
Role for panobinostat
MEF2D-rearranged leukemic cells produced high levels of HDAC9, which is targeted by panobinostat.
The researchers tested panobinostat in the lab and found the drug stopped proliferation of human MEF2D-rearranged leukemic cells.
Dr Mullighan said MEF2D-rearranged leukemic cells were exquisitely sensitive to panobinostat, which suggested the drug might function in a more targeted manner against cells with the rearrangement.
“If further testing of panobinostat, either alone or in combination therapy, confirms the anti-proliferative activity, that would lay the foundation for a clinical trial in patients, particularly patients with high-risk disease or those who have relapsed,” he said. ![]()
Flushing Lesion on 4-Month-Old Boy
IN THIS ARTICLE
- Clinical presentation
- Disease triggers
- Outcome for the case patient
A 4-month-old white boy is brought to the allergy office for evaluation of a lesion on his back, first noticed when he was one month old. His parents report that on two separate occasions he developed a bright red, whole-body rash that resolved on its own. He is described as a “fussy” baby who has been on both milk- and soy-based formulas. Aside from mild eczema and infantile seborrheic dermatitis, his medical history is unremarkable.
Physical examination reveals a reddish, 3-cm plaque on his left upper back that, when stroked, exhibits an immediate urticarial response. The patient is given cetirizine in the office and within 45 minutes, the flushing resolves. A normal tryptase level is obtained. The patient is diagnosed with a solitary mastocytoma.
DISCUSSION
Mast cells play a central role in allergic rhinitis, asthma, eczema, and anaphylaxis; they are also a vital component in the inflammatory process. Cutaneous mastocytosis is a pathologic increase in mast cells that, when degranulated, release histamine and tryptase.1 Histamine is produced equally by both mast cells and basophils, while the production of tryptase is relatively specific to mast cells. An elevated tryptase level is indicative of mast cell activation. The signs and symptoms associated with cutaneous mastocytosis are due to the release of these mediators.2
While there is no gender bias for cutaneous mastocytosis, there is a bimodal distribution: Children from birth to age 2 account for 55% of cases, whereas 35% of cases occur in those older than 15—the remaining 10% are between these ages.1 During the first year of life, 60% to 80% of patients with cutaneous mastocytosis will develop lesions. Familial cases are rare.2
Clinical presentation
The presentation of cutaneous mastocytosis can vary. It is often mistaken for common childhood rashes such as poison ivy, eczema, or hives. The effects of histamine and tryptase cause children to present with pruritus, flushing, and headache. Gastrointestinal symptoms, such as abdominal pain and diarrhea, can also occur as a result of histamine release. Symptoms can occur spontaneously or be induced by certain triggers (see Table).3

There are three types of cutaneous mastocytosis: urticarial pigmentosa (UP), solitary mastocytoma, and diffuse cutaneous mastocytosis (DCM). UP is the most common, representing 70% to 90% of all cases.3 It typically manifests as multiple red, brown, or yellow lesions that are small (usually < 2 cm). A positive Darier sign (the formation of a wheal and flare after stroking one or several lesions) is common in these patients.2,3
Solitary mastocytomas are single, indurated, red-brown macules, papules, or plaques.4 Frequently seen on the trunk, extremities, head, or neck, these lesions resemble UP but are larger (up to several centimeters in diameter).2,4 A positive Darier sign is elicited in approximately 50% of these patients.4 Solitary mastocytomas are seen in 10% to 15% of children with cutaneous mastocytosis.4
DCM accounts for only 1% to 3% of cases and typically involves the entire skin, which is usually thick, with a normal or yellowish brown color.3 Affected patients may experience blistering and bullae. Severe symptoms, including whole-body flushing, pruritus, diarrhea, intestinal bleeding, hypotension, anemia, and hypovolemic shock, can occur; deaths have been reported.2
Diagnostic evaluation
Following a thorough history and physical exam, the diagnosis of cutaneous mastocytosis can be made based on clinical findings.5 It can be confirmed by a punch biopsy of the lesion and measurement of serum tryptase levels; higher levels have been shown to correlate to the number of mast cells in the skin and the child’s cutaneous disease burden.2,4 Ordering baseline tryptase levels can also help distinguish children at risk for severe episodes of mast cell activation from those who may just have a mild case.6
If the patient exhibits symptoms suggestive of systemic mastocytosis (an abnormal increase in mast cells in extracutaneous organs, including bone marrow) or there is suspicion for malignancy, efforts must be made to rule out these more serious diagnoses. A complete white blood cell count with differential, metabolic profile with liver enzymes, and sedimentation rate should be obtained.4
Treatment
The treatment of cutaneous mastocytosis is symptomatic, as it is typically a benign disease. The goal of therapy is to prevent mast cell activation, and hence the symptoms that occur when mast cells release their mediators, by avoiding obvious triggers.4
NSAIDs have been reported to cause mast cell mediator release and should therefore be avoided. Since extremes in temperature—particularly heat—can lead to mast cell activation, it is important to control the temperature of a bath or swimming pool and to be wary of exposure to air conditioning. Though easier said than done, soothing crying babies and children is also helpful, as irritability is a known trigger. Management of anxiety and avoidance of stress when possible is recommended.2
Treatment usually begins with H1 and H2 blockers. Cetirizine and diphenhydramine work to control the itching, while ranitidine and famotidine help manage gastric acid secretion when there are symptoms of abdominal pain and peptic ulcer disease.3 Hydroxyzine is effective in controlling both itch and gastric acid secretion.4 Water-soluble cromolyn sodium cream helps to decrease the itch and flare of a mastocytoma; the oral form is also effective.3 Another option to relieve itching is to apply topical corticosteroids with an occlusive dressing.
An epinephrine auto-injector is recommended due to the risk for anaphylaxis; this risk, however, has not been clearly established in cutaneous mastocytosis, specifically in DCM. During an acute and severe flare that induces hypotension, wheezing, and/or laryngeal edema, epinephrine should be administered while the child is lying down.3,4
Surgical excision can be considered when other treatment options fail, or if the lesion is located on the scalp, flexure area, palm, or sole.3,4
Prognosis
The prognosis for a child with a solitary mastocytoma is extremely good. By the time the child reaches puberty, there is typically spontaneous regression.
If any type of mastocytoma persists beyond adolescence, or tryptase levels continue to rise after puberty, however, that raises concern for progression to systemic mastocytosis. Although this diagnosis is rare in children, bone marrow studies may be necessary to determine the patient’s course.1
OUTCOME FOR THE CASE PATIENT
The patient was placed on daily cetirizine. His parents were advised not to irritate the lesion and were educated on possible triggers, including the need to avoid NSAIDs. They were also trained in the use of an epinephrine auto-injector.
CONCLUSION
Cutaneous mastocytosis should be included in the differential diagnosis of rashes and skin lesions in pediatric patients. It is important to be able to recognize the presenting signs and symptoms of a mastocytoma and to monitor the lesion over time.4 Educating the child’s parents, teachers, and caregivers about potential triggers and treatment of a mast cell activation attack can help minimize the symptoms.
1. Frieri M, Quershi M. Pediatric mastocytosis: a review of the literature. Pediatr Allergy Immunol Pulmonol. 2013;26(4):175-180.
2. Castells M, Metcalfe DD, Escribano L. Guidelines for the diagnosis and treatment of cutaneous mastocytosis in children. Am J Clin Dermatol. 2011;12(4):259-270.
3. Castells M, Metcalfe DD, Escribano L. Diagnosis and treatment of cutaneous mastocytosis in children: practical recommendations. Am J Clin Dermatol. 2011;12(4):259-270.
4. Krishnan K, Ownby DR. A solitary mastocytoma presenting with urticaria and angioedema in a 14-year-old boy. Allergy Asthma Proc. 2010;31(6):520-523.
5. Fogelson SK, Dohil MA. Papular and nodular skin lesions in children. Semin Plast Surg. 2006;20(3):180-191.
6. Alvarez-Twose I, Vañó-Galván S, Sánchez-Muñoz L, et al. Increased serum baseline tryptase levels and extensive skin involvement are predictors for the severity of mast cell activation episodes in children with mastocytosis. Allergy. 2012;67(6):813-821.
IN THIS ARTICLE
- Clinical presentation
- Disease triggers
- Outcome for the case patient
A 4-month-old white boy is brought to the allergy office for evaluation of a lesion on his back, first noticed when he was one month old. His parents report that on two separate occasions he developed a bright red, whole-body rash that resolved on its own. He is described as a “fussy” baby who has been on both milk- and soy-based formulas. Aside from mild eczema and infantile seborrheic dermatitis, his medical history is unremarkable.
Physical examination reveals a reddish, 3-cm plaque on his left upper back that, when stroked, exhibits an immediate urticarial response. The patient is given cetirizine in the office and within 45 minutes, the flushing resolves. A normal tryptase level is obtained. The patient is diagnosed with a solitary mastocytoma.

DISCUSSION
Mast cells play a central role in allergic rhinitis, asthma, eczema, and anaphylaxis; they are also a vital component in the inflammatory process. Cutaneous mastocytosis is a pathologic increase in mast cells that, when degranulated, release histamine and tryptase.1 Histamine is produced equally by both mast cells and basophils, while the production of tryptase is relatively specific to mast cells. An elevated tryptase level is indicative of mast cell activation. The signs and symptoms associated with cutaneous mastocytosis are due to the release of these mediators.2
While there is no gender bias for cutaneous mastocytosis, there is a bimodal distribution: Children from birth to age 2 account for 55% of cases, whereas 35% of cases occur in those older than 15—the remaining 10% are between these ages.1 During the first year of life, 60% to 80% of patients with cutaneous mastocytosis will develop lesions. Familial cases are rare.2
Clinical presentation
The presentation of cutaneous mastocytosis can vary. It is often mistaken for common childhood rashes such as poison ivy, eczema, or hives. The effects of histamine and tryptase cause children to present with pruritus, flushing, and headache. Gastrointestinal symptoms, such as abdominal pain and diarrhea, can also occur as a result of histamine release. Symptoms can occur spontaneously or be induced by certain triggers (see Table).3

There are three types of cutaneous mastocytosis: urticarial pigmentosa (UP), solitary mastocytoma, and diffuse cutaneous mastocytosis (DCM). UP is the most common, representing 70% to 90% of all cases.3 It typically manifests as multiple red, brown, or yellow lesions that are small (usually < 2 cm). A positive Darier sign (the formation of a wheal and flare after stroking one or several lesions) is common in these patients.2,3
Solitary mastocytomas are single, indurated, red-brown macules, papules, or plaques.4 Frequently seen on the trunk, extremities, head, or neck, these lesions resemble UP but are larger (up to several centimeters in diameter).2,4 A positive Darier sign is elicited in approximately 50% of these patients.4 Solitary mastocytomas are seen in 10% to 15% of children with cutaneous mastocytosis.4
DCM accounts for only 1% to 3% of cases and typically involves the entire skin, which is usually thick, with a normal or yellowish brown color.3 Affected patients may experience blistering and bullae. Severe symptoms, including whole-body flushing, pruritus, diarrhea, intestinal bleeding, hypotension, anemia, and hypovolemic shock, can occur; deaths have been reported.2
Diagnostic evaluation
Following a thorough history and physical exam, the diagnosis of cutaneous mastocytosis can be made based on clinical findings.5 It can be confirmed by a punch biopsy of the lesion and measurement of serum tryptase levels; higher levels have been shown to correlate to the number of mast cells in the skin and the child’s cutaneous disease burden.2,4 Ordering baseline tryptase levels can also help distinguish children at risk for severe episodes of mast cell activation from those who may just have a mild case.6
If the patient exhibits symptoms suggestive of systemic mastocytosis (an abnormal increase in mast cells in extracutaneous organs, including bone marrow) or there is suspicion for malignancy, efforts must be made to rule out these more serious diagnoses. A complete white blood cell count with differential, metabolic profile with liver enzymes, and sedimentation rate should be obtained.4
Treatment
The treatment of cutaneous mastocytosis is symptomatic, as it is typically a benign disease. The goal of therapy is to prevent mast cell activation, and hence the symptoms that occur when mast cells release their mediators, by avoiding obvious triggers.4
NSAIDs have been reported to cause mast cell mediator release and should therefore be avoided. Since extremes in temperature—particularly heat—can lead to mast cell activation, it is important to control the temperature of a bath or swimming pool and to be wary of exposure to air conditioning. Though easier said than done, soothing crying babies and children is also helpful, as irritability is a known trigger. Management of anxiety and avoidance of stress when possible is recommended.2
Treatment usually begins with H1 and H2 blockers. Cetirizine and diphenhydramine work to control the itching, while ranitidine and famotidine help manage gastric acid secretion when there are symptoms of abdominal pain and peptic ulcer disease.3 Hydroxyzine is effective in controlling both itch and gastric acid secretion.4 Water-soluble cromolyn sodium cream helps to decrease the itch and flare of a mastocytoma; the oral form is also effective.3 Another option to relieve itching is to apply topical corticosteroids with an occlusive dressing.
An epinephrine auto-injector is recommended due to the risk for anaphylaxis; this risk, however, has not been clearly established in cutaneous mastocytosis, specifically in DCM. During an acute and severe flare that induces hypotension, wheezing, and/or laryngeal edema, epinephrine should be administered while the child is lying down.3,4
Surgical excision can be considered when other treatment options fail, or if the lesion is located on the scalp, flexure area, palm, or sole.3,4
Prognosis
The prognosis for a child with a solitary mastocytoma is extremely good. By the time the child reaches puberty, there is typically spontaneous regression.
If any type of mastocytoma persists beyond adolescence, or tryptase levels continue to rise after puberty, however, that raises concern for progression to systemic mastocytosis. Although this diagnosis is rare in children, bone marrow studies may be necessary to determine the patient’s course.1
OUTCOME FOR THE CASE PATIENT
The patient was placed on daily cetirizine. His parents were advised not to irritate the lesion and were educated on possible triggers, including the need to avoid NSAIDs. They were also trained in the use of an epinephrine auto-injector.
CONCLUSION
Cutaneous mastocytosis should be included in the differential diagnosis of rashes and skin lesions in pediatric patients. It is important to be able to recognize the presenting signs and symptoms of a mastocytoma and to monitor the lesion over time.4 Educating the child’s parents, teachers, and caregivers about potential triggers and treatment of a mast cell activation attack can help minimize the symptoms.
IN THIS ARTICLE
- Clinical presentation
- Disease triggers
- Outcome for the case patient
A 4-month-old white boy is brought to the allergy office for evaluation of a lesion on his back, first noticed when he was one month old. His parents report that on two separate occasions he developed a bright red, whole-body rash that resolved on its own. He is described as a “fussy” baby who has been on both milk- and soy-based formulas. Aside from mild eczema and infantile seborrheic dermatitis, his medical history is unremarkable.
Physical examination reveals a reddish, 3-cm plaque on his left upper back that, when stroked, exhibits an immediate urticarial response. The patient is given cetirizine in the office and within 45 minutes, the flushing resolves. A normal tryptase level is obtained. The patient is diagnosed with a solitary mastocytoma.

DISCUSSION
Mast cells play a central role in allergic rhinitis, asthma, eczema, and anaphylaxis; they are also a vital component in the inflammatory process. Cutaneous mastocytosis is a pathologic increase in mast cells that, when degranulated, release histamine and tryptase.1 Histamine is produced equally by both mast cells and basophils, while the production of tryptase is relatively specific to mast cells. An elevated tryptase level is indicative of mast cell activation. The signs and symptoms associated with cutaneous mastocytosis are due to the release of these mediators.2
While there is no gender bias for cutaneous mastocytosis, there is a bimodal distribution: Children from birth to age 2 account for 55% of cases, whereas 35% of cases occur in those older than 15—the remaining 10% are between these ages.1 During the first year of life, 60% to 80% of patients with cutaneous mastocytosis will develop lesions. Familial cases are rare.2
Clinical presentation
The presentation of cutaneous mastocytosis can vary. It is often mistaken for common childhood rashes such as poison ivy, eczema, or hives. The effects of histamine and tryptase cause children to present with pruritus, flushing, and headache. Gastrointestinal symptoms, such as abdominal pain and diarrhea, can also occur as a result of histamine release. Symptoms can occur spontaneously or be induced by certain triggers (see Table).3

There are three types of cutaneous mastocytosis: urticarial pigmentosa (UP), solitary mastocytoma, and diffuse cutaneous mastocytosis (DCM). UP is the most common, representing 70% to 90% of all cases.3 It typically manifests as multiple red, brown, or yellow lesions that are small (usually < 2 cm). A positive Darier sign (the formation of a wheal and flare after stroking one or several lesions) is common in these patients.2,3
Solitary mastocytomas are single, indurated, red-brown macules, papules, or plaques.4 Frequently seen on the trunk, extremities, head, or neck, these lesions resemble UP but are larger (up to several centimeters in diameter).2,4 A positive Darier sign is elicited in approximately 50% of these patients.4 Solitary mastocytomas are seen in 10% to 15% of children with cutaneous mastocytosis.4
DCM accounts for only 1% to 3% of cases and typically involves the entire skin, which is usually thick, with a normal or yellowish brown color.3 Affected patients may experience blistering and bullae. Severe symptoms, including whole-body flushing, pruritus, diarrhea, intestinal bleeding, hypotension, anemia, and hypovolemic shock, can occur; deaths have been reported.2
Diagnostic evaluation
Following a thorough history and physical exam, the diagnosis of cutaneous mastocytosis can be made based on clinical findings.5 It can be confirmed by a punch biopsy of the lesion and measurement of serum tryptase levels; higher levels have been shown to correlate to the number of mast cells in the skin and the child’s cutaneous disease burden.2,4 Ordering baseline tryptase levels can also help distinguish children at risk for severe episodes of mast cell activation from those who may just have a mild case.6
If the patient exhibits symptoms suggestive of systemic mastocytosis (an abnormal increase in mast cells in extracutaneous organs, including bone marrow) or there is suspicion for malignancy, efforts must be made to rule out these more serious diagnoses. A complete white blood cell count with differential, metabolic profile with liver enzymes, and sedimentation rate should be obtained.4
Treatment
The treatment of cutaneous mastocytosis is symptomatic, as it is typically a benign disease. The goal of therapy is to prevent mast cell activation, and hence the symptoms that occur when mast cells release their mediators, by avoiding obvious triggers.4
NSAIDs have been reported to cause mast cell mediator release and should therefore be avoided. Since extremes in temperature—particularly heat—can lead to mast cell activation, it is important to control the temperature of a bath or swimming pool and to be wary of exposure to air conditioning. Though easier said than done, soothing crying babies and children is also helpful, as irritability is a known trigger. Management of anxiety and avoidance of stress when possible is recommended.2
Treatment usually begins with H1 and H2 blockers. Cetirizine and diphenhydramine work to control the itching, while ranitidine and famotidine help manage gastric acid secretion when there are symptoms of abdominal pain and peptic ulcer disease.3 Hydroxyzine is effective in controlling both itch and gastric acid secretion.4 Water-soluble cromolyn sodium cream helps to decrease the itch and flare of a mastocytoma; the oral form is also effective.3 Another option to relieve itching is to apply topical corticosteroids with an occlusive dressing.
An epinephrine auto-injector is recommended due to the risk for anaphylaxis; this risk, however, has not been clearly established in cutaneous mastocytosis, specifically in DCM. During an acute and severe flare that induces hypotension, wheezing, and/or laryngeal edema, epinephrine should be administered while the child is lying down.3,4
Surgical excision can be considered when other treatment options fail, or if the lesion is located on the scalp, flexure area, palm, or sole.3,4
Prognosis
The prognosis for a child with a solitary mastocytoma is extremely good. By the time the child reaches puberty, there is typically spontaneous regression.
If any type of mastocytoma persists beyond adolescence, or tryptase levels continue to rise after puberty, however, that raises concern for progression to systemic mastocytosis. Although this diagnosis is rare in children, bone marrow studies may be necessary to determine the patient’s course.1
OUTCOME FOR THE CASE PATIENT
The patient was placed on daily cetirizine. His parents were advised not to irritate the lesion and were educated on possible triggers, including the need to avoid NSAIDs. They were also trained in the use of an epinephrine auto-injector.
CONCLUSION
Cutaneous mastocytosis should be included in the differential diagnosis of rashes and skin lesions in pediatric patients. It is important to be able to recognize the presenting signs and symptoms of a mastocytoma and to monitor the lesion over time.4 Educating the child’s parents, teachers, and caregivers about potential triggers and treatment of a mast cell activation attack can help minimize the symptoms.
1. Frieri M, Quershi M. Pediatric mastocytosis: a review of the literature. Pediatr Allergy Immunol Pulmonol. 2013;26(4):175-180.
2. Castells M, Metcalfe DD, Escribano L. Guidelines for the diagnosis and treatment of cutaneous mastocytosis in children. Am J Clin Dermatol. 2011;12(4):259-270.
3. Castells M, Metcalfe DD, Escribano L. Diagnosis and treatment of cutaneous mastocytosis in children: practical recommendations. Am J Clin Dermatol. 2011;12(4):259-270.
4. Krishnan K, Ownby DR. A solitary mastocytoma presenting with urticaria and angioedema in a 14-year-old boy. Allergy Asthma Proc. 2010;31(6):520-523.
5. Fogelson SK, Dohil MA. Papular and nodular skin lesions in children. Semin Plast Surg. 2006;20(3):180-191.
6. Alvarez-Twose I, Vañó-Galván S, Sánchez-Muñoz L, et al. Increased serum baseline tryptase levels and extensive skin involvement are predictors for the severity of mast cell activation episodes in children with mastocytosis. Allergy. 2012;67(6):813-821.
1. Frieri M, Quershi M. Pediatric mastocytosis: a review of the literature. Pediatr Allergy Immunol Pulmonol. 2013;26(4):175-180.
2. Castells M, Metcalfe DD, Escribano L. Guidelines for the diagnosis and treatment of cutaneous mastocytosis in children. Am J Clin Dermatol. 2011;12(4):259-270.
3. Castells M, Metcalfe DD, Escribano L. Diagnosis and treatment of cutaneous mastocytosis in children: practical recommendations. Am J Clin Dermatol. 2011;12(4):259-270.
4. Krishnan K, Ownby DR. A solitary mastocytoma presenting with urticaria and angioedema in a 14-year-old boy. Allergy Asthma Proc. 2010;31(6):520-523.
5. Fogelson SK, Dohil MA. Papular and nodular skin lesions in children. Semin Plast Surg. 2006;20(3):180-191.
6. Alvarez-Twose I, Vañó-Galván S, Sánchez-Muñoz L, et al. Increased serum baseline tryptase levels and extensive skin involvement are predictors for the severity of mast cell activation episodes in children with mastocytosis. Allergy. 2012;67(6):813-821.
Diabetes drugs with cardiovascular benefits broaden cardiology’s turf
The dramatic reduction in cardiovascular death and heart failure hospitalization seen during treatment with empagliflozin (Jardiance) in the EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) trial, for example, has prompted some cardiologists in the year since the first EMPA-REG report to become active prescribers of the drug to their patients who have type 2 diabetes and cardiovascular disease. The same evidence has driven other cardiologists who may not feel fully comfortable prescribing an antidiabetic drug on their own to enter into active partnerships with endocrinologists to work as a team to put diabetes patients with cardiovascular disease on empagliflozin.
In Dr. Fitchett’s practice, “if a patient with type 2 diabetes has an endocrinologist, then I will send a letter to that physician saying I think the patient should be on one of these drugs,” empagliflozin or liraglutide, he said. “If the patient is being treated by a primary care physician, then I will prescribe empagliflozin myself because most primary care physicians are not willing to prescribe it. I think more and more cardiologists are doing this. The great thing about empagliflozin and liraglutide is that they do not cause hypoglycemia and the adverse effect profiles are relatively good. As long as drug cost is not an issue, then as cardiologists we need to adjust glycemia control with cardiovascular benefit as we did years ago with statin treatment,” explained Dr. Fitchett, a cardiologist at St. Michael’s Hospital in Toronto and a senior collaborator and coauthor on the EMPA-REG study.
When results from the 4S [Scandinavian Simvastatin Survival Study] came out in 1994, proving that long-term statin treatment was both safe and increased survival in patients with coronary heart disease, “cardiologists took over lipid management from endocrinologists,” he recalled. “We now have a safe and simple treatment for glucose lowering that also cuts cardiovascular disease events, so cardiologists have to also be involved, at least to some extent. Their degree of involvement depends on their practice and who provides a patient’s primary diabetes care,” he said.
Cardiologists vary on empagliflozin
Other cardiologists are mixed in their take on personally prescribing antidiabetic drugs to high-risk patients with type 2 diabetes. Greg C. Fonarow, MD, has also aggressively taken to empagliflozin over the past year, especially for his patients with heart failure or at high risk for developing heart failure. The EMPA-REG results showed that empagliflozin’s potent impact on reducing cardiovascular death in patients linked closely with a reduction in heart failure hospitalizations. In his recent experience, endocrinologists as well as other physicians who care for patients with type 2 diabetes “are often reluctant to make any changes [in a patient’s hypoglycemic regimen], and in general they have not gravitated toward the treatments that have been shown to improve cardiovascular outcomes and instead focus solely on a patient’s hemoglobin A1c,” Dr. Fonarow said in an interview at the recent annual meeting of the Heart Failure Society of America.
He said he prescribes empagliflozin to patients with type 2 diabetes if they are hospitalized for heart failure or as outpatients, and he targets it to patients diagnosed with heart failure – including heart failure with preserved ejection fraction – as well as to patients with other forms of cardiovascular disease, closely following the EMPA-REG enrollment criteria. It’s too early in the experience with empagliflozin to use it preferentially in diabetes patients without cardiovascular disease or patients who in any other way fall outside the enrollment criteria for EMPA-REG, he said.
“I am happy to consult with their endocrinologist, or I tell patients to discuss this treatment with their endocrinologist. If the endocrinologist prescribes empagliflozin, great; if not, I feel an obligation to provide the best care I can to my patients. This is not a hard medication to use. The safety profile is good. Treatment with empagliflozin obviously has renal-function considerations, but that’s true for many drugs. The biggest challenge is what is covered by the patient’s insurance. We often need preauthorization.
“So far I have seen excellent responses in patients for both metabolic control and clinical responses in patients with heart failure. Their symptoms seem to improve,” said Dr. Fonarow, professor of medicine and co-chief of cardiology at the University of Southern California , Los Angeles.
While Dr. Fonarow cautioned that he also would not start empagliflozin in a patient with a HbA1c below 7%, he would seriously consider swapping out a patient’s drug for empagliflozin if it were a sulfonylurea or a dipeptidyl peptidase-4 inhibitor. He stopped short of suggesting a substitution of empagliflozin for metformin. In Dr. Fonarow’s opinion, the evidence for empagliflozin is also “more robust” than it has been for liraglutide or semaglutide. With what’s now known about the clinical impact of these drugs, he foresees a time when a combination between a SGLT-2 inhibitor, with its effect on heart failure, and a GLP-1 analogue, with its effect on atherosclerotic disease, may seem an ideal initial drug pairing for patients with type 2 diabetes and significant cardiovascular disease risk, with metformin relegated to a second-line role.
Other cardiologists endorsed a more collaborative approach to prescribing empagliflozin and liraglutide.
Another team-approach advocate is Robert O. Bonow, MD, cardiologist and professor of medicine at Northwestern University in Chicago. “Cardiologists are comfortable prescribing metformin and telling patients about lifestyle, but when it comes to newer antidiabetic drugs, that’s a new field, and a team approach may be best,” he said in an interview. “If possible, a cardiologist should have a friendly partnership with a diabetologist or endocrinologist who is expert in treating diabetes.” Many cardiologists now work in and for hospitals, and easy access to an endocrinologist is probably available, he noted.
But new analyses of the EMPA-REG data reported by Dr. Fitchett at the ESC congress showed that empagliflozin treatment exerted a similar benefit of reduced cardiovascular death regardless of whether patients had prevalent heart failure at entry into the study, incident heart failure during follow-up, or no heart failure of any sort.
Impact of heart failure in EMPA-REG
Roughly 10% of the 7,020 patients enrolled in EMPA-REG had heart failure at the time they entered the trial. During a median follow-up of just over 3 years, the incidence of new-onset heart failure – tallied as either a new heart failure hospitalization or a clinical episode deemed to be heart failure by an investigator – occurred in 4.6% of patients on empagliflozin and in 6.5% of patients in the placebo arm, a 1.9-percentage-point difference and a 30% relative risk reduction linked with empagliflozin use, Dr. Fitchett reported.
The main EMPA-REG outcome was a composite of cardiovascular death, nonfatal MI, and nonfatal stroke. This positive outcome in favor of empagliflozin treatment was primarily driven by a difference in the rate of cardiovascular death. In the new analysis, the relative reduction in cardiovascular deaths with empagliflozin compared with placebo was 29% among patients with prevalent heart failure at baseline, 35% among those who had an incident heart failure hospitalization during follow-up, 27% among patients with an incident heart failure episode diagnosed by an investigator during follow-up, 33% among the combined group of trial patients with any form of heart failure at trial entry or during the trial (those with prevalent heart failure at baseline plus those with an incident event), and 37% among the large number of patients in the trial who remained free from any indication of heart failure during follow-up.
In short, treatment with empagliflozin “reduced cardiovascular mortality by the same relative amount” regardless of whether patients did or did not have heart failure during the trial,” Dr. Fitchett concluded.
Additional secondary analyses from EMPA-REG reported at the ESC congress in August also documented that the benefit from empagliflozin treatment was roughly the same regardless of the age of patients enrolled in the trial and regardless of patients’ blood level of LDL cholesterol at entry into the study. These findings provide “confidence in the consistency of the effect” by empagliflozin, Dr. Fitchett said.
The endocrinologists’ view
“Most cardiologists are not thoroughly familiar with the full palette of medications for hyperglycemia. Selection of medication should not be made solely on the basis of results from a cardiovascular outcomes trial,” said Helena W. Rodbard, MD, a clinical endocrinologist in Rockville, Md.
“The EMPA-REG OUTCOMES and LEADER results are very exciting and encouraging. When all other factors are equal, the cardiovascular results could sway the decision about which medication to use. But an endocrinologist is in the best position to balance the many factors when choosing combination therapy and to set a target level for HbA1c, fasting blood glucose, and postprandial glucose, and to adjust therapy to minimize the risk of hypoglycemia,” Dr. Rodbard said in an interview.
He called empagliflozin a drug with “interesting promise,” especially for patients with incipient heart failure. The extra cardiovascular benefit from the GLP-1 analogues is “less settled,” although the liraglutide and semaglutide trial results are important and mean these drugs need more consideration and study. The EMPA-REG results were more clearly positive, he said.
“Metformin is still the initial drug” for most patients with type 2 diabetes, echoed Dr. Levy. Drugs like empagliflozin and liraglutide are usually used in combination with metformin.
“Like many endocrinologists, I have for some time used the oral SGLT-2 inhibitors and GLP-1 analogues in combination with metformin. It made sense before the recent cardiovascular data appeared, and it makes even more sense now,” said Dr. Jellinger, professor of clinical medicine and an endocrinologist at the University of Miami.
“Endocrinologists and diabetologists are aware that cardiologists have been taking a larger role in the care of patients with diabetes,” noted Dr. Rodbard. “I favor cardiologists and endocrinologists working in concert to improve the care of patients with diabetes.”
“Over the next few years, we will need to decide whether to treat patients with type 2 diabetes with an agent with proven benefits,” said Dr. Fitchett. “Until the results from EMPA-REG and the LEADER trial came out, there was no specific glucose-lowering agent that also reduced cardiovascular events. Some cardiologists might ask when they should get involved in managing patients with type 2 diabetes. What I would do for patients with a history of cardiovascular disease who develop new type 2 diabetes is start empagliflozin as their first drug,” Dr. Fitchett said, though he admitted that no evidence yet exists to back that approach.
The EMPA-REG trial was sponsored by Boehringer Ingelheim and by Eli Lilly, the companies that market empagliflozin. The LEADER trial was sponsored in part by Novo Nordisk, the company that markets liraglutide. Dr. Fitchett and Dr. Mentz were both researchers for EMPA-REG. Dr. Fitchett has been a consultant to AstraZeneca, Merck, and Amgen. Dr. Mentz has been an adviser to Boehringer Ingelheim. Dr. Fonarow has been an adviser to Amgen, Janssen, Novartis, and ZS Pharma. Dr. Bozkurt had no disclosures. Dr. Bonow has been a consultant to Gilead. Dr. Jellinger has been a speaker on behalf of Boehringer-Ingelheim, Novo Nordisk, Merck, and Janssen. Dr. Rodbard has been a consultant to or speaker for several drug companies including Boehringer-Ingelheim, Eli Lilly, and Novo Nordisk. Dr. Levy has been a speaker on behalf of Boehringer-Ingelheim, Eli Lilly, Novo Nordisk, and AstraZeneca. Dr. Hellman had no disclosures.
[email protected]
On Twitter @mitchelzoler
The dramatic reduction in cardiovascular death and heart failure hospitalization seen during treatment with empagliflozin (Jardiance) in the EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) trial, for example, has prompted some cardiologists in the year since the first EMPA-REG report to become active prescribers of the drug to their patients who have type 2 diabetes and cardiovascular disease. The same evidence has driven other cardiologists who may not feel fully comfortable prescribing an antidiabetic drug on their own to enter into active partnerships with endocrinologists to work as a team to put diabetes patients with cardiovascular disease on empagliflozin.
In Dr. Fitchett’s practice, “if a patient with type 2 diabetes has an endocrinologist, then I will send a letter to that physician saying I think the patient should be on one of these drugs,” empagliflozin or liraglutide, he said. “If the patient is being treated by a primary care physician, then I will prescribe empagliflozin myself because most primary care physicians are not willing to prescribe it. I think more and more cardiologists are doing this. The great thing about empagliflozin and liraglutide is that they do not cause hypoglycemia and the adverse effect profiles are relatively good. As long as drug cost is not an issue, then as cardiologists we need to adjust glycemia control with cardiovascular benefit as we did years ago with statin treatment,” explained Dr. Fitchett, a cardiologist at St. Michael’s Hospital in Toronto and a senior collaborator and coauthor on the EMPA-REG study.
When results from the 4S [Scandinavian Simvastatin Survival Study] came out in 1994, proving that long-term statin treatment was both safe and increased survival in patients with coronary heart disease, “cardiologists took over lipid management from endocrinologists,” he recalled. “We now have a safe and simple treatment for glucose lowering that also cuts cardiovascular disease events, so cardiologists have to also be involved, at least to some extent. Their degree of involvement depends on their practice and who provides a patient’s primary diabetes care,” he said.
Cardiologists vary on empagliflozin
Other cardiologists are mixed in their take on personally prescribing antidiabetic drugs to high-risk patients with type 2 diabetes. Greg C. Fonarow, MD, has also aggressively taken to empagliflozin over the past year, especially for his patients with heart failure or at high risk for developing heart failure. The EMPA-REG results showed that empagliflozin’s potent impact on reducing cardiovascular death in patients linked closely with a reduction in heart failure hospitalizations. In his recent experience, endocrinologists as well as other physicians who care for patients with type 2 diabetes “are often reluctant to make any changes [in a patient’s hypoglycemic regimen], and in general they have not gravitated toward the treatments that have been shown to improve cardiovascular outcomes and instead focus solely on a patient’s hemoglobin A1c,” Dr. Fonarow said in an interview at the recent annual meeting of the Heart Failure Society of America.
He said he prescribes empagliflozin to patients with type 2 diabetes if they are hospitalized for heart failure or as outpatients, and he targets it to patients diagnosed with heart failure – including heart failure with preserved ejection fraction – as well as to patients with other forms of cardiovascular disease, closely following the EMPA-REG enrollment criteria. It’s too early in the experience with empagliflozin to use it preferentially in diabetes patients without cardiovascular disease or patients who in any other way fall outside the enrollment criteria for EMPA-REG, he said.
“I am happy to consult with their endocrinologist, or I tell patients to discuss this treatment with their endocrinologist. If the endocrinologist prescribes empagliflozin, great; if not, I feel an obligation to provide the best care I can to my patients. This is not a hard medication to use. The safety profile is good. Treatment with empagliflozin obviously has renal-function considerations, but that’s true for many drugs. The biggest challenge is what is covered by the patient’s insurance. We often need preauthorization.
“So far I have seen excellent responses in patients for both metabolic control and clinical responses in patients with heart failure. Their symptoms seem to improve,” said Dr. Fonarow, professor of medicine and co-chief of cardiology at the University of Southern California , Los Angeles.
While Dr. Fonarow cautioned that he also would not start empagliflozin in a patient with a HbA1c below 7%, he would seriously consider swapping out a patient’s drug for empagliflozin if it were a sulfonylurea or a dipeptidyl peptidase-4 inhibitor. He stopped short of suggesting a substitution of empagliflozin for metformin. In Dr. Fonarow’s opinion, the evidence for empagliflozin is also “more robust” than it has been for liraglutide or semaglutide. With what’s now known about the clinical impact of these drugs, he foresees a time when a combination between a SGLT-2 inhibitor, with its effect on heart failure, and a GLP-1 analogue, with its effect on atherosclerotic disease, may seem an ideal initial drug pairing for patients with type 2 diabetes and significant cardiovascular disease risk, with metformin relegated to a second-line role.
Other cardiologists endorsed a more collaborative approach to prescribing empagliflozin and liraglutide.
Another team-approach advocate is Robert O. Bonow, MD, cardiologist and professor of medicine at Northwestern University in Chicago. “Cardiologists are comfortable prescribing metformin and telling patients about lifestyle, but when it comes to newer antidiabetic drugs, that’s a new field, and a team approach may be best,” he said in an interview. “If possible, a cardiologist should have a friendly partnership with a diabetologist or endocrinologist who is expert in treating diabetes.” Many cardiologists now work in and for hospitals, and easy access to an endocrinologist is probably available, he noted.
But new analyses of the EMPA-REG data reported by Dr. Fitchett at the ESC congress showed that empagliflozin treatment exerted a similar benefit of reduced cardiovascular death regardless of whether patients had prevalent heart failure at entry into the study, incident heart failure during follow-up, or no heart failure of any sort.
Impact of heart failure in EMPA-REG
Roughly 10% of the 7,020 patients enrolled in EMPA-REG had heart failure at the time they entered the trial. During a median follow-up of just over 3 years, the incidence of new-onset heart failure – tallied as either a new heart failure hospitalization or a clinical episode deemed to be heart failure by an investigator – occurred in 4.6% of patients on empagliflozin and in 6.5% of patients in the placebo arm, a 1.9-percentage-point difference and a 30% relative risk reduction linked with empagliflozin use, Dr. Fitchett reported.
The main EMPA-REG outcome was a composite of cardiovascular death, nonfatal MI, and nonfatal stroke. This positive outcome in favor of empagliflozin treatment was primarily driven by a difference in the rate of cardiovascular death. In the new analysis, the relative reduction in cardiovascular deaths with empagliflozin compared with placebo was 29% among patients with prevalent heart failure at baseline, 35% among those who had an incident heart failure hospitalization during follow-up, 27% among patients with an incident heart failure episode diagnosed by an investigator during follow-up, 33% among the combined group of trial patients with any form of heart failure at trial entry or during the trial (those with prevalent heart failure at baseline plus those with an incident event), and 37% among the large number of patients in the trial who remained free from any indication of heart failure during follow-up.
In short, treatment with empagliflozin “reduced cardiovascular mortality by the same relative amount” regardless of whether patients did or did not have heart failure during the trial,” Dr. Fitchett concluded.
Additional secondary analyses from EMPA-REG reported at the ESC congress in August also documented that the benefit from empagliflozin treatment was roughly the same regardless of the age of patients enrolled in the trial and regardless of patients’ blood level of LDL cholesterol at entry into the study. These findings provide “confidence in the consistency of the effect” by empagliflozin, Dr. Fitchett said.
The endocrinologists’ view
“Most cardiologists are not thoroughly familiar with the full palette of medications for hyperglycemia. Selection of medication should not be made solely on the basis of results from a cardiovascular outcomes trial,” said Helena W. Rodbard, MD, a clinical endocrinologist in Rockville, Md.
“The EMPA-REG OUTCOMES and LEADER results are very exciting and encouraging. When all other factors are equal, the cardiovascular results could sway the decision about which medication to use. But an endocrinologist is in the best position to balance the many factors when choosing combination therapy and to set a target level for HbA1c, fasting blood glucose, and postprandial glucose, and to adjust therapy to minimize the risk of hypoglycemia,” Dr. Rodbard said in an interview.
He called empagliflozin a drug with “interesting promise,” especially for patients with incipient heart failure. The extra cardiovascular benefit from the GLP-1 analogues is “less settled,” although the liraglutide and semaglutide trial results are important and mean these drugs need more consideration and study. The EMPA-REG results were more clearly positive, he said.
“Metformin is still the initial drug” for most patients with type 2 diabetes, echoed Dr. Levy. Drugs like empagliflozin and liraglutide are usually used in combination with metformin.
“Like many endocrinologists, I have for some time used the oral SGLT-2 inhibitors and GLP-1 analogues in combination with metformin. It made sense before the recent cardiovascular data appeared, and it makes even more sense now,” said Dr. Jellinger, professor of clinical medicine and an endocrinologist at the University of Miami.
“Endocrinologists and diabetologists are aware that cardiologists have been taking a larger role in the care of patients with diabetes,” noted Dr. Rodbard. “I favor cardiologists and endocrinologists working in concert to improve the care of patients with diabetes.”
“Over the next few years, we will need to decide whether to treat patients with type 2 diabetes with an agent with proven benefits,” said Dr. Fitchett. “Until the results from EMPA-REG and the LEADER trial came out, there was no specific glucose-lowering agent that also reduced cardiovascular events. Some cardiologists might ask when they should get involved in managing patients with type 2 diabetes. What I would do for patients with a history of cardiovascular disease who develop new type 2 diabetes is start empagliflozin as their first drug,” Dr. Fitchett said, though he admitted that no evidence yet exists to back that approach.
The EMPA-REG trial was sponsored by Boehringer Ingelheim and by Eli Lilly, the companies that market empagliflozin. The LEADER trial was sponsored in part by Novo Nordisk, the company that markets liraglutide. Dr. Fitchett and Dr. Mentz were both researchers for EMPA-REG. Dr. Fitchett has been a consultant to AstraZeneca, Merck, and Amgen. Dr. Mentz has been an adviser to Boehringer Ingelheim. Dr. Fonarow has been an adviser to Amgen, Janssen, Novartis, and ZS Pharma. Dr. Bozkurt had no disclosures. Dr. Bonow has been a consultant to Gilead. Dr. Jellinger has been a speaker on behalf of Boehringer-Ingelheim, Novo Nordisk, Merck, and Janssen. Dr. Rodbard has been a consultant to or speaker for several drug companies including Boehringer-Ingelheim, Eli Lilly, and Novo Nordisk. Dr. Levy has been a speaker on behalf of Boehringer-Ingelheim, Eli Lilly, Novo Nordisk, and AstraZeneca. Dr. Hellman had no disclosures.
[email protected]
On Twitter @mitchelzoler
The dramatic reduction in cardiovascular death and heart failure hospitalization seen during treatment with empagliflozin (Jardiance) in the EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) trial, for example, has prompted some cardiologists in the year since the first EMPA-REG report to become active prescribers of the drug to their patients who have type 2 diabetes and cardiovascular disease. The same evidence has driven other cardiologists who may not feel fully comfortable prescribing an antidiabetic drug on their own to enter into active partnerships with endocrinologists to work as a team to put diabetes patients with cardiovascular disease on empagliflozin.
In Dr. Fitchett’s practice, “if a patient with type 2 diabetes has an endocrinologist, then I will send a letter to that physician saying I think the patient should be on one of these drugs,” empagliflozin or liraglutide, he said. “If the patient is being treated by a primary care physician, then I will prescribe empagliflozin myself because most primary care physicians are not willing to prescribe it. I think more and more cardiologists are doing this. The great thing about empagliflozin and liraglutide is that they do not cause hypoglycemia and the adverse effect profiles are relatively good. As long as drug cost is not an issue, then as cardiologists we need to adjust glycemia control with cardiovascular benefit as we did years ago with statin treatment,” explained Dr. Fitchett, a cardiologist at St. Michael’s Hospital in Toronto and a senior collaborator and coauthor on the EMPA-REG study.
When results from the 4S [Scandinavian Simvastatin Survival Study] came out in 1994, proving that long-term statin treatment was both safe and increased survival in patients with coronary heart disease, “cardiologists took over lipid management from endocrinologists,” he recalled. “We now have a safe and simple treatment for glucose lowering that also cuts cardiovascular disease events, so cardiologists have to also be involved, at least to some extent. Their degree of involvement depends on their practice and who provides a patient’s primary diabetes care,” he said.
Cardiologists vary on empagliflozin
Other cardiologists are mixed in their take on personally prescribing antidiabetic drugs to high-risk patients with type 2 diabetes. Greg C. Fonarow, MD, has also aggressively taken to empagliflozin over the past year, especially for his patients with heart failure or at high risk for developing heart failure. The EMPA-REG results showed that empagliflozin’s potent impact on reducing cardiovascular death in patients linked closely with a reduction in heart failure hospitalizations. In his recent experience, endocrinologists as well as other physicians who care for patients with type 2 diabetes “are often reluctant to make any changes [in a patient’s hypoglycemic regimen], and in general they have not gravitated toward the treatments that have been shown to improve cardiovascular outcomes and instead focus solely on a patient’s hemoglobin A1c,” Dr. Fonarow said in an interview at the recent annual meeting of the Heart Failure Society of America.
He said he prescribes empagliflozin to patients with type 2 diabetes if they are hospitalized for heart failure or as outpatients, and he targets it to patients diagnosed with heart failure – including heart failure with preserved ejection fraction – as well as to patients with other forms of cardiovascular disease, closely following the EMPA-REG enrollment criteria. It’s too early in the experience with empagliflozin to use it preferentially in diabetes patients without cardiovascular disease or patients who in any other way fall outside the enrollment criteria for EMPA-REG, he said.
“I am happy to consult with their endocrinologist, or I tell patients to discuss this treatment with their endocrinologist. If the endocrinologist prescribes empagliflozin, great; if not, I feel an obligation to provide the best care I can to my patients. This is not a hard medication to use. The safety profile is good. Treatment with empagliflozin obviously has renal-function considerations, but that’s true for many drugs. The biggest challenge is what is covered by the patient’s insurance. We often need preauthorization.
“So far I have seen excellent responses in patients for both metabolic control and clinical responses in patients with heart failure. Their symptoms seem to improve,” said Dr. Fonarow, professor of medicine and co-chief of cardiology at the University of Southern California , Los Angeles.
While Dr. Fonarow cautioned that he also would not start empagliflozin in a patient with a HbA1c below 7%, he would seriously consider swapping out a patient’s drug for empagliflozin if it were a sulfonylurea or a dipeptidyl peptidase-4 inhibitor. He stopped short of suggesting a substitution of empagliflozin for metformin. In Dr. Fonarow’s opinion, the evidence for empagliflozin is also “more robust” than it has been for liraglutide or semaglutide. With what’s now known about the clinical impact of these drugs, he foresees a time when a combination between a SGLT-2 inhibitor, with its effect on heart failure, and a GLP-1 analogue, with its effect on atherosclerotic disease, may seem an ideal initial drug pairing for patients with type 2 diabetes and significant cardiovascular disease risk, with metformin relegated to a second-line role.
Other cardiologists endorsed a more collaborative approach to prescribing empagliflozin and liraglutide.
Another team-approach advocate is Robert O. Bonow, MD, cardiologist and professor of medicine at Northwestern University in Chicago. “Cardiologists are comfortable prescribing metformin and telling patients about lifestyle, but when it comes to newer antidiabetic drugs, that’s a new field, and a team approach may be best,” he said in an interview. “If possible, a cardiologist should have a friendly partnership with a diabetologist or endocrinologist who is expert in treating diabetes.” Many cardiologists now work in and for hospitals, and easy access to an endocrinologist is probably available, he noted.
But new analyses of the EMPA-REG data reported by Dr. Fitchett at the ESC congress showed that empagliflozin treatment exerted a similar benefit of reduced cardiovascular death regardless of whether patients had prevalent heart failure at entry into the study, incident heart failure during follow-up, or no heart failure of any sort.
Impact of heart failure in EMPA-REG
Roughly 10% of the 7,020 patients enrolled in EMPA-REG had heart failure at the time they entered the trial. During a median follow-up of just over 3 years, the incidence of new-onset heart failure – tallied as either a new heart failure hospitalization or a clinical episode deemed to be heart failure by an investigator – occurred in 4.6% of patients on empagliflozin and in 6.5% of patients in the placebo arm, a 1.9-percentage-point difference and a 30% relative risk reduction linked with empagliflozin use, Dr. Fitchett reported.
The main EMPA-REG outcome was a composite of cardiovascular death, nonfatal MI, and nonfatal stroke. This positive outcome in favor of empagliflozin treatment was primarily driven by a difference in the rate of cardiovascular death. In the new analysis, the relative reduction in cardiovascular deaths with empagliflozin compared with placebo was 29% among patients with prevalent heart failure at baseline, 35% among those who had an incident heart failure hospitalization during follow-up, 27% among patients with an incident heart failure episode diagnosed by an investigator during follow-up, 33% among the combined group of trial patients with any form of heart failure at trial entry or during the trial (those with prevalent heart failure at baseline plus those with an incident event), and 37% among the large number of patients in the trial who remained free from any indication of heart failure during follow-up.
In short, treatment with empagliflozin “reduced cardiovascular mortality by the same relative amount” regardless of whether patients did or did not have heart failure during the trial,” Dr. Fitchett concluded.
Additional secondary analyses from EMPA-REG reported at the ESC congress in August also documented that the benefit from empagliflozin treatment was roughly the same regardless of the age of patients enrolled in the trial and regardless of patients’ blood level of LDL cholesterol at entry into the study. These findings provide “confidence in the consistency of the effect” by empagliflozin, Dr. Fitchett said.
The endocrinologists’ view
“Most cardiologists are not thoroughly familiar with the full palette of medications for hyperglycemia. Selection of medication should not be made solely on the basis of results from a cardiovascular outcomes trial,” said Helena W. Rodbard, MD, a clinical endocrinologist in Rockville, Md.
“The EMPA-REG OUTCOMES and LEADER results are very exciting and encouraging. When all other factors are equal, the cardiovascular results could sway the decision about which medication to use. But an endocrinologist is in the best position to balance the many factors when choosing combination therapy and to set a target level for HbA1c, fasting blood glucose, and postprandial glucose, and to adjust therapy to minimize the risk of hypoglycemia,” Dr. Rodbard said in an interview.
He called empagliflozin a drug with “interesting promise,” especially for patients with incipient heart failure. The extra cardiovascular benefit from the GLP-1 analogues is “less settled,” although the liraglutide and semaglutide trial results are important and mean these drugs need more consideration and study. The EMPA-REG results were more clearly positive, he said.
“Metformin is still the initial drug” for most patients with type 2 diabetes, echoed Dr. Levy. Drugs like empagliflozin and liraglutide are usually used in combination with metformin.
“Like many endocrinologists, I have for some time used the oral SGLT-2 inhibitors and GLP-1 analogues in combination with metformin. It made sense before the recent cardiovascular data appeared, and it makes even more sense now,” said Dr. Jellinger, professor of clinical medicine and an endocrinologist at the University of Miami.
“Endocrinologists and diabetologists are aware that cardiologists have been taking a larger role in the care of patients with diabetes,” noted Dr. Rodbard. “I favor cardiologists and endocrinologists working in concert to improve the care of patients with diabetes.”
“Over the next few years, we will need to decide whether to treat patients with type 2 diabetes with an agent with proven benefits,” said Dr. Fitchett. “Until the results from EMPA-REG and the LEADER trial came out, there was no specific glucose-lowering agent that also reduced cardiovascular events. Some cardiologists might ask when they should get involved in managing patients with type 2 diabetes. What I would do for patients with a history of cardiovascular disease who develop new type 2 diabetes is start empagliflozin as their first drug,” Dr. Fitchett said, though he admitted that no evidence yet exists to back that approach.
The EMPA-REG trial was sponsored by Boehringer Ingelheim and by Eli Lilly, the companies that market empagliflozin. The LEADER trial was sponsored in part by Novo Nordisk, the company that markets liraglutide. Dr. Fitchett and Dr. Mentz were both researchers for EMPA-REG. Dr. Fitchett has been a consultant to AstraZeneca, Merck, and Amgen. Dr. Mentz has been an adviser to Boehringer Ingelheim. Dr. Fonarow has been an adviser to Amgen, Janssen, Novartis, and ZS Pharma. Dr. Bozkurt had no disclosures. Dr. Bonow has been a consultant to Gilead. Dr. Jellinger has been a speaker on behalf of Boehringer-Ingelheim, Novo Nordisk, Merck, and Janssen. Dr. Rodbard has been a consultant to or speaker for several drug companies including Boehringer-Ingelheim, Eli Lilly, and Novo Nordisk. Dr. Levy has been a speaker on behalf of Boehringer-Ingelheim, Eli Lilly, Novo Nordisk, and AstraZeneca. Dr. Hellman had no disclosures.
[email protected]
On Twitter @mitchelzoler