User login
Yes, pediatric practices can provide immunizations without going broke
SAN FRANCISCO – With a little number crunching and strategizing, pediatric practices can provide immunizations to their patients without getting financially soaked, according to Chip Hart, a pediatric practice management consultant.
He discussed various pitfalls and challenges when it comes to the business aspects of providing immunizations, and offered some solutions at the annual meeting of the American Academy of Pediatrics.
His company has collected data suggesting that as of 2015, revenue from vaccine products made up fully 21% of all revenue in private pediatric practices, a near doubling from the value in 2003. As a consultant today, “I try to find out how practices manage the vaccines because, after staff, it’s your biggest expense,” he noted.
Spotting hidden costs
In its business case, the AAP determined that direct and indirect expenses for vaccine product total to 17% to 28% of the cost. In other words, “if you buy a vaccine for $100, you need to collect somewhere between $117 and $128, on average, just to break even,” Mr. Hart explained.
What accounts for that extra expense? Carrying costs that are commonly overlooked, namely, those myriad costs of providing immunizations that accrue before a child is given any vaccine and that can add up quickly.
They include the costs of the refrigerator and examination table; the sharps and waste management; insurance to cover vaccine loss; vaccine wastage and denials; and opportunity cost, that is, the cost of not being able to invest the funds tied up in vaccine sitting in the fridge – some $75,000 to $100,000 for the average practice – elsewhere.
Add to those personnel costs; costs related to activities such as ordering, inventory and storage management, registry input, and temperature monitoring; and malpractice coverage. And not to be forgotten is the inability to collect payment for some vaccines.
“You’re not paid for carrying costs. Unfortunately, society or the American health care system has given pediatricians this burden,” Mr. Hart commented.
Doing the math
Pediatricians can get a handle on the true costs to their practice of providing immunizations by spending just an hour or two crunching some key numbers, according to Mr. Hart.
They should start by ascertaining those carrying costs. For example, assuming hazardous waste costs run $3,500 per year, vaccines account for 50% of the waste, and the practice gives 13,000 vaccines annually, it averages out to $0.13 per vaccine.
Similar calculations are done to determine the costs of administering the shot (preparing, administering, counseling, billing, recording, putting it in the registry, and so on), arriving at about $12 per vaccine. The largest share here comes from clinicians, so calculations focus on their hourly wages and the percent of their time spent on vaccines.
Next is a calculation of the cost of the vaccine product. This calculation starts with the hypothetical invoiced amount of $100, factors in units that are wasted or go unpaid (at least 5%, according to AAP data), and tacks on the distributed carrying costs, arriving finally at an actual cost to the practice of about $120.
Last, all of these data are loaded into a payer-specific spreadsheet. Commonly, payers go by Red Book values and will therefore cover, for example, only $98 of that $100 invoice cost of the vaccine. But they will pay roughly $27 for its administration.
Taken together, the math suggests the practice bears a total cost of $132 for this vaccine ($120 for the product and $12 for its administration) but will collect only $125 from this payer ($98 for the product and $27 for its administration).
“You see over and over again that the payers underpay for the vaccines and pay you well for the administration, and it very often makes up the difference,” Mr. Hart noted. “But even with that boost on the admin side, this practice is losing money on this vaccine – they get $125 for something that costs them $132.”
Practices strapped for time can use some estimates in their spreadsheets instead, he said. “If you use an assumption of 25% over your invoice” – roughly the midpoint between the AAP’s 17% and 28% – “and $12 to $15 on your administration” – based on the value found in a study using time-motion analysis (Pediatrics. 2009 Dec;124 Suppl 5:S492-8) – “for your costs, all you need is your fee schedule, and you can make a spreadsheet to find out whether it makes sense to continue giving immunizations to this payer’s kids.”
Striving for profitability
“In all honesty, from what I see nationally, pediatricians break even on vaccines. It’s a break-even situation, on average,” Mr. Hart commented. “But who wants to be average? No one. We want you to actually be profitable with vaccines because it’s the only way you can continue to give them.”
Practices can take a variety of steps toward that goal. First, they should negotiate payments with payers, using the AAP’s business case and other literature. “Don’t listen to anybody” who says you can’t negotiate, he stressed. “You can negotiate. I don’t care if you’re a solo practice or you’ve just opened. If a payer says they can’t negotiate, they are fibbing to you. The only payers who don’t negotiate are the state Medicaid and Medicare. Everyone else can and does.”
Second, practices should ensure that they are using proper Current Procedural Terminology codes when submitting claims to payers to maximize payment.
“I still see too many practices who don’t bill for these properly,” Mr. Hart commented. “If you have a typical pediatric practice and you use more 90471s and 90472s than 90460s and 90461s, and frankly, if [the latter] aren’t two to three to four to five times more common… you are losing a lot of money.”
Third, practices should join or confirm that they belong to an effective group purchasing organization (GPO) to reduce their vaccine costs, with data suggesting that doing so will save the practice $10,000 to $15,000 per physician each year.
“If you are solo, out on the furthest edge of Alaska, you can see Russia from your house, and you have no leverage whatsoever, you can sign up with one of these GPOs and you are as strong as any hospital,” he said. The AAP helps here as well, by maintaining a list of GPOs on its website.
Fourth, practices should review their vaccine delivery work flow to look for money leaks, Mr. Hart advised. For example, physicians who get caught up in tasks such as ordering and inventorying are losing revenue that could come in from seeing patients.
“This is the sort of thing that affects your bottom line substantially. And it’s exactly the sort of thing that is an invisible expense: the business owners don’t consider their time as part of the expense of doing this administration,” he said.
Additionally, legacy procedures should be re-evaluated to see if they can be streamlined. Gains also may be made here from investing in better technology, such as a refrigerator with a glass door that saves time by allowing ready identification of vaccines.
Finally, practices should join the AAP’s Section on Administration and Practice Management (SOAPM) as it’s an invaluable, interactive resource in this area when questions or challenges arise, Mr. Hart recommended.
[email protected]
SAN FRANCISCO – With a little number crunching and strategizing, pediatric practices can provide immunizations to their patients without getting financially soaked, according to Chip Hart, a pediatric practice management consultant.
He discussed various pitfalls and challenges when it comes to the business aspects of providing immunizations, and offered some solutions at the annual meeting of the American Academy of Pediatrics.
His company has collected data suggesting that as of 2015, revenue from vaccine products made up fully 21% of all revenue in private pediatric practices, a near doubling from the value in 2003. As a consultant today, “I try to find out how practices manage the vaccines because, after staff, it’s your biggest expense,” he noted.
Spotting hidden costs
In its business case, the AAP determined that direct and indirect expenses for vaccine product total to 17% to 28% of the cost. In other words, “if you buy a vaccine for $100, you need to collect somewhere between $117 and $128, on average, just to break even,” Mr. Hart explained.
What accounts for that extra expense? Carrying costs that are commonly overlooked, namely, those myriad costs of providing immunizations that accrue before a child is given any vaccine and that can add up quickly.
They include the costs of the refrigerator and examination table; the sharps and waste management; insurance to cover vaccine loss; vaccine wastage and denials; and opportunity cost, that is, the cost of not being able to invest the funds tied up in vaccine sitting in the fridge – some $75,000 to $100,000 for the average practice – elsewhere.
Add to those personnel costs; costs related to activities such as ordering, inventory and storage management, registry input, and temperature monitoring; and malpractice coverage. And not to be forgotten is the inability to collect payment for some vaccines.
“You’re not paid for carrying costs. Unfortunately, society or the American health care system has given pediatricians this burden,” Mr. Hart commented.
Doing the math
Pediatricians can get a handle on the true costs to their practice of providing immunizations by spending just an hour or two crunching some key numbers, according to Mr. Hart.
They should start by ascertaining those carrying costs. For example, assuming hazardous waste costs run $3,500 per year, vaccines account for 50% of the waste, and the practice gives 13,000 vaccines annually, it averages out to $0.13 per vaccine.
Similar calculations are done to determine the costs of administering the shot (preparing, administering, counseling, billing, recording, putting it in the registry, and so on), arriving at about $12 per vaccine. The largest share here comes from clinicians, so calculations focus on their hourly wages and the percent of their time spent on vaccines.
Next is a calculation of the cost of the vaccine product. This calculation starts with the hypothetical invoiced amount of $100, factors in units that are wasted or go unpaid (at least 5%, according to AAP data), and tacks on the distributed carrying costs, arriving finally at an actual cost to the practice of about $120.
Last, all of these data are loaded into a payer-specific spreadsheet. Commonly, payers go by Red Book values and will therefore cover, for example, only $98 of that $100 invoice cost of the vaccine. But they will pay roughly $27 for its administration.
Taken together, the math suggests the practice bears a total cost of $132 for this vaccine ($120 for the product and $12 for its administration) but will collect only $125 from this payer ($98 for the product and $27 for its administration).
“You see over and over again that the payers underpay for the vaccines and pay you well for the administration, and it very often makes up the difference,” Mr. Hart noted. “But even with that boost on the admin side, this practice is losing money on this vaccine – they get $125 for something that costs them $132.”
Practices strapped for time can use some estimates in their spreadsheets instead, he said. “If you use an assumption of 25% over your invoice” – roughly the midpoint between the AAP’s 17% and 28% – “and $12 to $15 on your administration” – based on the value found in a study using time-motion analysis (Pediatrics. 2009 Dec;124 Suppl 5:S492-8) – “for your costs, all you need is your fee schedule, and you can make a spreadsheet to find out whether it makes sense to continue giving immunizations to this payer’s kids.”
Striving for profitability
“In all honesty, from what I see nationally, pediatricians break even on vaccines. It’s a break-even situation, on average,” Mr. Hart commented. “But who wants to be average? No one. We want you to actually be profitable with vaccines because it’s the only way you can continue to give them.”
Practices can take a variety of steps toward that goal. First, they should negotiate payments with payers, using the AAP’s business case and other literature. “Don’t listen to anybody” who says you can’t negotiate, he stressed. “You can negotiate. I don’t care if you’re a solo practice or you’ve just opened. If a payer says they can’t negotiate, they are fibbing to you. The only payers who don’t negotiate are the state Medicaid and Medicare. Everyone else can and does.”
Second, practices should ensure that they are using proper Current Procedural Terminology codes when submitting claims to payers to maximize payment.
“I still see too many practices who don’t bill for these properly,” Mr. Hart commented. “If you have a typical pediatric practice and you use more 90471s and 90472s than 90460s and 90461s, and frankly, if [the latter] aren’t two to three to four to five times more common… you are losing a lot of money.”
Third, practices should join or confirm that they belong to an effective group purchasing organization (GPO) to reduce their vaccine costs, with data suggesting that doing so will save the practice $10,000 to $15,000 per physician each year.
“If you are solo, out on the furthest edge of Alaska, you can see Russia from your house, and you have no leverage whatsoever, you can sign up with one of these GPOs and you are as strong as any hospital,” he said. The AAP helps here as well, by maintaining a list of GPOs on its website.
Fourth, practices should review their vaccine delivery work flow to look for money leaks, Mr. Hart advised. For example, physicians who get caught up in tasks such as ordering and inventorying are losing revenue that could come in from seeing patients.
“This is the sort of thing that affects your bottom line substantially. And it’s exactly the sort of thing that is an invisible expense: the business owners don’t consider their time as part of the expense of doing this administration,” he said.
Additionally, legacy procedures should be re-evaluated to see if they can be streamlined. Gains also may be made here from investing in better technology, such as a refrigerator with a glass door that saves time by allowing ready identification of vaccines.
Finally, practices should join the AAP’s Section on Administration and Practice Management (SOAPM) as it’s an invaluable, interactive resource in this area when questions or challenges arise, Mr. Hart recommended.
[email protected]
SAN FRANCISCO – With a little number crunching and strategizing, pediatric practices can provide immunizations to their patients without getting financially soaked, according to Chip Hart, a pediatric practice management consultant.
He discussed various pitfalls and challenges when it comes to the business aspects of providing immunizations, and offered some solutions at the annual meeting of the American Academy of Pediatrics.
His company has collected data suggesting that as of 2015, revenue from vaccine products made up fully 21% of all revenue in private pediatric practices, a near doubling from the value in 2003. As a consultant today, “I try to find out how practices manage the vaccines because, after staff, it’s your biggest expense,” he noted.
Spotting hidden costs
In its business case, the AAP determined that direct and indirect expenses for vaccine product total to 17% to 28% of the cost. In other words, “if you buy a vaccine for $100, you need to collect somewhere between $117 and $128, on average, just to break even,” Mr. Hart explained.
What accounts for that extra expense? Carrying costs that are commonly overlooked, namely, those myriad costs of providing immunizations that accrue before a child is given any vaccine and that can add up quickly.
They include the costs of the refrigerator and examination table; the sharps and waste management; insurance to cover vaccine loss; vaccine wastage and denials; and opportunity cost, that is, the cost of not being able to invest the funds tied up in vaccine sitting in the fridge – some $75,000 to $100,000 for the average practice – elsewhere.
Add to those personnel costs; costs related to activities such as ordering, inventory and storage management, registry input, and temperature monitoring; and malpractice coverage. And not to be forgotten is the inability to collect payment for some vaccines.
“You’re not paid for carrying costs. Unfortunately, society or the American health care system has given pediatricians this burden,” Mr. Hart commented.
Doing the math
Pediatricians can get a handle on the true costs to their practice of providing immunizations by spending just an hour or two crunching some key numbers, according to Mr. Hart.
They should start by ascertaining those carrying costs. For example, assuming hazardous waste costs run $3,500 per year, vaccines account for 50% of the waste, and the practice gives 13,000 vaccines annually, it averages out to $0.13 per vaccine.
Similar calculations are done to determine the costs of administering the shot (preparing, administering, counseling, billing, recording, putting it in the registry, and so on), arriving at about $12 per vaccine. The largest share here comes from clinicians, so calculations focus on their hourly wages and the percent of their time spent on vaccines.
Next is a calculation of the cost of the vaccine product. This calculation starts with the hypothetical invoiced amount of $100, factors in units that are wasted or go unpaid (at least 5%, according to AAP data), and tacks on the distributed carrying costs, arriving finally at an actual cost to the practice of about $120.
Last, all of these data are loaded into a payer-specific spreadsheet. Commonly, payers go by Red Book values and will therefore cover, for example, only $98 of that $100 invoice cost of the vaccine. But they will pay roughly $27 for its administration.
Taken together, the math suggests the practice bears a total cost of $132 for this vaccine ($120 for the product and $12 for its administration) but will collect only $125 from this payer ($98 for the product and $27 for its administration).
“You see over and over again that the payers underpay for the vaccines and pay you well for the administration, and it very often makes up the difference,” Mr. Hart noted. “But even with that boost on the admin side, this practice is losing money on this vaccine – they get $125 for something that costs them $132.”
Practices strapped for time can use some estimates in their spreadsheets instead, he said. “If you use an assumption of 25% over your invoice” – roughly the midpoint between the AAP’s 17% and 28% – “and $12 to $15 on your administration” – based on the value found in a study using time-motion analysis (Pediatrics. 2009 Dec;124 Suppl 5:S492-8) – “for your costs, all you need is your fee schedule, and you can make a spreadsheet to find out whether it makes sense to continue giving immunizations to this payer’s kids.”
Striving for profitability
“In all honesty, from what I see nationally, pediatricians break even on vaccines. It’s a break-even situation, on average,” Mr. Hart commented. “But who wants to be average? No one. We want you to actually be profitable with vaccines because it’s the only way you can continue to give them.”
Practices can take a variety of steps toward that goal. First, they should negotiate payments with payers, using the AAP’s business case and other literature. “Don’t listen to anybody” who says you can’t negotiate, he stressed. “You can negotiate. I don’t care if you’re a solo practice or you’ve just opened. If a payer says they can’t negotiate, they are fibbing to you. The only payers who don’t negotiate are the state Medicaid and Medicare. Everyone else can and does.”
Second, practices should ensure that they are using proper Current Procedural Terminology codes when submitting claims to payers to maximize payment.
“I still see too many practices who don’t bill for these properly,” Mr. Hart commented. “If you have a typical pediatric practice and you use more 90471s and 90472s than 90460s and 90461s, and frankly, if [the latter] aren’t two to three to four to five times more common… you are losing a lot of money.”
Third, practices should join or confirm that they belong to an effective group purchasing organization (GPO) to reduce their vaccine costs, with data suggesting that doing so will save the practice $10,000 to $15,000 per physician each year.
“If you are solo, out on the furthest edge of Alaska, you can see Russia from your house, and you have no leverage whatsoever, you can sign up with one of these GPOs and you are as strong as any hospital,” he said. The AAP helps here as well, by maintaining a list of GPOs on its website.
Fourth, practices should review their vaccine delivery work flow to look for money leaks, Mr. Hart advised. For example, physicians who get caught up in tasks such as ordering and inventorying are losing revenue that could come in from seeing patients.
“This is the sort of thing that affects your bottom line substantially. And it’s exactly the sort of thing that is an invisible expense: the business owners don’t consider their time as part of the expense of doing this administration,” he said.
Additionally, legacy procedures should be re-evaluated to see if they can be streamlined. Gains also may be made here from investing in better technology, such as a refrigerator with a glass door that saves time by allowing ready identification of vaccines.
Finally, practices should join the AAP’s Section on Administration and Practice Management (SOAPM) as it’s an invaluable, interactive resource in this area when questions or challenges arise, Mr. Hart recommended.
[email protected]
AT AAP 16
Treating upper GI diseases: Where do we go from here?
WASHINGTON – The American Gastroenterological Association, the Food and Drug Administration, pharmaceutical companies, and patient advocacy groups came together for a first-of-its-kind meeting, a program of the AGA Center for Diagnostics and Therapeutics, to discuss new and emerging drugs for the treatment of four key GI diseases, highlighting the promise that these treatments show and the hurdles they face to gain approval.
“If we look at the AGA’s Burden of GI Disease Survey, published a few years ago in Gastroenterology, [you] can see that there are millions of visits to primary care and specialists for a variety of upper gastrointestinal symptoms, so clearly upper GI disorders still pose a major burden to our health care environment,” explained Colin W. Howden, MD, AGAF, chair of the AGA Center for Diagnostics and Therapeutics from the University of Tennessee in Memphis.

While proton pump inhibitors (PPIs) continue to be the first line of management for GERD, severe cases often require a stronger approach. Alternatives that are being investigated include potassium-competitive acid blockers, for which there have been clinical trials. However, no advantage over PPIs was demonstrated in any trials. Another alternative is bile salt binders, for which at least one trial is currently underway. Dr. Vela was unable to say when findings are expected to be published.
Another approach to managing GERD is to treat the acid pocket itself by using alginate. A randomized study by Rohof et al. investigated this in 2013, comparing Gaviscon and antacid; although the population size was small (n = 16), investigators concluded that “alginate-antacid raft localizes to the postprandial acid pocket and displaces it below the diaphragm to reduce postprandial acid reflux [making it] an appropriate therapy for postprandial acid reflux.”
Another new drug is lesogaberan, a GABA-B agonist that was examined in a 2010 randomized, double-blind crossover study by Boeckxstaens et al. While also a small trial (n = 21), the findings indicated that the drug is a good option for those with only partial response to PPIs, as it decreased the number of transient lower esophageal sphincter relaxations (TLESRs) and reflux episodes, and increased LES pressure [in] patients with reflux symptoms. Work to inhibit transient LES relaxations also is being done, but so far lesogaberan, arbaclofen, and ADX 10059 (an mGluR5 modulator) programs have all been halted because of side effects or insufficient efficacy findings.
Prokinetics are also being looked at, with drugs such as metoclopramide being examined for efficacy, although to this point, the drug has shown “no improvement in acid exposure or esophageal clearance [when] compared to placebo,” according to Dr. Vela. Other drugs that are available outside the United States include domperidone, itopride, and mosapride, but Dr. Vela, who led a 2014 report on these therapies, stated that benefits offered by these are modest, and studies investigating them are limited in number.
Also being looked at are rebamipide, which can be used a cytoprotective agent that increases prostaglandin production. A 2010 study by Yoshida et al. found that lansoprazole (15 mg/day), combined with 300 mg/day of rebamipide, was significantly better at preventing relapse within a year than was just the former medication taken on its own. Additionally, there are conceptual studies examining topical protection to maintain mucosal integrity, nociceptor blockades, and imipramine.
Regarding trials for GERD, Robyn Carson, director of health economics & outcomes research at Allergan stated that the company recently redefined what constitutes GERD in order to help refocus what its drugs were trying to do.
“I think we’ve operationalized it very recently in terms of exclusion and GERD,” she explained. “The way we define it is ‘active GERD’ with two or more episodes a week of heartburn, so it was very much focused on the heartburn and PPI use was acceptable.”
Following the GERD discussion, panelists talked about what’s coming up in the realm of EoE. Stuart J. Spechler, MD, of the University of Texas Southwestern in Dallas, discussed ongoing research into treating EoE as an antigen-driven disorder, noting that about half of all EoE patients have a history of atopic disease – such as rhinitis, asthma, and atopic dermatitis – and exhibit sensitization to food or other aeroallergens. Furthermore, about 3% of patients who undergo oral immunotherapy to treat a food allergy develop EoE.
But in terms of what to take EoE research forward, Dr. Spechler called for a shift away from trying to distinguish between EoE and GERD, arguing that GERD contributes to EoE pathogenesis, and vice versa. PPIs can and should be used in EoE for the same reasons that they’re used to treat GERD, he explained.
“We need a shift in focus [because] I don’t think it’s likely to be that productive a line of research,” Dr. Spechler said. “The two diseases often coexist.”
To attack gastroparesis, P. Jay Pasricha, MD explained that a number of trials examining several drugs have shown that they are either ineffective or “do not correlate with improvement in gastric emptying.” These drugs include cisapride, tegaserod, botulinum toxin, mitemcinal, camcinal, TZP 102, and relamorelin.
“I’m not going to talk about emerging biomarkers because there isn’t a lot to talk about with biomarkers that hasn’t already been said,” stated Dr. Pasricha of Johns Hopkins University in Baltimore adding that his focus would largely be focused on emerging therapies and treatment targets.
A 2013 study by Parkman et al. investigated the effects of nortriptyline on mitigating idiopathic gastroparesis symptoms, finding that there was no significant difference in symptoms among patients who took the drug, versus those who took a placebo. In terms of using antinauseants to alleviate symptoms, dopamine receptor antagonists continue to be commonly prescribed, but they have their limitations. Metoclopramide, though approved since 1986, can be used for only 12 weeks, has acute and chronic side effects such as mood and irreversible movement disorders, and a black box warning imposed on it by the FDA in 2009 for tardive dyskinesia. One drug approved in India, though not by the FDA, is domperidone, which has no side effects to the central nervous system but does raise cardiovascular concerns in patients with “mild hERG affinity.”
Currently, the APRON trial is investigating the efficacy of aprepitant to relieve chronic nausea and vomiting in gastroparesis patients. Those enrolled in the study are all at least 18 years old, have undergone gastric-emptying scintigraphy, and either a normal upper endoscopy or an upper GI series within the 2 years prior to enrollment, have symptoms of chronic nausea or vomiting consistent with a functional gastric disorder for at least 6 months before enrollment, and nausea defined as “significant” by a visual analog scale score of at least 25 mm.
“Continuing to focus solely on accelerating gastric emptying is a failed strategy,” said Dr. Pasricha, adding that research needs to focus on the unique aspects of the disease’s biology, including pathogenic similarities with functional dyspepsia.
To that end, the final disease covered was functional dyspepsia. In terms of ongoing or planned clinical studies, Jan Tack, MD, from the University of Leuven (Belgium), mentioned three. Two of them are multicenter controlled trials investigating acotiamide, one in Europe and the other in India, for the management of functional dyspepsia and postprandial distress syndrome, while the other is a multicenter study examining rikkunshito, a traditional Japanese medicine. Additionally, ongoing or planned mechanistic studies include single-center controlled trials in Belgium on the efficacy of acotiamide and rikkunshito for intragastric pressure, as well as another Belgian study analyzing the impact of monoacylglycerol lipase inhibitors on intragastric pressure in patients that have functional dyspepsia with “impaired accommodation.”
Meal-related symptoms, nutrient challenge tests, and intragastric pressure measurements should all become short-term pathophysiology and efficacy markers, said Dr. Tack, adding that it’s also important for new therapeutic targets to include gastric emptying, hypersensitivity, and duodenal alterations, if necessary.
Hurdles persist in getting drugs through the approval process, however. Juli Tomaino, MD, of the FDA’s Center for Drug Evaluation and Research, explained where many proposed drugs run into issues in the regulatory process.
“We really have to know what we’re diagnosing, so the regulatory pathway to any of these approvals will really depend on the independent patient population, it will depend on the mechanism of action of the drug, what the drug is able to do and not do, and how you’re going to design that trial to target whatever that drug can do,” Dr. Tomaino said.
The issue of labeling also factors in, according to her. “We know that patients with acid-mediated heartburn do well on PPIs, but if they’re having different symptoms due to different mechanisms of action, then you have to design that drug with that patient population in mind, and that’s what the labeling would look like,” she explained. “So I’m not saying that it would necessarily have to list all the enrollment criteria, all the enrichment techniques that we use in that trial, but it would be a description of the intended patient population and what the drug would do.”
Mrs. Carson also chimed in on the topic of trial difficulties, saying that “[Irritable bowel syndrome] and [chronic idiopathic constipation] became quite an impediment to recruitment, and I think as we get farther away from the complete overlapping conditions, I think that’s where in discussions with the [Qualification Review Team] at FDA, they recognize that [we should] track that and let this evidence drive the next step,” adding that “we’ll have data on that shortly.”
The AGA Center for Diagnostics and Therapeutics will be issuing white papers on each of the four upper GI disorders discussed at the meeting.
Dr. Howden disclosed that he is a consultant for Aralez, Ironwood, Allergan, Otsuka, and SynteractHCR; an expert witness for Allergan; and coeditor of Alimentary Pharmacology & Therapeutics. Dr. Spechler disclosed that he is a consultant for Interpace Diagnostics, Takeda Pharmaceuticals, and Ironwood Pharmaceuticals. Dr. Pasricha disclosed that he is the cofounder of Neurogastrx, OrphoMed, and ETX Pharma, and is a consultant for Vanda and Allergan. Dr. Tack disclosed that he is a consultant for Abide, Allergan, AstraZeneca, Danone, El Pharma, Menarini, Novartis, Ono, Shire, Takeda, Theravance, Tsumura, and Zeria, as well as being on several of their advisory boards and speakers bureaus. Dr. Vela is a consultant for Medtronic and Torax.*
*Additions were made to the story on 11/8/2016 and 11/18/2016.
WASHINGTON – The American Gastroenterological Association, the Food and Drug Administration, pharmaceutical companies, and patient advocacy groups came together for a first-of-its-kind meeting, a program of the AGA Center for Diagnostics and Therapeutics, to discuss new and emerging drugs for the treatment of four key GI diseases, highlighting the promise that these treatments show and the hurdles they face to gain approval.
“If we look at the AGA’s Burden of GI Disease Survey, published a few years ago in Gastroenterology, [you] can see that there are millions of visits to primary care and specialists for a variety of upper gastrointestinal symptoms, so clearly upper GI disorders still pose a major burden to our health care environment,” explained Colin W. Howden, MD, AGAF, chair of the AGA Center for Diagnostics and Therapeutics from the University of Tennessee in Memphis.

While proton pump inhibitors (PPIs) continue to be the first line of management for GERD, severe cases often require a stronger approach. Alternatives that are being investigated include potassium-competitive acid blockers, for which there have been clinical trials. However, no advantage over PPIs was demonstrated in any trials. Another alternative is bile salt binders, for which at least one trial is currently underway. Dr. Vela was unable to say when findings are expected to be published.
Another approach to managing GERD is to treat the acid pocket itself by using alginate. A randomized study by Rohof et al. investigated this in 2013, comparing Gaviscon and antacid; although the population size was small (n = 16), investigators concluded that “alginate-antacid raft localizes to the postprandial acid pocket and displaces it below the diaphragm to reduce postprandial acid reflux [making it] an appropriate therapy for postprandial acid reflux.”
Another new drug is lesogaberan, a GABA-B agonist that was examined in a 2010 randomized, double-blind crossover study by Boeckxstaens et al. While also a small trial (n = 21), the findings indicated that the drug is a good option for those with only partial response to PPIs, as it decreased the number of transient lower esophageal sphincter relaxations (TLESRs) and reflux episodes, and increased LES pressure [in] patients with reflux symptoms. Work to inhibit transient LES relaxations also is being done, but so far lesogaberan, arbaclofen, and ADX 10059 (an mGluR5 modulator) programs have all been halted because of side effects or insufficient efficacy findings.
Prokinetics are also being looked at, with drugs such as metoclopramide being examined for efficacy, although to this point, the drug has shown “no improvement in acid exposure or esophageal clearance [when] compared to placebo,” according to Dr. Vela. Other drugs that are available outside the United States include domperidone, itopride, and mosapride, but Dr. Vela, who led a 2014 report on these therapies, stated that benefits offered by these are modest, and studies investigating them are limited in number.
Also being looked at are rebamipide, which can be used a cytoprotective agent that increases prostaglandin production. A 2010 study by Yoshida et al. found that lansoprazole (15 mg/day), combined with 300 mg/day of rebamipide, was significantly better at preventing relapse within a year than was just the former medication taken on its own. Additionally, there are conceptual studies examining topical protection to maintain mucosal integrity, nociceptor blockades, and imipramine.
Regarding trials for GERD, Robyn Carson, director of health economics & outcomes research at Allergan stated that the company recently redefined what constitutes GERD in order to help refocus what its drugs were trying to do.
“I think we’ve operationalized it very recently in terms of exclusion and GERD,” she explained. “The way we define it is ‘active GERD’ with two or more episodes a week of heartburn, so it was very much focused on the heartburn and PPI use was acceptable.”
Following the GERD discussion, panelists talked about what’s coming up in the realm of EoE. Stuart J. Spechler, MD, of the University of Texas Southwestern in Dallas, discussed ongoing research into treating EoE as an antigen-driven disorder, noting that about half of all EoE patients have a history of atopic disease – such as rhinitis, asthma, and atopic dermatitis – and exhibit sensitization to food or other aeroallergens. Furthermore, about 3% of patients who undergo oral immunotherapy to treat a food allergy develop EoE.
But in terms of what to take EoE research forward, Dr. Spechler called for a shift away from trying to distinguish between EoE and GERD, arguing that GERD contributes to EoE pathogenesis, and vice versa. PPIs can and should be used in EoE for the same reasons that they’re used to treat GERD, he explained.
“We need a shift in focus [because] I don’t think it’s likely to be that productive a line of research,” Dr. Spechler said. “The two diseases often coexist.”
To attack gastroparesis, P. Jay Pasricha, MD explained that a number of trials examining several drugs have shown that they are either ineffective or “do not correlate with improvement in gastric emptying.” These drugs include cisapride, tegaserod, botulinum toxin, mitemcinal, camcinal, TZP 102, and relamorelin.
“I’m not going to talk about emerging biomarkers because there isn’t a lot to talk about with biomarkers that hasn’t already been said,” stated Dr. Pasricha of Johns Hopkins University in Baltimore adding that his focus would largely be focused on emerging therapies and treatment targets.
A 2013 study by Parkman et al. investigated the effects of nortriptyline on mitigating idiopathic gastroparesis symptoms, finding that there was no significant difference in symptoms among patients who took the drug, versus those who took a placebo. In terms of using antinauseants to alleviate symptoms, dopamine receptor antagonists continue to be commonly prescribed, but they have their limitations. Metoclopramide, though approved since 1986, can be used for only 12 weeks, has acute and chronic side effects such as mood and irreversible movement disorders, and a black box warning imposed on it by the FDA in 2009 for tardive dyskinesia. One drug approved in India, though not by the FDA, is domperidone, which has no side effects to the central nervous system but does raise cardiovascular concerns in patients with “mild hERG affinity.”
Currently, the APRON trial is investigating the efficacy of aprepitant to relieve chronic nausea and vomiting in gastroparesis patients. Those enrolled in the study are all at least 18 years old, have undergone gastric-emptying scintigraphy, and either a normal upper endoscopy or an upper GI series within the 2 years prior to enrollment, have symptoms of chronic nausea or vomiting consistent with a functional gastric disorder for at least 6 months before enrollment, and nausea defined as “significant” by a visual analog scale score of at least 25 mm.
“Continuing to focus solely on accelerating gastric emptying is a failed strategy,” said Dr. Pasricha, adding that research needs to focus on the unique aspects of the disease’s biology, including pathogenic similarities with functional dyspepsia.
To that end, the final disease covered was functional dyspepsia. In terms of ongoing or planned clinical studies, Jan Tack, MD, from the University of Leuven (Belgium), mentioned three. Two of them are multicenter controlled trials investigating acotiamide, one in Europe and the other in India, for the management of functional dyspepsia and postprandial distress syndrome, while the other is a multicenter study examining rikkunshito, a traditional Japanese medicine. Additionally, ongoing or planned mechanistic studies include single-center controlled trials in Belgium on the efficacy of acotiamide and rikkunshito for intragastric pressure, as well as another Belgian study analyzing the impact of monoacylglycerol lipase inhibitors on intragastric pressure in patients that have functional dyspepsia with “impaired accommodation.”
Meal-related symptoms, nutrient challenge tests, and intragastric pressure measurements should all become short-term pathophysiology and efficacy markers, said Dr. Tack, adding that it’s also important for new therapeutic targets to include gastric emptying, hypersensitivity, and duodenal alterations, if necessary.
Hurdles persist in getting drugs through the approval process, however. Juli Tomaino, MD, of the FDA’s Center for Drug Evaluation and Research, explained where many proposed drugs run into issues in the regulatory process.
“We really have to know what we’re diagnosing, so the regulatory pathway to any of these approvals will really depend on the independent patient population, it will depend on the mechanism of action of the drug, what the drug is able to do and not do, and how you’re going to design that trial to target whatever that drug can do,” Dr. Tomaino said.
The issue of labeling also factors in, according to her. “We know that patients with acid-mediated heartburn do well on PPIs, but if they’re having different symptoms due to different mechanisms of action, then you have to design that drug with that patient population in mind, and that’s what the labeling would look like,” she explained. “So I’m not saying that it would necessarily have to list all the enrollment criteria, all the enrichment techniques that we use in that trial, but it would be a description of the intended patient population and what the drug would do.”
Mrs. Carson also chimed in on the topic of trial difficulties, saying that “[Irritable bowel syndrome] and [chronic idiopathic constipation] became quite an impediment to recruitment, and I think as we get farther away from the complete overlapping conditions, I think that’s where in discussions with the [Qualification Review Team] at FDA, they recognize that [we should] track that and let this evidence drive the next step,” adding that “we’ll have data on that shortly.”
The AGA Center for Diagnostics and Therapeutics will be issuing white papers on each of the four upper GI disorders discussed at the meeting.
Dr. Howden disclosed that he is a consultant for Aralez, Ironwood, Allergan, Otsuka, and SynteractHCR; an expert witness for Allergan; and coeditor of Alimentary Pharmacology & Therapeutics. Dr. Spechler disclosed that he is a consultant for Interpace Diagnostics, Takeda Pharmaceuticals, and Ironwood Pharmaceuticals. Dr. Pasricha disclosed that he is the cofounder of Neurogastrx, OrphoMed, and ETX Pharma, and is a consultant for Vanda and Allergan. Dr. Tack disclosed that he is a consultant for Abide, Allergan, AstraZeneca, Danone, El Pharma, Menarini, Novartis, Ono, Shire, Takeda, Theravance, Tsumura, and Zeria, as well as being on several of their advisory boards and speakers bureaus. Dr. Vela is a consultant for Medtronic and Torax.*
*Additions were made to the story on 11/8/2016 and 11/18/2016.
WASHINGTON – The American Gastroenterological Association, the Food and Drug Administration, pharmaceutical companies, and patient advocacy groups came together for a first-of-its-kind meeting, a program of the AGA Center for Diagnostics and Therapeutics, to discuss new and emerging drugs for the treatment of four key GI diseases, highlighting the promise that these treatments show and the hurdles they face to gain approval.
“If we look at the AGA’s Burden of GI Disease Survey, published a few years ago in Gastroenterology, [you] can see that there are millions of visits to primary care and specialists for a variety of upper gastrointestinal symptoms, so clearly upper GI disorders still pose a major burden to our health care environment,” explained Colin W. Howden, MD, AGAF, chair of the AGA Center for Diagnostics and Therapeutics from the University of Tennessee in Memphis.

While proton pump inhibitors (PPIs) continue to be the first line of management for GERD, severe cases often require a stronger approach. Alternatives that are being investigated include potassium-competitive acid blockers, for which there have been clinical trials. However, no advantage over PPIs was demonstrated in any trials. Another alternative is bile salt binders, for which at least one trial is currently underway. Dr. Vela was unable to say when findings are expected to be published.
Another approach to managing GERD is to treat the acid pocket itself by using alginate. A randomized study by Rohof et al. investigated this in 2013, comparing Gaviscon and antacid; although the population size was small (n = 16), investigators concluded that “alginate-antacid raft localizes to the postprandial acid pocket and displaces it below the diaphragm to reduce postprandial acid reflux [making it] an appropriate therapy for postprandial acid reflux.”
Another new drug is lesogaberan, a GABA-B agonist that was examined in a 2010 randomized, double-blind crossover study by Boeckxstaens et al. While also a small trial (n = 21), the findings indicated that the drug is a good option for those with only partial response to PPIs, as it decreased the number of transient lower esophageal sphincter relaxations (TLESRs) and reflux episodes, and increased LES pressure [in] patients with reflux symptoms. Work to inhibit transient LES relaxations also is being done, but so far lesogaberan, arbaclofen, and ADX 10059 (an mGluR5 modulator) programs have all been halted because of side effects or insufficient efficacy findings.
Prokinetics are also being looked at, with drugs such as metoclopramide being examined for efficacy, although to this point, the drug has shown “no improvement in acid exposure or esophageal clearance [when] compared to placebo,” according to Dr. Vela. Other drugs that are available outside the United States include domperidone, itopride, and mosapride, but Dr. Vela, who led a 2014 report on these therapies, stated that benefits offered by these are modest, and studies investigating them are limited in number.
Also being looked at are rebamipide, which can be used a cytoprotective agent that increases prostaglandin production. A 2010 study by Yoshida et al. found that lansoprazole (15 mg/day), combined with 300 mg/day of rebamipide, was significantly better at preventing relapse within a year than was just the former medication taken on its own. Additionally, there are conceptual studies examining topical protection to maintain mucosal integrity, nociceptor blockades, and imipramine.
Regarding trials for GERD, Robyn Carson, director of health economics & outcomes research at Allergan stated that the company recently redefined what constitutes GERD in order to help refocus what its drugs were trying to do.
“I think we’ve operationalized it very recently in terms of exclusion and GERD,” she explained. “The way we define it is ‘active GERD’ with two or more episodes a week of heartburn, so it was very much focused on the heartburn and PPI use was acceptable.”
Following the GERD discussion, panelists talked about what’s coming up in the realm of EoE. Stuart J. Spechler, MD, of the University of Texas Southwestern in Dallas, discussed ongoing research into treating EoE as an antigen-driven disorder, noting that about half of all EoE patients have a history of atopic disease – such as rhinitis, asthma, and atopic dermatitis – and exhibit sensitization to food or other aeroallergens. Furthermore, about 3% of patients who undergo oral immunotherapy to treat a food allergy develop EoE.
But in terms of what to take EoE research forward, Dr. Spechler called for a shift away from trying to distinguish between EoE and GERD, arguing that GERD contributes to EoE pathogenesis, and vice versa. PPIs can and should be used in EoE for the same reasons that they’re used to treat GERD, he explained.
“We need a shift in focus [because] I don’t think it’s likely to be that productive a line of research,” Dr. Spechler said. “The two diseases often coexist.”
To attack gastroparesis, P. Jay Pasricha, MD explained that a number of trials examining several drugs have shown that they are either ineffective or “do not correlate with improvement in gastric emptying.” These drugs include cisapride, tegaserod, botulinum toxin, mitemcinal, camcinal, TZP 102, and relamorelin.
“I’m not going to talk about emerging biomarkers because there isn’t a lot to talk about with biomarkers that hasn’t already been said,” stated Dr. Pasricha of Johns Hopkins University in Baltimore adding that his focus would largely be focused on emerging therapies and treatment targets.
A 2013 study by Parkman et al. investigated the effects of nortriptyline on mitigating idiopathic gastroparesis symptoms, finding that there was no significant difference in symptoms among patients who took the drug, versus those who took a placebo. In terms of using antinauseants to alleviate symptoms, dopamine receptor antagonists continue to be commonly prescribed, but they have their limitations. Metoclopramide, though approved since 1986, can be used for only 12 weeks, has acute and chronic side effects such as mood and irreversible movement disorders, and a black box warning imposed on it by the FDA in 2009 for tardive dyskinesia. One drug approved in India, though not by the FDA, is domperidone, which has no side effects to the central nervous system but does raise cardiovascular concerns in patients with “mild hERG affinity.”
Currently, the APRON trial is investigating the efficacy of aprepitant to relieve chronic nausea and vomiting in gastroparesis patients. Those enrolled in the study are all at least 18 years old, have undergone gastric-emptying scintigraphy, and either a normal upper endoscopy or an upper GI series within the 2 years prior to enrollment, have symptoms of chronic nausea or vomiting consistent with a functional gastric disorder for at least 6 months before enrollment, and nausea defined as “significant” by a visual analog scale score of at least 25 mm.
“Continuing to focus solely on accelerating gastric emptying is a failed strategy,” said Dr. Pasricha, adding that research needs to focus on the unique aspects of the disease’s biology, including pathogenic similarities with functional dyspepsia.
To that end, the final disease covered was functional dyspepsia. In terms of ongoing or planned clinical studies, Jan Tack, MD, from the University of Leuven (Belgium), mentioned three. Two of them are multicenter controlled trials investigating acotiamide, one in Europe and the other in India, for the management of functional dyspepsia and postprandial distress syndrome, while the other is a multicenter study examining rikkunshito, a traditional Japanese medicine. Additionally, ongoing or planned mechanistic studies include single-center controlled trials in Belgium on the efficacy of acotiamide and rikkunshito for intragastric pressure, as well as another Belgian study analyzing the impact of monoacylglycerol lipase inhibitors on intragastric pressure in patients that have functional dyspepsia with “impaired accommodation.”
Meal-related symptoms, nutrient challenge tests, and intragastric pressure measurements should all become short-term pathophysiology and efficacy markers, said Dr. Tack, adding that it’s also important for new therapeutic targets to include gastric emptying, hypersensitivity, and duodenal alterations, if necessary.
Hurdles persist in getting drugs through the approval process, however. Juli Tomaino, MD, of the FDA’s Center for Drug Evaluation and Research, explained where many proposed drugs run into issues in the regulatory process.
“We really have to know what we’re diagnosing, so the regulatory pathway to any of these approvals will really depend on the independent patient population, it will depend on the mechanism of action of the drug, what the drug is able to do and not do, and how you’re going to design that trial to target whatever that drug can do,” Dr. Tomaino said.
The issue of labeling also factors in, according to her. “We know that patients with acid-mediated heartburn do well on PPIs, but if they’re having different symptoms due to different mechanisms of action, then you have to design that drug with that patient population in mind, and that’s what the labeling would look like,” she explained. “So I’m not saying that it would necessarily have to list all the enrollment criteria, all the enrichment techniques that we use in that trial, but it would be a description of the intended patient population and what the drug would do.”
Mrs. Carson also chimed in on the topic of trial difficulties, saying that “[Irritable bowel syndrome] and [chronic idiopathic constipation] became quite an impediment to recruitment, and I think as we get farther away from the complete overlapping conditions, I think that’s where in discussions with the [Qualification Review Team] at FDA, they recognize that [we should] track that and let this evidence drive the next step,” adding that “we’ll have data on that shortly.”
The AGA Center for Diagnostics and Therapeutics will be issuing white papers on each of the four upper GI disorders discussed at the meeting.
Dr. Howden disclosed that he is a consultant for Aralez, Ironwood, Allergan, Otsuka, and SynteractHCR; an expert witness for Allergan; and coeditor of Alimentary Pharmacology & Therapeutics. Dr. Spechler disclosed that he is a consultant for Interpace Diagnostics, Takeda Pharmaceuticals, and Ironwood Pharmaceuticals. Dr. Pasricha disclosed that he is the cofounder of Neurogastrx, OrphoMed, and ETX Pharma, and is a consultant for Vanda and Allergan. Dr. Tack disclosed that he is a consultant for Abide, Allergan, AstraZeneca, Danone, El Pharma, Menarini, Novartis, Ono, Shire, Takeda, Theravance, Tsumura, and Zeria, as well as being on several of their advisory boards and speakers bureaus. Dr. Vela is a consultant for Medtronic and Torax.*
*Additions were made to the story on 11/8/2016 and 11/18/2016.
AT THE AGA DRUG DEVELOPMENT CONFERENCE
Shared medical appointment model shows potential for fibromyalgia patients
Fibromyalgia patients who participated in shared medical appointments staffed by an interdisciplinary team at a rural academic medical center reported high satisfaction with the new care model, according to Nicole M. Orzechowski, DO, and her colleagues in the department of rheumatology at Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
The study of 67 referred patients, who were evaluated during a 1-year period with the shared medical appointment (SMA) model, revealed in post-session surveys that not only did all patients agree that the care model would assist in managing their condition, but 95% also thought that the peer-to-peer interaction was “extremely helpful.”
In the study, which Dr. Orzechowski will report at the annual meeting of the American College of Rheumatology in Washington, she and her associates noted that the 2.5-hour visits conducted by a rheumatologist, two nurse practitioners, a chaplain, and a secretary led to “clinical efficiency in assessing multiple new patients in a defined period of time,” improved wait times for fibromyalgia patients from 3 months to 1 month, and also freed up new patient consultation slots for other conditions. The investigators estimated that the SMA has generated an additional 113 work relative value units since its inception.
Prior to the SMA, each patient received a medical history questionnaire in the mail, and the investigators informed primary care physicians about the SMA and its format and asked them to perform specific labs if they had not been done already. Besides individual exams for confirming the diagnosis, measuring vital signs, and reconciling medications, the SMA consisted of a facilitated discussion by a trained chaplain, followed by a short presentation by the clinician (with time for discussion and questions), and then concluded with the chaplain’s demonstration of mindfulness techniques for managing chronic pain.
In the future, the investigators said that they hope to enhance and optimize the visit, expand its use, address referring provider satisfaction, and examine post-visit resource utilization.
None of the authors had disclosures to report.
Fibromyalgia patients who participated in shared medical appointments staffed by an interdisciplinary team at a rural academic medical center reported high satisfaction with the new care model, according to Nicole M. Orzechowski, DO, and her colleagues in the department of rheumatology at Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
The study of 67 referred patients, who were evaluated during a 1-year period with the shared medical appointment (SMA) model, revealed in post-session surveys that not only did all patients agree that the care model would assist in managing their condition, but 95% also thought that the peer-to-peer interaction was “extremely helpful.”
In the study, which Dr. Orzechowski will report at the annual meeting of the American College of Rheumatology in Washington, she and her associates noted that the 2.5-hour visits conducted by a rheumatologist, two nurse practitioners, a chaplain, and a secretary led to “clinical efficiency in assessing multiple new patients in a defined period of time,” improved wait times for fibromyalgia patients from 3 months to 1 month, and also freed up new patient consultation slots for other conditions. The investigators estimated that the SMA has generated an additional 113 work relative value units since its inception.
Prior to the SMA, each patient received a medical history questionnaire in the mail, and the investigators informed primary care physicians about the SMA and its format and asked them to perform specific labs if they had not been done already. Besides individual exams for confirming the diagnosis, measuring vital signs, and reconciling medications, the SMA consisted of a facilitated discussion by a trained chaplain, followed by a short presentation by the clinician (with time for discussion and questions), and then concluded with the chaplain’s demonstration of mindfulness techniques for managing chronic pain.
In the future, the investigators said that they hope to enhance and optimize the visit, expand its use, address referring provider satisfaction, and examine post-visit resource utilization.
None of the authors had disclosures to report.
Fibromyalgia patients who participated in shared medical appointments staffed by an interdisciplinary team at a rural academic medical center reported high satisfaction with the new care model, according to Nicole M. Orzechowski, DO, and her colleagues in the department of rheumatology at Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
The study of 67 referred patients, who were evaluated during a 1-year period with the shared medical appointment (SMA) model, revealed in post-session surveys that not only did all patients agree that the care model would assist in managing their condition, but 95% also thought that the peer-to-peer interaction was “extremely helpful.”
In the study, which Dr. Orzechowski will report at the annual meeting of the American College of Rheumatology in Washington, she and her associates noted that the 2.5-hour visits conducted by a rheumatologist, two nurse practitioners, a chaplain, and a secretary led to “clinical efficiency in assessing multiple new patients in a defined period of time,” improved wait times for fibromyalgia patients from 3 months to 1 month, and also freed up new patient consultation slots for other conditions. The investigators estimated that the SMA has generated an additional 113 work relative value units since its inception.
Prior to the SMA, each patient received a medical history questionnaire in the mail, and the investigators informed primary care physicians about the SMA and its format and asked them to perform specific labs if they had not been done already. Besides individual exams for confirming the diagnosis, measuring vital signs, and reconciling medications, the SMA consisted of a facilitated discussion by a trained chaplain, followed by a short presentation by the clinician (with time for discussion and questions), and then concluded with the chaplain’s demonstration of mindfulness techniques for managing chronic pain.
In the future, the investigators said that they hope to enhance and optimize the visit, expand its use, address referring provider satisfaction, and examine post-visit resource utilization.
None of the authors had disclosures to report.
FROM THE ACR ANNUAL MEETING
Liletta gets a new inserter: Steps for successful placement
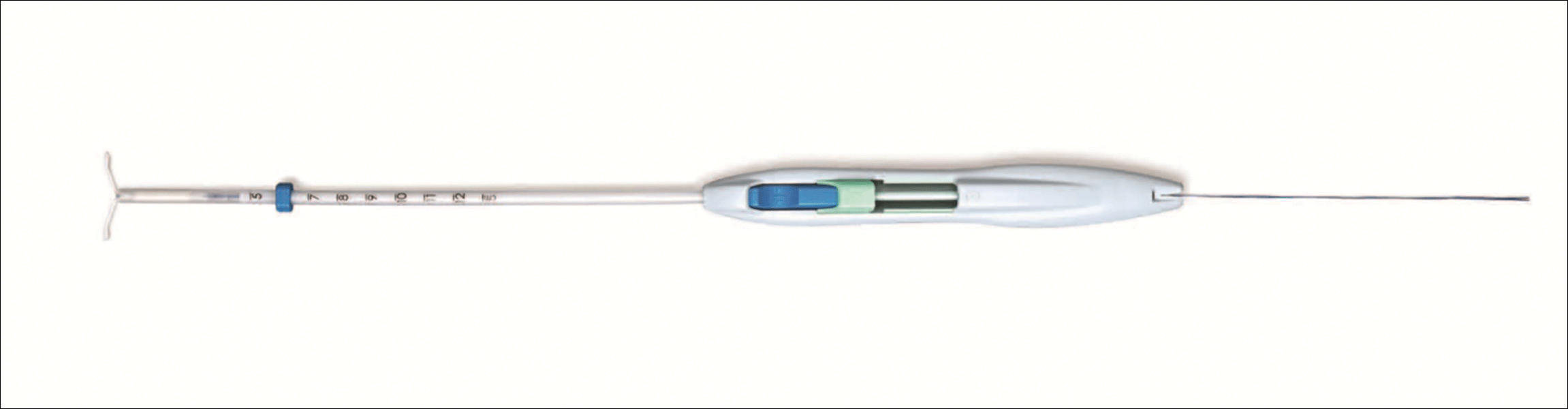
A new single-handed inserter (SHI) for placement of Liletta has been introduced and is currently available (FIGURE 1). Liletta is a levonorgestrel 52-mg intrauterine system (IUS) that is currently approved for 3 years of use as a contraceptive in the United States. The same product in Europe, known as Levosert, is approved for both contraception and the treatment of heavy menstrual bleeding. Liletta, as a branded contraceptive, is being studied through a large Phase 3 clinical trial called ACCESS IUS, which will continue to evaluate the contraceptive efficacy of Liletta for at least 7 years of use.1 A recent publication from the study showed that the levonorgestrel release rates from Liletta are almost identical to the rates reported for the other levonorgestrel 52-mg IUS on the market (Mirena) through 5 years and supports the continued study of the IUS for at least 7 years.2
Related article:
Benefit of self-administered vaginal lidocaine gel in IUD placement
The initial marketing and distribution of Liletta used a two-handed inserter that allowed for successful placement but required multiple steps. The SHI will make loading and placement of Liletta easier for all clinicians. Features of the new SHI include:
- A double-slider mechanism in which the first slider moves into the second slider during IUS placement, providing a tactile sense for the user during the insertion process
- The ability to reload the inserter if needed before placement
- A firm but bendable tube (that is 2 cm longer than other IUS inserters on the market). This length can be helpful for obese patients or postprocedure placements.
- Depth markings to 12 cm on both sides of the insertion tube.
Related article:
2016 Update on contraception
Liletta is an important and unique product in the US market. For most public sector providers and clinics, Liletta costs only $50, significantly less than other long-acting reversible contraceptives. The price of the IUS is only one aspect of its overall cost, however, as women still need to pay for any office visit or insertion fees. Liletta is unique in its pricing. Sales of Liletta in the private sector support the low price in the public sector. As a health care community, even if we do not directly care for women in public-sector settings, we all can help poor women access affordable, effective contraception. For providers, Liletta is a highly effective and lower-cost alternative to currently available hormonal IUS products.
How to insert Liletta
Insertion can be performed using a no-touch technique. The loading and placement technique outlined below should occur after speculum and tenaculum placement, sounding of the uterus, and vaginal preparation as would be performed for any intrauterine contraceptive insertion. Never force the uterine sound or insertion tube through the cervix; if necessary, stop and dilate the cervical canal. The steps below are the main points for successful Liletta placement; see the full product label for more details.3
Related article:
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
The key steps to remember are firm-gentle-firm
- Load the device. While pushing forward firmly on the blue slider (with the number 1), load Liletta by pulling down evenly on the threads. The IUS should be laid on a flat surface in the sterile tray to keep the arms parallel to the floor while pulling on the threads. Once loaded, the threads can be pulled up or down to be locked in the cleft. The tip of the IUS will form a hemispheric dome at the top of the insertion tube.
- Adjust the flange to the measured uterine depth based on sounding using the notch in the tray.
- Place inserter into the cervix. Apply gentle traction on the tenaculum to align the cervical canal and uterine cavity. While still holding firm forward pressure on the blue slider, place the tube through the cervical canal until the flange is approximately 1.5 to 2 cm from the cervix. (FIGURE 2.) Gently slide the blue slider downward until it is flush with the green slider (with the number 2; FIGURE 3). This step will allow the IUS arms to open in the lower uterine segment; wait 10 to 15 seconds for the arms to fully unfold. By sliding gently, you will feel when to stop moving the slider.
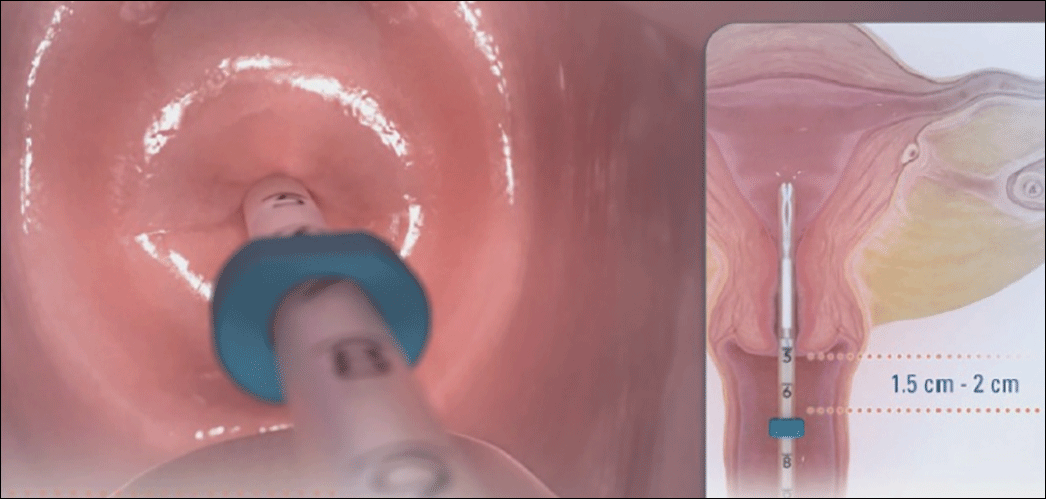 Image copyright Medicines360.
Image copyright Medicines360.Figure 2. Placing the inserter through the cervix 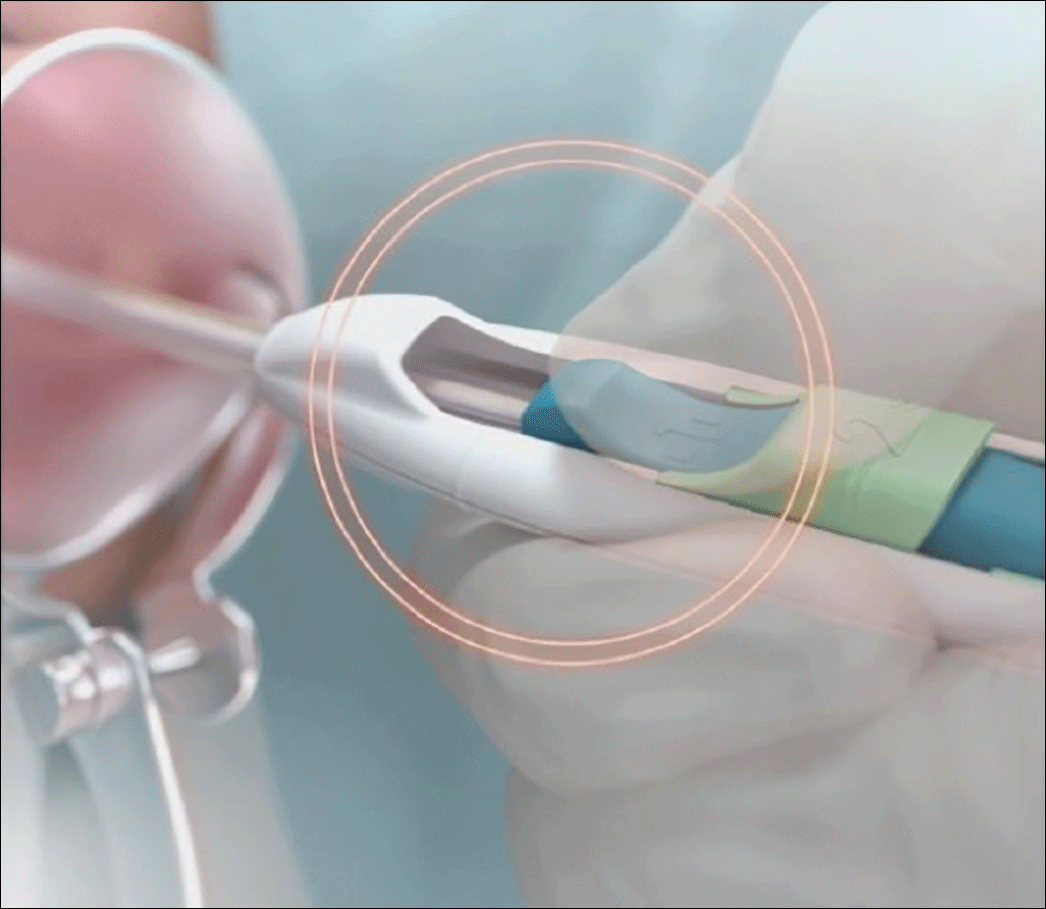 Image copyright Medicines360.
Image copyright Medicines360.Figure 3. Merging the blue and green sliders together to release the IUS arms - Advance the inserter. While holding traction on the tenaculum, advance the inserter to the fundus. You will feel slight resistance when the IUS is at the fundus and the flange should be flush against the cervix.
- Firmly slide both sliders together downward until a click is heard (FIGURE 4). A green indicator will be visible at the bottom of the handle to show that the threads have been ejected from the cleft.
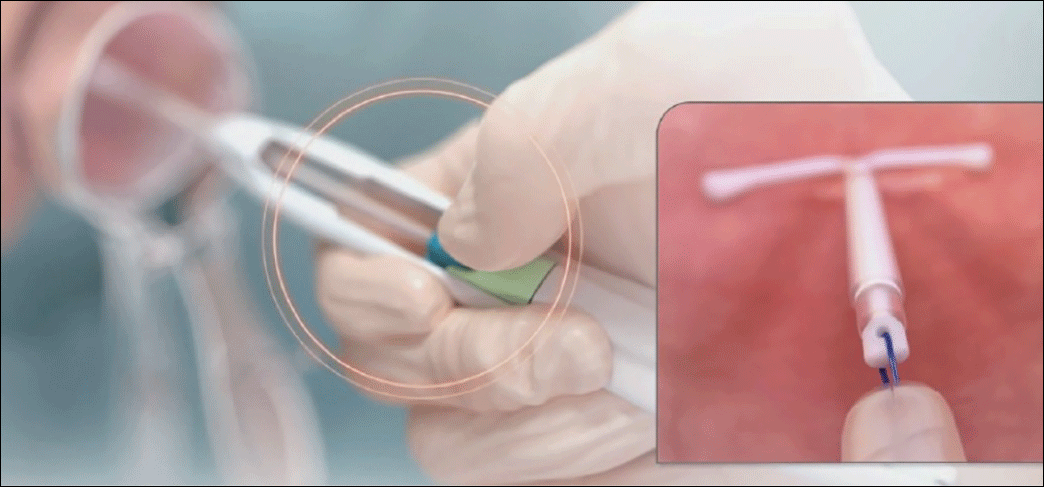 Image copyright Medicines360.
Image copyright Medicines360.Figure 4. Moving the blue and green sliders downward together to release the IUS - Withdraw the inserter from the uterus and cut the threads with blunt tipped scissors approximately 3 cm from the cervical os.
Technique video
Allergan and Medicines360 offer a video demonstrating proper Liletta insertion technique at www.lilettahcp.com. The video includes proper loading of the inserter as well as correct uterine depth measurement and release of Liletta from the inserter. Clinicians are reminded to use aseptic technique during the entire insertion procedure.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD. Three year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10-16.
- Creinin MD, Jansen R, Starr R, Gobburu J, Gopalakrishnan M, Olariu A. Levonorgestrel release rates over 5 years with the Liletta® 52 mg intrauterine system. Contraception. 2016;94(4):353-356.
- Liletta [package insert]. Irvine, California: Allergan USA, Inc., and San Francisco, California: Medicines360; 2016.
A new single-handed inserter (SHI) for placement of Liletta has been introduced and is currently available (FIGURE 1). Liletta is a levonorgestrel 52-mg intrauterine system (IUS) that is currently approved for 3 years of use as a contraceptive in the United States. The same product in Europe, known as Levosert, is approved for both contraception and the treatment of heavy menstrual bleeding. Liletta, as a branded contraceptive, is being studied through a large Phase 3 clinical trial called ACCESS IUS, which will continue to evaluate the contraceptive efficacy of Liletta for at least 7 years of use.1 A recent publication from the study showed that the levonorgestrel release rates from Liletta are almost identical to the rates reported for the other levonorgestrel 52-mg IUS on the market (Mirena) through 5 years and supports the continued study of the IUS for at least 7 years.2

Related article:
Benefit of self-administered vaginal lidocaine gel in IUD placement
The initial marketing and distribution of Liletta used a two-handed inserter that allowed for successful placement but required multiple steps. The SHI will make loading and placement of Liletta easier for all clinicians. Features of the new SHI include:
- A double-slider mechanism in which the first slider moves into the second slider during IUS placement, providing a tactile sense for the user during the insertion process
- The ability to reload the inserter if needed before placement
- A firm but bendable tube (that is 2 cm longer than other IUS inserters on the market). This length can be helpful for obese patients or postprocedure placements.
- Depth markings to 12 cm on both sides of the insertion tube.
Related article:
2016 Update on contraception
Liletta is an important and unique product in the US market. For most public sector providers and clinics, Liletta costs only $50, significantly less than other long-acting reversible contraceptives. The price of the IUS is only one aspect of its overall cost, however, as women still need to pay for any office visit or insertion fees. Liletta is unique in its pricing. Sales of Liletta in the private sector support the low price in the public sector. As a health care community, even if we do not directly care for women in public-sector settings, we all can help poor women access affordable, effective contraception. For providers, Liletta is a highly effective and lower-cost alternative to currently available hormonal IUS products.
How to insert Liletta
Insertion can be performed using a no-touch technique. The loading and placement technique outlined below should occur after speculum and tenaculum placement, sounding of the uterus, and vaginal preparation as would be performed for any intrauterine contraceptive insertion. Never force the uterine sound or insertion tube through the cervix; if necessary, stop and dilate the cervical canal. The steps below are the main points for successful Liletta placement; see the full product label for more details.3
Related article:
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
The key steps to remember are firm-gentle-firm
- Load the device. While pushing forward firmly on the blue slider (with the number 1), load Liletta by pulling down evenly on the threads. The IUS should be laid on a flat surface in the sterile tray to keep the arms parallel to the floor while pulling on the threads. Once loaded, the threads can be pulled up or down to be locked in the cleft. The tip of the IUS will form a hemispheric dome at the top of the insertion tube.
- Adjust the flange to the measured uterine depth based on sounding using the notch in the tray.
- Place inserter into the cervix. Apply gentle traction on the tenaculum to align the cervical canal and uterine cavity. While still holding firm forward pressure on the blue slider, place the tube through the cervical canal until the flange is approximately 1.5 to 2 cm from the cervix. (FIGURE 2.) Gently slide the blue slider downward until it is flush with the green slider (with the number 2; FIGURE 3). This step will allow the IUS arms to open in the lower uterine segment; wait 10 to 15 seconds for the arms to fully unfold. By sliding gently, you will feel when to stop moving the slider.
 Image copyright Medicines360.
Image copyright Medicines360.Figure 2. Placing the inserter through the cervix  Image copyright Medicines360.
Image copyright Medicines360.Figure 3. Merging the blue and green sliders together to release the IUS arms - Advance the inserter. While holding traction on the tenaculum, advance the inserter to the fundus. You will feel slight resistance when the IUS is at the fundus and the flange should be flush against the cervix.
- Firmly slide both sliders together downward until a click is heard (FIGURE 4). A green indicator will be visible at the bottom of the handle to show that the threads have been ejected from the cleft.
 Image copyright Medicines360.
Image copyright Medicines360.Figure 4. Moving the blue and green sliders downward together to release the IUS - Withdraw the inserter from the uterus and cut the threads with blunt tipped scissors approximately 3 cm from the cervical os.
Technique video
Allergan and Medicines360 offer a video demonstrating proper Liletta insertion technique at www.lilettahcp.com. The video includes proper loading of the inserter as well as correct uterine depth measurement and release of Liletta from the inserter. Clinicians are reminded to use aseptic technique during the entire insertion procedure.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
A new single-handed inserter (SHI) for placement of Liletta has been introduced and is currently available (FIGURE 1). Liletta is a levonorgestrel 52-mg intrauterine system (IUS) that is currently approved for 3 years of use as a contraceptive in the United States. The same product in Europe, known as Levosert, is approved for both contraception and the treatment of heavy menstrual bleeding. Liletta, as a branded contraceptive, is being studied through a large Phase 3 clinical trial called ACCESS IUS, which will continue to evaluate the contraceptive efficacy of Liletta for at least 7 years of use.1 A recent publication from the study showed that the levonorgestrel release rates from Liletta are almost identical to the rates reported for the other levonorgestrel 52-mg IUS on the market (Mirena) through 5 years and supports the continued study of the IUS for at least 7 years.2

Related article:
Benefit of self-administered vaginal lidocaine gel in IUD placement
The initial marketing and distribution of Liletta used a two-handed inserter that allowed for successful placement but required multiple steps. The SHI will make loading and placement of Liletta easier for all clinicians. Features of the new SHI include:
- A double-slider mechanism in which the first slider moves into the second slider during IUS placement, providing a tactile sense for the user during the insertion process
- The ability to reload the inserter if needed before placement
- A firm but bendable tube (that is 2 cm longer than other IUS inserters on the market). This length can be helpful for obese patients or postprocedure placements.
- Depth markings to 12 cm on both sides of the insertion tube.
Related article:
2016 Update on contraception
Liletta is an important and unique product in the US market. For most public sector providers and clinics, Liletta costs only $50, significantly less than other long-acting reversible contraceptives. The price of the IUS is only one aspect of its overall cost, however, as women still need to pay for any office visit or insertion fees. Liletta is unique in its pricing. Sales of Liletta in the private sector support the low price in the public sector. As a health care community, even if we do not directly care for women in public-sector settings, we all can help poor women access affordable, effective contraception. For providers, Liletta is a highly effective and lower-cost alternative to currently available hormonal IUS products.
How to insert Liletta
Insertion can be performed using a no-touch technique. The loading and placement technique outlined below should occur after speculum and tenaculum placement, sounding of the uterus, and vaginal preparation as would be performed for any intrauterine contraceptive insertion. Never force the uterine sound or insertion tube through the cervix; if necessary, stop and dilate the cervical canal. The steps below are the main points for successful Liletta placement; see the full product label for more details.3
Related article:
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
The key steps to remember are firm-gentle-firm
- Load the device. While pushing forward firmly on the blue slider (with the number 1), load Liletta by pulling down evenly on the threads. The IUS should be laid on a flat surface in the sterile tray to keep the arms parallel to the floor while pulling on the threads. Once loaded, the threads can be pulled up or down to be locked in the cleft. The tip of the IUS will form a hemispheric dome at the top of the insertion tube.
- Adjust the flange to the measured uterine depth based on sounding using the notch in the tray.
- Place inserter into the cervix. Apply gentle traction on the tenaculum to align the cervical canal and uterine cavity. While still holding firm forward pressure on the blue slider, place the tube through the cervical canal until the flange is approximately 1.5 to 2 cm from the cervix. (FIGURE 2.) Gently slide the blue slider downward until it is flush with the green slider (with the number 2; FIGURE 3). This step will allow the IUS arms to open in the lower uterine segment; wait 10 to 15 seconds for the arms to fully unfold. By sliding gently, you will feel when to stop moving the slider.
 Image copyright Medicines360.
Image copyright Medicines360.Figure 2. Placing the inserter through the cervix  Image copyright Medicines360.
Image copyright Medicines360.Figure 3. Merging the blue and green sliders together to release the IUS arms - Advance the inserter. While holding traction on the tenaculum, advance the inserter to the fundus. You will feel slight resistance when the IUS is at the fundus and the flange should be flush against the cervix.
- Firmly slide both sliders together downward until a click is heard (FIGURE 4). A green indicator will be visible at the bottom of the handle to show that the threads have been ejected from the cleft.
 Image copyright Medicines360.
Image copyright Medicines360.Figure 4. Moving the blue and green sliders downward together to release the IUS - Withdraw the inserter from the uterus and cut the threads with blunt tipped scissors approximately 3 cm from the cervical os.
Technique video
Allergan and Medicines360 offer a video demonstrating proper Liletta insertion technique at www.lilettahcp.com. The video includes proper loading of the inserter as well as correct uterine depth measurement and release of Liletta from the inserter. Clinicians are reminded to use aseptic technique during the entire insertion procedure.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD. Three year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10-16.
- Creinin MD, Jansen R, Starr R, Gobburu J, Gopalakrishnan M, Olariu A. Levonorgestrel release rates over 5 years with the Liletta® 52 mg intrauterine system. Contraception. 2016;94(4):353-356.
- Liletta [package insert]. Irvine, California: Allergan USA, Inc., and San Francisco, California: Medicines360; 2016.
- Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD. Three year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10-16.
- Creinin MD, Jansen R, Starr R, Gobburu J, Gopalakrishnan M, Olariu A. Levonorgestrel release rates over 5 years with the Liletta® 52 mg intrauterine system. Contraception. 2016;94(4):353-356.
- Liletta [package insert]. Irvine, California: Allergan USA, Inc., and San Francisco, California: Medicines360; 2016.
In this article
Obese IVF patients need higher HCG trigger dose
SALT LAKE CITY – Women who are obese should receive more than 5,000 IU of human chorionic gonadotropin (HCG) to trigger final oocyte maturation during in vitro fertilization, findings of a retrospective cohort study suggest.
Fully 23% of obese women had low beta-HCG levels the day after receiving a 5,000 IU HCG trigger dose, compared with 1% of non-obese women (odds ratio, 21.4; 95% confidence interval, 15.0-30.4), reported Mohamad Irani, MD, and his associates from Cornell University, New York. In contrast, 10,000 IU HCG triggered adequate beta-HCG levels in 81% of obese patients with a BMI of 30-40 kg/m2, and in 90% of those whose BMI exceeded 40 kg/m2.
Low beta-HCG levels decreased the chances of oocyte maturation, fertilization, and live birth in the study. “Patients’ BMI should be taken into consideration when determining the dose of HCG trigger,” the investigators concluded in a poster presented at the annual meeting of the American Society for Reproductive Medicine.
Patients with hypothalamic amenorrhea or who are undergoing stimulation with a gonadotropin-releasing hormone (GnRH) agonist are not candidates for a GnRH-agonist trigger and therefore need to receive HCG instead, the researchers noted. To understand how the dose of HCG affects the chances of final oocyte maturation, they studied 19,084 HCG trigger recipients at their center between 2004 and 2013.
By protocol, patients received 10,000 IU HCG if their serum estradiol (E2) level was less than 1,500 pg/mL on the day of trigger; 5,000 IU if it measured 1,501-2,500 pg/mL; 4,000 IU if it was 2,501-3,000 pg/mL; and 3,300 IU if it exceeded 3,000 pg/mL.
The day after HCG trigger, 18,666 patients had beta-HCG levels of at least 50 mIU/mL, while 418 patients had low beta-HCG levels of less than 50 mIU/mL. A comparison of the two groups showed that low beta-HCG was associated with significantly lower rates of oocyte maturation (77% vs. 81%; P less than .001) and fertilization (63% vs. 72%; P less than .001). It was also associated with more than a 30% lower chance of a live birth (adjusted OR, 0.67; 95% CI, 0.5-0.8), even after accounting for age, and the stage and number of embryos transferred.
The researchers also examined response to HCG trigger among non-obese patients. Among patients who were overweight (BMI 25 to 30 kg/m2), the chances of a low beta-HCG level the day after trigger were 14% when the HCG dose was 3,300 IU, 12.5% when it was 4,000 IU, 4.3% when it was 5,000 IU, and 0.4% when it was 10,000 IU.
Among healthy-weight patients (BMI 18.5-25 kg/m2), low beta-HCG levels occurred 3.6% of the time when the HCG dose was 3,300 IU, 2.3% of the time when it was 4,000 IU, 0.6% of the time when it was 5,000 IU, and 0.08% of the time when it was 10,000 IU. Notably, these same doses triggered adequate beta-HCG levels in all 660 patients who were underweight (BMI less than 18.5 kg/m2), the researchers reported.
Dr. Irani reported having no relevant financial disclosures.
SALT LAKE CITY – Women who are obese should receive more than 5,000 IU of human chorionic gonadotropin (HCG) to trigger final oocyte maturation during in vitro fertilization, findings of a retrospective cohort study suggest.
Fully 23% of obese women had low beta-HCG levels the day after receiving a 5,000 IU HCG trigger dose, compared with 1% of non-obese women (odds ratio, 21.4; 95% confidence interval, 15.0-30.4), reported Mohamad Irani, MD, and his associates from Cornell University, New York. In contrast, 10,000 IU HCG triggered adequate beta-HCG levels in 81% of obese patients with a BMI of 30-40 kg/m2, and in 90% of those whose BMI exceeded 40 kg/m2.
Low beta-HCG levels decreased the chances of oocyte maturation, fertilization, and live birth in the study. “Patients’ BMI should be taken into consideration when determining the dose of HCG trigger,” the investigators concluded in a poster presented at the annual meeting of the American Society for Reproductive Medicine.
Patients with hypothalamic amenorrhea or who are undergoing stimulation with a gonadotropin-releasing hormone (GnRH) agonist are not candidates for a GnRH-agonist trigger and therefore need to receive HCG instead, the researchers noted. To understand how the dose of HCG affects the chances of final oocyte maturation, they studied 19,084 HCG trigger recipients at their center between 2004 and 2013.
By protocol, patients received 10,000 IU HCG if their serum estradiol (E2) level was less than 1,500 pg/mL on the day of trigger; 5,000 IU if it measured 1,501-2,500 pg/mL; 4,000 IU if it was 2,501-3,000 pg/mL; and 3,300 IU if it exceeded 3,000 pg/mL.
The day after HCG trigger, 18,666 patients had beta-HCG levels of at least 50 mIU/mL, while 418 patients had low beta-HCG levels of less than 50 mIU/mL. A comparison of the two groups showed that low beta-HCG was associated with significantly lower rates of oocyte maturation (77% vs. 81%; P less than .001) and fertilization (63% vs. 72%; P less than .001). It was also associated with more than a 30% lower chance of a live birth (adjusted OR, 0.67; 95% CI, 0.5-0.8), even after accounting for age, and the stage and number of embryos transferred.
The researchers also examined response to HCG trigger among non-obese patients. Among patients who were overweight (BMI 25 to 30 kg/m2), the chances of a low beta-HCG level the day after trigger were 14% when the HCG dose was 3,300 IU, 12.5% when it was 4,000 IU, 4.3% when it was 5,000 IU, and 0.4% when it was 10,000 IU.
Among healthy-weight patients (BMI 18.5-25 kg/m2), low beta-HCG levels occurred 3.6% of the time when the HCG dose was 3,300 IU, 2.3% of the time when it was 4,000 IU, 0.6% of the time when it was 5,000 IU, and 0.08% of the time when it was 10,000 IU. Notably, these same doses triggered adequate beta-HCG levels in all 660 patients who were underweight (BMI less than 18.5 kg/m2), the researchers reported.
Dr. Irani reported having no relevant financial disclosures.
SALT LAKE CITY – Women who are obese should receive more than 5,000 IU of human chorionic gonadotropin (HCG) to trigger final oocyte maturation during in vitro fertilization, findings of a retrospective cohort study suggest.
Fully 23% of obese women had low beta-HCG levels the day after receiving a 5,000 IU HCG trigger dose, compared with 1% of non-obese women (odds ratio, 21.4; 95% confidence interval, 15.0-30.4), reported Mohamad Irani, MD, and his associates from Cornell University, New York. In contrast, 10,000 IU HCG triggered adequate beta-HCG levels in 81% of obese patients with a BMI of 30-40 kg/m2, and in 90% of those whose BMI exceeded 40 kg/m2.
Low beta-HCG levels decreased the chances of oocyte maturation, fertilization, and live birth in the study. “Patients’ BMI should be taken into consideration when determining the dose of HCG trigger,” the investigators concluded in a poster presented at the annual meeting of the American Society for Reproductive Medicine.
Patients with hypothalamic amenorrhea or who are undergoing stimulation with a gonadotropin-releasing hormone (GnRH) agonist are not candidates for a GnRH-agonist trigger and therefore need to receive HCG instead, the researchers noted. To understand how the dose of HCG affects the chances of final oocyte maturation, they studied 19,084 HCG trigger recipients at their center between 2004 and 2013.
By protocol, patients received 10,000 IU HCG if their serum estradiol (E2) level was less than 1,500 pg/mL on the day of trigger; 5,000 IU if it measured 1,501-2,500 pg/mL; 4,000 IU if it was 2,501-3,000 pg/mL; and 3,300 IU if it exceeded 3,000 pg/mL.
The day after HCG trigger, 18,666 patients had beta-HCG levels of at least 50 mIU/mL, while 418 patients had low beta-HCG levels of less than 50 mIU/mL. A comparison of the two groups showed that low beta-HCG was associated with significantly lower rates of oocyte maturation (77% vs. 81%; P less than .001) and fertilization (63% vs. 72%; P less than .001). It was also associated with more than a 30% lower chance of a live birth (adjusted OR, 0.67; 95% CI, 0.5-0.8), even after accounting for age, and the stage and number of embryos transferred.
The researchers also examined response to HCG trigger among non-obese patients. Among patients who were overweight (BMI 25 to 30 kg/m2), the chances of a low beta-HCG level the day after trigger were 14% when the HCG dose was 3,300 IU, 12.5% when it was 4,000 IU, 4.3% when it was 5,000 IU, and 0.4% when it was 10,000 IU.
Among healthy-weight patients (BMI 18.5-25 kg/m2), low beta-HCG levels occurred 3.6% of the time when the HCG dose was 3,300 IU, 2.3% of the time when it was 4,000 IU, 0.6% of the time when it was 5,000 IU, and 0.08% of the time when it was 10,000 IU. Notably, these same doses triggered adequate beta-HCG levels in all 660 patients who were underweight (BMI less than 18.5 kg/m2), the researchers reported.
Dr. Irani reported having no relevant financial disclosures.
AT 2016 ASRM
Key clinical point:
Major finding: Fully 23% of obese individuals had low beta-HCG levels the day after receiving a 5,000 IU HCG trigger, compared with 1% of non-obese patients (OR, 21.4).
Data source: A retrospective cohort study of 18,666 patients with beta-HCG levels of at least 50 mIU/mL and 418 patients with “low” levels of less than 50 mIU/mL the day after HCG trigger.
Disclosures: Dr. Irani reported having no relevant financial disclosures.
Acute Localized Exanthematous Pustulosis Caused by Flurbiprofen
To the Editor:
Acute generalized exanthematous pustulosis (AGEP) is an acute skin reaction that is characterized by generalized, nonfollicular, pinhead-sized, sterile pustules on an erythematous and edematous background. The eruption can be accompanied by fever and neutrophilic leukocytosis. Skin symptoms arise quickly (within a few hours), most commonly following drug administration. The medications most frequently responsible are beta-lactam antibiotics, macrolides, calcium channel blockers, and antimalarials. Pustules spontaneously resolve in 15 days and generalized desquamation occurs approximately 2 weeks later. The estimated incidence rate of AGEP is approximately 1 to 5 cases per million per year. Acute localized exanthematous pustulosis (ALEP) is a less common form of AGEP. We report a case of ALEP localized on the face that was caused by flurbiprofen, a propionic acid derivative from the family of nonsteroidal anti-inflammatory drugs (NSAIDs).
A 40-year-old woman was referred to the dermatology department due to the sudden onset of multiple pustules on the face. One week earlier she started oral flurbiprofen (8.75 mg daily) for a sore throat. After 3 days of therapy, multiple pruritic, erythematous and edematous lesions appeared abruptly on the face with associated multiple small nonfollicular pustules. At presentation the patient was febrile (temperature, 38.2°C) and presented with bilateral ocular edema and superficial small nonfollicular pustules on an erythematous background over the face, scalp, and oral mucosa (Figure 1). The rest of the body was not involved. The patient denied prior adverse reactions to other drugs. The white blood cell count was 15,000/μL (reference range, 4500–11,000/μL), with an increased neutrophil count (12,000/μL [reference range, 1800–7800/μL]). The erythrocyte sedimentation rate and C-reactive protein level was elevated (erythrocyte sedimentation rate, 53 mm/h [reference range, 0–20 mm/h]; C-reactive protein, 98 mg/dL [reference range, 0–5 mg/dL]). Bacterial and fungal cultures of skin lesions were negative. The results of a viral polymerase chain reaction analysis proved the absence of varicella-zoster virus or herpes simplex virus. Histopathology of a skin biopsy specimen showed subcorneal pustules composed of neutrophils and eosinophils, epidermal spongiosis, some necrotic keratinocytes, vacuolization of the basal layer, papillary edema, and a perivascular neutrophil and lymphocyte infiltrate (Figure 2). A leukocytoclastic infiltrate within and around the walls of blood vessels at the superficial level of the dermis and red cell extravasation in the epidermis was present. She discontinued use of flurbiprofen and was treated with a systemic corticosteroid (methylprednisolone 0.5 mg/kg daily). The pustules rapidly resolved within 7 days after discontinuation of flurbiprofen and were followed by transient scaling and discrete residual hyperpigmentation.
Acute localized exanthematous pustulosis is a less common form of a pustular drug eruption in which lesions are consistent with AGEP but typically are localized to the face, neck, or chest. The definition of ALEP was introduced by Prange et al1 to describe a woman who was diagnosed with a localized pustular eruption on the face without a generalized distribution as in AGEP. In the past, this localized eruption was described under different names (eg, localized pustular eruption, localized toxin follicular pustuloderma, nongeneralized acute exanthematic pustulosis).2-5 According to a PubMed search of articles indexed for MEDLINE using the terms localized pustulosis, localized pustular eruption, and localized pustuloderma, only 16 separate cases of ALEP have been documented since the report by Prange et al.1 The medications most frequently responsible are antibiotics. Three cases developed following administration of amoxicillin2,5,6; 2 cases of amoxicillin–clavulanic acid7,8; 1 of penicillin1; 1 of azithromycin9; 1 of levofloxacin10; and 1 of combination of cephalosporin, sulfamethoxazole-trimethoprim, and vancomycin.11 Other nonantibiotic causative drugs include sulfamethoxazole-trimethoprim,12 infliximab,13 sorafenib,14 docetaxel,15 finasteride,16 ibuprofen,17 and paracetamol.18 In reported cases, the lesions are consistent with the characteristics of AGEP both clinically and histopathologically but are localized typically to the face, neck, or chest. In the majority of patients with ALEP, the absence of fever has been observed, but it does not appear distinctive for diagnosis. Our patient represents another case of ALEP with flurbiprofen as the causative drug. The close relationship between the administration of the drug and the development of the pustules, the rapid acute resolution as soon as treatment was interrupted, and the histologic findings all supported the diagnosis of ALEP following administration of flurbiprofen. This NSAID—2-fluoro-α-methyl-(1,1'-biphenyl)-4-acetic acid—is a prostaglandin synthetase inhibitor with anti-inflammatory activity. It is a propionic acid derivative that is similar to ibuprofen, which was once involved in the occurrence of ALEP.17 In 2009, Rastogi et al17 reported a case of a 64-year-old woman with an acute outbreak of multiple pustular lesions and underlying erythema affecting the cheeks and chin without fever who had been taking ibuprofen for a toothache. The case is similar to ours and confirms that NSAIDs can induce ALEP. Compared with other NSAIDs, propionic acid derivatives are usually well tolerated and serious adverse reactions rarely have been documented.19
The physiopathologic mechanisms of ALEP are unknown but likely are similar to AGEP. The demonstration of drug-specific positive patch test responses and in vitro lymphocyte proliferative responses in patients with a history of AGEP strongly suggests that this adverse cutaneous reaction occurs via a drug-specific T cell–mediated process.20
Further study is needed to understand the etiopathogenesis of the localized form of the disease and to facilitate a correct diagnosis of this rare disorder.
- Prange B, Marini A, Kalke A, et al. Acute localized exanthematous pustulosis (ALEP). J Dtsch Dermatol Ges. 2005;3:210-212.
- Shuttleworth D. A localized, recurrent pustular eruption following amoxycillin administration. Clin Exp Dermatol. 1989;14:367-368.
- De Argila D, Ortiz-Frutos J, Rodriguez-Peralto JL, et al. An atypical case of non-generalized acute exanthematic pustulosis. Actas Dermosifiliogr. 1996;87:475-478.
- Corbalan-Velez R, Peon G, Ara M, et al. Localized toxic follicular pustuloderma. Int J Dermatol. 2000;39:209-211.
- Prieto A, de Barrio M, López-Sáez P, et al. Recurrent localized pustular eruption induced by amoxicillin. Allergy. 1997;52:777-778.
- Vickers JL, Matherne RJ, Mainous EG, et al. Acute localized exanthematous pustulosis: a cutaneous drug reaction in a dental setting. J Am Dent Assoc. 2008;139:1200-1203.
- Betto P, Germi L, Bonoldi E, et al. Acute localized exanthematous pustulosis (ALEP) caused by amoxicillin-clavulanic acid. Int J Dermatol. 2008;47:295-296.
- Ozkaya-Parlakay A, Azkur D, Kara A, et al. Localized acute generalized exanthematous pustulosis with amoxicillin and clavulanic acid. Turk J Pediatr. 2011;53:229-232.
- Zweegers J, Bovenschen HJ. A woman with skin abnormalities around the mouth [in Dutch]. Ned Tijdschr Geneeskd. 2012;156:A4613.
- Corral de la Calle M, Martín Díaz MA, Flores CR, et al. Acute localized exanthematous pustulosis secondary to levofloxacin. Br J Dermatol. 2005;152:1076-1077.
- Sim HS, Seol JE, Chun JS, et al. Acute localized exanthematous pustulosis on the face. Ann Dermatol. 2011;23(suppl 3):S3368-S3370.
- Lee I, Turner M, Lee CC. Acute patchy exanthematous pustulosis caused by sulfamethoxazole-trimethoprim. J Am Acad Dermatol. 2010;63:e41-e43.
- Lee HY, Pelivani N, Beltraminelli H, et al. Amicrobial pustulosis-like rash in a patient with Crohn’s disease under anti-TNF-alpha blocker. Dermatology. 2011;222:304-310.
- Liang CP, Yang CS, Shen JL, et al. Sorafenib-induced acute localized exanthematous pustulosis in a patient with hepatocellular carcinoma. Br J Dermatol. 2011;165:443-445.
- Kim SW, Lee UH, Jang SJ, et al. Acute localized exanthematous pustulosis induced by docetaxel. J Am Acad Dermatol. 2010;63:e44-e46.
- Tresch S, Cozzio A, Kamarashev J, et al. T cell-mediated acute localized exanthematous pustulosis caused by finasteride. J Allergy Clin Immunol. 2012;129:589-594.
- Rastogi S, Modi M, Dhawan V. Acute localized exanthematous pustulosis (ALEP) caused by Ibuprofen. a case report. Br J Oral Maxillofac Surg. 2009;47:132-134.
- Wohl Y, Goldberg I, Sharazi I, et al. A case of paracetamol-induced acute generalized exanthematous pustulosis in a pregnant woman localized in the neck region. Skinmed. 2004;3:47-49.
- Mehra KK, Rupawala AH, Gogtay NJ. Immediate hypersensitivity reaction to a single oral dose of flurbiprofen. J Postgrad Med. 2010;56:36-37.
- Girardi M, Duncan KO, Tigelaar RE, et al. Cross comparison of patch-test and lymphocyte proliferation responses in patients with a history of acute generalized exanthematous pustulosis. Am J Dermatopathol. 2005;27:343-346.
To the Editor:
Acute generalized exanthematous pustulosis (AGEP) is an acute skin reaction that is characterized by generalized, nonfollicular, pinhead-sized, sterile pustules on an erythematous and edematous background. The eruption can be accompanied by fever and neutrophilic leukocytosis. Skin symptoms arise quickly (within a few hours), most commonly following drug administration. The medications most frequently responsible are beta-lactam antibiotics, macrolides, calcium channel blockers, and antimalarials. Pustules spontaneously resolve in 15 days and generalized desquamation occurs approximately 2 weeks later. The estimated incidence rate of AGEP is approximately 1 to 5 cases per million per year. Acute localized exanthematous pustulosis (ALEP) is a less common form of AGEP. We report a case of ALEP localized on the face that was caused by flurbiprofen, a propionic acid derivative from the family of nonsteroidal anti-inflammatory drugs (NSAIDs).
A 40-year-old woman was referred to the dermatology department due to the sudden onset of multiple pustules on the face. One week earlier she started oral flurbiprofen (8.75 mg daily) for a sore throat. After 3 days of therapy, multiple pruritic, erythematous and edematous lesions appeared abruptly on the face with associated multiple small nonfollicular pustules. At presentation the patient was febrile (temperature, 38.2°C) and presented with bilateral ocular edema and superficial small nonfollicular pustules on an erythematous background over the face, scalp, and oral mucosa (Figure 1). The rest of the body was not involved. The patient denied prior adverse reactions to other drugs. The white blood cell count was 15,000/μL (reference range, 4500–11,000/μL), with an increased neutrophil count (12,000/μL [reference range, 1800–7800/μL]). The erythrocyte sedimentation rate and C-reactive protein level was elevated (erythrocyte sedimentation rate, 53 mm/h [reference range, 0–20 mm/h]; C-reactive protein, 98 mg/dL [reference range, 0–5 mg/dL]). Bacterial and fungal cultures of skin lesions were negative. The results of a viral polymerase chain reaction analysis proved the absence of varicella-zoster virus or herpes simplex virus. Histopathology of a skin biopsy specimen showed subcorneal pustules composed of neutrophils and eosinophils, epidermal spongiosis, some necrotic keratinocytes, vacuolization of the basal layer, papillary edema, and a perivascular neutrophil and lymphocyte infiltrate (Figure 2). A leukocytoclastic infiltrate within and around the walls of blood vessels at the superficial level of the dermis and red cell extravasation in the epidermis was present. She discontinued use of flurbiprofen and was treated with a systemic corticosteroid (methylprednisolone 0.5 mg/kg daily). The pustules rapidly resolved within 7 days after discontinuation of flurbiprofen and were followed by transient scaling and discrete residual hyperpigmentation.


Acute localized exanthematous pustulosis is a less common form of a pustular drug eruption in which lesions are consistent with AGEP but typically are localized to the face, neck, or chest. The definition of ALEP was introduced by Prange et al1 to describe a woman who was diagnosed with a localized pustular eruption on the face without a generalized distribution as in AGEP. In the past, this localized eruption was described under different names (eg, localized pustular eruption, localized toxin follicular pustuloderma, nongeneralized acute exanthematic pustulosis).2-5 According to a PubMed search of articles indexed for MEDLINE using the terms localized pustulosis, localized pustular eruption, and localized pustuloderma, only 16 separate cases of ALEP have been documented since the report by Prange et al.1 The medications most frequently responsible are antibiotics. Three cases developed following administration of amoxicillin2,5,6; 2 cases of amoxicillin–clavulanic acid7,8; 1 of penicillin1; 1 of azithromycin9; 1 of levofloxacin10; and 1 of combination of cephalosporin, sulfamethoxazole-trimethoprim, and vancomycin.11 Other nonantibiotic causative drugs include sulfamethoxazole-trimethoprim,12 infliximab,13 sorafenib,14 docetaxel,15 finasteride,16 ibuprofen,17 and paracetamol.18 In reported cases, the lesions are consistent with the characteristics of AGEP both clinically and histopathologically but are localized typically to the face, neck, or chest. In the majority of patients with ALEP, the absence of fever has been observed, but it does not appear distinctive for diagnosis. Our patient represents another case of ALEP with flurbiprofen as the causative drug. The close relationship between the administration of the drug and the development of the pustules, the rapid acute resolution as soon as treatment was interrupted, and the histologic findings all supported the diagnosis of ALEP following administration of flurbiprofen. This NSAID—2-fluoro-α-methyl-(1,1'-biphenyl)-4-acetic acid—is a prostaglandin synthetase inhibitor with anti-inflammatory activity. It is a propionic acid derivative that is similar to ibuprofen, which was once involved in the occurrence of ALEP.17 In 2009, Rastogi et al17 reported a case of a 64-year-old woman with an acute outbreak of multiple pustular lesions and underlying erythema affecting the cheeks and chin without fever who had been taking ibuprofen for a toothache. The case is similar to ours and confirms that NSAIDs can induce ALEP. Compared with other NSAIDs, propionic acid derivatives are usually well tolerated and serious adverse reactions rarely have been documented.19
The physiopathologic mechanisms of ALEP are unknown but likely are similar to AGEP. The demonstration of drug-specific positive patch test responses and in vitro lymphocyte proliferative responses in patients with a history of AGEP strongly suggests that this adverse cutaneous reaction occurs via a drug-specific T cell–mediated process.20
Further study is needed to understand the etiopathogenesis of the localized form of the disease and to facilitate a correct diagnosis of this rare disorder.
To the Editor:
Acute generalized exanthematous pustulosis (AGEP) is an acute skin reaction that is characterized by generalized, nonfollicular, pinhead-sized, sterile pustules on an erythematous and edematous background. The eruption can be accompanied by fever and neutrophilic leukocytosis. Skin symptoms arise quickly (within a few hours), most commonly following drug administration. The medications most frequently responsible are beta-lactam antibiotics, macrolides, calcium channel blockers, and antimalarials. Pustules spontaneously resolve in 15 days and generalized desquamation occurs approximately 2 weeks later. The estimated incidence rate of AGEP is approximately 1 to 5 cases per million per year. Acute localized exanthematous pustulosis (ALEP) is a less common form of AGEP. We report a case of ALEP localized on the face that was caused by flurbiprofen, a propionic acid derivative from the family of nonsteroidal anti-inflammatory drugs (NSAIDs).
A 40-year-old woman was referred to the dermatology department due to the sudden onset of multiple pustules on the face. One week earlier she started oral flurbiprofen (8.75 mg daily) for a sore throat. After 3 days of therapy, multiple pruritic, erythematous and edematous lesions appeared abruptly on the face with associated multiple small nonfollicular pustules. At presentation the patient was febrile (temperature, 38.2°C) and presented with bilateral ocular edema and superficial small nonfollicular pustules on an erythematous background over the face, scalp, and oral mucosa (Figure 1). The rest of the body was not involved. The patient denied prior adverse reactions to other drugs. The white blood cell count was 15,000/μL (reference range, 4500–11,000/μL), with an increased neutrophil count (12,000/μL [reference range, 1800–7800/μL]). The erythrocyte sedimentation rate and C-reactive protein level was elevated (erythrocyte sedimentation rate, 53 mm/h [reference range, 0–20 mm/h]; C-reactive protein, 98 mg/dL [reference range, 0–5 mg/dL]). Bacterial and fungal cultures of skin lesions were negative. The results of a viral polymerase chain reaction analysis proved the absence of varicella-zoster virus or herpes simplex virus. Histopathology of a skin biopsy specimen showed subcorneal pustules composed of neutrophils and eosinophils, epidermal spongiosis, some necrotic keratinocytes, vacuolization of the basal layer, papillary edema, and a perivascular neutrophil and lymphocyte infiltrate (Figure 2). A leukocytoclastic infiltrate within and around the walls of blood vessels at the superficial level of the dermis and red cell extravasation in the epidermis was present. She discontinued use of flurbiprofen and was treated with a systemic corticosteroid (methylprednisolone 0.5 mg/kg daily). The pustules rapidly resolved within 7 days after discontinuation of flurbiprofen and were followed by transient scaling and discrete residual hyperpigmentation.


Acute localized exanthematous pustulosis is a less common form of a pustular drug eruption in which lesions are consistent with AGEP but typically are localized to the face, neck, or chest. The definition of ALEP was introduced by Prange et al1 to describe a woman who was diagnosed with a localized pustular eruption on the face without a generalized distribution as in AGEP. In the past, this localized eruption was described under different names (eg, localized pustular eruption, localized toxin follicular pustuloderma, nongeneralized acute exanthematic pustulosis).2-5 According to a PubMed search of articles indexed for MEDLINE using the terms localized pustulosis, localized pustular eruption, and localized pustuloderma, only 16 separate cases of ALEP have been documented since the report by Prange et al.1 The medications most frequently responsible are antibiotics. Three cases developed following administration of amoxicillin2,5,6; 2 cases of amoxicillin–clavulanic acid7,8; 1 of penicillin1; 1 of azithromycin9; 1 of levofloxacin10; and 1 of combination of cephalosporin, sulfamethoxazole-trimethoprim, and vancomycin.11 Other nonantibiotic causative drugs include sulfamethoxazole-trimethoprim,12 infliximab,13 sorafenib,14 docetaxel,15 finasteride,16 ibuprofen,17 and paracetamol.18 In reported cases, the lesions are consistent with the characteristics of AGEP both clinically and histopathologically but are localized typically to the face, neck, or chest. In the majority of patients with ALEP, the absence of fever has been observed, but it does not appear distinctive for diagnosis. Our patient represents another case of ALEP with flurbiprofen as the causative drug. The close relationship between the administration of the drug and the development of the pustules, the rapid acute resolution as soon as treatment was interrupted, and the histologic findings all supported the diagnosis of ALEP following administration of flurbiprofen. This NSAID—2-fluoro-α-methyl-(1,1'-biphenyl)-4-acetic acid—is a prostaglandin synthetase inhibitor with anti-inflammatory activity. It is a propionic acid derivative that is similar to ibuprofen, which was once involved in the occurrence of ALEP.17 In 2009, Rastogi et al17 reported a case of a 64-year-old woman with an acute outbreak of multiple pustular lesions and underlying erythema affecting the cheeks and chin without fever who had been taking ibuprofen for a toothache. The case is similar to ours and confirms that NSAIDs can induce ALEP. Compared with other NSAIDs, propionic acid derivatives are usually well tolerated and serious adverse reactions rarely have been documented.19
The physiopathologic mechanisms of ALEP are unknown but likely are similar to AGEP. The demonstration of drug-specific positive patch test responses and in vitro lymphocyte proliferative responses in patients with a history of AGEP strongly suggests that this adverse cutaneous reaction occurs via a drug-specific T cell–mediated process.20
Further study is needed to understand the etiopathogenesis of the localized form of the disease and to facilitate a correct diagnosis of this rare disorder.
- Prange B, Marini A, Kalke A, et al. Acute localized exanthematous pustulosis (ALEP). J Dtsch Dermatol Ges. 2005;3:210-212.
- Shuttleworth D. A localized, recurrent pustular eruption following amoxycillin administration. Clin Exp Dermatol. 1989;14:367-368.
- De Argila D, Ortiz-Frutos J, Rodriguez-Peralto JL, et al. An atypical case of non-generalized acute exanthematic pustulosis. Actas Dermosifiliogr. 1996;87:475-478.
- Corbalan-Velez R, Peon G, Ara M, et al. Localized toxic follicular pustuloderma. Int J Dermatol. 2000;39:209-211.
- Prieto A, de Barrio M, López-Sáez P, et al. Recurrent localized pustular eruption induced by amoxicillin. Allergy. 1997;52:777-778.
- Vickers JL, Matherne RJ, Mainous EG, et al. Acute localized exanthematous pustulosis: a cutaneous drug reaction in a dental setting. J Am Dent Assoc. 2008;139:1200-1203.
- Betto P, Germi L, Bonoldi E, et al. Acute localized exanthematous pustulosis (ALEP) caused by amoxicillin-clavulanic acid. Int J Dermatol. 2008;47:295-296.
- Ozkaya-Parlakay A, Azkur D, Kara A, et al. Localized acute generalized exanthematous pustulosis with amoxicillin and clavulanic acid. Turk J Pediatr. 2011;53:229-232.
- Zweegers J, Bovenschen HJ. A woman with skin abnormalities around the mouth [in Dutch]. Ned Tijdschr Geneeskd. 2012;156:A4613.
- Corral de la Calle M, Martín Díaz MA, Flores CR, et al. Acute localized exanthematous pustulosis secondary to levofloxacin. Br J Dermatol. 2005;152:1076-1077.
- Sim HS, Seol JE, Chun JS, et al. Acute localized exanthematous pustulosis on the face. Ann Dermatol. 2011;23(suppl 3):S3368-S3370.
- Lee I, Turner M, Lee CC. Acute patchy exanthematous pustulosis caused by sulfamethoxazole-trimethoprim. J Am Acad Dermatol. 2010;63:e41-e43.
- Lee HY, Pelivani N, Beltraminelli H, et al. Amicrobial pustulosis-like rash in a patient with Crohn’s disease under anti-TNF-alpha blocker. Dermatology. 2011;222:304-310.
- Liang CP, Yang CS, Shen JL, et al. Sorafenib-induced acute localized exanthematous pustulosis in a patient with hepatocellular carcinoma. Br J Dermatol. 2011;165:443-445.
- Kim SW, Lee UH, Jang SJ, et al. Acute localized exanthematous pustulosis induced by docetaxel. J Am Acad Dermatol. 2010;63:e44-e46.
- Tresch S, Cozzio A, Kamarashev J, et al. T cell-mediated acute localized exanthematous pustulosis caused by finasteride. J Allergy Clin Immunol. 2012;129:589-594.
- Rastogi S, Modi M, Dhawan V. Acute localized exanthematous pustulosis (ALEP) caused by Ibuprofen. a case report. Br J Oral Maxillofac Surg. 2009;47:132-134.
- Wohl Y, Goldberg I, Sharazi I, et al. A case of paracetamol-induced acute generalized exanthematous pustulosis in a pregnant woman localized in the neck region. Skinmed. 2004;3:47-49.
- Mehra KK, Rupawala AH, Gogtay NJ. Immediate hypersensitivity reaction to a single oral dose of flurbiprofen. J Postgrad Med. 2010;56:36-37.
- Girardi M, Duncan KO, Tigelaar RE, et al. Cross comparison of patch-test and lymphocyte proliferation responses in patients with a history of acute generalized exanthematous pustulosis. Am J Dermatopathol. 2005;27:343-346.
- Prange B, Marini A, Kalke A, et al. Acute localized exanthematous pustulosis (ALEP). J Dtsch Dermatol Ges. 2005;3:210-212.
- Shuttleworth D. A localized, recurrent pustular eruption following amoxycillin administration. Clin Exp Dermatol. 1989;14:367-368.
- De Argila D, Ortiz-Frutos J, Rodriguez-Peralto JL, et al. An atypical case of non-generalized acute exanthematic pustulosis. Actas Dermosifiliogr. 1996;87:475-478.
- Corbalan-Velez R, Peon G, Ara M, et al. Localized toxic follicular pustuloderma. Int J Dermatol. 2000;39:209-211.
- Prieto A, de Barrio M, López-Sáez P, et al. Recurrent localized pustular eruption induced by amoxicillin. Allergy. 1997;52:777-778.
- Vickers JL, Matherne RJ, Mainous EG, et al. Acute localized exanthematous pustulosis: a cutaneous drug reaction in a dental setting. J Am Dent Assoc. 2008;139:1200-1203.
- Betto P, Germi L, Bonoldi E, et al. Acute localized exanthematous pustulosis (ALEP) caused by amoxicillin-clavulanic acid. Int J Dermatol. 2008;47:295-296.
- Ozkaya-Parlakay A, Azkur D, Kara A, et al. Localized acute generalized exanthematous pustulosis with amoxicillin and clavulanic acid. Turk J Pediatr. 2011;53:229-232.
- Zweegers J, Bovenschen HJ. A woman with skin abnormalities around the mouth [in Dutch]. Ned Tijdschr Geneeskd. 2012;156:A4613.
- Corral de la Calle M, Martín Díaz MA, Flores CR, et al. Acute localized exanthematous pustulosis secondary to levofloxacin. Br J Dermatol. 2005;152:1076-1077.
- Sim HS, Seol JE, Chun JS, et al. Acute localized exanthematous pustulosis on the face. Ann Dermatol. 2011;23(suppl 3):S3368-S3370.
- Lee I, Turner M, Lee CC. Acute patchy exanthematous pustulosis caused by sulfamethoxazole-trimethoprim. J Am Acad Dermatol. 2010;63:e41-e43.
- Lee HY, Pelivani N, Beltraminelli H, et al. Amicrobial pustulosis-like rash in a patient with Crohn’s disease under anti-TNF-alpha blocker. Dermatology. 2011;222:304-310.
- Liang CP, Yang CS, Shen JL, et al. Sorafenib-induced acute localized exanthematous pustulosis in a patient with hepatocellular carcinoma. Br J Dermatol. 2011;165:443-445.
- Kim SW, Lee UH, Jang SJ, et al. Acute localized exanthematous pustulosis induced by docetaxel. J Am Acad Dermatol. 2010;63:e44-e46.
- Tresch S, Cozzio A, Kamarashev J, et al. T cell-mediated acute localized exanthematous pustulosis caused by finasteride. J Allergy Clin Immunol. 2012;129:589-594.
- Rastogi S, Modi M, Dhawan V. Acute localized exanthematous pustulosis (ALEP) caused by Ibuprofen. a case report. Br J Oral Maxillofac Surg. 2009;47:132-134.
- Wohl Y, Goldberg I, Sharazi I, et al. A case of paracetamol-induced acute generalized exanthematous pustulosis in a pregnant woman localized in the neck region. Skinmed. 2004;3:47-49.
- Mehra KK, Rupawala AH, Gogtay NJ. Immediate hypersensitivity reaction to a single oral dose of flurbiprofen. J Postgrad Med. 2010;56:36-37.
- Girardi M, Duncan KO, Tigelaar RE, et al. Cross comparison of patch-test and lymphocyte proliferation responses in patients with a history of acute generalized exanthematous pustulosis. Am J Dermatopathol. 2005;27:343-346.
Practice Points
- Acute localized exanthematous pustulosis is a form of a pustular drug eruption in which lesions are consistent with acute generalized exanthematous pustulosis but typically localized in a single area.
- The medications most frequently responsible are antibiotics. Flurbiprofen, a propionic acid derivative, could be a rare causative agent of this disease.
Accuracy and Sources of Images From Direct Google Image Searches for Common Dermatology Terms
To the Editor:
Prior studies have assessed the quality of text-based dermatology information on the Internet using traditional search engine queries.1 However, little is understood about the sources, accuracy, and quality of online dermatology images derived from direct image searches. Previous work has shown that direct search engine image queries were largely accurate for 3 pediatric dermatology diagnosis searches: atopic dermatitis, lichen striatus, and subcutaneous fat necrosis.2 We assessed images obtained for common dermatologic conditions from a Google image search (GIS) compared to a traditional text-based Google web search (GWS).
Image results for 32 unique dermatologic search terms were analyzed (Table 1). These search terms were selected using the results of a prior study that identified the most common dermatologic diagnoses that led users to the 2 most popular dermatology-specific websites worldwide: the American Academy of Dermatology (www.aad.org) and DermNet New Zealand (www.dermnetnz.org).3 The Alexa directory (www.alexa.com), a large publicly available Internet analytics resource, was used to determine the most common dermatology search terms that led a user to either www.dermnetnz.org or www.aad.org. In addition, searches for the 3 most common types of skin cancer—melanoma, squamous cell carcinoma, and basal cell carcinoma—were included. Each term was entered into a GIS and a GWS. The first 10 results, which represent 92% of the websites ultimately visited by users,4 were analyzed. The source, diagnostic accuracy, and Fitzpatrick skin type of the images was determined. Website sources were organized into 11 categories. All data collection occurred within a 1-week period in August 2015.
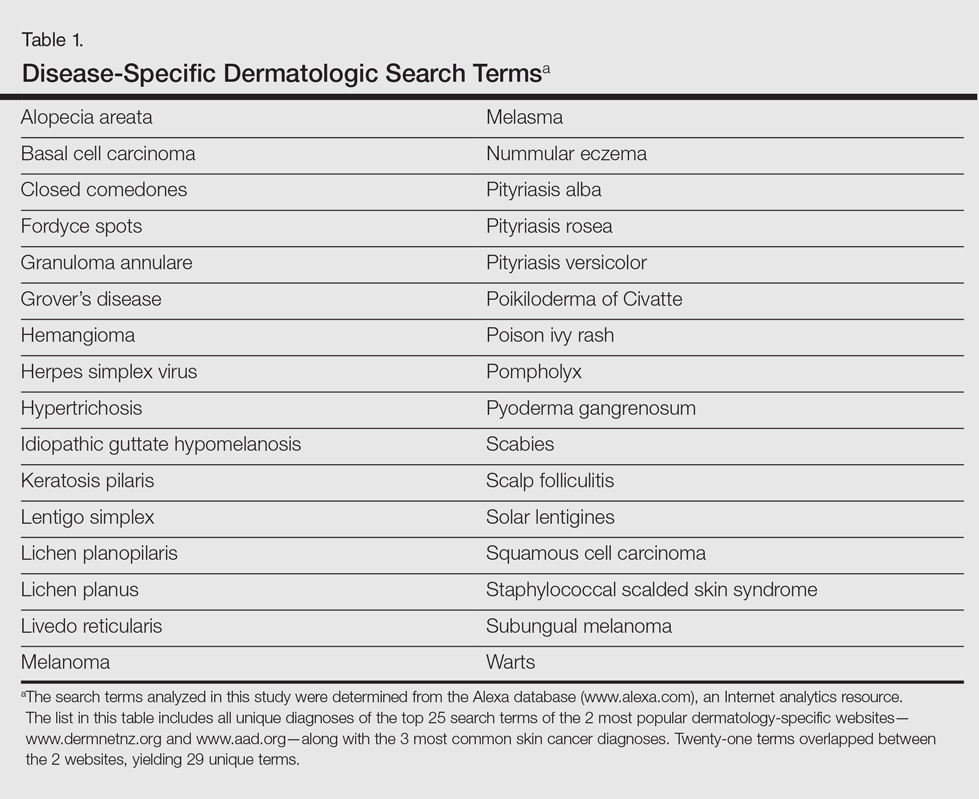
A total of 320 images were analyzed. In the GIS, private websites (36%), dermatology association websites (28%), and general health information websites (10%) were the 3 most common sources. In the GWS, health information websites (35%), private websites (21%), and dermatology association websites (20%) accounted for the most common sources (Table 2). The majority of images were of Fitzpatrick skin types I and II (89%) and nearly all images were diagnostically accurate (98%). There was no statistically significant difference in accuracy of diagnosis between physician-associated websites (100% accuracy) versus nonphysician-associated sites (98% accuracy, P=.25).
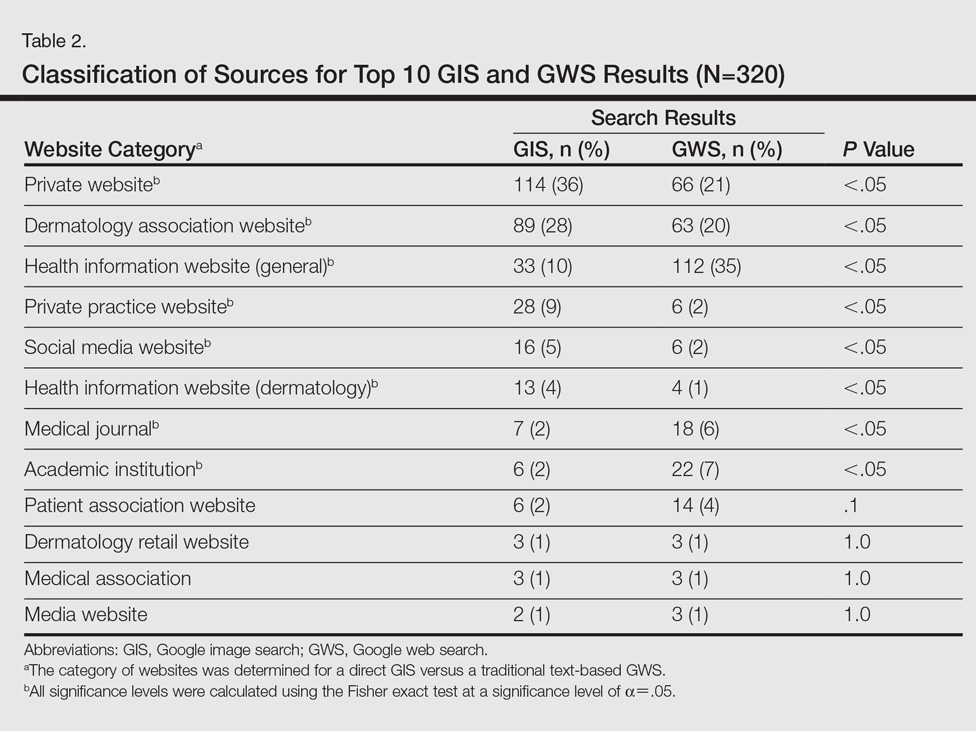
Our results showed high diagnostic accuracy among the top GIS results for common dermatology search terms. Diagnostic accuracy did not vary between websites that were physician associated versus those that were not. Our results are comparable to the reported accuracy of online dermatologic health information.1 In GIS results, the majority of images were provided by private websites, whereas the top websites in GWS results were health information websites.
Only 1% of images were of Fitzpatrick skin types VI and VII. Presentation of skin diseases is remarkably different based on the patient’s skin type.5 The shortage of readily accessible images of skin of color is in line with the lack of familiarity physicians and trainees have with dermatologic conditions in ethnic skin.6
Based on the results from this analysis, providers and patients searching for dermatologic conditions via a direct GIS should be cognizant of several considerations. Although our results showed that GIS was accurate, the searcher should note that image-based searches are not accompanied by relevant text that can help confirm relevancy and accuracy. Image searches depend on textual tags added by the source website. Websites that represent dermatological associations and academic centers can add an additional layer of confidence for users. Patients and clinicians also should be aware that the consideration of a patient’s Fitzpatrick skin type is critical when assessing the relevancy of a GIS result. In conclusion, search results via GIS queries are accurate for the dermatological diagnoses tested but may be lacking in skin of color variations, suggesting a potential unmet need based on our growing ethnic skin population.
- Jensen JD, Dunnick CA, Arbuckle HA, et al. Dermatology information on the Internet: an appraisal by dermatologists and dermatology residents. J Am Acad Dermatol. 2010;63:1101-1103.
- Cutrone M, Grimalt R. Dermatological image search engines on the Internet: do they work? J Eur Acad Dermatol Venereol. 2007;21:175-177.
- Xu S, Nault A, Bhatia A. Search and engagement analysis of association websites representing dermatologists—implications and opportunities for web visibility and patient education: website rankings of dermatology associations. Pract Dermatol. In press.
- comScore releases July 2015 U.S. desktop search engine rankings [press release]. Reston, VA: comScore, Inc; August 14, 2015. http://www.comscore.com/Insights/Market-Rankings/comScore-Releases-July-2015-U.S.-Desktop-Search-Engine-Rankings. Accessed October 18, 2016.
- Kundu RV, Patterson S. Dermatologic conditions in skin of color: part I. special considerations for common skin disorders. Am Fam Physician. 2013;87:850-856.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
To the Editor:
Prior studies have assessed the quality of text-based dermatology information on the Internet using traditional search engine queries.1 However, little is understood about the sources, accuracy, and quality of online dermatology images derived from direct image searches. Previous work has shown that direct search engine image queries were largely accurate for 3 pediatric dermatology diagnosis searches: atopic dermatitis, lichen striatus, and subcutaneous fat necrosis.2 We assessed images obtained for common dermatologic conditions from a Google image search (GIS) compared to a traditional text-based Google web search (GWS).
Image results for 32 unique dermatologic search terms were analyzed (Table 1). These search terms were selected using the results of a prior study that identified the most common dermatologic diagnoses that led users to the 2 most popular dermatology-specific websites worldwide: the American Academy of Dermatology (www.aad.org) and DermNet New Zealand (www.dermnetnz.org).3 The Alexa directory (www.alexa.com), a large publicly available Internet analytics resource, was used to determine the most common dermatology search terms that led a user to either www.dermnetnz.org or www.aad.org. In addition, searches for the 3 most common types of skin cancer—melanoma, squamous cell carcinoma, and basal cell carcinoma—were included. Each term was entered into a GIS and a GWS. The first 10 results, which represent 92% of the websites ultimately visited by users,4 were analyzed. The source, diagnostic accuracy, and Fitzpatrick skin type of the images was determined. Website sources were organized into 11 categories. All data collection occurred within a 1-week period in August 2015.

A total of 320 images were analyzed. In the GIS, private websites (36%), dermatology association websites (28%), and general health information websites (10%) were the 3 most common sources. In the GWS, health information websites (35%), private websites (21%), and dermatology association websites (20%) accounted for the most common sources (Table 2). The majority of images were of Fitzpatrick skin types I and II (89%) and nearly all images were diagnostically accurate (98%). There was no statistically significant difference in accuracy of diagnosis between physician-associated websites (100% accuracy) versus nonphysician-associated sites (98% accuracy, P=.25).

Our results showed high diagnostic accuracy among the top GIS results for common dermatology search terms. Diagnostic accuracy did not vary between websites that were physician associated versus those that were not. Our results are comparable to the reported accuracy of online dermatologic health information.1 In GIS results, the majority of images were provided by private websites, whereas the top websites in GWS results were health information websites.
Only 1% of images were of Fitzpatrick skin types VI and VII. Presentation of skin diseases is remarkably different based on the patient’s skin type.5 The shortage of readily accessible images of skin of color is in line with the lack of familiarity physicians and trainees have with dermatologic conditions in ethnic skin.6
Based on the results from this analysis, providers and patients searching for dermatologic conditions via a direct GIS should be cognizant of several considerations. Although our results showed that GIS was accurate, the searcher should note that image-based searches are not accompanied by relevant text that can help confirm relevancy and accuracy. Image searches depend on textual tags added by the source website. Websites that represent dermatological associations and academic centers can add an additional layer of confidence for users. Patients and clinicians also should be aware that the consideration of a patient’s Fitzpatrick skin type is critical when assessing the relevancy of a GIS result. In conclusion, search results via GIS queries are accurate for the dermatological diagnoses tested but may be lacking in skin of color variations, suggesting a potential unmet need based on our growing ethnic skin population.
To the Editor:
Prior studies have assessed the quality of text-based dermatology information on the Internet using traditional search engine queries.1 However, little is understood about the sources, accuracy, and quality of online dermatology images derived from direct image searches. Previous work has shown that direct search engine image queries were largely accurate for 3 pediatric dermatology diagnosis searches: atopic dermatitis, lichen striatus, and subcutaneous fat necrosis.2 We assessed images obtained for common dermatologic conditions from a Google image search (GIS) compared to a traditional text-based Google web search (GWS).
Image results for 32 unique dermatologic search terms were analyzed (Table 1). These search terms were selected using the results of a prior study that identified the most common dermatologic diagnoses that led users to the 2 most popular dermatology-specific websites worldwide: the American Academy of Dermatology (www.aad.org) and DermNet New Zealand (www.dermnetnz.org).3 The Alexa directory (www.alexa.com), a large publicly available Internet analytics resource, was used to determine the most common dermatology search terms that led a user to either www.dermnetnz.org or www.aad.org. In addition, searches for the 3 most common types of skin cancer—melanoma, squamous cell carcinoma, and basal cell carcinoma—were included. Each term was entered into a GIS and a GWS. The first 10 results, which represent 92% of the websites ultimately visited by users,4 were analyzed. The source, diagnostic accuracy, and Fitzpatrick skin type of the images was determined. Website sources were organized into 11 categories. All data collection occurred within a 1-week period in August 2015.

A total of 320 images were analyzed. In the GIS, private websites (36%), dermatology association websites (28%), and general health information websites (10%) were the 3 most common sources. In the GWS, health information websites (35%), private websites (21%), and dermatology association websites (20%) accounted for the most common sources (Table 2). The majority of images were of Fitzpatrick skin types I and II (89%) and nearly all images were diagnostically accurate (98%). There was no statistically significant difference in accuracy of diagnosis between physician-associated websites (100% accuracy) versus nonphysician-associated sites (98% accuracy, P=.25).

Our results showed high diagnostic accuracy among the top GIS results for common dermatology search terms. Diagnostic accuracy did not vary between websites that were physician associated versus those that were not. Our results are comparable to the reported accuracy of online dermatologic health information.1 In GIS results, the majority of images were provided by private websites, whereas the top websites in GWS results were health information websites.
Only 1% of images were of Fitzpatrick skin types VI and VII. Presentation of skin diseases is remarkably different based on the patient’s skin type.5 The shortage of readily accessible images of skin of color is in line with the lack of familiarity physicians and trainees have with dermatologic conditions in ethnic skin.6
Based on the results from this analysis, providers and patients searching for dermatologic conditions via a direct GIS should be cognizant of several considerations. Although our results showed that GIS was accurate, the searcher should note that image-based searches are not accompanied by relevant text that can help confirm relevancy and accuracy. Image searches depend on textual tags added by the source website. Websites that represent dermatological associations and academic centers can add an additional layer of confidence for users. Patients and clinicians also should be aware that the consideration of a patient’s Fitzpatrick skin type is critical when assessing the relevancy of a GIS result. In conclusion, search results via GIS queries are accurate for the dermatological diagnoses tested but may be lacking in skin of color variations, suggesting a potential unmet need based on our growing ethnic skin population.
- Jensen JD, Dunnick CA, Arbuckle HA, et al. Dermatology information on the Internet: an appraisal by dermatologists and dermatology residents. J Am Acad Dermatol. 2010;63:1101-1103.
- Cutrone M, Grimalt R. Dermatological image search engines on the Internet: do they work? J Eur Acad Dermatol Venereol. 2007;21:175-177.
- Xu S, Nault A, Bhatia A. Search and engagement analysis of association websites representing dermatologists—implications and opportunities for web visibility and patient education: website rankings of dermatology associations. Pract Dermatol. In press.
- comScore releases July 2015 U.S. desktop search engine rankings [press release]. Reston, VA: comScore, Inc; August 14, 2015. http://www.comscore.com/Insights/Market-Rankings/comScore-Releases-July-2015-U.S.-Desktop-Search-Engine-Rankings. Accessed October 18, 2016.
- Kundu RV, Patterson S. Dermatologic conditions in skin of color: part I. special considerations for common skin disorders. Am Fam Physician. 2013;87:850-856.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Jensen JD, Dunnick CA, Arbuckle HA, et al. Dermatology information on the Internet: an appraisal by dermatologists and dermatology residents. J Am Acad Dermatol. 2010;63:1101-1103.
- Cutrone M, Grimalt R. Dermatological image search engines on the Internet: do they work? J Eur Acad Dermatol Venereol. 2007;21:175-177.
- Xu S, Nault A, Bhatia A. Search and engagement analysis of association websites representing dermatologists—implications and opportunities for web visibility and patient education: website rankings of dermatology associations. Pract Dermatol. In press.
- comScore releases July 2015 U.S. desktop search engine rankings [press release]. Reston, VA: comScore, Inc; August 14, 2015. http://www.comscore.com/Insights/Market-Rankings/comScore-Releases-July-2015-U.S.-Desktop-Search-Engine-Rankings. Accessed October 18, 2016.
- Kundu RV, Patterson S. Dermatologic conditions in skin of color: part I. special considerations for common skin disorders. Am Fam Physician. 2013;87:850-856.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
Practice Points
- Direct Google image searches largely deliver accurate results for common dermatological diagnoses.
- Greater effort should be made to include more publicly available images for dermatological diseases in darker skin types.
Optimal MPE management requires early pulmonology referral
LOS ANGELES – About half of patients with symptomatic malignant pleural effusions at McGill University Health Centre in Montreal had unnecessary procedures and hospital admissions before definitive treatment with chemical pleurodesis or indwelling pleural catheters, according to researchers.
Instead of chest taps to relieve symptoms followed by referrals for definitive treatment, some patients got chest tubes – without pleurodesis – after presenting to the emergency department and being referred to radiology; they were then admitted to the hospital for a few days while the tubes were in place. In short, cancer patients were wasting what time they had left on medical care they didn’t need, and incurring unnecessary costs, said lead investigator Benjamin Shieh, MD, formerly at McGill but now an interventional pulmonology fellow at the University of Calgary.
McGill is a tertiary care center able to perform both definitive procedures, so “we should be a center of excellence. I imagine there are similar situations” at other hospitals, especially those without the resources of McGill, Dr. Shieh said at the annual meeting of the American College of Chest Physicians.
McGill has taken several steps to address the problem, including early ED referral to the pulmonology service and discouraging radiology from placing chest tubes for malignant pleural effusions (MPE). “I think we can avoid a big proportion of hospitalizations for MPE, and certainly a proportion of repeat [ED] visits,” said senior author Anne Gonzalez, MD, an attending pulmonologist at McGill.
The investigators looked into the issue after noting that a lot of their MPE cases had been hospitalized with chest tubes. They reviewed 72 symptomatic MPE cases in 69 patients treated in 2014 and 2015. Management was ideal in 36 cases (50%), meaning that, prior to definitive treatment, patients had no more than two pleural taps for symptom relief, no more than one ED visit, no chest tubes without pleurodesis, and no hospitalizations. “We thought this would be reasonable to try to achieve for MPE,” since there’s no definition of ideal management, Dr. Shieh said.
Nonideal patients had a mean of 2.5 pleural procedures – almost twice the number in the ideal group – before definitive palliation, with no respiratory consult beforehand. Chest tubes were placed in 27 cases (38%) for an average of 3.7 days; 28 cases (39%) were hospitalized. Nonideal patients were far more likely to present first to the ED, and ED presentations were more likely to get chest tubes and be admitted. All the cases were eventually treated definitively, 68 with indwelling pleural catheters and 4 by thoracoscopic talc insufflation. Time from initial presentation to definitive palliation was about 1 month in both groups. The investigators didn’t consider rate of effusion recurrence, which might help explain why the ideal group wasn’t treated sooner; they might not have needed it. The higher number of ED visits in the nonideal group suggests that they may have had quicker recurrences, and should have been treated sooner, Dr. Gonzalez said.
The patients were 70 years old, on average, and about 60% were women. Lung and breast were the most common cancers.
There was no industry funding for the work, and the investigators had no disclosures.
LOS ANGELES – About half of patients with symptomatic malignant pleural effusions at McGill University Health Centre in Montreal had unnecessary procedures and hospital admissions before definitive treatment with chemical pleurodesis or indwelling pleural catheters, according to researchers.
Instead of chest taps to relieve symptoms followed by referrals for definitive treatment, some patients got chest tubes – without pleurodesis – after presenting to the emergency department and being referred to radiology; they were then admitted to the hospital for a few days while the tubes were in place. In short, cancer patients were wasting what time they had left on medical care they didn’t need, and incurring unnecessary costs, said lead investigator Benjamin Shieh, MD, formerly at McGill but now an interventional pulmonology fellow at the University of Calgary.
McGill is a tertiary care center able to perform both definitive procedures, so “we should be a center of excellence. I imagine there are similar situations” at other hospitals, especially those without the resources of McGill, Dr. Shieh said at the annual meeting of the American College of Chest Physicians.
McGill has taken several steps to address the problem, including early ED referral to the pulmonology service and discouraging radiology from placing chest tubes for malignant pleural effusions (MPE). “I think we can avoid a big proportion of hospitalizations for MPE, and certainly a proportion of repeat [ED] visits,” said senior author Anne Gonzalez, MD, an attending pulmonologist at McGill.
The investigators looked into the issue after noting that a lot of their MPE cases had been hospitalized with chest tubes. They reviewed 72 symptomatic MPE cases in 69 patients treated in 2014 and 2015. Management was ideal in 36 cases (50%), meaning that, prior to definitive treatment, patients had no more than two pleural taps for symptom relief, no more than one ED visit, no chest tubes without pleurodesis, and no hospitalizations. “We thought this would be reasonable to try to achieve for MPE,” since there’s no definition of ideal management, Dr. Shieh said.
Nonideal patients had a mean of 2.5 pleural procedures – almost twice the number in the ideal group – before definitive palliation, with no respiratory consult beforehand. Chest tubes were placed in 27 cases (38%) for an average of 3.7 days; 28 cases (39%) were hospitalized. Nonideal patients were far more likely to present first to the ED, and ED presentations were more likely to get chest tubes and be admitted. All the cases were eventually treated definitively, 68 with indwelling pleural catheters and 4 by thoracoscopic talc insufflation. Time from initial presentation to definitive palliation was about 1 month in both groups. The investigators didn’t consider rate of effusion recurrence, which might help explain why the ideal group wasn’t treated sooner; they might not have needed it. The higher number of ED visits in the nonideal group suggests that they may have had quicker recurrences, and should have been treated sooner, Dr. Gonzalez said.
The patients were 70 years old, on average, and about 60% were women. Lung and breast were the most common cancers.
There was no industry funding for the work, and the investigators had no disclosures.
LOS ANGELES – About half of patients with symptomatic malignant pleural effusions at McGill University Health Centre in Montreal had unnecessary procedures and hospital admissions before definitive treatment with chemical pleurodesis or indwelling pleural catheters, according to researchers.
Instead of chest taps to relieve symptoms followed by referrals for definitive treatment, some patients got chest tubes – without pleurodesis – after presenting to the emergency department and being referred to radiology; they were then admitted to the hospital for a few days while the tubes were in place. In short, cancer patients were wasting what time they had left on medical care they didn’t need, and incurring unnecessary costs, said lead investigator Benjamin Shieh, MD, formerly at McGill but now an interventional pulmonology fellow at the University of Calgary.
McGill is a tertiary care center able to perform both definitive procedures, so “we should be a center of excellence. I imagine there are similar situations” at other hospitals, especially those without the resources of McGill, Dr. Shieh said at the annual meeting of the American College of Chest Physicians.
McGill has taken several steps to address the problem, including early ED referral to the pulmonology service and discouraging radiology from placing chest tubes for malignant pleural effusions (MPE). “I think we can avoid a big proportion of hospitalizations for MPE, and certainly a proportion of repeat [ED] visits,” said senior author Anne Gonzalez, MD, an attending pulmonologist at McGill.
The investigators looked into the issue after noting that a lot of their MPE cases had been hospitalized with chest tubes. They reviewed 72 symptomatic MPE cases in 69 patients treated in 2014 and 2015. Management was ideal in 36 cases (50%), meaning that, prior to definitive treatment, patients had no more than two pleural taps for symptom relief, no more than one ED visit, no chest tubes without pleurodesis, and no hospitalizations. “We thought this would be reasonable to try to achieve for MPE,” since there’s no definition of ideal management, Dr. Shieh said.
Nonideal patients had a mean of 2.5 pleural procedures – almost twice the number in the ideal group – before definitive palliation, with no respiratory consult beforehand. Chest tubes were placed in 27 cases (38%) for an average of 3.7 days; 28 cases (39%) were hospitalized. Nonideal patients were far more likely to present first to the ED, and ED presentations were more likely to get chest tubes and be admitted. All the cases were eventually treated definitively, 68 with indwelling pleural catheters and 4 by thoracoscopic talc insufflation. Time from initial presentation to definitive palliation was about 1 month in both groups. The investigators didn’t consider rate of effusion recurrence, which might help explain why the ideal group wasn’t treated sooner; they might not have needed it. The higher number of ED visits in the nonideal group suggests that they may have had quicker recurrences, and should have been treated sooner, Dr. Gonzalez said.
The patients were 70 years old, on average, and about 60% were women. Lung and breast were the most common cancers.
There was no industry funding for the work, and the investigators had no disclosures.
AT CHEST 2016
Key clinical point:
Major finding: Those patients had a mean of 2.5 pleural procedures before definitive palliation, with no respiratory consult beforehand. Chest tubes were placed for an average of 3.7 days.
Data source: Review of 72 MPE cases in 69 patients.
Disclosures: There was no industry funding for the work, and the investigators had no disclosures.
POEM procedure effective over the long term in achalasia
The therapeutic endoscopic procedure per-oral endoscopic myotomy (POEM) is safe and has a high clinical success rate at 2 years in patients with the rare esophageal motility disorder achalasia, according to results of the longest follow-up study to date.
The research team said that achalasia is characterized by the loss of enteric neurons, which results in impaired relaxation of the lower esophageal sphincter and absence of esophageal peristalsis.
The newer procedure, called POEM, is based on a technique of submucosal endoscopy and endoscopic myotomy. However, while short-term studies have shown it to be safe and effective for achalasia, long-term data have been limited.
“As achalasia is a chronic disease with a risk of recurrence of symptoms after treatment, long-term efficacy, rather than short-term data, is important for decision making surrounding the optimal therapeutic modalities,” wrote the investigators, led by Saowanee Ngamruengphong, MD, of Johns Hopkins Hospital, Baltimore (Gastrointest Endosc. 2016. doi: 10.1016/j.gie.2016.09.017).
The study involved 205 patients with achalasia from 10 centers in the United States, Europe, and Asia. Patients were included in the study if they had at least 2 years of follow-up data available.
Results showed that the overall clinical success – defined by a decrease in Eckardt score to 3 or lower – for the procedure was 91% at 2 years. The Eckardt score (maximum 12) is a composite measure of frequency of symptoms and severity of weight loss.
Overall, 18 patients (8.8%) were considered to have clinical failure (Eckardt score greater than 3) after POEM.
A history of prior pneumatic dilation was associated with long-term treatment failure (odds ratio, 3.41; 95% confidence interval, 1.25-9.23).
The researchers noted that the therapeutic success of POEM decreased over time as the clinical success rate of the procedure at 6 months’ follow-up had been 98%.
This is consistent with findings from other studies and also with other procedures such as Heller myotomy and pneumatic dilation.
“After POEM or other standard therapies, patients with achalasia require ongoing follow-up to assess recurrent symptoms with additional evaluation and treatment when indicated,” they wrote.
In terms of safety, the researchers reported procedure-related adverse events in 8.2% of patients, with one patient requiring surgical intervention.
Abnormal esophageal acid exposure and reflux esophagitis were also documented in 37.5% and 18% of patients, respectively.
Multivariate analysis revealed that full-thickness myotomy was independently associated with postoperative reflux esophagitis (adjusted OR, 3.99; 95% CI, 1.16-13.68; P = .002).
The authors noted that while reflux was common after POEM, almost all patients could be successfully treated with proton pump inhibitors.
“Prospective randomized studies comparing POEM and current standard therapy are awaited, but in the meantime, it can be considered an effective alternative option for patients with achalasia where expertise is available,” they concluded.
The therapeutic endoscopic procedure per-oral endoscopic myotomy (POEM) is safe and has a high clinical success rate at 2 years in patients with the rare esophageal motility disorder achalasia, according to results of the longest follow-up study to date.
The research team said that achalasia is characterized by the loss of enteric neurons, which results in impaired relaxation of the lower esophageal sphincter and absence of esophageal peristalsis.
The newer procedure, called POEM, is based on a technique of submucosal endoscopy and endoscopic myotomy. However, while short-term studies have shown it to be safe and effective for achalasia, long-term data have been limited.
“As achalasia is a chronic disease with a risk of recurrence of symptoms after treatment, long-term efficacy, rather than short-term data, is important for decision making surrounding the optimal therapeutic modalities,” wrote the investigators, led by Saowanee Ngamruengphong, MD, of Johns Hopkins Hospital, Baltimore (Gastrointest Endosc. 2016. doi: 10.1016/j.gie.2016.09.017).
The study involved 205 patients with achalasia from 10 centers in the United States, Europe, and Asia. Patients were included in the study if they had at least 2 years of follow-up data available.
Results showed that the overall clinical success – defined by a decrease in Eckardt score to 3 or lower – for the procedure was 91% at 2 years. The Eckardt score (maximum 12) is a composite measure of frequency of symptoms and severity of weight loss.
Overall, 18 patients (8.8%) were considered to have clinical failure (Eckardt score greater than 3) after POEM.
A history of prior pneumatic dilation was associated with long-term treatment failure (odds ratio, 3.41; 95% confidence interval, 1.25-9.23).
The researchers noted that the therapeutic success of POEM decreased over time as the clinical success rate of the procedure at 6 months’ follow-up had been 98%.
This is consistent with findings from other studies and also with other procedures such as Heller myotomy and pneumatic dilation.
“After POEM or other standard therapies, patients with achalasia require ongoing follow-up to assess recurrent symptoms with additional evaluation and treatment when indicated,” they wrote.
In terms of safety, the researchers reported procedure-related adverse events in 8.2% of patients, with one patient requiring surgical intervention.
Abnormal esophageal acid exposure and reflux esophagitis were also documented in 37.5% and 18% of patients, respectively.
Multivariate analysis revealed that full-thickness myotomy was independently associated with postoperative reflux esophagitis (adjusted OR, 3.99; 95% CI, 1.16-13.68; P = .002).
The authors noted that while reflux was common after POEM, almost all patients could be successfully treated with proton pump inhibitors.
“Prospective randomized studies comparing POEM and current standard therapy are awaited, but in the meantime, it can be considered an effective alternative option for patients with achalasia where expertise is available,” they concluded.
The therapeutic endoscopic procedure per-oral endoscopic myotomy (POEM) is safe and has a high clinical success rate at 2 years in patients with the rare esophageal motility disorder achalasia, according to results of the longest follow-up study to date.
The research team said that achalasia is characterized by the loss of enteric neurons, which results in impaired relaxation of the lower esophageal sphincter and absence of esophageal peristalsis.
The newer procedure, called POEM, is based on a technique of submucosal endoscopy and endoscopic myotomy. However, while short-term studies have shown it to be safe and effective for achalasia, long-term data have been limited.
“As achalasia is a chronic disease with a risk of recurrence of symptoms after treatment, long-term efficacy, rather than short-term data, is important for decision making surrounding the optimal therapeutic modalities,” wrote the investigators, led by Saowanee Ngamruengphong, MD, of Johns Hopkins Hospital, Baltimore (Gastrointest Endosc. 2016. doi: 10.1016/j.gie.2016.09.017).
The study involved 205 patients with achalasia from 10 centers in the United States, Europe, and Asia. Patients were included in the study if they had at least 2 years of follow-up data available.
Results showed that the overall clinical success – defined by a decrease in Eckardt score to 3 or lower – for the procedure was 91% at 2 years. The Eckardt score (maximum 12) is a composite measure of frequency of symptoms and severity of weight loss.
Overall, 18 patients (8.8%) were considered to have clinical failure (Eckardt score greater than 3) after POEM.
A history of prior pneumatic dilation was associated with long-term treatment failure (odds ratio, 3.41; 95% confidence interval, 1.25-9.23).
The researchers noted that the therapeutic success of POEM decreased over time as the clinical success rate of the procedure at 6 months’ follow-up had been 98%.
This is consistent with findings from other studies and also with other procedures such as Heller myotomy and pneumatic dilation.
“After POEM or other standard therapies, patients with achalasia require ongoing follow-up to assess recurrent symptoms with additional evaluation and treatment when indicated,” they wrote.
In terms of safety, the researchers reported procedure-related adverse events in 8.2% of patients, with one patient requiring surgical intervention.
Abnormal esophageal acid exposure and reflux esophagitis were also documented in 37.5% and 18% of patients, respectively.
Multivariate analysis revealed that full-thickness myotomy was independently associated with postoperative reflux esophagitis (adjusted OR, 3.99; 95% CI, 1.16-13.68; P = .002).
The authors noted that while reflux was common after POEM, almost all patients could be successfully treated with proton pump inhibitors.
“Prospective randomized studies comparing POEM and current standard therapy are awaited, but in the meantime, it can be considered an effective alternative option for patients with achalasia where expertise is available,” they concluded.
FROM GASTROINTESTINAL ENDOSCOPY
Key clinical point: The therapeutic endoscopic procedure per-oral endoscopic myotomy (POEM) has a high clinical success rate at 2 years in patients with achalasia.
Main finding: Overall clinical success for the procedure – defined by a decrease in Eckardt score to 3 or lower – was 91% at 2 years’ follow-up.
Data source: Retrospective study of 205 patients with achalasia from 10 centers across the United States, Europe, and Asia.
Disclosures: Several of the authors are consultants for Medtronic, Boston Scientific, and Sandhill Scientific, but no conflicts of interest were declared in relation to the current paper.
Targeting HER1/2 falls flat in bladder cancer trial
Patients with metastatic urothelial bladder cancer (UBC) overexpressing HER1 or HER2 did not benefit from a course of lapatinib maintenance therapy following chemotherapy, a U.K.-based research group reported.
The phase III study, led by Thomas Powles, MD, of Queen Mary University of London, randomized 232 patients (mean age 71, about 75% male) with HER1- or HER2-positive metastatic UBC who had not progressed during platinum-based chemotherapy to placebo or lapatinib (Tykerb), an oral medication that targets HER1 and HER2 and is marketed for use in some breast cancers. The lapatinib-treated group saw no significant gains in either progression-free (PFS) or overall survival (OS), Dr. Powles and associates reported (J Clin Oncol. 2016 Oct 31. doi: 10.1200/JCO.2015.66.3468).
The median PFS for patients receiving lapatinib 1,500 mg daily was 4.5 months, compared with 5.1 months for the placebo group (hazard ratio, 1.07; 95% CI, 0.81-1.43; P = .63), while OS after chemotherapy was 12.6 and 12 months, respectively (HR 0.96; 95% CI, 0.70-1.31; P = .80). A subgroup of patients strongly positive for either or both receptors did not see significant OS or PFS benefit associated with lapatinib, a finding that the investigators said reinforced a lack of benefit.
While previous studies have indicated roles for both HER1 and HER2 in bladder cancer progression, targeting them “may not be of clinical benefit in UBC,” Dr. Powles and his colleagues wrote.
Patients with metastatic UBC have short overall survival following first-line chemotherapy, and few proven second-line treatment options exist besides additional chemotherapy, whose benefit is controversial, the researchers noted.
Despite this trial’s negative result for postchemotherapy maintenance treatment with lapatinib, Dr. Powles and his colleagues said their study, which screened 446 patients with metastatic UBC before randomizing slightly more than half, nonetheless shed some light on this difficult-to-treat patient group, including identifying three prognostic factors associated with poor outcome: radiologic progression during chemotherapy, visceral metastasis, and poor performance status. Also, they noted, 61% of the screened patients received cisplatin chemotherapy, and 48% had visceral metastasis, “which gives some insight into the current population of patients who receive chemotherapy.”
GlaxoSmithKline and Cancer Research U.K. sponsored the study. Dr. Powles and several coauthors disclosed financial support from GlaxoSmithKline and other pharmaceutical firms.
Patients with metastatic urothelial bladder cancer (UBC) overexpressing HER1 or HER2 did not benefit from a course of lapatinib maintenance therapy following chemotherapy, a U.K.-based research group reported.
The phase III study, led by Thomas Powles, MD, of Queen Mary University of London, randomized 232 patients (mean age 71, about 75% male) with HER1- or HER2-positive metastatic UBC who had not progressed during platinum-based chemotherapy to placebo or lapatinib (Tykerb), an oral medication that targets HER1 and HER2 and is marketed for use in some breast cancers. The lapatinib-treated group saw no significant gains in either progression-free (PFS) or overall survival (OS), Dr. Powles and associates reported (J Clin Oncol. 2016 Oct 31. doi: 10.1200/JCO.2015.66.3468).
The median PFS for patients receiving lapatinib 1,500 mg daily was 4.5 months, compared with 5.1 months for the placebo group (hazard ratio, 1.07; 95% CI, 0.81-1.43; P = .63), while OS after chemotherapy was 12.6 and 12 months, respectively (HR 0.96; 95% CI, 0.70-1.31; P = .80). A subgroup of patients strongly positive for either or both receptors did not see significant OS or PFS benefit associated with lapatinib, a finding that the investigators said reinforced a lack of benefit.
While previous studies have indicated roles for both HER1 and HER2 in bladder cancer progression, targeting them “may not be of clinical benefit in UBC,” Dr. Powles and his colleagues wrote.
Patients with metastatic UBC have short overall survival following first-line chemotherapy, and few proven second-line treatment options exist besides additional chemotherapy, whose benefit is controversial, the researchers noted.
Despite this trial’s negative result for postchemotherapy maintenance treatment with lapatinib, Dr. Powles and his colleagues said their study, which screened 446 patients with metastatic UBC before randomizing slightly more than half, nonetheless shed some light on this difficult-to-treat patient group, including identifying three prognostic factors associated with poor outcome: radiologic progression during chemotherapy, visceral metastasis, and poor performance status. Also, they noted, 61% of the screened patients received cisplatin chemotherapy, and 48% had visceral metastasis, “which gives some insight into the current population of patients who receive chemotherapy.”
GlaxoSmithKline and Cancer Research U.K. sponsored the study. Dr. Powles and several coauthors disclosed financial support from GlaxoSmithKline and other pharmaceutical firms.
Patients with metastatic urothelial bladder cancer (UBC) overexpressing HER1 or HER2 did not benefit from a course of lapatinib maintenance therapy following chemotherapy, a U.K.-based research group reported.
The phase III study, led by Thomas Powles, MD, of Queen Mary University of London, randomized 232 patients (mean age 71, about 75% male) with HER1- or HER2-positive metastatic UBC who had not progressed during platinum-based chemotherapy to placebo or lapatinib (Tykerb), an oral medication that targets HER1 and HER2 and is marketed for use in some breast cancers. The lapatinib-treated group saw no significant gains in either progression-free (PFS) or overall survival (OS), Dr. Powles and associates reported (J Clin Oncol. 2016 Oct 31. doi: 10.1200/JCO.2015.66.3468).
The median PFS for patients receiving lapatinib 1,500 mg daily was 4.5 months, compared with 5.1 months for the placebo group (hazard ratio, 1.07; 95% CI, 0.81-1.43; P = .63), while OS after chemotherapy was 12.6 and 12 months, respectively (HR 0.96; 95% CI, 0.70-1.31; P = .80). A subgroup of patients strongly positive for either or both receptors did not see significant OS or PFS benefit associated with lapatinib, a finding that the investigators said reinforced a lack of benefit.
While previous studies have indicated roles for both HER1 and HER2 in bladder cancer progression, targeting them “may not be of clinical benefit in UBC,” Dr. Powles and his colleagues wrote.
Patients with metastatic UBC have short overall survival following first-line chemotherapy, and few proven second-line treatment options exist besides additional chemotherapy, whose benefit is controversial, the researchers noted.
Despite this trial’s negative result for postchemotherapy maintenance treatment with lapatinib, Dr. Powles and his colleagues said their study, which screened 446 patients with metastatic UBC before randomizing slightly more than half, nonetheless shed some light on this difficult-to-treat patient group, including identifying three prognostic factors associated with poor outcome: radiologic progression during chemotherapy, visceral metastasis, and poor performance status. Also, they noted, 61% of the screened patients received cisplatin chemotherapy, and 48% had visceral metastasis, “which gives some insight into the current population of patients who receive chemotherapy.”
GlaxoSmithKline and Cancer Research U.K. sponsored the study. Dr. Powles and several coauthors disclosed financial support from GlaxoSmithKline and other pharmaceutical firms.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Treatment with lapatinib after chemotherapy does not improve survival in people with HER1- or HER2-positive metastatic urothelial bladder cancer.
Major finding: Median progression-free survival for lapatinib was 4.5 months (95% CI, 2.8-5.4), compared with 5.1 (95% CI, 3.0-5.8) for placebo (HR, 1.07; 95% CI, 0.81-1.43; P = .063).
Data source: A randomized, placebo-controlled trial in which 232 patients with HER1- or HER2-positive disease were assigned treatment with lapatinib (n = 116) or placebo (n = 116) after platinum-based chemotherapy.
Disclosures: GlaxoSmithKline and Cancer Research U.K. sponsored the study. Dr. Powles and several coauthors disclosed financial support from GlaxoSmithKline and other pharmaceutical firms.