User login
Expert tips for working with aspiring LGBT parents
SALT LAKE CITY – An aspiring mother calls the sperm bank she has arranged to work with for insemination. “We can ship sperm across state lines,” she is told – until the clinic learns she has a wife and reverses its policy.
This is just one of many experiences faced by lesbian, gay, bisexual, and transgender (LGBT) individuals on the road to parenthood, Sarah R. Holley, PhD, in the psychology department bat San Francisco (Calif.) State University, said at the annual meeting of the American Society of Reproductive Medicine.
Such experiences harm LGBT persons seeking ART and convince them that clinics only care about their money, according to Dr. Holley. So how to do better? “In order to combat heteronormative bias, we first need to get out of problem-solving mode and offer emotional understanding,” she said. For example, providers should never assume that the uteri and eggs of two female partners are interchangeable, or that a woman who is infertile should not grieve, simply because her wife can conceive.
Providers also should be ready to discuss the downsides of tandem pregnancy with lesbian couples, said Angela K. Lawson, PhD, a psychologist at Northwestern University in Chicago. While few studies have examined specific outcomes, “Each woman has a different chance of a successful pregnancy,” she said. “What if one or both women have complications, or a medically challenged child, or they don’t get pregnant at same time?” By taking turns at IVF, couples can better cope with these potential outcomes, she suggested.
Increasingly, lesbian couples are pursuing “reciprocal in vitro fertilization,” in which one woman contributes her ovum for embryo formation, and her partner undergoes implantation. The correct terms here are “genetic mother” and “gestational mother,” not “egg donor” and “gestational carrier,” which have completely different emotional and legal implications, Dr. Lawson emphasized.
That difference makes it imperative for ART clinics to use properly worded consent forms, said Colleen M. Quinn, JD, of the Adoption and Surrogacy Law Center at Locke & Quinn in Richmond, Va. The U.S. Supreme Court decision recognizing same-sex marriage did not clarify legal parental status; birth certificates do not grant legal parental status or rescind the rights of sperm donors, she said. As a result, lesbian couples who separated have won or lost custody battles based on the wording of ART consent forms, she added. Clinics are ethically obligated to advise their LGBT clients to seek legal counsel and create their own agreements about intended parenthood, she emphasized.
For their own protection, clinics also should insist that couples create a legal property disposition agreement between themselves before creating or storing embryos on their behalf, Ms. Quinn said. “An informed consent document is not sufficient,” she added. “Neither is a disposition agreement with the clinic.”
Prospective transgender parents also merit empathic consideration, said Dr. Lawson. Self-identified women who are biologically male may face “profound sadness” because they cannot carry a pregnancy. Conversely, transgender men who become pregnant in order to fulfill dreams of children may nonetheless experience intense gender dysphoria. In at least one case, a transgender male secluded himself at home throughout his pregnancy to avoid public scrutiny, Dr. Lawson said. In all cases, it helps to identify local sources of support and information and refer patients appropriately, Dr. Holley noted.
The experts also briefly covered prospective gay male fathers, who may pay upward of $100,000 to work with a gestational carrier from an agency, they said. Couples who cannot afford to do so may work with a female friend or seek a gestational carrier in another country, each of which raises questions about parental involvement and legal rights. When same-sex male partners are both donating sperm for embryo formation, “One of the biggest controversies is what to do if only one embryo implants,” Dr. Lawson said. “Will the fathers pursue DNA testing of that child? This gets us into issues of intentional unknowing and secrecy, and we know that secrecy can destroy families, whereas privacy typically doesn’t. It’s in the best interest of children to know the story of their parentage.”
None of the experts acknowledged funding sources. Ms. Quinn had no disclosures.
SALT LAKE CITY – An aspiring mother calls the sperm bank she has arranged to work with for insemination. “We can ship sperm across state lines,” she is told – until the clinic learns she has a wife and reverses its policy.
This is just one of many experiences faced by lesbian, gay, bisexual, and transgender (LGBT) individuals on the road to parenthood, Sarah R. Holley, PhD, in the psychology department bat San Francisco (Calif.) State University, said at the annual meeting of the American Society of Reproductive Medicine.
Such experiences harm LGBT persons seeking ART and convince them that clinics only care about their money, according to Dr. Holley. So how to do better? “In order to combat heteronormative bias, we first need to get out of problem-solving mode and offer emotional understanding,” she said. For example, providers should never assume that the uteri and eggs of two female partners are interchangeable, or that a woman who is infertile should not grieve, simply because her wife can conceive.
Providers also should be ready to discuss the downsides of tandem pregnancy with lesbian couples, said Angela K. Lawson, PhD, a psychologist at Northwestern University in Chicago. While few studies have examined specific outcomes, “Each woman has a different chance of a successful pregnancy,” she said. “What if one or both women have complications, or a medically challenged child, or they don’t get pregnant at same time?” By taking turns at IVF, couples can better cope with these potential outcomes, she suggested.
Increasingly, lesbian couples are pursuing “reciprocal in vitro fertilization,” in which one woman contributes her ovum for embryo formation, and her partner undergoes implantation. The correct terms here are “genetic mother” and “gestational mother,” not “egg donor” and “gestational carrier,” which have completely different emotional and legal implications, Dr. Lawson emphasized.
That difference makes it imperative for ART clinics to use properly worded consent forms, said Colleen M. Quinn, JD, of the Adoption and Surrogacy Law Center at Locke & Quinn in Richmond, Va. The U.S. Supreme Court decision recognizing same-sex marriage did not clarify legal parental status; birth certificates do not grant legal parental status or rescind the rights of sperm donors, she said. As a result, lesbian couples who separated have won or lost custody battles based on the wording of ART consent forms, she added. Clinics are ethically obligated to advise their LGBT clients to seek legal counsel and create their own agreements about intended parenthood, she emphasized.
For their own protection, clinics also should insist that couples create a legal property disposition agreement between themselves before creating or storing embryos on their behalf, Ms. Quinn said. “An informed consent document is not sufficient,” she added. “Neither is a disposition agreement with the clinic.”
Prospective transgender parents also merit empathic consideration, said Dr. Lawson. Self-identified women who are biologically male may face “profound sadness” because they cannot carry a pregnancy. Conversely, transgender men who become pregnant in order to fulfill dreams of children may nonetheless experience intense gender dysphoria. In at least one case, a transgender male secluded himself at home throughout his pregnancy to avoid public scrutiny, Dr. Lawson said. In all cases, it helps to identify local sources of support and information and refer patients appropriately, Dr. Holley noted.
The experts also briefly covered prospective gay male fathers, who may pay upward of $100,000 to work with a gestational carrier from an agency, they said. Couples who cannot afford to do so may work with a female friend or seek a gestational carrier in another country, each of which raises questions about parental involvement and legal rights. When same-sex male partners are both donating sperm for embryo formation, “One of the biggest controversies is what to do if only one embryo implants,” Dr. Lawson said. “Will the fathers pursue DNA testing of that child? This gets us into issues of intentional unknowing and secrecy, and we know that secrecy can destroy families, whereas privacy typically doesn’t. It’s in the best interest of children to know the story of their parentage.”
None of the experts acknowledged funding sources. Ms. Quinn had no disclosures.
SALT LAKE CITY – An aspiring mother calls the sperm bank she has arranged to work with for insemination. “We can ship sperm across state lines,” she is told – until the clinic learns she has a wife and reverses its policy.
This is just one of many experiences faced by lesbian, gay, bisexual, and transgender (LGBT) individuals on the road to parenthood, Sarah R. Holley, PhD, in the psychology department bat San Francisco (Calif.) State University, said at the annual meeting of the American Society of Reproductive Medicine.
Such experiences harm LGBT persons seeking ART and convince them that clinics only care about their money, according to Dr. Holley. So how to do better? “In order to combat heteronormative bias, we first need to get out of problem-solving mode and offer emotional understanding,” she said. For example, providers should never assume that the uteri and eggs of two female partners are interchangeable, or that a woman who is infertile should not grieve, simply because her wife can conceive.
Providers also should be ready to discuss the downsides of tandem pregnancy with lesbian couples, said Angela K. Lawson, PhD, a psychologist at Northwestern University in Chicago. While few studies have examined specific outcomes, “Each woman has a different chance of a successful pregnancy,” she said. “What if one or both women have complications, or a medically challenged child, or they don’t get pregnant at same time?” By taking turns at IVF, couples can better cope with these potential outcomes, she suggested.
Increasingly, lesbian couples are pursuing “reciprocal in vitro fertilization,” in which one woman contributes her ovum for embryo formation, and her partner undergoes implantation. The correct terms here are “genetic mother” and “gestational mother,” not “egg donor” and “gestational carrier,” which have completely different emotional and legal implications, Dr. Lawson emphasized.
That difference makes it imperative for ART clinics to use properly worded consent forms, said Colleen M. Quinn, JD, of the Adoption and Surrogacy Law Center at Locke & Quinn in Richmond, Va. The U.S. Supreme Court decision recognizing same-sex marriage did not clarify legal parental status; birth certificates do not grant legal parental status or rescind the rights of sperm donors, she said. As a result, lesbian couples who separated have won or lost custody battles based on the wording of ART consent forms, she added. Clinics are ethically obligated to advise their LGBT clients to seek legal counsel and create their own agreements about intended parenthood, she emphasized.
For their own protection, clinics also should insist that couples create a legal property disposition agreement between themselves before creating or storing embryos on their behalf, Ms. Quinn said. “An informed consent document is not sufficient,” she added. “Neither is a disposition agreement with the clinic.”
Prospective transgender parents also merit empathic consideration, said Dr. Lawson. Self-identified women who are biologically male may face “profound sadness” because they cannot carry a pregnancy. Conversely, transgender men who become pregnant in order to fulfill dreams of children may nonetheless experience intense gender dysphoria. In at least one case, a transgender male secluded himself at home throughout his pregnancy to avoid public scrutiny, Dr. Lawson said. In all cases, it helps to identify local sources of support and information and refer patients appropriately, Dr. Holley noted.
The experts also briefly covered prospective gay male fathers, who may pay upward of $100,000 to work with a gestational carrier from an agency, they said. Couples who cannot afford to do so may work with a female friend or seek a gestational carrier in another country, each of which raises questions about parental involvement and legal rights. When same-sex male partners are both donating sperm for embryo formation, “One of the biggest controversies is what to do if only one embryo implants,” Dr. Lawson said. “Will the fathers pursue DNA testing of that child? This gets us into issues of intentional unknowing and secrecy, and we know that secrecy can destroy families, whereas privacy typically doesn’t. It’s in the best interest of children to know the story of their parentage.”
None of the experts acknowledged funding sources. Ms. Quinn had no disclosures.
EXPERT ANALYSIS FROM ASRM 2016
Improving the Care of Patients with COPD
In recognition of Chronic Obstructive Pulmonary Disease (COPD) Month, check out SHM’s free guide and toolkit to improve the care of patients hospitalized for an exacerbation of COPD. The toolkit can also help you make changes to COPD care at both the individual patient and institutional level.
Download the guide or view the toolkit today at www.hospitalmedicine.org/copd.
In recognition of Chronic Obstructive Pulmonary Disease (COPD) Month, check out SHM’s free guide and toolkit to improve the care of patients hospitalized for an exacerbation of COPD. The toolkit can also help you make changes to COPD care at both the individual patient and institutional level.
Download the guide or view the toolkit today at www.hospitalmedicine.org/copd.
In recognition of Chronic Obstructive Pulmonary Disease (COPD) Month, check out SHM’s free guide and toolkit to improve the care of patients hospitalized for an exacerbation of COPD. The toolkit can also help you make changes to COPD care at both the individual patient and institutional level.
Download the guide or view the toolkit today at www.hospitalmedicine.org/copd.
Combating drug resistance in FLT3-mutated AML
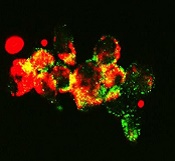
with autophagosomes (green),
a process that happens during
mitophagy in cancer cells
treated with FLT3 inhibitor
Image from Besim Ogretmen
and Mohammed Dany/MUSC
Research published in Blood has revealed a mechanism that confers treatment resistance in FLT3-mutated acute myeloid leukemia (AML), as well as a drug that might overcome that resistance.
Researchers found that ceramide-dependent mitophagy plays a key role in drug-mediated AML cell death.
“Ceramide, a pro-cell-death lipid, kills cancer cells by causing them to eat their own mitochondria,” explained study author Besim Ogretmen, PhD, of the Medical University of South Carolina (MUSC) Hollings Cancer Center in Charleston, South Carolina.
AML cells with FLT3-ITD inhibit ceramide synthesis and thereby become resistant to cell death. FLT3 inhibitors have been developed to combat this resistance, but they’ve fallen short of expectations.
“Unfortunately, regardless of the inhibitor, the problem of resistance to FLT3-targeted therapy has persisted,” said study author Mohammed Dany, an MD/PhD student at MUSC.
However, Dany, Dr Ogretmen, and their colleagues were able to overcome this resistance with a synthetic ceramide analogue known as LCL-461.
In vitro, the drug reactivated mitophagy and killed AML cells that were resistant to treatment with the FLT3 inhibitor crenolanib.
In mice with crenolanib-resistant human AML xenografts, LCL-461 eliminated AML cells from the bone marrow.
A positively charged molecule, LCL-461 is attracted to the mitochondria of cancer cells, which become negatively charged through the Warburg effect. The researchers said this limits off-target effects that can occur with less specific inhibitors of FLT3 signaling.
Furthermore, Dr Ogretmen’s lab has tested the safety of LCL-461 in previous studies and reported that it had no major side effects at therapeutically active doses.
Dr Ogretmen and his colleagues’ next step is to perform large animal studies with LCL-461.
“We are very excited about this,” Dr Ogretmen said. “Head and neck cancers also respond to this drug very well. What we are trying to do is really cure cancer one disease at a time, and we are digging and digging to understand the mechanisms of how these cancer cells escape therapeutic interventions so that we can find mechanism-based therapeutics to have more tools for treatment.”
LCL-461 was developed at MUSC. The MUSC Foundation for Research Development has patented the drug and licensed it to Charleston-based startup SphingoGene, Inc. ![]()

with autophagosomes (green),
a process that happens during
mitophagy in cancer cells
treated with FLT3 inhibitor
Image from Besim Ogretmen
and Mohammed Dany/MUSC
Research published in Blood has revealed a mechanism that confers treatment resistance in FLT3-mutated acute myeloid leukemia (AML), as well as a drug that might overcome that resistance.
Researchers found that ceramide-dependent mitophagy plays a key role in drug-mediated AML cell death.
“Ceramide, a pro-cell-death lipid, kills cancer cells by causing them to eat their own mitochondria,” explained study author Besim Ogretmen, PhD, of the Medical University of South Carolina (MUSC) Hollings Cancer Center in Charleston, South Carolina.
AML cells with FLT3-ITD inhibit ceramide synthesis and thereby become resistant to cell death. FLT3 inhibitors have been developed to combat this resistance, but they’ve fallen short of expectations.
“Unfortunately, regardless of the inhibitor, the problem of resistance to FLT3-targeted therapy has persisted,” said study author Mohammed Dany, an MD/PhD student at MUSC.
However, Dany, Dr Ogretmen, and their colleagues were able to overcome this resistance with a synthetic ceramide analogue known as LCL-461.
In vitro, the drug reactivated mitophagy and killed AML cells that were resistant to treatment with the FLT3 inhibitor crenolanib.
In mice with crenolanib-resistant human AML xenografts, LCL-461 eliminated AML cells from the bone marrow.
A positively charged molecule, LCL-461 is attracted to the mitochondria of cancer cells, which become negatively charged through the Warburg effect. The researchers said this limits off-target effects that can occur with less specific inhibitors of FLT3 signaling.
Furthermore, Dr Ogretmen’s lab has tested the safety of LCL-461 in previous studies and reported that it had no major side effects at therapeutically active doses.
Dr Ogretmen and his colleagues’ next step is to perform large animal studies with LCL-461.
“We are very excited about this,” Dr Ogretmen said. “Head and neck cancers also respond to this drug very well. What we are trying to do is really cure cancer one disease at a time, and we are digging and digging to understand the mechanisms of how these cancer cells escape therapeutic interventions so that we can find mechanism-based therapeutics to have more tools for treatment.”
LCL-461 was developed at MUSC. The MUSC Foundation for Research Development has patented the drug and licensed it to Charleston-based startup SphingoGene, Inc. ![]()

with autophagosomes (green),
a process that happens during
mitophagy in cancer cells
treated with FLT3 inhibitor
Image from Besim Ogretmen
and Mohammed Dany/MUSC
Research published in Blood has revealed a mechanism that confers treatment resistance in FLT3-mutated acute myeloid leukemia (AML), as well as a drug that might overcome that resistance.
Researchers found that ceramide-dependent mitophagy plays a key role in drug-mediated AML cell death.
“Ceramide, a pro-cell-death lipid, kills cancer cells by causing them to eat their own mitochondria,” explained study author Besim Ogretmen, PhD, of the Medical University of South Carolina (MUSC) Hollings Cancer Center in Charleston, South Carolina.
AML cells with FLT3-ITD inhibit ceramide synthesis and thereby become resistant to cell death. FLT3 inhibitors have been developed to combat this resistance, but they’ve fallen short of expectations.
“Unfortunately, regardless of the inhibitor, the problem of resistance to FLT3-targeted therapy has persisted,” said study author Mohammed Dany, an MD/PhD student at MUSC.
However, Dany, Dr Ogretmen, and their colleagues were able to overcome this resistance with a synthetic ceramide analogue known as LCL-461.
In vitro, the drug reactivated mitophagy and killed AML cells that were resistant to treatment with the FLT3 inhibitor crenolanib.
In mice with crenolanib-resistant human AML xenografts, LCL-461 eliminated AML cells from the bone marrow.
A positively charged molecule, LCL-461 is attracted to the mitochondria of cancer cells, which become negatively charged through the Warburg effect. The researchers said this limits off-target effects that can occur with less specific inhibitors of FLT3 signaling.
Furthermore, Dr Ogretmen’s lab has tested the safety of LCL-461 in previous studies and reported that it had no major side effects at therapeutically active doses.
Dr Ogretmen and his colleagues’ next step is to perform large animal studies with LCL-461.
“We are very excited about this,” Dr Ogretmen said. “Head and neck cancers also respond to this drug very well. What we are trying to do is really cure cancer one disease at a time, and we are digging and digging to understand the mechanisms of how these cancer cells escape therapeutic interventions so that we can find mechanism-based therapeutics to have more tools for treatment.”
LCL-461 was developed at MUSC. The MUSC Foundation for Research Development has patented the drug and licensed it to Charleston-based startup SphingoGene, Inc. ![]()
What good are biosimilars if patients won’t use them?
BOSTON – Biosimilar versions of disease-modifying antirheumatic drugs have arrived in the United States, but even the best, most efficacious drugs are worthless if patients don’t want to take them.
“The science is important, the medicine is important, but at the end of the day, acceptance and use is what’s going to measure success,” said Seth D. Ginsberg, at a biosimilars symposium sponsored by Corrona, a business that provides registry data and consulting services to biopharmaceutical companies.
He illustrated the value of biologic agents with this anecdote: “When we got started long, long ago, we used to hold patient events,” he said “and we usually set up for 100. The instructions to meeting planners were right before the event that it was protocol to pull the front 25, the front-right quarter of chairs. Why? To make room for those who can’t walk, to make room for the wheelchairs,” he said.
“Today, if we have one wheelchair at an event, it’s an outlier, and I can’t think of a better way to summarize the impact that biologics have had on our lives,” he said.
Biosimilar confidence
His group has launched “Operation: Biosimilar Confidence” which is designed to educate patients and physicians about the clinical value and scientific underpinnings of biosimilars, as well as the thorough development, review, and regulatory processes involved.
The goal of the project is to instill confidence in patients by helping them to understand the manufacturer’s safety track record, reliability of the biosimilar supply chain, and the availability to them of support services, if they make the switch to a biosimilar.
“Generics don’t have equivalent patient-support programs, and the projection is theoretically that [biosimilar] manufacturers won’t either. We will not accept that. We are going to do everything we can for those patients, to advocate for the continuation of the support programs that we rely on as patients,” he said.
Patient concerns
Surveys of patient concerns about biosimilars have highlighted four key areas:
- What is the manufacturer’s overall safety record in both biologic agents and small-molecule therapies?
- Supply-chain logistic – Will the manufacturer commit to consistent production and supply?
- Will biosimilar manufacturers provide patient support at levels equal to those offered by innovator biologic makers, and what kind of support will be available – phone, websites, social media, copays, etc.?
- Payer ethics – Will payers offer lower copays, deductibles, or premiums, and are payers as concerned as patients about product safety, supply chain, and support?
The implementation strategy for the campaign will focus on speaking directly to patients through CreakyJoints.org, partner Global Healthy Living Foundation, patient and physician organizations, social and conventional media, advertising, and one-on-one encounters.
“We have to talk directly and indirectly to employers and employee-advocacy groups. We have to let these big self-insured employers understand what the perspective of the patient is and what life is like thanks to these medicine, and why biosimilars are a critical component to the success of living with these conditions,” he said.
Advocates also have to work with the media to create “a surround-sound message that reaches all audiences with additional frequency.”
“We cannot allow Wall Street Journal business analysts to dictate the conversations about biosimilars. Why? They’re looking at one thing, and only one thing, and they’re ignoring the patient perspective,” Ginsberg said.
Lastly, patient groups need to work closely with payers, physician groups, and manufacturers to ensure that biosimilars can be smoothly integrated into the healthcare system, he emphasized.
“I want to be crystal clear here: We can’t wait for biosimilars. Bring it on! We want them,” he said.
BOSTON – Biosimilar versions of disease-modifying antirheumatic drugs have arrived in the United States, but even the best, most efficacious drugs are worthless if patients don’t want to take them.
“The science is important, the medicine is important, but at the end of the day, acceptance and use is what’s going to measure success,” said Seth D. Ginsberg, at a biosimilars symposium sponsored by Corrona, a business that provides registry data and consulting services to biopharmaceutical companies.
He illustrated the value of biologic agents with this anecdote: “When we got started long, long ago, we used to hold patient events,” he said “and we usually set up for 100. The instructions to meeting planners were right before the event that it was protocol to pull the front 25, the front-right quarter of chairs. Why? To make room for those who can’t walk, to make room for the wheelchairs,” he said.
“Today, if we have one wheelchair at an event, it’s an outlier, and I can’t think of a better way to summarize the impact that biologics have had on our lives,” he said.
Biosimilar confidence
His group has launched “Operation: Biosimilar Confidence” which is designed to educate patients and physicians about the clinical value and scientific underpinnings of biosimilars, as well as the thorough development, review, and regulatory processes involved.
The goal of the project is to instill confidence in patients by helping them to understand the manufacturer’s safety track record, reliability of the biosimilar supply chain, and the availability to them of support services, if they make the switch to a biosimilar.
“Generics don’t have equivalent patient-support programs, and the projection is theoretically that [biosimilar] manufacturers won’t either. We will not accept that. We are going to do everything we can for those patients, to advocate for the continuation of the support programs that we rely on as patients,” he said.
Patient concerns
Surveys of patient concerns about biosimilars have highlighted four key areas:
- What is the manufacturer’s overall safety record in both biologic agents and small-molecule therapies?
- Supply-chain logistic – Will the manufacturer commit to consistent production and supply?
- Will biosimilar manufacturers provide patient support at levels equal to those offered by innovator biologic makers, and what kind of support will be available – phone, websites, social media, copays, etc.?
- Payer ethics – Will payers offer lower copays, deductibles, or premiums, and are payers as concerned as patients about product safety, supply chain, and support?
The implementation strategy for the campaign will focus on speaking directly to patients through CreakyJoints.org, partner Global Healthy Living Foundation, patient and physician organizations, social and conventional media, advertising, and one-on-one encounters.
“We have to talk directly and indirectly to employers and employee-advocacy groups. We have to let these big self-insured employers understand what the perspective of the patient is and what life is like thanks to these medicine, and why biosimilars are a critical component to the success of living with these conditions,” he said.
Advocates also have to work with the media to create “a surround-sound message that reaches all audiences with additional frequency.”
“We cannot allow Wall Street Journal business analysts to dictate the conversations about biosimilars. Why? They’re looking at one thing, and only one thing, and they’re ignoring the patient perspective,” Ginsberg said.
Lastly, patient groups need to work closely with payers, physician groups, and manufacturers to ensure that biosimilars can be smoothly integrated into the healthcare system, he emphasized.
“I want to be crystal clear here: We can’t wait for biosimilars. Bring it on! We want them,” he said.
BOSTON – Biosimilar versions of disease-modifying antirheumatic drugs have arrived in the United States, but even the best, most efficacious drugs are worthless if patients don’t want to take them.
“The science is important, the medicine is important, but at the end of the day, acceptance and use is what’s going to measure success,” said Seth D. Ginsberg, at a biosimilars symposium sponsored by Corrona, a business that provides registry data and consulting services to biopharmaceutical companies.
He illustrated the value of biologic agents with this anecdote: “When we got started long, long ago, we used to hold patient events,” he said “and we usually set up for 100. The instructions to meeting planners were right before the event that it was protocol to pull the front 25, the front-right quarter of chairs. Why? To make room for those who can’t walk, to make room for the wheelchairs,” he said.
“Today, if we have one wheelchair at an event, it’s an outlier, and I can’t think of a better way to summarize the impact that biologics have had on our lives,” he said.
Biosimilar confidence
His group has launched “Operation: Biosimilar Confidence” which is designed to educate patients and physicians about the clinical value and scientific underpinnings of biosimilars, as well as the thorough development, review, and regulatory processes involved.
The goal of the project is to instill confidence in patients by helping them to understand the manufacturer’s safety track record, reliability of the biosimilar supply chain, and the availability to them of support services, if they make the switch to a biosimilar.
“Generics don’t have equivalent patient-support programs, and the projection is theoretically that [biosimilar] manufacturers won’t either. We will not accept that. We are going to do everything we can for those patients, to advocate for the continuation of the support programs that we rely on as patients,” he said.
Patient concerns
Surveys of patient concerns about biosimilars have highlighted four key areas:
- What is the manufacturer’s overall safety record in both biologic agents and small-molecule therapies?
- Supply-chain logistic – Will the manufacturer commit to consistent production and supply?
- Will biosimilar manufacturers provide patient support at levels equal to those offered by innovator biologic makers, and what kind of support will be available – phone, websites, social media, copays, etc.?
- Payer ethics – Will payers offer lower copays, deductibles, or premiums, and are payers as concerned as patients about product safety, supply chain, and support?
The implementation strategy for the campaign will focus on speaking directly to patients through CreakyJoints.org, partner Global Healthy Living Foundation, patient and physician organizations, social and conventional media, advertising, and one-on-one encounters.
“We have to talk directly and indirectly to employers and employee-advocacy groups. We have to let these big self-insured employers understand what the perspective of the patient is and what life is like thanks to these medicine, and why biosimilars are a critical component to the success of living with these conditions,” he said.
Advocates also have to work with the media to create “a surround-sound message that reaches all audiences with additional frequency.”
“We cannot allow Wall Street Journal business analysts to dictate the conversations about biosimilars. Why? They’re looking at one thing, and only one thing, and they’re ignoring the patient perspective,” Ginsberg said.
Lastly, patient groups need to work closely with payers, physician groups, and manufacturers to ensure that biosimilars can be smoothly integrated into the healthcare system, he emphasized.
“I want to be crystal clear here: We can’t wait for biosimilars. Bring it on! We want them,” he said.
EXPERT ANALYSIS FROM A BIOSIMILARS IN RHEUMATOLOGY SYMPOSIUM
CDC: Seven cases of multidrug resistant C. auris have occurred in United States
The Centers for Disease Control and Prevention have reported the first cases of the multidrug-resistant fungal infection Candida auris in the United States, with evidence suggesting transmission may have occurred within U.S. health care facilities.
The report, published in the Nov. 4 edition of Morbidity and Mortality Weekly Report, described seven cases of patients infected with C. auris, which was isolated from blood in five cases, urine in one, and the ear in one. All the patients with bloodstream infections had central venous catheters at the time of diagnosis, and four of these patients died in the weeks and months after diagnosis of the infection.
Patients’ underlying conditions usually involved immune system suppression resulting from corticisteroid therapy, malignancty, short gut syndrome, or parapleglia with a long-term, indwelling Foley catheter.
C. auris was first isolated in 2009 in Japan, but has since been reported in countries including Colombia, India, South Africa, Israel, and the United Kingdom. Snigdha Vallabhaneni, MD, of the mycotic diseases branch of CDC’s division of food water and environmental diseases, and her coauthors, said its appearance in the United States is a cause for serious concern (MMWR. 2016 Nov 4. doi: 0.15585/mmwr.mm6544e1).
“First, many isolates are multidrug resistant, with some strains having elevated minimum inhibitory concentrations to drugs in all three major classes of antifungal medications, a feature not found in other clinically relevant Candida species,” the authors wrote. All the patients with bloodstream infections were treated with antifungal echinocandins, and one also received liposomal amphotericin B.
“Second, C. auris is challenging to identify, requiring specialized methods such as matrix-assisted laser desorption/ionization time-of-flight or molecular identification based on sequencing the D1-D2 region of the 28s ribosomal DNA.”
They also highlighted that C. auris is known to cause outbreaks in health care settings. Samples taken from the mattress, bedside table, bed rail, chair, and windowsill in the room of one patient all tested positive for C. auris.
The authors also sequenced the genome of the isolates and found that isolates taken from patients admitted to the same hospital in New Jersey or the same Illinois hospital were nearly identical.
“Facilities should ensure thorough daily and terminal cleaning of rooms of patients with C. auris infections, including use of an [Environmental Protection Agency]–registered disinfectant with a fungal claim,” the authors wrote, stressing that facilities and laboratories should continue to report cases and forward suspicious unidentified Candida isolates to state or local health authorities and the CDC.
No conflicts of interest were declared.
The Centers for Disease Control and Prevention have reported the first cases of the multidrug-resistant fungal infection Candida auris in the United States, with evidence suggesting transmission may have occurred within U.S. health care facilities.
The report, published in the Nov. 4 edition of Morbidity and Mortality Weekly Report, described seven cases of patients infected with C. auris, which was isolated from blood in five cases, urine in one, and the ear in one. All the patients with bloodstream infections had central venous catheters at the time of diagnosis, and four of these patients died in the weeks and months after diagnosis of the infection.
Patients’ underlying conditions usually involved immune system suppression resulting from corticisteroid therapy, malignancty, short gut syndrome, or parapleglia with a long-term, indwelling Foley catheter.
C. auris was first isolated in 2009 in Japan, but has since been reported in countries including Colombia, India, South Africa, Israel, and the United Kingdom. Snigdha Vallabhaneni, MD, of the mycotic diseases branch of CDC’s division of food water and environmental diseases, and her coauthors, said its appearance in the United States is a cause for serious concern (MMWR. 2016 Nov 4. doi: 0.15585/mmwr.mm6544e1).
“First, many isolates are multidrug resistant, with some strains having elevated minimum inhibitory concentrations to drugs in all three major classes of antifungal medications, a feature not found in other clinically relevant Candida species,” the authors wrote. All the patients with bloodstream infections were treated with antifungal echinocandins, and one also received liposomal amphotericin B.
“Second, C. auris is challenging to identify, requiring specialized methods such as matrix-assisted laser desorption/ionization time-of-flight or molecular identification based on sequencing the D1-D2 region of the 28s ribosomal DNA.”
They also highlighted that C. auris is known to cause outbreaks in health care settings. Samples taken from the mattress, bedside table, bed rail, chair, and windowsill in the room of one patient all tested positive for C. auris.
The authors also sequenced the genome of the isolates and found that isolates taken from patients admitted to the same hospital in New Jersey or the same Illinois hospital were nearly identical.
“Facilities should ensure thorough daily and terminal cleaning of rooms of patients with C. auris infections, including use of an [Environmental Protection Agency]–registered disinfectant with a fungal claim,” the authors wrote, stressing that facilities and laboratories should continue to report cases and forward suspicious unidentified Candida isolates to state or local health authorities and the CDC.
No conflicts of interest were declared.
The Centers for Disease Control and Prevention have reported the first cases of the multidrug-resistant fungal infection Candida auris in the United States, with evidence suggesting transmission may have occurred within U.S. health care facilities.
The report, published in the Nov. 4 edition of Morbidity and Mortality Weekly Report, described seven cases of patients infected with C. auris, which was isolated from blood in five cases, urine in one, and the ear in one. All the patients with bloodstream infections had central venous catheters at the time of diagnosis, and four of these patients died in the weeks and months after diagnosis of the infection.
Patients’ underlying conditions usually involved immune system suppression resulting from corticisteroid therapy, malignancty, short gut syndrome, or parapleglia with a long-term, indwelling Foley catheter.
C. auris was first isolated in 2009 in Japan, but has since been reported in countries including Colombia, India, South Africa, Israel, and the United Kingdom. Snigdha Vallabhaneni, MD, of the mycotic diseases branch of CDC’s division of food water and environmental diseases, and her coauthors, said its appearance in the United States is a cause for serious concern (MMWR. 2016 Nov 4. doi: 0.15585/mmwr.mm6544e1).
“First, many isolates are multidrug resistant, with some strains having elevated minimum inhibitory concentrations to drugs in all three major classes of antifungal medications, a feature not found in other clinically relevant Candida species,” the authors wrote. All the patients with bloodstream infections were treated with antifungal echinocandins, and one also received liposomal amphotericin B.
“Second, C. auris is challenging to identify, requiring specialized methods such as matrix-assisted laser desorption/ionization time-of-flight or molecular identification based on sequencing the D1-D2 region of the 28s ribosomal DNA.”
They also highlighted that C. auris is known to cause outbreaks in health care settings. Samples taken from the mattress, bedside table, bed rail, chair, and windowsill in the room of one patient all tested positive for C. auris.
The authors also sequenced the genome of the isolates and found that isolates taken from patients admitted to the same hospital in New Jersey or the same Illinois hospital were nearly identical.
“Facilities should ensure thorough daily and terminal cleaning of rooms of patients with C. auris infections, including use of an [Environmental Protection Agency]–registered disinfectant with a fungal claim,” the authors wrote, stressing that facilities and laboratories should continue to report cases and forward suspicious unidentified Candida isolates to state or local health authorities and the CDC.
No conflicts of interest were declared.
Key clinical point: The first cases of the multidrug-resistant fungal infection C. auris have been reported in the United States.
Major finding: Seven cases of infection with the multidrug-resistant emerging fungal infection C. auris have been reported in the United States, five of which were bloodstream infections.
Data source: Case series.
Disclosures: No conflicts of interest were declared.
FDA: Etanercept first biologic approved for pediatric psoriasis
Etanercept has been received Food and Drug Administration approval for treating chronic moderate to severe plaque psoriasis in children and adolescents, aged 4-17 years, making this the first biologic and first systemic treatment approved in the United States for pediatric psoriasis.
Etanercept, a tumor necrosis factor blocker marketed as Enbrel, was approved in 1998 for treating moderately to severely active rheumatoid arthritis and has been approved for several other indications since then, including psoriatic arthritis and moderate to severe psoriasis in adults, and polyarticular juvenile idiopathic arthritis in patients aged 2 years and older.
Etanercept has been received Food and Drug Administration approval for treating chronic moderate to severe plaque psoriasis in children and adolescents, aged 4-17 years, making this the first biologic and first systemic treatment approved in the United States for pediatric psoriasis.
Etanercept, a tumor necrosis factor blocker marketed as Enbrel, was approved in 1998 for treating moderately to severely active rheumatoid arthritis and has been approved for several other indications since then, including psoriatic arthritis and moderate to severe psoriasis in adults, and polyarticular juvenile idiopathic arthritis in patients aged 2 years and older.
Etanercept has been received Food and Drug Administration approval for treating chronic moderate to severe plaque psoriasis in children and adolescents, aged 4-17 years, making this the first biologic and first systemic treatment approved in the United States for pediatric psoriasis.
Etanercept, a tumor necrosis factor blocker marketed as Enbrel, was approved in 1998 for treating moderately to severely active rheumatoid arthritis and has been approved for several other indications since then, including psoriatic arthritis and moderate to severe psoriasis in adults, and polyarticular juvenile idiopathic arthritis in patients aged 2 years and older.
CMS to pay more for care coordination, prevention
The Centers for Medicare & Medicaid Services plans to spend an extra $140 million on these services next year under the final physician fee schedule, released on Nov. 2 and scheduled for publication in the Federal Register on Nov. 15.
“Clinicians will additionally be able to bill and be paid more appropriately when they spend more time with their patients, serving their patients’ needs outside of the office visit, and better coordinate care,” they wrote.
CMS finalized a number of payment codes that “better identify and value primary care, care management, and cognitive services,” according to an agency fact sheet highlighting key provisions of the final physician fee schedule for 2017.
The coding changes allow for separate payments for non–face-to-face prolonged evaluation and management services; revalue existing codes for face-to-face prolonged services; separate payments for comprehensive assessment and care planning for patients with cognitive impairments such as dementia; and separate payments for chronic care management of complex patients.
“This final decision by CMS means individuals living with Alzheimer’s disease will finally have access to critical care and support services that can improve quality of life for the individual, their family, and caregivers,” Harry Johns, Alzheimer’s Association President and CEO, said in a statement. “Now that care-planning sessions will be available to them, individuals living with the disease will have access to much-needed information on treatments and services.”
“Geriatricians, internists, and family physicians provide core services for the Medicare program, including the kinds of care management and patient-centered care that are described by these new codes,” Mr. Slavitt and Dr. Conway wrote. “Over time, we estimate that the payment increases attributable to these new codes could be as much as 30% and 37%, respectively, to these specialties.”
CMS also finalized other coding changes, including:
- A separate code for moderate sedation services to account for changes in practice trends that report anesthesia separately from certain endoscopic procedures despite payment being built into the overall procedure payment.
- More payments for telehealth services, including for end-stage renal disease-related services for dialysis, advanced care planning; and critical care consultations.
The American College of Physicians applauded the final rule.
“The policies in the rule more accurately recognize the work of primary care physicians and other cognitive specialties to accommodate the changing needs of Medicare beneficiaries,” ACP President Nitin S. Damle, MD, said in a statement.
The Centers for Medicare & Medicaid Services plans to spend an extra $140 million on these services next year under the final physician fee schedule, released on Nov. 2 and scheduled for publication in the Federal Register on Nov. 15.
“Clinicians will additionally be able to bill and be paid more appropriately when they spend more time with their patients, serving their patients’ needs outside of the office visit, and better coordinate care,” they wrote.
CMS finalized a number of payment codes that “better identify and value primary care, care management, and cognitive services,” according to an agency fact sheet highlighting key provisions of the final physician fee schedule for 2017.
The coding changes allow for separate payments for non–face-to-face prolonged evaluation and management services; revalue existing codes for face-to-face prolonged services; separate payments for comprehensive assessment and care planning for patients with cognitive impairments such as dementia; and separate payments for chronic care management of complex patients.
“This final decision by CMS means individuals living with Alzheimer’s disease will finally have access to critical care and support services that can improve quality of life for the individual, their family, and caregivers,” Harry Johns, Alzheimer’s Association President and CEO, said in a statement. “Now that care-planning sessions will be available to them, individuals living with the disease will have access to much-needed information on treatments and services.”
“Geriatricians, internists, and family physicians provide core services for the Medicare program, including the kinds of care management and patient-centered care that are described by these new codes,” Mr. Slavitt and Dr. Conway wrote. “Over time, we estimate that the payment increases attributable to these new codes could be as much as 30% and 37%, respectively, to these specialties.”
CMS also finalized other coding changes, including:
- A separate code for moderate sedation services to account for changes in practice trends that report anesthesia separately from certain endoscopic procedures despite payment being built into the overall procedure payment.
- More payments for telehealth services, including for end-stage renal disease-related services for dialysis, advanced care planning; and critical care consultations.
The American College of Physicians applauded the final rule.
“The policies in the rule more accurately recognize the work of primary care physicians and other cognitive specialties to accommodate the changing needs of Medicare beneficiaries,” ACP President Nitin S. Damle, MD, said in a statement.
The Centers for Medicare & Medicaid Services plans to spend an extra $140 million on these services next year under the final physician fee schedule, released on Nov. 2 and scheduled for publication in the Federal Register on Nov. 15.
“Clinicians will additionally be able to bill and be paid more appropriately when they spend more time with their patients, serving their patients’ needs outside of the office visit, and better coordinate care,” they wrote.
CMS finalized a number of payment codes that “better identify and value primary care, care management, and cognitive services,” according to an agency fact sheet highlighting key provisions of the final physician fee schedule for 2017.
The coding changes allow for separate payments for non–face-to-face prolonged evaluation and management services; revalue existing codes for face-to-face prolonged services; separate payments for comprehensive assessment and care planning for patients with cognitive impairments such as dementia; and separate payments for chronic care management of complex patients.
“This final decision by CMS means individuals living with Alzheimer’s disease will finally have access to critical care and support services that can improve quality of life for the individual, their family, and caregivers,” Harry Johns, Alzheimer’s Association President and CEO, said in a statement. “Now that care-planning sessions will be available to them, individuals living with the disease will have access to much-needed information on treatments and services.”
“Geriatricians, internists, and family physicians provide core services for the Medicare program, including the kinds of care management and patient-centered care that are described by these new codes,” Mr. Slavitt and Dr. Conway wrote. “Over time, we estimate that the payment increases attributable to these new codes could be as much as 30% and 37%, respectively, to these specialties.”
CMS also finalized other coding changes, including:
- A separate code for moderate sedation services to account for changes in practice trends that report anesthesia separately from certain endoscopic procedures despite payment being built into the overall procedure payment.
- More payments for telehealth services, including for end-stage renal disease-related services for dialysis, advanced care planning; and critical care consultations.
The American College of Physicians applauded the final rule.
“The policies in the rule more accurately recognize the work of primary care physicians and other cognitive specialties to accommodate the changing needs of Medicare beneficiaries,” ACP President Nitin S. Damle, MD, said in a statement.
FDA’s Woodcock: Give biosimilars a chance
BOSTON – Biosimilar drugs are not identical twins of original biologic agents, but there are very strong family ties, and the newcomer is expected to look and behave very much like its older relative, said Janet Woodcock, MD, director of the Center for Drug Evaluation and Research at the Food and Drug Administration.
Although biosimilars differ from generic, small-molecule drugs, concerns about the development of biosimilars mirrors the kerfuffle over generic drugs surrounding the passage of the Hatch-Waxman (Drug Price Competition and Patent Term Restoration) Act in 1984.
She noted that clinicians today are asking the same questions about biosimilars that were asked about generics three decades ago:
- Are biosimilars as effective and as safe as the originally licensed biopharmaceuticals?
- If pharmacists substitute biosimilars for prescribed biologics, will patients be adversely affected?
- Can biosimilars reduce the high cost of biologic therapy?
“In certain specialties, this skepticism has persisted to this very day,” she said.
ACA mandate
Biosimilars owe their existence in large measure to the Biologics Price Competition and Innovation Act of 2009 (BPCI), passed as a part of the Affordable Care Act and signed into law by President Obama in 2010.
The act created an abbreviated licensure pathway for biologic products that can be shown to be either biosimilar to or interchangeable with an FDA-licensed reference drug.
A biosimilar is defined as a biological product that is highly similar to the reference product “notwithstanding minor differences in clinically inactive components,” and with no clinically meaningful differences between it and the reference product in terms of purity, safety, and potency.
To be interchangeable, a biosimilar must be expected to produce the same clinical results as the reference drug in any given patient, and “for a product that is administered more than once to an individual, the risk in terms of safety or diminished efficacy of alternating or switching between use of the product and its reference product is not greater than the risk of using the reference product without such alternation or switch.”
The definition of interchangeability includes the understanding that the prescriber’s approval is not necessary for substitution of a biosimilar for its reference product.
“If we’re going to have that kind of switching, if they are going to be interchangeable, then we have to have a very high bar,” Dr. Woodcock said.
She added that “any pressure people are feeling to push their patients to biosimilars is from the reimbursement system. There is no non-prescriber switching allowed currently; however, that doesn’t say there isn’t pressure on prescribers to write a different prescription.”
Faster track and bridge to approval
The biosimilar development and approval requires only convincing demonstration of biosimilarity to an existing agent, rather than an independent finding of safety or effectiveness, and the purpose of clinical studies in this case is to address “residual uncertainties,” Dr. Woodcock said.
Drug developers and regulatory authorities alike “are having trouble getting their mind around this concept,” she said.
The FDA requires manufacturers to provide data in their biosimilar drug license applications demonstrating biosimilarity based on analytical studies, animal studies that include toxicity assessments, and one or more clinical studies that include information on immunogenicity and pharmacokinetics (PK) or pharmacodynamics (PD) that is sufficient to demonstrate the safety, purity, and potency of the candidate biosimilar.
The FDA is also allowing manufacturers to submit data from animal studies and specified clinical studies comparing a proposed biosimilar product with a product not licensed in the United States, “as long as we are convinced that the reference product is equivalent to the U.S. product,” Dr. Woodcock said.
This “analytical bridge” process was requested by manufacturers who began development of biosimilars in Europe. The European Medicines Agency approved a biosimilar process in 2005, and gave the nod to the first biosimilar agents to existing erythropoietin products in 2007.
Current and pending
As of early October 2016, 66 programs were enrolled in FDA’s Biosimilar Product Development Program, and CDER has received requests for meetings with manufacturers to discuss what tests and documents are required for the development of biosimilars to 20 different reference products.
The FDA is prohibited from publicly discussing the existence of a pending application unless it has been previously disclosed or acknowledged publicly with the manufacturer’s permission, Dr. Woodcock noted, but as of Oct. 10, 2016, seven companies have announced a total of 10 biologic license applications for biosimilars to etanercept (Enbrel), adalimumab (Humira), pegfilgrastim (Neulasta), epoetin alfa (Epogen/Procrit), filgrastim (Neupogen), and infliximab (Remicade).
The FDA has granted licenses to four biosimilars to date (the four-letter suffix is intended to differentiate biosimilars agents from other biosimilars to the same reference product):
- Zarxio (filgrastim-sndz).
- Inflectra (infliximab-dyyb).
- Erelzi (etanercept-szzs).
- Amjevita (adalimumab-atto).
Physician perspective
“Everything I have heard suggests that biosimilars will be useful, but the scientist in me is a skeptic,” commented Donald Massenburg, MD, PhD, a rheumatologist at Wheaton Franciscan Healthcare in Franklin, Wisc., in an interview.
“I feel better now after learning that current biosimilars are not considered interchangeable, meaning that there can’t be substitutions made without the treating physician’s consent,” he said.
He acknowledged that “I wouldn’t be that excited” if a specific biosimilar was approved for interchangeability and was given to his patients without his knowledge.
Dr. Massenburg pointed to data from the NOR-SWITCH trial comparing the biosimilar Remsima to the reference product Remicade for treatment of rheumatic diseases, psoriasis, and inflammatory bowel disease. The trial showed that Remsima was noninferior to the reference product.
“I would like to be able to say whether a patient should be switched to a biosimilar or not just because of that potential risk,” Dr. Massenburg said.
A rheumatologist in private practice in New England said that what’s really needed in rheumatology is not the availability of more drugs that act like other drugs, but innovative research into therapies with better targeted mechanism of action.
“We’ve been through the ‘me-too’ hype; we did that with nonsteroidal anti-inflammatory drugs,” said J. Scott Toder, MD, director of the Toder Rheumatology and Osteoporosis Center, Providence, R.I.
“I think we need to concentrate on innovative therapies, and we may be able to do something about the escalating price of the biologics on the market by creating drugs with new mechanism of action to actually increase competition and hopefully control prices. I don’t think that having multiple drugs with the same mechanism of action is in the best interest of our patients,” he said in an interview.
Dr. Woodcock, Dr. Massenburg, and Dr. Toder reported having no relevant disclosures.
BOSTON – Biosimilar drugs are not identical twins of original biologic agents, but there are very strong family ties, and the newcomer is expected to look and behave very much like its older relative, said Janet Woodcock, MD, director of the Center for Drug Evaluation and Research at the Food and Drug Administration.
Although biosimilars differ from generic, small-molecule drugs, concerns about the development of biosimilars mirrors the kerfuffle over generic drugs surrounding the passage of the Hatch-Waxman (Drug Price Competition and Patent Term Restoration) Act in 1984.
She noted that clinicians today are asking the same questions about biosimilars that were asked about generics three decades ago:
- Are biosimilars as effective and as safe as the originally licensed biopharmaceuticals?
- If pharmacists substitute biosimilars for prescribed biologics, will patients be adversely affected?
- Can biosimilars reduce the high cost of biologic therapy?
“In certain specialties, this skepticism has persisted to this very day,” she said.
ACA mandate
Biosimilars owe their existence in large measure to the Biologics Price Competition and Innovation Act of 2009 (BPCI), passed as a part of the Affordable Care Act and signed into law by President Obama in 2010.
The act created an abbreviated licensure pathway for biologic products that can be shown to be either biosimilar to or interchangeable with an FDA-licensed reference drug.
A biosimilar is defined as a biological product that is highly similar to the reference product “notwithstanding minor differences in clinically inactive components,” and with no clinically meaningful differences between it and the reference product in terms of purity, safety, and potency.
To be interchangeable, a biosimilar must be expected to produce the same clinical results as the reference drug in any given patient, and “for a product that is administered more than once to an individual, the risk in terms of safety or diminished efficacy of alternating or switching between use of the product and its reference product is not greater than the risk of using the reference product without such alternation or switch.”
The definition of interchangeability includes the understanding that the prescriber’s approval is not necessary for substitution of a biosimilar for its reference product.
“If we’re going to have that kind of switching, if they are going to be interchangeable, then we have to have a very high bar,” Dr. Woodcock said.
She added that “any pressure people are feeling to push their patients to biosimilars is from the reimbursement system. There is no non-prescriber switching allowed currently; however, that doesn’t say there isn’t pressure on prescribers to write a different prescription.”
Faster track and bridge to approval
The biosimilar development and approval requires only convincing demonstration of biosimilarity to an existing agent, rather than an independent finding of safety or effectiveness, and the purpose of clinical studies in this case is to address “residual uncertainties,” Dr. Woodcock said.
Drug developers and regulatory authorities alike “are having trouble getting their mind around this concept,” she said.
The FDA requires manufacturers to provide data in their biosimilar drug license applications demonstrating biosimilarity based on analytical studies, animal studies that include toxicity assessments, and one or more clinical studies that include information on immunogenicity and pharmacokinetics (PK) or pharmacodynamics (PD) that is sufficient to demonstrate the safety, purity, and potency of the candidate biosimilar.
The FDA is also allowing manufacturers to submit data from animal studies and specified clinical studies comparing a proposed biosimilar product with a product not licensed in the United States, “as long as we are convinced that the reference product is equivalent to the U.S. product,” Dr. Woodcock said.
This “analytical bridge” process was requested by manufacturers who began development of biosimilars in Europe. The European Medicines Agency approved a biosimilar process in 2005, and gave the nod to the first biosimilar agents to existing erythropoietin products in 2007.
Current and pending
As of early October 2016, 66 programs were enrolled in FDA’s Biosimilar Product Development Program, and CDER has received requests for meetings with manufacturers to discuss what tests and documents are required for the development of biosimilars to 20 different reference products.
The FDA is prohibited from publicly discussing the existence of a pending application unless it has been previously disclosed or acknowledged publicly with the manufacturer’s permission, Dr. Woodcock noted, but as of Oct. 10, 2016, seven companies have announced a total of 10 biologic license applications for biosimilars to etanercept (Enbrel), adalimumab (Humira), pegfilgrastim (Neulasta), epoetin alfa (Epogen/Procrit), filgrastim (Neupogen), and infliximab (Remicade).
The FDA has granted licenses to four biosimilars to date (the four-letter suffix is intended to differentiate biosimilars agents from other biosimilars to the same reference product):
- Zarxio (filgrastim-sndz).
- Inflectra (infliximab-dyyb).
- Erelzi (etanercept-szzs).
- Amjevita (adalimumab-atto).
Physician perspective
“Everything I have heard suggests that biosimilars will be useful, but the scientist in me is a skeptic,” commented Donald Massenburg, MD, PhD, a rheumatologist at Wheaton Franciscan Healthcare in Franklin, Wisc., in an interview.
“I feel better now after learning that current biosimilars are not considered interchangeable, meaning that there can’t be substitutions made without the treating physician’s consent,” he said.
He acknowledged that “I wouldn’t be that excited” if a specific biosimilar was approved for interchangeability and was given to his patients without his knowledge.
Dr. Massenburg pointed to data from the NOR-SWITCH trial comparing the biosimilar Remsima to the reference product Remicade for treatment of rheumatic diseases, psoriasis, and inflammatory bowel disease. The trial showed that Remsima was noninferior to the reference product.
“I would like to be able to say whether a patient should be switched to a biosimilar or not just because of that potential risk,” Dr. Massenburg said.
A rheumatologist in private practice in New England said that what’s really needed in rheumatology is not the availability of more drugs that act like other drugs, but innovative research into therapies with better targeted mechanism of action.
“We’ve been through the ‘me-too’ hype; we did that with nonsteroidal anti-inflammatory drugs,” said J. Scott Toder, MD, director of the Toder Rheumatology and Osteoporosis Center, Providence, R.I.
“I think we need to concentrate on innovative therapies, and we may be able to do something about the escalating price of the biologics on the market by creating drugs with new mechanism of action to actually increase competition and hopefully control prices. I don’t think that having multiple drugs with the same mechanism of action is in the best interest of our patients,” he said in an interview.
Dr. Woodcock, Dr. Massenburg, and Dr. Toder reported having no relevant disclosures.
BOSTON – Biosimilar drugs are not identical twins of original biologic agents, but there are very strong family ties, and the newcomer is expected to look and behave very much like its older relative, said Janet Woodcock, MD, director of the Center for Drug Evaluation and Research at the Food and Drug Administration.
Although biosimilars differ from generic, small-molecule drugs, concerns about the development of biosimilars mirrors the kerfuffle over generic drugs surrounding the passage of the Hatch-Waxman (Drug Price Competition and Patent Term Restoration) Act in 1984.
She noted that clinicians today are asking the same questions about biosimilars that were asked about generics three decades ago:
- Are biosimilars as effective and as safe as the originally licensed biopharmaceuticals?
- If pharmacists substitute biosimilars for prescribed biologics, will patients be adversely affected?
- Can biosimilars reduce the high cost of biologic therapy?
“In certain specialties, this skepticism has persisted to this very day,” she said.
ACA mandate
Biosimilars owe their existence in large measure to the Biologics Price Competition and Innovation Act of 2009 (BPCI), passed as a part of the Affordable Care Act and signed into law by President Obama in 2010.
The act created an abbreviated licensure pathway for biologic products that can be shown to be either biosimilar to or interchangeable with an FDA-licensed reference drug.
A biosimilar is defined as a biological product that is highly similar to the reference product “notwithstanding minor differences in clinically inactive components,” and with no clinically meaningful differences between it and the reference product in terms of purity, safety, and potency.
To be interchangeable, a biosimilar must be expected to produce the same clinical results as the reference drug in any given patient, and “for a product that is administered more than once to an individual, the risk in terms of safety or diminished efficacy of alternating or switching between use of the product and its reference product is not greater than the risk of using the reference product without such alternation or switch.”
The definition of interchangeability includes the understanding that the prescriber’s approval is not necessary for substitution of a biosimilar for its reference product.
“If we’re going to have that kind of switching, if they are going to be interchangeable, then we have to have a very high bar,” Dr. Woodcock said.
She added that “any pressure people are feeling to push their patients to biosimilars is from the reimbursement system. There is no non-prescriber switching allowed currently; however, that doesn’t say there isn’t pressure on prescribers to write a different prescription.”
Faster track and bridge to approval
The biosimilar development and approval requires only convincing demonstration of biosimilarity to an existing agent, rather than an independent finding of safety or effectiveness, and the purpose of clinical studies in this case is to address “residual uncertainties,” Dr. Woodcock said.
Drug developers and regulatory authorities alike “are having trouble getting their mind around this concept,” she said.
The FDA requires manufacturers to provide data in their biosimilar drug license applications demonstrating biosimilarity based on analytical studies, animal studies that include toxicity assessments, and one or more clinical studies that include information on immunogenicity and pharmacokinetics (PK) or pharmacodynamics (PD) that is sufficient to demonstrate the safety, purity, and potency of the candidate biosimilar.
The FDA is also allowing manufacturers to submit data from animal studies and specified clinical studies comparing a proposed biosimilar product with a product not licensed in the United States, “as long as we are convinced that the reference product is equivalent to the U.S. product,” Dr. Woodcock said.
This “analytical bridge” process was requested by manufacturers who began development of biosimilars in Europe. The European Medicines Agency approved a biosimilar process in 2005, and gave the nod to the first biosimilar agents to existing erythropoietin products in 2007.
Current and pending
As of early October 2016, 66 programs were enrolled in FDA’s Biosimilar Product Development Program, and CDER has received requests for meetings with manufacturers to discuss what tests and documents are required for the development of biosimilars to 20 different reference products.
The FDA is prohibited from publicly discussing the existence of a pending application unless it has been previously disclosed or acknowledged publicly with the manufacturer’s permission, Dr. Woodcock noted, but as of Oct. 10, 2016, seven companies have announced a total of 10 biologic license applications for biosimilars to etanercept (Enbrel), adalimumab (Humira), pegfilgrastim (Neulasta), epoetin alfa (Epogen/Procrit), filgrastim (Neupogen), and infliximab (Remicade).
The FDA has granted licenses to four biosimilars to date (the four-letter suffix is intended to differentiate biosimilars agents from other biosimilars to the same reference product):
- Zarxio (filgrastim-sndz).
- Inflectra (infliximab-dyyb).
- Erelzi (etanercept-szzs).
- Amjevita (adalimumab-atto).
Physician perspective
“Everything I have heard suggests that biosimilars will be useful, but the scientist in me is a skeptic,” commented Donald Massenburg, MD, PhD, a rheumatologist at Wheaton Franciscan Healthcare in Franklin, Wisc., in an interview.
“I feel better now after learning that current biosimilars are not considered interchangeable, meaning that there can’t be substitutions made without the treating physician’s consent,” he said.
He acknowledged that “I wouldn’t be that excited” if a specific biosimilar was approved for interchangeability and was given to his patients without his knowledge.
Dr. Massenburg pointed to data from the NOR-SWITCH trial comparing the biosimilar Remsima to the reference product Remicade for treatment of rheumatic diseases, psoriasis, and inflammatory bowel disease. The trial showed that Remsima was noninferior to the reference product.
“I would like to be able to say whether a patient should be switched to a biosimilar or not just because of that potential risk,” Dr. Massenburg said.
A rheumatologist in private practice in New England said that what’s really needed in rheumatology is not the availability of more drugs that act like other drugs, but innovative research into therapies with better targeted mechanism of action.
“We’ve been through the ‘me-too’ hype; we did that with nonsteroidal anti-inflammatory drugs,” said J. Scott Toder, MD, director of the Toder Rheumatology and Osteoporosis Center, Providence, R.I.
“I think we need to concentrate on innovative therapies, and we may be able to do something about the escalating price of the biologics on the market by creating drugs with new mechanism of action to actually increase competition and hopefully control prices. I don’t think that having multiple drugs with the same mechanism of action is in the best interest of our patients,” he said in an interview.
Dr. Woodcock, Dr. Massenburg, and Dr. Toder reported having no relevant disclosures.
EXPERT ANALYSIS FROM A BIOSIMILARS IN RHEUMATOLOGY SYMPOSIUM
Hospitalists Need to Rethink the Way They Evaluate Students
Delivering feedback is a fundamental skill in medicine. Feedback ensures trainees remain on track to meet expected goals and standards. At some point in our careers, all of us have been on the receiving end of feedback. Many of us have likely had the opportunity to provide feedback to students or junior residents during our training. Moving from the role of trainee to supervisor presents a unique set of challenges and responsibilities to the young hospitalist.
Despite an extensive amount published on feedback, translation from theory to practice remains challenging.1 When surveyed, medical students and residents commonly perceive they do not receive enough feedback.2 Conversely, attendees of faculty development courses frequently indicate their greatest need is learning how to give feedback more effectively.3 Why does this performance gap exist?
The Issues
Careful exploration of our current training model reveals several systemic barriers to effective feedback. For one, many faculty members who supervise trainees are not formally trained educators. As such, they may lack the proper skills set to deliver feedback.1 Additionally, lack of time is often cited in the pressure to complete both clinical and academic duties within a packed workday. If learners aren’t directly observed by their supervisors, the impact and quality of feedback substantially diminishes.4 Likewise, if feedback is not embedded in the local culture and expected by both educator and learner, it can be perceived as a burden rather than a valuable exercise.
Feedback can evoke deep, sometimes subconscious emotional responses in both supervisor and recipient. During verbal interactions with trainees, dialogue tends to assume positive or neutral tones regardless of content.5 To avoid bruising a young learner’s ego, a well-intentioned educator may talk around the actual problem, using indirect statements in an attempt to “soften the blow.” Fearing a negative evaluation, the student may support and reinforce the teacher’s avoidance, further obscuring the message being sent. This concept is known as “vanishing feedback” and is a common barrier to the delivery of effective feedback.4 Educators additionally may shy away from giving constructive feedback because they fear reprisal on teaching evaluations.
Mounting evidence shows physicians, as a whole, tend to overestimate their abilities, and many are not skilled at self-assessment.6 When physician-learners receive feedback incongruent with their own self-perceptions, it may trigger feelings of anger, sadness, guilt, or self-doubt, which may block the receipt of any useful information. The so-called “millennial generation effect,” describing current medical school graduates, may further compound this issue. Millennials are “raised with an emphasis on being special; a previous absence of a balanced focus on weakness may present a barrier to accepting the validity of negative feedback.”1,5 As such, certain learners may intentionally avoid feedback as a method of self-preservation.
A New Approach
Many of us were taught to use the “feedback sandwich,” in which two positive statements surround a single negative corrective comment. This model, however, has some notable weaknesses. Given the ratio of positive to negative statements, educators may concentrate too heavily on the positive, diluting any constructive criticism and leaving learners with a false impression. Alternatively, trainees may learn to ignore positive comments while waiting for the other shoe to drop. As such, any initial positivity may feel insincere and artificial.7
Instead, we advocate using the “reflective feedback conversation,” a model that begins with self-assessment and places the onus on learners to identify their strengths and weaknesses.7 For example, a trainee might remark, “I struggle with controlling my temper when I am stressed.” The educator might reinforce that comment by stating, “I noticed you raised your voice last week when talking with the nurse because she forgot to administer Lasix.” To conclude the conversation, the teacher and student discuss shared goal setting and mutually agree on future improvements. Notably, this model does not facilitate conversation about problems a learner fails to detect. Hence, the educator must be prepared to deliver feedback outside of the learner’s own assessment.
Here are our favorite tips and tricks for delivering effective feedback:
- Establish a positive learning climate. Educators must partner with learners to generate an atmosphere of mutual trust and respect.1,3,4,8 An example of how to ally with learners is to announce early on, “As a teacher, I really value feedback. As such, I plan on giving feedback throughout the rotation because I want you to be the best doctor you can possibly be.”
- Require reflection. Effective feedback hinges on learners’ ability to self-assess.2,5,7 One approach is starting each feedback session with a simple open-ended question, such as, “How do you think you are doing?” Alternatively, you could be more specific, such as, “How do you think you did in managing the patient’s electrolytes when he went into diabetic ketoacidosis?”
- Be prompt. Feedback should be timely.1,4,7,8 An important distinction between feedback and evaluation is that feedback is formative, enabling learners to make needed changes before the end of a course, whereas evaluation is summative and presents a distinct judgment.1,4 If feedback is withheld until the end of the rotation, learners will not have an opportunity to remediate behaviors.
- Take advantage of different formats. Try a brief, concrete suggestion on the fly. A statement that might occur on bedside rounds is, “Allow me to show you a better technique to measure the liver span.” Or use a teachable event, such as a medical error or a particularly challenging case. Pulling interns aside after they deliver sobering news is a great opportunity to provide feedback in a semiformal fashion. Finally, formal sit-down feedback should be scheduled halfway through each rotation to ensure learners are on track and to address any major issues, such as professionalism or an inadequate clinical performance.2
- Be specific. Focus on behaviors and examples rather than judgments.1,2,4,7,8 For example, we have all experienced the inattentive student. Instead of framing feedback as, “It seems like you don’t care about medicine because you weren’t paying attention on rounds,” one could say, “I noticed you were fidgeting and looking at your phone during Aaron’s presentation.” Feedback should be based on firsthand observations and should be descriptive, utilizing neutral language.
- Avoid information overload. Feedback is best consumed in small snacks rather than an all-you-can-eat buffet.1,7 Your goal should not be to completely overhaul a learner but rather to focus on a few observable, correctable behaviors.
- Be empathetic. To make negative feedback less threatening, take yourself off the pedestal. An example of this could be saying, “As a third-year medical student, I struggled to remember all the right questions to ask, so performing a thorough review of systems helped me to catch the things I would miss.”
- Confirm understanding. It is important to know the learner has heard the feedback and to conclude the session with an action plan.
Just as hospitals engage in continuous quality improvement, as professionals, we should all strive for continuous self-improvement. Giving and receiving feedback is critical to personal growth. It is our hope that by using these tips, all of us will improve, creating a new generation of providers who give effective and useful feedback.
References
- Anderson PA. Giving feedback on clinical skills: are we starving our young? J Grad Med Educ. 2012;4(2):154-158. doi:10.4300/JGME-D-11-000295.1.
- Branch WT, Paranjape A. Feedback and reflection: teaching methods for clinical settings. Acad Med. 2002;77(12 Pt 1):1185-1188.
- Hewson MG, Little ML. Giving feedback in medical education: verification of recommended techniques. J Gen Intern Med. 1998;13(2):111-116.
- Ende J. Feedback in clinical medical education. JAMA. 1983;250(6):777-781.
- Bing-You RG, Trowbridge RL. Why medical educators may be failing at feedback. JAMA. 2009;302(12):1330-1331.
- Davis DA, Mazmanian PE, Fordis M, Van Harrison R, Thorpe KE, Perrier L. Accuracy of physician self-assessment compared with observed measures of competence: a systematic review. JAMA. 2006;296(9):1094-1102.
- Cantillon P, Sargeant J. Giving feedback in clinical settings. BMJ. 2008;337:a1961.
- Ramani S, Krackov SK. Twelve tips for giving feedback effectively in the clinical environment. Med Teach. 2012;34(10):787-791.
Delivering feedback is a fundamental skill in medicine. Feedback ensures trainees remain on track to meet expected goals and standards. At some point in our careers, all of us have been on the receiving end of feedback. Many of us have likely had the opportunity to provide feedback to students or junior residents during our training. Moving from the role of trainee to supervisor presents a unique set of challenges and responsibilities to the young hospitalist.
Despite an extensive amount published on feedback, translation from theory to practice remains challenging.1 When surveyed, medical students and residents commonly perceive they do not receive enough feedback.2 Conversely, attendees of faculty development courses frequently indicate their greatest need is learning how to give feedback more effectively.3 Why does this performance gap exist?
The Issues
Careful exploration of our current training model reveals several systemic barriers to effective feedback. For one, many faculty members who supervise trainees are not formally trained educators. As such, they may lack the proper skills set to deliver feedback.1 Additionally, lack of time is often cited in the pressure to complete both clinical and academic duties within a packed workday. If learners aren’t directly observed by their supervisors, the impact and quality of feedback substantially diminishes.4 Likewise, if feedback is not embedded in the local culture and expected by both educator and learner, it can be perceived as a burden rather than a valuable exercise.
Feedback can evoke deep, sometimes subconscious emotional responses in both supervisor and recipient. During verbal interactions with trainees, dialogue tends to assume positive or neutral tones regardless of content.5 To avoid bruising a young learner’s ego, a well-intentioned educator may talk around the actual problem, using indirect statements in an attempt to “soften the blow.” Fearing a negative evaluation, the student may support and reinforce the teacher’s avoidance, further obscuring the message being sent. This concept is known as “vanishing feedback” and is a common barrier to the delivery of effective feedback.4 Educators additionally may shy away from giving constructive feedback because they fear reprisal on teaching evaluations.
Mounting evidence shows physicians, as a whole, tend to overestimate their abilities, and many are not skilled at self-assessment.6 When physician-learners receive feedback incongruent with their own self-perceptions, it may trigger feelings of anger, sadness, guilt, or self-doubt, which may block the receipt of any useful information. The so-called “millennial generation effect,” describing current medical school graduates, may further compound this issue. Millennials are “raised with an emphasis on being special; a previous absence of a balanced focus on weakness may present a barrier to accepting the validity of negative feedback.”1,5 As such, certain learners may intentionally avoid feedback as a method of self-preservation.
A New Approach
Many of us were taught to use the “feedback sandwich,” in which two positive statements surround a single negative corrective comment. This model, however, has some notable weaknesses. Given the ratio of positive to negative statements, educators may concentrate too heavily on the positive, diluting any constructive criticism and leaving learners with a false impression. Alternatively, trainees may learn to ignore positive comments while waiting for the other shoe to drop. As such, any initial positivity may feel insincere and artificial.7
Instead, we advocate using the “reflective feedback conversation,” a model that begins with self-assessment and places the onus on learners to identify their strengths and weaknesses.7 For example, a trainee might remark, “I struggle with controlling my temper when I am stressed.” The educator might reinforce that comment by stating, “I noticed you raised your voice last week when talking with the nurse because she forgot to administer Lasix.” To conclude the conversation, the teacher and student discuss shared goal setting and mutually agree on future improvements. Notably, this model does not facilitate conversation about problems a learner fails to detect. Hence, the educator must be prepared to deliver feedback outside of the learner’s own assessment.
Here are our favorite tips and tricks for delivering effective feedback:
- Establish a positive learning climate. Educators must partner with learners to generate an atmosphere of mutual trust and respect.1,3,4,8 An example of how to ally with learners is to announce early on, “As a teacher, I really value feedback. As such, I plan on giving feedback throughout the rotation because I want you to be the best doctor you can possibly be.”
- Require reflection. Effective feedback hinges on learners’ ability to self-assess.2,5,7 One approach is starting each feedback session with a simple open-ended question, such as, “How do you think you are doing?” Alternatively, you could be more specific, such as, “How do you think you did in managing the patient’s electrolytes when he went into diabetic ketoacidosis?”
- Be prompt. Feedback should be timely.1,4,7,8 An important distinction between feedback and evaluation is that feedback is formative, enabling learners to make needed changes before the end of a course, whereas evaluation is summative and presents a distinct judgment.1,4 If feedback is withheld until the end of the rotation, learners will not have an opportunity to remediate behaviors.
- Take advantage of different formats. Try a brief, concrete suggestion on the fly. A statement that might occur on bedside rounds is, “Allow me to show you a better technique to measure the liver span.” Or use a teachable event, such as a medical error or a particularly challenging case. Pulling interns aside after they deliver sobering news is a great opportunity to provide feedback in a semiformal fashion. Finally, formal sit-down feedback should be scheduled halfway through each rotation to ensure learners are on track and to address any major issues, such as professionalism or an inadequate clinical performance.2
- Be specific. Focus on behaviors and examples rather than judgments.1,2,4,7,8 For example, we have all experienced the inattentive student. Instead of framing feedback as, “It seems like you don’t care about medicine because you weren’t paying attention on rounds,” one could say, “I noticed you were fidgeting and looking at your phone during Aaron’s presentation.” Feedback should be based on firsthand observations and should be descriptive, utilizing neutral language.
- Avoid information overload. Feedback is best consumed in small snacks rather than an all-you-can-eat buffet.1,7 Your goal should not be to completely overhaul a learner but rather to focus on a few observable, correctable behaviors.
- Be empathetic. To make negative feedback less threatening, take yourself off the pedestal. An example of this could be saying, “As a third-year medical student, I struggled to remember all the right questions to ask, so performing a thorough review of systems helped me to catch the things I would miss.”
- Confirm understanding. It is important to know the learner has heard the feedback and to conclude the session with an action plan.
Just as hospitals engage in continuous quality improvement, as professionals, we should all strive for continuous self-improvement. Giving and receiving feedback is critical to personal growth. It is our hope that by using these tips, all of us will improve, creating a new generation of providers who give effective and useful feedback.
References
- Anderson PA. Giving feedback on clinical skills: are we starving our young? J Grad Med Educ. 2012;4(2):154-158. doi:10.4300/JGME-D-11-000295.1.
- Branch WT, Paranjape A. Feedback and reflection: teaching methods for clinical settings. Acad Med. 2002;77(12 Pt 1):1185-1188.
- Hewson MG, Little ML. Giving feedback in medical education: verification of recommended techniques. J Gen Intern Med. 1998;13(2):111-116.
- Ende J. Feedback in clinical medical education. JAMA. 1983;250(6):777-781.
- Bing-You RG, Trowbridge RL. Why medical educators may be failing at feedback. JAMA. 2009;302(12):1330-1331.
- Davis DA, Mazmanian PE, Fordis M, Van Harrison R, Thorpe KE, Perrier L. Accuracy of physician self-assessment compared with observed measures of competence: a systematic review. JAMA. 2006;296(9):1094-1102.
- Cantillon P, Sargeant J. Giving feedback in clinical settings. BMJ. 2008;337:a1961.
- Ramani S, Krackov SK. Twelve tips for giving feedback effectively in the clinical environment. Med Teach. 2012;34(10):787-791.
Delivering feedback is a fundamental skill in medicine. Feedback ensures trainees remain on track to meet expected goals and standards. At some point in our careers, all of us have been on the receiving end of feedback. Many of us have likely had the opportunity to provide feedback to students or junior residents during our training. Moving from the role of trainee to supervisor presents a unique set of challenges and responsibilities to the young hospitalist.
Despite an extensive amount published on feedback, translation from theory to practice remains challenging.1 When surveyed, medical students and residents commonly perceive they do not receive enough feedback.2 Conversely, attendees of faculty development courses frequently indicate their greatest need is learning how to give feedback more effectively.3 Why does this performance gap exist?
The Issues
Careful exploration of our current training model reveals several systemic barriers to effective feedback. For one, many faculty members who supervise trainees are not formally trained educators. As such, they may lack the proper skills set to deliver feedback.1 Additionally, lack of time is often cited in the pressure to complete both clinical and academic duties within a packed workday. If learners aren’t directly observed by their supervisors, the impact and quality of feedback substantially diminishes.4 Likewise, if feedback is not embedded in the local culture and expected by both educator and learner, it can be perceived as a burden rather than a valuable exercise.
Feedback can evoke deep, sometimes subconscious emotional responses in both supervisor and recipient. During verbal interactions with trainees, dialogue tends to assume positive or neutral tones regardless of content.5 To avoid bruising a young learner’s ego, a well-intentioned educator may talk around the actual problem, using indirect statements in an attempt to “soften the blow.” Fearing a negative evaluation, the student may support and reinforce the teacher’s avoidance, further obscuring the message being sent. This concept is known as “vanishing feedback” and is a common barrier to the delivery of effective feedback.4 Educators additionally may shy away from giving constructive feedback because they fear reprisal on teaching evaluations.
Mounting evidence shows physicians, as a whole, tend to overestimate their abilities, and many are not skilled at self-assessment.6 When physician-learners receive feedback incongruent with their own self-perceptions, it may trigger feelings of anger, sadness, guilt, or self-doubt, which may block the receipt of any useful information. The so-called “millennial generation effect,” describing current medical school graduates, may further compound this issue. Millennials are “raised with an emphasis on being special; a previous absence of a balanced focus on weakness may present a barrier to accepting the validity of negative feedback.”1,5 As such, certain learners may intentionally avoid feedback as a method of self-preservation.
A New Approach
Many of us were taught to use the “feedback sandwich,” in which two positive statements surround a single negative corrective comment. This model, however, has some notable weaknesses. Given the ratio of positive to negative statements, educators may concentrate too heavily on the positive, diluting any constructive criticism and leaving learners with a false impression. Alternatively, trainees may learn to ignore positive comments while waiting for the other shoe to drop. As such, any initial positivity may feel insincere and artificial.7
Instead, we advocate using the “reflective feedback conversation,” a model that begins with self-assessment and places the onus on learners to identify their strengths and weaknesses.7 For example, a trainee might remark, “I struggle with controlling my temper when I am stressed.” The educator might reinforce that comment by stating, “I noticed you raised your voice last week when talking with the nurse because she forgot to administer Lasix.” To conclude the conversation, the teacher and student discuss shared goal setting and mutually agree on future improvements. Notably, this model does not facilitate conversation about problems a learner fails to detect. Hence, the educator must be prepared to deliver feedback outside of the learner’s own assessment.
Here are our favorite tips and tricks for delivering effective feedback:
- Establish a positive learning climate. Educators must partner with learners to generate an atmosphere of mutual trust and respect.1,3,4,8 An example of how to ally with learners is to announce early on, “As a teacher, I really value feedback. As such, I plan on giving feedback throughout the rotation because I want you to be the best doctor you can possibly be.”
- Require reflection. Effective feedback hinges on learners’ ability to self-assess.2,5,7 One approach is starting each feedback session with a simple open-ended question, such as, “How do you think you are doing?” Alternatively, you could be more specific, such as, “How do you think you did in managing the patient’s electrolytes when he went into diabetic ketoacidosis?”
- Be prompt. Feedback should be timely.1,4,7,8 An important distinction between feedback and evaluation is that feedback is formative, enabling learners to make needed changes before the end of a course, whereas evaluation is summative and presents a distinct judgment.1,4 If feedback is withheld until the end of the rotation, learners will not have an opportunity to remediate behaviors.
- Take advantage of different formats. Try a brief, concrete suggestion on the fly. A statement that might occur on bedside rounds is, “Allow me to show you a better technique to measure the liver span.” Or use a teachable event, such as a medical error or a particularly challenging case. Pulling interns aside after they deliver sobering news is a great opportunity to provide feedback in a semiformal fashion. Finally, formal sit-down feedback should be scheduled halfway through each rotation to ensure learners are on track and to address any major issues, such as professionalism or an inadequate clinical performance.2
- Be specific. Focus on behaviors and examples rather than judgments.1,2,4,7,8 For example, we have all experienced the inattentive student. Instead of framing feedback as, “It seems like you don’t care about medicine because you weren’t paying attention on rounds,” one could say, “I noticed you were fidgeting and looking at your phone during Aaron’s presentation.” Feedback should be based on firsthand observations and should be descriptive, utilizing neutral language.
- Avoid information overload. Feedback is best consumed in small snacks rather than an all-you-can-eat buffet.1,7 Your goal should not be to completely overhaul a learner but rather to focus on a few observable, correctable behaviors.
- Be empathetic. To make negative feedback less threatening, take yourself off the pedestal. An example of this could be saying, “As a third-year medical student, I struggled to remember all the right questions to ask, so performing a thorough review of systems helped me to catch the things I would miss.”
- Confirm understanding. It is important to know the learner has heard the feedback and to conclude the session with an action plan.
Just as hospitals engage in continuous quality improvement, as professionals, we should all strive for continuous self-improvement. Giving and receiving feedback is critical to personal growth. It is our hope that by using these tips, all of us will improve, creating a new generation of providers who give effective and useful feedback.
References
- Anderson PA. Giving feedback on clinical skills: are we starving our young? J Grad Med Educ. 2012;4(2):154-158. doi:10.4300/JGME-D-11-000295.1.
- Branch WT, Paranjape A. Feedback and reflection: teaching methods for clinical settings. Acad Med. 2002;77(12 Pt 1):1185-1188.
- Hewson MG, Little ML. Giving feedback in medical education: verification of recommended techniques. J Gen Intern Med. 1998;13(2):111-116.
- Ende J. Feedback in clinical medical education. JAMA. 1983;250(6):777-781.
- Bing-You RG, Trowbridge RL. Why medical educators may be failing at feedback. JAMA. 2009;302(12):1330-1331.
- Davis DA, Mazmanian PE, Fordis M, Van Harrison R, Thorpe KE, Perrier L. Accuracy of physician self-assessment compared with observed measures of competence: a systematic review. JAMA. 2006;296(9):1094-1102.
- Cantillon P, Sargeant J. Giving feedback in clinical settings. BMJ. 2008;337:a1961.
- Ramani S, Krackov SK. Twelve tips for giving feedback effectively in the clinical environment. Med Teach. 2012;34(10):787-791.
Team develops multicolor electron microscopy
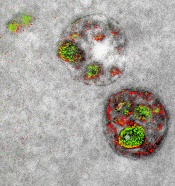
of endosomal uptake
of peptide proteins
Image by Adams et al/
Cell Chemical Biology 2016
Researchers say they have found a way to image specific cellular components using multicolor electron microscopy.
With their method, which the team worked on for nearly 15 years, up to 3 colors at a time (green, red, or yellow) can be used in an image.
A detector on the microscope captures electrons lost from metal ions painted over the specimen and records the metal’s energy loss signature as a color.
A technician must add the ionized metals one at a time and then lay the full color map over the still microscopy image.
The researchers described this method in detail in Cell Chemical Biology.
“This method has many potential applications in biology,” said study author Stephen Adams, PhD, of the University of California, San Diego in La Jolla.
“In the paper, we demonstrate how it can distinguish cellular compartments or track proteins and tag cells.”
For the multicolor effect to work, the researchers needed metal complexes that are stable enough to withstand application (meaning they don’t quickly deteriorate and blur the image) and have a distinct electron energy loss signature.
The researchers used ionized lanthanum (La), cerium (Ce), and praseodymium (Pr)—all metals in the lanthanide family. Each metal complex was laid down sequentially as a precipitate onto the specimen as it sat in the microscope.
“One challenge that kept us from publishing this much earlier, because we had the chemistry and we had an instrument that worked about 4 years ago, was we needed a way to deposit the metal compounds sequentially,” said study author Mark Ellisman, PhD, of the University of California, San Diego.
“We spent an awful lot of time trying to figure out how to deposit one of the lanthanides and then clear it so that it didn’t react when we deposited a second signal on the first site.”
Once the application process had been established, the researchers illustrated the power of multicolor electron microscopy by visualizing 2 brain cells sharing a single synapse. They also showed peptides entering through a cell membrane.
The researchers said there is more chemistry to be done to perfect the metal ion application process as well as produce images with more colors. There may also be ways to increase the amount of metal ions that can be deposited, which could help with resolution.
Many in the biochemical community should be able to begin using this technique right away, as it takes advantage of tools that are already found in laboratories.
This study is one of the last that Roger Tsien, PhD, who won a 2008 Nobel Prize in Chemistry for the discovery and application of green fluorescent protein to biochemical imaging, saw accepted by a journal before his death last August.
He did the first experiments to develop the chemical compounds needed for the multicolor imaging method nearly 15 years ago.
“One theme that has gone through all of Roger’s work is the desire to peer more closely into the workings of the cell,” Dr Adams said.
“With all of the fluorescence techniques that he’s introduced, he was able to do that in live cells and make action movies of them in vivid colors. But he always wanted to look closer, and now he’s left the beginnings for a method where we can add colors to electron microscopy.”
“This is clearly an example of Roger’s brilliance at chemistry and how he saw that, if we could do this, we would be able to enjoy the advantages of electron microscopy,” Dr Ellisman added.
“The biggest advantage of electron microscopy that we saw is that you have weak contrasts by the nature of the way that staining works, so color-specific labels give context to all of the rich information in the scene of which molecules are operating.” ![]()

of endosomal uptake
of peptide proteins
Image by Adams et al/
Cell Chemical Biology 2016
Researchers say they have found a way to image specific cellular components using multicolor electron microscopy.
With their method, which the team worked on for nearly 15 years, up to 3 colors at a time (green, red, or yellow) can be used in an image.
A detector on the microscope captures electrons lost from metal ions painted over the specimen and records the metal’s energy loss signature as a color.
A technician must add the ionized metals one at a time and then lay the full color map over the still microscopy image.
The researchers described this method in detail in Cell Chemical Biology.
“This method has many potential applications in biology,” said study author Stephen Adams, PhD, of the University of California, San Diego in La Jolla.
“In the paper, we demonstrate how it can distinguish cellular compartments or track proteins and tag cells.”
For the multicolor effect to work, the researchers needed metal complexes that are stable enough to withstand application (meaning they don’t quickly deteriorate and blur the image) and have a distinct electron energy loss signature.
The researchers used ionized lanthanum (La), cerium (Ce), and praseodymium (Pr)—all metals in the lanthanide family. Each metal complex was laid down sequentially as a precipitate onto the specimen as it sat in the microscope.
“One challenge that kept us from publishing this much earlier, because we had the chemistry and we had an instrument that worked about 4 years ago, was we needed a way to deposit the metal compounds sequentially,” said study author Mark Ellisman, PhD, of the University of California, San Diego.
“We spent an awful lot of time trying to figure out how to deposit one of the lanthanides and then clear it so that it didn’t react when we deposited a second signal on the first site.”
Once the application process had been established, the researchers illustrated the power of multicolor electron microscopy by visualizing 2 brain cells sharing a single synapse. They also showed peptides entering through a cell membrane.
The researchers said there is more chemistry to be done to perfect the metal ion application process as well as produce images with more colors. There may also be ways to increase the amount of metal ions that can be deposited, which could help with resolution.
Many in the biochemical community should be able to begin using this technique right away, as it takes advantage of tools that are already found in laboratories.
This study is one of the last that Roger Tsien, PhD, who won a 2008 Nobel Prize in Chemistry for the discovery and application of green fluorescent protein to biochemical imaging, saw accepted by a journal before his death last August.
He did the first experiments to develop the chemical compounds needed for the multicolor imaging method nearly 15 years ago.
“One theme that has gone through all of Roger’s work is the desire to peer more closely into the workings of the cell,” Dr Adams said.
“With all of the fluorescence techniques that he’s introduced, he was able to do that in live cells and make action movies of them in vivid colors. But he always wanted to look closer, and now he’s left the beginnings for a method where we can add colors to electron microscopy.”
“This is clearly an example of Roger’s brilliance at chemistry and how he saw that, if we could do this, we would be able to enjoy the advantages of electron microscopy,” Dr Ellisman added.
“The biggest advantage of electron microscopy that we saw is that you have weak contrasts by the nature of the way that staining works, so color-specific labels give context to all of the rich information in the scene of which molecules are operating.” ![]()

of endosomal uptake
of peptide proteins
Image by Adams et al/
Cell Chemical Biology 2016
Researchers say they have found a way to image specific cellular components using multicolor electron microscopy.
With their method, which the team worked on for nearly 15 years, up to 3 colors at a time (green, red, or yellow) can be used in an image.
A detector on the microscope captures electrons lost from metal ions painted over the specimen and records the metal’s energy loss signature as a color.
A technician must add the ionized metals one at a time and then lay the full color map over the still microscopy image.
The researchers described this method in detail in Cell Chemical Biology.
“This method has many potential applications in biology,” said study author Stephen Adams, PhD, of the University of California, San Diego in La Jolla.
“In the paper, we demonstrate how it can distinguish cellular compartments or track proteins and tag cells.”
For the multicolor effect to work, the researchers needed metal complexes that are stable enough to withstand application (meaning they don’t quickly deteriorate and blur the image) and have a distinct electron energy loss signature.
The researchers used ionized lanthanum (La), cerium (Ce), and praseodymium (Pr)—all metals in the lanthanide family. Each metal complex was laid down sequentially as a precipitate onto the specimen as it sat in the microscope.
“One challenge that kept us from publishing this much earlier, because we had the chemistry and we had an instrument that worked about 4 years ago, was we needed a way to deposit the metal compounds sequentially,” said study author Mark Ellisman, PhD, of the University of California, San Diego.
“We spent an awful lot of time trying to figure out how to deposit one of the lanthanides and then clear it so that it didn’t react when we deposited a second signal on the first site.”
Once the application process had been established, the researchers illustrated the power of multicolor electron microscopy by visualizing 2 brain cells sharing a single synapse. They also showed peptides entering through a cell membrane.
The researchers said there is more chemistry to be done to perfect the metal ion application process as well as produce images with more colors. There may also be ways to increase the amount of metal ions that can be deposited, which could help with resolution.
Many in the biochemical community should be able to begin using this technique right away, as it takes advantage of tools that are already found in laboratories.
This study is one of the last that Roger Tsien, PhD, who won a 2008 Nobel Prize in Chemistry for the discovery and application of green fluorescent protein to biochemical imaging, saw accepted by a journal before his death last August.
He did the first experiments to develop the chemical compounds needed for the multicolor imaging method nearly 15 years ago.
“One theme that has gone through all of Roger’s work is the desire to peer more closely into the workings of the cell,” Dr Adams said.
“With all of the fluorescence techniques that he’s introduced, he was able to do that in live cells and make action movies of them in vivid colors. But he always wanted to look closer, and now he’s left the beginnings for a method where we can add colors to electron microscopy.”
“This is clearly an example of Roger’s brilliance at chemistry and how he saw that, if we could do this, we would be able to enjoy the advantages of electron microscopy,” Dr Ellisman added.
“The biggest advantage of electron microscopy that we saw is that you have weak contrasts by the nature of the way that staining works, so color-specific labels give context to all of the rich information in the scene of which molecules are operating.” ![]()