User login
Treating Lower Arterial Occlusive Disease
How to proceed when a patient needs a bypass that can’t be done using the saphenous vein, and how to measure arterial flow to the foot are two novel areas that help improve a revascularization algorithm for lower arterial occlusive disease, according to Dr. Kenneth Ouriel, co-moderator of the Wednesday session, “Value of Deep Vein Grafts; New Concepts in Assessing Foot Perfusion and Improving It; More About the Angiosome Controversy; Some Ongoing CLI Trials.”
“A lot of patients don’t have a suitable bypass conduit to bring blood to the lower limb. But when the saphenous vein isn’t available, and you’re concerned an artificial graft won’t be as effective, there is another alternative,” said Dr. Ouriel, president and CEO of the New York–based clinical research firm, Syntactx.
Dr. Ouriel said “it’s also a vein that is not that long, so if you need a long vein for a bypass, from the top of the leg to the ankle, for example, it might not be long enough.”
Clinicians who attend this session also will be able to incorporate novel ways of quantifying perfusion to the foot, according to Dr. Ouriel. “The angiosome isn’t controversial so much as just new. Most of us didn’t learn about it in medical school, but we will explore it in depth during this session.”
The general principle of the angiosome is that arterial flow to the foot is “compartmentalized” into regions supplied by discrete vessels. This leads to seeing perfusion not only as a whole, but also in terms of specific regions, said Dr. Ouriel.
By focusing on the revascularization of the source artery to a specific angiosome, data indicate there are better rates of wound healing and limb salvage, according to Dr. Ouriel.
To round out the session are results and questions posed by a series of “exciting” trials both completed and still recruiting, including the LIBERTY, SPINACH, and BEST trials. The discussion of these trial findings will add nuance to the overall discussion about whether surgical reconstruction or peripheral intervention in patients with critical limb ischemia is appropriate and when to do it.
Session 30:
Value of Deep Vein Grafts; New Concepts in Assessing Foot Perfusion and Improving It; More About the Angiosome Controversy; Some Ongoing CLI Trials
Wednesday, 4:22 p.m. – 5:58 p.m.
Grand Ballroom East, 3rd Floor
How to proceed when a patient needs a bypass that can’t be done using the saphenous vein, and how to measure arterial flow to the foot are two novel areas that help improve a revascularization algorithm for lower arterial occlusive disease, according to Dr. Kenneth Ouriel, co-moderator of the Wednesday session, “Value of Deep Vein Grafts; New Concepts in Assessing Foot Perfusion and Improving It; More About the Angiosome Controversy; Some Ongoing CLI Trials.”
“A lot of patients don’t have a suitable bypass conduit to bring blood to the lower limb. But when the saphenous vein isn’t available, and you’re concerned an artificial graft won’t be as effective, there is another alternative,” said Dr. Ouriel, president and CEO of the New York–based clinical research firm, Syntactx.
Dr. Ouriel said “it’s also a vein that is not that long, so if you need a long vein for a bypass, from the top of the leg to the ankle, for example, it might not be long enough.”
Clinicians who attend this session also will be able to incorporate novel ways of quantifying perfusion to the foot, according to Dr. Ouriel. “The angiosome isn’t controversial so much as just new. Most of us didn’t learn about it in medical school, but we will explore it in depth during this session.”
The general principle of the angiosome is that arterial flow to the foot is “compartmentalized” into regions supplied by discrete vessels. This leads to seeing perfusion not only as a whole, but also in terms of specific regions, said Dr. Ouriel.
By focusing on the revascularization of the source artery to a specific angiosome, data indicate there are better rates of wound healing and limb salvage, according to Dr. Ouriel.
To round out the session are results and questions posed by a series of “exciting” trials both completed and still recruiting, including the LIBERTY, SPINACH, and BEST trials. The discussion of these trial findings will add nuance to the overall discussion about whether surgical reconstruction or peripheral intervention in patients with critical limb ischemia is appropriate and when to do it.
Session 30:
Value of Deep Vein Grafts; New Concepts in Assessing Foot Perfusion and Improving It; More About the Angiosome Controversy; Some Ongoing CLI Trials
Wednesday, 4:22 p.m. – 5:58 p.m.
Grand Ballroom East, 3rd Floor
How to proceed when a patient needs a bypass that can’t be done using the saphenous vein, and how to measure arterial flow to the foot are two novel areas that help improve a revascularization algorithm for lower arterial occlusive disease, according to Dr. Kenneth Ouriel, co-moderator of the Wednesday session, “Value of Deep Vein Grafts; New Concepts in Assessing Foot Perfusion and Improving It; More About the Angiosome Controversy; Some Ongoing CLI Trials.”
“A lot of patients don’t have a suitable bypass conduit to bring blood to the lower limb. But when the saphenous vein isn’t available, and you’re concerned an artificial graft won’t be as effective, there is another alternative,” said Dr. Ouriel, president and CEO of the New York–based clinical research firm, Syntactx.
Dr. Ouriel said “it’s also a vein that is not that long, so if you need a long vein for a bypass, from the top of the leg to the ankle, for example, it might not be long enough.”
Clinicians who attend this session also will be able to incorporate novel ways of quantifying perfusion to the foot, according to Dr. Ouriel. “The angiosome isn’t controversial so much as just new. Most of us didn’t learn about it in medical school, but we will explore it in depth during this session.”
The general principle of the angiosome is that arterial flow to the foot is “compartmentalized” into regions supplied by discrete vessels. This leads to seeing perfusion not only as a whole, but also in terms of specific regions, said Dr. Ouriel.
By focusing on the revascularization of the source artery to a specific angiosome, data indicate there are better rates of wound healing and limb salvage, according to Dr. Ouriel.
To round out the session are results and questions posed by a series of “exciting” trials both completed and still recruiting, including the LIBERTY, SPINACH, and BEST trials. The discussion of these trial findings will add nuance to the overall discussion about whether surgical reconstruction or peripheral intervention in patients with critical limb ischemia is appropriate and when to do it.
Session 30:
Value of Deep Vein Grafts; New Concepts in Assessing Foot Perfusion and Improving It; More About the Angiosome Controversy; Some Ongoing CLI Trials
Wednesday, 4:22 p.m. – 5:58 p.m.
Grand Ballroom East, 3rd Floor
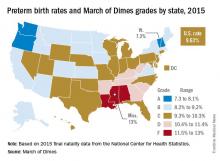
U.S. preterm birth rate rose slightly in 2015
The preterm birth rate in the United States for 2015 increased for the first time in 8 years, according to the March of Dimes.
The national preterm birth rate rose from 9.57% in 2014 to 9.63% last year, earning an overall grade of C on the March of Dimes 2016 Premature Birth Report Card.
The March of Dimes also ranked preterm births in the states by racial/ethnic disparity: Maine had the least disparity, followed by New Hampshire and Utah, while Hawaii had the greatest disparity, just ahead of Pennsylvania and Louisiana. Using an average of the 2012-2014 national preterm birth rates, the March of Dimes calculated that Asian/Pacific Islanders had the lowest preterm birth rate at 8.5%, compared with 9% for whites, 9.1% for Hispanics, 10.4% for American Indian/Alaska Natives, and 13.3% for blacks.
The report card shows “that there is an unfair burden of premature birth among specific racial and ethnic groups as well as geographic areas,” Jennifer L. Howse, PhD, president of the March of Dimes, said in a statement. “Babies in this country have different chances of surviving and thriving simply based on the circumstances of their birth.”
The preterm birth rate in the United States for 2015 increased for the first time in 8 years, according to the March of Dimes.
The national preterm birth rate rose from 9.57% in 2014 to 9.63% last year, earning an overall grade of C on the March of Dimes 2016 Premature Birth Report Card.
The March of Dimes also ranked preterm births in the states by racial/ethnic disparity: Maine had the least disparity, followed by New Hampshire and Utah, while Hawaii had the greatest disparity, just ahead of Pennsylvania and Louisiana. Using an average of the 2012-2014 national preterm birth rates, the March of Dimes calculated that Asian/Pacific Islanders had the lowest preterm birth rate at 8.5%, compared with 9% for whites, 9.1% for Hispanics, 10.4% for American Indian/Alaska Natives, and 13.3% for blacks.
The report card shows “that there is an unfair burden of premature birth among specific racial and ethnic groups as well as geographic areas,” Jennifer L. Howse, PhD, president of the March of Dimes, said in a statement. “Babies in this country have different chances of surviving and thriving simply based on the circumstances of their birth.”
The preterm birth rate in the United States for 2015 increased for the first time in 8 years, according to the March of Dimes.
The national preterm birth rate rose from 9.57% in 2014 to 9.63% last year, earning an overall grade of C on the March of Dimes 2016 Premature Birth Report Card.
The March of Dimes also ranked preterm births in the states by racial/ethnic disparity: Maine had the least disparity, followed by New Hampshire and Utah, while Hawaii had the greatest disparity, just ahead of Pennsylvania and Louisiana. Using an average of the 2012-2014 national preterm birth rates, the March of Dimes calculated that Asian/Pacific Islanders had the lowest preterm birth rate at 8.5%, compared with 9% for whites, 9.1% for Hispanics, 10.4% for American Indian/Alaska Natives, and 13.3% for blacks.
The report card shows “that there is an unfair burden of premature birth among specific racial and ethnic groups as well as geographic areas,” Jennifer L. Howse, PhD, president of the March of Dimes, said in a statement. “Babies in this country have different chances of surviving and thriving simply based on the circumstances of their birth.”
Switching Between Generic AEDs Not Linked to Hospital Visits for Seizure
Switching between generic versions of the same antiepileptic drug made by different manufacturers does not appear to change the risk of seizure-related events in patients with epilepsy, according to a population-based, case–crossover study of generic antiepileptic drug users published online ahead of print September 28 in Neurology. Delays and complications of the medication refilling process might increase a patient’s risk for a seizure, said Aaron Kesselheim, MD, JD, MPH, Associate Professor of Medicine at Harvard Medical School in Boston, and colleagues.
“These results add to the growing literature supporting the routine use of interchangeable generic [antiepileptic drugs] among patients with seizure disorders,” he added.
Although previous observational studies have demonstrated increased seizure activity following a switch from brand name to generic antiepileptic drugs, several recent randomized trials have found no
link between generic drug switching and seizure risk, said Dr. Kesselheim.
Investigators identified 59,344 patients with at least one refill of a prescription from the same manufacturer and 5,200 patients who switched from one generic to another from 2000 to 2010 in the Medicaid Analytic eXtract database and from 2005 to 2013 in a commercial health insurance database. Participants acted as their own controls in the study’s comparison of the effects of a refill or a refill with a switch in manufacturer on seizure-related events (ie, a seizure requiring an emergency department visit or hospitalization) during a hazard period, defined as days 2–36 preceding a seizure-related event, and a control period, defined as days 51–85 preceding the seizure-related event.
Overall, generic antiepileptic refilling of the same medication from the same manufacturer was associated with an 8% increase in the odds of having a seizure-related event. When the refill involved a switch to the same generic drug made by a different manufacturer, the odds of a seizure-related event rose by 9%. When the refill involved a change in the shape or color of the pill, the odds increased by 11% but did not increase when the switch was made to a pill with the same color and shape. The increased odds of seizure-related events became nonsignificant when the researchers adjusted these comparisons for the process of refilling, which “is often not straightforward,” said Dr. Kesselheim. “Patients have expressed frustration with delays and other complicating factors relating to refilling.… Greater work to enhance the refilling process, and to determine whether mail order pharmacies successfully improve outcomes on this point, is necessary.”
The study was not supported by any specific targeted funding. The investigators received support from various foundations and from programs within Harvard University and grants from the Agency for Healthcare Research and Quality and the FDA.The investigators also disclosed acting in a research support role for or receiving financial compensation from several pharmaceutical companies and other organizations.
—Jessica Craig
Suggested Reading
Kesselheim AS, Bykov K, Gagne JJ, et al. Switching generic antiepileptic drug manufacturer not linked to seizures: a case-crossover study. Neurology. 2016 Sep 28 [Epub ahead of print].
Krauss GL, Privitera M. More data on the safety of generic substitution: yes, the blue tablet is OK? Neurology. 2016 Sep 28 [Epub ahead of print].
Switching between generic versions of the same antiepileptic drug made by different manufacturers does not appear to change the risk of seizure-related events in patients with epilepsy, according to a population-based, case–crossover study of generic antiepileptic drug users published online ahead of print September 28 in Neurology. Delays and complications of the medication refilling process might increase a patient’s risk for a seizure, said Aaron Kesselheim, MD, JD, MPH, Associate Professor of Medicine at Harvard Medical School in Boston, and colleagues.
“These results add to the growing literature supporting the routine use of interchangeable generic [antiepileptic drugs] among patients with seizure disorders,” he added.
Although previous observational studies have demonstrated increased seizure activity following a switch from brand name to generic antiepileptic drugs, several recent randomized trials have found no
link between generic drug switching and seizure risk, said Dr. Kesselheim.
Investigators identified 59,344 patients with at least one refill of a prescription from the same manufacturer and 5,200 patients who switched from one generic to another from 2000 to 2010 in the Medicaid Analytic eXtract database and from 2005 to 2013 in a commercial health insurance database. Participants acted as their own controls in the study’s comparison of the effects of a refill or a refill with a switch in manufacturer on seizure-related events (ie, a seizure requiring an emergency department visit or hospitalization) during a hazard period, defined as days 2–36 preceding a seizure-related event, and a control period, defined as days 51–85 preceding the seizure-related event.
Overall, generic antiepileptic refilling of the same medication from the same manufacturer was associated with an 8% increase in the odds of having a seizure-related event. When the refill involved a switch to the same generic drug made by a different manufacturer, the odds of a seizure-related event rose by 9%. When the refill involved a change in the shape or color of the pill, the odds increased by 11% but did not increase when the switch was made to a pill with the same color and shape. The increased odds of seizure-related events became nonsignificant when the researchers adjusted these comparisons for the process of refilling, which “is often not straightforward,” said Dr. Kesselheim. “Patients have expressed frustration with delays and other complicating factors relating to refilling.… Greater work to enhance the refilling process, and to determine whether mail order pharmacies successfully improve outcomes on this point, is necessary.”
The study was not supported by any specific targeted funding. The investigators received support from various foundations and from programs within Harvard University and grants from the Agency for Healthcare Research and Quality and the FDA.The investigators also disclosed acting in a research support role for or receiving financial compensation from several pharmaceutical companies and other organizations.
—Jessica Craig
Suggested Reading
Kesselheim AS, Bykov K, Gagne JJ, et al. Switching generic antiepileptic drug manufacturer not linked to seizures: a case-crossover study. Neurology. 2016 Sep 28 [Epub ahead of print].
Krauss GL, Privitera M. More data on the safety of generic substitution: yes, the blue tablet is OK? Neurology. 2016 Sep 28 [Epub ahead of print].
Switching between generic versions of the same antiepileptic drug made by different manufacturers does not appear to change the risk of seizure-related events in patients with epilepsy, according to a population-based, case–crossover study of generic antiepileptic drug users published online ahead of print September 28 in Neurology. Delays and complications of the medication refilling process might increase a patient’s risk for a seizure, said Aaron Kesselheim, MD, JD, MPH, Associate Professor of Medicine at Harvard Medical School in Boston, and colleagues.
“These results add to the growing literature supporting the routine use of interchangeable generic [antiepileptic drugs] among patients with seizure disorders,” he added.
Although previous observational studies have demonstrated increased seizure activity following a switch from brand name to generic antiepileptic drugs, several recent randomized trials have found no
link between generic drug switching and seizure risk, said Dr. Kesselheim.
Investigators identified 59,344 patients with at least one refill of a prescription from the same manufacturer and 5,200 patients who switched from one generic to another from 2000 to 2010 in the Medicaid Analytic eXtract database and from 2005 to 2013 in a commercial health insurance database. Participants acted as their own controls in the study’s comparison of the effects of a refill or a refill with a switch in manufacturer on seizure-related events (ie, a seizure requiring an emergency department visit or hospitalization) during a hazard period, defined as days 2–36 preceding a seizure-related event, and a control period, defined as days 51–85 preceding the seizure-related event.
Overall, generic antiepileptic refilling of the same medication from the same manufacturer was associated with an 8% increase in the odds of having a seizure-related event. When the refill involved a switch to the same generic drug made by a different manufacturer, the odds of a seizure-related event rose by 9%. When the refill involved a change in the shape or color of the pill, the odds increased by 11% but did not increase when the switch was made to a pill with the same color and shape. The increased odds of seizure-related events became nonsignificant when the researchers adjusted these comparisons for the process of refilling, which “is often not straightforward,” said Dr. Kesselheim. “Patients have expressed frustration with delays and other complicating factors relating to refilling.… Greater work to enhance the refilling process, and to determine whether mail order pharmacies successfully improve outcomes on this point, is necessary.”
The study was not supported by any specific targeted funding. The investigators received support from various foundations and from programs within Harvard University and grants from the Agency for Healthcare Research and Quality and the FDA.The investigators also disclosed acting in a research support role for or receiving financial compensation from several pharmaceutical companies and other organizations.
—Jessica Craig
Suggested Reading
Kesselheim AS, Bykov K, Gagne JJ, et al. Switching generic antiepileptic drug manufacturer not linked to seizures: a case-crossover study. Neurology. 2016 Sep 28 [Epub ahead of print].
Krauss GL, Privitera M. More data on the safety of generic substitution: yes, the blue tablet is OK? Neurology. 2016 Sep 28 [Epub ahead of print].
COMMENTARY—Study Reaffirms FDA Findings
The study by Dr. Kesselheim and his colleagues is one of several recently aimed at reviewing the overall safety of generic drug switching. The FDA sponsored three clinical bioequivalence studies to determine the adequacy of average bioequivalence studies for ensuring safe conversion between different antiseizure products for patients with epilepsy. Taken together, these studies confirm that most patients can safely switch between generic formulations, even between tablets differing in appearance.
So why do patients and open series report seizures associated with formulation changes? Probably several things are happening:
• Patients want to find a reason for the near-random pattern of their seizures. Threshold cortical epileptogenic activity triggers seizures in near-random patterns, and their timing might be influenced by triggers such as missing doses, stress, and hormonal changes.
• Patients’ views of illness and treatments might influence their reporting of seizures and drug effects. This search for seizure explanations can even extend to pets with seizures.
• There is temporal variability in individual drug absorption and elimination. Food has a major effect on absorption of antiseizure medications and maximum concentration; this effect is particularly common with modified-release formulations. A small subgroup of patients in the FDA’s clinical bioequivalence studies had variability in concentrations during reexposure to the same product.
• A small group of patients may be outside the 90% confidence interval bioequivalence acceptance range and may experience product switching effects.
—Gregory L. Krauss, MD
Professor of Neurology
Johns Hopkins University, Baltimore
—Michael D. Privitera, MD
Professor of Neurology
University of Cincinnati
This commentary was adapted from Krauss GL, Privitera M. More data on the safety of generic substitution: yes, the blue tablet is OK? Neurology. 2016 Sep 28 [Epub ahead of print].
The study by Dr. Kesselheim and his colleagues is one of several recently aimed at reviewing the overall safety of generic drug switching. The FDA sponsored three clinical bioequivalence studies to determine the adequacy of average bioequivalence studies for ensuring safe conversion between different antiseizure products for patients with epilepsy. Taken together, these studies confirm that most patients can safely switch between generic formulations, even between tablets differing in appearance.
So why do patients and open series report seizures associated with formulation changes? Probably several things are happening:
• Patients want to find a reason for the near-random pattern of their seizures. Threshold cortical epileptogenic activity triggers seizures in near-random patterns, and their timing might be influenced by triggers such as missing doses, stress, and hormonal changes.
• Patients’ views of illness and treatments might influence their reporting of seizures and drug effects. This search for seizure explanations can even extend to pets with seizures.
• There is temporal variability in individual drug absorption and elimination. Food has a major effect on absorption of antiseizure medications and maximum concentration; this effect is particularly common with modified-release formulations. A small subgroup of patients in the FDA’s clinical bioequivalence studies had variability in concentrations during reexposure to the same product.
• A small group of patients may be outside the 90% confidence interval bioequivalence acceptance range and may experience product switching effects.
—Gregory L. Krauss, MD
Professor of Neurology
Johns Hopkins University, Baltimore
—Michael D. Privitera, MD
Professor of Neurology
University of Cincinnati
This commentary was adapted from Krauss GL, Privitera M. More data on the safety of generic substitution: yes, the blue tablet is OK? Neurology. 2016 Sep 28 [Epub ahead of print].
The study by Dr. Kesselheim and his colleagues is one of several recently aimed at reviewing the overall safety of generic drug switching. The FDA sponsored three clinical bioequivalence studies to determine the adequacy of average bioequivalence studies for ensuring safe conversion between different antiseizure products for patients with epilepsy. Taken together, these studies confirm that most patients can safely switch between generic formulations, even between tablets differing in appearance.
So why do patients and open series report seizures associated with formulation changes? Probably several things are happening:
• Patients want to find a reason for the near-random pattern of their seizures. Threshold cortical epileptogenic activity triggers seizures in near-random patterns, and their timing might be influenced by triggers such as missing doses, stress, and hormonal changes.
• Patients’ views of illness and treatments might influence their reporting of seizures and drug effects. This search for seizure explanations can even extend to pets with seizures.
• There is temporal variability in individual drug absorption and elimination. Food has a major effect on absorption of antiseizure medications and maximum concentration; this effect is particularly common with modified-release formulations. A small subgroup of patients in the FDA’s clinical bioequivalence studies had variability in concentrations during reexposure to the same product.
• A small group of patients may be outside the 90% confidence interval bioequivalence acceptance range and may experience product switching effects.
—Gregory L. Krauss, MD
Professor of Neurology
Johns Hopkins University, Baltimore
—Michael D. Privitera, MD
Professor of Neurology
University of Cincinnati
This commentary was adapted from Krauss GL, Privitera M. More data on the safety of generic substitution: yes, the blue tablet is OK? Neurology. 2016 Sep 28 [Epub ahead of print].
Treat ulcerative colitis ‘to target’
LAS VEGAS – In ulcerative colitis, symptoms aren’t everything. That’s the opinion of Stephen B. Hanauer, MD, medical director of Northwestern University’s Digestive Health Center, Chicago, who discussed the condition and the methods physicians use to assess patient improvement, at the annual meeting of the American College of Gastroenterology.
Many gastroenterologists accept patient reports of symptom improvement, but Dr. Hanauer advocates for a follow-up endoscopy, even when patients are making good progress.
Even in patients with improved symptoms, many endoscopic evaluations show inflammation and troubling histology that is in turn associated with worse quality of life and greater odds of hospitalizations and surgery (Dig Liver Dis. 2013 Dec;45:969-77).
So a better outcome is histological healing, characterized by the absence of neutrophils. A study presented at Digestive Disease Week® in 2015 (abstract 592) showed better relapse-free survival after histologic remission. “The deeper the remission that we achieve, the better the outcomes are going to be,” said Dr. Hanauer.
In fact, patients who report that they are asymptomatic may still be experiencing disease. “Many chronic patients accept a certain degree of symptoms as a new normal,” Dr. Hanauer said. Patients accept living with frequent nighttime awakenings to evacuate, or more frequent bowel movements. Further treatment could reduce or eliminate such problems. “There are subtle things you can improve upon, to convince them that the new normal is not the acceptable normal,” said Dr. Hanauer.
He suggests an endoscopy 3-12 months after patients report symptom abatement, although he admits that cost and other considerations can be barriers.
When an endoscopy is performed, it can lead to a conundrum if it shows residual inflammation despite a lack of symptoms. The physician might consider switching to a different class of drug, but that will usually raise cost and introduce new risks of adverse events. “We are doing that in some settings. For example, in postoperative Crohn’s disease, where patients have no active disease, we advocate prophylactically administering agents to prevent recurrence. So we are willing to do that in certain subsets of patients, but we have clinical trials to show the benefits of that,” said Dr. Hanauer. Unfortunately, there are no clinical trials demonstrating the benefits of such an approach in ulcerative colitis.
Biomarkers offer potential assistance for physicians who want to “treat to target,” as Dr. Hanauer put it, to achieve biological remission. Calprotectin – a protein secreted by neutrophils that mark the presence of these cells – are measurable in feces, and the levels correlate well with ulcerative colitis disease activity (Inflamm Bowel Dis. 2012 Nov;18:2011-7).
Dr. Hanauer’s advice to go beyond clinical remission is already occurring at the Food and Drug Administration, which no longer accepts clinical remission findings in testing new drugs for inflammatory bowel disease. “They are requiring patient-reported outcomes accompanied by endoscopic evidence of healing. I’m advocating what’s going on on a regulatory basis be applied on a clinical basis,” said Dr. Hanauer.
Dr. Hanauer had no relevant financial disclosures.
LAS VEGAS – In ulcerative colitis, symptoms aren’t everything. That’s the opinion of Stephen B. Hanauer, MD, medical director of Northwestern University’s Digestive Health Center, Chicago, who discussed the condition and the methods physicians use to assess patient improvement, at the annual meeting of the American College of Gastroenterology.
Many gastroenterologists accept patient reports of symptom improvement, but Dr. Hanauer advocates for a follow-up endoscopy, even when patients are making good progress.
Even in patients with improved symptoms, many endoscopic evaluations show inflammation and troubling histology that is in turn associated with worse quality of life and greater odds of hospitalizations and surgery (Dig Liver Dis. 2013 Dec;45:969-77).
So a better outcome is histological healing, characterized by the absence of neutrophils. A study presented at Digestive Disease Week® in 2015 (abstract 592) showed better relapse-free survival after histologic remission. “The deeper the remission that we achieve, the better the outcomes are going to be,” said Dr. Hanauer.
In fact, patients who report that they are asymptomatic may still be experiencing disease. “Many chronic patients accept a certain degree of symptoms as a new normal,” Dr. Hanauer said. Patients accept living with frequent nighttime awakenings to evacuate, or more frequent bowel movements. Further treatment could reduce or eliminate such problems. “There are subtle things you can improve upon, to convince them that the new normal is not the acceptable normal,” said Dr. Hanauer.
He suggests an endoscopy 3-12 months after patients report symptom abatement, although he admits that cost and other considerations can be barriers.
When an endoscopy is performed, it can lead to a conundrum if it shows residual inflammation despite a lack of symptoms. The physician might consider switching to a different class of drug, but that will usually raise cost and introduce new risks of adverse events. “We are doing that in some settings. For example, in postoperative Crohn’s disease, where patients have no active disease, we advocate prophylactically administering agents to prevent recurrence. So we are willing to do that in certain subsets of patients, but we have clinical trials to show the benefits of that,” said Dr. Hanauer. Unfortunately, there are no clinical trials demonstrating the benefits of such an approach in ulcerative colitis.
Biomarkers offer potential assistance for physicians who want to “treat to target,” as Dr. Hanauer put it, to achieve biological remission. Calprotectin – a protein secreted by neutrophils that mark the presence of these cells – are measurable in feces, and the levels correlate well with ulcerative colitis disease activity (Inflamm Bowel Dis. 2012 Nov;18:2011-7).
Dr. Hanauer’s advice to go beyond clinical remission is already occurring at the Food and Drug Administration, which no longer accepts clinical remission findings in testing new drugs for inflammatory bowel disease. “They are requiring patient-reported outcomes accompanied by endoscopic evidence of healing. I’m advocating what’s going on on a regulatory basis be applied on a clinical basis,” said Dr. Hanauer.
Dr. Hanauer had no relevant financial disclosures.
LAS VEGAS – In ulcerative colitis, symptoms aren’t everything. That’s the opinion of Stephen B. Hanauer, MD, medical director of Northwestern University’s Digestive Health Center, Chicago, who discussed the condition and the methods physicians use to assess patient improvement, at the annual meeting of the American College of Gastroenterology.
Many gastroenterologists accept patient reports of symptom improvement, but Dr. Hanauer advocates for a follow-up endoscopy, even when patients are making good progress.
Even in patients with improved symptoms, many endoscopic evaluations show inflammation and troubling histology that is in turn associated with worse quality of life and greater odds of hospitalizations and surgery (Dig Liver Dis. 2013 Dec;45:969-77).
So a better outcome is histological healing, characterized by the absence of neutrophils. A study presented at Digestive Disease Week® in 2015 (abstract 592) showed better relapse-free survival after histologic remission. “The deeper the remission that we achieve, the better the outcomes are going to be,” said Dr. Hanauer.
In fact, patients who report that they are asymptomatic may still be experiencing disease. “Many chronic patients accept a certain degree of symptoms as a new normal,” Dr. Hanauer said. Patients accept living with frequent nighttime awakenings to evacuate, or more frequent bowel movements. Further treatment could reduce or eliminate such problems. “There are subtle things you can improve upon, to convince them that the new normal is not the acceptable normal,” said Dr. Hanauer.
He suggests an endoscopy 3-12 months after patients report symptom abatement, although he admits that cost and other considerations can be barriers.
When an endoscopy is performed, it can lead to a conundrum if it shows residual inflammation despite a lack of symptoms. The physician might consider switching to a different class of drug, but that will usually raise cost and introduce new risks of adverse events. “We are doing that in some settings. For example, in postoperative Crohn’s disease, where patients have no active disease, we advocate prophylactically administering agents to prevent recurrence. So we are willing to do that in certain subsets of patients, but we have clinical trials to show the benefits of that,” said Dr. Hanauer. Unfortunately, there are no clinical trials demonstrating the benefits of such an approach in ulcerative colitis.
Biomarkers offer potential assistance for physicians who want to “treat to target,” as Dr. Hanauer put it, to achieve biological remission. Calprotectin – a protein secreted by neutrophils that mark the presence of these cells – are measurable in feces, and the levels correlate well with ulcerative colitis disease activity (Inflamm Bowel Dis. 2012 Nov;18:2011-7).
Dr. Hanauer’s advice to go beyond clinical remission is already occurring at the Food and Drug Administration, which no longer accepts clinical remission findings in testing new drugs for inflammatory bowel disease. “They are requiring patient-reported outcomes accompanied by endoscopic evidence of healing. I’m advocating what’s going on on a regulatory basis be applied on a clinical basis,” said Dr. Hanauer.
Dr. Hanauer had no relevant financial disclosures.
EXPERT ANALYSIS FROM ACG 2016
Innovations in treating AAA: Innovative Endovascular Approaches
Aortic aneurysms involving aortic branches pose significant challenges for open and endovascular repair. Tuesday morning’s session, “New Developments with Juxta and Pararenal Aortic Aneurysms and Thoracoabdominal Aneurysms,” will be a discussion of surgical experiences with the first fenestrated graft approved in the United States and a review several other approaches to treatment.
The session will provide “an important update about the most recent advances in this field,” Dr. Cao added. “It will be focused on the most recent adjuncts to be used not only in patients with a failed EVAR but also in preventing the failure as well as the indications for using endovascular versus an open approach.”
The session kicks off with presentations on the current status of ZFEN by Dr. Andres Schanzer of UMass Memorial Medical Center and on circumstances in when superior mesenteric artery (SMA) scallops can cause problems during F/EVAR with ZFEN by Dr. Carlos Timaran of the University of Texas Southwestern Medical School.
“The topics covered in this session will aid vascular surgeons in deciding the best approach to treat such patients, by emphasizing the strengths, weaknesses, and limitations of the various options,” he said. “Tips and tricks to address specific circumstances can be invaluable in optimizing patient outcomes.”
The last portion of the session will cover F/EVAR for failed EVAR, including when F/EVAR is indicated, adjuncts to improve outcomes of treatment for challenging AAAs, and branched EVAR (B/EVAR). The session will wrap with a presentation by Dr. Piotr Szopinski of the Medical University of Warsaw on early clinical results from the COLT endograft system for treatment of thoracoabdominal aortic aneurysms (TAAAs).
“Attendees will have the opportunity to compare the different treatment options,” Dr. Torsello said. “We look forward to an open discussion that will lead to practical guidelines for decision making. The audience can develop their own treatment algorithms for patients with hostile or absent aortic aneurysm neck.”
“We currently have multiple modalities to prevent and treat complications,” Dr. Cao said. “Vascular surgeons should be familiar with different techniques and should tailor their approach to each patient according to the morphology of the native aorta and access vessels, as well as the clinical conditions and risk factors for open surgery.”
Session 12:
New Developments with Juxta and Pararenal Aortic Aneurysms and Thoracoabdominal Aneurysms
Tuesday, 10:20 a.m. – 12:00 p.m.
Grand Ballroom West, 3 rd Floor
Aortic aneurysms involving aortic branches pose significant challenges for open and endovascular repair. Tuesday morning’s session, “New Developments with Juxta and Pararenal Aortic Aneurysms and Thoracoabdominal Aneurysms,” will be a discussion of surgical experiences with the first fenestrated graft approved in the United States and a review several other approaches to treatment.
The session will provide “an important update about the most recent advances in this field,” Dr. Cao added. “It will be focused on the most recent adjuncts to be used not only in patients with a failed EVAR but also in preventing the failure as well as the indications for using endovascular versus an open approach.”
The session kicks off with presentations on the current status of ZFEN by Dr. Andres Schanzer of UMass Memorial Medical Center and on circumstances in when superior mesenteric artery (SMA) scallops can cause problems during F/EVAR with ZFEN by Dr. Carlos Timaran of the University of Texas Southwestern Medical School.
“The topics covered in this session will aid vascular surgeons in deciding the best approach to treat such patients, by emphasizing the strengths, weaknesses, and limitations of the various options,” he said. “Tips and tricks to address specific circumstances can be invaluable in optimizing patient outcomes.”
The last portion of the session will cover F/EVAR for failed EVAR, including when F/EVAR is indicated, adjuncts to improve outcomes of treatment for challenging AAAs, and branched EVAR (B/EVAR). The session will wrap with a presentation by Dr. Piotr Szopinski of the Medical University of Warsaw on early clinical results from the COLT endograft system for treatment of thoracoabdominal aortic aneurysms (TAAAs).
“Attendees will have the opportunity to compare the different treatment options,” Dr. Torsello said. “We look forward to an open discussion that will lead to practical guidelines for decision making. The audience can develop their own treatment algorithms for patients with hostile or absent aortic aneurysm neck.”
“We currently have multiple modalities to prevent and treat complications,” Dr. Cao said. “Vascular surgeons should be familiar with different techniques and should tailor their approach to each patient according to the morphology of the native aorta and access vessels, as well as the clinical conditions and risk factors for open surgery.”
Session 12:
New Developments with Juxta and Pararenal Aortic Aneurysms and Thoracoabdominal Aneurysms
Tuesday, 10:20 a.m. – 12:00 p.m.
Grand Ballroom West, 3 rd Floor
Aortic aneurysms involving aortic branches pose significant challenges for open and endovascular repair. Tuesday morning’s session, “New Developments with Juxta and Pararenal Aortic Aneurysms and Thoracoabdominal Aneurysms,” will be a discussion of surgical experiences with the first fenestrated graft approved in the United States and a review several other approaches to treatment.
The session will provide “an important update about the most recent advances in this field,” Dr. Cao added. “It will be focused on the most recent adjuncts to be used not only in patients with a failed EVAR but also in preventing the failure as well as the indications for using endovascular versus an open approach.”
The session kicks off with presentations on the current status of ZFEN by Dr. Andres Schanzer of UMass Memorial Medical Center and on circumstances in when superior mesenteric artery (SMA) scallops can cause problems during F/EVAR with ZFEN by Dr. Carlos Timaran of the University of Texas Southwestern Medical School.
“The topics covered in this session will aid vascular surgeons in deciding the best approach to treat such patients, by emphasizing the strengths, weaknesses, and limitations of the various options,” he said. “Tips and tricks to address specific circumstances can be invaluable in optimizing patient outcomes.”
The last portion of the session will cover F/EVAR for failed EVAR, including when F/EVAR is indicated, adjuncts to improve outcomes of treatment for challenging AAAs, and branched EVAR (B/EVAR). The session will wrap with a presentation by Dr. Piotr Szopinski of the Medical University of Warsaw on early clinical results from the COLT endograft system for treatment of thoracoabdominal aortic aneurysms (TAAAs).
“Attendees will have the opportunity to compare the different treatment options,” Dr. Torsello said. “We look forward to an open discussion that will lead to practical guidelines for decision making. The audience can develop their own treatment algorithms for patients with hostile or absent aortic aneurysm neck.”
“We currently have multiple modalities to prevent and treat complications,” Dr. Cao said. “Vascular surgeons should be familiar with different techniques and should tailor their approach to each patient according to the morphology of the native aorta and access vessels, as well as the clinical conditions and risk factors for open surgery.”
Session 12:
New Developments with Juxta and Pararenal Aortic Aneurysms and Thoracoabdominal Aneurysms
Tuesday, 10:20 a.m. – 12:00 p.m.
Grand Ballroom West, 3 rd Floor
November 2016 Quiz 1
Q1: Answer: B
Rationale: Bile acid diarrhea (BAD) is becoming more widely recognized as a cause of chronic diarrhea. There are four associated types of BAD: Type 1 BAD is associated with ileal dysfunction with impaired reabsorption; type 2 BAD is considered primary or idiopathic BAD and occurs in the absence of ileal or obvious gastrointestinal disease; type 3 BAD is associated with gastrointestinal disorders, such as small intestinal bacterial overgrowth, celiac disease, or chronic pancreatitis; a fourth category of BAD may result from excessive hepatic bile acid synthesis caused by medications. This patient appears to have type 2 BAD; therefore, Answer B is correct. BAD has been shown to account for about one-third of patients with chronic diarrhea or irritable bowel syndrome with diarrhea.
References
1. Camilleri M. Bile acid diarrhea: prevalence, pathogenesis, and therapy. Gut Liver. 2015 May 23;9[3]:332-9.
2. Wedlake L., et al. Systematic review: The prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009;30:707-17.
Q1: Answer: B
Rationale: Bile acid diarrhea (BAD) is becoming more widely recognized as a cause of chronic diarrhea. There are four associated types of BAD: Type 1 BAD is associated with ileal dysfunction with impaired reabsorption; type 2 BAD is considered primary or idiopathic BAD and occurs in the absence of ileal or obvious gastrointestinal disease; type 3 BAD is associated with gastrointestinal disorders, such as small intestinal bacterial overgrowth, celiac disease, or chronic pancreatitis; a fourth category of BAD may result from excessive hepatic bile acid synthesis caused by medications. This patient appears to have type 2 BAD; therefore, Answer B is correct. BAD has been shown to account for about one-third of patients with chronic diarrhea or irritable bowel syndrome with diarrhea.
References
1. Camilleri M. Bile acid diarrhea: prevalence, pathogenesis, and therapy. Gut Liver. 2015 May 23;9[3]:332-9.
2. Wedlake L., et al. Systematic review: The prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009;30:707-17.
Q1: Answer: B
Rationale: Bile acid diarrhea (BAD) is becoming more widely recognized as a cause of chronic diarrhea. There are four associated types of BAD: Type 1 BAD is associated with ileal dysfunction with impaired reabsorption; type 2 BAD is considered primary or idiopathic BAD and occurs in the absence of ileal or obvious gastrointestinal disease; type 3 BAD is associated with gastrointestinal disorders, such as small intestinal bacterial overgrowth, celiac disease, or chronic pancreatitis; a fourth category of BAD may result from excessive hepatic bile acid synthesis caused by medications. This patient appears to have type 2 BAD; therefore, Answer B is correct. BAD has been shown to account for about one-third of patients with chronic diarrhea or irritable bowel syndrome with diarrhea.
References
1. Camilleri M. Bile acid diarrhea: prevalence, pathogenesis, and therapy. Gut Liver. 2015 May 23;9[3]:332-9.
2. Wedlake L., et al. Systematic review: The prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009;30:707-17.
A 44-year-old woman with no significant history presents for an evaluation of an 8-month history of meal-triggered diarrhea. Physical examination is normal. Thyroid function panel, celiac serology, C-reactive protein, lactose breath testing, stool studies for infectious pathogens, enterography imaging, and colonoscopy with biopsies are all negative. Trial of loperamide is not helpful. She is started on a bile acid sequestrant with prompt resolution of symptoms.
ACR annual meeting pediatric track highlights gene sequencing, brain disease
Will gene sequencing be one of the keys to unlocking previously mysterious inflammatory disorders in children?
Yes, according to new research to be presented at the annual meeting of the American College of Rheumatology in Washington. Gene and whole-exome sequencing will change the way these disorders are categorized, diagnosed, and managed, bringing new hope to the children who suffer from these rare, and devastating illnesses.
Program cochairs Anne M. Stevens, MD, and Anthony French, MD, PhD, agree: Gene sequencing is one of the most exciting and potentially practice-changing topics that ACR’s pediatric track will explore.
A session at 11:00 a.m. on Sunday, Nov. 13, is particularly intriguing, said Dr. Stevens of Seattle Children’s Hospital. “Early-Onset Monogenic Inflammatory Diseases” features two speakers.
Hal Hoffman, MD, chief of allergy, immunology and rheumatology in the department of pediatrics at the University of California, San Diego, will speak on inflammasome-associated disorders. He’ll present a case-based lecture, “so pediatric rheumatologists will be able to recognize these diseases, identify the genes underlying them, and make a clear genetic diagnosis,” Dr. Stevens said.
“These two speakers have been successful in identifying specific genes for these illnesses that have led to very specific treatments and, in some cases, complete resolution of symptoms,” Dr. Stevens noted.
“Advances in Clinical Care through Whole Exome Sequencing,” set for 11:00 a.m. on Tuesday, Nov. 15, will explore the practical impact of gene sequencing studies. Alexei A. Grom, MD, of Cincinnati Children’s Hospital, will discuss the practicalities of whole-exome sequencing: when to order it, how to interpret the findings, and how to use the results to help patients.
Jordan S. Orange, MD, PhD, of Baylor College of Medicine, Houston, will discuss his lab’s recent project: whole-exome sequencing of hundreds of pediatric rheumatology patients. “This study hasn’t focused on a specific disease or a specific gene but looked at the entire exome in an unbiased way,” Dr. Stevens said. “So far, they have identified genes associated with inflammatory disease in about 25% of this population.”
The session is meant to be practical as well as academic, Dr. French noted. “In the last few years, these tests have become much more accessible and easier to do in the clinic, not just in a research setting, and we’re going to focus on how to use them to individualize care.”
Dr. Stevens and Dr. French are also excited about the pediatric nephrology track, which kicks off with “What a Pediatric Rheumatologist May Want to Know About the Kidneys” at 8:30 a.m. on Monday, Nov. 14.
Mark M. Mitsnefes, MD, director of clinical and translational research at Cincinnati Children’s Hospital, will focus on treating hypertension in pediatric rheumatology patients. Bradley P. Dixon, MD, director of the nephrology clinical laboratory at Cincinnati Children’s, will review the thrombotic microangiopathies and discuss new treatment options. Stephen Marks, MD, a kidney transplant expert from London, will finish the session with a detailed discussion of lupus nephritis in children, focusing on early diagnosis.
Pediatric autoimmune brain disorders are on tap for a morning session on Tuesday, Nov. 14. Dr. Stevens is particularly looking forward to this session, which begins at 8:30 a.m.
“As pediatric rheumatologists, we are more and more often being asked to consult on new-onset seizures, psychoses, hallucinations, and stroke. It’s quite a challenge to figure out whether these are related to autoimmune disorders.”
The first lecture of the series focuses on the pathogenesis of autoimmune brain disease, and how to make the diagnosis. Russell Dale, MD, of the University of Sydney will talk about therapeutic decision making in these illnesses.
Josep Obrador Dalmau, MD, of the University of Pennsylvania, Philadelphia, will discuss anti–NMDA receptor encephalitis. Hermine I. Brunner, MD, director of rheumatology at Cincinnati Children’s, will close with a diagnostic and therapeutic update of neuropsychiatric systemic lupus erythematosus.
“This is a huge challenge for us,” Dr. Stevens said. “Children with lupus can have obvious central nervous system involvement, but also not-so-obvious involvement, including depression, anxiety, and cognitive difficulties.”
Will gene sequencing be one of the keys to unlocking previously mysterious inflammatory disorders in children?
Yes, according to new research to be presented at the annual meeting of the American College of Rheumatology in Washington. Gene and whole-exome sequencing will change the way these disorders are categorized, diagnosed, and managed, bringing new hope to the children who suffer from these rare, and devastating illnesses.
Program cochairs Anne M. Stevens, MD, and Anthony French, MD, PhD, agree: Gene sequencing is one of the most exciting and potentially practice-changing topics that ACR’s pediatric track will explore.
A session at 11:00 a.m. on Sunday, Nov. 13, is particularly intriguing, said Dr. Stevens of Seattle Children’s Hospital. “Early-Onset Monogenic Inflammatory Diseases” features two speakers.
Hal Hoffman, MD, chief of allergy, immunology and rheumatology in the department of pediatrics at the University of California, San Diego, will speak on inflammasome-associated disorders. He’ll present a case-based lecture, “so pediatric rheumatologists will be able to recognize these diseases, identify the genes underlying them, and make a clear genetic diagnosis,” Dr. Stevens said.
“These two speakers have been successful in identifying specific genes for these illnesses that have led to very specific treatments and, in some cases, complete resolution of symptoms,” Dr. Stevens noted.
“Advances in Clinical Care through Whole Exome Sequencing,” set for 11:00 a.m. on Tuesday, Nov. 15, will explore the practical impact of gene sequencing studies. Alexei A. Grom, MD, of Cincinnati Children’s Hospital, will discuss the practicalities of whole-exome sequencing: when to order it, how to interpret the findings, and how to use the results to help patients.
Jordan S. Orange, MD, PhD, of Baylor College of Medicine, Houston, will discuss his lab’s recent project: whole-exome sequencing of hundreds of pediatric rheumatology patients. “This study hasn’t focused on a specific disease or a specific gene but looked at the entire exome in an unbiased way,” Dr. Stevens said. “So far, they have identified genes associated with inflammatory disease in about 25% of this population.”
The session is meant to be practical as well as academic, Dr. French noted. “In the last few years, these tests have become much more accessible and easier to do in the clinic, not just in a research setting, and we’re going to focus on how to use them to individualize care.”
Dr. Stevens and Dr. French are also excited about the pediatric nephrology track, which kicks off with “What a Pediatric Rheumatologist May Want to Know About the Kidneys” at 8:30 a.m. on Monday, Nov. 14.
Mark M. Mitsnefes, MD, director of clinical and translational research at Cincinnati Children’s Hospital, will focus on treating hypertension in pediatric rheumatology patients. Bradley P. Dixon, MD, director of the nephrology clinical laboratory at Cincinnati Children’s, will review the thrombotic microangiopathies and discuss new treatment options. Stephen Marks, MD, a kidney transplant expert from London, will finish the session with a detailed discussion of lupus nephritis in children, focusing on early diagnosis.
Pediatric autoimmune brain disorders are on tap for a morning session on Tuesday, Nov. 14. Dr. Stevens is particularly looking forward to this session, which begins at 8:30 a.m.
“As pediatric rheumatologists, we are more and more often being asked to consult on new-onset seizures, psychoses, hallucinations, and stroke. It’s quite a challenge to figure out whether these are related to autoimmune disorders.”
The first lecture of the series focuses on the pathogenesis of autoimmune brain disease, and how to make the diagnosis. Russell Dale, MD, of the University of Sydney will talk about therapeutic decision making in these illnesses.
Josep Obrador Dalmau, MD, of the University of Pennsylvania, Philadelphia, will discuss anti–NMDA receptor encephalitis. Hermine I. Brunner, MD, director of rheumatology at Cincinnati Children’s, will close with a diagnostic and therapeutic update of neuropsychiatric systemic lupus erythematosus.
“This is a huge challenge for us,” Dr. Stevens said. “Children with lupus can have obvious central nervous system involvement, but also not-so-obvious involvement, including depression, anxiety, and cognitive difficulties.”
Will gene sequencing be one of the keys to unlocking previously mysterious inflammatory disorders in children?
Yes, according to new research to be presented at the annual meeting of the American College of Rheumatology in Washington. Gene and whole-exome sequencing will change the way these disorders are categorized, diagnosed, and managed, bringing new hope to the children who suffer from these rare, and devastating illnesses.
Program cochairs Anne M. Stevens, MD, and Anthony French, MD, PhD, agree: Gene sequencing is one of the most exciting and potentially practice-changing topics that ACR’s pediatric track will explore.
A session at 11:00 a.m. on Sunday, Nov. 13, is particularly intriguing, said Dr. Stevens of Seattle Children’s Hospital. “Early-Onset Monogenic Inflammatory Diseases” features two speakers.
Hal Hoffman, MD, chief of allergy, immunology and rheumatology in the department of pediatrics at the University of California, San Diego, will speak on inflammasome-associated disorders. He’ll present a case-based lecture, “so pediatric rheumatologists will be able to recognize these diseases, identify the genes underlying them, and make a clear genetic diagnosis,” Dr. Stevens said.
“These two speakers have been successful in identifying specific genes for these illnesses that have led to very specific treatments and, in some cases, complete resolution of symptoms,” Dr. Stevens noted.
“Advances in Clinical Care through Whole Exome Sequencing,” set for 11:00 a.m. on Tuesday, Nov. 15, will explore the practical impact of gene sequencing studies. Alexei A. Grom, MD, of Cincinnati Children’s Hospital, will discuss the practicalities of whole-exome sequencing: when to order it, how to interpret the findings, and how to use the results to help patients.
Jordan S. Orange, MD, PhD, of Baylor College of Medicine, Houston, will discuss his lab’s recent project: whole-exome sequencing of hundreds of pediatric rheumatology patients. “This study hasn’t focused on a specific disease or a specific gene but looked at the entire exome in an unbiased way,” Dr. Stevens said. “So far, they have identified genes associated with inflammatory disease in about 25% of this population.”
The session is meant to be practical as well as academic, Dr. French noted. “In the last few years, these tests have become much more accessible and easier to do in the clinic, not just in a research setting, and we’re going to focus on how to use them to individualize care.”
Dr. Stevens and Dr. French are also excited about the pediatric nephrology track, which kicks off with “What a Pediatric Rheumatologist May Want to Know About the Kidneys” at 8:30 a.m. on Monday, Nov. 14.
Mark M. Mitsnefes, MD, director of clinical and translational research at Cincinnati Children’s Hospital, will focus on treating hypertension in pediatric rheumatology patients. Bradley P. Dixon, MD, director of the nephrology clinical laboratory at Cincinnati Children’s, will review the thrombotic microangiopathies and discuss new treatment options. Stephen Marks, MD, a kidney transplant expert from London, will finish the session with a detailed discussion of lupus nephritis in children, focusing on early diagnosis.
Pediatric autoimmune brain disorders are on tap for a morning session on Tuesday, Nov. 14. Dr. Stevens is particularly looking forward to this session, which begins at 8:30 a.m.
“As pediatric rheumatologists, we are more and more often being asked to consult on new-onset seizures, psychoses, hallucinations, and stroke. It’s quite a challenge to figure out whether these are related to autoimmune disorders.”
The first lecture of the series focuses on the pathogenesis of autoimmune brain disease, and how to make the diagnosis. Russell Dale, MD, of the University of Sydney will talk about therapeutic decision making in these illnesses.
Josep Obrador Dalmau, MD, of the University of Pennsylvania, Philadelphia, will discuss anti–NMDA receptor encephalitis. Hermine I. Brunner, MD, director of rheumatology at Cincinnati Children’s, will close with a diagnostic and therapeutic update of neuropsychiatric systemic lupus erythematosus.
“This is a huge challenge for us,” Dr. Stevens said. “Children with lupus can have obvious central nervous system involvement, but also not-so-obvious involvement, including depression, anxiety, and cognitive difficulties.”
FROM THE ACR ANNUAL MEETING
Session Tackles Merits and Shortcomings of Drug Coated Balloons
Most vascular surgeons have a preferred method for managing femoropopliteal lesions, but drug-coated balloons are emerging as a option for lower extremity lesions.
The Wednesday morning session, “More on Lower Extremity Occlusive Disease: New Developments in Drug Coated Balloons (DCBs); Dealing with Complex Lesions and Trials,” will feature opposing views on how to manage the most severe disease morphology.
In this important session, world renown experts also will discuss the current status and immediate future of drug-coated balloons (DCB) for use on the lower extremity.
“The treatment with DCB is a story of great success. In general, the patency rate is increased and this is with no side effects due to the coating,” said Dr. Gunnar Tepe of Rosenheim Hospital in Rosenheim, Germany. “In the near future treatment with uncoated balloons will be mainly replaced by DCBs.” Dr. Tepe will present a paper on the current status and future prospects of DCBs.
Understanding important technical aspects remains essential to successful outcomes with DCBs, according to Dr. Tepe. “We have to know that treatment with DCBs is not a stand-alone therapy which can be successful in all lesions,” said Dr. Tepe. “If DCBs fail to obtain long-term patency, it is either due to recoil or drug uptake. For recoil, the combination of DCBs and stents is perfect, but for better drug uptake vessel, preparation is needed.”
Dr. Tepe and others will offer a detailed analysis of the potential shortcomings of DCBs, such as in long lesions, and in calcified arteries, and how to overcome them.
“The future of DCBs for use in patients with the potential threat of amputation is under development internationally,” said Dr. Schneider. “Practitioners will leave the session fully updated on the latest data and analysis of the value of DCBs in daily practice, since the most current results from randomized trials, and large registry datasets from the United States and around the world will be presented side by side.”
The presentations include the 3-year results from the international, randomized, controlled IN.PACT SFA Trial that compared the IN.PACT DCB against standard angioplasty. On hand to analyze and discuss the results will be co-principal investigators Dr. Schneider, Dr. Tepe, as well as panelist Dr. John R. Laird of the Vascular Center at the University of California, Davis. Co-moderator and study investigator Dr. Dierk Scheinert, MD, of the Leipzig University Hospital in Germany, will also join the discussion.
“I believe the data will show that DCBs have significantly changed how we practice and that by using them when possible, we can substantially improve what we can offer to patients in terms of long-term durability of the reconstructions we perform for their blocked and damaged arteries,” Dr. Tepe said.
The session will also cover second- and third-generation DCBs that are currently under development.
“As has been the tradition for many years, the VEITH planning committee has been able to bring together the world experts with the most important experience in these topics to educate, debate, inform the broader community,” said Dr. Schneider.
Session 26:
More on Lower Extremity Occlusive Disease: New Developments in Drug Coated Balloons (DCBs); Dealing with Complex Lesions and Trials
Wednesday 10:13 p.m. –12:00 p.m.
Grand Ballroom East, 3rd Floor
Most vascular surgeons have a preferred method for managing femoropopliteal lesions, but drug-coated balloons are emerging as a option for lower extremity lesions.
The Wednesday morning session, “More on Lower Extremity Occlusive Disease: New Developments in Drug Coated Balloons (DCBs); Dealing with Complex Lesions and Trials,” will feature opposing views on how to manage the most severe disease morphology.
In this important session, world renown experts also will discuss the current status and immediate future of drug-coated balloons (DCB) for use on the lower extremity.
“The treatment with DCB is a story of great success. In general, the patency rate is increased and this is with no side effects due to the coating,” said Dr. Gunnar Tepe of Rosenheim Hospital in Rosenheim, Germany. “In the near future treatment with uncoated balloons will be mainly replaced by DCBs.” Dr. Tepe will present a paper on the current status and future prospects of DCBs.
Understanding important technical aspects remains essential to successful outcomes with DCBs, according to Dr. Tepe. “We have to know that treatment with DCBs is not a stand-alone therapy which can be successful in all lesions,” said Dr. Tepe. “If DCBs fail to obtain long-term patency, it is either due to recoil or drug uptake. For recoil, the combination of DCBs and stents is perfect, but for better drug uptake vessel, preparation is needed.”
Dr. Tepe and others will offer a detailed analysis of the potential shortcomings of DCBs, such as in long lesions, and in calcified arteries, and how to overcome them.
“The future of DCBs for use in patients with the potential threat of amputation is under development internationally,” said Dr. Schneider. “Practitioners will leave the session fully updated on the latest data and analysis of the value of DCBs in daily practice, since the most current results from randomized trials, and large registry datasets from the United States and around the world will be presented side by side.”
The presentations include the 3-year results from the international, randomized, controlled IN.PACT SFA Trial that compared the IN.PACT DCB against standard angioplasty. On hand to analyze and discuss the results will be co-principal investigators Dr. Schneider, Dr. Tepe, as well as panelist Dr. John R. Laird of the Vascular Center at the University of California, Davis. Co-moderator and study investigator Dr. Dierk Scheinert, MD, of the Leipzig University Hospital in Germany, will also join the discussion.
“I believe the data will show that DCBs have significantly changed how we practice and that by using them when possible, we can substantially improve what we can offer to patients in terms of long-term durability of the reconstructions we perform for their blocked and damaged arteries,” Dr. Tepe said.
The session will also cover second- and third-generation DCBs that are currently under development.
“As has been the tradition for many years, the VEITH planning committee has been able to bring together the world experts with the most important experience in these topics to educate, debate, inform the broader community,” said Dr. Schneider.
Session 26:
More on Lower Extremity Occlusive Disease: New Developments in Drug Coated Balloons (DCBs); Dealing with Complex Lesions and Trials
Wednesday 10:13 p.m. –12:00 p.m.
Grand Ballroom East, 3rd Floor
Most vascular surgeons have a preferred method for managing femoropopliteal lesions, but drug-coated balloons are emerging as a option for lower extremity lesions.
The Wednesday morning session, “More on Lower Extremity Occlusive Disease: New Developments in Drug Coated Balloons (DCBs); Dealing with Complex Lesions and Trials,” will feature opposing views on how to manage the most severe disease morphology.
In this important session, world renown experts also will discuss the current status and immediate future of drug-coated balloons (DCB) for use on the lower extremity.
“The treatment with DCB is a story of great success. In general, the patency rate is increased and this is with no side effects due to the coating,” said Dr. Gunnar Tepe of Rosenheim Hospital in Rosenheim, Germany. “In the near future treatment with uncoated balloons will be mainly replaced by DCBs.” Dr. Tepe will present a paper on the current status and future prospects of DCBs.
Understanding important technical aspects remains essential to successful outcomes with DCBs, according to Dr. Tepe. “We have to know that treatment with DCBs is not a stand-alone therapy which can be successful in all lesions,” said Dr. Tepe. “If DCBs fail to obtain long-term patency, it is either due to recoil or drug uptake. For recoil, the combination of DCBs and stents is perfect, but for better drug uptake vessel, preparation is needed.”
Dr. Tepe and others will offer a detailed analysis of the potential shortcomings of DCBs, such as in long lesions, and in calcified arteries, and how to overcome them.
“The future of DCBs for use in patients with the potential threat of amputation is under development internationally,” said Dr. Schneider. “Practitioners will leave the session fully updated on the latest data and analysis of the value of DCBs in daily practice, since the most current results from randomized trials, and large registry datasets from the United States and around the world will be presented side by side.”
The presentations include the 3-year results from the international, randomized, controlled IN.PACT SFA Trial that compared the IN.PACT DCB against standard angioplasty. On hand to analyze and discuss the results will be co-principal investigators Dr. Schneider, Dr. Tepe, as well as panelist Dr. John R. Laird of the Vascular Center at the University of California, Davis. Co-moderator and study investigator Dr. Dierk Scheinert, MD, of the Leipzig University Hospital in Germany, will also join the discussion.
“I believe the data will show that DCBs have significantly changed how we practice and that by using them when possible, we can substantially improve what we can offer to patients in terms of long-term durability of the reconstructions we perform for their blocked and damaged arteries,” Dr. Tepe said.
The session will also cover second- and third-generation DCBs that are currently under development.
“As has been the tradition for many years, the VEITH planning committee has been able to bring together the world experts with the most important experience in these topics to educate, debate, inform the broader community,” said Dr. Schneider.
Session 26:
More on Lower Extremity Occlusive Disease: New Developments in Drug Coated Balloons (DCBs); Dealing with Complex Lesions and Trials
Wednesday 10:13 p.m. –12:00 p.m.
Grand Ballroom East, 3rd Floor
Remembering three giants in vascular surgery
This year the VEITHsymposium will be paying tribute to three influential vascular surgeons who are no longer with us: Dr. Allan Callow, Dr. Calvin Ernst, and Dr. John (Jack) Connolly.
Dr. Jerry Goldstone, MD, will have the honor of offering his thoughts on these extraordinary gentlemen during a session on Wednesday morning.
“I knew all three of these men quite well at various stages of my career,” Dr. Goldstone said.
Dr. Allan Callow, who died Dec. 22, 2015, at the age of 99, was considered a pioneer in vascular surgery. His contributions to vascular surgery include helping to perfect carotid endarterectomy.
He served in the U.S. Navy during World War II, retiring with the rank of rear admiral and was “an excellent speaker and had accumulated a very large personal experience with carotid artery disease which he was most recognized for as a clinician.”
Dr. Goldstone noted the different career path that this “great role model” followed.
“The most inspirational thing was his late-in-life switch to a basic science career,” Dr. Goldstone said. “Most of us in academics have our most intense basic research very early in our careers, but Allan’s late research career is inspiring and makes so much sense for a variety of reasons, not the least of which is it avoids the physical demands of clinical research.” Dr. Callow received an NIH RO1 grant at a time when contemporaries were retiring, Dr. Goldstone added.
Dr. Calvin Ernst “was probably best known as an educator, author and very dynamic Society of Vascular Surgery,” Dr. Goldstone said. “During his years on the SVS council, he was very actively involved in just about every activity that affected vascular surgery.”
Describing him as a “true Michigan guy” who was born in Detroit and attended the University of Michigan for undergraduate and medical school and stayed on for his surgery residency and then joined the faculty, Dr. Goldstone also remembered his accomplishments outside of SVS.
“Cal was a renowned surgeon and educator,” Dr. Goldstone recalled. “He was a prolific writer, authoring more than 300 papers and books, the best known probably being the first four editions of ‘Current Therapy in Vascular Surgery’ with Dr. James Stanley. ... He also served as the second co-editor of the Journal of Vascular Surgery and played a very important role in the early success of that journal.”
Dr. Ernst retired from practice in 2001 and died July 7, 2015 at the age of 81.
Dr. Goldstone noted that Dr. John (Jack) Connolly’s interests in cardiac and vascular surgery were broad and that he achieved world-wide fame for his presentations and lectures.
“He had a very prominent international influence and received countless invitations to speak abroad.”
He received fellowships were at the Royal College of Surgeons of England, Ireland and Edinburgh, as well as honorary membership in the Japan Vascular Society and the Vascular Surgical Society of Great Britain and Ireland. He also was honored by the University of California Irvine in 2012 when the institution established the John E. Connolly Endowed Chair in Surgery.
Dr. Goldstone remembered how “Jack” was “always a presence during my surgical training and career. Like Dr. Callow, he was still giving talks and writing papers well into his last years of life. He was very active internationally and was a vascular ambassador. He was charming, friendly, always willing to help younger surgeons and always had a genuine smile when he saw you.”
“For many,” Dr. Goldstone continued, “Jack will also be remembered for his friendship and unselfish support and mentoring.”
Dr. Connolly died Jan. 20, 2016, at the age of 92.
Session 34: Giants No Longer With Us: A Tribute To Allan Callow, Calvin Ernst And John (Jack) Connolly
Wednesday, 10:16 a.m. - 10:21 a.m.
Location: Grand Ballroom West, 3rd Floor
This year the VEITHsymposium will be paying tribute to three influential vascular surgeons who are no longer with us: Dr. Allan Callow, Dr. Calvin Ernst, and Dr. John (Jack) Connolly.
Dr. Jerry Goldstone, MD, will have the honor of offering his thoughts on these extraordinary gentlemen during a session on Wednesday morning.
“I knew all three of these men quite well at various stages of my career,” Dr. Goldstone said.
Dr. Allan Callow, who died Dec. 22, 2015, at the age of 99, was considered a pioneer in vascular surgery. His contributions to vascular surgery include helping to perfect carotid endarterectomy.
He served in the U.S. Navy during World War II, retiring with the rank of rear admiral and was “an excellent speaker and had accumulated a very large personal experience with carotid artery disease which he was most recognized for as a clinician.”
Dr. Goldstone noted the different career path that this “great role model” followed.
“The most inspirational thing was his late-in-life switch to a basic science career,” Dr. Goldstone said. “Most of us in academics have our most intense basic research very early in our careers, but Allan’s late research career is inspiring and makes so much sense for a variety of reasons, not the least of which is it avoids the physical demands of clinical research.” Dr. Callow received an NIH RO1 grant at a time when contemporaries were retiring, Dr. Goldstone added.
Dr. Calvin Ernst “was probably best known as an educator, author and very dynamic Society of Vascular Surgery,” Dr. Goldstone said. “During his years on the SVS council, he was very actively involved in just about every activity that affected vascular surgery.”
Describing him as a “true Michigan guy” who was born in Detroit and attended the University of Michigan for undergraduate and medical school and stayed on for his surgery residency and then joined the faculty, Dr. Goldstone also remembered his accomplishments outside of SVS.
“Cal was a renowned surgeon and educator,” Dr. Goldstone recalled. “He was a prolific writer, authoring more than 300 papers and books, the best known probably being the first four editions of ‘Current Therapy in Vascular Surgery’ with Dr. James Stanley. ... He also served as the second co-editor of the Journal of Vascular Surgery and played a very important role in the early success of that journal.”
Dr. Ernst retired from practice in 2001 and died July 7, 2015 at the age of 81.
Dr. Goldstone noted that Dr. John (Jack) Connolly’s interests in cardiac and vascular surgery were broad and that he achieved world-wide fame for his presentations and lectures.
“He had a very prominent international influence and received countless invitations to speak abroad.”
He received fellowships were at the Royal College of Surgeons of England, Ireland and Edinburgh, as well as honorary membership in the Japan Vascular Society and the Vascular Surgical Society of Great Britain and Ireland. He also was honored by the University of California Irvine in 2012 when the institution established the John E. Connolly Endowed Chair in Surgery.
Dr. Goldstone remembered how “Jack” was “always a presence during my surgical training and career. Like Dr. Callow, he was still giving talks and writing papers well into his last years of life. He was very active internationally and was a vascular ambassador. He was charming, friendly, always willing to help younger surgeons and always had a genuine smile when he saw you.”
“For many,” Dr. Goldstone continued, “Jack will also be remembered for his friendship and unselfish support and mentoring.”
Dr. Connolly died Jan. 20, 2016, at the age of 92.
Session 34: Giants No Longer With Us: A Tribute To Allan Callow, Calvin Ernst And John (Jack) Connolly
Wednesday, 10:16 a.m. - 10:21 a.m.
Location: Grand Ballroom West, 3rd Floor
This year the VEITHsymposium will be paying tribute to three influential vascular surgeons who are no longer with us: Dr. Allan Callow, Dr. Calvin Ernst, and Dr. John (Jack) Connolly.
Dr. Jerry Goldstone, MD, will have the honor of offering his thoughts on these extraordinary gentlemen during a session on Wednesday morning.
“I knew all three of these men quite well at various stages of my career,” Dr. Goldstone said.
Dr. Allan Callow, who died Dec. 22, 2015, at the age of 99, was considered a pioneer in vascular surgery. His contributions to vascular surgery include helping to perfect carotid endarterectomy.
He served in the U.S. Navy during World War II, retiring with the rank of rear admiral and was “an excellent speaker and had accumulated a very large personal experience with carotid artery disease which he was most recognized for as a clinician.”
Dr. Goldstone noted the different career path that this “great role model” followed.
“The most inspirational thing was his late-in-life switch to a basic science career,” Dr. Goldstone said. “Most of us in academics have our most intense basic research very early in our careers, but Allan’s late research career is inspiring and makes so much sense for a variety of reasons, not the least of which is it avoids the physical demands of clinical research.” Dr. Callow received an NIH RO1 grant at a time when contemporaries were retiring, Dr. Goldstone added.
Dr. Calvin Ernst “was probably best known as an educator, author and very dynamic Society of Vascular Surgery,” Dr. Goldstone said. “During his years on the SVS council, he was very actively involved in just about every activity that affected vascular surgery.”
Describing him as a “true Michigan guy” who was born in Detroit and attended the University of Michigan for undergraduate and medical school and stayed on for his surgery residency and then joined the faculty, Dr. Goldstone also remembered his accomplishments outside of SVS.
“Cal was a renowned surgeon and educator,” Dr. Goldstone recalled. “He was a prolific writer, authoring more than 300 papers and books, the best known probably being the first four editions of ‘Current Therapy in Vascular Surgery’ with Dr. James Stanley. ... He also served as the second co-editor of the Journal of Vascular Surgery and played a very important role in the early success of that journal.”
Dr. Ernst retired from practice in 2001 and died July 7, 2015 at the age of 81.
Dr. Goldstone noted that Dr. John (Jack) Connolly’s interests in cardiac and vascular surgery were broad and that he achieved world-wide fame for his presentations and lectures.
“He had a very prominent international influence and received countless invitations to speak abroad.”
He received fellowships were at the Royal College of Surgeons of England, Ireland and Edinburgh, as well as honorary membership in the Japan Vascular Society and the Vascular Surgical Society of Great Britain and Ireland. He also was honored by the University of California Irvine in 2012 when the institution established the John E. Connolly Endowed Chair in Surgery.
Dr. Goldstone remembered how “Jack” was “always a presence during my surgical training and career. Like Dr. Callow, he was still giving talks and writing papers well into his last years of life. He was very active internationally and was a vascular ambassador. He was charming, friendly, always willing to help younger surgeons and always had a genuine smile when he saw you.”
“For many,” Dr. Goldstone continued, “Jack will also be remembered for his friendship and unselfish support and mentoring.”
Dr. Connolly died Jan. 20, 2016, at the age of 92.
Session 34: Giants No Longer With Us: A Tribute To Allan Callow, Calvin Ernst And John (Jack) Connolly
Wednesday, 10:16 a.m. - 10:21 a.m.
Location: Grand Ballroom West, 3rd Floor