User login
Reassuring findings on neurodevelopmental outcomes in HIV-exposed children
DURBAN, SOUTH AFRICA – Children exposed to HIV in utero but uninfected at birth have neurodevelopmental test scores at age 24 months that are comparable with those of unexposed children, based on a study conducted in Botswana and presented by Jean Leidner at the 21st International AIDS Conference.
“These results provide reassurance regarding the potential effects of in-utero HIV and antiretroviral exposure,” declared Ms. Leidner, CEO of Goodtables Data Consulting in Norman, Okla., and the Botswana Harvard AIDS Institute Partnership.
The two groups of children had virtually identical scores on the cognitive, gross motor, fine motor, expressive language, and receptive language domains measured in the Bayley-III. The same was true for scores on the fine motor, locomotor, language, and personal-social elements of the Developmental Milestone Checklist.
The two groups of children differed in other ways; 17% of the uninfected children exposed to HIV in utero and 8% of the controls were low birth weight. The HIV-exposed children are being raised in a more challenging environment: just 49% have electricity in the home, compared with 64% of control families. Moreover, 53% of the HIV-exposed children and 33% of the controls live under conditions of moderate-to-severe food uncertainty.
Only 8% of the HIV-infected mothers breastfed, whereas breastfeeding was universal among the control group.
More than 99% of the HIV-infected mothers took antiretroviral medication antenatally. Roughly two-thirds were on zidovudine (Retrovir) monotherapy, the rest on a three-drug regimen of nevirapine (Viramune) plus lamivudine/zidovudine (Combivir). These are older antiretrovirals. Additional neurodevelopmental studies are warranted in children with in-utero exposure to newer agents, as well as in older children, Ms. Leidner said.
She reported having no financial conflicts regarding this study, which was funded by the National Institute of Mental Health.
DURBAN, SOUTH AFRICA – Children exposed to HIV in utero but uninfected at birth have neurodevelopmental test scores at age 24 months that are comparable with those of unexposed children, based on a study conducted in Botswana and presented by Jean Leidner at the 21st International AIDS Conference.
“These results provide reassurance regarding the potential effects of in-utero HIV and antiretroviral exposure,” declared Ms. Leidner, CEO of Goodtables Data Consulting in Norman, Okla., and the Botswana Harvard AIDS Institute Partnership.
The two groups of children had virtually identical scores on the cognitive, gross motor, fine motor, expressive language, and receptive language domains measured in the Bayley-III. The same was true for scores on the fine motor, locomotor, language, and personal-social elements of the Developmental Milestone Checklist.
The two groups of children differed in other ways; 17% of the uninfected children exposed to HIV in utero and 8% of the controls were low birth weight. The HIV-exposed children are being raised in a more challenging environment: just 49% have electricity in the home, compared with 64% of control families. Moreover, 53% of the HIV-exposed children and 33% of the controls live under conditions of moderate-to-severe food uncertainty.
Only 8% of the HIV-infected mothers breastfed, whereas breastfeeding was universal among the control group.
More than 99% of the HIV-infected mothers took antiretroviral medication antenatally. Roughly two-thirds were on zidovudine (Retrovir) monotherapy, the rest on a three-drug regimen of nevirapine (Viramune) plus lamivudine/zidovudine (Combivir). These are older antiretrovirals. Additional neurodevelopmental studies are warranted in children with in-utero exposure to newer agents, as well as in older children, Ms. Leidner said.
She reported having no financial conflicts regarding this study, which was funded by the National Institute of Mental Health.
DURBAN, SOUTH AFRICA – Children exposed to HIV in utero but uninfected at birth have neurodevelopmental test scores at age 24 months that are comparable with those of unexposed children, based on a study conducted in Botswana and presented by Jean Leidner at the 21st International AIDS Conference.
“These results provide reassurance regarding the potential effects of in-utero HIV and antiretroviral exposure,” declared Ms. Leidner, CEO of Goodtables Data Consulting in Norman, Okla., and the Botswana Harvard AIDS Institute Partnership.
The two groups of children had virtually identical scores on the cognitive, gross motor, fine motor, expressive language, and receptive language domains measured in the Bayley-III. The same was true for scores on the fine motor, locomotor, language, and personal-social elements of the Developmental Milestone Checklist.
The two groups of children differed in other ways; 17% of the uninfected children exposed to HIV in utero and 8% of the controls were low birth weight. The HIV-exposed children are being raised in a more challenging environment: just 49% have electricity in the home, compared with 64% of control families. Moreover, 53% of the HIV-exposed children and 33% of the controls live under conditions of moderate-to-severe food uncertainty.
Only 8% of the HIV-infected mothers breastfed, whereas breastfeeding was universal among the control group.
More than 99% of the HIV-infected mothers took antiretroviral medication antenatally. Roughly two-thirds were on zidovudine (Retrovir) monotherapy, the rest on a three-drug regimen of nevirapine (Viramune) plus lamivudine/zidovudine (Combivir). These are older antiretrovirals. Additional neurodevelopmental studies are warranted in children with in-utero exposure to newer agents, as well as in older children, Ms. Leidner said.
She reported having no financial conflicts regarding this study, which was funded by the National Institute of Mental Health.
Key clinical point:
Major finding: In-utero exposure to maternal HIV and antiretroviral drugs had no measurable adverse neurodevelopmental effects at age 24 months in uninfected children.
Data source: 337 uninfected children exposed to HIV in-utero and 387 children unexposed to HIV in utero.
Disclosures: The National Institute of Mental Health funded the study. The presenter reported having no financial conflicts of interest.
Striking the balance: Who should be screened for CP-CRE acquisition?
Carbapenem-resistant Enterobacteriaceae (CRE) are extremely drug-resistant organisms. According to the Centers for Disease Control and Prevention’s National Healthcare Safety Network, in 2014 in the United States, 3.6% of Enterobacteriaceae causing hospital-acquired infections were resistant to carbapenems.1 Antibiotic treatment options for CRE infections are severely limited, and mortality for invasive infections can be as high as 40%-50%.2
Resistance to carbapenems can be mediated by several mechanisms. From an epidemiologic standpoint, production of carbapenemases is the most-threatening mechanism because Enterobacteriaceae-harboring carbapenemases are highly transmissible.
Carbapenemase-producing CRE (CP-CRE) have caused large outbreaks throughout the world. Israel experienced a nationwide outbreak of CP-CRE, primarily Klebsiella pneumoniae carbapenemase–producing Klebsiella pneumoniae, in the mid-2000s. At the peak of the outbreak in 2007, there were 185 new cases per month (55.5/100,000 patient-days). A successful intervention at the national level dramatically decreased the incidence to 4.8/100,000 patient days in 2012.3
One component of the intervention (which is still ongoing) is active surveillance of high-risk groups using rectal swabs. Upon admission to the hospital, we screen patients who were recently in other hospitals or long-term care facilities. In addition, when a patient is newly diagnosed with CP-CRE (either asymptomatic carriage or clinical infection), we screen patients who had contact with that index case before isolation measures were implemented.
We recently published a study in Infection Control and Hospital Epidemiology that draws on our experience with CP-CRE screening of contacts at Tel Aviv Sourasky Medical Center.4 Both Israeli and International guidelines do not precisely define which contacts of a CP-CRE index case warrant screening. For example, should only roommates of index cases be screened or should we screen all patients on the same ward as the index case? Likewise, is there a minimum time of contact that should trigger screening?
Identifying which contacts are at high risk of acquiring CP-CRE is important for two reasons: We want to detect contacts who acquired CP-CRE so that they can be isolated before further transmission occurs, and we don’t want to waste resources and screen those at low risk. In our hospital, the criteria for being a contact are staying in the same ward and being treated by the same nursing staff as a newly identified CP-CRE patient.
This strategy appears to lead to overscreening, as we found that from October 2008 to June 2012, 3,158 screening tests were performed to detect 53 positive contacts (a yield of less than 2%). In order to screen more efficiently, our study aimed to determine risk factors for CP-CRE acquisition among patients exposed to a CP-CRE index patient.
We used a matched case-control design. The case group consisted of the 53 contacts who screened positive for CP-CRE. For each case we chose 2 controls: contacts who screened negative for CP-CRE. The basis for matching between the case and the 2 controls was that they were exposed to the same index patient. The benefit of matching this way was that it eliminated the question of whether a contact became positive because the index patient was more likely to transmit CP-CRE (e.g., because of diarrhea), and not because of characteristics of the contact patients themselves.
We found three factors that increased the risk that a contact would screen positive:
• Contact period of at least 3 days with the index case.
• Being on mechanical ventilation.
• Having a history of carriage or infection with another multidrug-resistant organism (such as methicillin-resistant Staphylococcus aureus).
Unexpectedly, sharing a room with the index patient or being debilitated did not significantly increase the risk of acquiring CP-CRE.
Many studies have identified antibiotic use as a risk factor for acquiring CP-CRE. In our study, no class of antibiotic increased the risk of CP-CRE acquisition, probably because only a small number of patients received each class. We were surprised to find that contacts who had taken cephalosporins were less likely to acquire CP-CRE. On further examination, when we compared patients who received only cephalosporins with patients who received no antibiotic, this protective effect disappeared. Nevertheless, compared with other antibiotics, it appears that cephalosporins might pose less of a risk for CP-CRE acquisition. More studies are needed to confirm our findings.
Our findings have practical implications for infection control. Using the risk factors we identified could help us to avoid excessive screening. We calculated that selective screening, based on our three risk factors, would have decreased the number of contacts screened by 30%, but 2 out of 53 positive contacts would have been missed. Institutions need to decide whether that is a trade-off they are willing to make.
Another way to apply our findings could be to add an additional layer of infection control by preemptively implementing contact precautions for patients at highest risk, for example, those with more than one risk factor.
1. Weiner LM, Fridkin SK, Aponte-Torres Z, Avery L, Coffin N, Dudeck MA, Edwards JR, Jernigan JA, Konnor R, Soe MM, Peterson K, Clifford McDonald L. Vital signs: preventing antibiotic-resistant infections in hospitals - United States, 2014. Am J Transplant. 2016 Jul;16(7):2224-30.
2. Centers for Disease Control and Prevention. Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE): November 2015 update – CRE Toolkit.
3. Schwaber MJ, Carmeli Y. An ongoing national intervention to contain the spread of carbapenem-resistant enterobacteriaceae. Clin Infect Dis. 2014 Mar;58(5):697-703.
Schwartz-Neiderman A, Braun T, Fallach N, Schwartz D, Carmeli Y, Schechner V. Risk factors for carbapenemase-producing carbapenem-resistant Enterobacteriaceae (CP-CRE) acquisition among contacts of newly diagnosed CP-CRE patients. Infect Control Hosp Epidemiol. 2016 Jul 25:1-7.
Vered Schechner, MD, MSc, is an infection control physician in the department of epidemiology at Tel Aviv Sourasky Medical Center.
Carbapenem-resistant Enterobacteriaceae (CRE) are extremely drug-resistant organisms. According to the Centers for Disease Control and Prevention’s National Healthcare Safety Network, in 2014 in the United States, 3.6% of Enterobacteriaceae causing hospital-acquired infections were resistant to carbapenems.1 Antibiotic treatment options for CRE infections are severely limited, and mortality for invasive infections can be as high as 40%-50%.2
Resistance to carbapenems can be mediated by several mechanisms. From an epidemiologic standpoint, production of carbapenemases is the most-threatening mechanism because Enterobacteriaceae-harboring carbapenemases are highly transmissible.
Carbapenemase-producing CRE (CP-CRE) have caused large outbreaks throughout the world. Israel experienced a nationwide outbreak of CP-CRE, primarily Klebsiella pneumoniae carbapenemase–producing Klebsiella pneumoniae, in the mid-2000s. At the peak of the outbreak in 2007, there were 185 new cases per month (55.5/100,000 patient-days). A successful intervention at the national level dramatically decreased the incidence to 4.8/100,000 patient days in 2012.3
One component of the intervention (which is still ongoing) is active surveillance of high-risk groups using rectal swabs. Upon admission to the hospital, we screen patients who were recently in other hospitals or long-term care facilities. In addition, when a patient is newly diagnosed with CP-CRE (either asymptomatic carriage or clinical infection), we screen patients who had contact with that index case before isolation measures were implemented.
We recently published a study in Infection Control and Hospital Epidemiology that draws on our experience with CP-CRE screening of contacts at Tel Aviv Sourasky Medical Center.4 Both Israeli and International guidelines do not precisely define which contacts of a CP-CRE index case warrant screening. For example, should only roommates of index cases be screened or should we screen all patients on the same ward as the index case? Likewise, is there a minimum time of contact that should trigger screening?
Identifying which contacts are at high risk of acquiring CP-CRE is important for two reasons: We want to detect contacts who acquired CP-CRE so that they can be isolated before further transmission occurs, and we don’t want to waste resources and screen those at low risk. In our hospital, the criteria for being a contact are staying in the same ward and being treated by the same nursing staff as a newly identified CP-CRE patient.
This strategy appears to lead to overscreening, as we found that from October 2008 to June 2012, 3,158 screening tests were performed to detect 53 positive contacts (a yield of less than 2%). In order to screen more efficiently, our study aimed to determine risk factors for CP-CRE acquisition among patients exposed to a CP-CRE index patient.
We used a matched case-control design. The case group consisted of the 53 contacts who screened positive for CP-CRE. For each case we chose 2 controls: contacts who screened negative for CP-CRE. The basis for matching between the case and the 2 controls was that they were exposed to the same index patient. The benefit of matching this way was that it eliminated the question of whether a contact became positive because the index patient was more likely to transmit CP-CRE (e.g., because of diarrhea), and not because of characteristics of the contact patients themselves.
We found three factors that increased the risk that a contact would screen positive:
• Contact period of at least 3 days with the index case.
• Being on mechanical ventilation.
• Having a history of carriage or infection with another multidrug-resistant organism (such as methicillin-resistant Staphylococcus aureus).
Unexpectedly, sharing a room with the index patient or being debilitated did not significantly increase the risk of acquiring CP-CRE.
Many studies have identified antibiotic use as a risk factor for acquiring CP-CRE. In our study, no class of antibiotic increased the risk of CP-CRE acquisition, probably because only a small number of patients received each class. We were surprised to find that contacts who had taken cephalosporins were less likely to acquire CP-CRE. On further examination, when we compared patients who received only cephalosporins with patients who received no antibiotic, this protective effect disappeared. Nevertheless, compared with other antibiotics, it appears that cephalosporins might pose less of a risk for CP-CRE acquisition. More studies are needed to confirm our findings.
Our findings have practical implications for infection control. Using the risk factors we identified could help us to avoid excessive screening. We calculated that selective screening, based on our three risk factors, would have decreased the number of contacts screened by 30%, but 2 out of 53 positive contacts would have been missed. Institutions need to decide whether that is a trade-off they are willing to make.
Another way to apply our findings could be to add an additional layer of infection control by preemptively implementing contact precautions for patients at highest risk, for example, those with more than one risk factor.
1. Weiner LM, Fridkin SK, Aponte-Torres Z, Avery L, Coffin N, Dudeck MA, Edwards JR, Jernigan JA, Konnor R, Soe MM, Peterson K, Clifford McDonald L. Vital signs: preventing antibiotic-resistant infections in hospitals - United States, 2014. Am J Transplant. 2016 Jul;16(7):2224-30.
2. Centers for Disease Control and Prevention. Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE): November 2015 update – CRE Toolkit.
3. Schwaber MJ, Carmeli Y. An ongoing national intervention to contain the spread of carbapenem-resistant enterobacteriaceae. Clin Infect Dis. 2014 Mar;58(5):697-703.
Schwartz-Neiderman A, Braun T, Fallach N, Schwartz D, Carmeli Y, Schechner V. Risk factors for carbapenemase-producing carbapenem-resistant Enterobacteriaceae (CP-CRE) acquisition among contacts of newly diagnosed CP-CRE patients. Infect Control Hosp Epidemiol. 2016 Jul 25:1-7.
Vered Schechner, MD, MSc, is an infection control physician in the department of epidemiology at Tel Aviv Sourasky Medical Center.
Carbapenem-resistant Enterobacteriaceae (CRE) are extremely drug-resistant organisms. According to the Centers for Disease Control and Prevention’s National Healthcare Safety Network, in 2014 in the United States, 3.6% of Enterobacteriaceae causing hospital-acquired infections were resistant to carbapenems.1 Antibiotic treatment options for CRE infections are severely limited, and mortality for invasive infections can be as high as 40%-50%.2
Resistance to carbapenems can be mediated by several mechanisms. From an epidemiologic standpoint, production of carbapenemases is the most-threatening mechanism because Enterobacteriaceae-harboring carbapenemases are highly transmissible.
Carbapenemase-producing CRE (CP-CRE) have caused large outbreaks throughout the world. Israel experienced a nationwide outbreak of CP-CRE, primarily Klebsiella pneumoniae carbapenemase–producing Klebsiella pneumoniae, in the mid-2000s. At the peak of the outbreak in 2007, there were 185 new cases per month (55.5/100,000 patient-days). A successful intervention at the national level dramatically decreased the incidence to 4.8/100,000 patient days in 2012.3
One component of the intervention (which is still ongoing) is active surveillance of high-risk groups using rectal swabs. Upon admission to the hospital, we screen patients who were recently in other hospitals or long-term care facilities. In addition, when a patient is newly diagnosed with CP-CRE (either asymptomatic carriage or clinical infection), we screen patients who had contact with that index case before isolation measures were implemented.
We recently published a study in Infection Control and Hospital Epidemiology that draws on our experience with CP-CRE screening of contacts at Tel Aviv Sourasky Medical Center.4 Both Israeli and International guidelines do not precisely define which contacts of a CP-CRE index case warrant screening. For example, should only roommates of index cases be screened or should we screen all patients on the same ward as the index case? Likewise, is there a minimum time of contact that should trigger screening?
Identifying which contacts are at high risk of acquiring CP-CRE is important for two reasons: We want to detect contacts who acquired CP-CRE so that they can be isolated before further transmission occurs, and we don’t want to waste resources and screen those at low risk. In our hospital, the criteria for being a contact are staying in the same ward and being treated by the same nursing staff as a newly identified CP-CRE patient.
This strategy appears to lead to overscreening, as we found that from October 2008 to June 2012, 3,158 screening tests were performed to detect 53 positive contacts (a yield of less than 2%). In order to screen more efficiently, our study aimed to determine risk factors for CP-CRE acquisition among patients exposed to a CP-CRE index patient.
We used a matched case-control design. The case group consisted of the 53 contacts who screened positive for CP-CRE. For each case we chose 2 controls: contacts who screened negative for CP-CRE. The basis for matching between the case and the 2 controls was that they were exposed to the same index patient. The benefit of matching this way was that it eliminated the question of whether a contact became positive because the index patient was more likely to transmit CP-CRE (e.g., because of diarrhea), and not because of characteristics of the contact patients themselves.
We found three factors that increased the risk that a contact would screen positive:
• Contact period of at least 3 days with the index case.
• Being on mechanical ventilation.
• Having a history of carriage or infection with another multidrug-resistant organism (such as methicillin-resistant Staphylococcus aureus).
Unexpectedly, sharing a room with the index patient or being debilitated did not significantly increase the risk of acquiring CP-CRE.
Many studies have identified antibiotic use as a risk factor for acquiring CP-CRE. In our study, no class of antibiotic increased the risk of CP-CRE acquisition, probably because only a small number of patients received each class. We were surprised to find that contacts who had taken cephalosporins were less likely to acquire CP-CRE. On further examination, when we compared patients who received only cephalosporins with patients who received no antibiotic, this protective effect disappeared. Nevertheless, compared with other antibiotics, it appears that cephalosporins might pose less of a risk for CP-CRE acquisition. More studies are needed to confirm our findings.
Our findings have practical implications for infection control. Using the risk factors we identified could help us to avoid excessive screening. We calculated that selective screening, based on our three risk factors, would have decreased the number of contacts screened by 30%, but 2 out of 53 positive contacts would have been missed. Institutions need to decide whether that is a trade-off they are willing to make.
Another way to apply our findings could be to add an additional layer of infection control by preemptively implementing contact precautions for patients at highest risk, for example, those with more than one risk factor.
1. Weiner LM, Fridkin SK, Aponte-Torres Z, Avery L, Coffin N, Dudeck MA, Edwards JR, Jernigan JA, Konnor R, Soe MM, Peterson K, Clifford McDonald L. Vital signs: preventing antibiotic-resistant infections in hospitals - United States, 2014. Am J Transplant. 2016 Jul;16(7):2224-30.
2. Centers for Disease Control and Prevention. Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE): November 2015 update – CRE Toolkit.
3. Schwaber MJ, Carmeli Y. An ongoing national intervention to contain the spread of carbapenem-resistant enterobacteriaceae. Clin Infect Dis. 2014 Mar;58(5):697-703.
Schwartz-Neiderman A, Braun T, Fallach N, Schwartz D, Carmeli Y, Schechner V. Risk factors for carbapenemase-producing carbapenem-resistant Enterobacteriaceae (CP-CRE) acquisition among contacts of newly diagnosed CP-CRE patients. Infect Control Hosp Epidemiol. 2016 Jul 25:1-7.
Vered Schechner, MD, MSc, is an infection control physician in the department of epidemiology at Tel Aviv Sourasky Medical Center.
Switching between generic antiepileptics not linked to hospital visits for seizure
Switching between generic versions of the same antiepileptic drug made by different manufacturers does not appear to change the risk of seizure-related events in patients with epilepsy, according to a population-based, case-crossover study of generic antiepileptic drug users.
Instead, the study indicates that delays and complications of the medication refilling process might increase a patient’s risk for a seizure, said first author Aaron Kesselheim, MD, and his associates from Brigham and Women’s Hospital and Harvard Medical School, Boston (Neurology. 2016 Sep 28. doi: 10.1212/WNL.0000000000003259).
Although the results of previous observational studies have demonstrated increased seizure activity following a switch from brand name to generic antiepileptic drugs, Dr. Kesselheim and his associates pointed out that several recent randomized trials have found no link between generic drug switching and seizure risk.
The investigators identified 59,344 patients who had at least one refill of a prescription from the same manufacturer and 5,200 patients who switched from one generic to another during 2000-2010 in the Medicaid Analytic eXtract database as well as during 2005-2013 in a commercial health insurance database. Participants acted as their own controls in the study’s comparison of the effects of a refill or a refill with a switch in manufacturer on seizure-related events (a seizure requiring an emergency department visit or hospitalization) during a hazard period, defined as days 2–36 preceding a seizure-related event, and a control period, defined as days 51–85 preceding the seizure-related event.
Overall, generic antiepileptic refilling of the same medication from the same manufacturer was associated with an 8% increase in the odds of having a seizure-related event (95% confidence interval, 1.06–1.11). When the refill involved a switch to the same generic drug made by a different manufacturer, the odds of a seizure-related event rose by 9% (95% CI, 1.03–1.15). When this involved a change in the shape or color of the pill, the odds increased by 11% (95% CI, 1.05–1.18) but did not increase when the switch was made to a pill with the same color and shape. The increased odds of seizure-related events became nonsignificant when the researchers adjusted these comparisons for the process of refilling, which the authors noted “is often not straightforward. [And] patients have expressed frustration with delays and other complicating factors relating to refilling. … Greater work to enhance the refilling process, and to determine whether mail order pharmacies successfully improve outcomes on this point, is necessary.”
The study was not supported by any specific targeted funding. The investigators reported receiving support from various foundations and from programs within Harvard University and grants from the Agency for Healthcare Research and Quality and the Food and Drug Administration; the investigators also disclosed acting in a research support role for and/or receiving financial compensation from several pharmaceutical companies and other organizations.
[email protected]
On Twitter @jessnicolecraig
The study by Dr. Kesselheim and his colleagues is one of several recently aimed at reviewing the overall safety of generic drug switching. The Food and Drug Administration sponsored three clinical bioequivalence studies to determine the adequacy of average bioequivalence studies for ensuring safe conversion between different antiseizure products for patients with epilepsy. Taken together, these studies confirm that most patients can safely switch between generic formulations, even between tablets differing in appearance.
• Patients want to find a reason for the near-random pattern of their seizures. Threshold cortical epileptogenic activity triggers seizures in near-random patterns, and their timing might be influenced by triggers such as missing doses, stress, and hormonal changes.
• Patients’ views toward illness and treatments might influence their reporting of seizures and drug effects. This search for seizure explanations can even extend to pets with seizures.
• A small group of patients may be outside the 90% confidence interval bioequivalence acceptance range and may experience product switching effects.
Gregory L. Krauss, MD, is a professor of neurology at the Johns Hopkins Hospital, Baltimore. Michael D. Privitera, MD, is a professor and director of the University of Cincinnati epilepsy center. Their comments are derived from an editorial accompanying the report by Dr. Kesselheim and his colleagues (Neurology. 2016 Sep 28. doi: 10.1212/WNL.0000000000003272). They reported having no relevant financial disclosures.
The study by Dr. Kesselheim and his colleagues is one of several recently aimed at reviewing the overall safety of generic drug switching. The Food and Drug Administration sponsored three clinical bioequivalence studies to determine the adequacy of average bioequivalence studies for ensuring safe conversion between different antiseizure products for patients with epilepsy. Taken together, these studies confirm that most patients can safely switch between generic formulations, even between tablets differing in appearance.
• Patients want to find a reason for the near-random pattern of their seizures. Threshold cortical epileptogenic activity triggers seizures in near-random patterns, and their timing might be influenced by triggers such as missing doses, stress, and hormonal changes.
• Patients’ views toward illness and treatments might influence their reporting of seizures and drug effects. This search for seizure explanations can even extend to pets with seizures.
• A small group of patients may be outside the 90% confidence interval bioequivalence acceptance range and may experience product switching effects.
Gregory L. Krauss, MD, is a professor of neurology at the Johns Hopkins Hospital, Baltimore. Michael D. Privitera, MD, is a professor and director of the University of Cincinnati epilepsy center. Their comments are derived from an editorial accompanying the report by Dr. Kesselheim and his colleagues (Neurology. 2016 Sep 28. doi: 10.1212/WNL.0000000000003272). They reported having no relevant financial disclosures.
The study by Dr. Kesselheim and his colleagues is one of several recently aimed at reviewing the overall safety of generic drug switching. The Food and Drug Administration sponsored three clinical bioequivalence studies to determine the adequacy of average bioequivalence studies for ensuring safe conversion between different antiseizure products for patients with epilepsy. Taken together, these studies confirm that most patients can safely switch between generic formulations, even between tablets differing in appearance.
• Patients want to find a reason for the near-random pattern of their seizures. Threshold cortical epileptogenic activity triggers seizures in near-random patterns, and their timing might be influenced by triggers such as missing doses, stress, and hormonal changes.
• Patients’ views toward illness and treatments might influence their reporting of seizures and drug effects. This search for seizure explanations can even extend to pets with seizures.
• A small group of patients may be outside the 90% confidence interval bioequivalence acceptance range and may experience product switching effects.
Gregory L. Krauss, MD, is a professor of neurology at the Johns Hopkins Hospital, Baltimore. Michael D. Privitera, MD, is a professor and director of the University of Cincinnati epilepsy center. Their comments are derived from an editorial accompanying the report by Dr. Kesselheim and his colleagues (Neurology. 2016 Sep 28. doi: 10.1212/WNL.0000000000003272). They reported having no relevant financial disclosures.
Switching between generic versions of the same antiepileptic drug made by different manufacturers does not appear to change the risk of seizure-related events in patients with epilepsy, according to a population-based, case-crossover study of generic antiepileptic drug users.
Instead, the study indicates that delays and complications of the medication refilling process might increase a patient’s risk for a seizure, said first author Aaron Kesselheim, MD, and his associates from Brigham and Women’s Hospital and Harvard Medical School, Boston (Neurology. 2016 Sep 28. doi: 10.1212/WNL.0000000000003259).
Although the results of previous observational studies have demonstrated increased seizure activity following a switch from brand name to generic antiepileptic drugs, Dr. Kesselheim and his associates pointed out that several recent randomized trials have found no link between generic drug switching and seizure risk.
The investigators identified 59,344 patients who had at least one refill of a prescription from the same manufacturer and 5,200 patients who switched from one generic to another during 2000-2010 in the Medicaid Analytic eXtract database as well as during 2005-2013 in a commercial health insurance database. Participants acted as their own controls in the study’s comparison of the effects of a refill or a refill with a switch in manufacturer on seizure-related events (a seizure requiring an emergency department visit or hospitalization) during a hazard period, defined as days 2–36 preceding a seizure-related event, and a control period, defined as days 51–85 preceding the seizure-related event.
Overall, generic antiepileptic refilling of the same medication from the same manufacturer was associated with an 8% increase in the odds of having a seizure-related event (95% confidence interval, 1.06–1.11). When the refill involved a switch to the same generic drug made by a different manufacturer, the odds of a seizure-related event rose by 9% (95% CI, 1.03–1.15). When this involved a change in the shape or color of the pill, the odds increased by 11% (95% CI, 1.05–1.18) but did not increase when the switch was made to a pill with the same color and shape. The increased odds of seizure-related events became nonsignificant when the researchers adjusted these comparisons for the process of refilling, which the authors noted “is often not straightforward. [And] patients have expressed frustration with delays and other complicating factors relating to refilling. … Greater work to enhance the refilling process, and to determine whether mail order pharmacies successfully improve outcomes on this point, is necessary.”
The study was not supported by any specific targeted funding. The investigators reported receiving support from various foundations and from programs within Harvard University and grants from the Agency for Healthcare Research and Quality and the Food and Drug Administration; the investigators also disclosed acting in a research support role for and/or receiving financial compensation from several pharmaceutical companies and other organizations.
[email protected]
On Twitter @jessnicolecraig
Switching between generic versions of the same antiepileptic drug made by different manufacturers does not appear to change the risk of seizure-related events in patients with epilepsy, according to a population-based, case-crossover study of generic antiepileptic drug users.
Instead, the study indicates that delays and complications of the medication refilling process might increase a patient’s risk for a seizure, said first author Aaron Kesselheim, MD, and his associates from Brigham and Women’s Hospital and Harvard Medical School, Boston (Neurology. 2016 Sep 28. doi: 10.1212/WNL.0000000000003259).
Although the results of previous observational studies have demonstrated increased seizure activity following a switch from brand name to generic antiepileptic drugs, Dr. Kesselheim and his associates pointed out that several recent randomized trials have found no link between generic drug switching and seizure risk.
The investigators identified 59,344 patients who had at least one refill of a prescription from the same manufacturer and 5,200 patients who switched from one generic to another during 2000-2010 in the Medicaid Analytic eXtract database as well as during 2005-2013 in a commercial health insurance database. Participants acted as their own controls in the study’s comparison of the effects of a refill or a refill with a switch in manufacturer on seizure-related events (a seizure requiring an emergency department visit or hospitalization) during a hazard period, defined as days 2–36 preceding a seizure-related event, and a control period, defined as days 51–85 preceding the seizure-related event.
Overall, generic antiepileptic refilling of the same medication from the same manufacturer was associated with an 8% increase in the odds of having a seizure-related event (95% confidence interval, 1.06–1.11). When the refill involved a switch to the same generic drug made by a different manufacturer, the odds of a seizure-related event rose by 9% (95% CI, 1.03–1.15). When this involved a change in the shape or color of the pill, the odds increased by 11% (95% CI, 1.05–1.18) but did not increase when the switch was made to a pill with the same color and shape. The increased odds of seizure-related events became nonsignificant when the researchers adjusted these comparisons for the process of refilling, which the authors noted “is often not straightforward. [And] patients have expressed frustration with delays and other complicating factors relating to refilling. … Greater work to enhance the refilling process, and to determine whether mail order pharmacies successfully improve outcomes on this point, is necessary.”
The study was not supported by any specific targeted funding. The investigators reported receiving support from various foundations and from programs within Harvard University and grants from the Agency for Healthcare Research and Quality and the Food and Drug Administration; the investigators also disclosed acting in a research support role for and/or receiving financial compensation from several pharmaceutical companies and other organizations.
[email protected]
On Twitter @jessnicolecraig
FROM NEUROLOGY
Key clinical point:
Major finding: Generic antiepileptic refilling was associated with an 8%-9% increase in the odds of having a seizure-related event, but this association disappeared after the investigators adjusted for the refilling process.
Data source: A population-based, case-crossover study of 64,544 generic antiepileptic drug users.
Disclosures: The study was not supported by any specific targeted funding. The investigators reported receiving support from various foundations and from programs within Harvard University and grants from the Agency for Healthcare Research and Quality and the Food and Drug Administration; the investigators also disclosed acting in a research support role for and/or receiving financial compensation from several pharmaceutical companies and other organizations.
Hypertension in children linked to lower neurocognitive performance
Children with primary hypertension demonstrated significantly lower performance on neurocognitive testing, compared with children without primary hypertension, according to Marc B. Lande, MD, of the University of Rochester (N.Y.), and his associates.
In the study, 75 children with newly diagnosed untreated primary hypertension and 75 normotensive control subjects were examined. Hypertension was linked with worse performances on neurocognitive measures of attention, learning, memory, and fine motor dexterity, compared with the controls. There also was an association between increased disordered sleep and worse executive function. This was more pronounced in children with hypertension than in normotensive children. Hypertension and control groups did not differ significantly in age, sex, maternal education, income, race, ethnicity, obesity, anxiety, depression, cholesterol, glucose, insulin, and C-reactive protein.
“These results suggest that hypertension in youth may have an impact on brain function, and perhaps brain development, in childhood,” the researchers concluded. “Future results from this study will assess the degree to which these effects can be minimized or reversed with antihypertensive therapies.”
Find the full study in the Journal of Pediatrics (2016 Sept 29. doi: 10.1016/j.jpeds.2016.08.076).
Children with primary hypertension demonstrated significantly lower performance on neurocognitive testing, compared with children without primary hypertension, according to Marc B. Lande, MD, of the University of Rochester (N.Y.), and his associates.
In the study, 75 children with newly diagnosed untreated primary hypertension and 75 normotensive control subjects were examined. Hypertension was linked with worse performances on neurocognitive measures of attention, learning, memory, and fine motor dexterity, compared with the controls. There also was an association between increased disordered sleep and worse executive function. This was more pronounced in children with hypertension than in normotensive children. Hypertension and control groups did not differ significantly in age, sex, maternal education, income, race, ethnicity, obesity, anxiety, depression, cholesterol, glucose, insulin, and C-reactive protein.
“These results suggest that hypertension in youth may have an impact on brain function, and perhaps brain development, in childhood,” the researchers concluded. “Future results from this study will assess the degree to which these effects can be minimized or reversed with antihypertensive therapies.”
Find the full study in the Journal of Pediatrics (2016 Sept 29. doi: 10.1016/j.jpeds.2016.08.076).
Children with primary hypertension demonstrated significantly lower performance on neurocognitive testing, compared with children without primary hypertension, according to Marc B. Lande, MD, of the University of Rochester (N.Y.), and his associates.
In the study, 75 children with newly diagnosed untreated primary hypertension and 75 normotensive control subjects were examined. Hypertension was linked with worse performances on neurocognitive measures of attention, learning, memory, and fine motor dexterity, compared with the controls. There also was an association between increased disordered sleep and worse executive function. This was more pronounced in children with hypertension than in normotensive children. Hypertension and control groups did not differ significantly in age, sex, maternal education, income, race, ethnicity, obesity, anxiety, depression, cholesterol, glucose, insulin, and C-reactive protein.
“These results suggest that hypertension in youth may have an impact on brain function, and perhaps brain development, in childhood,” the researchers concluded. “Future results from this study will assess the degree to which these effects can be minimized or reversed with antihypertensive therapies.”
Find the full study in the Journal of Pediatrics (2016 Sept 29. doi: 10.1016/j.jpeds.2016.08.076).
HIV research update: Late September 2016
A great volume of HIV and AIDS research enters the medical literature every month. It’s difficult to monitor everything, so here’s a quick look at some notable news items and journal articles published over the past few weeks.
Plasma lopinavir concentrations predicted viral outcomes in HIV-infected children receiving lopinavir-based antiretroviral therapy, a recent study demonstrated. Investigators said their findings support a minimum target concentration of greater than and equal to 1 mg/L of lopinavir to ensure sustained viral suppression.
Most antiretroviral-naive HIV-positive children experienced recovery of both weight for age and height for age over the 24 months following initiation of antiretroviral therapy (ART), according to results of a recent study. There was no significant difference between those receiving lopinavir/ritonavir and ART that was not based on nucleoside reverse transcriptase inhibitors (NNRTIs).
Even in an environment of easy access to antiretroviral therapy for HIV patients, many challenges still exist at the implementation stage of early ART, investigators for a study in AIDS Care reported. The authors said intense efforts in both patient and physician education will be required if the benefits of early ART are to be achieved at the individual and population level.
CD4+ and CD8+ T-cell immune activation and exhaustion are greater in HIV-infected youth, compared with matched controls, according to results of a recent study, while monocyte subpopulations are not changed even though there is a high soluble CD14 level.
The first documented HIV cure was based on a stem cell approach, and authors of a study in AIDS Research & Therapy say “there is reasonable hope that this unique case will not stand alone in the future.”
A study in HIV Clinical Trials found that telmisartan use is linked with an increase in circulating endothelial progenitor cells in older HIV-positive individuals who have cardiovascular disease risk factors.
The National Institutes of Health has given funding for a research network to promote the health and well-being of adolescents and young adults who are infected with HIV or at risk for HIV infection.
A study in the Lancet HIV found high levels of HIV pretreatment drug resistance in Mexico, and non-NNRTI pretreatment drug resistance significantly reduced the efficacy of first-line ART regimens that were based on these drugs.
The production of interleukin-1 beta by innate immune cells after stimulations of Toll-like receptors and bacillus Calmette-Guerin was correlated with different tuberculosis recurrence outcomes in ART-treated patients, according to a recent study.
Antiretroviral therapy during acute HIV infection, consisting of once daily emtricitabine/tenofovir/efavirenz, resulted in rapid and sustained viral suppression with high rates of patients staying in care and on ART, in a cohort including a large proportion of young men who have sex with men.
A recent study of cognitively impaired HIV-positive adults found that “higher self-efficacy, greater perception of treatment-related support, a stable medication regimen, stable stress levels, and absence of current stimulant use” predicted the best patient adherence to ART.
Viral suppression rates among HIV-infected children on ART in low- and middle-income countries were “low and were considerably poorer” than those previously found in adults in such countries and children in high-income countries, according to a recent study.
A study in Clinical Infectious Diseases found higher concentrations of inflammatory biomarkers among HIV RNA–suppressed men who reported less than 100% combination ART adherence, compared with more adherent men.
Any heavy alcohol consumption was associated with all-cause mortality among HIV-infected individuals, while only recent heavy consumption was associated with liver-related mortality, according to a study in HIV Medicine.
Family planning clinics, an important source of health care for young women, may be a natural setting for HIV preexposure prophylaxis discussion and roll-out, especially for women who have a history of intimate partner violence, according to a study in AIDS Care.
A relatively high rate of preliminary discontinuation of the antiretroviral dolutegravir (DGV) due to intolerability was detected in a recent study of combination ART. In particular, DGV was stopped more frequently if the regimen included abacavir.
Virologic failure rates in children and adolescents were high in a Tanzanian HIV study, with the majority of antiretroviral therapy–failing children harboring drug resistance–associated mutations (DRM) of HIV. The authors said viral load monitoring is urgently needed to maintain future treatment options for the millions of African children living with HIV.
In a recent study, virologically suppressed, HIV-infected adults with creatinine clearance 30-69 mL/min who switched from tenofovir disoproxil fumarate (TDF) to elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/C/F/TAF), had “stable creatinine clearance, significant and durable improvements in proteinuria, albuminuria, and tubular proteinuria, and significant increases in hip and spine bone mineral density.” It appears that the latter therapy is appropriate for HIV-infected individuals with mild to moderately impaired renal function.
Lower serum albumin and higher AST appear to be key mortality risk factors in HIV/HCV coinfection, but not as important in HIV-monoinfected individuals, according to a study in the journal AIDS.
A retrospective study of 11 European pediatric HIV cohorts found a high proportion of patients coinfected with hepatitis C virus who had progressive liver disease, which investigators said highlights the need for close monitoring and earlier and more efficacious hepatitis C virus therapy.
A study in Lancet Infectious Diseases confirmed that injecting drug use is a major contributor to the global burden of disease for HIV, hepatitis C, and hepatitis B. In 2013, an estimated 10 million disability-adjusted life-years were linked to exposure to HIV, hepatitis C, and hepatitis B via injecting drug use, the investigators reported.
[email protected]
On Twitter @richpizzi
A great volume of HIV and AIDS research enters the medical literature every month. It’s difficult to monitor everything, so here’s a quick look at some notable news items and journal articles published over the past few weeks.
Plasma lopinavir concentrations predicted viral outcomes in HIV-infected children receiving lopinavir-based antiretroviral therapy, a recent study demonstrated. Investigators said their findings support a minimum target concentration of greater than and equal to 1 mg/L of lopinavir to ensure sustained viral suppression.
Most antiretroviral-naive HIV-positive children experienced recovery of both weight for age and height for age over the 24 months following initiation of antiretroviral therapy (ART), according to results of a recent study. There was no significant difference between those receiving lopinavir/ritonavir and ART that was not based on nucleoside reverse transcriptase inhibitors (NNRTIs).
Even in an environment of easy access to antiretroviral therapy for HIV patients, many challenges still exist at the implementation stage of early ART, investigators for a study in AIDS Care reported. The authors said intense efforts in both patient and physician education will be required if the benefits of early ART are to be achieved at the individual and population level.
CD4+ and CD8+ T-cell immune activation and exhaustion are greater in HIV-infected youth, compared with matched controls, according to results of a recent study, while monocyte subpopulations are not changed even though there is a high soluble CD14 level.
The first documented HIV cure was based on a stem cell approach, and authors of a study in AIDS Research & Therapy say “there is reasonable hope that this unique case will not stand alone in the future.”
A study in HIV Clinical Trials found that telmisartan use is linked with an increase in circulating endothelial progenitor cells in older HIV-positive individuals who have cardiovascular disease risk factors.
The National Institutes of Health has given funding for a research network to promote the health and well-being of adolescents and young adults who are infected with HIV or at risk for HIV infection.
A study in the Lancet HIV found high levels of HIV pretreatment drug resistance in Mexico, and non-NNRTI pretreatment drug resistance significantly reduced the efficacy of first-line ART regimens that were based on these drugs.
The production of interleukin-1 beta by innate immune cells after stimulations of Toll-like receptors and bacillus Calmette-Guerin was correlated with different tuberculosis recurrence outcomes in ART-treated patients, according to a recent study.
Antiretroviral therapy during acute HIV infection, consisting of once daily emtricitabine/tenofovir/efavirenz, resulted in rapid and sustained viral suppression with high rates of patients staying in care and on ART, in a cohort including a large proportion of young men who have sex with men.
A recent study of cognitively impaired HIV-positive adults found that “higher self-efficacy, greater perception of treatment-related support, a stable medication regimen, stable stress levels, and absence of current stimulant use” predicted the best patient adherence to ART.
Viral suppression rates among HIV-infected children on ART in low- and middle-income countries were “low and were considerably poorer” than those previously found in adults in such countries and children in high-income countries, according to a recent study.
A study in Clinical Infectious Diseases found higher concentrations of inflammatory biomarkers among HIV RNA–suppressed men who reported less than 100% combination ART adherence, compared with more adherent men.
Any heavy alcohol consumption was associated with all-cause mortality among HIV-infected individuals, while only recent heavy consumption was associated with liver-related mortality, according to a study in HIV Medicine.
Family planning clinics, an important source of health care for young women, may be a natural setting for HIV preexposure prophylaxis discussion and roll-out, especially for women who have a history of intimate partner violence, according to a study in AIDS Care.
A relatively high rate of preliminary discontinuation of the antiretroviral dolutegravir (DGV) due to intolerability was detected in a recent study of combination ART. In particular, DGV was stopped more frequently if the regimen included abacavir.
Virologic failure rates in children and adolescents were high in a Tanzanian HIV study, with the majority of antiretroviral therapy–failing children harboring drug resistance–associated mutations (DRM) of HIV. The authors said viral load monitoring is urgently needed to maintain future treatment options for the millions of African children living with HIV.
In a recent study, virologically suppressed, HIV-infected adults with creatinine clearance 30-69 mL/min who switched from tenofovir disoproxil fumarate (TDF) to elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/C/F/TAF), had “stable creatinine clearance, significant and durable improvements in proteinuria, albuminuria, and tubular proteinuria, and significant increases in hip and spine bone mineral density.” It appears that the latter therapy is appropriate for HIV-infected individuals with mild to moderately impaired renal function.
Lower serum albumin and higher AST appear to be key mortality risk factors in HIV/HCV coinfection, but not as important in HIV-monoinfected individuals, according to a study in the journal AIDS.
A retrospective study of 11 European pediatric HIV cohorts found a high proportion of patients coinfected with hepatitis C virus who had progressive liver disease, which investigators said highlights the need for close monitoring and earlier and more efficacious hepatitis C virus therapy.
A study in Lancet Infectious Diseases confirmed that injecting drug use is a major contributor to the global burden of disease for HIV, hepatitis C, and hepatitis B. In 2013, an estimated 10 million disability-adjusted life-years were linked to exposure to HIV, hepatitis C, and hepatitis B via injecting drug use, the investigators reported.
[email protected]
On Twitter @richpizzi
A great volume of HIV and AIDS research enters the medical literature every month. It’s difficult to monitor everything, so here’s a quick look at some notable news items and journal articles published over the past few weeks.
Plasma lopinavir concentrations predicted viral outcomes in HIV-infected children receiving lopinavir-based antiretroviral therapy, a recent study demonstrated. Investigators said their findings support a minimum target concentration of greater than and equal to 1 mg/L of lopinavir to ensure sustained viral suppression.
Most antiretroviral-naive HIV-positive children experienced recovery of both weight for age and height for age over the 24 months following initiation of antiretroviral therapy (ART), according to results of a recent study. There was no significant difference between those receiving lopinavir/ritonavir and ART that was not based on nucleoside reverse transcriptase inhibitors (NNRTIs).
Even in an environment of easy access to antiretroviral therapy for HIV patients, many challenges still exist at the implementation stage of early ART, investigators for a study in AIDS Care reported. The authors said intense efforts in both patient and physician education will be required if the benefits of early ART are to be achieved at the individual and population level.
CD4+ and CD8+ T-cell immune activation and exhaustion are greater in HIV-infected youth, compared with matched controls, according to results of a recent study, while monocyte subpopulations are not changed even though there is a high soluble CD14 level.
The first documented HIV cure was based on a stem cell approach, and authors of a study in AIDS Research & Therapy say “there is reasonable hope that this unique case will not stand alone in the future.”
A study in HIV Clinical Trials found that telmisartan use is linked with an increase in circulating endothelial progenitor cells in older HIV-positive individuals who have cardiovascular disease risk factors.
The National Institutes of Health has given funding for a research network to promote the health and well-being of adolescents and young adults who are infected with HIV or at risk for HIV infection.
A study in the Lancet HIV found high levels of HIV pretreatment drug resistance in Mexico, and non-NNRTI pretreatment drug resistance significantly reduced the efficacy of first-line ART regimens that were based on these drugs.
The production of interleukin-1 beta by innate immune cells after stimulations of Toll-like receptors and bacillus Calmette-Guerin was correlated with different tuberculosis recurrence outcomes in ART-treated patients, according to a recent study.
Antiretroviral therapy during acute HIV infection, consisting of once daily emtricitabine/tenofovir/efavirenz, resulted in rapid and sustained viral suppression with high rates of patients staying in care and on ART, in a cohort including a large proportion of young men who have sex with men.
A recent study of cognitively impaired HIV-positive adults found that “higher self-efficacy, greater perception of treatment-related support, a stable medication regimen, stable stress levels, and absence of current stimulant use” predicted the best patient adherence to ART.
Viral suppression rates among HIV-infected children on ART in low- and middle-income countries were “low and were considerably poorer” than those previously found in adults in such countries and children in high-income countries, according to a recent study.
A study in Clinical Infectious Diseases found higher concentrations of inflammatory biomarkers among HIV RNA–suppressed men who reported less than 100% combination ART adherence, compared with more adherent men.
Any heavy alcohol consumption was associated with all-cause mortality among HIV-infected individuals, while only recent heavy consumption was associated with liver-related mortality, according to a study in HIV Medicine.
Family planning clinics, an important source of health care for young women, may be a natural setting for HIV preexposure prophylaxis discussion and roll-out, especially for women who have a history of intimate partner violence, according to a study in AIDS Care.
A relatively high rate of preliminary discontinuation of the antiretroviral dolutegravir (DGV) due to intolerability was detected in a recent study of combination ART. In particular, DGV was stopped more frequently if the regimen included abacavir.
Virologic failure rates in children and adolescents were high in a Tanzanian HIV study, with the majority of antiretroviral therapy–failing children harboring drug resistance–associated mutations (DRM) of HIV. The authors said viral load monitoring is urgently needed to maintain future treatment options for the millions of African children living with HIV.
In a recent study, virologically suppressed, HIV-infected adults with creatinine clearance 30-69 mL/min who switched from tenofovir disoproxil fumarate (TDF) to elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/C/F/TAF), had “stable creatinine clearance, significant and durable improvements in proteinuria, albuminuria, and tubular proteinuria, and significant increases in hip and spine bone mineral density.” It appears that the latter therapy is appropriate for HIV-infected individuals with mild to moderately impaired renal function.
Lower serum albumin and higher AST appear to be key mortality risk factors in HIV/HCV coinfection, but not as important in HIV-monoinfected individuals, according to a study in the journal AIDS.
A retrospective study of 11 European pediatric HIV cohorts found a high proportion of patients coinfected with hepatitis C virus who had progressive liver disease, which investigators said highlights the need for close monitoring and earlier and more efficacious hepatitis C virus therapy.
A study in Lancet Infectious Diseases confirmed that injecting drug use is a major contributor to the global burden of disease for HIV, hepatitis C, and hepatitis B. In 2013, an estimated 10 million disability-adjusted life-years were linked to exposure to HIV, hepatitis C, and hepatitis B via injecting drug use, the investigators reported.
[email protected]
On Twitter @richpizzi
Ostracism is a growing concern as mechanism of poor health outcomes in military
WASHINGTON – The role of ostracism in overall poor health outcomes in service personnel is a growing concern, according to a panel of military experts.
“Think about the primary mechanism of suicide in kids who are bullied: It’s ostracism,” Kate McGraw, PhD, said in an interview at the American Psychiatric Association’s Institute on Psychiatric Services. Dr. McGraw is the interim director of the Deployment Health Clinical Center, a Defense Centers of Excellence for Psychological Health and Traumatic Brain Injury.
Although the literature is scant at this point because the effects of being left out are “common sense,” said Dr. McGraw, “we need to take it seriously.”
While ostracism as a clinical term doesn’t actually exist and direct data on its impact are not numerous, its inherent psychological risks include suicide, depression, and trauma, according to another of the panelists, Jacqueline Garrick, acting director of the Defense Suicide Prevention Office in the Department of Defense.
Dr. McGraw defined ostracism as group behavior “designed to isolate or deprive another individual of being part of that group.”
Women in the military are particularly at risk for ostracism simply because they tend to be outnumbered by their male counterparts in a combat unit, according to Dr. McGraw. This, combined with a wariness of women after sexual assault awareness education, can exacerbate the segregation.
Add to the mix the separation from the male group that female biology can sometimes cause, whether due to menstrual cycles or toilet needs, Ms. Garrick said. This can widen the gap.
Additionally, service personnel – men or women – who report sexual assault are at risk of being isolated or can suffer retaliation, despite there being antiharassment and antibullying policies in place.
In the interview, Dr. McGraw said she recommends assessing the level of social support a serviceman or servicewoman has by asking directly: “How included do you feel in your group?” She also suggested looking for evidence of ostracism such as the patient endorsing a sense that they do not belong, or being friendless.
If a clinician suspects that a person who says “I am stressed” actually means, “My feelings are hurt,” Dr. McGraw suggested going deeper: Seek clues as to whether the person is experiencing ostracism either covertly, such as being bullied in private, or overtly such as not being given information that ends up making the person appear foolish or unprepared for a task.
“Ask some very pointed questions, such as ‘Are people behaving toward you in a certain way?’ and ‘Do you feel targeted?’ ”
The challenge, she said, is to maintain what is known as “military bearing” – essentially, cultivated stoicism, while also admitting that one’s functionality is suffering because of having been isolated. A dialogue between patient and clinician about being ostracized can lead to helping the person develop strategies for coping with its effects, such as making the commanding officer aware of what is happening.
“Most military personnel are not going to say their feelings are hurt, but they can address the behavior,” Dr. McGraw said.
Although Dr. McGraw admitted when asked that reporting the behavior to a superior could result in further ostracism, she said she has faith in the power of leadership to evoke cultural change. “In a military environment, if the leaders are aware of what is happening, and they take steps to mitigate or eliminate it as a unit, then they can create a healthier environment in the unit, improving morale and esprit de corps.”
None of the presenters had any relevant financial disclosures and said their presentations represented their own opinions, not those of the U.S. Armed Forces.
[email protected]
On Twitter @whitneymcknight
WASHINGTON – The role of ostracism in overall poor health outcomes in service personnel is a growing concern, according to a panel of military experts.
“Think about the primary mechanism of suicide in kids who are bullied: It’s ostracism,” Kate McGraw, PhD, said in an interview at the American Psychiatric Association’s Institute on Psychiatric Services. Dr. McGraw is the interim director of the Deployment Health Clinical Center, a Defense Centers of Excellence for Psychological Health and Traumatic Brain Injury.
Although the literature is scant at this point because the effects of being left out are “common sense,” said Dr. McGraw, “we need to take it seriously.”
While ostracism as a clinical term doesn’t actually exist and direct data on its impact are not numerous, its inherent psychological risks include suicide, depression, and trauma, according to another of the panelists, Jacqueline Garrick, acting director of the Defense Suicide Prevention Office in the Department of Defense.
Dr. McGraw defined ostracism as group behavior “designed to isolate or deprive another individual of being part of that group.”
Women in the military are particularly at risk for ostracism simply because they tend to be outnumbered by their male counterparts in a combat unit, according to Dr. McGraw. This, combined with a wariness of women after sexual assault awareness education, can exacerbate the segregation.
Add to the mix the separation from the male group that female biology can sometimes cause, whether due to menstrual cycles or toilet needs, Ms. Garrick said. This can widen the gap.
Additionally, service personnel – men or women – who report sexual assault are at risk of being isolated or can suffer retaliation, despite there being antiharassment and antibullying policies in place.
In the interview, Dr. McGraw said she recommends assessing the level of social support a serviceman or servicewoman has by asking directly: “How included do you feel in your group?” She also suggested looking for evidence of ostracism such as the patient endorsing a sense that they do not belong, or being friendless.
If a clinician suspects that a person who says “I am stressed” actually means, “My feelings are hurt,” Dr. McGraw suggested going deeper: Seek clues as to whether the person is experiencing ostracism either covertly, such as being bullied in private, or overtly such as not being given information that ends up making the person appear foolish or unprepared for a task.
“Ask some very pointed questions, such as ‘Are people behaving toward you in a certain way?’ and ‘Do you feel targeted?’ ”
The challenge, she said, is to maintain what is known as “military bearing” – essentially, cultivated stoicism, while also admitting that one’s functionality is suffering because of having been isolated. A dialogue between patient and clinician about being ostracized can lead to helping the person develop strategies for coping with its effects, such as making the commanding officer aware of what is happening.
“Most military personnel are not going to say their feelings are hurt, but they can address the behavior,” Dr. McGraw said.
Although Dr. McGraw admitted when asked that reporting the behavior to a superior could result in further ostracism, she said she has faith in the power of leadership to evoke cultural change. “In a military environment, if the leaders are aware of what is happening, and they take steps to mitigate or eliminate it as a unit, then they can create a healthier environment in the unit, improving morale and esprit de corps.”
None of the presenters had any relevant financial disclosures and said their presentations represented their own opinions, not those of the U.S. Armed Forces.
[email protected]
On Twitter @whitneymcknight
WASHINGTON – The role of ostracism in overall poor health outcomes in service personnel is a growing concern, according to a panel of military experts.
“Think about the primary mechanism of suicide in kids who are bullied: It’s ostracism,” Kate McGraw, PhD, said in an interview at the American Psychiatric Association’s Institute on Psychiatric Services. Dr. McGraw is the interim director of the Deployment Health Clinical Center, a Defense Centers of Excellence for Psychological Health and Traumatic Brain Injury.
Although the literature is scant at this point because the effects of being left out are “common sense,” said Dr. McGraw, “we need to take it seriously.”
While ostracism as a clinical term doesn’t actually exist and direct data on its impact are not numerous, its inherent psychological risks include suicide, depression, and trauma, according to another of the panelists, Jacqueline Garrick, acting director of the Defense Suicide Prevention Office in the Department of Defense.
Dr. McGraw defined ostracism as group behavior “designed to isolate or deprive another individual of being part of that group.”
Women in the military are particularly at risk for ostracism simply because they tend to be outnumbered by their male counterparts in a combat unit, according to Dr. McGraw. This, combined with a wariness of women after sexual assault awareness education, can exacerbate the segregation.
Add to the mix the separation from the male group that female biology can sometimes cause, whether due to menstrual cycles or toilet needs, Ms. Garrick said. This can widen the gap.
Additionally, service personnel – men or women – who report sexual assault are at risk of being isolated or can suffer retaliation, despite there being antiharassment and antibullying policies in place.
In the interview, Dr. McGraw said she recommends assessing the level of social support a serviceman or servicewoman has by asking directly: “How included do you feel in your group?” She also suggested looking for evidence of ostracism such as the patient endorsing a sense that they do not belong, or being friendless.
If a clinician suspects that a person who says “I am stressed” actually means, “My feelings are hurt,” Dr. McGraw suggested going deeper: Seek clues as to whether the person is experiencing ostracism either covertly, such as being bullied in private, or overtly such as not being given information that ends up making the person appear foolish or unprepared for a task.
“Ask some very pointed questions, such as ‘Are people behaving toward you in a certain way?’ and ‘Do you feel targeted?’ ”
The challenge, she said, is to maintain what is known as “military bearing” – essentially, cultivated stoicism, while also admitting that one’s functionality is suffering because of having been isolated. A dialogue between patient and clinician about being ostracized can lead to helping the person develop strategies for coping with its effects, such as making the commanding officer aware of what is happening.
“Most military personnel are not going to say their feelings are hurt, but they can address the behavior,” Dr. McGraw said.
Although Dr. McGraw admitted when asked that reporting the behavior to a superior could result in further ostracism, she said she has faith in the power of leadership to evoke cultural change. “In a military environment, if the leaders are aware of what is happening, and they take steps to mitigate or eliminate it as a unit, then they can create a healthier environment in the unit, improving morale and esprit de corps.”
None of the presenters had any relevant financial disclosures and said their presentations represented their own opinions, not those of the U.S. Armed Forces.
[email protected]
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM THE INSTITUTE ON PSYCHIATRIC SERVICES
Incidence of and Risk Factors for Symptomatic Venous Thromboembolism After Shoulder Arthroplasty
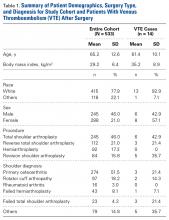
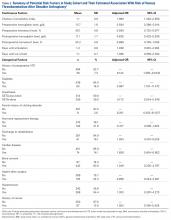
Venous thromboembolism (VTE) after shoulder arthroplasty (SA) is relatively uncommon. Reported rates of VTE development are highly variable, ranging from 0.2% to 13% (pulmonary embolism [PE], 0.2%-10.8%; deep venous thrombosis [DVT], 0.1%-13%).1-4 Sources of this variability include different methods of capturing cases (small clinical series vs large database studies, which capture mainly hospital readmissions), differences in defining or detecting VTE, and different patient populations (fracture vs osteoarthritis).1-3 Most studies have also tried to identify factors associated with increased risk for VTE. Risk factors associated with development of VTE after SA include history of VTE, advanced age, prolonged operating room time, higher body mass index (BMI), trauma, history of cancer, female sex, and raised Charlson Comorbidity Index (CCI).1-7 Limitations of clinical series include the smaller number of reporting institutions—a potential source of bias given regional variability.1,3,4,7 Limitations of large state or national databases include capturing only events coded during inpatient admission and capturing readmissions for complications at the same institution. This underreporting may lead to very conservative estimates of VTE incidence.2,5,6,8
In this study, we retrospectively identified all the SAs performed at a single institution over a 13-year period and evaluated the cases for development of VTE (DVT, PE). We hypothesized that the VTE rate would be lower than the very high rates reported by Hoxie and colleagues1 and Willis and colleagues4 but higher than those reported for large state or national databases.2,3 We also evaluated clotting risk factors, including many never analyzed before.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we searched our database for all SAs performed at our institution between January 1999 and May 2012 and identified cases in which symptomatic VTE developed within the first 90 days after surgery. Charts were reviewed for information on medical history, surgical procedure, and in-hospital and out-of-hospital care within the 90-day postoperative period. We recorded data on symptomatic VTE (DVT, PE) as documented by lower or upper extremity duplex ultrasonography (US) or chest computed tomography (CT) angiography. There had been no routine screening of patients; duplex US or CT angiography was performed only if a patient was clinically symptomatic (leg swelling, leg pain, shortness of breath, tachycardia, chest pain) for a potential DVT or PE. For a patient who had repeat SAs on the same shoulder or bilateral SAs at different times, only the first procedure was included in the analysis. Arthroplasties performed for fracture were excluded.
Study data were collected and managed with REDCap (Research Electronic Data Capture) tools hosted at the University of Utah School of Medicine.9 Continuous and discrete data collected on medical history and postoperative course included BMI, age at surgery, preoperative hemoglobin (Hb) and hematocrit (Hct) levels, days in hospital, days until out of bed and days until ambulation (both documented in nursing and physical therapy notes), postoperative Hb and Hct levels, and CCI. Categorical data included sex, diagnosis (primary osteoarthritis, rotator cuff arthropathy, rheumatoid arthritis, failed hemiarthroplasty [HA], failed total SA [TSA], others), attending surgeon, procedure (TSA, HA, reverse TSA, revision SA), anesthesia (general endotracheal anesthesia [GETA] alone, interscalene nerve block alone, GETA plus block), prophylactic use of aspirin after surgery, presence of various medical comorbidities (diabetes, hypertension, cardiac disease, clotting disorders, cancer), hormone replacement therapy, family history of a clotting disorder, and VTE consequences (cardiac events, death).
Statistical Analysis
Descriptive statistics were calculated to summarize aspects of the surgical procedures, the study cohort’s demographics and medical histories, and the incidence of VTE. Logistic regression analysis was performed to explore the association between development of VTE (DVT, PE) and potential risk factors. Unadjusted odds ratios (ORs) were estimated for the risk factors of age, BMI, revision SA, CCI, prophylactic use of aspirin after surgery, preoperative history of VTE, preoperative and postoperative Hb and Hct levels, diabetes, anesthesia (GETA with and without interscalene nerve block), family history of a clotting disorder, days until out of bed, hormone replacement therapy, race, discharge home or to rehabilitation, distance traveled for surgery, hypertension, cardiac disease, cement use, and history of cancer. In addition, ORs were adjusted for age, BMI, and revision SA. For all statistical tests, significance was set at P < .05. All analyses were performed with SAS Version 9.3 (SAS Institute).
Results
We identified 533 SAs: 245 anatomical TSAs, 112 reverse TSAs, 92 HAs, and 84 revision SAs. Three different surgeons performed the procedures, and no patients were lost to follow-up within the first 90 days after surgery. Although SAs were performed for various diagnoses, more than 50% (274) of the SAs were for primary osteoarthritis; 97 were performed for rotator cuff arthropathy, 16 for rheumatoid arthritis, 43 for failed HA, 23 for failed TSA, and 79 for other diagnoses.
Of the 533 patients, 288 were female and 245 were male. Mean age at surgery was 65.2 years (range, 16-93 years). Mean (SD) BMI was 29.2 (6.4) kg/m2. Mean (SD) preoperative Hb level was 13.7 (1.8) g/dL, and mean preoperative Hct level was 40.1% (4.8%). Mean (SD) length of hospital stay was 2.6 (1.5) days. Mean (SD) time before patients were out of bed was 1.1 (0.7) days. On postoperative day 1, mean Hb level was 11.1 (1.7) g/dL, and mean (SD) Hct level was 33.2% (4.8%). Mean (SD) CCI was 1.1 (0.9).
Anesthesia for the 533 patients consisted of GETA (209 patients, 39.0%), interscalene nerve block (2, 0.4%), or GETA with nerve block (314, 59.0%). After surgery, 125 patients (24.3%) received aspirin as prophylaxis. Diabetes was reported by 83 patients, hypertension by 286, cardiac disease by 74, a history of a clotting disorder by 2, a family history of a clotting disorder by 8, ongoing cancer by 4, a history of cancer by 67, and hormone replacement therapy by 104.
For the entire cohort of 533 patients, the symptomatic VTE rate was 2.6% (14 patients), the DVT rate was 0.9% (5), and the PE rate was 2.3% (12). Although VTE did not cause any deaths, there were 3 cardiac events.
Discussion
VTE after SA is rare. We report an overall VTE incidence of 2.6%, with DVT at 0.9% and PE at 2.3%. These rates are similar to those reported in clinical series and significantly higher than those reported for large institutional or national databases.2-7 Our results also support a previously reported trend: The ratio of PE to DVT for SA is significantly higher than historically reported ratios for lower extremity arthroplasty.2,6-8 We have identified many VTE risk factors: raised CCI, preoperative thrombotic event, lower preoperative Hb and Hct levels, lower postoperative Hb level, diabetes, use of GETA without interscalene nerve block, higher BMI, and revision SA. Results of other studies support 3 findings (higher BMI, raised CCI, preoperative thrombotic event); new findings include correlation with Hb and Hct levels, diabetes, type of anesthesia, and revision SA.6,7 Identification of these other factors may be useful in making treatment decisions in patients symptomatic after SA and in lowering the threshold for performing diagnostic tests in these patients at risk for VTE.
Reported rates of VTE after SA are highly variable, ranging from 0.2% to 13%.10 Our rationale for investigating VTE rates at a single institution was to estimate the rates that can be expected in a university-based practice and to determine whether these rates are high enough to warrant routine thromboprophylaxis. The rate variability seems to result in part from variability in the data sources. Most studies that have reported very low VTE rates typically used large state or national databases, which likely were subject to underreporting.
Lyman and colleagues6 found 0.5% DVT and 0.2% PE rates in a New York state hospital database, but only in-hospital immediate postoperative symptomatic complications were included; slightly delayed complications may have been missed. Farng and colleagues5 reported a 0.6% VTE rate, but only inpatient (immediate postoperative or readmission) events were included; all outpatient events were missed. Jameson and colleagues,2 using a national database that included only cases involving inpatient treatment, reported 0% DVT and 0.2% PE rates, again missing outpatient events, and relying on appropriate coding to capture events. Using electronic health records from a large healthcare system, Navarro and colleagues8 queried for VTE cases and reported 0.5% DVT and 0.5% PE rates. The inclusiveness of their data source for the outcome of interest was potentially improved relative to national or statewide databases—and the resulting data reported in their study should reflect that improvement. However, the authors relied on ICD–9 (International Classification of Diseases, Ninth Revision) coding to screen for VTE events and excluded patients with prior VTE, preoperative prophylaxis (enoxaparin or warfarin), or follow-up of <90 days. As patients with prior VTE are those most at risk (present study OR, 6-7), excluding them significantly reduces the overall incidence of clotting reported.
Only 4 studies specifically used information drawn directly from physicians’ clinic notes, vs data retrieved (using code-based queries) from databases.1,3,4,7 These studies may provide a better representation of the rate of VTE after SA, as they were not reliant on codes, included both inpatient and outpatient events, and were inclusive of outpatient follow-up of at least 3 months.
Three of the 4 studies used the Mayo Clinic Total Joint Registry.1,3,4 Hoxie and colleagues1 reported an 11% rate of PE after HA performed for fracture (we excluded SA for fracture). As several other investigators have reported an association between trauma and increased risk for VTE, postoperative anticoagulation should be considered in this patient population (though it was not the focus of the present study).6-8 Sperling and Cofield3 and Singh and colleagues7 reported on the risk for PE among SA patients at the Mayo Clinic. Sperling and Cofield3 included only those events that occurred within the first 7 days after surgery; Singh and colleagues7 included events out to 90 days after surgery. Sperling and Cofield3 reported a 0.17% PE rate; Singh and colleagues7 reported 0.6% PE and 0.1% DVT rates. Sperling and Cofield3 reported on 2885 SAs; Singh and colleagues7 reported on 4019 SAs from the same database. As it is unclear whether these 2 studies had complete information on all patients, underreporting may be an issue. Information was obtained through “clinic visits, medical records and/or standardized mailed and telephone-administered questionnaires.”7The fourth study, a prospective study of 100 patients by Willis and colleagues,4 had the best data on development of symptomatic PE after SA. The authors reported a 2% PE rate and a high (13%) DVT rate. Because US was not performed before the surgical procedures, the number of patients with new and existing DVT cases could not be determined. However, all PEs were new, and the 2% rate found there is similar to the 2.3% in our study. Therefore, we think these rates capture the data most accurately and avoid the underreporting that marks large databases.4Studies have identified various factors that increase the risk for VTE after SA. Singh and colleagues7 identified the risk factors of age over 70 years, female sex, higher BMI (25-29.9 kg/m2), CCI above 1, traumatic etiology, prior history of VTE, and HA. However, their use of univariate regression analysis may have confounded the effects—one factor may have become a surrogate for another (ie, trauma and HA, as most fractures treated with SA during the study period were treated with HA). Lyman and colleagues6 also found advanced age and trauma were associated with higher VTE risk, and reported prior history of cancer as a risk factor as well. Navarro and colleagues8 identified trauma as a risk factor, as in the other 2 studies.6,7 Our data support prior history of VTE, higher BMI, and raised CCI as increasing the risk for VTE.
Other factors identified in the present study are use of GETA without interscalene nerve block, lower preoperative and postoperative Hb levels, diabetes, and revision SA. Because of the limited number of events, only ORs with and without limited control of confounders were performed. Just as in the study by Singh and colleagues,7 uncontrolled confounding could have occurred. A nerve block may be protective, as less postoperative pain may allow patients quicker mobilization and therapy. Diabetes may be a surrogate for other medical comorbidities, as reflected by the higher overall risk with raised CCI. Lower preoperative and postoperative Hb levels were associated with clotting and may be representative of patients with poorer overall health and more complicated surgical procedures (eg, revision SA). In an earlier study, we found increased risk for transfusions in revision SA relative to primary SA.11 Lower preoperative Hb level correlated with development of VTE after lower extremity arthroplasty.12 Postoperative use of aspirin was not found to significantly reduce the incidence of clotting, though this finding may have resulted from lack of power. Therefore, from the present data, there is nothing to conclude about the efficacy of aspirin in preventing thrombosis.
Our findings can be placed in the context of the Virchow triad. Specifically, 3 categories of factors are thought to contribute to thrombosis: hypercoagulability, hemodynamic stasis, and endothelial injury. In grouping factors, we identified prior thrombotic event and obesity as increasing hypercoagulability; revision SA, more comorbidities, lower Hb and Hct levels, diabetes, and GETA as increasing hemodynamic stasis; and revision SA (longer operating room times) as leading to stasis. More comorbidities can be associated with delayed postoperative ambulation, and diabetes and lower Hb and Hct levels can be surrogates for more comorbidities. Surgery performed with the patient under GETA without interscalene nerve block can lead to higher levels of pain and less early mobility.
The present findings have made us more aware of patients at risk for VTE, and we have lowered our threshold for evaluating them for potential clots. Before this study, we used warfarin or enoxaparin for anticoagulation in patients with a history of VTE or active cancer. We are continuing this protocol, but not with other patients. Patients with many comorbidities, lower preoperative Hb level, revision SA, high BMI, or diabetes are carefully monitored for clots early in the postoperative course. Our new threshold for these high-risk patients is to order diagnostic testing, including duplex US or CT angiography. Now, even mild oxygen requirements or mild tachycardia within postoperative week 1 typically prompt a study in these patients. We hope this increased awareness will limit the potential negative consequences associated with development of VTE. Given the present data, we do not think the simple presence of increased comorbidities, lower preoperative Hb, revision SA, high BMI or diabetes should rule out performing SA; rather, it should increase surgeons’ postoperative vigilance in evaluating for potential clots.
Limitations of our study include its retrospective nature and reliance on clinic chart review. Patients were not directly questioned about venous thrombus at follow-up, so all events may not have been captured. Although retrospective review has its drawbacks, it allows for accurate identification of events, even uncoded events. Therefore, more events are likely to be captured with this technique than with large database analyses using only coding information. We tried to identify as many cases as possible by reviewing all outpatient records (orthopedic, nonorthopedic), inpatient records, radiologic studies, and scanned outside records. Another limitation is that having a small number of VTE events limited our ability to perform a multivariate analysis, and uncontrolled confounding likely resulted. Only a very large multi-institutional study can capture enough events to allow a multivariate analysis. A third limitation is that the small number of events may have underpowered the study. Having more patients would have allowed other potential factors to be identified as being significantly associated with VTE. Last, as the study captured only symptomatic VTE events, it may have underreported VTE events. Given our complete review of the medical records, however, most clinically significant events likely were captured.
Conclusion
VTE after SA is rare. In our single-institution study, the symptomatic DVT rate was 0.9%, and the symptomatic PE rate was 2.3%. Risk factors associated with clotting included prior VTE, higher BMI, lower preoperative and postoperative Hb levels, raised CCI, diabetes, use of GETA without interscalene nerve block, and revision SA. Risk factors can be used to identify patients who may benefit from a more scrutinized postoperative evaluation and from increased surgeon awareness of the potential for VTE development. Rates of VTE can be used to counsel SA patients regarding overall surgical risks.
Am J Orthop. 2016;45(6):E379-E385. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hoxie SC, Sperling JW, Cofield RH. Pulmonary embolism after operative treatment of proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(6):782-783.
2. Jameson SS, James P, Howcroft DW, et al. Venous thromboembolic events are rare after shoulder surgery: analysis of a national database. J Shoulder Elbow Surg. 2011;20(5):764-770.
3. Sperling JW, Cofield RH. Pulmonary embolism following shoulder arthroplasty. J Bone Joint Surg Am. 2002;84(11):1939-1941.
4. Willis AA, Warren RF, Craig EV, et al. Deep vein thrombosis after reconstructive shoulder arthroplasty: a prospective observational study. J Shoulder Elbow Surg. 2009;18(1):100-106.
5. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563.
6. Lyman S, Sherman S, Carter TI, Bach PB, Mandl LA, Marx RG. Prevalence and risk factors for symptomatic thromboembolic events after shoulder arthroplasty. Clin Orthop Relat Res. 2006;(448):152-156.
7. Singh JA, Sperling JW, Cofield RH. Cardiopulmonary complications after primary shoulder arthroplasty: a cohort study. Semin Arthritis Rheum. 2012;41(5):689-697.
8. Navarro RA, Inacio MC, Burke MF, Costouros JG, Yian EH. Risk of thromboembolism in shoulder arthroplasty: effect of implant type and traumatic indication. Clin Orthop Relat Res. 2013;471(5):1576-1581.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
10. Saleh HE, Pennings AL, ElMaraghy AW. Venous thromboembolism after shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2013;22(10):1440-1448.
11. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
12. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335-341.
Venous thromboembolism (VTE) after shoulder arthroplasty (SA) is relatively uncommon. Reported rates of VTE development are highly variable, ranging from 0.2% to 13% (pulmonary embolism [PE], 0.2%-10.8%; deep venous thrombosis [DVT], 0.1%-13%).1-4 Sources of this variability include different methods of capturing cases (small clinical series vs large database studies, which capture mainly hospital readmissions), differences in defining or detecting VTE, and different patient populations (fracture vs osteoarthritis).1-3 Most studies have also tried to identify factors associated with increased risk for VTE. Risk factors associated with development of VTE after SA include history of VTE, advanced age, prolonged operating room time, higher body mass index (BMI), trauma, history of cancer, female sex, and raised Charlson Comorbidity Index (CCI).1-7 Limitations of clinical series include the smaller number of reporting institutions—a potential source of bias given regional variability.1,3,4,7 Limitations of large state or national databases include capturing only events coded during inpatient admission and capturing readmissions for complications at the same institution. This underreporting may lead to very conservative estimates of VTE incidence.2,5,6,8
In this study, we retrospectively identified all the SAs performed at a single institution over a 13-year period and evaluated the cases for development of VTE (DVT, PE). We hypothesized that the VTE rate would be lower than the very high rates reported by Hoxie and colleagues1 and Willis and colleagues4 but higher than those reported for large state or national databases.2,3 We also evaluated clotting risk factors, including many never analyzed before.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we searched our database for all SAs performed at our institution between January 1999 and May 2012 and identified cases in which symptomatic VTE developed within the first 90 days after surgery. Charts were reviewed for information on medical history, surgical procedure, and in-hospital and out-of-hospital care within the 90-day postoperative period. We recorded data on symptomatic VTE (DVT, PE) as documented by lower or upper extremity duplex ultrasonography (US) or chest computed tomography (CT) angiography. There had been no routine screening of patients; duplex US or CT angiography was performed only if a patient was clinically symptomatic (leg swelling, leg pain, shortness of breath, tachycardia, chest pain) for a potential DVT or PE. For a patient who had repeat SAs on the same shoulder or bilateral SAs at different times, only the first procedure was included in the analysis. Arthroplasties performed for fracture were excluded.
Study data were collected and managed with REDCap (Research Electronic Data Capture) tools hosted at the University of Utah School of Medicine.9 Continuous and discrete data collected on medical history and postoperative course included BMI, age at surgery, preoperative hemoglobin (Hb) and hematocrit (Hct) levels, days in hospital, days until out of bed and days until ambulation (both documented in nursing and physical therapy notes), postoperative Hb and Hct levels, and CCI. Categorical data included sex, diagnosis (primary osteoarthritis, rotator cuff arthropathy, rheumatoid arthritis, failed hemiarthroplasty [HA], failed total SA [TSA], others), attending surgeon, procedure (TSA, HA, reverse TSA, revision SA), anesthesia (general endotracheal anesthesia [GETA] alone, interscalene nerve block alone, GETA plus block), prophylactic use of aspirin after surgery, presence of various medical comorbidities (diabetes, hypertension, cardiac disease, clotting disorders, cancer), hormone replacement therapy, family history of a clotting disorder, and VTE consequences (cardiac events, death).
Statistical Analysis
Descriptive statistics were calculated to summarize aspects of the surgical procedures, the study cohort’s demographics and medical histories, and the incidence of VTE. Logistic regression analysis was performed to explore the association between development of VTE (DVT, PE) and potential risk factors. Unadjusted odds ratios (ORs) were estimated for the risk factors of age, BMI, revision SA, CCI, prophylactic use of aspirin after surgery, preoperative history of VTE, preoperative and postoperative Hb and Hct levels, diabetes, anesthesia (GETA with and without interscalene nerve block), family history of a clotting disorder, days until out of bed, hormone replacement therapy, race, discharge home or to rehabilitation, distance traveled for surgery, hypertension, cardiac disease, cement use, and history of cancer. In addition, ORs were adjusted for age, BMI, and revision SA. For all statistical tests, significance was set at P < .05. All analyses were performed with SAS Version 9.3 (SAS Institute).
Results
We identified 533 SAs: 245 anatomical TSAs, 112 reverse TSAs, 92 HAs, and 84 revision SAs. Three different surgeons performed the procedures, and no patients were lost to follow-up within the first 90 days after surgery. Although SAs were performed for various diagnoses, more than 50% (274) of the SAs were for primary osteoarthritis; 97 were performed for rotator cuff arthropathy, 16 for rheumatoid arthritis, 43 for failed HA, 23 for failed TSA, and 79 for other diagnoses.
Of the 533 patients, 288 were female and 245 were male. Mean age at surgery was 65.2 years (range, 16-93 years). Mean (SD) BMI was 29.2 (6.4) kg/m2. Mean (SD) preoperative Hb level was 13.7 (1.8) g/dL, and mean preoperative Hct level was 40.1% (4.8%). Mean (SD) length of hospital stay was 2.6 (1.5) days. Mean (SD) time before patients were out of bed was 1.1 (0.7) days. On postoperative day 1, mean Hb level was 11.1 (1.7) g/dL, and mean (SD) Hct level was 33.2% (4.8%). Mean (SD) CCI was 1.1 (0.9).
Anesthesia for the 533 patients consisted of GETA (209 patients, 39.0%), interscalene nerve block (2, 0.4%), or GETA with nerve block (314, 59.0%). After surgery, 125 patients (24.3%) received aspirin as prophylaxis. Diabetes was reported by 83 patients, hypertension by 286, cardiac disease by 74, a history of a clotting disorder by 2, a family history of a clotting disorder by 8, ongoing cancer by 4, a history of cancer by 67, and hormone replacement therapy by 104.
For the entire cohort of 533 patients, the symptomatic VTE rate was 2.6% (14 patients), the DVT rate was 0.9% (5), and the PE rate was 2.3% (12). Although VTE did not cause any deaths, there were 3 cardiac events.
Discussion
VTE after SA is rare. We report an overall VTE incidence of 2.6%, with DVT at 0.9% and PE at 2.3%. These rates are similar to those reported in clinical series and significantly higher than those reported for large institutional or national databases.2-7 Our results also support a previously reported trend: The ratio of PE to DVT for SA is significantly higher than historically reported ratios for lower extremity arthroplasty.2,6-8 We have identified many VTE risk factors: raised CCI, preoperative thrombotic event, lower preoperative Hb and Hct levels, lower postoperative Hb level, diabetes, use of GETA without interscalene nerve block, higher BMI, and revision SA. Results of other studies support 3 findings (higher BMI, raised CCI, preoperative thrombotic event); new findings include correlation with Hb and Hct levels, diabetes, type of anesthesia, and revision SA.6,7 Identification of these other factors may be useful in making treatment decisions in patients symptomatic after SA and in lowering the threshold for performing diagnostic tests in these patients at risk for VTE.
Reported rates of VTE after SA are highly variable, ranging from 0.2% to 13%.10 Our rationale for investigating VTE rates at a single institution was to estimate the rates that can be expected in a university-based practice and to determine whether these rates are high enough to warrant routine thromboprophylaxis. The rate variability seems to result in part from variability in the data sources. Most studies that have reported very low VTE rates typically used large state or national databases, which likely were subject to underreporting.
Lyman and colleagues6 found 0.5% DVT and 0.2% PE rates in a New York state hospital database, but only in-hospital immediate postoperative symptomatic complications were included; slightly delayed complications may have been missed. Farng and colleagues5 reported a 0.6% VTE rate, but only inpatient (immediate postoperative or readmission) events were included; all outpatient events were missed. Jameson and colleagues,2 using a national database that included only cases involving inpatient treatment, reported 0% DVT and 0.2% PE rates, again missing outpatient events, and relying on appropriate coding to capture events. Using electronic health records from a large healthcare system, Navarro and colleagues8 queried for VTE cases and reported 0.5% DVT and 0.5% PE rates. The inclusiveness of their data source for the outcome of interest was potentially improved relative to national or statewide databases—and the resulting data reported in their study should reflect that improvement. However, the authors relied on ICD–9 (International Classification of Diseases, Ninth Revision) coding to screen for VTE events and excluded patients with prior VTE, preoperative prophylaxis (enoxaparin or warfarin), or follow-up of <90 days. As patients with prior VTE are those most at risk (present study OR, 6-7), excluding them significantly reduces the overall incidence of clotting reported.
Only 4 studies specifically used information drawn directly from physicians’ clinic notes, vs data retrieved (using code-based queries) from databases.1,3,4,7 These studies may provide a better representation of the rate of VTE after SA, as they were not reliant on codes, included both inpatient and outpatient events, and were inclusive of outpatient follow-up of at least 3 months.
Three of the 4 studies used the Mayo Clinic Total Joint Registry.1,3,4 Hoxie and colleagues1 reported an 11% rate of PE after HA performed for fracture (we excluded SA for fracture). As several other investigators have reported an association between trauma and increased risk for VTE, postoperative anticoagulation should be considered in this patient population (though it was not the focus of the present study).6-8 Sperling and Cofield3 and Singh and colleagues7 reported on the risk for PE among SA patients at the Mayo Clinic. Sperling and Cofield3 included only those events that occurred within the first 7 days after surgery; Singh and colleagues7 included events out to 90 days after surgery. Sperling and Cofield3 reported a 0.17% PE rate; Singh and colleagues7 reported 0.6% PE and 0.1% DVT rates. Sperling and Cofield3 reported on 2885 SAs; Singh and colleagues7 reported on 4019 SAs from the same database. As it is unclear whether these 2 studies had complete information on all patients, underreporting may be an issue. Information was obtained through “clinic visits, medical records and/or standardized mailed and telephone-administered questionnaires.”7The fourth study, a prospective study of 100 patients by Willis and colleagues,4 had the best data on development of symptomatic PE after SA. The authors reported a 2% PE rate and a high (13%) DVT rate. Because US was not performed before the surgical procedures, the number of patients with new and existing DVT cases could not be determined. However, all PEs were new, and the 2% rate found there is similar to the 2.3% in our study. Therefore, we think these rates capture the data most accurately and avoid the underreporting that marks large databases.4Studies have identified various factors that increase the risk for VTE after SA. Singh and colleagues7 identified the risk factors of age over 70 years, female sex, higher BMI (25-29.9 kg/m2), CCI above 1, traumatic etiology, prior history of VTE, and HA. However, their use of univariate regression analysis may have confounded the effects—one factor may have become a surrogate for another (ie, trauma and HA, as most fractures treated with SA during the study period were treated with HA). Lyman and colleagues6 also found advanced age and trauma were associated with higher VTE risk, and reported prior history of cancer as a risk factor as well. Navarro and colleagues8 identified trauma as a risk factor, as in the other 2 studies.6,7 Our data support prior history of VTE, higher BMI, and raised CCI as increasing the risk for VTE.
Other factors identified in the present study are use of GETA without interscalene nerve block, lower preoperative and postoperative Hb levels, diabetes, and revision SA. Because of the limited number of events, only ORs with and without limited control of confounders were performed. Just as in the study by Singh and colleagues,7 uncontrolled confounding could have occurred. A nerve block may be protective, as less postoperative pain may allow patients quicker mobilization and therapy. Diabetes may be a surrogate for other medical comorbidities, as reflected by the higher overall risk with raised CCI. Lower preoperative and postoperative Hb levels were associated with clotting and may be representative of patients with poorer overall health and more complicated surgical procedures (eg, revision SA). In an earlier study, we found increased risk for transfusions in revision SA relative to primary SA.11 Lower preoperative Hb level correlated with development of VTE after lower extremity arthroplasty.12 Postoperative use of aspirin was not found to significantly reduce the incidence of clotting, though this finding may have resulted from lack of power. Therefore, from the present data, there is nothing to conclude about the efficacy of aspirin in preventing thrombosis.
Our findings can be placed in the context of the Virchow triad. Specifically, 3 categories of factors are thought to contribute to thrombosis: hypercoagulability, hemodynamic stasis, and endothelial injury. In grouping factors, we identified prior thrombotic event and obesity as increasing hypercoagulability; revision SA, more comorbidities, lower Hb and Hct levels, diabetes, and GETA as increasing hemodynamic stasis; and revision SA (longer operating room times) as leading to stasis. More comorbidities can be associated with delayed postoperative ambulation, and diabetes and lower Hb and Hct levels can be surrogates for more comorbidities. Surgery performed with the patient under GETA without interscalene nerve block can lead to higher levels of pain and less early mobility.
The present findings have made us more aware of patients at risk for VTE, and we have lowered our threshold for evaluating them for potential clots. Before this study, we used warfarin or enoxaparin for anticoagulation in patients with a history of VTE or active cancer. We are continuing this protocol, but not with other patients. Patients with many comorbidities, lower preoperative Hb level, revision SA, high BMI, or diabetes are carefully monitored for clots early in the postoperative course. Our new threshold for these high-risk patients is to order diagnostic testing, including duplex US or CT angiography. Now, even mild oxygen requirements or mild tachycardia within postoperative week 1 typically prompt a study in these patients. We hope this increased awareness will limit the potential negative consequences associated with development of VTE. Given the present data, we do not think the simple presence of increased comorbidities, lower preoperative Hb, revision SA, high BMI or diabetes should rule out performing SA; rather, it should increase surgeons’ postoperative vigilance in evaluating for potential clots.
Limitations of our study include its retrospective nature and reliance on clinic chart review. Patients were not directly questioned about venous thrombus at follow-up, so all events may not have been captured. Although retrospective review has its drawbacks, it allows for accurate identification of events, even uncoded events. Therefore, more events are likely to be captured with this technique than with large database analyses using only coding information. We tried to identify as many cases as possible by reviewing all outpatient records (orthopedic, nonorthopedic), inpatient records, radiologic studies, and scanned outside records. Another limitation is that having a small number of VTE events limited our ability to perform a multivariate analysis, and uncontrolled confounding likely resulted. Only a very large multi-institutional study can capture enough events to allow a multivariate analysis. A third limitation is that the small number of events may have underpowered the study. Having more patients would have allowed other potential factors to be identified as being significantly associated with VTE. Last, as the study captured only symptomatic VTE events, it may have underreported VTE events. Given our complete review of the medical records, however, most clinically significant events likely were captured.
Conclusion
VTE after SA is rare. In our single-institution study, the symptomatic DVT rate was 0.9%, and the symptomatic PE rate was 2.3%. Risk factors associated with clotting included prior VTE, higher BMI, lower preoperative and postoperative Hb levels, raised CCI, diabetes, use of GETA without interscalene nerve block, and revision SA. Risk factors can be used to identify patients who may benefit from a more scrutinized postoperative evaluation and from increased surgeon awareness of the potential for VTE development. Rates of VTE can be used to counsel SA patients regarding overall surgical risks.
Am J Orthop. 2016;45(6):E379-E385. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Venous thromboembolism (VTE) after shoulder arthroplasty (SA) is relatively uncommon. Reported rates of VTE development are highly variable, ranging from 0.2% to 13% (pulmonary embolism [PE], 0.2%-10.8%; deep venous thrombosis [DVT], 0.1%-13%).1-4 Sources of this variability include different methods of capturing cases (small clinical series vs large database studies, which capture mainly hospital readmissions), differences in defining or detecting VTE, and different patient populations (fracture vs osteoarthritis).1-3 Most studies have also tried to identify factors associated with increased risk for VTE. Risk factors associated with development of VTE after SA include history of VTE, advanced age, prolonged operating room time, higher body mass index (BMI), trauma, history of cancer, female sex, and raised Charlson Comorbidity Index (CCI).1-7 Limitations of clinical series include the smaller number of reporting institutions—a potential source of bias given regional variability.1,3,4,7 Limitations of large state or national databases include capturing only events coded during inpatient admission and capturing readmissions for complications at the same institution. This underreporting may lead to very conservative estimates of VTE incidence.2,5,6,8
In this study, we retrospectively identified all the SAs performed at a single institution over a 13-year period and evaluated the cases for development of VTE (DVT, PE). We hypothesized that the VTE rate would be lower than the very high rates reported by Hoxie and colleagues1 and Willis and colleagues4 but higher than those reported for large state or national databases.2,3 We also evaluated clotting risk factors, including many never analyzed before.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we searched our database for all SAs performed at our institution between January 1999 and May 2012 and identified cases in which symptomatic VTE developed within the first 90 days after surgery. Charts were reviewed for information on medical history, surgical procedure, and in-hospital and out-of-hospital care within the 90-day postoperative period. We recorded data on symptomatic VTE (DVT, PE) as documented by lower or upper extremity duplex ultrasonography (US) or chest computed tomography (CT) angiography. There had been no routine screening of patients; duplex US or CT angiography was performed only if a patient was clinically symptomatic (leg swelling, leg pain, shortness of breath, tachycardia, chest pain) for a potential DVT or PE. For a patient who had repeat SAs on the same shoulder or bilateral SAs at different times, only the first procedure was included in the analysis. Arthroplasties performed for fracture were excluded.
Study data were collected and managed with REDCap (Research Electronic Data Capture) tools hosted at the University of Utah School of Medicine.9 Continuous and discrete data collected on medical history and postoperative course included BMI, age at surgery, preoperative hemoglobin (Hb) and hematocrit (Hct) levels, days in hospital, days until out of bed and days until ambulation (both documented in nursing and physical therapy notes), postoperative Hb and Hct levels, and CCI. Categorical data included sex, diagnosis (primary osteoarthritis, rotator cuff arthropathy, rheumatoid arthritis, failed hemiarthroplasty [HA], failed total SA [TSA], others), attending surgeon, procedure (TSA, HA, reverse TSA, revision SA), anesthesia (general endotracheal anesthesia [GETA] alone, interscalene nerve block alone, GETA plus block), prophylactic use of aspirin after surgery, presence of various medical comorbidities (diabetes, hypertension, cardiac disease, clotting disorders, cancer), hormone replacement therapy, family history of a clotting disorder, and VTE consequences (cardiac events, death).
Statistical Analysis
Descriptive statistics were calculated to summarize aspects of the surgical procedures, the study cohort’s demographics and medical histories, and the incidence of VTE. Logistic regression analysis was performed to explore the association between development of VTE (DVT, PE) and potential risk factors. Unadjusted odds ratios (ORs) were estimated for the risk factors of age, BMI, revision SA, CCI, prophylactic use of aspirin after surgery, preoperative history of VTE, preoperative and postoperative Hb and Hct levels, diabetes, anesthesia (GETA with and without interscalene nerve block), family history of a clotting disorder, days until out of bed, hormone replacement therapy, race, discharge home or to rehabilitation, distance traveled for surgery, hypertension, cardiac disease, cement use, and history of cancer. In addition, ORs were adjusted for age, BMI, and revision SA. For all statistical tests, significance was set at P < .05. All analyses were performed with SAS Version 9.3 (SAS Institute).
Results
We identified 533 SAs: 245 anatomical TSAs, 112 reverse TSAs, 92 HAs, and 84 revision SAs. Three different surgeons performed the procedures, and no patients were lost to follow-up within the first 90 days after surgery. Although SAs were performed for various diagnoses, more than 50% (274) of the SAs were for primary osteoarthritis; 97 were performed for rotator cuff arthropathy, 16 for rheumatoid arthritis, 43 for failed HA, 23 for failed TSA, and 79 for other diagnoses.
Of the 533 patients, 288 were female and 245 were male. Mean age at surgery was 65.2 years (range, 16-93 years). Mean (SD) BMI was 29.2 (6.4) kg/m2. Mean (SD) preoperative Hb level was 13.7 (1.8) g/dL, and mean preoperative Hct level was 40.1% (4.8%). Mean (SD) length of hospital stay was 2.6 (1.5) days. Mean (SD) time before patients were out of bed was 1.1 (0.7) days. On postoperative day 1, mean Hb level was 11.1 (1.7) g/dL, and mean (SD) Hct level was 33.2% (4.8%). Mean (SD) CCI was 1.1 (0.9).
Anesthesia for the 533 patients consisted of GETA (209 patients, 39.0%), interscalene nerve block (2, 0.4%), or GETA with nerve block (314, 59.0%). After surgery, 125 patients (24.3%) received aspirin as prophylaxis. Diabetes was reported by 83 patients, hypertension by 286, cardiac disease by 74, a history of a clotting disorder by 2, a family history of a clotting disorder by 8, ongoing cancer by 4, a history of cancer by 67, and hormone replacement therapy by 104.
For the entire cohort of 533 patients, the symptomatic VTE rate was 2.6% (14 patients), the DVT rate was 0.9% (5), and the PE rate was 2.3% (12). Although VTE did not cause any deaths, there were 3 cardiac events.
Discussion
VTE after SA is rare. We report an overall VTE incidence of 2.6%, with DVT at 0.9% and PE at 2.3%. These rates are similar to those reported in clinical series and significantly higher than those reported for large institutional or national databases.2-7 Our results also support a previously reported trend: The ratio of PE to DVT for SA is significantly higher than historically reported ratios for lower extremity arthroplasty.2,6-8 We have identified many VTE risk factors: raised CCI, preoperative thrombotic event, lower preoperative Hb and Hct levels, lower postoperative Hb level, diabetes, use of GETA without interscalene nerve block, higher BMI, and revision SA. Results of other studies support 3 findings (higher BMI, raised CCI, preoperative thrombotic event); new findings include correlation with Hb and Hct levels, diabetes, type of anesthesia, and revision SA.6,7 Identification of these other factors may be useful in making treatment decisions in patients symptomatic after SA and in lowering the threshold for performing diagnostic tests in these patients at risk for VTE.
Reported rates of VTE after SA are highly variable, ranging from 0.2% to 13%.10 Our rationale for investigating VTE rates at a single institution was to estimate the rates that can be expected in a university-based practice and to determine whether these rates are high enough to warrant routine thromboprophylaxis. The rate variability seems to result in part from variability in the data sources. Most studies that have reported very low VTE rates typically used large state or national databases, which likely were subject to underreporting.
Lyman and colleagues6 found 0.5% DVT and 0.2% PE rates in a New York state hospital database, but only in-hospital immediate postoperative symptomatic complications were included; slightly delayed complications may have been missed. Farng and colleagues5 reported a 0.6% VTE rate, but only inpatient (immediate postoperative or readmission) events were included; all outpatient events were missed. Jameson and colleagues,2 using a national database that included only cases involving inpatient treatment, reported 0% DVT and 0.2% PE rates, again missing outpatient events, and relying on appropriate coding to capture events. Using electronic health records from a large healthcare system, Navarro and colleagues8 queried for VTE cases and reported 0.5% DVT and 0.5% PE rates. The inclusiveness of their data source for the outcome of interest was potentially improved relative to national or statewide databases—and the resulting data reported in their study should reflect that improvement. However, the authors relied on ICD–9 (International Classification of Diseases, Ninth Revision) coding to screen for VTE events and excluded patients with prior VTE, preoperative prophylaxis (enoxaparin or warfarin), or follow-up of <90 days. As patients with prior VTE are those most at risk (present study OR, 6-7), excluding them significantly reduces the overall incidence of clotting reported.
Only 4 studies specifically used information drawn directly from physicians’ clinic notes, vs data retrieved (using code-based queries) from databases.1,3,4,7 These studies may provide a better representation of the rate of VTE after SA, as they were not reliant on codes, included both inpatient and outpatient events, and were inclusive of outpatient follow-up of at least 3 months.
Three of the 4 studies used the Mayo Clinic Total Joint Registry.1,3,4 Hoxie and colleagues1 reported an 11% rate of PE after HA performed for fracture (we excluded SA for fracture). As several other investigators have reported an association between trauma and increased risk for VTE, postoperative anticoagulation should be considered in this patient population (though it was not the focus of the present study).6-8 Sperling and Cofield3 and Singh and colleagues7 reported on the risk for PE among SA patients at the Mayo Clinic. Sperling and Cofield3 included only those events that occurred within the first 7 days after surgery; Singh and colleagues7 included events out to 90 days after surgery. Sperling and Cofield3 reported a 0.17% PE rate; Singh and colleagues7 reported 0.6% PE and 0.1% DVT rates. Sperling and Cofield3 reported on 2885 SAs; Singh and colleagues7 reported on 4019 SAs from the same database. As it is unclear whether these 2 studies had complete information on all patients, underreporting may be an issue. Information was obtained through “clinic visits, medical records and/or standardized mailed and telephone-administered questionnaires.”7The fourth study, a prospective study of 100 patients by Willis and colleagues,4 had the best data on development of symptomatic PE after SA. The authors reported a 2% PE rate and a high (13%) DVT rate. Because US was not performed before the surgical procedures, the number of patients with new and existing DVT cases could not be determined. However, all PEs were new, and the 2% rate found there is similar to the 2.3% in our study. Therefore, we think these rates capture the data most accurately and avoid the underreporting that marks large databases.4Studies have identified various factors that increase the risk for VTE after SA. Singh and colleagues7 identified the risk factors of age over 70 years, female sex, higher BMI (25-29.9 kg/m2), CCI above 1, traumatic etiology, prior history of VTE, and HA. However, their use of univariate regression analysis may have confounded the effects—one factor may have become a surrogate for another (ie, trauma and HA, as most fractures treated with SA during the study period were treated with HA). Lyman and colleagues6 also found advanced age and trauma were associated with higher VTE risk, and reported prior history of cancer as a risk factor as well. Navarro and colleagues8 identified trauma as a risk factor, as in the other 2 studies.6,7 Our data support prior history of VTE, higher BMI, and raised CCI as increasing the risk for VTE.
Other factors identified in the present study are use of GETA without interscalene nerve block, lower preoperative and postoperative Hb levels, diabetes, and revision SA. Because of the limited number of events, only ORs with and without limited control of confounders were performed. Just as in the study by Singh and colleagues,7 uncontrolled confounding could have occurred. A nerve block may be protective, as less postoperative pain may allow patients quicker mobilization and therapy. Diabetes may be a surrogate for other medical comorbidities, as reflected by the higher overall risk with raised CCI. Lower preoperative and postoperative Hb levels were associated with clotting and may be representative of patients with poorer overall health and more complicated surgical procedures (eg, revision SA). In an earlier study, we found increased risk for transfusions in revision SA relative to primary SA.11 Lower preoperative Hb level correlated with development of VTE after lower extremity arthroplasty.12 Postoperative use of aspirin was not found to significantly reduce the incidence of clotting, though this finding may have resulted from lack of power. Therefore, from the present data, there is nothing to conclude about the efficacy of aspirin in preventing thrombosis.
Our findings can be placed in the context of the Virchow triad. Specifically, 3 categories of factors are thought to contribute to thrombosis: hypercoagulability, hemodynamic stasis, and endothelial injury. In grouping factors, we identified prior thrombotic event and obesity as increasing hypercoagulability; revision SA, more comorbidities, lower Hb and Hct levels, diabetes, and GETA as increasing hemodynamic stasis; and revision SA (longer operating room times) as leading to stasis. More comorbidities can be associated with delayed postoperative ambulation, and diabetes and lower Hb and Hct levels can be surrogates for more comorbidities. Surgery performed with the patient under GETA without interscalene nerve block can lead to higher levels of pain and less early mobility.
The present findings have made us more aware of patients at risk for VTE, and we have lowered our threshold for evaluating them for potential clots. Before this study, we used warfarin or enoxaparin for anticoagulation in patients with a history of VTE or active cancer. We are continuing this protocol, but not with other patients. Patients with many comorbidities, lower preoperative Hb level, revision SA, high BMI, or diabetes are carefully monitored for clots early in the postoperative course. Our new threshold for these high-risk patients is to order diagnostic testing, including duplex US or CT angiography. Now, even mild oxygen requirements or mild tachycardia within postoperative week 1 typically prompt a study in these patients. We hope this increased awareness will limit the potential negative consequences associated with development of VTE. Given the present data, we do not think the simple presence of increased comorbidities, lower preoperative Hb, revision SA, high BMI or diabetes should rule out performing SA; rather, it should increase surgeons’ postoperative vigilance in evaluating for potential clots.
Limitations of our study include its retrospective nature and reliance on clinic chart review. Patients were not directly questioned about venous thrombus at follow-up, so all events may not have been captured. Although retrospective review has its drawbacks, it allows for accurate identification of events, even uncoded events. Therefore, more events are likely to be captured with this technique than with large database analyses using only coding information. We tried to identify as many cases as possible by reviewing all outpatient records (orthopedic, nonorthopedic), inpatient records, radiologic studies, and scanned outside records. Another limitation is that having a small number of VTE events limited our ability to perform a multivariate analysis, and uncontrolled confounding likely resulted. Only a very large multi-institutional study can capture enough events to allow a multivariate analysis. A third limitation is that the small number of events may have underpowered the study. Having more patients would have allowed other potential factors to be identified as being significantly associated with VTE. Last, as the study captured only symptomatic VTE events, it may have underreported VTE events. Given our complete review of the medical records, however, most clinically significant events likely were captured.
Conclusion
VTE after SA is rare. In our single-institution study, the symptomatic DVT rate was 0.9%, and the symptomatic PE rate was 2.3%. Risk factors associated with clotting included prior VTE, higher BMI, lower preoperative and postoperative Hb levels, raised CCI, diabetes, use of GETA without interscalene nerve block, and revision SA. Risk factors can be used to identify patients who may benefit from a more scrutinized postoperative evaluation and from increased surgeon awareness of the potential for VTE development. Rates of VTE can be used to counsel SA patients regarding overall surgical risks.
Am J Orthop. 2016;45(6):E379-E385. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hoxie SC, Sperling JW, Cofield RH. Pulmonary embolism after operative treatment of proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(6):782-783.
2. Jameson SS, James P, Howcroft DW, et al. Venous thromboembolic events are rare after shoulder surgery: analysis of a national database. J Shoulder Elbow Surg. 2011;20(5):764-770.
3. Sperling JW, Cofield RH. Pulmonary embolism following shoulder arthroplasty. J Bone Joint Surg Am. 2002;84(11):1939-1941.
4. Willis AA, Warren RF, Craig EV, et al. Deep vein thrombosis after reconstructive shoulder arthroplasty: a prospective observational study. J Shoulder Elbow Surg. 2009;18(1):100-106.
5. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563.
6. Lyman S, Sherman S, Carter TI, Bach PB, Mandl LA, Marx RG. Prevalence and risk factors for symptomatic thromboembolic events after shoulder arthroplasty. Clin Orthop Relat Res. 2006;(448):152-156.
7. Singh JA, Sperling JW, Cofield RH. Cardiopulmonary complications after primary shoulder arthroplasty: a cohort study. Semin Arthritis Rheum. 2012;41(5):689-697.
8. Navarro RA, Inacio MC, Burke MF, Costouros JG, Yian EH. Risk of thromboembolism in shoulder arthroplasty: effect of implant type and traumatic indication. Clin Orthop Relat Res. 2013;471(5):1576-1581.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
10. Saleh HE, Pennings AL, ElMaraghy AW. Venous thromboembolism after shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2013;22(10):1440-1448.
11. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
12. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335-341.
1. Hoxie SC, Sperling JW, Cofield RH. Pulmonary embolism after operative treatment of proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(6):782-783.
2. Jameson SS, James P, Howcroft DW, et al. Venous thromboembolic events are rare after shoulder surgery: analysis of a national database. J Shoulder Elbow Surg. 2011;20(5):764-770.
3. Sperling JW, Cofield RH. Pulmonary embolism following shoulder arthroplasty. J Bone Joint Surg Am. 2002;84(11):1939-1941.
4. Willis AA, Warren RF, Craig EV, et al. Deep vein thrombosis after reconstructive shoulder arthroplasty: a prospective observational study. J Shoulder Elbow Surg. 2009;18(1):100-106.
5. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563.
6. Lyman S, Sherman S, Carter TI, Bach PB, Mandl LA, Marx RG. Prevalence and risk factors for symptomatic thromboembolic events after shoulder arthroplasty. Clin Orthop Relat Res. 2006;(448):152-156.
7. Singh JA, Sperling JW, Cofield RH. Cardiopulmonary complications after primary shoulder arthroplasty: a cohort study. Semin Arthritis Rheum. 2012;41(5):689-697.
8. Navarro RA, Inacio MC, Burke MF, Costouros JG, Yian EH. Risk of thromboembolism in shoulder arthroplasty: effect of implant type and traumatic indication. Clin Orthop Relat Res. 2013;471(5):1576-1581.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
10. Saleh HE, Pennings AL, ElMaraghy AW. Venous thromboembolism after shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2013;22(10):1440-1448.
11. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
12. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335-341.
Laparoscopic sacrocolpopexy offers advantages over abdominal route
BOSTON – Laparoscopic sacrocolpopexy offers some distinct advantages over the abdominal route for treatment of pelvic organ prolapse, including reduced intraoperative blood loss and shorter hospital stays, according to findings from a new research review.
“We wanted to compare the efficiency and safety of abdominal sacral colpopexy and laparoscopic sacral colpopexy for the treatment of pelvic organ collapse,” Juan Liu, MD, of Guangzhou Medical University in China said at the annual Minimally Invasive Surgery Week, held by the Society of Laparoendoscopic Surgeons.
Analyses directly comparing the safety and effectiveness of the two surgical routes are low in number, Dr. Liu added.
The researchers looked at published articles, written in English or Chinese, that were either retrospective analyses or randomized controlled trial studies examining laparoscopic sacrocolpopexy (LSC) or abdominal sacrocolpopexy (ASC), with follow-up times of at least 30 days.
Studies that investigated robot-assisted sacrocolpopexy were excluded, as well as studies for which there were no specific feature data or for which the full text of the study was inaccessible. Of 1,807 articles identified, 10 studies containing 3,816 cases were included for the analysis.
The studies were used to compare laparoscopic and abdominal sacrocolpopexy on the following criteria: operating time; blood loss; hospital length of stay; intraoperative complications such as urinary, bladder, and rectal injury; and postoperative complications such as infection, intestinal obstruction, mesh exposure, new urinary incontinence, and dyspareunia. Weighted mean difference was calculated to account for the different sample sizes across the studies.
The weighted mean difference in intraoperative blood loss in the laparoscopic cohort, compared with the abdominal cohort, was –100.68 mL (P less than .01). Hospital length of stay was also significantly reduced in the laparoscopic cohort, with a weighted mean difference of –1.77 days (P less than .01). The odds ratio for gastrointestinal complications was 0.30 for the laparoscopic route, compared with the abdominal route (P less than .01).
Additionally, pulmonary complications and blood transfusions were also found to be reduced with laparoscopic sacrocolpopexy, compared with abdominal sacrocolpopexy, with an odds ratio of 0.59 (P = .02) and 0.47 (P = .03), respectively.
But the review found little difference in other areas. The weighted mean difference for operating time in the laparoscopic cohort was 0.06 minutes, compared with the abdominal cohort, which was not statistically significant (P= .84). And there was not a statistically significant difference between the two surgical approaches in urinary complications (OR, 0.41; P = .11), cardiovascular complications (OR, 0.31; P = .49), or mesh exposure (OR, 1.60, P = .18).
No funding source for this study was disclosed. Dr. Liu reported having no relevant financial disclosures.
BOSTON – Laparoscopic sacrocolpopexy offers some distinct advantages over the abdominal route for treatment of pelvic organ prolapse, including reduced intraoperative blood loss and shorter hospital stays, according to findings from a new research review.
“We wanted to compare the efficiency and safety of abdominal sacral colpopexy and laparoscopic sacral colpopexy for the treatment of pelvic organ collapse,” Juan Liu, MD, of Guangzhou Medical University in China said at the annual Minimally Invasive Surgery Week, held by the Society of Laparoendoscopic Surgeons.
Analyses directly comparing the safety and effectiveness of the two surgical routes are low in number, Dr. Liu added.
The researchers looked at published articles, written in English or Chinese, that were either retrospective analyses or randomized controlled trial studies examining laparoscopic sacrocolpopexy (LSC) or abdominal sacrocolpopexy (ASC), with follow-up times of at least 30 days.
Studies that investigated robot-assisted sacrocolpopexy were excluded, as well as studies for which there were no specific feature data or for which the full text of the study was inaccessible. Of 1,807 articles identified, 10 studies containing 3,816 cases were included for the analysis.
The studies were used to compare laparoscopic and abdominal sacrocolpopexy on the following criteria: operating time; blood loss; hospital length of stay; intraoperative complications such as urinary, bladder, and rectal injury; and postoperative complications such as infection, intestinal obstruction, mesh exposure, new urinary incontinence, and dyspareunia. Weighted mean difference was calculated to account for the different sample sizes across the studies.
The weighted mean difference in intraoperative blood loss in the laparoscopic cohort, compared with the abdominal cohort, was –100.68 mL (P less than .01). Hospital length of stay was also significantly reduced in the laparoscopic cohort, with a weighted mean difference of –1.77 days (P less than .01). The odds ratio for gastrointestinal complications was 0.30 for the laparoscopic route, compared with the abdominal route (P less than .01).
Additionally, pulmonary complications and blood transfusions were also found to be reduced with laparoscopic sacrocolpopexy, compared with abdominal sacrocolpopexy, with an odds ratio of 0.59 (P = .02) and 0.47 (P = .03), respectively.
But the review found little difference in other areas. The weighted mean difference for operating time in the laparoscopic cohort was 0.06 minutes, compared with the abdominal cohort, which was not statistically significant (P= .84). And there was not a statistically significant difference between the two surgical approaches in urinary complications (OR, 0.41; P = .11), cardiovascular complications (OR, 0.31; P = .49), or mesh exposure (OR, 1.60, P = .18).
No funding source for this study was disclosed. Dr. Liu reported having no relevant financial disclosures.
BOSTON – Laparoscopic sacrocolpopexy offers some distinct advantages over the abdominal route for treatment of pelvic organ prolapse, including reduced intraoperative blood loss and shorter hospital stays, according to findings from a new research review.
“We wanted to compare the efficiency and safety of abdominal sacral colpopexy and laparoscopic sacral colpopexy for the treatment of pelvic organ collapse,” Juan Liu, MD, of Guangzhou Medical University in China said at the annual Minimally Invasive Surgery Week, held by the Society of Laparoendoscopic Surgeons.
Analyses directly comparing the safety and effectiveness of the two surgical routes are low in number, Dr. Liu added.
The researchers looked at published articles, written in English or Chinese, that were either retrospective analyses or randomized controlled trial studies examining laparoscopic sacrocolpopexy (LSC) or abdominal sacrocolpopexy (ASC), with follow-up times of at least 30 days.
Studies that investigated robot-assisted sacrocolpopexy were excluded, as well as studies for which there were no specific feature data or for which the full text of the study was inaccessible. Of 1,807 articles identified, 10 studies containing 3,816 cases were included for the analysis.
The studies were used to compare laparoscopic and abdominal sacrocolpopexy on the following criteria: operating time; blood loss; hospital length of stay; intraoperative complications such as urinary, bladder, and rectal injury; and postoperative complications such as infection, intestinal obstruction, mesh exposure, new urinary incontinence, and dyspareunia. Weighted mean difference was calculated to account for the different sample sizes across the studies.
The weighted mean difference in intraoperative blood loss in the laparoscopic cohort, compared with the abdominal cohort, was –100.68 mL (P less than .01). Hospital length of stay was also significantly reduced in the laparoscopic cohort, with a weighted mean difference of –1.77 days (P less than .01). The odds ratio for gastrointestinal complications was 0.30 for the laparoscopic route, compared with the abdominal route (P less than .01).
Additionally, pulmonary complications and blood transfusions were also found to be reduced with laparoscopic sacrocolpopexy, compared with abdominal sacrocolpopexy, with an odds ratio of 0.59 (P = .02) and 0.47 (P = .03), respectively.
But the review found little difference in other areas. The weighted mean difference for operating time in the laparoscopic cohort was 0.06 minutes, compared with the abdominal cohort, which was not statistically significant (P= .84). And there was not a statistically significant difference between the two surgical approaches in urinary complications (OR, 0.41; P = .11), cardiovascular complications (OR, 0.31; P = .49), or mesh exposure (OR, 1.60, P = .18).
No funding source for this study was disclosed. Dr. Liu reported having no relevant financial disclosures.
AT MINIMALLY INVASIVE SURGERY WEEK
Key clinical point:
Major finding: The weighted mean difference in intraoperative blood loss in the laparoscopic cohort, compared with the abdominal cohort, was –100.68 mL (P less than .01).
Data source: Retrospective review of 10 studies involving 3,816 sacrocolpopexy cases.
Disclosures: Dr. Liu reported having no relevant financial disclosures.
Blaschkoid Unilateral Patch on the Chest
The Diagnosis: Lichen Striatus
Lichen striatus (LS) is an acquired and self-limited linear inflammatory dermatosis that most frequently occurs in children and less commonly in adults.1-3 Clinically, it is characterized by the sudden onset of an eruption consisting of slightly pigmented, erythematous, flat-topped papules with minimal scaling. These papules quickly coalesce to form a linear band that extends along a limb, the trunk, or the face, within Blaschko lines.1,4 In the adult form, patients tend to experience more diffuse lesions as well as severe pruritus with higher rates of relapse. It occasionally manifests in a dermatomal manner.1
The differential diagnosis includes other linear acquired inflammatory dermatoses such as blaschkitis, lichen planus, inflammatory linear verrucous epidermal nevus, and psoriasis. Blaschkitis has been described as a rare dermatosis that occurs along the Blaschko lines, affecting adults preferentially over children. Controversy exists whether blaschkitis and lichen striatus are the same disease or 2 separate entities.5 Clinically, both blaschkitis and lichen striatus can present with multiple linear papules and vesicles predominantly on the trunk. In blaschkitis, there is a predilection for males, with an older mean age at onset of 40 years.5 Lesions quickly resolve over months with frequent relapse compared to lichen striatus, which can persist for months to years.
Histopathologically, blaschkitis demonstrates spongiosis, usually without involvement of the adnexal structures. Lichenoid and spongiotic changes with adnexal extension are the hallmark features of lichen striatus. In our patient, biopsy showed several dense bandlike foci of lymphohistiocytic infiltrates along the dermoepidermal junction with spongiosis, basal cell liquefactive degeneration, and pigmentary incontinence (Figure 1). The focal areas were surfaced by parakeratotic and orthohyperkeratotic scale. Deep dermal perivascular and periadnexal extension was present (Figure 2). Periodic acid-Schiff stain was negative for fungi.
The pathogenesis of lichen striatus is not entirely understood, but it has been postulated that trauma, vaccinations, or viral infections may induce loss of immunologic tolerance to keratinocytes.1 This loss of tolerance can result in a T cell-mediated autoimmune reaction against malpighian cells, which show genetic mosaicism and are arranged along Blaschko lines.1,3 Familial cases also have been reported, suggesting that there may be an epigenetic mosaicism that contributes to this group of skin diseases.6,7
Lichen striatus tends to resolve on its own after approximately 6 to 9 months.8 Treatment typically consists of application of topical corticosteroids.1 Cases also have been successfully treated with tacrolimus and pimecrolimus.1,8 Our patient was treated with a midpotency topical steroid with improvement of the appearance but not complete resolution.
- Campanati A, Brandozzi G, Giangiacomi M, et al. Lichen striatus in adults and pimecrolimus: open, off-label clinical study. Int J Dermatol. 2008;47:732-736.
- Lee DY, Kim S, Kim CR, et al. Lichen striatus in an adult treated by a short course of low-dose systemic corticosteroid. J Dermatol. 2011;38:298-299.
- Hofer T. Lichen striatus in adults or "adult blaschkitis"? there is no need for a new naming. Dermatology. 2003;207:89-92.
- Shepherd V, Lun K, Strutton G. Lichen striatus in an adult following trauma. Australas J Dermatol. 2005;46:25-28.
- Müller CS, Schmaltz R, Vogt T, et al. Lichen striatus and blaschkitis reappraisal of the concept of blaschkolinear dermatoses. Br J Dermatol. 2011;164:257-262.
- Yaosaka M, Sawamura D, Iitoyo M, et al. Lichen striatus affecting a mother and her son. J Am Acad Dermatol. 2005;53:352-353.
- Jackson R. The lines of Blaschko: a review and reconsideration: observations of the cause of certain unusual linear conditions of the skin. Br J Dermatol. 1976;95:349-360.
- Sorgentini C, Allevato MA, Dahbar M, et al. Lichen striatus in an adult: successful treatment with tacrolimus. Br J Dermatol. 2004;150:776-777.
The Diagnosis: Lichen Striatus
Lichen striatus (LS) is an acquired and self-limited linear inflammatory dermatosis that most frequently occurs in children and less commonly in adults.1-3 Clinically, it is characterized by the sudden onset of an eruption consisting of slightly pigmented, erythematous, flat-topped papules with minimal scaling. These papules quickly coalesce to form a linear band that extends along a limb, the trunk, or the face, within Blaschko lines.1,4 In the adult form, patients tend to experience more diffuse lesions as well as severe pruritus with higher rates of relapse. It occasionally manifests in a dermatomal manner.1
The differential diagnosis includes other linear acquired inflammatory dermatoses such as blaschkitis, lichen planus, inflammatory linear verrucous epidermal nevus, and psoriasis. Blaschkitis has been described as a rare dermatosis that occurs along the Blaschko lines, affecting adults preferentially over children. Controversy exists whether blaschkitis and lichen striatus are the same disease or 2 separate entities.5 Clinically, both blaschkitis and lichen striatus can present with multiple linear papules and vesicles predominantly on the trunk. In blaschkitis, there is a predilection for males, with an older mean age at onset of 40 years.5 Lesions quickly resolve over months with frequent relapse compared to lichen striatus, which can persist for months to years.
Histopathologically, blaschkitis demonstrates spongiosis, usually without involvement of the adnexal structures. Lichenoid and spongiotic changes with adnexal extension are the hallmark features of lichen striatus. In our patient, biopsy showed several dense bandlike foci of lymphohistiocytic infiltrates along the dermoepidermal junction with spongiosis, basal cell liquefactive degeneration, and pigmentary incontinence (Figure 1). The focal areas were surfaced by parakeratotic and orthohyperkeratotic scale. Deep dermal perivascular and periadnexal extension was present (Figure 2). Periodic acid-Schiff stain was negative for fungi.


The pathogenesis of lichen striatus is not entirely understood, but it has been postulated that trauma, vaccinations, or viral infections may induce loss of immunologic tolerance to keratinocytes.1 This loss of tolerance can result in a T cell-mediated autoimmune reaction against malpighian cells, which show genetic mosaicism and are arranged along Blaschko lines.1,3 Familial cases also have been reported, suggesting that there may be an epigenetic mosaicism that contributes to this group of skin diseases.6,7
Lichen striatus tends to resolve on its own after approximately 6 to 9 months.8 Treatment typically consists of application of topical corticosteroids.1 Cases also have been successfully treated with tacrolimus and pimecrolimus.1,8 Our patient was treated with a midpotency topical steroid with improvement of the appearance but not complete resolution.
The Diagnosis: Lichen Striatus
Lichen striatus (LS) is an acquired and self-limited linear inflammatory dermatosis that most frequently occurs in children and less commonly in adults.1-3 Clinically, it is characterized by the sudden onset of an eruption consisting of slightly pigmented, erythematous, flat-topped papules with minimal scaling. These papules quickly coalesce to form a linear band that extends along a limb, the trunk, or the face, within Blaschko lines.1,4 In the adult form, patients tend to experience more diffuse lesions as well as severe pruritus with higher rates of relapse. It occasionally manifests in a dermatomal manner.1
The differential diagnosis includes other linear acquired inflammatory dermatoses such as blaschkitis, lichen planus, inflammatory linear verrucous epidermal nevus, and psoriasis. Blaschkitis has been described as a rare dermatosis that occurs along the Blaschko lines, affecting adults preferentially over children. Controversy exists whether blaschkitis and lichen striatus are the same disease or 2 separate entities.5 Clinically, both blaschkitis and lichen striatus can present with multiple linear papules and vesicles predominantly on the trunk. In blaschkitis, there is a predilection for males, with an older mean age at onset of 40 years.5 Lesions quickly resolve over months with frequent relapse compared to lichen striatus, which can persist for months to years.
Histopathologically, blaschkitis demonstrates spongiosis, usually without involvement of the adnexal structures. Lichenoid and spongiotic changes with adnexal extension are the hallmark features of lichen striatus. In our patient, biopsy showed several dense bandlike foci of lymphohistiocytic infiltrates along the dermoepidermal junction with spongiosis, basal cell liquefactive degeneration, and pigmentary incontinence (Figure 1). The focal areas were surfaced by parakeratotic and orthohyperkeratotic scale. Deep dermal perivascular and periadnexal extension was present (Figure 2). Periodic acid-Schiff stain was negative for fungi.


The pathogenesis of lichen striatus is not entirely understood, but it has been postulated that trauma, vaccinations, or viral infections may induce loss of immunologic tolerance to keratinocytes.1 This loss of tolerance can result in a T cell-mediated autoimmune reaction against malpighian cells, which show genetic mosaicism and are arranged along Blaschko lines.1,3 Familial cases also have been reported, suggesting that there may be an epigenetic mosaicism that contributes to this group of skin diseases.6,7
Lichen striatus tends to resolve on its own after approximately 6 to 9 months.8 Treatment typically consists of application of topical corticosteroids.1 Cases also have been successfully treated with tacrolimus and pimecrolimus.1,8 Our patient was treated with a midpotency topical steroid with improvement of the appearance but not complete resolution.
- Campanati A, Brandozzi G, Giangiacomi M, et al. Lichen striatus in adults and pimecrolimus: open, off-label clinical study. Int J Dermatol. 2008;47:732-736.
- Lee DY, Kim S, Kim CR, et al. Lichen striatus in an adult treated by a short course of low-dose systemic corticosteroid. J Dermatol. 2011;38:298-299.
- Hofer T. Lichen striatus in adults or "adult blaschkitis"? there is no need for a new naming. Dermatology. 2003;207:89-92.
- Shepherd V, Lun K, Strutton G. Lichen striatus in an adult following trauma. Australas J Dermatol. 2005;46:25-28.
- Müller CS, Schmaltz R, Vogt T, et al. Lichen striatus and blaschkitis reappraisal of the concept of blaschkolinear dermatoses. Br J Dermatol. 2011;164:257-262.
- Yaosaka M, Sawamura D, Iitoyo M, et al. Lichen striatus affecting a mother and her son. J Am Acad Dermatol. 2005;53:352-353.
- Jackson R. The lines of Blaschko: a review and reconsideration: observations of the cause of certain unusual linear conditions of the skin. Br J Dermatol. 1976;95:349-360.
- Sorgentini C, Allevato MA, Dahbar M, et al. Lichen striatus in an adult: successful treatment with tacrolimus. Br J Dermatol. 2004;150:776-777.
- Campanati A, Brandozzi G, Giangiacomi M, et al. Lichen striatus in adults and pimecrolimus: open, off-label clinical study. Int J Dermatol. 2008;47:732-736.
- Lee DY, Kim S, Kim CR, et al. Lichen striatus in an adult treated by a short course of low-dose systemic corticosteroid. J Dermatol. 2011;38:298-299.
- Hofer T. Lichen striatus in adults or "adult blaschkitis"? there is no need for a new naming. Dermatology. 2003;207:89-92.
- Shepherd V, Lun K, Strutton G. Lichen striatus in an adult following trauma. Australas J Dermatol. 2005;46:25-28.
- Müller CS, Schmaltz R, Vogt T, et al. Lichen striatus and blaschkitis reappraisal of the concept of blaschkolinear dermatoses. Br J Dermatol. 2011;164:257-262.
- Yaosaka M, Sawamura D, Iitoyo M, et al. Lichen striatus affecting a mother and her son. J Am Acad Dermatol. 2005;53:352-353.
- Jackson R. The lines of Blaschko: a review and reconsideration: observations of the cause of certain unusual linear conditions of the skin. Br J Dermatol. 1976;95:349-360.
- Sorgentini C, Allevato MA, Dahbar M, et al. Lichen striatus in an adult: successful treatment with tacrolimus. Br J Dermatol. 2004;150:776-777.
A 26-year-old man presented with erythematous, scaly, grouped papules along the right side of the chest of 3 weeks' duration, extending to the flank following a blaschkoid distribution on the right side of the chest and not crossing the midline. He reported occasional irritation but otherwise was asymptomatic. His medical history was unremarkable and he was not taking any medications. He also denied trauma to the area.
MRI measurements reveal effects of sleep deprivation
Lack of sleep significantly impacts the brain’s response to an attention task, and circadian rhythms play a role, according to functional magnetic resonance imaging data from 33 healthy adults that was published in Science. Study participants stayed awake for 42 hours and periodically performed a psychomotor vigilance task (PVT)—a visual reaction time task designed to measure attention—and an auditory n-back task. Overall, PVT performance was stable during the first day, but decreased significantly after sleep deprivation. For more on the study, see this article from Family Practice News: http://www.familypracticenews.com/specialty-focus/pulmonary-sleep-medicine/single-article-page/mri-measurements-reveal-effects-of-sleep-deprivation/e4929289d9671662155a4344b4429149.html.
Lack of sleep significantly impacts the brain’s response to an attention task, and circadian rhythms play a role, according to functional magnetic resonance imaging data from 33 healthy adults that was published in Science. Study participants stayed awake for 42 hours and periodically performed a psychomotor vigilance task (PVT)—a visual reaction time task designed to measure attention—and an auditory n-back task. Overall, PVT performance was stable during the first day, but decreased significantly after sleep deprivation. For more on the study, see this article from Family Practice News: http://www.familypracticenews.com/specialty-focus/pulmonary-sleep-medicine/single-article-page/mri-measurements-reveal-effects-of-sleep-deprivation/e4929289d9671662155a4344b4429149.html.
Lack of sleep significantly impacts the brain’s response to an attention task, and circadian rhythms play a role, according to functional magnetic resonance imaging data from 33 healthy adults that was published in Science. Study participants stayed awake for 42 hours and periodically performed a psychomotor vigilance task (PVT)—a visual reaction time task designed to measure attention—and an auditory n-back task. Overall, PVT performance was stable during the first day, but decreased significantly after sleep deprivation. For more on the study, see this article from Family Practice News: http://www.familypracticenews.com/specialty-focus/pulmonary-sleep-medicine/single-article-page/mri-measurements-reveal-effects-of-sleep-deprivation/e4929289d9671662155a4344b4429149.html.