User login
Patients with Epilepsy May Lack Essential Social Cognition Skills
Patients with epilepsy seem to have difficulty recognizing certain emotional states, according to a recent study that used video simulations to evaluate patients’ social cognition skills. When researchers administered the Awareness of Social Inference Test to 43 patients with focal epilepsy and 22 controls, using a video format, they found that neither group had trouble identifying positive emotional states like happiness; but patients with epilepsy had difficulty recognizing negative emotions such as anger, fear, and disgust. The study suggests that standard psychometric tools used to measure cognitive abilities in patients with epilepsy may need to be supplemented with a vehicle that evaluates social cognition.
Bujarski KA, Flashman L, Li Z, et al. Investigating social cognition in epilepsy using a naturalistic task. Epilepsia. 2016;57(9):1515-1520.
Patients with epilepsy seem to have difficulty recognizing certain emotional states, according to a recent study that used video simulations to evaluate patients’ social cognition skills. When researchers administered the Awareness of Social Inference Test to 43 patients with focal epilepsy and 22 controls, using a video format, they found that neither group had trouble identifying positive emotional states like happiness; but patients with epilepsy had difficulty recognizing negative emotions such as anger, fear, and disgust. The study suggests that standard psychometric tools used to measure cognitive abilities in patients with epilepsy may need to be supplemented with a vehicle that evaluates social cognition.
Bujarski KA, Flashman L, Li Z, et al. Investigating social cognition in epilepsy using a naturalistic task. Epilepsia. 2016;57(9):1515-1520.
Patients with epilepsy seem to have difficulty recognizing certain emotional states, according to a recent study that used video simulations to evaluate patients’ social cognition skills. When researchers administered the Awareness of Social Inference Test to 43 patients with focal epilepsy and 22 controls, using a video format, they found that neither group had trouble identifying positive emotional states like happiness; but patients with epilepsy had difficulty recognizing negative emotions such as anger, fear, and disgust. The study suggests that standard psychometric tools used to measure cognitive abilities in patients with epilepsy may need to be supplemented with a vehicle that evaluates social cognition.
Bujarski KA, Flashman L, Li Z, et al. Investigating social cognition in epilepsy using a naturalistic task. Epilepsia. 2016;57(9):1515-1520.
The Default Mode Network Plays Important Role in Pathology of Epilepsy
The default mode network (DMN), which connects brain regions such as precuneus/posterior cingulate cortex, medial prefrontal cortex, and medial, lateral, and inferior parietal cortex, plays an important role in temporal lobe epilepsy (TLE), according to a recent literature review. Among patients with TLE, the amplitude of the blood oxygenation-level dependent (BOLD) signal decreases during the interval between seizures. Investigators have also found that TLE patients have less anterograde connectivity from the anterior to the posterior DMN. Changes in the activity of the DMN in people with epilepsy, as well as several other neurological disorders, suggest that assessment of the network may help improve early detection and treatment, according to the researchers.
Mohan A, Roberto AJ, Mohan A, et al. The significance of the default mode network (DMN) in neurological and neuropsychiatric disorders: A review. Yale J Biol Med. 2016;89(1):49-57.
The default mode network (DMN), which connects brain regions such as precuneus/posterior cingulate cortex, medial prefrontal cortex, and medial, lateral, and inferior parietal cortex, plays an important role in temporal lobe epilepsy (TLE), according to a recent literature review. Among patients with TLE, the amplitude of the blood oxygenation-level dependent (BOLD) signal decreases during the interval between seizures. Investigators have also found that TLE patients have less anterograde connectivity from the anterior to the posterior DMN. Changes in the activity of the DMN in people with epilepsy, as well as several other neurological disorders, suggest that assessment of the network may help improve early detection and treatment, according to the researchers.
Mohan A, Roberto AJ, Mohan A, et al. The significance of the default mode network (DMN) in neurological and neuropsychiatric disorders: A review. Yale J Biol Med. 2016;89(1):49-57.
The default mode network (DMN), which connects brain regions such as precuneus/posterior cingulate cortex, medial prefrontal cortex, and medial, lateral, and inferior parietal cortex, plays an important role in temporal lobe epilepsy (TLE), according to a recent literature review. Among patients with TLE, the amplitude of the blood oxygenation-level dependent (BOLD) signal decreases during the interval between seizures. Investigators have also found that TLE patients have less anterograde connectivity from the anterior to the posterior DMN. Changes in the activity of the DMN in people with epilepsy, as well as several other neurological disorders, suggest that assessment of the network may help improve early detection and treatment, according to the researchers.
Mohan A, Roberto AJ, Mohan A, et al. The significance of the default mode network (DMN) in neurological and neuropsychiatric disorders: A review. Yale J Biol Med. 2016;89(1):49-57.
Using MRIs to Separate Rasmussen Encephalitis from Epilepsy
To help distinguish patients with Rasmussen encephalitis from patients with epilepsy not suffering from the syndrome, researchers performed quantitative volumetric MR imaging on 42 patients with Rasmussen syndrome and compared the readings to MRIs performed on 42 controls and 42 unaffected patients with epilepsy. Their analysis found that interhemispheric and frontal lobe ratios were the most effective way to differentiate Rasmussen encephalitis from the other 2 groups. They also found that the insula of Rasmussen encephalitis patients was significantly more atrophic, when compared with other cortical regions of the brain.
Wang Z, Krishnan B, Shattuck DW, et al. Automated MRI volumetric analysis in patients with Rasmussen syndrome [published online ahead of print September 8, 2016]. AJNR Am. J. Neuroradiol. 2016.
To help distinguish patients with Rasmussen encephalitis from patients with epilepsy not suffering from the syndrome, researchers performed quantitative volumetric MR imaging on 42 patients with Rasmussen syndrome and compared the readings to MRIs performed on 42 controls and 42 unaffected patients with epilepsy. Their analysis found that interhemispheric and frontal lobe ratios were the most effective way to differentiate Rasmussen encephalitis from the other 2 groups. They also found that the insula of Rasmussen encephalitis patients was significantly more atrophic, when compared with other cortical regions of the brain.
Wang Z, Krishnan B, Shattuck DW, et al. Automated MRI volumetric analysis in patients with Rasmussen syndrome [published online ahead of print September 8, 2016]. AJNR Am. J. Neuroradiol. 2016.
To help distinguish patients with Rasmussen encephalitis from patients with epilepsy not suffering from the syndrome, researchers performed quantitative volumetric MR imaging on 42 patients with Rasmussen syndrome and compared the readings to MRIs performed on 42 controls and 42 unaffected patients with epilepsy. Their analysis found that interhemispheric and frontal lobe ratios were the most effective way to differentiate Rasmussen encephalitis from the other 2 groups. They also found that the insula of Rasmussen encephalitis patients was significantly more atrophic, when compared with other cortical regions of the brain.
Wang Z, Krishnan B, Shattuck DW, et al. Automated MRI volumetric analysis in patients with Rasmussen syndrome [published online ahead of print September 8, 2016]. AJNR Am. J. Neuroradiol. 2016.
Long-term Intracranial Monitoring Reveals Circadian Pattern of Epileptic Discharges
Using the NeuroPace RNS system to monitor long-term epileptic-like activity, researchers have confirmed that there is a uniform circadian pattern to this brain activity. Studying 134 subjects, Spencer et al found the epileptiform activity peaked during normal sleeping hours. They also discovered a monophasic, nocturnally dominant rhythm in the neocortical areas of the brain and a more complex pattern, with a diurnal peak, in limbic sections of the brain. Some volunteers were also found to have a dual oscillator pattern to the brain activity, displaying a circadian and ultradian pattern.
Spencer D, Sun F, Brown S, Jobst, B, Wong V, Mirro E et al. Circadian and ultradian patterns of epileptiform discharges differ by seizure-onset location during long-term ambulatory intracranial monitoring. Epilepsia. 2016;57(9):1495-1502.
Using the NeuroPace RNS system to monitor long-term epileptic-like activity, researchers have confirmed that there is a uniform circadian pattern to this brain activity. Studying 134 subjects, Spencer et al found the epileptiform activity peaked during normal sleeping hours. They also discovered a monophasic, nocturnally dominant rhythm in the neocortical areas of the brain and a more complex pattern, with a diurnal peak, in limbic sections of the brain. Some volunteers were also found to have a dual oscillator pattern to the brain activity, displaying a circadian and ultradian pattern.
Spencer D, Sun F, Brown S, Jobst, B, Wong V, Mirro E et al. Circadian and ultradian patterns of epileptiform discharges differ by seizure-onset location during long-term ambulatory intracranial monitoring. Epilepsia. 2016;57(9):1495-1502.
Using the NeuroPace RNS system to monitor long-term epileptic-like activity, researchers have confirmed that there is a uniform circadian pattern to this brain activity. Studying 134 subjects, Spencer et al found the epileptiform activity peaked during normal sleeping hours. They also discovered a monophasic, nocturnally dominant rhythm in the neocortical areas of the brain and a more complex pattern, with a diurnal peak, in limbic sections of the brain. Some volunteers were also found to have a dual oscillator pattern to the brain activity, displaying a circadian and ultradian pattern.
Spencer D, Sun F, Brown S, Jobst, B, Wong V, Mirro E et al. Circadian and ultradian patterns of epileptiform discharges differ by seizure-onset location during long-term ambulatory intracranial monitoring. Epilepsia. 2016;57(9):1495-1502.
Finding the Links Between Tuberous Sclerosis Complex and Epilepsy
Patients with tuberous sclerosis complex (TSC) are at higher than average risk of developing epilepsy if they exhibit several systemic disease manifestations, according to a recent analysis of the TSC Natural History Database. After factoring out confounding variables like age, gender, and TSC mutation, Anna Jeong and Michael Wong of Washington University School of Medicine found that cardiac rhabdomyomas, retinal hamartomas, renal cysts, renal angiomyolipomas, shagreen patches, and facial angiofibromas increased the likelihood of TSC patients developing epilepsy.
Jeong A, Wong M. Systemic disease manifestations associated with epilepsy in tuberous sclerosis complex. Epilepsia. 2016;57(9):1443-1449.
Patients with tuberous sclerosis complex (TSC) are at higher than average risk of developing epilepsy if they exhibit several systemic disease manifestations, according to a recent analysis of the TSC Natural History Database. After factoring out confounding variables like age, gender, and TSC mutation, Anna Jeong and Michael Wong of Washington University School of Medicine found that cardiac rhabdomyomas, retinal hamartomas, renal cysts, renal angiomyolipomas, shagreen patches, and facial angiofibromas increased the likelihood of TSC patients developing epilepsy.
Jeong A, Wong M. Systemic disease manifestations associated with epilepsy in tuberous sclerosis complex. Epilepsia. 2016;57(9):1443-1449.
Patients with tuberous sclerosis complex (TSC) are at higher than average risk of developing epilepsy if they exhibit several systemic disease manifestations, according to a recent analysis of the TSC Natural History Database. After factoring out confounding variables like age, gender, and TSC mutation, Anna Jeong and Michael Wong of Washington University School of Medicine found that cardiac rhabdomyomas, retinal hamartomas, renal cysts, renal angiomyolipomas, shagreen patches, and facial angiofibromas increased the likelihood of TSC patients developing epilepsy.
Jeong A, Wong M. Systemic disease manifestations associated with epilepsy in tuberous sclerosis complex. Epilepsia. 2016;57(9):1443-1449.
Functional MRI Can Separate Types of Temporal Lobe Epilepsy
Performing resting state functioning MRIs can help distinguish temporal lobe epilepsy that’s accompanied by mesial temporal sclerosis (TLE-MTS) from temporal lobe epilepsy without the sclerosis. That conclusion was dreached by researchers who compared 34 TLE patients to 34 controls who were matched for age and gender and in whom the presence of mesial temporal sclerosis was definitively established by means of histologic examination of surgical tissue. More specifically, the investigators found that the fractional amplitude of low-frequency fluctuations (fALFF) in the blood oxygen level-dependent resting state fMRI was reduced in the ipsilateral amygdala and hippocampus among TLE patients with mesial temporal sclerosis. By contrast, among TLE patients without sclerosis, there was only marginally reduced fALFF in the ipsilateral amygdala but none in the hippocampus.
Reyes A, Thesen D, Wang X, Hahn D, Yoo D, Kuzniecky R et al. Resting-state functional MRI distinguishes temporal lobe epilepsy subtypes. Epilepsia. 2016;57(9):1475-1484.
Performing resting state functioning MRIs can help distinguish temporal lobe epilepsy that’s accompanied by mesial temporal sclerosis (TLE-MTS) from temporal lobe epilepsy without the sclerosis. That conclusion was dreached by researchers who compared 34 TLE patients to 34 controls who were matched for age and gender and in whom the presence of mesial temporal sclerosis was definitively established by means of histologic examination of surgical tissue. More specifically, the investigators found that the fractional amplitude of low-frequency fluctuations (fALFF) in the blood oxygen level-dependent resting state fMRI was reduced in the ipsilateral amygdala and hippocampus among TLE patients with mesial temporal sclerosis. By contrast, among TLE patients without sclerosis, there was only marginally reduced fALFF in the ipsilateral amygdala but none in the hippocampus.
Reyes A, Thesen D, Wang X, Hahn D, Yoo D, Kuzniecky R et al. Resting-state functional MRI distinguishes temporal lobe epilepsy subtypes. Epilepsia. 2016;57(9):1475-1484.
Performing resting state functioning MRIs can help distinguish temporal lobe epilepsy that’s accompanied by mesial temporal sclerosis (TLE-MTS) from temporal lobe epilepsy without the sclerosis. That conclusion was dreached by researchers who compared 34 TLE patients to 34 controls who were matched for age and gender and in whom the presence of mesial temporal sclerosis was definitively established by means of histologic examination of surgical tissue. More specifically, the investigators found that the fractional amplitude of low-frequency fluctuations (fALFF) in the blood oxygen level-dependent resting state fMRI was reduced in the ipsilateral amygdala and hippocampus among TLE patients with mesial temporal sclerosis. By contrast, among TLE patients without sclerosis, there was only marginally reduced fALFF in the ipsilateral amygdala but none in the hippocampus.
Reyes A, Thesen D, Wang X, Hahn D, Yoo D, Kuzniecky R et al. Resting-state functional MRI distinguishes temporal lobe epilepsy subtypes. Epilepsia. 2016;57(9):1475-1484.
Open Payments: Few dermatologists have significant conflicts of interest
A total of 8,333 dermatologists – 73% of those practicing in the United States – received $34.8 million from industry in 2014, mostly from pharmaceutical companies, according to an Oct. 5 report in JAMA Dermatology.
The bulk of the money went to a fraction of the dermatologists. Just 10% – 833 – collected $31.2 million. Eighty-three dermatologists – 1% of the total – pulled in about $15 million, each receiving at least $93,622. The 10 highest-paid dermatologists were mostly in private practice, not academia, and three were women (JAMA Dermatol. 2016 Oct 5. doi: 10.1001/jamadermatol.2016.3037).
Speaker fees accounted for 32% of the total payment amount, consulting fees for 22%, research payments for 17%, and food and beverage payments for 13%. Lesser amounts went towards travel and honoraria, among other things.
Almost $29 million came from pharmaceutical companies. AbbVie and Allergan led the way with payments of nearly $6 million each. Pharmaceutical company largesse is “not surprising” since companies have “financial incentives to promote their medications,” and having “thought leaders being advocates and spokespersons may help shift clinical practices,” said lead investigator Hao Feng, MD, a dermatology resident at New York University, and his colleagues. Recent studies “show that receipt of industry payments and industry-sponsored meals was associated with an increased rate of prescribing several class[es] of brand-name medications.”
The data come from the CMS Open Payments database, which records industry payments to physicians and is searchable by name. The investigators analyzed data from 2014 because it was the first year with a full 12 months of data.
For most dermatologists, industry payments didn’t amount to much: the overall median payment was $298, and 63% received less than $500. Almost 80% of the 208,613 payments in 2014 were for $50 or less.
The investigators were careful to note that industry payments are common in other specialties as well, with most of the money going to a select few. Dermatologists received 0.5% of the $6.5 billion that companies paid to U.S. physicians in 2014. Companies paid U.S. physicians $7.52 billion in 2015, according to CMS.
The Open Payments database was launched as part of the Affordable Care Act in the belief that transparency would combat the untoward effects of commercial interests and conflicts of interest on patient care, but it doesn’t catch everything. CMS only requires companies that make government-reimbursed products to report payments. For dermatologists, that means, for example, laser and other cosmetic device companies are exempt. Physicians also can direct payments to third parties. Such issues led Dr. Feng and his associates to conclude that CMS captures “only a fraction of … physician-industry financial relationships.”
Another problem, they said, is that CMS does not judge between industry payments that advance science and help patients and those that are “harmful,” so the database does little to counter public concerns about “dishonesty and selfishness” when doctors deal with industry. “Ultimately, the impact of financial disclosure from industry to dermatologists, and physicians in general, remains to be seen,” they wrote.
The investigators did not report any conflicts of interest.
Transparency for physician interactions with industry is important, especially given the findings showing individual consulting payments to dermatologists are as high as $249,643. However, the roll out of the Open Payments program has frustrated physicians and failed to provide the public with information of sufficient accuracy and meaning to use to make fair conclusions.
Some straightforward changes would substantially improve the situation. Physicians should have the opportunity to preview data before manufacturers transmit it to the CMS. The administrative burden inherent in the current CMS data review portal and the dispute process should be reduced. A common reporting method, including very clear definitions of meaningful categories of payments, should be standardized across companies. The CMS should issue clear guidance that reduces fear among manufacturers and decreases overreporting.
Jack Resneck Jr., MD, is professor and vice-chair of dermatology at the University of California, San Francisco. He serves on the American Medical Association Board of Trustees and made his comments in an editorial (JAMA Dermatol. 5 Oct. 2016).
Transparency for physician interactions with industry is important, especially given the findings showing individual consulting payments to dermatologists are as high as $249,643. However, the roll out of the Open Payments program has frustrated physicians and failed to provide the public with information of sufficient accuracy and meaning to use to make fair conclusions.
Some straightforward changes would substantially improve the situation. Physicians should have the opportunity to preview data before manufacturers transmit it to the CMS. The administrative burden inherent in the current CMS data review portal and the dispute process should be reduced. A common reporting method, including very clear definitions of meaningful categories of payments, should be standardized across companies. The CMS should issue clear guidance that reduces fear among manufacturers and decreases overreporting.
Jack Resneck Jr., MD, is professor and vice-chair of dermatology at the University of California, San Francisco. He serves on the American Medical Association Board of Trustees and made his comments in an editorial (JAMA Dermatol. 5 Oct. 2016).
Transparency for physician interactions with industry is important, especially given the findings showing individual consulting payments to dermatologists are as high as $249,643. However, the roll out of the Open Payments program has frustrated physicians and failed to provide the public with information of sufficient accuracy and meaning to use to make fair conclusions.
Some straightforward changes would substantially improve the situation. Physicians should have the opportunity to preview data before manufacturers transmit it to the CMS. The administrative burden inherent in the current CMS data review portal and the dispute process should be reduced. A common reporting method, including very clear definitions of meaningful categories of payments, should be standardized across companies. The CMS should issue clear guidance that reduces fear among manufacturers and decreases overreporting.
Jack Resneck Jr., MD, is professor and vice-chair of dermatology at the University of California, San Francisco. He serves on the American Medical Association Board of Trustees and made his comments in an editorial (JAMA Dermatol. 5 Oct. 2016).
A total of 8,333 dermatologists – 73% of those practicing in the United States – received $34.8 million from industry in 2014, mostly from pharmaceutical companies, according to an Oct. 5 report in JAMA Dermatology.
The bulk of the money went to a fraction of the dermatologists. Just 10% – 833 – collected $31.2 million. Eighty-three dermatologists – 1% of the total – pulled in about $15 million, each receiving at least $93,622. The 10 highest-paid dermatologists were mostly in private practice, not academia, and three were women (JAMA Dermatol. 2016 Oct 5. doi: 10.1001/jamadermatol.2016.3037).
Speaker fees accounted for 32% of the total payment amount, consulting fees for 22%, research payments for 17%, and food and beverage payments for 13%. Lesser amounts went towards travel and honoraria, among other things.
Almost $29 million came from pharmaceutical companies. AbbVie and Allergan led the way with payments of nearly $6 million each. Pharmaceutical company largesse is “not surprising” since companies have “financial incentives to promote their medications,” and having “thought leaders being advocates and spokespersons may help shift clinical practices,” said lead investigator Hao Feng, MD, a dermatology resident at New York University, and his colleagues. Recent studies “show that receipt of industry payments and industry-sponsored meals was associated with an increased rate of prescribing several class[es] of brand-name medications.”
The data come from the CMS Open Payments database, which records industry payments to physicians and is searchable by name. The investigators analyzed data from 2014 because it was the first year with a full 12 months of data.
For most dermatologists, industry payments didn’t amount to much: the overall median payment was $298, and 63% received less than $500. Almost 80% of the 208,613 payments in 2014 were for $50 or less.
The investigators were careful to note that industry payments are common in other specialties as well, with most of the money going to a select few. Dermatologists received 0.5% of the $6.5 billion that companies paid to U.S. physicians in 2014. Companies paid U.S. physicians $7.52 billion in 2015, according to CMS.
The Open Payments database was launched as part of the Affordable Care Act in the belief that transparency would combat the untoward effects of commercial interests and conflicts of interest on patient care, but it doesn’t catch everything. CMS only requires companies that make government-reimbursed products to report payments. For dermatologists, that means, for example, laser and other cosmetic device companies are exempt. Physicians also can direct payments to third parties. Such issues led Dr. Feng and his associates to conclude that CMS captures “only a fraction of … physician-industry financial relationships.”
Another problem, they said, is that CMS does not judge between industry payments that advance science and help patients and those that are “harmful,” so the database does little to counter public concerns about “dishonesty and selfishness” when doctors deal with industry. “Ultimately, the impact of financial disclosure from industry to dermatologists, and physicians in general, remains to be seen,” they wrote.
The investigators did not report any conflicts of interest.
A total of 8,333 dermatologists – 73% of those practicing in the United States – received $34.8 million from industry in 2014, mostly from pharmaceutical companies, according to an Oct. 5 report in JAMA Dermatology.
The bulk of the money went to a fraction of the dermatologists. Just 10% – 833 – collected $31.2 million. Eighty-three dermatologists – 1% of the total – pulled in about $15 million, each receiving at least $93,622. The 10 highest-paid dermatologists were mostly in private practice, not academia, and three were women (JAMA Dermatol. 2016 Oct 5. doi: 10.1001/jamadermatol.2016.3037).
Speaker fees accounted for 32% of the total payment amount, consulting fees for 22%, research payments for 17%, and food and beverage payments for 13%. Lesser amounts went towards travel and honoraria, among other things.
Almost $29 million came from pharmaceutical companies. AbbVie and Allergan led the way with payments of nearly $6 million each. Pharmaceutical company largesse is “not surprising” since companies have “financial incentives to promote their medications,” and having “thought leaders being advocates and spokespersons may help shift clinical practices,” said lead investigator Hao Feng, MD, a dermatology resident at New York University, and his colleagues. Recent studies “show that receipt of industry payments and industry-sponsored meals was associated with an increased rate of prescribing several class[es] of brand-name medications.”
The data come from the CMS Open Payments database, which records industry payments to physicians and is searchable by name. The investigators analyzed data from 2014 because it was the first year with a full 12 months of data.
For most dermatologists, industry payments didn’t amount to much: the overall median payment was $298, and 63% received less than $500. Almost 80% of the 208,613 payments in 2014 were for $50 or less.
The investigators were careful to note that industry payments are common in other specialties as well, with most of the money going to a select few. Dermatologists received 0.5% of the $6.5 billion that companies paid to U.S. physicians in 2014. Companies paid U.S. physicians $7.52 billion in 2015, according to CMS.
The Open Payments database was launched as part of the Affordable Care Act in the belief that transparency would combat the untoward effects of commercial interests and conflicts of interest on patient care, but it doesn’t catch everything. CMS only requires companies that make government-reimbursed products to report payments. For dermatologists, that means, for example, laser and other cosmetic device companies are exempt. Physicians also can direct payments to third parties. Such issues led Dr. Feng and his associates to conclude that CMS captures “only a fraction of … physician-industry financial relationships.”
Another problem, they said, is that CMS does not judge between industry payments that advance science and help patients and those that are “harmful,” so the database does little to counter public concerns about “dishonesty and selfishness” when doctors deal with industry. “Ultimately, the impact of financial disclosure from industry to dermatologists, and physicians in general, remains to be seen,” they wrote.
The investigators did not report any conflicts of interest.
Key clinical point:
Major finding: The bulk of the money went to 833 dermatologists who collected $31.2 million in 2014, mostly from pharmaceutical companies.
Data source: Review of the CMS Open Payments database
Disclosures: The investigators had no disclosures.
Tips for Sleep Hygiene
Click here to download the PDF.
Click here to download the PDF.
Click here to download the PDF.
VIDEO: The ‘artificial’ divide between biology and psychology
NEW YORK – “All psychology works through biology,” said the new head of the National Institute of Mental Health, Joshua A. Gordon, MD, PhD. “The divide is artificial at the level of neurocircuits.” All treatments for mental illness, from antidepressants to psychotherapy to emerging therapies, can be viewed through that lens.
In this video interview, conducted just days before he stepped into his new role, Dr. Gordon discusses how this biological view of the mind and brain will inform his approach to use of the Research Domain Criteria (RDoC) when reviewing grant applications, a process he said he is aware some researchers still resist.
“One thing that is important is that we really try to quantify and objectively evaluate behavior in the way that RDoC tries to do. RDoC essentially is a way to try to categorize behavior into its component building blocks, and then try to understand, yes, the biology underneath it,” Dr. Gordon said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @whitneymcknight
NEW YORK – “All psychology works through biology,” said the new head of the National Institute of Mental Health, Joshua A. Gordon, MD, PhD. “The divide is artificial at the level of neurocircuits.” All treatments for mental illness, from antidepressants to psychotherapy to emerging therapies, can be viewed through that lens.
In this video interview, conducted just days before he stepped into his new role, Dr. Gordon discusses how this biological view of the mind and brain will inform his approach to use of the Research Domain Criteria (RDoC) when reviewing grant applications, a process he said he is aware some researchers still resist.
“One thing that is important is that we really try to quantify and objectively evaluate behavior in the way that RDoC tries to do. RDoC essentially is a way to try to categorize behavior into its component building blocks, and then try to understand, yes, the biology underneath it,” Dr. Gordon said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @whitneymcknight
NEW YORK – “All psychology works through biology,” said the new head of the National Institute of Mental Health, Joshua A. Gordon, MD, PhD. “The divide is artificial at the level of neurocircuits.” All treatments for mental illness, from antidepressants to psychotherapy to emerging therapies, can be viewed through that lens.
In this video interview, conducted just days before he stepped into his new role, Dr. Gordon discusses how this biological view of the mind and brain will inform his approach to use of the Research Domain Criteria (RDoC) when reviewing grant applications, a process he said he is aware some researchers still resist.
“One thing that is important is that we really try to quantify and objectively evaluate behavior in the way that RDoC tries to do. RDoC essentially is a way to try to categorize behavior into its component building blocks, and then try to understand, yes, the biology underneath it,” Dr. Gordon said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @whitneymcknight
Emergency Imaging: Acute abdominal pain
An 89-year-old woman with a history of coronary artery disease, diabetes mellitus, hypertension, chronic constipation, and glaucoma presented to the ED for evaluation of chest pain and headache. Upon arrival at the ED, the patient also began to experience unrelenting abdominal pain. Abdominal examination showed mild tenderness in the right lower quadrant upon palpation. An abdominal radiograph and a computed tomography (CT) scan were ordered; representative images are presented above (Figure 1a-1d).
What is the diagnosis? What is the preferred management for this patient?
Answer
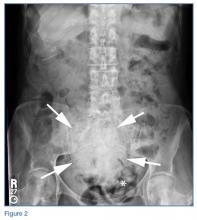
The abdominal radiograph showed no evidence of bowel obstruction. There was, however, a round area of increased density in the pelvis, suggesting the presence of a soft-tissue mass (white arrows, Figure 2) directly adjacent to the sigmoid colon (white asterisk, Figure 2).
Giant Colonic Diverticula
Giant colonic diverticula (GCD) are diverticula larger than 4 cm. This is a rare manifestation of diverticular disease of the bowel and most commonly occurs within the sigmoid colon. The majority of patients who develop GCD are older than age 60 years.1
The clinical presentation of GCD is nonspecific but can include abdominal pain, vomiting, nausea, and fever in the acute setting.2 Chronic presentations of GCD include intermittent abdominal pain, bloating, and constipation. In two-thirds of patients, a palpable abdominal mass is found on physical examination.3
Diagnosis
Due to the nonspecific presentation of GCD, imaging studies are typically required for diagnosis. Although radiographs may show a dilated air-filled structure in the abdomen, differentiation from a normal air-filled bowel may be difficult. Computed tomography is the imaging modality of choice based on its ability to demonstrate the presence of a smooth-walled gas-containing structure that communicates with the bowel lumen. In addition, CT has the ability to visualize the fluid and stool that are often present within the diverticulum. In cases of acute inflammation, diverticular wall thickening also may be present on CT.
Though no longer routinely used, barium enema is another option for diagnosing GCD because it can also demonstrate communication between the giant diverticula and the bowel lumen. However, barium enema is not often used in the emergency setting due to an increased risk of perforation and peritonitis.1
Management
Complications caused by GCD occur in 15% to 35% of cases and most commonly include perforation with associated peritonitis and abscess formation.4 Due to associated morbidity, the preferred treatment is surgical management—even when GCD is found incidentally in asymptomatic patients. In uncomplicated cases, surgical resection of the diverticulum and adjacent colon is performed with primary colic anastomosis. In some cases, a diverting ileostomy is created. In the presence of perforation and/or abscess, percutaneous catheter drainage and two-stage colectomy with colostomy typically is performed.5
1. Zeina AR, Mahamid A, Nachtigal A, Ashkenazi I, Shapira-Rootman M. Giant colonic diverticulum: radiographic and MDCT characteristics. Insights Imaging. 2015;6(6):659-664. doi: 10.1007/s13244-015-0433-x.
2. Custer TJ, Blevins DV, Vara TM. Giant colonic diverticulum: a rare manifestation of a common disease. J Gastrointest Surg. 1999;3(5):543-548.
3. de Oliveira NC, Welch JP. Giant diverticula of the colon: a clinical assessment. Am J Gastroenterol. 1997;92(7):1092-1096.
4. Majeski J, Durst G Jr. Obstructing giant colonic diverticulum. South Med J. 2000;93(8):797-799.
5. Nigri G, Petrucciani N, Giannini G, et al. Giant colonic diverticulum: clinical presentation, diagnosis and treatment: systematic review of 166 cases. World J Gastroenterol. 2015;21(1):360-368. doi: 10.3748/wjg.v21.i1.360.
An 89-year-old woman with a history of coronary artery disease, diabetes mellitus, hypertension, chronic constipation, and glaucoma presented to the ED for evaluation of chest pain and headache. Upon arrival at the ED, the patient also began to experience unrelenting abdominal pain. Abdominal examination showed mild tenderness in the right lower quadrant upon palpation. An abdominal radiograph and a computed tomography (CT) scan were ordered; representative images are presented above (Figure 1a-1d).
What is the diagnosis? What is the preferred management for this patient?
Answer
The abdominal radiograph showed no evidence of bowel obstruction. There was, however, a round area of increased density in the pelvis, suggesting the presence of a soft-tissue mass (white arrows, Figure 2) directly adjacent to the sigmoid colon (white asterisk, Figure 2).
Giant Colonic Diverticula
Giant colonic diverticula (GCD) are diverticula larger than 4 cm. This is a rare manifestation of diverticular disease of the bowel and most commonly occurs within the sigmoid colon. The majority of patients who develop GCD are older than age 60 years.1
The clinical presentation of GCD is nonspecific but can include abdominal pain, vomiting, nausea, and fever in the acute setting.2 Chronic presentations of GCD include intermittent abdominal pain, bloating, and constipation. In two-thirds of patients, a palpable abdominal mass is found on physical examination.3
Diagnosis
Due to the nonspecific presentation of GCD, imaging studies are typically required for diagnosis. Although radiographs may show a dilated air-filled structure in the abdomen, differentiation from a normal air-filled bowel may be difficult. Computed tomography is the imaging modality of choice based on its ability to demonstrate the presence of a smooth-walled gas-containing structure that communicates with the bowel lumen. In addition, CT has the ability to visualize the fluid and stool that are often present within the diverticulum. In cases of acute inflammation, diverticular wall thickening also may be present on CT.
Though no longer routinely used, barium enema is another option for diagnosing GCD because it can also demonstrate communication between the giant diverticula and the bowel lumen. However, barium enema is not often used in the emergency setting due to an increased risk of perforation and peritonitis.1
Management
Complications caused by GCD occur in 15% to 35% of cases and most commonly include perforation with associated peritonitis and abscess formation.4 Due to associated morbidity, the preferred treatment is surgical management—even when GCD is found incidentally in asymptomatic patients. In uncomplicated cases, surgical resection of the diverticulum and adjacent colon is performed with primary colic anastomosis. In some cases, a diverting ileostomy is created. In the presence of perforation and/or abscess, percutaneous catheter drainage and two-stage colectomy with colostomy typically is performed.5
An 89-year-old woman with a history of coronary artery disease, diabetes mellitus, hypertension, chronic constipation, and glaucoma presented to the ED for evaluation of chest pain and headache. Upon arrival at the ED, the patient also began to experience unrelenting abdominal pain. Abdominal examination showed mild tenderness in the right lower quadrant upon palpation. An abdominal radiograph and a computed tomography (CT) scan were ordered; representative images are presented above (Figure 1a-1d).
What is the diagnosis? What is the preferred management for this patient?
Answer
The abdominal radiograph showed no evidence of bowel obstruction. There was, however, a round area of increased density in the pelvis, suggesting the presence of a soft-tissue mass (white arrows, Figure 2) directly adjacent to the sigmoid colon (white asterisk, Figure 2).
Giant Colonic Diverticula
Giant colonic diverticula (GCD) are diverticula larger than 4 cm. This is a rare manifestation of diverticular disease of the bowel and most commonly occurs within the sigmoid colon. The majority of patients who develop GCD are older than age 60 years.1
The clinical presentation of GCD is nonspecific but can include abdominal pain, vomiting, nausea, and fever in the acute setting.2 Chronic presentations of GCD include intermittent abdominal pain, bloating, and constipation. In two-thirds of patients, a palpable abdominal mass is found on physical examination.3
Diagnosis
Due to the nonspecific presentation of GCD, imaging studies are typically required for diagnosis. Although radiographs may show a dilated air-filled structure in the abdomen, differentiation from a normal air-filled bowel may be difficult. Computed tomography is the imaging modality of choice based on its ability to demonstrate the presence of a smooth-walled gas-containing structure that communicates with the bowel lumen. In addition, CT has the ability to visualize the fluid and stool that are often present within the diverticulum. In cases of acute inflammation, diverticular wall thickening also may be present on CT.
Though no longer routinely used, barium enema is another option for diagnosing GCD because it can also demonstrate communication between the giant diverticula and the bowel lumen. However, barium enema is not often used in the emergency setting due to an increased risk of perforation and peritonitis.1
Management
Complications caused by GCD occur in 15% to 35% of cases and most commonly include perforation with associated peritonitis and abscess formation.4 Due to associated morbidity, the preferred treatment is surgical management—even when GCD is found incidentally in asymptomatic patients. In uncomplicated cases, surgical resection of the diverticulum and adjacent colon is performed with primary colic anastomosis. In some cases, a diverting ileostomy is created. In the presence of perforation and/or abscess, percutaneous catheter drainage and two-stage colectomy with colostomy typically is performed.5
1. Zeina AR, Mahamid A, Nachtigal A, Ashkenazi I, Shapira-Rootman M. Giant colonic diverticulum: radiographic and MDCT characteristics. Insights Imaging. 2015;6(6):659-664. doi: 10.1007/s13244-015-0433-x.
2. Custer TJ, Blevins DV, Vara TM. Giant colonic diverticulum: a rare manifestation of a common disease. J Gastrointest Surg. 1999;3(5):543-548.
3. de Oliveira NC, Welch JP. Giant diverticula of the colon: a clinical assessment. Am J Gastroenterol. 1997;92(7):1092-1096.
4. Majeski J, Durst G Jr. Obstructing giant colonic diverticulum. South Med J. 2000;93(8):797-799.
5. Nigri G, Petrucciani N, Giannini G, et al. Giant colonic diverticulum: clinical presentation, diagnosis and treatment: systematic review of 166 cases. World J Gastroenterol. 2015;21(1):360-368. doi: 10.3748/wjg.v21.i1.360.
1. Zeina AR, Mahamid A, Nachtigal A, Ashkenazi I, Shapira-Rootman M. Giant colonic diverticulum: radiographic and MDCT characteristics. Insights Imaging. 2015;6(6):659-664. doi: 10.1007/s13244-015-0433-x.
2. Custer TJ, Blevins DV, Vara TM. Giant colonic diverticulum: a rare manifestation of a common disease. J Gastrointest Surg. 1999;3(5):543-548.
3. de Oliveira NC, Welch JP. Giant diverticula of the colon: a clinical assessment. Am J Gastroenterol. 1997;92(7):1092-1096.
4. Majeski J, Durst G Jr. Obstructing giant colonic diverticulum. South Med J. 2000;93(8):797-799.
5. Nigri G, Petrucciani N, Giannini G, et al. Giant colonic diverticulum: clinical presentation, diagnosis and treatment: systematic review of 166 cases. World J Gastroenterol. 2015;21(1):360-368. doi: 10.3748/wjg.v21.i1.360.