User login
Clinical Challenges - October 2016: Boerhaave’s syndrome (spontaneous rupture of the esophagus)
What's Your Diagnosis?
The diagnosis
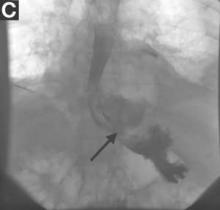
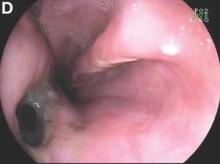
Gastrografin swallow (Figure C) demonstrated rupture of the distal esophagus, with leakage of gastrografin into the mediastinum (arrow). Upper gastrointestinal endoscopy confirmed rupture of the left posterolateral wall of the distal esophagus consistent with Boerhaave’s syndrome (Figure D), and a self-expanding covered metal stent was placed.
Broad-spectrum antibiotics and nasogastric feeding were commenced, and the left pleural effusion drained with a tube thoracostomy. Unfortunately, despite initial improvement, the patient subsequently deteriorated and died 30 days after admission.
Boerhaave’s is a rare clinical entity defined as spontaneous esophageal rupture, excluding perforations resulting from foreign bodies or iatrogenic instrumentation.
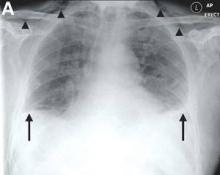
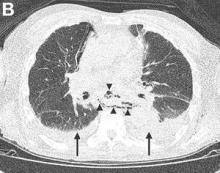
Mackler’s triad of vomiting, lower chest pain, and subcutaneous emphysema is the classical presentation but is seen in only a minority of cases; thus, diagnostic errors are common.2 Importantly, the chest radiograph is almost always abnormal, with pleural effusions or pneumomediastinum often seen.3 Surgical repair is the definitive treatment, but in patients considered unfit for surgery, conservative or endoscopic management is advocated. Mortality remains greater than 30%, and rises sharply if diagnosis is delayed,2 emphasizing the importance of awareness of this unusual diagnosis.
References
1. Lucendo, A.J., Fringal-Ruiz, A.B., Rodriguez, B. Boerhaave’s syndrome as the primary manifestation of adult eosinophilic esophagitis. (Two case reports and a review of the literature.) Dis Esophagus. 2011 Feb;24:E11-5.
2. Brauer, R.B., Liebermann-Meffert, D., Stein, H.J., et al. Boerhaave’s syndrome: Analysis of the literature and report of 18 new cases. Dis Esophagus. 1997 Jan;10:64-8.
3. Pate, J.W., Walker, W.A., Cole, F.H. Jr, et al. Spontaneous rupture of the esophagus: a 30-year experience. Ann Thorac Surg. 1989 May;47:689-92.
The diagnosis
Gastrografin swallow (Figure C) demonstrated rupture of the distal esophagus, with leakage of gastrografin into the mediastinum (arrow). Upper gastrointestinal endoscopy confirmed rupture of the left posterolateral wall of the distal esophagus consistent with Boerhaave’s syndrome (Figure D), and a self-expanding covered metal stent was placed.
Broad-spectrum antibiotics and nasogastric feeding were commenced, and the left pleural effusion drained with a tube thoracostomy. Unfortunately, despite initial improvement, the patient subsequently deteriorated and died 30 days after admission.
Boerhaave’s is a rare clinical entity defined as spontaneous esophageal rupture, excluding perforations resulting from foreign bodies or iatrogenic instrumentation.
Mackler’s triad of vomiting, lower chest pain, and subcutaneous emphysema is the classical presentation but is seen in only a minority of cases; thus, diagnostic errors are common.2 Importantly, the chest radiograph is almost always abnormal, with pleural effusions or pneumomediastinum often seen.3 Surgical repair is the definitive treatment, but in patients considered unfit for surgery, conservative or endoscopic management is advocated. Mortality remains greater than 30%, and rises sharply if diagnosis is delayed,2 emphasizing the importance of awareness of this unusual diagnosis.
References
1. Lucendo, A.J., Fringal-Ruiz, A.B., Rodriguez, B. Boerhaave’s syndrome as the primary manifestation of adult eosinophilic esophagitis. (Two case reports and a review of the literature.) Dis Esophagus. 2011 Feb;24:E11-5.
2. Brauer, R.B., Liebermann-Meffert, D., Stein, H.J., et al. Boerhaave’s syndrome: Analysis of the literature and report of 18 new cases. Dis Esophagus. 1997 Jan;10:64-8.
3. Pate, J.W., Walker, W.A., Cole, F.H. Jr, et al. Spontaneous rupture of the esophagus: a 30-year experience. Ann Thorac Surg. 1989 May;47:689-92.
The diagnosis
Gastrografin swallow (Figure C) demonstrated rupture of the distal esophagus, with leakage of gastrografin into the mediastinum (arrow). Upper gastrointestinal endoscopy confirmed rupture of the left posterolateral wall of the distal esophagus consistent with Boerhaave’s syndrome (Figure D), and a self-expanding covered metal stent was placed.
Broad-spectrum antibiotics and nasogastric feeding were commenced, and the left pleural effusion drained with a tube thoracostomy. Unfortunately, despite initial improvement, the patient subsequently deteriorated and died 30 days after admission.
Boerhaave’s is a rare clinical entity defined as spontaneous esophageal rupture, excluding perforations resulting from foreign bodies or iatrogenic instrumentation.
Mackler’s triad of vomiting, lower chest pain, and subcutaneous emphysema is the classical presentation but is seen in only a minority of cases; thus, diagnostic errors are common.2 Importantly, the chest radiograph is almost always abnormal, with pleural effusions or pneumomediastinum often seen.3 Surgical repair is the definitive treatment, but in patients considered unfit for surgery, conservative or endoscopic management is advocated. Mortality remains greater than 30%, and rises sharply if diagnosis is delayed,2 emphasizing the importance of awareness of this unusual diagnosis.
References
1. Lucendo, A.J., Fringal-Ruiz, A.B., Rodriguez, B. Boerhaave’s syndrome as the primary manifestation of adult eosinophilic esophagitis. (Two case reports and a review of the literature.) Dis Esophagus. 2011 Feb;24:E11-5.
2. Brauer, R.B., Liebermann-Meffert, D., Stein, H.J., et al. Boerhaave’s syndrome: Analysis of the literature and report of 18 new cases. Dis Esophagus. 1997 Jan;10:64-8.
3. Pate, J.W., Walker, W.A., Cole, F.H. Jr, et al. Spontaneous rupture of the esophagus: a 30-year experience. Ann Thorac Surg. 1989 May;47:689-92.
What's Your Diagnosis?
What's Your Diagnosis?
What's Your Diagnosis?
By Thomas P. Chapman, MD, David A. Gorard, MBBS, MD, and Emily A. Johns, MD. Published previously in Gastroenterology (2012;143:1438, 1692).
An 84-year-old man presented to the emergency department with acute left-sided chest pain, after a recent diarrheal and vomiting illness. He had a background of severe Alzheimer’s dementia and was a resident in a care home. On arrival in the emergency department, he was unable to give a clear history and was distressed by the chest pain.
Why Required Pediatric Hospital Medicine Fellowships Are Unnecessary
The Joint Council of Pediatric Hospital Medicine (JCPHM), successor to the Strategic Planning (STP) Committee, recently recommended submitting a petition for two-year pediatric hospital medicine (PHM) fellowship certification to the American Board of Pediatrics (ABP), which was completed in 2014. In December 2015, the ABP Board of Directors voted to (1) approve the proposal for a two-year PHM fellowship incorporating scholarly activity with the provision that entrustable professional activities (EPAs) be used as the framework for assessing competencies and (2) not require those who achieve and maintain PHM certification to maintain general pediatrics certification. The proposal for certification of a two-year PHM fellowship will now be submitted to the American Board of Medical Specialties (ABMS). Concerns regarding the formal certification of PHM as an ABMS-recognized subspecialty have been raised by many stakeholders, including community pediatric hospitalists, pediatric residency program directors, and med-peds physicians.
We feel that the “first, do no harm” guiding principle seems to have been forgotten by the ABP as it attempts to formalize the training of pediatric hospitalists. In December 2015, the ABP voted in favor of a two-year ACGME-accredited PHM fellowship. The intent was to “assure the best care of hospitalized children,” “assure the public,” “accelerate improvements and innovation in quality improvement,” and “raise the level of care of all hospitalized children by establishing best practices in clinical care.” To be clear, these goals are shared by all of us (although there is no indication that the public is seeking additional assurance). Prior to launching broad-scale, time-intensive, and financially costly initiatives, we should ensure that our efforts would achieve—rather than obstruct—their intended aims. In addition to a lack of evidence supporting that subspecialty certification will advance our path toward achieving these goals, there are numerous reasons a required PHM fellowship is unnecessary and potentially even harmful to the hospitalist workforce. The negative unintended consequences need to be weighed heavily.
We have found no data to support that children would receive inferior inpatient care from pediatric hospitalists due to lack of formal certification. Hospital medicine physicians are paving the way in quality improvement, high-value care, medical education, palliative care, and global health, supported in part through training in various non-accredited hospital medicine fellowships. There is nothing stopping pediatric hospitalists from establishing and disseminating best practices in clinical care. Hospitalists are already making strides in providing high-quality care at low costs, as demonstrated by the abundant PHM scholarly work described in the ABP application to the ABMS. The alleged problem of needing to build trust within the community is yet to be demonstrated, as we have leaders at local, regional, and national levels. The chief medical officer of the Centers for Medicare & Medicaid Services is a hospitalist as is our surgeon general. Hospital medicine is the fastest-growing specialty in the history of medicine,1 and we should seek to propel rather than fetter our future colleagues.
Below are our reasons for opposing this formal certification.
We already have a fellowship system.
As we all know, advanced training opportunities already exist for those interested in pursuing extra research and quality improvement training. Similar to other pediatric subspecialty fellowships, these PHM fellowships are undersubscribed (20% of PHM fellowships did not fill in 2016),2 with the majority of graduating pediatric residents transitioning to hospitalists opting not to pursue fellowship training. We should continue to let graduating pediatric residents vote with their feet without the undue influence of subspecialty certification.
Subspecialization has opportunity costs that may reduce the PHM pipeline.
Even if we assume an adequate number of fellowship programs could be developed and funded, our fear is that the decision to turn PHM into an accredited subspecialty could paradoxically reduce the pipeline of inpatient providers. Residency is already a three- to four-year endeavor (pediatrics and med-peds) that is poorly compensated and time-intensive. In the absence of evidence supporting the value of additional training, tacking on another two years seems unreasonable in the face of the student loan debt crisis, reduced compensation, and lost time for career advancement. These are significant opportunity costs. While most specialties lead to a significant pay raise to compensate for the added training time, pediatrics remains the lowest-paid physician specialty.3 Should PHM follow the trend of most pediatric subspecialties, pursuit of fellowship training would be a negative financial decision for residency graduates.4 For the health system, increasing debt-to-income ratios runs the risk of creating a medical education bubble market.5
More than 25% of med-peds graduates pursue careers in hospital medicine, a percentage that continues to grow, accounting for more than 100 new hospitalists per year.6 As a result, med-peds-trained hospitalists constitute more than 10% of the pediatric hospitalist workforce.6 Requiring PHM fellowship training may reduce this crucial pipeline of practitioners. In a 2014 unpublished survey of 225 med-peds practitioners, 78% of residents and 96% of attendings responded that they would not consider pursuing an ACGME-accredited PHM fellowship.7 This is compounded by a lack of parity with the practice of adult hospital medicine both in compensation and required training and is heightened by the fact that the training in question does not incorporate care for adult patients. There is clear consensus by 96% of med-peds hospitalists that the creation of an ACGME-certified PHM subspecialty will negatively affect the likelihood of med-peds providers pursuing PHM.7
Certification will pose a potential risk to specific patient populations.
We are also concerned that a reduced PHM workforce could disproportionately impact young adults with special healthcare needs and those children cared for in rural or community-based hospitals. Med-peds training equips providers to care for children with chronic diseases that then transition into adulthood; more than 25% provide care for young adults with special healthcare needs.6 With the increasing number of children with chronic health conditions surviving into adulthood,8 med-peds hospitalists serve essential roles in providing care and coordination for this vulnerable population. Furthermore, hospital medicine groups in medical systems that cannot support a full-time categorical pediatric hospitalist tend to employ med-peds physicians or family practitioners. Concerns with PHM certification are thus extended to those family medicine physicians who practice PHM.
Pediatric residency trains pediatricians in inpatient care.
We feel that the decision to move forward on PHM subspecialty certification calls into question the value of pediatric residency training. There is no evidence that clinical inpatient training in pediatrics residency is inadequate. If one leaves residency trained to do anything, it is practicing hospital medicine. A significant portion of residency takes place inpatient, both on wards and in the intensive care units. The 2009 ABP Foundation–funded study of PHM reported that 94% of pediatric hospitalist respondents rated their training in general clinical skills during residency as fully adequate, 85% rated their training in communication skills as fully adequate, and 73% did not believe any additional training beyond residency should be required.9 With respect to med-peds graduates, more than 90% feel equipped to care for children and adults upon residency completion.10 If the ABMS carries forward with this decision, the only clinical work one would be “certified” to do after residency is primary care. However, after completion of residency training, most of us feel at least as comfortable, if not more comfortable, caring for children in the inpatient setting.
Primary care should require subspecialty certification as well.
Furthermore, the decision to create a certified subspecialty begs the question as to why fellowship should not be mandated for those entering the field of primary care. Does the field of primary care not require research to move it forward? Does the field of primary care not require providers who can adeptly apply quality improvement methodologies to improve primary-care delivery? Does the public not require the same type of assurance? By these measures, primary care should require subspecialty certification as well. These arguments could easily be construed as an indictment of residency training.
The target should be residency training.
The PHM ABMS application describes a clinical curriculum consisting of eight core clinical rotations in various settings. That small number emphasizes the fact that extra clinical training is really not needed and that we do not require a complete overhaul of the current training system. The skills in question for the accredited PHM fellowship include communication, negotiation, leadership, quality improvement, pain management, sedation, procedures, transport, billing/coding, autonomous decision making, and scholarly practice. Are most of these not skills that we should foster in all practicing pediatricians? If graduating pediatric residents lack competence in core pediatric skills (e.g., communication, pain management, autonomous decision making), we should target improvements in residency education rather than require years of further training. Pediatrics residency training already requires training in quality improvement and is incorporating “tracks” that target areas of perceived deficiency. Those physicians who actually require specialized hospital-based skills (e.g., sedation, procedures, and transport) could receive core training during residency (e.g., through PHM tracks or electives) and further hone these skills through faculty development efforts. While non-PhD researchers may benefit from additional training in research methodologies, this training comes at the expense of time spent caring for patients on the wards and should not be required training for the majority of pediatric hospitalists pursuing purely clinical roles.
Broad-based support for a PHM subspecialty has not been demonstrated.
While approximately 40 pediatric hospitalists originated the PHM certification petition, we have not seen clear support for subspecialty certification from the community. PHM certification runs the risk of alienating the general pediatrics community, as many outpatient pediatricians continue to care for their patients in the inpatient setting. Furthermore, at tertiary-care medical centers, pediatric subspecialists often serve as hospitalists, yet this stakeholder group has not entered into this conversation. Importantly, the Association of Pediatric Program Directors (APPD) did not endorse this proposal. Many of the APPD members were quite concerned about the harm this certification could cause. While the APA Board and the AAP Board of Directors support PHM subspecialty certification, it is not clear that the rank-and-file members do. The Society of Hospital Medicine did not support or oppose certification. In an era of controversy surrounding certification requirements, prior to making a decision that will alter the direction of an entire field and impact all future residency graduates interested in entering that field, we should ensure there is broad-based support for this decision.
An alternative path has already been established and validated.
A more prudent, cost-effective, and universally acceptable approach would be to follow in the footsteps of the American Board of Internal Medicine (ABIM) and American Board of Family Medicine (ABFM) in establishing a Focused Practice in Pediatric Hospital Medicine program. This approach respects the unique body of knowledge required of those who care for hospitalized children while maintaining the required flexibility to nurture and help to mature existing training pipelines. Core hospital medicine skills should be further honed through residency curricular changes and faculty development efforts, while hospital-based physicians interested in developing niche skills could still do so via already existing fellowships.
When it comes to pediatric hospital medicine, first, do no harm.
Pediatric hospitalists are inpatient generalists by training and clinical approach. Our practices vary from large academic medical centers with every imaginable subspecialty consult service available to remote rural settings that require hospitalists to possess unique and specific skills. Some pediatric hospitalists participate in newborn care, some perform sedations, and some perform a variety of diagnostic and therapeutic procedures. The current system is meeting the needs of the vast majority of our PHM community. Changes to the residency curriculum that are already under way can address any clinical and quality improvement gaps. More than enough PHM fellowships are available to those who choose to pursue them. The public is not requesting reassurance, and the field is already advancing at a rapid rate both clinically and scholarly. Subspecialty recognition is not necessary and will likely lead to negative unintended consequences. Given the financial constraints on our current system and the need for pediatric hospitalists to be stewards of high-value care, we should make collective decisions that will clearly benefit our patients and health system. As medical professionals, our priority should always be first, do no harm.
Weijen W. Chang, MD, is chief of the Division of Pediatric Hospital Medicine at Baystate Children’s Hospital and associate professor of pediatrics at the University of Massachusetts Medical School.
Leonard Samuel Feldman, MD, is director of the Medicine-Pediatrics Urban Health Residency Program and associate professor of medicine and pediatrics at Johns Hopkins School of Medicine.
Bradley Monash, MD, is associate chief of medicine at University of California, San Francisco and assistant clinical professor of medicine and pediatrics at UCSF School of Medicine.
Archna Eniasivam, MD, is assistant clinical professor of medicine at UCSF School of Medicine.
References
- Chen C, Eagle S. “Should Pediatric HM Pursue Subspecialty Certification, Required Fellowship Training?” The Hospitalist. July 31, 2012
- Results and Data: Specialties Matching Service 2016 Appointment Year. National Resident Matching Program website. Accessed May 15, 2016.
- Medscape Pediatrician Compensation Report 2015. Medscape website. Accessed April 29, 2016.
- Rochlin JM, Simon HK. Does fellowship pay: what is the long-term financial impact of subspecialty training in pediatrics? Pediatrics. 2001;127(2):254-260.
- Asch DA, Nicholson S, Vujicic M. Are we in a medical education bubble market? N Engl J Med. 2013;369(21):1973-1975.
- O’Toole JK, Friedland AR, Gonzaga AM, et al. The practice patterns of recently graduated internal medicine-pediatric hospitalists. Hosp Pediatr. 2015;5(6):309-314.
- Society of Hospital Medicine: Survey of Med-Peds Physicians about PHM Certification. May 2014 (unpublished).
- Goodman DM, Hall M, Levin A, et al. Adults with chronic health conditions originating in childhood: inpatient experience in children’s hospitals. Pediatrics. 2011;128(1):5-13.
- Freed GL, Dunham KM, Research Advisory Committee of the American Board of P. Pediatric hospitalists: training, current practice, and career goals. J Hosp Med. 2009;4(3):179-186.
- Donnelly MJ, Lubrano L, Radabaugh CL, Lukela MP, Friedland AR, Ruch-Ross HS. The med-peds hospitalist workforce: results from the American Academy of Pediatrics Workforce Survey. Hosp Pediatr. 2015;5(11):574-579.
The Joint Council of Pediatric Hospital Medicine (JCPHM), successor to the Strategic Planning (STP) Committee, recently recommended submitting a petition for two-year pediatric hospital medicine (PHM) fellowship certification to the American Board of Pediatrics (ABP), which was completed in 2014. In December 2015, the ABP Board of Directors voted to (1) approve the proposal for a two-year PHM fellowship incorporating scholarly activity with the provision that entrustable professional activities (EPAs) be used as the framework for assessing competencies and (2) not require those who achieve and maintain PHM certification to maintain general pediatrics certification. The proposal for certification of a two-year PHM fellowship will now be submitted to the American Board of Medical Specialties (ABMS). Concerns regarding the formal certification of PHM as an ABMS-recognized subspecialty have been raised by many stakeholders, including community pediatric hospitalists, pediatric residency program directors, and med-peds physicians.
We feel that the “first, do no harm” guiding principle seems to have been forgotten by the ABP as it attempts to formalize the training of pediatric hospitalists. In December 2015, the ABP voted in favor of a two-year ACGME-accredited PHM fellowship. The intent was to “assure the best care of hospitalized children,” “assure the public,” “accelerate improvements and innovation in quality improvement,” and “raise the level of care of all hospitalized children by establishing best practices in clinical care.” To be clear, these goals are shared by all of us (although there is no indication that the public is seeking additional assurance). Prior to launching broad-scale, time-intensive, and financially costly initiatives, we should ensure that our efforts would achieve—rather than obstruct—their intended aims. In addition to a lack of evidence supporting that subspecialty certification will advance our path toward achieving these goals, there are numerous reasons a required PHM fellowship is unnecessary and potentially even harmful to the hospitalist workforce. The negative unintended consequences need to be weighed heavily.
We have found no data to support that children would receive inferior inpatient care from pediatric hospitalists due to lack of formal certification. Hospital medicine physicians are paving the way in quality improvement, high-value care, medical education, palliative care, and global health, supported in part through training in various non-accredited hospital medicine fellowships. There is nothing stopping pediatric hospitalists from establishing and disseminating best practices in clinical care. Hospitalists are already making strides in providing high-quality care at low costs, as demonstrated by the abundant PHM scholarly work described in the ABP application to the ABMS. The alleged problem of needing to build trust within the community is yet to be demonstrated, as we have leaders at local, regional, and national levels. The chief medical officer of the Centers for Medicare & Medicaid Services is a hospitalist as is our surgeon general. Hospital medicine is the fastest-growing specialty in the history of medicine,1 and we should seek to propel rather than fetter our future colleagues.
Below are our reasons for opposing this formal certification.
We already have a fellowship system.
As we all know, advanced training opportunities already exist for those interested in pursuing extra research and quality improvement training. Similar to other pediatric subspecialty fellowships, these PHM fellowships are undersubscribed (20% of PHM fellowships did not fill in 2016),2 with the majority of graduating pediatric residents transitioning to hospitalists opting not to pursue fellowship training. We should continue to let graduating pediatric residents vote with their feet without the undue influence of subspecialty certification.
Subspecialization has opportunity costs that may reduce the PHM pipeline.
Even if we assume an adequate number of fellowship programs could be developed and funded, our fear is that the decision to turn PHM into an accredited subspecialty could paradoxically reduce the pipeline of inpatient providers. Residency is already a three- to four-year endeavor (pediatrics and med-peds) that is poorly compensated and time-intensive. In the absence of evidence supporting the value of additional training, tacking on another two years seems unreasonable in the face of the student loan debt crisis, reduced compensation, and lost time for career advancement. These are significant opportunity costs. While most specialties lead to a significant pay raise to compensate for the added training time, pediatrics remains the lowest-paid physician specialty.3 Should PHM follow the trend of most pediatric subspecialties, pursuit of fellowship training would be a negative financial decision for residency graduates.4 For the health system, increasing debt-to-income ratios runs the risk of creating a medical education bubble market.5
More than 25% of med-peds graduates pursue careers in hospital medicine, a percentage that continues to grow, accounting for more than 100 new hospitalists per year.6 As a result, med-peds-trained hospitalists constitute more than 10% of the pediatric hospitalist workforce.6 Requiring PHM fellowship training may reduce this crucial pipeline of practitioners. In a 2014 unpublished survey of 225 med-peds practitioners, 78% of residents and 96% of attendings responded that they would not consider pursuing an ACGME-accredited PHM fellowship.7 This is compounded by a lack of parity with the practice of adult hospital medicine both in compensation and required training and is heightened by the fact that the training in question does not incorporate care for adult patients. There is clear consensus by 96% of med-peds hospitalists that the creation of an ACGME-certified PHM subspecialty will negatively affect the likelihood of med-peds providers pursuing PHM.7
Certification will pose a potential risk to specific patient populations.
We are also concerned that a reduced PHM workforce could disproportionately impact young adults with special healthcare needs and those children cared for in rural or community-based hospitals. Med-peds training equips providers to care for children with chronic diseases that then transition into adulthood; more than 25% provide care for young adults with special healthcare needs.6 With the increasing number of children with chronic health conditions surviving into adulthood,8 med-peds hospitalists serve essential roles in providing care and coordination for this vulnerable population. Furthermore, hospital medicine groups in medical systems that cannot support a full-time categorical pediatric hospitalist tend to employ med-peds physicians or family practitioners. Concerns with PHM certification are thus extended to those family medicine physicians who practice PHM.
Pediatric residency trains pediatricians in inpatient care.
We feel that the decision to move forward on PHM subspecialty certification calls into question the value of pediatric residency training. There is no evidence that clinical inpatient training in pediatrics residency is inadequate. If one leaves residency trained to do anything, it is practicing hospital medicine. A significant portion of residency takes place inpatient, both on wards and in the intensive care units. The 2009 ABP Foundation–funded study of PHM reported that 94% of pediatric hospitalist respondents rated their training in general clinical skills during residency as fully adequate, 85% rated their training in communication skills as fully adequate, and 73% did not believe any additional training beyond residency should be required.9 With respect to med-peds graduates, more than 90% feel equipped to care for children and adults upon residency completion.10 If the ABMS carries forward with this decision, the only clinical work one would be “certified” to do after residency is primary care. However, after completion of residency training, most of us feel at least as comfortable, if not more comfortable, caring for children in the inpatient setting.
Primary care should require subspecialty certification as well.
Furthermore, the decision to create a certified subspecialty begs the question as to why fellowship should not be mandated for those entering the field of primary care. Does the field of primary care not require research to move it forward? Does the field of primary care not require providers who can adeptly apply quality improvement methodologies to improve primary-care delivery? Does the public not require the same type of assurance? By these measures, primary care should require subspecialty certification as well. These arguments could easily be construed as an indictment of residency training.
The target should be residency training.
The PHM ABMS application describes a clinical curriculum consisting of eight core clinical rotations in various settings. That small number emphasizes the fact that extra clinical training is really not needed and that we do not require a complete overhaul of the current training system. The skills in question for the accredited PHM fellowship include communication, negotiation, leadership, quality improvement, pain management, sedation, procedures, transport, billing/coding, autonomous decision making, and scholarly practice. Are most of these not skills that we should foster in all practicing pediatricians? If graduating pediatric residents lack competence in core pediatric skills (e.g., communication, pain management, autonomous decision making), we should target improvements in residency education rather than require years of further training. Pediatrics residency training already requires training in quality improvement and is incorporating “tracks” that target areas of perceived deficiency. Those physicians who actually require specialized hospital-based skills (e.g., sedation, procedures, and transport) could receive core training during residency (e.g., through PHM tracks or electives) and further hone these skills through faculty development efforts. While non-PhD researchers may benefit from additional training in research methodologies, this training comes at the expense of time spent caring for patients on the wards and should not be required training for the majority of pediatric hospitalists pursuing purely clinical roles.
Broad-based support for a PHM subspecialty has not been demonstrated.
While approximately 40 pediatric hospitalists originated the PHM certification petition, we have not seen clear support for subspecialty certification from the community. PHM certification runs the risk of alienating the general pediatrics community, as many outpatient pediatricians continue to care for their patients in the inpatient setting. Furthermore, at tertiary-care medical centers, pediatric subspecialists often serve as hospitalists, yet this stakeholder group has not entered into this conversation. Importantly, the Association of Pediatric Program Directors (APPD) did not endorse this proposal. Many of the APPD members were quite concerned about the harm this certification could cause. While the APA Board and the AAP Board of Directors support PHM subspecialty certification, it is not clear that the rank-and-file members do. The Society of Hospital Medicine did not support or oppose certification. In an era of controversy surrounding certification requirements, prior to making a decision that will alter the direction of an entire field and impact all future residency graduates interested in entering that field, we should ensure there is broad-based support for this decision.
An alternative path has already been established and validated.
A more prudent, cost-effective, and universally acceptable approach would be to follow in the footsteps of the American Board of Internal Medicine (ABIM) and American Board of Family Medicine (ABFM) in establishing a Focused Practice in Pediatric Hospital Medicine program. This approach respects the unique body of knowledge required of those who care for hospitalized children while maintaining the required flexibility to nurture and help to mature existing training pipelines. Core hospital medicine skills should be further honed through residency curricular changes and faculty development efforts, while hospital-based physicians interested in developing niche skills could still do so via already existing fellowships.
When it comes to pediatric hospital medicine, first, do no harm.
Pediatric hospitalists are inpatient generalists by training and clinical approach. Our practices vary from large academic medical centers with every imaginable subspecialty consult service available to remote rural settings that require hospitalists to possess unique and specific skills. Some pediatric hospitalists participate in newborn care, some perform sedations, and some perform a variety of diagnostic and therapeutic procedures. The current system is meeting the needs of the vast majority of our PHM community. Changes to the residency curriculum that are already under way can address any clinical and quality improvement gaps. More than enough PHM fellowships are available to those who choose to pursue them. The public is not requesting reassurance, and the field is already advancing at a rapid rate both clinically and scholarly. Subspecialty recognition is not necessary and will likely lead to negative unintended consequences. Given the financial constraints on our current system and the need for pediatric hospitalists to be stewards of high-value care, we should make collective decisions that will clearly benefit our patients and health system. As medical professionals, our priority should always be first, do no harm.
Weijen W. Chang, MD, is chief of the Division of Pediatric Hospital Medicine at Baystate Children’s Hospital and associate professor of pediatrics at the University of Massachusetts Medical School.
Leonard Samuel Feldman, MD, is director of the Medicine-Pediatrics Urban Health Residency Program and associate professor of medicine and pediatrics at Johns Hopkins School of Medicine.
Bradley Monash, MD, is associate chief of medicine at University of California, San Francisco and assistant clinical professor of medicine and pediatrics at UCSF School of Medicine.
Archna Eniasivam, MD, is assistant clinical professor of medicine at UCSF School of Medicine.
References
- Chen C, Eagle S. “Should Pediatric HM Pursue Subspecialty Certification, Required Fellowship Training?” The Hospitalist. July 31, 2012
- Results and Data: Specialties Matching Service 2016 Appointment Year. National Resident Matching Program website. Accessed May 15, 2016.
- Medscape Pediatrician Compensation Report 2015. Medscape website. Accessed April 29, 2016.
- Rochlin JM, Simon HK. Does fellowship pay: what is the long-term financial impact of subspecialty training in pediatrics? Pediatrics. 2001;127(2):254-260.
- Asch DA, Nicholson S, Vujicic M. Are we in a medical education bubble market? N Engl J Med. 2013;369(21):1973-1975.
- O’Toole JK, Friedland AR, Gonzaga AM, et al. The practice patterns of recently graduated internal medicine-pediatric hospitalists. Hosp Pediatr. 2015;5(6):309-314.
- Society of Hospital Medicine: Survey of Med-Peds Physicians about PHM Certification. May 2014 (unpublished).
- Goodman DM, Hall M, Levin A, et al. Adults with chronic health conditions originating in childhood: inpatient experience in children’s hospitals. Pediatrics. 2011;128(1):5-13.
- Freed GL, Dunham KM, Research Advisory Committee of the American Board of P. Pediatric hospitalists: training, current practice, and career goals. J Hosp Med. 2009;4(3):179-186.
- Donnelly MJ, Lubrano L, Radabaugh CL, Lukela MP, Friedland AR, Ruch-Ross HS. The med-peds hospitalist workforce: results from the American Academy of Pediatrics Workforce Survey. Hosp Pediatr. 2015;5(11):574-579.
The Joint Council of Pediatric Hospital Medicine (JCPHM), successor to the Strategic Planning (STP) Committee, recently recommended submitting a petition for two-year pediatric hospital medicine (PHM) fellowship certification to the American Board of Pediatrics (ABP), which was completed in 2014. In December 2015, the ABP Board of Directors voted to (1) approve the proposal for a two-year PHM fellowship incorporating scholarly activity with the provision that entrustable professional activities (EPAs) be used as the framework for assessing competencies and (2) not require those who achieve and maintain PHM certification to maintain general pediatrics certification. The proposal for certification of a two-year PHM fellowship will now be submitted to the American Board of Medical Specialties (ABMS). Concerns regarding the formal certification of PHM as an ABMS-recognized subspecialty have been raised by many stakeholders, including community pediatric hospitalists, pediatric residency program directors, and med-peds physicians.
We feel that the “first, do no harm” guiding principle seems to have been forgotten by the ABP as it attempts to formalize the training of pediatric hospitalists. In December 2015, the ABP voted in favor of a two-year ACGME-accredited PHM fellowship. The intent was to “assure the best care of hospitalized children,” “assure the public,” “accelerate improvements and innovation in quality improvement,” and “raise the level of care of all hospitalized children by establishing best practices in clinical care.” To be clear, these goals are shared by all of us (although there is no indication that the public is seeking additional assurance). Prior to launching broad-scale, time-intensive, and financially costly initiatives, we should ensure that our efforts would achieve—rather than obstruct—their intended aims. In addition to a lack of evidence supporting that subspecialty certification will advance our path toward achieving these goals, there are numerous reasons a required PHM fellowship is unnecessary and potentially even harmful to the hospitalist workforce. The negative unintended consequences need to be weighed heavily.
We have found no data to support that children would receive inferior inpatient care from pediatric hospitalists due to lack of formal certification. Hospital medicine physicians are paving the way in quality improvement, high-value care, medical education, palliative care, and global health, supported in part through training in various non-accredited hospital medicine fellowships. There is nothing stopping pediatric hospitalists from establishing and disseminating best practices in clinical care. Hospitalists are already making strides in providing high-quality care at low costs, as demonstrated by the abundant PHM scholarly work described in the ABP application to the ABMS. The alleged problem of needing to build trust within the community is yet to be demonstrated, as we have leaders at local, regional, and national levels. The chief medical officer of the Centers for Medicare & Medicaid Services is a hospitalist as is our surgeon general. Hospital medicine is the fastest-growing specialty in the history of medicine,1 and we should seek to propel rather than fetter our future colleagues.
Below are our reasons for opposing this formal certification.
We already have a fellowship system.
As we all know, advanced training opportunities already exist for those interested in pursuing extra research and quality improvement training. Similar to other pediatric subspecialty fellowships, these PHM fellowships are undersubscribed (20% of PHM fellowships did not fill in 2016),2 with the majority of graduating pediatric residents transitioning to hospitalists opting not to pursue fellowship training. We should continue to let graduating pediatric residents vote with their feet without the undue influence of subspecialty certification.
Subspecialization has opportunity costs that may reduce the PHM pipeline.
Even if we assume an adequate number of fellowship programs could be developed and funded, our fear is that the decision to turn PHM into an accredited subspecialty could paradoxically reduce the pipeline of inpatient providers. Residency is already a three- to four-year endeavor (pediatrics and med-peds) that is poorly compensated and time-intensive. In the absence of evidence supporting the value of additional training, tacking on another two years seems unreasonable in the face of the student loan debt crisis, reduced compensation, and lost time for career advancement. These are significant opportunity costs. While most specialties lead to a significant pay raise to compensate for the added training time, pediatrics remains the lowest-paid physician specialty.3 Should PHM follow the trend of most pediatric subspecialties, pursuit of fellowship training would be a negative financial decision for residency graduates.4 For the health system, increasing debt-to-income ratios runs the risk of creating a medical education bubble market.5
More than 25% of med-peds graduates pursue careers in hospital medicine, a percentage that continues to grow, accounting for more than 100 new hospitalists per year.6 As a result, med-peds-trained hospitalists constitute more than 10% of the pediatric hospitalist workforce.6 Requiring PHM fellowship training may reduce this crucial pipeline of practitioners. In a 2014 unpublished survey of 225 med-peds practitioners, 78% of residents and 96% of attendings responded that they would not consider pursuing an ACGME-accredited PHM fellowship.7 This is compounded by a lack of parity with the practice of adult hospital medicine both in compensation and required training and is heightened by the fact that the training in question does not incorporate care for adult patients. There is clear consensus by 96% of med-peds hospitalists that the creation of an ACGME-certified PHM subspecialty will negatively affect the likelihood of med-peds providers pursuing PHM.7
Certification will pose a potential risk to specific patient populations.
We are also concerned that a reduced PHM workforce could disproportionately impact young adults with special healthcare needs and those children cared for in rural or community-based hospitals. Med-peds training equips providers to care for children with chronic diseases that then transition into adulthood; more than 25% provide care for young adults with special healthcare needs.6 With the increasing number of children with chronic health conditions surviving into adulthood,8 med-peds hospitalists serve essential roles in providing care and coordination for this vulnerable population. Furthermore, hospital medicine groups in medical systems that cannot support a full-time categorical pediatric hospitalist tend to employ med-peds physicians or family practitioners. Concerns with PHM certification are thus extended to those family medicine physicians who practice PHM.
Pediatric residency trains pediatricians in inpatient care.
We feel that the decision to move forward on PHM subspecialty certification calls into question the value of pediatric residency training. There is no evidence that clinical inpatient training in pediatrics residency is inadequate. If one leaves residency trained to do anything, it is practicing hospital medicine. A significant portion of residency takes place inpatient, both on wards and in the intensive care units. The 2009 ABP Foundation–funded study of PHM reported that 94% of pediatric hospitalist respondents rated their training in general clinical skills during residency as fully adequate, 85% rated their training in communication skills as fully adequate, and 73% did not believe any additional training beyond residency should be required.9 With respect to med-peds graduates, more than 90% feel equipped to care for children and adults upon residency completion.10 If the ABMS carries forward with this decision, the only clinical work one would be “certified” to do after residency is primary care. However, after completion of residency training, most of us feel at least as comfortable, if not more comfortable, caring for children in the inpatient setting.
Primary care should require subspecialty certification as well.
Furthermore, the decision to create a certified subspecialty begs the question as to why fellowship should not be mandated for those entering the field of primary care. Does the field of primary care not require research to move it forward? Does the field of primary care not require providers who can adeptly apply quality improvement methodologies to improve primary-care delivery? Does the public not require the same type of assurance? By these measures, primary care should require subspecialty certification as well. These arguments could easily be construed as an indictment of residency training.
The target should be residency training.
The PHM ABMS application describes a clinical curriculum consisting of eight core clinical rotations in various settings. That small number emphasizes the fact that extra clinical training is really not needed and that we do not require a complete overhaul of the current training system. The skills in question for the accredited PHM fellowship include communication, negotiation, leadership, quality improvement, pain management, sedation, procedures, transport, billing/coding, autonomous decision making, and scholarly practice. Are most of these not skills that we should foster in all practicing pediatricians? If graduating pediatric residents lack competence in core pediatric skills (e.g., communication, pain management, autonomous decision making), we should target improvements in residency education rather than require years of further training. Pediatrics residency training already requires training in quality improvement and is incorporating “tracks” that target areas of perceived deficiency. Those physicians who actually require specialized hospital-based skills (e.g., sedation, procedures, and transport) could receive core training during residency (e.g., through PHM tracks or electives) and further hone these skills through faculty development efforts. While non-PhD researchers may benefit from additional training in research methodologies, this training comes at the expense of time spent caring for patients on the wards and should not be required training for the majority of pediatric hospitalists pursuing purely clinical roles.
Broad-based support for a PHM subspecialty has not been demonstrated.
While approximately 40 pediatric hospitalists originated the PHM certification petition, we have not seen clear support for subspecialty certification from the community. PHM certification runs the risk of alienating the general pediatrics community, as many outpatient pediatricians continue to care for their patients in the inpatient setting. Furthermore, at tertiary-care medical centers, pediatric subspecialists often serve as hospitalists, yet this stakeholder group has not entered into this conversation. Importantly, the Association of Pediatric Program Directors (APPD) did not endorse this proposal. Many of the APPD members were quite concerned about the harm this certification could cause. While the APA Board and the AAP Board of Directors support PHM subspecialty certification, it is not clear that the rank-and-file members do. The Society of Hospital Medicine did not support or oppose certification. In an era of controversy surrounding certification requirements, prior to making a decision that will alter the direction of an entire field and impact all future residency graduates interested in entering that field, we should ensure there is broad-based support for this decision.
An alternative path has already been established and validated.
A more prudent, cost-effective, and universally acceptable approach would be to follow in the footsteps of the American Board of Internal Medicine (ABIM) and American Board of Family Medicine (ABFM) in establishing a Focused Practice in Pediatric Hospital Medicine program. This approach respects the unique body of knowledge required of those who care for hospitalized children while maintaining the required flexibility to nurture and help to mature existing training pipelines. Core hospital medicine skills should be further honed through residency curricular changes and faculty development efforts, while hospital-based physicians interested in developing niche skills could still do so via already existing fellowships.
When it comes to pediatric hospital medicine, first, do no harm.
Pediatric hospitalists are inpatient generalists by training and clinical approach. Our practices vary from large academic medical centers with every imaginable subspecialty consult service available to remote rural settings that require hospitalists to possess unique and specific skills. Some pediatric hospitalists participate in newborn care, some perform sedations, and some perform a variety of diagnostic and therapeutic procedures. The current system is meeting the needs of the vast majority of our PHM community. Changes to the residency curriculum that are already under way can address any clinical and quality improvement gaps. More than enough PHM fellowships are available to those who choose to pursue them. The public is not requesting reassurance, and the field is already advancing at a rapid rate both clinically and scholarly. Subspecialty recognition is not necessary and will likely lead to negative unintended consequences. Given the financial constraints on our current system and the need for pediatric hospitalists to be stewards of high-value care, we should make collective decisions that will clearly benefit our patients and health system. As medical professionals, our priority should always be first, do no harm.
Weijen W. Chang, MD, is chief of the Division of Pediatric Hospital Medicine at Baystate Children’s Hospital and associate professor of pediatrics at the University of Massachusetts Medical School.
Leonard Samuel Feldman, MD, is director of the Medicine-Pediatrics Urban Health Residency Program and associate professor of medicine and pediatrics at Johns Hopkins School of Medicine.
Bradley Monash, MD, is associate chief of medicine at University of California, San Francisco and assistant clinical professor of medicine and pediatrics at UCSF School of Medicine.
Archna Eniasivam, MD, is assistant clinical professor of medicine at UCSF School of Medicine.
References
- Chen C, Eagle S. “Should Pediatric HM Pursue Subspecialty Certification, Required Fellowship Training?” The Hospitalist. July 31, 2012
- Results and Data: Specialties Matching Service 2016 Appointment Year. National Resident Matching Program website. Accessed May 15, 2016.
- Medscape Pediatrician Compensation Report 2015. Medscape website. Accessed April 29, 2016.
- Rochlin JM, Simon HK. Does fellowship pay: what is the long-term financial impact of subspecialty training in pediatrics? Pediatrics. 2001;127(2):254-260.
- Asch DA, Nicholson S, Vujicic M. Are we in a medical education bubble market? N Engl J Med. 2013;369(21):1973-1975.
- O’Toole JK, Friedland AR, Gonzaga AM, et al. The practice patterns of recently graduated internal medicine-pediatric hospitalists. Hosp Pediatr. 2015;5(6):309-314.
- Society of Hospital Medicine: Survey of Med-Peds Physicians about PHM Certification. May 2014 (unpublished).
- Goodman DM, Hall M, Levin A, et al. Adults with chronic health conditions originating in childhood: inpatient experience in children’s hospitals. Pediatrics. 2011;128(1):5-13.
- Freed GL, Dunham KM, Research Advisory Committee of the American Board of P. Pediatric hospitalists: training, current practice, and career goals. J Hosp Med. 2009;4(3):179-186.
- Donnelly MJ, Lubrano L, Radabaugh CL, Lukela MP, Friedland AR, Ruch-Ross HS. The med-peds hospitalist workforce: results from the American Academy of Pediatrics Workforce Survey. Hosp Pediatr. 2015;5(11):574-579.
Supportive care isn’t palliative care, speaker says

Photo courtesy of NCI
NEW YORK—Two presentations at the NCCN 11th Annual Congress: Hematologic Malignancies addressed the importance of supportive care in the treatment of patients with T-cell lymphomas and multiple myeloma.
Erin Kopp, ACNP-BC, of City of Hope Comprehensive Cancer Center in Duarte, California, reminded the audience that supportive care is not palliative care.
Supportive care “complements critical care so that the patient doesn’t have to stop treatment,” she said.
Kopp focused primarily on cutaneous T-cell lymphoma (CTCL) in her presentation, with some recommendations for managing tumor lysis syndrome in patients undergoing therapy for peripheral T-cell lymphoma (PTCL).
And Kathleen Colson, RN, of the Dana-Farber Cancer Institute in Boston, Massachusetts, discussed supportive care for patients with multiple myeloma (MM).
T-cell lymphomas
Most T-cell lymphoma patients will require multiple treatment regimens over their lifetimes, Kopp said. And each type of therapy brings different treatment-related toxicities, which in turn require distinct supportive care measures to manage them.
Topical steroids, for example, may cause skin-thinning, stretch marks, skin irritation, and may be absorbed systemically when a high-potency formulation is used. So the lowest potency steroid that provides the maximum efficacy should be utilized. Practitioners should assess systemic effects if high-potency steroids are utilized.
Topical nitrogen mustard can darken the skin, which often occurs as the lesions resolve, Kopp said. She cautioned that patients experiencing hyperpigmentation often stop treatment without telling their physicians.
So Kopp recommends appropriate patient education to go along with the treatment. With nitrogen mustard, this includes applying a thin layer only to the affected areas and refrigerating the topical ointment to increase soothing.
Topical retinoids may cause redness, itching, warmth, swelling, burning, scaling or other irritation. They also increase the patients’ sensitivity to light. Kopp indicated that for the first week, topical retinoids should be applied once every other day and then titrated as tolerated.
Phototherapy with PUVA or narrowband-UVB may also cause itching, in addition to skin burn, nausea, and other side effects.
“Do not underestimate emollients,” Kopp said, for relief of pruritus. And skin baths with bleach significantly decrease infections that may result from treatment.
Systemic therapy with retinoids, interferon, cytotoxic agents, monoclonal antibodies, and HDAC inhibitors may also cause distinct reactions. For example, the retinoid bexarotene may cause primary hypothyroidism and major lipid abnormalities. Therefore, TSH, free T4, and triglycerides should be monitored every 8 weeks.
Cytotoxic agents such as pralatrexate and methotrexate significantly increase the risk for infection.
Monoclonal antibodies can reactivate previous viral infection, induce tumor lysis syndrome (TLS), and cause progressive multifocal leukoencephalopathy.
HDAC inhibitors such as vorinostat and romidepsin may cause QT prolongation and myelosuppression, among other side effects.
Practitioners need to assess symptoms and side effects thoroughly and often and provide options for supportive care management.
PTCL is an under recognized risk for TLS, Kopp said.
“It should be addressed aggressively,” she added, with monitoring and correction of electrolyte imbalance.
Patients should be rigorously hydrated, and allopurinol should be administered 2-3 days prior to treatment and adjusted based on the patient response and uric acid level.
Multiple myeloma
Colson described supportive care as “keeping all the pieces together.” MM itself can result in a broad spectrum of clinical manifestations, including renal compromise, neuropathy, infection, hypercalcemia, bone pain, lytic lesions, and anemia.
To preserve renal health, patients should drink plenty of water and avoid certain medications, such as IV contrast and nonsteroidal anti-inflammatory drugs.
Peripheral neuropathy can be a side effect of treatment or be caused by the disease itself. Bortezomib-related neuropathy can be reduced with weekly instead of twice weekly dosing and with subcutaneous administration.
Duration of higher doses of thalidomide treatment also impacts neuropathy. Carfilzomib and pomalidomide have a lower incidence of neuropathy.
Myeloma patients have a 15-fold increased risk of recurrent infection because white blood cell production is decreased and the normal immune role of plasma cells is lost.
Supportive therapy includes antibiotics and IVIG therapy. In addition, Colson said pneumonia and influenza vaccines should be considered, as well as prophylaxis for Pneumocystis carinii, herpes zoster, and fungal infections.
Hypercalcemia results from increased bone deterioration. Symptoms include loss of appetite, fatigue, vomiting, muscle weakness, confusion, constipation, increased thirst, and increased urine output. Supportive measures are adequate hydration, furosemide, bisphosphonates, and steroids.
Supportive therapy for bone pain includes bisphosphonates, radiation, pain medication, kyphoplasty, and vertebroplasty. Bisphosphonates, such as pamidronate and zoledronic acid, inhibit bone destruction and are recommended for all myeloma patients with bone disease. However, patients should be monitored for renal dysfunction and osteonecrosis of the jaw when taking bisphosphonates.
And Colson advises, “Hold bisphosphonate therapy if the patient needs a root canal or extraction.” Additionally, dental implants are not recommended for MM patients.
Anemia is another common presenting symptom of myeloma and may also be a result of decreased kidney function. Colson said the use of red blood cell supplements may be used with caution to ameliorate the symptom. Red blood cell transfusion may be considered and a reduction in the medication dose may be required.
MM is a hypercoagulable disease, and measures should be taken to avoid deep vein thrombosis (DVT) and pulmonary embolism (PE). Patients should wear anti-embolism stockings, exercise regularly, take low-dose aspirin, and move about frequently instead of sitting for long periods. Immunomodulatory medications may be adjusted to reduce the risk of a blot clot forming.
Infusion-related reactions are also a risk of therapy, and symptoms of a reaction need to be managed immediately and appropriately, with antihistamines, corticosteroids, interruption of the infusion, slowing of the infusion rate after symptom resolution, and permanent discontinuation in the case of grade 4 reactions.
The potential for longer survival exists, Colson said, due to appropriate supportive care measures. ![]()

Photo courtesy of NCI
NEW YORK—Two presentations at the NCCN 11th Annual Congress: Hematologic Malignancies addressed the importance of supportive care in the treatment of patients with T-cell lymphomas and multiple myeloma.
Erin Kopp, ACNP-BC, of City of Hope Comprehensive Cancer Center in Duarte, California, reminded the audience that supportive care is not palliative care.
Supportive care “complements critical care so that the patient doesn’t have to stop treatment,” she said.
Kopp focused primarily on cutaneous T-cell lymphoma (CTCL) in her presentation, with some recommendations for managing tumor lysis syndrome in patients undergoing therapy for peripheral T-cell lymphoma (PTCL).
And Kathleen Colson, RN, of the Dana-Farber Cancer Institute in Boston, Massachusetts, discussed supportive care for patients with multiple myeloma (MM).
T-cell lymphomas
Most T-cell lymphoma patients will require multiple treatment regimens over their lifetimes, Kopp said. And each type of therapy brings different treatment-related toxicities, which in turn require distinct supportive care measures to manage them.
Topical steroids, for example, may cause skin-thinning, stretch marks, skin irritation, and may be absorbed systemically when a high-potency formulation is used. So the lowest potency steroid that provides the maximum efficacy should be utilized. Practitioners should assess systemic effects if high-potency steroids are utilized.
Topical nitrogen mustard can darken the skin, which often occurs as the lesions resolve, Kopp said. She cautioned that patients experiencing hyperpigmentation often stop treatment without telling their physicians.
So Kopp recommends appropriate patient education to go along with the treatment. With nitrogen mustard, this includes applying a thin layer only to the affected areas and refrigerating the topical ointment to increase soothing.
Topical retinoids may cause redness, itching, warmth, swelling, burning, scaling or other irritation. They also increase the patients’ sensitivity to light. Kopp indicated that for the first week, topical retinoids should be applied once every other day and then titrated as tolerated.
Phototherapy with PUVA or narrowband-UVB may also cause itching, in addition to skin burn, nausea, and other side effects.
“Do not underestimate emollients,” Kopp said, for relief of pruritus. And skin baths with bleach significantly decrease infections that may result from treatment.
Systemic therapy with retinoids, interferon, cytotoxic agents, monoclonal antibodies, and HDAC inhibitors may also cause distinct reactions. For example, the retinoid bexarotene may cause primary hypothyroidism and major lipid abnormalities. Therefore, TSH, free T4, and triglycerides should be monitored every 8 weeks.
Cytotoxic agents such as pralatrexate and methotrexate significantly increase the risk for infection.
Monoclonal antibodies can reactivate previous viral infection, induce tumor lysis syndrome (TLS), and cause progressive multifocal leukoencephalopathy.
HDAC inhibitors such as vorinostat and romidepsin may cause QT prolongation and myelosuppression, among other side effects.
Practitioners need to assess symptoms and side effects thoroughly and often and provide options for supportive care management.
PTCL is an under recognized risk for TLS, Kopp said.
“It should be addressed aggressively,” she added, with monitoring and correction of electrolyte imbalance.
Patients should be rigorously hydrated, and allopurinol should be administered 2-3 days prior to treatment and adjusted based on the patient response and uric acid level.
Multiple myeloma
Colson described supportive care as “keeping all the pieces together.” MM itself can result in a broad spectrum of clinical manifestations, including renal compromise, neuropathy, infection, hypercalcemia, bone pain, lytic lesions, and anemia.
To preserve renal health, patients should drink plenty of water and avoid certain medications, such as IV contrast and nonsteroidal anti-inflammatory drugs.
Peripheral neuropathy can be a side effect of treatment or be caused by the disease itself. Bortezomib-related neuropathy can be reduced with weekly instead of twice weekly dosing and with subcutaneous administration.
Duration of higher doses of thalidomide treatment also impacts neuropathy. Carfilzomib and pomalidomide have a lower incidence of neuropathy.
Myeloma patients have a 15-fold increased risk of recurrent infection because white blood cell production is decreased and the normal immune role of plasma cells is lost.
Supportive therapy includes antibiotics and IVIG therapy. In addition, Colson said pneumonia and influenza vaccines should be considered, as well as prophylaxis for Pneumocystis carinii, herpes zoster, and fungal infections.
Hypercalcemia results from increased bone deterioration. Symptoms include loss of appetite, fatigue, vomiting, muscle weakness, confusion, constipation, increased thirst, and increased urine output. Supportive measures are adequate hydration, furosemide, bisphosphonates, and steroids.
Supportive therapy for bone pain includes bisphosphonates, radiation, pain medication, kyphoplasty, and vertebroplasty. Bisphosphonates, such as pamidronate and zoledronic acid, inhibit bone destruction and are recommended for all myeloma patients with bone disease. However, patients should be monitored for renal dysfunction and osteonecrosis of the jaw when taking bisphosphonates.
And Colson advises, “Hold bisphosphonate therapy if the patient needs a root canal or extraction.” Additionally, dental implants are not recommended for MM patients.
Anemia is another common presenting symptom of myeloma and may also be a result of decreased kidney function. Colson said the use of red blood cell supplements may be used with caution to ameliorate the symptom. Red blood cell transfusion may be considered and a reduction in the medication dose may be required.
MM is a hypercoagulable disease, and measures should be taken to avoid deep vein thrombosis (DVT) and pulmonary embolism (PE). Patients should wear anti-embolism stockings, exercise regularly, take low-dose aspirin, and move about frequently instead of sitting for long periods. Immunomodulatory medications may be adjusted to reduce the risk of a blot clot forming.
Infusion-related reactions are also a risk of therapy, and symptoms of a reaction need to be managed immediately and appropriately, with antihistamines, corticosteroids, interruption of the infusion, slowing of the infusion rate after symptom resolution, and permanent discontinuation in the case of grade 4 reactions.
The potential for longer survival exists, Colson said, due to appropriate supportive care measures. ![]()

Photo courtesy of NCI
NEW YORK—Two presentations at the NCCN 11th Annual Congress: Hematologic Malignancies addressed the importance of supportive care in the treatment of patients with T-cell lymphomas and multiple myeloma.
Erin Kopp, ACNP-BC, of City of Hope Comprehensive Cancer Center in Duarte, California, reminded the audience that supportive care is not palliative care.
Supportive care “complements critical care so that the patient doesn’t have to stop treatment,” she said.
Kopp focused primarily on cutaneous T-cell lymphoma (CTCL) in her presentation, with some recommendations for managing tumor lysis syndrome in patients undergoing therapy for peripheral T-cell lymphoma (PTCL).
And Kathleen Colson, RN, of the Dana-Farber Cancer Institute in Boston, Massachusetts, discussed supportive care for patients with multiple myeloma (MM).
T-cell lymphomas
Most T-cell lymphoma patients will require multiple treatment regimens over their lifetimes, Kopp said. And each type of therapy brings different treatment-related toxicities, which in turn require distinct supportive care measures to manage them.
Topical steroids, for example, may cause skin-thinning, stretch marks, skin irritation, and may be absorbed systemically when a high-potency formulation is used. So the lowest potency steroid that provides the maximum efficacy should be utilized. Practitioners should assess systemic effects if high-potency steroids are utilized.
Topical nitrogen mustard can darken the skin, which often occurs as the lesions resolve, Kopp said. She cautioned that patients experiencing hyperpigmentation often stop treatment without telling their physicians.
So Kopp recommends appropriate patient education to go along with the treatment. With nitrogen mustard, this includes applying a thin layer only to the affected areas and refrigerating the topical ointment to increase soothing.
Topical retinoids may cause redness, itching, warmth, swelling, burning, scaling or other irritation. They also increase the patients’ sensitivity to light. Kopp indicated that for the first week, topical retinoids should be applied once every other day and then titrated as tolerated.
Phototherapy with PUVA or narrowband-UVB may also cause itching, in addition to skin burn, nausea, and other side effects.
“Do not underestimate emollients,” Kopp said, for relief of pruritus. And skin baths with bleach significantly decrease infections that may result from treatment.
Systemic therapy with retinoids, interferon, cytotoxic agents, monoclonal antibodies, and HDAC inhibitors may also cause distinct reactions. For example, the retinoid bexarotene may cause primary hypothyroidism and major lipid abnormalities. Therefore, TSH, free T4, and triglycerides should be monitored every 8 weeks.
Cytotoxic agents such as pralatrexate and methotrexate significantly increase the risk for infection.
Monoclonal antibodies can reactivate previous viral infection, induce tumor lysis syndrome (TLS), and cause progressive multifocal leukoencephalopathy.
HDAC inhibitors such as vorinostat and romidepsin may cause QT prolongation and myelosuppression, among other side effects.
Practitioners need to assess symptoms and side effects thoroughly and often and provide options for supportive care management.
PTCL is an under recognized risk for TLS, Kopp said.
“It should be addressed aggressively,” she added, with monitoring and correction of electrolyte imbalance.
Patients should be rigorously hydrated, and allopurinol should be administered 2-3 days prior to treatment and adjusted based on the patient response and uric acid level.
Multiple myeloma
Colson described supportive care as “keeping all the pieces together.” MM itself can result in a broad spectrum of clinical manifestations, including renal compromise, neuropathy, infection, hypercalcemia, bone pain, lytic lesions, and anemia.
To preserve renal health, patients should drink plenty of water and avoid certain medications, such as IV contrast and nonsteroidal anti-inflammatory drugs.
Peripheral neuropathy can be a side effect of treatment or be caused by the disease itself. Bortezomib-related neuropathy can be reduced with weekly instead of twice weekly dosing and with subcutaneous administration.
Duration of higher doses of thalidomide treatment also impacts neuropathy. Carfilzomib and pomalidomide have a lower incidence of neuropathy.
Myeloma patients have a 15-fold increased risk of recurrent infection because white blood cell production is decreased and the normal immune role of plasma cells is lost.
Supportive therapy includes antibiotics and IVIG therapy. In addition, Colson said pneumonia and influenza vaccines should be considered, as well as prophylaxis for Pneumocystis carinii, herpes zoster, and fungal infections.
Hypercalcemia results from increased bone deterioration. Symptoms include loss of appetite, fatigue, vomiting, muscle weakness, confusion, constipation, increased thirst, and increased urine output. Supportive measures are adequate hydration, furosemide, bisphosphonates, and steroids.
Supportive therapy for bone pain includes bisphosphonates, radiation, pain medication, kyphoplasty, and vertebroplasty. Bisphosphonates, such as pamidronate and zoledronic acid, inhibit bone destruction and are recommended for all myeloma patients with bone disease. However, patients should be monitored for renal dysfunction and osteonecrosis of the jaw when taking bisphosphonates.
And Colson advises, “Hold bisphosphonate therapy if the patient needs a root canal or extraction.” Additionally, dental implants are not recommended for MM patients.
Anemia is another common presenting symptom of myeloma and may also be a result of decreased kidney function. Colson said the use of red blood cell supplements may be used with caution to ameliorate the symptom. Red blood cell transfusion may be considered and a reduction in the medication dose may be required.
MM is a hypercoagulable disease, and measures should be taken to avoid deep vein thrombosis (DVT) and pulmonary embolism (PE). Patients should wear anti-embolism stockings, exercise regularly, take low-dose aspirin, and move about frequently instead of sitting for long periods. Immunomodulatory medications may be adjusted to reduce the risk of a blot clot forming.
Infusion-related reactions are also a risk of therapy, and symptoms of a reaction need to be managed immediately and appropriately, with antihistamines, corticosteroids, interruption of the infusion, slowing of the infusion rate after symptom resolution, and permanent discontinuation in the case of grade 4 reactions.
The potential for longer survival exists, Colson said, due to appropriate supportive care measures. ![]()
Technology underused in psychiatry, but changes are ahead
Editors’ Note: The intent of this new column is to discuss topics at the intersection of technology and psychiatry – “Techiatry.” We’ve enlisted two leaders in this field to write for the column. Steven R. Daviss, MD, DFAPA (@HITshrink), is the chief medical informatics officer at M3 Information and chairs the American Psychiatric Association’s Committee on Mental Health Information Technology. James (Jay) H. Shore, MD, MPH, chairs the APA Committee on Telepsychiatry, is director of telemedicine at the Helen & Arthur E. Johnson Depression Center, and an associate professor of psychiatry at the University of Colorado at Denver, Aurora. Email them at [email protected].
Medicine is late to the game when it comes to technology, specifically information technology. And psychiatry, even more so. Jay will talk in future columns about early use of telepsychiatry in the 1960s and since. But here in 2016, a surprisingly low percentage of us are using it to deliver care, despite the fact that half of the counties in the United States lack psychiatrists – and telemedicine has been shown to improve access to care.
Nonetheless, telemedicine and other uses of technology across all specialties is growing quickly, as usability, mobile technology, economics, and policy-making all converge. The integration of mental health care (including addiction treatment) with primary care is one of the driving forces in expanding access to the expertise that physicians trained in psychiatry possess. The collaborative care model of integrated care has the most evidence, making regular access to psychiatric consultants a weekly event.
This exchange of information and knowledge between primary care and psychiatry is being formally incentivized by the Centers for Medicare & Medicaid Services (CMS) with proposed new codes to pay for this exchange, while the American Psychiatric Association has received a large grant from CMS to train 10% of its members in this care model.
Information technology is fundamental to this care model, because the efficient exchange of clinical information is important to optimize the capabilities and comprehensiveness of the clinical decision support provided by the psychiatrist to the primary care team.
As the team members learn what questions are asked and how the consultant arrives at her recommendations, they will become better at making these decisions on their own. They will learn how psychiatrists think and make decisions, weighing other medical, personal, social, family, and logistical aspects to guide the decision making process with the patient.
While this model of care is certainly helpful in expanding access to psychiatric expertise, there are other ways to achieve this access to expert knowledge. One of them is through electronic clinical decision support (CDS) tools. These are tools that apply clinical rules, algorithms, and other knowledge discovery processes to the information within the electronic health record (EHR) about a patient, with the goal of assessing and filling gaps in available patient information so that a set of possible recommendations can be delivered to the clinician.
Knowledge-based CDS tools apply clinical knowledge that comes from practice guidelines, textbooks, and the medical literature to what is known about the patient. The simplest CDS tool might be a rule that says, “IF patient is on lithium for bipolar disorder AND patient has current mood symptoms AND has not had a recent lithium level, THEN check a lithium level.” Applying and coding this rule into an EHR is fairly straightforward. A much more complex CDS tool could help the clinician think through the question, “What should I do next for this 32yo woman with hypertension and moderate depression who is symptomatic?”
Non–knowledge-based CDS tools use machine learning techniques, like neural networks, genetic algorithms, and natural language processing, to “learn” new clinical rules by going through a training process that inputs a large amount of clinical data and uses experts to “train” the system. Such a system was recently developed by IBM Watson and Memorial Sloan-Kettering Cancer Center to aid in developing recommendations for treating oncology patients.
The APA recently formed the CDS Product Workgroup (which I chair) to explore the feasibility of developing an electronic clinical decision support (CDS) tool that leverages the information and knowledge within the APA’s series of Practice Guidelines, DSM library, and other reference materials. This group will consider the necessary important clinical information sources – such as EHRs, personal health records, health information exchanges, claims and utilization data, patient generated data, mobile health apps, and clinical registries – from which to analyze patient-specific data and produce a set of ranked, evidence-based, annotated clinical suggestions.
The goal is to develop a CDS tool that is designed in a manner that ultimately benefits patients being treated by primary care practitioners, emergency practitioners, psychiatrists, and other medical specialists who treat patients with mental health and substance use disorders. Tools like this are being developed now in many specialties. Given the vast amount of psychiatric expertise within the APA, as well as the trove of content that exists within the publishing arm of the APA, the opportunity to make this more broadly available to medical practitioners is one that demands consideration.
Such an undertaking would require substantial time and commitment of resources, thus the task of the workgroup is to understand the pros and cons of developing this tool, and to explore its feasibility, including various business models to ensure that this CDS tool becomes a maintainable and sustainable product.
Bringing our expertise to the primary care settings, where most of our patients are treated, should greatly benefit the care of our patients, whether this is through collaborative care, clinical decision support, or telepsychiatry. In the same way that many people with diabetes do not require an endocrinologist, many with mental health conditions do not require a psychiatrist. Yet, primary care practitioners would certainly benefit from more help from us.
I will update readers of this column on our workgroup’s progress at the end of the year.
Editors’ Note: The intent of this new column is to discuss topics at the intersection of technology and psychiatry – “Techiatry.” We’ve enlisted two leaders in this field to write for the column. Steven R. Daviss, MD, DFAPA (@HITshrink), is the chief medical informatics officer at M3 Information and chairs the American Psychiatric Association’s Committee on Mental Health Information Technology. James (Jay) H. Shore, MD, MPH, chairs the APA Committee on Telepsychiatry, is director of telemedicine at the Helen & Arthur E. Johnson Depression Center, and an associate professor of psychiatry at the University of Colorado at Denver, Aurora. Email them at [email protected].
Medicine is late to the game when it comes to technology, specifically information technology. And psychiatry, even more so. Jay will talk in future columns about early use of telepsychiatry in the 1960s and since. But here in 2016, a surprisingly low percentage of us are using it to deliver care, despite the fact that half of the counties in the United States lack psychiatrists – and telemedicine has been shown to improve access to care.
Nonetheless, telemedicine and other uses of technology across all specialties is growing quickly, as usability, mobile technology, economics, and policy-making all converge. The integration of mental health care (including addiction treatment) with primary care is one of the driving forces in expanding access to the expertise that physicians trained in psychiatry possess. The collaborative care model of integrated care has the most evidence, making regular access to psychiatric consultants a weekly event.
This exchange of information and knowledge between primary care and psychiatry is being formally incentivized by the Centers for Medicare & Medicaid Services (CMS) with proposed new codes to pay for this exchange, while the American Psychiatric Association has received a large grant from CMS to train 10% of its members in this care model.
Information technology is fundamental to this care model, because the efficient exchange of clinical information is important to optimize the capabilities and comprehensiveness of the clinical decision support provided by the psychiatrist to the primary care team.
As the team members learn what questions are asked and how the consultant arrives at her recommendations, they will become better at making these decisions on their own. They will learn how psychiatrists think and make decisions, weighing other medical, personal, social, family, and logistical aspects to guide the decision making process with the patient.
While this model of care is certainly helpful in expanding access to psychiatric expertise, there are other ways to achieve this access to expert knowledge. One of them is through electronic clinical decision support (CDS) tools. These are tools that apply clinical rules, algorithms, and other knowledge discovery processes to the information within the electronic health record (EHR) about a patient, with the goal of assessing and filling gaps in available patient information so that a set of possible recommendations can be delivered to the clinician.
Knowledge-based CDS tools apply clinical knowledge that comes from practice guidelines, textbooks, and the medical literature to what is known about the patient. The simplest CDS tool might be a rule that says, “IF patient is on lithium for bipolar disorder AND patient has current mood symptoms AND has not had a recent lithium level, THEN check a lithium level.” Applying and coding this rule into an EHR is fairly straightforward. A much more complex CDS tool could help the clinician think through the question, “What should I do next for this 32yo woman with hypertension and moderate depression who is symptomatic?”
Non–knowledge-based CDS tools use machine learning techniques, like neural networks, genetic algorithms, and natural language processing, to “learn” new clinical rules by going through a training process that inputs a large amount of clinical data and uses experts to “train” the system. Such a system was recently developed by IBM Watson and Memorial Sloan-Kettering Cancer Center to aid in developing recommendations for treating oncology patients.
The APA recently formed the CDS Product Workgroup (which I chair) to explore the feasibility of developing an electronic clinical decision support (CDS) tool that leverages the information and knowledge within the APA’s series of Practice Guidelines, DSM library, and other reference materials. This group will consider the necessary important clinical information sources – such as EHRs, personal health records, health information exchanges, claims and utilization data, patient generated data, mobile health apps, and clinical registries – from which to analyze patient-specific data and produce a set of ranked, evidence-based, annotated clinical suggestions.
The goal is to develop a CDS tool that is designed in a manner that ultimately benefits patients being treated by primary care practitioners, emergency practitioners, psychiatrists, and other medical specialists who treat patients with mental health and substance use disorders. Tools like this are being developed now in many specialties. Given the vast amount of psychiatric expertise within the APA, as well as the trove of content that exists within the publishing arm of the APA, the opportunity to make this more broadly available to medical practitioners is one that demands consideration.
Such an undertaking would require substantial time and commitment of resources, thus the task of the workgroup is to understand the pros and cons of developing this tool, and to explore its feasibility, including various business models to ensure that this CDS tool becomes a maintainable and sustainable product.
Bringing our expertise to the primary care settings, where most of our patients are treated, should greatly benefit the care of our patients, whether this is through collaborative care, clinical decision support, or telepsychiatry. In the same way that many people with diabetes do not require an endocrinologist, many with mental health conditions do not require a psychiatrist. Yet, primary care practitioners would certainly benefit from more help from us.
I will update readers of this column on our workgroup’s progress at the end of the year.
Editors’ Note: The intent of this new column is to discuss topics at the intersection of technology and psychiatry – “Techiatry.” We’ve enlisted two leaders in this field to write for the column. Steven R. Daviss, MD, DFAPA (@HITshrink), is the chief medical informatics officer at M3 Information and chairs the American Psychiatric Association’s Committee on Mental Health Information Technology. James (Jay) H. Shore, MD, MPH, chairs the APA Committee on Telepsychiatry, is director of telemedicine at the Helen & Arthur E. Johnson Depression Center, and an associate professor of psychiatry at the University of Colorado at Denver, Aurora. Email them at [email protected].
Medicine is late to the game when it comes to technology, specifically information technology. And psychiatry, even more so. Jay will talk in future columns about early use of telepsychiatry in the 1960s and since. But here in 2016, a surprisingly low percentage of us are using it to deliver care, despite the fact that half of the counties in the United States lack psychiatrists – and telemedicine has been shown to improve access to care.
Nonetheless, telemedicine and other uses of technology across all specialties is growing quickly, as usability, mobile technology, economics, and policy-making all converge. The integration of mental health care (including addiction treatment) with primary care is one of the driving forces in expanding access to the expertise that physicians trained in psychiatry possess. The collaborative care model of integrated care has the most evidence, making regular access to psychiatric consultants a weekly event.
This exchange of information and knowledge between primary care and psychiatry is being formally incentivized by the Centers for Medicare & Medicaid Services (CMS) with proposed new codes to pay for this exchange, while the American Psychiatric Association has received a large grant from CMS to train 10% of its members in this care model.
Information technology is fundamental to this care model, because the efficient exchange of clinical information is important to optimize the capabilities and comprehensiveness of the clinical decision support provided by the psychiatrist to the primary care team.
As the team members learn what questions are asked and how the consultant arrives at her recommendations, they will become better at making these decisions on their own. They will learn how psychiatrists think and make decisions, weighing other medical, personal, social, family, and logistical aspects to guide the decision making process with the patient.
While this model of care is certainly helpful in expanding access to psychiatric expertise, there are other ways to achieve this access to expert knowledge. One of them is through electronic clinical decision support (CDS) tools. These are tools that apply clinical rules, algorithms, and other knowledge discovery processes to the information within the electronic health record (EHR) about a patient, with the goal of assessing and filling gaps in available patient information so that a set of possible recommendations can be delivered to the clinician.
Knowledge-based CDS tools apply clinical knowledge that comes from practice guidelines, textbooks, and the medical literature to what is known about the patient. The simplest CDS tool might be a rule that says, “IF patient is on lithium for bipolar disorder AND patient has current mood symptoms AND has not had a recent lithium level, THEN check a lithium level.” Applying and coding this rule into an EHR is fairly straightforward. A much more complex CDS tool could help the clinician think through the question, “What should I do next for this 32yo woman with hypertension and moderate depression who is symptomatic?”
Non–knowledge-based CDS tools use machine learning techniques, like neural networks, genetic algorithms, and natural language processing, to “learn” new clinical rules by going through a training process that inputs a large amount of clinical data and uses experts to “train” the system. Such a system was recently developed by IBM Watson and Memorial Sloan-Kettering Cancer Center to aid in developing recommendations for treating oncology patients.
The APA recently formed the CDS Product Workgroup (which I chair) to explore the feasibility of developing an electronic clinical decision support (CDS) tool that leverages the information and knowledge within the APA’s series of Practice Guidelines, DSM library, and other reference materials. This group will consider the necessary important clinical information sources – such as EHRs, personal health records, health information exchanges, claims and utilization data, patient generated data, mobile health apps, and clinical registries – from which to analyze patient-specific data and produce a set of ranked, evidence-based, annotated clinical suggestions.
The goal is to develop a CDS tool that is designed in a manner that ultimately benefits patients being treated by primary care practitioners, emergency practitioners, psychiatrists, and other medical specialists who treat patients with mental health and substance use disorders. Tools like this are being developed now in many specialties. Given the vast amount of psychiatric expertise within the APA, as well as the trove of content that exists within the publishing arm of the APA, the opportunity to make this more broadly available to medical practitioners is one that demands consideration.
Such an undertaking would require substantial time and commitment of resources, thus the task of the workgroup is to understand the pros and cons of developing this tool, and to explore its feasibility, including various business models to ensure that this CDS tool becomes a maintainable and sustainable product.
Bringing our expertise to the primary care settings, where most of our patients are treated, should greatly benefit the care of our patients, whether this is through collaborative care, clinical decision support, or telepsychiatry. In the same way that many people with diabetes do not require an endocrinologist, many with mental health conditions do not require a psychiatrist. Yet, primary care practitioners would certainly benefit from more help from us.
I will update readers of this column on our workgroup’s progress at the end of the year.
Glimmer of promise for nivolumab in neoadjuvant NSCLC therapy
COPENHAGEN – Neoadjuvant therapy with the PD-1 checkpoint inhibitor nivolumab is safe, does not delay surgery, and may offer clinical benefit in some patients with early-stage non–small cell lung cancer, preliminary results of a clinical trial show.
Among 17 patients with previously untreated stage I-IIIA non–small cell lung cancer (NSCLC) who had two courses of nivolumab (Opdivo) followed by surgical resection, 12 had pathologic evidence of tumor regression, including 7 who had what investigators termed a “major pathologic response,” defined as less than 10% residual viable tumor, reported Patrick M. Forde, MBBCh, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University in Baltimore.
To see whether immunotherapy could be safe and practical in the neoadjuvant setting, the investigators enrolled 18 patients with untreated, resectable disease and performed pretreatment tumor biopsies. Patients then received doses of nivolumab 3 mg/kg delivered at 4 weeks and 2 weeks prior to scheduled surgery. Following surgery, patients received adjuvant chemotherapy at the investigator’s discretion.
Treatment related adverse events included one grade 3 to 4 toxicity and one event leading to discontinuation. There were no adverse events requiring delay of surgery.
The investigators also conducted an exploratory analysis of response to treatment among 17 who had sufficient follow-up data for evaluation of the primary endpoints of safety and feasibility.
As noted, 12 patients had a measurable tumor response, and 7 had a major pathologic response. Of this latter group, three had no radiographic evidence of response, but tumor specimens from all 7 showed evidence of “substantial” T-cell infiltration, indicating an enhanced immune response, Dr. Forde said.
In all, 9 of the 17 patients had tumor regression of more than 50%, and 7 patients had pathologic downstaging from their pretreatment clinical stage.
Four of the seven tumors with the major pathologic response were tested with a programmed death–1 ligand immunohistochemical assay, and three were positive for the ligand.
The investigators also isolated both unique and shared T-cell clones from peripheral blood, and detected new infiltration of T-cell clones that were not seen in the tumor specimens taken prior to nivolumab therapy.
Based on these early results, the investigators plan to expand the study, with one cohort planned to receive a third presurgical dose of nivolumab, and a second cohort scheduled to receive both nivolumab and ipilimumab (Yervoy).
It remains to be seen, he said, whether patients should also receive maintenance therapy, and whether combined checkpoint inhibitors might be more effective.
However, Pieter Postmus, chair of thoracic oncology at the University of Liverpool (England), who was not involved in the study, said that he is reserving judgment on efficacy until further data are available.
“There is a potential for bias when comparing a small biopsy, which might not represent the whole tumor, with the resected tumor,” he said in a statement. “This is not a validated way to measure response to a treatment. It describes a biological effect but whether that has any clinical impact on survival is unproven.”
“Although we do not know for the time being if a major pathological response is correlated with improved survival, this method could first be validated in a cohort of patients with advanced disease by comparing the percentages of viable tumor cells in tumor biopsies taken before and 4 to 8 weeks after immunotherapy,” he added. “If in this way regression – as defined in the preoperative study – correlates with survival in patients with advanced cancer, it is likely to hold true in less advanced or resectable patients. Long-term survival data will be the ultimate test for these neoadjuvant immunotherapy strategies.”
COPENHAGEN – Neoadjuvant therapy with the PD-1 checkpoint inhibitor nivolumab is safe, does not delay surgery, and may offer clinical benefit in some patients with early-stage non–small cell lung cancer, preliminary results of a clinical trial show.
Among 17 patients with previously untreated stage I-IIIA non–small cell lung cancer (NSCLC) who had two courses of nivolumab (Opdivo) followed by surgical resection, 12 had pathologic evidence of tumor regression, including 7 who had what investigators termed a “major pathologic response,” defined as less than 10% residual viable tumor, reported Patrick M. Forde, MBBCh, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University in Baltimore.
To see whether immunotherapy could be safe and practical in the neoadjuvant setting, the investigators enrolled 18 patients with untreated, resectable disease and performed pretreatment tumor biopsies. Patients then received doses of nivolumab 3 mg/kg delivered at 4 weeks and 2 weeks prior to scheduled surgery. Following surgery, patients received adjuvant chemotherapy at the investigator’s discretion.
Treatment related adverse events included one grade 3 to 4 toxicity and one event leading to discontinuation. There were no adverse events requiring delay of surgery.
The investigators also conducted an exploratory analysis of response to treatment among 17 who had sufficient follow-up data for evaluation of the primary endpoints of safety and feasibility.
As noted, 12 patients had a measurable tumor response, and 7 had a major pathologic response. Of this latter group, three had no radiographic evidence of response, but tumor specimens from all 7 showed evidence of “substantial” T-cell infiltration, indicating an enhanced immune response, Dr. Forde said.
In all, 9 of the 17 patients had tumor regression of more than 50%, and 7 patients had pathologic downstaging from their pretreatment clinical stage.
Four of the seven tumors with the major pathologic response were tested with a programmed death–1 ligand immunohistochemical assay, and three were positive for the ligand.
The investigators also isolated both unique and shared T-cell clones from peripheral blood, and detected new infiltration of T-cell clones that were not seen in the tumor specimens taken prior to nivolumab therapy.
Based on these early results, the investigators plan to expand the study, with one cohort planned to receive a third presurgical dose of nivolumab, and a second cohort scheduled to receive both nivolumab and ipilimumab (Yervoy).
It remains to be seen, he said, whether patients should also receive maintenance therapy, and whether combined checkpoint inhibitors might be more effective.
However, Pieter Postmus, chair of thoracic oncology at the University of Liverpool (England), who was not involved in the study, said that he is reserving judgment on efficacy until further data are available.
“There is a potential for bias when comparing a small biopsy, which might not represent the whole tumor, with the resected tumor,” he said in a statement. “This is not a validated way to measure response to a treatment. It describes a biological effect but whether that has any clinical impact on survival is unproven.”
“Although we do not know for the time being if a major pathological response is correlated with improved survival, this method could first be validated in a cohort of patients with advanced disease by comparing the percentages of viable tumor cells in tumor biopsies taken before and 4 to 8 weeks after immunotherapy,” he added. “If in this way regression – as defined in the preoperative study – correlates with survival in patients with advanced cancer, it is likely to hold true in less advanced or resectable patients. Long-term survival data will be the ultimate test for these neoadjuvant immunotherapy strategies.”
COPENHAGEN – Neoadjuvant therapy with the PD-1 checkpoint inhibitor nivolumab is safe, does not delay surgery, and may offer clinical benefit in some patients with early-stage non–small cell lung cancer, preliminary results of a clinical trial show.
Among 17 patients with previously untreated stage I-IIIA non–small cell lung cancer (NSCLC) who had two courses of nivolumab (Opdivo) followed by surgical resection, 12 had pathologic evidence of tumor regression, including 7 who had what investigators termed a “major pathologic response,” defined as less than 10% residual viable tumor, reported Patrick M. Forde, MBBCh, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University in Baltimore.
To see whether immunotherapy could be safe and practical in the neoadjuvant setting, the investigators enrolled 18 patients with untreated, resectable disease and performed pretreatment tumor biopsies. Patients then received doses of nivolumab 3 mg/kg delivered at 4 weeks and 2 weeks prior to scheduled surgery. Following surgery, patients received adjuvant chemotherapy at the investigator’s discretion.
Treatment related adverse events included one grade 3 to 4 toxicity and one event leading to discontinuation. There were no adverse events requiring delay of surgery.
The investigators also conducted an exploratory analysis of response to treatment among 17 who had sufficient follow-up data for evaluation of the primary endpoints of safety and feasibility.
As noted, 12 patients had a measurable tumor response, and 7 had a major pathologic response. Of this latter group, three had no radiographic evidence of response, but tumor specimens from all 7 showed evidence of “substantial” T-cell infiltration, indicating an enhanced immune response, Dr. Forde said.
In all, 9 of the 17 patients had tumor regression of more than 50%, and 7 patients had pathologic downstaging from their pretreatment clinical stage.
Four of the seven tumors with the major pathologic response were tested with a programmed death–1 ligand immunohistochemical assay, and three were positive for the ligand.
The investigators also isolated both unique and shared T-cell clones from peripheral blood, and detected new infiltration of T-cell clones that were not seen in the tumor specimens taken prior to nivolumab therapy.
Based on these early results, the investigators plan to expand the study, with one cohort planned to receive a third presurgical dose of nivolumab, and a second cohort scheduled to receive both nivolumab and ipilimumab (Yervoy).
It remains to be seen, he said, whether patients should also receive maintenance therapy, and whether combined checkpoint inhibitors might be more effective.
However, Pieter Postmus, chair of thoracic oncology at the University of Liverpool (England), who was not involved in the study, said that he is reserving judgment on efficacy until further data are available.
“There is a potential for bias when comparing a small biopsy, which might not represent the whole tumor, with the resected tumor,” he said in a statement. “This is not a validated way to measure response to a treatment. It describes a biological effect but whether that has any clinical impact on survival is unproven.”
“Although we do not know for the time being if a major pathological response is correlated with improved survival, this method could first be validated in a cohort of patients with advanced disease by comparing the percentages of viable tumor cells in tumor biopsies taken before and 4 to 8 weeks after immunotherapy,” he added. “If in this way regression – as defined in the preoperative study – correlates with survival in patients with advanced cancer, it is likely to hold true in less advanced or resectable patients. Long-term survival data will be the ultimate test for these neoadjuvant immunotherapy strategies.”
AT ESMO 2016
Key clinical point: Checkpoint inhibitors have shown good efficacy for treatment of advanced NSCLC, but use in the neoadjuvant setting is still investigational.
Major finding: Seven of 17 patients had a major pathologic response (less than 10% viable tumor remaining).
Data source: Safety and feasibility study in 18 patients with stage I-IIIA non–small cell lung cancer.
Disclosures: The study was supported by the American Association for Cancer Research, the Cancer Research Institute, LUNGevity, and Stand Up to Cancer. Bristol-Myers Squibb donated nivolumab and provided funding for PD-L1 testing. Dr. Forde disclosed institution research grants from Bristol-Myers Squibb, Kyowa, Novartis, and uncompensated consulting for AstraZeneca and Celgene. Dr. Baas disclosed grants and research support from Bristol-Myers Squibb. Dr. Postmus had no disclosures.
Fluorescein, 10% dextrose topped other media for visualizing ureteral patency
DENVER – Ureteral jets were best visualized during cystoscopy with the help of 10% dextrose or sodium fluorescein, instead of phenazopyridine or normal saline, findings from a multicenter, randomized controlled trial suggested.
User satisfaction also was significantly higher with 10% dextrose and fluorescein, compared with the other interventions, Luis Espaillat-Rijo, MD, reported at Pelvic Floor Disorders Week, sponsored by the American Urogynecologic Society.
Average total cystoscopy time, time to first jet visualization, and rates of postoperative urinary tract infections were similar among groups, noted Dr. Espaillat-Rijo, who conducted the research at the Cleveland Clinic Florida in Weston.
Historically, surgeons used intravenous indigo carmine to evaluate ureteral patency during intraoperative cystoscopy, but the Food and Drug Administration announced a shortage in June 2014 that has not resolved.
Hypothesizing that not all alternatives confer equivalent visibility, the researchers randomly assigned 174 female cystoscopy patients aged 18 years or older to one of four interventions: intravenous sodium fluorescein, preoperative oral phenazopyridine (Pyridium), 10% dextrose solution, or normal saline (control).
The researchers excluded patients with kidney disease or a history of surgery for renal or ureteral obstruction. The primary outcome was visibility of the urine jet, which surgeons described as clearly visible, somewhat visible, or not at all visible. Surgeons also were asked how satisfied or confident they were in assessing ureteral patency with the test media, Dr. Espaillat-Rijo said.
The study groups were demographically similar. Fluorescein and 10% dextrose resulted in the highest visibility, with more than 80% of surgeons describing the ureteral jets as “clearly visible” with these modalities, compared with about 60% of cases in which phenazopyridine or saline was used (P = .001, for differences among groups).
Similarly, more than 80% of surgeons reported being very or somewhat satisfied with 10% dextrose and fluorescein, while about 60% of surgeons said they were very or somewhat satisfied with phenazopyridine and saline (P less than .001).
“It was interesting that phenazopyridine was not more visible than control saline,” Dr. Espaillat-Rijo said. “Surgeons also noted that it was harder to evaluate the uroepithelium when it was tinged with Pyridium.”
Use of these interventions did not add time to the procedure, he noted. Total cystoscopy time averaged 4.5 minutes and was similar among groups. Average time to detection of the first jet also was similar among all four interventions, ranging from 1.8 to 2.5 minutes.
None of the patients had allergic reactions or adverse events considered related to an intervention. The rates of urinary tract infection ranged from approximately 22% to 30% and were similar among groups. Three ureteral obstructions were correctly identified and released intraoperatively, and none was identified postoperatively. One patient had an episode of acute urinary retention 4 days after surgery that led to bladder rupture.
“Because we had no postoperative ureteral injuries, we were unable to detect the sensitivity or specificity for each method,” Dr. Espaillat-Rijo said. “Another limitation is that we did not have the power to find a difference in our secondary outcomes, and indigo carmine was not used as a control.”
The Cleveland Clinic Florida sponsored the study. The researchers reported having no relevant financial disclosures.
DENVER – Ureteral jets were best visualized during cystoscopy with the help of 10% dextrose or sodium fluorescein, instead of phenazopyridine or normal saline, findings from a multicenter, randomized controlled trial suggested.
User satisfaction also was significantly higher with 10% dextrose and fluorescein, compared with the other interventions, Luis Espaillat-Rijo, MD, reported at Pelvic Floor Disorders Week, sponsored by the American Urogynecologic Society.
Average total cystoscopy time, time to first jet visualization, and rates of postoperative urinary tract infections were similar among groups, noted Dr. Espaillat-Rijo, who conducted the research at the Cleveland Clinic Florida in Weston.
Historically, surgeons used intravenous indigo carmine to evaluate ureteral patency during intraoperative cystoscopy, but the Food and Drug Administration announced a shortage in June 2014 that has not resolved.
Hypothesizing that not all alternatives confer equivalent visibility, the researchers randomly assigned 174 female cystoscopy patients aged 18 years or older to one of four interventions: intravenous sodium fluorescein, preoperative oral phenazopyridine (Pyridium), 10% dextrose solution, or normal saline (control).
The researchers excluded patients with kidney disease or a history of surgery for renal or ureteral obstruction. The primary outcome was visibility of the urine jet, which surgeons described as clearly visible, somewhat visible, or not at all visible. Surgeons also were asked how satisfied or confident they were in assessing ureteral patency with the test media, Dr. Espaillat-Rijo said.
The study groups were demographically similar. Fluorescein and 10% dextrose resulted in the highest visibility, with more than 80% of surgeons describing the ureteral jets as “clearly visible” with these modalities, compared with about 60% of cases in which phenazopyridine or saline was used (P = .001, for differences among groups).
Similarly, more than 80% of surgeons reported being very or somewhat satisfied with 10% dextrose and fluorescein, while about 60% of surgeons said they were very or somewhat satisfied with phenazopyridine and saline (P less than .001).
“It was interesting that phenazopyridine was not more visible than control saline,” Dr. Espaillat-Rijo said. “Surgeons also noted that it was harder to evaluate the uroepithelium when it was tinged with Pyridium.”
Use of these interventions did not add time to the procedure, he noted. Total cystoscopy time averaged 4.5 minutes and was similar among groups. Average time to detection of the first jet also was similar among all four interventions, ranging from 1.8 to 2.5 minutes.
None of the patients had allergic reactions or adverse events considered related to an intervention. The rates of urinary tract infection ranged from approximately 22% to 30% and were similar among groups. Three ureteral obstructions were correctly identified and released intraoperatively, and none was identified postoperatively. One patient had an episode of acute urinary retention 4 days after surgery that led to bladder rupture.
“Because we had no postoperative ureteral injuries, we were unable to detect the sensitivity or specificity for each method,” Dr. Espaillat-Rijo said. “Another limitation is that we did not have the power to find a difference in our secondary outcomes, and indigo carmine was not used as a control.”
The Cleveland Clinic Florida sponsored the study. The researchers reported having no relevant financial disclosures.
DENVER – Ureteral jets were best visualized during cystoscopy with the help of 10% dextrose or sodium fluorescein, instead of phenazopyridine or normal saline, findings from a multicenter, randomized controlled trial suggested.
User satisfaction also was significantly higher with 10% dextrose and fluorescein, compared with the other interventions, Luis Espaillat-Rijo, MD, reported at Pelvic Floor Disorders Week, sponsored by the American Urogynecologic Society.
Average total cystoscopy time, time to first jet visualization, and rates of postoperative urinary tract infections were similar among groups, noted Dr. Espaillat-Rijo, who conducted the research at the Cleveland Clinic Florida in Weston.
Historically, surgeons used intravenous indigo carmine to evaluate ureteral patency during intraoperative cystoscopy, but the Food and Drug Administration announced a shortage in June 2014 that has not resolved.
Hypothesizing that not all alternatives confer equivalent visibility, the researchers randomly assigned 174 female cystoscopy patients aged 18 years or older to one of four interventions: intravenous sodium fluorescein, preoperative oral phenazopyridine (Pyridium), 10% dextrose solution, or normal saline (control).
The researchers excluded patients with kidney disease or a history of surgery for renal or ureteral obstruction. The primary outcome was visibility of the urine jet, which surgeons described as clearly visible, somewhat visible, or not at all visible. Surgeons also were asked how satisfied or confident they were in assessing ureteral patency with the test media, Dr. Espaillat-Rijo said.
The study groups were demographically similar. Fluorescein and 10% dextrose resulted in the highest visibility, with more than 80% of surgeons describing the ureteral jets as “clearly visible” with these modalities, compared with about 60% of cases in which phenazopyridine or saline was used (P = .001, for differences among groups).
Similarly, more than 80% of surgeons reported being very or somewhat satisfied with 10% dextrose and fluorescein, while about 60% of surgeons said they were very or somewhat satisfied with phenazopyridine and saline (P less than .001).
“It was interesting that phenazopyridine was not more visible than control saline,” Dr. Espaillat-Rijo said. “Surgeons also noted that it was harder to evaluate the uroepithelium when it was tinged with Pyridium.”
Use of these interventions did not add time to the procedure, he noted. Total cystoscopy time averaged 4.5 minutes and was similar among groups. Average time to detection of the first jet also was similar among all four interventions, ranging from 1.8 to 2.5 minutes.
None of the patients had allergic reactions or adverse events considered related to an intervention. The rates of urinary tract infection ranged from approximately 22% to 30% and were similar among groups. Three ureteral obstructions were correctly identified and released intraoperatively, and none was identified postoperatively. One patient had an episode of acute urinary retention 4 days after surgery that led to bladder rupture.
“Because we had no postoperative ureteral injuries, we were unable to detect the sensitivity or specificity for each method,” Dr. Espaillat-Rijo said. “Another limitation is that we did not have the power to find a difference in our secondary outcomes, and indigo carmine was not used as a control.”
The Cleveland Clinic Florida sponsored the study. The researchers reported having no relevant financial disclosures.
Key clinical point: Major finding: Visibility of the ureteral jet was significantly greater with 10% dextrose and oral phenazopyridine than with intravenous fluorescein or saline (P = .001).
Data source: A multicenter, randomized controlled trial of 174 women undergoing intraoperative cystoscopy.
Disclosures: The Cleveland Clinic Florida sponsored the study. The researchers reported having no relevant financial disclosures.
United States nears 2,500 Zika cases in pregnant women
The week also included a report of another live-born infant with Zika-related birth defects, bringing the U.S. total to 23 for the year: 22 in the 50 states and District of Columbia and 1 in the territories, the CDC reported Oct. 6. There also have been six Zika-related pregnancy losses reported so far in the states and territories, a number that has not changed since early August.
There have been 28,019 cases of Zika reported among all Americans as of Oct. 5, with the majority (86%) coming from the territories and the majority of those cases (98%) coming from Puerto Rico, according to an update from the CDC’s Arboviral Disease Branch.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
The week also included a report of another live-born infant with Zika-related birth defects, bringing the U.S. total to 23 for the year: 22 in the 50 states and District of Columbia and 1 in the territories, the CDC reported Oct. 6. There also have been six Zika-related pregnancy losses reported so far in the states and territories, a number that has not changed since early August.
There have been 28,019 cases of Zika reported among all Americans as of Oct. 5, with the majority (86%) coming from the territories and the majority of those cases (98%) coming from Puerto Rico, according to an update from the CDC’s Arboviral Disease Branch.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
The week also included a report of another live-born infant with Zika-related birth defects, bringing the U.S. total to 23 for the year: 22 in the 50 states and District of Columbia and 1 in the territories, the CDC reported Oct. 6. There also have been six Zika-related pregnancy losses reported so far in the states and territories, a number that has not changed since early August.
There have been 28,019 cases of Zika reported among all Americans as of Oct. 5, with the majority (86%) coming from the territories and the majority of those cases (98%) coming from Puerto Rico, according to an update from the CDC’s Arboviral Disease Branch.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
NAMS 2016 hormone therapy position statement
JoAnn Pinkerton, MD, Professor of Obstetrics and Gynecology at the University of Virginia, Executive Director of the North American Menopause Society (NAMS), and OBG Management Board of Editors Member, revealed the 2016 NAMS position statement on hormone therapy (HT) in Orlando, Florida, on Thursday, October 6, at the NAMS 2016 Annual Scientific Meeting.
The process of consensus among the more than 20 menopause experts who authored the 2016 statement was at times a challenge, indicated Pinkerton, given the variance in views on the significance of published clinical trial findings since the Society’s 20121 HT position statement. Over a 9-month period, the experts developed guidelines for clinicians, using levels of evidence to identify strength of the recommendations.
The clearest benefit for HT to treat hot flashes and prevent bone loss was found for women aged younger than 60 years and within 10 years of menopause onset.
According to the 2016 statement presented at NAMS:
Level I US Food and Drug Administration (FDA)-approved indications for HT include:
- as first-line therapy for women with vasomotor symptoms (VMS) of menopause without contraindications
- possible first-line therapy for prevention of bone loss and fracture in postmenopausal women at elevated risk for fracture (primarily for women aged younger than 60 years and within 10 years of menopause onset)
- low-dose vaginal estrogen as first-line treatment for women with isolated genitourinary symptoms caused by menopause (genitourinary syndrome of menopause [GSM]/vulvovaginal atrophy).
Level II FDA-approved indications for HT include:
- at least until age 52 (the median age of menopause onset) for women with early onset menopause (women with hypogonadism, primary ovarian insufficiency, or premature surgical menopause) and no HT contraindications.
Other level II indications, with observational data indicating benefit over risk, for HT include:
- at least until the median age of menopause for women with early onset menopause
- consideration among women with a family history of breast cancer, although family history is one risk among many for breast cancer that should be assessed
- benefit/risk consideration for women with a BRCA gene mutation who have undergone risk-reducing oophorectomy
- consideration of systemic use until the median age of menopause—after appropriate counseling and in the absence of HT contraindications, with longer duration of HT use individualized.
Level III indications for HT include:
- individualized decisions on use after the age of 60. (The position statement authors did not find that the current Beers criteria recommendation to routinely discontinue HT at age 65 was supported by data.)
The 2016 bottom line on HT
Overall, HT has clear benefits for the treatment of VMS and bone loss prevention, according to the presented position statement. These benefits are most favorable among women aged younger than 60 years who are within 10 years of menopause onset and have no contraindications to HT use. Women older than age 60 who initiate HT beyond 10 years of menopause onset appear to have a less favorable benefit-risk ratio because of elevated risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
The risks of HT vary among women depending on the HT type, duration of use, administration route, timing of treatment initiation, and whether a progestogen is needed. (With longer HT use, estrogen therapy is more favorable than estrogen-progestin therapy.) Therefore, HT should be individualized and reevaluated periodically to maximize the benefits as well as minimize the risks of use, according to the position statement.
Nonhormonal therapies for menopausal symptoms
The Society released its position on nonhormonal management of menopause-associated VMS in 2015.2 Based on examination of 340 original research articles and 105 systematic reviews, clinical and research experts categorized therapies as recommended, recommended with caution, and not recommended at this time.
Recommended non-HT to reduce VMS include:
- cognitive-behavioral therapy
- clinical hypnosis
- low-dose salt of paroxetine (FDA approved for menopausal VMS management)
- other SSRIs/SNRIs
- gabapentinoids
- clonidine.
Recommended-with-caution non-HT for VMS include:
- weight loss
- stress reduction (mindfulness based)
- S-equol derivatives of soy isoflavones
- stellate ganglion block.
Not recommended non-HT for VMS due to negative, insufficient, or inconclusive data include:
- cooling techniques
- avoidance of triggers
- exercise
- yoga
- paced respiration
- relaxation
- over-the-counter supplements and herbs
- acupuncture
- calibration of neural oscillations
- chiropractic interventions.
Note that the NAMS 2016 Hormone Therapy Position Statement was presented at the 2016 Annual Scientific Meeting of the North American Menopause Society, but the statement is not yet published.
- North American Menopause Society. The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause. 2012;19(3):257−271.
- Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015;(11):1155−1172.
JoAnn Pinkerton, MD, Professor of Obstetrics and Gynecology at the University of Virginia, Executive Director of the North American Menopause Society (NAMS), and OBG Management Board of Editors Member, revealed the 2016 NAMS position statement on hormone therapy (HT) in Orlando, Florida, on Thursday, October 6, at the NAMS 2016 Annual Scientific Meeting.
The process of consensus among the more than 20 menopause experts who authored the 2016 statement was at times a challenge, indicated Pinkerton, given the variance in views on the significance of published clinical trial findings since the Society’s 20121 HT position statement. Over a 9-month period, the experts developed guidelines for clinicians, using levels of evidence to identify strength of the recommendations.
The clearest benefit for HT to treat hot flashes and prevent bone loss was found for women aged younger than 60 years and within 10 years of menopause onset.
According to the 2016 statement presented at NAMS:
Level I US Food and Drug Administration (FDA)-approved indications for HT include:
- as first-line therapy for women with vasomotor symptoms (VMS) of menopause without contraindications
- possible first-line therapy for prevention of bone loss and fracture in postmenopausal women at elevated risk for fracture (primarily for women aged younger than 60 years and within 10 years of menopause onset)
- low-dose vaginal estrogen as first-line treatment for women with isolated genitourinary symptoms caused by menopause (genitourinary syndrome of menopause [GSM]/vulvovaginal atrophy).
Level II FDA-approved indications for HT include:
- at least until age 52 (the median age of menopause onset) for women with early onset menopause (women with hypogonadism, primary ovarian insufficiency, or premature surgical menopause) and no HT contraindications.
Other level II indications, with observational data indicating benefit over risk, for HT include:
- at least until the median age of menopause for women with early onset menopause
- consideration among women with a family history of breast cancer, although family history is one risk among many for breast cancer that should be assessed
- benefit/risk consideration for women with a BRCA gene mutation who have undergone risk-reducing oophorectomy
- consideration of systemic use until the median age of menopause—after appropriate counseling and in the absence of HT contraindications, with longer duration of HT use individualized.
Level III indications for HT include:
- individualized decisions on use after the age of 60. (The position statement authors did not find that the current Beers criteria recommendation to routinely discontinue HT at age 65 was supported by data.)
The 2016 bottom line on HT
Overall, HT has clear benefits for the treatment of VMS and bone loss prevention, according to the presented position statement. These benefits are most favorable among women aged younger than 60 years who are within 10 years of menopause onset and have no contraindications to HT use. Women older than age 60 who initiate HT beyond 10 years of menopause onset appear to have a less favorable benefit-risk ratio because of elevated risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
The risks of HT vary among women depending on the HT type, duration of use, administration route, timing of treatment initiation, and whether a progestogen is needed. (With longer HT use, estrogen therapy is more favorable than estrogen-progestin therapy.) Therefore, HT should be individualized and reevaluated periodically to maximize the benefits as well as minimize the risks of use, according to the position statement.
Nonhormonal therapies for menopausal symptoms
The Society released its position on nonhormonal management of menopause-associated VMS in 2015.2 Based on examination of 340 original research articles and 105 systematic reviews, clinical and research experts categorized therapies as recommended, recommended with caution, and not recommended at this time.
Recommended non-HT to reduce VMS include:
- cognitive-behavioral therapy
- clinical hypnosis
- low-dose salt of paroxetine (FDA approved for menopausal VMS management)
- other SSRIs/SNRIs
- gabapentinoids
- clonidine.
Recommended-with-caution non-HT for VMS include:
- weight loss
- stress reduction (mindfulness based)
- S-equol derivatives of soy isoflavones
- stellate ganglion block.
Not recommended non-HT for VMS due to negative, insufficient, or inconclusive data include:
- cooling techniques
- avoidance of triggers
- exercise
- yoga
- paced respiration
- relaxation
- over-the-counter supplements and herbs
- acupuncture
- calibration of neural oscillations
- chiropractic interventions.
Note that the NAMS 2016 Hormone Therapy Position Statement was presented at the 2016 Annual Scientific Meeting of the North American Menopause Society, but the statement is not yet published.
JoAnn Pinkerton, MD, Professor of Obstetrics and Gynecology at the University of Virginia, Executive Director of the North American Menopause Society (NAMS), and OBG Management Board of Editors Member, revealed the 2016 NAMS position statement on hormone therapy (HT) in Orlando, Florida, on Thursday, October 6, at the NAMS 2016 Annual Scientific Meeting.
The process of consensus among the more than 20 menopause experts who authored the 2016 statement was at times a challenge, indicated Pinkerton, given the variance in views on the significance of published clinical trial findings since the Society’s 20121 HT position statement. Over a 9-month period, the experts developed guidelines for clinicians, using levels of evidence to identify strength of the recommendations.
The clearest benefit for HT to treat hot flashes and prevent bone loss was found for women aged younger than 60 years and within 10 years of menopause onset.
According to the 2016 statement presented at NAMS:
Level I US Food and Drug Administration (FDA)-approved indications for HT include:
- as first-line therapy for women with vasomotor symptoms (VMS) of menopause without contraindications
- possible first-line therapy for prevention of bone loss and fracture in postmenopausal women at elevated risk for fracture (primarily for women aged younger than 60 years and within 10 years of menopause onset)
- low-dose vaginal estrogen as first-line treatment for women with isolated genitourinary symptoms caused by menopause (genitourinary syndrome of menopause [GSM]/vulvovaginal atrophy).
Level II FDA-approved indications for HT include:
- at least until age 52 (the median age of menopause onset) for women with early onset menopause (women with hypogonadism, primary ovarian insufficiency, or premature surgical menopause) and no HT contraindications.
Other level II indications, with observational data indicating benefit over risk, for HT include:
- at least until the median age of menopause for women with early onset menopause
- consideration among women with a family history of breast cancer, although family history is one risk among many for breast cancer that should be assessed
- benefit/risk consideration for women with a BRCA gene mutation who have undergone risk-reducing oophorectomy
- consideration of systemic use until the median age of menopause—after appropriate counseling and in the absence of HT contraindications, with longer duration of HT use individualized.
Level III indications for HT include:
- individualized decisions on use after the age of 60. (The position statement authors did not find that the current Beers criteria recommendation to routinely discontinue HT at age 65 was supported by data.)
The 2016 bottom line on HT
Overall, HT has clear benefits for the treatment of VMS and bone loss prevention, according to the presented position statement. These benefits are most favorable among women aged younger than 60 years who are within 10 years of menopause onset and have no contraindications to HT use. Women older than age 60 who initiate HT beyond 10 years of menopause onset appear to have a less favorable benefit-risk ratio because of elevated risks of coronary heart disease, stroke, venous thromboembolism, and dementia.
The risks of HT vary among women depending on the HT type, duration of use, administration route, timing of treatment initiation, and whether a progestogen is needed. (With longer HT use, estrogen therapy is more favorable than estrogen-progestin therapy.) Therefore, HT should be individualized and reevaluated periodically to maximize the benefits as well as minimize the risks of use, according to the position statement.
Nonhormonal therapies for menopausal symptoms
The Society released its position on nonhormonal management of menopause-associated VMS in 2015.2 Based on examination of 340 original research articles and 105 systematic reviews, clinical and research experts categorized therapies as recommended, recommended with caution, and not recommended at this time.
Recommended non-HT to reduce VMS include:
- cognitive-behavioral therapy
- clinical hypnosis
- low-dose salt of paroxetine (FDA approved for menopausal VMS management)
- other SSRIs/SNRIs
- gabapentinoids
- clonidine.
Recommended-with-caution non-HT for VMS include:
- weight loss
- stress reduction (mindfulness based)
- S-equol derivatives of soy isoflavones
- stellate ganglion block.
Not recommended non-HT for VMS due to negative, insufficient, or inconclusive data include:
- cooling techniques
- avoidance of triggers
- exercise
- yoga
- paced respiration
- relaxation
- over-the-counter supplements and herbs
- acupuncture
- calibration of neural oscillations
- chiropractic interventions.
Note that the NAMS 2016 Hormone Therapy Position Statement was presented at the 2016 Annual Scientific Meeting of the North American Menopause Society, but the statement is not yet published.
- North American Menopause Society. The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause. 2012;19(3):257−271.
- Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015;(11):1155−1172.
- North American Menopause Society. The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause. 2012;19(3):257−271.
- Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015;(11):1155−1172.
Cost may hinder timely TKI initiation for CML
Nearly a third of Medicare beneficiaries with chronic myeloid leukemia (CML) did not initiate tyrosine kinase inhibitor therapy within 6 months of diagnosis, according to a review of SEER-Medicare data.
The findings suggest that out-of-pocket costs might be a barrier to timely initiation of tyrosine kinase inhibitor (TKI) therapy in CML patients, Aaron N. Winn of the University of North Carolina at Chapel Hill and his colleagues reported online ahead of print in the Journal of Clinical Oncology.
Of 393 individuals diagnosed with CML between 2007 and 2011, only 68% initiated TKI therapy within 180 days (median, 75 days), and 61% of those patients were adherent. Earlier treatment initiation was associated with receipt of cost-sharing subsidies (hazard ratio, 1.35), more-recent diagnosis (HR, 1.14), and living in a big metropolitan area (HR, 1.80) or metropolitan area vs. an urban area (HR, 1.84), while later treatment initiation was associated with higher levels of comorbidity (HR, 0.81) and age older than 80 years vs. age younger than 70 years (HR, 0.53)
Multivariate analysis showed that therapy initiation within 180 days was significantly more likely among those with more-recent diagnosis (relative risk, 1.06) and those living in a large metropolitan area vs. an urban area (RR, 1.57), and was significantly less likely among those older than age 80 years vs. those younger than age 70 years (RR, 0.71). Adherence within 180 days of therapy initiation was higher for those diagnosed in more-recent years (RR, 1.07) and lower for patients aged 80 years or older vs. 66-69 years (RR, 0.74), the investigators found (J Clin Oncol. 2016 Oct 3. doi: 10/1200/JCO.2016.67.4184).
“Our findings highlight important gaps in TKI use among Medicare beneficiaries with CML and suggest that high cost sharing may result in delays in initiation of these life-saving medications,” they concluded.
This study was supported by a University of North Carolina Clinical and Translational Science award, the UNC School of Medicine, the Royster Society of Fellows at UNC Chapel Hill, and by grants from the National Institutes of Health, North Carolina Translational and Clinical Sciences Institute, and the National Cancer Institute. The authors reported having no disclosures.
Nearly a third of Medicare beneficiaries with chronic myeloid leukemia (CML) did not initiate tyrosine kinase inhibitor therapy within 6 months of diagnosis, according to a review of SEER-Medicare data.
The findings suggest that out-of-pocket costs might be a barrier to timely initiation of tyrosine kinase inhibitor (TKI) therapy in CML patients, Aaron N. Winn of the University of North Carolina at Chapel Hill and his colleagues reported online ahead of print in the Journal of Clinical Oncology.
Of 393 individuals diagnosed with CML between 2007 and 2011, only 68% initiated TKI therapy within 180 days (median, 75 days), and 61% of those patients were adherent. Earlier treatment initiation was associated with receipt of cost-sharing subsidies (hazard ratio, 1.35), more-recent diagnosis (HR, 1.14), and living in a big metropolitan area (HR, 1.80) or metropolitan area vs. an urban area (HR, 1.84), while later treatment initiation was associated with higher levels of comorbidity (HR, 0.81) and age older than 80 years vs. age younger than 70 years (HR, 0.53)
Multivariate analysis showed that therapy initiation within 180 days was significantly more likely among those with more-recent diagnosis (relative risk, 1.06) and those living in a large metropolitan area vs. an urban area (RR, 1.57), and was significantly less likely among those older than age 80 years vs. those younger than age 70 years (RR, 0.71). Adherence within 180 days of therapy initiation was higher for those diagnosed in more-recent years (RR, 1.07) and lower for patients aged 80 years or older vs. 66-69 years (RR, 0.74), the investigators found (J Clin Oncol. 2016 Oct 3. doi: 10/1200/JCO.2016.67.4184).
“Our findings highlight important gaps in TKI use among Medicare beneficiaries with CML and suggest that high cost sharing may result in delays in initiation of these life-saving medications,” they concluded.
This study was supported by a University of North Carolina Clinical and Translational Science award, the UNC School of Medicine, the Royster Society of Fellows at UNC Chapel Hill, and by grants from the National Institutes of Health, North Carolina Translational and Clinical Sciences Institute, and the National Cancer Institute. The authors reported having no disclosures.
Nearly a third of Medicare beneficiaries with chronic myeloid leukemia (CML) did not initiate tyrosine kinase inhibitor therapy within 6 months of diagnosis, according to a review of SEER-Medicare data.
The findings suggest that out-of-pocket costs might be a barrier to timely initiation of tyrosine kinase inhibitor (TKI) therapy in CML patients, Aaron N. Winn of the University of North Carolina at Chapel Hill and his colleagues reported online ahead of print in the Journal of Clinical Oncology.
Of 393 individuals diagnosed with CML between 2007 and 2011, only 68% initiated TKI therapy within 180 days (median, 75 days), and 61% of those patients were adherent. Earlier treatment initiation was associated with receipt of cost-sharing subsidies (hazard ratio, 1.35), more-recent diagnosis (HR, 1.14), and living in a big metropolitan area (HR, 1.80) or metropolitan area vs. an urban area (HR, 1.84), while later treatment initiation was associated with higher levels of comorbidity (HR, 0.81) and age older than 80 years vs. age younger than 70 years (HR, 0.53)
Multivariate analysis showed that therapy initiation within 180 days was significantly more likely among those with more-recent diagnosis (relative risk, 1.06) and those living in a large metropolitan area vs. an urban area (RR, 1.57), and was significantly less likely among those older than age 80 years vs. those younger than age 70 years (RR, 0.71). Adherence within 180 days of therapy initiation was higher for those diagnosed in more-recent years (RR, 1.07) and lower for patients aged 80 years or older vs. 66-69 years (RR, 0.74), the investigators found (J Clin Oncol. 2016 Oct 3. doi: 10/1200/JCO.2016.67.4184).
“Our findings highlight important gaps in TKI use among Medicare beneficiaries with CML and suggest that high cost sharing may result in delays in initiation of these life-saving medications,” they concluded.
This study was supported by a University of North Carolina Clinical and Translational Science award, the UNC School of Medicine, the Royster Society of Fellows at UNC Chapel Hill, and by grants from the National Institutes of Health, North Carolina Translational and Clinical Sciences Institute, and the National Cancer Institute. The authors reported having no disclosures.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: TKI initiation within 180 days was significantly less likely among those older than age 80 years vs. those under age 70 years (relative risk, 0.71).
Data source: A review of SEER-Medicare data for 393 patients.
Disclosures: This study was supported by a University of North Carolina Clinical and Translational Science award, the UNC School of Medicine, the Royster Society of Fellows at UNC Chapel Hill, and by grants from the National Institutes of Health, North Carolina Translational and Clinical Sciences Institute, and the National Cancer Institute. The authors reported having no disclosures.
Breast arterial calcifications predict atherosclerotic cardiovascular events
Longitudinal results of a prospective study of women with and without breast arterial calcifications showed that women with the calcifications were significantly more likely to have atherosclerotic cardiovascular events than were women without them.
In a 10-year follow-up of a cohort of women receiving screening mammograms, women who did not have cardiovascular disease at baseline and were positive for breast arterial calcifications (BACs) were three times more likely to develop atherosclerotic cardiovascular disease (ASCVD) as those who were BAC negative at baseline (9.8% vs. 3.3%; P = .001).
These results paralleled those found 5 years previously among the cohort. At that point, 6.3% of the BAC-positive group developed ASCVD, compared with 2.3% of the BAC-negative group (P = .003). At 10 years, “multiple logistic regression analysis found BAC to be strongly associated with ASCVD events, with an odds ratio of 2.29,” senior investigator Peter Schnatz, DO, said in an interview.
Based on these results, Dr. Schnatz and his coinvestigators are suggesting that BACs be routinely reported on mammograms and viewed as a marker for the development of cardiovascular disease.
Presenting the unpublished 10-year findings at the annual meeting of the North American Menopause Society, Dr. Schnatz, the society’s 2015-2016 president, said that the BAC-positive group was also more likely to develop risk factors for ASCVD (86.8% vs. 76.3; P = .01).
These risk factors were age, hypertension, hypercholesterolemia, diabetes, and menopause. The other results were significant after the investigators controlled for age, so the increased risk was not merely caused by the passage of time and the normal aging process.
The prospective study compared age-matched controls with and without BACs to determine whether BACs seen on routine mammography can predict the development of ASCVD, by tracking the presence of BACs and gathering information about ASCVD risk factors and events via patient self-report in an annual questionnaire. The events that were considered ASCVD markers were angina, myocardial infarction, an abnormal angiogram, coronary artery bypass grafting, and stroke.
The initial baseline study upon which the longitudinal prospective studies are based gathered data from 1,919 women with a median age of 56, plus or minus 12.7 years (range, 25-96), to determine whether women with BAC had an increased frequency of cardiovascular disease risk factors, and of ASCVD. Baseline findings showed an independent association of BAC with multiple risk factors and with ASCVD events, even after age was controlled for.
Dr. Schnatz, who is a professor of ob.gyn. and internal medicine at Thomas Jefferson University, Philadelphia, said in his presentation that BACs “appear to be a risk indicator of the presence of ASCVD. More importantly, in this first prospective analysis of BAC as a risk predictor, BAC appears to be a risk predictor for the future development of ASCVD.”
Patients were considered BAC positive if their mammograms showed BAC on at least one of two standard views of either or both breasts. The screening mammograms were read in a uniform fashion by 1 of 21 radiologists, who used identical and well-accepted criteria for BACs. Overall, 268 women (14%) were BAC positive, in line with the 9%-17.5% prevalence reported in the literature, Dr. Schnatz said.
BACs are diffuse calcifications of the arterial tunica media, which are common but often unreported by radiologists who find them on screening mammograms, though they are seen in up to 17.5% of mammograms.
Medial arterial calcifications, such as BACs, are fine-grained deposits seen in small- to medium-sized muscular arteries. Though they have been observed for some time, their significance has been unknown, and they are often seen as part of the normal aging process, Dr. Schnatz said. With the advent of newer mammography technology in the 2000s, much smaller calcifications could be seen, and research into the significance of BACs detected on screening mammogram was revived and refined, he said.
“If BAC has value as a marker for coronary artery disease, then mammograms could be a practical tool for detecting CAD risk in women,” Dr. Schnatz said. “This might contribute to earlier detection of vascular damage, especially important in women at high risk of CAD or with unrecognized heart disease.”
Dr. Schnatz said he plans to incorporate BAC status into validated cardiovascular risk predictors, and see how a prediction model that includes BAC fares.
Dr. Schnatz reported having no relevant financial disclosures.
[email protected]
On Twitter @karioakes
Longitudinal results of a prospective study of women with and without breast arterial calcifications showed that women with the calcifications were significantly more likely to have atherosclerotic cardiovascular events than were women without them.
In a 10-year follow-up of a cohort of women receiving screening mammograms, women who did not have cardiovascular disease at baseline and were positive for breast arterial calcifications (BACs) were three times more likely to develop atherosclerotic cardiovascular disease (ASCVD) as those who were BAC negative at baseline (9.8% vs. 3.3%; P = .001).
These results paralleled those found 5 years previously among the cohort. At that point, 6.3% of the BAC-positive group developed ASCVD, compared with 2.3% of the BAC-negative group (P = .003). At 10 years, “multiple logistic regression analysis found BAC to be strongly associated with ASCVD events, with an odds ratio of 2.29,” senior investigator Peter Schnatz, DO, said in an interview.
Based on these results, Dr. Schnatz and his coinvestigators are suggesting that BACs be routinely reported on mammograms and viewed as a marker for the development of cardiovascular disease.
Presenting the unpublished 10-year findings at the annual meeting of the North American Menopause Society, Dr. Schnatz, the society’s 2015-2016 president, said that the BAC-positive group was also more likely to develop risk factors for ASCVD (86.8% vs. 76.3; P = .01).
These risk factors were age, hypertension, hypercholesterolemia, diabetes, and menopause. The other results were significant after the investigators controlled for age, so the increased risk was not merely caused by the passage of time and the normal aging process.
The prospective study compared age-matched controls with and without BACs to determine whether BACs seen on routine mammography can predict the development of ASCVD, by tracking the presence of BACs and gathering information about ASCVD risk factors and events via patient self-report in an annual questionnaire. The events that were considered ASCVD markers were angina, myocardial infarction, an abnormal angiogram, coronary artery bypass grafting, and stroke.
The initial baseline study upon which the longitudinal prospective studies are based gathered data from 1,919 women with a median age of 56, plus or minus 12.7 years (range, 25-96), to determine whether women with BAC had an increased frequency of cardiovascular disease risk factors, and of ASCVD. Baseline findings showed an independent association of BAC with multiple risk factors and with ASCVD events, even after age was controlled for.
Dr. Schnatz, who is a professor of ob.gyn. and internal medicine at Thomas Jefferson University, Philadelphia, said in his presentation that BACs “appear to be a risk indicator of the presence of ASCVD. More importantly, in this first prospective analysis of BAC as a risk predictor, BAC appears to be a risk predictor for the future development of ASCVD.”
Patients were considered BAC positive if their mammograms showed BAC on at least one of two standard views of either or both breasts. The screening mammograms were read in a uniform fashion by 1 of 21 radiologists, who used identical and well-accepted criteria for BACs. Overall, 268 women (14%) were BAC positive, in line with the 9%-17.5% prevalence reported in the literature, Dr. Schnatz said.
BACs are diffuse calcifications of the arterial tunica media, which are common but often unreported by radiologists who find them on screening mammograms, though they are seen in up to 17.5% of mammograms.
Medial arterial calcifications, such as BACs, are fine-grained deposits seen in small- to medium-sized muscular arteries. Though they have been observed for some time, their significance has been unknown, and they are often seen as part of the normal aging process, Dr. Schnatz said. With the advent of newer mammography technology in the 2000s, much smaller calcifications could be seen, and research into the significance of BACs detected on screening mammogram was revived and refined, he said.
“If BAC has value as a marker for coronary artery disease, then mammograms could be a practical tool for detecting CAD risk in women,” Dr. Schnatz said. “This might contribute to earlier detection of vascular damage, especially important in women at high risk of CAD or with unrecognized heart disease.”
Dr. Schnatz said he plans to incorporate BAC status into validated cardiovascular risk predictors, and see how a prediction model that includes BAC fares.
Dr. Schnatz reported having no relevant financial disclosures.
[email protected]
On Twitter @karioakes
Longitudinal results of a prospective study of women with and without breast arterial calcifications showed that women with the calcifications were significantly more likely to have atherosclerotic cardiovascular events than were women without them.
In a 10-year follow-up of a cohort of women receiving screening mammograms, women who did not have cardiovascular disease at baseline and were positive for breast arterial calcifications (BACs) were three times more likely to develop atherosclerotic cardiovascular disease (ASCVD) as those who were BAC negative at baseline (9.8% vs. 3.3%; P = .001).
These results paralleled those found 5 years previously among the cohort. At that point, 6.3% of the BAC-positive group developed ASCVD, compared with 2.3% of the BAC-negative group (P = .003). At 10 years, “multiple logistic regression analysis found BAC to be strongly associated with ASCVD events, with an odds ratio of 2.29,” senior investigator Peter Schnatz, DO, said in an interview.
Based on these results, Dr. Schnatz and his coinvestigators are suggesting that BACs be routinely reported on mammograms and viewed as a marker for the development of cardiovascular disease.
Presenting the unpublished 10-year findings at the annual meeting of the North American Menopause Society, Dr. Schnatz, the society’s 2015-2016 president, said that the BAC-positive group was also more likely to develop risk factors for ASCVD (86.8% vs. 76.3; P = .01).
These risk factors were age, hypertension, hypercholesterolemia, diabetes, and menopause. The other results were significant after the investigators controlled for age, so the increased risk was not merely caused by the passage of time and the normal aging process.
The prospective study compared age-matched controls with and without BACs to determine whether BACs seen on routine mammography can predict the development of ASCVD, by tracking the presence of BACs and gathering information about ASCVD risk factors and events via patient self-report in an annual questionnaire. The events that were considered ASCVD markers were angina, myocardial infarction, an abnormal angiogram, coronary artery bypass grafting, and stroke.
The initial baseline study upon which the longitudinal prospective studies are based gathered data from 1,919 women with a median age of 56, plus or minus 12.7 years (range, 25-96), to determine whether women with BAC had an increased frequency of cardiovascular disease risk factors, and of ASCVD. Baseline findings showed an independent association of BAC with multiple risk factors and with ASCVD events, even after age was controlled for.
Dr. Schnatz, who is a professor of ob.gyn. and internal medicine at Thomas Jefferson University, Philadelphia, said in his presentation that BACs “appear to be a risk indicator of the presence of ASCVD. More importantly, in this first prospective analysis of BAC as a risk predictor, BAC appears to be a risk predictor for the future development of ASCVD.”
Patients were considered BAC positive if their mammograms showed BAC on at least one of two standard views of either or both breasts. The screening mammograms were read in a uniform fashion by 1 of 21 radiologists, who used identical and well-accepted criteria for BACs. Overall, 268 women (14%) were BAC positive, in line with the 9%-17.5% prevalence reported in the literature, Dr. Schnatz said.
BACs are diffuse calcifications of the arterial tunica media, which are common but often unreported by radiologists who find them on screening mammograms, though they are seen in up to 17.5% of mammograms.
Medial arterial calcifications, such as BACs, are fine-grained deposits seen in small- to medium-sized muscular arteries. Though they have been observed for some time, their significance has been unknown, and they are often seen as part of the normal aging process, Dr. Schnatz said. With the advent of newer mammography technology in the 2000s, much smaller calcifications could be seen, and research into the significance of BACs detected on screening mammogram was revived and refined, he said.
“If BAC has value as a marker for coronary artery disease, then mammograms could be a practical tool for detecting CAD risk in women,” Dr. Schnatz said. “This might contribute to earlier detection of vascular damage, especially important in women at high risk of CAD or with unrecognized heart disease.”
Dr. Schnatz said he plans to incorporate BAC status into validated cardiovascular risk predictors, and see how a prediction model that includes BAC fares.
Dr. Schnatz reported having no relevant financial disclosures.
[email protected]
On Twitter @karioakes
FROM THE NAMS 2016 ANNUAL MEETING
Key clinical point:
Major finding: BAC-positive women were three times more likely to develop ASCVD than were BAC-negative women (9.8% vs. 3.3%; P = .001)
Data source: A prospective longitudinal study of an initial cohort of 1,919 women receiving screening mammograms.
Disclosures: No outside funding source was reported. Dr. Schnatz reported having no relevant financial disclosures.