User login
Widow of Robin Williams places his suicide in context
Where – and how – do we even begin to talk about suicide? In psychiatry, we understand it as a product of mental illness: an act borne of the hopelessness of depression or as a way to escape psychic torment. In that sense, it is understandable and preventable: All we need to do is educate people about the symptoms and destigmatize the disorders so that those who have them will seek treatment. Suicide is an epidemic, and tens of thousands of people die this way every year. The figures quoted are that 90% of those who die from suicide suffer from a psychiatric illness, most often a mood disorder.
It’s a simple equation, and often the assumption is made that the suicidal person did not recognize his illness, did not know how to get help, did not believe treatment would work, was fearful of the stigma or consequences of seeking help, could not access care (because that is no simple task), or did not get the right care. It’s perplexing that suicide rates have continued to rise when the rates of antidepressant use also have risen. And while we don’t want to stigmatize mental illness, we do want to stigmatize suicide; it shouldn’t be anyone’s answer to life’s inevitable rough patches.
Soon after his death, it was made public that Robin Williams suffered from Parkinson’s disease, then later that was revised – he had Lewy body dementia.
On Sept. 27, his widow, Susan Schneider Williams, published an article called “The terrorist inside my husband’s brain” in the journal Neurology (2016. 87[13]:1308-11).
Mrs. Williams writes about the joy of their relationship, and she notes that many months before he died, her husband was under the care of doctors for a multitude of symptoms, including gastrointestinal problems, insomnia, and a tremor. His symptoms worsened, and he became plagued by anxiety and panic, memory difficulties, and delusions with paranoia. She describes a change in his personality and a preoccupation with his anxiety, physical failings, and memory problems that interfered with his ability to memorize movie lines. Robin Williams was changing and declining. He was treated with both psychotherapy and psychotropic medications. He went to Stanford for hypnosis to treat his anxiety. He exercised with a physical trainer. In May, he received the Parkinson’s disease diagnosis, and while he was told that it was early and mild, his wife wrote,
Robin was growing weary. The parkinsonian mask was ever present and his voice was weakened. His left hand tremor was continuous now and he had a slow, shuffling gait. He hated that he could not find the words he wanted in conversations. He would thrash at night and still had terrible insomnia. At times, he would find himself stuck in a frozen stance, unable to move, and frustrated when he came out of it. He was beginning to have trouble with visual and spatial abilities in the way of judging distance and depth. His loss of basic reasoning just added to his growing confusion.
Just months later, Robin Williams took his own life.
The story doesn’t fit the simple equation: Mr. Williams knew something was wrong, he sought help, he received psychiatric care, and he ended his life, anyway. Could more have been done? Of course, there are always more treatments that can be tried to address depression, but more may not have helped. The article notes that he was scheduled to have an inpatient neuropsychiatric assessment. But the truth is that even if a treatment were found that would have lifted his spirits, Robin Williams was suffering from a severe form of an incurable dementing illness, and his wife describes that he was in a great deal of distress with both his symptoms and his decline. This illness is a tragedy, but perhaps his suicide was a rational decision and not a preventable death. As a psychiatrist, it feels like taboo to suggest that suicide might ever be anything but the ultimate failure on both the part of the doctor and the patient, or that there isn’t always hope to be had. Robin Williams most certainly missed out on some good moments in the time he had remaining; his wife describes the pleasures of their last day together. But if he decided that he wanted to escape his suffering and avoid the undeniable decline and debility that he saw in his future, can we – or should we – blame him and call this a preventable tragedy? Is this the suicide that should be stigmatized and used for our “get help” slogans?
Obviously, I can’t know if Robin Williams was competent to make such a decision, or if his family would have suffered less if he’d lived out his natural life, but the truth is that competent or not, he made a choice and without anyone’s input, he took the action he chose.
The issue has become a heated one as some states have legalized physician-assisted suicide. In Belgium, intractable psychiatric illness is considered a valid reason for euthanasia, even in a young person. Make no mistake about my sentiments on this: Doctoring is about healing, and we have no business killing people or aiding in their deaths. Psychiatry, in particular, is about hope. Each person’s life has value, but each person’s life also ends. And while there is tremendous societal value in stigmatizing suicide, not all suicides are the same.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care,” which is due out this fall from Johns Hopkins University Press.
Where – and how – do we even begin to talk about suicide? In psychiatry, we understand it as a product of mental illness: an act borne of the hopelessness of depression or as a way to escape psychic torment. In that sense, it is understandable and preventable: All we need to do is educate people about the symptoms and destigmatize the disorders so that those who have them will seek treatment. Suicide is an epidemic, and tens of thousands of people die this way every year. The figures quoted are that 90% of those who die from suicide suffer from a psychiatric illness, most often a mood disorder.
It’s a simple equation, and often the assumption is made that the suicidal person did not recognize his illness, did not know how to get help, did not believe treatment would work, was fearful of the stigma or consequences of seeking help, could not access care (because that is no simple task), or did not get the right care. It’s perplexing that suicide rates have continued to rise when the rates of antidepressant use also have risen. And while we don’t want to stigmatize mental illness, we do want to stigmatize suicide; it shouldn’t be anyone’s answer to life’s inevitable rough patches.
Soon after his death, it was made public that Robin Williams suffered from Parkinson’s disease, then later that was revised – he had Lewy body dementia.
On Sept. 27, his widow, Susan Schneider Williams, published an article called “The terrorist inside my husband’s brain” in the journal Neurology (2016. 87[13]:1308-11).
Mrs. Williams writes about the joy of their relationship, and she notes that many months before he died, her husband was under the care of doctors for a multitude of symptoms, including gastrointestinal problems, insomnia, and a tremor. His symptoms worsened, and he became plagued by anxiety and panic, memory difficulties, and delusions with paranoia. She describes a change in his personality and a preoccupation with his anxiety, physical failings, and memory problems that interfered with his ability to memorize movie lines. Robin Williams was changing and declining. He was treated with both psychotherapy and psychotropic medications. He went to Stanford for hypnosis to treat his anxiety. He exercised with a physical trainer. In May, he received the Parkinson’s disease diagnosis, and while he was told that it was early and mild, his wife wrote,
Robin was growing weary. The parkinsonian mask was ever present and his voice was weakened. His left hand tremor was continuous now and he had a slow, shuffling gait. He hated that he could not find the words he wanted in conversations. He would thrash at night and still had terrible insomnia. At times, he would find himself stuck in a frozen stance, unable to move, and frustrated when he came out of it. He was beginning to have trouble with visual and spatial abilities in the way of judging distance and depth. His loss of basic reasoning just added to his growing confusion.
Just months later, Robin Williams took his own life.
The story doesn’t fit the simple equation: Mr. Williams knew something was wrong, he sought help, he received psychiatric care, and he ended his life, anyway. Could more have been done? Of course, there are always more treatments that can be tried to address depression, but more may not have helped. The article notes that he was scheduled to have an inpatient neuropsychiatric assessment. But the truth is that even if a treatment were found that would have lifted his spirits, Robin Williams was suffering from a severe form of an incurable dementing illness, and his wife describes that he was in a great deal of distress with both his symptoms and his decline. This illness is a tragedy, but perhaps his suicide was a rational decision and not a preventable death. As a psychiatrist, it feels like taboo to suggest that suicide might ever be anything but the ultimate failure on both the part of the doctor and the patient, or that there isn’t always hope to be had. Robin Williams most certainly missed out on some good moments in the time he had remaining; his wife describes the pleasures of their last day together. But if he decided that he wanted to escape his suffering and avoid the undeniable decline and debility that he saw in his future, can we – or should we – blame him and call this a preventable tragedy? Is this the suicide that should be stigmatized and used for our “get help” slogans?
Obviously, I can’t know if Robin Williams was competent to make such a decision, or if his family would have suffered less if he’d lived out his natural life, but the truth is that competent or not, he made a choice and without anyone’s input, he took the action he chose.
The issue has become a heated one as some states have legalized physician-assisted suicide. In Belgium, intractable psychiatric illness is considered a valid reason for euthanasia, even in a young person. Make no mistake about my sentiments on this: Doctoring is about healing, and we have no business killing people or aiding in their deaths. Psychiatry, in particular, is about hope. Each person’s life has value, but each person’s life also ends. And while there is tremendous societal value in stigmatizing suicide, not all suicides are the same.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care,” which is due out this fall from Johns Hopkins University Press.
Where – and how – do we even begin to talk about suicide? In psychiatry, we understand it as a product of mental illness: an act borne of the hopelessness of depression or as a way to escape psychic torment. In that sense, it is understandable and preventable: All we need to do is educate people about the symptoms and destigmatize the disorders so that those who have them will seek treatment. Suicide is an epidemic, and tens of thousands of people die this way every year. The figures quoted are that 90% of those who die from suicide suffer from a psychiatric illness, most often a mood disorder.
It’s a simple equation, and often the assumption is made that the suicidal person did not recognize his illness, did not know how to get help, did not believe treatment would work, was fearful of the stigma or consequences of seeking help, could not access care (because that is no simple task), or did not get the right care. It’s perplexing that suicide rates have continued to rise when the rates of antidepressant use also have risen. And while we don’t want to stigmatize mental illness, we do want to stigmatize suicide; it shouldn’t be anyone’s answer to life’s inevitable rough patches.
Soon after his death, it was made public that Robin Williams suffered from Parkinson’s disease, then later that was revised – he had Lewy body dementia.
On Sept. 27, his widow, Susan Schneider Williams, published an article called “The terrorist inside my husband’s brain” in the journal Neurology (2016. 87[13]:1308-11).
Mrs. Williams writes about the joy of their relationship, and she notes that many months before he died, her husband was under the care of doctors for a multitude of symptoms, including gastrointestinal problems, insomnia, and a tremor. His symptoms worsened, and he became plagued by anxiety and panic, memory difficulties, and delusions with paranoia. She describes a change in his personality and a preoccupation with his anxiety, physical failings, and memory problems that interfered with his ability to memorize movie lines. Robin Williams was changing and declining. He was treated with both psychotherapy and psychotropic medications. He went to Stanford for hypnosis to treat his anxiety. He exercised with a physical trainer. In May, he received the Parkinson’s disease diagnosis, and while he was told that it was early and mild, his wife wrote,
Robin was growing weary. The parkinsonian mask was ever present and his voice was weakened. His left hand tremor was continuous now and he had a slow, shuffling gait. He hated that he could not find the words he wanted in conversations. He would thrash at night and still had terrible insomnia. At times, he would find himself stuck in a frozen stance, unable to move, and frustrated when he came out of it. He was beginning to have trouble with visual and spatial abilities in the way of judging distance and depth. His loss of basic reasoning just added to his growing confusion.
Just months later, Robin Williams took his own life.
The story doesn’t fit the simple equation: Mr. Williams knew something was wrong, he sought help, he received psychiatric care, and he ended his life, anyway. Could more have been done? Of course, there are always more treatments that can be tried to address depression, but more may not have helped. The article notes that he was scheduled to have an inpatient neuropsychiatric assessment. But the truth is that even if a treatment were found that would have lifted his spirits, Robin Williams was suffering from a severe form of an incurable dementing illness, and his wife describes that he was in a great deal of distress with both his symptoms and his decline. This illness is a tragedy, but perhaps his suicide was a rational decision and not a preventable death. As a psychiatrist, it feels like taboo to suggest that suicide might ever be anything but the ultimate failure on both the part of the doctor and the patient, or that there isn’t always hope to be had. Robin Williams most certainly missed out on some good moments in the time he had remaining; his wife describes the pleasures of their last day together. But if he decided that he wanted to escape his suffering and avoid the undeniable decline and debility that he saw in his future, can we – or should we – blame him and call this a preventable tragedy? Is this the suicide that should be stigmatized and used for our “get help” slogans?
Obviously, I can’t know if Robin Williams was competent to make such a decision, or if his family would have suffered less if he’d lived out his natural life, but the truth is that competent or not, he made a choice and without anyone’s input, he took the action he chose.
The issue has become a heated one as some states have legalized physician-assisted suicide. In Belgium, intractable psychiatric illness is considered a valid reason for euthanasia, even in a young person. Make no mistake about my sentiments on this: Doctoring is about healing, and we have no business killing people or aiding in their deaths. Psychiatry, in particular, is about hope. Each person’s life has value, but each person’s life also ends. And while there is tremendous societal value in stigmatizing suicide, not all suicides are the same.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care,” which is due out this fall from Johns Hopkins University Press.
Acute Inflammatory Skin Reaction During Neutrophil Recovery After Antileukemic Therapy
To the Editor:
A 34-year-old man presented with fever, easy bruising, and pancytopenia with increased peripheral blasts of 77%. Bone marrow biopsy showed hypercellular marrow with 80% to 90% involvement by acute promyelocytic leukemia (APL) with complex cytogenetics: 47,XY,t(4;17;18)(p16;q21,q25;q21.1),+8, ins(15;17)(q22;q21q25). He underwent induction chemotherapy with all-trans retinoic acid (ATRA) and idarubicin, which was complicated by differentiation syndrome that presented with fever and fluid retention. Discontinuation of ATRA and initiation of dexamethasone led to resolution of the symptoms. Complete hematologic and molecular remission was achieved after the induction chemotherapy.
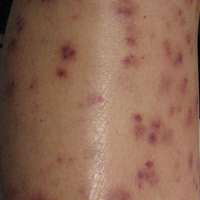
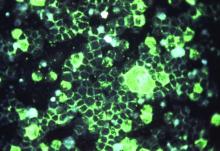
Following a risk-adapted treatment protocol for consolidation therapy,1 he underwent an uneventful first cycle of consolidation therapy. On day 15 of the second cycle of consolidation therapy with ATRA and mitoxantrone he was hospitalized with a fever (temperature, 38°C) in a setting of neutropenia (absolute neutrophil count [ANC], 0/µL [reference range, 1500–7200/µL]). He was empirically treated with ceftazidime and vancomycin and maintained on prophylactic acyclovir and fluconazole. Routine workup was negative for infection. He became afebrile within 24 hours. With negative infectious workup, vancomycin was discontinued on day 17. On day 33 he again developed a fever (temperature, 38.8°C) when the ANC started to recover (570/µL). A new skin rash was noted at this time. Physical examination revealed generalized, nonpruritic, tender, pink papules and plaques with dusky centers and central pustules on the trunk as well as the upper and lower extremities. The palms and soles were spared. The rash was somewhat reminiscent of Sweet syndrome (SS). No vesicles, bullae, or erosions were seen (Figure 1). Repeat blood and urine cultures and chest radiograph were unremarkable. Ceftazidime was discontinued due to concern of drug-associated rash. Within the next 48 hours, the patient developed rigors and a worsening rash that led to reinitiation of broad-spectrum antibiotic coverage with meropenem and vancomycin. Computed tomography of the chest, abdomen, and pelvis did not show any evidence of infection or other abnormalities. Skin biopsy showed an acute folliculitis and multiple foci of mixed granulomatous inflammation consisting of histiocytes, lymphocytes, and neutrophils with focal necrosis present in the dermis, dermis-subcutis junction, and subcutis (Figure 2). Diagnostic features of vasculitis were not seen. Viral cytopathic features were not identified. Tissue culture and special stains including Gram, acid-fast bacteria, and Grocott methenamine silver stains were negative for infectious organisms in the biopsy. Both direct fluorescent antibody study and cell cultures for varicella-zoster virus, cytomegalovirus, and herpes simplex virus also were negative.
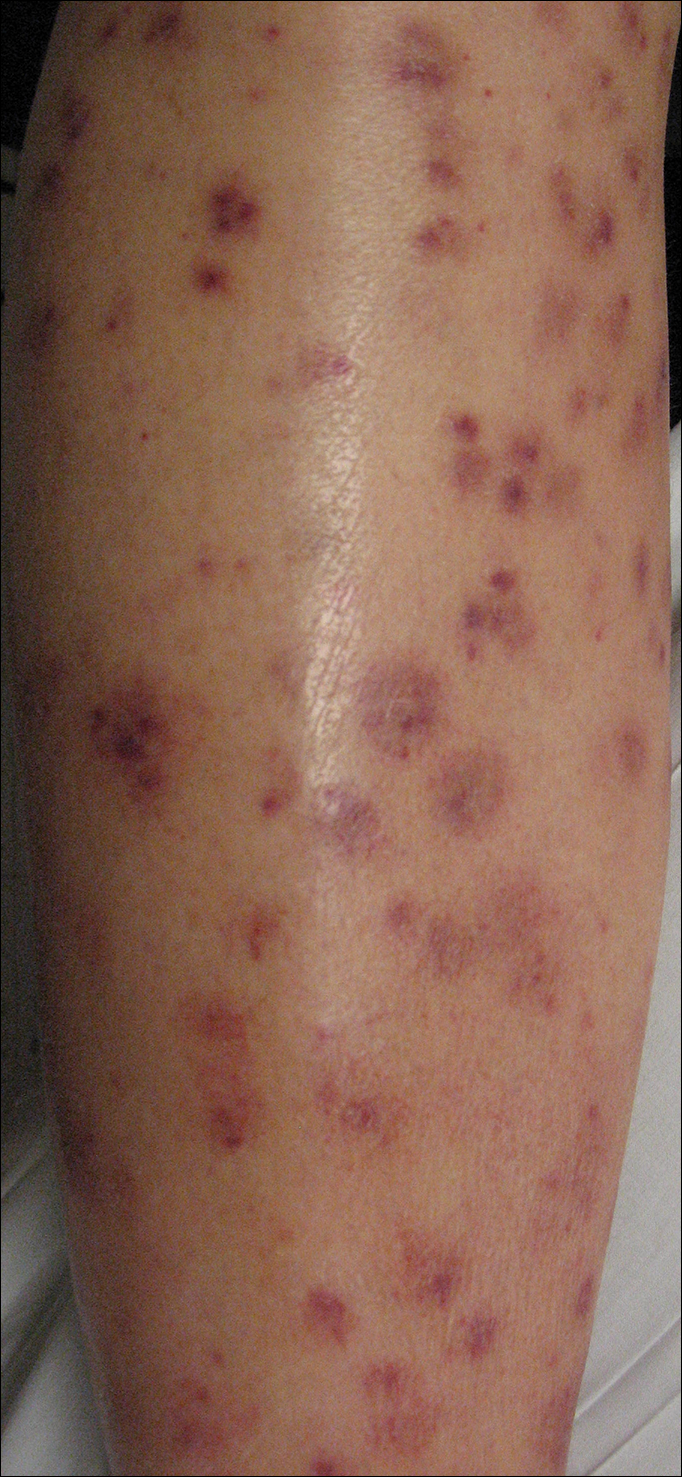
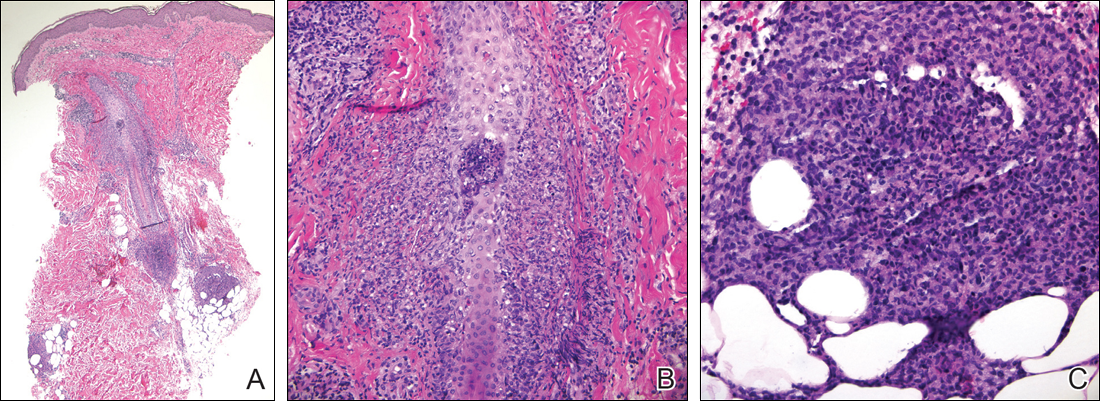
In the absence of microorganisms on skin biopsy and low clinical suspicion of infection, vancomycin and meropenem were discontinued on day 35 and empiric treatment with oral prednisone 40 mg daily was initiated on day 38, which resulted in a rapid improvement of the patient’s rash within 24 hours with complete resolution after a 7-day course of prednisone. Notably, the patient manifested concomitant recovery of the ANC. The patient completed his last cycle of consolidation therapy with ATRA and idarubicin without further complications and remains in molecular remission.
Neutrophilic dermatoses (NDs) are a group of disorders characterized by neutrophilic cutaneous infiltration without evidence of infection. These entities include SS, pyoderma gangrenosum, subcorneal pustular dermatosis, erythema elevatum diutinum, and neutrophilic eccrine hidradenitis.2 Neutrophilic dermatoses commonly present with acute onset of skin lesions and fever. Underlying systemic disease such as malignancy, inflammatory disease, autoimmune disease, pregnancy, and medications are known to be associated with ND. Although the rash clinically was reminiscent of SS, the histopathologic features were inconsistent with SS. Sweet syndrome typically presents with extensive monotonous neutrophilic infiltrates in the dermis. In this case, the neutrophilic infiltrates were localized and associated with the hair follicle, in the dermis and subcutis, and were accompanied by a granulomatous inflammation. Neutrophilic eccrine hidradenitis clinically is similar to SS and the distinction usually is made on the basis of histopathologic examination. Lack of the neutrophilic infiltrates within the eccrine secretary coils in our case did not support the diagnosis of neutrophilic eccrine hidradenitis.
Although the histopathologic features of the presented case were inconsistent with a particular subtype of ND, the clinical presentation and response to corticosteroids suggested that this unusual mixed inflammatory skin reaction might share a similar pathophysiologic mechanism.
A review of 20 patients with sterile neutrophilic folliculitis demonstrated an association with systemic diseases including cutaneous T-cell lymphoma, monoclonal gammopathy, Crohn disease, and autoimmune disorders.3 In acute myeloid leukemia, sterile neutrophilic folliculitis may be part of the initial presentation and responds to induction chemotherapy.4 An extensive search of PubMed articles indexed for MEDLINE using the search terms folliculitis, APL, and neutrophilic dermatoses did not reveal any prior reports of isolated neutrophilic folliculitis or mixed granulomatous reaction in patients with APL in molecular remission.
Although rare, cases of ATRA-induced SS have been reported. Some authors believe that SS in APL may represent a partial form of differentiation syndrome.5 Those cases usually occur during first induction. However, a recurrent episode of differentiation syndrome cannot be excluded in this patient.
A cutaneous reaction to chemotherapy with mitoxantrone as a cause also should be considered, given that the rash occurred only during the second cycle of consolidation therapy when mitoxantrone was used. However, this rash is rare in patients receiving mitoxantrone. The late onset of the rash from the time of last mitoxantrone administration argues against this diagnosis.
In summary, we describe an unusual presentation of a sterile mixed inflammatory skin reaction that occurred in a setting of neutrophil recovery following a second cycle of induction chemotherapy with ATRA and mitoxantrone for APL.
- Sanz MA, Montesinos P, Rayón C, et al; PETHEMA and HOVON Groups. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome [published online April 14, 2010]. Blood. 2010;115:5137-5146.
- Hensley CD, Caughman SW. Neutrophilic dermatoses associated with hematologic disorders. Clin Dermatol. 2000;18:355-367.
- Margro CM, Crowson AN. Sterile neutrophilic folliculitis with perifollicular vasculopathy: a distinctive cutaneous reaction pattern reflecting systemic disease. J Cutan Pathol. 1998;25:215-221.
- Inuzuka M, Tokura Y. Sterile suppurative folliculitis associated with acute myeloblastic leukaemia. Br J Dermatol. 2002;146:904-907.
- Astudillo L, Loche F, Reynish W, et al. Sweet’s syndrome associated with retinoic acid syndrome in a patient with promyelocytic leukemia [published online January 10, 2002]. Ann Hematol. 2002;81:111-114.
To the Editor:
A 34-year-old man presented with fever, easy bruising, and pancytopenia with increased peripheral blasts of 77%. Bone marrow biopsy showed hypercellular marrow with 80% to 90% involvement by acute promyelocytic leukemia (APL) with complex cytogenetics: 47,XY,t(4;17;18)(p16;q21,q25;q21.1),+8, ins(15;17)(q22;q21q25). He underwent induction chemotherapy with all-trans retinoic acid (ATRA) and idarubicin, which was complicated by differentiation syndrome that presented with fever and fluid retention. Discontinuation of ATRA and initiation of dexamethasone led to resolution of the symptoms. Complete hematologic and molecular remission was achieved after the induction chemotherapy.
Following a risk-adapted treatment protocol for consolidation therapy,1 he underwent an uneventful first cycle of consolidation therapy. On day 15 of the second cycle of consolidation therapy with ATRA and mitoxantrone he was hospitalized with a fever (temperature, 38°C) in a setting of neutropenia (absolute neutrophil count [ANC], 0/µL [reference range, 1500–7200/µL]). He was empirically treated with ceftazidime and vancomycin and maintained on prophylactic acyclovir and fluconazole. Routine workup was negative for infection. He became afebrile within 24 hours. With negative infectious workup, vancomycin was discontinued on day 17. On day 33 he again developed a fever (temperature, 38.8°C) when the ANC started to recover (570/µL). A new skin rash was noted at this time. Physical examination revealed generalized, nonpruritic, tender, pink papules and plaques with dusky centers and central pustules on the trunk as well as the upper and lower extremities. The palms and soles were spared. The rash was somewhat reminiscent of Sweet syndrome (SS). No vesicles, bullae, or erosions were seen (Figure 1). Repeat blood and urine cultures and chest radiograph were unremarkable. Ceftazidime was discontinued due to concern of drug-associated rash. Within the next 48 hours, the patient developed rigors and a worsening rash that led to reinitiation of broad-spectrum antibiotic coverage with meropenem and vancomycin. Computed tomography of the chest, abdomen, and pelvis did not show any evidence of infection or other abnormalities. Skin biopsy showed an acute folliculitis and multiple foci of mixed granulomatous inflammation consisting of histiocytes, lymphocytes, and neutrophils with focal necrosis present in the dermis, dermis-subcutis junction, and subcutis (Figure 2). Diagnostic features of vasculitis were not seen. Viral cytopathic features were not identified. Tissue culture and special stains including Gram, acid-fast bacteria, and Grocott methenamine silver stains were negative for infectious organisms in the biopsy. Both direct fluorescent antibody study and cell cultures for varicella-zoster virus, cytomegalovirus, and herpes simplex virus also were negative.


In the absence of microorganisms on skin biopsy and low clinical suspicion of infection, vancomycin and meropenem were discontinued on day 35 and empiric treatment with oral prednisone 40 mg daily was initiated on day 38, which resulted in a rapid improvement of the patient’s rash within 24 hours with complete resolution after a 7-day course of prednisone. Notably, the patient manifested concomitant recovery of the ANC. The patient completed his last cycle of consolidation therapy with ATRA and idarubicin without further complications and remains in molecular remission.
Neutrophilic dermatoses (NDs) are a group of disorders characterized by neutrophilic cutaneous infiltration without evidence of infection. These entities include SS, pyoderma gangrenosum, subcorneal pustular dermatosis, erythema elevatum diutinum, and neutrophilic eccrine hidradenitis.2 Neutrophilic dermatoses commonly present with acute onset of skin lesions and fever. Underlying systemic disease such as malignancy, inflammatory disease, autoimmune disease, pregnancy, and medications are known to be associated with ND. Although the rash clinically was reminiscent of SS, the histopathologic features were inconsistent with SS. Sweet syndrome typically presents with extensive monotonous neutrophilic infiltrates in the dermis. In this case, the neutrophilic infiltrates were localized and associated with the hair follicle, in the dermis and subcutis, and were accompanied by a granulomatous inflammation. Neutrophilic eccrine hidradenitis clinically is similar to SS and the distinction usually is made on the basis of histopathologic examination. Lack of the neutrophilic infiltrates within the eccrine secretary coils in our case did not support the diagnosis of neutrophilic eccrine hidradenitis.
Although the histopathologic features of the presented case were inconsistent with a particular subtype of ND, the clinical presentation and response to corticosteroids suggested that this unusual mixed inflammatory skin reaction might share a similar pathophysiologic mechanism.
A review of 20 patients with sterile neutrophilic folliculitis demonstrated an association with systemic diseases including cutaneous T-cell lymphoma, monoclonal gammopathy, Crohn disease, and autoimmune disorders.3 In acute myeloid leukemia, sterile neutrophilic folliculitis may be part of the initial presentation and responds to induction chemotherapy.4 An extensive search of PubMed articles indexed for MEDLINE using the search terms folliculitis, APL, and neutrophilic dermatoses did not reveal any prior reports of isolated neutrophilic folliculitis or mixed granulomatous reaction in patients with APL in molecular remission.
Although rare, cases of ATRA-induced SS have been reported. Some authors believe that SS in APL may represent a partial form of differentiation syndrome.5 Those cases usually occur during first induction. However, a recurrent episode of differentiation syndrome cannot be excluded in this patient.
A cutaneous reaction to chemotherapy with mitoxantrone as a cause also should be considered, given that the rash occurred only during the second cycle of consolidation therapy when mitoxantrone was used. However, this rash is rare in patients receiving mitoxantrone. The late onset of the rash from the time of last mitoxantrone administration argues against this diagnosis.
In summary, we describe an unusual presentation of a sterile mixed inflammatory skin reaction that occurred in a setting of neutrophil recovery following a second cycle of induction chemotherapy with ATRA and mitoxantrone for APL.
To the Editor:
A 34-year-old man presented with fever, easy bruising, and pancytopenia with increased peripheral blasts of 77%. Bone marrow biopsy showed hypercellular marrow with 80% to 90% involvement by acute promyelocytic leukemia (APL) with complex cytogenetics: 47,XY,t(4;17;18)(p16;q21,q25;q21.1),+8, ins(15;17)(q22;q21q25). He underwent induction chemotherapy with all-trans retinoic acid (ATRA) and idarubicin, which was complicated by differentiation syndrome that presented with fever and fluid retention. Discontinuation of ATRA and initiation of dexamethasone led to resolution of the symptoms. Complete hematologic and molecular remission was achieved after the induction chemotherapy.
Following a risk-adapted treatment protocol for consolidation therapy,1 he underwent an uneventful first cycle of consolidation therapy. On day 15 of the second cycle of consolidation therapy with ATRA and mitoxantrone he was hospitalized with a fever (temperature, 38°C) in a setting of neutropenia (absolute neutrophil count [ANC], 0/µL [reference range, 1500–7200/µL]). He was empirically treated with ceftazidime and vancomycin and maintained on prophylactic acyclovir and fluconazole. Routine workup was negative for infection. He became afebrile within 24 hours. With negative infectious workup, vancomycin was discontinued on day 17. On day 33 he again developed a fever (temperature, 38.8°C) when the ANC started to recover (570/µL). A new skin rash was noted at this time. Physical examination revealed generalized, nonpruritic, tender, pink papules and plaques with dusky centers and central pustules on the trunk as well as the upper and lower extremities. The palms and soles were spared. The rash was somewhat reminiscent of Sweet syndrome (SS). No vesicles, bullae, or erosions were seen (Figure 1). Repeat blood and urine cultures and chest radiograph were unremarkable. Ceftazidime was discontinued due to concern of drug-associated rash. Within the next 48 hours, the patient developed rigors and a worsening rash that led to reinitiation of broad-spectrum antibiotic coverage with meropenem and vancomycin. Computed tomography of the chest, abdomen, and pelvis did not show any evidence of infection or other abnormalities. Skin biopsy showed an acute folliculitis and multiple foci of mixed granulomatous inflammation consisting of histiocytes, lymphocytes, and neutrophils with focal necrosis present in the dermis, dermis-subcutis junction, and subcutis (Figure 2). Diagnostic features of vasculitis were not seen. Viral cytopathic features were not identified. Tissue culture and special stains including Gram, acid-fast bacteria, and Grocott methenamine silver stains were negative for infectious organisms in the biopsy. Both direct fluorescent antibody study and cell cultures for varicella-zoster virus, cytomegalovirus, and herpes simplex virus also were negative.


In the absence of microorganisms on skin biopsy and low clinical suspicion of infection, vancomycin and meropenem were discontinued on day 35 and empiric treatment with oral prednisone 40 mg daily was initiated on day 38, which resulted in a rapid improvement of the patient’s rash within 24 hours with complete resolution after a 7-day course of prednisone. Notably, the patient manifested concomitant recovery of the ANC. The patient completed his last cycle of consolidation therapy with ATRA and idarubicin without further complications and remains in molecular remission.
Neutrophilic dermatoses (NDs) are a group of disorders characterized by neutrophilic cutaneous infiltration without evidence of infection. These entities include SS, pyoderma gangrenosum, subcorneal pustular dermatosis, erythema elevatum diutinum, and neutrophilic eccrine hidradenitis.2 Neutrophilic dermatoses commonly present with acute onset of skin lesions and fever. Underlying systemic disease such as malignancy, inflammatory disease, autoimmune disease, pregnancy, and medications are known to be associated with ND. Although the rash clinically was reminiscent of SS, the histopathologic features were inconsistent with SS. Sweet syndrome typically presents with extensive monotonous neutrophilic infiltrates in the dermis. In this case, the neutrophilic infiltrates were localized and associated with the hair follicle, in the dermis and subcutis, and were accompanied by a granulomatous inflammation. Neutrophilic eccrine hidradenitis clinically is similar to SS and the distinction usually is made on the basis of histopathologic examination. Lack of the neutrophilic infiltrates within the eccrine secretary coils in our case did not support the diagnosis of neutrophilic eccrine hidradenitis.
Although the histopathologic features of the presented case were inconsistent with a particular subtype of ND, the clinical presentation and response to corticosteroids suggested that this unusual mixed inflammatory skin reaction might share a similar pathophysiologic mechanism.
A review of 20 patients with sterile neutrophilic folliculitis demonstrated an association with systemic diseases including cutaneous T-cell lymphoma, monoclonal gammopathy, Crohn disease, and autoimmune disorders.3 In acute myeloid leukemia, sterile neutrophilic folliculitis may be part of the initial presentation and responds to induction chemotherapy.4 An extensive search of PubMed articles indexed for MEDLINE using the search terms folliculitis, APL, and neutrophilic dermatoses did not reveal any prior reports of isolated neutrophilic folliculitis or mixed granulomatous reaction in patients with APL in molecular remission.
Although rare, cases of ATRA-induced SS have been reported. Some authors believe that SS in APL may represent a partial form of differentiation syndrome.5 Those cases usually occur during first induction. However, a recurrent episode of differentiation syndrome cannot be excluded in this patient.
A cutaneous reaction to chemotherapy with mitoxantrone as a cause also should be considered, given that the rash occurred only during the second cycle of consolidation therapy when mitoxantrone was used. However, this rash is rare in patients receiving mitoxantrone. The late onset of the rash from the time of last mitoxantrone administration argues against this diagnosis.
In summary, we describe an unusual presentation of a sterile mixed inflammatory skin reaction that occurred in a setting of neutrophil recovery following a second cycle of induction chemotherapy with ATRA and mitoxantrone for APL.
- Sanz MA, Montesinos P, Rayón C, et al; PETHEMA and HOVON Groups. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome [published online April 14, 2010]. Blood. 2010;115:5137-5146.
- Hensley CD, Caughman SW. Neutrophilic dermatoses associated with hematologic disorders. Clin Dermatol. 2000;18:355-367.
- Margro CM, Crowson AN. Sterile neutrophilic folliculitis with perifollicular vasculopathy: a distinctive cutaneous reaction pattern reflecting systemic disease. J Cutan Pathol. 1998;25:215-221.
- Inuzuka M, Tokura Y. Sterile suppurative folliculitis associated with acute myeloblastic leukaemia. Br J Dermatol. 2002;146:904-907.
- Astudillo L, Loche F, Reynish W, et al. Sweet’s syndrome associated with retinoic acid syndrome in a patient with promyelocytic leukemia [published online January 10, 2002]. Ann Hematol. 2002;81:111-114.
- Sanz MA, Montesinos P, Rayón C, et al; PETHEMA and HOVON Groups. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome [published online April 14, 2010]. Blood. 2010;115:5137-5146.
- Hensley CD, Caughman SW. Neutrophilic dermatoses associated with hematologic disorders. Clin Dermatol. 2000;18:355-367.
- Margro CM, Crowson AN. Sterile neutrophilic folliculitis with perifollicular vasculopathy: a distinctive cutaneous reaction pattern reflecting systemic disease. J Cutan Pathol. 1998;25:215-221.
- Inuzuka M, Tokura Y. Sterile suppurative folliculitis associated with acute myeloblastic leukaemia. Br J Dermatol. 2002;146:904-907.
- Astudillo L, Loche F, Reynish W, et al. Sweet’s syndrome associated with retinoic acid syndrome in a patient with promyelocytic leukemia [published online January 10, 2002]. Ann Hematol. 2002;81:111-114.
Practice Point
- Sterile mixed inflammatory skin reactions reminiscent of neutrophilic dermatoses may occur during neutrophil recovery in patients undergoing therapy for leukemias and need to be considered as part of the differential diagnosis.
HIV PrEP facing challenges, but implementation outlook positive
ATLANTA – Implementation of HIV preexposure prophylaxis (PrEP) by local health departments in the United States faces several challenges, but the majority of those already engaged plan to increase their participation soon, a study showed.
“For the purposes of this study, we very broadly defined engagement in PrEP implementation as anything from participating in a local or statewide working group, to planning and supporting implementation of PrEP, to doing community education and outreach, or working with providers to deliver PrEP via health department clinics,” explained Gretchen Weiss, director of HIV, STI, and viral hepatitis at the National Association of County and City Health Officials in Washington, D.C.
A total of 53% of LHDs now engaged in PrEP implementation anticipate expanding their engagement soon, 39% responded that they were unsure about expanding, and 8% said that they did not plan to expand PrEP engagement. Of the LHDs not currently implementing PrEP, 18% reported that they plan to implement PrEP within the next 4 years, while 46% were undecided, and 36% said they had no plans to implement PrEP, Ms. Weiss reported at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
Of the 109 LHDs using HIV PrEP, 75% reported that they are referring individuals at high risk for sexually transmitted diseases to PrEP, while 50% said that they conduct community outreach and education regarding the benefits of using PrEP. When asked what they viewed as their “optimal role” with regard to PrEP, 77% said that it was referring high-risk individuals for treatment. Sixty-five percent reported that their optimal role was to identify PrEP providers and develop referral lists, while 33% thought that delivering PrEP via health department clinics was the optimal role for their engagement in PrEP.
“In terms of the challenges faced by LHDs, 64% reported limited staff capacity, 57% reported concerns about financial access to PrEP, and 47% responded not having enough providers that are willing to provide PrEP,” Ms. Weiss said. “Thirteen percent reported that they didn’t face any significant challenges.”
No funding source for this study was disclosed. Ms. Weiss did not report any relevant financial disclosures.
ATLANTA – Implementation of HIV preexposure prophylaxis (PrEP) by local health departments in the United States faces several challenges, but the majority of those already engaged plan to increase their participation soon, a study showed.
“For the purposes of this study, we very broadly defined engagement in PrEP implementation as anything from participating in a local or statewide working group, to planning and supporting implementation of PrEP, to doing community education and outreach, or working with providers to deliver PrEP via health department clinics,” explained Gretchen Weiss, director of HIV, STI, and viral hepatitis at the National Association of County and City Health Officials in Washington, D.C.
A total of 53% of LHDs now engaged in PrEP implementation anticipate expanding their engagement soon, 39% responded that they were unsure about expanding, and 8% said that they did not plan to expand PrEP engagement. Of the LHDs not currently implementing PrEP, 18% reported that they plan to implement PrEP within the next 4 years, while 46% were undecided, and 36% said they had no plans to implement PrEP, Ms. Weiss reported at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
Of the 109 LHDs using HIV PrEP, 75% reported that they are referring individuals at high risk for sexually transmitted diseases to PrEP, while 50% said that they conduct community outreach and education regarding the benefits of using PrEP. When asked what they viewed as their “optimal role” with regard to PrEP, 77% said that it was referring high-risk individuals for treatment. Sixty-five percent reported that their optimal role was to identify PrEP providers and develop referral lists, while 33% thought that delivering PrEP via health department clinics was the optimal role for their engagement in PrEP.
“In terms of the challenges faced by LHDs, 64% reported limited staff capacity, 57% reported concerns about financial access to PrEP, and 47% responded not having enough providers that are willing to provide PrEP,” Ms. Weiss said. “Thirteen percent reported that they didn’t face any significant challenges.”
No funding source for this study was disclosed. Ms. Weiss did not report any relevant financial disclosures.
ATLANTA – Implementation of HIV preexposure prophylaxis (PrEP) by local health departments in the United States faces several challenges, but the majority of those already engaged plan to increase their participation soon, a study showed.
“For the purposes of this study, we very broadly defined engagement in PrEP implementation as anything from participating in a local or statewide working group, to planning and supporting implementation of PrEP, to doing community education and outreach, or working with providers to deliver PrEP via health department clinics,” explained Gretchen Weiss, director of HIV, STI, and viral hepatitis at the National Association of County and City Health Officials in Washington, D.C.
A total of 53% of LHDs now engaged in PrEP implementation anticipate expanding their engagement soon, 39% responded that they were unsure about expanding, and 8% said that they did not plan to expand PrEP engagement. Of the LHDs not currently implementing PrEP, 18% reported that they plan to implement PrEP within the next 4 years, while 46% were undecided, and 36% said they had no plans to implement PrEP, Ms. Weiss reported at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
Of the 109 LHDs using HIV PrEP, 75% reported that they are referring individuals at high risk for sexually transmitted diseases to PrEP, while 50% said that they conduct community outreach and education regarding the benefits of using PrEP. When asked what they viewed as their “optimal role” with regard to PrEP, 77% said that it was referring high-risk individuals for treatment. Sixty-five percent reported that their optimal role was to identify PrEP providers and develop referral lists, while 33% thought that delivering PrEP via health department clinics was the optimal role for their engagement in PrEP.
“In terms of the challenges faced by LHDs, 64% reported limited staff capacity, 57% reported concerns about financial access to PrEP, and 47% responded not having enough providers that are willing to provide PrEP,” Ms. Weiss said. “Thirteen percent reported that they didn’t face any significant challenges.”
No funding source for this study was disclosed. Ms. Weiss did not report any relevant financial disclosures.
Key clinical point:
Major finding: 53% of LHDs engaged in PrEP plan to increase participation; 18% of LHDs not currently engaged in PrEP plan to implement within the next 4 years.
Data source: A survey of 284 local health departments from across the United States.
Disclosures: Ms. Weiss did not report any relevant financial conflicts.
Promise of effective RSV vaccines on horizon
ATLANTA – A new vaccine for respiratory syncytial virus may truly be on the horizon, given recent advances in basic science and a marked increase in interest in the pharmaceutical industry.
That’s the conclusion of Larry Anderson, MD, professor of infectious disease in the Emory University department of pediatrics, who presented the most updated research and progress on a respiratory syncytial virus (RSV) vaccine during a conference sponsored by the Centers for Disease Control and Prevention.
The high hospitalization rates of infants with RSV, also associated with later development of reactive airway disease and asthma, highlight the challenge of developing a vaccine, Dr. Anderson said.
“The infant has an immature immune system less able to respond vigorously to a vaccine,” he said. “Also, it is highly susceptible to the disease of RSV, and therefore safety becomes an issue at least in terms of the live virus vaccine.” Furthermore, RSV causes multiple repeat infections throughout life, “which underlines the difficulty in inducing a protective immune response,” he added.
But Dr. Anderson said he believes there is light at the end of the tunnel when it comes to a vaccine for the virus.
“I think in terms of [the] potential of having an RSV vaccine in the near future, now is the most promising time, recognizing that work on an RSV vaccine has been going on for over 50 years without success to date,” he said. Significant advances in basic biology, immunology, and vaccinology have led to a better understanding of the virus, and new tools such as reverse genetics make “it possible to make any live virus you want as long as you know what you want,” he added.
Dr. Anderson provided an overview of published and preliminary data on the progress of more than five dozen groups working on an RSV vaccine. About 70% of these candidates remain in preclinical research, primarily in animal models. Of the dozen in phase I, several look promising, he said. Another six vaccines are in phase II or phase III testing, and MedImmune’s Synagis is market approved. But not all target infants.
“The first and highest priority is the young infant, particularly the under 2- to 4-month-old,” he said. In infants aged 4-6 months, it’s likely easier to induce an immune response, and there’s less susceptibility to disease with replication of the virus, he said. The elderly, also at high risk for RSV, would be another target population.
Potentially “the lowest apple on the tree for immunization,” Dr. Anderson said, would be pregnant women because a vaccine could prevent infection, disease, and transmission to their infant before he might be able to be vaccinated.
“There, the primary purpose is to increase the kind of antibody that is transferred across the placenta to the fetus to protect from RSV disease” in the infant after birth, he said. Data suggest it’s possible to increase titer antibodies in infants up to 4 months from maternal immunization, possibly longer, depending on how much the vaccine can induce antibodies in the woman.
For young children, he noted that five live attenuated RSV vaccines are in phase I testing, and four others are in phase I that use a virus vector to deliver the F protein – three using adenovirus and one with a modified vaccinia Ankara virus. A handful of subunit vaccines have reached phase II, and Novavax is furthest along in phase III, but these target older children and adults, including pregnant women.
“There’s going to be a lot of data in the coming year on completed clinical trials, and that’s going to tell us a lot about where we are,” Dr. Anderson said. “The young infant is the most challenging for a vaccine.” But, he added, “new information on protective immunity and disease pathogenesis should help achieve or improve vaccines in the future.”
Dr. Anderson has consulted on RSV vaccines for MedImmune, Novartis, Crucell Holland, and AVC, and has served on a Moderna Therapeutics scientific advisory board. His lab also has received grant funding from Trellis RSV Holdings, and he coinvented several RSV-related vaccine and treatment patents held by the CDC.
ATLANTA – A new vaccine for respiratory syncytial virus may truly be on the horizon, given recent advances in basic science and a marked increase in interest in the pharmaceutical industry.
That’s the conclusion of Larry Anderson, MD, professor of infectious disease in the Emory University department of pediatrics, who presented the most updated research and progress on a respiratory syncytial virus (RSV) vaccine during a conference sponsored by the Centers for Disease Control and Prevention.
The high hospitalization rates of infants with RSV, also associated with later development of reactive airway disease and asthma, highlight the challenge of developing a vaccine, Dr. Anderson said.
“The infant has an immature immune system less able to respond vigorously to a vaccine,” he said. “Also, it is highly susceptible to the disease of RSV, and therefore safety becomes an issue at least in terms of the live virus vaccine.” Furthermore, RSV causes multiple repeat infections throughout life, “which underlines the difficulty in inducing a protective immune response,” he added.
But Dr. Anderson said he believes there is light at the end of the tunnel when it comes to a vaccine for the virus.
“I think in terms of [the] potential of having an RSV vaccine in the near future, now is the most promising time, recognizing that work on an RSV vaccine has been going on for over 50 years without success to date,” he said. Significant advances in basic biology, immunology, and vaccinology have led to a better understanding of the virus, and new tools such as reverse genetics make “it possible to make any live virus you want as long as you know what you want,” he added.
Dr. Anderson provided an overview of published and preliminary data on the progress of more than five dozen groups working on an RSV vaccine. About 70% of these candidates remain in preclinical research, primarily in animal models. Of the dozen in phase I, several look promising, he said. Another six vaccines are in phase II or phase III testing, and MedImmune’s Synagis is market approved. But not all target infants.
“The first and highest priority is the young infant, particularly the under 2- to 4-month-old,” he said. In infants aged 4-6 months, it’s likely easier to induce an immune response, and there’s less susceptibility to disease with replication of the virus, he said. The elderly, also at high risk for RSV, would be another target population.
Potentially “the lowest apple on the tree for immunization,” Dr. Anderson said, would be pregnant women because a vaccine could prevent infection, disease, and transmission to their infant before he might be able to be vaccinated.
“There, the primary purpose is to increase the kind of antibody that is transferred across the placenta to the fetus to protect from RSV disease” in the infant after birth, he said. Data suggest it’s possible to increase titer antibodies in infants up to 4 months from maternal immunization, possibly longer, depending on how much the vaccine can induce antibodies in the woman.
For young children, he noted that five live attenuated RSV vaccines are in phase I testing, and four others are in phase I that use a virus vector to deliver the F protein – three using adenovirus and one with a modified vaccinia Ankara virus. A handful of subunit vaccines have reached phase II, and Novavax is furthest along in phase III, but these target older children and adults, including pregnant women.
“There’s going to be a lot of data in the coming year on completed clinical trials, and that’s going to tell us a lot about where we are,” Dr. Anderson said. “The young infant is the most challenging for a vaccine.” But, he added, “new information on protective immunity and disease pathogenesis should help achieve or improve vaccines in the future.”
Dr. Anderson has consulted on RSV vaccines for MedImmune, Novartis, Crucell Holland, and AVC, and has served on a Moderna Therapeutics scientific advisory board. His lab also has received grant funding from Trellis RSV Holdings, and he coinvented several RSV-related vaccine and treatment patents held by the CDC.
ATLANTA – A new vaccine for respiratory syncytial virus may truly be on the horizon, given recent advances in basic science and a marked increase in interest in the pharmaceutical industry.
That’s the conclusion of Larry Anderson, MD, professor of infectious disease in the Emory University department of pediatrics, who presented the most updated research and progress on a respiratory syncytial virus (RSV) vaccine during a conference sponsored by the Centers for Disease Control and Prevention.
The high hospitalization rates of infants with RSV, also associated with later development of reactive airway disease and asthma, highlight the challenge of developing a vaccine, Dr. Anderson said.
“The infant has an immature immune system less able to respond vigorously to a vaccine,” he said. “Also, it is highly susceptible to the disease of RSV, and therefore safety becomes an issue at least in terms of the live virus vaccine.” Furthermore, RSV causes multiple repeat infections throughout life, “which underlines the difficulty in inducing a protective immune response,” he added.
But Dr. Anderson said he believes there is light at the end of the tunnel when it comes to a vaccine for the virus.
“I think in terms of [the] potential of having an RSV vaccine in the near future, now is the most promising time, recognizing that work on an RSV vaccine has been going on for over 50 years without success to date,” he said. Significant advances in basic biology, immunology, and vaccinology have led to a better understanding of the virus, and new tools such as reverse genetics make “it possible to make any live virus you want as long as you know what you want,” he added.
Dr. Anderson provided an overview of published and preliminary data on the progress of more than five dozen groups working on an RSV vaccine. About 70% of these candidates remain in preclinical research, primarily in animal models. Of the dozen in phase I, several look promising, he said. Another six vaccines are in phase II or phase III testing, and MedImmune’s Synagis is market approved. But not all target infants.
“The first and highest priority is the young infant, particularly the under 2- to 4-month-old,” he said. In infants aged 4-6 months, it’s likely easier to induce an immune response, and there’s less susceptibility to disease with replication of the virus, he said. The elderly, also at high risk for RSV, would be another target population.
Potentially “the lowest apple on the tree for immunization,” Dr. Anderson said, would be pregnant women because a vaccine could prevent infection, disease, and transmission to their infant before he might be able to be vaccinated.
“There, the primary purpose is to increase the kind of antibody that is transferred across the placenta to the fetus to protect from RSV disease” in the infant after birth, he said. Data suggest it’s possible to increase titer antibodies in infants up to 4 months from maternal immunization, possibly longer, depending on how much the vaccine can induce antibodies in the woman.
For young children, he noted that five live attenuated RSV vaccines are in phase I testing, and four others are in phase I that use a virus vector to deliver the F protein – three using adenovirus and one with a modified vaccinia Ankara virus. A handful of subunit vaccines have reached phase II, and Novavax is furthest along in phase III, but these target older children and adults, including pregnant women.
“There’s going to be a lot of data in the coming year on completed clinical trials, and that’s going to tell us a lot about where we are,” Dr. Anderson said. “The young infant is the most challenging for a vaccine.” But, he added, “new information on protective immunity and disease pathogenesis should help achieve or improve vaccines in the future.”
Dr. Anderson has consulted on RSV vaccines for MedImmune, Novartis, Crucell Holland, and AVC, and has served on a Moderna Therapeutics scientific advisory board. His lab also has received grant funding from Trellis RSV Holdings, and he coinvented several RSV-related vaccine and treatment patents held by the CDC.
EXPERT ANALYSIS FROM THE NATIONAL IMMUNIZATION CONFERENCE
Key clinical point: A respiratory syncytial virus vaccine is closer to reality now than at any other time.
Major finding: 62 RSV vaccines are in development, with approximately 70% in preclinical studies.
Data source: Based on a review of the current state of research into an RSV vaccine and the burden of RSV disease.
Disclosures: Dr. Anderson has consulted on RSV vaccines for MedImmune, Novartis, Crucell Holland, and AVC, and has served on a Moderna Therapeutics scientific advisory board. His lab also has received grant funding from Trellis RSV Holdings, and he coinvented several RSV-related vaccine and treatment patents held by the CDC.
Partial-breast irradiation alternative to mastectomy following recurrence
BOSTON – In women with in-breast failures following breast-conserving surgery and whole-breast irradiation, partial-breast irradiation with 3D conformal radiation appears to be an effective and safe alternative to mastectomy, results of a phase II trial indicate.
At a median follow-up of 3.6 years, there were only two ipsilateral breast recurrences, and four mastectomies required among 58 women treated with partial-breast irradiation after a second lumpectomy, reported Douglas W. Arthur, MD, chair of radiation oncology at Virginia Commonwealth University in Richmond.
“I think this information adds to the growing data supporting this treatment approach as an alternative to mastectomy for those who continue to want to preserve the breast,” he said at a late-breaking abstracts session at the annual meeting of the American Society of Radiation Oncology.
The NRG/RTOG 1014 study is a prospective phase II trial designed to evaluate skin, breast, and chest wall events occurring within 1 year of re-irradiation. One-year toxicity results from the trial were reported at the 2015 ASTRO annual meeting.
Dr. Arthur presented 3-year efficacy results from the trial. The investigators enrolled women with in-breast recurrences more than 1 year after whole-breast irradiation (WBI), with tumors that were smaller than 3 cm, unifocal, and resected with negative margins. Axillary involvement was limited to pathologic NO or N1 without extracapsular extension.
Partial-breast irradiation was targeted to the surgical cavity with a safety margin of plus 1.5 cm for the clinical target volume, and an additional 1-cm expansion. The prescribed dose was 45 Gy in 1.5-Gy fractions delivered via 3D conformal radiotherapy in 30 treatments.
Of 65 patients accrued, 58 completed treatment and were evaluable for efficacy. The median age was 67.5 years. In all, 23 patients had ductal carcinoma in situ, and 35 had invasive cancers. All patients were node negative.
A total of four patients (6.9%) reported late grade 3 toxicities, which included breast infection, fibrous deep connective tissue, skin induration, and breast atrophy, pain, and volume loss. There were no grade 4 toxicities, and no treatment-related deaths.
Two patients had in-breast recurrences, which translated into a year estimate of 3.7%. Four patients underwent mastectomies, for a 3-year estimated mastectomy failure rate of 5.2%. Two of the mastectomies were for the in-breast recurrences, one for a nonhealing wound, and one occurred in a patient who developed cancer in the contralateral breast and opted for a bilateral mastectomy.
BOSTON – In women with in-breast failures following breast-conserving surgery and whole-breast irradiation, partial-breast irradiation with 3D conformal radiation appears to be an effective and safe alternative to mastectomy, results of a phase II trial indicate.
At a median follow-up of 3.6 years, there were only two ipsilateral breast recurrences, and four mastectomies required among 58 women treated with partial-breast irradiation after a second lumpectomy, reported Douglas W. Arthur, MD, chair of radiation oncology at Virginia Commonwealth University in Richmond.
“I think this information adds to the growing data supporting this treatment approach as an alternative to mastectomy for those who continue to want to preserve the breast,” he said at a late-breaking abstracts session at the annual meeting of the American Society of Radiation Oncology.
The NRG/RTOG 1014 study is a prospective phase II trial designed to evaluate skin, breast, and chest wall events occurring within 1 year of re-irradiation. One-year toxicity results from the trial were reported at the 2015 ASTRO annual meeting.
Dr. Arthur presented 3-year efficacy results from the trial. The investigators enrolled women with in-breast recurrences more than 1 year after whole-breast irradiation (WBI), with tumors that were smaller than 3 cm, unifocal, and resected with negative margins. Axillary involvement was limited to pathologic NO or N1 without extracapsular extension.
Partial-breast irradiation was targeted to the surgical cavity with a safety margin of plus 1.5 cm for the clinical target volume, and an additional 1-cm expansion. The prescribed dose was 45 Gy in 1.5-Gy fractions delivered via 3D conformal radiotherapy in 30 treatments.
Of 65 patients accrued, 58 completed treatment and were evaluable for efficacy. The median age was 67.5 years. In all, 23 patients had ductal carcinoma in situ, and 35 had invasive cancers. All patients were node negative.
A total of four patients (6.9%) reported late grade 3 toxicities, which included breast infection, fibrous deep connective tissue, skin induration, and breast atrophy, pain, and volume loss. There were no grade 4 toxicities, and no treatment-related deaths.
Two patients had in-breast recurrences, which translated into a year estimate of 3.7%. Four patients underwent mastectomies, for a 3-year estimated mastectomy failure rate of 5.2%. Two of the mastectomies were for the in-breast recurrences, one for a nonhealing wound, and one occurred in a patient who developed cancer in the contralateral breast and opted for a bilateral mastectomy.
BOSTON – In women with in-breast failures following breast-conserving surgery and whole-breast irradiation, partial-breast irradiation with 3D conformal radiation appears to be an effective and safe alternative to mastectomy, results of a phase II trial indicate.
At a median follow-up of 3.6 years, there were only two ipsilateral breast recurrences, and four mastectomies required among 58 women treated with partial-breast irradiation after a second lumpectomy, reported Douglas W. Arthur, MD, chair of radiation oncology at Virginia Commonwealth University in Richmond.
“I think this information adds to the growing data supporting this treatment approach as an alternative to mastectomy for those who continue to want to preserve the breast,” he said at a late-breaking abstracts session at the annual meeting of the American Society of Radiation Oncology.
The NRG/RTOG 1014 study is a prospective phase II trial designed to evaluate skin, breast, and chest wall events occurring within 1 year of re-irradiation. One-year toxicity results from the trial were reported at the 2015 ASTRO annual meeting.
Dr. Arthur presented 3-year efficacy results from the trial. The investigators enrolled women with in-breast recurrences more than 1 year after whole-breast irradiation (WBI), with tumors that were smaller than 3 cm, unifocal, and resected with negative margins. Axillary involvement was limited to pathologic NO or N1 without extracapsular extension.
Partial-breast irradiation was targeted to the surgical cavity with a safety margin of plus 1.5 cm for the clinical target volume, and an additional 1-cm expansion. The prescribed dose was 45 Gy in 1.5-Gy fractions delivered via 3D conformal radiotherapy in 30 treatments.
Of 65 patients accrued, 58 completed treatment and were evaluable for efficacy. The median age was 67.5 years. In all, 23 patients had ductal carcinoma in situ, and 35 had invasive cancers. All patients were node negative.
A total of four patients (6.9%) reported late grade 3 toxicities, which included breast infection, fibrous deep connective tissue, skin induration, and breast atrophy, pain, and volume loss. There were no grade 4 toxicities, and no treatment-related deaths.
Two patients had in-breast recurrences, which translated into a year estimate of 3.7%. Four patients underwent mastectomies, for a 3-year estimated mastectomy failure rate of 5.2%. Two of the mastectomies were for the in-breast recurrences, one for a nonhealing wound, and one occurred in a patient who developed cancer in the contralateral breast and opted for a bilateral mastectomy.
AT ASTRO 2016
Key clinical point: Re-irradiation following second lumpectomy after in-breast cancer recurrences appears to be a safe and effective alternative to mastectomy.
Major finding: The 3-year estimated in-breast recurrence rate was 3.7%.
Data source: Phase II nonrandomized study in 58 women with in-breast failures following breast-conserving surgery and whole-breast irradiation.
Disclosures: The study was supported by the National Cancer Institute. Dr. Arthur reported formerly serving on the medical advisory board of Impedimed,
CMS assures small-practice doctors they have a place in MACRA
WASHINGTON – Fear over how Medicare officials will operationalize the Medicare Access and CHIP Reauthorization Act – known as MACRA – should not be driving physicians in small and solo practices to become employees of large systems, a top federal health official said.
“If people want to sell their practices and they want to [be a] part of integrated care models for other reasons, that’s fine,” Andy Slavitt, acting administrator of the Centers for Medicare & Medicaid Services said during an Oct. 6 event hosted by the Association of Health Care Journalists. “I think we have to make a really big effort though to make sure that they are not being driven there because the world’s getting too complicated ... and they throw up their hands.”
“There are people throwing fear. There are consultants and there are hospitals saying MACRA is going to be impossible,” Mr. Slavitt said. “They don’t know the support that’s going to be provided. It is in certain people’s interest to put the story forward that small physician practices are doomed.”
One aspect of that support has already been announced – that physicians will have flexibility in terms of how much, or how little, they want to participate in the reporting requirements if they are selecting the Merit-based Incentive Payment System in 2017.
While it will be a challenge, Mr. Slavitt said the final MACRA regulations – due out in early November – will support small and solo practices so that they can be successful in the program.
“Health care has become more and more of a business and it becomes harder and harder if you don’t have those kinds of operations. But there are other ways to do it and I think we are going to be successful in a lot of ways,” he said.
WASHINGTON – Fear over how Medicare officials will operationalize the Medicare Access and CHIP Reauthorization Act – known as MACRA – should not be driving physicians in small and solo practices to become employees of large systems, a top federal health official said.
“If people want to sell their practices and they want to [be a] part of integrated care models for other reasons, that’s fine,” Andy Slavitt, acting administrator of the Centers for Medicare & Medicaid Services said during an Oct. 6 event hosted by the Association of Health Care Journalists. “I think we have to make a really big effort though to make sure that they are not being driven there because the world’s getting too complicated ... and they throw up their hands.”
“There are people throwing fear. There are consultants and there are hospitals saying MACRA is going to be impossible,” Mr. Slavitt said. “They don’t know the support that’s going to be provided. It is in certain people’s interest to put the story forward that small physician practices are doomed.”
One aspect of that support has already been announced – that physicians will have flexibility in terms of how much, or how little, they want to participate in the reporting requirements if they are selecting the Merit-based Incentive Payment System in 2017.
While it will be a challenge, Mr. Slavitt said the final MACRA regulations – due out in early November – will support small and solo practices so that they can be successful in the program.
“Health care has become more and more of a business and it becomes harder and harder if you don’t have those kinds of operations. But there are other ways to do it and I think we are going to be successful in a lot of ways,” he said.
WASHINGTON – Fear over how Medicare officials will operationalize the Medicare Access and CHIP Reauthorization Act – known as MACRA – should not be driving physicians in small and solo practices to become employees of large systems, a top federal health official said.
“If people want to sell their practices and they want to [be a] part of integrated care models for other reasons, that’s fine,” Andy Slavitt, acting administrator of the Centers for Medicare & Medicaid Services said during an Oct. 6 event hosted by the Association of Health Care Journalists. “I think we have to make a really big effort though to make sure that they are not being driven there because the world’s getting too complicated ... and they throw up their hands.”
“There are people throwing fear. There are consultants and there are hospitals saying MACRA is going to be impossible,” Mr. Slavitt said. “They don’t know the support that’s going to be provided. It is in certain people’s interest to put the story forward that small physician practices are doomed.”
One aspect of that support has already been announced – that physicians will have flexibility in terms of how much, or how little, they want to participate in the reporting requirements if they are selecting the Merit-based Incentive Payment System in 2017.
While it will be a challenge, Mr. Slavitt said the final MACRA regulations – due out in early November – will support small and solo practices so that they can be successful in the program.
“Health care has become more and more of a business and it becomes harder and harder if you don’t have those kinds of operations. But there are other ways to do it and I think we are going to be successful in a lot of ways,” he said.
Levosimendan does not reduce organ dysfunction risk in sepsis
Levosimendan does not reduce the likelihood of severe organ dysfunction in adults with sepsis, nor does it lower the mortality rate, according to research presented at the annual congress of the European Society of Intensive Care Medicine and published in the New England Journal of Medicine.
Levosimendan is a calcium-sensitizing drug with inotropic and vasodilatory properties, which is commonly used to treat decompensated heart failure. “Small studies that have investigated the use of levosimendan in patients with septic shock have shown improvements in hemodynamic variables, microcirculatory flow, and renal and hepatic function, as compared with dobutamine,” wrote Anthony C. Gordon, MD, of Imperial College London and Imperial College Healthcare NHS Trust and his coauthors.
In the Levosimendan for the Prevention of Acute Organ Dysfunction in Sepsis (LeoPARDS) trial, 516 patients were randomized to 24 hours of a blinded infusion either of levosimendan (.05-0.2 mcg per kilogram of body weight per minute) or placebo in addition to standard care.
Researchers saw no significant difference in the mean daily Sequential Organ Failure Assessment (SOFA) score between the two groups (mean difference, 0.61; 95% confidence interval, −0.07 to 1.29; P = .053). When the SOFA score was analyzed by system, the mean daily cardiovascular score was significantly higher in the levosimendan group, compared with the placebo group, indicating greater dysfunction in that system.
“The cardiovascular SOFA score was higher in the levosimendan group than in the placebo group, which reflects the higher doses of norepinephrine that were required to maintain the mean arterial pressure,” researchers reported.
There was no significant difference in 28-day mortality between the levosimendan and placebo groups (34.5% vs. 30.9%; 95% CI, −4.5 to 11.7; P = .43), and both groups had a similar number of catecholamine-free days. However, among the patients who required ventilation at baseline, those treated with levosimendan were less likely than those given placebo to be successfully weaned from ventilation over the 28-day follow-up.
Patients treated with levosimendan also had a higher incidence of serious adverse events, and supraventricular tachyarrhythmia was significantly more common in the levosimendan group than in the placebo group (3.1% vs. 0.4%; 95% CI, 0.1- 5.3; P = .04).
The two groups showed similar cardiac index, stroke volume, central venous oxygen saturations or pressure, the ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen, and serum creatinine and bilirubin levels.
The authors drew attention to several limitations of the study, including the fact that levosimendan was added to standard care rather than being compared with an alternative inotrope such as dobutamine.
“Less than 10% of the patients in the placebo group received dobutamine, although the rate of use in the placebo group was higher than in the levosimendan group and may explain in part why the cardiac index and stroke volume were not higher in the levosimendan group than in the placebo group,” they wrote.
The study did not include echocardiographic analysis to discover any changes in myocardial function with levosimendan, and there were only a small number of patients with low cardiac index.
“Therefore, this trial cannot provide guidance as to which inotrope is best to use in the management of sepsis if a low cardiac index is present,” the authors said. “The target mean arterial pressure of 65-70 mm Hg, which was recommended in the protocol and reiterated at investigator meetings, was frequently exceeded (as in other trials involving patients with shock), which suggests that the norepinephrine doses that were administered could have been reduced in the two trial groups.”
The study was supported by the Medical Research Council and National Institute for Health Research, United Kingdom, and Tenax Therapeutics. Four authors declared grants, personal fees, advisory board positions, and other funding from the pharmaceutical industry, including one author receiving support from Tenax Therapeutics. No other conflicts of interest were declared.
Levosimendan does not reduce the likelihood of severe organ dysfunction in adults with sepsis, nor does it lower the mortality rate, according to research presented at the annual congress of the European Society of Intensive Care Medicine and published in the New England Journal of Medicine.
Levosimendan is a calcium-sensitizing drug with inotropic and vasodilatory properties, which is commonly used to treat decompensated heart failure. “Small studies that have investigated the use of levosimendan in patients with septic shock have shown improvements in hemodynamic variables, microcirculatory flow, and renal and hepatic function, as compared with dobutamine,” wrote Anthony C. Gordon, MD, of Imperial College London and Imperial College Healthcare NHS Trust and his coauthors.
In the Levosimendan for the Prevention of Acute Organ Dysfunction in Sepsis (LeoPARDS) trial, 516 patients were randomized to 24 hours of a blinded infusion either of levosimendan (.05-0.2 mcg per kilogram of body weight per minute) or placebo in addition to standard care.
Researchers saw no significant difference in the mean daily Sequential Organ Failure Assessment (SOFA) score between the two groups (mean difference, 0.61; 95% confidence interval, −0.07 to 1.29; P = .053). When the SOFA score was analyzed by system, the mean daily cardiovascular score was significantly higher in the levosimendan group, compared with the placebo group, indicating greater dysfunction in that system.
“The cardiovascular SOFA score was higher in the levosimendan group than in the placebo group, which reflects the higher doses of norepinephrine that were required to maintain the mean arterial pressure,” researchers reported.
There was no significant difference in 28-day mortality between the levosimendan and placebo groups (34.5% vs. 30.9%; 95% CI, −4.5 to 11.7; P = .43), and both groups had a similar number of catecholamine-free days. However, among the patients who required ventilation at baseline, those treated with levosimendan were less likely than those given placebo to be successfully weaned from ventilation over the 28-day follow-up.
Patients treated with levosimendan also had a higher incidence of serious adverse events, and supraventricular tachyarrhythmia was significantly more common in the levosimendan group than in the placebo group (3.1% vs. 0.4%; 95% CI, 0.1- 5.3; P = .04).
The two groups showed similar cardiac index, stroke volume, central venous oxygen saturations or pressure, the ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen, and serum creatinine and bilirubin levels.
The authors drew attention to several limitations of the study, including the fact that levosimendan was added to standard care rather than being compared with an alternative inotrope such as dobutamine.
“Less than 10% of the patients in the placebo group received dobutamine, although the rate of use in the placebo group was higher than in the levosimendan group and may explain in part why the cardiac index and stroke volume were not higher in the levosimendan group than in the placebo group,” they wrote.
The study did not include echocardiographic analysis to discover any changes in myocardial function with levosimendan, and there were only a small number of patients with low cardiac index.
“Therefore, this trial cannot provide guidance as to which inotrope is best to use in the management of sepsis if a low cardiac index is present,” the authors said. “The target mean arterial pressure of 65-70 mm Hg, which was recommended in the protocol and reiterated at investigator meetings, was frequently exceeded (as in other trials involving patients with shock), which suggests that the norepinephrine doses that were administered could have been reduced in the two trial groups.”
The study was supported by the Medical Research Council and National Institute for Health Research, United Kingdom, and Tenax Therapeutics. Four authors declared grants, personal fees, advisory board positions, and other funding from the pharmaceutical industry, including one author receiving support from Tenax Therapeutics. No other conflicts of interest were declared.
Levosimendan does not reduce the likelihood of severe organ dysfunction in adults with sepsis, nor does it lower the mortality rate, according to research presented at the annual congress of the European Society of Intensive Care Medicine and published in the New England Journal of Medicine.
Levosimendan is a calcium-sensitizing drug with inotropic and vasodilatory properties, which is commonly used to treat decompensated heart failure. “Small studies that have investigated the use of levosimendan in patients with septic shock have shown improvements in hemodynamic variables, microcirculatory flow, and renal and hepatic function, as compared with dobutamine,” wrote Anthony C. Gordon, MD, of Imperial College London and Imperial College Healthcare NHS Trust and his coauthors.
In the Levosimendan for the Prevention of Acute Organ Dysfunction in Sepsis (LeoPARDS) trial, 516 patients were randomized to 24 hours of a blinded infusion either of levosimendan (.05-0.2 mcg per kilogram of body weight per minute) or placebo in addition to standard care.
Researchers saw no significant difference in the mean daily Sequential Organ Failure Assessment (SOFA) score between the two groups (mean difference, 0.61; 95% confidence interval, −0.07 to 1.29; P = .053). When the SOFA score was analyzed by system, the mean daily cardiovascular score was significantly higher in the levosimendan group, compared with the placebo group, indicating greater dysfunction in that system.
“The cardiovascular SOFA score was higher in the levosimendan group than in the placebo group, which reflects the higher doses of norepinephrine that were required to maintain the mean arterial pressure,” researchers reported.
There was no significant difference in 28-day mortality between the levosimendan and placebo groups (34.5% vs. 30.9%; 95% CI, −4.5 to 11.7; P = .43), and both groups had a similar number of catecholamine-free days. However, among the patients who required ventilation at baseline, those treated with levosimendan were less likely than those given placebo to be successfully weaned from ventilation over the 28-day follow-up.
Patients treated with levosimendan also had a higher incidence of serious adverse events, and supraventricular tachyarrhythmia was significantly more common in the levosimendan group than in the placebo group (3.1% vs. 0.4%; 95% CI, 0.1- 5.3; P = .04).
The two groups showed similar cardiac index, stroke volume, central venous oxygen saturations or pressure, the ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen, and serum creatinine and bilirubin levels.
The authors drew attention to several limitations of the study, including the fact that levosimendan was added to standard care rather than being compared with an alternative inotrope such as dobutamine.
“Less than 10% of the patients in the placebo group received dobutamine, although the rate of use in the placebo group was higher than in the levosimendan group and may explain in part why the cardiac index and stroke volume were not higher in the levosimendan group than in the placebo group,” they wrote.
The study did not include echocardiographic analysis to discover any changes in myocardial function with levosimendan, and there were only a small number of patients with low cardiac index.
“Therefore, this trial cannot provide guidance as to which inotrope is best to use in the management of sepsis if a low cardiac index is present,” the authors said. “The target mean arterial pressure of 65-70 mm Hg, which was recommended in the protocol and reiterated at investigator meetings, was frequently exceeded (as in other trials involving patients with shock), which suggests that the norepinephrine doses that were administered could have been reduced in the two trial groups.”
The study was supported by the Medical Research Council and National Institute for Health Research, United Kingdom, and Tenax Therapeutics. Four authors declared grants, personal fees, advisory board positions, and other funding from the pharmaceutical industry, including one author receiving support from Tenax Therapeutics. No other conflicts of interest were declared.
Key clinical point: Levosimendan does not reduce the likelihood of severe organ dysfunction or lower the mortality rate in adults with sepsis.
Major finding: There were no significant differences in mean daily Sequential Organ Failure Assessment score or mortality between patients treated with levosimendan or placebo in addition to standard care.
Data source: Randomized, placebo-controlled LeoPARDS trial in 516 adults with sepsis.
Disclosures: The study was supported by the Medical Research Council and National Institute for Health Research, United Kingdom, and Tenax Therapeutics. Four authors declared grants, personal fees, advisory board positions and other funding from the pharmaceutical industry, including one author receiving support from Tenax Therapeutics. No other conflicts of interest were declared.
Cough harder during stress urinary incontinence testing, study suggests
DENVER – Patients often do not cough hard enough during the cough stress test for it to identify urinary incontinence, Sudhi Trye, MD, reported at Pelvic Floor Disorders Week, sponsored by the American Urogynecologic Society.
“Based on our findings, 95% of patients with stress urinary incontinence will be missed on testing if abdominal pressure does not exceed 70 cm of water on the cough stress test,” said Dr. Trye, who is at Northwell Health, New Hyde Park, N.Y. In order to minimize false negatives, urodynamic operators should encourage patients to cough hard enough to exceed about 120-127 cm H2O in abdominal pressure, she said.
Patients were asked to cough at least three times with increasing force at volumes of 200 mL, 300 mL, 400 mL, and 500 mL. The median minimum abdominal leak point pressure ranged from 120 to 127 cm H2O, regardless of bladder volume, and did not differ significantly between premenopausal and postmenopausal women, Dr. Trye said. Only 5% had leakage at abdominal pressures of 70 cm of H2O or less. The same was true at the other end of the spectrum – only 5% of women had leakage at abdominal pressures above 200 cm H2O.
A total of 70% of patients were white; mean age was 65 years, with a standard deviation of 13.6 years; and average body mass index was 28.2 kg/m2 with a standard deviation of 5.4 kg/m2.
Although the findings merit replication in a larger and more diverse cohort, they suggest that patients should be encouraged to cough hard enough to achieve abdominal pressures above 120-127 cm H2O, Dr. Trye said. Since many patients have trouble coughing this hard, operators should model it rather than just tell patients to cough, she added.
Dr. Trye reported having no financial disclosures. One coinvestigator reported ties to Kimberly-Clark and Boston Scientific.
DENVER – Patients often do not cough hard enough during the cough stress test for it to identify urinary incontinence, Sudhi Trye, MD, reported at Pelvic Floor Disorders Week, sponsored by the American Urogynecologic Society.
“Based on our findings, 95% of patients with stress urinary incontinence will be missed on testing if abdominal pressure does not exceed 70 cm of water on the cough stress test,” said Dr. Trye, who is at Northwell Health, New Hyde Park, N.Y. In order to minimize false negatives, urodynamic operators should encourage patients to cough hard enough to exceed about 120-127 cm H2O in abdominal pressure, she said.
Patients were asked to cough at least three times with increasing force at volumes of 200 mL, 300 mL, 400 mL, and 500 mL. The median minimum abdominal leak point pressure ranged from 120 to 127 cm H2O, regardless of bladder volume, and did not differ significantly between premenopausal and postmenopausal women, Dr. Trye said. Only 5% had leakage at abdominal pressures of 70 cm of H2O or less. The same was true at the other end of the spectrum – only 5% of women had leakage at abdominal pressures above 200 cm H2O.
A total of 70% of patients were white; mean age was 65 years, with a standard deviation of 13.6 years; and average body mass index was 28.2 kg/m2 with a standard deviation of 5.4 kg/m2.
Although the findings merit replication in a larger and more diverse cohort, they suggest that patients should be encouraged to cough hard enough to achieve abdominal pressures above 120-127 cm H2O, Dr. Trye said. Since many patients have trouble coughing this hard, operators should model it rather than just tell patients to cough, she added.
Dr. Trye reported having no financial disclosures. One coinvestigator reported ties to Kimberly-Clark and Boston Scientific.
DENVER – Patients often do not cough hard enough during the cough stress test for it to identify urinary incontinence, Sudhi Trye, MD, reported at Pelvic Floor Disorders Week, sponsored by the American Urogynecologic Society.
“Based on our findings, 95% of patients with stress urinary incontinence will be missed on testing if abdominal pressure does not exceed 70 cm of water on the cough stress test,” said Dr. Trye, who is at Northwell Health, New Hyde Park, N.Y. In order to minimize false negatives, urodynamic operators should encourage patients to cough hard enough to exceed about 120-127 cm H2O in abdominal pressure, she said.
Patients were asked to cough at least three times with increasing force at volumes of 200 mL, 300 mL, 400 mL, and 500 mL. The median minimum abdominal leak point pressure ranged from 120 to 127 cm H2O, regardless of bladder volume, and did not differ significantly between premenopausal and postmenopausal women, Dr. Trye said. Only 5% had leakage at abdominal pressures of 70 cm of H2O or less. The same was true at the other end of the spectrum – only 5% of women had leakage at abdominal pressures above 200 cm H2O.
A total of 70% of patients were white; mean age was 65 years, with a standard deviation of 13.6 years; and average body mass index was 28.2 kg/m2 with a standard deviation of 5.4 kg/m2.
Although the findings merit replication in a larger and more diverse cohort, they suggest that patients should be encouraged to cough hard enough to achieve abdominal pressures above 120-127 cm H2O, Dr. Trye said. Since many patients have trouble coughing this hard, operators should model it rather than just tell patients to cough, she added.
Dr. Trye reported having no financial disclosures. One coinvestigator reported ties to Kimberly-Clark and Boston Scientific.
Key clinical point:
Major finding: The median minimum abdominal leak point pressure ranged from 120-127 cm H2O across all bladder volumes.
Data source: A retrospective study of 145 women with stress urinary incontinence based on urodynamic testing.
Disclosures: Dr. Trye reported having no financial disclosures. One coinvestigator reported ties to Kimberly-Clark and Boston Scientific.
Leadership Academy Helps SHM Member Improve Patient Flow, Satisfaction
This month, The Hospitalist spotlights G. Randy Smith Jr., MD, MS, SFHM, assistant professor in the Division of Hospital Medicine at the Northwestern University Feinberg School of Medicine and medical director of Unit 16 West at Northwestern Memorial Hospital in Chicago. Dr. Smith is an active member of SHM’s Practice Analysis Committee and a Leadership Academy veteran who has translated his learnings into more efficient rounding and patient-flow methodology.
Question: What inspired you to begin working in hospital medicine and later join SHM?
Answer: Interest in taking care of acutely ill patients inspired me to start working in hospital medicine. Evolution of this interest into care-delivery design inspired me to remain in hospital medicine. Joining SHM enabled me to make contacts nationally with people who share similar interests and engage in collaboration, which has been helpful for my growth as a physician.
Q: How has SHM provided you with resources to improve patient care during your time as a member?
A: The SHM annual meetings have provided a consistent framework for dissemination of clinically relevant innovations and discoveries. Each year I’ve attended, I’ve always learned something new from both the posters on display and from a quality improvement presentation. Last year at Hospital Medicine 2016, the HEADS-UP plenary abstract presentation was one very good example of a different approach to interdisciplinary rounding that I would not have been aware of without attending the SHM annual meeting.
Q: How did attending Leadership Academy help you grow to reach your medical director position at Northwestern Memorial?
A: Leadership Academy helped to open my mind to principles of negotiation and expectation management as well as self-awareness, which are not usually presented in medical school or residency. Many of the skills taught can be learned the hard way through the trials of life, but the Leadership Academy accelerated my real-world learning.
My hospital’s leadership recognized the skills I developed with the assistance of Leadership Academy, which helped me to maintain my effectiveness in my medical director role.
Skills obtained in the Leadership Academy helped me to incorporate ward-based afternoon throughput meetings into a hospital-wide patient-flow management network. I also learned to successfully negotiate procurement of chairs for our physicians to sit at the patient’s bedside in the hopes of improving patient satisfaction.
Q: How has your work on the Practice Analysis Committee impacted how you manage your hospital medicine teams?
A: My involvement in the Practice Analysis Committee is yet another example of an opportunity provided by SHM to develop a skills set I would not otherwise have the opportunity to develop. Working on the State of Hospital Medicine survey involves prioritizing information with the burden of the respondents’ time and effort in mind. Sensitivities to stakeholder interest play a major role as well.
Achieving balance between aspiring definitions of concepts to help drive the field and working definitions used heterogeneously throughout the country represents the hardest task of the committee members to sort; I’m very privileged to take the lessons learned through member dialogue and help colleagues apply the lessons locally.
Q: Hospital medicine is celebrating its 20th anniversary this year. How do you see the role of hospitalists evolving over the next 20 years?
A: Change has been a constant in hospital medicine since I began in 2004 and will continue to remain so. Because of the financial and documentary pressures placed on hospitals and physicians, hospital medicine finds itself in a state of flux at the moment. I believe these pressures will drive the 7-on/7-off hospitalist direct-care model as it exists today to evolve into something else. In particular, hospitalist groups, which engage solely in documentation and “decision in name” only and devolve all clinical decision making to another set of physicians in the hospital, are especially nonviable over the long term. I think many hospital administrators believe this as well. Hospital medicine must show value, including in the realm of direct clinical care.
One possibility is that we evolve into a “supervisor” model, where a program is composed of a few experienced hospitalists supervising numerous physician extenders, who in turn rely on multidisciplinary teams in the hospital for clinical decision-making input. Hospitalist physicians will slightly move away from direct clinical decision making in such a model.
Another possibility involves evolution of information support systems to a point where teams of providers organized around a single medical problem, e.g., congestive heart failure, can be replaced, leaving the hospitalist to make patient-centered clinical decisions with updated multidisciplinary input available electronically.
With information systems that provide equal access to evidence-driven guidance for optimal clinical practice, hospitalists will outperform subspecialists at the bedside on patient-centeredness, cost, and availability.
Regardless of how inpatient care evolves, hospital medicine will undoubtedly be at the epicenter of change for years to come. TH
This month, The Hospitalist spotlights G. Randy Smith Jr., MD, MS, SFHM, assistant professor in the Division of Hospital Medicine at the Northwestern University Feinberg School of Medicine and medical director of Unit 16 West at Northwestern Memorial Hospital in Chicago. Dr. Smith is an active member of SHM’s Practice Analysis Committee and a Leadership Academy veteran who has translated his learnings into more efficient rounding and patient-flow methodology.
Question: What inspired you to begin working in hospital medicine and later join SHM?
Answer: Interest in taking care of acutely ill patients inspired me to start working in hospital medicine. Evolution of this interest into care-delivery design inspired me to remain in hospital medicine. Joining SHM enabled me to make contacts nationally with people who share similar interests and engage in collaboration, which has been helpful for my growth as a physician.
Q: How has SHM provided you with resources to improve patient care during your time as a member?
A: The SHM annual meetings have provided a consistent framework for dissemination of clinically relevant innovations and discoveries. Each year I’ve attended, I’ve always learned something new from both the posters on display and from a quality improvement presentation. Last year at Hospital Medicine 2016, the HEADS-UP plenary abstract presentation was one very good example of a different approach to interdisciplinary rounding that I would not have been aware of without attending the SHM annual meeting.
Q: How did attending Leadership Academy help you grow to reach your medical director position at Northwestern Memorial?
A: Leadership Academy helped to open my mind to principles of negotiation and expectation management as well as self-awareness, which are not usually presented in medical school or residency. Many of the skills taught can be learned the hard way through the trials of life, but the Leadership Academy accelerated my real-world learning.
My hospital’s leadership recognized the skills I developed with the assistance of Leadership Academy, which helped me to maintain my effectiveness in my medical director role.
Skills obtained in the Leadership Academy helped me to incorporate ward-based afternoon throughput meetings into a hospital-wide patient-flow management network. I also learned to successfully negotiate procurement of chairs for our physicians to sit at the patient’s bedside in the hopes of improving patient satisfaction.
Q: How has your work on the Practice Analysis Committee impacted how you manage your hospital medicine teams?
A: My involvement in the Practice Analysis Committee is yet another example of an opportunity provided by SHM to develop a skills set I would not otherwise have the opportunity to develop. Working on the State of Hospital Medicine survey involves prioritizing information with the burden of the respondents’ time and effort in mind. Sensitivities to stakeholder interest play a major role as well.
Achieving balance between aspiring definitions of concepts to help drive the field and working definitions used heterogeneously throughout the country represents the hardest task of the committee members to sort; I’m very privileged to take the lessons learned through member dialogue and help colleagues apply the lessons locally.
Q: Hospital medicine is celebrating its 20th anniversary this year. How do you see the role of hospitalists evolving over the next 20 years?
A: Change has been a constant in hospital medicine since I began in 2004 and will continue to remain so. Because of the financial and documentary pressures placed on hospitals and physicians, hospital medicine finds itself in a state of flux at the moment. I believe these pressures will drive the 7-on/7-off hospitalist direct-care model as it exists today to evolve into something else. In particular, hospitalist groups, which engage solely in documentation and “decision in name” only and devolve all clinical decision making to another set of physicians in the hospital, are especially nonviable over the long term. I think many hospital administrators believe this as well. Hospital medicine must show value, including in the realm of direct clinical care.
One possibility is that we evolve into a “supervisor” model, where a program is composed of a few experienced hospitalists supervising numerous physician extenders, who in turn rely on multidisciplinary teams in the hospital for clinical decision-making input. Hospitalist physicians will slightly move away from direct clinical decision making in such a model.
Another possibility involves evolution of information support systems to a point where teams of providers organized around a single medical problem, e.g., congestive heart failure, can be replaced, leaving the hospitalist to make patient-centered clinical decisions with updated multidisciplinary input available electronically.
With information systems that provide equal access to evidence-driven guidance for optimal clinical practice, hospitalists will outperform subspecialists at the bedside on patient-centeredness, cost, and availability.
Regardless of how inpatient care evolves, hospital medicine will undoubtedly be at the epicenter of change for years to come. TH
This month, The Hospitalist spotlights G. Randy Smith Jr., MD, MS, SFHM, assistant professor in the Division of Hospital Medicine at the Northwestern University Feinberg School of Medicine and medical director of Unit 16 West at Northwestern Memorial Hospital in Chicago. Dr. Smith is an active member of SHM’s Practice Analysis Committee and a Leadership Academy veteran who has translated his learnings into more efficient rounding and patient-flow methodology.
Question: What inspired you to begin working in hospital medicine and later join SHM?
Answer: Interest in taking care of acutely ill patients inspired me to start working in hospital medicine. Evolution of this interest into care-delivery design inspired me to remain in hospital medicine. Joining SHM enabled me to make contacts nationally with people who share similar interests and engage in collaboration, which has been helpful for my growth as a physician.
Q: How has SHM provided you with resources to improve patient care during your time as a member?
A: The SHM annual meetings have provided a consistent framework for dissemination of clinically relevant innovations and discoveries. Each year I’ve attended, I’ve always learned something new from both the posters on display and from a quality improvement presentation. Last year at Hospital Medicine 2016, the HEADS-UP plenary abstract presentation was one very good example of a different approach to interdisciplinary rounding that I would not have been aware of without attending the SHM annual meeting.
Q: How did attending Leadership Academy help you grow to reach your medical director position at Northwestern Memorial?
A: Leadership Academy helped to open my mind to principles of negotiation and expectation management as well as self-awareness, which are not usually presented in medical school or residency. Many of the skills taught can be learned the hard way through the trials of life, but the Leadership Academy accelerated my real-world learning.
My hospital’s leadership recognized the skills I developed with the assistance of Leadership Academy, which helped me to maintain my effectiveness in my medical director role.
Skills obtained in the Leadership Academy helped me to incorporate ward-based afternoon throughput meetings into a hospital-wide patient-flow management network. I also learned to successfully negotiate procurement of chairs for our physicians to sit at the patient’s bedside in the hopes of improving patient satisfaction.
Q: How has your work on the Practice Analysis Committee impacted how you manage your hospital medicine teams?
A: My involvement in the Practice Analysis Committee is yet another example of an opportunity provided by SHM to develop a skills set I would not otherwise have the opportunity to develop. Working on the State of Hospital Medicine survey involves prioritizing information with the burden of the respondents’ time and effort in mind. Sensitivities to stakeholder interest play a major role as well.
Achieving balance between aspiring definitions of concepts to help drive the field and working definitions used heterogeneously throughout the country represents the hardest task of the committee members to sort; I’m very privileged to take the lessons learned through member dialogue and help colleagues apply the lessons locally.
Q: Hospital medicine is celebrating its 20th anniversary this year. How do you see the role of hospitalists evolving over the next 20 years?
A: Change has been a constant in hospital medicine since I began in 2004 and will continue to remain so. Because of the financial and documentary pressures placed on hospitals and physicians, hospital medicine finds itself in a state of flux at the moment. I believe these pressures will drive the 7-on/7-off hospitalist direct-care model as it exists today to evolve into something else. In particular, hospitalist groups, which engage solely in documentation and “decision in name” only and devolve all clinical decision making to another set of physicians in the hospital, are especially nonviable over the long term. I think many hospital administrators believe this as well. Hospital medicine must show value, including in the realm of direct clinical care.
One possibility is that we evolve into a “supervisor” model, where a program is composed of a few experienced hospitalists supervising numerous physician extenders, who in turn rely on multidisciplinary teams in the hospital for clinical decision-making input. Hospitalist physicians will slightly move away from direct clinical decision making in such a model.
Another possibility involves evolution of information support systems to a point where teams of providers organized around a single medical problem, e.g., congestive heart failure, can be replaced, leaving the hospitalist to make patient-centered clinical decisions with updated multidisciplinary input available electronically.
With information systems that provide equal access to evidence-driven guidance for optimal clinical practice, hospitalists will outperform subspecialists at the bedside on patient-centeredness, cost, and availability.
Regardless of how inpatient care evolves, hospital medicine will undoubtedly be at the epicenter of change for years to come. TH
MF assessment and treatment an ‘evolution’ from the past

© ASCO/Zach Boyden-Holmes
NEW YORK—The 11th NCCN Congress: Hematologic Malignancies coincided with the release of the inaugural edition of the NCCN treatment guideline on myeloproliferative neoplasms (MPNs), and Ruben A. Mesa, MD, took the opportunity to discuss the framework of the document and the evolving management of MPNs.
Dr Mesa, of the Mayo Clinic Cancer Center in Arizona, is the chair of the MPN guideline committee.
This initial version of the guideline focuses on the workup and diagnosis of primary myelofibrosis (MF), post-polycythemia vera MF, and post-essential thrombocythemia MF, and treatment guidelines for MF.
The committee decided to first tackle the treatment guidelines in MF because, as Dr Mesa explained, MF is “the most severe of the MPNs with the most unmet needs in terms of guidance.”
Treatment guidelines for polycythemia vera (PV) and essential thrombocythemia (ET) will be forthcoming in 2017, he said, as well as diagnosis and treatment of atypical MPNs, such as hypereosinophilic syndrome and systemic mast cell disease.
Workup of an MPN
The guideline committee focused on information regarding diagnosis—the time and place to consider bone marrow biopsies, when to use cytogenetics, and when to perform next-generation sequencing.
They stressed the importance of quantifying disease burden and utilizing the now-validated symptom assessment tools, particularly in MF.
“Finally, we leveraged the many prognostic scoring systems that have been developed for these diseases, particularly IPSS for myelofibrosis at diagnosis or the DIPSS and DIPSS-plus at subsequent time points,” Dr Mesa said.
In addition to clinical-based prognostic scoring systems, information regarding molecular features and their impact is evolving, he said.
The guideline provides a table of mutations with prognostic significance. JAK2, MPL, or CALR mutations are “brighter mutations,” he said, while adverse prognostic markers include ASXL1, EZH2, IDH1/2, SRSF2, and TP53.
“All of these have some significant prognostic implications in primary myelofibrosis, specifically,” he said.
Assessing MPN burden
“When treating these patients, it’s important to be mindful of the overall burden of the disease,” Dr Mesa said.
He called this emphasis on disease burden “an evolution from the past, where therapy was either supportive or primarily focused on prevention of thrombosis with ET or PV.”
Practitioners need to be additionally mindful of the risk of vascular events, progression, the impact of cytopenias, splenomegaly, the burden of symptoms, and the baseline degree of comorbidities.
“[W]e encourage the use of validated symptom tools that have been used now in the majority of clinical trials in this setting,” Dr Mesa added.
The MPN-10 assessment tool, included in the guideline, evaluates 10 symptoms—early satiety, abdominal discomfort, inactivity, problems concentrating, numbness/tingling, night sweats, itching, bone pain, fever, and unintentional weight loss—on a scale of 0 to 10.
Patients with MF are the most symptomatic, Dr Mesa commented, although “it is notable how frequent symptoms are present in patients with PV and ET.”
Response criteria
For a complete response, individuals must have marked improvement to near normalization in both bone marrow and peripheral blood in addition to resolution of their disease symptoms.
Partial response is basically “just shy” of the complete response level, Dr Mesa said, with bone marrow resolution not being required.
The guideline also outlines response criteria for progressive, stable, and relapsed disease, clinical improvement, and anemia, spleen, and symptom response.
“But I’ll highlight that the majority of the responses that are received currently with medical therapy are in the area of clinical improvement,” Dr Mesa said.
Treatment guidance
Specific treatment guidelines for low-risk, intermediate-1 risk, intermediate-2- or high-risk MF from the new guideline have been described in an earlier article and will not be discussed here.
Of note, however, Dr Mesa explained that ruxolitinib is a very important part of the treatment guideline because it is the only therapy approved by the US Food and Drug Administration (FDA) to treat MF.
“Over time, it’s been shown that there is an improvement in survival [with ruxolitinib],” Dr Mesa said, perhaps because of a decrease with treatment in the morbidity and the debilitation of patients.
Investigators presented the 5-year update of this information at ASCO 2016 and reported that patients maintained reduction in splenomegaly up through 5 years, both in those randomized to ruxolitinib and those crossing over from placebo.
“Long-term, we clearly looked to see whether there was a signal of new onset or late onset toxicities,” Dr Mesa said, which largely was not the case. "Toxicities have been well described in earlier studies, primarily around anemia, thrombocytopenia, and mild constitutional symptoms.”
However, long-term therapy increases the rate of development of shingles and non-melanoma skin cancer. Rates of transformation to acute myeloid leukemia were consistent with those published for similar patient populations with MF.
“If I see a patient stable on ruxolitinib who has a marked drop in their counts later on in the course of their disease,” Dr Mesa added, “I’m certainly suspicious of progression.”
He also works them up for other causes of anemia if it evolves out of the blue.
Support in MF-related anemia
“With anemia, we’re mindful of iron stores, EPO level, and being certain there’s not the presence of hemolysis or other contributors,” Dr Mesa said.
The guideline recommends stratifying patients based on serum EPO levels—those with less than 500 mU/mL and those with 500 mU/mL and higher.
“If we lower the EPO level, the greater the likelihood of response,” Dr Mesa said. “In my experience, the lower the transfusion burden, the greater the likelihood of response.”
Other than erythropoiesis-stimulating agents for individuals with lower serum levels, androgens and immunomodulatory drugs for those with higher levels have some benefit.
But “they all have their limitations,” Dr Mesa said, and “they tend to range in benefit from 20% to 30%.”
The future
Dr Mesa discussed a few agents in the pipeline that “might impact these guidelines,” such as the JAK2/FLT3 inhibitor pacritinib and the JAK1/JAK2 inhibitor momelotinib.
Pacritinib had a positive phase 3 study, but the mortality rate was higher than expected, and it was put on an FDA hold.
Data from a second phase 3 study (PERSIST-1) will be reviewed in the aggregate to evaluate the benefit of pacritinib and whether the mortality rate was associated with drug-related side effects or adverse patient selection, “which we suspect might be the case,” Dr Mesa said.
“This agent may come off hold, depending upon the data of that second phase 3 study,” he added.
Momelotinib is also in an advanced phase 3 program with 2 trials underway. Both trials have completed accrual.
One is an upfront study of momelotinib versus ruxolitinib (NCT01969838). The goal is to reduce anemia without inferiority of splenomegaly and MPN symptoms.
The other phase 3 momelotinib trial is a second-line study versus best alternative therapy, including ruxoltinib (NCT02101268).
Combination studies are ongoing with a ruxolitinib base and a variety of secondary agents, including danazol, pomalidomide, PEG IFN α2a, 5-AZA, panobinostat, BKM-120, and LDE-225. All agents appear to achieve improvement in splenomegaly and symptoms.
But “incremental benefit over ruxolitinib alone is not yet clear,” Dr Mesa said. “I would say there’s not yet a recommended off-label combination which is widely being used.”
Dr Mesa also highlighted PRM-151, an antifibrosing drug that had a favorable early stage study with several doses, and the telomerase inhibitor imetelstat, which had a deep set of molecular responses in about a third of patients with MF. Imetelstat is currently being evaluated in the second-line setting (NCT02426086).
Regarding the possible positioning of new therapies, Dr Mesa believes “momelotinib and pacritinib may play a role in front-line, depending on the final data from those studies.”
“In second-line for MF—this is where most of the activity is in the trials,” he said. “Momelotinib, pacritinib, PRM-151, and imetelstat have possibilities.” ![]()

© ASCO/Zach Boyden-Holmes
NEW YORK—The 11th NCCN Congress: Hematologic Malignancies coincided with the release of the inaugural edition of the NCCN treatment guideline on myeloproliferative neoplasms (MPNs), and Ruben A. Mesa, MD, took the opportunity to discuss the framework of the document and the evolving management of MPNs.
Dr Mesa, of the Mayo Clinic Cancer Center in Arizona, is the chair of the MPN guideline committee.
This initial version of the guideline focuses on the workup and diagnosis of primary myelofibrosis (MF), post-polycythemia vera MF, and post-essential thrombocythemia MF, and treatment guidelines for MF.
The committee decided to first tackle the treatment guidelines in MF because, as Dr Mesa explained, MF is “the most severe of the MPNs with the most unmet needs in terms of guidance.”
Treatment guidelines for polycythemia vera (PV) and essential thrombocythemia (ET) will be forthcoming in 2017, he said, as well as diagnosis and treatment of atypical MPNs, such as hypereosinophilic syndrome and systemic mast cell disease.
Workup of an MPN
The guideline committee focused on information regarding diagnosis—the time and place to consider bone marrow biopsies, when to use cytogenetics, and when to perform next-generation sequencing.
They stressed the importance of quantifying disease burden and utilizing the now-validated symptom assessment tools, particularly in MF.
“Finally, we leveraged the many prognostic scoring systems that have been developed for these diseases, particularly IPSS for myelofibrosis at diagnosis or the DIPSS and DIPSS-plus at subsequent time points,” Dr Mesa said.
In addition to clinical-based prognostic scoring systems, information regarding molecular features and their impact is evolving, he said.
The guideline provides a table of mutations with prognostic significance. JAK2, MPL, or CALR mutations are “brighter mutations,” he said, while adverse prognostic markers include ASXL1, EZH2, IDH1/2, SRSF2, and TP53.
“All of these have some significant prognostic implications in primary myelofibrosis, specifically,” he said.
Assessing MPN burden
“When treating these patients, it’s important to be mindful of the overall burden of the disease,” Dr Mesa said.
He called this emphasis on disease burden “an evolution from the past, where therapy was either supportive or primarily focused on prevention of thrombosis with ET or PV.”
Practitioners need to be additionally mindful of the risk of vascular events, progression, the impact of cytopenias, splenomegaly, the burden of symptoms, and the baseline degree of comorbidities.
“[W]e encourage the use of validated symptom tools that have been used now in the majority of clinical trials in this setting,” Dr Mesa added.
The MPN-10 assessment tool, included in the guideline, evaluates 10 symptoms—early satiety, abdominal discomfort, inactivity, problems concentrating, numbness/tingling, night sweats, itching, bone pain, fever, and unintentional weight loss—on a scale of 0 to 10.
Patients with MF are the most symptomatic, Dr Mesa commented, although “it is notable how frequent symptoms are present in patients with PV and ET.”
Response criteria
For a complete response, individuals must have marked improvement to near normalization in both bone marrow and peripheral blood in addition to resolution of their disease symptoms.
Partial response is basically “just shy” of the complete response level, Dr Mesa said, with bone marrow resolution not being required.
The guideline also outlines response criteria for progressive, stable, and relapsed disease, clinical improvement, and anemia, spleen, and symptom response.
“But I’ll highlight that the majority of the responses that are received currently with medical therapy are in the area of clinical improvement,” Dr Mesa said.
Treatment guidance
Specific treatment guidelines for low-risk, intermediate-1 risk, intermediate-2- or high-risk MF from the new guideline have been described in an earlier article and will not be discussed here.
Of note, however, Dr Mesa explained that ruxolitinib is a very important part of the treatment guideline because it is the only therapy approved by the US Food and Drug Administration (FDA) to treat MF.
“Over time, it’s been shown that there is an improvement in survival [with ruxolitinib],” Dr Mesa said, perhaps because of a decrease with treatment in the morbidity and the debilitation of patients.
Investigators presented the 5-year update of this information at ASCO 2016 and reported that patients maintained reduction in splenomegaly up through 5 years, both in those randomized to ruxolitinib and those crossing over from placebo.
“Long-term, we clearly looked to see whether there was a signal of new onset or late onset toxicities,” Dr Mesa said, which largely was not the case. "Toxicities have been well described in earlier studies, primarily around anemia, thrombocytopenia, and mild constitutional symptoms.”
However, long-term therapy increases the rate of development of shingles and non-melanoma skin cancer. Rates of transformation to acute myeloid leukemia were consistent with those published for similar patient populations with MF.
“If I see a patient stable on ruxolitinib who has a marked drop in their counts later on in the course of their disease,” Dr Mesa added, “I’m certainly suspicious of progression.”
He also works them up for other causes of anemia if it evolves out of the blue.
Support in MF-related anemia
“With anemia, we’re mindful of iron stores, EPO level, and being certain there’s not the presence of hemolysis or other contributors,” Dr Mesa said.
The guideline recommends stratifying patients based on serum EPO levels—those with less than 500 mU/mL and those with 500 mU/mL and higher.
“If we lower the EPO level, the greater the likelihood of response,” Dr Mesa said. “In my experience, the lower the transfusion burden, the greater the likelihood of response.”
Other than erythropoiesis-stimulating agents for individuals with lower serum levels, androgens and immunomodulatory drugs for those with higher levels have some benefit.
But “they all have their limitations,” Dr Mesa said, and “they tend to range in benefit from 20% to 30%.”
The future
Dr Mesa discussed a few agents in the pipeline that “might impact these guidelines,” such as the JAK2/FLT3 inhibitor pacritinib and the JAK1/JAK2 inhibitor momelotinib.
Pacritinib had a positive phase 3 study, but the mortality rate was higher than expected, and it was put on an FDA hold.
Data from a second phase 3 study (PERSIST-1) will be reviewed in the aggregate to evaluate the benefit of pacritinib and whether the mortality rate was associated with drug-related side effects or adverse patient selection, “which we suspect might be the case,” Dr Mesa said.
“This agent may come off hold, depending upon the data of that second phase 3 study,” he added.
Momelotinib is also in an advanced phase 3 program with 2 trials underway. Both trials have completed accrual.
One is an upfront study of momelotinib versus ruxolitinib (NCT01969838). The goal is to reduce anemia without inferiority of splenomegaly and MPN symptoms.
The other phase 3 momelotinib trial is a second-line study versus best alternative therapy, including ruxoltinib (NCT02101268).
Combination studies are ongoing with a ruxolitinib base and a variety of secondary agents, including danazol, pomalidomide, PEG IFN α2a, 5-AZA, panobinostat, BKM-120, and LDE-225. All agents appear to achieve improvement in splenomegaly and symptoms.
But “incremental benefit over ruxolitinib alone is not yet clear,” Dr Mesa said. “I would say there’s not yet a recommended off-label combination which is widely being used.”
Dr Mesa also highlighted PRM-151, an antifibrosing drug that had a favorable early stage study with several doses, and the telomerase inhibitor imetelstat, which had a deep set of molecular responses in about a third of patients with MF. Imetelstat is currently being evaluated in the second-line setting (NCT02426086).
Regarding the possible positioning of new therapies, Dr Mesa believes “momelotinib and pacritinib may play a role in front-line, depending on the final data from those studies.”
“In second-line for MF—this is where most of the activity is in the trials,” he said. “Momelotinib, pacritinib, PRM-151, and imetelstat have possibilities.” ![]()

© ASCO/Zach Boyden-Holmes
NEW YORK—The 11th NCCN Congress: Hematologic Malignancies coincided with the release of the inaugural edition of the NCCN treatment guideline on myeloproliferative neoplasms (MPNs), and Ruben A. Mesa, MD, took the opportunity to discuss the framework of the document and the evolving management of MPNs.
Dr Mesa, of the Mayo Clinic Cancer Center in Arizona, is the chair of the MPN guideline committee.
This initial version of the guideline focuses on the workup and diagnosis of primary myelofibrosis (MF), post-polycythemia vera MF, and post-essential thrombocythemia MF, and treatment guidelines for MF.
The committee decided to first tackle the treatment guidelines in MF because, as Dr Mesa explained, MF is “the most severe of the MPNs with the most unmet needs in terms of guidance.”
Treatment guidelines for polycythemia vera (PV) and essential thrombocythemia (ET) will be forthcoming in 2017, he said, as well as diagnosis and treatment of atypical MPNs, such as hypereosinophilic syndrome and systemic mast cell disease.
Workup of an MPN
The guideline committee focused on information regarding diagnosis—the time and place to consider bone marrow biopsies, when to use cytogenetics, and when to perform next-generation sequencing.
They stressed the importance of quantifying disease burden and utilizing the now-validated symptom assessment tools, particularly in MF.
“Finally, we leveraged the many prognostic scoring systems that have been developed for these diseases, particularly IPSS for myelofibrosis at diagnosis or the DIPSS and DIPSS-plus at subsequent time points,” Dr Mesa said.
In addition to clinical-based prognostic scoring systems, information regarding molecular features and their impact is evolving, he said.
The guideline provides a table of mutations with prognostic significance. JAK2, MPL, or CALR mutations are “brighter mutations,” he said, while adverse prognostic markers include ASXL1, EZH2, IDH1/2, SRSF2, and TP53.
“All of these have some significant prognostic implications in primary myelofibrosis, specifically,” he said.
Assessing MPN burden
“When treating these patients, it’s important to be mindful of the overall burden of the disease,” Dr Mesa said.
He called this emphasis on disease burden “an evolution from the past, where therapy was either supportive or primarily focused on prevention of thrombosis with ET or PV.”
Practitioners need to be additionally mindful of the risk of vascular events, progression, the impact of cytopenias, splenomegaly, the burden of symptoms, and the baseline degree of comorbidities.
“[W]e encourage the use of validated symptom tools that have been used now in the majority of clinical trials in this setting,” Dr Mesa added.
The MPN-10 assessment tool, included in the guideline, evaluates 10 symptoms—early satiety, abdominal discomfort, inactivity, problems concentrating, numbness/tingling, night sweats, itching, bone pain, fever, and unintentional weight loss—on a scale of 0 to 10.
Patients with MF are the most symptomatic, Dr Mesa commented, although “it is notable how frequent symptoms are present in patients with PV and ET.”
Response criteria
For a complete response, individuals must have marked improvement to near normalization in both bone marrow and peripheral blood in addition to resolution of their disease symptoms.
Partial response is basically “just shy” of the complete response level, Dr Mesa said, with bone marrow resolution not being required.
The guideline also outlines response criteria for progressive, stable, and relapsed disease, clinical improvement, and anemia, spleen, and symptom response.
“But I’ll highlight that the majority of the responses that are received currently with medical therapy are in the area of clinical improvement,” Dr Mesa said.
Treatment guidance
Specific treatment guidelines for low-risk, intermediate-1 risk, intermediate-2- or high-risk MF from the new guideline have been described in an earlier article and will not be discussed here.
Of note, however, Dr Mesa explained that ruxolitinib is a very important part of the treatment guideline because it is the only therapy approved by the US Food and Drug Administration (FDA) to treat MF.
“Over time, it’s been shown that there is an improvement in survival [with ruxolitinib],” Dr Mesa said, perhaps because of a decrease with treatment in the morbidity and the debilitation of patients.
Investigators presented the 5-year update of this information at ASCO 2016 and reported that patients maintained reduction in splenomegaly up through 5 years, both in those randomized to ruxolitinib and those crossing over from placebo.
“Long-term, we clearly looked to see whether there was a signal of new onset or late onset toxicities,” Dr Mesa said, which largely was not the case. "Toxicities have been well described in earlier studies, primarily around anemia, thrombocytopenia, and mild constitutional symptoms.”
However, long-term therapy increases the rate of development of shingles and non-melanoma skin cancer. Rates of transformation to acute myeloid leukemia were consistent with those published for similar patient populations with MF.
“If I see a patient stable on ruxolitinib who has a marked drop in their counts later on in the course of their disease,” Dr Mesa added, “I’m certainly suspicious of progression.”
He also works them up for other causes of anemia if it evolves out of the blue.
Support in MF-related anemia
“With anemia, we’re mindful of iron stores, EPO level, and being certain there’s not the presence of hemolysis or other contributors,” Dr Mesa said.
The guideline recommends stratifying patients based on serum EPO levels—those with less than 500 mU/mL and those with 500 mU/mL and higher.
“If we lower the EPO level, the greater the likelihood of response,” Dr Mesa said. “In my experience, the lower the transfusion burden, the greater the likelihood of response.”
Other than erythropoiesis-stimulating agents for individuals with lower serum levels, androgens and immunomodulatory drugs for those with higher levels have some benefit.
But “they all have their limitations,” Dr Mesa said, and “they tend to range in benefit from 20% to 30%.”
The future
Dr Mesa discussed a few agents in the pipeline that “might impact these guidelines,” such as the JAK2/FLT3 inhibitor pacritinib and the JAK1/JAK2 inhibitor momelotinib.
Pacritinib had a positive phase 3 study, but the mortality rate was higher than expected, and it was put on an FDA hold.
Data from a second phase 3 study (PERSIST-1) will be reviewed in the aggregate to evaluate the benefit of pacritinib and whether the mortality rate was associated with drug-related side effects or adverse patient selection, “which we suspect might be the case,” Dr Mesa said.
“This agent may come off hold, depending upon the data of that second phase 3 study,” he added.
Momelotinib is also in an advanced phase 3 program with 2 trials underway. Both trials have completed accrual.
One is an upfront study of momelotinib versus ruxolitinib (NCT01969838). The goal is to reduce anemia without inferiority of splenomegaly and MPN symptoms.
The other phase 3 momelotinib trial is a second-line study versus best alternative therapy, including ruxoltinib (NCT02101268).
Combination studies are ongoing with a ruxolitinib base and a variety of secondary agents, including danazol, pomalidomide, PEG IFN α2a, 5-AZA, panobinostat, BKM-120, and LDE-225. All agents appear to achieve improvement in splenomegaly and symptoms.
But “incremental benefit over ruxolitinib alone is not yet clear,” Dr Mesa said. “I would say there’s not yet a recommended off-label combination which is widely being used.”
Dr Mesa also highlighted PRM-151, an antifibrosing drug that had a favorable early stage study with several doses, and the telomerase inhibitor imetelstat, which had a deep set of molecular responses in about a third of patients with MF. Imetelstat is currently being evaluated in the second-line setting (NCT02426086).
Regarding the possible positioning of new therapies, Dr Mesa believes “momelotinib and pacritinib may play a role in front-line, depending on the final data from those studies.”
“In second-line for MF—this is where most of the activity is in the trials,” he said. “Momelotinib, pacritinib, PRM-151, and imetelstat have possibilities.” ![]()