User login
Tetravalent dengue vaccine shows efficacy against asymptomatic dengue
A tetravalent dengue vaccine reduced symptomatic and asymptomatic dengue infection in Asian and Latin American children aged 2-16 years, according to Gustavo Olivera-Botello, PhD, and his associates at Sanofi Pasteur.
They analyzed data from 3,736 individuals in two phase III clinical trials. Patients received either the dengue vaccine CYD-TDV or placebo at month 0, month 6, and month 12, and had immunologic results from months 13 to 25. The seroconversion rate in individuals with virologically confirmed dengue between 13 and 25 months after vaccination was 88% in the vaccine group, and 98% in the placebo group.
In individuals without virologically confirmed dengue, 219 of 2,485 participants in the vaccine group and 157 of 1,184 in the placebo group seroconverted during months 13-25. Total vaccine efficacy against asymptomatic dengue was 33.5%, and was higher in children aged 9-16, with vaccine efficacy at 38.6%.
“Since about 80% of DENV [dengue virus] infections are asymptomatic, it is likely that they contribute significantly to viral transmission to mosquitoes and thus to other human hosts. Consequently, providing simultaneous protection against both asymptomatic and symptomatic infections could contribute to reduced transmission and thus to indirect protection if the vaccine coverage rates are sufficient,” the investigators wrote.
Find the full study in the Journal of Infectious Disease (doi: 10.1093/infdis/jiw297)
A tetravalent dengue vaccine reduced symptomatic and asymptomatic dengue infection in Asian and Latin American children aged 2-16 years, according to Gustavo Olivera-Botello, PhD, and his associates at Sanofi Pasteur.
They analyzed data from 3,736 individuals in two phase III clinical trials. Patients received either the dengue vaccine CYD-TDV or placebo at month 0, month 6, and month 12, and had immunologic results from months 13 to 25. The seroconversion rate in individuals with virologically confirmed dengue between 13 and 25 months after vaccination was 88% in the vaccine group, and 98% in the placebo group.
In individuals without virologically confirmed dengue, 219 of 2,485 participants in the vaccine group and 157 of 1,184 in the placebo group seroconverted during months 13-25. Total vaccine efficacy against asymptomatic dengue was 33.5%, and was higher in children aged 9-16, with vaccine efficacy at 38.6%.
“Since about 80% of DENV [dengue virus] infections are asymptomatic, it is likely that they contribute significantly to viral transmission to mosquitoes and thus to other human hosts. Consequently, providing simultaneous protection against both asymptomatic and symptomatic infections could contribute to reduced transmission and thus to indirect protection if the vaccine coverage rates are sufficient,” the investigators wrote.
Find the full study in the Journal of Infectious Disease (doi: 10.1093/infdis/jiw297)
A tetravalent dengue vaccine reduced symptomatic and asymptomatic dengue infection in Asian and Latin American children aged 2-16 years, according to Gustavo Olivera-Botello, PhD, and his associates at Sanofi Pasteur.
They analyzed data from 3,736 individuals in two phase III clinical trials. Patients received either the dengue vaccine CYD-TDV or placebo at month 0, month 6, and month 12, and had immunologic results from months 13 to 25. The seroconversion rate in individuals with virologically confirmed dengue between 13 and 25 months after vaccination was 88% in the vaccine group, and 98% in the placebo group.
In individuals without virologically confirmed dengue, 219 of 2,485 participants in the vaccine group and 157 of 1,184 in the placebo group seroconverted during months 13-25. Total vaccine efficacy against asymptomatic dengue was 33.5%, and was higher in children aged 9-16, with vaccine efficacy at 38.6%.
“Since about 80% of DENV [dengue virus] infections are asymptomatic, it is likely that they contribute significantly to viral transmission to mosquitoes and thus to other human hosts. Consequently, providing simultaneous protection against both asymptomatic and symptomatic infections could contribute to reduced transmission and thus to indirect protection if the vaccine coverage rates are sufficient,” the investigators wrote.
Find the full study in the Journal of Infectious Disease (doi: 10.1093/infdis/jiw297)
FROM THE JOURNAL OF INFECTIOUS DISEASES
Cutting routine glucometer readings saves time and money
Eliminating routine glucometer readings makes primary care visits more efficient, according to a 6-month review of activity at a primary care clinic. The results were published online Sept. 26 in JAMA Internal Medicine.
“The routine tasks that are components of rooming the clinic patient are increasing in number,” wrote James L Wofford, MD, of Wake Forest University, Winston-Salem, N.C., and his colleagues (JAMA Intern Med. 2016 Sep 26. doi: 10.1001/jamainternmed.2016.5769). Routine glucometer readings are costly in terms of time, money, and mental energy of clinicians, and do not add value, they wrote.
The researchers compared data from a primary care clinic in North Carolina for 3 months before and 3 months after the clinic eliminated routine glucometer readings (Jan. 1, 2015, to March 15, 2015, and March 16, 2015, to June 30, 2015). After a 1-week trial during which no routine clinical glucometry was performed, the option remained available at the request of a nurse or patient.
The number of glucometer readings decreased from approximately 400 per month to 100 per month after the change in policy, yielded a cost savings of at least $2,000 per month, and time saving of 25 hours of nursing time per month.
“Despite the fear of missing an occasional markedly elevated glucose level, clinicians gradually grew comfortable and never reconsidered reinstitution of routine glucometer readings,” Dr. Wofford and his associates wrote. However, some patient education was needed to reassure those who were disappointed by the change.
“As important as the lesson that routine glucometer readings in the clinic is a wasteful practice, the more important lesson is that examining office routines for foolish consistencies should be a regular component of making primary care more efficient,” the researchers added.
They had no financial conflicts to disclose.
Cutting down on unnecessary procedures allows primary care physicians to optimize their time with patients, and curbing routine glucose testing saves time and money, Adam J. Schoenfeld, MD, and Patrick G. O’Malley, MD, wrote in an editorial.
“Changing this policy to leave glucose testing to the discretion of the patient and nurse resulted in a decrease of 300 glucometer tests per month, saving the clinic $2, 000 and 25 hours of nursing time,” they wrote.
“Patients without infectious symptoms likely do not need their temperature taken, and we probably do not need to elicit a pain severity scale in patients without an active complaint of pain,” they noted. “Instead, other issues could take priority, such as medication reconciliation; collection of important psychosocial information, such as screening for commonly undiagnosed illnesses like depression; discussing advanced directives; and preparing the patient to be more active and engaged in addressing their agenda for the visit.”
Dr. Schoenfeld is affiliated with the University of California, San Francisco, and Dr. O’Malley is associated with the Uniformed Services University, Bethesda, Md. They had no financial conflicts to disclose.
Cutting down on unnecessary procedures allows primary care physicians to optimize their time with patients, and curbing routine glucose testing saves time and money, Adam J. Schoenfeld, MD, and Patrick G. O’Malley, MD, wrote in an editorial.
“Changing this policy to leave glucose testing to the discretion of the patient and nurse resulted in a decrease of 300 glucometer tests per month, saving the clinic $2, 000 and 25 hours of nursing time,” they wrote.
“Patients without infectious symptoms likely do not need their temperature taken, and we probably do not need to elicit a pain severity scale in patients without an active complaint of pain,” they noted. “Instead, other issues could take priority, such as medication reconciliation; collection of important psychosocial information, such as screening for commonly undiagnosed illnesses like depression; discussing advanced directives; and preparing the patient to be more active and engaged in addressing their agenda for the visit.”
Dr. Schoenfeld is affiliated with the University of California, San Francisco, and Dr. O’Malley is associated with the Uniformed Services University, Bethesda, Md. They had no financial conflicts to disclose.
Cutting down on unnecessary procedures allows primary care physicians to optimize their time with patients, and curbing routine glucose testing saves time and money, Adam J. Schoenfeld, MD, and Patrick G. O’Malley, MD, wrote in an editorial.
“Changing this policy to leave glucose testing to the discretion of the patient and nurse resulted in a decrease of 300 glucometer tests per month, saving the clinic $2, 000 and 25 hours of nursing time,” they wrote.
“Patients without infectious symptoms likely do not need their temperature taken, and we probably do not need to elicit a pain severity scale in patients without an active complaint of pain,” they noted. “Instead, other issues could take priority, such as medication reconciliation; collection of important psychosocial information, such as screening for commonly undiagnosed illnesses like depression; discussing advanced directives; and preparing the patient to be more active and engaged in addressing their agenda for the visit.”
Dr. Schoenfeld is affiliated with the University of California, San Francisco, and Dr. O’Malley is associated with the Uniformed Services University, Bethesda, Md. They had no financial conflicts to disclose.
Eliminating routine glucometer readings makes primary care visits more efficient, according to a 6-month review of activity at a primary care clinic. The results were published online Sept. 26 in JAMA Internal Medicine.
“The routine tasks that are components of rooming the clinic patient are increasing in number,” wrote James L Wofford, MD, of Wake Forest University, Winston-Salem, N.C., and his colleagues (JAMA Intern Med. 2016 Sep 26. doi: 10.1001/jamainternmed.2016.5769). Routine glucometer readings are costly in terms of time, money, and mental energy of clinicians, and do not add value, they wrote.
The researchers compared data from a primary care clinic in North Carolina for 3 months before and 3 months after the clinic eliminated routine glucometer readings (Jan. 1, 2015, to March 15, 2015, and March 16, 2015, to June 30, 2015). After a 1-week trial during which no routine clinical glucometry was performed, the option remained available at the request of a nurse or patient.
The number of glucometer readings decreased from approximately 400 per month to 100 per month after the change in policy, yielded a cost savings of at least $2,000 per month, and time saving of 25 hours of nursing time per month.
“Despite the fear of missing an occasional markedly elevated glucose level, clinicians gradually grew comfortable and never reconsidered reinstitution of routine glucometer readings,” Dr. Wofford and his associates wrote. However, some patient education was needed to reassure those who were disappointed by the change.
“As important as the lesson that routine glucometer readings in the clinic is a wasteful practice, the more important lesson is that examining office routines for foolish consistencies should be a regular component of making primary care more efficient,” the researchers added.
They had no financial conflicts to disclose.
Eliminating routine glucometer readings makes primary care visits more efficient, according to a 6-month review of activity at a primary care clinic. The results were published online Sept. 26 in JAMA Internal Medicine.
“The routine tasks that are components of rooming the clinic patient are increasing in number,” wrote James L Wofford, MD, of Wake Forest University, Winston-Salem, N.C., and his colleagues (JAMA Intern Med. 2016 Sep 26. doi: 10.1001/jamainternmed.2016.5769). Routine glucometer readings are costly in terms of time, money, and mental energy of clinicians, and do not add value, they wrote.
The researchers compared data from a primary care clinic in North Carolina for 3 months before and 3 months after the clinic eliminated routine glucometer readings (Jan. 1, 2015, to March 15, 2015, and March 16, 2015, to June 30, 2015). After a 1-week trial during which no routine clinical glucometry was performed, the option remained available at the request of a nurse or patient.
The number of glucometer readings decreased from approximately 400 per month to 100 per month after the change in policy, yielded a cost savings of at least $2,000 per month, and time saving of 25 hours of nursing time per month.
“Despite the fear of missing an occasional markedly elevated glucose level, clinicians gradually grew comfortable and never reconsidered reinstitution of routine glucometer readings,” Dr. Wofford and his associates wrote. However, some patient education was needed to reassure those who were disappointed by the change.
“As important as the lesson that routine glucometer readings in the clinic is a wasteful practice, the more important lesson is that examining office routines for foolish consistencies should be a regular component of making primary care more efficient,” the researchers added.
They had no financial conflicts to disclose.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Routine glucometer readings are unnecessary as part of a primary care visit.
Major finding: Decreasing glucometer readings from approximately 400 per month to approximately 100 per month saved more than $2,000 and 25 hours of nursing time.
Data source: A comparison of 3 months before and after a policy change eliminating routine glucometer readings in a primary care clinic.
Disclosures: The researchers had no financial conflicts to disclose.
Morning sickness linked to lower risk of pregnancy loss
Nausea and vomiting in early pregnancy were associated with a substantially reduced risk of pregnancy loss in a prospective preconception cohort of almost 800 pregnant women.
Although there has long been the suggestion that nausea is a sign of a healthy pregnancy, the evidence supporting this idea has been limited.
“Much of the published literature reports on studies that enrolled women after a clinically recognized pregnancy, thereby failing to include women with early pregnancy losses or relying on participant recall of nausea and/or loss,” wrote Stefanie N. Hinkle, PhD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md., and her colleagues.
In this study, the researchers examined data from 797 women with one or two prior pregnancy losses and a current pregnancy confirmed by an HCG pregnancy test. They all were enrolled in a randomized clinical trial on the effects of aspirin on gestation and reproduction (JAMA Intern Med. 2016 Sep 26. doi: 10.1001/jamainternmed.2016.5641).
Participants kept a daily record of nausea and vomiting symptoms for gestational weeks 2-8 and then monthly after that. At week 12, 86% of the women reported nausea and 35% reported nausea with vomiting at least once a week in the previous 4 weeks.
Overall, women with nausea and vomiting in any given week had a 75% lower risk of pregnancy loss during that week (hazard ratio, 0.25), while those with only nausea had a 50% reduction in pregnancy loss (HR, 0.50), compared with women with neither symptom.
For women who had a peri-implantation pregnancy loss, the researchers found a similar association but it did not reach statistical significance. The hazard ratio was 0.59 for women who had nausea only and 0.51 for women who experienced nausea with vomiting.
Among women who did not experience a peri-implantation pregnancy loss, nausea only and nausea with vomiting were associated with a 66% (HR, 0.44) and 80% (HR, 0.20) reduction in risk for pregnancy loss, respectively, compared with women with neither symptom. These reductions in risk were similar when the analysis was limited to first-trimester pregnancy loss and persisted even after accounting for lifestyle and fetal factors, such as number of prior pregnancy losses, body-mass index, fetal karyotype, and multiple fetal gestations.
“These findings overcome prior analytic and design limitations and represent the most definitive data available, to our knowledge, indicating the protective association of nausea and vomiting in early pregnancy on the risk for pregnancy loss and thus may provide reassurance to women experiencing these difficult symptoms in pregnancy,” researchers wrote.
The study was supported by the National Institutes of Health. The researchers reported having no financial disclosures.
This study’s contribution to the existing literature is valuable because investigators were able to compare nausea and vomiting among women who experienced an early pregnancy loss with those whose pregnancies continued. Furthermore, by collecting daily urine samples, it was possible to identify all pregnancies very early in gestation and thus definitively quantify the losses that occurred around the time of implantation in addition to those that occurred after ultrasonography.
As common as nausea and vomiting are in the first trimester, researchers and clinicians should be cautious about deeming it to have a protective effect against pregnancy loss. Although such a designation may provide reassurance to some women, they should not be discouraged from seeking treatment for a condition that can have a considerable negative effect on their quality of life.
Siripanth Nippita, MD, and Laura E. Dodge, ScD, are with the department of obstetrics and gynecology at Beth Israel Deaconess Medical Center, Boston. These comments are adapted from an accompanying editorial (JAMA Intern Med. 2016 Sep 26. doi: 10.1001/jamainternmed.2016.6101). They reported having no financial disclosures.
This study’s contribution to the existing literature is valuable because investigators were able to compare nausea and vomiting among women who experienced an early pregnancy loss with those whose pregnancies continued. Furthermore, by collecting daily urine samples, it was possible to identify all pregnancies very early in gestation and thus definitively quantify the losses that occurred around the time of implantation in addition to those that occurred after ultrasonography.
As common as nausea and vomiting are in the first trimester, researchers and clinicians should be cautious about deeming it to have a protective effect against pregnancy loss. Although such a designation may provide reassurance to some women, they should not be discouraged from seeking treatment for a condition that can have a considerable negative effect on their quality of life.
Siripanth Nippita, MD, and Laura E. Dodge, ScD, are with the department of obstetrics and gynecology at Beth Israel Deaconess Medical Center, Boston. These comments are adapted from an accompanying editorial (JAMA Intern Med. 2016 Sep 26. doi: 10.1001/jamainternmed.2016.6101). They reported having no financial disclosures.
This study’s contribution to the existing literature is valuable because investigators were able to compare nausea and vomiting among women who experienced an early pregnancy loss with those whose pregnancies continued. Furthermore, by collecting daily urine samples, it was possible to identify all pregnancies very early in gestation and thus definitively quantify the losses that occurred around the time of implantation in addition to those that occurred after ultrasonography.
As common as nausea and vomiting are in the first trimester, researchers and clinicians should be cautious about deeming it to have a protective effect against pregnancy loss. Although such a designation may provide reassurance to some women, they should not be discouraged from seeking treatment for a condition that can have a considerable negative effect on their quality of life.
Siripanth Nippita, MD, and Laura E. Dodge, ScD, are with the department of obstetrics and gynecology at Beth Israel Deaconess Medical Center, Boston. These comments are adapted from an accompanying editorial (JAMA Intern Med. 2016 Sep 26. doi: 10.1001/jamainternmed.2016.6101). They reported having no financial disclosures.
Nausea and vomiting in early pregnancy were associated with a substantially reduced risk of pregnancy loss in a prospective preconception cohort of almost 800 pregnant women.
Although there has long been the suggestion that nausea is a sign of a healthy pregnancy, the evidence supporting this idea has been limited.
“Much of the published literature reports on studies that enrolled women after a clinically recognized pregnancy, thereby failing to include women with early pregnancy losses or relying on participant recall of nausea and/or loss,” wrote Stefanie N. Hinkle, PhD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md., and her colleagues.
In this study, the researchers examined data from 797 women with one or two prior pregnancy losses and a current pregnancy confirmed by an HCG pregnancy test. They all were enrolled in a randomized clinical trial on the effects of aspirin on gestation and reproduction (JAMA Intern Med. 2016 Sep 26. doi: 10.1001/jamainternmed.2016.5641).
Participants kept a daily record of nausea and vomiting symptoms for gestational weeks 2-8 and then monthly after that. At week 12, 86% of the women reported nausea and 35% reported nausea with vomiting at least once a week in the previous 4 weeks.
Overall, women with nausea and vomiting in any given week had a 75% lower risk of pregnancy loss during that week (hazard ratio, 0.25), while those with only nausea had a 50% reduction in pregnancy loss (HR, 0.50), compared with women with neither symptom.
For women who had a peri-implantation pregnancy loss, the researchers found a similar association but it did not reach statistical significance. The hazard ratio was 0.59 for women who had nausea only and 0.51 for women who experienced nausea with vomiting.
Among women who did not experience a peri-implantation pregnancy loss, nausea only and nausea with vomiting were associated with a 66% (HR, 0.44) and 80% (HR, 0.20) reduction in risk for pregnancy loss, respectively, compared with women with neither symptom. These reductions in risk were similar when the analysis was limited to first-trimester pregnancy loss and persisted even after accounting for lifestyle and fetal factors, such as number of prior pregnancy losses, body-mass index, fetal karyotype, and multiple fetal gestations.
“These findings overcome prior analytic and design limitations and represent the most definitive data available, to our knowledge, indicating the protective association of nausea and vomiting in early pregnancy on the risk for pregnancy loss and thus may provide reassurance to women experiencing these difficult symptoms in pregnancy,” researchers wrote.
The study was supported by the National Institutes of Health. The researchers reported having no financial disclosures.
Nausea and vomiting in early pregnancy were associated with a substantially reduced risk of pregnancy loss in a prospective preconception cohort of almost 800 pregnant women.
Although there has long been the suggestion that nausea is a sign of a healthy pregnancy, the evidence supporting this idea has been limited.
“Much of the published literature reports on studies that enrolled women after a clinically recognized pregnancy, thereby failing to include women with early pregnancy losses or relying on participant recall of nausea and/or loss,” wrote Stefanie N. Hinkle, PhD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md., and her colleagues.
In this study, the researchers examined data from 797 women with one or two prior pregnancy losses and a current pregnancy confirmed by an HCG pregnancy test. They all were enrolled in a randomized clinical trial on the effects of aspirin on gestation and reproduction (JAMA Intern Med. 2016 Sep 26. doi: 10.1001/jamainternmed.2016.5641).
Participants kept a daily record of nausea and vomiting symptoms for gestational weeks 2-8 and then monthly after that. At week 12, 86% of the women reported nausea and 35% reported nausea with vomiting at least once a week in the previous 4 weeks.
Overall, women with nausea and vomiting in any given week had a 75% lower risk of pregnancy loss during that week (hazard ratio, 0.25), while those with only nausea had a 50% reduction in pregnancy loss (HR, 0.50), compared with women with neither symptom.
For women who had a peri-implantation pregnancy loss, the researchers found a similar association but it did not reach statistical significance. The hazard ratio was 0.59 for women who had nausea only and 0.51 for women who experienced nausea with vomiting.
Among women who did not experience a peri-implantation pregnancy loss, nausea only and nausea with vomiting were associated with a 66% (HR, 0.44) and 80% (HR, 0.20) reduction in risk for pregnancy loss, respectively, compared with women with neither symptom. These reductions in risk were similar when the analysis was limited to first-trimester pregnancy loss and persisted even after accounting for lifestyle and fetal factors, such as number of prior pregnancy losses, body-mass index, fetal karyotype, and multiple fetal gestations.
“These findings overcome prior analytic and design limitations and represent the most definitive data available, to our knowledge, indicating the protective association of nausea and vomiting in early pregnancy on the risk for pregnancy loss and thus may provide reassurance to women experiencing these difficult symptoms in pregnancy,” researchers wrote.
The study was supported by the National Institutes of Health. The researchers reported having no financial disclosures.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Nausea and vomiting in early pregnancy are associated with a reduced risk of pregnancy loss.
Major finding: Women with nausea and vomiting in any given week had a 75% lower risk of pregnancy loss during that week, while those with nausea only had a 50% reduction in pregnancy loss, compared with women with neither symptom.
Data source: Analysis of data from 797 pregnant women enrolled in a randomized clinical trial on the effects of aspirin on gestation and reproduction.
Disclosures: The study was supported by the National Institutes of Health. The researchers reported having no financial disclosures.
Too many infants with congenital hypothyroidism go undetected, untreated
DENVER – An alarming percentage of infants born in Utah from 2006 to 2015 with primary congenital hypothyroidism were either lost to follow-up or inadequately treated.
If such a thing can happen in Utah with its highly functioning public health system, it probably can happen in the rest of the United States as well, Joel Ehrenkranz, MD, said at the American Thyroid Association annual meeting. “We just have not looked for it yet.”
Screening for and treating congenital hypothyroidism in infants is one of the great public health successes of the 20th century in the United States. It deserves to have the same level of importance as eradication of polio and smallpox in this country, noted Dr. Ehrenkranz, an endocrinologist in private practice in Glenwood Springs, Colo. At the time of this research, Dr. Ehrenkranz was with Intermountain Healthcare in Murray, Utah.
The American Academy of Pediatrics recommends diagnosis of congenital hypothyroidism by the 14th day of life and that the baby be biochemically euthyroid by week 6 (Pediatrics. 2006 June. doi: 10.1542/peds.2006-0915), he said.
The cohort included 4,394 children from birth to 24 months of age. The screening test was done by a third-generation bioluminescence serum TSH assay, not dried blood blot. Of these infants, 2% (82 babies) had a TSH level greater than or equal to 20 mIU/L at their initial test. That TSH was still high by day 14 in 42 infants (23 girls). But of all the babies with primary congenital hypothyroidism, 50% had a delayed diagnosis, he reported.
Twelve children (15%) were never retested; 34% reached AAP goals of having a TSH level less than 5 mIU/L within 28 days after starting treatment; half of the children with primary congenital hypothyropidism did not meet the treatment goal: “They were inadequately treated,” he said.
Of particular interest were 16 infants who had a TSH level of less than 20 mIU/L but on retesting had one of 20 or higher. Three of these infants had multiple TSH levels greater than 20, perhaps representing a subset with a late onset form of the disorder.
“We are not doing as well as we could do,” he said; 50% of affected babies have a delayed diagnosis with consequences of a delayed maturation of the pituitary thyroid axis. Logistics are a challenge. And 50% of babies did not meet treatment guidelines.
In comparison to the state of screening and management in Utah, moderator Alex S. Stagnaro-Green, MD, noted that many pediatricians and endocrinologists operate under the presumption that screening for primary congenital hypothyroidism is “a well-oiled machine and that these babies are being taken care of.”
Utah has a very functional public health infrastructure. Of note, Utah’s birth rate is the highest in the country. The birth rates in several of its counties rival the highest rates found in the world, according to Dr. Ehrenkranz.
“So I think we have a very significant problem nationwide that just hasn’t been looked at,” he noted in response to the question. He undertook looking at newborn TSH levels in the first place as part of a project with the Food and Drug Administration. It was only then that he and his associates were struck by how many babies had low serum TSH levels, he said.
He had no relevant financial relationships to disclose.
DENVER – An alarming percentage of infants born in Utah from 2006 to 2015 with primary congenital hypothyroidism were either lost to follow-up or inadequately treated.
If such a thing can happen in Utah with its highly functioning public health system, it probably can happen in the rest of the United States as well, Joel Ehrenkranz, MD, said at the American Thyroid Association annual meeting. “We just have not looked for it yet.”
Screening for and treating congenital hypothyroidism in infants is one of the great public health successes of the 20th century in the United States. It deserves to have the same level of importance as eradication of polio and smallpox in this country, noted Dr. Ehrenkranz, an endocrinologist in private practice in Glenwood Springs, Colo. At the time of this research, Dr. Ehrenkranz was with Intermountain Healthcare in Murray, Utah.
The American Academy of Pediatrics recommends diagnosis of congenital hypothyroidism by the 14th day of life and that the baby be biochemically euthyroid by week 6 (Pediatrics. 2006 June. doi: 10.1542/peds.2006-0915), he said.
The cohort included 4,394 children from birth to 24 months of age. The screening test was done by a third-generation bioluminescence serum TSH assay, not dried blood blot. Of these infants, 2% (82 babies) had a TSH level greater than or equal to 20 mIU/L at their initial test. That TSH was still high by day 14 in 42 infants (23 girls). But of all the babies with primary congenital hypothyroidism, 50% had a delayed diagnosis, he reported.
Twelve children (15%) were never retested; 34% reached AAP goals of having a TSH level less than 5 mIU/L within 28 days after starting treatment; half of the children with primary congenital hypothyropidism did not meet the treatment goal: “They were inadequately treated,” he said.
Of particular interest were 16 infants who had a TSH level of less than 20 mIU/L but on retesting had one of 20 or higher. Three of these infants had multiple TSH levels greater than 20, perhaps representing a subset with a late onset form of the disorder.
“We are not doing as well as we could do,” he said; 50% of affected babies have a delayed diagnosis with consequences of a delayed maturation of the pituitary thyroid axis. Logistics are a challenge. And 50% of babies did not meet treatment guidelines.
In comparison to the state of screening and management in Utah, moderator Alex S. Stagnaro-Green, MD, noted that many pediatricians and endocrinologists operate under the presumption that screening for primary congenital hypothyroidism is “a well-oiled machine and that these babies are being taken care of.”
Utah has a very functional public health infrastructure. Of note, Utah’s birth rate is the highest in the country. The birth rates in several of its counties rival the highest rates found in the world, according to Dr. Ehrenkranz.
“So I think we have a very significant problem nationwide that just hasn’t been looked at,” he noted in response to the question. He undertook looking at newborn TSH levels in the first place as part of a project with the Food and Drug Administration. It was only then that he and his associates were struck by how many babies had low serum TSH levels, he said.
He had no relevant financial relationships to disclose.
DENVER – An alarming percentage of infants born in Utah from 2006 to 2015 with primary congenital hypothyroidism were either lost to follow-up or inadequately treated.
If such a thing can happen in Utah with its highly functioning public health system, it probably can happen in the rest of the United States as well, Joel Ehrenkranz, MD, said at the American Thyroid Association annual meeting. “We just have not looked for it yet.”
Screening for and treating congenital hypothyroidism in infants is one of the great public health successes of the 20th century in the United States. It deserves to have the same level of importance as eradication of polio and smallpox in this country, noted Dr. Ehrenkranz, an endocrinologist in private practice in Glenwood Springs, Colo. At the time of this research, Dr. Ehrenkranz was with Intermountain Healthcare in Murray, Utah.
The American Academy of Pediatrics recommends diagnosis of congenital hypothyroidism by the 14th day of life and that the baby be biochemically euthyroid by week 6 (Pediatrics. 2006 June. doi: 10.1542/peds.2006-0915), he said.
The cohort included 4,394 children from birth to 24 months of age. The screening test was done by a third-generation bioluminescence serum TSH assay, not dried blood blot. Of these infants, 2% (82 babies) had a TSH level greater than or equal to 20 mIU/L at their initial test. That TSH was still high by day 14 in 42 infants (23 girls). But of all the babies with primary congenital hypothyroidism, 50% had a delayed diagnosis, he reported.
Twelve children (15%) were never retested; 34% reached AAP goals of having a TSH level less than 5 mIU/L within 28 days after starting treatment; half of the children with primary congenital hypothyropidism did not meet the treatment goal: “They were inadequately treated,” he said.
Of particular interest were 16 infants who had a TSH level of less than 20 mIU/L but on retesting had one of 20 or higher. Three of these infants had multiple TSH levels greater than 20, perhaps representing a subset with a late onset form of the disorder.
“We are not doing as well as we could do,” he said; 50% of affected babies have a delayed diagnosis with consequences of a delayed maturation of the pituitary thyroid axis. Logistics are a challenge. And 50% of babies did not meet treatment guidelines.
In comparison to the state of screening and management in Utah, moderator Alex S. Stagnaro-Green, MD, noted that many pediatricians and endocrinologists operate under the presumption that screening for primary congenital hypothyroidism is “a well-oiled machine and that these babies are being taken care of.”
Utah has a very functional public health infrastructure. Of note, Utah’s birth rate is the highest in the country. The birth rates in several of its counties rival the highest rates found in the world, according to Dr. Ehrenkranz.
“So I think we have a very significant problem nationwide that just hasn’t been looked at,” he noted in response to the question. He undertook looking at newborn TSH levels in the first place as part of a project with the Food and Drug Administration. It was only then that he and his associates were struck by how many babies had low serum TSH levels, he said.
He had no relevant financial relationships to disclose.
AT THE ATA ANNUAL MEETING
Key clinical point: A large percentage of babies with congenital hypothyroidism are falling through the cracks in Utah, and likely throughout the United States.
Major finding: Almost 2% of 4,395 babies had TSH levels equal to or above 20 mIU/L when assessed after birth; of those, a significant share were lost to follow-up or inadequately treated.
Data source: A review of TSH measurements in all babies born in Utah between 2006 and 2015.
Disclosures: Dr. Ehrenkranz had no relevant financial disclosures.
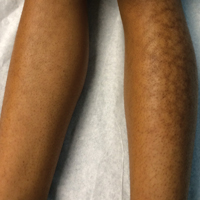
Reticular Hyperpigmentation on the Lower Legs
The Diagnosis: Erythema Ab Igne
Given the patient's reticulated hyperpigmented lesions in the setting of recent space heater use with heater closer to the more affected leg, erythema ab igne was diagnosed. Patient education was provided and moving the heater away from the lower extremities was advised.
Erythema ab igne first was described by German dermatologist Abraham Buschke as hitze melanose, meaning melanosis induced by heat. The classic skin findings were first observed on the lower legs of patients who worked in front of open fires or coal stoves.1 Over the years, new causes of erythema ab igne secondary to prolonged thermal radiation exposure have been reported.1 In the elderly, hospitalized, and chronic pain patients, erythema ab igne has been observed in areas treated with heating pads and blankets.2 Other triggers such as frequent hot bathing, furniture, steam radiators, space heaters, and laptops also have been reported.3-6 Laptop-induced erythema ab igne is a diagnosis that has been reported in the last decade and its incidence likely will increase in the future.6
The clinical manifestations of erythema ab igne correlate with the frequency and duration of heat exposure. Acutely, a mild and transient erythema develops in the affected area. With chronic heat exposure, these areas subsequently develop a permanent reticulated hyperpigmented pattern and may eventually become atrophic.2,6 All body surfaces are at risk, but erythema ab igne classically involves the legs, lower back, and/or abdomen. Lesions typically are asymptomatic; however, burning and pruritus can be present.2,6 Bullous erythema ab igne, though rare, has been reported,7 suggesting a potential transition from erythema ab igne to burns.6
Biopsy is not recommended for diagnosis; however, the histopathologic changes of erythema ab igne include hyperkeratosis, interface dermatitis, epidermal atrophy with apoptotic keratinocytes, and melanin incontinence. Although this condition typically is benign, histologic findings could resemble actinic keratosis, suggesting that chronic changes induced by infrared thermal radiation may lead to squamous cell carcinoma or rarely Merkel cell carcinoma. The latency for developing carcinoma appears to extend 30 years, with a 30% tendency for recurrence or metastasis. Given the possibility of an increase in erythema ab igne in the pediatric population in the upcoming years, as displayed by our patient, and increasing laptop and electronic use in children and adolescents, it is important to be aware of this skin condition and the potential complications of it going undiagnosed.2,6
No specific therapy for erythema ab igne exists. Treatment is centered on eliminating exposure to the heat source. With appropriate removal, the reticulated hyperpigmented lesions will resolve, sometimes taking several months.
Differential diagnosis includes livedo reticularis, livedoid vasculopathy, and cutis marmorata. The reticulated purpuric lesions of livedo reticularis involving the extremities often mimic erythema ab igne's cutaneous morphology; however, livedo reticularis frequently is associated with conditions such as drug reactions, infections, thrombosis, and vasculitides,2 as opposed to erythema ab igne, which frequently is associated with conditions causing pain or decreased body temperature, thus necessitating use of heating devices, as seen in our patient. Livedoid vasculopathy is characterized by purpuric macules involving the lower legs and feet that progress to recurrent leg ulcers. Our patient's asymptomatic lesions and absence of ulcers excluded this diagnosis.8 Lastly, cutis marmorata, a congenital condition, is characterized by blue-violet vascular networks that often display ulceration and atrophy of the involved skin as well as hypertrophy or atrophy of the involved limb9; these clinical findings were not present in our patient and this diagnosis would not explain the relationship between the cutaneous lesions and heat exposure.
- Nilic M, Adams BB. Erythema ab igne induced by a laptop computer. J Am Acad Dermatol. 2004;50:973-974.
- Riahi RR, Cohen PR, Robinson FW, et al. Erythema ab igne mimicking livedo reticularis. Int J Dermatol. 2010;49:1314-1317.
- Lin SJ, Hsu CJ, Chiu HC. Erythema ab igne caused by frequent hot bathing. Acta Derm Venereol. 2002;82:478-479.
- Meffert JJ, Davis BM. Furniture-induced erythema ab igne. J Am Acad Dermatol. 1996;34:516-517.
- Kligman LH, Kligman AM. Reflections on heat. Br J Dermatol. 1984;110:369-375.
- Arnold AW, Itin PH. Laptop computer−induced erythema ab igne in a child and review of the literature [published online October 4, 2010]. Pediatrics. 2010;126:e1227-e1230.
- Kokturk A, Kaya TI, Baz K, et al. Bullous erythema ab igne. Dermatol Online J. 2003;9:18.
- Khenifer S, Thomas L, Balme B, et al. Livedoid vasculopathy: thrombotic or inflammatory disease? Clin Exp Dermatol. 2009;35:693-698.
- Pernet C, Guillot B, Bigorre M, et al. Focal and atrophic cutis marmorata telangiectatica congenital. J Am Acad Dermatol. 2013;69:e268-e269.
The Diagnosis: Erythema Ab Igne
Given the patient's reticulated hyperpigmented lesions in the setting of recent space heater use with heater closer to the more affected leg, erythema ab igne was diagnosed. Patient education was provided and moving the heater away from the lower extremities was advised.
Erythema ab igne first was described by German dermatologist Abraham Buschke as hitze melanose, meaning melanosis induced by heat. The classic skin findings were first observed on the lower legs of patients who worked in front of open fires or coal stoves.1 Over the years, new causes of erythema ab igne secondary to prolonged thermal radiation exposure have been reported.1 In the elderly, hospitalized, and chronic pain patients, erythema ab igne has been observed in areas treated with heating pads and blankets.2 Other triggers such as frequent hot bathing, furniture, steam radiators, space heaters, and laptops also have been reported.3-6 Laptop-induced erythema ab igne is a diagnosis that has been reported in the last decade and its incidence likely will increase in the future.6
The clinical manifestations of erythema ab igne correlate with the frequency and duration of heat exposure. Acutely, a mild and transient erythema develops in the affected area. With chronic heat exposure, these areas subsequently develop a permanent reticulated hyperpigmented pattern and may eventually become atrophic.2,6 All body surfaces are at risk, but erythema ab igne classically involves the legs, lower back, and/or abdomen. Lesions typically are asymptomatic; however, burning and pruritus can be present.2,6 Bullous erythema ab igne, though rare, has been reported,7 suggesting a potential transition from erythema ab igne to burns.6
Biopsy is not recommended for diagnosis; however, the histopathologic changes of erythema ab igne include hyperkeratosis, interface dermatitis, epidermal atrophy with apoptotic keratinocytes, and melanin incontinence. Although this condition typically is benign, histologic findings could resemble actinic keratosis, suggesting that chronic changes induced by infrared thermal radiation may lead to squamous cell carcinoma or rarely Merkel cell carcinoma. The latency for developing carcinoma appears to extend 30 years, with a 30% tendency for recurrence or metastasis. Given the possibility of an increase in erythema ab igne in the pediatric population in the upcoming years, as displayed by our patient, and increasing laptop and electronic use in children and adolescents, it is important to be aware of this skin condition and the potential complications of it going undiagnosed.2,6
No specific therapy for erythema ab igne exists. Treatment is centered on eliminating exposure to the heat source. With appropriate removal, the reticulated hyperpigmented lesions will resolve, sometimes taking several months.
Differential diagnosis includes livedo reticularis, livedoid vasculopathy, and cutis marmorata. The reticulated purpuric lesions of livedo reticularis involving the extremities often mimic erythema ab igne's cutaneous morphology; however, livedo reticularis frequently is associated with conditions such as drug reactions, infections, thrombosis, and vasculitides,2 as opposed to erythema ab igne, which frequently is associated with conditions causing pain or decreased body temperature, thus necessitating use of heating devices, as seen in our patient. Livedoid vasculopathy is characterized by purpuric macules involving the lower legs and feet that progress to recurrent leg ulcers. Our patient's asymptomatic lesions and absence of ulcers excluded this diagnosis.8 Lastly, cutis marmorata, a congenital condition, is characterized by blue-violet vascular networks that often display ulceration and atrophy of the involved skin as well as hypertrophy or atrophy of the involved limb9; these clinical findings were not present in our patient and this diagnosis would not explain the relationship between the cutaneous lesions and heat exposure.
The Diagnosis: Erythema Ab Igne
Given the patient's reticulated hyperpigmented lesions in the setting of recent space heater use with heater closer to the more affected leg, erythema ab igne was diagnosed. Patient education was provided and moving the heater away from the lower extremities was advised.
Erythema ab igne first was described by German dermatologist Abraham Buschke as hitze melanose, meaning melanosis induced by heat. The classic skin findings were first observed on the lower legs of patients who worked in front of open fires or coal stoves.1 Over the years, new causes of erythema ab igne secondary to prolonged thermal radiation exposure have been reported.1 In the elderly, hospitalized, and chronic pain patients, erythema ab igne has been observed in areas treated with heating pads and blankets.2 Other triggers such as frequent hot bathing, furniture, steam radiators, space heaters, and laptops also have been reported.3-6 Laptop-induced erythema ab igne is a diagnosis that has been reported in the last decade and its incidence likely will increase in the future.6
The clinical manifestations of erythema ab igne correlate with the frequency and duration of heat exposure. Acutely, a mild and transient erythema develops in the affected area. With chronic heat exposure, these areas subsequently develop a permanent reticulated hyperpigmented pattern and may eventually become atrophic.2,6 All body surfaces are at risk, but erythema ab igne classically involves the legs, lower back, and/or abdomen. Lesions typically are asymptomatic; however, burning and pruritus can be present.2,6 Bullous erythema ab igne, though rare, has been reported,7 suggesting a potential transition from erythema ab igne to burns.6
Biopsy is not recommended for diagnosis; however, the histopathologic changes of erythema ab igne include hyperkeratosis, interface dermatitis, epidermal atrophy with apoptotic keratinocytes, and melanin incontinence. Although this condition typically is benign, histologic findings could resemble actinic keratosis, suggesting that chronic changes induced by infrared thermal radiation may lead to squamous cell carcinoma or rarely Merkel cell carcinoma. The latency for developing carcinoma appears to extend 30 years, with a 30% tendency for recurrence or metastasis. Given the possibility of an increase in erythema ab igne in the pediatric population in the upcoming years, as displayed by our patient, and increasing laptop and electronic use in children and adolescents, it is important to be aware of this skin condition and the potential complications of it going undiagnosed.2,6
No specific therapy for erythema ab igne exists. Treatment is centered on eliminating exposure to the heat source. With appropriate removal, the reticulated hyperpigmented lesions will resolve, sometimes taking several months.
Differential diagnosis includes livedo reticularis, livedoid vasculopathy, and cutis marmorata. The reticulated purpuric lesions of livedo reticularis involving the extremities often mimic erythema ab igne's cutaneous morphology; however, livedo reticularis frequently is associated with conditions such as drug reactions, infections, thrombosis, and vasculitides,2 as opposed to erythema ab igne, which frequently is associated with conditions causing pain or decreased body temperature, thus necessitating use of heating devices, as seen in our patient. Livedoid vasculopathy is characterized by purpuric macules involving the lower legs and feet that progress to recurrent leg ulcers. Our patient's asymptomatic lesions and absence of ulcers excluded this diagnosis.8 Lastly, cutis marmorata, a congenital condition, is characterized by blue-violet vascular networks that often display ulceration and atrophy of the involved skin as well as hypertrophy or atrophy of the involved limb9; these clinical findings were not present in our patient and this diagnosis would not explain the relationship between the cutaneous lesions and heat exposure.
- Nilic M, Adams BB. Erythema ab igne induced by a laptop computer. J Am Acad Dermatol. 2004;50:973-974.
- Riahi RR, Cohen PR, Robinson FW, et al. Erythema ab igne mimicking livedo reticularis. Int J Dermatol. 2010;49:1314-1317.
- Lin SJ, Hsu CJ, Chiu HC. Erythema ab igne caused by frequent hot bathing. Acta Derm Venereol. 2002;82:478-479.
- Meffert JJ, Davis BM. Furniture-induced erythema ab igne. J Am Acad Dermatol. 1996;34:516-517.
- Kligman LH, Kligman AM. Reflections on heat. Br J Dermatol. 1984;110:369-375.
- Arnold AW, Itin PH. Laptop computer−induced erythema ab igne in a child and review of the literature [published online October 4, 2010]. Pediatrics. 2010;126:e1227-e1230.
- Kokturk A, Kaya TI, Baz K, et al. Bullous erythema ab igne. Dermatol Online J. 2003;9:18.
- Khenifer S, Thomas L, Balme B, et al. Livedoid vasculopathy: thrombotic or inflammatory disease? Clin Exp Dermatol. 2009;35:693-698.
- Pernet C, Guillot B, Bigorre M, et al. Focal and atrophic cutis marmorata telangiectatica congenital. J Am Acad Dermatol. 2013;69:e268-e269.
- Nilic M, Adams BB. Erythema ab igne induced by a laptop computer. J Am Acad Dermatol. 2004;50:973-974.
- Riahi RR, Cohen PR, Robinson FW, et al. Erythema ab igne mimicking livedo reticularis. Int J Dermatol. 2010;49:1314-1317.
- Lin SJ, Hsu CJ, Chiu HC. Erythema ab igne caused by frequent hot bathing. Acta Derm Venereol. 2002;82:478-479.
- Meffert JJ, Davis BM. Furniture-induced erythema ab igne. J Am Acad Dermatol. 1996;34:516-517.
- Kligman LH, Kligman AM. Reflections on heat. Br J Dermatol. 1984;110:369-375.
- Arnold AW, Itin PH. Laptop computer−induced erythema ab igne in a child and review of the literature [published online October 4, 2010]. Pediatrics. 2010;126:e1227-e1230.
- Kokturk A, Kaya TI, Baz K, et al. Bullous erythema ab igne. Dermatol Online J. 2003;9:18.
- Khenifer S, Thomas L, Balme B, et al. Livedoid vasculopathy: thrombotic or inflammatory disease? Clin Exp Dermatol. 2009;35:693-698.
- Pernet C, Guillot B, Bigorre M, et al. Focal and atrophic cutis marmorata telangiectatica congenital. J Am Acad Dermatol. 2013;69:e268-e269.
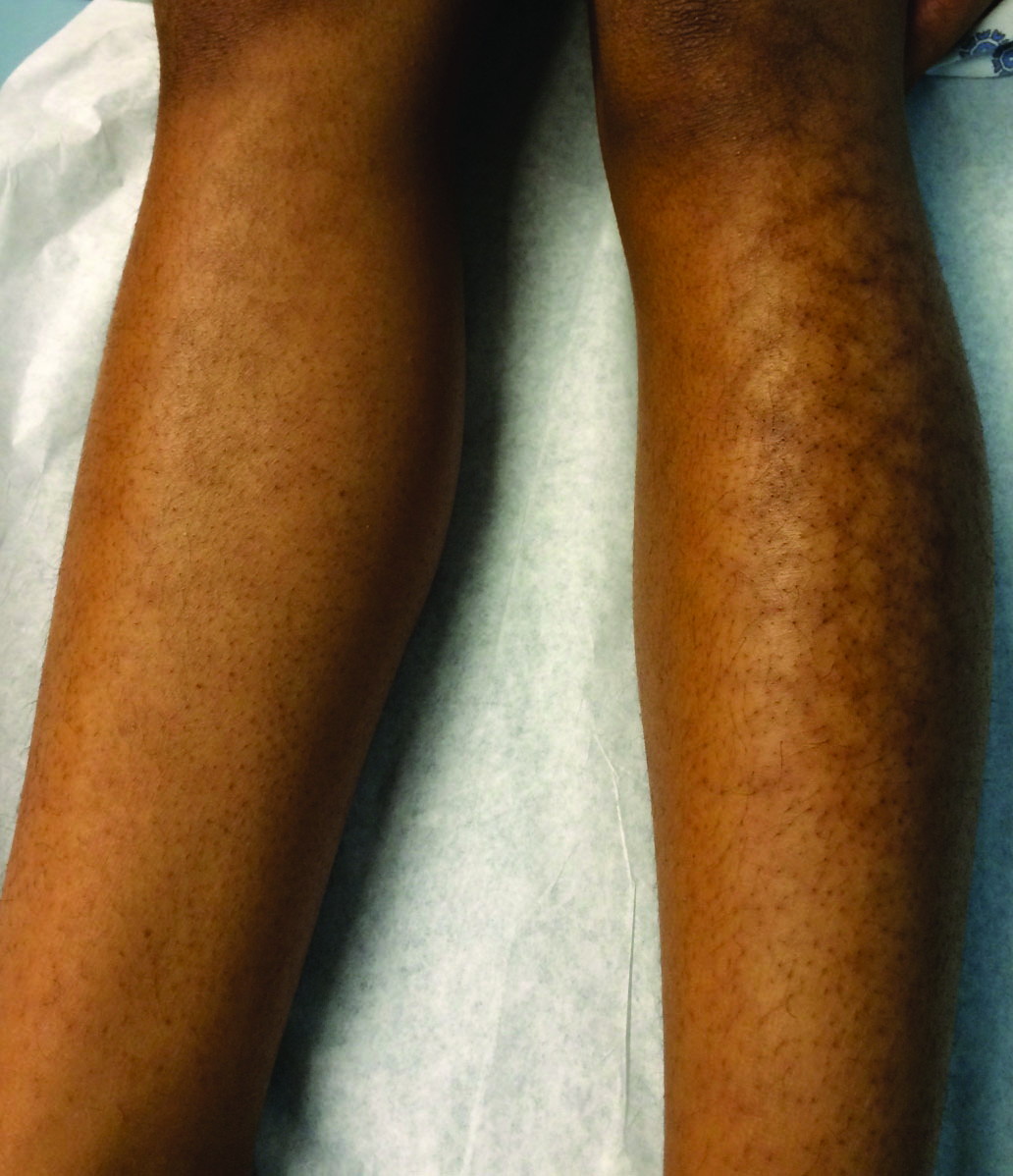
A 13-year-old otherwise healthy adolescent girl presented to the pediatric dermatology clinic for evaluation of a rash on the legs. The patient noticed the rash 1 month prior to presentation. The rash initially involved the left shin and gradually spread to involve the shins bilaterally. The rash was asymptomatic with no pain, pruritus, or muscular asymmetry of the legs. She denied recent fevers, chills, or travel. The patient reported using a space heater daily that was directed at the legs, approximately 0.5 m away. Physical examination revealed a well-nourished adolescent girl in no acute distress with reticular hyperpigmentation of the lower extremities located on the left anterior shin and knee, with mild involvement of the right shin. The reticulated hyperpigmented areas were arranged in a rectangular distribution. Lower extremity musculoskeletal examination was symmetric.
Older adults are using more cannabis, to uncertain effect
SAN FRANCISCO – An absence of guidelines on cannabis in older adults makes it difficult for clinicians to advise seniors, particularly those who use it for medical reasons, Adrienne Withall, PhD, said at the 2016 congress of the International Psychogeriatric Association.
“We don’t really know the impacts of cannabis use on older people. They may be positive or negative, depending on what it’s being used for,” said Dr. Withall of the University of New South Wales in Sydney.
Questions about how cannabis affects older people are growing as the boomer generation ages and cannabis becomes easier to legally obtain.
“Our picture of the cannabis user as a young adult or teen is inaccurate,” Dr. Withall said. “Many reports show that adults aged 50 and up are using cannabis for pain management or other therapeutic reasons, although we suspect they are also using it for recreation.” In the United States, regular use of cannabis rose by 455% among 55- to 64-year-olds between 2002 and 2014, according to data from the U.S. National Survey on Drug Use and Health. In Australia, studies of middle-aged and older chronic pain patients, including those without cancer, suggest that about 15% have used cannabis for pain, Dr. Withall said.
Older patients may ask if cannabis causes cognitive deficits. “Anecdotally, the answer is yes. There is certainly evidence in younger cohorts that cannabis affects cognition, although this remains fiercely debated,” Dr. Withall said. She cited a 25-year longitudinal cohort study of persistent cannabis users in which habitual cannabis use during adolescence led to significant cognitive impairment in executive function, information processing speed, and other cognitive domains in adulthood. Importantly, those deficits did not fully reverse after users stopped. In contrast, users who started as adults developed milder cognitive deficits and greater restoration of cognitive function after cessation. In another study that is currently under review, 42% of cannabis-using older adults had significant cognitive impairment (scores of less than 88 on the ACE-R [Addenbrooke’s Cognitive Examination-Revised]), Dr. Withall said.
These users did have comorbid substance abuse, depression, and other potential confounders, but nonetheless, more frequent cannabis use approached statistical significance as a negative predictor of ACE-R scores, she added.
The effects of various cannabis products depend on their ratio of cannabidiol (CBD) and tetrahydrocannabinol (THC), Dr. Withall emphasized. “There is increasing preclinical evidence that the endocannabinoid system regulates neurodegenerative processes common to various dementias, including excess glutamate, glial activation, oxidative stress, and neuroinflammation,” she said. Some studies suggest a neuroprotective role for CBD, while in safety studies, THC was well tolerated in patients with dementia but did not improve cognition. In another 4-week, uncontrolled, open-label trial of 10 patients with Alzheimer’s disease, adding medical cannabis oil to regular care was associated with significant decreases in delusions, agitation or aggression, irritability, apathy, sleep, and caregiver distress, Dr. Withall said (J Alzheimers Dis. 2016;51[1]15-9).
So what to tell patients who ask about cannabis or are habitual users?
“At the moment, we are trying to encourage people to minimize use of cannabis, but we don’t have enough information,” she concluded. “Even though we suspect it is having a detrimental effect on patients, it may be that certain groups are showing benefits. There is just not enough to hang a hat on yet.”
Dr. Withall disclosed no funding sources or conflicts of interest.
SAN FRANCISCO – An absence of guidelines on cannabis in older adults makes it difficult for clinicians to advise seniors, particularly those who use it for medical reasons, Adrienne Withall, PhD, said at the 2016 congress of the International Psychogeriatric Association.
“We don’t really know the impacts of cannabis use on older people. They may be positive or negative, depending on what it’s being used for,” said Dr. Withall of the University of New South Wales in Sydney.
Questions about how cannabis affects older people are growing as the boomer generation ages and cannabis becomes easier to legally obtain.
“Our picture of the cannabis user as a young adult or teen is inaccurate,” Dr. Withall said. “Many reports show that adults aged 50 and up are using cannabis for pain management or other therapeutic reasons, although we suspect they are also using it for recreation.” In the United States, regular use of cannabis rose by 455% among 55- to 64-year-olds between 2002 and 2014, according to data from the U.S. National Survey on Drug Use and Health. In Australia, studies of middle-aged and older chronic pain patients, including those without cancer, suggest that about 15% have used cannabis for pain, Dr. Withall said.
Older patients may ask if cannabis causes cognitive deficits. “Anecdotally, the answer is yes. There is certainly evidence in younger cohorts that cannabis affects cognition, although this remains fiercely debated,” Dr. Withall said. She cited a 25-year longitudinal cohort study of persistent cannabis users in which habitual cannabis use during adolescence led to significant cognitive impairment in executive function, information processing speed, and other cognitive domains in adulthood. Importantly, those deficits did not fully reverse after users stopped. In contrast, users who started as adults developed milder cognitive deficits and greater restoration of cognitive function after cessation. In another study that is currently under review, 42% of cannabis-using older adults had significant cognitive impairment (scores of less than 88 on the ACE-R [Addenbrooke’s Cognitive Examination-Revised]), Dr. Withall said.
These users did have comorbid substance abuse, depression, and other potential confounders, but nonetheless, more frequent cannabis use approached statistical significance as a negative predictor of ACE-R scores, she added.
The effects of various cannabis products depend on their ratio of cannabidiol (CBD) and tetrahydrocannabinol (THC), Dr. Withall emphasized. “There is increasing preclinical evidence that the endocannabinoid system regulates neurodegenerative processes common to various dementias, including excess glutamate, glial activation, oxidative stress, and neuroinflammation,” she said. Some studies suggest a neuroprotective role for CBD, while in safety studies, THC was well tolerated in patients with dementia but did not improve cognition. In another 4-week, uncontrolled, open-label trial of 10 patients with Alzheimer’s disease, adding medical cannabis oil to regular care was associated with significant decreases in delusions, agitation or aggression, irritability, apathy, sleep, and caregiver distress, Dr. Withall said (J Alzheimers Dis. 2016;51[1]15-9).
So what to tell patients who ask about cannabis or are habitual users?
“At the moment, we are trying to encourage people to minimize use of cannabis, but we don’t have enough information,” she concluded. “Even though we suspect it is having a detrimental effect on patients, it may be that certain groups are showing benefits. There is just not enough to hang a hat on yet.”
Dr. Withall disclosed no funding sources or conflicts of interest.
SAN FRANCISCO – An absence of guidelines on cannabis in older adults makes it difficult for clinicians to advise seniors, particularly those who use it for medical reasons, Adrienne Withall, PhD, said at the 2016 congress of the International Psychogeriatric Association.
“We don’t really know the impacts of cannabis use on older people. They may be positive or negative, depending on what it’s being used for,” said Dr. Withall of the University of New South Wales in Sydney.
Questions about how cannabis affects older people are growing as the boomer generation ages and cannabis becomes easier to legally obtain.
“Our picture of the cannabis user as a young adult or teen is inaccurate,” Dr. Withall said. “Many reports show that adults aged 50 and up are using cannabis for pain management or other therapeutic reasons, although we suspect they are also using it for recreation.” In the United States, regular use of cannabis rose by 455% among 55- to 64-year-olds between 2002 and 2014, according to data from the U.S. National Survey on Drug Use and Health. In Australia, studies of middle-aged and older chronic pain patients, including those without cancer, suggest that about 15% have used cannabis for pain, Dr. Withall said.
Older patients may ask if cannabis causes cognitive deficits. “Anecdotally, the answer is yes. There is certainly evidence in younger cohorts that cannabis affects cognition, although this remains fiercely debated,” Dr. Withall said. She cited a 25-year longitudinal cohort study of persistent cannabis users in which habitual cannabis use during adolescence led to significant cognitive impairment in executive function, information processing speed, and other cognitive domains in adulthood. Importantly, those deficits did not fully reverse after users stopped. In contrast, users who started as adults developed milder cognitive deficits and greater restoration of cognitive function after cessation. In another study that is currently under review, 42% of cannabis-using older adults had significant cognitive impairment (scores of less than 88 on the ACE-R [Addenbrooke’s Cognitive Examination-Revised]), Dr. Withall said.
These users did have comorbid substance abuse, depression, and other potential confounders, but nonetheless, more frequent cannabis use approached statistical significance as a negative predictor of ACE-R scores, she added.
The effects of various cannabis products depend on their ratio of cannabidiol (CBD) and tetrahydrocannabinol (THC), Dr. Withall emphasized. “There is increasing preclinical evidence that the endocannabinoid system regulates neurodegenerative processes common to various dementias, including excess glutamate, glial activation, oxidative stress, and neuroinflammation,” she said. Some studies suggest a neuroprotective role for CBD, while in safety studies, THC was well tolerated in patients with dementia but did not improve cognition. In another 4-week, uncontrolled, open-label trial of 10 patients with Alzheimer’s disease, adding medical cannabis oil to regular care was associated with significant decreases in delusions, agitation or aggression, irritability, apathy, sleep, and caregiver distress, Dr. Withall said (J Alzheimers Dis. 2016;51[1]15-9).
So what to tell patients who ask about cannabis or are habitual users?
“At the moment, we are trying to encourage people to minimize use of cannabis, but we don’t have enough information,” she concluded. “Even though we suspect it is having a detrimental effect on patients, it may be that certain groups are showing benefits. There is just not enough to hang a hat on yet.”
Dr. Withall disclosed no funding sources or conflicts of interest.
AT IPA 2016
Psychiatric risks quantified in siblings of mental disorder patients
VIENNA – The brothers and sisters of patients hospitalized for schizophrenia, bipolar disorder, or unipolar depression are themselves at strikingly high risk of subsequently developing not only the same disorder as their sibling, but other forms of major mental illness as well, Mark Weiser, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
He presented the results of the first comprehensive national population-based study to examine in this fashion the extent to which heritability contributes to schizophrenia and affective disorders. This nested case-control study included all siblings of 6,111 Israeli patients hospitalized for schizophrenia, schizoaffective disorder, bipolar disorder, or unipolar depression. The siblings’ rates of and reasons for subsequent psychiatric hospitalization were compared with those of 74,988 age- and gender-matched Israeli controls. All admission and discharge diagnoses were made by board-certified psychiatrists.
Siblings of individuals with schizophrenia were at 9.4-fold increased risk of subsequent hospitalization for schizophrenia, 8.5-fold relative risk for schizoaffective disorder, and 7.7-fold increased risk for bipolar disorder, compared with controls.
Moreover, siblings of patients with bipolar disorder were not only at 8.4-fold increased risk of subsequent hospitalization for that disease, they also were at 4.2-fold greater risk than controls for schizophrenia and 7.6-fold increased risk for hospitalization for other psychiatric disorders, a grab bag category that included anxiety disorders, dissociative disorder, post-traumatic stress disorder, eating disorders, pervasive developmental disorders, and personality disorders, according to Dr. Weiser, professor of psychiatry at Tel Aviv University.
Siblings of patients hospitalized for unipolar depression were at 6.2-fold relative risk of subsequent hospitalization for schizophrenia and 9.7-fold increased risk of hospitalization of other psychiatric disorders. “The bottom line of our study is it’s not a one gene/one disorder model. There’s not a gene for schizophrenia and a different gene for bipolar disorder. There are probably a bunch of different genes that increase the risk for schizophrenia but also increase risk for bipolar disorder, and the other way around,” the psychiatrist explained in an interview.
“Clinically it’s well known from the literature that if I have schizophrenia, there’s an increased chance that my brother will have it as well, so when my brother comes in having trouble, you obviously suggest that he might be developing schizophrenia. What these data imply is that if the brother of a schizophrenia patient comes in seeking help, it might not be schizophrenia, because he’s also at increased risk for bipolar disorder. So your index of suspicion should be much broader, not only for the one specific illness but for the whole idea of psychopathology in general. It’s a challenge. It demands for clinicians to be more broad-minded and to understand that these genes we’re looking for in large studies are not specific for one particular illness,” Dr. Weiser said.
This study was made possible because Israel, like Denmark, maintains multiple comprehensive national registries in which health care researchers are able to tap into and connect.
“A study like this can’t be done in the United States,” he said. “No how, no way.”
Dr. Weiser reported having no financial conflicts of interest regarding this study, which was conducted without external funding.
VIENNA – The brothers and sisters of patients hospitalized for schizophrenia, bipolar disorder, or unipolar depression are themselves at strikingly high risk of subsequently developing not only the same disorder as their sibling, but other forms of major mental illness as well, Mark Weiser, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
He presented the results of the first comprehensive national population-based study to examine in this fashion the extent to which heritability contributes to schizophrenia and affective disorders. This nested case-control study included all siblings of 6,111 Israeli patients hospitalized for schizophrenia, schizoaffective disorder, bipolar disorder, or unipolar depression. The siblings’ rates of and reasons for subsequent psychiatric hospitalization were compared with those of 74,988 age- and gender-matched Israeli controls. All admission and discharge diagnoses were made by board-certified psychiatrists.
Siblings of individuals with schizophrenia were at 9.4-fold increased risk of subsequent hospitalization for schizophrenia, 8.5-fold relative risk for schizoaffective disorder, and 7.7-fold increased risk for bipolar disorder, compared with controls.
Moreover, siblings of patients with bipolar disorder were not only at 8.4-fold increased risk of subsequent hospitalization for that disease, they also were at 4.2-fold greater risk than controls for schizophrenia and 7.6-fold increased risk for hospitalization for other psychiatric disorders, a grab bag category that included anxiety disorders, dissociative disorder, post-traumatic stress disorder, eating disorders, pervasive developmental disorders, and personality disorders, according to Dr. Weiser, professor of psychiatry at Tel Aviv University.
Siblings of patients hospitalized for unipolar depression were at 6.2-fold relative risk of subsequent hospitalization for schizophrenia and 9.7-fold increased risk of hospitalization of other psychiatric disorders. “The bottom line of our study is it’s not a one gene/one disorder model. There’s not a gene for schizophrenia and a different gene for bipolar disorder. There are probably a bunch of different genes that increase the risk for schizophrenia but also increase risk for bipolar disorder, and the other way around,” the psychiatrist explained in an interview.
“Clinically it’s well known from the literature that if I have schizophrenia, there’s an increased chance that my brother will have it as well, so when my brother comes in having trouble, you obviously suggest that he might be developing schizophrenia. What these data imply is that if the brother of a schizophrenia patient comes in seeking help, it might not be schizophrenia, because he’s also at increased risk for bipolar disorder. So your index of suspicion should be much broader, not only for the one specific illness but for the whole idea of psychopathology in general. It’s a challenge. It demands for clinicians to be more broad-minded and to understand that these genes we’re looking for in large studies are not specific for one particular illness,” Dr. Weiser said.
This study was made possible because Israel, like Denmark, maintains multiple comprehensive national registries in which health care researchers are able to tap into and connect.
“A study like this can’t be done in the United States,” he said. “No how, no way.”
Dr. Weiser reported having no financial conflicts of interest regarding this study, which was conducted without external funding.
VIENNA – The brothers and sisters of patients hospitalized for schizophrenia, bipolar disorder, or unipolar depression are themselves at strikingly high risk of subsequently developing not only the same disorder as their sibling, but other forms of major mental illness as well, Mark Weiser, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
He presented the results of the first comprehensive national population-based study to examine in this fashion the extent to which heritability contributes to schizophrenia and affective disorders. This nested case-control study included all siblings of 6,111 Israeli patients hospitalized for schizophrenia, schizoaffective disorder, bipolar disorder, or unipolar depression. The siblings’ rates of and reasons for subsequent psychiatric hospitalization were compared with those of 74,988 age- and gender-matched Israeli controls. All admission and discharge diagnoses were made by board-certified psychiatrists.
Siblings of individuals with schizophrenia were at 9.4-fold increased risk of subsequent hospitalization for schizophrenia, 8.5-fold relative risk for schizoaffective disorder, and 7.7-fold increased risk for bipolar disorder, compared with controls.
Moreover, siblings of patients with bipolar disorder were not only at 8.4-fold increased risk of subsequent hospitalization for that disease, they also were at 4.2-fold greater risk than controls for schizophrenia and 7.6-fold increased risk for hospitalization for other psychiatric disorders, a grab bag category that included anxiety disorders, dissociative disorder, post-traumatic stress disorder, eating disorders, pervasive developmental disorders, and personality disorders, according to Dr. Weiser, professor of psychiatry at Tel Aviv University.
Siblings of patients hospitalized for unipolar depression were at 6.2-fold relative risk of subsequent hospitalization for schizophrenia and 9.7-fold increased risk of hospitalization of other psychiatric disorders. “The bottom line of our study is it’s not a one gene/one disorder model. There’s not a gene for schizophrenia and a different gene for bipolar disorder. There are probably a bunch of different genes that increase the risk for schizophrenia but also increase risk for bipolar disorder, and the other way around,” the psychiatrist explained in an interview.
“Clinically it’s well known from the literature that if I have schizophrenia, there’s an increased chance that my brother will have it as well, so when my brother comes in having trouble, you obviously suggest that he might be developing schizophrenia. What these data imply is that if the brother of a schizophrenia patient comes in seeking help, it might not be schizophrenia, because he’s also at increased risk for bipolar disorder. So your index of suspicion should be much broader, not only for the one specific illness but for the whole idea of psychopathology in general. It’s a challenge. It demands for clinicians to be more broad-minded and to understand that these genes we’re looking for in large studies are not specific for one particular illness,” Dr. Weiser said.
This study was made possible because Israel, like Denmark, maintains multiple comprehensive national registries in which health care researchers are able to tap into and connect.
“A study like this can’t be done in the United States,” he said. “No how, no way.”
Dr. Weiser reported having no financial conflicts of interest regarding this study, which was conducted without external funding.
AT THE ECNP CONGRESS
Key clinical point: Siblings of patients with schizophrenia or bipolar disorder are at sharply increased risk of subsequent hospitalization for a range of psychiatric disorders, not just what their sibling has.
Major finding: Siblings of schizophrenia patients were not only at 9.4-fold increased risk of subsequent hospitalization for schizophrenia in a national study, but also at 7.7-fold greater risk for bipolar disorder.
Data source: This nested case-control study compared psychiatric hospitalization rates for all siblings of 6,111 Israeli patients with schizophrenia, bipolar disorder, or unipolar depression to nearly 75,000 matched controls.
Disclosures: The study was conducted without external funding. The presenter reported having no financial conflicts of interest.
VIDEO: When is it time to jump into MACRA with both feet?
LAS VEGAS – Change in federal reimbursement for physicians is coming. Though the change is inevitable, physicians still have to weigh choices about when they might want to jump in with both feet, since entry into the full incentive payment system will be optional – for a time.
The Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) is “basically a reorganization of all of these disparate reward and penalty systems” that have existed within the federal health care reimbursement landscape, said Joseph S. Eastern, MD. “The idea was to collect them all within one system.”
The new system is called the Medicare Incentive Payment System, or MIPS. Physicians are already familiar with many MIPS components, including meaningful use of the electronic health record, “which everybody thought was going away, but it isn’t,” said Dr. Eastern, a dermatologist in private practice in Belleville, N.J., who’s affiliated with Seton Hall University, South Orange, N.J. Also included are the Physician Quality Reimbursement System (PQRS) and the value-based modifier system.
MIPS is designed so that “you’ll either get a reward or a penalty depending on how well you do, compared with other physicians,” said Dr. Eastern, speaking at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
The alternative, he said, is to opt for one of the Alternative Payment Models, or APMs. However, details about APMs are “really up in the air, because a lot of them have either not been doing very well, or have not been very well defined,” so that physicians often don’t currently have enough data to make an informed choice. He expects the APM landscape to sort out over the next year or two.
Opting not to comply and take the 1%-3% cut in Medicare reimbursement associated with noncompliance might make sense for just a few physicians, though it might seem tempting, Dr. Eastern said in a video interview. Since the penalties will escalate significantly over the next few years, he feels that only physicians who are considering retiring soon or selling their practices should consider opting out.
Global Academy for Medical Education and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @karioakes
LAS VEGAS – Change in federal reimbursement for physicians is coming. Though the change is inevitable, physicians still have to weigh choices about when they might want to jump in with both feet, since entry into the full incentive payment system will be optional – for a time.
The Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) is “basically a reorganization of all of these disparate reward and penalty systems” that have existed within the federal health care reimbursement landscape, said Joseph S. Eastern, MD. “The idea was to collect them all within one system.”
The new system is called the Medicare Incentive Payment System, or MIPS. Physicians are already familiar with many MIPS components, including meaningful use of the electronic health record, “which everybody thought was going away, but it isn’t,” said Dr. Eastern, a dermatologist in private practice in Belleville, N.J., who’s affiliated with Seton Hall University, South Orange, N.J. Also included are the Physician Quality Reimbursement System (PQRS) and the value-based modifier system.
MIPS is designed so that “you’ll either get a reward or a penalty depending on how well you do, compared with other physicians,” said Dr. Eastern, speaking at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
The alternative, he said, is to opt for one of the Alternative Payment Models, or APMs. However, details about APMs are “really up in the air, because a lot of them have either not been doing very well, or have not been very well defined,” so that physicians often don’t currently have enough data to make an informed choice. He expects the APM landscape to sort out over the next year or two.
Opting not to comply and take the 1%-3% cut in Medicare reimbursement associated with noncompliance might make sense for just a few physicians, though it might seem tempting, Dr. Eastern said in a video interview. Since the penalties will escalate significantly over the next few years, he feels that only physicians who are considering retiring soon or selling their practices should consider opting out.
Global Academy for Medical Education and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @karioakes
LAS VEGAS – Change in federal reimbursement for physicians is coming. Though the change is inevitable, physicians still have to weigh choices about when they might want to jump in with both feet, since entry into the full incentive payment system will be optional – for a time.
The Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) is “basically a reorganization of all of these disparate reward and penalty systems” that have existed within the federal health care reimbursement landscape, said Joseph S. Eastern, MD. “The idea was to collect them all within one system.”
The new system is called the Medicare Incentive Payment System, or MIPS. Physicians are already familiar with many MIPS components, including meaningful use of the electronic health record, “which everybody thought was going away, but it isn’t,” said Dr. Eastern, a dermatologist in private practice in Belleville, N.J., who’s affiliated with Seton Hall University, South Orange, N.J. Also included are the Physician Quality Reimbursement System (PQRS) and the value-based modifier system.
MIPS is designed so that “you’ll either get a reward or a penalty depending on how well you do, compared with other physicians,” said Dr. Eastern, speaking at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
The alternative, he said, is to opt for one of the Alternative Payment Models, or APMs. However, details about APMs are “really up in the air, because a lot of them have either not been doing very well, or have not been very well defined,” so that physicians often don’t currently have enough data to make an informed choice. He expects the APM landscape to sort out over the next year or two.
Opting not to comply and take the 1%-3% cut in Medicare reimbursement associated with noncompliance might make sense for just a few physicians, though it might seem tempting, Dr. Eastern said in a video interview. Since the penalties will escalate significantly over the next few years, he feels that only physicians who are considering retiring soon or selling their practices should consider opting out.
Global Academy for Medical Education and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @karioakes
EXPERT ANALYSIS FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
Children having an anaphylactic attack at school may not get proper treatment
Children experiencing an anaphylactic event at school may frequently encounter staff members who are not permitted to administer potentially life-saving epinephrine, according to recent survey study.
Of 6,574 surveys submitted by schools, there were 1,140 anaphylactic events reported in 736 schools.
A total of 6,088 schools provided data on staff training for recognizing anaphylaxis recognition: 30% provided training for all staff, 28% for most staff, 37% for the school nurse and select staff, and 2% for just the school nurse. Of 6,053 schools providing data on who is permitted to administer epinephrine to treat anaphylaxis, 22% permitted all staff, 16% permitted most staff, 55% permitted the school nurse and select trained staff, and 3% permitted the school nurse only, reported Martha V. White, MD, Institute for Asthma and Allergy, Wheaton, Md., and her associates (Pediatr Allerg Immunol Pulmonol. 2016. doi: 10.1089/ped.2016.0675).
These findings “suggest that there may be an opportunity to improve school staff training programs. Only 58.6% of schools surveyed trained all or most staff members to recognize the signs and symptoms of anaphylaxis. Similarly,only 37% of responding schools permitted all or most staff to administer epinephrine. ... School policies should be designed to allow for prompt administration of epinephrine during the early stages of an anaphylactic attack,” Dr. White and her associates concluded.
Among students having events for whom grade information was available, 33% occurred in elementary school students, 19% occurred in middle school students, and 45% occurred in high school students. In 1,049 anaphylactic events, the allergy history was known: 68% of events occurred in students with known allergies and 25% were in students with no known allergies.
When triggers were identified (in 78% of cases), food was the most common trigger, occurring in 60%, followed by insect bites or stings in 8%; environmental, medication, or health related triggers in 9%; and latex in 1%.
Data on use of epinephrine autoinjectors (EAIs) was available in 1,059 cases. EAIs were administered in 76% of anaphylactic events, were not administered in 23% cases, and it was unknown whether EAIs were given in the remaining 1%.
This study was supported by Mylan Specialty. Dr. White has served as a consultant for Mylan and Merck, and has received grants, fees, or support from numerous pharmaceutical companies. Christopher Herrem, PhD, is a paid employee of Mylan and may hold stock within the company. The remaining authors reported that they had no conflicts of interest.
Children experiencing an anaphylactic event at school may frequently encounter staff members who are not permitted to administer potentially life-saving epinephrine, according to recent survey study.
Of 6,574 surveys submitted by schools, there were 1,140 anaphylactic events reported in 736 schools.
A total of 6,088 schools provided data on staff training for recognizing anaphylaxis recognition: 30% provided training for all staff, 28% for most staff, 37% for the school nurse and select staff, and 2% for just the school nurse. Of 6,053 schools providing data on who is permitted to administer epinephrine to treat anaphylaxis, 22% permitted all staff, 16% permitted most staff, 55% permitted the school nurse and select trained staff, and 3% permitted the school nurse only, reported Martha V. White, MD, Institute for Asthma and Allergy, Wheaton, Md., and her associates (Pediatr Allerg Immunol Pulmonol. 2016. doi: 10.1089/ped.2016.0675).
These findings “suggest that there may be an opportunity to improve school staff training programs. Only 58.6% of schools surveyed trained all or most staff members to recognize the signs and symptoms of anaphylaxis. Similarly,only 37% of responding schools permitted all or most staff to administer epinephrine. ... School policies should be designed to allow for prompt administration of epinephrine during the early stages of an anaphylactic attack,” Dr. White and her associates concluded.
Among students having events for whom grade information was available, 33% occurred in elementary school students, 19% occurred in middle school students, and 45% occurred in high school students. In 1,049 anaphylactic events, the allergy history was known: 68% of events occurred in students with known allergies and 25% were in students with no known allergies.
When triggers were identified (in 78% of cases), food was the most common trigger, occurring in 60%, followed by insect bites or stings in 8%; environmental, medication, or health related triggers in 9%; and latex in 1%.
Data on use of epinephrine autoinjectors (EAIs) was available in 1,059 cases. EAIs were administered in 76% of anaphylactic events, were not administered in 23% cases, and it was unknown whether EAIs were given in the remaining 1%.
This study was supported by Mylan Specialty. Dr. White has served as a consultant for Mylan and Merck, and has received grants, fees, or support from numerous pharmaceutical companies. Christopher Herrem, PhD, is a paid employee of Mylan and may hold stock within the company. The remaining authors reported that they had no conflicts of interest.
Children experiencing an anaphylactic event at school may frequently encounter staff members who are not permitted to administer potentially life-saving epinephrine, according to recent survey study.
Of 6,574 surveys submitted by schools, there were 1,140 anaphylactic events reported in 736 schools.
A total of 6,088 schools provided data on staff training for recognizing anaphylaxis recognition: 30% provided training for all staff, 28% for most staff, 37% for the school nurse and select staff, and 2% for just the school nurse. Of 6,053 schools providing data on who is permitted to administer epinephrine to treat anaphylaxis, 22% permitted all staff, 16% permitted most staff, 55% permitted the school nurse and select trained staff, and 3% permitted the school nurse only, reported Martha V. White, MD, Institute for Asthma and Allergy, Wheaton, Md., and her associates (Pediatr Allerg Immunol Pulmonol. 2016. doi: 10.1089/ped.2016.0675).
These findings “suggest that there may be an opportunity to improve school staff training programs. Only 58.6% of schools surveyed trained all or most staff members to recognize the signs and symptoms of anaphylaxis. Similarly,only 37% of responding schools permitted all or most staff to administer epinephrine. ... School policies should be designed to allow for prompt administration of epinephrine during the early stages of an anaphylactic attack,” Dr. White and her associates concluded.
Among students having events for whom grade information was available, 33% occurred in elementary school students, 19% occurred in middle school students, and 45% occurred in high school students. In 1,049 anaphylactic events, the allergy history was known: 68% of events occurred in students with known allergies and 25% were in students with no known allergies.
When triggers were identified (in 78% of cases), food was the most common trigger, occurring in 60%, followed by insect bites or stings in 8%; environmental, medication, or health related triggers in 9%; and latex in 1%.
Data on use of epinephrine autoinjectors (EAIs) was available in 1,059 cases. EAIs were administered in 76% of anaphylactic events, were not administered in 23% cases, and it was unknown whether EAIs were given in the remaining 1%.
This study was supported by Mylan Specialty. Dr. White has served as a consultant for Mylan and Merck, and has received grants, fees, or support from numerous pharmaceutical companies. Christopher Herrem, PhD, is a paid employee of Mylan and may hold stock within the company. The remaining authors reported that they had no conflicts of interest.
FROM PEDIATRIC ALLERGY, IMMUNOLOGY, AND PULMONOLOGY
Key clinical point: School staff members are often not allowed to administer potentially life-saving epinephrine to children having an anaphylactic event.
Major finding: Of 6,053 schools providing data on who is permitted to administer epinephrine to treat anaphylaxis, 22% permitted all staff, 16% permitted most staff, 55% permitted the school nurse and select trained staff, and 3% permitted the school nurse only.
Data source: A survey of 6,574 schools.
Disclosures: This study was supported by Mylan Specialty. Dr. White has served as a consultant for Mylan and Merck and has received grants, fees, or support from numerous pharmaceutical companies. Christopher Herrem, PhD, is a paid employee of Mylan and may hold stock within the company. The remaining authors reported having no conflicts of interest.
VIDEO: Consider immunogenicity when choosing biologics
LAS VEGAS – Although biologics have transformed treatment of many rheumatologic disorders, their structure and mechanism of action can come with immunogenic baggage. “They’re proteins; consequently, one can expect immunogenicity,” said Daniel Furst, MD, speaking at the annual Perspectives in Rheumatic Diseases held by the Global Academy for Medical Education.
Immunogenicity can increase clearance of drugs; it can also interfere with the function of the drug. Although physicians may be tempted to think that developing an antidrug antibody to a specific drug is the principal cause for lack of efficacy in an individual patient, “in fact, it’s not that simple,” said Dr. Furst. “There are comorbidities, other drugs, which can affect the immunogenicity itself; there’s even the specific epitope that’s being affected by the drug antibody, which may or may not result in neutralization and an effect.”
In the broad class of tumor necrosis factor inhibitors (TNFIs), about 30% of patients taking adalimumab or infliximab will develop autoantibodies. This is higher than the 10% rate of autoantibody development for other TNFIs.
For non-TNFIs, including abatacept, tocilizumab, and rituximab, “the incidence of immunogenicity is significantly lower,” on the order of 1%-5%, said Dr. Furst, who has appointments at the University of California, Los Angeles, the University of Washington, Seattle, and the University of Florence, Italy.
Still, “there’s reasonable evidence that antidrug antibodies to TNFIs decrease efficacy and increase toxicity, particularly skin reactions,” he said.
Global Academy for Medical Education and this organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @karioakes
LAS VEGAS – Although biologics have transformed treatment of many rheumatologic disorders, their structure and mechanism of action can come with immunogenic baggage. “They’re proteins; consequently, one can expect immunogenicity,” said Daniel Furst, MD, speaking at the annual Perspectives in Rheumatic Diseases held by the Global Academy for Medical Education.
Immunogenicity can increase clearance of drugs; it can also interfere with the function of the drug. Although physicians may be tempted to think that developing an antidrug antibody to a specific drug is the principal cause for lack of efficacy in an individual patient, “in fact, it’s not that simple,” said Dr. Furst. “There are comorbidities, other drugs, which can affect the immunogenicity itself; there’s even the specific epitope that’s being affected by the drug antibody, which may or may not result in neutralization and an effect.”
In the broad class of tumor necrosis factor inhibitors (TNFIs), about 30% of patients taking adalimumab or infliximab will develop autoantibodies. This is higher than the 10% rate of autoantibody development for other TNFIs.
For non-TNFIs, including abatacept, tocilizumab, and rituximab, “the incidence of immunogenicity is significantly lower,” on the order of 1%-5%, said Dr. Furst, who has appointments at the University of California, Los Angeles, the University of Washington, Seattle, and the University of Florence, Italy.
Still, “there’s reasonable evidence that antidrug antibodies to TNFIs decrease efficacy and increase toxicity, particularly skin reactions,” he said.
Global Academy for Medical Education and this organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @karioakes
LAS VEGAS – Although biologics have transformed treatment of many rheumatologic disorders, their structure and mechanism of action can come with immunogenic baggage. “They’re proteins; consequently, one can expect immunogenicity,” said Daniel Furst, MD, speaking at the annual Perspectives in Rheumatic Diseases held by the Global Academy for Medical Education.
Immunogenicity can increase clearance of drugs; it can also interfere with the function of the drug. Although physicians may be tempted to think that developing an antidrug antibody to a specific drug is the principal cause for lack of efficacy in an individual patient, “in fact, it’s not that simple,” said Dr. Furst. “There are comorbidities, other drugs, which can affect the immunogenicity itself; there’s even the specific epitope that’s being affected by the drug antibody, which may or may not result in neutralization and an effect.”
In the broad class of tumor necrosis factor inhibitors (TNFIs), about 30% of patients taking adalimumab or infliximab will develop autoantibodies. This is higher than the 10% rate of autoantibody development for other TNFIs.
For non-TNFIs, including abatacept, tocilizumab, and rituximab, “the incidence of immunogenicity is significantly lower,” on the order of 1%-5%, said Dr. Furst, who has appointments at the University of California, Los Angeles, the University of Washington, Seattle, and the University of Florence, Italy.
Still, “there’s reasonable evidence that antidrug antibodies to TNFIs decrease efficacy and increase toxicity, particularly skin reactions,” he said.
Global Academy for Medical Education and this organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @karioakes
EXPERT ANALYSIS FROM PERSPECTIVES IN RHEUMATIC DISEASES