User login
Glenohumeral Joint Sepsis Caused by Streptococcus mitis: A Case Report
Septic arthritis predominantly involves the weight-bearing joints of the hip and knee, which account for nearly 60% of cases.1 In contrast, the shoulder joint is involved in 10% to 15% of cases, though this number may be higher among intravenous (IV) drug users.2 The most common causative organisms are the Staphylococcus species, followed closely by β-hemolytic streptococci, with these 2 groups accounting for more than 90% of all cases.3 The Streptococcus viridans group belongs to normal oral flora residing predominantly on the surface of teeth. Although well known for its ability to colonize heart valves and frequently cause bacterial endocarditis, this group has rarely been associated with septic arthritis. Furthermore, Streptococcus mitis, a subgroup of S viridans, has been implicated even less commonly.
In this article, we report a case of glenohumeral joint septic arthritis caused by S mitis. To our knowledge, such a case has not been previously reported in the English literature. Given the low virulence of this orally based bacterium, treating physicians must maintain clinical suspicion for the organism in the setting of persistent joint effusion and pain in association with periodontal disease or trauma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A right-hand-dominant 54-year-old man presented to Dr. Gruson with complaints of persistent right shoulder pain associated with worsening range of motion (ROM). Three weeks earlier, the patient reported being assaulted and noted progressive swelling about the right shoulder. He denied fevers, chills, or prior shoulder problems. Although his past medical history was remarkable for hepatitis C and diabetes, he was not taking any diabetic medications at that time. A review of systems was remarkable for poor dental hygiene, and the patient was missing several teeth, which he said had been knocked out during the assault. Physical examination revealed diffuse tenderness about the right shoulder and severe pain with all passive movement. The shoulder was pseudoparalyzed. There were no subcutaneous collections, wounds, or ecchymosis about the shoulder. Mild calor was noted on the right shoulder relative to the left. Radiographs of the right shoulder showed no acute osseous abnormalities.
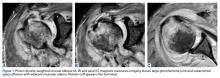
Magnetic resonance imaging (MRI), which was urgently obtained to assess the integrity of the rotator cuff and the location of the effusion, showed a large subacromial and glenohumeral joint effusion as well as diffuse muscular edema (Figures 1A-1C).
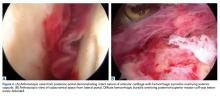
In light of the elevated infection findings of the laboratory tests and the positive culture, urgent arthroscopic irrigation and débridement of the right shoulder were indicated. Given the organism identified, transesophageal echocardiography was performed; there were no valvular vegetations. Creation of the posterior glenohumeral portal resulted in egress of turbid fluid, which was sent for culture. The subacromial space and the glenohumeral joint were thoroughly lavaged and the copious hemorrhagic synovitis débrided (Figures 2A, 2B).
The 8-week course of antibiotics normalized the patient’s ESR to 13 mm/h. Follow-up MRI showed improvement in the soft-tissue edema. Clinically, the patient reported minimal shoulder pain. He was undergoing physical therapy to regain strength and ROM.
Discussion
Staphylococcus aureus is the leading causative organism of septic arthritis, accounting for more than 60% of all cases.4 Conversely, the Streptococcus viridans group is rarely implicated in septic arthritis, accounting for <1% of cases.4S viridans is part of the commensal oral flora and has low virulence. This heterogeneous group is subdivided into S mitis, S salivarius, S anginosus, S mutans, and S bovis. The S mitis group is further subdivided into S sanguinis (formerly known as S sanguis) and S mitis. Infection by an organism of the S viridans group usually occurs on a previously injured focus, and the organism is a causative agent of bacterial endocarditis.5 Reported cases of septic arthritis caused by S viridans have predominantly involved the knee joint—with severe osteoarthritis, poor dental hygiene, and prior IV drug use identified as risk factors.5-7The shoulder joint is seldom involved in septic arthritis; estimated incidence is under 8%.8 Although overall incidence may rise in an increasingly elderly patient population, incidence of shoulder infection remains low.2,9
The main routes for developing septic arthritis include direct inoculation secondary to penetrating trauma or hematologic spread.10 Coatsworth and colleagues11 reported on iatrogenic S mitis septic arthritis of a shoulder arthroplasty during ultrasonography-guided aspiration by a technician who was not wearing a mask. Our institutional policy is to perform joint aspiration under strictly sterile conditions, which were adhered to in the present case. We surmise our patient developed transient bacteremia from the loss of several teeth, particularly given his poor dentition. Yombi and colleagues5 documented 2 cases of septic arthritis caused by Streptococcus gordonii, a relative of S sanguinis. One involved a previously replaced knee, and the other a native knee joint. Other cases of S viridans group septic arthritis have involved the knee,6,7,12,13 the sternoclavicular joint,14-16 and the acromioclavicular joint.17S sanguinis6,7,12,15,16 and S gordonii5 have been implicated in most cases, and an unspeciated S viridans in others.13,14,17 Concomitant periodontal disease has been reported in most cases as well,6,7,12,15 including our patient’s case. In the English-language literature, we found no other reports of S mitis as the causative agent of acute septic glenohumeral joint arthritis from hematogenous spread.
There should be no delay in diagnosing septic arthritis, and infected material should be removed from the joint. In animal models, complete joint destruction occurred only 5 weeks after inoculation with Staphylococcus aureus.10 Garofalo and colleagues18 reported a trend toward improved functional outcomes after earlier operative treatment. The choice of open surgical drainage vs repeat needle aspiration seems to be of little consequence, as both have good long-term outcomes, but open surgical drainage seems to result in better long-term functional ROM.2,9 However, results of a recent study suggested surgical treatment is not always superior to medical treatment for septic arthritis in native joints.19 In some cases involving S viridans species, treatment consisted of a combination of IV antibiotics and onetime or repeat aspiration;6,12-15 treatment in the remaining cases was surgical débridement.5,7,16,17 Given that S viridans is associated with bacterial endocarditis, echocardiography is essential if this organism is to be identified. Medical management and antibiotic treatment should be initiated after consultation with medical and infectious disease specialists.19We have reported a case of septic shoulder caused by S mitis, a low-virulence organism seldom associated with joint infection. The patient’s infection likely resulted from hematogenous spread from the oral cavity (dentition was poor). Urgent aspiration of the joint and baseline infection laboratory tests are recommended. MRI of the shoulder may show an effusion. Urgent arthroscopic irrigation and débridement can yield good clinical outcomes.
Am J Orthop. 2016;45(6):E343-E346. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Mathews CJ, Kingsley G, Field M, et al. Management of septic arthritis: a systematic review. Ann Rheum Dis. 2007;66(4):440-445.
2. Leslie BM, Harris JM 3rd, Driscoll D. Septic arthritis of the shoulder in adults. J Bone Joint Surg Am. 1989;71(10):1516-1522.
3. Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology. 2001;40(1):24-30.
4. Dubost JJ, Soubrier M, De Champs C, Ristori JM, Bussiere JL, Sauvezie B. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis. 2002;61(3):267-269.
5. Yombi J, Belkhir L, Jonckheere S, et al. Streptococcus gordonii septic arthritis: two cases and review of literature. BMC Infect Dis. 2012;12:215.
6. Papaioannides D, Boniatsi L, Korantzopoulos P, Sinapidis D, Giotis C. Acute septic arthritis due to Streptococcus sanguis. Med Princ Pract. 2006;15(1):77-79.
7. Edson RS, Osmon DR, Berry DJ. Septic arthritis due to Streptococcus sanguis. Mayo Clin Proc. 2002;77(7):709-710.
8. Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK health district 1982-1991. Ann Rheum Dis. 1999;58(4):214-219.
9. Lossos IS, Yossepowitch O, Kandel L, Yardeni D, Arber N. Septic arthritis of the glenohumeral joint. A report of 11 cases and review of the literature. Medicine. 1998;77(3):177-187.
10. Esterhai JL Jr, Gelb I. Adult septic arthritis. Orthop Clin North Am. 1991;22(3):503-514.
11. Coatsworth NR, Huntington PG, Giuffre B, Kotsiou G. The doctor and the mask: iatrogenic septic arthritis caused by Streptoccocus mitis. Med J Aust. 2013;198(5):285-286.
12. Patrick MR, Lewis D. Short of a length: Streptococcus sanguis knee infection from dental source. Br J Rheumatol. 1992;31(8):569.
13. Barbadillo C, Trujillo A, Cuende E, Mazzucchelli R, Mulero J, Andreu JL. Septic arthritis due to Streptococcus viridans. Clin Exp Rheumatol. 1990;8(5):520-521.
14. Mata P, Molins A, de Oya M. Sternal arthritis caused by Streptococcus viridans in a heroin addict [in Spanish]. Med Clin. 1984;83(16):689.
15. Mandac I, Prkacin I, Sabljar Matovinovic M, Sustercic D. Septic arthritis due to Streptococcus sanguis. Coll Antropol. 2010;34(2):661-664.
16. Nitsche JF, Vaughan JH, Williams G, Curd JG. Septic sternoclavicular arthritis with Pasteurella multocida and Streptococcus sanguis. Arthritis Rheum. 1982;25(4):467-469.
17. Blankstein A, Amsallem JL, Rubenstein E, Horoszowski H, Farin I. Septic arthritis of the acromioclavicular joint. Arch Orthop Trauma Surg. 1985;103(6):417-418.
18. Garofalo R, Flanagin B, Cesari E, Vinci E, Conti M, Castagna A. Destructive septic arthritis of shoulder in adults. Musculoskelet Surg. 2014;98(supp 1):S35-S39.
19. Ravindran V, Logan I, Bourke BE. Medical vs surgical treatment for the native joint in septic arthritis: a 6-year, single UK academic centre experience. Rheumatology. 2009;48(10):1320-1322.
Septic arthritis predominantly involves the weight-bearing joints of the hip and knee, which account for nearly 60% of cases.1 In contrast, the shoulder joint is involved in 10% to 15% of cases, though this number may be higher among intravenous (IV) drug users.2 The most common causative organisms are the Staphylococcus species, followed closely by β-hemolytic streptococci, with these 2 groups accounting for more than 90% of all cases.3 The Streptococcus viridans group belongs to normal oral flora residing predominantly on the surface of teeth. Although well known for its ability to colonize heart valves and frequently cause bacterial endocarditis, this group has rarely been associated with septic arthritis. Furthermore, Streptococcus mitis, a subgroup of S viridans, has been implicated even less commonly.
In this article, we report a case of glenohumeral joint septic arthritis caused by S mitis. To our knowledge, such a case has not been previously reported in the English literature. Given the low virulence of this orally based bacterium, treating physicians must maintain clinical suspicion for the organism in the setting of persistent joint effusion and pain in association with periodontal disease or trauma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A right-hand-dominant 54-year-old man presented to Dr. Gruson with complaints of persistent right shoulder pain associated with worsening range of motion (ROM). Three weeks earlier, the patient reported being assaulted and noted progressive swelling about the right shoulder. He denied fevers, chills, or prior shoulder problems. Although his past medical history was remarkable for hepatitis C and diabetes, he was not taking any diabetic medications at that time. A review of systems was remarkable for poor dental hygiene, and the patient was missing several teeth, which he said had been knocked out during the assault. Physical examination revealed diffuse tenderness about the right shoulder and severe pain with all passive movement. The shoulder was pseudoparalyzed. There were no subcutaneous collections, wounds, or ecchymosis about the shoulder. Mild calor was noted on the right shoulder relative to the left. Radiographs of the right shoulder showed no acute osseous abnormalities.
Magnetic resonance imaging (MRI), which was urgently obtained to assess the integrity of the rotator cuff and the location of the effusion, showed a large subacromial and glenohumeral joint effusion as well as diffuse muscular edema (Figures 1A-1C).
In light of the elevated infection findings of the laboratory tests and the positive culture, urgent arthroscopic irrigation and débridement of the right shoulder were indicated. Given the organism identified, transesophageal echocardiography was performed; there were no valvular vegetations. Creation of the posterior glenohumeral portal resulted in egress of turbid fluid, which was sent for culture. The subacromial space and the glenohumeral joint were thoroughly lavaged and the copious hemorrhagic synovitis débrided (Figures 2A, 2B).
The 8-week course of antibiotics normalized the patient’s ESR to 13 mm/h. Follow-up MRI showed improvement in the soft-tissue edema. Clinically, the patient reported minimal shoulder pain. He was undergoing physical therapy to regain strength and ROM.
Discussion
Staphylococcus aureus is the leading causative organism of septic arthritis, accounting for more than 60% of all cases.4 Conversely, the Streptococcus viridans group is rarely implicated in septic arthritis, accounting for <1% of cases.4S viridans is part of the commensal oral flora and has low virulence. This heterogeneous group is subdivided into S mitis, S salivarius, S anginosus, S mutans, and S bovis. The S mitis group is further subdivided into S sanguinis (formerly known as S sanguis) and S mitis. Infection by an organism of the S viridans group usually occurs on a previously injured focus, and the organism is a causative agent of bacterial endocarditis.5 Reported cases of septic arthritis caused by S viridans have predominantly involved the knee joint—with severe osteoarthritis, poor dental hygiene, and prior IV drug use identified as risk factors.5-7The shoulder joint is seldom involved in septic arthritis; estimated incidence is under 8%.8 Although overall incidence may rise in an increasingly elderly patient population, incidence of shoulder infection remains low.2,9
The main routes for developing septic arthritis include direct inoculation secondary to penetrating trauma or hematologic spread.10 Coatsworth and colleagues11 reported on iatrogenic S mitis septic arthritis of a shoulder arthroplasty during ultrasonography-guided aspiration by a technician who was not wearing a mask. Our institutional policy is to perform joint aspiration under strictly sterile conditions, which were adhered to in the present case. We surmise our patient developed transient bacteremia from the loss of several teeth, particularly given his poor dentition. Yombi and colleagues5 documented 2 cases of septic arthritis caused by Streptococcus gordonii, a relative of S sanguinis. One involved a previously replaced knee, and the other a native knee joint. Other cases of S viridans group septic arthritis have involved the knee,6,7,12,13 the sternoclavicular joint,14-16 and the acromioclavicular joint.17S sanguinis6,7,12,15,16 and S gordonii5 have been implicated in most cases, and an unspeciated S viridans in others.13,14,17 Concomitant periodontal disease has been reported in most cases as well,6,7,12,15 including our patient’s case. In the English-language literature, we found no other reports of S mitis as the causative agent of acute septic glenohumeral joint arthritis from hematogenous spread.
There should be no delay in diagnosing septic arthritis, and infected material should be removed from the joint. In animal models, complete joint destruction occurred only 5 weeks after inoculation with Staphylococcus aureus.10 Garofalo and colleagues18 reported a trend toward improved functional outcomes after earlier operative treatment. The choice of open surgical drainage vs repeat needle aspiration seems to be of little consequence, as both have good long-term outcomes, but open surgical drainage seems to result in better long-term functional ROM.2,9 However, results of a recent study suggested surgical treatment is not always superior to medical treatment for septic arthritis in native joints.19 In some cases involving S viridans species, treatment consisted of a combination of IV antibiotics and onetime or repeat aspiration;6,12-15 treatment in the remaining cases was surgical débridement.5,7,16,17 Given that S viridans is associated with bacterial endocarditis, echocardiography is essential if this organism is to be identified. Medical management and antibiotic treatment should be initiated after consultation with medical and infectious disease specialists.19We have reported a case of septic shoulder caused by S mitis, a low-virulence organism seldom associated with joint infection. The patient’s infection likely resulted from hematogenous spread from the oral cavity (dentition was poor). Urgent aspiration of the joint and baseline infection laboratory tests are recommended. MRI of the shoulder may show an effusion. Urgent arthroscopic irrigation and débridement can yield good clinical outcomes.
Am J Orthop. 2016;45(6):E343-E346. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Septic arthritis predominantly involves the weight-bearing joints of the hip and knee, which account for nearly 60% of cases.1 In contrast, the shoulder joint is involved in 10% to 15% of cases, though this number may be higher among intravenous (IV) drug users.2 The most common causative organisms are the Staphylococcus species, followed closely by β-hemolytic streptococci, with these 2 groups accounting for more than 90% of all cases.3 The Streptococcus viridans group belongs to normal oral flora residing predominantly on the surface of teeth. Although well known for its ability to colonize heart valves and frequently cause bacterial endocarditis, this group has rarely been associated with septic arthritis. Furthermore, Streptococcus mitis, a subgroup of S viridans, has been implicated even less commonly.
In this article, we report a case of glenohumeral joint septic arthritis caused by S mitis. To our knowledge, such a case has not been previously reported in the English literature. Given the low virulence of this orally based bacterium, treating physicians must maintain clinical suspicion for the organism in the setting of persistent joint effusion and pain in association with periodontal disease or trauma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A right-hand-dominant 54-year-old man presented to Dr. Gruson with complaints of persistent right shoulder pain associated with worsening range of motion (ROM). Three weeks earlier, the patient reported being assaulted and noted progressive swelling about the right shoulder. He denied fevers, chills, or prior shoulder problems. Although his past medical history was remarkable for hepatitis C and diabetes, he was not taking any diabetic medications at that time. A review of systems was remarkable for poor dental hygiene, and the patient was missing several teeth, which he said had been knocked out during the assault. Physical examination revealed diffuse tenderness about the right shoulder and severe pain with all passive movement. The shoulder was pseudoparalyzed. There were no subcutaneous collections, wounds, or ecchymosis about the shoulder. Mild calor was noted on the right shoulder relative to the left. Radiographs of the right shoulder showed no acute osseous abnormalities.
Magnetic resonance imaging (MRI), which was urgently obtained to assess the integrity of the rotator cuff and the location of the effusion, showed a large subacromial and glenohumeral joint effusion as well as diffuse muscular edema (Figures 1A-1C).
In light of the elevated infection findings of the laboratory tests and the positive culture, urgent arthroscopic irrigation and débridement of the right shoulder were indicated. Given the organism identified, transesophageal echocardiography was performed; there were no valvular vegetations. Creation of the posterior glenohumeral portal resulted in egress of turbid fluid, which was sent for culture. The subacromial space and the glenohumeral joint were thoroughly lavaged and the copious hemorrhagic synovitis débrided (Figures 2A, 2B).
The 8-week course of antibiotics normalized the patient’s ESR to 13 mm/h. Follow-up MRI showed improvement in the soft-tissue edema. Clinically, the patient reported minimal shoulder pain. He was undergoing physical therapy to regain strength and ROM.
Discussion
Staphylococcus aureus is the leading causative organism of septic arthritis, accounting for more than 60% of all cases.4 Conversely, the Streptococcus viridans group is rarely implicated in septic arthritis, accounting for <1% of cases.4S viridans is part of the commensal oral flora and has low virulence. This heterogeneous group is subdivided into S mitis, S salivarius, S anginosus, S mutans, and S bovis. The S mitis group is further subdivided into S sanguinis (formerly known as S sanguis) and S mitis. Infection by an organism of the S viridans group usually occurs on a previously injured focus, and the organism is a causative agent of bacterial endocarditis.5 Reported cases of septic arthritis caused by S viridans have predominantly involved the knee joint—with severe osteoarthritis, poor dental hygiene, and prior IV drug use identified as risk factors.5-7The shoulder joint is seldom involved in septic arthritis; estimated incidence is under 8%.8 Although overall incidence may rise in an increasingly elderly patient population, incidence of shoulder infection remains low.2,9
The main routes for developing septic arthritis include direct inoculation secondary to penetrating trauma or hematologic spread.10 Coatsworth and colleagues11 reported on iatrogenic S mitis septic arthritis of a shoulder arthroplasty during ultrasonography-guided aspiration by a technician who was not wearing a mask. Our institutional policy is to perform joint aspiration under strictly sterile conditions, which were adhered to in the present case. We surmise our patient developed transient bacteremia from the loss of several teeth, particularly given his poor dentition. Yombi and colleagues5 documented 2 cases of septic arthritis caused by Streptococcus gordonii, a relative of S sanguinis. One involved a previously replaced knee, and the other a native knee joint. Other cases of S viridans group septic arthritis have involved the knee,6,7,12,13 the sternoclavicular joint,14-16 and the acromioclavicular joint.17S sanguinis6,7,12,15,16 and S gordonii5 have been implicated in most cases, and an unspeciated S viridans in others.13,14,17 Concomitant periodontal disease has been reported in most cases as well,6,7,12,15 including our patient’s case. In the English-language literature, we found no other reports of S mitis as the causative agent of acute septic glenohumeral joint arthritis from hematogenous spread.
There should be no delay in diagnosing septic arthritis, and infected material should be removed from the joint. In animal models, complete joint destruction occurred only 5 weeks after inoculation with Staphylococcus aureus.10 Garofalo and colleagues18 reported a trend toward improved functional outcomes after earlier operative treatment. The choice of open surgical drainage vs repeat needle aspiration seems to be of little consequence, as both have good long-term outcomes, but open surgical drainage seems to result in better long-term functional ROM.2,9 However, results of a recent study suggested surgical treatment is not always superior to medical treatment for septic arthritis in native joints.19 In some cases involving S viridans species, treatment consisted of a combination of IV antibiotics and onetime or repeat aspiration;6,12-15 treatment in the remaining cases was surgical débridement.5,7,16,17 Given that S viridans is associated with bacterial endocarditis, echocardiography is essential if this organism is to be identified. Medical management and antibiotic treatment should be initiated after consultation with medical and infectious disease specialists.19We have reported a case of septic shoulder caused by S mitis, a low-virulence organism seldom associated with joint infection. The patient’s infection likely resulted from hematogenous spread from the oral cavity (dentition was poor). Urgent aspiration of the joint and baseline infection laboratory tests are recommended. MRI of the shoulder may show an effusion. Urgent arthroscopic irrigation and débridement can yield good clinical outcomes.
Am J Orthop. 2016;45(6):E343-E346. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Mathews CJ, Kingsley G, Field M, et al. Management of septic arthritis: a systematic review. Ann Rheum Dis. 2007;66(4):440-445.
2. Leslie BM, Harris JM 3rd, Driscoll D. Septic arthritis of the shoulder in adults. J Bone Joint Surg Am. 1989;71(10):1516-1522.
3. Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology. 2001;40(1):24-30.
4. Dubost JJ, Soubrier M, De Champs C, Ristori JM, Bussiere JL, Sauvezie B. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis. 2002;61(3):267-269.
5. Yombi J, Belkhir L, Jonckheere S, et al. Streptococcus gordonii septic arthritis: two cases and review of literature. BMC Infect Dis. 2012;12:215.
6. Papaioannides D, Boniatsi L, Korantzopoulos P, Sinapidis D, Giotis C. Acute septic arthritis due to Streptococcus sanguis. Med Princ Pract. 2006;15(1):77-79.
7. Edson RS, Osmon DR, Berry DJ. Septic arthritis due to Streptococcus sanguis. Mayo Clin Proc. 2002;77(7):709-710.
8. Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK health district 1982-1991. Ann Rheum Dis. 1999;58(4):214-219.
9. Lossos IS, Yossepowitch O, Kandel L, Yardeni D, Arber N. Septic arthritis of the glenohumeral joint. A report of 11 cases and review of the literature. Medicine. 1998;77(3):177-187.
10. Esterhai JL Jr, Gelb I. Adult septic arthritis. Orthop Clin North Am. 1991;22(3):503-514.
11. Coatsworth NR, Huntington PG, Giuffre B, Kotsiou G. The doctor and the mask: iatrogenic septic arthritis caused by Streptoccocus mitis. Med J Aust. 2013;198(5):285-286.
12. Patrick MR, Lewis D. Short of a length: Streptococcus sanguis knee infection from dental source. Br J Rheumatol. 1992;31(8):569.
13. Barbadillo C, Trujillo A, Cuende E, Mazzucchelli R, Mulero J, Andreu JL. Septic arthritis due to Streptococcus viridans. Clin Exp Rheumatol. 1990;8(5):520-521.
14. Mata P, Molins A, de Oya M. Sternal arthritis caused by Streptococcus viridans in a heroin addict [in Spanish]. Med Clin. 1984;83(16):689.
15. Mandac I, Prkacin I, Sabljar Matovinovic M, Sustercic D. Septic arthritis due to Streptococcus sanguis. Coll Antropol. 2010;34(2):661-664.
16. Nitsche JF, Vaughan JH, Williams G, Curd JG. Septic sternoclavicular arthritis with Pasteurella multocida and Streptococcus sanguis. Arthritis Rheum. 1982;25(4):467-469.
17. Blankstein A, Amsallem JL, Rubenstein E, Horoszowski H, Farin I. Septic arthritis of the acromioclavicular joint. Arch Orthop Trauma Surg. 1985;103(6):417-418.
18. Garofalo R, Flanagin B, Cesari E, Vinci E, Conti M, Castagna A. Destructive septic arthritis of shoulder in adults. Musculoskelet Surg. 2014;98(supp 1):S35-S39.
19. Ravindran V, Logan I, Bourke BE. Medical vs surgical treatment for the native joint in septic arthritis: a 6-year, single UK academic centre experience. Rheumatology. 2009;48(10):1320-1322.
1. Mathews CJ, Kingsley G, Field M, et al. Management of septic arthritis: a systematic review. Ann Rheum Dis. 2007;66(4):440-445.
2. Leslie BM, Harris JM 3rd, Driscoll D. Septic arthritis of the shoulder in adults. J Bone Joint Surg Am. 1989;71(10):1516-1522.
3. Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology. 2001;40(1):24-30.
4. Dubost JJ, Soubrier M, De Champs C, Ristori JM, Bussiere JL, Sauvezie B. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis. 2002;61(3):267-269.
5. Yombi J, Belkhir L, Jonckheere S, et al. Streptococcus gordonii septic arthritis: two cases and review of literature. BMC Infect Dis. 2012;12:215.
6. Papaioannides D, Boniatsi L, Korantzopoulos P, Sinapidis D, Giotis C. Acute septic arthritis due to Streptococcus sanguis. Med Princ Pract. 2006;15(1):77-79.
7. Edson RS, Osmon DR, Berry DJ. Septic arthritis due to Streptococcus sanguis. Mayo Clin Proc. 2002;77(7):709-710.
8. Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK health district 1982-1991. Ann Rheum Dis. 1999;58(4):214-219.
9. Lossos IS, Yossepowitch O, Kandel L, Yardeni D, Arber N. Septic arthritis of the glenohumeral joint. A report of 11 cases and review of the literature. Medicine. 1998;77(3):177-187.
10. Esterhai JL Jr, Gelb I. Adult septic arthritis. Orthop Clin North Am. 1991;22(3):503-514.
11. Coatsworth NR, Huntington PG, Giuffre B, Kotsiou G. The doctor and the mask: iatrogenic septic arthritis caused by Streptoccocus mitis. Med J Aust. 2013;198(5):285-286.
12. Patrick MR, Lewis D. Short of a length: Streptococcus sanguis knee infection from dental source. Br J Rheumatol. 1992;31(8):569.
13. Barbadillo C, Trujillo A, Cuende E, Mazzucchelli R, Mulero J, Andreu JL. Septic arthritis due to Streptococcus viridans. Clin Exp Rheumatol. 1990;8(5):520-521.
14. Mata P, Molins A, de Oya M. Sternal arthritis caused by Streptococcus viridans in a heroin addict [in Spanish]. Med Clin. 1984;83(16):689.
15. Mandac I, Prkacin I, Sabljar Matovinovic M, Sustercic D. Septic arthritis due to Streptococcus sanguis. Coll Antropol. 2010;34(2):661-664.
16. Nitsche JF, Vaughan JH, Williams G, Curd JG. Septic sternoclavicular arthritis with Pasteurella multocida and Streptococcus sanguis. Arthritis Rheum. 1982;25(4):467-469.
17. Blankstein A, Amsallem JL, Rubenstein E, Horoszowski H, Farin I. Septic arthritis of the acromioclavicular joint. Arch Orthop Trauma Surg. 1985;103(6):417-418.
18. Garofalo R, Flanagin B, Cesari E, Vinci E, Conti M, Castagna A. Destructive septic arthritis of shoulder in adults. Musculoskelet Surg. 2014;98(supp 1):S35-S39.
19. Ravindran V, Logan I, Bourke BE. Medical vs surgical treatment for the native joint in septic arthritis: a 6-year, single UK academic centre experience. Rheumatology. 2009;48(10):1320-1322.
Experts offer tips on anxiety, depression, and psychosis in Parkinson’s
SAN FRANCISCO – Depression and psychosis were strongly correlated in Parkinson’s disease, while the presence of clinical anxiety upped the odds of psychosis by a statistically significant 8%, in a cross-sectional study presented at the 2016 congress of the International Psychogeriatric Association.
Felicia C. Goldstein, PhD, professor of neurology at Emory University, Atlanta, compared 48 patients with Parkinson’s disease and psychosis with 96 nonpsychotic controls who also had Parkinson’s disease. The groups were similar in terms of age, age of disease onset, educational level, Montreal Cognitive Assessment score (MoCA), and Unified Parkinson’s Disease Rating Scale (UPDRS) score, although patients with psychosis had about a 1.5-year longer mean duration of Parkinson’s disease than did controls (8.8 years vs. 7.3 years; P = .06).
Patients with psychosis were significantly more likely than controls to meet DSM-5 and Beck Depression Inventory II criteria for depression, with odds ratios of 8.0 (95% confidence interval, 2.5-25.6; P = .001) and 1.1 (1.02-1.1; P = .01), respectively. Patients with psychosis also were significantly more likely to have a positive result on the Beck Anxiety Inventory (OR, 1.1; 95% CI, 1.01-1.15; P = .01), and met DSM-5 criteria for anxiety more often than did controls (OR, 3.0; 95% CI, 0.9-9.5), although the latter correlation did not reach statistical significance (P = .07).
“The association between psychosis and anxiety has not been previously reported,” Dr. Goldstein noted. The findings underscore the link between psychosis and mood disorders in Parkinson’s disease and the need to treat these comorbidities, she said.
Neuropsychiatric symptoms in Parkinson’s disease also merit close monitoring and treatment, because they correlate with greater disability, faster progression of motor symptoms, and increased mortality, Adriana P. Hermida, MD, said in a separate oral presentation at the congress. In particular, depression is “the elephant in the room when it comes to Parkinson’s disease,” she said. “It is there, it is underrecognized, and it is undertreated.” Suicidal ideation is common, and patients should be treated even if they do not meet all criteria for a depressive disorder, added Dr. Hermida of the department of psychiatry and behavioral sciences at Emory.
For depression in Parkinson’s disease, Dr. Hermida said she typically starts with a selective serotonin reuptake inhibitor, most often escitalopram or sertraline. If the patient has a partial response, she adds another antidepressant, but if there is no response, she switches antidepressants. Second-line options for add-ons and switches include mirtazapine, which improves sleep and appetite and may improve tremor; venlafaxine extended release, which can raise blood pressure and may benefit hypotensive patients; and bupropion extended release, which is best for patients who need more activation, do not have substantial concerns with anxiety, and have REM sleep behavior disorder, she said. She said she also will consider dopamine agonists such as pramipexole, but they can increase the risk of psychosis, impulse control disorders, and dopamine dysregulation syndrome. She also noted that electroconvulsive therapy can rapidly improve both depression and motor symptoms, and should not be reserved for last-resort cases. Parkinson’s medications should be held the day of ECT, and cognition should be monitored afterward, she said.
Approximately 30% of Parkinson’s patients meet DSM-5 criteria for an anxiety disorder, and more than half have significant symptoms of anxiety, Dr. Hermida continued. Anxiety, like other signs and symptoms of Parkinson’s disease, can fluctuate throughout the day and tends to occur most frequently during “off” periods. No randomized controlled trials have examined anxiolytics in Parkinson’s disease patients, but studies of mindfulness-based cognitive therapy have yielded good results, she noted. Benzodiazepines “should be used sparingly, if at all,” as they increase the risk of confusion, gait abnormalities, and falls.
Psychosis should be treated if symptoms are ego-dystonic, she said. She said she uses first-line clozapine, which is more effective for delusions than quetiapine and has fewer adverse motor effects. She said she has not yet used pimavanserin (Nuplazid), a selective 5-HT2A inverse agonist that in April 2016 became the first drug approved by the Food and Drug Administration for treating hallucinations and delusions in Parkinson’s disease. The pivotal trial lasted 6 weeks and included 199 patients; those who received pimavanserin had a median 5.8-point drop on the Scale to Access Psychosis in Parkinson’s Disease (SAPS-PD), compared with 2.7 for placebo (P = .001), Dr. Hermida noted. Patients did not experience sedation or motor impairment, which are common adverse effects of other antipsychotics in this population.
Dr. Goldstein and Dr. Hermida reported no funding sources or conflicts of interest.
SAN FRANCISCO – Depression and psychosis were strongly correlated in Parkinson’s disease, while the presence of clinical anxiety upped the odds of psychosis by a statistically significant 8%, in a cross-sectional study presented at the 2016 congress of the International Psychogeriatric Association.
Felicia C. Goldstein, PhD, professor of neurology at Emory University, Atlanta, compared 48 patients with Parkinson’s disease and psychosis with 96 nonpsychotic controls who also had Parkinson’s disease. The groups were similar in terms of age, age of disease onset, educational level, Montreal Cognitive Assessment score (MoCA), and Unified Parkinson’s Disease Rating Scale (UPDRS) score, although patients with psychosis had about a 1.5-year longer mean duration of Parkinson’s disease than did controls (8.8 years vs. 7.3 years; P = .06).
Patients with psychosis were significantly more likely than controls to meet DSM-5 and Beck Depression Inventory II criteria for depression, with odds ratios of 8.0 (95% confidence interval, 2.5-25.6; P = .001) and 1.1 (1.02-1.1; P = .01), respectively. Patients with psychosis also were significantly more likely to have a positive result on the Beck Anxiety Inventory (OR, 1.1; 95% CI, 1.01-1.15; P = .01), and met DSM-5 criteria for anxiety more often than did controls (OR, 3.0; 95% CI, 0.9-9.5), although the latter correlation did not reach statistical significance (P = .07).
“The association between psychosis and anxiety has not been previously reported,” Dr. Goldstein noted. The findings underscore the link between psychosis and mood disorders in Parkinson’s disease and the need to treat these comorbidities, she said.
Neuropsychiatric symptoms in Parkinson’s disease also merit close monitoring and treatment, because they correlate with greater disability, faster progression of motor symptoms, and increased mortality, Adriana P. Hermida, MD, said in a separate oral presentation at the congress. In particular, depression is “the elephant in the room when it comes to Parkinson’s disease,” she said. “It is there, it is underrecognized, and it is undertreated.” Suicidal ideation is common, and patients should be treated even if they do not meet all criteria for a depressive disorder, added Dr. Hermida of the department of psychiatry and behavioral sciences at Emory.
For depression in Parkinson’s disease, Dr. Hermida said she typically starts with a selective serotonin reuptake inhibitor, most often escitalopram or sertraline. If the patient has a partial response, she adds another antidepressant, but if there is no response, she switches antidepressants. Second-line options for add-ons and switches include mirtazapine, which improves sleep and appetite and may improve tremor; venlafaxine extended release, which can raise blood pressure and may benefit hypotensive patients; and bupropion extended release, which is best for patients who need more activation, do not have substantial concerns with anxiety, and have REM sleep behavior disorder, she said. She said she also will consider dopamine agonists such as pramipexole, but they can increase the risk of psychosis, impulse control disorders, and dopamine dysregulation syndrome. She also noted that electroconvulsive therapy can rapidly improve both depression and motor symptoms, and should not be reserved for last-resort cases. Parkinson’s medications should be held the day of ECT, and cognition should be monitored afterward, she said.
Approximately 30% of Parkinson’s patients meet DSM-5 criteria for an anxiety disorder, and more than half have significant symptoms of anxiety, Dr. Hermida continued. Anxiety, like other signs and symptoms of Parkinson’s disease, can fluctuate throughout the day and tends to occur most frequently during “off” periods. No randomized controlled trials have examined anxiolytics in Parkinson’s disease patients, but studies of mindfulness-based cognitive therapy have yielded good results, she noted. Benzodiazepines “should be used sparingly, if at all,” as they increase the risk of confusion, gait abnormalities, and falls.
Psychosis should be treated if symptoms are ego-dystonic, she said. She said she uses first-line clozapine, which is more effective for delusions than quetiapine and has fewer adverse motor effects. She said she has not yet used pimavanserin (Nuplazid), a selective 5-HT2A inverse agonist that in April 2016 became the first drug approved by the Food and Drug Administration for treating hallucinations and delusions in Parkinson’s disease. The pivotal trial lasted 6 weeks and included 199 patients; those who received pimavanserin had a median 5.8-point drop on the Scale to Access Psychosis in Parkinson’s Disease (SAPS-PD), compared with 2.7 for placebo (P = .001), Dr. Hermida noted. Patients did not experience sedation or motor impairment, which are common adverse effects of other antipsychotics in this population.
Dr. Goldstein and Dr. Hermida reported no funding sources or conflicts of interest.
SAN FRANCISCO – Depression and psychosis were strongly correlated in Parkinson’s disease, while the presence of clinical anxiety upped the odds of psychosis by a statistically significant 8%, in a cross-sectional study presented at the 2016 congress of the International Psychogeriatric Association.
Felicia C. Goldstein, PhD, professor of neurology at Emory University, Atlanta, compared 48 patients with Parkinson’s disease and psychosis with 96 nonpsychotic controls who also had Parkinson’s disease. The groups were similar in terms of age, age of disease onset, educational level, Montreal Cognitive Assessment score (MoCA), and Unified Parkinson’s Disease Rating Scale (UPDRS) score, although patients with psychosis had about a 1.5-year longer mean duration of Parkinson’s disease than did controls (8.8 years vs. 7.3 years; P = .06).
Patients with psychosis were significantly more likely than controls to meet DSM-5 and Beck Depression Inventory II criteria for depression, with odds ratios of 8.0 (95% confidence interval, 2.5-25.6; P = .001) and 1.1 (1.02-1.1; P = .01), respectively. Patients with psychosis also were significantly more likely to have a positive result on the Beck Anxiety Inventory (OR, 1.1; 95% CI, 1.01-1.15; P = .01), and met DSM-5 criteria for anxiety more often than did controls (OR, 3.0; 95% CI, 0.9-9.5), although the latter correlation did not reach statistical significance (P = .07).
“The association between psychosis and anxiety has not been previously reported,” Dr. Goldstein noted. The findings underscore the link between psychosis and mood disorders in Parkinson’s disease and the need to treat these comorbidities, she said.
Neuropsychiatric symptoms in Parkinson’s disease also merit close monitoring and treatment, because they correlate with greater disability, faster progression of motor symptoms, and increased mortality, Adriana P. Hermida, MD, said in a separate oral presentation at the congress. In particular, depression is “the elephant in the room when it comes to Parkinson’s disease,” she said. “It is there, it is underrecognized, and it is undertreated.” Suicidal ideation is common, and patients should be treated even if they do not meet all criteria for a depressive disorder, added Dr. Hermida of the department of psychiatry and behavioral sciences at Emory.
For depression in Parkinson’s disease, Dr. Hermida said she typically starts with a selective serotonin reuptake inhibitor, most often escitalopram or sertraline. If the patient has a partial response, she adds another antidepressant, but if there is no response, she switches antidepressants. Second-line options for add-ons and switches include mirtazapine, which improves sleep and appetite and may improve tremor; venlafaxine extended release, which can raise blood pressure and may benefit hypotensive patients; and bupropion extended release, which is best for patients who need more activation, do not have substantial concerns with anxiety, and have REM sleep behavior disorder, she said. She said she also will consider dopamine agonists such as pramipexole, but they can increase the risk of psychosis, impulse control disorders, and dopamine dysregulation syndrome. She also noted that electroconvulsive therapy can rapidly improve both depression and motor symptoms, and should not be reserved for last-resort cases. Parkinson’s medications should be held the day of ECT, and cognition should be monitored afterward, she said.
Approximately 30% of Parkinson’s patients meet DSM-5 criteria for an anxiety disorder, and more than half have significant symptoms of anxiety, Dr. Hermida continued. Anxiety, like other signs and symptoms of Parkinson’s disease, can fluctuate throughout the day and tends to occur most frequently during “off” periods. No randomized controlled trials have examined anxiolytics in Parkinson’s disease patients, but studies of mindfulness-based cognitive therapy have yielded good results, she noted. Benzodiazepines “should be used sparingly, if at all,” as they increase the risk of confusion, gait abnormalities, and falls.
Psychosis should be treated if symptoms are ego-dystonic, she said. She said she uses first-line clozapine, which is more effective for delusions than quetiapine and has fewer adverse motor effects. She said she has not yet used pimavanserin (Nuplazid), a selective 5-HT2A inverse agonist that in April 2016 became the first drug approved by the Food and Drug Administration for treating hallucinations and delusions in Parkinson’s disease. The pivotal trial lasted 6 weeks and included 199 patients; those who received pimavanserin had a median 5.8-point drop on the Scale to Access Psychosis in Parkinson’s Disease (SAPS-PD), compared with 2.7 for placebo (P = .001), Dr. Hermida noted. Patients did not experience sedation or motor impairment, which are common adverse effects of other antipsychotics in this population.
Dr. Goldstein and Dr. Hermida reported no funding sources or conflicts of interest.
AT IPA 2016
Key clinical point: A cross-sectional study uncovered a statistically significant link between anxiety and psychosis in Parkinson’s disease.
Major finding: Patients with psychosis also were significantly more likely to have a positive result on the Beck Anxiety Inventory (odds ratio, 1.1; 95% confidence interval, 1.01-1.15; P = .01).
Data source: A cross-sectional study of 48 patients with Parkinson’s disease and psychosis and 96 nonpsychotic controls who also had Parkinson’s disease.
Disclosures: Dr. Goldstein and Dr. Hermida disclosed no funding sources or conflicts of interest.
2016 GYN coding changes to note for your maximized reimbursement
In the August 2016 issue of OBG
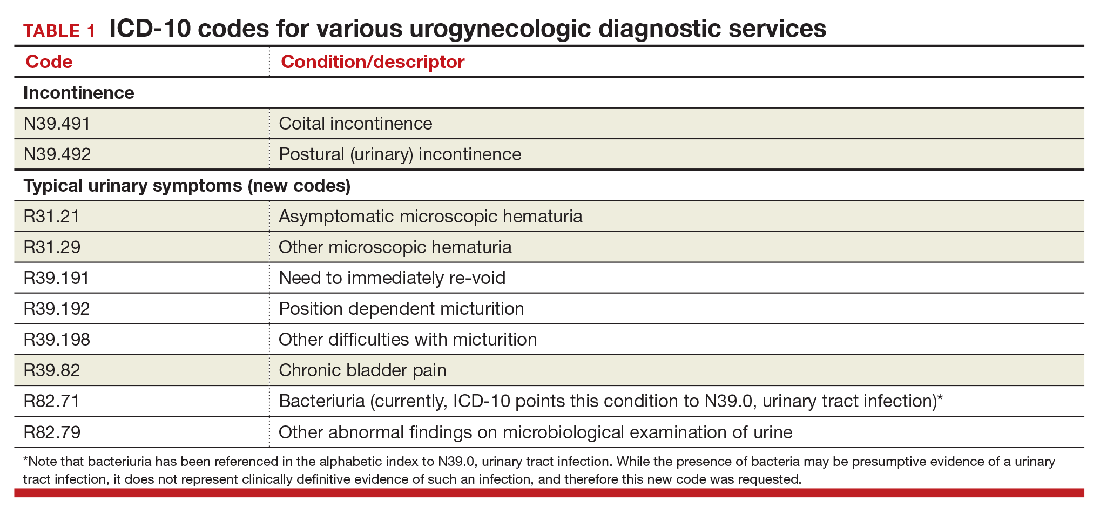
Urogynecology diagnostic codes
Urogynecologists will find a large number of changes to codes they can select on October 1, 2016. While some codes improve reporting for conditions or symptoms related to urinary issues, many more concern postoperative complications following surgery for devices and grafts applied to the genitourinary system.
The American Urological Association requested new codes to align with a 2009 joint report on the terminology for female pelvic floor dysfunction.1 These codes, along with others, are listed in TABLE 1.
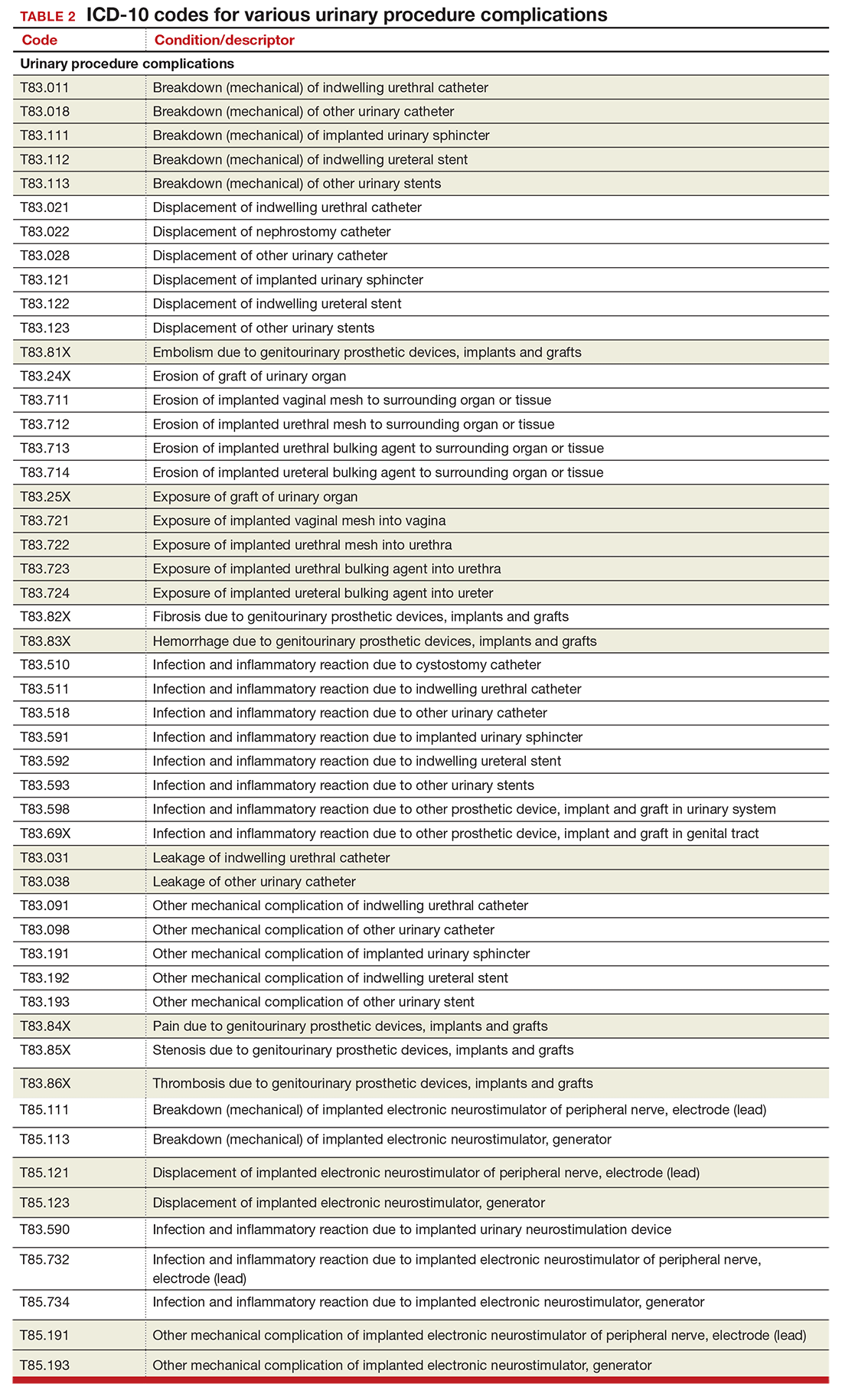
Urinary procedure complication codes
Not every urogynecologist will have an issue after surgery for incontinence, but if they do, there are tons of new and revised codes to address every possible complication the patient may have (TABLE 2). Each of these codes is reported based on whether the complication is being actively treated (initial encounter: final character is A), is being followed up after treatment (subsequent encounter: final character is D), or is caused by another condition (sequela: final character is S).
Gynecology-related diagnostic codes
Laterality
If there are 2 organs in the genitourinary system, the chances are good that there is now a right and a left designation code in ICD-10. Documentation should be clear, of course, and if the condition exists on both the right and the left side (even if only one side is being treated actively), list both codes, as there is no bilateral designation in the codes (TABLE 3). And while there is a code for “unspecified side,” providers normally do know which side, so use of this code should be avoided, if possible.
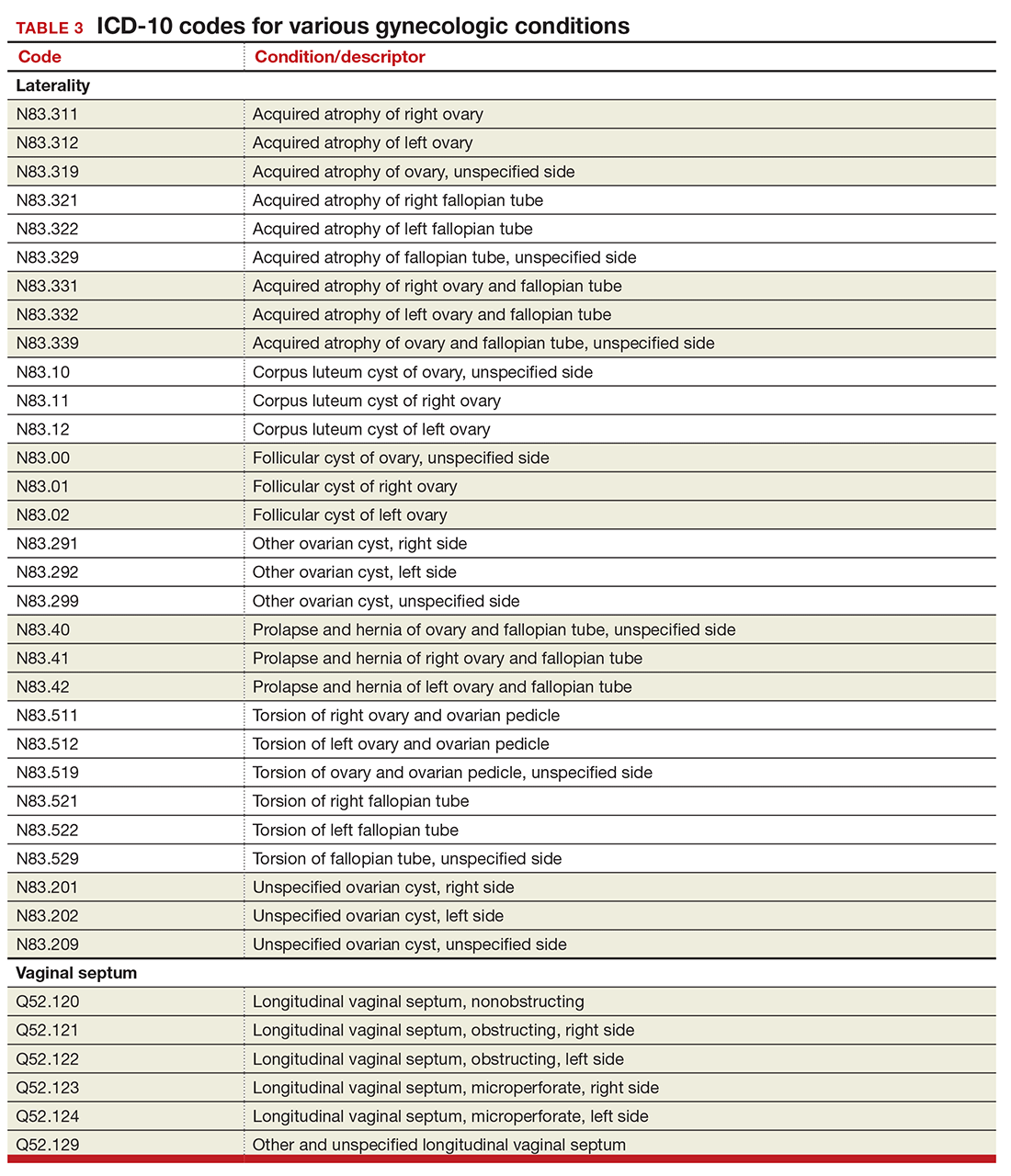
Vaginal septum
The right and left designations extend to the congenital codes for vaginal septum, but they go one step further. The American Congress of Obstetricians and Gynecologists (ACOG) requested that the codes for longitudinal vaginal septum be expanded to differentiate a nonobstructing vaginal septum from an obstructing vaginal septum, in addition to adding laterality to these codes (TABLE 3).2
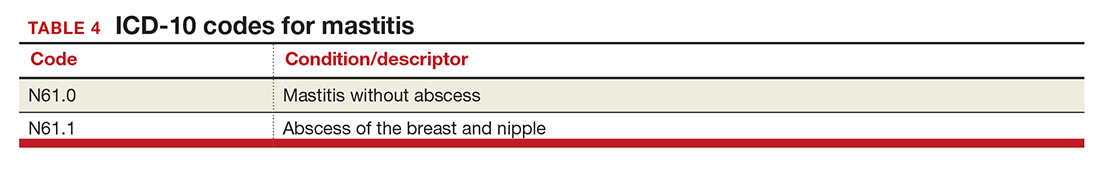
Mastitis
There are now 2 new codes for inflammation of the breast; one describes mastitis without abscess, while the other code includes an abscess of the breast and nipple (TABLE 4). Once again, documentation will lead to the most specific code to describe the findings.
Childhood and prepubertal concerns
The single code for hypertrophy of the vulva has been expanded to include asymmetric labium majus enlargement. This code was requested by ACOG because this is a known clinical diagnosis and the currently available vulvar codes are inadequate for capturing this condition. The vulvar enlargement appears to be in response to hormonal surges during prepuberty and early puberty. Adult hypertrophy, either congenital or acquired from childbirth or as a late result of an old injury, would be reported by the “other specified” code (TABLE 5).
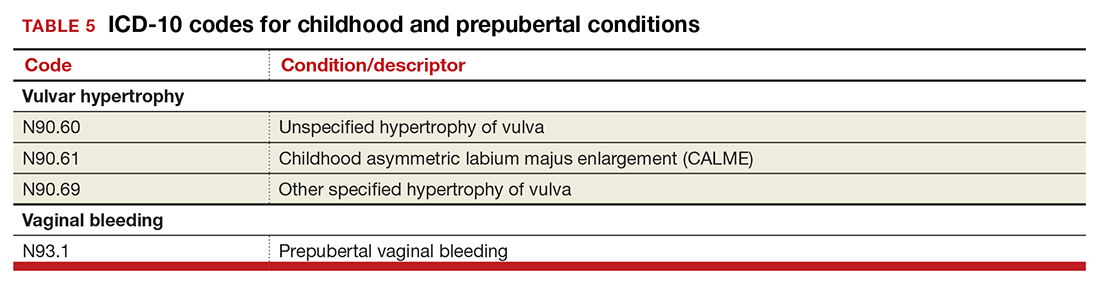
The causes of prepubertal bleeding vary and include the presence of a foreign object, tumors, or fluctuations in hormones, but prepubertal bleeding differs from the bleeding associated with normal menstruation. For that reason, ACOG requested a code that better captures the nature of the condition before a thorough work-up has pinpointed the cause (TABLE 5). Once the cause of the bleeding is known, a more specific diagnosis would then be reported (eg, D28.1, Benign neoplasm of vagina, or T19.2XXA, Foreign body in vulva and vagina, initial encounter).
Dypareunia
Additional documentation for dyspareunia will now be required to ensure that the most specific code is reported. In this case, the clinician should identify whether the pain is superficial or deep to better report on female pelvic floor dysfunction and to support the different treatments based on the location of the pain (TABLE 6). Deep dyspareunia would be felt in the mid or upper vagina.

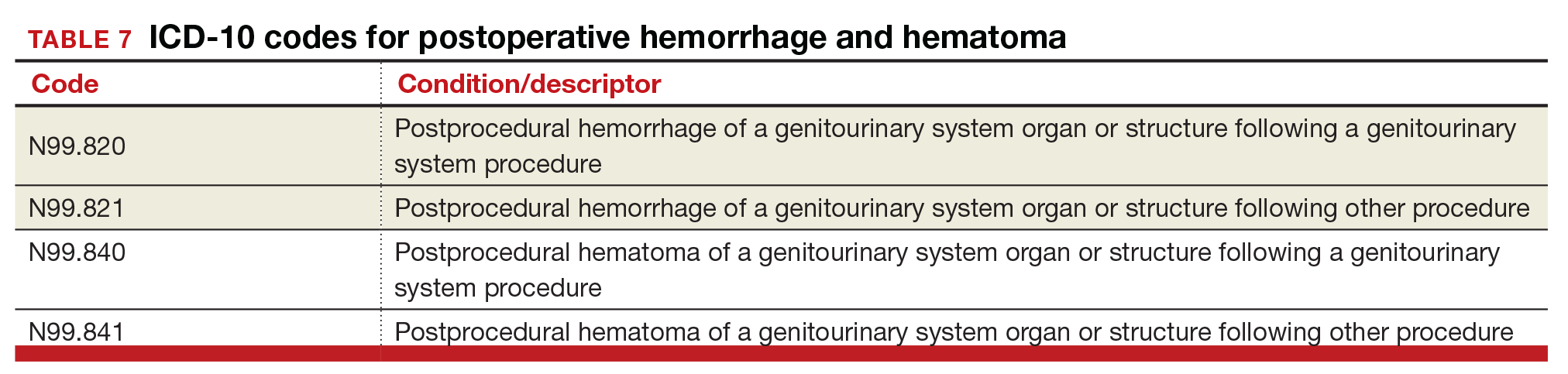
Postoperative hemorrhage and hematoma
The codes for postprocedural hemorrhage and hematomas have received a face-lift: the single codes for these 2 complications will be split so that each can be reported separately (TABLE 7). Note that the new codes require that the condition be found following the initial surgery, and the code selected depends on whether the surgery involved the genitourinary system or another system.
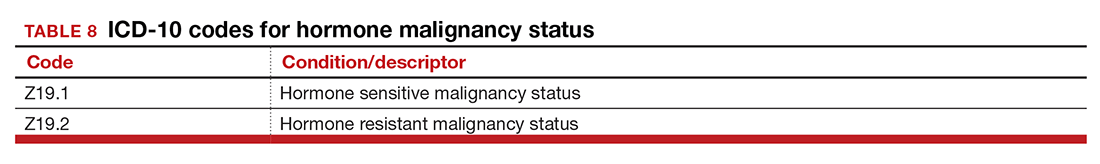
Hormone malignancy status
The new Z19 codes will augment information known about a patient’s neoplasm (TABLE 8). The ICD-10 rule states that the type and location of the neoplasm are always coded first, followed by one of the new Z19 codes, if known.
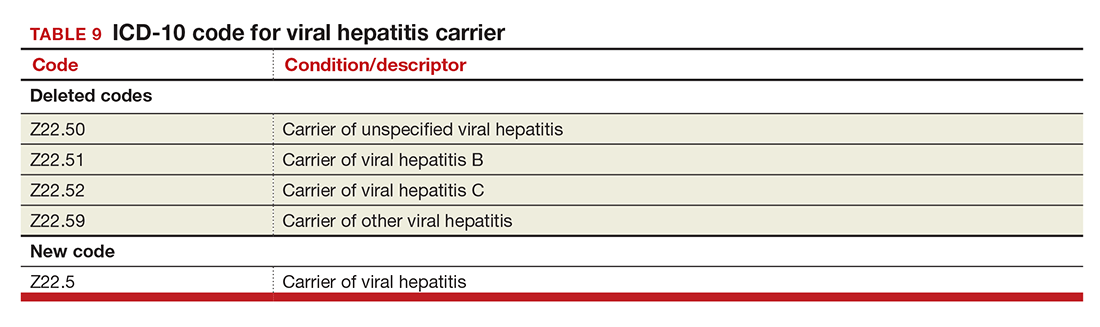
Viral hepatitis carrier
The more specific code for type of viral hepatitis the patient is a carrier of has been bundled into a single code for viral hepatitis (TABLE 9). Carrier status in ICD-10 is defined as a person who harbors the specific organisms of a disease, does not currently have any symptoms, but is capable of transmitting the infection.
Contraception
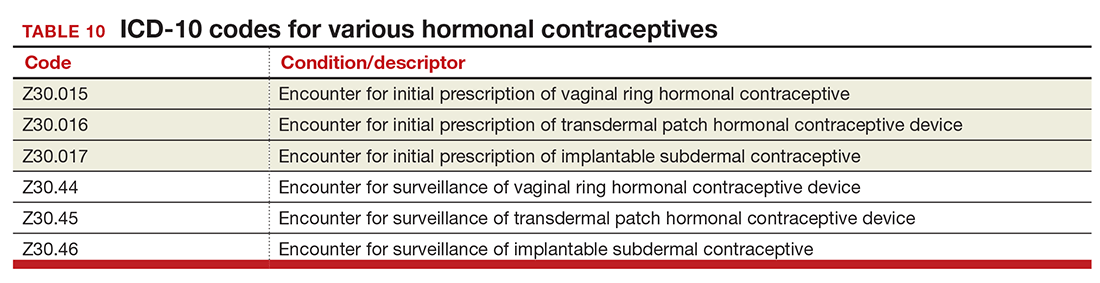
The good news is that the ICD-9 code for contraceptive subdermal implants has been added to ICD-10 coding. In addition, the codes for contraceptive methods have been expanded to also include vaginal rings and transdermal patches (TABLE 10).
Miscellaneous code changes
Counseling a patient prior to pregnancy just got easier with the addition of a code for gestational carriers. Also, the old ICD-9 code that let a payer know that a procedure was converted from a laparoscopic to an open abdominal procedure is back (TABLE 11).

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Centers for Disease Control and Prevention. ICD-10 Coordination and Maintenance Committee Meeting: diagnosis agenda. September 23–24, 2014;28–29. https://www.cdc.gov/nchs/data/icd/topic_packet_09_23_2012.pdf. Accessed August 30, 2016.
- Centers for Disease Control and Prevention. ICD-10 Coordination and Maintenance Committee Meeting: diagnosis agenda. March 18–19, 2015. http://www.cdc.gov/nchs/data/icd/Tentative_Agenda_March%202015_Final.pdf. Accessed August 30, 2016.
In the August 2016 issue of OBG
Urogynecology diagnostic codes
Urogynecologists will find a large number of changes to codes they can select on October 1, 2016. While some codes improve reporting for conditions or symptoms related to urinary issues, many more concern postoperative complications following surgery for devices and grafts applied to the genitourinary system.
The American Urological Association requested new codes to align with a 2009 joint report on the terminology for female pelvic floor dysfunction.1 These codes, along with others, are listed in TABLE 1.

Urinary procedure complication codes
Not every urogynecologist will have an issue after surgery for incontinence, but if they do, there are tons of new and revised codes to address every possible complication the patient may have (TABLE 2). Each of these codes is reported based on whether the complication is being actively treated (initial encounter: final character is A), is being followed up after treatment (subsequent encounter: final character is D), or is caused by another condition (sequela: final character is S).

Gynecology-related diagnostic codes
Laterality
If there are 2 organs in the genitourinary system, the chances are good that there is now a right and a left designation code in ICD-10. Documentation should be clear, of course, and if the condition exists on both the right and the left side (even if only one side is being treated actively), list both codes, as there is no bilateral designation in the codes (TABLE 3). And while there is a code for “unspecified side,” providers normally do know which side, so use of this code should be avoided, if possible.

Vaginal septum
The right and left designations extend to the congenital codes for vaginal septum, but they go one step further. The American Congress of Obstetricians and Gynecologists (ACOG) requested that the codes for longitudinal vaginal septum be expanded to differentiate a nonobstructing vaginal septum from an obstructing vaginal septum, in addition to adding laterality to these codes (TABLE 3).2
Mastitis
There are now 2 new codes for inflammation of the breast; one describes mastitis without abscess, while the other code includes an abscess of the breast and nipple (TABLE 4). Once again, documentation will lead to the most specific code to describe the findings.

Childhood and prepubertal concerns
The single code for hypertrophy of the vulva has been expanded to include asymmetric labium majus enlargement. This code was requested by ACOG because this is a known clinical diagnosis and the currently available vulvar codes are inadequate for capturing this condition. The vulvar enlargement appears to be in response to hormonal surges during prepuberty and early puberty. Adult hypertrophy, either congenital or acquired from childbirth or as a late result of an old injury, would be reported by the “other specified” code (TABLE 5).

The causes of prepubertal bleeding vary and include the presence of a foreign object, tumors, or fluctuations in hormones, but prepubertal bleeding differs from the bleeding associated with normal menstruation. For that reason, ACOG requested a code that better captures the nature of the condition before a thorough work-up has pinpointed the cause (TABLE 5). Once the cause of the bleeding is known, a more specific diagnosis would then be reported (eg, D28.1, Benign neoplasm of vagina, or T19.2XXA, Foreign body in vulva and vagina, initial encounter).
Dypareunia
Additional documentation for dyspareunia will now be required to ensure that the most specific code is reported. In this case, the clinician should identify whether the pain is superficial or deep to better report on female pelvic floor dysfunction and to support the different treatments based on the location of the pain (TABLE 6). Deep dyspareunia would be felt in the mid or upper vagina.

Postoperative hemorrhage and hematoma
The codes for postprocedural hemorrhage and hematomas have received a face-lift: the single codes for these 2 complications will be split so that each can be reported separately (TABLE 7). Note that the new codes require that the condition be found following the initial surgery, and the code selected depends on whether the surgery involved the genitourinary system or another system.

Hormone malignancy status
The new Z19 codes will augment information known about a patient’s neoplasm (TABLE 8). The ICD-10 rule states that the type and location of the neoplasm are always coded first, followed by one of the new Z19 codes, if known.

Viral hepatitis carrier
The more specific code for type of viral hepatitis the patient is a carrier of has been bundled into a single code for viral hepatitis (TABLE 9). Carrier status in ICD-10 is defined as a person who harbors the specific organisms of a disease, does not currently have any symptoms, but is capable of transmitting the infection.

Contraception
The good news is that the ICD-9 code for contraceptive subdermal implants has been added to ICD-10 coding. In addition, the codes for contraceptive methods have been expanded to also include vaginal rings and transdermal patches (TABLE 10).

Miscellaneous code changes
Counseling a patient prior to pregnancy just got easier with the addition of a code for gestational carriers. Also, the old ICD-9 code that let a payer know that a procedure was converted from a laparoscopic to an open abdominal procedure is back (TABLE 11).

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In the August 2016 issue of OBG
Urogynecology diagnostic codes
Urogynecologists will find a large number of changes to codes they can select on October 1, 2016. While some codes improve reporting for conditions or symptoms related to urinary issues, many more concern postoperative complications following surgery for devices and grafts applied to the genitourinary system.
The American Urological Association requested new codes to align with a 2009 joint report on the terminology for female pelvic floor dysfunction.1 These codes, along with others, are listed in TABLE 1.

Urinary procedure complication codes
Not every urogynecologist will have an issue after surgery for incontinence, but if they do, there are tons of new and revised codes to address every possible complication the patient may have (TABLE 2). Each of these codes is reported based on whether the complication is being actively treated (initial encounter: final character is A), is being followed up after treatment (subsequent encounter: final character is D), or is caused by another condition (sequela: final character is S).

Gynecology-related diagnostic codes
Laterality
If there are 2 organs in the genitourinary system, the chances are good that there is now a right and a left designation code in ICD-10. Documentation should be clear, of course, and if the condition exists on both the right and the left side (even if only one side is being treated actively), list both codes, as there is no bilateral designation in the codes (TABLE 3). And while there is a code for “unspecified side,” providers normally do know which side, so use of this code should be avoided, if possible.

Vaginal septum
The right and left designations extend to the congenital codes for vaginal septum, but they go one step further. The American Congress of Obstetricians and Gynecologists (ACOG) requested that the codes for longitudinal vaginal septum be expanded to differentiate a nonobstructing vaginal septum from an obstructing vaginal septum, in addition to adding laterality to these codes (TABLE 3).2
Mastitis
There are now 2 new codes for inflammation of the breast; one describes mastitis without abscess, while the other code includes an abscess of the breast and nipple (TABLE 4). Once again, documentation will lead to the most specific code to describe the findings.

Childhood and prepubertal concerns
The single code for hypertrophy of the vulva has been expanded to include asymmetric labium majus enlargement. This code was requested by ACOG because this is a known clinical diagnosis and the currently available vulvar codes are inadequate for capturing this condition. The vulvar enlargement appears to be in response to hormonal surges during prepuberty and early puberty. Adult hypertrophy, either congenital or acquired from childbirth or as a late result of an old injury, would be reported by the “other specified” code (TABLE 5).

The causes of prepubertal bleeding vary and include the presence of a foreign object, tumors, or fluctuations in hormones, but prepubertal bleeding differs from the bleeding associated with normal menstruation. For that reason, ACOG requested a code that better captures the nature of the condition before a thorough work-up has pinpointed the cause (TABLE 5). Once the cause of the bleeding is known, a more specific diagnosis would then be reported (eg, D28.1, Benign neoplasm of vagina, or T19.2XXA, Foreign body in vulva and vagina, initial encounter).
Dypareunia
Additional documentation for dyspareunia will now be required to ensure that the most specific code is reported. In this case, the clinician should identify whether the pain is superficial or deep to better report on female pelvic floor dysfunction and to support the different treatments based on the location of the pain (TABLE 6). Deep dyspareunia would be felt in the mid or upper vagina.

Postoperative hemorrhage and hematoma
The codes for postprocedural hemorrhage and hematomas have received a face-lift: the single codes for these 2 complications will be split so that each can be reported separately (TABLE 7). Note that the new codes require that the condition be found following the initial surgery, and the code selected depends on whether the surgery involved the genitourinary system or another system.

Hormone malignancy status
The new Z19 codes will augment information known about a patient’s neoplasm (TABLE 8). The ICD-10 rule states that the type and location of the neoplasm are always coded first, followed by one of the new Z19 codes, if known.

Viral hepatitis carrier
The more specific code for type of viral hepatitis the patient is a carrier of has been bundled into a single code for viral hepatitis (TABLE 9). Carrier status in ICD-10 is defined as a person who harbors the specific organisms of a disease, does not currently have any symptoms, but is capable of transmitting the infection.

Contraception
The good news is that the ICD-9 code for contraceptive subdermal implants has been added to ICD-10 coding. In addition, the codes for contraceptive methods have been expanded to also include vaginal rings and transdermal patches (TABLE 10).

Miscellaneous code changes
Counseling a patient prior to pregnancy just got easier with the addition of a code for gestational carriers. Also, the old ICD-9 code that let a payer know that a procedure was converted from a laparoscopic to an open abdominal procedure is back (TABLE 11).

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Centers for Disease Control and Prevention. ICD-10 Coordination and Maintenance Committee Meeting: diagnosis agenda. September 23–24, 2014;28–29. https://www.cdc.gov/nchs/data/icd/topic_packet_09_23_2012.pdf. Accessed August 30, 2016.
- Centers for Disease Control and Prevention. ICD-10 Coordination and Maintenance Committee Meeting: diagnosis agenda. March 18–19, 2015. http://www.cdc.gov/nchs/data/icd/Tentative_Agenda_March%202015_Final.pdf. Accessed August 30, 2016.
- Centers for Disease Control and Prevention. ICD-10 Coordination and Maintenance Committee Meeting: diagnosis agenda. September 23–24, 2014;28–29. https://www.cdc.gov/nchs/data/icd/topic_packet_09_23_2012.pdf. Accessed August 30, 2016.
- Centers for Disease Control and Prevention. ICD-10 Coordination and Maintenance Committee Meeting: diagnosis agenda. March 18–19, 2015. http://www.cdc.gov/nchs/data/icd/Tentative_Agenda_March%202015_Final.pdf. Accessed August 30, 2016.
In this Article
- Urogynecology dx codes
- Gynecology dx codes
- Codes for hormonal contraceptives
Medicare’s revaluation of gastrointestinal endoscopic procedures: Implications for academic and community-based practices
No sentient gastroenterologist has missed the fact that, over the past 3 years, Medicare revalued our endoscopy codes. The impact of those reimbursement changes has been felt both by community gastroenterologists and by those practicing in academic centers. Impacts are different, however, because funds flow, opportunities for ancillary income and compensation formulas all are different for private versus academic physicians. In this month’s Road Ahead column, I have invited leaders from both camps (private practice and academic GI) to describe how reduced procedural reimbursement is affecting their practices. I was impressed and surprised at the level of detail and analysis provided by Dr. Dorn and Dr. Vesy. There are few other sources of financial data that are embedded in real-world experience. We all are concerned about our futures, and this article should spur us into serious discussions about practice strategies going forward. As I wrote in a recent article in Gastroenterology (2016;150:295-9), this is “No Time for WIMPs.”
Gastroenterology practices generate the bulk of their revenue from endoscopic procedures. Over the past decade, the professional fees Medicare pays for these procedures have generally declined. Meanwhile, associated hospital outpatient facility fees have increased while ambulatory surgery center (ASC) fees remain below 2007 levels. This article surveys these changes and examines their significant impact on academic and private gastroenterology practices.
Professional fees for endoscopic procedures
Since 1992 physician professional fees have been linked to the Medicare Physician Fee Schedule, which assigns each service a certain number of relative value units (RVUs). First, the work RVU (wRVU) is based on the estimated physician time, mental effort, technical skill, and psychological stress required to provide a service. Second, a practice expense RVU (PE RVU) reflects the direct and indirect costs of providing the service. For procedures performed in office-based settings the PE RVU includes rent, nonclinician staff, equipment, and supplies, on average amounting to 44% of the total RVU. For procedures performed in hospital outpatient departments (HOPDs) and ASCs, the PE RVU is much lower, because most costs are incurred by the facility (which receives a separate facility fee), rather than the physician practice. Third, a small proportion of the overall RVU is linked to malpractice insurance costs (MP RVU). The RVU components are geographically adjusted, combined, and then multiplied by a conversion factor (CF, which in 2016 is $35.80) to determine actual Medicare payment (Payment = [wRVU + PE RVU + MP RVU] × CF).1
To address potential distortions in this physician fee schedule, The Affordable Care Act directed the Secretary of Health and Human Services to establish a formal process to review potentially misvalued procedure codes. During 2012 and 2014, multiple gastroenterological and surgical societies surveyed practicing physicians on the physician work, time, and intensity required to perform more than 120 services in question, including esophagoscopy, esophagogastroduodenoscopy, endoscopic retrograde cholangiopancreatography, flexible sigmoidoscopy and ileoscopy, pouchoscopy, and colonoscopy. At the same time, these societies assembled an expert panel of practicing physicians to determine the practice expenses associated with these procedures. The societies analyzed the results and presented recommendations to the American Medical Association/Specialty Society Relative Value Scale Update Committee, which, in turn, presented their recommendations to the Centers for Medicare and Medicaid Services.2 In 2014, CMS accepted approximately three-quarters of the Relative Value Scale Update Committee’s recommendations, ultimately decreasing wRVUs, increasing PE RVUs for procedures performed in office-based settings, and leaving MP RVUs unchanged for most upper endoscopy and endoscopic retrograde cholangiopancreatography procedures. These changes translated into significant 2015 payment reductions for esophagoscopy and esophagogastroduodenoscopy (4%-42%), endoscopic ultrasound (10%-35%), and endoscopic retrograde cholangiopancreatography (0%-37%) performed within facilities, with less effect for those performed in office-based settings. At that time, “in light of the substantial nature of [the colonoscopy] code revision and its relationship to the policies on moderate sedation,” CMS delayed revaluation of the lower gastrointestinal (GI) endoscopy codes.3 This reprieve is now over: The 2016 Medicare Physician Fee Schedule Final Rule includes up to 17% cuts (12% on average) to the wRVU associated with these lower GI procedures (Table 1). For office-based procedures (but not facility-based procedures) these wRVU cuts are buffered (and sometimes offset) by general increases in PE RVUs.
Facility fees for endoscopic procedures
Compared with the small percentage of endoscopic procedures that are performed in office-based settings, those performed in HOPDs and ASCs entail a lower professional fee plus a separate facility fee. Since 2000 CMS has paid for services provided in HOPDs using the outpatient prospective payment system (OPPS). Clinical services are first classified into ambulatory payment classifications (APC) on the basis of clinical and cost similarity. Next, services within an APC are assigned a single relative payment rate, which is linked to the resources required to perform the service. The APC payment rate is geographically adjusted and then multiplied by a CF to determine an actual dollar amount.4
Since 2008, CMS has used a nearly identical mechanism to pay for facility services provided in ASCs. Services are classified using the same APCs and same relative weights as the OPPS. The difference is that the ASC CF is less than the OPPS CF (the 2016 ASC CF is 58% of the OPPS CF), translating into lower dollar payments for ASC services.5 Of note, in 2008 ASC rates were cut significantly when CMS adopted this methodology for determining ASC facility fees (previously, ASC rates were approximately 85% of HOPD rates).
CMS reviews the APCs and their relative weights annually, and may adjust how specific services are classified and how APCs are weighed. Since 2006, HOPD rates for the 10 common procedures listed in Table 1 have increased by 26%-93% real (i.e., Consumer Price Index–adjusted) dollars. Meanwhile, given the steep 2008 ASC fee cuts the 2016 ASC fees are still significantly lower than they were 1 decade ago, especially when accounting for inflation. In fact, ASC fees for the most common procedures have decreased by 20% in real dollars (Supplementary Tables 1 and 2 at http://dx.doi.org/10.1016/j.cgh.2016.03.032).
Putting these changes in context
It is important to consider these changes within a broader context. First, the full economic impact of these changes on an individual gastroenterology practice depends on where it performs its services and whether it collects associated facility fees, and fees for anesthesia and pathology services. Second, the Medicare population is growing by more than 10,000 people each day.6 Third, beyond Medicare, most commercial insurers peg their reimbursement rates to a percentage of the Medicare Fee Schedule. Although the details of specific contracts vary, gastroenterologists should expect to see commercial rates move in a similar direction within the next 1–2 years. Fourth, in the 2016 Fee Schedule CMS described its future intention to remove the value of moderate sedation from all GI procedures valued with moderate sedation inherent to the procedure. The more that moderate sedation is ultimately valued, the less that endoscopic procedures will be valued. Consequently, gastroenterologists who rely on anesthesiology professionals to sedate their patients will receive less revenue per procedure. Finally, the 2015 Medicare Access and Children’s Health Insurance Program Reauthorization Act (MACRA) links a higher proportion of these dwindling fee-for-service payments to performance. Starting in 2018, physicians who elect to remain on a fee-for-service tract will receive a composite Merit-Based Incentive Payment System (MIPS) score that will translate into either performance bonuses or penalties (as much as 4% in 2019 and going up to 9% in 2022). Alternatively, providers who “sufficiently” participate in “two-sided” (i.e., risk-bearing) alternative payment models (e.g., bundled payments and accountable care organizations) instead of fee-for-service payments will receive 5% across-the-board bonuses. In sum, it is possible that fee-for-service payments may eventually become so unattractive that many gastroenterologists decide (or hope) to move to alternative payment models that combine both professional and facility fees, such as the CMS now-mandatory Comprehensive Care for Joint Replacement (CJR) program.7
Impact on academic practices
For most academic gastroenterology practices, clinical revenue far exceeds all other sources of funding, including research grants, teaching support, philanthropy, and partnerships with industry. Although a select few academic GI divisions have managed to build their own ASCs to share facility fees, for most academic practices, clinical revenue comes solely from the professional fees for endoscopic procedures and, less so, from office and hospital-based consultations and management of established patients.8 Thus, steep cuts to professional fees for endoscopic procedures, the leading source of overall revenue, will severely strain most academic gastroenterology practices.
In one of our practices (SDD), the 10 common procedures listed in Table 1 accounted for 27% of total direct clinical revenue over the past year. Roughly one-fourth of our patients are Medicare beneficiaries. Assuming no change in volume, the 2016 fee schedule cuts will amount to a 0.9% drop in direct clinical revenue. If all other payers follow with identical cuts, then direct clinical revenue will drop by 3.7%. Although our practice is fortunate to have other well-developed clinical and nonclinical revenue streams, these cuts are not insignificant.
How can academic practices continue to provide accessible clinical care in the face of these fee cuts? It can help to first consider how revenue is distributed for a Medicare beneficiary who undergoes a diagnostic colonoscopy with biopsy to evaluate unexplained diarrhea in an academic medical center’s hospital-based facility. The academic gastroenterologist receives $212 (CPT 45380). From this $212, the academic gastroenterology practice must pay assessments to various entities (sometimes including the school of medicine, department of medicine, and faculty practice plan) that may amount to more than 30% of total revenue. The roughly $150 that remains is used to pay faculty member salaries (the median salary for an academic gastroenterologist is $300,009) and benefits (estimated at $74,000/year); MP insurance (estimated at $2,275/year, higher in certain parts of the United States); and overhead for support staff, supplies, and other expenses (estimated at $50,550/year). Thus, a purely clinical academic gastroenterologist who is paid at the Medical Group Management Association (MGMA) median must generate $610,179 in preassessment revenue. If this hypothetical academic gastroenterologist solely treated Medicare beneficiaries, at $35.82 per RVU he or she would need to generate a staggering 17,035 RVUs per year, an amount that far exceeds the MGMA median (6,445) and 90th percentile (10,991) for academic gastroenterologists. Of course, real-world academic gastroenterologists also treat commercially insured patients and many spend time on nonclinical activities (although clinical income typically supports time devoted to research and teaching, not vice versa). Still, this example highlights a clear fact: Academic gastroenterology practices take a major financial loss delivering services to Medicare beneficiaries. Meanwhile, the HOPD charges more than $2,200 for the procedure. Although it receives $752 facility fee from Medicare (Disproportionate Share Hospitals and NCI Cancer Centers receive more), with a cost-to-charge ratio of roughly 0.2, revenue still clearly exceeds expenses. Finally, the anesthesia professional (if any) receives roughly $198 (CPT 00810) and the pathologist $74 (CPT 88305).
Although some may argue that academic gastroenterologists should simply accept a pay cut, lower salaries may drive many away from academia. To hire enough new (or even maintain enough existing) faculty members to maintain and grow volume, academic gastroenterology practices must find ways to supplement declining professional fees. One option is for academic practices to open their own ASCs, either alone or jointly with their health care system. But this assumes, often erroneously, that the health care system is willing to share facility fees. A second option is to develop incentive programs that transfer revenue from the health care system to the physicians. Importantly, these must be at fair market value and for nonemployed physicians cannot be linked to volume.10 Examples include medical directorships and non–volume-based performance bonuses. A third option is to consider alternative payment models, such as bundled payments that include a single lump sum payment for both professional and facility fees. The practice and health system then negotiates how the bundle is shared. Bundles should motivate hospitals and academic practices to work together to improve care and reduce overall expenses. Along these lines, CMS recently announced the CJR program under which hospitals and physicians in 75 locations will be required to participate. Although AGA recently published a bundled payment framework for screening and surveillance colonoscopy,11 bundles for other endoscopic procedures remain to be defined. But, a clearly defined, attractively priced, and skillfully negotiated bundle could be a means for redistributing revenue in ways that are more favorable to academic practices. No matter the approach, it is critical for academic gastroenterology practices and their health care systems to align their goals and integrate their services. But this is easier said than done.
Impact on private gastroenterology practices
The impact of these professional and facility fee changes on private practice depends on the practice’s payer mix, and whether it owns an ASC and directly provides anesthesia and pathology services. Consider Texas Digestive Disease Consultants (TDDC), an 80-gastroenterologist practice that provides GI care and endoscopic procedures throughout north and central Texas. TDDC revenue comes solely from professional fees generated by gastroenterologists and the pathologists that the practice employs. TDDC operating expenses include employee salaries and benefits, rent, and taxes. Based on an expected work-year of 2,080 hours, each TDDC gastroenterologist must generate $219 per hour to cover practice expenses. Physicians receive income only after practice revenue exceeds $219 per hour.
In the 2016 Medicare Physician Fee Schedule, colonoscopy code 45387 is assigned 3.36 RVUs, based on 67 minutes in total time. Based on this information, 2016 CMS payment for a screening colonoscopy ($200) will result in a loss of $45 in practice revenue over expenses. Across the TDDC practices in 2015, CMS patients represented 29% of all colonoscopies performed, but only 11% of revenue from those procedures. In aggregate, assuming no change in volume, the 2016 fee schedule cuts will translate into $5,472 less physician income for each gastroenterologist. If all other payers follow with identical cuts then each gastroenterologist would forfeit $50,896 of income.
For pathology services, code 88305 reimburses a global service per specimen. The average number of specimens per outpatient GI procedure at TDDC is 1.5. Based on CMS’ per specimen payment of $73 (down from $107 in 2012), average pathology reimbursement per a CMS endoscopic procedure is $110.
What about facility fees? Many TDDC physicians separately own and operate ASCs and anesthesia practices. Because the physician owners of these facilities funded, developed, and operate these facilities on their own, any revenue over expenses from these facilities flow directly to the ASC, rather than to TDDC. Since 2008, when ASC payments were severely reduced, ASC revenue over expenses for a Medicare colonoscopy has ranged from $0 to $70. For anesthesia services, the ASC receives approximately $159 per screening colonoscopy, which is not enough to actually cover the costs of providing anesthesia services. In sum, when TDDC gastroenterologists perform colonoscopies on Medicare patients at an HOPD the physician loses money. When the same procedure is performed at an ASC the physician barely break even.
How can private practice gastroenterologists respond to these fee cuts? First, some gastroenterologists may be forced to accept lower salaries. Second, practices can offset fee cuts by improving efficiency and reducing overall cost of care, assuming they are not already maximally efficient. Third, some private practices can increase Medicare professional payments by reclassifying as nonparticipating with Medicare. Nonparticipating status allows practices to charge the patient up to approximately 109.25% of the Medicare approved rate, with the patient submitting the claim directly to Medicare (“balance billing”). The downside is that patients shoulder the additional cost and may potentially decide to seek care elsewhere. Fourth, private practices should explore alternative payment models, such as bundled payment as described previously, either on their own or by partnering with local health systems to share risk. Finally, some practices may sell equity to and become employees of local health systems.
Conclusions
Changes to Medicare professional and facility fee payments for endoscopic procedures significantly affect academic and private gastroenterology practices. Dwindling professional fees – alongside increasing HOPD facility fees – make academic gastroenterology practices increasingly reliant on support from their parent health care systems. Like academic practices, private gastroenterology practices experience financial losses when treating Medicare beneficiaries. Academic and private gastroenterology practices should consider several potential responses. Although beyond the scope of this article, all practices must continuously strive to improve the quality and reduce the costs of the endoscopic procedures they perform.
Acknowledgments
The authors thank Leslie Narramore, MPA, for her insights and help with reimbursement data. They also thank Bryan Rhodes for providing financial data and Shivan Mehta, MD, MBA, for his feedback on the Relative Value Scale Update Committee process.
References
1. MEDPAC. Payment basics: physician and other health professional payment system. Medicare Payment Advisory Commission, Washington, DC; 2015.
2. Mehta, S.J., Brill, J.V. What is the RUC and how does it impact gastroenterology?. Clin Gastroenterol Hepatol. 2014;12:1208-11.
3. American College of Gastroenterology, American Gastroenterological Association, American Society for Gastrointestinal Endoscopy. Key Provisions in the CY 2015 Medicare Physician Fee Schedule Final Rule, 2015. Available at: http://gi.org/wp-content/uploads/2014/11/Tri-Soc_CY15_MPFS_Final_Rule_Summary.pdf.
4. MEDPAC. Payment basics: Outpatient hospital services payment system. Medicare Payment Advisory Commission, Washington, DC; 2015.
5. MEDPAC. Payment basics: Ambulatory surgery center services payment system. Medicare Payment Advisory Commission, Washington, DC; 2015.
6. Diamond, D. 10,000 people are now enrolling in Medicare – every day. Forbes; 2015 (Available at: http://www.forbes.com/sites/dandiamond/2015/07/13/aging-in-america-10000-people-enroll-in-medicare-every-day/#5177f1f35e07. Accessed January 3, 2016)
7. Mechanic, R.E. Mandatory Medicare bundled payment: Is it ready for prime time?. N Engl J Med. 2015;373:1291-3.
8. Rustgi, A.K., Allen, J.I. The house of gastrointestinal medicine: How academic medical centers can build a sustainable economic clinical model. Clin Gastroenterol Hepatol. 2013;11:1370-3.
9. Academic Practice Compensation and Production Report. Medical Group Management Association, Englewood, Colo.; 2015.
10. Becker S., Townshend G., Carnell H., et al. Physician compensation: 10 core legal and regulatory concepts. Becker’s Hospital Review Available at: http://www.beckershospitalreview.com/legal-regulatory-issues/physician-compensation-10-core-legal-and-regulatory-concepts.html. Accessed Jan. 20, 2016.
11. Brill, J.V., Jain, R., Margolis, P.S., et al. A bundled payment framework for colonoscopy performed for colorectal cancer screening or surveillance. Gastroenterology. 2014;146:849-53, e9.
Dr. Dorn is vice chief, division of gastroenterology and hepatology, associate professor of medicine, health policy & management, University of North Carolina at Chapel Hill; he has received honoraria for consulting and presentations on health reform from AbbVie and Olympus. Dr. Vesy is a gastroenterologist affiliated with Baylor University Medical Center, Dallas.
No sentient gastroenterologist has missed the fact that, over the past 3 years, Medicare revalued our endoscopy codes. The impact of those reimbursement changes has been felt both by community gastroenterologists and by those practicing in academic centers. Impacts are different, however, because funds flow, opportunities for ancillary income and compensation formulas all are different for private versus academic physicians. In this month’s Road Ahead column, I have invited leaders from both camps (private practice and academic GI) to describe how reduced procedural reimbursement is affecting their practices. I was impressed and surprised at the level of detail and analysis provided by Dr. Dorn and Dr. Vesy. There are few other sources of financial data that are embedded in real-world experience. We all are concerned about our futures, and this article should spur us into serious discussions about practice strategies going forward. As I wrote in a recent article in Gastroenterology (2016;150:295-9), this is “No Time for WIMPs.”
Gastroenterology practices generate the bulk of their revenue from endoscopic procedures. Over the past decade, the professional fees Medicare pays for these procedures have generally declined. Meanwhile, associated hospital outpatient facility fees have increased while ambulatory surgery center (ASC) fees remain below 2007 levels. This article surveys these changes and examines their significant impact on academic and private gastroenterology practices.
Professional fees for endoscopic procedures
Since 1992 physician professional fees have been linked to the Medicare Physician Fee Schedule, which assigns each service a certain number of relative value units (RVUs). First, the work RVU (wRVU) is based on the estimated physician time, mental effort, technical skill, and psychological stress required to provide a service. Second, a practice expense RVU (PE RVU) reflects the direct and indirect costs of providing the service. For procedures performed in office-based settings the PE RVU includes rent, nonclinician staff, equipment, and supplies, on average amounting to 44% of the total RVU. For procedures performed in hospital outpatient departments (HOPDs) and ASCs, the PE RVU is much lower, because most costs are incurred by the facility (which receives a separate facility fee), rather than the physician practice. Third, a small proportion of the overall RVU is linked to malpractice insurance costs (MP RVU). The RVU components are geographically adjusted, combined, and then multiplied by a conversion factor (CF, which in 2016 is $35.80) to determine actual Medicare payment (Payment = [wRVU + PE RVU + MP RVU] × CF).1
To address potential distortions in this physician fee schedule, The Affordable Care Act directed the Secretary of Health and Human Services to establish a formal process to review potentially misvalued procedure codes. During 2012 and 2014, multiple gastroenterological and surgical societies surveyed practicing physicians on the physician work, time, and intensity required to perform more than 120 services in question, including esophagoscopy, esophagogastroduodenoscopy, endoscopic retrograde cholangiopancreatography, flexible sigmoidoscopy and ileoscopy, pouchoscopy, and colonoscopy. At the same time, these societies assembled an expert panel of practicing physicians to determine the practice expenses associated with these procedures. The societies analyzed the results and presented recommendations to the American Medical Association/Specialty Society Relative Value Scale Update Committee, which, in turn, presented their recommendations to the Centers for Medicare and Medicaid Services.2 In 2014, CMS accepted approximately three-quarters of the Relative Value Scale Update Committee’s recommendations, ultimately decreasing wRVUs, increasing PE RVUs for procedures performed in office-based settings, and leaving MP RVUs unchanged for most upper endoscopy and endoscopic retrograde cholangiopancreatography procedures. These changes translated into significant 2015 payment reductions for esophagoscopy and esophagogastroduodenoscopy (4%-42%), endoscopic ultrasound (10%-35%), and endoscopic retrograde cholangiopancreatography (0%-37%) performed within facilities, with less effect for those performed in office-based settings. At that time, “in light of the substantial nature of [the colonoscopy] code revision and its relationship to the policies on moderate sedation,” CMS delayed revaluation of the lower gastrointestinal (GI) endoscopy codes.3 This reprieve is now over: The 2016 Medicare Physician Fee Schedule Final Rule includes up to 17% cuts (12% on average) to the wRVU associated with these lower GI procedures (Table 1). For office-based procedures (but not facility-based procedures) these wRVU cuts are buffered (and sometimes offset) by general increases in PE RVUs.
Facility fees for endoscopic procedures
Compared with the small percentage of endoscopic procedures that are performed in office-based settings, those performed in HOPDs and ASCs entail a lower professional fee plus a separate facility fee. Since 2000 CMS has paid for services provided in HOPDs using the outpatient prospective payment system (OPPS). Clinical services are first classified into ambulatory payment classifications (APC) on the basis of clinical and cost similarity. Next, services within an APC are assigned a single relative payment rate, which is linked to the resources required to perform the service. The APC payment rate is geographically adjusted and then multiplied by a CF to determine an actual dollar amount.4
Since 2008, CMS has used a nearly identical mechanism to pay for facility services provided in ASCs. Services are classified using the same APCs and same relative weights as the OPPS. The difference is that the ASC CF is less than the OPPS CF (the 2016 ASC CF is 58% of the OPPS CF), translating into lower dollar payments for ASC services.5 Of note, in 2008 ASC rates were cut significantly when CMS adopted this methodology for determining ASC facility fees (previously, ASC rates were approximately 85% of HOPD rates).
CMS reviews the APCs and their relative weights annually, and may adjust how specific services are classified and how APCs are weighed. Since 2006, HOPD rates for the 10 common procedures listed in Table 1 have increased by 26%-93% real (i.e., Consumer Price Index–adjusted) dollars. Meanwhile, given the steep 2008 ASC fee cuts the 2016 ASC fees are still significantly lower than they were 1 decade ago, especially when accounting for inflation. In fact, ASC fees for the most common procedures have decreased by 20% in real dollars (Supplementary Tables 1 and 2 at http://dx.doi.org/10.1016/j.cgh.2016.03.032).
Putting these changes in context
It is important to consider these changes within a broader context. First, the full economic impact of these changes on an individual gastroenterology practice depends on where it performs its services and whether it collects associated facility fees, and fees for anesthesia and pathology services. Second, the Medicare population is growing by more than 10,000 people each day.6 Third, beyond Medicare, most commercial insurers peg their reimbursement rates to a percentage of the Medicare Fee Schedule. Although the details of specific contracts vary, gastroenterologists should expect to see commercial rates move in a similar direction within the next 1–2 years. Fourth, in the 2016 Fee Schedule CMS described its future intention to remove the value of moderate sedation from all GI procedures valued with moderate sedation inherent to the procedure. The more that moderate sedation is ultimately valued, the less that endoscopic procedures will be valued. Consequently, gastroenterologists who rely on anesthesiology professionals to sedate their patients will receive less revenue per procedure. Finally, the 2015 Medicare Access and Children’s Health Insurance Program Reauthorization Act (MACRA) links a higher proportion of these dwindling fee-for-service payments to performance. Starting in 2018, physicians who elect to remain on a fee-for-service tract will receive a composite Merit-Based Incentive Payment System (MIPS) score that will translate into either performance bonuses or penalties (as much as 4% in 2019 and going up to 9% in 2022). Alternatively, providers who “sufficiently” participate in “two-sided” (i.e., risk-bearing) alternative payment models (e.g., bundled payments and accountable care organizations) instead of fee-for-service payments will receive 5% across-the-board bonuses. In sum, it is possible that fee-for-service payments may eventually become so unattractive that many gastroenterologists decide (or hope) to move to alternative payment models that combine both professional and facility fees, such as the CMS now-mandatory Comprehensive Care for Joint Replacement (CJR) program.7
Impact on academic practices
For most academic gastroenterology practices, clinical revenue far exceeds all other sources of funding, including research grants, teaching support, philanthropy, and partnerships with industry. Although a select few academic GI divisions have managed to build their own ASCs to share facility fees, for most academic practices, clinical revenue comes solely from the professional fees for endoscopic procedures and, less so, from office and hospital-based consultations and management of established patients.8 Thus, steep cuts to professional fees for endoscopic procedures, the leading source of overall revenue, will severely strain most academic gastroenterology practices.
In one of our practices (SDD), the 10 common procedures listed in Table 1 accounted for 27% of total direct clinical revenue over the past year. Roughly one-fourth of our patients are Medicare beneficiaries. Assuming no change in volume, the 2016 fee schedule cuts will amount to a 0.9% drop in direct clinical revenue. If all other payers follow with identical cuts, then direct clinical revenue will drop by 3.7%. Although our practice is fortunate to have other well-developed clinical and nonclinical revenue streams, these cuts are not insignificant.
How can academic practices continue to provide accessible clinical care in the face of these fee cuts? It can help to first consider how revenue is distributed for a Medicare beneficiary who undergoes a diagnostic colonoscopy with biopsy to evaluate unexplained diarrhea in an academic medical center’s hospital-based facility. The academic gastroenterologist receives $212 (CPT 45380). From this $212, the academic gastroenterology practice must pay assessments to various entities (sometimes including the school of medicine, department of medicine, and faculty practice plan) that may amount to more than 30% of total revenue. The roughly $150 that remains is used to pay faculty member salaries (the median salary for an academic gastroenterologist is $300,009) and benefits (estimated at $74,000/year); MP insurance (estimated at $2,275/year, higher in certain parts of the United States); and overhead for support staff, supplies, and other expenses (estimated at $50,550/year). Thus, a purely clinical academic gastroenterologist who is paid at the Medical Group Management Association (MGMA) median must generate $610,179 in preassessment revenue. If this hypothetical academic gastroenterologist solely treated Medicare beneficiaries, at $35.82 per RVU he or she would need to generate a staggering 17,035 RVUs per year, an amount that far exceeds the MGMA median (6,445) and 90th percentile (10,991) for academic gastroenterologists. Of course, real-world academic gastroenterologists also treat commercially insured patients and many spend time on nonclinical activities (although clinical income typically supports time devoted to research and teaching, not vice versa). Still, this example highlights a clear fact: Academic gastroenterology practices take a major financial loss delivering services to Medicare beneficiaries. Meanwhile, the HOPD charges more than $2,200 for the procedure. Although it receives $752 facility fee from Medicare (Disproportionate Share Hospitals and NCI Cancer Centers receive more), with a cost-to-charge ratio of roughly 0.2, revenue still clearly exceeds expenses. Finally, the anesthesia professional (if any) receives roughly $198 (CPT 00810) and the pathologist $74 (CPT 88305).
Although some may argue that academic gastroenterologists should simply accept a pay cut, lower salaries may drive many away from academia. To hire enough new (or even maintain enough existing) faculty members to maintain and grow volume, academic gastroenterology practices must find ways to supplement declining professional fees. One option is for academic practices to open their own ASCs, either alone or jointly with their health care system. But this assumes, often erroneously, that the health care system is willing to share facility fees. A second option is to develop incentive programs that transfer revenue from the health care system to the physicians. Importantly, these must be at fair market value and for nonemployed physicians cannot be linked to volume.10 Examples include medical directorships and non–volume-based performance bonuses. A third option is to consider alternative payment models, such as bundled payments that include a single lump sum payment for both professional and facility fees. The practice and health system then negotiates how the bundle is shared. Bundles should motivate hospitals and academic practices to work together to improve care and reduce overall expenses. Along these lines, CMS recently announced the CJR program under which hospitals and physicians in 75 locations will be required to participate. Although AGA recently published a bundled payment framework for screening and surveillance colonoscopy,11 bundles for other endoscopic procedures remain to be defined. But, a clearly defined, attractively priced, and skillfully negotiated bundle could be a means for redistributing revenue in ways that are more favorable to academic practices. No matter the approach, it is critical for academic gastroenterology practices and their health care systems to align their goals and integrate their services. But this is easier said than done.
Impact on private gastroenterology practices
The impact of these professional and facility fee changes on private practice depends on the practice’s payer mix, and whether it owns an ASC and directly provides anesthesia and pathology services. Consider Texas Digestive Disease Consultants (TDDC), an 80-gastroenterologist practice that provides GI care and endoscopic procedures throughout north and central Texas. TDDC revenue comes solely from professional fees generated by gastroenterologists and the pathologists that the practice employs. TDDC operating expenses include employee salaries and benefits, rent, and taxes. Based on an expected work-year of 2,080 hours, each TDDC gastroenterologist must generate $219 per hour to cover practice expenses. Physicians receive income only after practice revenue exceeds $219 per hour.
In the 2016 Medicare Physician Fee Schedule, colonoscopy code 45387 is assigned 3.36 RVUs, based on 67 minutes in total time. Based on this information, 2016 CMS payment for a screening colonoscopy ($200) will result in a loss of $45 in practice revenue over expenses. Across the TDDC practices in 2015, CMS patients represented 29% of all colonoscopies performed, but only 11% of revenue from those procedures. In aggregate, assuming no change in volume, the 2016 fee schedule cuts will translate into $5,472 less physician income for each gastroenterologist. If all other payers follow with identical cuts then each gastroenterologist would forfeit $50,896 of income.
For pathology services, code 88305 reimburses a global service per specimen. The average number of specimens per outpatient GI procedure at TDDC is 1.5. Based on CMS’ per specimen payment of $73 (down from $107 in 2012), average pathology reimbursement per a CMS endoscopic procedure is $110.
What about facility fees? Many TDDC physicians separately own and operate ASCs and anesthesia practices. Because the physician owners of these facilities funded, developed, and operate these facilities on their own, any revenue over expenses from these facilities flow directly to the ASC, rather than to TDDC. Since 2008, when ASC payments were severely reduced, ASC revenue over expenses for a Medicare colonoscopy has ranged from $0 to $70. For anesthesia services, the ASC receives approximately $159 per screening colonoscopy, which is not enough to actually cover the costs of providing anesthesia services. In sum, when TDDC gastroenterologists perform colonoscopies on Medicare patients at an HOPD the physician loses money. When the same procedure is performed at an ASC the physician barely break even.
How can private practice gastroenterologists respond to these fee cuts? First, some gastroenterologists may be forced to accept lower salaries. Second, practices can offset fee cuts by improving efficiency and reducing overall cost of care, assuming they are not already maximally efficient. Third, some private practices can increase Medicare professional payments by reclassifying as nonparticipating with Medicare. Nonparticipating status allows practices to charge the patient up to approximately 109.25% of the Medicare approved rate, with the patient submitting the claim directly to Medicare (“balance billing”). The downside is that patients shoulder the additional cost and may potentially decide to seek care elsewhere. Fourth, private practices should explore alternative payment models, such as bundled payment as described previously, either on their own or by partnering with local health systems to share risk. Finally, some practices may sell equity to and become employees of local health systems.
Conclusions
Changes to Medicare professional and facility fee payments for endoscopic procedures significantly affect academic and private gastroenterology practices. Dwindling professional fees – alongside increasing HOPD facility fees – make academic gastroenterology practices increasingly reliant on support from their parent health care systems. Like academic practices, private gastroenterology practices experience financial losses when treating Medicare beneficiaries. Academic and private gastroenterology practices should consider several potential responses. Although beyond the scope of this article, all practices must continuously strive to improve the quality and reduce the costs of the endoscopic procedures they perform.
Acknowledgments
The authors thank Leslie Narramore, MPA, for her insights and help with reimbursement data. They also thank Bryan Rhodes for providing financial data and Shivan Mehta, MD, MBA, for his feedback on the Relative Value Scale Update Committee process.
References
1. MEDPAC. Payment basics: physician and other health professional payment system. Medicare Payment Advisory Commission, Washington, DC; 2015.
2. Mehta, S.J., Brill, J.V. What is the RUC and how does it impact gastroenterology?. Clin Gastroenterol Hepatol. 2014;12:1208-11.
3. American College of Gastroenterology, American Gastroenterological Association, American Society for Gastrointestinal Endoscopy. Key Provisions in the CY 2015 Medicare Physician Fee Schedule Final Rule, 2015. Available at: http://gi.org/wp-content/uploads/2014/11/Tri-Soc_CY15_MPFS_Final_Rule_Summary.pdf.
4. MEDPAC. Payment basics: Outpatient hospital services payment system. Medicare Payment Advisory Commission, Washington, DC; 2015.
5. MEDPAC. Payment basics: Ambulatory surgery center services payment system. Medicare Payment Advisory Commission, Washington, DC; 2015.
6. Diamond, D. 10,000 people are now enrolling in Medicare – every day. Forbes; 2015 (Available at: http://www.forbes.com/sites/dandiamond/2015/07/13/aging-in-america-10000-people-enroll-in-medicare-every-day/#5177f1f35e07. Accessed January 3, 2016)
7. Mechanic, R.E. Mandatory Medicare bundled payment: Is it ready for prime time?. N Engl J Med. 2015;373:1291-3.
8. Rustgi, A.K., Allen, J.I. The house of gastrointestinal medicine: How academic medical centers can build a sustainable economic clinical model. Clin Gastroenterol Hepatol. 2013;11:1370-3.
9. Academic Practice Compensation and Production Report. Medical Group Management Association, Englewood, Colo.; 2015.
10. Becker S., Townshend G., Carnell H., et al. Physician compensation: 10 core legal and regulatory concepts. Becker’s Hospital Review Available at: http://www.beckershospitalreview.com/legal-regulatory-issues/physician-compensation-10-core-legal-and-regulatory-concepts.html. Accessed Jan. 20, 2016.
11. Brill, J.V., Jain, R., Margolis, P.S., et al. A bundled payment framework for colonoscopy performed for colorectal cancer screening or surveillance. Gastroenterology. 2014;146:849-53, e9.
Dr. Dorn is vice chief, division of gastroenterology and hepatology, associate professor of medicine, health policy & management, University of North Carolina at Chapel Hill; he has received honoraria for consulting and presentations on health reform from AbbVie and Olympus. Dr. Vesy is a gastroenterologist affiliated with Baylor University Medical Center, Dallas.
No sentient gastroenterologist has missed the fact that, over the past 3 years, Medicare revalued our endoscopy codes. The impact of those reimbursement changes has been felt both by community gastroenterologists and by those practicing in academic centers. Impacts are different, however, because funds flow, opportunities for ancillary income and compensation formulas all are different for private versus academic physicians. In this month’s Road Ahead column, I have invited leaders from both camps (private practice and academic GI) to describe how reduced procedural reimbursement is affecting their practices. I was impressed and surprised at the level of detail and analysis provided by Dr. Dorn and Dr. Vesy. There are few other sources of financial data that are embedded in real-world experience. We all are concerned about our futures, and this article should spur us into serious discussions about practice strategies going forward. As I wrote in a recent article in Gastroenterology (2016;150:295-9), this is “No Time for WIMPs.”
Gastroenterology practices generate the bulk of their revenue from endoscopic procedures. Over the past decade, the professional fees Medicare pays for these procedures have generally declined. Meanwhile, associated hospital outpatient facility fees have increased while ambulatory surgery center (ASC) fees remain below 2007 levels. This article surveys these changes and examines their significant impact on academic and private gastroenterology practices.
Professional fees for endoscopic procedures
Since 1992 physician professional fees have been linked to the Medicare Physician Fee Schedule, which assigns each service a certain number of relative value units (RVUs). First, the work RVU (wRVU) is based on the estimated physician time, mental effort, technical skill, and psychological stress required to provide a service. Second, a practice expense RVU (PE RVU) reflects the direct and indirect costs of providing the service. For procedures performed in office-based settings the PE RVU includes rent, nonclinician staff, equipment, and supplies, on average amounting to 44% of the total RVU. For procedures performed in hospital outpatient departments (HOPDs) and ASCs, the PE RVU is much lower, because most costs are incurred by the facility (which receives a separate facility fee), rather than the physician practice. Third, a small proportion of the overall RVU is linked to malpractice insurance costs (MP RVU). The RVU components are geographically adjusted, combined, and then multiplied by a conversion factor (CF, which in 2016 is $35.80) to determine actual Medicare payment (Payment = [wRVU + PE RVU + MP RVU] × CF).1
To address potential distortions in this physician fee schedule, The Affordable Care Act directed the Secretary of Health and Human Services to establish a formal process to review potentially misvalued procedure codes. During 2012 and 2014, multiple gastroenterological and surgical societies surveyed practicing physicians on the physician work, time, and intensity required to perform more than 120 services in question, including esophagoscopy, esophagogastroduodenoscopy, endoscopic retrograde cholangiopancreatography, flexible sigmoidoscopy and ileoscopy, pouchoscopy, and colonoscopy. At the same time, these societies assembled an expert panel of practicing physicians to determine the practice expenses associated with these procedures. The societies analyzed the results and presented recommendations to the American Medical Association/Specialty Society Relative Value Scale Update Committee, which, in turn, presented their recommendations to the Centers for Medicare and Medicaid Services.2 In 2014, CMS accepted approximately three-quarters of the Relative Value Scale Update Committee’s recommendations, ultimately decreasing wRVUs, increasing PE RVUs for procedures performed in office-based settings, and leaving MP RVUs unchanged for most upper endoscopy and endoscopic retrograde cholangiopancreatography procedures. These changes translated into significant 2015 payment reductions for esophagoscopy and esophagogastroduodenoscopy (4%-42%), endoscopic ultrasound (10%-35%), and endoscopic retrograde cholangiopancreatography (0%-37%) performed within facilities, with less effect for those performed in office-based settings. At that time, “in light of the substantial nature of [the colonoscopy] code revision and its relationship to the policies on moderate sedation,” CMS delayed revaluation of the lower gastrointestinal (GI) endoscopy codes.3 This reprieve is now over: The 2016 Medicare Physician Fee Schedule Final Rule includes up to 17% cuts (12% on average) to the wRVU associated with these lower GI procedures (Table 1). For office-based procedures (but not facility-based procedures) these wRVU cuts are buffered (and sometimes offset) by general increases in PE RVUs.
Facility fees for endoscopic procedures
Compared with the small percentage of endoscopic procedures that are performed in office-based settings, those performed in HOPDs and ASCs entail a lower professional fee plus a separate facility fee. Since 2000 CMS has paid for services provided in HOPDs using the outpatient prospective payment system (OPPS). Clinical services are first classified into ambulatory payment classifications (APC) on the basis of clinical and cost similarity. Next, services within an APC are assigned a single relative payment rate, which is linked to the resources required to perform the service. The APC payment rate is geographically adjusted and then multiplied by a CF to determine an actual dollar amount.4
Since 2008, CMS has used a nearly identical mechanism to pay for facility services provided in ASCs. Services are classified using the same APCs and same relative weights as the OPPS. The difference is that the ASC CF is less than the OPPS CF (the 2016 ASC CF is 58% of the OPPS CF), translating into lower dollar payments for ASC services.5 Of note, in 2008 ASC rates were cut significantly when CMS adopted this methodology for determining ASC facility fees (previously, ASC rates were approximately 85% of HOPD rates).
CMS reviews the APCs and their relative weights annually, and may adjust how specific services are classified and how APCs are weighed. Since 2006, HOPD rates for the 10 common procedures listed in Table 1 have increased by 26%-93% real (i.e., Consumer Price Index–adjusted) dollars. Meanwhile, given the steep 2008 ASC fee cuts the 2016 ASC fees are still significantly lower than they were 1 decade ago, especially when accounting for inflation. In fact, ASC fees for the most common procedures have decreased by 20% in real dollars (Supplementary Tables 1 and 2 at http://dx.doi.org/10.1016/j.cgh.2016.03.032).
Putting these changes in context
It is important to consider these changes within a broader context. First, the full economic impact of these changes on an individual gastroenterology practice depends on where it performs its services and whether it collects associated facility fees, and fees for anesthesia and pathology services. Second, the Medicare population is growing by more than 10,000 people each day.6 Third, beyond Medicare, most commercial insurers peg their reimbursement rates to a percentage of the Medicare Fee Schedule. Although the details of specific contracts vary, gastroenterologists should expect to see commercial rates move in a similar direction within the next 1–2 years. Fourth, in the 2016 Fee Schedule CMS described its future intention to remove the value of moderate sedation from all GI procedures valued with moderate sedation inherent to the procedure. The more that moderate sedation is ultimately valued, the less that endoscopic procedures will be valued. Consequently, gastroenterologists who rely on anesthesiology professionals to sedate their patients will receive less revenue per procedure. Finally, the 2015 Medicare Access and Children’s Health Insurance Program Reauthorization Act (MACRA) links a higher proportion of these dwindling fee-for-service payments to performance. Starting in 2018, physicians who elect to remain on a fee-for-service tract will receive a composite Merit-Based Incentive Payment System (MIPS) score that will translate into either performance bonuses or penalties (as much as 4% in 2019 and going up to 9% in 2022). Alternatively, providers who “sufficiently” participate in “two-sided” (i.e., risk-bearing) alternative payment models (e.g., bundled payments and accountable care organizations) instead of fee-for-service payments will receive 5% across-the-board bonuses. In sum, it is possible that fee-for-service payments may eventually become so unattractive that many gastroenterologists decide (or hope) to move to alternative payment models that combine both professional and facility fees, such as the CMS now-mandatory Comprehensive Care for Joint Replacement (CJR) program.7
Impact on academic practices
For most academic gastroenterology practices, clinical revenue far exceeds all other sources of funding, including research grants, teaching support, philanthropy, and partnerships with industry. Although a select few academic GI divisions have managed to build their own ASCs to share facility fees, for most academic practices, clinical revenue comes solely from the professional fees for endoscopic procedures and, less so, from office and hospital-based consultations and management of established patients.8 Thus, steep cuts to professional fees for endoscopic procedures, the leading source of overall revenue, will severely strain most academic gastroenterology practices.
In one of our practices (SDD), the 10 common procedures listed in Table 1 accounted for 27% of total direct clinical revenue over the past year. Roughly one-fourth of our patients are Medicare beneficiaries. Assuming no change in volume, the 2016 fee schedule cuts will amount to a 0.9% drop in direct clinical revenue. If all other payers follow with identical cuts, then direct clinical revenue will drop by 3.7%. Although our practice is fortunate to have other well-developed clinical and nonclinical revenue streams, these cuts are not insignificant.
How can academic practices continue to provide accessible clinical care in the face of these fee cuts? It can help to first consider how revenue is distributed for a Medicare beneficiary who undergoes a diagnostic colonoscopy with biopsy to evaluate unexplained diarrhea in an academic medical center’s hospital-based facility. The academic gastroenterologist receives $212 (CPT 45380). From this $212, the academic gastroenterology practice must pay assessments to various entities (sometimes including the school of medicine, department of medicine, and faculty practice plan) that may amount to more than 30% of total revenue. The roughly $150 that remains is used to pay faculty member salaries (the median salary for an academic gastroenterologist is $300,009) and benefits (estimated at $74,000/year); MP insurance (estimated at $2,275/year, higher in certain parts of the United States); and overhead for support staff, supplies, and other expenses (estimated at $50,550/year). Thus, a purely clinical academic gastroenterologist who is paid at the Medical Group Management Association (MGMA) median must generate $610,179 in preassessment revenue. If this hypothetical academic gastroenterologist solely treated Medicare beneficiaries, at $35.82 per RVU he or she would need to generate a staggering 17,035 RVUs per year, an amount that far exceeds the MGMA median (6,445) and 90th percentile (10,991) for academic gastroenterologists. Of course, real-world academic gastroenterologists also treat commercially insured patients and many spend time on nonclinical activities (although clinical income typically supports time devoted to research and teaching, not vice versa). Still, this example highlights a clear fact: Academic gastroenterology practices take a major financial loss delivering services to Medicare beneficiaries. Meanwhile, the HOPD charges more than $2,200 for the procedure. Although it receives $752 facility fee from Medicare (Disproportionate Share Hospitals and NCI Cancer Centers receive more), with a cost-to-charge ratio of roughly 0.2, revenue still clearly exceeds expenses. Finally, the anesthesia professional (if any) receives roughly $198 (CPT 00810) and the pathologist $74 (CPT 88305).
Although some may argue that academic gastroenterologists should simply accept a pay cut, lower salaries may drive many away from academia. To hire enough new (or even maintain enough existing) faculty members to maintain and grow volume, academic gastroenterology practices must find ways to supplement declining professional fees. One option is for academic practices to open their own ASCs, either alone or jointly with their health care system. But this assumes, often erroneously, that the health care system is willing to share facility fees. A second option is to develop incentive programs that transfer revenue from the health care system to the physicians. Importantly, these must be at fair market value and for nonemployed physicians cannot be linked to volume.10 Examples include medical directorships and non–volume-based performance bonuses. A third option is to consider alternative payment models, such as bundled payments that include a single lump sum payment for both professional and facility fees. The practice and health system then negotiates how the bundle is shared. Bundles should motivate hospitals and academic practices to work together to improve care and reduce overall expenses. Along these lines, CMS recently announced the CJR program under which hospitals and physicians in 75 locations will be required to participate. Although AGA recently published a bundled payment framework for screening and surveillance colonoscopy,11 bundles for other endoscopic procedures remain to be defined. But, a clearly defined, attractively priced, and skillfully negotiated bundle could be a means for redistributing revenue in ways that are more favorable to academic practices. No matter the approach, it is critical for academic gastroenterology practices and their health care systems to align their goals and integrate their services. But this is easier said than done.
Impact on private gastroenterology practices
The impact of these professional and facility fee changes on private practice depends on the practice’s payer mix, and whether it owns an ASC and directly provides anesthesia and pathology services. Consider Texas Digestive Disease Consultants (TDDC), an 80-gastroenterologist practice that provides GI care and endoscopic procedures throughout north and central Texas. TDDC revenue comes solely from professional fees generated by gastroenterologists and the pathologists that the practice employs. TDDC operating expenses include employee salaries and benefits, rent, and taxes. Based on an expected work-year of 2,080 hours, each TDDC gastroenterologist must generate $219 per hour to cover practice expenses. Physicians receive income only after practice revenue exceeds $219 per hour.
In the 2016 Medicare Physician Fee Schedule, colonoscopy code 45387 is assigned 3.36 RVUs, based on 67 minutes in total time. Based on this information, 2016 CMS payment for a screening colonoscopy ($200) will result in a loss of $45 in practice revenue over expenses. Across the TDDC practices in 2015, CMS patients represented 29% of all colonoscopies performed, but only 11% of revenue from those procedures. In aggregate, assuming no change in volume, the 2016 fee schedule cuts will translate into $5,472 less physician income for each gastroenterologist. If all other payers follow with identical cuts then each gastroenterologist would forfeit $50,896 of income.
For pathology services, code 88305 reimburses a global service per specimen. The average number of specimens per outpatient GI procedure at TDDC is 1.5. Based on CMS’ per specimen payment of $73 (down from $107 in 2012), average pathology reimbursement per a CMS endoscopic procedure is $110.
What about facility fees? Many TDDC physicians separately own and operate ASCs and anesthesia practices. Because the physician owners of these facilities funded, developed, and operate these facilities on their own, any revenue over expenses from these facilities flow directly to the ASC, rather than to TDDC. Since 2008, when ASC payments were severely reduced, ASC revenue over expenses for a Medicare colonoscopy has ranged from $0 to $70. For anesthesia services, the ASC receives approximately $159 per screening colonoscopy, which is not enough to actually cover the costs of providing anesthesia services. In sum, when TDDC gastroenterologists perform colonoscopies on Medicare patients at an HOPD the physician loses money. When the same procedure is performed at an ASC the physician barely break even.
How can private practice gastroenterologists respond to these fee cuts? First, some gastroenterologists may be forced to accept lower salaries. Second, practices can offset fee cuts by improving efficiency and reducing overall cost of care, assuming they are not already maximally efficient. Third, some private practices can increase Medicare professional payments by reclassifying as nonparticipating with Medicare. Nonparticipating status allows practices to charge the patient up to approximately 109.25% of the Medicare approved rate, with the patient submitting the claim directly to Medicare (“balance billing”). The downside is that patients shoulder the additional cost and may potentially decide to seek care elsewhere. Fourth, private practices should explore alternative payment models, such as bundled payment as described previously, either on their own or by partnering with local health systems to share risk. Finally, some practices may sell equity to and become employees of local health systems.
Conclusions
Changes to Medicare professional and facility fee payments for endoscopic procedures significantly affect academic and private gastroenterology practices. Dwindling professional fees – alongside increasing HOPD facility fees – make academic gastroenterology practices increasingly reliant on support from their parent health care systems. Like academic practices, private gastroenterology practices experience financial losses when treating Medicare beneficiaries. Academic and private gastroenterology practices should consider several potential responses. Although beyond the scope of this article, all practices must continuously strive to improve the quality and reduce the costs of the endoscopic procedures they perform.
Acknowledgments
The authors thank Leslie Narramore, MPA, for her insights and help with reimbursement data. They also thank Bryan Rhodes for providing financial data and Shivan Mehta, MD, MBA, for his feedback on the Relative Value Scale Update Committee process.
References
1. MEDPAC. Payment basics: physician and other health professional payment system. Medicare Payment Advisory Commission, Washington, DC; 2015.
2. Mehta, S.J., Brill, J.V. What is the RUC and how does it impact gastroenterology?. Clin Gastroenterol Hepatol. 2014;12:1208-11.
3. American College of Gastroenterology, American Gastroenterological Association, American Society for Gastrointestinal Endoscopy. Key Provisions in the CY 2015 Medicare Physician Fee Schedule Final Rule, 2015. Available at: http://gi.org/wp-content/uploads/2014/11/Tri-Soc_CY15_MPFS_Final_Rule_Summary.pdf.
4. MEDPAC. Payment basics: Outpatient hospital services payment system. Medicare Payment Advisory Commission, Washington, DC; 2015.
5. MEDPAC. Payment basics: Ambulatory surgery center services payment system. Medicare Payment Advisory Commission, Washington, DC; 2015.
6. Diamond, D. 10,000 people are now enrolling in Medicare – every day. Forbes; 2015 (Available at: http://www.forbes.com/sites/dandiamond/2015/07/13/aging-in-america-10000-people-enroll-in-medicare-every-day/#5177f1f35e07. Accessed January 3, 2016)
7. Mechanic, R.E. Mandatory Medicare bundled payment: Is it ready for prime time?. N Engl J Med. 2015;373:1291-3.
8. Rustgi, A.K., Allen, J.I. The house of gastrointestinal medicine: How academic medical centers can build a sustainable economic clinical model. Clin Gastroenterol Hepatol. 2013;11:1370-3.
9. Academic Practice Compensation and Production Report. Medical Group Management Association, Englewood, Colo.; 2015.
10. Becker S., Townshend G., Carnell H., et al. Physician compensation: 10 core legal and regulatory concepts. Becker’s Hospital Review Available at: http://www.beckershospitalreview.com/legal-regulatory-issues/physician-compensation-10-core-legal-and-regulatory-concepts.html. Accessed Jan. 20, 2016.
11. Brill, J.V., Jain, R., Margolis, P.S., et al. A bundled payment framework for colonoscopy performed for colorectal cancer screening or surveillance. Gastroenterology. 2014;146:849-53, e9.
Dr. Dorn is vice chief, division of gastroenterology and hepatology, associate professor of medicine, health policy & management, University of North Carolina at Chapel Hill; he has received honoraria for consulting and presentations on health reform from AbbVie and Olympus. Dr. Vesy is a gastroenterologist affiliated with Baylor University Medical Center, Dallas.
Merck Manual available as free app
The Merck Manual professional app is now available for free for use on either Android or Apple devices.
“In today’s mobile world, health care professionals expect critical health information at their fingertips, with or without an Internet connection,” said Robert S. Porter, MD, Merck Manuals’ editor-in-chief.
The app contains more than 1,000 photos and illustrations, and offline content can be stored on the device. A Wi-Fi connection is not needed to access the manual. When a cell phone is connected to the Internet, the app allows access to features such as videos of procedures, quizzes, medical news, editorials, and drug reference information.
The Merck Manual professional app is now available for free for use on either Android or Apple devices.
“In today’s mobile world, health care professionals expect critical health information at their fingertips, with or without an Internet connection,” said Robert S. Porter, MD, Merck Manuals’ editor-in-chief.
The app contains more than 1,000 photos and illustrations, and offline content can be stored on the device. A Wi-Fi connection is not needed to access the manual. When a cell phone is connected to the Internet, the app allows access to features such as videos of procedures, quizzes, medical news, editorials, and drug reference information.
The Merck Manual professional app is now available for free for use on either Android or Apple devices.
“In today’s mobile world, health care professionals expect critical health information at their fingertips, with or without an Internet connection,” said Robert S. Porter, MD, Merck Manuals’ editor-in-chief.
The app contains more than 1,000 photos and illustrations, and offline content can be stored on the device. A Wi-Fi connection is not needed to access the manual. When a cell phone is connected to the Internet, the app allows access to features such as videos of procedures, quizzes, medical news, editorials, and drug reference information.
Preparing for the GI boards
The latest installment of AGA’s Digestive Diseases Self Education Program® (DDSEP®) 8 not only helps prepare you for the in-depth gastroenterology and hepatology material on the GI board exam, but also for the test-taking process itself, thanks to a helpful piece by Suzanne Rose, MD, MSEd, and Norma Saks, EdD.
Dr. Rose, professor of medicine and senior associate dean for education at the University of Connecticut School of Medicine, and Dr. Saks, professor of the department of psychiatry, assistant dean for educational programs and director of the Cognitive Skills Program at the Robert Wood Johnson Medical School, compiled their experience and knowledge into a helpful and comprehensive list of test-taking tips for practitioners taking the GI board exams.
The article, “How to Prepare for the Gastroenterology Boards,” delves into each stage of test preparation, including studying, practice testing, and taking the test itself. Below are just a few summaries of the many tips they provide. You can access the full article by purchasing DDSEP® 8. To learn more about AGA’s DDSEP® offerings, visit http://buyddsep8.gastro.org.
Before the exam
Get to know the rules and familiarize yourself with the American Board of Internal Medicine’s (ABIM) certification process. Make sure you allow enough time to prepare for the exam when you’re signing up, and also get familiar with the location, scheduling, and potential penalties of the exam.
Preparing for the exam
While studying, Dr. Rose and Dr. Saks recommend first identifying your strengths and weaknesses so that you can take the time to prepare where you need it. Also, make a study schedule and take practice tests so that you can better identify topics you should spend more time studying for. For instance, even though you might think something’s a test strength, practicing could help show you that you’re overthinking topics you’re most comfortable with. Also, consider working with a partner or larger group to help increase learning retention and keep you on track with your study schedule.
Taking the exam
In addition to obvious things like double-checking ABIM’s website before the test day and getting a good night’s sleep, the authors provide some helpful time management suggestions. Some topics they address include guessing, pacing yourself during the exam and how to best address those difficult questions. One big tip they offer: don’t leave questions blank, as those will automatically be counted as incorrect answers.
The latest installment of AGA’s Digestive Diseases Self Education Program® (DDSEP®) 8 not only helps prepare you for the in-depth gastroenterology and hepatology material on the GI board exam, but also for the test-taking process itself, thanks to a helpful piece by Suzanne Rose, MD, MSEd, and Norma Saks, EdD.
Dr. Rose, professor of medicine and senior associate dean for education at the University of Connecticut School of Medicine, and Dr. Saks, professor of the department of psychiatry, assistant dean for educational programs and director of the Cognitive Skills Program at the Robert Wood Johnson Medical School, compiled their experience and knowledge into a helpful and comprehensive list of test-taking tips for practitioners taking the GI board exams.
The article, “How to Prepare for the Gastroenterology Boards,” delves into each stage of test preparation, including studying, practice testing, and taking the test itself. Below are just a few summaries of the many tips they provide. You can access the full article by purchasing DDSEP® 8. To learn more about AGA’s DDSEP® offerings, visit http://buyddsep8.gastro.org.
Before the exam
Get to know the rules and familiarize yourself with the American Board of Internal Medicine’s (ABIM) certification process. Make sure you allow enough time to prepare for the exam when you’re signing up, and also get familiar with the location, scheduling, and potential penalties of the exam.
Preparing for the exam
While studying, Dr. Rose and Dr. Saks recommend first identifying your strengths and weaknesses so that you can take the time to prepare where you need it. Also, make a study schedule and take practice tests so that you can better identify topics you should spend more time studying for. For instance, even though you might think something’s a test strength, practicing could help show you that you’re overthinking topics you’re most comfortable with. Also, consider working with a partner or larger group to help increase learning retention and keep you on track with your study schedule.
Taking the exam
In addition to obvious things like double-checking ABIM’s website before the test day and getting a good night’s sleep, the authors provide some helpful time management suggestions. Some topics they address include guessing, pacing yourself during the exam and how to best address those difficult questions. One big tip they offer: don’t leave questions blank, as those will automatically be counted as incorrect answers.
The latest installment of AGA’s Digestive Diseases Self Education Program® (DDSEP®) 8 not only helps prepare you for the in-depth gastroenterology and hepatology material on the GI board exam, but also for the test-taking process itself, thanks to a helpful piece by Suzanne Rose, MD, MSEd, and Norma Saks, EdD.
Dr. Rose, professor of medicine and senior associate dean for education at the University of Connecticut School of Medicine, and Dr. Saks, professor of the department of psychiatry, assistant dean for educational programs and director of the Cognitive Skills Program at the Robert Wood Johnson Medical School, compiled their experience and knowledge into a helpful and comprehensive list of test-taking tips for practitioners taking the GI board exams.
The article, “How to Prepare for the Gastroenterology Boards,” delves into each stage of test preparation, including studying, practice testing, and taking the test itself. Below are just a few summaries of the many tips they provide. You can access the full article by purchasing DDSEP® 8. To learn more about AGA’s DDSEP® offerings, visit http://buyddsep8.gastro.org.
Before the exam
Get to know the rules and familiarize yourself with the American Board of Internal Medicine’s (ABIM) certification process. Make sure you allow enough time to prepare for the exam when you’re signing up, and also get familiar with the location, scheduling, and potential penalties of the exam.
Preparing for the exam
While studying, Dr. Rose and Dr. Saks recommend first identifying your strengths and weaknesses so that you can take the time to prepare where you need it. Also, make a study schedule and take practice tests so that you can better identify topics you should spend more time studying for. For instance, even though you might think something’s a test strength, practicing could help show you that you’re overthinking topics you’re most comfortable with. Also, consider working with a partner or larger group to help increase learning retention and keep you on track with your study schedule.
Taking the exam
In addition to obvious things like double-checking ABIM’s website before the test day and getting a good night’s sleep, the authors provide some helpful time management suggestions. Some topics they address include guessing, pacing yourself during the exam and how to best address those difficult questions. One big tip they offer: don’t leave questions blank, as those will automatically be counted as incorrect answers.
PAD Toolkit Available
For SVS members, every month is PAD month. But with September PAD Awareness Month, it's a good time to remind members that SVS offers a digital toolkit to share with colleagues, patients and referring physicians.
If you have ever wished you had a YouTube playlist of video information on PAD that patients can watch, we’ve got it. We also provide links to printable brochures, articles, practice guidelines and more.
Visit the SVS PAD Resources page here.
For SVS members, every month is PAD month. But with September PAD Awareness Month, it's a good time to remind members that SVS offers a digital toolkit to share with colleagues, patients and referring physicians.
If you have ever wished you had a YouTube playlist of video information on PAD that patients can watch, we’ve got it. We also provide links to printable brochures, articles, practice guidelines and more.
Visit the SVS PAD Resources page here.
For SVS members, every month is PAD month. But with September PAD Awareness Month, it's a good time to remind members that SVS offers a digital toolkit to share with colleagues, patients and referring physicians.
If you have ever wished you had a YouTube playlist of video information on PAD that patients can watch, we’ve got it. We also provide links to printable brochures, articles, practice guidelines and more.
Visit the SVS PAD Resources page here.
NHLBI Strategic Vision Completed with SVS Input
By helping to formulate the new NHLBI Strategic Vision, the SVS successfully advocated for prioritization of disease processes which challenge vascular surgeons. “With continued advocacy and support, we can better position our members for successful NIH funding in the future,” said the SVS Research Council, in a summer document on the Strategic Vision.
NHLBI recently released "Charting the Future Together: The NHLBI Strategic Vision." More than a year ago, the SVS, spearheaded by the Research Council, participated in the strategic visioning process by contributing compelling questions and critical challenges and providing feedback on the draft report. SVS delivered a key message that “it is essential to prioritize disease processes which challenge vascular surgeons and vascular physicians.” The Society’s involvement was critical because the Strategic Vision defines research areas deemed to be the most important, timely and feasible for NHLBI to address in its targeted/solicited research in the next decade.
The Research Council has prepared a brief summary of the NHLBI Strategic Vision.
By helping to formulate the new NHLBI Strategic Vision, the SVS successfully advocated for prioritization of disease processes which challenge vascular surgeons. “With continued advocacy and support, we can better position our members for successful NIH funding in the future,” said the SVS Research Council, in a summer document on the Strategic Vision.
NHLBI recently released "Charting the Future Together: The NHLBI Strategic Vision." More than a year ago, the SVS, spearheaded by the Research Council, participated in the strategic visioning process by contributing compelling questions and critical challenges and providing feedback on the draft report. SVS delivered a key message that “it is essential to prioritize disease processes which challenge vascular surgeons and vascular physicians.” The Society’s involvement was critical because the Strategic Vision defines research areas deemed to be the most important, timely and feasible for NHLBI to address in its targeted/solicited research in the next decade.
The Research Council has prepared a brief summary of the NHLBI Strategic Vision.
By helping to formulate the new NHLBI Strategic Vision, the SVS successfully advocated for prioritization of disease processes which challenge vascular surgeons. “With continued advocacy and support, we can better position our members for successful NIH funding in the future,” said the SVS Research Council, in a summer document on the Strategic Vision.
NHLBI recently released "Charting the Future Together: The NHLBI Strategic Vision." More than a year ago, the SVS, spearheaded by the Research Council, participated in the strategic visioning process by contributing compelling questions and critical challenges and providing feedback on the draft report. SVS delivered a key message that “it is essential to prioritize disease processes which challenge vascular surgeons and vascular physicians.” The Society’s involvement was critical because the Strategic Vision defines research areas deemed to be the most important, timely and feasible for NHLBI to address in its targeted/solicited research in the next decade.
The Research Council has prepared a brief summary of the NHLBI Strategic Vision.
Affect the Future; Donate to the SVS Foundation
The 2016 SVS Foundation Annual Appeal is underway, with donations sought to fund the research that leads to breakthroughs that ultimately will improve patient care.
Dr. Bruce Perler, SVS Foundation chair, has written a letter that reminds all members that contributions to the SVS Foundation - which funds basic and clinical research grants - are vital to the continuation of innovation that has always been and remains integral to our specialty.
Donations to the SVS Foundation - which funds basic and clinical research grants - are an opportunity for all members to make a significant difference in not only the future of SVS but also that of vascular disease patients.
The 2016 SVS Foundation Annual Appeal is underway, with donations sought to fund the research that leads to breakthroughs that ultimately will improve patient care.
Dr. Bruce Perler, SVS Foundation chair, has written a letter that reminds all members that contributions to the SVS Foundation - which funds basic and clinical research grants - are vital to the continuation of innovation that has always been and remains integral to our specialty.
Donations to the SVS Foundation - which funds basic and clinical research grants - are an opportunity for all members to make a significant difference in not only the future of SVS but also that of vascular disease patients.
The 2016 SVS Foundation Annual Appeal is underway, with donations sought to fund the research that leads to breakthroughs that ultimately will improve patient care.
Dr. Bruce Perler, SVS Foundation chair, has written a letter that reminds all members that contributions to the SVS Foundation - which funds basic and clinical research grants - are vital to the continuation of innovation that has always been and remains integral to our specialty.
Donations to the SVS Foundation - which funds basic and clinical research grants - are an opportunity for all members to make a significant difference in not only the future of SVS but also that of vascular disease patients.
Male libido problems successfully treated with bright light
VIENNA – Daily exposure to high-intensity light early in the morning resulted in significantly improved sexual satisfaction scores and a boost in testosterone levels in men with reduced libido or erection difficulty in a randomized, placebo-controlled clinical trial, Andrea Fagiolini, MD., said at the annual meeting of the American Thyroid Association.
“We found a very strong difference in sexual satisfaction and also in blood testosterone levels with intense light therapy. Patients seemed to respond to it really well, and our placebo group didn’t respond at all,” according to Dr. Fagiolini, professor and chairman of the department of psychiatry at the University of Siena (Italy).
“It’s still early to make a general recommendation. This was a small study. We need a larger trial with more patients. But if these results are confirmed, it would be very helpful because as of now we don’t have many tools to treat sexual dysfunction. So if light therapy works, I think it may be a better option than medications like Viagra [sildenafil] or similar drugs, which have side effects,” he said in an interview.
The trial included 38 men seen at the university urology clinic with a diagnosis of primary hypoactive sexual desire or sexual arousal disorder. Half were randomized to daily use of a white fluorescent light box for 30 minutes in the morning, preferably between 7 and 8 a.m. The light box, equipped with a UV filter, was the same as that used in treating seasonal affective disorder. Light therapy for seasonal affective disorder is an established therapy in the psychiatric world, one that has proved as effective as antidepressant therapy for that condition. It is “absolutely safe and tolerable,” except in patients with eye conditions or on photosensitizing medications, the psychiatrist noted.
The control group was on the same treatment schedule, but with light boxes that had been modified through the use of a neutral-density gel filter to provide a dose of just 100 lux instead of the 10,000 lux at a distance of 1 m from the light source to the cornea generated by the active therapy light box.
The study endpoints were change from baseline through 2 weeks of therapy on a self-administered rating of sexual satisfaction on a scale of 1-10, as well as testosterone levels.
From a baseline mean self-rated sexual satisfaction score of about 2, the group exposed to daily bright light for 2 weeks had a more than threefold increase to a score of 6.3, while the controls showed no significant change.
In addition, testosterone levels in the bright light group increased from 2.1 ng/mL to 3.6 ng/mL after 2 weeks, while remaining unchanged in controls.
“Our idea for this study came from the observation that sexual satisfaction is known to increase during the spring and summer, compared to fall and winter. We thought that may have something to do with the natural daylight,” Dr. Fagiolini said.
The most likely mechanism of benefit springs from the established finding that bright light stimulates the pituitary gland to produce leutinizing hormone. This results in increased levels of testosterone and inhibition of melatonin production by the pineal gland. Decreased plasma melatonin is thought to lead to reduced serum prolactin – and high prolactin has been linked to sexual dysfunction, he explained.
The trial was conducted in the fall and winter months to avoid confounding by more natural daylight during the other seasons. But if the study results are confirmed, this therapy might well be used year-round, the psychiatrist said.
He reported having no financial conflicts of interest regarding the study, which was conducted without external funding.
VIENNA – Daily exposure to high-intensity light early in the morning resulted in significantly improved sexual satisfaction scores and a boost in testosterone levels in men with reduced libido or erection difficulty in a randomized, placebo-controlled clinical trial, Andrea Fagiolini, MD., said at the annual meeting of the American Thyroid Association.
“We found a very strong difference in sexual satisfaction and also in blood testosterone levels with intense light therapy. Patients seemed to respond to it really well, and our placebo group didn’t respond at all,” according to Dr. Fagiolini, professor and chairman of the department of psychiatry at the University of Siena (Italy).
“It’s still early to make a general recommendation. This was a small study. We need a larger trial with more patients. But if these results are confirmed, it would be very helpful because as of now we don’t have many tools to treat sexual dysfunction. So if light therapy works, I think it may be a better option than medications like Viagra [sildenafil] or similar drugs, which have side effects,” he said in an interview.
The trial included 38 men seen at the university urology clinic with a diagnosis of primary hypoactive sexual desire or sexual arousal disorder. Half were randomized to daily use of a white fluorescent light box for 30 minutes in the morning, preferably between 7 and 8 a.m. The light box, equipped with a UV filter, was the same as that used in treating seasonal affective disorder. Light therapy for seasonal affective disorder is an established therapy in the psychiatric world, one that has proved as effective as antidepressant therapy for that condition. It is “absolutely safe and tolerable,” except in patients with eye conditions or on photosensitizing medications, the psychiatrist noted.
The control group was on the same treatment schedule, but with light boxes that had been modified through the use of a neutral-density gel filter to provide a dose of just 100 lux instead of the 10,000 lux at a distance of 1 m from the light source to the cornea generated by the active therapy light box.
The study endpoints were change from baseline through 2 weeks of therapy on a self-administered rating of sexual satisfaction on a scale of 1-10, as well as testosterone levels.
From a baseline mean self-rated sexual satisfaction score of about 2, the group exposed to daily bright light for 2 weeks had a more than threefold increase to a score of 6.3, while the controls showed no significant change.
In addition, testosterone levels in the bright light group increased from 2.1 ng/mL to 3.6 ng/mL after 2 weeks, while remaining unchanged in controls.
“Our idea for this study came from the observation that sexual satisfaction is known to increase during the spring and summer, compared to fall and winter. We thought that may have something to do with the natural daylight,” Dr. Fagiolini said.
The most likely mechanism of benefit springs from the established finding that bright light stimulates the pituitary gland to produce leutinizing hormone. This results in increased levels of testosterone and inhibition of melatonin production by the pineal gland. Decreased plasma melatonin is thought to lead to reduced serum prolactin – and high prolactin has been linked to sexual dysfunction, he explained.
The trial was conducted in the fall and winter months to avoid confounding by more natural daylight during the other seasons. But if the study results are confirmed, this therapy might well be used year-round, the psychiatrist said.
He reported having no financial conflicts of interest regarding the study, which was conducted without external funding.
VIENNA – Daily exposure to high-intensity light early in the morning resulted in significantly improved sexual satisfaction scores and a boost in testosterone levels in men with reduced libido or erection difficulty in a randomized, placebo-controlled clinical trial, Andrea Fagiolini, MD., said at the annual meeting of the American Thyroid Association.
“We found a very strong difference in sexual satisfaction and also in blood testosterone levels with intense light therapy. Patients seemed to respond to it really well, and our placebo group didn’t respond at all,” according to Dr. Fagiolini, professor and chairman of the department of psychiatry at the University of Siena (Italy).
“It’s still early to make a general recommendation. This was a small study. We need a larger trial with more patients. But if these results are confirmed, it would be very helpful because as of now we don’t have many tools to treat sexual dysfunction. So if light therapy works, I think it may be a better option than medications like Viagra [sildenafil] or similar drugs, which have side effects,” he said in an interview.
The trial included 38 men seen at the university urology clinic with a diagnosis of primary hypoactive sexual desire or sexual arousal disorder. Half were randomized to daily use of a white fluorescent light box for 30 minutes in the morning, preferably between 7 and 8 a.m. The light box, equipped with a UV filter, was the same as that used in treating seasonal affective disorder. Light therapy for seasonal affective disorder is an established therapy in the psychiatric world, one that has proved as effective as antidepressant therapy for that condition. It is “absolutely safe and tolerable,” except in patients with eye conditions or on photosensitizing medications, the psychiatrist noted.
The control group was on the same treatment schedule, but with light boxes that had been modified through the use of a neutral-density gel filter to provide a dose of just 100 lux instead of the 10,000 lux at a distance of 1 m from the light source to the cornea generated by the active therapy light box.
The study endpoints were change from baseline through 2 weeks of therapy on a self-administered rating of sexual satisfaction on a scale of 1-10, as well as testosterone levels.
From a baseline mean self-rated sexual satisfaction score of about 2, the group exposed to daily bright light for 2 weeks had a more than threefold increase to a score of 6.3, while the controls showed no significant change.
In addition, testosterone levels in the bright light group increased from 2.1 ng/mL to 3.6 ng/mL after 2 weeks, while remaining unchanged in controls.
“Our idea for this study came from the observation that sexual satisfaction is known to increase during the spring and summer, compared to fall and winter. We thought that may have something to do with the natural daylight,” Dr. Fagiolini said.
The most likely mechanism of benefit springs from the established finding that bright light stimulates the pituitary gland to produce leutinizing hormone. This results in increased levels of testosterone and inhibition of melatonin production by the pineal gland. Decreased plasma melatonin is thought to lead to reduced serum prolactin – and high prolactin has been linked to sexual dysfunction, he explained.
The trial was conducted in the fall and winter months to avoid confounding by more natural daylight during the other seasons. But if the study results are confirmed, this therapy might well be used year-round, the psychiatrist said.
He reported having no financial conflicts of interest regarding the study, which was conducted without external funding.
AT THE ECNP CONGRESS
Key clinical point: Daily exposure to intense white light, an established treatment for seasonal affective disorder, also may improve male sexual dysfunction.
Major finding: After 2 weeks of daily bright light therapy, men with low libido or erection difficulty showed an improvement in self-rated sexual satisfaction from 2.1 to 6.3 on a 1-10 scale.
Data source: A randomized trial that included 38 men diagnosed with hypoactive sexual desire disorder or sexual arousal disorder who received 2 weeks of high-intensity light therapy for 30 minutes per morning or a sham placebo involving exposure to far less intense light.
Disclosures: The presenter reported having no financial conflicts of interest regarding the study, which was conducted without external funding.