User login
Hospital managers identify barriers to antimicrobial stewardship
Antimicrobial stewardship programs are being introduced in hospitals internationally amidst the problem of escalating antimicrobial resistance. But sustained behavioral change in the area of antibiotic prescribing has been difficult to achieve.
While we have an understanding of doctors’ roles in antibiotic optimization within hospital contexts, the role of hospital management in successes or failures of antimicrobial stewardship programs (and optimization of antibiotics more broadly) has not been explored. Our new study published in the Journal of Hospital Infection examines this very question – the role of the manager as an enabler, or indeed a barrier, to antibiotic optimization.
Researchers in the study performed semistructured interviews with 23 hospital managers at three hospitals in two different states in Australia to specifically examine their opinions on antibiotic resistance, antibiotic governance, and their roles as senior management. The results illustrate how hospital managers prioritize financial pressures and immediate clinical demands over longer-term issues such as antibiotic prescribing and resistance. Here is an example of those pressures, described by one manager:
“I think the problem is [antimicrobial stewardship] in a competitive market. Are the waiting lists more newsworthy than antibiotic prescribing? Absolutely. You get more adverse events happening because of the waiting lists. … So, of course it’s not going to be the [antibiotic] prescribing that comes up to the top of that.” –Departmental Director
The study results also showed how managers find it challenging to comprehend, or act on the basis of, antibiotic-prescribing audits and had little faith in the value of data on antibiotic use and appropriateness. Other clinical areas with more clearly defined targets (and consequences for failing to meet targets) were prioritized over antibiotic prescribing in medical management decision making. Managers also found it difficult to influence the behavior of doctors and thought that it was a clinical responsibility to improve practice. In the words of one:
“I am a believer in delegated accountability and people on the shop floor knowing what they’re doing and being held accountable for it.” – Divisional Director
Managers perceived that there was limited accountability among doctors for antibiotic use and limited education and feedback to doctors:
“Those figures [on suboptimal prescribing] you give me, I haven’t heard them before. So, that in itself is a problem, and I would suggest you’d probably find a large number of medical staff haven’t been exposed to that.”
– Divisional Director
This study was performed in three hospitals with active antimicrobial stewardship programs. In Australia, as is becoming the pattern in countries within the Organisation for Economic Co-operation and Development (OECD), there is a legislative requirement for hospitals to have an effective antimicrobial stewardship program. And yet, meaningful sustained change in antibiotic prescribing is elusive, as evidenced by national antibiotic-prescribing data. The study results raise the important question of who is perceived as responsible for antibiotic-prescribing improvement and the actual and potential role of hospital managers in enacting change. It seems likely that both “top-down” influence (by managers and executive) and “bottom-up” influence (clinician-driven processes) will be required for effective and sustained practice change.
It is also clear from the results of this study that hospital managers do not perceive clear or immediate consequences for failing to improve antibiotic prescribing, and the perceived “distant” threat of antimicrobial resistance is not prioritized among other competing pressures. In addition, the widespread nature of antibiotic use makes it difficult to audit and even more difficult to communicate the extent of the problem.
These data would suggest that to move forward we need to look at an incentive structure for antibiotic-prescribing improvements or consequences in the short term for failing to optimize antibiotic use, and clearly defined goals for antibiotic optimization in hospitals.
Jennifer Broom, MBChB, PhD, is an infectious diseases physician at the Sunshine Coast Hospital and Health Service and an associate professor of medicine at the University of Queensland, Brisbane, Australia. Alex Broom, PhD, is professor of sociology in the School of Social Sciences at the University of New South Wales, Sydney.
Antimicrobial stewardship programs are being introduced in hospitals internationally amidst the problem of escalating antimicrobial resistance. But sustained behavioral change in the area of antibiotic prescribing has been difficult to achieve.
While we have an understanding of doctors’ roles in antibiotic optimization within hospital contexts, the role of hospital management in successes or failures of antimicrobial stewardship programs (and optimization of antibiotics more broadly) has not been explored. Our new study published in the Journal of Hospital Infection examines this very question – the role of the manager as an enabler, or indeed a barrier, to antibiotic optimization.
Researchers in the study performed semistructured interviews with 23 hospital managers at three hospitals in two different states in Australia to specifically examine their opinions on antibiotic resistance, antibiotic governance, and their roles as senior management. The results illustrate how hospital managers prioritize financial pressures and immediate clinical demands over longer-term issues such as antibiotic prescribing and resistance. Here is an example of those pressures, described by one manager:
“I think the problem is [antimicrobial stewardship] in a competitive market. Are the waiting lists more newsworthy than antibiotic prescribing? Absolutely. You get more adverse events happening because of the waiting lists. … So, of course it’s not going to be the [antibiotic] prescribing that comes up to the top of that.” –Departmental Director
The study results also showed how managers find it challenging to comprehend, or act on the basis of, antibiotic-prescribing audits and had little faith in the value of data on antibiotic use and appropriateness. Other clinical areas with more clearly defined targets (and consequences for failing to meet targets) were prioritized over antibiotic prescribing in medical management decision making. Managers also found it difficult to influence the behavior of doctors and thought that it was a clinical responsibility to improve practice. In the words of one:
“I am a believer in delegated accountability and people on the shop floor knowing what they’re doing and being held accountable for it.” – Divisional Director
Managers perceived that there was limited accountability among doctors for antibiotic use and limited education and feedback to doctors:
“Those figures [on suboptimal prescribing] you give me, I haven’t heard them before. So, that in itself is a problem, and I would suggest you’d probably find a large number of medical staff haven’t been exposed to that.”
– Divisional Director
This study was performed in three hospitals with active antimicrobial stewardship programs. In Australia, as is becoming the pattern in countries within the Organisation for Economic Co-operation and Development (OECD), there is a legislative requirement for hospitals to have an effective antimicrobial stewardship program. And yet, meaningful sustained change in antibiotic prescribing is elusive, as evidenced by national antibiotic-prescribing data. The study results raise the important question of who is perceived as responsible for antibiotic-prescribing improvement and the actual and potential role of hospital managers in enacting change. It seems likely that both “top-down” influence (by managers and executive) and “bottom-up” influence (clinician-driven processes) will be required for effective and sustained practice change.
It is also clear from the results of this study that hospital managers do not perceive clear or immediate consequences for failing to improve antibiotic prescribing, and the perceived “distant” threat of antimicrobial resistance is not prioritized among other competing pressures. In addition, the widespread nature of antibiotic use makes it difficult to audit and even more difficult to communicate the extent of the problem.
These data would suggest that to move forward we need to look at an incentive structure for antibiotic-prescribing improvements or consequences in the short term for failing to optimize antibiotic use, and clearly defined goals for antibiotic optimization in hospitals.
Jennifer Broom, MBChB, PhD, is an infectious diseases physician at the Sunshine Coast Hospital and Health Service and an associate professor of medicine at the University of Queensland, Brisbane, Australia. Alex Broom, PhD, is professor of sociology in the School of Social Sciences at the University of New South Wales, Sydney.
Antimicrobial stewardship programs are being introduced in hospitals internationally amidst the problem of escalating antimicrobial resistance. But sustained behavioral change in the area of antibiotic prescribing has been difficult to achieve.
While we have an understanding of doctors’ roles in antibiotic optimization within hospital contexts, the role of hospital management in successes or failures of antimicrobial stewardship programs (and optimization of antibiotics more broadly) has not been explored. Our new study published in the Journal of Hospital Infection examines this very question – the role of the manager as an enabler, or indeed a barrier, to antibiotic optimization.
Researchers in the study performed semistructured interviews with 23 hospital managers at three hospitals in two different states in Australia to specifically examine their opinions on antibiotic resistance, antibiotic governance, and their roles as senior management. The results illustrate how hospital managers prioritize financial pressures and immediate clinical demands over longer-term issues such as antibiotic prescribing and resistance. Here is an example of those pressures, described by one manager:
“I think the problem is [antimicrobial stewardship] in a competitive market. Are the waiting lists more newsworthy than antibiotic prescribing? Absolutely. You get more adverse events happening because of the waiting lists. … So, of course it’s not going to be the [antibiotic] prescribing that comes up to the top of that.” –Departmental Director
The study results also showed how managers find it challenging to comprehend, or act on the basis of, antibiotic-prescribing audits and had little faith in the value of data on antibiotic use and appropriateness. Other clinical areas with more clearly defined targets (and consequences for failing to meet targets) were prioritized over antibiotic prescribing in medical management decision making. Managers also found it difficult to influence the behavior of doctors and thought that it was a clinical responsibility to improve practice. In the words of one:
“I am a believer in delegated accountability and people on the shop floor knowing what they’re doing and being held accountable for it.” – Divisional Director
Managers perceived that there was limited accountability among doctors for antibiotic use and limited education and feedback to doctors:
“Those figures [on suboptimal prescribing] you give me, I haven’t heard them before. So, that in itself is a problem, and I would suggest you’d probably find a large number of medical staff haven’t been exposed to that.”
– Divisional Director
This study was performed in three hospitals with active antimicrobial stewardship programs. In Australia, as is becoming the pattern in countries within the Organisation for Economic Co-operation and Development (OECD), there is a legislative requirement for hospitals to have an effective antimicrobial stewardship program. And yet, meaningful sustained change in antibiotic prescribing is elusive, as evidenced by national antibiotic-prescribing data. The study results raise the important question of who is perceived as responsible for antibiotic-prescribing improvement and the actual and potential role of hospital managers in enacting change. It seems likely that both “top-down” influence (by managers and executive) and “bottom-up” influence (clinician-driven processes) will be required for effective and sustained practice change.
It is also clear from the results of this study that hospital managers do not perceive clear or immediate consequences for failing to improve antibiotic prescribing, and the perceived “distant” threat of antimicrobial resistance is not prioritized among other competing pressures. In addition, the widespread nature of antibiotic use makes it difficult to audit and even more difficult to communicate the extent of the problem.
These data would suggest that to move forward we need to look at an incentive structure for antibiotic-prescribing improvements or consequences in the short term for failing to optimize antibiotic use, and clearly defined goals for antibiotic optimization in hospitals.
Jennifer Broom, MBChB, PhD, is an infectious diseases physician at the Sunshine Coast Hospital and Health Service and an associate professor of medicine at the University of Queensland, Brisbane, Australia. Alex Broom, PhD, is professor of sociology in the School of Social Sciences at the University of New South Wales, Sydney.
CABG best for diabetes patients with CKD – or is it?
ROME – The use of coronary artery bypass graft surgery for revascularization in patients with multivessel CAD and comorbid diabetes plus chronic kidney disease was associated with a significantly lower risk of major cardiovascular and cerebrovascular events than was PCI with first-generation drug-eluting stents in a new secondary analysis from the landmark FREEDOM trial.
“The reason for this presentation is that even though chronic kidney disease is common in patients with diabetes, until now there has not been a large study of the efficacy and safety of coronary revascularization with drug-eluting stents versus CABG in this population in a randomized trial cohort,” explained Usman Baber, MD, who reported the results at the annual congress of the European Society of Cardiology.
FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) randomized 1,900 diabetic patients with multivessel CAD to PCI or CABG. As previously reported, CABG proved superior to PCI, with a significantly lower rate of the composite primary endpoint composed of all-cause mortality, MI, or stroke (N Engl J Med. 2012 Dec 20;367[25]:2375-84).
Dr. Baber presented a post hoc analysis of the 451 FREEDOM participants with baseline comorbid chronic kidney disease (CKD). Their mean SYNTAX score was 27, and their mean baseline estimated glomerular filtration rate was 44 mL/min per 1.73 m2, indicative of mild to moderate CKD.
“Only 28 patients in the FREEDOM trial had an estimated GFR below 30, therefore we can’t make any inferences about revascularization in that setting, which I think is a completely different population,” he noted.
The 5-year rate of major adverse cardiovascular and cerebrovascular events in patients with CKD was 26% in the CABG group, an absolute 9.4% less than the 35.6% rate in subjects randomized to PCI.
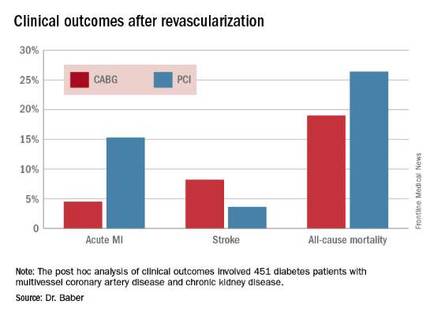
Roughly one-quarter of FREEDOM participants had CKD. They fared significantly worse than did those without CKD. The 5-year incidence of major adverse cardiovascular and cerebrovascular events was 30.8% in patients with CKD and 20.1% in patients without renal impairment. In a multivariate analysis adjusted for age, gender, hypertension, peripheral vascular disease, and other potential confounders, the risk of all-cause mortality was twofold higher in the CKD group. Their risk of cardiac death was increased 1.8-fold, and they were at 1.9-fold increased risk for stroke. Interestingly, however, the acute MI risk did not differ between patients with or without CKD, Dr. Baber observed.
Drilling deeper into the data, the cardiologist reported that CABG was associated with significantly lower rates of MI and a nonsignificant trend for fewer deaths, but with a significantly higher stroke rate than PCI.
One audience member rose to complain that this information won’t be helpful in counseling his diabetic patients with CKD and multivessel CAD because the choices look so grim: a higher risk of MI with percutaneous therapy, and a greater risk of stroke with surgery.
Dr. Baber replied by pointing out that the 10.8% absolute reduction in the risk of MI with CABG compared with PCI was more than twice as large as the absolute 4.6% increase in stroke risk with surgery.
“Most people would say that a heart attack is an inconvenience, and a stroke is a life-changing experience for them and their family,” said session cochair Kim A. Williams, MD, professor of medicine and chairman of cardiology at Rush University Medical Center in Chicago.
At that, Dr. Baber backtracked a bit, observing that since this was a post hoc analysis, the FREEDOM findings in patients with CKD must be viewed as hypothesis-generating rather than definitive. And, of course, contemporary second-generation drug-eluting stents have a better risk/benefit profile than do those used in FREEDOM.
“The number needed to treat/number needed to harm ratio for CABG and PCI probably ends up being roughly equal. The pertinence of an analysis like this is if you look at real-world registry-based data, you find a therapeutic nihilism that’s highly prevalent in CKD patients, where many patients who might benefit are not provided with revascularization therapy. It’s clear that we as clinicians – either because we don’t know there is a benefit or we are too concerned about potential harm – deprive patients of a treatment that might be beneficial. This analysis makes clinicians who might be concerned feel somewhat comforted that there is not unacceptable harm and that there is benefit,” Dr. Baber said.
Follow-up of FREEDOM participants continues and will be the subject of future reports, he added.
The FREEDOM trial was sponsored by the National Heart, Lung and Blood Institute. Dr. Baber reported having no financial conflicts of interest.
ROME – The use of coronary artery bypass graft surgery for revascularization in patients with multivessel CAD and comorbid diabetes plus chronic kidney disease was associated with a significantly lower risk of major cardiovascular and cerebrovascular events than was PCI with first-generation drug-eluting stents in a new secondary analysis from the landmark FREEDOM trial.
“The reason for this presentation is that even though chronic kidney disease is common in patients with diabetes, until now there has not been a large study of the efficacy and safety of coronary revascularization with drug-eluting stents versus CABG in this population in a randomized trial cohort,” explained Usman Baber, MD, who reported the results at the annual congress of the European Society of Cardiology.
FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) randomized 1,900 diabetic patients with multivessel CAD to PCI or CABG. As previously reported, CABG proved superior to PCI, with a significantly lower rate of the composite primary endpoint composed of all-cause mortality, MI, or stroke (N Engl J Med. 2012 Dec 20;367[25]:2375-84).
Dr. Baber presented a post hoc analysis of the 451 FREEDOM participants with baseline comorbid chronic kidney disease (CKD). Their mean SYNTAX score was 27, and their mean baseline estimated glomerular filtration rate was 44 mL/min per 1.73 m2, indicative of mild to moderate CKD.
“Only 28 patients in the FREEDOM trial had an estimated GFR below 30, therefore we can’t make any inferences about revascularization in that setting, which I think is a completely different population,” he noted.
The 5-year rate of major adverse cardiovascular and cerebrovascular events in patients with CKD was 26% in the CABG group, an absolute 9.4% less than the 35.6% rate in subjects randomized to PCI.

Roughly one-quarter of FREEDOM participants had CKD. They fared significantly worse than did those without CKD. The 5-year incidence of major adverse cardiovascular and cerebrovascular events was 30.8% in patients with CKD and 20.1% in patients without renal impairment. In a multivariate analysis adjusted for age, gender, hypertension, peripheral vascular disease, and other potential confounders, the risk of all-cause mortality was twofold higher in the CKD group. Their risk of cardiac death was increased 1.8-fold, and they were at 1.9-fold increased risk for stroke. Interestingly, however, the acute MI risk did not differ between patients with or without CKD, Dr. Baber observed.
Drilling deeper into the data, the cardiologist reported that CABG was associated with significantly lower rates of MI and a nonsignificant trend for fewer deaths, but with a significantly higher stroke rate than PCI.
One audience member rose to complain that this information won’t be helpful in counseling his diabetic patients with CKD and multivessel CAD because the choices look so grim: a higher risk of MI with percutaneous therapy, and a greater risk of stroke with surgery.
Dr. Baber replied by pointing out that the 10.8% absolute reduction in the risk of MI with CABG compared with PCI was more than twice as large as the absolute 4.6% increase in stroke risk with surgery.
“Most people would say that a heart attack is an inconvenience, and a stroke is a life-changing experience for them and their family,” said session cochair Kim A. Williams, MD, professor of medicine and chairman of cardiology at Rush University Medical Center in Chicago.
At that, Dr. Baber backtracked a bit, observing that since this was a post hoc analysis, the FREEDOM findings in patients with CKD must be viewed as hypothesis-generating rather than definitive. And, of course, contemporary second-generation drug-eluting stents have a better risk/benefit profile than do those used in FREEDOM.
“The number needed to treat/number needed to harm ratio for CABG and PCI probably ends up being roughly equal. The pertinence of an analysis like this is if you look at real-world registry-based data, you find a therapeutic nihilism that’s highly prevalent in CKD patients, where many patients who might benefit are not provided with revascularization therapy. It’s clear that we as clinicians – either because we don’t know there is a benefit or we are too concerned about potential harm – deprive patients of a treatment that might be beneficial. This analysis makes clinicians who might be concerned feel somewhat comforted that there is not unacceptable harm and that there is benefit,” Dr. Baber said.
Follow-up of FREEDOM participants continues and will be the subject of future reports, he added.
The FREEDOM trial was sponsored by the National Heart, Lung and Blood Institute. Dr. Baber reported having no financial conflicts of interest.
ROME – The use of coronary artery bypass graft surgery for revascularization in patients with multivessel CAD and comorbid diabetes plus chronic kidney disease was associated with a significantly lower risk of major cardiovascular and cerebrovascular events than was PCI with first-generation drug-eluting stents in a new secondary analysis from the landmark FREEDOM trial.
“The reason for this presentation is that even though chronic kidney disease is common in patients with diabetes, until now there has not been a large study of the efficacy and safety of coronary revascularization with drug-eluting stents versus CABG in this population in a randomized trial cohort,” explained Usman Baber, MD, who reported the results at the annual congress of the European Society of Cardiology.
FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) randomized 1,900 diabetic patients with multivessel CAD to PCI or CABG. As previously reported, CABG proved superior to PCI, with a significantly lower rate of the composite primary endpoint composed of all-cause mortality, MI, or stroke (N Engl J Med. 2012 Dec 20;367[25]:2375-84).
Dr. Baber presented a post hoc analysis of the 451 FREEDOM participants with baseline comorbid chronic kidney disease (CKD). Their mean SYNTAX score was 27, and their mean baseline estimated glomerular filtration rate was 44 mL/min per 1.73 m2, indicative of mild to moderate CKD.
“Only 28 patients in the FREEDOM trial had an estimated GFR below 30, therefore we can’t make any inferences about revascularization in that setting, which I think is a completely different population,” he noted.
The 5-year rate of major adverse cardiovascular and cerebrovascular events in patients with CKD was 26% in the CABG group, an absolute 9.4% less than the 35.6% rate in subjects randomized to PCI.

Roughly one-quarter of FREEDOM participants had CKD. They fared significantly worse than did those without CKD. The 5-year incidence of major adverse cardiovascular and cerebrovascular events was 30.8% in patients with CKD and 20.1% in patients without renal impairment. In a multivariate analysis adjusted for age, gender, hypertension, peripheral vascular disease, and other potential confounders, the risk of all-cause mortality was twofold higher in the CKD group. Their risk of cardiac death was increased 1.8-fold, and they were at 1.9-fold increased risk for stroke. Interestingly, however, the acute MI risk did not differ between patients with or without CKD, Dr. Baber observed.
Drilling deeper into the data, the cardiologist reported that CABG was associated with significantly lower rates of MI and a nonsignificant trend for fewer deaths, but with a significantly higher stroke rate than PCI.
One audience member rose to complain that this information won’t be helpful in counseling his diabetic patients with CKD and multivessel CAD because the choices look so grim: a higher risk of MI with percutaneous therapy, and a greater risk of stroke with surgery.
Dr. Baber replied by pointing out that the 10.8% absolute reduction in the risk of MI with CABG compared with PCI was more than twice as large as the absolute 4.6% increase in stroke risk with surgery.
“Most people would say that a heart attack is an inconvenience, and a stroke is a life-changing experience for them and their family,” said session cochair Kim A. Williams, MD, professor of medicine and chairman of cardiology at Rush University Medical Center in Chicago.
At that, Dr. Baber backtracked a bit, observing that since this was a post hoc analysis, the FREEDOM findings in patients with CKD must be viewed as hypothesis-generating rather than definitive. And, of course, contemporary second-generation drug-eluting stents have a better risk/benefit profile than do those used in FREEDOM.
“The number needed to treat/number needed to harm ratio for CABG and PCI probably ends up being roughly equal. The pertinence of an analysis like this is if you look at real-world registry-based data, you find a therapeutic nihilism that’s highly prevalent in CKD patients, where many patients who might benefit are not provided with revascularization therapy. It’s clear that we as clinicians – either because we don’t know there is a benefit or we are too concerned about potential harm – deprive patients of a treatment that might be beneficial. This analysis makes clinicians who might be concerned feel somewhat comforted that there is not unacceptable harm and that there is benefit,” Dr. Baber said.
Follow-up of FREEDOM participants continues and will be the subject of future reports, he added.
The FREEDOM trial was sponsored by the National Heart, Lung and Blood Institute. Dr. Baber reported having no financial conflicts of interest.
AT THE ESC CONGRESS 2016
Key clinical point: Coronary artery bypass graft surgery resulted in fewer myocardial infarctions but more strokes than did percutaneous coronary intervention at 5 years of follow-up in diabetic patients with multivessel coronary artery disease and chronic kidney disease.
Major finding: The cumulative MI rates in patients randomized to CABG versus PCI were 4.5% and 15.3%, respectively, while the stroke rates were 8.2% versus 3.6%.
Data source: A post hoc analysis of clinical outcomes in 451 diabetic patients with multivessel CAD and chronic kidney disease who were randomized to CABG or PCI in the prospective multicenter FREEDOM trial.
Disclosures: The FREEDOM trial was sponsored by the National Heart, Lung, and Blood Institute. The presenter reported having no financial conflicts of interest.
Parkinson’s cell transplant trials require better standardization
PORTLAND, ORE. – Implantation of dopamine-producing cells to replace those lost in the substantia nigra has been seen as the cure for Parkinson’s disease, with many attempts in patients over the years. Along the way, a few successes have occurred, but in general, cell-based therapies have produced mixed results.
“When it works well, it works very well with fetal dopamine cells, but it does not always work well,” said Roger Barker, MBBS, PhD, of the department of clinical neurosciences at the University of Cambridge (England). In his lecture to a large, capacity crowd at the World Parkinson Congress, he reviewed the field going back more than three decades and drew “lessons learned” from the results of animal experiments and human trials.
He said dopamine-producing cell therapy will never cure the disease, although it works well early in the disease “but creates problems later,” with off-target and nonphysiologic effects in other parts of the brain, such as overstimulation. One exception was the use of fetal dopamine cells in an animal model, showing that cells could survive long-term, connect and integrate into the brain, release dopamine, and restore behaviors to normal if cells from the same species were used at the right developmental stage of the animal and implanted where dopamine normally works, that is, in the striatum.
Other problems reported from human trials using embryonic dopamine neurons or fetal nigral transplants have been graft-induced dyskinesias, but without clinical benefit, as well as Lewy bodies in grafted neurons, suggesting host-to-graft disease propagation. However, in another report, a patient had a clinical benefit and showed extensive graft-derived dopaminergic innervation 24 years after transplantation, at which time the patient died.
Lessons learned over time
Dr. Barker explained that the variable results that have been achieved in various trials using different protocols and nonstandardized approaches over the years make it “extremely difficult to make any conclusions.” These variations included performing tests on different kinds of patients and using different doses of cells, delivery approaches, immunosuppression, primary endpoints of the trials, and levels of follow-up.
The age of the patient, disease stage, and graft technique emerged as key issues in data gathered from the trials, he said. The best chance of success occurred in younger patients with less advanced disease, when grafting occurred across the whole striatum evenly, and when contamination with 5-hydroxytryptamine (serotonin) neurons was avoided.
Going forward, trials in Parkinson’s disease are planned or underway using embryonic/fetal stem cells or adult stem cells. Such cells provide logistical advantages in that their differentiation can be controlled more easily and they are a defined product. Nigral dopaminergic cells can be produced from human embryonic stem cells that behave like human fetal ventral midbrain dopamine cells in vitro and in vivo in rats and show similar efficacy and potency.
The Center for iPS Cell Research and Application in Kyoto, Japan, will conduct a trial using induced pluripotent stem (iPS) cells beginning next year, the New York State Stem Cell Science Consortia (NYSTEM) will use human embryonic stem cells beginning in 2018, and around the same time the European Union’s NeuroStemCellRepair network will also use human embryonic stem cells. In addition, the European TRANSEURO trial, coordinated by Dr. Barker, is planning a single-arm, multicenter, dose-escalation trial for 2018/2019 using intracerebral neurotransplantation of dopaminergic neurons derived from human embryonic stem cells. Participating patients will be younger than 65 years with less than 10 years disease duration, no significant levodopa-induced dyskinesia, and no cognitive or psychiatric problems.
From these trials, scientists hope to eventually produce a human embryonic stem cell–derived dopaminergic cell product made under GMP (good manufacturing practice) conditions that can be tested for safety and efficacy and put into clinical trials, with an ultimate goal of production and commercialization.
Finally, Dr. Barker alerted physicians to published guidance from the International Society for Stem Cell Research that can help them critically evaluate any cell-based clinical trial that they may be asked to run. And for patients, large numbers of whom attended the conference, he advised avoiding any “trial” that would charge them to participate because legitimate research trials do not charge for experimental therapies.
Asked to comment on the field, Peter LeWitt, MD, director of the Parkinson’s Disease and Movement Disorders Program at Henry Ford Hospital, West Bloomfield, Mich., and professor of neurology at Wayne State University, Detroit, said, “The jury is out and is coming back on stem cells and its alternative, such as fetal tissue. It’s pointing to the bright future of restoring the brain and not just using drugs to mask symptoms.”
He said the critical review of evidence from past trials “sounds like it’s heading towards a more focused view of how to enhance the successes that have occurred already – the 24-year outcomes [of] the patients who have met all biological plausibility improvements at this point. ... The science has moved along to improve their techniques of judging success and failure and sorting out partial benefits.”
Dr. Barker reported that he sits on an advisory board of Teva-Lundbeck and has advised and received honoraria from Solvay, GSK, Eli Lilly, and Pfizer, receives royalties from Springer, Wiley, and Cambridge University Press, and receives grant support from various institutes and foundations, Dr. LeWitt reported no relevant conflicts of interest.
PORTLAND, ORE. – Implantation of dopamine-producing cells to replace those lost in the substantia nigra has been seen as the cure for Parkinson’s disease, with many attempts in patients over the years. Along the way, a few successes have occurred, but in general, cell-based therapies have produced mixed results.
“When it works well, it works very well with fetal dopamine cells, but it does not always work well,” said Roger Barker, MBBS, PhD, of the department of clinical neurosciences at the University of Cambridge (England). In his lecture to a large, capacity crowd at the World Parkinson Congress, he reviewed the field going back more than three decades and drew “lessons learned” from the results of animal experiments and human trials.
He said dopamine-producing cell therapy will never cure the disease, although it works well early in the disease “but creates problems later,” with off-target and nonphysiologic effects in other parts of the brain, such as overstimulation. One exception was the use of fetal dopamine cells in an animal model, showing that cells could survive long-term, connect and integrate into the brain, release dopamine, and restore behaviors to normal if cells from the same species were used at the right developmental stage of the animal and implanted where dopamine normally works, that is, in the striatum.
Other problems reported from human trials using embryonic dopamine neurons or fetal nigral transplants have been graft-induced dyskinesias, but without clinical benefit, as well as Lewy bodies in grafted neurons, suggesting host-to-graft disease propagation. However, in another report, a patient had a clinical benefit and showed extensive graft-derived dopaminergic innervation 24 years after transplantation, at which time the patient died.
Lessons learned over time
Dr. Barker explained that the variable results that have been achieved in various trials using different protocols and nonstandardized approaches over the years make it “extremely difficult to make any conclusions.” These variations included performing tests on different kinds of patients and using different doses of cells, delivery approaches, immunosuppression, primary endpoints of the trials, and levels of follow-up.
The age of the patient, disease stage, and graft technique emerged as key issues in data gathered from the trials, he said. The best chance of success occurred in younger patients with less advanced disease, when grafting occurred across the whole striatum evenly, and when contamination with 5-hydroxytryptamine (serotonin) neurons was avoided.
Going forward, trials in Parkinson’s disease are planned or underway using embryonic/fetal stem cells or adult stem cells. Such cells provide logistical advantages in that their differentiation can be controlled more easily and they are a defined product. Nigral dopaminergic cells can be produced from human embryonic stem cells that behave like human fetal ventral midbrain dopamine cells in vitro and in vivo in rats and show similar efficacy and potency.
The Center for iPS Cell Research and Application in Kyoto, Japan, will conduct a trial using induced pluripotent stem (iPS) cells beginning next year, the New York State Stem Cell Science Consortia (NYSTEM) will use human embryonic stem cells beginning in 2018, and around the same time the European Union’s NeuroStemCellRepair network will also use human embryonic stem cells. In addition, the European TRANSEURO trial, coordinated by Dr. Barker, is planning a single-arm, multicenter, dose-escalation trial for 2018/2019 using intracerebral neurotransplantation of dopaminergic neurons derived from human embryonic stem cells. Participating patients will be younger than 65 years with less than 10 years disease duration, no significant levodopa-induced dyskinesia, and no cognitive or psychiatric problems.
From these trials, scientists hope to eventually produce a human embryonic stem cell–derived dopaminergic cell product made under GMP (good manufacturing practice) conditions that can be tested for safety and efficacy and put into clinical trials, with an ultimate goal of production and commercialization.
Finally, Dr. Barker alerted physicians to published guidance from the International Society for Stem Cell Research that can help them critically evaluate any cell-based clinical trial that they may be asked to run. And for patients, large numbers of whom attended the conference, he advised avoiding any “trial” that would charge them to participate because legitimate research trials do not charge for experimental therapies.
Asked to comment on the field, Peter LeWitt, MD, director of the Parkinson’s Disease and Movement Disorders Program at Henry Ford Hospital, West Bloomfield, Mich., and professor of neurology at Wayne State University, Detroit, said, “The jury is out and is coming back on stem cells and its alternative, such as fetal tissue. It’s pointing to the bright future of restoring the brain and not just using drugs to mask symptoms.”
He said the critical review of evidence from past trials “sounds like it’s heading towards a more focused view of how to enhance the successes that have occurred already – the 24-year outcomes [of] the patients who have met all biological plausibility improvements at this point. ... The science has moved along to improve their techniques of judging success and failure and sorting out partial benefits.”
Dr. Barker reported that he sits on an advisory board of Teva-Lundbeck and has advised and received honoraria from Solvay, GSK, Eli Lilly, and Pfizer, receives royalties from Springer, Wiley, and Cambridge University Press, and receives grant support from various institutes and foundations, Dr. LeWitt reported no relevant conflicts of interest.
PORTLAND, ORE. – Implantation of dopamine-producing cells to replace those lost in the substantia nigra has been seen as the cure for Parkinson’s disease, with many attempts in patients over the years. Along the way, a few successes have occurred, but in general, cell-based therapies have produced mixed results.
“When it works well, it works very well with fetal dopamine cells, but it does not always work well,” said Roger Barker, MBBS, PhD, of the department of clinical neurosciences at the University of Cambridge (England). In his lecture to a large, capacity crowd at the World Parkinson Congress, he reviewed the field going back more than three decades and drew “lessons learned” from the results of animal experiments and human trials.
He said dopamine-producing cell therapy will never cure the disease, although it works well early in the disease “but creates problems later,” with off-target and nonphysiologic effects in other parts of the brain, such as overstimulation. One exception was the use of fetal dopamine cells in an animal model, showing that cells could survive long-term, connect and integrate into the brain, release dopamine, and restore behaviors to normal if cells from the same species were used at the right developmental stage of the animal and implanted where dopamine normally works, that is, in the striatum.
Other problems reported from human trials using embryonic dopamine neurons or fetal nigral transplants have been graft-induced dyskinesias, but without clinical benefit, as well as Lewy bodies in grafted neurons, suggesting host-to-graft disease propagation. However, in another report, a patient had a clinical benefit and showed extensive graft-derived dopaminergic innervation 24 years after transplantation, at which time the patient died.
Lessons learned over time
Dr. Barker explained that the variable results that have been achieved in various trials using different protocols and nonstandardized approaches over the years make it “extremely difficult to make any conclusions.” These variations included performing tests on different kinds of patients and using different doses of cells, delivery approaches, immunosuppression, primary endpoints of the trials, and levels of follow-up.
The age of the patient, disease stage, and graft technique emerged as key issues in data gathered from the trials, he said. The best chance of success occurred in younger patients with less advanced disease, when grafting occurred across the whole striatum evenly, and when contamination with 5-hydroxytryptamine (serotonin) neurons was avoided.
Going forward, trials in Parkinson’s disease are planned or underway using embryonic/fetal stem cells or adult stem cells. Such cells provide logistical advantages in that their differentiation can be controlled more easily and they are a defined product. Nigral dopaminergic cells can be produced from human embryonic stem cells that behave like human fetal ventral midbrain dopamine cells in vitro and in vivo in rats and show similar efficacy and potency.
The Center for iPS Cell Research and Application in Kyoto, Japan, will conduct a trial using induced pluripotent stem (iPS) cells beginning next year, the New York State Stem Cell Science Consortia (NYSTEM) will use human embryonic stem cells beginning in 2018, and around the same time the European Union’s NeuroStemCellRepair network will also use human embryonic stem cells. In addition, the European TRANSEURO trial, coordinated by Dr. Barker, is planning a single-arm, multicenter, dose-escalation trial for 2018/2019 using intracerebral neurotransplantation of dopaminergic neurons derived from human embryonic stem cells. Participating patients will be younger than 65 years with less than 10 years disease duration, no significant levodopa-induced dyskinesia, and no cognitive or psychiatric problems.
From these trials, scientists hope to eventually produce a human embryonic stem cell–derived dopaminergic cell product made under GMP (good manufacturing practice) conditions that can be tested for safety and efficacy and put into clinical trials, with an ultimate goal of production and commercialization.
Finally, Dr. Barker alerted physicians to published guidance from the International Society for Stem Cell Research that can help them critically evaluate any cell-based clinical trial that they may be asked to run. And for patients, large numbers of whom attended the conference, he advised avoiding any “trial” that would charge them to participate because legitimate research trials do not charge for experimental therapies.
Asked to comment on the field, Peter LeWitt, MD, director of the Parkinson’s Disease and Movement Disorders Program at Henry Ford Hospital, West Bloomfield, Mich., and professor of neurology at Wayne State University, Detroit, said, “The jury is out and is coming back on stem cells and its alternative, such as fetal tissue. It’s pointing to the bright future of restoring the brain and not just using drugs to mask symptoms.”
He said the critical review of evidence from past trials “sounds like it’s heading towards a more focused view of how to enhance the successes that have occurred already – the 24-year outcomes [of] the patients who have met all biological plausibility improvements at this point. ... The science has moved along to improve their techniques of judging success and failure and sorting out partial benefits.”
Dr. Barker reported that he sits on an advisory board of Teva-Lundbeck and has advised and received honoraria from Solvay, GSK, Eli Lilly, and Pfizer, receives royalties from Springer, Wiley, and Cambridge University Press, and receives grant support from various institutes and foundations, Dr. LeWitt reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM WPC 2016
Americas are declared measles free by PAHO
The Americas are measles free.
The region is the first in the world to have eradicated the disease, according to Pan-American Health Organization officials who spoke during a media briefing.
“Bye-bye measles,” said PAHO director Carissa F. Etienne, MD, during the briefing.
In 1994, countries across the Americas collectively declared their determination to rid the region of endemic measles transmission by 2000, agreeing to implement PAHO-recommended surveillance and immunization strategies, including the introduction and expanded use of the triple viral vaccine (MMR) against measles, mumps, and rubella.
In 2007, an expert international committee began verifying absence of the disease regionally. Although the disease was largely thought to have been wiped out in the Americas by 2002, officials postponed making an announcement until elimination of both measles and rubella could be declared jointly. An outbreak of measles in 2013 thwarted this plan, with the last confirmed case of reported endemic measles in the Americas not occurring until July 2015 in Brazil. In all, five vaccine-preventable diseases have been eliminated in the region, according to PAHO: smallpox in 1971, polio in 1994, and rubella and congenital rubella syndrome in 2015.
“The tasks of verification was not without its challenges, and countries overcame a number of geopolitical difficulties, including [maintaining] high vaccination coverage for very mobile populations, limited – and often no – access to deprived areas, and the presence of conflict situations,” Merceline Dahl-Regis, MD, the Bahamas’ chief medical officer and chair of PAHO’s elimination verification committee, said during the media briefing. She credited the expansion of national immunization programs, the “stellar work” conducted by various national commissions, and excellent communication between the nations’ respective governments, local and federal, as critical to the success of the effort. Also credited by other officials is PAHO’s revolving vaccine fund, which provides five-dose vials of MMR for about 1 dollar each.
Globally, since use of the MMR vaccine began, there has been a 95% drop in cases over a 35-year period, from 4.5 million cases in 1980 to approximately 244,700 in 2015. At the time the initiative to eradicate the disease was announced, measles was among the top five most common killers of children under the age of 5 years worldwide. The vaccine-preventable disease took the lives of 114,900 children globally in 2014, according to the World Health Organization. Worldwide, about 85% of children are vaccinated against measles by their first birthday, up from 73% in 2000.
Keeping vaccination rates high and immediately reporting any imported cases of the disease are essential to maintaining this milestone, according to PAHO officials.
“Importation of measles from other parts of the world into the Americas will continue, and we must be ready to detect and respond quickly, but today, let us celebrate,” Capt. Mary Agocs, MD, a Red Cross senior adviser to the elimination initiative, said during the briefing.
On Twitter @whitneymcknight
The Americas are measles free.
The region is the first in the world to have eradicated the disease, according to Pan-American Health Organization officials who spoke during a media briefing.
“Bye-bye measles,” said PAHO director Carissa F. Etienne, MD, during the briefing.
In 1994, countries across the Americas collectively declared their determination to rid the region of endemic measles transmission by 2000, agreeing to implement PAHO-recommended surveillance and immunization strategies, including the introduction and expanded use of the triple viral vaccine (MMR) against measles, mumps, and rubella.
In 2007, an expert international committee began verifying absence of the disease regionally. Although the disease was largely thought to have been wiped out in the Americas by 2002, officials postponed making an announcement until elimination of both measles and rubella could be declared jointly. An outbreak of measles in 2013 thwarted this plan, with the last confirmed case of reported endemic measles in the Americas not occurring until July 2015 in Brazil. In all, five vaccine-preventable diseases have been eliminated in the region, according to PAHO: smallpox in 1971, polio in 1994, and rubella and congenital rubella syndrome in 2015.
“The tasks of verification was not without its challenges, and countries overcame a number of geopolitical difficulties, including [maintaining] high vaccination coverage for very mobile populations, limited – and often no – access to deprived areas, and the presence of conflict situations,” Merceline Dahl-Regis, MD, the Bahamas’ chief medical officer and chair of PAHO’s elimination verification committee, said during the media briefing. She credited the expansion of national immunization programs, the “stellar work” conducted by various national commissions, and excellent communication between the nations’ respective governments, local and federal, as critical to the success of the effort. Also credited by other officials is PAHO’s revolving vaccine fund, which provides five-dose vials of MMR for about 1 dollar each.
Globally, since use of the MMR vaccine began, there has been a 95% drop in cases over a 35-year period, from 4.5 million cases in 1980 to approximately 244,700 in 2015. At the time the initiative to eradicate the disease was announced, measles was among the top five most common killers of children under the age of 5 years worldwide. The vaccine-preventable disease took the lives of 114,900 children globally in 2014, according to the World Health Organization. Worldwide, about 85% of children are vaccinated against measles by their first birthday, up from 73% in 2000.
Keeping vaccination rates high and immediately reporting any imported cases of the disease are essential to maintaining this milestone, according to PAHO officials.
“Importation of measles from other parts of the world into the Americas will continue, and we must be ready to detect and respond quickly, but today, let us celebrate,” Capt. Mary Agocs, MD, a Red Cross senior adviser to the elimination initiative, said during the briefing.
On Twitter @whitneymcknight
The Americas are measles free.
The region is the first in the world to have eradicated the disease, according to Pan-American Health Organization officials who spoke during a media briefing.
“Bye-bye measles,” said PAHO director Carissa F. Etienne, MD, during the briefing.
In 1994, countries across the Americas collectively declared their determination to rid the region of endemic measles transmission by 2000, agreeing to implement PAHO-recommended surveillance and immunization strategies, including the introduction and expanded use of the triple viral vaccine (MMR) against measles, mumps, and rubella.
In 2007, an expert international committee began verifying absence of the disease regionally. Although the disease was largely thought to have been wiped out in the Americas by 2002, officials postponed making an announcement until elimination of both measles and rubella could be declared jointly. An outbreak of measles in 2013 thwarted this plan, with the last confirmed case of reported endemic measles in the Americas not occurring until July 2015 in Brazil. In all, five vaccine-preventable diseases have been eliminated in the region, according to PAHO: smallpox in 1971, polio in 1994, and rubella and congenital rubella syndrome in 2015.
“The tasks of verification was not without its challenges, and countries overcame a number of geopolitical difficulties, including [maintaining] high vaccination coverage for very mobile populations, limited – and often no – access to deprived areas, and the presence of conflict situations,” Merceline Dahl-Regis, MD, the Bahamas’ chief medical officer and chair of PAHO’s elimination verification committee, said during the media briefing. She credited the expansion of national immunization programs, the “stellar work” conducted by various national commissions, and excellent communication between the nations’ respective governments, local and federal, as critical to the success of the effort. Also credited by other officials is PAHO’s revolving vaccine fund, which provides five-dose vials of MMR for about 1 dollar each.
Globally, since use of the MMR vaccine began, there has been a 95% drop in cases over a 35-year period, from 4.5 million cases in 1980 to approximately 244,700 in 2015. At the time the initiative to eradicate the disease was announced, measles was among the top five most common killers of children under the age of 5 years worldwide. The vaccine-preventable disease took the lives of 114,900 children globally in 2014, according to the World Health Organization. Worldwide, about 85% of children are vaccinated against measles by their first birthday, up from 73% in 2000.
Keeping vaccination rates high and immediately reporting any imported cases of the disease are essential to maintaining this milestone, according to PAHO officials.
“Importation of measles from other parts of the world into the Americas will continue, and we must be ready to detect and respond quickly, but today, let us celebrate,” Capt. Mary Agocs, MD, a Red Cross senior adviser to the elimination initiative, said during the briefing.
On Twitter @whitneymcknight
FROM A PAHO MEDIA BRIEFING
Aspirin not prescribed appropriately to cut cardiovascular risk in diabetes
MUNICH – Many patients with diabetes who could benefit from low-dose aspirin therapy may not be getting it – and many who are getting aspirin should not be, according to data presented at the annual meeting of the European Association for the Study of Diabetes.
A large, randomized trial concluded that 21% of diabetes patients who qualified for aspirin therapy for cardiovascular risk reduction were not getting it, and that it was contraindicated in almost 60% of those who were taking it, Lauren Crain, PhD, reported at the meeting.
Balancing the risks and benefits of aspirin therapy is not an easy challenge, said Dr. Crain, a health behavior researcher at HealthPartners Institute, Minneapolis. The clinical information necessary for the assessment is “rather lengthy, and not always readily available in primary care settings,” she said, and it’s clear from this study that clinicians could use some help in this area. Unfortunately, the electronic algorithm tested, which was meant to improve appropriate aspirin prescribing, didn’t improve the situation very much.
“At the final visit in the diabetes group [after the algorithm was employed], the total proportion of patients using aspirin was higher than at the first visit,” Dr. Crain noted. “However, that was the case regardless of whether patients were over- or underusing aspirin at the first visit.”
The aspirin findings were part of a large, randomized trial testing the algorithm as a way to reduce cardiovascular risk factors. The study was conducted in 19 primary care practices.
The decision-making algorithm, Cardiovascular Wizard, uses electronic health records to identify and advise patients with uncontrolled cardiovascular risk factors. Priorities and clinical recommendations are displayed for the provider and patient in the hope of facilitating shared decision making, Dr. Crain said.
One of the Wizard’s algorithms concerns aspirin prescribing. It is programmed with data from the United States Preventive Services Task Force, and recommends aspirin if cardiovascular risk scores are high and if consistent with providing a benefit greater than the risk of gastrointestinal bleeding. Aspirin is not recommended if the benefit is determined to be low or if major contraindications are present, including anticoagulant use or history of intracerebral hemorrhage.
The tool also alerts providers to the presence of other potential risks including aspirin allergy or intolerance, history of GI bleeds or risk conditions, and the concomitant use of nonsteroidal anti-inflammatory drugs.
The study comprised 11,000 adults, 4,000 of whom had diabetes. The remainder had high-risk, reversible cardiovascular risk factors (hypertension, dyslipidemia, or tobacco use). Each group was randomized to either cardiovascular risk assessment by usual care or with the Cardiovascular Wizard program.
The aspirin substudy looked at aspirin use at the baseline visit and the patient’s final, 1-year follow-up visit. At both visits, aspirin use was documented, and the clinician used the Wizard to assess whether or not it was indicated.
At the baseline visit, 71% of the diabetes group was using aspirin. However, according to the Wizard tool, more than one-third of them should not have been taking it – and among these, 57% were doing so. Among the remaining two-thirds of patients, all of whom should have been using aspirin, 21% were not taking it, Dr. Crain said.
Among the patients with reversible high-risk factors, 27% were using aspirin. However, according to the Wizard tool, the drug was contraindicated in 34% of those patients. “Most importantly, however, among those for whom aspirin was indicated, only 25% were using it – so, we’re talking about a 75% underusage,” Dr. Crain said.
By the 1-year follow-up visit, the situation was not much changed, despite the tool’s recommendations. Among those with diabetes, 56% in the usual care group and 60% in Wizard group were still overusing aspirin. Underuse was occurring in 21% of the usual care group and 17% of the Wizard group.
Patients with reversible high-risk factors fared a little better at 1 year, especially those who, at baseline, should have been taking aspirin but were not. Among these, 10% in the usual care group and 13% in the Wizard group had started taking aspirin.
The results were a bit of a disappointment, Dr. Crain said, but they don’t invalidate the investigators’ faith in an algorithmic advising system.
“We do think that electronic health record tools like this can help providers follow guidelines and improve the quality of their aspirin recommendations and prescribing, and hopefully reduce cardiovascular events and aspirin-related hazards,” she said. “Unfortunately, that didn’t happen here in the diabetes patients,” and the results in the second group were not stellar.
She added that the Wizard development team will be tweaking the tool to clarify some of the choices available as it guides patients and providers through the algorithm, in hopes of improving its efficacy.
Dr. Crain made no financial disclosures.
On Twitter @alz_gal
MUNICH – Many patients with diabetes who could benefit from low-dose aspirin therapy may not be getting it – and many who are getting aspirin should not be, according to data presented at the annual meeting of the European Association for the Study of Diabetes.
A large, randomized trial concluded that 21% of diabetes patients who qualified for aspirin therapy for cardiovascular risk reduction were not getting it, and that it was contraindicated in almost 60% of those who were taking it, Lauren Crain, PhD, reported at the meeting.
Balancing the risks and benefits of aspirin therapy is not an easy challenge, said Dr. Crain, a health behavior researcher at HealthPartners Institute, Minneapolis. The clinical information necessary for the assessment is “rather lengthy, and not always readily available in primary care settings,” she said, and it’s clear from this study that clinicians could use some help in this area. Unfortunately, the electronic algorithm tested, which was meant to improve appropriate aspirin prescribing, didn’t improve the situation very much.
“At the final visit in the diabetes group [after the algorithm was employed], the total proportion of patients using aspirin was higher than at the first visit,” Dr. Crain noted. “However, that was the case regardless of whether patients were over- or underusing aspirin at the first visit.”
The aspirin findings were part of a large, randomized trial testing the algorithm as a way to reduce cardiovascular risk factors. The study was conducted in 19 primary care practices.
The decision-making algorithm, Cardiovascular Wizard, uses electronic health records to identify and advise patients with uncontrolled cardiovascular risk factors. Priorities and clinical recommendations are displayed for the provider and patient in the hope of facilitating shared decision making, Dr. Crain said.
One of the Wizard’s algorithms concerns aspirin prescribing. It is programmed with data from the United States Preventive Services Task Force, and recommends aspirin if cardiovascular risk scores are high and if consistent with providing a benefit greater than the risk of gastrointestinal bleeding. Aspirin is not recommended if the benefit is determined to be low or if major contraindications are present, including anticoagulant use or history of intracerebral hemorrhage.
The tool also alerts providers to the presence of other potential risks including aspirin allergy or intolerance, history of GI bleeds or risk conditions, and the concomitant use of nonsteroidal anti-inflammatory drugs.
The study comprised 11,000 adults, 4,000 of whom had diabetes. The remainder had high-risk, reversible cardiovascular risk factors (hypertension, dyslipidemia, or tobacco use). Each group was randomized to either cardiovascular risk assessment by usual care or with the Cardiovascular Wizard program.
The aspirin substudy looked at aspirin use at the baseline visit and the patient’s final, 1-year follow-up visit. At both visits, aspirin use was documented, and the clinician used the Wizard to assess whether or not it was indicated.
At the baseline visit, 71% of the diabetes group was using aspirin. However, according to the Wizard tool, more than one-third of them should not have been taking it – and among these, 57% were doing so. Among the remaining two-thirds of patients, all of whom should have been using aspirin, 21% were not taking it, Dr. Crain said.
Among the patients with reversible high-risk factors, 27% were using aspirin. However, according to the Wizard tool, the drug was contraindicated in 34% of those patients. “Most importantly, however, among those for whom aspirin was indicated, only 25% were using it – so, we’re talking about a 75% underusage,” Dr. Crain said.
By the 1-year follow-up visit, the situation was not much changed, despite the tool’s recommendations. Among those with diabetes, 56% in the usual care group and 60% in Wizard group were still overusing aspirin. Underuse was occurring in 21% of the usual care group and 17% of the Wizard group.
Patients with reversible high-risk factors fared a little better at 1 year, especially those who, at baseline, should have been taking aspirin but were not. Among these, 10% in the usual care group and 13% in the Wizard group had started taking aspirin.
The results were a bit of a disappointment, Dr. Crain said, but they don’t invalidate the investigators’ faith in an algorithmic advising system.
“We do think that electronic health record tools like this can help providers follow guidelines and improve the quality of their aspirin recommendations and prescribing, and hopefully reduce cardiovascular events and aspirin-related hazards,” she said. “Unfortunately, that didn’t happen here in the diabetes patients,” and the results in the second group were not stellar.
She added that the Wizard development team will be tweaking the tool to clarify some of the choices available as it guides patients and providers through the algorithm, in hopes of improving its efficacy.
Dr. Crain made no financial disclosures.
On Twitter @alz_gal
MUNICH – Many patients with diabetes who could benefit from low-dose aspirin therapy may not be getting it – and many who are getting aspirin should not be, according to data presented at the annual meeting of the European Association for the Study of Diabetes.
A large, randomized trial concluded that 21% of diabetes patients who qualified for aspirin therapy for cardiovascular risk reduction were not getting it, and that it was contraindicated in almost 60% of those who were taking it, Lauren Crain, PhD, reported at the meeting.
Balancing the risks and benefits of aspirin therapy is not an easy challenge, said Dr. Crain, a health behavior researcher at HealthPartners Institute, Minneapolis. The clinical information necessary for the assessment is “rather lengthy, and not always readily available in primary care settings,” she said, and it’s clear from this study that clinicians could use some help in this area. Unfortunately, the electronic algorithm tested, which was meant to improve appropriate aspirin prescribing, didn’t improve the situation very much.
“At the final visit in the diabetes group [after the algorithm was employed], the total proportion of patients using aspirin was higher than at the first visit,” Dr. Crain noted. “However, that was the case regardless of whether patients were over- or underusing aspirin at the first visit.”
The aspirin findings were part of a large, randomized trial testing the algorithm as a way to reduce cardiovascular risk factors. The study was conducted in 19 primary care practices.
The decision-making algorithm, Cardiovascular Wizard, uses electronic health records to identify and advise patients with uncontrolled cardiovascular risk factors. Priorities and clinical recommendations are displayed for the provider and patient in the hope of facilitating shared decision making, Dr. Crain said.
One of the Wizard’s algorithms concerns aspirin prescribing. It is programmed with data from the United States Preventive Services Task Force, and recommends aspirin if cardiovascular risk scores are high and if consistent with providing a benefit greater than the risk of gastrointestinal bleeding. Aspirin is not recommended if the benefit is determined to be low or if major contraindications are present, including anticoagulant use or history of intracerebral hemorrhage.
The tool also alerts providers to the presence of other potential risks including aspirin allergy or intolerance, history of GI bleeds or risk conditions, and the concomitant use of nonsteroidal anti-inflammatory drugs.
The study comprised 11,000 adults, 4,000 of whom had diabetes. The remainder had high-risk, reversible cardiovascular risk factors (hypertension, dyslipidemia, or tobacco use). Each group was randomized to either cardiovascular risk assessment by usual care or with the Cardiovascular Wizard program.
The aspirin substudy looked at aspirin use at the baseline visit and the patient’s final, 1-year follow-up visit. At both visits, aspirin use was documented, and the clinician used the Wizard to assess whether or not it was indicated.
At the baseline visit, 71% of the diabetes group was using aspirin. However, according to the Wizard tool, more than one-third of them should not have been taking it – and among these, 57% were doing so. Among the remaining two-thirds of patients, all of whom should have been using aspirin, 21% were not taking it, Dr. Crain said.
Among the patients with reversible high-risk factors, 27% were using aspirin. However, according to the Wizard tool, the drug was contraindicated in 34% of those patients. “Most importantly, however, among those for whom aspirin was indicated, only 25% were using it – so, we’re talking about a 75% underusage,” Dr. Crain said.
By the 1-year follow-up visit, the situation was not much changed, despite the tool’s recommendations. Among those with diabetes, 56% in the usual care group and 60% in Wizard group were still overusing aspirin. Underuse was occurring in 21% of the usual care group and 17% of the Wizard group.
Patients with reversible high-risk factors fared a little better at 1 year, especially those who, at baseline, should have been taking aspirin but were not. Among these, 10% in the usual care group and 13% in the Wizard group had started taking aspirin.
The results were a bit of a disappointment, Dr. Crain said, but they don’t invalidate the investigators’ faith in an algorithmic advising system.
“We do think that electronic health record tools like this can help providers follow guidelines and improve the quality of their aspirin recommendations and prescribing, and hopefully reduce cardiovascular events and aspirin-related hazards,” she said. “Unfortunately, that didn’t happen here in the diabetes patients,” and the results in the second group were not stellar.
She added that the Wizard development team will be tweaking the tool to clarify some of the choices available as it guides patients and providers through the algorithm, in hopes of improving its efficacy.
Dr. Crain made no financial disclosures.
On Twitter @alz_gal
AT EASD 2016
Key clinical point: Many diabetes patients who should be taking aspirin for cardiovascular risk reduction are not doing so, and many who should not be taking it are.
Major finding: Aspirin was underused in 21% of diabetes patients and overused in 57% of patients.
Data source: A randomized study of 11,000 patients.
Disclosures: Dr. Lauren Crain had no financial disclosures.
Nearly half of patients readmitted after liver transplant
Nearly half of patients were readmitted to the hospital within 90 days of liver transplantation, according to a single-center retrospective study.
“As readmission portends decreased survival, an emphasis should be placed on identifying and optimizing those at increased risk. If readmission does occur, however, it presents an opportunity to intervene, as virtually no patients died during initial readmission,” Madhukar S. Patel, MD, and his associates at Massachusetts General Hospital, Boston, wrote online in HPB.
Long wait times for liver transplantation in this part of the country lead to “high patient acuity,” the researchers noted. To better understand the correlates and consequences of posttransplant readmissions, they reviewed the records for 325 adults who underwent liver transplantation at their hospital between 2005 and 2015. Patients averaged 56 years old and had awaited transplant for a mean of 1 year (standard deviation, 506 days). Their average MELD (Model for End-Stage Liver Disease) scores were 30.3 at transplant (SD, 5.8), and 16.9 (SD, 9.4) on postoperative day 5. Their average hospital length of stay was 12 days, the investigators reported (HPB. 2016 Sep 15. doi: 10.1016/j.hpb.2016.08.003). A total of 149 patients (46%) were readmitted within 90 days of discharge, most often for infections (28% of readmissions), followed by medication issues (19%) and biliary complications (11%). The strongest predictor of posttransplant readmission was hepatitis C virus (HCV) infection, which more than doubled the odds of readmission, compared with alcoholic liver disease (odds ratio, 2.37; 95% confidence interval, 1.44-3.91; P = .001). Transplantees with HCV might benefit from closer outpatient follow-up to detect worsening liver function, diagnostic algorithms to help prevent unnecessary readmissions, and associated nosocomial infections, and pre- and posttransplant direct-acting antiviral therapy, “although the impact [of direct-acting antiviral] therapy on readmissions] is unknown at this time,” the investigators said.
The multivariable analysis also linked readmissions to longer hospital stays (OR, 1.03; P = .04), while age and male sex were protective factors, the investigators said. “Although speculative, it is possible that these factors may be protective due to differences in social support structures upon discharge,” they wrote, noting that women are more likely than men to outlive their partners and thus to live alone in later life.
Readmission within 90 days was associated with a significantly lower rate of survival at 5 years (75% vs. 88% for patients who were not readmitted; P = .008). But only one patient died during the initial readmission, “suggesting that when readmission does occur, it may be an opportunity to intervene,” the researchers said. Strategies include earlier extubation and removal of indwelling catheters, decreasing levels of immunosuppression, lowering treatment thresholds, and shifting patients with laboratory abnormalities to the outpatient setting, they noted. “At our center a process has been initiated in which the inpatient transplant attending surgeon directly passes off discharged patients to the outpatient team,” the investigators wrote. “Additionally, for patients discharged to an acute rehabilitation facility, a specific transplant physician point of contact is provided to the team at the rehab center in case any questions or issues arise [after] discharge. Although these strategies are a reasonable starting point, follow-up studies remain necessary in order to evaluate the impact of these interventions in this patient cohort.”
The researchers reported no funding sources and had no relevant financial disclosures.
Nearly half of patients were readmitted to the hospital within 90 days of liver transplantation, according to a single-center retrospective study.
“As readmission portends decreased survival, an emphasis should be placed on identifying and optimizing those at increased risk. If readmission does occur, however, it presents an opportunity to intervene, as virtually no patients died during initial readmission,” Madhukar S. Patel, MD, and his associates at Massachusetts General Hospital, Boston, wrote online in HPB.
Long wait times for liver transplantation in this part of the country lead to “high patient acuity,” the researchers noted. To better understand the correlates and consequences of posttransplant readmissions, they reviewed the records for 325 adults who underwent liver transplantation at their hospital between 2005 and 2015. Patients averaged 56 years old and had awaited transplant for a mean of 1 year (standard deviation, 506 days). Their average MELD (Model for End-Stage Liver Disease) scores were 30.3 at transplant (SD, 5.8), and 16.9 (SD, 9.4) on postoperative day 5. Their average hospital length of stay was 12 days, the investigators reported (HPB. 2016 Sep 15. doi: 10.1016/j.hpb.2016.08.003). A total of 149 patients (46%) were readmitted within 90 days of discharge, most often for infections (28% of readmissions), followed by medication issues (19%) and biliary complications (11%). The strongest predictor of posttransplant readmission was hepatitis C virus (HCV) infection, which more than doubled the odds of readmission, compared with alcoholic liver disease (odds ratio, 2.37; 95% confidence interval, 1.44-3.91; P = .001). Transplantees with HCV might benefit from closer outpatient follow-up to detect worsening liver function, diagnostic algorithms to help prevent unnecessary readmissions, and associated nosocomial infections, and pre- and posttransplant direct-acting antiviral therapy, “although the impact [of direct-acting antiviral] therapy on readmissions] is unknown at this time,” the investigators said.
The multivariable analysis also linked readmissions to longer hospital stays (OR, 1.03; P = .04), while age and male sex were protective factors, the investigators said. “Although speculative, it is possible that these factors may be protective due to differences in social support structures upon discharge,” they wrote, noting that women are more likely than men to outlive their partners and thus to live alone in later life.
Readmission within 90 days was associated with a significantly lower rate of survival at 5 years (75% vs. 88% for patients who were not readmitted; P = .008). But only one patient died during the initial readmission, “suggesting that when readmission does occur, it may be an opportunity to intervene,” the researchers said. Strategies include earlier extubation and removal of indwelling catheters, decreasing levels of immunosuppression, lowering treatment thresholds, and shifting patients with laboratory abnormalities to the outpatient setting, they noted. “At our center a process has been initiated in which the inpatient transplant attending surgeon directly passes off discharged patients to the outpatient team,” the investigators wrote. “Additionally, for patients discharged to an acute rehabilitation facility, a specific transplant physician point of contact is provided to the team at the rehab center in case any questions or issues arise [after] discharge. Although these strategies are a reasonable starting point, follow-up studies remain necessary in order to evaluate the impact of these interventions in this patient cohort.”
The researchers reported no funding sources and had no relevant financial disclosures.
Nearly half of patients were readmitted to the hospital within 90 days of liver transplantation, according to a single-center retrospective study.
“As readmission portends decreased survival, an emphasis should be placed on identifying and optimizing those at increased risk. If readmission does occur, however, it presents an opportunity to intervene, as virtually no patients died during initial readmission,” Madhukar S. Patel, MD, and his associates at Massachusetts General Hospital, Boston, wrote online in HPB.
Long wait times for liver transplantation in this part of the country lead to “high patient acuity,” the researchers noted. To better understand the correlates and consequences of posttransplant readmissions, they reviewed the records for 325 adults who underwent liver transplantation at their hospital between 2005 and 2015. Patients averaged 56 years old and had awaited transplant for a mean of 1 year (standard deviation, 506 days). Their average MELD (Model for End-Stage Liver Disease) scores were 30.3 at transplant (SD, 5.8), and 16.9 (SD, 9.4) on postoperative day 5. Their average hospital length of stay was 12 days, the investigators reported (HPB. 2016 Sep 15. doi: 10.1016/j.hpb.2016.08.003). A total of 149 patients (46%) were readmitted within 90 days of discharge, most often for infections (28% of readmissions), followed by medication issues (19%) and biliary complications (11%). The strongest predictor of posttransplant readmission was hepatitis C virus (HCV) infection, which more than doubled the odds of readmission, compared with alcoholic liver disease (odds ratio, 2.37; 95% confidence interval, 1.44-3.91; P = .001). Transplantees with HCV might benefit from closer outpatient follow-up to detect worsening liver function, diagnostic algorithms to help prevent unnecessary readmissions, and associated nosocomial infections, and pre- and posttransplant direct-acting antiviral therapy, “although the impact [of direct-acting antiviral] therapy on readmissions] is unknown at this time,” the investigators said.
The multivariable analysis also linked readmissions to longer hospital stays (OR, 1.03; P = .04), while age and male sex were protective factors, the investigators said. “Although speculative, it is possible that these factors may be protective due to differences in social support structures upon discharge,” they wrote, noting that women are more likely than men to outlive their partners and thus to live alone in later life.
Readmission within 90 days was associated with a significantly lower rate of survival at 5 years (75% vs. 88% for patients who were not readmitted; P = .008). But only one patient died during the initial readmission, “suggesting that when readmission does occur, it may be an opportunity to intervene,” the researchers said. Strategies include earlier extubation and removal of indwelling catheters, decreasing levels of immunosuppression, lowering treatment thresholds, and shifting patients with laboratory abnormalities to the outpatient setting, they noted. “At our center a process has been initiated in which the inpatient transplant attending surgeon directly passes off discharged patients to the outpatient team,” the investigators wrote. “Additionally, for patients discharged to an acute rehabilitation facility, a specific transplant physician point of contact is provided to the team at the rehab center in case any questions or issues arise [after] discharge. Although these strategies are a reasonable starting point, follow-up studies remain necessary in order to evaluate the impact of these interventions in this patient cohort.”
The researchers reported no funding sources and had no relevant financial disclosures.
FROM HPB
Key clinical point: Nearly half of liver recipients at Massachusetts General Hospital were readmitted within 90 days.
Major finding: A total of 46% of patients were readmitted, with hepatitis C virus infection being the strongest predictor of readmission (odds ratio, 2.37, compared with alcoholic liver disease).
Data source: A single-center retrospective study of 325 liver transplant patients between 2005 and 2015.
Disclosures: The researchers reported no funding sources and had no relevant financial disclosures.
AATS Submission Opportunities
Current and future CT surgery division chiefs/department chairs are invited to apply for the AATS Leadership Academy Program.
Friday, April 28, 2017
AATS Centennial
Boston, MA
This intensive, didactic and interactive program brings together up to 20 international surgeons who have demonstrated significant promise as potential future division chiefs or have recent assumed that role. The program provides participants with administrative, interpersonal, mentoring and negotiating skills, as well as the opportunity to network with well-known thoracic surgeon leaders and potential mentors.
Deadline: November 30, 2016
Qualifications/More Information
Don’t miss the opportunity to submit to one of these AATS scholarship programs.
Deadline: January 20, 2017
AATS Member for a Day
North American medical students, and general and up to third year integrated CT Surgery
(I-6) surgery residents can accompany an AATS Member during portions of the AATS Centennial as an AATS Member for a Day.
The meeting takes place April 29-May 3, 2017 in Boston, MA.
Those selected will receive free hotel accommodations for three to four night in an AATS Centennial hotel. They will also be given a $250 meal and $500 travel stipend at the end of the meeting.
Eligibility/More information
Summer Internship Opportunity for First/Second Year Medical Students
First and second year medical students can spend the summer being exposed to cardiothoracic surgery thanks to the AATS Summer Intern Scholarship. For eight weeks (June – September), students will work in the CT department of an AATS member.
Those chosen receive $2,500 for living expenses. They also will be able to attend the AATS Centennial gratis.
The meeting takes place April 29 – May 3, 2017 in Boston, MA.
More information
AATS Resident Poster Competition
International cardiothoracic surgery residents and/or congenital heart surgery fellows: Take advantage of this opportunity to represent your institution and present a scientific poster of your clinical/investigative research at the AATS Centennial.
The meeting will take place April 29 - May 3, 2017 in Boston, MA.
Awardee institutions get a $500 stipend to offset meal/travel costs. Each winner receives free registration to the AATS Centennial and access to the Skills Course (April 30) and Postgraduate Course (May 1).
More information
Non-MD CT Surgical Team Scientific Poster Competition
Non-MD cardiothoracic team professionals can submit a scientific poster for the Perioperative/Team-Based Care Poster Competition.
Winning posters will be displayed at the AATS Centennial, April 29 – May 3, 2017 in Boston, MA.
The competition winner will receive a $1,000 stipend to offset travel and accommodation costs.
More information
Share:
Current and future CT surgery division chiefs/department chairs are invited to apply for the AATS Leadership Academy Program.
Friday, April 28, 2017
AATS Centennial
Boston, MA
This intensive, didactic and interactive program brings together up to 20 international surgeons who have demonstrated significant promise as potential future division chiefs or have recent assumed that role. The program provides participants with administrative, interpersonal, mentoring and negotiating skills, as well as the opportunity to network with well-known thoracic surgeon leaders and potential mentors.
Deadline: November 30, 2016
Qualifications/More Information
Don’t miss the opportunity to submit to one of these AATS scholarship programs.
Deadline: January 20, 2017
AATS Member for a Day
North American medical students, and general and up to third year integrated CT Surgery
(I-6) surgery residents can accompany an AATS Member during portions of the AATS Centennial as an AATS Member for a Day.
The meeting takes place April 29-May 3, 2017 in Boston, MA.
Those selected will receive free hotel accommodations for three to four night in an AATS Centennial hotel. They will also be given a $250 meal and $500 travel stipend at the end of the meeting.
Eligibility/More information
Summer Internship Opportunity for First/Second Year Medical Students
First and second year medical students can spend the summer being exposed to cardiothoracic surgery thanks to the AATS Summer Intern Scholarship. For eight weeks (June – September), students will work in the CT department of an AATS member.
Those chosen receive $2,500 for living expenses. They also will be able to attend the AATS Centennial gratis.
The meeting takes place April 29 – May 3, 2017 in Boston, MA.
More information
AATS Resident Poster Competition
International cardiothoracic surgery residents and/or congenital heart surgery fellows: Take advantage of this opportunity to represent your institution and present a scientific poster of your clinical/investigative research at the AATS Centennial.
The meeting will take place April 29 - May 3, 2017 in Boston, MA.
Awardee institutions get a $500 stipend to offset meal/travel costs. Each winner receives free registration to the AATS Centennial and access to the Skills Course (April 30) and Postgraduate Course (May 1).
More information
Non-MD CT Surgical Team Scientific Poster Competition
Non-MD cardiothoracic team professionals can submit a scientific poster for the Perioperative/Team-Based Care Poster Competition.
Winning posters will be displayed at the AATS Centennial, April 29 – May 3, 2017 in Boston, MA.
The competition winner will receive a $1,000 stipend to offset travel and accommodation costs.
More information
Share:
Current and future CT surgery division chiefs/department chairs are invited to apply for the AATS Leadership Academy Program.
Friday, April 28, 2017
AATS Centennial
Boston, MA
This intensive, didactic and interactive program brings together up to 20 international surgeons who have demonstrated significant promise as potential future division chiefs or have recent assumed that role. The program provides participants with administrative, interpersonal, mentoring and negotiating skills, as well as the opportunity to network with well-known thoracic surgeon leaders and potential mentors.
Deadline: November 30, 2016
Qualifications/More Information
Don’t miss the opportunity to submit to one of these AATS scholarship programs.
Deadline: January 20, 2017
AATS Member for a Day
North American medical students, and general and up to third year integrated CT Surgery
(I-6) surgery residents can accompany an AATS Member during portions of the AATS Centennial as an AATS Member for a Day.
The meeting takes place April 29-May 3, 2017 in Boston, MA.
Those selected will receive free hotel accommodations for three to four night in an AATS Centennial hotel. They will also be given a $250 meal and $500 travel stipend at the end of the meeting.
Eligibility/More information
Summer Internship Opportunity for First/Second Year Medical Students
First and second year medical students can spend the summer being exposed to cardiothoracic surgery thanks to the AATS Summer Intern Scholarship. For eight weeks (June – September), students will work in the CT department of an AATS member.
Those chosen receive $2,500 for living expenses. They also will be able to attend the AATS Centennial gratis.
The meeting takes place April 29 – May 3, 2017 in Boston, MA.
More information
AATS Resident Poster Competition
International cardiothoracic surgery residents and/or congenital heart surgery fellows: Take advantage of this opportunity to represent your institution and present a scientific poster of your clinical/investigative research at the AATS Centennial.
The meeting will take place April 29 - May 3, 2017 in Boston, MA.
Awardee institutions get a $500 stipend to offset meal/travel costs. Each winner receives free registration to the AATS Centennial and access to the Skills Course (April 30) and Postgraduate Course (May 1).
More information
Non-MD CT Surgical Team Scientific Poster Competition
Non-MD cardiothoracic team professionals can submit a scientific poster for the Perioperative/Team-Based Care Poster Competition.
Winning posters will be displayed at the AATS Centennial, April 29 – May 3, 2017 in Boston, MA.
The competition winner will receive a $1,000 stipend to offset travel and accommodation costs.
More information
Share:
IVIg, corticosteroids appear comparable for ITP in pregnancy
Most pregnant women with a history of immune thrombocytopenia purpura need no treatment. Of those who do, neonatal outcomes were comparable for mothers who received treatment with intravenous immunoglobulin and for those who received corticosteroids, results of an observational study indicate.
Though limited by its retrospective design and low event rates that limit inferences about treatment effects, this observational study – the first to compare the effectiveness of treatment with IVIg to treatment with corticosteroids for immune thrombocytopenia purpura (ITP) in pregnancy – shows that outcomes did not significantly differ with the two regimens. The findings also highlight the need for ongoing neonatal platelet count monitoring throughout the first week of life, regardless of the maternal platelet count, the investigators said.
A review of medical records at two tertiary care centers identified 235 pregnancies in 195 women with a history of ITP. No treatment was required in 137 pregnancies. Of the remaining 98 pregnancies in 91 women, 47 (48%) were treated initially with intravenous immunoglobulin (IVIg) and 51 were treated initially with corticosteroids, reported Dongmei Sun, MD, of University of Western Ontario, London, and colleagues (Blood. 2016;128[10]:1329-35).
The two treatment groups had similar mean maternal platelet counts at birth (68.7 x 109/L and 77.3 x 109/L for IVIg and corticosteroids, respectively). The proportion of mothers who achieved a platelet count response did not significantly differ (38% vs. 39%), the researchers reported.
“The sole difference between treatments was a higher maternal composite outcome noted in the IVIg group,” they wrote, referring to a secondary composite outcome of postpartum hemorrhage, predelivery platelet transfusion, peripartum transfusion of any blood product, or postpartum reduction in the hemoglobin concentration of 30 g/L or more. That composite outcome occurred in 46.8% of pregnancies in the IVIg group and 23.5% in the corticosteroid group.
Adverse events were reported in 13% of cases in each group, and included hemolytic anemia, headache, and “other” in those treated with IVIg, and hyperglycemia requiring treatment, hyperglycemia with neonatal hypoglycemia, infection, and “other” in those receiving corticosteroids.
No severe or fatal maternal, fetal, or neonatal hemorrhages occurred, but two newborns did experience intracranial hemorrhage. No maternal or neonatal deaths occurred, the investigators said.
In 203 neonates for whom platelet counts were available, 56 (28%) had a count of less than 150 x 109/L and 18 (9%) had a count of less than 50 x 109/L.
Nadir platelet counts occurred at birth for 30% of neonates. In two cases, the nadir occurred as late as day 6 postnatally. Of note, a drop in platelets to below 150 x 109/L was found in 9 (11%) of 129 neonates who had a normal cord platelet count and had a repeat count.
ITP occurs in 1 to 10 of every 10,000 pregnancies, and about one-third of cases require intervention. IVIg and corticosteroids are acceptable treatments, but most data on their effectiveness are extrapolated from nonpregnant patients and the treatments have not been adequately assessed in pregnancy, the investigators said. The current study was designed to compare the efficacy of the two treatments for maternal ITP.
Study subjects were women with singleton pregnancies with an ITP diagnosis either before or during pregnancy.
At less than 40% in both groups, the response to ITP therapies was lower than has been reported for nonpregnant patients, the investigators noted.
“Our observation of a relative resistance to ITP treatment during pregnancy requires further validation in prospective studies. We speculate that increased potency of antiplatelet antibodies during pregnancy, pregnancy-associated changes in platelet turnover, or altered drug metabolism may contribute to the lower response rates we observed,” they wrote.
With respect to corticosteroids, this “may warrant consideration of starting therapy earlier in the third trimester to maximize the likelihood of reaching target platelet counts in time for delivery and raises the possibility that lower corticosteroid doses are ineffective in this setting,” they added.
“Also of significance is the finding that 9 neonates (11%) with normal cord platelet counts were found to have a reduction in their platelet count on repeat measurement. These findings highlight the need for determination of cord platelet counts in all neonates born to mothers with active or previous ITP and the need for continued monitoring of the neonatal platelet count during the first week of life, despite normal cord platelet counts,” they wrote.
“Prospective studies are needed to better characterize the safety of these regimens, to determine the optimal dose of corticosteroids, to identify risk factors for neonatal thrombocytopenia, and to explore new therapeutic options,” they concluded.
Among the options worth exploring are rituximab, a monoclonal antibody against B-cell surface antigen CD20, for which “pregnancy data are accumulating,” and romiplostim, a thrombopoietin receptor agonist, which has been used in a few cases without reported fetal complications, they noted.
This study was supported by Canadian Blood Services Small Projects Fund and Canadian Institute of Health Research/Canadian Blood Services New Investigator Award. Individual authors reported receiving support from the Canadian Institutes of Health Research and McMaster University. The authors reported having no other disclosures.
Most pregnant women with a history of immune thrombocytopenia purpura need no treatment. Of those who do, neonatal outcomes were comparable for mothers who received treatment with intravenous immunoglobulin and for those who received corticosteroids, results of an observational study indicate.
Though limited by its retrospective design and low event rates that limit inferences about treatment effects, this observational study – the first to compare the effectiveness of treatment with IVIg to treatment with corticosteroids for immune thrombocytopenia purpura (ITP) in pregnancy – shows that outcomes did not significantly differ with the two regimens. The findings also highlight the need for ongoing neonatal platelet count monitoring throughout the first week of life, regardless of the maternal platelet count, the investigators said.
A review of medical records at two tertiary care centers identified 235 pregnancies in 195 women with a history of ITP. No treatment was required in 137 pregnancies. Of the remaining 98 pregnancies in 91 women, 47 (48%) were treated initially with intravenous immunoglobulin (IVIg) and 51 were treated initially with corticosteroids, reported Dongmei Sun, MD, of University of Western Ontario, London, and colleagues (Blood. 2016;128[10]:1329-35).
The two treatment groups had similar mean maternal platelet counts at birth (68.7 x 109/L and 77.3 x 109/L for IVIg and corticosteroids, respectively). The proportion of mothers who achieved a platelet count response did not significantly differ (38% vs. 39%), the researchers reported.
“The sole difference between treatments was a higher maternal composite outcome noted in the IVIg group,” they wrote, referring to a secondary composite outcome of postpartum hemorrhage, predelivery platelet transfusion, peripartum transfusion of any blood product, or postpartum reduction in the hemoglobin concentration of 30 g/L or more. That composite outcome occurred in 46.8% of pregnancies in the IVIg group and 23.5% in the corticosteroid group.
Adverse events were reported in 13% of cases in each group, and included hemolytic anemia, headache, and “other” in those treated with IVIg, and hyperglycemia requiring treatment, hyperglycemia with neonatal hypoglycemia, infection, and “other” in those receiving corticosteroids.
No severe or fatal maternal, fetal, or neonatal hemorrhages occurred, but two newborns did experience intracranial hemorrhage. No maternal or neonatal deaths occurred, the investigators said.
In 203 neonates for whom platelet counts were available, 56 (28%) had a count of less than 150 x 109/L and 18 (9%) had a count of less than 50 x 109/L.
Nadir platelet counts occurred at birth for 30% of neonates. In two cases, the nadir occurred as late as day 6 postnatally. Of note, a drop in platelets to below 150 x 109/L was found in 9 (11%) of 129 neonates who had a normal cord platelet count and had a repeat count.
ITP occurs in 1 to 10 of every 10,000 pregnancies, and about one-third of cases require intervention. IVIg and corticosteroids are acceptable treatments, but most data on their effectiveness are extrapolated from nonpregnant patients and the treatments have not been adequately assessed in pregnancy, the investigators said. The current study was designed to compare the efficacy of the two treatments for maternal ITP.
Study subjects were women with singleton pregnancies with an ITP diagnosis either before or during pregnancy.
At less than 40% in both groups, the response to ITP therapies was lower than has been reported for nonpregnant patients, the investigators noted.
“Our observation of a relative resistance to ITP treatment during pregnancy requires further validation in prospective studies. We speculate that increased potency of antiplatelet antibodies during pregnancy, pregnancy-associated changes in platelet turnover, or altered drug metabolism may contribute to the lower response rates we observed,” they wrote.
With respect to corticosteroids, this “may warrant consideration of starting therapy earlier in the third trimester to maximize the likelihood of reaching target platelet counts in time for delivery and raises the possibility that lower corticosteroid doses are ineffective in this setting,” they added.
“Also of significance is the finding that 9 neonates (11%) with normal cord platelet counts were found to have a reduction in their platelet count on repeat measurement. These findings highlight the need for determination of cord platelet counts in all neonates born to mothers with active or previous ITP and the need for continued monitoring of the neonatal platelet count during the first week of life, despite normal cord platelet counts,” they wrote.
“Prospective studies are needed to better characterize the safety of these regimens, to determine the optimal dose of corticosteroids, to identify risk factors for neonatal thrombocytopenia, and to explore new therapeutic options,” they concluded.
Among the options worth exploring are rituximab, a monoclonal antibody against B-cell surface antigen CD20, for which “pregnancy data are accumulating,” and romiplostim, a thrombopoietin receptor agonist, which has been used in a few cases without reported fetal complications, they noted.
This study was supported by Canadian Blood Services Small Projects Fund and Canadian Institute of Health Research/Canadian Blood Services New Investigator Award. Individual authors reported receiving support from the Canadian Institutes of Health Research and McMaster University. The authors reported having no other disclosures.
Most pregnant women with a history of immune thrombocytopenia purpura need no treatment. Of those who do, neonatal outcomes were comparable for mothers who received treatment with intravenous immunoglobulin and for those who received corticosteroids, results of an observational study indicate.
Though limited by its retrospective design and low event rates that limit inferences about treatment effects, this observational study – the first to compare the effectiveness of treatment with IVIg to treatment with corticosteroids for immune thrombocytopenia purpura (ITP) in pregnancy – shows that outcomes did not significantly differ with the two regimens. The findings also highlight the need for ongoing neonatal platelet count monitoring throughout the first week of life, regardless of the maternal platelet count, the investigators said.
A review of medical records at two tertiary care centers identified 235 pregnancies in 195 women with a history of ITP. No treatment was required in 137 pregnancies. Of the remaining 98 pregnancies in 91 women, 47 (48%) were treated initially with intravenous immunoglobulin (IVIg) and 51 were treated initially with corticosteroids, reported Dongmei Sun, MD, of University of Western Ontario, London, and colleagues (Blood. 2016;128[10]:1329-35).
The two treatment groups had similar mean maternal platelet counts at birth (68.7 x 109/L and 77.3 x 109/L for IVIg and corticosteroids, respectively). The proportion of mothers who achieved a platelet count response did not significantly differ (38% vs. 39%), the researchers reported.
“The sole difference between treatments was a higher maternal composite outcome noted in the IVIg group,” they wrote, referring to a secondary composite outcome of postpartum hemorrhage, predelivery platelet transfusion, peripartum transfusion of any blood product, or postpartum reduction in the hemoglobin concentration of 30 g/L or more. That composite outcome occurred in 46.8% of pregnancies in the IVIg group and 23.5% in the corticosteroid group.
Adverse events were reported in 13% of cases in each group, and included hemolytic anemia, headache, and “other” in those treated with IVIg, and hyperglycemia requiring treatment, hyperglycemia with neonatal hypoglycemia, infection, and “other” in those receiving corticosteroids.
No severe or fatal maternal, fetal, or neonatal hemorrhages occurred, but two newborns did experience intracranial hemorrhage. No maternal or neonatal deaths occurred, the investigators said.
In 203 neonates for whom platelet counts were available, 56 (28%) had a count of less than 150 x 109/L and 18 (9%) had a count of less than 50 x 109/L.
Nadir platelet counts occurred at birth for 30% of neonates. In two cases, the nadir occurred as late as day 6 postnatally. Of note, a drop in platelets to below 150 x 109/L was found in 9 (11%) of 129 neonates who had a normal cord platelet count and had a repeat count.
ITP occurs in 1 to 10 of every 10,000 pregnancies, and about one-third of cases require intervention. IVIg and corticosteroids are acceptable treatments, but most data on their effectiveness are extrapolated from nonpregnant patients and the treatments have not been adequately assessed in pregnancy, the investigators said. The current study was designed to compare the efficacy of the two treatments for maternal ITP.
Study subjects were women with singleton pregnancies with an ITP diagnosis either before or during pregnancy.
At less than 40% in both groups, the response to ITP therapies was lower than has been reported for nonpregnant patients, the investigators noted.
“Our observation of a relative resistance to ITP treatment during pregnancy requires further validation in prospective studies. We speculate that increased potency of antiplatelet antibodies during pregnancy, pregnancy-associated changes in platelet turnover, or altered drug metabolism may contribute to the lower response rates we observed,” they wrote.
With respect to corticosteroids, this “may warrant consideration of starting therapy earlier in the third trimester to maximize the likelihood of reaching target platelet counts in time for delivery and raises the possibility that lower corticosteroid doses are ineffective in this setting,” they added.
“Also of significance is the finding that 9 neonates (11%) with normal cord platelet counts were found to have a reduction in their platelet count on repeat measurement. These findings highlight the need for determination of cord platelet counts in all neonates born to mothers with active or previous ITP and the need for continued monitoring of the neonatal platelet count during the first week of life, despite normal cord platelet counts,” they wrote.
“Prospective studies are needed to better characterize the safety of these regimens, to determine the optimal dose of corticosteroids, to identify risk factors for neonatal thrombocytopenia, and to explore new therapeutic options,” they concluded.
Among the options worth exploring are rituximab, a monoclonal antibody against B-cell surface antigen CD20, for which “pregnancy data are accumulating,” and romiplostim, a thrombopoietin receptor agonist, which has been used in a few cases without reported fetal complications, they noted.
This study was supported by Canadian Blood Services Small Projects Fund and Canadian Institute of Health Research/Canadian Blood Services New Investigator Award. Individual authors reported receiving support from the Canadian Institutes of Health Research and McMaster University. The authors reported having no other disclosures.
FROM BLOOD
Key clinical point: Most pregnant women with a history of immune thrombocytopenia purpura in an observational study needed no treatment, but neonatal outcomes were comparable for mothers who received treatment with intravenous immunoglobulin and those who received corticosteroids.
Major finding: Groups given IVIg or corticosteroids had similar mean maternal platelet counts at birth (68.7 x 109/L and 77.3 x 109/L for IVIg and corticosteroids, respectively), and no difference was seen in the proportion of mothers who achieved a platelet count response (38% and 39%, respectively).
Data source: A retrospective observational study of 235 pregnancies.
Disclosures: This study was supported by Canadian Blood Services Small Projects Fund and Canadian Institute of Health Research/Canadian Blood Services New Investigator Award. Individual authors reported receiving support from the Canadian Institutes of Health Research and McMaster University. The authors reported having no other disclosures.
Adding foot screening to eye clinic catches diabetic neuropathy
MUNICH – A combined eye, foot, and renal screening clinic identified undiagnosed painful diabetic neuropathy in 12% of diabetic patients who attended.
The clinic in Sheffield, England, was well attended and well liked, with 85% patient approval. The patients appreciated that the visit assured attention to their feet and also let them combine several individual clinic visits for diabetes complications screening, Solomon Tesfaye, MD, said at the annual meeting of the European Association for the Study of Diabetes.
“Twenty percent of our attendants said they had never even had a foot exam before,” said Dr. Tesfaye, an endocrinologist at the University of Sheffield. “And almost half had never had any kind of diabetic foot education.”
Dr. Tesfaye said this lack of attention to foot care in diabetes patients in the United Kingdom is “completely unacceptable.” The country is facing an epidemic of diabetes-related foot amputations, he said.
“We have unacceptable numbers of amputations, on the order of 135 each week, and unfortunately that number is rising. Why is that? Because in the U.K., we tend to diagnose diabetic neuropathy mainly with the 10-g monofilament test, because that is reimbursed. And while it’s a good way of screening for foot ulceration risk, it’s not a good model of diagnosing neuropathy early. So we are now unfortunately diagnosing it late, when it’s advanced and completely irreversible.”
The consequences of foot damage are far-reaching, Dr. Tesfaye said. “Patients who have to be referred into a foot clinic have very high mortality, approaching 50% at 5 years. In the U.K., we’re adding more and more foot clinics every year, and this is not a measure of success. It’s a measure of failure.”
Diabetic retinopathy, however, is the country’s good news story. More than 90% of people – diabetic or not – attend an annual eye screening as part of their wellness visits. As a result, diabetic eye disease is no longer the leading cause of blindness in adults in the United Kingdom.
“Everyone attends this annual eye screening, which uses retinal photography, and anyone with early changes is referred to specialist care,” Dr. Tesfaye said. “This has resulted in a paradigm shift in blindness, and that’s fantastic news.”
It just made sense, he said, to use the popular eye screening visit as a chance to also intervene early in undiagnosed diabetic neuropathy. A recent German study underscored the importance of early intervention (Diabetes 2014 Jul;63[7]:2454-63).
This study demonstrated that intraepidermal nerve fibers were already significantly reduced in 20% of patients within a year of their diabetes diagnosis. Nerve conduction values and amplitude were already impacted as well.
“The neuropathic process starts early, but we are using very insensitive measures to diagnose it,” Dr. Tesfaye said.
The combination clinic used several devices to test nerve function in feet, in addition to the 10-g monofilament test:
• A handheld device called DPN-Check, which measures sural nerve conduction velocity and response amplitude.
• Sudoscan, which measures sudomotor dysfunction – one of the earliest neurophysiologic changes in distal small fiber neuropathies.
• The Toronto Clinical Scoring System, a clinical tool that assesses symptoms, reflexes, and sensory function.
The entire foot exam takes 15 minutes and is done after the patient receives the eyedrops necessary for the ocular exam. The renal screening consists of a blood draw for tests of renal function.
The study group comprised 180 patients, with a mean age of 64 years. Type 1 diabetes was present in 6%; the rest had type 2 disease.
The Toronto score was 5 or higher, indicating the presence of diabetic neuropathy, in almost 32%.
The Sudoscan identified small nerve dysfunction consistent with neuropathy in 40%. The DPN-Check score identified neuropathy in 55%. However, the monofilament test was positive in 12%.
In addition to the increased number of neuropathy patients identified, “we also had new diagnoses of painful diabetic neuropathy in about 12% of the group,” Dr. Tesfaye said.
The devices had very good individual diagnostic accuracy, and when the devices were combined, the results were “really staggering,” he said. The Sudoscan alone had a sensitivity of 79% and a specificity of 60%; the DPN-Check alone, a sensitivity of 91% and a specificity of 73%. But when combined, the two diagnostic tools yielded an overall sensitivity of 94% and specificity of 63%. This correlated very well with the Toronto Clinical Scoring System, he added.
“It’s obvious that the 10-g monofilament test grossly underestimated the true presence of diabetic neuropathy. But using these combined point-of-care devices, we were able to detect it with an extremely high sensitivity. This service enables our patients to be referred early to podiatric services. Whether this early referral will result in the reversal of these neuropathic changes is yet to be determined.”
Dr. Tesfaye reported relationships with NeuroMetrix, Impeto Medical, Eli Lilly, Pfizer, Worwag Pharma, and TRIGOcare International.
MUNICH – A combined eye, foot, and renal screening clinic identified undiagnosed painful diabetic neuropathy in 12% of diabetic patients who attended.
The clinic in Sheffield, England, was well attended and well liked, with 85% patient approval. The patients appreciated that the visit assured attention to their feet and also let them combine several individual clinic visits for diabetes complications screening, Solomon Tesfaye, MD, said at the annual meeting of the European Association for the Study of Diabetes.
“Twenty percent of our attendants said they had never even had a foot exam before,” said Dr. Tesfaye, an endocrinologist at the University of Sheffield. “And almost half had never had any kind of diabetic foot education.”
Dr. Tesfaye said this lack of attention to foot care in diabetes patients in the United Kingdom is “completely unacceptable.” The country is facing an epidemic of diabetes-related foot amputations, he said.
“We have unacceptable numbers of amputations, on the order of 135 each week, and unfortunately that number is rising. Why is that? Because in the U.K., we tend to diagnose diabetic neuropathy mainly with the 10-g monofilament test, because that is reimbursed. And while it’s a good way of screening for foot ulceration risk, it’s not a good model of diagnosing neuropathy early. So we are now unfortunately diagnosing it late, when it’s advanced and completely irreversible.”
The consequences of foot damage are far-reaching, Dr. Tesfaye said. “Patients who have to be referred into a foot clinic have very high mortality, approaching 50% at 5 years. In the U.K., we’re adding more and more foot clinics every year, and this is not a measure of success. It’s a measure of failure.”
Diabetic retinopathy, however, is the country’s good news story. More than 90% of people – diabetic or not – attend an annual eye screening as part of their wellness visits. As a result, diabetic eye disease is no longer the leading cause of blindness in adults in the United Kingdom.
“Everyone attends this annual eye screening, which uses retinal photography, and anyone with early changes is referred to specialist care,” Dr. Tesfaye said. “This has resulted in a paradigm shift in blindness, and that’s fantastic news.”
It just made sense, he said, to use the popular eye screening visit as a chance to also intervene early in undiagnosed diabetic neuropathy. A recent German study underscored the importance of early intervention (Diabetes 2014 Jul;63[7]:2454-63).
This study demonstrated that intraepidermal nerve fibers were already significantly reduced in 20% of patients within a year of their diabetes diagnosis. Nerve conduction values and amplitude were already impacted as well.
“The neuropathic process starts early, but we are using very insensitive measures to diagnose it,” Dr. Tesfaye said.
The combination clinic used several devices to test nerve function in feet, in addition to the 10-g monofilament test:
• A handheld device called DPN-Check, which measures sural nerve conduction velocity and response amplitude.
• Sudoscan, which measures sudomotor dysfunction – one of the earliest neurophysiologic changes in distal small fiber neuropathies.
• The Toronto Clinical Scoring System, a clinical tool that assesses symptoms, reflexes, and sensory function.
The entire foot exam takes 15 minutes and is done after the patient receives the eyedrops necessary for the ocular exam. The renal screening consists of a blood draw for tests of renal function.
The study group comprised 180 patients, with a mean age of 64 years. Type 1 diabetes was present in 6%; the rest had type 2 disease.
The Toronto score was 5 or higher, indicating the presence of diabetic neuropathy, in almost 32%.
The Sudoscan identified small nerve dysfunction consistent with neuropathy in 40%. The DPN-Check score identified neuropathy in 55%. However, the monofilament test was positive in 12%.
In addition to the increased number of neuropathy patients identified, “we also had new diagnoses of painful diabetic neuropathy in about 12% of the group,” Dr. Tesfaye said.
The devices had very good individual diagnostic accuracy, and when the devices were combined, the results were “really staggering,” he said. The Sudoscan alone had a sensitivity of 79% and a specificity of 60%; the DPN-Check alone, a sensitivity of 91% and a specificity of 73%. But when combined, the two diagnostic tools yielded an overall sensitivity of 94% and specificity of 63%. This correlated very well with the Toronto Clinical Scoring System, he added.
“It’s obvious that the 10-g monofilament test grossly underestimated the true presence of diabetic neuropathy. But using these combined point-of-care devices, we were able to detect it with an extremely high sensitivity. This service enables our patients to be referred early to podiatric services. Whether this early referral will result in the reversal of these neuropathic changes is yet to be determined.”
Dr. Tesfaye reported relationships with NeuroMetrix, Impeto Medical, Eli Lilly, Pfizer, Worwag Pharma, and TRIGOcare International.
MUNICH – A combined eye, foot, and renal screening clinic identified undiagnosed painful diabetic neuropathy in 12% of diabetic patients who attended.
The clinic in Sheffield, England, was well attended and well liked, with 85% patient approval. The patients appreciated that the visit assured attention to their feet and also let them combine several individual clinic visits for diabetes complications screening, Solomon Tesfaye, MD, said at the annual meeting of the European Association for the Study of Diabetes.
“Twenty percent of our attendants said they had never even had a foot exam before,” said Dr. Tesfaye, an endocrinologist at the University of Sheffield. “And almost half had never had any kind of diabetic foot education.”
Dr. Tesfaye said this lack of attention to foot care in diabetes patients in the United Kingdom is “completely unacceptable.” The country is facing an epidemic of diabetes-related foot amputations, he said.
“We have unacceptable numbers of amputations, on the order of 135 each week, and unfortunately that number is rising. Why is that? Because in the U.K., we tend to diagnose diabetic neuropathy mainly with the 10-g monofilament test, because that is reimbursed. And while it’s a good way of screening for foot ulceration risk, it’s not a good model of diagnosing neuropathy early. So we are now unfortunately diagnosing it late, when it’s advanced and completely irreversible.”
The consequences of foot damage are far-reaching, Dr. Tesfaye said. “Patients who have to be referred into a foot clinic have very high mortality, approaching 50% at 5 years. In the U.K., we’re adding more and more foot clinics every year, and this is not a measure of success. It’s a measure of failure.”
Diabetic retinopathy, however, is the country’s good news story. More than 90% of people – diabetic or not – attend an annual eye screening as part of their wellness visits. As a result, diabetic eye disease is no longer the leading cause of blindness in adults in the United Kingdom.
“Everyone attends this annual eye screening, which uses retinal photography, and anyone with early changes is referred to specialist care,” Dr. Tesfaye said. “This has resulted in a paradigm shift in blindness, and that’s fantastic news.”
It just made sense, he said, to use the popular eye screening visit as a chance to also intervene early in undiagnosed diabetic neuropathy. A recent German study underscored the importance of early intervention (Diabetes 2014 Jul;63[7]:2454-63).
This study demonstrated that intraepidermal nerve fibers were already significantly reduced in 20% of patients within a year of their diabetes diagnosis. Nerve conduction values and amplitude were already impacted as well.
“The neuropathic process starts early, but we are using very insensitive measures to diagnose it,” Dr. Tesfaye said.
The combination clinic used several devices to test nerve function in feet, in addition to the 10-g monofilament test:
• A handheld device called DPN-Check, which measures sural nerve conduction velocity and response amplitude.
• Sudoscan, which measures sudomotor dysfunction – one of the earliest neurophysiologic changes in distal small fiber neuropathies.
• The Toronto Clinical Scoring System, a clinical tool that assesses symptoms, reflexes, and sensory function.
The entire foot exam takes 15 minutes and is done after the patient receives the eyedrops necessary for the ocular exam. The renal screening consists of a blood draw for tests of renal function.
The study group comprised 180 patients, with a mean age of 64 years. Type 1 diabetes was present in 6%; the rest had type 2 disease.
The Toronto score was 5 or higher, indicating the presence of diabetic neuropathy, in almost 32%.
The Sudoscan identified small nerve dysfunction consistent with neuropathy in 40%. The DPN-Check score identified neuropathy in 55%. However, the monofilament test was positive in 12%.
In addition to the increased number of neuropathy patients identified, “we also had new diagnoses of painful diabetic neuropathy in about 12% of the group,” Dr. Tesfaye said.
The devices had very good individual diagnostic accuracy, and when the devices were combined, the results were “really staggering,” he said. The Sudoscan alone had a sensitivity of 79% and a specificity of 60%; the DPN-Check alone, a sensitivity of 91% and a specificity of 73%. But when combined, the two diagnostic tools yielded an overall sensitivity of 94% and specificity of 63%. This correlated very well with the Toronto Clinical Scoring System, he added.
“It’s obvious that the 10-g monofilament test grossly underestimated the true presence of diabetic neuropathy. But using these combined point-of-care devices, we were able to detect it with an extremely high sensitivity. This service enables our patients to be referred early to podiatric services. Whether this early referral will result in the reversal of these neuropathic changes is yet to be determined.”
Dr. Tesfaye reported relationships with NeuroMetrix, Impeto Medical, Eli Lilly, Pfizer, Worwag Pharma, and TRIGOcare International.
AT EASD 2016
Key clinical point: The addition of diabetic foot screening to an eye clinic boosted the diagnosis of diabetic neuropathy.
Major finding: Painful diabetic neuropathy was diagnosed in 12% of those who attended.
Data source: A prospective study involving 180 patients.
Disclosures: Dr. Tesfaye disclosed relationships with numerous drug and device manufacturers.
Benefits of early endovascular thrombectomy outlined in five trials
For patients with large-vessel ischemic stroke, endovascular thrombectomy produces better functional outcomes at 90 days than does optimal medical therapy, as long as the procedure is started within 7.3 hours of symptom onset, according to a report published online Sept. 27 in JAMA.
The benefit of thrombectomy was greatest when the procedure was begun under 2 hours from symptom onset, and it became nonsignificant after 7 hours and 18 minutes elapsed. This emphasizes “the importance of programs to enhance patient awareness, out-of-hospital care, and in-hospital management to shorten symptom onset-to-treatment times,” wrote Jeffrey L. Saver, MD, of the University of California, Los Angeles, and his associates.
Five major randomized trials have demonstrated the benefit of second-generation endovascular recanalization therapies over medical therapy in this patient population, but uncertainties persist regarding the timing of the intervention. For example, practice guidelines in the United States recommend thrombectomy until 6 hours after symptom onset, but the Food and Drug Administration allows thrombectomy devices to be used up to 8 hours after symptom onset and Canadian guidelines recommend the procedure for selected patients up to 12 hours after symptom onset.
The investigators for the five trials formed the Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials (HERMES) collaboration to pool their individual patient data and perform a meta-analysis to clarify the issue of timing. They assessed patients’ functional independence at 90 days using the modified Rankin Score (mRS). The study participants included 634 patients who had been randomly assigned to endovascular thrombectomy and 653 randomly assigned to medical therapy.
The intervention correlated with a substantially lower degree of patient disability at 90 days than did medical therapy: the mean mRS was 2.9 in the thrombectomy group and 3.6 in the medical therapy group. In addition, increasing delays in treatment were associated with higher levels of residual disability in the thrombectomy group but not in the medical therapy group, the investigators reported.
“Based on the current study, and assuming the findings are generalizable to the population of patients with acute ischemic stroke due to large-vessel occlusion, among every 1,000 patients achieving substantial endovascular reperfusion, for every 15-minute faster ED door-to-reperfusion time, an estimated 39 patients would have a less-disabled outcome at 3 months, including 25 more who would achieve functional independence (mRS 0-2),” Dr. Saver and his associates wrote (JAMA. 2016;316[12]:1279-88).
These findings reinforce current recommendations to attempt endovascular thrombectomy when the procedure can be initiated within 6 hours of symptom onset, and they also “provide evidence that potentially supports strengthening of the recommendation for treatment from 6 through 7.3 hours after symptom onset,” they added.
No specific sponsor of this study was cited. Dr. Saver reported ties to Medtronic, Stryker, Cognition Medical, Covidien, Neuravi, BrainsGate, Pfizer, Bristol-Myers Squibb, Boehringer Ingelheim, ZZ Biotech, St. Jude Medical, and Genentech. His associates reported ties to numerous industry sources.
Earlier thrombectomy has such a profound effect on stroke patients’ outcomes that substantial changes in the current medical system are warranted to shorten these times even further. In this study, median time from symptom onset to randomization was approximately 3 hours, median time to arterial puncture was approximately 4 hours, and median time to reperfusion was nearly 5 hours.
Reducing the number of patients who are transferred from community hospitals to facilities with stroke centers would shorten door-to-reperfusion time a great deal. It is estimated that direct transport to stroke centers would allow endovascular thrombectomy for an additional 13% of stroke patients. Telemedicine, mobile stroke units, and out-of-hospital administration of tissue plasminogen activator are other possibilities that should be investigated.
Steven Warach, MD, PhD, and S. Claiborne Johnston, MD, PhD, are with the University of Texas at Austin. They reported having no relevant financial disclosures. Dr. Warach and Dr. Johnston made these remarks in an editorial (JAMA. 2016;316[12]:1265-6) accompanying Dr. Saver’s report.
Earlier thrombectomy has such a profound effect on stroke patients’ outcomes that substantial changes in the current medical system are warranted to shorten these times even further. In this study, median time from symptom onset to randomization was approximately 3 hours, median time to arterial puncture was approximately 4 hours, and median time to reperfusion was nearly 5 hours.
Reducing the number of patients who are transferred from community hospitals to facilities with stroke centers would shorten door-to-reperfusion time a great deal. It is estimated that direct transport to stroke centers would allow endovascular thrombectomy for an additional 13% of stroke patients. Telemedicine, mobile stroke units, and out-of-hospital administration of tissue plasminogen activator are other possibilities that should be investigated.
Steven Warach, MD, PhD, and S. Claiborne Johnston, MD, PhD, are with the University of Texas at Austin. They reported having no relevant financial disclosures. Dr. Warach and Dr. Johnston made these remarks in an editorial (JAMA. 2016;316[12]:1265-6) accompanying Dr. Saver’s report.
Earlier thrombectomy has such a profound effect on stroke patients’ outcomes that substantial changes in the current medical system are warranted to shorten these times even further. In this study, median time from symptom onset to randomization was approximately 3 hours, median time to arterial puncture was approximately 4 hours, and median time to reperfusion was nearly 5 hours.
Reducing the number of patients who are transferred from community hospitals to facilities with stroke centers would shorten door-to-reperfusion time a great deal. It is estimated that direct transport to stroke centers would allow endovascular thrombectomy for an additional 13% of stroke patients. Telemedicine, mobile stroke units, and out-of-hospital administration of tissue plasminogen activator are other possibilities that should be investigated.
Steven Warach, MD, PhD, and S. Claiborne Johnston, MD, PhD, are with the University of Texas at Austin. They reported having no relevant financial disclosures. Dr. Warach and Dr. Johnston made these remarks in an editorial (JAMA. 2016;316[12]:1265-6) accompanying Dr. Saver’s report.
For patients with large-vessel ischemic stroke, endovascular thrombectomy produces better functional outcomes at 90 days than does optimal medical therapy, as long as the procedure is started within 7.3 hours of symptom onset, according to a report published online Sept. 27 in JAMA.
The benefit of thrombectomy was greatest when the procedure was begun under 2 hours from symptom onset, and it became nonsignificant after 7 hours and 18 minutes elapsed. This emphasizes “the importance of programs to enhance patient awareness, out-of-hospital care, and in-hospital management to shorten symptom onset-to-treatment times,” wrote Jeffrey L. Saver, MD, of the University of California, Los Angeles, and his associates.
Five major randomized trials have demonstrated the benefit of second-generation endovascular recanalization therapies over medical therapy in this patient population, but uncertainties persist regarding the timing of the intervention. For example, practice guidelines in the United States recommend thrombectomy until 6 hours after symptom onset, but the Food and Drug Administration allows thrombectomy devices to be used up to 8 hours after symptom onset and Canadian guidelines recommend the procedure for selected patients up to 12 hours after symptom onset.
The investigators for the five trials formed the Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials (HERMES) collaboration to pool their individual patient data and perform a meta-analysis to clarify the issue of timing. They assessed patients’ functional independence at 90 days using the modified Rankin Score (mRS). The study participants included 634 patients who had been randomly assigned to endovascular thrombectomy and 653 randomly assigned to medical therapy.
The intervention correlated with a substantially lower degree of patient disability at 90 days than did medical therapy: the mean mRS was 2.9 in the thrombectomy group and 3.6 in the medical therapy group. In addition, increasing delays in treatment were associated with higher levels of residual disability in the thrombectomy group but not in the medical therapy group, the investigators reported.
“Based on the current study, and assuming the findings are generalizable to the population of patients with acute ischemic stroke due to large-vessel occlusion, among every 1,000 patients achieving substantial endovascular reperfusion, for every 15-minute faster ED door-to-reperfusion time, an estimated 39 patients would have a less-disabled outcome at 3 months, including 25 more who would achieve functional independence (mRS 0-2),” Dr. Saver and his associates wrote (JAMA. 2016;316[12]:1279-88).
These findings reinforce current recommendations to attempt endovascular thrombectomy when the procedure can be initiated within 6 hours of symptom onset, and they also “provide evidence that potentially supports strengthening of the recommendation for treatment from 6 through 7.3 hours after symptom onset,” they added.
No specific sponsor of this study was cited. Dr. Saver reported ties to Medtronic, Stryker, Cognition Medical, Covidien, Neuravi, BrainsGate, Pfizer, Bristol-Myers Squibb, Boehringer Ingelheim, ZZ Biotech, St. Jude Medical, and Genentech. His associates reported ties to numerous industry sources.
For patients with large-vessel ischemic stroke, endovascular thrombectomy produces better functional outcomes at 90 days than does optimal medical therapy, as long as the procedure is started within 7.3 hours of symptom onset, according to a report published online Sept. 27 in JAMA.
The benefit of thrombectomy was greatest when the procedure was begun under 2 hours from symptom onset, and it became nonsignificant after 7 hours and 18 minutes elapsed. This emphasizes “the importance of programs to enhance patient awareness, out-of-hospital care, and in-hospital management to shorten symptom onset-to-treatment times,” wrote Jeffrey L. Saver, MD, of the University of California, Los Angeles, and his associates.
Five major randomized trials have demonstrated the benefit of second-generation endovascular recanalization therapies over medical therapy in this patient population, but uncertainties persist regarding the timing of the intervention. For example, practice guidelines in the United States recommend thrombectomy until 6 hours after symptom onset, but the Food and Drug Administration allows thrombectomy devices to be used up to 8 hours after symptom onset and Canadian guidelines recommend the procedure for selected patients up to 12 hours after symptom onset.
The investigators for the five trials formed the Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials (HERMES) collaboration to pool their individual patient data and perform a meta-analysis to clarify the issue of timing. They assessed patients’ functional independence at 90 days using the modified Rankin Score (mRS). The study participants included 634 patients who had been randomly assigned to endovascular thrombectomy and 653 randomly assigned to medical therapy.
The intervention correlated with a substantially lower degree of patient disability at 90 days than did medical therapy: the mean mRS was 2.9 in the thrombectomy group and 3.6 in the medical therapy group. In addition, increasing delays in treatment were associated with higher levels of residual disability in the thrombectomy group but not in the medical therapy group, the investigators reported.
“Based on the current study, and assuming the findings are generalizable to the population of patients with acute ischemic stroke due to large-vessel occlusion, among every 1,000 patients achieving substantial endovascular reperfusion, for every 15-minute faster ED door-to-reperfusion time, an estimated 39 patients would have a less-disabled outcome at 3 months, including 25 more who would achieve functional independence (mRS 0-2),” Dr. Saver and his associates wrote (JAMA. 2016;316[12]:1279-88).
These findings reinforce current recommendations to attempt endovascular thrombectomy when the procedure can be initiated within 6 hours of symptom onset, and they also “provide evidence that potentially supports strengthening of the recommendation for treatment from 6 through 7.3 hours after symptom onset,” they added.
No specific sponsor of this study was cited. Dr. Saver reported ties to Medtronic, Stryker, Cognition Medical, Covidien, Neuravi, BrainsGate, Pfizer, Bristol-Myers Squibb, Boehringer Ingelheim, ZZ Biotech, St. Jude Medical, and Genentech. His associates reported ties to numerous industry sources.
FROM JAMA
Key clinical point: Endovascular thrombectomy started within 7.3 hours of symptom onset for large-vessel ischemic stroke produces better outcomes than does optimal medical therapy.
Major finding: For every 15-minute shorter door-to-reperfusion time, an estimated 39 patients would have a less-disabled outcome at 3 months, including 25 more who would achieve functional independence.
Data source: A meta-analysis of pooled data from five randomized clinical trials involving 1,287 patients.
Disclosures: No specific sponsor of this study was cited. Dr. Saver reported ties to Medtronic, Stryker, Cognition Medical, Covidien, Neuravi, BrainsGate, Pfizer, Bristol Myers Squibb, Boehringer Ingelheim, ZZ Biotech, St. Jude Medical, and Genentech. His associates reported ties to numerous industry sources.