User login
Study: C-section increases risk of VTE

Photo by Nina Matthews
Research has shown that women have an increased risk of venous thromboembolism (VTE) after giving birth, but it’s believed that a cesarean section (CS) leaves a woman more vulnerable to VTE than a vaginal delivery (VD).
A meta-analysis published in CHEST supports this idea. The data showed that CS carried a greater risk of VTE than VD, and emergency CS was associated with a greater risk than elective CS.
“We found that CS is an important, independent risk factor for the development of VTE in the postpartum period and that approximately 3 VTE will occur for every 1000 CS performed, with greater risks for nonscheduled, emergency CS,” said study investigator Marc Blondon, MD, of Geneva University Hospitals in Geneva, Switzerland.
Analysis results
Dr Blondon and his colleagues evaluated 28 studies (most of them retrospective) comparing the risk of VTE after CS and VD (>53,000 VTEs) and 32 prospective studies reporting the risk of VTE after CS (218 VTEs).
In unadjusted analyses of the individual studies, the risk of VTE was 1 to 22 times higher after CS than after VD. The pooled random effect odds ratio (OR) was 3.7 (95% CI, 3.0-4.6).
The investigators said adjusting analyses for at least maternal age had a marginal effect in the 7 studies that included both univariate and multivariate analyses. The OR comparing CS with VD was 3.3 (95% CI, 2.5-4.5) in univariate analyses and 2.8 (95% CI, 2.1-3.8) in adjusted analyses.
Similarly, adjusting analyses for both maternal age and body mass index had a slight effect in the 4 studies in which researchers adjusted for at least these 2 factors. The pooled OR was 2.8 (95% CI, 2.1-3.7) in crude analyses and 2.5 (95% CI, 1.8-3.1) in adjusted analyses.
When the investigators combined all 7 studies reporting adjusted risk estimates, the OR was 2.7 (95% CI, 2.2-3.3).
The data also showed an increased risk of VTE for both elective and emergency CS when compared to VD. The pooled ORs were 2.3 (95% CI, 1.7-3.1) for elective CS and 3.6 (95% CI, 2.8-4.7) for emergency CS.
After adjustment (in 6 studies), the pooled ORs were 2.1 for elective CS and 2.8 for emergency CS.
Explanations and implications
The investigators noted that pregnant women become more susceptible to VTE due to a variety of factors, including venous stasis and trauma associated with delivery. In addition, hemostatic changes drive increases in some coagulation factors while decreasing bleeding inhibitors.
For some reason, these changes seem to be worse for women who deliver via CS.
“In the postpartum period specifically, women following CS exhibit greater activation of coagulation than women following VD, as reflected by greater D-dimer levels,” Dr Blondon explained.
“This outcome may be a result of the conditions leading to the CS or to the procedure itself, similar to the increased VTE risk following non-obstetric surgery. Furthermore, physical activity is reduced following CS compared with following VD, with delayed recovery of mobility occurring in the first 2 days following delivery.”
Dr Blondon and his colleagues said this study helps shed some light on VTE risks associated with CS. Practitioners should be aware of the risks, and further research is needed to plot the best course of action and inform future guidelines concerning thromboprophylaxis.
“Thromboprophylaxis [after CS] seems widely underutilized in the United States,” Dr Blondon said. “It is estimated that 75% of women following CS do not receive any prophylaxis in the postpartum period. This scenario may arise from a lack of recognition by care providers of the risk of VTE following CS.”
“Preventing postpartum VTE following CS may lead to an important reduction of its associated morbidity and mortality from a public health perspective. In this setting, further observational studies and randomized trials are needed to better appreciate the risks of VTE in specific groups following CS and to define the efficacy and safety of thromboprophylaxis.” ![]()

Photo by Nina Matthews
Research has shown that women have an increased risk of venous thromboembolism (VTE) after giving birth, but it’s believed that a cesarean section (CS) leaves a woman more vulnerable to VTE than a vaginal delivery (VD).
A meta-analysis published in CHEST supports this idea. The data showed that CS carried a greater risk of VTE than VD, and emergency CS was associated with a greater risk than elective CS.
“We found that CS is an important, independent risk factor for the development of VTE in the postpartum period and that approximately 3 VTE will occur for every 1000 CS performed, with greater risks for nonscheduled, emergency CS,” said study investigator Marc Blondon, MD, of Geneva University Hospitals in Geneva, Switzerland.
Analysis results
Dr Blondon and his colleagues evaluated 28 studies (most of them retrospective) comparing the risk of VTE after CS and VD (>53,000 VTEs) and 32 prospective studies reporting the risk of VTE after CS (218 VTEs).
In unadjusted analyses of the individual studies, the risk of VTE was 1 to 22 times higher after CS than after VD. The pooled random effect odds ratio (OR) was 3.7 (95% CI, 3.0-4.6).
The investigators said adjusting analyses for at least maternal age had a marginal effect in the 7 studies that included both univariate and multivariate analyses. The OR comparing CS with VD was 3.3 (95% CI, 2.5-4.5) in univariate analyses and 2.8 (95% CI, 2.1-3.8) in adjusted analyses.
Similarly, adjusting analyses for both maternal age and body mass index had a slight effect in the 4 studies in which researchers adjusted for at least these 2 factors. The pooled OR was 2.8 (95% CI, 2.1-3.7) in crude analyses and 2.5 (95% CI, 1.8-3.1) in adjusted analyses.
When the investigators combined all 7 studies reporting adjusted risk estimates, the OR was 2.7 (95% CI, 2.2-3.3).
The data also showed an increased risk of VTE for both elective and emergency CS when compared to VD. The pooled ORs were 2.3 (95% CI, 1.7-3.1) for elective CS and 3.6 (95% CI, 2.8-4.7) for emergency CS.
After adjustment (in 6 studies), the pooled ORs were 2.1 for elective CS and 2.8 for emergency CS.
Explanations and implications
The investigators noted that pregnant women become more susceptible to VTE due to a variety of factors, including venous stasis and trauma associated with delivery. In addition, hemostatic changes drive increases in some coagulation factors while decreasing bleeding inhibitors.
For some reason, these changes seem to be worse for women who deliver via CS.
“In the postpartum period specifically, women following CS exhibit greater activation of coagulation than women following VD, as reflected by greater D-dimer levels,” Dr Blondon explained.
“This outcome may be a result of the conditions leading to the CS or to the procedure itself, similar to the increased VTE risk following non-obstetric surgery. Furthermore, physical activity is reduced following CS compared with following VD, with delayed recovery of mobility occurring in the first 2 days following delivery.”
Dr Blondon and his colleagues said this study helps shed some light on VTE risks associated with CS. Practitioners should be aware of the risks, and further research is needed to plot the best course of action and inform future guidelines concerning thromboprophylaxis.
“Thromboprophylaxis [after CS] seems widely underutilized in the United States,” Dr Blondon said. “It is estimated that 75% of women following CS do not receive any prophylaxis in the postpartum period. This scenario may arise from a lack of recognition by care providers of the risk of VTE following CS.”
“Preventing postpartum VTE following CS may lead to an important reduction of its associated morbidity and mortality from a public health perspective. In this setting, further observational studies and randomized trials are needed to better appreciate the risks of VTE in specific groups following CS and to define the efficacy and safety of thromboprophylaxis.” ![]()

Photo by Nina Matthews
Research has shown that women have an increased risk of venous thromboembolism (VTE) after giving birth, but it’s believed that a cesarean section (CS) leaves a woman more vulnerable to VTE than a vaginal delivery (VD).
A meta-analysis published in CHEST supports this idea. The data showed that CS carried a greater risk of VTE than VD, and emergency CS was associated with a greater risk than elective CS.
“We found that CS is an important, independent risk factor for the development of VTE in the postpartum period and that approximately 3 VTE will occur for every 1000 CS performed, with greater risks for nonscheduled, emergency CS,” said study investigator Marc Blondon, MD, of Geneva University Hospitals in Geneva, Switzerland.
Analysis results
Dr Blondon and his colleagues evaluated 28 studies (most of them retrospective) comparing the risk of VTE after CS and VD (>53,000 VTEs) and 32 prospective studies reporting the risk of VTE after CS (218 VTEs).
In unadjusted analyses of the individual studies, the risk of VTE was 1 to 22 times higher after CS than after VD. The pooled random effect odds ratio (OR) was 3.7 (95% CI, 3.0-4.6).
The investigators said adjusting analyses for at least maternal age had a marginal effect in the 7 studies that included both univariate and multivariate analyses. The OR comparing CS with VD was 3.3 (95% CI, 2.5-4.5) in univariate analyses and 2.8 (95% CI, 2.1-3.8) in adjusted analyses.
Similarly, adjusting analyses for both maternal age and body mass index had a slight effect in the 4 studies in which researchers adjusted for at least these 2 factors. The pooled OR was 2.8 (95% CI, 2.1-3.7) in crude analyses and 2.5 (95% CI, 1.8-3.1) in adjusted analyses.
When the investigators combined all 7 studies reporting adjusted risk estimates, the OR was 2.7 (95% CI, 2.2-3.3).
The data also showed an increased risk of VTE for both elective and emergency CS when compared to VD. The pooled ORs were 2.3 (95% CI, 1.7-3.1) for elective CS and 3.6 (95% CI, 2.8-4.7) for emergency CS.
After adjustment (in 6 studies), the pooled ORs were 2.1 for elective CS and 2.8 for emergency CS.
Explanations and implications
The investigators noted that pregnant women become more susceptible to VTE due to a variety of factors, including venous stasis and trauma associated with delivery. In addition, hemostatic changes drive increases in some coagulation factors while decreasing bleeding inhibitors.
For some reason, these changes seem to be worse for women who deliver via CS.
“In the postpartum period specifically, women following CS exhibit greater activation of coagulation than women following VD, as reflected by greater D-dimer levels,” Dr Blondon explained.
“This outcome may be a result of the conditions leading to the CS or to the procedure itself, similar to the increased VTE risk following non-obstetric surgery. Furthermore, physical activity is reduced following CS compared with following VD, with delayed recovery of mobility occurring in the first 2 days following delivery.”
Dr Blondon and his colleagues said this study helps shed some light on VTE risks associated with CS. Practitioners should be aware of the risks, and further research is needed to plot the best course of action and inform future guidelines concerning thromboprophylaxis.
“Thromboprophylaxis [after CS] seems widely underutilized in the United States,” Dr Blondon said. “It is estimated that 75% of women following CS do not receive any prophylaxis in the postpartum period. This scenario may arise from a lack of recognition by care providers of the risk of VTE following CS.”
“Preventing postpartum VTE following CS may lead to an important reduction of its associated morbidity and mortality from a public health perspective. In this setting, further observational studies and randomized trials are needed to better appreciate the risks of VTE in specific groups following CS and to define the efficacy and safety of thromboprophylaxis.” ![]()
Multiple Chronic Conditions: Continuing Public Health Issue
In 2012, a survey found that 1 in 4 American adults had > 1 chronic health condition. In 2014, the National Health Interview Survey of 36,697 adults found the same thing. The stable prevalence of multiple chronic conditions (MCC) indicates a continuing public health issue, according to an article in the July 29, 2016 Morbidity and Mortality Weekly Report.
The prevalence varied across the country, from 19% in Colorado to 38% in Kentucky. Prevalence was higher than the national average percentage in Alabama, West Virginia, Mississippi, Montana, New Mexico, Maine, Michigan, Ohio, and Pennsylvania.
Several states with higher prevalence of MCC overlapped the so-called stroke belt, which includes much of the southern U.S. Similarly, MCC prevalence also overlapped the diabetes belt—again taking in much of the South and parts of the Midwest and West.
The survey covered 10 conditions: arthritis, asthma, cancer, chronic obstructive pulmonary disease, coronary artery disease, diabetes, hepatitis, hypertension, stroke, and weak or failing kidneys. But those are only 10 of the 20 conditions HHS has identified for inclusion in studies of MCC. Moreover, no data on mental health conditions, undiagnosed conditions, or adults in long-term care or congregant facilities were included, so the findings are limited in generalizability. Nonetheless, the MMWR report authors say the findings further HHS research and surveillance objectives. Geographic disparities in MCC prevalence can inform state-level surveillance programs and groups targeting service delivery or allocating resources.
In 2012, a survey found that 1 in 4 American adults had > 1 chronic health condition. In 2014, the National Health Interview Survey of 36,697 adults found the same thing. The stable prevalence of multiple chronic conditions (MCC) indicates a continuing public health issue, according to an article in the July 29, 2016 Morbidity and Mortality Weekly Report.
The prevalence varied across the country, from 19% in Colorado to 38% in Kentucky. Prevalence was higher than the national average percentage in Alabama, West Virginia, Mississippi, Montana, New Mexico, Maine, Michigan, Ohio, and Pennsylvania.
Several states with higher prevalence of MCC overlapped the so-called stroke belt, which includes much of the southern U.S. Similarly, MCC prevalence also overlapped the diabetes belt—again taking in much of the South and parts of the Midwest and West.
The survey covered 10 conditions: arthritis, asthma, cancer, chronic obstructive pulmonary disease, coronary artery disease, diabetes, hepatitis, hypertension, stroke, and weak or failing kidneys. But those are only 10 of the 20 conditions HHS has identified for inclusion in studies of MCC. Moreover, no data on mental health conditions, undiagnosed conditions, or adults in long-term care or congregant facilities were included, so the findings are limited in generalizability. Nonetheless, the MMWR report authors say the findings further HHS research and surveillance objectives. Geographic disparities in MCC prevalence can inform state-level surveillance programs and groups targeting service delivery or allocating resources.
In 2012, a survey found that 1 in 4 American adults had > 1 chronic health condition. In 2014, the National Health Interview Survey of 36,697 adults found the same thing. The stable prevalence of multiple chronic conditions (MCC) indicates a continuing public health issue, according to an article in the July 29, 2016 Morbidity and Mortality Weekly Report.
The prevalence varied across the country, from 19% in Colorado to 38% in Kentucky. Prevalence was higher than the national average percentage in Alabama, West Virginia, Mississippi, Montana, New Mexico, Maine, Michigan, Ohio, and Pennsylvania.
Several states with higher prevalence of MCC overlapped the so-called stroke belt, which includes much of the southern U.S. Similarly, MCC prevalence also overlapped the diabetes belt—again taking in much of the South and parts of the Midwest and West.
The survey covered 10 conditions: arthritis, asthma, cancer, chronic obstructive pulmonary disease, coronary artery disease, diabetes, hepatitis, hypertension, stroke, and weak or failing kidneys. But those are only 10 of the 20 conditions HHS has identified for inclusion in studies of MCC. Moreover, no data on mental health conditions, undiagnosed conditions, or adults in long-term care or congregant facilities were included, so the findings are limited in generalizability. Nonetheless, the MMWR report authors say the findings further HHS research and surveillance objectives. Geographic disparities in MCC prevalence can inform state-level surveillance programs and groups targeting service delivery or allocating resources.
Honor a loved one and support the AGA Research Awards Program
Did you know that you can honor a family member, friend, or colleague through a gift to the AGA Research Foundation? A simple, flexible, and versatile way to ensure the AGA Research Foundation can continue our work for years to come is a gift in your will or living trust, known as a charitable bequest. To make a charitable gift in a will, you need a current will or living trust.
You can include a gift in your will or living trust stating that a specific asset, certain dollar amount, or – more commonly – a percentage of your estate will pass to the AGA following your death in honor of your loved one. A gift of $50,000 or more in your will qualifies for membership in the AGA Legacy Society.
We hope you’ll consider including a gift to the AGA Research Foundation in your will or living trust. It’s simple – just a few sentences in your will or trust are all that is needed. The official bequest language for the AGA Research Foundation is: “I, [name], of [city, state, ZIP], give, devise and bequeath to the AGA Research Foundation [written amount or percentage of the estate or description of property] for its unrestricted use and purpose.”
For more information on how to give and recognize your loved one, contact Harmony Excellent at 301-272-1602 or [email protected] or visit http://gastro.planmylegacy.org/.
Did you know that you can honor a family member, friend, or colleague through a gift to the AGA Research Foundation? A simple, flexible, and versatile way to ensure the AGA Research Foundation can continue our work for years to come is a gift in your will or living trust, known as a charitable bequest. To make a charitable gift in a will, you need a current will or living trust.
You can include a gift in your will or living trust stating that a specific asset, certain dollar amount, or – more commonly – a percentage of your estate will pass to the AGA following your death in honor of your loved one. A gift of $50,000 or more in your will qualifies for membership in the AGA Legacy Society.
We hope you’ll consider including a gift to the AGA Research Foundation in your will or living trust. It’s simple – just a few sentences in your will or trust are all that is needed. The official bequest language for the AGA Research Foundation is: “I, [name], of [city, state, ZIP], give, devise and bequeath to the AGA Research Foundation [written amount or percentage of the estate or description of property] for its unrestricted use and purpose.”
For more information on how to give and recognize your loved one, contact Harmony Excellent at 301-272-1602 or [email protected] or visit http://gastro.planmylegacy.org/.
Did you know that you can honor a family member, friend, or colleague through a gift to the AGA Research Foundation? A simple, flexible, and versatile way to ensure the AGA Research Foundation can continue our work for years to come is a gift in your will or living trust, known as a charitable bequest. To make a charitable gift in a will, you need a current will or living trust.
You can include a gift in your will or living trust stating that a specific asset, certain dollar amount, or – more commonly – a percentage of your estate will pass to the AGA following your death in honor of your loved one. A gift of $50,000 or more in your will qualifies for membership in the AGA Legacy Society.
We hope you’ll consider including a gift to the AGA Research Foundation in your will or living trust. It’s simple – just a few sentences in your will or trust are all that is needed. The official bequest language for the AGA Research Foundation is: “I, [name], of [city, state, ZIP], give, devise and bequeath to the AGA Research Foundation [written amount or percentage of the estate or description of property] for its unrestricted use and purpose.”
For more information on how to give and recognize your loved one, contact Harmony Excellent at 301-272-1602 or [email protected] or visit http://gastro.planmylegacy.org/.
CMGH now in PubMed
Cellular and Molecular Gastroenterology and Hepatology (CMGH) – AGA’s newest scientific journal, which launched in January 2015 – has been accepted to PubMed Central. This means that all CMGH articles, beginning with the very first issue of the journal, will now be available when you search in the PubMed database. Articles should be available by the end of September.
Being searchable in PubMed will increase the visibility of the high-quality digestive biology research published in CMGH, and will also allow easier access to journal content.
Visit the journal at www.cmghjournal.org.
Cellular and Molecular Gastroenterology and Hepatology (CMGH) – AGA’s newest scientific journal, which launched in January 2015 – has been accepted to PubMed Central. This means that all CMGH articles, beginning with the very first issue of the journal, will now be available when you search in the PubMed database. Articles should be available by the end of September.
Being searchable in PubMed will increase the visibility of the high-quality digestive biology research published in CMGH, and will also allow easier access to journal content.
Visit the journal at www.cmghjournal.org.
Cellular and Molecular Gastroenterology and Hepatology (CMGH) – AGA’s newest scientific journal, which launched in January 2015 – has been accepted to PubMed Central. This means that all CMGH articles, beginning with the very first issue of the journal, will now be available when you search in the PubMed database. Articles should be available by the end of September.
Being searchable in PubMed will increase the visibility of the high-quality digestive biology research published in CMGH, and will also allow easier access to journal content.
Visit the journal at www.cmghjournal.org.
AGA: Preparing Practicing GIs for the New World of Reimbursement
Progress toward value-based care by Medicare and commercial payors will continue regardless of the election outcome in November. The health care market will continue to move away from fee-for-service and toward reimbursement systems that reward physicians for improving the quality of patient care and controlling costs. AGA is shaping the future of practice. We continue to be at the forefront of the value-based movement, creating the tools gastroenterologists need for success in new reimbursement environments and preparing our members for a successful future.
Did you know?
• AGA has created bundle and episode payment models for GI issues, including colonoscopy screening and surveillance and GERD, with obesity to launch in 2016 and more to come. These alternative payment models reward providers for identifying efficiency gains, effectively coordinating patient care and improving quality. By the time the Medicare Access and CHIP Reauthorization Act (MACRA) was enacted in 2015, AGA was ready with fully developed, practical alternative payment model solutions for GI.
• AGA is the only GI society to advocate for Medicare to recognize established GI payment models as advanced alternative payment models (APMs) so that gastroenterologists will have a pathway to greater earnings than under the Merit-based Incentive Payment System (MIPS). Not sure what MACRA, MIPS, and APMs are? AGA can help.
• AGA is also the only GI society to develop a MACRA education tool that explains, in plain language, MACRA’s impact on GIs and offers a customized plan for how to prepare based on your practice situation.
• AGA has established relationships with Medicare and commercial payors that allow us to provide input on coverage decisions. We recognize that payor coverage of procedures and technology plays a vital role in reimbursement success and we take every opportunity to provide payors with input from AGA member experts.
• In addition to AGA’s rigorous, evidence-based clinical practice guidelines, we provide expert reviews and other clinical practice guidance documents to provide best practice advice for physicians in areas in which there is not yet enough literature to produce a clinical guideline. Additionally, Technology Coverage Statements provide support when working with payors on coverage and reimbursement for proven procedures, diagnostics, and therapies that advance the science and practice of gastroenterology and improve care for the patients you treat.
AGA works hard for the gastroenterology field to ensure that you are poised for success under new reimbursement models and will continue to do so. Learn more at http://www.gastro.org/practice-management.
Progress toward value-based care by Medicare and commercial payors will continue regardless of the election outcome in November. The health care market will continue to move away from fee-for-service and toward reimbursement systems that reward physicians for improving the quality of patient care and controlling costs. AGA is shaping the future of practice. We continue to be at the forefront of the value-based movement, creating the tools gastroenterologists need for success in new reimbursement environments and preparing our members for a successful future.
Did you know?
• AGA has created bundle and episode payment models for GI issues, including colonoscopy screening and surveillance and GERD, with obesity to launch in 2016 and more to come. These alternative payment models reward providers for identifying efficiency gains, effectively coordinating patient care and improving quality. By the time the Medicare Access and CHIP Reauthorization Act (MACRA) was enacted in 2015, AGA was ready with fully developed, practical alternative payment model solutions for GI.
• AGA is the only GI society to advocate for Medicare to recognize established GI payment models as advanced alternative payment models (APMs) so that gastroenterologists will have a pathway to greater earnings than under the Merit-based Incentive Payment System (MIPS). Not sure what MACRA, MIPS, and APMs are? AGA can help.
• AGA is also the only GI society to develop a MACRA education tool that explains, in plain language, MACRA’s impact on GIs and offers a customized plan for how to prepare based on your practice situation.
• AGA has established relationships with Medicare and commercial payors that allow us to provide input on coverage decisions. We recognize that payor coverage of procedures and technology plays a vital role in reimbursement success and we take every opportunity to provide payors with input from AGA member experts.
• In addition to AGA’s rigorous, evidence-based clinical practice guidelines, we provide expert reviews and other clinical practice guidance documents to provide best practice advice for physicians in areas in which there is not yet enough literature to produce a clinical guideline. Additionally, Technology Coverage Statements provide support when working with payors on coverage and reimbursement for proven procedures, diagnostics, and therapies that advance the science and practice of gastroenterology and improve care for the patients you treat.
AGA works hard for the gastroenterology field to ensure that you are poised for success under new reimbursement models and will continue to do so. Learn more at http://www.gastro.org/practice-management.
Progress toward value-based care by Medicare and commercial payors will continue regardless of the election outcome in November. The health care market will continue to move away from fee-for-service and toward reimbursement systems that reward physicians for improving the quality of patient care and controlling costs. AGA is shaping the future of practice. We continue to be at the forefront of the value-based movement, creating the tools gastroenterologists need for success in new reimbursement environments and preparing our members for a successful future.
Did you know?
• AGA has created bundle and episode payment models for GI issues, including colonoscopy screening and surveillance and GERD, with obesity to launch in 2016 and more to come. These alternative payment models reward providers for identifying efficiency gains, effectively coordinating patient care and improving quality. By the time the Medicare Access and CHIP Reauthorization Act (MACRA) was enacted in 2015, AGA was ready with fully developed, practical alternative payment model solutions for GI.
• AGA is the only GI society to advocate for Medicare to recognize established GI payment models as advanced alternative payment models (APMs) so that gastroenterologists will have a pathway to greater earnings than under the Merit-based Incentive Payment System (MIPS). Not sure what MACRA, MIPS, and APMs are? AGA can help.
• AGA is also the only GI society to develop a MACRA education tool that explains, in plain language, MACRA’s impact on GIs and offers a customized plan for how to prepare based on your practice situation.
• AGA has established relationships with Medicare and commercial payors that allow us to provide input on coverage decisions. We recognize that payor coverage of procedures and technology plays a vital role in reimbursement success and we take every opportunity to provide payors with input from AGA member experts.
• In addition to AGA’s rigorous, evidence-based clinical practice guidelines, we provide expert reviews and other clinical practice guidance documents to provide best practice advice for physicians in areas in which there is not yet enough literature to produce a clinical guideline. Additionally, Technology Coverage Statements provide support when working with payors on coverage and reimbursement for proven procedures, diagnostics, and therapies that advance the science and practice of gastroenterology and improve care for the patients you treat.
AGA works hard for the gastroenterology field to ensure that you are poised for success under new reimbursement models and will continue to do so. Learn more at http://www.gastro.org/practice-management.
VIDEO: Consider chikungunya for unexplained seronegative arthritis
LAS VEGAS – When rheumatologists consider a differential diagnosis that includes seronegative rheumatoid arthritis, they should also consider chikungunya, according to Len Calabrese, DO.
The patient who presents with weeks to months of unexplained arthralgia and perhaps arthritis and a negative autoimmune panel deserves consideration of chikungunya or another arbovirus, said Dr. Calabrese, speaking at the annual Perspectives in Rheumatic Diseases held by the Global Academy for Medical Education.
Among the mosquito-borne arboviruses now in play in the Western Hemisphere, chikungunya is particularly likely to cause long-lasting and sometimes debilitating joint pain weeks and even months after initial infection.
An alphavirus, chikungunya virus makes most affected individuals quite ill, and serum IgG and IgM titers persist long after infection. Testing is straightforward, as long as the virus is a candidate diagnosis, Dr. Calabrese said.
In addition to obtaining an accurate travel history, said Dr. Calabrese, physicians should consider the possibility of autochthonous transmission, which occurs when an infected individual who returns from an endemic area is bitten by mosquitoes once home. Flares of autochthonous transmission can result in pockets of locally heavy transmission far from the zones where chikungunya usually resides.
Dr. Calabrese is chair of clinical immunology and chair of osteopathic research and education at the Cleveland Clinic, and he reported no relevant financial disclosures.
Global Academy for Medical Education and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @karioakes
LAS VEGAS – When rheumatologists consider a differential diagnosis that includes seronegative rheumatoid arthritis, they should also consider chikungunya, according to Len Calabrese, DO.
The patient who presents with weeks to months of unexplained arthralgia and perhaps arthritis and a negative autoimmune panel deserves consideration of chikungunya or another arbovirus, said Dr. Calabrese, speaking at the annual Perspectives in Rheumatic Diseases held by the Global Academy for Medical Education.
Among the mosquito-borne arboviruses now in play in the Western Hemisphere, chikungunya is particularly likely to cause long-lasting and sometimes debilitating joint pain weeks and even months after initial infection.
An alphavirus, chikungunya virus makes most affected individuals quite ill, and serum IgG and IgM titers persist long after infection. Testing is straightforward, as long as the virus is a candidate diagnosis, Dr. Calabrese said.
In addition to obtaining an accurate travel history, said Dr. Calabrese, physicians should consider the possibility of autochthonous transmission, which occurs when an infected individual who returns from an endemic area is bitten by mosquitoes once home. Flares of autochthonous transmission can result in pockets of locally heavy transmission far from the zones where chikungunya usually resides.
Dr. Calabrese is chair of clinical immunology and chair of osteopathic research and education at the Cleveland Clinic, and he reported no relevant financial disclosures.
Global Academy for Medical Education and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @karioakes
LAS VEGAS – When rheumatologists consider a differential diagnosis that includes seronegative rheumatoid arthritis, they should also consider chikungunya, according to Len Calabrese, DO.
The patient who presents with weeks to months of unexplained arthralgia and perhaps arthritis and a negative autoimmune panel deserves consideration of chikungunya or another arbovirus, said Dr. Calabrese, speaking at the annual Perspectives in Rheumatic Diseases held by the Global Academy for Medical Education.
Among the mosquito-borne arboviruses now in play in the Western Hemisphere, chikungunya is particularly likely to cause long-lasting and sometimes debilitating joint pain weeks and even months after initial infection.
An alphavirus, chikungunya virus makes most affected individuals quite ill, and serum IgG and IgM titers persist long after infection. Testing is straightforward, as long as the virus is a candidate diagnosis, Dr. Calabrese said.
In addition to obtaining an accurate travel history, said Dr. Calabrese, physicians should consider the possibility of autochthonous transmission, which occurs when an infected individual who returns from an endemic area is bitten by mosquitoes once home. Flares of autochthonous transmission can result in pockets of locally heavy transmission far from the zones where chikungunya usually resides.
Dr. Calabrese is chair of clinical immunology and chair of osteopathic research and education at the Cleveland Clinic, and he reported no relevant financial disclosures.
Global Academy for Medical Education and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @karioakes
EXPERT ANALYSIS FROM ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
Safety and Efficacy of Five Years of Levodopa–Carbidopa Intestinal Gel Treatment
PORTLAND, OR—Most patients with advanced Parkinson’s disease who received levodopa–carbidopa intestinal gel in an open-label, continued-access-to-treatment study had, at five years, transitioned to using the therapy outside of the study when it became commercially available or continued to participate in the extension study, according to data presented at the Fourth World Parkinson Congress.
Forty-two percent of the patients in the phase III extension study transitioned to commercially available drug (designated as carbidopa–levodopa enteral suspension in the United States), and 24% of patients remained in the multinational study, said Hubert H. Fernandez, MD, Head of Movement Disorders at the Center for Neurological Restoration at the Cleveland Clinic in Ohio, and colleagues. Patients may remain in the extension study until a commercially available product is available in the country where they live.
Levodopa–carbidopa intestinal gel is infused continuously directly to the jejunum using a portable pump during approximately 16 hours of wakefulness. The therapy is designed to overcome some of the limitations of oral levodopa, which may lose effectiveness as Parkinson’s disease progresses.
Although investigators observed a high incidence of adverse events, long-term use of levodopa–carbidopa intestinal gel was well tolerated, the researchers said. The average discontinuation rate of 9.6% per year, including all causes of death, was relatively low, Dr. Fernandez and colleagues said.
The most frequently reported adverse events were associated with complications related to percutaneous gastrojejunostomy, such as stoma site maintenance. Other adverse events were associated with advanced Parkinson’s disease, aging, or levodopa. Most patients experienced device malfunctions and required pump replacement during the study.
The extension study began in November 2009. Data through September 30, 2015, were used in the present study. The study enrolled 262 patients with advanced Parkinson’s disease from 11 countries who had completed a 12-week double-blind study and its 52-week open-label extension or who had completed a separate 54-week open-label study. Participants attended scheduled study visits every six months.
Patients had a mean age of 64, 62% were male, and mean disease duration was 11.4 years. Mean exposure to levodopa–carbidopa intestinal gel was 3.1 years in the present study. Patients’ mean total exposure to the treatment was 4.1 years. Fifty-six percent of patients were exposed to the treatment for at least five years.
Adverse events led to discontinuation in 62 patients (24%). Device complaints occurred in 244 patients (93%). The most common device complaints included device malfunction (85%), device occlusion (57%), and device dislocation (56%). Thirty-eight patients died during the study. Two patients died as a result of intestinal dilatation and cardiac arrest, which an investigator considered possibly related to the treatment.
As part of an amended study protocol, patients in the United States began completing efficacy assessments in December 2013 (ie, Parkinson’s disease diary, Unified Parkinson’s Disease Rating Scale, and the Parkinson’s Disease Questionnaire). Patients in the United States showed sustained and clinically meaningful benefits of treatment that were demonstrated by decreased off time and increased on time without troublesome dyskinesia, the researchers concluded.
—Jake Remaly
PORTLAND, OR—Most patients with advanced Parkinson’s disease who received levodopa–carbidopa intestinal gel in an open-label, continued-access-to-treatment study had, at five years, transitioned to using the therapy outside of the study when it became commercially available or continued to participate in the extension study, according to data presented at the Fourth World Parkinson Congress.
Forty-two percent of the patients in the phase III extension study transitioned to commercially available drug (designated as carbidopa–levodopa enteral suspension in the United States), and 24% of patients remained in the multinational study, said Hubert H. Fernandez, MD, Head of Movement Disorders at the Center for Neurological Restoration at the Cleveland Clinic in Ohio, and colleagues. Patients may remain in the extension study until a commercially available product is available in the country where they live.
Levodopa–carbidopa intestinal gel is infused continuously directly to the jejunum using a portable pump during approximately 16 hours of wakefulness. The therapy is designed to overcome some of the limitations of oral levodopa, which may lose effectiveness as Parkinson’s disease progresses.
Although investigators observed a high incidence of adverse events, long-term use of levodopa–carbidopa intestinal gel was well tolerated, the researchers said. The average discontinuation rate of 9.6% per year, including all causes of death, was relatively low, Dr. Fernandez and colleagues said.
The most frequently reported adverse events were associated with complications related to percutaneous gastrojejunostomy, such as stoma site maintenance. Other adverse events were associated with advanced Parkinson’s disease, aging, or levodopa. Most patients experienced device malfunctions and required pump replacement during the study.
The extension study began in November 2009. Data through September 30, 2015, were used in the present study. The study enrolled 262 patients with advanced Parkinson’s disease from 11 countries who had completed a 12-week double-blind study and its 52-week open-label extension or who had completed a separate 54-week open-label study. Participants attended scheduled study visits every six months.
Patients had a mean age of 64, 62% were male, and mean disease duration was 11.4 years. Mean exposure to levodopa–carbidopa intestinal gel was 3.1 years in the present study. Patients’ mean total exposure to the treatment was 4.1 years. Fifty-six percent of patients were exposed to the treatment for at least five years.
Adverse events led to discontinuation in 62 patients (24%). Device complaints occurred in 244 patients (93%). The most common device complaints included device malfunction (85%), device occlusion (57%), and device dislocation (56%). Thirty-eight patients died during the study. Two patients died as a result of intestinal dilatation and cardiac arrest, which an investigator considered possibly related to the treatment.
As part of an amended study protocol, patients in the United States began completing efficacy assessments in December 2013 (ie, Parkinson’s disease diary, Unified Parkinson’s Disease Rating Scale, and the Parkinson’s Disease Questionnaire). Patients in the United States showed sustained and clinically meaningful benefits of treatment that were demonstrated by decreased off time and increased on time without troublesome dyskinesia, the researchers concluded.
—Jake Remaly
PORTLAND, OR—Most patients with advanced Parkinson’s disease who received levodopa–carbidopa intestinal gel in an open-label, continued-access-to-treatment study had, at five years, transitioned to using the therapy outside of the study when it became commercially available or continued to participate in the extension study, according to data presented at the Fourth World Parkinson Congress.
Forty-two percent of the patients in the phase III extension study transitioned to commercially available drug (designated as carbidopa–levodopa enteral suspension in the United States), and 24% of patients remained in the multinational study, said Hubert H. Fernandez, MD, Head of Movement Disorders at the Center for Neurological Restoration at the Cleveland Clinic in Ohio, and colleagues. Patients may remain in the extension study until a commercially available product is available in the country where they live.
Levodopa–carbidopa intestinal gel is infused continuously directly to the jejunum using a portable pump during approximately 16 hours of wakefulness. The therapy is designed to overcome some of the limitations of oral levodopa, which may lose effectiveness as Parkinson’s disease progresses.
Although investigators observed a high incidence of adverse events, long-term use of levodopa–carbidopa intestinal gel was well tolerated, the researchers said. The average discontinuation rate of 9.6% per year, including all causes of death, was relatively low, Dr. Fernandez and colleagues said.
The most frequently reported adverse events were associated with complications related to percutaneous gastrojejunostomy, such as stoma site maintenance. Other adverse events were associated with advanced Parkinson’s disease, aging, or levodopa. Most patients experienced device malfunctions and required pump replacement during the study.
The extension study began in November 2009. Data through September 30, 2015, were used in the present study. The study enrolled 262 patients with advanced Parkinson’s disease from 11 countries who had completed a 12-week double-blind study and its 52-week open-label extension or who had completed a separate 54-week open-label study. Participants attended scheduled study visits every six months.
Patients had a mean age of 64, 62% were male, and mean disease duration was 11.4 years. Mean exposure to levodopa–carbidopa intestinal gel was 3.1 years in the present study. Patients’ mean total exposure to the treatment was 4.1 years. Fifty-six percent of patients were exposed to the treatment for at least five years.
Adverse events led to discontinuation in 62 patients (24%). Device complaints occurred in 244 patients (93%). The most common device complaints included device malfunction (85%), device occlusion (57%), and device dislocation (56%). Thirty-eight patients died during the study. Two patients died as a result of intestinal dilatation and cardiac arrest, which an investigator considered possibly related to the treatment.
As part of an amended study protocol, patients in the United States began completing efficacy assessments in December 2013 (ie, Parkinson’s disease diary, Unified Parkinson’s Disease Rating Scale, and the Parkinson’s Disease Questionnaire). Patients in the United States showed sustained and clinically meaningful benefits of treatment that were demonstrated by decreased off time and increased on time without troublesome dyskinesia, the researchers concluded.
—Jake Remaly
Parenting: Tips on discussing a tough but important topic
It seems like the field of psychiatry has been all over the map when it comes to viewing the importance of parenting with regard to child behavioral problems and disorders. For decades, we heard that parents, particularly mothers, were to blame for everything from childhood autism to excessive temper tantrums.1 Then, parenting somehow got somewhat pushed aside as the genetic and biological underpinnings of behavior became increasingly appreciated. For a while, parenting was nearly relegated to epiphenomenon status – that is, an almost irrelevant reaction to genetically driven child behavior.
More recently, it appears that some semblance of balance has been restored with parenting behavior being appreciated as critically important in the development of a child, but in the context of many other mutually interacting factors.2 There also is a far greater understanding that child behavior and parent behavior is very much a two-way street.
These more nuanced and neuroscience-backed perspectives, however, don’t make bringing up the subject of parenting any easier. In part because of how seriously most mothers, fathers, and other caretakers take their job as a parent, it can be easy to put parents on the defensive, especially when one of their children is struggling behaviorally. At the same time, taking the easy way out by giving boilerplate advice, or even avoiding the topic of parenting completely, is a huge missed opportunity to engage families who often are desperately seeking some guidance.
Case summary
Emily is a healthy 6-year-old girl who comes in with her single mother and her two younger siblings for an annual exam. Her mother proudly reports that she is doing great at school, but seems reluctant to say much about her home life. The mother seems somewhat frazzled, and the interview is difficult because the three siblings are arguing with each other. After Emily and her sister fight over reading the same book, the mother suddenly and quite loudly says, “Can you just let me talk for 1 second!”
Discussion
Pediatricians often have strong suspicions that parents are struggling with a child’s behavior but can have trouble knowing how exactly to bring up the subject of parenting. Some specific suggestions for having productive discussions on parenting include the following:
• Think about the statements embedded in your questions. A screening question about parenting such as, “Can you tell me about the areas of parenting that you are most proud of and the areas where you feel you need the most help?” helps a parent understand that you assume that no parent is perfect and that everyone has areas of strength and weakness.
• Compliment when you can. Related to the above, find those areas of positive parenting, even if it involves effort more than results, and communicate that you have noticed them. This can make talking about the weaknesses a little easier to hear for the parent.
• Frame the issue in terms of surpluses rather than deficits. Instead of coming from the perspective that a parent is deficient in their basic parenting skills, reframe the challenge as someone needing “superparent” skills to manage multiple or more challenging children. The often-heard statements that “kids don’t come with instruction books” or “you need to earn a license to drive a car but not raise a child” are almost cliché these days, but still convey to parents that you understand how difficult parenting can be. In some cases, it may be appropriate to disclose some parenting challenges you have experienced firsthand.
• Get details. Before launching into specific recommendations, ask yourself if you are able to really see the issue a parent is describing. Rather than reviewing a laundry list of sleep hygiene recommendations, for example, it can be very worthwhile to ask, “How exactly does bedtime work at your home?” Getting all the details can not only build empathy, but allow you to really see specific areas for improvement. If you can’t paint a picture of how a scene might really look at this patient’s home, there likely is more to learn.
Of course, one of the key challenges here is time. Really giving these parenting concerns the time they deserve usually means going beyond the precious few minutes pediatricians have for a well visit. In these instances, it may be worth scheduling a future appointment that is exclusively devoted to this issue. Alternatively, a referral can be made to a therapist, counselor, or parent “coach” to give a family greater opportunity to work 1:1 with a professional. When you do this, be clear that you are looking for a therapist to work with the whole family, ideally using many of the evidence-based techniques that have been shown to be effective. A list of manual-based treatments as well as some books that parents could read on their own to address oppositional-defiant behavior is available, including a guide for families from the American Academy from Child and Adolescent Psychiatry.3
Case follow-up
The pediatrician finds another book to satisfy the younger sibling and says to the mother, “I’m glad to see that at least they are fighting over a book. That’s great that you have taught them to like reading.” They commiserate about how difficult it is to raise three young children as a single parent, and the mother then begins to open up about Emily’s defiant and disrespectful behavior at home that the mother blames on herself. The pediatrician offers a referral to see a local family therapist, which the mother gratefully accepts.
References
1. Am J Orthopsychiatry. 1985 Jul;55(3):345-53.
2. Child Adolesc Psychiatr Clin N Am. 2016 Apr;25(2):167-78.
3. American Academy of Child and Adolescent Psychiatry. (2009). Oppositional Defiant Disorder: A Guide for Families.
Dr. Rettew is associate professor of psychiatry and pediatrics at the University of Vermont, Burlington. Email him at [email protected].
It seems like the field of psychiatry has been all over the map when it comes to viewing the importance of parenting with regard to child behavioral problems and disorders. For decades, we heard that parents, particularly mothers, were to blame for everything from childhood autism to excessive temper tantrums.1 Then, parenting somehow got somewhat pushed aside as the genetic and biological underpinnings of behavior became increasingly appreciated. For a while, parenting was nearly relegated to epiphenomenon status – that is, an almost irrelevant reaction to genetically driven child behavior.
More recently, it appears that some semblance of balance has been restored with parenting behavior being appreciated as critically important in the development of a child, but in the context of many other mutually interacting factors.2 There also is a far greater understanding that child behavior and parent behavior is very much a two-way street.
These more nuanced and neuroscience-backed perspectives, however, don’t make bringing up the subject of parenting any easier. In part because of how seriously most mothers, fathers, and other caretakers take their job as a parent, it can be easy to put parents on the defensive, especially when one of their children is struggling behaviorally. At the same time, taking the easy way out by giving boilerplate advice, or even avoiding the topic of parenting completely, is a huge missed opportunity to engage families who often are desperately seeking some guidance.
Case summary
Emily is a healthy 6-year-old girl who comes in with her single mother and her two younger siblings for an annual exam. Her mother proudly reports that she is doing great at school, but seems reluctant to say much about her home life. The mother seems somewhat frazzled, and the interview is difficult because the three siblings are arguing with each other. After Emily and her sister fight over reading the same book, the mother suddenly and quite loudly says, “Can you just let me talk for 1 second!”
Discussion
Pediatricians often have strong suspicions that parents are struggling with a child’s behavior but can have trouble knowing how exactly to bring up the subject of parenting. Some specific suggestions for having productive discussions on parenting include the following:
• Think about the statements embedded in your questions. A screening question about parenting such as, “Can you tell me about the areas of parenting that you are most proud of and the areas where you feel you need the most help?” helps a parent understand that you assume that no parent is perfect and that everyone has areas of strength and weakness.
• Compliment when you can. Related to the above, find those areas of positive parenting, even if it involves effort more than results, and communicate that you have noticed them. This can make talking about the weaknesses a little easier to hear for the parent.
• Frame the issue in terms of surpluses rather than deficits. Instead of coming from the perspective that a parent is deficient in their basic parenting skills, reframe the challenge as someone needing “superparent” skills to manage multiple or more challenging children. The often-heard statements that “kids don’t come with instruction books” or “you need to earn a license to drive a car but not raise a child” are almost cliché these days, but still convey to parents that you understand how difficult parenting can be. In some cases, it may be appropriate to disclose some parenting challenges you have experienced firsthand.
• Get details. Before launching into specific recommendations, ask yourself if you are able to really see the issue a parent is describing. Rather than reviewing a laundry list of sleep hygiene recommendations, for example, it can be very worthwhile to ask, “How exactly does bedtime work at your home?” Getting all the details can not only build empathy, but allow you to really see specific areas for improvement. If you can’t paint a picture of how a scene might really look at this patient’s home, there likely is more to learn.
Of course, one of the key challenges here is time. Really giving these parenting concerns the time they deserve usually means going beyond the precious few minutes pediatricians have for a well visit. In these instances, it may be worth scheduling a future appointment that is exclusively devoted to this issue. Alternatively, a referral can be made to a therapist, counselor, or parent “coach” to give a family greater opportunity to work 1:1 with a professional. When you do this, be clear that you are looking for a therapist to work with the whole family, ideally using many of the evidence-based techniques that have been shown to be effective. A list of manual-based treatments as well as some books that parents could read on their own to address oppositional-defiant behavior is available, including a guide for families from the American Academy from Child and Adolescent Psychiatry.3
Case follow-up
The pediatrician finds another book to satisfy the younger sibling and says to the mother, “I’m glad to see that at least they are fighting over a book. That’s great that you have taught them to like reading.” They commiserate about how difficult it is to raise three young children as a single parent, and the mother then begins to open up about Emily’s defiant and disrespectful behavior at home that the mother blames on herself. The pediatrician offers a referral to see a local family therapist, which the mother gratefully accepts.
References
1. Am J Orthopsychiatry. 1985 Jul;55(3):345-53.
2. Child Adolesc Psychiatr Clin N Am. 2016 Apr;25(2):167-78.
3. American Academy of Child and Adolescent Psychiatry. (2009). Oppositional Defiant Disorder: A Guide for Families.
Dr. Rettew is associate professor of psychiatry and pediatrics at the University of Vermont, Burlington. Email him at [email protected].
It seems like the field of psychiatry has been all over the map when it comes to viewing the importance of parenting with regard to child behavioral problems and disorders. For decades, we heard that parents, particularly mothers, were to blame for everything from childhood autism to excessive temper tantrums.1 Then, parenting somehow got somewhat pushed aside as the genetic and biological underpinnings of behavior became increasingly appreciated. For a while, parenting was nearly relegated to epiphenomenon status – that is, an almost irrelevant reaction to genetically driven child behavior.
More recently, it appears that some semblance of balance has been restored with parenting behavior being appreciated as critically important in the development of a child, but in the context of many other mutually interacting factors.2 There also is a far greater understanding that child behavior and parent behavior is very much a two-way street.
These more nuanced and neuroscience-backed perspectives, however, don’t make bringing up the subject of parenting any easier. In part because of how seriously most mothers, fathers, and other caretakers take their job as a parent, it can be easy to put parents on the defensive, especially when one of their children is struggling behaviorally. At the same time, taking the easy way out by giving boilerplate advice, or even avoiding the topic of parenting completely, is a huge missed opportunity to engage families who often are desperately seeking some guidance.
Case summary
Emily is a healthy 6-year-old girl who comes in with her single mother and her two younger siblings for an annual exam. Her mother proudly reports that she is doing great at school, but seems reluctant to say much about her home life. The mother seems somewhat frazzled, and the interview is difficult because the three siblings are arguing with each other. After Emily and her sister fight over reading the same book, the mother suddenly and quite loudly says, “Can you just let me talk for 1 second!”
Discussion
Pediatricians often have strong suspicions that parents are struggling with a child’s behavior but can have trouble knowing how exactly to bring up the subject of parenting. Some specific suggestions for having productive discussions on parenting include the following:
• Think about the statements embedded in your questions. A screening question about parenting such as, “Can you tell me about the areas of parenting that you are most proud of and the areas where you feel you need the most help?” helps a parent understand that you assume that no parent is perfect and that everyone has areas of strength and weakness.
• Compliment when you can. Related to the above, find those areas of positive parenting, even if it involves effort more than results, and communicate that you have noticed them. This can make talking about the weaknesses a little easier to hear for the parent.
• Frame the issue in terms of surpluses rather than deficits. Instead of coming from the perspective that a parent is deficient in their basic parenting skills, reframe the challenge as someone needing “superparent” skills to manage multiple or more challenging children. The often-heard statements that “kids don’t come with instruction books” or “you need to earn a license to drive a car but not raise a child” are almost cliché these days, but still convey to parents that you understand how difficult parenting can be. In some cases, it may be appropriate to disclose some parenting challenges you have experienced firsthand.
• Get details. Before launching into specific recommendations, ask yourself if you are able to really see the issue a parent is describing. Rather than reviewing a laundry list of sleep hygiene recommendations, for example, it can be very worthwhile to ask, “How exactly does bedtime work at your home?” Getting all the details can not only build empathy, but allow you to really see specific areas for improvement. If you can’t paint a picture of how a scene might really look at this patient’s home, there likely is more to learn.
Of course, one of the key challenges here is time. Really giving these parenting concerns the time they deserve usually means going beyond the precious few minutes pediatricians have for a well visit. In these instances, it may be worth scheduling a future appointment that is exclusively devoted to this issue. Alternatively, a referral can be made to a therapist, counselor, or parent “coach” to give a family greater opportunity to work 1:1 with a professional. When you do this, be clear that you are looking for a therapist to work with the whole family, ideally using many of the evidence-based techniques that have been shown to be effective. A list of manual-based treatments as well as some books that parents could read on their own to address oppositional-defiant behavior is available, including a guide for families from the American Academy from Child and Adolescent Psychiatry.3
Case follow-up
The pediatrician finds another book to satisfy the younger sibling and says to the mother, “I’m glad to see that at least they are fighting over a book. That’s great that you have taught them to like reading.” They commiserate about how difficult it is to raise three young children as a single parent, and the mother then begins to open up about Emily’s defiant and disrespectful behavior at home that the mother blames on herself. The pediatrician offers a referral to see a local family therapist, which the mother gratefully accepts.
References
1. Am J Orthopsychiatry. 1985 Jul;55(3):345-53.
2. Child Adolesc Psychiatr Clin N Am. 2016 Apr;25(2):167-78.
3. American Academy of Child and Adolescent Psychiatry. (2009). Oppositional Defiant Disorder: A Guide for Families.
Dr. Rettew is associate professor of psychiatry and pediatrics at the University of Vermont, Burlington. Email him at [email protected].
The thyroid takes a beating during PCI in about 3% of patients
DENVER – Undergoing percutaneous coronary intervention (PCI) may impair thyroid function and change the gland’s morphology, probably because of the cumulative effects of handling and exposure to radiation and iodine in the contrast dye, Samir Naim Assaad, MD, said during a poster presentation at the annual meeting of the American Thyroid Association.
Cardiologists should inform their patients of these possible effects as part of their pre- and post-PCI counseling so that they won’t be alarmed by the changes in how they feel, Dr. Assaad, chief of the division of endocrinology at Alexandria (Egypt) University, said in an interview.
Similarly, when a formerly euthyroid patient presents to an endocrinologist with sudden-onset hyperthyroidism, “Have you had a PCI recently?” should be one of the first questions asked. If the answer is yes, then further testing and imaging should be delayed at least 3 months, he noted.
Dr. Assaad and his associates examined 113 clinically euthyroid patients both before and several months after they underwent PCI for management of stable coronary artery disease. The cohort included 93 men, and patients’ ages ranged from 32 years to 73 years.
All the patients underwent a series of tests immediately before PCI, 24 hours after, and 3 months later. Those tests included serum free triiodothyronine (FT3), free thyroxine (FT4), thyroid-stimulating hormone (TSH), free T3/T4 ratio, antithyroperoxidase (anti-TPO), and high-sensitivity C-reactive protein.
The gland’s morphology, including volume, vascularity, nodules, and echogenicity, were assessed on the same timetable using ultrasonography.
One day after PCI, there was a significant increase in serum FT3 (5.2-0.5 vs. 3.3-0.7 pg/mL, P less than .001), and serum FT4 (1.3 – 0.5 vs 1.2 – 0.3 ng/dL, P = .04), with no significant change in serum TSH.
Three months after PCI, there was a further significant increase in serum FT4 (1.5 – 0.3 ng/dL), decrease in serum FT3 returning to baseline (3.2 – 1.3 pg/mL), and a significant increase in serum TSH, compared with just before PCI (mean, 3.2-5 vs. 1.5-2.1 mIU/L, P less than .001). Serum anti-TPO (AU/mL) showed a significant increase 3 months after PCI.
There was a significant increase in thyroid gland volume 3 months after PCI (13.6-3.9 vs. 13.1-3.5 cm3, P = .02). The measured echogenicity of the thyroid gland showed a significant decrease 3 months after PCI (67.1-10.9 vs. 88.7-25.6 GWE, P less than .001).
Thyroid radiation had a negative effect on serum TSH, anti-TPO, FT3, and FT3/FT4 ratio, and an inverse correlation of dye injection time with serum anti-TPO and TSH, judging from the findings of a regression analysis model.
Dr. Assaad was not included on the list of presenters with relevant financial disclosures that was provided by the American Thyroid Association.
DENVER – Undergoing percutaneous coronary intervention (PCI) may impair thyroid function and change the gland’s morphology, probably because of the cumulative effects of handling and exposure to radiation and iodine in the contrast dye, Samir Naim Assaad, MD, said during a poster presentation at the annual meeting of the American Thyroid Association.
Cardiologists should inform their patients of these possible effects as part of their pre- and post-PCI counseling so that they won’t be alarmed by the changes in how they feel, Dr. Assaad, chief of the division of endocrinology at Alexandria (Egypt) University, said in an interview.
Similarly, when a formerly euthyroid patient presents to an endocrinologist with sudden-onset hyperthyroidism, “Have you had a PCI recently?” should be one of the first questions asked. If the answer is yes, then further testing and imaging should be delayed at least 3 months, he noted.
Dr. Assaad and his associates examined 113 clinically euthyroid patients both before and several months after they underwent PCI for management of stable coronary artery disease. The cohort included 93 men, and patients’ ages ranged from 32 years to 73 years.
All the patients underwent a series of tests immediately before PCI, 24 hours after, and 3 months later. Those tests included serum free triiodothyronine (FT3), free thyroxine (FT4), thyroid-stimulating hormone (TSH), free T3/T4 ratio, antithyroperoxidase (anti-TPO), and high-sensitivity C-reactive protein.
The gland’s morphology, including volume, vascularity, nodules, and echogenicity, were assessed on the same timetable using ultrasonography.
One day after PCI, there was a significant increase in serum FT3 (5.2-0.5 vs. 3.3-0.7 pg/mL, P less than .001), and serum FT4 (1.3 – 0.5 vs 1.2 – 0.3 ng/dL, P = .04), with no significant change in serum TSH.
Three months after PCI, there was a further significant increase in serum FT4 (1.5 – 0.3 ng/dL), decrease in serum FT3 returning to baseline (3.2 – 1.3 pg/mL), and a significant increase in serum TSH, compared with just before PCI (mean, 3.2-5 vs. 1.5-2.1 mIU/L, P less than .001). Serum anti-TPO (AU/mL) showed a significant increase 3 months after PCI.
There was a significant increase in thyroid gland volume 3 months after PCI (13.6-3.9 vs. 13.1-3.5 cm3, P = .02). The measured echogenicity of the thyroid gland showed a significant decrease 3 months after PCI (67.1-10.9 vs. 88.7-25.6 GWE, P less than .001).
Thyroid radiation had a negative effect on serum TSH, anti-TPO, FT3, and FT3/FT4 ratio, and an inverse correlation of dye injection time with serum anti-TPO and TSH, judging from the findings of a regression analysis model.
Dr. Assaad was not included on the list of presenters with relevant financial disclosures that was provided by the American Thyroid Association.
DENVER – Undergoing percutaneous coronary intervention (PCI) may impair thyroid function and change the gland’s morphology, probably because of the cumulative effects of handling and exposure to radiation and iodine in the contrast dye, Samir Naim Assaad, MD, said during a poster presentation at the annual meeting of the American Thyroid Association.
Cardiologists should inform their patients of these possible effects as part of their pre- and post-PCI counseling so that they won’t be alarmed by the changes in how they feel, Dr. Assaad, chief of the division of endocrinology at Alexandria (Egypt) University, said in an interview.
Similarly, when a formerly euthyroid patient presents to an endocrinologist with sudden-onset hyperthyroidism, “Have you had a PCI recently?” should be one of the first questions asked. If the answer is yes, then further testing and imaging should be delayed at least 3 months, he noted.
Dr. Assaad and his associates examined 113 clinically euthyroid patients both before and several months after they underwent PCI for management of stable coronary artery disease. The cohort included 93 men, and patients’ ages ranged from 32 years to 73 years.
All the patients underwent a series of tests immediately before PCI, 24 hours after, and 3 months later. Those tests included serum free triiodothyronine (FT3), free thyroxine (FT4), thyroid-stimulating hormone (TSH), free T3/T4 ratio, antithyroperoxidase (anti-TPO), and high-sensitivity C-reactive protein.
The gland’s morphology, including volume, vascularity, nodules, and echogenicity, were assessed on the same timetable using ultrasonography.
One day after PCI, there was a significant increase in serum FT3 (5.2-0.5 vs. 3.3-0.7 pg/mL, P less than .001), and serum FT4 (1.3 – 0.5 vs 1.2 – 0.3 ng/dL, P = .04), with no significant change in serum TSH.
Three months after PCI, there was a further significant increase in serum FT4 (1.5 – 0.3 ng/dL), decrease in serum FT3 returning to baseline (3.2 – 1.3 pg/mL), and a significant increase in serum TSH, compared with just before PCI (mean, 3.2-5 vs. 1.5-2.1 mIU/L, P less than .001). Serum anti-TPO (AU/mL) showed a significant increase 3 months after PCI.
There was a significant increase in thyroid gland volume 3 months after PCI (13.6-3.9 vs. 13.1-3.5 cm3, P = .02). The measured echogenicity of the thyroid gland showed a significant decrease 3 months after PCI (67.1-10.9 vs. 88.7-25.6 GWE, P less than .001).
Thyroid radiation had a negative effect on serum TSH, anti-TPO, FT3, and FT3/FT4 ratio, and an inverse correlation of dye injection time with serum anti-TPO and TSH, judging from the findings of a regression analysis model.
Dr. Assaad was not included on the list of presenters with relevant financial disclosures that was provided by the American Thyroid Association.
AT THE ATA ANNUAL MEETING
Key clinical point: The thyroid function and morphology of any patient undergoing PCI may be altered by the procedure; but most changes normalize within several months.
Major finding: Thyroid function changes in close to 3% of patients undergoing PCI.
Data source: A study of 113 euthyroid patients who had PCI for coronary artery disease.
Disclosures: Dr. Assaad was not included on the list of presenters with relevant financial disclosures provided by the American Thyroid Association.
Zika cases in pregnant women hit new weekly high
The weekly number of pregnant women in the United States with laboratory evidence of Zika virus infection topped 200 for the first time during the week ending Sept. 15, according to the Centers for Disease Control and Prevention.
With the 50 states and the District of Columbia reporting 18 new cases for the week and the U.S. territories reporting 192, there were 210 more pregnant women with Zika for the week ending Sept. 15, the CDC reported Sept. 22. The previous weekly high had been 199 for the week ending Aug. 25.
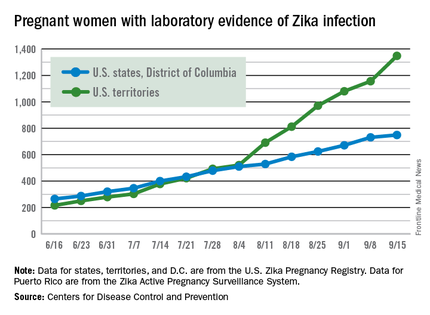
The CDC also reported two new cases of liveborn infants – both in the 50 states and D.C. – with Zika-related birth defects. No infants with Zika-related birth defects were reported in the territories for the week, and there were no new reports of pregnancy losses related to Zika. The number of pregnancy losses holds at six for the year so far, but the number of U.S. liveborn infants with Zika-related birth defects is now 21, with 20 cases in the states/D.C. and one in the territories, the CDC said. State- or territorial-level data are not being reported to protect the privacy of affected women and children.
There were 182 new cases of Zika infection reported among all Americans in the states/D.C. for the week ending Sept. 21, along with 2,083 new cases in the territories – almost all in Puerto Rico, which continues to retroactively report cases, the CDC noted Sept. 22. The U.S. total for 2015-2016 is 23,135 cases: 3,358 reported in the states/D.C. and 19,777 in the territories. Puerto Rico represents 98% of the territorial total, the CDC said.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
The weekly number of pregnant women in the United States with laboratory evidence of Zika virus infection topped 200 for the first time during the week ending Sept. 15, according to the Centers for Disease Control and Prevention.
With the 50 states and the District of Columbia reporting 18 new cases for the week and the U.S. territories reporting 192, there were 210 more pregnant women with Zika for the week ending Sept. 15, the CDC reported Sept. 22. The previous weekly high had been 199 for the week ending Aug. 25.

The CDC also reported two new cases of liveborn infants – both in the 50 states and D.C. – with Zika-related birth defects. No infants with Zika-related birth defects were reported in the territories for the week, and there were no new reports of pregnancy losses related to Zika. The number of pregnancy losses holds at six for the year so far, but the number of U.S. liveborn infants with Zika-related birth defects is now 21, with 20 cases in the states/D.C. and one in the territories, the CDC said. State- or territorial-level data are not being reported to protect the privacy of affected women and children.
There were 182 new cases of Zika infection reported among all Americans in the states/D.C. for the week ending Sept. 21, along with 2,083 new cases in the territories – almost all in Puerto Rico, which continues to retroactively report cases, the CDC noted Sept. 22. The U.S. total for 2015-2016 is 23,135 cases: 3,358 reported in the states/D.C. and 19,777 in the territories. Puerto Rico represents 98% of the territorial total, the CDC said.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
The weekly number of pregnant women in the United States with laboratory evidence of Zika virus infection topped 200 for the first time during the week ending Sept. 15, according to the Centers for Disease Control and Prevention.
With the 50 states and the District of Columbia reporting 18 new cases for the week and the U.S. territories reporting 192, there were 210 more pregnant women with Zika for the week ending Sept. 15, the CDC reported Sept. 22. The previous weekly high had been 199 for the week ending Aug. 25.

The CDC also reported two new cases of liveborn infants – both in the 50 states and D.C. – with Zika-related birth defects. No infants with Zika-related birth defects were reported in the territories for the week, and there were no new reports of pregnancy losses related to Zika. The number of pregnancy losses holds at six for the year so far, but the number of U.S. liveborn infants with Zika-related birth defects is now 21, with 20 cases in the states/D.C. and one in the territories, the CDC said. State- or territorial-level data are not being reported to protect the privacy of affected women and children.
There were 182 new cases of Zika infection reported among all Americans in the states/D.C. for the week ending Sept. 21, along with 2,083 new cases in the territories – almost all in Puerto Rico, which continues to retroactively report cases, the CDC noted Sept. 22. The U.S. total for 2015-2016 is 23,135 cases: 3,358 reported in the states/D.C. and 19,777 in the territories. Puerto Rico represents 98% of the territorial total, the CDC said.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.






