User login
Decision rule identifies unprovoked VTE patients who can halt anticoagulation
ROME – Half of all women who experience a first unprovoked venous thromboembolism (VTE) can safely be spared lifelong anticoagulation through application of the newly validated HERDOO2 decision rule, Marc A. Rodger, MD, reported at the annual congress of the European Society of Cardiology.
“We’ve validated that a simple, memorable decision rule on anticoagulation applied at the clinically relevant time point works. And it is the only clinical decision rule that has now been prospectively validated,” said Dr. Rodger, professor of medicine, chief and chair of the division of hematology, and head of the thrombosis program at the University of Ottawa.
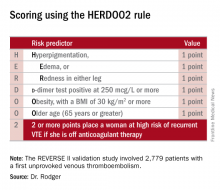
He presented the results of the validation study, known as the REVERSE II study, which included 2,779 patients with a first unprovoked VTE at 44 centers in seven countries. The full name of the decision rule is “Men Continue and HERDOO2,” a name that says it all: the rule posits that all men as well as those women with a HERDOO2 (Hyperpigmentation, Edema, Redness, d-dimer, Obesity, Older age, 2 or more points) score of at least 2 out of a possible 4 points need to stay on anticoagulation indefinitely because their risk of a recurrent VTE off-therapy clearly exceeds that of a bleeding event on-therapy. In contrast, women with a HERDOO2 score of 0 or 1 can safely stop anticoagulation after the standard 3-6 months of acute short-term therapy.
“Sorry, gentlemen, but we could find no low-risk group of men. They were all high risk,” he said. “But 50% of women with unprovoked vein blood clots can be spared the burdens, costs, and risks of lifelong blood thinners.”
Dr. Rodger and coinvestigators began work on developing a multivariate clinical decision rule in 2001. They examined 69 risk predictors, eventually winnowing down to a manageable four potent risk predictors identified by the acronym HERDOO2.
The derivation study was published 8 years ago (CMAJ. 2008;Aug 26;179[5]:417-26). It showed that women with a HERDOO2 score of 2 or more as well as all men had roughly a 14% rate of recurrent VTE in the first year after stopping anticoagulation, while women with a score of 0 or 1 had about a 1.6% risk. The International Society on Thrombosis and Haemostasis suggests that it’s safe to discontinue anticoagulants if the risk of recurrent thrombosis at 1 year off-therapy is less than 5%, given the significant risk of serious bleeding on-therapy and the fact that a serious bleed event is two to three times more likely than a VTE to be fatal.
Dr. Rodger and coinvestigators recognized that a clinical decision rule needs to be externally validated before it’s ready for prime-time use in clinical practice. Thus, they conducted the REVERSE II study, in which the decision rule was applied after the 2,799 participants had been on anticoagulation for 5-12 months. All had a first proximal deep vein thrombosis and/or a segmental or greater pulmonary embolism. Patients were still on anticoagulation at the time the rule was applied, which is why the cut point for a positive d-dimer test in HERDOO2 is 250 mcg/L, half of the threshold value for a positive test in patients not on anticoagulation.
They identified 631 women as low risk, with a HERDOO2 score of 0 or 1. They and their physicians were instructed to stop anticoagulation at that time. The 2,148 high-risk subjects – that is, all of the men and the high-risk women – were advised to remain on anticoagulation. The primary study endpoint was the rate of recurrent VTE in the 12 months following testing and patient guidance. The lost-to-follow-up rate was 2.2%.
The recurrent VTE rate was 3% in the 591 low-risk women who discontinued anticoagulants and zero in 31 others who elected to stay on medication. In the high-risk group identified by the HERDOO2 rule, the recurrent VTE rate at 12 months was 8.1% in the 323 who opted to discontinue anticoagulants and just 1.6% in 1,802 who continued on therapy as advised, a finding that underscores the effectiveness of selectively applied long-term anticoagulation therapy, he continued.
The recurrent VTE rate among the 291 women with a HERDOO2 score of 0 or 1 who were on exogenous estrogen was 1.4%, while in high-risk women taking estrogen the rate was more than doubled at 3.1%. But in women aged 50-64 identified by the HERDOO2 rule as being low risk, the actual recurrent VTE rate was 5.7%, a finding that raised a red flag for the investigators.
“There may be an evolution of the HERDOO2 decision rule to a lower age cut point. But that’s something that requires further study in postmenopausal women,” according to Dr. Rodger.
The investigators defined a first unprovoked VTE as one occurring in the absence during the previous 90 days of major surgery, a fracture or cast, more than 3 days of immobilization, or malignancy within the last 5 years.
Venous thromboembolism is the second most common cardiovascular disorder and the third most common cause of cardiovascular death. Unprovoked VTEs account for half of all VTEs. Their management has been a controversial subject. Both the American College of Chest Physicians and the European Society of Cardiology recommend continuing anticoagulation indefinitely in patients who aren’t at high bleeding risk.
“But this is a relatively weak 2B recommendation because of the tightly balanced competing risks of recurrent thrombosis off anticoagulation and major bleeding on anticoagulation,” Dr. Rodger said. He added that he considers REVERSE II to be practice changing, and predicted that once the results are published the guidelines will be revised.
Discussant Giancarlo Agnelli, MD, was a tough critic who gave fair warning.
“I am friends with many of the authors of this paper, and in this country we are usually gentle with enemies and nasty with friends,” declared Dr. Agnelli, professor of internal medicine and director of internal and cardiovascular medicine and the stroke unit at the University of Perugia, Italy.
He didn’t find the REVERSE II study or the HERDOO2 rule persuasive. On the plus side, he said, the HERDOO2 rule has now been validated, unlike the proposed DASH and Vienna rules. And it was tested in a diverse multinational patient population. But the fact that the HERDOO2 rule is only applicable in women is a major limitation. And REVERSE II was not a randomized trial, Dr. Agnelli noted.
Moreover, 1 year of follow-up seems insufficient, he continued. He cited a French multicenter trial in which patients with a first unprovoked VTE received 6 months of anticoagulants and were then randomized to another 18 months of anticoagulation or placebo. During that 18 months, the group on anticoagulants had a significantly lower rate of the composite endpoint comprised of recurrent VTE or major bleeding, but once that period was over they experienced catchup. By the time the study ended at 42 months, the two study arms didn’t differ significantly in the composite endpoint (JAMA. 2015 Jul 7;314[1]:31-40).
More broadly, Dr. Agnelli also questioned the need for an anticoagulation discontinuation rule in the contemporary era of new oral anticoagulants (NOACs). He was lead investigator in the AMPLIFY study, a major randomized trial of fixed-dose apixaban (Eliquis) versus conventional therapy with subcutaneous enoxaparin (Lovenox) bridging to warfarin in 5,395 patients with acute VTE. The NOAC was associated with a 69% reduction in the relative risk of bleeding and was noninferior to standard therapy in the risk of recurrent VTE (N Engl J Med. 2013 Aug 29;369[9]:799-808).
“Why should we think about withholding anticoagulation in some patients when we now have such a safe approach?” he asked.
Dr. Rodger reported receiving research grants from the French government as well as from Biomerieux, which funded the REVERSE II study. Dr. Agnelli reported having no financial conflicts.
ROME – Half of all women who experience a first unprovoked venous thromboembolism (VTE) can safely be spared lifelong anticoagulation through application of the newly validated HERDOO2 decision rule, Marc A. Rodger, MD, reported at the annual congress of the European Society of Cardiology.
“We’ve validated that a simple, memorable decision rule on anticoagulation applied at the clinically relevant time point works. And it is the only clinical decision rule that has now been prospectively validated,” said Dr. Rodger, professor of medicine, chief and chair of the division of hematology, and head of the thrombosis program at the University of Ottawa.
He presented the results of the validation study, known as the REVERSE II study, which included 2,779 patients with a first unprovoked VTE at 44 centers in seven countries. The full name of the decision rule is “Men Continue and HERDOO2,” a name that says it all: the rule posits that all men as well as those women with a HERDOO2 (Hyperpigmentation, Edema, Redness, d-dimer, Obesity, Older age, 2 or more points) score of at least 2 out of a possible 4 points need to stay on anticoagulation indefinitely because their risk of a recurrent VTE off-therapy clearly exceeds that of a bleeding event on-therapy. In contrast, women with a HERDOO2 score of 0 or 1 can safely stop anticoagulation after the standard 3-6 months of acute short-term therapy.
“Sorry, gentlemen, but we could find no low-risk group of men. They were all high risk,” he said. “But 50% of women with unprovoked vein blood clots can be spared the burdens, costs, and risks of lifelong blood thinners.”
Dr. Rodger and coinvestigators began work on developing a multivariate clinical decision rule in 2001. They examined 69 risk predictors, eventually winnowing down to a manageable four potent risk predictors identified by the acronym HERDOO2.
The derivation study was published 8 years ago (CMAJ. 2008;Aug 26;179[5]:417-26). It showed that women with a HERDOO2 score of 2 or more as well as all men had roughly a 14% rate of recurrent VTE in the first year after stopping anticoagulation, while women with a score of 0 or 1 had about a 1.6% risk. The International Society on Thrombosis and Haemostasis suggests that it’s safe to discontinue anticoagulants if the risk of recurrent thrombosis at 1 year off-therapy is less than 5%, given the significant risk of serious bleeding on-therapy and the fact that a serious bleed event is two to three times more likely than a VTE to be fatal.
Dr. Rodger and coinvestigators recognized that a clinical decision rule needs to be externally validated before it’s ready for prime-time use in clinical practice. Thus, they conducted the REVERSE II study, in which the decision rule was applied after the 2,799 participants had been on anticoagulation for 5-12 months. All had a first proximal deep vein thrombosis and/or a segmental or greater pulmonary embolism. Patients were still on anticoagulation at the time the rule was applied, which is why the cut point for a positive d-dimer test in HERDOO2 is 250 mcg/L, half of the threshold value for a positive test in patients not on anticoagulation.
They identified 631 women as low risk, with a HERDOO2 score of 0 or 1. They and their physicians were instructed to stop anticoagulation at that time. The 2,148 high-risk subjects – that is, all of the men and the high-risk women – were advised to remain on anticoagulation. The primary study endpoint was the rate of recurrent VTE in the 12 months following testing and patient guidance. The lost-to-follow-up rate was 2.2%.
The recurrent VTE rate was 3% in the 591 low-risk women who discontinued anticoagulants and zero in 31 others who elected to stay on medication. In the high-risk group identified by the HERDOO2 rule, the recurrent VTE rate at 12 months was 8.1% in the 323 who opted to discontinue anticoagulants and just 1.6% in 1,802 who continued on therapy as advised, a finding that underscores the effectiveness of selectively applied long-term anticoagulation therapy, he continued.
The recurrent VTE rate among the 291 women with a HERDOO2 score of 0 or 1 who were on exogenous estrogen was 1.4%, while in high-risk women taking estrogen the rate was more than doubled at 3.1%. But in women aged 50-64 identified by the HERDOO2 rule as being low risk, the actual recurrent VTE rate was 5.7%, a finding that raised a red flag for the investigators.
“There may be an evolution of the HERDOO2 decision rule to a lower age cut point. But that’s something that requires further study in postmenopausal women,” according to Dr. Rodger.
The investigators defined a first unprovoked VTE as one occurring in the absence during the previous 90 days of major surgery, a fracture or cast, more than 3 days of immobilization, or malignancy within the last 5 years.
Venous thromboembolism is the second most common cardiovascular disorder and the third most common cause of cardiovascular death. Unprovoked VTEs account for half of all VTEs. Their management has been a controversial subject. Both the American College of Chest Physicians and the European Society of Cardiology recommend continuing anticoagulation indefinitely in patients who aren’t at high bleeding risk.
“But this is a relatively weak 2B recommendation because of the tightly balanced competing risks of recurrent thrombosis off anticoagulation and major bleeding on anticoagulation,” Dr. Rodger said. He added that he considers REVERSE II to be practice changing, and predicted that once the results are published the guidelines will be revised.
Discussant Giancarlo Agnelli, MD, was a tough critic who gave fair warning.
“I am friends with many of the authors of this paper, and in this country we are usually gentle with enemies and nasty with friends,” declared Dr. Agnelli, professor of internal medicine and director of internal and cardiovascular medicine and the stroke unit at the University of Perugia, Italy.
He didn’t find the REVERSE II study or the HERDOO2 rule persuasive. On the plus side, he said, the HERDOO2 rule has now been validated, unlike the proposed DASH and Vienna rules. And it was tested in a diverse multinational patient population. But the fact that the HERDOO2 rule is only applicable in women is a major limitation. And REVERSE II was not a randomized trial, Dr. Agnelli noted.
Moreover, 1 year of follow-up seems insufficient, he continued. He cited a French multicenter trial in which patients with a first unprovoked VTE received 6 months of anticoagulants and were then randomized to another 18 months of anticoagulation or placebo. During that 18 months, the group on anticoagulants had a significantly lower rate of the composite endpoint comprised of recurrent VTE or major bleeding, but once that period was over they experienced catchup. By the time the study ended at 42 months, the two study arms didn’t differ significantly in the composite endpoint (JAMA. 2015 Jul 7;314[1]:31-40).
More broadly, Dr. Agnelli also questioned the need for an anticoagulation discontinuation rule in the contemporary era of new oral anticoagulants (NOACs). He was lead investigator in the AMPLIFY study, a major randomized trial of fixed-dose apixaban (Eliquis) versus conventional therapy with subcutaneous enoxaparin (Lovenox) bridging to warfarin in 5,395 patients with acute VTE. The NOAC was associated with a 69% reduction in the relative risk of bleeding and was noninferior to standard therapy in the risk of recurrent VTE (N Engl J Med. 2013 Aug 29;369[9]:799-808).
“Why should we think about withholding anticoagulation in some patients when we now have such a safe approach?” he asked.
Dr. Rodger reported receiving research grants from the French government as well as from Biomerieux, which funded the REVERSE II study. Dr. Agnelli reported having no financial conflicts.
ROME – Half of all women who experience a first unprovoked venous thromboembolism (VTE) can safely be spared lifelong anticoagulation through application of the newly validated HERDOO2 decision rule, Marc A. Rodger, MD, reported at the annual congress of the European Society of Cardiology.
“We’ve validated that a simple, memorable decision rule on anticoagulation applied at the clinically relevant time point works. And it is the only clinical decision rule that has now been prospectively validated,” said Dr. Rodger, professor of medicine, chief and chair of the division of hematology, and head of the thrombosis program at the University of Ottawa.
He presented the results of the validation study, known as the REVERSE II study, which included 2,779 patients with a first unprovoked VTE at 44 centers in seven countries. The full name of the decision rule is “Men Continue and HERDOO2,” a name that says it all: the rule posits that all men as well as those women with a HERDOO2 (Hyperpigmentation, Edema, Redness, d-dimer, Obesity, Older age, 2 or more points) score of at least 2 out of a possible 4 points need to stay on anticoagulation indefinitely because their risk of a recurrent VTE off-therapy clearly exceeds that of a bleeding event on-therapy. In contrast, women with a HERDOO2 score of 0 or 1 can safely stop anticoagulation after the standard 3-6 months of acute short-term therapy.
“Sorry, gentlemen, but we could find no low-risk group of men. They were all high risk,” he said. “But 50% of women with unprovoked vein blood clots can be spared the burdens, costs, and risks of lifelong blood thinners.”
Dr. Rodger and coinvestigators began work on developing a multivariate clinical decision rule in 2001. They examined 69 risk predictors, eventually winnowing down to a manageable four potent risk predictors identified by the acronym HERDOO2.
The derivation study was published 8 years ago (CMAJ. 2008;Aug 26;179[5]:417-26). It showed that women with a HERDOO2 score of 2 or more as well as all men had roughly a 14% rate of recurrent VTE in the first year after stopping anticoagulation, while women with a score of 0 or 1 had about a 1.6% risk. The International Society on Thrombosis and Haemostasis suggests that it’s safe to discontinue anticoagulants if the risk of recurrent thrombosis at 1 year off-therapy is less than 5%, given the significant risk of serious bleeding on-therapy and the fact that a serious bleed event is two to three times more likely than a VTE to be fatal.
Dr. Rodger and coinvestigators recognized that a clinical decision rule needs to be externally validated before it’s ready for prime-time use in clinical practice. Thus, they conducted the REVERSE II study, in which the decision rule was applied after the 2,799 participants had been on anticoagulation for 5-12 months. All had a first proximal deep vein thrombosis and/or a segmental or greater pulmonary embolism. Patients were still on anticoagulation at the time the rule was applied, which is why the cut point for a positive d-dimer test in HERDOO2 is 250 mcg/L, half of the threshold value for a positive test in patients not on anticoagulation.
They identified 631 women as low risk, with a HERDOO2 score of 0 or 1. They and their physicians were instructed to stop anticoagulation at that time. The 2,148 high-risk subjects – that is, all of the men and the high-risk women – were advised to remain on anticoagulation. The primary study endpoint was the rate of recurrent VTE in the 12 months following testing and patient guidance. The lost-to-follow-up rate was 2.2%.
The recurrent VTE rate was 3% in the 591 low-risk women who discontinued anticoagulants and zero in 31 others who elected to stay on medication. In the high-risk group identified by the HERDOO2 rule, the recurrent VTE rate at 12 months was 8.1% in the 323 who opted to discontinue anticoagulants and just 1.6% in 1,802 who continued on therapy as advised, a finding that underscores the effectiveness of selectively applied long-term anticoagulation therapy, he continued.
The recurrent VTE rate among the 291 women with a HERDOO2 score of 0 or 1 who were on exogenous estrogen was 1.4%, while in high-risk women taking estrogen the rate was more than doubled at 3.1%. But in women aged 50-64 identified by the HERDOO2 rule as being low risk, the actual recurrent VTE rate was 5.7%, a finding that raised a red flag for the investigators.
“There may be an evolution of the HERDOO2 decision rule to a lower age cut point. But that’s something that requires further study in postmenopausal women,” according to Dr. Rodger.
The investigators defined a first unprovoked VTE as one occurring in the absence during the previous 90 days of major surgery, a fracture or cast, more than 3 days of immobilization, or malignancy within the last 5 years.
Venous thromboembolism is the second most common cardiovascular disorder and the third most common cause of cardiovascular death. Unprovoked VTEs account for half of all VTEs. Their management has been a controversial subject. Both the American College of Chest Physicians and the European Society of Cardiology recommend continuing anticoagulation indefinitely in patients who aren’t at high bleeding risk.
“But this is a relatively weak 2B recommendation because of the tightly balanced competing risks of recurrent thrombosis off anticoagulation and major bleeding on anticoagulation,” Dr. Rodger said. He added that he considers REVERSE II to be practice changing, and predicted that once the results are published the guidelines will be revised.
Discussant Giancarlo Agnelli, MD, was a tough critic who gave fair warning.
“I am friends with many of the authors of this paper, and in this country we are usually gentle with enemies and nasty with friends,” declared Dr. Agnelli, professor of internal medicine and director of internal and cardiovascular medicine and the stroke unit at the University of Perugia, Italy.
He didn’t find the REVERSE II study or the HERDOO2 rule persuasive. On the plus side, he said, the HERDOO2 rule has now been validated, unlike the proposed DASH and Vienna rules. And it was tested in a diverse multinational patient population. But the fact that the HERDOO2 rule is only applicable in women is a major limitation. And REVERSE II was not a randomized trial, Dr. Agnelli noted.
Moreover, 1 year of follow-up seems insufficient, he continued. He cited a French multicenter trial in which patients with a first unprovoked VTE received 6 months of anticoagulants and were then randomized to another 18 months of anticoagulation or placebo. During that 18 months, the group on anticoagulants had a significantly lower rate of the composite endpoint comprised of recurrent VTE or major bleeding, but once that period was over they experienced catchup. By the time the study ended at 42 months, the two study arms didn’t differ significantly in the composite endpoint (JAMA. 2015 Jul 7;314[1]:31-40).
More broadly, Dr. Agnelli also questioned the need for an anticoagulation discontinuation rule in the contemporary era of new oral anticoagulants (NOACs). He was lead investigator in the AMPLIFY study, a major randomized trial of fixed-dose apixaban (Eliquis) versus conventional therapy with subcutaneous enoxaparin (Lovenox) bridging to warfarin in 5,395 patients with acute VTE. The NOAC was associated with a 69% reduction in the relative risk of bleeding and was noninferior to standard therapy in the risk of recurrent VTE (N Engl J Med. 2013 Aug 29;369[9]:799-808).
“Why should we think about withholding anticoagulation in some patients when we now have such a safe approach?” he asked.
Dr. Rodger reported receiving research grants from the French government as well as from Biomerieux, which funded the REVERSE II study. Dr. Agnelli reported having no financial conflicts.
AT THE ESC CONGRESS 2016
Key clinical point: Half of women who have a first unprovoked venous thromboembolism can safely be spared lifelong anticoagulation through application of the newly validated HERDOO2 decision rule.
Major finding: Women with a first unprovoked venous thromboembolism identified as being at low risk of recurrence on the basis of the HERDOO2 decision rule had a 3% recurrence rate in the year after stopping anticoagulation therapy, while those identified as high risk had an 8.1% recurrence rate if they discontinued anticoagulants.
Data source: This was a prospective, multinational, observational study involving 2,779 patients with a first unprovoked venous thromboembolism.
Disclosures: The presenter reported receiving research grants from the French government as well as from Biomerieux, which funded the REVERSE II study.
Simtuzumab did not help IPF patients
LONDON – Despite very promising activity in animal models of idiopathic pulmonary fibrosis (IPF), a monoclonal antibody targeted at an enzyme considered to be important to collagen cross-linking did not produce any improvement in progression-free survival (PFS), according to results of a multicenter study presented at the annual congress of the European Respiratory Society.
“This was such a negative study, there is no point in doing another,” reported Ganesh Raghu, MD, director of the Pulmonary Fibrosis Program at the University of Washington Medical Center, Seattle.
The focus of this study was simtuzumab, a monoclonal antibody targeted at lysyl oxidase like 2 (LOXL2), an enzyme which catalyzes a step in the formation of collagen crosslinks, which are thought to be important in fibrosis formation. Simtuzumab has been entered into clinical trials for treatment of several forms of fibrosis, including fibrosis in the liver.
“In animal models, simtuzumab has demonstrated efficacy in reducing fibrosis when administered prior to fibrosis formation or after the process has already begun,” Dr. Raghu explained. He said a large trial was initiated in IPF because the agent seemed so promising and because a large study was thought to be the best strategy to arrive at a definitive answer regarding safety and efficacy.
The drug was found safe but not effective. The independent data monitoring and safety committee terminated the trial early for futility.
In the study, 544 IPF patients were randomized to 125 mg simtuzumab or placebo administered subcutaneously once weekly. The primary endpoint was PFS, but there were a large number of secondary endpoints including hospitalization for progressive disease, change in 6-minute walk distance (6MWD), and overall survival.
For the endpoint of PFS, “there was absolutely no difference” between the groups receiving simtuzumab or placebo. When the patients were stratified for demonstrating above or below median expression of LOXL2, which was a prespecified analysis for the trial, there was still no difference between groups. Even when those in the top quarter percentile of LOXL2 expression were compared with those with less [expression of the enzyme], there was still “absolutely no difference.”
There was also no significant evidence of benefit for simtuzumab observed on key secondary endpoints, such as overall survival. When patients were stratified by baseline lung function as expressed by percentage of predicted forced expiratory volume in 1 second (FEV1), there was no signal of benefit for those with severe, moderate, or mild impairment.
One criticism of this study raised after the presentation was that patients with 26% or greater of predicted FEV1 were permitted into the study. It was suggested that such patients would be expected to already have a high degree of fibrosis and therefore would be less likely to benefit from an antifibrosis therapy. Dr. Raghu acknowledged this criticism, but he said it was important to include patients with advanced disease in order to generate an adequate event rate. Even with inclusion of patients with severe lung impairment, the mortality rate was less than 10%.
He concluded that there was no signal of benefit even among those with the greatest expression of the target.
“We absolutely need better markers for IPF,” Dr. Raghu maintained. While other members of the LOXL family of enzymes may still prove to be valuable markers of IPF risk and targets of therapy, these data appear to rule out a therapeutic role for blocking LOXL2.
Dr. Raghu is a consultant for Boehringer Ingelheim, Biogen, FibroGen, Gilead, Janssen, MedImmune, Promedior, Sanofi-Aventis, and Veracyte.
LONDON – Despite very promising activity in animal models of idiopathic pulmonary fibrosis (IPF), a monoclonal antibody targeted at an enzyme considered to be important to collagen cross-linking did not produce any improvement in progression-free survival (PFS), according to results of a multicenter study presented at the annual congress of the European Respiratory Society.
“This was such a negative study, there is no point in doing another,” reported Ganesh Raghu, MD, director of the Pulmonary Fibrosis Program at the University of Washington Medical Center, Seattle.
The focus of this study was simtuzumab, a monoclonal antibody targeted at lysyl oxidase like 2 (LOXL2), an enzyme which catalyzes a step in the formation of collagen crosslinks, which are thought to be important in fibrosis formation. Simtuzumab has been entered into clinical trials for treatment of several forms of fibrosis, including fibrosis in the liver.
“In animal models, simtuzumab has demonstrated efficacy in reducing fibrosis when administered prior to fibrosis formation or after the process has already begun,” Dr. Raghu explained. He said a large trial was initiated in IPF because the agent seemed so promising and because a large study was thought to be the best strategy to arrive at a definitive answer regarding safety and efficacy.
The drug was found safe but not effective. The independent data monitoring and safety committee terminated the trial early for futility.
In the study, 544 IPF patients were randomized to 125 mg simtuzumab or placebo administered subcutaneously once weekly. The primary endpoint was PFS, but there were a large number of secondary endpoints including hospitalization for progressive disease, change in 6-minute walk distance (6MWD), and overall survival.
For the endpoint of PFS, “there was absolutely no difference” between the groups receiving simtuzumab or placebo. When the patients were stratified for demonstrating above or below median expression of LOXL2, which was a prespecified analysis for the trial, there was still no difference between groups. Even when those in the top quarter percentile of LOXL2 expression were compared with those with less [expression of the enzyme], there was still “absolutely no difference.”
There was also no significant evidence of benefit for simtuzumab observed on key secondary endpoints, such as overall survival. When patients were stratified by baseline lung function as expressed by percentage of predicted forced expiratory volume in 1 second (FEV1), there was no signal of benefit for those with severe, moderate, or mild impairment.
One criticism of this study raised after the presentation was that patients with 26% or greater of predicted FEV1 were permitted into the study. It was suggested that such patients would be expected to already have a high degree of fibrosis and therefore would be less likely to benefit from an antifibrosis therapy. Dr. Raghu acknowledged this criticism, but he said it was important to include patients with advanced disease in order to generate an adequate event rate. Even with inclusion of patients with severe lung impairment, the mortality rate was less than 10%.
He concluded that there was no signal of benefit even among those with the greatest expression of the target.
“We absolutely need better markers for IPF,” Dr. Raghu maintained. While other members of the LOXL family of enzymes may still prove to be valuable markers of IPF risk and targets of therapy, these data appear to rule out a therapeutic role for blocking LOXL2.
Dr. Raghu is a consultant for Boehringer Ingelheim, Biogen, FibroGen, Gilead, Janssen, MedImmune, Promedior, Sanofi-Aventis, and Veracyte.
LONDON – Despite very promising activity in animal models of idiopathic pulmonary fibrosis (IPF), a monoclonal antibody targeted at an enzyme considered to be important to collagen cross-linking did not produce any improvement in progression-free survival (PFS), according to results of a multicenter study presented at the annual congress of the European Respiratory Society.
“This was such a negative study, there is no point in doing another,” reported Ganesh Raghu, MD, director of the Pulmonary Fibrosis Program at the University of Washington Medical Center, Seattle.
The focus of this study was simtuzumab, a monoclonal antibody targeted at lysyl oxidase like 2 (LOXL2), an enzyme which catalyzes a step in the formation of collagen crosslinks, which are thought to be important in fibrosis formation. Simtuzumab has been entered into clinical trials for treatment of several forms of fibrosis, including fibrosis in the liver.
“In animal models, simtuzumab has demonstrated efficacy in reducing fibrosis when administered prior to fibrosis formation or after the process has already begun,” Dr. Raghu explained. He said a large trial was initiated in IPF because the agent seemed so promising and because a large study was thought to be the best strategy to arrive at a definitive answer regarding safety and efficacy.
The drug was found safe but not effective. The independent data monitoring and safety committee terminated the trial early for futility.
In the study, 544 IPF patients were randomized to 125 mg simtuzumab or placebo administered subcutaneously once weekly. The primary endpoint was PFS, but there were a large number of secondary endpoints including hospitalization for progressive disease, change in 6-minute walk distance (6MWD), and overall survival.
For the endpoint of PFS, “there was absolutely no difference” between the groups receiving simtuzumab or placebo. When the patients were stratified for demonstrating above or below median expression of LOXL2, which was a prespecified analysis for the trial, there was still no difference between groups. Even when those in the top quarter percentile of LOXL2 expression were compared with those with less [expression of the enzyme], there was still “absolutely no difference.”
There was also no significant evidence of benefit for simtuzumab observed on key secondary endpoints, such as overall survival. When patients were stratified by baseline lung function as expressed by percentage of predicted forced expiratory volume in 1 second (FEV1), there was no signal of benefit for those with severe, moderate, or mild impairment.
One criticism of this study raised after the presentation was that patients with 26% or greater of predicted FEV1 were permitted into the study. It was suggested that such patients would be expected to already have a high degree of fibrosis and therefore would be less likely to benefit from an antifibrosis therapy. Dr. Raghu acknowledged this criticism, but he said it was important to include patients with advanced disease in order to generate an adequate event rate. Even with inclusion of patients with severe lung impairment, the mortality rate was less than 10%.
He concluded that there was no signal of benefit even among those with the greatest expression of the target.
“We absolutely need better markers for IPF,” Dr. Raghu maintained. While other members of the LOXL family of enzymes may still prove to be valuable markers of IPF risk and targets of therapy, these data appear to rule out a therapeutic role for blocking LOXL2.
Dr. Raghu is a consultant for Boehringer Ingelheim, Biogen, FibroGen, Gilead, Janssen, MedImmune, Promedior, Sanofi-Aventis, and Veracyte.
AT THE ERS CONGRESS 2016
Key clinical point: A large multicenter trial with simtuzumab in idiopathic pulmonary fibrosis failed to generate a hint of benefit.
Major finding: In this study, efficacy was not seen even in those with high expression of the simtuzumab target, lysyl oxidase like 2 (LOXL2).
Data source: Phase II multicenter, placebo-controlled trial.
Disclosures: Dr. Raghu is a consultant for Boehringer Ingelheim, Biogen, FibroGen, Gilead, Janssen, MedImmune, Promedior, Sanofi-Aventis, and Veracyte.
More TOPCAT flaws back spironolactone’s HFpEF efficacy
ORLANDO – Spironolactone inched a little closer toward becoming the first and only agent with proven efficacy for treating patients with heart failure with preserved ejection fraction based on further evidence for the drug’s efficacy in a subgroup of patients enrolled in the TOPCAT trial.
In 2014, the initial TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) report showed that spironolactone treatment of patients with heart failure with preserved ejection fraction (HFpEF) for 3 years produced a small, 11% relative reduction in the primary risk endpoint, compared with placebo that was not statistically significant (N Engl J Med. 2014 Apr 10;370[15]:1383-92).
But a follow-up post hoc analysis a year later showed evidence that the roughly half of patients in TOPCAT enrolled at centers in Russia and the Republic of Georgia may not have had HFpEF and also may not have received the planned dosage of spironolactone (Circulation. 2015 Jan 6;131[1]:34-42). An analysis that focused only on the 1,767 HFpEF patients (51% of the total TOPCAT cohort) enrolled in the Americas (United States, Canada, Argentina, and Brazil) showed that, compared with placebo, treatment with spironolactone cut the combined rate of cardiovascular death, nonfatal cardiac arrest, and heart failure hospitalization by 4.5 percentage points, an 18% relative risk reduction that was statistically significant. In the Americas, spironolactone also cut cardiovascular death alone by a relative 26%, and reduced heart failure hospitalization by a relative 18%, both statistically significant.
Additional analysis reported at the annual scientific meeting of the Heart Failure Society of America further supported the idea that many TOPCAT patients enrolled in Russia did not receive a physiologically meaningful dosage of spironolactone. Among 66 Russian patients randomized to the spironolactone arm who reported taking their drug as prescribed and who participated in a random draw of blood specimens 1 year into the study, 20 (30%) failed to show detectable blood levels of canrenone, a characteristic spironolactone metabolite, reported Eileen O’Meara, MD, at the meeting. In contrast, 2 (3%) of 76 enrolled U.S. patients failed to show detectable blood levels of the canrenone metabolite, a 10-fold difference said Dr. O’Meara, a cardiologist at the Montreal Heart Institute.The tested U.S. patients also showed a clear dose-response relationship between their reported spironolactone dosage and their canrenone levels, something not seen in the Russian patients. These new findings, plus the evidence cited in the 2015 analysis, create a compelling case that “actual use of spironolactone in Russia was lower than reported” by the trial participants in Russia and probably in Georgia as well, Dr. O’Meara said. The implication is that spironolactone’s real impact on HFpEF patients is best represented in the 51% of TOPCAT patients from the Americas, she added.
“We believe these findings emphasize the reliability of the Americas data,” said Marc A. Pfeffer, MD, a coinvestigator for TOPCAT and lead author of the 2015 post hoc analysis. “Until someone comes up with a better treatment for patients with HFpEF, we should pay attention to this. People need to get this message. And spironolactone costs 7 cents a day,” said Dr. Pfeffer, professor of medicine at Harvard Medical School in Boston.Currently, no agent is considered proven effective for improving outcomes in HFpEF patients. The 2016 guidelinesfor heart failure treatment from the European Society of Cardiology said “no treatment has yet been shown, convincingly, to reduce morbidity or mortality in patients with HFpEF.”
After the TOPCAT results and post hoc analysis came out in 2014 and 2015 and word spread of spironolactone’s apparent efficacy in the American half of the trial, use of spironolactone to treat HFpEF patient has increased, commented Margaret M. Redfield, MD, a heart failure physician and professor at the Mayo Clinic in Rochester, Minn. She said she often prescribes spironolactone patients to HFpEF patients who require potassium supplementation, generally because of their diuretic treatment. These are the “safest” HFpEF patients for spironolactone treatment, she said, because they face the lowest risk for hyperkalemia, the major adverse effect from spironolactone.
On Twitter @mitchelzoler
This is extraordinarily important information from an extraordinarily important study. I’m strongly persuaded that the data from the Americas in TOPCAT show that spironolactone worked. The new data presented on canrenone levels make me even more ready to exclude from consideration the TOPCAT data from Russia and Georgia.
 |
| Mitchel L. Zoler/Frontline Medical News Dr. Barry H. Greenberg |
The heart failure community is left to decide what conclusions to draw from TOPCAT. I think guideline committees will struggle over what to make of the TOPCAT evidence. Any recommendation in favor of spironolactone needs to be somewhat guarded, but if a group made recommendations in support of spironolactone it would add an impetus for using it.
There has been long-standing interest in treating patients with heart failure with preserved ejection fraction with spironolactone. Currently, about a quarter of these patients take spironolactone. I’m not sure this level of use will increase dramatically because of what we now know about TOPCAT.
Dr. Barry H. Greenberg is professor of medicine and director of the advanced heart failure treatment program at the University of California, San Diego. He had no relevant disclosures. He made these comments in an interview.
This is extraordinarily important information from an extraordinarily important study. I’m strongly persuaded that the data from the Americas in TOPCAT show that spironolactone worked. The new data presented on canrenone levels make me even more ready to exclude from consideration the TOPCAT data from Russia and Georgia.
 |
| Mitchel L. Zoler/Frontline Medical News Dr. Barry H. Greenberg |
The heart failure community is left to decide what conclusions to draw from TOPCAT. I think guideline committees will struggle over what to make of the TOPCAT evidence. Any recommendation in favor of spironolactone needs to be somewhat guarded, but if a group made recommendations in support of spironolactone it would add an impetus for using it.
There has been long-standing interest in treating patients with heart failure with preserved ejection fraction with spironolactone. Currently, about a quarter of these patients take spironolactone. I’m not sure this level of use will increase dramatically because of what we now know about TOPCAT.
Dr. Barry H. Greenberg is professor of medicine and director of the advanced heart failure treatment program at the University of California, San Diego. He had no relevant disclosures. He made these comments in an interview.
This is extraordinarily important information from an extraordinarily important study. I’m strongly persuaded that the data from the Americas in TOPCAT show that spironolactone worked. The new data presented on canrenone levels make me even more ready to exclude from consideration the TOPCAT data from Russia and Georgia.
 |
| Mitchel L. Zoler/Frontline Medical News Dr. Barry H. Greenberg |
The heart failure community is left to decide what conclusions to draw from TOPCAT. I think guideline committees will struggle over what to make of the TOPCAT evidence. Any recommendation in favor of spironolactone needs to be somewhat guarded, but if a group made recommendations in support of spironolactone it would add an impetus for using it.
There has been long-standing interest in treating patients with heart failure with preserved ejection fraction with spironolactone. Currently, about a quarter of these patients take spironolactone. I’m not sure this level of use will increase dramatically because of what we now know about TOPCAT.
Dr. Barry H. Greenberg is professor of medicine and director of the advanced heart failure treatment program at the University of California, San Diego. He had no relevant disclosures. He made these comments in an interview.
ORLANDO – Spironolactone inched a little closer toward becoming the first and only agent with proven efficacy for treating patients with heart failure with preserved ejection fraction based on further evidence for the drug’s efficacy in a subgroup of patients enrolled in the TOPCAT trial.
In 2014, the initial TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) report showed that spironolactone treatment of patients with heart failure with preserved ejection fraction (HFpEF) for 3 years produced a small, 11% relative reduction in the primary risk endpoint, compared with placebo that was not statistically significant (N Engl J Med. 2014 Apr 10;370[15]:1383-92).
But a follow-up post hoc analysis a year later showed evidence that the roughly half of patients in TOPCAT enrolled at centers in Russia and the Republic of Georgia may not have had HFpEF and also may not have received the planned dosage of spironolactone (Circulation. 2015 Jan 6;131[1]:34-42). An analysis that focused only on the 1,767 HFpEF patients (51% of the total TOPCAT cohort) enrolled in the Americas (United States, Canada, Argentina, and Brazil) showed that, compared with placebo, treatment with spironolactone cut the combined rate of cardiovascular death, nonfatal cardiac arrest, and heart failure hospitalization by 4.5 percentage points, an 18% relative risk reduction that was statistically significant. In the Americas, spironolactone also cut cardiovascular death alone by a relative 26%, and reduced heart failure hospitalization by a relative 18%, both statistically significant.
Additional analysis reported at the annual scientific meeting of the Heart Failure Society of America further supported the idea that many TOPCAT patients enrolled in Russia did not receive a physiologically meaningful dosage of spironolactone. Among 66 Russian patients randomized to the spironolactone arm who reported taking their drug as prescribed and who participated in a random draw of blood specimens 1 year into the study, 20 (30%) failed to show detectable blood levels of canrenone, a characteristic spironolactone metabolite, reported Eileen O’Meara, MD, at the meeting. In contrast, 2 (3%) of 76 enrolled U.S. patients failed to show detectable blood levels of the canrenone metabolite, a 10-fold difference said Dr. O’Meara, a cardiologist at the Montreal Heart Institute.The tested U.S. patients also showed a clear dose-response relationship between their reported spironolactone dosage and their canrenone levels, something not seen in the Russian patients. These new findings, plus the evidence cited in the 2015 analysis, create a compelling case that “actual use of spironolactone in Russia was lower than reported” by the trial participants in Russia and probably in Georgia as well, Dr. O’Meara said. The implication is that spironolactone’s real impact on HFpEF patients is best represented in the 51% of TOPCAT patients from the Americas, she added.
“We believe these findings emphasize the reliability of the Americas data,” said Marc A. Pfeffer, MD, a coinvestigator for TOPCAT and lead author of the 2015 post hoc analysis. “Until someone comes up with a better treatment for patients with HFpEF, we should pay attention to this. People need to get this message. And spironolactone costs 7 cents a day,” said Dr. Pfeffer, professor of medicine at Harvard Medical School in Boston.Currently, no agent is considered proven effective for improving outcomes in HFpEF patients. The 2016 guidelinesfor heart failure treatment from the European Society of Cardiology said “no treatment has yet been shown, convincingly, to reduce morbidity or mortality in patients with HFpEF.”
After the TOPCAT results and post hoc analysis came out in 2014 and 2015 and word spread of spironolactone’s apparent efficacy in the American half of the trial, use of spironolactone to treat HFpEF patient has increased, commented Margaret M. Redfield, MD, a heart failure physician and professor at the Mayo Clinic in Rochester, Minn. She said she often prescribes spironolactone patients to HFpEF patients who require potassium supplementation, generally because of their diuretic treatment. These are the “safest” HFpEF patients for spironolactone treatment, she said, because they face the lowest risk for hyperkalemia, the major adverse effect from spironolactone.
On Twitter @mitchelzoler
ORLANDO – Spironolactone inched a little closer toward becoming the first and only agent with proven efficacy for treating patients with heart failure with preserved ejection fraction based on further evidence for the drug’s efficacy in a subgroup of patients enrolled in the TOPCAT trial.
In 2014, the initial TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) report showed that spironolactone treatment of patients with heart failure with preserved ejection fraction (HFpEF) for 3 years produced a small, 11% relative reduction in the primary risk endpoint, compared with placebo that was not statistically significant (N Engl J Med. 2014 Apr 10;370[15]:1383-92).
But a follow-up post hoc analysis a year later showed evidence that the roughly half of patients in TOPCAT enrolled at centers in Russia and the Republic of Georgia may not have had HFpEF and also may not have received the planned dosage of spironolactone (Circulation. 2015 Jan 6;131[1]:34-42). An analysis that focused only on the 1,767 HFpEF patients (51% of the total TOPCAT cohort) enrolled in the Americas (United States, Canada, Argentina, and Brazil) showed that, compared with placebo, treatment with spironolactone cut the combined rate of cardiovascular death, nonfatal cardiac arrest, and heart failure hospitalization by 4.5 percentage points, an 18% relative risk reduction that was statistically significant. In the Americas, spironolactone also cut cardiovascular death alone by a relative 26%, and reduced heart failure hospitalization by a relative 18%, both statistically significant.
Additional analysis reported at the annual scientific meeting of the Heart Failure Society of America further supported the idea that many TOPCAT patients enrolled in Russia did not receive a physiologically meaningful dosage of spironolactone. Among 66 Russian patients randomized to the spironolactone arm who reported taking their drug as prescribed and who participated in a random draw of blood specimens 1 year into the study, 20 (30%) failed to show detectable blood levels of canrenone, a characteristic spironolactone metabolite, reported Eileen O’Meara, MD, at the meeting. In contrast, 2 (3%) of 76 enrolled U.S. patients failed to show detectable blood levels of the canrenone metabolite, a 10-fold difference said Dr. O’Meara, a cardiologist at the Montreal Heart Institute.The tested U.S. patients also showed a clear dose-response relationship between their reported spironolactone dosage and their canrenone levels, something not seen in the Russian patients. These new findings, plus the evidence cited in the 2015 analysis, create a compelling case that “actual use of spironolactone in Russia was lower than reported” by the trial participants in Russia and probably in Georgia as well, Dr. O’Meara said. The implication is that spironolactone’s real impact on HFpEF patients is best represented in the 51% of TOPCAT patients from the Americas, she added.
“We believe these findings emphasize the reliability of the Americas data,” said Marc A. Pfeffer, MD, a coinvestigator for TOPCAT and lead author of the 2015 post hoc analysis. “Until someone comes up with a better treatment for patients with HFpEF, we should pay attention to this. People need to get this message. And spironolactone costs 7 cents a day,” said Dr. Pfeffer, professor of medicine at Harvard Medical School in Boston.Currently, no agent is considered proven effective for improving outcomes in HFpEF patients. The 2016 guidelinesfor heart failure treatment from the European Society of Cardiology said “no treatment has yet been shown, convincingly, to reduce morbidity or mortality in patients with HFpEF.”
After the TOPCAT results and post hoc analysis came out in 2014 and 2015 and word spread of spironolactone’s apparent efficacy in the American half of the trial, use of spironolactone to treat HFpEF patient has increased, commented Margaret M. Redfield, MD, a heart failure physician and professor at the Mayo Clinic in Rochester, Minn. She said she often prescribes spironolactone patients to HFpEF patients who require potassium supplementation, generally because of their diuretic treatment. These are the “safest” HFpEF patients for spironolactone treatment, she said, because they face the lowest risk for hyperkalemia, the major adverse effect from spironolactone.
On Twitter @mitchelzoler
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point: A post hoc analysis of spironolactone use among TOPCAT participants further fueled the idea that spironolactone provides real benefit to patients with HFpEF.
Major finding: Among patients reportedly taking spironolactone, 30% of tested Russians and 3% of tested Americans did not have detectable canrenone levels.
Data source: TOPCAT, a multicenter, randomized trial with 3,445 HFpEF patients.
Disclosures: TOPCAT received no commercial funding. Dr. O’Meara, Dr. Pfeffer, and Dr. Redfield had no relevant disclosures.
Data point to optimal window for endoscopy in sicker patients with peptic ulcer bleeding
The timing of endoscopy may make the difference between life and death in sicker patients with peptic ulcer bleeding, according to an analysis of more than 12,000 patients treated in Denmark.
Patients who were hemodynamically stable but had a higher level of comorbidity were about half as likely to die during their hospital stay if they underwent endoscopy within 12-36 hours of presentation as compared with sooner or later, results showed (Gastrointest Endosc. 2016 Sep 10. doi: 10.1016/j.gie.2016.08.049). And hemodynamically unstable patients had a roughly one-fourth reduction in the odds of death if they underwent the procedure within 6-24 hours.
[[{"attributes":{},"fields":{}}]]
“Although caution should be applied when interpreting these data, the current recommendation of endoscopy within 0-24 hours may not be optimal for all patients,” wrote the investigators, who were led by Stig B. Laursen, PhD, department of medical gastroenterology, Odense (Denmark) University Hospital.
“Our data may suggest that in patients with major comorbidities, the first few hours of hospital admission might be best used for optimising treatment of comorbidities, which may include correction of severe anaemia, reversal of anticoagulants, and investigation for possible infection that requires rapid treatment with antibiotics,” they elaborate. “Likewise, in patients with hemodynamic instability, endoscopy between 6 and 24 hours from time of admission to hospital allows time for optimal resuscitation and initiating treatment of comorbid diseases before endoscopy. However, these data should not lead to delayed endoscopy in patients with severe hemodynamic instability not responding to intensive resuscitation.”
The investigators analyzed data from 12,601 consecutive patients with peptic ulcer bleeding admitted between January 2005 and September 2013 to Danish hospitals, where all patients had access to 24-hour endoscopy. Time to endoscopy was assessed from hospital admission, defined as arrival in the emergency department, or from symptom onset in patients who developed bleeding when already hospitalized.
For analyses, the patients were stratified by hemodynamic status (a marker for the severity of bleeding) and by American Society of Anesthesiologists score (a marker for the extent of comorbidity).
[[{"attributes":{},"fields":{}}]]
The timing of endoscopy did not significantly influence in-hospital or 30-day mortality in hemodynamically stable patients with an American Society of Anesthesiologists score of 1-2 as a whole, Dr. Laursen and his colleagues report. Subgroup analyses suggested a reduction of in-hospital mortality when it was done between 0 and 24 hours in those patients whose bleeding began outside the hospital (adjusted odds ratio, 0.48).
In contrast, analyses revealed a U-shaped association between timing and mortality for hemodynamically stable patients with an American Society of Anesthesiologists score of 3-5. For this group, in-hospital mortality was significantly lower when endoscopy was performed within 12-36 hours as compared with times outside this window (adjusted OR, 0.48), and 30-day mortality tended to be lower as well.
Similarly, timing appeared to influence outcome for hemodynamically unstable patients, having both systolic blood pressure below 100 mm Hg and heart rate above 100 beats/min. For this group, performance of endoscopy within 6-24 hours was associated with significantly lower in-hospital mortality (adjusted OR, 0.73) and also 30-day mortality (adjusted OR, 0.66). Patients’ American Society of Anesthesiologists score did not appear to play a role here.
The study’s findings may have been affected by unmeasured and unknown confounders, acknowledge the investigators, who declared that they have no competing interests related to the research.
“Although a well-powered randomized controlled trial represents the best way to account for these problems, randomizing patients with [peptic ulcer bleeding] to early versus late endoscopy will be very difficult, including from an ethical and methodological point of view,” they note.
The timing of endoscopy may make the difference between life and death in sicker patients with peptic ulcer bleeding, according to an analysis of more than 12,000 patients treated in Denmark.
Patients who were hemodynamically stable but had a higher level of comorbidity were about half as likely to die during their hospital stay if they underwent endoscopy within 12-36 hours of presentation as compared with sooner or later, results showed (Gastrointest Endosc. 2016 Sep 10. doi: 10.1016/j.gie.2016.08.049). And hemodynamically unstable patients had a roughly one-fourth reduction in the odds of death if they underwent the procedure within 6-24 hours.
[[{"attributes":{},"fields":{}}]]
“Although caution should be applied when interpreting these data, the current recommendation of endoscopy within 0-24 hours may not be optimal for all patients,” wrote the investigators, who were led by Stig B. Laursen, PhD, department of medical gastroenterology, Odense (Denmark) University Hospital.
“Our data may suggest that in patients with major comorbidities, the first few hours of hospital admission might be best used for optimising treatment of comorbidities, which may include correction of severe anaemia, reversal of anticoagulants, and investigation for possible infection that requires rapid treatment with antibiotics,” they elaborate. “Likewise, in patients with hemodynamic instability, endoscopy between 6 and 24 hours from time of admission to hospital allows time for optimal resuscitation and initiating treatment of comorbid diseases before endoscopy. However, these data should not lead to delayed endoscopy in patients with severe hemodynamic instability not responding to intensive resuscitation.”
The investigators analyzed data from 12,601 consecutive patients with peptic ulcer bleeding admitted between January 2005 and September 2013 to Danish hospitals, where all patients had access to 24-hour endoscopy. Time to endoscopy was assessed from hospital admission, defined as arrival in the emergency department, or from symptom onset in patients who developed bleeding when already hospitalized.
For analyses, the patients were stratified by hemodynamic status (a marker for the severity of bleeding) and by American Society of Anesthesiologists score (a marker for the extent of comorbidity).
[[{"attributes":{},"fields":{}}]]
The timing of endoscopy did not significantly influence in-hospital or 30-day mortality in hemodynamically stable patients with an American Society of Anesthesiologists score of 1-2 as a whole, Dr. Laursen and his colleagues report. Subgroup analyses suggested a reduction of in-hospital mortality when it was done between 0 and 24 hours in those patients whose bleeding began outside the hospital (adjusted odds ratio, 0.48).
In contrast, analyses revealed a U-shaped association between timing and mortality for hemodynamically stable patients with an American Society of Anesthesiologists score of 3-5. For this group, in-hospital mortality was significantly lower when endoscopy was performed within 12-36 hours as compared with times outside this window (adjusted OR, 0.48), and 30-day mortality tended to be lower as well.
Similarly, timing appeared to influence outcome for hemodynamically unstable patients, having both systolic blood pressure below 100 mm Hg and heart rate above 100 beats/min. For this group, performance of endoscopy within 6-24 hours was associated with significantly lower in-hospital mortality (adjusted OR, 0.73) and also 30-day mortality (adjusted OR, 0.66). Patients’ American Society of Anesthesiologists score did not appear to play a role here.
The study’s findings may have been affected by unmeasured and unknown confounders, acknowledge the investigators, who declared that they have no competing interests related to the research.
“Although a well-powered randomized controlled trial represents the best way to account for these problems, randomizing patients with [peptic ulcer bleeding] to early versus late endoscopy will be very difficult, including from an ethical and methodological point of view,” they note.
The timing of endoscopy may make the difference between life and death in sicker patients with peptic ulcer bleeding, according to an analysis of more than 12,000 patients treated in Denmark.
Patients who were hemodynamically stable but had a higher level of comorbidity were about half as likely to die during their hospital stay if they underwent endoscopy within 12-36 hours of presentation as compared with sooner or later, results showed (Gastrointest Endosc. 2016 Sep 10. doi: 10.1016/j.gie.2016.08.049). And hemodynamically unstable patients had a roughly one-fourth reduction in the odds of death if they underwent the procedure within 6-24 hours.
[[{"attributes":{},"fields":{}}]]
“Although caution should be applied when interpreting these data, the current recommendation of endoscopy within 0-24 hours may not be optimal for all patients,” wrote the investigators, who were led by Stig B. Laursen, PhD, department of medical gastroenterology, Odense (Denmark) University Hospital.
“Our data may suggest that in patients with major comorbidities, the first few hours of hospital admission might be best used for optimising treatment of comorbidities, which may include correction of severe anaemia, reversal of anticoagulants, and investigation for possible infection that requires rapid treatment with antibiotics,” they elaborate. “Likewise, in patients with hemodynamic instability, endoscopy between 6 and 24 hours from time of admission to hospital allows time for optimal resuscitation and initiating treatment of comorbid diseases before endoscopy. However, these data should not lead to delayed endoscopy in patients with severe hemodynamic instability not responding to intensive resuscitation.”
The investigators analyzed data from 12,601 consecutive patients with peptic ulcer bleeding admitted between January 2005 and September 2013 to Danish hospitals, where all patients had access to 24-hour endoscopy. Time to endoscopy was assessed from hospital admission, defined as arrival in the emergency department, or from symptom onset in patients who developed bleeding when already hospitalized.
For analyses, the patients were stratified by hemodynamic status (a marker for the severity of bleeding) and by American Society of Anesthesiologists score (a marker for the extent of comorbidity).
[[{"attributes":{},"fields":{}}]]
The timing of endoscopy did not significantly influence in-hospital or 30-day mortality in hemodynamically stable patients with an American Society of Anesthesiologists score of 1-2 as a whole, Dr. Laursen and his colleagues report. Subgroup analyses suggested a reduction of in-hospital mortality when it was done between 0 and 24 hours in those patients whose bleeding began outside the hospital (adjusted odds ratio, 0.48).
In contrast, analyses revealed a U-shaped association between timing and mortality for hemodynamically stable patients with an American Society of Anesthesiologists score of 3-5. For this group, in-hospital mortality was significantly lower when endoscopy was performed within 12-36 hours as compared with times outside this window (adjusted OR, 0.48), and 30-day mortality tended to be lower as well.
Similarly, timing appeared to influence outcome for hemodynamically unstable patients, having both systolic blood pressure below 100 mm Hg and heart rate above 100 beats/min. For this group, performance of endoscopy within 6-24 hours was associated with significantly lower in-hospital mortality (adjusted OR, 0.73) and also 30-day mortality (adjusted OR, 0.66). Patients’ American Society of Anesthesiologists score did not appear to play a role here.
The study’s findings may have been affected by unmeasured and unknown confounders, acknowledge the investigators, who declared that they have no competing interests related to the research.
“Although a well-powered randomized controlled trial represents the best way to account for these problems, randomizing patients with [peptic ulcer bleeding] to early versus late endoscopy will be very difficult, including from an ethical and methodological point of view,” they note.
FROM GASTROINTESTINAL ENDOSCOPY
Key clinical point:
Major finding: In-hospital mortality was lower when endoscopy was performed within 12-36 hours in hemodynamically stable patients with higher comorbidity (odds ratio, 0.48) and within 6-24 hours in hemodynamically unstable patients (OR, 0.73).
Data source: A nationwide cohort study of 12,601 consecutive patients admitted to Danish hospitals with peptic ulcer bleeding.
Disclosures: The investigators declare that they do not have any competing interests.
FDA modifies dosage regimen for nivolumab
The Food and Drug Administration has modified the dosage regimen for nivolumab for indications of renal cell carcinoma, metastatic melanoma, and non–small cell lung cancer.
The single-dose regimen of nivolumab (3 mg/kg IV every 2 weeks) is replaced with the new recommended regimen of 240 mg IV every 2 weeks until disease progression or intolerable toxicity, the FDA said in a written statement.
The nivolumab (Opdivo) dosing regimen in combination with ipilimumab for melanoma will stay the same (nivolumab 1 mg/kg IV, followed by ipilimumab on the same day, every 3 weeks for four doses); however, after completion of ipilimumab, the recommended nivolumab dose is modified to 240 mg every 2 weeks until disease progression or intolerable toxicity. The recommended dose for classical Hodgkin lymphoma remains at 3 mg/kg IV every 2 weeks until disease progression or intolerable toxicity.
The change was made based on analyses demonstrating the comparability of the pharmacokinetics exposure, safety, and efficacy of the proposed new dosing regimen with the previously approved regimen. “Based on simulations by the population pharmacokinetics model, [the] FDA determined that the overall exposure at 240 mg every 2 weeks flat dose is similar (less than 6% difference) to 3 mg/kg every 2 weeks. These differences in exposure are not likely to have a clinically meaningful effect on safety and efficacy, since dose/exposure response relationships appear to be relatively flat in these three indications,” the FDA said.
The Food and Drug Administration has modified the dosage regimen for nivolumab for indications of renal cell carcinoma, metastatic melanoma, and non–small cell lung cancer.
The single-dose regimen of nivolumab (3 mg/kg IV every 2 weeks) is replaced with the new recommended regimen of 240 mg IV every 2 weeks until disease progression or intolerable toxicity, the FDA said in a written statement.
The nivolumab (Opdivo) dosing regimen in combination with ipilimumab for melanoma will stay the same (nivolumab 1 mg/kg IV, followed by ipilimumab on the same day, every 3 weeks for four doses); however, after completion of ipilimumab, the recommended nivolumab dose is modified to 240 mg every 2 weeks until disease progression or intolerable toxicity. The recommended dose for classical Hodgkin lymphoma remains at 3 mg/kg IV every 2 weeks until disease progression or intolerable toxicity.
The change was made based on analyses demonstrating the comparability of the pharmacokinetics exposure, safety, and efficacy of the proposed new dosing regimen with the previously approved regimen. “Based on simulations by the population pharmacokinetics model, [the] FDA determined that the overall exposure at 240 mg every 2 weeks flat dose is similar (less than 6% difference) to 3 mg/kg every 2 weeks. These differences in exposure are not likely to have a clinically meaningful effect on safety and efficacy, since dose/exposure response relationships appear to be relatively flat in these three indications,” the FDA said.
The Food and Drug Administration has modified the dosage regimen for nivolumab for indications of renal cell carcinoma, metastatic melanoma, and non–small cell lung cancer.
The single-dose regimen of nivolumab (3 mg/kg IV every 2 weeks) is replaced with the new recommended regimen of 240 mg IV every 2 weeks until disease progression or intolerable toxicity, the FDA said in a written statement.
The nivolumab (Opdivo) dosing regimen in combination with ipilimumab for melanoma will stay the same (nivolumab 1 mg/kg IV, followed by ipilimumab on the same day, every 3 weeks for four doses); however, after completion of ipilimumab, the recommended nivolumab dose is modified to 240 mg every 2 weeks until disease progression or intolerable toxicity. The recommended dose for classical Hodgkin lymphoma remains at 3 mg/kg IV every 2 weeks until disease progression or intolerable toxicity.
The change was made based on analyses demonstrating the comparability of the pharmacokinetics exposure, safety, and efficacy of the proposed new dosing regimen with the previously approved regimen. “Based on simulations by the population pharmacokinetics model, [the] FDA determined that the overall exposure at 240 mg every 2 weeks flat dose is similar (less than 6% difference) to 3 mg/kg every 2 weeks. These differences in exposure are not likely to have a clinically meaningful effect on safety and efficacy, since dose/exposure response relationships appear to be relatively flat in these three indications,” the FDA said.
Global Polio Vaccine “Switch” a Success
Type 2 circulating vaccine-derived polioviruses (cVDPV) have caused hundreds of cases of paralytic poliomyelitis and now accounts for > 94% of polio cases since 2006. To address cVDPV and the risk of vaccine-derived polioviruses, the World Health Organization (WHO) scheduled the type 2 component of oral poliovirus vaccine (OPV) for global withdrawal and planned a synchronized switch from trivalent oral poliovirus vaccine (tOPV) to bivalent oral poliovirus vaccine (bOPV), which only types 1 and 3 attenuated polioviruses. The switch is one step in the WHO Polio Eradication and Endgame Strategic Plan 2013-2018, which describes specific steps to take to successfully achieve eradication.
Related: Comparing Pneumococcal Vaccines
The 155 countries and territories that used OPV in immunization programs now report that they completely stopped using tOPV in May 2016. (All manufacturers of OPV ended production of tOPV before the switch.) All countries not already using inactivated polio vaccine (IPV) have committed to introducing it. As of August 2016, 173 of 194 WHO countries introduced IPV into their immunization programs—despite a global shortage of IPV.
According to the CDC, the global cooperation in stopping tOPV use has gone smoothly and is, in fact, “unprecedented.” Although this represents a milestone in the effort to eradicate polio, the CDC warns that vigilance is still needed. For example, clinicians should destroy any remaining tOPV found in a vaccine storage refrigerator or freezer. All remaining type 2 polioviruses, including type 2 wild poliovirus, type 2 vaccine-derived polioviruses, and the type 2 Sabin polioviruses used in tOPV and monovalent OPV type 2, also should be destroyed or appropriately contained in certified poliovirus-essential facilities.
Related: Can Hepatitis B and C Be Eliminated?
If type 2 poliovirus outbreaks occur, the United Nations Children’s Fund has a global stockpile of approximately 36 million doses of monovalent OPV type 2, with 100 million more to become available soon. Hundreds of millions of doses stored in bulk form also are available for conversion, the CDC says.
Ultimately, the CDC claims that it will not know how well the process went until it knows the number of polio cases caused by cVDPV2s that arise after the tOPV withdrawal, “with fewer cases indicating a greater success.” As of August 31, 2016, no new cVDPV outbreaks have been identified in 2016.
Type 2 circulating vaccine-derived polioviruses (cVDPV) have caused hundreds of cases of paralytic poliomyelitis and now accounts for > 94% of polio cases since 2006. To address cVDPV and the risk of vaccine-derived polioviruses, the World Health Organization (WHO) scheduled the type 2 component of oral poliovirus vaccine (OPV) for global withdrawal and planned a synchronized switch from trivalent oral poliovirus vaccine (tOPV) to bivalent oral poliovirus vaccine (bOPV), which only types 1 and 3 attenuated polioviruses. The switch is one step in the WHO Polio Eradication and Endgame Strategic Plan 2013-2018, which describes specific steps to take to successfully achieve eradication.
Related: Comparing Pneumococcal Vaccines
The 155 countries and territories that used OPV in immunization programs now report that they completely stopped using tOPV in May 2016. (All manufacturers of OPV ended production of tOPV before the switch.) All countries not already using inactivated polio vaccine (IPV) have committed to introducing it. As of August 2016, 173 of 194 WHO countries introduced IPV into their immunization programs—despite a global shortage of IPV.
According to the CDC, the global cooperation in stopping tOPV use has gone smoothly and is, in fact, “unprecedented.” Although this represents a milestone in the effort to eradicate polio, the CDC warns that vigilance is still needed. For example, clinicians should destroy any remaining tOPV found in a vaccine storage refrigerator or freezer. All remaining type 2 polioviruses, including type 2 wild poliovirus, type 2 vaccine-derived polioviruses, and the type 2 Sabin polioviruses used in tOPV and monovalent OPV type 2, also should be destroyed or appropriately contained in certified poliovirus-essential facilities.
Related: Can Hepatitis B and C Be Eliminated?
If type 2 poliovirus outbreaks occur, the United Nations Children’s Fund has a global stockpile of approximately 36 million doses of monovalent OPV type 2, with 100 million more to become available soon. Hundreds of millions of doses stored in bulk form also are available for conversion, the CDC says.
Ultimately, the CDC claims that it will not know how well the process went until it knows the number of polio cases caused by cVDPV2s that arise after the tOPV withdrawal, “with fewer cases indicating a greater success.” As of August 31, 2016, no new cVDPV outbreaks have been identified in 2016.
Type 2 circulating vaccine-derived polioviruses (cVDPV) have caused hundreds of cases of paralytic poliomyelitis and now accounts for > 94% of polio cases since 2006. To address cVDPV and the risk of vaccine-derived polioviruses, the World Health Organization (WHO) scheduled the type 2 component of oral poliovirus vaccine (OPV) for global withdrawal and planned a synchronized switch from trivalent oral poliovirus vaccine (tOPV) to bivalent oral poliovirus vaccine (bOPV), which only types 1 and 3 attenuated polioviruses. The switch is one step in the WHO Polio Eradication and Endgame Strategic Plan 2013-2018, which describes specific steps to take to successfully achieve eradication.
Related: Comparing Pneumococcal Vaccines
The 155 countries and territories that used OPV in immunization programs now report that they completely stopped using tOPV in May 2016. (All manufacturers of OPV ended production of tOPV before the switch.) All countries not already using inactivated polio vaccine (IPV) have committed to introducing it. As of August 2016, 173 of 194 WHO countries introduced IPV into their immunization programs—despite a global shortage of IPV.
According to the CDC, the global cooperation in stopping tOPV use has gone smoothly and is, in fact, “unprecedented.” Although this represents a milestone in the effort to eradicate polio, the CDC warns that vigilance is still needed. For example, clinicians should destroy any remaining tOPV found in a vaccine storage refrigerator or freezer. All remaining type 2 polioviruses, including type 2 wild poliovirus, type 2 vaccine-derived polioviruses, and the type 2 Sabin polioviruses used in tOPV and monovalent OPV type 2, also should be destroyed or appropriately contained in certified poliovirus-essential facilities.
Related: Can Hepatitis B and C Be Eliminated?
If type 2 poliovirus outbreaks occur, the United Nations Children’s Fund has a global stockpile of approximately 36 million doses of monovalent OPV type 2, with 100 million more to become available soon. Hundreds of millions of doses stored in bulk form also are available for conversion, the CDC says.
Ultimately, the CDC claims that it will not know how well the process went until it knows the number of polio cases caused by cVDPV2s that arise after the tOPV withdrawal, “with fewer cases indicating a greater success.” As of August 31, 2016, no new cVDPV outbreaks have been identified in 2016.
Development of Bullous Pemphigoid in a Patient With Psoriasis and Metabolic Syndrome
Bullous pemphigoid (BP) is an autoimmune subepidermal blistering disease.1 The majority of BP cases are idiopathic and occur in patients older than 60 years. The disease is characterized by the development of circulating IgG autoantibodies reacting with the BP180 antigen of the basement membrane zone.1 Psoriasis vulgaris (PV) is a common, chronic, immune-mediated disease affecting approximately 2% of the world’s population including children and adults.2 Both entities may coexist with internal disorders such as hypertension, diabetes mellitus, coronary heart disease, congestive heart failure, hyperlipidemia, and cerebrovascular accident. It has been postulated that BP more often coexists with neurological disorders, such as stroke and Parkinson disease,3 whereas PV usually is associated with cardiovascular disorders and diabetes mellitus.2 We report the case of a 35-year-old man with chronic PV and metabolic syndrome who developed BP that was successfully treated with methotrexate (MTX).
Case Report
A 35-year-old man with a 15-year history of PV, class 3 obesity (body mass index, 69.2), and thrombosis of the left leg was referred to the dermatology department due to a sudden extensive erythematous and bullous eruption located on the trunk, arms, and legs with involvement of the oral mucosa that had started 4 weeks prior. The skin lesions were accompanied by severe pruritus. On admission to the hospital, the patient presented with stable psoriatic plaques located on the trunk, arms, and proximal part of the lower legs with a psoriasis area severity index score of 11.8 (Figure 1A). He also had disseminated tense blisters and erosions partially arranged in an annular pattern located on the border of the psoriatic plaques as well as on an erythematous base or within unaffected skin (Figure 1B). Additionally, a few small erosions were present on the oral mucosa.
The patient’s father had a history of PV, but there was no family history of obesity or autoimmune blistering disorders. On physical examination, central obesity was noted with a waist circumference of 180 cm and a body mass index of 69.2; his blood pressure was 220/150 mm Hg. Laboratory tests revealed leukocytosis (20.06×109/L [reference range, 4.5–11.0×109/L]) with neutrophilia (16.2×109/L [reference range, 1.6–7.6×109/L]; 80.9% [reference range, 40.0%–70.0%]), eosinophilia (1.01×109/L [reference range, 0–0.5×109/L]), elevated C-reactive protein levels (49.4 mg/L [reference range, 0.0–9.0 mg/L]), elevated erythrocyte sedimentation rate (35 mm/h [reference range, 0–12 mm/h]), elevated γ-glutamyltransferase (66 U/L [reference range, 0–55 U/L]), decreased high-density lipoprotein levels (38 mg/dL [reference range, ≥40 mg/dL]), elevated fasting plasma glucose (116 mg/dL or 6.4 mmol/L [reference range, 70–99 mg/dL or 3.9–5.5 mmol/L]), elevated total IgE (1540 µg/L [reference range, 0–1000 µg/L]), elevated D-dimer (3.21 µg/mL [reference range, <0.5 µg/mL]), and low free triiodothyronine levels (130 pg/dL [reference range, 171–371 pg/dL]). The total protein level was 6.5 g/dL (reference range, 6.0–8.0 g/dL) and albumin level was 3.2 g/dL (reference range, 4.02–4.76 g/dL). A chest radiograph showed no abnormalities.
Based on the physical examination and laboratory testing, it was determined that the patient fulfilled 4 of 5 criteria for metabolic syndrome described by the International Diabetes Federation in 2006 (Table).4 Direct immunofluorescence performed on normal-appearing perilesional skin demonstrated linear IgG and C3 deposits along the basement membrane zone. Indirect immunofluorescence detected circulating IgG autoantibodies at a titer of 1:80. Serum studies using biochip mosaics5 revealed the reactivity of circulating IgG antibodies to the epidermal side of salt-split skin and with antigen dots of tetrameric BP180-NC16a, which prompted the diagnosis of BP (Figure 2).

Oral treatment with MTX 12.5 mg once weekly with clobetasol propionate cream applied to affected skin was initiated for 4 weeks. The PV resolved completely and blister formation stopped. A few weeks later BP reappeared, even though the patient was still taking MTX. The treatment failure may have been related to the patient’s class 3 obesity; therefore, the dose was increased to 20 mg once weekly for 8 weeks, which led to rapid healing of BP erosions. The patient was monitored for 2 months with no symptoms of recurrence.
Comment
Psoriasis Comorbidities
The correlation between PV and cardiovascular disorders such as myocardial infarction, cerebrovascular accident, and pulmonary embolism has been well established and is widely accepted.2 It also has been documented that the risk for metabolic syndrome with components such as diabetes mellitus, hypertension, lipid abnormalities, obesity, and arteriosclerosis is notably increased in PV patients.6 Moreover, associated internal disorders are responsible for a 3- to 4-year reduction in life expectancy in patients with moderate to severe PV.7
Correlation of PV and BP
Psoriasis also may coexist with autoimmune disorders such as rheumatoid arthritis, lupus erythematosus, and blistering disorders.8 There are more than 60 known cases reporting PV in association with various types of subepidermal blistering diseases, including pemphigus vulgaris, epidermolysis bullosa acquisita, anti-p200 pemphigoid, and BP.8,9 The pathogenetic relationship between BP and PV remains obscure. In most published cases, PV preceded BP by 5 to 30 years, possibly ascribable to patients being diagnosed with PV at a younger age.9 In general, patients with BP and PV are younger than patients with BP only, with a mean age of 62 years.9 Because our patient was in his mid-30s when he developed BP, in such cases physicians should take under consideration any triggering factors (eg, drugs). Physical examination and detailed laboratory findings allowed us to make the patient aware of the potential for development of metabolic syndrome. This condition in combination with PV could be a predisposing factor for BP development. According to more recent research, PV is considered a generalized inflammatory process rather than a disorder limited to the skin and joints.10 The chronic inflammatory process in psoriatic skin results in exposure of autoantigens, leading to an immune response and the production of BP antibodies. The neutrophil elastase enzyme present in psoriatic lesions also may take part in dermoepidermal junction degradation and blister formation of BP.11 According to other observations, some antipsoriatic therapies (eg, psoralen plus UVA, UVB, dithranol, coal tar) could be associated with development of BP.12 Moreover, it was shown that psoralen plus UVA therapy, which is widely used in PV treatment, alters the cytokine profile from helper T cells TH1 to TH2.12 TH2-dependent cytokines predominate the sera and erosions in BP patients and seem to be notably relevant to the pathophysiology of the disease.13 The history of our patient’s psoriatic treatment included only topical corticosteroids, keratolytic agents, and occasionally dithranol and coal tar; however, UV phototherapy or any other systemic therapies had never been utilized. Three previously reported cases of patients with PV and BP also revealed no history of UV phototherapy,8,9 which suggests that mechanisms responsible for coexistence of PV and BP are more complex. It has been proven that proinflammatory cytokines secreted by TH1 and TH17 cells, in particular tumor necrosis factor α, IL-17, IL-22, and IL-23, play an important role in the development of psoriatic lesions.10 On the other hand, these cytokines are known to contribute to vascular inflammation, leading to development of arteriosclerosis, as well as to regulate adipogenesis and obesity.14,15 Arakawa et al16 reported increased expression of IL-17 in lesional skin in BP. They concluded that IL-17 may contribute to the recruitment of eosinophils and neutrophils and tissue damage in BP. Therefore, it is highly likely that IL-17 might be a common factor underlying the coexistence of BP with PV and metabolic syndrome. More such reports are required for better understanding this association.
BP Treatment
Selecting a therapy for BP with coexistent PV is challenging, especially in patients with extreme obesity and metabolic syndrome. It is well established that obesity correlates with a higher incidence of PV and more severe disease. On the other hand, obesity also influences response to therapy. Systemic corticosteroids are contraindicated in psoriasis patients because of severe side effects, such as rebound phenomenon of psoriatic lesions and risk for development of generalized pustular PV. Although systemic corticosteroids are effective in BP, high-dose therapy may potentially be life-threatening, particularly in these obese patients with conditions such as hypertension and diabetes mellitus, among others,1 as was observed in our case. Taking into consideration the above mentioned conditions and our experience on such cases, the current patient had received MTX (12.5 mg once weekly) and clobetasol propionate cream, which led to the rapid healing of the psoriatic plaques, whereas BP was more resistant to this therapy. This response may be explained by our patient’s class 3 obesity (body mass index, 69.2). Therefore, the dose of MTX was increased to 20 mg once weekly and was successful. The decision to use MTX was supported by evidence that this medicine may reduce the risk for arteriosclerosis and cardiovascular disorders.17
There are some alternative therapeutic options for patients with coexisting BP and PV, such as cyclosporine,18 combination low-dose cyclosporine and low-dose systemic corticosteroids,19 dapsone,20 azathioprine,21 mycophenolate mofetil,22 and acitretin.23 It also has been shown that biologics (eg, ustekinumab) may be a successful solution in patients with PV and antilaminin-γ1 pemphigoid.24 However, these alternative therapeutic regimens could not be considered in our patient because of serious coexisting internal disorders.
Conclusion
We present a case of concomitant BP and PV in a patient with metabolic syndrome. Although the pathogenic role of this unique coexistence is not fully understood, MTX proved suitable and effective in this single case. Further studies should be performed to elucidate the pathogenic relationship and therapeutic solutions for cases with coexisting PV, BP, and metabolic syndrome.
- Rzany B, Partscht K, Jung M, et al. Risk factors for lethal outcome in patients with bullous pemphigoid: low serum albumin level, high dosage of gluco-corticosteroids, and old age. Arch Dermatol. 2002;138:903-908.
- Pietrzak A, Bartosinska J, Chodorowska G, et al. Cardiovascular aspects of psoriasis vulgaris. Int J Dermatol. 2013;52:153-162.
- Stinco G, Codutti R, Scarbolo M, et al. A retrospective epidemiological study on the association of bullous pemphigoid and neurological diseases. Acta Derm Venereol. 2005;85:136-139.
- International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Brussels, Belgium: International Diabetes Foundation; 2006. http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf. Accessed September 14, 2016.
- Van Beek N, Rentzsch K, Probst C, et al. Serological diagnosis of autoimmune bullous skin diseases: prospective comparison of the BIOCHIP mosaic-based indirect immunofluorescence technique with the conventional multi-step single test strategy. Orphanet J Rare Dis. 2012;7:49.
- Sommer DM, Jenisch S, Suchan M, et al. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298:321-328.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Lazarczyk M, Wozniak K, Ishii N, et al. Coexistence of psoriasis and pemphigoid—only a coincidence? Int J Mol Med. 2006;18:619-623.
- Yasuda H, Tomita Y, Shibaki A, et al. Two cases of subepidermal blistering disease with anti-p200 or 180-kD bullous pemphigoid antigen associated with psoriasis. Dermatology. 2004;209:149-155.
- Malakouti M, Brown GE, Wang E, et al. The role of IL-17 in psoriasis [published online February 20, 2014]. J Dermatolog Treat. 2015;26:41-44.
- Glinski W, Jarzabek-Chorzelska M, Pierozynska-Dubowska M, et al. Basement membrane zone as a target for human neutrophil elastase in psoriasis. Arch Dermatol Res. 1990;282:506-511.
- Klosner G, Trautinger F, Knobler R, et al. Treatment of peripheral blood mononuclear cells with 8-methoxypsoralen plus ultraviolet A radiation induces a shift in cytokine expression from a Th1 to a Th2 response. J Invest Dermatol. 2001;116:459-462.
- Gounni AS, Wellemans V, Agouli M, et al. Increased expression of Th2-associated chemokines in bullous pemphigoid disease. role of eosinophils in the production and release of these chemokines. Clin Immunol. 2006;120:220-231.
- Gao Q, Jiang Y, Ma T, et al. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol. 2010;185:5820-5827.
- Zúñiga LA, Shen WJ, Joyce-Shaikh B, et al. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947-6959.
- Arakawa M, Dainichi T, Ishii N, et al. Lesional Th17 cells and regulatory T cells in bullous pemphigoid. Exp Dermatol. 2011;20:1022-1024.
- Everett BM, Pradhan AD, Solomon DH, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199-207.
- Boixeda JP, Soria C, Medina S, et al. Bullous pemphigoid and psoriasis: treatment with cyclosporine. J Am Acad Dermatol. 1991;24:152.
- Bianchi L, Gatti S, Nini G. Bullous pemphigoid and severe erythrodermic psoriasis: combined low-dose treatment with cyclosporine and systemic steroids. J Am Acad Dermatol. 1992;27(2, pt 1):278.
- Hisler BM, Blumenthal NC, Aronson PJ, et al. Bullous pemphigoid in psoriatic lesions. J Am Acad Dermatol. 1989;20:683-684.
- Primka EJ III, Camisa C. Psoriasis and bullous pemphigoid treated with azathioprine. J Am Acad Dermatol. 1998;39:121-123.
- Nousari HC, Sragovich A, Kimyai-Asadi A, et al. Mycophenolate mofetil in autoimmune and inflammatory skin disorders. J Am Acad Dermatol. 1999;40:265-268.
- Kobayashi TT, Elston DM, Libow LF, et al. A case of bullous pemphigoid limited to psoriatic plaques. Cutis. 2002;70:283-287.
- Maijima Y, Yagi H, Tateishi C, et al. A successful treatment with ustekinumab in case of antilaminin-γ1 pemphigoid associated with psoriasis. Br J Dermatol. 2013;168:1367-1369.
Bullous pemphigoid (BP) is an autoimmune subepidermal blistering disease.1 The majority of BP cases are idiopathic and occur in patients older than 60 years. The disease is characterized by the development of circulating IgG autoantibodies reacting with the BP180 antigen of the basement membrane zone.1 Psoriasis vulgaris (PV) is a common, chronic, immune-mediated disease affecting approximately 2% of the world’s population including children and adults.2 Both entities may coexist with internal disorders such as hypertension, diabetes mellitus, coronary heart disease, congestive heart failure, hyperlipidemia, and cerebrovascular accident. It has been postulated that BP more often coexists with neurological disorders, such as stroke and Parkinson disease,3 whereas PV usually is associated with cardiovascular disorders and diabetes mellitus.2 We report the case of a 35-year-old man with chronic PV and metabolic syndrome who developed BP that was successfully treated with methotrexate (MTX).
Case Report
A 35-year-old man with a 15-year history of PV, class 3 obesity (body mass index, 69.2), and thrombosis of the left leg was referred to the dermatology department due to a sudden extensive erythematous and bullous eruption located on the trunk, arms, and legs with involvement of the oral mucosa that had started 4 weeks prior. The skin lesions were accompanied by severe pruritus. On admission to the hospital, the patient presented with stable psoriatic plaques located on the trunk, arms, and proximal part of the lower legs with a psoriasis area severity index score of 11.8 (Figure 1A). He also had disseminated tense blisters and erosions partially arranged in an annular pattern located on the border of the psoriatic plaques as well as on an erythematous base or within unaffected skin (Figure 1B). Additionally, a few small erosions were present on the oral mucosa.

The patient’s father had a history of PV, but there was no family history of obesity or autoimmune blistering disorders. On physical examination, central obesity was noted with a waist circumference of 180 cm and a body mass index of 69.2; his blood pressure was 220/150 mm Hg. Laboratory tests revealed leukocytosis (20.06×109/L [reference range, 4.5–11.0×109/L]) with neutrophilia (16.2×109/L [reference range, 1.6–7.6×109/L]; 80.9% [reference range, 40.0%–70.0%]), eosinophilia (1.01×109/L [reference range, 0–0.5×109/L]), elevated C-reactive protein levels (49.4 mg/L [reference range, 0.0–9.0 mg/L]), elevated erythrocyte sedimentation rate (35 mm/h [reference range, 0–12 mm/h]), elevated γ-glutamyltransferase (66 U/L [reference range, 0–55 U/L]), decreased high-density lipoprotein levels (38 mg/dL [reference range, ≥40 mg/dL]), elevated fasting plasma glucose (116 mg/dL or 6.4 mmol/L [reference range, 70–99 mg/dL or 3.9–5.5 mmol/L]), elevated total IgE (1540 µg/L [reference range, 0–1000 µg/L]), elevated D-dimer (3.21 µg/mL [reference range, <0.5 µg/mL]), and low free triiodothyronine levels (130 pg/dL [reference range, 171–371 pg/dL]). The total protein level was 6.5 g/dL (reference range, 6.0–8.0 g/dL) and albumin level was 3.2 g/dL (reference range, 4.02–4.76 g/dL). A chest radiograph showed no abnormalities.
Based on the physical examination and laboratory testing, it was determined that the patient fulfilled 4 of 5 criteria for metabolic syndrome described by the International Diabetes Federation in 2006 (Table).4 Direct immunofluorescence performed on normal-appearing perilesional skin demonstrated linear IgG and C3 deposits along the basement membrane zone. Indirect immunofluorescence detected circulating IgG autoantibodies at a titer of 1:80. Serum studies using biochip mosaics5 revealed the reactivity of circulating IgG antibodies to the epidermal side of salt-split skin and with antigen dots of tetrameric BP180-NC16a, which prompted the diagnosis of BP (Figure 2).

Oral treatment with MTX 12.5 mg once weekly with clobetasol propionate cream applied to affected skin was initiated for 4 weeks. The PV resolved completely and blister formation stopped. A few weeks later BP reappeared, even though the patient was still taking MTX. The treatment failure may have been related to the patient’s class 3 obesity; therefore, the dose was increased to 20 mg once weekly for 8 weeks, which led to rapid healing of BP erosions. The patient was monitored for 2 months with no symptoms of recurrence.
Comment
Psoriasis Comorbidities
The correlation between PV and cardiovascular disorders such as myocardial infarction, cerebrovascular accident, and pulmonary embolism has been well established and is widely accepted.2 It also has been documented that the risk for metabolic syndrome with components such as diabetes mellitus, hypertension, lipid abnormalities, obesity, and arteriosclerosis is notably increased in PV patients.6 Moreover, associated internal disorders are responsible for a 3- to 4-year reduction in life expectancy in patients with moderate to severe PV.7
Correlation of PV and BP
Psoriasis also may coexist with autoimmune disorders such as rheumatoid arthritis, lupus erythematosus, and blistering disorders.8 There are more than 60 known cases reporting PV in association with various types of subepidermal blistering diseases, including pemphigus vulgaris, epidermolysis bullosa acquisita, anti-p200 pemphigoid, and BP.8,9 The pathogenetic relationship between BP and PV remains obscure. In most published cases, PV preceded BP by 5 to 30 years, possibly ascribable to patients being diagnosed with PV at a younger age.9 In general, patients with BP and PV are younger than patients with BP only, with a mean age of 62 years.9 Because our patient was in his mid-30s when he developed BP, in such cases physicians should take under consideration any triggering factors (eg, drugs). Physical examination and detailed laboratory findings allowed us to make the patient aware of the potential for development of metabolic syndrome. This condition in combination with PV could be a predisposing factor for BP development. According to more recent research, PV is considered a generalized inflammatory process rather than a disorder limited to the skin and joints.10 The chronic inflammatory process in psoriatic skin results in exposure of autoantigens, leading to an immune response and the production of BP antibodies. The neutrophil elastase enzyme present in psoriatic lesions also may take part in dermoepidermal junction degradation and blister formation of BP.11 According to other observations, some antipsoriatic therapies (eg, psoralen plus UVA, UVB, dithranol, coal tar) could be associated with development of BP.12 Moreover, it was shown that psoralen plus UVA therapy, which is widely used in PV treatment, alters the cytokine profile from helper T cells TH1 to TH2.12 TH2-dependent cytokines predominate the sera and erosions in BP patients and seem to be notably relevant to the pathophysiology of the disease.13 The history of our patient’s psoriatic treatment included only topical corticosteroids, keratolytic agents, and occasionally dithranol and coal tar; however, UV phototherapy or any other systemic therapies had never been utilized. Three previously reported cases of patients with PV and BP also revealed no history of UV phototherapy,8,9 which suggests that mechanisms responsible for coexistence of PV and BP are more complex. It has been proven that proinflammatory cytokines secreted by TH1 and TH17 cells, in particular tumor necrosis factor α, IL-17, IL-22, and IL-23, play an important role in the development of psoriatic lesions.10 On the other hand, these cytokines are known to contribute to vascular inflammation, leading to development of arteriosclerosis, as well as to regulate adipogenesis and obesity.14,15 Arakawa et al16 reported increased expression of IL-17 in lesional skin in BP. They concluded that IL-17 may contribute to the recruitment of eosinophils and neutrophils and tissue damage in BP. Therefore, it is highly likely that IL-17 might be a common factor underlying the coexistence of BP with PV and metabolic syndrome. More such reports are required for better understanding this association.
BP Treatment
Selecting a therapy for BP with coexistent PV is challenging, especially in patients with extreme obesity and metabolic syndrome. It is well established that obesity correlates with a higher incidence of PV and more severe disease. On the other hand, obesity also influences response to therapy. Systemic corticosteroids are contraindicated in psoriasis patients because of severe side effects, such as rebound phenomenon of psoriatic lesions and risk for development of generalized pustular PV. Although systemic corticosteroids are effective in BP, high-dose therapy may potentially be life-threatening, particularly in these obese patients with conditions such as hypertension and diabetes mellitus, among others,1 as was observed in our case. Taking into consideration the above mentioned conditions and our experience on such cases, the current patient had received MTX (12.5 mg once weekly) and clobetasol propionate cream, which led to the rapid healing of the psoriatic plaques, whereas BP was more resistant to this therapy. This response may be explained by our patient’s class 3 obesity (body mass index, 69.2). Therefore, the dose of MTX was increased to 20 mg once weekly and was successful. The decision to use MTX was supported by evidence that this medicine may reduce the risk for arteriosclerosis and cardiovascular disorders.17
There are some alternative therapeutic options for patients with coexisting BP and PV, such as cyclosporine,18 combination low-dose cyclosporine and low-dose systemic corticosteroids,19 dapsone,20 azathioprine,21 mycophenolate mofetil,22 and acitretin.23 It also has been shown that biologics (eg, ustekinumab) may be a successful solution in patients with PV and antilaminin-γ1 pemphigoid.24 However, these alternative therapeutic regimens could not be considered in our patient because of serious coexisting internal disorders.
Conclusion
We present a case of concomitant BP and PV in a patient with metabolic syndrome. Although the pathogenic role of this unique coexistence is not fully understood, MTX proved suitable and effective in this single case. Further studies should be performed to elucidate the pathogenic relationship and therapeutic solutions for cases with coexisting PV, BP, and metabolic syndrome.
Bullous pemphigoid (BP) is an autoimmune subepidermal blistering disease.1 The majority of BP cases are idiopathic and occur in patients older than 60 years. The disease is characterized by the development of circulating IgG autoantibodies reacting with the BP180 antigen of the basement membrane zone.1 Psoriasis vulgaris (PV) is a common, chronic, immune-mediated disease affecting approximately 2% of the world’s population including children and adults.2 Both entities may coexist with internal disorders such as hypertension, diabetes mellitus, coronary heart disease, congestive heart failure, hyperlipidemia, and cerebrovascular accident. It has been postulated that BP more often coexists with neurological disorders, such as stroke and Parkinson disease,3 whereas PV usually is associated with cardiovascular disorders and diabetes mellitus.2 We report the case of a 35-year-old man with chronic PV and metabolic syndrome who developed BP that was successfully treated with methotrexate (MTX).
Case Report
A 35-year-old man with a 15-year history of PV, class 3 obesity (body mass index, 69.2), and thrombosis of the left leg was referred to the dermatology department due to a sudden extensive erythematous and bullous eruption located on the trunk, arms, and legs with involvement of the oral mucosa that had started 4 weeks prior. The skin lesions were accompanied by severe pruritus. On admission to the hospital, the patient presented with stable psoriatic plaques located on the trunk, arms, and proximal part of the lower legs with a psoriasis area severity index score of 11.8 (Figure 1A). He also had disseminated tense blisters and erosions partially arranged in an annular pattern located on the border of the psoriatic plaques as well as on an erythematous base or within unaffected skin (Figure 1B). Additionally, a few small erosions were present on the oral mucosa.

The patient’s father had a history of PV, but there was no family history of obesity or autoimmune blistering disorders. On physical examination, central obesity was noted with a waist circumference of 180 cm and a body mass index of 69.2; his blood pressure was 220/150 mm Hg. Laboratory tests revealed leukocytosis (20.06×109/L [reference range, 4.5–11.0×109/L]) with neutrophilia (16.2×109/L [reference range, 1.6–7.6×109/L]; 80.9% [reference range, 40.0%–70.0%]), eosinophilia (1.01×109/L [reference range, 0–0.5×109/L]), elevated C-reactive protein levels (49.4 mg/L [reference range, 0.0–9.0 mg/L]), elevated erythrocyte sedimentation rate (35 mm/h [reference range, 0–12 mm/h]), elevated γ-glutamyltransferase (66 U/L [reference range, 0–55 U/L]), decreased high-density lipoprotein levels (38 mg/dL [reference range, ≥40 mg/dL]), elevated fasting plasma glucose (116 mg/dL or 6.4 mmol/L [reference range, 70–99 mg/dL or 3.9–5.5 mmol/L]), elevated total IgE (1540 µg/L [reference range, 0–1000 µg/L]), elevated D-dimer (3.21 µg/mL [reference range, <0.5 µg/mL]), and low free triiodothyronine levels (130 pg/dL [reference range, 171–371 pg/dL]). The total protein level was 6.5 g/dL (reference range, 6.0–8.0 g/dL) and albumin level was 3.2 g/dL (reference range, 4.02–4.76 g/dL). A chest radiograph showed no abnormalities.
Based on the physical examination and laboratory testing, it was determined that the patient fulfilled 4 of 5 criteria for metabolic syndrome described by the International Diabetes Federation in 2006 (Table).4 Direct immunofluorescence performed on normal-appearing perilesional skin demonstrated linear IgG and C3 deposits along the basement membrane zone. Indirect immunofluorescence detected circulating IgG autoantibodies at a titer of 1:80. Serum studies using biochip mosaics5 revealed the reactivity of circulating IgG antibodies to the epidermal side of salt-split skin and with antigen dots of tetrameric BP180-NC16a, which prompted the diagnosis of BP (Figure 2).

Oral treatment with MTX 12.5 mg once weekly with clobetasol propionate cream applied to affected skin was initiated for 4 weeks. The PV resolved completely and blister formation stopped. A few weeks later BP reappeared, even though the patient was still taking MTX. The treatment failure may have been related to the patient’s class 3 obesity; therefore, the dose was increased to 20 mg once weekly for 8 weeks, which led to rapid healing of BP erosions. The patient was monitored for 2 months with no symptoms of recurrence.
Comment
Psoriasis Comorbidities
The correlation between PV and cardiovascular disorders such as myocardial infarction, cerebrovascular accident, and pulmonary embolism has been well established and is widely accepted.2 It also has been documented that the risk for metabolic syndrome with components such as diabetes mellitus, hypertension, lipid abnormalities, obesity, and arteriosclerosis is notably increased in PV patients.6 Moreover, associated internal disorders are responsible for a 3- to 4-year reduction in life expectancy in patients with moderate to severe PV.7
Correlation of PV and BP
Psoriasis also may coexist with autoimmune disorders such as rheumatoid arthritis, lupus erythematosus, and blistering disorders.8 There are more than 60 known cases reporting PV in association with various types of subepidermal blistering diseases, including pemphigus vulgaris, epidermolysis bullosa acquisita, anti-p200 pemphigoid, and BP.8,9 The pathogenetic relationship between BP and PV remains obscure. In most published cases, PV preceded BP by 5 to 30 years, possibly ascribable to patients being diagnosed with PV at a younger age.9 In general, patients with BP and PV are younger than patients with BP only, with a mean age of 62 years.9 Because our patient was in his mid-30s when he developed BP, in such cases physicians should take under consideration any triggering factors (eg, drugs). Physical examination and detailed laboratory findings allowed us to make the patient aware of the potential for development of metabolic syndrome. This condition in combination with PV could be a predisposing factor for BP development. According to more recent research, PV is considered a generalized inflammatory process rather than a disorder limited to the skin and joints.10 The chronic inflammatory process in psoriatic skin results in exposure of autoantigens, leading to an immune response and the production of BP antibodies. The neutrophil elastase enzyme present in psoriatic lesions also may take part in dermoepidermal junction degradation and blister formation of BP.11 According to other observations, some antipsoriatic therapies (eg, psoralen plus UVA, UVB, dithranol, coal tar) could be associated with development of BP.12 Moreover, it was shown that psoralen plus UVA therapy, which is widely used in PV treatment, alters the cytokine profile from helper T cells TH1 to TH2.12 TH2-dependent cytokines predominate the sera and erosions in BP patients and seem to be notably relevant to the pathophysiology of the disease.13 The history of our patient’s psoriatic treatment included only topical corticosteroids, keratolytic agents, and occasionally dithranol and coal tar; however, UV phototherapy or any other systemic therapies had never been utilized. Three previously reported cases of patients with PV and BP also revealed no history of UV phototherapy,8,9 which suggests that mechanisms responsible for coexistence of PV and BP are more complex. It has been proven that proinflammatory cytokines secreted by TH1 and TH17 cells, in particular tumor necrosis factor α, IL-17, IL-22, and IL-23, play an important role in the development of psoriatic lesions.10 On the other hand, these cytokines are known to contribute to vascular inflammation, leading to development of arteriosclerosis, as well as to regulate adipogenesis and obesity.14,15 Arakawa et al16 reported increased expression of IL-17 in lesional skin in BP. They concluded that IL-17 may contribute to the recruitment of eosinophils and neutrophils and tissue damage in BP. Therefore, it is highly likely that IL-17 might be a common factor underlying the coexistence of BP with PV and metabolic syndrome. More such reports are required for better understanding this association.
BP Treatment
Selecting a therapy for BP with coexistent PV is challenging, especially in patients with extreme obesity and metabolic syndrome. It is well established that obesity correlates with a higher incidence of PV and more severe disease. On the other hand, obesity also influences response to therapy. Systemic corticosteroids are contraindicated in psoriasis patients because of severe side effects, such as rebound phenomenon of psoriatic lesions and risk for development of generalized pustular PV. Although systemic corticosteroids are effective in BP, high-dose therapy may potentially be life-threatening, particularly in these obese patients with conditions such as hypertension and diabetes mellitus, among others,1 as was observed in our case. Taking into consideration the above mentioned conditions and our experience on such cases, the current patient had received MTX (12.5 mg once weekly) and clobetasol propionate cream, which led to the rapid healing of the psoriatic plaques, whereas BP was more resistant to this therapy. This response may be explained by our patient’s class 3 obesity (body mass index, 69.2). Therefore, the dose of MTX was increased to 20 mg once weekly and was successful. The decision to use MTX was supported by evidence that this medicine may reduce the risk for arteriosclerosis and cardiovascular disorders.17
There are some alternative therapeutic options for patients with coexisting BP and PV, such as cyclosporine,18 combination low-dose cyclosporine and low-dose systemic corticosteroids,19 dapsone,20 azathioprine,21 mycophenolate mofetil,22 and acitretin.23 It also has been shown that biologics (eg, ustekinumab) may be a successful solution in patients with PV and antilaminin-γ1 pemphigoid.24 However, these alternative therapeutic regimens could not be considered in our patient because of serious coexisting internal disorders.
Conclusion
We present a case of concomitant BP and PV in a patient with metabolic syndrome. Although the pathogenic role of this unique coexistence is not fully understood, MTX proved suitable and effective in this single case. Further studies should be performed to elucidate the pathogenic relationship and therapeutic solutions for cases with coexisting PV, BP, and metabolic syndrome.
- Rzany B, Partscht K, Jung M, et al. Risk factors for lethal outcome in patients with bullous pemphigoid: low serum albumin level, high dosage of gluco-corticosteroids, and old age. Arch Dermatol. 2002;138:903-908.
- Pietrzak A, Bartosinska J, Chodorowska G, et al. Cardiovascular aspects of psoriasis vulgaris. Int J Dermatol. 2013;52:153-162.
- Stinco G, Codutti R, Scarbolo M, et al. A retrospective epidemiological study on the association of bullous pemphigoid and neurological diseases. Acta Derm Venereol. 2005;85:136-139.
- International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Brussels, Belgium: International Diabetes Foundation; 2006. http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf. Accessed September 14, 2016.
- Van Beek N, Rentzsch K, Probst C, et al. Serological diagnosis of autoimmune bullous skin diseases: prospective comparison of the BIOCHIP mosaic-based indirect immunofluorescence technique with the conventional multi-step single test strategy. Orphanet J Rare Dis. 2012;7:49.
- Sommer DM, Jenisch S, Suchan M, et al. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298:321-328.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Lazarczyk M, Wozniak K, Ishii N, et al. Coexistence of psoriasis and pemphigoid—only a coincidence? Int J Mol Med. 2006;18:619-623.
- Yasuda H, Tomita Y, Shibaki A, et al. Two cases of subepidermal blistering disease with anti-p200 or 180-kD bullous pemphigoid antigen associated with psoriasis. Dermatology. 2004;209:149-155.
- Malakouti M, Brown GE, Wang E, et al. The role of IL-17 in psoriasis [published online February 20, 2014]. J Dermatolog Treat. 2015;26:41-44.
- Glinski W, Jarzabek-Chorzelska M, Pierozynska-Dubowska M, et al. Basement membrane zone as a target for human neutrophil elastase in psoriasis. Arch Dermatol Res. 1990;282:506-511.
- Klosner G, Trautinger F, Knobler R, et al. Treatment of peripheral blood mononuclear cells with 8-methoxypsoralen plus ultraviolet A radiation induces a shift in cytokine expression from a Th1 to a Th2 response. J Invest Dermatol. 2001;116:459-462.
- Gounni AS, Wellemans V, Agouli M, et al. Increased expression of Th2-associated chemokines in bullous pemphigoid disease. role of eosinophils in the production and release of these chemokines. Clin Immunol. 2006;120:220-231.
- Gao Q, Jiang Y, Ma T, et al. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol. 2010;185:5820-5827.
- Zúñiga LA, Shen WJ, Joyce-Shaikh B, et al. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947-6959.
- Arakawa M, Dainichi T, Ishii N, et al. Lesional Th17 cells and regulatory T cells in bullous pemphigoid. Exp Dermatol. 2011;20:1022-1024.
- Everett BM, Pradhan AD, Solomon DH, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199-207.
- Boixeda JP, Soria C, Medina S, et al. Bullous pemphigoid and psoriasis: treatment with cyclosporine. J Am Acad Dermatol. 1991;24:152.
- Bianchi L, Gatti S, Nini G. Bullous pemphigoid and severe erythrodermic psoriasis: combined low-dose treatment with cyclosporine and systemic steroids. J Am Acad Dermatol. 1992;27(2, pt 1):278.
- Hisler BM, Blumenthal NC, Aronson PJ, et al. Bullous pemphigoid in psoriatic lesions. J Am Acad Dermatol. 1989;20:683-684.
- Primka EJ III, Camisa C. Psoriasis and bullous pemphigoid treated with azathioprine. J Am Acad Dermatol. 1998;39:121-123.
- Nousari HC, Sragovich A, Kimyai-Asadi A, et al. Mycophenolate mofetil in autoimmune and inflammatory skin disorders. J Am Acad Dermatol. 1999;40:265-268.
- Kobayashi TT, Elston DM, Libow LF, et al. A case of bullous pemphigoid limited to psoriatic plaques. Cutis. 2002;70:283-287.
- Maijima Y, Yagi H, Tateishi C, et al. A successful treatment with ustekinumab in case of antilaminin-γ1 pemphigoid associated with psoriasis. Br J Dermatol. 2013;168:1367-1369.
- Rzany B, Partscht K, Jung M, et al. Risk factors for lethal outcome in patients with bullous pemphigoid: low serum albumin level, high dosage of gluco-corticosteroids, and old age. Arch Dermatol. 2002;138:903-908.
- Pietrzak A, Bartosinska J, Chodorowska G, et al. Cardiovascular aspects of psoriasis vulgaris. Int J Dermatol. 2013;52:153-162.
- Stinco G, Codutti R, Scarbolo M, et al. A retrospective epidemiological study on the association of bullous pemphigoid and neurological diseases. Acta Derm Venereol. 2005;85:136-139.
- International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Brussels, Belgium: International Diabetes Foundation; 2006. http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf. Accessed September 14, 2016.
- Van Beek N, Rentzsch K, Probst C, et al. Serological diagnosis of autoimmune bullous skin diseases: prospective comparison of the BIOCHIP mosaic-based indirect immunofluorescence technique with the conventional multi-step single test strategy. Orphanet J Rare Dis. 2012;7:49.
- Sommer DM, Jenisch S, Suchan M, et al. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298:321-328.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Lazarczyk M, Wozniak K, Ishii N, et al. Coexistence of psoriasis and pemphigoid—only a coincidence? Int J Mol Med. 2006;18:619-623.
- Yasuda H, Tomita Y, Shibaki A, et al. Two cases of subepidermal blistering disease with anti-p200 or 180-kD bullous pemphigoid antigen associated with psoriasis. Dermatology. 2004;209:149-155.
- Malakouti M, Brown GE, Wang E, et al. The role of IL-17 in psoriasis [published online February 20, 2014]. J Dermatolog Treat. 2015;26:41-44.
- Glinski W, Jarzabek-Chorzelska M, Pierozynska-Dubowska M, et al. Basement membrane zone as a target for human neutrophil elastase in psoriasis. Arch Dermatol Res. 1990;282:506-511.
- Klosner G, Trautinger F, Knobler R, et al. Treatment of peripheral blood mononuclear cells with 8-methoxypsoralen plus ultraviolet A radiation induces a shift in cytokine expression from a Th1 to a Th2 response. J Invest Dermatol. 2001;116:459-462.
- Gounni AS, Wellemans V, Agouli M, et al. Increased expression of Th2-associated chemokines in bullous pemphigoid disease. role of eosinophils in the production and release of these chemokines. Clin Immunol. 2006;120:220-231.
- Gao Q, Jiang Y, Ma T, et al. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol. 2010;185:5820-5827.
- Zúñiga LA, Shen WJ, Joyce-Shaikh B, et al. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947-6959.
- Arakawa M, Dainichi T, Ishii N, et al. Lesional Th17 cells and regulatory T cells in bullous pemphigoid. Exp Dermatol. 2011;20:1022-1024.
- Everett BM, Pradhan AD, Solomon DH, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199-207.
- Boixeda JP, Soria C, Medina S, et al. Bullous pemphigoid and psoriasis: treatment with cyclosporine. J Am Acad Dermatol. 1991;24:152.
- Bianchi L, Gatti S, Nini G. Bullous pemphigoid and severe erythrodermic psoriasis: combined low-dose treatment with cyclosporine and systemic steroids. J Am Acad Dermatol. 1992;27(2, pt 1):278.
- Hisler BM, Blumenthal NC, Aronson PJ, et al. Bullous pemphigoid in psoriatic lesions. J Am Acad Dermatol. 1989;20:683-684.
- Primka EJ III, Camisa C. Psoriasis and bullous pemphigoid treated with azathioprine. J Am Acad Dermatol. 1998;39:121-123.
- Nousari HC, Sragovich A, Kimyai-Asadi A, et al. Mycophenolate mofetil in autoimmune and inflammatory skin disorders. J Am Acad Dermatol. 1999;40:265-268.
- Kobayashi TT, Elston DM, Libow LF, et al. A case of bullous pemphigoid limited to psoriatic plaques. Cutis. 2002;70:283-287.
- Maijima Y, Yagi H, Tateishi C, et al. A successful treatment with ustekinumab in case of antilaminin-γ1 pemphigoid associated with psoriasis. Br J Dermatol. 2013;168:1367-1369.
Practice Points
- Metabolic syndrome and psoriasis vulgaris (PV) may promote development of bullous pemphigoid (BP) in patients younger than 60 years.
- Methotrexate may be a therapeutic solution for BP coexisting with PV and metabolic syndrome.
COPD patient characteristics predict response to maintenance drug
LONDON – Maintenance azithromycin may be best reserved for patients with mild to moderate chronic obstructive pulmonary disease (COPD) who also have few symptoms, according to an analysis from the COLUMBUS randomized controlled trial.
Significantly fewer exacerbations (1.06 vs. 2.62; P = .02) occurred at 1 year in patients treated with the macrolide antibiotic azithromycin rather than placebo if they were classified as having GOLD [Global Initiative for Chronic Obstructive Lung Disease] stage 1 or 2 versus stage 4.
Study participants who were classified as being part of GOLD group C (which includes patients with a high risk of COPD exacerbation but a low level of COPD symptoms) who were treated with maintenance azithromycin were also more likely to have fewer exacerbations at 1 year, compared with patients classified as being part of GOLD group D (which includes patients with a high risk of COPD exacerbation and a high level of COPD symptoms), who took the same antibiotic (0.45 vs. 2.18; P less than .01).
Having a high serum eosinophil level (2% or higher) was a third factor found in COPD patients that was predictive of fewer exacerbations following azithromycin use (1.26 vs. 2.5; P = .02).
“Azithromycin maintenance therapy should not be given to every COPD patient,” Remco Djamin, MD, of Amphia Hospital Breda in the Netherlands said in an interview at the annual congress of the European Respiratory Society. There is, of course, the concern over antibiotic resistance developing and macrolide antibiotic use has been linked with heart problems such as arrhythmia.
These data show, however, that there are certain predictors that might help clinicians decide if long-term antibiotic therapy might be beneficial for their patients who are experiencing frequent acute exacerbations of COPD.
Further research should look at the dosing and duration of azithromycin, Dr. Djamin suggested. Perhaps reducing the dose by half to 250 mg three times per week would be just as good; maybe 6 months’ rather than 12 months’ treatment would be sufficient, or perhaps it could be given intermittently. The aim is to ensure that patients are not being exposed unnecessarily, as there is concern over antibiotic resistance.
The use of azithromycin is not currently recommended in guidelines for COPD management to prevent exacerbations, but it is something that is likely to be added to the guidelines, as the evidence for its benefit mounts, Dr. Djamin said.
In addition to COLUMBUS, there have been at least two other studies looking at long-term antibiotic use to prevent exacerbations in patients with COPD. One (Am J Respir Crit Care Med. 2008;178:1139-47) showed erythromycin could decrease the exacerbation rate at 1 year by 36%, compared with placebo, while the other (N Engl J Med. 2011;365:689-8) again showed a benefit for azithromycin, with a 27% decrease in the 1-year exacerbation rate.
In COLUMBUS, 92 patients who had experienced at least three or more acute COPD exacerbations in the previous year were randomized to treatment with azithromycin 500 mg or placebo, taken three times per week for 12 months. This was a single-center, double-blind trial conducted in the Netherlands that showed a 42% reduction in the 1-year exacerbation rate could be achieved with the antibiotic treatment (Lancet Respir Med. 2014;2:361-8).
An additional benefit to using the antibiotic was seen in patients with GOLD stage 1-2 over patients with GOLD stage 4 and in patients with a higher percentage of serum eosinophils. The GOLD stage 1-2 patients experienced fewer exacerbations leading to hospitalization, compared with patients with GOLD stage 4 (0.31 vs. 1.00; P = .04), while the patients with higher levels of eosinophils experienced fewer exacerbations requiring hospitalization than those patients with lower percentages of eosinophils (0.26 vs. 1.07; P = 0.01).
“What you should consider is that this is a group of patients who have frequent exacerbations, and most of these exacerbations are caused by infections,” Dr. Djamin said, during a poster presentation at the conference. “Their exacerbations are often already being treated with antibiotics and so maintaining treatment has become one possible way of perhaps preventing exacerbations in the future.”
The study received no industry funding. Dr. Djamin had no competing interests to disclose.
LONDON – Maintenance azithromycin may be best reserved for patients with mild to moderate chronic obstructive pulmonary disease (COPD) who also have few symptoms, according to an analysis from the COLUMBUS randomized controlled trial.
Significantly fewer exacerbations (1.06 vs. 2.62; P = .02) occurred at 1 year in patients treated with the macrolide antibiotic azithromycin rather than placebo if they were classified as having GOLD [Global Initiative for Chronic Obstructive Lung Disease] stage 1 or 2 versus stage 4.
Study participants who were classified as being part of GOLD group C (which includes patients with a high risk of COPD exacerbation but a low level of COPD symptoms) who were treated with maintenance azithromycin were also more likely to have fewer exacerbations at 1 year, compared with patients classified as being part of GOLD group D (which includes patients with a high risk of COPD exacerbation and a high level of COPD symptoms), who took the same antibiotic (0.45 vs. 2.18; P less than .01).
Having a high serum eosinophil level (2% or higher) was a third factor found in COPD patients that was predictive of fewer exacerbations following azithromycin use (1.26 vs. 2.5; P = .02).
“Azithromycin maintenance therapy should not be given to every COPD patient,” Remco Djamin, MD, of Amphia Hospital Breda in the Netherlands said in an interview at the annual congress of the European Respiratory Society. There is, of course, the concern over antibiotic resistance developing and macrolide antibiotic use has been linked with heart problems such as arrhythmia.
These data show, however, that there are certain predictors that might help clinicians decide if long-term antibiotic therapy might be beneficial for their patients who are experiencing frequent acute exacerbations of COPD.
Further research should look at the dosing and duration of azithromycin, Dr. Djamin suggested. Perhaps reducing the dose by half to 250 mg three times per week would be just as good; maybe 6 months’ rather than 12 months’ treatment would be sufficient, or perhaps it could be given intermittently. The aim is to ensure that patients are not being exposed unnecessarily, as there is concern over antibiotic resistance.
The use of azithromycin is not currently recommended in guidelines for COPD management to prevent exacerbations, but it is something that is likely to be added to the guidelines, as the evidence for its benefit mounts, Dr. Djamin said.
In addition to COLUMBUS, there have been at least two other studies looking at long-term antibiotic use to prevent exacerbations in patients with COPD. One (Am J Respir Crit Care Med. 2008;178:1139-47) showed erythromycin could decrease the exacerbation rate at 1 year by 36%, compared with placebo, while the other (N Engl J Med. 2011;365:689-8) again showed a benefit for azithromycin, with a 27% decrease in the 1-year exacerbation rate.
In COLUMBUS, 92 patients who had experienced at least three or more acute COPD exacerbations in the previous year were randomized to treatment with azithromycin 500 mg or placebo, taken three times per week for 12 months. This was a single-center, double-blind trial conducted in the Netherlands that showed a 42% reduction in the 1-year exacerbation rate could be achieved with the antibiotic treatment (Lancet Respir Med. 2014;2:361-8).
An additional benefit to using the antibiotic was seen in patients with GOLD stage 1-2 over patients with GOLD stage 4 and in patients with a higher percentage of serum eosinophils. The GOLD stage 1-2 patients experienced fewer exacerbations leading to hospitalization, compared with patients with GOLD stage 4 (0.31 vs. 1.00; P = .04), while the patients with higher levels of eosinophils experienced fewer exacerbations requiring hospitalization than those patients with lower percentages of eosinophils (0.26 vs. 1.07; P = 0.01).
“What you should consider is that this is a group of patients who have frequent exacerbations, and most of these exacerbations are caused by infections,” Dr. Djamin said, during a poster presentation at the conference. “Their exacerbations are often already being treated with antibiotics and so maintaining treatment has become one possible way of perhaps preventing exacerbations in the future.”
The study received no industry funding. Dr. Djamin had no competing interests to disclose.
LONDON – Maintenance azithromycin may be best reserved for patients with mild to moderate chronic obstructive pulmonary disease (COPD) who also have few symptoms, according to an analysis from the COLUMBUS randomized controlled trial.
Significantly fewer exacerbations (1.06 vs. 2.62; P = .02) occurred at 1 year in patients treated with the macrolide antibiotic azithromycin rather than placebo if they were classified as having GOLD [Global Initiative for Chronic Obstructive Lung Disease] stage 1 or 2 versus stage 4.
Study participants who were classified as being part of GOLD group C (which includes patients with a high risk of COPD exacerbation but a low level of COPD symptoms) who were treated with maintenance azithromycin were also more likely to have fewer exacerbations at 1 year, compared with patients classified as being part of GOLD group D (which includes patients with a high risk of COPD exacerbation and a high level of COPD symptoms), who took the same antibiotic (0.45 vs. 2.18; P less than .01).
Having a high serum eosinophil level (2% or higher) was a third factor found in COPD patients that was predictive of fewer exacerbations following azithromycin use (1.26 vs. 2.5; P = .02).
“Azithromycin maintenance therapy should not be given to every COPD patient,” Remco Djamin, MD, of Amphia Hospital Breda in the Netherlands said in an interview at the annual congress of the European Respiratory Society. There is, of course, the concern over antibiotic resistance developing and macrolide antibiotic use has been linked with heart problems such as arrhythmia.
These data show, however, that there are certain predictors that might help clinicians decide if long-term antibiotic therapy might be beneficial for their patients who are experiencing frequent acute exacerbations of COPD.
Further research should look at the dosing and duration of azithromycin, Dr. Djamin suggested. Perhaps reducing the dose by half to 250 mg three times per week would be just as good; maybe 6 months’ rather than 12 months’ treatment would be sufficient, or perhaps it could be given intermittently. The aim is to ensure that patients are not being exposed unnecessarily, as there is concern over antibiotic resistance.
The use of azithromycin is not currently recommended in guidelines for COPD management to prevent exacerbations, but it is something that is likely to be added to the guidelines, as the evidence for its benefit mounts, Dr. Djamin said.
In addition to COLUMBUS, there have been at least two other studies looking at long-term antibiotic use to prevent exacerbations in patients with COPD. One (Am J Respir Crit Care Med. 2008;178:1139-47) showed erythromycin could decrease the exacerbation rate at 1 year by 36%, compared with placebo, while the other (N Engl J Med. 2011;365:689-8) again showed a benefit for azithromycin, with a 27% decrease in the 1-year exacerbation rate.
In COLUMBUS, 92 patients who had experienced at least three or more acute COPD exacerbations in the previous year were randomized to treatment with azithromycin 500 mg or placebo, taken three times per week for 12 months. This was a single-center, double-blind trial conducted in the Netherlands that showed a 42% reduction in the 1-year exacerbation rate could be achieved with the antibiotic treatment (Lancet Respir Med. 2014;2:361-8).
An additional benefit to using the antibiotic was seen in patients with GOLD stage 1-2 over patients with GOLD stage 4 and in patients with a higher percentage of serum eosinophils. The GOLD stage 1-2 patients experienced fewer exacerbations leading to hospitalization, compared with patients with GOLD stage 4 (0.31 vs. 1.00; P = .04), while the patients with higher levels of eosinophils experienced fewer exacerbations requiring hospitalization than those patients with lower percentages of eosinophils (0.26 vs. 1.07; P = 0.01).
“What you should consider is that this is a group of patients who have frequent exacerbations, and most of these exacerbations are caused by infections,” Dr. Djamin said, during a poster presentation at the conference. “Their exacerbations are often already being treated with antibiotics and so maintaining treatment has become one possible way of perhaps preventing exacerbations in the future.”
The study received no industry funding. Dr. Djamin had no competing interests to disclose.
AT THE ERS CONGRESS 2016
Key clinical point: Maintenance azithromycin may be best reserved for patients with more mild to moderate chronic obstructive pulmonary disease and few symptoms.
Major finding: Fewer exacerbations at 1 year occurred in patients with higher vs. lower serum eosinophil levels, GOLD stage 1-2 vs. GOLD stage 4, and GOLD group C vs. group D COPD.
Data source: Analysis of the COLUMBUS randomized, double-blind, placebo-controlled trial of 92 COPD patients with frequent exacerbations who were treated with maintenance azithromycin or placebo for 1 year.
Disclosures: The study received no industry funding. Dr. Djamin had no competing interests to disclose.
Biologic mesh for ventral hernia repair compared for recurrence, cost
The porcine acellular dermal mesh product Strattice was associated with significantly lower odds of hernia recurrence, compared with several other biologic mesh products, in a study of 223 patients who underwent open ventral hernia repair.
Prospective operative outcomes data from a tertiary referral hernia center showed that at a mean follow-up of 18.2 months, the rate of hernia recurrence was 35% in 40 patients who were treated with Alloderm (LifeCell Corporation), 34.5% in 23 patients treated with AlloMax (Bard/Davol), 37.1% in 70 patients treated with FlexHD (Ethicon), and 59.1% in 22 patients treated with Xenmatrix (Bard/Davol), compared with 14.7% in 68 patients treated with Strattice (LifeCell Corporation). Alloderm, AlloMax, and FlexHD are all human acellular dermal mesh products, and Strattice and Xenmatrix are both porcine acellular dermal mesh products, Ciara R. Huntington, MD, and her colleagues at the Carolinas Medical Center in Charlotte, N.C., reported.
 |
| Photo courtesy Acelity. STRATTICETM Reconstructive Tissue Matrix |
After multivariate analysis to adjust for factors such as comorbidities, hernia size, and intraoperative techniques, the odds ratios for recurrence with each product as compared with Strattice were 2.4 with Alloderm, 2.9 with FlexHD, 3.4 with AlloMax, and 7.8 with Xenmatrix. The odds for recurrence were significantly greater with all except Alloderm, the investigators said (Surgery. 2016. doi: 10.1016/j.surg.2016.07.008).
The significant differences between the two porcine acellular dermal meshes (Xenmatrix and Strattice) may reflect variation in tissue processing and design in biomesh engineering, they noted.
Study subjects were adults with a mean age of 57.7 years and mean body mass index of 34.8 kg/m2. Overall, 9.8% had an American Society of Anesthesiology classification of 4, 54.6% had a classification of 3, and 35.6% had a classification of 1 or 2. Average operative time was 241 minutes with estimated blood loss of 202 mL.
Average hernia defect size was 257 cm2, with average mesh size of 384 cm2.
“Component separation was performed in 47.5% of cases, and abdomen was left open prior to definitive closure in 10.7%. Biologic mesh was used to bridge fascial defects in 19.6% of cases. The mesh was placed in the preperitoneal space in 38.2% of cases,” the investigators wrote, noting that a concomitant procedure was performed in 82% of cases.
Sepsis developed in 6.7% of patients, 36.3% had a wound infection, and 24.3% required a negative pressure dressing for healing. The inpatient mortality rate was 1.4%.
However, mesh infections requiring explantation occurred in less than 1% of cases.
On adjusted analysis, Xenmatrix was the most expensive mesh and AlloMax was the least expensive (mean of $59,122 and $22,304, respectively). Strattice costs averaged $40,490.
Ventral hernia repair (VHR) is a common operation, with about 350,000 performed each year. Rates of postoperative wound infection and hernia recurrence vary widely, but may be improved with appropriate mesh selection. However, prospective data to guide selection are lacking, the investigators said.
“The great number of meshes available for use complicates the debate surrounding the best timing and use of biologic mesh in VHR, and the search for the better mesh for use in the abdominal wall reconstruction continues. Biologic mesh usually is reserved for the patients at the highest risk for developing a postoperative wound complication, and although there is a current dearth of high-level evidence supporting its use, this report confirms that complications are low despite obvious surgical complexity presented herein,” they wrote.
The findings of this study – the largest report of outcomes with biologic mesh in ventral hernia repair to date, according to the authors – support the safety of using biologic mesh in high-risk patients, they said.
They noted, however, that the study may still be underpowered to make final clinical decisions.
“Although our study provides useful information to the practicing surgeon, there is much work to be done regarding the selection of biologic mesh,” they wrote, adding that while “a well-performing biologic mesh should be in the toolkit of every general surgeon who may face complex abdominal walls requiring reconstruction in patients that are at high risk for a postoperative wound complication,” additional research is necessary to further clarify the role of biologic mesh in these operations.
Dr. Huntington reported having no disclosures. Other authors reported having been awarded honoraria, speaking fees, surgical research funding, and education grants from W.L. Gore and Associates, Ethicon, Novadaq, Bard/Davol, and LifeCell Corporation.
The porcine acellular dermal mesh product Strattice was associated with significantly lower odds of hernia recurrence, compared with several other biologic mesh products, in a study of 223 patients who underwent open ventral hernia repair.
Prospective operative outcomes data from a tertiary referral hernia center showed that at a mean follow-up of 18.2 months, the rate of hernia recurrence was 35% in 40 patients who were treated with Alloderm (LifeCell Corporation), 34.5% in 23 patients treated with AlloMax (Bard/Davol), 37.1% in 70 patients treated with FlexHD (Ethicon), and 59.1% in 22 patients treated with Xenmatrix (Bard/Davol), compared with 14.7% in 68 patients treated with Strattice (LifeCell Corporation). Alloderm, AlloMax, and FlexHD are all human acellular dermal mesh products, and Strattice and Xenmatrix are both porcine acellular dermal mesh products, Ciara R. Huntington, MD, and her colleagues at the Carolinas Medical Center in Charlotte, N.C., reported.
 |
| Photo courtesy Acelity. STRATTICETM Reconstructive Tissue Matrix |
After multivariate analysis to adjust for factors such as comorbidities, hernia size, and intraoperative techniques, the odds ratios for recurrence with each product as compared with Strattice were 2.4 with Alloderm, 2.9 with FlexHD, 3.4 with AlloMax, and 7.8 with Xenmatrix. The odds for recurrence were significantly greater with all except Alloderm, the investigators said (Surgery. 2016. doi: 10.1016/j.surg.2016.07.008).
The significant differences between the two porcine acellular dermal meshes (Xenmatrix and Strattice) may reflect variation in tissue processing and design in biomesh engineering, they noted.
Study subjects were adults with a mean age of 57.7 years and mean body mass index of 34.8 kg/m2. Overall, 9.8% had an American Society of Anesthesiology classification of 4, 54.6% had a classification of 3, and 35.6% had a classification of 1 or 2. Average operative time was 241 minutes with estimated blood loss of 202 mL.
Average hernia defect size was 257 cm2, with average mesh size of 384 cm2.
“Component separation was performed in 47.5% of cases, and abdomen was left open prior to definitive closure in 10.7%. Biologic mesh was used to bridge fascial defects in 19.6% of cases. The mesh was placed in the preperitoneal space in 38.2% of cases,” the investigators wrote, noting that a concomitant procedure was performed in 82% of cases.
Sepsis developed in 6.7% of patients, 36.3% had a wound infection, and 24.3% required a negative pressure dressing for healing. The inpatient mortality rate was 1.4%.
However, mesh infections requiring explantation occurred in less than 1% of cases.
On adjusted analysis, Xenmatrix was the most expensive mesh and AlloMax was the least expensive (mean of $59,122 and $22,304, respectively). Strattice costs averaged $40,490.
Ventral hernia repair (VHR) is a common operation, with about 350,000 performed each year. Rates of postoperative wound infection and hernia recurrence vary widely, but may be improved with appropriate mesh selection. However, prospective data to guide selection are lacking, the investigators said.
“The great number of meshes available for use complicates the debate surrounding the best timing and use of biologic mesh in VHR, and the search for the better mesh for use in the abdominal wall reconstruction continues. Biologic mesh usually is reserved for the patients at the highest risk for developing a postoperative wound complication, and although there is a current dearth of high-level evidence supporting its use, this report confirms that complications are low despite obvious surgical complexity presented herein,” they wrote.
The findings of this study – the largest report of outcomes with biologic mesh in ventral hernia repair to date, according to the authors – support the safety of using biologic mesh in high-risk patients, they said.
They noted, however, that the study may still be underpowered to make final clinical decisions.
“Although our study provides useful information to the practicing surgeon, there is much work to be done regarding the selection of biologic mesh,” they wrote, adding that while “a well-performing biologic mesh should be in the toolkit of every general surgeon who may face complex abdominal walls requiring reconstruction in patients that are at high risk for a postoperative wound complication,” additional research is necessary to further clarify the role of biologic mesh in these operations.
Dr. Huntington reported having no disclosures. Other authors reported having been awarded honoraria, speaking fees, surgical research funding, and education grants from W.L. Gore and Associates, Ethicon, Novadaq, Bard/Davol, and LifeCell Corporation.
The porcine acellular dermal mesh product Strattice was associated with significantly lower odds of hernia recurrence, compared with several other biologic mesh products, in a study of 223 patients who underwent open ventral hernia repair.
Prospective operative outcomes data from a tertiary referral hernia center showed that at a mean follow-up of 18.2 months, the rate of hernia recurrence was 35% in 40 patients who were treated with Alloderm (LifeCell Corporation), 34.5% in 23 patients treated with AlloMax (Bard/Davol), 37.1% in 70 patients treated with FlexHD (Ethicon), and 59.1% in 22 patients treated with Xenmatrix (Bard/Davol), compared with 14.7% in 68 patients treated with Strattice (LifeCell Corporation). Alloderm, AlloMax, and FlexHD are all human acellular dermal mesh products, and Strattice and Xenmatrix are both porcine acellular dermal mesh products, Ciara R. Huntington, MD, and her colleagues at the Carolinas Medical Center in Charlotte, N.C., reported.
 |
| Photo courtesy Acelity. STRATTICETM Reconstructive Tissue Matrix |
After multivariate analysis to adjust for factors such as comorbidities, hernia size, and intraoperative techniques, the odds ratios for recurrence with each product as compared with Strattice were 2.4 with Alloderm, 2.9 with FlexHD, 3.4 with AlloMax, and 7.8 with Xenmatrix. The odds for recurrence were significantly greater with all except Alloderm, the investigators said (Surgery. 2016. doi: 10.1016/j.surg.2016.07.008).
The significant differences between the two porcine acellular dermal meshes (Xenmatrix and Strattice) may reflect variation in tissue processing and design in biomesh engineering, they noted.
Study subjects were adults with a mean age of 57.7 years and mean body mass index of 34.8 kg/m2. Overall, 9.8% had an American Society of Anesthesiology classification of 4, 54.6% had a classification of 3, and 35.6% had a classification of 1 or 2. Average operative time was 241 minutes with estimated blood loss of 202 mL.
Average hernia defect size was 257 cm2, with average mesh size of 384 cm2.
“Component separation was performed in 47.5% of cases, and abdomen was left open prior to definitive closure in 10.7%. Biologic mesh was used to bridge fascial defects in 19.6% of cases. The mesh was placed in the preperitoneal space in 38.2% of cases,” the investigators wrote, noting that a concomitant procedure was performed in 82% of cases.
Sepsis developed in 6.7% of patients, 36.3% had a wound infection, and 24.3% required a negative pressure dressing for healing. The inpatient mortality rate was 1.4%.
However, mesh infections requiring explantation occurred in less than 1% of cases.
On adjusted analysis, Xenmatrix was the most expensive mesh and AlloMax was the least expensive (mean of $59,122 and $22,304, respectively). Strattice costs averaged $40,490.
Ventral hernia repair (VHR) is a common operation, with about 350,000 performed each year. Rates of postoperative wound infection and hernia recurrence vary widely, but may be improved with appropriate mesh selection. However, prospective data to guide selection are lacking, the investigators said.
“The great number of meshes available for use complicates the debate surrounding the best timing and use of biologic mesh in VHR, and the search for the better mesh for use in the abdominal wall reconstruction continues. Biologic mesh usually is reserved for the patients at the highest risk for developing a postoperative wound complication, and although there is a current dearth of high-level evidence supporting its use, this report confirms that complications are low despite obvious surgical complexity presented herein,” they wrote.
The findings of this study – the largest report of outcomes with biologic mesh in ventral hernia repair to date, according to the authors – support the safety of using biologic mesh in high-risk patients, they said.
They noted, however, that the study may still be underpowered to make final clinical decisions.
“Although our study provides useful information to the practicing surgeon, there is much work to be done regarding the selection of biologic mesh,” they wrote, adding that while “a well-performing biologic mesh should be in the toolkit of every general surgeon who may face complex abdominal walls requiring reconstruction in patients that are at high risk for a postoperative wound complication,” additional research is necessary to further clarify the role of biologic mesh in these operations.
Dr. Huntington reported having no disclosures. Other authors reported having been awarded honoraria, speaking fees, surgical research funding, and education grants from W.L. Gore and Associates, Ethicon, Novadaq, Bard/Davol, and LifeCell Corporation.
FROM SURGERY
Key clinical point: The porcine acellular dermal mesh product Strattice was associated with significantly lower odds of hernia recurrence, compared with several other biologic mesh products, in a study of 223 patients who underwent open ventral hernia repair.
Major finding: The adjusted odds ratios for recurrence, compared with Strattice, were 2.4 with Alloderm, 2.9 with FlexHD, 3.4 with AlloMax, and 7.8 with Xenmatrix.
Data source: 223 cases from a prospective operative outcomes database.
Disclosures: Dr. Huntington reported having no disclosures. Other authors reported having been awarded honoraria, speaking fees, surgical research funding, and education grants from W.L. Gore and Associates, Ethicon, Novadaq, Bard/Davol, and LifeCell Corporation.
‘Unprecedented’ Study Sheds Light on Origins of Diabetes
An international study “unprecedented in both scale and scope” has provided some important answers to the mysteries of how diabetes develops—not least, the answer to a century-old debate about whether genetic differences are shared and common or rare and individual.
Led by researchers from University of Michigan, University of Oxford, Massachusetts Institute of Technology (MIT), Harvard University, and Massachusetts General Hospital, more than 300 scientists from 22 countries used DNA from 120,000 individuals to pinpoint genes and their variants.
Related: A Window Into the Genetics Behind Diabetes
The collaboration brought together 2 research projects: GoT2D and T2D-DENES. The research teams completed whole genome sequencing of more than 2,600 people and exome sequencing of 12,940, as well as genome- or exomewide array genotyping of 111,548 people. Unlike most previous studies, which involved people only of European ancestry, this study included people with ancestral origins in Europe, South and East Asia, the Americas, and Africa.
The researchers were able to highlight “with unprecedented precision” a number of genes directly involved in the development of type 2 diabetes mellitus (T2DM)—promising new avenues for research into treatment or prevention. They also identified more than a dozen genetic regions that harbor variants that influence risk of T2DM. Most were common to all human populations and had previously been detected by other genomewide association studies.
Related: Confronting the Diabetes Epidemic
“While rare variants certainly influence type 2 diabetes risk, our results demonstrate that common variants shared across populations explain most of the genetic risk,” said Michael Boehnke, director of the Center for Statistical Genetics at the University of Michigan School of Public Health and one of the study’s 3 senior authors.
“The conclusions seem clear,” agreed Mark McCarthy, Group Head, Wellcome Trust Centre for Human Genetics. He noted, “[T]he evidence is increasingly stacking up in favor of the view that most of the genetic risk of T2D can be attributed to common alleles that are widely shared within, and between, human populations.”
Related: Thanks to IHS Funding Program, “Sustained Achievements” in Diabetes Prevention
“While this large range of genetic risk may challenge our efforts at precision medicine,” said Jason Flannick, co-lead author and senior group leader at the Broad Institute of Harvard and MIT and research associate at the Massachusetts General Hospital, “our consortium also offers a publicly accessible dataset, unprecedented in scope, for researchers around the world to advance our molecular understanding of type 2 diabetes.”
An international study “unprecedented in both scale and scope” has provided some important answers to the mysteries of how diabetes develops—not least, the answer to a century-old debate about whether genetic differences are shared and common or rare and individual.
Led by researchers from University of Michigan, University of Oxford, Massachusetts Institute of Technology (MIT), Harvard University, and Massachusetts General Hospital, more than 300 scientists from 22 countries used DNA from 120,000 individuals to pinpoint genes and their variants.
Related: A Window Into the Genetics Behind Diabetes
The collaboration brought together 2 research projects: GoT2D and T2D-DENES. The research teams completed whole genome sequencing of more than 2,600 people and exome sequencing of 12,940, as well as genome- or exomewide array genotyping of 111,548 people. Unlike most previous studies, which involved people only of European ancestry, this study included people with ancestral origins in Europe, South and East Asia, the Americas, and Africa.
The researchers were able to highlight “with unprecedented precision” a number of genes directly involved in the development of type 2 diabetes mellitus (T2DM)—promising new avenues for research into treatment or prevention. They also identified more than a dozen genetic regions that harbor variants that influence risk of T2DM. Most were common to all human populations and had previously been detected by other genomewide association studies.
Related: Confronting the Diabetes Epidemic
“While rare variants certainly influence type 2 diabetes risk, our results demonstrate that common variants shared across populations explain most of the genetic risk,” said Michael Boehnke, director of the Center for Statistical Genetics at the University of Michigan School of Public Health and one of the study’s 3 senior authors.
“The conclusions seem clear,” agreed Mark McCarthy, Group Head, Wellcome Trust Centre for Human Genetics. He noted, “[T]he evidence is increasingly stacking up in favor of the view that most of the genetic risk of T2D can be attributed to common alleles that are widely shared within, and between, human populations.”
Related: Thanks to IHS Funding Program, “Sustained Achievements” in Diabetes Prevention
“While this large range of genetic risk may challenge our efforts at precision medicine,” said Jason Flannick, co-lead author and senior group leader at the Broad Institute of Harvard and MIT and research associate at the Massachusetts General Hospital, “our consortium also offers a publicly accessible dataset, unprecedented in scope, for researchers around the world to advance our molecular understanding of type 2 diabetes.”
An international study “unprecedented in both scale and scope” has provided some important answers to the mysteries of how diabetes develops—not least, the answer to a century-old debate about whether genetic differences are shared and common or rare and individual.
Led by researchers from University of Michigan, University of Oxford, Massachusetts Institute of Technology (MIT), Harvard University, and Massachusetts General Hospital, more than 300 scientists from 22 countries used DNA from 120,000 individuals to pinpoint genes and their variants.
Related: A Window Into the Genetics Behind Diabetes
The collaboration brought together 2 research projects: GoT2D and T2D-DENES. The research teams completed whole genome sequencing of more than 2,600 people and exome sequencing of 12,940, as well as genome- or exomewide array genotyping of 111,548 people. Unlike most previous studies, which involved people only of European ancestry, this study included people with ancestral origins in Europe, South and East Asia, the Americas, and Africa.
The researchers were able to highlight “with unprecedented precision” a number of genes directly involved in the development of type 2 diabetes mellitus (T2DM)—promising new avenues for research into treatment or prevention. They also identified more than a dozen genetic regions that harbor variants that influence risk of T2DM. Most were common to all human populations and had previously been detected by other genomewide association studies.
Related: Confronting the Diabetes Epidemic
“While rare variants certainly influence type 2 diabetes risk, our results demonstrate that common variants shared across populations explain most of the genetic risk,” said Michael Boehnke, director of the Center for Statistical Genetics at the University of Michigan School of Public Health and one of the study’s 3 senior authors.
“The conclusions seem clear,” agreed Mark McCarthy, Group Head, Wellcome Trust Centre for Human Genetics. He noted, “[T]he evidence is increasingly stacking up in favor of the view that most of the genetic risk of T2D can be attributed to common alleles that are widely shared within, and between, human populations.”
Related: Thanks to IHS Funding Program, “Sustained Achievements” in Diabetes Prevention
“While this large range of genetic risk may challenge our efforts at precision medicine,” said Jason Flannick, co-lead author and senior group leader at the Broad Institute of Harvard and MIT and research associate at the Massachusetts General Hospital, “our consortium also offers a publicly accessible dataset, unprecedented in scope, for researchers around the world to advance our molecular understanding of type 2 diabetes.”