User login
A Melting Pot of Mail
VAPING DANGERS: CLEARING THE AIR
The liquid base of an e-cigarette contains either vegetable glycerin (VG) or propylene glycol, or more commonly, a proprietary combination of both. Each of these ingredients has varying effects on the body.
However, the first paragraph of Randy D. Danielsen’s editorial alluded to what I consider a bigger concern regarding the future medical complications of vaping. The description of a “… huge puff of cherry-scented smoke …” indicates that vapes are not puffed on the way cigarettes are.
Cigarette smoking is similar to drinking through a straw—the smoke is first captured in the mouth, then cooled and inhaled. In contrast, vaping involves inhaling smoke directly into the lungs. This action, along with the thick VG base, produces a high volume of smoke. Vape shops even sponsor contests to see who can produce the largest cloud of smoke.
Therefore, my concern regarding vaping is not limited to the toxicity of the ingredients; it extends to how the toxicants are delivered to the poor, unsuspecting alveoli.
Gary Dula, FNP-C
Houston, TX
Continue for Millenials: Not All Sitting at the Kids' Table >>
MILLENIALS: NOT ALL SITTING AT THE KIDS' TABLES
I received my master’s degree in 2015 and am nearing completion of a year-long FNP fellowship program. I was an Army nurse for four years and a float nurse at various hospitals for five. I am a “millennial”—and, according to the published letters about precepting, am hated by older nurses because of it. Considering I have practiced with many hard-working people my age who would lay down their lives for this country, I find this unprecedented.
I work hard, but the school I attended for my FNP did not prepare me well; it was difficult to get people to teach and precept me during school. This led me to apply for my current fellowship.
Throughout my nine-year nursing career, I have precepted many nurses, including those with associate degrees. I will continue to mentor and precept as an APRN. I take issue with the portrayal of millennials as lazy and unable to work hard. Why? Because we will not work for free, would like to collaboratively learn, and need help to develop our skills?
One day, you will grow old and need someone to take care of you. Why on earth would you berate the people who will be doing just that? Complaining about this generation is not going to change the fact that they are here and present in the workforce. We need more providers, and chastising the younger generation is not going to solve that problem.
Stephanie Butler-Cleland, FNP-BC
Colorado Springs, CO
Continue for The Pros of Precepting >>
THE PROS OF PRECEPTING
I am an urgent care NP in urban communities on the West Coast of Florida. I had taken a break from precepting as a result of negative experiences, but I recently resumed to precept my first NP student in years.
Prior to accepting the student I precepted, I received requests from two other students. One asked if I could change my schedule to be closer to where she lived. The other clearly didn’t want to commit to the drive or the hours I was available, and asked if I would work more weekends to accommodate her schedule. Needless to say, I refused both students.
Instead, I precepted a smart 28-year-old student from my alma mater, one of the Florida state universities. She was attentive, prepared, and eager. I was very, very impressed with her. She had been a nurse for four years and was a second-semester student. It was a pleasure to have her; I like being questioned and challenged. It was fun to see her enjoying my job, and it reminded me of why I love what I do.
Anne Conklin, MS, ARNP-C
Bradenton, FL
Continue for A Scheming Industry >>
A SCHEMING INDUSTRY
Intelligent health care policy has been frustrated by the enormous amount of money brought to bear on Congress by the insurance and pharmaceutical industries. Each dollar paid to an insurance company is used to construct buildings, hire workers, create a sales staff, and ultimately pay their shareholders a profit.
Since the insurance industry obtained an antitrust exemption in the 1940s, they are essentially immune from prosecution for price collusion. Until recently, it was difficult to know how much of the money paid was returned in the form of medical benefits. In order to keep profits rising, they must enroll more people. Promising coverage while impeding medical workups and care, making great profits, and needing more and more enrollees fits the definition of a Ponzi scheme.
Several years ago in California, the state insurance commission (under threat of decertification) got an industry representative to admit that the maximum percentage of dollars used for services was 70%. In other words, for each dollar spent, a patient would be lucky to get 70 cents worth of services.
All of us who practice know how the companies do this: We request a needed diagnostic test or treatment and are denied. I have interrupted my schedule on many days to call for a “peer to peer” review—only once was I denied. This is a roadblock that many busy practitioners will not challenge. Since insurance companies market how great their coverage is, patients often get angry at the provider.
The repeated argument is that the market forces will lower medical costs. This fallacy is easily debunked by noting the ever-escalating costs and comparing health care costs as a percent of gross domestic product (GDP) in our country versus others. France, for example, expends 12% of GDP on health and ranks first in health care outcomes by world standards. In the US, we are approaching 20% of GDP.
Since insurance adds nothing to care and increases costs dramatically (every provider has to have billers for the various insurance companies, since each has its own requirements), a single-payer system is the only system that will lower costs. Those who benefit from the current system declare that we can’t have “socialized medicine.” To which I would respond, fine; we’ll continue to pay 30% to 50% more so that insurance companies can have their profits at our expense.
Nelson Herilhy, PA-C, MHS
Concord, CA
VAPING DANGERS: CLEARING THE AIR
The liquid base of an e-cigarette contains either vegetable glycerin (VG) or propylene glycol, or more commonly, a proprietary combination of both. Each of these ingredients has varying effects on the body.
However, the first paragraph of Randy D. Danielsen’s editorial alluded to what I consider a bigger concern regarding the future medical complications of vaping. The description of a “… huge puff of cherry-scented smoke …” indicates that vapes are not puffed on the way cigarettes are.
Cigarette smoking is similar to drinking through a straw—the smoke is first captured in the mouth, then cooled and inhaled. In contrast, vaping involves inhaling smoke directly into the lungs. This action, along with the thick VG base, produces a high volume of smoke. Vape shops even sponsor contests to see who can produce the largest cloud of smoke.
Therefore, my concern regarding vaping is not limited to the toxicity of the ingredients; it extends to how the toxicants are delivered to the poor, unsuspecting alveoli.
Gary Dula, FNP-C
Houston, TX
Continue for Millenials: Not All Sitting at the Kids' Table >>
MILLENIALS: NOT ALL SITTING AT THE KIDS' TABLES
I received my master’s degree in 2015 and am nearing completion of a year-long FNP fellowship program. I was an Army nurse for four years and a float nurse at various hospitals for five. I am a “millennial”—and, according to the published letters about precepting, am hated by older nurses because of it. Considering I have practiced with many hard-working people my age who would lay down their lives for this country, I find this unprecedented.
I work hard, but the school I attended for my FNP did not prepare me well; it was difficult to get people to teach and precept me during school. This led me to apply for my current fellowship.
Throughout my nine-year nursing career, I have precepted many nurses, including those with associate degrees. I will continue to mentor and precept as an APRN. I take issue with the portrayal of millennials as lazy and unable to work hard. Why? Because we will not work for free, would like to collaboratively learn, and need help to develop our skills?
One day, you will grow old and need someone to take care of you. Why on earth would you berate the people who will be doing just that? Complaining about this generation is not going to change the fact that they are here and present in the workforce. We need more providers, and chastising the younger generation is not going to solve that problem.
Stephanie Butler-Cleland, FNP-BC
Colorado Springs, CO
Continue for The Pros of Precepting >>
THE PROS OF PRECEPTING
I am an urgent care NP in urban communities on the West Coast of Florida. I had taken a break from precepting as a result of negative experiences, but I recently resumed to precept my first NP student in years.
Prior to accepting the student I precepted, I received requests from two other students. One asked if I could change my schedule to be closer to where she lived. The other clearly didn’t want to commit to the drive or the hours I was available, and asked if I would work more weekends to accommodate her schedule. Needless to say, I refused both students.
Instead, I precepted a smart 28-year-old student from my alma mater, one of the Florida state universities. She was attentive, prepared, and eager. I was very, very impressed with her. She had been a nurse for four years and was a second-semester student. It was a pleasure to have her; I like being questioned and challenged. It was fun to see her enjoying my job, and it reminded me of why I love what I do.
Anne Conklin, MS, ARNP-C
Bradenton, FL
Continue for A Scheming Industry >>
A SCHEMING INDUSTRY
Intelligent health care policy has been frustrated by the enormous amount of money brought to bear on Congress by the insurance and pharmaceutical industries. Each dollar paid to an insurance company is used to construct buildings, hire workers, create a sales staff, and ultimately pay their shareholders a profit.
Since the insurance industry obtained an antitrust exemption in the 1940s, they are essentially immune from prosecution for price collusion. Until recently, it was difficult to know how much of the money paid was returned in the form of medical benefits. In order to keep profits rising, they must enroll more people. Promising coverage while impeding medical workups and care, making great profits, and needing more and more enrollees fits the definition of a Ponzi scheme.
Several years ago in California, the state insurance commission (under threat of decertification) got an industry representative to admit that the maximum percentage of dollars used for services was 70%. In other words, for each dollar spent, a patient would be lucky to get 70 cents worth of services.
All of us who practice know how the companies do this: We request a needed diagnostic test or treatment and are denied. I have interrupted my schedule on many days to call for a “peer to peer” review—only once was I denied. This is a roadblock that many busy practitioners will not challenge. Since insurance companies market how great their coverage is, patients often get angry at the provider.
The repeated argument is that the market forces will lower medical costs. This fallacy is easily debunked by noting the ever-escalating costs and comparing health care costs as a percent of gross domestic product (GDP) in our country versus others. France, for example, expends 12% of GDP on health and ranks first in health care outcomes by world standards. In the US, we are approaching 20% of GDP.
Since insurance adds nothing to care and increases costs dramatically (every provider has to have billers for the various insurance companies, since each has its own requirements), a single-payer system is the only system that will lower costs. Those who benefit from the current system declare that we can’t have “socialized medicine.” To which I would respond, fine; we’ll continue to pay 30% to 50% more so that insurance companies can have their profits at our expense.
Nelson Herilhy, PA-C, MHS
Concord, CA
VAPING DANGERS: CLEARING THE AIR
The liquid base of an e-cigarette contains either vegetable glycerin (VG) or propylene glycol, or more commonly, a proprietary combination of both. Each of these ingredients has varying effects on the body.
However, the first paragraph of Randy D. Danielsen’s editorial alluded to what I consider a bigger concern regarding the future medical complications of vaping. The description of a “… huge puff of cherry-scented smoke …” indicates that vapes are not puffed on the way cigarettes are.
Cigarette smoking is similar to drinking through a straw—the smoke is first captured in the mouth, then cooled and inhaled. In contrast, vaping involves inhaling smoke directly into the lungs. This action, along with the thick VG base, produces a high volume of smoke. Vape shops even sponsor contests to see who can produce the largest cloud of smoke.
Therefore, my concern regarding vaping is not limited to the toxicity of the ingredients; it extends to how the toxicants are delivered to the poor, unsuspecting alveoli.
Gary Dula, FNP-C
Houston, TX
Continue for Millenials: Not All Sitting at the Kids' Table >>
MILLENIALS: NOT ALL SITTING AT THE KIDS' TABLES
I received my master’s degree in 2015 and am nearing completion of a year-long FNP fellowship program. I was an Army nurse for four years and a float nurse at various hospitals for five. I am a “millennial”—and, according to the published letters about precepting, am hated by older nurses because of it. Considering I have practiced with many hard-working people my age who would lay down their lives for this country, I find this unprecedented.
I work hard, but the school I attended for my FNP did not prepare me well; it was difficult to get people to teach and precept me during school. This led me to apply for my current fellowship.
Throughout my nine-year nursing career, I have precepted many nurses, including those with associate degrees. I will continue to mentor and precept as an APRN. I take issue with the portrayal of millennials as lazy and unable to work hard. Why? Because we will not work for free, would like to collaboratively learn, and need help to develop our skills?
One day, you will grow old and need someone to take care of you. Why on earth would you berate the people who will be doing just that? Complaining about this generation is not going to change the fact that they are here and present in the workforce. We need more providers, and chastising the younger generation is not going to solve that problem.
Stephanie Butler-Cleland, FNP-BC
Colorado Springs, CO
Continue for The Pros of Precepting >>
THE PROS OF PRECEPTING
I am an urgent care NP in urban communities on the West Coast of Florida. I had taken a break from precepting as a result of negative experiences, but I recently resumed to precept my first NP student in years.
Prior to accepting the student I precepted, I received requests from two other students. One asked if I could change my schedule to be closer to where she lived. The other clearly didn’t want to commit to the drive or the hours I was available, and asked if I would work more weekends to accommodate her schedule. Needless to say, I refused both students.
Instead, I precepted a smart 28-year-old student from my alma mater, one of the Florida state universities. She was attentive, prepared, and eager. I was very, very impressed with her. She had been a nurse for four years and was a second-semester student. It was a pleasure to have her; I like being questioned and challenged. It was fun to see her enjoying my job, and it reminded me of why I love what I do.
Anne Conklin, MS, ARNP-C
Bradenton, FL
Continue for A Scheming Industry >>
A SCHEMING INDUSTRY
Intelligent health care policy has been frustrated by the enormous amount of money brought to bear on Congress by the insurance and pharmaceutical industries. Each dollar paid to an insurance company is used to construct buildings, hire workers, create a sales staff, and ultimately pay their shareholders a profit.
Since the insurance industry obtained an antitrust exemption in the 1940s, they are essentially immune from prosecution for price collusion. Until recently, it was difficult to know how much of the money paid was returned in the form of medical benefits. In order to keep profits rising, they must enroll more people. Promising coverage while impeding medical workups and care, making great profits, and needing more and more enrollees fits the definition of a Ponzi scheme.
Several years ago in California, the state insurance commission (under threat of decertification) got an industry representative to admit that the maximum percentage of dollars used for services was 70%. In other words, for each dollar spent, a patient would be lucky to get 70 cents worth of services.
All of us who practice know how the companies do this: We request a needed diagnostic test or treatment and are denied. I have interrupted my schedule on many days to call for a “peer to peer” review—only once was I denied. This is a roadblock that many busy practitioners will not challenge. Since insurance companies market how great their coverage is, patients often get angry at the provider.
The repeated argument is that the market forces will lower medical costs. This fallacy is easily debunked by noting the ever-escalating costs and comparing health care costs as a percent of gross domestic product (GDP) in our country versus others. France, for example, expends 12% of GDP on health and ranks first in health care outcomes by world standards. In the US, we are approaching 20% of GDP.
Since insurance adds nothing to care and increases costs dramatically (every provider has to have billers for the various insurance companies, since each has its own requirements), a single-payer system is the only system that will lower costs. Those who benefit from the current system declare that we can’t have “socialized medicine.” To which I would respond, fine; we’ll continue to pay 30% to 50% more so that insurance companies can have their profits at our expense.
Nelson Herilhy, PA-C, MHS
Concord, CA
“Unprecedented” VA Proposal? We Don’t Think So
On May 25, 2016, the Department of Veterans Affairs (VA) published a proposed rule change in the Federal Register under the simple heading “Advanced Practice Registered Nurses.” From such modest beginnings stemmed a potential game-changer for advanced practice clinicians in this country: In summary, the VA proposed to “amend its medical regulations to permit full practice authority of all VA advanced practice registered nurses (APRNs) when they are acting within the scope of their VA employment.”1
The impetus for the VA’s proposal is that 505,000 veterans wait 30 days to access care within the VA system—and 300,000 wait between 31 and 60 days for health services.2 Granting plenary practice to VA APRNs would enable them to respond to this backlog of patients, since veterans would have direct access to APRNs who practice within the VA system, regardless of their state of licensure.
More than 4,800 NPs work within the VA; they provide clinical assessments, order appropriate tests and medications, and develop patient-centered care plans.2,3 Research has documented that outcomes for patients whose care is managed by NPs are equal to or better than outcomes for similar patients who are managed by physicians.4 As Major General Vincent Boles of the US Army (retired) stated, “Veterans rely on VA health care to take care of them, and the VA’s nurse practitioners are qualified to provide our veterans with the care they need and deserve.”4
Allowing veterans access to high-quality care is a 21st century solution that is “zero risk, zero cost, zero delay,” according to Dr. Cindy Cooke, President of the American Association of Nurse Practitioners (AANP).4 And it is not just the AANP that supports this rule change. Ninety-one percent of US households that are home to a veteran, and 88% of Americans overall, express support for the VA proposal. In a Mellman Group survey of more than 1,000 adults, strong support was noted across party lines (91% of Republicans; 90% of Democrats)—a rarity in our current political climate.4
Support for full practice authority for NPs at the VA has come from more than 60 organizations, including the Military Officers’ Association of America, the Air Force Sergeants Association, AARP (with 3.7 million veteran households in its membership), and 80 bipartisan members of Congress.5 At the AANP annual conference in San Antonio, Dr. Cooke was joined by leaders from the local American Legion and retired military officers who announced their support for this “change in practice.”3
However, among the more than 162,000 comments received by the VA during the public comment period, there were dissenting opinions. On July 13, 2016, Dr. Robert Wergin, Chair of the Board of the American Academy of Family Physicians (AAFP), sent a letter to Dr. David Shulkin, the Undersecretary of Health in the VA, stating that there were “significant concerns” about the rule change. His main point was that granting full practice authority to NPs would “alter the consistent standards of care for veterans over nonveterans in the states; further fragment the health care system; and dismantle physician-led team-based health care models.” He also stated that “the AAFP strongly opposes the unprecedented proposal to dismiss state practice authority regarding the authority of NPs.”6
Unprecedented? I don’t think so. I practiced as a family NP in the Navy for more than 20 years. I had my own patient panel, cared for active duty members and their families, and evaluated outcomes the same way my physician colleagues did. We practiced collaboratively and respectfully. We discussed patient plan issues, provided peer review on one another’s charts, and accepted new patients into our panels. It was a true collaborative practice.
Military nurses only need to be licensed in one state. The guidelines for NP practice were not based on the rules of the state in which we were licensed but were established by our professional practice association—just as the guidelines for physician practice were not based on the rules extant in their licensing state. I practiced successfully in many states and overseas, although I was licensed in a state that did not recognize plenary practice at the time.
The VA is attempting to respond to veterans’ need for access to care by adopting a model similar to what the military employs. It’s not a matter of superseding state regulations; it’s a matter of recognizing the education and training of health care professionals who can improve patient outcomes.
The opportunity to respond to the proposed amendment has now closed. Through its grassroots Veterans Deserve Care campaign, the AANP and its partners and supporters—clinicians, veterans, families, and others—submitted nearly 60,000 comments.2 Now we wait for the VA to review the abundance of feedback and issue their final decision.
I am hopeful that the VA will acknowledge the overwhelming evidence that our veterans deserve access to care led by highly qualified professionals. The old system isn’t working. Einstein said that the definition of insanity was to do the same thing over and over and expect a different outcome; maintaining a faulty system fits that description. NPs have a well-tested, evidence-based, high-quality education that encourages their ability to lead health care teams, perform collaboratively, and improve outcomes for those who have served our country.
Caring for active duty military and veterans is in the DNA of nurses. Florence Nightingale spent much of her post-Crimea life using evidence-based proposals and political influence to improve the health care of the soldiers and veterans of the British Empire. In Notes on Nursing, she spurred nurses to political action: “Let whoever [sic] is in charge keep this simple question in her [sic] head (not how can I always do this right thing myself, but) how can I provide for this right thing to be always done?”7 This advice should be taken to heart by all health care professionals: We can honor our veterans by advocating for and providing the health care access they need.
To share your thoughts, please contact us at [email protected]
1. Advanced practice registered nurses [2016-12338]. Fed Regist. May 25, 2016. https://federalregister.gov/a/2016-12338.
2. American Association of Nurse Practitioners. AANP and Air Force Sergeants Association urge VA to swiftly enact proposed rule. July 25, 2016. www.aanp.org/legislation-regu lation/federal-legislation/va-proposed-rule/173-press-room/2016-press-releases/ 1987-aanp-and-air-force-sergeants-associa tion-urge-va-to-swiftly-enact-proposed-rule. Accessed August 9, 2016.
3. American Association of Nurse Practitioners. AANP and veterans groups call for streamlined access to veteran’s health care. June 23, 2016. www.aanp.org/press-room/press-releases/173-press-room/2016-press-releases/1959-aanp-veteran-groups-call-for-streamlined-access-to-veterans-health-care. Accessed August 9, 2016.
4. American Association of Nurse Practitioners. National survey finds overwhelming support for VA rule granting veterans direct access to nurse practitioner care. July 20, 2016. www.aanp.org/press-room/press-releases/173-press-room/2016-press-releases/1986-national-survey-finds-overwhelming-support-for-va-rule-granting-veterans-direct-access-to-nurse-practition er-care. Accessed August 9, 2016.
5. American Association of Nurse Anesthetists. Nursing coalition and veterans groups join forces in unprecedented response to VA proposed rule to increase veterans’ access to care. June 28, 2016. www.aana.com/newsandjournal/News/Pages/062816-Nursing-Coalition-and-Veterans-Groups-Join-Forces-in-Unprecedented-Response-to-VA-Proposed-Rule.aspx. Accessed August 9, 2016.
6. Wergin RL. Letter to David Shulkin. July 13, 2016. www.aafp.org/dam/AAFP/docu ments/advocacy/workforce/scope/LT-VHA-APRN-071316.pdf. Accessed August 9, 2016 .
7. Nightingale F. Notes on Nursing: What It Is and What It Is Not. New York, NY: D. Appleton and Company; 1860.
On May 25, 2016, the Department of Veterans Affairs (VA) published a proposed rule change in the Federal Register under the simple heading “Advanced Practice Registered Nurses.” From such modest beginnings stemmed a potential game-changer for advanced practice clinicians in this country: In summary, the VA proposed to “amend its medical regulations to permit full practice authority of all VA advanced practice registered nurses (APRNs) when they are acting within the scope of their VA employment.”1
The impetus for the VA’s proposal is that 505,000 veterans wait 30 days to access care within the VA system—and 300,000 wait between 31 and 60 days for health services.2 Granting plenary practice to VA APRNs would enable them to respond to this backlog of patients, since veterans would have direct access to APRNs who practice within the VA system, regardless of their state of licensure.
More than 4,800 NPs work within the VA; they provide clinical assessments, order appropriate tests and medications, and develop patient-centered care plans.2,3 Research has documented that outcomes for patients whose care is managed by NPs are equal to or better than outcomes for similar patients who are managed by physicians.4 As Major General Vincent Boles of the US Army (retired) stated, “Veterans rely on VA health care to take care of them, and the VA’s nurse practitioners are qualified to provide our veterans with the care they need and deserve.”4
Allowing veterans access to high-quality care is a 21st century solution that is “zero risk, zero cost, zero delay,” according to Dr. Cindy Cooke, President of the American Association of Nurse Practitioners (AANP).4 And it is not just the AANP that supports this rule change. Ninety-one percent of US households that are home to a veteran, and 88% of Americans overall, express support for the VA proposal. In a Mellman Group survey of more than 1,000 adults, strong support was noted across party lines (91% of Republicans; 90% of Democrats)—a rarity in our current political climate.4
Support for full practice authority for NPs at the VA has come from more than 60 organizations, including the Military Officers’ Association of America, the Air Force Sergeants Association, AARP (with 3.7 million veteran households in its membership), and 80 bipartisan members of Congress.5 At the AANP annual conference in San Antonio, Dr. Cooke was joined by leaders from the local American Legion and retired military officers who announced their support for this “change in practice.”3
However, among the more than 162,000 comments received by the VA during the public comment period, there were dissenting opinions. On July 13, 2016, Dr. Robert Wergin, Chair of the Board of the American Academy of Family Physicians (AAFP), sent a letter to Dr. David Shulkin, the Undersecretary of Health in the VA, stating that there were “significant concerns” about the rule change. His main point was that granting full practice authority to NPs would “alter the consistent standards of care for veterans over nonveterans in the states; further fragment the health care system; and dismantle physician-led team-based health care models.” He also stated that “the AAFP strongly opposes the unprecedented proposal to dismiss state practice authority regarding the authority of NPs.”6
Unprecedented? I don’t think so. I practiced as a family NP in the Navy for more than 20 years. I had my own patient panel, cared for active duty members and their families, and evaluated outcomes the same way my physician colleagues did. We practiced collaboratively and respectfully. We discussed patient plan issues, provided peer review on one another’s charts, and accepted new patients into our panels. It was a true collaborative practice.
Military nurses only need to be licensed in one state. The guidelines for NP practice were not based on the rules of the state in which we were licensed but were established by our professional practice association—just as the guidelines for physician practice were not based on the rules extant in their licensing state. I practiced successfully in many states and overseas, although I was licensed in a state that did not recognize plenary practice at the time.
The VA is attempting to respond to veterans’ need for access to care by adopting a model similar to what the military employs. It’s not a matter of superseding state regulations; it’s a matter of recognizing the education and training of health care professionals who can improve patient outcomes.
The opportunity to respond to the proposed amendment has now closed. Through its grassroots Veterans Deserve Care campaign, the AANP and its partners and supporters—clinicians, veterans, families, and others—submitted nearly 60,000 comments.2 Now we wait for the VA to review the abundance of feedback and issue their final decision.
I am hopeful that the VA will acknowledge the overwhelming evidence that our veterans deserve access to care led by highly qualified professionals. The old system isn’t working. Einstein said that the definition of insanity was to do the same thing over and over and expect a different outcome; maintaining a faulty system fits that description. NPs have a well-tested, evidence-based, high-quality education that encourages their ability to lead health care teams, perform collaboratively, and improve outcomes for those who have served our country.
Caring for active duty military and veterans is in the DNA of nurses. Florence Nightingale spent much of her post-Crimea life using evidence-based proposals and political influence to improve the health care of the soldiers and veterans of the British Empire. In Notes on Nursing, she spurred nurses to political action: “Let whoever [sic] is in charge keep this simple question in her [sic] head (not how can I always do this right thing myself, but) how can I provide for this right thing to be always done?”7 This advice should be taken to heart by all health care professionals: We can honor our veterans by advocating for and providing the health care access they need.
To share your thoughts, please contact us at [email protected]
On May 25, 2016, the Department of Veterans Affairs (VA) published a proposed rule change in the Federal Register under the simple heading “Advanced Practice Registered Nurses.” From such modest beginnings stemmed a potential game-changer for advanced practice clinicians in this country: In summary, the VA proposed to “amend its medical regulations to permit full practice authority of all VA advanced practice registered nurses (APRNs) when they are acting within the scope of their VA employment.”1
The impetus for the VA’s proposal is that 505,000 veterans wait 30 days to access care within the VA system—and 300,000 wait between 31 and 60 days for health services.2 Granting plenary practice to VA APRNs would enable them to respond to this backlog of patients, since veterans would have direct access to APRNs who practice within the VA system, regardless of their state of licensure.
More than 4,800 NPs work within the VA; they provide clinical assessments, order appropriate tests and medications, and develop patient-centered care plans.2,3 Research has documented that outcomes for patients whose care is managed by NPs are equal to or better than outcomes for similar patients who are managed by physicians.4 As Major General Vincent Boles of the US Army (retired) stated, “Veterans rely on VA health care to take care of them, and the VA’s nurse practitioners are qualified to provide our veterans with the care they need and deserve.”4
Allowing veterans access to high-quality care is a 21st century solution that is “zero risk, zero cost, zero delay,” according to Dr. Cindy Cooke, President of the American Association of Nurse Practitioners (AANP).4 And it is not just the AANP that supports this rule change. Ninety-one percent of US households that are home to a veteran, and 88% of Americans overall, express support for the VA proposal. In a Mellman Group survey of more than 1,000 adults, strong support was noted across party lines (91% of Republicans; 90% of Democrats)—a rarity in our current political climate.4
Support for full practice authority for NPs at the VA has come from more than 60 organizations, including the Military Officers’ Association of America, the Air Force Sergeants Association, AARP (with 3.7 million veteran households in its membership), and 80 bipartisan members of Congress.5 At the AANP annual conference in San Antonio, Dr. Cooke was joined by leaders from the local American Legion and retired military officers who announced their support for this “change in practice.”3
However, among the more than 162,000 comments received by the VA during the public comment period, there were dissenting opinions. On July 13, 2016, Dr. Robert Wergin, Chair of the Board of the American Academy of Family Physicians (AAFP), sent a letter to Dr. David Shulkin, the Undersecretary of Health in the VA, stating that there were “significant concerns” about the rule change. His main point was that granting full practice authority to NPs would “alter the consistent standards of care for veterans over nonveterans in the states; further fragment the health care system; and dismantle physician-led team-based health care models.” He also stated that “the AAFP strongly opposes the unprecedented proposal to dismiss state practice authority regarding the authority of NPs.”6
Unprecedented? I don’t think so. I practiced as a family NP in the Navy for more than 20 years. I had my own patient panel, cared for active duty members and their families, and evaluated outcomes the same way my physician colleagues did. We practiced collaboratively and respectfully. We discussed patient plan issues, provided peer review on one another’s charts, and accepted new patients into our panels. It was a true collaborative practice.
Military nurses only need to be licensed in one state. The guidelines for NP practice were not based on the rules of the state in which we were licensed but were established by our professional practice association—just as the guidelines for physician practice were not based on the rules extant in their licensing state. I practiced successfully in many states and overseas, although I was licensed in a state that did not recognize plenary practice at the time.
The VA is attempting to respond to veterans’ need for access to care by adopting a model similar to what the military employs. It’s not a matter of superseding state regulations; it’s a matter of recognizing the education and training of health care professionals who can improve patient outcomes.
The opportunity to respond to the proposed amendment has now closed. Through its grassroots Veterans Deserve Care campaign, the AANP and its partners and supporters—clinicians, veterans, families, and others—submitted nearly 60,000 comments.2 Now we wait for the VA to review the abundance of feedback and issue their final decision.
I am hopeful that the VA will acknowledge the overwhelming evidence that our veterans deserve access to care led by highly qualified professionals. The old system isn’t working. Einstein said that the definition of insanity was to do the same thing over and over and expect a different outcome; maintaining a faulty system fits that description. NPs have a well-tested, evidence-based, high-quality education that encourages their ability to lead health care teams, perform collaboratively, and improve outcomes for those who have served our country.
Caring for active duty military and veterans is in the DNA of nurses. Florence Nightingale spent much of her post-Crimea life using evidence-based proposals and political influence to improve the health care of the soldiers and veterans of the British Empire. In Notes on Nursing, she spurred nurses to political action: “Let whoever [sic] is in charge keep this simple question in her [sic] head (not how can I always do this right thing myself, but) how can I provide for this right thing to be always done?”7 This advice should be taken to heart by all health care professionals: We can honor our veterans by advocating for and providing the health care access they need.
To share your thoughts, please contact us at [email protected]
1. Advanced practice registered nurses [2016-12338]. Fed Regist. May 25, 2016. https://federalregister.gov/a/2016-12338.
2. American Association of Nurse Practitioners. AANP and Air Force Sergeants Association urge VA to swiftly enact proposed rule. July 25, 2016. www.aanp.org/legislation-regu lation/federal-legislation/va-proposed-rule/173-press-room/2016-press-releases/ 1987-aanp-and-air-force-sergeants-associa tion-urge-va-to-swiftly-enact-proposed-rule. Accessed August 9, 2016.
3. American Association of Nurse Practitioners. AANP and veterans groups call for streamlined access to veteran’s health care. June 23, 2016. www.aanp.org/press-room/press-releases/173-press-room/2016-press-releases/1959-aanp-veteran-groups-call-for-streamlined-access-to-veterans-health-care. Accessed August 9, 2016.
4. American Association of Nurse Practitioners. National survey finds overwhelming support for VA rule granting veterans direct access to nurse practitioner care. July 20, 2016. www.aanp.org/press-room/press-releases/173-press-room/2016-press-releases/1986-national-survey-finds-overwhelming-support-for-va-rule-granting-veterans-direct-access-to-nurse-practition er-care. Accessed August 9, 2016.
5. American Association of Nurse Anesthetists. Nursing coalition and veterans groups join forces in unprecedented response to VA proposed rule to increase veterans’ access to care. June 28, 2016. www.aana.com/newsandjournal/News/Pages/062816-Nursing-Coalition-and-Veterans-Groups-Join-Forces-in-Unprecedented-Response-to-VA-Proposed-Rule.aspx. Accessed August 9, 2016.
6. Wergin RL. Letter to David Shulkin. July 13, 2016. www.aafp.org/dam/AAFP/docu ments/advocacy/workforce/scope/LT-VHA-APRN-071316.pdf. Accessed August 9, 2016 .
7. Nightingale F. Notes on Nursing: What It Is and What It Is Not. New York, NY: D. Appleton and Company; 1860.
1. Advanced practice registered nurses [2016-12338]. Fed Regist. May 25, 2016. https://federalregister.gov/a/2016-12338.
2. American Association of Nurse Practitioners. AANP and Air Force Sergeants Association urge VA to swiftly enact proposed rule. July 25, 2016. www.aanp.org/legislation-regu lation/federal-legislation/va-proposed-rule/173-press-room/2016-press-releases/ 1987-aanp-and-air-force-sergeants-associa tion-urge-va-to-swiftly-enact-proposed-rule. Accessed August 9, 2016.
3. American Association of Nurse Practitioners. AANP and veterans groups call for streamlined access to veteran’s health care. June 23, 2016. www.aanp.org/press-room/press-releases/173-press-room/2016-press-releases/1959-aanp-veteran-groups-call-for-streamlined-access-to-veterans-health-care. Accessed August 9, 2016.
4. American Association of Nurse Practitioners. National survey finds overwhelming support for VA rule granting veterans direct access to nurse practitioner care. July 20, 2016. www.aanp.org/press-room/press-releases/173-press-room/2016-press-releases/1986-national-survey-finds-overwhelming-support-for-va-rule-granting-veterans-direct-access-to-nurse-practition er-care. Accessed August 9, 2016.
5. American Association of Nurse Anesthetists. Nursing coalition and veterans groups join forces in unprecedented response to VA proposed rule to increase veterans’ access to care. June 28, 2016. www.aana.com/newsandjournal/News/Pages/062816-Nursing-Coalition-and-Veterans-Groups-Join-Forces-in-Unprecedented-Response-to-VA-Proposed-Rule.aspx. Accessed August 9, 2016.
6. Wergin RL. Letter to David Shulkin. July 13, 2016. www.aafp.org/dam/AAFP/docu ments/advocacy/workforce/scope/LT-VHA-APRN-071316.pdf. Accessed August 9, 2016 .
7. Nightingale F. Notes on Nursing: What It Is and What It Is Not. New York, NY: D. Appleton and Company; 1860.
When Man’s Legs “Give Out,” His Buttocks Takes the Brunt
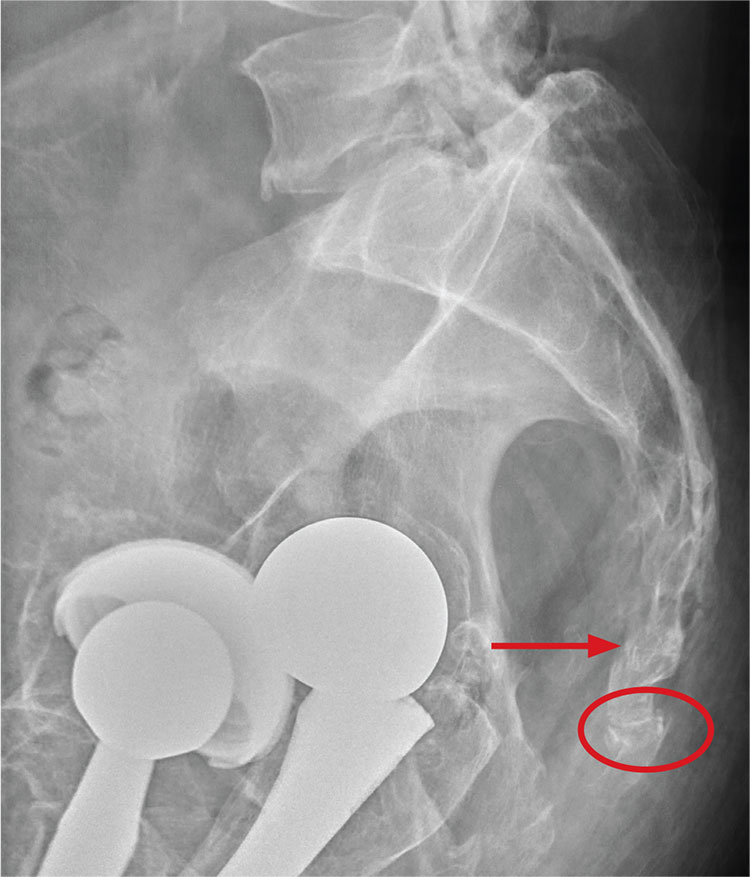
ANSWER

There are degenerative changes present. Bilateral hip prostheses are noted. Within the coccyx, there is bone remodeling and angulation that are likely chronic and related to remote trauma or injury (arrow). Below this, some cortical lucency (circled) is noted, most likely consistent with an acute fracture. The patient was prescribed a nonsteroidal medication and a mild narcotic pain medication.

ANSWER

There are degenerative changes present. Bilateral hip prostheses are noted. Within the coccyx, there is bone remodeling and angulation that are likely chronic and related to remote trauma or injury (arrow). Below this, some cortical lucency (circled) is noted, most likely consistent with an acute fracture. The patient was prescribed a nonsteroidal medication and a mild narcotic pain medication.

ANSWER

There are degenerative changes present. Bilateral hip prostheses are noted. Within the coccyx, there is bone remodeling and angulation that are likely chronic and related to remote trauma or injury (arrow). Below this, some cortical lucency (circled) is noted, most likely consistent with an acute fracture. The patient was prescribed a nonsteroidal medication and a mild narcotic pain medication.
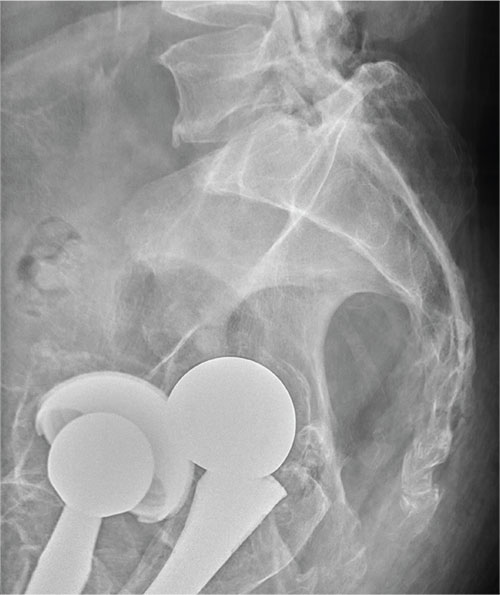
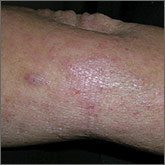
A 75-year-old man presents to the urgent care center for evaluation of pain in his buttocks after a fall. He states he was walking when his “legs gave out” and he hit the ground. He landed squarely on his buttocks, causing immediate pain. He was eventually able to get up with some assistance. He denies current weakness or any bowel or bladder complaints.
His medical/surgical history is significant for coronary artery disease, hypertension, and bilateral hip replacements. Physical exam reveals an elderly male who is uncomfortable but in no obvious distress. His vital signs are stable. He has moderate point tenderness over his sacrum but is able to move all his extremities well, with normal strength.
Radiograph of his sacrum/coccyx is shown. What is your impression?
Maybe it is all in your head

Origin of pain: Brain vs body. Recent research provides strong evidence that in some cases of intractable chronic pain, the origin of the pain signal is in the brain—rather than the body. In this issue of JFP, Davis and Vanderah discuss this type of pain as “a third kind” that needs to be treated in a manner that completely differs from that for peripherally generated pain. They refer to the traditional kinds of pain as either nociceptive (resulting from tissue damage or insult), or neuropathic (due to dysfunctional stimulation of peripheral nerves). The neurophysiology of the third kind of pain, which I will call “centrally generated pain,” is not fully understood, but neuroimaging and other sophisticated methods are identifying areas of the brain that become activated by psychological trauma, leading to significant painful suffering in the absence of tissue damage, or that is far out of proportion to physical insult.
The bad news for primary care physicians is that this third kind of pain is difficult, if not impossible, to treat with our traditional armamentarium of pain medications and physical modalities. In fact, these patients are often at risk for addiction, as doses of ineffective narcotics are escalated.
The good news is that clinical researchers have begun to identify ways to effectively treat centrally generated pain. For example, Schubiner et al used a novel psychological approach that involved helping patients "learn that their pain is influenced primarily by central nervous system psychological processes, and to enhance awareness and expression of emotions related to psychological trauma or conflict."1 Thirty percent of the 72 participants in the preliminary, uncontrolled trial experienced a 70% reduction in pain. Dr. Schubiner’s research is ongoing and supported by funding from the National Institutes of Health.
Proper diagnosis is paramount. Of course, proper diagnosis is paramount because an individual may suffer from more than one of the 3 kinds of pain and require different approaches for each. Thorough evaluation at a multidisciplinary pain clinic is a good place to start. Once the diagnoses are sorted out, it will then be possible to treat each component of pain appropriately.
Dr. Schubiner’s methods and other new and developing treatment approaches to chronic pain will help us better relieve patients’ suffering, reduce narcotic overuse, and relieve our own anxiety about caring for these challenging patients.
1. Burger AJ, Lumley MA, Carty JN, et al. The effects of a novel psychological attribution and emotional awareness and expression therapy for chronic musculoskeletal pain: a preliminary, uncontrolled trial. J Psychosom Res. 2016;81:1-8.

Origin of pain: Brain vs body. Recent research provides strong evidence that in some cases of intractable chronic pain, the origin of the pain signal is in the brain—rather than the body. In this issue of JFP, Davis and Vanderah discuss this type of pain as “a third kind” that needs to be treated in a manner that completely differs from that for peripherally generated pain. They refer to the traditional kinds of pain as either nociceptive (resulting from tissue damage or insult), or neuropathic (due to dysfunctional stimulation of peripheral nerves). The neurophysiology of the third kind of pain, which I will call “centrally generated pain,” is not fully understood, but neuroimaging and other sophisticated methods are identifying areas of the brain that become activated by psychological trauma, leading to significant painful suffering in the absence of tissue damage, or that is far out of proportion to physical insult.
The bad news for primary care physicians is that this third kind of pain is difficult, if not impossible, to treat with our traditional armamentarium of pain medications and physical modalities. In fact, these patients are often at risk for addiction, as doses of ineffective narcotics are escalated.
The good news is that clinical researchers have begun to identify ways to effectively treat centrally generated pain. For example, Schubiner et al used a novel psychological approach that involved helping patients "learn that their pain is influenced primarily by central nervous system psychological processes, and to enhance awareness and expression of emotions related to psychological trauma or conflict."1 Thirty percent of the 72 participants in the preliminary, uncontrolled trial experienced a 70% reduction in pain. Dr. Schubiner’s research is ongoing and supported by funding from the National Institutes of Health.
Proper diagnosis is paramount. Of course, proper diagnosis is paramount because an individual may suffer from more than one of the 3 kinds of pain and require different approaches for each. Thorough evaluation at a multidisciplinary pain clinic is a good place to start. Once the diagnoses are sorted out, it will then be possible to treat each component of pain appropriately.
Dr. Schubiner’s methods and other new and developing treatment approaches to chronic pain will help us better relieve patients’ suffering, reduce narcotic overuse, and relieve our own anxiety about caring for these challenging patients.
1. Burger AJ, Lumley MA, Carty JN, et al. The effects of a novel psychological attribution and emotional awareness and expression therapy for chronic musculoskeletal pain: a preliminary, uncontrolled trial. J Psychosom Res. 2016;81:1-8.

Origin of pain: Brain vs body. Recent research provides strong evidence that in some cases of intractable chronic pain, the origin of the pain signal is in the brain—rather than the body. In this issue of JFP, Davis and Vanderah discuss this type of pain as “a third kind” that needs to be treated in a manner that completely differs from that for peripherally generated pain. They refer to the traditional kinds of pain as either nociceptive (resulting from tissue damage or insult), or neuropathic (due to dysfunctional stimulation of peripheral nerves). The neurophysiology of the third kind of pain, which I will call “centrally generated pain,” is not fully understood, but neuroimaging and other sophisticated methods are identifying areas of the brain that become activated by psychological trauma, leading to significant painful suffering in the absence of tissue damage, or that is far out of proportion to physical insult.
The bad news for primary care physicians is that this third kind of pain is difficult, if not impossible, to treat with our traditional armamentarium of pain medications and physical modalities. In fact, these patients are often at risk for addiction, as doses of ineffective narcotics are escalated.
The good news is that clinical researchers have begun to identify ways to effectively treat centrally generated pain. For example, Schubiner et al used a novel psychological approach that involved helping patients "learn that their pain is influenced primarily by central nervous system psychological processes, and to enhance awareness and expression of emotions related to psychological trauma or conflict."1 Thirty percent of the 72 participants in the preliminary, uncontrolled trial experienced a 70% reduction in pain. Dr. Schubiner’s research is ongoing and supported by funding from the National Institutes of Health.
Proper diagnosis is paramount. Of course, proper diagnosis is paramount because an individual may suffer from more than one of the 3 kinds of pain and require different approaches for each. Thorough evaluation at a multidisciplinary pain clinic is a good place to start. Once the diagnoses are sorted out, it will then be possible to treat each component of pain appropriately.
Dr. Schubiner’s methods and other new and developing treatment approaches to chronic pain will help us better relieve patients’ suffering, reduce narcotic overuse, and relieve our own anxiety about caring for these challenging patients.
1. Burger AJ, Lumley MA, Carty JN, et al. The effects of a novel psychological attribution and emotional awareness and expression therapy for chronic musculoskeletal pain: a preliminary, uncontrolled trial. J Psychosom Res. 2016;81:1-8.
Why did testing stop at EKG—especially given family history? ... More
Why did testing stop at EKG—especially given family history?
AFTER COMPLAINING OF CHEST PAIN, a 37-year-old man underwent an electrocardiogram (EKG) examination. The doctor concluded that the pain was not cardiac in nature. Two years later, the patient died of a sudden cardiac event associated with coronary atherosclerotic disease.
PLAINTIFF’S CLAIM The decedent suffered from high cholesterol and had a family history of cardiac issues, yet no additional testing was performed when the patient’s complaints continued.
THE DEFENSE No information on the defense is available.
VERDICT $3 million settlement.
COMMENT This is déjà vu for me. A colleague of mine had a nearly identical case a few years ago, but the patient died several days later. In the case described here, the high cholesterol and family history were red flags. A normal EKG does not rule out angina. I do wonder what happened, however, in the 2 years between the office visit and the patient’s sudden death. The chest pain at the office visit may well have been non-cardiac, but it appears the jury was not convinced.
2 FPs overlook boy’s proteinuria; delay in Dx costs him a kidney
AN 11-YEAR-OLD BOY underwent a laparoscopic appendectomy that included a urinalysis. Following the surgery, the surgeon notified the family physician (FP) that the patient’s urinalysis showed >300 mg/dL of protein. The result was unusual and required follow-up. The surgeon felt that the urinalysis result might be related to the proximity of the appendicitis to the boy’s ureter. The boy was evaluated on several other occasions by the FP, but no work-up was performed.
Three years later, the boy saw a different FP, who noted that the child had elevated blood pressure and blurry vision—among other symptoms. The boy’s renal function tests were documented as abnormal; however, the patient and his mother were never notified of this. Also, the patient was never referred to a nephrologist or neurologist and there was no intervention for a potential kidney abnormality.
Two years later, an associate of the FP ordered further blood tests that showed a clear abnormality with regard to the integrity of the child’s kidney function. The boy was evaluated at a hospital and diagnosed with end-stage renal disease. He received a kidney transplant 3 months later and requires lifetime medical care as a result of the transplant. The boy will likely require further transplants in 10-year increments.
PLAINTIFF’S CLAIM Both FPs deviated from the accepted standard of care when they failed to order further testing as a result of the abnormal laboratory tests. Earlier intervention may have prolonged the life of the boy’s kidney, thereby postponing the need for kidney replacements.
THE DEFENSE No information on the defense is available.
VERDICT $1.25 million Massachusetts settlement.
COMMENT 300 mg/dL is a significant amount of proteinuria and requires further testing. Why didn’t the FP follow up? Was a summary of the hospitalization sent to him/her? Certainly the diagnosis should have been made by the second FP, and the patient should’ve been referred to a nephrologist. A lawsuit would most likely have been averted had this happened. Delayed diagnosis accounts for a high proportion of malpractice suits against FPs.
Duodenal ulcer mistakenly attributed to Crohn’s disease
A 47-YEAR-OLD MAN with a history of Crohn’s disease began experiencing persistent abdominal pain. He hadn’t had symptoms of his Crohn’s disease in over 12 years. Nevertheless, doctors diagnosed his pain as an aggravation of the disease and gave him treatment based on this diagnosis. In fact, though, the man had an acute duodenal ulcer that had progressed and perforated. The patient underwent 12 surgeries (with complications) and almost 2 years of near-constant hospitalization as a result of the misdiagnosis. He now requires 24-hour care in all aspects of his life.
PLAINTIFF’S CLAIM The doctors were negligent in their failure to consider and diagnose a peptic ulcer when the plaintiff’s symptoms indicated issues other than Crohn’s disease.
THE DEFENSE No information on the defense is available.
VERDICT $28 million Maryland verdict.
COMMENT I suspect this was a tough diagnosis, given the patient’s prior history of Crohn’s disease. We are not told the nature of the abdominal pain. If the patient had classic epigastric pain, peptic ulcer disease should have been investigated. This case serves as a reminder that patients can have more than one disease of an organ system, and it reminds us of the need for a careful history and close follow-up if a complaint does not resolve.
Why did testing stop at EKG—especially given family history?
AFTER COMPLAINING OF CHEST PAIN, a 37-year-old man underwent an electrocardiogram (EKG) examination. The doctor concluded that the pain was not cardiac in nature. Two years later, the patient died of a sudden cardiac event associated with coronary atherosclerotic disease.
PLAINTIFF’S CLAIM The decedent suffered from high cholesterol and had a family history of cardiac issues, yet no additional testing was performed when the patient’s complaints continued.
THE DEFENSE No information on the defense is available.
VERDICT $3 million settlement.
COMMENT This is déjà vu for me. A colleague of mine had a nearly identical case a few years ago, but the patient died several days later. In the case described here, the high cholesterol and family history were red flags. A normal EKG does not rule out angina. I do wonder what happened, however, in the 2 years between the office visit and the patient’s sudden death. The chest pain at the office visit may well have been non-cardiac, but it appears the jury was not convinced.
2 FPs overlook boy’s proteinuria; delay in Dx costs him a kidney
AN 11-YEAR-OLD BOY underwent a laparoscopic appendectomy that included a urinalysis. Following the surgery, the surgeon notified the family physician (FP) that the patient’s urinalysis showed >300 mg/dL of protein. The result was unusual and required follow-up. The surgeon felt that the urinalysis result might be related to the proximity of the appendicitis to the boy’s ureter. The boy was evaluated on several other occasions by the FP, but no work-up was performed.
Three years later, the boy saw a different FP, who noted that the child had elevated blood pressure and blurry vision—among other symptoms. The boy’s renal function tests were documented as abnormal; however, the patient and his mother were never notified of this. Also, the patient was never referred to a nephrologist or neurologist and there was no intervention for a potential kidney abnormality.
Two years later, an associate of the FP ordered further blood tests that showed a clear abnormality with regard to the integrity of the child’s kidney function. The boy was evaluated at a hospital and diagnosed with end-stage renal disease. He received a kidney transplant 3 months later and requires lifetime medical care as a result of the transplant. The boy will likely require further transplants in 10-year increments.
PLAINTIFF’S CLAIM Both FPs deviated from the accepted standard of care when they failed to order further testing as a result of the abnormal laboratory tests. Earlier intervention may have prolonged the life of the boy’s kidney, thereby postponing the need for kidney replacements.
THE DEFENSE No information on the defense is available.
VERDICT $1.25 million Massachusetts settlement.
COMMENT 300 mg/dL is a significant amount of proteinuria and requires further testing. Why didn’t the FP follow up? Was a summary of the hospitalization sent to him/her? Certainly the diagnosis should have been made by the second FP, and the patient should’ve been referred to a nephrologist. A lawsuit would most likely have been averted had this happened. Delayed diagnosis accounts for a high proportion of malpractice suits against FPs.
Duodenal ulcer mistakenly attributed to Crohn’s disease
A 47-YEAR-OLD MAN with a history of Crohn’s disease began experiencing persistent abdominal pain. He hadn’t had symptoms of his Crohn’s disease in over 12 years. Nevertheless, doctors diagnosed his pain as an aggravation of the disease and gave him treatment based on this diagnosis. In fact, though, the man had an acute duodenal ulcer that had progressed and perforated. The patient underwent 12 surgeries (with complications) and almost 2 years of near-constant hospitalization as a result of the misdiagnosis. He now requires 24-hour care in all aspects of his life.
PLAINTIFF’S CLAIM The doctors were negligent in their failure to consider and diagnose a peptic ulcer when the plaintiff’s symptoms indicated issues other than Crohn’s disease.
THE DEFENSE No information on the defense is available.
VERDICT $28 million Maryland verdict.
COMMENT I suspect this was a tough diagnosis, given the patient’s prior history of Crohn’s disease. We are not told the nature of the abdominal pain. If the patient had classic epigastric pain, peptic ulcer disease should have been investigated. This case serves as a reminder that patients can have more than one disease of an organ system, and it reminds us of the need for a careful history and close follow-up if a complaint does not resolve.
Why did testing stop at EKG—especially given family history?
AFTER COMPLAINING OF CHEST PAIN, a 37-year-old man underwent an electrocardiogram (EKG) examination. The doctor concluded that the pain was not cardiac in nature. Two years later, the patient died of a sudden cardiac event associated with coronary atherosclerotic disease.
PLAINTIFF’S CLAIM The decedent suffered from high cholesterol and had a family history of cardiac issues, yet no additional testing was performed when the patient’s complaints continued.
THE DEFENSE No information on the defense is available.
VERDICT $3 million settlement.
COMMENT This is déjà vu for me. A colleague of mine had a nearly identical case a few years ago, but the patient died several days later. In the case described here, the high cholesterol and family history were red flags. A normal EKG does not rule out angina. I do wonder what happened, however, in the 2 years between the office visit and the patient’s sudden death. The chest pain at the office visit may well have been non-cardiac, but it appears the jury was not convinced.
2 FPs overlook boy’s proteinuria; delay in Dx costs him a kidney
AN 11-YEAR-OLD BOY underwent a laparoscopic appendectomy that included a urinalysis. Following the surgery, the surgeon notified the family physician (FP) that the patient’s urinalysis showed >300 mg/dL of protein. The result was unusual and required follow-up. The surgeon felt that the urinalysis result might be related to the proximity of the appendicitis to the boy’s ureter. The boy was evaluated on several other occasions by the FP, but no work-up was performed.
Three years later, the boy saw a different FP, who noted that the child had elevated blood pressure and blurry vision—among other symptoms. The boy’s renal function tests were documented as abnormal; however, the patient and his mother were never notified of this. Also, the patient was never referred to a nephrologist or neurologist and there was no intervention for a potential kidney abnormality.
Two years later, an associate of the FP ordered further blood tests that showed a clear abnormality with regard to the integrity of the child’s kidney function. The boy was evaluated at a hospital and diagnosed with end-stage renal disease. He received a kidney transplant 3 months later and requires lifetime medical care as a result of the transplant. The boy will likely require further transplants in 10-year increments.
PLAINTIFF’S CLAIM Both FPs deviated from the accepted standard of care when they failed to order further testing as a result of the abnormal laboratory tests. Earlier intervention may have prolonged the life of the boy’s kidney, thereby postponing the need for kidney replacements.
THE DEFENSE No information on the defense is available.
VERDICT $1.25 million Massachusetts settlement.
COMMENT 300 mg/dL is a significant amount of proteinuria and requires further testing. Why didn’t the FP follow up? Was a summary of the hospitalization sent to him/her? Certainly the diagnosis should have been made by the second FP, and the patient should’ve been referred to a nephrologist. A lawsuit would most likely have been averted had this happened. Delayed diagnosis accounts for a high proportion of malpractice suits against FPs.
Duodenal ulcer mistakenly attributed to Crohn’s disease
A 47-YEAR-OLD MAN with a history of Crohn’s disease began experiencing persistent abdominal pain. He hadn’t had symptoms of his Crohn’s disease in over 12 years. Nevertheless, doctors diagnosed his pain as an aggravation of the disease and gave him treatment based on this diagnosis. In fact, though, the man had an acute duodenal ulcer that had progressed and perforated. The patient underwent 12 surgeries (with complications) and almost 2 years of near-constant hospitalization as a result of the misdiagnosis. He now requires 24-hour care in all aspects of his life.
PLAINTIFF’S CLAIM The doctors were negligent in their failure to consider and diagnose a peptic ulcer when the plaintiff’s symptoms indicated issues other than Crohn’s disease.
THE DEFENSE No information on the defense is available.
VERDICT $28 million Maryland verdict.
COMMENT I suspect this was a tough diagnosis, given the patient’s prior history of Crohn’s disease. We are not told the nature of the abdominal pain. If the patient had classic epigastric pain, peptic ulcer disease should have been investigated. This case serves as a reminder that patients can have more than one disease of an organ system, and it reminds us of the need for a careful history and close follow-up if a complaint does not resolve.
Need-to-know information for the 2016-2017 flu season
The Advisory Committee on Immunization Practices (ACIP) took the unusual step at its June 2016 meeting of recommending against using a currently licensed vaccine, live attenuated influenza vaccine (LAIV), in the 2016-2017 influenza season.1 ACIP based its recommendation on surveillance data collected by the US Influenza Vaccine Effectiveness Network of the Centers for Disease Control and Prevention (CDC), which showed poor effectiveness by the LAIV vaccine among children and adolescents during the past 3 years.
The US Food and Drug Administration (FDA), however, has chosen not to take any action on this matter, saying on its Web site it “has determined that specific regulatory action is not warranted at this time. This determination is based on FDA’s review of manufacturing and clinical data supporting licensure … the totality of the evidence presented at the ACIP meeting, taking into account the inherent limitations of observational studies conducted to evaluate influenza vaccine effectiveness, as well as the well-known variability of influenza vaccine effectiveness across influenza seasons.”2
CDC data for the 2015-2016 flu season showed the effectiveness of LAIV to be just 3% among children 2 years through 17 years of age.3 The reason for this apparent lack of effectiveness is unknown. Other LAIV-effectiveness studies conducted in the 2015-2016 season—one each, in the United States, United Kingdom, and Finland—had results that differed from the CDC surveillance data, with effectiveness ranging from 46% to 58% against all strains combined.2 These results are comparable to vaccine effectiveness found in observational studies in children for both LAIV and inactivated influenza vaccines (IIV) in prior seasons.2
Vaccine manufacturers had projected that 171 to 176 million doses of flu vaccine, in all forms, would be available in the United States during the 2016-2017 season.3 LAIV accounts for about 8% of the total supply of influenza vaccine in the United States,3 and ACIP’s recommendation is not expected to create shortages of other options for the upcoming season. However, the LAIV accounts for one-third of flu vaccines administered to children, and clinicians who provide vaccinations to children have already ordered their vaccine supplies for the upcoming season. Also, it is not clear if children who have previously received the LAIV product will now accept other options for influenza vaccination—all of which involve an injection.
Whether the recommendation against LAIV will continue after this season is also unknown.
What happened during the 2015-2016 influenza season?
The 2015-2016 influenza season was relatively mild with the peak activity occurring in March, somewhat later than in previous years. The circulating influenza strains matched closely to those in the vaccine, making it more effective than the previous year’s vaccine. The predominant circulating strain was A (H1N1), accounting for 58% of illness; A (H3N2) caused 6% of cases and all B types together accounted for 34%.4 The hospitalization rate for all ages was 31.3/100,000 compared with 64.1 the year before.5 There were 85 pediatric deaths compared with 148 in 2014-2015.6
Vaccine effectiveness among all age groups and against all circulating strains was 47%.4 No major vaccine safety concerns were detected. Among those who received IIV3, there was a slight increase in the incidence of Guillain-Barré syndrome of 2.6 cases per one million vaccines.7
Other recommendations for 2016-2017
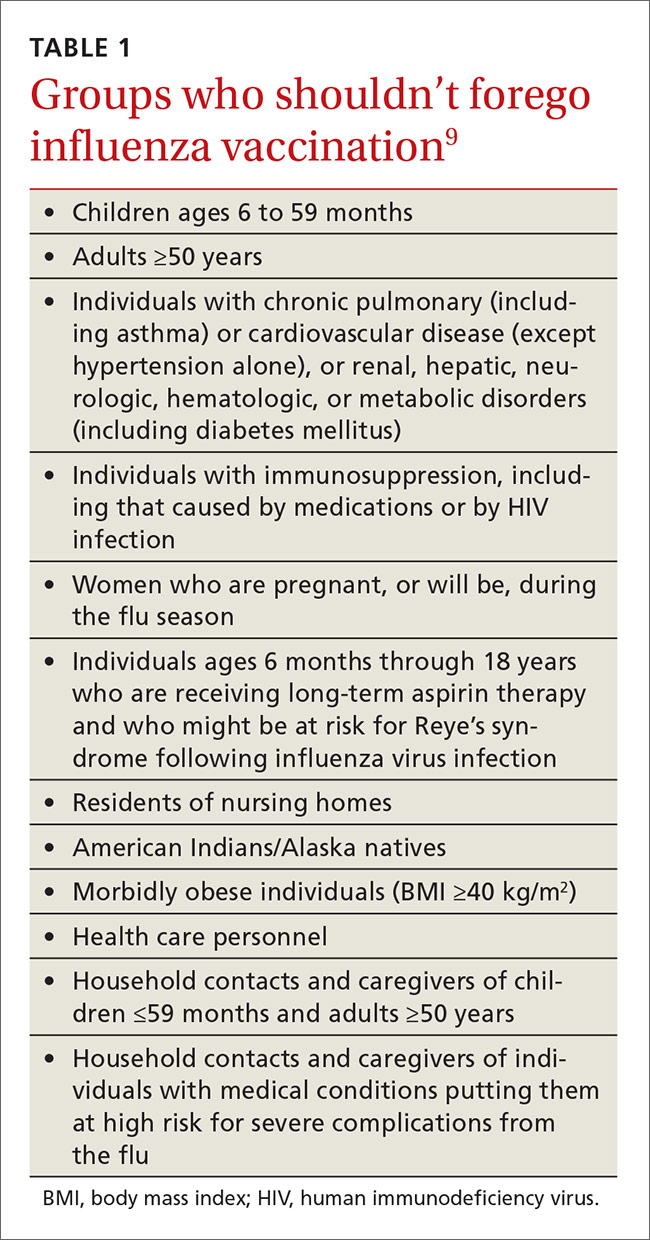
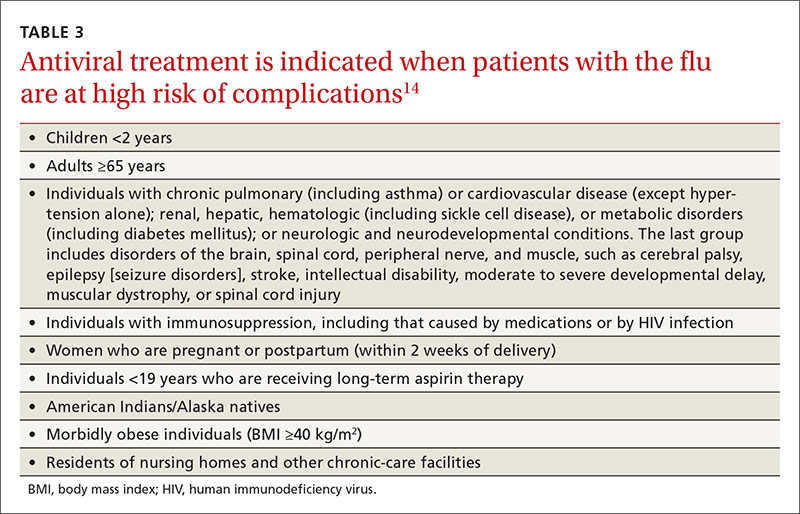
Once again, ACIP recommends influenza vaccine for all individuals 6 months and older.8 The CDC additionally specifies particular groups that should not skip vaccination given that they are at high risk of complications from influenza infection or because they could expose high-risk individuals to infection (TABLE 1).9
There will continue to be a selection of trivalent and quadrivalent influenza vaccine products in 2016-2017. Trivalent products will contain 3 viral strains: A/California/7/2009 (H1N1), A/Hong Kong/4801/2014 (H3N2) and B/Brisbane/60/2008.10 The quadrivalent products will contain those 3 antigens plus B/Phuket/3073/2013.10 The H3N2 strain is different from the one in last year’s vaccine. Each year, influenza experts analyze surveillance data to predict which circulating strains will predominate in North America, and these antigens constitute the vaccine formulation. The accuracy of this prediction in large part determines how effective the vaccine will be that season.
Two new vaccines have been approved for use in the United States. A quadrivalent cell culture inactivated vaccine (CCIV4), Flucelvax, was licensed in May 2016. It is prepared from virus propagated in canine kidney cells, not with an egg-based production process. It is approved for use in individuals 4 years of age and older.8 Fluad, an adjuvanted trivalent inactivated influenza vaccine, was licensed in late 2015 for individuals 65 years of age and older.8 This is the first adjuvanted influenza vaccine licensed in the United States and will compete with high-dose quadrivalent vaccine for use in older adults. ACIP does not express a preference for any vaccine in this age group.
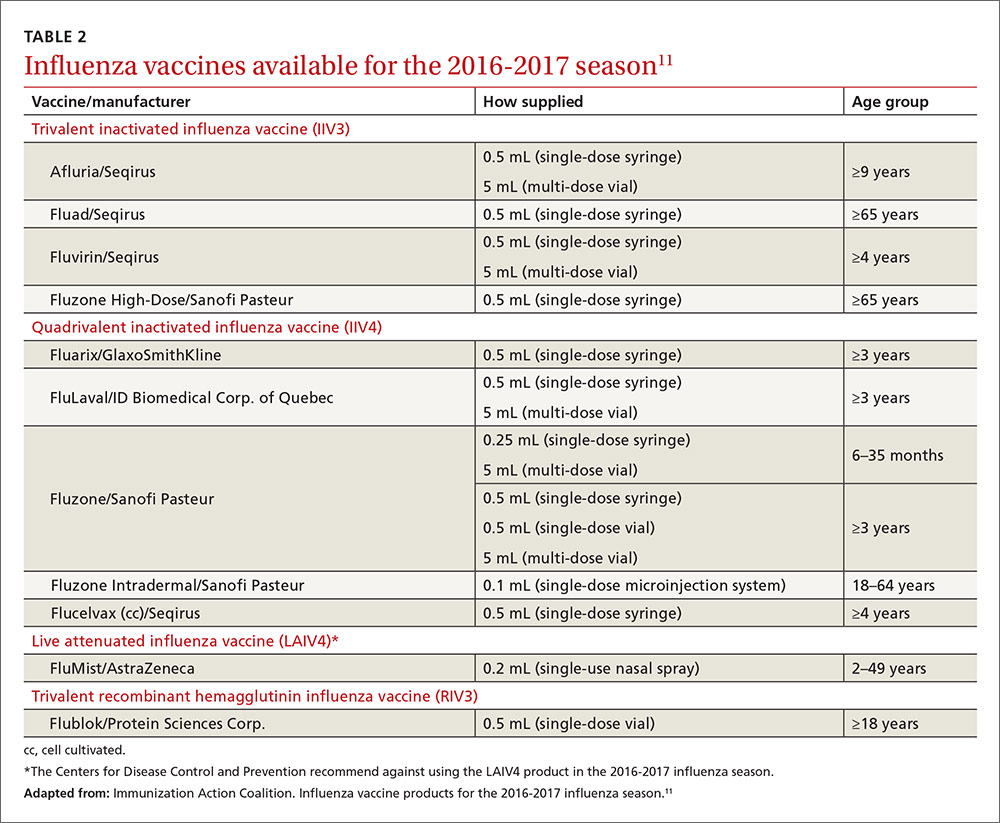
Two other vaccines should also be available by this fall: Flublok, a quadrivalent recombinant influenza vaccine for individuals 18 years and older, and Flulaval, a quadrivalent inactivated influenza vaccine, for individuals 6 months of age and older. TABLE 211 lists approved influenza vaccines.
Issues specific to children
Deciding how many vaccine doses children need has been further simplified. Children younger than 9 years need 2 doses if they have received fewer than 2 doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The interval between the 2 doses should be at least 4 weeks. The 2 doses do not have to be the same product; importantly, do not delay a second dose just to obtain the same product used for the first dose. Also, one dose can be trivalent and the other one quadrivalent, although this offers less-than-optimal protection against the B-virus that is only in the quadrivalent product.
Children younger than 9 years require only one dose if they have received 2 or more total doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The 2 previous doses need not have been received during the same influenza season or consecutive influenza seasons.
In children ages 6 through 23 months there is a slight increased risk of febrile seizure if the influenza vaccine is co-administered with other vaccines, specifically pneumococcal conjugate vaccine (PCV 13) and diphtheria-tetanus-acellular-pertussis (DTaP). The 3 vaccines administered at the same time result in 30 febrile seizures per 100,000 children;12 the rate is lower when influenza vaccine is co-administered with only one of the others. ACIP believes that the risk of a febrile seizure, which does no long-term harm, does not warrant delaying vaccines that could be co-administered.13
Egg allergy requires no special precautions
Evidence continues to grow that influenza vaccine products do not contain enough egg protein to cause significant problems in those with a history of egg allergies. This year’s recommendations state that no special precautions are needed regarding the anatomic site of immunization or the length of observation after administering influenza vaccine in those with a history of allergies to eggs, no matter how severe. All vaccine-administration facilities should be able to respond to any hypersensitivity reaction, and the standard waiting time for observation after all vaccinations is 15 minutes.
Antiviral medications for treatment or prevention
Most influenza strains circulating in 2016-2017 are expected to remain sensitive to oseltamivir and zanamivir, which can be used for treatment or disease prevention. A third neuraminidase inhibitor, peramivir, is available for intravenous use in adults 18 and older. Treatment is recommended for those who have confirmed or suspected influenza and are at high risk for complications (TABLE 3).14 Consideration of antiviral chemoprevention is recommended under certain circumstances (TABLE 4).15,16 The CDC influenza Web site lists recommended doses and duration for each antiviral for treatment and chemoprevention.15

1. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2016-17 influenza season. MMWR Recomm Rep. 2016;65:1-54.
2. U.S. Food and Drug Administration. FDA information regarding FluMist quadrivalent vaccine. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm508761.htm. Accessed July 13, 2016.
3. Centers for Disease Control and Prevention. ACIP votes down use of LAIV for 2016-2017 flu season. Available at: http://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Accessed July 13, 2016.
4. Flannery B, Chung J. Influenza vaccine effectiveness, including LAIV vs IIV in children and adolescents, US Flu VE Network, 2015-2016. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-05-flannery.pdf. Accessed July 22, 2016.
5. Centers for Disease Control and Prevention. FluView. Laboratory-confirmed influenza hospitalizations. Available at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html. Accessed July 25, 2016.
6. Centers for Disease Control and Prevention. FluView. Number of influenza-associated pediatric deaths by week of death. Available at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html. Accessed July 25, 2016.
7. Shimabukuro T. End-of-season update: 2015-2016 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-04-shimabukuro.pdf. Accessed July 22, 2016.
8. Grohskopf L. Proposed recommendations 2016-2017 influenza season. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-08-grohskopf.pdf. Accessed July 22, 2016.
9. Centers for Disease Control and Prevention. Influenza vaccination: a summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/vaccination/vax-summary.htm. Accessed July 13, 2016.
10. Centers for Disease Control and Prevention. What you should know for the 2016-2017 influenza season. Available at: http://www.cdc.gov/flu/about/season/flu-season-2016-2017.htm. Accessed July 13, 2016.
11. Immunization Action Coalition. Influenza vaccine products for the 2016-2017 influenza season. Available at: http://www.immunize.org/catg.d/p4072.pdf. Accessed July 13, 2016.
12. Duffy J, Weintraub E, Hambidge SJ, et al. Febrile seizure risk after vaccination in children 6 to 23 months. Pediatrics. 2016;138.
13. Centers for Disease Control and Prevention. Childhood vaccines and febrile seizures. Available at: http://www.cdc.gov/vaccinesafety/concerns/febrile-seizures.html. Accessed August 11, 2016.
14. Centers for Disease Control and Prevention. Use of antivirals. Background and guidance on the use of influenza antiviral agents. Available at: http://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm. Accessed July 13, 2016.
15. Centers for Disease Control and Prevention. Influenza antiviral medications: summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed July 13, 2016.
16. American Academy of Pediatrics. Recommendations for prevention and control of influenza in children, 2015-2016. Pediatrics. 2015;136:792-808.
The Advisory Committee on Immunization Practices (ACIP) took the unusual step at its June 2016 meeting of recommending against using a currently licensed vaccine, live attenuated influenza vaccine (LAIV), in the 2016-2017 influenza season.1 ACIP based its recommendation on surveillance data collected by the US Influenza Vaccine Effectiveness Network of the Centers for Disease Control and Prevention (CDC), which showed poor effectiveness by the LAIV vaccine among children and adolescents during the past 3 years.
The US Food and Drug Administration (FDA), however, has chosen not to take any action on this matter, saying on its Web site it “has determined that specific regulatory action is not warranted at this time. This determination is based on FDA’s review of manufacturing and clinical data supporting licensure … the totality of the evidence presented at the ACIP meeting, taking into account the inherent limitations of observational studies conducted to evaluate influenza vaccine effectiveness, as well as the well-known variability of influenza vaccine effectiveness across influenza seasons.”2
CDC data for the 2015-2016 flu season showed the effectiveness of LAIV to be just 3% among children 2 years through 17 years of age.3 The reason for this apparent lack of effectiveness is unknown. Other LAIV-effectiveness studies conducted in the 2015-2016 season—one each, in the United States, United Kingdom, and Finland—had results that differed from the CDC surveillance data, with effectiveness ranging from 46% to 58% against all strains combined.2 These results are comparable to vaccine effectiveness found in observational studies in children for both LAIV and inactivated influenza vaccines (IIV) in prior seasons.2
Vaccine manufacturers had projected that 171 to 176 million doses of flu vaccine, in all forms, would be available in the United States during the 2016-2017 season.3 LAIV accounts for about 8% of the total supply of influenza vaccine in the United States,3 and ACIP’s recommendation is not expected to create shortages of other options for the upcoming season. However, the LAIV accounts for one-third of flu vaccines administered to children, and clinicians who provide vaccinations to children have already ordered their vaccine supplies for the upcoming season. Also, it is not clear if children who have previously received the LAIV product will now accept other options for influenza vaccination—all of which involve an injection.
Whether the recommendation against LAIV will continue after this season is also unknown.
What happened during the 2015-2016 influenza season?
The 2015-2016 influenza season was relatively mild with the peak activity occurring in March, somewhat later than in previous years. The circulating influenza strains matched closely to those in the vaccine, making it more effective than the previous year’s vaccine. The predominant circulating strain was A (H1N1), accounting for 58% of illness; A (H3N2) caused 6% of cases and all B types together accounted for 34%.4 The hospitalization rate for all ages was 31.3/100,000 compared with 64.1 the year before.5 There were 85 pediatric deaths compared with 148 in 2014-2015.6
Vaccine effectiveness among all age groups and against all circulating strains was 47%.4 No major vaccine safety concerns were detected. Among those who received IIV3, there was a slight increase in the incidence of Guillain-Barré syndrome of 2.6 cases per one million vaccines.7
Other recommendations for 2016-2017
Once again, ACIP recommends influenza vaccine for all individuals 6 months and older.8 The CDC additionally specifies particular groups that should not skip vaccination given that they are at high risk of complications from influenza infection or because they could expose high-risk individuals to infection (TABLE 1).9

There will continue to be a selection of trivalent and quadrivalent influenza vaccine products in 2016-2017. Trivalent products will contain 3 viral strains: A/California/7/2009 (H1N1), A/Hong Kong/4801/2014 (H3N2) and B/Brisbane/60/2008.10 The quadrivalent products will contain those 3 antigens plus B/Phuket/3073/2013.10 The H3N2 strain is different from the one in last year’s vaccine. Each year, influenza experts analyze surveillance data to predict which circulating strains will predominate in North America, and these antigens constitute the vaccine formulation. The accuracy of this prediction in large part determines how effective the vaccine will be that season.
Two new vaccines have been approved for use in the United States. A quadrivalent cell culture inactivated vaccine (CCIV4), Flucelvax, was licensed in May 2016. It is prepared from virus propagated in canine kidney cells, not with an egg-based production process. It is approved for use in individuals 4 years of age and older.8 Fluad, an adjuvanted trivalent inactivated influenza vaccine, was licensed in late 2015 for individuals 65 years of age and older.8 This is the first adjuvanted influenza vaccine licensed in the United States and will compete with high-dose quadrivalent vaccine for use in older adults. ACIP does not express a preference for any vaccine in this age group.
Two other vaccines should also be available by this fall: Flublok, a quadrivalent recombinant influenza vaccine for individuals 18 years and older, and Flulaval, a quadrivalent inactivated influenza vaccine, for individuals 6 months of age and older. TABLE 211 lists approved influenza vaccines.

Issues specific to children
Deciding how many vaccine doses children need has been further simplified. Children younger than 9 years need 2 doses if they have received fewer than 2 doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The interval between the 2 doses should be at least 4 weeks. The 2 doses do not have to be the same product; importantly, do not delay a second dose just to obtain the same product used for the first dose. Also, one dose can be trivalent and the other one quadrivalent, although this offers less-than-optimal protection against the B-virus that is only in the quadrivalent product.
Children younger than 9 years require only one dose if they have received 2 or more total doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The 2 previous doses need not have been received during the same influenza season or consecutive influenza seasons.
In children ages 6 through 23 months there is a slight increased risk of febrile seizure if the influenza vaccine is co-administered with other vaccines, specifically pneumococcal conjugate vaccine (PCV 13) and diphtheria-tetanus-acellular-pertussis (DTaP). The 3 vaccines administered at the same time result in 30 febrile seizures per 100,000 children;12 the rate is lower when influenza vaccine is co-administered with only one of the others. ACIP believes that the risk of a febrile seizure, which does no long-term harm, does not warrant delaying vaccines that could be co-administered.13
Egg allergy requires no special precautions
Evidence continues to grow that influenza vaccine products do not contain enough egg protein to cause significant problems in those with a history of egg allergies. This year’s recommendations state that no special precautions are needed regarding the anatomic site of immunization or the length of observation after administering influenza vaccine in those with a history of allergies to eggs, no matter how severe. All vaccine-administration facilities should be able to respond to any hypersensitivity reaction, and the standard waiting time for observation after all vaccinations is 15 minutes.

Antiviral medications for treatment or prevention
Most influenza strains circulating in 2016-2017 are expected to remain sensitive to oseltamivir and zanamivir, which can be used for treatment or disease prevention. A third neuraminidase inhibitor, peramivir, is available for intravenous use in adults 18 and older. Treatment is recommended for those who have confirmed or suspected influenza and are at high risk for complications (TABLE 3).14 Consideration of antiviral chemoprevention is recommended under certain circumstances (TABLE 4).15,16 The CDC influenza Web site lists recommended doses and duration for each antiviral for treatment and chemoprevention.15

The Advisory Committee on Immunization Practices (ACIP) took the unusual step at its June 2016 meeting of recommending against using a currently licensed vaccine, live attenuated influenza vaccine (LAIV), in the 2016-2017 influenza season.1 ACIP based its recommendation on surveillance data collected by the US Influenza Vaccine Effectiveness Network of the Centers for Disease Control and Prevention (CDC), which showed poor effectiveness by the LAIV vaccine among children and adolescents during the past 3 years.
The US Food and Drug Administration (FDA), however, has chosen not to take any action on this matter, saying on its Web site it “has determined that specific regulatory action is not warranted at this time. This determination is based on FDA’s review of manufacturing and clinical data supporting licensure … the totality of the evidence presented at the ACIP meeting, taking into account the inherent limitations of observational studies conducted to evaluate influenza vaccine effectiveness, as well as the well-known variability of influenza vaccine effectiveness across influenza seasons.”2
CDC data for the 2015-2016 flu season showed the effectiveness of LAIV to be just 3% among children 2 years through 17 years of age.3 The reason for this apparent lack of effectiveness is unknown. Other LAIV-effectiveness studies conducted in the 2015-2016 season—one each, in the United States, United Kingdom, and Finland—had results that differed from the CDC surveillance data, with effectiveness ranging from 46% to 58% against all strains combined.2 These results are comparable to vaccine effectiveness found in observational studies in children for both LAIV and inactivated influenza vaccines (IIV) in prior seasons.2
Vaccine manufacturers had projected that 171 to 176 million doses of flu vaccine, in all forms, would be available in the United States during the 2016-2017 season.3 LAIV accounts for about 8% of the total supply of influenza vaccine in the United States,3 and ACIP’s recommendation is not expected to create shortages of other options for the upcoming season. However, the LAIV accounts for one-third of flu vaccines administered to children, and clinicians who provide vaccinations to children have already ordered their vaccine supplies for the upcoming season. Also, it is not clear if children who have previously received the LAIV product will now accept other options for influenza vaccination—all of which involve an injection.
Whether the recommendation against LAIV will continue after this season is also unknown.
What happened during the 2015-2016 influenza season?
The 2015-2016 influenza season was relatively mild with the peak activity occurring in March, somewhat later than in previous years. The circulating influenza strains matched closely to those in the vaccine, making it more effective than the previous year’s vaccine. The predominant circulating strain was A (H1N1), accounting for 58% of illness; A (H3N2) caused 6% of cases and all B types together accounted for 34%.4 The hospitalization rate for all ages was 31.3/100,000 compared with 64.1 the year before.5 There were 85 pediatric deaths compared with 148 in 2014-2015.6
Vaccine effectiveness among all age groups and against all circulating strains was 47%.4 No major vaccine safety concerns were detected. Among those who received IIV3, there was a slight increase in the incidence of Guillain-Barré syndrome of 2.6 cases per one million vaccines.7
Other recommendations for 2016-2017
Once again, ACIP recommends influenza vaccine for all individuals 6 months and older.8 The CDC additionally specifies particular groups that should not skip vaccination given that they are at high risk of complications from influenza infection or because they could expose high-risk individuals to infection (TABLE 1).9

There will continue to be a selection of trivalent and quadrivalent influenza vaccine products in 2016-2017. Trivalent products will contain 3 viral strains: A/California/7/2009 (H1N1), A/Hong Kong/4801/2014 (H3N2) and B/Brisbane/60/2008.10 The quadrivalent products will contain those 3 antigens plus B/Phuket/3073/2013.10 The H3N2 strain is different from the one in last year’s vaccine. Each year, influenza experts analyze surveillance data to predict which circulating strains will predominate in North America, and these antigens constitute the vaccine formulation. The accuracy of this prediction in large part determines how effective the vaccine will be that season.
Two new vaccines have been approved for use in the United States. A quadrivalent cell culture inactivated vaccine (CCIV4), Flucelvax, was licensed in May 2016. It is prepared from virus propagated in canine kidney cells, not with an egg-based production process. It is approved for use in individuals 4 years of age and older.8 Fluad, an adjuvanted trivalent inactivated influenza vaccine, was licensed in late 2015 for individuals 65 years of age and older.8 This is the first adjuvanted influenza vaccine licensed in the United States and will compete with high-dose quadrivalent vaccine for use in older adults. ACIP does not express a preference for any vaccine in this age group.
Two other vaccines should also be available by this fall: Flublok, a quadrivalent recombinant influenza vaccine for individuals 18 years and older, and Flulaval, a quadrivalent inactivated influenza vaccine, for individuals 6 months of age and older. TABLE 211 lists approved influenza vaccines.

Issues specific to children
Deciding how many vaccine doses children need has been further simplified. Children younger than 9 years need 2 doses if they have received fewer than 2 doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The interval between the 2 doses should be at least 4 weeks. The 2 doses do not have to be the same product; importantly, do not delay a second dose just to obtain the same product used for the first dose. Also, one dose can be trivalent and the other one quadrivalent, although this offers less-than-optimal protection against the B-virus that is only in the quadrivalent product.
Children younger than 9 years require only one dose if they have received 2 or more total doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The 2 previous doses need not have been received during the same influenza season or consecutive influenza seasons.
In children ages 6 through 23 months there is a slight increased risk of febrile seizure if the influenza vaccine is co-administered with other vaccines, specifically pneumococcal conjugate vaccine (PCV 13) and diphtheria-tetanus-acellular-pertussis (DTaP). The 3 vaccines administered at the same time result in 30 febrile seizures per 100,000 children;12 the rate is lower when influenza vaccine is co-administered with only one of the others. ACIP believes that the risk of a febrile seizure, which does no long-term harm, does not warrant delaying vaccines that could be co-administered.13
Egg allergy requires no special precautions
Evidence continues to grow that influenza vaccine products do not contain enough egg protein to cause significant problems in those with a history of egg allergies. This year’s recommendations state that no special precautions are needed regarding the anatomic site of immunization or the length of observation after administering influenza vaccine in those with a history of allergies to eggs, no matter how severe. All vaccine-administration facilities should be able to respond to any hypersensitivity reaction, and the standard waiting time for observation after all vaccinations is 15 minutes.

Antiviral medications for treatment or prevention
Most influenza strains circulating in 2016-2017 are expected to remain sensitive to oseltamivir and zanamivir, which can be used for treatment or disease prevention. A third neuraminidase inhibitor, peramivir, is available for intravenous use in adults 18 and older. Treatment is recommended for those who have confirmed or suspected influenza and are at high risk for complications (TABLE 3).14 Consideration of antiviral chemoprevention is recommended under certain circumstances (TABLE 4).15,16 The CDC influenza Web site lists recommended doses and duration for each antiviral for treatment and chemoprevention.15

1. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2016-17 influenza season. MMWR Recomm Rep. 2016;65:1-54.
2. U.S. Food and Drug Administration. FDA information regarding FluMist quadrivalent vaccine. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm508761.htm. Accessed July 13, 2016.
3. Centers for Disease Control and Prevention. ACIP votes down use of LAIV for 2016-2017 flu season. Available at: http://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Accessed July 13, 2016.
4. Flannery B, Chung J. Influenza vaccine effectiveness, including LAIV vs IIV in children and adolescents, US Flu VE Network, 2015-2016. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-05-flannery.pdf. Accessed July 22, 2016.
5. Centers for Disease Control and Prevention. FluView. Laboratory-confirmed influenza hospitalizations. Available at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html. Accessed July 25, 2016.
6. Centers for Disease Control and Prevention. FluView. Number of influenza-associated pediatric deaths by week of death. Available at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html. Accessed July 25, 2016.
7. Shimabukuro T. End-of-season update: 2015-2016 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-04-shimabukuro.pdf. Accessed July 22, 2016.
8. Grohskopf L. Proposed recommendations 2016-2017 influenza season. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-08-grohskopf.pdf. Accessed July 22, 2016.
9. Centers for Disease Control and Prevention. Influenza vaccination: a summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/vaccination/vax-summary.htm. Accessed July 13, 2016.
10. Centers for Disease Control and Prevention. What you should know for the 2016-2017 influenza season. Available at: http://www.cdc.gov/flu/about/season/flu-season-2016-2017.htm. Accessed July 13, 2016.
11. Immunization Action Coalition. Influenza vaccine products for the 2016-2017 influenza season. Available at: http://www.immunize.org/catg.d/p4072.pdf. Accessed July 13, 2016.
12. Duffy J, Weintraub E, Hambidge SJ, et al. Febrile seizure risk after vaccination in children 6 to 23 months. Pediatrics. 2016;138.
13. Centers for Disease Control and Prevention. Childhood vaccines and febrile seizures. Available at: http://www.cdc.gov/vaccinesafety/concerns/febrile-seizures.html. Accessed August 11, 2016.
14. Centers for Disease Control and Prevention. Use of antivirals. Background and guidance on the use of influenza antiviral agents. Available at: http://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm. Accessed July 13, 2016.
15. Centers for Disease Control and Prevention. Influenza antiviral medications: summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed July 13, 2016.
16. American Academy of Pediatrics. Recommendations for prevention and control of influenza in children, 2015-2016. Pediatrics. 2015;136:792-808.
1. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2016-17 influenza season. MMWR Recomm Rep. 2016;65:1-54.
2. U.S. Food and Drug Administration. FDA information regarding FluMist quadrivalent vaccine. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm508761.htm. Accessed July 13, 2016.
3. Centers for Disease Control and Prevention. ACIP votes down use of LAIV for 2016-2017 flu season. Available at: http://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Accessed July 13, 2016.
4. Flannery B, Chung J. Influenza vaccine effectiveness, including LAIV vs IIV in children and adolescents, US Flu VE Network, 2015-2016. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-05-flannery.pdf. Accessed July 22, 2016.
5. Centers for Disease Control and Prevention. FluView. Laboratory-confirmed influenza hospitalizations. Available at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html. Accessed July 25, 2016.
6. Centers for Disease Control and Prevention. FluView. Number of influenza-associated pediatric deaths by week of death. Available at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html. Accessed July 25, 2016.
7. Shimabukuro T. End-of-season update: 2015-2016 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-04-shimabukuro.pdf. Accessed July 22, 2016.
8. Grohskopf L. Proposed recommendations 2016-2017 influenza season. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-08-grohskopf.pdf. Accessed July 22, 2016.
9. Centers for Disease Control and Prevention. Influenza vaccination: a summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/vaccination/vax-summary.htm. Accessed July 13, 2016.
10. Centers for Disease Control and Prevention. What you should know for the 2016-2017 influenza season. Available at: http://www.cdc.gov/flu/about/season/flu-season-2016-2017.htm. Accessed July 13, 2016.
11. Immunization Action Coalition. Influenza vaccine products for the 2016-2017 influenza season. Available at: http://www.immunize.org/catg.d/p4072.pdf. Accessed July 13, 2016.
12. Duffy J, Weintraub E, Hambidge SJ, et al. Febrile seizure risk after vaccination in children 6 to 23 months. Pediatrics. 2016;138.
13. Centers for Disease Control and Prevention. Childhood vaccines and febrile seizures. Available at: http://www.cdc.gov/vaccinesafety/concerns/febrile-seizures.html. Accessed August 11, 2016.
14. Centers for Disease Control and Prevention. Use of antivirals. Background and guidance on the use of influenza antiviral agents. Available at: http://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm. Accessed July 13, 2016.
15. Centers for Disease Control and Prevention. Influenza antiviral medications: summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed July 13, 2016.
16. American Academy of Pediatrics. Recommendations for prevention and control of influenza in children, 2015-2016. Pediatrics. 2015;136:792-808.
Pruritus since childhood
A 48-year-old woman experiencing homelessness presented to our clinic with a 4-week history of an intensely pruritic rash on her upper back and bilateral upper extremities. She reported that she had experienced exacerbations and remissions of the rash in similar locations for the past several years and during childhood. Factors that exacerbated the rash included being outdoors and being exposed to heat. Her pruritus was intensified by scratching the skin and was significantly worse at night. Previous doctors had diagnosed her with psoriasis and prescribed a short trial of hydrocortisone cream and oral antihistamines, but they provided minimal relief.
The patient indicated that the itching interrupted her sleep and her skin’s appearance made it difficult to get a job. The physical exam revealed excoriated and erythematous papules and patches on her upper back, the extensor and flexor aspects of her bilateral forearms, and the dorsal surface of her bilateral wrists, hands, and fingers (FIGURE 1). Her skin was dry and scaly with pigmentary changes and skin thickening (FIGURE 2). She denied any other systemic symptoms. Her hair and nails were normal, she had no palpable lymph nodes, and she was afebrile. She reported suffering from seasonal allergies, but wasn’t aware of a family history of skin disorders.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Chronic atopic dermatitis
Although the patient was told she had psoriasis by previous doctors, we diagnosed her condition as atopic dermatitis based on its clinical appearance. There is no single test that can establish a diagnosis of atopic dermatitis. While serum total IgE levels are often elevated, testing is not currently recommended.
The United Kingdom working group on atopic dermatitis published diagnostic criteria based on clinical history and physical exam that include pruritic skin in addition to the presence of 3 or more of the following: skin crease involvement, chronically dry skin, symptom onset before 2 years of age, and visible evidence of dermatitis involving flexural surfaces.1 Our patient fulfilled all but one condition, as she wasn’t sure if her symptoms began before age 2.
Atopic dermatitis is a chronic and inflammatory cutaneous disease that affects approximately 10% to 12% of children and less than 1% of adults in the United States.2 Approximately 90% of cases present before the age of 5 and the literature demonstrates a slight female predominance.3,4
Disease severity is classified as mild, moderate, or severe.5 Mild disease is characterized by dry skin and minimal itching with little impairment of the patient’s physical and psychological wellbeing. Moderate disease includes frequent pruritus and erythema with or without secondary skin changes and a moderate impact on physical and mental health. In severe disease, extensive secondary skin changes exist and the patient’s daily activities, sleep, and mental health may be severely impaired.
Etiology is multifactorial. Causes of atopic dermatitis include abnormalities in the epidermal stratum corneum and tight junctions, a heightened type-2 helper T-cell response to environmental antigens, innate immunity defects, and altered microbial skin flora.6,7
Genetic influences appear to play a substantial role in disease development. Approximately 70% of patients have a positive family history of an atopic disease such as eczema, asthma, or allergic rhinitis.8 Genetic defects are believed to be related to defective proteins and lipids in the epidermis that lead to disruption of the epidermal barrier and subsequent cutaneous inflammation.6,7
Clinical presentation: Lesion distribution varies with age
Intense pruritus and dry scaly skin occur in both children and adults, although the distribution of lesions may vary with age. Children typically exhibit erythematous patches with papules and crusting on the face, scalp, extremities, or trunk. In adults, lesions are primarily located on the hands and feet, but may also present on the face, wrists, forearms, and flexural areas.3
Adults also frequently present with secondary skin changes such as thickened skin, pigmentation changes, lichenification, and excoriated papules due to chronic rubbing or scratching.3 Our patient presented with significant lichenification and hyperpigmentation of the skin that was most prominent on the wrists and forearms.
Additional clinical features consistent with atopic dermatitis include a personal history of allergic conditions and a disease course characterized by exacerbations and remissions. Exacerbations may be caused by heat exposure, dry climates, anxiety, rapid temperature variations, contact with certain chemical substances, or microbial infections.8
Differential Dx includes psoriasis and scabies
The differential diagnosis of chronic atopic dermatitis consists of allergic or irritant contact dermatitis, plaque psoriasis, seborrheic dermatitis, scabies, and drug eruptions. Early diagnosis of atopic dermatitis is imperative to prevent sleep disturbances, chronic secondary skin changes, scarring, and the development of skin infections.
Allergic or irritant contact dermatitis is a cutaneous inflammation occurring after contact with an allergen or irritant. The lesions include erythematous, scaling areas with marked borders that are commonly pruritic. Acute cases often present with vesicles and bullae, while lichenification with cracks and fissures are common among chronic cases. Patch testing may be performed if the diagnosis is suspected.
Plaque psoriasis is characterized by areas of dry, erythematous, and well-demarcated plaques with silver scales that are most commonly found on the knees, elbows, scalp, and lower back. Systemic manifestations can include joint pain and joint swelling. Nail pitting and onycholysis are also common. While our patient had skin thickening, it was from the lichenification that is common in atopic dermatitis.
Psoriasis and atopic dermatitis are often confused. Psoriasis has discrete plaques on extensor surfaces and is often associated with nail changes, while the thickening of the skin that comes with chronic itch and scratching of atopic dermatitis is often less well circumscribed and found on flexor surfaces. Family physicians are frequently the first to encounter patients with atopic dermatitis and psoriasis and must be able to distinguish these conditions, as their treatments differ.
Seborrheic dermatitis is a chronic, relapsing inflammatory condition characterized by pruritic, erythematous, greasy, scaly patches on sebum-rich skin such as the scalp, face, and upper trunk. Seborrheic dermatitis is a clinical diagnosis.
Scabies is a pruritic skin condition caused by Sarcoptes scabiei var hominis. Characteristic linear burrows often appear as serpiginous, gray, threadlike elevations in the webbed spaces of the fingers, scrotum, areolae, elbows, axillae, feet, and wrist flexors. Secondary lesions from scratching or inflammation include excoriations, erythema, and hyperpigmentation. The diagnosis is made clinically and confirmed by dermoscopy, when available. Alternatively, mites or eggs may be observed on skin scrapings using light microscopy.
Drug eruptions should be considered in individuals taking medications who develop acute, symmetric cutaneous eruptions that may be morbilliform, urticarial, papulosquamous, pustular, or bullous in nature.
Treatment depends on severity, area of involvement, and patient’s age
Components of atopic dermatitis treatment include skin hydration, negative stimuli avoidance, pharmacologic modalities, and patient education. Improved skin hydration can be achieved by applying thick emollients containing little to no water at least twice daily and after bathing.
Topical corticosteroids are added when emollient use alone fails. Potency selection is based upon the patient’s age, involved body region, and the severity of skin inflammation.8 In order to reduce cutaneous atrophy, only low-potency corticosteroids should be applied to the face, groin, and axillae. Patients with mild disease may apply desonide 0.05% or hydrocortisone 2.5% cream or ointment once or twice daily for 2 to 4 weeks.8 Patients without improvement or with moderate disease may need medium- to high-potency steroids such as fluocinolone 0.025% or triamcinolone 0.1%.
Patients with atopic dermatitis on the face, eyelids, neck, and skin folds or those who do not obtain relief from combined emollients and topical corticosteroids may benefit from topical calcineurin inhibitors such as pimecrolimus or tacrolimus.9 However, these agents should be utilized only for short periods of time and avoided in immunocompromised patients and those younger than 2 years of age.9
Patients with uncontrolled moderate to severe refractory disease may consider a trial of phototherapy or cyclosporine if phototherapy is ineffective or unavailable.10 A meta-analysis has shown that once remission is achieved, intermittent therapy with moderate- to high-potency corticosteroids or tacrolimus may be effective in reducing subsequent flares.11
In all cases, sedating antihistamines such as diphenhydramine or hydroxyzine can be utilized for pruritic relief, particularly at night. Additionally, signs suggestive of infection should prompt antibiotic treatment with agents that provide coverage for Staphylococcus and Streptococcus species. Lastly, patients must be adequately educated on stimuli avoidance (eg, hot water, wool) and counseled on the medical and psychological issues that often accompany atopic dermatitis.
Our patient was placed on triamcinolone 0.1% for 4 weeks and her condition improved.
CORRESPONDENCE
Andrea Richardson, MD, MPH, 7414 Carriage Bay, San Antonio, TX 78249; [email protected].
1. Williams HC, Burney PG, Pembroke AC, et al. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. III. Independent hospital validation. Br J Dermatol. 1994;131:406-416.
2. Horii KA, Simon SD, Liu DY, et al. Atopic dermatitis in children in the United States, 1997-2004: visit trends, patient and provider characteristics, and prescribing patterns. Pediatrics. 2007;120:e527-e534.
3. Rudikoff D, Lebwohl M. Atopic dermatitis. Lancet. 1998;351:1715-1721.
4. Kang K, Polster AM, Nedorost ST, et al. Atopic dermatitis. In: Dermatology. Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Mosby, New York;2003:199.
5. Lewis-Jones S, Mugglestone MA; Guideline Development Group. Management of atopic eczema in children aged up to 12 years: summary of NICE guidance. BMJ. 2007;335:1263-1264.
6. Kuo IH, Yoshida T, De Benedetto A, et al. The cutaneous innate immune response in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:266-278.
7. Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233-246.
8. Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
9. Ashcroft DM, Dimmock P, Garside R, et al. Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis: meta-analysis of randomised controlled trials. BMJ. 2005;330:516.
10. Garritsen FM, Brouwer MW, Limpens J, et al. Photo(chemo)therapy in the management of atopic dermatitis: an updated systematic review with implications for practice and research. Br J Dermatol. 2014;170:501-513.
11. Schmitt J, von Kobyletzki L, Svensson A, et al. Efficacy and tolerability of proactive treatment with topical corticosteroids and calcineurin inhibitors for atopic eczema: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol. 2011;164:415-428.
A 48-year-old woman experiencing homelessness presented to our clinic with a 4-week history of an intensely pruritic rash on her upper back and bilateral upper extremities. She reported that she had experienced exacerbations and remissions of the rash in similar locations for the past several years and during childhood. Factors that exacerbated the rash included being outdoors and being exposed to heat. Her pruritus was intensified by scratching the skin and was significantly worse at night. Previous doctors had diagnosed her with psoriasis and prescribed a short trial of hydrocortisone cream and oral antihistamines, but they provided minimal relief.
The patient indicated that the itching interrupted her sleep and her skin’s appearance made it difficult to get a job. The physical exam revealed excoriated and erythematous papules and patches on her upper back, the extensor and flexor aspects of her bilateral forearms, and the dorsal surface of her bilateral wrists, hands, and fingers (FIGURE 1). Her skin was dry and scaly with pigmentary changes and skin thickening (FIGURE 2). She denied any other systemic symptoms. Her hair and nails were normal, she had no palpable lymph nodes, and she was afebrile. She reported suffering from seasonal allergies, but wasn’t aware of a family history of skin disorders.


WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Chronic atopic dermatitis
Although the patient was told she had psoriasis by previous doctors, we diagnosed her condition as atopic dermatitis based on its clinical appearance. There is no single test that can establish a diagnosis of atopic dermatitis. While serum total IgE levels are often elevated, testing is not currently recommended.
The United Kingdom working group on atopic dermatitis published diagnostic criteria based on clinical history and physical exam that include pruritic skin in addition to the presence of 3 or more of the following: skin crease involvement, chronically dry skin, symptom onset before 2 years of age, and visible evidence of dermatitis involving flexural surfaces.1 Our patient fulfilled all but one condition, as she wasn’t sure if her symptoms began before age 2.
Atopic dermatitis is a chronic and inflammatory cutaneous disease that affects approximately 10% to 12% of children and less than 1% of adults in the United States.2 Approximately 90% of cases present before the age of 5 and the literature demonstrates a slight female predominance.3,4
Disease severity is classified as mild, moderate, or severe.5 Mild disease is characterized by dry skin and minimal itching with little impairment of the patient’s physical and psychological wellbeing. Moderate disease includes frequent pruritus and erythema with or without secondary skin changes and a moderate impact on physical and mental health. In severe disease, extensive secondary skin changes exist and the patient’s daily activities, sleep, and mental health may be severely impaired.
Etiology is multifactorial. Causes of atopic dermatitis include abnormalities in the epidermal stratum corneum and tight junctions, a heightened type-2 helper T-cell response to environmental antigens, innate immunity defects, and altered microbial skin flora.6,7
Genetic influences appear to play a substantial role in disease development. Approximately 70% of patients have a positive family history of an atopic disease such as eczema, asthma, or allergic rhinitis.8 Genetic defects are believed to be related to defective proteins and lipids in the epidermis that lead to disruption of the epidermal barrier and subsequent cutaneous inflammation.6,7
Clinical presentation: Lesion distribution varies with age
Intense pruritus and dry scaly skin occur in both children and adults, although the distribution of lesions may vary with age. Children typically exhibit erythematous patches with papules and crusting on the face, scalp, extremities, or trunk. In adults, lesions are primarily located on the hands and feet, but may also present on the face, wrists, forearms, and flexural areas.3
Adults also frequently present with secondary skin changes such as thickened skin, pigmentation changes, lichenification, and excoriated papules due to chronic rubbing or scratching.3 Our patient presented with significant lichenification and hyperpigmentation of the skin that was most prominent on the wrists and forearms.
Additional clinical features consistent with atopic dermatitis include a personal history of allergic conditions and a disease course characterized by exacerbations and remissions. Exacerbations may be caused by heat exposure, dry climates, anxiety, rapid temperature variations, contact with certain chemical substances, or microbial infections.8
Differential Dx includes psoriasis and scabies
The differential diagnosis of chronic atopic dermatitis consists of allergic or irritant contact dermatitis, plaque psoriasis, seborrheic dermatitis, scabies, and drug eruptions. Early diagnosis of atopic dermatitis is imperative to prevent sleep disturbances, chronic secondary skin changes, scarring, and the development of skin infections.
Allergic or irritant contact dermatitis is a cutaneous inflammation occurring after contact with an allergen or irritant. The lesions include erythematous, scaling areas with marked borders that are commonly pruritic. Acute cases often present with vesicles and bullae, while lichenification with cracks and fissures are common among chronic cases. Patch testing may be performed if the diagnosis is suspected.
Plaque psoriasis is characterized by areas of dry, erythematous, and well-demarcated plaques with silver scales that are most commonly found on the knees, elbows, scalp, and lower back. Systemic manifestations can include joint pain and joint swelling. Nail pitting and onycholysis are also common. While our patient had skin thickening, it was from the lichenification that is common in atopic dermatitis.
Psoriasis and atopic dermatitis are often confused. Psoriasis has discrete plaques on extensor surfaces and is often associated with nail changes, while the thickening of the skin that comes with chronic itch and scratching of atopic dermatitis is often less well circumscribed and found on flexor surfaces. Family physicians are frequently the first to encounter patients with atopic dermatitis and psoriasis and must be able to distinguish these conditions, as their treatments differ.
Seborrheic dermatitis is a chronic, relapsing inflammatory condition characterized by pruritic, erythematous, greasy, scaly patches on sebum-rich skin such as the scalp, face, and upper trunk. Seborrheic dermatitis is a clinical diagnosis.
Scabies is a pruritic skin condition caused by Sarcoptes scabiei var hominis. Characteristic linear burrows often appear as serpiginous, gray, threadlike elevations in the webbed spaces of the fingers, scrotum, areolae, elbows, axillae, feet, and wrist flexors. Secondary lesions from scratching or inflammation include excoriations, erythema, and hyperpigmentation. The diagnosis is made clinically and confirmed by dermoscopy, when available. Alternatively, mites or eggs may be observed on skin scrapings using light microscopy.
Drug eruptions should be considered in individuals taking medications who develop acute, symmetric cutaneous eruptions that may be morbilliform, urticarial, papulosquamous, pustular, or bullous in nature.
Treatment depends on severity, area of involvement, and patient’s age
Components of atopic dermatitis treatment include skin hydration, negative stimuli avoidance, pharmacologic modalities, and patient education. Improved skin hydration can be achieved by applying thick emollients containing little to no water at least twice daily and after bathing.
Topical corticosteroids are added when emollient use alone fails. Potency selection is based upon the patient’s age, involved body region, and the severity of skin inflammation.8 In order to reduce cutaneous atrophy, only low-potency corticosteroids should be applied to the face, groin, and axillae. Patients with mild disease may apply desonide 0.05% or hydrocortisone 2.5% cream or ointment once or twice daily for 2 to 4 weeks.8 Patients without improvement or with moderate disease may need medium- to high-potency steroids such as fluocinolone 0.025% or triamcinolone 0.1%.
Patients with atopic dermatitis on the face, eyelids, neck, and skin folds or those who do not obtain relief from combined emollients and topical corticosteroids may benefit from topical calcineurin inhibitors such as pimecrolimus or tacrolimus.9 However, these agents should be utilized only for short periods of time and avoided in immunocompromised patients and those younger than 2 years of age.9
Patients with uncontrolled moderate to severe refractory disease may consider a trial of phototherapy or cyclosporine if phototherapy is ineffective or unavailable.10 A meta-analysis has shown that once remission is achieved, intermittent therapy with moderate- to high-potency corticosteroids or tacrolimus may be effective in reducing subsequent flares.11
In all cases, sedating antihistamines such as diphenhydramine or hydroxyzine can be utilized for pruritic relief, particularly at night. Additionally, signs suggestive of infection should prompt antibiotic treatment with agents that provide coverage for Staphylococcus and Streptococcus species. Lastly, patients must be adequately educated on stimuli avoidance (eg, hot water, wool) and counseled on the medical and psychological issues that often accompany atopic dermatitis.
Our patient was placed on triamcinolone 0.1% for 4 weeks and her condition improved.
CORRESPONDENCE
Andrea Richardson, MD, MPH, 7414 Carriage Bay, San Antonio, TX 78249; [email protected].
A 48-year-old woman experiencing homelessness presented to our clinic with a 4-week history of an intensely pruritic rash on her upper back and bilateral upper extremities. She reported that she had experienced exacerbations and remissions of the rash in similar locations for the past several years and during childhood. Factors that exacerbated the rash included being outdoors and being exposed to heat. Her pruritus was intensified by scratching the skin and was significantly worse at night. Previous doctors had diagnosed her with psoriasis and prescribed a short trial of hydrocortisone cream and oral antihistamines, but they provided minimal relief.
The patient indicated that the itching interrupted her sleep and her skin’s appearance made it difficult to get a job. The physical exam revealed excoriated and erythematous papules and patches on her upper back, the extensor and flexor aspects of her bilateral forearms, and the dorsal surface of her bilateral wrists, hands, and fingers (FIGURE 1). Her skin was dry and scaly with pigmentary changes and skin thickening (FIGURE 2). She denied any other systemic symptoms. Her hair and nails were normal, she had no palpable lymph nodes, and she was afebrile. She reported suffering from seasonal allergies, but wasn’t aware of a family history of skin disorders.


WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Chronic atopic dermatitis
Although the patient was told she had psoriasis by previous doctors, we diagnosed her condition as atopic dermatitis based on its clinical appearance. There is no single test that can establish a diagnosis of atopic dermatitis. While serum total IgE levels are often elevated, testing is not currently recommended.
The United Kingdom working group on atopic dermatitis published diagnostic criteria based on clinical history and physical exam that include pruritic skin in addition to the presence of 3 or more of the following: skin crease involvement, chronically dry skin, symptom onset before 2 years of age, and visible evidence of dermatitis involving flexural surfaces.1 Our patient fulfilled all but one condition, as she wasn’t sure if her symptoms began before age 2.
Atopic dermatitis is a chronic and inflammatory cutaneous disease that affects approximately 10% to 12% of children and less than 1% of adults in the United States.2 Approximately 90% of cases present before the age of 5 and the literature demonstrates a slight female predominance.3,4
Disease severity is classified as mild, moderate, or severe.5 Mild disease is characterized by dry skin and minimal itching with little impairment of the patient’s physical and psychological wellbeing. Moderate disease includes frequent pruritus and erythema with or without secondary skin changes and a moderate impact on physical and mental health. In severe disease, extensive secondary skin changes exist and the patient’s daily activities, sleep, and mental health may be severely impaired.
Etiology is multifactorial. Causes of atopic dermatitis include abnormalities in the epidermal stratum corneum and tight junctions, a heightened type-2 helper T-cell response to environmental antigens, innate immunity defects, and altered microbial skin flora.6,7
Genetic influences appear to play a substantial role in disease development. Approximately 70% of patients have a positive family history of an atopic disease such as eczema, asthma, or allergic rhinitis.8 Genetic defects are believed to be related to defective proteins and lipids in the epidermis that lead to disruption of the epidermal barrier and subsequent cutaneous inflammation.6,7
Clinical presentation: Lesion distribution varies with age
Intense pruritus and dry scaly skin occur in both children and adults, although the distribution of lesions may vary with age. Children typically exhibit erythematous patches with papules and crusting on the face, scalp, extremities, or trunk. In adults, lesions are primarily located on the hands and feet, but may also present on the face, wrists, forearms, and flexural areas.3
Adults also frequently present with secondary skin changes such as thickened skin, pigmentation changes, lichenification, and excoriated papules due to chronic rubbing or scratching.3 Our patient presented with significant lichenification and hyperpigmentation of the skin that was most prominent on the wrists and forearms.
Additional clinical features consistent with atopic dermatitis include a personal history of allergic conditions and a disease course characterized by exacerbations and remissions. Exacerbations may be caused by heat exposure, dry climates, anxiety, rapid temperature variations, contact with certain chemical substances, or microbial infections.8
Differential Dx includes psoriasis and scabies
The differential diagnosis of chronic atopic dermatitis consists of allergic or irritant contact dermatitis, plaque psoriasis, seborrheic dermatitis, scabies, and drug eruptions. Early diagnosis of atopic dermatitis is imperative to prevent sleep disturbances, chronic secondary skin changes, scarring, and the development of skin infections.
Allergic or irritant contact dermatitis is a cutaneous inflammation occurring after contact with an allergen or irritant. The lesions include erythematous, scaling areas with marked borders that are commonly pruritic. Acute cases often present with vesicles and bullae, while lichenification with cracks and fissures are common among chronic cases. Patch testing may be performed if the diagnosis is suspected.
Plaque psoriasis is characterized by areas of dry, erythematous, and well-demarcated plaques with silver scales that are most commonly found on the knees, elbows, scalp, and lower back. Systemic manifestations can include joint pain and joint swelling. Nail pitting and onycholysis are also common. While our patient had skin thickening, it was from the lichenification that is common in atopic dermatitis.
Psoriasis and atopic dermatitis are often confused. Psoriasis has discrete plaques on extensor surfaces and is often associated with nail changes, while the thickening of the skin that comes with chronic itch and scratching of atopic dermatitis is often less well circumscribed and found on flexor surfaces. Family physicians are frequently the first to encounter patients with atopic dermatitis and psoriasis and must be able to distinguish these conditions, as their treatments differ.
Seborrheic dermatitis is a chronic, relapsing inflammatory condition characterized by pruritic, erythematous, greasy, scaly patches on sebum-rich skin such as the scalp, face, and upper trunk. Seborrheic dermatitis is a clinical diagnosis.
Scabies is a pruritic skin condition caused by Sarcoptes scabiei var hominis. Characteristic linear burrows often appear as serpiginous, gray, threadlike elevations in the webbed spaces of the fingers, scrotum, areolae, elbows, axillae, feet, and wrist flexors. Secondary lesions from scratching or inflammation include excoriations, erythema, and hyperpigmentation. The diagnosis is made clinically and confirmed by dermoscopy, when available. Alternatively, mites or eggs may be observed on skin scrapings using light microscopy.
Drug eruptions should be considered in individuals taking medications who develop acute, symmetric cutaneous eruptions that may be morbilliform, urticarial, papulosquamous, pustular, or bullous in nature.
Treatment depends on severity, area of involvement, and patient’s age
Components of atopic dermatitis treatment include skin hydration, negative stimuli avoidance, pharmacologic modalities, and patient education. Improved skin hydration can be achieved by applying thick emollients containing little to no water at least twice daily and after bathing.
Topical corticosteroids are added when emollient use alone fails. Potency selection is based upon the patient’s age, involved body region, and the severity of skin inflammation.8 In order to reduce cutaneous atrophy, only low-potency corticosteroids should be applied to the face, groin, and axillae. Patients with mild disease may apply desonide 0.05% or hydrocortisone 2.5% cream or ointment once or twice daily for 2 to 4 weeks.8 Patients without improvement or with moderate disease may need medium- to high-potency steroids such as fluocinolone 0.025% or triamcinolone 0.1%.
Patients with atopic dermatitis on the face, eyelids, neck, and skin folds or those who do not obtain relief from combined emollients and topical corticosteroids may benefit from topical calcineurin inhibitors such as pimecrolimus or tacrolimus.9 However, these agents should be utilized only for short periods of time and avoided in immunocompromised patients and those younger than 2 years of age.9
Patients with uncontrolled moderate to severe refractory disease may consider a trial of phototherapy or cyclosporine if phototherapy is ineffective or unavailable.10 A meta-analysis has shown that once remission is achieved, intermittent therapy with moderate- to high-potency corticosteroids or tacrolimus may be effective in reducing subsequent flares.11
In all cases, sedating antihistamines such as diphenhydramine or hydroxyzine can be utilized for pruritic relief, particularly at night. Additionally, signs suggestive of infection should prompt antibiotic treatment with agents that provide coverage for Staphylococcus and Streptococcus species. Lastly, patients must be adequately educated on stimuli avoidance (eg, hot water, wool) and counseled on the medical and psychological issues that often accompany atopic dermatitis.
Our patient was placed on triamcinolone 0.1% for 4 weeks and her condition improved.
CORRESPONDENCE
Andrea Richardson, MD, MPH, 7414 Carriage Bay, San Antonio, TX 78249; [email protected].
1. Williams HC, Burney PG, Pembroke AC, et al. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. III. Independent hospital validation. Br J Dermatol. 1994;131:406-416.
2. Horii KA, Simon SD, Liu DY, et al. Atopic dermatitis in children in the United States, 1997-2004: visit trends, patient and provider characteristics, and prescribing patterns. Pediatrics. 2007;120:e527-e534.
3. Rudikoff D, Lebwohl M. Atopic dermatitis. Lancet. 1998;351:1715-1721.
4. Kang K, Polster AM, Nedorost ST, et al. Atopic dermatitis. In: Dermatology. Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Mosby, New York;2003:199.
5. Lewis-Jones S, Mugglestone MA; Guideline Development Group. Management of atopic eczema in children aged up to 12 years: summary of NICE guidance. BMJ. 2007;335:1263-1264.
6. Kuo IH, Yoshida T, De Benedetto A, et al. The cutaneous innate immune response in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:266-278.
7. Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233-246.
8. Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
9. Ashcroft DM, Dimmock P, Garside R, et al. Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis: meta-analysis of randomised controlled trials. BMJ. 2005;330:516.
10. Garritsen FM, Brouwer MW, Limpens J, et al. Photo(chemo)therapy in the management of atopic dermatitis: an updated systematic review with implications for practice and research. Br J Dermatol. 2014;170:501-513.
11. Schmitt J, von Kobyletzki L, Svensson A, et al. Efficacy and tolerability of proactive treatment with topical corticosteroids and calcineurin inhibitors for atopic eczema: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol. 2011;164:415-428.
1. Williams HC, Burney PG, Pembroke AC, et al. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. III. Independent hospital validation. Br J Dermatol. 1994;131:406-416.
2. Horii KA, Simon SD, Liu DY, et al. Atopic dermatitis in children in the United States, 1997-2004: visit trends, patient and provider characteristics, and prescribing patterns. Pediatrics. 2007;120:e527-e534.
3. Rudikoff D, Lebwohl M. Atopic dermatitis. Lancet. 1998;351:1715-1721.
4. Kang K, Polster AM, Nedorost ST, et al. Atopic dermatitis. In: Dermatology. Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Mosby, New York;2003:199.
5. Lewis-Jones S, Mugglestone MA; Guideline Development Group. Management of atopic eczema in children aged up to 12 years: summary of NICE guidance. BMJ. 2007;335:1263-1264.
6. Kuo IH, Yoshida T, De Benedetto A, et al. The cutaneous innate immune response in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:266-278.
7. Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233-246.
8. Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
9. Ashcroft DM, Dimmock P, Garside R, et al. Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis: meta-analysis of randomised controlled trials. BMJ. 2005;330:516.
10. Garritsen FM, Brouwer MW, Limpens J, et al. Photo(chemo)therapy in the management of atopic dermatitis: an updated systematic review with implications for practice and research. Br J Dermatol. 2014;170:501-513.
11. Schmitt J, von Kobyletzki L, Svensson A, et al. Efficacy and tolerability of proactive treatment with topical corticosteroids and calcineurin inhibitors for atopic eczema: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol. 2011;164:415-428.
Depigmented patches, mild scaling on newborn • Dx?
THE CASE
A 21-year-old G3P2 mother gave birth to an African American girl via vaginal delivery. Labor had been induced due to gestational hypertension at term. She’d also had a stillborn at term at the age of 16 followed by a second live term birth 3 years ago. During this most recent pregnancy, she’d received adequate prenatal care and had been treated for chlamydia with a single dose of oral azithromycin 1 g.
The newborn had an Apgar score of 9 out of 9 and weighed 6.7 pounds at birth. During a physical examination in the nursery, the infant was found to have large areas of smooth depigmentation on her forehead, right forearm, lower abdomen, and left thigh, with surrounding areas of thickened skin that had mild scaling and hyperpigmentation (FIGURE). The depigmented areas involved approximately 15% of the newborn’s body. The father and paternal grandmother, who were present at the time of delivery, also had depigmented areas of their skin.
The newborn’s tongue was pink and her mucus membranes were moist. No macules or patches were noted on either the oral or vaginal mucosa. Cardiac, pulmonary, and ocular examinations (including evaluation of the retina by ophthalmoscopy) were normal. There was no nystagmus or strabismus. The newborn’s extremities were normal, symmetric, and moveable, and she was easily consoled.
THE DIAGNOSIS
We diagnosed the newborn with piebaldism based on her appearance. Piebaldism consists of hypopigmented/depigmented areas and is a clinical diagnosis; no testing is required.
Concerned about the areas of hyperpigmentation, we decided to get a dermatologist’s opinion. The dermatology team briefly considered the diagnosis of a large melanocytic nevus with sparing of some areas, but a skin biopsy of a hyperpigmented area on the left leg came back with a normal number of melanocytes.
DISCUSSION
Piebaldism is a rare autosomal dominant disorder characterized by the congenital absence of melanocytes in affected areas of the skin and hair due to mutations of the c-kit gene. The c-kit gene affects the differentiation and migration of melanoblasts from the neural crest during embryonic life.1 The incidence of piebaldism is estimated to be less than one in 20,000.2 Both males and females are equally affected, and no race is spared.2,3
Affected individuals present with a white forelock and relatively stable, persistent depigmentation of skin with a characteristic distribution from birth.3 A white forelock arising from a triangular, elongated, or diamond-shaped midline or depigmented macule on the forehead may be the only manifestation in 80% to 90% of cases.3 The characteristic distribution of depigmented macules includes a central macule on the forehead, the anterior abdomen extending to the chest, the lateral trunk sparing the dorsal spine, and the mid-arms and legs sparing the hands and feet.2
Depigmented macules are rectangular, rhomboid, or irregular in shape and usually have a symmetrical distribution. Typically, islands of hyperpigmentation are present within and at the border of depigmented areas.4 Piebaldism is associated in rare instances with neurofibromatosis type 1, Hirschsprung’s disease, hearing loss, and Waardenburg syndrome.4
Histologically, melanocytes are absent or considerably reduced in the depigmented areas and are normal in number in the hyperpigmented areas.5
The differential diagnosis of piebaldism includes mosaicism, albinism, and vitiligo.
Cutaneous mosaicism stems from a gene mutation that occurs during embryogenesis and the lesions are distributed along certain patterns and forms. A chromosomal analysis of our patient showed a normal female karyotype that excluded mosaicism.
Albinism is a genetically inherited disorder characterized by partial or complete absence of melanin production in the skin, hair, and eyes.6 Eye and fundus examinations were normal in our patient, which excluded albinism.
Vitiligo is rarely present at birth but is usually acquired later in life. It results from an immune-mediated destruction of melanocytes and is not genetically inherited, although familial incidence has been reported.7
There are no effective therapies
A combination of dermabrasion and grafting of pigmented skin into depigmented areas, with or without phototherapy, has been used in select patients, although no solid data are available on its effectiveness.3 The lack of effective and safe therapies can make treatment challenging. Piebaldism is usually not medically harmful, but the emotional and psychological effects on the family and the patient as they grow up can be devastating. Therefore, supportive counseling is recommended.
Our patient. Supportive counseling and a follow-up appointment with a dermatologist was planned for our patient and her family.
THE TAKEAWAY
The clinical diagnosis of piebaldism is straightforward based on the presence of a white forelock in the frontal region, the appearance of depigmented macules since birth that stay relatively stable, and the presence of a similar pattern of depigmented macules in other family members. Histologic or genetic testing is not necessary to establish the diagnosis. Rarely, cases of piebaldism are associated with hearing loss, necessitating a hearing assessment and an audiology exam. Unfortunately, there are no effective treatments for piebaldism.
1. Ward KA, Moss C, Sanders DS. Human piebaldism: relationship between phenotype and site of kit gene mutation. Br J Dermatol. 1995;132:929-935.
2. Agarwal S, Ojha A. Piebaldism: A brief report and review of the literature. Indian Dermatol Online J. 2012;3:144-147.
3. Oiso N, Fukai K, Kawada A, et al. Piebaldism. J Dermatol. 2013;40:330-335.
4. Spritz RA, Itin PH, Gutmann DH. Piebaldism and neurofibromatosis type 1: horses of very different colors. J Invest Dermatol. 2004;122:xxxiv-xxxv.
5. Makino T, Yanagihara M, Oiso N, et al. Repigmentation of the epidermis around the acrosyringium in piebald skin: an ultrastructural examination. Br J Dermatol. 2013;168:910-912.
6. Karaman A. Oculocutaneous albinism type 1A: a case report. Dermatol Online J. 2008;14:13.
7. Plensdorf S, Martinez J. Common pigmentation disorders. Am Fam Physician. 2009;79:109-116.
THE CASE
A 21-year-old G3P2 mother gave birth to an African American girl via vaginal delivery. Labor had been induced due to gestational hypertension at term. She’d also had a stillborn at term at the age of 16 followed by a second live term birth 3 years ago. During this most recent pregnancy, she’d received adequate prenatal care and had been treated for chlamydia with a single dose of oral azithromycin 1 g.
The newborn had an Apgar score of 9 out of 9 and weighed 6.7 pounds at birth. During a physical examination in the nursery, the infant was found to have large areas of smooth depigmentation on her forehead, right forearm, lower abdomen, and left thigh, with surrounding areas of thickened skin that had mild scaling and hyperpigmentation (FIGURE). The depigmented areas involved approximately 15% of the newborn’s body. The father and paternal grandmother, who were present at the time of delivery, also had depigmented areas of their skin.
The newborn’s tongue was pink and her mucus membranes were moist. No macules or patches were noted on either the oral or vaginal mucosa. Cardiac, pulmonary, and ocular examinations (including evaluation of the retina by ophthalmoscopy) were normal. There was no nystagmus or strabismus. The newborn’s extremities were normal, symmetric, and moveable, and she was easily consoled.
THE DIAGNOSIS
We diagnosed the newborn with piebaldism based on her appearance. Piebaldism consists of hypopigmented/depigmented areas and is a clinical diagnosis; no testing is required.
Concerned about the areas of hyperpigmentation, we decided to get a dermatologist’s opinion. The dermatology team briefly considered the diagnosis of a large melanocytic nevus with sparing of some areas, but a skin biopsy of a hyperpigmented area on the left leg came back with a normal number of melanocytes.
DISCUSSION
Piebaldism is a rare autosomal dominant disorder characterized by the congenital absence of melanocytes in affected areas of the skin and hair due to mutations of the c-kit gene. The c-kit gene affects the differentiation and migration of melanoblasts from the neural crest during embryonic life.1 The incidence of piebaldism is estimated to be less than one in 20,000.2 Both males and females are equally affected, and no race is spared.2,3
Affected individuals present with a white forelock and relatively stable, persistent depigmentation of skin with a characteristic distribution from birth.3 A white forelock arising from a triangular, elongated, or diamond-shaped midline or depigmented macule on the forehead may be the only manifestation in 80% to 90% of cases.3 The characteristic distribution of depigmented macules includes a central macule on the forehead, the anterior abdomen extending to the chest, the lateral trunk sparing the dorsal spine, and the mid-arms and legs sparing the hands and feet.2
Depigmented macules are rectangular, rhomboid, or irregular in shape and usually have a symmetrical distribution. Typically, islands of hyperpigmentation are present within and at the border of depigmented areas.4 Piebaldism is associated in rare instances with neurofibromatosis type 1, Hirschsprung’s disease, hearing loss, and Waardenburg syndrome.4
Histologically, melanocytes are absent or considerably reduced in the depigmented areas and are normal in number in the hyperpigmented areas.5
The differential diagnosis of piebaldism includes mosaicism, albinism, and vitiligo.
Cutaneous mosaicism stems from a gene mutation that occurs during embryogenesis and the lesions are distributed along certain patterns and forms. A chromosomal analysis of our patient showed a normal female karyotype that excluded mosaicism.
Albinism is a genetically inherited disorder characterized by partial or complete absence of melanin production in the skin, hair, and eyes.6 Eye and fundus examinations were normal in our patient, which excluded albinism.
Vitiligo is rarely present at birth but is usually acquired later in life. It results from an immune-mediated destruction of melanocytes and is not genetically inherited, although familial incidence has been reported.7
There are no effective therapies
A combination of dermabrasion and grafting of pigmented skin into depigmented areas, with or without phototherapy, has been used in select patients, although no solid data are available on its effectiveness.3 The lack of effective and safe therapies can make treatment challenging. Piebaldism is usually not medically harmful, but the emotional and psychological effects on the family and the patient as they grow up can be devastating. Therefore, supportive counseling is recommended.
Our patient. Supportive counseling and a follow-up appointment with a dermatologist was planned for our patient and her family.
THE TAKEAWAY
The clinical diagnosis of piebaldism is straightforward based on the presence of a white forelock in the frontal region, the appearance of depigmented macules since birth that stay relatively stable, and the presence of a similar pattern of depigmented macules in other family members. Histologic or genetic testing is not necessary to establish the diagnosis. Rarely, cases of piebaldism are associated with hearing loss, necessitating a hearing assessment and an audiology exam. Unfortunately, there are no effective treatments for piebaldism.
THE CASE
A 21-year-old G3P2 mother gave birth to an African American girl via vaginal delivery. Labor had been induced due to gestational hypertension at term. She’d also had a stillborn at term at the age of 16 followed by a second live term birth 3 years ago. During this most recent pregnancy, she’d received adequate prenatal care and had been treated for chlamydia with a single dose of oral azithromycin 1 g.
The newborn had an Apgar score of 9 out of 9 and weighed 6.7 pounds at birth. During a physical examination in the nursery, the infant was found to have large areas of smooth depigmentation on her forehead, right forearm, lower abdomen, and left thigh, with surrounding areas of thickened skin that had mild scaling and hyperpigmentation (FIGURE). The depigmented areas involved approximately 15% of the newborn’s body. The father and paternal grandmother, who were present at the time of delivery, also had depigmented areas of their skin.
The newborn’s tongue was pink and her mucus membranes were moist. No macules or patches were noted on either the oral or vaginal mucosa. Cardiac, pulmonary, and ocular examinations (including evaluation of the retina by ophthalmoscopy) were normal. There was no nystagmus or strabismus. The newborn’s extremities were normal, symmetric, and moveable, and she was easily consoled.
THE DIAGNOSIS
We diagnosed the newborn with piebaldism based on her appearance. Piebaldism consists of hypopigmented/depigmented areas and is a clinical diagnosis; no testing is required.
Concerned about the areas of hyperpigmentation, we decided to get a dermatologist’s opinion. The dermatology team briefly considered the diagnosis of a large melanocytic nevus with sparing of some areas, but a skin biopsy of a hyperpigmented area on the left leg came back with a normal number of melanocytes.
DISCUSSION
Piebaldism is a rare autosomal dominant disorder characterized by the congenital absence of melanocytes in affected areas of the skin and hair due to mutations of the c-kit gene. The c-kit gene affects the differentiation and migration of melanoblasts from the neural crest during embryonic life.1 The incidence of piebaldism is estimated to be less than one in 20,000.2 Both males and females are equally affected, and no race is spared.2,3
Affected individuals present with a white forelock and relatively stable, persistent depigmentation of skin with a characteristic distribution from birth.3 A white forelock arising from a triangular, elongated, or diamond-shaped midline or depigmented macule on the forehead may be the only manifestation in 80% to 90% of cases.3 The characteristic distribution of depigmented macules includes a central macule on the forehead, the anterior abdomen extending to the chest, the lateral trunk sparing the dorsal spine, and the mid-arms and legs sparing the hands and feet.2
Depigmented macules are rectangular, rhomboid, or irregular in shape and usually have a symmetrical distribution. Typically, islands of hyperpigmentation are present within and at the border of depigmented areas.4 Piebaldism is associated in rare instances with neurofibromatosis type 1, Hirschsprung’s disease, hearing loss, and Waardenburg syndrome.4
Histologically, melanocytes are absent or considerably reduced in the depigmented areas and are normal in number in the hyperpigmented areas.5
The differential diagnosis of piebaldism includes mosaicism, albinism, and vitiligo.
Cutaneous mosaicism stems from a gene mutation that occurs during embryogenesis and the lesions are distributed along certain patterns and forms. A chromosomal analysis of our patient showed a normal female karyotype that excluded mosaicism.
Albinism is a genetically inherited disorder characterized by partial or complete absence of melanin production in the skin, hair, and eyes.6 Eye and fundus examinations were normal in our patient, which excluded albinism.
Vitiligo is rarely present at birth but is usually acquired later in life. It results from an immune-mediated destruction of melanocytes and is not genetically inherited, although familial incidence has been reported.7
There are no effective therapies
A combination of dermabrasion and grafting of pigmented skin into depigmented areas, with or without phototherapy, has been used in select patients, although no solid data are available on its effectiveness.3 The lack of effective and safe therapies can make treatment challenging. Piebaldism is usually not medically harmful, but the emotional and psychological effects on the family and the patient as they grow up can be devastating. Therefore, supportive counseling is recommended.
Our patient. Supportive counseling and a follow-up appointment with a dermatologist was planned for our patient and her family.
THE TAKEAWAY
The clinical diagnosis of piebaldism is straightforward based on the presence of a white forelock in the frontal region, the appearance of depigmented macules since birth that stay relatively stable, and the presence of a similar pattern of depigmented macules in other family members. Histologic or genetic testing is not necessary to establish the diagnosis. Rarely, cases of piebaldism are associated with hearing loss, necessitating a hearing assessment and an audiology exam. Unfortunately, there are no effective treatments for piebaldism.
1. Ward KA, Moss C, Sanders DS. Human piebaldism: relationship between phenotype and site of kit gene mutation. Br J Dermatol. 1995;132:929-935.
2. Agarwal S, Ojha A. Piebaldism: A brief report and review of the literature. Indian Dermatol Online J. 2012;3:144-147.
3. Oiso N, Fukai K, Kawada A, et al. Piebaldism. J Dermatol. 2013;40:330-335.
4. Spritz RA, Itin PH, Gutmann DH. Piebaldism and neurofibromatosis type 1: horses of very different colors. J Invest Dermatol. 2004;122:xxxiv-xxxv.
5. Makino T, Yanagihara M, Oiso N, et al. Repigmentation of the epidermis around the acrosyringium in piebald skin: an ultrastructural examination. Br J Dermatol. 2013;168:910-912.
6. Karaman A. Oculocutaneous albinism type 1A: a case report. Dermatol Online J. 2008;14:13.
7. Plensdorf S, Martinez J. Common pigmentation disorders. Am Fam Physician. 2009;79:109-116.
1. Ward KA, Moss C, Sanders DS. Human piebaldism: relationship between phenotype and site of kit gene mutation. Br J Dermatol. 1995;132:929-935.
2. Agarwal S, Ojha A. Piebaldism: A brief report and review of the literature. Indian Dermatol Online J. 2012;3:144-147.
3. Oiso N, Fukai K, Kawada A, et al. Piebaldism. J Dermatol. 2013;40:330-335.
4. Spritz RA, Itin PH, Gutmann DH. Piebaldism and neurofibromatosis type 1: horses of very different colors. J Invest Dermatol. 2004;122:xxxiv-xxxv.
5. Makino T, Yanagihara M, Oiso N, et al. Repigmentation of the epidermis around the acrosyringium in piebald skin: an ultrastructural examination. Br J Dermatol. 2013;168:910-912.
6. Karaman A. Oculocutaneous albinism type 1A: a case report. Dermatol Online J. 2008;14:13.
7. Plensdorf S, Martinez J. Common pigmentation disorders. Am Fam Physician. 2009;79:109-116.
Bone disease in patients with kidney disease: A tricky interplay
PRACTICE RECOMMENDATIONS
› Perform laboratory testing for chronic kidney disease (CKD)-induced bone disease at CKD stage 3. B
› Avoid calcium-based phosphate binders in patients with known vascular calcifications. B
› Consider the use of phosphate binders in non-dialysis patients on a case-by-case basis, particularly in those with hyperphosphatemia not controlled by dietary measures. B
› Prescribe native vitamin D (ergocalciferol or cholecalciferol) to patients with CKD stages 3 to 4 who have secondary hyperparathyroidism and vitamin D deficiency. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
About 14% of the US general population has chronic kidney disease (CKD).1 Limited data exist regarding the exact prevalence of CKD-mineral and bone disorder (MBD), but abnormal mineral metabolism is believed to start in stage 3 CKD, implying that 8% of the adult US population could be at risk for, or already have established, CKD-MBD.2 Although the disorder has traditionally been managed by nephrologists, this earlier onset suggests that many patients should be screened and treated by their primary care physicians.
Because CKD-MBD can lead to significant morbidity (ie, increased fracture risk) and mortality, identification and treatment are of utmost importance.3 This review provides information from the current literature and the KDIGO (Kidney Disease: Improving Global Outcomes) guidelines, and focuses primarily on the non-dialysis CKD population.
CKD-MBD: A broad spectrum of disorders
CKD-MBD is defined as a systemic disorder of mineral and bone metabolism due to CKD. Traditionally referred to as renal osteodystrophy, the term CKD-MBD is meant to indicate and describe a broad clinical spectrum of CKD-associated bone mineral metabolism disorders that manifest from one or a combination of the following:
- Abnormalities of calcium, phosphorus, parathyroid hormone (PTH), or vitamin D metabolism
- Abnormalities in bone turnover, mineralization, volume, linear growth, or strength
- Vascular or other soft-tissue calcification.4
Renal bone disease can be divided into low bone turnover (adynamic bone disease) and high bone turnover states. Both can lead to a decrease in bone strength and an increase in pathological fractures.5
Pathophysiology: Difficult to know where the cascade begins
Understanding the pathophysiology and treatment of bone disease in patients with CKD can be challenging. Because of abnormalities of mineral metabolism and changes in hormones and cytokines, bone remodeling is severely disrupted in patients with CKD, and it remains unclear where this cascade begins.
As an adaptive response to decreased kidney function, PTH levels increase. Elevations of both fibroblast growth factor 23 (FGF23) lower blood phosphate levels by inhibiting phosphate reabsorption in the kidneys, thus increasing urinary excretion of phosphorus. Secondary hyperparathyroidism (SHPT), driven by hypocalcemia, responds to normalize serum calcium levels by increasing the number and size of osteoclasts actively breaking down bone matrix. This escalates fracture risk. In addition, the inability of damaged kidneys to convert vitamin D to an active form further deranges calcium and phosphate homeostasis.
Successful management of serum levels begins with monitoring
KDIGO, an independent nonprofit foundation that seeks to improve the care and outcomes of kidney disease patients worldwide, developed guidelines for the diagnosis, evaluation, prevention, and treatment of CKD-MBD in 2009.6 These guidelines recommend that treatment of CKD-MBD be aimed at managing serum phosphate, PTH, and calcium levels. The recommended frequency for laboratory monitoring of these levels varies by stage of CKD and is described in TABLE 1.6 (For more on chronic kidney disease staging, see KDIGO’s 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease, available at: http://www.kdigo.org/clinical_practice_guidelines/pdf/CKD/KDIGO_2012_CKD_GL.pdf.)
Because of the interrelated nature of these minerals and hormones, drug therapy aimed at treating one may impact the others. This must be considered when designing treatment regimens.
Hyperphosphatemia: Manage with diet, drugs, dialysis
Observational studies have shown an association between higher serum phosphate levels and mortality.6-8 KDIGO recommends maintaining serum phosphorus levels within the normal range of the assay in patients with CKD who are not receiving dialysis.6 For dialyzed patients, the recommendation is to lower the phosphorus level toward the normal range as much as possible.6 Maintaining an appropriate phosphorus level is accomplished through dietary phosphate restriction, the use of phosphate binders, and, in dialyzed patients, dialytic removal of phosphate.6
Dietary phosphate restriction is often challenging for patients, in part, because phosphorous content is not always included on food labels in the United States.9 Phosphorus is highly absorbed from additives in processed food (approaching 100% absorption), less absorbed from animal sources such as meat and dairy products (40%-60%), and is the least absorbed from plant sources such as beans and nuts (20%-40%).10 Advise patients to avoid fast food, processed foods, cheese, frozen meals, colas, and certain ready-to-eat cereals and prepared meats, as these products may have additives from which phosphorus is readily absorbed.11 A patient-friendly list of high-phosphorus foods, as well as other dietary advice and recipes, can be found on the National Kidney Foundation Web site at https://www.kidney.org/atoz/content/phosphorus. Tables listing the phosphorus content of common foods are also available in the literature and online.11,12 Keep in mind that not all resources take into account phosphate bioavailability. Dietician referral may be helpful to assure that patients maintain adequate protein intake while restricting dietary phosphate.
Phosphate binders are recommended by the KDIGO guidelines for use in patients with kidney disease and hyperphosphatemia.6 Most of the data to support the use of phosphate binders was gleaned from the dialysis population. The use of phosphate binders in non-dialyzed patients with CKD has both proponents and opponents, with literature supporting both positions.13,14 A recent KDIGO conference on controversies in CKD-MBD identified this as an area that should be evaluated further for the next guideline update.15
Phosphate binders—which bind the phosphorus in food to prevent absorption—should be taken with meals or high-phosphorus snacks. Products and formulations of commonly used phosphate binders are shown in TABLE 2.16,17 Taste, formulation, adverse effects, pill burden, and cost are issues to discuss with patients when initiating or adjusting phosphate binder therapy. It’s estimated that more than half of all patients receiving dialysis do not adhere to their prescribed phosphate binder regimen, highlighting the need to assess adherence before adjusting dose and to involve the patient in the decision-making process to select a phosphate binder product.18
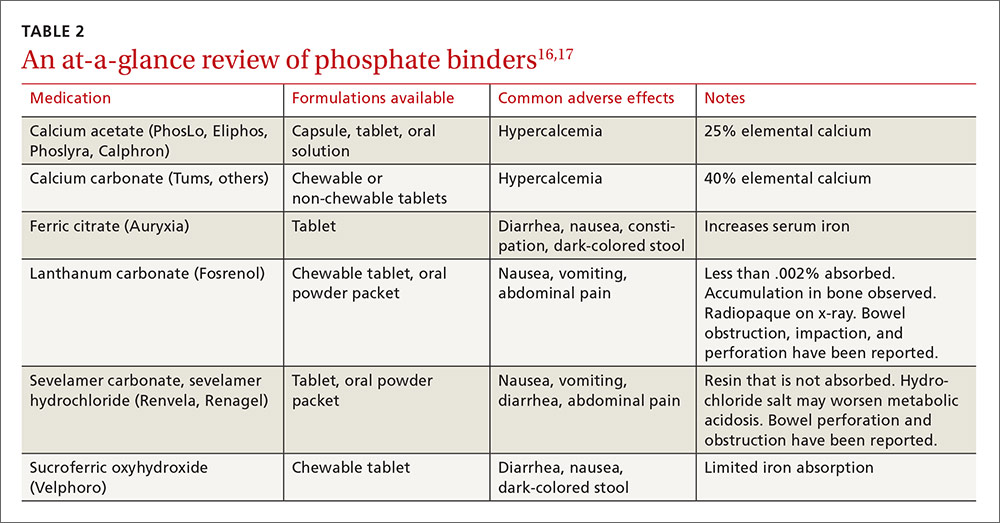
Avoid calcium-based binders? The risk of hypercalcemia and the potentially increased risk of vascular calcifications with calcium-based binders have led some nephrologists to favor non-calcium-based products. Two recent meta-analyses found a reduced risk of all-cause mortality with the non-calcium-based binders sevelamer or lanthanum as compared to calcium-based binders.19,20 Current KDIGO guidelines were published prior to these meta-analyses and do not recommend one phosphate binder over another. They do, however, recommend restricting the dose of calcium-based binders in the setting of persistent or recurrent hypercalcemia, known vascular calcification, or low PTH levels.6
Secondary hyperparathyroidism
Due to a lack of data, the goal PTH level in patients not receiving dialysis is unknown.6 A reasonable approach in non-dialyzed patients, however, is to correct 25-OH vitamin D (25[OH]D) deficiency, elevations in serum phosphate, and hypocalcemia when the level of intact PTH (iPTH) exceeds the normal range for the assay because correcting these derangements may result in a decline in iPTH.6,21 If this approach fails and PTH levels continue to rise, use of calcitriol or vitamin D analogues is recommended.6 Characteristics of medications used to treat SHPT are presented in TABLE 3.16,17
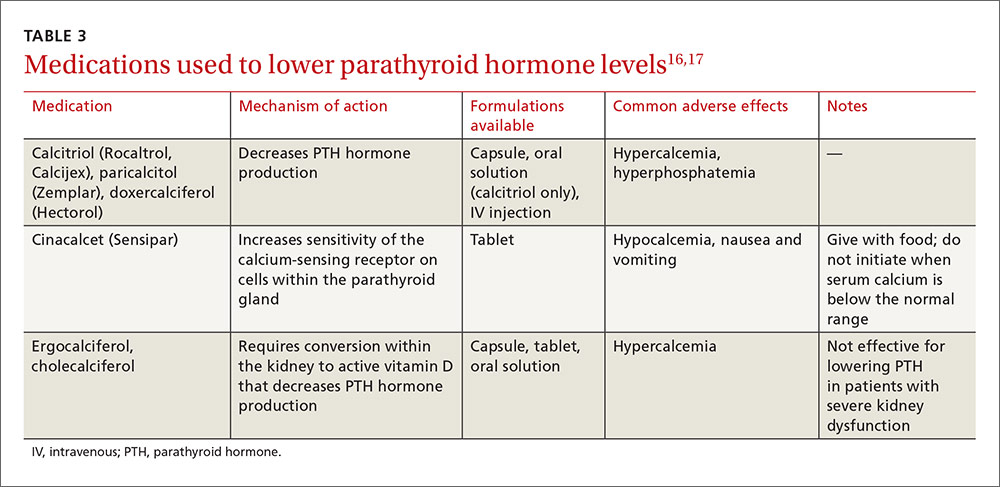
In dialysis patients, the target iPTH range suggested by KDIGO is 2 to 9 times the upper limit of normal for the assay.6 Elevated PTH levels in the dialysis population may be managed with activated vitamin D and/or cinacalcet.
Native vitamin D (ergocalciferol, cholecalciferol) and activated vitamin D analogs (calcitriol, doxercalciferol, paricalcitol). Native vitamin D products are recommended for non-dialyzed patients with CKD to correct vitamin D deficiencies. Although many approaches may be used clinically to replenish low vitamin D stores, one reasonable recommendation in patients with a 25(OH)D level <30 ng/mL is to prescribe ergocalciferol 50,000 units/week for 8 weeks and then to repeat the serum 25-OH vitamin D test. If the level is still <30 ng/mL, a second 8-week course of weekly ergocalciferol 50,000 IU may be administered.21
Following repletion with ergocalciferol, maintenance doses of cholecalciferol (1000-2000 IU/d) or ergocalciferol (50,000 IU/-month) may be initiated.21 Discontinue native vitamin D in patients who develop hypercalcemia.
Native vitamin D becomes less effective at reducing PTH levels as kidney disease advances. This is likely due to a decline in renal conversion of 25(OH)D to 1,25-(OH)2vitamin D (1,25[OH]2D), the most active form of vitamin D and the form of vitamin D that decreases PTH production. By stage 5 CKD, it is unlikely that native vitamin D will significantly decrease PTH levels; treatment with activated vitamin D products or cinacalcet is generally required.
Because the enzyme responsible for converting 25(OH)D into the most active form can be found in multiple tissues outside of the kidney, and the 1,25(OH)2D converted for use by these organs may help prevent such conditions/events as hypertension, type 2 diabetes, myocardial infarction, and stroke (in patients with and without kidney disease), some specialists prescribe native vitamin D to patients with CKD for reasons unrelated to PTH suppression. There are no data, however, confirming that 25(OH)D supplementation mitigates these outcomes.21
Don’t forget calcium
All of the active vitamin D products can increase serum calcium and phosphate levels. Calcitriol, however, may cause more hypercalcemia than paricalcitol.22 If hypercalcemia develops, you may need to stop, or reduce the dose of, vitamin D analogues. Or you may need to switch patients from calcium-based to non-calcium-based phosphate binders. If hyperphosphatemia develops, intensify phosphate binder therapy or reduce the dose of, or stop, vitamin D analogues. If iPTH levels go below the target range, reduce the dose of the vitamin D analogue to avoid iatrogenic adynamic bone disease.
Avoid this agent in the non-dialyzed patient. Cinacalcet effectively treats SHPT in patients receiving dialysis, but is not recommended for use in undialyzed patients.23 That’s because unacceptably high rates of hypocalcemia have been observed in non-dialyzed patients who were taking the drug.23,24 In addition, while cinacalcet neutrally affects, or causes a slight decrease in, serum phosphate in patients receiving dialysis, it increases serum phosphate in patients who are not.24,25
Drug therapy for osteoporosis
Therapy to prevent and treat fractures in patients with CKD is controversial because patients with CKD stage 3 to 5 with and without MBD were excluded from clinical trials of commercially available treatments. Furthermore, in adynamic bone disease, bones are capable of neither breaking down nor building (ie, reduced resorption). Bisphosphonates and other antiresorptive therapies are more effective at decreasing fractures in patients who are in a state of increased bone resorption, such as menopausal women, so the benefits of these medications in terms of their ability to reduce fractures in CKD patients are questionable, as is their safety.26,27
In addition, while dual-energy x-ray absorptiometry (DXA) is typically used to identify patients who would benefit from these agents, studies have recently demonstrated that femoral neck bone density measured via DXA may underestimate fracture risk in patients with CKD-MBD (ie, bone density may actually be lower than measured).26,28
Antiresorptive agents and teriparatide
Osteoporosis treatments include antiresorptive agents (ie, the bisphosphonates, raloxifene, denosumab), and the anabolic bone agent teriparatide.
Evidence supports treating patients with stage 1 to 3 CKD the same as patients without CKD.15 Bisphosphonates are labeled as contraindicated in patients with a glomerular filtration rate (GFR) <30 mL/min/1.73m2, due to concerns arising from animal trials and subsequent human case reports (both with intravenous formulations only) regarding acute kidney injury.27
While raloxifene lacks a warning regarding use in patients with stage 3 to 5 CKD, it has not been shown to prevent hip fractures in any population.29
Denosumab is not contraindicated for use in patients with CKD stage 3 to 5 without MBD, but it can worsen hypocalcemia, particularly in patients receiving dialysis.30
Teriparatide is contraindicated in patients with CKD and SHPT,31 and there are no studies of its use in patients with CKD-MBD.
What the guidelines say about antiresorptive treatment
For patients with stage 3 to 5 CKD with manifestations of MBD, 2009 KDIGO guidelines recommend a bone biopsy to evaluate for adynamic bone disease before initiating antiresorptive treatment.6 Because few physicians in most communities are trained to conduct and evaluate bone biopsies, this recommendation is infrequently followed. Without a bone biopsy to rule out adynamic bone disease, options to prevent or treat fractures in the setting of CKD-MBD are limited.
CORRESPONDENCE
Karly Pippitt, MD, Department of Family and Preventive Medicine, University of Utah School of Medicine, 375 Chipeta Way, Suite A, Salt Lake City, UT 84108; [email protected].
1. United States Renal Data System. Chapter 1: CKD in the general population. Available at: https://www.usrds.org/2015/download/vol1_01_General_Pop_15.pdf. Accessed July 27, 2016.
2. Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038-2047.
3. Uhlig K, Berns JS, Kestenbaum B, et al. KDOQI US commentary on the 2009 KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of CKD-Mineral and Bone Disorder (CKD-MBD). Am J Kidney Dis. 2010;55:773-799.
4. Martin KJ, Gonzalez EA. Metabolic bone disease in chronic kidney disease. J Am Soc Nephrol. 2007;18:875-885.
5. Roberts DM, Singer RF. Management of renal bone disease. Aust Prescr. 2010;33:34-37.
6. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009:S1-130.
7. Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011;305:1119-1127.
8. Cannata-Andia JB, Martin KJ. The challenge of controlling phosphorus in chronic kidney disease. Nephrol Dial Transplant. 2016;31:541-547.
9. US Food and Drug Administration. Food Labeling Guide. Available at: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/ucm2006828.htm. Accessed July 25, 2016.
10. Kalantar-Zadeh K. Patient education for phosphorus management in chronic kidney disease. Patient Prefer Adherence. 2013;7:379-390.
11. Kalantar-Zadeh K, Gutekunst L, Mehrotra R, et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:519-530.
12. USDA National Nutrient Database for Standard Reference. 2015; Available at: https://ndb.nal.usda.gov. Accessed April 25, 2016.
13. Bellasi A. Pro: Should phosphate binders be used in chronic kidney disease stage 3-4? Nephrol Dial Transplant. 2016;31:184-188.
14. Kestenbaum B. Con: Phosphate binders in chronic kidney disease. Nephrol Dial Transplant. 2016;31:189-194.
15. Ketteler M, Elder GJ, Evenepoel P, et al. Revisiting KDIGO clinical practice guideline on chronic kidney disease-mineral and bone disorder: a commentary from a Kidney Disease: Improving Global Outcomes controversies conference. Kidney Int. 2015;87:502-528.
16. Wolters Kluwer. Lexicomp. Clinical Drug Information. Available at: http://www.wolterskluwercdi.com/lexicomp-online/. Accessed April 26, 2016.
17. Truven Health Analytics. Micromedex Solutions. Available at: http://micromedex.com/. Accessed April 26, 2016.
18. Wang S, Anum EA, Ramakrishnan K, et al. Reasons for phosphate binder discontinuation vary by binder type. J Ren Nutr. 2014;24:105-109.
19. Patel L, Bernard LM, Elder GJ. Sevelamer versus calcium-based binders for treatment of hyperphosphatemia in CKD: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol. 2016;11:232-244.
20. Jamal SA, Vandermeer B, Raggi P, et al. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet. 2013;382:1268-1277.
21. Nigwekar SU, Bhan I, Thadhani R. Ergocalciferol and cholecalciferol in CKD. Am J Kidney Dis. 2012;60:139-156.
22. Teng M, Wolf M, Lowrie E, et al. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446-456.
23. Sensipar package insert. Thousand Oaks, California: Amgen Pharmaceuticals; 2014. Available at: http://pi.amgen.com/united_states/sensipar/sensipar_pi_hcp_english.pdf. Accessed April 25, 2016.
24. Chonchol M, Locatelli F, Abboud HE, et al. A randomized, double-blind, placebo-controlled study to assess the efficacy and safety of cinacalcet HCl in participants with CKD not receiving dialysis. Am J Kidney Dis. 2009;53:197-207.
25. Ballinger AE, Palmer SC, Nistor I,et al. Calcimimetics for secondary hyperparathyroidism in chronic kidney disease patients. Cochrane Database Syst Rev. 2014;12:CD006254.
26. Miller PD. Bone disease in CKD: a focus on osteoporosis diagnosis and management. Am J Kidney Dis. 2014;64:290-304.
27. Ott SM. Bisphosphonate safety and efficacy in chronic kidney disease. Kidney Int. 2012;82:833-835.
28. Yencheck RH, Ix JH, Shlipak MG, et al. Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol. 2012;7:1130-1136.
29. Crandall CJ, Newberry SJ, Diamant A, et al. Treatments to prevent fractures in men and women with low bone density or osteoporosis: update of a 2007 report. Comparative Effectiveness Reviews, No. 53. Rockville, MD: Agency for Healthcare Research and Quality; March 2012. Available at: www.effectivehealthcare.ahrq.gov/lbd.cfm. Accessed August 14, 2016.
30. Amgen. Prolia package insert. Available at: http://pi.amgen.com/united_states/prolia/prolia_pi.pdf. Accessed April 26, 2016.
31. Eli Lilly and Company. Fortio package insert. Available at: https://pi.lilly.com/us/forteo-pi.pdf. Accessed April 26, 2016.
PRACTICE RECOMMENDATIONS
› Perform laboratory testing for chronic kidney disease (CKD)-induced bone disease at CKD stage 3. B
› Avoid calcium-based phosphate binders in patients with known vascular calcifications. B
› Consider the use of phosphate binders in non-dialysis patients on a case-by-case basis, particularly in those with hyperphosphatemia not controlled by dietary measures. B
› Prescribe native vitamin D (ergocalciferol or cholecalciferol) to patients with CKD stages 3 to 4 who have secondary hyperparathyroidism and vitamin D deficiency. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
About 14% of the US general population has chronic kidney disease (CKD).1 Limited data exist regarding the exact prevalence of CKD-mineral and bone disorder (MBD), but abnormal mineral metabolism is believed to start in stage 3 CKD, implying that 8% of the adult US population could be at risk for, or already have established, CKD-MBD.2 Although the disorder has traditionally been managed by nephrologists, this earlier onset suggests that many patients should be screened and treated by their primary care physicians.
Because CKD-MBD can lead to significant morbidity (ie, increased fracture risk) and mortality, identification and treatment are of utmost importance.3 This review provides information from the current literature and the KDIGO (Kidney Disease: Improving Global Outcomes) guidelines, and focuses primarily on the non-dialysis CKD population.
CKD-MBD: A broad spectrum of disorders
CKD-MBD is defined as a systemic disorder of mineral and bone metabolism due to CKD. Traditionally referred to as renal osteodystrophy, the term CKD-MBD is meant to indicate and describe a broad clinical spectrum of CKD-associated bone mineral metabolism disorders that manifest from one or a combination of the following:
- Abnormalities of calcium, phosphorus, parathyroid hormone (PTH), or vitamin D metabolism
- Abnormalities in bone turnover, mineralization, volume, linear growth, or strength
- Vascular or other soft-tissue calcification.4
Renal bone disease can be divided into low bone turnover (adynamic bone disease) and high bone turnover states. Both can lead to a decrease in bone strength and an increase in pathological fractures.5
Pathophysiology: Difficult to know where the cascade begins
Understanding the pathophysiology and treatment of bone disease in patients with CKD can be challenging. Because of abnormalities of mineral metabolism and changes in hormones and cytokines, bone remodeling is severely disrupted in patients with CKD, and it remains unclear where this cascade begins.
As an adaptive response to decreased kidney function, PTH levels increase. Elevations of both fibroblast growth factor 23 (FGF23) lower blood phosphate levels by inhibiting phosphate reabsorption in the kidneys, thus increasing urinary excretion of phosphorus. Secondary hyperparathyroidism (SHPT), driven by hypocalcemia, responds to normalize serum calcium levels by increasing the number and size of osteoclasts actively breaking down bone matrix. This escalates fracture risk. In addition, the inability of damaged kidneys to convert vitamin D to an active form further deranges calcium and phosphate homeostasis.
Successful management of serum levels begins with monitoring
KDIGO, an independent nonprofit foundation that seeks to improve the care and outcomes of kidney disease patients worldwide, developed guidelines for the diagnosis, evaluation, prevention, and treatment of CKD-MBD in 2009.6 These guidelines recommend that treatment of CKD-MBD be aimed at managing serum phosphate, PTH, and calcium levels. The recommended frequency for laboratory monitoring of these levels varies by stage of CKD and is described in TABLE 1.6 (For more on chronic kidney disease staging, see KDIGO’s 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease, available at: http://www.kdigo.org/clinical_practice_guidelines/pdf/CKD/KDIGO_2012_CKD_GL.pdf.)

Because of the interrelated nature of these minerals and hormones, drug therapy aimed at treating one may impact the others. This must be considered when designing treatment regimens.
Hyperphosphatemia: Manage with diet, drugs, dialysis
Observational studies have shown an association between higher serum phosphate levels and mortality.6-8 KDIGO recommends maintaining serum phosphorus levels within the normal range of the assay in patients with CKD who are not receiving dialysis.6 For dialyzed patients, the recommendation is to lower the phosphorus level toward the normal range as much as possible.6 Maintaining an appropriate phosphorus level is accomplished through dietary phosphate restriction, the use of phosphate binders, and, in dialyzed patients, dialytic removal of phosphate.6
Dietary phosphate restriction is often challenging for patients, in part, because phosphorous content is not always included on food labels in the United States.9 Phosphorus is highly absorbed from additives in processed food (approaching 100% absorption), less absorbed from animal sources such as meat and dairy products (40%-60%), and is the least absorbed from plant sources such as beans and nuts (20%-40%).10 Advise patients to avoid fast food, processed foods, cheese, frozen meals, colas, and certain ready-to-eat cereals and prepared meats, as these products may have additives from which phosphorus is readily absorbed.11 A patient-friendly list of high-phosphorus foods, as well as other dietary advice and recipes, can be found on the National Kidney Foundation Web site at https://www.kidney.org/atoz/content/phosphorus. Tables listing the phosphorus content of common foods are also available in the literature and online.11,12 Keep in mind that not all resources take into account phosphate bioavailability. Dietician referral may be helpful to assure that patients maintain adequate protein intake while restricting dietary phosphate.
Phosphate binders are recommended by the KDIGO guidelines for use in patients with kidney disease and hyperphosphatemia.6 Most of the data to support the use of phosphate binders was gleaned from the dialysis population. The use of phosphate binders in non-dialyzed patients with CKD has both proponents and opponents, with literature supporting both positions.13,14 A recent KDIGO conference on controversies in CKD-MBD identified this as an area that should be evaluated further for the next guideline update.15
Phosphate binders—which bind the phosphorus in food to prevent absorption—should be taken with meals or high-phosphorus snacks. Products and formulations of commonly used phosphate binders are shown in TABLE 2.16,17 Taste, formulation, adverse effects, pill burden, and cost are issues to discuss with patients when initiating or adjusting phosphate binder therapy. It’s estimated that more than half of all patients receiving dialysis do not adhere to their prescribed phosphate binder regimen, highlighting the need to assess adherence before adjusting dose and to involve the patient in the decision-making process to select a phosphate binder product.18

Avoid calcium-based binders? The risk of hypercalcemia and the potentially increased risk of vascular calcifications with calcium-based binders have led some nephrologists to favor non-calcium-based products. Two recent meta-analyses found a reduced risk of all-cause mortality with the non-calcium-based binders sevelamer or lanthanum as compared to calcium-based binders.19,20 Current KDIGO guidelines were published prior to these meta-analyses and do not recommend one phosphate binder over another. They do, however, recommend restricting the dose of calcium-based binders in the setting of persistent or recurrent hypercalcemia, known vascular calcification, or low PTH levels.6
Secondary hyperparathyroidism
Due to a lack of data, the goal PTH level in patients not receiving dialysis is unknown.6 A reasonable approach in non-dialyzed patients, however, is to correct 25-OH vitamin D (25[OH]D) deficiency, elevations in serum phosphate, and hypocalcemia when the level of intact PTH (iPTH) exceeds the normal range for the assay because correcting these derangements may result in a decline in iPTH.6,21 If this approach fails and PTH levels continue to rise, use of calcitriol or vitamin D analogues is recommended.6 Characteristics of medications used to treat SHPT are presented in TABLE 3.16,17

In dialysis patients, the target iPTH range suggested by KDIGO is 2 to 9 times the upper limit of normal for the assay.6 Elevated PTH levels in the dialysis population may be managed with activated vitamin D and/or cinacalcet.
Native vitamin D (ergocalciferol, cholecalciferol) and activated vitamin D analogs (calcitriol, doxercalciferol, paricalcitol). Native vitamin D products are recommended for non-dialyzed patients with CKD to correct vitamin D deficiencies. Although many approaches may be used clinically to replenish low vitamin D stores, one reasonable recommendation in patients with a 25(OH)D level <30 ng/mL is to prescribe ergocalciferol 50,000 units/week for 8 weeks and then to repeat the serum 25-OH vitamin D test. If the level is still <30 ng/mL, a second 8-week course of weekly ergocalciferol 50,000 IU may be administered.21
Following repletion with ergocalciferol, maintenance doses of cholecalciferol (1000-2000 IU/d) or ergocalciferol (50,000 IU/-month) may be initiated.21 Discontinue native vitamin D in patients who develop hypercalcemia.
Native vitamin D becomes less effective at reducing PTH levels as kidney disease advances. This is likely due to a decline in renal conversion of 25(OH)D to 1,25-(OH)2vitamin D (1,25[OH]2D), the most active form of vitamin D and the form of vitamin D that decreases PTH production. By stage 5 CKD, it is unlikely that native vitamin D will significantly decrease PTH levels; treatment with activated vitamin D products or cinacalcet is generally required.
Because the enzyme responsible for converting 25(OH)D into the most active form can be found in multiple tissues outside of the kidney, and the 1,25(OH)2D converted for use by these organs may help prevent such conditions/events as hypertension, type 2 diabetes, myocardial infarction, and stroke (in patients with and without kidney disease), some specialists prescribe native vitamin D to patients with CKD for reasons unrelated to PTH suppression. There are no data, however, confirming that 25(OH)D supplementation mitigates these outcomes.21
Don’t forget calcium
All of the active vitamin D products can increase serum calcium and phosphate levels. Calcitriol, however, may cause more hypercalcemia than paricalcitol.22 If hypercalcemia develops, you may need to stop, or reduce the dose of, vitamin D analogues. Or you may need to switch patients from calcium-based to non-calcium-based phosphate binders. If hyperphosphatemia develops, intensify phosphate binder therapy or reduce the dose of, or stop, vitamin D analogues. If iPTH levels go below the target range, reduce the dose of the vitamin D analogue to avoid iatrogenic adynamic bone disease.
Avoid this agent in the non-dialyzed patient. Cinacalcet effectively treats SHPT in patients receiving dialysis, but is not recommended for use in undialyzed patients.23 That’s because unacceptably high rates of hypocalcemia have been observed in non-dialyzed patients who were taking the drug.23,24 In addition, while cinacalcet neutrally affects, or causes a slight decrease in, serum phosphate in patients receiving dialysis, it increases serum phosphate in patients who are not.24,25
Drug therapy for osteoporosis
Therapy to prevent and treat fractures in patients with CKD is controversial because patients with CKD stage 3 to 5 with and without MBD were excluded from clinical trials of commercially available treatments. Furthermore, in adynamic bone disease, bones are capable of neither breaking down nor building (ie, reduced resorption). Bisphosphonates and other antiresorptive therapies are more effective at decreasing fractures in patients who are in a state of increased bone resorption, such as menopausal women, so the benefits of these medications in terms of their ability to reduce fractures in CKD patients are questionable, as is their safety.26,27
In addition, while dual-energy x-ray absorptiometry (DXA) is typically used to identify patients who would benefit from these agents, studies have recently demonstrated that femoral neck bone density measured via DXA may underestimate fracture risk in patients with CKD-MBD (ie, bone density may actually be lower than measured).26,28
Antiresorptive agents and teriparatide
Osteoporosis treatments include antiresorptive agents (ie, the bisphosphonates, raloxifene, denosumab), and the anabolic bone agent teriparatide.
Evidence supports treating patients with stage 1 to 3 CKD the same as patients without CKD.15 Bisphosphonates are labeled as contraindicated in patients with a glomerular filtration rate (GFR) <30 mL/min/1.73m2, due to concerns arising from animal trials and subsequent human case reports (both with intravenous formulations only) regarding acute kidney injury.27
While raloxifene lacks a warning regarding use in patients with stage 3 to 5 CKD, it has not been shown to prevent hip fractures in any population.29
Denosumab is not contraindicated for use in patients with CKD stage 3 to 5 without MBD, but it can worsen hypocalcemia, particularly in patients receiving dialysis.30
Teriparatide is contraindicated in patients with CKD and SHPT,31 and there are no studies of its use in patients with CKD-MBD.
What the guidelines say about antiresorptive treatment
For patients with stage 3 to 5 CKD with manifestations of MBD, 2009 KDIGO guidelines recommend a bone biopsy to evaluate for adynamic bone disease before initiating antiresorptive treatment.6 Because few physicians in most communities are trained to conduct and evaluate bone biopsies, this recommendation is infrequently followed. Without a bone biopsy to rule out adynamic bone disease, options to prevent or treat fractures in the setting of CKD-MBD are limited.
CORRESPONDENCE
Karly Pippitt, MD, Department of Family and Preventive Medicine, University of Utah School of Medicine, 375 Chipeta Way, Suite A, Salt Lake City, UT 84108; [email protected].
PRACTICE RECOMMENDATIONS
› Perform laboratory testing for chronic kidney disease (CKD)-induced bone disease at CKD stage 3. B
› Avoid calcium-based phosphate binders in patients with known vascular calcifications. B
› Consider the use of phosphate binders in non-dialysis patients on a case-by-case basis, particularly in those with hyperphosphatemia not controlled by dietary measures. B
› Prescribe native vitamin D (ergocalciferol or cholecalciferol) to patients with CKD stages 3 to 4 who have secondary hyperparathyroidism and vitamin D deficiency. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
About 14% of the US general population has chronic kidney disease (CKD).1 Limited data exist regarding the exact prevalence of CKD-mineral and bone disorder (MBD), but abnormal mineral metabolism is believed to start in stage 3 CKD, implying that 8% of the adult US population could be at risk for, or already have established, CKD-MBD.2 Although the disorder has traditionally been managed by nephrologists, this earlier onset suggests that many patients should be screened and treated by their primary care physicians.
Because CKD-MBD can lead to significant morbidity (ie, increased fracture risk) and mortality, identification and treatment are of utmost importance.3 This review provides information from the current literature and the KDIGO (Kidney Disease: Improving Global Outcomes) guidelines, and focuses primarily on the non-dialysis CKD population.
CKD-MBD: A broad spectrum of disorders
CKD-MBD is defined as a systemic disorder of mineral and bone metabolism due to CKD. Traditionally referred to as renal osteodystrophy, the term CKD-MBD is meant to indicate and describe a broad clinical spectrum of CKD-associated bone mineral metabolism disorders that manifest from one or a combination of the following:
- Abnormalities of calcium, phosphorus, parathyroid hormone (PTH), or vitamin D metabolism
- Abnormalities in bone turnover, mineralization, volume, linear growth, or strength
- Vascular or other soft-tissue calcification.4
Renal bone disease can be divided into low bone turnover (adynamic bone disease) and high bone turnover states. Both can lead to a decrease in bone strength and an increase in pathological fractures.5
Pathophysiology: Difficult to know where the cascade begins
Understanding the pathophysiology and treatment of bone disease in patients with CKD can be challenging. Because of abnormalities of mineral metabolism and changes in hormones and cytokines, bone remodeling is severely disrupted in patients with CKD, and it remains unclear where this cascade begins.
As an adaptive response to decreased kidney function, PTH levels increase. Elevations of both fibroblast growth factor 23 (FGF23) lower blood phosphate levels by inhibiting phosphate reabsorption in the kidneys, thus increasing urinary excretion of phosphorus. Secondary hyperparathyroidism (SHPT), driven by hypocalcemia, responds to normalize serum calcium levels by increasing the number and size of osteoclasts actively breaking down bone matrix. This escalates fracture risk. In addition, the inability of damaged kidneys to convert vitamin D to an active form further deranges calcium and phosphate homeostasis.
Successful management of serum levels begins with monitoring
KDIGO, an independent nonprofit foundation that seeks to improve the care and outcomes of kidney disease patients worldwide, developed guidelines for the diagnosis, evaluation, prevention, and treatment of CKD-MBD in 2009.6 These guidelines recommend that treatment of CKD-MBD be aimed at managing serum phosphate, PTH, and calcium levels. The recommended frequency for laboratory monitoring of these levels varies by stage of CKD and is described in TABLE 1.6 (For more on chronic kidney disease staging, see KDIGO’s 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease, available at: http://www.kdigo.org/clinical_practice_guidelines/pdf/CKD/KDIGO_2012_CKD_GL.pdf.)

Because of the interrelated nature of these minerals and hormones, drug therapy aimed at treating one may impact the others. This must be considered when designing treatment regimens.
Hyperphosphatemia: Manage with diet, drugs, dialysis
Observational studies have shown an association between higher serum phosphate levels and mortality.6-8 KDIGO recommends maintaining serum phosphorus levels within the normal range of the assay in patients with CKD who are not receiving dialysis.6 For dialyzed patients, the recommendation is to lower the phosphorus level toward the normal range as much as possible.6 Maintaining an appropriate phosphorus level is accomplished through dietary phosphate restriction, the use of phosphate binders, and, in dialyzed patients, dialytic removal of phosphate.6
Dietary phosphate restriction is often challenging for patients, in part, because phosphorous content is not always included on food labels in the United States.9 Phosphorus is highly absorbed from additives in processed food (approaching 100% absorption), less absorbed from animal sources such as meat and dairy products (40%-60%), and is the least absorbed from plant sources such as beans and nuts (20%-40%).10 Advise patients to avoid fast food, processed foods, cheese, frozen meals, colas, and certain ready-to-eat cereals and prepared meats, as these products may have additives from which phosphorus is readily absorbed.11 A patient-friendly list of high-phosphorus foods, as well as other dietary advice and recipes, can be found on the National Kidney Foundation Web site at https://www.kidney.org/atoz/content/phosphorus. Tables listing the phosphorus content of common foods are also available in the literature and online.11,12 Keep in mind that not all resources take into account phosphate bioavailability. Dietician referral may be helpful to assure that patients maintain adequate protein intake while restricting dietary phosphate.
Phosphate binders are recommended by the KDIGO guidelines for use in patients with kidney disease and hyperphosphatemia.6 Most of the data to support the use of phosphate binders was gleaned from the dialysis population. The use of phosphate binders in non-dialyzed patients with CKD has both proponents and opponents, with literature supporting both positions.13,14 A recent KDIGO conference on controversies in CKD-MBD identified this as an area that should be evaluated further for the next guideline update.15
Phosphate binders—which bind the phosphorus in food to prevent absorption—should be taken with meals or high-phosphorus snacks. Products and formulations of commonly used phosphate binders are shown in TABLE 2.16,17 Taste, formulation, adverse effects, pill burden, and cost are issues to discuss with patients when initiating or adjusting phosphate binder therapy. It’s estimated that more than half of all patients receiving dialysis do not adhere to their prescribed phosphate binder regimen, highlighting the need to assess adherence before adjusting dose and to involve the patient in the decision-making process to select a phosphate binder product.18

Avoid calcium-based binders? The risk of hypercalcemia and the potentially increased risk of vascular calcifications with calcium-based binders have led some nephrologists to favor non-calcium-based products. Two recent meta-analyses found a reduced risk of all-cause mortality with the non-calcium-based binders sevelamer or lanthanum as compared to calcium-based binders.19,20 Current KDIGO guidelines were published prior to these meta-analyses and do not recommend one phosphate binder over another. They do, however, recommend restricting the dose of calcium-based binders in the setting of persistent or recurrent hypercalcemia, known vascular calcification, or low PTH levels.6
Secondary hyperparathyroidism
Due to a lack of data, the goal PTH level in patients not receiving dialysis is unknown.6 A reasonable approach in non-dialyzed patients, however, is to correct 25-OH vitamin D (25[OH]D) deficiency, elevations in serum phosphate, and hypocalcemia when the level of intact PTH (iPTH) exceeds the normal range for the assay because correcting these derangements may result in a decline in iPTH.6,21 If this approach fails and PTH levels continue to rise, use of calcitriol or vitamin D analogues is recommended.6 Characteristics of medications used to treat SHPT are presented in TABLE 3.16,17

In dialysis patients, the target iPTH range suggested by KDIGO is 2 to 9 times the upper limit of normal for the assay.6 Elevated PTH levels in the dialysis population may be managed with activated vitamin D and/or cinacalcet.
Native vitamin D (ergocalciferol, cholecalciferol) and activated vitamin D analogs (calcitriol, doxercalciferol, paricalcitol). Native vitamin D products are recommended for non-dialyzed patients with CKD to correct vitamin D deficiencies. Although many approaches may be used clinically to replenish low vitamin D stores, one reasonable recommendation in patients with a 25(OH)D level <30 ng/mL is to prescribe ergocalciferol 50,000 units/week for 8 weeks and then to repeat the serum 25-OH vitamin D test. If the level is still <30 ng/mL, a second 8-week course of weekly ergocalciferol 50,000 IU may be administered.21
Following repletion with ergocalciferol, maintenance doses of cholecalciferol (1000-2000 IU/d) or ergocalciferol (50,000 IU/-month) may be initiated.21 Discontinue native vitamin D in patients who develop hypercalcemia.
Native vitamin D becomes less effective at reducing PTH levels as kidney disease advances. This is likely due to a decline in renal conversion of 25(OH)D to 1,25-(OH)2vitamin D (1,25[OH]2D), the most active form of vitamin D and the form of vitamin D that decreases PTH production. By stage 5 CKD, it is unlikely that native vitamin D will significantly decrease PTH levels; treatment with activated vitamin D products or cinacalcet is generally required.
Because the enzyme responsible for converting 25(OH)D into the most active form can be found in multiple tissues outside of the kidney, and the 1,25(OH)2D converted for use by these organs may help prevent such conditions/events as hypertension, type 2 diabetes, myocardial infarction, and stroke (in patients with and without kidney disease), some specialists prescribe native vitamin D to patients with CKD for reasons unrelated to PTH suppression. There are no data, however, confirming that 25(OH)D supplementation mitigates these outcomes.21
Don’t forget calcium
All of the active vitamin D products can increase serum calcium and phosphate levels. Calcitriol, however, may cause more hypercalcemia than paricalcitol.22 If hypercalcemia develops, you may need to stop, or reduce the dose of, vitamin D analogues. Or you may need to switch patients from calcium-based to non-calcium-based phosphate binders. If hyperphosphatemia develops, intensify phosphate binder therapy or reduce the dose of, or stop, vitamin D analogues. If iPTH levels go below the target range, reduce the dose of the vitamin D analogue to avoid iatrogenic adynamic bone disease.
Avoid this agent in the non-dialyzed patient. Cinacalcet effectively treats SHPT in patients receiving dialysis, but is not recommended for use in undialyzed patients.23 That’s because unacceptably high rates of hypocalcemia have been observed in non-dialyzed patients who were taking the drug.23,24 In addition, while cinacalcet neutrally affects, or causes a slight decrease in, serum phosphate in patients receiving dialysis, it increases serum phosphate in patients who are not.24,25
Drug therapy for osteoporosis
Therapy to prevent and treat fractures in patients with CKD is controversial because patients with CKD stage 3 to 5 with and without MBD were excluded from clinical trials of commercially available treatments. Furthermore, in adynamic bone disease, bones are capable of neither breaking down nor building (ie, reduced resorption). Bisphosphonates and other antiresorptive therapies are more effective at decreasing fractures in patients who are in a state of increased bone resorption, such as menopausal women, so the benefits of these medications in terms of their ability to reduce fractures in CKD patients are questionable, as is their safety.26,27
In addition, while dual-energy x-ray absorptiometry (DXA) is typically used to identify patients who would benefit from these agents, studies have recently demonstrated that femoral neck bone density measured via DXA may underestimate fracture risk in patients with CKD-MBD (ie, bone density may actually be lower than measured).26,28
Antiresorptive agents and teriparatide
Osteoporosis treatments include antiresorptive agents (ie, the bisphosphonates, raloxifene, denosumab), and the anabolic bone agent teriparatide.
Evidence supports treating patients with stage 1 to 3 CKD the same as patients without CKD.15 Bisphosphonates are labeled as contraindicated in patients with a glomerular filtration rate (GFR) <30 mL/min/1.73m2, due to concerns arising from animal trials and subsequent human case reports (both with intravenous formulations only) regarding acute kidney injury.27
While raloxifene lacks a warning regarding use in patients with stage 3 to 5 CKD, it has not been shown to prevent hip fractures in any population.29
Denosumab is not contraindicated for use in patients with CKD stage 3 to 5 without MBD, but it can worsen hypocalcemia, particularly in patients receiving dialysis.30
Teriparatide is contraindicated in patients with CKD and SHPT,31 and there are no studies of its use in patients with CKD-MBD.
What the guidelines say about antiresorptive treatment
For patients with stage 3 to 5 CKD with manifestations of MBD, 2009 KDIGO guidelines recommend a bone biopsy to evaluate for adynamic bone disease before initiating antiresorptive treatment.6 Because few physicians in most communities are trained to conduct and evaluate bone biopsies, this recommendation is infrequently followed. Without a bone biopsy to rule out adynamic bone disease, options to prevent or treat fractures in the setting of CKD-MBD are limited.
CORRESPONDENCE
Karly Pippitt, MD, Department of Family and Preventive Medicine, University of Utah School of Medicine, 375 Chipeta Way, Suite A, Salt Lake City, UT 84108; [email protected].
1. United States Renal Data System. Chapter 1: CKD in the general population. Available at: https://www.usrds.org/2015/download/vol1_01_General_Pop_15.pdf. Accessed July 27, 2016.
2. Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038-2047.
3. Uhlig K, Berns JS, Kestenbaum B, et al. KDOQI US commentary on the 2009 KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of CKD-Mineral and Bone Disorder (CKD-MBD). Am J Kidney Dis. 2010;55:773-799.
4. Martin KJ, Gonzalez EA. Metabolic bone disease in chronic kidney disease. J Am Soc Nephrol. 2007;18:875-885.
5. Roberts DM, Singer RF. Management of renal bone disease. Aust Prescr. 2010;33:34-37.
6. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009:S1-130.
7. Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011;305:1119-1127.
8. Cannata-Andia JB, Martin KJ. The challenge of controlling phosphorus in chronic kidney disease. Nephrol Dial Transplant. 2016;31:541-547.
9. US Food and Drug Administration. Food Labeling Guide. Available at: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/ucm2006828.htm. Accessed July 25, 2016.
10. Kalantar-Zadeh K. Patient education for phosphorus management in chronic kidney disease. Patient Prefer Adherence. 2013;7:379-390.
11. Kalantar-Zadeh K, Gutekunst L, Mehrotra R, et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:519-530.
12. USDA National Nutrient Database for Standard Reference. 2015; Available at: https://ndb.nal.usda.gov. Accessed April 25, 2016.
13. Bellasi A. Pro: Should phosphate binders be used in chronic kidney disease stage 3-4? Nephrol Dial Transplant. 2016;31:184-188.
14. Kestenbaum B. Con: Phosphate binders in chronic kidney disease. Nephrol Dial Transplant. 2016;31:189-194.
15. Ketteler M, Elder GJ, Evenepoel P, et al. Revisiting KDIGO clinical practice guideline on chronic kidney disease-mineral and bone disorder: a commentary from a Kidney Disease: Improving Global Outcomes controversies conference. Kidney Int. 2015;87:502-528.
16. Wolters Kluwer. Lexicomp. Clinical Drug Information. Available at: http://www.wolterskluwercdi.com/lexicomp-online/. Accessed April 26, 2016.
17. Truven Health Analytics. Micromedex Solutions. Available at: http://micromedex.com/. Accessed April 26, 2016.
18. Wang S, Anum EA, Ramakrishnan K, et al. Reasons for phosphate binder discontinuation vary by binder type. J Ren Nutr. 2014;24:105-109.
19. Patel L, Bernard LM, Elder GJ. Sevelamer versus calcium-based binders for treatment of hyperphosphatemia in CKD: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol. 2016;11:232-244.
20. Jamal SA, Vandermeer B, Raggi P, et al. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet. 2013;382:1268-1277.
21. Nigwekar SU, Bhan I, Thadhani R. Ergocalciferol and cholecalciferol in CKD. Am J Kidney Dis. 2012;60:139-156.
22. Teng M, Wolf M, Lowrie E, et al. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446-456.
23. Sensipar package insert. Thousand Oaks, California: Amgen Pharmaceuticals; 2014. Available at: http://pi.amgen.com/united_states/sensipar/sensipar_pi_hcp_english.pdf. Accessed April 25, 2016.
24. Chonchol M, Locatelli F, Abboud HE, et al. A randomized, double-blind, placebo-controlled study to assess the efficacy and safety of cinacalcet HCl in participants with CKD not receiving dialysis. Am J Kidney Dis. 2009;53:197-207.
25. Ballinger AE, Palmer SC, Nistor I,et al. Calcimimetics for secondary hyperparathyroidism in chronic kidney disease patients. Cochrane Database Syst Rev. 2014;12:CD006254.
26. Miller PD. Bone disease in CKD: a focus on osteoporosis diagnosis and management. Am J Kidney Dis. 2014;64:290-304.
27. Ott SM. Bisphosphonate safety and efficacy in chronic kidney disease. Kidney Int. 2012;82:833-835.
28. Yencheck RH, Ix JH, Shlipak MG, et al. Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol. 2012;7:1130-1136.
29. Crandall CJ, Newberry SJ, Diamant A, et al. Treatments to prevent fractures in men and women with low bone density or osteoporosis: update of a 2007 report. Comparative Effectiveness Reviews, No. 53. Rockville, MD: Agency for Healthcare Research and Quality; March 2012. Available at: www.effectivehealthcare.ahrq.gov/lbd.cfm. Accessed August 14, 2016.
30. Amgen. Prolia package insert. Available at: http://pi.amgen.com/united_states/prolia/prolia_pi.pdf. Accessed April 26, 2016.
31. Eli Lilly and Company. Fortio package insert. Available at: https://pi.lilly.com/us/forteo-pi.pdf. Accessed April 26, 2016.
1. United States Renal Data System. Chapter 1: CKD in the general population. Available at: https://www.usrds.org/2015/download/vol1_01_General_Pop_15.pdf. Accessed July 27, 2016.
2. Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038-2047.
3. Uhlig K, Berns JS, Kestenbaum B, et al. KDOQI US commentary on the 2009 KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of CKD-Mineral and Bone Disorder (CKD-MBD). Am J Kidney Dis. 2010;55:773-799.
4. Martin KJ, Gonzalez EA. Metabolic bone disease in chronic kidney disease. J Am Soc Nephrol. 2007;18:875-885.
5. Roberts DM, Singer RF. Management of renal bone disease. Aust Prescr. 2010;33:34-37.
6. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009:S1-130.
7. Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011;305:1119-1127.
8. Cannata-Andia JB, Martin KJ. The challenge of controlling phosphorus in chronic kidney disease. Nephrol Dial Transplant. 2016;31:541-547.
9. US Food and Drug Administration. Food Labeling Guide. Available at: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/ucm2006828.htm. Accessed July 25, 2016.
10. Kalantar-Zadeh K. Patient education for phosphorus management in chronic kidney disease. Patient Prefer Adherence. 2013;7:379-390.
11. Kalantar-Zadeh K, Gutekunst L, Mehrotra R, et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:519-530.
12. USDA National Nutrient Database for Standard Reference. 2015; Available at: https://ndb.nal.usda.gov. Accessed April 25, 2016.
13. Bellasi A. Pro: Should phosphate binders be used in chronic kidney disease stage 3-4? Nephrol Dial Transplant. 2016;31:184-188.
14. Kestenbaum B. Con: Phosphate binders in chronic kidney disease. Nephrol Dial Transplant. 2016;31:189-194.
15. Ketteler M, Elder GJ, Evenepoel P, et al. Revisiting KDIGO clinical practice guideline on chronic kidney disease-mineral and bone disorder: a commentary from a Kidney Disease: Improving Global Outcomes controversies conference. Kidney Int. 2015;87:502-528.
16. Wolters Kluwer. Lexicomp. Clinical Drug Information. Available at: http://www.wolterskluwercdi.com/lexicomp-online/. Accessed April 26, 2016.
17. Truven Health Analytics. Micromedex Solutions. Available at: http://micromedex.com/. Accessed April 26, 2016.
18. Wang S, Anum EA, Ramakrishnan K, et al. Reasons for phosphate binder discontinuation vary by binder type. J Ren Nutr. 2014;24:105-109.
19. Patel L, Bernard LM, Elder GJ. Sevelamer versus calcium-based binders for treatment of hyperphosphatemia in CKD: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol. 2016;11:232-244.
20. Jamal SA, Vandermeer B, Raggi P, et al. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet. 2013;382:1268-1277.
21. Nigwekar SU, Bhan I, Thadhani R. Ergocalciferol and cholecalciferol in CKD. Am J Kidney Dis. 2012;60:139-156.
22. Teng M, Wolf M, Lowrie E, et al. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446-456.
23. Sensipar package insert. Thousand Oaks, California: Amgen Pharmaceuticals; 2014. Available at: http://pi.amgen.com/united_states/sensipar/sensipar_pi_hcp_english.pdf. Accessed April 25, 2016.
24. Chonchol M, Locatelli F, Abboud HE, et al. A randomized, double-blind, placebo-controlled study to assess the efficacy and safety of cinacalcet HCl in participants with CKD not receiving dialysis. Am J Kidney Dis. 2009;53:197-207.
25. Ballinger AE, Palmer SC, Nistor I,et al. Calcimimetics for secondary hyperparathyroidism in chronic kidney disease patients. Cochrane Database Syst Rev. 2014;12:CD006254.
26. Miller PD. Bone disease in CKD: a focus on osteoporosis diagnosis and management. Am J Kidney Dis. 2014;64:290-304.
27. Ott SM. Bisphosphonate safety and efficacy in chronic kidney disease. Kidney Int. 2012;82:833-835.
28. Yencheck RH, Ix JH, Shlipak MG, et al. Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol. 2012;7:1130-1136.
29. Crandall CJ, Newberry SJ, Diamant A, et al. Treatments to prevent fractures in men and women with low bone density or osteoporosis: update of a 2007 report. Comparative Effectiveness Reviews, No. 53. Rockville, MD: Agency for Healthcare Research and Quality; March 2012. Available at: www.effectivehealthcare.ahrq.gov/lbd.cfm. Accessed August 14, 2016.
30. Amgen. Prolia package insert. Available at: http://pi.amgen.com/united_states/prolia/prolia_pi.pdf. Accessed April 26, 2016.
31. Eli Lilly and Company. Fortio package insert. Available at: https://pi.lilly.com/us/forteo-pi.pdf. Accessed April 26, 2016.
From The Journal of Family Practice | 2016;65(9):606-608,610-612
Is an SGLT2 inhibitor right for your patient with type 2 diabetes?
› Consider sodium-glucose cotransporter 2 (SGLT2) inhibitors as second-line agents in patients with type 2 diabetes mellitus who need mild hemoglobin A1c reductions (≤1%) and who would benefit from mild to modest weight and blood pressure reductions. A
› Avoid using SGLT2 inhibitors in patients with a history of recurrent genital mycotic or urinary tract infections. B
› Use SGLT2 inhibitors with caution in patients at risk for volume-related adverse effects (dizziness and hypotension), such as the elderly, those with moderate renal dysfunction, and those taking concomitant diuretic therapy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Joe S is a 41-year-old African-American man who comes to your clinic after his employee health screening revealed elevated triglycerides. The patient has a 3-year history of type 2 diabetes mellitus (T2DM); he also has a history of hypertension, gastroesophageal reflux disease, and obstructive sleep apnea. Mr. S tells you he takes metformin 1000 mg twice daily, but stopped taking his glipizide because he didn’t think it was helping his blood sugar. His last hemoglobin (Hb) A1c result was 8.8%, and he is very resistant to starting insulin therapy.
The patient’s other medications include enalapril 10 mg/d, atorvastatin 10 mg/d, and omeprazole 20 mg/d. Mr. S weighs 255.6 lbs (body mass index=34.7), his BP is 140/88 mm Hg, and his heart rate is 82 beats per minute. Laboratory values include: serum creatinine, 1.01 mg/dL; estimated glomerular filtration rate (eGFR) >100 mL/min/1.73 m2; potassium (K), 4.3 mmol/L; serum phosphorous (Phos), 2.8 mg/dL; magnesium (Mg), 1.9 mg/dL; total cholesterol, 167 mg/dL; low-density lipoprotein (LDL), 78 mg/dL; high-density lipoprotein (HDL), 38 mg/dL; and triglycerides, 256 mg/dL.
CASE 2 › Susan R, a 68-year-old Caucasian woman, returns to your clinic for a follow-up visit 3 months after you prescribed dapagliflozin 10 mg/d for her T2DM. Her glucose levels have improved, but she complains of vaginal pruritus and is worried that she has a yeast infection.
You diagnose vulvovaginal candidiasis in this patient and prescribe a single dose of fluconazole 150 mg. After reviewing her laboratory test results, you notice that since starting the dapagliflozin, her HbA1c level has improved slightly from 9.8% to 9.3%, but is still not where it needs to be. Her eGFR is 49 mL/min/1.73 m2.
What would you recommend to improve control of these patients’ blood glucose levels?
When to consider an SGLT2 inhibitor
Consider therapy with SGLT2 inhibitors in adult patients with T2DM who:3-9,13-15,17-24
- have an HbA1c between 7% and 9%
- would benefit from weight and/or blood pressure reductions
- have metabolic syndrome
- have adequate means to pay for the medication (ie, prescription coverage or the ability to afford it).
In addition, consider an SGLT2 inhibitor as initial monotherapy if metformin is contraindicated or not tolerated, or as add-on therapy to metformin, sulfonylureas, thiazolidinediones, dipeptidyl peptidase IV inhibitors, or insulin.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are the newest class of agents to enter the T2DM management arena. They act in the proximal renal tubules to decrease the reabsorption of glucose by targeting the SGLT2 transmembrane protein, which reabsorbs about 90% of the body’s glucose.1,2 The class is currently made up of 3 agents—canagliflozin, dapagliflozin, and empagliflozin—all of which are approved by the US Food and Drug Administration (FDA) for the treatment of T2DM (TABLE 1).2
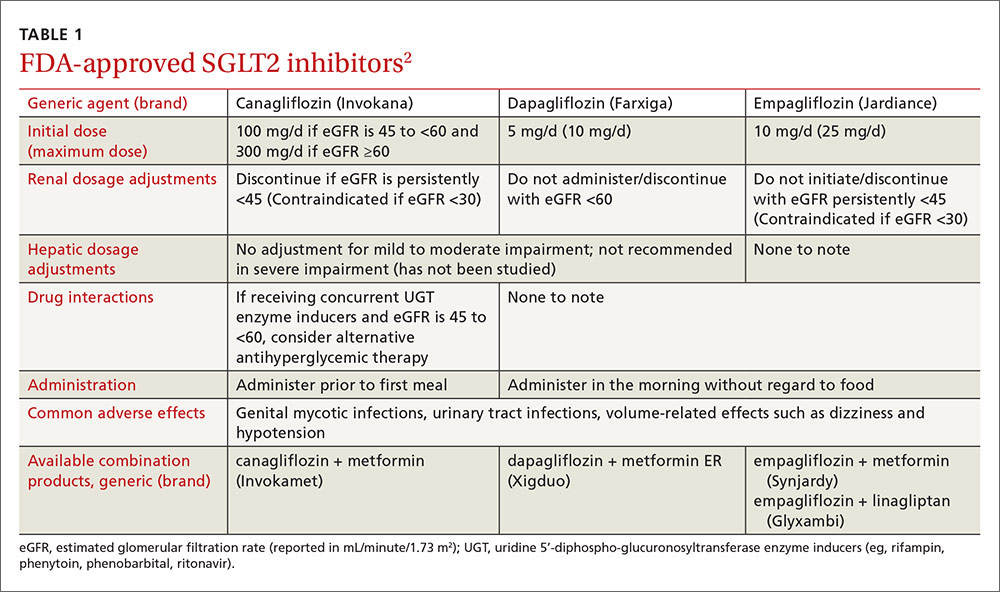
The American Diabetes Association and the European Association for the Study of Diabetes published updated guidelines for T2DM management in 2015.1 In addition to lifestyle modifications, the guidelines recommend the use of metformin as first-line therapy unless it is contraindicated or patients are unable to tolerate it (eg, because of gastrointestinal adverse effects). They recommend other pharmacologic therapies as second-line options based on specific patient characteristics. Thus, SGLT2 inhibitors may be used as add-on therapy after metformin, or as a first-line option if metformin is contraindicated or not tolerated. Because the mechanism of action of SGLT2 inhibitors is independent of insulin secretion, these agents may be used at any stage of the diabetes continuum.
SGLT2 agents as monotherapy, or as add-on therapy
All SGLT2 agents have been studied as monotherapy accompanied by diet and exercise and shown to produce HbA1c reductions of 0.34% to 1.11%.3-6 In trials, the effect was similar regardless of study duration (18-104 weeks); generally, higher doses corresponded with larger HbA1c reductions.3-6
SGLT2 inhibitors have also been studied as add-on therapy to several oral agents including metformin, sulfonylureas, thiazolidinediones (TZDs), and the combination of metformin plus sulfonylureas or TZDs or dipeptidyl peptidase IV (DPP-IV) inhibitors.1 When used in any of these combinations, each SGLT2 agent demonstrated a consistent HbA1c lowering effect of 0.62% to 1.19%.7-14
Additionally, SGLT2 inhibitors have been studied in combination with insulin therapy (median or mean daily doses >60 units), which yielded further reductions in HbA1c of 0.58% to 1.02% without significant insulin adjustments or an increase in major hypoglycemia events.15-17 Patients receiving insulin and an SGLT2 inhibitor had lower insulin doses and more weight loss compared to placebo groups.
SGLT2 inhibitors offer additional benefits
Secondary analyses of most studies of SGLT2 inhibitors include changes in BP and weight from baseline as well as minor changes (some positive, some not) in several lipid parameters.3-5,7-9,13-15,17-24 In general, these effects do not appear to be dose-dependent (with the exception of canagliflozin and its associated lipid effects25) and are similar among the 3 medications.3-5,7-9,13-15,17-24 (For more on who would benefit from these agents, see “When to consider an SGLT2 inhibitor” above.)
BP reduction. Although the mean baseline BP was controlled in most studies, SGLT2 inhibitors have been shown to significantly reduce BP. Reductions in BP with all 3 SGLT2 medications range from approximately 2 to 5 mm Hg systolic and 0.5 to 2.5 mm Hg diastolic, which may be due to weight loss and diuresis.4-8,10-16,20-23 While the reductions were modest at best, one study involving empagliflozin reported that more than one-quarter of patients with uncontrolled BP at baseline achieved a BP <130/80 mm Hg 24 weeks later.5 While these agents should not be used solely for their BP lowering effects, they may help a small number of patients with mildly elevated BP achieve their goal without an additional antihypertensive agent.
Weight reduction. Modest weight loss, likely due to the loss of calories through urine, was seen with SGLT2 inhibitors in most studies, with reductions persisting beyond one year of use. In most studies, including those involving obese patients on insulin therapy,15,17,21 patients’ body weights were reduced by approximately 2 to 4 kg from baseline.3-16,18,21-23,26
Lipid effects. Although the mechanism is unclear, use of SGLT2 inhibitors can have varying effects on lipid panels. In most studies, total and LDL cholesterol levels were increased with elevations ranging from 0.7 to 10 mg/dL.3,7,8,18,19,22,23 Conversely, at least one study demonstrated mild reductions in total and LDL cholesterol levels with higher doses of empagliflozin.13 Additionally, modest reductions in triglycerides and increases in HDL across all doses of canagliflozin, dapagliflozin, and empagliflozin have been seen.8,9,13,15,19 While the clinical relevance of these lipid changes is unknown, monitoring is recommended.2
These agents are well tolerated
SGLT2 inhibitors were generally well tolerated in studies. The most common adverse effects include mycotic infections (2.4%-21.6%) and urinary tract infections (UTIs) (4.0%-19.6%) (both with higher incidences in females); volume-related effects such as dizziness and hypotension (0.3%-8.3%); and nasopharyngitis (5.4%-18.3%).4-14,16-23,26-28 Hypoglycemia was observed more often when an SGLT2 inhibitor was used in combination with a sulfonylurea or insulin therapy.4-14,16-23,26-28 The number of times adverse events led to discontinuation was low and similar to that in control groups.4-14,16-23,26-28
Mycotic and urinary infections should be diagnosed and treated according to current standards of care and do not require discontinuation of the SGLT2 inhibitor. Canagli-flozin therapy was associated with electrolyte abnormalities including hyperkalemia, hypermagnesemia, and hyperphosphatemia.25 Thus, levels should be monitored periodically, especially in patients predisposed to elevations due to other conditions or medications.25
Two additional warnings are worth noting
Diabetic ketoacidosis (DKA) has been reported with all 3 agents, and bone fractures have been reported with canagliflozin.
The FDA issued a warning in May 2015 regarding the increased risk of DKA with the use of SGLT2 inhibitor single and combination products.29 This warning was prompted by several case reports of DKA with uncharacteristically mild to moderate glucose elevations in patients with type 1 diabetes mellitus (T1DM) and T2DM who were taking an SGLT2 inhibitor. The absence of significant hyperglycemia delayed diagnosis in many cases. Therefore, patients should be counseled on the signs and symptoms of DKA, as well as when to seek medical attention.
Patients with diabetes and symptoms of ketoacidosis (eg, difficulty breathing, nausea, vomiting, abdominal pain, confusion, and fatigue) should be evaluated regardless of current blood glucose levels, and SGLT2 inhibitors should be discontinued if acidosis is confirmed. Identified potential triggers include illness, reduced food and fluid intake, reduced insulin dose, and history of alcohol intake. Use of SGLT2 inhibitors should be avoided in patients with T1DM until safety and efficacy are established in large randomized controlled trials. The European Medicines Agency announced that a thorough review of all currently approved SGLT2 agents is underway to evaluate the risk for DKA.30
In addition, the FDA called for a revision of the label of canagliflozin to reflect a strengthened warning about an increased risk of bone fractures and decreased bone mineral density (BMD).31 Fractures can occur as early as 12 weeks after initiating treatment and with only minor trauma.31
Over a 2-year period, canagliflozin also significantly decreased BMD in the hip and lower spine compared to placebo.31 Patients should be evaluated for additional risk factors for fracture before taking canagliflozin.31 The FDA is continuing to evaluate whether the other approved SGLT2 inhibitors are associated with an increased risk for fractures.
Drug interactions: Proceed carefully with diuretics
The number of drugs that interact with SGLT2 inhibitors is minimal. Because these agents can cause volume-related effects such as hypotension, dizziness, and osmotic diuresis, patients—particularly the elderly and those with renal impairment—taking concomitant diuretics, especially loop diuretics, may be at increased risk for these effects and should be monitored accordingly.2,25
Canagliflozin is primarily metabolized via glucuronidation by the uridine 5'-diphospho-glucuronosyltransferase (UGT) enzymes. Therefore, UGT enzyme inducers (eg, rifampin, phenytoin, phenobarbital, ritonavir) decrease canagliflozin’s serum concentration. If a patient has an eGFR >60 mL/min/1.73 m2 and is tolerating a dose of 100 mg/d, consider increasing the dose to 300 mg/d during concomitant treatment.
In addition, researchers have found that canagliflozin increases serum levels of digoxin by between 20% and 36%.25 Experts suspect this occurs because canagliflozin inhibits P-glycoprotein efflux of digoxin. Although monitoring of digoxin levels is recommended, this interaction is considered to be minor.25
Cost consideration: SGLT2 inhibitors are more expensive
The SGLT2 inhibitors are available only as brand name products and are more expensive than agents that have generic options (eg, metformin, sulfonylureas, TZDs). The average wholesale cost is approximately $400 for a 30-day supply of all SGLT2 agents.32 When considering an SGLT2 inhibitor, the patient should ideally have medication prescription coverage. Depending on the specific insurance plan, these agents are classified as tier 2 to 4, which is comparable to other oral brand name options.
Research looks at CV outcomes and cancer risk
Cardiovascular (CV) risk reduction. To date, only one study evaluating the effect of SGLT2 inhibitors on CV outcomes is complete.33 Two large randomized controlled trials involving canagliflozin and dapagliflozin designed to evaluate treatment effects on major CV endpoints are ongoing.34,35
In the EMPA-REG OUTCOME trial,33 researchers found that empagliflozin had beneficial effects on CV outcomes, making it one of the only antidiabetic agents on the market to have such benefits. The study, which involved more than 7000 patients with a history of T2DM and existing cardiovascular disease (CVD), found that 10.5% of patients in the empagliflozin group vs 12.1% in the placebo group died from a CV cause or experienced a nonfatal myocardial infarction or stroke over a median of 3.1 years. Results were similar with both doses (10 mg vs 25 mg) of empagliflozin. The mechanisms behind the CV benefits are likely multifactorial and may be related to reductions in weight and BP,33 but additional research is needed to fully elucidate the role of empagliflozin in this population.
Canagliflozin is being evaluated in the Canagliflozin Cardiovascular Assessment Study (CANVAS) for its effect on major CV events—CV death, nonfatal myocardial infarction, and nonfatal stroke—in patients with either a history of CVD or who are at increased risk of CVD and have uncontrolled diabetes.34 The trial is expected to wrap up in June 2017.
And dapagliflozin is being studied in the DECLARE-TIMI 58 trial (the Effect of Dapagliflozin on the Incidence of Cardiovascular Events) in patients with T2DM and either known CVD or at least 2 risk factors for CVD.35 The study is designed to assess dapagliflozin’s effect on the incidence of CV death, myocardial infarction, and ischemic stroke and has an estimated completion date of April 2019, which will provide a median follow-up of 4.5 years.
Cancer. All 3 agents have been examined for any possible carcinogenic links. In 2011, the FDA issued a request for further investigation surrounding the risk of cancer associated with dapagliflozin.36 As of November 2013, 10 of 6045 patients treated with dapagliflozin developed bladder cancer compared to 1 of 3512 controls.36 Furthermore, 9 of 2223 patients treated with dapagliflozin developed breast cancer compared to 1 of 1053 controls.36
Although the trials were not designed to detect an increase in risk, the number of observed cases warranted further investigation. No official warning for breast cancer exists since the characteristics of the malignancies led the FDA to believe dapagliflozin was unlikely the cause.36
Given what we know to date, it appears to be prudent to avoid prescribing SGLT2 inhibitors in patients with active bladder cancer, and to use them with caution in those with a history of the disease.2
Other studies. Initially, animal studies suggested an increased risk of various malignancies associated with canagliflozin use in rats,37 but consistent results were not seen in human studies. Similarly, at least one study found that empagliflozin was associated with lung cancer and melanoma, but closer examination found that most patients who developed these cancers had risk factors.38 Large, long-term studies of these agents in various populations are needed to thoroughly investigate possible carcinogenicity.
Additional considerations: Kidney function, age, and pregnancy
Consider avoiding SGLT2 inhibitors in patients with moderate kidney dysfunction (eGFR 30-59 mL/min/1.73 m2). Studies have shown that SGLT2 inhibitors are not as effective at lowering blood glucose in those with reduced eGFR, although adverse events were similar to those in placebo groups.24,39,40 Dapagliflozin is not recommended in patients with an eGFR <60 mL/min/1.73 m2 due to lack of efficacy.2,24 Empagliflozin does not require dose adjustments if eGFR is ≥45 mL/min/1.73 m2. A lower dose of canagliflozin (ie, 100 mg/d) is recommended in those with an eGFR of 45 to 59 mL/min/1.73 m2.2 All agents are contraindicated in patients with severe renal impairment (eGFR <30 mL/min/1.73 m2).
Older patients are at higher risk for dehydration, hypotension, and falls; therefore, SGLT2 inhibitors should be used with caution in this population. Similarly, they should not be used in patients with T1DM and should be avoided in those with active, or a history of, DKA.
There are no data on the use of SGLT2 inhibitors in pregnancy; thus, these agents should be avoided unless the potential benefits outweigh the potential risks to the unborn fetus.2
CASE 1 › An SGLT2 inhibitor is an acceptable option for Mr. S. Because he is resistant to starting insulin therapy and his HbA1c is <9%, an additional oral medication is reasonable. Adding an SGLT2 inhibitor may reduce his HbA1c up to ~1%, and education on lifestyle modifications may help bring him to goal. An SGLT2 inhibitor may also benefit his BP and weight, both of which could be improved.
Given the drugs he’s taking, drug interactions should not be an issue, and his renal function and pertinent labs (K, Phos, Mg) are within normal limits. Nevertheless, monitor these labs periodically and monitor Mr. S for adverse effects, such as UTIs, although these are more common in women. Canagliflozin is the preferred SGLT2 inhibitor on his insurance formulary, so you could initiate therapy at 100 mg/d, administered prior to the first meal, and increase to 300 mg/d if needed. As an alternative, consider prescribing the metformin/canagliflozin combination agent.
CASE 2 › Ms. R is likely experiencing a yeast infection as an adverse effect of the dapagliflozin. Although one yeast infection is insufficient grounds for discontinuation of the drug, recurrent infections should prompt a risk-to-benefit analysis to determine whether it’s worth continuing the medication. Her recent eGFR (<60 mL/min/1.73 m2) is, however, a contraindication to dapagliflozin, and therapy should be discontinued. Canagliflozin and empagliflozin may be considered since her eGFR is >45 mL/min/1.73 m2, but given her current HbA1c and recent adverse drug event, alternative therapies, such as basal insulin, are more appropriate treatment choices.
CORRESPONDENCE
Katelin M. Lisenby, PharmD, BCPS, University of Alabama College of Community Health Sciences, University Medical Center, Box 870374, Tuscaloosa, AL 35487; [email protected].
1. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2015;38:140-149.
2. Canagliflozin, dapagliflozin, empagliflozin. Lexicomp, Inc. (Lexi-Drugs®). Accessed October 12, 2015.
3. Stenlöf K, Cefalu WT, Kim KA, et al. Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes mellitus inadequately controlled with diet and exercise: findings from the 52-week CANTATA-M study. Curr Med Res Opin. 2014;30:163-175.
4. Ferrannini E, Ramos SJ, Salsali A, et al. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010:33:2217-2224.
5. Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1:208-219.
6. Ferrannini E, Berk A, Hantel S, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care. 2013;36:4015-4021.
7. Wilding JPH, Charpentier G, Hollander P, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013;67:1267-1282.
8. Forst T, Guthrie R, Goldenberg R, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. 2014;16:467-477.
9. Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. 2013;36:2508-2515.
10. Bristol-Myers Squibb [press release]. New phase III data showed dapagliflozin significantly reduced HbA1c compared to placebo at 24 weeks in patients with type 2 diabetes inadequately controlled with the combination of metformin plus sulfonylurea. Available at: http://news.bms.com/press-release/rd-news/new-phase-iii-data-showed-dapagliflozin-significantly-reduced-hba1c-compared-p&t=635156160653787526. Accessed September 17, 2015.
11. Jabbour SA, Hardy E, Sugg J, et al. Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: a 24- week, multicenter, randomized, double-blind, placebo-controlled study. Diabetes Care. 2014;37:740-750.
12. DeFronzo RA, Lewin A, Patel S, et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care. 2015;38:384-393.
13. Kovacs CS, Seshiah V, Merker L, et al. Empagliflozin as add-on therapy to pioglitazone with or without metformin in patients with type 2 diabetes mellitus. Clin Ther. 2015;37:1773-1788.
14. Haring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized double-blind, placebo-controlled trial. Diabetes Care. 2013;36:3396-3404.
15. Neal B, Percovik V, de Zeeuw D, et al. Efficacy and safety of canagliflozin, an inhibitor of sodium–glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38:403-411.
16. Wilding JPH, Woo V, Soler NG, et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156:405-415.
17. Rosenstock J, Jelaska A, Frappin G, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37:1815-1823.
18. Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941-950.
19. Bailey CJ, Gross JL, Pieters A, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010:375:2223-2233.
20. Bailey CJ, Gross JL, Hennicken D, et al. Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial. BMC Med. 2013;11:43.
21. Rosenstock J, Vico M, Wei L, et al. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35:1473-1478.
22. Merker L, Häring HU, Christiansen AV, et al. Empagliflozin as add-on to metformin in people with type 2 diabetes. Diabet Med. 2015;32:1555-1567.
23. Ridderstråle M, Anderson KR, Zeller C, et al. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691-700.
24. Kohan DE, Fioretto P, Tang W, et al. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962-971.
25. Invokana (canagliflozin) tablets [product information]. Titusville, NJ: Janssen Pharmaceuticals Inc. Available at: https://www.invokana.com. Accessed March 15, 2013.
26. Lavalle-González FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56:2582-2592.
27. Strojek K, Yoon KH, Hruba V, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011;13:928-938.
28. Leiter LA, Yoon KH, Arias P, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care. 2015;38:355-364.
29. US Food and Drug Administration. FDA drug safety communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm. Accessed July 11, 2016.
30. Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38:1638-1642.
31. US Food and Drug Administration. FDA drug safety communication: FDA revises label of diabetes drug canagliflozin (Invokana, Invokamet) to include updates on bone fracture risk and new information on decreased bone mineral density. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm461449.htm. Acces-sed July 11, 2016.
32. Canagliflozin, dapagliflozin, empagliflozin. In: RED BOOK [AUHSOP intranet database]. Greenwood Village, CO: Truven Health Analytics; [updated daily]. Available at: http://www.micromedexsolutions.com/micromedex2/librarian/ND_T/evidencexpert/ND_PR/evidencexpert/CS/BB1644/ND_AppProduct/evidencexpert/DUPLICATIONSHIELDSYNC/FAF693/ND_PG/evidencexpert/ND_B/evidencexpert/ND_P/evidencexpert/PFActionId/redbook.ShowProductSearchResults?SearchTerm=JARDIANCE&searchType=redbookProductName&searchTermId=42798&searchContent=%24searchContent&searchFilterAD=filterADActive&searchFilterRepackager=filterExcludeRepackager&searchPattern=%5Ejard. Accessed March 15, 2016.
33. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
34. CANagliflozin cardioVascular Assessment Study (CANVAS). Available at: http://clinicaltrials.gov/show/NCT01032629. Accessed October 12, 2015.
35. Multicenter trial to evaluate the effect of dapagliflozin on the incidence of cardiovascular events (DECLARE-TIMI 58). Available at: http://clinicaltrials.gov/show/NCT01730534. Accessed October 12, 2015.
36. FDA background document. BMS-512148 NDA 202293. In: Proceedings of the US Food and Drug Administration Endocrinologic & Metabolic Drug Advisory Committee Meeting, 2013. Available at: http://www.fda.gov/downloads/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm378079.pdf. Accessed October 12, 2015.
37. Lin HW, Tseng CH. A review of the relationship between SGLT2 inhibitors and cancer. Int J Endocrinol. 2014;2014:719578.
38. Center for Drug Evaluation and Research. Risk assessment and risk mitigation review(s). July 28, 2014. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/ 204629Orig1s000RiskR.pdf. Accessed September 21, 2015.
39. Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2014;16:1016-1027.
40. Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2: 369-384.
› Consider sodium-glucose cotransporter 2 (SGLT2) inhibitors as second-line agents in patients with type 2 diabetes mellitus who need mild hemoglobin A1c reductions (≤1%) and who would benefit from mild to modest weight and blood pressure reductions. A
› Avoid using SGLT2 inhibitors in patients with a history of recurrent genital mycotic or urinary tract infections. B
› Use SGLT2 inhibitors with caution in patients at risk for volume-related adverse effects (dizziness and hypotension), such as the elderly, those with moderate renal dysfunction, and those taking concomitant diuretic therapy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Joe S is a 41-year-old African-American man who comes to your clinic after his employee health screening revealed elevated triglycerides. The patient has a 3-year history of type 2 diabetes mellitus (T2DM); he also has a history of hypertension, gastroesophageal reflux disease, and obstructive sleep apnea. Mr. S tells you he takes metformin 1000 mg twice daily, but stopped taking his glipizide because he didn’t think it was helping his blood sugar. His last hemoglobin (Hb) A1c result was 8.8%, and he is very resistant to starting insulin therapy.
The patient’s other medications include enalapril 10 mg/d, atorvastatin 10 mg/d, and omeprazole 20 mg/d. Mr. S weighs 255.6 lbs (body mass index=34.7), his BP is 140/88 mm Hg, and his heart rate is 82 beats per minute. Laboratory values include: serum creatinine, 1.01 mg/dL; estimated glomerular filtration rate (eGFR) >100 mL/min/1.73 m2; potassium (K), 4.3 mmol/L; serum phosphorous (Phos), 2.8 mg/dL; magnesium (Mg), 1.9 mg/dL; total cholesterol, 167 mg/dL; low-density lipoprotein (LDL), 78 mg/dL; high-density lipoprotein (HDL), 38 mg/dL; and triglycerides, 256 mg/dL.
CASE 2 › Susan R, a 68-year-old Caucasian woman, returns to your clinic for a follow-up visit 3 months after you prescribed dapagliflozin 10 mg/d for her T2DM. Her glucose levels have improved, but she complains of vaginal pruritus and is worried that she has a yeast infection.
You diagnose vulvovaginal candidiasis in this patient and prescribe a single dose of fluconazole 150 mg. After reviewing her laboratory test results, you notice that since starting the dapagliflozin, her HbA1c level has improved slightly from 9.8% to 9.3%, but is still not where it needs to be. Her eGFR is 49 mL/min/1.73 m2.
What would you recommend to improve control of these patients’ blood glucose levels?
When to consider an SGLT2 inhibitor
Consider therapy with SGLT2 inhibitors in adult patients with T2DM who:3-9,13-15,17-24
- have an HbA1c between 7% and 9%
- would benefit from weight and/or blood pressure reductions
- have metabolic syndrome
- have adequate means to pay for the medication (ie, prescription coverage or the ability to afford it).
In addition, consider an SGLT2 inhibitor as initial monotherapy if metformin is contraindicated or not tolerated, or as add-on therapy to metformin, sulfonylureas, thiazolidinediones, dipeptidyl peptidase IV inhibitors, or insulin.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are the newest class of agents to enter the T2DM management arena. They act in the proximal renal tubules to decrease the reabsorption of glucose by targeting the SGLT2 transmembrane protein, which reabsorbs about 90% of the body’s glucose.1,2 The class is currently made up of 3 agents—canagliflozin, dapagliflozin, and empagliflozin—all of which are approved by the US Food and Drug Administration (FDA) for the treatment of T2DM (TABLE 1).2

The American Diabetes Association and the European Association for the Study of Diabetes published updated guidelines for T2DM management in 2015.1 In addition to lifestyle modifications, the guidelines recommend the use of metformin as first-line therapy unless it is contraindicated or patients are unable to tolerate it (eg, because of gastrointestinal adverse effects). They recommend other pharmacologic therapies as second-line options based on specific patient characteristics. Thus, SGLT2 inhibitors may be used as add-on therapy after metformin, or as a first-line option if metformin is contraindicated or not tolerated. Because the mechanism of action of SGLT2 inhibitors is independent of insulin secretion, these agents may be used at any stage of the diabetes continuum.
SGLT2 agents as monotherapy, or as add-on therapy
All SGLT2 agents have been studied as monotherapy accompanied by diet and exercise and shown to produce HbA1c reductions of 0.34% to 1.11%.3-6 In trials, the effect was similar regardless of study duration (18-104 weeks); generally, higher doses corresponded with larger HbA1c reductions.3-6
SGLT2 inhibitors have also been studied as add-on therapy to several oral agents including metformin, sulfonylureas, thiazolidinediones (TZDs), and the combination of metformin plus sulfonylureas or TZDs or dipeptidyl peptidase IV (DPP-IV) inhibitors.1 When used in any of these combinations, each SGLT2 agent demonstrated a consistent HbA1c lowering effect of 0.62% to 1.19%.7-14
Additionally, SGLT2 inhibitors have been studied in combination with insulin therapy (median or mean daily doses >60 units), which yielded further reductions in HbA1c of 0.58% to 1.02% without significant insulin adjustments or an increase in major hypoglycemia events.15-17 Patients receiving insulin and an SGLT2 inhibitor had lower insulin doses and more weight loss compared to placebo groups.
SGLT2 inhibitors offer additional benefits
Secondary analyses of most studies of SGLT2 inhibitors include changes in BP and weight from baseline as well as minor changes (some positive, some not) in several lipid parameters.3-5,7-9,13-15,17-24 In general, these effects do not appear to be dose-dependent (with the exception of canagliflozin and its associated lipid effects25) and are similar among the 3 medications.3-5,7-9,13-15,17-24 (For more on who would benefit from these agents, see “When to consider an SGLT2 inhibitor” above.)
BP reduction. Although the mean baseline BP was controlled in most studies, SGLT2 inhibitors have been shown to significantly reduce BP. Reductions in BP with all 3 SGLT2 medications range from approximately 2 to 5 mm Hg systolic and 0.5 to 2.5 mm Hg diastolic, which may be due to weight loss and diuresis.4-8,10-16,20-23 While the reductions were modest at best, one study involving empagliflozin reported that more than one-quarter of patients with uncontrolled BP at baseline achieved a BP <130/80 mm Hg 24 weeks later.5 While these agents should not be used solely for their BP lowering effects, they may help a small number of patients with mildly elevated BP achieve their goal without an additional antihypertensive agent.
Weight reduction. Modest weight loss, likely due to the loss of calories through urine, was seen with SGLT2 inhibitors in most studies, with reductions persisting beyond one year of use. In most studies, including those involving obese patients on insulin therapy,15,17,21 patients’ body weights were reduced by approximately 2 to 4 kg from baseline.3-16,18,21-23,26
Lipid effects. Although the mechanism is unclear, use of SGLT2 inhibitors can have varying effects on lipid panels. In most studies, total and LDL cholesterol levels were increased with elevations ranging from 0.7 to 10 mg/dL.3,7,8,18,19,22,23 Conversely, at least one study demonstrated mild reductions in total and LDL cholesterol levels with higher doses of empagliflozin.13 Additionally, modest reductions in triglycerides and increases in HDL across all doses of canagliflozin, dapagliflozin, and empagliflozin have been seen.8,9,13,15,19 While the clinical relevance of these lipid changes is unknown, monitoring is recommended.2
These agents are well tolerated
SGLT2 inhibitors were generally well tolerated in studies. The most common adverse effects include mycotic infections (2.4%-21.6%) and urinary tract infections (UTIs) (4.0%-19.6%) (both with higher incidences in females); volume-related effects such as dizziness and hypotension (0.3%-8.3%); and nasopharyngitis (5.4%-18.3%).4-14,16-23,26-28 Hypoglycemia was observed more often when an SGLT2 inhibitor was used in combination with a sulfonylurea or insulin therapy.4-14,16-23,26-28 The number of times adverse events led to discontinuation was low and similar to that in control groups.4-14,16-23,26-28
Mycotic and urinary infections should be diagnosed and treated according to current standards of care and do not require discontinuation of the SGLT2 inhibitor. Canagli-flozin therapy was associated with electrolyte abnormalities including hyperkalemia, hypermagnesemia, and hyperphosphatemia.25 Thus, levels should be monitored periodically, especially in patients predisposed to elevations due to other conditions or medications.25
Two additional warnings are worth noting
Diabetic ketoacidosis (DKA) has been reported with all 3 agents, and bone fractures have been reported with canagliflozin.
The FDA issued a warning in May 2015 regarding the increased risk of DKA with the use of SGLT2 inhibitor single and combination products.29 This warning was prompted by several case reports of DKA with uncharacteristically mild to moderate glucose elevations in patients with type 1 diabetes mellitus (T1DM) and T2DM who were taking an SGLT2 inhibitor. The absence of significant hyperglycemia delayed diagnosis in many cases. Therefore, patients should be counseled on the signs and symptoms of DKA, as well as when to seek medical attention.
Patients with diabetes and symptoms of ketoacidosis (eg, difficulty breathing, nausea, vomiting, abdominal pain, confusion, and fatigue) should be evaluated regardless of current blood glucose levels, and SGLT2 inhibitors should be discontinued if acidosis is confirmed. Identified potential triggers include illness, reduced food and fluid intake, reduced insulin dose, and history of alcohol intake. Use of SGLT2 inhibitors should be avoided in patients with T1DM until safety and efficacy are established in large randomized controlled trials. The European Medicines Agency announced that a thorough review of all currently approved SGLT2 agents is underway to evaluate the risk for DKA.30
In addition, the FDA called for a revision of the label of canagliflozin to reflect a strengthened warning about an increased risk of bone fractures and decreased bone mineral density (BMD).31 Fractures can occur as early as 12 weeks after initiating treatment and with only minor trauma.31
Over a 2-year period, canagliflozin also significantly decreased BMD in the hip and lower spine compared to placebo.31 Patients should be evaluated for additional risk factors for fracture before taking canagliflozin.31 The FDA is continuing to evaluate whether the other approved SGLT2 inhibitors are associated with an increased risk for fractures.
Drug interactions: Proceed carefully with diuretics
The number of drugs that interact with SGLT2 inhibitors is minimal. Because these agents can cause volume-related effects such as hypotension, dizziness, and osmotic diuresis, patients—particularly the elderly and those with renal impairment—taking concomitant diuretics, especially loop diuretics, may be at increased risk for these effects and should be monitored accordingly.2,25
Canagliflozin is primarily metabolized via glucuronidation by the uridine 5'-diphospho-glucuronosyltransferase (UGT) enzymes. Therefore, UGT enzyme inducers (eg, rifampin, phenytoin, phenobarbital, ritonavir) decrease canagliflozin’s serum concentration. If a patient has an eGFR >60 mL/min/1.73 m2 and is tolerating a dose of 100 mg/d, consider increasing the dose to 300 mg/d during concomitant treatment.
In addition, researchers have found that canagliflozin increases serum levels of digoxin by between 20% and 36%.25 Experts suspect this occurs because canagliflozin inhibits P-glycoprotein efflux of digoxin. Although monitoring of digoxin levels is recommended, this interaction is considered to be minor.25
Cost consideration: SGLT2 inhibitors are more expensive
The SGLT2 inhibitors are available only as brand name products and are more expensive than agents that have generic options (eg, metformin, sulfonylureas, TZDs). The average wholesale cost is approximately $400 for a 30-day supply of all SGLT2 agents.32 When considering an SGLT2 inhibitor, the patient should ideally have medication prescription coverage. Depending on the specific insurance plan, these agents are classified as tier 2 to 4, which is comparable to other oral brand name options.
Research looks at CV outcomes and cancer risk
Cardiovascular (CV) risk reduction. To date, only one study evaluating the effect of SGLT2 inhibitors on CV outcomes is complete.33 Two large randomized controlled trials involving canagliflozin and dapagliflozin designed to evaluate treatment effects on major CV endpoints are ongoing.34,35
In the EMPA-REG OUTCOME trial,33 researchers found that empagliflozin had beneficial effects on CV outcomes, making it one of the only antidiabetic agents on the market to have such benefits. The study, which involved more than 7000 patients with a history of T2DM and existing cardiovascular disease (CVD), found that 10.5% of patients in the empagliflozin group vs 12.1% in the placebo group died from a CV cause or experienced a nonfatal myocardial infarction or stroke over a median of 3.1 years. Results were similar with both doses (10 mg vs 25 mg) of empagliflozin. The mechanisms behind the CV benefits are likely multifactorial and may be related to reductions in weight and BP,33 but additional research is needed to fully elucidate the role of empagliflozin in this population.
Canagliflozin is being evaluated in the Canagliflozin Cardiovascular Assessment Study (CANVAS) for its effect on major CV events—CV death, nonfatal myocardial infarction, and nonfatal stroke—in patients with either a history of CVD or who are at increased risk of CVD and have uncontrolled diabetes.34 The trial is expected to wrap up in June 2017.
And dapagliflozin is being studied in the DECLARE-TIMI 58 trial (the Effect of Dapagliflozin on the Incidence of Cardiovascular Events) in patients with T2DM and either known CVD or at least 2 risk factors for CVD.35 The study is designed to assess dapagliflozin’s effect on the incidence of CV death, myocardial infarction, and ischemic stroke and has an estimated completion date of April 2019, which will provide a median follow-up of 4.5 years.
Cancer. All 3 agents have been examined for any possible carcinogenic links. In 2011, the FDA issued a request for further investigation surrounding the risk of cancer associated with dapagliflozin.36 As of November 2013, 10 of 6045 patients treated with dapagliflozin developed bladder cancer compared to 1 of 3512 controls.36 Furthermore, 9 of 2223 patients treated with dapagliflozin developed breast cancer compared to 1 of 1053 controls.36
Although the trials were not designed to detect an increase in risk, the number of observed cases warranted further investigation. No official warning for breast cancer exists since the characteristics of the malignancies led the FDA to believe dapagliflozin was unlikely the cause.36
Given what we know to date, it appears to be prudent to avoid prescribing SGLT2 inhibitors in patients with active bladder cancer, and to use them with caution in those with a history of the disease.2
Other studies. Initially, animal studies suggested an increased risk of various malignancies associated with canagliflozin use in rats,37 but consistent results were not seen in human studies. Similarly, at least one study found that empagliflozin was associated with lung cancer and melanoma, but closer examination found that most patients who developed these cancers had risk factors.38 Large, long-term studies of these agents in various populations are needed to thoroughly investigate possible carcinogenicity.
Additional considerations: Kidney function, age, and pregnancy
Consider avoiding SGLT2 inhibitors in patients with moderate kidney dysfunction (eGFR 30-59 mL/min/1.73 m2). Studies have shown that SGLT2 inhibitors are not as effective at lowering blood glucose in those with reduced eGFR, although adverse events were similar to those in placebo groups.24,39,40 Dapagliflozin is not recommended in patients with an eGFR <60 mL/min/1.73 m2 due to lack of efficacy.2,24 Empagliflozin does not require dose adjustments if eGFR is ≥45 mL/min/1.73 m2. A lower dose of canagliflozin (ie, 100 mg/d) is recommended in those with an eGFR of 45 to 59 mL/min/1.73 m2.2 All agents are contraindicated in patients with severe renal impairment (eGFR <30 mL/min/1.73 m2).
Older patients are at higher risk for dehydration, hypotension, and falls; therefore, SGLT2 inhibitors should be used with caution in this population. Similarly, they should not be used in patients with T1DM and should be avoided in those with active, or a history of, DKA.
There are no data on the use of SGLT2 inhibitors in pregnancy; thus, these agents should be avoided unless the potential benefits outweigh the potential risks to the unborn fetus.2
CASE 1 › An SGLT2 inhibitor is an acceptable option for Mr. S. Because he is resistant to starting insulin therapy and his HbA1c is <9%, an additional oral medication is reasonable. Adding an SGLT2 inhibitor may reduce his HbA1c up to ~1%, and education on lifestyle modifications may help bring him to goal. An SGLT2 inhibitor may also benefit his BP and weight, both of which could be improved.
Given the drugs he’s taking, drug interactions should not be an issue, and his renal function and pertinent labs (K, Phos, Mg) are within normal limits. Nevertheless, monitor these labs periodically and monitor Mr. S for adverse effects, such as UTIs, although these are more common in women. Canagliflozin is the preferred SGLT2 inhibitor on his insurance formulary, so you could initiate therapy at 100 mg/d, administered prior to the first meal, and increase to 300 mg/d if needed. As an alternative, consider prescribing the metformin/canagliflozin combination agent.
CASE 2 › Ms. R is likely experiencing a yeast infection as an adverse effect of the dapagliflozin. Although one yeast infection is insufficient grounds for discontinuation of the drug, recurrent infections should prompt a risk-to-benefit analysis to determine whether it’s worth continuing the medication. Her recent eGFR (<60 mL/min/1.73 m2) is, however, a contraindication to dapagliflozin, and therapy should be discontinued. Canagliflozin and empagliflozin may be considered since her eGFR is >45 mL/min/1.73 m2, but given her current HbA1c and recent adverse drug event, alternative therapies, such as basal insulin, are more appropriate treatment choices.
CORRESPONDENCE
Katelin M. Lisenby, PharmD, BCPS, University of Alabama College of Community Health Sciences, University Medical Center, Box 870374, Tuscaloosa, AL 35487; [email protected].
› Consider sodium-glucose cotransporter 2 (SGLT2) inhibitors as second-line agents in patients with type 2 diabetes mellitus who need mild hemoglobin A1c reductions (≤1%) and who would benefit from mild to modest weight and blood pressure reductions. A
› Avoid using SGLT2 inhibitors in patients with a history of recurrent genital mycotic or urinary tract infections. B
› Use SGLT2 inhibitors with caution in patients at risk for volume-related adverse effects (dizziness and hypotension), such as the elderly, those with moderate renal dysfunction, and those taking concomitant diuretic therapy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Joe S is a 41-year-old African-American man who comes to your clinic after his employee health screening revealed elevated triglycerides. The patient has a 3-year history of type 2 diabetes mellitus (T2DM); he also has a history of hypertension, gastroesophageal reflux disease, and obstructive sleep apnea. Mr. S tells you he takes metformin 1000 mg twice daily, but stopped taking his glipizide because he didn’t think it was helping his blood sugar. His last hemoglobin (Hb) A1c result was 8.8%, and he is very resistant to starting insulin therapy.
The patient’s other medications include enalapril 10 mg/d, atorvastatin 10 mg/d, and omeprazole 20 mg/d. Mr. S weighs 255.6 lbs (body mass index=34.7), his BP is 140/88 mm Hg, and his heart rate is 82 beats per minute. Laboratory values include: serum creatinine, 1.01 mg/dL; estimated glomerular filtration rate (eGFR) >100 mL/min/1.73 m2; potassium (K), 4.3 mmol/L; serum phosphorous (Phos), 2.8 mg/dL; magnesium (Mg), 1.9 mg/dL; total cholesterol, 167 mg/dL; low-density lipoprotein (LDL), 78 mg/dL; high-density lipoprotein (HDL), 38 mg/dL; and triglycerides, 256 mg/dL.
CASE 2 › Susan R, a 68-year-old Caucasian woman, returns to your clinic for a follow-up visit 3 months after you prescribed dapagliflozin 10 mg/d for her T2DM. Her glucose levels have improved, but she complains of vaginal pruritus and is worried that she has a yeast infection.
You diagnose vulvovaginal candidiasis in this patient and prescribe a single dose of fluconazole 150 mg. After reviewing her laboratory test results, you notice that since starting the dapagliflozin, her HbA1c level has improved slightly from 9.8% to 9.3%, but is still not where it needs to be. Her eGFR is 49 mL/min/1.73 m2.
What would you recommend to improve control of these patients’ blood glucose levels?
When to consider an SGLT2 inhibitor
Consider therapy with SGLT2 inhibitors in adult patients with T2DM who:3-9,13-15,17-24
- have an HbA1c between 7% and 9%
- would benefit from weight and/or blood pressure reductions
- have metabolic syndrome
- have adequate means to pay for the medication (ie, prescription coverage or the ability to afford it).
In addition, consider an SGLT2 inhibitor as initial monotherapy if metformin is contraindicated or not tolerated, or as add-on therapy to metformin, sulfonylureas, thiazolidinediones, dipeptidyl peptidase IV inhibitors, or insulin.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are the newest class of agents to enter the T2DM management arena. They act in the proximal renal tubules to decrease the reabsorption of glucose by targeting the SGLT2 transmembrane protein, which reabsorbs about 90% of the body’s glucose.1,2 The class is currently made up of 3 agents—canagliflozin, dapagliflozin, and empagliflozin—all of which are approved by the US Food and Drug Administration (FDA) for the treatment of T2DM (TABLE 1).2

The American Diabetes Association and the European Association for the Study of Diabetes published updated guidelines for T2DM management in 2015.1 In addition to lifestyle modifications, the guidelines recommend the use of metformin as first-line therapy unless it is contraindicated or patients are unable to tolerate it (eg, because of gastrointestinal adverse effects). They recommend other pharmacologic therapies as second-line options based on specific patient characteristics. Thus, SGLT2 inhibitors may be used as add-on therapy after metformin, or as a first-line option if metformin is contraindicated or not tolerated. Because the mechanism of action of SGLT2 inhibitors is independent of insulin secretion, these agents may be used at any stage of the diabetes continuum.
SGLT2 agents as monotherapy, or as add-on therapy
All SGLT2 agents have been studied as monotherapy accompanied by diet and exercise and shown to produce HbA1c reductions of 0.34% to 1.11%.3-6 In trials, the effect was similar regardless of study duration (18-104 weeks); generally, higher doses corresponded with larger HbA1c reductions.3-6
SGLT2 inhibitors have also been studied as add-on therapy to several oral agents including metformin, sulfonylureas, thiazolidinediones (TZDs), and the combination of metformin plus sulfonylureas or TZDs or dipeptidyl peptidase IV (DPP-IV) inhibitors.1 When used in any of these combinations, each SGLT2 agent demonstrated a consistent HbA1c lowering effect of 0.62% to 1.19%.7-14
Additionally, SGLT2 inhibitors have been studied in combination with insulin therapy (median or mean daily doses >60 units), which yielded further reductions in HbA1c of 0.58% to 1.02% without significant insulin adjustments or an increase in major hypoglycemia events.15-17 Patients receiving insulin and an SGLT2 inhibitor had lower insulin doses and more weight loss compared to placebo groups.
SGLT2 inhibitors offer additional benefits
Secondary analyses of most studies of SGLT2 inhibitors include changes in BP and weight from baseline as well as minor changes (some positive, some not) in several lipid parameters.3-5,7-9,13-15,17-24 In general, these effects do not appear to be dose-dependent (with the exception of canagliflozin and its associated lipid effects25) and are similar among the 3 medications.3-5,7-9,13-15,17-24 (For more on who would benefit from these agents, see “When to consider an SGLT2 inhibitor” above.)
BP reduction. Although the mean baseline BP was controlled in most studies, SGLT2 inhibitors have been shown to significantly reduce BP. Reductions in BP with all 3 SGLT2 medications range from approximately 2 to 5 mm Hg systolic and 0.5 to 2.5 mm Hg diastolic, which may be due to weight loss and diuresis.4-8,10-16,20-23 While the reductions were modest at best, one study involving empagliflozin reported that more than one-quarter of patients with uncontrolled BP at baseline achieved a BP <130/80 mm Hg 24 weeks later.5 While these agents should not be used solely for their BP lowering effects, they may help a small number of patients with mildly elevated BP achieve their goal without an additional antihypertensive agent.
Weight reduction. Modest weight loss, likely due to the loss of calories through urine, was seen with SGLT2 inhibitors in most studies, with reductions persisting beyond one year of use. In most studies, including those involving obese patients on insulin therapy,15,17,21 patients’ body weights were reduced by approximately 2 to 4 kg from baseline.3-16,18,21-23,26
Lipid effects. Although the mechanism is unclear, use of SGLT2 inhibitors can have varying effects on lipid panels. In most studies, total and LDL cholesterol levels were increased with elevations ranging from 0.7 to 10 mg/dL.3,7,8,18,19,22,23 Conversely, at least one study demonstrated mild reductions in total and LDL cholesterol levels with higher doses of empagliflozin.13 Additionally, modest reductions in triglycerides and increases in HDL across all doses of canagliflozin, dapagliflozin, and empagliflozin have been seen.8,9,13,15,19 While the clinical relevance of these lipid changes is unknown, monitoring is recommended.2
These agents are well tolerated
SGLT2 inhibitors were generally well tolerated in studies. The most common adverse effects include mycotic infections (2.4%-21.6%) and urinary tract infections (UTIs) (4.0%-19.6%) (both with higher incidences in females); volume-related effects such as dizziness and hypotension (0.3%-8.3%); and nasopharyngitis (5.4%-18.3%).4-14,16-23,26-28 Hypoglycemia was observed more often when an SGLT2 inhibitor was used in combination with a sulfonylurea or insulin therapy.4-14,16-23,26-28 The number of times adverse events led to discontinuation was low and similar to that in control groups.4-14,16-23,26-28
Mycotic and urinary infections should be diagnosed and treated according to current standards of care and do not require discontinuation of the SGLT2 inhibitor. Canagli-flozin therapy was associated with electrolyte abnormalities including hyperkalemia, hypermagnesemia, and hyperphosphatemia.25 Thus, levels should be monitored periodically, especially in patients predisposed to elevations due to other conditions or medications.25
Two additional warnings are worth noting
Diabetic ketoacidosis (DKA) has been reported with all 3 agents, and bone fractures have been reported with canagliflozin.
The FDA issued a warning in May 2015 regarding the increased risk of DKA with the use of SGLT2 inhibitor single and combination products.29 This warning was prompted by several case reports of DKA with uncharacteristically mild to moderate glucose elevations in patients with type 1 diabetes mellitus (T1DM) and T2DM who were taking an SGLT2 inhibitor. The absence of significant hyperglycemia delayed diagnosis in many cases. Therefore, patients should be counseled on the signs and symptoms of DKA, as well as when to seek medical attention.
Patients with diabetes and symptoms of ketoacidosis (eg, difficulty breathing, nausea, vomiting, abdominal pain, confusion, and fatigue) should be evaluated regardless of current blood glucose levels, and SGLT2 inhibitors should be discontinued if acidosis is confirmed. Identified potential triggers include illness, reduced food and fluid intake, reduced insulin dose, and history of alcohol intake. Use of SGLT2 inhibitors should be avoided in patients with T1DM until safety and efficacy are established in large randomized controlled trials. The European Medicines Agency announced that a thorough review of all currently approved SGLT2 agents is underway to evaluate the risk for DKA.30
In addition, the FDA called for a revision of the label of canagliflozin to reflect a strengthened warning about an increased risk of bone fractures and decreased bone mineral density (BMD).31 Fractures can occur as early as 12 weeks after initiating treatment and with only minor trauma.31
Over a 2-year period, canagliflozin also significantly decreased BMD in the hip and lower spine compared to placebo.31 Patients should be evaluated for additional risk factors for fracture before taking canagliflozin.31 The FDA is continuing to evaluate whether the other approved SGLT2 inhibitors are associated with an increased risk for fractures.
Drug interactions: Proceed carefully with diuretics
The number of drugs that interact with SGLT2 inhibitors is minimal. Because these agents can cause volume-related effects such as hypotension, dizziness, and osmotic diuresis, patients—particularly the elderly and those with renal impairment—taking concomitant diuretics, especially loop diuretics, may be at increased risk for these effects and should be monitored accordingly.2,25
Canagliflozin is primarily metabolized via glucuronidation by the uridine 5'-diphospho-glucuronosyltransferase (UGT) enzymes. Therefore, UGT enzyme inducers (eg, rifampin, phenytoin, phenobarbital, ritonavir) decrease canagliflozin’s serum concentration. If a patient has an eGFR >60 mL/min/1.73 m2 and is tolerating a dose of 100 mg/d, consider increasing the dose to 300 mg/d during concomitant treatment.
In addition, researchers have found that canagliflozin increases serum levels of digoxin by between 20% and 36%.25 Experts suspect this occurs because canagliflozin inhibits P-glycoprotein efflux of digoxin. Although monitoring of digoxin levels is recommended, this interaction is considered to be minor.25
Cost consideration: SGLT2 inhibitors are more expensive
The SGLT2 inhibitors are available only as brand name products and are more expensive than agents that have generic options (eg, metformin, sulfonylureas, TZDs). The average wholesale cost is approximately $400 for a 30-day supply of all SGLT2 agents.32 When considering an SGLT2 inhibitor, the patient should ideally have medication prescription coverage. Depending on the specific insurance plan, these agents are classified as tier 2 to 4, which is comparable to other oral brand name options.
Research looks at CV outcomes and cancer risk
Cardiovascular (CV) risk reduction. To date, only one study evaluating the effect of SGLT2 inhibitors on CV outcomes is complete.33 Two large randomized controlled trials involving canagliflozin and dapagliflozin designed to evaluate treatment effects on major CV endpoints are ongoing.34,35
In the EMPA-REG OUTCOME trial,33 researchers found that empagliflozin had beneficial effects on CV outcomes, making it one of the only antidiabetic agents on the market to have such benefits. The study, which involved more than 7000 patients with a history of T2DM and existing cardiovascular disease (CVD), found that 10.5% of patients in the empagliflozin group vs 12.1% in the placebo group died from a CV cause or experienced a nonfatal myocardial infarction or stroke over a median of 3.1 years. Results were similar with both doses (10 mg vs 25 mg) of empagliflozin. The mechanisms behind the CV benefits are likely multifactorial and may be related to reductions in weight and BP,33 but additional research is needed to fully elucidate the role of empagliflozin in this population.
Canagliflozin is being evaluated in the Canagliflozin Cardiovascular Assessment Study (CANVAS) for its effect on major CV events—CV death, nonfatal myocardial infarction, and nonfatal stroke—in patients with either a history of CVD or who are at increased risk of CVD and have uncontrolled diabetes.34 The trial is expected to wrap up in June 2017.
And dapagliflozin is being studied in the DECLARE-TIMI 58 trial (the Effect of Dapagliflozin on the Incidence of Cardiovascular Events) in patients with T2DM and either known CVD or at least 2 risk factors for CVD.35 The study is designed to assess dapagliflozin’s effect on the incidence of CV death, myocardial infarction, and ischemic stroke and has an estimated completion date of April 2019, which will provide a median follow-up of 4.5 years.
Cancer. All 3 agents have been examined for any possible carcinogenic links. In 2011, the FDA issued a request for further investigation surrounding the risk of cancer associated with dapagliflozin.36 As of November 2013, 10 of 6045 patients treated with dapagliflozin developed bladder cancer compared to 1 of 3512 controls.36 Furthermore, 9 of 2223 patients treated with dapagliflozin developed breast cancer compared to 1 of 1053 controls.36
Although the trials were not designed to detect an increase in risk, the number of observed cases warranted further investigation. No official warning for breast cancer exists since the characteristics of the malignancies led the FDA to believe dapagliflozin was unlikely the cause.36
Given what we know to date, it appears to be prudent to avoid prescribing SGLT2 inhibitors in patients with active bladder cancer, and to use them with caution in those with a history of the disease.2
Other studies. Initially, animal studies suggested an increased risk of various malignancies associated with canagliflozin use in rats,37 but consistent results were not seen in human studies. Similarly, at least one study found that empagliflozin was associated with lung cancer and melanoma, but closer examination found that most patients who developed these cancers had risk factors.38 Large, long-term studies of these agents in various populations are needed to thoroughly investigate possible carcinogenicity.
Additional considerations: Kidney function, age, and pregnancy
Consider avoiding SGLT2 inhibitors in patients with moderate kidney dysfunction (eGFR 30-59 mL/min/1.73 m2). Studies have shown that SGLT2 inhibitors are not as effective at lowering blood glucose in those with reduced eGFR, although adverse events were similar to those in placebo groups.24,39,40 Dapagliflozin is not recommended in patients with an eGFR <60 mL/min/1.73 m2 due to lack of efficacy.2,24 Empagliflozin does not require dose adjustments if eGFR is ≥45 mL/min/1.73 m2. A lower dose of canagliflozin (ie, 100 mg/d) is recommended in those with an eGFR of 45 to 59 mL/min/1.73 m2.2 All agents are contraindicated in patients with severe renal impairment (eGFR <30 mL/min/1.73 m2).
Older patients are at higher risk for dehydration, hypotension, and falls; therefore, SGLT2 inhibitors should be used with caution in this population. Similarly, they should not be used in patients with T1DM and should be avoided in those with active, or a history of, DKA.
There are no data on the use of SGLT2 inhibitors in pregnancy; thus, these agents should be avoided unless the potential benefits outweigh the potential risks to the unborn fetus.2
CASE 1 › An SGLT2 inhibitor is an acceptable option for Mr. S. Because he is resistant to starting insulin therapy and his HbA1c is <9%, an additional oral medication is reasonable. Adding an SGLT2 inhibitor may reduce his HbA1c up to ~1%, and education on lifestyle modifications may help bring him to goal. An SGLT2 inhibitor may also benefit his BP and weight, both of which could be improved.
Given the drugs he’s taking, drug interactions should not be an issue, and his renal function and pertinent labs (K, Phos, Mg) are within normal limits. Nevertheless, monitor these labs periodically and monitor Mr. S for adverse effects, such as UTIs, although these are more common in women. Canagliflozin is the preferred SGLT2 inhibitor on his insurance formulary, so you could initiate therapy at 100 mg/d, administered prior to the first meal, and increase to 300 mg/d if needed. As an alternative, consider prescribing the metformin/canagliflozin combination agent.
CASE 2 › Ms. R is likely experiencing a yeast infection as an adverse effect of the dapagliflozin. Although one yeast infection is insufficient grounds for discontinuation of the drug, recurrent infections should prompt a risk-to-benefit analysis to determine whether it’s worth continuing the medication. Her recent eGFR (<60 mL/min/1.73 m2) is, however, a contraindication to dapagliflozin, and therapy should be discontinued. Canagliflozin and empagliflozin may be considered since her eGFR is >45 mL/min/1.73 m2, but given her current HbA1c and recent adverse drug event, alternative therapies, such as basal insulin, are more appropriate treatment choices.
CORRESPONDENCE
Katelin M. Lisenby, PharmD, BCPS, University of Alabama College of Community Health Sciences, University Medical Center, Box 870374, Tuscaloosa, AL 35487; [email protected].
1. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2015;38:140-149.
2. Canagliflozin, dapagliflozin, empagliflozin. Lexicomp, Inc. (Lexi-Drugs®). Accessed October 12, 2015.
3. Stenlöf K, Cefalu WT, Kim KA, et al. Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes mellitus inadequately controlled with diet and exercise: findings from the 52-week CANTATA-M study. Curr Med Res Opin. 2014;30:163-175.
4. Ferrannini E, Ramos SJ, Salsali A, et al. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010:33:2217-2224.
5. Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1:208-219.
6. Ferrannini E, Berk A, Hantel S, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care. 2013;36:4015-4021.
7. Wilding JPH, Charpentier G, Hollander P, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013;67:1267-1282.
8. Forst T, Guthrie R, Goldenberg R, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. 2014;16:467-477.
9. Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. 2013;36:2508-2515.
10. Bristol-Myers Squibb [press release]. New phase III data showed dapagliflozin significantly reduced HbA1c compared to placebo at 24 weeks in patients with type 2 diabetes inadequately controlled with the combination of metformin plus sulfonylurea. Available at: http://news.bms.com/press-release/rd-news/new-phase-iii-data-showed-dapagliflozin-significantly-reduced-hba1c-compared-p&t=635156160653787526. Accessed September 17, 2015.
11. Jabbour SA, Hardy E, Sugg J, et al. Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: a 24- week, multicenter, randomized, double-blind, placebo-controlled study. Diabetes Care. 2014;37:740-750.
12. DeFronzo RA, Lewin A, Patel S, et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care. 2015;38:384-393.
13. Kovacs CS, Seshiah V, Merker L, et al. Empagliflozin as add-on therapy to pioglitazone with or without metformin in patients with type 2 diabetes mellitus. Clin Ther. 2015;37:1773-1788.
14. Haring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized double-blind, placebo-controlled trial. Diabetes Care. 2013;36:3396-3404.
15. Neal B, Percovik V, de Zeeuw D, et al. Efficacy and safety of canagliflozin, an inhibitor of sodium–glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38:403-411.
16. Wilding JPH, Woo V, Soler NG, et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156:405-415.
17. Rosenstock J, Jelaska A, Frappin G, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37:1815-1823.
18. Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941-950.
19. Bailey CJ, Gross JL, Pieters A, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010:375:2223-2233.
20. Bailey CJ, Gross JL, Hennicken D, et al. Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial. BMC Med. 2013;11:43.
21. Rosenstock J, Vico M, Wei L, et al. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35:1473-1478.
22. Merker L, Häring HU, Christiansen AV, et al. Empagliflozin as add-on to metformin in people with type 2 diabetes. Diabet Med. 2015;32:1555-1567.
23. Ridderstråle M, Anderson KR, Zeller C, et al. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691-700.
24. Kohan DE, Fioretto P, Tang W, et al. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962-971.
25. Invokana (canagliflozin) tablets [product information]. Titusville, NJ: Janssen Pharmaceuticals Inc. Available at: https://www.invokana.com. Accessed March 15, 2013.
26. Lavalle-González FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56:2582-2592.
27. Strojek K, Yoon KH, Hruba V, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011;13:928-938.
28. Leiter LA, Yoon KH, Arias P, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care. 2015;38:355-364.
29. US Food and Drug Administration. FDA drug safety communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm. Accessed July 11, 2016.
30. Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38:1638-1642.
31. US Food and Drug Administration. FDA drug safety communication: FDA revises label of diabetes drug canagliflozin (Invokana, Invokamet) to include updates on bone fracture risk and new information on decreased bone mineral density. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm461449.htm. Acces-sed July 11, 2016.
32. Canagliflozin, dapagliflozin, empagliflozin. In: RED BOOK [AUHSOP intranet database]. Greenwood Village, CO: Truven Health Analytics; [updated daily]. Available at: http://www.micromedexsolutions.com/micromedex2/librarian/ND_T/evidencexpert/ND_PR/evidencexpert/CS/BB1644/ND_AppProduct/evidencexpert/DUPLICATIONSHIELDSYNC/FAF693/ND_PG/evidencexpert/ND_B/evidencexpert/ND_P/evidencexpert/PFActionId/redbook.ShowProductSearchResults?SearchTerm=JARDIANCE&searchType=redbookProductName&searchTermId=42798&searchContent=%24searchContent&searchFilterAD=filterADActive&searchFilterRepackager=filterExcludeRepackager&searchPattern=%5Ejard. Accessed March 15, 2016.
33. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
34. CANagliflozin cardioVascular Assessment Study (CANVAS). Available at: http://clinicaltrials.gov/show/NCT01032629. Accessed October 12, 2015.
35. Multicenter trial to evaluate the effect of dapagliflozin on the incidence of cardiovascular events (DECLARE-TIMI 58). Available at: http://clinicaltrials.gov/show/NCT01730534. Accessed October 12, 2015.
36. FDA background document. BMS-512148 NDA 202293. In: Proceedings of the US Food and Drug Administration Endocrinologic & Metabolic Drug Advisory Committee Meeting, 2013. Available at: http://www.fda.gov/downloads/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm378079.pdf. Accessed October 12, 2015.
37. Lin HW, Tseng CH. A review of the relationship between SGLT2 inhibitors and cancer. Int J Endocrinol. 2014;2014:719578.
38. Center for Drug Evaluation and Research. Risk assessment and risk mitigation review(s). July 28, 2014. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/ 204629Orig1s000RiskR.pdf. Accessed September 21, 2015.
39. Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2014;16:1016-1027.
40. Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2: 369-384.
1. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2015;38:140-149.
2. Canagliflozin, dapagliflozin, empagliflozin. Lexicomp, Inc. (Lexi-Drugs®). Accessed October 12, 2015.
3. Stenlöf K, Cefalu WT, Kim KA, et al. Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes mellitus inadequately controlled with diet and exercise: findings from the 52-week CANTATA-M study. Curr Med Res Opin. 2014;30:163-175.
4. Ferrannini E, Ramos SJ, Salsali A, et al. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010:33:2217-2224.
5. Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1:208-219.
6. Ferrannini E, Berk A, Hantel S, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care. 2013;36:4015-4021.
7. Wilding JPH, Charpentier G, Hollander P, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013;67:1267-1282.
8. Forst T, Guthrie R, Goldenberg R, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. 2014;16:467-477.
9. Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. 2013;36:2508-2515.
10. Bristol-Myers Squibb [press release]. New phase III data showed dapagliflozin significantly reduced HbA1c compared to placebo at 24 weeks in patients with type 2 diabetes inadequately controlled with the combination of metformin plus sulfonylurea. Available at: http://news.bms.com/press-release/rd-news/new-phase-iii-data-showed-dapagliflozin-significantly-reduced-hba1c-compared-p&t=635156160653787526. Accessed September 17, 2015.
11. Jabbour SA, Hardy E, Sugg J, et al. Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: a 24- week, multicenter, randomized, double-blind, placebo-controlled study. Diabetes Care. 2014;37:740-750.
12. DeFronzo RA, Lewin A, Patel S, et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care. 2015;38:384-393.
13. Kovacs CS, Seshiah V, Merker L, et al. Empagliflozin as add-on therapy to pioglitazone with or without metformin in patients with type 2 diabetes mellitus. Clin Ther. 2015;37:1773-1788.
14. Haring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized double-blind, placebo-controlled trial. Diabetes Care. 2013;36:3396-3404.
15. Neal B, Percovik V, de Zeeuw D, et al. Efficacy and safety of canagliflozin, an inhibitor of sodium–glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38:403-411.
16. Wilding JPH, Woo V, Soler NG, et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156:405-415.
17. Rosenstock J, Jelaska A, Frappin G, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37:1815-1823.
18. Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941-950.
19. Bailey CJ, Gross JL, Pieters A, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010:375:2223-2233.
20. Bailey CJ, Gross JL, Hennicken D, et al. Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial. BMC Med. 2013;11:43.
21. Rosenstock J, Vico M, Wei L, et al. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35:1473-1478.
22. Merker L, Häring HU, Christiansen AV, et al. Empagliflozin as add-on to metformin in people with type 2 diabetes. Diabet Med. 2015;32:1555-1567.
23. Ridderstråle M, Anderson KR, Zeller C, et al. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691-700.
24. Kohan DE, Fioretto P, Tang W, et al. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962-971.
25. Invokana (canagliflozin) tablets [product information]. Titusville, NJ: Janssen Pharmaceuticals Inc. Available at: https://www.invokana.com. Accessed March 15, 2013.
26. Lavalle-González FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56:2582-2592.
27. Strojek K, Yoon KH, Hruba V, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011;13:928-938.
28. Leiter LA, Yoon KH, Arias P, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care. 2015;38:355-364.
29. US Food and Drug Administration. FDA drug safety communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm. Accessed July 11, 2016.
30. Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38:1638-1642.
31. US Food and Drug Administration. FDA drug safety communication: FDA revises label of diabetes drug canagliflozin (Invokana, Invokamet) to include updates on bone fracture risk and new information on decreased bone mineral density. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm461449.htm. Acces-sed July 11, 2016.
32. Canagliflozin, dapagliflozin, empagliflozin. In: RED BOOK [AUHSOP intranet database]. Greenwood Village, CO: Truven Health Analytics; [updated daily]. Available at: http://www.micromedexsolutions.com/micromedex2/librarian/ND_T/evidencexpert/ND_PR/evidencexpert/CS/BB1644/ND_AppProduct/evidencexpert/DUPLICATIONSHIELDSYNC/FAF693/ND_PG/evidencexpert/ND_B/evidencexpert/ND_P/evidencexpert/PFActionId/redbook.ShowProductSearchResults?SearchTerm=JARDIANCE&searchType=redbookProductName&searchTermId=42798&searchContent=%24searchContent&searchFilterAD=filterADActive&searchFilterRepackager=filterExcludeRepackager&searchPattern=%5Ejard. Accessed March 15, 2016.
33. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
34. CANagliflozin cardioVascular Assessment Study (CANVAS). Available at: http://clinicaltrials.gov/show/NCT01032629. Accessed October 12, 2015.
35. Multicenter trial to evaluate the effect of dapagliflozin on the incidence of cardiovascular events (DECLARE-TIMI 58). Available at: http://clinicaltrials.gov/show/NCT01730534. Accessed October 12, 2015.
36. FDA background document. BMS-512148 NDA 202293. In: Proceedings of the US Food and Drug Administration Endocrinologic & Metabolic Drug Advisory Committee Meeting, 2013. Available at: http://www.fda.gov/downloads/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm378079.pdf. Accessed October 12, 2015.
37. Lin HW, Tseng CH. A review of the relationship between SGLT2 inhibitors and cancer. Int J Endocrinol. 2014;2014:719578.
38. Center for Drug Evaluation and Research. Risk assessment and risk mitigation review(s). July 28, 2014. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/ 204629Orig1s000RiskR.pdf. Accessed September 21, 2015.
39. Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2014;16:1016-1027.
40. Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2: 369-384.