User login
Anemia linked to risk of death after stroke

Anemia may increase the risk of death in older adults who have had a stroke, according to research published in the Journal of the American Heart Association.
An initial analysis of more than 8000 patients showed that anemia was associated with a higher risk of death for up to 1 year following ischemic or hemorrhagic stroke.
A second analysis of nearly 30,000 patients suggested the risk of dying from ischemic stroke is about 2 times higher in patients with anemia than those without it, and the risk of death from hemorrhagic stroke is about 1.5 times higher in anemic patients.
“So there’s the potential for a much poorer outcome if somebody comes in with stroke and they’re also anemic,” said study author Phyo Myint, MD, of the University of Aberdeen in Scotland.
Dr Myint and his colleagues first examined data from the UK Regional Stroke Register. This included 8013 patients with an average age of 78 who were admitted to the hospital with acute stroke between 2003 and 2015.
The team assessed the impact of anemia and hemoglobin levels at admission on death at different time points—inpatient, 7 days, 14 days, 1 month, 3 months, 6 months, and 1 year after stroke.
Anemia was associated with higher odds of death at most of the time points examined. And elevated hemoglobin was associated with a higher risk of death, mainly within the first month.
In addition to analyzing data from the UK Regional Stroke Registry, the researchers systematically reviewed relevant literature published to date. They compiled data from 20 previous studies, increasing the study population to 29,943 stroke patients.
In analyzing these patients, the researchers found that anemia on admission was associated with an increased risk of mortality in both ischemic stroke and hemorrhagic stroke. The odds ratios were 1.97 and 1.46, respectively.
The researchers believe this study emphasizes the impact of anemia on stroke outcomes and the need for increased awareness and interventions for stroke patients with anemia.
“One example of an intervention might be treating the underlying causes of anemia, such as iron deficiency, which is common in this age group,” said study author Raphae Barlas, a medical student at the University of Aberdeen.
“As the study has convincingly demonstrated, anemia does worsen the outcome of stroke, so it is very important that we identify at-risk patients and optimize the management.” ![]()

Anemia may increase the risk of death in older adults who have had a stroke, according to research published in the Journal of the American Heart Association.
An initial analysis of more than 8000 patients showed that anemia was associated with a higher risk of death for up to 1 year following ischemic or hemorrhagic stroke.
A second analysis of nearly 30,000 patients suggested the risk of dying from ischemic stroke is about 2 times higher in patients with anemia than those without it, and the risk of death from hemorrhagic stroke is about 1.5 times higher in anemic patients.
“So there’s the potential for a much poorer outcome if somebody comes in with stroke and they’re also anemic,” said study author Phyo Myint, MD, of the University of Aberdeen in Scotland.
Dr Myint and his colleagues first examined data from the UK Regional Stroke Register. This included 8013 patients with an average age of 78 who were admitted to the hospital with acute stroke between 2003 and 2015.
The team assessed the impact of anemia and hemoglobin levels at admission on death at different time points—inpatient, 7 days, 14 days, 1 month, 3 months, 6 months, and 1 year after stroke.
Anemia was associated with higher odds of death at most of the time points examined. And elevated hemoglobin was associated with a higher risk of death, mainly within the first month.
In addition to analyzing data from the UK Regional Stroke Registry, the researchers systematically reviewed relevant literature published to date. They compiled data from 20 previous studies, increasing the study population to 29,943 stroke patients.
In analyzing these patients, the researchers found that anemia on admission was associated with an increased risk of mortality in both ischemic stroke and hemorrhagic stroke. The odds ratios were 1.97 and 1.46, respectively.
The researchers believe this study emphasizes the impact of anemia on stroke outcomes and the need for increased awareness and interventions for stroke patients with anemia.
“One example of an intervention might be treating the underlying causes of anemia, such as iron deficiency, which is common in this age group,” said study author Raphae Barlas, a medical student at the University of Aberdeen.
“As the study has convincingly demonstrated, anemia does worsen the outcome of stroke, so it is very important that we identify at-risk patients and optimize the management.” ![]()

Anemia may increase the risk of death in older adults who have had a stroke, according to research published in the Journal of the American Heart Association.
An initial analysis of more than 8000 patients showed that anemia was associated with a higher risk of death for up to 1 year following ischemic or hemorrhagic stroke.
A second analysis of nearly 30,000 patients suggested the risk of dying from ischemic stroke is about 2 times higher in patients with anemia than those without it, and the risk of death from hemorrhagic stroke is about 1.5 times higher in anemic patients.
“So there’s the potential for a much poorer outcome if somebody comes in with stroke and they’re also anemic,” said study author Phyo Myint, MD, of the University of Aberdeen in Scotland.
Dr Myint and his colleagues first examined data from the UK Regional Stroke Register. This included 8013 patients with an average age of 78 who were admitted to the hospital with acute stroke between 2003 and 2015.
The team assessed the impact of anemia and hemoglobin levels at admission on death at different time points—inpatient, 7 days, 14 days, 1 month, 3 months, 6 months, and 1 year after stroke.
Anemia was associated with higher odds of death at most of the time points examined. And elevated hemoglobin was associated with a higher risk of death, mainly within the first month.
In addition to analyzing data from the UK Regional Stroke Registry, the researchers systematically reviewed relevant literature published to date. They compiled data from 20 previous studies, increasing the study population to 29,943 stroke patients.
In analyzing these patients, the researchers found that anemia on admission was associated with an increased risk of mortality in both ischemic stroke and hemorrhagic stroke. The odds ratios were 1.97 and 1.46, respectively.
The researchers believe this study emphasizes the impact of anemia on stroke outcomes and the need for increased awareness and interventions for stroke patients with anemia.
“One example of an intervention might be treating the underlying causes of anemia, such as iron deficiency, which is common in this age group,” said study author Raphae Barlas, a medical student at the University of Aberdeen.
“As the study has convincingly demonstrated, anemia does worsen the outcome of stroke, so it is very important that we identify at-risk patients and optimize the management.” ![]()
Music may alleviate cancer patients’ symptoms

Photo by Lars Frantzen
Results of a systematic review suggest music can help alleviate symptoms of anxiety, pain, and fatigue in cancer patients.
The review included more than 50 studies investigating the impact of music therapy—a personalized music experience offered by trained music therapists—and music medicine—listening to pre-recorded music provided by a doctor or nurse—on psychological and physical outcomes in people with cancer.
“We found that music therapy interventions specifically help improve patients’ quality of life,” said study author Joke Bradt, PhD, of Drexel University in Philadelphia, Pennsylvania.
“These are important findings, as these outcomes play an important role in patients’ overall well-being.”
Dr Bradt and her colleagues reported their findings in Cochrane Database of Systematic Reviews.
The researchers examined 52 trials including 3731 cancer patients. The music interventions were classified as music therapy in 23 of the trials and as music medicine in 29 trials.
Analyses suggested that both types of music interventions positively impacted patients. The interventions had a moderate-to-strong effect on anxiety, a strong effect on pain reduction, and a small-to-moderate effect on fatigue.
Small reductions in heart and respiratory rates, as well as lowered blood pressure, were linked to the interventions as well.
In addition, the researchers observed a moderate increase in patients’ quality of life with music therapy but not music medicine.
The team could not determine the effect of music interventions on depression due to the low quality of evidence. And there was no evidence that the interventions improve mood, distress, or physical functioning, but there were few trials investigating these outcomes.
Similarly, the researchers said they could not draw any conclusions about the effect of music interventions on immunologic functioning, coping, resilience, or communication because there were not enough trials evaluating these outcomes.
Still, the researchers hope music interventions will become more widely used, in light of the potential benefits to cancer patients.
“We hope that the findings of this review will encourage healthcare providers in medical settings to seriously consider the use of music therapy in the psychosocial care of people with cancer,” Dr Bradt said. ![]()

Photo by Lars Frantzen
Results of a systematic review suggest music can help alleviate symptoms of anxiety, pain, and fatigue in cancer patients.
The review included more than 50 studies investigating the impact of music therapy—a personalized music experience offered by trained music therapists—and music medicine—listening to pre-recorded music provided by a doctor or nurse—on psychological and physical outcomes in people with cancer.
“We found that music therapy interventions specifically help improve patients’ quality of life,” said study author Joke Bradt, PhD, of Drexel University in Philadelphia, Pennsylvania.
“These are important findings, as these outcomes play an important role in patients’ overall well-being.”
Dr Bradt and her colleagues reported their findings in Cochrane Database of Systematic Reviews.
The researchers examined 52 trials including 3731 cancer patients. The music interventions were classified as music therapy in 23 of the trials and as music medicine in 29 trials.
Analyses suggested that both types of music interventions positively impacted patients. The interventions had a moderate-to-strong effect on anxiety, a strong effect on pain reduction, and a small-to-moderate effect on fatigue.
Small reductions in heart and respiratory rates, as well as lowered blood pressure, were linked to the interventions as well.
In addition, the researchers observed a moderate increase in patients’ quality of life with music therapy but not music medicine.
The team could not determine the effect of music interventions on depression due to the low quality of evidence. And there was no evidence that the interventions improve mood, distress, or physical functioning, but there were few trials investigating these outcomes.
Similarly, the researchers said they could not draw any conclusions about the effect of music interventions on immunologic functioning, coping, resilience, or communication because there were not enough trials evaluating these outcomes.
Still, the researchers hope music interventions will become more widely used, in light of the potential benefits to cancer patients.
“We hope that the findings of this review will encourage healthcare providers in medical settings to seriously consider the use of music therapy in the psychosocial care of people with cancer,” Dr Bradt said. ![]()

Photo by Lars Frantzen
Results of a systematic review suggest music can help alleviate symptoms of anxiety, pain, and fatigue in cancer patients.
The review included more than 50 studies investigating the impact of music therapy—a personalized music experience offered by trained music therapists—and music medicine—listening to pre-recorded music provided by a doctor or nurse—on psychological and physical outcomes in people with cancer.
“We found that music therapy interventions specifically help improve patients’ quality of life,” said study author Joke Bradt, PhD, of Drexel University in Philadelphia, Pennsylvania.
“These are important findings, as these outcomes play an important role in patients’ overall well-being.”
Dr Bradt and her colleagues reported their findings in Cochrane Database of Systematic Reviews.
The researchers examined 52 trials including 3731 cancer patients. The music interventions were classified as music therapy in 23 of the trials and as music medicine in 29 trials.
Analyses suggested that both types of music interventions positively impacted patients. The interventions had a moderate-to-strong effect on anxiety, a strong effect on pain reduction, and a small-to-moderate effect on fatigue.
Small reductions in heart and respiratory rates, as well as lowered blood pressure, were linked to the interventions as well.
In addition, the researchers observed a moderate increase in patients’ quality of life with music therapy but not music medicine.
The team could not determine the effect of music interventions on depression due to the low quality of evidence. And there was no evidence that the interventions improve mood, distress, or physical functioning, but there were few trials investigating these outcomes.
Similarly, the researchers said they could not draw any conclusions about the effect of music interventions on immunologic functioning, coping, resilience, or communication because there were not enough trials evaluating these outcomes.
Still, the researchers hope music interventions will become more widely used, in light of the potential benefits to cancer patients.
“We hope that the findings of this review will encourage healthcare providers in medical settings to seriously consider the use of music therapy in the psychosocial care of people with cancer,” Dr Bradt said. ![]()
State Medicaid Expansion Status
On January 1, 2014, several major provisions of the Affordable Care Act (ACA) took effect, including introduction of the individual mandate for health insurance coverage, opening of the Health Insurance Marketplace, and expansion of Medicaid eligibility to Americans earning up to 133% of the federal poverty level.[1] Nearly 9 million US adults have enrolled in Medicaid since that time, primarily in the 31 states and Washington, DC that have opted into Medicaid expansion.[2, 3] ACA implementation has also had a significant impact on hospital payer mix, primarily by reducing the volume of uncompensated care in Medicaid‐expansion states.[4, 5]
The differential shift in payer mix in Medicaid‐expansion versus nonexpansion states may be relevant to hospitals beyond reimbursement. Medicaid insurance has historically been associated with longer hospitalizations and higher in‐hospital mortality in diverse patient populations, more so than commercial insurance and often even uninsured payer status.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15] The disparity in outcomes between patients with Medicaid versus other insurance persists even after adjustment for disease severity and baseline comorbidities. Insurance type may influence the delivery of inpatient care through variation in access to invasive procedures and adherence to guideline‐concordant medical therapies.[9, 10, 11, 12] Medicaid patients may be more likely than uninsured patients to remain hospitalized pending postacute care placement rather than be discharged home with family support.[16] Medicaid patients are also less likely to leave against medical advice than uninsured patients.[17]
Currently, little is known about the impact of state Medicaid expansion status on length of stay (LOS) or mortality nationally. It is possible that hospitals in Medicaid‐expansion states have experienced relative worsening in LOS and mortality as their share of Medicaid patients has grown. Determining the impact of ACA implementation on payer mix and patient outcomes is particularly important for academic medical centers (AMCs), as they traditionally care for the greatest percentage of both Medicaid and uninsured patients.[18] We sought to characterize the impact of state Medicaid expansion status on payer mix, LOS, and in‐hospital mortality for general medicine patients at AMCs in the United States.
METHODS
The University HealthSystem Consortium (UHC) is an alliance of 117 AMCs and 310 affiliated hospitals, representing >90% of such institutions in the US. We queried the online UHC Clinical Data Base/Resource Manager (CDB/RM) to obtain hospital‐level insurance, LOS, and mortality data for inpatients discharged from a general medicine service between October 1, 2012 and September 30, 2015. We excluded hospitals that were missing data for any month within the study period. No patient‐level data were accessed.
Our outcomes of interest were the proportion of discharges by primary payer (Medicare, commercial, Medicaid, uninsured, or other [eg, Tri‐Care or Workers' Compensation]), as well as the LOS index and mortality index. Both indices were defined as the ratio of the observed to expected values. To determine the expected LOS and mortality, the UHC 2015 risk adjustment models were applied to all cases, adjusting for variables such as patient demographics, low socioeconomic status, admit source and status, severity of illness, and comorbid conditions, as described by International Classification of Diseases, Ninth Revision codes. These models have been validated and are used for research and quality benchmarking for member institutions.[19]
We next stratified hospitals according to state Medicaid expansion status. We defined Medicaid‐expansion states as those that had expanded Medicaid by the end of the study period: Arizona, Arkansas, California, Colorado, Connecticut, Illinois, Indiana, Iowa, Kentucky, Maryland, Massachusetts, Michigan, Minnesota, Nevada, New Hampshire, New Jersey, New Mexico, New York, Ohio, Oregon, Pennsylvania, Rhode Island, Washington, Washington DC, and West Virginia. Nonexpansion states included Alabama, Florida, Georgia, Kansas, Louisiana, Missouri, Nebraska, North Carolina, South Carolina, Tennessee, Texas, Utah, Virginia, and Wisconsin. We excluded 12 states due to incomplete data: Alaska, Delaware, Hawaii, Idaho, North Dakota, Maine, Mississippi, Montana, Oklahoma, South Dakota, Vermont, and Wyoming.
We then identified our pre‐ and post‐ACA implementation periods. Medicaid coverage expansion took effect in all expansion states on January 1, 2014, with the exception of Michigan (April 1, 2014), New Hampshire (August 15, 2014), Pennsylvania (January 1, 2015), and Indiana (February 1, 2015).[3] We therefore defined October 1, 2012 to December 31, 2013 as the pre‐ACA implementation period and January 1, 2014 to September 30, 2015 as the post‐ACA implementation period for all states except for Michigan, New Hampshire, Pennsylvania, and Indiana. For these 4 states, we customized the pre‐ and post‐ACA implementation periods to their respective dates of Medicaid expansion; for New Hampshire, we designated October 1, 2012 to July 31, 2014 as the pre‐ACA implementation period and September 1, 2014 to September 30, 2015 as the post‐ACA implementation period, as we were unable to distinguish before versus after data in August 2014 based on the midmonth expansion of Medicaid.
After stratifying hospitals into groups based on whether they were located in Medicaid‐expansion or nonexpansion states, the proportion of discharges by payer was compared between pre‐ and post‐ACA implementation periods both graphically by quarter and using linear regression models weighted for the number of cases from each hospital. Next, for both Medicaid‐expansion and nonexpansion hospitals, LOS index and mortality index were compared before and after ACA implementation using linear regression models weighted for the number of cases from each hospital, both overall and by payer. Difference‐in‐differences estimations were then completed to compare the proportion of discharges by payer, LOS index, and mortality index between Medicaid‐expansion and nonexpansion hospitals before and after ACA implementation. Post hoc linear regression analyses were completed to evaluate the effect of clustering by state level strata on payer mix and LOS and mortality indices. A 2‐sided P value of 0.05 was considered statistically significant. Data analyses were performed using Stata 12.0 (StataCorp, College Station, TX).
RESULTS
We identified 4,258,952 discharges among general medicine patients from 211 hospitals in 38 states and Washington, DC between October 1, 2012, and September 30, 2015. This included 3,144,488 discharges from 156 hospitals in 24 Medicaid‐expansion states and Washington, DC and 1,114,464 discharges from 55 hospitals in 14 nonexpansion states.
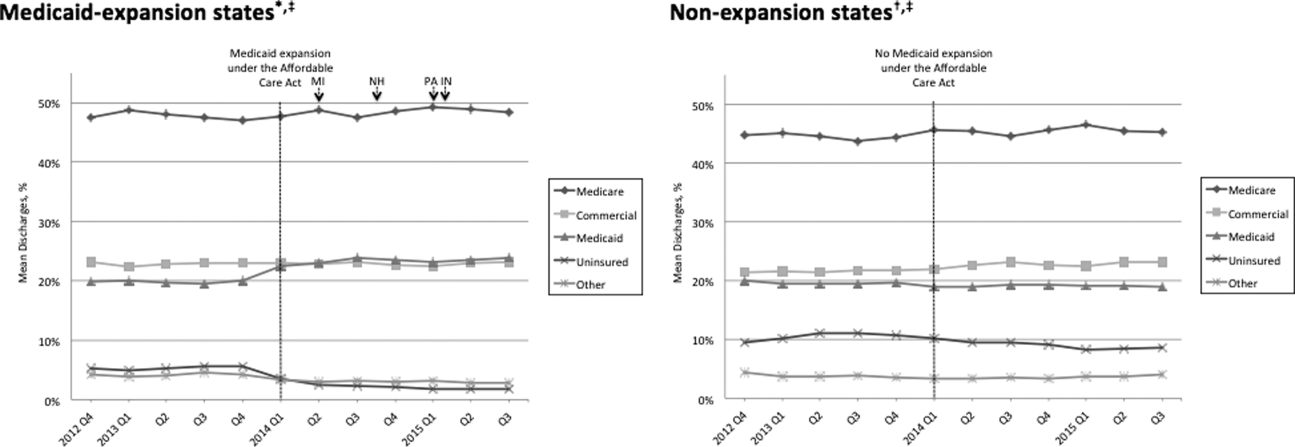
Figure 1 shows the trends in payer mix over time for hospitals in both Medicaid‐expansion and nonexpansion states. As summarized in Table 1, hospitals in Medicaid‐expansion states experienced a significant 3.7‐percentage point increase in Medicaid discharges (P = 0.013) and 2.9‐percentage point decrease in uninsured discharges (P 0.001) after ACA implementation. This represented an approximately 19% jump and 60% drop in Medicaid and uninsured discharges, respectively. Hospitals in nonexpansion states saw no significant change in the proportion of discharges by payer after ACA implementation. In the difference‐in‐differences analysis, there was a trend toward a greater change in the proportion of Medicaid discharges pre‐ to post‐ACA implementation among hospitals in Medicaid‐expansion states compared to hospitals in nonexpansion states (mean difference‐in‐differences 4.1%, 95% confidence interval [CI]: 0.3%, 8.6%, P = 0.070).
| Medicaid‐expansion n=156 hospitals; 3,144,488 cases | Non‐expansion n=55 hospitals; 1,114,464 cases | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre‐ACA Implementation (1,453,090 Cases) | Post‐ACA Implementation (1,691,398 Cases) | Mean Difference | P Value | Pre‐ACA Implementation (455,440 Cases) | Post‐ACA Implementation (659,024 Cases) | Mean Difference | P Value | Mean Difference‐in‐Differences | P Value | |
| ||||||||||
| Payer mix, % (95% CI) | ||||||||||
| Medicare | 48.6 (46.2, 51.0)* | 48.3 (45.9, 50.7) | 0.3 (3.6, 3.1) | 0.865 | 44.3 (40.7, 47.7)* | 45.3 (41.9, 48.6) | 1.0 (3.8, 5.8) | 0.671 | 1.3 (7.1, 4.5) | 0.655 |
| Commercial | 23.1 (21.4, 24.7) | 23.2 (21.8, 24.6) | 0.2 (2.0, 2.3) | 0.882 | 21.5 (18.5, 24.6) | 22.7 (19.7, 25.8) | 1.2 (3.0, 5.4) | 0.574 | 1.0 (5.7, 3.6) | 0.662 |
| Medicaid | 19.6 (17.6, 21.6) | 23.3 (21.2, 25.5) | 3.7 (0.8, 6.6) | 0.013 | 19.4 (16.9, 21.9) | 19.0 (16.5, 21.4) | 0.4 (3.8, 3.0) | 0.812 | 4.1 (0.3, 8.6) | 0.070 |
| Uninsured | 5.0 (4.0, 5.9) | 2.0 (1.7, 2.3) | 2.9 (3.9, 2.0) | 0.001 | 10.9 (8.1, 13.7) | 9.4 (7.0, 11.7) | 1.5 (5.1, 2.1) | 0.407 | 1.4 (5.1, 2.2) | 0.442 |
| Other | 3.8 (2.6, 4.9) | 3.1 (2.0, 4.3) | 0.7 (2.3, 1.0) | 0.435 | 4.0 (2.9, 5.0) | 3.7 (2.6, 4.7) | 0.3 (1.7, 1.1) | 0.662 | 0.3 (2.5, 1.8) | 0.762 |
| LOS index, mean (95% CI) | ||||||||||
| Overall | 1.017 (0.996, 1.038) | 1.006 (0.981, 1.031) | 0.011 (0.044, 0.021) | 0.488 | 1.008 (0.974, 1.042) | 0.995 (0.961, 1.029) | 0.013 (0.061, 0.034) | 0.574 | 0.002 (0.055, 0.059) | 0.943 |
| Medicare | 1.012 (0.989, 1.035) | 0.999 (0.971, 1.027) | 0.013 (0.049, 0.023) | 0.488 | 0.982 (0.946, 1.017) | 0.979 (0.944, 1.013) | 0.003 (0.052, 0.046) | 0.899 | 0.010 (0.070, 0.051) | 0.754 |
| Commercial | 0.993 (0.974, 1.012) | 0.977 (0.955, 0.998) | 0.016 (0.045, 0.013) | 0.271 | 1.009 (0.978, 1.039) | 0.986 (0.956, 1.016) | 0.022 (0.065, 0.020) | 0.298 | 0.006 (0.044, 0.057) | 0.809 |
| Medicaid | 1.059 (1.036, 1.082) | 1.043 (1.018, 1.067) | 0.016 (0.049, 0.017) | 0.349 | 1.064 (1.020, 1.108) | 1.060 (1.015, 1.106) | 0.004 (0.066, 0.059) | 0.911 | 0.012 (0.082, 0.057) | 0.727 |
| Uninsured | 0.960 (0.933, 0.988) | 0.925 (0.890, 0.961) | 0.035 (0.080, 0.010) | 0.126 | 0.972 (0.935, 1.009) | 0.944 (0.909, 0.979) | 0.028 (0.078, 0.022) | 0.273 | 0.007 (0.074, 0.060) | 0.835 |
| Other | 0.988 (0.960, 1.017) | 0.984 (0.952, 1.015) | 0.005 (0.047, 0.037) | 0.822 | 1.022 (0.973, 1.071) | 0.984 (0.944, 1.024) | 0.038 (0.100, 0.024) | 0.232 | 0.033 (0.042, 0.107) | 0.386 |
| Mortality index, mean (95% CI) | ||||||||||
| Overall | 1.000 (0.955, 1.045) | 0.878 (0.836, 0.921) | 0.122 (0.183, 0.061) | 0.001 | 0.997 (0.931, 1.062) | 0.850 (0.800, 0.900) | 0.147 (0.227, 0.066) | 0.001 | 0.025 (0.076, 0.125) | 0.628 |
| Medicare | 0.990 (0.942, 1.038) | 0.871 (0.826, 0.917) | 0.119 (0.185, 0.053) | 0.001 | 1.000 (0.925, 1.076) | 0.844 (0.788, 0.900) | 0.156 (0.249, 0.064) | 0.001 | 0.038 (0.075, 0.150) | 0.513 |
| Commercial | 1.045 (0.934, 1.155) | 0.908 (0.842, 0.975) | 0.136 (0.264, 0.008) | 0.037 | 1.023 (0.935, 1.111) | 0.820 (0.758, 0.883) | 0.203 (0.309, 0.096) | 0.001 | 0.067 (0.099, 0.232) | 0.430 |
| Medicaid | 0.894 (0.845, 0.942) | 0.786 (0.748, 0.824) | 0.107 (0.168, 0.046) | 0.001 | 0.937 (0.861, 1.013) | 0.789 (0.733, 0.844) | 0.148 (0.242, 0.055) | 0.002 | 0.041 (0.069, 0.151) | 0.464 |
| Uninsured | 1.172 (1.007, 1.337)∥ | 1.136 (0.968, 1.303) | 0.037 (0.271, 0.197) | 0.758 | 0.868 (0.768, 0.968)∥ | 0.850 (0.761, 0.939) | 0.017 (0.149, 0.115) | 0.795 | 0.019 (0.287, 0.248) | 0.887 |
| Other | 1.376 (1.052, 1.700)# | 1.156 (0.910, 1.402) | 0.220 (0.624, 0.184) | 0.285 | 1.009 (0.868, 1.150) # | 0.874 (0.682, 1.066) | 0.135 (0.369, 0.099) | 0.254 | 0.085 (0.555, 0.380) | 0.720 |
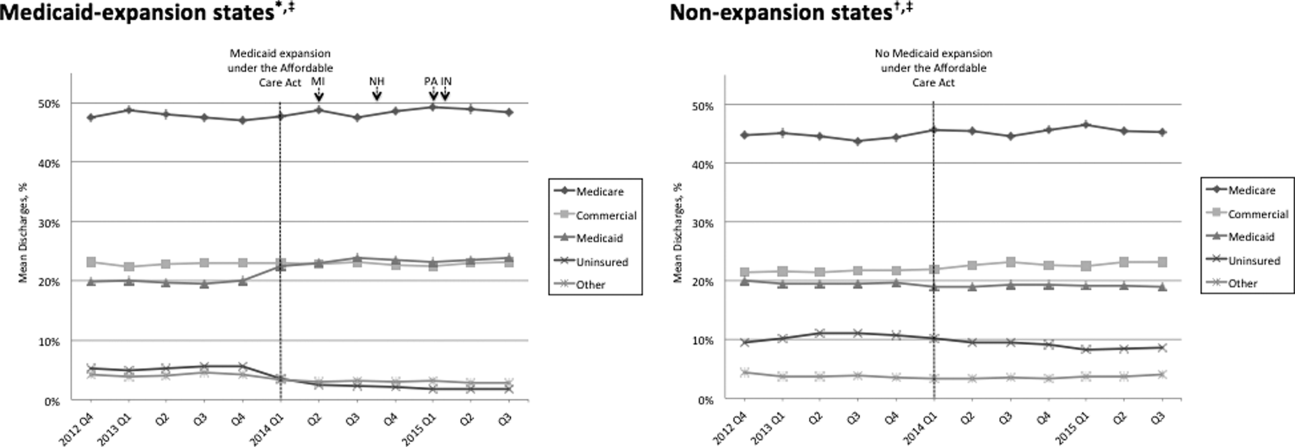
Table 1 shows that the overall LOS index remained unchanged pre‐ to post‐ACA implementation for both Medicaid‐expansion (1.017 to 1.006, P = 0.488) and nonexpansion hospitals (1.008 to 0.995, P = 0.574). LOS indices for each payer type also remained unchanged. The overall mortality index significantly improved pre‐ to post‐ACA implementation for both Medicaid‐expansion (1.000 to 0.878, P 0.001) and nonexpansion hospitals (0.997 to 0.850, P = 0.001). Among both Medicaid‐expansion and nonexpansion hospitals, the mortality index significantly improved for Medicare, commercial, and Medicaid discharges but not for uninsured or other discharges. In the difference‐in‐differences analysis, the changes in LOS indices and mortality indices pre‐ to post‐ACA implementation did not differ significantly between hospitals in Medicaid‐expansion versus nonexpansion states.
In post hoc linear regression analyses of payer mix and LOS and mortality indices clustered by state‐level strata, point estimates were minimally changed. Although 95% CIs were slightly wider, statistical significance was unchanged from our primary analyses (data not shown).
DISCUSSION
We found that ACA implementation had a significant impact on payer mix for general medicine patients at AMCs in the United States, primarily by increasing the number of Medicaid beneficiaries and by decreasing the number of uninsured patients in Medicaid‐expansion states. State Medicaid expansion status did not appear to influence either LOS or in‐hospital mortality.
Our study offers some of the longest‐term data currently available on the impact of ACA implementation on payer mix trends and encompasses more states than others have previously. Although we uniquely focused on general medicine patients at AMCs, our results are similar to those seen for US hospitals overall. Nikpay and colleagues evaluated payer mix trends for non‐Medicare adult inpatient stays in 16 states through the second quarter of 2014 using the Healthcare Cost and Utilization Project database through the Agency for Healthcare Research and Quality.[4] They found a relative 20% increase and 50% decrease in Medicaid and uninsured discharges in Medicaid‐expansion states, along with nonsignificant changes in nonexpansion states. Hempstead and Cantor assessed payer mix for non‐Medicare discharges using state hospital association data from 21 states through the fourth quarter of 2014 and found a significant increase in Medicaid patients as well as a nearly significant decrease in uninsured patients in expansion states relative to nonexpansion states.[5] The Department of Health and Human Services also reported that uninsured/self‐pay discharges fell substantially (65%73%) in Medicaid‐expansion states by the end of 2014, with slight decreases in nonexpansion states.[20]
In contrast to our hypothesis, the overall LOS and in‐hospital mortality indices were not influenced by state Medicaid expansion status. From a purely mathematical standpoint, the contribution of Medicaid patients to the overall LOS and mortality indices may have been eclipsed by Medicare and commercially insured patients, who represented a higher proportion of total discharges. The lack of impact of state Medicaid expansion status on overall LOS and mortality indices did not appear to occur as a result of indices for Medicaid patients trending toward the mean. As predicted based on observational studies, Medicaid patients in our study tended to have a higher LOS index than those with other insurance types. Medicaid patients actually tended to have a lower mortality index in our analysis; the reason for this latter finding is unclear and in contrast to other published studies.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 21]
To our knowledge, no other studies have evaluated the effect of payer mix changes under the ACA on inpatient outcomes. However, new evidence is emerging on outpatient outcomes. Low‐income adults in Medicaid‐expansion states have reported greater gains in access to primary care services and in the diagnosis of certain chronic health conditions than those in nonexpansion states as a result of ACA implementation.[22, 23] Such improvements in the outpatient setting might be expected to reduce patient acuity on admission. However, they would not necessarily translate to relative improvements in LOS or mortality indices for Medicaid‐expansion hospitals, as the UHC risk adjustment models controlled for disease severity on admission.
Similarly, few studies have assessed the impact of payer mix changes under previous state Medicaid expansions on inpatient outcomes. After Massachusetts expanded Medicaid and enacted near‐universal healthcare coverage in 2006, a minimal LOS reduction of just 0.05 days was observed.[24] New York expanded Medicaid eligibility to nondisabled childless adults with incomes below 100% of the federal poverty level in September 2001, whereas Arizona did so in November 2001 and Maine in October 2002. A study comparing outcomes in these states to 4 neighboring nonexpansion states found a relative reduction in annual all‐cause mortality of 6.1% population wide; however, it did not assess in‐hospital mortality.[25] The Oregon Health Insurance Experiment that randomized low‐income adults to expanded Medicaid coverage or not in 2008 has also reported on outpatient rather than inpatient outcomes.[26]
Our findings have potential implications for health policymakers. That Medicaid expansion status had a neutral effect on both LOS and mortality indices in our analysis should be reassuring for states contemplating Medicaid expansion in the future. Our results also highlight the need for further efforts to reduce disparities in inpatient care based on payer status. For example, although Medicare, commercially insured, and Medicaid patients witnessed significant improvements in mortality indices pre‐ to post‐ACA implementation in hospitals in both Medicaid‐expansion and nonexpansion states, uninsured patients did not.
This study has several limitations. First, our analysis of the impact of ACA implementation on payer mix did not account for concurrent socioeconomic trends that may have influenced insurance coverage across the United States. However, the main goal of this analysis was to demonstrate that changes in payer mix did in fact occur over time, to provide rationale for our subsequent LOS and mortality analyses. Second, we could not control for variation in the design and implementation of Medicaid expansions across states as permitted under the federal Section 1115 waiver process. Third, we only had access to hospital‐level data through the UHC CDB/RM, rather than individual patient data. We attempted to mitigate this limitation by weighting data according to the number of cases per hospital. Lastly, additional patient‐level factors that may influence LOS or mortality may not be included in the UHC risk adjustment models.
In summary, the differential shift in payer mix between Medicaid‐expansion and nonexpansion states did not influence overall LOS or in‐hospital mortality for general medicine patients at AMCs in the United States. Additional research could help to determine the impact of ACA implementation on other patient outcomes that may be dependent on insurance status, such as readmissions or hospital‐acquired complications.
Disclosures: M.E.A. conceived of the study concept and design, assisted with data acquisition, and drafted the manuscript. J.J.G. assisted with study design and made critical revisions to the manuscript. D.A. assisted with study design and made critical revisions to the manuscript. R.P. assisted with study design and made critical revisions to the manuscript. M.L. assisted with study design and data acquisition and made critical revisions to the manuscript. C.D.J. assisted with study design, performed data analyses, and made critical revisions to the manuscript. A modified abstract was presented in poster format at the University HealthSystem Consortium Annual Conference held September 30 to October 2, 2015 in Orlando, Florida, as well as at the Society of Hospital Medicine Research, Innovations, and Vignettes 2016 Annual Meeting held March 69, 2016, in San Diego, California. The authors report no conflicts of interest.
- Department of Health and Human Services. Key features of the Affordable Care Act by year. Available at: http://www.hhs.gov/healthcare/facts‐and‐features/key‐features‐of‐aca‐by‐year/index.html#2014. Accessed April 4, 2016.
- Centers for Medicare and Medicaid Services. Medicaid enrollment data collected through MBES. Available at: https://www.medicaid.gov/medicaid‐chip‐program‐information/program‐information/medicaid‐and‐chip‐enrollment‐data/medicaid‐enrollment‐data‐collected‐through‐mbes.html. Accessed April 4, 2016.
- The Henry J. Kaiser Family Foundation. Status of state action on the Medicaid expansion decision. Available at: http://kff.org/health‐reform/state‐indicator/state‐activity‐around‐expanding‐medicaid‐under‐the‐affordable‐care‐act. Accessed April 4, 2016.
- , , . Affordable Care Act Medicaid expansion reduced uninsured hospital stays in 2014. Health Aff (Millwood). 2016;35(1):106–110.
- , . State Medicaid expansion and changes in hospital volume according to payer. N Engl J Med. 2016;374(2):196–198.
- , , , , , . Understanding predictors of prolonged hospitalizations among general medicine patients: a guide and preliminary analysis. J Hosp Med. 2015;10(9):623–626.
- , , , . Impact of insurance and hospital ownership on hospital length of stay among patients with ambulatory care‐sensitive conditions. Ann Fam Med. 2011;9:489–495.
- , , . Insurance status and hospital care for myocardial infarction, stroke, and pneumonia. J Hosp Med. 2010;5:452–459.
- , , , , , . Payment source, quality of care, and outcomes in patients hospitalized with heart failure. J Am Coll Cardiol. 2011;58(14):1465–1471.
- , , , , . The inpatient experience and predictors of length of stay for patients hospitalized with systolic heart failure: comparison by commercial, Medicaid, and Medicare payer type. J Med Econ. 2013;16(1):43–54.
- , , et al. Insurance coverage and care of patients with non‐ST‐segment elevation acute coronary syndromes. Ann Intern Med. 2006;145:739–748.
- , , , et al. Association of insurance status with inpatient treatment for coronary artery disease: findings from the Get with the Guidelines Program. Am Heart J. 2010;159:1026–1036.
- , , , et al. Primary payer status affects mortality for major surgical operations. Ann Surg. 2010;252:544–551.
- , , . Medicaid payer status is associated with in‐hospital morbidity and resource utilization following primary total joint arthroplasty. J Bone Joint Surg Am. 2014;96(21):e180.
- , , . The quality of care delivered to patients within the same hospital varies by insurance type. Health Aff (Millwood). 2013;32(10):1731–1739.
- , , , , . Effect of insurance status on postacute care among working age stroke survivors. Neurology. 2012;78(20):1590–1595.
- , , , . Hospitalizations in which patients leave the hospital against medical advice (AMA), 2007. HCUP statistical brief #78. August 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2009. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb78.pdf. Accessed May 12, 2016.
- , , , . Characteristics of Medicaid and uninsured hospitalizations, 2012. HCUP statistical brief #182. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb182‐Medicaid‐Uninsured‐Hospitalizations‐2012.pdf. Accessed March 9, 2016.
- Agency for Healthcare Research and Quality. Mortality measurement: mortality risk adjustment methodology for University HealthSystem Consortium. Available at: http://archive.ahrq.gov/professionals/quality‐patient‐safety/quality‐resources/tools/mortality/Meurer.pdf. Accessed May 10, 2016.
- Department of Health and Human Services. Insurance expansion, hospital uncompensated care, and the Affordable Care Act. Available at: https://aspe.hhs.gov/pdf‐report/insurance‐expansion‐hospital‐uncompensated‐care‐and‐affordable‐care‐act. Accessed May 27, 2016.
- , , , . Our flawed but beneficial Medicaid program. N Engl J Med. 2011;364(16):e31.
- , , , . Changes in self‐reported insurance coverage, access to care, and health under the Affordable Care Act. JAMA. 2015;314(4):366–374.
- , . Early coverage, access, utilization, and health effects associated with the Affordable Care Act Medicaid Expansions: a quasi‐experimental study. Ann Intern Med. 2016;164(12):795–803.
- , . The impact of health care reform on hospital and preventive care: evidence from Massachusetts. J Public Econ. 2012;96(11–12):909–929.
- , , . Mortality and access to care among adults after state Medicaid expansions. N Engl J Med. 2012;367:1025–1034.
- , , , et al. The Oregon Experiment—effects of Medicaid on clinical outcomes. N Engl J Med. 2013;368(18):1713–1722.
On January 1, 2014, several major provisions of the Affordable Care Act (ACA) took effect, including introduction of the individual mandate for health insurance coverage, opening of the Health Insurance Marketplace, and expansion of Medicaid eligibility to Americans earning up to 133% of the federal poverty level.[1] Nearly 9 million US adults have enrolled in Medicaid since that time, primarily in the 31 states and Washington, DC that have opted into Medicaid expansion.[2, 3] ACA implementation has also had a significant impact on hospital payer mix, primarily by reducing the volume of uncompensated care in Medicaid‐expansion states.[4, 5]
The differential shift in payer mix in Medicaid‐expansion versus nonexpansion states may be relevant to hospitals beyond reimbursement. Medicaid insurance has historically been associated with longer hospitalizations and higher in‐hospital mortality in diverse patient populations, more so than commercial insurance and often even uninsured payer status.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15] The disparity in outcomes between patients with Medicaid versus other insurance persists even after adjustment for disease severity and baseline comorbidities. Insurance type may influence the delivery of inpatient care through variation in access to invasive procedures and adherence to guideline‐concordant medical therapies.[9, 10, 11, 12] Medicaid patients may be more likely than uninsured patients to remain hospitalized pending postacute care placement rather than be discharged home with family support.[16] Medicaid patients are also less likely to leave against medical advice than uninsured patients.[17]
Currently, little is known about the impact of state Medicaid expansion status on length of stay (LOS) or mortality nationally. It is possible that hospitals in Medicaid‐expansion states have experienced relative worsening in LOS and mortality as their share of Medicaid patients has grown. Determining the impact of ACA implementation on payer mix and patient outcomes is particularly important for academic medical centers (AMCs), as they traditionally care for the greatest percentage of both Medicaid and uninsured patients.[18] We sought to characterize the impact of state Medicaid expansion status on payer mix, LOS, and in‐hospital mortality for general medicine patients at AMCs in the United States.
METHODS
The University HealthSystem Consortium (UHC) is an alliance of 117 AMCs and 310 affiliated hospitals, representing >90% of such institutions in the US. We queried the online UHC Clinical Data Base/Resource Manager (CDB/RM) to obtain hospital‐level insurance, LOS, and mortality data for inpatients discharged from a general medicine service between October 1, 2012 and September 30, 2015. We excluded hospitals that were missing data for any month within the study period. No patient‐level data were accessed.
Our outcomes of interest were the proportion of discharges by primary payer (Medicare, commercial, Medicaid, uninsured, or other [eg, Tri‐Care or Workers' Compensation]), as well as the LOS index and mortality index. Both indices were defined as the ratio of the observed to expected values. To determine the expected LOS and mortality, the UHC 2015 risk adjustment models were applied to all cases, adjusting for variables such as patient demographics, low socioeconomic status, admit source and status, severity of illness, and comorbid conditions, as described by International Classification of Diseases, Ninth Revision codes. These models have been validated and are used for research and quality benchmarking for member institutions.[19]
We next stratified hospitals according to state Medicaid expansion status. We defined Medicaid‐expansion states as those that had expanded Medicaid by the end of the study period: Arizona, Arkansas, California, Colorado, Connecticut, Illinois, Indiana, Iowa, Kentucky, Maryland, Massachusetts, Michigan, Minnesota, Nevada, New Hampshire, New Jersey, New Mexico, New York, Ohio, Oregon, Pennsylvania, Rhode Island, Washington, Washington DC, and West Virginia. Nonexpansion states included Alabama, Florida, Georgia, Kansas, Louisiana, Missouri, Nebraska, North Carolina, South Carolina, Tennessee, Texas, Utah, Virginia, and Wisconsin. We excluded 12 states due to incomplete data: Alaska, Delaware, Hawaii, Idaho, North Dakota, Maine, Mississippi, Montana, Oklahoma, South Dakota, Vermont, and Wyoming.
We then identified our pre‐ and post‐ACA implementation periods. Medicaid coverage expansion took effect in all expansion states on January 1, 2014, with the exception of Michigan (April 1, 2014), New Hampshire (August 15, 2014), Pennsylvania (January 1, 2015), and Indiana (February 1, 2015).[3] We therefore defined October 1, 2012 to December 31, 2013 as the pre‐ACA implementation period and January 1, 2014 to September 30, 2015 as the post‐ACA implementation period for all states except for Michigan, New Hampshire, Pennsylvania, and Indiana. For these 4 states, we customized the pre‐ and post‐ACA implementation periods to their respective dates of Medicaid expansion; for New Hampshire, we designated October 1, 2012 to July 31, 2014 as the pre‐ACA implementation period and September 1, 2014 to September 30, 2015 as the post‐ACA implementation period, as we were unable to distinguish before versus after data in August 2014 based on the midmonth expansion of Medicaid.
After stratifying hospitals into groups based on whether they were located in Medicaid‐expansion or nonexpansion states, the proportion of discharges by payer was compared between pre‐ and post‐ACA implementation periods both graphically by quarter and using linear regression models weighted for the number of cases from each hospital. Next, for both Medicaid‐expansion and nonexpansion hospitals, LOS index and mortality index were compared before and after ACA implementation using linear regression models weighted for the number of cases from each hospital, both overall and by payer. Difference‐in‐differences estimations were then completed to compare the proportion of discharges by payer, LOS index, and mortality index between Medicaid‐expansion and nonexpansion hospitals before and after ACA implementation. Post hoc linear regression analyses were completed to evaluate the effect of clustering by state level strata on payer mix and LOS and mortality indices. A 2‐sided P value of 0.05 was considered statistically significant. Data analyses were performed using Stata 12.0 (StataCorp, College Station, TX).
RESULTS
We identified 4,258,952 discharges among general medicine patients from 211 hospitals in 38 states and Washington, DC between October 1, 2012, and September 30, 2015. This included 3,144,488 discharges from 156 hospitals in 24 Medicaid‐expansion states and Washington, DC and 1,114,464 discharges from 55 hospitals in 14 nonexpansion states.
Figure 1 shows the trends in payer mix over time for hospitals in both Medicaid‐expansion and nonexpansion states. As summarized in Table 1, hospitals in Medicaid‐expansion states experienced a significant 3.7‐percentage point increase in Medicaid discharges (P = 0.013) and 2.9‐percentage point decrease in uninsured discharges (P 0.001) after ACA implementation. This represented an approximately 19% jump and 60% drop in Medicaid and uninsured discharges, respectively. Hospitals in nonexpansion states saw no significant change in the proportion of discharges by payer after ACA implementation. In the difference‐in‐differences analysis, there was a trend toward a greater change in the proportion of Medicaid discharges pre‐ to post‐ACA implementation among hospitals in Medicaid‐expansion states compared to hospitals in nonexpansion states (mean difference‐in‐differences 4.1%, 95% confidence interval [CI]: 0.3%, 8.6%, P = 0.070).
| Medicaid‐expansion n=156 hospitals; 3,144,488 cases | Non‐expansion n=55 hospitals; 1,114,464 cases | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre‐ACA Implementation (1,453,090 Cases) | Post‐ACA Implementation (1,691,398 Cases) | Mean Difference | P Value | Pre‐ACA Implementation (455,440 Cases) | Post‐ACA Implementation (659,024 Cases) | Mean Difference | P Value | Mean Difference‐in‐Differences | P Value | |
| ||||||||||
| Payer mix, % (95% CI) | ||||||||||
| Medicare | 48.6 (46.2, 51.0)* | 48.3 (45.9, 50.7) | 0.3 (3.6, 3.1) | 0.865 | 44.3 (40.7, 47.7)* | 45.3 (41.9, 48.6) | 1.0 (3.8, 5.8) | 0.671 | 1.3 (7.1, 4.5) | 0.655 |
| Commercial | 23.1 (21.4, 24.7) | 23.2 (21.8, 24.6) | 0.2 (2.0, 2.3) | 0.882 | 21.5 (18.5, 24.6) | 22.7 (19.7, 25.8) | 1.2 (3.0, 5.4) | 0.574 | 1.0 (5.7, 3.6) | 0.662 |
| Medicaid | 19.6 (17.6, 21.6) | 23.3 (21.2, 25.5) | 3.7 (0.8, 6.6) | 0.013 | 19.4 (16.9, 21.9) | 19.0 (16.5, 21.4) | 0.4 (3.8, 3.0) | 0.812 | 4.1 (0.3, 8.6) | 0.070 |
| Uninsured | 5.0 (4.0, 5.9) | 2.0 (1.7, 2.3) | 2.9 (3.9, 2.0) | 0.001 | 10.9 (8.1, 13.7) | 9.4 (7.0, 11.7) | 1.5 (5.1, 2.1) | 0.407 | 1.4 (5.1, 2.2) | 0.442 |
| Other | 3.8 (2.6, 4.9) | 3.1 (2.0, 4.3) | 0.7 (2.3, 1.0) | 0.435 | 4.0 (2.9, 5.0) | 3.7 (2.6, 4.7) | 0.3 (1.7, 1.1) | 0.662 | 0.3 (2.5, 1.8) | 0.762 |
| LOS index, mean (95% CI) | ||||||||||
| Overall | 1.017 (0.996, 1.038) | 1.006 (0.981, 1.031) | 0.011 (0.044, 0.021) | 0.488 | 1.008 (0.974, 1.042) | 0.995 (0.961, 1.029) | 0.013 (0.061, 0.034) | 0.574 | 0.002 (0.055, 0.059) | 0.943 |
| Medicare | 1.012 (0.989, 1.035) | 0.999 (0.971, 1.027) | 0.013 (0.049, 0.023) | 0.488 | 0.982 (0.946, 1.017) | 0.979 (0.944, 1.013) | 0.003 (0.052, 0.046) | 0.899 | 0.010 (0.070, 0.051) | 0.754 |
| Commercial | 0.993 (0.974, 1.012) | 0.977 (0.955, 0.998) | 0.016 (0.045, 0.013) | 0.271 | 1.009 (0.978, 1.039) | 0.986 (0.956, 1.016) | 0.022 (0.065, 0.020) | 0.298 | 0.006 (0.044, 0.057) | 0.809 |
| Medicaid | 1.059 (1.036, 1.082) | 1.043 (1.018, 1.067) | 0.016 (0.049, 0.017) | 0.349 | 1.064 (1.020, 1.108) | 1.060 (1.015, 1.106) | 0.004 (0.066, 0.059) | 0.911 | 0.012 (0.082, 0.057) | 0.727 |
| Uninsured | 0.960 (0.933, 0.988) | 0.925 (0.890, 0.961) | 0.035 (0.080, 0.010) | 0.126 | 0.972 (0.935, 1.009) | 0.944 (0.909, 0.979) | 0.028 (0.078, 0.022) | 0.273 | 0.007 (0.074, 0.060) | 0.835 |
| Other | 0.988 (0.960, 1.017) | 0.984 (0.952, 1.015) | 0.005 (0.047, 0.037) | 0.822 | 1.022 (0.973, 1.071) | 0.984 (0.944, 1.024) | 0.038 (0.100, 0.024) | 0.232 | 0.033 (0.042, 0.107) | 0.386 |
| Mortality index, mean (95% CI) | ||||||||||
| Overall | 1.000 (0.955, 1.045) | 0.878 (0.836, 0.921) | 0.122 (0.183, 0.061) | 0.001 | 0.997 (0.931, 1.062) | 0.850 (0.800, 0.900) | 0.147 (0.227, 0.066) | 0.001 | 0.025 (0.076, 0.125) | 0.628 |
| Medicare | 0.990 (0.942, 1.038) | 0.871 (0.826, 0.917) | 0.119 (0.185, 0.053) | 0.001 | 1.000 (0.925, 1.076) | 0.844 (0.788, 0.900) | 0.156 (0.249, 0.064) | 0.001 | 0.038 (0.075, 0.150) | 0.513 |
| Commercial | 1.045 (0.934, 1.155) | 0.908 (0.842, 0.975) | 0.136 (0.264, 0.008) | 0.037 | 1.023 (0.935, 1.111) | 0.820 (0.758, 0.883) | 0.203 (0.309, 0.096) | 0.001 | 0.067 (0.099, 0.232) | 0.430 |
| Medicaid | 0.894 (0.845, 0.942) | 0.786 (0.748, 0.824) | 0.107 (0.168, 0.046) | 0.001 | 0.937 (0.861, 1.013) | 0.789 (0.733, 0.844) | 0.148 (0.242, 0.055) | 0.002 | 0.041 (0.069, 0.151) | 0.464 |
| Uninsured | 1.172 (1.007, 1.337)∥ | 1.136 (0.968, 1.303) | 0.037 (0.271, 0.197) | 0.758 | 0.868 (0.768, 0.968)∥ | 0.850 (0.761, 0.939) | 0.017 (0.149, 0.115) | 0.795 | 0.019 (0.287, 0.248) | 0.887 |
| Other | 1.376 (1.052, 1.700)# | 1.156 (0.910, 1.402) | 0.220 (0.624, 0.184) | 0.285 | 1.009 (0.868, 1.150) # | 0.874 (0.682, 1.066) | 0.135 (0.369, 0.099) | 0.254 | 0.085 (0.555, 0.380) | 0.720 |

Table 1 shows that the overall LOS index remained unchanged pre‐ to post‐ACA implementation for both Medicaid‐expansion (1.017 to 1.006, P = 0.488) and nonexpansion hospitals (1.008 to 0.995, P = 0.574). LOS indices for each payer type also remained unchanged. The overall mortality index significantly improved pre‐ to post‐ACA implementation for both Medicaid‐expansion (1.000 to 0.878, P 0.001) and nonexpansion hospitals (0.997 to 0.850, P = 0.001). Among both Medicaid‐expansion and nonexpansion hospitals, the mortality index significantly improved for Medicare, commercial, and Medicaid discharges but not for uninsured or other discharges. In the difference‐in‐differences analysis, the changes in LOS indices and mortality indices pre‐ to post‐ACA implementation did not differ significantly between hospitals in Medicaid‐expansion versus nonexpansion states.
In post hoc linear regression analyses of payer mix and LOS and mortality indices clustered by state‐level strata, point estimates were minimally changed. Although 95% CIs were slightly wider, statistical significance was unchanged from our primary analyses (data not shown).
DISCUSSION
We found that ACA implementation had a significant impact on payer mix for general medicine patients at AMCs in the United States, primarily by increasing the number of Medicaid beneficiaries and by decreasing the number of uninsured patients in Medicaid‐expansion states. State Medicaid expansion status did not appear to influence either LOS or in‐hospital mortality.
Our study offers some of the longest‐term data currently available on the impact of ACA implementation on payer mix trends and encompasses more states than others have previously. Although we uniquely focused on general medicine patients at AMCs, our results are similar to those seen for US hospitals overall. Nikpay and colleagues evaluated payer mix trends for non‐Medicare adult inpatient stays in 16 states through the second quarter of 2014 using the Healthcare Cost and Utilization Project database through the Agency for Healthcare Research and Quality.[4] They found a relative 20% increase and 50% decrease in Medicaid and uninsured discharges in Medicaid‐expansion states, along with nonsignificant changes in nonexpansion states. Hempstead and Cantor assessed payer mix for non‐Medicare discharges using state hospital association data from 21 states through the fourth quarter of 2014 and found a significant increase in Medicaid patients as well as a nearly significant decrease in uninsured patients in expansion states relative to nonexpansion states.[5] The Department of Health and Human Services also reported that uninsured/self‐pay discharges fell substantially (65%73%) in Medicaid‐expansion states by the end of 2014, with slight decreases in nonexpansion states.[20]
In contrast to our hypothesis, the overall LOS and in‐hospital mortality indices were not influenced by state Medicaid expansion status. From a purely mathematical standpoint, the contribution of Medicaid patients to the overall LOS and mortality indices may have been eclipsed by Medicare and commercially insured patients, who represented a higher proportion of total discharges. The lack of impact of state Medicaid expansion status on overall LOS and mortality indices did not appear to occur as a result of indices for Medicaid patients trending toward the mean. As predicted based on observational studies, Medicaid patients in our study tended to have a higher LOS index than those with other insurance types. Medicaid patients actually tended to have a lower mortality index in our analysis; the reason for this latter finding is unclear and in contrast to other published studies.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 21]
To our knowledge, no other studies have evaluated the effect of payer mix changes under the ACA on inpatient outcomes. However, new evidence is emerging on outpatient outcomes. Low‐income adults in Medicaid‐expansion states have reported greater gains in access to primary care services and in the diagnosis of certain chronic health conditions than those in nonexpansion states as a result of ACA implementation.[22, 23] Such improvements in the outpatient setting might be expected to reduce patient acuity on admission. However, they would not necessarily translate to relative improvements in LOS or mortality indices for Medicaid‐expansion hospitals, as the UHC risk adjustment models controlled for disease severity on admission.
Similarly, few studies have assessed the impact of payer mix changes under previous state Medicaid expansions on inpatient outcomes. After Massachusetts expanded Medicaid and enacted near‐universal healthcare coverage in 2006, a minimal LOS reduction of just 0.05 days was observed.[24] New York expanded Medicaid eligibility to nondisabled childless adults with incomes below 100% of the federal poverty level in September 2001, whereas Arizona did so in November 2001 and Maine in October 2002. A study comparing outcomes in these states to 4 neighboring nonexpansion states found a relative reduction in annual all‐cause mortality of 6.1% population wide; however, it did not assess in‐hospital mortality.[25] The Oregon Health Insurance Experiment that randomized low‐income adults to expanded Medicaid coverage or not in 2008 has also reported on outpatient rather than inpatient outcomes.[26]
Our findings have potential implications for health policymakers. That Medicaid expansion status had a neutral effect on both LOS and mortality indices in our analysis should be reassuring for states contemplating Medicaid expansion in the future. Our results also highlight the need for further efforts to reduce disparities in inpatient care based on payer status. For example, although Medicare, commercially insured, and Medicaid patients witnessed significant improvements in mortality indices pre‐ to post‐ACA implementation in hospitals in both Medicaid‐expansion and nonexpansion states, uninsured patients did not.
This study has several limitations. First, our analysis of the impact of ACA implementation on payer mix did not account for concurrent socioeconomic trends that may have influenced insurance coverage across the United States. However, the main goal of this analysis was to demonstrate that changes in payer mix did in fact occur over time, to provide rationale for our subsequent LOS and mortality analyses. Second, we could not control for variation in the design and implementation of Medicaid expansions across states as permitted under the federal Section 1115 waiver process. Third, we only had access to hospital‐level data through the UHC CDB/RM, rather than individual patient data. We attempted to mitigate this limitation by weighting data according to the number of cases per hospital. Lastly, additional patient‐level factors that may influence LOS or mortality may not be included in the UHC risk adjustment models.
In summary, the differential shift in payer mix between Medicaid‐expansion and nonexpansion states did not influence overall LOS or in‐hospital mortality for general medicine patients at AMCs in the United States. Additional research could help to determine the impact of ACA implementation on other patient outcomes that may be dependent on insurance status, such as readmissions or hospital‐acquired complications.
Disclosures: M.E.A. conceived of the study concept and design, assisted with data acquisition, and drafted the manuscript. J.J.G. assisted with study design and made critical revisions to the manuscript. D.A. assisted with study design and made critical revisions to the manuscript. R.P. assisted with study design and made critical revisions to the manuscript. M.L. assisted with study design and data acquisition and made critical revisions to the manuscript. C.D.J. assisted with study design, performed data analyses, and made critical revisions to the manuscript. A modified abstract was presented in poster format at the University HealthSystem Consortium Annual Conference held September 30 to October 2, 2015 in Orlando, Florida, as well as at the Society of Hospital Medicine Research, Innovations, and Vignettes 2016 Annual Meeting held March 69, 2016, in San Diego, California. The authors report no conflicts of interest.
On January 1, 2014, several major provisions of the Affordable Care Act (ACA) took effect, including introduction of the individual mandate for health insurance coverage, opening of the Health Insurance Marketplace, and expansion of Medicaid eligibility to Americans earning up to 133% of the federal poverty level.[1] Nearly 9 million US adults have enrolled in Medicaid since that time, primarily in the 31 states and Washington, DC that have opted into Medicaid expansion.[2, 3] ACA implementation has also had a significant impact on hospital payer mix, primarily by reducing the volume of uncompensated care in Medicaid‐expansion states.[4, 5]
The differential shift in payer mix in Medicaid‐expansion versus nonexpansion states may be relevant to hospitals beyond reimbursement. Medicaid insurance has historically been associated with longer hospitalizations and higher in‐hospital mortality in diverse patient populations, more so than commercial insurance and often even uninsured payer status.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15] The disparity in outcomes between patients with Medicaid versus other insurance persists even after adjustment for disease severity and baseline comorbidities. Insurance type may influence the delivery of inpatient care through variation in access to invasive procedures and adherence to guideline‐concordant medical therapies.[9, 10, 11, 12] Medicaid patients may be more likely than uninsured patients to remain hospitalized pending postacute care placement rather than be discharged home with family support.[16] Medicaid patients are also less likely to leave against medical advice than uninsured patients.[17]
Currently, little is known about the impact of state Medicaid expansion status on length of stay (LOS) or mortality nationally. It is possible that hospitals in Medicaid‐expansion states have experienced relative worsening in LOS and mortality as their share of Medicaid patients has grown. Determining the impact of ACA implementation on payer mix and patient outcomes is particularly important for academic medical centers (AMCs), as they traditionally care for the greatest percentage of both Medicaid and uninsured patients.[18] We sought to characterize the impact of state Medicaid expansion status on payer mix, LOS, and in‐hospital mortality for general medicine patients at AMCs in the United States.
METHODS
The University HealthSystem Consortium (UHC) is an alliance of 117 AMCs and 310 affiliated hospitals, representing >90% of such institutions in the US. We queried the online UHC Clinical Data Base/Resource Manager (CDB/RM) to obtain hospital‐level insurance, LOS, and mortality data for inpatients discharged from a general medicine service between October 1, 2012 and September 30, 2015. We excluded hospitals that were missing data for any month within the study period. No patient‐level data were accessed.
Our outcomes of interest were the proportion of discharges by primary payer (Medicare, commercial, Medicaid, uninsured, or other [eg, Tri‐Care or Workers' Compensation]), as well as the LOS index and mortality index. Both indices were defined as the ratio of the observed to expected values. To determine the expected LOS and mortality, the UHC 2015 risk adjustment models were applied to all cases, adjusting for variables such as patient demographics, low socioeconomic status, admit source and status, severity of illness, and comorbid conditions, as described by International Classification of Diseases, Ninth Revision codes. These models have been validated and are used for research and quality benchmarking for member institutions.[19]
We next stratified hospitals according to state Medicaid expansion status. We defined Medicaid‐expansion states as those that had expanded Medicaid by the end of the study period: Arizona, Arkansas, California, Colorado, Connecticut, Illinois, Indiana, Iowa, Kentucky, Maryland, Massachusetts, Michigan, Minnesota, Nevada, New Hampshire, New Jersey, New Mexico, New York, Ohio, Oregon, Pennsylvania, Rhode Island, Washington, Washington DC, and West Virginia. Nonexpansion states included Alabama, Florida, Georgia, Kansas, Louisiana, Missouri, Nebraska, North Carolina, South Carolina, Tennessee, Texas, Utah, Virginia, and Wisconsin. We excluded 12 states due to incomplete data: Alaska, Delaware, Hawaii, Idaho, North Dakota, Maine, Mississippi, Montana, Oklahoma, South Dakota, Vermont, and Wyoming.
We then identified our pre‐ and post‐ACA implementation periods. Medicaid coverage expansion took effect in all expansion states on January 1, 2014, with the exception of Michigan (April 1, 2014), New Hampshire (August 15, 2014), Pennsylvania (January 1, 2015), and Indiana (February 1, 2015).[3] We therefore defined October 1, 2012 to December 31, 2013 as the pre‐ACA implementation period and January 1, 2014 to September 30, 2015 as the post‐ACA implementation period for all states except for Michigan, New Hampshire, Pennsylvania, and Indiana. For these 4 states, we customized the pre‐ and post‐ACA implementation periods to their respective dates of Medicaid expansion; for New Hampshire, we designated October 1, 2012 to July 31, 2014 as the pre‐ACA implementation period and September 1, 2014 to September 30, 2015 as the post‐ACA implementation period, as we were unable to distinguish before versus after data in August 2014 based on the midmonth expansion of Medicaid.
After stratifying hospitals into groups based on whether they were located in Medicaid‐expansion or nonexpansion states, the proportion of discharges by payer was compared between pre‐ and post‐ACA implementation periods both graphically by quarter and using linear regression models weighted for the number of cases from each hospital. Next, for both Medicaid‐expansion and nonexpansion hospitals, LOS index and mortality index were compared before and after ACA implementation using linear regression models weighted for the number of cases from each hospital, both overall and by payer. Difference‐in‐differences estimations were then completed to compare the proportion of discharges by payer, LOS index, and mortality index between Medicaid‐expansion and nonexpansion hospitals before and after ACA implementation. Post hoc linear regression analyses were completed to evaluate the effect of clustering by state level strata on payer mix and LOS and mortality indices. A 2‐sided P value of 0.05 was considered statistically significant. Data analyses were performed using Stata 12.0 (StataCorp, College Station, TX).
RESULTS
We identified 4,258,952 discharges among general medicine patients from 211 hospitals in 38 states and Washington, DC between October 1, 2012, and September 30, 2015. This included 3,144,488 discharges from 156 hospitals in 24 Medicaid‐expansion states and Washington, DC and 1,114,464 discharges from 55 hospitals in 14 nonexpansion states.
Figure 1 shows the trends in payer mix over time for hospitals in both Medicaid‐expansion and nonexpansion states. As summarized in Table 1, hospitals in Medicaid‐expansion states experienced a significant 3.7‐percentage point increase in Medicaid discharges (P = 0.013) and 2.9‐percentage point decrease in uninsured discharges (P 0.001) after ACA implementation. This represented an approximately 19% jump and 60% drop in Medicaid and uninsured discharges, respectively. Hospitals in nonexpansion states saw no significant change in the proportion of discharges by payer after ACA implementation. In the difference‐in‐differences analysis, there was a trend toward a greater change in the proportion of Medicaid discharges pre‐ to post‐ACA implementation among hospitals in Medicaid‐expansion states compared to hospitals in nonexpansion states (mean difference‐in‐differences 4.1%, 95% confidence interval [CI]: 0.3%, 8.6%, P = 0.070).
| Medicaid‐expansion n=156 hospitals; 3,144,488 cases | Non‐expansion n=55 hospitals; 1,114,464 cases | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre‐ACA Implementation (1,453,090 Cases) | Post‐ACA Implementation (1,691,398 Cases) | Mean Difference | P Value | Pre‐ACA Implementation (455,440 Cases) | Post‐ACA Implementation (659,024 Cases) | Mean Difference | P Value | Mean Difference‐in‐Differences | P Value | |
| ||||||||||
| Payer mix, % (95% CI) | ||||||||||
| Medicare | 48.6 (46.2, 51.0)* | 48.3 (45.9, 50.7) | 0.3 (3.6, 3.1) | 0.865 | 44.3 (40.7, 47.7)* | 45.3 (41.9, 48.6) | 1.0 (3.8, 5.8) | 0.671 | 1.3 (7.1, 4.5) | 0.655 |
| Commercial | 23.1 (21.4, 24.7) | 23.2 (21.8, 24.6) | 0.2 (2.0, 2.3) | 0.882 | 21.5 (18.5, 24.6) | 22.7 (19.7, 25.8) | 1.2 (3.0, 5.4) | 0.574 | 1.0 (5.7, 3.6) | 0.662 |
| Medicaid | 19.6 (17.6, 21.6) | 23.3 (21.2, 25.5) | 3.7 (0.8, 6.6) | 0.013 | 19.4 (16.9, 21.9) | 19.0 (16.5, 21.4) | 0.4 (3.8, 3.0) | 0.812 | 4.1 (0.3, 8.6) | 0.070 |
| Uninsured | 5.0 (4.0, 5.9) | 2.0 (1.7, 2.3) | 2.9 (3.9, 2.0) | 0.001 | 10.9 (8.1, 13.7) | 9.4 (7.0, 11.7) | 1.5 (5.1, 2.1) | 0.407 | 1.4 (5.1, 2.2) | 0.442 |
| Other | 3.8 (2.6, 4.9) | 3.1 (2.0, 4.3) | 0.7 (2.3, 1.0) | 0.435 | 4.0 (2.9, 5.0) | 3.7 (2.6, 4.7) | 0.3 (1.7, 1.1) | 0.662 | 0.3 (2.5, 1.8) | 0.762 |
| LOS index, mean (95% CI) | ||||||||||
| Overall | 1.017 (0.996, 1.038) | 1.006 (0.981, 1.031) | 0.011 (0.044, 0.021) | 0.488 | 1.008 (0.974, 1.042) | 0.995 (0.961, 1.029) | 0.013 (0.061, 0.034) | 0.574 | 0.002 (0.055, 0.059) | 0.943 |
| Medicare | 1.012 (0.989, 1.035) | 0.999 (0.971, 1.027) | 0.013 (0.049, 0.023) | 0.488 | 0.982 (0.946, 1.017) | 0.979 (0.944, 1.013) | 0.003 (0.052, 0.046) | 0.899 | 0.010 (0.070, 0.051) | 0.754 |
| Commercial | 0.993 (0.974, 1.012) | 0.977 (0.955, 0.998) | 0.016 (0.045, 0.013) | 0.271 | 1.009 (0.978, 1.039) | 0.986 (0.956, 1.016) | 0.022 (0.065, 0.020) | 0.298 | 0.006 (0.044, 0.057) | 0.809 |
| Medicaid | 1.059 (1.036, 1.082) | 1.043 (1.018, 1.067) | 0.016 (0.049, 0.017) | 0.349 | 1.064 (1.020, 1.108) | 1.060 (1.015, 1.106) | 0.004 (0.066, 0.059) | 0.911 | 0.012 (0.082, 0.057) | 0.727 |
| Uninsured | 0.960 (0.933, 0.988) | 0.925 (0.890, 0.961) | 0.035 (0.080, 0.010) | 0.126 | 0.972 (0.935, 1.009) | 0.944 (0.909, 0.979) | 0.028 (0.078, 0.022) | 0.273 | 0.007 (0.074, 0.060) | 0.835 |
| Other | 0.988 (0.960, 1.017) | 0.984 (0.952, 1.015) | 0.005 (0.047, 0.037) | 0.822 | 1.022 (0.973, 1.071) | 0.984 (0.944, 1.024) | 0.038 (0.100, 0.024) | 0.232 | 0.033 (0.042, 0.107) | 0.386 |
| Mortality index, mean (95% CI) | ||||||||||
| Overall | 1.000 (0.955, 1.045) | 0.878 (0.836, 0.921) | 0.122 (0.183, 0.061) | 0.001 | 0.997 (0.931, 1.062) | 0.850 (0.800, 0.900) | 0.147 (0.227, 0.066) | 0.001 | 0.025 (0.076, 0.125) | 0.628 |
| Medicare | 0.990 (0.942, 1.038) | 0.871 (0.826, 0.917) | 0.119 (0.185, 0.053) | 0.001 | 1.000 (0.925, 1.076) | 0.844 (0.788, 0.900) | 0.156 (0.249, 0.064) | 0.001 | 0.038 (0.075, 0.150) | 0.513 |
| Commercial | 1.045 (0.934, 1.155) | 0.908 (0.842, 0.975) | 0.136 (0.264, 0.008) | 0.037 | 1.023 (0.935, 1.111) | 0.820 (0.758, 0.883) | 0.203 (0.309, 0.096) | 0.001 | 0.067 (0.099, 0.232) | 0.430 |
| Medicaid | 0.894 (0.845, 0.942) | 0.786 (0.748, 0.824) | 0.107 (0.168, 0.046) | 0.001 | 0.937 (0.861, 1.013) | 0.789 (0.733, 0.844) | 0.148 (0.242, 0.055) | 0.002 | 0.041 (0.069, 0.151) | 0.464 |
| Uninsured | 1.172 (1.007, 1.337)∥ | 1.136 (0.968, 1.303) | 0.037 (0.271, 0.197) | 0.758 | 0.868 (0.768, 0.968)∥ | 0.850 (0.761, 0.939) | 0.017 (0.149, 0.115) | 0.795 | 0.019 (0.287, 0.248) | 0.887 |
| Other | 1.376 (1.052, 1.700)# | 1.156 (0.910, 1.402) | 0.220 (0.624, 0.184) | 0.285 | 1.009 (0.868, 1.150) # | 0.874 (0.682, 1.066) | 0.135 (0.369, 0.099) | 0.254 | 0.085 (0.555, 0.380) | 0.720 |

Table 1 shows that the overall LOS index remained unchanged pre‐ to post‐ACA implementation for both Medicaid‐expansion (1.017 to 1.006, P = 0.488) and nonexpansion hospitals (1.008 to 0.995, P = 0.574). LOS indices for each payer type also remained unchanged. The overall mortality index significantly improved pre‐ to post‐ACA implementation for both Medicaid‐expansion (1.000 to 0.878, P 0.001) and nonexpansion hospitals (0.997 to 0.850, P = 0.001). Among both Medicaid‐expansion and nonexpansion hospitals, the mortality index significantly improved for Medicare, commercial, and Medicaid discharges but not for uninsured or other discharges. In the difference‐in‐differences analysis, the changes in LOS indices and mortality indices pre‐ to post‐ACA implementation did not differ significantly between hospitals in Medicaid‐expansion versus nonexpansion states.
In post hoc linear regression analyses of payer mix and LOS and mortality indices clustered by state‐level strata, point estimates were minimally changed. Although 95% CIs were slightly wider, statistical significance was unchanged from our primary analyses (data not shown).
DISCUSSION
We found that ACA implementation had a significant impact on payer mix for general medicine patients at AMCs in the United States, primarily by increasing the number of Medicaid beneficiaries and by decreasing the number of uninsured patients in Medicaid‐expansion states. State Medicaid expansion status did not appear to influence either LOS or in‐hospital mortality.
Our study offers some of the longest‐term data currently available on the impact of ACA implementation on payer mix trends and encompasses more states than others have previously. Although we uniquely focused on general medicine patients at AMCs, our results are similar to those seen for US hospitals overall. Nikpay and colleagues evaluated payer mix trends for non‐Medicare adult inpatient stays in 16 states through the second quarter of 2014 using the Healthcare Cost and Utilization Project database through the Agency for Healthcare Research and Quality.[4] They found a relative 20% increase and 50% decrease in Medicaid and uninsured discharges in Medicaid‐expansion states, along with nonsignificant changes in nonexpansion states. Hempstead and Cantor assessed payer mix for non‐Medicare discharges using state hospital association data from 21 states through the fourth quarter of 2014 and found a significant increase in Medicaid patients as well as a nearly significant decrease in uninsured patients in expansion states relative to nonexpansion states.[5] The Department of Health and Human Services also reported that uninsured/self‐pay discharges fell substantially (65%73%) in Medicaid‐expansion states by the end of 2014, with slight decreases in nonexpansion states.[20]
In contrast to our hypothesis, the overall LOS and in‐hospital mortality indices were not influenced by state Medicaid expansion status. From a purely mathematical standpoint, the contribution of Medicaid patients to the overall LOS and mortality indices may have been eclipsed by Medicare and commercially insured patients, who represented a higher proportion of total discharges. The lack of impact of state Medicaid expansion status on overall LOS and mortality indices did not appear to occur as a result of indices for Medicaid patients trending toward the mean. As predicted based on observational studies, Medicaid patients in our study tended to have a higher LOS index than those with other insurance types. Medicaid patients actually tended to have a lower mortality index in our analysis; the reason for this latter finding is unclear and in contrast to other published studies.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 21]
To our knowledge, no other studies have evaluated the effect of payer mix changes under the ACA on inpatient outcomes. However, new evidence is emerging on outpatient outcomes. Low‐income adults in Medicaid‐expansion states have reported greater gains in access to primary care services and in the diagnosis of certain chronic health conditions than those in nonexpansion states as a result of ACA implementation.[22, 23] Such improvements in the outpatient setting might be expected to reduce patient acuity on admission. However, they would not necessarily translate to relative improvements in LOS or mortality indices for Medicaid‐expansion hospitals, as the UHC risk adjustment models controlled for disease severity on admission.
Similarly, few studies have assessed the impact of payer mix changes under previous state Medicaid expansions on inpatient outcomes. After Massachusetts expanded Medicaid and enacted near‐universal healthcare coverage in 2006, a minimal LOS reduction of just 0.05 days was observed.[24] New York expanded Medicaid eligibility to nondisabled childless adults with incomes below 100% of the federal poverty level in September 2001, whereas Arizona did so in November 2001 and Maine in October 2002. A study comparing outcomes in these states to 4 neighboring nonexpansion states found a relative reduction in annual all‐cause mortality of 6.1% population wide; however, it did not assess in‐hospital mortality.[25] The Oregon Health Insurance Experiment that randomized low‐income adults to expanded Medicaid coverage or not in 2008 has also reported on outpatient rather than inpatient outcomes.[26]
Our findings have potential implications for health policymakers. That Medicaid expansion status had a neutral effect on both LOS and mortality indices in our analysis should be reassuring for states contemplating Medicaid expansion in the future. Our results also highlight the need for further efforts to reduce disparities in inpatient care based on payer status. For example, although Medicare, commercially insured, and Medicaid patients witnessed significant improvements in mortality indices pre‐ to post‐ACA implementation in hospitals in both Medicaid‐expansion and nonexpansion states, uninsured patients did not.
This study has several limitations. First, our analysis of the impact of ACA implementation on payer mix did not account for concurrent socioeconomic trends that may have influenced insurance coverage across the United States. However, the main goal of this analysis was to demonstrate that changes in payer mix did in fact occur over time, to provide rationale for our subsequent LOS and mortality analyses. Second, we could not control for variation in the design and implementation of Medicaid expansions across states as permitted under the federal Section 1115 waiver process. Third, we only had access to hospital‐level data through the UHC CDB/RM, rather than individual patient data. We attempted to mitigate this limitation by weighting data according to the number of cases per hospital. Lastly, additional patient‐level factors that may influence LOS or mortality may not be included in the UHC risk adjustment models.
In summary, the differential shift in payer mix between Medicaid‐expansion and nonexpansion states did not influence overall LOS or in‐hospital mortality for general medicine patients at AMCs in the United States. Additional research could help to determine the impact of ACA implementation on other patient outcomes that may be dependent on insurance status, such as readmissions or hospital‐acquired complications.
Disclosures: M.E.A. conceived of the study concept and design, assisted with data acquisition, and drafted the manuscript. J.J.G. assisted with study design and made critical revisions to the manuscript. D.A. assisted with study design and made critical revisions to the manuscript. R.P. assisted with study design and made critical revisions to the manuscript. M.L. assisted with study design and data acquisition and made critical revisions to the manuscript. C.D.J. assisted with study design, performed data analyses, and made critical revisions to the manuscript. A modified abstract was presented in poster format at the University HealthSystem Consortium Annual Conference held September 30 to October 2, 2015 in Orlando, Florida, as well as at the Society of Hospital Medicine Research, Innovations, and Vignettes 2016 Annual Meeting held March 69, 2016, in San Diego, California. The authors report no conflicts of interest.
- Department of Health and Human Services. Key features of the Affordable Care Act by year. Available at: http://www.hhs.gov/healthcare/facts‐and‐features/key‐features‐of‐aca‐by‐year/index.html#2014. Accessed April 4, 2016.
- Centers for Medicare and Medicaid Services. Medicaid enrollment data collected through MBES. Available at: https://www.medicaid.gov/medicaid‐chip‐program‐information/program‐information/medicaid‐and‐chip‐enrollment‐data/medicaid‐enrollment‐data‐collected‐through‐mbes.html. Accessed April 4, 2016.
- The Henry J. Kaiser Family Foundation. Status of state action on the Medicaid expansion decision. Available at: http://kff.org/health‐reform/state‐indicator/state‐activity‐around‐expanding‐medicaid‐under‐the‐affordable‐care‐act. Accessed April 4, 2016.
- , , . Affordable Care Act Medicaid expansion reduced uninsured hospital stays in 2014. Health Aff (Millwood). 2016;35(1):106–110.
- , . State Medicaid expansion and changes in hospital volume according to payer. N Engl J Med. 2016;374(2):196–198.
- , , , , , . Understanding predictors of prolonged hospitalizations among general medicine patients: a guide and preliminary analysis. J Hosp Med. 2015;10(9):623–626.
- , , , . Impact of insurance and hospital ownership on hospital length of stay among patients with ambulatory care‐sensitive conditions. Ann Fam Med. 2011;9:489–495.
- , , . Insurance status and hospital care for myocardial infarction, stroke, and pneumonia. J Hosp Med. 2010;5:452–459.
- , , , , , . Payment source, quality of care, and outcomes in patients hospitalized with heart failure. J Am Coll Cardiol. 2011;58(14):1465–1471.
- , , , , . The inpatient experience and predictors of length of stay for patients hospitalized with systolic heart failure: comparison by commercial, Medicaid, and Medicare payer type. J Med Econ. 2013;16(1):43–54.
- , , et al. Insurance coverage and care of patients with non‐ST‐segment elevation acute coronary syndromes. Ann Intern Med. 2006;145:739–748.
- , , , et al. Association of insurance status with inpatient treatment for coronary artery disease: findings from the Get with the Guidelines Program. Am Heart J. 2010;159:1026–1036.
- , , , et al. Primary payer status affects mortality for major surgical operations. Ann Surg. 2010;252:544–551.
- , , . Medicaid payer status is associated with in‐hospital morbidity and resource utilization following primary total joint arthroplasty. J Bone Joint Surg Am. 2014;96(21):e180.
- , , . The quality of care delivered to patients within the same hospital varies by insurance type. Health Aff (Millwood). 2013;32(10):1731–1739.
- , , , , . Effect of insurance status on postacute care among working age stroke survivors. Neurology. 2012;78(20):1590–1595.
- , , , . Hospitalizations in which patients leave the hospital against medical advice (AMA), 2007. HCUP statistical brief #78. August 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2009. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb78.pdf. Accessed May 12, 2016.
- , , , . Characteristics of Medicaid and uninsured hospitalizations, 2012. HCUP statistical brief #182. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb182‐Medicaid‐Uninsured‐Hospitalizations‐2012.pdf. Accessed March 9, 2016.
- Agency for Healthcare Research and Quality. Mortality measurement: mortality risk adjustment methodology for University HealthSystem Consortium. Available at: http://archive.ahrq.gov/professionals/quality‐patient‐safety/quality‐resources/tools/mortality/Meurer.pdf. Accessed May 10, 2016.
- Department of Health and Human Services. Insurance expansion, hospital uncompensated care, and the Affordable Care Act. Available at: https://aspe.hhs.gov/pdf‐report/insurance‐expansion‐hospital‐uncompensated‐care‐and‐affordable‐care‐act. Accessed May 27, 2016.
- , , , . Our flawed but beneficial Medicaid program. N Engl J Med. 2011;364(16):e31.
- , , , . Changes in self‐reported insurance coverage, access to care, and health under the Affordable Care Act. JAMA. 2015;314(4):366–374.
- , . Early coverage, access, utilization, and health effects associated with the Affordable Care Act Medicaid Expansions: a quasi‐experimental study. Ann Intern Med. 2016;164(12):795–803.
- , . The impact of health care reform on hospital and preventive care: evidence from Massachusetts. J Public Econ. 2012;96(11–12):909–929.
- , , . Mortality and access to care among adults after state Medicaid expansions. N Engl J Med. 2012;367:1025–1034.
- , , , et al. The Oregon Experiment—effects of Medicaid on clinical outcomes. N Engl J Med. 2013;368(18):1713–1722.
- Department of Health and Human Services. Key features of the Affordable Care Act by year. Available at: http://www.hhs.gov/healthcare/facts‐and‐features/key‐features‐of‐aca‐by‐year/index.html#2014. Accessed April 4, 2016.
- Centers for Medicare and Medicaid Services. Medicaid enrollment data collected through MBES. Available at: https://www.medicaid.gov/medicaid‐chip‐program‐information/program‐information/medicaid‐and‐chip‐enrollment‐data/medicaid‐enrollment‐data‐collected‐through‐mbes.html. Accessed April 4, 2016.
- The Henry J. Kaiser Family Foundation. Status of state action on the Medicaid expansion decision. Available at: http://kff.org/health‐reform/state‐indicator/state‐activity‐around‐expanding‐medicaid‐under‐the‐affordable‐care‐act. Accessed April 4, 2016.
- , , . Affordable Care Act Medicaid expansion reduced uninsured hospital stays in 2014. Health Aff (Millwood). 2016;35(1):106–110.
- , . State Medicaid expansion and changes in hospital volume according to payer. N Engl J Med. 2016;374(2):196–198.
- , , , , , . Understanding predictors of prolonged hospitalizations among general medicine patients: a guide and preliminary analysis. J Hosp Med. 2015;10(9):623–626.
- , , , . Impact of insurance and hospital ownership on hospital length of stay among patients with ambulatory care‐sensitive conditions. Ann Fam Med. 2011;9:489–495.
- , , . Insurance status and hospital care for myocardial infarction, stroke, and pneumonia. J Hosp Med. 2010;5:452–459.
- , , , , , . Payment source, quality of care, and outcomes in patients hospitalized with heart failure. J Am Coll Cardiol. 2011;58(14):1465–1471.
- , , , , . The inpatient experience and predictors of length of stay for patients hospitalized with systolic heart failure: comparison by commercial, Medicaid, and Medicare payer type. J Med Econ. 2013;16(1):43–54.
- , , et al. Insurance coverage and care of patients with non‐ST‐segment elevation acute coronary syndromes. Ann Intern Med. 2006;145:739–748.
- , , , et al. Association of insurance status with inpatient treatment for coronary artery disease: findings from the Get with the Guidelines Program. Am Heart J. 2010;159:1026–1036.
- , , , et al. Primary payer status affects mortality for major surgical operations. Ann Surg. 2010;252:544–551.
- , , . Medicaid payer status is associated with in‐hospital morbidity and resource utilization following primary total joint arthroplasty. J Bone Joint Surg Am. 2014;96(21):e180.
- , , . The quality of care delivered to patients within the same hospital varies by insurance type. Health Aff (Millwood). 2013;32(10):1731–1739.
- , , , , . Effect of insurance status on postacute care among working age stroke survivors. Neurology. 2012;78(20):1590–1595.
- , , , . Hospitalizations in which patients leave the hospital against medical advice (AMA), 2007. HCUP statistical brief #78. August 2009. Rockville, MD: Agency for Healthcare Research and Quality; 2009. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb78.pdf. Accessed May 12, 2016.
- , , , . Characteristics of Medicaid and uninsured hospitalizations, 2012. HCUP statistical brief #182. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb182‐Medicaid‐Uninsured‐Hospitalizations‐2012.pdf. Accessed March 9, 2016.
- Agency for Healthcare Research and Quality. Mortality measurement: mortality risk adjustment methodology for University HealthSystem Consortium. Available at: http://archive.ahrq.gov/professionals/quality‐patient‐safety/quality‐resources/tools/mortality/Meurer.pdf. Accessed May 10, 2016.
- Department of Health and Human Services. Insurance expansion, hospital uncompensated care, and the Affordable Care Act. Available at: https://aspe.hhs.gov/pdf‐report/insurance‐expansion‐hospital‐uncompensated‐care‐and‐affordable‐care‐act. Accessed May 27, 2016.
- , , , . Our flawed but beneficial Medicaid program. N Engl J Med. 2011;364(16):e31.
- , , , . Changes in self‐reported insurance coverage, access to care, and health under the Affordable Care Act. JAMA. 2015;314(4):366–374.
- , . Early coverage, access, utilization, and health effects associated with the Affordable Care Act Medicaid Expansions: a quasi‐experimental study. Ann Intern Med. 2016;164(12):795–803.
- , . The impact of health care reform on hospital and preventive care: evidence from Massachusetts. J Public Econ. 2012;96(11–12):909–929.
- , , . Mortality and access to care among adults after state Medicaid expansions. N Engl J Med. 2012;367:1025–1034.
- , , , et al. The Oregon Experiment—effects of Medicaid on clinical outcomes. N Engl J Med. 2013;368(18):1713–1722.
Myth of the Month: Vaccinations in patients with Guillain-Barré syndrome
A 66-year-old woman presents as a new patient for a clinic visit. She has a history of Guillain-Barré syndrome 10 years ago. The last immunization she received was a tetanus-diphtheria 12 years ago.
What do you recommend for her to receive over the next year?
A. Pneumococcal 13/Pneumococcal 23/Tdap/influenza vaccines.
B. Pneumococcal 13/Pneumococcal 23/Tdap vaccines.
C. Influenza vaccine.
D. No vaccines.
Guillain-Barré syndrome (GBS) is a rare, acute, immune-mediated polyneuropathy that has an incidence of about 2 cases per 100,000 people each year.1 Most cases of GBS follow an infectious event (usually an upper respiratory infection or gastrointestinal infection). In 1976, administration of the swine flu vaccine was associated with an up to eightfold increased risk of GBS.2,3 Many patients who have had GBS have been advised not to – or are fearful to – receive influenza vaccine or any vaccine.
Is there good evidence for patients with a history of GBS to avoid influenza vaccines or vaccinations in general?
The initial concern over the increased risk of GBS following the large-scale influenza vaccination in 1976 has not been realized with subsequent influenza vaccines. In a study by Baxter and colleagues, GBS cases from Kaiser Permanente Northern California from 1995 to 2006 were reviewed.4 They looked at whether patients had received influenza vaccine in the 6 weeks prior to GBS, compared with vaccination within the prior 9 months.
The odds ratio for influenza vaccination in the 6 weeks prior to GBS was 1.1 (95% confidence interval, 0.4-3.1). The odds ratio for receiving tetanus diphtheria vaccine in the 6 weeks prior to GBS was 1.4 (95% CI, 0.3-4.5); pneumococcal 23 vaccine, 0.7 (95% CI, 0.1-2.9); and all vaccines combined, 1.3 (95% CI, 0.8-2.3).
Shahed Iqbal, MBBS, et al. looked at the relationship between influenza illness, pneumonia, influenza vaccination, and GBS.5 They found that although influenza vaccine coverage increased from 20% to 36% over the study period, there was not an increase in GBS hospitalizations over the same period. There was a significant correlation between hospitalizations for pneumonia and influenza and GBS hospitalizations in the same month.
In a simulation study, Steven Hawken, PhD, and his colleagues concluded that under typical conditions (influenza incidence greater than 5% and vaccine effectiveness greater than 60%), influenza vaccination reduced GBS risk.6
There are fewer data on vaccination in patients who have previously had GBS, but there is enough evidence to help guide us.
Roger Baxter, MD, and colleagues, using the database in reference 4, looked at outcome of patients with GBS who received vaccinations subsequent to recovery from GBS.7 A total of 279 patient with previous GBS received a total of 989 vaccinations, including 405 trivalent influenza vaccinations. None of the patients with GBS who received vaccinations had a recurrence of GBS.
Krista Kuitwaard, MD, et al. reported identical findings in a survey of patients with a history of GBS or chronic inflammatory demyelinating polyradiculoneuropathy (CIDP).8 A total of 245 patients with GBS responded to the survey. A total of 106 GBS patients had received influenza vaccine following their GBS diagnosis (a total of 775 vaccinations in those patients). None of the patients with a history of GBS who received influenza vaccination had a recurrence of their GBS.
The current position of the GBS/CIDP Foundation on vaccination for patients with GBS is as follows: The GBS/CIDP Foundation recommends avoiding immunizations that a GBS patient had received within 6 weeks of developing their initial symptoms.9
I think the current evidence is enough to guide us in this issue. Vaccinations, including influenza vaccine, are likely safe for patients with a history of GBS. The recommendation of the GBS/CIDP foundation is reasonable – to avoid immunizations that appeared to have potentially triggered the initial GBS (ones that had been received within 6 weeks of onset of symptoms).
In the case presented above, I think that choice A – receiving all the recommended immunizations – would be appropriate.
References
1. Neuroepidemiology 2011; 36(2):123-33.
2. Am J Epidemiol. 1979 Aug;110(2):105-23.
3. Clin Infect Dis. 2014 Apr;58(8):1149-55.
4. Clin Infect Dis. 2013 Jul;57(2):197-204.
5. Vaccine. 2015 Apr 21;33(17):2045-9.
6. Emerg Infect Dis. 2015 Feb;21(2):224-31.
7. Clin Infect Dis. 2012 Mar;54(6):800-4.
8. J Peripher Nerv Syst. 2009 Dec;14(4):310-5.
9. GBS/CIDP Foundation International, Position on Flu Shots and Vaccinations.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A 66-year-old woman presents as a new patient for a clinic visit. She has a history of Guillain-Barré syndrome 10 years ago. The last immunization she received was a tetanus-diphtheria 12 years ago.
What do you recommend for her to receive over the next year?
A. Pneumococcal 13/Pneumococcal 23/Tdap/influenza vaccines.
B. Pneumococcal 13/Pneumococcal 23/Tdap vaccines.
C. Influenza vaccine.
D. No vaccines.
Guillain-Barré syndrome (GBS) is a rare, acute, immune-mediated polyneuropathy that has an incidence of about 2 cases per 100,000 people each year.1 Most cases of GBS follow an infectious event (usually an upper respiratory infection or gastrointestinal infection). In 1976, administration of the swine flu vaccine was associated with an up to eightfold increased risk of GBS.2,3 Many patients who have had GBS have been advised not to – or are fearful to – receive influenza vaccine or any vaccine.
Is there good evidence for patients with a history of GBS to avoid influenza vaccines or vaccinations in general?
The initial concern over the increased risk of GBS following the large-scale influenza vaccination in 1976 has not been realized with subsequent influenza vaccines. In a study by Baxter and colleagues, GBS cases from Kaiser Permanente Northern California from 1995 to 2006 were reviewed.4 They looked at whether patients had received influenza vaccine in the 6 weeks prior to GBS, compared with vaccination within the prior 9 months.
The odds ratio for influenza vaccination in the 6 weeks prior to GBS was 1.1 (95% confidence interval, 0.4-3.1). The odds ratio for receiving tetanus diphtheria vaccine in the 6 weeks prior to GBS was 1.4 (95% CI, 0.3-4.5); pneumococcal 23 vaccine, 0.7 (95% CI, 0.1-2.9); and all vaccines combined, 1.3 (95% CI, 0.8-2.3).
Shahed Iqbal, MBBS, et al. looked at the relationship between influenza illness, pneumonia, influenza vaccination, and GBS.5 They found that although influenza vaccine coverage increased from 20% to 36% over the study period, there was not an increase in GBS hospitalizations over the same period. There was a significant correlation between hospitalizations for pneumonia and influenza and GBS hospitalizations in the same month.
In a simulation study, Steven Hawken, PhD, and his colleagues concluded that under typical conditions (influenza incidence greater than 5% and vaccine effectiveness greater than 60%), influenza vaccination reduced GBS risk.6
There are fewer data on vaccination in patients who have previously had GBS, but there is enough evidence to help guide us.
Roger Baxter, MD, and colleagues, using the database in reference 4, looked at outcome of patients with GBS who received vaccinations subsequent to recovery from GBS.7 A total of 279 patient with previous GBS received a total of 989 vaccinations, including 405 trivalent influenza vaccinations. None of the patients with GBS who received vaccinations had a recurrence of GBS.
Krista Kuitwaard, MD, et al. reported identical findings in a survey of patients with a history of GBS or chronic inflammatory demyelinating polyradiculoneuropathy (CIDP).8 A total of 245 patients with GBS responded to the survey. A total of 106 GBS patients had received influenza vaccine following their GBS diagnosis (a total of 775 vaccinations in those patients). None of the patients with a history of GBS who received influenza vaccination had a recurrence of their GBS.
The current position of the GBS/CIDP Foundation on vaccination for patients with GBS is as follows: The GBS/CIDP Foundation recommends avoiding immunizations that a GBS patient had received within 6 weeks of developing their initial symptoms.9
I think the current evidence is enough to guide us in this issue. Vaccinations, including influenza vaccine, are likely safe for patients with a history of GBS. The recommendation of the GBS/CIDP foundation is reasonable – to avoid immunizations that appeared to have potentially triggered the initial GBS (ones that had been received within 6 weeks of onset of symptoms).
In the case presented above, I think that choice A – receiving all the recommended immunizations – would be appropriate.
References
1. Neuroepidemiology 2011; 36(2):123-33.
2. Am J Epidemiol. 1979 Aug;110(2):105-23.
3. Clin Infect Dis. 2014 Apr;58(8):1149-55.
4. Clin Infect Dis. 2013 Jul;57(2):197-204.
5. Vaccine. 2015 Apr 21;33(17):2045-9.
6. Emerg Infect Dis. 2015 Feb;21(2):224-31.
7. Clin Infect Dis. 2012 Mar;54(6):800-4.
8. J Peripher Nerv Syst. 2009 Dec;14(4):310-5.
9. GBS/CIDP Foundation International, Position on Flu Shots and Vaccinations.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A 66-year-old woman presents as a new patient for a clinic visit. She has a history of Guillain-Barré syndrome 10 years ago. The last immunization she received was a tetanus-diphtheria 12 years ago.
What do you recommend for her to receive over the next year?
A. Pneumococcal 13/Pneumococcal 23/Tdap/influenza vaccines.
B. Pneumococcal 13/Pneumococcal 23/Tdap vaccines.
C. Influenza vaccine.
D. No vaccines.
Guillain-Barré syndrome (GBS) is a rare, acute, immune-mediated polyneuropathy that has an incidence of about 2 cases per 100,000 people each year.1 Most cases of GBS follow an infectious event (usually an upper respiratory infection or gastrointestinal infection). In 1976, administration of the swine flu vaccine was associated with an up to eightfold increased risk of GBS.2,3 Many patients who have had GBS have been advised not to – or are fearful to – receive influenza vaccine or any vaccine.
Is there good evidence for patients with a history of GBS to avoid influenza vaccines or vaccinations in general?
The initial concern over the increased risk of GBS following the large-scale influenza vaccination in 1976 has not been realized with subsequent influenza vaccines. In a study by Baxter and colleagues, GBS cases from Kaiser Permanente Northern California from 1995 to 2006 were reviewed.4 They looked at whether patients had received influenza vaccine in the 6 weeks prior to GBS, compared with vaccination within the prior 9 months.
The odds ratio for influenza vaccination in the 6 weeks prior to GBS was 1.1 (95% confidence interval, 0.4-3.1). The odds ratio for receiving tetanus diphtheria vaccine in the 6 weeks prior to GBS was 1.4 (95% CI, 0.3-4.5); pneumococcal 23 vaccine, 0.7 (95% CI, 0.1-2.9); and all vaccines combined, 1.3 (95% CI, 0.8-2.3).
Shahed Iqbal, MBBS, et al. looked at the relationship between influenza illness, pneumonia, influenza vaccination, and GBS.5 They found that although influenza vaccine coverage increased from 20% to 36% over the study period, there was not an increase in GBS hospitalizations over the same period. There was a significant correlation between hospitalizations for pneumonia and influenza and GBS hospitalizations in the same month.
In a simulation study, Steven Hawken, PhD, and his colleagues concluded that under typical conditions (influenza incidence greater than 5% and vaccine effectiveness greater than 60%), influenza vaccination reduced GBS risk.6
There are fewer data on vaccination in patients who have previously had GBS, but there is enough evidence to help guide us.
Roger Baxter, MD, and colleagues, using the database in reference 4, looked at outcome of patients with GBS who received vaccinations subsequent to recovery from GBS.7 A total of 279 patient with previous GBS received a total of 989 vaccinations, including 405 trivalent influenza vaccinations. None of the patients with GBS who received vaccinations had a recurrence of GBS.
Krista Kuitwaard, MD, et al. reported identical findings in a survey of patients with a history of GBS or chronic inflammatory demyelinating polyradiculoneuropathy (CIDP).8 A total of 245 patients with GBS responded to the survey. A total of 106 GBS patients had received influenza vaccine following their GBS diagnosis (a total of 775 vaccinations in those patients). None of the patients with a history of GBS who received influenza vaccination had a recurrence of their GBS.
The current position of the GBS/CIDP Foundation on vaccination for patients with GBS is as follows: The GBS/CIDP Foundation recommends avoiding immunizations that a GBS patient had received within 6 weeks of developing their initial symptoms.9
I think the current evidence is enough to guide us in this issue. Vaccinations, including influenza vaccine, are likely safe for patients with a history of GBS. The recommendation of the GBS/CIDP foundation is reasonable – to avoid immunizations that appeared to have potentially triggered the initial GBS (ones that had been received within 6 weeks of onset of symptoms).
In the case presented above, I think that choice A – receiving all the recommended immunizations – would be appropriate.
References
1. Neuroepidemiology 2011; 36(2):123-33.
2. Am J Epidemiol. 1979 Aug;110(2):105-23.
3. Clin Infect Dis. 2014 Apr;58(8):1149-55.
4. Clin Infect Dis. 2013 Jul;57(2):197-204.
5. Vaccine. 2015 Apr 21;33(17):2045-9.
6. Emerg Infect Dis. 2015 Feb;21(2):224-31.
7. Clin Infect Dis. 2012 Mar;54(6):800-4.
8. J Peripher Nerv Syst. 2009 Dec;14(4):310-5.
9. GBS/CIDP Foundation International, Position on Flu Shots and Vaccinations.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
Acetaminophen doesn’t exacerbate asthma in young children
As-needed use of acetaminophen for fever or pain does not exacerbate mild persistent asthma in young children, according to a report published online August 18 in the New England Journal of Medicine.
In a prospective, randomized, double-blind clinical trial performed at 18 U.S. medical centers, neither acetaminophen nor ibuprofen raised the rate of exacerbations or impaired asthma control among 300 children aged 1-5 years. This result refutes those of observational and post hoc data that linked acetaminophen to increased asthma exacerbations, daily symptoms, and need for bronchodilators in children and adults. Those findings “have led to much controversy and even alarm,” with some physicians recommending that acetaminophen be completely avoided in children with asthma until more safety data became available, said William J. Sheehan, MD, of the division of allergy and immunology, Boston Children’s Hospital and Harvard Medical School, Boston, and his associates.
The investigators performed this 2-year study to obtain such safety data. The children (median age, 40 months) were randomly assigned to receive either liquid acetaminophen (150 patients) or matching liquid ibuprofen (150 patients) as needed for pain, fever, or discomfort and were followed for 46 weeks. All the participants received standard asthma-control therapies including inhaled glucocorticoids, oral leukotriene-receptor antagonists, and as-needed inhaled glucocorticoids.
The primary outcome – the mean number of asthma exacerbations – was 0.81 in the acetaminophen group and 0.87 in the ibuprofen group, a nonsignificant difference. The rate of exacerbations also did not differ between acetaminophen and ibuprofen in the subgroup of 226 children who completed the entire trial or in the subgroup of 200 who received a study medication for pain or fever at least once during follow-up, Dr. Sheehan and his associates said (N Engl J Med. 2016 Aug 18. doi: 10.1056/NEJMoa1515990).
There also were no significant differences between the two study groups in time to first asthma exacerbation, percentage of days of good asthma control (85.8% vs. 86.8% of days), use of rescue albuterol (2.8 vs. 3.0 inhalations per week), or unscheduled health care visits for asthma (0.75 vs. 0.76 visits). No between-group differences occurred regarding adverse events or serious adverse events.
Some experts have suggested that the observational studies reporting a link between acetaminophen and asthma exacerbations may have been flawed by “confounding by indication,” because children with asthma have more symptomatic respiratory infections than do those without asthma and use more acetaminophen for fever and malaise. “We [also] observed that greater use of antipyretic, analgesic medications was associated with more apparent respiratory illnesses and that the reported respiratory illnesses were associated with asthma exacerbations.
“However, we found no evidence that acetaminophen, when used during periods of respiratory illness, was associated with a higher risk of asthma exacerbations or other asthma-related complications than was ibuprofen,” Dr. Sheehan and his associates wrote.
This study was funded by the National Institutes of Health and the National Heart, Lung, and Blood Institute. Dr. Sheehan reported having no relevant financial disclosures; his associates reported numerous ties to industry sources.
The findings of Sheehan et al. should reassure clinicians and parents who care for young children taking asthma-controlling medications that the use of acetaminophen in usual, as-needed doses will not worsen the condition.
Acetaminophen and ibuprofen can be used similarly in situations for which they are indicated.
Augusto A. Litonjua, MD, is in the Channing Division of Network Medicine, Brigham and Women’s Hospital, and at Harvard Medical School, both in Boston. Dr. Litonjua made these remarks in an editorial accompanying Dr. Sheehan’s report (N Engl J Med. 2016 Aug 18. doi: 10.1056/NEJMe1607629). He reported receiving personal fees from UpToDate, Springer Humana Press, and AstraZeneca outside this editorial.
The findings of Sheehan et al. should reassure clinicians and parents who care for young children taking asthma-controlling medications that the use of acetaminophen in usual, as-needed doses will not worsen the condition.
Acetaminophen and ibuprofen can be used similarly in situations for which they are indicated.
Augusto A. Litonjua, MD, is in the Channing Division of Network Medicine, Brigham and Women’s Hospital, and at Harvard Medical School, both in Boston. Dr. Litonjua made these remarks in an editorial accompanying Dr. Sheehan’s report (N Engl J Med. 2016 Aug 18. doi: 10.1056/NEJMe1607629). He reported receiving personal fees from UpToDate, Springer Humana Press, and AstraZeneca outside this editorial.
The findings of Sheehan et al. should reassure clinicians and parents who care for young children taking asthma-controlling medications that the use of acetaminophen in usual, as-needed doses will not worsen the condition.
Acetaminophen and ibuprofen can be used similarly in situations for which they are indicated.
Augusto A. Litonjua, MD, is in the Channing Division of Network Medicine, Brigham and Women’s Hospital, and at Harvard Medical School, both in Boston. Dr. Litonjua made these remarks in an editorial accompanying Dr. Sheehan’s report (N Engl J Med. 2016 Aug 18. doi: 10.1056/NEJMe1607629). He reported receiving personal fees from UpToDate, Springer Humana Press, and AstraZeneca outside this editorial.
As-needed use of acetaminophen for fever or pain does not exacerbate mild persistent asthma in young children, according to a report published online August 18 in the New England Journal of Medicine.
In a prospective, randomized, double-blind clinical trial performed at 18 U.S. medical centers, neither acetaminophen nor ibuprofen raised the rate of exacerbations or impaired asthma control among 300 children aged 1-5 years. This result refutes those of observational and post hoc data that linked acetaminophen to increased asthma exacerbations, daily symptoms, and need for bronchodilators in children and adults. Those findings “have led to much controversy and even alarm,” with some physicians recommending that acetaminophen be completely avoided in children with asthma until more safety data became available, said William J. Sheehan, MD, of the division of allergy and immunology, Boston Children’s Hospital and Harvard Medical School, Boston, and his associates.
The investigators performed this 2-year study to obtain such safety data. The children (median age, 40 months) were randomly assigned to receive either liquid acetaminophen (150 patients) or matching liquid ibuprofen (150 patients) as needed for pain, fever, or discomfort and were followed for 46 weeks. All the participants received standard asthma-control therapies including inhaled glucocorticoids, oral leukotriene-receptor antagonists, and as-needed inhaled glucocorticoids.
The primary outcome – the mean number of asthma exacerbations – was 0.81 in the acetaminophen group and 0.87 in the ibuprofen group, a nonsignificant difference. The rate of exacerbations also did not differ between acetaminophen and ibuprofen in the subgroup of 226 children who completed the entire trial or in the subgroup of 200 who received a study medication for pain or fever at least once during follow-up, Dr. Sheehan and his associates said (N Engl J Med. 2016 Aug 18. doi: 10.1056/NEJMoa1515990).
There also were no significant differences between the two study groups in time to first asthma exacerbation, percentage of days of good asthma control (85.8% vs. 86.8% of days), use of rescue albuterol (2.8 vs. 3.0 inhalations per week), or unscheduled health care visits for asthma (0.75 vs. 0.76 visits). No between-group differences occurred regarding adverse events or serious adverse events.
Some experts have suggested that the observational studies reporting a link between acetaminophen and asthma exacerbations may have been flawed by “confounding by indication,” because children with asthma have more symptomatic respiratory infections than do those without asthma and use more acetaminophen for fever and malaise. “We [also] observed that greater use of antipyretic, analgesic medications was associated with more apparent respiratory illnesses and that the reported respiratory illnesses were associated with asthma exacerbations.
“However, we found no evidence that acetaminophen, when used during periods of respiratory illness, was associated with a higher risk of asthma exacerbations or other asthma-related complications than was ibuprofen,” Dr. Sheehan and his associates wrote.
This study was funded by the National Institutes of Health and the National Heart, Lung, and Blood Institute. Dr. Sheehan reported having no relevant financial disclosures; his associates reported numerous ties to industry sources.
As-needed use of acetaminophen for fever or pain does not exacerbate mild persistent asthma in young children, according to a report published online August 18 in the New England Journal of Medicine.
In a prospective, randomized, double-blind clinical trial performed at 18 U.S. medical centers, neither acetaminophen nor ibuprofen raised the rate of exacerbations or impaired asthma control among 300 children aged 1-5 years. This result refutes those of observational and post hoc data that linked acetaminophen to increased asthma exacerbations, daily symptoms, and need for bronchodilators in children and adults. Those findings “have led to much controversy and even alarm,” with some physicians recommending that acetaminophen be completely avoided in children with asthma until more safety data became available, said William J. Sheehan, MD, of the division of allergy and immunology, Boston Children’s Hospital and Harvard Medical School, Boston, and his associates.
The investigators performed this 2-year study to obtain such safety data. The children (median age, 40 months) were randomly assigned to receive either liquid acetaminophen (150 patients) or matching liquid ibuprofen (150 patients) as needed for pain, fever, or discomfort and were followed for 46 weeks. All the participants received standard asthma-control therapies including inhaled glucocorticoids, oral leukotriene-receptor antagonists, and as-needed inhaled glucocorticoids.
The primary outcome – the mean number of asthma exacerbations – was 0.81 in the acetaminophen group and 0.87 in the ibuprofen group, a nonsignificant difference. The rate of exacerbations also did not differ between acetaminophen and ibuprofen in the subgroup of 226 children who completed the entire trial or in the subgroup of 200 who received a study medication for pain or fever at least once during follow-up, Dr. Sheehan and his associates said (N Engl J Med. 2016 Aug 18. doi: 10.1056/NEJMoa1515990).
There also were no significant differences between the two study groups in time to first asthma exacerbation, percentage of days of good asthma control (85.8% vs. 86.8% of days), use of rescue albuterol (2.8 vs. 3.0 inhalations per week), or unscheduled health care visits for asthma (0.75 vs. 0.76 visits). No between-group differences occurred regarding adverse events or serious adverse events.
Some experts have suggested that the observational studies reporting a link between acetaminophen and asthma exacerbations may have been flawed by “confounding by indication,” because children with asthma have more symptomatic respiratory infections than do those without asthma and use more acetaminophen for fever and malaise. “We [also] observed that greater use of antipyretic, analgesic medications was associated with more apparent respiratory illnesses and that the reported respiratory illnesses were associated with asthma exacerbations.
“However, we found no evidence that acetaminophen, when used during periods of respiratory illness, was associated with a higher risk of asthma exacerbations or other asthma-related complications than was ibuprofen,” Dr. Sheehan and his associates wrote.
This study was funded by the National Institutes of Health and the National Heart, Lung, and Blood Institute. Dr. Sheehan reported having no relevant financial disclosures; his associates reported numerous ties to industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Using acetaminophen for fever or pain doesn’t exacerbate mild persistent asthma in young children.
Major finding: The mean number of asthma exacerbations was 0.81 in the acetaminophen group and 0.87 in the ibuprofen group, a nonsignificant difference.
Data source: A 2-year multicenter, double-blind, randomized clinical trial involving 300 children aged 1-5 years.
Disclosures: This study was funded by the National Institutes of Health and the National Heart, Lung, and Blood Institute. Dr. Sheehan reported having no relevant financial disclosures; his associates reported numerous ties to industry sources.
Primary biliary cholangitis: Obeticholic acid reduces biomarkers
Obeticholic acid reduced alkaline phosphatase level, total bilirubin level, and other biomarkers of cholestasis, hepatocellular injury, inflammation, and apoptosis in patients with primary biliary cholangitis participating in a phase III clinical trial, which was reported online August 17 in the New England Journal of Medicine.
A study to assess the drug’s effects on clinical outcomes is currently enrolling patients (NCT02308111), said Frederik Nevens, MD, PhD, of University Hospitals Leuven (Belgium) and his associates.
They assessed treatment with obeticholic acid, a selective farnesoid X receptor agonist, in a manufacturer-sponsored double-blind randomized trial involving 217 patients treated at 59 sites in 13 countries. Participants were assigned to receive a once-daily oral placebo (73 patients), obeticholic acid at an initial dose of 5 mg with escalation to 10 mg if possible (71 patients), or a daily 10-mg dose of obeticholic acid (73 patients). All were also given standard ursodiol (13-15 mg per kg) at the same time, with the expectation that many would have to discontinue that drug due to adverse events. The study participants were treated for 1 year, when the double-blind phase of the study was completed and all were offered entry into a 5-year open-label phase.
The primary composite endpoint was 1) an alkaline phosphatase level of less than 1.67 times the upper limit of the normal range, with a reduction of at least 15% below baseline level, and 2) a total bilirubin level at or below the upper limit of the normal range. After 1 year of treatment, 46% of the 5-mg group and 47% of the 10-mg group achieved this endpoint, compared with only 10% of the placebo group. The response to treatment was rapid, with patients in the active-treatment groups showing significantly lower alkaline phosphatase and bilirubin levels than the placebo group within 2 weeks of starting therapy.
In addition, the percentage of patients who showed at least a 15% reduction in alkaline phosphatase level was significantly higher with 5-10 mg active treatment (77%) and with 10 mg active treatment (77%) than with placebo (29%). And total bilirubin level progressively decreased in both active treatment groups but progressively increased in the placebo group, Dr. Nevens and his associates said (N Engl J Med. 2016 Aug 18. doi: 10.1056/NEJMoa1509840). Obeticholic acid also reduced levels of IgM and interleukin-12, as well as levels of gamma-glutamyl transferase, alanine aminotransferase, aspartate aminotransferase, and conjugated bilirubin. It did not induce significant symptom relief as measured by a disease-specific quality of life questionnaire, and it didn’t reduce liver fibrosis as measured by noninvasive techniques.
In the open-label extension phase of the study, treatment efficacy was similar.
The most common adverse event was pruritus, which is also characteristic of the disease itself. The incidence was higher in both active-treatment groups (56% with 5-10-mg therapy and 68% with 10-mg therapy) than in the placebo group (38%). One patient (1%) in the 5-10-mg group and seven patients (10%) in the 10-mg group discontinued treatment because of pruritus, compared with no patients in the placebo group.
The treatment’s beneficial effects persisted for 2 years, but that is a relatively short follow-up for a chronic disease that will require lifelong therapy. Future research must address longer-term benefits as well as adverse events, the investigators said.
The results of this phase III clinical trial are encouraging, but we still do not know whether or how obeticholic acid impacts clinical endpoints such as hepatic decompensation, progression to cirrhosis, symptoms, or survival.
Also unknown is whether patients with this chronic disease will be able to take obeticholic acid indefinitely. And we don’t know whether to continue treating those who don’t show a biochemical response to the drug within 1 year, who comprise approximately 50% of patients, according to the results of this trial.
The price of obeticholic acid is also a concern. At present it costs approximately $70,000 per year.
Daniel S. Pratt, MD, is at the Autoimmune and Cholestatic Liver Center at Massachusetts General Hospital and at Harvard Medical School, both in Boston. He reported having no relevant financial disclosures. Dr. Pratt made these remarks in an editorial accompanying Dr. Nevens’ report (N Engl J Med. 2016 Aug 18. doi: 10.1056/NEJMe1607744).
The results of this phase III clinical trial are encouraging, but we still do not know whether or how obeticholic acid impacts clinical endpoints such as hepatic decompensation, progression to cirrhosis, symptoms, or survival.
Also unknown is whether patients with this chronic disease will be able to take obeticholic acid indefinitely. And we don’t know whether to continue treating those who don’t show a biochemical response to the drug within 1 year, who comprise approximately 50% of patients, according to the results of this trial.
The price of obeticholic acid is also a concern. At present it costs approximately $70,000 per year.
Daniel S. Pratt, MD, is at the Autoimmune and Cholestatic Liver Center at Massachusetts General Hospital and at Harvard Medical School, both in Boston. He reported having no relevant financial disclosures. Dr. Pratt made these remarks in an editorial accompanying Dr. Nevens’ report (N Engl J Med. 2016 Aug 18. doi: 10.1056/NEJMe1607744).
The results of this phase III clinical trial are encouraging, but we still do not know whether or how obeticholic acid impacts clinical endpoints such as hepatic decompensation, progression to cirrhosis, symptoms, or survival.
Also unknown is whether patients with this chronic disease will be able to take obeticholic acid indefinitely. And we don’t know whether to continue treating those who don’t show a biochemical response to the drug within 1 year, who comprise approximately 50% of patients, according to the results of this trial.
The price of obeticholic acid is also a concern. At present it costs approximately $70,000 per year.
Daniel S. Pratt, MD, is at the Autoimmune and Cholestatic Liver Center at Massachusetts General Hospital and at Harvard Medical School, both in Boston. He reported having no relevant financial disclosures. Dr. Pratt made these remarks in an editorial accompanying Dr. Nevens’ report (N Engl J Med. 2016 Aug 18. doi: 10.1056/NEJMe1607744).
Obeticholic acid reduced alkaline phosphatase level, total bilirubin level, and other biomarkers of cholestasis, hepatocellular injury, inflammation, and apoptosis in patients with primary biliary cholangitis participating in a phase III clinical trial, which was reported online August 17 in the New England Journal of Medicine.
A study to assess the drug’s effects on clinical outcomes is currently enrolling patients (NCT02308111), said Frederik Nevens, MD, PhD, of University Hospitals Leuven (Belgium) and his associates.
They assessed treatment with obeticholic acid, a selective farnesoid X receptor agonist, in a manufacturer-sponsored double-blind randomized trial involving 217 patients treated at 59 sites in 13 countries. Participants were assigned to receive a once-daily oral placebo (73 patients), obeticholic acid at an initial dose of 5 mg with escalation to 10 mg if possible (71 patients), or a daily 10-mg dose of obeticholic acid (73 patients). All were also given standard ursodiol (13-15 mg per kg) at the same time, with the expectation that many would have to discontinue that drug due to adverse events. The study participants were treated for 1 year, when the double-blind phase of the study was completed and all were offered entry into a 5-year open-label phase.
The primary composite endpoint was 1) an alkaline phosphatase level of less than 1.67 times the upper limit of the normal range, with a reduction of at least 15% below baseline level, and 2) a total bilirubin level at or below the upper limit of the normal range. After 1 year of treatment, 46% of the 5-mg group and 47% of the 10-mg group achieved this endpoint, compared with only 10% of the placebo group. The response to treatment was rapid, with patients in the active-treatment groups showing significantly lower alkaline phosphatase and bilirubin levels than the placebo group within 2 weeks of starting therapy.
In addition, the percentage of patients who showed at least a 15% reduction in alkaline phosphatase level was significantly higher with 5-10 mg active treatment (77%) and with 10 mg active treatment (77%) than with placebo (29%). And total bilirubin level progressively decreased in both active treatment groups but progressively increased in the placebo group, Dr. Nevens and his associates said (N Engl J Med. 2016 Aug 18. doi: 10.1056/NEJMoa1509840). Obeticholic acid also reduced levels of IgM and interleukin-12, as well as levels of gamma-glutamyl transferase, alanine aminotransferase, aspartate aminotransferase, and conjugated bilirubin. It did not induce significant symptom relief as measured by a disease-specific quality of life questionnaire, and it didn’t reduce liver fibrosis as measured by noninvasive techniques.
In the open-label extension phase of the study, treatment efficacy was similar.
The most common adverse event was pruritus, which is also characteristic of the disease itself. The incidence was higher in both active-treatment groups (56% with 5-10-mg therapy and 68% with 10-mg therapy) than in the placebo group (38%). One patient (1%) in the 5-10-mg group and seven patients (10%) in the 10-mg group discontinued treatment because of pruritus, compared with no patients in the placebo group.
The treatment’s beneficial effects persisted for 2 years, but that is a relatively short follow-up for a chronic disease that will require lifelong therapy. Future research must address longer-term benefits as well as adverse events, the investigators said.
Obeticholic acid reduced alkaline phosphatase level, total bilirubin level, and other biomarkers of cholestasis, hepatocellular injury, inflammation, and apoptosis in patients with primary biliary cholangitis participating in a phase III clinical trial, which was reported online August 17 in the New England Journal of Medicine.
A study to assess the drug’s effects on clinical outcomes is currently enrolling patients (NCT02308111), said Frederik Nevens, MD, PhD, of University Hospitals Leuven (Belgium) and his associates.
They assessed treatment with obeticholic acid, a selective farnesoid X receptor agonist, in a manufacturer-sponsored double-blind randomized trial involving 217 patients treated at 59 sites in 13 countries. Participants were assigned to receive a once-daily oral placebo (73 patients), obeticholic acid at an initial dose of 5 mg with escalation to 10 mg if possible (71 patients), or a daily 10-mg dose of obeticholic acid (73 patients). All were also given standard ursodiol (13-15 mg per kg) at the same time, with the expectation that many would have to discontinue that drug due to adverse events. The study participants were treated for 1 year, when the double-blind phase of the study was completed and all were offered entry into a 5-year open-label phase.
The primary composite endpoint was 1) an alkaline phosphatase level of less than 1.67 times the upper limit of the normal range, with a reduction of at least 15% below baseline level, and 2) a total bilirubin level at or below the upper limit of the normal range. After 1 year of treatment, 46% of the 5-mg group and 47% of the 10-mg group achieved this endpoint, compared with only 10% of the placebo group. The response to treatment was rapid, with patients in the active-treatment groups showing significantly lower alkaline phosphatase and bilirubin levels than the placebo group within 2 weeks of starting therapy.
In addition, the percentage of patients who showed at least a 15% reduction in alkaline phosphatase level was significantly higher with 5-10 mg active treatment (77%) and with 10 mg active treatment (77%) than with placebo (29%). And total bilirubin level progressively decreased in both active treatment groups but progressively increased in the placebo group, Dr. Nevens and his associates said (N Engl J Med. 2016 Aug 18. doi: 10.1056/NEJMoa1509840). Obeticholic acid also reduced levels of IgM and interleukin-12, as well as levels of gamma-glutamyl transferase, alanine aminotransferase, aspartate aminotransferase, and conjugated bilirubin. It did not induce significant symptom relief as measured by a disease-specific quality of life questionnaire, and it didn’t reduce liver fibrosis as measured by noninvasive techniques.
In the open-label extension phase of the study, treatment efficacy was similar.
The most common adverse event was pruritus, which is also characteristic of the disease itself. The incidence was higher in both active-treatment groups (56% with 5-10-mg therapy and 68% with 10-mg therapy) than in the placebo group (38%). One patient (1%) in the 5-10-mg group and seven patients (10%) in the 10-mg group discontinued treatment because of pruritus, compared with no patients in the placebo group.
The treatment’s beneficial effects persisted for 2 years, but that is a relatively short follow-up for a chronic disease that will require lifelong therapy. Future research must address longer-term benefits as well as adverse events, the investigators said.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Obeticholic acid therapy reduced alkaline phosphatase level, total bilirubin level, and other biomarkers in primary biliary cholangitis.
Major finding: 46% of the 5-mg group and 47% of the 10-mg group achieved the primary end point, compared with only 10% of the placebo group.
Data source: A 1-year international randomized double-blind placebo-controlled phase III clinical trial involving 217 adults with primary biliary cholangitis.
Disclosures: This study was supported by Intercept Pharmaceuticals. Dr. Nevens reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
A dual Y-shaped stent can improve QOL with airway fistulas
Airway fistula is a rare but life-threatening complication of esophageal surgery, but an innovative technique using two custom-made, Y-shaped metallic stents can preserve airway patency, researchers at Zhengzhou University in China reported in the August issue of the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2016;152:557-63).
The study involved 10 patients who received Y-shaped stents to treat gastrotracheal or gastrobronchial fistulas (GTFs and GBFs, respectively) after esophageal surgery from 2010 through 2014. “Our patients tolerated the stents well and had good palliation of their symptoms,” wrote Teng-Fei Li, MD, and colleagues.
Six patients died within 8 months for unrelated reasons – either tumors (four patients), or hemoptysis or pulmonary infection (one each). In one patient, the carinal fistula enlarged 4 months after stenting, but the researchers successfully placed an additional small Y-shaped stent. At the publication of the paper, this patient and three others had survived, Dr. Li and colleagues said.
After esophagectomy, fistulas can form between the tracheobronchial tree and stomach for a variety of reasons. A metallic stent would seem the logical choice after fistula formation, but it can be problematic, Dr. Li and colleagues pointed out. “Most often the clinician faces a situation in which the esophageal stent should have a larger diameter on the gastric side, making stenting the alimentary side of the fistula insufficient,” they said. The risk of stent migration is high, and the bifurcated structure of the trachea and main bronchi can cause leakage and stent displacement.
The researchers noted that Y-shaped self-expanding stents have been used for sealing airway fistulas, but they don’t always fully seal large GTFs and GBFs. Their primary objective in studying the combined-type Y-shaped covered metallic stent was to determine the safety and feasibility of the technique; the secondary aim was to evaluate long-term patency and complication rates.
They designed a Y-shaped stent delivery system (Micro-Tech) and used a combined bundle-and-push to insert the main body of the stent. In all, they inserted 20 Y-shaped stents in the 10 patients, although two stents did not fully expand and were dilated with a balloon. The researchers reported resolution of coughing during eating, toleration of liquid or semiliquid diet, and no complications after insertion.
Dr. Li and colleagues also developed strategies to avoid complications of Y-shaped stents, which have been known to retain secretions because they hinder cilia function. “To avoid this, we provided sputum suction and administered continuous high-concentration oxygen during the procedure,” they noted. Also, speed and agility in placement are important. “The operation should be performed as rapidly and gently as possible to avoid irritation to the airway,” Dr. Li and colleagues wrote. The postoperative course involved IV expectorants and antiasthma agents and aerosol inhalation of terbutaline. Surveillance bronchoscopes and debridement of granulation tissue helped avoid stent obstruction.
Nonetheless, the researches acknowledged limitations of the retrospective study, namely its small sample size and lack of a control group.
Dr. Li and colleagues had no financial relationships to disclose.
The Zhengzhou University investigators provide an opportunity to “think outside the box” when managing complex airway fistulas, Waël C. Hanna, MDCM, of McMaster University and St. Joseph’s Healthcare in Hamilton, Ontario, said in his invited commentary (J Thorac Cardiovasc Surg. 2016;152:564).

|
Dr. Waël Hanna |
Dr. Hanna credited a couple of innovations in their technique to overcome the challenge of Y stents that “remain notoriously difficult to position”: eliminating rigid bronchoscopy and using angiography-guided oral delivery; and developing the hybrid deployment mechanism.
Dr. Hanna also noted two “important nuances” of the technique: The stents are custom-made based on the length and location of the fistula; and the routine placement of two stents, with a limb of the smaller Y stent projecting through a limb of the larger Y stent to seal the entire airway. “This Y-en-Y technique using perfectly fitted stents is likely what caused the excellent outcomes that are reported in this series,” Dr. Hanna said.
But their approach may not be a practical solution to complex airway fistulas soon, he said. “Most of us who see the occasional case are unlikely to be able to commission custom-made Y stents,” he said. What’s more, the deployment mechanism is complicated, and the effect on patient quality of life is unclear.
Dr. Hanna had no financial relationships to disclose.
The Zhengzhou University investigators provide an opportunity to “think outside the box” when managing complex airway fistulas, Waël C. Hanna, MDCM, of McMaster University and St. Joseph’s Healthcare in Hamilton, Ontario, said in his invited commentary (J Thorac Cardiovasc Surg. 2016;152:564).

|
Dr. Waël Hanna |
Dr. Hanna credited a couple of innovations in their technique to overcome the challenge of Y stents that “remain notoriously difficult to position”: eliminating rigid bronchoscopy and using angiography-guided oral delivery; and developing the hybrid deployment mechanism.
Dr. Hanna also noted two “important nuances” of the technique: The stents are custom-made based on the length and location of the fistula; and the routine placement of two stents, with a limb of the smaller Y stent projecting through a limb of the larger Y stent to seal the entire airway. “This Y-en-Y technique using perfectly fitted stents is likely what caused the excellent outcomes that are reported in this series,” Dr. Hanna said.
But their approach may not be a practical solution to complex airway fistulas soon, he said. “Most of us who see the occasional case are unlikely to be able to commission custom-made Y stents,” he said. What’s more, the deployment mechanism is complicated, and the effect on patient quality of life is unclear.
Dr. Hanna had no financial relationships to disclose.
The Zhengzhou University investigators provide an opportunity to “think outside the box” when managing complex airway fistulas, Waël C. Hanna, MDCM, of McMaster University and St. Joseph’s Healthcare in Hamilton, Ontario, said in his invited commentary (J Thorac Cardiovasc Surg. 2016;152:564).

|
Dr. Waël Hanna |
Dr. Hanna credited a couple of innovations in their technique to overcome the challenge of Y stents that “remain notoriously difficult to position”: eliminating rigid bronchoscopy and using angiography-guided oral delivery; and developing the hybrid deployment mechanism.
Dr. Hanna also noted two “important nuances” of the technique: The stents are custom-made based on the length and location of the fistula; and the routine placement of two stents, with a limb of the smaller Y stent projecting through a limb of the larger Y stent to seal the entire airway. “This Y-en-Y technique using perfectly fitted stents is likely what caused the excellent outcomes that are reported in this series,” Dr. Hanna said.
But their approach may not be a practical solution to complex airway fistulas soon, he said. “Most of us who see the occasional case are unlikely to be able to commission custom-made Y stents,” he said. What’s more, the deployment mechanism is complicated, and the effect on patient quality of life is unclear.
Dr. Hanna had no financial relationships to disclose.
Airway fistula is a rare but life-threatening complication of esophageal surgery, but an innovative technique using two custom-made, Y-shaped metallic stents can preserve airway patency, researchers at Zhengzhou University in China reported in the August issue of the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2016;152:557-63).
The study involved 10 patients who received Y-shaped stents to treat gastrotracheal or gastrobronchial fistulas (GTFs and GBFs, respectively) after esophageal surgery from 2010 through 2014. “Our patients tolerated the stents well and had good palliation of their symptoms,” wrote Teng-Fei Li, MD, and colleagues.
Six patients died within 8 months for unrelated reasons – either tumors (four patients), or hemoptysis or pulmonary infection (one each). In one patient, the carinal fistula enlarged 4 months after stenting, but the researchers successfully placed an additional small Y-shaped stent. At the publication of the paper, this patient and three others had survived, Dr. Li and colleagues said.
After esophagectomy, fistulas can form between the tracheobronchial tree and stomach for a variety of reasons. A metallic stent would seem the logical choice after fistula formation, but it can be problematic, Dr. Li and colleagues pointed out. “Most often the clinician faces a situation in which the esophageal stent should have a larger diameter on the gastric side, making stenting the alimentary side of the fistula insufficient,” they said. The risk of stent migration is high, and the bifurcated structure of the trachea and main bronchi can cause leakage and stent displacement.
The researchers noted that Y-shaped self-expanding stents have been used for sealing airway fistulas, but they don’t always fully seal large GTFs and GBFs. Their primary objective in studying the combined-type Y-shaped covered metallic stent was to determine the safety and feasibility of the technique; the secondary aim was to evaluate long-term patency and complication rates.
They designed a Y-shaped stent delivery system (Micro-Tech) and used a combined bundle-and-push to insert the main body of the stent. In all, they inserted 20 Y-shaped stents in the 10 patients, although two stents did not fully expand and were dilated with a balloon. The researchers reported resolution of coughing during eating, toleration of liquid or semiliquid diet, and no complications after insertion.
Dr. Li and colleagues also developed strategies to avoid complications of Y-shaped stents, which have been known to retain secretions because they hinder cilia function. “To avoid this, we provided sputum suction and administered continuous high-concentration oxygen during the procedure,” they noted. Also, speed and agility in placement are important. “The operation should be performed as rapidly and gently as possible to avoid irritation to the airway,” Dr. Li and colleagues wrote. The postoperative course involved IV expectorants and antiasthma agents and aerosol inhalation of terbutaline. Surveillance bronchoscopes and debridement of granulation tissue helped avoid stent obstruction.
Nonetheless, the researches acknowledged limitations of the retrospective study, namely its small sample size and lack of a control group.
Dr. Li and colleagues had no financial relationships to disclose.
Airway fistula is a rare but life-threatening complication of esophageal surgery, but an innovative technique using two custom-made, Y-shaped metallic stents can preserve airway patency, researchers at Zhengzhou University in China reported in the August issue of the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2016;152:557-63).
The study involved 10 patients who received Y-shaped stents to treat gastrotracheal or gastrobronchial fistulas (GTFs and GBFs, respectively) after esophageal surgery from 2010 through 2014. “Our patients tolerated the stents well and had good palliation of their symptoms,” wrote Teng-Fei Li, MD, and colleagues.
Six patients died within 8 months for unrelated reasons – either tumors (four patients), or hemoptysis or pulmonary infection (one each). In one patient, the carinal fistula enlarged 4 months after stenting, but the researchers successfully placed an additional small Y-shaped stent. At the publication of the paper, this patient and three others had survived, Dr. Li and colleagues said.
After esophagectomy, fistulas can form between the tracheobronchial tree and stomach for a variety of reasons. A metallic stent would seem the logical choice after fistula formation, but it can be problematic, Dr. Li and colleagues pointed out. “Most often the clinician faces a situation in which the esophageal stent should have a larger diameter on the gastric side, making stenting the alimentary side of the fistula insufficient,” they said. The risk of stent migration is high, and the bifurcated structure of the trachea and main bronchi can cause leakage and stent displacement.
The researchers noted that Y-shaped self-expanding stents have been used for sealing airway fistulas, but they don’t always fully seal large GTFs and GBFs. Their primary objective in studying the combined-type Y-shaped covered metallic stent was to determine the safety and feasibility of the technique; the secondary aim was to evaluate long-term patency and complication rates.
They designed a Y-shaped stent delivery system (Micro-Tech) and used a combined bundle-and-push to insert the main body of the stent. In all, they inserted 20 Y-shaped stents in the 10 patients, although two stents did not fully expand and were dilated with a balloon. The researchers reported resolution of coughing during eating, toleration of liquid or semiliquid diet, and no complications after insertion.
Dr. Li and colleagues also developed strategies to avoid complications of Y-shaped stents, which have been known to retain secretions because they hinder cilia function. “To avoid this, we provided sputum suction and administered continuous high-concentration oxygen during the procedure,” they noted. Also, speed and agility in placement are important. “The operation should be performed as rapidly and gently as possible to avoid irritation to the airway,” Dr. Li and colleagues wrote. The postoperative course involved IV expectorants and antiasthma agents and aerosol inhalation of terbutaline. Surveillance bronchoscopes and debridement of granulation tissue helped avoid stent obstruction.
Nonetheless, the researches acknowledged limitations of the retrospective study, namely its small sample size and lack of a control group.
Dr. Li and colleagues had no financial relationships to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: A combined type Y-shaped self-expandable coated metallic stent is a new approach for treatment of airway fistulas.
Major finding: Ten patients received the stents; all of them reported improved quality of life. Six died within 8 months because of unrelated factors.
Data source: Single-institution retrospective review of 10 patients with gastrotracheal or gastrobronchial fistulas who received the stent to reopen the airway.
Disclosures: Dr. Li and coauthors had no financial relationships to disclose. The study received support from the National High-Tech R&D Program of China.
Guideline recommends 2-mm negative margins for DCIS
Surgical excision with 2-mm clear margins combined with whole-breast irradiation may be the optimal standard to reduce recurrence in patients with ductal carcinoma in situ (DCIS), according to a multidisciplinary consensus panel.
Despite the widespread use of surgical excision in breast-conserving therapy among patients with DCIS, there is no consensus on the optimal negative margin to prevent recurrence and re-excision. Therefore, the Society of Surgical Oncology, the American Society for Radiation Oncology, and the American Society of Clinical Oncology convened a panel to answer the following question: What margin width minimizes the risk of ipsilateral breast tumor recurrence in patients with DCIS receiving breast-conserving surgery?
The guideline panel reviewed 20 studies including 7,883 DCIS patients. A median of 100% of patients received whole-breast radiation therapy and a median of 21% received endocrine therapy. Patients were followed for a median of 6.5 years and the median incidence of recurrence was 8.3%.
“There is no debate that a positive margin ... implies a potentially incomplete resection and is associated with a higher rate of [recurrence],” according to Monica Morrow, MD, of Memorial Sloan-Kettering Cancer Center, and her fellow panelists. Further, the addition of whole-breast irradiation did not negate this increased risk, the panelists noted (J Clin Oncol. 2016 Aug. doi: 10.1200/JCO.2016.68.3573).
According to the meta-analysis, patients with 2-mm negative margins plus whole-breast irradiation were significantly less likely to experience ipsilateral breast tumor recurrence compared with patients who had excisions with positive margins.
“Margins of at least 2 mm are associated with a reduced risk of [recurrence] relative to narrower negative margin widths in patients receiving [whole-breast radiotherapy]. The routine practice of obtaining negative margin widths wider than 2 mm is not supported by the evidence,” they wrote.
The panel also noted that treatment with excision alone is associated with higher rates of recurrence compared with treatment involving with both excision and whole-breast radiation therapy. However, if patients are treated with excision alone, the optimal margin, which is unknown for this subset of patients, should be at least 2 millimeters, according to the guideline.
Due to variability in specimen sampling and in margin evaluation and assessment, “clinical judgment must be used in determining whether patients with smaller negative margin widths (0 or 1 mm) require re-excision,” the panelists noted. “The findings should not be extrapolated to DCIS patients treated with [accelerated partial breast irradiation] or those with invasive carcinoma for whom a separate guideline has been developed,” Dr. Morrow and her associates wrote.
The guideline development process was funded by the Susan G. Komen Foundation. The authors had no relevant disclosures to report.
On Twitter @jessnicolecraig
Surgical excision with 2-mm clear margins combined with whole-breast irradiation may be the optimal standard to reduce recurrence in patients with ductal carcinoma in situ (DCIS), according to a multidisciplinary consensus panel.
Despite the widespread use of surgical excision in breast-conserving therapy among patients with DCIS, there is no consensus on the optimal negative margin to prevent recurrence and re-excision. Therefore, the Society of Surgical Oncology, the American Society for Radiation Oncology, and the American Society of Clinical Oncology convened a panel to answer the following question: What margin width minimizes the risk of ipsilateral breast tumor recurrence in patients with DCIS receiving breast-conserving surgery?
The guideline panel reviewed 20 studies including 7,883 DCIS patients. A median of 100% of patients received whole-breast radiation therapy and a median of 21% received endocrine therapy. Patients were followed for a median of 6.5 years and the median incidence of recurrence was 8.3%.
“There is no debate that a positive margin ... implies a potentially incomplete resection and is associated with a higher rate of [recurrence],” according to Monica Morrow, MD, of Memorial Sloan-Kettering Cancer Center, and her fellow panelists. Further, the addition of whole-breast irradiation did not negate this increased risk, the panelists noted (J Clin Oncol. 2016 Aug. doi: 10.1200/JCO.2016.68.3573).
According to the meta-analysis, patients with 2-mm negative margins plus whole-breast irradiation were significantly less likely to experience ipsilateral breast tumor recurrence compared with patients who had excisions with positive margins.
“Margins of at least 2 mm are associated with a reduced risk of [recurrence] relative to narrower negative margin widths in patients receiving [whole-breast radiotherapy]. The routine practice of obtaining negative margin widths wider than 2 mm is not supported by the evidence,” they wrote.
The panel also noted that treatment with excision alone is associated with higher rates of recurrence compared with treatment involving with both excision and whole-breast radiation therapy. However, if patients are treated with excision alone, the optimal margin, which is unknown for this subset of patients, should be at least 2 millimeters, according to the guideline.
Due to variability in specimen sampling and in margin evaluation and assessment, “clinical judgment must be used in determining whether patients with smaller negative margin widths (0 or 1 mm) require re-excision,” the panelists noted. “The findings should not be extrapolated to DCIS patients treated with [accelerated partial breast irradiation] or those with invasive carcinoma for whom a separate guideline has been developed,” Dr. Morrow and her associates wrote.
The guideline development process was funded by the Susan G. Komen Foundation. The authors had no relevant disclosures to report.
On Twitter @jessnicolecraig
Surgical excision with 2-mm clear margins combined with whole-breast irradiation may be the optimal standard to reduce recurrence in patients with ductal carcinoma in situ (DCIS), according to a multidisciplinary consensus panel.
Despite the widespread use of surgical excision in breast-conserving therapy among patients with DCIS, there is no consensus on the optimal negative margin to prevent recurrence and re-excision. Therefore, the Society of Surgical Oncology, the American Society for Radiation Oncology, and the American Society of Clinical Oncology convened a panel to answer the following question: What margin width minimizes the risk of ipsilateral breast tumor recurrence in patients with DCIS receiving breast-conserving surgery?
The guideline panel reviewed 20 studies including 7,883 DCIS patients. A median of 100% of patients received whole-breast radiation therapy and a median of 21% received endocrine therapy. Patients were followed for a median of 6.5 years and the median incidence of recurrence was 8.3%.
“There is no debate that a positive margin ... implies a potentially incomplete resection and is associated with a higher rate of [recurrence],” according to Monica Morrow, MD, of Memorial Sloan-Kettering Cancer Center, and her fellow panelists. Further, the addition of whole-breast irradiation did not negate this increased risk, the panelists noted (J Clin Oncol. 2016 Aug. doi: 10.1200/JCO.2016.68.3573).
According to the meta-analysis, patients with 2-mm negative margins plus whole-breast irradiation were significantly less likely to experience ipsilateral breast tumor recurrence compared with patients who had excisions with positive margins.
“Margins of at least 2 mm are associated with a reduced risk of [recurrence] relative to narrower negative margin widths in patients receiving [whole-breast radiotherapy]. The routine practice of obtaining negative margin widths wider than 2 mm is not supported by the evidence,” they wrote.
The panel also noted that treatment with excision alone is associated with higher rates of recurrence compared with treatment involving with both excision and whole-breast radiation therapy. However, if patients are treated with excision alone, the optimal margin, which is unknown for this subset of patients, should be at least 2 millimeters, according to the guideline.
Due to variability in specimen sampling and in margin evaluation and assessment, “clinical judgment must be used in determining whether patients with smaller negative margin widths (0 or 1 mm) require re-excision,” the panelists noted. “The findings should not be extrapolated to DCIS patients treated with [accelerated partial breast irradiation] or those with invasive carcinoma for whom a separate guideline has been developed,” Dr. Morrow and her associates wrote.
The guideline development process was funded by the Susan G. Komen Foundation. The authors had no relevant disclosures to report.
On Twitter @jessnicolecraig
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Surgical excision with 2-mm clear margins combined with whole-breast irradiation may be the optimal standard to reduce recurrence in patients with ductal carcinoma in situ.
Major finding: Patients with 2-mm clear margins were significantly less likely to experience ipsilateral breast tumor recurrence compared with patients who had excisions with positive margins.
Data source: Meta-analysis of 20 studies and other published literature.
Disclosures: The Susan G. Komen Foundation funded the guideline development process. The panel members had no relevant disclosures.
Pilot program helps children better understand food allergies
Elementary school students had improved attitudes toward food allergies and felt more confident in taking action during a food allergy emergency after completing an education program on the subject, results of a pilot study in Japan suggest.
At present there is no standard school curriculum for children regarding food allergy, wrote Dr. Kiwako Yamamoto-Hanada of the National Center for Child Health and Development, Tokyo, and associates, so they developed such a program consisting of two 60-minute sessions. A total of 36 elementary school children, 8 of whom had a history of food allergies, filled out questionnaires before and after participating in the program.
After completing the program, 79% of the students stated that it should be included in the school curriculum. Students also demonstrated improved knowledge about food allergies, with a greater percentage knowing what an EpiPen is (100% vs. 0%), understanding that food allergy is related to death (100% vs. 43%), and feeling confident that they could take immediate action if they saw a food allergy emergency (61% vs. 4%). “This is the first report to find that a [food allergy] program for elementary schoolchildren was well tolerated and that perceptions and attitudes toward [food allergies] improved,” the investigators wrote.
The authors stated that they had no financial conflicts of interest.
Read the full story here: http://dx.doi.org/10.1016/j.anai.2016.06.018
Elementary school students had improved attitudes toward food allergies and felt more confident in taking action during a food allergy emergency after completing an education program on the subject, results of a pilot study in Japan suggest.
At present there is no standard school curriculum for children regarding food allergy, wrote Dr. Kiwako Yamamoto-Hanada of the National Center for Child Health and Development, Tokyo, and associates, so they developed such a program consisting of two 60-minute sessions. A total of 36 elementary school children, 8 of whom had a history of food allergies, filled out questionnaires before and after participating in the program.
After completing the program, 79% of the students stated that it should be included in the school curriculum. Students also demonstrated improved knowledge about food allergies, with a greater percentage knowing what an EpiPen is (100% vs. 0%), understanding that food allergy is related to death (100% vs. 43%), and feeling confident that they could take immediate action if they saw a food allergy emergency (61% vs. 4%). “This is the first report to find that a [food allergy] program for elementary schoolchildren was well tolerated and that perceptions and attitudes toward [food allergies] improved,” the investigators wrote.
The authors stated that they had no financial conflicts of interest.
Read the full story here: http://dx.doi.org/10.1016/j.anai.2016.06.018
Elementary school students had improved attitudes toward food allergies and felt more confident in taking action during a food allergy emergency after completing an education program on the subject, results of a pilot study in Japan suggest.
At present there is no standard school curriculum for children regarding food allergy, wrote Dr. Kiwako Yamamoto-Hanada of the National Center for Child Health and Development, Tokyo, and associates, so they developed such a program consisting of two 60-minute sessions. A total of 36 elementary school children, 8 of whom had a history of food allergies, filled out questionnaires before and after participating in the program.
After completing the program, 79% of the students stated that it should be included in the school curriculum. Students also demonstrated improved knowledge about food allergies, with a greater percentage knowing what an EpiPen is (100% vs. 0%), understanding that food allergy is related to death (100% vs. 43%), and feeling confident that they could take immediate action if they saw a food allergy emergency (61% vs. 4%). “This is the first report to find that a [food allergy] program for elementary schoolchildren was well tolerated and that perceptions and attitudes toward [food allergies] improved,” the investigators wrote.
The authors stated that they had no financial conflicts of interest.
Read the full story here: http://dx.doi.org/10.1016/j.anai.2016.06.018
FROM ANNALS OF ALLERGY, ASTHMA & IMMUNOLOGY
Antipsychotics in pregnancy pose no meaningful malformation risk
Antipsychotic use early in pregnancy does not appear to meaningfully increase the risk of congenital malformations, according to findings from a large Medicaid pregnancy cohort.
A possible exception is with the use of risperidone; a small increase in the risk for malformations seen with the drug should be viewed as “an initial safety signal that will require confirmation in other studies,” Krista F. Huybrechts, PhD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, and her colleagues reported online Aug. 17 in JAMA Psychiatry.
In a nationwide sample of more than 1.3 million pregnant women with a live-born infant between January 1, 2000 and December 31, 2010, the rate of congenital malformations identified within 90 days after delivery was 32.7 per 1,000 births among 1,331,724 women with no antipsychotic (AP) exposure, compared with a rate of 44.5 per 1,000 births among 9,258 women who filled at least one prescription for an atypical AP during their first trimester, and 38.2 per 1,000 in 733 women who filled at least one prescription for a typical AP during their first trimester, the researchers found (JAMA Psychiatry. 2016 Aug 17. doi: 10.1001/jamapsychiatry.2016.1520).
On unadjusted analyses, an overall risk was seen with atypical AP exposure (relative risk, 1.36), but not with typical AP exposure (RR, 1.17). After adjusting for confounding variables, the risk was not statistically significant (RR, 1.05 and 0.90, respectively). The findings were similar for cardiac malformations, the researchers noted.
When agents were analyzed individually, a small increased risk in overall malformations and cardiac malformations was found with risperidone (RR, 1.26 for each).
“The findings for risperidone should be viewed as an initial safety signal that will require confirmation in other studies,” the researchers wrote.
The study was supported by grants from the National Institutes of Health and the Swiss National Science Foundation. Dr. Huybrechts reported having no financial disclosures. Other authors disclosed receiving consulting fees and/or grant support from AstraZeneca, UCB, Alkermes, Bristol-Myers Squibb/Otsuka, Sunovion, Bayer, Ortho-McNeil Janssen, Pfizer, Forest Laboratories, Cephalon, GlaxoSmithKline, Takeda/Lundbeck, the National Institutes of Health, JDS Therapeutics, Noven Pharmaceuticals, and PamLab.
This “landmark report” by Huybrechts et al involves the largest population of women exposed to APs published to date, and addresses a “major source of concern for women and prescribers,” Katherine L. Wisner, MD, and her colleagues wrote in an editorial.
With the exception of risperidone, which was found to be associated with a 26% increase in the risk of overall and cardiac malformations, and possibly even greater risk among those who filled at least two AP prescriptions – and thus merits further exploration – AP use was found in the study to be safe.
“This study increases the field’s appetite for additional high-quality data on other maternal and offspring outcomes to improve the sophistication of risk-benefit decision making,” the authors wrote.
Nowhere in medicine is there a greater need for personalization of care, particularly when it comes to the treatment of serious mental illness with consideration of “pregnancy physiology and the mother’s capacity to provide sustenance for the growing fetus and newborn,” they wrote.
Dr. Wisner is with Northwestern University, Chicago. She reported that her department at Northwestern received contractual fees for her consultation to Quinn Emanuel Urquhart & Sullivan, LLP, who represented Pfizer Pharmaceutical Company in 2015. A coauthor, Christina Chambers, PhD, reported receiving research funding from AbbVie, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Pfizer, Sandoz, and Teva. No other disclosures were reported. Their comments are excerpted from an accompanying editorial (JAMA Psychiatry. 2016 Aug 17. doi: 10.1001/jamapsychiatry.2016.1538).
This “landmark report” by Huybrechts et al involves the largest population of women exposed to APs published to date, and addresses a “major source of concern for women and prescribers,” Katherine L. Wisner, MD, and her colleagues wrote in an editorial.
With the exception of risperidone, which was found to be associated with a 26% increase in the risk of overall and cardiac malformations, and possibly even greater risk among those who filled at least two AP prescriptions – and thus merits further exploration – AP use was found in the study to be safe.
“This study increases the field’s appetite for additional high-quality data on other maternal and offspring outcomes to improve the sophistication of risk-benefit decision making,” the authors wrote.
Nowhere in medicine is there a greater need for personalization of care, particularly when it comes to the treatment of serious mental illness with consideration of “pregnancy physiology and the mother’s capacity to provide sustenance for the growing fetus and newborn,” they wrote.
Dr. Wisner is with Northwestern University, Chicago. She reported that her department at Northwestern received contractual fees for her consultation to Quinn Emanuel Urquhart & Sullivan, LLP, who represented Pfizer Pharmaceutical Company in 2015. A coauthor, Christina Chambers, PhD, reported receiving research funding from AbbVie, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Pfizer, Sandoz, and Teva. No other disclosures were reported. Their comments are excerpted from an accompanying editorial (JAMA Psychiatry. 2016 Aug 17. doi: 10.1001/jamapsychiatry.2016.1538).
This “landmark report” by Huybrechts et al involves the largest population of women exposed to APs published to date, and addresses a “major source of concern for women and prescribers,” Katherine L. Wisner, MD, and her colleagues wrote in an editorial.
With the exception of risperidone, which was found to be associated with a 26% increase in the risk of overall and cardiac malformations, and possibly even greater risk among those who filled at least two AP prescriptions – and thus merits further exploration – AP use was found in the study to be safe.
“This study increases the field’s appetite for additional high-quality data on other maternal and offspring outcomes to improve the sophistication of risk-benefit decision making,” the authors wrote.
Nowhere in medicine is there a greater need for personalization of care, particularly when it comes to the treatment of serious mental illness with consideration of “pregnancy physiology and the mother’s capacity to provide sustenance for the growing fetus and newborn,” they wrote.
Dr. Wisner is with Northwestern University, Chicago. She reported that her department at Northwestern received contractual fees for her consultation to Quinn Emanuel Urquhart & Sullivan, LLP, who represented Pfizer Pharmaceutical Company in 2015. A coauthor, Christina Chambers, PhD, reported receiving research funding from AbbVie, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Pfizer, Sandoz, and Teva. No other disclosures were reported. Their comments are excerpted from an accompanying editorial (JAMA Psychiatry. 2016 Aug 17. doi: 10.1001/jamapsychiatry.2016.1538).
Antipsychotic use early in pregnancy does not appear to meaningfully increase the risk of congenital malformations, according to findings from a large Medicaid pregnancy cohort.
A possible exception is with the use of risperidone; a small increase in the risk for malformations seen with the drug should be viewed as “an initial safety signal that will require confirmation in other studies,” Krista F. Huybrechts, PhD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, and her colleagues reported online Aug. 17 in JAMA Psychiatry.
In a nationwide sample of more than 1.3 million pregnant women with a live-born infant between January 1, 2000 and December 31, 2010, the rate of congenital malformations identified within 90 days after delivery was 32.7 per 1,000 births among 1,331,724 women with no antipsychotic (AP) exposure, compared with a rate of 44.5 per 1,000 births among 9,258 women who filled at least one prescription for an atypical AP during their first trimester, and 38.2 per 1,000 in 733 women who filled at least one prescription for a typical AP during their first trimester, the researchers found (JAMA Psychiatry. 2016 Aug 17. doi: 10.1001/jamapsychiatry.2016.1520).
On unadjusted analyses, an overall risk was seen with atypical AP exposure (relative risk, 1.36), but not with typical AP exposure (RR, 1.17). After adjusting for confounding variables, the risk was not statistically significant (RR, 1.05 and 0.90, respectively). The findings were similar for cardiac malformations, the researchers noted.
When agents were analyzed individually, a small increased risk in overall malformations and cardiac malformations was found with risperidone (RR, 1.26 for each).
“The findings for risperidone should be viewed as an initial safety signal that will require confirmation in other studies,” the researchers wrote.
The study was supported by grants from the National Institutes of Health and the Swiss National Science Foundation. Dr. Huybrechts reported having no financial disclosures. Other authors disclosed receiving consulting fees and/or grant support from AstraZeneca, UCB, Alkermes, Bristol-Myers Squibb/Otsuka, Sunovion, Bayer, Ortho-McNeil Janssen, Pfizer, Forest Laboratories, Cephalon, GlaxoSmithKline, Takeda/Lundbeck, the National Institutes of Health, JDS Therapeutics, Noven Pharmaceuticals, and PamLab.
Antipsychotic use early in pregnancy does not appear to meaningfully increase the risk of congenital malformations, according to findings from a large Medicaid pregnancy cohort.
A possible exception is with the use of risperidone; a small increase in the risk for malformations seen with the drug should be viewed as “an initial safety signal that will require confirmation in other studies,” Krista F. Huybrechts, PhD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, and her colleagues reported online Aug. 17 in JAMA Psychiatry.
In a nationwide sample of more than 1.3 million pregnant women with a live-born infant between January 1, 2000 and December 31, 2010, the rate of congenital malformations identified within 90 days after delivery was 32.7 per 1,000 births among 1,331,724 women with no antipsychotic (AP) exposure, compared with a rate of 44.5 per 1,000 births among 9,258 women who filled at least one prescription for an atypical AP during their first trimester, and 38.2 per 1,000 in 733 women who filled at least one prescription for a typical AP during their first trimester, the researchers found (JAMA Psychiatry. 2016 Aug 17. doi: 10.1001/jamapsychiatry.2016.1520).
On unadjusted analyses, an overall risk was seen with atypical AP exposure (relative risk, 1.36), but not with typical AP exposure (RR, 1.17). After adjusting for confounding variables, the risk was not statistically significant (RR, 1.05 and 0.90, respectively). The findings were similar for cardiac malformations, the researchers noted.
When agents were analyzed individually, a small increased risk in overall malformations and cardiac malformations was found with risperidone (RR, 1.26 for each).
“The findings for risperidone should be viewed as an initial safety signal that will require confirmation in other studies,” the researchers wrote.
The study was supported by grants from the National Institutes of Health and the Swiss National Science Foundation. Dr. Huybrechts reported having no financial disclosures. Other authors disclosed receiving consulting fees and/or grant support from AstraZeneca, UCB, Alkermes, Bristol-Myers Squibb/Otsuka, Sunovion, Bayer, Ortho-McNeil Janssen, Pfizer, Forest Laboratories, Cephalon, GlaxoSmithKline, Takeda/Lundbeck, the National Institutes of Health, JDS Therapeutics, Noven Pharmaceuticals, and PamLab.
FROM JAMA PSYCHIATRY
Key clinical point: Antipsychotic use in the first trimester does not appear to meaningfully increase the risk of congenital malformations.
Major finding: The adjusted relative risk for malformations is 1.05 and 0.90 with atypical and typical antipsychotic drug exposure, respectively.
Data source: A total of 1,341,715 pregnancies from the Medicaid Analytic Extract database pregnancy cohort.
Disclosures: The study was supported by grants from the National Institutes of Health and the Swiss National Science Foundation. Dr. Huybrechts reported having no financial disclosures. Other authors disclosed receiving consulting fees and/or grant support from AstraZeneca, UCB, Alkermes, Bristol-Myers Squibb/Otsuka, Sunovion, Bayer, Ortho-McNeil Janssen, Pfizer, Forest Laboratories, Cephalon, GlaxoSmithKline, Takeda/Lundbeck, the National Institutes of Health, JDS Therapeutics, Noven Pharmaceuticals, and PamLab.