User login
Cancer trends shifting in HIV-positive patients
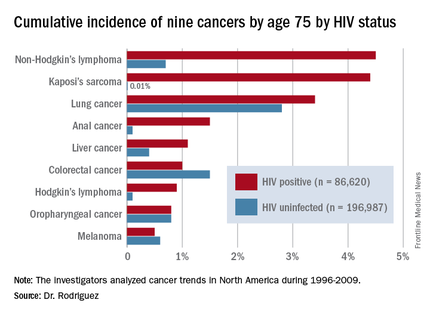
DURBAN, SOUTH AFRICA – The rates of the AIDS-defining cancers Kaposi’s sarcoma and non-Hodgkin’s lymphoma have plummeted in the antiretroviral era, yet they are still the two top cancers in terms of cumulative incidence in HIV-infected patients by age 75, Benigno Rodriguez, MD, said at the 21st International AIDS Conference.
Lung cancer also remains a major concern in the HIV-infected population. Each of these three cancers carries about a 1 in 25 lifetime risk to age 75, the estimated lifespan of HIV-positive patients on combination antiretroviral therapy (ART), according to Dr. Rodriguez of Case Western Reserve University in Cleveland.
ART has resulted in a marked change in cancer trends among HIV-infected patients. The incidence of AIDS-defining cancers has decreased “massively,” Dr. Rodriguez observed; but because ART has extended the lifespan, patients are now living long enough to get other cancers. And they do so at a higher rate than that of the general population because of their impaired immune function, higher rate of risk factors such as smoking, and greater prevalence of oncogenic viral coinfections such as hepatitis B and C and Epstein-Barr virus.
The increase in the incidence and risk of colorectal, liver, and anal cancers among HIV-positive individuals since the introduction of combination ART can largely be explained by the population’s longer exposure to risk due to increasing survival, he said.
Dr. Rodriguez presented highlights of an analysis of cancer trends in North America during 1996-2009. The study was based on 86,620 HIV-infected persons with 475,660 person-years of follow-up and 196,987 subjects without HIV infection and with more than 1.8 million person-years of follow-up. Trends over time were assessed for nine cancers: Kaposi’s sarcoma, non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, and melanoma, as well as anal, lung, colorectal, liver, and oropharyngeal cancers (Ann Intern Med. 2015;163:507-18).
Examining trends in three periods – 1996-1999, 2000-2004, and 2005-2009 – the investigators looked at the impact of ART over time on rates of AIDS-related and non–AIDS-related cancers in HIV-infected patients and compared them to results in the general HIV-uninfected population.
By conducting a competing risk analysis, Dr. Rodriguez and his coworkers were able to estimate the cumulative lifetime risk of the various cancers by age 75, a metric that provides readily understandable information for counseling HIV-infected patients about their long-term cancer risk.
The measure “is more intuitive than using incidence rates,” Dr. Rodriguez said. In a study of 1,578 HIV-infected patients who received the hepatitis B vaccine, for example, those patients whose immune function did not to respond to the vaccine were more likely to be among the 6% of patients who subsequently developed cancer during up to 20 years of follow-up.
The findings on cancer trends in the ART era have clinical implications for cancer screening and prevention in HIV-infected patients. The high rates of smoking and lung cancer in this population make HIV-positive smokers a logical target for lung cancer screening. The rising risk of colorectal cancer – the cumulative lifetime risk to age 75 was 0.4% in 1996-1999, 0.7% in 2000-2004, and 1.3% in 2005-2009 – suggests a need for increased colorectal cancer screening in the older HIV-positive population.
Early and sustained HIV suppression with combination ART remains the only known method of preventing AIDS-defining cancers. Dr. Rodriguez and his coinvestigators in the Centers for AIDS Research Network of Integrated Clinical Systems demonstrated the crucial role of suppressing HIV in a study of 6,036 HIV-infected patients who started on ART and were followed for more than 21,000 person-years. Compared with HIV-infected patients with a 3-month lagged HIV viremia of no more than 50 copies/mL, patients’ risk of developing non-Hodgkin’s lymphoma was 2.1-fold greater if their 3-month lagged HIV viremia was 51-500 copies/mL and 3.56-fold greater if it exceeded 500 copies/mL (Clin Infect Dis. 2014 Jun;58[11]:1599-606).
The study on cancer trends over time was funded by the National Institutes of Health. Dr. Rodriguez reported receiving honoraria from Gilead Sciences.
DURBAN, SOUTH AFRICA – The rates of the AIDS-defining cancers Kaposi’s sarcoma and non-Hodgkin’s lymphoma have plummeted in the antiretroviral era, yet they are still the two top cancers in terms of cumulative incidence in HIV-infected patients by age 75, Benigno Rodriguez, MD, said at the 21st International AIDS Conference.
Lung cancer also remains a major concern in the HIV-infected population. Each of these three cancers carries about a 1 in 25 lifetime risk to age 75, the estimated lifespan of HIV-positive patients on combination antiretroviral therapy (ART), according to Dr. Rodriguez of Case Western Reserve University in Cleveland.
ART has resulted in a marked change in cancer trends among HIV-infected patients. The incidence of AIDS-defining cancers has decreased “massively,” Dr. Rodriguez observed; but because ART has extended the lifespan, patients are now living long enough to get other cancers. And they do so at a higher rate than that of the general population because of their impaired immune function, higher rate of risk factors such as smoking, and greater prevalence of oncogenic viral coinfections such as hepatitis B and C and Epstein-Barr virus.
The increase in the incidence and risk of colorectal, liver, and anal cancers among HIV-positive individuals since the introduction of combination ART can largely be explained by the population’s longer exposure to risk due to increasing survival, he said.
Dr. Rodriguez presented highlights of an analysis of cancer trends in North America during 1996-2009. The study was based on 86,620 HIV-infected persons with 475,660 person-years of follow-up and 196,987 subjects without HIV infection and with more than 1.8 million person-years of follow-up. Trends over time were assessed for nine cancers: Kaposi’s sarcoma, non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, and melanoma, as well as anal, lung, colorectal, liver, and oropharyngeal cancers (Ann Intern Med. 2015;163:507-18).
Examining trends in three periods – 1996-1999, 2000-2004, and 2005-2009 – the investigators looked at the impact of ART over time on rates of AIDS-related and non–AIDS-related cancers in HIV-infected patients and compared them to results in the general HIV-uninfected population.

By conducting a competing risk analysis, Dr. Rodriguez and his coworkers were able to estimate the cumulative lifetime risk of the various cancers by age 75, a metric that provides readily understandable information for counseling HIV-infected patients about their long-term cancer risk.
The measure “is more intuitive than using incidence rates,” Dr. Rodriguez said. In a study of 1,578 HIV-infected patients who received the hepatitis B vaccine, for example, those patients whose immune function did not to respond to the vaccine were more likely to be among the 6% of patients who subsequently developed cancer during up to 20 years of follow-up.
The findings on cancer trends in the ART era have clinical implications for cancer screening and prevention in HIV-infected patients. The high rates of smoking and lung cancer in this population make HIV-positive smokers a logical target for lung cancer screening. The rising risk of colorectal cancer – the cumulative lifetime risk to age 75 was 0.4% in 1996-1999, 0.7% in 2000-2004, and 1.3% in 2005-2009 – suggests a need for increased colorectal cancer screening in the older HIV-positive population.
Early and sustained HIV suppression with combination ART remains the only known method of preventing AIDS-defining cancers. Dr. Rodriguez and his coinvestigators in the Centers for AIDS Research Network of Integrated Clinical Systems demonstrated the crucial role of suppressing HIV in a study of 6,036 HIV-infected patients who started on ART and were followed for more than 21,000 person-years. Compared with HIV-infected patients with a 3-month lagged HIV viremia of no more than 50 copies/mL, patients’ risk of developing non-Hodgkin’s lymphoma was 2.1-fold greater if their 3-month lagged HIV viremia was 51-500 copies/mL and 3.56-fold greater if it exceeded 500 copies/mL (Clin Infect Dis. 2014 Jun;58[11]:1599-606).
The study on cancer trends over time was funded by the National Institutes of Health. Dr. Rodriguez reported receiving honoraria from Gilead Sciences.
DURBAN, SOUTH AFRICA – The rates of the AIDS-defining cancers Kaposi’s sarcoma and non-Hodgkin’s lymphoma have plummeted in the antiretroviral era, yet they are still the two top cancers in terms of cumulative incidence in HIV-infected patients by age 75, Benigno Rodriguez, MD, said at the 21st International AIDS Conference.
Lung cancer also remains a major concern in the HIV-infected population. Each of these three cancers carries about a 1 in 25 lifetime risk to age 75, the estimated lifespan of HIV-positive patients on combination antiretroviral therapy (ART), according to Dr. Rodriguez of Case Western Reserve University in Cleveland.
ART has resulted in a marked change in cancer trends among HIV-infected patients. The incidence of AIDS-defining cancers has decreased “massively,” Dr. Rodriguez observed; but because ART has extended the lifespan, patients are now living long enough to get other cancers. And they do so at a higher rate than that of the general population because of their impaired immune function, higher rate of risk factors such as smoking, and greater prevalence of oncogenic viral coinfections such as hepatitis B and C and Epstein-Barr virus.
The increase in the incidence and risk of colorectal, liver, and anal cancers among HIV-positive individuals since the introduction of combination ART can largely be explained by the population’s longer exposure to risk due to increasing survival, he said.
Dr. Rodriguez presented highlights of an analysis of cancer trends in North America during 1996-2009. The study was based on 86,620 HIV-infected persons with 475,660 person-years of follow-up and 196,987 subjects without HIV infection and with more than 1.8 million person-years of follow-up. Trends over time were assessed for nine cancers: Kaposi’s sarcoma, non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, and melanoma, as well as anal, lung, colorectal, liver, and oropharyngeal cancers (Ann Intern Med. 2015;163:507-18).
Examining trends in three periods – 1996-1999, 2000-2004, and 2005-2009 – the investigators looked at the impact of ART over time on rates of AIDS-related and non–AIDS-related cancers in HIV-infected patients and compared them to results in the general HIV-uninfected population.

By conducting a competing risk analysis, Dr. Rodriguez and his coworkers were able to estimate the cumulative lifetime risk of the various cancers by age 75, a metric that provides readily understandable information for counseling HIV-infected patients about their long-term cancer risk.
The measure “is more intuitive than using incidence rates,” Dr. Rodriguez said. In a study of 1,578 HIV-infected patients who received the hepatitis B vaccine, for example, those patients whose immune function did not to respond to the vaccine were more likely to be among the 6% of patients who subsequently developed cancer during up to 20 years of follow-up.
The findings on cancer trends in the ART era have clinical implications for cancer screening and prevention in HIV-infected patients. The high rates of smoking and lung cancer in this population make HIV-positive smokers a logical target for lung cancer screening. The rising risk of colorectal cancer – the cumulative lifetime risk to age 75 was 0.4% in 1996-1999, 0.7% in 2000-2004, and 1.3% in 2005-2009 – suggests a need for increased colorectal cancer screening in the older HIV-positive population.
Early and sustained HIV suppression with combination ART remains the only known method of preventing AIDS-defining cancers. Dr. Rodriguez and his coinvestigators in the Centers for AIDS Research Network of Integrated Clinical Systems demonstrated the crucial role of suppressing HIV in a study of 6,036 HIV-infected patients who started on ART and were followed for more than 21,000 person-years. Compared with HIV-infected patients with a 3-month lagged HIV viremia of no more than 50 copies/mL, patients’ risk of developing non-Hodgkin’s lymphoma was 2.1-fold greater if their 3-month lagged HIV viremia was 51-500 copies/mL and 3.56-fold greater if it exceeded 500 copies/mL (Clin Infect Dis. 2014 Jun;58[11]:1599-606).
The study on cancer trends over time was funded by the National Institutes of Health. Dr. Rodriguez reported receiving honoraria from Gilead Sciences.
AT AIDS 2016
Key clinical point: HIV-infected persons in North America have roughly a 1 in 25 cumulative lifetime risk of developing lung cancer, Kaposi’s sarcoma, or non-Hodgkin’s lymphoma.
Major finding: The cumulative lifetime risk of developing non-Hodgkin’s lymphoma in HIV-infected patients on combination antiretroviral therapy is sevenfold greater than the risk in the general population.
Data source: A competing risk analysis for nine cancers based upon 86,620 HIV-infected persons followed for 475,660 person-years and 196,987 subjects not infected with HIV and with more than 1.8 million person-years of follow-up.
Disclosures: The National Institutes of Health funded the study. The presenter reported receiving honoraria from Gilead Sciences.
Ankylosing spondylitis patients develop multiple comorbidities after diagnosis
DENVER – Evidence continues to mount that ankylosing spondylitis patients are at increased risk for developing various comorbidities, compared with the general adult population.
Patients newly diagnosed with ankylosing spondylitis (AS) had twice the rate of new-onset depression during the first 3 years following diagnosis, compared with matched people from the general population in a study of more than 21,000 American adults. Patients with newly diagnosed AS also had a 60% higher rate of developing a new cardiovascular disease, compared with the matched general population, Jessica A. Walsh, MD, said at the annual meeting of the Spondyloarthritis Research and Treatment Network.
“We need to figure out what to do about all the comorbidities. Rheumatologists need to either screen their AS patients for comorbidities, or they need to be sure their patients are plugged in with another physician who will screen them,” said Dr. Walsh, director of the spondyloarthritis clinic at the University of Utah in Salt Lake City.
Her analysis used data from the Truven Health MarketScan databases for U.S. patients covered by Medicare or commercial health insurance, and included 6,370 patients with newly diagnosed AS and 14,988 adults matched by age and sex. The analysis only included AS patients who were free from comorbidities during the 2 years prior to their AS diagnosis. The average age of the people in the study was 52 years, and 54% were men.
During an average follow-up of 2.9 years following initial AS diagnosis, the most common comorbidity among the AS patients was uveitis, which occurred nearly 15-fold more frequently among the AS patients than in the controls. Other common incident comorbidities included inflammatory bowel disease, nearly sixfold more common among the AS patients, and osteoporosis, which was nearly threefold more common during follow-up after AS diagnosis, compared with the controls.
Other comorbidities with increased incidence in the AS patients included sleep apnea (80% more common among the AS patients during follow-up), asthma (50% more often), hypertension (44% more common), malignancy (23% more common), diabetes (20% more common), and dyslipidemia (11% more common). All these incidence rates represented statistically significant increases in the AS patients, compared with the controls.
A related analysis reported by Dr. Walsh also used data from the Truven Health databases for a somewhat larger group of AS patients, 6,679 followed for 1 year after their AS diagnosis, and 19,951 matched controls. The AS patients had a significantly higher rate of hospital admissions – 12%, compared with 6% among the controls – and a significantly higher rate of emergency department visits, at 23%, compared with 15% among the controls. The AS patients also had double the rate of physician office visits and prescribed medications.
“Obviously, the AS patients are not as healthy,” Dr. Walsh said in an interview. “We adjusted for their comorbidities, but that did not affect the hospitalization rates. We need to look into this more; I don’t know why the AS patients are being hospitalized. Typically AS itself does not lead to hospitalization, so I suspect it’s because of comorbidities, or perhaps because of adverse events from treatment.”
Dr. Walsh is a consultant to AbbVie and Novartis.
On Twitter @mitchelzoler
DENVER – Evidence continues to mount that ankylosing spondylitis patients are at increased risk for developing various comorbidities, compared with the general adult population.
Patients newly diagnosed with ankylosing spondylitis (AS) had twice the rate of new-onset depression during the first 3 years following diagnosis, compared with matched people from the general population in a study of more than 21,000 American adults. Patients with newly diagnosed AS also had a 60% higher rate of developing a new cardiovascular disease, compared with the matched general population, Jessica A. Walsh, MD, said at the annual meeting of the Spondyloarthritis Research and Treatment Network.
“We need to figure out what to do about all the comorbidities. Rheumatologists need to either screen their AS patients for comorbidities, or they need to be sure their patients are plugged in with another physician who will screen them,” said Dr. Walsh, director of the spondyloarthritis clinic at the University of Utah in Salt Lake City.
Her analysis used data from the Truven Health MarketScan databases for U.S. patients covered by Medicare or commercial health insurance, and included 6,370 patients with newly diagnosed AS and 14,988 adults matched by age and sex. The analysis only included AS patients who were free from comorbidities during the 2 years prior to their AS diagnosis. The average age of the people in the study was 52 years, and 54% were men.
During an average follow-up of 2.9 years following initial AS diagnosis, the most common comorbidity among the AS patients was uveitis, which occurred nearly 15-fold more frequently among the AS patients than in the controls. Other common incident comorbidities included inflammatory bowel disease, nearly sixfold more common among the AS patients, and osteoporosis, which was nearly threefold more common during follow-up after AS diagnosis, compared with the controls.
Other comorbidities with increased incidence in the AS patients included sleep apnea (80% more common among the AS patients during follow-up), asthma (50% more often), hypertension (44% more common), malignancy (23% more common), diabetes (20% more common), and dyslipidemia (11% more common). All these incidence rates represented statistically significant increases in the AS patients, compared with the controls.
A related analysis reported by Dr. Walsh also used data from the Truven Health databases for a somewhat larger group of AS patients, 6,679 followed for 1 year after their AS diagnosis, and 19,951 matched controls. The AS patients had a significantly higher rate of hospital admissions – 12%, compared with 6% among the controls – and a significantly higher rate of emergency department visits, at 23%, compared with 15% among the controls. The AS patients also had double the rate of physician office visits and prescribed medications.
“Obviously, the AS patients are not as healthy,” Dr. Walsh said in an interview. “We adjusted for their comorbidities, but that did not affect the hospitalization rates. We need to look into this more; I don’t know why the AS patients are being hospitalized. Typically AS itself does not lead to hospitalization, so I suspect it’s because of comorbidities, or perhaps because of adverse events from treatment.”
Dr. Walsh is a consultant to AbbVie and Novartis.
On Twitter @mitchelzoler
DENVER – Evidence continues to mount that ankylosing spondylitis patients are at increased risk for developing various comorbidities, compared with the general adult population.
Patients newly diagnosed with ankylosing spondylitis (AS) had twice the rate of new-onset depression during the first 3 years following diagnosis, compared with matched people from the general population in a study of more than 21,000 American adults. Patients with newly diagnosed AS also had a 60% higher rate of developing a new cardiovascular disease, compared with the matched general population, Jessica A. Walsh, MD, said at the annual meeting of the Spondyloarthritis Research and Treatment Network.
“We need to figure out what to do about all the comorbidities. Rheumatologists need to either screen their AS patients for comorbidities, or they need to be sure their patients are plugged in with another physician who will screen them,” said Dr. Walsh, director of the spondyloarthritis clinic at the University of Utah in Salt Lake City.
Her analysis used data from the Truven Health MarketScan databases for U.S. patients covered by Medicare or commercial health insurance, and included 6,370 patients with newly diagnosed AS and 14,988 adults matched by age and sex. The analysis only included AS patients who were free from comorbidities during the 2 years prior to their AS diagnosis. The average age of the people in the study was 52 years, and 54% were men.
During an average follow-up of 2.9 years following initial AS diagnosis, the most common comorbidity among the AS patients was uveitis, which occurred nearly 15-fold more frequently among the AS patients than in the controls. Other common incident comorbidities included inflammatory bowel disease, nearly sixfold more common among the AS patients, and osteoporosis, which was nearly threefold more common during follow-up after AS diagnosis, compared with the controls.
Other comorbidities with increased incidence in the AS patients included sleep apnea (80% more common among the AS patients during follow-up), asthma (50% more often), hypertension (44% more common), malignancy (23% more common), diabetes (20% more common), and dyslipidemia (11% more common). All these incidence rates represented statistically significant increases in the AS patients, compared with the controls.
A related analysis reported by Dr. Walsh also used data from the Truven Health databases for a somewhat larger group of AS patients, 6,679 followed for 1 year after their AS diagnosis, and 19,951 matched controls. The AS patients had a significantly higher rate of hospital admissions – 12%, compared with 6% among the controls – and a significantly higher rate of emergency department visits, at 23%, compared with 15% among the controls. The AS patients also had double the rate of physician office visits and prescribed medications.
“Obviously, the AS patients are not as healthy,” Dr. Walsh said in an interview. “We adjusted for their comorbidities, but that did not affect the hospitalization rates. We need to look into this more; I don’t know why the AS patients are being hospitalized. Typically AS itself does not lead to hospitalization, so I suspect it’s because of comorbidities, or perhaps because of adverse events from treatment.”
Dr. Walsh is a consultant to AbbVie and Novartis.
On Twitter @mitchelzoler
AT THE 2016 SPARTAN ANNUAL MEETING
Key clinical point: In the 3 years after initial ankylosing spondylitis diagnosis, the incidence of several comorbidities rises significantly above those in the general population.
Major finding: The incidence of new-onset depression among newly diagnosed ankylosing spondylitis patients was twice as high as it was among matched controls.
Data source: Observational data collected by Truven Health MarketScan, with a total of 21,358 patients and controls.
Disclosures: Dr. Walsh is a consultant to AbbVie and Novartis.
BM transplants provide better quality of life

Photo by Chad McNeeley
Patients receiving hematopoietic stem cell transplants from unrelated donors have better quality of life if they receive cells derived from bone marrow (BM) rather than peripheral blood (PB), according to a large study.
Recipients of BM transplants reported better psychological well-being, had fewer symptoms of graft-versus-host disease (GVHD), and were more likely to be back at work 5 years after their transplant.
However, there was no significant difference in overall survival, treatment-related death, or relapse between patients who received BM grafts and those who received PB transplants.
Stephanie Lee, MD, of Fred Hutchinson Cancer Research Center in Seattle, Washington, and her colleagues reported these results in JAMA Oncology.
“We’re hoping that, once we provide information about long-term quality of life and recovery, patients and their doctors can take this into account when they’re planning their transplants,” Dr Lee said.
She noted, however, that the results would only be applicable to transplant patients who are similar to those enrolled in this study.
The study included 551 patients, ages 16 to 66, who were receiving transplants from unrelated donors to treat hematologic neoplasms. The patients were randomly assigned to receive PB or BM grafts.
From 6 months to 5 years after transplant, researchers called the patients periodically to assess how they were doing.
At the 5-year mark, 102 BM recipients and 93 PB recipients were still alive and eligible for assessment.
At a median follow-up of 73 months (range, 30-121 months), there was no significant difference in survival rate, relapse incidence, or treatment-related mortality between BM and PB recipients.
The survival rate was 40% for BM recipients and 39% for PB recipients (P=0.84). The relapse rates were 32% and 29%, respectively (P=0.47). And the treatment-related mortality rates were 29% and 32%, respectively (P=0.44).
However, BM recipients were more likely to report better psychological well-being, earning higher Mental Health Inventory Psychological Well-Being scores than PB recipients. The mean scores were 78.9 and 72.2, respectively (P=0.01).
In addition, BM recipients had fewer symptoms of GVHD, earning lower Lee Chronic GVHD symptom scores than PB recipients. The mean scores were 13.1 and 19.3, respectively (P=0.004).
The researchers suspected, but could not confirm, that BM recipients had better psychological well-being because they experienced fewer self-reported symptoms of chronic GVHD.
The researchers also found that BM recipients were more likely than PB recipients to be working full-time or part-time 5 years after transplant. When the team adjusted for work status before transplant, the odds ratio was 1.5 (P=0.002).
“Results of this study set bone marrow as the standard source of stem cells for transplantation from unrelated donors,” said study author Claudio Anasetti, MD, of Moffitt Cancer Center in Tampa, Florida.
“When both your disease and the recommended treatment are life-threatening, I don’t think people are necessarily asking, ‘Which treatment is going to give me better quality of life years from now?’” Dr Lee added.
“Yet, if you’re going to make it through, as many patients do, you want to do it with good quality of life. That’s the whole point of having the transplant. It’s not just to cure your disease but also to try to get back to as normal a lifestyle as you can.” ![]()

Photo by Chad McNeeley
Patients receiving hematopoietic stem cell transplants from unrelated donors have better quality of life if they receive cells derived from bone marrow (BM) rather than peripheral blood (PB), according to a large study.
Recipients of BM transplants reported better psychological well-being, had fewer symptoms of graft-versus-host disease (GVHD), and were more likely to be back at work 5 years after their transplant.
However, there was no significant difference in overall survival, treatment-related death, or relapse between patients who received BM grafts and those who received PB transplants.
Stephanie Lee, MD, of Fred Hutchinson Cancer Research Center in Seattle, Washington, and her colleagues reported these results in JAMA Oncology.
“We’re hoping that, once we provide information about long-term quality of life and recovery, patients and their doctors can take this into account when they’re planning their transplants,” Dr Lee said.
She noted, however, that the results would only be applicable to transplant patients who are similar to those enrolled in this study.
The study included 551 patients, ages 16 to 66, who were receiving transplants from unrelated donors to treat hematologic neoplasms. The patients were randomly assigned to receive PB or BM grafts.
From 6 months to 5 years after transplant, researchers called the patients periodically to assess how they were doing.
At the 5-year mark, 102 BM recipients and 93 PB recipients were still alive and eligible for assessment.
At a median follow-up of 73 months (range, 30-121 months), there was no significant difference in survival rate, relapse incidence, or treatment-related mortality between BM and PB recipients.
The survival rate was 40% for BM recipients and 39% for PB recipients (P=0.84). The relapse rates were 32% and 29%, respectively (P=0.47). And the treatment-related mortality rates were 29% and 32%, respectively (P=0.44).
However, BM recipients were more likely to report better psychological well-being, earning higher Mental Health Inventory Psychological Well-Being scores than PB recipients. The mean scores were 78.9 and 72.2, respectively (P=0.01).
In addition, BM recipients had fewer symptoms of GVHD, earning lower Lee Chronic GVHD symptom scores than PB recipients. The mean scores were 13.1 and 19.3, respectively (P=0.004).
The researchers suspected, but could not confirm, that BM recipients had better psychological well-being because they experienced fewer self-reported symptoms of chronic GVHD.
The researchers also found that BM recipients were more likely than PB recipients to be working full-time or part-time 5 years after transplant. When the team adjusted for work status before transplant, the odds ratio was 1.5 (P=0.002).
“Results of this study set bone marrow as the standard source of stem cells for transplantation from unrelated donors,” said study author Claudio Anasetti, MD, of Moffitt Cancer Center in Tampa, Florida.
“When both your disease and the recommended treatment are life-threatening, I don’t think people are necessarily asking, ‘Which treatment is going to give me better quality of life years from now?’” Dr Lee added.
“Yet, if you’re going to make it through, as many patients do, you want to do it with good quality of life. That’s the whole point of having the transplant. It’s not just to cure your disease but also to try to get back to as normal a lifestyle as you can.” ![]()

Photo by Chad McNeeley
Patients receiving hematopoietic stem cell transplants from unrelated donors have better quality of life if they receive cells derived from bone marrow (BM) rather than peripheral blood (PB), according to a large study.
Recipients of BM transplants reported better psychological well-being, had fewer symptoms of graft-versus-host disease (GVHD), and were more likely to be back at work 5 years after their transplant.
However, there was no significant difference in overall survival, treatment-related death, or relapse between patients who received BM grafts and those who received PB transplants.
Stephanie Lee, MD, of Fred Hutchinson Cancer Research Center in Seattle, Washington, and her colleagues reported these results in JAMA Oncology.
“We’re hoping that, once we provide information about long-term quality of life and recovery, patients and their doctors can take this into account when they’re planning their transplants,” Dr Lee said.
She noted, however, that the results would only be applicable to transplant patients who are similar to those enrolled in this study.
The study included 551 patients, ages 16 to 66, who were receiving transplants from unrelated donors to treat hematologic neoplasms. The patients were randomly assigned to receive PB or BM grafts.
From 6 months to 5 years after transplant, researchers called the patients periodically to assess how they were doing.
At the 5-year mark, 102 BM recipients and 93 PB recipients were still alive and eligible for assessment.
At a median follow-up of 73 months (range, 30-121 months), there was no significant difference in survival rate, relapse incidence, or treatment-related mortality between BM and PB recipients.
The survival rate was 40% for BM recipients and 39% for PB recipients (P=0.84). The relapse rates were 32% and 29%, respectively (P=0.47). And the treatment-related mortality rates were 29% and 32%, respectively (P=0.44).
However, BM recipients were more likely to report better psychological well-being, earning higher Mental Health Inventory Psychological Well-Being scores than PB recipients. The mean scores were 78.9 and 72.2, respectively (P=0.01).
In addition, BM recipients had fewer symptoms of GVHD, earning lower Lee Chronic GVHD symptom scores than PB recipients. The mean scores were 13.1 and 19.3, respectively (P=0.004).
The researchers suspected, but could not confirm, that BM recipients had better psychological well-being because they experienced fewer self-reported symptoms of chronic GVHD.
The researchers also found that BM recipients were more likely than PB recipients to be working full-time or part-time 5 years after transplant. When the team adjusted for work status before transplant, the odds ratio was 1.5 (P=0.002).
“Results of this study set bone marrow as the standard source of stem cells for transplantation from unrelated donors,” said study author Claudio Anasetti, MD, of Moffitt Cancer Center in Tampa, Florida.
“When both your disease and the recommended treatment are life-threatening, I don’t think people are necessarily asking, ‘Which treatment is going to give me better quality of life years from now?’” Dr Lee added.
“Yet, if you’re going to make it through, as many patients do, you want to do it with good quality of life. That’s the whole point of having the transplant. It’s not just to cure your disease but also to try to get back to as normal a lifestyle as you can.” ![]()
Assay can aid blood biomarker research

Photo by Graham Colm
A new assay can overcome a limitation in blood biomarker research, according to a study published in Scientific Reports.
Researchers noted that transcriptome sequencing of whole-blood RNA holds promise for the identification and tracking of biomarkers.
Unfortunately, the high globin mRNA (gmRNA) content of erythrocytes can be problematic, causing technical bias and leaving biologically relevant molecules undetectable.
With this in mind, the researchers developed an assay known as GlobinLock, which is designed to preserve RNA quality by reducing globin content.
“The globin reduction rate of GlobinLock is sufficient for any application,” said study author Kaarel Krjutškov, PhD, of Karolinska Institutet in Huddinge, Sweden.
“It reduces the globin prevalence from 63% . . . to 5%, which makes it an effective tool for biotechnology companies as an additive to their kits.”
GlobinLock consists of a pair of gmRNA-specific oligonucleotides that silence the majority of globin RNA molecules by highly specific binding.
The oligonucleotides are introduced to a purified RNA sample and, according to the researchers, are effective immediately after RNA denaturation.
“We show that globin locking is fully effective not only for human samples but also for widely used animal models, like mouse and rat, cow, dog, and even zebrafish,” said study author Juha Kere, MD, PhD, of Karolinska Institutet. ![]()

Photo by Graham Colm
A new assay can overcome a limitation in blood biomarker research, according to a study published in Scientific Reports.
Researchers noted that transcriptome sequencing of whole-blood RNA holds promise for the identification and tracking of biomarkers.
Unfortunately, the high globin mRNA (gmRNA) content of erythrocytes can be problematic, causing technical bias and leaving biologically relevant molecules undetectable.
With this in mind, the researchers developed an assay known as GlobinLock, which is designed to preserve RNA quality by reducing globin content.
“The globin reduction rate of GlobinLock is sufficient for any application,” said study author Kaarel Krjutškov, PhD, of Karolinska Institutet in Huddinge, Sweden.
“It reduces the globin prevalence from 63% . . . to 5%, which makes it an effective tool for biotechnology companies as an additive to their kits.”
GlobinLock consists of a pair of gmRNA-specific oligonucleotides that silence the majority of globin RNA molecules by highly specific binding.
The oligonucleotides are introduced to a purified RNA sample and, according to the researchers, are effective immediately after RNA denaturation.
“We show that globin locking is fully effective not only for human samples but also for widely used animal models, like mouse and rat, cow, dog, and even zebrafish,” said study author Juha Kere, MD, PhD, of Karolinska Institutet. ![]()

Photo by Graham Colm
A new assay can overcome a limitation in blood biomarker research, according to a study published in Scientific Reports.
Researchers noted that transcriptome sequencing of whole-blood RNA holds promise for the identification and tracking of biomarkers.
Unfortunately, the high globin mRNA (gmRNA) content of erythrocytes can be problematic, causing technical bias and leaving biologically relevant molecules undetectable.
With this in mind, the researchers developed an assay known as GlobinLock, which is designed to preserve RNA quality by reducing globin content.
“The globin reduction rate of GlobinLock is sufficient for any application,” said study author Kaarel Krjutškov, PhD, of Karolinska Institutet in Huddinge, Sweden.
“It reduces the globin prevalence from 63% . . . to 5%, which makes it an effective tool for biotechnology companies as an additive to their kits.”
GlobinLock consists of a pair of gmRNA-specific oligonucleotides that silence the majority of globin RNA molecules by highly specific binding.
The oligonucleotides are introduced to a purified RNA sample and, according to the researchers, are effective immediately after RNA denaturation.
“We show that globin locking is fully effective not only for human samples but also for widely used animal models, like mouse and rat, cow, dog, and even zebrafish,” said study author Juha Kere, MD, PhD, of Karolinska Institutet. ![]()
Drug can worsen allo-HSCT outcomes in ATLL

Photo by Chad McNeeley
Results of a large, retrospective study suggest that receiving mogamulizumab before allogeneic hematopoietic stem cell transplant (allo-HSCT) can worsen outcomes in patients with adult T-cell leukemia/lymphoma (ATLL).
Patients who received mogamulizumab had a higher risk of grade 3/4 acute graft-versus-host disease (GVHD), a higher incidence of nonrelapse mortality, and worse overall survival than patients who did not take the drug.
Researchers reported these findings in the Journal of Clinical Oncology.
Previous research suggested that pre-HSCT mogamulizumab can produce adverse effects, but these studies had small patient numbers. Researchers have suggested the adverse effects may occur because mogamulizumab depletes regulatory T cells for several months, but there has been no direct evidence supporting this idea.
To investigate the issue, Shigeo Fuji, MD, of National Cancer Center Hospital in Tokyo, Japan, and colleagues assessed the impact of pre-HSCT mogamulizumab in a large group of ATLL patients undergoing allo-HSCT.
The study included 996 patients age 70 and younger. Patients had aggressive ATLL diagnosed between 2000 and 2013. They received intensive chemotherapy as first-line treatment.
Eighty-two of the patients received mogamulizumab before HSCT, with a median of 45 days from the last mogamulizumab treatment to allo-HSCT.
Pre-HSCT mogamulizumab was associated with an increased risk of grade 3/4 acute GVHD, with a relative risk of 1.80 (P<0.01).
Patients who received mogamulizumab were also more likely to be refractory to the systemic corticosteroids given to treat acute GVHD, with a relative risk of 2.09 (P<0.01).
The 1-year cumulative incidence of nonrelapse mortality was significantly higher among patients who received mogamulizumab than among those who did not—43.7% and 25.1%, respectively (P<0.01).
And the probability of 1-year overall survival was significantly lower in patients who received mogamulizumab than in those who did not—32.3% and 49.4%, respectively (P<0.01).
The researchers noted that outcomes were particularly poor when patients received mogamulizumab within less than 50 days of allo-HSCT.
The team said this study appears to confirm that pre-HSCT mogamulizumab significantly worsens clinical outcomes, mainly because of an increased risk of severe/corticosteroid-refractory acute GVHD. And the results support the idea that the drug depletes regulatory T cells.
The researchers concluded that mogamulizumab should be used with caution in ATLL patients who are eligible for allo-HSCT. And the possibility of intensifying GVHD prophylaxis in patients who do receive pre-HSCT mogamulizumab should be explored. ![]()

Photo by Chad McNeeley
Results of a large, retrospective study suggest that receiving mogamulizumab before allogeneic hematopoietic stem cell transplant (allo-HSCT) can worsen outcomes in patients with adult T-cell leukemia/lymphoma (ATLL).
Patients who received mogamulizumab had a higher risk of grade 3/4 acute graft-versus-host disease (GVHD), a higher incidence of nonrelapse mortality, and worse overall survival than patients who did not take the drug.
Researchers reported these findings in the Journal of Clinical Oncology.
Previous research suggested that pre-HSCT mogamulizumab can produce adverse effects, but these studies had small patient numbers. Researchers have suggested the adverse effects may occur because mogamulizumab depletes regulatory T cells for several months, but there has been no direct evidence supporting this idea.
To investigate the issue, Shigeo Fuji, MD, of National Cancer Center Hospital in Tokyo, Japan, and colleagues assessed the impact of pre-HSCT mogamulizumab in a large group of ATLL patients undergoing allo-HSCT.
The study included 996 patients age 70 and younger. Patients had aggressive ATLL diagnosed between 2000 and 2013. They received intensive chemotherapy as first-line treatment.
Eighty-two of the patients received mogamulizumab before HSCT, with a median of 45 days from the last mogamulizumab treatment to allo-HSCT.
Pre-HSCT mogamulizumab was associated with an increased risk of grade 3/4 acute GVHD, with a relative risk of 1.80 (P<0.01).
Patients who received mogamulizumab were also more likely to be refractory to the systemic corticosteroids given to treat acute GVHD, with a relative risk of 2.09 (P<0.01).
The 1-year cumulative incidence of nonrelapse mortality was significantly higher among patients who received mogamulizumab than among those who did not—43.7% and 25.1%, respectively (P<0.01).
And the probability of 1-year overall survival was significantly lower in patients who received mogamulizumab than in those who did not—32.3% and 49.4%, respectively (P<0.01).
The researchers noted that outcomes were particularly poor when patients received mogamulizumab within less than 50 days of allo-HSCT.
The team said this study appears to confirm that pre-HSCT mogamulizumab significantly worsens clinical outcomes, mainly because of an increased risk of severe/corticosteroid-refractory acute GVHD. And the results support the idea that the drug depletes regulatory T cells.
The researchers concluded that mogamulizumab should be used with caution in ATLL patients who are eligible for allo-HSCT. And the possibility of intensifying GVHD prophylaxis in patients who do receive pre-HSCT mogamulizumab should be explored. ![]()

Photo by Chad McNeeley
Results of a large, retrospective study suggest that receiving mogamulizumab before allogeneic hematopoietic stem cell transplant (allo-HSCT) can worsen outcomes in patients with adult T-cell leukemia/lymphoma (ATLL).
Patients who received mogamulizumab had a higher risk of grade 3/4 acute graft-versus-host disease (GVHD), a higher incidence of nonrelapse mortality, and worse overall survival than patients who did not take the drug.
Researchers reported these findings in the Journal of Clinical Oncology.
Previous research suggested that pre-HSCT mogamulizumab can produce adverse effects, but these studies had small patient numbers. Researchers have suggested the adverse effects may occur because mogamulizumab depletes regulatory T cells for several months, but there has been no direct evidence supporting this idea.
To investigate the issue, Shigeo Fuji, MD, of National Cancer Center Hospital in Tokyo, Japan, and colleagues assessed the impact of pre-HSCT mogamulizumab in a large group of ATLL patients undergoing allo-HSCT.
The study included 996 patients age 70 and younger. Patients had aggressive ATLL diagnosed between 2000 and 2013. They received intensive chemotherapy as first-line treatment.
Eighty-two of the patients received mogamulizumab before HSCT, with a median of 45 days from the last mogamulizumab treatment to allo-HSCT.
Pre-HSCT mogamulizumab was associated with an increased risk of grade 3/4 acute GVHD, with a relative risk of 1.80 (P<0.01).
Patients who received mogamulizumab were also more likely to be refractory to the systemic corticosteroids given to treat acute GVHD, with a relative risk of 2.09 (P<0.01).
The 1-year cumulative incidence of nonrelapse mortality was significantly higher among patients who received mogamulizumab than among those who did not—43.7% and 25.1%, respectively (P<0.01).
And the probability of 1-year overall survival was significantly lower in patients who received mogamulizumab than in those who did not—32.3% and 49.4%, respectively (P<0.01).
The researchers noted that outcomes were particularly poor when patients received mogamulizumab within less than 50 days of allo-HSCT.
The team said this study appears to confirm that pre-HSCT mogamulizumab significantly worsens clinical outcomes, mainly because of an increased risk of severe/corticosteroid-refractory acute GVHD. And the results support the idea that the drug depletes regulatory T cells.
The researchers concluded that mogamulizumab should be used with caution in ATLL patients who are eligible for allo-HSCT. And the possibility of intensifying GVHD prophylaxis in patients who do receive pre-HSCT mogamulizumab should be explored. ![]()
Mortality rates higher among influenza B patients than influenza A patients
Influenza-attributable mortality was significantly greater in children with influenza B, compared with influenza A, investigators found.
Among those with influenza B, patients aged 10-16 years were most likely to require ICU admission, suggesting this subpopulation may be a target for immunization programs.
The percentage of clinical cases attributed to influenza B range from less than 1% to 44%, according to data published by the Centers for Disease Control and Prevention. However, influenza B is considered less virulent and less capable of causing pandemics and has therefore been less studied and outcomes of its disease less characterized, Dat Tran, MD, MSc, of the Hospital for Sick Children in Canada and his associates reported (Pediatrics. 2016 August. doi: 10.1542/peds.2015-4643).
The purpose of this study was to further understand the prevalence and severity of influenza B cases in comparison with influenza A and to identify pediatric subpopulations most at risk for contracting influenza B.
Children aged 16 years or younger hospitalized from laboratory-confirmed influenza A or B from September 2004 to June 2013 (excluding the pandemic year 2009-2010) were identified through active surveillance of admissions at the 12 pediatric referral centers of the Canadian Immunization Monitoring Program Active (IMPACT), a national surveillance initiative. Information regarding demographics, health status, vaccination status, presenting signs and symptoms, illness severity and mortality, treatment regimens, and ICU admission were collected and analyzed.
Of 4,155 influenza-related admissions during this time period, influenza B accounted for 1,510 (36.3%) cases and influenza A accounted for 2,645 (63.7%) cases.
Children admitted with influenza B tended to be older with a median age 3.9 years (interquartile range, 1.4-7.2), compared with a median of 2 years (IQR, 0.6-4.8 years) for children admitted with influenza A.
Children admitted with influenza B, compared with influenza A, had higher odds of having a vaccine-indicated condition (odds ratio, 1.30; 95% confidence interval, 1.14-1.47) and lower odds of having no underlying medical condition (OR, 0.80; 95% CI, 0.71-0.91), Dr. Tran and his associates reported.
“Compared with influenza A cases, children admitted with influenza B had greater adjusted odds of presenting with headache, abdominal pain, and myalgia, ranging from 1.38 for abdominal pain to 3.19 for myalgia,” they added. “There were no significant differences in antiviral or antibiotic prescription or use between influenza A and B cases.”
There was no significant difference in the proportion of influenza A or B patients admitted to the ICU (12.7% vs. 12.6%). Rather, multivariate modeling identified age and presence of an underlying condition as independent predictors of ICU admission.
Finally, influenza-attributable mortality was significantly greater in children with influenza B (adjusted OR, 2.65; 95% CI, 1.18-5.94). Influenza-attributable mortality occurred in 16 (1.1%) children with influenza B and only 10 (0.4%) children with influenza A. All-cause mortality followed a similar trend.
“Among hospitalized children, influenza A and B infections resulted in similar morbidity while mortality was greater for influenza B disease. Among healthy children hospitalized with influenza B, those aged 10-16 years were most likely to require ICU admission,” the investigators summarized.
“These children should be considered at high risk for complicated influenza B infection and be specifically targeted by immunization programs to receive influenza vaccination, and in particular, a [quadrivalent influenza vaccine],” they recommended.
This study was funded by GlaxoSmithKline Biologicals SA. The Canadian Immunization Monitoring Program Active is funded by the Public Health Agency of Canada. The investigators reported having no relevant disclosures.
Influenza-attributable mortality was significantly greater in children with influenza B, compared with influenza A, investigators found.
Among those with influenza B, patients aged 10-16 years were most likely to require ICU admission, suggesting this subpopulation may be a target for immunization programs.
The percentage of clinical cases attributed to influenza B range from less than 1% to 44%, according to data published by the Centers for Disease Control and Prevention. However, influenza B is considered less virulent and less capable of causing pandemics and has therefore been less studied and outcomes of its disease less characterized, Dat Tran, MD, MSc, of the Hospital for Sick Children in Canada and his associates reported (Pediatrics. 2016 August. doi: 10.1542/peds.2015-4643).
The purpose of this study was to further understand the prevalence and severity of influenza B cases in comparison with influenza A and to identify pediatric subpopulations most at risk for contracting influenza B.
Children aged 16 years or younger hospitalized from laboratory-confirmed influenza A or B from September 2004 to June 2013 (excluding the pandemic year 2009-2010) were identified through active surveillance of admissions at the 12 pediatric referral centers of the Canadian Immunization Monitoring Program Active (IMPACT), a national surveillance initiative. Information regarding demographics, health status, vaccination status, presenting signs and symptoms, illness severity and mortality, treatment regimens, and ICU admission were collected and analyzed.
Of 4,155 influenza-related admissions during this time period, influenza B accounted for 1,510 (36.3%) cases and influenza A accounted for 2,645 (63.7%) cases.
Children admitted with influenza B tended to be older with a median age 3.9 years (interquartile range, 1.4-7.2), compared with a median of 2 years (IQR, 0.6-4.8 years) for children admitted with influenza A.
Children admitted with influenza B, compared with influenza A, had higher odds of having a vaccine-indicated condition (odds ratio, 1.30; 95% confidence interval, 1.14-1.47) and lower odds of having no underlying medical condition (OR, 0.80; 95% CI, 0.71-0.91), Dr. Tran and his associates reported.
“Compared with influenza A cases, children admitted with influenza B had greater adjusted odds of presenting with headache, abdominal pain, and myalgia, ranging from 1.38 for abdominal pain to 3.19 for myalgia,” they added. “There were no significant differences in antiviral or antibiotic prescription or use between influenza A and B cases.”
There was no significant difference in the proportion of influenza A or B patients admitted to the ICU (12.7% vs. 12.6%). Rather, multivariate modeling identified age and presence of an underlying condition as independent predictors of ICU admission.
Finally, influenza-attributable mortality was significantly greater in children with influenza B (adjusted OR, 2.65; 95% CI, 1.18-5.94). Influenza-attributable mortality occurred in 16 (1.1%) children with influenza B and only 10 (0.4%) children with influenza A. All-cause mortality followed a similar trend.
“Among hospitalized children, influenza A and B infections resulted in similar morbidity while mortality was greater for influenza B disease. Among healthy children hospitalized with influenza B, those aged 10-16 years were most likely to require ICU admission,” the investigators summarized.
“These children should be considered at high risk for complicated influenza B infection and be specifically targeted by immunization programs to receive influenza vaccination, and in particular, a [quadrivalent influenza vaccine],” they recommended.
This study was funded by GlaxoSmithKline Biologicals SA. The Canadian Immunization Monitoring Program Active is funded by the Public Health Agency of Canada. The investigators reported having no relevant disclosures.
Influenza-attributable mortality was significantly greater in children with influenza B, compared with influenza A, investigators found.
Among those with influenza B, patients aged 10-16 years were most likely to require ICU admission, suggesting this subpopulation may be a target for immunization programs.
The percentage of clinical cases attributed to influenza B range from less than 1% to 44%, according to data published by the Centers for Disease Control and Prevention. However, influenza B is considered less virulent and less capable of causing pandemics and has therefore been less studied and outcomes of its disease less characterized, Dat Tran, MD, MSc, of the Hospital for Sick Children in Canada and his associates reported (Pediatrics. 2016 August. doi: 10.1542/peds.2015-4643).
The purpose of this study was to further understand the prevalence and severity of influenza B cases in comparison with influenza A and to identify pediatric subpopulations most at risk for contracting influenza B.
Children aged 16 years or younger hospitalized from laboratory-confirmed influenza A or B from September 2004 to June 2013 (excluding the pandemic year 2009-2010) were identified through active surveillance of admissions at the 12 pediatric referral centers of the Canadian Immunization Monitoring Program Active (IMPACT), a national surveillance initiative. Information regarding demographics, health status, vaccination status, presenting signs and symptoms, illness severity and mortality, treatment regimens, and ICU admission were collected and analyzed.
Of 4,155 influenza-related admissions during this time period, influenza B accounted for 1,510 (36.3%) cases and influenza A accounted for 2,645 (63.7%) cases.
Children admitted with influenza B tended to be older with a median age 3.9 years (interquartile range, 1.4-7.2), compared with a median of 2 years (IQR, 0.6-4.8 years) for children admitted with influenza A.
Children admitted with influenza B, compared with influenza A, had higher odds of having a vaccine-indicated condition (odds ratio, 1.30; 95% confidence interval, 1.14-1.47) and lower odds of having no underlying medical condition (OR, 0.80; 95% CI, 0.71-0.91), Dr. Tran and his associates reported.
“Compared with influenza A cases, children admitted with influenza B had greater adjusted odds of presenting with headache, abdominal pain, and myalgia, ranging from 1.38 for abdominal pain to 3.19 for myalgia,” they added. “There were no significant differences in antiviral or antibiotic prescription or use between influenza A and B cases.”
There was no significant difference in the proportion of influenza A or B patients admitted to the ICU (12.7% vs. 12.6%). Rather, multivariate modeling identified age and presence of an underlying condition as independent predictors of ICU admission.
Finally, influenza-attributable mortality was significantly greater in children with influenza B (adjusted OR, 2.65; 95% CI, 1.18-5.94). Influenza-attributable mortality occurred in 16 (1.1%) children with influenza B and only 10 (0.4%) children with influenza A. All-cause mortality followed a similar trend.
“Among hospitalized children, influenza A and B infections resulted in similar morbidity while mortality was greater for influenza B disease. Among healthy children hospitalized with influenza B, those aged 10-16 years were most likely to require ICU admission,” the investigators summarized.
“These children should be considered at high risk for complicated influenza B infection and be specifically targeted by immunization programs to receive influenza vaccination, and in particular, a [quadrivalent influenza vaccine],” they recommended.
This study was funded by GlaxoSmithKline Biologicals SA. The Canadian Immunization Monitoring Program Active is funded by the Public Health Agency of Canada. The investigators reported having no relevant disclosures.
FROM PEDIATRICS
Key clinical point: Influenza-attributable mortality was significantly greater in children with influenza B, compared with influenza A.
Major finding: Influenza-attributable mortality occurred in 16 (1.1%) children with influenza B and only 10 (0.4%) children with influenza A. Influenza-attributable mortality was significantly greater in children with influenza B (adjusted odds ratio, 2.65, 95% confidence interval, 1.18-5.94).
Data source: An observational study of 4,155 children admitted to the hospital with influenza A or B during nonpandemic years between September 2004 and June 2013.
Disclosures: This study was funded by GlaxoSmithKline Biologicals SA. The Canadian Immunization Monitoring Program Active is funded by the Public Health Agency of Canada. The investigators reported having no relevant disclosures.
Providing Effective Palliative Care in the Era of Value
Although effective palliative care has always been a must-have for patients and caregivers facing serious illness, it hasn’t always been readily available. With the emergence of value-based healthcare models—and their potent incentives to reduce avoidable readmissions—there is renewed hope that such care will be accessible to those who need it.
Palliative and end-of-life care have long been promoted as core skills for hospitalists. The topic has regularly been included at SHM annual meetings and other prominent hospital medicine conferences, in the American Board of Internal Medicine blueprint for recognition of focused practice in hospital medicine, and in a number of influential references for hospitalists. Still, as I look at hospitalist programs around the country, there is a clear need to improve hospitalists’ delivery of palliative and end-of-life care.
Care of patients with chronic illness in their last two years of life accounts for a third of all Medicare spending.1 As hospitalists, we encounter many of these patients as they are hospitalized—and often re-hospitalized. Palliative care, which can improve quality of life and decrease costs for patients while leading to increased satisfaction and better outcomes for caregivers, can help alleviate unneeded and unwanted aggressive interventions like hospitalization.2,3
In its 2014 report, Dying in America, the Institute of Medicine (IOM) identified several areas for improvement, including better advance care planning and payment systems supporting high quality end-of-life care.4 As I write this column in mid 2016, there are two notable achievements since the IOM report: two E&M codes for advance care planning and a substantial and growing number of hospitalist patients in alternative payment models like bundled payments or ACOs.5 I believe we are entering a time when the availability of good palliative care will be accelerated due to broader forces in healthcare that for the first time align incentives between patients’ wishes and how care is paid for.
Palliative Care Skills for Hospitalists
The following are key actions for physicians in addressing palliative care for the hospitalized patient. At the risk of oversimplifying the discipline, I offer a few key actions for hospitalists to keep in mind.
Identify patients who would benefit from palliative care. The surprise question—“Would I be surprised if this patient died in the next year?”—has the ability to predict which patients would benefit from palliative care. In one observation from a group of patients with cancer, a “no” answer identified 60% of patients who died within a year.6 The surprise question has previously been shown to be predictive in other cancer and non-cancer populations.7,8
Weisman and Meier suggest using the following in a checklist at the time of hospital admission as “primary criteria to screen for unmet palliative care needs”:9
- The surprise question
- Frequent admissions
- Admission prompted by difficult-to-control physical or psychological symptoms
- Complex care requirements
- Decline in function, feeding intolerance, or unintended decline in weight
Hold a “goals of care” meeting. A notable step forward for supporting conversations between physicians and patients occurred on Jan. 1, when the Centers for Medicare & Medicaid Services (CMS) announced the Advance Care Planning E&M codes. These are CPT codes 99497 and 99498. They can be used on the same day as other E&M codes and cover discussions regarding advance care planning issues including discussing advance directives, appointing a healthcare proxy or durable power of attorney, discussing a living will, or addressing orders for life-sustaining treatment like the role of hydration or future hospitalizations. (For more information on how to use them, visit the CMS website and search for the FAQ.)
What should hospitalists concentrate on when having “goals of care” conversations with patients and caregivers? Ariadne Labs, a Harvard-affiliated health innovation group, offers the following as elements of a serious illness conversation:10
- Patients’ understanding of their illness
- Patients’ preferences for information and for family involvement
- Personal life goals, fears, and anxieties
- Trade-offs they are willing to accept
For hospitalists, an important area to pay particular attention to is the role of future hospitalizations in patients’ wishes for care, as some patients, if offered appropriate symptom control, would prefer to remain at home.
Two other crucial elements of inpatient palliative care—offer psychosocial support and symptom relief and hand off patient to effective post-hospital palliative care—are outside the scope of this article. However, they should be kept in mind and, of course, applied.
Understand the role of the palliative care consultation. Busy hospitalists might reasonably think, “I simply don’t have time to address palliative care in patients who aren’t likely to die during this hospitalization or soon after.” The palliative care consult service, if available, should be accessed when patients are identified as palliative care candidates but the primary hospitalist does not have the time or resources—including specialized knowledge in some cases—to deliver adequate palliative care. Palliative care specialists can also help bridge the gap between inpatient and outpatient palliative care resources.
In sum, the move to value-based payment models and the new advance care planning E&M codes provide a renewed focus—with more aligned incentives—and the opportunity to provide good palliative care to all who need it.
For hospitalists, identifying those who would benefit from palliative care and working with the healthcare team to ensure the care is delivered are at the heart of our professional mission. TH
References
- End-of-life care. The Darmouth Atlas of Health Care website. Accessed June 23, 2016.
- Gade G, Venohr I, Conner D, et al. Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med. 2008;11(2):180-190.
- Morrison RS, Penrod JD, Cassel JB, et al. Cost savings associated with US hospital palliative care consultation programs. Arch Int Med. 2008;168(16):1783-1790.
- Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences near the End of Life. 2014.
- BPCI Model 2: Retrospective acute & post acute care episode. Centers for Medicare & Medicaid Services website. Accessed June 24, 2016.
- Vick JB, Pertsch N, Hutchings M, et al. The utility of the surprise question in identifying patients most at risk of death. J Clin Oncol. 2015;33(suppl):8.
- Moss AH, Ganjoo J, Sharma S, et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3:1379-1384.
- Moss AH, Lunney JR, Culp S, et al. Prognostic significance of the “surprise” question in cancer patients. J Palliat Med. 2010;13(7):837-840.
- Weissman D, Meier C. Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17-23.
- Serious illness care resources. Ariadne Labs website. Accessed June 24, 2016.
Although effective palliative care has always been a must-have for patients and caregivers facing serious illness, it hasn’t always been readily available. With the emergence of value-based healthcare models—and their potent incentives to reduce avoidable readmissions—there is renewed hope that such care will be accessible to those who need it.
Palliative and end-of-life care have long been promoted as core skills for hospitalists. The topic has regularly been included at SHM annual meetings and other prominent hospital medicine conferences, in the American Board of Internal Medicine blueprint for recognition of focused practice in hospital medicine, and in a number of influential references for hospitalists. Still, as I look at hospitalist programs around the country, there is a clear need to improve hospitalists’ delivery of palliative and end-of-life care.
Care of patients with chronic illness in their last two years of life accounts for a third of all Medicare spending.1 As hospitalists, we encounter many of these patients as they are hospitalized—and often re-hospitalized. Palliative care, which can improve quality of life and decrease costs for patients while leading to increased satisfaction and better outcomes for caregivers, can help alleviate unneeded and unwanted aggressive interventions like hospitalization.2,3
In its 2014 report, Dying in America, the Institute of Medicine (IOM) identified several areas for improvement, including better advance care planning and payment systems supporting high quality end-of-life care.4 As I write this column in mid 2016, there are two notable achievements since the IOM report: two E&M codes for advance care planning and a substantial and growing number of hospitalist patients in alternative payment models like bundled payments or ACOs.5 I believe we are entering a time when the availability of good palliative care will be accelerated due to broader forces in healthcare that for the first time align incentives between patients’ wishes and how care is paid for.
Palliative Care Skills for Hospitalists
The following are key actions for physicians in addressing palliative care for the hospitalized patient. At the risk of oversimplifying the discipline, I offer a few key actions for hospitalists to keep in mind.
Identify patients who would benefit from palliative care. The surprise question—“Would I be surprised if this patient died in the next year?”—has the ability to predict which patients would benefit from palliative care. In one observation from a group of patients with cancer, a “no” answer identified 60% of patients who died within a year.6 The surprise question has previously been shown to be predictive in other cancer and non-cancer populations.7,8
Weisman and Meier suggest using the following in a checklist at the time of hospital admission as “primary criteria to screen for unmet palliative care needs”:9
- The surprise question
- Frequent admissions
- Admission prompted by difficult-to-control physical or psychological symptoms
- Complex care requirements
- Decline in function, feeding intolerance, or unintended decline in weight
Hold a “goals of care” meeting. A notable step forward for supporting conversations between physicians and patients occurred on Jan. 1, when the Centers for Medicare & Medicaid Services (CMS) announced the Advance Care Planning E&M codes. These are CPT codes 99497 and 99498. They can be used on the same day as other E&M codes and cover discussions regarding advance care planning issues including discussing advance directives, appointing a healthcare proxy or durable power of attorney, discussing a living will, or addressing orders for life-sustaining treatment like the role of hydration or future hospitalizations. (For more information on how to use them, visit the CMS website and search for the FAQ.)
What should hospitalists concentrate on when having “goals of care” conversations with patients and caregivers? Ariadne Labs, a Harvard-affiliated health innovation group, offers the following as elements of a serious illness conversation:10
- Patients’ understanding of their illness
- Patients’ preferences for information and for family involvement
- Personal life goals, fears, and anxieties
- Trade-offs they are willing to accept
For hospitalists, an important area to pay particular attention to is the role of future hospitalizations in patients’ wishes for care, as some patients, if offered appropriate symptom control, would prefer to remain at home.
Two other crucial elements of inpatient palliative care—offer psychosocial support and symptom relief and hand off patient to effective post-hospital palliative care—are outside the scope of this article. However, they should be kept in mind and, of course, applied.
Understand the role of the palliative care consultation. Busy hospitalists might reasonably think, “I simply don’t have time to address palliative care in patients who aren’t likely to die during this hospitalization or soon after.” The palliative care consult service, if available, should be accessed when patients are identified as palliative care candidates but the primary hospitalist does not have the time or resources—including specialized knowledge in some cases—to deliver adequate palliative care. Palliative care specialists can also help bridge the gap between inpatient and outpatient palliative care resources.
In sum, the move to value-based payment models and the new advance care planning E&M codes provide a renewed focus—with more aligned incentives—and the opportunity to provide good palliative care to all who need it.
For hospitalists, identifying those who would benefit from palliative care and working with the healthcare team to ensure the care is delivered are at the heart of our professional mission. TH
References
- End-of-life care. The Darmouth Atlas of Health Care website. Accessed June 23, 2016.
- Gade G, Venohr I, Conner D, et al. Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med. 2008;11(2):180-190.
- Morrison RS, Penrod JD, Cassel JB, et al. Cost savings associated with US hospital palliative care consultation programs. Arch Int Med. 2008;168(16):1783-1790.
- Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences near the End of Life. 2014.
- BPCI Model 2: Retrospective acute & post acute care episode. Centers for Medicare & Medicaid Services website. Accessed June 24, 2016.
- Vick JB, Pertsch N, Hutchings M, et al. The utility of the surprise question in identifying patients most at risk of death. J Clin Oncol. 2015;33(suppl):8.
- Moss AH, Ganjoo J, Sharma S, et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3:1379-1384.
- Moss AH, Lunney JR, Culp S, et al. Prognostic significance of the “surprise” question in cancer patients. J Palliat Med. 2010;13(7):837-840.
- Weissman D, Meier C. Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17-23.
- Serious illness care resources. Ariadne Labs website. Accessed June 24, 2016.
Although effective palliative care has always been a must-have for patients and caregivers facing serious illness, it hasn’t always been readily available. With the emergence of value-based healthcare models—and their potent incentives to reduce avoidable readmissions—there is renewed hope that such care will be accessible to those who need it.
Palliative and end-of-life care have long been promoted as core skills for hospitalists. The topic has regularly been included at SHM annual meetings and other prominent hospital medicine conferences, in the American Board of Internal Medicine blueprint for recognition of focused practice in hospital medicine, and in a number of influential references for hospitalists. Still, as I look at hospitalist programs around the country, there is a clear need to improve hospitalists’ delivery of palliative and end-of-life care.
Care of patients with chronic illness in their last two years of life accounts for a third of all Medicare spending.1 As hospitalists, we encounter many of these patients as they are hospitalized—and often re-hospitalized. Palliative care, which can improve quality of life and decrease costs for patients while leading to increased satisfaction and better outcomes for caregivers, can help alleviate unneeded and unwanted aggressive interventions like hospitalization.2,3
In its 2014 report, Dying in America, the Institute of Medicine (IOM) identified several areas for improvement, including better advance care planning and payment systems supporting high quality end-of-life care.4 As I write this column in mid 2016, there are two notable achievements since the IOM report: two E&M codes for advance care planning and a substantial and growing number of hospitalist patients in alternative payment models like bundled payments or ACOs.5 I believe we are entering a time when the availability of good palliative care will be accelerated due to broader forces in healthcare that for the first time align incentives between patients’ wishes and how care is paid for.
Palliative Care Skills for Hospitalists
The following are key actions for physicians in addressing palliative care for the hospitalized patient. At the risk of oversimplifying the discipline, I offer a few key actions for hospitalists to keep in mind.
Identify patients who would benefit from palliative care. The surprise question—“Would I be surprised if this patient died in the next year?”—has the ability to predict which patients would benefit from palliative care. In one observation from a group of patients with cancer, a “no” answer identified 60% of patients who died within a year.6 The surprise question has previously been shown to be predictive in other cancer and non-cancer populations.7,8
Weisman and Meier suggest using the following in a checklist at the time of hospital admission as “primary criteria to screen for unmet palliative care needs”:9
- The surprise question
- Frequent admissions
- Admission prompted by difficult-to-control physical or psychological symptoms
- Complex care requirements
- Decline in function, feeding intolerance, or unintended decline in weight
Hold a “goals of care” meeting. A notable step forward for supporting conversations between physicians and patients occurred on Jan. 1, when the Centers for Medicare & Medicaid Services (CMS) announced the Advance Care Planning E&M codes. These are CPT codes 99497 and 99498. They can be used on the same day as other E&M codes and cover discussions regarding advance care planning issues including discussing advance directives, appointing a healthcare proxy or durable power of attorney, discussing a living will, or addressing orders for life-sustaining treatment like the role of hydration or future hospitalizations. (For more information on how to use them, visit the CMS website and search for the FAQ.)
What should hospitalists concentrate on when having “goals of care” conversations with patients and caregivers? Ariadne Labs, a Harvard-affiliated health innovation group, offers the following as elements of a serious illness conversation:10
- Patients’ understanding of their illness
- Patients’ preferences for information and for family involvement
- Personal life goals, fears, and anxieties
- Trade-offs they are willing to accept
For hospitalists, an important area to pay particular attention to is the role of future hospitalizations in patients’ wishes for care, as some patients, if offered appropriate symptom control, would prefer to remain at home.
Two other crucial elements of inpatient palliative care—offer psychosocial support and symptom relief and hand off patient to effective post-hospital palliative care—are outside the scope of this article. However, they should be kept in mind and, of course, applied.
Understand the role of the palliative care consultation. Busy hospitalists might reasonably think, “I simply don’t have time to address palliative care in patients who aren’t likely to die during this hospitalization or soon after.” The palliative care consult service, if available, should be accessed when patients are identified as palliative care candidates but the primary hospitalist does not have the time or resources—including specialized knowledge in some cases—to deliver adequate palliative care. Palliative care specialists can also help bridge the gap between inpatient and outpatient palliative care resources.
In sum, the move to value-based payment models and the new advance care planning E&M codes provide a renewed focus—with more aligned incentives—and the opportunity to provide good palliative care to all who need it.
For hospitalists, identifying those who would benefit from palliative care and working with the healthcare team to ensure the care is delivered are at the heart of our professional mission. TH
References
- End-of-life care. The Darmouth Atlas of Health Care website. Accessed June 23, 2016.
- Gade G, Venohr I, Conner D, et al. Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med. 2008;11(2):180-190.
- Morrison RS, Penrod JD, Cassel JB, et al. Cost savings associated with US hospital palliative care consultation programs. Arch Int Med. 2008;168(16):1783-1790.
- Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences near the End of Life. 2014.
- BPCI Model 2: Retrospective acute & post acute care episode. Centers for Medicare & Medicaid Services website. Accessed June 24, 2016.
- Vick JB, Pertsch N, Hutchings M, et al. The utility of the surprise question in identifying patients most at risk of death. J Clin Oncol. 2015;33(suppl):8.
- Moss AH, Ganjoo J, Sharma S, et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3:1379-1384.
- Moss AH, Lunney JR, Culp S, et al. Prognostic significance of the “surprise” question in cancer patients. J Palliat Med. 2010;13(7):837-840.
- Weissman D, Meier C. Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17-23.
- Serious illness care resources. Ariadne Labs website. Accessed June 24, 2016.
5 tips to surviving Meaningful Use audits
The Meaningful Use (MU) program as doctors know it may soon be ending, but audits of past attestations are slated to continue for at least the next 6 years.
Experts offer the following guidance on how to successfully manage audits and subsequent appeals.
1. Expect an audit.
Assume that at some point, you will be audited, said Joshua J. Freemire, a health law attorney based in Baltimore who specializes in regulatory compliance matters.
“Have good procedures in place to ensure the audit is brought to the attention of management and responded to promptly,” Mr. Freemire said in an interview. “Similarly, it is important that practices understand, in advance, how information created by their EHR is stored and managed.”
Audits are not limited to the current or most recent year, he noted. Being able to promptly access the appropriate information is an important part of drafting appropriate responses.
2. Save records
Keep all relevant records electronically for at least 6 years after attestation, advised Carmiña Nitzki, a senior consultant with GE Healthcare Camden Group.
Some documentation, such as your security risk analysis, should be maintained in paper form, she added. It is also helpful to take screen shots of certain measures that address the functionality of your system.
Records should follow when a physician moves a practice or changes employment. Ms. Nitzki recently assisted a case in which an early adopter physician dissolved his practice, became employed, and moved offices. His 2011 attestation was later audited, and he did not have all of the requested information. The doctor came to Ms. Nitzki after failing an audit and being assessed an $18,000 recoupment payment. Together, they were able to recreate and locate much of the necessary records through old emails and past documentation.
“We won the appeal,” said Ms. Nitzki, who spoke about meaningful use audits at a recent American Bar Association meeting. “The takeaway is if you’re going to respond to an audit, make sure you understand everything that they’re asking for and that you’re confident that you’re responding with all of the appropriate documentation,” she said.
3. Meet deadlines
Promptly responding to audits and quickly addressing failed audits are critical, Mr. Freemire said.
“The [MU] program is very strict with regard to deadlines, and an appeal cannot be filed once the applicable deadline has passed,” he said.
Mr. Freemire said that he routinely encounters health providers who have missed deadlines or let too much time lapse after being contacted by the government. Failure to meet deadlines can result from various reasons, including that notices are not promptly brought to the physician’s attention, delays investigating the availability of requested materials, or a false assumption that the requested information is readily at hand, he said.
Ensure that all audit deadlines are recorded and work with staff and leadership to file responses on time. Factor in additional time if there could be difficulty in locating records, experts stressed.
“I see difficulty in effectively responding to audits where records are simply not maintained,” he said. “Again, this can happen for many reasons, including personnel turnover, but it can be difficult or impossible to effectively respond to audit requests” in this situation.
4. Appeal effectively
Swiftly and thoroughly appeal a failed audit.
When a doctor fails an audit, it’s usually because of incomplete responses or missing information, Ms. Nitzki said. Only those measures that were rejected or that failed to meet standards need to be addressed during an appeal. Sending a cover letter to CMS with the appeal, explaining why the doctor may have failed initially can strengthen the case, she said.
“The appeals process is done directly with CMS, so they’re a little more reasonable,” she said. “They tend to look at things on broader scale.”
Don’t wait to appeal, Mr. Freemire said. Practices have 30 days from the date of an adverse audit determination letter to submit an appeal. If time runs out, physicians must pay back any money requested by auditors, and CMS expects the recoupment in full.
“Following a negative decision, the most important step is prompt attention,” he said. “The sooner that process begins, the sooner it can be completed. The time line for audit appeals is not generous and providers need to ensure they react promptly to a negative finding.”
5. Get help
Ms. Nitzki recommends that physicians obtain professional assistance when responding to an audit.
Practices can hire a consulting company or find audit resources from their specialty society or state medical association. The American Academy of Family Physicians provides a tip sheet on responding to audits. Some state organizations, such as the Texas Medical Association, offer a helpline for doctors to call and ask questions related to meaningful use.
“Unless they have a very savvy person in their office who has taken care all of their meaningful use attestations and documentation, we recommend they get help because it’s in the details where they could fail,” she said.
Seeking help after a failed audit is also essential, notes Mr. Freemire. Experts who are familiar with the program and its requirements and procedures can be of great assistance when it comes organizing the facts into a persuasive argument, he said.
“A cost/benefit is necessary – as it is with all service providers,” he said. “But a provider’s chances for a successful appeal are increased when that appeal is prepared by someone who understands the history and intention behind program requirements, who can best identify and present the necessary evidence of compliance, and who can make a compelling argument as to the provider’s satisfaction of program requirements.”
On Twitter @legal_med
The Meaningful Use (MU) program as doctors know it may soon be ending, but audits of past attestations are slated to continue for at least the next 6 years.
Experts offer the following guidance on how to successfully manage audits and subsequent appeals.
1. Expect an audit.
Assume that at some point, you will be audited, said Joshua J. Freemire, a health law attorney based in Baltimore who specializes in regulatory compliance matters.
“Have good procedures in place to ensure the audit is brought to the attention of management and responded to promptly,” Mr. Freemire said in an interview. “Similarly, it is important that practices understand, in advance, how information created by their EHR is stored and managed.”
Audits are not limited to the current or most recent year, he noted. Being able to promptly access the appropriate information is an important part of drafting appropriate responses.
2. Save records
Keep all relevant records electronically for at least 6 years after attestation, advised Carmiña Nitzki, a senior consultant with GE Healthcare Camden Group.
Some documentation, such as your security risk analysis, should be maintained in paper form, she added. It is also helpful to take screen shots of certain measures that address the functionality of your system.
Records should follow when a physician moves a practice or changes employment. Ms. Nitzki recently assisted a case in which an early adopter physician dissolved his practice, became employed, and moved offices. His 2011 attestation was later audited, and he did not have all of the requested information. The doctor came to Ms. Nitzki after failing an audit and being assessed an $18,000 recoupment payment. Together, they were able to recreate and locate much of the necessary records through old emails and past documentation.
“We won the appeal,” said Ms. Nitzki, who spoke about meaningful use audits at a recent American Bar Association meeting. “The takeaway is if you’re going to respond to an audit, make sure you understand everything that they’re asking for and that you’re confident that you’re responding with all of the appropriate documentation,” she said.
3. Meet deadlines
Promptly responding to audits and quickly addressing failed audits are critical, Mr. Freemire said.
“The [MU] program is very strict with regard to deadlines, and an appeal cannot be filed once the applicable deadline has passed,” he said.
Mr. Freemire said that he routinely encounters health providers who have missed deadlines or let too much time lapse after being contacted by the government. Failure to meet deadlines can result from various reasons, including that notices are not promptly brought to the physician’s attention, delays investigating the availability of requested materials, or a false assumption that the requested information is readily at hand, he said.
Ensure that all audit deadlines are recorded and work with staff and leadership to file responses on time. Factor in additional time if there could be difficulty in locating records, experts stressed.
“I see difficulty in effectively responding to audits where records are simply not maintained,” he said. “Again, this can happen for many reasons, including personnel turnover, but it can be difficult or impossible to effectively respond to audit requests” in this situation.
4. Appeal effectively
Swiftly and thoroughly appeal a failed audit.
When a doctor fails an audit, it’s usually because of incomplete responses or missing information, Ms. Nitzki said. Only those measures that were rejected or that failed to meet standards need to be addressed during an appeal. Sending a cover letter to CMS with the appeal, explaining why the doctor may have failed initially can strengthen the case, she said.
“The appeals process is done directly with CMS, so they’re a little more reasonable,” she said. “They tend to look at things on broader scale.”
Don’t wait to appeal, Mr. Freemire said. Practices have 30 days from the date of an adverse audit determination letter to submit an appeal. If time runs out, physicians must pay back any money requested by auditors, and CMS expects the recoupment in full.
“Following a negative decision, the most important step is prompt attention,” he said. “The sooner that process begins, the sooner it can be completed. The time line for audit appeals is not generous and providers need to ensure they react promptly to a negative finding.”
5. Get help
Ms. Nitzki recommends that physicians obtain professional assistance when responding to an audit.
Practices can hire a consulting company or find audit resources from their specialty society or state medical association. The American Academy of Family Physicians provides a tip sheet on responding to audits. Some state organizations, such as the Texas Medical Association, offer a helpline for doctors to call and ask questions related to meaningful use.
“Unless they have a very savvy person in their office who has taken care all of their meaningful use attestations and documentation, we recommend they get help because it’s in the details where they could fail,” she said.
Seeking help after a failed audit is also essential, notes Mr. Freemire. Experts who are familiar with the program and its requirements and procedures can be of great assistance when it comes organizing the facts into a persuasive argument, he said.
“A cost/benefit is necessary – as it is with all service providers,” he said. “But a provider’s chances for a successful appeal are increased when that appeal is prepared by someone who understands the history and intention behind program requirements, who can best identify and present the necessary evidence of compliance, and who can make a compelling argument as to the provider’s satisfaction of program requirements.”
On Twitter @legal_med
The Meaningful Use (MU) program as doctors know it may soon be ending, but audits of past attestations are slated to continue for at least the next 6 years.
Experts offer the following guidance on how to successfully manage audits and subsequent appeals.
1. Expect an audit.
Assume that at some point, you will be audited, said Joshua J. Freemire, a health law attorney based in Baltimore who specializes in regulatory compliance matters.
“Have good procedures in place to ensure the audit is brought to the attention of management and responded to promptly,” Mr. Freemire said in an interview. “Similarly, it is important that practices understand, in advance, how information created by their EHR is stored and managed.”
Audits are not limited to the current or most recent year, he noted. Being able to promptly access the appropriate information is an important part of drafting appropriate responses.
2. Save records
Keep all relevant records electronically for at least 6 years after attestation, advised Carmiña Nitzki, a senior consultant with GE Healthcare Camden Group.
Some documentation, such as your security risk analysis, should be maintained in paper form, she added. It is also helpful to take screen shots of certain measures that address the functionality of your system.
Records should follow when a physician moves a practice or changes employment. Ms. Nitzki recently assisted a case in which an early adopter physician dissolved his practice, became employed, and moved offices. His 2011 attestation was later audited, and he did not have all of the requested information. The doctor came to Ms. Nitzki after failing an audit and being assessed an $18,000 recoupment payment. Together, they were able to recreate and locate much of the necessary records through old emails and past documentation.
“We won the appeal,” said Ms. Nitzki, who spoke about meaningful use audits at a recent American Bar Association meeting. “The takeaway is if you’re going to respond to an audit, make sure you understand everything that they’re asking for and that you’re confident that you’re responding with all of the appropriate documentation,” she said.
3. Meet deadlines
Promptly responding to audits and quickly addressing failed audits are critical, Mr. Freemire said.
“The [MU] program is very strict with regard to deadlines, and an appeal cannot be filed once the applicable deadline has passed,” he said.
Mr. Freemire said that he routinely encounters health providers who have missed deadlines or let too much time lapse after being contacted by the government. Failure to meet deadlines can result from various reasons, including that notices are not promptly brought to the physician’s attention, delays investigating the availability of requested materials, or a false assumption that the requested information is readily at hand, he said.
Ensure that all audit deadlines are recorded and work with staff and leadership to file responses on time. Factor in additional time if there could be difficulty in locating records, experts stressed.
“I see difficulty in effectively responding to audits where records are simply not maintained,” he said. “Again, this can happen for many reasons, including personnel turnover, but it can be difficult or impossible to effectively respond to audit requests” in this situation.
4. Appeal effectively
Swiftly and thoroughly appeal a failed audit.
When a doctor fails an audit, it’s usually because of incomplete responses or missing information, Ms. Nitzki said. Only those measures that were rejected or that failed to meet standards need to be addressed during an appeal. Sending a cover letter to CMS with the appeal, explaining why the doctor may have failed initially can strengthen the case, she said.
“The appeals process is done directly with CMS, so they’re a little more reasonable,” she said. “They tend to look at things on broader scale.”
Don’t wait to appeal, Mr. Freemire said. Practices have 30 days from the date of an adverse audit determination letter to submit an appeal. If time runs out, physicians must pay back any money requested by auditors, and CMS expects the recoupment in full.
“Following a negative decision, the most important step is prompt attention,” he said. “The sooner that process begins, the sooner it can be completed. The time line for audit appeals is not generous and providers need to ensure they react promptly to a negative finding.”
5. Get help
Ms. Nitzki recommends that physicians obtain professional assistance when responding to an audit.
Practices can hire a consulting company or find audit resources from their specialty society or state medical association. The American Academy of Family Physicians provides a tip sheet on responding to audits. Some state organizations, such as the Texas Medical Association, offer a helpline for doctors to call and ask questions related to meaningful use.
“Unless they have a very savvy person in their office who has taken care all of their meaningful use attestations and documentation, we recommend they get help because it’s in the details where they could fail,” she said.
Seeking help after a failed audit is also essential, notes Mr. Freemire. Experts who are familiar with the program and its requirements and procedures can be of great assistance when it comes organizing the facts into a persuasive argument, he said.
“A cost/benefit is necessary – as it is with all service providers,” he said. “But a provider’s chances for a successful appeal are increased when that appeal is prepared by someone who understands the history and intention behind program requirements, who can best identify and present the necessary evidence of compliance, and who can make a compelling argument as to the provider’s satisfaction of program requirements.”
On Twitter @legal_med
Routine screening unwarranted in siblings of food-allergic children
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
Siblings of children with food allergy should not be screened routinely for such allergies before food introduction, results of a large cohort study suggest.
In a study of 478 food-allergic children and 642 of their siblings, 53% of the siblings were sensitized without clinical reactivity and only 13.6% were found to have a clinically reactive food allergy, wrote Ruchi S. Gupta, MD, of Northwestern University, Chicago, and her associates.
The investigators noted that their findings support current guidelines from the National Institute of Allergy and Infectious Diseases to not screen siblings based on another sibling having a food allergy.
“Given the lack of a dramatically increased risk of food allergy in siblings, compared with that of the general population, as well as the high rate of what are falsely positive diagnostic test results among siblings of a food allergic child, [these siblings] should not have routine screening for food allergy before food introduction,” the investigators concluded. “Such siblings are likely to be mislabeled as allergic when they are actually tolerant to the food, which may lead to an increased risk of developing allergy via avoidance,” and both quality of life and nutrition may be adversely impacted.
Dr. Gupta has received research support from Mylan, Food Allergy Research and Education, and United Health Care.
Read the full study here (http://dx.doi.org/10.1016/j.jaip.2016.04.009)
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
Siblings of children with food allergy should not be screened routinely for such allergies before food introduction, results of a large cohort study suggest.
In a study of 478 food-allergic children and 642 of their siblings, 53% of the siblings were sensitized without clinical reactivity and only 13.6% were found to have a clinically reactive food allergy, wrote Ruchi S. Gupta, MD, of Northwestern University, Chicago, and her associates.
The investigators noted that their findings support current guidelines from the National Institute of Allergy and Infectious Diseases to not screen siblings based on another sibling having a food allergy.
“Given the lack of a dramatically increased risk of food allergy in siblings, compared with that of the general population, as well as the high rate of what are falsely positive diagnostic test results among siblings of a food allergic child, [these siblings] should not have routine screening for food allergy before food introduction,” the investigators concluded. “Such siblings are likely to be mislabeled as allergic when they are actually tolerant to the food, which may lead to an increased risk of developing allergy via avoidance,” and both quality of life and nutrition may be adversely impacted.
Dr. Gupta has received research support from Mylan, Food Allergy Research and Education, and United Health Care.
Read the full study here (http://dx.doi.org/10.1016/j.jaip.2016.04.009)
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
Siblings of children with food allergy should not be screened routinely for such allergies before food introduction, results of a large cohort study suggest.
In a study of 478 food-allergic children and 642 of their siblings, 53% of the siblings were sensitized without clinical reactivity and only 13.6% were found to have a clinically reactive food allergy, wrote Ruchi S. Gupta, MD, of Northwestern University, Chicago, and her associates.
The investigators noted that their findings support current guidelines from the National Institute of Allergy and Infectious Diseases to not screen siblings based on another sibling having a food allergy.
“Given the lack of a dramatically increased risk of food allergy in siblings, compared with that of the general population, as well as the high rate of what are falsely positive diagnostic test results among siblings of a food allergic child, [these siblings] should not have routine screening for food allergy before food introduction,” the investigators concluded. “Such siblings are likely to be mislabeled as allergic when they are actually tolerant to the food, which may lead to an increased risk of developing allergy via avoidance,” and both quality of life and nutrition may be adversely impacted.
Dr. Gupta has received research support from Mylan, Food Allergy Research and Education, and United Health Care.
Read the full study here (http://dx.doi.org/10.1016/j.jaip.2016.04.009)
Cardiologists underrecognize angina about half the time
Angina is underrecognized by cardiologists in clinic visits, compared with assessments made by the patients themselves, suggesting that a standard screening tool be used to improve accuracy.
Researchers conducted a survey of 1,257 patients and their 155 doctors at 25 cardiology outpatient practices in 19 states. All patients had documented coronary artery disease and completed the Seattle Angina Questionnaire (SAQ) before their office visits to assess angina symptoms and frequency. Right after they left, their cardiologists estimated and recorded their idea of how often their patients had heart pain and what their symptoms were.
Patient and physician estimates didn’t match: 411 patients (33%) reported at least one bout of angina in the previous month, but their physicians estimated a frequency of 14% (173 patients). Heart failure patients were more than three times as likely to have their angina underestimated, and patients who reported a single bout of angina per month were about 70% less likely than those who reported daily or weekly attacks.
Years in practice and the number of angina patients in the case load didn’t seem to make a difference. Patient demographics, atypical symptoms, and comorbidities besides heart failure didn’t either. “Some physicians were much better at recognizing the frequency of patients’ angina,” said lead investigator Suzanne Arnold, MD, a cardiologist at Saint Luke’s Mid America Heart Institute in Kansas City, Mo., and her associates.
“Evaluation of angina is subject to limitations inherent in history taking, including pre-existing biases and time constraints on the part of physicians and patients. ... Under-recognition of angina by physicians may result in under treatment with revascularization or medications that could improve patients’ quality of life,” and it wastes healthcare dollars, they said.
The team concluded that angina needs to be assessed directly from patients with a standardized survey like the Seattle Angina Questionnaire (SAQ). “We still routinely depend solely on an unstructured interview instead of directly asking patients using standardized assessments,” the investigators noted. “A more systematic approach is needed for eliciting a history and assessing angina in patients with coronary artery disease to appropriately guide further testing and treatment. ... The use of a validated, patient-centered tool for eliciting patients’ angina, such as the SAQ, should be tested in routine clinical care to see if it improves angina recognition, treatment, and outcomes,” the study team said (Circ Cardiovasc Qual Outcomes. 2016 AUG 16;doi: 10.1161/CIRCOUTCOMES.116.002781).
Patients in the study were, on average, 69 years old, and the majority were white men. About 40% reported previous myocardial infarctions, and more than half were stented. Well over three quarters were on beta-blockers for angina.
The senior investigator, John Spertus, MD, holds a copyright on the SAQ used in the study. Gilead Sciences funded the work. Investigators reported ties to Gilead, Abbvie, Genentech, Glumetrics, Maquet, Sanofi, AstraZeneca, Edwards Life Sciences, Roche, St. Jude Medical, Regeneron, Lilly, and ZS Pharma.
Angina is underrecognized by cardiologists in clinic visits, compared with assessments made by the patients themselves, suggesting that a standard screening tool be used to improve accuracy.
Researchers conducted a survey of 1,257 patients and their 155 doctors at 25 cardiology outpatient practices in 19 states. All patients had documented coronary artery disease and completed the Seattle Angina Questionnaire (SAQ) before their office visits to assess angina symptoms and frequency. Right after they left, their cardiologists estimated and recorded their idea of how often their patients had heart pain and what their symptoms were.
Patient and physician estimates didn’t match: 411 patients (33%) reported at least one bout of angina in the previous month, but their physicians estimated a frequency of 14% (173 patients). Heart failure patients were more than three times as likely to have their angina underestimated, and patients who reported a single bout of angina per month were about 70% less likely than those who reported daily or weekly attacks.
Years in practice and the number of angina patients in the case load didn’t seem to make a difference. Patient demographics, atypical symptoms, and comorbidities besides heart failure didn’t either. “Some physicians were much better at recognizing the frequency of patients’ angina,” said lead investigator Suzanne Arnold, MD, a cardiologist at Saint Luke’s Mid America Heart Institute in Kansas City, Mo., and her associates.
“Evaluation of angina is subject to limitations inherent in history taking, including pre-existing biases and time constraints on the part of physicians and patients. ... Under-recognition of angina by physicians may result in under treatment with revascularization or medications that could improve patients’ quality of life,” and it wastes healthcare dollars, they said.
The team concluded that angina needs to be assessed directly from patients with a standardized survey like the Seattle Angina Questionnaire (SAQ). “We still routinely depend solely on an unstructured interview instead of directly asking patients using standardized assessments,” the investigators noted. “A more systematic approach is needed for eliciting a history and assessing angina in patients with coronary artery disease to appropriately guide further testing and treatment. ... The use of a validated, patient-centered tool for eliciting patients’ angina, such as the SAQ, should be tested in routine clinical care to see if it improves angina recognition, treatment, and outcomes,” the study team said (Circ Cardiovasc Qual Outcomes. 2016 AUG 16;doi: 10.1161/CIRCOUTCOMES.116.002781).
Patients in the study were, on average, 69 years old, and the majority were white men. About 40% reported previous myocardial infarctions, and more than half were stented. Well over three quarters were on beta-blockers for angina.
The senior investigator, John Spertus, MD, holds a copyright on the SAQ used in the study. Gilead Sciences funded the work. Investigators reported ties to Gilead, Abbvie, Genentech, Glumetrics, Maquet, Sanofi, AstraZeneca, Edwards Life Sciences, Roche, St. Jude Medical, Regeneron, Lilly, and ZS Pharma.
Angina is underrecognized by cardiologists in clinic visits, compared with assessments made by the patients themselves, suggesting that a standard screening tool be used to improve accuracy.
Researchers conducted a survey of 1,257 patients and their 155 doctors at 25 cardiology outpatient practices in 19 states. All patients had documented coronary artery disease and completed the Seattle Angina Questionnaire (SAQ) before their office visits to assess angina symptoms and frequency. Right after they left, their cardiologists estimated and recorded their idea of how often their patients had heart pain and what their symptoms were.
Patient and physician estimates didn’t match: 411 patients (33%) reported at least one bout of angina in the previous month, but their physicians estimated a frequency of 14% (173 patients). Heart failure patients were more than three times as likely to have their angina underestimated, and patients who reported a single bout of angina per month were about 70% less likely than those who reported daily or weekly attacks.
Years in practice and the number of angina patients in the case load didn’t seem to make a difference. Patient demographics, atypical symptoms, and comorbidities besides heart failure didn’t either. “Some physicians were much better at recognizing the frequency of patients’ angina,” said lead investigator Suzanne Arnold, MD, a cardiologist at Saint Luke’s Mid America Heart Institute in Kansas City, Mo., and her associates.
“Evaluation of angina is subject to limitations inherent in history taking, including pre-existing biases and time constraints on the part of physicians and patients. ... Under-recognition of angina by physicians may result in under treatment with revascularization or medications that could improve patients’ quality of life,” and it wastes healthcare dollars, they said.
The team concluded that angina needs to be assessed directly from patients with a standardized survey like the Seattle Angina Questionnaire (SAQ). “We still routinely depend solely on an unstructured interview instead of directly asking patients using standardized assessments,” the investigators noted. “A more systematic approach is needed for eliciting a history and assessing angina in patients with coronary artery disease to appropriately guide further testing and treatment. ... The use of a validated, patient-centered tool for eliciting patients’ angina, such as the SAQ, should be tested in routine clinical care to see if it improves angina recognition, treatment, and outcomes,” the study team said (Circ Cardiovasc Qual Outcomes. 2016 AUG 16;doi: 10.1161/CIRCOUTCOMES.116.002781).
Patients in the study were, on average, 69 years old, and the majority were white men. About 40% reported previous myocardial infarctions, and more than half were stented. Well over three quarters were on beta-blockers for angina.
The senior investigator, John Spertus, MD, holds a copyright on the SAQ used in the study. Gilead Sciences funded the work. Investigators reported ties to Gilead, Abbvie, Genentech, Glumetrics, Maquet, Sanofi, AstraZeneca, Edwards Life Sciences, Roche, St. Jude Medical, Regeneron, Lilly, and ZS Pharma.
FROM CIRCULATION CARDIOVASCULAR QUALITY AND OUTCOMES
Key clinical point: Cardiologists miss angina nearly half of the time.
Major finding: Patient and physician estimates don’t match up; 411 patients (33%) reported at least one bout of angina in the previous month, but their physicians estimated a frequency of 14% (173 patients).
Data source: A survey of 1,257 patients and their 155 doctors at 25 cardiology outpatient practices in 19 states.
Disclosures: The senior investigator, John Spertus, MD, holds a copyright on the Seattle Angina Questionnaire used in the study. Gilead Sciences funded the work. Investigators reported ties to Gilead, Abbvie, Genentech, Glumetrics, Maquet, Sanofi, AstraZeneca, Edwards Life Sciences, Roche, St. Jude Medical, Regeneron, Lilly, and ZS Pharma.