User login
Don’t delay treatment for patients with TB and HIV
Clinicians should treat patients diagnosed with HIV and tuberculosis for both conditions immediately, according to new guidelines on the treatment of drug-susceptible tuberculosis.
The clinical practice guidelines were issued collectively by three organizations: the American Thoracic Society (ATS), the U.S. Centers for Disease Control and Prevention (CDC), and the Infectious Diseases Society of America (IDSA), and published online in Clinical Infectious Diseases.
The guidelines recommend starting TB treatment for all patients as soon as an infection is suspected, rather than waiting for test results, and focusing on daily therapy to reduce the risk of relapse. In addition, all TB patients should receive comprehensive care, including direct observed therapy (DOT) when appropriate (Clin Infect Dis. 2016 Aug 10. doi: 10.1093/cid/ciw376).
“Treatment of tuberculosis is focused on both curing the individual patient and minimizing the transmission,” wrote Payam Nahid, MD, professor of medicine at the University of California, San Francisco, and his colleagues on the guidelines committee.
The guidelines’ section on treatment of tuberculosis in special situations addresses management of TB in patients with conditions including HIV, extrapulmonary TB, culture-negative pulmonary TB, pregnancy, renal disease, and hepatic disease, as well as treatment of children and the elderly.
With regard to HIV, the guidelines recommend the standard 6-month daily TB treatment for HIV patients on antiretroviral therapy. This treatment includes 2 months of isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), and ethambutol (EMB), followed by a continuation phase of 4 months of INH and RIF.
“Patients with HIV infection and tuberculosis are at an increased risk of developing paradoxical worsening of symptoms, signs, or clinical manifestations of tuberculosis after beginning antituberculosis and antiretroviral treatments,” according to the guidelines. These responses are defined as Immune Reconstitution Inflammatory Syndrome (IRIS). However, IRIS does not appear to impact the simultaneous treatment of TB and HIV, and the condition can be managed symptomatically if it occurs, the researchers noted.
The guidelines identified several areas in need of further study, including new TB drugs and treatment plans; the effects of biomarkers to help design individual therapy; TB in special populations including HIV patients, pregnant women, and children; and treatment delivery strategies.
The guidelines also are endorsed by the European Respiratory Society (ERS) and the U.S. National Tuberculosis Controllers Association (NCTA).
The American Thoracic Society, the Infections Diseases Society of America, and the Centers for Disease Control and Prevention provided financial support. Lead author Dr. Nahid had no financial conflicts to disclose.
Clinicians should treat patients diagnosed with HIV and tuberculosis for both conditions immediately, according to new guidelines on the treatment of drug-susceptible tuberculosis.
The clinical practice guidelines were issued collectively by three organizations: the American Thoracic Society (ATS), the U.S. Centers for Disease Control and Prevention (CDC), and the Infectious Diseases Society of America (IDSA), and published online in Clinical Infectious Diseases.
The guidelines recommend starting TB treatment for all patients as soon as an infection is suspected, rather than waiting for test results, and focusing on daily therapy to reduce the risk of relapse. In addition, all TB patients should receive comprehensive care, including direct observed therapy (DOT) when appropriate (Clin Infect Dis. 2016 Aug 10. doi: 10.1093/cid/ciw376).
“Treatment of tuberculosis is focused on both curing the individual patient and minimizing the transmission,” wrote Payam Nahid, MD, professor of medicine at the University of California, San Francisco, and his colleagues on the guidelines committee.
The guidelines’ section on treatment of tuberculosis in special situations addresses management of TB in patients with conditions including HIV, extrapulmonary TB, culture-negative pulmonary TB, pregnancy, renal disease, and hepatic disease, as well as treatment of children and the elderly.
With regard to HIV, the guidelines recommend the standard 6-month daily TB treatment for HIV patients on antiretroviral therapy. This treatment includes 2 months of isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), and ethambutol (EMB), followed by a continuation phase of 4 months of INH and RIF.
“Patients with HIV infection and tuberculosis are at an increased risk of developing paradoxical worsening of symptoms, signs, or clinical manifestations of tuberculosis after beginning antituberculosis and antiretroviral treatments,” according to the guidelines. These responses are defined as Immune Reconstitution Inflammatory Syndrome (IRIS). However, IRIS does not appear to impact the simultaneous treatment of TB and HIV, and the condition can be managed symptomatically if it occurs, the researchers noted.
The guidelines identified several areas in need of further study, including new TB drugs and treatment plans; the effects of biomarkers to help design individual therapy; TB in special populations including HIV patients, pregnant women, and children; and treatment delivery strategies.
The guidelines also are endorsed by the European Respiratory Society (ERS) and the U.S. National Tuberculosis Controllers Association (NCTA).
The American Thoracic Society, the Infections Diseases Society of America, and the Centers for Disease Control and Prevention provided financial support. Lead author Dr. Nahid had no financial conflicts to disclose.
Clinicians should treat patients diagnosed with HIV and tuberculosis for both conditions immediately, according to new guidelines on the treatment of drug-susceptible tuberculosis.
The clinical practice guidelines were issued collectively by three organizations: the American Thoracic Society (ATS), the U.S. Centers for Disease Control and Prevention (CDC), and the Infectious Diseases Society of America (IDSA), and published online in Clinical Infectious Diseases.
The guidelines recommend starting TB treatment for all patients as soon as an infection is suspected, rather than waiting for test results, and focusing on daily therapy to reduce the risk of relapse. In addition, all TB patients should receive comprehensive care, including direct observed therapy (DOT) when appropriate (Clin Infect Dis. 2016 Aug 10. doi: 10.1093/cid/ciw376).
“Treatment of tuberculosis is focused on both curing the individual patient and minimizing the transmission,” wrote Payam Nahid, MD, professor of medicine at the University of California, San Francisco, and his colleagues on the guidelines committee.
The guidelines’ section on treatment of tuberculosis in special situations addresses management of TB in patients with conditions including HIV, extrapulmonary TB, culture-negative pulmonary TB, pregnancy, renal disease, and hepatic disease, as well as treatment of children and the elderly.
With regard to HIV, the guidelines recommend the standard 6-month daily TB treatment for HIV patients on antiretroviral therapy. This treatment includes 2 months of isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), and ethambutol (EMB), followed by a continuation phase of 4 months of INH and RIF.
“Patients with HIV infection and tuberculosis are at an increased risk of developing paradoxical worsening of symptoms, signs, or clinical manifestations of tuberculosis after beginning antituberculosis and antiretroviral treatments,” according to the guidelines. These responses are defined as Immune Reconstitution Inflammatory Syndrome (IRIS). However, IRIS does not appear to impact the simultaneous treatment of TB and HIV, and the condition can be managed symptomatically if it occurs, the researchers noted.
The guidelines identified several areas in need of further study, including new TB drugs and treatment plans; the effects of biomarkers to help design individual therapy; TB in special populations including HIV patients, pregnant women, and children; and treatment delivery strategies.
The guidelines also are endorsed by the European Respiratory Society (ERS) and the U.S. National Tuberculosis Controllers Association (NCTA).
The American Thoracic Society, the Infections Diseases Society of America, and the Centers for Disease Control and Prevention provided financial support. Lead author Dr. Nahid had no financial conflicts to disclose.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: Clinicians should treat patients diagnosed with HIV and tuberculosis for both conditions immediately.
Major finding: A four-drug regimen of INH, RIF, PZA, and EMB remains the preferred initial treatment for drug-susceptible pulmonary tuberculosis. Treatment should be initiated promptly even before diagnostic test results are known in patients with high likelihood of having tuberculosis.
Data source: Nine PICO (population, intervention, comparators, outcomes) questions and associated recommendations for the treatment of patients diagnosed with both HIV and TB, developed based on the evidence appraised using GRADE (Grading of Recommendations Assessment, Development, and Evaluation) methodology.
Disclosures: The American Thoracic Society, the Infections Diseases Society of America, and the Centers for Disease Control and Prevention provided financial support. Lead author Dr. Nahid had no financial conflicts to disclose.
CMV viremia not culprit in high mortality of TB/HIV coinfection
DURBAN, SOUTH AFRICA – Cytomegalovirus viremia is common among patients hospitalized for HIV-associated tuberculosis, but it appears to be a bystander rather than a contributor to the high mortality seen in this population, Amy Ward, MD, said at the 21st International AIDS Conference.
“CMV [cytomegalovirus] viremia is likely a marker of more severe immunodeficiency rather than a direct contributor to mortality,” she concluded based upon the findings of her prospective cohort study. The finding means therapies for CMV viremia will not open up a new avenue of potentially life-saving treatments for these patients.
In other severe immunodeficiency conditions, such as after organ transplant, CMV viremia is directly related to increased mortality, and ganciclovir therapy can prevent progression to clinical disease and death, explained Dr. Ward of the University of Cape Town, South Africa.
She presented a prospective cohort study of 256 HIV-infected South African adults, median age 36 years, who were hospitalized with a new diagnosis of TB. At enrollment, their median CD4 count was 64 cells/mm3. Only 35% were on antiretroviral therapy (ART); 44% had previously been on ART, 21% were ART-naive, and 41% had a positive TB blood culture.
CMV viremia was present in 31%, and CMV viral load was 1,000 copies/mL or more in half of them. None had CMV retinitis, based on panoptic fundoscopy at enrollment. HIV-related retinal pathologies at enrollment included disseminated cryptococcal disease, ocular TB granules, and HIV retinitis.
The primary endpoint of the study was mortality at 12 weeks on anti-TB therapy. The mortality rate was 38% in the CMV viremic group, significantly higher than the 17.8% mortality rate seen in the CMV-negative patients.
In a univariate Cox proportional hazards regression analysis, CMV viremia was associated with a 2.1-fold increased risk for 12-week mortality. But advancing age, a low CD4 count, and decreasing serum albumin were also risk factors.
When these variables were incorporated in a multivariate regression analysis along with HIV viral load, tuberculosis blood culture results, and gender, CMV viremia was no longer a significant risk factor for 12-week mortality. Age was the sole significant predictor of death. Patients who were at least 36 years old had a 32.8% mortality rate, compared with a 14.1% rate in those who were younger. The CD4 count didn’t differ significantly by age; however, the prevalence of CMV viremia was 38% in the older group and 26.3% in patients under age 36.
“Those patients who were 36 years old and above had a higher mortality and were more likely to have CMV viremia, both findings perhaps reflecting premature aging of the immune system,” Dr. Ward said.
Also, no dose-response was seen between CMV viral load and mortality risk. The 12-week mortality rate was 33.3% in patients with a CMV viral load below 1,000 copies/mL and similar at 34.1% in those with a viral load above that cutpoint, she noted.
The study was funded by the Wellcome Trust and the South African Medical Research Council. Dr. Ward reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – Cytomegalovirus viremia is common among patients hospitalized for HIV-associated tuberculosis, but it appears to be a bystander rather than a contributor to the high mortality seen in this population, Amy Ward, MD, said at the 21st International AIDS Conference.
“CMV [cytomegalovirus] viremia is likely a marker of more severe immunodeficiency rather than a direct contributor to mortality,” she concluded based upon the findings of her prospective cohort study. The finding means therapies for CMV viremia will not open up a new avenue of potentially life-saving treatments for these patients.
In other severe immunodeficiency conditions, such as after organ transplant, CMV viremia is directly related to increased mortality, and ganciclovir therapy can prevent progression to clinical disease and death, explained Dr. Ward of the University of Cape Town, South Africa.
She presented a prospective cohort study of 256 HIV-infected South African adults, median age 36 years, who were hospitalized with a new diagnosis of TB. At enrollment, their median CD4 count was 64 cells/mm3. Only 35% were on antiretroviral therapy (ART); 44% had previously been on ART, 21% were ART-naive, and 41% had a positive TB blood culture.
CMV viremia was present in 31%, and CMV viral load was 1,000 copies/mL or more in half of them. None had CMV retinitis, based on panoptic fundoscopy at enrollment. HIV-related retinal pathologies at enrollment included disseminated cryptococcal disease, ocular TB granules, and HIV retinitis.
The primary endpoint of the study was mortality at 12 weeks on anti-TB therapy. The mortality rate was 38% in the CMV viremic group, significantly higher than the 17.8% mortality rate seen in the CMV-negative patients.
In a univariate Cox proportional hazards regression analysis, CMV viremia was associated with a 2.1-fold increased risk for 12-week mortality. But advancing age, a low CD4 count, and decreasing serum albumin were also risk factors.
When these variables were incorporated in a multivariate regression analysis along with HIV viral load, tuberculosis blood culture results, and gender, CMV viremia was no longer a significant risk factor for 12-week mortality. Age was the sole significant predictor of death. Patients who were at least 36 years old had a 32.8% mortality rate, compared with a 14.1% rate in those who were younger. The CD4 count didn’t differ significantly by age; however, the prevalence of CMV viremia was 38% in the older group and 26.3% in patients under age 36.
“Those patients who were 36 years old and above had a higher mortality and were more likely to have CMV viremia, both findings perhaps reflecting premature aging of the immune system,” Dr. Ward said.
Also, no dose-response was seen between CMV viral load and mortality risk. The 12-week mortality rate was 33.3% in patients with a CMV viral load below 1,000 copies/mL and similar at 34.1% in those with a viral load above that cutpoint, she noted.
The study was funded by the Wellcome Trust and the South African Medical Research Council. Dr. Ward reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – Cytomegalovirus viremia is common among patients hospitalized for HIV-associated tuberculosis, but it appears to be a bystander rather than a contributor to the high mortality seen in this population, Amy Ward, MD, said at the 21st International AIDS Conference.
“CMV [cytomegalovirus] viremia is likely a marker of more severe immunodeficiency rather than a direct contributor to mortality,” she concluded based upon the findings of her prospective cohort study. The finding means therapies for CMV viremia will not open up a new avenue of potentially life-saving treatments for these patients.
In other severe immunodeficiency conditions, such as after organ transplant, CMV viremia is directly related to increased mortality, and ganciclovir therapy can prevent progression to clinical disease and death, explained Dr. Ward of the University of Cape Town, South Africa.
She presented a prospective cohort study of 256 HIV-infected South African adults, median age 36 years, who were hospitalized with a new diagnosis of TB. At enrollment, their median CD4 count was 64 cells/mm3. Only 35% were on antiretroviral therapy (ART); 44% had previously been on ART, 21% were ART-naive, and 41% had a positive TB blood culture.
CMV viremia was present in 31%, and CMV viral load was 1,000 copies/mL or more in half of them. None had CMV retinitis, based on panoptic fundoscopy at enrollment. HIV-related retinal pathologies at enrollment included disseminated cryptococcal disease, ocular TB granules, and HIV retinitis.
The primary endpoint of the study was mortality at 12 weeks on anti-TB therapy. The mortality rate was 38% in the CMV viremic group, significantly higher than the 17.8% mortality rate seen in the CMV-negative patients.
In a univariate Cox proportional hazards regression analysis, CMV viremia was associated with a 2.1-fold increased risk for 12-week mortality. But advancing age, a low CD4 count, and decreasing serum albumin were also risk factors.
When these variables were incorporated in a multivariate regression analysis along with HIV viral load, tuberculosis blood culture results, and gender, CMV viremia was no longer a significant risk factor for 12-week mortality. Age was the sole significant predictor of death. Patients who were at least 36 years old had a 32.8% mortality rate, compared with a 14.1% rate in those who were younger. The CD4 count didn’t differ significantly by age; however, the prevalence of CMV viremia was 38% in the older group and 26.3% in patients under age 36.
“Those patients who were 36 years old and above had a higher mortality and were more likely to have CMV viremia, both findings perhaps reflecting premature aging of the immune system,” Dr. Ward said.
Also, no dose-response was seen between CMV viral load and mortality risk. The 12-week mortality rate was 33.3% in patients with a CMV viral load below 1,000 copies/mL and similar at 34.1% in those with a viral load above that cutpoint, she noted.
The study was funded by the Wellcome Trust and the South African Medical Research Council. Dr. Ward reported having no financial conflicts of interest.
AT AIDS 2016
Key clinical point: Cytomegalovirus viremia is common in patients hospitalized for HIV-associated tuberculosis, but treating the CMV infection is unlikely to reduce the coinfected group’s high mortality rate.
Major finding: Cytomegalovirus viremia was present in nearly one-third of a group of hospitalized patients with HIV infection and tuberculosis, but was not an independent risk factor for their 23% mortality rate at 12 weeks.
Data source: This was a prospective cohort study including 256 hospitalized patients coinfected with HIV and newly diagnosed tuberculosis.
Disclosures: The study was funded by the Wellcome Trust and the South African Medical Research Council. The presenter reported having no financial conflicts of interest.
Healthcare spending doesn’t impact cancer outcomes

receiving treatment
Photo by Rhoda Baer
A new study suggests that higher healthcare spending is not associated with better cancer outcomes in the US, but state-level wealth is.
Researchers found that higher gross domestic product (GDP) per capita was associated with lower mortality for all cancers, colorectal cancer, and breast cancer.
However, higher healthcare spending was only associated with lower mortality for breast cancer—not colorectal cancer or all cancers combined.
Jad Chahoud, MD, of The University of Texas MD Anderson Cancer Center in Houston, and his colleagues reported these findings in JNCCN.
To investigate the implications of socioeconomic status and health expenditures on cancer outcomes, the researchers conducted an ecological study at the state level for 3 distinct patient populations: breast cancer, colorectal cancer, and all-cancer patients.
The team extracted data on GDP and healthcare spending per capita from the 2009 Bureau of Economic Analysis and the Centers for Medicare & Medicaid Services, respectively.
Using data from the National Cancer Institute, the researchers retrieved breast, colorectal, and all-cancer age-adjusted rates and computed mortality/incidence (M/I) ratios for each population.
The team found that higher GDP per capita was significantly associated with lower M/I ratios for all cancers (rho=–0.4406; P=0.0017), breast cancer (rho=–0.3605; P=0.0118), and colorectal cancer (rho=–0.3612; P=0.0117).
But higher healthcare spending was only associated with a lower M/I ratio for breast cancer (rho=–0.4237; P=0.0027).
In a related editorial, Melissa A. Simon, MD, of Northwestern University Feinberg School of Medicine in Chicago, Illinois, and her colleagues pointed out that the data in this study predate the Affordable Care Act. So the results may not reflect the current state of affairs in the US.
The authors also said these data should not be used to guide—or misguide—policy makers to cap or decrease spending for certain health issues.
“Increased spending does not necessarily improve quality of care, but capping or cutting spending on healthcare does not necessarily solve problems either,” the authors wrote.
In a counterpoint editorial, Dr Chahoud and his colleagues said the goal of their study was not to misguide policy makers.
The team doesn’t recommend capping healthcare spending. Rather, they want to see “smart” spending that will have an impact on patient outcomes. ![]()

receiving treatment
Photo by Rhoda Baer
A new study suggests that higher healthcare spending is not associated with better cancer outcomes in the US, but state-level wealth is.
Researchers found that higher gross domestic product (GDP) per capita was associated with lower mortality for all cancers, colorectal cancer, and breast cancer.
However, higher healthcare spending was only associated with lower mortality for breast cancer—not colorectal cancer or all cancers combined.
Jad Chahoud, MD, of The University of Texas MD Anderson Cancer Center in Houston, and his colleagues reported these findings in JNCCN.
To investigate the implications of socioeconomic status and health expenditures on cancer outcomes, the researchers conducted an ecological study at the state level for 3 distinct patient populations: breast cancer, colorectal cancer, and all-cancer patients.
The team extracted data on GDP and healthcare spending per capita from the 2009 Bureau of Economic Analysis and the Centers for Medicare & Medicaid Services, respectively.
Using data from the National Cancer Institute, the researchers retrieved breast, colorectal, and all-cancer age-adjusted rates and computed mortality/incidence (M/I) ratios for each population.
The team found that higher GDP per capita was significantly associated with lower M/I ratios for all cancers (rho=–0.4406; P=0.0017), breast cancer (rho=–0.3605; P=0.0118), and colorectal cancer (rho=–0.3612; P=0.0117).
But higher healthcare spending was only associated with a lower M/I ratio for breast cancer (rho=–0.4237; P=0.0027).
In a related editorial, Melissa A. Simon, MD, of Northwestern University Feinberg School of Medicine in Chicago, Illinois, and her colleagues pointed out that the data in this study predate the Affordable Care Act. So the results may not reflect the current state of affairs in the US.
The authors also said these data should not be used to guide—or misguide—policy makers to cap or decrease spending for certain health issues.
“Increased spending does not necessarily improve quality of care, but capping or cutting spending on healthcare does not necessarily solve problems either,” the authors wrote.
In a counterpoint editorial, Dr Chahoud and his colleagues said the goal of their study was not to misguide policy makers.
The team doesn’t recommend capping healthcare spending. Rather, they want to see “smart” spending that will have an impact on patient outcomes. ![]()

receiving treatment
Photo by Rhoda Baer
A new study suggests that higher healthcare spending is not associated with better cancer outcomes in the US, but state-level wealth is.
Researchers found that higher gross domestic product (GDP) per capita was associated with lower mortality for all cancers, colorectal cancer, and breast cancer.
However, higher healthcare spending was only associated with lower mortality for breast cancer—not colorectal cancer or all cancers combined.
Jad Chahoud, MD, of The University of Texas MD Anderson Cancer Center in Houston, and his colleagues reported these findings in JNCCN.
To investigate the implications of socioeconomic status and health expenditures on cancer outcomes, the researchers conducted an ecological study at the state level for 3 distinct patient populations: breast cancer, colorectal cancer, and all-cancer patients.
The team extracted data on GDP and healthcare spending per capita from the 2009 Bureau of Economic Analysis and the Centers for Medicare & Medicaid Services, respectively.
Using data from the National Cancer Institute, the researchers retrieved breast, colorectal, and all-cancer age-adjusted rates and computed mortality/incidence (M/I) ratios for each population.
The team found that higher GDP per capita was significantly associated with lower M/I ratios for all cancers (rho=–0.4406; P=0.0017), breast cancer (rho=–0.3605; P=0.0118), and colorectal cancer (rho=–0.3612; P=0.0117).
But higher healthcare spending was only associated with a lower M/I ratio for breast cancer (rho=–0.4237; P=0.0027).
In a related editorial, Melissa A. Simon, MD, of Northwestern University Feinberg School of Medicine in Chicago, Illinois, and her colleagues pointed out that the data in this study predate the Affordable Care Act. So the results may not reflect the current state of affairs in the US.
The authors also said these data should not be used to guide—or misguide—policy makers to cap or decrease spending for certain health issues.
“Increased spending does not necessarily improve quality of care, but capping or cutting spending on healthcare does not necessarily solve problems either,” the authors wrote.
In a counterpoint editorial, Dr Chahoud and his colleagues said the goal of their study was not to misguide policy makers.
The team doesn’t recommend capping healthcare spending. Rather, they want to see “smart” spending that will have an impact on patient outcomes. ![]()
Why Residents Order Unnecessary Inpatient Laboratory Tests
Resident physicians routinely order inpatient laboratory tests,[1] and there is evidence to suggest that many of these tests are unnecessary[2] and potentially harmful.[3] The Society of Hospital Medicine has identified reducing the unnecessary ordering of inpatient laboratory testing as part of the Choosing Wisely campaign.[4] Hospitalists at academic medical centers face growing pressures to develop processes to reduce low‐value care and train residents to be stewards of healthcare resources.[5] Studies[6, 7, 8, 9] have described that institutional and training factors drive residents' resource utilization patterns, but, to our knowledge, none have described what factors contribute to residents' unnecessary laboratory testing. To better understand the factors associated with residents' ordering patterns, we conducted a qualitative analysis of internal medicine (IM) and general surgery (GS) residents at a large academic medical center in order to describe residents' perception of the: (1) frequency of ordering unnecessary inpatient laboratory tests, (2) factors contributing to that behavior, and (3) potential interventions to change it. We also explored differences in responses by specialty and training level.
METHODS
In October 2014, we surveyed all IM and GS residents at the Hospital of the University of Pennsylvania. We reviewed the literature and conducted focus groups with residents to formulate items for the survey instrument. A draft of the survey was administered to 8 residents from both specialties, and their feedback was collated and incorporated into the final version of the instrument. The final 15‐question survey was comprised of 4 components: (1) training information such as specialty and postgraduate year (PGY), (2) self‐reported frequency of perceived unnecessary ordering of inpatient laboratory tests, (3) perception of factors contributing to unnecessary ordering, and (4) potential interventions to reduce unnecessary ordering. An unnecessary test was defined as a test that would not change management regardless of its result. To increase response rates, participants were entered into drawings for $5 gift cards, a $200 air travel voucher, and an iPad mini.
Descriptive statistics and 2tests were conducted with Stata version 13 (StataCorp LP, College Station, TX) to explore differences in the frequency of responses by specialty and training level. To identify themes that emerged from free‐text responses, two independent reviewers (M.S.S. and E.J.K.) performed qualitative content analysis using grounded theory.[10] Reviewers read 10% of responses to create a coding guide. Another 10% of the responses were randomly selected to assess inter‐rater reliability by calculating scores. The reviewers independently coded the remaining 80% of responses. Discrepancies were adjudicated by consensus between the reviewers. The University of Pennsylvania Institutional Review Board deemed this study exempt from review.
RESULTS
The sample comprised 57.0% (85/149) of IM and 54.4% (31/57) of GS residents (Table 1). Among respondents, perceived unnecessary inpatient laboratory test ordering was self‐reported by 88.2% of IM and 67.7% of GS residents. This behavior was reported to occur on a daily basis by 43.5% and 32.3% of responding IM and GS residents, respectively. Across both specialties, the most commonly reported factors contributing to these behaviors were learned practice habit/routine (90.5%), a lack of understanding of the costs associated with lab tests (86.2%), diagnostic uncertainty (82.8%), and fear of not having the lab result information when requested by an attending (75.9%). There were no significant differences in any of these contributing factors by specialty or PGY level. Among respondents who completed a free‐text response (IM: 76 of 85; GS: 21 of 31), the most commonly proposed interventions to address these issues were increasing cost transparency (IM 40.8%; GS 33.3%), improvements to faculty role modeling (IM 30.2%; GS 33.3%), and computerized reminders or decision support (IM 21.1%; GS 28.6%) (Table 2).
| Residents (n = 116)* | |
|---|---|
| |
| Reported he or she orders unnecessary routine labs, no. (%) | 96 (82.8) |
| Frequency of ordering unnecessary labs, no. (%) | |
| Daily | 47 (49.0) |
| 23 times/week | 44 (45.8) |
| 1 time/week or less | 5 (5.2) |
| Agreement with statement as factors contributing to ordering unnecessary labs, no. (%) | |
| Practice habit; I am trained to order repeating daily labs | 105 (90.5) |
| Lack of cost transparency of labs | 100 (86.2) |
| Discomfort with diagnostic uncertainty | 96 (82.8) |
| Concern that the attending will ask for the data and I will not have it | 88 (75.9) |
| Lack of role modeling of cost conscious care | 78 (67.2) |
| Lack of cost conscious culture at our institution | 76 (65.5) |
| Lack of experience | 72 (62.1) |
| Ease of ordering repeating labs in EHR | 60 (51.7) |
| Fear of litigation from missed diagnosis related to lab data | 44 (37.9) |
| Categories* | Representative Quotes | IM, n = 76, No. (%) | GS, n = 21, No. (%) |
|---|---|---|---|
| |||
| Cost transparency | Let us know the costs of what we order and train us to remember that a patient gets a bill and we are contributing to a possible bankruptcy or hardship. | 31 (40.8) | 7 (33.3) |
| Display the cost of labs when [we're] ordering them [in the EHR]. | |||
| Post the prices so that MDs understand how much everything costs. | |||
| Role modeling restrain | Train attendings to be more critical about necessity of labs and overordering. Make it part of rounding practice to decide on the labs truly needed for each patient the next day. | 23 (30.2) | 7 (33.3) |
| Attendings could review daily lab orders and briefly explain which they do not believe we need. This would allow residents to learn from their experience and their thought processes. | |||
| Encouragement and modeling of this practice from the faculty perhaps by laying out more clear expectations for which clinical situations warrant daily labs and which do not. | |||
| Computerized or decision support | When someone orders labs and the previous day's lab was normal or labs were stable for 2 days, an alert should pop up to reconsider. | 16 (21.1) | 6 (28.6) |
| Prevent us from being able to order repeating [or standing] labs. | |||
| Track how many times labs changed management, and restrict certain labslike LFTs/coags. | |||
| High‐value care educational curricula | Increase awareness of issue by having a noon conference about it or some other forum for residents to discuss the issue. | 12 (15.8) | 4 (19.0) |
| Establish guidelines for housestaff to learn/follow from start of residency. | |||
| Integrate cost conscious care into training program curricula. | |||
| System improvements | Make it easier to get labs later [in the day] | 6 (7.9) | 2 (9.5) |
| Improve timeliness of phlebotomy/laboratory systems. | |||
| More responsive phlebotomy. | |||
DISCUSSION
A significant portion of inpatient laboratory testing is unnecessary,[2] creating an opportunity to reduce utilization and associated costs. Our findings demonstrate that these behaviors occur at high levels among residents (IM 88.2%; GS 67.7%) at a large academic medical center. These findings also reveal that residents attribute this behavior to practice habit, lack of access to cost data, and perceived expectations for daily lab ordering by faculty. Interventions to change these behaviors will need to involve changes to the health system culture, increasing transparency of the costs associated with healthcare services, and shifting to a model of education that celebrates restraint.[11]
Our study adds to the emerging quest for delivering value in healthcare and provides several important insights for hospitalists and medical educators at academic centers. First, our findings reflect the significant role that the clinical learning environment plays in influencing practice behaviors among residents. Residency training is a critical time when physicians begin to form habits that imprint upon their future practice patterns,[5] and our residents are aware that their behavior to order what they perceive to be unnecessary laboratory tests is driven by habit. Studies[6, 7] have shown that residents may implicitly accept certain styles of practice as correct and are more likely to adopt those styles during the early years of their training. In our institution, for example, the process of ordering standing or daily morning labs using a repeated copy‐forward function in the electronic health record is a common, learned practice (a ritual) that is passed down from senior to junior residents year after year. This practice is common across both training specialties. There is a need to better understand, measure, and change the culture in the clinical learning environment to demonstrate practices and values that model high‐value care for residents. Multipronged interventions that address culture, oversight, and systems change[12] are necessary to facilitate effective physician stewardship of inpatient laboratory testing and attack a problem so deeply ingrained in habit.
Second, residents in our study believe that access to cost information will better equip them to reduce unnecessary lab ordering. Two recent systematic reviews[13, 14] have shown that having real‐time access to charges changes physician ordering and prescribing behavior. Increasing cost transparency may not only be an important intervention for hospitals to reduce overuse and control cost, but also better arm resident physicians with the information they need to make higher‐value recommendations for their patients and be stewards of healthcare resources.
Third, our study highlights that residents' unnecessary laboratory utilization is driven by perceived, unspoken expectations for such ordering by faculty. This reflects an important undercurrent in the medical education system that has historically emphasized and rewarded thoroughness while often penalizing restraint.[11] Hospitalists can play a major role in changing these behaviors by sharing their expectations regarding test ordering at the beginning of teaching rotations, including teaching points that discourage overutilization during rounds, and role modeling high‐value care in their own practice. Taken together and practiced routinely, these hospitalist behaviors could prevent poor habits from forming in our trainees and discourage overinvestigation. Hospitalists must be responsible to disseminate the practice of restraint to achieve more cost‐effective care. Purposeful faculty development efforts in the area of high‐value care are needed. Additionally, supporting physician leaders that serve as the institutional bridge between graduate medical education and the health system[15] could foster an environment conducive to coaching residents and faculty to reduce unnecessary practice variation.
This study is subject to several limitations. First, the survey was conducted at a single academic medical center, with a modest response rate, and thus our findings may not be generalizable to other settings or residents of different training programs. Second, we did not validate residents' perception of whether or not tests were, in fact, unnecessary. We also did not validate residents' self‐reporting of their own behavior, which may vary from actual behavior. Lack of validation at the level of the tests and at the level of the residents' behavior are two distinct but inter‐related limitations. Although self‐reported responses among residents are an important indicator of their practice, validating these data with objective measures, such as a measure of necessity of ordered lab tests as determined by an expert physician or group of experienced physicians or the number of inpatient labs ordered by residents, may add further insights. Ordering of perceived unnecessary tests may be even more common if there was under‐reporting of this behavior. Third, although we provided a definition within the survey, interpretation among survey respondents of the term unnecessary may vary, and this variation may contribute to our findings. However, we did provide a clear definition in the survey and we attempted to mitigate this with feedback from residents on our preliminary pilot.
In conclusion, this is one of the first qualitative evaluations to explore residents' perceptions on why they order unnecessary inpatient laboratory tests. Our findings offer a rich understanding of residents' beliefs about their own role in unnecessary lab ordering and explore possible solutions through the lens of the resident. Yet, it is unclear whether tests deemed unnecessary by residents would also be considered unnecessary by attending physicians or even patients. Future efforts are needed to better define which inpatient tests are unnecessary from multiple perspectives including clinicians and patients.
Acknowledgements
The authors thank Patrick J. Brennan, MD, Jeffery S. Berns, MD, Lisa M. Bellini, MD, Jon B. Morris, MD, and Irving Nachamkin, DrPH, MPH, all from the Hospital of the University of Pennsylvania, for supporting this work. They received no compensation.
Disclosures: This work was presented in part at the AAMC Integrating Quality Meeting, June 11, 2015, Chicago, Illinois; and the Alliance for Academic Internal Medicine Fall Meeting, October 9, 2015, Atlanta, Georgia. The authors report no conflicts of interest.
- , , , . The impact of residents, interns, and attendings on inpatient laboratory ordering patterns: a report from one university's hospitalist service. Acad Med. 2011;86(1):139–145.
- , , , , . The landscape of inappropriate laboratory testing: a 15‐year meta‐analysis. PLoS One. 2013;8(11):e78962.
- , , , et al. Diagnostic blood loss from phlebotomy and hospital‐acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171(18):1646–1653.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- . Charting the route to high‐value care the role of medical education. JAMA. 2016;314(22):2359–2361.
- , , , , . Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385–2393.
- , , , . The association between residency training and internists' ability to practice conservatively. JAMA Intern Med. 2014;174(10):1640–1648.
- , , , , . Effect of attending practice style on generic medication prescribing by residents in the clinic setting: an observational study. J Gen Intern Med. 2015;30(9):1286–1293.
- , , , . Role‐modeling cost‐conscious care—a national evaluation of perceptions of faculty at teaching hospitals in the United States. J Gen Intern Med. 2015;30(9):1294–1298.
- , . The discovery of grounded theory. Int J Qual Methods. 1967;5:1–10.
- , . A new model for medical education: celebrating restraint. JAMA. 2012;308(13):1329–1330.
- , , . A framework for the frontline: how hospitalists can improve healthcare value. J Hosp Med. 2016;11(4):297–302.
- , , , . The effect of charge display on cost of care and physician practice behaviors: a systematic review. J Gen Intern Med. 2015;30(6):835–842.
- , , , . Impact of price display on provider ordering: a systematic review. J Hosp Med. 2016;11(1):65–76.
- , . Merging the health system and education silos to better educate future physicians. JAMA. 2015;314(22):2349–2350.
Resident physicians routinely order inpatient laboratory tests,[1] and there is evidence to suggest that many of these tests are unnecessary[2] and potentially harmful.[3] The Society of Hospital Medicine has identified reducing the unnecessary ordering of inpatient laboratory testing as part of the Choosing Wisely campaign.[4] Hospitalists at academic medical centers face growing pressures to develop processes to reduce low‐value care and train residents to be stewards of healthcare resources.[5] Studies[6, 7, 8, 9] have described that institutional and training factors drive residents' resource utilization patterns, but, to our knowledge, none have described what factors contribute to residents' unnecessary laboratory testing. To better understand the factors associated with residents' ordering patterns, we conducted a qualitative analysis of internal medicine (IM) and general surgery (GS) residents at a large academic medical center in order to describe residents' perception of the: (1) frequency of ordering unnecessary inpatient laboratory tests, (2) factors contributing to that behavior, and (3) potential interventions to change it. We also explored differences in responses by specialty and training level.
METHODS
In October 2014, we surveyed all IM and GS residents at the Hospital of the University of Pennsylvania. We reviewed the literature and conducted focus groups with residents to formulate items for the survey instrument. A draft of the survey was administered to 8 residents from both specialties, and their feedback was collated and incorporated into the final version of the instrument. The final 15‐question survey was comprised of 4 components: (1) training information such as specialty and postgraduate year (PGY), (2) self‐reported frequency of perceived unnecessary ordering of inpatient laboratory tests, (3) perception of factors contributing to unnecessary ordering, and (4) potential interventions to reduce unnecessary ordering. An unnecessary test was defined as a test that would not change management regardless of its result. To increase response rates, participants were entered into drawings for $5 gift cards, a $200 air travel voucher, and an iPad mini.
Descriptive statistics and 2tests were conducted with Stata version 13 (StataCorp LP, College Station, TX) to explore differences in the frequency of responses by specialty and training level. To identify themes that emerged from free‐text responses, two independent reviewers (M.S.S. and E.J.K.) performed qualitative content analysis using grounded theory.[10] Reviewers read 10% of responses to create a coding guide. Another 10% of the responses were randomly selected to assess inter‐rater reliability by calculating scores. The reviewers independently coded the remaining 80% of responses. Discrepancies were adjudicated by consensus between the reviewers. The University of Pennsylvania Institutional Review Board deemed this study exempt from review.
RESULTS
The sample comprised 57.0% (85/149) of IM and 54.4% (31/57) of GS residents (Table 1). Among respondents, perceived unnecessary inpatient laboratory test ordering was self‐reported by 88.2% of IM and 67.7% of GS residents. This behavior was reported to occur on a daily basis by 43.5% and 32.3% of responding IM and GS residents, respectively. Across both specialties, the most commonly reported factors contributing to these behaviors were learned practice habit/routine (90.5%), a lack of understanding of the costs associated with lab tests (86.2%), diagnostic uncertainty (82.8%), and fear of not having the lab result information when requested by an attending (75.9%). There were no significant differences in any of these contributing factors by specialty or PGY level. Among respondents who completed a free‐text response (IM: 76 of 85; GS: 21 of 31), the most commonly proposed interventions to address these issues were increasing cost transparency (IM 40.8%; GS 33.3%), improvements to faculty role modeling (IM 30.2%; GS 33.3%), and computerized reminders or decision support (IM 21.1%; GS 28.6%) (Table 2).
| Residents (n = 116)* | |
|---|---|
| |
| Reported he or she orders unnecessary routine labs, no. (%) | 96 (82.8) |
| Frequency of ordering unnecessary labs, no. (%) | |
| Daily | 47 (49.0) |
| 23 times/week | 44 (45.8) |
| 1 time/week or less | 5 (5.2) |
| Agreement with statement as factors contributing to ordering unnecessary labs, no. (%) | |
| Practice habit; I am trained to order repeating daily labs | 105 (90.5) |
| Lack of cost transparency of labs | 100 (86.2) |
| Discomfort with diagnostic uncertainty | 96 (82.8) |
| Concern that the attending will ask for the data and I will not have it | 88 (75.9) |
| Lack of role modeling of cost conscious care | 78 (67.2) |
| Lack of cost conscious culture at our institution | 76 (65.5) |
| Lack of experience | 72 (62.1) |
| Ease of ordering repeating labs in EHR | 60 (51.7) |
| Fear of litigation from missed diagnosis related to lab data | 44 (37.9) |
| Categories* | Representative Quotes | IM, n = 76, No. (%) | GS, n = 21, No. (%) |
|---|---|---|---|
| |||
| Cost transparency | Let us know the costs of what we order and train us to remember that a patient gets a bill and we are contributing to a possible bankruptcy or hardship. | 31 (40.8) | 7 (33.3) |
| Display the cost of labs when [we're] ordering them [in the EHR]. | |||
| Post the prices so that MDs understand how much everything costs. | |||
| Role modeling restrain | Train attendings to be more critical about necessity of labs and overordering. Make it part of rounding practice to decide on the labs truly needed for each patient the next day. | 23 (30.2) | 7 (33.3) |
| Attendings could review daily lab orders and briefly explain which they do not believe we need. This would allow residents to learn from their experience and their thought processes. | |||
| Encouragement and modeling of this practice from the faculty perhaps by laying out more clear expectations for which clinical situations warrant daily labs and which do not. | |||
| Computerized or decision support | When someone orders labs and the previous day's lab was normal or labs were stable for 2 days, an alert should pop up to reconsider. | 16 (21.1) | 6 (28.6) |
| Prevent us from being able to order repeating [or standing] labs. | |||
| Track how many times labs changed management, and restrict certain labslike LFTs/coags. | |||
| High‐value care educational curricula | Increase awareness of issue by having a noon conference about it or some other forum for residents to discuss the issue. | 12 (15.8) | 4 (19.0) |
| Establish guidelines for housestaff to learn/follow from start of residency. | |||
| Integrate cost conscious care into training program curricula. | |||
| System improvements | Make it easier to get labs later [in the day] | 6 (7.9) | 2 (9.5) |
| Improve timeliness of phlebotomy/laboratory systems. | |||
| More responsive phlebotomy. | |||
DISCUSSION
A significant portion of inpatient laboratory testing is unnecessary,[2] creating an opportunity to reduce utilization and associated costs. Our findings demonstrate that these behaviors occur at high levels among residents (IM 88.2%; GS 67.7%) at a large academic medical center. These findings also reveal that residents attribute this behavior to practice habit, lack of access to cost data, and perceived expectations for daily lab ordering by faculty. Interventions to change these behaviors will need to involve changes to the health system culture, increasing transparency of the costs associated with healthcare services, and shifting to a model of education that celebrates restraint.[11]
Our study adds to the emerging quest for delivering value in healthcare and provides several important insights for hospitalists and medical educators at academic centers. First, our findings reflect the significant role that the clinical learning environment plays in influencing practice behaviors among residents. Residency training is a critical time when physicians begin to form habits that imprint upon their future practice patterns,[5] and our residents are aware that their behavior to order what they perceive to be unnecessary laboratory tests is driven by habit. Studies[6, 7] have shown that residents may implicitly accept certain styles of practice as correct and are more likely to adopt those styles during the early years of their training. In our institution, for example, the process of ordering standing or daily morning labs using a repeated copy‐forward function in the electronic health record is a common, learned practice (a ritual) that is passed down from senior to junior residents year after year. This practice is common across both training specialties. There is a need to better understand, measure, and change the culture in the clinical learning environment to demonstrate practices and values that model high‐value care for residents. Multipronged interventions that address culture, oversight, and systems change[12] are necessary to facilitate effective physician stewardship of inpatient laboratory testing and attack a problem so deeply ingrained in habit.
Second, residents in our study believe that access to cost information will better equip them to reduce unnecessary lab ordering. Two recent systematic reviews[13, 14] have shown that having real‐time access to charges changes physician ordering and prescribing behavior. Increasing cost transparency may not only be an important intervention for hospitals to reduce overuse and control cost, but also better arm resident physicians with the information they need to make higher‐value recommendations for their patients and be stewards of healthcare resources.
Third, our study highlights that residents' unnecessary laboratory utilization is driven by perceived, unspoken expectations for such ordering by faculty. This reflects an important undercurrent in the medical education system that has historically emphasized and rewarded thoroughness while often penalizing restraint.[11] Hospitalists can play a major role in changing these behaviors by sharing their expectations regarding test ordering at the beginning of teaching rotations, including teaching points that discourage overutilization during rounds, and role modeling high‐value care in their own practice. Taken together and practiced routinely, these hospitalist behaviors could prevent poor habits from forming in our trainees and discourage overinvestigation. Hospitalists must be responsible to disseminate the practice of restraint to achieve more cost‐effective care. Purposeful faculty development efforts in the area of high‐value care are needed. Additionally, supporting physician leaders that serve as the institutional bridge between graduate medical education and the health system[15] could foster an environment conducive to coaching residents and faculty to reduce unnecessary practice variation.
This study is subject to several limitations. First, the survey was conducted at a single academic medical center, with a modest response rate, and thus our findings may not be generalizable to other settings or residents of different training programs. Second, we did not validate residents' perception of whether or not tests were, in fact, unnecessary. We also did not validate residents' self‐reporting of their own behavior, which may vary from actual behavior. Lack of validation at the level of the tests and at the level of the residents' behavior are two distinct but inter‐related limitations. Although self‐reported responses among residents are an important indicator of their practice, validating these data with objective measures, such as a measure of necessity of ordered lab tests as determined by an expert physician or group of experienced physicians or the number of inpatient labs ordered by residents, may add further insights. Ordering of perceived unnecessary tests may be even more common if there was under‐reporting of this behavior. Third, although we provided a definition within the survey, interpretation among survey respondents of the term unnecessary may vary, and this variation may contribute to our findings. However, we did provide a clear definition in the survey and we attempted to mitigate this with feedback from residents on our preliminary pilot.
In conclusion, this is one of the first qualitative evaluations to explore residents' perceptions on why they order unnecessary inpatient laboratory tests. Our findings offer a rich understanding of residents' beliefs about their own role in unnecessary lab ordering and explore possible solutions through the lens of the resident. Yet, it is unclear whether tests deemed unnecessary by residents would also be considered unnecessary by attending physicians or even patients. Future efforts are needed to better define which inpatient tests are unnecessary from multiple perspectives including clinicians and patients.
Acknowledgements
The authors thank Patrick J. Brennan, MD, Jeffery S. Berns, MD, Lisa M. Bellini, MD, Jon B. Morris, MD, and Irving Nachamkin, DrPH, MPH, all from the Hospital of the University of Pennsylvania, for supporting this work. They received no compensation.
Disclosures: This work was presented in part at the AAMC Integrating Quality Meeting, June 11, 2015, Chicago, Illinois; and the Alliance for Academic Internal Medicine Fall Meeting, October 9, 2015, Atlanta, Georgia. The authors report no conflicts of interest.
Resident physicians routinely order inpatient laboratory tests,[1] and there is evidence to suggest that many of these tests are unnecessary[2] and potentially harmful.[3] The Society of Hospital Medicine has identified reducing the unnecessary ordering of inpatient laboratory testing as part of the Choosing Wisely campaign.[4] Hospitalists at academic medical centers face growing pressures to develop processes to reduce low‐value care and train residents to be stewards of healthcare resources.[5] Studies[6, 7, 8, 9] have described that institutional and training factors drive residents' resource utilization patterns, but, to our knowledge, none have described what factors contribute to residents' unnecessary laboratory testing. To better understand the factors associated with residents' ordering patterns, we conducted a qualitative analysis of internal medicine (IM) and general surgery (GS) residents at a large academic medical center in order to describe residents' perception of the: (1) frequency of ordering unnecessary inpatient laboratory tests, (2) factors contributing to that behavior, and (3) potential interventions to change it. We also explored differences in responses by specialty and training level.
METHODS
In October 2014, we surveyed all IM and GS residents at the Hospital of the University of Pennsylvania. We reviewed the literature and conducted focus groups with residents to formulate items for the survey instrument. A draft of the survey was administered to 8 residents from both specialties, and their feedback was collated and incorporated into the final version of the instrument. The final 15‐question survey was comprised of 4 components: (1) training information such as specialty and postgraduate year (PGY), (2) self‐reported frequency of perceived unnecessary ordering of inpatient laboratory tests, (3) perception of factors contributing to unnecessary ordering, and (4) potential interventions to reduce unnecessary ordering. An unnecessary test was defined as a test that would not change management regardless of its result. To increase response rates, participants were entered into drawings for $5 gift cards, a $200 air travel voucher, and an iPad mini.
Descriptive statistics and 2tests were conducted with Stata version 13 (StataCorp LP, College Station, TX) to explore differences in the frequency of responses by specialty and training level. To identify themes that emerged from free‐text responses, two independent reviewers (M.S.S. and E.J.K.) performed qualitative content analysis using grounded theory.[10] Reviewers read 10% of responses to create a coding guide. Another 10% of the responses were randomly selected to assess inter‐rater reliability by calculating scores. The reviewers independently coded the remaining 80% of responses. Discrepancies were adjudicated by consensus between the reviewers. The University of Pennsylvania Institutional Review Board deemed this study exempt from review.
RESULTS
The sample comprised 57.0% (85/149) of IM and 54.4% (31/57) of GS residents (Table 1). Among respondents, perceived unnecessary inpatient laboratory test ordering was self‐reported by 88.2% of IM and 67.7% of GS residents. This behavior was reported to occur on a daily basis by 43.5% and 32.3% of responding IM and GS residents, respectively. Across both specialties, the most commonly reported factors contributing to these behaviors were learned practice habit/routine (90.5%), a lack of understanding of the costs associated with lab tests (86.2%), diagnostic uncertainty (82.8%), and fear of not having the lab result information when requested by an attending (75.9%). There were no significant differences in any of these contributing factors by specialty or PGY level. Among respondents who completed a free‐text response (IM: 76 of 85; GS: 21 of 31), the most commonly proposed interventions to address these issues were increasing cost transparency (IM 40.8%; GS 33.3%), improvements to faculty role modeling (IM 30.2%; GS 33.3%), and computerized reminders or decision support (IM 21.1%; GS 28.6%) (Table 2).
| Residents (n = 116)* | |
|---|---|
| |
| Reported he or she orders unnecessary routine labs, no. (%) | 96 (82.8) |
| Frequency of ordering unnecessary labs, no. (%) | |
| Daily | 47 (49.0) |
| 23 times/week | 44 (45.8) |
| 1 time/week or less | 5 (5.2) |
| Agreement with statement as factors contributing to ordering unnecessary labs, no. (%) | |
| Practice habit; I am trained to order repeating daily labs | 105 (90.5) |
| Lack of cost transparency of labs | 100 (86.2) |
| Discomfort with diagnostic uncertainty | 96 (82.8) |
| Concern that the attending will ask for the data and I will not have it | 88 (75.9) |
| Lack of role modeling of cost conscious care | 78 (67.2) |
| Lack of cost conscious culture at our institution | 76 (65.5) |
| Lack of experience | 72 (62.1) |
| Ease of ordering repeating labs in EHR | 60 (51.7) |
| Fear of litigation from missed diagnosis related to lab data | 44 (37.9) |
| Categories* | Representative Quotes | IM, n = 76, No. (%) | GS, n = 21, No. (%) |
|---|---|---|---|
| |||
| Cost transparency | Let us know the costs of what we order and train us to remember that a patient gets a bill and we are contributing to a possible bankruptcy or hardship. | 31 (40.8) | 7 (33.3) |
| Display the cost of labs when [we're] ordering them [in the EHR]. | |||
| Post the prices so that MDs understand how much everything costs. | |||
| Role modeling restrain | Train attendings to be more critical about necessity of labs and overordering. Make it part of rounding practice to decide on the labs truly needed for each patient the next day. | 23 (30.2) | 7 (33.3) |
| Attendings could review daily lab orders and briefly explain which they do not believe we need. This would allow residents to learn from their experience and their thought processes. | |||
| Encouragement and modeling of this practice from the faculty perhaps by laying out more clear expectations for which clinical situations warrant daily labs and which do not. | |||
| Computerized or decision support | When someone orders labs and the previous day's lab was normal or labs were stable for 2 days, an alert should pop up to reconsider. | 16 (21.1) | 6 (28.6) |
| Prevent us from being able to order repeating [or standing] labs. | |||
| Track how many times labs changed management, and restrict certain labslike LFTs/coags. | |||
| High‐value care educational curricula | Increase awareness of issue by having a noon conference about it or some other forum for residents to discuss the issue. | 12 (15.8) | 4 (19.0) |
| Establish guidelines for housestaff to learn/follow from start of residency. | |||
| Integrate cost conscious care into training program curricula. | |||
| System improvements | Make it easier to get labs later [in the day] | 6 (7.9) | 2 (9.5) |
| Improve timeliness of phlebotomy/laboratory systems. | |||
| More responsive phlebotomy. | |||
DISCUSSION
A significant portion of inpatient laboratory testing is unnecessary,[2] creating an opportunity to reduce utilization and associated costs. Our findings demonstrate that these behaviors occur at high levels among residents (IM 88.2%; GS 67.7%) at a large academic medical center. These findings also reveal that residents attribute this behavior to practice habit, lack of access to cost data, and perceived expectations for daily lab ordering by faculty. Interventions to change these behaviors will need to involve changes to the health system culture, increasing transparency of the costs associated with healthcare services, and shifting to a model of education that celebrates restraint.[11]
Our study adds to the emerging quest for delivering value in healthcare and provides several important insights for hospitalists and medical educators at academic centers. First, our findings reflect the significant role that the clinical learning environment plays in influencing practice behaviors among residents. Residency training is a critical time when physicians begin to form habits that imprint upon their future practice patterns,[5] and our residents are aware that their behavior to order what they perceive to be unnecessary laboratory tests is driven by habit. Studies[6, 7] have shown that residents may implicitly accept certain styles of practice as correct and are more likely to adopt those styles during the early years of their training. In our institution, for example, the process of ordering standing or daily morning labs using a repeated copy‐forward function in the electronic health record is a common, learned practice (a ritual) that is passed down from senior to junior residents year after year. This practice is common across both training specialties. There is a need to better understand, measure, and change the culture in the clinical learning environment to demonstrate practices and values that model high‐value care for residents. Multipronged interventions that address culture, oversight, and systems change[12] are necessary to facilitate effective physician stewardship of inpatient laboratory testing and attack a problem so deeply ingrained in habit.
Second, residents in our study believe that access to cost information will better equip them to reduce unnecessary lab ordering. Two recent systematic reviews[13, 14] have shown that having real‐time access to charges changes physician ordering and prescribing behavior. Increasing cost transparency may not only be an important intervention for hospitals to reduce overuse and control cost, but also better arm resident physicians with the information they need to make higher‐value recommendations for their patients and be stewards of healthcare resources.
Third, our study highlights that residents' unnecessary laboratory utilization is driven by perceived, unspoken expectations for such ordering by faculty. This reflects an important undercurrent in the medical education system that has historically emphasized and rewarded thoroughness while often penalizing restraint.[11] Hospitalists can play a major role in changing these behaviors by sharing their expectations regarding test ordering at the beginning of teaching rotations, including teaching points that discourage overutilization during rounds, and role modeling high‐value care in their own practice. Taken together and practiced routinely, these hospitalist behaviors could prevent poor habits from forming in our trainees and discourage overinvestigation. Hospitalists must be responsible to disseminate the practice of restraint to achieve more cost‐effective care. Purposeful faculty development efforts in the area of high‐value care are needed. Additionally, supporting physician leaders that serve as the institutional bridge between graduate medical education and the health system[15] could foster an environment conducive to coaching residents and faculty to reduce unnecessary practice variation.
This study is subject to several limitations. First, the survey was conducted at a single academic medical center, with a modest response rate, and thus our findings may not be generalizable to other settings or residents of different training programs. Second, we did not validate residents' perception of whether or not tests were, in fact, unnecessary. We also did not validate residents' self‐reporting of their own behavior, which may vary from actual behavior. Lack of validation at the level of the tests and at the level of the residents' behavior are two distinct but inter‐related limitations. Although self‐reported responses among residents are an important indicator of their practice, validating these data with objective measures, such as a measure of necessity of ordered lab tests as determined by an expert physician or group of experienced physicians or the number of inpatient labs ordered by residents, may add further insights. Ordering of perceived unnecessary tests may be even more common if there was under‐reporting of this behavior. Third, although we provided a definition within the survey, interpretation among survey respondents of the term unnecessary may vary, and this variation may contribute to our findings. However, we did provide a clear definition in the survey and we attempted to mitigate this with feedback from residents on our preliminary pilot.
In conclusion, this is one of the first qualitative evaluations to explore residents' perceptions on why they order unnecessary inpatient laboratory tests. Our findings offer a rich understanding of residents' beliefs about their own role in unnecessary lab ordering and explore possible solutions through the lens of the resident. Yet, it is unclear whether tests deemed unnecessary by residents would also be considered unnecessary by attending physicians or even patients. Future efforts are needed to better define which inpatient tests are unnecessary from multiple perspectives including clinicians and patients.
Acknowledgements
The authors thank Patrick J. Brennan, MD, Jeffery S. Berns, MD, Lisa M. Bellini, MD, Jon B. Morris, MD, and Irving Nachamkin, DrPH, MPH, all from the Hospital of the University of Pennsylvania, for supporting this work. They received no compensation.
Disclosures: This work was presented in part at the AAMC Integrating Quality Meeting, June 11, 2015, Chicago, Illinois; and the Alliance for Academic Internal Medicine Fall Meeting, October 9, 2015, Atlanta, Georgia. The authors report no conflicts of interest.
- , , , . The impact of residents, interns, and attendings on inpatient laboratory ordering patterns: a report from one university's hospitalist service. Acad Med. 2011;86(1):139–145.
- , , , , . The landscape of inappropriate laboratory testing: a 15‐year meta‐analysis. PLoS One. 2013;8(11):e78962.
- , , , et al. Diagnostic blood loss from phlebotomy and hospital‐acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171(18):1646–1653.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- . Charting the route to high‐value care the role of medical education. JAMA. 2016;314(22):2359–2361.
- , , , , . Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385–2393.
- , , , . The association between residency training and internists' ability to practice conservatively. JAMA Intern Med. 2014;174(10):1640–1648.
- , , , , . Effect of attending practice style on generic medication prescribing by residents in the clinic setting: an observational study. J Gen Intern Med. 2015;30(9):1286–1293.
- , , , . Role‐modeling cost‐conscious care—a national evaluation of perceptions of faculty at teaching hospitals in the United States. J Gen Intern Med. 2015;30(9):1294–1298.
- , . The discovery of grounded theory. Int J Qual Methods. 1967;5:1–10.
- , . A new model for medical education: celebrating restraint. JAMA. 2012;308(13):1329–1330.
- , , . A framework for the frontline: how hospitalists can improve healthcare value. J Hosp Med. 2016;11(4):297–302.
- , , , . The effect of charge display on cost of care and physician practice behaviors: a systematic review. J Gen Intern Med. 2015;30(6):835–842.
- , , , . Impact of price display on provider ordering: a systematic review. J Hosp Med. 2016;11(1):65–76.
- , . Merging the health system and education silos to better educate future physicians. JAMA. 2015;314(22):2349–2350.
- , , , . The impact of residents, interns, and attendings on inpatient laboratory ordering patterns: a report from one university's hospitalist service. Acad Med. 2011;86(1):139–145.
- , , , , . The landscape of inappropriate laboratory testing: a 15‐year meta‐analysis. PLoS One. 2013;8(11):e78962.
- , , , et al. Diagnostic blood loss from phlebotomy and hospital‐acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171(18):1646–1653.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- . Charting the route to high‐value care the role of medical education. JAMA. 2016;314(22):2359–2361.
- , , , , . Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385–2393.
- , , , . The association between residency training and internists' ability to practice conservatively. JAMA Intern Med. 2014;174(10):1640–1648.
- , , , , . Effect of attending practice style on generic medication prescribing by residents in the clinic setting: an observational study. J Gen Intern Med. 2015;30(9):1286–1293.
- , , , . Role‐modeling cost‐conscious care—a national evaluation of perceptions of faculty at teaching hospitals in the United States. J Gen Intern Med. 2015;30(9):1294–1298.
- , . The discovery of grounded theory. Int J Qual Methods. 1967;5:1–10.
- , . A new model for medical education: celebrating restraint. JAMA. 2012;308(13):1329–1330.
- , , . A framework for the frontline: how hospitalists can improve healthcare value. J Hosp Med. 2016;11(4):297–302.
- , , , . The effect of charge display on cost of care and physician practice behaviors: a systematic review. J Gen Intern Med. 2015;30(6):835–842.
- , , , . Impact of price display on provider ordering: a systematic review. J Hosp Med. 2016;11(1):65–76.
- , . Merging the health system and education silos to better educate future physicians. JAMA. 2015;314(22):2349–2350.
Comportment and Communication Score
In 2014, there were more than 40,000 hospitalists in the United States, and approximately 20% were employed by academic medical centers.[1] Hospitalist physicians groups are committed to delivering excellent patient care. However, the published literature is limited with respect to defining optimal care in hospital medicine.
Patient satisfaction surveys, such as Press Ganey (PG)[2] and Hospital Consumer Assessment of Healthcare Providers and Systems,[3] are being used to assess patients' contentment with the quality of care they receive while hospitalized. The Society of Hospital Medicine, the largest professional medical society representing hospitalists, encourages the use of patient satisfaction surveys to measure hospitalist providers' quality of patient care.[4] There are, however, several problems with the current methods. First, the attribution to specific providers is questionable. Second, recall about the provider by the patients may be poor because surveys are sent to patients days after they return home. Third, the patients' recovery and health outcomes are likely to influence their assessment of the doctor. Finally, feedback is known to be most valuable and transformative when it is specific and given in real time. Thus, a tool that is able to provide feedback at the encounter level should be more helpful than a tool that offers assessment at the level of the admission, particularly when it can be also delivered immediately after the data are collected.
Comportment has been used to describe both the way a person behaves and also the way she carries herself (ie, her general manner).[5] Excellent comportment and communication can serve as the foundation for delivering patient‐centered care.[6, 7, 8] Patient centeredness has been shown to improve the patient experience and clinical outcomes, including compliance with therapeutic plans.[9, 10, 11] Respectful behavior, etiquette‐based medicine, and effective communication also lay the foundation upon which the therapeutic alliance between a doctor and patient can be built.
The goal of this study was to establish a metric that could comprehensively assess a hospitalist provider's comportment and communication skills during an encounter with a hospitalized patient.
METHODS
Study Design and Setting
An observational study of hospitalist physicians was conducted between June 2013 and December 2013 at 5 hospitals in Maryland and Washington DC. Two are academic medical centers (Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center [JHBMC]), and the others are community hospitals (Howard County General Hospital [HCGH], Sibley Memorial Hospital [SMC], and Suburban Hospital). These 5 hospitals, across 2 large cities, have distinct culture and leadership, each serving different populations.
Subjects
In developing a tool to measure communication and comportment, we needed to observe physicianpatient encounters wherein there would be a good deal of variability in performance. During pilot testing, when following a few of the most senior and respected hospitalists, we noted encounters during which they excelled and others where they performed less optimally. Further, in following some less‐experienced providers, their skills were less developed and they were uniformly missing most of the behaviors on the tool that were believed to be associated with optimal communication and comportment. Because of this, we decided to purposively sample the strongest clinicians at each of the 5 hospitals in hopes of seeing a range of scores on the tool.
The chiefs of hospital medicine at the 5 hospitals were contacted and asked to identify their most clinically excellent hospitalists, namely those who they thought were most clinically skilled within their groups. Because our goal was to observe the top tier (approximately 20%) of the hospitalists within each group, we asked each chief to name a specific number of physicians (eg, 3 names for 1 group with 15 hospitalists, and 8 from another group with 40 physicians). No precise definition of most clinically excellent hospitalists was provided to the chiefs. It was believed that they were well positioned to select their best clinicians because of both subjective feedback and objective data that flow to them. This postulate may have been corroborated by the fact that each of them efficiently sent a list of their top choices without any questions being asked.
The 29 hospitalists (named by their chiefs) were in turn emailed and invited to participate in the study. All but 3 hospitalists consented to participate in the study; this resulted in a cohort of 26 who would be observed.
Tool Development
A team was assembled to develop the hospital medicine comportment and communication observation tool (HMCCOT). All team members had extensive clinical experience, several had published articles on clinical excellence, had won clinical awards, and all had been teaching clinical skills for many years. The team's development of the HMCCOT was extensively informed by a review of the literature. Two articles that most heavily influenced the HMCCOT's development were Christmas et al.'s paper describing 7 core domains of excellence, 2 of which are intimately linked to communication and comportment,[12] and Kahn's text that delineates behaviors to be performed upon entering the patient's room, termed etiquette‐based medicine.[6] The team also considered the work from prior timemotion studies in hospital medicine,[7, 13] which led to the inclusion of temporal measurements during the observations. The tool was also presented at academic conferences in the Division of General Internal Medicine at Johns Hopkins and iteratively revised based on the feedback. Feedback was sought from people who have spent their entire career studying physicianpatient relationships and who are members of the American Academy on Communication in Healthcare. These methods established content validity evidence for the tool under development. The goal of the HMCCOT was to assess behaviors believed to be associated with optimal comportment and communication in hospital medicine.
The HMCCOT was pilot tested by observing different JHBMC hospitalists patient encounters and it was iteratively revised. On multiple occasions, 2 authors/emnvestigators spent time observing JHBMC hospitalists together and compared data capture and levels of agreement across all elements. Then, for formal assessment of inter‐rater reliability, 2 authors observed 5 different hospitalists across 25 patient encounters; the coefficient was 0.91 (standard error = 0.04). This step helped to establish internal structure validity evidence for the tool.
The initial version of the HMCCOT contained 36 elements, and it was organized sequentially to allow the observer to document behaviors in the order that they were likely to occur so as to facilitate the process and to minimize oversight. A few examples of the elements were as follows: open‐ended versus a close‐ended statement at the beginning of the encounter, hospitalist introduces himself/herself, and whether the provider smiles at any point during the patient encounter.
Data Collection
One author scheduled a time to observe each hospitalist physician during their routine clinical care of patients when they were not working with medical learners. Hospitalists were naturally aware that they were being observed but were not aware of the specific data elements or behaviors that were being recorded.
The study was approved by the institutional review board at the Johns Hopkins University School of Medicine, and by each of the research review committees at HCGH, SMC, and Suburban hospitals.
Data Analysis
After data collection, all data were deidentified so that the researchers were blinded to the identities of the physicians. Respondent characteristics are presented as proportions and means. Unpaired t test and 2 tests were used to compare demographic information, and stratified by mean HMCCOT score. The survey data were analyzed using Stata statistical software version 12.1 (StataCorp LP, College Station, TX).
Further Validation of the HMCCOT
Upon reviewing the distribution of data after observing the 26 physicians with their patients, we excluded 13 variables from the initial version of the tool that lacked discriminatory value (eg, 100% or 0% of physicians performed the observed behavior during the encounters); this left 23 variables that were judged to be most clinically relevant in the final version of the HMCCOT. Two examples of the variables that were excluded were: uses technology/literature to educate patients (not witnessed in any encounter), and obeys posted contact precautions (done uniformly by all). The HMCCOT score represents the proportion of observed behaviors (out of the 23 behaviors). It was computed for each hospitalist for every patient encounter. Finally, relation to other variables validity evidence would be established by comparing the mean HMCCOT scores of the physicians to their PG scores from the same time period to evaluate the correlation between the 2 scores. This association was assessed using Pearson correlations.
RESULTS
The average clinical experience of the 26 hospitalist physicians studied was 6 years (Table 1). Their mean age was 38 years, 13 (50%) were female, and 16 (62%) were of nonwhite race. Fourteen hospitalists (54%) worked at 1 of the nonacademic hospitals. In terms of clinical workload, most physicians (n = 17, 65%) devoted more than 70% of their time working in direct patient care. Mean time spent observing each physician was 280 minutes. During this time, the 26 physicians were observed for 181 separate clinical encounters; 54% of these patients were new encounters, patients who were not previously known to the physician. The average time each physician spent in a patient room was 10.8 minutes. Mean number of observed patient encounters per hospitalist was 7.
| Total Study Population, n = 26 | HMCCOT Score 60, n = 14 | HMCCOT Score >60, n = 12 | P Value* | |
|---|---|---|---|---|
| ||||
| Age, mean (SD) | 38 (5.6) | 37.9 (5.6) | 38.1 (5.7) | 0.95 |
| Female, n (%) | 13 (50) | 6 (43) | 7 (58) | 0.43 |
| Race, n (%) | ||||
| Caucasian | 10 (38) | 5 (36) | 5 (41) | 0.31 |
| Asian | 13 (50) | 8 (57) | 5 (41) | |
| African/African American | 2 (8) | 0 (0) | 2 (17) | |
| Other | 1 (4) | 1 (7) | 0 (0) | |
| Clinical experience >6 years, n (%) | 12 (46) | 6 (43) | 6 (50) | 0.72 |
| Clinical workload >70% | 17 (65) | 10 (71) | 7 (58) | 0.48 |
| Academic hospitalist, n (%) | 12 (46) | 5 (36) | 7 (58) | 0.25 |
| Hospital | 0.47 | |||
| JHBMC | 8 (31) | 3 (21.4) | 5 (41) | |
| JHH | 4 (15) | 2 (14.3) | 2 (17) | |
| HCGH | 5 (19) | 3 (21.4) | 2 (17) | |
| Suburban | 6 (23) | 3 (21.4) | 3 (25) | |
| SMC | 3 (12) | 3 (21.4) | 0 (0) | |
| Minutes spent observing hospitalist per shift, mean (SD) | 280 (104.5) | 280.4 (115.5) | 281.4 (95.3) | 0.98 |
| Average time spent per patient encounter in minutes, mean (SD) | 10.8 (8.9) | 8.7 (9.1) | 13 (8.1) | 0.001 |
| Proportion of observed patients who were new to provider, % | 97 (53.5) | 37 (39.7) | 60 (68.1) | 0.001 |
The distribution of HMCCOT scores was not statistically significantly different when analyzed by age, gender, race, amount of clinical experience, clinical workload of the hospitalist, hospital, time spent observing the hospitalist (all P > 0.05). The distribution of HMCCOT scores was statistically different in new patient encounters compared to follow‐ups (68.1% vs 39.7%, P 0.001). Encounters with patients that generated HMCCOT scores above versus below the mean were longer (13 minutes vs 8.7 minutes, P 0.001).
The mean HMCCOT score was 61 (standard deviation [SD] = 10.6), and it was normally distributed (Figure 1). Table 2 shows the data for the 23 behaviors that were objectively assessed as part of the HMCCOT for the 181 patient encounters. The most frequently observed behaviors were physicians washing hands after leaving the patient's room in 170 (94%) of the encounters and smiling (83%). The behaviors that were observed with the least regularity were using an empathic statement (26% of encounters), and employing teach‐back (13% of encounters). A common method of demonstrating interest in the patient as a person, seen in 41% of encounters, involved physicians asking about patients' personal histories and their interests.
| Variables | All Visits Combined, n = 181 | HMCCOT Score 60, n = 93 | HMCCOT Score >60, n = 88 | P Value* |
|---|---|---|---|---|
| ||||
| Objective observations, n (%) | ||||
| Washes hands after leaving room | 170 (94) | 83 (89) | 87 (99) | 0.007 |
| Discusses plan for the day | 163 (91) | 78 (84) | 85 (99) | 0.001 |
| Does not interrupt the patient | 159 (88) | 79 (85) | 80 (91) | 0.21 |
| Smiles | 149 (83) | 71 (77) | 78 (89) | 0.04 |
| Washes hands before entering | 139 (77) | 64 (69) | 75 (85) | 0.009 |
| Begins with open‐ended question | 134 (77) | 68 (76) | 66 (78) | 0.74 |
| Knocks before entering the room | 127 (76) | 57 (65) | 70 (89) | 0.001 |
| Introduces him/herself to the patient | 122 (67) | 45 (48) | 77 (88) | 0.001 |
| Explains his/her role | 120 (66) | 44 (47) | 76 (86) | 0.001 |
| Asks about pain | 110 (61) | 45 (49) | 65 (74) | 0.001 |
| Asks permission prior to examining | 106 (61) | 43 (50) | 63 (72) | 0.002 |
| Uncovers body area for the physical exam | 100 (57) | 34 (38) | 66 (77) | 0.001 |
| Discusses discharge plan | 99 (55) | 38 (41) | 61 (71) | 0.001 |
| Sits down in the patient room | 74 (41) | 24 (26) | 50 (57) | 0.001 |
| Asks about patient's feelings | 58 (33) | 17 (19) | 41 (47) | 0.001 |
| Shakes hands with the patient | 57 (32) | 17 (18) | 40 (46) | 0.001 |
| Uses teach‐back | 24 (13) | 4 (4.3) | 20 (24) | 0.001 |
| Subjective observations, n (%) | ||||
| Avoids medical jargon | 160 (89) | 85 (91) | 83 (95) | 0.28 |
| Demonstrates interest in patient as a person | 72 (41) | 16 (18) | 56 (66) | 0.001 |
| Touches appropriately | 62 (34) | 21 (23) | 41 (47) | 0.001 |
| Shows sensitivity to patient modesty | 57 (93) | 15 (79) | 42 (100) | 0.002 |
| Engages in nonmedical conversation | 54 (30) | 10 (11) | 44 (51) | 0.001 |
| Uses empathic statement | 47 (26) | 9 (10) | 38 (43) | 0.001 |
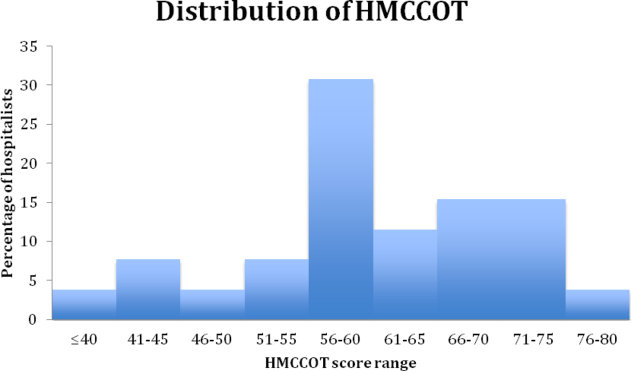
The average composite PG scores for the physician sample was 38.95 (SD=39.64). A moderate correlation was found between the HMCCOT score and PG score (adjusted Pearson correlation: 0.45, P = 0.047).
DISCUSSION
In this study, we followed 26 hospitalist physicians during routine clinical care, and we focused intently on their communication and their comportment with patients at the bedside. Even among clinically respected hospitalists, the results reveal that there is wide variability in comportment and communication practices and behaviors at the bedside. The physicians' HMCCOT scores were associated with their PG scores. These findings suggest that improved bedside communication and comportment with patients might translate into enhanced patient satisfaction.
This is the first study that honed in on hospitalist communication and comportment. With validity evidence established for the HMCCOT, some may elect to more explicitly perform these behaviors themselves, and others may wish to watch other hospitalists to give them feedback that is tied to specific behaviors. Beginning with the basics, the hospitalists we studied introduced themselves to their patients at the initial encounter 78% of the time, less frequently than is done by primary care clinicians (89%) but more consistently than do emergency department providers (64%).[7] Other variables that stood out in the HMCCOT was that teach‐back was employed in only 13% of the encounters. Previous studies have shown that teach‐back corroborates patient comprehension and can be used to engage patients (and caregivers) in realistic goal setting and optimal health service utilization.[14] Further, patients who clearly understand their postdischarge plan are 30% less likely to be readmitted or visit the emergency department.[14] The data for our group have helped us to see areas of strengths, such as hand washing, where we are above compliance rates across hospitals in the United States,[15] as well as those matters that represent opportunities for improvement such as connecting more deeply with our patients.
Tackett et al. have looked at encounter length and its association with performance of etiquette‐based medicine behaviors.[7] Similar to their study, we found a positive correlation between spending more time with patients and higher HMCCOT scores. We also found that HMCCOT scores were higher when providers were caring for new patients. Patients' complaints about doctors often relate to feeling rushed, that their physicians did not listen to them, or that information was not conveyed in a clear manner.[16] Such challenges in physicianpatient communication are ubiquitous across clinical settings.[16] When successfully achieved, patient‐centered communication has been associated with improved clinical outcomes, including adherence to recommended treatment and better self‐management of chronic disease.[17, 18, 19, 20, 21, 22, 23, 24, 25, 26] Many of the components of the HMCCOT described in this article are at the heart of patient‐centered care.
Several limitations of the study should be considered. First, physicians may have behaved differently while they were being observed, which is known as the Hawthorne effect. We observed them for many hours and across multiple patient encounters, and the physicians were not aware of the specific types of data that we were collecting. These factors may have limited the biases along such lines. Second, there may be elements of optimal comportment and communication that were not captured by the HMCCOT. Hopefully, there are not big gaps, as we used multiple methods and an iterative process in the refinement of the HMCCOT metric. Third, one investigator did all of the observing, and it is possible that he might have missed certain behaviors. Through extensive pilot testing and comparisons with other raters, the observer became very skilled and facile with such data collection and the tool. Fourth, we did not survey the same patients that were cared for to compare their perspectives to the HMCCOT scores following the clinical encounters. For patient perspectives, we relied only on PG scores. Fifth, quality of care is a broad and multidimensional construct. The HMCCOT focuses exclusively on hospitalists' comportment and communication at the bedside; therefore, it does not comprehensively assess care quality. Sixth, with our goal to optimally validate the HMCCOT, we tested it on the top tier of hospitalists within each group. We may have observed different results had we randomly selected hospitalists from each hospital or had we conducted the study at hospitals in other geographic regions. Finally, all of the doctors observed worked at hospitals in the Mid‐Atlantic region. However, these five distinct hospitals each have their own cultures, and they are led by different administrators. We purposively chose to sample both academic as well as community settings.
In conclusion, this study reports on the development of a comportment and communication tool that was established and validated by following clinically excellent hospitalists at the bedside. Future studies are necessary to determine whether hospitalists of all levels of experience and clinical skill can improve when given data and feedback using the HMCCOT. Larger studies will then be needed to assess whether enhancing comportment and communication can truly improve patient satisfaction and clinical outcomes in the hospital.
Disclosures: Dr. Wright is a Miller‐Coulson Family Scholar and is supported through the Johns Hopkins Center for Innovative Medicine. Susrutha Kotwal, MD, and Waseem Khaliq, MD, contributed equally to this work. The authors report no conflicts of interest.
- 2014 state of hospital medicine report. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org/Web/Practice_Management/State_of_HM_Surveys/2014.aspx. Accessed January 10, 2015.
- Press Ganey website. Available at: http://www.pressganey.com/home. Accessed December 15, 2015.
- Hospital Consumer Assessment of Healthcare Providers and Systems website. Available at: http://www.hcahpsonline.org/home.aspx. Accessed February 2, 2016.
- Membership committee guidelines for hospitalists patient satisfaction surveys. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed February 2, 2016.
- Definition of comportment. Available at: http://www.vocabulary.com/dictionary/comportment. Accessed December 15, 2015.
- . Etiquette‐based medicine. N Engl J Med. 2008;358(19):1988–1989.
- , , , , . Appraising the practice of etiquette‐based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908–913.
- , , . Developing physician communication skills for patient‐centered care. Health Aff (Millwood). 2010;29(7):1310–1318.
- . The impact on patient health outcomes of interventions targeting the patient–physician relationship. Patient. 2009;2(2):77–84.
- , , , , , . Effect on health‐related outcomes of interventions to alter the interaction between patients and practitioners: a systematic review of trials. Ann Fam Med. 2004;2(6):595–608.
- , , , . How does communication heal? Pathways linking clinician–patient communication to health outcomes. Patient Educ Couns. 2009;74(3):295–301.
- , , , . Clinical excellence in academia: perspectives from masterful academic clinicians. Mayo Clin Proc. 2008;83(9):989–994.
- , , , et al. Where did the day go?—a time‐motion study of hospitalists. J Hosp Med. 2010;5(6):323–328.
- , , , et al. Reducing readmissions using teach‐back: enhancing patient and family education. J Nurs Adm. 2015;45(1):35–42.
- , , . Hand hygiene compliance rates in the United States—a one‐year multicenter collaboration using product/volume usage measurement and feedback. Am J Med Qual. 2009;24(3):205–213.
- , , , et al. Obstetricians' prior malpractice experience and patients' satisfaction with care. JAMA. 1994;272(20):1583–1587.
- , . Patient‐Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. NIH publication no. 07–6225. Bethesda, MD: National Cancer Institute; 2007.
- . Interacting with cancer patients: the significance of physicians' communication behavior. Soc Sci Med. 2003;57(5):791–806.
- , , . Expanding patient involvement in care: effects on patient outcomes. Ann Intern Med. 1985;102(4):520–528.
- , . Measuring patient‐centeredness: a comparison of three observation‐based instruments. Patient Educ Couns. 2000;39(1):71–80.
- , , , . Doctor‐patient communication: a review of the literature. Soc Sci Med. 1995;40(7):903–918.
- , , , , , . Linking primary care performance to outcomes of care. J Fam Pract. 1998;47(3):213–220.
- , , , et al. The impact of patient‐centered care on outcomes. J Fam Pract. 2000;49(9):796–804.
- , , , et al. Measuring patient‐centered communication in patient‐physician consultations: theoretical and practical issues. Soc Sci Med. 2005;61(7):1516–1528.
- , . Patient‐centered consultations and outcomes in primary care: a review of the literature. Patient Educ Couns. 2002;48(1):51–61.
- , , . Doctor‐patient communication and satisfaction with care in oncology. Curr Opin Oncol. 2005;17(4):351–354.
In 2014, there were more than 40,000 hospitalists in the United States, and approximately 20% were employed by academic medical centers.[1] Hospitalist physicians groups are committed to delivering excellent patient care. However, the published literature is limited with respect to defining optimal care in hospital medicine.
Patient satisfaction surveys, such as Press Ganey (PG)[2] and Hospital Consumer Assessment of Healthcare Providers and Systems,[3] are being used to assess patients' contentment with the quality of care they receive while hospitalized. The Society of Hospital Medicine, the largest professional medical society representing hospitalists, encourages the use of patient satisfaction surveys to measure hospitalist providers' quality of patient care.[4] There are, however, several problems with the current methods. First, the attribution to specific providers is questionable. Second, recall about the provider by the patients may be poor because surveys are sent to patients days after they return home. Third, the patients' recovery and health outcomes are likely to influence their assessment of the doctor. Finally, feedback is known to be most valuable and transformative when it is specific and given in real time. Thus, a tool that is able to provide feedback at the encounter level should be more helpful than a tool that offers assessment at the level of the admission, particularly when it can be also delivered immediately after the data are collected.
Comportment has been used to describe both the way a person behaves and also the way she carries herself (ie, her general manner).[5] Excellent comportment and communication can serve as the foundation for delivering patient‐centered care.[6, 7, 8] Patient centeredness has been shown to improve the patient experience and clinical outcomes, including compliance with therapeutic plans.[9, 10, 11] Respectful behavior, etiquette‐based medicine, and effective communication also lay the foundation upon which the therapeutic alliance between a doctor and patient can be built.
The goal of this study was to establish a metric that could comprehensively assess a hospitalist provider's comportment and communication skills during an encounter with a hospitalized patient.
METHODS
Study Design and Setting
An observational study of hospitalist physicians was conducted between June 2013 and December 2013 at 5 hospitals in Maryland and Washington DC. Two are academic medical centers (Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center [JHBMC]), and the others are community hospitals (Howard County General Hospital [HCGH], Sibley Memorial Hospital [SMC], and Suburban Hospital). These 5 hospitals, across 2 large cities, have distinct culture and leadership, each serving different populations.
Subjects
In developing a tool to measure communication and comportment, we needed to observe physicianpatient encounters wherein there would be a good deal of variability in performance. During pilot testing, when following a few of the most senior and respected hospitalists, we noted encounters during which they excelled and others where they performed less optimally. Further, in following some less‐experienced providers, their skills were less developed and they were uniformly missing most of the behaviors on the tool that were believed to be associated with optimal communication and comportment. Because of this, we decided to purposively sample the strongest clinicians at each of the 5 hospitals in hopes of seeing a range of scores on the tool.
The chiefs of hospital medicine at the 5 hospitals were contacted and asked to identify their most clinically excellent hospitalists, namely those who they thought were most clinically skilled within their groups. Because our goal was to observe the top tier (approximately 20%) of the hospitalists within each group, we asked each chief to name a specific number of physicians (eg, 3 names for 1 group with 15 hospitalists, and 8 from another group with 40 physicians). No precise definition of most clinically excellent hospitalists was provided to the chiefs. It was believed that they were well positioned to select their best clinicians because of both subjective feedback and objective data that flow to them. This postulate may have been corroborated by the fact that each of them efficiently sent a list of their top choices without any questions being asked.
The 29 hospitalists (named by their chiefs) were in turn emailed and invited to participate in the study. All but 3 hospitalists consented to participate in the study; this resulted in a cohort of 26 who would be observed.
Tool Development
A team was assembled to develop the hospital medicine comportment and communication observation tool (HMCCOT). All team members had extensive clinical experience, several had published articles on clinical excellence, had won clinical awards, and all had been teaching clinical skills for many years. The team's development of the HMCCOT was extensively informed by a review of the literature. Two articles that most heavily influenced the HMCCOT's development were Christmas et al.'s paper describing 7 core domains of excellence, 2 of which are intimately linked to communication and comportment,[12] and Kahn's text that delineates behaviors to be performed upon entering the patient's room, termed etiquette‐based medicine.[6] The team also considered the work from prior timemotion studies in hospital medicine,[7, 13] which led to the inclusion of temporal measurements during the observations. The tool was also presented at academic conferences in the Division of General Internal Medicine at Johns Hopkins and iteratively revised based on the feedback. Feedback was sought from people who have spent their entire career studying physicianpatient relationships and who are members of the American Academy on Communication in Healthcare. These methods established content validity evidence for the tool under development. The goal of the HMCCOT was to assess behaviors believed to be associated with optimal comportment and communication in hospital medicine.
The HMCCOT was pilot tested by observing different JHBMC hospitalists patient encounters and it was iteratively revised. On multiple occasions, 2 authors/emnvestigators spent time observing JHBMC hospitalists together and compared data capture and levels of agreement across all elements. Then, for formal assessment of inter‐rater reliability, 2 authors observed 5 different hospitalists across 25 patient encounters; the coefficient was 0.91 (standard error = 0.04). This step helped to establish internal structure validity evidence for the tool.
The initial version of the HMCCOT contained 36 elements, and it was organized sequentially to allow the observer to document behaviors in the order that they were likely to occur so as to facilitate the process and to minimize oversight. A few examples of the elements were as follows: open‐ended versus a close‐ended statement at the beginning of the encounter, hospitalist introduces himself/herself, and whether the provider smiles at any point during the patient encounter.
Data Collection
One author scheduled a time to observe each hospitalist physician during their routine clinical care of patients when they were not working with medical learners. Hospitalists were naturally aware that they were being observed but were not aware of the specific data elements or behaviors that were being recorded.
The study was approved by the institutional review board at the Johns Hopkins University School of Medicine, and by each of the research review committees at HCGH, SMC, and Suburban hospitals.
Data Analysis
After data collection, all data were deidentified so that the researchers were blinded to the identities of the physicians. Respondent characteristics are presented as proportions and means. Unpaired t test and 2 tests were used to compare demographic information, and stratified by mean HMCCOT score. The survey data were analyzed using Stata statistical software version 12.1 (StataCorp LP, College Station, TX).
Further Validation of the HMCCOT
Upon reviewing the distribution of data after observing the 26 physicians with their patients, we excluded 13 variables from the initial version of the tool that lacked discriminatory value (eg, 100% or 0% of physicians performed the observed behavior during the encounters); this left 23 variables that were judged to be most clinically relevant in the final version of the HMCCOT. Two examples of the variables that were excluded were: uses technology/literature to educate patients (not witnessed in any encounter), and obeys posted contact precautions (done uniformly by all). The HMCCOT score represents the proportion of observed behaviors (out of the 23 behaviors). It was computed for each hospitalist for every patient encounter. Finally, relation to other variables validity evidence would be established by comparing the mean HMCCOT scores of the physicians to their PG scores from the same time period to evaluate the correlation between the 2 scores. This association was assessed using Pearson correlations.
RESULTS
The average clinical experience of the 26 hospitalist physicians studied was 6 years (Table 1). Their mean age was 38 years, 13 (50%) were female, and 16 (62%) were of nonwhite race. Fourteen hospitalists (54%) worked at 1 of the nonacademic hospitals. In terms of clinical workload, most physicians (n = 17, 65%) devoted more than 70% of their time working in direct patient care. Mean time spent observing each physician was 280 minutes. During this time, the 26 physicians were observed for 181 separate clinical encounters; 54% of these patients were new encounters, patients who were not previously known to the physician. The average time each physician spent in a patient room was 10.8 minutes. Mean number of observed patient encounters per hospitalist was 7.
| Total Study Population, n = 26 | HMCCOT Score 60, n = 14 | HMCCOT Score >60, n = 12 | P Value* | |
|---|---|---|---|---|
| ||||
| Age, mean (SD) | 38 (5.6) | 37.9 (5.6) | 38.1 (5.7) | 0.95 |
| Female, n (%) | 13 (50) | 6 (43) | 7 (58) | 0.43 |
| Race, n (%) | ||||
| Caucasian | 10 (38) | 5 (36) | 5 (41) | 0.31 |
| Asian | 13 (50) | 8 (57) | 5 (41) | |
| African/African American | 2 (8) | 0 (0) | 2 (17) | |
| Other | 1 (4) | 1 (7) | 0 (0) | |
| Clinical experience >6 years, n (%) | 12 (46) | 6 (43) | 6 (50) | 0.72 |
| Clinical workload >70% | 17 (65) | 10 (71) | 7 (58) | 0.48 |
| Academic hospitalist, n (%) | 12 (46) | 5 (36) | 7 (58) | 0.25 |
| Hospital | 0.47 | |||
| JHBMC | 8 (31) | 3 (21.4) | 5 (41) | |
| JHH | 4 (15) | 2 (14.3) | 2 (17) | |
| HCGH | 5 (19) | 3 (21.4) | 2 (17) | |
| Suburban | 6 (23) | 3 (21.4) | 3 (25) | |
| SMC | 3 (12) | 3 (21.4) | 0 (0) | |
| Minutes spent observing hospitalist per shift, mean (SD) | 280 (104.5) | 280.4 (115.5) | 281.4 (95.3) | 0.98 |
| Average time spent per patient encounter in minutes, mean (SD) | 10.8 (8.9) | 8.7 (9.1) | 13 (8.1) | 0.001 |
| Proportion of observed patients who were new to provider, % | 97 (53.5) | 37 (39.7) | 60 (68.1) | 0.001 |
The distribution of HMCCOT scores was not statistically significantly different when analyzed by age, gender, race, amount of clinical experience, clinical workload of the hospitalist, hospital, time spent observing the hospitalist (all P > 0.05). The distribution of HMCCOT scores was statistically different in new patient encounters compared to follow‐ups (68.1% vs 39.7%, P 0.001). Encounters with patients that generated HMCCOT scores above versus below the mean were longer (13 minutes vs 8.7 minutes, P 0.001).
The mean HMCCOT score was 61 (standard deviation [SD] = 10.6), and it was normally distributed (Figure 1). Table 2 shows the data for the 23 behaviors that were objectively assessed as part of the HMCCOT for the 181 patient encounters. The most frequently observed behaviors were physicians washing hands after leaving the patient's room in 170 (94%) of the encounters and smiling (83%). The behaviors that were observed with the least regularity were using an empathic statement (26% of encounters), and employing teach‐back (13% of encounters). A common method of demonstrating interest in the patient as a person, seen in 41% of encounters, involved physicians asking about patients' personal histories and their interests.
| Variables | All Visits Combined, n = 181 | HMCCOT Score 60, n = 93 | HMCCOT Score >60, n = 88 | P Value* |
|---|---|---|---|---|
| ||||
| Objective observations, n (%) | ||||
| Washes hands after leaving room | 170 (94) | 83 (89) | 87 (99) | 0.007 |
| Discusses plan for the day | 163 (91) | 78 (84) | 85 (99) | 0.001 |
| Does not interrupt the patient | 159 (88) | 79 (85) | 80 (91) | 0.21 |
| Smiles | 149 (83) | 71 (77) | 78 (89) | 0.04 |
| Washes hands before entering | 139 (77) | 64 (69) | 75 (85) | 0.009 |
| Begins with open‐ended question | 134 (77) | 68 (76) | 66 (78) | 0.74 |
| Knocks before entering the room | 127 (76) | 57 (65) | 70 (89) | 0.001 |
| Introduces him/herself to the patient | 122 (67) | 45 (48) | 77 (88) | 0.001 |
| Explains his/her role | 120 (66) | 44 (47) | 76 (86) | 0.001 |
| Asks about pain | 110 (61) | 45 (49) | 65 (74) | 0.001 |
| Asks permission prior to examining | 106 (61) | 43 (50) | 63 (72) | 0.002 |
| Uncovers body area for the physical exam | 100 (57) | 34 (38) | 66 (77) | 0.001 |
| Discusses discharge plan | 99 (55) | 38 (41) | 61 (71) | 0.001 |
| Sits down in the patient room | 74 (41) | 24 (26) | 50 (57) | 0.001 |
| Asks about patient's feelings | 58 (33) | 17 (19) | 41 (47) | 0.001 |
| Shakes hands with the patient | 57 (32) | 17 (18) | 40 (46) | 0.001 |
| Uses teach‐back | 24 (13) | 4 (4.3) | 20 (24) | 0.001 |
| Subjective observations, n (%) | ||||
| Avoids medical jargon | 160 (89) | 85 (91) | 83 (95) | 0.28 |
| Demonstrates interest in patient as a person | 72 (41) | 16 (18) | 56 (66) | 0.001 |
| Touches appropriately | 62 (34) | 21 (23) | 41 (47) | 0.001 |
| Shows sensitivity to patient modesty | 57 (93) | 15 (79) | 42 (100) | 0.002 |
| Engages in nonmedical conversation | 54 (30) | 10 (11) | 44 (51) | 0.001 |
| Uses empathic statement | 47 (26) | 9 (10) | 38 (43) | 0.001 |

The average composite PG scores for the physician sample was 38.95 (SD=39.64). A moderate correlation was found between the HMCCOT score and PG score (adjusted Pearson correlation: 0.45, P = 0.047).
DISCUSSION
In this study, we followed 26 hospitalist physicians during routine clinical care, and we focused intently on their communication and their comportment with patients at the bedside. Even among clinically respected hospitalists, the results reveal that there is wide variability in comportment and communication practices and behaviors at the bedside. The physicians' HMCCOT scores were associated with their PG scores. These findings suggest that improved bedside communication and comportment with patients might translate into enhanced patient satisfaction.
This is the first study that honed in on hospitalist communication and comportment. With validity evidence established for the HMCCOT, some may elect to more explicitly perform these behaviors themselves, and others may wish to watch other hospitalists to give them feedback that is tied to specific behaviors. Beginning with the basics, the hospitalists we studied introduced themselves to their patients at the initial encounter 78% of the time, less frequently than is done by primary care clinicians (89%) but more consistently than do emergency department providers (64%).[7] Other variables that stood out in the HMCCOT was that teach‐back was employed in only 13% of the encounters. Previous studies have shown that teach‐back corroborates patient comprehension and can be used to engage patients (and caregivers) in realistic goal setting and optimal health service utilization.[14] Further, patients who clearly understand their postdischarge plan are 30% less likely to be readmitted or visit the emergency department.[14] The data for our group have helped us to see areas of strengths, such as hand washing, where we are above compliance rates across hospitals in the United States,[15] as well as those matters that represent opportunities for improvement such as connecting more deeply with our patients.
Tackett et al. have looked at encounter length and its association with performance of etiquette‐based medicine behaviors.[7] Similar to their study, we found a positive correlation between spending more time with patients and higher HMCCOT scores. We also found that HMCCOT scores were higher when providers were caring for new patients. Patients' complaints about doctors often relate to feeling rushed, that their physicians did not listen to them, or that information was not conveyed in a clear manner.[16] Such challenges in physicianpatient communication are ubiquitous across clinical settings.[16] When successfully achieved, patient‐centered communication has been associated with improved clinical outcomes, including adherence to recommended treatment and better self‐management of chronic disease.[17, 18, 19, 20, 21, 22, 23, 24, 25, 26] Many of the components of the HMCCOT described in this article are at the heart of patient‐centered care.
Several limitations of the study should be considered. First, physicians may have behaved differently while they were being observed, which is known as the Hawthorne effect. We observed them for many hours and across multiple patient encounters, and the physicians were not aware of the specific types of data that we were collecting. These factors may have limited the biases along such lines. Second, there may be elements of optimal comportment and communication that were not captured by the HMCCOT. Hopefully, there are not big gaps, as we used multiple methods and an iterative process in the refinement of the HMCCOT metric. Third, one investigator did all of the observing, and it is possible that he might have missed certain behaviors. Through extensive pilot testing and comparisons with other raters, the observer became very skilled and facile with such data collection and the tool. Fourth, we did not survey the same patients that were cared for to compare their perspectives to the HMCCOT scores following the clinical encounters. For patient perspectives, we relied only on PG scores. Fifth, quality of care is a broad and multidimensional construct. The HMCCOT focuses exclusively on hospitalists' comportment and communication at the bedside; therefore, it does not comprehensively assess care quality. Sixth, with our goal to optimally validate the HMCCOT, we tested it on the top tier of hospitalists within each group. We may have observed different results had we randomly selected hospitalists from each hospital or had we conducted the study at hospitals in other geographic regions. Finally, all of the doctors observed worked at hospitals in the Mid‐Atlantic region. However, these five distinct hospitals each have their own cultures, and they are led by different administrators. We purposively chose to sample both academic as well as community settings.
In conclusion, this study reports on the development of a comportment and communication tool that was established and validated by following clinically excellent hospitalists at the bedside. Future studies are necessary to determine whether hospitalists of all levels of experience and clinical skill can improve when given data and feedback using the HMCCOT. Larger studies will then be needed to assess whether enhancing comportment and communication can truly improve patient satisfaction and clinical outcomes in the hospital.
Disclosures: Dr. Wright is a Miller‐Coulson Family Scholar and is supported through the Johns Hopkins Center for Innovative Medicine. Susrutha Kotwal, MD, and Waseem Khaliq, MD, contributed equally to this work. The authors report no conflicts of interest.
In 2014, there were more than 40,000 hospitalists in the United States, and approximately 20% were employed by academic medical centers.[1] Hospitalist physicians groups are committed to delivering excellent patient care. However, the published literature is limited with respect to defining optimal care in hospital medicine.
Patient satisfaction surveys, such as Press Ganey (PG)[2] and Hospital Consumer Assessment of Healthcare Providers and Systems,[3] are being used to assess patients' contentment with the quality of care they receive while hospitalized. The Society of Hospital Medicine, the largest professional medical society representing hospitalists, encourages the use of patient satisfaction surveys to measure hospitalist providers' quality of patient care.[4] There are, however, several problems with the current methods. First, the attribution to specific providers is questionable. Second, recall about the provider by the patients may be poor because surveys are sent to patients days after they return home. Third, the patients' recovery and health outcomes are likely to influence their assessment of the doctor. Finally, feedback is known to be most valuable and transformative when it is specific and given in real time. Thus, a tool that is able to provide feedback at the encounter level should be more helpful than a tool that offers assessment at the level of the admission, particularly when it can be also delivered immediately after the data are collected.
Comportment has been used to describe both the way a person behaves and also the way she carries herself (ie, her general manner).[5] Excellent comportment and communication can serve as the foundation for delivering patient‐centered care.[6, 7, 8] Patient centeredness has been shown to improve the patient experience and clinical outcomes, including compliance with therapeutic plans.[9, 10, 11] Respectful behavior, etiquette‐based medicine, and effective communication also lay the foundation upon which the therapeutic alliance between a doctor and patient can be built.
The goal of this study was to establish a metric that could comprehensively assess a hospitalist provider's comportment and communication skills during an encounter with a hospitalized patient.
METHODS
Study Design and Setting
An observational study of hospitalist physicians was conducted between June 2013 and December 2013 at 5 hospitals in Maryland and Washington DC. Two are academic medical centers (Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center [JHBMC]), and the others are community hospitals (Howard County General Hospital [HCGH], Sibley Memorial Hospital [SMC], and Suburban Hospital). These 5 hospitals, across 2 large cities, have distinct culture and leadership, each serving different populations.
Subjects
In developing a tool to measure communication and comportment, we needed to observe physicianpatient encounters wherein there would be a good deal of variability in performance. During pilot testing, when following a few of the most senior and respected hospitalists, we noted encounters during which they excelled and others where they performed less optimally. Further, in following some less‐experienced providers, their skills were less developed and they were uniformly missing most of the behaviors on the tool that were believed to be associated with optimal communication and comportment. Because of this, we decided to purposively sample the strongest clinicians at each of the 5 hospitals in hopes of seeing a range of scores on the tool.
The chiefs of hospital medicine at the 5 hospitals were contacted and asked to identify their most clinically excellent hospitalists, namely those who they thought were most clinically skilled within their groups. Because our goal was to observe the top tier (approximately 20%) of the hospitalists within each group, we asked each chief to name a specific number of physicians (eg, 3 names for 1 group with 15 hospitalists, and 8 from another group with 40 physicians). No precise definition of most clinically excellent hospitalists was provided to the chiefs. It was believed that they were well positioned to select their best clinicians because of both subjective feedback and objective data that flow to them. This postulate may have been corroborated by the fact that each of them efficiently sent a list of their top choices without any questions being asked.
The 29 hospitalists (named by their chiefs) were in turn emailed and invited to participate in the study. All but 3 hospitalists consented to participate in the study; this resulted in a cohort of 26 who would be observed.
Tool Development
A team was assembled to develop the hospital medicine comportment and communication observation tool (HMCCOT). All team members had extensive clinical experience, several had published articles on clinical excellence, had won clinical awards, and all had been teaching clinical skills for many years. The team's development of the HMCCOT was extensively informed by a review of the literature. Two articles that most heavily influenced the HMCCOT's development were Christmas et al.'s paper describing 7 core domains of excellence, 2 of which are intimately linked to communication and comportment,[12] and Kahn's text that delineates behaviors to be performed upon entering the patient's room, termed etiquette‐based medicine.[6] The team also considered the work from prior timemotion studies in hospital medicine,[7, 13] which led to the inclusion of temporal measurements during the observations. The tool was also presented at academic conferences in the Division of General Internal Medicine at Johns Hopkins and iteratively revised based on the feedback. Feedback was sought from people who have spent their entire career studying physicianpatient relationships and who are members of the American Academy on Communication in Healthcare. These methods established content validity evidence for the tool under development. The goal of the HMCCOT was to assess behaviors believed to be associated with optimal comportment and communication in hospital medicine.
The HMCCOT was pilot tested by observing different JHBMC hospitalists patient encounters and it was iteratively revised. On multiple occasions, 2 authors/emnvestigators spent time observing JHBMC hospitalists together and compared data capture and levels of agreement across all elements. Then, for formal assessment of inter‐rater reliability, 2 authors observed 5 different hospitalists across 25 patient encounters; the coefficient was 0.91 (standard error = 0.04). This step helped to establish internal structure validity evidence for the tool.
The initial version of the HMCCOT contained 36 elements, and it was organized sequentially to allow the observer to document behaviors in the order that they were likely to occur so as to facilitate the process and to minimize oversight. A few examples of the elements were as follows: open‐ended versus a close‐ended statement at the beginning of the encounter, hospitalist introduces himself/herself, and whether the provider smiles at any point during the patient encounter.
Data Collection
One author scheduled a time to observe each hospitalist physician during their routine clinical care of patients when they were not working with medical learners. Hospitalists were naturally aware that they were being observed but were not aware of the specific data elements or behaviors that were being recorded.
The study was approved by the institutional review board at the Johns Hopkins University School of Medicine, and by each of the research review committees at HCGH, SMC, and Suburban hospitals.
Data Analysis
After data collection, all data were deidentified so that the researchers were blinded to the identities of the physicians. Respondent characteristics are presented as proportions and means. Unpaired t test and 2 tests were used to compare demographic information, and stratified by mean HMCCOT score. The survey data were analyzed using Stata statistical software version 12.1 (StataCorp LP, College Station, TX).
Further Validation of the HMCCOT
Upon reviewing the distribution of data after observing the 26 physicians with their patients, we excluded 13 variables from the initial version of the tool that lacked discriminatory value (eg, 100% or 0% of physicians performed the observed behavior during the encounters); this left 23 variables that were judged to be most clinically relevant in the final version of the HMCCOT. Two examples of the variables that were excluded were: uses technology/literature to educate patients (not witnessed in any encounter), and obeys posted contact precautions (done uniformly by all). The HMCCOT score represents the proportion of observed behaviors (out of the 23 behaviors). It was computed for each hospitalist for every patient encounter. Finally, relation to other variables validity evidence would be established by comparing the mean HMCCOT scores of the physicians to their PG scores from the same time period to evaluate the correlation between the 2 scores. This association was assessed using Pearson correlations.
RESULTS
The average clinical experience of the 26 hospitalist physicians studied was 6 years (Table 1). Their mean age was 38 years, 13 (50%) were female, and 16 (62%) were of nonwhite race. Fourteen hospitalists (54%) worked at 1 of the nonacademic hospitals. In terms of clinical workload, most physicians (n = 17, 65%) devoted more than 70% of their time working in direct patient care. Mean time spent observing each physician was 280 minutes. During this time, the 26 physicians were observed for 181 separate clinical encounters; 54% of these patients were new encounters, patients who were not previously known to the physician. The average time each physician spent in a patient room was 10.8 minutes. Mean number of observed patient encounters per hospitalist was 7.
| Total Study Population, n = 26 | HMCCOT Score 60, n = 14 | HMCCOT Score >60, n = 12 | P Value* | |
|---|---|---|---|---|
| ||||
| Age, mean (SD) | 38 (5.6) | 37.9 (5.6) | 38.1 (5.7) | 0.95 |
| Female, n (%) | 13 (50) | 6 (43) | 7 (58) | 0.43 |
| Race, n (%) | ||||
| Caucasian | 10 (38) | 5 (36) | 5 (41) | 0.31 |
| Asian | 13 (50) | 8 (57) | 5 (41) | |
| African/African American | 2 (8) | 0 (0) | 2 (17) | |
| Other | 1 (4) | 1 (7) | 0 (0) | |
| Clinical experience >6 years, n (%) | 12 (46) | 6 (43) | 6 (50) | 0.72 |
| Clinical workload >70% | 17 (65) | 10 (71) | 7 (58) | 0.48 |
| Academic hospitalist, n (%) | 12 (46) | 5 (36) | 7 (58) | 0.25 |
| Hospital | 0.47 | |||
| JHBMC | 8 (31) | 3 (21.4) | 5 (41) | |
| JHH | 4 (15) | 2 (14.3) | 2 (17) | |
| HCGH | 5 (19) | 3 (21.4) | 2 (17) | |
| Suburban | 6 (23) | 3 (21.4) | 3 (25) | |
| SMC | 3 (12) | 3 (21.4) | 0 (0) | |
| Minutes spent observing hospitalist per shift, mean (SD) | 280 (104.5) | 280.4 (115.5) | 281.4 (95.3) | 0.98 |
| Average time spent per patient encounter in minutes, mean (SD) | 10.8 (8.9) | 8.7 (9.1) | 13 (8.1) | 0.001 |
| Proportion of observed patients who were new to provider, % | 97 (53.5) | 37 (39.7) | 60 (68.1) | 0.001 |
The distribution of HMCCOT scores was not statistically significantly different when analyzed by age, gender, race, amount of clinical experience, clinical workload of the hospitalist, hospital, time spent observing the hospitalist (all P > 0.05). The distribution of HMCCOT scores was statistically different in new patient encounters compared to follow‐ups (68.1% vs 39.7%, P 0.001). Encounters with patients that generated HMCCOT scores above versus below the mean were longer (13 minutes vs 8.7 minutes, P 0.001).
The mean HMCCOT score was 61 (standard deviation [SD] = 10.6), and it was normally distributed (Figure 1). Table 2 shows the data for the 23 behaviors that were objectively assessed as part of the HMCCOT for the 181 patient encounters. The most frequently observed behaviors were physicians washing hands after leaving the patient's room in 170 (94%) of the encounters and smiling (83%). The behaviors that were observed with the least regularity were using an empathic statement (26% of encounters), and employing teach‐back (13% of encounters). A common method of demonstrating interest in the patient as a person, seen in 41% of encounters, involved physicians asking about patients' personal histories and their interests.
| Variables | All Visits Combined, n = 181 | HMCCOT Score 60, n = 93 | HMCCOT Score >60, n = 88 | P Value* |
|---|---|---|---|---|
| ||||
| Objective observations, n (%) | ||||
| Washes hands after leaving room | 170 (94) | 83 (89) | 87 (99) | 0.007 |
| Discusses plan for the day | 163 (91) | 78 (84) | 85 (99) | 0.001 |
| Does not interrupt the patient | 159 (88) | 79 (85) | 80 (91) | 0.21 |
| Smiles | 149 (83) | 71 (77) | 78 (89) | 0.04 |
| Washes hands before entering | 139 (77) | 64 (69) | 75 (85) | 0.009 |
| Begins with open‐ended question | 134 (77) | 68 (76) | 66 (78) | 0.74 |
| Knocks before entering the room | 127 (76) | 57 (65) | 70 (89) | 0.001 |
| Introduces him/herself to the patient | 122 (67) | 45 (48) | 77 (88) | 0.001 |
| Explains his/her role | 120 (66) | 44 (47) | 76 (86) | 0.001 |
| Asks about pain | 110 (61) | 45 (49) | 65 (74) | 0.001 |
| Asks permission prior to examining | 106 (61) | 43 (50) | 63 (72) | 0.002 |
| Uncovers body area for the physical exam | 100 (57) | 34 (38) | 66 (77) | 0.001 |
| Discusses discharge plan | 99 (55) | 38 (41) | 61 (71) | 0.001 |
| Sits down in the patient room | 74 (41) | 24 (26) | 50 (57) | 0.001 |
| Asks about patient's feelings | 58 (33) | 17 (19) | 41 (47) | 0.001 |
| Shakes hands with the patient | 57 (32) | 17 (18) | 40 (46) | 0.001 |
| Uses teach‐back | 24 (13) | 4 (4.3) | 20 (24) | 0.001 |
| Subjective observations, n (%) | ||||
| Avoids medical jargon | 160 (89) | 85 (91) | 83 (95) | 0.28 |
| Demonstrates interest in patient as a person | 72 (41) | 16 (18) | 56 (66) | 0.001 |
| Touches appropriately | 62 (34) | 21 (23) | 41 (47) | 0.001 |
| Shows sensitivity to patient modesty | 57 (93) | 15 (79) | 42 (100) | 0.002 |
| Engages in nonmedical conversation | 54 (30) | 10 (11) | 44 (51) | 0.001 |
| Uses empathic statement | 47 (26) | 9 (10) | 38 (43) | 0.001 |

The average composite PG scores for the physician sample was 38.95 (SD=39.64). A moderate correlation was found between the HMCCOT score and PG score (adjusted Pearson correlation: 0.45, P = 0.047).
DISCUSSION
In this study, we followed 26 hospitalist physicians during routine clinical care, and we focused intently on their communication and their comportment with patients at the bedside. Even among clinically respected hospitalists, the results reveal that there is wide variability in comportment and communication practices and behaviors at the bedside. The physicians' HMCCOT scores were associated with their PG scores. These findings suggest that improved bedside communication and comportment with patients might translate into enhanced patient satisfaction.
This is the first study that honed in on hospitalist communication and comportment. With validity evidence established for the HMCCOT, some may elect to more explicitly perform these behaviors themselves, and others may wish to watch other hospitalists to give them feedback that is tied to specific behaviors. Beginning with the basics, the hospitalists we studied introduced themselves to their patients at the initial encounter 78% of the time, less frequently than is done by primary care clinicians (89%) but more consistently than do emergency department providers (64%).[7] Other variables that stood out in the HMCCOT was that teach‐back was employed in only 13% of the encounters. Previous studies have shown that teach‐back corroborates patient comprehension and can be used to engage patients (and caregivers) in realistic goal setting and optimal health service utilization.[14] Further, patients who clearly understand their postdischarge plan are 30% less likely to be readmitted or visit the emergency department.[14] The data for our group have helped us to see areas of strengths, such as hand washing, where we are above compliance rates across hospitals in the United States,[15] as well as those matters that represent opportunities for improvement such as connecting more deeply with our patients.
Tackett et al. have looked at encounter length and its association with performance of etiquette‐based medicine behaviors.[7] Similar to their study, we found a positive correlation between spending more time with patients and higher HMCCOT scores. We also found that HMCCOT scores were higher when providers were caring for new patients. Patients' complaints about doctors often relate to feeling rushed, that their physicians did not listen to them, or that information was not conveyed in a clear manner.[16] Such challenges in physicianpatient communication are ubiquitous across clinical settings.[16] When successfully achieved, patient‐centered communication has been associated with improved clinical outcomes, including adherence to recommended treatment and better self‐management of chronic disease.[17, 18, 19, 20, 21, 22, 23, 24, 25, 26] Many of the components of the HMCCOT described in this article are at the heart of patient‐centered care.
Several limitations of the study should be considered. First, physicians may have behaved differently while they were being observed, which is known as the Hawthorne effect. We observed them for many hours and across multiple patient encounters, and the physicians were not aware of the specific types of data that we were collecting. These factors may have limited the biases along such lines. Second, there may be elements of optimal comportment and communication that were not captured by the HMCCOT. Hopefully, there are not big gaps, as we used multiple methods and an iterative process in the refinement of the HMCCOT metric. Third, one investigator did all of the observing, and it is possible that he might have missed certain behaviors. Through extensive pilot testing and comparisons with other raters, the observer became very skilled and facile with such data collection and the tool. Fourth, we did not survey the same patients that were cared for to compare their perspectives to the HMCCOT scores following the clinical encounters. For patient perspectives, we relied only on PG scores. Fifth, quality of care is a broad and multidimensional construct. The HMCCOT focuses exclusively on hospitalists' comportment and communication at the bedside; therefore, it does not comprehensively assess care quality. Sixth, with our goal to optimally validate the HMCCOT, we tested it on the top tier of hospitalists within each group. We may have observed different results had we randomly selected hospitalists from each hospital or had we conducted the study at hospitals in other geographic regions. Finally, all of the doctors observed worked at hospitals in the Mid‐Atlantic region. However, these five distinct hospitals each have their own cultures, and they are led by different administrators. We purposively chose to sample both academic as well as community settings.
In conclusion, this study reports on the development of a comportment and communication tool that was established and validated by following clinically excellent hospitalists at the bedside. Future studies are necessary to determine whether hospitalists of all levels of experience and clinical skill can improve when given data and feedback using the HMCCOT. Larger studies will then be needed to assess whether enhancing comportment and communication can truly improve patient satisfaction and clinical outcomes in the hospital.
Disclosures: Dr. Wright is a Miller‐Coulson Family Scholar and is supported through the Johns Hopkins Center for Innovative Medicine. Susrutha Kotwal, MD, and Waseem Khaliq, MD, contributed equally to this work. The authors report no conflicts of interest.
- 2014 state of hospital medicine report. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org/Web/Practice_Management/State_of_HM_Surveys/2014.aspx. Accessed January 10, 2015.
- Press Ganey website. Available at: http://www.pressganey.com/home. Accessed December 15, 2015.
- Hospital Consumer Assessment of Healthcare Providers and Systems website. Available at: http://www.hcahpsonline.org/home.aspx. Accessed February 2, 2016.
- Membership committee guidelines for hospitalists patient satisfaction surveys. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed February 2, 2016.
- Definition of comportment. Available at: http://www.vocabulary.com/dictionary/comportment. Accessed December 15, 2015.
- . Etiquette‐based medicine. N Engl J Med. 2008;358(19):1988–1989.
- , , , , . Appraising the practice of etiquette‐based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908–913.
- , , . Developing physician communication skills for patient‐centered care. Health Aff (Millwood). 2010;29(7):1310–1318.
- . The impact on patient health outcomes of interventions targeting the patient–physician relationship. Patient. 2009;2(2):77–84.
- , , , , , . Effect on health‐related outcomes of interventions to alter the interaction between patients and practitioners: a systematic review of trials. Ann Fam Med. 2004;2(6):595–608.
- , , , . How does communication heal? Pathways linking clinician–patient communication to health outcomes. Patient Educ Couns. 2009;74(3):295–301.
- , , , . Clinical excellence in academia: perspectives from masterful academic clinicians. Mayo Clin Proc. 2008;83(9):989–994.
- , , , et al. Where did the day go?—a time‐motion study of hospitalists. J Hosp Med. 2010;5(6):323–328.
- , , , et al. Reducing readmissions using teach‐back: enhancing patient and family education. J Nurs Adm. 2015;45(1):35–42.
- , , . Hand hygiene compliance rates in the United States—a one‐year multicenter collaboration using product/volume usage measurement and feedback. Am J Med Qual. 2009;24(3):205–213.
- , , , et al. Obstetricians' prior malpractice experience and patients' satisfaction with care. JAMA. 1994;272(20):1583–1587.
- , . Patient‐Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. NIH publication no. 07–6225. Bethesda, MD: National Cancer Institute; 2007.
- . Interacting with cancer patients: the significance of physicians' communication behavior. Soc Sci Med. 2003;57(5):791–806.
- , , . Expanding patient involvement in care: effects on patient outcomes. Ann Intern Med. 1985;102(4):520–528.
- , . Measuring patient‐centeredness: a comparison of three observation‐based instruments. Patient Educ Couns. 2000;39(1):71–80.
- , , , . Doctor‐patient communication: a review of the literature. Soc Sci Med. 1995;40(7):903–918.
- , , , , , . Linking primary care performance to outcomes of care. J Fam Pract. 1998;47(3):213–220.
- , , , et al. The impact of patient‐centered care on outcomes. J Fam Pract. 2000;49(9):796–804.
- , , , et al. Measuring patient‐centered communication in patient‐physician consultations: theoretical and practical issues. Soc Sci Med. 2005;61(7):1516–1528.
- , . Patient‐centered consultations and outcomes in primary care: a review of the literature. Patient Educ Couns. 2002;48(1):51–61.
- , , . Doctor‐patient communication and satisfaction with care in oncology. Curr Opin Oncol. 2005;17(4):351–354.
- 2014 state of hospital medicine report. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org/Web/Practice_Management/State_of_HM_Surveys/2014.aspx. Accessed January 10, 2015.
- Press Ganey website. Available at: http://www.pressganey.com/home. Accessed December 15, 2015.
- Hospital Consumer Assessment of Healthcare Providers and Systems website. Available at: http://www.hcahpsonline.org/home.aspx. Accessed February 2, 2016.
- Membership committee guidelines for hospitalists patient satisfaction surveys. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed February 2, 2016.
- Definition of comportment. Available at: http://www.vocabulary.com/dictionary/comportment. Accessed December 15, 2015.
- . Etiquette‐based medicine. N Engl J Med. 2008;358(19):1988–1989.
- , , , , . Appraising the practice of etiquette‐based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908–913.
- , , . Developing physician communication skills for patient‐centered care. Health Aff (Millwood). 2010;29(7):1310–1318.
- . The impact on patient health outcomes of interventions targeting the patient–physician relationship. Patient. 2009;2(2):77–84.
- , , , , , . Effect on health‐related outcomes of interventions to alter the interaction between patients and practitioners: a systematic review of trials. Ann Fam Med. 2004;2(6):595–608.
- , , , . How does communication heal? Pathways linking clinician–patient communication to health outcomes. Patient Educ Couns. 2009;74(3):295–301.
- , , , . Clinical excellence in academia: perspectives from masterful academic clinicians. Mayo Clin Proc. 2008;83(9):989–994.
- , , , et al. Where did the day go?—a time‐motion study of hospitalists. J Hosp Med. 2010;5(6):323–328.
- , , , et al. Reducing readmissions using teach‐back: enhancing patient and family education. J Nurs Adm. 2015;45(1):35–42.
- , , . Hand hygiene compliance rates in the United States—a one‐year multicenter collaboration using product/volume usage measurement and feedback. Am J Med Qual. 2009;24(3):205–213.
- , , , et al. Obstetricians' prior malpractice experience and patients' satisfaction with care. JAMA. 1994;272(20):1583–1587.
- , . Patient‐Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. NIH publication no. 07–6225. Bethesda, MD: National Cancer Institute; 2007.
- . Interacting with cancer patients: the significance of physicians' communication behavior. Soc Sci Med. 2003;57(5):791–806.
- , , . Expanding patient involvement in care: effects on patient outcomes. Ann Intern Med. 1985;102(4):520–528.
- , . Measuring patient‐centeredness: a comparison of three observation‐based instruments. Patient Educ Couns. 2000;39(1):71–80.
- , , , . Doctor‐patient communication: a review of the literature. Soc Sci Med. 1995;40(7):903–918.
- , , , , , . Linking primary care performance to outcomes of care. J Fam Pract. 1998;47(3):213–220.
- , , , et al. The impact of patient‐centered care on outcomes. J Fam Pract. 2000;49(9):796–804.
- , , , et al. Measuring patient‐centered communication in patient‐physician consultations: theoretical and practical issues. Soc Sci Med. 2005;61(7):1516–1528.
- , . Patient‐centered consultations and outcomes in primary care: a review of the literature. Patient Educ Couns. 2002;48(1):51–61.
- , , . Doctor‐patient communication and satisfaction with care in oncology. Curr Opin Oncol. 2005;17(4):351–354.
How Common is Coexisting Epilepsy/PNES?
Researchers examined 1567 patient medical records from the Vanderbilt University Medical Center Adult EMU and found a 5.2% prevalence rate of coexisting epilepsy/psychogenic nonepileptic spells (PNES). Other findings include:
· Epileptic seizures were preceded by a PNES event in 94.4% of epilepsy/PNES patients
· Patients with epilepsy/PNES had a higher presence of epilepsy risk factors
· Abnormal brain MRI and abnormal neurological examination were more common in the epilepsy/PNES group.
Chen-Block S, Abou-Khalil BW, Arain A, et al. Video-EEG results and clinical characteristics in patients with psychogenic nonepileptic spells: the effect of a coexistent epilepsy. Epilepsy Behav. 2016;62:62-65.
Researchers examined 1567 patient medical records from the Vanderbilt University Medical Center Adult EMU and found a 5.2% prevalence rate of coexisting epilepsy/psychogenic nonepileptic spells (PNES). Other findings include:
· Epileptic seizures were preceded by a PNES event in 94.4% of epilepsy/PNES patients
· Patients with epilepsy/PNES had a higher presence of epilepsy risk factors
· Abnormal brain MRI and abnormal neurological examination were more common in the epilepsy/PNES group.
Chen-Block S, Abou-Khalil BW, Arain A, et al. Video-EEG results and clinical characteristics in patients with psychogenic nonepileptic spells: the effect of a coexistent epilepsy. Epilepsy Behav. 2016;62:62-65.
Researchers examined 1567 patient medical records from the Vanderbilt University Medical Center Adult EMU and found a 5.2% prevalence rate of coexisting epilepsy/psychogenic nonepileptic spells (PNES). Other findings include:
· Epileptic seizures were preceded by a PNES event in 94.4% of epilepsy/PNES patients
· Patients with epilepsy/PNES had a higher presence of epilepsy risk factors
· Abnormal brain MRI and abnormal neurological examination were more common in the epilepsy/PNES group.
Chen-Block S, Abou-Khalil BW, Arain A, et al. Video-EEG results and clinical characteristics in patients with psychogenic nonepileptic spells: the effect of a coexistent epilepsy. Epilepsy Behav. 2016;62:62-65.
Hospitalized Patients With Epilepsy at Risk for Specific Safety-Related Adverse Events
People with epilepsy are at an increased risk of specific safety-related adverse events while in the hospital. Researchers found that hospitalized patients with epilepsy were at a greater risk for fall with hip fracture, respiratory failure, sepsis, and preventable postoperative death. The authors also reported that adverse events were associated with a prolonged length of stay, as well as an increase in the odds of inpatient death and an increase in high-level post-acute care.
Mendizabal A, Thibault DP, Willis AW. Patient safety events in hospital care of individuals with epilepsy [published online ahead of print June 28, 2016]. Epilepsia. 2016;doi:10.1111/epi.13440.
People with epilepsy are at an increased risk of specific safety-related adverse events while in the hospital. Researchers found that hospitalized patients with epilepsy were at a greater risk for fall with hip fracture, respiratory failure, sepsis, and preventable postoperative death. The authors also reported that adverse events were associated with a prolonged length of stay, as well as an increase in the odds of inpatient death and an increase in high-level post-acute care.
Mendizabal A, Thibault DP, Willis AW. Patient safety events in hospital care of individuals with epilepsy [published online ahead of print June 28, 2016]. Epilepsia. 2016;doi:10.1111/epi.13440.
People with epilepsy are at an increased risk of specific safety-related adverse events while in the hospital. Researchers found that hospitalized patients with epilepsy were at a greater risk for fall with hip fracture, respiratory failure, sepsis, and preventable postoperative death. The authors also reported that adverse events were associated with a prolonged length of stay, as well as an increase in the odds of inpatient death and an increase in high-level post-acute care.
Mendizabal A, Thibault DP, Willis AW. Patient safety events in hospital care of individuals with epilepsy [published online ahead of print June 28, 2016]. Epilepsia. 2016;doi:10.1111/epi.13440.
Systemic Disease Manifestations of TSC Strongly Associated With Epilepsy
In a study of 1816 patients with tuberous sclerosis complex (TSC), researchers found that specific disease manifestations—cardiac rhabodmyomas, retinal hemartomas, renal cysts, renal angiomyolipipomas, and facial angiofibromas—were associated with a higher likelihood of epilepsy development. The authors posit that this research can help identify patients who will benefit from novel, targeted, preventative treatments.
Jeong A, Wong M. Systemic disease manifestations associated with epilepsy in tuberous sclerosis complex [published online ahead of print July 15, 2016]. Epilepsia. 2016;doi:10.1111/epi.13467.
In a study of 1816 patients with tuberous sclerosis complex (TSC), researchers found that specific disease manifestations—cardiac rhabodmyomas, retinal hemartomas, renal cysts, renal angiomyolipipomas, and facial angiofibromas—were associated with a higher likelihood of epilepsy development. The authors posit that this research can help identify patients who will benefit from novel, targeted, preventative treatments.
Jeong A, Wong M. Systemic disease manifestations associated with epilepsy in tuberous sclerosis complex [published online ahead of print July 15, 2016]. Epilepsia. 2016;doi:10.1111/epi.13467.
In a study of 1816 patients with tuberous sclerosis complex (TSC), researchers found that specific disease manifestations—cardiac rhabodmyomas, retinal hemartomas, renal cysts, renal angiomyolipipomas, and facial angiofibromas—were associated with a higher likelihood of epilepsy development. The authors posit that this research can help identify patients who will benefit from novel, targeted, preventative treatments.
Jeong A, Wong M. Systemic disease manifestations associated with epilepsy in tuberous sclerosis complex [published online ahead of print July 15, 2016]. Epilepsia. 2016;doi:10.1111/epi.13467.
A Second Look at Head MRIs Demonstrates the Value of Re-Review
To determine if patients with epilepsy are appropriate candidates for resective surgery, presurgical conferences are conducted to review magnetic resonance images (MRIs) of the patient’s head. Kenney and associates analyzed repeat reviews of MRIs at presurgical epilepsy conferences to assess their impact on the decision-making process. Among the 233 patients whose charts were re-reviewed, 94 patients (40.3%) had the resective surgery performed, and the analysis revealed that 41 patients (17.6%) had previously undiagnosed findings; 18 of the 41 patients had the surgery. However, among 4 of the 41 patients (9.8%), the re-reviews found abnormalities that did not warrant surgical resection, including autoimmunity and bilateral pathology.
Kenney DL, Kelly-Williams KM, Krecke KN et al. Usefulness of Repeat Review of Head Magnetic Resonance Images During Presurgical Epilepsy Conferences. Epilepsy Res. 2016. In press. http://dx.doi.org/10.1016/j.eplepsyres.2016.06.005.
To determine if patients with epilepsy are appropriate candidates for resective surgery, presurgical conferences are conducted to review magnetic resonance images (MRIs) of the patient’s head. Kenney and associates analyzed repeat reviews of MRIs at presurgical epilepsy conferences to assess their impact on the decision-making process. Among the 233 patients whose charts were re-reviewed, 94 patients (40.3%) had the resective surgery performed, and the analysis revealed that 41 patients (17.6%) had previously undiagnosed findings; 18 of the 41 patients had the surgery. However, among 4 of the 41 patients (9.8%), the re-reviews found abnormalities that did not warrant surgical resection, including autoimmunity and bilateral pathology.
Kenney DL, Kelly-Williams KM, Krecke KN et al. Usefulness of Repeat Review of Head Magnetic Resonance Images During Presurgical Epilepsy Conferences. Epilepsy Res. 2016. In press. http://dx.doi.org/10.1016/j.eplepsyres.2016.06.005.
To determine if patients with epilepsy are appropriate candidates for resective surgery, presurgical conferences are conducted to review magnetic resonance images (MRIs) of the patient’s head. Kenney and associates analyzed repeat reviews of MRIs at presurgical epilepsy conferences to assess their impact on the decision-making process. Among the 233 patients whose charts were re-reviewed, 94 patients (40.3%) had the resective surgery performed, and the analysis revealed that 41 patients (17.6%) had previously undiagnosed findings; 18 of the 41 patients had the surgery. However, among 4 of the 41 patients (9.8%), the re-reviews found abnormalities that did not warrant surgical resection, including autoimmunity and bilateral pathology.
Kenney DL, Kelly-Williams KM, Krecke KN et al. Usefulness of Repeat Review of Head Magnetic Resonance Images During Presurgical Epilepsy Conferences. Epilepsy Res. 2016. In press. http://dx.doi.org/10.1016/j.eplepsyres.2016.06.005.
Stimulation-identified Cortical Naming Sites Pose Unexpected Challenges
Before surgeons perform a resection involving the language-dominant hemisphere of a patient with epilepsy, they may do electrical stimulation mapping to identify a patient’s language-dominant hemisphere. Typically they will ask patients to identify objects to help locate the language cortex and then avoid resection in an area of the brain in which electrical stimulation makes it difficult for patients to name said objects. But because word production involves mechanisms that may be centered in more than one area of the brain, Hamberger et al tested locations that have been identified by stimulation as naming sites to look for disparities. Testing patients with refractory temporal lobe epilepsy who had subdural electrodes implanted, they discovered that stimulating naming sites in the superior temporary lobe was more likely to disrupt phonological processing but did not affect a patient’s ability to process semantic information. Stimulating the inferior temporal naming sites was more likely to impair semantic processing.
Hamberger MJ, Miozzo M, Schevon CA, et al. Functional differences among stimulation-identified cortical naming sites in the temporal region. Epilepsy Behav. 2016;60:124-129.
Before surgeons perform a resection involving the language-dominant hemisphere of a patient with epilepsy, they may do electrical stimulation mapping to identify a patient’s language-dominant hemisphere. Typically they will ask patients to identify objects to help locate the language cortex and then avoid resection in an area of the brain in which electrical stimulation makes it difficult for patients to name said objects. But because word production involves mechanisms that may be centered in more than one area of the brain, Hamberger et al tested locations that have been identified by stimulation as naming sites to look for disparities. Testing patients with refractory temporal lobe epilepsy who had subdural electrodes implanted, they discovered that stimulating naming sites in the superior temporary lobe was more likely to disrupt phonological processing but did not affect a patient’s ability to process semantic information. Stimulating the inferior temporal naming sites was more likely to impair semantic processing.
Hamberger MJ, Miozzo M, Schevon CA, et al. Functional differences among stimulation-identified cortical naming sites in the temporal region. Epilepsy Behav. 2016;60:124-129.
Before surgeons perform a resection involving the language-dominant hemisphere of a patient with epilepsy, they may do electrical stimulation mapping to identify a patient’s language-dominant hemisphere. Typically they will ask patients to identify objects to help locate the language cortex and then avoid resection in an area of the brain in which electrical stimulation makes it difficult for patients to name said objects. But because word production involves mechanisms that may be centered in more than one area of the brain, Hamberger et al tested locations that have been identified by stimulation as naming sites to look for disparities. Testing patients with refractory temporal lobe epilepsy who had subdural electrodes implanted, they discovered that stimulating naming sites in the superior temporary lobe was more likely to disrupt phonological processing but did not affect a patient’s ability to process semantic information. Stimulating the inferior temporal naming sites was more likely to impair semantic processing.
Hamberger MJ, Miozzo M, Schevon CA, et al. Functional differences among stimulation-identified cortical naming sites in the temporal region. Epilepsy Behav. 2016;60:124-129.