User login
Studies highlight diagnostic and treatment challenges in hidradenitis suppurativa
SCOTTSDALE, ARIZ. – Patients with hidradenitis suppurativa (HS) may be misdiagnosed when they see providers who are not dermatologists – as is usually the case during the initial years of their disease, according to a large analysis of medical claims data.
The findings highlight the need for visual diagnostic aids and specific guidelines for treating HS that target nondermatologists, Melissa Butt, MPH, of Penn State Hershey (Pa.) Medical Center, said during an interview at the annual meeting of the Society for Investigative Dermatology. She presented the findings during a poster session at the meeting.
HS is a chronic inflammatory disease of the hair follicles that affects 0.5%-4% of people in the United States. In past studies, up to 12 years elapsed between disease onset and diagnosis, in part because patients often cannot readily access dermatologists, Ms. Butt said. To better understand patterns of health care use during the years leading up to HS diagnosis, she and her colleagues used MarketScan data to identify 1,733 patients with HS-specific medical care claims filed in 2012 and 2013. Then they looked back at medical claims for these patients during 2008 through 2011, before the patients were diagnosed with HS. The cohort averaged 37 years of age (standard deviation, 15 years), and 73% were female.
Among 239,892 claims filed before patients were diagnosed with HS, 11,381 (4.7%) included codes for other diseases of the skin and subcutaneous tissues, Ms. Butt said. Dermatologists filed only 31% of these skin-specific claims, while 69% were filed by other providers, such as family practitioners, internists, emergency department physicians, and acute care hospitalists.
Notably, about two-thirds of the skin-specific diagnostic codes could have represented a misdiagnosis of HS. These codes included conditions such as abscesses, carbuncles, local infections, ulcers, and diseases of the sebaceous glands.
The fact that 78% of visits occurred in offices and other outpatient settings further underscores the need to improve the detection and care of HS in these environments, Ms. Butt said. Given current national shortages of dermatologists, visual HS diagnostic aids and “detailed, multistep clinical practice guidelines” for nondermatologists could help improve care of HS while patients wait to see the specialists, she added.
A second poster presented at the meeting provided results of a study on the use and impact of antibiotics in the treatment of HS. Alexander Fischer of Johns Hopkins University, Baltimore, and his associates studied antibiotic prescriptions and bacterial cultures from the lesions of 239 patients with HS who were treated at Johns Hopkins medical facilities between 2010 and 2015. Not only were 51% of HS patients on antibiotics at the time of culture, but these patients’ lesions were significantly more likely to contain antibiotic-resistant bacteria than were those of patients not on antibiotics.
Strikingly, Proteus species were isolated from nearly half of patients on trimethoprim-sulfamethoxazole (TMP/SMX), and 88% of colonies were resistant to TMP/SMX, while only 13% of cultures from untreated patients grew Proteus (P less than .001) and all were TMP/SMX-susceptible (P less than .001). Likewise, 100% of methicillin-resistant Staphylococcus aureus (MRSA) strains from patients prescribed ciprofloxacin were resistant to it, compared with a 10% background rate of ciprofloxacin resistance among MRSA from patients not taking antibiotics (P = .04). In addition, the proportion of other S. aureus strains that were clindamycin-resistant was higher when patients were taking this antibiotic than when they were not (63% versus 17%; P = .03).
The results “raise questions” about whether antibiotics should be used in HS patients who are not clearly benefiting from them, according to the researchers.
The authors of both studies reported no funding sources and had no disclosures.
SCOTTSDALE, ARIZ. – Patients with hidradenitis suppurativa (HS) may be misdiagnosed when they see providers who are not dermatologists – as is usually the case during the initial years of their disease, according to a large analysis of medical claims data.
The findings highlight the need for visual diagnostic aids and specific guidelines for treating HS that target nondermatologists, Melissa Butt, MPH, of Penn State Hershey (Pa.) Medical Center, said during an interview at the annual meeting of the Society for Investigative Dermatology. She presented the findings during a poster session at the meeting.
HS is a chronic inflammatory disease of the hair follicles that affects 0.5%-4% of people in the United States. In past studies, up to 12 years elapsed between disease onset and diagnosis, in part because patients often cannot readily access dermatologists, Ms. Butt said. To better understand patterns of health care use during the years leading up to HS diagnosis, she and her colleagues used MarketScan data to identify 1,733 patients with HS-specific medical care claims filed in 2012 and 2013. Then they looked back at medical claims for these patients during 2008 through 2011, before the patients were diagnosed with HS. The cohort averaged 37 years of age (standard deviation, 15 years), and 73% were female.
Among 239,892 claims filed before patients were diagnosed with HS, 11,381 (4.7%) included codes for other diseases of the skin and subcutaneous tissues, Ms. Butt said. Dermatologists filed only 31% of these skin-specific claims, while 69% were filed by other providers, such as family practitioners, internists, emergency department physicians, and acute care hospitalists.
Notably, about two-thirds of the skin-specific diagnostic codes could have represented a misdiagnosis of HS. These codes included conditions such as abscesses, carbuncles, local infections, ulcers, and diseases of the sebaceous glands.
The fact that 78% of visits occurred in offices and other outpatient settings further underscores the need to improve the detection and care of HS in these environments, Ms. Butt said. Given current national shortages of dermatologists, visual HS diagnostic aids and “detailed, multistep clinical practice guidelines” for nondermatologists could help improve care of HS while patients wait to see the specialists, she added.
A second poster presented at the meeting provided results of a study on the use and impact of antibiotics in the treatment of HS. Alexander Fischer of Johns Hopkins University, Baltimore, and his associates studied antibiotic prescriptions and bacterial cultures from the lesions of 239 patients with HS who were treated at Johns Hopkins medical facilities between 2010 and 2015. Not only were 51% of HS patients on antibiotics at the time of culture, but these patients’ lesions were significantly more likely to contain antibiotic-resistant bacteria than were those of patients not on antibiotics.
Strikingly, Proteus species were isolated from nearly half of patients on trimethoprim-sulfamethoxazole (TMP/SMX), and 88% of colonies were resistant to TMP/SMX, while only 13% of cultures from untreated patients grew Proteus (P less than .001) and all were TMP/SMX-susceptible (P less than .001). Likewise, 100% of methicillin-resistant Staphylococcus aureus (MRSA) strains from patients prescribed ciprofloxacin were resistant to it, compared with a 10% background rate of ciprofloxacin resistance among MRSA from patients not taking antibiotics (P = .04). In addition, the proportion of other S. aureus strains that were clindamycin-resistant was higher when patients were taking this antibiotic than when they were not (63% versus 17%; P = .03).
The results “raise questions” about whether antibiotics should be used in HS patients who are not clearly benefiting from them, according to the researchers.
The authors of both studies reported no funding sources and had no disclosures.
SCOTTSDALE, ARIZ. – Patients with hidradenitis suppurativa (HS) may be misdiagnosed when they see providers who are not dermatologists – as is usually the case during the initial years of their disease, according to a large analysis of medical claims data.
The findings highlight the need for visual diagnostic aids and specific guidelines for treating HS that target nondermatologists, Melissa Butt, MPH, of Penn State Hershey (Pa.) Medical Center, said during an interview at the annual meeting of the Society for Investigative Dermatology. She presented the findings during a poster session at the meeting.
HS is a chronic inflammatory disease of the hair follicles that affects 0.5%-4% of people in the United States. In past studies, up to 12 years elapsed between disease onset and diagnosis, in part because patients often cannot readily access dermatologists, Ms. Butt said. To better understand patterns of health care use during the years leading up to HS diagnosis, she and her colleagues used MarketScan data to identify 1,733 patients with HS-specific medical care claims filed in 2012 and 2013. Then they looked back at medical claims for these patients during 2008 through 2011, before the patients were diagnosed with HS. The cohort averaged 37 years of age (standard deviation, 15 years), and 73% were female.
Among 239,892 claims filed before patients were diagnosed with HS, 11,381 (4.7%) included codes for other diseases of the skin and subcutaneous tissues, Ms. Butt said. Dermatologists filed only 31% of these skin-specific claims, while 69% were filed by other providers, such as family practitioners, internists, emergency department physicians, and acute care hospitalists.
Notably, about two-thirds of the skin-specific diagnostic codes could have represented a misdiagnosis of HS. These codes included conditions such as abscesses, carbuncles, local infections, ulcers, and diseases of the sebaceous glands.
The fact that 78% of visits occurred in offices and other outpatient settings further underscores the need to improve the detection and care of HS in these environments, Ms. Butt said. Given current national shortages of dermatologists, visual HS diagnostic aids and “detailed, multistep clinical practice guidelines” for nondermatologists could help improve care of HS while patients wait to see the specialists, she added.
A second poster presented at the meeting provided results of a study on the use and impact of antibiotics in the treatment of HS. Alexander Fischer of Johns Hopkins University, Baltimore, and his associates studied antibiotic prescriptions and bacterial cultures from the lesions of 239 patients with HS who were treated at Johns Hopkins medical facilities between 2010 and 2015. Not only were 51% of HS patients on antibiotics at the time of culture, but these patients’ lesions were significantly more likely to contain antibiotic-resistant bacteria than were those of patients not on antibiotics.
Strikingly, Proteus species were isolated from nearly half of patients on trimethoprim-sulfamethoxazole (TMP/SMX), and 88% of colonies were resistant to TMP/SMX, while only 13% of cultures from untreated patients grew Proteus (P less than .001) and all were TMP/SMX-susceptible (P less than .001). Likewise, 100% of methicillin-resistant Staphylococcus aureus (MRSA) strains from patients prescribed ciprofloxacin were resistant to it, compared with a 10% background rate of ciprofloxacin resistance among MRSA from patients not taking antibiotics (P = .04). In addition, the proportion of other S. aureus strains that were clindamycin-resistant was higher when patients were taking this antibiotic than when they were not (63% versus 17%; P = .03).
The results “raise questions” about whether antibiotics should be used in HS patients who are not clearly benefiting from them, according to the researchers.
The authors of both studies reported no funding sources and had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Two studies underscored current challenges in diagnosing and treating hidradenitis suppurativa (HS).
Major finding: HS was usually diagnosed in outpatient settings by nondermatologists who often initially filed claims for carbuncles, ulcers, and other conditions that are confused with HS. In a separate study, antibiotic-resistant bacteria were significantly more prevalent in the lesions of HS patients who were receiving antibiotics than in patients who were not taking antibiotics.
Data source: A medical claims analysis of 1,733 patients with HS, and a study of antibiotic prescriptions and bacterial cultures from 239 patients with HS.
Disclosures: The authors of both studies reported no funding sources and had no disclosures.
Are Periodic Endoscopies Useful for Patients Under 40?
Endoscopies do not necessarily help catch early gastric cancer in young people, say researchers from Severance Hospital, Seoul, and Hanyang University, Guri, both in Korea.
In countries such as Korea and Japan, where the researchers say Helicobacter pylori is endemic and gastric cancer is prevalent, screening for gastric cancer is recommended for people aged ≥ 40 years. In a previous study, they found that biennial endoscopies increased the diagnosis of gastric neoplasms, including gastric cancer and adenoma, at an early stage in people aged ≥ 40 years. Noting that disease-free status and overall survival of younger patients with gastric cancer are dependent on the cancer’s stage at diagnosis, as is the case with middle-aged patients, they wanted to find out whether earlier periodic endoscopies would be useful.
Related:Clarifying the Links Between Gallbladder Disease and Cancer
In their study, 101 patients underwent screening endoscopy within 24 months after receiving their gastric cancer diagnosis. Another 463 had not had an endoscopy within 2 years. Overall, 65% to 68% had early gastric cancer, but the researchers found no significant difference between the 2 groups. However, the proportion of lesions that were treated with endoscopic submucosal dissection (ESD) differed according to the interval: 9.8% for ≤ 24 and 4.5% for ≥ 24 months.
The only factor associated with early gastric cancer was gastrointestinal symptoms at the time of diagnosis.
Related: How Much Is Too Much Cancer Screening?
Performing an earlier endoscopy detected gastric cancer at a smaller size (23.8 mm in the ≤ 24-month group vs 30.5 mm in the ≥ 24-month group), which could facilitate treatment with ESD, the researchers say. They suggest that although not necessarily influencing early diagnosis, periodic endoscopies may help increase the proportion of lesions treated with ESD.
Source:
Park CH, Kim EH, Chung H, et al. PLoS One. 2016;11(7):e0159759.
doi: 10.1371/journal.pone.0159759.
Endoscopies do not necessarily help catch early gastric cancer in young people, say researchers from Severance Hospital, Seoul, and Hanyang University, Guri, both in Korea.
In countries such as Korea and Japan, where the researchers say Helicobacter pylori is endemic and gastric cancer is prevalent, screening for gastric cancer is recommended for people aged ≥ 40 years. In a previous study, they found that biennial endoscopies increased the diagnosis of gastric neoplasms, including gastric cancer and adenoma, at an early stage in people aged ≥ 40 years. Noting that disease-free status and overall survival of younger patients with gastric cancer are dependent on the cancer’s stage at diagnosis, as is the case with middle-aged patients, they wanted to find out whether earlier periodic endoscopies would be useful.
Related:Clarifying the Links Between Gallbladder Disease and Cancer
In their study, 101 patients underwent screening endoscopy within 24 months after receiving their gastric cancer diagnosis. Another 463 had not had an endoscopy within 2 years. Overall, 65% to 68% had early gastric cancer, but the researchers found no significant difference between the 2 groups. However, the proportion of lesions that were treated with endoscopic submucosal dissection (ESD) differed according to the interval: 9.8% for ≤ 24 and 4.5% for ≥ 24 months.
The only factor associated with early gastric cancer was gastrointestinal symptoms at the time of diagnosis.
Related: How Much Is Too Much Cancer Screening?
Performing an earlier endoscopy detected gastric cancer at a smaller size (23.8 mm in the ≤ 24-month group vs 30.5 mm in the ≥ 24-month group), which could facilitate treatment with ESD, the researchers say. They suggest that although not necessarily influencing early diagnosis, periodic endoscopies may help increase the proportion of lesions treated with ESD.
Source:
Park CH, Kim EH, Chung H, et al. PLoS One. 2016;11(7):e0159759.
doi: 10.1371/journal.pone.0159759.
Endoscopies do not necessarily help catch early gastric cancer in young people, say researchers from Severance Hospital, Seoul, and Hanyang University, Guri, both in Korea.
In countries such as Korea and Japan, where the researchers say Helicobacter pylori is endemic and gastric cancer is prevalent, screening for gastric cancer is recommended for people aged ≥ 40 years. In a previous study, they found that biennial endoscopies increased the diagnosis of gastric neoplasms, including gastric cancer and adenoma, at an early stage in people aged ≥ 40 years. Noting that disease-free status and overall survival of younger patients with gastric cancer are dependent on the cancer’s stage at diagnosis, as is the case with middle-aged patients, they wanted to find out whether earlier periodic endoscopies would be useful.
Related:Clarifying the Links Between Gallbladder Disease and Cancer
In their study, 101 patients underwent screening endoscopy within 24 months after receiving their gastric cancer diagnosis. Another 463 had not had an endoscopy within 2 years. Overall, 65% to 68% had early gastric cancer, but the researchers found no significant difference between the 2 groups. However, the proportion of lesions that were treated with endoscopic submucosal dissection (ESD) differed according to the interval: 9.8% for ≤ 24 and 4.5% for ≥ 24 months.
The only factor associated with early gastric cancer was gastrointestinal symptoms at the time of diagnosis.
Related: How Much Is Too Much Cancer Screening?
Performing an earlier endoscopy detected gastric cancer at a smaller size (23.8 mm in the ≤ 24-month group vs 30.5 mm in the ≥ 24-month group), which could facilitate treatment with ESD, the researchers say. They suggest that although not necessarily influencing early diagnosis, periodic endoscopies may help increase the proportion of lesions treated with ESD.
Source:
Park CH, Kim EH, Chung H, et al. PLoS One. 2016;11(7):e0159759.
doi: 10.1371/journal.pone.0159759.
Emergency Imaging: Shortness of breath
A 79-year-old woman presented to the ED with acute shortness of breath. Of note, she had been recently discharged from our hospital after an open reduction and internal fixation of an intertrochanteric fracture of the right hip. The patient’s postoperative course was uncomplicated, and she was discharged home after a brief inpatient stay.
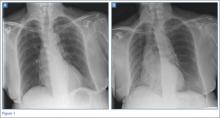
On physical examination, the patient was diaphoretic and tachypneic; oxygen saturation was 68% on room air, but increased to 100% saturation with supplemental oxygen through a nonrebreather mask. Radiographs from the patient’s inpatient hospital stay (Figure 1a) as well as ED visit (Figure 1b) were reviewed; representative images are shown above.
What is the diagnosis? What additional imaging tests may be useful to confirm the diagnosis?
Answer
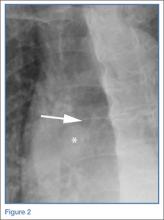
The radiographs taken at the time of the patient’s discharge were normal. The radiograph of the chest obtained in the ED, however, demonstrated a distinct cut-off of the right mainstem bronchus, referred to as a bronchial cut-off sign (white arrow, Figure 2), with a rounded density projecting over the right mainstem bronchus (white asterisk, Figure 2). These radiographic appearances suggested the presence of an aspirated foreign body.
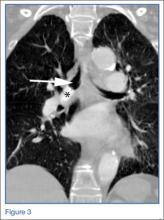
A computed tomography (CT) scan of the chest with contrast was performed to further evaluate the radiographic opacity and to exclude pulmonary embolism (PE), as this patient was at risk for such. The CT scan revealed no evidence of PE but confirmed the diagnosis of an aspirated foreign body. A high-density tablet (black asterisk, Figure 3) was noted to be completely occluding the right mainstem bronchus (white arrow, Figure 3) with resultant mild hyperinflation of the right lung. Upon further questioning, the patient stated that she had choked on a calcium tablet earlier in the day, but thought that the pill had finally “gone down.”
Since aspiration of foreign bodies is far more common in children,1,2 the diagnosis often is not considered in adults who present with acute onset of shortness of breath. In adults, the most significant predisposing factor to aspiration is alcoholism. However, foreign body aspiration may arise in various clinical scenarios, including in patients with structural abnormalities, in those with neuromuscular disease, and in the postoperative setting. The most common aspirated foreign bodies are food and broken tooth fragments/periodontal devices (eg, periodontal splint).2
Presentation is varied and depends upon the nature and volume of the aspirated foreign body, which may contribute to the airway obstruction or an inflammatory bronchopneumonia. The posterior segment of the upper lobes and the superior segments of the lower lobes are the most commonly involved sites, with the right lung preferentially involved over the left lung.3
The diagnosis of foreign body aspiration begins with an appropriate clinical history. Given our patient’s recent orthopedic surgery, PE was an understandable diagnostic consideration. As with any patient acutely short of breath, radiographs are the initial diagnostic imaging study of choice. An abrupt truncation of a bronchus on radiography suggests obstruction related to a mucous plugging, cancer, or foreign body aspiration. Other findings of foreign body aspiration include segmental/lobar hyperinflation and/or atelectasis.3 In many scenarios, the aspirated foreign body may not be radiodense, which limits the utility and diagnostic accuracy of radiography. Computed tomography improves diagnostic precision and time to diagnosis by directly visualizing the airway lumen and improving visualization of radiolucent objects.4
Treatment for obstructive aspiration depends upon the location and nature of the aspirated object. However, bedside bronchoscopy and extraction of the foreign object is the mainstay of treatment, and is how this patient was treated. Rapid diagnosis and treatment is key to alleviating obstruction and preventing potential complications of hemoptysis and infection.
1. Marom EM, McAdams HP, Erasmus JJ, Goodman PC. The many faces of pulmonary aspiration. AJR Am J Roentgenol. 1999;172(1):121-128.
2. McGuirt WF, Holmes KD, Feehs R, Browne JD. Tracheobronchial foreign bodies. Laryngoscope. 1988;98(6 Pt 1):615-618.
3. Franquet T, Giménez A, Rosón N, Torrubia S, Sabaté JM, Pérez C. Aspiration diseases: Findings, pitfalls, and differential diagnosis. Radiographics. 2000;20(3):673-685.
4. Newton JP, Abel RW, Lloyd CH, Yemm R. The use of computed tomography in the detection of radiolucent denture base material in the chest. J Oral Rehabil. 1987;14(2):193-202.
A 79-year-old woman presented to the ED with acute shortness of breath. Of note, she had been recently discharged from our hospital after an open reduction and internal fixation of an intertrochanteric fracture of the right hip. The patient’s postoperative course was uncomplicated, and she was discharged home after a brief inpatient stay.
On physical examination, the patient was diaphoretic and tachypneic; oxygen saturation was 68% on room air, but increased to 100% saturation with supplemental oxygen through a nonrebreather mask. Radiographs from the patient’s inpatient hospital stay (Figure 1a) as well as ED visit (Figure 1b) were reviewed; representative images are shown above.
What is the diagnosis? What additional imaging tests may be useful to confirm the diagnosis?
Answer
The radiographs taken at the time of the patient’s discharge were normal. The radiograph of the chest obtained in the ED, however, demonstrated a distinct cut-off of the right mainstem bronchus, referred to as a bronchial cut-off sign (white arrow, Figure 2), with a rounded density projecting over the right mainstem bronchus (white asterisk, Figure 2). These radiographic appearances suggested the presence of an aspirated foreign body.
A computed tomography (CT) scan of the chest with contrast was performed to further evaluate the radiographic opacity and to exclude pulmonary embolism (PE), as this patient was at risk for such. The CT scan revealed no evidence of PE but confirmed the diagnosis of an aspirated foreign body. A high-density tablet (black asterisk, Figure 3) was noted to be completely occluding the right mainstem bronchus (white arrow, Figure 3) with resultant mild hyperinflation of the right lung. Upon further questioning, the patient stated that she had choked on a calcium tablet earlier in the day, but thought that the pill had finally “gone down.”
Since aspiration of foreign bodies is far more common in children,1,2 the diagnosis often is not considered in adults who present with acute onset of shortness of breath. In adults, the most significant predisposing factor to aspiration is alcoholism. However, foreign body aspiration may arise in various clinical scenarios, including in patients with structural abnormalities, in those with neuromuscular disease, and in the postoperative setting. The most common aspirated foreign bodies are food and broken tooth fragments/periodontal devices (eg, periodontal splint).2
Presentation is varied and depends upon the nature and volume of the aspirated foreign body, which may contribute to the airway obstruction or an inflammatory bronchopneumonia. The posterior segment of the upper lobes and the superior segments of the lower lobes are the most commonly involved sites, with the right lung preferentially involved over the left lung.3
The diagnosis of foreign body aspiration begins with an appropriate clinical history. Given our patient’s recent orthopedic surgery, PE was an understandable diagnostic consideration. As with any patient acutely short of breath, radiographs are the initial diagnostic imaging study of choice. An abrupt truncation of a bronchus on radiography suggests obstruction related to a mucous plugging, cancer, or foreign body aspiration. Other findings of foreign body aspiration include segmental/lobar hyperinflation and/or atelectasis.3 In many scenarios, the aspirated foreign body may not be radiodense, which limits the utility and diagnostic accuracy of radiography. Computed tomography improves diagnostic precision and time to diagnosis by directly visualizing the airway lumen and improving visualization of radiolucent objects.4
Treatment for obstructive aspiration depends upon the location and nature of the aspirated object. However, bedside bronchoscopy and extraction of the foreign object is the mainstay of treatment, and is how this patient was treated. Rapid diagnosis and treatment is key to alleviating obstruction and preventing potential complications of hemoptysis and infection.
A 79-year-old woman presented to the ED with acute shortness of breath. Of note, she had been recently discharged from our hospital after an open reduction and internal fixation of an intertrochanteric fracture of the right hip. The patient’s postoperative course was uncomplicated, and she was discharged home after a brief inpatient stay.
On physical examination, the patient was diaphoretic and tachypneic; oxygen saturation was 68% on room air, but increased to 100% saturation with supplemental oxygen through a nonrebreather mask. Radiographs from the patient’s inpatient hospital stay (Figure 1a) as well as ED visit (Figure 1b) were reviewed; representative images are shown above.
What is the diagnosis? What additional imaging tests may be useful to confirm the diagnosis?
Answer
The radiographs taken at the time of the patient’s discharge were normal. The radiograph of the chest obtained in the ED, however, demonstrated a distinct cut-off of the right mainstem bronchus, referred to as a bronchial cut-off sign (white arrow, Figure 2), with a rounded density projecting over the right mainstem bronchus (white asterisk, Figure 2). These radiographic appearances suggested the presence of an aspirated foreign body.
A computed tomography (CT) scan of the chest with contrast was performed to further evaluate the radiographic opacity and to exclude pulmonary embolism (PE), as this patient was at risk for such. The CT scan revealed no evidence of PE but confirmed the diagnosis of an aspirated foreign body. A high-density tablet (black asterisk, Figure 3) was noted to be completely occluding the right mainstem bronchus (white arrow, Figure 3) with resultant mild hyperinflation of the right lung. Upon further questioning, the patient stated that she had choked on a calcium tablet earlier in the day, but thought that the pill had finally “gone down.”
Since aspiration of foreign bodies is far more common in children,1,2 the diagnosis often is not considered in adults who present with acute onset of shortness of breath. In adults, the most significant predisposing factor to aspiration is alcoholism. However, foreign body aspiration may arise in various clinical scenarios, including in patients with structural abnormalities, in those with neuromuscular disease, and in the postoperative setting. The most common aspirated foreign bodies are food and broken tooth fragments/periodontal devices (eg, periodontal splint).2
Presentation is varied and depends upon the nature and volume of the aspirated foreign body, which may contribute to the airway obstruction or an inflammatory bronchopneumonia. The posterior segment of the upper lobes and the superior segments of the lower lobes are the most commonly involved sites, with the right lung preferentially involved over the left lung.3
The diagnosis of foreign body aspiration begins with an appropriate clinical history. Given our patient’s recent orthopedic surgery, PE was an understandable diagnostic consideration. As with any patient acutely short of breath, radiographs are the initial diagnostic imaging study of choice. An abrupt truncation of a bronchus on radiography suggests obstruction related to a mucous plugging, cancer, or foreign body aspiration. Other findings of foreign body aspiration include segmental/lobar hyperinflation and/or atelectasis.3 In many scenarios, the aspirated foreign body may not be radiodense, which limits the utility and diagnostic accuracy of radiography. Computed tomography improves diagnostic precision and time to diagnosis by directly visualizing the airway lumen and improving visualization of radiolucent objects.4
Treatment for obstructive aspiration depends upon the location and nature of the aspirated object. However, bedside bronchoscopy and extraction of the foreign object is the mainstay of treatment, and is how this patient was treated. Rapid diagnosis and treatment is key to alleviating obstruction and preventing potential complications of hemoptysis and infection.
1. Marom EM, McAdams HP, Erasmus JJ, Goodman PC. The many faces of pulmonary aspiration. AJR Am J Roentgenol. 1999;172(1):121-128.
2. McGuirt WF, Holmes KD, Feehs R, Browne JD. Tracheobronchial foreign bodies. Laryngoscope. 1988;98(6 Pt 1):615-618.
3. Franquet T, Giménez A, Rosón N, Torrubia S, Sabaté JM, Pérez C. Aspiration diseases: Findings, pitfalls, and differential diagnosis. Radiographics. 2000;20(3):673-685.
4. Newton JP, Abel RW, Lloyd CH, Yemm R. The use of computed tomography in the detection of radiolucent denture base material in the chest. J Oral Rehabil. 1987;14(2):193-202.
1. Marom EM, McAdams HP, Erasmus JJ, Goodman PC. The many faces of pulmonary aspiration. AJR Am J Roentgenol. 1999;172(1):121-128.
2. McGuirt WF, Holmes KD, Feehs R, Browne JD. Tracheobronchial foreign bodies. Laryngoscope. 1988;98(6 Pt 1):615-618.
3. Franquet T, Giménez A, Rosón N, Torrubia S, Sabaté JM, Pérez C. Aspiration diseases: Findings, pitfalls, and differential diagnosis. Radiographics. 2000;20(3):673-685.
4. Newton JP, Abel RW, Lloyd CH, Yemm R. The use of computed tomography in the detection of radiolucent denture base material in the chest. J Oral Rehabil. 1987;14(2):193-202.
An Unusual Cause of Syncope With T-Wave Abnormalities
Case
A 34-year-old man presented to our ED via emergency medical services (EMS) following a syncopal episode. The patient stated that as he was getting ready for work earlier that morning, he experienced sudden lightheadedness and passed out, whereupon his wife immediately called EMS. The patient denied any previous history of syncope, but said he had been experiencing frequent episodes of nausea and vomiting over the past week. He also complained of a mild occipital headache that acetaminophen had failed to relieve.
The patient had been seen at a different ED 3 days earlier for nausea and vomiting. After evaluating the patient, the emergency physician (EP) at this facility felt the most likely cause of the patient’s gastrointestinal issues was related to hydralazine, his antihypertensive medication, and advised the patient to discontinue its use.
During evaluation at our ED, the patient denied fever, chills, neck stiffness, numbness, weakness, tingling of the extremities, or difficulty walking. He also denied chest pain, shortness of breath, or urinary symptoms. The patient’s medical history was significant only for hypertension; he had not taken any antihypertensive or other medications for the past 3 days, as previously instructed by the EP at the other ED. The patient denied alcohol or drug abuse.
On physical examination, the patient’s vital signs were: temperature, 98.6°F; heart rate, 58 beats/minute; blood pressure, 130/90 mm Hg; and respiratory rate, 16 breaths/minute. Oxygen saturation was 100% on room air. Examination of the head was normal and without evidence of trauma. Both pupils measured 4 mm and were equally round and reactive to light; the patient’s extraocular movements were intact. The remainder of the head, eyes, ears, nose, and throat examination was normal. The neck was supple, without masses or meningeal signs. The cardiopulmonary and abdominal examinations were all normal. On neurological examination, the patient was awake, alert, and oriented to person, place, and time. Cranial nerves II through XII were intact, and the patient had 5/5 motor strength in all four extremities and a normal gait.
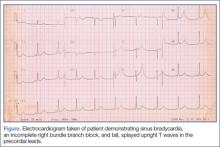
Because we were concerned about the patient’s unexplained syncopal episode, we ordered laboratory tests, including a complete blood count (CBC), evaluation of electrolytes and glucose levels, and kidney function. In addition, we also ordered an electrocardiogram (ECG) and a noncontrast computed tomography (CT) scan of the head. All laboratory test results were within normal range. The ECG, however, demonstrated sinus bradycardia (approximately 58 beats/minute), a normal PR and QRS interval, a normal axis, and an incomplete right bundle branch block with tall, large, splayed upright T waves in the precordial leads (Figure). Based on the abnormal ECG results, we ordered serum cardiac marker studies, the values of which were all within normal range. The noncontrast CT scan of the head revealed a low-density posterior fossa mass compressing the fourth ventricle with secondary hydrocephalus.
The patient was placed with his head in an upright position and given 1 g/kg mannitol and 10 mg dexamethasone intravenously (IV). Neurosurgery services were consulted, and the patient underwent surgery the following morning. Surgery confirmed the presence of a hemangioblastoma. The hemangioblastoma was successfully excised, and the patient had an uneventful recovery. Interestingly, the significant T-wave changes in the precordial leads were no longer present postoperatively.
Discussion
Syncope and near-syncope are common reasons for ED visits. Syncope is a syndrome characterized by a transient, self-limited episode of loss of consciousness occurring as a result of a brief interruption of the oxygen supply to the brain.1 This interruption is almost always due to a transient cessation of blood flow.1 In true syncope (as opposed to seizures or hypoglycemia), the episode is characterized by a rapid onset of loss of consciousness—with or without warning symptoms.1 It is important to determine the cause of syncope, because 7% to 23% of such patients will suffer serious outcomes within 7 to 30 days of their ED visit—either within a hospital setting or at home.2
Etiology
There are many causes of syncope. In most cases, the etiology falls under one of three broad categories: neurally mediated (or reflex mediated), orthostatic hypotensive-mediated, or cardiovascular (CV)-mediated. Less common causes of syncope include cerebrovascular injury.1 The Table outlines both common and uncommon causes of syncope.
On presentation, our patient had several possible causes for his syncopal episode: an abnormal ECG (CV); multiple episodes of emesis (volume depletion); and headache (cerebrovascular). The EP worked up all three of these signs and symptoms simultaneously as is the appropriate protocol when evaluating an ED patient presenting with undifferentiated syncope.
Signs and Symptoms
Patients with undiagnosed brain tumors normally present with headache, seizures, nausea, vomiting, focal neurological deficits, or an altered mental status.3 Syncope is a very rare manifestation of a brain tumor3; however, our patient did complain of headache, nausea, and vomiting.
In addition to the unusual cause of the syncope, the abnormally large upright T waves make this case even more notable. T-wave changes are the most common ECG abnormality, seen in about 50% of abnormal tracings reviewed in a hospital population and in 2.4% of all ECGs.4
In general, T-wave changes are a result of local changes in the duration of repolarization. T-wave inversion is the most common T-wave abnormality and is typically observed in the setting of ischemia, post-ingestion of food, following an episode of tachycardia or anxiety, and autonomic dysfunction.5 However, in patients who have a cerebral etiology (usually hemorrhage), the T-wave changes may be either upright (as in our case) or inverted.5 Historically, subarachnoid hemorrhage (SAH) has been associated with ST-segment elevation and T-wave inversion. Hypothalamic stimulation and autonomic dysfunction have been linked to abnormal T-waves, but this has not been conclusively proven to be the cause of the abnormality.6 For all of the aforementioned reasons, the specificity for a given cause of T-wave changes is exceedingly low.5
Hyperacute T-wave amplitude, with prominent symmetrical T waves in at least two continuous leads, may be the earliest sign of acute transmural myocardial infarction (MI).7 It usually persists for only a brief time before other ECG findings of acute MI are observed. Other common causes of hyperacute T waves include hyperkalemia (usually narrow-based, and peaked), early repolarization, left ventricular hypertrophy, and acute myocarditis.8 Less common causes of prominent T waves include pre-excitation syndromes, pericarditis, and scorpion stings.9,10
Summary
It is unclear why our patient, who had a hemangioblastoma, demonstrated hyperacute T-wave abnormality on ECG. The abnormal upright T waves may have occurred secondary to the same theories regarding SAH, hypothalamic stimulation, or autonomic dysfunction. Regardless of the underlying etiology, this case serves as a reminder to the EP that not all T-wave changes on ECG are cardiac in origin.
1. Puppala VK, Dickinson O, Benditt DG. Syncope: classification and risk stratification. J Cardiol. 2014;63(3):171-177.
2. Thiruganasambandamoorthy V, Stiell IG, Sivilotti ML, et al. Risk stratification of adult emergency department syncope patients to predict short-term serious outcomes after discharge (RiSEDS) study. BMC Emerg Med. 2014;14(1):8.
3. van der Sluijs BM, Renier WO, Kappelle AC. Brain tumor as a rare cause of cardiac syncope. J Neurooncol. 2004;67(1-2):241-244.
4. Friedberg CK, Zagar A. Nonspecific ST and T-wave changes. Circulation. 1961;23:665-661.
5. Fisch C. T wave abnormalities due to extracardiac “functional” causes. ACC Curr J Rev. 1997;6(2):101-104.
6. Chatterjee S. ECG changes in subarachnoid hemorrhage: a synopsis. Neth Heart J. 2011;19(1):31-47.
7. Vojáčeka, J, Janskýb P, Janotac T. Third universal definition of myocardial infarction. Cor Vasa. 2013;55:e228-e235.
8. Levis JT. ECG diagnosis: hyperacute T waves. Perm J. 2015;19(3):79.
9. Somers MP, Brady WJ, Perron AD, Mattu A. The prominent T wave: electrocardiographic differential diagnosis. Am J Emerg Med. 2002;20(3):243-251.
10. Kumar MR, Bharath RV, Subrahmanyam BV, Rammohan P, Agrawal A. Scorpion envenomation and its management in adults. Sahel Med J. 2013;16(2):60-63.
Case
A 34-year-old man presented to our ED via emergency medical services (EMS) following a syncopal episode. The patient stated that as he was getting ready for work earlier that morning, he experienced sudden lightheadedness and passed out, whereupon his wife immediately called EMS. The patient denied any previous history of syncope, but said he had been experiencing frequent episodes of nausea and vomiting over the past week. He also complained of a mild occipital headache that acetaminophen had failed to relieve.
The patient had been seen at a different ED 3 days earlier for nausea and vomiting. After evaluating the patient, the emergency physician (EP) at this facility felt the most likely cause of the patient’s gastrointestinal issues was related to hydralazine, his antihypertensive medication, and advised the patient to discontinue its use.
During evaluation at our ED, the patient denied fever, chills, neck stiffness, numbness, weakness, tingling of the extremities, or difficulty walking. He also denied chest pain, shortness of breath, or urinary symptoms. The patient’s medical history was significant only for hypertension; he had not taken any antihypertensive or other medications for the past 3 days, as previously instructed by the EP at the other ED. The patient denied alcohol or drug abuse.
On physical examination, the patient’s vital signs were: temperature, 98.6°F; heart rate, 58 beats/minute; blood pressure, 130/90 mm Hg; and respiratory rate, 16 breaths/minute. Oxygen saturation was 100% on room air. Examination of the head was normal and without evidence of trauma. Both pupils measured 4 mm and were equally round and reactive to light; the patient’s extraocular movements were intact. The remainder of the head, eyes, ears, nose, and throat examination was normal. The neck was supple, without masses or meningeal signs. The cardiopulmonary and abdominal examinations were all normal. On neurological examination, the patient was awake, alert, and oriented to person, place, and time. Cranial nerves II through XII were intact, and the patient had 5/5 motor strength in all four extremities and a normal gait.
Because we were concerned about the patient’s unexplained syncopal episode, we ordered laboratory tests, including a complete blood count (CBC), evaluation of electrolytes and glucose levels, and kidney function. In addition, we also ordered an electrocardiogram (ECG) and a noncontrast computed tomography (CT) scan of the head. All laboratory test results were within normal range. The ECG, however, demonstrated sinus bradycardia (approximately 58 beats/minute), a normal PR and QRS interval, a normal axis, and an incomplete right bundle branch block with tall, large, splayed upright T waves in the precordial leads (Figure). Based on the abnormal ECG results, we ordered serum cardiac marker studies, the values of which were all within normal range. The noncontrast CT scan of the head revealed a low-density posterior fossa mass compressing the fourth ventricle with secondary hydrocephalus.
The patient was placed with his head in an upright position and given 1 g/kg mannitol and 10 mg dexamethasone intravenously (IV). Neurosurgery services were consulted, and the patient underwent surgery the following morning. Surgery confirmed the presence of a hemangioblastoma. The hemangioblastoma was successfully excised, and the patient had an uneventful recovery. Interestingly, the significant T-wave changes in the precordial leads were no longer present postoperatively.
Discussion
Syncope and near-syncope are common reasons for ED visits. Syncope is a syndrome characterized by a transient, self-limited episode of loss of consciousness occurring as a result of a brief interruption of the oxygen supply to the brain.1 This interruption is almost always due to a transient cessation of blood flow.1 In true syncope (as opposed to seizures or hypoglycemia), the episode is characterized by a rapid onset of loss of consciousness—with or without warning symptoms.1 It is important to determine the cause of syncope, because 7% to 23% of such patients will suffer serious outcomes within 7 to 30 days of their ED visit—either within a hospital setting or at home.2
Etiology
There are many causes of syncope. In most cases, the etiology falls under one of three broad categories: neurally mediated (or reflex mediated), orthostatic hypotensive-mediated, or cardiovascular (CV)-mediated. Less common causes of syncope include cerebrovascular injury.1 The Table outlines both common and uncommon causes of syncope.
On presentation, our patient had several possible causes for his syncopal episode: an abnormal ECG (CV); multiple episodes of emesis (volume depletion); and headache (cerebrovascular). The EP worked up all three of these signs and symptoms simultaneously as is the appropriate protocol when evaluating an ED patient presenting with undifferentiated syncope.
Signs and Symptoms
Patients with undiagnosed brain tumors normally present with headache, seizures, nausea, vomiting, focal neurological deficits, or an altered mental status.3 Syncope is a very rare manifestation of a brain tumor3; however, our patient did complain of headache, nausea, and vomiting.
In addition to the unusual cause of the syncope, the abnormally large upright T waves make this case even more notable. T-wave changes are the most common ECG abnormality, seen in about 50% of abnormal tracings reviewed in a hospital population and in 2.4% of all ECGs.4
In general, T-wave changes are a result of local changes in the duration of repolarization. T-wave inversion is the most common T-wave abnormality and is typically observed in the setting of ischemia, post-ingestion of food, following an episode of tachycardia or anxiety, and autonomic dysfunction.5 However, in patients who have a cerebral etiology (usually hemorrhage), the T-wave changes may be either upright (as in our case) or inverted.5 Historically, subarachnoid hemorrhage (SAH) has been associated with ST-segment elevation and T-wave inversion. Hypothalamic stimulation and autonomic dysfunction have been linked to abnormal T-waves, but this has not been conclusively proven to be the cause of the abnormality.6 For all of the aforementioned reasons, the specificity for a given cause of T-wave changes is exceedingly low.5
Hyperacute T-wave amplitude, with prominent symmetrical T waves in at least two continuous leads, may be the earliest sign of acute transmural myocardial infarction (MI).7 It usually persists for only a brief time before other ECG findings of acute MI are observed. Other common causes of hyperacute T waves include hyperkalemia (usually narrow-based, and peaked), early repolarization, left ventricular hypertrophy, and acute myocarditis.8 Less common causes of prominent T waves include pre-excitation syndromes, pericarditis, and scorpion stings.9,10
Summary
It is unclear why our patient, who had a hemangioblastoma, demonstrated hyperacute T-wave abnormality on ECG. The abnormal upright T waves may have occurred secondary to the same theories regarding SAH, hypothalamic stimulation, or autonomic dysfunction. Regardless of the underlying etiology, this case serves as a reminder to the EP that not all T-wave changes on ECG are cardiac in origin.
Case
A 34-year-old man presented to our ED via emergency medical services (EMS) following a syncopal episode. The patient stated that as he was getting ready for work earlier that morning, he experienced sudden lightheadedness and passed out, whereupon his wife immediately called EMS. The patient denied any previous history of syncope, but said he had been experiencing frequent episodes of nausea and vomiting over the past week. He also complained of a mild occipital headache that acetaminophen had failed to relieve.
The patient had been seen at a different ED 3 days earlier for nausea and vomiting. After evaluating the patient, the emergency physician (EP) at this facility felt the most likely cause of the patient’s gastrointestinal issues was related to hydralazine, his antihypertensive medication, and advised the patient to discontinue its use.
During evaluation at our ED, the patient denied fever, chills, neck stiffness, numbness, weakness, tingling of the extremities, or difficulty walking. He also denied chest pain, shortness of breath, or urinary symptoms. The patient’s medical history was significant only for hypertension; he had not taken any antihypertensive or other medications for the past 3 days, as previously instructed by the EP at the other ED. The patient denied alcohol or drug abuse.
On physical examination, the patient’s vital signs were: temperature, 98.6°F; heart rate, 58 beats/minute; blood pressure, 130/90 mm Hg; and respiratory rate, 16 breaths/minute. Oxygen saturation was 100% on room air. Examination of the head was normal and without evidence of trauma. Both pupils measured 4 mm and were equally round and reactive to light; the patient’s extraocular movements were intact. The remainder of the head, eyes, ears, nose, and throat examination was normal. The neck was supple, without masses or meningeal signs. The cardiopulmonary and abdominal examinations were all normal. On neurological examination, the patient was awake, alert, and oriented to person, place, and time. Cranial nerves II through XII were intact, and the patient had 5/5 motor strength in all four extremities and a normal gait.
Because we were concerned about the patient’s unexplained syncopal episode, we ordered laboratory tests, including a complete blood count (CBC), evaluation of electrolytes and glucose levels, and kidney function. In addition, we also ordered an electrocardiogram (ECG) and a noncontrast computed tomography (CT) scan of the head. All laboratory test results were within normal range. The ECG, however, demonstrated sinus bradycardia (approximately 58 beats/minute), a normal PR and QRS interval, a normal axis, and an incomplete right bundle branch block with tall, large, splayed upright T waves in the precordial leads (Figure). Based on the abnormal ECG results, we ordered serum cardiac marker studies, the values of which were all within normal range. The noncontrast CT scan of the head revealed a low-density posterior fossa mass compressing the fourth ventricle with secondary hydrocephalus.
The patient was placed with his head in an upright position and given 1 g/kg mannitol and 10 mg dexamethasone intravenously (IV). Neurosurgery services were consulted, and the patient underwent surgery the following morning. Surgery confirmed the presence of a hemangioblastoma. The hemangioblastoma was successfully excised, and the patient had an uneventful recovery. Interestingly, the significant T-wave changes in the precordial leads were no longer present postoperatively.
Discussion
Syncope and near-syncope are common reasons for ED visits. Syncope is a syndrome characterized by a transient, self-limited episode of loss of consciousness occurring as a result of a brief interruption of the oxygen supply to the brain.1 This interruption is almost always due to a transient cessation of blood flow.1 In true syncope (as opposed to seizures or hypoglycemia), the episode is characterized by a rapid onset of loss of consciousness—with or without warning symptoms.1 It is important to determine the cause of syncope, because 7% to 23% of such patients will suffer serious outcomes within 7 to 30 days of their ED visit—either within a hospital setting or at home.2
Etiology
There are many causes of syncope. In most cases, the etiology falls under one of three broad categories: neurally mediated (or reflex mediated), orthostatic hypotensive-mediated, or cardiovascular (CV)-mediated. Less common causes of syncope include cerebrovascular injury.1 The Table outlines both common and uncommon causes of syncope.
On presentation, our patient had several possible causes for his syncopal episode: an abnormal ECG (CV); multiple episodes of emesis (volume depletion); and headache (cerebrovascular). The EP worked up all three of these signs and symptoms simultaneously as is the appropriate protocol when evaluating an ED patient presenting with undifferentiated syncope.
Signs and Symptoms
Patients with undiagnosed brain tumors normally present with headache, seizures, nausea, vomiting, focal neurological deficits, or an altered mental status.3 Syncope is a very rare manifestation of a brain tumor3; however, our patient did complain of headache, nausea, and vomiting.
In addition to the unusual cause of the syncope, the abnormally large upright T waves make this case even more notable. T-wave changes are the most common ECG abnormality, seen in about 50% of abnormal tracings reviewed in a hospital population and in 2.4% of all ECGs.4
In general, T-wave changes are a result of local changes in the duration of repolarization. T-wave inversion is the most common T-wave abnormality and is typically observed in the setting of ischemia, post-ingestion of food, following an episode of tachycardia or anxiety, and autonomic dysfunction.5 However, in patients who have a cerebral etiology (usually hemorrhage), the T-wave changes may be either upright (as in our case) or inverted.5 Historically, subarachnoid hemorrhage (SAH) has been associated with ST-segment elevation and T-wave inversion. Hypothalamic stimulation and autonomic dysfunction have been linked to abnormal T-waves, but this has not been conclusively proven to be the cause of the abnormality.6 For all of the aforementioned reasons, the specificity for a given cause of T-wave changes is exceedingly low.5
Hyperacute T-wave amplitude, with prominent symmetrical T waves in at least two continuous leads, may be the earliest sign of acute transmural myocardial infarction (MI).7 It usually persists for only a brief time before other ECG findings of acute MI are observed. Other common causes of hyperacute T waves include hyperkalemia (usually narrow-based, and peaked), early repolarization, left ventricular hypertrophy, and acute myocarditis.8 Less common causes of prominent T waves include pre-excitation syndromes, pericarditis, and scorpion stings.9,10
Summary
It is unclear why our patient, who had a hemangioblastoma, demonstrated hyperacute T-wave abnormality on ECG. The abnormal upright T waves may have occurred secondary to the same theories regarding SAH, hypothalamic stimulation, or autonomic dysfunction. Regardless of the underlying etiology, this case serves as a reminder to the EP that not all T-wave changes on ECG are cardiac in origin.
1. Puppala VK, Dickinson O, Benditt DG. Syncope: classification and risk stratification. J Cardiol. 2014;63(3):171-177.
2. Thiruganasambandamoorthy V, Stiell IG, Sivilotti ML, et al. Risk stratification of adult emergency department syncope patients to predict short-term serious outcomes after discharge (RiSEDS) study. BMC Emerg Med. 2014;14(1):8.
3. van der Sluijs BM, Renier WO, Kappelle AC. Brain tumor as a rare cause of cardiac syncope. J Neurooncol. 2004;67(1-2):241-244.
4. Friedberg CK, Zagar A. Nonspecific ST and T-wave changes. Circulation. 1961;23:665-661.
5. Fisch C. T wave abnormalities due to extracardiac “functional” causes. ACC Curr J Rev. 1997;6(2):101-104.
6. Chatterjee S. ECG changes in subarachnoid hemorrhage: a synopsis. Neth Heart J. 2011;19(1):31-47.
7. Vojáčeka, J, Janskýb P, Janotac T. Third universal definition of myocardial infarction. Cor Vasa. 2013;55:e228-e235.
8. Levis JT. ECG diagnosis: hyperacute T waves. Perm J. 2015;19(3):79.
9. Somers MP, Brady WJ, Perron AD, Mattu A. The prominent T wave: electrocardiographic differential diagnosis. Am J Emerg Med. 2002;20(3):243-251.
10. Kumar MR, Bharath RV, Subrahmanyam BV, Rammohan P, Agrawal A. Scorpion envenomation and its management in adults. Sahel Med J. 2013;16(2):60-63.
1. Puppala VK, Dickinson O, Benditt DG. Syncope: classification and risk stratification. J Cardiol. 2014;63(3):171-177.
2. Thiruganasambandamoorthy V, Stiell IG, Sivilotti ML, et al. Risk stratification of adult emergency department syncope patients to predict short-term serious outcomes after discharge (RiSEDS) study. BMC Emerg Med. 2014;14(1):8.
3. van der Sluijs BM, Renier WO, Kappelle AC. Brain tumor as a rare cause of cardiac syncope. J Neurooncol. 2004;67(1-2):241-244.
4. Friedberg CK, Zagar A. Nonspecific ST and T-wave changes. Circulation. 1961;23:665-661.
5. Fisch C. T wave abnormalities due to extracardiac “functional” causes. ACC Curr J Rev. 1997;6(2):101-104.
6. Chatterjee S. ECG changes in subarachnoid hemorrhage: a synopsis. Neth Heart J. 2011;19(1):31-47.
7. Vojáčeka, J, Janskýb P, Janotac T. Third universal definition of myocardial infarction. Cor Vasa. 2013;55:e228-e235.
8. Levis JT. ECG diagnosis: hyperacute T waves. Perm J. 2015;19(3):79.
9. Somers MP, Brady WJ, Perron AD, Mattu A. The prominent T wave: electrocardiographic differential diagnosis. Am J Emerg Med. 2002;20(3):243-251.
10. Kumar MR, Bharath RV, Subrahmanyam BV, Rammohan P, Agrawal A. Scorpion envenomation and its management in adults. Sahel Med J. 2013;16(2):60-63.
A Rare Case of Traumatic Tension Pneumo-orbitum
Traumatic eye injuries ranging from mild corneal abrasions to penetrating globe injuries are commonly seen in the ED, and ocular trauma accounts for nearly 1% of all complaints in the ED.1 Up to 29% of facial fractures have associated eye injuries.2 Emergency physicians (EPs) must be aware of possible eye injuries, including traumatic vision loss, and the indicators for emergent interventions.
Tension pneumo-orbitum following facial trauma is rarely reported. We present a case of orbital compartment syndrome (OCS) in an elderly woman who sustained an orbital floor fracture and required emergent lateral canthotomy to preserve vision.
Case
A 76-year-old woman presented to the ED for evaluation of pain, swelling, and loss of vision in the right eye. She said she had been sitting in a chair tying her shoes when she lost her balance and fell forward, striking her head and the right side of her face against the floor. She experienced no loss of consciousness and denied any neck pain, jaw pain, or dizziness. She also denied any chest pain, shortness of breath, weakness, or loss of function in either her arms or legs. She did, however, note a small nosebleed that had stopped before she arrived at the ED. The patient’s primary complaint was a possible nasal bone fracture.
Her medical history was significant for hypertension and coronary artery disease. Her medications include amitriptyline, an antihistamine, aspirin, clopidogrel, diltiazem, folic acid, furosemide, hydralazine, levothyroxine, prednisone, and zolpidem. She stated that she was allergic to amoxicillin and sulfa drugs.
The patient’s vital signs at presentation were: blood pressure (BP), 193/82 mm Hg; heart rate, 71 beats/minute; respiratory rate, 16 breaths/minute; and temperature, 97°F. She was alert, oriented, and in no distress. Her head and neck examination showed no scalp lacerations or swelling. There was, however, significant swelling and ecchymosis around the right eye and swelling and ecchymosis around the nose, with dried blood in both nares. No septal hematoma was present. The patient had tenderness to palpation over the infraorbital area and nose. No gross facial instability was present, and Battle sign was not appreciated. No jaw or dental abnormalities were noted.
The patient’s right pupil was fixed and dilated, and she could not perceive light. She did have upward and lateral movement of the eye, but was unable to look down. A minimal amount of proptosis was noted. Her intraocular pressure (IOP) was elevated at 54 mm Hg (normal range, 10-20 mm Hg). The remainder of the examination, including the neurological examination, was unremarkable.
The patient received emergent head and facial computed tomography (CT) scans. The head CT showed no acute intracranial hemorrhage, mass, or infarct. The facial CT was read as a right orbital floor fracture with intraorbital air, and a right maxillary sinus hematoma. Laboratory evaluation revealed a hematocrit of 38% and a platelet count of 544,000/mcL (normal range, 150,000-450,000/mcL). The prothrombin time was 10.9 seconds (normal range, 11-13.5 seconds); the international normalized ratio was 0.8 (normal range, 0.8-1.1); and the partial thromboplastin time was 22.5 seconds (normal range, 25-35 seconds).
Because the patient was at risk for permanent visual impairment due to increased IOP from the injury, a lateral canthotomy was immediately performed. A small amount of air was released, and the proptosis was notably reduced.
At this point, the ophthalmologist arrived and used an 18-gauge needle to explore the retrobulbar space. Two pockets of air were released, which markedly reduced the tactile pressure of the globe. Repeat tonography of the globe was 28 mm Hg. The wound was left open to drain, and the patient was started on azithromycin. She was discharged home to follow up with ophthalmology.
The patient presented to the ED 2 months later for an unrelated condition. At that time, she reported a complete return of her vision with no deficits and no noticeable scarring around the eye.
Discussion
The orbit is an enclosed space, bordered by bone laterally and posteriorly—the orbital septa superiorly and inferiorly, and the globe anteriorly.3 The lateral canthus is a combined tendon-ligament that helps attach the tarsal plates of the lids and the orbicularis oculi muscles to the lateral orbital wall and zygoma, which forms the posterior orbital wall.3,4 The lateral canthal tendon is located beneath the lateral canthus and is comprised of the inferior and superior crus, which attaches to the inner aspect of the lateral orbital wall, forming a structure called Whitnall’s tubercle.3,4
Other than globe injuries, the most common findings in patients with orbital trauma are periocular lacerations (96%), orbital fractures (16%), and retrobulbar hemorrhage (8%).5 The most common cause of retrobulbar hemorrhage is ocular trauma, but it is also observed in facial fractures, orbital surgery, retrobulbar injections, venous anomalies, atherosclerosis, intraorbital aneurysm of the ophthalmic artery, lacerated ophthalmic artery, hypertension, hemophilia, leukemia, von Willebrand disease, and straining.3,6,7
In retrobulbar hemorrhage, an increased pressure in the orbital space can lead to optic nerve compression and vascular compromise.6 Important alternative diagnoses to consider include orbital cellulitis, orbital fracture, and globe rupture.3 Retrobulbar hemorrhage should be suspected in the clinical setting of exophthalmos, proptosis, diffuse subconjunctival hemorrhage, pain, visual loss or diplopia, periorbital edema, partial or complete ophthalmoplegia, resistance to retropulsion, increased IOP, a blanched ophthalmic artery on funduscopic examination, and an afferent pupillary defect.3,4,8,9 Less commonly, periorbital crepitus and infraorbital hypoesthesia can be appreciated.3
If a patient with a retrobulbar hemorrhage is experiencing diminished vision, an emergent lateral canthotomy should be attempted. Retrobulbar hemorrhage can be difficult to diagnose in the setting of trauma. There can be damage to the optic nerve with associated edema and vision loss that is not associated with a retrobulbar hemorrhage and does not require a lateral canthotomy.3,7 A dedicated CT scan of the orbits can aid in the diagnosis, but treatment should not be delayed.8
Patients with retrobulbar hemorrhage may initially present to the ED with intact visual acuity, but as the pressure behind the globe increases, vision will diminish.3 Although the medical literature has not established a definitive timeframe, it is believed that permanent visual compromise develops between 1 to 3 hours after ischemia develops.6 Animal studies show that visual loss due to central retinal artery ischemia may be reversible up to 100 minutes.3
Not all cases of retrobulbar hemorrhage are associated with vision loss. In patients without diminished vision, conservative treatments such as bed rest, elevation of the head of the bed, ice packs, analgesia, lowering BP, and sedatives should be attempted first.5 Acetazolamide and mannitol can also be considered in consultation with an ophthalmologist.
Pneumo-orbitum
The presence of pneumo-orbitum should alert the clinician to either a communication with a paranasal sinus, a gas-forming organism, or (rarely) Munchausen syndrome.10 Unlike most case presentations, most causes of pneumo-orbitum do not involve OCS and are self-limited.11 Traumatic pneumo-orbitum without OCS has been reported in the literature.12-15 However, traumatic tension pneumo-orbitum is rare.12-16 One case report involved an elderly man with an orbital floor fracture who developed recurrent tension pneumo-orbitum after blowing his nose while intoxicated.12 Another case involved a boy with tension pneumo-orbitum that required surgical decompression.16
In a patient who has experienced trauma, the combination of proptosis, elevated IOP, and vision loss likely represent a retrobulbar hematoma or OCS. A lateral canthotomy can help relieve IOP from either condition.17,18 Orbital compartment syndrome can be caused by edema, emphysema, and caroticocavernous fistula, leading to increased orbital pressure and decreased perfusion.17,18
In a review of 10 trauma patients with OCS, all cases were intubated due to the severity of the head trauma, and all had OCS due to edema.17 In a review of eight trauma patients with OCS, all had eye pain, reduced visual acuity, and proptosis.18 Most of the patients had periorbital edema, ophthalmoparesis, a relative afferent pupillary defect (as compared to a fixed and dilated pupil), and chemosis.18,19 All of the patients with OCS required cantholysis or a lateral canthotomy.18
Lateral Canthotomy
Although EPs rarely perform lateral canthotomy, knowledge of this procedure is important, because it can prevent vision loss in the appropriate clinical setting. To perform a lateral canthotomy, the area around the affected eye is cleaned with saline irrigation.4 One percent or 2% lidocaine with epinephrine is then injected into the lateral canthus of the affected eye.4,10 A straight hemostat is applied between the upper and lower lids, producing a crush injury along the site of local anesthesia for 1 to 2 minutes.3,4,10 This is done to reduce the risk of bleeding by devitalizing the tissue.4 Straight scissors are then used to make a 1-cm horizontal incision from the lateral canthal tendon to the lateral orbital rim.4 This initial incision exposes the orbicularis muscle, orbital septum, palpebral conjunctiva, and an area called Eisler’s pocket that sits anterior to the lateral canthal tendon.3
Cantholysis can then be performed by blunt dissection.10 The inferior crus of the lateral canthus is identified either visually or by palpation, and a 1- to 2-cm inferior-posterior cut of the inferior crus accomplishes the lateral canthotomy.3-4 After cutting the inferior crus, the lower lid should be pulled away easily, and if this does not occur, repeated attempts at cutting the inferior crus should be made.3 Pulling the lower eyelid down and away from the lateral orbital rim separates the skin and conjunctiva, aiding in visualization.4
After cutting the inferior crus, only a small amount of blood or air typically is expressed, but this is usually enough to prevent vision loss.3 When the procedure is performed correctly, the practitioner should be able to palpate a difference in the pressure of the globe, and tonography will show a reduced IOP. If the ocular pressure is still significantly elevated, the physician can proceed to cut the superior canthus of the lateral canthal tendon in a manner similar to cutting the inferior crus of the tendon.4 After the procedure is performed, urgent ophthalmologic consultation is required.
The risks of performing a lateral canthotomy include mechanical injury, hemorrhage, and infection.4 The incision from a lateral canthotomy generally does not need suturing and will heal without significant scarring.4 If the scissors are aimed superiorly instead of inferiorly for the inferior crus of the lateral canthal tendon, there is risk of injuring the levator aponeurosis leading to ptosis, as well as a small risk of injury to the lacrimal gland and lacrimal artery.3
Conclusion
Our patient demonstrates a case of traumatic OCS, a vision-threatening medical condition that requires rapid diagnosis and lateral canthotomy to lower IOP and reduce the risk of permanent vision loss. While an orbital CT scan may assist in confirming the diagnosis, treatment of IOP should not be delayed.
1. McCaig LF, Burt CW. National Hospital Ambulatory Medical Care Survey: 2001 Emergency Department Summary. Advance Data from Vital Health and Statistics; No. 335. https://www.cdc.gov/nchs/data/ad/ad335.pdf. Accessed July 22, 2016.
2. Knoop KJ, Dennis WR. Eye trauma. In: Wolfson AB, Hendy GW, Hendy PL, et al (eds). Harwood-Nuss’ Clinical Practice of Emergency Medicine. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
3. Vassallo S, Hartstein M, Howard D, Stetz J. Traumatic retrobulbar hemorrhage: emergent decompression by lateral canthotomy and cantholysis. J Emerg Med. 2002;22(3):251-256.
4. Roberts JR, Hedges JR (eds). Clinical Procedures in Emergency Medicine, 4th ed. Philadelphia, PA: Saunders; 2004.
5. Hatton MP, Thakker MM, Ray S. Orbital and adnexal trauma associated with open-globe injuries. Ophthal Plast Reconstr Surg. 2002;18(6):458-461.
6. Suner S, Simmons W, Savitt DL. A porcine model for instruction of lateral canthotomy. Acad Emerg Med. 2000;7(7):837-838.
7. Goodall KL, Brahma A, Bates A, Leatherbarrow B. Lateral canthotomy and inferior cantholysis: an effective method of urgent orbital decompression for sight threatening acute retrobulbar hemorrhage. Injury. 1999;30(7):485-490.
8. Gerbino G, Ramieri GA, Nasi A. Diagnosis and treatment of retrobulbar haematomas following blunt orbital trauma: a description of eight cases. Int J Oral Maxillofac Surg. 2005;34(2):127-131.
9. Machado RA, Silveira RL, Borges HO, Filho AM, de Oliveira GM. Retrobulbar hemorrhage: A case report. J Contemp Dent Pract. 2006;7(2):130-136.
10. Winans JM, House LR, Robinson HE. Self-induced orbital emphysema as a presenting sign of Munchausen’s syndrome. Laryngoscope. 1983;93(9):1209-1211.
11. Zimmer-Galler IE, Bartley GB. Orbital emphysema: case reports and review of the literature. Mayo Clin Proc. 1994;69(2):115-121.
12. Ahnood D, Toft PB. Recurrent orbital compartment syndrome caused by a blow-out fracture and accumulation of air; management by orbital punctures. Acta Ophthalmol. 2012;90(12):199-200.
13. Martin PW, Williams AC. Supraorbital emphysema: report of a case. J Oral Surg. 1972;30(12):901-902.
14. Per BL, Sanders BB. Post-traumatic intraorbital pneumatocele--a rare case of unilateral exophthalmos. Br J Radio. 1971;44(519):214-215.
15. Haller ML, Brackup AH, Shiffman F. Intraorbital aerocele. Arch Ophthalmol. 1980;98(9):1612-1613.
16. Chaudhry IA, Al-Amri A, Shamsi FA, Al-Rashed W. Visual recovery after evacuation of orbital emphysema. Orbit. 2007;26(4):283-285.
17. Perry M. Acute proptosis in trauma: retrobulbar hemorrhage or orbital compartment syndrome—does it really matter? J Oral Maxillofac Surg. 2008;66(9):1913-1920.
18. Sun MT, Chan WO, Selva D. Traumatic orbital compartment syndrome: importance of the lateral canthotomy and cantholysis. Emerg Med Australas. 2014;26(3):274-278.
19. Belliveau MJ, Johnson D. Orbital compartment syndrome after head trauma. Lancet Neurol. 2015;14(2):136-137.
Traumatic eye injuries ranging from mild corneal abrasions to penetrating globe injuries are commonly seen in the ED, and ocular trauma accounts for nearly 1% of all complaints in the ED.1 Up to 29% of facial fractures have associated eye injuries.2 Emergency physicians (EPs) must be aware of possible eye injuries, including traumatic vision loss, and the indicators for emergent interventions.
Tension pneumo-orbitum following facial trauma is rarely reported. We present a case of orbital compartment syndrome (OCS) in an elderly woman who sustained an orbital floor fracture and required emergent lateral canthotomy to preserve vision.
Case
A 76-year-old woman presented to the ED for evaluation of pain, swelling, and loss of vision in the right eye. She said she had been sitting in a chair tying her shoes when she lost her balance and fell forward, striking her head and the right side of her face against the floor. She experienced no loss of consciousness and denied any neck pain, jaw pain, or dizziness. She also denied any chest pain, shortness of breath, weakness, or loss of function in either her arms or legs. She did, however, note a small nosebleed that had stopped before she arrived at the ED. The patient’s primary complaint was a possible nasal bone fracture.
Her medical history was significant for hypertension and coronary artery disease. Her medications include amitriptyline, an antihistamine, aspirin, clopidogrel, diltiazem, folic acid, furosemide, hydralazine, levothyroxine, prednisone, and zolpidem. She stated that she was allergic to amoxicillin and sulfa drugs.
The patient’s vital signs at presentation were: blood pressure (BP), 193/82 mm Hg; heart rate, 71 beats/minute; respiratory rate, 16 breaths/minute; and temperature, 97°F. She was alert, oriented, and in no distress. Her head and neck examination showed no scalp lacerations or swelling. There was, however, significant swelling and ecchymosis around the right eye and swelling and ecchymosis around the nose, with dried blood in both nares. No septal hematoma was present. The patient had tenderness to palpation over the infraorbital area and nose. No gross facial instability was present, and Battle sign was not appreciated. No jaw or dental abnormalities were noted.
The patient’s right pupil was fixed and dilated, and she could not perceive light. She did have upward and lateral movement of the eye, but was unable to look down. A minimal amount of proptosis was noted. Her intraocular pressure (IOP) was elevated at 54 mm Hg (normal range, 10-20 mm Hg). The remainder of the examination, including the neurological examination, was unremarkable.
The patient received emergent head and facial computed tomography (CT) scans. The head CT showed no acute intracranial hemorrhage, mass, or infarct. The facial CT was read as a right orbital floor fracture with intraorbital air, and a right maxillary sinus hematoma. Laboratory evaluation revealed a hematocrit of 38% and a platelet count of 544,000/mcL (normal range, 150,000-450,000/mcL). The prothrombin time was 10.9 seconds (normal range, 11-13.5 seconds); the international normalized ratio was 0.8 (normal range, 0.8-1.1); and the partial thromboplastin time was 22.5 seconds (normal range, 25-35 seconds).
Because the patient was at risk for permanent visual impairment due to increased IOP from the injury, a lateral canthotomy was immediately performed. A small amount of air was released, and the proptosis was notably reduced.
At this point, the ophthalmologist arrived and used an 18-gauge needle to explore the retrobulbar space. Two pockets of air were released, which markedly reduced the tactile pressure of the globe. Repeat tonography of the globe was 28 mm Hg. The wound was left open to drain, and the patient was started on azithromycin. She was discharged home to follow up with ophthalmology.
The patient presented to the ED 2 months later for an unrelated condition. At that time, she reported a complete return of her vision with no deficits and no noticeable scarring around the eye.
Discussion
The orbit is an enclosed space, bordered by bone laterally and posteriorly—the orbital septa superiorly and inferiorly, and the globe anteriorly.3 The lateral canthus is a combined tendon-ligament that helps attach the tarsal plates of the lids and the orbicularis oculi muscles to the lateral orbital wall and zygoma, which forms the posterior orbital wall.3,4 The lateral canthal tendon is located beneath the lateral canthus and is comprised of the inferior and superior crus, which attaches to the inner aspect of the lateral orbital wall, forming a structure called Whitnall’s tubercle.3,4
Other than globe injuries, the most common findings in patients with orbital trauma are periocular lacerations (96%), orbital fractures (16%), and retrobulbar hemorrhage (8%).5 The most common cause of retrobulbar hemorrhage is ocular trauma, but it is also observed in facial fractures, orbital surgery, retrobulbar injections, venous anomalies, atherosclerosis, intraorbital aneurysm of the ophthalmic artery, lacerated ophthalmic artery, hypertension, hemophilia, leukemia, von Willebrand disease, and straining.3,6,7
In retrobulbar hemorrhage, an increased pressure in the orbital space can lead to optic nerve compression and vascular compromise.6 Important alternative diagnoses to consider include orbital cellulitis, orbital fracture, and globe rupture.3 Retrobulbar hemorrhage should be suspected in the clinical setting of exophthalmos, proptosis, diffuse subconjunctival hemorrhage, pain, visual loss or diplopia, periorbital edema, partial or complete ophthalmoplegia, resistance to retropulsion, increased IOP, a blanched ophthalmic artery on funduscopic examination, and an afferent pupillary defect.3,4,8,9 Less commonly, periorbital crepitus and infraorbital hypoesthesia can be appreciated.3
If a patient with a retrobulbar hemorrhage is experiencing diminished vision, an emergent lateral canthotomy should be attempted. Retrobulbar hemorrhage can be difficult to diagnose in the setting of trauma. There can be damage to the optic nerve with associated edema and vision loss that is not associated with a retrobulbar hemorrhage and does not require a lateral canthotomy.3,7 A dedicated CT scan of the orbits can aid in the diagnosis, but treatment should not be delayed.8
Patients with retrobulbar hemorrhage may initially present to the ED with intact visual acuity, but as the pressure behind the globe increases, vision will diminish.3 Although the medical literature has not established a definitive timeframe, it is believed that permanent visual compromise develops between 1 to 3 hours after ischemia develops.6 Animal studies show that visual loss due to central retinal artery ischemia may be reversible up to 100 minutes.3
Not all cases of retrobulbar hemorrhage are associated with vision loss. In patients without diminished vision, conservative treatments such as bed rest, elevation of the head of the bed, ice packs, analgesia, lowering BP, and sedatives should be attempted first.5 Acetazolamide and mannitol can also be considered in consultation with an ophthalmologist.
Pneumo-orbitum
The presence of pneumo-orbitum should alert the clinician to either a communication with a paranasal sinus, a gas-forming organism, or (rarely) Munchausen syndrome.10 Unlike most case presentations, most causes of pneumo-orbitum do not involve OCS and are self-limited.11 Traumatic pneumo-orbitum without OCS has been reported in the literature.12-15 However, traumatic tension pneumo-orbitum is rare.12-16 One case report involved an elderly man with an orbital floor fracture who developed recurrent tension pneumo-orbitum after blowing his nose while intoxicated.12 Another case involved a boy with tension pneumo-orbitum that required surgical decompression.16
In a patient who has experienced trauma, the combination of proptosis, elevated IOP, and vision loss likely represent a retrobulbar hematoma or OCS. A lateral canthotomy can help relieve IOP from either condition.17,18 Orbital compartment syndrome can be caused by edema, emphysema, and caroticocavernous fistula, leading to increased orbital pressure and decreased perfusion.17,18
In a review of 10 trauma patients with OCS, all cases were intubated due to the severity of the head trauma, and all had OCS due to edema.17 In a review of eight trauma patients with OCS, all had eye pain, reduced visual acuity, and proptosis.18 Most of the patients had periorbital edema, ophthalmoparesis, a relative afferent pupillary defect (as compared to a fixed and dilated pupil), and chemosis.18,19 All of the patients with OCS required cantholysis or a lateral canthotomy.18
Lateral Canthotomy
Although EPs rarely perform lateral canthotomy, knowledge of this procedure is important, because it can prevent vision loss in the appropriate clinical setting. To perform a lateral canthotomy, the area around the affected eye is cleaned with saline irrigation.4 One percent or 2% lidocaine with epinephrine is then injected into the lateral canthus of the affected eye.4,10 A straight hemostat is applied between the upper and lower lids, producing a crush injury along the site of local anesthesia for 1 to 2 minutes.3,4,10 This is done to reduce the risk of bleeding by devitalizing the tissue.4 Straight scissors are then used to make a 1-cm horizontal incision from the lateral canthal tendon to the lateral orbital rim.4 This initial incision exposes the orbicularis muscle, orbital septum, palpebral conjunctiva, and an area called Eisler’s pocket that sits anterior to the lateral canthal tendon.3
Cantholysis can then be performed by blunt dissection.10 The inferior crus of the lateral canthus is identified either visually or by palpation, and a 1- to 2-cm inferior-posterior cut of the inferior crus accomplishes the lateral canthotomy.3-4 After cutting the inferior crus, the lower lid should be pulled away easily, and if this does not occur, repeated attempts at cutting the inferior crus should be made.3 Pulling the lower eyelid down and away from the lateral orbital rim separates the skin and conjunctiva, aiding in visualization.4
After cutting the inferior crus, only a small amount of blood or air typically is expressed, but this is usually enough to prevent vision loss.3 When the procedure is performed correctly, the practitioner should be able to palpate a difference in the pressure of the globe, and tonography will show a reduced IOP. If the ocular pressure is still significantly elevated, the physician can proceed to cut the superior canthus of the lateral canthal tendon in a manner similar to cutting the inferior crus of the tendon.4 After the procedure is performed, urgent ophthalmologic consultation is required.
The risks of performing a lateral canthotomy include mechanical injury, hemorrhage, and infection.4 The incision from a lateral canthotomy generally does not need suturing and will heal without significant scarring.4 If the scissors are aimed superiorly instead of inferiorly for the inferior crus of the lateral canthal tendon, there is risk of injuring the levator aponeurosis leading to ptosis, as well as a small risk of injury to the lacrimal gland and lacrimal artery.3
Conclusion
Our patient demonstrates a case of traumatic OCS, a vision-threatening medical condition that requires rapid diagnosis and lateral canthotomy to lower IOP and reduce the risk of permanent vision loss. While an orbital CT scan may assist in confirming the diagnosis, treatment of IOP should not be delayed.
Traumatic eye injuries ranging from mild corneal abrasions to penetrating globe injuries are commonly seen in the ED, and ocular trauma accounts for nearly 1% of all complaints in the ED.1 Up to 29% of facial fractures have associated eye injuries.2 Emergency physicians (EPs) must be aware of possible eye injuries, including traumatic vision loss, and the indicators for emergent interventions.
Tension pneumo-orbitum following facial trauma is rarely reported. We present a case of orbital compartment syndrome (OCS) in an elderly woman who sustained an orbital floor fracture and required emergent lateral canthotomy to preserve vision.
Case
A 76-year-old woman presented to the ED for evaluation of pain, swelling, and loss of vision in the right eye. She said she had been sitting in a chair tying her shoes when she lost her balance and fell forward, striking her head and the right side of her face against the floor. She experienced no loss of consciousness and denied any neck pain, jaw pain, or dizziness. She also denied any chest pain, shortness of breath, weakness, or loss of function in either her arms or legs. She did, however, note a small nosebleed that had stopped before she arrived at the ED. The patient’s primary complaint was a possible nasal bone fracture.
Her medical history was significant for hypertension and coronary artery disease. Her medications include amitriptyline, an antihistamine, aspirin, clopidogrel, diltiazem, folic acid, furosemide, hydralazine, levothyroxine, prednisone, and zolpidem. She stated that she was allergic to amoxicillin and sulfa drugs.
The patient’s vital signs at presentation were: blood pressure (BP), 193/82 mm Hg; heart rate, 71 beats/minute; respiratory rate, 16 breaths/minute; and temperature, 97°F. She was alert, oriented, and in no distress. Her head and neck examination showed no scalp lacerations or swelling. There was, however, significant swelling and ecchymosis around the right eye and swelling and ecchymosis around the nose, with dried blood in both nares. No septal hematoma was present. The patient had tenderness to palpation over the infraorbital area and nose. No gross facial instability was present, and Battle sign was not appreciated. No jaw or dental abnormalities were noted.
The patient’s right pupil was fixed and dilated, and she could not perceive light. She did have upward and lateral movement of the eye, but was unable to look down. A minimal amount of proptosis was noted. Her intraocular pressure (IOP) was elevated at 54 mm Hg (normal range, 10-20 mm Hg). The remainder of the examination, including the neurological examination, was unremarkable.
The patient received emergent head and facial computed tomography (CT) scans. The head CT showed no acute intracranial hemorrhage, mass, or infarct. The facial CT was read as a right orbital floor fracture with intraorbital air, and a right maxillary sinus hematoma. Laboratory evaluation revealed a hematocrit of 38% and a platelet count of 544,000/mcL (normal range, 150,000-450,000/mcL). The prothrombin time was 10.9 seconds (normal range, 11-13.5 seconds); the international normalized ratio was 0.8 (normal range, 0.8-1.1); and the partial thromboplastin time was 22.5 seconds (normal range, 25-35 seconds).
Because the patient was at risk for permanent visual impairment due to increased IOP from the injury, a lateral canthotomy was immediately performed. A small amount of air was released, and the proptosis was notably reduced.
At this point, the ophthalmologist arrived and used an 18-gauge needle to explore the retrobulbar space. Two pockets of air were released, which markedly reduced the tactile pressure of the globe. Repeat tonography of the globe was 28 mm Hg. The wound was left open to drain, and the patient was started on azithromycin. She was discharged home to follow up with ophthalmology.
The patient presented to the ED 2 months later for an unrelated condition. At that time, she reported a complete return of her vision with no deficits and no noticeable scarring around the eye.
Discussion
The orbit is an enclosed space, bordered by bone laterally and posteriorly—the orbital septa superiorly and inferiorly, and the globe anteriorly.3 The lateral canthus is a combined tendon-ligament that helps attach the tarsal plates of the lids and the orbicularis oculi muscles to the lateral orbital wall and zygoma, which forms the posterior orbital wall.3,4 The lateral canthal tendon is located beneath the lateral canthus and is comprised of the inferior and superior crus, which attaches to the inner aspect of the lateral orbital wall, forming a structure called Whitnall’s tubercle.3,4
Other than globe injuries, the most common findings in patients with orbital trauma are periocular lacerations (96%), orbital fractures (16%), and retrobulbar hemorrhage (8%).5 The most common cause of retrobulbar hemorrhage is ocular trauma, but it is also observed in facial fractures, orbital surgery, retrobulbar injections, venous anomalies, atherosclerosis, intraorbital aneurysm of the ophthalmic artery, lacerated ophthalmic artery, hypertension, hemophilia, leukemia, von Willebrand disease, and straining.3,6,7
In retrobulbar hemorrhage, an increased pressure in the orbital space can lead to optic nerve compression and vascular compromise.6 Important alternative diagnoses to consider include orbital cellulitis, orbital fracture, and globe rupture.3 Retrobulbar hemorrhage should be suspected in the clinical setting of exophthalmos, proptosis, diffuse subconjunctival hemorrhage, pain, visual loss or diplopia, periorbital edema, partial or complete ophthalmoplegia, resistance to retropulsion, increased IOP, a blanched ophthalmic artery on funduscopic examination, and an afferent pupillary defect.3,4,8,9 Less commonly, periorbital crepitus and infraorbital hypoesthesia can be appreciated.3
If a patient with a retrobulbar hemorrhage is experiencing diminished vision, an emergent lateral canthotomy should be attempted. Retrobulbar hemorrhage can be difficult to diagnose in the setting of trauma. There can be damage to the optic nerve with associated edema and vision loss that is not associated with a retrobulbar hemorrhage and does not require a lateral canthotomy.3,7 A dedicated CT scan of the orbits can aid in the diagnosis, but treatment should not be delayed.8
Patients with retrobulbar hemorrhage may initially present to the ED with intact visual acuity, but as the pressure behind the globe increases, vision will diminish.3 Although the medical literature has not established a definitive timeframe, it is believed that permanent visual compromise develops between 1 to 3 hours after ischemia develops.6 Animal studies show that visual loss due to central retinal artery ischemia may be reversible up to 100 minutes.3
Not all cases of retrobulbar hemorrhage are associated with vision loss. In patients without diminished vision, conservative treatments such as bed rest, elevation of the head of the bed, ice packs, analgesia, lowering BP, and sedatives should be attempted first.5 Acetazolamide and mannitol can also be considered in consultation with an ophthalmologist.
Pneumo-orbitum
The presence of pneumo-orbitum should alert the clinician to either a communication with a paranasal sinus, a gas-forming organism, or (rarely) Munchausen syndrome.10 Unlike most case presentations, most causes of pneumo-orbitum do not involve OCS and are self-limited.11 Traumatic pneumo-orbitum without OCS has been reported in the literature.12-15 However, traumatic tension pneumo-orbitum is rare.12-16 One case report involved an elderly man with an orbital floor fracture who developed recurrent tension pneumo-orbitum after blowing his nose while intoxicated.12 Another case involved a boy with tension pneumo-orbitum that required surgical decompression.16
In a patient who has experienced trauma, the combination of proptosis, elevated IOP, and vision loss likely represent a retrobulbar hematoma or OCS. A lateral canthotomy can help relieve IOP from either condition.17,18 Orbital compartment syndrome can be caused by edema, emphysema, and caroticocavernous fistula, leading to increased orbital pressure and decreased perfusion.17,18
In a review of 10 trauma patients with OCS, all cases were intubated due to the severity of the head trauma, and all had OCS due to edema.17 In a review of eight trauma patients with OCS, all had eye pain, reduced visual acuity, and proptosis.18 Most of the patients had periorbital edema, ophthalmoparesis, a relative afferent pupillary defect (as compared to a fixed and dilated pupil), and chemosis.18,19 All of the patients with OCS required cantholysis or a lateral canthotomy.18
Lateral Canthotomy
Although EPs rarely perform lateral canthotomy, knowledge of this procedure is important, because it can prevent vision loss in the appropriate clinical setting. To perform a lateral canthotomy, the area around the affected eye is cleaned with saline irrigation.4 One percent or 2% lidocaine with epinephrine is then injected into the lateral canthus of the affected eye.4,10 A straight hemostat is applied between the upper and lower lids, producing a crush injury along the site of local anesthesia for 1 to 2 minutes.3,4,10 This is done to reduce the risk of bleeding by devitalizing the tissue.4 Straight scissors are then used to make a 1-cm horizontal incision from the lateral canthal tendon to the lateral orbital rim.4 This initial incision exposes the orbicularis muscle, orbital septum, palpebral conjunctiva, and an area called Eisler’s pocket that sits anterior to the lateral canthal tendon.3
Cantholysis can then be performed by blunt dissection.10 The inferior crus of the lateral canthus is identified either visually or by palpation, and a 1- to 2-cm inferior-posterior cut of the inferior crus accomplishes the lateral canthotomy.3-4 After cutting the inferior crus, the lower lid should be pulled away easily, and if this does not occur, repeated attempts at cutting the inferior crus should be made.3 Pulling the lower eyelid down and away from the lateral orbital rim separates the skin and conjunctiva, aiding in visualization.4
After cutting the inferior crus, only a small amount of blood or air typically is expressed, but this is usually enough to prevent vision loss.3 When the procedure is performed correctly, the practitioner should be able to palpate a difference in the pressure of the globe, and tonography will show a reduced IOP. If the ocular pressure is still significantly elevated, the physician can proceed to cut the superior canthus of the lateral canthal tendon in a manner similar to cutting the inferior crus of the tendon.4 After the procedure is performed, urgent ophthalmologic consultation is required.
The risks of performing a lateral canthotomy include mechanical injury, hemorrhage, and infection.4 The incision from a lateral canthotomy generally does not need suturing and will heal without significant scarring.4 If the scissors are aimed superiorly instead of inferiorly for the inferior crus of the lateral canthal tendon, there is risk of injuring the levator aponeurosis leading to ptosis, as well as a small risk of injury to the lacrimal gland and lacrimal artery.3
Conclusion
Our patient demonstrates a case of traumatic OCS, a vision-threatening medical condition that requires rapid diagnosis and lateral canthotomy to lower IOP and reduce the risk of permanent vision loss. While an orbital CT scan may assist in confirming the diagnosis, treatment of IOP should not be delayed.
1. McCaig LF, Burt CW. National Hospital Ambulatory Medical Care Survey: 2001 Emergency Department Summary. Advance Data from Vital Health and Statistics; No. 335. https://www.cdc.gov/nchs/data/ad/ad335.pdf. Accessed July 22, 2016.
2. Knoop KJ, Dennis WR. Eye trauma. In: Wolfson AB, Hendy GW, Hendy PL, et al (eds). Harwood-Nuss’ Clinical Practice of Emergency Medicine. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
3. Vassallo S, Hartstein M, Howard D, Stetz J. Traumatic retrobulbar hemorrhage: emergent decompression by lateral canthotomy and cantholysis. J Emerg Med. 2002;22(3):251-256.
4. Roberts JR, Hedges JR (eds). Clinical Procedures in Emergency Medicine, 4th ed. Philadelphia, PA: Saunders; 2004.
5. Hatton MP, Thakker MM, Ray S. Orbital and adnexal trauma associated with open-globe injuries. Ophthal Plast Reconstr Surg. 2002;18(6):458-461.
6. Suner S, Simmons W, Savitt DL. A porcine model for instruction of lateral canthotomy. Acad Emerg Med. 2000;7(7):837-838.
7. Goodall KL, Brahma A, Bates A, Leatherbarrow B. Lateral canthotomy and inferior cantholysis: an effective method of urgent orbital decompression for sight threatening acute retrobulbar hemorrhage. Injury. 1999;30(7):485-490.
8. Gerbino G, Ramieri GA, Nasi A. Diagnosis and treatment of retrobulbar haematomas following blunt orbital trauma: a description of eight cases. Int J Oral Maxillofac Surg. 2005;34(2):127-131.
9. Machado RA, Silveira RL, Borges HO, Filho AM, de Oliveira GM. Retrobulbar hemorrhage: A case report. J Contemp Dent Pract. 2006;7(2):130-136.
10. Winans JM, House LR, Robinson HE. Self-induced orbital emphysema as a presenting sign of Munchausen’s syndrome. Laryngoscope. 1983;93(9):1209-1211.
11. Zimmer-Galler IE, Bartley GB. Orbital emphysema: case reports and review of the literature. Mayo Clin Proc. 1994;69(2):115-121.
12. Ahnood D, Toft PB. Recurrent orbital compartment syndrome caused by a blow-out fracture and accumulation of air; management by orbital punctures. Acta Ophthalmol. 2012;90(12):199-200.
13. Martin PW, Williams AC. Supraorbital emphysema: report of a case. J Oral Surg. 1972;30(12):901-902.
14. Per BL, Sanders BB. Post-traumatic intraorbital pneumatocele--a rare case of unilateral exophthalmos. Br J Radio. 1971;44(519):214-215.
15. Haller ML, Brackup AH, Shiffman F. Intraorbital aerocele. Arch Ophthalmol. 1980;98(9):1612-1613.
16. Chaudhry IA, Al-Amri A, Shamsi FA, Al-Rashed W. Visual recovery after evacuation of orbital emphysema. Orbit. 2007;26(4):283-285.
17. Perry M. Acute proptosis in trauma: retrobulbar hemorrhage or orbital compartment syndrome—does it really matter? J Oral Maxillofac Surg. 2008;66(9):1913-1920.
18. Sun MT, Chan WO, Selva D. Traumatic orbital compartment syndrome: importance of the lateral canthotomy and cantholysis. Emerg Med Australas. 2014;26(3):274-278.
19. Belliveau MJ, Johnson D. Orbital compartment syndrome after head trauma. Lancet Neurol. 2015;14(2):136-137.
1. McCaig LF, Burt CW. National Hospital Ambulatory Medical Care Survey: 2001 Emergency Department Summary. Advance Data from Vital Health and Statistics; No. 335. https://www.cdc.gov/nchs/data/ad/ad335.pdf. Accessed July 22, 2016.
2. Knoop KJ, Dennis WR. Eye trauma. In: Wolfson AB, Hendy GW, Hendy PL, et al (eds). Harwood-Nuss’ Clinical Practice of Emergency Medicine. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
3. Vassallo S, Hartstein M, Howard D, Stetz J. Traumatic retrobulbar hemorrhage: emergent decompression by lateral canthotomy and cantholysis. J Emerg Med. 2002;22(3):251-256.
4. Roberts JR, Hedges JR (eds). Clinical Procedures in Emergency Medicine, 4th ed. Philadelphia, PA: Saunders; 2004.
5. Hatton MP, Thakker MM, Ray S. Orbital and adnexal trauma associated with open-globe injuries. Ophthal Plast Reconstr Surg. 2002;18(6):458-461.
6. Suner S, Simmons W, Savitt DL. A porcine model for instruction of lateral canthotomy. Acad Emerg Med. 2000;7(7):837-838.
7. Goodall KL, Brahma A, Bates A, Leatherbarrow B. Lateral canthotomy and inferior cantholysis: an effective method of urgent orbital decompression for sight threatening acute retrobulbar hemorrhage. Injury. 1999;30(7):485-490.
8. Gerbino G, Ramieri GA, Nasi A. Diagnosis and treatment of retrobulbar haematomas following blunt orbital trauma: a description of eight cases. Int J Oral Maxillofac Surg. 2005;34(2):127-131.
9. Machado RA, Silveira RL, Borges HO, Filho AM, de Oliveira GM. Retrobulbar hemorrhage: A case report. J Contemp Dent Pract. 2006;7(2):130-136.
10. Winans JM, House LR, Robinson HE. Self-induced orbital emphysema as a presenting sign of Munchausen’s syndrome. Laryngoscope. 1983;93(9):1209-1211.
11. Zimmer-Galler IE, Bartley GB. Orbital emphysema: case reports and review of the literature. Mayo Clin Proc. 1994;69(2):115-121.
12. Ahnood D, Toft PB. Recurrent orbital compartment syndrome caused by a blow-out fracture and accumulation of air; management by orbital punctures. Acta Ophthalmol. 2012;90(12):199-200.
13. Martin PW, Williams AC. Supraorbital emphysema: report of a case. J Oral Surg. 1972;30(12):901-902.
14. Per BL, Sanders BB. Post-traumatic intraorbital pneumatocele--a rare case of unilateral exophthalmos. Br J Radio. 1971;44(519):214-215.
15. Haller ML, Brackup AH, Shiffman F. Intraorbital aerocele. Arch Ophthalmol. 1980;98(9):1612-1613.
16. Chaudhry IA, Al-Amri A, Shamsi FA, Al-Rashed W. Visual recovery after evacuation of orbital emphysema. Orbit. 2007;26(4):283-285.
17. Perry M. Acute proptosis in trauma: retrobulbar hemorrhage or orbital compartment syndrome—does it really matter? J Oral Maxillofac Surg. 2008;66(9):1913-1920.
18. Sun MT, Chan WO, Selva D. Traumatic orbital compartment syndrome: importance of the lateral canthotomy and cantholysis. Emerg Med Australas. 2014;26(3):274-278.
19. Belliveau MJ, Johnson D. Orbital compartment syndrome after head trauma. Lancet Neurol. 2015;14(2):136-137.
The Orlando Nightclub Shooting: Firsthand Accounts and Lessons Learned
Orlando Regional Medical Center (ORMC) is an urban, academic, Level 1 trauma center with an ED that treats in excess of 85,000 patients each year. There are more than 4,500 annual trauma admissions, including penetrating and blunt mechanism injuries. We have a 3-year emergency medicine (EM) residency program, originally established in 1986, that now has 14 residents per year. The events that we experienced on the early morning of June 12, 2016 were tragic and unexpected and tested our community, our teams, and our organization. The following is a description of activity perspectives, personal reflections and feelings, and important lessons learned from which other EDs may benefit.
Prehospital/Rescue Period
The prehospital approach to a mass-casualty incident (MCI) caused by gunfire has changed significantly over the last decade, with lessons gleaned from the experience of military and domestic attacks. The Orange County (Florida) Emergency Medical Services (EMS) System, including the agencies involved in the response to the June 12, 2016 Pulse nightclub shooting incident (Orlando Fire Department, Rural Metro Ambulance, and Orange County Fire Rescue), have trained specifically on the evolving priorities during an active shooter incident. This includes targeted hemorrhage control, swift extrication, minimal interventions, and immediate transport to the appropriate receiving center. In June 2015, 1 year prior to the Pulse nightclub shooting, a protocol variance was written by medical directors, explaining that “EMS providers may engage in initial patient care with only the supplies deemed absolutely necessary for rapid evaluation and removal into a safe triage zone.” This variance applies when there is an active shooter scenario. In addition, in 2014, 2015, and 2016, we conducted large scale, multiagency community exercises, encompassing shooting/active shooter scenarios and drills.
On June 12, 2016, EMS units were on scene of the Pulse nightclub in downtown Orlando less than 5 minutes after shooting began. It became immediately obvious that additional resources would be necessary. Soon after the incident, an MCI alert was activated from the Orlando Fire Department Communications Center, utilizing a software system to notify area hospitals and the medical directors that up to 20 patients had been shot, and requesting status on bed availability. Christopher L. Hunter, MD, PhD, one of our associate EMS medical directors, was working in the ED at a hospital several miles away from the incident and began coordinating efforts to distribute patients to the appropriate destination via radio and phone communication between the scene, the communications center, and the trauma center.
On scene, a group of law enforcement officers were engaging the shooter while others assisted in extracting victims from the club and surrounding area. Injured victims were brought to a casualty collection point, under cover, across the street and were transported as units became available. Initially, law enforcement vehicles and ambulances would make the two-block drive from the scene to ORMC carrying as many patients as they safely could, and return immediately after offload. Per previous training, minimal interventions were performed, and unlike standard procedure, EMS could offer no prearrival report to the hospital. The decision was made to triage only patients meeting Florida’s state trauma alert criteria to ORMC, and funnel other patients to nearby nontrauma centers. Dr Hunter attempted to notify the hospitals of patients as they were transported off the scene; however, the extremely short transport times made this process difficult.
Over the course of the incident, on-scene commands designated a casualty collection point, a transport unit staging area, and a hospital liaison. Emergency medical service responders cooperated with law enforcement to transport injured patients throughout the response. By the end of the event, nearly 100 EMS providers utilizing dozens of vehicles had responded to the scene.
The ED Period
The overnight shift of Saturday, June 11 was slower than usual in the ORMC ED, and triage housed the typical Saturday-night complaints. One of our mid-level providers, Brian Clayton, ARNP, worked in the fast track pod treating lower acuity illnesses, while the four senior residents discussed who might receive the honor of leaving early. Even the senior attending, Gary A. Parrish, MD, wrapped up his patients and was ready to leave right on time. The 11:00 pm to 7:00 am attending, Kathryn J. Bondani, MD, was looking forward to an easy shift with four graduating senior residents. We had just called in an order from a popular nearby sandwich shop when suddenly, a slew of police cars flew past the hospital—not unusual given our proximity to downtown. The radio squawked “Multiple gunshots wounds en route.” Someone said “a club downtown got shot up.” In anticipation of multiple patient arrivals, resident Amanda M. Stone, MD, and the attending hurried to the trauma/ressuscitation bay. Another resident, Amanda F. Tarkowski, MD, called the trauma attending to rally his team downstairs, informing him that “A club downtown has a shooter and we have multiple patients with a 2-minute ETA.” Multiple gunshot wounds (GSWs) at once is not unusual for our Level 1 trauma center.
The first patient arrived shortly after 2:00 am and had multiple GSWs to the abdomen, but was awake and talking. He was mildly tachycardic, but his blood pressure (BP) was stable. We all gaped at the fist-sized wound on his back—some of us had never seen a GSW like this before. It was apparent that he needed to go to the operating room, but before preparations could be made, three more patients rolled into the ED. Instead of our usual organized, methodical EMS report, these patients were dropped off by a police pickup truck and rolled in on our own stretchers by nurses and technicians. Soon, all six of our trauma bays were filled with critical patients. Christopher H. Ponder, MD, recalled, “I heard an overhead page for ‘all available trauma nurses to the trauma bay’ shortly followed by a more concerned-sounding ‘all available staff to the trauma bay.’” All four senior EM residents, both EM attendings, the trauma attending, four trauma residents, multiple ED nurses, technicians, and various ancillary staff quickly descended on the patients. Chest tubes were placed, and multiple patients were intubated. Several thoracotomies were performed at the initial point when the team was unaware of the exact number of patients who would ultimately follow. Blood bags were hung and tranexamic acid was administered liberally. Unfortunately, some of these initial attempts were unsuccessful, as the first wave contained the most critically injured patients.
We barely had time to reflect on the dead as more patients filled the hallways. In the midst of all of this, triage decisions came quickly—awake and talking patients with an acceptable BP were moved out of the trauma bay in favor of less stable patients. Intubation and chest-tube placement decisions were made instantly. There was no time for routine X-rays or laboratory evaluation. Nurses, technicians, and doctors crowded the trauma bay desperately trying to stabilize the critically injured. Vital signs were taken manually. Dr Ponder called his colleague Thomas N. Smith, MD, who was also a graduating senior EM resident, and who happened to be staffing the children’s ED across the street that night. “Is there any way you can make it over here right now?” he shouted into the phone. Dr Smith quickly grabbed several trauma supplies and hitched a ride with security to the ORMC ED to assist. He was confronted with the scene of the previously mentioned “first wave” and grabbed an ultrasound machine to help triage these unfortunate patients. In addition to Dr Smith, unit clerks continued making calls to additional off-duty medical, nursing, and support staff. Critical care attendings and fellows responded from upstairs to assist in the ED. The Hospital Incident Command System (HICS) was initiated to provide hospital and corporate coordination of services. Timothy B. Bullard, MD, another EM attending physician and medical staff director of HICS, was en route to the ED to assist in the response.
Amidst the overwhelming mass of mortally and critically wounded patients, we were told stories of terror from just down the road. The sense of horror was almost contagious, and we all wondered if the violence would spread to the hospital.
About an hour into the ordeal, we heard another page overhead “Code Silver, ED Triage. Code Silver, ED Triage.” Everyone in the trauma bay froze. We heard someone shout from the hallway “Shots fired in triage!” After a few seconds, those nearest the trauma bay doors pushed them shut. One of the trauma surgeons shouted, “Keep caring for your patients, push the portable X-ray machines in front of the doors.” That is exactly what happened, and we worked in the barricaded trauma room for the longest few minutes of the night. We would later learn that this report was false, but fearing for our own lives as well as the lives of our patients is an experience that few, if any, of us had previously been through. The fact that we continued to work in such a situation illustrates everyone’s dedication to their patients and mission.
After the first wave of patients, it was clear that reassessing patients was now our greatest challenge. There were multiple patients with stable vital signs but who had GSWs to the abdomen, pelvis, and thorax and required surgery. Having realized that the ultrasound machine and focused assessment with sonography for trauma (FAST) examinations were the most practical rapid imaging modality, Drs Tarkowski, Ponder, and Smith grabbed the machine and went from room to room repeating the FAST examinations, vital signs, and assessments for missed wounds. A portable computer allowed us to order X-ray orders for patients with extremity wounds. Several patients who initially had negative FASTs had a repeat examination that was positive for free fluid. The operating room triage list shuffled based on these examinations.
At this point, there seemed to be a never-ending list of “Doe” names on the electronic medical record (EMR) tracking board. Tracking the location of patients was a dynamic process, and updating the tracking board was difficult. Patients were continually cycled in and out of the trauma bay, shuffled in and out of treatment rooms, lined up in the hallways, and transported up to the intensive care unit (ICU). Some of those “Doe” names belonged to patients who had succumbed to their injuries. Some patients had empty, bloody charts next to them on their stretcher. Every patient had a wristband. Dr Tarkowski improvised a rapid documentation system using quarter sheets of scrap paper that were taped either to the stretcher or door with a patient name, brief list of injuries, FAST examination result, pending tests, and medications given. This system was an efficient way of identifying which patients had been evaluated, what had been done, and what was pending.
While we were “rounding” on our patients, down the street, law enforcement had broken down the club’s wall, and a second wave of patients began to arrive. Two residents completed the secondary evaluations while the other three resuscitated new patients. The second wave seemed like a repeat of the first. Several extremity-only injuries were triaged directly to the hallways to be seen by the two residents outside of the trauma bay. By this time, the orthopedic surgery service had come down to the ED and was washing out wounds, splinting, and making plans for surgery. The internal medicine service and the medical critical care team were helping reassess patients as well.
There were many emotional moments. Dr Ponder remembered, “One of the first few patients I saw was pulseless, and as I went to start chest compressions, I was stopped by a trauma surgeon who said, ‘He’s gone, focus on the ones we can help.’ That’s when I realized the gravity of our situation. For almost 2 hours, each resident cycled through patients.” Dr Stone recounted, “I just went down the line of patients, from head of the bed to head of the bed, some patients still on EMS stretchers, intubating many of them. It was surreal to see that many severely injured in one place.” Tory L. Weatherford, MD, recalled, “It was controlled chaos. My training kicked in, and it became about just trying to do anything possible to help.”
Dr Bondani, the overnight attending, said she does not remember many specifics from the event. “Faces and injuries blurred together. I remember looking in one young man’s eyes and telling him, ‘We are going to help you, just hang in there,’ and telling another panicking woman, ‘You’re talking, you can feel pain, you’re alive. Calm down.’ It was organized chaos as we swept from patient to patient moving as quickly as possible. Your training kicks in and you do what needs to be done in the moment.”
We were fortunate to have the EM team we did, and to be in the place that we were. On duty, we had five senior graduating residents, essentially with attending-level skills and training, who had been together since day one. “We gave everything we had; there was no time to stop. We went where our hands could be helpful—it didn’t matter if it was your traditional role or not,” said Dr Weatherford.
Dr Tarkowski remembered what it was like leaving the walls of the ED later that morning. “Leaving the hospital didn’t feel like a success. We knew the work we did was good, that we did everything we could, but it didn’t feel like it. It felt heavy. It felt empty.” Afterward, the emotional toll set in. We gathered up the names of the deceased and looked at a status board littered with “Doe” names, and we tried hard not to break down at the violence and the pain we witnessed.
Immediate Recovery Period (Prehospital Setting)
In the aftermath of the initial rescue operation, stabilization of injured victims, and demobilization of resources, a second “event” unfolded—hundreds of family members flooded to ORMC looking for unaccounted loved ones. At this point, there were dozens of deceased and critically injured patients who remained unidentified, and addressing the needs of both the victims and the families was becoming overwhelming. With the cooperation of federal, state, county, city, and private resources, a family reunification center (FRC; a family staging area/family reception center) was created and managed initially within the hospital. At this site, grief counselors, victim advocates, law enforcement and medical examiner’s officials, hospital chaplains, and translators gathered with the loved ones of those missing to synchronize efforts to identify and reunite them. The Emergency Operations Center quickly created a telephone hotline and Web site to guide those in need to this resource. Food and housing for those in need were provided by the generosity of our community—which cannot be overstated in the wake of this tragedy. As days passed, the FRC transitioned to a new location as the Orlando United Assistance Center, and will continue to serve as a navigation point for those who are and will be affected by the event for months to come.
Hospital Incident Command System
The HICS was activated shortly past 3:00 am, just after the initial wave of victims flooded our ED and the gravity of the situation became apparent. The ED and trauma services were already near full staff due to timing, rapid response, and communication between the traumatologists, as well as a bit of luck. Because of the time of the disaster, our normal notification process for incident command and all personnel was severely limited. In retrospect, this turned out to be a blessing. While everyone who normally would respond to HICS was not available, individuals serving in key positions were reached by personal phone calls and were on-site quickly. One of the main functions of HICS is to control the internal chaos that arises when a disaster occurs and all personnel want to assist in some manner. We have spent hours during drills crafting communications that target key personnel necessary to meet the mission, while controlling well-intentioned but unnecessary personnel. In our drills, this is not an issue because everyone knows the exercise is not reality; however, in a real disaster, everyone wants to help. This was confirmed by the guilt that so many of our team members expressed at not being involved that Sunday.
With an initial skeleton crew in incident command, it was easy to focus on the immediate needs of patient care. The strong leadership and cool heads of our incident command leadership led to rapid role definition and responsibility, and set forth an easy path for execution. Hospital command personnel adapted their usual roles and performed functions and assignments as needed. Many HICS staff had direct face-to-face contact with frontline providers in the ED and other vital areas. This was possible because of the close proximity of the hospital command headquarters to the ED. The need for additional resources was rapidly identified, and the hospital command leaders assumed direct responsibility for procuring them instead of delegating. A great example of this was when Orlando Health’s chief executive officer and chief operating officer went to our nearby hospitals to gather additional chest tubes after learning that we were running in short supply. Their main responsibilities lay ahead of them, and they were willing to help in any manner they could at the time. Some of the medical personnel were able to switch roles and pitch in to treat the second wave of victims that arrived at around 5:00 am.
As the flow of victims to the ED subsided and order began to be restored, HICS shifted gears and took on a much broader role: coordinating activities with multiple agencies, including local law enforcement, the Federal Bureau of Investigation, news media, and an array of patient and family services. Again, the timing of this tragic event allowed the needs and functions of our hospital command to concentrate the focus in a more structured manner than might have been possible during regular operational hours.
Lessons Learned and Recommendations
As with any MCI, it is important to perform after-incident debriefings to reflect upon the prehospital, ED, and hospital care to assess for areas in need of improvement. Obviously, depending on the number and type of patients who are received and the resources available at the institution, such events can stress EDs that are already at or above their capacity. At the time of this writing (less than 2 months after the incident), although we are still in the early stages of our post-incident debriefing process, we offer the following suggestions and recommendations that we believe will be a benefit to other institutions faced with similar challenges.
Dedicated Disaster Preparedness Program
Depending on the size of the institution and whether or not it is a trauma-receiving center, it is vitally important to have a team of individuals dedicated to the development and maintenance of a disaster readiness program. Eric Alberts, manager of Emergency Preparedness at Orlando Heath, emphasized the need to harness and leverage preparedness efforts and relationships with community partners, law enforcement, and EMS. In addition, he noted that these trained individuals can provide education and coordination for mass-casualty drills, assist in developing and maintaining policies and protocols, and coordinate with hospital incident command during actual events.
Practice, Practice, Practice
In order for medical staff, nursing staff, and support personnel to understand their roles and responsibilities if an MCI occurs, they must practice simulated drills. Tabletop and full-scale emergency intake drills, with a range of scenarios occurring at different times of the day and night, will improve teamwork and coordination. At least once a year, a large, full-scale community exercise that involves scene casualty collection points, law enforcement and EMS involvement, multiple EDs, and hospital-wide integration will educate staff members on their duties. There should be enough patients in these drills to stress the entire system—both ED and in-hospital. Physician involvement in these exercises is crucial. In March 2016, a full-scale community exercise was performed in the Central Florida area in which more than 500 volunteers, 50 agencies, and 15 hospitals participated. A segment of that exercise involved an active shooter impersonating a patient brought to the ED. We feel this recent exercise, and others like it, were helpful in managing our actual mass casualty event.
Notification
When the shootings at the Pulse nightclub started, we were advised that there might be as many as 20 victims, but over the next few hours, we received more than twice as many injured patients. In any scenario where it is felt additional ED resources and personnel are needed, it is advantageous to begin the notification process as early as possible. Ideally, there are redundant methods in place to notify in-house and off-duty personnel, preferably in a multilayered system of electronic and voice communications that provides feedback as to staff availability. During daytime hours, it is easier for off-duty staff to learn of an event through news and social media. However, during early morning hours, such as when these shootings occurred, it may be difficult to notify sleeping staff that they are needed. With the trend of using mobile devices instead of landline phones, and with the ability to silence those devices during nighttime sleep hours, it has become increasingly difficult to “break through” the silence. In our event, group e-mail notifications were activated, but individual phone calls were also required. Initially, some calls to staff went to voice mail rather than being
answered directly.
Communication
Communication is an area of opportunity for improvement in almost any mass-casualty event. Redundant methods of electronic, voice, telephone, and radio communications are crucial for personnel to coordinate efforts. It is imperative that HICS and ED personnel receive updates about events on the scene and the status of potential incoming patients. An infrastructure of communications is paramount for the coordination that is needed between prehospital, ED, and in-house resources.
Throughput
Facilitating ED and hospital throughput and improving operational efficiencies are regular topics of discussion in most busy EDs. However, when it becomes necessary to intake large numbers of patients in an ED within a short period of time, the need to move patients out of the department intensifies. In many cases, to continue intake of patients, it will be necessary to quickly find areas outside of the ED—such as operating rooms, ICUs, and general floors—to transport patients to. At our facility, we normally have two operating rooms staffed throughout the night for traumas. On the night of the shootings, a total of six operating rooms were activated quickly to manage the penetrating injuries of these patients. Previously admitted medical patients were retrieved by in-house nursing staff to free up ED beds.
Media
It is important to recognize the intense social, print, online, and television media exposure that will occur with such tragedies, and have personnel and systems in place to manage it. The Pulse nightclub is located only a few short blocks south of ORMC, and the majority of all injured patients, including those with the most severe injuries, presented to our facility. As a result, the intense media coverage at our hospital physically overlapped the intense media coverage at the scene. Nearby businesses were shut down, access to several main thoroughfare roads were blocked, and some individuals mistakenly thought the hospital was closed. Our media relations department felt the most efficient way to manage information dissemination was to hold a “presser”—a press conference with a few of the health professionals involved in the care of the patients. The press conference helped to relieve some of the media presence and pressure, and provided welcomed transparency to the community.
Expect the Unexpected
Even when physicians and staff conduct regular training exercises and are familiar with policies and procedures, the controlled chaos that is typical of a true mass-casualty event will likely result in some unexpected occurrences. After injured patients began arriving at our ED, during a period when patient influx and medical care was intense, there was a brief time when we were concerned an active shooter was also in our ED. Since the mass shootings were only a few blocks from the ED, and we did not know the scope or number of shooters, it was a reasonable assumption that a perpetrator could present to the ED as a patient or active shooter. During an event such as this, it is the practice at ORMC to have security officers place the facility on lockdown; however; the added concern that an active shooter was physically present in the ED required additional law enforcement officers to methodically search the entire facility until the threat of a shooter was eliminated. Although this did not directly impact patient care, it did create another level of complexity and stress to the already challenging situation.
Emotional Impact
One should not underestimate the emotional impact of a mass-casualty event. Emergency physicians and personnel are well equipped to manage illness, injury, and death. However, an event of this magnitude, with the number and manner of injuries and deaths that presented to our ED, along with the closeness to a scene of bloodshed where many young lives were taken in a senseless act of violence, will likely have a lasting impression on many staff members. Any institution encountering such an event should have behavioral health and support counselors readily available as soon as possible.
Conclusion
When patients present in truckloads, as they did in our ambulance bay in the early hours of June 12, an ED may be inundated with injured patients without notice or preparation. Teams need to trust their instincts, their training, and one another. It is unlikely that your normal ED practice patterns will be sufficient to take care of a large surge of patients, and you will need to turn to your ingenuity, creativity, and resourcefulness to do what you think is best to save the lives of as many patients as possible. As Sarah Duran, BSN, the trauma nurse who was working as the ED charge nurse the night of the shootings, stated, “I don’t think anyone can fully prepare for anything on the scale of what happened in Orlando, but with a good foundation of protocols in place, strong set of staff, constant vigilance, and great teamwork, any hospital can be successful in handling a mass casualty incident.”
Orlando Regional Medical Center (ORMC) is an urban, academic, Level 1 trauma center with an ED that treats in excess of 85,000 patients each year. There are more than 4,500 annual trauma admissions, including penetrating and blunt mechanism injuries. We have a 3-year emergency medicine (EM) residency program, originally established in 1986, that now has 14 residents per year. The events that we experienced on the early morning of June 12, 2016 were tragic and unexpected and tested our community, our teams, and our organization. The following is a description of activity perspectives, personal reflections and feelings, and important lessons learned from which other EDs may benefit.
Prehospital/Rescue Period
The prehospital approach to a mass-casualty incident (MCI) caused by gunfire has changed significantly over the last decade, with lessons gleaned from the experience of military and domestic attacks. The Orange County (Florida) Emergency Medical Services (EMS) System, including the agencies involved in the response to the June 12, 2016 Pulse nightclub shooting incident (Orlando Fire Department, Rural Metro Ambulance, and Orange County Fire Rescue), have trained specifically on the evolving priorities during an active shooter incident. This includes targeted hemorrhage control, swift extrication, minimal interventions, and immediate transport to the appropriate receiving center. In June 2015, 1 year prior to the Pulse nightclub shooting, a protocol variance was written by medical directors, explaining that “EMS providers may engage in initial patient care with only the supplies deemed absolutely necessary for rapid evaluation and removal into a safe triage zone.” This variance applies when there is an active shooter scenario. In addition, in 2014, 2015, and 2016, we conducted large scale, multiagency community exercises, encompassing shooting/active shooter scenarios and drills.
On June 12, 2016, EMS units were on scene of the Pulse nightclub in downtown Orlando less than 5 minutes after shooting began. It became immediately obvious that additional resources would be necessary. Soon after the incident, an MCI alert was activated from the Orlando Fire Department Communications Center, utilizing a software system to notify area hospitals and the medical directors that up to 20 patients had been shot, and requesting status on bed availability. Christopher L. Hunter, MD, PhD, one of our associate EMS medical directors, was working in the ED at a hospital several miles away from the incident and began coordinating efforts to distribute patients to the appropriate destination via radio and phone communication between the scene, the communications center, and the trauma center.
On scene, a group of law enforcement officers were engaging the shooter while others assisted in extracting victims from the club and surrounding area. Injured victims were brought to a casualty collection point, under cover, across the street and were transported as units became available. Initially, law enforcement vehicles and ambulances would make the two-block drive from the scene to ORMC carrying as many patients as they safely could, and return immediately after offload. Per previous training, minimal interventions were performed, and unlike standard procedure, EMS could offer no prearrival report to the hospital. The decision was made to triage only patients meeting Florida’s state trauma alert criteria to ORMC, and funnel other patients to nearby nontrauma centers. Dr Hunter attempted to notify the hospitals of patients as they were transported off the scene; however, the extremely short transport times made this process difficult.
Over the course of the incident, on-scene commands designated a casualty collection point, a transport unit staging area, and a hospital liaison. Emergency medical service responders cooperated with law enforcement to transport injured patients throughout the response. By the end of the event, nearly 100 EMS providers utilizing dozens of vehicles had responded to the scene.
The ED Period
The overnight shift of Saturday, June 11 was slower than usual in the ORMC ED, and triage housed the typical Saturday-night complaints. One of our mid-level providers, Brian Clayton, ARNP, worked in the fast track pod treating lower acuity illnesses, while the four senior residents discussed who might receive the honor of leaving early. Even the senior attending, Gary A. Parrish, MD, wrapped up his patients and was ready to leave right on time. The 11:00 pm to 7:00 am attending, Kathryn J. Bondani, MD, was looking forward to an easy shift with four graduating senior residents. We had just called in an order from a popular nearby sandwich shop when suddenly, a slew of police cars flew past the hospital—not unusual given our proximity to downtown. The radio squawked “Multiple gunshots wounds en route.” Someone said “a club downtown got shot up.” In anticipation of multiple patient arrivals, resident Amanda M. Stone, MD, and the attending hurried to the trauma/ressuscitation bay. Another resident, Amanda F. Tarkowski, MD, called the trauma attending to rally his team downstairs, informing him that “A club downtown has a shooter and we have multiple patients with a 2-minute ETA.” Multiple gunshot wounds (GSWs) at once is not unusual for our Level 1 trauma center.
The first patient arrived shortly after 2:00 am and had multiple GSWs to the abdomen, but was awake and talking. He was mildly tachycardic, but his blood pressure (BP) was stable. We all gaped at the fist-sized wound on his back—some of us had never seen a GSW like this before. It was apparent that he needed to go to the operating room, but before preparations could be made, three more patients rolled into the ED. Instead of our usual organized, methodical EMS report, these patients were dropped off by a police pickup truck and rolled in on our own stretchers by nurses and technicians. Soon, all six of our trauma bays were filled with critical patients. Christopher H. Ponder, MD, recalled, “I heard an overhead page for ‘all available trauma nurses to the trauma bay’ shortly followed by a more concerned-sounding ‘all available staff to the trauma bay.’” All four senior EM residents, both EM attendings, the trauma attending, four trauma residents, multiple ED nurses, technicians, and various ancillary staff quickly descended on the patients. Chest tubes were placed, and multiple patients were intubated. Several thoracotomies were performed at the initial point when the team was unaware of the exact number of patients who would ultimately follow. Blood bags were hung and tranexamic acid was administered liberally. Unfortunately, some of these initial attempts were unsuccessful, as the first wave contained the most critically injured patients.
We barely had time to reflect on the dead as more patients filled the hallways. In the midst of all of this, triage decisions came quickly—awake and talking patients with an acceptable BP were moved out of the trauma bay in favor of less stable patients. Intubation and chest-tube placement decisions were made instantly. There was no time for routine X-rays or laboratory evaluation. Nurses, technicians, and doctors crowded the trauma bay desperately trying to stabilize the critically injured. Vital signs were taken manually. Dr Ponder called his colleague Thomas N. Smith, MD, who was also a graduating senior EM resident, and who happened to be staffing the children’s ED across the street that night. “Is there any way you can make it over here right now?” he shouted into the phone. Dr Smith quickly grabbed several trauma supplies and hitched a ride with security to the ORMC ED to assist. He was confronted with the scene of the previously mentioned “first wave” and grabbed an ultrasound machine to help triage these unfortunate patients. In addition to Dr Smith, unit clerks continued making calls to additional off-duty medical, nursing, and support staff. Critical care attendings and fellows responded from upstairs to assist in the ED. The Hospital Incident Command System (HICS) was initiated to provide hospital and corporate coordination of services. Timothy B. Bullard, MD, another EM attending physician and medical staff director of HICS, was en route to the ED to assist in the response.
Amidst the overwhelming mass of mortally and critically wounded patients, we were told stories of terror from just down the road. The sense of horror was almost contagious, and we all wondered if the violence would spread to the hospital.
About an hour into the ordeal, we heard another page overhead “Code Silver, ED Triage. Code Silver, ED Triage.” Everyone in the trauma bay froze. We heard someone shout from the hallway “Shots fired in triage!” After a few seconds, those nearest the trauma bay doors pushed them shut. One of the trauma surgeons shouted, “Keep caring for your patients, push the portable X-ray machines in front of the doors.” That is exactly what happened, and we worked in the barricaded trauma room for the longest few minutes of the night. We would later learn that this report was false, but fearing for our own lives as well as the lives of our patients is an experience that few, if any, of us had previously been through. The fact that we continued to work in such a situation illustrates everyone’s dedication to their patients and mission.
After the first wave of patients, it was clear that reassessing patients was now our greatest challenge. There were multiple patients with stable vital signs but who had GSWs to the abdomen, pelvis, and thorax and required surgery. Having realized that the ultrasound machine and focused assessment with sonography for trauma (FAST) examinations were the most practical rapid imaging modality, Drs Tarkowski, Ponder, and Smith grabbed the machine and went from room to room repeating the FAST examinations, vital signs, and assessments for missed wounds. A portable computer allowed us to order X-ray orders for patients with extremity wounds. Several patients who initially had negative FASTs had a repeat examination that was positive for free fluid. The operating room triage list shuffled based on these examinations.
At this point, there seemed to be a never-ending list of “Doe” names on the electronic medical record (EMR) tracking board. Tracking the location of patients was a dynamic process, and updating the tracking board was difficult. Patients were continually cycled in and out of the trauma bay, shuffled in and out of treatment rooms, lined up in the hallways, and transported up to the intensive care unit (ICU). Some of those “Doe” names belonged to patients who had succumbed to their injuries. Some patients had empty, bloody charts next to them on their stretcher. Every patient had a wristband. Dr Tarkowski improvised a rapid documentation system using quarter sheets of scrap paper that were taped either to the stretcher or door with a patient name, brief list of injuries, FAST examination result, pending tests, and medications given. This system was an efficient way of identifying which patients had been evaluated, what had been done, and what was pending.
While we were “rounding” on our patients, down the street, law enforcement had broken down the club’s wall, and a second wave of patients began to arrive. Two residents completed the secondary evaluations while the other three resuscitated new patients. The second wave seemed like a repeat of the first. Several extremity-only injuries were triaged directly to the hallways to be seen by the two residents outside of the trauma bay. By this time, the orthopedic surgery service had come down to the ED and was washing out wounds, splinting, and making plans for surgery. The internal medicine service and the medical critical care team were helping reassess patients as well.
There were many emotional moments. Dr Ponder remembered, “One of the first few patients I saw was pulseless, and as I went to start chest compressions, I was stopped by a trauma surgeon who said, ‘He’s gone, focus on the ones we can help.’ That’s when I realized the gravity of our situation. For almost 2 hours, each resident cycled through patients.” Dr Stone recounted, “I just went down the line of patients, from head of the bed to head of the bed, some patients still on EMS stretchers, intubating many of them. It was surreal to see that many severely injured in one place.” Tory L. Weatherford, MD, recalled, “It was controlled chaos. My training kicked in, and it became about just trying to do anything possible to help.”
Dr Bondani, the overnight attending, said she does not remember many specifics from the event. “Faces and injuries blurred together. I remember looking in one young man’s eyes and telling him, ‘We are going to help you, just hang in there,’ and telling another panicking woman, ‘You’re talking, you can feel pain, you’re alive. Calm down.’ It was organized chaos as we swept from patient to patient moving as quickly as possible. Your training kicks in and you do what needs to be done in the moment.”
We were fortunate to have the EM team we did, and to be in the place that we were. On duty, we had five senior graduating residents, essentially with attending-level skills and training, who had been together since day one. “We gave everything we had; there was no time to stop. We went where our hands could be helpful—it didn’t matter if it was your traditional role or not,” said Dr Weatherford.
Dr Tarkowski remembered what it was like leaving the walls of the ED later that morning. “Leaving the hospital didn’t feel like a success. We knew the work we did was good, that we did everything we could, but it didn’t feel like it. It felt heavy. It felt empty.” Afterward, the emotional toll set in. We gathered up the names of the deceased and looked at a status board littered with “Doe” names, and we tried hard not to break down at the violence and the pain we witnessed.
Immediate Recovery Period (Prehospital Setting)
In the aftermath of the initial rescue operation, stabilization of injured victims, and demobilization of resources, a second “event” unfolded—hundreds of family members flooded to ORMC looking for unaccounted loved ones. At this point, there were dozens of deceased and critically injured patients who remained unidentified, and addressing the needs of both the victims and the families was becoming overwhelming. With the cooperation of federal, state, county, city, and private resources, a family reunification center (FRC; a family staging area/family reception center) was created and managed initially within the hospital. At this site, grief counselors, victim advocates, law enforcement and medical examiner’s officials, hospital chaplains, and translators gathered with the loved ones of those missing to synchronize efforts to identify and reunite them. The Emergency Operations Center quickly created a telephone hotline and Web site to guide those in need to this resource. Food and housing for those in need were provided by the generosity of our community—which cannot be overstated in the wake of this tragedy. As days passed, the FRC transitioned to a new location as the Orlando United Assistance Center, and will continue to serve as a navigation point for those who are and will be affected by the event for months to come.
Hospital Incident Command System
The HICS was activated shortly past 3:00 am, just after the initial wave of victims flooded our ED and the gravity of the situation became apparent. The ED and trauma services were already near full staff due to timing, rapid response, and communication between the traumatologists, as well as a bit of luck. Because of the time of the disaster, our normal notification process for incident command and all personnel was severely limited. In retrospect, this turned out to be a blessing. While everyone who normally would respond to HICS was not available, individuals serving in key positions were reached by personal phone calls and were on-site quickly. One of the main functions of HICS is to control the internal chaos that arises when a disaster occurs and all personnel want to assist in some manner. We have spent hours during drills crafting communications that target key personnel necessary to meet the mission, while controlling well-intentioned but unnecessary personnel. In our drills, this is not an issue because everyone knows the exercise is not reality; however, in a real disaster, everyone wants to help. This was confirmed by the guilt that so many of our team members expressed at not being involved that Sunday.
With an initial skeleton crew in incident command, it was easy to focus on the immediate needs of patient care. The strong leadership and cool heads of our incident command leadership led to rapid role definition and responsibility, and set forth an easy path for execution. Hospital command personnel adapted their usual roles and performed functions and assignments as needed. Many HICS staff had direct face-to-face contact with frontline providers in the ED and other vital areas. This was possible because of the close proximity of the hospital command headquarters to the ED. The need for additional resources was rapidly identified, and the hospital command leaders assumed direct responsibility for procuring them instead of delegating. A great example of this was when Orlando Health’s chief executive officer and chief operating officer went to our nearby hospitals to gather additional chest tubes after learning that we were running in short supply. Their main responsibilities lay ahead of them, and they were willing to help in any manner they could at the time. Some of the medical personnel were able to switch roles and pitch in to treat the second wave of victims that arrived at around 5:00 am.
As the flow of victims to the ED subsided and order began to be restored, HICS shifted gears and took on a much broader role: coordinating activities with multiple agencies, including local law enforcement, the Federal Bureau of Investigation, news media, and an array of patient and family services. Again, the timing of this tragic event allowed the needs and functions of our hospital command to concentrate the focus in a more structured manner than might have been possible during regular operational hours.
Lessons Learned and Recommendations
As with any MCI, it is important to perform after-incident debriefings to reflect upon the prehospital, ED, and hospital care to assess for areas in need of improvement. Obviously, depending on the number and type of patients who are received and the resources available at the institution, such events can stress EDs that are already at or above their capacity. At the time of this writing (less than 2 months after the incident), although we are still in the early stages of our post-incident debriefing process, we offer the following suggestions and recommendations that we believe will be a benefit to other institutions faced with similar challenges.
Dedicated Disaster Preparedness Program
Depending on the size of the institution and whether or not it is a trauma-receiving center, it is vitally important to have a team of individuals dedicated to the development and maintenance of a disaster readiness program. Eric Alberts, manager of Emergency Preparedness at Orlando Heath, emphasized the need to harness and leverage preparedness efforts and relationships with community partners, law enforcement, and EMS. In addition, he noted that these trained individuals can provide education and coordination for mass-casualty drills, assist in developing and maintaining policies and protocols, and coordinate with hospital incident command during actual events.
Practice, Practice, Practice
In order for medical staff, nursing staff, and support personnel to understand their roles and responsibilities if an MCI occurs, they must practice simulated drills. Tabletop and full-scale emergency intake drills, with a range of scenarios occurring at different times of the day and night, will improve teamwork and coordination. At least once a year, a large, full-scale community exercise that involves scene casualty collection points, law enforcement and EMS involvement, multiple EDs, and hospital-wide integration will educate staff members on their duties. There should be enough patients in these drills to stress the entire system—both ED and in-hospital. Physician involvement in these exercises is crucial. In March 2016, a full-scale community exercise was performed in the Central Florida area in which more than 500 volunteers, 50 agencies, and 15 hospitals participated. A segment of that exercise involved an active shooter impersonating a patient brought to the ED. We feel this recent exercise, and others like it, were helpful in managing our actual mass casualty event.
Notification
When the shootings at the Pulse nightclub started, we were advised that there might be as many as 20 victims, but over the next few hours, we received more than twice as many injured patients. In any scenario where it is felt additional ED resources and personnel are needed, it is advantageous to begin the notification process as early as possible. Ideally, there are redundant methods in place to notify in-house and off-duty personnel, preferably in a multilayered system of electronic and voice communications that provides feedback as to staff availability. During daytime hours, it is easier for off-duty staff to learn of an event through news and social media. However, during early morning hours, such as when these shootings occurred, it may be difficult to notify sleeping staff that they are needed. With the trend of using mobile devices instead of landline phones, and with the ability to silence those devices during nighttime sleep hours, it has become increasingly difficult to “break through” the silence. In our event, group e-mail notifications were activated, but individual phone calls were also required. Initially, some calls to staff went to voice mail rather than being
answered directly.
Communication
Communication is an area of opportunity for improvement in almost any mass-casualty event. Redundant methods of electronic, voice, telephone, and radio communications are crucial for personnel to coordinate efforts. It is imperative that HICS and ED personnel receive updates about events on the scene and the status of potential incoming patients. An infrastructure of communications is paramount for the coordination that is needed between prehospital, ED, and in-house resources.
Throughput
Facilitating ED and hospital throughput and improving operational efficiencies are regular topics of discussion in most busy EDs. However, when it becomes necessary to intake large numbers of patients in an ED within a short period of time, the need to move patients out of the department intensifies. In many cases, to continue intake of patients, it will be necessary to quickly find areas outside of the ED—such as operating rooms, ICUs, and general floors—to transport patients to. At our facility, we normally have two operating rooms staffed throughout the night for traumas. On the night of the shootings, a total of six operating rooms were activated quickly to manage the penetrating injuries of these patients. Previously admitted medical patients were retrieved by in-house nursing staff to free up ED beds.
Media
It is important to recognize the intense social, print, online, and television media exposure that will occur with such tragedies, and have personnel and systems in place to manage it. The Pulse nightclub is located only a few short blocks south of ORMC, and the majority of all injured patients, including those with the most severe injuries, presented to our facility. As a result, the intense media coverage at our hospital physically overlapped the intense media coverage at the scene. Nearby businesses were shut down, access to several main thoroughfare roads were blocked, and some individuals mistakenly thought the hospital was closed. Our media relations department felt the most efficient way to manage information dissemination was to hold a “presser”—a press conference with a few of the health professionals involved in the care of the patients. The press conference helped to relieve some of the media presence and pressure, and provided welcomed transparency to the community.
Expect the Unexpected
Even when physicians and staff conduct regular training exercises and are familiar with policies and procedures, the controlled chaos that is typical of a true mass-casualty event will likely result in some unexpected occurrences. After injured patients began arriving at our ED, during a period when patient influx and medical care was intense, there was a brief time when we were concerned an active shooter was also in our ED. Since the mass shootings were only a few blocks from the ED, and we did not know the scope or number of shooters, it was a reasonable assumption that a perpetrator could present to the ED as a patient or active shooter. During an event such as this, it is the practice at ORMC to have security officers place the facility on lockdown; however; the added concern that an active shooter was physically present in the ED required additional law enforcement officers to methodically search the entire facility until the threat of a shooter was eliminated. Although this did not directly impact patient care, it did create another level of complexity and stress to the already challenging situation.
Emotional Impact
One should not underestimate the emotional impact of a mass-casualty event. Emergency physicians and personnel are well equipped to manage illness, injury, and death. However, an event of this magnitude, with the number and manner of injuries and deaths that presented to our ED, along with the closeness to a scene of bloodshed where many young lives were taken in a senseless act of violence, will likely have a lasting impression on many staff members. Any institution encountering such an event should have behavioral health and support counselors readily available as soon as possible.
Conclusion
When patients present in truckloads, as they did in our ambulance bay in the early hours of June 12, an ED may be inundated with injured patients without notice or preparation. Teams need to trust their instincts, their training, and one another. It is unlikely that your normal ED practice patterns will be sufficient to take care of a large surge of patients, and you will need to turn to your ingenuity, creativity, and resourcefulness to do what you think is best to save the lives of as many patients as possible. As Sarah Duran, BSN, the trauma nurse who was working as the ED charge nurse the night of the shootings, stated, “I don’t think anyone can fully prepare for anything on the scale of what happened in Orlando, but with a good foundation of protocols in place, strong set of staff, constant vigilance, and great teamwork, any hospital can be successful in handling a mass casualty incident.”
Orlando Regional Medical Center (ORMC) is an urban, academic, Level 1 trauma center with an ED that treats in excess of 85,000 patients each year. There are more than 4,500 annual trauma admissions, including penetrating and blunt mechanism injuries. We have a 3-year emergency medicine (EM) residency program, originally established in 1986, that now has 14 residents per year. The events that we experienced on the early morning of June 12, 2016 were tragic and unexpected and tested our community, our teams, and our organization. The following is a description of activity perspectives, personal reflections and feelings, and important lessons learned from which other EDs may benefit.
Prehospital/Rescue Period
The prehospital approach to a mass-casualty incident (MCI) caused by gunfire has changed significantly over the last decade, with lessons gleaned from the experience of military and domestic attacks. The Orange County (Florida) Emergency Medical Services (EMS) System, including the agencies involved in the response to the June 12, 2016 Pulse nightclub shooting incident (Orlando Fire Department, Rural Metro Ambulance, and Orange County Fire Rescue), have trained specifically on the evolving priorities during an active shooter incident. This includes targeted hemorrhage control, swift extrication, minimal interventions, and immediate transport to the appropriate receiving center. In June 2015, 1 year prior to the Pulse nightclub shooting, a protocol variance was written by medical directors, explaining that “EMS providers may engage in initial patient care with only the supplies deemed absolutely necessary for rapid evaluation and removal into a safe triage zone.” This variance applies when there is an active shooter scenario. In addition, in 2014, 2015, and 2016, we conducted large scale, multiagency community exercises, encompassing shooting/active shooter scenarios and drills.
On June 12, 2016, EMS units were on scene of the Pulse nightclub in downtown Orlando less than 5 minutes after shooting began. It became immediately obvious that additional resources would be necessary. Soon after the incident, an MCI alert was activated from the Orlando Fire Department Communications Center, utilizing a software system to notify area hospitals and the medical directors that up to 20 patients had been shot, and requesting status on bed availability. Christopher L. Hunter, MD, PhD, one of our associate EMS medical directors, was working in the ED at a hospital several miles away from the incident and began coordinating efforts to distribute patients to the appropriate destination via radio and phone communication between the scene, the communications center, and the trauma center.
On scene, a group of law enforcement officers were engaging the shooter while others assisted in extracting victims from the club and surrounding area. Injured victims were brought to a casualty collection point, under cover, across the street and were transported as units became available. Initially, law enforcement vehicles and ambulances would make the two-block drive from the scene to ORMC carrying as many patients as they safely could, and return immediately after offload. Per previous training, minimal interventions were performed, and unlike standard procedure, EMS could offer no prearrival report to the hospital. The decision was made to triage only patients meeting Florida’s state trauma alert criteria to ORMC, and funnel other patients to nearby nontrauma centers. Dr Hunter attempted to notify the hospitals of patients as they were transported off the scene; however, the extremely short transport times made this process difficult.
Over the course of the incident, on-scene commands designated a casualty collection point, a transport unit staging area, and a hospital liaison. Emergency medical service responders cooperated with law enforcement to transport injured patients throughout the response. By the end of the event, nearly 100 EMS providers utilizing dozens of vehicles had responded to the scene.
The ED Period
The overnight shift of Saturday, June 11 was slower than usual in the ORMC ED, and triage housed the typical Saturday-night complaints. One of our mid-level providers, Brian Clayton, ARNP, worked in the fast track pod treating lower acuity illnesses, while the four senior residents discussed who might receive the honor of leaving early. Even the senior attending, Gary A. Parrish, MD, wrapped up his patients and was ready to leave right on time. The 11:00 pm to 7:00 am attending, Kathryn J. Bondani, MD, was looking forward to an easy shift with four graduating senior residents. We had just called in an order from a popular nearby sandwich shop when suddenly, a slew of police cars flew past the hospital—not unusual given our proximity to downtown. The radio squawked “Multiple gunshots wounds en route.” Someone said “a club downtown got shot up.” In anticipation of multiple patient arrivals, resident Amanda M. Stone, MD, and the attending hurried to the trauma/ressuscitation bay. Another resident, Amanda F. Tarkowski, MD, called the trauma attending to rally his team downstairs, informing him that “A club downtown has a shooter and we have multiple patients with a 2-minute ETA.” Multiple gunshot wounds (GSWs) at once is not unusual for our Level 1 trauma center.
The first patient arrived shortly after 2:00 am and had multiple GSWs to the abdomen, but was awake and talking. He was mildly tachycardic, but his blood pressure (BP) was stable. We all gaped at the fist-sized wound on his back—some of us had never seen a GSW like this before. It was apparent that he needed to go to the operating room, but before preparations could be made, three more patients rolled into the ED. Instead of our usual organized, methodical EMS report, these patients were dropped off by a police pickup truck and rolled in on our own stretchers by nurses and technicians. Soon, all six of our trauma bays were filled with critical patients. Christopher H. Ponder, MD, recalled, “I heard an overhead page for ‘all available trauma nurses to the trauma bay’ shortly followed by a more concerned-sounding ‘all available staff to the trauma bay.’” All four senior EM residents, both EM attendings, the trauma attending, four trauma residents, multiple ED nurses, technicians, and various ancillary staff quickly descended on the patients. Chest tubes were placed, and multiple patients were intubated. Several thoracotomies were performed at the initial point when the team was unaware of the exact number of patients who would ultimately follow. Blood bags were hung and tranexamic acid was administered liberally. Unfortunately, some of these initial attempts were unsuccessful, as the first wave contained the most critically injured patients.
We barely had time to reflect on the dead as more patients filled the hallways. In the midst of all of this, triage decisions came quickly—awake and talking patients with an acceptable BP were moved out of the trauma bay in favor of less stable patients. Intubation and chest-tube placement decisions were made instantly. There was no time for routine X-rays or laboratory evaluation. Nurses, technicians, and doctors crowded the trauma bay desperately trying to stabilize the critically injured. Vital signs were taken manually. Dr Ponder called his colleague Thomas N. Smith, MD, who was also a graduating senior EM resident, and who happened to be staffing the children’s ED across the street that night. “Is there any way you can make it over here right now?” he shouted into the phone. Dr Smith quickly grabbed several trauma supplies and hitched a ride with security to the ORMC ED to assist. He was confronted with the scene of the previously mentioned “first wave” and grabbed an ultrasound machine to help triage these unfortunate patients. In addition to Dr Smith, unit clerks continued making calls to additional off-duty medical, nursing, and support staff. Critical care attendings and fellows responded from upstairs to assist in the ED. The Hospital Incident Command System (HICS) was initiated to provide hospital and corporate coordination of services. Timothy B. Bullard, MD, another EM attending physician and medical staff director of HICS, was en route to the ED to assist in the response.
Amidst the overwhelming mass of mortally and critically wounded patients, we were told stories of terror from just down the road. The sense of horror was almost contagious, and we all wondered if the violence would spread to the hospital.
About an hour into the ordeal, we heard another page overhead “Code Silver, ED Triage. Code Silver, ED Triage.” Everyone in the trauma bay froze. We heard someone shout from the hallway “Shots fired in triage!” After a few seconds, those nearest the trauma bay doors pushed them shut. One of the trauma surgeons shouted, “Keep caring for your patients, push the portable X-ray machines in front of the doors.” That is exactly what happened, and we worked in the barricaded trauma room for the longest few minutes of the night. We would later learn that this report was false, but fearing for our own lives as well as the lives of our patients is an experience that few, if any, of us had previously been through. The fact that we continued to work in such a situation illustrates everyone’s dedication to their patients and mission.
After the first wave of patients, it was clear that reassessing patients was now our greatest challenge. There were multiple patients with stable vital signs but who had GSWs to the abdomen, pelvis, and thorax and required surgery. Having realized that the ultrasound machine and focused assessment with sonography for trauma (FAST) examinations were the most practical rapid imaging modality, Drs Tarkowski, Ponder, and Smith grabbed the machine and went from room to room repeating the FAST examinations, vital signs, and assessments for missed wounds. A portable computer allowed us to order X-ray orders for patients with extremity wounds. Several patients who initially had negative FASTs had a repeat examination that was positive for free fluid. The operating room triage list shuffled based on these examinations.
At this point, there seemed to be a never-ending list of “Doe” names on the electronic medical record (EMR) tracking board. Tracking the location of patients was a dynamic process, and updating the tracking board was difficult. Patients were continually cycled in and out of the trauma bay, shuffled in and out of treatment rooms, lined up in the hallways, and transported up to the intensive care unit (ICU). Some of those “Doe” names belonged to patients who had succumbed to their injuries. Some patients had empty, bloody charts next to them on their stretcher. Every patient had a wristband. Dr Tarkowski improvised a rapid documentation system using quarter sheets of scrap paper that were taped either to the stretcher or door with a patient name, brief list of injuries, FAST examination result, pending tests, and medications given. This system was an efficient way of identifying which patients had been evaluated, what had been done, and what was pending.
While we were “rounding” on our patients, down the street, law enforcement had broken down the club’s wall, and a second wave of patients began to arrive. Two residents completed the secondary evaluations while the other three resuscitated new patients. The second wave seemed like a repeat of the first. Several extremity-only injuries were triaged directly to the hallways to be seen by the two residents outside of the trauma bay. By this time, the orthopedic surgery service had come down to the ED and was washing out wounds, splinting, and making plans for surgery. The internal medicine service and the medical critical care team were helping reassess patients as well.
There were many emotional moments. Dr Ponder remembered, “One of the first few patients I saw was pulseless, and as I went to start chest compressions, I was stopped by a trauma surgeon who said, ‘He’s gone, focus on the ones we can help.’ That’s when I realized the gravity of our situation. For almost 2 hours, each resident cycled through patients.” Dr Stone recounted, “I just went down the line of patients, from head of the bed to head of the bed, some patients still on EMS stretchers, intubating many of them. It was surreal to see that many severely injured in one place.” Tory L. Weatherford, MD, recalled, “It was controlled chaos. My training kicked in, and it became about just trying to do anything possible to help.”
Dr Bondani, the overnight attending, said she does not remember many specifics from the event. “Faces and injuries blurred together. I remember looking in one young man’s eyes and telling him, ‘We are going to help you, just hang in there,’ and telling another panicking woman, ‘You’re talking, you can feel pain, you’re alive. Calm down.’ It was organized chaos as we swept from patient to patient moving as quickly as possible. Your training kicks in and you do what needs to be done in the moment.”
We were fortunate to have the EM team we did, and to be in the place that we were. On duty, we had five senior graduating residents, essentially with attending-level skills and training, who had been together since day one. “We gave everything we had; there was no time to stop. We went where our hands could be helpful—it didn’t matter if it was your traditional role or not,” said Dr Weatherford.
Dr Tarkowski remembered what it was like leaving the walls of the ED later that morning. “Leaving the hospital didn’t feel like a success. We knew the work we did was good, that we did everything we could, but it didn’t feel like it. It felt heavy. It felt empty.” Afterward, the emotional toll set in. We gathered up the names of the deceased and looked at a status board littered with “Doe” names, and we tried hard not to break down at the violence and the pain we witnessed.
Immediate Recovery Period (Prehospital Setting)
In the aftermath of the initial rescue operation, stabilization of injured victims, and demobilization of resources, a second “event” unfolded—hundreds of family members flooded to ORMC looking for unaccounted loved ones. At this point, there were dozens of deceased and critically injured patients who remained unidentified, and addressing the needs of both the victims and the families was becoming overwhelming. With the cooperation of federal, state, county, city, and private resources, a family reunification center (FRC; a family staging area/family reception center) was created and managed initially within the hospital. At this site, grief counselors, victim advocates, law enforcement and medical examiner’s officials, hospital chaplains, and translators gathered with the loved ones of those missing to synchronize efforts to identify and reunite them. The Emergency Operations Center quickly created a telephone hotline and Web site to guide those in need to this resource. Food and housing for those in need were provided by the generosity of our community—which cannot be overstated in the wake of this tragedy. As days passed, the FRC transitioned to a new location as the Orlando United Assistance Center, and will continue to serve as a navigation point for those who are and will be affected by the event for months to come.
Hospital Incident Command System
The HICS was activated shortly past 3:00 am, just after the initial wave of victims flooded our ED and the gravity of the situation became apparent. The ED and trauma services were already near full staff due to timing, rapid response, and communication between the traumatologists, as well as a bit of luck. Because of the time of the disaster, our normal notification process for incident command and all personnel was severely limited. In retrospect, this turned out to be a blessing. While everyone who normally would respond to HICS was not available, individuals serving in key positions were reached by personal phone calls and were on-site quickly. One of the main functions of HICS is to control the internal chaos that arises when a disaster occurs and all personnel want to assist in some manner. We have spent hours during drills crafting communications that target key personnel necessary to meet the mission, while controlling well-intentioned but unnecessary personnel. In our drills, this is not an issue because everyone knows the exercise is not reality; however, in a real disaster, everyone wants to help. This was confirmed by the guilt that so many of our team members expressed at not being involved that Sunday.
With an initial skeleton crew in incident command, it was easy to focus on the immediate needs of patient care. The strong leadership and cool heads of our incident command leadership led to rapid role definition and responsibility, and set forth an easy path for execution. Hospital command personnel adapted their usual roles and performed functions and assignments as needed. Many HICS staff had direct face-to-face contact with frontline providers in the ED and other vital areas. This was possible because of the close proximity of the hospital command headquarters to the ED. The need for additional resources was rapidly identified, and the hospital command leaders assumed direct responsibility for procuring them instead of delegating. A great example of this was when Orlando Health’s chief executive officer and chief operating officer went to our nearby hospitals to gather additional chest tubes after learning that we were running in short supply. Their main responsibilities lay ahead of them, and they were willing to help in any manner they could at the time. Some of the medical personnel were able to switch roles and pitch in to treat the second wave of victims that arrived at around 5:00 am.
As the flow of victims to the ED subsided and order began to be restored, HICS shifted gears and took on a much broader role: coordinating activities with multiple agencies, including local law enforcement, the Federal Bureau of Investigation, news media, and an array of patient and family services. Again, the timing of this tragic event allowed the needs and functions of our hospital command to concentrate the focus in a more structured manner than might have been possible during regular operational hours.
Lessons Learned and Recommendations
As with any MCI, it is important to perform after-incident debriefings to reflect upon the prehospital, ED, and hospital care to assess for areas in need of improvement. Obviously, depending on the number and type of patients who are received and the resources available at the institution, such events can stress EDs that are already at or above their capacity. At the time of this writing (less than 2 months after the incident), although we are still in the early stages of our post-incident debriefing process, we offer the following suggestions and recommendations that we believe will be a benefit to other institutions faced with similar challenges.
Dedicated Disaster Preparedness Program
Depending on the size of the institution and whether or not it is a trauma-receiving center, it is vitally important to have a team of individuals dedicated to the development and maintenance of a disaster readiness program. Eric Alberts, manager of Emergency Preparedness at Orlando Heath, emphasized the need to harness and leverage preparedness efforts and relationships with community partners, law enforcement, and EMS. In addition, he noted that these trained individuals can provide education and coordination for mass-casualty drills, assist in developing and maintaining policies and protocols, and coordinate with hospital incident command during actual events.
Practice, Practice, Practice
In order for medical staff, nursing staff, and support personnel to understand their roles and responsibilities if an MCI occurs, they must practice simulated drills. Tabletop and full-scale emergency intake drills, with a range of scenarios occurring at different times of the day and night, will improve teamwork and coordination. At least once a year, a large, full-scale community exercise that involves scene casualty collection points, law enforcement and EMS involvement, multiple EDs, and hospital-wide integration will educate staff members on their duties. There should be enough patients in these drills to stress the entire system—both ED and in-hospital. Physician involvement in these exercises is crucial. In March 2016, a full-scale community exercise was performed in the Central Florida area in which more than 500 volunteers, 50 agencies, and 15 hospitals participated. A segment of that exercise involved an active shooter impersonating a patient brought to the ED. We feel this recent exercise, and others like it, were helpful in managing our actual mass casualty event.
Notification
When the shootings at the Pulse nightclub started, we were advised that there might be as many as 20 victims, but over the next few hours, we received more than twice as many injured patients. In any scenario where it is felt additional ED resources and personnel are needed, it is advantageous to begin the notification process as early as possible. Ideally, there are redundant methods in place to notify in-house and off-duty personnel, preferably in a multilayered system of electronic and voice communications that provides feedback as to staff availability. During daytime hours, it is easier for off-duty staff to learn of an event through news and social media. However, during early morning hours, such as when these shootings occurred, it may be difficult to notify sleeping staff that they are needed. With the trend of using mobile devices instead of landline phones, and with the ability to silence those devices during nighttime sleep hours, it has become increasingly difficult to “break through” the silence. In our event, group e-mail notifications were activated, but individual phone calls were also required. Initially, some calls to staff went to voice mail rather than being
answered directly.
Communication
Communication is an area of opportunity for improvement in almost any mass-casualty event. Redundant methods of electronic, voice, telephone, and radio communications are crucial for personnel to coordinate efforts. It is imperative that HICS and ED personnel receive updates about events on the scene and the status of potential incoming patients. An infrastructure of communications is paramount for the coordination that is needed between prehospital, ED, and in-house resources.
Throughput
Facilitating ED and hospital throughput and improving operational efficiencies are regular topics of discussion in most busy EDs. However, when it becomes necessary to intake large numbers of patients in an ED within a short period of time, the need to move patients out of the department intensifies. In many cases, to continue intake of patients, it will be necessary to quickly find areas outside of the ED—such as operating rooms, ICUs, and general floors—to transport patients to. At our facility, we normally have two operating rooms staffed throughout the night for traumas. On the night of the shootings, a total of six operating rooms were activated quickly to manage the penetrating injuries of these patients. Previously admitted medical patients were retrieved by in-house nursing staff to free up ED beds.
Media
It is important to recognize the intense social, print, online, and television media exposure that will occur with such tragedies, and have personnel and systems in place to manage it. The Pulse nightclub is located only a few short blocks south of ORMC, and the majority of all injured patients, including those with the most severe injuries, presented to our facility. As a result, the intense media coverage at our hospital physically overlapped the intense media coverage at the scene. Nearby businesses were shut down, access to several main thoroughfare roads were blocked, and some individuals mistakenly thought the hospital was closed. Our media relations department felt the most efficient way to manage information dissemination was to hold a “presser”—a press conference with a few of the health professionals involved in the care of the patients. The press conference helped to relieve some of the media presence and pressure, and provided welcomed transparency to the community.
Expect the Unexpected
Even when physicians and staff conduct regular training exercises and are familiar with policies and procedures, the controlled chaos that is typical of a true mass-casualty event will likely result in some unexpected occurrences. After injured patients began arriving at our ED, during a period when patient influx and medical care was intense, there was a brief time when we were concerned an active shooter was also in our ED. Since the mass shootings were only a few blocks from the ED, and we did not know the scope or number of shooters, it was a reasonable assumption that a perpetrator could present to the ED as a patient or active shooter. During an event such as this, it is the practice at ORMC to have security officers place the facility on lockdown; however; the added concern that an active shooter was physically present in the ED required additional law enforcement officers to methodically search the entire facility until the threat of a shooter was eliminated. Although this did not directly impact patient care, it did create another level of complexity and stress to the already challenging situation.
Emotional Impact
One should not underestimate the emotional impact of a mass-casualty event. Emergency physicians and personnel are well equipped to manage illness, injury, and death. However, an event of this magnitude, with the number and manner of injuries and deaths that presented to our ED, along with the closeness to a scene of bloodshed where many young lives were taken in a senseless act of violence, will likely have a lasting impression on many staff members. Any institution encountering such an event should have behavioral health and support counselors readily available as soon as possible.
Conclusion
When patients present in truckloads, as they did in our ambulance bay in the early hours of June 12, an ED may be inundated with injured patients without notice or preparation. Teams need to trust their instincts, their training, and one another. It is unlikely that your normal ED practice patterns will be sufficient to take care of a large surge of patients, and you will need to turn to your ingenuity, creativity, and resourcefulness to do what you think is best to save the lives of as many patients as possible. As Sarah Duran, BSN, the trauma nurse who was working as the ED charge nurse the night of the shootings, stated, “I don’t think anyone can fully prepare for anything on the scale of what happened in Orlando, but with a good foundation of protocols in place, strong set of staff, constant vigilance, and great teamwork, any hospital can be successful in handling a mass casualty incident.”
Experts assess soft filler procedures for hand rejuvenation
Cosmetic dermatology patients are increasingly seeking hand rejuvenation procedures to eliminate appearance discrepancies between the face and hands, reported Ramin Fathi, MD, and Joel L. Cohen, MD, FAAD, who reviewed the benefits and complications of soft filler methods.
Pre-procedure evaluation includes assessing volume of lost fat from the skin via the Merz hand grading scale. Prior to augmentation, a detailed patient history, including medications, medical problems, and bleeding abnormalities, is typically taken, as well as assessment of patient lifestyle issues involving extensive hand activity, such as typing on a keyboard or playing piano. The authors reviewed three hand rejuvenation methods: autologous fat grafting, hyaluronic acid (HA), and poly-L-lactic acid (PLLA).
Although autologous fat grafting is still done in some practices, it is more invasive compared with soft tissue fillers, and can result in complications such as infection, cyst formation, temporary dysesthesia, and significant edema, the authors noted. PLLA is not approved for dorsal hand augmentation, and is used off label.
Hyaluronic acid (HA) fillers such as Restylane, Belotero, and Juvederm are approved only in the United States for facial soft tissue augmentation, and are also used off label for hand rejuvenation. The authors cited a small study of 16 patients evaluating small gel particle HA in the dorsal hand, in which vascular, tendon, bony prominence, and skin turgor were improved by 60.9%, 65.2%, 73.7%, and 26.3%, respectively, 2 weeks after treatment. “A distinct advantage of HA is that imperfections or undesired product can usually be reversed in the short term using hyaluronidase enzyme,” they added.
Despite the common adverse effects of erythema, pruritus, ecchymosis, and edema, “hand augmentation with soft tissue filler, alone or in conjunction with other rejuvenation modalities, can have a significant impact on improving appearance of the dorsal hands,” satisfying patient cosmetic objectives, the authors concluded.
Read the full article in the Journal of Drugs in Dermatology (J Drugs Dermatol. 2016;15[7]:809-15).
Cosmetic dermatology patients are increasingly seeking hand rejuvenation procedures to eliminate appearance discrepancies between the face and hands, reported Ramin Fathi, MD, and Joel L. Cohen, MD, FAAD, who reviewed the benefits and complications of soft filler methods.
Pre-procedure evaluation includes assessing volume of lost fat from the skin via the Merz hand grading scale. Prior to augmentation, a detailed patient history, including medications, medical problems, and bleeding abnormalities, is typically taken, as well as assessment of patient lifestyle issues involving extensive hand activity, such as typing on a keyboard or playing piano. The authors reviewed three hand rejuvenation methods: autologous fat grafting, hyaluronic acid (HA), and poly-L-lactic acid (PLLA).
Although autologous fat grafting is still done in some practices, it is more invasive compared with soft tissue fillers, and can result in complications such as infection, cyst formation, temporary dysesthesia, and significant edema, the authors noted. PLLA is not approved for dorsal hand augmentation, and is used off label.
Hyaluronic acid (HA) fillers such as Restylane, Belotero, and Juvederm are approved only in the United States for facial soft tissue augmentation, and are also used off label for hand rejuvenation. The authors cited a small study of 16 patients evaluating small gel particle HA in the dorsal hand, in which vascular, tendon, bony prominence, and skin turgor were improved by 60.9%, 65.2%, 73.7%, and 26.3%, respectively, 2 weeks after treatment. “A distinct advantage of HA is that imperfections or undesired product can usually be reversed in the short term using hyaluronidase enzyme,” they added.
Despite the common adverse effects of erythema, pruritus, ecchymosis, and edema, “hand augmentation with soft tissue filler, alone or in conjunction with other rejuvenation modalities, can have a significant impact on improving appearance of the dorsal hands,” satisfying patient cosmetic objectives, the authors concluded.
Read the full article in the Journal of Drugs in Dermatology (J Drugs Dermatol. 2016;15[7]:809-15).
Cosmetic dermatology patients are increasingly seeking hand rejuvenation procedures to eliminate appearance discrepancies between the face and hands, reported Ramin Fathi, MD, and Joel L. Cohen, MD, FAAD, who reviewed the benefits and complications of soft filler methods.
Pre-procedure evaluation includes assessing volume of lost fat from the skin via the Merz hand grading scale. Prior to augmentation, a detailed patient history, including medications, medical problems, and bleeding abnormalities, is typically taken, as well as assessment of patient lifestyle issues involving extensive hand activity, such as typing on a keyboard or playing piano. The authors reviewed three hand rejuvenation methods: autologous fat grafting, hyaluronic acid (HA), and poly-L-lactic acid (PLLA).
Although autologous fat grafting is still done in some practices, it is more invasive compared with soft tissue fillers, and can result in complications such as infection, cyst formation, temporary dysesthesia, and significant edema, the authors noted. PLLA is not approved for dorsal hand augmentation, and is used off label.
Hyaluronic acid (HA) fillers such as Restylane, Belotero, and Juvederm are approved only in the United States for facial soft tissue augmentation, and are also used off label for hand rejuvenation. The authors cited a small study of 16 patients evaluating small gel particle HA in the dorsal hand, in which vascular, tendon, bony prominence, and skin turgor were improved by 60.9%, 65.2%, 73.7%, and 26.3%, respectively, 2 weeks after treatment. “A distinct advantage of HA is that imperfections or undesired product can usually be reversed in the short term using hyaluronidase enzyme,” they added.
Despite the common adverse effects of erythema, pruritus, ecchymosis, and edema, “hand augmentation with soft tissue filler, alone or in conjunction with other rejuvenation modalities, can have a significant impact on improving appearance of the dorsal hands,” satisfying patient cosmetic objectives, the authors concluded.
Read the full article in the Journal of Drugs in Dermatology (J Drugs Dermatol. 2016;15[7]:809-15).
FROM THE JOURNAL OF DRUGS IN DERMATOLOGY
Malpractice Counsel: Retained foreign body, ruptured esophagus
Retained Foreign Body
A 15-year-old male adolescent was brought to the ED by his father for evaluation of lacerations on the teenager’s left forearm, which were caused by a shattered glass door. The accident happened approximately 45 minutes prior to the patient’s arrival at the ED. The patient was up to date on all of his immunizations, including tetanus, and had no significant medical history.
On physical examination, the patient’s vital signs were all normal. He was noted to have two lacerations on the volar aspect of the distal one-third of his left forearm. One laceration measured 2.5 cm, running diagonally on the forearm; the other laceration was approximately 2 cm, running horizontally on the forearm. The bleeding from both wound sites was easily controlled with pressure.
The emergency physician (EP) did not document a neurological examination of the left wrist and hand. He did, however, note that the patient had a 2+ radial pulse and good capillary refill. The EP irrigated the wounds thoroughly and sutured the two lacerations. There was no documentation on file of wound exploration or imaging studies. The patient returned 1 week after discharge from the ED for a wound check, and again 6 weeks later. On both occasions, he continued to complain of pain and decreased function of his left thumb and index finger.
Since the patient’s condition did not improve, his father took him to an orthopedic surgeon. The orthopedist ordered a magnetic resonance imaging (MRI) study of the left forearm, which demonstrated a complete tear of one of the patient’s flexor tendons. The orthopedist thought it was too late to repair the tendon and referred the patient to physical therapy. As the patient continued to complain of pain and decreased function of his left thumb, he consulted a second orthopedist, who decided to surgically explore the wound to determine the cause of the patient’s continued pain and loss of thumb function. Surgical exploration revealed a piece of glass measuring 3.5 x 2 cm retained in the patient’s forearm. The orthopedist removed the glass, irrigated the wound thoroughly, and closed the incision, after which the patient’s thumb function improved considerably and his pain resolved.
The patient’s family sued the EP and the hospital, arguing that the wound should have been explored and the glass removed on the initial ED visit. They further stated that if these steps were performed initially, the patient would not have required multiple imaging studies and surgery. At trial, the jury returned a defense verdict.
Discussion
Approximately 11 million wounds are treated in US EDs each year.1 Proper management of lacerations and wounds requires more than sutures or staples. The EP must also evaluate for associated injuries (eg, tendon laceration, vascular injury), and the possibility of a retained foreign body. It is also important to ensure the patient is up to date on his or her tetanus immunization.
As with most areas of medicine, a good history and physical examination are essential. The mechanism of injury will often be the first clue to the risk of a retained foreign body. For example, shattered glass or porcelain carries a much higher risk of retention compared to a laceration from a box cutter.
The age of the injury is also important in determining the best management approach and the risk of infection. In a study by Brancto,1 wounds closed within 19 hours of injury had a 92% rate of healing without infection, compared to only 77% of those closed after 19 hours. In addition, determination of a patient’s allergy status to anesthetics and antibiotics ensures safe and appropriate treatment.
On physical examination, the wound should be described in sufficient detail (eg, length, shape), and a distal neurovascular examination should be completed and documented. This involves testing the patient’s motor strength, sensation, adequacy of pulses, and capillary refill. When examining the extremities, flexion and extension strength should also be assessed and documented.
After a wound is prepped and anesthetized, it should be explored. Often a patient may have excellent flexor or extensor strength on testing, but have a near-complete tendon laceration on visual inspection. Similarly, the wound should be explored for foreign bodies. It is important to identify and remove foreign bodies because of the associated increased risk of infection, pain, and delayed healing.1 Occasionally, a wound may need to be extended to remove a foreign body.
Unfortunately, visual inspection of a wound, especially a deep one, is not highly sensitive. If a physician has a high index of suspicion for a retained foreign body but is unable to identify one on examination, imaging studies should be ordered. Conventional plain radiography, ultrasonography, computed tomography (CT), and MRI studies can all be used to identify foreign bodies. Each of these modalities has its unique advantages and disadvantages. A recent study by Pattamapaspong et al2 compared the accuracy of radiography, CT, and MRI in detecting foreign bodies in the foot. In this study, researchers placed various types of foreign bodies, including fresh wood, dry wood, glass, porcelain, and plastic—all measuring 5 x 2 mm— in cadaver feet.2 The overall sensitivity and specificity for foreign body detection was 29% and 100%, respectively, for radiographs; 63% and 98%, respectively, for CT; and 58% and 100%, respectively, for MRI.2 Interestingly, CT was superior to MRI in identifying water-rich fresh wood.2 A similar study by Aras et al3 compared the sensitivity of plain radiographs, CT, and ultrasound in detecting foreign bodies in the face. The foreign bodies used in this study measured 1 x 1 x 1 cm and included metal, glass, wood, stone, acrylic, graphite, and polyoxybenzylmethylenglycolanhydride (ie, Bakelite).3 In this study, ultrasound identified superficial foreign bodies with low radiopacity in body tissues more effectively than CT or plain radiographs.3 In a review by Karabay4 of traumatic wrist and hand injuries, ultrasound was considered the best modality to identify and locate both opaque and radiolucent foreign bodies in the soft tissue.
If a foreign body is identified but cannot be removed, consultation with a surgical service is required. Depending on the local referral pattern, this might be general surgery, plastic surgery, or hand surgery. Unless there is an acute nerve or vascular injury, patients rarely require immediate surgery. In most cases, the wound can be closed loosely until the surgeon can remove the foreign body in the operating room and/or with aid of fluoroscopy at a later time. Depending on the size, material, and location of the foreign body, the surgeon might even elect to simply observe.
The bottom-line lesson from this case: depending on the mechanism of injury, EPs must maintain a high index of suspicion for retained foreign bodies in traumatic wounds. In addition to wound exploration, imaging studies should be used in patients at high risk for a retained foreign body, such as those injured with broken glass or porcelain, but in whom no foreign body is found on wound exploration.
Ruptured Esophagus
A 78-year-old man presented to the ED with symptoms of choking and chest discomfort. The patient stated that he had experienced a sudden onset of difficulty swallowing, along with chest pain, while he was eating dinner at a restaurant earlier that evening. The patient initially thought he had a piece of carrot stuck in his throat. He denied any previous history of similar symptoms. He complained of mild shortness of breath, but denied any drooling or vomiting. His medical history was significant for hypertension, which was controlled with medication. He denied tobacco or alcohol use and had no known drug allergies.
On physical examination, the patient’s vital signs were: heart rate (HR), 106 beats/minute; blood pressure (BP), 144/82 mm Hg; respiratory rate, 22 breaths/minute, and temperature, 98.6°F. Oxygen saturation was 95% on room air. The patient’s oropharynx appeared normal and without foreign body obstruction; his lungs were clear to auscultation bilaterally; and his HR was tachycardic but with a regular rhythm. Other than mild diaphoresis, the remainder of the physical examination was normal.
The EP ordered a complete blood count (CBC), a basic metabolic profile (BMP), and a portable chest X-ray, which the EP interpreted as normal. In addition, an intravenous (IV) saline lock was placed, and the patient was given morphine 4 mg IV and ondansetron 4 mg IV. He was also placed on 2 L of oxygen via nasal cannula. Since the patient continued to complain of chest pain and dysphagia, the EP consulted with a gastroenterologist; unfortunately, there was no documentation of this.
The EP admitted the patient to the floor with a diagnosis of esophageal obstruction, probably secondary to a piece of carrot. During the night, the patient’s shortness of breath worsened, requiring an increase in supplemental oxygen. The next morning, the patient’s HR increased to 120 beats/minute; his BP dropped to 96/50 mm Hg, and he developed a low-grade fever. He was transferred to the intensive care unit, where he was started on IV fluid resuscitation with normal saline and broad spectrum antibiotics. A CT scan of the chest was also ordered, which revealed an esophageal perforation. The patient was taken immediately to the operating room; surgery revealed a large esophageal perforation with evidence of mediastinitis and gross contamination of the left hemithorax. The patient died 2 days later.
The patient’s family sued the EP for failure to diagnose and treat the esophageal perforation in a timely manner. The EP argued that the patient’s symptoms were consistent with an obstruction, not esophageal perforation. The defendant also argued that the initial chest X-ray was normal. The case was resolved for $800,000 prior to going to trial.
Discussion
Esophageal perforation is a true medical emergency that requires timely diagnosis and management because morbidity and mortality are directly related to the time to treatment. Unfortunately, esophageal perforation can be a difficult diagnosis due to its relative rarity and variability in clinical presentation.
More than 50% of all esophageal perforations are iatrogenic, primarily as a complication of endoscopy.1 Other causes of perforation include spontaneous perforation or Boerhaave syndrome (15%), foreign body (12%), trauma (9%), and malignancy (1%).1 Anatomically, perforation tends to occur in the areas of the esophagus that are most narrow—eg, cricopharyngeus muscle, area of broncho-aortic constriction, and esophagogastric junction.1
Food impactions, not surprisingly, tend to occur in these same areas of the esophagus. In addition, there are structural esophageal abnormalities that increase the risk of food impaction, including diverticula, webs, rings, strictures, achalasia, and tumors.2 Since food impaction can result in an esophageal perforation, there is a significant overlap in the initial presentation of these two conditions. However, in cases of perforation, signs and symptoms of shock predominate as time progresses due to esophageal contents leaking into the mediastinal and pleural spaces.
Patients with a food impaction will often complain of an acute onset of dysphagia, difficulty in handling secretions, choking, drooling, retrosternal fullness, regurgitation of undigested food, and wheezing.2 Perforation can cause severe chest pain, tachypnea, dyspnea, fever, and shock.2
A chest X-ray is typically the initial imaging study for suspected esophageal perforation. Since most spontaneous perforations occur through the left posterolateral wall of the distal esophagus, a new left pleural effusion can frequently be seen on X-ray. Mediastinal emphysema is highly suspicious for perforation, but the condition takes time to develop; therefore, its absence on X-ray does not exclude perforation. In the setting of a normal chest X-ray and ongoing esophageal symptoms, further investigation is required, usually via CT scan or endoscopy. Computed tomography, because of its availability and speed, is usually the preferred study to confirm the diagnosis.
Once an esophageal perforation is confirmed or is highly suspected, the patient will require IV fluid resuscitation, IV broad-spectrum antibiotic treatment, and emergency surgical consultation. As previously stated, esophageal perforation is associated with a high mortality rate, and time is critical to successful management.
- Retained Foreign Body
1. Brancto JC. Minor wound preparation and irrigation. http://www.uptodate.com/contents/minor-wound-preparation-and-irrigation. Accessed June 1, 2016.
2. Pattamapaspong N, Srisuwan T, Sivasomboon C, et al. Accuracy of radiography, computed tomography and magnetic resonance imaging in diagnosing foreign bodies in the foot. Radiol Med. 2013;118(2):303-310.
3. Aras MH, Miloglu O, Barutcugil C, Kantarci M, Ozcan E, Harorli A. Comparison of the sensitivity for detecting foreign bodies among conventional plain radiography, computed tomography and ultrasonography. Dentomaxillofac Radiol. 2010;39(2):72-78.
4. Karabay N. US findings in traumatic wrist and hand injuries. Diagn Interv Radiol. 2013;19(4):320-325.
- Ruptured Esophagus
1. Raymond DP, Jones C. Surgical management of esophageal perforation. http://www.uptodate.com/contents/surgical-management-of-esophageal-perforation. Accessed June 27, 2016.
2. Triadafilopoulos G. Ingested foreign bodies and food impaction in adults. http://www.uptodate.com/contents/ingested-foreign-bodies-and-food-impactions-in-adults. Accessed June 27, 2016.
Retained Foreign Body
A 15-year-old male adolescent was brought to the ED by his father for evaluation of lacerations on the teenager’s left forearm, which were caused by a shattered glass door. The accident happened approximately 45 minutes prior to the patient’s arrival at the ED. The patient was up to date on all of his immunizations, including tetanus, and had no significant medical history.
On physical examination, the patient’s vital signs were all normal. He was noted to have two lacerations on the volar aspect of the distal one-third of his left forearm. One laceration measured 2.5 cm, running diagonally on the forearm; the other laceration was approximately 2 cm, running horizontally on the forearm. The bleeding from both wound sites was easily controlled with pressure.
The emergency physician (EP) did not document a neurological examination of the left wrist and hand. He did, however, note that the patient had a 2+ radial pulse and good capillary refill. The EP irrigated the wounds thoroughly and sutured the two lacerations. There was no documentation on file of wound exploration or imaging studies. The patient returned 1 week after discharge from the ED for a wound check, and again 6 weeks later. On both occasions, he continued to complain of pain and decreased function of his left thumb and index finger.
Since the patient’s condition did not improve, his father took him to an orthopedic surgeon. The orthopedist ordered a magnetic resonance imaging (MRI) study of the left forearm, which demonstrated a complete tear of one of the patient’s flexor tendons. The orthopedist thought it was too late to repair the tendon and referred the patient to physical therapy. As the patient continued to complain of pain and decreased function of his left thumb, he consulted a second orthopedist, who decided to surgically explore the wound to determine the cause of the patient’s continued pain and loss of thumb function. Surgical exploration revealed a piece of glass measuring 3.5 x 2 cm retained in the patient’s forearm. The orthopedist removed the glass, irrigated the wound thoroughly, and closed the incision, after which the patient’s thumb function improved considerably and his pain resolved.
The patient’s family sued the EP and the hospital, arguing that the wound should have been explored and the glass removed on the initial ED visit. They further stated that if these steps were performed initially, the patient would not have required multiple imaging studies and surgery. At trial, the jury returned a defense verdict.
Discussion
Approximately 11 million wounds are treated in US EDs each year.1 Proper management of lacerations and wounds requires more than sutures or staples. The EP must also evaluate for associated injuries (eg, tendon laceration, vascular injury), and the possibility of a retained foreign body. It is also important to ensure the patient is up to date on his or her tetanus immunization.
As with most areas of medicine, a good history and physical examination are essential. The mechanism of injury will often be the first clue to the risk of a retained foreign body. For example, shattered glass or porcelain carries a much higher risk of retention compared to a laceration from a box cutter.
The age of the injury is also important in determining the best management approach and the risk of infection. In a study by Brancto,1 wounds closed within 19 hours of injury had a 92% rate of healing without infection, compared to only 77% of those closed after 19 hours. In addition, determination of a patient’s allergy status to anesthetics and antibiotics ensures safe and appropriate treatment.
On physical examination, the wound should be described in sufficient detail (eg, length, shape), and a distal neurovascular examination should be completed and documented. This involves testing the patient’s motor strength, sensation, adequacy of pulses, and capillary refill. When examining the extremities, flexion and extension strength should also be assessed and documented.
After a wound is prepped and anesthetized, it should be explored. Often a patient may have excellent flexor or extensor strength on testing, but have a near-complete tendon laceration on visual inspection. Similarly, the wound should be explored for foreign bodies. It is important to identify and remove foreign bodies because of the associated increased risk of infection, pain, and delayed healing.1 Occasionally, a wound may need to be extended to remove a foreign body.
Unfortunately, visual inspection of a wound, especially a deep one, is not highly sensitive. If a physician has a high index of suspicion for a retained foreign body but is unable to identify one on examination, imaging studies should be ordered. Conventional plain radiography, ultrasonography, computed tomography (CT), and MRI studies can all be used to identify foreign bodies. Each of these modalities has its unique advantages and disadvantages. A recent study by Pattamapaspong et al2 compared the accuracy of radiography, CT, and MRI in detecting foreign bodies in the foot. In this study, researchers placed various types of foreign bodies, including fresh wood, dry wood, glass, porcelain, and plastic—all measuring 5 x 2 mm— in cadaver feet.2 The overall sensitivity and specificity for foreign body detection was 29% and 100%, respectively, for radiographs; 63% and 98%, respectively, for CT; and 58% and 100%, respectively, for MRI.2 Interestingly, CT was superior to MRI in identifying water-rich fresh wood.2 A similar study by Aras et al3 compared the sensitivity of plain radiographs, CT, and ultrasound in detecting foreign bodies in the face. The foreign bodies used in this study measured 1 x 1 x 1 cm and included metal, glass, wood, stone, acrylic, graphite, and polyoxybenzylmethylenglycolanhydride (ie, Bakelite).3 In this study, ultrasound identified superficial foreign bodies with low radiopacity in body tissues more effectively than CT or plain radiographs.3 In a review by Karabay4 of traumatic wrist and hand injuries, ultrasound was considered the best modality to identify and locate both opaque and radiolucent foreign bodies in the soft tissue.
If a foreign body is identified but cannot be removed, consultation with a surgical service is required. Depending on the local referral pattern, this might be general surgery, plastic surgery, or hand surgery. Unless there is an acute nerve or vascular injury, patients rarely require immediate surgery. In most cases, the wound can be closed loosely until the surgeon can remove the foreign body in the operating room and/or with aid of fluoroscopy at a later time. Depending on the size, material, and location of the foreign body, the surgeon might even elect to simply observe.
The bottom-line lesson from this case: depending on the mechanism of injury, EPs must maintain a high index of suspicion for retained foreign bodies in traumatic wounds. In addition to wound exploration, imaging studies should be used in patients at high risk for a retained foreign body, such as those injured with broken glass or porcelain, but in whom no foreign body is found on wound exploration.
Ruptured Esophagus
A 78-year-old man presented to the ED with symptoms of choking and chest discomfort. The patient stated that he had experienced a sudden onset of difficulty swallowing, along with chest pain, while he was eating dinner at a restaurant earlier that evening. The patient initially thought he had a piece of carrot stuck in his throat. He denied any previous history of similar symptoms. He complained of mild shortness of breath, but denied any drooling or vomiting. His medical history was significant for hypertension, which was controlled with medication. He denied tobacco or alcohol use and had no known drug allergies.
On physical examination, the patient’s vital signs were: heart rate (HR), 106 beats/minute; blood pressure (BP), 144/82 mm Hg; respiratory rate, 22 breaths/minute, and temperature, 98.6°F. Oxygen saturation was 95% on room air. The patient’s oropharynx appeared normal and without foreign body obstruction; his lungs were clear to auscultation bilaterally; and his HR was tachycardic but with a regular rhythm. Other than mild diaphoresis, the remainder of the physical examination was normal.
The EP ordered a complete blood count (CBC), a basic metabolic profile (BMP), and a portable chest X-ray, which the EP interpreted as normal. In addition, an intravenous (IV) saline lock was placed, and the patient was given morphine 4 mg IV and ondansetron 4 mg IV. He was also placed on 2 L of oxygen via nasal cannula. Since the patient continued to complain of chest pain and dysphagia, the EP consulted with a gastroenterologist; unfortunately, there was no documentation of this.
The EP admitted the patient to the floor with a diagnosis of esophageal obstruction, probably secondary to a piece of carrot. During the night, the patient’s shortness of breath worsened, requiring an increase in supplemental oxygen. The next morning, the patient’s HR increased to 120 beats/minute; his BP dropped to 96/50 mm Hg, and he developed a low-grade fever. He was transferred to the intensive care unit, where he was started on IV fluid resuscitation with normal saline and broad spectrum antibiotics. A CT scan of the chest was also ordered, which revealed an esophageal perforation. The patient was taken immediately to the operating room; surgery revealed a large esophageal perforation with evidence of mediastinitis and gross contamination of the left hemithorax. The patient died 2 days later.
The patient’s family sued the EP for failure to diagnose and treat the esophageal perforation in a timely manner. The EP argued that the patient’s symptoms were consistent with an obstruction, not esophageal perforation. The defendant also argued that the initial chest X-ray was normal. The case was resolved for $800,000 prior to going to trial.
Discussion
Esophageal perforation is a true medical emergency that requires timely diagnosis and management because morbidity and mortality are directly related to the time to treatment. Unfortunately, esophageal perforation can be a difficult diagnosis due to its relative rarity and variability in clinical presentation.
More than 50% of all esophageal perforations are iatrogenic, primarily as a complication of endoscopy.1 Other causes of perforation include spontaneous perforation or Boerhaave syndrome (15%), foreign body (12%), trauma (9%), and malignancy (1%).1 Anatomically, perforation tends to occur in the areas of the esophagus that are most narrow—eg, cricopharyngeus muscle, area of broncho-aortic constriction, and esophagogastric junction.1
Food impactions, not surprisingly, tend to occur in these same areas of the esophagus. In addition, there are structural esophageal abnormalities that increase the risk of food impaction, including diverticula, webs, rings, strictures, achalasia, and tumors.2 Since food impaction can result in an esophageal perforation, there is a significant overlap in the initial presentation of these two conditions. However, in cases of perforation, signs and symptoms of shock predominate as time progresses due to esophageal contents leaking into the mediastinal and pleural spaces.
Patients with a food impaction will often complain of an acute onset of dysphagia, difficulty in handling secretions, choking, drooling, retrosternal fullness, regurgitation of undigested food, and wheezing.2 Perforation can cause severe chest pain, tachypnea, dyspnea, fever, and shock.2
A chest X-ray is typically the initial imaging study for suspected esophageal perforation. Since most spontaneous perforations occur through the left posterolateral wall of the distal esophagus, a new left pleural effusion can frequently be seen on X-ray. Mediastinal emphysema is highly suspicious for perforation, but the condition takes time to develop; therefore, its absence on X-ray does not exclude perforation. In the setting of a normal chest X-ray and ongoing esophageal symptoms, further investigation is required, usually via CT scan or endoscopy. Computed tomography, because of its availability and speed, is usually the preferred study to confirm the diagnosis.
Once an esophageal perforation is confirmed or is highly suspected, the patient will require IV fluid resuscitation, IV broad-spectrum antibiotic treatment, and emergency surgical consultation. As previously stated, esophageal perforation is associated with a high mortality rate, and time is critical to successful management.
Retained Foreign Body
A 15-year-old male adolescent was brought to the ED by his father for evaluation of lacerations on the teenager’s left forearm, which were caused by a shattered glass door. The accident happened approximately 45 minutes prior to the patient’s arrival at the ED. The patient was up to date on all of his immunizations, including tetanus, and had no significant medical history.
On physical examination, the patient’s vital signs were all normal. He was noted to have two lacerations on the volar aspect of the distal one-third of his left forearm. One laceration measured 2.5 cm, running diagonally on the forearm; the other laceration was approximately 2 cm, running horizontally on the forearm. The bleeding from both wound sites was easily controlled with pressure.
The emergency physician (EP) did not document a neurological examination of the left wrist and hand. He did, however, note that the patient had a 2+ radial pulse and good capillary refill. The EP irrigated the wounds thoroughly and sutured the two lacerations. There was no documentation on file of wound exploration or imaging studies. The patient returned 1 week after discharge from the ED for a wound check, and again 6 weeks later. On both occasions, he continued to complain of pain and decreased function of his left thumb and index finger.
Since the patient’s condition did not improve, his father took him to an orthopedic surgeon. The orthopedist ordered a magnetic resonance imaging (MRI) study of the left forearm, which demonstrated a complete tear of one of the patient’s flexor tendons. The orthopedist thought it was too late to repair the tendon and referred the patient to physical therapy. As the patient continued to complain of pain and decreased function of his left thumb, he consulted a second orthopedist, who decided to surgically explore the wound to determine the cause of the patient’s continued pain and loss of thumb function. Surgical exploration revealed a piece of glass measuring 3.5 x 2 cm retained in the patient’s forearm. The orthopedist removed the glass, irrigated the wound thoroughly, and closed the incision, after which the patient’s thumb function improved considerably and his pain resolved.
The patient’s family sued the EP and the hospital, arguing that the wound should have been explored and the glass removed on the initial ED visit. They further stated that if these steps were performed initially, the patient would not have required multiple imaging studies and surgery. At trial, the jury returned a defense verdict.
Discussion
Approximately 11 million wounds are treated in US EDs each year.1 Proper management of lacerations and wounds requires more than sutures or staples. The EP must also evaluate for associated injuries (eg, tendon laceration, vascular injury), and the possibility of a retained foreign body. It is also important to ensure the patient is up to date on his or her tetanus immunization.
As with most areas of medicine, a good history and physical examination are essential. The mechanism of injury will often be the first clue to the risk of a retained foreign body. For example, shattered glass or porcelain carries a much higher risk of retention compared to a laceration from a box cutter.
The age of the injury is also important in determining the best management approach and the risk of infection. In a study by Brancto,1 wounds closed within 19 hours of injury had a 92% rate of healing without infection, compared to only 77% of those closed after 19 hours. In addition, determination of a patient’s allergy status to anesthetics and antibiotics ensures safe and appropriate treatment.
On physical examination, the wound should be described in sufficient detail (eg, length, shape), and a distal neurovascular examination should be completed and documented. This involves testing the patient’s motor strength, sensation, adequacy of pulses, and capillary refill. When examining the extremities, flexion and extension strength should also be assessed and documented.
After a wound is prepped and anesthetized, it should be explored. Often a patient may have excellent flexor or extensor strength on testing, but have a near-complete tendon laceration on visual inspection. Similarly, the wound should be explored for foreign bodies. It is important to identify and remove foreign bodies because of the associated increased risk of infection, pain, and delayed healing.1 Occasionally, a wound may need to be extended to remove a foreign body.
Unfortunately, visual inspection of a wound, especially a deep one, is not highly sensitive. If a physician has a high index of suspicion for a retained foreign body but is unable to identify one on examination, imaging studies should be ordered. Conventional plain radiography, ultrasonography, computed tomography (CT), and MRI studies can all be used to identify foreign bodies. Each of these modalities has its unique advantages and disadvantages. A recent study by Pattamapaspong et al2 compared the accuracy of radiography, CT, and MRI in detecting foreign bodies in the foot. In this study, researchers placed various types of foreign bodies, including fresh wood, dry wood, glass, porcelain, and plastic—all measuring 5 x 2 mm— in cadaver feet.2 The overall sensitivity and specificity for foreign body detection was 29% and 100%, respectively, for radiographs; 63% and 98%, respectively, for CT; and 58% and 100%, respectively, for MRI.2 Interestingly, CT was superior to MRI in identifying water-rich fresh wood.2 A similar study by Aras et al3 compared the sensitivity of plain radiographs, CT, and ultrasound in detecting foreign bodies in the face. The foreign bodies used in this study measured 1 x 1 x 1 cm and included metal, glass, wood, stone, acrylic, graphite, and polyoxybenzylmethylenglycolanhydride (ie, Bakelite).3 In this study, ultrasound identified superficial foreign bodies with low radiopacity in body tissues more effectively than CT or plain radiographs.3 In a review by Karabay4 of traumatic wrist and hand injuries, ultrasound was considered the best modality to identify and locate both opaque and radiolucent foreign bodies in the soft tissue.
If a foreign body is identified but cannot be removed, consultation with a surgical service is required. Depending on the local referral pattern, this might be general surgery, plastic surgery, or hand surgery. Unless there is an acute nerve or vascular injury, patients rarely require immediate surgery. In most cases, the wound can be closed loosely until the surgeon can remove the foreign body in the operating room and/or with aid of fluoroscopy at a later time. Depending on the size, material, and location of the foreign body, the surgeon might even elect to simply observe.
The bottom-line lesson from this case: depending on the mechanism of injury, EPs must maintain a high index of suspicion for retained foreign bodies in traumatic wounds. In addition to wound exploration, imaging studies should be used in patients at high risk for a retained foreign body, such as those injured with broken glass or porcelain, but in whom no foreign body is found on wound exploration.
Ruptured Esophagus
A 78-year-old man presented to the ED with symptoms of choking and chest discomfort. The patient stated that he had experienced a sudden onset of difficulty swallowing, along with chest pain, while he was eating dinner at a restaurant earlier that evening. The patient initially thought he had a piece of carrot stuck in his throat. He denied any previous history of similar symptoms. He complained of mild shortness of breath, but denied any drooling or vomiting. His medical history was significant for hypertension, which was controlled with medication. He denied tobacco or alcohol use and had no known drug allergies.
On physical examination, the patient’s vital signs were: heart rate (HR), 106 beats/minute; blood pressure (BP), 144/82 mm Hg; respiratory rate, 22 breaths/minute, and temperature, 98.6°F. Oxygen saturation was 95% on room air. The patient’s oropharynx appeared normal and without foreign body obstruction; his lungs were clear to auscultation bilaterally; and his HR was tachycardic but with a regular rhythm. Other than mild diaphoresis, the remainder of the physical examination was normal.
The EP ordered a complete blood count (CBC), a basic metabolic profile (BMP), and a portable chest X-ray, which the EP interpreted as normal. In addition, an intravenous (IV) saline lock was placed, and the patient was given morphine 4 mg IV and ondansetron 4 mg IV. He was also placed on 2 L of oxygen via nasal cannula. Since the patient continued to complain of chest pain and dysphagia, the EP consulted with a gastroenterologist; unfortunately, there was no documentation of this.
The EP admitted the patient to the floor with a diagnosis of esophageal obstruction, probably secondary to a piece of carrot. During the night, the patient’s shortness of breath worsened, requiring an increase in supplemental oxygen. The next morning, the patient’s HR increased to 120 beats/minute; his BP dropped to 96/50 mm Hg, and he developed a low-grade fever. He was transferred to the intensive care unit, where he was started on IV fluid resuscitation with normal saline and broad spectrum antibiotics. A CT scan of the chest was also ordered, which revealed an esophageal perforation. The patient was taken immediately to the operating room; surgery revealed a large esophageal perforation with evidence of mediastinitis and gross contamination of the left hemithorax. The patient died 2 days later.
The patient’s family sued the EP for failure to diagnose and treat the esophageal perforation in a timely manner. The EP argued that the patient’s symptoms were consistent with an obstruction, not esophageal perforation. The defendant also argued that the initial chest X-ray was normal. The case was resolved for $800,000 prior to going to trial.
Discussion
Esophageal perforation is a true medical emergency that requires timely diagnosis and management because morbidity and mortality are directly related to the time to treatment. Unfortunately, esophageal perforation can be a difficult diagnosis due to its relative rarity and variability in clinical presentation.
More than 50% of all esophageal perforations are iatrogenic, primarily as a complication of endoscopy.1 Other causes of perforation include spontaneous perforation or Boerhaave syndrome (15%), foreign body (12%), trauma (9%), and malignancy (1%).1 Anatomically, perforation tends to occur in the areas of the esophagus that are most narrow—eg, cricopharyngeus muscle, area of broncho-aortic constriction, and esophagogastric junction.1
Food impactions, not surprisingly, tend to occur in these same areas of the esophagus. In addition, there are structural esophageal abnormalities that increase the risk of food impaction, including diverticula, webs, rings, strictures, achalasia, and tumors.2 Since food impaction can result in an esophageal perforation, there is a significant overlap in the initial presentation of these two conditions. However, in cases of perforation, signs and symptoms of shock predominate as time progresses due to esophageal contents leaking into the mediastinal and pleural spaces.
Patients with a food impaction will often complain of an acute onset of dysphagia, difficulty in handling secretions, choking, drooling, retrosternal fullness, regurgitation of undigested food, and wheezing.2 Perforation can cause severe chest pain, tachypnea, dyspnea, fever, and shock.2
A chest X-ray is typically the initial imaging study for suspected esophageal perforation. Since most spontaneous perforations occur through the left posterolateral wall of the distal esophagus, a new left pleural effusion can frequently be seen on X-ray. Mediastinal emphysema is highly suspicious for perforation, but the condition takes time to develop; therefore, its absence on X-ray does not exclude perforation. In the setting of a normal chest X-ray and ongoing esophageal symptoms, further investigation is required, usually via CT scan or endoscopy. Computed tomography, because of its availability and speed, is usually the preferred study to confirm the diagnosis.
Once an esophageal perforation is confirmed or is highly suspected, the patient will require IV fluid resuscitation, IV broad-spectrum antibiotic treatment, and emergency surgical consultation. As previously stated, esophageal perforation is associated with a high mortality rate, and time is critical to successful management.
- Retained Foreign Body
1. Brancto JC. Minor wound preparation and irrigation. http://www.uptodate.com/contents/minor-wound-preparation-and-irrigation. Accessed June 1, 2016.
2. Pattamapaspong N, Srisuwan T, Sivasomboon C, et al. Accuracy of radiography, computed tomography and magnetic resonance imaging in diagnosing foreign bodies in the foot. Radiol Med. 2013;118(2):303-310.
3. Aras MH, Miloglu O, Barutcugil C, Kantarci M, Ozcan E, Harorli A. Comparison of the sensitivity for detecting foreign bodies among conventional plain radiography, computed tomography and ultrasonography. Dentomaxillofac Radiol. 2010;39(2):72-78.
4. Karabay N. US findings in traumatic wrist and hand injuries. Diagn Interv Radiol. 2013;19(4):320-325.
- Ruptured Esophagus
1. Raymond DP, Jones C. Surgical management of esophageal perforation. http://www.uptodate.com/contents/surgical-management-of-esophageal-perforation. Accessed June 27, 2016.
2. Triadafilopoulos G. Ingested foreign bodies and food impaction in adults. http://www.uptodate.com/contents/ingested-foreign-bodies-and-food-impactions-in-adults. Accessed June 27, 2016.
- Retained Foreign Body
1. Brancto JC. Minor wound preparation and irrigation. http://www.uptodate.com/contents/minor-wound-preparation-and-irrigation. Accessed June 1, 2016.
2. Pattamapaspong N, Srisuwan T, Sivasomboon C, et al. Accuracy of radiography, computed tomography and magnetic resonance imaging in diagnosing foreign bodies in the foot. Radiol Med. 2013;118(2):303-310.
3. Aras MH, Miloglu O, Barutcugil C, Kantarci M, Ozcan E, Harorli A. Comparison of the sensitivity for detecting foreign bodies among conventional plain radiography, computed tomography and ultrasonography. Dentomaxillofac Radiol. 2010;39(2):72-78.
4. Karabay N. US findings in traumatic wrist and hand injuries. Diagn Interv Radiol. 2013;19(4):320-325.
- Ruptured Esophagus
1. Raymond DP, Jones C. Surgical management of esophageal perforation. http://www.uptodate.com/contents/surgical-management-of-esophageal-perforation. Accessed June 27, 2016.
2. Triadafilopoulos G. Ingested foreign bodies and food impaction in adults. http://www.uptodate.com/contents/ingested-foreign-bodies-and-food-impactions-in-adults. Accessed June 27, 2016.
AAP Proposes Update to Evaluating, Managing Febrile Infants Guideline
Presenter: Kenneth Roberts, MD
One of PHM16’s most highly attended sessions was about anticipated updated from the American Academy of Pediatrics (AAP) to its guidelines for febrile infants ages 7¬–90 days. The proposed update stresses the need to separate individual components of serious bacterial infections (UTI, bacteremia, and meningitis) as the incidence and clinical course can vary greatly in this population.
The proposed inclusion criteria for infants for this algorithm require an infant to be full-term (37–43 weeks’ gestation), aged 7–90 days, well-appearing, and presenting with a temperature of 38°C. Proposed exclusion criteria include perinatal/prenatal/neonatal maternal fever, infection, or antimicrobial treatment; the presence of any evident infection; being technology-dependent; and the presence of congenital anomalies.
The proposed guideline update will aim to stratify management by ages 7–28 days, 29–60 days, and 61–90 days to provide the most appropriate and directed treatment. It will also include a role for inflammatory markers and allow for a “kinder, gentler” approach, including withholding certain treatments and procedures if infants are at low risk of infection. An active need for observation may be appropriate for certain infants as well.
Key Takeaway
It is likely that the AAP will update its algorithm for the management of well-appearing febrile infants ages 7–28 days, 29–60 days and 60–90 days. It will help standardize care in this population but should not be used as a substitute for clinical judgment. The new guidelines are expected to be published in early 2017.
Chandani DeZure, MD, FAAP, is a pediatric hospitalist at Children’s National Health System and instructor of pediatrics at George Washington University School of Medicine & Health Sciences in Washington, D.C.
Presenter: Kenneth Roberts, MD
One of PHM16’s most highly attended sessions was about anticipated updated from the American Academy of Pediatrics (AAP) to its guidelines for febrile infants ages 7¬–90 days. The proposed update stresses the need to separate individual components of serious bacterial infections (UTI, bacteremia, and meningitis) as the incidence and clinical course can vary greatly in this population.
The proposed inclusion criteria for infants for this algorithm require an infant to be full-term (37–43 weeks’ gestation), aged 7–90 days, well-appearing, and presenting with a temperature of 38°C. Proposed exclusion criteria include perinatal/prenatal/neonatal maternal fever, infection, or antimicrobial treatment; the presence of any evident infection; being technology-dependent; and the presence of congenital anomalies.
The proposed guideline update will aim to stratify management by ages 7–28 days, 29–60 days, and 61–90 days to provide the most appropriate and directed treatment. It will also include a role for inflammatory markers and allow for a “kinder, gentler” approach, including withholding certain treatments and procedures if infants are at low risk of infection. An active need for observation may be appropriate for certain infants as well.
Key Takeaway
It is likely that the AAP will update its algorithm for the management of well-appearing febrile infants ages 7–28 days, 29–60 days and 60–90 days. It will help standardize care in this population but should not be used as a substitute for clinical judgment. The new guidelines are expected to be published in early 2017.
Chandani DeZure, MD, FAAP, is a pediatric hospitalist at Children’s National Health System and instructor of pediatrics at George Washington University School of Medicine & Health Sciences in Washington, D.C.
Presenter: Kenneth Roberts, MD
One of PHM16’s most highly attended sessions was about anticipated updated from the American Academy of Pediatrics (AAP) to its guidelines for febrile infants ages 7¬–90 days. The proposed update stresses the need to separate individual components of serious bacterial infections (UTI, bacteremia, and meningitis) as the incidence and clinical course can vary greatly in this population.
The proposed inclusion criteria for infants for this algorithm require an infant to be full-term (37–43 weeks’ gestation), aged 7–90 days, well-appearing, and presenting with a temperature of 38°C. Proposed exclusion criteria include perinatal/prenatal/neonatal maternal fever, infection, or antimicrobial treatment; the presence of any evident infection; being technology-dependent; and the presence of congenital anomalies.
The proposed guideline update will aim to stratify management by ages 7–28 days, 29–60 days, and 61–90 days to provide the most appropriate and directed treatment. It will also include a role for inflammatory markers and allow for a “kinder, gentler” approach, including withholding certain treatments and procedures if infants are at low risk of infection. An active need for observation may be appropriate for certain infants as well.
Key Takeaway
It is likely that the AAP will update its algorithm for the management of well-appearing febrile infants ages 7–28 days, 29–60 days and 60–90 days. It will help standardize care in this population but should not be used as a substitute for clinical judgment. The new guidelines are expected to be published in early 2017.
Chandani DeZure, MD, FAAP, is a pediatric hospitalist at Children’s National Health System and instructor of pediatrics at George Washington University School of Medicine & Health Sciences in Washington, D.C.
First EDition: News for and about the practice of emergency medicine
Rate of ED Deaths Drops by Nearly 50%
BY JEFF BAUER
FROM HEALTH AFFAIRS
The rate of ED deaths dropped by almost half from 1997 to 2011, according to recent analysis of a large database of ED visits.
Researchers evaluated data from the National Hospital Ambulatory Medical Care Survey, which is conducted annually by the National Center for Health Statistics and is the largest nationally representative database of information about emergency conditions in the United States. For the 1997 through 2011 study period, it provided data on 1.3 billion ED visits by patients ages 18 years and older. Using data from the US Census Bureau, researchers calculated the ED mortality rate per 1,000 US adults. Death in the ED also included individuals who were dead upon arrival.
There were 1.48 ED deaths per 1,000 adults in 1997 and .77 deaths per 1,000 adults in 2011, the most recent year for which data are available. This is a 48% reduction. Compared to patients who survived to ED discharge or hospital admission, those who died in the ED were older, had higher triage scores, and were more likely to be male and white. The proportion of patients who visited a rural ED or an ED in the South was higher among those who died than among those who survived. For roughly 63% of ED deaths, the patient was in cardiopulmonary arrest, unconscious, or dead on arrival.
The study was not designed to identify possible reasons for the reduction in ED deaths. The authors suggested it was likely due to multiple causes, including an increase in palliative care, less frequent use of resuscitation efforts in the prehospital setting, and improvements in emergency medicine and public health.
1. Kanazaria HK, Probst MA, Hsia RY. Emergency department death rates dropped by nearly 50 percent, 1997-2011. Health Aff (Millwood). 2016;35(7):1303-1308.
AHA Issues Statement on Drugs That May Cause or Exacerbate Heart Failure
BY MITCHEL L. ZOLER
FRONTLINE MEDICAL NEWS
Many commonly used prescription drugs, many over-the-counter (OTC) agents, and several complementary or alternative medications can either trigger heart failure or exacerbate the disease in patients with existing heart failure, according to a scientific statement written by a committee of the American Heart Association (AHA).
This first-ever authoritative US overview of what is known about drugs that can affect heart failure was compiled to address an important practice issue for the large and growing number of US patients with heart failure, which is estimated to be nearly 6 million Americans, and “provide some guidance to healthcare providers in how to minimize polypharmacy, improve medication safety, as well as identify the medications that could exacerbate or cause heart failure,” said Robert L. Page II, PharmD, chair of the committee and a professor of clinical pharmacy at the University of Colorado at Denver, Aurora.
Although the comprehensive statement lists 88 distinct prescription drugs or drug classes as agents that pose major or moderate threats for causing or worsening heart failure, “from the American public’s perspective, importance should be placed on educating patients regarding the impact that OTC medications can have on their heart failure,” Dr Page said in an interview. “For example, nonsteroidal anti-inflammatory drugs like ibuprofen or naproxen can cause sodium and water retention and antagonize the effects of evidence-based heart failure pharmacotherapies. Additionally, OTC medications like pseudoephedrine, which many cough and cold products contain, can increase blood pressure and afterload,” he noted. The risks these drugs pose become even greater when they are taken at higher doses.
The full text of the AHA statement is available at http://bit.ly/29ELCXz.
1. Page RL 2nd, O’Bryant CL, Cheng D, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016 Jul 11. [Epub ahead of print]
Rate of ED Deaths Drops by Nearly 50%
BY JEFF BAUER
FROM HEALTH AFFAIRS
The rate of ED deaths dropped by almost half from 1997 to 2011, according to recent analysis of a large database of ED visits.
Researchers evaluated data from the National Hospital Ambulatory Medical Care Survey, which is conducted annually by the National Center for Health Statistics and is the largest nationally representative database of information about emergency conditions in the United States. For the 1997 through 2011 study period, it provided data on 1.3 billion ED visits by patients ages 18 years and older. Using data from the US Census Bureau, researchers calculated the ED mortality rate per 1,000 US adults. Death in the ED also included individuals who were dead upon arrival.
There were 1.48 ED deaths per 1,000 adults in 1997 and .77 deaths per 1,000 adults in 2011, the most recent year for which data are available. This is a 48% reduction. Compared to patients who survived to ED discharge or hospital admission, those who died in the ED were older, had higher triage scores, and were more likely to be male and white. The proportion of patients who visited a rural ED or an ED in the South was higher among those who died than among those who survived. For roughly 63% of ED deaths, the patient was in cardiopulmonary arrest, unconscious, or dead on arrival.
The study was not designed to identify possible reasons for the reduction in ED deaths. The authors suggested it was likely due to multiple causes, including an increase in palliative care, less frequent use of resuscitation efforts in the prehospital setting, and improvements in emergency medicine and public health.
1. Kanazaria HK, Probst MA, Hsia RY. Emergency department death rates dropped by nearly 50 percent, 1997-2011. Health Aff (Millwood). 2016;35(7):1303-1308.
AHA Issues Statement on Drugs That May Cause or Exacerbate Heart Failure
BY MITCHEL L. ZOLER
FRONTLINE MEDICAL NEWS
Many commonly used prescription drugs, many over-the-counter (OTC) agents, and several complementary or alternative medications can either trigger heart failure or exacerbate the disease in patients with existing heart failure, according to a scientific statement written by a committee of the American Heart Association (AHA).
This first-ever authoritative US overview of what is known about drugs that can affect heart failure was compiled to address an important practice issue for the large and growing number of US patients with heart failure, which is estimated to be nearly 6 million Americans, and “provide some guidance to healthcare providers in how to minimize polypharmacy, improve medication safety, as well as identify the medications that could exacerbate or cause heart failure,” said Robert L. Page II, PharmD, chair of the committee and a professor of clinical pharmacy at the University of Colorado at Denver, Aurora.
Although the comprehensive statement lists 88 distinct prescription drugs or drug classes as agents that pose major or moderate threats for causing or worsening heart failure, “from the American public’s perspective, importance should be placed on educating patients regarding the impact that OTC medications can have on their heart failure,” Dr Page said in an interview. “For example, nonsteroidal anti-inflammatory drugs like ibuprofen or naproxen can cause sodium and water retention and antagonize the effects of evidence-based heart failure pharmacotherapies. Additionally, OTC medications like pseudoephedrine, which many cough and cold products contain, can increase blood pressure and afterload,” he noted. The risks these drugs pose become even greater when they are taken at higher doses.
The full text of the AHA statement is available at http://bit.ly/29ELCXz.
1. Page RL 2nd, O’Bryant CL, Cheng D, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016 Jul 11. [Epub ahead of print]
Rate of ED Deaths Drops by Nearly 50%
BY JEFF BAUER
FROM HEALTH AFFAIRS
The rate of ED deaths dropped by almost half from 1997 to 2011, according to recent analysis of a large database of ED visits.
Researchers evaluated data from the National Hospital Ambulatory Medical Care Survey, which is conducted annually by the National Center for Health Statistics and is the largest nationally representative database of information about emergency conditions in the United States. For the 1997 through 2011 study period, it provided data on 1.3 billion ED visits by patients ages 18 years and older. Using data from the US Census Bureau, researchers calculated the ED mortality rate per 1,000 US adults. Death in the ED also included individuals who were dead upon arrival.
There were 1.48 ED deaths per 1,000 adults in 1997 and .77 deaths per 1,000 adults in 2011, the most recent year for which data are available. This is a 48% reduction. Compared to patients who survived to ED discharge or hospital admission, those who died in the ED were older, had higher triage scores, and were more likely to be male and white. The proportion of patients who visited a rural ED or an ED in the South was higher among those who died than among those who survived. For roughly 63% of ED deaths, the patient was in cardiopulmonary arrest, unconscious, or dead on arrival.
The study was not designed to identify possible reasons for the reduction in ED deaths. The authors suggested it was likely due to multiple causes, including an increase in palliative care, less frequent use of resuscitation efforts in the prehospital setting, and improvements in emergency medicine and public health.
1. Kanazaria HK, Probst MA, Hsia RY. Emergency department death rates dropped by nearly 50 percent, 1997-2011. Health Aff (Millwood). 2016;35(7):1303-1308.
AHA Issues Statement on Drugs That May Cause or Exacerbate Heart Failure
BY MITCHEL L. ZOLER
FRONTLINE MEDICAL NEWS
Many commonly used prescription drugs, many over-the-counter (OTC) agents, and several complementary or alternative medications can either trigger heart failure or exacerbate the disease in patients with existing heart failure, according to a scientific statement written by a committee of the American Heart Association (AHA).
This first-ever authoritative US overview of what is known about drugs that can affect heart failure was compiled to address an important practice issue for the large and growing number of US patients with heart failure, which is estimated to be nearly 6 million Americans, and “provide some guidance to healthcare providers in how to minimize polypharmacy, improve medication safety, as well as identify the medications that could exacerbate or cause heart failure,” said Robert L. Page II, PharmD, chair of the committee and a professor of clinical pharmacy at the University of Colorado at Denver, Aurora.
Although the comprehensive statement lists 88 distinct prescription drugs or drug classes as agents that pose major or moderate threats for causing or worsening heart failure, “from the American public’s perspective, importance should be placed on educating patients regarding the impact that OTC medications can have on their heart failure,” Dr Page said in an interview. “For example, nonsteroidal anti-inflammatory drugs like ibuprofen or naproxen can cause sodium and water retention and antagonize the effects of evidence-based heart failure pharmacotherapies. Additionally, OTC medications like pseudoephedrine, which many cough and cold products contain, can increase blood pressure and afterload,” he noted. The risks these drugs pose become even greater when they are taken at higher doses.
The full text of the AHA statement is available at http://bit.ly/29ELCXz.
1. Page RL 2nd, O’Bryant CL, Cheng D, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016 Jul 11. [Epub ahead of print]