User login
Ali Rezai, MD
Psychotropic drug use more likely with ASD diagnosis at older age
Older age at the time of autism spectrum disorder diagnosis was associated with a higher likelihood of psychotropic medication in children aged 6-11, according to Katharine Zuckerman, MD, and her associates.
The mean age at diagnosis for the 722 children included in the study was 4.4 years, with a mean diagnostic delay of 2.2 years. The adjusted odds ratio for psychotropic drug use in children who were diagnosed with autism spectrum disorder (ASD) after the age of 4 was 3.09, compared with children diagnosed before the age of 4. Children diagnosed at a later age were less likely to receive behavioral intervention (BI) therapy (adjusted odds ratio = 0.44), complementary and alternative medicine (aOR = 0.63), and school-based therapy (aOR = 0.38).
Children who experienced at least 2 years of delay before diagnosis were also significantly more likely to use psychotropic drugs (aOR = 1.77), and less likely to receive BI therapy (aOR = 0.54) and school-based therapy (aOR = 0.59). However, a delay of at least 2 years was associated with a greater chance of receiving complementary and alternative medicine (aOR = 2.81).
“Results suggest that if long-term ASD therapy use is a priority, payers and policymakers may need to proactively accelerate diagnosis by incentivizing screening or enhancing case management of children at high risk of diagnostic delays. From a population standpoint, as children receive earlier ASD diagnoses, payers may expect changes in service use patterns toward more therapy use and less pharmacology,” the investigators wrote.
Find the full study online in Psychiatric Services (doi:10.1176/appi.ps.201500549).
Older age at the time of autism spectrum disorder diagnosis was associated with a higher likelihood of psychotropic medication in children aged 6-11, according to Katharine Zuckerman, MD, and her associates.
The mean age at diagnosis for the 722 children included in the study was 4.4 years, with a mean diagnostic delay of 2.2 years. The adjusted odds ratio for psychotropic drug use in children who were diagnosed with autism spectrum disorder (ASD) after the age of 4 was 3.09, compared with children diagnosed before the age of 4. Children diagnosed at a later age were less likely to receive behavioral intervention (BI) therapy (adjusted odds ratio = 0.44), complementary and alternative medicine (aOR = 0.63), and school-based therapy (aOR = 0.38).
Children who experienced at least 2 years of delay before diagnosis were also significantly more likely to use psychotropic drugs (aOR = 1.77), and less likely to receive BI therapy (aOR = 0.54) and school-based therapy (aOR = 0.59). However, a delay of at least 2 years was associated with a greater chance of receiving complementary and alternative medicine (aOR = 2.81).
“Results suggest that if long-term ASD therapy use is a priority, payers and policymakers may need to proactively accelerate diagnosis by incentivizing screening or enhancing case management of children at high risk of diagnostic delays. From a population standpoint, as children receive earlier ASD diagnoses, payers may expect changes in service use patterns toward more therapy use and less pharmacology,” the investigators wrote.
Find the full study online in Psychiatric Services (doi:10.1176/appi.ps.201500549).
Older age at the time of autism spectrum disorder diagnosis was associated with a higher likelihood of psychotropic medication in children aged 6-11, according to Katharine Zuckerman, MD, and her associates.
The mean age at diagnosis for the 722 children included in the study was 4.4 years, with a mean diagnostic delay of 2.2 years. The adjusted odds ratio for psychotropic drug use in children who were diagnosed with autism spectrum disorder (ASD) after the age of 4 was 3.09, compared with children diagnosed before the age of 4. Children diagnosed at a later age were less likely to receive behavioral intervention (BI) therapy (adjusted odds ratio = 0.44), complementary and alternative medicine (aOR = 0.63), and school-based therapy (aOR = 0.38).
Children who experienced at least 2 years of delay before diagnosis were also significantly more likely to use psychotropic drugs (aOR = 1.77), and less likely to receive BI therapy (aOR = 0.54) and school-based therapy (aOR = 0.59). However, a delay of at least 2 years was associated with a greater chance of receiving complementary and alternative medicine (aOR = 2.81).
“Results suggest that if long-term ASD therapy use is a priority, payers and policymakers may need to proactively accelerate diagnosis by incentivizing screening or enhancing case management of children at high risk of diagnostic delays. From a population standpoint, as children receive earlier ASD diagnoses, payers may expect changes in service use patterns toward more therapy use and less pharmacology,” the investigators wrote.
Find the full study online in Psychiatric Services (doi:10.1176/appi.ps.201500549).
FROM PSYCHIATRIC SERVICES
Malpractice cases serve to warn about careful use of EHRs
Buy our EHR. Come to a dinner and see our EHR. Our EHR is the best.
I hear that a lot, but am not rushing out to get one. Yes, I have an EHR, but it’s one I designed myself. It works very well for my dinky little practice, and I’m happy with it.
I get notes from practices that use commercial EHRs all the time. They’re usually a pile of gobbledygook, mixing labs, vital signs, medication lists, and ICD-10 codes that tell you absolutely nothing about the patient or the physician’s thought process.
Recently, while reviewing malpractice cases, I saw some where I thought that the EHR was contributing to the problem. Medication lists that showed everything a patient had ever taken, without showing when some were discontinued. Prescribing lists that allowed you to enter only certain doses or schedules, therefore blocking the physician from tailoring them to a specific person’s needs. Physical exam forms that allowed only positive or negative answers, with nowhere to comment on unusual findings.
Of course, I’m just one person, so I thought “maybe it’s just me.” After all, a lot of practices out there use EHRs.
But apparently I’m not the only one. A recent report by one of the nation’s largest doctor-owned malpractice companies found that EHRs may increase the risks of liability and medical errors. In 2013, The Doctor’s Company found 28 closed claims where the EHR contributed, and 26 in just the first half of 2014.
These include both technological problems with the systems and human error in their use. Troubles included erroneous medication dose and schedules, diagnosis-related errors, and the sadly common pitfall of cutting and pasting previous notes without modification.
Medicine has always been, and will always be, a thinking profession, and trying to have computers take part of this job off of us is never good. EHRs, while they have advantages in convenience and space savings, are as imperfect as those who write them.
Patients are not one-size-fits-all, and individual treatments have to be customized in ways that current systems often don’t recognize, sometimes with sad consequences for all involved.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Buy our EHR. Come to a dinner and see our EHR. Our EHR is the best.
I hear that a lot, but am not rushing out to get one. Yes, I have an EHR, but it’s one I designed myself. It works very well for my dinky little practice, and I’m happy with it.
I get notes from practices that use commercial EHRs all the time. They’re usually a pile of gobbledygook, mixing labs, vital signs, medication lists, and ICD-10 codes that tell you absolutely nothing about the patient or the physician’s thought process.
Recently, while reviewing malpractice cases, I saw some where I thought that the EHR was contributing to the problem. Medication lists that showed everything a patient had ever taken, without showing when some were discontinued. Prescribing lists that allowed you to enter only certain doses or schedules, therefore blocking the physician from tailoring them to a specific person’s needs. Physical exam forms that allowed only positive or negative answers, with nowhere to comment on unusual findings.
Of course, I’m just one person, so I thought “maybe it’s just me.” After all, a lot of practices out there use EHRs.
But apparently I’m not the only one. A recent report by one of the nation’s largest doctor-owned malpractice companies found that EHRs may increase the risks of liability and medical errors. In 2013, The Doctor’s Company found 28 closed claims where the EHR contributed, and 26 in just the first half of 2014.
These include both technological problems with the systems and human error in their use. Troubles included erroneous medication dose and schedules, diagnosis-related errors, and the sadly common pitfall of cutting and pasting previous notes without modification.
Medicine has always been, and will always be, a thinking profession, and trying to have computers take part of this job off of us is never good. EHRs, while they have advantages in convenience and space savings, are as imperfect as those who write them.
Patients are not one-size-fits-all, and individual treatments have to be customized in ways that current systems often don’t recognize, sometimes with sad consequences for all involved.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Buy our EHR. Come to a dinner and see our EHR. Our EHR is the best.
I hear that a lot, but am not rushing out to get one. Yes, I have an EHR, but it’s one I designed myself. It works very well for my dinky little practice, and I’m happy with it.
I get notes from practices that use commercial EHRs all the time. They’re usually a pile of gobbledygook, mixing labs, vital signs, medication lists, and ICD-10 codes that tell you absolutely nothing about the patient or the physician’s thought process.
Recently, while reviewing malpractice cases, I saw some where I thought that the EHR was contributing to the problem. Medication lists that showed everything a patient had ever taken, without showing when some were discontinued. Prescribing lists that allowed you to enter only certain doses or schedules, therefore blocking the physician from tailoring them to a specific person’s needs. Physical exam forms that allowed only positive or negative answers, with nowhere to comment on unusual findings.
Of course, I’m just one person, so I thought “maybe it’s just me.” After all, a lot of practices out there use EHRs.
But apparently I’m not the only one. A recent report by one of the nation’s largest doctor-owned malpractice companies found that EHRs may increase the risks of liability and medical errors. In 2013, The Doctor’s Company found 28 closed claims where the EHR contributed, and 26 in just the first half of 2014.
These include both technological problems with the systems and human error in their use. Troubles included erroneous medication dose and schedules, diagnosis-related errors, and the sadly common pitfall of cutting and pasting previous notes without modification.
Medicine has always been, and will always be, a thinking profession, and trying to have computers take part of this job off of us is never good. EHRs, while they have advantages in convenience and space savings, are as imperfect as those who write them.
Patients are not one-size-fits-all, and individual treatments have to be customized in ways that current systems often don’t recognize, sometimes with sad consequences for all involved.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Using transcatheter aortic valves for severe mitral annular calcification
PARIS – Transcatheter mitral valve replacement using a repurposed transcatheter aortic valve in patients with severe symptomatic native mitral valve disease and severe mitral annular calcification is feasible and may be an option in carefully selected patients who aren’t candidates for surgery, Mayra Guerrero, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
However, at this early point in development, the procedure is associated with an exceptionally steep learning curve, said Dr. Guerrero, director of cardiac structural interventions at the NorthShore University Health System in Evanston, Ill.
She presented the procedural and 30-day outcomes for the first 104 patients entered into a global registry encompassing 47 centers in 11 countries. Nearly 90% of patients received an Edwards SAPIEN XT or SAPIEN 3 valve. The EuroPCR results update an earlier report on the first 64 patients in the registry (JACC Cardiovasc Interv. 2016 Jul 11;9[13]:1361-71).
The results, she said, are reminiscent of the early days in transcatheter aortic valve replacement, which were marked by an initial very high early mortality rate that fell dramatically as technology and techniques improved.
“We know that there are important things we need to improve. Left ventricular outflow tract obstruction is the Achilles heel of this procedure; we need to work on its prevention and management. We need better annulus sizing methods. We need to find the best delivery method and improve our patient selection in order to avoid taking on patients who are too sick. Still, even during this early experience, the technical success rate has improved, and 30-day mortality continues to drop,” Dr. Guerrero said.
Indeed, the 30-day all-cause mortality rate of 25% in the first 104 patients doesn’t tell the whole story. The rate was 37.5% in the first third of patients, fell to 21.9% in the second tertile, and then to 15% in the most recent tertile.
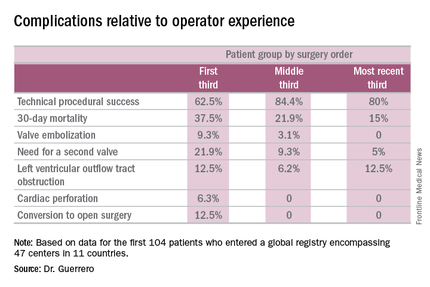
Similarly, the technical success rate of the procedure according to Mitral Valve Academic Research Consortium criteria improved from 62.5% in the first tertile of patients to 84.4% and 80% in the second and third, respectively, she continued.
The rates of almost all complications went down with greater operator experience, too. The notable exception was left ventricular outflow tract obstruction (LVOTO). It occurred in 12.5% of patients in the first tertile and remained unchanged in the third.
It’s noteworthy that the majority of deaths were noncardiac in nature. Patients with mitral annular calcification are a high-risk group even before they develop valvular dysfunction. They are typically older and have multiple comorbidities. Participants in the global registry had a mean Society of Thoracic Surgeons score of 14.4; 38% had diabetes, 45% had chronic obstructive pulmonary disease, 35% had heart failure, 34% had previously undergone coronary artery bypass surgery, and roughly half of patients had a prior aortic valve replacement.
Causes of noncardiac mortality within 30 days included multiorgan failure in 8.6% of subjects, pneumonia in 2.9%, infection in 1.9%, and one fatal thoracentesis-related bleeding complication.
Cardiovascular deaths included two cases due to left ventricular perforation, two fatal strokes, an MI due to air emboli, a lethal complete atrioventricular block, and three deaths owing to LVOTO.
Alcohol ablation met with some success as a bailout treatment in cases of LVOTO with hemodynamic compromise after transmitral valve replacement in the global registry. All six treated patients had significant improvement initially, although the LVOTO recurred the next day in one instance. Four of the six patients were discharged from the hospital. One patient died because of atrioventricular block, and another from multiorgan failure 3 weeks after alcohol ablation of the LVOTO.
Dr. Guerrero has been a leader in this new field. She reported the first percutaneous implantation of a balloon expandable transcatheter valve in a native mitral valve without a surgical incision (Catheter Cardiovasc Interv. 2014 Jun 1;83[7]:E287-91), and more recently, together with coworkers developed a percutaneous alcohol ablation technique for acute reduction of LVOTO due to transcatheter mitral valve replacement (Catheter Cardiovasc Interv. 2016 Jul 5. doi:10.1002/ccd.26649).
She is now the principal investigator in the ongoing Mitral Implantation of Transcatheter Valves (MITRAL) trial, a physician-sponsored, 90-patient pilot study underway at six U.S. sites. MITRAL is recruiting three patient populations for transcatheter mitral valve replacement: patients like those in the global registry, with native mitral valve disease and severe mitral annular calcification; those with a symptomatic failing surgical ring with severe mitral regurgitation or stenosis; and patients with a symptomatic failing surgical bioprosthesis with severe mitral regurgitation or stenosis.
Discussant Nicolo Piazza, MD, of McGill University, Montreal, said transcatheter mitral valve replacement in mitral valve disease with severe mitral annular calcification in patients unsuitable for surgery “definitely represents an unmet clinical need in our practice today.” But he urged caution in interpreting the global registry data.
“This is a real world registry study with inherent selection bias and physician reporting bias,” the cardiologist said.
“We are leveraging a therapy from the aortic field into the mitral field. Of course, we do not have dedicated devices yet to treat these patients. The main finding of this study is that the procedure is actually feasible,” Dr. Piazza said.
Still, it’s sobering that at least 1 in 10 treated patients experiences LVOTO, 1 in 10 requires a second valve, and technical success is achieved in 3 out of 4 patients, he added.
Dr. Piazza predicted that multislice CT scans will be “extremely important” in refining patient selection criteria for the procedure, and echocardiography will be helpful in understanding the optimal procedural techniques and viewing angles. Work also needs to be done on developing optimal anticoagulation protocols in order to avoid valve thrombosis.
Dr. Guerrero reported serving as a consultant to Edwards Lifesciences. Dr. Piazza is a consultant to Medtronic and MicroPort.
PARIS – Transcatheter mitral valve replacement using a repurposed transcatheter aortic valve in patients with severe symptomatic native mitral valve disease and severe mitral annular calcification is feasible and may be an option in carefully selected patients who aren’t candidates for surgery, Mayra Guerrero, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
However, at this early point in development, the procedure is associated with an exceptionally steep learning curve, said Dr. Guerrero, director of cardiac structural interventions at the NorthShore University Health System in Evanston, Ill.
She presented the procedural and 30-day outcomes for the first 104 patients entered into a global registry encompassing 47 centers in 11 countries. Nearly 90% of patients received an Edwards SAPIEN XT or SAPIEN 3 valve. The EuroPCR results update an earlier report on the first 64 patients in the registry (JACC Cardiovasc Interv. 2016 Jul 11;9[13]:1361-71).
The results, she said, are reminiscent of the early days in transcatheter aortic valve replacement, which were marked by an initial very high early mortality rate that fell dramatically as technology and techniques improved.
“We know that there are important things we need to improve. Left ventricular outflow tract obstruction is the Achilles heel of this procedure; we need to work on its prevention and management. We need better annulus sizing methods. We need to find the best delivery method and improve our patient selection in order to avoid taking on patients who are too sick. Still, even during this early experience, the technical success rate has improved, and 30-day mortality continues to drop,” Dr. Guerrero said.
Indeed, the 30-day all-cause mortality rate of 25% in the first 104 patients doesn’t tell the whole story. The rate was 37.5% in the first third of patients, fell to 21.9% in the second tertile, and then to 15% in the most recent tertile.

Similarly, the technical success rate of the procedure according to Mitral Valve Academic Research Consortium criteria improved from 62.5% in the first tertile of patients to 84.4% and 80% in the second and third, respectively, she continued.
The rates of almost all complications went down with greater operator experience, too. The notable exception was left ventricular outflow tract obstruction (LVOTO). It occurred in 12.5% of patients in the first tertile and remained unchanged in the third.
It’s noteworthy that the majority of deaths were noncardiac in nature. Patients with mitral annular calcification are a high-risk group even before they develop valvular dysfunction. They are typically older and have multiple comorbidities. Participants in the global registry had a mean Society of Thoracic Surgeons score of 14.4; 38% had diabetes, 45% had chronic obstructive pulmonary disease, 35% had heart failure, 34% had previously undergone coronary artery bypass surgery, and roughly half of patients had a prior aortic valve replacement.
Causes of noncardiac mortality within 30 days included multiorgan failure in 8.6% of subjects, pneumonia in 2.9%, infection in 1.9%, and one fatal thoracentesis-related bleeding complication.
Cardiovascular deaths included two cases due to left ventricular perforation, two fatal strokes, an MI due to air emboli, a lethal complete atrioventricular block, and three deaths owing to LVOTO.
Alcohol ablation met with some success as a bailout treatment in cases of LVOTO with hemodynamic compromise after transmitral valve replacement in the global registry. All six treated patients had significant improvement initially, although the LVOTO recurred the next day in one instance. Four of the six patients were discharged from the hospital. One patient died because of atrioventricular block, and another from multiorgan failure 3 weeks after alcohol ablation of the LVOTO.
Dr. Guerrero has been a leader in this new field. She reported the first percutaneous implantation of a balloon expandable transcatheter valve in a native mitral valve without a surgical incision (Catheter Cardiovasc Interv. 2014 Jun 1;83[7]:E287-91), and more recently, together with coworkers developed a percutaneous alcohol ablation technique for acute reduction of LVOTO due to transcatheter mitral valve replacement (Catheter Cardiovasc Interv. 2016 Jul 5. doi:10.1002/ccd.26649).
She is now the principal investigator in the ongoing Mitral Implantation of Transcatheter Valves (MITRAL) trial, a physician-sponsored, 90-patient pilot study underway at six U.S. sites. MITRAL is recruiting three patient populations for transcatheter mitral valve replacement: patients like those in the global registry, with native mitral valve disease and severe mitral annular calcification; those with a symptomatic failing surgical ring with severe mitral regurgitation or stenosis; and patients with a symptomatic failing surgical bioprosthesis with severe mitral regurgitation or stenosis.
Discussant Nicolo Piazza, MD, of McGill University, Montreal, said transcatheter mitral valve replacement in mitral valve disease with severe mitral annular calcification in patients unsuitable for surgery “definitely represents an unmet clinical need in our practice today.” But he urged caution in interpreting the global registry data.
“This is a real world registry study with inherent selection bias and physician reporting bias,” the cardiologist said.
“We are leveraging a therapy from the aortic field into the mitral field. Of course, we do not have dedicated devices yet to treat these patients. The main finding of this study is that the procedure is actually feasible,” Dr. Piazza said.
Still, it’s sobering that at least 1 in 10 treated patients experiences LVOTO, 1 in 10 requires a second valve, and technical success is achieved in 3 out of 4 patients, he added.
Dr. Piazza predicted that multislice CT scans will be “extremely important” in refining patient selection criteria for the procedure, and echocardiography will be helpful in understanding the optimal procedural techniques and viewing angles. Work also needs to be done on developing optimal anticoagulation protocols in order to avoid valve thrombosis.
Dr. Guerrero reported serving as a consultant to Edwards Lifesciences. Dr. Piazza is a consultant to Medtronic and MicroPort.
PARIS – Transcatheter mitral valve replacement using a repurposed transcatheter aortic valve in patients with severe symptomatic native mitral valve disease and severe mitral annular calcification is feasible and may be an option in carefully selected patients who aren’t candidates for surgery, Mayra Guerrero, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
However, at this early point in development, the procedure is associated with an exceptionally steep learning curve, said Dr. Guerrero, director of cardiac structural interventions at the NorthShore University Health System in Evanston, Ill.
She presented the procedural and 30-day outcomes for the first 104 patients entered into a global registry encompassing 47 centers in 11 countries. Nearly 90% of patients received an Edwards SAPIEN XT or SAPIEN 3 valve. The EuroPCR results update an earlier report on the first 64 patients in the registry (JACC Cardiovasc Interv. 2016 Jul 11;9[13]:1361-71).
The results, she said, are reminiscent of the early days in transcatheter aortic valve replacement, which were marked by an initial very high early mortality rate that fell dramatically as technology and techniques improved.
“We know that there are important things we need to improve. Left ventricular outflow tract obstruction is the Achilles heel of this procedure; we need to work on its prevention and management. We need better annulus sizing methods. We need to find the best delivery method and improve our patient selection in order to avoid taking on patients who are too sick. Still, even during this early experience, the technical success rate has improved, and 30-day mortality continues to drop,” Dr. Guerrero said.
Indeed, the 30-day all-cause mortality rate of 25% in the first 104 patients doesn’t tell the whole story. The rate was 37.5% in the first third of patients, fell to 21.9% in the second tertile, and then to 15% in the most recent tertile.

Similarly, the technical success rate of the procedure according to Mitral Valve Academic Research Consortium criteria improved from 62.5% in the first tertile of patients to 84.4% and 80% in the second and third, respectively, she continued.
The rates of almost all complications went down with greater operator experience, too. The notable exception was left ventricular outflow tract obstruction (LVOTO). It occurred in 12.5% of patients in the first tertile and remained unchanged in the third.
It’s noteworthy that the majority of deaths were noncardiac in nature. Patients with mitral annular calcification are a high-risk group even before they develop valvular dysfunction. They are typically older and have multiple comorbidities. Participants in the global registry had a mean Society of Thoracic Surgeons score of 14.4; 38% had diabetes, 45% had chronic obstructive pulmonary disease, 35% had heart failure, 34% had previously undergone coronary artery bypass surgery, and roughly half of patients had a prior aortic valve replacement.
Causes of noncardiac mortality within 30 days included multiorgan failure in 8.6% of subjects, pneumonia in 2.9%, infection in 1.9%, and one fatal thoracentesis-related bleeding complication.
Cardiovascular deaths included two cases due to left ventricular perforation, two fatal strokes, an MI due to air emboli, a lethal complete atrioventricular block, and three deaths owing to LVOTO.
Alcohol ablation met with some success as a bailout treatment in cases of LVOTO with hemodynamic compromise after transmitral valve replacement in the global registry. All six treated patients had significant improvement initially, although the LVOTO recurred the next day in one instance. Four of the six patients were discharged from the hospital. One patient died because of atrioventricular block, and another from multiorgan failure 3 weeks after alcohol ablation of the LVOTO.
Dr. Guerrero has been a leader in this new field. She reported the first percutaneous implantation of a balloon expandable transcatheter valve in a native mitral valve without a surgical incision (Catheter Cardiovasc Interv. 2014 Jun 1;83[7]:E287-91), and more recently, together with coworkers developed a percutaneous alcohol ablation technique for acute reduction of LVOTO due to transcatheter mitral valve replacement (Catheter Cardiovasc Interv. 2016 Jul 5. doi:10.1002/ccd.26649).
She is now the principal investigator in the ongoing Mitral Implantation of Transcatheter Valves (MITRAL) trial, a physician-sponsored, 90-patient pilot study underway at six U.S. sites. MITRAL is recruiting three patient populations for transcatheter mitral valve replacement: patients like those in the global registry, with native mitral valve disease and severe mitral annular calcification; those with a symptomatic failing surgical ring with severe mitral regurgitation or stenosis; and patients with a symptomatic failing surgical bioprosthesis with severe mitral regurgitation or stenosis.
Discussant Nicolo Piazza, MD, of McGill University, Montreal, said transcatheter mitral valve replacement in mitral valve disease with severe mitral annular calcification in patients unsuitable for surgery “definitely represents an unmet clinical need in our practice today.” But he urged caution in interpreting the global registry data.
“This is a real world registry study with inherent selection bias and physician reporting bias,” the cardiologist said.
“We are leveraging a therapy from the aortic field into the mitral field. Of course, we do not have dedicated devices yet to treat these patients. The main finding of this study is that the procedure is actually feasible,” Dr. Piazza said.
Still, it’s sobering that at least 1 in 10 treated patients experiences LVOTO, 1 in 10 requires a second valve, and technical success is achieved in 3 out of 4 patients, he added.
Dr. Piazza predicted that multislice CT scans will be “extremely important” in refining patient selection criteria for the procedure, and echocardiography will be helpful in understanding the optimal procedural techniques and viewing angles. Work also needs to be done on developing optimal anticoagulation protocols in order to avoid valve thrombosis.
Dr. Guerrero reported serving as a consultant to Edwards Lifesciences. Dr. Piazza is a consultant to Medtronic and MicroPort.
AT EUROPCR 2016
Key clinical point: A report from a global registry of transcatheter aortic valve implantation in the mitral position shows the procedure is feasible.
Major finding: Thirty-day mortality fell from 37.5% in the first third of treated patients to 15% in the most recent tertile.
Data source: A real world registry that includes 104 patients at 47 centers in 11 countries to date.
Disclosures: The study presenter reported serving as a consultant to Edwards Lifesciences.
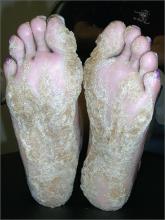
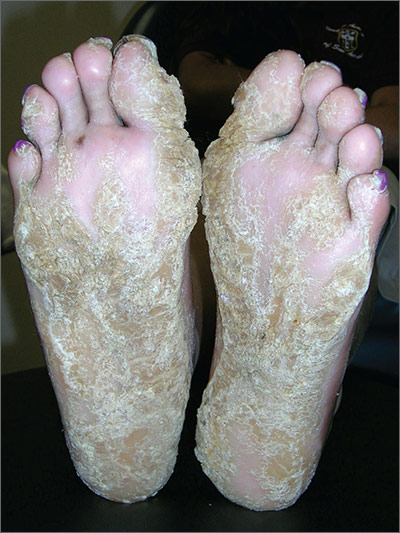
Thickening of skin on feet
The FP recognized this as diffuse palmoplantar keratoderma of the palms and soles. This is an inherited genodermatosis that may be autosomal dominant or sporadic. Palmoplantar keratoderma includes a rare heterogeneous group of disorders that are characterized by thickening of the palms and the soles that can also be an associated feature of some very rare syndromes. The patient in this case had diffuse hyperkeratosis, which can be distributed over most of the plantar surface.
This condition can be differentiated from plantar warts as it is present on more diffuse locations on the palmoplantar surfaces (including palms and soles) and it lacks the black dots of thrombosed capillaries that are seen in plantar warts.
There is no cure for this genetic condition and the goals of treatment are functional and cosmetic improvement. The FP prescribed 12% ammonium lactate as an emollient and keratolytic. The patient applied this twice daily with some symptomatic relief. The family hoped that there would be some genetic treatment for this in the future.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ. Plantar warts. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:766-770.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP recognized this as diffuse palmoplantar keratoderma of the palms and soles. This is an inherited genodermatosis that may be autosomal dominant or sporadic. Palmoplantar keratoderma includes a rare heterogeneous group of disorders that are characterized by thickening of the palms and the soles that can also be an associated feature of some very rare syndromes. The patient in this case had diffuse hyperkeratosis, which can be distributed over most of the plantar surface.
This condition can be differentiated from plantar warts as it is present on more diffuse locations on the palmoplantar surfaces (including palms and soles) and it lacks the black dots of thrombosed capillaries that are seen in plantar warts.
There is no cure for this genetic condition and the goals of treatment are functional and cosmetic improvement. The FP prescribed 12% ammonium lactate as an emollient and keratolytic. The patient applied this twice daily with some symptomatic relief. The family hoped that there would be some genetic treatment for this in the future.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ. Plantar warts. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:766-770.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP recognized this as diffuse palmoplantar keratoderma of the palms and soles. This is an inherited genodermatosis that may be autosomal dominant or sporadic. Palmoplantar keratoderma includes a rare heterogeneous group of disorders that are characterized by thickening of the palms and the soles that can also be an associated feature of some very rare syndromes. The patient in this case had diffuse hyperkeratosis, which can be distributed over most of the plantar surface.
This condition can be differentiated from plantar warts as it is present on more diffuse locations on the palmoplantar surfaces (including palms and soles) and it lacks the black dots of thrombosed capillaries that are seen in plantar warts.
There is no cure for this genetic condition and the goals of treatment are functional and cosmetic improvement. The FP prescribed 12% ammonium lactate as an emollient and keratolytic. The patient applied this twice daily with some symptomatic relief. The family hoped that there would be some genetic treatment for this in the future.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ. Plantar warts. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:766-770.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
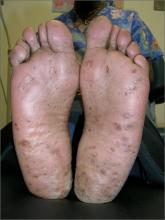
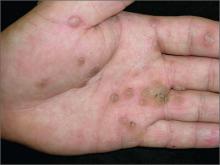
Scaly palms and soles
The FP recognized this as focal palmoplantar keratoderma of the palms and soles, which is an inherited genodermatosis that is autosomal dominant. The lesions are located mainly on high pressure areas and spare the arches of the feet.
Palmoplantar keratoderma includes a rare heterogeneous group of disorders that are characterized by thickening of the palms and the soles that can also be an associated feature of some very rare syndromes. The patient in this case had focal hyperkeratosis, which is located mainly on pressure points and sites of recurrent friction.
Palmoplantar keratoderma can be differentiated from plantar warts as it is present on more diffuse locations on the palmoplantar surfaces (including palms and soles) and it lacks the black dots of thrombosed capillaries that are seen in plantar warts.
There is no cure for this genetic condition and the goals of treatment are functional and cosmetic improvement. The FP prescribed 12% ammonium lactate as an emollient and keratolytic. The patient applied this twice daily with some symptomatic relief.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ. Plantar warts. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:766-770.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP recognized this as focal palmoplantar keratoderma of the palms and soles, which is an inherited genodermatosis that is autosomal dominant. The lesions are located mainly on high pressure areas and spare the arches of the feet.
Palmoplantar keratoderma includes a rare heterogeneous group of disorders that are characterized by thickening of the palms and the soles that can also be an associated feature of some very rare syndromes. The patient in this case had focal hyperkeratosis, which is located mainly on pressure points and sites of recurrent friction.
Palmoplantar keratoderma can be differentiated from plantar warts as it is present on more diffuse locations on the palmoplantar surfaces (including palms and soles) and it lacks the black dots of thrombosed capillaries that are seen in plantar warts.
There is no cure for this genetic condition and the goals of treatment are functional and cosmetic improvement. The FP prescribed 12% ammonium lactate as an emollient and keratolytic. The patient applied this twice daily with some symptomatic relief.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ. Plantar warts. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:766-770.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP recognized this as focal palmoplantar keratoderma of the palms and soles, which is an inherited genodermatosis that is autosomal dominant. The lesions are located mainly on high pressure areas and spare the arches of the feet.
Palmoplantar keratoderma includes a rare heterogeneous group of disorders that are characterized by thickening of the palms and the soles that can also be an associated feature of some very rare syndromes. The patient in this case had focal hyperkeratosis, which is located mainly on pressure points and sites of recurrent friction.
Palmoplantar keratoderma can be differentiated from plantar warts as it is present on more diffuse locations on the palmoplantar surfaces (including palms and soles) and it lacks the black dots of thrombosed capillaries that are seen in plantar warts.
There is no cure for this genetic condition and the goals of treatment are functional and cosmetic improvement. The FP prescribed 12% ammonium lactate as an emollient and keratolytic. The patient applied this twice daily with some symptomatic relief.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ. Plantar warts. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:766-770.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
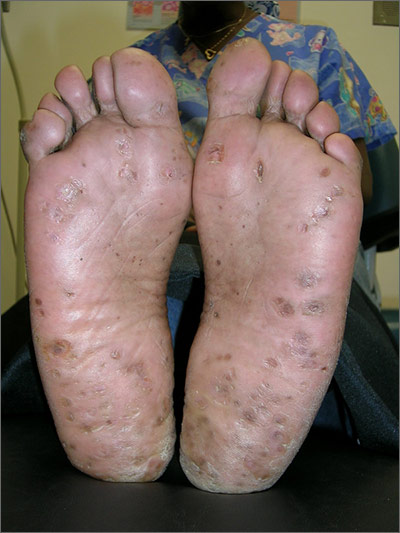
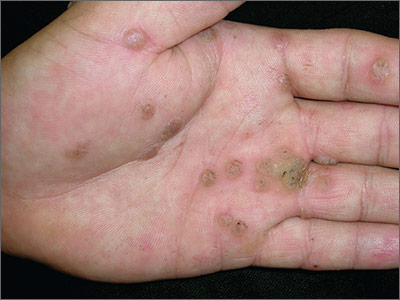
Growths on palms
The FP diagnosed this patient with palmar warts, which are similar in morphology to plantar warts. Plantar (or palmar) warts are lesions caused by human papillomavirus (HPV) that occur on the soles of the feet and palms of the hands. Plantar warts occur mostly in adolescents and young adults and affect up to 10% of people in these age groups.
Plantar warts usually occur at points of maximum pressure, such as on the heels or over the heads of the metatarsal bones, but may appear anywhere on the plantar surface, including the tips of the toes. A cluster of multiple warts that appear to fuse together is referred to as a mosaic wart. HIV is a risk factor for any type of HPV infection.
Distinguish warts from calluses by noting the skin lines; warts lack skin lines crossing their surface, whereas normal skin lines cross through a callus without any disturbance. Additionally, plantar/palmar warts may have a highly organized mosaic pattern on the surface when examined with a hand lens and have prominent black dots (thrombosed capillaries).
The most common treatments for warts include topical salicylic acid and cryotherapy. Topical salicylic acid preparations are nonscarring, minimally painful, and relatively effective, but require persistent application of medication once a day for weeks to months. Cryotherapy with liquid nitrogen therapy is commonly used, but plantar warts are more resistant than other HPV lesions. The liquid nitrogen is applied to form a freeze ball that covers the lesion and 2 mm of surrounding normal tissue for usually 10 to 20 seconds per treatment. This can be performed as a single long freeze or divided into 2 freezing episodes with thawing in between. This allows for more freeze time in a way that is less painful to the patient.
In this case, the patient chose cryotherapy and arranged for a follow-up appointment in 3 to 4 weeks for a second round.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ. Plantar warts. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:766-770.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed this patient with palmar warts, which are similar in morphology to plantar warts. Plantar (or palmar) warts are lesions caused by human papillomavirus (HPV) that occur on the soles of the feet and palms of the hands. Plantar warts occur mostly in adolescents and young adults and affect up to 10% of people in these age groups.
Plantar warts usually occur at points of maximum pressure, such as on the heels or over the heads of the metatarsal bones, but may appear anywhere on the plantar surface, including the tips of the toes. A cluster of multiple warts that appear to fuse together is referred to as a mosaic wart. HIV is a risk factor for any type of HPV infection.
Distinguish warts from calluses by noting the skin lines; warts lack skin lines crossing their surface, whereas normal skin lines cross through a callus without any disturbance. Additionally, plantar/palmar warts may have a highly organized mosaic pattern on the surface when examined with a hand lens and have prominent black dots (thrombosed capillaries).
The most common treatments for warts include topical salicylic acid and cryotherapy. Topical salicylic acid preparations are nonscarring, minimally painful, and relatively effective, but require persistent application of medication once a day for weeks to months. Cryotherapy with liquid nitrogen therapy is commonly used, but plantar warts are more resistant than other HPV lesions. The liquid nitrogen is applied to form a freeze ball that covers the lesion and 2 mm of surrounding normal tissue for usually 10 to 20 seconds per treatment. This can be performed as a single long freeze or divided into 2 freezing episodes with thawing in between. This allows for more freeze time in a way that is less painful to the patient.
In this case, the patient chose cryotherapy and arranged for a follow-up appointment in 3 to 4 weeks for a second round.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ. Plantar warts. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:766-770.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed this patient with palmar warts, which are similar in morphology to plantar warts. Plantar (or palmar) warts are lesions caused by human papillomavirus (HPV) that occur on the soles of the feet and palms of the hands. Plantar warts occur mostly in adolescents and young adults and affect up to 10% of people in these age groups.
Plantar warts usually occur at points of maximum pressure, such as on the heels or over the heads of the metatarsal bones, but may appear anywhere on the plantar surface, including the tips of the toes. A cluster of multiple warts that appear to fuse together is referred to as a mosaic wart. HIV is a risk factor for any type of HPV infection.
Distinguish warts from calluses by noting the skin lines; warts lack skin lines crossing their surface, whereas normal skin lines cross through a callus without any disturbance. Additionally, plantar/palmar warts may have a highly organized mosaic pattern on the surface when examined with a hand lens and have prominent black dots (thrombosed capillaries).
The most common treatments for warts include topical salicylic acid and cryotherapy. Topical salicylic acid preparations are nonscarring, minimally painful, and relatively effective, but require persistent application of medication once a day for weeks to months. Cryotherapy with liquid nitrogen therapy is commonly used, but plantar warts are more resistant than other HPV lesions. The liquid nitrogen is applied to form a freeze ball that covers the lesion and 2 mm of surrounding normal tissue for usually 10 to 20 seconds per treatment. This can be performed as a single long freeze or divided into 2 freezing episodes with thawing in between. This allows for more freeze time in a way that is less painful to the patient.
In this case, the patient chose cryotherapy and arranged for a follow-up appointment in 3 to 4 weeks for a second round.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ. Plantar warts. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:766-770.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Rises in LDL and HDL Cholesterol, Triglycerides Tied to Lower Diabetes Risk
Higher levels of LDL cholesterol, HDL cholesterol, and triglycerides over a lifetime are protective against type 2 diabetes, a Mendelian randomization study has shown.
The study also bolstered established evidence that LDL cholesterol and triglycerides boost the risk of coronary artery disease (CAD) but showed no contribution of HDL cholesterol to that risk.
Investigators sought to shed light on the role of the most commonly measured lipid fractions – LDL cholesterol, HDL cholesterol, and triglycerides – in the development of CAD and diabetes, particularly the observed link between statin therapy and an increased risk of diabetes.
Because genotype is not modifiable by disease, a genetic instrument can be used as an model for an exposure, and “Mendelian randomization generates unbiased, unconfounded effect estimates that are sometimes taken as evidence of a causal role,” Jon White, PhD, of University College London and his coinvestigators explained.
They used data from three genome-wide association studies involving 188,577 persons with blood lipid measures, 63,158 CAD cases, and 34,840 diabetes cases. All involved only people of European ancestry. Summary-level data for lipids were from the Global Lipids Genetics Consortium (Nat Genet. 2013;45[11]:1274-83), diabetes data came from the Diabetes Genetics Replication and Meta-analysis (Nat Genet. 2012;44[9]:981-90), and CAD data were from the Coronary Artery Disease Genome-wide Replication and Meta-analysis plus Coronary Artery Disease Genetics (Nat Genet. 2013;45[1]:25-33). From these, the investigators constructed genetic instruments comprised of single-nucleotide polymorphisms (SNPs) and conducted Mendelian randomizations designed to adjust for the SNPs’ possible associations with other traits, or pleiotropy.
The results showed that two lipid fractions were associated with reduced risk for type 2 diabetes and one had no discernible effect. LDL cholesterol showed the strongest association: An increase of 1 standard deviation, equivalent to 38 mg/dL, was tied to a 21% reduction in risk (odds ratio, 0.79) of diabetes. For HDL, a 1-SD rise of 16 mg/DL in HDL was associated with a 17% lower risk (OR, 0.83). A 1-SD rise of triglycerides, 89 mg/dL, also reduced risk by 17% (OR, 0.83), but there were statistical inconsistencies between analyses.
The associations between 1-SD increases and CAD were consistent with conventional wisdom: For LDL cholesterol, CAD risk rose by 68%; for triglycerides, the increase was 28%; and for HDL cholesterol, the risk was slightly reduced by 5% but was not statistically significant (JAMA Cardiol. 2016 Aug 3. doi: 10.1001/jamacardio.2016.1884).
These results can help to identify the potential effects of lipid-modifying drugs, yet “although all three lipids were associated with reduced risk of diabetes, it does not necessarily follow that lowering of LDL cholesterol or triglyceride levels through use of drugs that target specific proteins (eg, PCSK9) will alter the risk of diabetes,” Dr. White and his colleagues wrote. Large-scale genetic and clinical trials are needed to determine such dysglycemic associations.
This study was conducted by the Clinical Trial Service Unit of the University of Oxford through a grant by Merck Sharp & Dohme, with additional funding from numerous academic and research institutions. The funding sources had no role in the design or conduct of the study. Two of the investigators had ties to pharmaceutical companies.
The findings from Dr. White and his associates will no doubt fuel the controversy on the causal association of major plasma lipids with type 2 diabetes.
Because prior studies have shown that lowering LDL cholesterol with statins is associated with a 21% increased risk of diabetes, the finding that increasing LDL lowers the risk of diabetes is not inconsistent. The magnitude of risk, however, was much lower (absolute increase of 9%) in the statin trials. Nonetheless, if LDL is indeed shown to be protective against diabetes, this will have major implications for all lipid-lowering drugs, not just statins.
Regarding HDL cholesterol, the inconclusive findings of Dr. White and his coinvestigators, combined with prior research showing both direct and inverse associations between elevated HDL and type 2 diabetes, shed scant light on the role of HDL in diabetes.
The findings on the association of genetically mediated triglyceride levels and type 2 diabetes from this study are the most counterintuitive. They are opposite to previous epidemiological reports showing that elevated triglyceride levels are associated with higher risk for diabetes. If true, the implications of this are substantial, including the potential that intervention to reduce triglyceride levels could paradoxically increase the risk for type 2 diabetes.
This study, using Mendelian randomization and sophisticated analyses to adjust for pleiotropic effects, advances our knowledge; however, it seems that other approaches are required to further evaluate the causal relevance of each of these lipid fractions in association with type 2 diabetes.
Danish Saleheen, MBBS, PhD; Daniel J. Rader, MD; and Benjamin F. Voight, PhD, of the University of Pennsylvania, Philadelphia, made these comments in an accompanying editorial (JAMA Cardiol. 2016 Aug 3. doi: 10.1001/jamacardio.2016.2298). They had no disclosures to report.
The findings from Dr. White and his associates will no doubt fuel the controversy on the causal association of major plasma lipids with type 2 diabetes.
Because prior studies have shown that lowering LDL cholesterol with statins is associated with a 21% increased risk of diabetes, the finding that increasing LDL lowers the risk of diabetes is not inconsistent. The magnitude of risk, however, was much lower (absolute increase of 9%) in the statin trials. Nonetheless, if LDL is indeed shown to be protective against diabetes, this will have major implications for all lipid-lowering drugs, not just statins.
Regarding HDL cholesterol, the inconclusive findings of Dr. White and his coinvestigators, combined with prior research showing both direct and inverse associations between elevated HDL and type 2 diabetes, shed scant light on the role of HDL in diabetes.
The findings on the association of genetically mediated triglyceride levels and type 2 diabetes from this study are the most counterintuitive. They are opposite to previous epidemiological reports showing that elevated triglyceride levels are associated with higher risk for diabetes. If true, the implications of this are substantial, including the potential that intervention to reduce triglyceride levels could paradoxically increase the risk for type 2 diabetes.
This study, using Mendelian randomization and sophisticated analyses to adjust for pleiotropic effects, advances our knowledge; however, it seems that other approaches are required to further evaluate the causal relevance of each of these lipid fractions in association with type 2 diabetes.
Danish Saleheen, MBBS, PhD; Daniel J. Rader, MD; and Benjamin F. Voight, PhD, of the University of Pennsylvania, Philadelphia, made these comments in an accompanying editorial (JAMA Cardiol. 2016 Aug 3. doi: 10.1001/jamacardio.2016.2298). They had no disclosures to report.
The findings from Dr. White and his associates will no doubt fuel the controversy on the causal association of major plasma lipids with type 2 diabetes.
Because prior studies have shown that lowering LDL cholesterol with statins is associated with a 21% increased risk of diabetes, the finding that increasing LDL lowers the risk of diabetes is not inconsistent. The magnitude of risk, however, was much lower (absolute increase of 9%) in the statin trials. Nonetheless, if LDL is indeed shown to be protective against diabetes, this will have major implications for all lipid-lowering drugs, not just statins.
Regarding HDL cholesterol, the inconclusive findings of Dr. White and his coinvestigators, combined with prior research showing both direct and inverse associations between elevated HDL and type 2 diabetes, shed scant light on the role of HDL in diabetes.
The findings on the association of genetically mediated triglyceride levels and type 2 diabetes from this study are the most counterintuitive. They are opposite to previous epidemiological reports showing that elevated triglyceride levels are associated with higher risk for diabetes. If true, the implications of this are substantial, including the potential that intervention to reduce triglyceride levels could paradoxically increase the risk for type 2 diabetes.
This study, using Mendelian randomization and sophisticated analyses to adjust for pleiotropic effects, advances our knowledge; however, it seems that other approaches are required to further evaluate the causal relevance of each of these lipid fractions in association with type 2 diabetes.
Danish Saleheen, MBBS, PhD; Daniel J. Rader, MD; and Benjamin F. Voight, PhD, of the University of Pennsylvania, Philadelphia, made these comments in an accompanying editorial (JAMA Cardiol. 2016 Aug 3. doi: 10.1001/jamacardio.2016.2298). They had no disclosures to report.
Higher levels of LDL cholesterol, HDL cholesterol, and triglycerides over a lifetime are protective against type 2 diabetes, a Mendelian randomization study has shown.
The study also bolstered established evidence that LDL cholesterol and triglycerides boost the risk of coronary artery disease (CAD) but showed no contribution of HDL cholesterol to that risk.
Investigators sought to shed light on the role of the most commonly measured lipid fractions – LDL cholesterol, HDL cholesterol, and triglycerides – in the development of CAD and diabetes, particularly the observed link between statin therapy and an increased risk of diabetes.
Because genotype is not modifiable by disease, a genetic instrument can be used as an model for an exposure, and “Mendelian randomization generates unbiased, unconfounded effect estimates that are sometimes taken as evidence of a causal role,” Jon White, PhD, of University College London and his coinvestigators explained.
They used data from three genome-wide association studies involving 188,577 persons with blood lipid measures, 63,158 CAD cases, and 34,840 diabetes cases. All involved only people of European ancestry. Summary-level data for lipids were from the Global Lipids Genetics Consortium (Nat Genet. 2013;45[11]:1274-83), diabetes data came from the Diabetes Genetics Replication and Meta-analysis (Nat Genet. 2012;44[9]:981-90), and CAD data were from the Coronary Artery Disease Genome-wide Replication and Meta-analysis plus Coronary Artery Disease Genetics (Nat Genet. 2013;45[1]:25-33). From these, the investigators constructed genetic instruments comprised of single-nucleotide polymorphisms (SNPs) and conducted Mendelian randomizations designed to adjust for the SNPs’ possible associations with other traits, or pleiotropy.
The results showed that two lipid fractions were associated with reduced risk for type 2 diabetes and one had no discernible effect. LDL cholesterol showed the strongest association: An increase of 1 standard deviation, equivalent to 38 mg/dL, was tied to a 21% reduction in risk (odds ratio, 0.79) of diabetes. For HDL, a 1-SD rise of 16 mg/DL in HDL was associated with a 17% lower risk (OR, 0.83). A 1-SD rise of triglycerides, 89 mg/dL, also reduced risk by 17% (OR, 0.83), but there were statistical inconsistencies between analyses.
The associations between 1-SD increases and CAD were consistent with conventional wisdom: For LDL cholesterol, CAD risk rose by 68%; for triglycerides, the increase was 28%; and for HDL cholesterol, the risk was slightly reduced by 5% but was not statistically significant (JAMA Cardiol. 2016 Aug 3. doi: 10.1001/jamacardio.2016.1884).
These results can help to identify the potential effects of lipid-modifying drugs, yet “although all three lipids were associated with reduced risk of diabetes, it does not necessarily follow that lowering of LDL cholesterol or triglyceride levels through use of drugs that target specific proteins (eg, PCSK9) will alter the risk of diabetes,” Dr. White and his colleagues wrote. Large-scale genetic and clinical trials are needed to determine such dysglycemic associations.
This study was conducted by the Clinical Trial Service Unit of the University of Oxford through a grant by Merck Sharp & Dohme, with additional funding from numerous academic and research institutions. The funding sources had no role in the design or conduct of the study. Two of the investigators had ties to pharmaceutical companies.
Higher levels of LDL cholesterol, HDL cholesterol, and triglycerides over a lifetime are protective against type 2 diabetes, a Mendelian randomization study has shown.
The study also bolstered established evidence that LDL cholesterol and triglycerides boost the risk of coronary artery disease (CAD) but showed no contribution of HDL cholesterol to that risk.
Investigators sought to shed light on the role of the most commonly measured lipid fractions – LDL cholesterol, HDL cholesterol, and triglycerides – in the development of CAD and diabetes, particularly the observed link between statin therapy and an increased risk of diabetes.
Because genotype is not modifiable by disease, a genetic instrument can be used as an model for an exposure, and “Mendelian randomization generates unbiased, unconfounded effect estimates that are sometimes taken as evidence of a causal role,” Jon White, PhD, of University College London and his coinvestigators explained.
They used data from three genome-wide association studies involving 188,577 persons with blood lipid measures, 63,158 CAD cases, and 34,840 diabetes cases. All involved only people of European ancestry. Summary-level data for lipids were from the Global Lipids Genetics Consortium (Nat Genet. 2013;45[11]:1274-83), diabetes data came from the Diabetes Genetics Replication and Meta-analysis (Nat Genet. 2012;44[9]:981-90), and CAD data were from the Coronary Artery Disease Genome-wide Replication and Meta-analysis plus Coronary Artery Disease Genetics (Nat Genet. 2013;45[1]:25-33). From these, the investigators constructed genetic instruments comprised of single-nucleotide polymorphisms (SNPs) and conducted Mendelian randomizations designed to adjust for the SNPs’ possible associations with other traits, or pleiotropy.
The results showed that two lipid fractions were associated with reduced risk for type 2 diabetes and one had no discernible effect. LDL cholesterol showed the strongest association: An increase of 1 standard deviation, equivalent to 38 mg/dL, was tied to a 21% reduction in risk (odds ratio, 0.79) of diabetes. For HDL, a 1-SD rise of 16 mg/DL in HDL was associated with a 17% lower risk (OR, 0.83). A 1-SD rise of triglycerides, 89 mg/dL, also reduced risk by 17% (OR, 0.83), but there were statistical inconsistencies between analyses.
The associations between 1-SD increases and CAD were consistent with conventional wisdom: For LDL cholesterol, CAD risk rose by 68%; for triglycerides, the increase was 28%; and for HDL cholesterol, the risk was slightly reduced by 5% but was not statistically significant (JAMA Cardiol. 2016 Aug 3. doi: 10.1001/jamacardio.2016.1884).
These results can help to identify the potential effects of lipid-modifying drugs, yet “although all three lipids were associated with reduced risk of diabetes, it does not necessarily follow that lowering of LDL cholesterol or triglyceride levels through use of drugs that target specific proteins (eg, PCSK9) will alter the risk of diabetes,” Dr. White and his colleagues wrote. Large-scale genetic and clinical trials are needed to determine such dysglycemic associations.
This study was conducted by the Clinical Trial Service Unit of the University of Oxford through a grant by Merck Sharp & Dohme, with additional funding from numerous academic and research institutions. The funding sources had no role in the design or conduct of the study. Two of the investigators had ties to pharmaceutical companies.
FROM JAMA CARDIOLOGY
Rises in LDL and HDL cholesterol, triglycerides tied to lower diabetes risk
Higher levels of LDL cholesterol, HDL cholesterol, and triglycerides over a lifetime are protective against type 2 diabetes, a Mendelian randomization study has shown.
The study also bolstered established evidence that LDL cholesterol and triglycerides boost the risk of coronary artery disease (CAD) but showed no contribution of HDL cholesterol to that risk.
Investigators sought to shed light on the role of the most commonly measured lipid fractions – LDL cholesterol, HDL cholesterol, and triglycerides – in the development of CAD and diabetes, particularly the observed link between statin therapy and an increased risk of diabetes.
Because genotype is not modifiable by disease, a genetic instrument can be used as an model for an exposure, and “Mendelian randomization generates unbiased, unconfounded effect estimates that are sometimes taken as evidence of a causal role,” Jon White, PhD, of University College London and his coinvestigators explained.
They used data from three genome-wide association studies involving 188,577 persons with blood lipid measures, 63,158 CAD cases, and 34,840 diabetes cases. All involved only people of European ancestry. Summary-level data for lipids were from the Global Lipids Genetics Consortium (Nat Genet. 2013;45[11]:1274-83), diabetes data came from the Diabetes Genetics Replication and Meta-analysis (Nat Genet. 2012;44[9]:981-90), and CAD data were from the Coronary Artery Disease Genome-wide Replication and Meta-analysis plus Coronary Artery Disease Genetics (Nat Genet. 2013;45[1]:25-33). From these, the investigators constructed genetic instruments comprised of single-nucleotide polymorphisms (SNPs) and conducted Mendelian randomizations designed to adjust for the SNPs’ possible associations with other traits, or pleiotropy.
The results showed that two lipid fractions were associated with reduced risk for type 2 diabetes and one had no discernible effect. LDL cholesterol showed the strongest association: An increase of 1 standard deviation, equivalent to 38 mg/dL, was tied to a 21% reduction in risk (odds ratio, 0.79) of diabetes. For HDL, a 1-SD rise of 16 mg/DL in HDL was associated with a 17% lower risk (OR, 0.83). A 1-SD rise of triglycerides, 89 mg/dL, also reduced risk by 17% (OR, 0.83), but there were statistical inconsistencies between analyses.
The associations between 1-SD increases and CAD were consistent with conventional wisdom: For LDL cholesterol, CAD risk rose by 68%; for triglycerides, the increase was 28%; and for HDL cholesterol, the risk was slightly reduced by 5% but was not statistically significant (JAMA Cardiol. 2016 Aug 3. doi: 10.1001/jamacardio.2016.1884).
These results can help to identify the potential effects of lipid-modifying drugs, yet “although all three lipids were associated with reduced risk of diabetes, it does not necessarily follow that lowering of LDL cholesterol or triglyceride levels through use of drugs that target specific proteins (eg, PCSK9) will alter the risk of diabetes,” Dr. White and his colleagues wrote. Large-scale genetic and clinical trials are needed to determine such dysglycemic associations.
This study was conducted by the Clinical Trial Service Unit of the University of Oxford through a grant by Merck Sharp & Dohme, with additional funding from numerous academic and research institutions. The funding sources had no role in the design or conduct of the study. Two of the investigators had ties to pharmaceutical companies.
The findings from Dr. White and his associates will no doubt fuel the controversy on the causal association of major plasma lipids with type 2 diabetes.
Because prior studies have shown that lowering LDL cholesterol with statins is associated with a 21% increased risk of diabetes, the finding that increasing LDL lowers the risk of diabetes is not inconsistent. The magnitude of risk, however, was much lower (absolute increase of 9%) in the statin trials. Nonetheless, if LDL is indeed shown to be protective against diabetes, this will have major implications for all lipid-lowering drugs, not just statins.
Regarding HDL cholesterol, the inconclusive findings of Dr. White and his coinvestigators, combined with prior research showing both direct and inverse associations between elevated HDL and type 2 diabetes, shed scant light on the role of HDL in diabetes.
The findings on the association of genetically mediated triglyceride levels and type 2 diabetes from this study are the most counterintuitive. They are opposite to previous epidemiological reports showing that elevated triglyceride levels are associated with higher risk for diabetes. If true, the implications of this are substantial, including the potential that intervention to reduce triglyceride levels could paradoxically increase the risk for type 2 diabetes.
This study, using Mendelian randomization and sophisticated analyses to adjust for pleiotropic effects, advances our knowledge; however, it seems that other approaches are required to further evaluate the causal relevance of each of these lipid fractions in association with type 2 diabetes.
Danish Saleheen, MBBS, PhD; Daniel J. Rader, MD; and Benjamin F. Voight, PhD, of the University of Pennsylvania, Philadelphia, made these comments in an accompanying editorial (JAMA Cardiol. 2016 Aug 3. doi: 10.1001/jamacardio.2016.2298). They had no disclosures to report.
The findings from Dr. White and his associates will no doubt fuel the controversy on the causal association of major plasma lipids with type 2 diabetes.
Because prior studies have shown that lowering LDL cholesterol with statins is associated with a 21% increased risk of diabetes, the finding that increasing LDL lowers the risk of diabetes is not inconsistent. The magnitude of risk, however, was much lower (absolute increase of 9%) in the statin trials. Nonetheless, if LDL is indeed shown to be protective against diabetes, this will have major implications for all lipid-lowering drugs, not just statins.
Regarding HDL cholesterol, the inconclusive findings of Dr. White and his coinvestigators, combined with prior research showing both direct and inverse associations between elevated HDL and type 2 diabetes, shed scant light on the role of HDL in diabetes.
The findings on the association of genetically mediated triglyceride levels and type 2 diabetes from this study are the most counterintuitive. They are opposite to previous epidemiological reports showing that elevated triglyceride levels are associated with higher risk for diabetes. If true, the implications of this are substantial, including the potential that intervention to reduce triglyceride levels could paradoxically increase the risk for type 2 diabetes.
This study, using Mendelian randomization and sophisticated analyses to adjust for pleiotropic effects, advances our knowledge; however, it seems that other approaches are required to further evaluate the causal relevance of each of these lipid fractions in association with type 2 diabetes.
Danish Saleheen, MBBS, PhD; Daniel J. Rader, MD; and Benjamin F. Voight, PhD, of the University of Pennsylvania, Philadelphia, made these comments in an accompanying editorial (JAMA Cardiol. 2016 Aug 3. doi: 10.1001/jamacardio.2016.2298). They had no disclosures to report.
The findings from Dr. White and his associates will no doubt fuel the controversy on the causal association of major plasma lipids with type 2 diabetes.
Because prior studies have shown that lowering LDL cholesterol with statins is associated with a 21% increased risk of diabetes, the finding that increasing LDL lowers the risk of diabetes is not inconsistent. The magnitude of risk, however, was much lower (absolute increase of 9%) in the statin trials. Nonetheless, if LDL is indeed shown to be protective against diabetes, this will have major implications for all lipid-lowering drugs, not just statins.
Regarding HDL cholesterol, the inconclusive findings of Dr. White and his coinvestigators, combined with prior research showing both direct and inverse associations between elevated HDL and type 2 diabetes, shed scant light on the role of HDL in diabetes.
The findings on the association of genetically mediated triglyceride levels and type 2 diabetes from this study are the most counterintuitive. They are opposite to previous epidemiological reports showing that elevated triglyceride levels are associated with higher risk for diabetes. If true, the implications of this are substantial, including the potential that intervention to reduce triglyceride levels could paradoxically increase the risk for type 2 diabetes.
This study, using Mendelian randomization and sophisticated analyses to adjust for pleiotropic effects, advances our knowledge; however, it seems that other approaches are required to further evaluate the causal relevance of each of these lipid fractions in association with type 2 diabetes.
Danish Saleheen, MBBS, PhD; Daniel J. Rader, MD; and Benjamin F. Voight, PhD, of the University of Pennsylvania, Philadelphia, made these comments in an accompanying editorial (JAMA Cardiol. 2016 Aug 3. doi: 10.1001/jamacardio.2016.2298). They had no disclosures to report.
Higher levels of LDL cholesterol, HDL cholesterol, and triglycerides over a lifetime are protective against type 2 diabetes, a Mendelian randomization study has shown.
The study also bolstered established evidence that LDL cholesterol and triglycerides boost the risk of coronary artery disease (CAD) but showed no contribution of HDL cholesterol to that risk.
Investigators sought to shed light on the role of the most commonly measured lipid fractions – LDL cholesterol, HDL cholesterol, and triglycerides – in the development of CAD and diabetes, particularly the observed link between statin therapy and an increased risk of diabetes.
Because genotype is not modifiable by disease, a genetic instrument can be used as an model for an exposure, and “Mendelian randomization generates unbiased, unconfounded effect estimates that are sometimes taken as evidence of a causal role,” Jon White, PhD, of University College London and his coinvestigators explained.
They used data from three genome-wide association studies involving 188,577 persons with blood lipid measures, 63,158 CAD cases, and 34,840 diabetes cases. All involved only people of European ancestry. Summary-level data for lipids were from the Global Lipids Genetics Consortium (Nat Genet. 2013;45[11]:1274-83), diabetes data came from the Diabetes Genetics Replication and Meta-analysis (Nat Genet. 2012;44[9]:981-90), and CAD data were from the Coronary Artery Disease Genome-wide Replication and Meta-analysis plus Coronary Artery Disease Genetics (Nat Genet. 2013;45[1]:25-33). From these, the investigators constructed genetic instruments comprised of single-nucleotide polymorphisms (SNPs) and conducted Mendelian randomizations designed to adjust for the SNPs’ possible associations with other traits, or pleiotropy.
The results showed that two lipid fractions were associated with reduced risk for type 2 diabetes and one had no discernible effect. LDL cholesterol showed the strongest association: An increase of 1 standard deviation, equivalent to 38 mg/dL, was tied to a 21% reduction in risk (odds ratio, 0.79) of diabetes. For HDL, a 1-SD rise of 16 mg/DL in HDL was associated with a 17% lower risk (OR, 0.83). A 1-SD rise of triglycerides, 89 mg/dL, also reduced risk by 17% (OR, 0.83), but there were statistical inconsistencies between analyses.
The associations between 1-SD increases and CAD were consistent with conventional wisdom: For LDL cholesterol, CAD risk rose by 68%; for triglycerides, the increase was 28%; and for HDL cholesterol, the risk was slightly reduced by 5% but was not statistically significant (JAMA Cardiol. 2016 Aug 3. doi: 10.1001/jamacardio.2016.1884).
These results can help to identify the potential effects of lipid-modifying drugs, yet “although all three lipids were associated with reduced risk of diabetes, it does not necessarily follow that lowering of LDL cholesterol or triglyceride levels through use of drugs that target specific proteins (eg, PCSK9) will alter the risk of diabetes,” Dr. White and his colleagues wrote. Large-scale genetic and clinical trials are needed to determine such dysglycemic associations.
This study was conducted by the Clinical Trial Service Unit of the University of Oxford through a grant by Merck Sharp & Dohme, with additional funding from numerous academic and research institutions. The funding sources had no role in the design or conduct of the study. Two of the investigators had ties to pharmaceutical companies.
Higher levels of LDL cholesterol, HDL cholesterol, and triglycerides over a lifetime are protective against type 2 diabetes, a Mendelian randomization study has shown.
The study also bolstered established evidence that LDL cholesterol and triglycerides boost the risk of coronary artery disease (CAD) but showed no contribution of HDL cholesterol to that risk.
Investigators sought to shed light on the role of the most commonly measured lipid fractions – LDL cholesterol, HDL cholesterol, and triglycerides – in the development of CAD and diabetes, particularly the observed link between statin therapy and an increased risk of diabetes.
Because genotype is not modifiable by disease, a genetic instrument can be used as an model for an exposure, and “Mendelian randomization generates unbiased, unconfounded effect estimates that are sometimes taken as evidence of a causal role,” Jon White, PhD, of University College London and his coinvestigators explained.
They used data from three genome-wide association studies involving 188,577 persons with blood lipid measures, 63,158 CAD cases, and 34,840 diabetes cases. All involved only people of European ancestry. Summary-level data for lipids were from the Global Lipids Genetics Consortium (Nat Genet. 2013;45[11]:1274-83), diabetes data came from the Diabetes Genetics Replication and Meta-analysis (Nat Genet. 2012;44[9]:981-90), and CAD data were from the Coronary Artery Disease Genome-wide Replication and Meta-analysis plus Coronary Artery Disease Genetics (Nat Genet. 2013;45[1]:25-33). From these, the investigators constructed genetic instruments comprised of single-nucleotide polymorphisms (SNPs) and conducted Mendelian randomizations designed to adjust for the SNPs’ possible associations with other traits, or pleiotropy.
The results showed that two lipid fractions were associated with reduced risk for type 2 diabetes and one had no discernible effect. LDL cholesterol showed the strongest association: An increase of 1 standard deviation, equivalent to 38 mg/dL, was tied to a 21% reduction in risk (odds ratio, 0.79) of diabetes. For HDL, a 1-SD rise of 16 mg/DL in HDL was associated with a 17% lower risk (OR, 0.83). A 1-SD rise of triglycerides, 89 mg/dL, also reduced risk by 17% (OR, 0.83), but there were statistical inconsistencies between analyses.
The associations between 1-SD increases and CAD were consistent with conventional wisdom: For LDL cholesterol, CAD risk rose by 68%; for triglycerides, the increase was 28%; and for HDL cholesterol, the risk was slightly reduced by 5% but was not statistically significant (JAMA Cardiol. 2016 Aug 3. doi: 10.1001/jamacardio.2016.1884).
These results can help to identify the potential effects of lipid-modifying drugs, yet “although all three lipids were associated with reduced risk of diabetes, it does not necessarily follow that lowering of LDL cholesterol or triglyceride levels through use of drugs that target specific proteins (eg, PCSK9) will alter the risk of diabetes,” Dr. White and his colleagues wrote. Large-scale genetic and clinical trials are needed to determine such dysglycemic associations.
This study was conducted by the Clinical Trial Service Unit of the University of Oxford through a grant by Merck Sharp & Dohme, with additional funding from numerous academic and research institutions. The funding sources had no role in the design or conduct of the study. Two of the investigators had ties to pharmaceutical companies.
FROM JAMA CARDIOLOGY
Key clinical point: Elevated LDL cholesterol and triglyceride levels increase the risk of coronary artery disease but are linked with a lower risk of diabetes.
Major finding: Rises in LDL cholesterol and triglycerides were associated with decreases in diabetes risk of 21% and 17%, respectively.
Data source: A Mendelian randomization analysis using three genome-wide association studies.
Disclosures: This study was conducted by the Clinical Trial Service Unit of the University of Oxford through a grant by Merck Sharp & Dohme, with additional funding from numerous academic and research institutions. The funding sources had no role in the design or conduct of the study. Two of the investigators had ties to pharmaceutical companies.
Find SHM’s eLearning Initiatives in One Convenient Location
Seven-Module Anticoagulation Series
This comprehensive series of recorded webinars reviews best practices for inpatient anticoagulation, including the evidence-based management of atrial fibrillation, venous thromboembolism, perioperative anticoagulation, and anticoagulant-related bleeding. Features include:
- Seven 60-minute webinar-based sessions accessible on-demand
- Brief introductory materials accompanying each webinar as well as a post-test
- Links to additional resources with each webinar
- 6.5 CME credits for all seven sessions
This module is free for SHM members and non-members.
Acute Coronary Syndrome Performance Improvement CME Activity
Improve quality improvement strategies around acute coronary syndrome (ACS) while earning CME. This tool is a free, self-directed, web-based, yearlong activity designed to help you evaluate your practice. Upon completion of the activity you will receive 20 CME credits. The activity features three stages, including completing a performance assessment measurement through a self-evaluation, building an action plan for personal improvement, and using a performance analysis tool to compare performance from the previous stages.
The deadline to participate is fall 2016. This module is free for SHM members and non-members.
Adolescent and Young Adult Inpatient Care: Not a Kid Anymore
This online case-based module allows participants to:
- Identify appropriate interview techniques with the adolescent inpatient
- Recognize the components in a complete psychosocial interview with an adolescent inpatient
- Define the necessary elements of a pelvic examination in an adolescent female inpatient
- Select the appropriate diagnosis, treatment, and partner notification for STI in the inpatient setting
- Earn 10 self-evaluation points when enrolled in the American Board of Pediatrics’ MOC program and 3.0 AMA PRA Category 1 Credits upon completion of the activity
This module is free for SHM members and $50 for non-members.
Updated Hospital Quality and Patient Safety MOC Self-Assessment (Adult and Pediatrics)
- Quality improvement and patient safety principles such as QI theory, system processes, measurement tools for implementation and error types, disclosure, and prevention strategies
- 26 multiple-choice questions with accompanying answers, rationales, and references
- 8 self-evaluation points for diplomates enrolled in ABIM’s MOC program as well as 2.0 AMA PRA Category 1 Credits
SHM members receive a special price of $65; the non-member price is $100.
Seven-Module Anticoagulation Series
This comprehensive series of recorded webinars reviews best practices for inpatient anticoagulation, including the evidence-based management of atrial fibrillation, venous thromboembolism, perioperative anticoagulation, and anticoagulant-related bleeding. Features include:
- Seven 60-minute webinar-based sessions accessible on-demand
- Brief introductory materials accompanying each webinar as well as a post-test
- Links to additional resources with each webinar
- 6.5 CME credits for all seven sessions
This module is free for SHM members and non-members.
Acute Coronary Syndrome Performance Improvement CME Activity
Improve quality improvement strategies around acute coronary syndrome (ACS) while earning CME. This tool is a free, self-directed, web-based, yearlong activity designed to help you evaluate your practice. Upon completion of the activity you will receive 20 CME credits. The activity features three stages, including completing a performance assessment measurement through a self-evaluation, building an action plan for personal improvement, and using a performance analysis tool to compare performance from the previous stages.
The deadline to participate is fall 2016. This module is free for SHM members and non-members.
Adolescent and Young Adult Inpatient Care: Not a Kid Anymore
This online case-based module allows participants to:
- Identify appropriate interview techniques with the adolescent inpatient
- Recognize the components in a complete psychosocial interview with an adolescent inpatient
- Define the necessary elements of a pelvic examination in an adolescent female inpatient
- Select the appropriate diagnosis, treatment, and partner notification for STI in the inpatient setting
- Earn 10 self-evaluation points when enrolled in the American Board of Pediatrics’ MOC program and 3.0 AMA PRA Category 1 Credits upon completion of the activity
This module is free for SHM members and $50 for non-members.
Updated Hospital Quality and Patient Safety MOC Self-Assessment (Adult and Pediatrics)
- Quality improvement and patient safety principles such as QI theory, system processes, measurement tools for implementation and error types, disclosure, and prevention strategies
- 26 multiple-choice questions with accompanying answers, rationales, and references
- 8 self-evaluation points for diplomates enrolled in ABIM’s MOC program as well as 2.0 AMA PRA Category 1 Credits
SHM members receive a special price of $65; the non-member price is $100.
Seven-Module Anticoagulation Series
This comprehensive series of recorded webinars reviews best practices for inpatient anticoagulation, including the evidence-based management of atrial fibrillation, venous thromboembolism, perioperative anticoagulation, and anticoagulant-related bleeding. Features include:
- Seven 60-minute webinar-based sessions accessible on-demand
- Brief introductory materials accompanying each webinar as well as a post-test
- Links to additional resources with each webinar
- 6.5 CME credits for all seven sessions
This module is free for SHM members and non-members.
Acute Coronary Syndrome Performance Improvement CME Activity
Improve quality improvement strategies around acute coronary syndrome (ACS) while earning CME. This tool is a free, self-directed, web-based, yearlong activity designed to help you evaluate your practice. Upon completion of the activity you will receive 20 CME credits. The activity features three stages, including completing a performance assessment measurement through a self-evaluation, building an action plan for personal improvement, and using a performance analysis tool to compare performance from the previous stages.
The deadline to participate is fall 2016. This module is free for SHM members and non-members.
Adolescent and Young Adult Inpatient Care: Not a Kid Anymore
This online case-based module allows participants to:
- Identify appropriate interview techniques with the adolescent inpatient
- Recognize the components in a complete psychosocial interview with an adolescent inpatient
- Define the necessary elements of a pelvic examination in an adolescent female inpatient
- Select the appropriate diagnosis, treatment, and partner notification for STI in the inpatient setting
- Earn 10 self-evaluation points when enrolled in the American Board of Pediatrics’ MOC program and 3.0 AMA PRA Category 1 Credits upon completion of the activity
This module is free for SHM members and $50 for non-members.
Updated Hospital Quality and Patient Safety MOC Self-Assessment (Adult and Pediatrics)
- Quality improvement and patient safety principles such as QI theory, system processes, measurement tools for implementation and error types, disclosure, and prevention strategies
- 26 multiple-choice questions with accompanying answers, rationales, and references
- 8 self-evaluation points for diplomates enrolled in ABIM’s MOC program as well as 2.0 AMA PRA Category 1 Credits
SHM members receive a special price of $65; the non-member price is $100.