User login
Thyroid tumor reclassification eases burden, raises diagnostic questions
The recent reclassification of encapsulated follicular variant of papillary thyroid carcinoma (EFVPTC) as noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) – an action taken to better reflect the very low risk of adverse events associated with these tumors – has important clinical and psychological implications for patients.
“Even though physicians know that most thyroid cancers have an excellent prognosis, the impact on a patient of being given a diagnosis of cancer should not be underestimated,” Dr. Peter Angelos, professor of surgery and chief of endocrine surgery at the University of Chicago, said in an interview. “It is, however, critical for doctors and patients to understand that this change from ‘thyroid cancer’ to a ‘benign thyroid nodule,’ is not something that can be determined prior to surgery. Patients will still need thyroid operations to determine if their indeterminate nodules have cancer in them or not.”
The change in nomenclature followed an international, multidisciplinary, retrospective study of patients with thyroid nodules diagnosed as EFVPTC. Such patients are usually treated as having conventional thyroid cancer. The study included 109 patients with noninvasive EFVPTC who were followed for 10-26 years, and 101 with invasive EFVPTC who were followed for 1-18 years. At median follow-up of 13 years, all of the 109 patients with noninvasive EFVPTC were alive, and based on consensus diagnostic criteria developed by an Endocrine Pathology Society working group – a multinational panel of 24 thyroid pathologists – they had no evidence of disease, reported Dr. Yuri E. Nikiforov of the University of Pittsburgh and colleagues (JAMA Oncol. 2016 April 14. doi: 10.1001/jamaoncol.2016.0386).
Most of those patients (67%) were treated only with lobectomy, and none received radioiodine (RAI) treatment.
Of the 101 with invasive EFVPTC, 12 experienced an adverse event, including 5 who developed distant (lung and/or bone) metastases. Two died from the disease, one had a lymph node recurrence, one had persistent disease, and five had detectable serum thyroglobulin and were considered to have indeterminate or biochemically incomplete response to therapy, the investigators said.
Based on the findings in the noninvasive EFVPTC patients, the recommended nomenclature change was adopted to reflect the main morphological features of, and lack of invasion of, the benign tumors as well as their very low risk of adverse outcome. To assist in the diagnosis of NIFTP in routine pathology practice, a simplified three-point diagnostic nuclear scoring scheme based on the six main consensus nuclear features of the tumors was developed and validated; the scoring scheme yielded sensitivity of 98.6%, specificity of 90.1%, and overall classification accuracy of 94.3% for NIFTP.
The study involved a review of digitized histologic slides collected at 13 sites in 5 countries. The pathologists who composed the working group conducted the review and consulted in a series of teleconferences and face-to-face meetings to establish the consensus criteria. They measured the frequency of adverse outcomes, including death from disease, distant or locoregional metastases, and structural or biochemical recurrence.
The findings suggest that “clinical management of patients with NIFTP can be deescalated because they are unlikely to benefit from immediate completion thyroidectomy and RAI therapy,” the investigators said.
“Staging would be unnecessary. In addition to eliminating the psychological impact of the diagnosis of cancer, this would reduce complications of total thyroidectomy, risk of secondary tumors following RAI therapy, and the overall cost of health care. Avoidance of RAI treatment alone would save between $5,000 and $8,500 per patient (based on U.S. cost),” they wrote, adding that an estimated 45,000 patients worldwide each year will be affected by this reclassification, resulting in significant reduction in “psychological burden, medical overtreatment and expense, and other clinical consequences associated with a cancer diagnosis.”
Dr. Martha A. Zeiger, professor of surgery at Johns Hopkins University, Baltimore, agreed that the change has important implications for patients.
“With the advent of new nomenclature for encapsulated follicular variant of papillary thyroid cancer, namely that it is now considered a benign tumor, thousands of patients who have carried this original diagnosis of cancer can breathe a sigh of relief. Our new understanding will also decrease the number of patients undergoing more extensive surgery and many can now be treated with a thyroid lobectomy only,” she said in an interview.
One thing the new nomenclature doesn’t do, however, is solve the problem of the suspicious or indeterminate thyroid fine needle aspiration diagnosis, she noted.
“Clouding the landscape even further is the fact that many of our commonly used molecular diagnostics were based on studies in which encapsulated follicular variant of papillary thyroid cancer was considered malignant, and were included in the analysis. Because of this, diagnostic molecular tools will likely now require a renewed scrutiny as to their true efficacy in differentiating benign from malignant tumors,” she said.
Dr. Nikiforov is a consultant for Quest Diagnostics. A coauthor, Dr. Sylvia Asa, is a member of the medical advisory board of Leica Aperio, and another coauthor, Dr. Virginia LiVolsi, is a consultant for Veracyte Inc. The project used a facility supported by the National Cancer Institute, and molecular analysis was supported in part by funds from the University of Pittsburgh Cancer Institute and the University of Pittsburgh Medical Center. The Endocrine Pathology Society working group conference was supported by a grant from CBLPath Inc. Dr. Angelos and Dr. Zeiger reported having no disclosures.
The recent reclassification of encapsulated follicular variant of papillary thyroid carcinoma (EFVPTC) as noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) – an action taken to better reflect the very low risk of adverse events associated with these tumors – has important clinical and psychological implications for patients.
“Even though physicians know that most thyroid cancers have an excellent prognosis, the impact on a patient of being given a diagnosis of cancer should not be underestimated,” Dr. Peter Angelos, professor of surgery and chief of endocrine surgery at the University of Chicago, said in an interview. “It is, however, critical for doctors and patients to understand that this change from ‘thyroid cancer’ to a ‘benign thyroid nodule,’ is not something that can be determined prior to surgery. Patients will still need thyroid operations to determine if their indeterminate nodules have cancer in them or not.”
The change in nomenclature followed an international, multidisciplinary, retrospective study of patients with thyroid nodules diagnosed as EFVPTC. Such patients are usually treated as having conventional thyroid cancer. The study included 109 patients with noninvasive EFVPTC who were followed for 10-26 years, and 101 with invasive EFVPTC who were followed for 1-18 years. At median follow-up of 13 years, all of the 109 patients with noninvasive EFVPTC were alive, and based on consensus diagnostic criteria developed by an Endocrine Pathology Society working group – a multinational panel of 24 thyroid pathologists – they had no evidence of disease, reported Dr. Yuri E. Nikiforov of the University of Pittsburgh and colleagues (JAMA Oncol. 2016 April 14. doi: 10.1001/jamaoncol.2016.0386).
Most of those patients (67%) were treated only with lobectomy, and none received radioiodine (RAI) treatment.
Of the 101 with invasive EFVPTC, 12 experienced an adverse event, including 5 who developed distant (lung and/or bone) metastases. Two died from the disease, one had a lymph node recurrence, one had persistent disease, and five had detectable serum thyroglobulin and were considered to have indeterminate or biochemically incomplete response to therapy, the investigators said.
Based on the findings in the noninvasive EFVPTC patients, the recommended nomenclature change was adopted to reflect the main morphological features of, and lack of invasion of, the benign tumors as well as their very low risk of adverse outcome. To assist in the diagnosis of NIFTP in routine pathology practice, a simplified three-point diagnostic nuclear scoring scheme based on the six main consensus nuclear features of the tumors was developed and validated; the scoring scheme yielded sensitivity of 98.6%, specificity of 90.1%, and overall classification accuracy of 94.3% for NIFTP.
The study involved a review of digitized histologic slides collected at 13 sites in 5 countries. The pathologists who composed the working group conducted the review and consulted in a series of teleconferences and face-to-face meetings to establish the consensus criteria. They measured the frequency of adverse outcomes, including death from disease, distant or locoregional metastases, and structural or biochemical recurrence.
The findings suggest that “clinical management of patients with NIFTP can be deescalated because they are unlikely to benefit from immediate completion thyroidectomy and RAI therapy,” the investigators said.
“Staging would be unnecessary. In addition to eliminating the psychological impact of the diagnosis of cancer, this would reduce complications of total thyroidectomy, risk of secondary tumors following RAI therapy, and the overall cost of health care. Avoidance of RAI treatment alone would save between $5,000 and $8,500 per patient (based on U.S. cost),” they wrote, adding that an estimated 45,000 patients worldwide each year will be affected by this reclassification, resulting in significant reduction in “psychological burden, medical overtreatment and expense, and other clinical consequences associated with a cancer diagnosis.”
Dr. Martha A. Zeiger, professor of surgery at Johns Hopkins University, Baltimore, agreed that the change has important implications for patients.
“With the advent of new nomenclature for encapsulated follicular variant of papillary thyroid cancer, namely that it is now considered a benign tumor, thousands of patients who have carried this original diagnosis of cancer can breathe a sigh of relief. Our new understanding will also decrease the number of patients undergoing more extensive surgery and many can now be treated with a thyroid lobectomy only,” she said in an interview.
One thing the new nomenclature doesn’t do, however, is solve the problem of the suspicious or indeterminate thyroid fine needle aspiration diagnosis, she noted.
“Clouding the landscape even further is the fact that many of our commonly used molecular diagnostics were based on studies in which encapsulated follicular variant of papillary thyroid cancer was considered malignant, and were included in the analysis. Because of this, diagnostic molecular tools will likely now require a renewed scrutiny as to their true efficacy in differentiating benign from malignant tumors,” she said.
Dr. Nikiforov is a consultant for Quest Diagnostics. A coauthor, Dr. Sylvia Asa, is a member of the medical advisory board of Leica Aperio, and another coauthor, Dr. Virginia LiVolsi, is a consultant for Veracyte Inc. The project used a facility supported by the National Cancer Institute, and molecular analysis was supported in part by funds from the University of Pittsburgh Cancer Institute and the University of Pittsburgh Medical Center. The Endocrine Pathology Society working group conference was supported by a grant from CBLPath Inc. Dr. Angelos and Dr. Zeiger reported having no disclosures.
The recent reclassification of encapsulated follicular variant of papillary thyroid carcinoma (EFVPTC) as noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) – an action taken to better reflect the very low risk of adverse events associated with these tumors – has important clinical and psychological implications for patients.
“Even though physicians know that most thyroid cancers have an excellent prognosis, the impact on a patient of being given a diagnosis of cancer should not be underestimated,” Dr. Peter Angelos, professor of surgery and chief of endocrine surgery at the University of Chicago, said in an interview. “It is, however, critical for doctors and patients to understand that this change from ‘thyroid cancer’ to a ‘benign thyroid nodule,’ is not something that can be determined prior to surgery. Patients will still need thyroid operations to determine if their indeterminate nodules have cancer in them or not.”
The change in nomenclature followed an international, multidisciplinary, retrospective study of patients with thyroid nodules diagnosed as EFVPTC. Such patients are usually treated as having conventional thyroid cancer. The study included 109 patients with noninvasive EFVPTC who were followed for 10-26 years, and 101 with invasive EFVPTC who were followed for 1-18 years. At median follow-up of 13 years, all of the 109 patients with noninvasive EFVPTC were alive, and based on consensus diagnostic criteria developed by an Endocrine Pathology Society working group – a multinational panel of 24 thyroid pathologists – they had no evidence of disease, reported Dr. Yuri E. Nikiforov of the University of Pittsburgh and colleagues (JAMA Oncol. 2016 April 14. doi: 10.1001/jamaoncol.2016.0386).
Most of those patients (67%) were treated only with lobectomy, and none received radioiodine (RAI) treatment.
Of the 101 with invasive EFVPTC, 12 experienced an adverse event, including 5 who developed distant (lung and/or bone) metastases. Two died from the disease, one had a lymph node recurrence, one had persistent disease, and five had detectable serum thyroglobulin and were considered to have indeterminate or biochemically incomplete response to therapy, the investigators said.
Based on the findings in the noninvasive EFVPTC patients, the recommended nomenclature change was adopted to reflect the main morphological features of, and lack of invasion of, the benign tumors as well as their very low risk of adverse outcome. To assist in the diagnosis of NIFTP in routine pathology practice, a simplified three-point diagnostic nuclear scoring scheme based on the six main consensus nuclear features of the tumors was developed and validated; the scoring scheme yielded sensitivity of 98.6%, specificity of 90.1%, and overall classification accuracy of 94.3% for NIFTP.
The study involved a review of digitized histologic slides collected at 13 sites in 5 countries. The pathologists who composed the working group conducted the review and consulted in a series of teleconferences and face-to-face meetings to establish the consensus criteria. They measured the frequency of adverse outcomes, including death from disease, distant or locoregional metastases, and structural or biochemical recurrence.
The findings suggest that “clinical management of patients with NIFTP can be deescalated because they are unlikely to benefit from immediate completion thyroidectomy and RAI therapy,” the investigators said.
“Staging would be unnecessary. In addition to eliminating the psychological impact of the diagnosis of cancer, this would reduce complications of total thyroidectomy, risk of secondary tumors following RAI therapy, and the overall cost of health care. Avoidance of RAI treatment alone would save between $5,000 and $8,500 per patient (based on U.S. cost),” they wrote, adding that an estimated 45,000 patients worldwide each year will be affected by this reclassification, resulting in significant reduction in “psychological burden, medical overtreatment and expense, and other clinical consequences associated with a cancer diagnosis.”
Dr. Martha A. Zeiger, professor of surgery at Johns Hopkins University, Baltimore, agreed that the change has important implications for patients.
“With the advent of new nomenclature for encapsulated follicular variant of papillary thyroid cancer, namely that it is now considered a benign tumor, thousands of patients who have carried this original diagnosis of cancer can breathe a sigh of relief. Our new understanding will also decrease the number of patients undergoing more extensive surgery and many can now be treated with a thyroid lobectomy only,” she said in an interview.
One thing the new nomenclature doesn’t do, however, is solve the problem of the suspicious or indeterminate thyroid fine needle aspiration diagnosis, she noted.
“Clouding the landscape even further is the fact that many of our commonly used molecular diagnostics were based on studies in which encapsulated follicular variant of papillary thyroid cancer was considered malignant, and were included in the analysis. Because of this, diagnostic molecular tools will likely now require a renewed scrutiny as to their true efficacy in differentiating benign from malignant tumors,” she said.
Dr. Nikiforov is a consultant for Quest Diagnostics. A coauthor, Dr. Sylvia Asa, is a member of the medical advisory board of Leica Aperio, and another coauthor, Dr. Virginia LiVolsi, is a consultant for Veracyte Inc. The project used a facility supported by the National Cancer Institute, and molecular analysis was supported in part by funds from the University of Pittsburgh Cancer Institute and the University of Pittsburgh Medical Center. The Endocrine Pathology Society working group conference was supported by a grant from CBLPath Inc. Dr. Angelos and Dr. Zeiger reported having no disclosures.
FROM JAMA ONCOLOGY
Key clinical point: The recent reclassification of EFVPTC as noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) has important clinical and psychological implications for patients.
Major finding: 109 patients with noninvasive EFVPTC were alive and had no evidence of disease at a median of 13 years follow-up. Most of those patients (67%) were treated only with lobectomy, and none received radioiodine treatment.
Data source: A retrospective study of 210 patients.
Disclosures: Dr. Nikiforov is a consultant for Quest Diagnostics. A coauthor, Dr. Sylvia Asa, is a member of the medical advisory board of Leica Aperio, and another coauthor, Dr. Virginia LiVolsi, is a consultant for Veracyte Inc. The project used a facility supported by the National Cancer Institute, and molecular analysis was supported in part by funds from the University of Pittsburgh Cancer Institute and the University of Pittsburgh Medical Center. The Endocrine Pathology Society working group conference was supported by a grant from CBLPath Inc. Dr. Angelos and Dr. Zeiger reported having no disclosures.
Mentally ill and behind bars
The measure of a country’s greatness, Mahatma Gandhi said, should be based on how well it cares for its most vulnerable. Recently, I had the opportunity to work with members of a vulnerable population: men and women who have a mental illness and languish in jails and prisons around the country. My experience was eye-opening and heartbreaking.
Widespread incarceration of the mentally ill in a developed country such as the United States should be a national embarrassment. But this tragedy, which has reached an epidemic level, has been effectively shut out of the national conversation.
The problem has grown, and is enormous
By the estimate of the U.S. Department of Justice, more than one-half of people incarcerated in the United States are mentally ill and approximately 20% suffer from a serious mental illness.1,2 In fact, there are now 3 times as many mentally ill people in jail and prison as there are occupying psychiatric beds in hospitals.3 These numbers represent a considerable increase over the past 6 decades, and can be attributed to 2 major factors:
- A program of deinstitutionalization set in motion by the federal government in the 1950s called for shuttering of state psychiatric facilities around the country. This was a period of renewed national discourse on civil rights; for many people, the practice of institutionalization was considered a violation of civil rights. (Coincidentally, chlorpromazine was introduced about this time, and many experts believed that the drug would revolutionize outpatient management of psychiatric disorders.)
- More recently, heavy criminal penalties have been attached to convictions for possession and distribution of illegal substances—part of the government’s “war on drugs.”
As a consequence of these programs and policies, the United States has come full circle—routinely incarcerating the mentally ill as it did in the early 19th century, before reforms were initiated in response to the lobbying efforts of activist Dorothea Dix and her contemporaries.
My distressing, eye-opening experience
The time I spent with the incarcerated mentally ill was limited to a 6-month period at a county jail during residency. Yet the contrast between services provided to this population and those that are available to people in the community was immediately evident—and stark. The sheer number of adults in jails and prisons who require mental health care is such that the ratio of patients to psychiatrists, psychologists, and other mental health clinicians is shockingly skewed.
It does not take years of experience to figure out that a brief interview with an 18-year-old who is being jailed for the first time, has never seen a psychiatrist, and suffers panic attacks (or hallucinations, or suicidal thoughts) is a less-than-ideal clinical situation. Making that situation even more hazardous is that inmates have a high risk of suicide, particularly in the first 24 to 48 hours of incarceration.4
Other ethical issues arose during my stint in the correctional system: My patients frequently would be charged with prison-rule violations (there is evidence that mentally ill inmates are more likely to be charged with such violations2); on many such occasions, they would be placed in solitary confinement (“the hole”), a practice the United Nations has called “cruel, inhuman, and degrading: for the mentally ill5 and that, in turn, exacerbates the inmate’s psychiatric illness.6-11
Last, there are restrictions on the types of formulations of medications that can be prescribed, involuntary treatment, and other critical aspects of care that make the experience of providing care in this system frustrating for mental health providers.
Are there solutions?
One way to tackle this crisis might be to insert more psychiatrists and psychologists into the correctional system. A more sensible approach, however, would be to tackle the root cause and divert the mentally ill away from incarceration and into treatment—moving from a model of retribution and incapacitation to one of rehabilitation. For example:
- Several counties nationwide have adopted diversion programs that include so-called mental health courts and drug courts, with encouraging results12
- Police departments are establishing Crisis Intervention Teams
- Assisted outpatient treatment programs are growing in popularity.
Far more needs to be done, however. In the absence of a national debate on the problem of the incarcerated mentally ill, there is real risk that this population will continue to be ignored and that our mental health care infrastructure will remain inadequate for meeting their need for services.
1. American Psychiatric Association. Psychiatric services in jails and prisons: a task force report of the American Psychiatric Association. 2nd ed. Washington, DC: American Psychiatric Association; 2000:XIX.
2. U.S. Department of Justice. Bureau of Justice Statistics: special report. Mental health problems of prison and jail inmates. http://www.bjs.gov/content/pub/pdf/mhppji.pdf. Updated December 14, 2006. Accessed April 8, 2016.|
3. Torrey FE, Kennard AD, Eslinger D, et al. More mentally ill persons are in jails and prisons than hospitals: a survey of the states. http://www.treatmentadvocacycenter.org/storage/documents/final_jails_v_hospitals_study.pdf. Published May 2010. Accessed April 8, 2016.
4. U.S. Department of Justice. National study of jail suicide: 20 years later. http://static.nicic.gov/Library/024308.pdf. Published April 2010. Accessed April 8, 2016.
5. Méndez JE. Special Rapporteur on torture and other cruel, inhuman or degrading treatment or punishment. http://www.ohchr.org/EN/Issues/Torture/SRTorture/Pages/SRTortureIndex.aspx. Published 2011. Accessed April 8, 2016.
6. Daniel AE. Preventing suicide in prison: a collaborative responsibility of administrative, custodial, and clinical staff. J Am Acad Psychiatry Law. 2006;34(2):165-175.
7. White TW, Schimmel DJ, Frickey R. A comprehensive analysis of suicide in federal prisons: a fifteen-year review. J Correct Health Care. 2002;9(3):321-345.
8. Smith PS. The effects of solitary confinement on prison inmates: a brief history and review of the literature, crime and justice. Crime and Justice. 2006;34(1):441-528.
9. Grassian S. Psychopathological effects of solitary confinement. Am J Psychiatry. 1983;140(11):1450-1454.
10. Patterson RF, Hughes K. Review of completed suicides in the California Department of Corrections and Rehabilitation, 1999 to 2004. Psychiatr Serv. 2008;59(6):676-682.
11. Kaba F, Lewis A, Glowa-Kollisch S, et al. Solitary confinement and risk of self-harm among jail inmates. Am J Public Health. 2014;104(3):442-447.
12. McNiel DE, Binder RL. Effectiveness of a mental health court in reducing criminal recidivism and violence. Am J Psychiatry. 2007;164(9):1395-1403.
The measure of a country’s greatness, Mahatma Gandhi said, should be based on how well it cares for its most vulnerable. Recently, I had the opportunity to work with members of a vulnerable population: men and women who have a mental illness and languish in jails and prisons around the country. My experience was eye-opening and heartbreaking.
Widespread incarceration of the mentally ill in a developed country such as the United States should be a national embarrassment. But this tragedy, which has reached an epidemic level, has been effectively shut out of the national conversation.
The problem has grown, and is enormous
By the estimate of the U.S. Department of Justice, more than one-half of people incarcerated in the United States are mentally ill and approximately 20% suffer from a serious mental illness.1,2 In fact, there are now 3 times as many mentally ill people in jail and prison as there are occupying psychiatric beds in hospitals.3 These numbers represent a considerable increase over the past 6 decades, and can be attributed to 2 major factors:
- A program of deinstitutionalization set in motion by the federal government in the 1950s called for shuttering of state psychiatric facilities around the country. This was a period of renewed national discourse on civil rights; for many people, the practice of institutionalization was considered a violation of civil rights. (Coincidentally, chlorpromazine was introduced about this time, and many experts believed that the drug would revolutionize outpatient management of psychiatric disorders.)
- More recently, heavy criminal penalties have been attached to convictions for possession and distribution of illegal substances—part of the government’s “war on drugs.”
As a consequence of these programs and policies, the United States has come full circle—routinely incarcerating the mentally ill as it did in the early 19th century, before reforms were initiated in response to the lobbying efforts of activist Dorothea Dix and her contemporaries.
My distressing, eye-opening experience
The time I spent with the incarcerated mentally ill was limited to a 6-month period at a county jail during residency. Yet the contrast between services provided to this population and those that are available to people in the community was immediately evident—and stark. The sheer number of adults in jails and prisons who require mental health care is such that the ratio of patients to psychiatrists, psychologists, and other mental health clinicians is shockingly skewed.
It does not take years of experience to figure out that a brief interview with an 18-year-old who is being jailed for the first time, has never seen a psychiatrist, and suffers panic attacks (or hallucinations, or suicidal thoughts) is a less-than-ideal clinical situation. Making that situation even more hazardous is that inmates have a high risk of suicide, particularly in the first 24 to 48 hours of incarceration.4
Other ethical issues arose during my stint in the correctional system: My patients frequently would be charged with prison-rule violations (there is evidence that mentally ill inmates are more likely to be charged with such violations2); on many such occasions, they would be placed in solitary confinement (“the hole”), a practice the United Nations has called “cruel, inhuman, and degrading: for the mentally ill5 and that, in turn, exacerbates the inmate’s psychiatric illness.6-11
Last, there are restrictions on the types of formulations of medications that can be prescribed, involuntary treatment, and other critical aspects of care that make the experience of providing care in this system frustrating for mental health providers.
Are there solutions?
One way to tackle this crisis might be to insert more psychiatrists and psychologists into the correctional system. A more sensible approach, however, would be to tackle the root cause and divert the mentally ill away from incarceration and into treatment—moving from a model of retribution and incapacitation to one of rehabilitation. For example:
- Several counties nationwide have adopted diversion programs that include so-called mental health courts and drug courts, with encouraging results12
- Police departments are establishing Crisis Intervention Teams
- Assisted outpatient treatment programs are growing in popularity.
Far more needs to be done, however. In the absence of a national debate on the problem of the incarcerated mentally ill, there is real risk that this population will continue to be ignored and that our mental health care infrastructure will remain inadequate for meeting their need for services.
The measure of a country’s greatness, Mahatma Gandhi said, should be based on how well it cares for its most vulnerable. Recently, I had the opportunity to work with members of a vulnerable population: men and women who have a mental illness and languish in jails and prisons around the country. My experience was eye-opening and heartbreaking.
Widespread incarceration of the mentally ill in a developed country such as the United States should be a national embarrassment. But this tragedy, which has reached an epidemic level, has been effectively shut out of the national conversation.
The problem has grown, and is enormous
By the estimate of the U.S. Department of Justice, more than one-half of people incarcerated in the United States are mentally ill and approximately 20% suffer from a serious mental illness.1,2 In fact, there are now 3 times as many mentally ill people in jail and prison as there are occupying psychiatric beds in hospitals.3 These numbers represent a considerable increase over the past 6 decades, and can be attributed to 2 major factors:
- A program of deinstitutionalization set in motion by the federal government in the 1950s called for shuttering of state psychiatric facilities around the country. This was a period of renewed national discourse on civil rights; for many people, the practice of institutionalization was considered a violation of civil rights. (Coincidentally, chlorpromazine was introduced about this time, and many experts believed that the drug would revolutionize outpatient management of psychiatric disorders.)
- More recently, heavy criminal penalties have been attached to convictions for possession and distribution of illegal substances—part of the government’s “war on drugs.”
As a consequence of these programs and policies, the United States has come full circle—routinely incarcerating the mentally ill as it did in the early 19th century, before reforms were initiated in response to the lobbying efforts of activist Dorothea Dix and her contemporaries.
My distressing, eye-opening experience
The time I spent with the incarcerated mentally ill was limited to a 6-month period at a county jail during residency. Yet the contrast between services provided to this population and those that are available to people in the community was immediately evident—and stark. The sheer number of adults in jails and prisons who require mental health care is such that the ratio of patients to psychiatrists, psychologists, and other mental health clinicians is shockingly skewed.
It does not take years of experience to figure out that a brief interview with an 18-year-old who is being jailed for the first time, has never seen a psychiatrist, and suffers panic attacks (or hallucinations, or suicidal thoughts) is a less-than-ideal clinical situation. Making that situation even more hazardous is that inmates have a high risk of suicide, particularly in the first 24 to 48 hours of incarceration.4
Other ethical issues arose during my stint in the correctional system: My patients frequently would be charged with prison-rule violations (there is evidence that mentally ill inmates are more likely to be charged with such violations2); on many such occasions, they would be placed in solitary confinement (“the hole”), a practice the United Nations has called “cruel, inhuman, and degrading: for the mentally ill5 and that, in turn, exacerbates the inmate’s psychiatric illness.6-11
Last, there are restrictions on the types of formulations of medications that can be prescribed, involuntary treatment, and other critical aspects of care that make the experience of providing care in this system frustrating for mental health providers.
Are there solutions?
One way to tackle this crisis might be to insert more psychiatrists and psychologists into the correctional system. A more sensible approach, however, would be to tackle the root cause and divert the mentally ill away from incarceration and into treatment—moving from a model of retribution and incapacitation to one of rehabilitation. For example:
- Several counties nationwide have adopted diversion programs that include so-called mental health courts and drug courts, with encouraging results12
- Police departments are establishing Crisis Intervention Teams
- Assisted outpatient treatment programs are growing in popularity.
Far more needs to be done, however. In the absence of a national debate on the problem of the incarcerated mentally ill, there is real risk that this population will continue to be ignored and that our mental health care infrastructure will remain inadequate for meeting their need for services.
1. American Psychiatric Association. Psychiatric services in jails and prisons: a task force report of the American Psychiatric Association. 2nd ed. Washington, DC: American Psychiatric Association; 2000:XIX.
2. U.S. Department of Justice. Bureau of Justice Statistics: special report. Mental health problems of prison and jail inmates. http://www.bjs.gov/content/pub/pdf/mhppji.pdf. Updated December 14, 2006. Accessed April 8, 2016.|
3. Torrey FE, Kennard AD, Eslinger D, et al. More mentally ill persons are in jails and prisons than hospitals: a survey of the states. http://www.treatmentadvocacycenter.org/storage/documents/final_jails_v_hospitals_study.pdf. Published May 2010. Accessed April 8, 2016.
4. U.S. Department of Justice. National study of jail suicide: 20 years later. http://static.nicic.gov/Library/024308.pdf. Published April 2010. Accessed April 8, 2016.
5. Méndez JE. Special Rapporteur on torture and other cruel, inhuman or degrading treatment or punishment. http://www.ohchr.org/EN/Issues/Torture/SRTorture/Pages/SRTortureIndex.aspx. Published 2011. Accessed April 8, 2016.
6. Daniel AE. Preventing suicide in prison: a collaborative responsibility of administrative, custodial, and clinical staff. J Am Acad Psychiatry Law. 2006;34(2):165-175.
7. White TW, Schimmel DJ, Frickey R. A comprehensive analysis of suicide in federal prisons: a fifteen-year review. J Correct Health Care. 2002;9(3):321-345.
8. Smith PS. The effects of solitary confinement on prison inmates: a brief history and review of the literature, crime and justice. Crime and Justice. 2006;34(1):441-528.
9. Grassian S. Psychopathological effects of solitary confinement. Am J Psychiatry. 1983;140(11):1450-1454.
10. Patterson RF, Hughes K. Review of completed suicides in the California Department of Corrections and Rehabilitation, 1999 to 2004. Psychiatr Serv. 2008;59(6):676-682.
11. Kaba F, Lewis A, Glowa-Kollisch S, et al. Solitary confinement and risk of self-harm among jail inmates. Am J Public Health. 2014;104(3):442-447.
12. McNiel DE, Binder RL. Effectiveness of a mental health court in reducing criminal recidivism and violence. Am J Psychiatry. 2007;164(9):1395-1403.
1. American Psychiatric Association. Psychiatric services in jails and prisons: a task force report of the American Psychiatric Association. 2nd ed. Washington, DC: American Psychiatric Association; 2000:XIX.
2. U.S. Department of Justice. Bureau of Justice Statistics: special report. Mental health problems of prison and jail inmates. http://www.bjs.gov/content/pub/pdf/mhppji.pdf. Updated December 14, 2006. Accessed April 8, 2016.|
3. Torrey FE, Kennard AD, Eslinger D, et al. More mentally ill persons are in jails and prisons than hospitals: a survey of the states. http://www.treatmentadvocacycenter.org/storage/documents/final_jails_v_hospitals_study.pdf. Published May 2010. Accessed April 8, 2016.
4. U.S. Department of Justice. National study of jail suicide: 20 years later. http://static.nicic.gov/Library/024308.pdf. Published April 2010. Accessed April 8, 2016.
5. Méndez JE. Special Rapporteur on torture and other cruel, inhuman or degrading treatment or punishment. http://www.ohchr.org/EN/Issues/Torture/SRTorture/Pages/SRTortureIndex.aspx. Published 2011. Accessed April 8, 2016.
6. Daniel AE. Preventing suicide in prison: a collaborative responsibility of administrative, custodial, and clinical staff. J Am Acad Psychiatry Law. 2006;34(2):165-175.
7. White TW, Schimmel DJ, Frickey R. A comprehensive analysis of suicide in federal prisons: a fifteen-year review. J Correct Health Care. 2002;9(3):321-345.
8. Smith PS. The effects of solitary confinement on prison inmates: a brief history and review of the literature, crime and justice. Crime and Justice. 2006;34(1):441-528.
9. Grassian S. Psychopathological effects of solitary confinement. Am J Psychiatry. 1983;140(11):1450-1454.
10. Patterson RF, Hughes K. Review of completed suicides in the California Department of Corrections and Rehabilitation, 1999 to 2004. Psychiatr Serv. 2008;59(6):676-682.
11. Kaba F, Lewis A, Glowa-Kollisch S, et al. Solitary confinement and risk of self-harm among jail inmates. Am J Public Health. 2014;104(3):442-447.
12. McNiel DE, Binder RL. Effectiveness of a mental health court in reducing criminal recidivism and violence. Am J Psychiatry. 2007;164(9):1395-1403.
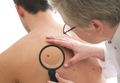
Early Detection of Melanoma in Men
Men do not know as much about skin cancer prevention and detection techniques as women, according to a recent survey conducted by the American Academy of Dermatology (AAD). This lack of knowledge may delay or prevent early diagnosis and treatment of melanoma and other nonmelanoma skin cancers in this patient population.
The survey results showed that only 56% of men versus 76% of women know there is no such thing as a healthy tan, and only 54% of men versus 70% of women know that getting a base tan is not a healthy way to protect skin from the sun. Furthermore, only 56% of men surveyed were aware that skin cancer could occur on areas of the skin not typically exposed to the sun compared to 65% of women.
“While our survey results indicate that men don’t know as much about skin cancer prevention and detection as women, men over 50 have a higher risk of developing melanoma, so it’s especially important for them to be vigilant about protecting and monitoring their skin,” said AAD President Abel Torres, MD, JD.
May is skin cancer awareness month and the AAD is encouraging patients to make sure their skin is “Looking Good in 2016” by using sun protection and regularly examining skin for signs of skin cancer. The campaign features a public service announcement encouraging men to check their skin for signs of skin cancer and find a partner to help. The AAD also released a new infographic with tips on performing a skin cancer self-examination that dermatologists can share with patients to promote early detection of skin cancer.
Dermatologist intervention in catching skin cancers when they are easier to treat also is key. At the 74th Annual Meeting of the American Academy of Dermatology in Washington, DC, Dr. Orit Markowitz discussed noninvasive imaging tools that can help dermatologists diagnose skin cancers earlier. She noted that even when a lesion looks very small, tools such as dermoscopy can reveal features that indicate it already has depth and therefore may progress to a more serious malignancy. Early detection is particularly crucial in cases of rare aggressive tumors such as amelanotic melanoma. “If something is very pink clinically and then suddenly has pigmentation dermoscopically, you really have to be considering biopsying that lesion because you may be looking at an early amelanotic melanoma,” Dr. Markowitz explained. By the time the lesion develops more obvious clinical features suggesting malignancy, the tumor progression may be far more advanced.
Men do not know as much about skin cancer prevention and detection techniques as women, according to a recent survey conducted by the American Academy of Dermatology (AAD). This lack of knowledge may delay or prevent early diagnosis and treatment of melanoma and other nonmelanoma skin cancers in this patient population.
The survey results showed that only 56% of men versus 76% of women know there is no such thing as a healthy tan, and only 54% of men versus 70% of women know that getting a base tan is not a healthy way to protect skin from the sun. Furthermore, only 56% of men surveyed were aware that skin cancer could occur on areas of the skin not typically exposed to the sun compared to 65% of women.
“While our survey results indicate that men don’t know as much about skin cancer prevention and detection as women, men over 50 have a higher risk of developing melanoma, so it’s especially important for them to be vigilant about protecting and monitoring their skin,” said AAD President Abel Torres, MD, JD.
May is skin cancer awareness month and the AAD is encouraging patients to make sure their skin is “Looking Good in 2016” by using sun protection and regularly examining skin for signs of skin cancer. The campaign features a public service announcement encouraging men to check their skin for signs of skin cancer and find a partner to help. The AAD also released a new infographic with tips on performing a skin cancer self-examination that dermatologists can share with patients to promote early detection of skin cancer.
Dermatologist intervention in catching skin cancers when they are easier to treat also is key. At the 74th Annual Meeting of the American Academy of Dermatology in Washington, DC, Dr. Orit Markowitz discussed noninvasive imaging tools that can help dermatologists diagnose skin cancers earlier. She noted that even when a lesion looks very small, tools such as dermoscopy can reveal features that indicate it already has depth and therefore may progress to a more serious malignancy. Early detection is particularly crucial in cases of rare aggressive tumors such as amelanotic melanoma. “If something is very pink clinically and then suddenly has pigmentation dermoscopically, you really have to be considering biopsying that lesion because you may be looking at an early amelanotic melanoma,” Dr. Markowitz explained. By the time the lesion develops more obvious clinical features suggesting malignancy, the tumor progression may be far more advanced.
Men do not know as much about skin cancer prevention and detection techniques as women, according to a recent survey conducted by the American Academy of Dermatology (AAD). This lack of knowledge may delay or prevent early diagnosis and treatment of melanoma and other nonmelanoma skin cancers in this patient population.
The survey results showed that only 56% of men versus 76% of women know there is no such thing as a healthy tan, and only 54% of men versus 70% of women know that getting a base tan is not a healthy way to protect skin from the sun. Furthermore, only 56% of men surveyed were aware that skin cancer could occur on areas of the skin not typically exposed to the sun compared to 65% of women.
“While our survey results indicate that men don’t know as much about skin cancer prevention and detection as women, men over 50 have a higher risk of developing melanoma, so it’s especially important for them to be vigilant about protecting and monitoring their skin,” said AAD President Abel Torres, MD, JD.
May is skin cancer awareness month and the AAD is encouraging patients to make sure their skin is “Looking Good in 2016” by using sun protection and regularly examining skin for signs of skin cancer. The campaign features a public service announcement encouraging men to check their skin for signs of skin cancer and find a partner to help. The AAD also released a new infographic with tips on performing a skin cancer self-examination that dermatologists can share with patients to promote early detection of skin cancer.
Dermatologist intervention in catching skin cancers when they are easier to treat also is key. At the 74th Annual Meeting of the American Academy of Dermatology in Washington, DC, Dr. Orit Markowitz discussed noninvasive imaging tools that can help dermatologists diagnose skin cancers earlier. She noted that even when a lesion looks very small, tools such as dermoscopy can reveal features that indicate it already has depth and therefore may progress to a more serious malignancy. Early detection is particularly crucial in cases of rare aggressive tumors such as amelanotic melanoma. “If something is very pink clinically and then suddenly has pigmentation dermoscopically, you really have to be considering biopsying that lesion because you may be looking at an early amelanotic melanoma,” Dr. Markowitz explained. By the time the lesion develops more obvious clinical features suggesting malignancy, the tumor progression may be far more advanced.
Law & Medicine: Locality rule
Question: An injured patient alleges that her eye doctor was negligent in failing to adhere to national treatment guidelines and in not using modern medical equipment. The encounter took place in a rural setting, with the nearest hospital 100 miles away.
In her malpractice lawsuit, which of the following choices is incorrect?
A. One of the doctor’s defenses may be the locality rule.
B. The plaintiff’s strongest argument is that community standards should parallel national standards for a specialist doctor such as an ophthalmologist.
C. Her expert witness must be a practicing ophthalmologist from the area.
D. The expert must be familiar with the local standards but does not have to practice there.
E. It all depends on what the state statute says, because the locality rule is not uniform settled law.
Answer: C. In traditional medical tort law, courts would rely on the standard of the particular locale where the tortious act took place, the so-called locality rule. This was based on the belief that different standards of care were applicable in different areas of the country, e.g., urban vs. rural. The rule can be traced to Small v. Howard,1 an 1880 Massachusetts opinion, which was subsequently overruled in 1968.
Factors favoring the trend away from a local standard toward a national standard include conformity in medical school and residency curricula, and prescribed board certification requirements. Internet access and telemedicine have further propagated this uniformity. Finally, two additional facets of modern medicine – continuing medical education and published clinical practice guidelines – are at odds with a rule geared toward local standards.
One argument against the locality rule is that undue reliance on an outdated mode of practice will perpetuate substandard care. In an older New York malpractice case where a newborn became blind, the pediatrician cited local custom to defend the prolonged use of oxygen to treat preterm infants, despite evidence that this practice might have serious consequences. However, the court of appeals held that the pediatrician’s superior knowledge of the increased risk of hyperoxygenation should have enabled him to use his best judgment instead of relying on the indefensible local custom.
Under a strict version of the locality rule, otherwise qualified expert witnesses may be excluded if they are not practitioners in the locale in question. Still, some courts may allow out-of-state experts to offer their opinions. This has been especially helpful to plaintiffs who are far less likely to be able to secure willing local experts, given the reluctance of many physicians to testify against a fellow doctor in their community.
Take Tennessee as an example. It once excluded the expert testimony of an orthopedic surgeon from Johnson City because the expert witness testified about the national standard and did not have actual knowledge of the standard of care in Nashville, the community where the alleged malpractice occurred.
The Tennessee Court of Appeals later ruled that expert witnesses had to have “personal” or “firsthand knowledge” of the community standard of care, and that interviewing other physicians in the area did not suffice. It subsequently clarified that an expert witness need not actually practice in the same or similar locale, and that professional contact with physicians from comparable communities, such as through referrals, would be acceptable.
Two well-known cases touching on the locality rule bear summarizing: In Swink v. Weintraub,2 Mrs. Swink bled into her pericardium during repair of a defective pacemaker electric lead. She died as a result, and her family pursued a wrongful death action, alleging negligence including delayed pericardiocentesis and surgical intervention.
The jury returned a verdict for the plaintiff, awarding damages in the amount of $1,047,732.20. On appeal, the defendants argued that North Carolina’s locality rule extended to all aspects of a negligence action, and that the trial court erred in admitting expert opinions without regard to whether those opinions reflected the “same or similar community” standard of care.
But the court of appeals disagreed, emphasizing that compliance with the “same or similar community” standard does not necessarily exonerate a defendant from an allegation of medical negligence. The court said liability can be established if the defendant did not exercise his “best judgment” in the treatment of the patient or if the defendant failed to use “reasonable care and diligence” in his efforts to render medical assistance.
In McClure v. Inova Medical Group, a Virginia jury found that a family practice resident had failed to meet the Virginia community standard of care when he did not order the prostate-specific antigen (PSA) test in a 53-year-old patient who was subsequently diagnosed with prostate cancer. The doctor had discussed the risks and benefits of PSA testing, but the patient declined taking the test. Jurors sided with the plaintiff’s argument that according to the local or statewide standard, Virginia doctors simply ordered the test as a matter of routine for men older than 50 years without necessarily discussing risks and benefits. The court awarded $1 million to the patient.3
Although the majority of jurisdictions have abandoned the locality rule, several continue to adhere to either a strict or modified version.4 Examples are Arizona, Idaho, New York, Tennessee, Virginia, and Washington. A modified rule exists in Louisiana, which holds general practitioners to a community standard and specialists to a national standard.
Finally, many authors have recommended a narrowly constructed rule based not on geographic boundaries, but on the availability of local resources. Courts would then look at the totality of circumstances, but remember that there is always the duty to refer or transfer to an available specialist/facility – and that the failure to do so may form the basis of liability.
As one physician put it: Location should not come into play with respect to the knowledge or skill of the treating physician; and even if a physician may not have the facilities to perform an emergency cesarean section, he or she should still know when it’s called for.
References
1. Small v. Howard, 128 Mass 131 (1880).
2. Swink v. Weintraub, 672 S.E.2d 53 (N.C. Court of Appeals 2009).
3. JAMA. 2004 Jan 7;291(1):15-6.
4. JAMA. 2007 Jun 20;297(23):2633-7.
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
Question: An injured patient alleges that her eye doctor was negligent in failing to adhere to national treatment guidelines and in not using modern medical equipment. The encounter took place in a rural setting, with the nearest hospital 100 miles away.
In her malpractice lawsuit, which of the following choices is incorrect?
A. One of the doctor’s defenses may be the locality rule.
B. The plaintiff’s strongest argument is that community standards should parallel national standards for a specialist doctor such as an ophthalmologist.
C. Her expert witness must be a practicing ophthalmologist from the area.
D. The expert must be familiar with the local standards but does not have to practice there.
E. It all depends on what the state statute says, because the locality rule is not uniform settled law.
Answer: C. In traditional medical tort law, courts would rely on the standard of the particular locale where the tortious act took place, the so-called locality rule. This was based on the belief that different standards of care were applicable in different areas of the country, e.g., urban vs. rural. The rule can be traced to Small v. Howard,1 an 1880 Massachusetts opinion, which was subsequently overruled in 1968.
Factors favoring the trend away from a local standard toward a national standard include conformity in medical school and residency curricula, and prescribed board certification requirements. Internet access and telemedicine have further propagated this uniformity. Finally, two additional facets of modern medicine – continuing medical education and published clinical practice guidelines – are at odds with a rule geared toward local standards.
One argument against the locality rule is that undue reliance on an outdated mode of practice will perpetuate substandard care. In an older New York malpractice case where a newborn became blind, the pediatrician cited local custom to defend the prolonged use of oxygen to treat preterm infants, despite evidence that this practice might have serious consequences. However, the court of appeals held that the pediatrician’s superior knowledge of the increased risk of hyperoxygenation should have enabled him to use his best judgment instead of relying on the indefensible local custom.
Under a strict version of the locality rule, otherwise qualified expert witnesses may be excluded if they are not practitioners in the locale in question. Still, some courts may allow out-of-state experts to offer their opinions. This has been especially helpful to plaintiffs who are far less likely to be able to secure willing local experts, given the reluctance of many physicians to testify against a fellow doctor in their community.
Take Tennessee as an example. It once excluded the expert testimony of an orthopedic surgeon from Johnson City because the expert witness testified about the national standard and did not have actual knowledge of the standard of care in Nashville, the community where the alleged malpractice occurred.
The Tennessee Court of Appeals later ruled that expert witnesses had to have “personal” or “firsthand knowledge” of the community standard of care, and that interviewing other physicians in the area did not suffice. It subsequently clarified that an expert witness need not actually practice in the same or similar locale, and that professional contact with physicians from comparable communities, such as through referrals, would be acceptable.
Two well-known cases touching on the locality rule bear summarizing: In Swink v. Weintraub,2 Mrs. Swink bled into her pericardium during repair of a defective pacemaker electric lead. She died as a result, and her family pursued a wrongful death action, alleging negligence including delayed pericardiocentesis and surgical intervention.
The jury returned a verdict for the plaintiff, awarding damages in the amount of $1,047,732.20. On appeal, the defendants argued that North Carolina’s locality rule extended to all aspects of a negligence action, and that the trial court erred in admitting expert opinions without regard to whether those opinions reflected the “same or similar community” standard of care.
But the court of appeals disagreed, emphasizing that compliance with the “same or similar community” standard does not necessarily exonerate a defendant from an allegation of medical negligence. The court said liability can be established if the defendant did not exercise his “best judgment” in the treatment of the patient or if the defendant failed to use “reasonable care and diligence” in his efforts to render medical assistance.
In McClure v. Inova Medical Group, a Virginia jury found that a family practice resident had failed to meet the Virginia community standard of care when he did not order the prostate-specific antigen (PSA) test in a 53-year-old patient who was subsequently diagnosed with prostate cancer. The doctor had discussed the risks and benefits of PSA testing, but the patient declined taking the test. Jurors sided with the plaintiff’s argument that according to the local or statewide standard, Virginia doctors simply ordered the test as a matter of routine for men older than 50 years without necessarily discussing risks and benefits. The court awarded $1 million to the patient.3
Although the majority of jurisdictions have abandoned the locality rule, several continue to adhere to either a strict or modified version.4 Examples are Arizona, Idaho, New York, Tennessee, Virginia, and Washington. A modified rule exists in Louisiana, which holds general practitioners to a community standard and specialists to a national standard.
Finally, many authors have recommended a narrowly constructed rule based not on geographic boundaries, but on the availability of local resources. Courts would then look at the totality of circumstances, but remember that there is always the duty to refer or transfer to an available specialist/facility – and that the failure to do so may form the basis of liability.
As one physician put it: Location should not come into play with respect to the knowledge or skill of the treating physician; and even if a physician may not have the facilities to perform an emergency cesarean section, he or she should still know when it’s called for.
References
1. Small v. Howard, 128 Mass 131 (1880).
2. Swink v. Weintraub, 672 S.E.2d 53 (N.C. Court of Appeals 2009).
3. JAMA. 2004 Jan 7;291(1):15-6.
4. JAMA. 2007 Jun 20;297(23):2633-7.
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
Question: An injured patient alleges that her eye doctor was negligent in failing to adhere to national treatment guidelines and in not using modern medical equipment. The encounter took place in a rural setting, with the nearest hospital 100 miles away.
In her malpractice lawsuit, which of the following choices is incorrect?
A. One of the doctor’s defenses may be the locality rule.
B. The plaintiff’s strongest argument is that community standards should parallel national standards for a specialist doctor such as an ophthalmologist.
C. Her expert witness must be a practicing ophthalmologist from the area.
D. The expert must be familiar with the local standards but does not have to practice there.
E. It all depends on what the state statute says, because the locality rule is not uniform settled law.
Answer: C. In traditional medical tort law, courts would rely on the standard of the particular locale where the tortious act took place, the so-called locality rule. This was based on the belief that different standards of care were applicable in different areas of the country, e.g., urban vs. rural. The rule can be traced to Small v. Howard,1 an 1880 Massachusetts opinion, which was subsequently overruled in 1968.
Factors favoring the trend away from a local standard toward a national standard include conformity in medical school and residency curricula, and prescribed board certification requirements. Internet access and telemedicine have further propagated this uniformity. Finally, two additional facets of modern medicine – continuing medical education and published clinical practice guidelines – are at odds with a rule geared toward local standards.
One argument against the locality rule is that undue reliance on an outdated mode of practice will perpetuate substandard care. In an older New York malpractice case where a newborn became blind, the pediatrician cited local custom to defend the prolonged use of oxygen to treat preterm infants, despite evidence that this practice might have serious consequences. However, the court of appeals held that the pediatrician’s superior knowledge of the increased risk of hyperoxygenation should have enabled him to use his best judgment instead of relying on the indefensible local custom.
Under a strict version of the locality rule, otherwise qualified expert witnesses may be excluded if they are not practitioners in the locale in question. Still, some courts may allow out-of-state experts to offer their opinions. This has been especially helpful to plaintiffs who are far less likely to be able to secure willing local experts, given the reluctance of many physicians to testify against a fellow doctor in their community.
Take Tennessee as an example. It once excluded the expert testimony of an orthopedic surgeon from Johnson City because the expert witness testified about the national standard and did not have actual knowledge of the standard of care in Nashville, the community where the alleged malpractice occurred.
The Tennessee Court of Appeals later ruled that expert witnesses had to have “personal” or “firsthand knowledge” of the community standard of care, and that interviewing other physicians in the area did not suffice. It subsequently clarified that an expert witness need not actually practice in the same or similar locale, and that professional contact with physicians from comparable communities, such as through referrals, would be acceptable.
Two well-known cases touching on the locality rule bear summarizing: In Swink v. Weintraub,2 Mrs. Swink bled into her pericardium during repair of a defective pacemaker electric lead. She died as a result, and her family pursued a wrongful death action, alleging negligence including delayed pericardiocentesis and surgical intervention.
The jury returned a verdict for the plaintiff, awarding damages in the amount of $1,047,732.20. On appeal, the defendants argued that North Carolina’s locality rule extended to all aspects of a negligence action, and that the trial court erred in admitting expert opinions without regard to whether those opinions reflected the “same or similar community” standard of care.
But the court of appeals disagreed, emphasizing that compliance with the “same or similar community” standard does not necessarily exonerate a defendant from an allegation of medical negligence. The court said liability can be established if the defendant did not exercise his “best judgment” in the treatment of the patient or if the defendant failed to use “reasonable care and diligence” in his efforts to render medical assistance.
In McClure v. Inova Medical Group, a Virginia jury found that a family practice resident had failed to meet the Virginia community standard of care when he did not order the prostate-specific antigen (PSA) test in a 53-year-old patient who was subsequently diagnosed with prostate cancer. The doctor had discussed the risks and benefits of PSA testing, but the patient declined taking the test. Jurors sided with the plaintiff’s argument that according to the local or statewide standard, Virginia doctors simply ordered the test as a matter of routine for men older than 50 years without necessarily discussing risks and benefits. The court awarded $1 million to the patient.3
Although the majority of jurisdictions have abandoned the locality rule, several continue to adhere to either a strict or modified version.4 Examples are Arizona, Idaho, New York, Tennessee, Virginia, and Washington. A modified rule exists in Louisiana, which holds general practitioners to a community standard and specialists to a national standard.
Finally, many authors have recommended a narrowly constructed rule based not on geographic boundaries, but on the availability of local resources. Courts would then look at the totality of circumstances, but remember that there is always the duty to refer or transfer to an available specialist/facility – and that the failure to do so may form the basis of liability.
As one physician put it: Location should not come into play with respect to the knowledge or skill of the treating physician; and even if a physician may not have the facilities to perform an emergency cesarean section, he or she should still know when it’s called for.
References
1. Small v. Howard, 128 Mass 131 (1880).
2. Swink v. Weintraub, 672 S.E.2d 53 (N.C. Court of Appeals 2009).
3. JAMA. 2004 Jan 7;291(1):15-6.
4. JAMA. 2007 Jun 20;297(23):2633-7.
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
Ultrasound improves early diagnosis of ventilator-associated pneumonia
The use of lung ultrasound, both alone and in combination with clinical and microbiologic data, can improve the early diagnosis of ventilator-associated pneumonia (VAP), according to the results of a study published in Chest.
The early diagnosis of VAP is challenging, and leaves intensivists with two options. The first is waiting for positive results from patients’ specimens, which delays treatment and increases mortality risk. The other is to administer antibiotics to all patients suspected of having VAP, which may be inappropriate and can lead to the development of multiresistant bacteria. “A pressing need therefore exists for reliable diagnostic tools to diagnose VAP early so that antibiotics can be promptly initiated, avoiding two extreme approaches,” wrote Dr. Silvia Mongodi of the Fondazione IRCCS Policlinico San Matteo in Pavia, Italy, and her colleagues.
Based on the results of previous research, the investigators hypothesized that lung ultrasound (LUS) could be used to diagnose VAP early and help to avoid treatment delays or mistakes. To test this hypothesis, the diagnostic performance of LUS alone and in combination with clinical and microbiologic data was evaluated prospectively in 99 patients with suspected VAP in ICUs at Saint Joseph Hospital (Paris), Fondazione IRCCS Policlinico San Matteo, and Centre Hospitalier de l’Université de Montréal (Chest. 2016 Apr;149[4]:969-80. doi: 10.1016/j.chest.2015.12.012).
The study results showed that subpleural consolidations and dynamic linear/arborescent air bronchograms were the principal LUS signs of VAP, and that the presence of both in the same individual made the diagnosis highly specific (88%), with a high positive predictive value (86%) and a positive likelihood ratio of 2.9. Furthermore, the addition of data from either of two different endotracheal aspirate assessment techniques (EAgram [direct Gram stain examination] or EAquant [direct Gram stain culture]) to the data from the principal LUS signs showed 97% specificity with each technique and positive likelihood ratios of 6.6 and 7.1, respectively, Dr. Mongodi and her associates reported.
Dr. Mongodi and her colleagues said that their results were encouraging but would need to be validated in larger clinical trials.
No funding was received for this study. The authors reported no conflicts of interest.
Dr. Daniel Ouellette, FCCP comments: Ultrasound techniques are increasingly being used in the intensive care unit to direct physician decisions. A report by Mongodi and colleagues suggests that ultrasound may be employed to diagnose ventilator-associated pneumonia in critically ill patients. While promising, this study is limited by small patient numbers and by the fact that reliable criteria to diagnose VAP are lacking. Further research is needed before this technique can be used reliably in the ICU.
Dr. Daniel Ouellette, FCCP comments: Ultrasound techniques are increasingly being used in the intensive care unit to direct physician decisions. A report by Mongodi and colleagues suggests that ultrasound may be employed to diagnose ventilator-associated pneumonia in critically ill patients. While promising, this study is limited by small patient numbers and by the fact that reliable criteria to diagnose VAP are lacking. Further research is needed before this technique can be used reliably in the ICU.
Dr. Daniel Ouellette, FCCP comments: Ultrasound techniques are increasingly being used in the intensive care unit to direct physician decisions. A report by Mongodi and colleagues suggests that ultrasound may be employed to diagnose ventilator-associated pneumonia in critically ill patients. While promising, this study is limited by small patient numbers and by the fact that reliable criteria to diagnose VAP are lacking. Further research is needed before this technique can be used reliably in the ICU.
The use of lung ultrasound, both alone and in combination with clinical and microbiologic data, can improve the early diagnosis of ventilator-associated pneumonia (VAP), according to the results of a study published in Chest.
The early diagnosis of VAP is challenging, and leaves intensivists with two options. The first is waiting for positive results from patients’ specimens, which delays treatment and increases mortality risk. The other is to administer antibiotics to all patients suspected of having VAP, which may be inappropriate and can lead to the development of multiresistant bacteria. “A pressing need therefore exists for reliable diagnostic tools to diagnose VAP early so that antibiotics can be promptly initiated, avoiding two extreme approaches,” wrote Dr. Silvia Mongodi of the Fondazione IRCCS Policlinico San Matteo in Pavia, Italy, and her colleagues.
Based on the results of previous research, the investigators hypothesized that lung ultrasound (LUS) could be used to diagnose VAP early and help to avoid treatment delays or mistakes. To test this hypothesis, the diagnostic performance of LUS alone and in combination with clinical and microbiologic data was evaluated prospectively in 99 patients with suspected VAP in ICUs at Saint Joseph Hospital (Paris), Fondazione IRCCS Policlinico San Matteo, and Centre Hospitalier de l’Université de Montréal (Chest. 2016 Apr;149[4]:969-80. doi: 10.1016/j.chest.2015.12.012).
The study results showed that subpleural consolidations and dynamic linear/arborescent air bronchograms were the principal LUS signs of VAP, and that the presence of both in the same individual made the diagnosis highly specific (88%), with a high positive predictive value (86%) and a positive likelihood ratio of 2.9. Furthermore, the addition of data from either of two different endotracheal aspirate assessment techniques (EAgram [direct Gram stain examination] or EAquant [direct Gram stain culture]) to the data from the principal LUS signs showed 97% specificity with each technique and positive likelihood ratios of 6.6 and 7.1, respectively, Dr. Mongodi and her associates reported.
Dr. Mongodi and her colleagues said that their results were encouraging but would need to be validated in larger clinical trials.
No funding was received for this study. The authors reported no conflicts of interest.
The use of lung ultrasound, both alone and in combination with clinical and microbiologic data, can improve the early diagnosis of ventilator-associated pneumonia (VAP), according to the results of a study published in Chest.
The early diagnosis of VAP is challenging, and leaves intensivists with two options. The first is waiting for positive results from patients’ specimens, which delays treatment and increases mortality risk. The other is to administer antibiotics to all patients suspected of having VAP, which may be inappropriate and can lead to the development of multiresistant bacteria. “A pressing need therefore exists for reliable diagnostic tools to diagnose VAP early so that antibiotics can be promptly initiated, avoiding two extreme approaches,” wrote Dr. Silvia Mongodi of the Fondazione IRCCS Policlinico San Matteo in Pavia, Italy, and her colleagues.
Based on the results of previous research, the investigators hypothesized that lung ultrasound (LUS) could be used to diagnose VAP early and help to avoid treatment delays or mistakes. To test this hypothesis, the diagnostic performance of LUS alone and in combination with clinical and microbiologic data was evaluated prospectively in 99 patients with suspected VAP in ICUs at Saint Joseph Hospital (Paris), Fondazione IRCCS Policlinico San Matteo, and Centre Hospitalier de l’Université de Montréal (Chest. 2016 Apr;149[4]:969-80. doi: 10.1016/j.chest.2015.12.012).
The study results showed that subpleural consolidations and dynamic linear/arborescent air bronchograms were the principal LUS signs of VAP, and that the presence of both in the same individual made the diagnosis highly specific (88%), with a high positive predictive value (86%) and a positive likelihood ratio of 2.9. Furthermore, the addition of data from either of two different endotracheal aspirate assessment techniques (EAgram [direct Gram stain examination] or EAquant [direct Gram stain culture]) to the data from the principal LUS signs showed 97% specificity with each technique and positive likelihood ratios of 6.6 and 7.1, respectively, Dr. Mongodi and her associates reported.
Dr. Mongodi and her colleagues said that their results were encouraging but would need to be validated in larger clinical trials.
No funding was received for this study. The authors reported no conflicts of interest.
Key clinical point: The specificity of the examination for ventilator-associated pneumonia diagnosis could be increased with daily lung-ultrasound monitoring of ICU patients.
Major finding: Lung ultrasound reliably improved the diagnosis of ventilator-associated pneumonia with high specificity (88%), high positive predictive value (86%), and a positive likelihood ratio of 2.9.
Data sources: Patients with suspected ventilator-associated pneumonia in ICUs in France, Italy, and Canada.
Disclosures: No funding was received for this study. The authors reported no conflicts of interest.
VIDEO: SCOTUS decision sends contraception mandate to lower courts
WASHINGTON – It will be up to the lower courts to decide how to work out religious exemptions under the Affordable Care Act’s contraception mandate, following the Supreme Court’s decision to remand Zubik v. Burwell back to the U.S. Court of Appeals for the 3rd, 5th, 10th, and District of Columbia Circuits.
In an unusual move, on May 16 the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and has remanded the case back to the four appeals courts that had originally ruled on the issue.
At issue in the case is the implementation of the Affordable Care Act’s contraception mandate and specifically how nonprofit religious employers can opt out of directly paying for their employees’ contraception. The federal government had created a workaround that required employers to submit a form stating that they have religious objections, but the plaintiffs asserted that the process itself was a violation of their religious freedom.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In March, the high court asked all parties in the case to submit additional briefs outlining how contraception could be provided without requiring notice on the part of the employers. After reviewing the briefs, the Supreme Court justices concluded that “such an option is feasible.”
“Given the gravity of the dispute and the substantial clarification and refinement in the positions of the parties, the parties on remand should be afforded an opportunity to arrive at an approach going forward that accommodates petitioners’ religious exercise while at the same time ensuring that women covered by petitioners’ health plans ‘receive full and equal health coverage, including contraceptive coverage,’ ” the justices wrote in the decision. “We anticipate that the Courts of Appeals will allow the parties sufficient time to resolve any outstanding issues between them.”
The Supreme Court made no decision about the merits of Zubik v. Burwell.
Dr. Sara Imershein, a clinical professor at George Washington University and an ob.gyn. at Planned Parenthood in Washington, said the decision was a disappointment because it requires the courts to sort out a workaround to the contraception mandate when the government has already put one in place. Dr. Imershein, who is a reproductive rights advocate, commented on the news in a video interview while attending the annual meeting of the American College of Obstetricians and Gynecologists.
Dr. Mark S. DeFrancesco, ACOG president, expressed the college’s disappointment in the Supreme Court’s decision.
“ACOG strongly believes that contraception is an essential part of women’s preventive care, and that any accommodation to employers’ beliefs must not impose barriers to women’s ability to access contraception,” Dr. DeFrancesco said in a statement. “We encourage the lower courts to adopt a solution that ensures that coverage is provided seamlessly ‘through petitioner’s insurance companies.’”
On Twitter @maryellenny
WASHINGTON – It will be up to the lower courts to decide how to work out religious exemptions under the Affordable Care Act’s contraception mandate, following the Supreme Court’s decision to remand Zubik v. Burwell back to the U.S. Court of Appeals for the 3rd, 5th, 10th, and District of Columbia Circuits.
In an unusual move, on May 16 the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and has remanded the case back to the four appeals courts that had originally ruled on the issue.
At issue in the case is the implementation of the Affordable Care Act’s contraception mandate and specifically how nonprofit religious employers can opt out of directly paying for their employees’ contraception. The federal government had created a workaround that required employers to submit a form stating that they have religious objections, but the plaintiffs asserted that the process itself was a violation of their religious freedom.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In March, the high court asked all parties in the case to submit additional briefs outlining how contraception could be provided without requiring notice on the part of the employers. After reviewing the briefs, the Supreme Court justices concluded that “such an option is feasible.”
“Given the gravity of the dispute and the substantial clarification and refinement in the positions of the parties, the parties on remand should be afforded an opportunity to arrive at an approach going forward that accommodates petitioners’ religious exercise while at the same time ensuring that women covered by petitioners’ health plans ‘receive full and equal health coverage, including contraceptive coverage,’ ” the justices wrote in the decision. “We anticipate that the Courts of Appeals will allow the parties sufficient time to resolve any outstanding issues between them.”
The Supreme Court made no decision about the merits of Zubik v. Burwell.
Dr. Sara Imershein, a clinical professor at George Washington University and an ob.gyn. at Planned Parenthood in Washington, said the decision was a disappointment because it requires the courts to sort out a workaround to the contraception mandate when the government has already put one in place. Dr. Imershein, who is a reproductive rights advocate, commented on the news in a video interview while attending the annual meeting of the American College of Obstetricians and Gynecologists.
Dr. Mark S. DeFrancesco, ACOG president, expressed the college’s disappointment in the Supreme Court’s decision.
“ACOG strongly believes that contraception is an essential part of women’s preventive care, and that any accommodation to employers’ beliefs must not impose barriers to women’s ability to access contraception,” Dr. DeFrancesco said in a statement. “We encourage the lower courts to adopt a solution that ensures that coverage is provided seamlessly ‘through petitioner’s insurance companies.’”
On Twitter @maryellenny
WASHINGTON – It will be up to the lower courts to decide how to work out religious exemptions under the Affordable Care Act’s contraception mandate, following the Supreme Court’s decision to remand Zubik v. Burwell back to the U.S. Court of Appeals for the 3rd, 5th, 10th, and District of Columbia Circuits.
In an unusual move, on May 16 the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and has remanded the case back to the four appeals courts that had originally ruled on the issue.
At issue in the case is the implementation of the Affordable Care Act’s contraception mandate and specifically how nonprofit religious employers can opt out of directly paying for their employees’ contraception. The federal government had created a workaround that required employers to submit a form stating that they have religious objections, but the plaintiffs asserted that the process itself was a violation of their religious freedom.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In March, the high court asked all parties in the case to submit additional briefs outlining how contraception could be provided without requiring notice on the part of the employers. After reviewing the briefs, the Supreme Court justices concluded that “such an option is feasible.”
“Given the gravity of the dispute and the substantial clarification and refinement in the positions of the parties, the parties on remand should be afforded an opportunity to arrive at an approach going forward that accommodates petitioners’ religious exercise while at the same time ensuring that women covered by petitioners’ health plans ‘receive full and equal health coverage, including contraceptive coverage,’ ” the justices wrote in the decision. “We anticipate that the Courts of Appeals will allow the parties sufficient time to resolve any outstanding issues between them.”
The Supreme Court made no decision about the merits of Zubik v. Burwell.
Dr. Sara Imershein, a clinical professor at George Washington University and an ob.gyn. at Planned Parenthood in Washington, said the decision was a disappointment because it requires the courts to sort out a workaround to the contraception mandate when the government has already put one in place. Dr. Imershein, who is a reproductive rights advocate, commented on the news in a video interview while attending the annual meeting of the American College of Obstetricians and Gynecologists.
Dr. Mark S. DeFrancesco, ACOG president, expressed the college’s disappointment in the Supreme Court’s decision.
“ACOG strongly believes that contraception is an essential part of women’s preventive care, and that any accommodation to employers’ beliefs must not impose barriers to women’s ability to access contraception,” Dr. DeFrancesco said in a statement. “We encourage the lower courts to adopt a solution that ensures that coverage is provided seamlessly ‘through petitioner’s insurance companies.’”
On Twitter @maryellenny
AT ACOG 2016
Team describes new approach to cancer immunotherapy

Image by Kathryn T. Iacono
A new approach to cancer immunotherapy may avoid some of the shortcomings associated with other methods, according to researchers.
The group found that eliminating a key protein in regulatory T cells (Tregs) makes them so unstable that they become effector T cells (Teff) and begin to attack the cancer.
And this conversion from Treg to Teff occurs only in the inflammatory conditions that prevail within many tumors.
As a result, Tregs embedded in normal tissue throughout the body continue to have a restraining effect on their local Teffs, protecting healthy organs and tissues from attack.
The researchers said this raises the prospect of therapies that concentrate the immune system’s firepower on tumors without producing residual damage and harmful side effects.
“Many current approaches to immunotherapy involve depleting or blocking Tregs in order to shift the balance toward Teff cells,” said Harvey Cantor, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“This, however, runs the risk of triggering an autoimmune response in which the Teff cells attack normal as well as malignant tissue. The key to our approach is that it singles out the Tregs inside a tumor for conversion, leaving Tregs elsewhere in the body unchanged.”
Dr Cantor and his colleagues described the approach in PNAS.
The study builds on research published last year in Science. That study showed that Tregs maintain their immune-suppressive properties under inflammatory conditions as long as they have high enough levels of a protein called Helios. Depriving Tregs of sufficient Helios caused them to lose that stability and turn into Teff cells.
The new study explored whether this convertibility could be harnessed for therapeutic purposes in cancers.
The first set of experiments involved mice engineered to lack Helios in their Tregs. When the animals were injected with melanoma or colon cancer cells, they developed tumors far more slowly than animals with normal Tregs.
“Inspection of the animals’ tumor tissue showed an unstable set of T regulatory cells, many of which had converted into Teffs,” said Hye-Jung Kim, PhD, also of the Dana-Farber Cancer Institute.
The researchers then explored whether stanching Helios production in tumor-dwelling Tregs could have the same effect. They tested several antibodies that bind to key receptors on Tregs and cause a downturn in Helios production.
The team chose an antibody that worked well, DTA-1, and tested it in mice with Treg-laden tumors. When they analyzed the tumor tissue, it was clear that DTA-1 had triggered conversion of Tregs to Teffs.
“This represents a next stage in cancer immunotherapy,” Dr Cantor said. “We now have a very specific, targeted way of inducing a T-effector-cell attack on cancer while lowering the risk of adverse effects on healthy tissue. The next step will be to organize a clinical trial using this approach in patients.” ![]()

Image by Kathryn T. Iacono
A new approach to cancer immunotherapy may avoid some of the shortcomings associated with other methods, according to researchers.
The group found that eliminating a key protein in regulatory T cells (Tregs) makes them so unstable that they become effector T cells (Teff) and begin to attack the cancer.
And this conversion from Treg to Teff occurs only in the inflammatory conditions that prevail within many tumors.
As a result, Tregs embedded in normal tissue throughout the body continue to have a restraining effect on their local Teffs, protecting healthy organs and tissues from attack.
The researchers said this raises the prospect of therapies that concentrate the immune system’s firepower on tumors without producing residual damage and harmful side effects.
“Many current approaches to immunotherapy involve depleting or blocking Tregs in order to shift the balance toward Teff cells,” said Harvey Cantor, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“This, however, runs the risk of triggering an autoimmune response in which the Teff cells attack normal as well as malignant tissue. The key to our approach is that it singles out the Tregs inside a tumor for conversion, leaving Tregs elsewhere in the body unchanged.”
Dr Cantor and his colleagues described the approach in PNAS.
The study builds on research published last year in Science. That study showed that Tregs maintain their immune-suppressive properties under inflammatory conditions as long as they have high enough levels of a protein called Helios. Depriving Tregs of sufficient Helios caused them to lose that stability and turn into Teff cells.
The new study explored whether this convertibility could be harnessed for therapeutic purposes in cancers.
The first set of experiments involved mice engineered to lack Helios in their Tregs. When the animals were injected with melanoma or colon cancer cells, they developed tumors far more slowly than animals with normal Tregs.
“Inspection of the animals’ tumor tissue showed an unstable set of T regulatory cells, many of which had converted into Teffs,” said Hye-Jung Kim, PhD, also of the Dana-Farber Cancer Institute.
The researchers then explored whether stanching Helios production in tumor-dwelling Tregs could have the same effect. They tested several antibodies that bind to key receptors on Tregs and cause a downturn in Helios production.
The team chose an antibody that worked well, DTA-1, and tested it in mice with Treg-laden tumors. When they analyzed the tumor tissue, it was clear that DTA-1 had triggered conversion of Tregs to Teffs.
“This represents a next stage in cancer immunotherapy,” Dr Cantor said. “We now have a very specific, targeted way of inducing a T-effector-cell attack on cancer while lowering the risk of adverse effects on healthy tissue. The next step will be to organize a clinical trial using this approach in patients.” ![]()

Image by Kathryn T. Iacono
A new approach to cancer immunotherapy may avoid some of the shortcomings associated with other methods, according to researchers.
The group found that eliminating a key protein in regulatory T cells (Tregs) makes them so unstable that they become effector T cells (Teff) and begin to attack the cancer.
And this conversion from Treg to Teff occurs only in the inflammatory conditions that prevail within many tumors.
As a result, Tregs embedded in normal tissue throughout the body continue to have a restraining effect on their local Teffs, protecting healthy organs and tissues from attack.
The researchers said this raises the prospect of therapies that concentrate the immune system’s firepower on tumors without producing residual damage and harmful side effects.
“Many current approaches to immunotherapy involve depleting or blocking Tregs in order to shift the balance toward Teff cells,” said Harvey Cantor, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“This, however, runs the risk of triggering an autoimmune response in which the Teff cells attack normal as well as malignant tissue. The key to our approach is that it singles out the Tregs inside a tumor for conversion, leaving Tregs elsewhere in the body unchanged.”
Dr Cantor and his colleagues described the approach in PNAS.
The study builds on research published last year in Science. That study showed that Tregs maintain their immune-suppressive properties under inflammatory conditions as long as they have high enough levels of a protein called Helios. Depriving Tregs of sufficient Helios caused them to lose that stability and turn into Teff cells.
The new study explored whether this convertibility could be harnessed for therapeutic purposes in cancers.
The first set of experiments involved mice engineered to lack Helios in their Tregs. When the animals were injected with melanoma or colon cancer cells, they developed tumors far more slowly than animals with normal Tregs.
“Inspection of the animals’ tumor tissue showed an unstable set of T regulatory cells, many of which had converted into Teffs,” said Hye-Jung Kim, PhD, also of the Dana-Farber Cancer Institute.
The researchers then explored whether stanching Helios production in tumor-dwelling Tregs could have the same effect. They tested several antibodies that bind to key receptors on Tregs and cause a downturn in Helios production.
The team chose an antibody that worked well, DTA-1, and tested it in mice with Treg-laden tumors. When they analyzed the tumor tissue, it was clear that DTA-1 had triggered conversion of Tregs to Teffs.
“This represents a next stage in cancer immunotherapy,” Dr Cantor said. “We now have a very specific, targeted way of inducing a T-effector-cell attack on cancer while lowering the risk of adverse effects on healthy tissue. The next step will be to organize a clinical trial using this approach in patients.” ![]()
Physical activity may lower risk of some cancers

Photo by K. Johansson
Being physically active during leisure time may lower a person’s risk of certain cancers, according to a new study.
A high level of physical activity was associated with a 20% lower risk of myeloid leukemia, a 17% lower risk of myeloma, a 9% lower risk of non-Hodgkin lymphoma, and a 7% lower risk of cancer in general.
On the other hand, a high level of physical activity was also associated with a higher risk of malignant melanoma and prostate cancer.
Steven C. Moore, PhD, of the National Cancer Institute in Bethesda, Maryland, and his colleagues reported these findings in JAMA Internal Medicine.
The researchers pooled data from 12 US and European study cohorts with self-reported physical activity (1987-2004). And they analyzed associations between physical activity and 26 types of cancer.
The study included 1.4 million participants, and 186,932 cancers were identified during a median of 11 years of follow-up.
Compared with the lowest level of leisure-time physical activity (10th percentile), the highest level of activity (90th percentile) had strong inverse associations (a 20% or greater reduction in risk) for 7 cancer types:
- Myeloid leukemia (hazard ratio [HR]=0.80 [95% CI, 0.70-0.92])
- Esophageal adenocarcinoma (HR=0.58 [95% CI, 0.37-0.89])
- Liver cancer (HR=0.73 [95% CI, 0.55-0.98])
- Lung cancer (HR=0.74 [95% CI, 0.71-0.77])
- Kidney cancer (HR=0.77 [95% CI, 0.70-0.85])
- Gastric cardia (HR=0.78 [95% CI, 0.64-0.95])
- Endometrial cancer (HR=0.79 [95% CI, 0.68-0.92]).
There were moderate inverse associations (a 10% to 20% reduction in risk) between the highest level of activity and 6 cancers:
- Myeloma (HR=0.83 [95% CI, 0.72-0.95])
- Colon cancer (HR=0.84 [95% CI, 0.77-0.91])
- Head and neck cancer (HR=0.85 [95% CI, 0.78-0.93])
- Rectal cancer (HR=0.87 [95% CI, 0.80-0.95])
- Bladder cancer (HR=0.87 [95% CI, 0.82-0.92])
- Breast cancer (HR=0.90 [95% CI, 0.87-0.93]).
And there were suggestive inverse associations between the highest level of activity and 3 cancers:
- Non-Hodgkin lymphoma (HR=0.91 [95% CI, 0.83-1.00])
- Gallbladder cancer (HR=0.72 [95% CI, 0.51-1.01])
- Small intestine cancer (HR=0.78 [95% CI, 0.60-1.00]).
However, the highest level of activity was also associated with an increased risk of prostate cancer (HR=1.05 [95% CI, 1.03-1.08]) and malignant melanoma (HR=1.27 [95% CI, 1.16-1.40]).
The researchers said the main limitation of this study is that they cannot fully exclude the possibility that diet, smoking, and other factors may have affected these results. Also, the study used self-reported physical activity, which can mean errors in recall.
Still, the team said these findings support promoting physical activity as a key component of population-wide cancer prevention and control efforts. ![]()

Photo by K. Johansson
Being physically active during leisure time may lower a person’s risk of certain cancers, according to a new study.
A high level of physical activity was associated with a 20% lower risk of myeloid leukemia, a 17% lower risk of myeloma, a 9% lower risk of non-Hodgkin lymphoma, and a 7% lower risk of cancer in general.
On the other hand, a high level of physical activity was also associated with a higher risk of malignant melanoma and prostate cancer.
Steven C. Moore, PhD, of the National Cancer Institute in Bethesda, Maryland, and his colleagues reported these findings in JAMA Internal Medicine.
The researchers pooled data from 12 US and European study cohorts with self-reported physical activity (1987-2004). And they analyzed associations between physical activity and 26 types of cancer.
The study included 1.4 million participants, and 186,932 cancers were identified during a median of 11 years of follow-up.
Compared with the lowest level of leisure-time physical activity (10th percentile), the highest level of activity (90th percentile) had strong inverse associations (a 20% or greater reduction in risk) for 7 cancer types:
- Myeloid leukemia (hazard ratio [HR]=0.80 [95% CI, 0.70-0.92])
- Esophageal adenocarcinoma (HR=0.58 [95% CI, 0.37-0.89])
- Liver cancer (HR=0.73 [95% CI, 0.55-0.98])
- Lung cancer (HR=0.74 [95% CI, 0.71-0.77])
- Kidney cancer (HR=0.77 [95% CI, 0.70-0.85])
- Gastric cardia (HR=0.78 [95% CI, 0.64-0.95])
- Endometrial cancer (HR=0.79 [95% CI, 0.68-0.92]).
There were moderate inverse associations (a 10% to 20% reduction in risk) between the highest level of activity and 6 cancers:
- Myeloma (HR=0.83 [95% CI, 0.72-0.95])
- Colon cancer (HR=0.84 [95% CI, 0.77-0.91])
- Head and neck cancer (HR=0.85 [95% CI, 0.78-0.93])
- Rectal cancer (HR=0.87 [95% CI, 0.80-0.95])
- Bladder cancer (HR=0.87 [95% CI, 0.82-0.92])
- Breast cancer (HR=0.90 [95% CI, 0.87-0.93]).
And there were suggestive inverse associations between the highest level of activity and 3 cancers:
- Non-Hodgkin lymphoma (HR=0.91 [95% CI, 0.83-1.00])
- Gallbladder cancer (HR=0.72 [95% CI, 0.51-1.01])
- Small intestine cancer (HR=0.78 [95% CI, 0.60-1.00]).
However, the highest level of activity was also associated with an increased risk of prostate cancer (HR=1.05 [95% CI, 1.03-1.08]) and malignant melanoma (HR=1.27 [95% CI, 1.16-1.40]).
The researchers said the main limitation of this study is that they cannot fully exclude the possibility that diet, smoking, and other factors may have affected these results. Also, the study used self-reported physical activity, which can mean errors in recall.
Still, the team said these findings support promoting physical activity as a key component of population-wide cancer prevention and control efforts. ![]()

Photo by K. Johansson
Being physically active during leisure time may lower a person’s risk of certain cancers, according to a new study.
A high level of physical activity was associated with a 20% lower risk of myeloid leukemia, a 17% lower risk of myeloma, a 9% lower risk of non-Hodgkin lymphoma, and a 7% lower risk of cancer in general.
On the other hand, a high level of physical activity was also associated with a higher risk of malignant melanoma and prostate cancer.
Steven C. Moore, PhD, of the National Cancer Institute in Bethesda, Maryland, and his colleagues reported these findings in JAMA Internal Medicine.
The researchers pooled data from 12 US and European study cohorts with self-reported physical activity (1987-2004). And they analyzed associations between physical activity and 26 types of cancer.
The study included 1.4 million participants, and 186,932 cancers were identified during a median of 11 years of follow-up.
Compared with the lowest level of leisure-time physical activity (10th percentile), the highest level of activity (90th percentile) had strong inverse associations (a 20% or greater reduction in risk) for 7 cancer types:
- Myeloid leukemia (hazard ratio [HR]=0.80 [95% CI, 0.70-0.92])
- Esophageal adenocarcinoma (HR=0.58 [95% CI, 0.37-0.89])
- Liver cancer (HR=0.73 [95% CI, 0.55-0.98])
- Lung cancer (HR=0.74 [95% CI, 0.71-0.77])
- Kidney cancer (HR=0.77 [95% CI, 0.70-0.85])
- Gastric cardia (HR=0.78 [95% CI, 0.64-0.95])
- Endometrial cancer (HR=0.79 [95% CI, 0.68-0.92]).
There were moderate inverse associations (a 10% to 20% reduction in risk) between the highest level of activity and 6 cancers:
- Myeloma (HR=0.83 [95% CI, 0.72-0.95])
- Colon cancer (HR=0.84 [95% CI, 0.77-0.91])
- Head and neck cancer (HR=0.85 [95% CI, 0.78-0.93])
- Rectal cancer (HR=0.87 [95% CI, 0.80-0.95])
- Bladder cancer (HR=0.87 [95% CI, 0.82-0.92])
- Breast cancer (HR=0.90 [95% CI, 0.87-0.93]).
And there were suggestive inverse associations between the highest level of activity and 3 cancers:
- Non-Hodgkin lymphoma (HR=0.91 [95% CI, 0.83-1.00])
- Gallbladder cancer (HR=0.72 [95% CI, 0.51-1.01])
- Small intestine cancer (HR=0.78 [95% CI, 0.60-1.00]).
However, the highest level of activity was also associated with an increased risk of prostate cancer (HR=1.05 [95% CI, 1.03-1.08]) and malignant melanoma (HR=1.27 [95% CI, 1.16-1.40]).
The researchers said the main limitation of this study is that they cannot fully exclude the possibility that diet, smoking, and other factors may have affected these results. Also, the study used self-reported physical activity, which can mean errors in recall.
Still, the team said these findings support promoting physical activity as a key component of population-wide cancer prevention and control efforts. ![]()
Reversal agent granted conditional approval in Canada

to prevent thrombosis after
knee replacement surgery
© Boehringer Ingelheim
Health Canada has granted conditional approval for idarucizumab (Praxbind), a humanized antibody fragment designed to reverse the anticoagulant effects of dabigatran etexilate (Pradaxa) in cases of emergency surgery/urgent procedures or in situations of life-threatening or uncontrolled bleeding.
The conditional approval of idarucizumab reflects the promising nature of the available clinical evidence.
For the drug to gain full approval, Boehringer Ingelheim—the company that markets both idarucizumab and dabigatran—must provide Health Canada with data confirming that idarucizumab provides a clinical benefit.
To date, study results have demonstrated that 5g of idarucizumab provides immediate, complete, and sustained reversal of the anticoagulant effects of dabigatran in most patients.
In the ongoing phase 3 RE-VERSE AD trial, researchers are evaluating idarucizumab in emergency settings.
Interim results from this trial showed that idarucizumab normalized diluted thrombin time and ecarin clotting time in a majority of dabigatran-treated patients with uncontrolled or life-threatening bleeding complications and most patients who required emergency surgery or an invasive procedure.
Researchers said there were no safety concerns related to idarucizumab. However, 23% of patients in this trial experienced serious adverse events, 20% of patients died, and several patients had thrombotic or bleeding events. ![]()

to prevent thrombosis after
knee replacement surgery
© Boehringer Ingelheim
Health Canada has granted conditional approval for idarucizumab (Praxbind), a humanized antibody fragment designed to reverse the anticoagulant effects of dabigatran etexilate (Pradaxa) in cases of emergency surgery/urgent procedures or in situations of life-threatening or uncontrolled bleeding.
The conditional approval of idarucizumab reflects the promising nature of the available clinical evidence.
For the drug to gain full approval, Boehringer Ingelheim—the company that markets both idarucizumab and dabigatran—must provide Health Canada with data confirming that idarucizumab provides a clinical benefit.
To date, study results have demonstrated that 5g of idarucizumab provides immediate, complete, and sustained reversal of the anticoagulant effects of dabigatran in most patients.
In the ongoing phase 3 RE-VERSE AD trial, researchers are evaluating idarucizumab in emergency settings.
Interim results from this trial showed that idarucizumab normalized diluted thrombin time and ecarin clotting time in a majority of dabigatran-treated patients with uncontrolled or life-threatening bleeding complications and most patients who required emergency surgery or an invasive procedure.
Researchers said there were no safety concerns related to idarucizumab. However, 23% of patients in this trial experienced serious adverse events, 20% of patients died, and several patients had thrombotic or bleeding events. ![]()

to prevent thrombosis after
knee replacement surgery
© Boehringer Ingelheim
Health Canada has granted conditional approval for idarucizumab (Praxbind), a humanized antibody fragment designed to reverse the anticoagulant effects of dabigatran etexilate (Pradaxa) in cases of emergency surgery/urgent procedures or in situations of life-threatening or uncontrolled bleeding.
The conditional approval of idarucizumab reflects the promising nature of the available clinical evidence.
For the drug to gain full approval, Boehringer Ingelheim—the company that markets both idarucizumab and dabigatran—must provide Health Canada with data confirming that idarucizumab provides a clinical benefit.
To date, study results have demonstrated that 5g of idarucizumab provides immediate, complete, and sustained reversal of the anticoagulant effects of dabigatran in most patients.
In the ongoing phase 3 RE-VERSE AD trial, researchers are evaluating idarucizumab in emergency settings.
Interim results from this trial showed that idarucizumab normalized diluted thrombin time and ecarin clotting time in a majority of dabigatran-treated patients with uncontrolled or life-threatening bleeding complications and most patients who required emergency surgery or an invasive procedure.
Researchers said there were no safety concerns related to idarucizumab. However, 23% of patients in this trial experienced serious adverse events, 20% of patients died, and several patients had thrombotic or bleeding events. ![]()
Improving NK cell therapy

Image by Joshua Stokes
New findings published in PNAS may help scientists improve the efficacy of natural killer (NK) cell therapy for patients with leukemia.
The preclinical research revealed a tolerance mechanism that restrains the activity of NK cells, as well as a potential way to overcome this problem.
Investigators found that a transcription factor, Kruppel-like factor 2 (KFL2), is critical for NK cell expansion and survival.
Specifically, KLF2 limits immature NK cell proliferation and instructs mature NK cells to home to niches rich in interleukin 15 (IL-15), which is necessary for their continued survival.
“This is the same process likely used by cancer cells to avoid destruction by NK cells,” said study author Eric Sebzda, PhD, of Vanderbilt University Medical Center in Nashville, Tennessee.
In particular, tumors may avoid immune clearance by promoting KLF2 destruction within the NK cell population, thereby starving these cells of IL-15.
Dr Sebzda and his colleagues noted that increased expression of IL-15 can improve immune responses against tumors. Unfortunately, it’s not easy to introduce the cytokine only within a tumor microenvironment, and high systemic levels of IL-15 can be toxic.
Recruiting cells that transpresent IL-15 to the tumor microenvironment may overcome this barrier and therefore improve NK cell-mediated cancer therapy, the investigators said. However, the methodology hasn’t been worked out yet.
“Our paper should encourage this line of inquiry,” Dr Sebzda concluded. ![]()

Image by Joshua Stokes
New findings published in PNAS may help scientists improve the efficacy of natural killer (NK) cell therapy for patients with leukemia.
The preclinical research revealed a tolerance mechanism that restrains the activity of NK cells, as well as a potential way to overcome this problem.
Investigators found that a transcription factor, Kruppel-like factor 2 (KFL2), is critical for NK cell expansion and survival.
Specifically, KLF2 limits immature NK cell proliferation and instructs mature NK cells to home to niches rich in interleukin 15 (IL-15), which is necessary for their continued survival.
“This is the same process likely used by cancer cells to avoid destruction by NK cells,” said study author Eric Sebzda, PhD, of Vanderbilt University Medical Center in Nashville, Tennessee.
In particular, tumors may avoid immune clearance by promoting KLF2 destruction within the NK cell population, thereby starving these cells of IL-15.
Dr Sebzda and his colleagues noted that increased expression of IL-15 can improve immune responses against tumors. Unfortunately, it’s not easy to introduce the cytokine only within a tumor microenvironment, and high systemic levels of IL-15 can be toxic.
Recruiting cells that transpresent IL-15 to the tumor microenvironment may overcome this barrier and therefore improve NK cell-mediated cancer therapy, the investigators said. However, the methodology hasn’t been worked out yet.
“Our paper should encourage this line of inquiry,” Dr Sebzda concluded. ![]()

Image by Joshua Stokes
New findings published in PNAS may help scientists improve the efficacy of natural killer (NK) cell therapy for patients with leukemia.
The preclinical research revealed a tolerance mechanism that restrains the activity of NK cells, as well as a potential way to overcome this problem.
Investigators found that a transcription factor, Kruppel-like factor 2 (KFL2), is critical for NK cell expansion and survival.
Specifically, KLF2 limits immature NK cell proliferation and instructs mature NK cells to home to niches rich in interleukin 15 (IL-15), which is necessary for their continued survival.
“This is the same process likely used by cancer cells to avoid destruction by NK cells,” said study author Eric Sebzda, PhD, of Vanderbilt University Medical Center in Nashville, Tennessee.
In particular, tumors may avoid immune clearance by promoting KLF2 destruction within the NK cell population, thereby starving these cells of IL-15.
Dr Sebzda and his colleagues noted that increased expression of IL-15 can improve immune responses against tumors. Unfortunately, it’s not easy to introduce the cytokine only within a tumor microenvironment, and high systemic levels of IL-15 can be toxic.
Recruiting cells that transpresent IL-15 to the tumor microenvironment may overcome this barrier and therefore improve NK cell-mediated cancer therapy, the investigators said. However, the methodology hasn’t been worked out yet.
“Our paper should encourage this line of inquiry,” Dr Sebzda concluded. ![]()