User login
Members Attending VAM in for a “Capitol” Treat!
When in D.C., well, pay attention to politics! Especially in an election year ...
Attendees at the Vascular Annual Meeting will be treated to a political comedy show from 8:30 to 9:30 p.m. Friday, June 10. The “Capitol Steps” group will poke its traditional fun at politics, politicians and the world at large as it has since the Reagan administration.
The event is free and open to all registered attendees, guests and exhibitors.
The group mixes standup comedy with song parodies, exploring the hot topics of the day, from the Democratic debates to “Apple vs. the FBI” to “If There were No Rich Men” and “Kasich is the Hardest Rhyme.” With the conventions scheduled for later this year, who knows what the Capitol Steps will be talking about, come June?
Come and find out. And be prepared to laugh heartily.
When in D.C., well, pay attention to politics! Especially in an election year ...
Attendees at the Vascular Annual Meeting will be treated to a political comedy show from 8:30 to 9:30 p.m. Friday, June 10. The “Capitol Steps” group will poke its traditional fun at politics, politicians and the world at large as it has since the Reagan administration.
The event is free and open to all registered attendees, guests and exhibitors.
The group mixes standup comedy with song parodies, exploring the hot topics of the day, from the Democratic debates to “Apple vs. the FBI” to “If There were No Rich Men” and “Kasich is the Hardest Rhyme.” With the conventions scheduled for later this year, who knows what the Capitol Steps will be talking about, come June?
Come and find out. And be prepared to laugh heartily.
When in D.C., well, pay attention to politics! Especially in an election year ...
Attendees at the Vascular Annual Meeting will be treated to a political comedy show from 8:30 to 9:30 p.m. Friday, June 10. The “Capitol Steps” group will poke its traditional fun at politics, politicians and the world at large as it has since the Reagan administration.
The event is free and open to all registered attendees, guests and exhibitors.
The group mixes standup comedy with song parodies, exploring the hot topics of the day, from the Democratic debates to “Apple vs. the FBI” to “If There were No Rich Men” and “Kasich is the Hardest Rhyme.” With the conventions scheduled for later this year, who knows what the Capitol Steps will be talking about, come June?
Come and find out. And be prepared to laugh heartily.
Apply for International Scholarships by June 17
Up to four $5,000 scholarships to attend the 2017 Vascular Annual Meeting in San Diego are available to qualified young vascular surgeons living outside of North America.
In addition to the meeting, scholars also will visit clinical, teaching and research programs in the United States and Canada. Applications can be completed online.
The application deadline is June 17, 2016. For questions, email [email protected] or call 312-334-2300. Click here for more information.
Up to four $5,000 scholarships to attend the 2017 Vascular Annual Meeting in San Diego are available to qualified young vascular surgeons living outside of North America.
In addition to the meeting, scholars also will visit clinical, teaching and research programs in the United States and Canada. Applications can be completed online.
The application deadline is June 17, 2016. For questions, email [email protected] or call 312-334-2300. Click here for more information.
Up to four $5,000 scholarships to attend the 2017 Vascular Annual Meeting in San Diego are available to qualified young vascular surgeons living outside of North America.
In addition to the meeting, scholars also will visit clinical, teaching and research programs in the United States and Canada. Applications can be completed online.
The application deadline is June 17, 2016. For questions, email [email protected] or call 312-334-2300. Click here for more information.
VIDEO: Sutureless Heart Valve: A Critical Innovation
BALTIMORE – The sutureless heart valve may now be a “necessity” for contemporary cardiothoracic surgeons, said an expert at the annual meeting of the American Association for Thoracic Surgery.
In an interview at the event, Niv Ad, MD, chief of cardiac surgery at Inova Heart and Vascular Institute, Falls Church, Va., discussed the results of an international trial evaluating clinical outcomes of two patient subgroups implanted with a sutureless valve prosthesis. He was enthusiastic about the trial’s results and the future promise of the sutureless valve.
The study, "Clinical Outcomes in Low and Intermediate-High Risk Groups with a Sutureless Heart Valve," will be presented at 7:30 a.m. on Wednesday.
“This study teaches us that the technology is safe,” he said. “This is really great news for surgeons, cardiologists, and patients. Complications associated with implantation of the valves were fairly low in their incidence, and the survival rates at 1 year were high.”
The study, led by Axel Haverich, MD, of Hannover Medical School in Germany, confirmed the safety and performance of the valve in both patient groups – high and low risk – regardless of the preoperative risk score. Dr. Ad said that sutureless valve technology would likely replace suture technology in all cases, although perhaps not in the near term.
Dr. Ad reported no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @richpizzi
BALTIMORE – The sutureless heart valve may now be a “necessity” for contemporary cardiothoracic surgeons, said an expert at the annual meeting of the American Association for Thoracic Surgery.
In an interview at the event, Niv Ad, MD, chief of cardiac surgery at Inova Heart and Vascular Institute, Falls Church, Va., discussed the results of an international trial evaluating clinical outcomes of two patient subgroups implanted with a sutureless valve prosthesis. He was enthusiastic about the trial’s results and the future promise of the sutureless valve.
The study, "Clinical Outcomes in Low and Intermediate-High Risk Groups with a Sutureless Heart Valve," will be presented at 7:30 a.m. on Wednesday.
“This study teaches us that the technology is safe,” he said. “This is really great news for surgeons, cardiologists, and patients. Complications associated with implantation of the valves were fairly low in their incidence, and the survival rates at 1 year were high.”
The study, led by Axel Haverich, MD, of Hannover Medical School in Germany, confirmed the safety and performance of the valve in both patient groups – high and low risk – regardless of the preoperative risk score. Dr. Ad said that sutureless valve technology would likely replace suture technology in all cases, although perhaps not in the near term.
Dr. Ad reported no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @richpizzi
BALTIMORE – The sutureless heart valve may now be a “necessity” for contemporary cardiothoracic surgeons, said an expert at the annual meeting of the American Association for Thoracic Surgery.
In an interview at the event, Niv Ad, MD, chief of cardiac surgery at Inova Heart and Vascular Institute, Falls Church, Va., discussed the results of an international trial evaluating clinical outcomes of two patient subgroups implanted with a sutureless valve prosthesis. He was enthusiastic about the trial’s results and the future promise of the sutureless valve.
The study, "Clinical Outcomes in Low and Intermediate-High Risk Groups with a Sutureless Heart Valve," will be presented at 7:30 a.m. on Wednesday.
“This study teaches us that the technology is safe,” he said. “This is really great news for surgeons, cardiologists, and patients. Complications associated with implantation of the valves were fairly low in their incidence, and the survival rates at 1 year were high.”
The study, led by Axel Haverich, MD, of Hannover Medical School in Germany, confirmed the safety and performance of the valve in both patient groups – high and low risk – regardless of the preoperative risk score. Dr. Ad said that sutureless valve technology would likely replace suture technology in all cases, although perhaps not in the near term.
Dr. Ad reported no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @richpizzi
AT THE AATS ANNUAL MEETING
Some improvements seen in neurocognition post-bariatric surgery
ATLANTA – Some patients experienced improvement in at least one neurocognitive domain up to 3 years after having bariatric surgery, a small, systematic review has shown.
The most significant improvements were reported in memory, with nine studies showing some statistically significant improvement in a post-bariatric surgery cohort. Four studies showed statistically significant improvement in attention and executive function, and two did so in language.
Dr. Gurneet S. Thiara, a psychiatry resident at the University of Toronto, presented the findings during a scientific session at this year’s annual meeting of the American Psychiatric Association.
Because the studies that form the basis of the analysis did not follow a standard pre-surgery neurocognitive assessment, the actual scope of bariatric surgery’s impact on neurocognition is hard to determine. This shortcoming provides evidence that instituting a standardized method of psychiatric assessment pre-bariatric surgery could help clinicians better anticipate overall neurocognitive outcomes, he said.
“It’s hard to pinpoint the one domain that affects [this cohort] most,” said Dr. Thiara.
One study included in the analysis showed no neurocognitive improvement, although Dr. Thiara noted this was possibly due to the under- or non-reporting of negative outcomes by researchers who conducted studies that might have met his inclusion criteria.
Dr. Thiara and his colleagues were not able to draw conclusions as to which patients would be affected in which domains and by what mechanism of action. Their analysis did suggest possible relationships between gastric bypass and changes in metabolism, levels of leptin and ghrelin, vascular function, hypoperfusion in the brain, and even shifts in the gut microbiome.
Dr. Thiara sought studies with bariatric surgery patients whose neurocognitive and psychological outcomes were followed anywhere from one to three years post-surgery. After analyzing 422 studies published between January 1990 and August 2015, only ten studies, with patient sample sizes ranging from 10 to 156, met the criteria.
The study was not intended to determine a relationship between neurocognitive outcomes and type of bypass surgery performed, but Dr. Thiara said the majority of the procedures analyzed tended to be Roux-en-Y rather than the gastric bypass sleeve.
On Twitter @whitneymcknight
ATLANTA – Some patients experienced improvement in at least one neurocognitive domain up to 3 years after having bariatric surgery, a small, systematic review has shown.
The most significant improvements were reported in memory, with nine studies showing some statistically significant improvement in a post-bariatric surgery cohort. Four studies showed statistically significant improvement in attention and executive function, and two did so in language.
Dr. Gurneet S. Thiara, a psychiatry resident at the University of Toronto, presented the findings during a scientific session at this year’s annual meeting of the American Psychiatric Association.
Because the studies that form the basis of the analysis did not follow a standard pre-surgery neurocognitive assessment, the actual scope of bariatric surgery’s impact on neurocognition is hard to determine. This shortcoming provides evidence that instituting a standardized method of psychiatric assessment pre-bariatric surgery could help clinicians better anticipate overall neurocognitive outcomes, he said.
“It’s hard to pinpoint the one domain that affects [this cohort] most,” said Dr. Thiara.
One study included in the analysis showed no neurocognitive improvement, although Dr. Thiara noted this was possibly due to the under- or non-reporting of negative outcomes by researchers who conducted studies that might have met his inclusion criteria.
Dr. Thiara and his colleagues were not able to draw conclusions as to which patients would be affected in which domains and by what mechanism of action. Their analysis did suggest possible relationships between gastric bypass and changes in metabolism, levels of leptin and ghrelin, vascular function, hypoperfusion in the brain, and even shifts in the gut microbiome.
Dr. Thiara sought studies with bariatric surgery patients whose neurocognitive and psychological outcomes were followed anywhere from one to three years post-surgery. After analyzing 422 studies published between January 1990 and August 2015, only ten studies, with patient sample sizes ranging from 10 to 156, met the criteria.
The study was not intended to determine a relationship between neurocognitive outcomes and type of bypass surgery performed, but Dr. Thiara said the majority of the procedures analyzed tended to be Roux-en-Y rather than the gastric bypass sleeve.
On Twitter @whitneymcknight
ATLANTA – Some patients experienced improvement in at least one neurocognitive domain up to 3 years after having bariatric surgery, a small, systematic review has shown.
The most significant improvements were reported in memory, with nine studies showing some statistically significant improvement in a post-bariatric surgery cohort. Four studies showed statistically significant improvement in attention and executive function, and two did so in language.
Dr. Gurneet S. Thiara, a psychiatry resident at the University of Toronto, presented the findings during a scientific session at this year’s annual meeting of the American Psychiatric Association.
Because the studies that form the basis of the analysis did not follow a standard pre-surgery neurocognitive assessment, the actual scope of bariatric surgery’s impact on neurocognition is hard to determine. This shortcoming provides evidence that instituting a standardized method of psychiatric assessment pre-bariatric surgery could help clinicians better anticipate overall neurocognitive outcomes, he said.
“It’s hard to pinpoint the one domain that affects [this cohort] most,” said Dr. Thiara.
One study included in the analysis showed no neurocognitive improvement, although Dr. Thiara noted this was possibly due to the under- or non-reporting of negative outcomes by researchers who conducted studies that might have met his inclusion criteria.
Dr. Thiara and his colleagues were not able to draw conclusions as to which patients would be affected in which domains and by what mechanism of action. Their analysis did suggest possible relationships between gastric bypass and changes in metabolism, levels of leptin and ghrelin, vascular function, hypoperfusion in the brain, and even shifts in the gut microbiome.
Dr. Thiara sought studies with bariatric surgery patients whose neurocognitive and psychological outcomes were followed anywhere from one to three years post-surgery. After analyzing 422 studies published between January 1990 and August 2015, only ten studies, with patient sample sizes ranging from 10 to 156, met the criteria.
The study was not intended to determine a relationship between neurocognitive outcomes and type of bypass surgery performed, but Dr. Thiara said the majority of the procedures analyzed tended to be Roux-en-Y rather than the gastric bypass sleeve.
On Twitter @whitneymcknight
AT APA 2016
Key clinical point: Neurocognitive testing in patients before bariatric surgery could be a useful tool for tracking overall psychosocial outcomes.
Major finding: Improvements in neurocognitive function were found across several domains in some patients in the years after bariatric surgery.
Data source: Systematic review of neurocognitive outcomes in post-bariatric surgery patients followed for at least 1 year in 10 studies of between 10 and 156 patients.
Disclosures: Dr. Thiara had no relevant disclosures. This study was sponsored in part by the Toronto Western Hospital Bariatric Psychosocial Surgery Program, part of the University Health Network, Toronto, Ont.
Data support safety of MS drugs before, during early pregnancy
VANCOUVER – Three types of drugs commonly used to treat multiple sclerosis (MS) appear to have a generally good safety profile when administered before and during early pregnancy, suggest a trio of studies reported at the annual meeting of the American Academy of Neurology.
Exposure to interferon-beta during the first trimester did not increase the risks of miscarriage, congenital anomalies, or birth weight. And although use of natalizumab led to higher odds of spontaneous abortion as compared with interferon-beta or no therapy, the absolute rate still fell within that of the general population. Pregnancies occurring weeks to years after treatment with alemtuzumab also had rates of spontaneous abortion, birth defects, and stillbirth on par with population values.
All three drugs fall in a gray area when it comes to use in pregnancy, with a Food and Drug Administration designation of category C, indicating that animal studies have shown adverse effects on the fetus and adequate, quality human data are lacking, but potential benefits may outweigh potential harms in pregnancy. The studies’ findings are therefore likely to be informative to women of reproductive age, a group disproportionately affected by MS.
“It’s very important to collect as much data as possible about potential exposure risks with disease-modifying therapy in pregnancy,” session comoderator Dr. Jennifer Graves, a neurologist at the Multiple Sclerosis Center at University of California–San Francisco Medical Center and UCSF Benioff Children’s Hospital, San Francisco, said in an interview.
“However, all studies have the limitation of sample size,” she added. “The majority of serious adverse effects – teratogenicity, major birth defects – may be less than 1 in 500 [in frequency]. So this is something that puts all of these studies into context because we just don’t have that many pregnancies that have been exposed to some of these agents.” The method whereby miscarriages are ascertained can also influence findings.
Nonetheless, this research is critical for women and their physicians when it comes to making decisions about treatment, Dr. Graves maintained. “Although pregnancy may be protective for many women with MS, many do need treatment during their pregnancy to prevent severe relapses due to various factors. Collecting this type of information is important.”
Interferon-beta safety
In the first study, investigators led by Dr. Sandra Thiel of Ruhr University Bochum and the Heinrich Heine University Düsseldorf, both in Germany, analyzed data from the German Multiple Sclerosis and Pregnancy Registry.
They studied pregnancies among women who had at least 12 months of postpartum follow-up. Exposure to interferon-beta (brand names Rebif, Avonex, Betaseron, and Extavia) was defined as injection of the drug at any time after the last menstrual period.
In all, 251 pregnancies exposed to interferon-beta were compared with 194 pregnancies not exposed to any disease-modifying drugs. The median duration of exposure in the former group was 32 days, indicating that most women stopped the drug soon after discovering they were pregnant, Dr. Thiel noted.
Study results, reported at the meeting and recently published (Mult Scler. 2016 Feb 26. doi: 10.1177/1352458516634872), showed that the rate of miscarriage was 9.96% in the exposed group and 7.73% in the unexposed group, and the rate of congenital anomalies among live births was 3.08% and 5.52%.
In analyses using propensity score adjustment, there were no significant differences between groups in the odds of live birth, spontaneous abortion, congenital anomalies, preterm birth, cesarean section, or small for gestational age, or in mean infant birth weight.
Of note, the women whose pregnancies were not exposed to any disease-modifying therapy had a higher rate of relapse during pregnancy when compared with counterparts whose pregnancies were exposed to interferon-beta (27.3% vs. 14.3%).
“Taken together with the existing literature, our study provides further reassurance that interferon-beta treatment can be safely continued up until the time when women with MS become pregnant,” Dr. Thiel concluded. The safety profile seen “is consistent with the pharmacologically plausible safety of interferon-beta, as interferon-beta is a huge molecule that cannot pass the placental barrier.”
“Since the vast majority of women stopped the interferon-beta treatment during the first trimester of pregnancy, we cannot draw any conclusions about the safety of interferon-beta later in pregnancy,” she cautioned. “Another limitation is the variability in the gestational week of entry into the cohort, as later than first-trimester inclusion can lead to an underestimation of early events, particularly spontaneous abortions.”
Natalizumab safety
In the second study, Dr. Maria Pia Amato, Department of NEUROFARBA, Section of Neurosciences, University of Florence, Italy, and her colleagues with the Italian MS Study Group assessed pregnancy outcomes after exposure to natalizumab (Tysabri). This antibody targets alpha-4 integrins, which play a role in a variety of pregnancy processes and in fetal hematopoiesis and cardiac development, she noted.
They identified women for the study using two sources: the prospective Italian Pregnancy Dataset of consecutive female patients with MS referred to 25 centers and a cohort of women from an Italian interferon-beta study.
In all, they compared 65 pregnancies exposed to natalizumab (any treatment from 8 weeks before the start of the last menstrual period onward), 88 exposed to interferon-beta as a control, and 339 not exposed to either. The mean duration of natalizumab exposure was 1.16 weeks, and the mean duration of interferon-beta exposure was 4.6 weeks.
Results showed that the rate of spontaneous abortion was higher for natalizumab-exposed pregnancies, at 18.5%, than for interferon-beta–exposed pregnancies, at 8%, and for nonexposed pregnancies, at 6.5% (P = .006). But the timing of these abortions was similar, at about 8 weeks of gestation.
In adjusted analyses, natalizumab exposure was still associated with an elevated risk of spontaneous abortion when compared with interferon-beta or no exposure (odds ratio, 3.9). However, the 18.5% rate seen with the antibody fell within the range for the Italian general population of 4.8% to 21.1%.
Infants in the natalizumab group had the lowest birth weight and length, while infants in the interferon-beta group had the youngest gestational age. The groups did not differ significantly with respect to the incidence of birth defects; however, with a single defect seen in each, the study was underpowered to assess differences in this outcome.
Data additionally showed that discontinuation of natalizumab in advance of pregnancy led to an uptick in relapses, with 30% of women having a relapse, according to Dr. Amato. “This increase started during the first trimester of pregnancy and culminated in the second trimester of pregnancy. All the women were retreated soon after delivery with natalizumab, and disease activity returned to the pre-pregnancy period level with resumption of the drug.”
“Patients and clinicians should discuss together and balance the potential risks to the fetus with natalizumab exposure with the potential risks to the mother of disease reactivation during pregnancy,” she recommended, cautioning that the findings were based on first-trimester exposure of short duration. “Decisions should be made case by case on the basis of the disease activity in a specific patient and the availability of alternative treatment, and whether to use a conservative approach, stopping the drug and respecting the washout period, or in a few cases, an active approach, continuing the drug till conception or even discuss continuing the drug during pregnancy.”
Alemtuzumab safety
In the third study, investigators led by Dr. Jiwon Oh of the division of neurology at the University of Toronto analyzed outcomes of pregnancies among women who had been treated with alemtuzumab (Lemtrada), an antibody that targets CD52.
Treatment with alemtuzumab leads to depletion of lymphocytes followed by reconstitution of the immune system with a less inflammatory profile. “This is thought to contribute to the durable efficacy in the absence of continuous treatment with alemtuzumab,” she explained. “This durable efficacy and the fact that you may not need to redose is relevant when you are thinking about pregnancy in patients with MS, just because this is a drug that is given in two different cycles and may not need to be readministered.”
Although the antibody becomes undetectable in serum about 30 days after administration, it is unclear whether the ongoing immune reconstitution has any impact on subsequent pregnancy, Dr. Oh said.
The investigators pooled data from the phase II CAMMS223 trial and the phase III CARE-MS I and CARE-MS II trials of alemtuzumab and their extension phases. Women enrolled in the trials were required to use effective contraception during treatment and for the next 6 months.
Dr. Oh reported interim results based on 200 pregnancies among 137 women. Most pregnancies occurred at least 4 months after the last dose of alemtuzumab; only four occurred within 1 month and another four occurred 1 to 3 months after the last dose.
Among the 181 completed pregnancies with known outcomes, 67.4% resulted in live births. Another 21.5% ended in spontaneous abortion. “Although this is on the higher end of normal, it is still in keeping with what is seen in the general population,” Dr. Oh noted.
The rate of stillbirth was 0.6%, also at the upper end of the range seen for the general population. Finally, 10.5% of the pregnancies ended in elective abortion.
There were no congenital anomalies or birth defects among the live-born infants. One was seen in an electively terminated pregnancy (26 months after the last dose), and another was seen in a stillbirth (4 years after the last dose).
“Two out of 200 is 1%, and this is actually lower than what is normally seen in the general population, approximately 3% to 7%,” Dr. Oh commented.
“To date, there is no indication of an increased risk for congenital anomalies or birth defects in infants,” she summarized. “There has also been no indication of increased rates of spontaneous abortion in women who become pregnant, but obviously, these data are limited because we don’t necessarily have a control group.”
“Based on the pharmacokinetics of alemtuzumab and labeling guidelines, women of childbearing potential should continue to use contraception for 4 months after receiving a course of alemtuzumab,” Dr. Oh concluded. “There is an international Alemtuzumab Pregnancy Exposure Registry that is open and enrolling patients who become pregnant between the first dose of alemtuzumab and 4 months after the last infusion, and hopefully this will give us more information to confirm some of the observations that we see here.”
Dr. Thiel disclosed that she had no relevant conflicts of interest; the German Multiple Sclerosis and Pregnancy registry was partly supported by Bayer Healthcare, Biogen Idec Germany, Merck Serono, Novartis Pharma, Teva Pharma, and Sanofi-Aventis/Genzyme Pharmaceuticals. Dr. Amato disclosed that she has received research grants and honoraria as a speaker from and is a member of advisory boards for Bayer, Biogen Idec, Merck Serono, Novartis, Sanofi Genzyme, Teva, Almirall, and Roche; the study did not receive any financial support. Dr. Oh disclosed that she serves on the scientific advisory boards or is a speaker for Biogen Idec, EMD Serono, Genzyme, Novartis, Roche, and Teva; the study was supported by Genzyme and Bayer Healthcare.
VANCOUVER – Three types of drugs commonly used to treat multiple sclerosis (MS) appear to have a generally good safety profile when administered before and during early pregnancy, suggest a trio of studies reported at the annual meeting of the American Academy of Neurology.
Exposure to interferon-beta during the first trimester did not increase the risks of miscarriage, congenital anomalies, or birth weight. And although use of natalizumab led to higher odds of spontaneous abortion as compared with interferon-beta or no therapy, the absolute rate still fell within that of the general population. Pregnancies occurring weeks to years after treatment with alemtuzumab also had rates of spontaneous abortion, birth defects, and stillbirth on par with population values.
All three drugs fall in a gray area when it comes to use in pregnancy, with a Food and Drug Administration designation of category C, indicating that animal studies have shown adverse effects on the fetus and adequate, quality human data are lacking, but potential benefits may outweigh potential harms in pregnancy. The studies’ findings are therefore likely to be informative to women of reproductive age, a group disproportionately affected by MS.
“It’s very important to collect as much data as possible about potential exposure risks with disease-modifying therapy in pregnancy,” session comoderator Dr. Jennifer Graves, a neurologist at the Multiple Sclerosis Center at University of California–San Francisco Medical Center and UCSF Benioff Children’s Hospital, San Francisco, said in an interview.
“However, all studies have the limitation of sample size,” she added. “The majority of serious adverse effects – teratogenicity, major birth defects – may be less than 1 in 500 [in frequency]. So this is something that puts all of these studies into context because we just don’t have that many pregnancies that have been exposed to some of these agents.” The method whereby miscarriages are ascertained can also influence findings.
Nonetheless, this research is critical for women and their physicians when it comes to making decisions about treatment, Dr. Graves maintained. “Although pregnancy may be protective for many women with MS, many do need treatment during their pregnancy to prevent severe relapses due to various factors. Collecting this type of information is important.”
Interferon-beta safety
In the first study, investigators led by Dr. Sandra Thiel of Ruhr University Bochum and the Heinrich Heine University Düsseldorf, both in Germany, analyzed data from the German Multiple Sclerosis and Pregnancy Registry.
They studied pregnancies among women who had at least 12 months of postpartum follow-up. Exposure to interferon-beta (brand names Rebif, Avonex, Betaseron, and Extavia) was defined as injection of the drug at any time after the last menstrual period.
In all, 251 pregnancies exposed to interferon-beta were compared with 194 pregnancies not exposed to any disease-modifying drugs. The median duration of exposure in the former group was 32 days, indicating that most women stopped the drug soon after discovering they were pregnant, Dr. Thiel noted.
Study results, reported at the meeting and recently published (Mult Scler. 2016 Feb 26. doi: 10.1177/1352458516634872), showed that the rate of miscarriage was 9.96% in the exposed group and 7.73% in the unexposed group, and the rate of congenital anomalies among live births was 3.08% and 5.52%.
In analyses using propensity score adjustment, there were no significant differences between groups in the odds of live birth, spontaneous abortion, congenital anomalies, preterm birth, cesarean section, or small for gestational age, or in mean infant birth weight.
Of note, the women whose pregnancies were not exposed to any disease-modifying therapy had a higher rate of relapse during pregnancy when compared with counterparts whose pregnancies were exposed to interferon-beta (27.3% vs. 14.3%).
“Taken together with the existing literature, our study provides further reassurance that interferon-beta treatment can be safely continued up until the time when women with MS become pregnant,” Dr. Thiel concluded. The safety profile seen “is consistent with the pharmacologically plausible safety of interferon-beta, as interferon-beta is a huge molecule that cannot pass the placental barrier.”
“Since the vast majority of women stopped the interferon-beta treatment during the first trimester of pregnancy, we cannot draw any conclusions about the safety of interferon-beta later in pregnancy,” she cautioned. “Another limitation is the variability in the gestational week of entry into the cohort, as later than first-trimester inclusion can lead to an underestimation of early events, particularly spontaneous abortions.”
Natalizumab safety
In the second study, Dr. Maria Pia Amato, Department of NEUROFARBA, Section of Neurosciences, University of Florence, Italy, and her colleagues with the Italian MS Study Group assessed pregnancy outcomes after exposure to natalizumab (Tysabri). This antibody targets alpha-4 integrins, which play a role in a variety of pregnancy processes and in fetal hematopoiesis and cardiac development, she noted.
They identified women for the study using two sources: the prospective Italian Pregnancy Dataset of consecutive female patients with MS referred to 25 centers and a cohort of women from an Italian interferon-beta study.
In all, they compared 65 pregnancies exposed to natalizumab (any treatment from 8 weeks before the start of the last menstrual period onward), 88 exposed to interferon-beta as a control, and 339 not exposed to either. The mean duration of natalizumab exposure was 1.16 weeks, and the mean duration of interferon-beta exposure was 4.6 weeks.
Results showed that the rate of spontaneous abortion was higher for natalizumab-exposed pregnancies, at 18.5%, than for interferon-beta–exposed pregnancies, at 8%, and for nonexposed pregnancies, at 6.5% (P = .006). But the timing of these abortions was similar, at about 8 weeks of gestation.
In adjusted analyses, natalizumab exposure was still associated with an elevated risk of spontaneous abortion when compared with interferon-beta or no exposure (odds ratio, 3.9). However, the 18.5% rate seen with the antibody fell within the range for the Italian general population of 4.8% to 21.1%.
Infants in the natalizumab group had the lowest birth weight and length, while infants in the interferon-beta group had the youngest gestational age. The groups did not differ significantly with respect to the incidence of birth defects; however, with a single defect seen in each, the study was underpowered to assess differences in this outcome.
Data additionally showed that discontinuation of natalizumab in advance of pregnancy led to an uptick in relapses, with 30% of women having a relapse, according to Dr. Amato. “This increase started during the first trimester of pregnancy and culminated in the second trimester of pregnancy. All the women were retreated soon after delivery with natalizumab, and disease activity returned to the pre-pregnancy period level with resumption of the drug.”
“Patients and clinicians should discuss together and balance the potential risks to the fetus with natalizumab exposure with the potential risks to the mother of disease reactivation during pregnancy,” she recommended, cautioning that the findings were based on first-trimester exposure of short duration. “Decisions should be made case by case on the basis of the disease activity in a specific patient and the availability of alternative treatment, and whether to use a conservative approach, stopping the drug and respecting the washout period, or in a few cases, an active approach, continuing the drug till conception or even discuss continuing the drug during pregnancy.”
Alemtuzumab safety
In the third study, investigators led by Dr. Jiwon Oh of the division of neurology at the University of Toronto analyzed outcomes of pregnancies among women who had been treated with alemtuzumab (Lemtrada), an antibody that targets CD52.
Treatment with alemtuzumab leads to depletion of lymphocytes followed by reconstitution of the immune system with a less inflammatory profile. “This is thought to contribute to the durable efficacy in the absence of continuous treatment with alemtuzumab,” she explained. “This durable efficacy and the fact that you may not need to redose is relevant when you are thinking about pregnancy in patients with MS, just because this is a drug that is given in two different cycles and may not need to be readministered.”
Although the antibody becomes undetectable in serum about 30 days after administration, it is unclear whether the ongoing immune reconstitution has any impact on subsequent pregnancy, Dr. Oh said.
The investigators pooled data from the phase II CAMMS223 trial and the phase III CARE-MS I and CARE-MS II trials of alemtuzumab and their extension phases. Women enrolled in the trials were required to use effective contraception during treatment and for the next 6 months.
Dr. Oh reported interim results based on 200 pregnancies among 137 women. Most pregnancies occurred at least 4 months after the last dose of alemtuzumab; only four occurred within 1 month and another four occurred 1 to 3 months after the last dose.
Among the 181 completed pregnancies with known outcomes, 67.4% resulted in live births. Another 21.5% ended in spontaneous abortion. “Although this is on the higher end of normal, it is still in keeping with what is seen in the general population,” Dr. Oh noted.
The rate of stillbirth was 0.6%, also at the upper end of the range seen for the general population. Finally, 10.5% of the pregnancies ended in elective abortion.
There were no congenital anomalies or birth defects among the live-born infants. One was seen in an electively terminated pregnancy (26 months after the last dose), and another was seen in a stillbirth (4 years after the last dose).
“Two out of 200 is 1%, and this is actually lower than what is normally seen in the general population, approximately 3% to 7%,” Dr. Oh commented.
“To date, there is no indication of an increased risk for congenital anomalies or birth defects in infants,” she summarized. “There has also been no indication of increased rates of spontaneous abortion in women who become pregnant, but obviously, these data are limited because we don’t necessarily have a control group.”
“Based on the pharmacokinetics of alemtuzumab and labeling guidelines, women of childbearing potential should continue to use contraception for 4 months after receiving a course of alemtuzumab,” Dr. Oh concluded. “There is an international Alemtuzumab Pregnancy Exposure Registry that is open and enrolling patients who become pregnant between the first dose of alemtuzumab and 4 months after the last infusion, and hopefully this will give us more information to confirm some of the observations that we see here.”
Dr. Thiel disclosed that she had no relevant conflicts of interest; the German Multiple Sclerosis and Pregnancy registry was partly supported by Bayer Healthcare, Biogen Idec Germany, Merck Serono, Novartis Pharma, Teva Pharma, and Sanofi-Aventis/Genzyme Pharmaceuticals. Dr. Amato disclosed that she has received research grants and honoraria as a speaker from and is a member of advisory boards for Bayer, Biogen Idec, Merck Serono, Novartis, Sanofi Genzyme, Teva, Almirall, and Roche; the study did not receive any financial support. Dr. Oh disclosed that she serves on the scientific advisory boards or is a speaker for Biogen Idec, EMD Serono, Genzyme, Novartis, Roche, and Teva; the study was supported by Genzyme and Bayer Healthcare.
VANCOUVER – Three types of drugs commonly used to treat multiple sclerosis (MS) appear to have a generally good safety profile when administered before and during early pregnancy, suggest a trio of studies reported at the annual meeting of the American Academy of Neurology.
Exposure to interferon-beta during the first trimester did not increase the risks of miscarriage, congenital anomalies, or birth weight. And although use of natalizumab led to higher odds of spontaneous abortion as compared with interferon-beta or no therapy, the absolute rate still fell within that of the general population. Pregnancies occurring weeks to years after treatment with alemtuzumab also had rates of spontaneous abortion, birth defects, and stillbirth on par with population values.
All three drugs fall in a gray area when it comes to use in pregnancy, with a Food and Drug Administration designation of category C, indicating that animal studies have shown adverse effects on the fetus and adequate, quality human data are lacking, but potential benefits may outweigh potential harms in pregnancy. The studies’ findings are therefore likely to be informative to women of reproductive age, a group disproportionately affected by MS.
“It’s very important to collect as much data as possible about potential exposure risks with disease-modifying therapy in pregnancy,” session comoderator Dr. Jennifer Graves, a neurologist at the Multiple Sclerosis Center at University of California–San Francisco Medical Center and UCSF Benioff Children’s Hospital, San Francisco, said in an interview.
“However, all studies have the limitation of sample size,” she added. “The majority of serious adverse effects – teratogenicity, major birth defects – may be less than 1 in 500 [in frequency]. So this is something that puts all of these studies into context because we just don’t have that many pregnancies that have been exposed to some of these agents.” The method whereby miscarriages are ascertained can also influence findings.
Nonetheless, this research is critical for women and their physicians when it comes to making decisions about treatment, Dr. Graves maintained. “Although pregnancy may be protective for many women with MS, many do need treatment during their pregnancy to prevent severe relapses due to various factors. Collecting this type of information is important.”
Interferon-beta safety
In the first study, investigators led by Dr. Sandra Thiel of Ruhr University Bochum and the Heinrich Heine University Düsseldorf, both in Germany, analyzed data from the German Multiple Sclerosis and Pregnancy Registry.
They studied pregnancies among women who had at least 12 months of postpartum follow-up. Exposure to interferon-beta (brand names Rebif, Avonex, Betaseron, and Extavia) was defined as injection of the drug at any time after the last menstrual period.
In all, 251 pregnancies exposed to interferon-beta were compared with 194 pregnancies not exposed to any disease-modifying drugs. The median duration of exposure in the former group was 32 days, indicating that most women stopped the drug soon after discovering they were pregnant, Dr. Thiel noted.
Study results, reported at the meeting and recently published (Mult Scler. 2016 Feb 26. doi: 10.1177/1352458516634872), showed that the rate of miscarriage was 9.96% in the exposed group and 7.73% in the unexposed group, and the rate of congenital anomalies among live births was 3.08% and 5.52%.
In analyses using propensity score adjustment, there were no significant differences between groups in the odds of live birth, spontaneous abortion, congenital anomalies, preterm birth, cesarean section, or small for gestational age, or in mean infant birth weight.
Of note, the women whose pregnancies were not exposed to any disease-modifying therapy had a higher rate of relapse during pregnancy when compared with counterparts whose pregnancies were exposed to interferon-beta (27.3% vs. 14.3%).
“Taken together with the existing literature, our study provides further reassurance that interferon-beta treatment can be safely continued up until the time when women with MS become pregnant,” Dr. Thiel concluded. The safety profile seen “is consistent with the pharmacologically plausible safety of interferon-beta, as interferon-beta is a huge molecule that cannot pass the placental barrier.”
“Since the vast majority of women stopped the interferon-beta treatment during the first trimester of pregnancy, we cannot draw any conclusions about the safety of interferon-beta later in pregnancy,” she cautioned. “Another limitation is the variability in the gestational week of entry into the cohort, as later than first-trimester inclusion can lead to an underestimation of early events, particularly spontaneous abortions.”
Natalizumab safety
In the second study, Dr. Maria Pia Amato, Department of NEUROFARBA, Section of Neurosciences, University of Florence, Italy, and her colleagues with the Italian MS Study Group assessed pregnancy outcomes after exposure to natalizumab (Tysabri). This antibody targets alpha-4 integrins, which play a role in a variety of pregnancy processes and in fetal hematopoiesis and cardiac development, she noted.
They identified women for the study using two sources: the prospective Italian Pregnancy Dataset of consecutive female patients with MS referred to 25 centers and a cohort of women from an Italian interferon-beta study.
In all, they compared 65 pregnancies exposed to natalizumab (any treatment from 8 weeks before the start of the last menstrual period onward), 88 exposed to interferon-beta as a control, and 339 not exposed to either. The mean duration of natalizumab exposure was 1.16 weeks, and the mean duration of interferon-beta exposure was 4.6 weeks.
Results showed that the rate of spontaneous abortion was higher for natalizumab-exposed pregnancies, at 18.5%, than for interferon-beta–exposed pregnancies, at 8%, and for nonexposed pregnancies, at 6.5% (P = .006). But the timing of these abortions was similar, at about 8 weeks of gestation.
In adjusted analyses, natalizumab exposure was still associated with an elevated risk of spontaneous abortion when compared with interferon-beta or no exposure (odds ratio, 3.9). However, the 18.5% rate seen with the antibody fell within the range for the Italian general population of 4.8% to 21.1%.
Infants in the natalizumab group had the lowest birth weight and length, while infants in the interferon-beta group had the youngest gestational age. The groups did not differ significantly with respect to the incidence of birth defects; however, with a single defect seen in each, the study was underpowered to assess differences in this outcome.
Data additionally showed that discontinuation of natalizumab in advance of pregnancy led to an uptick in relapses, with 30% of women having a relapse, according to Dr. Amato. “This increase started during the first trimester of pregnancy and culminated in the second trimester of pregnancy. All the women were retreated soon after delivery with natalizumab, and disease activity returned to the pre-pregnancy period level with resumption of the drug.”
“Patients and clinicians should discuss together and balance the potential risks to the fetus with natalizumab exposure with the potential risks to the mother of disease reactivation during pregnancy,” she recommended, cautioning that the findings were based on first-trimester exposure of short duration. “Decisions should be made case by case on the basis of the disease activity in a specific patient and the availability of alternative treatment, and whether to use a conservative approach, stopping the drug and respecting the washout period, or in a few cases, an active approach, continuing the drug till conception or even discuss continuing the drug during pregnancy.”
Alemtuzumab safety
In the third study, investigators led by Dr. Jiwon Oh of the division of neurology at the University of Toronto analyzed outcomes of pregnancies among women who had been treated with alemtuzumab (Lemtrada), an antibody that targets CD52.
Treatment with alemtuzumab leads to depletion of lymphocytes followed by reconstitution of the immune system with a less inflammatory profile. “This is thought to contribute to the durable efficacy in the absence of continuous treatment with alemtuzumab,” she explained. “This durable efficacy and the fact that you may not need to redose is relevant when you are thinking about pregnancy in patients with MS, just because this is a drug that is given in two different cycles and may not need to be readministered.”
Although the antibody becomes undetectable in serum about 30 days after administration, it is unclear whether the ongoing immune reconstitution has any impact on subsequent pregnancy, Dr. Oh said.
The investigators pooled data from the phase II CAMMS223 trial and the phase III CARE-MS I and CARE-MS II trials of alemtuzumab and their extension phases. Women enrolled in the trials were required to use effective contraception during treatment and for the next 6 months.
Dr. Oh reported interim results based on 200 pregnancies among 137 women. Most pregnancies occurred at least 4 months after the last dose of alemtuzumab; only four occurred within 1 month and another four occurred 1 to 3 months after the last dose.
Among the 181 completed pregnancies with known outcomes, 67.4% resulted in live births. Another 21.5% ended in spontaneous abortion. “Although this is on the higher end of normal, it is still in keeping with what is seen in the general population,” Dr. Oh noted.
The rate of stillbirth was 0.6%, also at the upper end of the range seen for the general population. Finally, 10.5% of the pregnancies ended in elective abortion.
There were no congenital anomalies or birth defects among the live-born infants. One was seen in an electively terminated pregnancy (26 months after the last dose), and another was seen in a stillbirth (4 years after the last dose).
“Two out of 200 is 1%, and this is actually lower than what is normally seen in the general population, approximately 3% to 7%,” Dr. Oh commented.
“To date, there is no indication of an increased risk for congenital anomalies or birth defects in infants,” she summarized. “There has also been no indication of increased rates of spontaneous abortion in women who become pregnant, but obviously, these data are limited because we don’t necessarily have a control group.”
“Based on the pharmacokinetics of alemtuzumab and labeling guidelines, women of childbearing potential should continue to use contraception for 4 months after receiving a course of alemtuzumab,” Dr. Oh concluded. “There is an international Alemtuzumab Pregnancy Exposure Registry that is open and enrolling patients who become pregnant between the first dose of alemtuzumab and 4 months after the last infusion, and hopefully this will give us more information to confirm some of the observations that we see here.”
Dr. Thiel disclosed that she had no relevant conflicts of interest; the German Multiple Sclerosis and Pregnancy registry was partly supported by Bayer Healthcare, Biogen Idec Germany, Merck Serono, Novartis Pharma, Teva Pharma, and Sanofi-Aventis/Genzyme Pharmaceuticals. Dr. Amato disclosed that she has received research grants and honoraria as a speaker from and is a member of advisory boards for Bayer, Biogen Idec, Merck Serono, Novartis, Sanofi Genzyme, Teva, Almirall, and Roche; the study did not receive any financial support. Dr. Oh disclosed that she serves on the scientific advisory boards or is a speaker for Biogen Idec, EMD Serono, Genzyme, Novartis, Roche, and Teva; the study was supported by Genzyme and Bayer Healthcare.
AT THE AAN 2016 ANNUAL MEETING
FDA warns against routine fluoroquinolone use
The U.S. Food and Drug Administration has issued a warning to health care providers against the routine prescribing of fluoroquinolone antibiotics to those patients with sinusitis, bronchitis, and uncomplicated urinary tract infections who have other treatment options.
After conducting a safety review of the drugs, the FDA concluded that fluoroquinolone should be reserved for those patients who do not have alternative treatment options, in light of the findings that the antibiotics – when used systemically in either tablet, capsule, or injectable form – are associated with “disabling and potentially permanent serious side effects” that can occur together. These side effects can involve the tendons, muscles, joints, nerves, and central nervous system, according to the agency.
The FDA says health care providers should stop systemic fluoroquinolone treatment immediately in patients reporting serious side effects and switch to a non-fluoroquinolone antibacterial drug to complete the patient’s treatment course. The drug labels and medication guides for all fluoroquinolone antibiotics will be updated to reflect the new safety information.
The FDA had previously communicated safety information associated with systemic fluoroquinolone antibacterial drugs in August 2013 and July 2008, and the safety issues described in the current warning were discussed at an FDA Advisory Committee meeting in November 2015.
On Twitter @richpizzi
The U.S. Food and Drug Administration has issued a warning to health care providers against the routine prescribing of fluoroquinolone antibiotics to those patients with sinusitis, bronchitis, and uncomplicated urinary tract infections who have other treatment options.
After conducting a safety review of the drugs, the FDA concluded that fluoroquinolone should be reserved for those patients who do not have alternative treatment options, in light of the findings that the antibiotics – when used systemically in either tablet, capsule, or injectable form – are associated with “disabling and potentially permanent serious side effects” that can occur together. These side effects can involve the tendons, muscles, joints, nerves, and central nervous system, according to the agency.
The FDA says health care providers should stop systemic fluoroquinolone treatment immediately in patients reporting serious side effects and switch to a non-fluoroquinolone antibacterial drug to complete the patient’s treatment course. The drug labels and medication guides for all fluoroquinolone antibiotics will be updated to reflect the new safety information.
The FDA had previously communicated safety information associated with systemic fluoroquinolone antibacterial drugs in August 2013 and July 2008, and the safety issues described in the current warning were discussed at an FDA Advisory Committee meeting in November 2015.
On Twitter @richpizzi
The U.S. Food and Drug Administration has issued a warning to health care providers against the routine prescribing of fluoroquinolone antibiotics to those patients with sinusitis, bronchitis, and uncomplicated urinary tract infections who have other treatment options.
After conducting a safety review of the drugs, the FDA concluded that fluoroquinolone should be reserved for those patients who do not have alternative treatment options, in light of the findings that the antibiotics – when used systemically in either tablet, capsule, or injectable form – are associated with “disabling and potentially permanent serious side effects” that can occur together. These side effects can involve the tendons, muscles, joints, nerves, and central nervous system, according to the agency.
The FDA says health care providers should stop systemic fluoroquinolone treatment immediately in patients reporting serious side effects and switch to a non-fluoroquinolone antibacterial drug to complete the patient’s treatment course. The drug labels and medication guides for all fluoroquinolone antibiotics will be updated to reflect the new safety information.
The FDA had previously communicated safety information associated with systemic fluoroquinolone antibacterial drugs in August 2013 and July 2008, and the safety issues described in the current warning were discussed at an FDA Advisory Committee meeting in November 2015.
On Twitter @richpizzi
FDA warns of counterfeit BiCNU
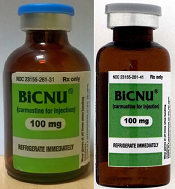
shown on the left and a
counterfeit vial on the right
Photo courtesy of the FDA
The US Food and Drug Administration (FDA) is warning healthcare professionals that a counterfeit version of BiCNU (carmustine for injection) 100 mg has been detected in some foreign countries.
The agency said there is no indication that counterfeit BiCNU has entered the legitimate US drug supply chain and no indication that any US patients have received counterfeit BiCNU.
Still, the FDA is advising that healthcare professionals inspect BiCNU vials as an added precaution to ensure the product administered to patients is authentic.
BiCNU is approved to treat brain cancers, multiple myeloma, and lymphoma. It is manufactured by Emcure Pharmaceuticals Ltd. and distributed in the US by Heritage Pharmaceuticals Inc.
Heritage previously announced that counterfeit BiCNU had been found in India, Ireland, and Israel.
How to identify counterfeit BiCNU
BiCNU is available as a vial of BiCNU and dehydrated alcohol co-packaged together.
While the NDC on the outer package of the authentic and counterfeit versions might match, the best way to distinguish a counterfeit is to look at the BiCNU vial inside the packaging. The authentic product has a blue flip top, while the counterfeit product may have a gray flip top.
The product may also be counterfeit if the vial displays the following lot numbers, batch numbers, manufacturing dates, and expiration dates.
| Product | Expiration
date |
Manufacturing
date |
Lot number | Batch number |
| BiCNU | 01/18 | 2/16 | BCEM771322 | EM/BC20161990 |
| Diluent | 01/18 | 2/16 | SBCDA224736 | EM/BCD2220 |
| BiCNU | 12/17 | 1/16 | BCEM771318 | EM/BC20151896 |
| Diluent | 12/17 | 1/16 | SBCDA224732 | EM/BCD2216 |
| BiCNU | 10/17 | 11/15 | BCEM771317 | EM/BC20151895 |
| Diluent | 10/17 | 11/15 | SBCDA224731 | EM/BCD2215 |
The FDA urges healthcare professionals to purchase drug products only from legitimate suppliers.
Healthcare professionals are encouraged to report sales solicitation of suspect drug products by calling the FDA’s Office of Criminal Investigations (OCI) at 800-551-3989, reporting via OCI’s website, or emailing [email protected].
Healthcare professionals and patients should report adverse events related to the use of any suspect medications to the FDA’s MedWatch Adverse Event Reporting Program. ![]()

shown on the left and a
counterfeit vial on the right
Photo courtesy of the FDA
The US Food and Drug Administration (FDA) is warning healthcare professionals that a counterfeit version of BiCNU (carmustine for injection) 100 mg has been detected in some foreign countries.
The agency said there is no indication that counterfeit BiCNU has entered the legitimate US drug supply chain and no indication that any US patients have received counterfeit BiCNU.
Still, the FDA is advising that healthcare professionals inspect BiCNU vials as an added precaution to ensure the product administered to patients is authentic.
BiCNU is approved to treat brain cancers, multiple myeloma, and lymphoma. It is manufactured by Emcure Pharmaceuticals Ltd. and distributed in the US by Heritage Pharmaceuticals Inc.
Heritage previously announced that counterfeit BiCNU had been found in India, Ireland, and Israel.
How to identify counterfeit BiCNU
BiCNU is available as a vial of BiCNU and dehydrated alcohol co-packaged together.
While the NDC on the outer package of the authentic and counterfeit versions might match, the best way to distinguish a counterfeit is to look at the BiCNU vial inside the packaging. The authentic product has a blue flip top, while the counterfeit product may have a gray flip top.
The product may also be counterfeit if the vial displays the following lot numbers, batch numbers, manufacturing dates, and expiration dates.
| Product | Expiration
date |
Manufacturing
date |
Lot number | Batch number |
| BiCNU | 01/18 | 2/16 | BCEM771322 | EM/BC20161990 |
| Diluent | 01/18 | 2/16 | SBCDA224736 | EM/BCD2220 |
| BiCNU | 12/17 | 1/16 | BCEM771318 | EM/BC20151896 |
| Diluent | 12/17 | 1/16 | SBCDA224732 | EM/BCD2216 |
| BiCNU | 10/17 | 11/15 | BCEM771317 | EM/BC20151895 |
| Diluent | 10/17 | 11/15 | SBCDA224731 | EM/BCD2215 |
The FDA urges healthcare professionals to purchase drug products only from legitimate suppliers.
Healthcare professionals are encouraged to report sales solicitation of suspect drug products by calling the FDA’s Office of Criminal Investigations (OCI) at 800-551-3989, reporting via OCI’s website, or emailing [email protected].
Healthcare professionals and patients should report adverse events related to the use of any suspect medications to the FDA’s MedWatch Adverse Event Reporting Program. ![]()

shown on the left and a
counterfeit vial on the right
Photo courtesy of the FDA
The US Food and Drug Administration (FDA) is warning healthcare professionals that a counterfeit version of BiCNU (carmustine for injection) 100 mg has been detected in some foreign countries.
The agency said there is no indication that counterfeit BiCNU has entered the legitimate US drug supply chain and no indication that any US patients have received counterfeit BiCNU.
Still, the FDA is advising that healthcare professionals inspect BiCNU vials as an added precaution to ensure the product administered to patients is authentic.
BiCNU is approved to treat brain cancers, multiple myeloma, and lymphoma. It is manufactured by Emcure Pharmaceuticals Ltd. and distributed in the US by Heritage Pharmaceuticals Inc.
Heritage previously announced that counterfeit BiCNU had been found in India, Ireland, and Israel.
How to identify counterfeit BiCNU
BiCNU is available as a vial of BiCNU and dehydrated alcohol co-packaged together.
While the NDC on the outer package of the authentic and counterfeit versions might match, the best way to distinguish a counterfeit is to look at the BiCNU vial inside the packaging. The authentic product has a blue flip top, while the counterfeit product may have a gray flip top.
The product may also be counterfeit if the vial displays the following lot numbers, batch numbers, manufacturing dates, and expiration dates.
| Product | Expiration
date |
Manufacturing
date |
Lot number | Batch number |
| BiCNU | 01/18 | 2/16 | BCEM771322 | EM/BC20161990 |
| Diluent | 01/18 | 2/16 | SBCDA224736 | EM/BCD2220 |
| BiCNU | 12/17 | 1/16 | BCEM771318 | EM/BC20151896 |
| Diluent | 12/17 | 1/16 | SBCDA224732 | EM/BCD2216 |
| BiCNU | 10/17 | 11/15 | BCEM771317 | EM/BC20151895 |
| Diluent | 10/17 | 11/15 | SBCDA224731 | EM/BCD2215 |
The FDA urges healthcare professionals to purchase drug products only from legitimate suppliers.
Healthcare professionals are encouraged to report sales solicitation of suspect drug products by calling the FDA’s Office of Criminal Investigations (OCI) at 800-551-3989, reporting via OCI’s website, or emailing [email protected].
Healthcare professionals and patients should report adverse events related to the use of any suspect medications to the FDA’s MedWatch Adverse Event Reporting Program. ![]()
PVI redo at 2 months drops 1 year AF recurrence by 30%
SAN FRANCISCO – Repeat, invasive electrophysiology studies conducted 2 months after pulmonary vein isolation – regardless of symptoms and, if necessary, with repeat ablations – substantially reduce atrial fibrillation recurrence and improve quality of life at 1 year, according to an investigation conducted at the Liverpool (Eng.) Heart and Chest Hospital.
After initial pulmonary vein isolation (PVI), 40 patients with drug-refractory, paroxysmal atrial fibrillation (AF) were randomized to the repeat approach, and 40 others to the current standard of care (SC), meaning repeat PVI based on recurrent AF symptoms.
Pulmonary vein reconnections were found in 25 patients (63%) checked at 2 months, and all 25 had repeat PVIs without complications. Meanwhile, nine (23%) patients in the SC group had repeat PVIs for clinical recurrence at a mean of about 7 months.
At one year, 33 patients in the repeat group (82.5%), but only 23 in the SC group (57.5%), were free of atrial tachyarrhythmia (AT) (P = .03), and total group AT burden was lower (91 versus 127 days, P = .03). Quality of life scores on the Atrial Fibrillation Effect on Quality of Life (AFEQT) questionnaire were higher in the repeat study group, too (mean 92.2 versus 79.1 out of 126 points, P = .030).
“A strategy of early assessment with re-isolation of PV reconnections can be deployed safely and improves freedom from AT recurrence and quality of life compared with current standard care. While the gold standard remains durable PVI from the initial procedure, until rates of this can be substantially improved, early re-intervention could be considered as a reasonable strategy to improve outcomes,” the investigators concluded.
“If it was my dad and I was doing a PVI today, I’d say, ‘Dad, let’s bring you back and look at how you’re doing in 2 months,’” lead investigator Dr. Dhiraj Gupta said during an interview after his presentation at the Heart Rhythm Society annual meeting.
“We can’t really afford to do everybody twice, but this has certainly lowered our threshold for reintervention,” said Dr. Gupta, a cardiologist at the Liverpool hospital. “We used to try antiarrhythmic drugs” for recurrence; “we don’t do that anymore. We complete the job that [we] set out to do in the first place. Our threshold is any recurrence beyond 1 month. A surprising number of patients agree with this [recheck] strategy, which is one of the reasons we didn’t have a single drop out in this study. I tell them that it’s highly likely that some of the pulmonary veins I isolated for them are going to reconnect.”
Audience members were concerned that 15 patients in the study arm (38%) ended up having an invasive test they didn’t need. Dr. Gupta said it’s a “glass half full or half empty” situation. “I see it as half full. These repeat procedures are short, safe, and quick [about 80 minutes], and even shorter for those patients who don’t require pulmonary reisolation.” For those patients who do, only a few have symptoms; the rest would have had to wait for remergent symptoms to trigger a second procedure. “I believe that these repeat procedures have become so safe that the risk is more than made up” for by the benefits.
There were no complications with early reinterventions, and there were just two complications with the original PVI; one patient who ended up in the repeat group had a spontaneously resolving phrenic nerve palsy with the first procedure, and one SC patient had a transient ischemic attack. In short, “the complication rates for the two groups were identical,” Dr. Gupta said.
Patients were split about evenly between men and women in both study arms, and patients were in their early 60s, on average. The mean baseline AFEQT score was 46.8 points; 78% in the standard care group, but 55% in the early reintervention group, were on baseline anticoagulants.
All of the subjects were given portable ECG recorders after their initial PVIs, and told to take a daily 30-second recording, and to record if they felt any heart symptoms. They followed the instructions and made more than 32,000 recordings.
Every PVI patient at the Liverpool hospital now gets a recorder at discharge, and physicians there base early interventions on the results, whether or not patients are symptomatic. “We’ve bought lots of them, and tell patients to have a low threshold for recording. I believe 24-hours Holters are a bit outdated,” Dr. Gupta said.
All the PVIs in the study were contact-force guided and used wide area circumferential ablation with the help of 3-D mapping and automated lesion tagging. Entrance and exit block were demonstrated, and adenosine was administered to unmask dormant reconnections after a waiting period of at least 20 minutes. Antiarrhythmic drugs were stopped at 4 weeks.
Biosense Webster funded the work. Dr. Gupta is a speaker for and receives research and fellowship funding from the company.
This is a provocative study, but the redo rate was very low in the standard of care arm [23%], much lower than we typically see. In my mind, they should have been more aggressive with that group. I would love to see them repeat this study but with a redo procedure in the standard of care arm with the first recurrence after 2 months.
Dr. John Day is the director of Intermountain Heart Rhythm Specialists in Murray, Utah, and the current president of the Hearth Rhythm Society. He has no disclosures.
This is a provocative study, but the redo rate was very low in the standard of care arm [23%], much lower than we typically see. In my mind, they should have been more aggressive with that group. I would love to see them repeat this study but with a redo procedure in the standard of care arm with the first recurrence after 2 months.
Dr. John Day is the director of Intermountain Heart Rhythm Specialists in Murray, Utah, and the current president of the Hearth Rhythm Society. He has no disclosures.
This is a provocative study, but the redo rate was very low in the standard of care arm [23%], much lower than we typically see. In my mind, they should have been more aggressive with that group. I would love to see them repeat this study but with a redo procedure in the standard of care arm with the first recurrence after 2 months.
Dr. John Day is the director of Intermountain Heart Rhythm Specialists in Murray, Utah, and the current president of the Hearth Rhythm Society. He has no disclosures.
SAN FRANCISCO – Repeat, invasive electrophysiology studies conducted 2 months after pulmonary vein isolation – regardless of symptoms and, if necessary, with repeat ablations – substantially reduce atrial fibrillation recurrence and improve quality of life at 1 year, according to an investigation conducted at the Liverpool (Eng.) Heart and Chest Hospital.
After initial pulmonary vein isolation (PVI), 40 patients with drug-refractory, paroxysmal atrial fibrillation (AF) were randomized to the repeat approach, and 40 others to the current standard of care (SC), meaning repeat PVI based on recurrent AF symptoms.
Pulmonary vein reconnections were found in 25 patients (63%) checked at 2 months, and all 25 had repeat PVIs without complications. Meanwhile, nine (23%) patients in the SC group had repeat PVIs for clinical recurrence at a mean of about 7 months.
At one year, 33 patients in the repeat group (82.5%), but only 23 in the SC group (57.5%), were free of atrial tachyarrhythmia (AT) (P = .03), and total group AT burden was lower (91 versus 127 days, P = .03). Quality of life scores on the Atrial Fibrillation Effect on Quality of Life (AFEQT) questionnaire were higher in the repeat study group, too (mean 92.2 versus 79.1 out of 126 points, P = .030).
“A strategy of early assessment with re-isolation of PV reconnections can be deployed safely and improves freedom from AT recurrence and quality of life compared with current standard care. While the gold standard remains durable PVI from the initial procedure, until rates of this can be substantially improved, early re-intervention could be considered as a reasonable strategy to improve outcomes,” the investigators concluded.
“If it was my dad and I was doing a PVI today, I’d say, ‘Dad, let’s bring you back and look at how you’re doing in 2 months,’” lead investigator Dr. Dhiraj Gupta said during an interview after his presentation at the Heart Rhythm Society annual meeting.
“We can’t really afford to do everybody twice, but this has certainly lowered our threshold for reintervention,” said Dr. Gupta, a cardiologist at the Liverpool hospital. “We used to try antiarrhythmic drugs” for recurrence; “we don’t do that anymore. We complete the job that [we] set out to do in the first place. Our threshold is any recurrence beyond 1 month. A surprising number of patients agree with this [recheck] strategy, which is one of the reasons we didn’t have a single drop out in this study. I tell them that it’s highly likely that some of the pulmonary veins I isolated for them are going to reconnect.”
Audience members were concerned that 15 patients in the study arm (38%) ended up having an invasive test they didn’t need. Dr. Gupta said it’s a “glass half full or half empty” situation. “I see it as half full. These repeat procedures are short, safe, and quick [about 80 minutes], and even shorter for those patients who don’t require pulmonary reisolation.” For those patients who do, only a few have symptoms; the rest would have had to wait for remergent symptoms to trigger a second procedure. “I believe that these repeat procedures have become so safe that the risk is more than made up” for by the benefits.
There were no complications with early reinterventions, and there were just two complications with the original PVI; one patient who ended up in the repeat group had a spontaneously resolving phrenic nerve palsy with the first procedure, and one SC patient had a transient ischemic attack. In short, “the complication rates for the two groups were identical,” Dr. Gupta said.
Patients were split about evenly between men and women in both study arms, and patients were in their early 60s, on average. The mean baseline AFEQT score was 46.8 points; 78% in the standard care group, but 55% in the early reintervention group, were on baseline anticoagulants.
All of the subjects were given portable ECG recorders after their initial PVIs, and told to take a daily 30-second recording, and to record if they felt any heart symptoms. They followed the instructions and made more than 32,000 recordings.
Every PVI patient at the Liverpool hospital now gets a recorder at discharge, and physicians there base early interventions on the results, whether or not patients are symptomatic. “We’ve bought lots of them, and tell patients to have a low threshold for recording. I believe 24-hours Holters are a bit outdated,” Dr. Gupta said.
All the PVIs in the study were contact-force guided and used wide area circumferential ablation with the help of 3-D mapping and automated lesion tagging. Entrance and exit block were demonstrated, and adenosine was administered to unmask dormant reconnections after a waiting period of at least 20 minutes. Antiarrhythmic drugs were stopped at 4 weeks.
Biosense Webster funded the work. Dr. Gupta is a speaker for and receives research and fellowship funding from the company.
SAN FRANCISCO – Repeat, invasive electrophysiology studies conducted 2 months after pulmonary vein isolation – regardless of symptoms and, if necessary, with repeat ablations – substantially reduce atrial fibrillation recurrence and improve quality of life at 1 year, according to an investigation conducted at the Liverpool (Eng.) Heart and Chest Hospital.
After initial pulmonary vein isolation (PVI), 40 patients with drug-refractory, paroxysmal atrial fibrillation (AF) were randomized to the repeat approach, and 40 others to the current standard of care (SC), meaning repeat PVI based on recurrent AF symptoms.
Pulmonary vein reconnections were found in 25 patients (63%) checked at 2 months, and all 25 had repeat PVIs without complications. Meanwhile, nine (23%) patients in the SC group had repeat PVIs for clinical recurrence at a mean of about 7 months.
At one year, 33 patients in the repeat group (82.5%), but only 23 in the SC group (57.5%), were free of atrial tachyarrhythmia (AT) (P = .03), and total group AT burden was lower (91 versus 127 days, P = .03). Quality of life scores on the Atrial Fibrillation Effect on Quality of Life (AFEQT) questionnaire were higher in the repeat study group, too (mean 92.2 versus 79.1 out of 126 points, P = .030).
“A strategy of early assessment with re-isolation of PV reconnections can be deployed safely and improves freedom from AT recurrence and quality of life compared with current standard care. While the gold standard remains durable PVI from the initial procedure, until rates of this can be substantially improved, early re-intervention could be considered as a reasonable strategy to improve outcomes,” the investigators concluded.
“If it was my dad and I was doing a PVI today, I’d say, ‘Dad, let’s bring you back and look at how you’re doing in 2 months,’” lead investigator Dr. Dhiraj Gupta said during an interview after his presentation at the Heart Rhythm Society annual meeting.
“We can’t really afford to do everybody twice, but this has certainly lowered our threshold for reintervention,” said Dr. Gupta, a cardiologist at the Liverpool hospital. “We used to try antiarrhythmic drugs” for recurrence; “we don’t do that anymore. We complete the job that [we] set out to do in the first place. Our threshold is any recurrence beyond 1 month. A surprising number of patients agree with this [recheck] strategy, which is one of the reasons we didn’t have a single drop out in this study. I tell them that it’s highly likely that some of the pulmonary veins I isolated for them are going to reconnect.”
Audience members were concerned that 15 patients in the study arm (38%) ended up having an invasive test they didn’t need. Dr. Gupta said it’s a “glass half full or half empty” situation. “I see it as half full. These repeat procedures are short, safe, and quick [about 80 minutes], and even shorter for those patients who don’t require pulmonary reisolation.” For those patients who do, only a few have symptoms; the rest would have had to wait for remergent symptoms to trigger a second procedure. “I believe that these repeat procedures have become so safe that the risk is more than made up” for by the benefits.
There were no complications with early reinterventions, and there were just two complications with the original PVI; one patient who ended up in the repeat group had a spontaneously resolving phrenic nerve palsy with the first procedure, and one SC patient had a transient ischemic attack. In short, “the complication rates for the two groups were identical,” Dr. Gupta said.
Patients were split about evenly between men and women in both study arms, and patients were in their early 60s, on average. The mean baseline AFEQT score was 46.8 points; 78% in the standard care group, but 55% in the early reintervention group, were on baseline anticoagulants.
All of the subjects were given portable ECG recorders after their initial PVIs, and told to take a daily 30-second recording, and to record if they felt any heart symptoms. They followed the instructions and made more than 32,000 recordings.
Every PVI patient at the Liverpool hospital now gets a recorder at discharge, and physicians there base early interventions on the results, whether or not patients are symptomatic. “We’ve bought lots of them, and tell patients to have a low threshold for recording. I believe 24-hours Holters are a bit outdated,” Dr. Gupta said.
All the PVIs in the study were contact-force guided and used wide area circumferential ablation with the help of 3-D mapping and automated lesion tagging. Entrance and exit block were demonstrated, and adenosine was administered to unmask dormant reconnections after a waiting period of at least 20 minutes. Antiarrhythmic drugs were stopped at 4 weeks.
Biosense Webster funded the work. Dr. Gupta is a speaker for and receives research and fellowship funding from the company.
AT HEART RHYTHM 2016
Key clinical point: Repeat, invasive electrophysiology studies 2 months after pulmonary vein isolation – regardless of symptoms and, if necessary, with repeat ablations – substantially reduce atrial fibrillation recurrence and improve quality of life at 1 year.
Major finding: Thirty-three patients in the repeat group (82.5%), but only 23 in the SC group (57.5%), were free of atrial tachyarrhythmias at 12 months (P = .03).
Data source: Randomized study in 80 drug-refractory, paroxysmal atrial fibrillation patients
Disclosures: Biosense Webster funded the work. Dr. Gupta is a speaker for and receives research and fellowship funding from Biosense Webster.
Myth of the Month: NPO good for people with pancreatitis?
A 60-year-old man presents to the emergency department with nausea and abdominal pain, and is admitted with pancreatitis due to alcohol. In the evening after receiving pain medication, his abdominal pain is diminished but still present. He has an appetite and asks for food.
What do you recommend?
A. Nil per os (NPO) until pain is resolved.
B. NPO until amylase/lipase have normalized.
C. Nasogastric tube placement.
D. Okay to start feeding.
Myth: Treatment of pancreatitis includes early avoidance of food.
The conventional management of acute pancreatitis involves an NPO regimen until the pain and nausea have resolved.1 This dogma is offered because of the concern that food intake will stimulate pancreatic enzyme release in an already inflamed/injured pancreas.
The approach of NPO and slowly reintroducing feeding after prolonged periods of being without food is associated with pain relapses and increased length of hospitalizations.2 Nasojejunal feedings have become well accepted in patients with severe pancreatitis requiring ICU care.3
Are there data to show that oral feeding of patients with mild pancreatitis causes worse outcomes?
Dr. Niels Teich and colleagues randomized 143 hospitalized patients with mild pancreatitis to eating when they felt ready to (69 patients) vs. a group that were kept NPO until lipase levels returned to normal.4 The patients who started eating when they were ready left the hospital a day earlier than the patients who were fed only when lipase levels normalized (7 days vs. 8 days). There was no difference in abdominal pain between the two groups.
Dr. Maxim Petrov and colleagues looked at whether nasogastric tube feeding was preferable to NPO in patients with mild to moderate pancreatitis.5 In a randomized trial of 35 patients with pancreatitis, 17 received nasogastric feedings within 24 hours of admission, and 18 were NPO. The patients who received early nasogastric feedings had lower pain scores at 72 hours, compared with the NPO group (1 vs. 3 on a visual analog 10-point scale, P = .036). The number of patients who did not require opiates at 48 hours was also significantly less in the nasogastric feeding group (9 vs. 3, P = .024).
I think the most striking difference was in patients’ ability to tolerate oral feeding. Patients in both groups received oral food at an average of 4 days; only 1 of 17 patients in the nasogastric feeding group could not tolerate feeding, compared with 9 of 18 patients in the NPO group.
Dr. Gunilla Eckerwall and colleagues studied the outcome of immediate oral feeding in patients with mild pancreatitis.6 Sixty patients with mild acute pancreatitis, defined by amylase greater than 3 times normal and APACHE scores less than 8, were randomized to either immediate oral feeding (30 patients) or fasting (30 patients). Key outcome measures in the study were amylase, systemic inflammatory response, and length of hospital stay.
There were no differences in amylase levels, labs measuring systemic inflammatory response, or gastrointestinal symptoms between the two groups. The immediate oral feeding group had a significantly shorter length of hospital stay than the fasting group (4 days vs. 6 days, P less than .05).
So, what does all this tell us about feeding patients with acute pancreatitis? For mild to moderate acute pancreatitis, the outcomes appear to be no worse when patients are fed early. There may be a trend to quicker hospital discharge in those who get fed earlier. The studies have all been small, and a large multicenter trial would be welcome.
References
1. Gastroenterology. 2007 May;132(5):2022-44.
3. Am J Gastroenterol. 2006 Oct;101(10):2379-400.
4. Pancreas. 2010 Oct;39(7):1088-92.
5. Clin Nutr. 2013 Oct;32(5):697-703.
6. Clin Nutr. 2007 Dec;26(6):758-63.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A 60-year-old man presents to the emergency department with nausea and abdominal pain, and is admitted with pancreatitis due to alcohol. In the evening after receiving pain medication, his abdominal pain is diminished but still present. He has an appetite and asks for food.
What do you recommend?
A. Nil per os (NPO) until pain is resolved.
B. NPO until amylase/lipase have normalized.
C. Nasogastric tube placement.
D. Okay to start feeding.
Myth: Treatment of pancreatitis includes early avoidance of food.
The conventional management of acute pancreatitis involves an NPO regimen until the pain and nausea have resolved.1 This dogma is offered because of the concern that food intake will stimulate pancreatic enzyme release in an already inflamed/injured pancreas.
The approach of NPO and slowly reintroducing feeding after prolonged periods of being without food is associated with pain relapses and increased length of hospitalizations.2 Nasojejunal feedings have become well accepted in patients with severe pancreatitis requiring ICU care.3
Are there data to show that oral feeding of patients with mild pancreatitis causes worse outcomes?
Dr. Niels Teich and colleagues randomized 143 hospitalized patients with mild pancreatitis to eating when they felt ready to (69 patients) vs. a group that were kept NPO until lipase levels returned to normal.4 The patients who started eating when they were ready left the hospital a day earlier than the patients who were fed only when lipase levels normalized (7 days vs. 8 days). There was no difference in abdominal pain between the two groups.
Dr. Maxim Petrov and colleagues looked at whether nasogastric tube feeding was preferable to NPO in patients with mild to moderate pancreatitis.5 In a randomized trial of 35 patients with pancreatitis, 17 received nasogastric feedings within 24 hours of admission, and 18 were NPO. The patients who received early nasogastric feedings had lower pain scores at 72 hours, compared with the NPO group (1 vs. 3 on a visual analog 10-point scale, P = .036). The number of patients who did not require opiates at 48 hours was also significantly less in the nasogastric feeding group (9 vs. 3, P = .024).
I think the most striking difference was in patients’ ability to tolerate oral feeding. Patients in both groups received oral food at an average of 4 days; only 1 of 17 patients in the nasogastric feeding group could not tolerate feeding, compared with 9 of 18 patients in the NPO group.
Dr. Gunilla Eckerwall and colleagues studied the outcome of immediate oral feeding in patients with mild pancreatitis.6 Sixty patients with mild acute pancreatitis, defined by amylase greater than 3 times normal and APACHE scores less than 8, were randomized to either immediate oral feeding (30 patients) or fasting (30 patients). Key outcome measures in the study were amylase, systemic inflammatory response, and length of hospital stay.
There were no differences in amylase levels, labs measuring systemic inflammatory response, or gastrointestinal symptoms between the two groups. The immediate oral feeding group had a significantly shorter length of hospital stay than the fasting group (4 days vs. 6 days, P less than .05).
So, what does all this tell us about feeding patients with acute pancreatitis? For mild to moderate acute pancreatitis, the outcomes appear to be no worse when patients are fed early. There may be a trend to quicker hospital discharge in those who get fed earlier. The studies have all been small, and a large multicenter trial would be welcome.
References
1. Gastroenterology. 2007 May;132(5):2022-44.
3. Am J Gastroenterol. 2006 Oct;101(10):2379-400.
4. Pancreas. 2010 Oct;39(7):1088-92.
5. Clin Nutr. 2013 Oct;32(5):697-703.
6. Clin Nutr. 2007 Dec;26(6):758-63.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A 60-year-old man presents to the emergency department with nausea and abdominal pain, and is admitted with pancreatitis due to alcohol. In the evening after receiving pain medication, his abdominal pain is diminished but still present. He has an appetite and asks for food.
What do you recommend?
A. Nil per os (NPO) until pain is resolved.
B. NPO until amylase/lipase have normalized.
C. Nasogastric tube placement.
D. Okay to start feeding.
Myth: Treatment of pancreatitis includes early avoidance of food.
The conventional management of acute pancreatitis involves an NPO regimen until the pain and nausea have resolved.1 This dogma is offered because of the concern that food intake will stimulate pancreatic enzyme release in an already inflamed/injured pancreas.
The approach of NPO and slowly reintroducing feeding after prolonged periods of being without food is associated with pain relapses and increased length of hospitalizations.2 Nasojejunal feedings have become well accepted in patients with severe pancreatitis requiring ICU care.3
Are there data to show that oral feeding of patients with mild pancreatitis causes worse outcomes?
Dr. Niels Teich and colleagues randomized 143 hospitalized patients with mild pancreatitis to eating when they felt ready to (69 patients) vs. a group that were kept NPO until lipase levels returned to normal.4 The patients who started eating when they were ready left the hospital a day earlier than the patients who were fed only when lipase levels normalized (7 days vs. 8 days). There was no difference in abdominal pain between the two groups.
Dr. Maxim Petrov and colleagues looked at whether nasogastric tube feeding was preferable to NPO in patients with mild to moderate pancreatitis.5 In a randomized trial of 35 patients with pancreatitis, 17 received nasogastric feedings within 24 hours of admission, and 18 were NPO. The patients who received early nasogastric feedings had lower pain scores at 72 hours, compared with the NPO group (1 vs. 3 on a visual analog 10-point scale, P = .036). The number of patients who did not require opiates at 48 hours was also significantly less in the nasogastric feeding group (9 vs. 3, P = .024).
I think the most striking difference was in patients’ ability to tolerate oral feeding. Patients in both groups received oral food at an average of 4 days; only 1 of 17 patients in the nasogastric feeding group could not tolerate feeding, compared with 9 of 18 patients in the NPO group.
Dr. Gunilla Eckerwall and colleagues studied the outcome of immediate oral feeding in patients with mild pancreatitis.6 Sixty patients with mild acute pancreatitis, defined by amylase greater than 3 times normal and APACHE scores less than 8, were randomized to either immediate oral feeding (30 patients) or fasting (30 patients). Key outcome measures in the study were amylase, systemic inflammatory response, and length of hospital stay.
There were no differences in amylase levels, labs measuring systemic inflammatory response, or gastrointestinal symptoms between the two groups. The immediate oral feeding group had a significantly shorter length of hospital stay than the fasting group (4 days vs. 6 days, P less than .05).
So, what does all this tell us about feeding patients with acute pancreatitis? For mild to moderate acute pancreatitis, the outcomes appear to be no worse when patients are fed early. There may be a trend to quicker hospital discharge in those who get fed earlier. The studies have all been small, and a large multicenter trial would be welcome.
References
1. Gastroenterology. 2007 May;132(5):2022-44.
3. Am J Gastroenterol. 2006 Oct;101(10):2379-400.
4. Pancreas. 2010 Oct;39(7):1088-92.
5. Clin Nutr. 2013 Oct;32(5):697-703.
6. Clin Nutr. 2007 Dec;26(6):758-63.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
Crisaborole’s safety holds up in long-term atopic dermatitis trial
SCOTTSDALE, ARIZ. – The phosphodiesterase-4 inhibitor crisaborole was well tolerated over 6 to 12 months, yielding no major safety signals during a multicenter, open-label extension study of patients with mild-to-moderate atopic dermatitis.
These safety results held up across age groups and over time, said Dr. Lawrence Eichenfield, a dermatologist at Children’s Hospital, San Diego, and at the University of San Diego School of Medicine. “The majority of treatment-emergent adverse events were considered mild to moderate and not related to treatment. There were no reports of long-term cutaneous reactions, such as atrophy or telangiectasia,” he and his associates added.
Atopic dermatitis has lacked widely accepted treatment options. Despite attempts to educate patients and parents about topical steroids, many are afraid to use them, and topical calcineurin inhibitors have a black box warning for cancer risk. Not surprisingly, therefore, a 2% ointment of crisaborole made headlines in 2015 after meeting its efficacy and safety endpoints in two pivotal phase III trials of patients with mild-to-moderate atopic dermatitis. Based on those results, Anacor Pharmaceuticals filed a new drug application for the novel boron-based small molecule in January 2016.
The pivotal trials lasted just 28 days, so to assess long-term safety, Dr. Eichenfield and his associates enrolled a subgroup of 517 patients aged 2 to 72 years into a single-arm, open-label, 48-week extension study of crisaborole. About 31% of participants had received the control vehicle during the pivotal trials, while the rest had received crisaborole and tolerated it well enough to continue using it. Patients applied crisaborole twice daily during treatment cycles of 28 days, and were evaluated on days 1, 8, and 29 for up to 12 treatment cycles. Patients whose skin became clear or almost clear went off treatment, but they were still assessed for adverse effects at the same frequency.
In all, 396 patients used crisaborole for at least 6 months, and 271 completed 12 months of treatment, the researchers reported at the annual meeting of the Society for Investigative Dermatology. Only nine (1.7%) patients stopped treatment during the extension study because of treatment-emergent adverse effects. A total of 65% of patients had at least one treatment-emergent adverse event during the initial phase III trials, the extension study, or both. These were usually mildly or moderately severe and included nasopharyngitis, upper respiratory infections, cough, and/or fever, all of which were considered unrelated to treatment.
Treatment-related adverse events included flares of atopic dermatitis, burning or stinging at the application site, and application site infection, which affected 3.1%, 2.3%, and 1.2%, respectively, of patients in the extension study. None of these events were considered serious. Notably, 11% of patients experienced atopic dermatitis flares in the original phase III trials, the researchers reported. Patients who could not tolerate crisaborole were excluded from the extension study, which might help explain the lower flare rate (3%) with long-term treatment.
“Crisaborole topical ointment, 2%, demonstrated a favorable long-term safety profile for the treatment of patients aged 2 years and older with mild-to-moderate atopic dermatitis,” the researchers concluded. The Food and Drug Administration accepted the new drug application in March.
Anacor Pharmaceuticals makes crisaborole and funded the study. Dr. Eichenfield has served as an investigator and consultant to Anacor. Three coinvestigators also reported affiliations with Anacor.
SCOTTSDALE, ARIZ. – The phosphodiesterase-4 inhibitor crisaborole was well tolerated over 6 to 12 months, yielding no major safety signals during a multicenter, open-label extension study of patients with mild-to-moderate atopic dermatitis.
These safety results held up across age groups and over time, said Dr. Lawrence Eichenfield, a dermatologist at Children’s Hospital, San Diego, and at the University of San Diego School of Medicine. “The majority of treatment-emergent adverse events were considered mild to moderate and not related to treatment. There were no reports of long-term cutaneous reactions, such as atrophy or telangiectasia,” he and his associates added.
Atopic dermatitis has lacked widely accepted treatment options. Despite attempts to educate patients and parents about topical steroids, many are afraid to use them, and topical calcineurin inhibitors have a black box warning for cancer risk. Not surprisingly, therefore, a 2% ointment of crisaborole made headlines in 2015 after meeting its efficacy and safety endpoints in two pivotal phase III trials of patients with mild-to-moderate atopic dermatitis. Based on those results, Anacor Pharmaceuticals filed a new drug application for the novel boron-based small molecule in January 2016.
The pivotal trials lasted just 28 days, so to assess long-term safety, Dr. Eichenfield and his associates enrolled a subgroup of 517 patients aged 2 to 72 years into a single-arm, open-label, 48-week extension study of crisaborole. About 31% of participants had received the control vehicle during the pivotal trials, while the rest had received crisaborole and tolerated it well enough to continue using it. Patients applied crisaborole twice daily during treatment cycles of 28 days, and were evaluated on days 1, 8, and 29 for up to 12 treatment cycles. Patients whose skin became clear or almost clear went off treatment, but they were still assessed for adverse effects at the same frequency.
In all, 396 patients used crisaborole for at least 6 months, and 271 completed 12 months of treatment, the researchers reported at the annual meeting of the Society for Investigative Dermatology. Only nine (1.7%) patients stopped treatment during the extension study because of treatment-emergent adverse effects. A total of 65% of patients had at least one treatment-emergent adverse event during the initial phase III trials, the extension study, or both. These were usually mildly or moderately severe and included nasopharyngitis, upper respiratory infections, cough, and/or fever, all of which were considered unrelated to treatment.
Treatment-related adverse events included flares of atopic dermatitis, burning or stinging at the application site, and application site infection, which affected 3.1%, 2.3%, and 1.2%, respectively, of patients in the extension study. None of these events were considered serious. Notably, 11% of patients experienced atopic dermatitis flares in the original phase III trials, the researchers reported. Patients who could not tolerate crisaborole were excluded from the extension study, which might help explain the lower flare rate (3%) with long-term treatment.
“Crisaborole topical ointment, 2%, demonstrated a favorable long-term safety profile for the treatment of patients aged 2 years and older with mild-to-moderate atopic dermatitis,” the researchers concluded. The Food and Drug Administration accepted the new drug application in March.
Anacor Pharmaceuticals makes crisaborole and funded the study. Dr. Eichenfield has served as an investigator and consultant to Anacor. Three coinvestigators also reported affiliations with Anacor.
SCOTTSDALE, ARIZ. – The phosphodiesterase-4 inhibitor crisaborole was well tolerated over 6 to 12 months, yielding no major safety signals during a multicenter, open-label extension study of patients with mild-to-moderate atopic dermatitis.
These safety results held up across age groups and over time, said Dr. Lawrence Eichenfield, a dermatologist at Children’s Hospital, San Diego, and at the University of San Diego School of Medicine. “The majority of treatment-emergent adverse events were considered mild to moderate and not related to treatment. There were no reports of long-term cutaneous reactions, such as atrophy or telangiectasia,” he and his associates added.
Atopic dermatitis has lacked widely accepted treatment options. Despite attempts to educate patients and parents about topical steroids, many are afraid to use them, and topical calcineurin inhibitors have a black box warning for cancer risk. Not surprisingly, therefore, a 2% ointment of crisaborole made headlines in 2015 after meeting its efficacy and safety endpoints in two pivotal phase III trials of patients with mild-to-moderate atopic dermatitis. Based on those results, Anacor Pharmaceuticals filed a new drug application for the novel boron-based small molecule in January 2016.
The pivotal trials lasted just 28 days, so to assess long-term safety, Dr. Eichenfield and his associates enrolled a subgroup of 517 patients aged 2 to 72 years into a single-arm, open-label, 48-week extension study of crisaborole. About 31% of participants had received the control vehicle during the pivotal trials, while the rest had received crisaborole and tolerated it well enough to continue using it. Patients applied crisaborole twice daily during treatment cycles of 28 days, and were evaluated on days 1, 8, and 29 for up to 12 treatment cycles. Patients whose skin became clear or almost clear went off treatment, but they were still assessed for adverse effects at the same frequency.
In all, 396 patients used crisaborole for at least 6 months, and 271 completed 12 months of treatment, the researchers reported at the annual meeting of the Society for Investigative Dermatology. Only nine (1.7%) patients stopped treatment during the extension study because of treatment-emergent adverse effects. A total of 65% of patients had at least one treatment-emergent adverse event during the initial phase III trials, the extension study, or both. These were usually mildly or moderately severe and included nasopharyngitis, upper respiratory infections, cough, and/or fever, all of which were considered unrelated to treatment.
Treatment-related adverse events included flares of atopic dermatitis, burning or stinging at the application site, and application site infection, which affected 3.1%, 2.3%, and 1.2%, respectively, of patients in the extension study. None of these events were considered serious. Notably, 11% of patients experienced atopic dermatitis flares in the original phase III trials, the researchers reported. Patients who could not tolerate crisaborole were excluded from the extension study, which might help explain the lower flare rate (3%) with long-term treatment.
“Crisaborole topical ointment, 2%, demonstrated a favorable long-term safety profile for the treatment of patients aged 2 years and older with mild-to-moderate atopic dermatitis,” the researchers concluded. The Food and Drug Administration accepted the new drug application in March.
Anacor Pharmaceuticals makes crisaborole and funded the study. Dr. Eichenfield has served as an investigator and consultant to Anacor. Three coinvestigators also reported affiliations with Anacor.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: The topical phosphodiesterase-4 inhibitor crisaborole was safe and well tolerated for up to 48 weeks in patients with mild-to-moderate atopic dermatitis.
Major finding: The most common treatment–related adverse events were atopic dermatitis flare (3%), stinging and burning at the application site (2%), and application site infection (1%). None were serious.
Data source: A single-arm, multicenter, open-label, 48-week extension study of 517 patients with mild-to-moderate atopic dermatitis.
Disclosures: Anacor Pharmaceuticals makes crisaborole and funded the study. Dr. Eichenfield has served as an investigator and consultant to Anacor. Three coinvestigators also reported affiliations with Anacor.












