User login
What is the best approach to a high systolic pulmonary artery pressure on echocardiography?
The incidental finding of high systolic pulmonary artery pressure on echocardiography is common. What we should do about it varies according to clinical presentation, comorbidities, and results of other tests, including assessment of the right ventricle. Thus, the optimal approach ranges from no further investigation to right heart catheterization and, in some cases, referral to a pulmonary hypertension center.
THE TWO MEASUREMENTS COMPARED
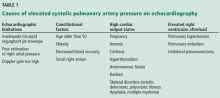
Although it raises concern, the finding of high systolic pulmonary artery pressure is not enough to diagnose pulmonary hypertension. In fact, several other conditions are associated with high systolic pulmonary artery pressure on echocardiography (Table 1). The diagnosis must be confirmed with right heart catheterization.1
Echocardiography provides an estimate of the systolic pulmonary artery pressure that is calculated from other values, whereas right heart catheterization gives a direct measurement of the mean pulmonary artery pressure, which is necessary for diagnosing pulmonary hypertension. The two values are correlated, but the differences are noteworthy.
WHAT IS PULMONARY HYPERTENSION?
Pulmonary hypertension is defined by a resting mean pulmonary artery pressure 25 mm Hg or greater during right heart catheterization.1 The large number of conditions associated with pulmonary hypertension can be divided into five groups2:
- Group 1, pulmonary artery hypertension
- Group 2, pulmonary hypertension associated with left heart disease
- Group 3, pulmonary hypertension due to chronic lung disease or hypoxia
- Group 4, chronic thromboembolic pulmonary hypertension
- Group 5, pulmonary hypertension due to unclear multifactorial mechanisms.2
Pulmonary artery hypertension (group 1) is a syndrome characterized by a restricted flow of small pulmonary arteries that can be idiopathic, heritable, or induced by anorexigens, connective tissue disease, congenital heart disease, portal hypertension, human immunodeficiency virus (HIV), or schistosomiasis.2,3 In spite of significant advances in therapy in the last 3 decades, pulmonary artery hypertension continues to lead to right heart failure and death,4 and the diagnosis has adverse prognostic implications. Therefore, it is essential to be attentive when reviewing the echocardiogram, since an elevated systolic pulmonary artery pressure may be an important clue to pulmonary hypertension.
ESTIMATED PRESSURE: HOW HIGH IS TOO HIGH?
There is no consensus on the optimal cutoff of echocardiographic systolic pulmonary artery pressure to trigger a further evaluation for pulmonary hypertension.
A retrospective evaluation of nearly 16,000 normal echocardiograms found that the 95% upper limit for systolic pulmonary artery pressure was 37 mm Hg.5
European guidelines6 propose that pulmonary hypertension is unlikely if the estimated systolic pulmonary artery pressure is 36 mm Hg or lower, possible if it is 37 to 50 mm Hg, and likely if it is higher than 50 mm Hg.6
The 2009 consensus document of the American College of Cardiology Foundation and American Heart Association3 recommends a systolic pulmonary artery pressure greater than 40 mm Hg as the threshold to suggest further evaluation in a patient with unexplained dyspnea.
Converting the systolic pulmonary artery pressure to the mean pressure
Although not validated to use with echocardiography, the most accurate estimate of mean pulmonary artery pressure was shown in one study7 to be obtained with the equation:
0.61 × systolic pulmonary artery pressure
+ 2 mm Hg
Using this formula, a systolic pulmonary artery pressure of 37 mm Hg would correspond to a mean pulmonary artery pressure of 24.6 mm Hg. A systolic pulmonary artery pressure of 40 mm Hg would correspond to a mean pulmonary artery pressure of 26.4 mm Hg.
Estimated systolic pulmonary artery pressure depends on several variables
Systolic pulmonary artery pressure is estimated using the simplified Bernoulli equation8:
4 × tricuspid regurgitation jet velocity2 (m/s)
+ right atrial pressure (mm Hg)
Tricuspid regurgitation is present in over 75% of the normal population. The regurgitation velocity across the tricuspid valve must be measured to estimate the pressure gradient between the right ventricle and the right atrium. The right atrial pressure is estimated from the diameter of the inferior vena cava and the degree of inspiratory collapse with the sniff test. As the right atrial pressure increases, the inferior vena cava dilates and inspiratory collapse decreases.8 If there is no gradient across the right ventricular outflow tract or pulmonary valve, the right ventricular systolic pressure is equal to the systolic pulmonary artery pressure.
Since tricuspid regurgitation velocity is squared and then multiplied by 4, small deviations of this measurement lead to markedly different systolic pulmonary artery pressure values. To avoid this problem, the tricuspid regurgitation velocity needs to be looked at in multiple echocardiographic views to find the best alignment with the flow and an adequate envelope.
Many causes of high estimated systolic pulmonary artery pressure
Table 1 shows conditions associated with a high estimated systolic pulmonary artery pressure. Echocardiographic limitations, constitutional factors, and high cardiac output states can lead to an apparent elevation in systolic pulmonary artery pressure, which is not confirmed later during right heart catheterization.
Systolic pulmonary artery pressure increases with age and body mass index as a result of worsening left ventricular diastolic dysfunction.8 In fact, an estimated pressure greater than 40 mm Hg is found5 in 6% of people over age 50 and in 5% of people with a body mass index greater than 30 kg/m2. It can also be high in conditions in which there is an increase in cardiac output, such as pregnancy, anemia (sickle cell disease, thalassemia), cirrhosis, and arteriovenous fistula.
The estimated systolic value often differs from the measured value
Studies have compared the systolic pulmonary artery pressure measured during right heart catheterization with the estimated value on echocardiography.9,10 These studies noted a reasonable degree of agreement between the tests but a substantial variability.
Both underestimation and overestimation of the systolic pulmonary artery pressure by echocardiography were common, with 95% limits of agreement ranging from minus 40 mm Hg to plus 40 mm Hg.9,10 A difference of plus or minus 10 mm Hg in systolic pulmonary artery pressure between echocardiography and catheterization was observed in 48% to 51% of patients with pulmonary hypertension, particularly in those with higher systolic pulmonary artery pressure.9,10
An important reason for overestimation of systolic pulmonary artery pressure is the inaccurate estimation of the right atrial pressure by echocardiography.9,10 Indeed, this factor may account for half of the cases in which the systolic pulmonary artery pressure is overestimated.10 Although the traditional methods to estimate the right atrial pressure have been revisited,8,11 this estimation is less reliable for intermediate pressure values, for patients on mechanical ventilation, and for young athletes.8
Other explanations for the variability between measured and estimated systolic pulmonary artery pressure include suboptimal alignment between the Doppler beam and the regurgitant jet, severe tricuspid regurgitation, arrhythmias, and limitations inherent to the simplified Bernoulli equation.12 The estimated value is particularly inaccurate in patients with advanced lung disease, possibly owing to lung hyperinflation and alteration in the thoracic cavity and position of the heart—all factors that limit visualization and measurement of the tricuspid regurgitant jet.13
OTHER SIGNS OF PULMONARY HYPERTENSION ON ECHOCARDIOGRAPHY
Echocardiography provides information that is useful in assessing the accuracy of the estimated systolic pulmonary artery pressure, particularly right ventricular size and function.
As pulmonary hypertension progresses, the right ventricle dilates, and its function is compromised. Therefore, it is important to determine the right ventricular size and function by using objective echocardiographic findings such as right ventricular diameters (basal, mid, apical) and area, right ventricular fractional area change, tricuspid annular plane systolic excursion, myocardial performance index, and the pulsed tissue Doppler tricuspid annular peak systolic excursion velocity.8
Other echocardiographic features that suggest pulmonary hypertension include a dilated right atrial area, flattening of the interventricular septum, notching of the right ventricular outflow tract flow, and dilation of the main pulmonary artery. Interestingly, left ventricular diastolic dysfunction of the impaired relaxation type (grade I) is commonly observed in pulmonary hypertension14; however, more advanced degrees of diastolic dysfunction, ie, pseudonormalization (grade II) or restrictive left ventricular filling (grade III),15 particularly when associated with a left atrial enlargement, suggest pulmonary hypertension associated with left heart disease and not pulmonary artery hypertension.
WHAT TO DO IF ECHOCARDIOGRAPHY INDICATES PULMONARY HYPERTENSION
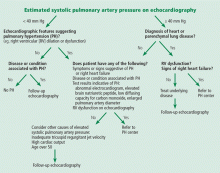
An algorithm showing the approach to an elevated systolic pulmonary artery pressure on echocardiography is presented in Figure 1.
In the appropriate clinical setting, if the systolic pulmonary artery pressure is 40 mm Hg or greater or if other echocardiographic variables suggest pulmonary hypertension, our practice is to proceed with right heart catheterization.
Clinical variables that suggest pulmonary hypertension include progressive dyspnea, chest pain, presyncope-syncope, lower extremity edema, hepatomegaly, jugular vein distention, hepatojugular reflux, sternal heave, loud second heart sound (P2), murmur of tricuspid or pulmonary regurgitation, and right ventricular third heart sound.16 These are of particular interest when associated with conditions known to cause pulmonary hypertension,2such as connective tissue disease, portal hypertension, congenital heart disease, HIV infection, and certain drugs and toxins.
Other tests that raise suspicion of pulmonary hypertension are an electrocardiogram suggesting a dilated right atrium or ventricle, an elevated brain natriuretic peptide level, a low carbon monoxide diffusing capacity on pulmonary function testing, and an enlarged pulmonary artery diameter on imaging.
Given the high prevalence of pulmonary hypertension, the Fifth World Symposium on Pulmonary Hypertension recommended first considering heart or parenchymal lung disease when an echocardiogram suggests pulmonary hypertension.6 If there are signs of severe pulmonary hypertension or right ventricular dysfunction, referral to a center specializing in pulmonary hypertension is recommended. Referral is also appropriate when there is no major heart or lung disease and the echocardiogram shows an elevated systolic pulmonary artery pressure, particularly when the clinical presentation or results of other testing suggest pulmonary hypertension.
TAKE-HOME POINTS
In the appropriate context, a high systolic pulmonary artery pressure on echocardiography suggests pulmonary hypertension, but right heart catheterization is needed to confirm the diagnosis. Estimating the systolic pulmonary artery pressure with echocardiography has limitations, including false-positive results, predominantly when the pretest probability of pulmonary hypertension is low.
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D42–D50.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D34–D41.
- McLaughlin VV, Archer SL, Badesch DB, et al; American College of Cardiology Foundation Task Force on Expert Consensus Documents; American Heart Association; American College of Chest Physicians; American Thoracic Society, Inc; Pulmonary Hypertension Association. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53:1573–1619.
- Tonelli AR, Arelli V, Minai OA, et al. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med 2013; 188:365–369.
- McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 2001; 104:2797–2802.
- Galiè N, Hoeper MM, Humbert M, et al; ESC Committee for Practice Guidelines (CPG). Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30:2493–2537.
- Chemla D, Castelain V, Provencher S, Humbert M, Simonneau G, Herve P. Evaluation of various empirical formulas for estimating mean pulmonary artery pressure by using systolic pulmonary artery pressure in adults. Chest 2009; 135:760–768.
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23:685–713.
- Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest 2011; 139:988–993.
- Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009; 179:615–621.
- Brennan JM, Blair JE, Goonewardena S, et al. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J Am Soc Echocardiogr 2007; 20:857–861.
- Giardini A, Tacy TA. Non-invasive estimation of pressure gradients in regurgitant jets: an overdue consideration. Eur J Echocardiogr 2008; 9:578–584.
- Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003; 167:735–740.
- Tonelli AR, Plana JC, Heresi GA, Dweik RA. Prevalence and prognostic value of left ventricular diastolic dysfunction in idiopathic and heritable pulmonary arterial hypertension. Chest 2012; 141:1457–1465.
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009; 22:107–133.
- Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43(suppl S):40S–47S.
The incidental finding of high systolic pulmonary artery pressure on echocardiography is common. What we should do about it varies according to clinical presentation, comorbidities, and results of other tests, including assessment of the right ventricle. Thus, the optimal approach ranges from no further investigation to right heart catheterization and, in some cases, referral to a pulmonary hypertension center.
THE TWO MEASUREMENTS COMPARED
Although it raises concern, the finding of high systolic pulmonary artery pressure is not enough to diagnose pulmonary hypertension. In fact, several other conditions are associated with high systolic pulmonary artery pressure on echocardiography (Table 1). The diagnosis must be confirmed with right heart catheterization.1
Echocardiography provides an estimate of the systolic pulmonary artery pressure that is calculated from other values, whereas right heart catheterization gives a direct measurement of the mean pulmonary artery pressure, which is necessary for diagnosing pulmonary hypertension. The two values are correlated, but the differences are noteworthy.
WHAT IS PULMONARY HYPERTENSION?
Pulmonary hypertension is defined by a resting mean pulmonary artery pressure 25 mm Hg or greater during right heart catheterization.1 The large number of conditions associated with pulmonary hypertension can be divided into five groups2:
- Group 1, pulmonary artery hypertension
- Group 2, pulmonary hypertension associated with left heart disease
- Group 3, pulmonary hypertension due to chronic lung disease or hypoxia
- Group 4, chronic thromboembolic pulmonary hypertension
- Group 5, pulmonary hypertension due to unclear multifactorial mechanisms.2
Pulmonary artery hypertension (group 1) is a syndrome characterized by a restricted flow of small pulmonary arteries that can be idiopathic, heritable, or induced by anorexigens, connective tissue disease, congenital heart disease, portal hypertension, human immunodeficiency virus (HIV), or schistosomiasis.2,3 In spite of significant advances in therapy in the last 3 decades, pulmonary artery hypertension continues to lead to right heart failure and death,4 and the diagnosis has adverse prognostic implications. Therefore, it is essential to be attentive when reviewing the echocardiogram, since an elevated systolic pulmonary artery pressure may be an important clue to pulmonary hypertension.
ESTIMATED PRESSURE: HOW HIGH IS TOO HIGH?
There is no consensus on the optimal cutoff of echocardiographic systolic pulmonary artery pressure to trigger a further evaluation for pulmonary hypertension.
A retrospective evaluation of nearly 16,000 normal echocardiograms found that the 95% upper limit for systolic pulmonary artery pressure was 37 mm Hg.5
European guidelines6 propose that pulmonary hypertension is unlikely if the estimated systolic pulmonary artery pressure is 36 mm Hg or lower, possible if it is 37 to 50 mm Hg, and likely if it is higher than 50 mm Hg.6
The 2009 consensus document of the American College of Cardiology Foundation and American Heart Association3 recommends a systolic pulmonary artery pressure greater than 40 mm Hg as the threshold to suggest further evaluation in a patient with unexplained dyspnea.
Converting the systolic pulmonary artery pressure to the mean pressure
Although not validated to use with echocardiography, the most accurate estimate of mean pulmonary artery pressure was shown in one study7 to be obtained with the equation:
0.61 × systolic pulmonary artery pressure
+ 2 mm Hg
Using this formula, a systolic pulmonary artery pressure of 37 mm Hg would correspond to a mean pulmonary artery pressure of 24.6 mm Hg. A systolic pulmonary artery pressure of 40 mm Hg would correspond to a mean pulmonary artery pressure of 26.4 mm Hg.
Estimated systolic pulmonary artery pressure depends on several variables
Systolic pulmonary artery pressure is estimated using the simplified Bernoulli equation8:
4 × tricuspid regurgitation jet velocity2 (m/s)
+ right atrial pressure (mm Hg)
Tricuspid regurgitation is present in over 75% of the normal population. The regurgitation velocity across the tricuspid valve must be measured to estimate the pressure gradient between the right ventricle and the right atrium. The right atrial pressure is estimated from the diameter of the inferior vena cava and the degree of inspiratory collapse with the sniff test. As the right atrial pressure increases, the inferior vena cava dilates and inspiratory collapse decreases.8 If there is no gradient across the right ventricular outflow tract or pulmonary valve, the right ventricular systolic pressure is equal to the systolic pulmonary artery pressure.
Since tricuspid regurgitation velocity is squared and then multiplied by 4, small deviations of this measurement lead to markedly different systolic pulmonary artery pressure values. To avoid this problem, the tricuspid regurgitation velocity needs to be looked at in multiple echocardiographic views to find the best alignment with the flow and an adequate envelope.
Many causes of high estimated systolic pulmonary artery pressure
Table 1 shows conditions associated with a high estimated systolic pulmonary artery pressure. Echocardiographic limitations, constitutional factors, and high cardiac output states can lead to an apparent elevation in systolic pulmonary artery pressure, which is not confirmed later during right heart catheterization.
Systolic pulmonary artery pressure increases with age and body mass index as a result of worsening left ventricular diastolic dysfunction.8 In fact, an estimated pressure greater than 40 mm Hg is found5 in 6% of people over age 50 and in 5% of people with a body mass index greater than 30 kg/m2. It can also be high in conditions in which there is an increase in cardiac output, such as pregnancy, anemia (sickle cell disease, thalassemia), cirrhosis, and arteriovenous fistula.
The estimated systolic value often differs from the measured value
Studies have compared the systolic pulmonary artery pressure measured during right heart catheterization with the estimated value on echocardiography.9,10 These studies noted a reasonable degree of agreement between the tests but a substantial variability.
Both underestimation and overestimation of the systolic pulmonary artery pressure by echocardiography were common, with 95% limits of agreement ranging from minus 40 mm Hg to plus 40 mm Hg.9,10 A difference of plus or minus 10 mm Hg in systolic pulmonary artery pressure between echocardiography and catheterization was observed in 48% to 51% of patients with pulmonary hypertension, particularly in those with higher systolic pulmonary artery pressure.9,10
An important reason for overestimation of systolic pulmonary artery pressure is the inaccurate estimation of the right atrial pressure by echocardiography.9,10 Indeed, this factor may account for half of the cases in which the systolic pulmonary artery pressure is overestimated.10 Although the traditional methods to estimate the right atrial pressure have been revisited,8,11 this estimation is less reliable for intermediate pressure values, for patients on mechanical ventilation, and for young athletes.8
Other explanations for the variability between measured and estimated systolic pulmonary artery pressure include suboptimal alignment between the Doppler beam and the regurgitant jet, severe tricuspid regurgitation, arrhythmias, and limitations inherent to the simplified Bernoulli equation.12 The estimated value is particularly inaccurate in patients with advanced lung disease, possibly owing to lung hyperinflation and alteration in the thoracic cavity and position of the heart—all factors that limit visualization and measurement of the tricuspid regurgitant jet.13
OTHER SIGNS OF PULMONARY HYPERTENSION ON ECHOCARDIOGRAPHY
Echocardiography provides information that is useful in assessing the accuracy of the estimated systolic pulmonary artery pressure, particularly right ventricular size and function.
As pulmonary hypertension progresses, the right ventricle dilates, and its function is compromised. Therefore, it is important to determine the right ventricular size and function by using objective echocardiographic findings such as right ventricular diameters (basal, mid, apical) and area, right ventricular fractional area change, tricuspid annular plane systolic excursion, myocardial performance index, and the pulsed tissue Doppler tricuspid annular peak systolic excursion velocity.8
Other echocardiographic features that suggest pulmonary hypertension include a dilated right atrial area, flattening of the interventricular septum, notching of the right ventricular outflow tract flow, and dilation of the main pulmonary artery. Interestingly, left ventricular diastolic dysfunction of the impaired relaxation type (grade I) is commonly observed in pulmonary hypertension14; however, more advanced degrees of diastolic dysfunction, ie, pseudonormalization (grade II) or restrictive left ventricular filling (grade III),15 particularly when associated with a left atrial enlargement, suggest pulmonary hypertension associated with left heart disease and not pulmonary artery hypertension.
WHAT TO DO IF ECHOCARDIOGRAPHY INDICATES PULMONARY HYPERTENSION
An algorithm showing the approach to an elevated systolic pulmonary artery pressure on echocardiography is presented in Figure 1.
In the appropriate clinical setting, if the systolic pulmonary artery pressure is 40 mm Hg or greater or if other echocardiographic variables suggest pulmonary hypertension, our practice is to proceed with right heart catheterization.
Clinical variables that suggest pulmonary hypertension include progressive dyspnea, chest pain, presyncope-syncope, lower extremity edema, hepatomegaly, jugular vein distention, hepatojugular reflux, sternal heave, loud second heart sound (P2), murmur of tricuspid or pulmonary regurgitation, and right ventricular third heart sound.16 These are of particular interest when associated with conditions known to cause pulmonary hypertension,2such as connective tissue disease, portal hypertension, congenital heart disease, HIV infection, and certain drugs and toxins.
Other tests that raise suspicion of pulmonary hypertension are an electrocardiogram suggesting a dilated right atrium or ventricle, an elevated brain natriuretic peptide level, a low carbon monoxide diffusing capacity on pulmonary function testing, and an enlarged pulmonary artery diameter on imaging.
Given the high prevalence of pulmonary hypertension, the Fifth World Symposium on Pulmonary Hypertension recommended first considering heart or parenchymal lung disease when an echocardiogram suggests pulmonary hypertension.6 If there are signs of severe pulmonary hypertension or right ventricular dysfunction, referral to a center specializing in pulmonary hypertension is recommended. Referral is also appropriate when there is no major heart or lung disease and the echocardiogram shows an elevated systolic pulmonary artery pressure, particularly when the clinical presentation or results of other testing suggest pulmonary hypertension.
TAKE-HOME POINTS
In the appropriate context, a high systolic pulmonary artery pressure on echocardiography suggests pulmonary hypertension, but right heart catheterization is needed to confirm the diagnosis. Estimating the systolic pulmonary artery pressure with echocardiography has limitations, including false-positive results, predominantly when the pretest probability of pulmonary hypertension is low.
The incidental finding of high systolic pulmonary artery pressure on echocardiography is common. What we should do about it varies according to clinical presentation, comorbidities, and results of other tests, including assessment of the right ventricle. Thus, the optimal approach ranges from no further investigation to right heart catheterization and, in some cases, referral to a pulmonary hypertension center.
THE TWO MEASUREMENTS COMPARED
Although it raises concern, the finding of high systolic pulmonary artery pressure is not enough to diagnose pulmonary hypertension. In fact, several other conditions are associated with high systolic pulmonary artery pressure on echocardiography (Table 1). The diagnosis must be confirmed with right heart catheterization.1
Echocardiography provides an estimate of the systolic pulmonary artery pressure that is calculated from other values, whereas right heart catheterization gives a direct measurement of the mean pulmonary artery pressure, which is necessary for diagnosing pulmonary hypertension. The two values are correlated, but the differences are noteworthy.
WHAT IS PULMONARY HYPERTENSION?
Pulmonary hypertension is defined by a resting mean pulmonary artery pressure 25 mm Hg or greater during right heart catheterization.1 The large number of conditions associated with pulmonary hypertension can be divided into five groups2:
- Group 1, pulmonary artery hypertension
- Group 2, pulmonary hypertension associated with left heart disease
- Group 3, pulmonary hypertension due to chronic lung disease or hypoxia
- Group 4, chronic thromboembolic pulmonary hypertension
- Group 5, pulmonary hypertension due to unclear multifactorial mechanisms.2
Pulmonary artery hypertension (group 1) is a syndrome characterized by a restricted flow of small pulmonary arteries that can be idiopathic, heritable, or induced by anorexigens, connective tissue disease, congenital heart disease, portal hypertension, human immunodeficiency virus (HIV), or schistosomiasis.2,3 In spite of significant advances in therapy in the last 3 decades, pulmonary artery hypertension continues to lead to right heart failure and death,4 and the diagnosis has adverse prognostic implications. Therefore, it is essential to be attentive when reviewing the echocardiogram, since an elevated systolic pulmonary artery pressure may be an important clue to pulmonary hypertension.
ESTIMATED PRESSURE: HOW HIGH IS TOO HIGH?
There is no consensus on the optimal cutoff of echocardiographic systolic pulmonary artery pressure to trigger a further evaluation for pulmonary hypertension.
A retrospective evaluation of nearly 16,000 normal echocardiograms found that the 95% upper limit for systolic pulmonary artery pressure was 37 mm Hg.5
European guidelines6 propose that pulmonary hypertension is unlikely if the estimated systolic pulmonary artery pressure is 36 mm Hg or lower, possible if it is 37 to 50 mm Hg, and likely if it is higher than 50 mm Hg.6
The 2009 consensus document of the American College of Cardiology Foundation and American Heart Association3 recommends a systolic pulmonary artery pressure greater than 40 mm Hg as the threshold to suggest further evaluation in a patient with unexplained dyspnea.
Converting the systolic pulmonary artery pressure to the mean pressure
Although not validated to use with echocardiography, the most accurate estimate of mean pulmonary artery pressure was shown in one study7 to be obtained with the equation:
0.61 × systolic pulmonary artery pressure
+ 2 mm Hg
Using this formula, a systolic pulmonary artery pressure of 37 mm Hg would correspond to a mean pulmonary artery pressure of 24.6 mm Hg. A systolic pulmonary artery pressure of 40 mm Hg would correspond to a mean pulmonary artery pressure of 26.4 mm Hg.
Estimated systolic pulmonary artery pressure depends on several variables
Systolic pulmonary artery pressure is estimated using the simplified Bernoulli equation8:
4 × tricuspid regurgitation jet velocity2 (m/s)
+ right atrial pressure (mm Hg)
Tricuspid regurgitation is present in over 75% of the normal population. The regurgitation velocity across the tricuspid valve must be measured to estimate the pressure gradient between the right ventricle and the right atrium. The right atrial pressure is estimated from the diameter of the inferior vena cava and the degree of inspiratory collapse with the sniff test. As the right atrial pressure increases, the inferior vena cava dilates and inspiratory collapse decreases.8 If there is no gradient across the right ventricular outflow tract or pulmonary valve, the right ventricular systolic pressure is equal to the systolic pulmonary artery pressure.
Since tricuspid regurgitation velocity is squared and then multiplied by 4, small deviations of this measurement lead to markedly different systolic pulmonary artery pressure values. To avoid this problem, the tricuspid regurgitation velocity needs to be looked at in multiple echocardiographic views to find the best alignment with the flow and an adequate envelope.
Many causes of high estimated systolic pulmonary artery pressure
Table 1 shows conditions associated with a high estimated systolic pulmonary artery pressure. Echocardiographic limitations, constitutional factors, and high cardiac output states can lead to an apparent elevation in systolic pulmonary artery pressure, which is not confirmed later during right heart catheterization.
Systolic pulmonary artery pressure increases with age and body mass index as a result of worsening left ventricular diastolic dysfunction.8 In fact, an estimated pressure greater than 40 mm Hg is found5 in 6% of people over age 50 and in 5% of people with a body mass index greater than 30 kg/m2. It can also be high in conditions in which there is an increase in cardiac output, such as pregnancy, anemia (sickle cell disease, thalassemia), cirrhosis, and arteriovenous fistula.
The estimated systolic value often differs from the measured value
Studies have compared the systolic pulmonary artery pressure measured during right heart catheterization with the estimated value on echocardiography.9,10 These studies noted a reasonable degree of agreement between the tests but a substantial variability.
Both underestimation and overestimation of the systolic pulmonary artery pressure by echocardiography were common, with 95% limits of agreement ranging from minus 40 mm Hg to plus 40 mm Hg.9,10 A difference of plus or minus 10 mm Hg in systolic pulmonary artery pressure between echocardiography and catheterization was observed in 48% to 51% of patients with pulmonary hypertension, particularly in those with higher systolic pulmonary artery pressure.9,10
An important reason for overestimation of systolic pulmonary artery pressure is the inaccurate estimation of the right atrial pressure by echocardiography.9,10 Indeed, this factor may account for half of the cases in which the systolic pulmonary artery pressure is overestimated.10 Although the traditional methods to estimate the right atrial pressure have been revisited,8,11 this estimation is less reliable for intermediate pressure values, for patients on mechanical ventilation, and for young athletes.8
Other explanations for the variability between measured and estimated systolic pulmonary artery pressure include suboptimal alignment between the Doppler beam and the regurgitant jet, severe tricuspid regurgitation, arrhythmias, and limitations inherent to the simplified Bernoulli equation.12 The estimated value is particularly inaccurate in patients with advanced lung disease, possibly owing to lung hyperinflation and alteration in the thoracic cavity and position of the heart—all factors that limit visualization and measurement of the tricuspid regurgitant jet.13
OTHER SIGNS OF PULMONARY HYPERTENSION ON ECHOCARDIOGRAPHY
Echocardiography provides information that is useful in assessing the accuracy of the estimated systolic pulmonary artery pressure, particularly right ventricular size and function.
As pulmonary hypertension progresses, the right ventricle dilates, and its function is compromised. Therefore, it is important to determine the right ventricular size and function by using objective echocardiographic findings such as right ventricular diameters (basal, mid, apical) and area, right ventricular fractional area change, tricuspid annular plane systolic excursion, myocardial performance index, and the pulsed tissue Doppler tricuspid annular peak systolic excursion velocity.8
Other echocardiographic features that suggest pulmonary hypertension include a dilated right atrial area, flattening of the interventricular septum, notching of the right ventricular outflow tract flow, and dilation of the main pulmonary artery. Interestingly, left ventricular diastolic dysfunction of the impaired relaxation type (grade I) is commonly observed in pulmonary hypertension14; however, more advanced degrees of diastolic dysfunction, ie, pseudonormalization (grade II) or restrictive left ventricular filling (grade III),15 particularly when associated with a left atrial enlargement, suggest pulmonary hypertension associated with left heart disease and not pulmonary artery hypertension.
WHAT TO DO IF ECHOCARDIOGRAPHY INDICATES PULMONARY HYPERTENSION
An algorithm showing the approach to an elevated systolic pulmonary artery pressure on echocardiography is presented in Figure 1.
In the appropriate clinical setting, if the systolic pulmonary artery pressure is 40 mm Hg or greater or if other echocardiographic variables suggest pulmonary hypertension, our practice is to proceed with right heart catheterization.
Clinical variables that suggest pulmonary hypertension include progressive dyspnea, chest pain, presyncope-syncope, lower extremity edema, hepatomegaly, jugular vein distention, hepatojugular reflux, sternal heave, loud second heart sound (P2), murmur of tricuspid or pulmonary regurgitation, and right ventricular third heart sound.16 These are of particular interest when associated with conditions known to cause pulmonary hypertension,2such as connective tissue disease, portal hypertension, congenital heart disease, HIV infection, and certain drugs and toxins.
Other tests that raise suspicion of pulmonary hypertension are an electrocardiogram suggesting a dilated right atrium or ventricle, an elevated brain natriuretic peptide level, a low carbon monoxide diffusing capacity on pulmonary function testing, and an enlarged pulmonary artery diameter on imaging.
Given the high prevalence of pulmonary hypertension, the Fifth World Symposium on Pulmonary Hypertension recommended first considering heart or parenchymal lung disease when an echocardiogram suggests pulmonary hypertension.6 If there are signs of severe pulmonary hypertension or right ventricular dysfunction, referral to a center specializing in pulmonary hypertension is recommended. Referral is also appropriate when there is no major heart or lung disease and the echocardiogram shows an elevated systolic pulmonary artery pressure, particularly when the clinical presentation or results of other testing suggest pulmonary hypertension.
TAKE-HOME POINTS
In the appropriate context, a high systolic pulmonary artery pressure on echocardiography suggests pulmonary hypertension, but right heart catheterization is needed to confirm the diagnosis. Estimating the systolic pulmonary artery pressure with echocardiography has limitations, including false-positive results, predominantly when the pretest probability of pulmonary hypertension is low.
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D42–D50.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D34–D41.
- McLaughlin VV, Archer SL, Badesch DB, et al; American College of Cardiology Foundation Task Force on Expert Consensus Documents; American Heart Association; American College of Chest Physicians; American Thoracic Society, Inc; Pulmonary Hypertension Association. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53:1573–1619.
- Tonelli AR, Arelli V, Minai OA, et al. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med 2013; 188:365–369.
- McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 2001; 104:2797–2802.
- Galiè N, Hoeper MM, Humbert M, et al; ESC Committee for Practice Guidelines (CPG). Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30:2493–2537.
- Chemla D, Castelain V, Provencher S, Humbert M, Simonneau G, Herve P. Evaluation of various empirical formulas for estimating mean pulmonary artery pressure by using systolic pulmonary artery pressure in adults. Chest 2009; 135:760–768.
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23:685–713.
- Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest 2011; 139:988–993.
- Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009; 179:615–621.
- Brennan JM, Blair JE, Goonewardena S, et al. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J Am Soc Echocardiogr 2007; 20:857–861.
- Giardini A, Tacy TA. Non-invasive estimation of pressure gradients in regurgitant jets: an overdue consideration. Eur J Echocardiogr 2008; 9:578–584.
- Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003; 167:735–740.
- Tonelli AR, Plana JC, Heresi GA, Dweik RA. Prevalence and prognostic value of left ventricular diastolic dysfunction in idiopathic and heritable pulmonary arterial hypertension. Chest 2012; 141:1457–1465.
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009; 22:107–133.
- Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43(suppl S):40S–47S.
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D42–D50.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(suppl D):D34–D41.
- McLaughlin VV, Archer SL, Badesch DB, et al; American College of Cardiology Foundation Task Force on Expert Consensus Documents; American Heart Association; American College of Chest Physicians; American Thoracic Society, Inc; Pulmonary Hypertension Association. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53:1573–1619.
- Tonelli AR, Arelli V, Minai OA, et al. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med 2013; 188:365–369.
- McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 2001; 104:2797–2802.
- Galiè N, Hoeper MM, Humbert M, et al; ESC Committee for Practice Guidelines (CPG). Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30:2493–2537.
- Chemla D, Castelain V, Provencher S, Humbert M, Simonneau G, Herve P. Evaluation of various empirical formulas for estimating mean pulmonary artery pressure by using systolic pulmonary artery pressure in adults. Chest 2009; 135:760–768.
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23:685–713.
- Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest 2011; 139:988–993.
- Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009; 179:615–621.
- Brennan JM, Blair JE, Goonewardena S, et al. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J Am Soc Echocardiogr 2007; 20:857–861.
- Giardini A, Tacy TA. Non-invasive estimation of pressure gradients in regurgitant jets: an overdue consideration. Eur J Echocardiogr 2008; 9:578–584.
- Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003; 167:735–740.
- Tonelli AR, Plana JC, Heresi GA, Dweik RA. Prevalence and prognostic value of left ventricular diastolic dysfunction in idiopathic and heritable pulmonary arterial hypertension. Chest 2012; 141:1457–1465.
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009; 22:107–133.
- Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43(suppl S):40S–47S.
Drugs that may harm bone: Mitigating the risk
Drug-induced osteoporosis is common, and the list of drugs that can harm bone continues to grow. As part of routine health maintenance, practitioners should recognize the drugs that increase bone loss and take measures to mitigate these effects to help avoid osteopenia and osteoporosis.
Osteoporosis, a silent systemic disease defined by low bone mineral density and changes in skeletal microstructure, leads to a higher risk of fragility fractures. Some of the risk factors are well described, but less well known is the role of pharmacologic therapy. The implicated drugs (Table 1) have important therapeutic roles, so the benefits of using them must be weighed against their risks, including their potential effects on bone.
This review focuses on a few drugs known to increase fracture risk, their mechanisms of bone loss, and management considerations (Table 2).
GLUCOCORTICOIDS
Glucocorticoids are used to treat many medical conditions, including allergic, rheumatic, and other inflammatory diseases, and as immunosuppressive therapy after solid organ and bone marrow transplant. They are the most common cause of drug-induced bone loss and related secondary osteoporosis.
Multiple effects on bone
Glucocorticoids both increase bone resorption and decrease bone formation by a variety of mechanisms.1 They reduce intestinal calcium absorption, increase urinary excretion of calcium, and enhance osteocyte apoptosis, leading to deterioration of the bone microarchitecture and bone mineral density.2 They also affect sex hormones, decreasing testosterone production in men and estrogen in women, leading to increased bone resorption, altered bone architecture, and poorer bone quality.3,4 The bone loss is greater in trabecular bone (eg, the femoral neck and vertebral bodies) than in cortical bone (eg, the forearm).5
Glucocorticoids have other systemic effects that increase fracture risk. For example, they cause muscle weakness and atrophy, increasing the risk of falls.4 Additionally, many of the inflammatory conditions for which they are prescribed (eg, rheumatoid arthritis) also increase the risk of osteoporosis by means of proinflammatory cytokine production, which may contribute to systemic and local effects on bone.4,6
Bone mineral density declines quickly
Bone mineral density declines within the first 3 months after starting oral glucocorticoids, with the rate of bone loss peaking at 6 months. Up to 12% of bone mass is lost in the first year. In subsequent years of continued use, bone loss progresses at a slower, steadier rate, averaging 2% to 3% annually.5,7,8
Oral therapy increases fracture risk
Kanis et al9 performed a meta-analysis of seven prospective cohort studies in 40,000 patients and found that the current or previous use of an oral glucocorticoid increased the risk of fragility fractures, and that men and women were affected about equally.9
Van Staa et al10,11 reported that daily doses of glucocorticoids equivalent to more than 2.5 mg of prednisone were associated with an increased risk of vertebral and hip fractures; fracture risk was related mainly to daily dosage rather than cumulative dose.
Van Staa et al,12 in a retrospective cohort study, compared nearly 250,000 adult users of oral glucocorticoids from general medical practice settings with the same number of controls matched for sex, age, and medical practice. The relative risks for fractures and 95% confidence intervals (CIs) during oral glucocorticoid treatment were as follows:
- Forearm fracture 1.09 (1.01–1.17)
- Nonvertebral fracture 1.33 (1.29–1.38)
- Hip fracture 1.61 (1.47–1.76)
- Vertebral fracture 2.60 (2.31–2.92).
The risk was dose-dependent. For a low daily dose (< 2.5 mg/day of prednisolone), the relative risks were:
- Hip fracture 0.99 (0.82–1.20)
- Vertebral fracture 1.55 (1.20–2.01) .
For a medium daily dose (2.5–7.5 mg/day), the relative risks were:
- Hip fracture 1.77 (1.55–2.02)
- Vertebral fracture 2.59 (2.16–3.10) .
For a high daily dose (> 7.5 mg/day), the relative risks were:
- Hip fracture 2.27 (1.94–2.66)
- Vertebral fracture 5.18 (4.25–6.31).
Fracture risk rapidly declined toward baseline after the patients stopped taking oral glucocorticoids but did not return to baseline levels. The lessening of excess fracture risk occurred mainly within the first year after stopping therapy.
Other studies5,9,13 have suggested that the increased fracture risk is mostly independent of bone mineral density, and that other mechanisms are at play. One study14 found that oral glucocorticoid users with a prevalent vertebral fracture actually had higher bone mineral density than patients with a fracture not taking glucocorticoids, although this finding was not confirmed in a subsequent study.15
Inhaled glucocorticoids have less effect on bone
Inhaled glucocorticoids are commonly used to treat chronic obstructive pulmonary disease and asthma. They do not have the same systemic bioavailability as oral glucocorticoids, so the risk of adverse effects is lower.
Data are inconsistent among several studies that evaluated the relationship between inhaled glucocorticoids, bone mineral density, osteoporosis, and fragility fracture. The inconsistencies may be due to heterogeneity of the study populations, self-reporting of fractures, and different methods of assessing chronic obstructive pulmonary disease severity.16
A Cochrane review17 in 2002 evaluated seven randomized controlled trials that compared the use of inhaled glucocorticoids vs placebo in nearly 2,000 patients with mild asthma or chronic obstructive pulmonary disease and found no evidence for decreased bone mineral density, increased bone turnover, or increased vertebral fracture incidence in the glucocorticoid users at 2 to 3 years of follow-up (odds ratio for fracture 1.87, 95% CI 0.5–7.0).
The Evaluation of Obstructive Lung Disease and Osteoporosis study,16 a multicenter Italian observational epidemiologic study, reported that patients taking the highest daily doses of inhaled glucocorticoids (> 1,500 μg of beclomethasone or its equivalent) had a significantly higher risk of vertebral fracture (odds ratio 1.4, 95% CI 1.04–1.89).16
A meta-analysis18 of five case-control studies (43,783 cases and 259,936 controls) identified a possible dose-dependent relationship, with a relative risk for nonvertebral fracture of 1.12 (95% CI 1.0–1.26) for each 1,000-μg increase in beclomethasone-equivalent inhaled glucocorticoid per day.
In summary, the effects of inhaled glucocorticoids in adults are uncertain, although trends toward increased fracture risk and decreased bone mineral density are evident with chronic therapy at moderate to high dosages. The risks and benefits of treatment should be carefully considered in patients with osteoporosis and baseline elevated fracture risk.19
Managing the risk of glucocorticoid-induced osteoporosis
In 2010, the American College of Rheumatology published recommendations for preventing and treating glucocorticoid-induced osteoporosis, which were endorsed by the American Society for Bone and Mineral Research.20 To lessen the risk of osteoporosis, the recommendations are as follows:
Limit exposure. Patients receiving glucocorticoids should be given the smallest dosage for the shortest duration possible.
Advise lifestyle changes. Patients should be counseled to limit their alcohol intake to no more than two drinks per day, to quit smoking, to engage in weight-bearing exercise, and to ingest enough calcium (1,200–1,500 mg/day, through diet and supplements) and vitamin D.
Monitor bone mineral density. Patients starting glucocorticoids at any dosage for an expected duration of at least 3 months should have their bone mineral density measured at baseline. The frequency of subsequent measurements should be based on the presence of other risk factors for fracture, results of previous bone density testing, glucocorticoid dosage, whether therapy for bone health has been initiated, and the rate of change in bone mineral density. If warranted and if the results would lead to a change in management, patients can undergo dual-energy x-ray absorptiometry more often than usual, ie, more often than every 2 years. Prevalent and incident fragility fractures, height measurements, fall risk assessments, laboratory measurements of 25-hydroxyvitamin D, and consideration of vertebral fracture assessment or other imaging of the spine, as necessary, should be part of counseling and monitoring.
Osteoporosis treatment. For patients who will be taking glucocorticoids for at least 3 months, alendronate, risedronate, zoledronic acid, or teriparatide can be initiated to prevent or treat osteoporosis in the following groups:
- Postmenopausal women and men over age 50 if the daily glucocorticoid dosage is at least 7.5 mg/day or if the World Health Organization Fracture Risk Assessment Tool (FRAX) score is more than 10% (the threshold for medium fracture risk)
- Premenopausal women and men younger than 50 if they have a history of fragility fracture, the FRAX score is more than 20% (the threshold for high fracture risk), or the T score is less than –2.5.
Certain clinical factors can also put a patient into a higher-risk category. These include current tobacco use, low body mass index, parental history of hip fracture, consuming more than three alcoholic drinks daily, higher daily or cumulative glucocorticoid dosage, intravenous pulse glucocorticoid usage, or a decline in central bone mineral density that exceeds the least significant change according to the scanner used.20
The FRAX tool accounts for bone density only at the femoral neck, and while useful, it cannot replace clinical judgment in stratifying risk. Moreover, it does not apply to premenopausal women or men under age 40.
The long-term risks of medications to treat glucocorticoid-induced osteoporosis are not well defined for premenopausal women (or their unborn children) or in men younger than 40, so treatment is recommended in those groups only for those with prevalent fragility fractures who are clearly at higher risk of additional fractures.20
PROTON PUMP INHIBITORS
Proton pump inhibitors are available by prescription and over the counter for gastric acid-related conditions. Concerns have been raised that these highly effective drugs are overused.21 Several of their adverse effects are self-limited and minor, but long-term use may entail serious risks, including propensity to bone fracture.22
Low acid leads to poor calcium absorption
Why fracture risk increases with proton pump inhibitors is controversial and may relate to their desired effect of suppressing gastric acid production: calcium salts, including carbonate and chloride, are poorly soluble and require an acidic environment to increase calcium ionization and thus absorption.23 For this reason, if patients taking a proton pump inhibitor take a calcium supplement, it should be calcium citrate, which unlike calcium carbonate does not require an acid environment for absorption.
Higher risk in older patients, with longer use, and with higher dosage
Since the first reports on proton pump inhibitors and fracture risk were published in 2006,24,25 a number of studies have reported this association, including several systematic reviews.
In 2011, the US Food and Drug Administration (FDA) updated a 2010 safety communication based on seven epidemiologic studies reporting an increased risk of fractures of the spine, hip, and wrist with proton pump inhibitors.24–31 Time of exposure to a proton pump inhibitor in these studies varied from 1 to 12 years. Fracture risk was higher in older patients,26 with higher doses,24,29 and with longer duration of drug use.24,27 On the other hand, one study that included only patients without other major fracture risk factors failed to find an association between the use of proton pump inhibitors and fractures.28
Is evidence sufficient for changing use?
The FDA report included a disclaimer that they had no access to study data or protocols and so could not verify the findings.26 Moreover, the studies used claims data from computerized databases to evaluate the risk of fractures in patients treated with proton pump inhibitors compared with those not using these drugs.24–31 Information was often incomplete regarding potentially important factors (eg, falls, family history of osteoporosis, calcium and vitamin D intake, smoking, alcohol use, reason for medication use), as well as the timing of drug use related to the onset or worsening of osteoporosis.26
Although 34 published studies evaluated the association of fracture risk and proton pump inhibitors, Leontiadis and Moayyedi32 argued that insufficient evidence exists to change our prescribing habits for these drugs based on fracture risk, as the studies varied considerably in their designs and results, a clear dose-response relationship is lacking, and the modest association is likely related to multiple confounders.
Bottom line: Use with caution
Although the increased fracture risk associated with proton pump inhibitors is likely multifactorial and is not fully delineated, it appears to be real. These drugs should be used only if there is a clear indication for them and if their benefits likely outweigh their risks. The lowest effective dose should be used, and the need for continuing use should be frequently reassessed.
SELECTIVE SEROTONIN REUPTAKE INHIBITORS
Depression affects 1 in 10 people in the United States, is especially common in the elderly, and leads to significant morbidity and reduced quality of life.33 Selective serotonin reuptake inhibitors (SSRIs) are often prescribed and are generally considered first-line agents for treating depression.
Complex bone effects
SSRIs antagonize the serotonin transporter, which normally assists serotonin uptake from the extracellular space. The serotonin transporter is found in all main types of bone cells, including osteoclasts, osteoblasts, and osteocytes.33 Serotonin is made by different genes in the brain than in the periphery, causing opposing effects on bone biology: when generated peripherally, it acts as a hormone to inhibit bone formation, while when generated in the brain, it acts as a neurotransmitter to create a major and positive effect on bone mass accrual by enhancing bone formation and limiting bone resorption.34,35
Potential confounders complicate the effect of SSRIs on bone health, as depression itself may be a risk factor for fracture. Patients with depression tend to have increased inflammation and cortisol, decreased gonadal steroids, more behavioral risk factors such as tobacco and increased alcohol use, and less physical activity, all of which can contribute to low bone density and risk of falls and fractures.33
Daily use of SSRIs increases fracture risk
A 2012 meta-analysis36 of 12 studies (seven case-control and five cohort), showed that SSRI users had a higher overall risk of fracture (adjusted odds ratio 1.69, 95% CI 1.51–1.90). By anatomic site, pooled odds ratios and 95% CIs were:
- Vertebral fractures 1.34 (1.13–1.59)
- Wrist or forearm fractures 1.51 (1.26–1.82)
- Hip or femur fractures 2.06 (1.84–2.30).
A 2013 meta-analysis37 of 34 studies with more than 1 million patients found that the random-effects pooled relative risk of all fracture types in users of antidepressants (including but not limited to SSRIs) was 1.39 (95% CI 1.32–1.47) compared with nonusers. Relative risks and 95% CIs in antidepressant users were:
- Vertebral fractures 1.38 (1.19–1.61)
- Nonvertebral fractures 1.42 (1.34–1.51)
- Hip fractures 1.47 (1.36–1.58).
A population-based, prospective cohort study38 of 5,008 community-dwelling adults age 50 and older, followed for 5 years, found that the daily use of SSRIs was associated with a twofold increased risk of clinical fragility fractures (defined as minimal trauma fractures that were clinically reported and radiographically confirmed) after adjusting for potential covariates. Daily SSRI use was also associated with an increased risk of falling (odds ratio 2.2, 95% CI 1.4–3.5), lower bone mineral density at the hip, and a trend toward lower bone mineral density at the spine. These effects were dose-dependent and were similar for those who reported taking SSRIs at baseline and at 5 years.
Bottom line: Counsel bone health
Although no guidelines exist for preventing or treating SSRI-induced bone loss, providers should discuss with patients the potential effect of these medications on bone health, taking into account patient age, severity of depression, sex, duration of use, length of SSRI treatment, and other clinical risk factors for osteoporosis.34 Given the widespread use of these medications for treating depression, more study into this association is needed to further guide providers.
ANTIEPILEPTIC DRUGS
Antiepileptic drugs are used to treat not only seizure disorders but also migraine headaches, neuropathy, and psychiatric and pain disorders. Many studies have linked their use to an increased risk of fractures.
The mechanism of this effect remains controversial. Early studies reported that inducers of cytochrome P450 enzymes (eg, phenobarbital, phenytoin) lead to increased vitamin D degradation, causing osteomalacia.39 Another study suggested that changes in calcium metabolism and reduced bone mineral density occur without vitamin D deficiency and that drugs such as valproate that do not induce cytochrome P450 enzymes may also affect bone health.40 Other bone effects may include direct inhibition of intestinal calcium absorption (seen in animal studies) and the induction of a high remodeling state leading to osteomalacia.41,42
Epilepsy itself increases risk of fractures
Patients with seizure disorders may also have an increased risk of fractures because of falls, trauma, impaired balance, use of glucocorticoids and benzodiazepines, and comorbid conditions (eg, mental retardation, cerebral palsy, and brain neoplasm).43
A 2005 meta-analysis43 of 11 studies of epilepsy and fracture risk and 12 studies of epilepsy and bone mineral density found that the risks of fractures were increased. The following relative risks and 95% CIs were noted:
- Any fracture 2.2 (1.9–2.5), in five studies
- Forearm 1.7 (1.2–2.3), in six studies
- Hip 5.3 (3.2–8.8), six studies
- Spine 6.2 (2.5–15.5), in three studies.
A large proportion of fractures (35%) seemed related to seizures.
Certain drugs increase risk
A large 2004 population-based, case-control, study44 (124,655 fracture cases and 373,962 controls) found an association between the use of antiepileptic drugs and increased fracture risk. After adjusting for current or prior use of glucocorticoids, prior fracture, social variables, comorbid conditions, and epilepsy diagnosis, excess fracture risk was found to be associated with the following drugs (odds ratios and 95% CIs):
- Oxcarbazepine 1.14 (1.03–1.26)
- Valproate 1.15 (1.05–1.26)
- Carbamazepine 1.18 (1.10-1.26)
- Phenobarbital 1.79 (1.64–1.95).
The risk was higher with higher doses. Fracture risk was higher with cytochrome P450 enzyme-inducing drugs (carbamazepine, oxcarbazepine, phenobarbital, phenytoin, and primidone; odds ratio 1.38, 95% CI 1.31–1.45) than for noninducing drugs (clonazepam, ethosuximide, lamotrigine, tiagabine, topiramate, valproate, and vigabatrin; odds ratio 1.19, 95% CI 1.11–1.27). No significant increased risk of fracture was found with use of phenytoin, tiagabine, topiramate, ethosuximide, lamotrigine, vigabatrin, or primidone after adjusting for confounders.
Bottom line: Monitor bone health
With antiepileptic drugs, the benefit of preventing seizures outweighs the risks of fractures. Patients on long-term antiepileptic drug therapy should be monitored for bone mineral density and vitamin D levels and receive counseling on lifestyle measures including tobacco cessation, alcohol moderation, and fall prevention.45 As there are no evidence-based guidelines for bone health in patients on antiepileptic drugs, management should be based on current guidelines for treating osteoporosis.
AROMATASE INHIBITORS
Breast cancer is the most common cancer in women and is the second-leading cause of cancer-associated deaths in women after lung cancer. Aromatase inhibitors, such as anastrozole, letrozole, and exemestane, are the standard of care in adjuvant treatment for hormone-receptor-positive breast cancer, leading to longer disease-free survival.
However, aromatase inhibitors increase bone loss and fracture risk, and only partial recovery of bone mineral density occurs after treatment is stopped. The drugs deter the aromatization of androgens and their conversion to estrogens in peripheral tissue, leading to reduced estrogen levels and resulting bone loss.46 Anastrozole and letrozole have been found to reduce bone mineral density, increase bone turnover, and increase the relative risk for nonvertebral and vertebral fractures in postmenopausal women by 40% compared with tamoxifen.47,48
Base osteoporosis treatment on risk
Several groups have issued guidelines for preventing and treating bone loss in postmenopausal women being treated with an aromatase inhibitor. When initiating treatment, women should be counseled about modifiable risk factors, exercise, and calcium and vitamin D supplementation.
Baseline bone mineral testing should also be obtained when starting treatment. Hadji et al,49 in a review article, recommend starting bone-directed therapy if the patient’s T score is less than –2.0 (using the lowest score from three sites) or if she has any of at least two of the following fracture risk factors:
- T score less than –1.5
- Age over 65
- Family history of hip fracture
- Personal history of fragility fracture after age 50
- Low body mass index (< 20 kg/m2)
- Current or prior history of tobacco use
- Oral glucocorticoid use for longer than 6 months.
Patients with a T score at or above –2.0 and no risk factors should have bone mineral density reassessed after 1 to 2 years. Antiresorptive therapy with intravenous zoledronic acid and evaluation for other secondary causes of bone loss should be initiated for either:
- An annual decrease of at least 10% or
- An annual decrease of at least 4% in patients with osteopenia at baseline.49
In 2003, the American Society of Clinical Oncology updated its recommendations on the role of bisphosphonates and bone health in women with breast cancer.50 They recommend the following:
- If the T score is –2.5 or less, prescribe a bisphosphonate (alendronate, risedronate, or zoledronic acid)
- If the T score is –1.0 to –2.5, tailor treatment individually and monitor bone mineral density annually
- If the T score is greater than –1.0, monitor bone mineral density annually.
All patients should receive lifestyle counseling, calcium and vitamin D supplementation, and monitoring of additional risk factors for osteoporosis as appropriate.50
- Canalis E, Delany AM. Mechanisms of glucocorticoid action in bone. Ann N Y Acad Sci 2002; 966:73–81.
- Manolagas SC. Corticosteroids and fractures: a close encounter of the third cell kind. J Bone Miner Res 2000; 15:1001–1005.
- Papaioannou A, Ferko NC, Adachi JD. Corticosteroids and the skeletal system. In: Lin AN, Paget SA, eds. Principles of corticosteroid therapy. New York, NY: Arnold Publishers; 2002:69–86.
- Van Staa TP. The pathogenesis, epidemiology, and management of glucocorticoid-induced osteoporosis. Calcif Tissue Int 2006; 79:129–137.
- Van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporosis Int 2002; 13:777–787.
- Clowes JA, Riggs BL, Khosla S. The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev 2005; 208:207–227.
- LoCascio V, Bonucci E, Imbimbo B, et al. Bone loss in response to long-term glucocorticoid therapy. Bone Miner 1990; 8:39–51.
- Lane NE, Lukert B. The science and therapy of glucocorticoid-induced bone loss. Endocrinol Metab Clin North Am 1998; 27:465–483.
- Kanis JA, Johansson H, Oden A, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res 2004; 19:893–899.
- Van Staa TP, Geusens P, Pols HA, de Laet C, Leufkens HG, Cooper C. A simple score for estimating the long-term risk of fracture in patients using oral glucocorticoids. QJM 2005; 98:191–198.
- Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology (Oxford) 2000; 39:1383–1389.
- Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of corticosteroids and risk of fractures. J Bone Miner Res 2000; 15:993–1000.
- Van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 2003; 48:3224–3229.
- Luengo M, Picado C, Del Rio L, Guanabens N, Montserrat JM, Setoain J. Vertebral fractures in steroid dependent asthma and involutional osteoporosis: a comparative study. Thorax 1991; 46:803–806.
- Selby PL, Halsey JP, Adams KRH, et al. Corticosteroids do not alter the threshold for vertebral fracture. J Bone Miner Res 2000; 15:952–956.
- Gonnelli S, Caffarelli C, Maggi S, et al. Effect of inhaled glucocorticoids and beta(2) agonists on vertebral fracture risk in COPD patients: the EOLO study. Calcif Tissue Int 2010; 87:137–143.
- Jones A, Fay JK, Burr M, Stone M, Hood K, Roberts G. Inhaled corticosteroid effects on bone metabolism in asthma and mild chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2002; 1:CD003537.
- Weatherall M, James K, Clay J, et al. Dose-response relationship for risk of non-vertebral fracture with inhaled corticosteroids. Clin Exp Allergy 2008; 38:1451–1458
- Buehring B, Viswanathan R, Binkley N, Busse W. Glucocorticoid-induced osteoporosis: an update on effects and management. J Allergy Clin Immunol 2013; 132:1019–1030.
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2010; 62:1515–1526.
- Naunton M, Peterson GM, Bleasel MD. Overuse of proton pump inhibitors. J Clin Pharm Ther 2000; 25:333–340.
- Wilhelm SM, Rjater RG, Kale-Pradhan PB. Perils and pitfalls of long-term effects of proton pump inhibitors. Expert Rev Clin Pharmacol 2013; 6:443–451.
- Sheikh MS, Santa Ana CA, Nicar MJ, Schiller LR, Fordtran JS. Gastrointestinal absorption of calcium from milk and calcium salts. N Engl J Med 1987; 317:532–536.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int 2006; 79:76–83.
- Food and Drug Administration (FDA). FDA drug safety communication: possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm213206.htm. Accessed March 7, 2016.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ 2008; 179:319–326.
- Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy 2008; 28:951–959.
- Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology 2010; 139:93–101.
- Gray SL, LaCroix AZ, Larson J, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med 2010; 170:765–771.
- Yu EW, Blackwell T, Ensrud KE, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int 2008; 83:251–259.
- Leontiadis GI, Moayyedi P. Proton pump inhibitors and risk of bone fractures. Curr Treat Options Gastroenterol 2014; 12:414–423.
- Chen F, Hahn TJ, Weintraub NT. Do SSRIs play a role in decreasing bone mineral density? J Am Med Dir Assoc 2012; 13:413–417.
- Bruyere O, Reginster JY. Osteoporosis in patients taking selective serotonin reuptake inhibitors: a focus on fracture outcome. Endocrine 2015; 48:65–68.
- Ducy P, Karsenty G. The two faces of serotonin in bone biology. J Cell Biol 2010; 191:7–13.
- Eom CS, Lee HK, Ye S, Park SM, Cho KH. Use of selective serotonin reuptake inhibitors and risk of fracture: a systematic review and meta-analysis. J Bone Miner Res 2012; 27:1186–1195.
- Rabenda V, Nicolet D, Beaudart C, Bruyere O, Reginster JY. Relationship between use of antidepressants and risk of fractures: a meta-analysis. Osteoporosis Int 2013; 24:121–137.
- Richards JB, Papaioannou A, Adachi JD, et al; Canadian Multicentre Osteoporosis Study Research Group. Effect of selective serotonin reuptake inhibitors on the risk of fracture. Arch Intern Med 2007; 22;167:188–194.
- Hahn TJ, Hendin BA, Scharp CR, Boisseau VC, Haddad JG Jr. Serum 25-hydroxycalciferol levels and bone mass in children on chronic anticonvulsant therapy. N Engl J Med 1975; 292:550–554.
- Weinstein RS, Bryce GF, Sappington LJ, King DW, Gallagher BB. Decreased serum ionized calcium and normal vitamin D metabolite levels with anticonvulsant drug treatment. J Clin Endocrinol Metab 1984; 58:1003–1009.
- Koch HU, Kraft D, von Herrath D, Schaefer K. Influence of diphenylhydantoin and phenobarbital on intestinal calcium transport in the rat. Epilepsia 1972; 13:829–834.
- Shane E. Osteoporosis associated with illness and medications. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. San Diego, CA: Academic Press; 1996.
- Vestergaard P. Epilepsy, osteoporosis and fracture risk—a meta-analysis. Acta Neurol Scand 2005; 112:277–286.
- Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with use of antiepileptic drugs. Epilepsia 2004; 45:1330–1337.
- Petty SJ, O’Brien TJ, Wark JD. Anti-epileptic medication and bone health. Osteoporos Int 2007; 18:129–142.
- Mazziotti G, Canalis E, Giustina A. Drug-induced osteoporosis: mechanisms and clinical implications. Am J Med 2010; 123:877–884.
- Rabaglio M, Sun Z, Price KN, et al; BIG 1-98 Collaborative and International Breast Cancer Study Groups. Bone fractures among postmenopausal patients with endocrine-responsive early breast cancer treated with 5 years of letrozole or tamoxifen in the BIG 1-98 trial. Ann Oncol 2009; 20:1489–1498.
- Khan MN, Khan AA. Cancer treatment-related bone loss: a review and synthesis of the literature. Curr Oncol 2008; 15:S30–S40.
- Hadji P, Aapro MS, Body JJ, et al. Management of aromatase inhibitor-associated bone loss in postmenopausal women with breast cancer: practical guidance for prevention and treatment. Ann Oncol 2011; 22:2546–2555.
- Hillner BE, Ingle JN, Chlebowski RT, et al; American Society of Clinical Oncology. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in breast cancer. J Clin Oncol 2003; 21:4042–4057.
Drug-induced osteoporosis is common, and the list of drugs that can harm bone continues to grow. As part of routine health maintenance, practitioners should recognize the drugs that increase bone loss and take measures to mitigate these effects to help avoid osteopenia and osteoporosis.
Osteoporosis, a silent systemic disease defined by low bone mineral density and changes in skeletal microstructure, leads to a higher risk of fragility fractures. Some of the risk factors are well described, but less well known is the role of pharmacologic therapy. The implicated drugs (Table 1) have important therapeutic roles, so the benefits of using them must be weighed against their risks, including their potential effects on bone.
This review focuses on a few drugs known to increase fracture risk, their mechanisms of bone loss, and management considerations (Table 2).
GLUCOCORTICOIDS
Glucocorticoids are used to treat many medical conditions, including allergic, rheumatic, and other inflammatory diseases, and as immunosuppressive therapy after solid organ and bone marrow transplant. They are the most common cause of drug-induced bone loss and related secondary osteoporosis.
Multiple effects on bone
Glucocorticoids both increase bone resorption and decrease bone formation by a variety of mechanisms.1 They reduce intestinal calcium absorption, increase urinary excretion of calcium, and enhance osteocyte apoptosis, leading to deterioration of the bone microarchitecture and bone mineral density.2 They also affect sex hormones, decreasing testosterone production in men and estrogen in women, leading to increased bone resorption, altered bone architecture, and poorer bone quality.3,4 The bone loss is greater in trabecular bone (eg, the femoral neck and vertebral bodies) than in cortical bone (eg, the forearm).5
Glucocorticoids have other systemic effects that increase fracture risk. For example, they cause muscle weakness and atrophy, increasing the risk of falls.4 Additionally, many of the inflammatory conditions for which they are prescribed (eg, rheumatoid arthritis) also increase the risk of osteoporosis by means of proinflammatory cytokine production, which may contribute to systemic and local effects on bone.4,6
Bone mineral density declines quickly
Bone mineral density declines within the first 3 months after starting oral glucocorticoids, with the rate of bone loss peaking at 6 months. Up to 12% of bone mass is lost in the first year. In subsequent years of continued use, bone loss progresses at a slower, steadier rate, averaging 2% to 3% annually.5,7,8
Oral therapy increases fracture risk
Kanis et al9 performed a meta-analysis of seven prospective cohort studies in 40,000 patients and found that the current or previous use of an oral glucocorticoid increased the risk of fragility fractures, and that men and women were affected about equally.9
Van Staa et al10,11 reported that daily doses of glucocorticoids equivalent to more than 2.5 mg of prednisone were associated with an increased risk of vertebral and hip fractures; fracture risk was related mainly to daily dosage rather than cumulative dose.
Van Staa et al,12 in a retrospective cohort study, compared nearly 250,000 adult users of oral glucocorticoids from general medical practice settings with the same number of controls matched for sex, age, and medical practice. The relative risks for fractures and 95% confidence intervals (CIs) during oral glucocorticoid treatment were as follows:
- Forearm fracture 1.09 (1.01–1.17)
- Nonvertebral fracture 1.33 (1.29–1.38)
- Hip fracture 1.61 (1.47–1.76)
- Vertebral fracture 2.60 (2.31–2.92).
The risk was dose-dependent. For a low daily dose (< 2.5 mg/day of prednisolone), the relative risks were:
- Hip fracture 0.99 (0.82–1.20)
- Vertebral fracture 1.55 (1.20–2.01) .
For a medium daily dose (2.5–7.5 mg/day), the relative risks were:
- Hip fracture 1.77 (1.55–2.02)
- Vertebral fracture 2.59 (2.16–3.10) .
For a high daily dose (> 7.5 mg/day), the relative risks were:
- Hip fracture 2.27 (1.94–2.66)
- Vertebral fracture 5.18 (4.25–6.31).
Fracture risk rapidly declined toward baseline after the patients stopped taking oral glucocorticoids but did not return to baseline levels. The lessening of excess fracture risk occurred mainly within the first year after stopping therapy.
Other studies5,9,13 have suggested that the increased fracture risk is mostly independent of bone mineral density, and that other mechanisms are at play. One study14 found that oral glucocorticoid users with a prevalent vertebral fracture actually had higher bone mineral density than patients with a fracture not taking glucocorticoids, although this finding was not confirmed in a subsequent study.15
Inhaled glucocorticoids have less effect on bone
Inhaled glucocorticoids are commonly used to treat chronic obstructive pulmonary disease and asthma. They do not have the same systemic bioavailability as oral glucocorticoids, so the risk of adverse effects is lower.
Data are inconsistent among several studies that evaluated the relationship between inhaled glucocorticoids, bone mineral density, osteoporosis, and fragility fracture. The inconsistencies may be due to heterogeneity of the study populations, self-reporting of fractures, and different methods of assessing chronic obstructive pulmonary disease severity.16
A Cochrane review17 in 2002 evaluated seven randomized controlled trials that compared the use of inhaled glucocorticoids vs placebo in nearly 2,000 patients with mild asthma or chronic obstructive pulmonary disease and found no evidence for decreased bone mineral density, increased bone turnover, or increased vertebral fracture incidence in the glucocorticoid users at 2 to 3 years of follow-up (odds ratio for fracture 1.87, 95% CI 0.5–7.0).
The Evaluation of Obstructive Lung Disease and Osteoporosis study,16 a multicenter Italian observational epidemiologic study, reported that patients taking the highest daily doses of inhaled glucocorticoids (> 1,500 μg of beclomethasone or its equivalent) had a significantly higher risk of vertebral fracture (odds ratio 1.4, 95% CI 1.04–1.89).16
A meta-analysis18 of five case-control studies (43,783 cases and 259,936 controls) identified a possible dose-dependent relationship, with a relative risk for nonvertebral fracture of 1.12 (95% CI 1.0–1.26) for each 1,000-μg increase in beclomethasone-equivalent inhaled glucocorticoid per day.
In summary, the effects of inhaled glucocorticoids in adults are uncertain, although trends toward increased fracture risk and decreased bone mineral density are evident with chronic therapy at moderate to high dosages. The risks and benefits of treatment should be carefully considered in patients with osteoporosis and baseline elevated fracture risk.19
Managing the risk of glucocorticoid-induced osteoporosis
In 2010, the American College of Rheumatology published recommendations for preventing and treating glucocorticoid-induced osteoporosis, which were endorsed by the American Society for Bone and Mineral Research.20 To lessen the risk of osteoporosis, the recommendations are as follows:
Limit exposure. Patients receiving glucocorticoids should be given the smallest dosage for the shortest duration possible.
Advise lifestyle changes. Patients should be counseled to limit their alcohol intake to no more than two drinks per day, to quit smoking, to engage in weight-bearing exercise, and to ingest enough calcium (1,200–1,500 mg/day, through diet and supplements) and vitamin D.
Monitor bone mineral density. Patients starting glucocorticoids at any dosage for an expected duration of at least 3 months should have their bone mineral density measured at baseline. The frequency of subsequent measurements should be based on the presence of other risk factors for fracture, results of previous bone density testing, glucocorticoid dosage, whether therapy for bone health has been initiated, and the rate of change in bone mineral density. If warranted and if the results would lead to a change in management, patients can undergo dual-energy x-ray absorptiometry more often than usual, ie, more often than every 2 years. Prevalent and incident fragility fractures, height measurements, fall risk assessments, laboratory measurements of 25-hydroxyvitamin D, and consideration of vertebral fracture assessment or other imaging of the spine, as necessary, should be part of counseling and monitoring.
Osteoporosis treatment. For patients who will be taking glucocorticoids for at least 3 months, alendronate, risedronate, zoledronic acid, or teriparatide can be initiated to prevent or treat osteoporosis in the following groups:
- Postmenopausal women and men over age 50 if the daily glucocorticoid dosage is at least 7.5 mg/day or if the World Health Organization Fracture Risk Assessment Tool (FRAX) score is more than 10% (the threshold for medium fracture risk)
- Premenopausal women and men younger than 50 if they have a history of fragility fracture, the FRAX score is more than 20% (the threshold for high fracture risk), or the T score is less than –2.5.
Certain clinical factors can also put a patient into a higher-risk category. These include current tobacco use, low body mass index, parental history of hip fracture, consuming more than three alcoholic drinks daily, higher daily or cumulative glucocorticoid dosage, intravenous pulse glucocorticoid usage, or a decline in central bone mineral density that exceeds the least significant change according to the scanner used.20
The FRAX tool accounts for bone density only at the femoral neck, and while useful, it cannot replace clinical judgment in stratifying risk. Moreover, it does not apply to premenopausal women or men under age 40.
The long-term risks of medications to treat glucocorticoid-induced osteoporosis are not well defined for premenopausal women (or their unborn children) or in men younger than 40, so treatment is recommended in those groups only for those with prevalent fragility fractures who are clearly at higher risk of additional fractures.20
PROTON PUMP INHIBITORS
Proton pump inhibitors are available by prescription and over the counter for gastric acid-related conditions. Concerns have been raised that these highly effective drugs are overused.21 Several of their adverse effects are self-limited and minor, but long-term use may entail serious risks, including propensity to bone fracture.22
Low acid leads to poor calcium absorption
Why fracture risk increases with proton pump inhibitors is controversial and may relate to their desired effect of suppressing gastric acid production: calcium salts, including carbonate and chloride, are poorly soluble and require an acidic environment to increase calcium ionization and thus absorption.23 For this reason, if patients taking a proton pump inhibitor take a calcium supplement, it should be calcium citrate, which unlike calcium carbonate does not require an acid environment for absorption.
Higher risk in older patients, with longer use, and with higher dosage
Since the first reports on proton pump inhibitors and fracture risk were published in 2006,24,25 a number of studies have reported this association, including several systematic reviews.
In 2011, the US Food and Drug Administration (FDA) updated a 2010 safety communication based on seven epidemiologic studies reporting an increased risk of fractures of the spine, hip, and wrist with proton pump inhibitors.24–31 Time of exposure to a proton pump inhibitor in these studies varied from 1 to 12 years. Fracture risk was higher in older patients,26 with higher doses,24,29 and with longer duration of drug use.24,27 On the other hand, one study that included only patients without other major fracture risk factors failed to find an association between the use of proton pump inhibitors and fractures.28
Is evidence sufficient for changing use?
The FDA report included a disclaimer that they had no access to study data or protocols and so could not verify the findings.26 Moreover, the studies used claims data from computerized databases to evaluate the risk of fractures in patients treated with proton pump inhibitors compared with those not using these drugs.24–31 Information was often incomplete regarding potentially important factors (eg, falls, family history of osteoporosis, calcium and vitamin D intake, smoking, alcohol use, reason for medication use), as well as the timing of drug use related to the onset or worsening of osteoporosis.26
Although 34 published studies evaluated the association of fracture risk and proton pump inhibitors, Leontiadis and Moayyedi32 argued that insufficient evidence exists to change our prescribing habits for these drugs based on fracture risk, as the studies varied considerably in their designs and results, a clear dose-response relationship is lacking, and the modest association is likely related to multiple confounders.
Bottom line: Use with caution
Although the increased fracture risk associated with proton pump inhibitors is likely multifactorial and is not fully delineated, it appears to be real. These drugs should be used only if there is a clear indication for them and if their benefits likely outweigh their risks. The lowest effective dose should be used, and the need for continuing use should be frequently reassessed.
SELECTIVE SEROTONIN REUPTAKE INHIBITORS
Depression affects 1 in 10 people in the United States, is especially common in the elderly, and leads to significant morbidity and reduced quality of life.33 Selective serotonin reuptake inhibitors (SSRIs) are often prescribed and are generally considered first-line agents for treating depression.
Complex bone effects
SSRIs antagonize the serotonin transporter, which normally assists serotonin uptake from the extracellular space. The serotonin transporter is found in all main types of bone cells, including osteoclasts, osteoblasts, and osteocytes.33 Serotonin is made by different genes in the brain than in the periphery, causing opposing effects on bone biology: when generated peripherally, it acts as a hormone to inhibit bone formation, while when generated in the brain, it acts as a neurotransmitter to create a major and positive effect on bone mass accrual by enhancing bone formation and limiting bone resorption.34,35
Potential confounders complicate the effect of SSRIs on bone health, as depression itself may be a risk factor for fracture. Patients with depression tend to have increased inflammation and cortisol, decreased gonadal steroids, more behavioral risk factors such as tobacco and increased alcohol use, and less physical activity, all of which can contribute to low bone density and risk of falls and fractures.33
Daily use of SSRIs increases fracture risk
A 2012 meta-analysis36 of 12 studies (seven case-control and five cohort), showed that SSRI users had a higher overall risk of fracture (adjusted odds ratio 1.69, 95% CI 1.51–1.90). By anatomic site, pooled odds ratios and 95% CIs were:
- Vertebral fractures 1.34 (1.13–1.59)
- Wrist or forearm fractures 1.51 (1.26–1.82)
- Hip or femur fractures 2.06 (1.84–2.30).
A 2013 meta-analysis37 of 34 studies with more than 1 million patients found that the random-effects pooled relative risk of all fracture types in users of antidepressants (including but not limited to SSRIs) was 1.39 (95% CI 1.32–1.47) compared with nonusers. Relative risks and 95% CIs in antidepressant users were:
- Vertebral fractures 1.38 (1.19–1.61)
- Nonvertebral fractures 1.42 (1.34–1.51)
- Hip fractures 1.47 (1.36–1.58).
A population-based, prospective cohort study38 of 5,008 community-dwelling adults age 50 and older, followed for 5 years, found that the daily use of SSRIs was associated with a twofold increased risk of clinical fragility fractures (defined as minimal trauma fractures that were clinically reported and radiographically confirmed) after adjusting for potential covariates. Daily SSRI use was also associated with an increased risk of falling (odds ratio 2.2, 95% CI 1.4–3.5), lower bone mineral density at the hip, and a trend toward lower bone mineral density at the spine. These effects were dose-dependent and were similar for those who reported taking SSRIs at baseline and at 5 years.
Bottom line: Counsel bone health
Although no guidelines exist for preventing or treating SSRI-induced bone loss, providers should discuss with patients the potential effect of these medications on bone health, taking into account patient age, severity of depression, sex, duration of use, length of SSRI treatment, and other clinical risk factors for osteoporosis.34 Given the widespread use of these medications for treating depression, more study into this association is needed to further guide providers.
ANTIEPILEPTIC DRUGS
Antiepileptic drugs are used to treat not only seizure disorders but also migraine headaches, neuropathy, and psychiatric and pain disorders. Many studies have linked their use to an increased risk of fractures.
The mechanism of this effect remains controversial. Early studies reported that inducers of cytochrome P450 enzymes (eg, phenobarbital, phenytoin) lead to increased vitamin D degradation, causing osteomalacia.39 Another study suggested that changes in calcium metabolism and reduced bone mineral density occur without vitamin D deficiency and that drugs such as valproate that do not induce cytochrome P450 enzymes may also affect bone health.40 Other bone effects may include direct inhibition of intestinal calcium absorption (seen in animal studies) and the induction of a high remodeling state leading to osteomalacia.41,42
Epilepsy itself increases risk of fractures
Patients with seizure disorders may also have an increased risk of fractures because of falls, trauma, impaired balance, use of glucocorticoids and benzodiazepines, and comorbid conditions (eg, mental retardation, cerebral palsy, and brain neoplasm).43
A 2005 meta-analysis43 of 11 studies of epilepsy and fracture risk and 12 studies of epilepsy and bone mineral density found that the risks of fractures were increased. The following relative risks and 95% CIs were noted:
- Any fracture 2.2 (1.9–2.5), in five studies
- Forearm 1.7 (1.2–2.3), in six studies
- Hip 5.3 (3.2–8.8), six studies
- Spine 6.2 (2.5–15.5), in three studies.
A large proportion of fractures (35%) seemed related to seizures.
Certain drugs increase risk
A large 2004 population-based, case-control, study44 (124,655 fracture cases and 373,962 controls) found an association between the use of antiepileptic drugs and increased fracture risk. After adjusting for current or prior use of glucocorticoids, prior fracture, social variables, comorbid conditions, and epilepsy diagnosis, excess fracture risk was found to be associated with the following drugs (odds ratios and 95% CIs):
- Oxcarbazepine 1.14 (1.03–1.26)
- Valproate 1.15 (1.05–1.26)
- Carbamazepine 1.18 (1.10-1.26)
- Phenobarbital 1.79 (1.64–1.95).
The risk was higher with higher doses. Fracture risk was higher with cytochrome P450 enzyme-inducing drugs (carbamazepine, oxcarbazepine, phenobarbital, phenytoin, and primidone; odds ratio 1.38, 95% CI 1.31–1.45) than for noninducing drugs (clonazepam, ethosuximide, lamotrigine, tiagabine, topiramate, valproate, and vigabatrin; odds ratio 1.19, 95% CI 1.11–1.27). No significant increased risk of fracture was found with use of phenytoin, tiagabine, topiramate, ethosuximide, lamotrigine, vigabatrin, or primidone after adjusting for confounders.
Bottom line: Monitor bone health
With antiepileptic drugs, the benefit of preventing seizures outweighs the risks of fractures. Patients on long-term antiepileptic drug therapy should be monitored for bone mineral density and vitamin D levels and receive counseling on lifestyle measures including tobacco cessation, alcohol moderation, and fall prevention.45 As there are no evidence-based guidelines for bone health in patients on antiepileptic drugs, management should be based on current guidelines for treating osteoporosis.
AROMATASE INHIBITORS
Breast cancer is the most common cancer in women and is the second-leading cause of cancer-associated deaths in women after lung cancer. Aromatase inhibitors, such as anastrozole, letrozole, and exemestane, are the standard of care in adjuvant treatment for hormone-receptor-positive breast cancer, leading to longer disease-free survival.
However, aromatase inhibitors increase bone loss and fracture risk, and only partial recovery of bone mineral density occurs after treatment is stopped. The drugs deter the aromatization of androgens and their conversion to estrogens in peripheral tissue, leading to reduced estrogen levels and resulting bone loss.46 Anastrozole and letrozole have been found to reduce bone mineral density, increase bone turnover, and increase the relative risk for nonvertebral and vertebral fractures in postmenopausal women by 40% compared with tamoxifen.47,48
Base osteoporosis treatment on risk
Several groups have issued guidelines for preventing and treating bone loss in postmenopausal women being treated with an aromatase inhibitor. When initiating treatment, women should be counseled about modifiable risk factors, exercise, and calcium and vitamin D supplementation.
Baseline bone mineral testing should also be obtained when starting treatment. Hadji et al,49 in a review article, recommend starting bone-directed therapy if the patient’s T score is less than –2.0 (using the lowest score from three sites) or if she has any of at least two of the following fracture risk factors:
- T score less than –1.5
- Age over 65
- Family history of hip fracture
- Personal history of fragility fracture after age 50
- Low body mass index (< 20 kg/m2)
- Current or prior history of tobacco use
- Oral glucocorticoid use for longer than 6 months.
Patients with a T score at or above –2.0 and no risk factors should have bone mineral density reassessed after 1 to 2 years. Antiresorptive therapy with intravenous zoledronic acid and evaluation for other secondary causes of bone loss should be initiated for either:
- An annual decrease of at least 10% or
- An annual decrease of at least 4% in patients with osteopenia at baseline.49
In 2003, the American Society of Clinical Oncology updated its recommendations on the role of bisphosphonates and bone health in women with breast cancer.50 They recommend the following:
- If the T score is –2.5 or less, prescribe a bisphosphonate (alendronate, risedronate, or zoledronic acid)
- If the T score is –1.0 to –2.5, tailor treatment individually and monitor bone mineral density annually
- If the T score is greater than –1.0, monitor bone mineral density annually.
All patients should receive lifestyle counseling, calcium and vitamin D supplementation, and monitoring of additional risk factors for osteoporosis as appropriate.50
Drug-induced osteoporosis is common, and the list of drugs that can harm bone continues to grow. As part of routine health maintenance, practitioners should recognize the drugs that increase bone loss and take measures to mitigate these effects to help avoid osteopenia and osteoporosis.
Osteoporosis, a silent systemic disease defined by low bone mineral density and changes in skeletal microstructure, leads to a higher risk of fragility fractures. Some of the risk factors are well described, but less well known is the role of pharmacologic therapy. The implicated drugs (Table 1) have important therapeutic roles, so the benefits of using them must be weighed against their risks, including their potential effects on bone.
This review focuses on a few drugs known to increase fracture risk, their mechanisms of bone loss, and management considerations (Table 2).
GLUCOCORTICOIDS
Glucocorticoids are used to treat many medical conditions, including allergic, rheumatic, and other inflammatory diseases, and as immunosuppressive therapy after solid organ and bone marrow transplant. They are the most common cause of drug-induced bone loss and related secondary osteoporosis.
Multiple effects on bone
Glucocorticoids both increase bone resorption and decrease bone formation by a variety of mechanisms.1 They reduce intestinal calcium absorption, increase urinary excretion of calcium, and enhance osteocyte apoptosis, leading to deterioration of the bone microarchitecture and bone mineral density.2 They also affect sex hormones, decreasing testosterone production in men and estrogen in women, leading to increased bone resorption, altered bone architecture, and poorer bone quality.3,4 The bone loss is greater in trabecular bone (eg, the femoral neck and vertebral bodies) than in cortical bone (eg, the forearm).5
Glucocorticoids have other systemic effects that increase fracture risk. For example, they cause muscle weakness and atrophy, increasing the risk of falls.4 Additionally, many of the inflammatory conditions for which they are prescribed (eg, rheumatoid arthritis) also increase the risk of osteoporosis by means of proinflammatory cytokine production, which may contribute to systemic and local effects on bone.4,6
Bone mineral density declines quickly
Bone mineral density declines within the first 3 months after starting oral glucocorticoids, with the rate of bone loss peaking at 6 months. Up to 12% of bone mass is lost in the first year. In subsequent years of continued use, bone loss progresses at a slower, steadier rate, averaging 2% to 3% annually.5,7,8
Oral therapy increases fracture risk
Kanis et al9 performed a meta-analysis of seven prospective cohort studies in 40,000 patients and found that the current or previous use of an oral glucocorticoid increased the risk of fragility fractures, and that men and women were affected about equally.9
Van Staa et al10,11 reported that daily doses of glucocorticoids equivalent to more than 2.5 mg of prednisone were associated with an increased risk of vertebral and hip fractures; fracture risk was related mainly to daily dosage rather than cumulative dose.
Van Staa et al,12 in a retrospective cohort study, compared nearly 250,000 adult users of oral glucocorticoids from general medical practice settings with the same number of controls matched for sex, age, and medical practice. The relative risks for fractures and 95% confidence intervals (CIs) during oral glucocorticoid treatment were as follows:
- Forearm fracture 1.09 (1.01–1.17)
- Nonvertebral fracture 1.33 (1.29–1.38)
- Hip fracture 1.61 (1.47–1.76)
- Vertebral fracture 2.60 (2.31–2.92).
The risk was dose-dependent. For a low daily dose (< 2.5 mg/day of prednisolone), the relative risks were:
- Hip fracture 0.99 (0.82–1.20)
- Vertebral fracture 1.55 (1.20–2.01) .
For a medium daily dose (2.5–7.5 mg/day), the relative risks were:
- Hip fracture 1.77 (1.55–2.02)
- Vertebral fracture 2.59 (2.16–3.10) .
For a high daily dose (> 7.5 mg/day), the relative risks were:
- Hip fracture 2.27 (1.94–2.66)
- Vertebral fracture 5.18 (4.25–6.31).
Fracture risk rapidly declined toward baseline after the patients stopped taking oral glucocorticoids but did not return to baseline levels. The lessening of excess fracture risk occurred mainly within the first year after stopping therapy.
Other studies5,9,13 have suggested that the increased fracture risk is mostly independent of bone mineral density, and that other mechanisms are at play. One study14 found that oral glucocorticoid users with a prevalent vertebral fracture actually had higher bone mineral density than patients with a fracture not taking glucocorticoids, although this finding was not confirmed in a subsequent study.15
Inhaled glucocorticoids have less effect on bone
Inhaled glucocorticoids are commonly used to treat chronic obstructive pulmonary disease and asthma. They do not have the same systemic bioavailability as oral glucocorticoids, so the risk of adverse effects is lower.
Data are inconsistent among several studies that evaluated the relationship between inhaled glucocorticoids, bone mineral density, osteoporosis, and fragility fracture. The inconsistencies may be due to heterogeneity of the study populations, self-reporting of fractures, and different methods of assessing chronic obstructive pulmonary disease severity.16
A Cochrane review17 in 2002 evaluated seven randomized controlled trials that compared the use of inhaled glucocorticoids vs placebo in nearly 2,000 patients with mild asthma or chronic obstructive pulmonary disease and found no evidence for decreased bone mineral density, increased bone turnover, or increased vertebral fracture incidence in the glucocorticoid users at 2 to 3 years of follow-up (odds ratio for fracture 1.87, 95% CI 0.5–7.0).
The Evaluation of Obstructive Lung Disease and Osteoporosis study,16 a multicenter Italian observational epidemiologic study, reported that patients taking the highest daily doses of inhaled glucocorticoids (> 1,500 μg of beclomethasone or its equivalent) had a significantly higher risk of vertebral fracture (odds ratio 1.4, 95% CI 1.04–1.89).16
A meta-analysis18 of five case-control studies (43,783 cases and 259,936 controls) identified a possible dose-dependent relationship, with a relative risk for nonvertebral fracture of 1.12 (95% CI 1.0–1.26) for each 1,000-μg increase in beclomethasone-equivalent inhaled glucocorticoid per day.
In summary, the effects of inhaled glucocorticoids in adults are uncertain, although trends toward increased fracture risk and decreased bone mineral density are evident with chronic therapy at moderate to high dosages. The risks and benefits of treatment should be carefully considered in patients with osteoporosis and baseline elevated fracture risk.19
Managing the risk of glucocorticoid-induced osteoporosis
In 2010, the American College of Rheumatology published recommendations for preventing and treating glucocorticoid-induced osteoporosis, which were endorsed by the American Society for Bone and Mineral Research.20 To lessen the risk of osteoporosis, the recommendations are as follows:
Limit exposure. Patients receiving glucocorticoids should be given the smallest dosage for the shortest duration possible.
Advise lifestyle changes. Patients should be counseled to limit their alcohol intake to no more than two drinks per day, to quit smoking, to engage in weight-bearing exercise, and to ingest enough calcium (1,200–1,500 mg/day, through diet and supplements) and vitamin D.
Monitor bone mineral density. Patients starting glucocorticoids at any dosage for an expected duration of at least 3 months should have their bone mineral density measured at baseline. The frequency of subsequent measurements should be based on the presence of other risk factors for fracture, results of previous bone density testing, glucocorticoid dosage, whether therapy for bone health has been initiated, and the rate of change in bone mineral density. If warranted and if the results would lead to a change in management, patients can undergo dual-energy x-ray absorptiometry more often than usual, ie, more often than every 2 years. Prevalent and incident fragility fractures, height measurements, fall risk assessments, laboratory measurements of 25-hydroxyvitamin D, and consideration of vertebral fracture assessment or other imaging of the spine, as necessary, should be part of counseling and monitoring.
Osteoporosis treatment. For patients who will be taking glucocorticoids for at least 3 months, alendronate, risedronate, zoledronic acid, or teriparatide can be initiated to prevent or treat osteoporosis in the following groups:
- Postmenopausal women and men over age 50 if the daily glucocorticoid dosage is at least 7.5 mg/day or if the World Health Organization Fracture Risk Assessment Tool (FRAX) score is more than 10% (the threshold for medium fracture risk)
- Premenopausal women and men younger than 50 if they have a history of fragility fracture, the FRAX score is more than 20% (the threshold for high fracture risk), or the T score is less than –2.5.
Certain clinical factors can also put a patient into a higher-risk category. These include current tobacco use, low body mass index, parental history of hip fracture, consuming more than three alcoholic drinks daily, higher daily or cumulative glucocorticoid dosage, intravenous pulse glucocorticoid usage, or a decline in central bone mineral density that exceeds the least significant change according to the scanner used.20
The FRAX tool accounts for bone density only at the femoral neck, and while useful, it cannot replace clinical judgment in stratifying risk. Moreover, it does not apply to premenopausal women or men under age 40.
The long-term risks of medications to treat glucocorticoid-induced osteoporosis are not well defined for premenopausal women (or their unborn children) or in men younger than 40, so treatment is recommended in those groups only for those with prevalent fragility fractures who are clearly at higher risk of additional fractures.20
PROTON PUMP INHIBITORS
Proton pump inhibitors are available by prescription and over the counter for gastric acid-related conditions. Concerns have been raised that these highly effective drugs are overused.21 Several of their adverse effects are self-limited and minor, but long-term use may entail serious risks, including propensity to bone fracture.22
Low acid leads to poor calcium absorption
Why fracture risk increases with proton pump inhibitors is controversial and may relate to their desired effect of suppressing gastric acid production: calcium salts, including carbonate and chloride, are poorly soluble and require an acidic environment to increase calcium ionization and thus absorption.23 For this reason, if patients taking a proton pump inhibitor take a calcium supplement, it should be calcium citrate, which unlike calcium carbonate does not require an acid environment for absorption.
Higher risk in older patients, with longer use, and with higher dosage
Since the first reports on proton pump inhibitors and fracture risk were published in 2006,24,25 a number of studies have reported this association, including several systematic reviews.
In 2011, the US Food and Drug Administration (FDA) updated a 2010 safety communication based on seven epidemiologic studies reporting an increased risk of fractures of the spine, hip, and wrist with proton pump inhibitors.24–31 Time of exposure to a proton pump inhibitor in these studies varied from 1 to 12 years. Fracture risk was higher in older patients,26 with higher doses,24,29 and with longer duration of drug use.24,27 On the other hand, one study that included only patients without other major fracture risk factors failed to find an association between the use of proton pump inhibitors and fractures.28
Is evidence sufficient for changing use?
The FDA report included a disclaimer that they had no access to study data or protocols and so could not verify the findings.26 Moreover, the studies used claims data from computerized databases to evaluate the risk of fractures in patients treated with proton pump inhibitors compared with those not using these drugs.24–31 Information was often incomplete regarding potentially important factors (eg, falls, family history of osteoporosis, calcium and vitamin D intake, smoking, alcohol use, reason for medication use), as well as the timing of drug use related to the onset or worsening of osteoporosis.26
Although 34 published studies evaluated the association of fracture risk and proton pump inhibitors, Leontiadis and Moayyedi32 argued that insufficient evidence exists to change our prescribing habits for these drugs based on fracture risk, as the studies varied considerably in their designs and results, a clear dose-response relationship is lacking, and the modest association is likely related to multiple confounders.
Bottom line: Use with caution
Although the increased fracture risk associated with proton pump inhibitors is likely multifactorial and is not fully delineated, it appears to be real. These drugs should be used only if there is a clear indication for them and if their benefits likely outweigh their risks. The lowest effective dose should be used, and the need for continuing use should be frequently reassessed.
SELECTIVE SEROTONIN REUPTAKE INHIBITORS
Depression affects 1 in 10 people in the United States, is especially common in the elderly, and leads to significant morbidity and reduced quality of life.33 Selective serotonin reuptake inhibitors (SSRIs) are often prescribed and are generally considered first-line agents for treating depression.
Complex bone effects
SSRIs antagonize the serotonin transporter, which normally assists serotonin uptake from the extracellular space. The serotonin transporter is found in all main types of bone cells, including osteoclasts, osteoblasts, and osteocytes.33 Serotonin is made by different genes in the brain than in the periphery, causing opposing effects on bone biology: when generated peripherally, it acts as a hormone to inhibit bone formation, while when generated in the brain, it acts as a neurotransmitter to create a major and positive effect on bone mass accrual by enhancing bone formation and limiting bone resorption.34,35
Potential confounders complicate the effect of SSRIs on bone health, as depression itself may be a risk factor for fracture. Patients with depression tend to have increased inflammation and cortisol, decreased gonadal steroids, more behavioral risk factors such as tobacco and increased alcohol use, and less physical activity, all of which can contribute to low bone density and risk of falls and fractures.33
Daily use of SSRIs increases fracture risk
A 2012 meta-analysis36 of 12 studies (seven case-control and five cohort), showed that SSRI users had a higher overall risk of fracture (adjusted odds ratio 1.69, 95% CI 1.51–1.90). By anatomic site, pooled odds ratios and 95% CIs were:
- Vertebral fractures 1.34 (1.13–1.59)
- Wrist or forearm fractures 1.51 (1.26–1.82)
- Hip or femur fractures 2.06 (1.84–2.30).
A 2013 meta-analysis37 of 34 studies with more than 1 million patients found that the random-effects pooled relative risk of all fracture types in users of antidepressants (including but not limited to SSRIs) was 1.39 (95% CI 1.32–1.47) compared with nonusers. Relative risks and 95% CIs in antidepressant users were:
- Vertebral fractures 1.38 (1.19–1.61)
- Nonvertebral fractures 1.42 (1.34–1.51)
- Hip fractures 1.47 (1.36–1.58).
A population-based, prospective cohort study38 of 5,008 community-dwelling adults age 50 and older, followed for 5 years, found that the daily use of SSRIs was associated with a twofold increased risk of clinical fragility fractures (defined as minimal trauma fractures that were clinically reported and radiographically confirmed) after adjusting for potential covariates. Daily SSRI use was also associated with an increased risk of falling (odds ratio 2.2, 95% CI 1.4–3.5), lower bone mineral density at the hip, and a trend toward lower bone mineral density at the spine. These effects were dose-dependent and were similar for those who reported taking SSRIs at baseline and at 5 years.
Bottom line: Counsel bone health
Although no guidelines exist for preventing or treating SSRI-induced bone loss, providers should discuss with patients the potential effect of these medications on bone health, taking into account patient age, severity of depression, sex, duration of use, length of SSRI treatment, and other clinical risk factors for osteoporosis.34 Given the widespread use of these medications for treating depression, more study into this association is needed to further guide providers.
ANTIEPILEPTIC DRUGS
Antiepileptic drugs are used to treat not only seizure disorders but also migraine headaches, neuropathy, and psychiatric and pain disorders. Many studies have linked their use to an increased risk of fractures.
The mechanism of this effect remains controversial. Early studies reported that inducers of cytochrome P450 enzymes (eg, phenobarbital, phenytoin) lead to increased vitamin D degradation, causing osteomalacia.39 Another study suggested that changes in calcium metabolism and reduced bone mineral density occur without vitamin D deficiency and that drugs such as valproate that do not induce cytochrome P450 enzymes may also affect bone health.40 Other bone effects may include direct inhibition of intestinal calcium absorption (seen in animal studies) and the induction of a high remodeling state leading to osteomalacia.41,42
Epilepsy itself increases risk of fractures
Patients with seizure disorders may also have an increased risk of fractures because of falls, trauma, impaired balance, use of glucocorticoids and benzodiazepines, and comorbid conditions (eg, mental retardation, cerebral palsy, and brain neoplasm).43
A 2005 meta-analysis43 of 11 studies of epilepsy and fracture risk and 12 studies of epilepsy and bone mineral density found that the risks of fractures were increased. The following relative risks and 95% CIs were noted:
- Any fracture 2.2 (1.9–2.5), in five studies
- Forearm 1.7 (1.2–2.3), in six studies
- Hip 5.3 (3.2–8.8), six studies
- Spine 6.2 (2.5–15.5), in three studies.
A large proportion of fractures (35%) seemed related to seizures.
Certain drugs increase risk
A large 2004 population-based, case-control, study44 (124,655 fracture cases and 373,962 controls) found an association between the use of antiepileptic drugs and increased fracture risk. After adjusting for current or prior use of glucocorticoids, prior fracture, social variables, comorbid conditions, and epilepsy diagnosis, excess fracture risk was found to be associated with the following drugs (odds ratios and 95% CIs):
- Oxcarbazepine 1.14 (1.03–1.26)
- Valproate 1.15 (1.05–1.26)
- Carbamazepine 1.18 (1.10-1.26)
- Phenobarbital 1.79 (1.64–1.95).
The risk was higher with higher doses. Fracture risk was higher with cytochrome P450 enzyme-inducing drugs (carbamazepine, oxcarbazepine, phenobarbital, phenytoin, and primidone; odds ratio 1.38, 95% CI 1.31–1.45) than for noninducing drugs (clonazepam, ethosuximide, lamotrigine, tiagabine, topiramate, valproate, and vigabatrin; odds ratio 1.19, 95% CI 1.11–1.27). No significant increased risk of fracture was found with use of phenytoin, tiagabine, topiramate, ethosuximide, lamotrigine, vigabatrin, or primidone after adjusting for confounders.
Bottom line: Monitor bone health
With antiepileptic drugs, the benefit of preventing seizures outweighs the risks of fractures. Patients on long-term antiepileptic drug therapy should be monitored for bone mineral density and vitamin D levels and receive counseling on lifestyle measures including tobacco cessation, alcohol moderation, and fall prevention.45 As there are no evidence-based guidelines for bone health in patients on antiepileptic drugs, management should be based on current guidelines for treating osteoporosis.
AROMATASE INHIBITORS
Breast cancer is the most common cancer in women and is the second-leading cause of cancer-associated deaths in women after lung cancer. Aromatase inhibitors, such as anastrozole, letrozole, and exemestane, are the standard of care in adjuvant treatment for hormone-receptor-positive breast cancer, leading to longer disease-free survival.
However, aromatase inhibitors increase bone loss and fracture risk, and only partial recovery of bone mineral density occurs after treatment is stopped. The drugs deter the aromatization of androgens and their conversion to estrogens in peripheral tissue, leading to reduced estrogen levels and resulting bone loss.46 Anastrozole and letrozole have been found to reduce bone mineral density, increase bone turnover, and increase the relative risk for nonvertebral and vertebral fractures in postmenopausal women by 40% compared with tamoxifen.47,48
Base osteoporosis treatment on risk
Several groups have issued guidelines for preventing and treating bone loss in postmenopausal women being treated with an aromatase inhibitor. When initiating treatment, women should be counseled about modifiable risk factors, exercise, and calcium and vitamin D supplementation.
Baseline bone mineral testing should also be obtained when starting treatment. Hadji et al,49 in a review article, recommend starting bone-directed therapy if the patient’s T score is less than –2.0 (using the lowest score from three sites) or if she has any of at least two of the following fracture risk factors:
- T score less than –1.5
- Age over 65
- Family history of hip fracture
- Personal history of fragility fracture after age 50
- Low body mass index (< 20 kg/m2)
- Current or prior history of tobacco use
- Oral glucocorticoid use for longer than 6 months.
Patients with a T score at or above –2.0 and no risk factors should have bone mineral density reassessed after 1 to 2 years. Antiresorptive therapy with intravenous zoledronic acid and evaluation for other secondary causes of bone loss should be initiated for either:
- An annual decrease of at least 10% or
- An annual decrease of at least 4% in patients with osteopenia at baseline.49
In 2003, the American Society of Clinical Oncology updated its recommendations on the role of bisphosphonates and bone health in women with breast cancer.50 They recommend the following:
- If the T score is –2.5 or less, prescribe a bisphosphonate (alendronate, risedronate, or zoledronic acid)
- If the T score is –1.0 to –2.5, tailor treatment individually and monitor bone mineral density annually
- If the T score is greater than –1.0, monitor bone mineral density annually.
All patients should receive lifestyle counseling, calcium and vitamin D supplementation, and monitoring of additional risk factors for osteoporosis as appropriate.50
- Canalis E, Delany AM. Mechanisms of glucocorticoid action in bone. Ann N Y Acad Sci 2002; 966:73–81.
- Manolagas SC. Corticosteroids and fractures: a close encounter of the third cell kind. J Bone Miner Res 2000; 15:1001–1005.
- Papaioannou A, Ferko NC, Adachi JD. Corticosteroids and the skeletal system. In: Lin AN, Paget SA, eds. Principles of corticosteroid therapy. New York, NY: Arnold Publishers; 2002:69–86.
- Van Staa TP. The pathogenesis, epidemiology, and management of glucocorticoid-induced osteoporosis. Calcif Tissue Int 2006; 79:129–137.
- Van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporosis Int 2002; 13:777–787.
- Clowes JA, Riggs BL, Khosla S. The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev 2005; 208:207–227.
- LoCascio V, Bonucci E, Imbimbo B, et al. Bone loss in response to long-term glucocorticoid therapy. Bone Miner 1990; 8:39–51.
- Lane NE, Lukert B. The science and therapy of glucocorticoid-induced bone loss. Endocrinol Metab Clin North Am 1998; 27:465–483.
- Kanis JA, Johansson H, Oden A, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res 2004; 19:893–899.
- Van Staa TP, Geusens P, Pols HA, de Laet C, Leufkens HG, Cooper C. A simple score for estimating the long-term risk of fracture in patients using oral glucocorticoids. QJM 2005; 98:191–198.
- Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology (Oxford) 2000; 39:1383–1389.
- Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of corticosteroids and risk of fractures. J Bone Miner Res 2000; 15:993–1000.
- Van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 2003; 48:3224–3229.
- Luengo M, Picado C, Del Rio L, Guanabens N, Montserrat JM, Setoain J. Vertebral fractures in steroid dependent asthma and involutional osteoporosis: a comparative study. Thorax 1991; 46:803–806.
- Selby PL, Halsey JP, Adams KRH, et al. Corticosteroids do not alter the threshold for vertebral fracture. J Bone Miner Res 2000; 15:952–956.
- Gonnelli S, Caffarelli C, Maggi S, et al. Effect of inhaled glucocorticoids and beta(2) agonists on vertebral fracture risk in COPD patients: the EOLO study. Calcif Tissue Int 2010; 87:137–143.
- Jones A, Fay JK, Burr M, Stone M, Hood K, Roberts G. Inhaled corticosteroid effects on bone metabolism in asthma and mild chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2002; 1:CD003537.
- Weatherall M, James K, Clay J, et al. Dose-response relationship for risk of non-vertebral fracture with inhaled corticosteroids. Clin Exp Allergy 2008; 38:1451–1458
- Buehring B, Viswanathan R, Binkley N, Busse W. Glucocorticoid-induced osteoporosis: an update on effects and management. J Allergy Clin Immunol 2013; 132:1019–1030.
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2010; 62:1515–1526.
- Naunton M, Peterson GM, Bleasel MD. Overuse of proton pump inhibitors. J Clin Pharm Ther 2000; 25:333–340.
- Wilhelm SM, Rjater RG, Kale-Pradhan PB. Perils and pitfalls of long-term effects of proton pump inhibitors. Expert Rev Clin Pharmacol 2013; 6:443–451.
- Sheikh MS, Santa Ana CA, Nicar MJ, Schiller LR, Fordtran JS. Gastrointestinal absorption of calcium from milk and calcium salts. N Engl J Med 1987; 317:532–536.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int 2006; 79:76–83.
- Food and Drug Administration (FDA). FDA drug safety communication: possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm213206.htm. Accessed March 7, 2016.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ 2008; 179:319–326.
- Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy 2008; 28:951–959.
- Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology 2010; 139:93–101.
- Gray SL, LaCroix AZ, Larson J, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med 2010; 170:765–771.
- Yu EW, Blackwell T, Ensrud KE, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int 2008; 83:251–259.
- Leontiadis GI, Moayyedi P. Proton pump inhibitors and risk of bone fractures. Curr Treat Options Gastroenterol 2014; 12:414–423.
- Chen F, Hahn TJ, Weintraub NT. Do SSRIs play a role in decreasing bone mineral density? J Am Med Dir Assoc 2012; 13:413–417.
- Bruyere O, Reginster JY. Osteoporosis in patients taking selective serotonin reuptake inhibitors: a focus on fracture outcome. Endocrine 2015; 48:65–68.
- Ducy P, Karsenty G. The two faces of serotonin in bone biology. J Cell Biol 2010; 191:7–13.
- Eom CS, Lee HK, Ye S, Park SM, Cho KH. Use of selective serotonin reuptake inhibitors and risk of fracture: a systematic review and meta-analysis. J Bone Miner Res 2012; 27:1186–1195.
- Rabenda V, Nicolet D, Beaudart C, Bruyere O, Reginster JY. Relationship between use of antidepressants and risk of fractures: a meta-analysis. Osteoporosis Int 2013; 24:121–137.
- Richards JB, Papaioannou A, Adachi JD, et al; Canadian Multicentre Osteoporosis Study Research Group. Effect of selective serotonin reuptake inhibitors on the risk of fracture. Arch Intern Med 2007; 22;167:188–194.
- Hahn TJ, Hendin BA, Scharp CR, Boisseau VC, Haddad JG Jr. Serum 25-hydroxycalciferol levels and bone mass in children on chronic anticonvulsant therapy. N Engl J Med 1975; 292:550–554.
- Weinstein RS, Bryce GF, Sappington LJ, King DW, Gallagher BB. Decreased serum ionized calcium and normal vitamin D metabolite levels with anticonvulsant drug treatment. J Clin Endocrinol Metab 1984; 58:1003–1009.
- Koch HU, Kraft D, von Herrath D, Schaefer K. Influence of diphenylhydantoin and phenobarbital on intestinal calcium transport in the rat. Epilepsia 1972; 13:829–834.
- Shane E. Osteoporosis associated with illness and medications. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. San Diego, CA: Academic Press; 1996.
- Vestergaard P. Epilepsy, osteoporosis and fracture risk—a meta-analysis. Acta Neurol Scand 2005; 112:277–286.
- Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with use of antiepileptic drugs. Epilepsia 2004; 45:1330–1337.
- Petty SJ, O’Brien TJ, Wark JD. Anti-epileptic medication and bone health. Osteoporos Int 2007; 18:129–142.
- Mazziotti G, Canalis E, Giustina A. Drug-induced osteoporosis: mechanisms and clinical implications. Am J Med 2010; 123:877–884.
- Rabaglio M, Sun Z, Price KN, et al; BIG 1-98 Collaborative and International Breast Cancer Study Groups. Bone fractures among postmenopausal patients with endocrine-responsive early breast cancer treated with 5 years of letrozole or tamoxifen in the BIG 1-98 trial. Ann Oncol 2009; 20:1489–1498.
- Khan MN, Khan AA. Cancer treatment-related bone loss: a review and synthesis of the literature. Curr Oncol 2008; 15:S30–S40.
- Hadji P, Aapro MS, Body JJ, et al. Management of aromatase inhibitor-associated bone loss in postmenopausal women with breast cancer: practical guidance for prevention and treatment. Ann Oncol 2011; 22:2546–2555.
- Hillner BE, Ingle JN, Chlebowski RT, et al; American Society of Clinical Oncology. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in breast cancer. J Clin Oncol 2003; 21:4042–4057.
- Canalis E, Delany AM. Mechanisms of glucocorticoid action in bone. Ann N Y Acad Sci 2002; 966:73–81.
- Manolagas SC. Corticosteroids and fractures: a close encounter of the third cell kind. J Bone Miner Res 2000; 15:1001–1005.
- Papaioannou A, Ferko NC, Adachi JD. Corticosteroids and the skeletal system. In: Lin AN, Paget SA, eds. Principles of corticosteroid therapy. New York, NY: Arnold Publishers; 2002:69–86.
- Van Staa TP. The pathogenesis, epidemiology, and management of glucocorticoid-induced osteoporosis. Calcif Tissue Int 2006; 79:129–137.
- Van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporosis Int 2002; 13:777–787.
- Clowes JA, Riggs BL, Khosla S. The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev 2005; 208:207–227.
- LoCascio V, Bonucci E, Imbimbo B, et al. Bone loss in response to long-term glucocorticoid therapy. Bone Miner 1990; 8:39–51.
- Lane NE, Lukert B. The science and therapy of glucocorticoid-induced bone loss. Endocrinol Metab Clin North Am 1998; 27:465–483.
- Kanis JA, Johansson H, Oden A, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res 2004; 19:893–899.
- Van Staa TP, Geusens P, Pols HA, de Laet C, Leufkens HG, Cooper C. A simple score for estimating the long-term risk of fracture in patients using oral glucocorticoids. QJM 2005; 98:191–198.
- Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology (Oxford) 2000; 39:1383–1389.
- Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of corticosteroids and risk of fractures. J Bone Miner Res 2000; 15:993–1000.
- Van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 2003; 48:3224–3229.
- Luengo M, Picado C, Del Rio L, Guanabens N, Montserrat JM, Setoain J. Vertebral fractures in steroid dependent asthma and involutional osteoporosis: a comparative study. Thorax 1991; 46:803–806.
- Selby PL, Halsey JP, Adams KRH, et al. Corticosteroids do not alter the threshold for vertebral fracture. J Bone Miner Res 2000; 15:952–956.
- Gonnelli S, Caffarelli C, Maggi S, et al. Effect of inhaled glucocorticoids and beta(2) agonists on vertebral fracture risk in COPD patients: the EOLO study. Calcif Tissue Int 2010; 87:137–143.
- Jones A, Fay JK, Burr M, Stone M, Hood K, Roberts G. Inhaled corticosteroid effects on bone metabolism in asthma and mild chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2002; 1:CD003537.
- Weatherall M, James K, Clay J, et al. Dose-response relationship for risk of non-vertebral fracture with inhaled corticosteroids. Clin Exp Allergy 2008; 38:1451–1458
- Buehring B, Viswanathan R, Binkley N, Busse W. Glucocorticoid-induced osteoporosis: an update on effects and management. J Allergy Clin Immunol 2013; 132:1019–1030.
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2010; 62:1515–1526.
- Naunton M, Peterson GM, Bleasel MD. Overuse of proton pump inhibitors. J Clin Pharm Ther 2000; 25:333–340.
- Wilhelm SM, Rjater RG, Kale-Pradhan PB. Perils and pitfalls of long-term effects of proton pump inhibitors. Expert Rev Clin Pharmacol 2013; 6:443–451.
- Sheikh MS, Santa Ana CA, Nicar MJ, Schiller LR, Fordtran JS. Gastrointestinal absorption of calcium from milk and calcium salts. N Engl J Med 1987; 317:532–536.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int 2006; 79:76–83.
- Food and Drug Administration (FDA). FDA drug safety communication: possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm213206.htm. Accessed March 7, 2016.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ 2008; 179:319–326.
- Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy 2008; 28:951–959.
- Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology 2010; 139:93–101.
- Gray SL, LaCroix AZ, Larson J, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med 2010; 170:765–771.
- Yu EW, Blackwell T, Ensrud KE, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int 2008; 83:251–259.
- Leontiadis GI, Moayyedi P. Proton pump inhibitors and risk of bone fractures. Curr Treat Options Gastroenterol 2014; 12:414–423.
- Chen F, Hahn TJ, Weintraub NT. Do SSRIs play a role in decreasing bone mineral density? J Am Med Dir Assoc 2012; 13:413–417.
- Bruyere O, Reginster JY. Osteoporosis in patients taking selective serotonin reuptake inhibitors: a focus on fracture outcome. Endocrine 2015; 48:65–68.
- Ducy P, Karsenty G. The two faces of serotonin in bone biology. J Cell Biol 2010; 191:7–13.
- Eom CS, Lee HK, Ye S, Park SM, Cho KH. Use of selective serotonin reuptake inhibitors and risk of fracture: a systematic review and meta-analysis. J Bone Miner Res 2012; 27:1186–1195.
- Rabenda V, Nicolet D, Beaudart C, Bruyere O, Reginster JY. Relationship between use of antidepressants and risk of fractures: a meta-analysis. Osteoporosis Int 2013; 24:121–137.
- Richards JB, Papaioannou A, Adachi JD, et al; Canadian Multicentre Osteoporosis Study Research Group. Effect of selective serotonin reuptake inhibitors on the risk of fracture. Arch Intern Med 2007; 22;167:188–194.
- Hahn TJ, Hendin BA, Scharp CR, Boisseau VC, Haddad JG Jr. Serum 25-hydroxycalciferol levels and bone mass in children on chronic anticonvulsant therapy. N Engl J Med 1975; 292:550–554.
- Weinstein RS, Bryce GF, Sappington LJ, King DW, Gallagher BB. Decreased serum ionized calcium and normal vitamin D metabolite levels with anticonvulsant drug treatment. J Clin Endocrinol Metab 1984; 58:1003–1009.
- Koch HU, Kraft D, von Herrath D, Schaefer K. Influence of diphenylhydantoin and phenobarbital on intestinal calcium transport in the rat. Epilepsia 1972; 13:829–834.
- Shane E. Osteoporosis associated with illness and medications. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. San Diego, CA: Academic Press; 1996.
- Vestergaard P. Epilepsy, osteoporosis and fracture risk—a meta-analysis. Acta Neurol Scand 2005; 112:277–286.
- Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with use of antiepileptic drugs. Epilepsia 2004; 45:1330–1337.
- Petty SJ, O’Brien TJ, Wark JD. Anti-epileptic medication and bone health. Osteoporos Int 2007; 18:129–142.
- Mazziotti G, Canalis E, Giustina A. Drug-induced osteoporosis: mechanisms and clinical implications. Am J Med 2010; 123:877–884.
- Rabaglio M, Sun Z, Price KN, et al; BIG 1-98 Collaborative and International Breast Cancer Study Groups. Bone fractures among postmenopausal patients with endocrine-responsive early breast cancer treated with 5 years of letrozole or tamoxifen in the BIG 1-98 trial. Ann Oncol 2009; 20:1489–1498.
- Khan MN, Khan AA. Cancer treatment-related bone loss: a review and synthesis of the literature. Curr Oncol 2008; 15:S30–S40.
- Hadji P, Aapro MS, Body JJ, et al. Management of aromatase inhibitor-associated bone loss in postmenopausal women with breast cancer: practical guidance for prevention and treatment. Ann Oncol 2011; 22:2546–2555.
- Hillner BE, Ingle JN, Chlebowski RT, et al; American Society of Clinical Oncology. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in breast cancer. J Clin Oncol 2003; 21:4042–4057.
KEY POINTS
- Professional society guidelines advise initiating treatment for bone loss in patients starting glucocorticoid therapy expected to last at least 3 months and for women taking an aromatase inhibitor.
- If patients taking a proton pump inhibitor take a calcium supplement, they should take calcium citrate.
- Daily SSRI use nearly doubles the risk of hip fracture in people over age 50.
- Many drugs for epilepsy are associated with increased fracture risk, but so are seizures (which may confound the issue).
Cocaine-induced ecchymotic rash
A 50-year-old man presented with a painful rash over his extremities for the past 2 days (Figure 1). He said he had been in his usual state of health until the day he woke up with the rash. The rash was initially limited to his upper and lower extremities, but the next day he noticed similar lesions over his cheek and hard palate. He was a smoker and was known to have hepatitis C virus infection. He denied recent trauma, fever, or chills. He said he had snorted cocaine about 24 hours before the rash first appeared.
On examination, his vital signs were normal. He had an extensive retiform rash involving the upper and lower extremities, earlobes, right cheek, and hard palate. Otherwise, the physical examination was normal.
Initial laboratory evaluation showed:
- Hemoglobin 11.5 g/dL (reference range 14.0–17.5)
- White blood cell count 2.1 × 109/L (4.5–11.0)
- Platelet count 168 × 109/L (150–350)
- Absolute neutrophil count 0.9 × 109/L (≥ 1.5)
- Urine toxicology screen positive for cocaine.
He was admitted to the hospital and was started on intravenous vancomycin and piperacillin-sulbactam for a presumed infectious cause of the rash.
One day later, testing for myeloperoxidase-specific antineutrophil cytoplasmic antibodies (p-ANCA) was strongly positive. Skin biopsy revealed leukocytoclastic vasculitis with small-vessel thrombosis. These findings, along with the timing of the appearance of the rash after his cocaine use, led to a diagnosis of levamisole-adulterated cocaine-induced vasculitis.
LEVAMISOLE AND VASCULITIS
Levamisole used to be used as an antihelminthic and as an adjuvant chemotherapeutic agent, but it is also added to cocaine to increase its euphoric and psychotropic effects.1 It has been withdrawn from the market for human use because of toxic side effects including agranulocytosis, vasculitis, and autoantibody positivity.
Levamisole-adulterated cocaine has been known to induce ANCA-associated vasculitis.2 Symptoms of levamisole-induced vasculitis usually start a few hours to a few days after the last dose of cocaine. Almost all patients with this condition present with a characteristic retiform purpuric rash, which has a predilection for the ears, nose, cheeks, and extremities. It also causes neutropenia and agranulocytosis (as well as autoantibodies, including antinuclear antibodies and antiphospholipid antibodies).
The characteristic lesions tend to be in a stellate pattern with erythematous borders. They often but not always have a central necrotic area. The location of the rash and the fact that it resolves after discontinuation of the offending agent help distinguish this condition from other types of vasculitis. Usually, antibodies against myeloperoxidase are present.3
Leukopenia does not typically occur in patients with primary vasculitis syndromes. The literature is mixed on the presence or absence of specific ANCAs.
Inflammatory and noninflammatory vasculopathic disorders that can present similarly include disseminated intravascular coagulation, antiphospholipid syndrome, warfarin-induced skin necrosis, paroxysmal nocturnal hemoglobinuria, cryoglobulinemia, vasculitis, and calciphylaxis.
Treatment is mainly supportive, but emollients and steroids4 have been reported to alleviate the symptoms. Surgical debridement is rarely needed unless skin necrosis is extensive.
Levamisole-induced vasculitis is a diagnosis of exclusion but should be strongly considered when the rash is associated with recent cocaine use, retiform purpura, or bullae with skin involvement, leukopenia, and positive ANCA in high titers.3 If cocaine use is discontinued, the syndrome is expected to resolve.
- Tervaert JW, Stegeman CA. A difficult diagnosis. Lancet 2004; 364:1313–1314.
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin Pharmacol Ther 2010; 88:408–411.
- Gross RL, Brucker J, Bahce-Altuntas A, et al. A novel cutaneous vasculitis syndrome induced by levamisole-contaminated cocaine. Clin Rheumatol 2011; 30:1385–1392.
- Carter MR, Amirhaeri S. p-ANCA-associated vasculitis caused by levamisole-adulterated cocaine: a case report. Case Rep Emerg Med 2013; 2013:878903.
A 50-year-old man presented with a painful rash over his extremities for the past 2 days (Figure 1). He said he had been in his usual state of health until the day he woke up with the rash. The rash was initially limited to his upper and lower extremities, but the next day he noticed similar lesions over his cheek and hard palate. He was a smoker and was known to have hepatitis C virus infection. He denied recent trauma, fever, or chills. He said he had snorted cocaine about 24 hours before the rash first appeared.
On examination, his vital signs were normal. He had an extensive retiform rash involving the upper and lower extremities, earlobes, right cheek, and hard palate. Otherwise, the physical examination was normal.
Initial laboratory evaluation showed:
- Hemoglobin 11.5 g/dL (reference range 14.0–17.5)
- White blood cell count 2.1 × 109/L (4.5–11.0)
- Platelet count 168 × 109/L (150–350)
- Absolute neutrophil count 0.9 × 109/L (≥ 1.5)
- Urine toxicology screen positive for cocaine.
He was admitted to the hospital and was started on intravenous vancomycin and piperacillin-sulbactam for a presumed infectious cause of the rash.
One day later, testing for myeloperoxidase-specific antineutrophil cytoplasmic antibodies (p-ANCA) was strongly positive. Skin biopsy revealed leukocytoclastic vasculitis with small-vessel thrombosis. These findings, along with the timing of the appearance of the rash after his cocaine use, led to a diagnosis of levamisole-adulterated cocaine-induced vasculitis.
LEVAMISOLE AND VASCULITIS
Levamisole used to be used as an antihelminthic and as an adjuvant chemotherapeutic agent, but it is also added to cocaine to increase its euphoric and psychotropic effects.1 It has been withdrawn from the market for human use because of toxic side effects including agranulocytosis, vasculitis, and autoantibody positivity.
Levamisole-adulterated cocaine has been known to induce ANCA-associated vasculitis.2 Symptoms of levamisole-induced vasculitis usually start a few hours to a few days after the last dose of cocaine. Almost all patients with this condition present with a characteristic retiform purpuric rash, which has a predilection for the ears, nose, cheeks, and extremities. It also causes neutropenia and agranulocytosis (as well as autoantibodies, including antinuclear antibodies and antiphospholipid antibodies).
The characteristic lesions tend to be in a stellate pattern with erythematous borders. They often but not always have a central necrotic area. The location of the rash and the fact that it resolves after discontinuation of the offending agent help distinguish this condition from other types of vasculitis. Usually, antibodies against myeloperoxidase are present.3
Leukopenia does not typically occur in patients with primary vasculitis syndromes. The literature is mixed on the presence or absence of specific ANCAs.
Inflammatory and noninflammatory vasculopathic disorders that can present similarly include disseminated intravascular coagulation, antiphospholipid syndrome, warfarin-induced skin necrosis, paroxysmal nocturnal hemoglobinuria, cryoglobulinemia, vasculitis, and calciphylaxis.
Treatment is mainly supportive, but emollients and steroids4 have been reported to alleviate the symptoms. Surgical debridement is rarely needed unless skin necrosis is extensive.
Levamisole-induced vasculitis is a diagnosis of exclusion but should be strongly considered when the rash is associated with recent cocaine use, retiform purpura, or bullae with skin involvement, leukopenia, and positive ANCA in high titers.3 If cocaine use is discontinued, the syndrome is expected to resolve.
A 50-year-old man presented with a painful rash over his extremities for the past 2 days (Figure 1). He said he had been in his usual state of health until the day he woke up with the rash. The rash was initially limited to his upper and lower extremities, but the next day he noticed similar lesions over his cheek and hard palate. He was a smoker and was known to have hepatitis C virus infection. He denied recent trauma, fever, or chills. He said he had snorted cocaine about 24 hours before the rash first appeared.
On examination, his vital signs were normal. He had an extensive retiform rash involving the upper and lower extremities, earlobes, right cheek, and hard palate. Otherwise, the physical examination was normal.
Initial laboratory evaluation showed:
- Hemoglobin 11.5 g/dL (reference range 14.0–17.5)
- White blood cell count 2.1 × 109/L (4.5–11.0)
- Platelet count 168 × 109/L (150–350)
- Absolute neutrophil count 0.9 × 109/L (≥ 1.5)
- Urine toxicology screen positive for cocaine.
He was admitted to the hospital and was started on intravenous vancomycin and piperacillin-sulbactam for a presumed infectious cause of the rash.
One day later, testing for myeloperoxidase-specific antineutrophil cytoplasmic antibodies (p-ANCA) was strongly positive. Skin biopsy revealed leukocytoclastic vasculitis with small-vessel thrombosis. These findings, along with the timing of the appearance of the rash after his cocaine use, led to a diagnosis of levamisole-adulterated cocaine-induced vasculitis.
LEVAMISOLE AND VASCULITIS
Levamisole used to be used as an antihelminthic and as an adjuvant chemotherapeutic agent, but it is also added to cocaine to increase its euphoric and psychotropic effects.1 It has been withdrawn from the market for human use because of toxic side effects including agranulocytosis, vasculitis, and autoantibody positivity.
Levamisole-adulterated cocaine has been known to induce ANCA-associated vasculitis.2 Symptoms of levamisole-induced vasculitis usually start a few hours to a few days after the last dose of cocaine. Almost all patients with this condition present with a characteristic retiform purpuric rash, which has a predilection for the ears, nose, cheeks, and extremities. It also causes neutropenia and agranulocytosis (as well as autoantibodies, including antinuclear antibodies and antiphospholipid antibodies).
The characteristic lesions tend to be in a stellate pattern with erythematous borders. They often but not always have a central necrotic area. The location of the rash and the fact that it resolves after discontinuation of the offending agent help distinguish this condition from other types of vasculitis. Usually, antibodies against myeloperoxidase are present.3
Leukopenia does not typically occur in patients with primary vasculitis syndromes. The literature is mixed on the presence or absence of specific ANCAs.
Inflammatory and noninflammatory vasculopathic disorders that can present similarly include disseminated intravascular coagulation, antiphospholipid syndrome, warfarin-induced skin necrosis, paroxysmal nocturnal hemoglobinuria, cryoglobulinemia, vasculitis, and calciphylaxis.
Treatment is mainly supportive, but emollients and steroids4 have been reported to alleviate the symptoms. Surgical debridement is rarely needed unless skin necrosis is extensive.
Levamisole-induced vasculitis is a diagnosis of exclusion but should be strongly considered when the rash is associated with recent cocaine use, retiform purpura, or bullae with skin involvement, leukopenia, and positive ANCA in high titers.3 If cocaine use is discontinued, the syndrome is expected to resolve.
- Tervaert JW, Stegeman CA. A difficult diagnosis. Lancet 2004; 364:1313–1314.
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin Pharmacol Ther 2010; 88:408–411.
- Gross RL, Brucker J, Bahce-Altuntas A, et al. A novel cutaneous vasculitis syndrome induced by levamisole-contaminated cocaine. Clin Rheumatol 2011; 30:1385–1392.
- Carter MR, Amirhaeri S. p-ANCA-associated vasculitis caused by levamisole-adulterated cocaine: a case report. Case Rep Emerg Med 2013; 2013:878903.
- Tervaert JW, Stegeman CA. A difficult diagnosis. Lancet 2004; 364:1313–1314.
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin Pharmacol Ther 2010; 88:408–411.
- Gross RL, Brucker J, Bahce-Altuntas A, et al. A novel cutaneous vasculitis syndrome induced by levamisole-contaminated cocaine. Clin Rheumatol 2011; 30:1385–1392.
- Carter MR, Amirhaeri S. p-ANCA-associated vasculitis caused by levamisole-adulterated cocaine: a case report. Case Rep Emerg Med 2013; 2013:878903.
When does asymptomatic aortic stenosis warrant surgery? Assessment techniques
Aortic stenosis is the most common valvular heart condition in the developed world, affecting 3% of people between ages 75 and 851 and 4% of people over age 85.2 Aortic valve replacement remains the only treatment proven to reduce the rates of mortality and morbidity in this condition.3 Under current guidelines,4,5 the onset of symptoms of exertional angina, syncope, or dyspnea in a patient who has severe aortic stenosis is a class I indication for surgery—ie, surgery should be performed.
However, high-gradient, severe aortic stenosis that is asymptomatic often poses a dilemma. The annual rate of sudden death in patients with this condition is estimated at 1% to 3%,6–9 but the surgical mortality rate in aortic valve replacement has been as high as 6% in Medicare patients (varying by center and comorbidities).10 Therefore, the traditional teaching was to not surgically replace the valve in asymptomatic patients, based on an adverse risk-benefit ratio. But with improvements in surgical techniques and prostheses, these rates have been reduced to 2.41% at high-volume centers11 (and to less than 1% at some hospitals),12 arguing in favor of earlier intervention.
Complicating the issue, transcatheter aortic valve replacement has become widely available, but further investigation into its use in this patient cohort is warranted.
Furthermore, many patients with severe but apparently asymptomatic aortic stenosis and normal left ventricular ejection fraction may actually have impaired exercise capacity, or they may have structural left ventricular changes such as severe hypertrophy or reduction in global strain, which may worsen the long-term survival rate.13,14
A prospective trial in patients with severe aortic stenosis found that mortality rates were significantly lower in those who underwent surgery early than in those who received conventional treatment, ie, watchful waiting (no specific medical treatment for aortic stenosis is available).15
Patients with asymptomatic severe aortic stenosis are a diverse group; some have a far worse prognosis than others, with or without surgery.
This paper reviews the guidelines for valve replacement in this patient group and the factors useful in establishing who should be considered for early intervention even if they have no classic symptoms (Figure 1).
SIGNS AND SYMPTOMS OF STENOSIS
Aortic stenosis is often first suspected when a patient presents with angina, dyspnea, and syncope, or when an ejection systolic murmur is heard incidentally on physical examination—typically a high-pitched, crescendo-decrescendo, midsystolic ejection murmur that is best heard at the right upper sternal border and that radiates to the carotid arteries.
Several physical findings may help in assessing the severity of aortic stenosis. In mild stenosis, the murmur peaks in early systole, but as the disease progresses the peak moves later into systole. The corollary of this phenomenon is a weak and delayed carotid upstroke known as “pulsus parvus et tardus.” This can be assessed by palpating the carotid artery while auscultating the heart.
The second heart sound becomes progressively softer as the stenosis advances until it is no longer audible. If a fourth heart sound is present, it may be due to concentric left ventricular hypertrophy with reduced left ventricular compliance, and a third heart sound indicates severe left ventricular dysfunction. Both of these findings suggest severe aortic stenosis.
ECHOCARDIOGRAPHIC MEASURES OF SEVERITY
Echocardiography is the best established and most important initial investigation in the assessment of a patient with suspected aortic stenosis. It usually provides accurate information on the severity and the mechanism of stenosis. The following findings indicate severe aortic stenosis:
- Mean pressure gradient > 40 mm Hg
- Peak aortic jet velocity > 4.0 m/s
- Aortic valve area < 1 cm2.
RECOMMENDATIONS FOR SURGERY BASED ON SEVERITY AND SYMPTOMS
The American College of Cardiology and American Heart Association (ACC/AHA)4 have issued the following recommendations for aortic valve replacement, based on the severity of stenosis and on whether the patient has symptoms (Figure 2):
Severe stenosis, with symptoms: class I recommendation (surgery should be done). Without surgery, these patients have a very poor prognosis, with an overall mortality rate of 75% at 3 years.3
Severe stenosis, no symptoms, in patients undergoing cardiac surgery for another indication (eg, coronary artery bypass grafting, ascending aortic surgery, or surgery on other valves): class I recommendation for concomitant aortic valve replacement.
Moderate stenosis, no symptoms, in patients undergoing cardiac surgery for another indication: class IIa recommendation (ie, aortic valve replacement “is reasonable”).
Very severe stenosis (aortic peak velocity > 5.0 m/s or mean pressure gradient ≥ 60 mm Hg), no symptoms, and low risk of death during surgery: class IIa recommendation.
Severe stenosis, no symptoms, and an increase in transaortic velocity of 0.3 m/s or more per year on serial testing or in patients considered to be at high risk for rapid disease progression, such as elderly patients with severe calcification: class IIb recommendation (surgery “can be considered”). The threshold to replace the valve is lower for patients who cannot make serial follow-up appointments because they live far away or lack transportation, or because they have problems with compliance.
Surgery for those with left ventricular dysfunction
Echocardiography also provides information on left ventricular function, and patients with left ventricular dysfunction have significantly worse outcomes. Studies have shown substantial differences in survival in patients who had an ejection fraction of less than 50% before valve replacement compared with those with a normal ejection fraction.3
Thus, the ACC/AHA guidelines recommend immediate referral for aortic valve replacement in asymptomatic patients whose left ventricular ejection fraction is less than 50% (class I recommendation, level of evidence B) in the hope of preventing irreversible ventricular dysfunction.4
TREADMILL EXERCISE TESTING UNMASKS SYMPTOMS
In the past, severe aortic stenosis was considered a contraindication to stress testing because of concerns of precipitating severe, life-threatening complications. However, studies over the past 10 years have shown that a supervised modified Bruce protocol is safe in patients with severe asymptomatic aortic stenosis.16,17
However, treadmill exercise testing clearly is absolutely contraindicated in patients with severe symptomatic aortic stenosis because of the risk of syncope or of precipitating a malignant arrhythmia. Nevertheless, it may play an essential role in the workup of a physically active patient with no symptoms.
Symptoms can develop insidiously in patients with chronic valve disease and may often go unrecognized by patients and their physicians. Many patients who state they have no symptoms may actually be subconsciously limiting their exercise to avoid symptoms.
Amato et al13 examined the exercise capacity of 66 patients reported to have severe asymptomatic aortic stenosis. Treadmill exercise testing was considered positive in this study if the patient developed symptoms or complex ventricular arrhythmias, had blood pressure that failed to rise by 20 mm Hg, or developed horizontal or down-sloping ST depression (≥ 1 mm in men, ≥ 2 mm in women). Twenty (30.3%) of the 66 patients developed symptoms during exercise testing, and they had a significantly worse prognosis: the 2-year event-free survival rate was only 19% in those with a positive test compared with 85% in those with a negative test.13 This study highlights the problem of patients subconsciously reducing their level of activity, thereby masking their true symptoms.
A meta-analysis by Rafique et al18 found that asymptomatic patients with abnormal results on exercise testing had a risk of cardiac events during follow-up that was eight times higher than normal, and a risk of sudden death 5.5 times higher.
With trials demonstrating that exercise testing is safe and prognostically useful in patients with aortic stenosis, the ACC/AHA guidelines emphasize its role, giving a class I recommendation for aortic valve replacement in patients who develop symptoms on exercise testing, and a class IIa recommendation in asymptomatic patients with decreased exercise tolerance or an exercise-related fall in blood pressure (Figure 2).4
STRESS ECHOCARDIOGRAPHY
Stress echocardiography has been used since the 1980s to assess the hemodynamic consequences of valvular heart disease, and many studies highlight its prognostic usefulness in patients with asymptomatic aortic stenosis.
In a 2005 study by Lancellotti et al,19 69 patients with severe asymptomatic aortic stenosis underwent a symptom-limited bicycle exercise stress test using quantitative Doppler echocardiography both at rest and at peak exercise, and a number of independent predictors of poor outcome (ie, symptoms, aortic valve replacement, death) were identified. These predictors included an abnormal test result, defined as any of the following: angina, dyspnea, ST-segment depression of 2 mm Hg or more, a fall or a small (< 20 mm Hg) rise in systolic blood pressure during the test, an aortic valve area of 0.75 cm2 or less, or a mean increase in valve gradient of 18 mm Hg or more.
Subsequently, a multicenter prospective trial assessed the value of exercise stress echocardiography in 186 patients with asymptomatic moderate or severe aortic stenosis.20 A mean increase in the aortic valve gradient of 20 mm Hg or more after exercise was associated with a rate of cardiovascular events (death, aortic valve replacement) 3.8 times higher, independent of other risk factors and whether moderate or severe stenosis was present (Table 1).20
Exercise-induced changes in systolic pulmonary artery pressure, which can be assessed using stress echocardiography, also have prognostic utility. Elevated systolic pulmonary artery pressure (> 50 mm Hg) seems to portend a poorer prognosis21,22 and a higher mortality rate after valve replacement,23 making it an independent predictor of hospital mortality and postoperative major adverse cardiovascular and cerebrovascular events (Table 1).
Exercise echocardiography also can be used to assess the patient’s contractile reserve. Left ventricular contractile reserve can be defined as an exercise-induced increase in left ventricular ejection fraction. In a study by Maréchaux et al24 in 50 patients with asymptomatic aortic stenosis and a normal resting left ventricular ejection fraction (> 50%), 40% of patients did not have left ventricular contractile reserve. In fact, their left ventricular ejection fraction decreased with exercise (from 64 ± 10% to 53 ± 12%). The subgroup of patients without contractile reserve developed symptoms more frequently during exercise and had lower event-free survival (Table 1).
Stress echocardiography has recently been introduced into the European Society of Cardiology guidelines, which give a class IIb indication for aortic valve replacement in asymptomatic patients who have severe aortic stenosis, a normal ejection fraction, and a greater than 20-mm Hg increase in mean gradient on exercise.5 But it has yet to be introduced into the ACC/AHA guidelines as a consideration for surgery.
LEFT VENTRICULAR FUNCTION: BEYOND EJECTION FRACTION
Left ventricular dysfunction is a bad sign for patients with aortic stenosis. Struggling to empty its contents through the narrowed aortic valve, the left ventricle is subjected to increased wall stress and eventually develops hypertrophy. The hypertrophied heart muscle requires more oxygen but receives less perfusion. Eventually, myocardial fibrosis develops, leading to systolic dysfunction and a reduction in the ejection fraction. As described above, patients with asymptomatic aortic stenosis and a left ventricular ejection fraction less than 50% have a poor prognosis,14 and therefore the ACC/AHA guidelines give this condition a class I recommendation for surgery.4
However, the ejection fraction has limitations as a marker of left ventricular function. It reflects changes in left ventricular cavity volume but not in the complex structure of the left ventricle. Several studies show that up to one-third of patients with severe aortic stenosis have considerable impairment of intrinsic myocardial systolic function despite a preserved ejection fraction.8,25,26
Thus, other variables such as left atrial size, left ventricular hypertrophy, myocardial deformation (assessed using strain imaging), and B-type natriuretic peptide (BNP) level may also be considered in assessing the effect of severe aortic stenosis on left ventricular function in the context of a normal ejection fraction (Table 2).
Left ventricular hypertrophy
The development of left ventricular hypertrophy is one of the earliest compensatory responses of the ventricle to the increase in afterload. This leads to impaired myocardial relaxation and reduced myocardial compliance, with resultant diastolic dysfunction with increased filling pressures.
Cioffi et al,27 in a study in 209 patients with severe but asymptomatic aortic stenosis, found that inappropriately high left ventricular mass (> 110% of that expected for body size, sex, and wall stress) portended a 4.5-times higher risk of death, independent of other risk factors.
Severe left ventricular hypertrophy may have a long-term effect on prognosis irrespective of valve replacement. An observational study14 of 3,049 patients who underwent aortic valve replacement for severe aortic stenosis showed that the 10-year survival rate was 45% in those whose left ventricular mass was greater than 185 g/m2, compared with 65% in patients whose left ventricular mass was less than 100 g/m2.
Thus, as surgical mortality and morbidity rates decrease, the impact of these structural changes in left ventricular wall thickness may affect the decision to intervene earlier in order to improve longer-term outcomes in select asymptomatic patients with high-risk features.
Left atrial size
Diastolic dysfunction is caused by increased afterload and results in elevated left ventricular end-diastolic pressure and elevated left atrial pressure. The left atrium responds by dilating, which increases the risk of atrial fibrillation.
Lancellotti et al8 investigated the negative prognostic implications of a large indexed left atrial area in asymptomatic patients with severe aortic stenosis. They found that patients with an indexed left atrial area greater than 12.2 cm2/m2 had a 77% 2-year probability of aortic valve replacement or death.
Beach et al28 examined cardiac remodeling after surgery and found that the left atrial diameter did not decrease after aortic valve replacement, even after left ventricular hypertrophy reversed. This observation has major prognostic implications. Patients with a severely enlarged left atrium (> 5.0 cm in diameter) had considerably lower survival rates than patients with a diameter less than 3.55 cm at 5 years (61% vs 85%) and at 10 years (28% vs 62%) after aortic valve replacement.
Therefore, left atrial size appears to have an important long-term impact on prognosis in patients with aortic stenosis even after aortic valve replacement and adds valuable information when assessing the effect of aortic stenosis on myocardial function.
B-type natriuretic peptide
Natriuretic peptides are cardiac hormones released in response to myocyte stretch. In aortic stenosis, increased afterload induces significant expression of BNP, N-terminal proBNP,29 and atrial natriuretic peptide,30 with numerous studies showing a good correlation between plasma natriuretic peptide levels and severity of aortic stenosis.31–34
Bergler-Klein et al33 showed that patients with asymptomatic aortic stenosis who developed symptoms during follow-up had higher levels of these biomarkers than patients who remained asymptomatic. Of note, patients with BNP levels lower than 130 pg/mL had significantly better symptom-free survival than those with higher levels, 66% vs 34% at 12 months.
However, these biomarkers are not specific to aortic stenosis and can be elevated in any condition that increases left ventricular stress. Nevertheless, they offer an easy and low-cost way to assess left ventricular function and may give an indication of the total burden of disease on the left ventricle.
Global left ventricular longitudinal strain
In view of the limitations of the left ventricular ejection fraction in identifying changes in the structure of the heart and in early detection of myocardial dysfunction, assessment of myocardial deformation using strain imaging is proving an attractive alternative.
Strain is the normalized, dimensionless measure of deformation of a solid object (such as a segment of myocardium) in response to an applied force or stress.35 A novel echocardiographic technique allows assessment of segmental myocardial deformation and thereby overcomes the limitation of tethering, which limits other echocardiographic techniques in the assessment of systolic function. Strain can be circumferential, longitudinal, or radial and is generally assessed using either tissue Doppler velocities or 2D echocardiographic speckle-tracking techniques. Longitudinal strain has proven to be a more sensitive method than left ventricular ejection fraction in detecting subclinical myocardial dysfunction and is a superior prognosticator in a variety of clinical conditions.36,37
Abnormal strain develops very early in the disease process and can even be seen in patients with mild aortic stenosis.
A study by Kearney et al38 in 146 patients with various degrees of aortic stenosis (26% mild, 21% moderate, and 53% severe) and preserved left ventricular ejection fraction demonstrated that global longitudinal strain worsened with increasing severity of aortic stenosis. Furthermore, global longitudinal strain was a strong independent predictor of all-cause mortality (hazard ratio 1.38, P < .001).
Similarly, in a study by Lancellotti et al8 in 163 patients with at least moderate to severe asymptomatic aortic stenosis, impaired longitudinal myocardial strain was an independent predictor of survival. Patients with longitudinal strain greater than 15.9% had significantly better outcomes than patients with strain of 15.9% or less (4-year survival 63% vs 22%, P < .001).
Hence, left ventricular global longitudinal strain offers an alternative—perhaps a superior alternative—to left ventricular ejection fraction in detecting and quantifying left ventricular dysfunction in asymptomatic aortic stenosis. It is an exciting new marker for the future in aortic stenosis, with a threshold of strain below 15.9% as a possible cutoff for those at higher risk of poorer outcomes.
WHERE ARE WE NOW? WHERE ARE WE GOING?
Aortic valve replacement in patients with severe but asymptomatic aortic stenosis remains a topic of debate, but support is growing for earlier intervention.
Now that concerns over the safety of exercise stress testing in patients with severe asymptomatic aortic stenosis have subsided following multiple studies,16,17 exercise testing should be performed in patients with asymptomatic severe aortic stenosis suspected of having reduced exercise capacity, with stress echocardiography providing added prognostic information through its assessment of exercise-induced changes in mean pressure gradient19 and systolic pulmonary artery pressure.21–23
Assessing left ventricular function provides important information about prognosis, with left ventricular ejection fraction, left ventricular diameter, left atrial size, BNP, and global longitudinal strain all helping identify asymptomatic patients at higher risk of death. Surgical intervention in asymptomatic patients with severe aortic stenosis may be considered when there is evidence of higher longer-term mortality risk based on reduced functional capacity, excess left ventricular hypertrophy, and abnormal left ventricular function as detected by ancillary methods such as global longitudinal strain and BNP elevation despite a normal left ventricular ejection fraction.
Figure 3 shows a possible algorithm to define which patients would benefit from earlier intervention. However, left ventricular hypertrophy, left atrial diameter, BNP, left ventricular longitudinal strain, and changes in systolic pulmonary artery pressure are not included in the current ACC/AHA guidelines for the management of asymptomatic patients with severe aortic stenosis. Further study is needed to determine whether earlier intervention in those with adverse risk profiles based on the newer evaluation techniques described above leads to better long-term outcomes.
Intervention should especially be considered in those in whom the measured surgical risk is low and in surgical centers at which the mortality rate is low.
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006; 368:1005–1011.
- Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997; 29:630–634.
- Schwarz F, Baumann P, Manthey J, et al. The effect of aortic valve replacement on survival. Circulation 1982; 66:1105–1110.
- Nishimura RA, Otto CM, Bonow RO, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:e57–e185.
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS); Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012; 33:2451–2496.
- Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000; 343:611–617.
- Rosenhek R, Zilberszac R, Schemper M, at al. Natural history of very severe aortic stenosis. Circulation 2010; 121:151–156.
- Lancellotti P, Donal E, Magne J, et al. Risk stratification in asymptomatic moderate to severe aortic stenosis: the importance of the valvular, arterial and ventricular interplay. Heart 2010; 96:1364–1371.
- Pai R, Kapoor N, Bansal RC, Varadarajan P. Natural malignant history of asymptomatic severe aortic stenosis: benefit of aortic valve replacement. Ann Thorac Surg 2006; 82:2116–2122.
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease); Society of Cardiovascular Anesthesiologists; Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006; 48:e1–e148.
- Patel HJ, Herbert MA, Drake DH, et al. Aortic valve replacement: using a statewide cardiac surgical database identifies a procedural volume hinge point. Ann Thorac Surg 2013; 96:1560–1566.
- Johnston DR, Roselli EE. Minimally invasive aortic valve surgery: Cleveland Clinic experience. Ann Cardiothorac Surg 2015;4:140–147.
- Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 2001; 86:381–386.
- Mihaljevic T, Nowicki ER, Rajeswaran J, et al. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg 2008; 135:1270–1279.
- Kang DH, Park SJ, Rim JH, et al. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation 2010; 121:1502–1509.
- Alborino D, Hoffmann JL, Fournet PC, Bloch A. Value of exercise testing to evaluate the indication for surgery in asymptomatic patients with valvular aortic stenosis. J Heart Valve Dis 2002; 11:204–209.
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005; 26:1309–1313.
- Rafique AM, Biner S, Ray I, Forrester JS, Tolstrup K, Siegel RJ. Meta-analysis of prognostic value of stress testing in patients with asymptomatic severe aortic stenosis. Am J Cardiol 2009; 104:972–977.
- Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation 2005; 112(suppl I):I377–I382.
- Marechaux S, Hachicha Z, Bellouin A, et al. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J 2010; 31:1390–1397.
- Cooper R, Ghali J, Simmons BE, Castaner A. Elevated pulmonary artery pressure. An independent predictor of mortality. Chest 1991; 99:112–120.
- McHenry MM, Rice J, Matlof HJ, Flamm MD Jr. Pulmonary hypertension and sudden death in aortic stenosis. Br Heart J 1979; 41:463–467.
- Copeland JG, Griepp RB, Stinson EB, Shumway NE. Long-term follow-up after isolated aortic valve replacement. J Thorac Cardiovasc Surg 1977; 74: 875–889.
- Maréchaux S, Ennezat PV, LeJemtel TH, et al. Left ventricular response to exercise in aortic stenosis: an exercise echocardiographic study. Echocardiography 2007; 24:955–959.
- Cramariuc D, Cioffi G, Rieck AE, et al. Low-flow aortic stenosis in asymptomatic patients: valvular arterial impedance and systolic function from the SEAS substudy. JACC Cardiovasc Imaging 2009; 2:390–399.
- Dumesnil JG, Shoucri RM, Laurenceau JL, Turcot J. A mathematical model of the dynamic geometry of the intact left ventricle and its application to clinical data. Circulation 1979; 59:1024–1034.
- Cioffi G, Faggiano P, Vizzardi E, et al. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart 2011; 97:301–307.
- Beach JM, Mihaljevic T, Rajeswaran J, et al. Ventricular hypertrophy and left atrial dilatation persist and are associated with reduced survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg 2014; 147:362–369.e8.
- Vanderheyden M, Goethals M, Verstreken S, et al. Wall stress modulates brain natriuretic peptide production in pressure overload cardiomyopathy. J Am Coll Cardiol 2004; 44:2349–2354.
- Ikeda T, Matsuda K, Itoh H, et al. Plasma levels of brain and atrial natriuretic peptides elevate in proportion to left ventricular end-systolic wall stress in patients with aortic stenosis. Am Heart J 1997; 133:307–314.
- Qi W, Mathisen P, Kjekshus J, et al. Natriuretic peptides in patients with aortic stenosis. Am Heart J 2001; 142:725–732.
- Weber M, Arnold R, Rau M, et al. Relation of N-terminal pro-B-type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol 2004; 94:740–745.
- Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109:2302–2308.
- Lim P, Monin JL, Monchi M, et al. Predictors of outcome in patients with severe aortic stenosis and normal left ventricular function: role of B-type natriuretic peptide. Eur Heart J 2004; 25:2048–2053.
- Holt B. Strain and strain rate echocardiography and coronary artery disease. Circ Cardiovasc Imaging 2011; 4:179–190.
- Ng AC, Delgado V, Bertini M, et al. Alterations in multidirectional myocardial functions in patients with aortic stenosis and preserved ejection fraction: a two-dimensional speckle tracking analysis. Eur Heart J 2011; 32:1542–1550.
- Ng AC, Delgado V, Bertini M, et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol 2009; 104:1398–1401
- Kearney LG, Lu K, Ord M, et al. Global longitudinal strain is a strong independent predictor of all-cause mortality in patients with aortic stenosis. Eur Heart J Cardiovasc Imag 2012; 13:827–833.
Aortic stenosis is the most common valvular heart condition in the developed world, affecting 3% of people between ages 75 and 851 and 4% of people over age 85.2 Aortic valve replacement remains the only treatment proven to reduce the rates of mortality and morbidity in this condition.3 Under current guidelines,4,5 the onset of symptoms of exertional angina, syncope, or dyspnea in a patient who has severe aortic stenosis is a class I indication for surgery—ie, surgery should be performed.
However, high-gradient, severe aortic stenosis that is asymptomatic often poses a dilemma. The annual rate of sudden death in patients with this condition is estimated at 1% to 3%,6–9 but the surgical mortality rate in aortic valve replacement has been as high as 6% in Medicare patients (varying by center and comorbidities).10 Therefore, the traditional teaching was to not surgically replace the valve in asymptomatic patients, based on an adverse risk-benefit ratio. But with improvements in surgical techniques and prostheses, these rates have been reduced to 2.41% at high-volume centers11 (and to less than 1% at some hospitals),12 arguing in favor of earlier intervention.
Complicating the issue, transcatheter aortic valve replacement has become widely available, but further investigation into its use in this patient cohort is warranted.
Furthermore, many patients with severe but apparently asymptomatic aortic stenosis and normal left ventricular ejection fraction may actually have impaired exercise capacity, or they may have structural left ventricular changes such as severe hypertrophy or reduction in global strain, which may worsen the long-term survival rate.13,14
A prospective trial in patients with severe aortic stenosis found that mortality rates were significantly lower in those who underwent surgery early than in those who received conventional treatment, ie, watchful waiting (no specific medical treatment for aortic stenosis is available).15
Patients with asymptomatic severe aortic stenosis are a diverse group; some have a far worse prognosis than others, with or without surgery.
This paper reviews the guidelines for valve replacement in this patient group and the factors useful in establishing who should be considered for early intervention even if they have no classic symptoms (Figure 1).
SIGNS AND SYMPTOMS OF STENOSIS
Aortic stenosis is often first suspected when a patient presents with angina, dyspnea, and syncope, or when an ejection systolic murmur is heard incidentally on physical examination—typically a high-pitched, crescendo-decrescendo, midsystolic ejection murmur that is best heard at the right upper sternal border and that radiates to the carotid arteries.
Several physical findings may help in assessing the severity of aortic stenosis. In mild stenosis, the murmur peaks in early systole, but as the disease progresses the peak moves later into systole. The corollary of this phenomenon is a weak and delayed carotid upstroke known as “pulsus parvus et tardus.” This can be assessed by palpating the carotid artery while auscultating the heart.
The second heart sound becomes progressively softer as the stenosis advances until it is no longer audible. If a fourth heart sound is present, it may be due to concentric left ventricular hypertrophy with reduced left ventricular compliance, and a third heart sound indicates severe left ventricular dysfunction. Both of these findings suggest severe aortic stenosis.
ECHOCARDIOGRAPHIC MEASURES OF SEVERITY
Echocardiography is the best established and most important initial investigation in the assessment of a patient with suspected aortic stenosis. It usually provides accurate information on the severity and the mechanism of stenosis. The following findings indicate severe aortic stenosis:
- Mean pressure gradient > 40 mm Hg
- Peak aortic jet velocity > 4.0 m/s
- Aortic valve area < 1 cm2.
RECOMMENDATIONS FOR SURGERY BASED ON SEVERITY AND SYMPTOMS
The American College of Cardiology and American Heart Association (ACC/AHA)4 have issued the following recommendations for aortic valve replacement, based on the severity of stenosis and on whether the patient has symptoms (Figure 2):
Severe stenosis, with symptoms: class I recommendation (surgery should be done). Without surgery, these patients have a very poor prognosis, with an overall mortality rate of 75% at 3 years.3
Severe stenosis, no symptoms, in patients undergoing cardiac surgery for another indication (eg, coronary artery bypass grafting, ascending aortic surgery, or surgery on other valves): class I recommendation for concomitant aortic valve replacement.
Moderate stenosis, no symptoms, in patients undergoing cardiac surgery for another indication: class IIa recommendation (ie, aortic valve replacement “is reasonable”).
Very severe stenosis (aortic peak velocity > 5.0 m/s or mean pressure gradient ≥ 60 mm Hg), no symptoms, and low risk of death during surgery: class IIa recommendation.
Severe stenosis, no symptoms, and an increase in transaortic velocity of 0.3 m/s or more per year on serial testing or in patients considered to be at high risk for rapid disease progression, such as elderly patients with severe calcification: class IIb recommendation (surgery “can be considered”). The threshold to replace the valve is lower for patients who cannot make serial follow-up appointments because they live far away or lack transportation, or because they have problems with compliance.
Surgery for those with left ventricular dysfunction
Echocardiography also provides information on left ventricular function, and patients with left ventricular dysfunction have significantly worse outcomes. Studies have shown substantial differences in survival in patients who had an ejection fraction of less than 50% before valve replacement compared with those with a normal ejection fraction.3
Thus, the ACC/AHA guidelines recommend immediate referral for aortic valve replacement in asymptomatic patients whose left ventricular ejection fraction is less than 50% (class I recommendation, level of evidence B) in the hope of preventing irreversible ventricular dysfunction.4
TREADMILL EXERCISE TESTING UNMASKS SYMPTOMS
In the past, severe aortic stenosis was considered a contraindication to stress testing because of concerns of precipitating severe, life-threatening complications. However, studies over the past 10 years have shown that a supervised modified Bruce protocol is safe in patients with severe asymptomatic aortic stenosis.16,17
However, treadmill exercise testing clearly is absolutely contraindicated in patients with severe symptomatic aortic stenosis because of the risk of syncope or of precipitating a malignant arrhythmia. Nevertheless, it may play an essential role in the workup of a physically active patient with no symptoms.
Symptoms can develop insidiously in patients with chronic valve disease and may often go unrecognized by patients and their physicians. Many patients who state they have no symptoms may actually be subconsciously limiting their exercise to avoid symptoms.
Amato et al13 examined the exercise capacity of 66 patients reported to have severe asymptomatic aortic stenosis. Treadmill exercise testing was considered positive in this study if the patient developed symptoms or complex ventricular arrhythmias, had blood pressure that failed to rise by 20 mm Hg, or developed horizontal or down-sloping ST depression (≥ 1 mm in men, ≥ 2 mm in women). Twenty (30.3%) of the 66 patients developed symptoms during exercise testing, and they had a significantly worse prognosis: the 2-year event-free survival rate was only 19% in those with a positive test compared with 85% in those with a negative test.13 This study highlights the problem of patients subconsciously reducing their level of activity, thereby masking their true symptoms.
A meta-analysis by Rafique et al18 found that asymptomatic patients with abnormal results on exercise testing had a risk of cardiac events during follow-up that was eight times higher than normal, and a risk of sudden death 5.5 times higher.
With trials demonstrating that exercise testing is safe and prognostically useful in patients with aortic stenosis, the ACC/AHA guidelines emphasize its role, giving a class I recommendation for aortic valve replacement in patients who develop symptoms on exercise testing, and a class IIa recommendation in asymptomatic patients with decreased exercise tolerance or an exercise-related fall in blood pressure (Figure 2).4
STRESS ECHOCARDIOGRAPHY
Stress echocardiography has been used since the 1980s to assess the hemodynamic consequences of valvular heart disease, and many studies highlight its prognostic usefulness in patients with asymptomatic aortic stenosis.
In a 2005 study by Lancellotti et al,19 69 patients with severe asymptomatic aortic stenosis underwent a symptom-limited bicycle exercise stress test using quantitative Doppler echocardiography both at rest and at peak exercise, and a number of independent predictors of poor outcome (ie, symptoms, aortic valve replacement, death) were identified. These predictors included an abnormal test result, defined as any of the following: angina, dyspnea, ST-segment depression of 2 mm Hg or more, a fall or a small (< 20 mm Hg) rise in systolic blood pressure during the test, an aortic valve area of 0.75 cm2 or less, or a mean increase in valve gradient of 18 mm Hg or more.
Subsequently, a multicenter prospective trial assessed the value of exercise stress echocardiography in 186 patients with asymptomatic moderate or severe aortic stenosis.20 A mean increase in the aortic valve gradient of 20 mm Hg or more after exercise was associated with a rate of cardiovascular events (death, aortic valve replacement) 3.8 times higher, independent of other risk factors and whether moderate or severe stenosis was present (Table 1).20
Exercise-induced changes in systolic pulmonary artery pressure, which can be assessed using stress echocardiography, also have prognostic utility. Elevated systolic pulmonary artery pressure (> 50 mm Hg) seems to portend a poorer prognosis21,22 and a higher mortality rate after valve replacement,23 making it an independent predictor of hospital mortality and postoperative major adverse cardiovascular and cerebrovascular events (Table 1).
Exercise echocardiography also can be used to assess the patient’s contractile reserve. Left ventricular contractile reserve can be defined as an exercise-induced increase in left ventricular ejection fraction. In a study by Maréchaux et al24 in 50 patients with asymptomatic aortic stenosis and a normal resting left ventricular ejection fraction (> 50%), 40% of patients did not have left ventricular contractile reserve. In fact, their left ventricular ejection fraction decreased with exercise (from 64 ± 10% to 53 ± 12%). The subgroup of patients without contractile reserve developed symptoms more frequently during exercise and had lower event-free survival (Table 1).
Stress echocardiography has recently been introduced into the European Society of Cardiology guidelines, which give a class IIb indication for aortic valve replacement in asymptomatic patients who have severe aortic stenosis, a normal ejection fraction, and a greater than 20-mm Hg increase in mean gradient on exercise.5 But it has yet to be introduced into the ACC/AHA guidelines as a consideration for surgery.
LEFT VENTRICULAR FUNCTION: BEYOND EJECTION FRACTION
Left ventricular dysfunction is a bad sign for patients with aortic stenosis. Struggling to empty its contents through the narrowed aortic valve, the left ventricle is subjected to increased wall stress and eventually develops hypertrophy. The hypertrophied heart muscle requires more oxygen but receives less perfusion. Eventually, myocardial fibrosis develops, leading to systolic dysfunction and a reduction in the ejection fraction. As described above, patients with asymptomatic aortic stenosis and a left ventricular ejection fraction less than 50% have a poor prognosis,14 and therefore the ACC/AHA guidelines give this condition a class I recommendation for surgery.4
However, the ejection fraction has limitations as a marker of left ventricular function. It reflects changes in left ventricular cavity volume but not in the complex structure of the left ventricle. Several studies show that up to one-third of patients with severe aortic stenosis have considerable impairment of intrinsic myocardial systolic function despite a preserved ejection fraction.8,25,26
Thus, other variables such as left atrial size, left ventricular hypertrophy, myocardial deformation (assessed using strain imaging), and B-type natriuretic peptide (BNP) level may also be considered in assessing the effect of severe aortic stenosis on left ventricular function in the context of a normal ejection fraction (Table 2).
Left ventricular hypertrophy
The development of left ventricular hypertrophy is one of the earliest compensatory responses of the ventricle to the increase in afterload. This leads to impaired myocardial relaxation and reduced myocardial compliance, with resultant diastolic dysfunction with increased filling pressures.
Cioffi et al,27 in a study in 209 patients with severe but asymptomatic aortic stenosis, found that inappropriately high left ventricular mass (> 110% of that expected for body size, sex, and wall stress) portended a 4.5-times higher risk of death, independent of other risk factors.
Severe left ventricular hypertrophy may have a long-term effect on prognosis irrespective of valve replacement. An observational study14 of 3,049 patients who underwent aortic valve replacement for severe aortic stenosis showed that the 10-year survival rate was 45% in those whose left ventricular mass was greater than 185 g/m2, compared with 65% in patients whose left ventricular mass was less than 100 g/m2.
Thus, as surgical mortality and morbidity rates decrease, the impact of these structural changes in left ventricular wall thickness may affect the decision to intervene earlier in order to improve longer-term outcomes in select asymptomatic patients with high-risk features.
Left atrial size
Diastolic dysfunction is caused by increased afterload and results in elevated left ventricular end-diastolic pressure and elevated left atrial pressure. The left atrium responds by dilating, which increases the risk of atrial fibrillation.
Lancellotti et al8 investigated the negative prognostic implications of a large indexed left atrial area in asymptomatic patients with severe aortic stenosis. They found that patients with an indexed left atrial area greater than 12.2 cm2/m2 had a 77% 2-year probability of aortic valve replacement or death.
Beach et al28 examined cardiac remodeling after surgery and found that the left atrial diameter did not decrease after aortic valve replacement, even after left ventricular hypertrophy reversed. This observation has major prognostic implications. Patients with a severely enlarged left atrium (> 5.0 cm in diameter) had considerably lower survival rates than patients with a diameter less than 3.55 cm at 5 years (61% vs 85%) and at 10 years (28% vs 62%) after aortic valve replacement.
Therefore, left atrial size appears to have an important long-term impact on prognosis in patients with aortic stenosis even after aortic valve replacement and adds valuable information when assessing the effect of aortic stenosis on myocardial function.
B-type natriuretic peptide
Natriuretic peptides are cardiac hormones released in response to myocyte stretch. In aortic stenosis, increased afterload induces significant expression of BNP, N-terminal proBNP,29 and atrial natriuretic peptide,30 with numerous studies showing a good correlation between plasma natriuretic peptide levels and severity of aortic stenosis.31–34
Bergler-Klein et al33 showed that patients with asymptomatic aortic stenosis who developed symptoms during follow-up had higher levels of these biomarkers than patients who remained asymptomatic. Of note, patients with BNP levels lower than 130 pg/mL had significantly better symptom-free survival than those with higher levels, 66% vs 34% at 12 months.
However, these biomarkers are not specific to aortic stenosis and can be elevated in any condition that increases left ventricular stress. Nevertheless, they offer an easy and low-cost way to assess left ventricular function and may give an indication of the total burden of disease on the left ventricle.
Global left ventricular longitudinal strain
In view of the limitations of the left ventricular ejection fraction in identifying changes in the structure of the heart and in early detection of myocardial dysfunction, assessment of myocardial deformation using strain imaging is proving an attractive alternative.
Strain is the normalized, dimensionless measure of deformation of a solid object (such as a segment of myocardium) in response to an applied force or stress.35 A novel echocardiographic technique allows assessment of segmental myocardial deformation and thereby overcomes the limitation of tethering, which limits other echocardiographic techniques in the assessment of systolic function. Strain can be circumferential, longitudinal, or radial and is generally assessed using either tissue Doppler velocities or 2D echocardiographic speckle-tracking techniques. Longitudinal strain has proven to be a more sensitive method than left ventricular ejection fraction in detecting subclinical myocardial dysfunction and is a superior prognosticator in a variety of clinical conditions.36,37
Abnormal strain develops very early in the disease process and can even be seen in patients with mild aortic stenosis.
A study by Kearney et al38 in 146 patients with various degrees of aortic stenosis (26% mild, 21% moderate, and 53% severe) and preserved left ventricular ejection fraction demonstrated that global longitudinal strain worsened with increasing severity of aortic stenosis. Furthermore, global longitudinal strain was a strong independent predictor of all-cause mortality (hazard ratio 1.38, P < .001).
Similarly, in a study by Lancellotti et al8 in 163 patients with at least moderate to severe asymptomatic aortic stenosis, impaired longitudinal myocardial strain was an independent predictor of survival. Patients with longitudinal strain greater than 15.9% had significantly better outcomes than patients with strain of 15.9% or less (4-year survival 63% vs 22%, P < .001).
Hence, left ventricular global longitudinal strain offers an alternative—perhaps a superior alternative—to left ventricular ejection fraction in detecting and quantifying left ventricular dysfunction in asymptomatic aortic stenosis. It is an exciting new marker for the future in aortic stenosis, with a threshold of strain below 15.9% as a possible cutoff for those at higher risk of poorer outcomes.
WHERE ARE WE NOW? WHERE ARE WE GOING?
Aortic valve replacement in patients with severe but asymptomatic aortic stenosis remains a topic of debate, but support is growing for earlier intervention.
Now that concerns over the safety of exercise stress testing in patients with severe asymptomatic aortic stenosis have subsided following multiple studies,16,17 exercise testing should be performed in patients with asymptomatic severe aortic stenosis suspected of having reduced exercise capacity, with stress echocardiography providing added prognostic information through its assessment of exercise-induced changes in mean pressure gradient19 and systolic pulmonary artery pressure.21–23
Assessing left ventricular function provides important information about prognosis, with left ventricular ejection fraction, left ventricular diameter, left atrial size, BNP, and global longitudinal strain all helping identify asymptomatic patients at higher risk of death. Surgical intervention in asymptomatic patients with severe aortic stenosis may be considered when there is evidence of higher longer-term mortality risk based on reduced functional capacity, excess left ventricular hypertrophy, and abnormal left ventricular function as detected by ancillary methods such as global longitudinal strain and BNP elevation despite a normal left ventricular ejection fraction.
Figure 3 shows a possible algorithm to define which patients would benefit from earlier intervention. However, left ventricular hypertrophy, left atrial diameter, BNP, left ventricular longitudinal strain, and changes in systolic pulmonary artery pressure are not included in the current ACC/AHA guidelines for the management of asymptomatic patients with severe aortic stenosis. Further study is needed to determine whether earlier intervention in those with adverse risk profiles based on the newer evaluation techniques described above leads to better long-term outcomes.
Intervention should especially be considered in those in whom the measured surgical risk is low and in surgical centers at which the mortality rate is low.
Aortic stenosis is the most common valvular heart condition in the developed world, affecting 3% of people between ages 75 and 851 and 4% of people over age 85.2 Aortic valve replacement remains the only treatment proven to reduce the rates of mortality and morbidity in this condition.3 Under current guidelines,4,5 the onset of symptoms of exertional angina, syncope, or dyspnea in a patient who has severe aortic stenosis is a class I indication for surgery—ie, surgery should be performed.
However, high-gradient, severe aortic stenosis that is asymptomatic often poses a dilemma. The annual rate of sudden death in patients with this condition is estimated at 1% to 3%,6–9 but the surgical mortality rate in aortic valve replacement has been as high as 6% in Medicare patients (varying by center and comorbidities).10 Therefore, the traditional teaching was to not surgically replace the valve in asymptomatic patients, based on an adverse risk-benefit ratio. But with improvements in surgical techniques and prostheses, these rates have been reduced to 2.41% at high-volume centers11 (and to less than 1% at some hospitals),12 arguing in favor of earlier intervention.
Complicating the issue, transcatheter aortic valve replacement has become widely available, but further investigation into its use in this patient cohort is warranted.
Furthermore, many patients with severe but apparently asymptomatic aortic stenosis and normal left ventricular ejection fraction may actually have impaired exercise capacity, or they may have structural left ventricular changes such as severe hypertrophy or reduction in global strain, which may worsen the long-term survival rate.13,14
A prospective trial in patients with severe aortic stenosis found that mortality rates were significantly lower in those who underwent surgery early than in those who received conventional treatment, ie, watchful waiting (no specific medical treatment for aortic stenosis is available).15
Patients with asymptomatic severe aortic stenosis are a diverse group; some have a far worse prognosis than others, with or without surgery.
This paper reviews the guidelines for valve replacement in this patient group and the factors useful in establishing who should be considered for early intervention even if they have no classic symptoms (Figure 1).
SIGNS AND SYMPTOMS OF STENOSIS
Aortic stenosis is often first suspected when a patient presents with angina, dyspnea, and syncope, or when an ejection systolic murmur is heard incidentally on physical examination—typically a high-pitched, crescendo-decrescendo, midsystolic ejection murmur that is best heard at the right upper sternal border and that radiates to the carotid arteries.
Several physical findings may help in assessing the severity of aortic stenosis. In mild stenosis, the murmur peaks in early systole, but as the disease progresses the peak moves later into systole. The corollary of this phenomenon is a weak and delayed carotid upstroke known as “pulsus parvus et tardus.” This can be assessed by palpating the carotid artery while auscultating the heart.
The second heart sound becomes progressively softer as the stenosis advances until it is no longer audible. If a fourth heart sound is present, it may be due to concentric left ventricular hypertrophy with reduced left ventricular compliance, and a third heart sound indicates severe left ventricular dysfunction. Both of these findings suggest severe aortic stenosis.
ECHOCARDIOGRAPHIC MEASURES OF SEVERITY
Echocardiography is the best established and most important initial investigation in the assessment of a patient with suspected aortic stenosis. It usually provides accurate information on the severity and the mechanism of stenosis. The following findings indicate severe aortic stenosis:
- Mean pressure gradient > 40 mm Hg
- Peak aortic jet velocity > 4.0 m/s
- Aortic valve area < 1 cm2.
RECOMMENDATIONS FOR SURGERY BASED ON SEVERITY AND SYMPTOMS
The American College of Cardiology and American Heart Association (ACC/AHA)4 have issued the following recommendations for aortic valve replacement, based on the severity of stenosis and on whether the patient has symptoms (Figure 2):
Severe stenosis, with symptoms: class I recommendation (surgery should be done). Without surgery, these patients have a very poor prognosis, with an overall mortality rate of 75% at 3 years.3
Severe stenosis, no symptoms, in patients undergoing cardiac surgery for another indication (eg, coronary artery bypass grafting, ascending aortic surgery, or surgery on other valves): class I recommendation for concomitant aortic valve replacement.
Moderate stenosis, no symptoms, in patients undergoing cardiac surgery for another indication: class IIa recommendation (ie, aortic valve replacement “is reasonable”).
Very severe stenosis (aortic peak velocity > 5.0 m/s or mean pressure gradient ≥ 60 mm Hg), no symptoms, and low risk of death during surgery: class IIa recommendation.
Severe stenosis, no symptoms, and an increase in transaortic velocity of 0.3 m/s or more per year on serial testing or in patients considered to be at high risk for rapid disease progression, such as elderly patients with severe calcification: class IIb recommendation (surgery “can be considered”). The threshold to replace the valve is lower for patients who cannot make serial follow-up appointments because they live far away or lack transportation, or because they have problems with compliance.
Surgery for those with left ventricular dysfunction
Echocardiography also provides information on left ventricular function, and patients with left ventricular dysfunction have significantly worse outcomes. Studies have shown substantial differences in survival in patients who had an ejection fraction of less than 50% before valve replacement compared with those with a normal ejection fraction.3
Thus, the ACC/AHA guidelines recommend immediate referral for aortic valve replacement in asymptomatic patients whose left ventricular ejection fraction is less than 50% (class I recommendation, level of evidence B) in the hope of preventing irreversible ventricular dysfunction.4
TREADMILL EXERCISE TESTING UNMASKS SYMPTOMS
In the past, severe aortic stenosis was considered a contraindication to stress testing because of concerns of precipitating severe, life-threatening complications. However, studies over the past 10 years have shown that a supervised modified Bruce protocol is safe in patients with severe asymptomatic aortic stenosis.16,17
However, treadmill exercise testing clearly is absolutely contraindicated in patients with severe symptomatic aortic stenosis because of the risk of syncope or of precipitating a malignant arrhythmia. Nevertheless, it may play an essential role in the workup of a physically active patient with no symptoms.
Symptoms can develop insidiously in patients with chronic valve disease and may often go unrecognized by patients and their physicians. Many patients who state they have no symptoms may actually be subconsciously limiting their exercise to avoid symptoms.
Amato et al13 examined the exercise capacity of 66 patients reported to have severe asymptomatic aortic stenosis. Treadmill exercise testing was considered positive in this study if the patient developed symptoms or complex ventricular arrhythmias, had blood pressure that failed to rise by 20 mm Hg, or developed horizontal or down-sloping ST depression (≥ 1 mm in men, ≥ 2 mm in women). Twenty (30.3%) of the 66 patients developed symptoms during exercise testing, and they had a significantly worse prognosis: the 2-year event-free survival rate was only 19% in those with a positive test compared with 85% in those with a negative test.13 This study highlights the problem of patients subconsciously reducing their level of activity, thereby masking their true symptoms.
A meta-analysis by Rafique et al18 found that asymptomatic patients with abnormal results on exercise testing had a risk of cardiac events during follow-up that was eight times higher than normal, and a risk of sudden death 5.5 times higher.
With trials demonstrating that exercise testing is safe and prognostically useful in patients with aortic stenosis, the ACC/AHA guidelines emphasize its role, giving a class I recommendation for aortic valve replacement in patients who develop symptoms on exercise testing, and a class IIa recommendation in asymptomatic patients with decreased exercise tolerance or an exercise-related fall in blood pressure (Figure 2).4
STRESS ECHOCARDIOGRAPHY
Stress echocardiography has been used since the 1980s to assess the hemodynamic consequences of valvular heart disease, and many studies highlight its prognostic usefulness in patients with asymptomatic aortic stenosis.
In a 2005 study by Lancellotti et al,19 69 patients with severe asymptomatic aortic stenosis underwent a symptom-limited bicycle exercise stress test using quantitative Doppler echocardiography both at rest and at peak exercise, and a number of independent predictors of poor outcome (ie, symptoms, aortic valve replacement, death) were identified. These predictors included an abnormal test result, defined as any of the following: angina, dyspnea, ST-segment depression of 2 mm Hg or more, a fall or a small (< 20 mm Hg) rise in systolic blood pressure during the test, an aortic valve area of 0.75 cm2 or less, or a mean increase in valve gradient of 18 mm Hg or more.
Subsequently, a multicenter prospective trial assessed the value of exercise stress echocardiography in 186 patients with asymptomatic moderate or severe aortic stenosis.20 A mean increase in the aortic valve gradient of 20 mm Hg or more after exercise was associated with a rate of cardiovascular events (death, aortic valve replacement) 3.8 times higher, independent of other risk factors and whether moderate or severe stenosis was present (Table 1).20
Exercise-induced changes in systolic pulmonary artery pressure, which can be assessed using stress echocardiography, also have prognostic utility. Elevated systolic pulmonary artery pressure (> 50 mm Hg) seems to portend a poorer prognosis21,22 and a higher mortality rate after valve replacement,23 making it an independent predictor of hospital mortality and postoperative major adverse cardiovascular and cerebrovascular events (Table 1).
Exercise echocardiography also can be used to assess the patient’s contractile reserve. Left ventricular contractile reserve can be defined as an exercise-induced increase in left ventricular ejection fraction. In a study by Maréchaux et al24 in 50 patients with asymptomatic aortic stenosis and a normal resting left ventricular ejection fraction (> 50%), 40% of patients did not have left ventricular contractile reserve. In fact, their left ventricular ejection fraction decreased with exercise (from 64 ± 10% to 53 ± 12%). The subgroup of patients without contractile reserve developed symptoms more frequently during exercise and had lower event-free survival (Table 1).
Stress echocardiography has recently been introduced into the European Society of Cardiology guidelines, which give a class IIb indication for aortic valve replacement in asymptomatic patients who have severe aortic stenosis, a normal ejection fraction, and a greater than 20-mm Hg increase in mean gradient on exercise.5 But it has yet to be introduced into the ACC/AHA guidelines as a consideration for surgery.
LEFT VENTRICULAR FUNCTION: BEYOND EJECTION FRACTION
Left ventricular dysfunction is a bad sign for patients with aortic stenosis. Struggling to empty its contents through the narrowed aortic valve, the left ventricle is subjected to increased wall stress and eventually develops hypertrophy. The hypertrophied heart muscle requires more oxygen but receives less perfusion. Eventually, myocardial fibrosis develops, leading to systolic dysfunction and a reduction in the ejection fraction. As described above, patients with asymptomatic aortic stenosis and a left ventricular ejection fraction less than 50% have a poor prognosis,14 and therefore the ACC/AHA guidelines give this condition a class I recommendation for surgery.4
However, the ejection fraction has limitations as a marker of left ventricular function. It reflects changes in left ventricular cavity volume but not in the complex structure of the left ventricle. Several studies show that up to one-third of patients with severe aortic stenosis have considerable impairment of intrinsic myocardial systolic function despite a preserved ejection fraction.8,25,26
Thus, other variables such as left atrial size, left ventricular hypertrophy, myocardial deformation (assessed using strain imaging), and B-type natriuretic peptide (BNP) level may also be considered in assessing the effect of severe aortic stenosis on left ventricular function in the context of a normal ejection fraction (Table 2).
Left ventricular hypertrophy
The development of left ventricular hypertrophy is one of the earliest compensatory responses of the ventricle to the increase in afterload. This leads to impaired myocardial relaxation and reduced myocardial compliance, with resultant diastolic dysfunction with increased filling pressures.
Cioffi et al,27 in a study in 209 patients with severe but asymptomatic aortic stenosis, found that inappropriately high left ventricular mass (> 110% of that expected for body size, sex, and wall stress) portended a 4.5-times higher risk of death, independent of other risk factors.
Severe left ventricular hypertrophy may have a long-term effect on prognosis irrespective of valve replacement. An observational study14 of 3,049 patients who underwent aortic valve replacement for severe aortic stenosis showed that the 10-year survival rate was 45% in those whose left ventricular mass was greater than 185 g/m2, compared with 65% in patients whose left ventricular mass was less than 100 g/m2.
Thus, as surgical mortality and morbidity rates decrease, the impact of these structural changes in left ventricular wall thickness may affect the decision to intervene earlier in order to improve longer-term outcomes in select asymptomatic patients with high-risk features.
Left atrial size
Diastolic dysfunction is caused by increased afterload and results in elevated left ventricular end-diastolic pressure and elevated left atrial pressure. The left atrium responds by dilating, which increases the risk of atrial fibrillation.
Lancellotti et al8 investigated the negative prognostic implications of a large indexed left atrial area in asymptomatic patients with severe aortic stenosis. They found that patients with an indexed left atrial area greater than 12.2 cm2/m2 had a 77% 2-year probability of aortic valve replacement or death.
Beach et al28 examined cardiac remodeling after surgery and found that the left atrial diameter did not decrease after aortic valve replacement, even after left ventricular hypertrophy reversed. This observation has major prognostic implications. Patients with a severely enlarged left atrium (> 5.0 cm in diameter) had considerably lower survival rates than patients with a diameter less than 3.55 cm at 5 years (61% vs 85%) and at 10 years (28% vs 62%) after aortic valve replacement.
Therefore, left atrial size appears to have an important long-term impact on prognosis in patients with aortic stenosis even after aortic valve replacement and adds valuable information when assessing the effect of aortic stenosis on myocardial function.
B-type natriuretic peptide
Natriuretic peptides are cardiac hormones released in response to myocyte stretch. In aortic stenosis, increased afterload induces significant expression of BNP, N-terminal proBNP,29 and atrial natriuretic peptide,30 with numerous studies showing a good correlation between plasma natriuretic peptide levels and severity of aortic stenosis.31–34
Bergler-Klein et al33 showed that patients with asymptomatic aortic stenosis who developed symptoms during follow-up had higher levels of these biomarkers than patients who remained asymptomatic. Of note, patients with BNP levels lower than 130 pg/mL had significantly better symptom-free survival than those with higher levels, 66% vs 34% at 12 months.
However, these biomarkers are not specific to aortic stenosis and can be elevated in any condition that increases left ventricular stress. Nevertheless, they offer an easy and low-cost way to assess left ventricular function and may give an indication of the total burden of disease on the left ventricle.
Global left ventricular longitudinal strain
In view of the limitations of the left ventricular ejection fraction in identifying changes in the structure of the heart and in early detection of myocardial dysfunction, assessment of myocardial deformation using strain imaging is proving an attractive alternative.
Strain is the normalized, dimensionless measure of deformation of a solid object (such as a segment of myocardium) in response to an applied force or stress.35 A novel echocardiographic technique allows assessment of segmental myocardial deformation and thereby overcomes the limitation of tethering, which limits other echocardiographic techniques in the assessment of systolic function. Strain can be circumferential, longitudinal, or radial and is generally assessed using either tissue Doppler velocities or 2D echocardiographic speckle-tracking techniques. Longitudinal strain has proven to be a more sensitive method than left ventricular ejection fraction in detecting subclinical myocardial dysfunction and is a superior prognosticator in a variety of clinical conditions.36,37
Abnormal strain develops very early in the disease process and can even be seen in patients with mild aortic stenosis.
A study by Kearney et al38 in 146 patients with various degrees of aortic stenosis (26% mild, 21% moderate, and 53% severe) and preserved left ventricular ejection fraction demonstrated that global longitudinal strain worsened with increasing severity of aortic stenosis. Furthermore, global longitudinal strain was a strong independent predictor of all-cause mortality (hazard ratio 1.38, P < .001).
Similarly, in a study by Lancellotti et al8 in 163 patients with at least moderate to severe asymptomatic aortic stenosis, impaired longitudinal myocardial strain was an independent predictor of survival. Patients with longitudinal strain greater than 15.9% had significantly better outcomes than patients with strain of 15.9% or less (4-year survival 63% vs 22%, P < .001).
Hence, left ventricular global longitudinal strain offers an alternative—perhaps a superior alternative—to left ventricular ejection fraction in detecting and quantifying left ventricular dysfunction in asymptomatic aortic stenosis. It is an exciting new marker for the future in aortic stenosis, with a threshold of strain below 15.9% as a possible cutoff for those at higher risk of poorer outcomes.
WHERE ARE WE NOW? WHERE ARE WE GOING?
Aortic valve replacement in patients with severe but asymptomatic aortic stenosis remains a topic of debate, but support is growing for earlier intervention.
Now that concerns over the safety of exercise stress testing in patients with severe asymptomatic aortic stenosis have subsided following multiple studies,16,17 exercise testing should be performed in patients with asymptomatic severe aortic stenosis suspected of having reduced exercise capacity, with stress echocardiography providing added prognostic information through its assessment of exercise-induced changes in mean pressure gradient19 and systolic pulmonary artery pressure.21–23
Assessing left ventricular function provides important information about prognosis, with left ventricular ejection fraction, left ventricular diameter, left atrial size, BNP, and global longitudinal strain all helping identify asymptomatic patients at higher risk of death. Surgical intervention in asymptomatic patients with severe aortic stenosis may be considered when there is evidence of higher longer-term mortality risk based on reduced functional capacity, excess left ventricular hypertrophy, and abnormal left ventricular function as detected by ancillary methods such as global longitudinal strain and BNP elevation despite a normal left ventricular ejection fraction.
Figure 3 shows a possible algorithm to define which patients would benefit from earlier intervention. However, left ventricular hypertrophy, left atrial diameter, BNP, left ventricular longitudinal strain, and changes in systolic pulmonary artery pressure are not included in the current ACC/AHA guidelines for the management of asymptomatic patients with severe aortic stenosis. Further study is needed to determine whether earlier intervention in those with adverse risk profiles based on the newer evaluation techniques described above leads to better long-term outcomes.
Intervention should especially be considered in those in whom the measured surgical risk is low and in surgical centers at which the mortality rate is low.
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006; 368:1005–1011.
- Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997; 29:630–634.
- Schwarz F, Baumann P, Manthey J, et al. The effect of aortic valve replacement on survival. Circulation 1982; 66:1105–1110.
- Nishimura RA, Otto CM, Bonow RO, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:e57–e185.
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS); Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012; 33:2451–2496.
- Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000; 343:611–617.
- Rosenhek R, Zilberszac R, Schemper M, at al. Natural history of very severe aortic stenosis. Circulation 2010; 121:151–156.
- Lancellotti P, Donal E, Magne J, et al. Risk stratification in asymptomatic moderate to severe aortic stenosis: the importance of the valvular, arterial and ventricular interplay. Heart 2010; 96:1364–1371.
- Pai R, Kapoor N, Bansal RC, Varadarajan P. Natural malignant history of asymptomatic severe aortic stenosis: benefit of aortic valve replacement. Ann Thorac Surg 2006; 82:2116–2122.
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease); Society of Cardiovascular Anesthesiologists; Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006; 48:e1–e148.
- Patel HJ, Herbert MA, Drake DH, et al. Aortic valve replacement: using a statewide cardiac surgical database identifies a procedural volume hinge point. Ann Thorac Surg 2013; 96:1560–1566.
- Johnston DR, Roselli EE. Minimally invasive aortic valve surgery: Cleveland Clinic experience. Ann Cardiothorac Surg 2015;4:140–147.
- Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 2001; 86:381–386.
- Mihaljevic T, Nowicki ER, Rajeswaran J, et al. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg 2008; 135:1270–1279.
- Kang DH, Park SJ, Rim JH, et al. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation 2010; 121:1502–1509.
- Alborino D, Hoffmann JL, Fournet PC, Bloch A. Value of exercise testing to evaluate the indication for surgery in asymptomatic patients with valvular aortic stenosis. J Heart Valve Dis 2002; 11:204–209.
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005; 26:1309–1313.
- Rafique AM, Biner S, Ray I, Forrester JS, Tolstrup K, Siegel RJ. Meta-analysis of prognostic value of stress testing in patients with asymptomatic severe aortic stenosis. Am J Cardiol 2009; 104:972–977.
- Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation 2005; 112(suppl I):I377–I382.
- Marechaux S, Hachicha Z, Bellouin A, et al. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J 2010; 31:1390–1397.
- Cooper R, Ghali J, Simmons BE, Castaner A. Elevated pulmonary artery pressure. An independent predictor of mortality. Chest 1991; 99:112–120.
- McHenry MM, Rice J, Matlof HJ, Flamm MD Jr. Pulmonary hypertension and sudden death in aortic stenosis. Br Heart J 1979; 41:463–467.
- Copeland JG, Griepp RB, Stinson EB, Shumway NE. Long-term follow-up after isolated aortic valve replacement. J Thorac Cardiovasc Surg 1977; 74: 875–889.
- Maréchaux S, Ennezat PV, LeJemtel TH, et al. Left ventricular response to exercise in aortic stenosis: an exercise echocardiographic study. Echocardiography 2007; 24:955–959.
- Cramariuc D, Cioffi G, Rieck AE, et al. Low-flow aortic stenosis in asymptomatic patients: valvular arterial impedance and systolic function from the SEAS substudy. JACC Cardiovasc Imaging 2009; 2:390–399.
- Dumesnil JG, Shoucri RM, Laurenceau JL, Turcot J. A mathematical model of the dynamic geometry of the intact left ventricle and its application to clinical data. Circulation 1979; 59:1024–1034.
- Cioffi G, Faggiano P, Vizzardi E, et al. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart 2011; 97:301–307.
- Beach JM, Mihaljevic T, Rajeswaran J, et al. Ventricular hypertrophy and left atrial dilatation persist and are associated with reduced survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg 2014; 147:362–369.e8.
- Vanderheyden M, Goethals M, Verstreken S, et al. Wall stress modulates brain natriuretic peptide production in pressure overload cardiomyopathy. J Am Coll Cardiol 2004; 44:2349–2354.
- Ikeda T, Matsuda K, Itoh H, et al. Plasma levels of brain and atrial natriuretic peptides elevate in proportion to left ventricular end-systolic wall stress in patients with aortic stenosis. Am Heart J 1997; 133:307–314.
- Qi W, Mathisen P, Kjekshus J, et al. Natriuretic peptides in patients with aortic stenosis. Am Heart J 2001; 142:725–732.
- Weber M, Arnold R, Rau M, et al. Relation of N-terminal pro-B-type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol 2004; 94:740–745.
- Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109:2302–2308.
- Lim P, Monin JL, Monchi M, et al. Predictors of outcome in patients with severe aortic stenosis and normal left ventricular function: role of B-type natriuretic peptide. Eur Heart J 2004; 25:2048–2053.
- Holt B. Strain and strain rate echocardiography and coronary artery disease. Circ Cardiovasc Imaging 2011; 4:179–190.
- Ng AC, Delgado V, Bertini M, et al. Alterations in multidirectional myocardial functions in patients with aortic stenosis and preserved ejection fraction: a two-dimensional speckle tracking analysis. Eur Heart J 2011; 32:1542–1550.
- Ng AC, Delgado V, Bertini M, et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol 2009; 104:1398–1401
- Kearney LG, Lu K, Ord M, et al. Global longitudinal strain is a strong independent predictor of all-cause mortality in patients with aortic stenosis. Eur Heart J Cardiovasc Imag 2012; 13:827–833.
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006; 368:1005–1011.
- Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997; 29:630–634.
- Schwarz F, Baumann P, Manthey J, et al. The effect of aortic valve replacement on survival. Circulation 1982; 66:1105–1110.
- Nishimura RA, Otto CM, Bonow RO, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:e57–e185.
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS); Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012; 33:2451–2496.
- Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000; 343:611–617.
- Rosenhek R, Zilberszac R, Schemper M, at al. Natural history of very severe aortic stenosis. Circulation 2010; 121:151–156.
- Lancellotti P, Donal E, Magne J, et al. Risk stratification in asymptomatic moderate to severe aortic stenosis: the importance of the valvular, arterial and ventricular interplay. Heart 2010; 96:1364–1371.
- Pai R, Kapoor N, Bansal RC, Varadarajan P. Natural malignant history of asymptomatic severe aortic stenosis: benefit of aortic valve replacement. Ann Thorac Surg 2006; 82:2116–2122.
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease); Society of Cardiovascular Anesthesiologists; Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006; 48:e1–e148.
- Patel HJ, Herbert MA, Drake DH, et al. Aortic valve replacement: using a statewide cardiac surgical database identifies a procedural volume hinge point. Ann Thorac Surg 2013; 96:1560–1566.
- Johnston DR, Roselli EE. Minimally invasive aortic valve surgery: Cleveland Clinic experience. Ann Cardiothorac Surg 2015;4:140–147.
- Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 2001; 86:381–386.
- Mihaljevic T, Nowicki ER, Rajeswaran J, et al. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg 2008; 135:1270–1279.
- Kang DH, Park SJ, Rim JH, et al. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation 2010; 121:1502–1509.
- Alborino D, Hoffmann JL, Fournet PC, Bloch A. Value of exercise testing to evaluate the indication for surgery in asymptomatic patients with valvular aortic stenosis. J Heart Valve Dis 2002; 11:204–209.
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005; 26:1309–1313.
- Rafique AM, Biner S, Ray I, Forrester JS, Tolstrup K, Siegel RJ. Meta-analysis of prognostic value of stress testing in patients with asymptomatic severe aortic stenosis. Am J Cardiol 2009; 104:972–977.
- Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation 2005; 112(suppl I):I377–I382.
- Marechaux S, Hachicha Z, Bellouin A, et al. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J 2010; 31:1390–1397.
- Cooper R, Ghali J, Simmons BE, Castaner A. Elevated pulmonary artery pressure. An independent predictor of mortality. Chest 1991; 99:112–120.
- McHenry MM, Rice J, Matlof HJ, Flamm MD Jr. Pulmonary hypertension and sudden death in aortic stenosis. Br Heart J 1979; 41:463–467.
- Copeland JG, Griepp RB, Stinson EB, Shumway NE. Long-term follow-up after isolated aortic valve replacement. J Thorac Cardiovasc Surg 1977; 74: 875–889.
- Maréchaux S, Ennezat PV, LeJemtel TH, et al. Left ventricular response to exercise in aortic stenosis: an exercise echocardiographic study. Echocardiography 2007; 24:955–959.
- Cramariuc D, Cioffi G, Rieck AE, et al. Low-flow aortic stenosis in asymptomatic patients: valvular arterial impedance and systolic function from the SEAS substudy. JACC Cardiovasc Imaging 2009; 2:390–399.
- Dumesnil JG, Shoucri RM, Laurenceau JL, Turcot J. A mathematical model of the dynamic geometry of the intact left ventricle and its application to clinical data. Circulation 1979; 59:1024–1034.
- Cioffi G, Faggiano P, Vizzardi E, et al. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart 2011; 97:301–307.
- Beach JM, Mihaljevic T, Rajeswaran J, et al. Ventricular hypertrophy and left atrial dilatation persist and are associated with reduced survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg 2014; 147:362–369.e8.
- Vanderheyden M, Goethals M, Verstreken S, et al. Wall stress modulates brain natriuretic peptide production in pressure overload cardiomyopathy. J Am Coll Cardiol 2004; 44:2349–2354.
- Ikeda T, Matsuda K, Itoh H, et al. Plasma levels of brain and atrial natriuretic peptides elevate in proportion to left ventricular end-systolic wall stress in patients with aortic stenosis. Am Heart J 1997; 133:307–314.
- Qi W, Mathisen P, Kjekshus J, et al. Natriuretic peptides in patients with aortic stenosis. Am Heart J 2001; 142:725–732.
- Weber M, Arnold R, Rau M, et al. Relation of N-terminal pro-B-type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol 2004; 94:740–745.
- Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109:2302–2308.
- Lim P, Monin JL, Monchi M, et al. Predictors of outcome in patients with severe aortic stenosis and normal left ventricular function: role of B-type natriuretic peptide. Eur Heart J 2004; 25:2048–2053.
- Holt B. Strain and strain rate echocardiography and coronary artery disease. Circ Cardiovasc Imaging 2011; 4:179–190.
- Ng AC, Delgado V, Bertini M, et al. Alterations in multidirectional myocardial functions in patients with aortic stenosis and preserved ejection fraction: a two-dimensional speckle tracking analysis. Eur Heart J 2011; 32:1542–1550.
- Ng AC, Delgado V, Bertini M, et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol 2009; 104:1398–1401
- Kearney LG, Lu K, Ord M, et al. Global longitudinal strain is a strong independent predictor of all-cause mortality in patients with aortic stenosis. Eur Heart J Cardiovasc Imag 2012; 13:827–833.
KEY POINTS
- Echocardiography is the best established and most important initial test in patients with suspected aortic stenosis.
- Traditional echocardiographic variables used in assessing aortic stenosis and the need for surgery are the pressure gradient across the valve, the velocity through the valve, the valve area, and the left ventricular ejection fraction.
- Aortic valve replacement is recommended for severe aortic stenosis if the patient has symptoms. It is also recommended if the left ventricular ejection fraction is less than 50%, if the patient is undergoing other cardiac surgery, or if symptoms arise on exercise stress testing.
- Novel assessment variables include left ventricular hypertrophy, left atrial size, B-type natriuretic peptide level, and global left ventricular longitudinal strain.
Phrenic nerve paralysis induced by brachial plexus block
A 72-year-old man underwent elective ambulatory arthroscopic repair of the right shoulder rotator cuff. To manage postoperative pain, a supraclavicular catheter was placed for brachial plexus block, and he was sent home with a ropivacaine infusion pump.
The next day, he presented to the emergency department with right-sided chest pain and mild shortness of breath. He had normal vital signs and adequate oxygen saturation on room air. On physical examination, breath sounds were decreased at the right lung base, and chest radiography (Figure 1) revealed an isolated elevated right hemidiaphragm, a clear indication of phrenic nerve paralysis from local infiltration of the infusion.
The ropivacaine infusion was stopped, and the supraclavicular catheter was removed under anesthesia. He was admitted to the hospital for observation, and over the course of 8 to 12 hours his shortness of breath resolved, and his findings on lung examination normalized. Repeat chest radiography 24 hours after his emergency room presentation showed regular positioning of his diaphragm (Figure 2).
RECOGNIZING AND MANAGING PHRENIC NERVE PARALYSIS
The scenario described here illustrates the importance of recognizing symptomatic phrenic nerve paralysis as a result of local infiltration of anesthetic from supraclavicular brachial plexus block. Regional anesthesia is commonly used for perioperative analgesia for minor shoulder surgeries. Because these blocks anesthetize the trunks formed by the C5–T1 nerve roots, infiltration of the anesthetic agent to the proximal nerve roots resulting in phrenic nerve paralysis is a common complication.
Although phrenic nerve paralysis has been reported to some degree in nearly all patients, reports of significant shortness of breath and radiographic evidence of hemidiaphragm are few.1–4 When it occurs, the analgesic regimen must be changed from regional anesthesia to oral or parenteral pain medications. Resolution of symptoms and radiographic abnormalities usually occurs spontaneously.
When available, an ultrasonographically guided approach for supraclavicular brachial plexus blocks is preferred over a blind approach and is associated with a higher success rate and a lower rate of complications.5,6
A potentially life-threatening complication of brachial plexus block is pneumothorax.
Contraindications to brachial plexus block include severe lung disease and previous surgery or interventions with the potential for phrenic nerve injury that could result in bilateral paralysis of the diaphragm. Ultimately, preprocedural chest radiography in selected patients at high risk should be considered to mitigate this risk.
- Tran QH, Clemente A, Doan J, Finlayson RJ. Brachial plexus blocks: a review of approaches and techniques. Can J Anaesth 2007; 54:662–674.
- Mian A, Chaudhry I, Huang R, Rizk E, Tubbs RS, Loukas M. Brachial plexus anesthesia: a review of the relevant anatomy, complications, and anatomical variations. Clin Anat 2014; 27:210–221.
- Knoblanche GE. The incidence and aetiology of phrenic nerve blockade associated with supraclavicular brachial plexus block. Anaesth Intensive Care 1979; 7:346–349.
- Urmey WF, Talts KH, Sharrock NE. One hundred percent incidence of hemidiaphragmatic paresis associated with interscalene brachial plexus anesthesia as diagnosed by ultrasonography. Anesth Analg 1991; 72:498–503.
- Gelfand HJ, Ouanes JP, Lesley MR, et al. Analgesic efficacy of ultrasound-guided regional anesthesia: a meta-analysis. J Clin Anesth 2011; 23:90–96.
- Sandhu NS, Capan LM. Ultrasound-guided infraclavicular brachial plexus block. Br J Anaesth 2002; 89:254–259.
A 72-year-old man underwent elective ambulatory arthroscopic repair of the right shoulder rotator cuff. To manage postoperative pain, a supraclavicular catheter was placed for brachial plexus block, and he was sent home with a ropivacaine infusion pump.
The next day, he presented to the emergency department with right-sided chest pain and mild shortness of breath. He had normal vital signs and adequate oxygen saturation on room air. On physical examination, breath sounds were decreased at the right lung base, and chest radiography (Figure 1) revealed an isolated elevated right hemidiaphragm, a clear indication of phrenic nerve paralysis from local infiltration of the infusion.
The ropivacaine infusion was stopped, and the supraclavicular catheter was removed under anesthesia. He was admitted to the hospital for observation, and over the course of 8 to 12 hours his shortness of breath resolved, and his findings on lung examination normalized. Repeat chest radiography 24 hours after his emergency room presentation showed regular positioning of his diaphragm (Figure 2).
RECOGNIZING AND MANAGING PHRENIC NERVE PARALYSIS
The scenario described here illustrates the importance of recognizing symptomatic phrenic nerve paralysis as a result of local infiltration of anesthetic from supraclavicular brachial plexus block. Regional anesthesia is commonly used for perioperative analgesia for minor shoulder surgeries. Because these blocks anesthetize the trunks formed by the C5–T1 nerve roots, infiltration of the anesthetic agent to the proximal nerve roots resulting in phrenic nerve paralysis is a common complication.
Although phrenic nerve paralysis has been reported to some degree in nearly all patients, reports of significant shortness of breath and radiographic evidence of hemidiaphragm are few.1–4 When it occurs, the analgesic regimen must be changed from regional anesthesia to oral or parenteral pain medications. Resolution of symptoms and radiographic abnormalities usually occurs spontaneously.
When available, an ultrasonographically guided approach for supraclavicular brachial plexus blocks is preferred over a blind approach and is associated with a higher success rate and a lower rate of complications.5,6
A potentially life-threatening complication of brachial plexus block is pneumothorax.
Contraindications to brachial plexus block include severe lung disease and previous surgery or interventions with the potential for phrenic nerve injury that could result in bilateral paralysis of the diaphragm. Ultimately, preprocedural chest radiography in selected patients at high risk should be considered to mitigate this risk.
A 72-year-old man underwent elective ambulatory arthroscopic repair of the right shoulder rotator cuff. To manage postoperative pain, a supraclavicular catheter was placed for brachial plexus block, and he was sent home with a ropivacaine infusion pump.
The next day, he presented to the emergency department with right-sided chest pain and mild shortness of breath. He had normal vital signs and adequate oxygen saturation on room air. On physical examination, breath sounds were decreased at the right lung base, and chest radiography (Figure 1) revealed an isolated elevated right hemidiaphragm, a clear indication of phrenic nerve paralysis from local infiltration of the infusion.
The ropivacaine infusion was stopped, and the supraclavicular catheter was removed under anesthesia. He was admitted to the hospital for observation, and over the course of 8 to 12 hours his shortness of breath resolved, and his findings on lung examination normalized. Repeat chest radiography 24 hours after his emergency room presentation showed regular positioning of his diaphragm (Figure 2).
RECOGNIZING AND MANAGING PHRENIC NERVE PARALYSIS
The scenario described here illustrates the importance of recognizing symptomatic phrenic nerve paralysis as a result of local infiltration of anesthetic from supraclavicular brachial plexus block. Regional anesthesia is commonly used for perioperative analgesia for minor shoulder surgeries. Because these blocks anesthetize the trunks formed by the C5–T1 nerve roots, infiltration of the anesthetic agent to the proximal nerve roots resulting in phrenic nerve paralysis is a common complication.
Although phrenic nerve paralysis has been reported to some degree in nearly all patients, reports of significant shortness of breath and radiographic evidence of hemidiaphragm are few.1–4 When it occurs, the analgesic regimen must be changed from regional anesthesia to oral or parenteral pain medications. Resolution of symptoms and radiographic abnormalities usually occurs spontaneously.
When available, an ultrasonographically guided approach for supraclavicular brachial plexus blocks is preferred over a blind approach and is associated with a higher success rate and a lower rate of complications.5,6
A potentially life-threatening complication of brachial plexus block is pneumothorax.
Contraindications to brachial plexus block include severe lung disease and previous surgery or interventions with the potential for phrenic nerve injury that could result in bilateral paralysis of the diaphragm. Ultimately, preprocedural chest radiography in selected patients at high risk should be considered to mitigate this risk.
- Tran QH, Clemente A, Doan J, Finlayson RJ. Brachial plexus blocks: a review of approaches and techniques. Can J Anaesth 2007; 54:662–674.
- Mian A, Chaudhry I, Huang R, Rizk E, Tubbs RS, Loukas M. Brachial plexus anesthesia: a review of the relevant anatomy, complications, and anatomical variations. Clin Anat 2014; 27:210–221.
- Knoblanche GE. The incidence and aetiology of phrenic nerve blockade associated with supraclavicular brachial plexus block. Anaesth Intensive Care 1979; 7:346–349.
- Urmey WF, Talts KH, Sharrock NE. One hundred percent incidence of hemidiaphragmatic paresis associated with interscalene brachial plexus anesthesia as diagnosed by ultrasonography. Anesth Analg 1991; 72:498–503.
- Gelfand HJ, Ouanes JP, Lesley MR, et al. Analgesic efficacy of ultrasound-guided regional anesthesia: a meta-analysis. J Clin Anesth 2011; 23:90–96.
- Sandhu NS, Capan LM. Ultrasound-guided infraclavicular brachial plexus block. Br J Anaesth 2002; 89:254–259.
- Tran QH, Clemente A, Doan J, Finlayson RJ. Brachial plexus blocks: a review of approaches and techniques. Can J Anaesth 2007; 54:662–674.
- Mian A, Chaudhry I, Huang R, Rizk E, Tubbs RS, Loukas M. Brachial plexus anesthesia: a review of the relevant anatomy, complications, and anatomical variations. Clin Anat 2014; 27:210–221.
- Knoblanche GE. The incidence and aetiology of phrenic nerve blockade associated with supraclavicular brachial plexus block. Anaesth Intensive Care 1979; 7:346–349.
- Urmey WF, Talts KH, Sharrock NE. One hundred percent incidence of hemidiaphragmatic paresis associated with interscalene brachial plexus anesthesia as diagnosed by ultrasonography. Anesth Analg 1991; 72:498–503.
- Gelfand HJ, Ouanes JP, Lesley MR, et al. Analgesic efficacy of ultrasound-guided regional anesthesia: a meta-analysis. J Clin Anesth 2011; 23:90–96.
- Sandhu NS, Capan LM. Ultrasound-guided infraclavicular brachial plexus block. Br J Anaesth 2002; 89:254–259.
VIDEO: Anesthesia services during colonoscopy increase risk of near-term complications
Receiving anesthesia services while undergoing a colonoscopy may not be in your patients’ best interest, as doing so could significantly increase the likelihood of patients experiencing serious complications within 30 days of the procedure.
This is according to a new study published in the April issue of Gastroenterology, in which Dr. Karen J. Wernli and her coinvestigators analyzed claims data, collected from the Truven Health MarketScan Research Database, related to 3,168,228 colonoscopy procedures that took place between 2008 and 2011, to determine whether patients who received anesthesia were at a higher risk of developing complications after the procedure (doi: 10.1053/j.gastro.2015.12.018).
Source: American Gastroenterological Association
“The involvement of anesthesia services for colonoscopy sedation, mainly to administer propofol, has increased accordingly, from 11.0% of colonoscopies in 2001 to 23.4% in 2006, with projections of more than 50% in 2015,” wrote Dr. Wernli of the Group Health Research Institute in Seattle, and her coauthors. “Whether the use of propofol is associated with higher rates of short-term complications compared with standard sedation is not well understood.”
Men and women whose data was included in the study were between 40 and 64 years of age; men accounted for 46.8% of those receiving standard sedation (53.2% women) and 46.5% of those receiving anesthesia services (53.5% women). A total of 4,939,993 individuals were initially screened for enrollment, with 39,784 excluded because of a previous colorectal cancer diagnosis, 240,038 for “noncancer exclusions,” and 1,491,943 for being enrolled in the study less than 1 year.
Standard sedation was done in 2,079,784 (65.6%) of the procedures included in the study, while the other 1,088,444 (34.4%) colonoscopies involved anesthesia services. Use of anesthesia services resulted in a 13% increase in likelihood for patients to experience some kind of complication within 30 days of colonoscopy (95% confidence interval, 1.12-1.14). The most common complications were perforation (odds ratio, 1.07; 95% CI, 1.00-1.15), hemorrhage (OR, 1.28; 95% CI, 1.27-1.30), abdominal pain (OR, 1.07; 95% CI, 1.05-1.08), complications secondary to anesthesia (OR, 1.15; 95% CI, 1.05-1.28), and “stroke and other central nervous system events” (OR, 1.04; 95% CI, 1.00-1.08).
Analysis of geographic distribution of colonoscopies performed with and without anesthesia services showed that all areas of the United States had a higher likelihood of postcolonoscopy complications associated with anesthesia except in the Southeast, where there was no association between the two. Additionally, in the western U.S., use of anesthesia services was less common than in any other geographic area, but was associated with a staggering 60% higher chance of complication within 30 days for patients who did opt for it.
“Although the use of anesthesia agents can directly impact colonoscopy outcomes, it is not solely the anesthesia agent that could lead to additional complications,” the study authors wrote. “In the absence of patient feedback, increased colonic-wall tension from colonoscopy pressure may not be identified by the endoscopist, and, consistent with our results, could lead to increased risks of colonic complications, such as perforation and abdominal pain.”
Dr. Wernli and her coauthors did not report any relevant financial disclosures.
We are approaching a time when half of all colonoscopies are performed with anesthesia assistance, most using propofol. Undeniably, some patients require anesthesia support for medical reasons, or because they do not sedate adequately with opiate-benzodiazepine combinations endoscopists can administer. The popularity of propofol-based anesthesia for routine colonoscopy, however, is based on several perceived benefits: patient demand for a discomfort-free procedure, rapid sedation followed by quick recovery, and good reimbursement for the anesthesia service itself, added to the benefits of faster overall procedure turnaround time. And presently, there is no disincentive — financial or otherwise — to continuing or expanding this practice. Colonoscopy with anesthesia looks like a win-win for both patient and endoscopist, as long as the added cost of anesthesia can be justified.
However, while anesthesia-assisted colonoscopy appears to possess several advantages, growing evidence suggests that a lower risk of complications is not one of them.
A smaller study (165,000 colonoscopies) using NCI SEER registry data suggested that adding anesthesia to colonoscopy may increase some adverse events. Cooper et al. (JAMA Intern Med. 2013;173:551-6) showed an increase in overall complications and, specifically, aspiration, although not in technical complications of colonoscopy, including perforation and splenic rupture. However, this study did not include patients who underwent polypectomy. Wernli, et al. now show evidence derived from over 3 million patients demonstrating that adding anesthesia to colonoscopy increases complications significantly — not only aspiration, but also technical aspects of colonoscopy, including perforation, bleeding, and abdominal pain.
Colonoscopy is extremely safe, so complications are infrequent. Thus, data sets of colonoscopy complications large enough to be statistically meaningful for studies of this type require an extraordinarily large patient pool. For this prospective, observational cohort study, the authors obtained the large sample size by mining administrative claims data for 3 years, not through examining clinical data. As a result, several assumptions were made. These 3 million colonoscopies represented all indications — not just colorectal cancer screening. Billing claims for anesthesia represented surrogate markers for administration of propofol-based anesthesia. While anesthesia assistance was associated with increased risk of perforation, hemorrhage, abdominal pain, anesthesia complications, and stroke; risk of perforation associated with anesthesia was increased only in patients who underwent polypectomy.
Study methodology and confounding variables aside, it is hard to ignore the core message here: a large body of data analyzed rigorously demonstrate that anesthesia support for colonoscopy increases risk of procedure-related complications.
Patients who are ill, have certain cardiopulmonary issues, or do not sedate adequately with moderate sedation benefit from anesthesia assistance for colonoscopy. But for patients undergoing routine colonoscopy, without such issues, who could safely undergo colonoscopy under moderate sedation without unreasonable discomfort, we must now ask ourselves and discuss with our patients honestly, not only whether the added cost of anesthesia is reasonable — but also whether the apparent added risk of anesthesia justifies perceived benefits.
Dr. John A. Martin is senior associate consultant and associate professor, associate chair for endoscopy, Mayo Clinic, Rochester, Minn. He has no conflicts of interest to disclose.
We are approaching a time when half of all colonoscopies are performed with anesthesia assistance, most using propofol. Undeniably, some patients require anesthesia support for medical reasons, or because they do not sedate adequately with opiate-benzodiazepine combinations endoscopists can administer. The popularity of propofol-based anesthesia for routine colonoscopy, however, is based on several perceived benefits: patient demand for a discomfort-free procedure, rapid sedation followed by quick recovery, and good reimbursement for the anesthesia service itself, added to the benefits of faster overall procedure turnaround time. And presently, there is no disincentive — financial or otherwise — to continuing or expanding this practice. Colonoscopy with anesthesia looks like a win-win for both patient and endoscopist, as long as the added cost of anesthesia can be justified.
However, while anesthesia-assisted colonoscopy appears to possess several advantages, growing evidence suggests that a lower risk of complications is not one of them.
A smaller study (165,000 colonoscopies) using NCI SEER registry data suggested that adding anesthesia to colonoscopy may increase some adverse events. Cooper et al. (JAMA Intern Med. 2013;173:551-6) showed an increase in overall complications and, specifically, aspiration, although not in technical complications of colonoscopy, including perforation and splenic rupture. However, this study did not include patients who underwent polypectomy. Wernli, et al. now show evidence derived from over 3 million patients demonstrating that adding anesthesia to colonoscopy increases complications significantly — not only aspiration, but also technical aspects of colonoscopy, including perforation, bleeding, and abdominal pain.
Colonoscopy is extremely safe, so complications are infrequent. Thus, data sets of colonoscopy complications large enough to be statistically meaningful for studies of this type require an extraordinarily large patient pool. For this prospective, observational cohort study, the authors obtained the large sample size by mining administrative claims data for 3 years, not through examining clinical data. As a result, several assumptions were made. These 3 million colonoscopies represented all indications — not just colorectal cancer screening. Billing claims for anesthesia represented surrogate markers for administration of propofol-based anesthesia. While anesthesia assistance was associated with increased risk of perforation, hemorrhage, abdominal pain, anesthesia complications, and stroke; risk of perforation associated with anesthesia was increased only in patients who underwent polypectomy.
Study methodology and confounding variables aside, it is hard to ignore the core message here: a large body of data analyzed rigorously demonstrate that anesthesia support for colonoscopy increases risk of procedure-related complications.
Patients who are ill, have certain cardiopulmonary issues, or do not sedate adequately with moderate sedation benefit from anesthesia assistance for colonoscopy. But for patients undergoing routine colonoscopy, without such issues, who could safely undergo colonoscopy under moderate sedation without unreasonable discomfort, we must now ask ourselves and discuss with our patients honestly, not only whether the added cost of anesthesia is reasonable — but also whether the apparent added risk of anesthesia justifies perceived benefits.
Dr. John A. Martin is senior associate consultant and associate professor, associate chair for endoscopy, Mayo Clinic, Rochester, Minn. He has no conflicts of interest to disclose.
We are approaching a time when half of all colonoscopies are performed with anesthesia assistance, most using propofol. Undeniably, some patients require anesthesia support for medical reasons, or because they do not sedate adequately with opiate-benzodiazepine combinations endoscopists can administer. The popularity of propofol-based anesthesia for routine colonoscopy, however, is based on several perceived benefits: patient demand for a discomfort-free procedure, rapid sedation followed by quick recovery, and good reimbursement for the anesthesia service itself, added to the benefits of faster overall procedure turnaround time. And presently, there is no disincentive — financial or otherwise — to continuing or expanding this practice. Colonoscopy with anesthesia looks like a win-win for both patient and endoscopist, as long as the added cost of anesthesia can be justified.
However, while anesthesia-assisted colonoscopy appears to possess several advantages, growing evidence suggests that a lower risk of complications is not one of them.
A smaller study (165,000 colonoscopies) using NCI SEER registry data suggested that adding anesthesia to colonoscopy may increase some adverse events. Cooper et al. (JAMA Intern Med. 2013;173:551-6) showed an increase in overall complications and, specifically, aspiration, although not in technical complications of colonoscopy, including perforation and splenic rupture. However, this study did not include patients who underwent polypectomy. Wernli, et al. now show evidence derived from over 3 million patients demonstrating that adding anesthesia to colonoscopy increases complications significantly — not only aspiration, but also technical aspects of colonoscopy, including perforation, bleeding, and abdominal pain.
Colonoscopy is extremely safe, so complications are infrequent. Thus, data sets of colonoscopy complications large enough to be statistically meaningful for studies of this type require an extraordinarily large patient pool. For this prospective, observational cohort study, the authors obtained the large sample size by mining administrative claims data for 3 years, not through examining clinical data. As a result, several assumptions were made. These 3 million colonoscopies represented all indications — not just colorectal cancer screening. Billing claims for anesthesia represented surrogate markers for administration of propofol-based anesthesia. While anesthesia assistance was associated with increased risk of perforation, hemorrhage, abdominal pain, anesthesia complications, and stroke; risk of perforation associated with anesthesia was increased only in patients who underwent polypectomy.
Study methodology and confounding variables aside, it is hard to ignore the core message here: a large body of data analyzed rigorously demonstrate that anesthesia support for colonoscopy increases risk of procedure-related complications.
Patients who are ill, have certain cardiopulmonary issues, or do not sedate adequately with moderate sedation benefit from anesthesia assistance for colonoscopy. But for patients undergoing routine colonoscopy, without such issues, who could safely undergo colonoscopy under moderate sedation without unreasonable discomfort, we must now ask ourselves and discuss with our patients honestly, not only whether the added cost of anesthesia is reasonable — but also whether the apparent added risk of anesthesia justifies perceived benefits.
Dr. John A. Martin is senior associate consultant and associate professor, associate chair for endoscopy, Mayo Clinic, Rochester, Minn. He has no conflicts of interest to disclose.
Receiving anesthesia services while undergoing a colonoscopy may not be in your patients’ best interest, as doing so could significantly increase the likelihood of patients experiencing serious complications within 30 days of the procedure.
This is according to a new study published in the April issue of Gastroenterology, in which Dr. Karen J. Wernli and her coinvestigators analyzed claims data, collected from the Truven Health MarketScan Research Database, related to 3,168,228 colonoscopy procedures that took place between 2008 and 2011, to determine whether patients who received anesthesia were at a higher risk of developing complications after the procedure (doi: 10.1053/j.gastro.2015.12.018).
Source: American Gastroenterological Association
“The involvement of anesthesia services for colonoscopy sedation, mainly to administer propofol, has increased accordingly, from 11.0% of colonoscopies in 2001 to 23.4% in 2006, with projections of more than 50% in 2015,” wrote Dr. Wernli of the Group Health Research Institute in Seattle, and her coauthors. “Whether the use of propofol is associated with higher rates of short-term complications compared with standard sedation is not well understood.”
Men and women whose data was included in the study were between 40 and 64 years of age; men accounted for 46.8% of those receiving standard sedation (53.2% women) and 46.5% of those receiving anesthesia services (53.5% women). A total of 4,939,993 individuals were initially screened for enrollment, with 39,784 excluded because of a previous colorectal cancer diagnosis, 240,038 for “noncancer exclusions,” and 1,491,943 for being enrolled in the study less than 1 year.
Standard sedation was done in 2,079,784 (65.6%) of the procedures included in the study, while the other 1,088,444 (34.4%) colonoscopies involved anesthesia services. Use of anesthesia services resulted in a 13% increase in likelihood for patients to experience some kind of complication within 30 days of colonoscopy (95% confidence interval, 1.12-1.14). The most common complications were perforation (odds ratio, 1.07; 95% CI, 1.00-1.15), hemorrhage (OR, 1.28; 95% CI, 1.27-1.30), abdominal pain (OR, 1.07; 95% CI, 1.05-1.08), complications secondary to anesthesia (OR, 1.15; 95% CI, 1.05-1.28), and “stroke and other central nervous system events” (OR, 1.04; 95% CI, 1.00-1.08).
Analysis of geographic distribution of colonoscopies performed with and without anesthesia services showed that all areas of the United States had a higher likelihood of postcolonoscopy complications associated with anesthesia except in the Southeast, where there was no association between the two. Additionally, in the western U.S., use of anesthesia services was less common than in any other geographic area, but was associated with a staggering 60% higher chance of complication within 30 days for patients who did opt for it.
“Although the use of anesthesia agents can directly impact colonoscopy outcomes, it is not solely the anesthesia agent that could lead to additional complications,” the study authors wrote. “In the absence of patient feedback, increased colonic-wall tension from colonoscopy pressure may not be identified by the endoscopist, and, consistent with our results, could lead to increased risks of colonic complications, such as perforation and abdominal pain.”
Dr. Wernli and her coauthors did not report any relevant financial disclosures.
Receiving anesthesia services while undergoing a colonoscopy may not be in your patients’ best interest, as doing so could significantly increase the likelihood of patients experiencing serious complications within 30 days of the procedure.
This is according to a new study published in the April issue of Gastroenterology, in which Dr. Karen J. Wernli and her coinvestigators analyzed claims data, collected from the Truven Health MarketScan Research Database, related to 3,168,228 colonoscopy procedures that took place between 2008 and 2011, to determine whether patients who received anesthesia were at a higher risk of developing complications after the procedure (doi: 10.1053/j.gastro.2015.12.018).
Source: American Gastroenterological Association
“The involvement of anesthesia services for colonoscopy sedation, mainly to administer propofol, has increased accordingly, from 11.0% of colonoscopies in 2001 to 23.4% in 2006, with projections of more than 50% in 2015,” wrote Dr. Wernli of the Group Health Research Institute in Seattle, and her coauthors. “Whether the use of propofol is associated with higher rates of short-term complications compared with standard sedation is not well understood.”
Men and women whose data was included in the study were between 40 and 64 years of age; men accounted for 46.8% of those receiving standard sedation (53.2% women) and 46.5% of those receiving anesthesia services (53.5% women). A total of 4,939,993 individuals were initially screened for enrollment, with 39,784 excluded because of a previous colorectal cancer diagnosis, 240,038 for “noncancer exclusions,” and 1,491,943 for being enrolled in the study less than 1 year.
Standard sedation was done in 2,079,784 (65.6%) of the procedures included in the study, while the other 1,088,444 (34.4%) colonoscopies involved anesthesia services. Use of anesthesia services resulted in a 13% increase in likelihood for patients to experience some kind of complication within 30 days of colonoscopy (95% confidence interval, 1.12-1.14). The most common complications were perforation (odds ratio, 1.07; 95% CI, 1.00-1.15), hemorrhage (OR, 1.28; 95% CI, 1.27-1.30), abdominal pain (OR, 1.07; 95% CI, 1.05-1.08), complications secondary to anesthesia (OR, 1.15; 95% CI, 1.05-1.28), and “stroke and other central nervous system events” (OR, 1.04; 95% CI, 1.00-1.08).
Analysis of geographic distribution of colonoscopies performed with and without anesthesia services showed that all areas of the United States had a higher likelihood of postcolonoscopy complications associated with anesthesia except in the Southeast, where there was no association between the two. Additionally, in the western U.S., use of anesthesia services was less common than in any other geographic area, but was associated with a staggering 60% higher chance of complication within 30 days for patients who did opt for it.
“Although the use of anesthesia agents can directly impact colonoscopy outcomes, it is not solely the anesthesia agent that could lead to additional complications,” the study authors wrote. “In the absence of patient feedback, increased colonic-wall tension from colonoscopy pressure may not be identified by the endoscopist, and, consistent with our results, could lead to increased risks of colonic complications, such as perforation and abdominal pain.”
Dr. Wernli and her coauthors did not report any relevant financial disclosures.
FROM GASTROENTEROLOGY
Key clinical point: Using anesthesia services on individuals receiving colonoscopy increases the overall risk of complications associated with the procedure.
Major finding: Colonoscopy patients who received anesthesia had a 13% higher risk of complication within 30 days, including perforation, hemorrhage, abdominal pain, and stroke.
Data source: A prospective cohort study of claims data from 3,168,228 colonoscopy procedures in the Truven Health MarketScan Research Databases from 2008 to 2011.
Disclosures: Funding provided by the Agency for Healthcare Research and Quality and the National Institutes of Health. Dr. Wernli and her coauthors did not report any relevant financial disclosures.
Zika virus: A primer for clinicians
On February 1, 2016, the World Health Organization declared Zika virus a public health emergency of international concern due to clusters of microcephaly and neurologic manifestations in areas of Zika virus transmission.1 On February 8, the US Centers for Disease Control and Prevention (CDC) elevated its response to level 1, its highest.2
Case reports and guidelines have been published to help clinicians better understand the epidemiology, risk, and pathogenesis of Zika virus infection, but much is still unknown. Clinicians must be ready to address the concerns of international travelers and must also consider Zika virus in the differential diagnosis of fever in the returned traveler.
FLAVIVIRUSES: DENGUE, WEST NILE … ZIKA
Zika virus, a single-stranded RNA arthropod-borne virus (arbovirus), is transmitted by mosquitoes. It is a member of the flavivirus family, which consists of over 70 viruses including some well known for causing diseases in humans, such as dengue, yellow fever, Japanese encephalitis, and West Nile virus.3
Phylogenetically, Zika virus is most similar to and included in a clade with Spondweni virus, which, like Zika, originated in Africa.4 Genomic analysis has revealed an African and an Asian lineage. The Asian lineage is responsible for the current epidemic in the Pacific and the Western Hemisphere.4–6
OUT OF AFRICA AND ASIA
Zika virus is named after a forested area in present-day Uganda, where it was first isolated in a febrile rhesus monkey that was being used to study yellow fever.7 Further studies in the 1950s confirmed its transmission to humans, as 6% of the sera tested in Ugandans showed evidence of specific antibodies to the virus.8 In 1978, antibody prevalence studies showed that up to 40% of Nigerians had Zika virus-neutralizing antibodies.9 Over the next 38 years, scattered case reports and seroprevalence studies showed infections occurring throughout Africa and Asia.9–11
In 2007, the first case of Zika virus transmission outside of Asia and Africa occurred on Yap Island in the Federated States of Micronesia.10–12 No further transmission in the Pacific was noted for 6 years until an outbreak occurred in French Polynesia in 2013.13–15 The first time Zika virus was found in the Western Hemisphere was in January 2014, when an outbreak occurred on Chile’s Easter Island.16 Genomic analysis of the Zika virus isolated on Easter Island indicated it was most closely related to isolates from French Polynesia.16 In 2014, additional cases of Zika virus infection were reported in New Caledonia and the Cook Islands.13,14
In May 2015, the World Health Organization issued an epidemiologic alert in response to dramatic increases in the spread of Zika virus in Brazil.17 From Brazil, Zika virus has rapidly spread to most countries in South and Central America and the Caribbean (Figure 1).2,5,6
TRANSMITTED BY MOSQUITO
The Aedes (Stegomyia) genus of mosquitoes is a well-known source of transmission for several arboviruses, including yellow fever, dengue, chikungunya, and now Zika virus.18,19 Zika virus was originally isolated in Uganda from Aedes africanus mosquitoes.7,20 Subsequently, other species of Aedes mosquitoes have been shown to transmit Zika virus, with Aedes aegypti being the most important human vector.7,8,19–21
Another species, Aedes albopictus has been identified as a human vector in Gabon and is also suspected of being a vector in the Brazilian outbreak.22 Spread of A albopictus from Asia to Europe, the Mediterranean region, and the Americas, including 32 states in the United States, has increased the fear of potential spread of Zika virus infection to a more expansive geographic range.13,18,19 Local transmission may become established if local mosquitoes become infected when infected travelers return from endemic areas.23
OTHER ROUTES OF TRANSMISSION
While mosquito-borne transmission is the most common route of infection with Zika virus, human-to-human transmission has been documented. Potential routes of transmission include sexual intercourse, blood transfusions, and vertical (mother-to-child) transmission.
Sexual transmission. Replicative Zika virus particles were identified in the semen of a patient who presented with hematospermia in French Polynesia.24
Previously, there was a report of Zika virus being sexually transmitted from a US man who had returned from Senegal to his spouse, who had not traveled to a Zika virus-endemic region. Both patients became ill following vaginal intercourse, with the onset of the wife’s illness occurring 5 days after the onset of the husband’s illness. The husband was noted to have hematospermia.25 Neutralization testing for both patients confirmed infection with Zika virus.25
The first reported case of sexual transmission in the current outbreak in the United States occurred in a traveler returning to Texas from Venezuela.26 The CDC is currently investigating several other potential cases and an additional two laboratory-confirmed cases. All cases were in symptomatic male travelers who had condomless vaginal intercourse with their female partners after return from Zika virus-endemic areas.27
Blood transfusions. Several arboviruses are known to be transmitted via blood.
In French Polynesia, Zika virus RNA was present in 3% of blood donors.28,29 These blood donors had been screened and were asymptomatic at the time of donation. Twenty-six percent of donors who had Zika RNA reported an illness compatible with Zika virus infection in the 3 to 10 days before donation.28
Brazil has reported two cases of Zika virus infection through blood transfusion.30
In May 2015, the European Centers for Disease Control recommended that travelers to affected areas defer blood donation for 28 days.31 The Association of American Blood Banks has also recommended that travelers self-defer donating blood for 28 days after travel to an endemic area.32 Most recently the US Food and Drug Administration recommended a 4-week deferral for travelers to Zika virus-endemic areas and after resolution of symptoms for those who have had Zika virus infection.33 Additional guidance for donors who have had sexual contact with Zika virus-infected persons and areas with active transmission of Zika virus is also available.33
Vertical transmission. Perinatal and transplacental transmission have also been documented.34,35 The extent and frequency of the clinical manifestations of these infections are still being elucidated in light of reports of association with fetal abnormalities.
Although Zika virus has been detected in breast milk, no cases of transmission through breastfeeding have been reported. Currently, women are advised to continue to breastfeed in areas of known Zika virus transmission.34,36,37
IS USUALLY ASYMPTOMATIC OR CAUSES MILD SYMPTOMS
Most Zika virus infections are asymptomatic, as illustrated by reports from the Yap Island outbreak, where only 19% of those with immunoglobulin M (IgM) antibodies to Zika virus had symptoms.12 The illness in symptomatic patients is often mild and self-limited, and most manifestations resolve by 7 days.12,25,38,39
Initial descriptions in the 1950s and 1960s of the clinical features of Zika virus infection in Africa included fever and headache as the most prominent symptoms.38,40 Description of the outbreak on Yap in 2007 characterized the predominant symptoms as rash, fever, arthralgia/arthritis, and nonpurulent conjunctivitis in 31 patients,12 and the current CDC case definition includes at least two of these four symptoms.41 The arthralgia and arthritis are usually of the small joints of the hands and feet and can persist for as long as a month.25,42 The rash can be pruritic.15,33,42,43
Less commonly reported manifestations of Zika virus infection include malaise, stomachaches, dizziness, anorexia, retro-orbital pain, aphthous ulcers, hematospermia, and prostatitis.14,15,24,25,44,45
The initial reports from eight patients in the outbreak in Brazil noted rash and joint pain as the most common manifestations. The maculopapular rash was present in all patients and the joint pain was characterized as severe, with the hands, ankles, elbows, knees, and wrists most consistently described.43
The clinical presentation is similar to those of dengue and chikungunya virus infections, confounding diagnosis, as these viruses may be cocirculating in the same geographic regions (and indeed are transmitted by the same mosquito vectors).11,12,15 The conjunctivitis present in Zika virus infections can also be present in chikungunya but is much less commonly a clinical feature of dengue.15,46,47 See Table 1 for the differential diagnosis of Zika virus infection.
Severe manifestations requiring hospitalization or resulting in death are thought to be uncommon, although neurologic and fetal complications have recently been described.12,29,43,48,49
CLINICAL ASSOCIATIONS
Primary infection with Zika virus is relatively benign. The greatest and most recent concerns are related to postinfectious complications and those that may occur in pregnant women.
Guillain-Barré syndrome
During the Zika virus outbreak in French Polynesia in 2013–2014, the incidence of Guillain-Barré syndrome was multiplied by a factor of 20.50 Prior to the first hospitalization of a patient with Zika virus infection and associated Guillain-Barré syndrome in French Polynesia, there had been no reported hospitalizations for Zika virus infection.50
This same association is now being seen in the recent outbreak in the Americas.50 In July 2015, Brazilian health officials in the State of Bahia reported 76 patients with neurologic syndromes, of whom 55% had Guillain-Barré syndrome.51 A history consistent with Zika virus infection was found in 62%.48
In January 2016, El Salvador also reported an unusual increase in Guillain-Barré syndrome cases since early December 2015.51 Between December 1, 2015, and January 6, 2016, there were 46 Guillain-Barré syndrome cases reported, compared with a baseline of 14 cases per month.51
Other countries where Zika virus infection is endemic are also currently investigating similar trends.51
Microcephaly
On November 17, 2015, the Pan American Health Organization issued an epidemiologic alert because of increased reports of microcephaly in the Pernambuco State of Brazil. Whereas there are typically about 10 cases per year, there had been 141 in the previous 11 months.51 Other states in Brazil such as Paraiba and Rio Grande del Norte also reported increases in the diagnosis of microcephaly. A physician alert published in Brazil described two infants from the Paraiba state who were diagnosed with fetal microcephaly.35 Testing for Zika virus by polymerase chain reaction (PCR) was negative in the maternal blood, but PCR of amniotic fluid was positive in both infants.35
In January 2016, the Brazil Ministry of Health reported that Zika virus had been detected by real-time PCR (RT-PCR) in four infants with congenital malformations in Rio Grande del Norte. Two of these cases were miscarriages and two were infants who died within 24 hours of birth. Immunohistochemistry of tissues from these infants was positive for Zika virus.
A February 2016 case report describes a European woman who developed Zika virus infection at 13 weeks gestation while working in Northeast Brazil and upon return to Europe elected to terminate the pregnancy after ultrasonography showed cerebral calcifications with microcephaly. The infant was found to have a very small brain, hypoplasia of the brainstem and spinal cord with degeneration of spinal tracts, complete absence of cerebral gyri, and severe dilatation of lateral ventricles as well as calcifications throughout the cerebral cortex.49 No genetic abnormalities or evidence of other etiologies was found, and large amounts of Zika virus RNA were found in the brain.
The CDC also recently reported confirmation of Zika virus infection from fetal tissues of two miscarriages (fetal loss at 11 and 13 weeks) and two fetal deaths (36 and 38 weeks) received from the state of Rio Grande do Norte in Brazil.52 All four mothers reported clinical signs of fever and rash during their first trimester of pregnancy.52 Additional testing for toxoplasmosis, rubella, cytomegalovirus, herpes simplex, and human immunodeficiency virus were all negative in the mothers who had miscarriages.52
Of critical note, the causality of Zika virus and microcephaly remains under investigation. See Table 2 for other causes of microcephaly.53
Macular atrophy
In January 2016, a case series of three infants with microcephaly and macular atrophy was reported.54 These infants were tested for toxoplasmosis, rubella, cytomegalovirus, herpes simplex, syphilis, and human immunodeficiency virus (HIV), and all the results were negative. The detection of Zika virus fulfilled the Brazilian Ministry of Health’s definition of vertical transmission of Zika virus, and laboratory diagnostic tests for Zika virus were not performed. In this series, one mother reported an illness with rash and arthralgias during the first trimester.54
LABORATORY DIAGNOSTIC METHODS
The diagnosis of Zika virus infection is challenging. The low viremia at initial presentation and cross-reactivity of serologic testing with other flaviviruses, especially dengue, can contribute to misdiagnosis.40,50
In the first 7 days of Zika virus infection, the diagnosis is based on detection of viral RNA in serum by RT-PCR.12,55,56 RT-PCR is very specific for Zika virus and is an important tool in differentiating between Zika virus and other flaviviruses often present in areas where Zika virus is circulating.12,56 After 3 to 4 days, viremia may decrease to levels that may be below the assay’s level of detection.40–42,45
While Zika virus RNA may be undetectable in the serum, other samples such as saliva, urine, and semen may be positive for longer.28,42,57 For example, urine samples were positive by RT-PCR up to 7 days beyond blood RT-PCR in the outbreak in New Caledonia.42 A recent report found semen remaining positive on RT-PCR for 62 days after the onset of confirmed Zika virus illness in a traveler returning to the United Kingdom from the Cook Islands in 2014.58
Because RT-PCR of blood is only useful early in infection, the current diagnostic guidelines recommend testing an acute-phase serum sample for Zika virus IgM collected as early as possible after the onset of illness and repeated 2 to 3 weeks after the initial set. These IgM antibodies typically develop toward the end of the first week of illness and are expected to be present for up to 12 weeks, based on experience with other flaviviruses.41 Cross-reactivity with other flaviviruses circulating in the area can occur and has been problematic in areas where dengue is circulating.12,41,45,56 IgM-positive specimens should be further tested, by plaque-reduction neutralization, to confirm the presence of Zika virus-specific neutralizing antibodies. Results can be difficult to interpret, especially in those who have been previously infected or vaccinated against other flaviviruses.12,41
If amniocentesis is done, these specimens should be tested by RT-PCR. However, the sensitivity of PCR in amniotic fluid is currently unknown.41
In infants with findings of cerebral calcifications and microcephaly, IgM serologies with RT-PCR are also recommended and should be drawn within 2 days of birth. Specimens should be drawn concurrently as it is not known which test is most reliable in infants.23 Additionally, placenta and umbilical cord samples should be collected for immunohistochemical staining at specialized laboratories.36
In the United States, providers should contact their state health departments to determine where tests can be run reliably. Refined diagnostic assays are in development at the time of this publication and are likely to be made available through CDC’s Laboratory Response Network.
See Figure 2 and Table 3 for a summary of diagnostic tests.
IMPLICATIONS, RECOMMENDATIONS
Pregnant women
The CDC now recommends that asymptomatic pregnant women who returned from travel to a Zika virus-endemic zone in the last 2 to 12 weeks be offered serologic testing.41 This includes women who may be living in an area with ongoing Zika virus transmission; however, these women should also have testing at the initiation of prenatal care and then follow-up testing in the middle of the second trimester. Of importance, these results may be difficult to interpret due to potential cross-reactivity between Zika virus and other flaviviruses, and false-positive results in recipients of yellow fever and Japanese encephalitis vaccines.41,59
If a pregnant woman with a positive travel history is symptomatic, testing should be offered during the first week of illness. After day 4 of the illness, testing should include both RT-PCR and IgM serology.41,59
A screening ultrasound scan is recommended for any pregnant woman who has traveled to a Zika virus-affected area to determine if microcephaly or cerebral or intracranial calcifications are present. Those women with confirmed Zika virus infection should continue to have monthly screening ultrasounds, while those who are negative for Zika virus should have another ultrasound at the end of the second trimester or the beginning of the third trimester to ensure that no abnormalities had developed.41,59
At present, pregnant women and women of childbearing age who may become pregnant are advised by the CDC to postpone travel to affected areas until more information becomes available about mother-to-child transmission.59
Algorithms for the care of pregnant women and women of childbearing age who may have been exposed to Zika virus are available from the CDC41 at www.cdc.gov/mmwr/volumes/65/wr/mm6505e2.htm.
Male partners of pregnant women
Since the length of time that Zika virus remains viable in semen is not known, men who have traveled to Zika virus-endemic areas and who have pregnant partners should refrain from having sex or use a condom with every sexual encounter through the duration of the pregnancy.60
Guidelines for prevention of sexual transmission of Zika virus are available from the CDC59 at www.cdc.gov/mmwr/volumes/65/wr/mm6505e1er.htm.
Infants with possible congenital Zika virus infection
Zika virus testing is recommended for any infant born with microcephaly or intracranial calcifications or whose mother has positive or inconclusive testing if the mother had visited an endemic area during her pregnancy.
Zika virus testing in infants consists of serologic IgM determination and RT-PCR for both dengue and Zika virus drawn concurrently in the first 2 days of life.36 Umbilical cord blood can be used. In addition, if cerebrospinal fluid is being collected for other reasons, it can also be tested for Zika virus. The placenta and umbilical cord should be saved for immunohistochemistry testing for Zika virus.61
An infant who tests positive or inconclusive for Zika virus, regardless of the presence of microcephaly or intracranial calcifications, should have a complete physical examination specifically evaluating growth parameters, estimated gestational age, and signs of neurologic disease, skin rashes, hepatosplenomegaly, or any dysmorphic features. Additional evaluation includes an ophthalmologic examination in the first month of life to evaluate for macular atrophy.36 An ultrasound scan of the head should be completed if it has not been done. Hearing is screened in all newborns, and hearing testing should be repeated at 6 months of age.36
Infants with microcephaly or intracranial calcifications should also have consultations with specialists in genetics, neurology, and pediatric infectious diseases.61 These infants should have blood work including complete blood cell counts and liver function testing that includes alanine aminotransferase, aspartate aminotransferase, and bilirubin levels.36
All infants with possible congenital Zika virus infection should be followed long-term with close attention to developmental milestones and growth parameters including occipital frontal head circumference measurements.61,62
Infants without microcephaly or calcifications whose mothers had negative Zika virus test results or were not tested for Zika virus should have routine care.37
Guidelines for the care of infants with Zika virus infection are available from the CDC36 at www.cdc.gov/mmwr/volumes/65/wr/mm6503e3.htm.
TREATMENT
There is no treatment for Zika virus infection, and care is supportive. Most infections are mild and self-limited.12,15 Avoidance of aspirin and other nonsteroidal anti-inflammatory drugs that may affect platelets is important until dengue infection has been ruled out.
PREVENTION
There is currently no vaccine to prevent Zika virus infection. Woman who are pregnant should avoid travel to any area where Zika virus transmission is occurring.41,59 The CDC advises pregnant women and women of childbearing age who may become pregnant to postpone travel to Zika virus-affected areas.59 Patients can find travel alerts for specific areas at wwwnc.cdc.gov/travel/notices/alert/zika-virus-south-america.
Avoiding mosquito bites is the best way to prevent the spread of Zika virus. Aedes aegypti and A albopictus, the most common vectors of Zika virus, can bite at night but are known more for being aggressive daytime biters.63 Travelers should apply an Environmental Protection Agency-registered insect repellent as directed, wear long-sleeved shirts and long pants, use permethrin-treated clothing and gear, and stay in places with screens or air conditioning. Any containers with standing water should be eliminated as they are breeding areas for mosquitoes. It is also important that symptomatic people in the first week of illness use mosquito precautions to prevent the spread of Zika virus.
Patient handouts and posters for mosquito bite prevention can be found at www.cdc.gov/zika/fs-posters/index.html.
WATCH FOR UPDATES
Many questions remain regarding the epidemiology of this infection and its relationship to neurologic and pregnancy complications. However, due to its rapid spread across the Western hemisphere and its potential for significant complications, much is being done at the local and international levels to better understand the virus and halt its spread. More information will continue to be available as results from ongoing studies are conducted and potential associations are investigated. Until more is known, providers should familiarize themselves with the latest guidelines in order to better counsel their patients who may live in or travel to Zika virus endemic areas. We advise clinicians to follow the CDC’s web site, www.cdc.gov/zika/.
- World Health Organization. Zika virus fact sheet. www.who.int/mediacentre/factsheets/zika/en/. Accessed February 24, 2016.
- Centers for Disease Control and Prevention. Areas with Zika. www.cdc.gov/zika/geo/index.html. Accessed February 24, 2016.
- Rice CM. Flaviviruses. In: Fields BN, Knipe DM, Howley PM, Chanock RM, editors. Fields Virology, 3rd ed. Philadelphia: Lippincott-Raven, 1996:961–1034.
- Kuno G, Chang GJ, Tsuchiya KR, Karabatsos N, Cropp CB. Phylogeny of the genus Flavivirus. J Virol 1998; 72:73–83.
- Haddow AD, Schuh AJ, Yasuda CY, et al. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis 2012; 6:e1477.
- Faye O, Freire CC, Iamarino A, et al. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis 2014; 8:e2636.
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952; 46:509–520.
- Dick GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg 1952; 46:521–534.
- Fagbami AH. Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State. J Hyg (Lond) 1979; 83:213–219.
- Hayes EB. Zika virus outside Africa. Emerg Infect Dis 2009; 15:1347–1350.
- Heang V, Yasuda CY, Sovann L, et al. Zika virus infection, Cambodia, 2010. Emerg Infect Dis 2012; 18:349–351.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360:2536–2543.
- Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infec 2014; 20:O595–O596.
- Cao-Lormeau VM, Roche C, Teissier A, et al. Zika virus, French polynesia, South Pacific, 2013. Emerg Infect Dis 2014; 20:1085–1086.
- Ioos S, Mallet HP, Leparc Goffart I, Gauthier V, Cardoso T, Herida M. Current Zika virus epidemiology and recent epidemics. Med Mal Infect 2014; 44:302–307.
- Tognarelli J, Ulloa S, Villagra E, et al. A report on the outbreak of Zika virus on Easter Island, South Pacific, 2014. Arch Virol Nov 26 2015 [Epub ahead of print].
- Pan American Health Organization/World Health Organization, Regional Office for the Americas. Zika virus infection. 7 May 2015. www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=30075=en. Accessed February 24, 2016.
- Fauci AS, Morens DM. Zika virus in the Americas—yet another arbovirus threat. N Engl J Med 2016; 347:601–604.
- Marcondes CB, Ximenes MF. Zika virus in Brazil and the danger of infestation by Aedes (Stegomyia) mosquitoes. Rev Soc Bras Med Trop. Dec 22 2015. pii: S0037-86822015005003102. [Epub ahead of print]
- Weinbren MP, Williams MC. Zika virus: further isolations in the Zika area, and some studies on the strains isolated. Trans R Soc Trop Med Hyg 1958; 52:263–268.
- Diallo D, Sall AA, Diagne CT, et al. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS One 2014; 9:e109442.
- Grard G, Caron M, Mombo IM, et al. Zika virus in Gabon (Central Africa)—2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis 2014; 8:e2681.
- Hennessey M, Fischer M, Staples JE. Zika virus spreads to new areas—region of the Americas, May 2015–January 2016. MMWR 2016; 65:55–58.
- Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis 2015; 21:359–361.
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 2011; 17:880–882.
- Smith J, Woldai S, Chung W. Health advisory: sexual transmission of Zika virus. Dallas Country Department of Health and Human Services, February 2, 2016. http://walnuthillobgyn.com/wp-content/uploads/2012/05/zika-transmission.pdf. Accessed February 24, 2016.
- Hills SL, Russell K, Hennessey M, et al. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission—continental United States, 2016. MMWR Early release February 26, 2016. www.cdc.gov/mmwr/volumes/65/wr/mm6508e2er.htm Accessed February 29, 2016.
- Musso D, Nhan T, Robin E, et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill 2014; 19(14). pii: 20761. Erratum in Euro Surveill 2014; 19(15). pii/20771.
- Marano G, Pupella S, Vaglio S, Liumbruno GM, Grazzini G. Zika virus and the never-ending story of emerging pathogens and transfusion medicine. Blood Transfus 2015; Nov 5:1–6. doi: 10.2450/2015.0066-15. [Epub ahead of print]
- European Centre for Disease Prevention and Control. Epidemiological update: complications potentially linked to Zika virus outbreak, Brazil and French Polynesia. November 27, 2015. http://ecdc.europa.eu/en/press/news/_layouts/forms/News_DispForm.aspx?ID=1332&List=8db7286c-fe2d-476c-9133-18ff4cb1b568&Source=http%3A%2F%2Fecdc%2Eeuropa%2Eeu%2Fen%2Fpress%2Fepidemiological%5Fupdates%2FPages%2Fepidemiological%5Fupdates%2Easpx. Accessed February 24, 2016
- European Centre for Disease Prevention and Control. Rapid risk assessment. Zika virus infection outbreak, Brazil and the Pacific region 25 May 2015. http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-Zika%20virus-south-america-Brazil-2015.pdf. Accessed February 24, 2016
- Regan DM, Markowitz MA. Association Bulletin #16-03. Re: Zika, dengue, and chikungunya viruses. American Association of Blood Banks, February 1, 2016. www.aabb.org/programs/publications/bulletins/Documents/ab16-03.pdf. Accessed February 24, 2016.
- US Food and Drug Administration (FDA). Recommendations for donor screening, deferral, and product management to reduce the risk of transfusion-transmission of Zika virus. Guidance for industry. February, 2016. www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/UCM486360.pdf. Accessed February 24, 2016.
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill 2014; 19(13). pii: 20751.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol 2016; 47:6–7.
- Staples JE, Dziuban EJ, Fischer M, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection—United States, 2016. MMWR 2016; 65:63–67.
- Fleming-Dutra K, Nelson J, Fischer M, Staples J, Mateusz P, et al. Update: interim guidelines for health care providers caring for infants and children with possible Zika virus infection—United States, February 2016. MMWR 2016; 65:1–6.
- Simpson DI. Zika virus infection in man. Trans R Soc Trop Med Hyg Jul 1964; 58:335–338.
- Olson JG, Ksiazek TG, Suhandiman, Triwibowo. Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg 1981; 75:389–393.
- Bearcroft WG. Zika virus infection experimentally induced in a human volunteer. Trans R Soc Trop Med Hyg 1956; 50:442–448.
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR 2016; 65:122–127.
- Gourinat AC, O’Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis 2015; 21:84–86.
- Zanluca C, de Melo VC, Mosimann AL, Dos Santos GI, Dos Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz 2015; 110:569–572.
- Alera MT, Hermann L, Tac-An IA, et al. Zika virus infection, Philippines, 2012. Emerg Infect Dis 2015; 21:722–724.
- Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14:1232–1239.
- Centers for Disease Control and Prevention. Chikungunya virus. Clinical evaluation & disease. www.cdc.gov/chikungunya/hc/clinicalevaluation.html. Accessed February 24, 2016.
- Centers for Disease Control and Prevention. Clinical guidance. Dengue virus. www.cdc.gov/dengue/clinicalLab/clinical.html. Accessed February 24, 2016.
- Pan American Health Organization/World Health Organization. Epidemiological alert. Increase in microcephaly in the northeast of Brazil. November 17, 2015. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32636&lang=en. Accessed February 24, 2016.
- Rubin EJ, Greene MF, Baden LR. Zika virus and microcephaly. N Engl J Med 2016; Feb 10 [Epub ahead of print].
- Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barré syndrome—case report, French Polynesia, December 2013. Euro Surveill 2014; 19(9). pii: 20720.
- Pan American Health Organization/World Health Organization. Epidemiological alert. Neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. December 1, 2015. www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32405&lang=en. Accessed February 24, 2016.
- Martines R, Bhatnagar J, Keating M, et al. Notes from the field: evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses—Brazil, 2015. MMRW 2016; 65:159–160.
- Ashwal S, Michelson D, Plawner L, Dobyns WB; Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Practice parameter: evaluation of the child with microcephaly (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2009; 73:887–897.
- Ventura CV, Maia M, Bravo-Filho V, Góis AL, Belfort R Jr. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet 2016; 387:228.
- Centers for Disease Control and Prevention. Updated diagnostic testing for Zika, chikungunya, and dengue viruses in US Public Health Laboratories. http://stacks.cdc.gov/view/cdc/37594. Accessed February 24, 2016.
- Faye O, Faye O, Diallo D, Diallo M, Weidmann M, Sall AA. Quantitative real-time PCR detection of Zika virus and evaluation with field-caught mosquitoes. Virol J 2013; 10:311.
- Musso D, Roche C, Nhan TX, Robin E, Teissier A, Cao-Lormeau VM. Detection of Zika virus in saliva. J Clin Virol 2015; 68:53–55.
- Atkinson B, Hearn P, Afrough B, et al. Detection of Zika virus in semen [letter]. Emerg Infect Dis 2016 May. http://wwwnc.cdc.gov/eid/article/22/5/16-0107_article. Accessed February 24, 2016.
- Petersen EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR 2016; 65:30–33.
- Oster AM, Brooks JT, Stryker JE, et al. Interim guidelines for prevention of sexual transmission of Zika virus—United States, 2016. MMWR 2016; 65:120–121.
- Staples JE, Dziuban EJ, Fischer M, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection—United States, 2016. MMWR 2016; 65:63–67.
- Centers for Disease Control and Prevention. Zika virus clinical evaluation and disease. www.cdc.gov/zika/hc-providers/clinicalevaluation.html. Accessed February 24, 2016.
- Centers for Disease Control and Prevention. Zika virus. Transmission & risks. www.cdc.gov/zika/transmission/index.html. Accessed February 29, 2016.
On February 1, 2016, the World Health Organization declared Zika virus a public health emergency of international concern due to clusters of microcephaly and neurologic manifestations in areas of Zika virus transmission.1 On February 8, the US Centers for Disease Control and Prevention (CDC) elevated its response to level 1, its highest.2
Case reports and guidelines have been published to help clinicians better understand the epidemiology, risk, and pathogenesis of Zika virus infection, but much is still unknown. Clinicians must be ready to address the concerns of international travelers and must also consider Zika virus in the differential diagnosis of fever in the returned traveler.
FLAVIVIRUSES: DENGUE, WEST NILE … ZIKA
Zika virus, a single-stranded RNA arthropod-borne virus (arbovirus), is transmitted by mosquitoes. It is a member of the flavivirus family, which consists of over 70 viruses including some well known for causing diseases in humans, such as dengue, yellow fever, Japanese encephalitis, and West Nile virus.3
Phylogenetically, Zika virus is most similar to and included in a clade with Spondweni virus, which, like Zika, originated in Africa.4 Genomic analysis has revealed an African and an Asian lineage. The Asian lineage is responsible for the current epidemic in the Pacific and the Western Hemisphere.4–6
OUT OF AFRICA AND ASIA
Zika virus is named after a forested area in present-day Uganda, where it was first isolated in a febrile rhesus monkey that was being used to study yellow fever.7 Further studies in the 1950s confirmed its transmission to humans, as 6% of the sera tested in Ugandans showed evidence of specific antibodies to the virus.8 In 1978, antibody prevalence studies showed that up to 40% of Nigerians had Zika virus-neutralizing antibodies.9 Over the next 38 years, scattered case reports and seroprevalence studies showed infections occurring throughout Africa and Asia.9–11
In 2007, the first case of Zika virus transmission outside of Asia and Africa occurred on Yap Island in the Federated States of Micronesia.10–12 No further transmission in the Pacific was noted for 6 years until an outbreak occurred in French Polynesia in 2013.13–15 The first time Zika virus was found in the Western Hemisphere was in January 2014, when an outbreak occurred on Chile’s Easter Island.16 Genomic analysis of the Zika virus isolated on Easter Island indicated it was most closely related to isolates from French Polynesia.16 In 2014, additional cases of Zika virus infection were reported in New Caledonia and the Cook Islands.13,14
In May 2015, the World Health Organization issued an epidemiologic alert in response to dramatic increases in the spread of Zika virus in Brazil.17 From Brazil, Zika virus has rapidly spread to most countries in South and Central America and the Caribbean (Figure 1).2,5,6
TRANSMITTED BY MOSQUITO
The Aedes (Stegomyia) genus of mosquitoes is a well-known source of transmission for several arboviruses, including yellow fever, dengue, chikungunya, and now Zika virus.18,19 Zika virus was originally isolated in Uganda from Aedes africanus mosquitoes.7,20 Subsequently, other species of Aedes mosquitoes have been shown to transmit Zika virus, with Aedes aegypti being the most important human vector.7,8,19–21
Another species, Aedes albopictus has been identified as a human vector in Gabon and is also suspected of being a vector in the Brazilian outbreak.22 Spread of A albopictus from Asia to Europe, the Mediterranean region, and the Americas, including 32 states in the United States, has increased the fear of potential spread of Zika virus infection to a more expansive geographic range.13,18,19 Local transmission may become established if local mosquitoes become infected when infected travelers return from endemic areas.23
OTHER ROUTES OF TRANSMISSION
While mosquito-borne transmission is the most common route of infection with Zika virus, human-to-human transmission has been documented. Potential routes of transmission include sexual intercourse, blood transfusions, and vertical (mother-to-child) transmission.
Sexual transmission. Replicative Zika virus particles were identified in the semen of a patient who presented with hematospermia in French Polynesia.24
Previously, there was a report of Zika virus being sexually transmitted from a US man who had returned from Senegal to his spouse, who had not traveled to a Zika virus-endemic region. Both patients became ill following vaginal intercourse, with the onset of the wife’s illness occurring 5 days after the onset of the husband’s illness. The husband was noted to have hematospermia.25 Neutralization testing for both patients confirmed infection with Zika virus.25
The first reported case of sexual transmission in the current outbreak in the United States occurred in a traveler returning to Texas from Venezuela.26 The CDC is currently investigating several other potential cases and an additional two laboratory-confirmed cases. All cases were in symptomatic male travelers who had condomless vaginal intercourse with their female partners after return from Zika virus-endemic areas.27
Blood transfusions. Several arboviruses are known to be transmitted via blood.
In French Polynesia, Zika virus RNA was present in 3% of blood donors.28,29 These blood donors had been screened and were asymptomatic at the time of donation. Twenty-six percent of donors who had Zika RNA reported an illness compatible with Zika virus infection in the 3 to 10 days before donation.28
Brazil has reported two cases of Zika virus infection through blood transfusion.30
In May 2015, the European Centers for Disease Control recommended that travelers to affected areas defer blood donation for 28 days.31 The Association of American Blood Banks has also recommended that travelers self-defer donating blood for 28 days after travel to an endemic area.32 Most recently the US Food and Drug Administration recommended a 4-week deferral for travelers to Zika virus-endemic areas and after resolution of symptoms for those who have had Zika virus infection.33 Additional guidance for donors who have had sexual contact with Zika virus-infected persons and areas with active transmission of Zika virus is also available.33
Vertical transmission. Perinatal and transplacental transmission have also been documented.34,35 The extent and frequency of the clinical manifestations of these infections are still being elucidated in light of reports of association with fetal abnormalities.
Although Zika virus has been detected in breast milk, no cases of transmission through breastfeeding have been reported. Currently, women are advised to continue to breastfeed in areas of known Zika virus transmission.34,36,37
IS USUALLY ASYMPTOMATIC OR CAUSES MILD SYMPTOMS
Most Zika virus infections are asymptomatic, as illustrated by reports from the Yap Island outbreak, where only 19% of those with immunoglobulin M (IgM) antibodies to Zika virus had symptoms.12 The illness in symptomatic patients is often mild and self-limited, and most manifestations resolve by 7 days.12,25,38,39
Initial descriptions in the 1950s and 1960s of the clinical features of Zika virus infection in Africa included fever and headache as the most prominent symptoms.38,40 Description of the outbreak on Yap in 2007 characterized the predominant symptoms as rash, fever, arthralgia/arthritis, and nonpurulent conjunctivitis in 31 patients,12 and the current CDC case definition includes at least two of these four symptoms.41 The arthralgia and arthritis are usually of the small joints of the hands and feet and can persist for as long as a month.25,42 The rash can be pruritic.15,33,42,43
Less commonly reported manifestations of Zika virus infection include malaise, stomachaches, dizziness, anorexia, retro-orbital pain, aphthous ulcers, hematospermia, and prostatitis.14,15,24,25,44,45
The initial reports from eight patients in the outbreak in Brazil noted rash and joint pain as the most common manifestations. The maculopapular rash was present in all patients and the joint pain was characterized as severe, with the hands, ankles, elbows, knees, and wrists most consistently described.43
The clinical presentation is similar to those of dengue and chikungunya virus infections, confounding diagnosis, as these viruses may be cocirculating in the same geographic regions (and indeed are transmitted by the same mosquito vectors).11,12,15 The conjunctivitis present in Zika virus infections can also be present in chikungunya but is much less commonly a clinical feature of dengue.15,46,47 See Table 1 for the differential diagnosis of Zika virus infection.
Severe manifestations requiring hospitalization or resulting in death are thought to be uncommon, although neurologic and fetal complications have recently been described.12,29,43,48,49
CLINICAL ASSOCIATIONS
Primary infection with Zika virus is relatively benign. The greatest and most recent concerns are related to postinfectious complications and those that may occur in pregnant women.
Guillain-Barré syndrome
During the Zika virus outbreak in French Polynesia in 2013–2014, the incidence of Guillain-Barré syndrome was multiplied by a factor of 20.50 Prior to the first hospitalization of a patient with Zika virus infection and associated Guillain-Barré syndrome in French Polynesia, there had been no reported hospitalizations for Zika virus infection.50
This same association is now being seen in the recent outbreak in the Americas.50 In July 2015, Brazilian health officials in the State of Bahia reported 76 patients with neurologic syndromes, of whom 55% had Guillain-Barré syndrome.51 A history consistent with Zika virus infection was found in 62%.48
In January 2016, El Salvador also reported an unusual increase in Guillain-Barré syndrome cases since early December 2015.51 Between December 1, 2015, and January 6, 2016, there were 46 Guillain-Barré syndrome cases reported, compared with a baseline of 14 cases per month.51
Other countries where Zika virus infection is endemic are also currently investigating similar trends.51
Microcephaly
On November 17, 2015, the Pan American Health Organization issued an epidemiologic alert because of increased reports of microcephaly in the Pernambuco State of Brazil. Whereas there are typically about 10 cases per year, there had been 141 in the previous 11 months.51 Other states in Brazil such as Paraiba and Rio Grande del Norte also reported increases in the diagnosis of microcephaly. A physician alert published in Brazil described two infants from the Paraiba state who were diagnosed with fetal microcephaly.35 Testing for Zika virus by polymerase chain reaction (PCR) was negative in the maternal blood, but PCR of amniotic fluid was positive in both infants.35
In January 2016, the Brazil Ministry of Health reported that Zika virus had been detected by real-time PCR (RT-PCR) in four infants with congenital malformations in Rio Grande del Norte. Two of these cases were miscarriages and two were infants who died within 24 hours of birth. Immunohistochemistry of tissues from these infants was positive for Zika virus.
A February 2016 case report describes a European woman who developed Zika virus infection at 13 weeks gestation while working in Northeast Brazil and upon return to Europe elected to terminate the pregnancy after ultrasonography showed cerebral calcifications with microcephaly. The infant was found to have a very small brain, hypoplasia of the brainstem and spinal cord with degeneration of spinal tracts, complete absence of cerebral gyri, and severe dilatation of lateral ventricles as well as calcifications throughout the cerebral cortex.49 No genetic abnormalities or evidence of other etiologies was found, and large amounts of Zika virus RNA were found in the brain.
The CDC also recently reported confirmation of Zika virus infection from fetal tissues of two miscarriages (fetal loss at 11 and 13 weeks) and two fetal deaths (36 and 38 weeks) received from the state of Rio Grande do Norte in Brazil.52 All four mothers reported clinical signs of fever and rash during their first trimester of pregnancy.52 Additional testing for toxoplasmosis, rubella, cytomegalovirus, herpes simplex, and human immunodeficiency virus were all negative in the mothers who had miscarriages.52
Of critical note, the causality of Zika virus and microcephaly remains under investigation. See Table 2 for other causes of microcephaly.53
Macular atrophy
In January 2016, a case series of three infants with microcephaly and macular atrophy was reported.54 These infants were tested for toxoplasmosis, rubella, cytomegalovirus, herpes simplex, syphilis, and human immunodeficiency virus (HIV), and all the results were negative. The detection of Zika virus fulfilled the Brazilian Ministry of Health’s definition of vertical transmission of Zika virus, and laboratory diagnostic tests for Zika virus were not performed. In this series, one mother reported an illness with rash and arthralgias during the first trimester.54
LABORATORY DIAGNOSTIC METHODS
The diagnosis of Zika virus infection is challenging. The low viremia at initial presentation and cross-reactivity of serologic testing with other flaviviruses, especially dengue, can contribute to misdiagnosis.40,50
In the first 7 days of Zika virus infection, the diagnosis is based on detection of viral RNA in serum by RT-PCR.12,55,56 RT-PCR is very specific for Zika virus and is an important tool in differentiating between Zika virus and other flaviviruses often present in areas where Zika virus is circulating.12,56 After 3 to 4 days, viremia may decrease to levels that may be below the assay’s level of detection.40–42,45
While Zika virus RNA may be undetectable in the serum, other samples such as saliva, urine, and semen may be positive for longer.28,42,57 For example, urine samples were positive by RT-PCR up to 7 days beyond blood RT-PCR in the outbreak in New Caledonia.42 A recent report found semen remaining positive on RT-PCR for 62 days after the onset of confirmed Zika virus illness in a traveler returning to the United Kingdom from the Cook Islands in 2014.58
Because RT-PCR of blood is only useful early in infection, the current diagnostic guidelines recommend testing an acute-phase serum sample for Zika virus IgM collected as early as possible after the onset of illness and repeated 2 to 3 weeks after the initial set. These IgM antibodies typically develop toward the end of the first week of illness and are expected to be present for up to 12 weeks, based on experience with other flaviviruses.41 Cross-reactivity with other flaviviruses circulating in the area can occur and has been problematic in areas where dengue is circulating.12,41,45,56 IgM-positive specimens should be further tested, by plaque-reduction neutralization, to confirm the presence of Zika virus-specific neutralizing antibodies. Results can be difficult to interpret, especially in those who have been previously infected or vaccinated against other flaviviruses.12,41
If amniocentesis is done, these specimens should be tested by RT-PCR. However, the sensitivity of PCR in amniotic fluid is currently unknown.41
In infants with findings of cerebral calcifications and microcephaly, IgM serologies with RT-PCR are also recommended and should be drawn within 2 days of birth. Specimens should be drawn concurrently as it is not known which test is most reliable in infants.23 Additionally, placenta and umbilical cord samples should be collected for immunohistochemical staining at specialized laboratories.36
In the United States, providers should contact their state health departments to determine where tests can be run reliably. Refined diagnostic assays are in development at the time of this publication and are likely to be made available through CDC’s Laboratory Response Network.
See Figure 2 and Table 3 for a summary of diagnostic tests.
IMPLICATIONS, RECOMMENDATIONS
Pregnant women
The CDC now recommends that asymptomatic pregnant women who returned from travel to a Zika virus-endemic zone in the last 2 to 12 weeks be offered serologic testing.41 This includes women who may be living in an area with ongoing Zika virus transmission; however, these women should also have testing at the initiation of prenatal care and then follow-up testing in the middle of the second trimester. Of importance, these results may be difficult to interpret due to potential cross-reactivity between Zika virus and other flaviviruses, and false-positive results in recipients of yellow fever and Japanese encephalitis vaccines.41,59
If a pregnant woman with a positive travel history is symptomatic, testing should be offered during the first week of illness. After day 4 of the illness, testing should include both RT-PCR and IgM serology.41,59
A screening ultrasound scan is recommended for any pregnant woman who has traveled to a Zika virus-affected area to determine if microcephaly or cerebral or intracranial calcifications are present. Those women with confirmed Zika virus infection should continue to have monthly screening ultrasounds, while those who are negative for Zika virus should have another ultrasound at the end of the second trimester or the beginning of the third trimester to ensure that no abnormalities had developed.41,59
At present, pregnant women and women of childbearing age who may become pregnant are advised by the CDC to postpone travel to affected areas until more information becomes available about mother-to-child transmission.59
Algorithms for the care of pregnant women and women of childbearing age who may have been exposed to Zika virus are available from the CDC41 at www.cdc.gov/mmwr/volumes/65/wr/mm6505e2.htm.
Male partners of pregnant women
Since the length of time that Zika virus remains viable in semen is not known, men who have traveled to Zika virus-endemic areas and who have pregnant partners should refrain from having sex or use a condom with every sexual encounter through the duration of the pregnancy.60
Guidelines for prevention of sexual transmission of Zika virus are available from the CDC59 at www.cdc.gov/mmwr/volumes/65/wr/mm6505e1er.htm.
Infants with possible congenital Zika virus infection
Zika virus testing is recommended for any infant born with microcephaly or intracranial calcifications or whose mother has positive or inconclusive testing if the mother had visited an endemic area during her pregnancy.
Zika virus testing in infants consists of serologic IgM determination and RT-PCR for both dengue and Zika virus drawn concurrently in the first 2 days of life.36 Umbilical cord blood can be used. In addition, if cerebrospinal fluid is being collected for other reasons, it can also be tested for Zika virus. The placenta and umbilical cord should be saved for immunohistochemistry testing for Zika virus.61
An infant who tests positive or inconclusive for Zika virus, regardless of the presence of microcephaly or intracranial calcifications, should have a complete physical examination specifically evaluating growth parameters, estimated gestational age, and signs of neurologic disease, skin rashes, hepatosplenomegaly, or any dysmorphic features. Additional evaluation includes an ophthalmologic examination in the first month of life to evaluate for macular atrophy.36 An ultrasound scan of the head should be completed if it has not been done. Hearing is screened in all newborns, and hearing testing should be repeated at 6 months of age.36
Infants with microcephaly or intracranial calcifications should also have consultations with specialists in genetics, neurology, and pediatric infectious diseases.61 These infants should have blood work including complete blood cell counts and liver function testing that includes alanine aminotransferase, aspartate aminotransferase, and bilirubin levels.36
All infants with possible congenital Zika virus infection should be followed long-term with close attention to developmental milestones and growth parameters including occipital frontal head circumference measurements.61,62
Infants without microcephaly or calcifications whose mothers had negative Zika virus test results or were not tested for Zika virus should have routine care.37
Guidelines for the care of infants with Zika virus infection are available from the CDC36 at www.cdc.gov/mmwr/volumes/65/wr/mm6503e3.htm.
TREATMENT
There is no treatment for Zika virus infection, and care is supportive. Most infections are mild and self-limited.12,15 Avoidance of aspirin and other nonsteroidal anti-inflammatory drugs that may affect platelets is important until dengue infection has been ruled out.
PREVENTION
There is currently no vaccine to prevent Zika virus infection. Woman who are pregnant should avoid travel to any area where Zika virus transmission is occurring.41,59 The CDC advises pregnant women and women of childbearing age who may become pregnant to postpone travel to Zika virus-affected areas.59 Patients can find travel alerts for specific areas at wwwnc.cdc.gov/travel/notices/alert/zika-virus-south-america.
Avoiding mosquito bites is the best way to prevent the spread of Zika virus. Aedes aegypti and A albopictus, the most common vectors of Zika virus, can bite at night but are known more for being aggressive daytime biters.63 Travelers should apply an Environmental Protection Agency-registered insect repellent as directed, wear long-sleeved shirts and long pants, use permethrin-treated clothing and gear, and stay in places with screens or air conditioning. Any containers with standing water should be eliminated as they are breeding areas for mosquitoes. It is also important that symptomatic people in the first week of illness use mosquito precautions to prevent the spread of Zika virus.
Patient handouts and posters for mosquito bite prevention can be found at www.cdc.gov/zika/fs-posters/index.html.
WATCH FOR UPDATES
Many questions remain regarding the epidemiology of this infection and its relationship to neurologic and pregnancy complications. However, due to its rapid spread across the Western hemisphere and its potential for significant complications, much is being done at the local and international levels to better understand the virus and halt its spread. More information will continue to be available as results from ongoing studies are conducted and potential associations are investigated. Until more is known, providers should familiarize themselves with the latest guidelines in order to better counsel their patients who may live in or travel to Zika virus endemic areas. We advise clinicians to follow the CDC’s web site, www.cdc.gov/zika/.
On February 1, 2016, the World Health Organization declared Zika virus a public health emergency of international concern due to clusters of microcephaly and neurologic manifestations in areas of Zika virus transmission.1 On February 8, the US Centers for Disease Control and Prevention (CDC) elevated its response to level 1, its highest.2
Case reports and guidelines have been published to help clinicians better understand the epidemiology, risk, and pathogenesis of Zika virus infection, but much is still unknown. Clinicians must be ready to address the concerns of international travelers and must also consider Zika virus in the differential diagnosis of fever in the returned traveler.
FLAVIVIRUSES: DENGUE, WEST NILE … ZIKA
Zika virus, a single-stranded RNA arthropod-borne virus (arbovirus), is transmitted by mosquitoes. It is a member of the flavivirus family, which consists of over 70 viruses including some well known for causing diseases in humans, such as dengue, yellow fever, Japanese encephalitis, and West Nile virus.3
Phylogenetically, Zika virus is most similar to and included in a clade with Spondweni virus, which, like Zika, originated in Africa.4 Genomic analysis has revealed an African and an Asian lineage. The Asian lineage is responsible for the current epidemic in the Pacific and the Western Hemisphere.4–6
OUT OF AFRICA AND ASIA
Zika virus is named after a forested area in present-day Uganda, where it was first isolated in a febrile rhesus monkey that was being used to study yellow fever.7 Further studies in the 1950s confirmed its transmission to humans, as 6% of the sera tested in Ugandans showed evidence of specific antibodies to the virus.8 In 1978, antibody prevalence studies showed that up to 40% of Nigerians had Zika virus-neutralizing antibodies.9 Over the next 38 years, scattered case reports and seroprevalence studies showed infections occurring throughout Africa and Asia.9–11
In 2007, the first case of Zika virus transmission outside of Asia and Africa occurred on Yap Island in the Federated States of Micronesia.10–12 No further transmission in the Pacific was noted for 6 years until an outbreak occurred in French Polynesia in 2013.13–15 The first time Zika virus was found in the Western Hemisphere was in January 2014, when an outbreak occurred on Chile’s Easter Island.16 Genomic analysis of the Zika virus isolated on Easter Island indicated it was most closely related to isolates from French Polynesia.16 In 2014, additional cases of Zika virus infection were reported in New Caledonia and the Cook Islands.13,14
In May 2015, the World Health Organization issued an epidemiologic alert in response to dramatic increases in the spread of Zika virus in Brazil.17 From Brazil, Zika virus has rapidly spread to most countries in South and Central America and the Caribbean (Figure 1).2,5,6
TRANSMITTED BY MOSQUITO
The Aedes (Stegomyia) genus of mosquitoes is a well-known source of transmission for several arboviruses, including yellow fever, dengue, chikungunya, and now Zika virus.18,19 Zika virus was originally isolated in Uganda from Aedes africanus mosquitoes.7,20 Subsequently, other species of Aedes mosquitoes have been shown to transmit Zika virus, with Aedes aegypti being the most important human vector.7,8,19–21
Another species, Aedes albopictus has been identified as a human vector in Gabon and is also suspected of being a vector in the Brazilian outbreak.22 Spread of A albopictus from Asia to Europe, the Mediterranean region, and the Americas, including 32 states in the United States, has increased the fear of potential spread of Zika virus infection to a more expansive geographic range.13,18,19 Local transmission may become established if local mosquitoes become infected when infected travelers return from endemic areas.23
OTHER ROUTES OF TRANSMISSION
While mosquito-borne transmission is the most common route of infection with Zika virus, human-to-human transmission has been documented. Potential routes of transmission include sexual intercourse, blood transfusions, and vertical (mother-to-child) transmission.
Sexual transmission. Replicative Zika virus particles were identified in the semen of a patient who presented with hematospermia in French Polynesia.24
Previously, there was a report of Zika virus being sexually transmitted from a US man who had returned from Senegal to his spouse, who had not traveled to a Zika virus-endemic region. Both patients became ill following vaginal intercourse, with the onset of the wife’s illness occurring 5 days after the onset of the husband’s illness. The husband was noted to have hematospermia.25 Neutralization testing for both patients confirmed infection with Zika virus.25
The first reported case of sexual transmission in the current outbreak in the United States occurred in a traveler returning to Texas from Venezuela.26 The CDC is currently investigating several other potential cases and an additional two laboratory-confirmed cases. All cases were in symptomatic male travelers who had condomless vaginal intercourse with their female partners after return from Zika virus-endemic areas.27
Blood transfusions. Several arboviruses are known to be transmitted via blood.
In French Polynesia, Zika virus RNA was present in 3% of blood donors.28,29 These blood donors had been screened and were asymptomatic at the time of donation. Twenty-six percent of donors who had Zika RNA reported an illness compatible with Zika virus infection in the 3 to 10 days before donation.28
Brazil has reported two cases of Zika virus infection through blood transfusion.30
In May 2015, the European Centers for Disease Control recommended that travelers to affected areas defer blood donation for 28 days.31 The Association of American Blood Banks has also recommended that travelers self-defer donating blood for 28 days after travel to an endemic area.32 Most recently the US Food and Drug Administration recommended a 4-week deferral for travelers to Zika virus-endemic areas and after resolution of symptoms for those who have had Zika virus infection.33 Additional guidance for donors who have had sexual contact with Zika virus-infected persons and areas with active transmission of Zika virus is also available.33
Vertical transmission. Perinatal and transplacental transmission have also been documented.34,35 The extent and frequency of the clinical manifestations of these infections are still being elucidated in light of reports of association with fetal abnormalities.
Although Zika virus has been detected in breast milk, no cases of transmission through breastfeeding have been reported. Currently, women are advised to continue to breastfeed in areas of known Zika virus transmission.34,36,37
IS USUALLY ASYMPTOMATIC OR CAUSES MILD SYMPTOMS
Most Zika virus infections are asymptomatic, as illustrated by reports from the Yap Island outbreak, where only 19% of those with immunoglobulin M (IgM) antibodies to Zika virus had symptoms.12 The illness in symptomatic patients is often mild and self-limited, and most manifestations resolve by 7 days.12,25,38,39
Initial descriptions in the 1950s and 1960s of the clinical features of Zika virus infection in Africa included fever and headache as the most prominent symptoms.38,40 Description of the outbreak on Yap in 2007 characterized the predominant symptoms as rash, fever, arthralgia/arthritis, and nonpurulent conjunctivitis in 31 patients,12 and the current CDC case definition includes at least two of these four symptoms.41 The arthralgia and arthritis are usually of the small joints of the hands and feet and can persist for as long as a month.25,42 The rash can be pruritic.15,33,42,43
Less commonly reported manifestations of Zika virus infection include malaise, stomachaches, dizziness, anorexia, retro-orbital pain, aphthous ulcers, hematospermia, and prostatitis.14,15,24,25,44,45
The initial reports from eight patients in the outbreak in Brazil noted rash and joint pain as the most common manifestations. The maculopapular rash was present in all patients and the joint pain was characterized as severe, with the hands, ankles, elbows, knees, and wrists most consistently described.43
The clinical presentation is similar to those of dengue and chikungunya virus infections, confounding diagnosis, as these viruses may be cocirculating in the same geographic regions (and indeed are transmitted by the same mosquito vectors).11,12,15 The conjunctivitis present in Zika virus infections can also be present in chikungunya but is much less commonly a clinical feature of dengue.15,46,47 See Table 1 for the differential diagnosis of Zika virus infection.
Severe manifestations requiring hospitalization or resulting in death are thought to be uncommon, although neurologic and fetal complications have recently been described.12,29,43,48,49
CLINICAL ASSOCIATIONS
Primary infection with Zika virus is relatively benign. The greatest and most recent concerns are related to postinfectious complications and those that may occur in pregnant women.
Guillain-Barré syndrome
During the Zika virus outbreak in French Polynesia in 2013–2014, the incidence of Guillain-Barré syndrome was multiplied by a factor of 20.50 Prior to the first hospitalization of a patient with Zika virus infection and associated Guillain-Barré syndrome in French Polynesia, there had been no reported hospitalizations for Zika virus infection.50
This same association is now being seen in the recent outbreak in the Americas.50 In July 2015, Brazilian health officials in the State of Bahia reported 76 patients with neurologic syndromes, of whom 55% had Guillain-Barré syndrome.51 A history consistent with Zika virus infection was found in 62%.48
In January 2016, El Salvador also reported an unusual increase in Guillain-Barré syndrome cases since early December 2015.51 Between December 1, 2015, and January 6, 2016, there were 46 Guillain-Barré syndrome cases reported, compared with a baseline of 14 cases per month.51
Other countries where Zika virus infection is endemic are also currently investigating similar trends.51
Microcephaly
On November 17, 2015, the Pan American Health Organization issued an epidemiologic alert because of increased reports of microcephaly in the Pernambuco State of Brazil. Whereas there are typically about 10 cases per year, there had been 141 in the previous 11 months.51 Other states in Brazil such as Paraiba and Rio Grande del Norte also reported increases in the diagnosis of microcephaly. A physician alert published in Brazil described two infants from the Paraiba state who were diagnosed with fetal microcephaly.35 Testing for Zika virus by polymerase chain reaction (PCR) was negative in the maternal blood, but PCR of amniotic fluid was positive in both infants.35
In January 2016, the Brazil Ministry of Health reported that Zika virus had been detected by real-time PCR (RT-PCR) in four infants with congenital malformations in Rio Grande del Norte. Two of these cases were miscarriages and two were infants who died within 24 hours of birth. Immunohistochemistry of tissues from these infants was positive for Zika virus.
A February 2016 case report describes a European woman who developed Zika virus infection at 13 weeks gestation while working in Northeast Brazil and upon return to Europe elected to terminate the pregnancy after ultrasonography showed cerebral calcifications with microcephaly. The infant was found to have a very small brain, hypoplasia of the brainstem and spinal cord with degeneration of spinal tracts, complete absence of cerebral gyri, and severe dilatation of lateral ventricles as well as calcifications throughout the cerebral cortex.49 No genetic abnormalities or evidence of other etiologies was found, and large amounts of Zika virus RNA were found in the brain.
The CDC also recently reported confirmation of Zika virus infection from fetal tissues of two miscarriages (fetal loss at 11 and 13 weeks) and two fetal deaths (36 and 38 weeks) received from the state of Rio Grande do Norte in Brazil.52 All four mothers reported clinical signs of fever and rash during their first trimester of pregnancy.52 Additional testing for toxoplasmosis, rubella, cytomegalovirus, herpes simplex, and human immunodeficiency virus were all negative in the mothers who had miscarriages.52
Of critical note, the causality of Zika virus and microcephaly remains under investigation. See Table 2 for other causes of microcephaly.53
Macular atrophy
In January 2016, a case series of three infants with microcephaly and macular atrophy was reported.54 These infants were tested for toxoplasmosis, rubella, cytomegalovirus, herpes simplex, syphilis, and human immunodeficiency virus (HIV), and all the results were negative. The detection of Zika virus fulfilled the Brazilian Ministry of Health’s definition of vertical transmission of Zika virus, and laboratory diagnostic tests for Zika virus were not performed. In this series, one mother reported an illness with rash and arthralgias during the first trimester.54
LABORATORY DIAGNOSTIC METHODS
The diagnosis of Zika virus infection is challenging. The low viremia at initial presentation and cross-reactivity of serologic testing with other flaviviruses, especially dengue, can contribute to misdiagnosis.40,50
In the first 7 days of Zika virus infection, the diagnosis is based on detection of viral RNA in serum by RT-PCR.12,55,56 RT-PCR is very specific for Zika virus and is an important tool in differentiating between Zika virus and other flaviviruses often present in areas where Zika virus is circulating.12,56 After 3 to 4 days, viremia may decrease to levels that may be below the assay’s level of detection.40–42,45
While Zika virus RNA may be undetectable in the serum, other samples such as saliva, urine, and semen may be positive for longer.28,42,57 For example, urine samples were positive by RT-PCR up to 7 days beyond blood RT-PCR in the outbreak in New Caledonia.42 A recent report found semen remaining positive on RT-PCR for 62 days after the onset of confirmed Zika virus illness in a traveler returning to the United Kingdom from the Cook Islands in 2014.58
Because RT-PCR of blood is only useful early in infection, the current diagnostic guidelines recommend testing an acute-phase serum sample for Zika virus IgM collected as early as possible after the onset of illness and repeated 2 to 3 weeks after the initial set. These IgM antibodies typically develop toward the end of the first week of illness and are expected to be present for up to 12 weeks, based on experience with other flaviviruses.41 Cross-reactivity with other flaviviruses circulating in the area can occur and has been problematic in areas where dengue is circulating.12,41,45,56 IgM-positive specimens should be further tested, by plaque-reduction neutralization, to confirm the presence of Zika virus-specific neutralizing antibodies. Results can be difficult to interpret, especially in those who have been previously infected or vaccinated against other flaviviruses.12,41
If amniocentesis is done, these specimens should be tested by RT-PCR. However, the sensitivity of PCR in amniotic fluid is currently unknown.41
In infants with findings of cerebral calcifications and microcephaly, IgM serologies with RT-PCR are also recommended and should be drawn within 2 days of birth. Specimens should be drawn concurrently as it is not known which test is most reliable in infants.23 Additionally, placenta and umbilical cord samples should be collected for immunohistochemical staining at specialized laboratories.36
In the United States, providers should contact their state health departments to determine where tests can be run reliably. Refined diagnostic assays are in development at the time of this publication and are likely to be made available through CDC’s Laboratory Response Network.
See Figure 2 and Table 3 for a summary of diagnostic tests.
IMPLICATIONS, RECOMMENDATIONS
Pregnant women
The CDC now recommends that asymptomatic pregnant women who returned from travel to a Zika virus-endemic zone in the last 2 to 12 weeks be offered serologic testing.41 This includes women who may be living in an area with ongoing Zika virus transmission; however, these women should also have testing at the initiation of prenatal care and then follow-up testing in the middle of the second trimester. Of importance, these results may be difficult to interpret due to potential cross-reactivity between Zika virus and other flaviviruses, and false-positive results in recipients of yellow fever and Japanese encephalitis vaccines.41,59
If a pregnant woman with a positive travel history is symptomatic, testing should be offered during the first week of illness. After day 4 of the illness, testing should include both RT-PCR and IgM serology.41,59
A screening ultrasound scan is recommended for any pregnant woman who has traveled to a Zika virus-affected area to determine if microcephaly or cerebral or intracranial calcifications are present. Those women with confirmed Zika virus infection should continue to have monthly screening ultrasounds, while those who are negative for Zika virus should have another ultrasound at the end of the second trimester or the beginning of the third trimester to ensure that no abnormalities had developed.41,59
At present, pregnant women and women of childbearing age who may become pregnant are advised by the CDC to postpone travel to affected areas until more information becomes available about mother-to-child transmission.59
Algorithms for the care of pregnant women and women of childbearing age who may have been exposed to Zika virus are available from the CDC41 at www.cdc.gov/mmwr/volumes/65/wr/mm6505e2.htm.
Male partners of pregnant women
Since the length of time that Zika virus remains viable in semen is not known, men who have traveled to Zika virus-endemic areas and who have pregnant partners should refrain from having sex or use a condom with every sexual encounter through the duration of the pregnancy.60
Guidelines for prevention of sexual transmission of Zika virus are available from the CDC59 at www.cdc.gov/mmwr/volumes/65/wr/mm6505e1er.htm.
Infants with possible congenital Zika virus infection
Zika virus testing is recommended for any infant born with microcephaly or intracranial calcifications or whose mother has positive or inconclusive testing if the mother had visited an endemic area during her pregnancy.
Zika virus testing in infants consists of serologic IgM determination and RT-PCR for both dengue and Zika virus drawn concurrently in the first 2 days of life.36 Umbilical cord blood can be used. In addition, if cerebrospinal fluid is being collected for other reasons, it can also be tested for Zika virus. The placenta and umbilical cord should be saved for immunohistochemistry testing for Zika virus.61
An infant who tests positive or inconclusive for Zika virus, regardless of the presence of microcephaly or intracranial calcifications, should have a complete physical examination specifically evaluating growth parameters, estimated gestational age, and signs of neurologic disease, skin rashes, hepatosplenomegaly, or any dysmorphic features. Additional evaluation includes an ophthalmologic examination in the first month of life to evaluate for macular atrophy.36 An ultrasound scan of the head should be completed if it has not been done. Hearing is screened in all newborns, and hearing testing should be repeated at 6 months of age.36
Infants with microcephaly or intracranial calcifications should also have consultations with specialists in genetics, neurology, and pediatric infectious diseases.61 These infants should have blood work including complete blood cell counts and liver function testing that includes alanine aminotransferase, aspartate aminotransferase, and bilirubin levels.36
All infants with possible congenital Zika virus infection should be followed long-term with close attention to developmental milestones and growth parameters including occipital frontal head circumference measurements.61,62
Infants without microcephaly or calcifications whose mothers had negative Zika virus test results or were not tested for Zika virus should have routine care.37
Guidelines for the care of infants with Zika virus infection are available from the CDC36 at www.cdc.gov/mmwr/volumes/65/wr/mm6503e3.htm.
TREATMENT
There is no treatment for Zika virus infection, and care is supportive. Most infections are mild and self-limited.12,15 Avoidance of aspirin and other nonsteroidal anti-inflammatory drugs that may affect platelets is important until dengue infection has been ruled out.
PREVENTION
There is currently no vaccine to prevent Zika virus infection. Woman who are pregnant should avoid travel to any area where Zika virus transmission is occurring.41,59 The CDC advises pregnant women and women of childbearing age who may become pregnant to postpone travel to Zika virus-affected areas.59 Patients can find travel alerts for specific areas at wwwnc.cdc.gov/travel/notices/alert/zika-virus-south-america.
Avoiding mosquito bites is the best way to prevent the spread of Zika virus. Aedes aegypti and A albopictus, the most common vectors of Zika virus, can bite at night but are known more for being aggressive daytime biters.63 Travelers should apply an Environmental Protection Agency-registered insect repellent as directed, wear long-sleeved shirts and long pants, use permethrin-treated clothing and gear, and stay in places with screens or air conditioning. Any containers with standing water should be eliminated as they are breeding areas for mosquitoes. It is also important that symptomatic people in the first week of illness use mosquito precautions to prevent the spread of Zika virus.
Patient handouts and posters for mosquito bite prevention can be found at www.cdc.gov/zika/fs-posters/index.html.
WATCH FOR UPDATES
Many questions remain regarding the epidemiology of this infection and its relationship to neurologic and pregnancy complications. However, due to its rapid spread across the Western hemisphere and its potential for significant complications, much is being done at the local and international levels to better understand the virus and halt its spread. More information will continue to be available as results from ongoing studies are conducted and potential associations are investigated. Until more is known, providers should familiarize themselves with the latest guidelines in order to better counsel their patients who may live in or travel to Zika virus endemic areas. We advise clinicians to follow the CDC’s web site, www.cdc.gov/zika/.
- World Health Organization. Zika virus fact sheet. www.who.int/mediacentre/factsheets/zika/en/. Accessed February 24, 2016.
- Centers for Disease Control and Prevention. Areas with Zika. www.cdc.gov/zika/geo/index.html. Accessed February 24, 2016.
- Rice CM. Flaviviruses. In: Fields BN, Knipe DM, Howley PM, Chanock RM, editors. Fields Virology, 3rd ed. Philadelphia: Lippincott-Raven, 1996:961–1034.
- Kuno G, Chang GJ, Tsuchiya KR, Karabatsos N, Cropp CB. Phylogeny of the genus Flavivirus. J Virol 1998; 72:73–83.
- Haddow AD, Schuh AJ, Yasuda CY, et al. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis 2012; 6:e1477.
- Faye O, Freire CC, Iamarino A, et al. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis 2014; 8:e2636.
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952; 46:509–520.
- Dick GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg 1952; 46:521–534.
- Fagbami AH. Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State. J Hyg (Lond) 1979; 83:213–219.
- Hayes EB. Zika virus outside Africa. Emerg Infect Dis 2009; 15:1347–1350.
- Heang V, Yasuda CY, Sovann L, et al. Zika virus infection, Cambodia, 2010. Emerg Infect Dis 2012; 18:349–351.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360:2536–2543.
- Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infec 2014; 20:O595–O596.
- Cao-Lormeau VM, Roche C, Teissier A, et al. Zika virus, French polynesia, South Pacific, 2013. Emerg Infect Dis 2014; 20:1085–1086.
- Ioos S, Mallet HP, Leparc Goffart I, Gauthier V, Cardoso T, Herida M. Current Zika virus epidemiology and recent epidemics. Med Mal Infect 2014; 44:302–307.
- Tognarelli J, Ulloa S, Villagra E, et al. A report on the outbreak of Zika virus on Easter Island, South Pacific, 2014. Arch Virol Nov 26 2015 [Epub ahead of print].
- Pan American Health Organization/World Health Organization, Regional Office for the Americas. Zika virus infection. 7 May 2015. www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=30075=en. Accessed February 24, 2016.
- Fauci AS, Morens DM. Zika virus in the Americas—yet another arbovirus threat. N Engl J Med 2016; 347:601–604.
- Marcondes CB, Ximenes MF. Zika virus in Brazil and the danger of infestation by Aedes (Stegomyia) mosquitoes. Rev Soc Bras Med Trop. Dec 22 2015. pii: S0037-86822015005003102. [Epub ahead of print]
- Weinbren MP, Williams MC. Zika virus: further isolations in the Zika area, and some studies on the strains isolated. Trans R Soc Trop Med Hyg 1958; 52:263–268.
- Diallo D, Sall AA, Diagne CT, et al. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS One 2014; 9:e109442.
- Grard G, Caron M, Mombo IM, et al. Zika virus in Gabon (Central Africa)—2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis 2014; 8:e2681.
- Hennessey M, Fischer M, Staples JE. Zika virus spreads to new areas—region of the Americas, May 2015–January 2016. MMWR 2016; 65:55–58.
- Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis 2015; 21:359–361.
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 2011; 17:880–882.
- Smith J, Woldai S, Chung W. Health advisory: sexual transmission of Zika virus. Dallas Country Department of Health and Human Services, February 2, 2016. http://walnuthillobgyn.com/wp-content/uploads/2012/05/zika-transmission.pdf. Accessed February 24, 2016.
- Hills SL, Russell K, Hennessey M, et al. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission—continental United States, 2016. MMWR Early release February 26, 2016. www.cdc.gov/mmwr/volumes/65/wr/mm6508e2er.htm Accessed February 29, 2016.
- Musso D, Nhan T, Robin E, et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill 2014; 19(14). pii: 20761. Erratum in Euro Surveill 2014; 19(15). pii/20771.
- Marano G, Pupella S, Vaglio S, Liumbruno GM, Grazzini G. Zika virus and the never-ending story of emerging pathogens and transfusion medicine. Blood Transfus 2015; Nov 5:1–6. doi: 10.2450/2015.0066-15. [Epub ahead of print]
- European Centre for Disease Prevention and Control. Epidemiological update: complications potentially linked to Zika virus outbreak, Brazil and French Polynesia. November 27, 2015. http://ecdc.europa.eu/en/press/news/_layouts/forms/News_DispForm.aspx?ID=1332&List=8db7286c-fe2d-476c-9133-18ff4cb1b568&Source=http%3A%2F%2Fecdc%2Eeuropa%2Eeu%2Fen%2Fpress%2Fepidemiological%5Fupdates%2FPages%2Fepidemiological%5Fupdates%2Easpx. Accessed February 24, 2016
- European Centre for Disease Prevention and Control. Rapid risk assessment. Zika virus infection outbreak, Brazil and the Pacific region 25 May 2015. http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-Zika%20virus-south-america-Brazil-2015.pdf. Accessed February 24, 2016
- Regan DM, Markowitz MA. Association Bulletin #16-03. Re: Zika, dengue, and chikungunya viruses. American Association of Blood Banks, February 1, 2016. www.aabb.org/programs/publications/bulletins/Documents/ab16-03.pdf. Accessed February 24, 2016.
- US Food and Drug Administration (FDA). Recommendations for donor screening, deferral, and product management to reduce the risk of transfusion-transmission of Zika virus. Guidance for industry. February, 2016. www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/UCM486360.pdf. Accessed February 24, 2016.
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill 2014; 19(13). pii: 20751.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol 2016; 47:6–7.
- Staples JE, Dziuban EJ, Fischer M, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection—United States, 2016. MMWR 2016; 65:63–67.
- Fleming-Dutra K, Nelson J, Fischer M, Staples J, Mateusz P, et al. Update: interim guidelines for health care providers caring for infants and children with possible Zika virus infection—United States, February 2016. MMWR 2016; 65:1–6.
- Simpson DI. Zika virus infection in man. Trans R Soc Trop Med Hyg Jul 1964; 58:335–338.
- Olson JG, Ksiazek TG, Suhandiman, Triwibowo. Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg 1981; 75:389–393.
- Bearcroft WG. Zika virus infection experimentally induced in a human volunteer. Trans R Soc Trop Med Hyg 1956; 50:442–448.
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR 2016; 65:122–127.
- Gourinat AC, O’Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis 2015; 21:84–86.
- Zanluca C, de Melo VC, Mosimann AL, Dos Santos GI, Dos Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz 2015; 110:569–572.
- Alera MT, Hermann L, Tac-An IA, et al. Zika virus infection, Philippines, 2012. Emerg Infect Dis 2015; 21:722–724.
- Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14:1232–1239.
- Centers for Disease Control and Prevention. Chikungunya virus. Clinical evaluation & disease. www.cdc.gov/chikungunya/hc/clinicalevaluation.html. Accessed February 24, 2016.
- Centers for Disease Control and Prevention. Clinical guidance. Dengue virus. www.cdc.gov/dengue/clinicalLab/clinical.html. Accessed February 24, 2016.
- Pan American Health Organization/World Health Organization. Epidemiological alert. Increase in microcephaly in the northeast of Brazil. November 17, 2015. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32636&lang=en. Accessed February 24, 2016.
- Rubin EJ, Greene MF, Baden LR. Zika virus and microcephaly. N Engl J Med 2016; Feb 10 [Epub ahead of print].
- Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barré syndrome—case report, French Polynesia, December 2013. Euro Surveill 2014; 19(9). pii: 20720.
- Pan American Health Organization/World Health Organization. Epidemiological alert. Neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. December 1, 2015. www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32405&lang=en. Accessed February 24, 2016.
- Martines R, Bhatnagar J, Keating M, et al. Notes from the field: evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses—Brazil, 2015. MMRW 2016; 65:159–160.
- Ashwal S, Michelson D, Plawner L, Dobyns WB; Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Practice parameter: evaluation of the child with microcephaly (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2009; 73:887–897.
- Ventura CV, Maia M, Bravo-Filho V, Góis AL, Belfort R Jr. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet 2016; 387:228.
- Centers for Disease Control and Prevention. Updated diagnostic testing for Zika, chikungunya, and dengue viruses in US Public Health Laboratories. http://stacks.cdc.gov/view/cdc/37594. Accessed February 24, 2016.
- Faye O, Faye O, Diallo D, Diallo M, Weidmann M, Sall AA. Quantitative real-time PCR detection of Zika virus and evaluation with field-caught mosquitoes. Virol J 2013; 10:311.
- Musso D, Roche C, Nhan TX, Robin E, Teissier A, Cao-Lormeau VM. Detection of Zika virus in saliva. J Clin Virol 2015; 68:53–55.
- Atkinson B, Hearn P, Afrough B, et al. Detection of Zika virus in semen [letter]. Emerg Infect Dis 2016 May. http://wwwnc.cdc.gov/eid/article/22/5/16-0107_article. Accessed February 24, 2016.
- Petersen EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR 2016; 65:30–33.
- Oster AM, Brooks JT, Stryker JE, et al. Interim guidelines for prevention of sexual transmission of Zika virus—United States, 2016. MMWR 2016; 65:120–121.
- Staples JE, Dziuban EJ, Fischer M, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection—United States, 2016. MMWR 2016; 65:63–67.
- Centers for Disease Control and Prevention. Zika virus clinical evaluation and disease. www.cdc.gov/zika/hc-providers/clinicalevaluation.html. Accessed February 24, 2016.
- Centers for Disease Control and Prevention. Zika virus. Transmission & risks. www.cdc.gov/zika/transmission/index.html. Accessed February 29, 2016.
- World Health Organization. Zika virus fact sheet. www.who.int/mediacentre/factsheets/zika/en/. Accessed February 24, 2016.
- Centers for Disease Control and Prevention. Areas with Zika. www.cdc.gov/zika/geo/index.html. Accessed February 24, 2016.
- Rice CM. Flaviviruses. In: Fields BN, Knipe DM, Howley PM, Chanock RM, editors. Fields Virology, 3rd ed. Philadelphia: Lippincott-Raven, 1996:961–1034.
- Kuno G, Chang GJ, Tsuchiya KR, Karabatsos N, Cropp CB. Phylogeny of the genus Flavivirus. J Virol 1998; 72:73–83.
- Haddow AD, Schuh AJ, Yasuda CY, et al. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis 2012; 6:e1477.
- Faye O, Freire CC, Iamarino A, et al. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis 2014; 8:e2636.
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952; 46:509–520.
- Dick GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg 1952; 46:521–534.
- Fagbami AH. Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State. J Hyg (Lond) 1979; 83:213–219.
- Hayes EB. Zika virus outside Africa. Emerg Infect Dis 2009; 15:1347–1350.
- Heang V, Yasuda CY, Sovann L, et al. Zika virus infection, Cambodia, 2010. Emerg Infect Dis 2012; 18:349–351.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360:2536–2543.
- Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infec 2014; 20:O595–O596.
- Cao-Lormeau VM, Roche C, Teissier A, et al. Zika virus, French polynesia, South Pacific, 2013. Emerg Infect Dis 2014; 20:1085–1086.
- Ioos S, Mallet HP, Leparc Goffart I, Gauthier V, Cardoso T, Herida M. Current Zika virus epidemiology and recent epidemics. Med Mal Infect 2014; 44:302–307.
- Tognarelli J, Ulloa S, Villagra E, et al. A report on the outbreak of Zika virus on Easter Island, South Pacific, 2014. Arch Virol Nov 26 2015 [Epub ahead of print].
- Pan American Health Organization/World Health Organization, Regional Office for the Americas. Zika virus infection. 7 May 2015. www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=30075=en. Accessed February 24, 2016.
- Fauci AS, Morens DM. Zika virus in the Americas—yet another arbovirus threat. N Engl J Med 2016; 347:601–604.
- Marcondes CB, Ximenes MF. Zika virus in Brazil and the danger of infestation by Aedes (Stegomyia) mosquitoes. Rev Soc Bras Med Trop. Dec 22 2015. pii: S0037-86822015005003102. [Epub ahead of print]
- Weinbren MP, Williams MC. Zika virus: further isolations in the Zika area, and some studies on the strains isolated. Trans R Soc Trop Med Hyg 1958; 52:263–268.
- Diallo D, Sall AA, Diagne CT, et al. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS One 2014; 9:e109442.
- Grard G, Caron M, Mombo IM, et al. Zika virus in Gabon (Central Africa)—2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis 2014; 8:e2681.
- Hennessey M, Fischer M, Staples JE. Zika virus spreads to new areas—region of the Americas, May 2015–January 2016. MMWR 2016; 65:55–58.
- Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis 2015; 21:359–361.
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 2011; 17:880–882.
- Smith J, Woldai S, Chung W. Health advisory: sexual transmission of Zika virus. Dallas Country Department of Health and Human Services, February 2, 2016. http://walnuthillobgyn.com/wp-content/uploads/2012/05/zika-transmission.pdf. Accessed February 24, 2016.
- Hills SL, Russell K, Hennessey M, et al. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission—continental United States, 2016. MMWR Early release February 26, 2016. www.cdc.gov/mmwr/volumes/65/wr/mm6508e2er.htm Accessed February 29, 2016.
- Musso D, Nhan T, Robin E, et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill 2014; 19(14). pii: 20761. Erratum in Euro Surveill 2014; 19(15). pii/20771.
- Marano G, Pupella S, Vaglio S, Liumbruno GM, Grazzini G. Zika virus and the never-ending story of emerging pathogens and transfusion medicine. Blood Transfus 2015; Nov 5:1–6. doi: 10.2450/2015.0066-15. [Epub ahead of print]
- European Centre for Disease Prevention and Control. Epidemiological update: complications potentially linked to Zika virus outbreak, Brazil and French Polynesia. November 27, 2015. http://ecdc.europa.eu/en/press/news/_layouts/forms/News_DispForm.aspx?ID=1332&List=8db7286c-fe2d-476c-9133-18ff4cb1b568&Source=http%3A%2F%2Fecdc%2Eeuropa%2Eeu%2Fen%2Fpress%2Fepidemiological%5Fupdates%2FPages%2Fepidemiological%5Fupdates%2Easpx. Accessed February 24, 2016
- European Centre for Disease Prevention and Control. Rapid risk assessment. Zika virus infection outbreak, Brazil and the Pacific region 25 May 2015. http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-Zika%20virus-south-america-Brazil-2015.pdf. Accessed February 24, 2016
- Regan DM, Markowitz MA. Association Bulletin #16-03. Re: Zika, dengue, and chikungunya viruses. American Association of Blood Banks, February 1, 2016. www.aabb.org/programs/publications/bulletins/Documents/ab16-03.pdf. Accessed February 24, 2016.
- US Food and Drug Administration (FDA). Recommendations for donor screening, deferral, and product management to reduce the risk of transfusion-transmission of Zika virus. Guidance for industry. February, 2016. www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/UCM486360.pdf. Accessed February 24, 2016.
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill 2014; 19(13). pii: 20751.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol 2016; 47:6–7.
- Staples JE, Dziuban EJ, Fischer M, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection—United States, 2016. MMWR 2016; 65:63–67.
- Fleming-Dutra K, Nelson J, Fischer M, Staples J, Mateusz P, et al. Update: interim guidelines for health care providers caring for infants and children with possible Zika virus infection—United States, February 2016. MMWR 2016; 65:1–6.
- Simpson DI. Zika virus infection in man. Trans R Soc Trop Med Hyg Jul 1964; 58:335–338.
- Olson JG, Ksiazek TG, Suhandiman, Triwibowo. Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg 1981; 75:389–393.
- Bearcroft WG. Zika virus infection experimentally induced in a human volunteer. Trans R Soc Trop Med Hyg 1956; 50:442–448.
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR 2016; 65:122–127.
- Gourinat AC, O’Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis 2015; 21:84–86.
- Zanluca C, de Melo VC, Mosimann AL, Dos Santos GI, Dos Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz 2015; 110:569–572.
- Alera MT, Hermann L, Tac-An IA, et al. Zika virus infection, Philippines, 2012. Emerg Infect Dis 2015; 21:722–724.
- Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14:1232–1239.
- Centers for Disease Control and Prevention. Chikungunya virus. Clinical evaluation & disease. www.cdc.gov/chikungunya/hc/clinicalevaluation.html. Accessed February 24, 2016.
- Centers for Disease Control and Prevention. Clinical guidance. Dengue virus. www.cdc.gov/dengue/clinicalLab/clinical.html. Accessed February 24, 2016.
- Pan American Health Organization/World Health Organization. Epidemiological alert. Increase in microcephaly in the northeast of Brazil. November 17, 2015. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32636&lang=en. Accessed February 24, 2016.
- Rubin EJ, Greene MF, Baden LR. Zika virus and microcephaly. N Engl J Med 2016; Feb 10 [Epub ahead of print].
- Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barré syndrome—case report, French Polynesia, December 2013. Euro Surveill 2014; 19(9). pii: 20720.
- Pan American Health Organization/World Health Organization. Epidemiological alert. Neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. December 1, 2015. www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32405&lang=en. Accessed February 24, 2016.
- Martines R, Bhatnagar J, Keating M, et al. Notes from the field: evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses—Brazil, 2015. MMRW 2016; 65:159–160.
- Ashwal S, Michelson D, Plawner L, Dobyns WB; Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Practice parameter: evaluation of the child with microcephaly (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2009; 73:887–897.
- Ventura CV, Maia M, Bravo-Filho V, Góis AL, Belfort R Jr. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet 2016; 387:228.
- Centers for Disease Control and Prevention. Updated diagnostic testing for Zika, chikungunya, and dengue viruses in US Public Health Laboratories. http://stacks.cdc.gov/view/cdc/37594. Accessed February 24, 2016.
- Faye O, Faye O, Diallo D, Diallo M, Weidmann M, Sall AA. Quantitative real-time PCR detection of Zika virus and evaluation with field-caught mosquitoes. Virol J 2013; 10:311.
- Musso D, Roche C, Nhan TX, Robin E, Teissier A, Cao-Lormeau VM. Detection of Zika virus in saliva. J Clin Virol 2015; 68:53–55.
- Atkinson B, Hearn P, Afrough B, et al. Detection of Zika virus in semen [letter]. Emerg Infect Dis 2016 May. http://wwwnc.cdc.gov/eid/article/22/5/16-0107_article. Accessed February 24, 2016.
- Petersen EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR 2016; 65:30–33.
- Oster AM, Brooks JT, Stryker JE, et al. Interim guidelines for prevention of sexual transmission of Zika virus—United States, 2016. MMWR 2016; 65:120–121.
- Staples JE, Dziuban EJ, Fischer M, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection—United States, 2016. MMWR 2016; 65:63–67.
- Centers for Disease Control and Prevention. Zika virus clinical evaluation and disease. www.cdc.gov/zika/hc-providers/clinicalevaluation.html. Accessed February 24, 2016.
- Centers for Disease Control and Prevention. Zika virus. Transmission & risks. www.cdc.gov/zika/transmission/index.html. Accessed February 29, 2016.
KEY POINTS
- Zika virus infection is spread by the bite of infected mosquitoes and also through sexual contact, blood transfusions, and vertical transmission.
- Most Zika virus infections are asymptomatic, and symptomatic cases are often mild and self-limited, with rash, fever, joint pain, and nonpurulent conjunctivitis the most common symptoms.
- Polymerase chain reaction testing can detect viral RNA in the blood, but only in the first few days after the onset of symptoms. Immunoglobulin M against the virus becomes detectable at approximately 1 week and persists for about 12 weeks, but cross-reactivity with other viruses is a problem with serologic testing.
- As yet, there is no vaccine and no specific treatment.
- Pregnant women and women who may become pregnant are advised to defer travel to areas where Zika virus is endemic.
Zika—a new continent and new complications?
The latest reminders that we live in a medically connected global community are the appearance of the Africa-born Zika virus infection in Brazil and other areas within the Western hemisphere and the subsequent apparent transmission of the disease to female sexual contacts of infected males in the United States. Zika virus’ geographic travels are most certainly of interest; they can be traced from sub-Saharan Africa, where serologically identified outbreaks have continued since 1947, through Asia, Micronesia, Polynesia, and now South and Central America. But what may turn out to be even more interesting than the virus’s travel itinerary is what we may learn about the Zika virus-human host interaction and the subsequent spectrum of clinical disease.
The primary clinical illness following serologically defined infection seems to be relatively uncommon and generally mild: a fairly nondistinctive febrile episode with mild rash, small- and large-joint arthralgias or arthritis, and nonpurulent conjunctivitis. But what has fostered the greatest concern is the epidemiologic association of Zika infection with the neurologic complications of microcephaly and Guillain-Barré syndrome (GBS).
During the 2013–2014 outbreak of Zika infection in French Polynesia, 42 patients with GBS were identified, 100% of whom had serologic evidence suggestive of recent Zika infection, compared with 56% of control patients without GBS.1 Serologic determination of recent infection can be difficult due to cross-reactivity with other flaviviruses, but it seems that in the Polynesian outbreak the risk of GBS might be much less than 1 in 1,000 patients. This is not unlike the incidence of GBS following influenza, Campylobacter, and cytomegalovirus. One explanation for why GBS may follow certain infections is that the infection can trigger antibodies that cross-react with neuronal membrane components. However, those antiganglioside antibodies were not uniformly present in the Polynesian patients who developed GBS following Zika infection. Thus, this may provide an opportunity to further understand the mechanism by which GBS is associated with some infections, in selected patients.
Patients with post-Zika GBS seem to fare well, with a very good prognosis for complete recovery. That is not the case, however, for infants born with microcephaly, another epidemiologically linked complication of Zika infection. In Brazil, the exact incidence rate remains to be determined, and it is not yet certain whether the rate is higher than in the previous Polynesian epidemic (the number of infections is far greater in Brazil, and thus the accuracy of estimated frequency may also be greater), but there may have been a significantly increased frequency of microcephaly in the Polynesian outbreak as well. Like the related West Nile, Saint Louis encephalitis, and Japanese encephalitis viruses, Zika virus has the ability to directly attack certain neurons, and the Zika genome has been detected in brains of infected babies at autopsy. So this particularly devastating aspect of Zika infection may turn out to be relatively easy to understand—perhaps the portal for viral infection of specific neurons is expressed only at certain times during brain development. I’m sure these investigations are under way at a feverish pitch.
Recognizing that new information is being released virtually daily, Flores et al provide a current overview of our understanding of the virus and some practical advice regarding diagnosis and prevention.
As laboratories gear up to devise rapid and more specific diagnostic tests and develop effective anti-Zika vaccines, we hope to learn more about how a seemingly minimally relevant virus, when introduced into a new environment, can wreak clinical havoc. Possible explanations abound—genetic differences in the population, altered immunologic background of infected patients due to prior infection with related viruses such as dengue, or the direct impact of other coinfections. Or, with careful study, it may be discovered that these neurologic issues have been present elsewhere all along, but not previously linked to the Zika virus.
- Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 2016 Feb 29. pii: S0140-6736(16)00562-6. doi: 10.1016/S0140-6736(16)00562-6. [Epub ahead of print].
The latest reminders that we live in a medically connected global community are the appearance of the Africa-born Zika virus infection in Brazil and other areas within the Western hemisphere and the subsequent apparent transmission of the disease to female sexual contacts of infected males in the United States. Zika virus’ geographic travels are most certainly of interest; they can be traced from sub-Saharan Africa, where serologically identified outbreaks have continued since 1947, through Asia, Micronesia, Polynesia, and now South and Central America. But what may turn out to be even more interesting than the virus’s travel itinerary is what we may learn about the Zika virus-human host interaction and the subsequent spectrum of clinical disease.
The primary clinical illness following serologically defined infection seems to be relatively uncommon and generally mild: a fairly nondistinctive febrile episode with mild rash, small- and large-joint arthralgias or arthritis, and nonpurulent conjunctivitis. But what has fostered the greatest concern is the epidemiologic association of Zika infection with the neurologic complications of microcephaly and Guillain-Barré syndrome (GBS).
During the 2013–2014 outbreak of Zika infection in French Polynesia, 42 patients with GBS were identified, 100% of whom had serologic evidence suggestive of recent Zika infection, compared with 56% of control patients without GBS.1 Serologic determination of recent infection can be difficult due to cross-reactivity with other flaviviruses, but it seems that in the Polynesian outbreak the risk of GBS might be much less than 1 in 1,000 patients. This is not unlike the incidence of GBS following influenza, Campylobacter, and cytomegalovirus. One explanation for why GBS may follow certain infections is that the infection can trigger antibodies that cross-react with neuronal membrane components. However, those antiganglioside antibodies were not uniformly present in the Polynesian patients who developed GBS following Zika infection. Thus, this may provide an opportunity to further understand the mechanism by which GBS is associated with some infections, in selected patients.
Patients with post-Zika GBS seem to fare well, with a very good prognosis for complete recovery. That is not the case, however, for infants born with microcephaly, another epidemiologically linked complication of Zika infection. In Brazil, the exact incidence rate remains to be determined, and it is not yet certain whether the rate is higher than in the previous Polynesian epidemic (the number of infections is far greater in Brazil, and thus the accuracy of estimated frequency may also be greater), but there may have been a significantly increased frequency of microcephaly in the Polynesian outbreak as well. Like the related West Nile, Saint Louis encephalitis, and Japanese encephalitis viruses, Zika virus has the ability to directly attack certain neurons, and the Zika genome has been detected in brains of infected babies at autopsy. So this particularly devastating aspect of Zika infection may turn out to be relatively easy to understand—perhaps the portal for viral infection of specific neurons is expressed only at certain times during brain development. I’m sure these investigations are under way at a feverish pitch.
Recognizing that new information is being released virtually daily, Flores et al provide a current overview of our understanding of the virus and some practical advice regarding diagnosis and prevention.
As laboratories gear up to devise rapid and more specific diagnostic tests and develop effective anti-Zika vaccines, we hope to learn more about how a seemingly minimally relevant virus, when introduced into a new environment, can wreak clinical havoc. Possible explanations abound—genetic differences in the population, altered immunologic background of infected patients due to prior infection with related viruses such as dengue, or the direct impact of other coinfections. Or, with careful study, it may be discovered that these neurologic issues have been present elsewhere all along, but not previously linked to the Zika virus.
The latest reminders that we live in a medically connected global community are the appearance of the Africa-born Zika virus infection in Brazil and other areas within the Western hemisphere and the subsequent apparent transmission of the disease to female sexual contacts of infected males in the United States. Zika virus’ geographic travels are most certainly of interest; they can be traced from sub-Saharan Africa, where serologically identified outbreaks have continued since 1947, through Asia, Micronesia, Polynesia, and now South and Central America. But what may turn out to be even more interesting than the virus’s travel itinerary is what we may learn about the Zika virus-human host interaction and the subsequent spectrum of clinical disease.
The primary clinical illness following serologically defined infection seems to be relatively uncommon and generally mild: a fairly nondistinctive febrile episode with mild rash, small- and large-joint arthralgias or arthritis, and nonpurulent conjunctivitis. But what has fostered the greatest concern is the epidemiologic association of Zika infection with the neurologic complications of microcephaly and Guillain-Barré syndrome (GBS).
During the 2013–2014 outbreak of Zika infection in French Polynesia, 42 patients with GBS were identified, 100% of whom had serologic evidence suggestive of recent Zika infection, compared with 56% of control patients without GBS.1 Serologic determination of recent infection can be difficult due to cross-reactivity with other flaviviruses, but it seems that in the Polynesian outbreak the risk of GBS might be much less than 1 in 1,000 patients. This is not unlike the incidence of GBS following influenza, Campylobacter, and cytomegalovirus. One explanation for why GBS may follow certain infections is that the infection can trigger antibodies that cross-react with neuronal membrane components. However, those antiganglioside antibodies were not uniformly present in the Polynesian patients who developed GBS following Zika infection. Thus, this may provide an opportunity to further understand the mechanism by which GBS is associated with some infections, in selected patients.
Patients with post-Zika GBS seem to fare well, with a very good prognosis for complete recovery. That is not the case, however, for infants born with microcephaly, another epidemiologically linked complication of Zika infection. In Brazil, the exact incidence rate remains to be determined, and it is not yet certain whether the rate is higher than in the previous Polynesian epidemic (the number of infections is far greater in Brazil, and thus the accuracy of estimated frequency may also be greater), but there may have been a significantly increased frequency of microcephaly in the Polynesian outbreak as well. Like the related West Nile, Saint Louis encephalitis, and Japanese encephalitis viruses, Zika virus has the ability to directly attack certain neurons, and the Zika genome has been detected in brains of infected babies at autopsy. So this particularly devastating aspect of Zika infection may turn out to be relatively easy to understand—perhaps the portal for viral infection of specific neurons is expressed only at certain times during brain development. I’m sure these investigations are under way at a feverish pitch.
Recognizing that new information is being released virtually daily, Flores et al provide a current overview of our understanding of the virus and some practical advice regarding diagnosis and prevention.
As laboratories gear up to devise rapid and more specific diagnostic tests and develop effective anti-Zika vaccines, we hope to learn more about how a seemingly minimally relevant virus, when introduced into a new environment, can wreak clinical havoc. Possible explanations abound—genetic differences in the population, altered immunologic background of infected patients due to prior infection with related viruses such as dengue, or the direct impact of other coinfections. Or, with careful study, it may be discovered that these neurologic issues have been present elsewhere all along, but not previously linked to the Zika virus.
- Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 2016 Feb 29. pii: S0140-6736(16)00562-6. doi: 10.1016/S0140-6736(16)00562-6. [Epub ahead of print].
- Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 2016 Feb 29. pii: S0140-6736(16)00562-6. doi: 10.1016/S0140-6736(16)00562-6. [Epub ahead of print].
Breast cancer treatment linked to mild systolic dysfunction
AMSTERDAM – Breast cancer patients who underwent chemotherapy or radiotherapy had about a two-fold increased prevalence of mild systolic cardiac dysfunction a median of 10 years after treatment, compared with age-matched controls in a study that included a total of 700 people.
But even longer follow-up of treated breast cancer patients is needed to determine whether the excess mild cardiac dysfunction seen in this analysis eventually progresses to more severe cardiac impairment, Liselotte M. Boerman said at the European Breast Cancer Conference.
Data from the Breast Cancer Long-term Outcome of Cardiac Dysfunction (BLOC) study showed that 175 breast cancer patients who received chemotherapy (and may have also received radiotherapy) had a 2.5-fold higher prevalence of a left ventricular ejection fraction (LVEF) below 54% (95% confidence interval, 1.2-5.4) when measured by echocardiography a median of 10 years after treatment, compared with an equal number of age-matched individuals from the general population.
A separate group of 175 patients treated with radiotherapy only and evaluated by echocardiography a median of 10 years later had a 2.3-fold increased prevalence (1.1-4.7) of a LVEF below 54% when compared with an equal number of age-matched individuals, said Ms. Boerman, an epidemiology researcher at the University of Groningen (the Netherlands).
This degree of left-ventricular dysfunction was found in 15% of the chemotherapy patients and 6% of their controls, and in 16% of the radiotherapy patients and 8% of their controls.
However, the treated breast cancer patients had no long-term increase in their prevalence of more significant systolic cardiac dysfunction, defined as a LVEF of less than 45%, compared with the controls, and the overall rate of systolic dysfunction of this severity was low, affecting fewer than 1% of patients.
Also, the chemotherapy and radiotherapy patients showed no significant increase in the prevalence of diastolic cardiac dysfunction, defined as delayed cardiac relaxation beyond the age-appropriate range. Treated patients did show, after 10 years, a suggestion of an increased prevalence of diagnosed cardiovascular disease, which was 2.3-fold higher (1.0-4.9) in the chemotherapy-receiving patients, compared with their controls; and 70% higher (0.9-3.4) among the patients treated with radiotherapy, compared with their controls, Ms. Boerman said.
The study used data collected from breast cancer patients younger than 80 years old treated after 1980 and controls seen by general practice Dutch physicians. The chemotherapy patients were diagnosed at an average age of 49 years old (range 26-66 years old). About 78% had received treatment with an anthracycline agent and 7% had received trastuzumab (Herceptin). Radiotherapy had also been administered to 70%, while 62% had also received hormonal therapy, and 7% either had a recurrence or developed a tumor in their contralateral breast.
None of the radiotherapy-only patients had received chemotherapy, but 21% had also received hormonal therapy. Their average age at diagnosis was 54 years old (range 32-79 years old), and 10% had a recurrence or a contralateral tumor.
Follow-up echocardiography occurred 5-34 years after the index treatment, at a median age of 60 years old. Echocardiography follow-up occurred in 70% of the chemotherapy breast cancer patients contacted, and in 63% of those who received radiotherapy only. Among controls, about half of those selected by age matching, and contacted, agreed to participate.
Rates of cardiovascular-disease risk factors – dyslipidemia, hypertension, and diabetes – were at roughly similar levels in the cases and controls at the time of breast cancer diagnosis. But the rate of current smoking at the time of diagnosis appeared higher in the cases (30% among those who received chemotherapy and 33% among those on radiotherapy), compared with their respective control groups (22% and 30%). Ms. Boerman said that a multivariate analysis had not yet been run on the data but should occur soon.
“The prevalence of cardiac dysfunction was higher [in treated patients] than I would have expected, but there is potential bias as only 70% of invited patients actually participated,” commented Dr. Robert Mansel, a professor at the Institute of Cancer & Genetics at Cardiff University (Wales). He also noted the very low rate of patients who developed severe cardiac dysfunction.
Ms. Boerman and Dr. Mansel reported having no financial disclosures.
On Twitter @mitchelzoler
AMSTERDAM – Breast cancer patients who underwent chemotherapy or radiotherapy had about a two-fold increased prevalence of mild systolic cardiac dysfunction a median of 10 years after treatment, compared with age-matched controls in a study that included a total of 700 people.
But even longer follow-up of treated breast cancer patients is needed to determine whether the excess mild cardiac dysfunction seen in this analysis eventually progresses to more severe cardiac impairment, Liselotte M. Boerman said at the European Breast Cancer Conference.
Data from the Breast Cancer Long-term Outcome of Cardiac Dysfunction (BLOC) study showed that 175 breast cancer patients who received chemotherapy (and may have also received radiotherapy) had a 2.5-fold higher prevalence of a left ventricular ejection fraction (LVEF) below 54% (95% confidence interval, 1.2-5.4) when measured by echocardiography a median of 10 years after treatment, compared with an equal number of age-matched individuals from the general population.
A separate group of 175 patients treated with radiotherapy only and evaluated by echocardiography a median of 10 years later had a 2.3-fold increased prevalence (1.1-4.7) of a LVEF below 54% when compared with an equal number of age-matched individuals, said Ms. Boerman, an epidemiology researcher at the University of Groningen (the Netherlands).
This degree of left-ventricular dysfunction was found in 15% of the chemotherapy patients and 6% of their controls, and in 16% of the radiotherapy patients and 8% of their controls.
However, the treated breast cancer patients had no long-term increase in their prevalence of more significant systolic cardiac dysfunction, defined as a LVEF of less than 45%, compared with the controls, and the overall rate of systolic dysfunction of this severity was low, affecting fewer than 1% of patients.
Also, the chemotherapy and radiotherapy patients showed no significant increase in the prevalence of diastolic cardiac dysfunction, defined as delayed cardiac relaxation beyond the age-appropriate range. Treated patients did show, after 10 years, a suggestion of an increased prevalence of diagnosed cardiovascular disease, which was 2.3-fold higher (1.0-4.9) in the chemotherapy-receiving patients, compared with their controls; and 70% higher (0.9-3.4) among the patients treated with radiotherapy, compared with their controls, Ms. Boerman said.
The study used data collected from breast cancer patients younger than 80 years old treated after 1980 and controls seen by general practice Dutch physicians. The chemotherapy patients were diagnosed at an average age of 49 years old (range 26-66 years old). About 78% had received treatment with an anthracycline agent and 7% had received trastuzumab (Herceptin). Radiotherapy had also been administered to 70%, while 62% had also received hormonal therapy, and 7% either had a recurrence or developed a tumor in their contralateral breast.
None of the radiotherapy-only patients had received chemotherapy, but 21% had also received hormonal therapy. Their average age at diagnosis was 54 years old (range 32-79 years old), and 10% had a recurrence or a contralateral tumor.
Follow-up echocardiography occurred 5-34 years after the index treatment, at a median age of 60 years old. Echocardiography follow-up occurred in 70% of the chemotherapy breast cancer patients contacted, and in 63% of those who received radiotherapy only. Among controls, about half of those selected by age matching, and contacted, agreed to participate.
Rates of cardiovascular-disease risk factors – dyslipidemia, hypertension, and diabetes – were at roughly similar levels in the cases and controls at the time of breast cancer diagnosis. But the rate of current smoking at the time of diagnosis appeared higher in the cases (30% among those who received chemotherapy and 33% among those on radiotherapy), compared with their respective control groups (22% and 30%). Ms. Boerman said that a multivariate analysis had not yet been run on the data but should occur soon.
“The prevalence of cardiac dysfunction was higher [in treated patients] than I would have expected, but there is potential bias as only 70% of invited patients actually participated,” commented Dr. Robert Mansel, a professor at the Institute of Cancer & Genetics at Cardiff University (Wales). He also noted the very low rate of patients who developed severe cardiac dysfunction.
Ms. Boerman and Dr. Mansel reported having no financial disclosures.
On Twitter @mitchelzoler
AMSTERDAM – Breast cancer patients who underwent chemotherapy or radiotherapy had about a two-fold increased prevalence of mild systolic cardiac dysfunction a median of 10 years after treatment, compared with age-matched controls in a study that included a total of 700 people.
But even longer follow-up of treated breast cancer patients is needed to determine whether the excess mild cardiac dysfunction seen in this analysis eventually progresses to more severe cardiac impairment, Liselotte M. Boerman said at the European Breast Cancer Conference.
Data from the Breast Cancer Long-term Outcome of Cardiac Dysfunction (BLOC) study showed that 175 breast cancer patients who received chemotherapy (and may have also received radiotherapy) had a 2.5-fold higher prevalence of a left ventricular ejection fraction (LVEF) below 54% (95% confidence interval, 1.2-5.4) when measured by echocardiography a median of 10 years after treatment, compared with an equal number of age-matched individuals from the general population.
A separate group of 175 patients treated with radiotherapy only and evaluated by echocardiography a median of 10 years later had a 2.3-fold increased prevalence (1.1-4.7) of a LVEF below 54% when compared with an equal number of age-matched individuals, said Ms. Boerman, an epidemiology researcher at the University of Groningen (the Netherlands).
This degree of left-ventricular dysfunction was found in 15% of the chemotherapy patients and 6% of their controls, and in 16% of the radiotherapy patients and 8% of their controls.
However, the treated breast cancer patients had no long-term increase in their prevalence of more significant systolic cardiac dysfunction, defined as a LVEF of less than 45%, compared with the controls, and the overall rate of systolic dysfunction of this severity was low, affecting fewer than 1% of patients.
Also, the chemotherapy and radiotherapy patients showed no significant increase in the prevalence of diastolic cardiac dysfunction, defined as delayed cardiac relaxation beyond the age-appropriate range. Treated patients did show, after 10 years, a suggestion of an increased prevalence of diagnosed cardiovascular disease, which was 2.3-fold higher (1.0-4.9) in the chemotherapy-receiving patients, compared with their controls; and 70% higher (0.9-3.4) among the patients treated with radiotherapy, compared with their controls, Ms. Boerman said.
The study used data collected from breast cancer patients younger than 80 years old treated after 1980 and controls seen by general practice Dutch physicians. The chemotherapy patients were diagnosed at an average age of 49 years old (range 26-66 years old). About 78% had received treatment with an anthracycline agent and 7% had received trastuzumab (Herceptin). Radiotherapy had also been administered to 70%, while 62% had also received hormonal therapy, and 7% either had a recurrence or developed a tumor in their contralateral breast.
None of the radiotherapy-only patients had received chemotherapy, but 21% had also received hormonal therapy. Their average age at diagnosis was 54 years old (range 32-79 years old), and 10% had a recurrence or a contralateral tumor.
Follow-up echocardiography occurred 5-34 years after the index treatment, at a median age of 60 years old. Echocardiography follow-up occurred in 70% of the chemotherapy breast cancer patients contacted, and in 63% of those who received radiotherapy only. Among controls, about half of those selected by age matching, and contacted, agreed to participate.
Rates of cardiovascular-disease risk factors – dyslipidemia, hypertension, and diabetes – were at roughly similar levels in the cases and controls at the time of breast cancer diagnosis. But the rate of current smoking at the time of diagnosis appeared higher in the cases (30% among those who received chemotherapy and 33% among those on radiotherapy), compared with their respective control groups (22% and 30%). Ms. Boerman said that a multivariate analysis had not yet been run on the data but should occur soon.
“The prevalence of cardiac dysfunction was higher [in treated patients] than I would have expected, but there is potential bias as only 70% of invited patients actually participated,” commented Dr. Robert Mansel, a professor at the Institute of Cancer & Genetics at Cardiff University (Wales). He also noted the very low rate of patients who developed severe cardiac dysfunction.
Ms. Boerman and Dr. Mansel reported having no financial disclosures.
On Twitter @mitchelzoler
AT EBCC10
Key clinical point: Breast cancer patients treated with chemotherapy or radiotherapy showed a doubled rate of mild left-ventricular dysfunction, compared with matched controls 10 years after treatment.
Major finding: Mildly reduced left-ventricular function occurred in 15% of post-chemotherapy patients, compared with 6% of controls.
Data source: Echocardiography examinations conducted on 350 Dutch breast cancer patients and an equal number of age-matched controls.
Disclosures: Ms. Boerman and Dr. Mansel reported having no financial disclosures.
Drug for conditioning AML patients for transplant gets orphan drug designation
A radioimmunotherapeutic drug for conditioning relapsed and refractory acute myeloid leukemia (AML) patients for a hematopoietic stem cell transplant has been granted orphan drug designation by the Food and Drug Administration.
Iomab-B is a radioimmunoconjugate consisting of the murine monoclonal antibody BC8 and an iodine-131 radioisotope. The Fred Hutchinson Cancer Research Center developed BC8 to target CD45, a panleukocytic antigen widely expressed on white blood cells. “When labeled with radioactive isotopes, BC8 carries radioactivity directly to the site of cancerous growth and bone marrow while avoiding effects of radiation on most healthy tissues,” says a statement from Actinium Pharmaceuticals, which would market the drug.
Iomab-B will be tested in a multicenter trial that will include 150 patients over age 55 with refractory and relapsed AML. “There has not been a new drug approved for relapsed and refractory AML patients over the age of 55 in decades and with Iomab-B being the only therapy of its kind, we are pleased to have achieved this important milestone,” Sandesh Seth, executive chairman of Actinium, said in the statement.
A radioimmunotherapeutic drug for conditioning relapsed and refractory acute myeloid leukemia (AML) patients for a hematopoietic stem cell transplant has been granted orphan drug designation by the Food and Drug Administration.
Iomab-B is a radioimmunoconjugate consisting of the murine monoclonal antibody BC8 and an iodine-131 radioisotope. The Fred Hutchinson Cancer Research Center developed BC8 to target CD45, a panleukocytic antigen widely expressed on white blood cells. “When labeled with radioactive isotopes, BC8 carries radioactivity directly to the site of cancerous growth and bone marrow while avoiding effects of radiation on most healthy tissues,” says a statement from Actinium Pharmaceuticals, which would market the drug.
Iomab-B will be tested in a multicenter trial that will include 150 patients over age 55 with refractory and relapsed AML. “There has not been a new drug approved for relapsed and refractory AML patients over the age of 55 in decades and with Iomab-B being the only therapy of its kind, we are pleased to have achieved this important milestone,” Sandesh Seth, executive chairman of Actinium, said in the statement.
A radioimmunotherapeutic drug for conditioning relapsed and refractory acute myeloid leukemia (AML) patients for a hematopoietic stem cell transplant has been granted orphan drug designation by the Food and Drug Administration.
Iomab-B is a radioimmunoconjugate consisting of the murine monoclonal antibody BC8 and an iodine-131 radioisotope. The Fred Hutchinson Cancer Research Center developed BC8 to target CD45, a panleukocytic antigen widely expressed on white blood cells. “When labeled with radioactive isotopes, BC8 carries radioactivity directly to the site of cancerous growth and bone marrow while avoiding effects of radiation on most healthy tissues,” says a statement from Actinium Pharmaceuticals, which would market the drug.
Iomab-B will be tested in a multicenter trial that will include 150 patients over age 55 with refractory and relapsed AML. “There has not been a new drug approved for relapsed and refractory AML patients over the age of 55 in decades and with Iomab-B being the only therapy of its kind, we are pleased to have achieved this important milestone,” Sandesh Seth, executive chairman of Actinium, said in the statement.