User login
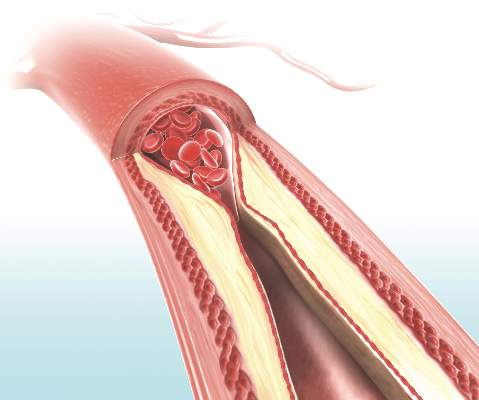
Only ‘early’ estradiol limits atherosclerosis progression
Hormone therapy – estradiol with or without progesterone – only limits the progression of subclinical atherosclerosis if it is initiated within 6 years of menopause onset, according to a report published online March 30 in the New England Journal of Medicine.
The “hormone-timing hypothesis” posits that hormone therapy’s beneficial effects on atherosclerosis depend on the timing of initiating that therapy relative to menopause. To test this hypothesis, researchers began the ELITE study (Early versus Late Intervention Trial with Estradiol) in 2002, using serial noninvasive measurements of carotid-artery intima-media thickness (CIMT) as a marker of atherosclerosis progression.
Several other studies since 2002 have reported that the timing hypothesis appears to be valid, wrote Dr. Howard N. Hodis of the Atherosclerosis Research Unit, University of Southern California, Los Angeles, and his associates.
Their single-center trial involved 643 healthy postmenopausal women who had no diabetes and no evidence of cardiovascular disease at baseline, and who were randomly assigned to receive either daily oral estradiol or a matching placebo for 5 years. Women who had an intact uterus and took active estradiol also received a 4% micronized progesterone vaginal gel, while those who had an intact uterus and took placebo also received a matching placebo gel.
The participants were stratified according to the number of years they were past menopause: less than 6 years (271 women in the “early” group) or more than 10 years (372 in the “late” group).
A total of 137 women in the early group and 186 women in the late group were assigned to active estradiol, while 134 women in the early group and 186 women in the late group were assigned to placebo. As expected, serum estradiol levels were at least 3 times higher among women assigned to active treatment, compared with those assigned to placebo.
The primary outcome – the effect of hormone therapy on CIMT progression – differed by timing of the initiation of treatment. In the “early” group, the mean CIMT progression rate was decreased by 0.0034 mm per year with estradiol, compared with placebo.
In contrast, in the “late” group, the rates of CIMT progression were not significantly different between estradiol and placebo, the investigators wrote (N Engl J Med. 2016;374:1221-31. doi: 10.1056/NEJMoa1505241).
This beneficial effect remained significant in a sensitivity analysis restricted only to study participants who showed at least 80% adherence to their assigned treatment. The benefit also remained significant in a post-hoc analysis comparing women who took estradiol alone against those who took estradiol plus progestogen, as well as in a separate analysis comparing women who used lipid-lowering and/or hypertensive medications against those who did not.
The findings add further evidence in favor of the hormone timing hypothesis. The effect of estradiol therapy on CIMT progression was significantly modified by time since menopause (P = .007 for the interaction), the researchers wrote.
Cardiac computer tomography (CT) was used as a different method of assessing coronary atherosclerosis in a subgroup of 167 women in the early group (88 receiving estradiol and 79 receiving placebo) and 214 in the late group (101 receiving estradiol and 113 receiving placebo). The timing of estradiol treatment did not affect coronary artery calcium and other cardiac CT measures. This is consistent with previous reports that hormone therapy has no significant effect on established lesions in the coronary arteries, the researchers wrote.
The ELITE trial was funded by the National Institute on Aging. Dr. Hodis reported having no relevant financial disclosures; two of his associates reported ties to GE and TherapeuticsMD.
Despite the favorable effect of estrogen on atherosclerosis in early postmenopausal women in the ELITE trial, the relevance of these results to clinical coronary heart disease events remains questionable. The trial assessed only surrogate measures of coronary heart disease and was not designed or powered to assess clinical events. The occurrence of myocardial infarction and stroke involves not only atherosclerotic plaque formation but also plaque rupture and thrombosis. Any changes in these latter two phenomena would not be captured by the CIMT measurements in ELITE — a point of particular interest, given that postmenopausal hormone therapy may promote thrombosis and inflammation. A final caution is that the available clinical data in support of the timing hypothesis are suggestive but inconsistent.
Guidelines from various professional organizations currently caution against using postmenopausal hormone therapy for the purpose of preventing cardiovascular events. Although the ELITE trial results support the hypothesis that postmenopausal hormone therapy may have more favorable effects on atherosclerosis when initiated soon after menopause, extrapolation of these results to clinical events would be premature, and the present guidance remains prudent.
Dr. John F. Keaney, Jr., is at the University of Massachusetts, Worcester and is an associate editor at the New England Journal of Medicine, and Dr. Caren G. Solomon is a deputy editor at the New England Journal of Medicine. They reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (N Engl J Med. 2016 Mar 30. doi: 10.1056/NEJMe1602846).
Despite the favorable effect of estrogen on atherosclerosis in early postmenopausal women in the ELITE trial, the relevance of these results to clinical coronary heart disease events remains questionable. The trial assessed only surrogate measures of coronary heart disease and was not designed or powered to assess clinical events. The occurrence of myocardial infarction and stroke involves not only atherosclerotic plaque formation but also plaque rupture and thrombosis. Any changes in these latter two phenomena would not be captured by the CIMT measurements in ELITE — a point of particular interest, given that postmenopausal hormone therapy may promote thrombosis and inflammation. A final caution is that the available clinical data in support of the timing hypothesis are suggestive but inconsistent.
Guidelines from various professional organizations currently caution against using postmenopausal hormone therapy for the purpose of preventing cardiovascular events. Although the ELITE trial results support the hypothesis that postmenopausal hormone therapy may have more favorable effects on atherosclerosis when initiated soon after menopause, extrapolation of these results to clinical events would be premature, and the present guidance remains prudent.
Dr. John F. Keaney, Jr., is at the University of Massachusetts, Worcester and is an associate editor at the New England Journal of Medicine, and Dr. Caren G. Solomon is a deputy editor at the New England Journal of Medicine. They reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (N Engl J Med. 2016 Mar 30. doi: 10.1056/NEJMe1602846).
Despite the favorable effect of estrogen on atherosclerosis in early postmenopausal women in the ELITE trial, the relevance of these results to clinical coronary heart disease events remains questionable. The trial assessed only surrogate measures of coronary heart disease and was not designed or powered to assess clinical events. The occurrence of myocardial infarction and stroke involves not only atherosclerotic plaque formation but also plaque rupture and thrombosis. Any changes in these latter two phenomena would not be captured by the CIMT measurements in ELITE — a point of particular interest, given that postmenopausal hormone therapy may promote thrombosis and inflammation. A final caution is that the available clinical data in support of the timing hypothesis are suggestive but inconsistent.
Guidelines from various professional organizations currently caution against using postmenopausal hormone therapy for the purpose of preventing cardiovascular events. Although the ELITE trial results support the hypothesis that postmenopausal hormone therapy may have more favorable effects on atherosclerosis when initiated soon after menopause, extrapolation of these results to clinical events would be premature, and the present guidance remains prudent.
Dr. John F. Keaney, Jr., is at the University of Massachusetts, Worcester and is an associate editor at the New England Journal of Medicine, and Dr. Caren G. Solomon is a deputy editor at the New England Journal of Medicine. They reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (N Engl J Med. 2016 Mar 30. doi: 10.1056/NEJMe1602846).
Hormone therapy – estradiol with or without progesterone – only limits the progression of subclinical atherosclerosis if it is initiated within 6 years of menopause onset, according to a report published online March 30 in the New England Journal of Medicine.
The “hormone-timing hypothesis” posits that hormone therapy’s beneficial effects on atherosclerosis depend on the timing of initiating that therapy relative to menopause. To test this hypothesis, researchers began the ELITE study (Early versus Late Intervention Trial with Estradiol) in 2002, using serial noninvasive measurements of carotid-artery intima-media thickness (CIMT) as a marker of atherosclerosis progression.
Several other studies since 2002 have reported that the timing hypothesis appears to be valid, wrote Dr. Howard N. Hodis of the Atherosclerosis Research Unit, University of Southern California, Los Angeles, and his associates.
Their single-center trial involved 643 healthy postmenopausal women who had no diabetes and no evidence of cardiovascular disease at baseline, and who were randomly assigned to receive either daily oral estradiol or a matching placebo for 5 years. Women who had an intact uterus and took active estradiol also received a 4% micronized progesterone vaginal gel, while those who had an intact uterus and took placebo also received a matching placebo gel.
The participants were stratified according to the number of years they were past menopause: less than 6 years (271 women in the “early” group) or more than 10 years (372 in the “late” group).
A total of 137 women in the early group and 186 women in the late group were assigned to active estradiol, while 134 women in the early group and 186 women in the late group were assigned to placebo. As expected, serum estradiol levels were at least 3 times higher among women assigned to active treatment, compared with those assigned to placebo.
The primary outcome – the effect of hormone therapy on CIMT progression – differed by timing of the initiation of treatment. In the “early” group, the mean CIMT progression rate was decreased by 0.0034 mm per year with estradiol, compared with placebo.
In contrast, in the “late” group, the rates of CIMT progression were not significantly different between estradiol and placebo, the investigators wrote (N Engl J Med. 2016;374:1221-31. doi: 10.1056/NEJMoa1505241).
This beneficial effect remained significant in a sensitivity analysis restricted only to study participants who showed at least 80% adherence to their assigned treatment. The benefit also remained significant in a post-hoc analysis comparing women who took estradiol alone against those who took estradiol plus progestogen, as well as in a separate analysis comparing women who used lipid-lowering and/or hypertensive medications against those who did not.
The findings add further evidence in favor of the hormone timing hypothesis. The effect of estradiol therapy on CIMT progression was significantly modified by time since menopause (P = .007 for the interaction), the researchers wrote.
Cardiac computer tomography (CT) was used as a different method of assessing coronary atherosclerosis in a subgroup of 167 women in the early group (88 receiving estradiol and 79 receiving placebo) and 214 in the late group (101 receiving estradiol and 113 receiving placebo). The timing of estradiol treatment did not affect coronary artery calcium and other cardiac CT measures. This is consistent with previous reports that hormone therapy has no significant effect on established lesions in the coronary arteries, the researchers wrote.
The ELITE trial was funded by the National Institute on Aging. Dr. Hodis reported having no relevant financial disclosures; two of his associates reported ties to GE and TherapeuticsMD.
Hormone therapy – estradiol with or without progesterone – only limits the progression of subclinical atherosclerosis if it is initiated within 6 years of menopause onset, according to a report published online March 30 in the New England Journal of Medicine.
The “hormone-timing hypothesis” posits that hormone therapy’s beneficial effects on atherosclerosis depend on the timing of initiating that therapy relative to menopause. To test this hypothesis, researchers began the ELITE study (Early versus Late Intervention Trial with Estradiol) in 2002, using serial noninvasive measurements of carotid-artery intima-media thickness (CIMT) as a marker of atherosclerosis progression.
Several other studies since 2002 have reported that the timing hypothesis appears to be valid, wrote Dr. Howard N. Hodis of the Atherosclerosis Research Unit, University of Southern California, Los Angeles, and his associates.
Their single-center trial involved 643 healthy postmenopausal women who had no diabetes and no evidence of cardiovascular disease at baseline, and who were randomly assigned to receive either daily oral estradiol or a matching placebo for 5 years. Women who had an intact uterus and took active estradiol also received a 4% micronized progesterone vaginal gel, while those who had an intact uterus and took placebo also received a matching placebo gel.
The participants were stratified according to the number of years they were past menopause: less than 6 years (271 women in the “early” group) or more than 10 years (372 in the “late” group).
A total of 137 women in the early group and 186 women in the late group were assigned to active estradiol, while 134 women in the early group and 186 women in the late group were assigned to placebo. As expected, serum estradiol levels were at least 3 times higher among women assigned to active treatment, compared with those assigned to placebo.
The primary outcome – the effect of hormone therapy on CIMT progression – differed by timing of the initiation of treatment. In the “early” group, the mean CIMT progression rate was decreased by 0.0034 mm per year with estradiol, compared with placebo.
In contrast, in the “late” group, the rates of CIMT progression were not significantly different between estradiol and placebo, the investigators wrote (N Engl J Med. 2016;374:1221-31. doi: 10.1056/NEJMoa1505241).
This beneficial effect remained significant in a sensitivity analysis restricted only to study participants who showed at least 80% adherence to their assigned treatment. The benefit also remained significant in a post-hoc analysis comparing women who took estradiol alone against those who took estradiol plus progestogen, as well as in a separate analysis comparing women who used lipid-lowering and/or hypertensive medications against those who did not.
The findings add further evidence in favor of the hormone timing hypothesis. The effect of estradiol therapy on CIMT progression was significantly modified by time since menopause (P = .007 for the interaction), the researchers wrote.
Cardiac computer tomography (CT) was used as a different method of assessing coronary atherosclerosis in a subgroup of 167 women in the early group (88 receiving estradiol and 79 receiving placebo) and 214 in the late group (101 receiving estradiol and 113 receiving placebo). The timing of estradiol treatment did not affect coronary artery calcium and other cardiac CT measures. This is consistent with previous reports that hormone therapy has no significant effect on established lesions in the coronary arteries, the researchers wrote.
The ELITE trial was funded by the National Institute on Aging. Dr. Hodis reported having no relevant financial disclosures; two of his associates reported ties to GE and TherapeuticsMD.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Estradiol only limits the progression of subclinical atherosclerosis if it is initiated within 6 years of menopause, not later.
Major finding: The mean CIMT progression rate was decreased by 0.0034 mm per year with estradiol, compared with placebo, but only in women who initiated hormone therapy within 6 years of menopause onset.
Data source: A single-center randomized, double-blind, placebo-controlled trial involving 643 healthy postmenopausal women treated for 5 years.
Disclosures: The ELITE trial was funded by the National Institute on Aging. Dr. Hodis reported having no relevant financial disclosures; two of his associates reported ties to GE and TherapeuticsMD.
Can the Mediterranean Diet Reduce the Risk of Hip Fracture?
Eating a Mediterranean diet full of fruits, vegetables, fish, nuts, legumes, and whole grains is associated with a slightly lower risk of hip fracture in women, according to a study published online ahead of print in JAMA Internal Medicine.
Researchers analyzed data on diet and fracture risk in more than 90,000 postmenopausal women (average age, 63.6 years) who were followed for an average of almost 16 years. Diet quality and adherence were assessed by scores on 4 scales: the alternate Mediterranean Diet (aMED); the Healthy Eating Index 2010 (HEI-2010); the Alternate Healthy Eating Index 2010 (AHEI-2010); and the Dietary Approaches to Stop Hypertension (DASH) diet.
Women who scored the highest for adherence to a Mediterranean diet were at lower risk for hip fractures, although the absolute risk reduction was 0.29%. There was no association between a Mediterranean diet and total fracture risk.
A higher HEI-2010 or DASH score was inversely related to the risk of hip fracture, but the finding was not statistically significant. There was no association between HEI-2010, DASH and total fracture risk. The highest scores for AHEI-2010 were not significantly associated with hip or total fracture risk.
Suggested Reading
Haring B, Crandall CJ, Wu C, et al. Dietary patterns and fractures in postmenopausal women: results from the women's health initiative. JAMA Intern Med. 2016 Mar 28. [Epub ahead of print]
Eating a Mediterranean diet full of fruits, vegetables, fish, nuts, legumes, and whole grains is associated with a slightly lower risk of hip fracture in women, according to a study published online ahead of print in JAMA Internal Medicine.
Researchers analyzed data on diet and fracture risk in more than 90,000 postmenopausal women (average age, 63.6 years) who were followed for an average of almost 16 years. Diet quality and adherence were assessed by scores on 4 scales: the alternate Mediterranean Diet (aMED); the Healthy Eating Index 2010 (HEI-2010); the Alternate Healthy Eating Index 2010 (AHEI-2010); and the Dietary Approaches to Stop Hypertension (DASH) diet.
Women who scored the highest for adherence to a Mediterranean diet were at lower risk for hip fractures, although the absolute risk reduction was 0.29%. There was no association between a Mediterranean diet and total fracture risk.
A higher HEI-2010 or DASH score was inversely related to the risk of hip fracture, but the finding was not statistically significant. There was no association between HEI-2010, DASH and total fracture risk. The highest scores for AHEI-2010 were not significantly associated with hip or total fracture risk.
Eating a Mediterranean diet full of fruits, vegetables, fish, nuts, legumes, and whole grains is associated with a slightly lower risk of hip fracture in women, according to a study published online ahead of print in JAMA Internal Medicine.
Researchers analyzed data on diet and fracture risk in more than 90,000 postmenopausal women (average age, 63.6 years) who were followed for an average of almost 16 years. Diet quality and adherence were assessed by scores on 4 scales: the alternate Mediterranean Diet (aMED); the Healthy Eating Index 2010 (HEI-2010); the Alternate Healthy Eating Index 2010 (AHEI-2010); and the Dietary Approaches to Stop Hypertension (DASH) diet.
Women who scored the highest for adherence to a Mediterranean diet were at lower risk for hip fractures, although the absolute risk reduction was 0.29%. There was no association between a Mediterranean diet and total fracture risk.
A higher HEI-2010 or DASH score was inversely related to the risk of hip fracture, but the finding was not statistically significant. There was no association between HEI-2010, DASH and total fracture risk. The highest scores for AHEI-2010 were not significantly associated with hip or total fracture risk.
Suggested Reading
Haring B, Crandall CJ, Wu C, et al. Dietary patterns and fractures in postmenopausal women: results from the women's health initiative. JAMA Intern Med. 2016 Mar 28. [Epub ahead of print]
Suggested Reading
Haring B, Crandall CJ, Wu C, et al. Dietary patterns and fractures in postmenopausal women: results from the women's health initiative. JAMA Intern Med. 2016 Mar 28. [Epub ahead of print]
Rate of Tommy John Surgeries in Young Athletes Is on the Rise
An increasing number of adolescents are undergoing ulnar lateral ligament (UCL) surgery to repair a pitching-related elbow injury, according to a study published online ahead of print in the March issue of the American Journal of Sports Medicine.
Analyzing a database of all ambulatory discharges in New York state, researchers found that 444 patients underwent surgery to repair their UCL between 2002 and 2011. The median age of study participants was 21; most were male.
The total volume of UCL surgeries increased nearly 200% during that time, while the number of UCL reconstructions per 100,000 people tripled from 0.15 to 0.45. Almost all of the growth occurred in two age groups, 17- to 18-year-olds and 19- to 20-year-olds. Patients who had private insurance were 25 times more likely to undergo UCL construction than those with Medicaid.
Suggested Reading
Hodgins JL, Vitale M, Arons RR, et al. Epidemiology of Medial Ulnar Collateral Ligament Reconstruction. Am J Sports Med. 2016 Mar;44(3):729-34. [Epub ahead of print].
An increasing number of adolescents are undergoing ulnar lateral ligament (UCL) surgery to repair a pitching-related elbow injury, according to a study published online ahead of print in the March issue of the American Journal of Sports Medicine.
Analyzing a database of all ambulatory discharges in New York state, researchers found that 444 patients underwent surgery to repair their UCL between 2002 and 2011. The median age of study participants was 21; most were male.
The total volume of UCL surgeries increased nearly 200% during that time, while the number of UCL reconstructions per 100,000 people tripled from 0.15 to 0.45. Almost all of the growth occurred in two age groups, 17- to 18-year-olds and 19- to 20-year-olds. Patients who had private insurance were 25 times more likely to undergo UCL construction than those with Medicaid.
An increasing number of adolescents are undergoing ulnar lateral ligament (UCL) surgery to repair a pitching-related elbow injury, according to a study published online ahead of print in the March issue of the American Journal of Sports Medicine.
Analyzing a database of all ambulatory discharges in New York state, researchers found that 444 patients underwent surgery to repair their UCL between 2002 and 2011. The median age of study participants was 21; most were male.
The total volume of UCL surgeries increased nearly 200% during that time, while the number of UCL reconstructions per 100,000 people tripled from 0.15 to 0.45. Almost all of the growth occurred in two age groups, 17- to 18-year-olds and 19- to 20-year-olds. Patients who had private insurance were 25 times more likely to undergo UCL construction than those with Medicaid.
Suggested Reading
Hodgins JL, Vitale M, Arons RR, et al. Epidemiology of Medial Ulnar Collateral Ligament Reconstruction. Am J Sports Med. 2016 Mar;44(3):729-34. [Epub ahead of print].
Suggested Reading
Hodgins JL, Vitale M, Arons RR, et al. Epidemiology of Medial Ulnar Collateral Ligament Reconstruction. Am J Sports Med. 2016 Mar;44(3):729-34. [Epub ahead of print].
ADHD Medications Are Linked to Diminished Bone Density in Young Patients
Children and adolescents who take medication for attention-deficit hyperactivity disorder (ADHD) show decreased bone density, according to a large cross-sectional study presented at the 2016 Annual Meeting of the American Academy of Orthopaedic Surgeons in Orlando.
Researchers identified 5,315 pediatric patients in the Center for Disease Control and Prevention’s National Health and Nutrition Examination Survey (NHANES) and compared children who were taking an ADHD medication (methylphenidate, desmethylphenidate, dextroamphetamine, atomoxetine, or lisdexamfetamine) with those who were not.
Children taking ADHD medication had lower bone mineral density in the femur, femoral neck, and lumbar spine. Approximately 25% of survey participants who were taking ADHD medication met criteria for osteopenia.
Researchers were able to rule out other potential causes of low bone density in these children, including age, sex, race/ethnicity, and poverty levels. However, the study did not include information on dose, duration of use, or changes in therapy because of the limitations of the NHANES survey data.
Children and adolescents who take medication for attention-deficit hyperactivity disorder (ADHD) show decreased bone density, according to a large cross-sectional study presented at the 2016 Annual Meeting of the American Academy of Orthopaedic Surgeons in Orlando.
Researchers identified 5,315 pediatric patients in the Center for Disease Control and Prevention’s National Health and Nutrition Examination Survey (NHANES) and compared children who were taking an ADHD medication (methylphenidate, desmethylphenidate, dextroamphetamine, atomoxetine, or lisdexamfetamine) with those who were not.
Children taking ADHD medication had lower bone mineral density in the femur, femoral neck, and lumbar spine. Approximately 25% of survey participants who were taking ADHD medication met criteria for osteopenia.
Researchers were able to rule out other potential causes of low bone density in these children, including age, sex, race/ethnicity, and poverty levels. However, the study did not include information on dose, duration of use, or changes in therapy because of the limitations of the NHANES survey data.
Children and adolescents who take medication for attention-deficit hyperactivity disorder (ADHD) show decreased bone density, according to a large cross-sectional study presented at the 2016 Annual Meeting of the American Academy of Orthopaedic Surgeons in Orlando.
Researchers identified 5,315 pediatric patients in the Center for Disease Control and Prevention’s National Health and Nutrition Examination Survey (NHANES) and compared children who were taking an ADHD medication (methylphenidate, desmethylphenidate, dextroamphetamine, atomoxetine, or lisdexamfetamine) with those who were not.
Children taking ADHD medication had lower bone mineral density in the femur, femoral neck, and lumbar spine. Approximately 25% of survey participants who were taking ADHD medication met criteria for osteopenia.
Researchers were able to rule out other potential causes of low bone density in these children, including age, sex, race/ethnicity, and poverty levels. However, the study did not include information on dose, duration of use, or changes in therapy because of the limitations of the NHANES survey data.
April 2016 Quiz 2
Q2: ANSWER: D
Critique
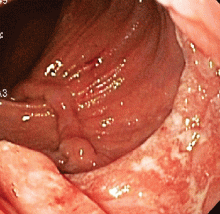
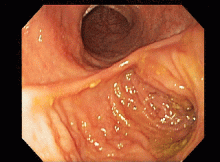
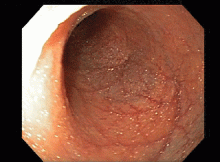
This series of endoscopic findings shows changes consistent with inflammation of the cuff.
Up to 20%-30% of patients with ulcerative colitis ultimately require colectomy due to medically refractory colitis or to the development of dysplasia. A total abdominal protocolectomy with ileal pouch anal anastomosis has become the surgical procedure of choice for most ulcerative colitis patients who require surgery.
There are both early and late complications of this type of surgery. Late complications include anastomotic stricture, pouchitis, abscesses, inflammation of the cuff or cuffitis, and functional difficulties such as irritable pouch syndrome, anal pain, and pouch stasis. Patients may also present with inflammation of the pouch and the prepouch ileum that is consistent with Crohn’s disease. Endoscopy can help to narrow down the differential diagnosis of these pouch complications.
In this series of photos the prepouch ileum has no inflammation. The pouch also has no ulcerations or evidence of pouchitis or Crohn’s disease. The cuff is inflamed consistent with a diagnosis of cuffitis. If the inflammation becomes difficult to manage, immunosuppressive therapy may be required but at this point it is reasonable to start with topical therapy to the inflamed area.
Reference
- Li, Y. Shen, B. Evaluating pouch problems. Gastroenterology Clinics North Am. 2012;41:355-78.
- Shen, G. Diagnosis and management of post-operative ileal pouch disorders. Clin Colon Rectal Surg. 2010;23:259-68.
Q2: ANSWER: D
Critique
This series of endoscopic findings shows changes consistent with inflammation of the cuff.
Up to 20%-30% of patients with ulcerative colitis ultimately require colectomy due to medically refractory colitis or to the development of dysplasia. A total abdominal protocolectomy with ileal pouch anal anastomosis has become the surgical procedure of choice for most ulcerative colitis patients who require surgery.
There are both early and late complications of this type of surgery. Late complications include anastomotic stricture, pouchitis, abscesses, inflammation of the cuff or cuffitis, and functional difficulties such as irritable pouch syndrome, anal pain, and pouch stasis. Patients may also present with inflammation of the pouch and the prepouch ileum that is consistent with Crohn’s disease. Endoscopy can help to narrow down the differential diagnosis of these pouch complications.
In this series of photos the prepouch ileum has no inflammation. The pouch also has no ulcerations or evidence of pouchitis or Crohn’s disease. The cuff is inflamed consistent with a diagnosis of cuffitis. If the inflammation becomes difficult to manage, immunosuppressive therapy may be required but at this point it is reasonable to start with topical therapy to the inflamed area.
Reference
- Li, Y. Shen, B. Evaluating pouch problems. Gastroenterology Clinics North Am. 2012;41:355-78.
- Shen, G. Diagnosis and management of post-operative ileal pouch disorders. Clin Colon Rectal Surg. 2010;23:259-68.
Q2: ANSWER: D
Critique
This series of endoscopic findings shows changes consistent with inflammation of the cuff.
Up to 20%-30% of patients with ulcerative colitis ultimately require colectomy due to medically refractory colitis or to the development of dysplasia. A total abdominal protocolectomy with ileal pouch anal anastomosis has become the surgical procedure of choice for most ulcerative colitis patients who require surgery.
There are both early and late complications of this type of surgery. Late complications include anastomotic stricture, pouchitis, abscesses, inflammation of the cuff or cuffitis, and functional difficulties such as irritable pouch syndrome, anal pain, and pouch stasis. Patients may also present with inflammation of the pouch and the prepouch ileum that is consistent with Crohn’s disease. Endoscopy can help to narrow down the differential diagnosis of these pouch complications.
In this series of photos the prepouch ileum has no inflammation. The pouch also has no ulcerations or evidence of pouchitis or Crohn’s disease. The cuff is inflamed consistent with a diagnosis of cuffitis. If the inflammation becomes difficult to manage, immunosuppressive therapy may be required but at this point it is reasonable to start with topical therapy to the inflamed area.
Reference
- Li, Y. Shen, B. Evaluating pouch problems. Gastroenterology Clinics North Am. 2012;41:355-78.
- Shen, G. Diagnosis and management of post-operative ileal pouch disorders. Clin Colon Rectal Surg. 2010;23:259-68.
Why the AMA is (now) worth joining
Until recently, I was not a member of the American Medical Association (AMA). For the past 30 years, I chose not to join because I was troubled by the organization’s direction and the way it seemed to be dominated by special interests. But things have changed—and so has its focus.
The AMA has a new strategy entitled, “A vision for a healthier nation.” Well aligned with the needs of family physicians, the campaign addresses 3 specific areas: better patient health, smarter medical training, and sustainable practices.
Better patient health. The AMA is partnering with several public health-oriented organizations, including the Centers for Disease Control and Prevention, to reach out to individuals with diabetes, cardiovascular disease, and cardiac risk factors to promote primary and secondary prevention strategies at a population level. This is a very different posture than the AMA assumed in the early 20th century, when it was more likely to resist public health programs.
Smarter medical training. Under the direction of family physician Susan Skochelak, MD, MPH, group vice president for medical education, the AMA has provided grant funding to 31 US medical schools to assist with curricular redesign and innovation to train physicians to be effective leaders in the health care system of the future.
Sustainable practices. This area houses what is perhaps the AMA’s most meaningful new program for primary care clinicians. Under the leadership of general internist Christine A. Sinsky, MD, PhD, vice president for professional satisfaction, the AMA has developed a suite of Web tools to help physicians improve the quality and efficiency of their clinical practices.
Specifically, the AMA is offering the STEPS Forward program, a collection of interactive, educational modules developed by physicians for physicians to help address common practice challenges and to achieve the quadruple aim of a better patient experience, better population health, lower overall costs, and improved professional satisfaction.1 The 27 modules are self-directed, group learning exercises that encompass a wide range of thorny issues we deal with on a daily basis. A sampling of topics includes: preparing your practice for change, revenue cycle management, synchronized prescription renewals, and creating a strong team culture.
Programs like these are evidence that the new AMA is a different organization from the one I chose not to join for the past 30 years. I am now a member. Check it out; it might be time for you to join, too.
1. STEPS Forward Series and CME Accreditation. American Medical Association Web site. Available at http://www.ama-assn.org/ama/pub/about-ama/strategic-focus/physician-practices/steps-forward.page. Accessed March 16, 2016.
Until recently, I was not a member of the American Medical Association (AMA). For the past 30 years, I chose not to join because I was troubled by the organization’s direction and the way it seemed to be dominated by special interests. But things have changed—and so has its focus.
The AMA has a new strategy entitled, “A vision for a healthier nation.” Well aligned with the needs of family physicians, the campaign addresses 3 specific areas: better patient health, smarter medical training, and sustainable practices.
Better patient health. The AMA is partnering with several public health-oriented organizations, including the Centers for Disease Control and Prevention, to reach out to individuals with diabetes, cardiovascular disease, and cardiac risk factors to promote primary and secondary prevention strategies at a population level. This is a very different posture than the AMA assumed in the early 20th century, when it was more likely to resist public health programs.
Smarter medical training. Under the direction of family physician Susan Skochelak, MD, MPH, group vice president for medical education, the AMA has provided grant funding to 31 US medical schools to assist with curricular redesign and innovation to train physicians to be effective leaders in the health care system of the future.
Sustainable practices. This area houses what is perhaps the AMA’s most meaningful new program for primary care clinicians. Under the leadership of general internist Christine A. Sinsky, MD, PhD, vice president for professional satisfaction, the AMA has developed a suite of Web tools to help physicians improve the quality and efficiency of their clinical practices.
Specifically, the AMA is offering the STEPS Forward program, a collection of interactive, educational modules developed by physicians for physicians to help address common practice challenges and to achieve the quadruple aim of a better patient experience, better population health, lower overall costs, and improved professional satisfaction.1 The 27 modules are self-directed, group learning exercises that encompass a wide range of thorny issues we deal with on a daily basis. A sampling of topics includes: preparing your practice for change, revenue cycle management, synchronized prescription renewals, and creating a strong team culture.
Programs like these are evidence that the new AMA is a different organization from the one I chose not to join for the past 30 years. I am now a member. Check it out; it might be time for you to join, too.
Until recently, I was not a member of the American Medical Association (AMA). For the past 30 years, I chose not to join because I was troubled by the organization’s direction and the way it seemed to be dominated by special interests. But things have changed—and so has its focus.
The AMA has a new strategy entitled, “A vision for a healthier nation.” Well aligned with the needs of family physicians, the campaign addresses 3 specific areas: better patient health, smarter medical training, and sustainable practices.
Better patient health. The AMA is partnering with several public health-oriented organizations, including the Centers for Disease Control and Prevention, to reach out to individuals with diabetes, cardiovascular disease, and cardiac risk factors to promote primary and secondary prevention strategies at a population level. This is a very different posture than the AMA assumed in the early 20th century, when it was more likely to resist public health programs.
Smarter medical training. Under the direction of family physician Susan Skochelak, MD, MPH, group vice president for medical education, the AMA has provided grant funding to 31 US medical schools to assist with curricular redesign and innovation to train physicians to be effective leaders in the health care system of the future.
Sustainable practices. This area houses what is perhaps the AMA’s most meaningful new program for primary care clinicians. Under the leadership of general internist Christine A. Sinsky, MD, PhD, vice president for professional satisfaction, the AMA has developed a suite of Web tools to help physicians improve the quality and efficiency of their clinical practices.
Specifically, the AMA is offering the STEPS Forward program, a collection of interactive, educational modules developed by physicians for physicians to help address common practice challenges and to achieve the quadruple aim of a better patient experience, better population health, lower overall costs, and improved professional satisfaction.1 The 27 modules are self-directed, group learning exercises that encompass a wide range of thorny issues we deal with on a daily basis. A sampling of topics includes: preparing your practice for change, revenue cycle management, synchronized prescription renewals, and creating a strong team culture.
Programs like these are evidence that the new AMA is a different organization from the one I chose not to join for the past 30 years. I am now a member. Check it out; it might be time for you to join, too.
1. STEPS Forward Series and CME Accreditation. American Medical Association Web site. Available at http://www.ama-assn.org/ama/pub/about-ama/strategic-focus/physician-practices/steps-forward.page. Accessed March 16, 2016.
1. STEPS Forward Series and CME Accreditation. American Medical Association Web site. Available at http://www.ama-assn.org/ama/pub/about-ama/strategic-focus/physician-practices/steps-forward.page. Accessed March 16, 2016.
Flamin’ hots
If you have been practicing for a while, you’re probably old enough to remember the Life cereal commercial that featured Mikey, the “he likes it” kid, who would eat anything. It was falsely rumored that he died from eating too many Pop Rocks candy with soda pop, causing his stomach to explode. Although there was no truth to any of it, it was the rumor of the day. Well, today there is a new snack on the block that is sending many children and teens to the emergency department.
Flamin’ Hots characterize several brands of chips in which the snacks are covered in a chili pepper mixture. As the name implies, the chips are very hot, which only adds to the excitement associated with them. But we have seen an increase in ED visits by teens for severe abdominal pain and red stools.
If you examine the label, it lists “natural flavors,” which is the industry’s code word for their secret formula. In fact, nowhere on the label is chili or any other spicy seasoning listed. Even more interesting is that you can’t find anywhere on the Internet what makes Flamin’ Hot chips so hot. There is evidence to support that red pepper and chili peppers are related to gastric ulcers (Crit Rev Food Sci Nutr. 2006;46[4]:275-328).
Examining the food label, one might be misled into thinking it’s is actually a reasonable snack. The serving size is listed as 21 pieces, but the likelihood that anyone stops at 21 pieces is small. In fact, there are reports that there is an addictive component. As the chili pepper hits the stomach, it causes pain, which causes a release of endorphins. This leads to a feeling of pleasure, which in turns encourages the person to want more chips, hence the ingestion of multiple bags prior to the trip to the ED.
There have been many warnings of the deleterious effects of the chips. In 2011 California, and soon after that Illinois, banned it from schools, stating it was unhealthy. Many schools since then have followed suit. But despite all the hype, kids are eating them by the dozen. Although there are no data to support that there is a cause and effect relationship with Flamin’ Hots and ulcers, it is safe to assume with the number of ED visits and the diet of the average teen, that at least gastritis is an issue.
Beyond stomach pain, prolonged intake of spicy hot snacks will contribute to high cholesterol and obesity. Many children are under the false assumption that because they don’t eat big meals, they are eating well. But because so many of these unhealthy snacks are empty calories and increase fat intake (N Engl J Med. 2006 Apr 13;354[15]:1601-13), children’s body mass indices continue to rise. Informing parents about the harmful effect is important so they can monitor intake as well as encourage healthier snacks. Understanding how to read a food label is another important thing to teach during well visits so that parents can understand how much fat is in these snacks and can adjust accordingly.
Dr. Pearce is a pediatrician in Frankfort, Ill. Email her at [email protected].
If you have been practicing for a while, you’re probably old enough to remember the Life cereal commercial that featured Mikey, the “he likes it” kid, who would eat anything. It was falsely rumored that he died from eating too many Pop Rocks candy with soda pop, causing his stomach to explode. Although there was no truth to any of it, it was the rumor of the day. Well, today there is a new snack on the block that is sending many children and teens to the emergency department.
Flamin’ Hots characterize several brands of chips in which the snacks are covered in a chili pepper mixture. As the name implies, the chips are very hot, which only adds to the excitement associated with them. But we have seen an increase in ED visits by teens for severe abdominal pain and red stools.
If you examine the label, it lists “natural flavors,” which is the industry’s code word for their secret formula. In fact, nowhere on the label is chili or any other spicy seasoning listed. Even more interesting is that you can’t find anywhere on the Internet what makes Flamin’ Hot chips so hot. There is evidence to support that red pepper and chili peppers are related to gastric ulcers (Crit Rev Food Sci Nutr. 2006;46[4]:275-328).
Examining the food label, one might be misled into thinking it’s is actually a reasonable snack. The serving size is listed as 21 pieces, but the likelihood that anyone stops at 21 pieces is small. In fact, there are reports that there is an addictive component. As the chili pepper hits the stomach, it causes pain, which causes a release of endorphins. This leads to a feeling of pleasure, which in turns encourages the person to want more chips, hence the ingestion of multiple bags prior to the trip to the ED.
There have been many warnings of the deleterious effects of the chips. In 2011 California, and soon after that Illinois, banned it from schools, stating it was unhealthy. Many schools since then have followed suit. But despite all the hype, kids are eating them by the dozen. Although there are no data to support that there is a cause and effect relationship with Flamin’ Hots and ulcers, it is safe to assume with the number of ED visits and the diet of the average teen, that at least gastritis is an issue.
Beyond stomach pain, prolonged intake of spicy hot snacks will contribute to high cholesterol and obesity. Many children are under the false assumption that because they don’t eat big meals, they are eating well. But because so many of these unhealthy snacks are empty calories and increase fat intake (N Engl J Med. 2006 Apr 13;354[15]:1601-13), children’s body mass indices continue to rise. Informing parents about the harmful effect is important so they can monitor intake as well as encourage healthier snacks. Understanding how to read a food label is another important thing to teach during well visits so that parents can understand how much fat is in these snacks and can adjust accordingly.
Dr. Pearce is a pediatrician in Frankfort, Ill. Email her at [email protected].
If you have been practicing for a while, you’re probably old enough to remember the Life cereal commercial that featured Mikey, the “he likes it” kid, who would eat anything. It was falsely rumored that he died from eating too many Pop Rocks candy with soda pop, causing his stomach to explode. Although there was no truth to any of it, it was the rumor of the day. Well, today there is a new snack on the block that is sending many children and teens to the emergency department.
Flamin’ Hots characterize several brands of chips in which the snacks are covered in a chili pepper mixture. As the name implies, the chips are very hot, which only adds to the excitement associated with them. But we have seen an increase in ED visits by teens for severe abdominal pain and red stools.
If you examine the label, it lists “natural flavors,” which is the industry’s code word for their secret formula. In fact, nowhere on the label is chili or any other spicy seasoning listed. Even more interesting is that you can’t find anywhere on the Internet what makes Flamin’ Hot chips so hot. There is evidence to support that red pepper and chili peppers are related to gastric ulcers (Crit Rev Food Sci Nutr. 2006;46[4]:275-328).
Examining the food label, one might be misled into thinking it’s is actually a reasonable snack. The serving size is listed as 21 pieces, but the likelihood that anyone stops at 21 pieces is small. In fact, there are reports that there is an addictive component. As the chili pepper hits the stomach, it causes pain, which causes a release of endorphins. This leads to a feeling of pleasure, which in turns encourages the person to want more chips, hence the ingestion of multiple bags prior to the trip to the ED.
There have been many warnings of the deleterious effects of the chips. In 2011 California, and soon after that Illinois, banned it from schools, stating it was unhealthy. Many schools since then have followed suit. But despite all the hype, kids are eating them by the dozen. Although there are no data to support that there is a cause and effect relationship with Flamin’ Hots and ulcers, it is safe to assume with the number of ED visits and the diet of the average teen, that at least gastritis is an issue.
Beyond stomach pain, prolonged intake of spicy hot snacks will contribute to high cholesterol and obesity. Many children are under the false assumption that because they don’t eat big meals, they are eating well. But because so many of these unhealthy snacks are empty calories and increase fat intake (N Engl J Med. 2006 Apr 13;354[15]:1601-13), children’s body mass indices continue to rise. Informing parents about the harmful effect is important so they can monitor intake as well as encourage healthier snacks. Understanding how to read a food label is another important thing to teach during well visits so that parents can understand how much fat is in these snacks and can adjust accordingly.
Dr. Pearce is a pediatrician in Frankfort, Ill. Email her at [email protected].
Biologics for Psoriasis
Review the PDF of the fact sheet on biologics for psoriasis with board-relevant, easy-to-review material. This month's fact sheet discusses the current US Food and Drug Administration–approved biologic medications for psoriasis and psoriatic arthritis, including the mechanism of action, dosing, and side effects.
Practice Questions
1. Which biologic is administered as an intravenous infusion?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
2. Which biologic is dosed based on body weight?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
3. Which biologic has been shown to worsen existing Crohn disease?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
4. Which biologic is a fusion protein?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
5. Which biologic has been shown to cause reversible posterior leukoencephalopathy syndrome?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
Answers to practice questions provided on next page
Practice Question Answers
1. Which biologic is administered as an intravenous infusion?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
2. Which biologic is dosed based on body weight?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
3. Which biologic has been shown to worsen existing Crohn disease?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
4. Which biologic is a fusion protein?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
5. Which biologic has been shown to cause reversible posterior leukoencephalopathy syndrome?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
Review the PDF of the fact sheet on biologics for psoriasis with board-relevant, easy-to-review material. This month's fact sheet discusses the current US Food and Drug Administration–approved biologic medications for psoriasis and psoriatic arthritis, including the mechanism of action, dosing, and side effects.
Practice Questions
1. Which biologic is administered as an intravenous infusion?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
2. Which biologic is dosed based on body weight?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
3. Which biologic has been shown to worsen existing Crohn disease?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
4. Which biologic is a fusion protein?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
5. Which biologic has been shown to cause reversible posterior leukoencephalopathy syndrome?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
Answers to practice questions provided on next page
Practice Question Answers
1. Which biologic is administered as an intravenous infusion?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
2. Which biologic is dosed based on body weight?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
3. Which biologic has been shown to worsen existing Crohn disease?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
4. Which biologic is a fusion protein?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
5. Which biologic has been shown to cause reversible posterior leukoencephalopathy syndrome?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
Review the PDF of the fact sheet on biologics for psoriasis with board-relevant, easy-to-review material. This month's fact sheet discusses the current US Food and Drug Administration–approved biologic medications for psoriasis and psoriatic arthritis, including the mechanism of action, dosing, and side effects.
Practice Questions
1. Which biologic is administered as an intravenous infusion?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
2. Which biologic is dosed based on body weight?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
3. Which biologic has been shown to worsen existing Crohn disease?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
4. Which biologic is a fusion protein?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
5. Which biologic has been shown to cause reversible posterior leukoencephalopathy syndrome?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
Answers to practice questions provided on next page
Practice Question Answers
1. Which biologic is administered as an intravenous infusion?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
2. Which biologic is dosed based on body weight?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
3. Which biologic has been shown to worsen existing Crohn disease?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
4. Which biologic is a fusion protein?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
5. Which biologic has been shown to cause reversible posterior leukoencephalopathy syndrome?
a. adalimumab
b. etanercept
c. infliximab
d. secukinumab
e. ustekinumab
Statins inversely linked to colorectal cancer in patients with IBD
Patients with inflammatory bowel disease who were prescribed statins had 65% lower odds of subsequent colorectal cancer, compared with other IBD patients, even after controlling for multiple potential confounders, researchers reported in Clinical Gastroenterology and Hepatology.
“Further confirmation from other cohorts may provide support for the use of statins as a chemopreventive in patients with IBD,” said Dr. Ashwin Ananthakrishnan of Massachusetts General Hospital in Boston, and his associates.
Patients with long-standing ulcerative colitis or colonic Crohn’s disease have about twice the risk of colorectal cancer (CRC), compared with the general population, and up to an 18% lifetime risk of CRC by 30 years after diagnosis, the researchers noted. Early results supporting mesalamine as chemoprophylaxis did not hold up in later trials. Although several studies suggested that statins might help prevent sporadic colon cancer, the only such study in IBD patients was small and did not control for key covariates such as smoking, the investigators added. Therefore, they collected data from 11,001 patients with IBD who were seen at Boston area hospitals between 1998 and 2010. They identified CRC diagnoses based on ICD-9 codes, and analyzed electronic prescriptions to see whether and when patients had used statins (Clin Gastroenterol Hepatol. 2016 Feb 21. doi: 10.1016/j.cgh.2016.02.017).
A total of 1,376 patients (12.5%) were prescribed at least one statin. Over 9 years of follow-up, 2% of statin users developed CRC, compared with 3% of nonusers (age-adjusted odds ratio, 0.35; 95% confidence interval, 0.24-0.53). Statin users were more likely to be older, male, white, smokers, and had more comorbidities than nonusers. Nonetheless, the protective effect of statins remained significant after controlling for demographic factors, smoking status, number of colonoscopies, use of steroids and immunomodulators, the presence of primary sclerosing cholangitis, and increases in inflammatory biomarkers (OR, 0.42; 95% CI, 0.28-0.62). The effect occurred for both Crohn’s disease and ulcerative colitis. Notably, the inverse association was even stronger among patients who had been prescribed at least two statins or who had at least a 2-year interval between statin use and CRC diagnosis.
Statins might help prevent CRC through HMG-CoA reductase inhibition and other mechanisms, according to the researchers. By inhibiting HMG-CoA reductase, statins lower production of farnesyl pyrophosphate and geranylgeranyl pyrophosphate, which are needed for post-translational activation of Ras, Rho, and other proteins that are overexpressed in CRC and that have been linked to tumor invasion. Statins also might help prevent CRC through antioxidant effects or by inhibiting inflammation, cell adhesion, and angiogenesis, the investigators added. “Although we did not see a difference in median C-reactive protein levels between statin users and nonusers, statin users were less likely to require immunomodulator or biologic therapy for their IBD, supporting a potential anti-inflammatory role for statins.”
Because patients mainly were treated at two tertiary referral hospitals, they may have had more severe disease than the general population of patients with IBD, the investigators acknowledged. They noted that in some meta-analyses, referral center studies yielded chemopreventive effects that did not hold up in population-based cohorts.
The study was funded by the National Institutes of Health, the American Gastroenterological Association, and the Harold and Duval Bowen Fund. The researchers had no disclosures.
Patients with inflammatory bowel disease who were prescribed statins had 65% lower odds of subsequent colorectal cancer, compared with other IBD patients, even after controlling for multiple potential confounders, researchers reported in Clinical Gastroenterology and Hepatology.
“Further confirmation from other cohorts may provide support for the use of statins as a chemopreventive in patients with IBD,” said Dr. Ashwin Ananthakrishnan of Massachusetts General Hospital in Boston, and his associates.
Patients with long-standing ulcerative colitis or colonic Crohn’s disease have about twice the risk of colorectal cancer (CRC), compared with the general population, and up to an 18% lifetime risk of CRC by 30 years after diagnosis, the researchers noted. Early results supporting mesalamine as chemoprophylaxis did not hold up in later trials. Although several studies suggested that statins might help prevent sporadic colon cancer, the only such study in IBD patients was small and did not control for key covariates such as smoking, the investigators added. Therefore, they collected data from 11,001 patients with IBD who were seen at Boston area hospitals between 1998 and 2010. They identified CRC diagnoses based on ICD-9 codes, and analyzed electronic prescriptions to see whether and when patients had used statins (Clin Gastroenterol Hepatol. 2016 Feb 21. doi: 10.1016/j.cgh.2016.02.017).
A total of 1,376 patients (12.5%) were prescribed at least one statin. Over 9 years of follow-up, 2% of statin users developed CRC, compared with 3% of nonusers (age-adjusted odds ratio, 0.35; 95% confidence interval, 0.24-0.53). Statin users were more likely to be older, male, white, smokers, and had more comorbidities than nonusers. Nonetheless, the protective effect of statins remained significant after controlling for demographic factors, smoking status, number of colonoscopies, use of steroids and immunomodulators, the presence of primary sclerosing cholangitis, and increases in inflammatory biomarkers (OR, 0.42; 95% CI, 0.28-0.62). The effect occurred for both Crohn’s disease and ulcerative colitis. Notably, the inverse association was even stronger among patients who had been prescribed at least two statins or who had at least a 2-year interval between statin use and CRC diagnosis.
Statins might help prevent CRC through HMG-CoA reductase inhibition and other mechanisms, according to the researchers. By inhibiting HMG-CoA reductase, statins lower production of farnesyl pyrophosphate and geranylgeranyl pyrophosphate, which are needed for post-translational activation of Ras, Rho, and other proteins that are overexpressed in CRC and that have been linked to tumor invasion. Statins also might help prevent CRC through antioxidant effects or by inhibiting inflammation, cell adhesion, and angiogenesis, the investigators added. “Although we did not see a difference in median C-reactive protein levels between statin users and nonusers, statin users were less likely to require immunomodulator or biologic therapy for their IBD, supporting a potential anti-inflammatory role for statins.”
Because patients mainly were treated at two tertiary referral hospitals, they may have had more severe disease than the general population of patients with IBD, the investigators acknowledged. They noted that in some meta-analyses, referral center studies yielded chemopreventive effects that did not hold up in population-based cohorts.
The study was funded by the National Institutes of Health, the American Gastroenterological Association, and the Harold and Duval Bowen Fund. The researchers had no disclosures.
Patients with inflammatory bowel disease who were prescribed statins had 65% lower odds of subsequent colorectal cancer, compared with other IBD patients, even after controlling for multiple potential confounders, researchers reported in Clinical Gastroenterology and Hepatology.
“Further confirmation from other cohorts may provide support for the use of statins as a chemopreventive in patients with IBD,” said Dr. Ashwin Ananthakrishnan of Massachusetts General Hospital in Boston, and his associates.
Patients with long-standing ulcerative colitis or colonic Crohn’s disease have about twice the risk of colorectal cancer (CRC), compared with the general population, and up to an 18% lifetime risk of CRC by 30 years after diagnosis, the researchers noted. Early results supporting mesalamine as chemoprophylaxis did not hold up in later trials. Although several studies suggested that statins might help prevent sporadic colon cancer, the only such study in IBD patients was small and did not control for key covariates such as smoking, the investigators added. Therefore, they collected data from 11,001 patients with IBD who were seen at Boston area hospitals between 1998 and 2010. They identified CRC diagnoses based on ICD-9 codes, and analyzed electronic prescriptions to see whether and when patients had used statins (Clin Gastroenterol Hepatol. 2016 Feb 21. doi: 10.1016/j.cgh.2016.02.017).
A total of 1,376 patients (12.5%) were prescribed at least one statin. Over 9 years of follow-up, 2% of statin users developed CRC, compared with 3% of nonusers (age-adjusted odds ratio, 0.35; 95% confidence interval, 0.24-0.53). Statin users were more likely to be older, male, white, smokers, and had more comorbidities than nonusers. Nonetheless, the protective effect of statins remained significant after controlling for demographic factors, smoking status, number of colonoscopies, use of steroids and immunomodulators, the presence of primary sclerosing cholangitis, and increases in inflammatory biomarkers (OR, 0.42; 95% CI, 0.28-0.62). The effect occurred for both Crohn’s disease and ulcerative colitis. Notably, the inverse association was even stronger among patients who had been prescribed at least two statins or who had at least a 2-year interval between statin use and CRC diagnosis.
Statins might help prevent CRC through HMG-CoA reductase inhibition and other mechanisms, according to the researchers. By inhibiting HMG-CoA reductase, statins lower production of farnesyl pyrophosphate and geranylgeranyl pyrophosphate, which are needed for post-translational activation of Ras, Rho, and other proteins that are overexpressed in CRC and that have been linked to tumor invasion. Statins also might help prevent CRC through antioxidant effects or by inhibiting inflammation, cell adhesion, and angiogenesis, the investigators added. “Although we did not see a difference in median C-reactive protein levels between statin users and nonusers, statin users were less likely to require immunomodulator or biologic therapy for their IBD, supporting a potential anti-inflammatory role for statins.”
Because patients mainly were treated at two tertiary referral hospitals, they may have had more severe disease than the general population of patients with IBD, the investigators acknowledged. They noted that in some meta-analyses, referral center studies yielded chemopreventive effects that did not hold up in population-based cohorts.
The study was funded by the National Institutes of Health, the American Gastroenterological Association, and the Harold and Duval Bowen Fund. The researchers had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Preliminary evidence suggests that statins might help prevent colorectal cancer in patients with inflammatory bowel disease.
Major finding: A total of 2% of statin users developed CRC over 9 years of follow-up, compared with 3% of nonusers (age-adjusted odds ratio, 0.35).
Data source: An analysis of ICD-9 codes and electronic prescription data for 11,001 patients with IBD.
Disclosures: The study was funded by the National Institutes of Health, the American Gastroenterological Association, and the Harold and Duval Bowen Fund. The researchers had no disclosures.
Surgery vs conservative management for AC joint repair: How do the 2 compare?
When not considering the grade of acromioclavicular (AC) joint dislocation, both conservative and surgical management lead to positive outcomes, although surgically managed patients require more time out of work (strength of recommendation [SOR]: B, Cochrane review of low-quality randomized controlled trials [RCTs]).
For Rockwood grade III dislocations, surgical intervention provides a better cosmetic outcome but increases infection risk (SOR: B, meta-analysis of retrospective case series).
Consensus guidelines suggest conservative management for Rockwood grade I to II dislocations and surgical repair for Rockwood grade IV to VI dislocations (SOR: C, expert opinion).
Similar outcomes, except when it comes to return to work
A 2010 Cochrane review of 2 RCTs and one quasi-randomized trial (174 patients, 93% male, moderate to high risk of bias) compared surgical intervention with conservative management of acute AC separations of unspecified Rockwood classification.1 Surgeries included coracoclavicular fixation with a cancellous screw or transfixation of the AC joint with Steinmann pins or Kirschner wires.
Conservative treatment included immobilization of the shoulder using an arm sling for 2 to 4 weeks. Patients were evaluated for a minimum of 12 months with a nonvalidated scoring system that measured pain, motion, and function or strength.
At one year, 63 of 76 patients (83%) in the post-surgical group and 74 of 84 patients (88%) in the conservative intervention group had either good or excellent results with no significant difference in unsatisfactory outcomes (relative risk [RR]=1.49; 95% confidence interval [CI], 0.75-2.95). (Fourteen patients—7 in each group—were lost to follow-up.) Moreover, the review found no significant difference in treatment failures requiring a subsequent operation between the groups—11 of 83 (13%) surgical patients and 7 of 91 (8%) conservatively managed patients (RR=1.72; 95% CI, 0.72-4.12).
Notably, regardless of activity level, surgical patients consistently returned to previous work functions later than patients managed conservatively. The mean convalescence time ranged from 8 to 11 weeks for surgical patients and 4 to 6 weeks for conservatively managed patients (P<.05).
A look at cosmetic results and risk of infection
A 2011 meta-analysis of 6 retrospective case series (379 patients, approximately 88% male) compared operative with nonoperative management in patients with acute, closed Rockwood grade III AC dislocations.2 Operative techniques varied; nonoperative patients each received physiotherapy or rehabilitation therapy and most were treated with a sling. Patient follow-up varied from 32 months to 10.8 years.
Four of the included studies suggested that nonoperative management resulted in poorer cosmetic results (methods not defined) compared with the operative group (11 of 115 surgical patients [10%], 74 of 88 nonoperative patients [84%]; risk difference [RD]=−0.79; 95% CI, −0.92 to −0.66; number needed to harm [NNH]=>2). Two of the studies evaluated the duration of sick leave and found a longer leave with operative management (50 operative and 54 nonoperative patients; mean difference=3.3; 95% CI, 2.1-4.5).
Five of the studies observed an increased risk of infection following operative management (8 of 175 [5%] operative patients compared with 0 of 152 [0%] nonoperative patients; RD=0.05; 95% CI, 0.01-0.09; NNH=20).
Recommendations depend on the grade of the injury
The American College of Occupational and Environmental Medicine recommends against routine surgical repair for Grade III AC joint separations.3 The College also recommends nonoperative management for patients with grade I to II AC dislocations and surgical repair for patients with grades IV to VI and select grade III AC dislocations.
1. Tamaoki MJS, Belloti JC, Lenza M, et al. Surgical versus conservative interventions for treating acromioclavicular dislocation of the shoulder in adults. Cochrane Database Syst Rev. 2010;(8):CD007429.
2. Smith TO, Chester R, Pearse EO, et al. Operative versus non-operative management following Rockwood grade III acromioclavicular separation: a meta-analysis of the current evidence base. J Orthopaed Traumatol. 2011;12:19–27.
3. Hegmann KT. Shoulder disorders. In: Occupational Medicine Practice Guidelines. Evaluation and Management of Common Health Problems and Functional Recovery in Workers. 3rd ed. Elk Grove Village, IL: American College of Occupational and Environmental Medicine; 2011:1-297.
When not considering the grade of acromioclavicular (AC) joint dislocation, both conservative and surgical management lead to positive outcomes, although surgically managed patients require more time out of work (strength of recommendation [SOR]: B, Cochrane review of low-quality randomized controlled trials [RCTs]).
For Rockwood grade III dislocations, surgical intervention provides a better cosmetic outcome but increases infection risk (SOR: B, meta-analysis of retrospective case series).
Consensus guidelines suggest conservative management for Rockwood grade I to II dislocations and surgical repair for Rockwood grade IV to VI dislocations (SOR: C, expert opinion).
Similar outcomes, except when it comes to return to work
A 2010 Cochrane review of 2 RCTs and one quasi-randomized trial (174 patients, 93% male, moderate to high risk of bias) compared surgical intervention with conservative management of acute AC separations of unspecified Rockwood classification.1 Surgeries included coracoclavicular fixation with a cancellous screw or transfixation of the AC joint with Steinmann pins or Kirschner wires.
Conservative treatment included immobilization of the shoulder using an arm sling for 2 to 4 weeks. Patients were evaluated for a minimum of 12 months with a nonvalidated scoring system that measured pain, motion, and function or strength.
At one year, 63 of 76 patients (83%) in the post-surgical group and 74 of 84 patients (88%) in the conservative intervention group had either good or excellent results with no significant difference in unsatisfactory outcomes (relative risk [RR]=1.49; 95% confidence interval [CI], 0.75-2.95). (Fourteen patients—7 in each group—were lost to follow-up.) Moreover, the review found no significant difference in treatment failures requiring a subsequent operation between the groups—11 of 83 (13%) surgical patients and 7 of 91 (8%) conservatively managed patients (RR=1.72; 95% CI, 0.72-4.12).
Notably, regardless of activity level, surgical patients consistently returned to previous work functions later than patients managed conservatively. The mean convalescence time ranged from 8 to 11 weeks for surgical patients and 4 to 6 weeks for conservatively managed patients (P<.05).
A look at cosmetic results and risk of infection
A 2011 meta-analysis of 6 retrospective case series (379 patients, approximately 88% male) compared operative with nonoperative management in patients with acute, closed Rockwood grade III AC dislocations.2 Operative techniques varied; nonoperative patients each received physiotherapy or rehabilitation therapy and most were treated with a sling. Patient follow-up varied from 32 months to 10.8 years.
Four of the included studies suggested that nonoperative management resulted in poorer cosmetic results (methods not defined) compared with the operative group (11 of 115 surgical patients [10%], 74 of 88 nonoperative patients [84%]; risk difference [RD]=−0.79; 95% CI, −0.92 to −0.66; number needed to harm [NNH]=>2). Two of the studies evaluated the duration of sick leave and found a longer leave with operative management (50 operative and 54 nonoperative patients; mean difference=3.3; 95% CI, 2.1-4.5).
Five of the studies observed an increased risk of infection following operative management (8 of 175 [5%] operative patients compared with 0 of 152 [0%] nonoperative patients; RD=0.05; 95% CI, 0.01-0.09; NNH=20).
Recommendations depend on the grade of the injury
The American College of Occupational and Environmental Medicine recommends against routine surgical repair for Grade III AC joint separations.3 The College also recommends nonoperative management for patients with grade I to II AC dislocations and surgical repair for patients with grades IV to VI and select grade III AC dislocations.
When not considering the grade of acromioclavicular (AC) joint dislocation, both conservative and surgical management lead to positive outcomes, although surgically managed patients require more time out of work (strength of recommendation [SOR]: B, Cochrane review of low-quality randomized controlled trials [RCTs]).
For Rockwood grade III dislocations, surgical intervention provides a better cosmetic outcome but increases infection risk (SOR: B, meta-analysis of retrospective case series).
Consensus guidelines suggest conservative management for Rockwood grade I to II dislocations and surgical repair for Rockwood grade IV to VI dislocations (SOR: C, expert opinion).
Similar outcomes, except when it comes to return to work
A 2010 Cochrane review of 2 RCTs and one quasi-randomized trial (174 patients, 93% male, moderate to high risk of bias) compared surgical intervention with conservative management of acute AC separations of unspecified Rockwood classification.1 Surgeries included coracoclavicular fixation with a cancellous screw or transfixation of the AC joint with Steinmann pins or Kirschner wires.
Conservative treatment included immobilization of the shoulder using an arm sling for 2 to 4 weeks. Patients were evaluated for a minimum of 12 months with a nonvalidated scoring system that measured pain, motion, and function or strength.
At one year, 63 of 76 patients (83%) in the post-surgical group and 74 of 84 patients (88%) in the conservative intervention group had either good or excellent results with no significant difference in unsatisfactory outcomes (relative risk [RR]=1.49; 95% confidence interval [CI], 0.75-2.95). (Fourteen patients—7 in each group—were lost to follow-up.) Moreover, the review found no significant difference in treatment failures requiring a subsequent operation between the groups—11 of 83 (13%) surgical patients and 7 of 91 (8%) conservatively managed patients (RR=1.72; 95% CI, 0.72-4.12).
Notably, regardless of activity level, surgical patients consistently returned to previous work functions later than patients managed conservatively. The mean convalescence time ranged from 8 to 11 weeks for surgical patients and 4 to 6 weeks for conservatively managed patients (P<.05).
A look at cosmetic results and risk of infection
A 2011 meta-analysis of 6 retrospective case series (379 patients, approximately 88% male) compared operative with nonoperative management in patients with acute, closed Rockwood grade III AC dislocations.2 Operative techniques varied; nonoperative patients each received physiotherapy or rehabilitation therapy and most were treated with a sling. Patient follow-up varied from 32 months to 10.8 years.
Four of the included studies suggested that nonoperative management resulted in poorer cosmetic results (methods not defined) compared with the operative group (11 of 115 surgical patients [10%], 74 of 88 nonoperative patients [84%]; risk difference [RD]=−0.79; 95% CI, −0.92 to −0.66; number needed to harm [NNH]=>2). Two of the studies evaluated the duration of sick leave and found a longer leave with operative management (50 operative and 54 nonoperative patients; mean difference=3.3; 95% CI, 2.1-4.5).
Five of the studies observed an increased risk of infection following operative management (8 of 175 [5%] operative patients compared with 0 of 152 [0%] nonoperative patients; RD=0.05; 95% CI, 0.01-0.09; NNH=20).
Recommendations depend on the grade of the injury
The American College of Occupational and Environmental Medicine recommends against routine surgical repair for Grade III AC joint separations.3 The College also recommends nonoperative management for patients with grade I to II AC dislocations and surgical repair for patients with grades IV to VI and select grade III AC dislocations.
1. Tamaoki MJS, Belloti JC, Lenza M, et al. Surgical versus conservative interventions for treating acromioclavicular dislocation of the shoulder in adults. Cochrane Database Syst Rev. 2010;(8):CD007429.
2. Smith TO, Chester R, Pearse EO, et al. Operative versus non-operative management following Rockwood grade III acromioclavicular separation: a meta-analysis of the current evidence base. J Orthopaed Traumatol. 2011;12:19–27.
3. Hegmann KT. Shoulder disorders. In: Occupational Medicine Practice Guidelines. Evaluation and Management of Common Health Problems and Functional Recovery in Workers. 3rd ed. Elk Grove Village, IL: American College of Occupational and Environmental Medicine; 2011:1-297.
1. Tamaoki MJS, Belloti JC, Lenza M, et al. Surgical versus conservative interventions for treating acromioclavicular dislocation of the shoulder in adults. Cochrane Database Syst Rev. 2010;(8):CD007429.
2. Smith TO, Chester R, Pearse EO, et al. Operative versus non-operative management following Rockwood grade III acromioclavicular separation: a meta-analysis of the current evidence base. J Orthopaed Traumatol. 2011;12:19–27.
3. Hegmann KT. Shoulder disorders. In: Occupational Medicine Practice Guidelines. Evaluation and Management of Common Health Problems and Functional Recovery in Workers. 3rd ed. Elk Grove Village, IL: American College of Occupational and Environmental Medicine; 2011:1-297.
Evidence-based answers from the Family Physicians Inquiries Network