User login
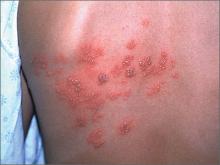
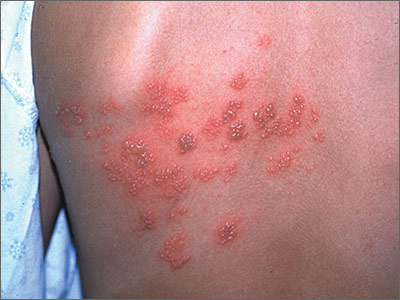
Severe Psoriasis, Kidney Disease Linked
WASHINGTON – Another population-based study has found a link between severe psoriasis and kidney disease – this one discovering almost a fivefold increase in the risk of immunoglobulin A nephropathy (IgAN) and a doubling in the risk of glomerular disease.
The findings suggest yet again that psoriasis is a systemic illness, and not something that affects only the skin, Sungat Grewal said at the annual meeting of the American Academy of Dermatology.
“Numerous case reports have generated a hypothesis that psoriasis may be associated,” with an increased risk of IgAN, said Ms. Grewal, of the department of dermatology at the University of Pennsylvania, Philadelphia. “Our study is the first to test this, and it supports the notion that this is no coincidence. Now we need further research to determine if this association is due to causality or to a shared pathophysiology.”
The link between psoriasis and kidney disease has long been noted, but the first study formally investigating this association was published in 2013 (BMJ. 2013 Oct;347:f5961). The study, also conducted by University of Pennsylvania investigators, used a large patient database in the United Kingdom, matched about 143,000 patients with psoriasis with up to five controls without psoriasis each, and found the risk of chronic kidney disease was nearly doubled for those with severe psoriasis (hazard ratio, 1.93).
A similar finding emerged from Taiwan in 2015. Using the national healthcare database, researchers matched about 4,600 patients with psoriasis with about 923,000 controls. They found that having severe psoriasis was associated with almost a doubling in the risk of chronic kidney disease (HR, 1.90) and almost a tripling in the risk of end stage renal disease (HR, 2.97), after adjusting for age, gender, comorbidities, and use of nonsteroidal anti-inflammatory drugs (J Dermatol Sci. 2015 Jun;78[3]:232-8).
Ms. Grewal and her coinvestigators used data from The Health Improvement Network in the United Kingdom – the same database used in the 2013 study. The study group comprised 206,000 patients with psoriasis and about 1 million controls.
In the overall group of patients, the risk of IgAN was not significantly increased. Nor was there a significant overall association with glomerular disease. And when the group was divided by disease severity, there were no significant associations with either IgAN or glomerular disease in the group with mild psoriasis.
Among those with severe psoriasis, however, the risk of IgAN was almost five times higher (HR, 4.75) and the risk of glomerular disease was doubled (HR, 2.05).
But although the hazard ratios look impressive, the clinical reality shouldn’t spark too much concern, Ms. Grewal said. “To keep things in context, it’s very important to remember that the excess risk of nephropathy attributed to severe psoriasis was still quite small – similar to the chance of a spontaneous pregnancy resulting in triplets.”
Still, she said, the link is intriguing, and something clinicians should keep in mind when managing patients with severe psoriasis.
Ms. Grewal had no financial disclosures. She is a medical student at the Commonwealth Medical College (Scranton, Pa.), and is currently spending a year at the Gelfand Clinical Research Lab at the University of Pennsylvania, Philadelphia.
WASHINGTON – Another population-based study has found a link between severe psoriasis and kidney disease – this one discovering almost a fivefold increase in the risk of immunoglobulin A nephropathy (IgAN) and a doubling in the risk of glomerular disease.
The findings suggest yet again that psoriasis is a systemic illness, and not something that affects only the skin, Sungat Grewal said at the annual meeting of the American Academy of Dermatology.
“Numerous case reports have generated a hypothesis that psoriasis may be associated,” with an increased risk of IgAN, said Ms. Grewal, of the department of dermatology at the University of Pennsylvania, Philadelphia. “Our study is the first to test this, and it supports the notion that this is no coincidence. Now we need further research to determine if this association is due to causality or to a shared pathophysiology.”
The link between psoriasis and kidney disease has long been noted, but the first study formally investigating this association was published in 2013 (BMJ. 2013 Oct;347:f5961). The study, also conducted by University of Pennsylvania investigators, used a large patient database in the United Kingdom, matched about 143,000 patients with psoriasis with up to five controls without psoriasis each, and found the risk of chronic kidney disease was nearly doubled for those with severe psoriasis (hazard ratio, 1.93).
A similar finding emerged from Taiwan in 2015. Using the national healthcare database, researchers matched about 4,600 patients with psoriasis with about 923,000 controls. They found that having severe psoriasis was associated with almost a doubling in the risk of chronic kidney disease (HR, 1.90) and almost a tripling in the risk of end stage renal disease (HR, 2.97), after adjusting for age, gender, comorbidities, and use of nonsteroidal anti-inflammatory drugs (J Dermatol Sci. 2015 Jun;78[3]:232-8).
Ms. Grewal and her coinvestigators used data from The Health Improvement Network in the United Kingdom – the same database used in the 2013 study. The study group comprised 206,000 patients with psoriasis and about 1 million controls.
In the overall group of patients, the risk of IgAN was not significantly increased. Nor was there a significant overall association with glomerular disease. And when the group was divided by disease severity, there were no significant associations with either IgAN or glomerular disease in the group with mild psoriasis.
Among those with severe psoriasis, however, the risk of IgAN was almost five times higher (HR, 4.75) and the risk of glomerular disease was doubled (HR, 2.05).
But although the hazard ratios look impressive, the clinical reality shouldn’t spark too much concern, Ms. Grewal said. “To keep things in context, it’s very important to remember that the excess risk of nephropathy attributed to severe psoriasis was still quite small – similar to the chance of a spontaneous pregnancy resulting in triplets.”
Still, she said, the link is intriguing, and something clinicians should keep in mind when managing patients with severe psoriasis.
Ms. Grewal had no financial disclosures. She is a medical student at the Commonwealth Medical College (Scranton, Pa.), and is currently spending a year at the Gelfand Clinical Research Lab at the University of Pennsylvania, Philadelphia.
WASHINGTON – Another population-based study has found a link between severe psoriasis and kidney disease – this one discovering almost a fivefold increase in the risk of immunoglobulin A nephropathy (IgAN) and a doubling in the risk of glomerular disease.
The findings suggest yet again that psoriasis is a systemic illness, and not something that affects only the skin, Sungat Grewal said at the annual meeting of the American Academy of Dermatology.
“Numerous case reports have generated a hypothesis that psoriasis may be associated,” with an increased risk of IgAN, said Ms. Grewal, of the department of dermatology at the University of Pennsylvania, Philadelphia. “Our study is the first to test this, and it supports the notion that this is no coincidence. Now we need further research to determine if this association is due to causality or to a shared pathophysiology.”
The link between psoriasis and kidney disease has long been noted, but the first study formally investigating this association was published in 2013 (BMJ. 2013 Oct;347:f5961). The study, also conducted by University of Pennsylvania investigators, used a large patient database in the United Kingdom, matched about 143,000 patients with psoriasis with up to five controls without psoriasis each, and found the risk of chronic kidney disease was nearly doubled for those with severe psoriasis (hazard ratio, 1.93).
A similar finding emerged from Taiwan in 2015. Using the national healthcare database, researchers matched about 4,600 patients with psoriasis with about 923,000 controls. They found that having severe psoriasis was associated with almost a doubling in the risk of chronic kidney disease (HR, 1.90) and almost a tripling in the risk of end stage renal disease (HR, 2.97), after adjusting for age, gender, comorbidities, and use of nonsteroidal anti-inflammatory drugs (J Dermatol Sci. 2015 Jun;78[3]:232-8).
Ms. Grewal and her coinvestigators used data from The Health Improvement Network in the United Kingdom – the same database used in the 2013 study. The study group comprised 206,000 patients with psoriasis and about 1 million controls.
In the overall group of patients, the risk of IgAN was not significantly increased. Nor was there a significant overall association with glomerular disease. And when the group was divided by disease severity, there were no significant associations with either IgAN or glomerular disease in the group with mild psoriasis.
Among those with severe psoriasis, however, the risk of IgAN was almost five times higher (HR, 4.75) and the risk of glomerular disease was doubled (HR, 2.05).
But although the hazard ratios look impressive, the clinical reality shouldn’t spark too much concern, Ms. Grewal said. “To keep things in context, it’s very important to remember that the excess risk of nephropathy attributed to severe psoriasis was still quite small – similar to the chance of a spontaneous pregnancy resulting in triplets.”
Still, she said, the link is intriguing, and something clinicians should keep in mind when managing patients with severe psoriasis.
Ms. Grewal had no financial disclosures. She is a medical student at the Commonwealth Medical College (Scranton, Pa.), and is currently spending a year at the Gelfand Clinical Research Lab at the University of Pennsylvania, Philadelphia.
AT AAD 16
Severe psoriasis, kidney disease linked
WASHINGTON – Another population-based study has found a link between severe psoriasis and kidney disease – this one discovering almost a fivefold increase in the risk of immunoglobulin A nephropathy (IgAN) and a doubling in the risk of glomerular disease.
The findings suggest yet again that psoriasis is a systemic illness, and not something that affects only the skin, Sungat Grewal said at the annual meeting of the American Academy of Dermatology.
“Numerous case reports have generated a hypothesis that psoriasis may be associated,” with an increased risk of IgAN, said Ms. Grewal, of the department of dermatology at the University of Pennsylvania, Philadelphia. “Our study is the first to test this, and it supports the notion that this is no coincidence. Now we need further research to determine if this association is due to causality or to a shared pathophysiology.”
The link between psoriasis and kidney disease has long been noted, but the first study formally investigating this association was published in 2013 (BMJ. 2013 Oct;347:f5961). The study, also conducted by University of Pennsylvania investigators, used a large patient database in the United Kingdom, matched about 143,000 patients with psoriasis with up to five controls without psoriasis each, and found the risk of chronic kidney disease was nearly doubled for those with severe psoriasis (hazard ratio, 1.93).
A similar finding emerged from Taiwan in 2015. Using the national healthcare database, researchers matched about 4,600 patients with psoriasis with about 923,000 controls. They found that having severe psoriasis was associated with almost a doubling in the risk of chronic kidney disease (HR, 1.90) and almost a tripling in the risk of end stage renal disease (HR, 2.97), after adjusting for age, gender, comorbidities, and use of nonsteroidal anti-inflammatory drugs (J Dermatol Sci. 2015 Jun;78[3]:232-8).
Ms. Grewal and her coinvestigators used data from The Health Improvement Network in the United Kingdom – the same database used in the 2013 study. The study group comprised 206,000 patients with psoriasis and about 1 million controls.
In the overall group of patients, the risk of IgAN was not significantly increased. Nor was there a significant overall association with glomerular disease. And when the group was divided by disease severity, there were no significant associations with either IgAN or glomerular disease in the group with mild psoriasis.
Among those with severe psoriasis, however, the risk of IgAN was almost five times higher (HR, 4.75) and the risk of glomerular disease was doubled (HR, 2.05).
But although the hazard ratios look impressive, the clinical reality shouldn’t spark too much concern, Ms. Grewal said. “To keep things in context, it’s very important to remember that the excess risk of nephropathy attributed to severe psoriasis was still quite small – similar to the chance of a spontaneous pregnancy resulting in triplets.”
Still, she said, the link is intriguing, and something clinicians should keep in mind when managing patients with severe psoriasis.
Ms. Grewal had no financial disclosures. She is a medical student at the Commonwealth Medical College (Scranton, Pa.), and is currently spending a year at the Gelfand Clinical Research Lab at the University of Pennsylvania, Philadelphia.
WASHINGTON – Another population-based study has found a link between severe psoriasis and kidney disease – this one discovering almost a fivefold increase in the risk of immunoglobulin A nephropathy (IgAN) and a doubling in the risk of glomerular disease.
The findings suggest yet again that psoriasis is a systemic illness, and not something that affects only the skin, Sungat Grewal said at the annual meeting of the American Academy of Dermatology.
“Numerous case reports have generated a hypothesis that psoriasis may be associated,” with an increased risk of IgAN, said Ms. Grewal, of the department of dermatology at the University of Pennsylvania, Philadelphia. “Our study is the first to test this, and it supports the notion that this is no coincidence. Now we need further research to determine if this association is due to causality or to a shared pathophysiology.”
The link between psoriasis and kidney disease has long been noted, but the first study formally investigating this association was published in 2013 (BMJ. 2013 Oct;347:f5961). The study, also conducted by University of Pennsylvania investigators, used a large patient database in the United Kingdom, matched about 143,000 patients with psoriasis with up to five controls without psoriasis each, and found the risk of chronic kidney disease was nearly doubled for those with severe psoriasis (hazard ratio, 1.93).
A similar finding emerged from Taiwan in 2015. Using the national healthcare database, researchers matched about 4,600 patients with psoriasis with about 923,000 controls. They found that having severe psoriasis was associated with almost a doubling in the risk of chronic kidney disease (HR, 1.90) and almost a tripling in the risk of end stage renal disease (HR, 2.97), after adjusting for age, gender, comorbidities, and use of nonsteroidal anti-inflammatory drugs (J Dermatol Sci. 2015 Jun;78[3]:232-8).
Ms. Grewal and her coinvestigators used data from The Health Improvement Network in the United Kingdom – the same database used in the 2013 study. The study group comprised 206,000 patients with psoriasis and about 1 million controls.
In the overall group of patients, the risk of IgAN was not significantly increased. Nor was there a significant overall association with glomerular disease. And when the group was divided by disease severity, there were no significant associations with either IgAN or glomerular disease in the group with mild psoriasis.
Among those with severe psoriasis, however, the risk of IgAN was almost five times higher (HR, 4.75) and the risk of glomerular disease was doubled (HR, 2.05).
But although the hazard ratios look impressive, the clinical reality shouldn’t spark too much concern, Ms. Grewal said. “To keep things in context, it’s very important to remember that the excess risk of nephropathy attributed to severe psoriasis was still quite small – similar to the chance of a spontaneous pregnancy resulting in triplets.”
Still, she said, the link is intriguing, and something clinicians should keep in mind when managing patients with severe psoriasis.
Ms. Grewal had no financial disclosures. She is a medical student at the Commonwealth Medical College (Scranton, Pa.), and is currently spending a year at the Gelfand Clinical Research Lab at the University of Pennsylvania, Philadelphia.
WASHINGTON – Another population-based study has found a link between severe psoriasis and kidney disease – this one discovering almost a fivefold increase in the risk of immunoglobulin A nephropathy (IgAN) and a doubling in the risk of glomerular disease.
The findings suggest yet again that psoriasis is a systemic illness, and not something that affects only the skin, Sungat Grewal said at the annual meeting of the American Academy of Dermatology.
“Numerous case reports have generated a hypothesis that psoriasis may be associated,” with an increased risk of IgAN, said Ms. Grewal, of the department of dermatology at the University of Pennsylvania, Philadelphia. “Our study is the first to test this, and it supports the notion that this is no coincidence. Now we need further research to determine if this association is due to causality or to a shared pathophysiology.”
The link between psoriasis and kidney disease has long been noted, but the first study formally investigating this association was published in 2013 (BMJ. 2013 Oct;347:f5961). The study, also conducted by University of Pennsylvania investigators, used a large patient database in the United Kingdom, matched about 143,000 patients with psoriasis with up to five controls without psoriasis each, and found the risk of chronic kidney disease was nearly doubled for those with severe psoriasis (hazard ratio, 1.93).
A similar finding emerged from Taiwan in 2015. Using the national healthcare database, researchers matched about 4,600 patients with psoriasis with about 923,000 controls. They found that having severe psoriasis was associated with almost a doubling in the risk of chronic kidney disease (HR, 1.90) and almost a tripling in the risk of end stage renal disease (HR, 2.97), after adjusting for age, gender, comorbidities, and use of nonsteroidal anti-inflammatory drugs (J Dermatol Sci. 2015 Jun;78[3]:232-8).
Ms. Grewal and her coinvestigators used data from The Health Improvement Network in the United Kingdom – the same database used in the 2013 study. The study group comprised 206,000 patients with psoriasis and about 1 million controls.
In the overall group of patients, the risk of IgAN was not significantly increased. Nor was there a significant overall association with glomerular disease. And when the group was divided by disease severity, there were no significant associations with either IgAN or glomerular disease in the group with mild psoriasis.
Among those with severe psoriasis, however, the risk of IgAN was almost five times higher (HR, 4.75) and the risk of glomerular disease was doubled (HR, 2.05).
But although the hazard ratios look impressive, the clinical reality shouldn’t spark too much concern, Ms. Grewal said. “To keep things in context, it’s very important to remember that the excess risk of nephropathy attributed to severe psoriasis was still quite small – similar to the chance of a spontaneous pregnancy resulting in triplets.”
Still, she said, the link is intriguing, and something clinicians should keep in mind when managing patients with severe psoriasis.
Ms. Grewal had no financial disclosures. She is a medical student at the Commonwealth Medical College (Scranton, Pa.), and is currently spending a year at the Gelfand Clinical Research Lab at the University of Pennsylvania, Philadelphia.
AT AAD 16
Key clinical point: Severe psoriasis appears to increase the risk of both immunoglobulin A glomerulonephritis and glomerular disease.
Major finding: The risk of glomerulonephritis was five-fold higher and the risk of glomerular disease doubled in those with severe psoriasis.
Data source: A population based cohort study comprised about 1.2 million subjects.
Disclosures: Ms. Sungat Grewal had no financial disclosures.
Prepsychosis links with elevated metabolic syndrome
MADRID – Untreated people at high risk for developing psychosis also showed an increased prevalence of certain components of metabolic syndrome in data collected from 163 German study participants, a finding that gives new insight into the well-documented but poorly delineated link between schizophrenia and metabolic syndrome.
“The findings point out that a high risk for schizophrenia implies a certain risk for patients to develop metabolic syndrome independent of treatment effects,” said Dr. Joachim Cordes, a psychiatrist at the LVR Clinic of the Heinrich-Heine University in Düsseldorf, Germany. He assumed that genetic factors underlie the shared risk some people face for both developing schizophrenia and metabolic syndrome. “I think there is a direct connection between schizophrenia and metabolic syndrome, an inherent factor like a genetic factor,” Dr. Cordes said in an interview. This understanding should influence how patients with newly diagnosed schizophrenia or those at risk for psychosis are managed, he added.
Dr. Cordes’s report was one of several at the meeting sponsored by the European Psychiatric Association that examined different facets of the complex links that tie schizophrenia to metabolic syndrome, an association that already had lots of evidence, including a recent meta-analysis (Schizophr Bull. 2013 March;39[2]:306-18).
He used data collected on 163 people enrolled in the PREVENT study and at high risk for a first psychotic episode. Run at nine German centers, PREVENT primarily tested very early intervention with drug and behavioral therapy to improve outcomes. Dr. Cordes took data collected from these prepsychosis, high-risk patients to assess their prevalence of metabolic syndrome and of the various individual features that define metabolic syndrome, using a definition published by the American Heart Association and the U.S. National Heart, Lung, and Blood Institute (Circulation. 2005 Oct 18;112[17]:2735-52). He compared these metabolic syndrome rates with the general German population, using data from 35,869 randomly selected German adults in more than 1,500 German primary care practices, the German Metabolic and Cardiovascular Risk Project (GEMCAS).
The findings showed a 9.2% prevalence of metabolic syndrome in the prepsychosis group and a 7.4% rate among the general adult population, Dr. Cordes reported. Among men in the prepsychosis group, the metabolic syndrome definers with the largest increments in prevalence were low HDL, in 21% of the prepsychosis people and in 12% of the general population, and elevated blood glucose in 11%, compared with 6%. Among women, the metabolic syndrome definers with the greatest between-group differences were elevated waist circumference, in 30% of those with prepsychosis, compared with 17% in the general population, and low HDL in 19%, compared with 14%.
This apparently inherent link between a tendency toward psychosis and schizophrenia and a tendency to develop features of metabolic syndrome suggests that patients with newly diagnosed schizophrenia need a preventive approach to weight management, Dr. Cordes said. He also suggested prescribing antipsychotic medications that pose the lowest risk for causing further metabolic derangements in patients.
A second report at the meeting came from an assessment of cognitive function and its relationship to metabolic syndrome in 54 women diagnosed with schizophrenia and on stable treatment. The schizophrenia patients with metabolic syndrome, nearly half of the total group, performed significantly worse than those without metabolic syndrome in tests of verbal memory, executive function, and attention and processing speed, findings that support an increased incidence of selective cognitive impairment in patients with schizophrenia and metabolic syndrome, said Dr. Adela C. Botis, a psychiatrist and researcher at the University of Medicine and Pharmacy in Cluj-Napoca, Romania.
Dr. Botis and her associates studied 54 women diagnosed with schizophrenia who had remitted symptoms for at least 6 months on stable antipsychotic treatment. Using the metabolic syndrome definition of the International Diabetes Federation 25 (46%) had metabolic syndrome, and the other 29 (54%) did not. These numbers document the high prevalence of metabolic syndrome in schizophrenia patients.
A multivariate analysis identified demographic and metabolic factors that significantly linked with decrements in several cognitive domains. Economic status and living situation linked with deficits in verbal memory; elevated systolic blood pressure significantly linked with worsened attention and processing speed; high body mass index linked with loss of motor speed; and less education significantly linked with all these increments as well as four other domains,
A third report used a post-hoc analysis of data from two separate trials to show that treatment with a relatively new antipsychotic drug, lurasidone (Latuda), produced less metabolic syndrome, compared with risperidone or extended-release quetiapine (Seroquel XR), said Dr. Andrei Pikalov, head of global medical affairs at Sunovion Pharmaceuticals, the company that markets Latuda. Lurasidone received approval for treating schizophrenia in 2010.
He took data from two studies designed to assess lurasidone’s efficacy for treating adults with schizophrenia for 12 months, compared with either risperidone in a study with 621 patients, or with quetiapine XR in a study with 292 patients. He applied the same metabolic syndrome definition used by Dr. Cordes to clinical measurements taken at baseline and after 12 months on treatment.
The results showed that treatment with lurasidone produced less than half the rate of new metabolic syndrome cases, compared with risperidone, a statistically significant difference, and less than two-thirds the rate of quetiapine XR, a difference that did not reach statistical significance.
Dr. Cordes said he has been a speaker for Servier. Dr. Botis had no disclosures. Dr. Pikalov is an employee of Sunovion, which markets lurasidone (Latuda).
On Twitter @mitchelzoler
MADRID – Untreated people at high risk for developing psychosis also showed an increased prevalence of certain components of metabolic syndrome in data collected from 163 German study participants, a finding that gives new insight into the well-documented but poorly delineated link between schizophrenia and metabolic syndrome.
“The findings point out that a high risk for schizophrenia implies a certain risk for patients to develop metabolic syndrome independent of treatment effects,” said Dr. Joachim Cordes, a psychiatrist at the LVR Clinic of the Heinrich-Heine University in Düsseldorf, Germany. He assumed that genetic factors underlie the shared risk some people face for both developing schizophrenia and metabolic syndrome. “I think there is a direct connection between schizophrenia and metabolic syndrome, an inherent factor like a genetic factor,” Dr. Cordes said in an interview. This understanding should influence how patients with newly diagnosed schizophrenia or those at risk for psychosis are managed, he added.
Dr. Cordes’s report was one of several at the meeting sponsored by the European Psychiatric Association that examined different facets of the complex links that tie schizophrenia to metabolic syndrome, an association that already had lots of evidence, including a recent meta-analysis (Schizophr Bull. 2013 March;39[2]:306-18).
He used data collected on 163 people enrolled in the PREVENT study and at high risk for a first psychotic episode. Run at nine German centers, PREVENT primarily tested very early intervention with drug and behavioral therapy to improve outcomes. Dr. Cordes took data collected from these prepsychosis, high-risk patients to assess their prevalence of metabolic syndrome and of the various individual features that define metabolic syndrome, using a definition published by the American Heart Association and the U.S. National Heart, Lung, and Blood Institute (Circulation. 2005 Oct 18;112[17]:2735-52). He compared these metabolic syndrome rates with the general German population, using data from 35,869 randomly selected German adults in more than 1,500 German primary care practices, the German Metabolic and Cardiovascular Risk Project (GEMCAS).
The findings showed a 9.2% prevalence of metabolic syndrome in the prepsychosis group and a 7.4% rate among the general adult population, Dr. Cordes reported. Among men in the prepsychosis group, the metabolic syndrome definers with the largest increments in prevalence were low HDL, in 21% of the prepsychosis people and in 12% of the general population, and elevated blood glucose in 11%, compared with 6%. Among women, the metabolic syndrome definers with the greatest between-group differences were elevated waist circumference, in 30% of those with prepsychosis, compared with 17% in the general population, and low HDL in 19%, compared with 14%.
This apparently inherent link between a tendency toward psychosis and schizophrenia and a tendency to develop features of metabolic syndrome suggests that patients with newly diagnosed schizophrenia need a preventive approach to weight management, Dr. Cordes said. He also suggested prescribing antipsychotic medications that pose the lowest risk for causing further metabolic derangements in patients.
A second report at the meeting came from an assessment of cognitive function and its relationship to metabolic syndrome in 54 women diagnosed with schizophrenia and on stable treatment. The schizophrenia patients with metabolic syndrome, nearly half of the total group, performed significantly worse than those without metabolic syndrome in tests of verbal memory, executive function, and attention and processing speed, findings that support an increased incidence of selective cognitive impairment in patients with schizophrenia and metabolic syndrome, said Dr. Adela C. Botis, a psychiatrist and researcher at the University of Medicine and Pharmacy in Cluj-Napoca, Romania.
Dr. Botis and her associates studied 54 women diagnosed with schizophrenia who had remitted symptoms for at least 6 months on stable antipsychotic treatment. Using the metabolic syndrome definition of the International Diabetes Federation 25 (46%) had metabolic syndrome, and the other 29 (54%) did not. These numbers document the high prevalence of metabolic syndrome in schizophrenia patients.
A multivariate analysis identified demographic and metabolic factors that significantly linked with decrements in several cognitive domains. Economic status and living situation linked with deficits in verbal memory; elevated systolic blood pressure significantly linked with worsened attention and processing speed; high body mass index linked with loss of motor speed; and less education significantly linked with all these increments as well as four other domains,
A third report used a post-hoc analysis of data from two separate trials to show that treatment with a relatively new antipsychotic drug, lurasidone (Latuda), produced less metabolic syndrome, compared with risperidone or extended-release quetiapine (Seroquel XR), said Dr. Andrei Pikalov, head of global medical affairs at Sunovion Pharmaceuticals, the company that markets Latuda. Lurasidone received approval for treating schizophrenia in 2010.
He took data from two studies designed to assess lurasidone’s efficacy for treating adults with schizophrenia for 12 months, compared with either risperidone in a study with 621 patients, or with quetiapine XR in a study with 292 patients. He applied the same metabolic syndrome definition used by Dr. Cordes to clinical measurements taken at baseline and after 12 months on treatment.
The results showed that treatment with lurasidone produced less than half the rate of new metabolic syndrome cases, compared with risperidone, a statistically significant difference, and less than two-thirds the rate of quetiapine XR, a difference that did not reach statistical significance.
Dr. Cordes said he has been a speaker for Servier. Dr. Botis had no disclosures. Dr. Pikalov is an employee of Sunovion, which markets lurasidone (Latuda).
On Twitter @mitchelzoler
MADRID – Untreated people at high risk for developing psychosis also showed an increased prevalence of certain components of metabolic syndrome in data collected from 163 German study participants, a finding that gives new insight into the well-documented but poorly delineated link between schizophrenia and metabolic syndrome.
“The findings point out that a high risk for schizophrenia implies a certain risk for patients to develop metabolic syndrome independent of treatment effects,” said Dr. Joachim Cordes, a psychiatrist at the LVR Clinic of the Heinrich-Heine University in Düsseldorf, Germany. He assumed that genetic factors underlie the shared risk some people face for both developing schizophrenia and metabolic syndrome. “I think there is a direct connection between schizophrenia and metabolic syndrome, an inherent factor like a genetic factor,” Dr. Cordes said in an interview. This understanding should influence how patients with newly diagnosed schizophrenia or those at risk for psychosis are managed, he added.
Dr. Cordes’s report was one of several at the meeting sponsored by the European Psychiatric Association that examined different facets of the complex links that tie schizophrenia to metabolic syndrome, an association that already had lots of evidence, including a recent meta-analysis (Schizophr Bull. 2013 March;39[2]:306-18).
He used data collected on 163 people enrolled in the PREVENT study and at high risk for a first psychotic episode. Run at nine German centers, PREVENT primarily tested very early intervention with drug and behavioral therapy to improve outcomes. Dr. Cordes took data collected from these prepsychosis, high-risk patients to assess their prevalence of metabolic syndrome and of the various individual features that define metabolic syndrome, using a definition published by the American Heart Association and the U.S. National Heart, Lung, and Blood Institute (Circulation. 2005 Oct 18;112[17]:2735-52). He compared these metabolic syndrome rates with the general German population, using data from 35,869 randomly selected German adults in more than 1,500 German primary care practices, the German Metabolic and Cardiovascular Risk Project (GEMCAS).
The findings showed a 9.2% prevalence of metabolic syndrome in the prepsychosis group and a 7.4% rate among the general adult population, Dr. Cordes reported. Among men in the prepsychosis group, the metabolic syndrome definers with the largest increments in prevalence were low HDL, in 21% of the prepsychosis people and in 12% of the general population, and elevated blood glucose in 11%, compared with 6%. Among women, the metabolic syndrome definers with the greatest between-group differences were elevated waist circumference, in 30% of those with prepsychosis, compared with 17% in the general population, and low HDL in 19%, compared with 14%.
This apparently inherent link between a tendency toward psychosis and schizophrenia and a tendency to develop features of metabolic syndrome suggests that patients with newly diagnosed schizophrenia need a preventive approach to weight management, Dr. Cordes said. He also suggested prescribing antipsychotic medications that pose the lowest risk for causing further metabolic derangements in patients.
A second report at the meeting came from an assessment of cognitive function and its relationship to metabolic syndrome in 54 women diagnosed with schizophrenia and on stable treatment. The schizophrenia patients with metabolic syndrome, nearly half of the total group, performed significantly worse than those without metabolic syndrome in tests of verbal memory, executive function, and attention and processing speed, findings that support an increased incidence of selective cognitive impairment in patients with schizophrenia and metabolic syndrome, said Dr. Adela C. Botis, a psychiatrist and researcher at the University of Medicine and Pharmacy in Cluj-Napoca, Romania.
Dr. Botis and her associates studied 54 women diagnosed with schizophrenia who had remitted symptoms for at least 6 months on stable antipsychotic treatment. Using the metabolic syndrome definition of the International Diabetes Federation 25 (46%) had metabolic syndrome, and the other 29 (54%) did not. These numbers document the high prevalence of metabolic syndrome in schizophrenia patients.
A multivariate analysis identified demographic and metabolic factors that significantly linked with decrements in several cognitive domains. Economic status and living situation linked with deficits in verbal memory; elevated systolic blood pressure significantly linked with worsened attention and processing speed; high body mass index linked with loss of motor speed; and less education significantly linked with all these increments as well as four other domains,
A third report used a post-hoc analysis of data from two separate trials to show that treatment with a relatively new antipsychotic drug, lurasidone (Latuda), produced less metabolic syndrome, compared with risperidone or extended-release quetiapine (Seroquel XR), said Dr. Andrei Pikalov, head of global medical affairs at Sunovion Pharmaceuticals, the company that markets Latuda. Lurasidone received approval for treating schizophrenia in 2010.
He took data from two studies designed to assess lurasidone’s efficacy for treating adults with schizophrenia for 12 months, compared with either risperidone in a study with 621 patients, or with quetiapine XR in a study with 292 patients. He applied the same metabolic syndrome definition used by Dr. Cordes to clinical measurements taken at baseline and after 12 months on treatment.
The results showed that treatment with lurasidone produced less than half the rate of new metabolic syndrome cases, compared with risperidone, a statistically significant difference, and less than two-thirds the rate of quetiapine XR, a difference that did not reach statistical significance.
Dr. Cordes said he has been a speaker for Servier. Dr. Botis had no disclosures. Dr. Pikalov is an employee of Sunovion, which markets lurasidone (Latuda).
On Twitter @mitchelzoler
AT THE EUROPEAN CONGRESS OF PSYCHIATRY
Key clinical point: People at high risk for a first psychosis episode had an increased prevalence of certain metabolic syndrome components, compared with the general population.
Major finding: Metabolic syndrome was prevalent in 9.2% of the prepsychosis group and in 7.4% of the general population.
Data source: Post-hoc analysis of data from 163 German people with prepsychosis.
Disclosures: Dr. Cordes said he has been a speaker for Servier. Dr. Botis had no disclosures. Dr. Pikalov is an employee of Sunovion, which markets lurasidone (Latuda).
Early estrogen likely prevents bone fractures in Turner syndrome
BOSTON – The longer that estrogen therapy is delayed in girls with Turner syndrome, the lower their bone density will be in subsequent years, based on results of a retrospective, cross-sectional study from Monash University, in Melbourne, Australia.
For every year after age 11 that Turner patients went without estrogen – generally due to delayed initiation, but sometimes noncompliance – there was a significant reduction in bone mineral density in both the lumbar spine (Beta -0.582, P less than 0.001) and femoral neck (Beta -0.383, P = 0.008).
Estrogen deficiency and subsequent suboptimal bone mass accrual are known to contribute to the increased risk of osteoporosis in women with Turner syndrome, and about a doubling of the risk of fragility fractures, mostly of the forearm. About a third of the 76 women in the study had at least one fracture, explained investigator Dr. Amanda Vincent, head of the Midlife Health and Menopause Program at Monash.
“Avoiding estrogen deficiency is important to optimize bone health in Turner syndrome.” It “depends on early diagnosis, age-appropriate pubertal induction, and optimization of compliance,” Dr. Vincent said at the Endocrine Society annual meeting.
The median age of Turner syndrome diagnosis was 11 years, but estrogen treatments didn’t begin until a median age of 15. The women in the study were a median of about 30 years old, which means that they were adolescents at the time when estrogen treatment was often delayed in the mistaken belief that growth hormone therapy would be more effective before puberty was induced.
It’s now known that estrogen replacement works synergistically with, and even potentiates, the effects of growth hormone. Current guidelines recommend pubertal induction by age 13 (J Clin Endocrinol Metab. 2007 Jan;92(1):10-25).
The women had at least one dual-energy x-ray absorptiometry scan at Monash since 1998. Z-scores below - 2, indicating low bone density, were found in the lumbar spines of about a quarter the subjects, and in the femoral necks of about 8%. Primary amenorrhea and premature menopause, followed by vitamin D deficiency, were the most common risk factors for low bone mass. Almost 40% of the women reported non-continuous use of estrogen. About half had undergone growth hormone therapy.
At a median height of 149 cm, the subjects were about 15 cm shorter than age-matched, healthy controls, and also had a slightly higher median body mass index of 25.6 kg/m2. Lumbar spine bone area, bone mineral content, areal bone mineral density, and bone mineral apparent density were significantly lower in Turner syndrome patients. In the femoral neck, areal bone mineral density was significantly lower.
There was no relationship between bone markers and growth hormone use or Turner syndrome karyotype; the predominant karyotype was 45XO, but the study also included mosaic karyotypes.
The investigators had no disclosures.
These are important observations. The bottom line is early recognition and early referral. It’s clear from this study and others that earlier institution of estrogen is beneficial for height, bone density, and fracture risk throughout life. It’s not just an issue of a 20 year old with low bone density; that 20 year old later becomes a 60 year old with low bone density.

|
Dr. Michael Levine |
[However,] we still have a problem with delayed recognition and referral of young girls with Turner syndrome. Most girls with Turner syndrome have some typical phenotypic features, but some do not, so the diagnosis is often made too late. [To get around that problem,] we recommend that all children below the 5th percentile for height – or who flatten out too early on growth curves – be referred to rule out Turner syndrome and other problems.
Dr. Michael Levine is chief of the Division of Endocrinology at The Children’s Hospital of Philadelphia. He made his comments after the study presentation, and was not involved in the work.
These are important observations. The bottom line is early recognition and early referral. It’s clear from this study and others that earlier institution of estrogen is beneficial for height, bone density, and fracture risk throughout life. It’s not just an issue of a 20 year old with low bone density; that 20 year old later becomes a 60 year old with low bone density.

|
Dr. Michael Levine |
[However,] we still have a problem with delayed recognition and referral of young girls with Turner syndrome. Most girls with Turner syndrome have some typical phenotypic features, but some do not, so the diagnosis is often made too late. [To get around that problem,] we recommend that all children below the 5th percentile for height – or who flatten out too early on growth curves – be referred to rule out Turner syndrome and other problems.
Dr. Michael Levine is chief of the Division of Endocrinology at The Children’s Hospital of Philadelphia. He made his comments after the study presentation, and was not involved in the work.
These are important observations. The bottom line is early recognition and early referral. It’s clear from this study and others that earlier institution of estrogen is beneficial for height, bone density, and fracture risk throughout life. It’s not just an issue of a 20 year old with low bone density; that 20 year old later becomes a 60 year old with low bone density.

|
Dr. Michael Levine |
[However,] we still have a problem with delayed recognition and referral of young girls with Turner syndrome. Most girls with Turner syndrome have some typical phenotypic features, but some do not, so the diagnosis is often made too late. [To get around that problem,] we recommend that all children below the 5th percentile for height – or who flatten out too early on growth curves – be referred to rule out Turner syndrome and other problems.
Dr. Michael Levine is chief of the Division of Endocrinology at The Children’s Hospital of Philadelphia. He made his comments after the study presentation, and was not involved in the work.
BOSTON – The longer that estrogen therapy is delayed in girls with Turner syndrome, the lower their bone density will be in subsequent years, based on results of a retrospective, cross-sectional study from Monash University, in Melbourne, Australia.
For every year after age 11 that Turner patients went without estrogen – generally due to delayed initiation, but sometimes noncompliance – there was a significant reduction in bone mineral density in both the lumbar spine (Beta -0.582, P less than 0.001) and femoral neck (Beta -0.383, P = 0.008).
Estrogen deficiency and subsequent suboptimal bone mass accrual are known to contribute to the increased risk of osteoporosis in women with Turner syndrome, and about a doubling of the risk of fragility fractures, mostly of the forearm. About a third of the 76 women in the study had at least one fracture, explained investigator Dr. Amanda Vincent, head of the Midlife Health and Menopause Program at Monash.
“Avoiding estrogen deficiency is important to optimize bone health in Turner syndrome.” It “depends on early diagnosis, age-appropriate pubertal induction, and optimization of compliance,” Dr. Vincent said at the Endocrine Society annual meeting.
The median age of Turner syndrome diagnosis was 11 years, but estrogen treatments didn’t begin until a median age of 15. The women in the study were a median of about 30 years old, which means that they were adolescents at the time when estrogen treatment was often delayed in the mistaken belief that growth hormone therapy would be more effective before puberty was induced.
It’s now known that estrogen replacement works synergistically with, and even potentiates, the effects of growth hormone. Current guidelines recommend pubertal induction by age 13 (J Clin Endocrinol Metab. 2007 Jan;92(1):10-25).
The women had at least one dual-energy x-ray absorptiometry scan at Monash since 1998. Z-scores below - 2, indicating low bone density, were found in the lumbar spines of about a quarter the subjects, and in the femoral necks of about 8%. Primary amenorrhea and premature menopause, followed by vitamin D deficiency, were the most common risk factors for low bone mass. Almost 40% of the women reported non-continuous use of estrogen. About half had undergone growth hormone therapy.
At a median height of 149 cm, the subjects were about 15 cm shorter than age-matched, healthy controls, and also had a slightly higher median body mass index of 25.6 kg/m2. Lumbar spine bone area, bone mineral content, areal bone mineral density, and bone mineral apparent density were significantly lower in Turner syndrome patients. In the femoral neck, areal bone mineral density was significantly lower.
There was no relationship between bone markers and growth hormone use or Turner syndrome karyotype; the predominant karyotype was 45XO, but the study also included mosaic karyotypes.
The investigators had no disclosures.
BOSTON – The longer that estrogen therapy is delayed in girls with Turner syndrome, the lower their bone density will be in subsequent years, based on results of a retrospective, cross-sectional study from Monash University, in Melbourne, Australia.
For every year after age 11 that Turner patients went without estrogen – generally due to delayed initiation, but sometimes noncompliance – there was a significant reduction in bone mineral density in both the lumbar spine (Beta -0.582, P less than 0.001) and femoral neck (Beta -0.383, P = 0.008).
Estrogen deficiency and subsequent suboptimal bone mass accrual are known to contribute to the increased risk of osteoporosis in women with Turner syndrome, and about a doubling of the risk of fragility fractures, mostly of the forearm. About a third of the 76 women in the study had at least one fracture, explained investigator Dr. Amanda Vincent, head of the Midlife Health and Menopause Program at Monash.
“Avoiding estrogen deficiency is important to optimize bone health in Turner syndrome.” It “depends on early diagnosis, age-appropriate pubertal induction, and optimization of compliance,” Dr. Vincent said at the Endocrine Society annual meeting.
The median age of Turner syndrome diagnosis was 11 years, but estrogen treatments didn’t begin until a median age of 15. The women in the study were a median of about 30 years old, which means that they were adolescents at the time when estrogen treatment was often delayed in the mistaken belief that growth hormone therapy would be more effective before puberty was induced.
It’s now known that estrogen replacement works synergistically with, and even potentiates, the effects of growth hormone. Current guidelines recommend pubertal induction by age 13 (J Clin Endocrinol Metab. 2007 Jan;92(1):10-25).
The women had at least one dual-energy x-ray absorptiometry scan at Monash since 1998. Z-scores below - 2, indicating low bone density, were found in the lumbar spines of about a quarter the subjects, and in the femoral necks of about 8%. Primary amenorrhea and premature menopause, followed by vitamin D deficiency, were the most common risk factors for low bone mass. Almost 40% of the women reported non-continuous use of estrogen. About half had undergone growth hormone therapy.
At a median height of 149 cm, the subjects were about 15 cm shorter than age-matched, healthy controls, and also had a slightly higher median body mass index of 25.6 kg/m2. Lumbar spine bone area, bone mineral content, areal bone mineral density, and bone mineral apparent density were significantly lower in Turner syndrome patients. In the femoral neck, areal bone mineral density was significantly lower.
There was no relationship between bone markers and growth hormone use or Turner syndrome karyotype; the predominant karyotype was 45XO, but the study also included mosaic karyotypes.
The investigators had no disclosures.
AT ENDO 2016
Key clinical point: Induce puberty by age 13 in Turner syndrome.
Major finding: For every year after age 11 that Turner patients went without estrogen – generally due to delayed initiation, but sometimes noncompliance – there was a significant reduction in bone mineral density in both the lubar spine (Beta -0.582, P less than 0.001) and femoral neck (Beta -0.383, P = 0.008).
Data source: Retrospective, cross-section study of 76 Turner syndrome patients
Disclosures: The investigators had no disclosures.
HM16 Speakers Focus on Public Health, Leadership, Future of Hospital Medicine
SAN DIEGO — Hospital medicine’s annual extravaganza nestled into the southwestern corner of the country in March, with a record 4,000 hospitalists and others expanding their knowledge of clinical care, management, leadership, technology, and quality improvement.
They listened, they laughed, they learned.
Read more about the knowledge, experiences hospitalists shared at HM16.
Between the nitty-gritty of the workshops, expert panels, and forums, three high-profile speakers offered broad and insightful perspectives:
- U.S. Surgeon General Vivek Murthy, MD, MBA, a hospitalist by training, on his experiences as a hospitalist and his thoughts on the importance of public health in America;
- New SHM President Brian Harte, MD, SFHM, on the role of hospital medicine in cultivating leadership; and
- Hospitalist pioneer Robert Wachter, MD, MHM, on the future of hospital medicine as it reaches its 20th year since he introduced the term “hospitalist” in a New England Journal of Medicine article.
Dr. Murthy, formerly a hospitalist at Brigham and Women’s Hospital in Boston who in 2009 founded Doctors for America, an organization for healthcare improvement in the U.S., said his career as a hospitalist came as a surprise to him.
“When I was in medical school, I didn’t even know what a hospitalist was,” he said. “When I became a hospitalist, I thought it would be a temporary gig, something I did for a couple of years while I figured out what I really wanted to do. But as it turned out, I really loved what I did as a hospitalist. I love teaching. I love caring for patients. I love being part of a tight-knit team.”
He called good health “the key to opportunity,” explaining health is “intrinsically connected to the American dream.”
Hospitalists can play a role in building a “foundation for health,” he said. Four ingredients to this, he said, are creating a culture in which “healthy is equated with happiness” rather than associated with an attitude of “suck it up and eat your spinach”; changing our environment, such as adding sidewalks to encourage walking, to promote healthy behavior change; focusing on the spirit and mind as well as the body; and cultivating our ability to give and receive kindness, which he called “a source of healing.”
Dr. Harte described hospital medicine as “fertile ground” for leadership development.
“Our day-to-day experiences provide a leadership incubator that really no other specialty can claim,” he said.
He said he hopes that over the next several years, hospitalists and SHM make strides in these areas:
- Continuing and expanding membership;
- Continuing to push members and projects to focus on the Triple Aim, particularly patient- and family-centered care; and
- Better understanding hospitalists’ role in the era of risk.
“We need to clarify our position regarding specialty training and our training programs,” he added.
Dr. Harte recognized that such a discussion can get “difficult and contentious and political,” but that “when we look at what we have to do to be clinically effective, and what our current training programs and family medicine, internal medicine, and pediatrics provide for us, that gap to me only appears to be increasing.”
He said SHM has and will “continue to step up with curricula to fill those gaps.” However, he also said hospitalists “have to question what is the best way to train physicians for the roles of providers in the acute-care setting.”
Dr. Wachter, keeping his tradition of giving the final talk of the four-day conference, retraced the roots and successes of the field over the last 20 years. It was part history lesson, part report card, and part prognostication.
What the field has gotten wrong, so far, amounts to “an amazingly short list,” he said, but it’s not a nonexistent list.
“I think one thing we got wrong was a 7-days-on/7-days-off schedule,” he said, drawing applause. While it might be appealing to a 35-year-old doctor, he added, “I don’t believe this is a viable schedule for a 60-year-old.”
HM modeled itself after its closest cousin, emergency medicine, in which doctors frequently work 10- to 12-hour shifts every other day. Since that every-other-day schedule is not good for continuity, HM essentially strung together shifts for as many consecutive days as possible, leading to the 7-on/7-off. Now, many clinicians won’t consider positions without such a schedule even though it’s not a schedule suitable for everyone.
“I think we’ve shot ourselves in the foot,” he said. “Because what it means is you take all the work that needs to be done and you shove it into a very small amount of space. Therefore, the amount of intensity in that work that you have is, I think, undoable over time. I hope we rethink that.”
He cautioned that SHM is near the age when, all too often, societies begin to be complacent and needs to guard against the instinct to keep doing things as they have always been done.
“We need to instinctively say, ‘Wait a second, am I turning into all of those other societies that have become irrelevant—or less relevant—because of that reflex?’” he questioned.
He predicted that, even though value in care is now becoming an obsession, the digitization of healthcare ultimately will have a deeper impact on medicine.
“You ask me 10 years from now, I’m guessing that the fact that we’ve just gone from analog to digital will have turned out to be a bigger transformation,” he explained. “And the reason I say that is if you look at the history of every other industry that went from analog to digital, eventually the industries got turned upside down.”
Burnout, a prominent topic at the meeting, still doesn’t seem to be worse in hospital medicine than in many other specialties, he said, but it is a concern.
“We need to rethink this. We need to come up with some new practice models using information technology in new ways, collaborating with members of the team in different ways,” he said. “We have to take this issue and figure out a way of solving it.”
Dr. Wachter said hospital medicine needs to keep innovating and finding ways to add value; otherwise, the financial support hospitals give to hospital medicine could begin to shrink.
The field is facing challenges, he said, but he is clearly proud of its accomplishments. He said that before he went out on the stage at an early conference he organized in 1998, during the early days of hospital medicine, his wife asked him, “Are you sure this is a good idea?”
“What I said to her was, ‘It is a good idea, and it will be a good idea if we are successful in recruiting and retaining young people, innovative people who want to change the world,’” he said. “I think we have done that, and I thank all of you for turning this into a good idea.” TH
Thomas R. Collins is a freelance writer in South Florida.
SAN DIEGO — Hospital medicine’s annual extravaganza nestled into the southwestern corner of the country in March, with a record 4,000 hospitalists and others expanding their knowledge of clinical care, management, leadership, technology, and quality improvement.
They listened, they laughed, they learned.
Read more about the knowledge, experiences hospitalists shared at HM16.
Between the nitty-gritty of the workshops, expert panels, and forums, three high-profile speakers offered broad and insightful perspectives:
- U.S. Surgeon General Vivek Murthy, MD, MBA, a hospitalist by training, on his experiences as a hospitalist and his thoughts on the importance of public health in America;
- New SHM President Brian Harte, MD, SFHM, on the role of hospital medicine in cultivating leadership; and
- Hospitalist pioneer Robert Wachter, MD, MHM, on the future of hospital medicine as it reaches its 20th year since he introduced the term “hospitalist” in a New England Journal of Medicine article.
Dr. Murthy, formerly a hospitalist at Brigham and Women’s Hospital in Boston who in 2009 founded Doctors for America, an organization for healthcare improvement in the U.S., said his career as a hospitalist came as a surprise to him.
“When I was in medical school, I didn’t even know what a hospitalist was,” he said. “When I became a hospitalist, I thought it would be a temporary gig, something I did for a couple of years while I figured out what I really wanted to do. But as it turned out, I really loved what I did as a hospitalist. I love teaching. I love caring for patients. I love being part of a tight-knit team.”
He called good health “the key to opportunity,” explaining health is “intrinsically connected to the American dream.”
Hospitalists can play a role in building a “foundation for health,” he said. Four ingredients to this, he said, are creating a culture in which “healthy is equated with happiness” rather than associated with an attitude of “suck it up and eat your spinach”; changing our environment, such as adding sidewalks to encourage walking, to promote healthy behavior change; focusing on the spirit and mind as well as the body; and cultivating our ability to give and receive kindness, which he called “a source of healing.”
Dr. Harte described hospital medicine as “fertile ground” for leadership development.
“Our day-to-day experiences provide a leadership incubator that really no other specialty can claim,” he said.
He said he hopes that over the next several years, hospitalists and SHM make strides in these areas:
- Continuing and expanding membership;
- Continuing to push members and projects to focus on the Triple Aim, particularly patient- and family-centered care; and
- Better understanding hospitalists’ role in the era of risk.
“We need to clarify our position regarding specialty training and our training programs,” he added.
Dr. Harte recognized that such a discussion can get “difficult and contentious and political,” but that “when we look at what we have to do to be clinically effective, and what our current training programs and family medicine, internal medicine, and pediatrics provide for us, that gap to me only appears to be increasing.”
He said SHM has and will “continue to step up with curricula to fill those gaps.” However, he also said hospitalists “have to question what is the best way to train physicians for the roles of providers in the acute-care setting.”
Dr. Wachter, keeping his tradition of giving the final talk of the four-day conference, retraced the roots and successes of the field over the last 20 years. It was part history lesson, part report card, and part prognostication.
What the field has gotten wrong, so far, amounts to “an amazingly short list,” he said, but it’s not a nonexistent list.
“I think one thing we got wrong was a 7-days-on/7-days-off schedule,” he said, drawing applause. While it might be appealing to a 35-year-old doctor, he added, “I don’t believe this is a viable schedule for a 60-year-old.”
HM modeled itself after its closest cousin, emergency medicine, in which doctors frequently work 10- to 12-hour shifts every other day. Since that every-other-day schedule is not good for continuity, HM essentially strung together shifts for as many consecutive days as possible, leading to the 7-on/7-off. Now, many clinicians won’t consider positions without such a schedule even though it’s not a schedule suitable for everyone.
“I think we’ve shot ourselves in the foot,” he said. “Because what it means is you take all the work that needs to be done and you shove it into a very small amount of space. Therefore, the amount of intensity in that work that you have is, I think, undoable over time. I hope we rethink that.”
He cautioned that SHM is near the age when, all too often, societies begin to be complacent and needs to guard against the instinct to keep doing things as they have always been done.
“We need to instinctively say, ‘Wait a second, am I turning into all of those other societies that have become irrelevant—or less relevant—because of that reflex?’” he questioned.
He predicted that, even though value in care is now becoming an obsession, the digitization of healthcare ultimately will have a deeper impact on medicine.
“You ask me 10 years from now, I’m guessing that the fact that we’ve just gone from analog to digital will have turned out to be a bigger transformation,” he explained. “And the reason I say that is if you look at the history of every other industry that went from analog to digital, eventually the industries got turned upside down.”
Burnout, a prominent topic at the meeting, still doesn’t seem to be worse in hospital medicine than in many other specialties, he said, but it is a concern.
“We need to rethink this. We need to come up with some new practice models using information technology in new ways, collaborating with members of the team in different ways,” he said. “We have to take this issue and figure out a way of solving it.”
Dr. Wachter said hospital medicine needs to keep innovating and finding ways to add value; otherwise, the financial support hospitals give to hospital medicine could begin to shrink.
The field is facing challenges, he said, but he is clearly proud of its accomplishments. He said that before he went out on the stage at an early conference he organized in 1998, during the early days of hospital medicine, his wife asked him, “Are you sure this is a good idea?”
“What I said to her was, ‘It is a good idea, and it will be a good idea if we are successful in recruiting and retaining young people, innovative people who want to change the world,’” he said. “I think we have done that, and I thank all of you for turning this into a good idea.” TH
Thomas R. Collins is a freelance writer in South Florida.
SAN DIEGO — Hospital medicine’s annual extravaganza nestled into the southwestern corner of the country in March, with a record 4,000 hospitalists and others expanding their knowledge of clinical care, management, leadership, technology, and quality improvement.
They listened, they laughed, they learned.
Read more about the knowledge, experiences hospitalists shared at HM16.
Between the nitty-gritty of the workshops, expert panels, and forums, three high-profile speakers offered broad and insightful perspectives:
- U.S. Surgeon General Vivek Murthy, MD, MBA, a hospitalist by training, on his experiences as a hospitalist and his thoughts on the importance of public health in America;
- New SHM President Brian Harte, MD, SFHM, on the role of hospital medicine in cultivating leadership; and
- Hospitalist pioneer Robert Wachter, MD, MHM, on the future of hospital medicine as it reaches its 20th year since he introduced the term “hospitalist” in a New England Journal of Medicine article.
Dr. Murthy, formerly a hospitalist at Brigham and Women’s Hospital in Boston who in 2009 founded Doctors for America, an organization for healthcare improvement in the U.S., said his career as a hospitalist came as a surprise to him.
“When I was in medical school, I didn’t even know what a hospitalist was,” he said. “When I became a hospitalist, I thought it would be a temporary gig, something I did for a couple of years while I figured out what I really wanted to do. But as it turned out, I really loved what I did as a hospitalist. I love teaching. I love caring for patients. I love being part of a tight-knit team.”
He called good health “the key to opportunity,” explaining health is “intrinsically connected to the American dream.”
Hospitalists can play a role in building a “foundation for health,” he said. Four ingredients to this, he said, are creating a culture in which “healthy is equated with happiness” rather than associated with an attitude of “suck it up and eat your spinach”; changing our environment, such as adding sidewalks to encourage walking, to promote healthy behavior change; focusing on the spirit and mind as well as the body; and cultivating our ability to give and receive kindness, which he called “a source of healing.”
Dr. Harte described hospital medicine as “fertile ground” for leadership development.
“Our day-to-day experiences provide a leadership incubator that really no other specialty can claim,” he said.
He said he hopes that over the next several years, hospitalists and SHM make strides in these areas:
- Continuing and expanding membership;
- Continuing to push members and projects to focus on the Triple Aim, particularly patient- and family-centered care; and
- Better understanding hospitalists’ role in the era of risk.
“We need to clarify our position regarding specialty training and our training programs,” he added.
Dr. Harte recognized that such a discussion can get “difficult and contentious and political,” but that “when we look at what we have to do to be clinically effective, and what our current training programs and family medicine, internal medicine, and pediatrics provide for us, that gap to me only appears to be increasing.”
He said SHM has and will “continue to step up with curricula to fill those gaps.” However, he also said hospitalists “have to question what is the best way to train physicians for the roles of providers in the acute-care setting.”
Dr. Wachter, keeping his tradition of giving the final talk of the four-day conference, retraced the roots and successes of the field over the last 20 years. It was part history lesson, part report card, and part prognostication.
What the field has gotten wrong, so far, amounts to “an amazingly short list,” he said, but it’s not a nonexistent list.
“I think one thing we got wrong was a 7-days-on/7-days-off schedule,” he said, drawing applause. While it might be appealing to a 35-year-old doctor, he added, “I don’t believe this is a viable schedule for a 60-year-old.”
HM modeled itself after its closest cousin, emergency medicine, in which doctors frequently work 10- to 12-hour shifts every other day. Since that every-other-day schedule is not good for continuity, HM essentially strung together shifts for as many consecutive days as possible, leading to the 7-on/7-off. Now, many clinicians won’t consider positions without such a schedule even though it’s not a schedule suitable for everyone.
“I think we’ve shot ourselves in the foot,” he said. “Because what it means is you take all the work that needs to be done and you shove it into a very small amount of space. Therefore, the amount of intensity in that work that you have is, I think, undoable over time. I hope we rethink that.”
He cautioned that SHM is near the age when, all too often, societies begin to be complacent and needs to guard against the instinct to keep doing things as they have always been done.
“We need to instinctively say, ‘Wait a second, am I turning into all of those other societies that have become irrelevant—or less relevant—because of that reflex?’” he questioned.
He predicted that, even though value in care is now becoming an obsession, the digitization of healthcare ultimately will have a deeper impact on medicine.
“You ask me 10 years from now, I’m guessing that the fact that we’ve just gone from analog to digital will have turned out to be a bigger transformation,” he explained. “And the reason I say that is if you look at the history of every other industry that went from analog to digital, eventually the industries got turned upside down.”
Burnout, a prominent topic at the meeting, still doesn’t seem to be worse in hospital medicine than in many other specialties, he said, but it is a concern.
“We need to rethink this. We need to come up with some new practice models using information technology in new ways, collaborating with members of the team in different ways,” he said. “We have to take this issue and figure out a way of solving it.”
Dr. Wachter said hospital medicine needs to keep innovating and finding ways to add value; otherwise, the financial support hospitals give to hospital medicine could begin to shrink.
The field is facing challenges, he said, but he is clearly proud of its accomplishments. He said that before he went out on the stage at an early conference he organized in 1998, during the early days of hospital medicine, his wife asked him, “Are you sure this is a good idea?”
“What I said to her was, ‘It is a good idea, and it will be a good idea if we are successful in recruiting and retaining young people, innovative people who want to change the world,’” he said. “I think we have done that, and I thank all of you for turning this into a good idea.” TH
Thomas R. Collins is a freelance writer in South Florida.
CHMP recommends daratumumab for MM

Photo courtesy of Janssen
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended conditional marketing authorization for daratumumab (Darzalex), a first-in-class monoclonal antibody targeting CD38.
The recommended indication for daratumumab is as monotherapy for adults with relapsed and refractory multiple myeloma (MM).
The patients must have progressed on their last therapy and have received treatment with both a proteasome inhibitor and an immunomodulatory agent.
The CHMP’s positive opinion will now be reviewed by the European Commission, which has the authority to grant marketing authorization for medicines in the European Economic Area.
The European Commission’s final decision on daratumumab is anticipated in the coming months.
About conditional authorization
A product may receive conditional marketing authorization if the CHMP finds that, although comprehensive clinical data on the safety and efficacy of the product are not available, all of the following requirements are met:
- The risk-benefit balance of the product is positive
- The company developing the product will likely be in a position to provide comprehensive clinical data in the future
- Unmet medical needs will be fulfilled
- The benefit to public health of the immediate availability of the product outweighs the risk inherent in the fact that additional data are still required.
Conditional marketing authorizations are valid for 1 year, on a renewable basis. The holder will be required to complete ongoing studies or to conduct new studies with a view to confirming that the benefit-risk balance of a product is positive. In addition, specific obligations may be imposed in relation to the collection of pharmacovigilance data.
About daratumumab
Daratumumab is the first CD38-directed monoclonal antibody recommended for approval in Europe. It works by binding to CD38, a signaling molecule highly expressed on the surface of MM cells regardless of stage of disease.
In binding to CD38, daratumumab triggers the patient’s own immune system to attack the cancer cells, resulting in rapid tumor cell death through multiple, immune-mediated mechanisms of action and through immunomodulatory effects, in addition to direct tumor cell death via apoptosis.
The CHMP’s positive opinion of daratumumab was based on a review of data from the phase 2 SIRIUS study, the phase 1/2 GEN501 study, and 3 additional supportive studies.
The GEN501 study enrolled 102 patients with relapsed MM or relapsed MM that was refractory to 2 or more prior lines of therapy. The patients received daratumumab at a range of doses and on a number of different schedules.
The results suggested daratumumab is most effective at a dose of 16 mg/kg. At this dose, the overall response rate was 36%. Most adverse events in this study were grade 1 or 2, although serious events did occur.
The SIRIUS study enrolled 124 MM patients who had received 3 or more prior lines of therapy. They received daratumumab at different doses and on different schedules, but 106 patients received the drug at 16 mg/kg.
Twenty-nine percent of the 106 patients responded to treatment, and the median duration of response was 7 months. Thirty percent of patients experienced serious adverse events.
Findings from a combined efficacy analysis of the GEN501 and SIRIUS trials demonstrated that, after a mean follow-up of 14.8 months, the estimated median overall survival for patients who received single-agent daratumumab at 16 mg/kg was 20 months.
Five phase 3 clinical studies with daratumumab in MM patients—in relapsed and frontline settings—are ongoing. Additional studies are ongoing or planned to assess the drug’s potential in other malignant and pre-malignant diseases in which CD38 is expressed.
Janssen has exclusive worldwide rights to the development, manufacturing, and commercialization of daratumumab for all potential indications. Janssen licensed daratumumab from Genmab A/S in August 2012. ![]()

Photo courtesy of Janssen
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended conditional marketing authorization for daratumumab (Darzalex), a first-in-class monoclonal antibody targeting CD38.
The recommended indication for daratumumab is as monotherapy for adults with relapsed and refractory multiple myeloma (MM).
The patients must have progressed on their last therapy and have received treatment with both a proteasome inhibitor and an immunomodulatory agent.
The CHMP’s positive opinion will now be reviewed by the European Commission, which has the authority to grant marketing authorization for medicines in the European Economic Area.
The European Commission’s final decision on daratumumab is anticipated in the coming months.
About conditional authorization
A product may receive conditional marketing authorization if the CHMP finds that, although comprehensive clinical data on the safety and efficacy of the product are not available, all of the following requirements are met:
- The risk-benefit balance of the product is positive
- The company developing the product will likely be in a position to provide comprehensive clinical data in the future
- Unmet medical needs will be fulfilled
- The benefit to public health of the immediate availability of the product outweighs the risk inherent in the fact that additional data are still required.
Conditional marketing authorizations are valid for 1 year, on a renewable basis. The holder will be required to complete ongoing studies or to conduct new studies with a view to confirming that the benefit-risk balance of a product is positive. In addition, specific obligations may be imposed in relation to the collection of pharmacovigilance data.
About daratumumab
Daratumumab is the first CD38-directed monoclonal antibody recommended for approval in Europe. It works by binding to CD38, a signaling molecule highly expressed on the surface of MM cells regardless of stage of disease.
In binding to CD38, daratumumab triggers the patient’s own immune system to attack the cancer cells, resulting in rapid tumor cell death through multiple, immune-mediated mechanisms of action and through immunomodulatory effects, in addition to direct tumor cell death via apoptosis.
The CHMP’s positive opinion of daratumumab was based on a review of data from the phase 2 SIRIUS study, the phase 1/2 GEN501 study, and 3 additional supportive studies.
The GEN501 study enrolled 102 patients with relapsed MM or relapsed MM that was refractory to 2 or more prior lines of therapy. The patients received daratumumab at a range of doses and on a number of different schedules.
The results suggested daratumumab is most effective at a dose of 16 mg/kg. At this dose, the overall response rate was 36%. Most adverse events in this study were grade 1 or 2, although serious events did occur.
The SIRIUS study enrolled 124 MM patients who had received 3 or more prior lines of therapy. They received daratumumab at different doses and on different schedules, but 106 patients received the drug at 16 mg/kg.
Twenty-nine percent of the 106 patients responded to treatment, and the median duration of response was 7 months. Thirty percent of patients experienced serious adverse events.
Findings from a combined efficacy analysis of the GEN501 and SIRIUS trials demonstrated that, after a mean follow-up of 14.8 months, the estimated median overall survival for patients who received single-agent daratumumab at 16 mg/kg was 20 months.
Five phase 3 clinical studies with daratumumab in MM patients—in relapsed and frontline settings—are ongoing. Additional studies are ongoing or planned to assess the drug’s potential in other malignant and pre-malignant diseases in which CD38 is expressed.
Janssen has exclusive worldwide rights to the development, manufacturing, and commercialization of daratumumab for all potential indications. Janssen licensed daratumumab from Genmab A/S in August 2012. ![]()

Photo courtesy of Janssen
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended conditional marketing authorization for daratumumab (Darzalex), a first-in-class monoclonal antibody targeting CD38.
The recommended indication for daratumumab is as monotherapy for adults with relapsed and refractory multiple myeloma (MM).
The patients must have progressed on their last therapy and have received treatment with both a proteasome inhibitor and an immunomodulatory agent.
The CHMP’s positive opinion will now be reviewed by the European Commission, which has the authority to grant marketing authorization for medicines in the European Economic Area.
The European Commission’s final decision on daratumumab is anticipated in the coming months.
About conditional authorization
A product may receive conditional marketing authorization if the CHMP finds that, although comprehensive clinical data on the safety and efficacy of the product are not available, all of the following requirements are met:
- The risk-benefit balance of the product is positive
- The company developing the product will likely be in a position to provide comprehensive clinical data in the future
- Unmet medical needs will be fulfilled
- The benefit to public health of the immediate availability of the product outweighs the risk inherent in the fact that additional data are still required.
Conditional marketing authorizations are valid for 1 year, on a renewable basis. The holder will be required to complete ongoing studies or to conduct new studies with a view to confirming that the benefit-risk balance of a product is positive. In addition, specific obligations may be imposed in relation to the collection of pharmacovigilance data.
About daratumumab
Daratumumab is the first CD38-directed monoclonal antibody recommended for approval in Europe. It works by binding to CD38, a signaling molecule highly expressed on the surface of MM cells regardless of stage of disease.
In binding to CD38, daratumumab triggers the patient’s own immune system to attack the cancer cells, resulting in rapid tumor cell death through multiple, immune-mediated mechanisms of action and through immunomodulatory effects, in addition to direct tumor cell death via apoptosis.
The CHMP’s positive opinion of daratumumab was based on a review of data from the phase 2 SIRIUS study, the phase 1/2 GEN501 study, and 3 additional supportive studies.
The GEN501 study enrolled 102 patients with relapsed MM or relapsed MM that was refractory to 2 or more prior lines of therapy. The patients received daratumumab at a range of doses and on a number of different schedules.
The results suggested daratumumab is most effective at a dose of 16 mg/kg. At this dose, the overall response rate was 36%. Most adverse events in this study were grade 1 or 2, although serious events did occur.
The SIRIUS study enrolled 124 MM patients who had received 3 or more prior lines of therapy. They received daratumumab at different doses and on different schedules, but 106 patients received the drug at 16 mg/kg.
Twenty-nine percent of the 106 patients responded to treatment, and the median duration of response was 7 months. Thirty percent of patients experienced serious adverse events.
Findings from a combined efficacy analysis of the GEN501 and SIRIUS trials demonstrated that, after a mean follow-up of 14.8 months, the estimated median overall survival for patients who received single-agent daratumumab at 16 mg/kg was 20 months.
Five phase 3 clinical studies with daratumumab in MM patients—in relapsed and frontline settings—are ongoing. Additional studies are ongoing or planned to assess the drug’s potential in other malignant and pre-malignant diseases in which CD38 is expressed.
Janssen has exclusive worldwide rights to the development, manufacturing, and commercialization of daratumumab for all potential indications. Janssen licensed daratumumab from Genmab A/S in August 2012. ![]()
FDA: CT scans safe for patients with electronic medical devices
There’s no need to let fear of electronic interference between computed tomography and electronic medical devices preclude the ordering of such scans for patients with insulin pumps, cardiac implantable electronic devices, or neurostimulators, the Food and Drug Administration said in a written notification.
“The probability of an adverse event being caused by exposing these devices to CT irradiation is extremely low, and it is greatly outweighed by the clinical benefit of a medically indicated CT examination,” according to the new notification, which updates and replaces a preliminary health notification released on July 14, 2008.
The preliminary notification said there was a “possibility that the x-rays used during CT examinations may cause some implanted and external electronic medical devices to malfunction.” It also included recommendations to reduce the potential risk of such events from occurring and cited adverse events experienced by a few patients with medical devices who had undergone CT scanning, including unintended shocks from neurostimulators, malfunctions of insulin infusion pumps, and transient changes in pacemaker output pulse rate.
The new notification says there is an extremely low probability that a CT scanner directly irradiating the circuitry of certain implantable or wearable electronic medical devices can cause sufficient electronic interference to affect the function and operation of the medical device, and this probability is even lower when the radiation dose and the radiation dose rate are reduced. The FDA also notes that the interference is completely avoided when the medical device is outside of the primary x-ray beam of the CT scanner.
The update, which provides additional reports of adverse events by patients with electronic medical devices who had CT scans, states that the number of such events was small, compared with the number of patients with insulin pumps, cardiac implantable electronic devices, and neurostimulators who were scanned without adverse effects.
The FDA encourages health care providers and patients who suspect a problem with a medical imaging device to file a voluntary report through MedWatch, the FDA Safety Information and Adverse Event Reporting Program.
There’s no need to let fear of electronic interference between computed tomography and electronic medical devices preclude the ordering of such scans for patients with insulin pumps, cardiac implantable electronic devices, or neurostimulators, the Food and Drug Administration said in a written notification.
“The probability of an adverse event being caused by exposing these devices to CT irradiation is extremely low, and it is greatly outweighed by the clinical benefit of a medically indicated CT examination,” according to the new notification, which updates and replaces a preliminary health notification released on July 14, 2008.
The preliminary notification said there was a “possibility that the x-rays used during CT examinations may cause some implanted and external electronic medical devices to malfunction.” It also included recommendations to reduce the potential risk of such events from occurring and cited adverse events experienced by a few patients with medical devices who had undergone CT scanning, including unintended shocks from neurostimulators, malfunctions of insulin infusion pumps, and transient changes in pacemaker output pulse rate.
The new notification says there is an extremely low probability that a CT scanner directly irradiating the circuitry of certain implantable or wearable electronic medical devices can cause sufficient electronic interference to affect the function and operation of the medical device, and this probability is even lower when the radiation dose and the radiation dose rate are reduced. The FDA also notes that the interference is completely avoided when the medical device is outside of the primary x-ray beam of the CT scanner.
The update, which provides additional reports of adverse events by patients with electronic medical devices who had CT scans, states that the number of such events was small, compared with the number of patients with insulin pumps, cardiac implantable electronic devices, and neurostimulators who were scanned without adverse effects.
The FDA encourages health care providers and patients who suspect a problem with a medical imaging device to file a voluntary report through MedWatch, the FDA Safety Information and Adverse Event Reporting Program.
There’s no need to let fear of electronic interference between computed tomography and electronic medical devices preclude the ordering of such scans for patients with insulin pumps, cardiac implantable electronic devices, or neurostimulators, the Food and Drug Administration said in a written notification.
“The probability of an adverse event being caused by exposing these devices to CT irradiation is extremely low, and it is greatly outweighed by the clinical benefit of a medically indicated CT examination,” according to the new notification, which updates and replaces a preliminary health notification released on July 14, 2008.
The preliminary notification said there was a “possibility that the x-rays used during CT examinations may cause some implanted and external electronic medical devices to malfunction.” It also included recommendations to reduce the potential risk of such events from occurring and cited adverse events experienced by a few patients with medical devices who had undergone CT scanning, including unintended shocks from neurostimulators, malfunctions of insulin infusion pumps, and transient changes in pacemaker output pulse rate.
The new notification says there is an extremely low probability that a CT scanner directly irradiating the circuitry of certain implantable or wearable electronic medical devices can cause sufficient electronic interference to affect the function and operation of the medical device, and this probability is even lower when the radiation dose and the radiation dose rate are reduced. The FDA also notes that the interference is completely avoided when the medical device is outside of the primary x-ray beam of the CT scanner.
The update, which provides additional reports of adverse events by patients with electronic medical devices who had CT scans, states that the number of such events was small, compared with the number of patients with insulin pumps, cardiac implantable electronic devices, and neurostimulators who were scanned without adverse effects.
The FDA encourages health care providers and patients who suspect a problem with a medical imaging device to file a voluntary report through MedWatch, the FDA Safety Information and Adverse Event Reporting Program.
Blisters on face and ear
The FP diagnosed herpes zoster with possible Ramsay Hunt syndrome in this patient. The zoster also appeared superinfected by a bacterial infection, which was causing the extensive erythema, weeping of yellow fluid, and some honey crusting.
Herpes zoster oticus (Ramsay Hunt syndrome) includes the triad of ipsilateral facial paralysis, ear pain, and vesicles in the auditory canal and auricle. Disturbances in taste perception, hearing (tinnitus, hyperacusis), lacrimation, and vestibular function (vertigo) may occur. Fortunately, the child did not have facial paralysis, but did have ear pain and vesicles on the auricle. The child also admitted to some ringing in the right ear.
The FP prescribed oral acyclovir and cultured the weeping fluid to determine if this was truly a bacterial superinfection. The child was started on oral cephalexin 3 times a day to cover Streptococcus pyogenes and Staphylococcus aureus. Oral acetaminophen was prescribed for the pain and fever. The physician called his ear, nose, and throat (ENT) colleague who agreed to see the patient later that day.
The FP and ENT physician decided to follow her without immediate hospitalization since she was taking fluids well and did not appear systemically ill. The following day, the patient was already improving and later that week the culture grew out Staphylococcus aureus that was methicillin sensitive. The child fully recovered from the superinfected herpes zoster without any sequelae. The tinnitus resolved, as well.
Photo courtesy of UTHSCSA Dermatology division. Text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Zoster. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:712-717.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed herpes zoster with possible Ramsay Hunt syndrome in this patient. The zoster also appeared superinfected by a bacterial infection, which was causing the extensive erythema, weeping of yellow fluid, and some honey crusting.
Herpes zoster oticus (Ramsay Hunt syndrome) includes the triad of ipsilateral facial paralysis, ear pain, and vesicles in the auditory canal and auricle. Disturbances in taste perception, hearing (tinnitus, hyperacusis), lacrimation, and vestibular function (vertigo) may occur. Fortunately, the child did not have facial paralysis, but did have ear pain and vesicles on the auricle. The child also admitted to some ringing in the right ear.
The FP prescribed oral acyclovir and cultured the weeping fluid to determine if this was truly a bacterial superinfection. The child was started on oral cephalexin 3 times a day to cover Streptococcus pyogenes and Staphylococcus aureus. Oral acetaminophen was prescribed for the pain and fever. The physician called his ear, nose, and throat (ENT) colleague who agreed to see the patient later that day.
The FP and ENT physician decided to follow her without immediate hospitalization since she was taking fluids well and did not appear systemically ill. The following day, the patient was already improving and later that week the culture grew out Staphylococcus aureus that was methicillin sensitive. The child fully recovered from the superinfected herpes zoster without any sequelae. The tinnitus resolved, as well.
Photo courtesy of UTHSCSA Dermatology division. Text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Zoster. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:712-717.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed herpes zoster with possible Ramsay Hunt syndrome in this patient. The zoster also appeared superinfected by a bacterial infection, which was causing the extensive erythema, weeping of yellow fluid, and some honey crusting.
Herpes zoster oticus (Ramsay Hunt syndrome) includes the triad of ipsilateral facial paralysis, ear pain, and vesicles in the auditory canal and auricle. Disturbances in taste perception, hearing (tinnitus, hyperacusis), lacrimation, and vestibular function (vertigo) may occur. Fortunately, the child did not have facial paralysis, but did have ear pain and vesicles on the auricle. The child also admitted to some ringing in the right ear.
The FP prescribed oral acyclovir and cultured the weeping fluid to determine if this was truly a bacterial superinfection. The child was started on oral cephalexin 3 times a day to cover Streptococcus pyogenes and Staphylococcus aureus. Oral acetaminophen was prescribed for the pain and fever. The physician called his ear, nose, and throat (ENT) colleague who agreed to see the patient later that day.
The FP and ENT physician decided to follow her without immediate hospitalization since she was taking fluids well and did not appear systemically ill. The following day, the patient was already improving and later that week the culture grew out Staphylococcus aureus that was methicillin sensitive. The child fully recovered from the superinfected herpes zoster without any sequelae. The tinnitus resolved, as well.
Photo courtesy of UTHSCSA Dermatology division. Text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Zoster. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:712-717.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Blisters on thigh
The FP diagnosed the boy with herpes zoster that was found in multiple dermatomes. He did not think it was disseminated because it was still localized to one area and there were not 20 or more lesions outside the primary zoster. However, the FP was very concerned about HIV and the boy was tested. A rapid HIV test came back positive.
The FP discussed the results of his findings with the child's grandmother, who was now caring for the child. An attempt was made to obtain oral or intravenous acyclovir but it was not available in the village or local health center. The child was given oral liquid acetaminophen for the pain and was added to the list for the local HIV clinic. Fortunately, the zoster resolved without antiviral medications and the child began to receive care for his HIV infection.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Zoster. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:712-717.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed the boy with herpes zoster that was found in multiple dermatomes. He did not think it was disseminated because it was still localized to one area and there were not 20 or more lesions outside the primary zoster. However, the FP was very concerned about HIV and the boy was tested. A rapid HIV test came back positive.
The FP discussed the results of his findings with the child's grandmother, who was now caring for the child. An attempt was made to obtain oral or intravenous acyclovir but it was not available in the village or local health center. The child was given oral liquid acetaminophen for the pain and was added to the list for the local HIV clinic. Fortunately, the zoster resolved without antiviral medications and the child began to receive care for his HIV infection.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Zoster. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:712-717.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed the boy with herpes zoster that was found in multiple dermatomes. He did not think it was disseminated because it was still localized to one area and there were not 20 or more lesions outside the primary zoster. However, the FP was very concerned about HIV and the boy was tested. A rapid HIV test came back positive.
The FP discussed the results of his findings with the child's grandmother, who was now caring for the child. An attempt was made to obtain oral or intravenous acyclovir but it was not available in the village or local health center. The child was given oral liquid acetaminophen for the pain and was added to the list for the local HIV clinic. Fortunately, the zoster resolved without antiviral medications and the child began to receive care for his HIV infection.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Zoster. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:712-717.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Pruritic rash on 16-year-old girl
Despite the fact that some of the vesicles crossed the midline (which is suggestive of disseminated zoster), the FP felt confident in diagnosing herpes zoster (shingles) in this patient. The FP knew that the varicella-zoster virus (VZV) leaves the dorsal root ganglion to travel down the spinal nerves to the cutaneous nerves of the skin. But she also knew that the vesicles could cross the midline by a few centimeters because the posterior primary ramus of the spinal nerve includes a small cutaneous medial branch that reaches across the midline.1
After a primary infection with either chickenpox or vaccine-type VZV, a latent infection is established in the sensory dorsal root ganglia. Reactivation of this latent VZV infection results in herpes zoster. Both sensory ganglia neurons and satellite cells surrounding the neurons serve as sites of VZV latent infection. Once reactivated, the virus spreads to other cells within the ganglion. The dermatomal distribution of the rash corresponds to the sensory fields of the infected neurons within the specific ganglion.
The pain associated with zoster infections and postherpetic neuralgia (PHN) is thought to result from injury to the peripheral nerves and altered central nervous system processing. PHN occurs more commonly in individuals older than age 60 and in immunosuppressed individuals. The lesions typically crust in approximately a week, with complete resolution within 3 to 4 weeks. If there are more than 20 lesions distributed outside the affected dermatome, the patient has disseminated zoster.
The treatment of herpes zoster includes antiviral agents, such as acyclovir, famciclovir, and valacyclovir. The evidence only supports their use if started within 72 hours of rash onset. Pain can be managed with nonprescription analgesics or narcotics and should be treated aggressively. This may actually prevent or lessen the severity of PHN.
The FP in this case was also concerned about whether the patient might be positive for human immunodeficiency virus (HIV) or have some type of immunosuppression. The patient denied sexual activity and use of intravenous drugs (even when her mom wasn’t in the room). She was otherwise healthy, so no further workup for HIV or immunosuppression was ordered. The FP told the teen to stay home from school until the lesions crusted over. A follow-up visit in one month was scheduled. The zoster resolved and the girl returned to her usual state of good health.
1. Usatine RP, Clemente C. Is herpes zoster unilateral? West J Med. 1999;170:263.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Zoster. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:712-717.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Despite the fact that some of the vesicles crossed the midline (which is suggestive of disseminated zoster), the FP felt confident in diagnosing herpes zoster (shingles) in this patient. The FP knew that the varicella-zoster virus (VZV) leaves the dorsal root ganglion to travel down the spinal nerves to the cutaneous nerves of the skin. But she also knew that the vesicles could cross the midline by a few centimeters because the posterior primary ramus of the spinal nerve includes a small cutaneous medial branch that reaches across the midline.1
After a primary infection with either chickenpox or vaccine-type VZV, a latent infection is established in the sensory dorsal root ganglia. Reactivation of this latent VZV infection results in herpes zoster. Both sensory ganglia neurons and satellite cells surrounding the neurons serve as sites of VZV latent infection. Once reactivated, the virus spreads to other cells within the ganglion. The dermatomal distribution of the rash corresponds to the sensory fields of the infected neurons within the specific ganglion.
The pain associated with zoster infections and postherpetic neuralgia (PHN) is thought to result from injury to the peripheral nerves and altered central nervous system processing. PHN occurs more commonly in individuals older than age 60 and in immunosuppressed individuals. The lesions typically crust in approximately a week, with complete resolution within 3 to 4 weeks. If there are more than 20 lesions distributed outside the affected dermatome, the patient has disseminated zoster.
The treatment of herpes zoster includes antiviral agents, such as acyclovir, famciclovir, and valacyclovir. The evidence only supports their use if started within 72 hours of rash onset. Pain can be managed with nonprescription analgesics or narcotics and should be treated aggressively. This may actually prevent or lessen the severity of PHN.
The FP in this case was also concerned about whether the patient might be positive for human immunodeficiency virus (HIV) or have some type of immunosuppression. The patient denied sexual activity and use of intravenous drugs (even when her mom wasn’t in the room). She was otherwise healthy, so no further workup for HIV or immunosuppression was ordered. The FP told the teen to stay home from school until the lesions crusted over. A follow-up visit in one month was scheduled. The zoster resolved and the girl returned to her usual state of good health.
1. Usatine RP, Clemente C. Is herpes zoster unilateral? West J Med. 1999;170:263.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Zoster. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:712-717.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Despite the fact that some of the vesicles crossed the midline (which is suggestive of disseminated zoster), the FP felt confident in diagnosing herpes zoster (shingles) in this patient. The FP knew that the varicella-zoster virus (VZV) leaves the dorsal root ganglion to travel down the spinal nerves to the cutaneous nerves of the skin. But she also knew that the vesicles could cross the midline by a few centimeters because the posterior primary ramus of the spinal nerve includes a small cutaneous medial branch that reaches across the midline.1
After a primary infection with either chickenpox or vaccine-type VZV, a latent infection is established in the sensory dorsal root ganglia. Reactivation of this latent VZV infection results in herpes zoster. Both sensory ganglia neurons and satellite cells surrounding the neurons serve as sites of VZV latent infection. Once reactivated, the virus spreads to other cells within the ganglion. The dermatomal distribution of the rash corresponds to the sensory fields of the infected neurons within the specific ganglion.
The pain associated with zoster infections and postherpetic neuralgia (PHN) is thought to result from injury to the peripheral nerves and altered central nervous system processing. PHN occurs more commonly in individuals older than age 60 and in immunosuppressed individuals. The lesions typically crust in approximately a week, with complete resolution within 3 to 4 weeks. If there are more than 20 lesions distributed outside the affected dermatome, the patient has disseminated zoster.
The treatment of herpes zoster includes antiviral agents, such as acyclovir, famciclovir, and valacyclovir. The evidence only supports their use if started within 72 hours of rash onset. Pain can be managed with nonprescription analgesics or narcotics and should be treated aggressively. This may actually prevent or lessen the severity of PHN.
The FP in this case was also concerned about whether the patient might be positive for human immunodeficiency virus (HIV) or have some type of immunosuppression. The patient denied sexual activity and use of intravenous drugs (even when her mom wasn’t in the room). She was otherwise healthy, so no further workup for HIV or immunosuppression was ordered. The FP told the teen to stay home from school until the lesions crusted over. A follow-up visit in one month was scheduled. The zoster resolved and the girl returned to her usual state of good health.
1. Usatine RP, Clemente C. Is herpes zoster unilateral? West J Med. 1999;170:263.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R. Zoster. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:712-717.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com