User login
Does caffeine intake during pregnancy affect birth weight?
No. Reducing caffeinated coffee consumption by 180 mg of caffeine (the equivalent of 2 cups) per day after 16 weeks’ gestation doesn’t affect birth weight. Consuming more than 300 mg of caffeine per day is associated with a clinically trivial, and statistically insignificant (less than 1 ounce), reduction in birth weight, compared with consuming no caffeine (strength of recommendation: B, randomized controlled trial [RCT] and large prospective cohort study).
EVIDENCE SUMMARY
A Cochrane systematic review of the effects of caffeine on pregnancy identified 2 studies, only one of which addressed the question of maternal caffeine intake and infant birth weight.1 The double-blind RCT evaluating caffeine intake during pregnancy found no significant differences in birth weight or length of gestation between women who drank regular coffee and women who drank decaffeinated coffee.2
At 16 weeks’ gestation, investigators randomized 1207 pregnant women who reported daily intake of at least 3 cups of regular coffee to drink unlabeled instant coffee (which was either regular or decaffeinated) for the rest of their pregnancy. The women were allowed to request as much of their assigned instant coffee as they wanted.
Subjects were recruited from among all women with uncomplicated, singleton pregnancies who were expected to deliver at a Danish university hospital during the study period. Investigators interviewed the women at 20, 25, and 34 weeks to determine coffee consumption (including both coffee provided by the investigators and other coffee), consumption of other caffeinated beverages, and smoking status.
The difference in caffeine intake between the groups didn’t correspond to significant differences in birth weight (16 g lighter with caffeinated coffee; 95% confidence interval [CI], −40 g to 73 g; P=.48) or birth length (0.03 cm longer with caffeinated coffee; 95% CI, −0.29 to 0.22) among infants born to the 1150 women who completed the study.
Limitations of the study include randomizing women after 16 weeks’ gestation and the observation that many women correctly guessed which type of coffee they received (35% of women drinking caffeinated coffee and 49% of women drinking decaf).
A caffeine effect, but with study limitations
The Cochrane systematic review (described above) and a meta-analysis of 9 prospective cohort studies with a total of 90,000 patients that evaluated maternal caffeine intake found that it was associated with increased low birth weight, intrauterine growth restriction (IUGR), or small for gestational age (SGA) infants.3
Researchers assessed caffeine consumption from coffee or other sources either by questionnaire (5 studies) or interview (4 studies) at various times during pregnancy, mostly in the first or second trimester, and assigned subjects to 4 intake categories: none, low (50-149 mg/d), moderate (150-349 mg/d), and high (>350 mg/d).
Compared with no caffeine, all levels of caffeine intake were associated with increased rates of low birth weight, IUGR, or SGA (low intake: relative risk [RR]=1.13; 95% CI, 1.06-1.21; moderate intake: RR=1.38; 95% CI, 1.18-1.62; high intake: RR=1.60; 95% CI, 1.24-2.08).
A major limitation of the meta-analysis was that 8 of the included studies were identified by the reviewers as having quality problems. The reviewers also identified additional cohort studies, not included in the meta-analysis, which failed to show any association between caffeine intake and poor pregnancy outcomes.
Results of best-quality study prove clinically trivial
The best-quality prospective cohort study in the review described above was also the largest, comprising two-thirds of the total patients. It found a statistically significant, but clinically trivial, association between caffeine intake and birth weight.4
Investigators from Norway’s Institute of Public Health mailed surveys to 106,707 pregnant Norwegian women and recruited 59,123 with uncomplicated singleton pregnancies. The survey assessed diet and lifestyle at several stages of pregnancy and correlated caffeine intake with birth weight, gestational length, and SGA deliveries. Investigators calculated caffeine intake from coffee and other dietary sources (tea and chocolate).
Higher caffeine intake was associated with a small reduction in birth weight (8 g/100 mg/d of additional caffeine intake; 95% CI, −10 to −6 g/100 mg/d). Higher intake was also associated with increasing likelihood of SGA birth, a finding of borderline significance (odds ratio [OR]=1.18; 95% CI, 1.00-1.38, comparing intake <50 mg/d with 51-200 mg/d; OR=1.62; 95% CI, 1.26-2.29, comparing <50 mg/d with 201-300 mg/d; and OR=1.62; 95% CI, 1.15-2.29, comparing <50 mg/d with >300 mg/d).
1. Jahanfar S, Jaafar SH. Effects of restricted caffeine intake by mother on fetal, neonatal and pregnancy outcome. Cochrane Database Syst Rev. 2013;(2):CD006965.
2. Bech BH, Obel C, Henriksen TB, et al. Effect of reducing caffeine intake on birth weight and length of gestation: randomised controlled trial. BMJ. 2007;334:409.
3. Chen LW, Wu Y, Neelakantan N, et al. Maternal caffeine intake during pregnancy is associated with risk of low birth weight: a systematic review and dose-response meta-analysis. BMC Medicine. 2014;12:174-176.
4. Sengpiel V, Elind E, Bacelis J, et al. Maternal caffeine intake during pregnancy is associated with birth weight but not with gestational length: results form a large prospective observational cohort trial. BMC Medicine. 2013;11:42.
No. Reducing caffeinated coffee consumption by 180 mg of caffeine (the equivalent of 2 cups) per day after 16 weeks’ gestation doesn’t affect birth weight. Consuming more than 300 mg of caffeine per day is associated with a clinically trivial, and statistically insignificant (less than 1 ounce), reduction in birth weight, compared with consuming no caffeine (strength of recommendation: B, randomized controlled trial [RCT] and large prospective cohort study).
EVIDENCE SUMMARY
A Cochrane systematic review of the effects of caffeine on pregnancy identified 2 studies, only one of which addressed the question of maternal caffeine intake and infant birth weight.1 The double-blind RCT evaluating caffeine intake during pregnancy found no significant differences in birth weight or length of gestation between women who drank regular coffee and women who drank decaffeinated coffee.2
At 16 weeks’ gestation, investigators randomized 1207 pregnant women who reported daily intake of at least 3 cups of regular coffee to drink unlabeled instant coffee (which was either regular or decaffeinated) for the rest of their pregnancy. The women were allowed to request as much of their assigned instant coffee as they wanted.
Subjects were recruited from among all women with uncomplicated, singleton pregnancies who were expected to deliver at a Danish university hospital during the study period. Investigators interviewed the women at 20, 25, and 34 weeks to determine coffee consumption (including both coffee provided by the investigators and other coffee), consumption of other caffeinated beverages, and smoking status.
The difference in caffeine intake between the groups didn’t correspond to significant differences in birth weight (16 g lighter with caffeinated coffee; 95% confidence interval [CI], −40 g to 73 g; P=.48) or birth length (0.03 cm longer with caffeinated coffee; 95% CI, −0.29 to 0.22) among infants born to the 1150 women who completed the study.
Limitations of the study include randomizing women after 16 weeks’ gestation and the observation that many women correctly guessed which type of coffee they received (35% of women drinking caffeinated coffee and 49% of women drinking decaf).
A caffeine effect, but with study limitations
The Cochrane systematic review (described above) and a meta-analysis of 9 prospective cohort studies with a total of 90,000 patients that evaluated maternal caffeine intake found that it was associated with increased low birth weight, intrauterine growth restriction (IUGR), or small for gestational age (SGA) infants.3
Researchers assessed caffeine consumption from coffee or other sources either by questionnaire (5 studies) or interview (4 studies) at various times during pregnancy, mostly in the first or second trimester, and assigned subjects to 4 intake categories: none, low (50-149 mg/d), moderate (150-349 mg/d), and high (>350 mg/d).
Compared with no caffeine, all levels of caffeine intake were associated with increased rates of low birth weight, IUGR, or SGA (low intake: relative risk [RR]=1.13; 95% CI, 1.06-1.21; moderate intake: RR=1.38; 95% CI, 1.18-1.62; high intake: RR=1.60; 95% CI, 1.24-2.08).
A major limitation of the meta-analysis was that 8 of the included studies were identified by the reviewers as having quality problems. The reviewers also identified additional cohort studies, not included in the meta-analysis, which failed to show any association between caffeine intake and poor pregnancy outcomes.
Results of best-quality study prove clinically trivial
The best-quality prospective cohort study in the review described above was also the largest, comprising two-thirds of the total patients. It found a statistically significant, but clinically trivial, association between caffeine intake and birth weight.4
Investigators from Norway’s Institute of Public Health mailed surveys to 106,707 pregnant Norwegian women and recruited 59,123 with uncomplicated singleton pregnancies. The survey assessed diet and lifestyle at several stages of pregnancy and correlated caffeine intake with birth weight, gestational length, and SGA deliveries. Investigators calculated caffeine intake from coffee and other dietary sources (tea and chocolate).
Higher caffeine intake was associated with a small reduction in birth weight (8 g/100 mg/d of additional caffeine intake; 95% CI, −10 to −6 g/100 mg/d). Higher intake was also associated with increasing likelihood of SGA birth, a finding of borderline significance (odds ratio [OR]=1.18; 95% CI, 1.00-1.38, comparing intake <50 mg/d with 51-200 mg/d; OR=1.62; 95% CI, 1.26-2.29, comparing <50 mg/d with 201-300 mg/d; and OR=1.62; 95% CI, 1.15-2.29, comparing <50 mg/d with >300 mg/d).
No. Reducing caffeinated coffee consumption by 180 mg of caffeine (the equivalent of 2 cups) per day after 16 weeks’ gestation doesn’t affect birth weight. Consuming more than 300 mg of caffeine per day is associated with a clinically trivial, and statistically insignificant (less than 1 ounce), reduction in birth weight, compared with consuming no caffeine (strength of recommendation: B, randomized controlled trial [RCT] and large prospective cohort study).
EVIDENCE SUMMARY
A Cochrane systematic review of the effects of caffeine on pregnancy identified 2 studies, only one of which addressed the question of maternal caffeine intake and infant birth weight.1 The double-blind RCT evaluating caffeine intake during pregnancy found no significant differences in birth weight or length of gestation between women who drank regular coffee and women who drank decaffeinated coffee.2
At 16 weeks’ gestation, investigators randomized 1207 pregnant women who reported daily intake of at least 3 cups of regular coffee to drink unlabeled instant coffee (which was either regular or decaffeinated) for the rest of their pregnancy. The women were allowed to request as much of their assigned instant coffee as they wanted.
Subjects were recruited from among all women with uncomplicated, singleton pregnancies who were expected to deliver at a Danish university hospital during the study period. Investigators interviewed the women at 20, 25, and 34 weeks to determine coffee consumption (including both coffee provided by the investigators and other coffee), consumption of other caffeinated beverages, and smoking status.
The difference in caffeine intake between the groups didn’t correspond to significant differences in birth weight (16 g lighter with caffeinated coffee; 95% confidence interval [CI], −40 g to 73 g; P=.48) or birth length (0.03 cm longer with caffeinated coffee; 95% CI, −0.29 to 0.22) among infants born to the 1150 women who completed the study.
Limitations of the study include randomizing women after 16 weeks’ gestation and the observation that many women correctly guessed which type of coffee they received (35% of women drinking caffeinated coffee and 49% of women drinking decaf).
A caffeine effect, but with study limitations
The Cochrane systematic review (described above) and a meta-analysis of 9 prospective cohort studies with a total of 90,000 patients that evaluated maternal caffeine intake found that it was associated with increased low birth weight, intrauterine growth restriction (IUGR), or small for gestational age (SGA) infants.3
Researchers assessed caffeine consumption from coffee or other sources either by questionnaire (5 studies) or interview (4 studies) at various times during pregnancy, mostly in the first or second trimester, and assigned subjects to 4 intake categories: none, low (50-149 mg/d), moderate (150-349 mg/d), and high (>350 mg/d).
Compared with no caffeine, all levels of caffeine intake were associated with increased rates of low birth weight, IUGR, or SGA (low intake: relative risk [RR]=1.13; 95% CI, 1.06-1.21; moderate intake: RR=1.38; 95% CI, 1.18-1.62; high intake: RR=1.60; 95% CI, 1.24-2.08).
A major limitation of the meta-analysis was that 8 of the included studies were identified by the reviewers as having quality problems. The reviewers also identified additional cohort studies, not included in the meta-analysis, which failed to show any association between caffeine intake and poor pregnancy outcomes.
Results of best-quality study prove clinically trivial
The best-quality prospective cohort study in the review described above was also the largest, comprising two-thirds of the total patients. It found a statistically significant, but clinically trivial, association between caffeine intake and birth weight.4
Investigators from Norway’s Institute of Public Health mailed surveys to 106,707 pregnant Norwegian women and recruited 59,123 with uncomplicated singleton pregnancies. The survey assessed diet and lifestyle at several stages of pregnancy and correlated caffeine intake with birth weight, gestational length, and SGA deliveries. Investigators calculated caffeine intake from coffee and other dietary sources (tea and chocolate).
Higher caffeine intake was associated with a small reduction in birth weight (8 g/100 mg/d of additional caffeine intake; 95% CI, −10 to −6 g/100 mg/d). Higher intake was also associated with increasing likelihood of SGA birth, a finding of borderline significance (odds ratio [OR]=1.18; 95% CI, 1.00-1.38, comparing intake <50 mg/d with 51-200 mg/d; OR=1.62; 95% CI, 1.26-2.29, comparing <50 mg/d with 201-300 mg/d; and OR=1.62; 95% CI, 1.15-2.29, comparing <50 mg/d with >300 mg/d).
1. Jahanfar S, Jaafar SH. Effects of restricted caffeine intake by mother on fetal, neonatal and pregnancy outcome. Cochrane Database Syst Rev. 2013;(2):CD006965.
2. Bech BH, Obel C, Henriksen TB, et al. Effect of reducing caffeine intake on birth weight and length of gestation: randomised controlled trial. BMJ. 2007;334:409.
3. Chen LW, Wu Y, Neelakantan N, et al. Maternal caffeine intake during pregnancy is associated with risk of low birth weight: a systematic review and dose-response meta-analysis. BMC Medicine. 2014;12:174-176.
4. Sengpiel V, Elind E, Bacelis J, et al. Maternal caffeine intake during pregnancy is associated with birth weight but not with gestational length: results form a large prospective observational cohort trial. BMC Medicine. 2013;11:42.
1. Jahanfar S, Jaafar SH. Effects of restricted caffeine intake by mother on fetal, neonatal and pregnancy outcome. Cochrane Database Syst Rev. 2013;(2):CD006965.
2. Bech BH, Obel C, Henriksen TB, et al. Effect of reducing caffeine intake on birth weight and length of gestation: randomised controlled trial. BMJ. 2007;334:409.
3. Chen LW, Wu Y, Neelakantan N, et al. Maternal caffeine intake during pregnancy is associated with risk of low birth weight: a systematic review and dose-response meta-analysis. BMC Medicine. 2014;12:174-176.
4. Sengpiel V, Elind E, Bacelis J, et al. Maternal caffeine intake during pregnancy is associated with birth weight but not with gestational length: results form a large prospective observational cohort trial. BMC Medicine. 2013;11:42.
Evidence-based answers from the Family Physicians Inquiries Network
Do corticosteroids relieve Bell’s palsy?
Yes, but not severe disease. Corticosteroids likely improve facial motor function in adults with mild to moderate Bell’s palsy (strength of recommendation [SOR]: B, meta-analysis of heterogeneous randomized controlled trials [RCTs]). Corticosteroids are probably ineffective in treating cosmetically disabling or severe disease (SOR: A, meta-analysis and large RCT).
Improvement seen with corticosteroids in mild to moderate palsy
A 2010 Cochrane review of 8 RCTs (7 double-blind) compared corticosteroids with placebo in 1569 patients with Bell’s palsy, 24 months to 84 years of age.1 The definition of mild and moderate severity of symptoms differed across studies, as did corticosteroid doses. Only 6 trials required initiation of therapy within 3 days.
More patients in the corticosteroid group had completely recovered facial motor function at 6 months than patients taking placebo (77% vs 65%; 7 trials, 1507 patients; relative risk [RR]=0.71; 95% confidence interval [CI], 0.61-0.81; number needed to treat=10). Improvement in cosmetically disabling or severe disease wasn’t significant (5 trials, 668 patients; RR=0.97; 95% CI, 0.44-2.2).
Prednisolone with and without an antiviral reduces facial weakness
A 2012 prospective, randomized, double-blind, placebo-controlled, multicenter trial evaluated prednisolone (60 mg/day for 5 days, tapered for 5 days) in 829 adults, 18 to 75 years of age.2 Patients were randomized to one of 4 groups: placebo plus placebo, prednisolone plus placebo, valacyclovir plus placebo, and prednisolone plus valacyclovir. Facial function was assessed over 12 months using the Sunnybrook grading system (scored from 0 to 100; 0=complete paralysis, 100=normal function).
Compared to the groups not receiving any prednisolone, the 2 groups that received prednisolone, either with placebo or valacyclovir, had significantly less facial weakness at 12 months for both mild and moderate palsy (Sunnybrook scores <90: 184 patients; difference= −10.3%; 95% CI, −15.9 to −4.7; P<.001; Sunnybrook score <80: 134 patients; difference= −6.9%; 95% CI, −11.9 to −1.9; P=.01; Sunnybrook score <70: 98 patients; difference= −7.8%; 95% CI, −12.1 to −3.4; P<.001). Patients with severe disease (Sunnybrook score <50) didn’t show significant improvement (56 patients; difference= –2.9%; CI, −6.4 to 0.5; P=.10).
Guideline recommends corticosteroids for Bell’s palsy
The 2014 American Academy of Neurology evidence-based guideline reviewed all studies of the use of steroids in Bell’s palsy published after the original 2001 guideline.3 They found 2 high-quality RCTs, both of which are included in the 2010 Cochrane review. The 2014 guideline recommends corticosteroids for every patient who develops Bell’s palsy unless a medical contraindication exists (2 Class 1 studies [RCTs], Level A [must prescribe or offer]).
1. Salinas RA, Alvarez G, Daly F, et al. Corticosteroids for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst Rev. 2010;(3):CD001942.
2. Berg T, Bylund N, Marsk E, et al. The effect of prednisolone on sequelae in Bell’s palsy. Arch Otolaryngol Head Neck Surg. 2012;138:445-449.
3. Gronseth G, Paduga R, American Academy of Neurology. Evidence-based guideline update: steroids and antivirals for Bell’s palsy: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2012;79:2209-2213.
Yes, but not severe disease. Corticosteroids likely improve facial motor function in adults with mild to moderate Bell’s palsy (strength of recommendation [SOR]: B, meta-analysis of heterogeneous randomized controlled trials [RCTs]). Corticosteroids are probably ineffective in treating cosmetically disabling or severe disease (SOR: A, meta-analysis and large RCT).
Improvement seen with corticosteroids in mild to moderate palsy
A 2010 Cochrane review of 8 RCTs (7 double-blind) compared corticosteroids with placebo in 1569 patients with Bell’s palsy, 24 months to 84 years of age.1 The definition of mild and moderate severity of symptoms differed across studies, as did corticosteroid doses. Only 6 trials required initiation of therapy within 3 days.
More patients in the corticosteroid group had completely recovered facial motor function at 6 months than patients taking placebo (77% vs 65%; 7 trials, 1507 patients; relative risk [RR]=0.71; 95% confidence interval [CI], 0.61-0.81; number needed to treat=10). Improvement in cosmetically disabling or severe disease wasn’t significant (5 trials, 668 patients; RR=0.97; 95% CI, 0.44-2.2).
Prednisolone with and without an antiviral reduces facial weakness
A 2012 prospective, randomized, double-blind, placebo-controlled, multicenter trial evaluated prednisolone (60 mg/day for 5 days, tapered for 5 days) in 829 adults, 18 to 75 years of age.2 Patients were randomized to one of 4 groups: placebo plus placebo, prednisolone plus placebo, valacyclovir plus placebo, and prednisolone plus valacyclovir. Facial function was assessed over 12 months using the Sunnybrook grading system (scored from 0 to 100; 0=complete paralysis, 100=normal function).
Compared to the groups not receiving any prednisolone, the 2 groups that received prednisolone, either with placebo or valacyclovir, had significantly less facial weakness at 12 months for both mild and moderate palsy (Sunnybrook scores <90: 184 patients; difference= −10.3%; 95% CI, −15.9 to −4.7; P<.001; Sunnybrook score <80: 134 patients; difference= −6.9%; 95% CI, −11.9 to −1.9; P=.01; Sunnybrook score <70: 98 patients; difference= −7.8%; 95% CI, −12.1 to −3.4; P<.001). Patients with severe disease (Sunnybrook score <50) didn’t show significant improvement (56 patients; difference= –2.9%; CI, −6.4 to 0.5; P=.10).
Guideline recommends corticosteroids for Bell’s palsy
The 2014 American Academy of Neurology evidence-based guideline reviewed all studies of the use of steroids in Bell’s palsy published after the original 2001 guideline.3 They found 2 high-quality RCTs, both of which are included in the 2010 Cochrane review. The 2014 guideline recommends corticosteroids for every patient who develops Bell’s palsy unless a medical contraindication exists (2 Class 1 studies [RCTs], Level A [must prescribe or offer]).
Yes, but not severe disease. Corticosteroids likely improve facial motor function in adults with mild to moderate Bell’s palsy (strength of recommendation [SOR]: B, meta-analysis of heterogeneous randomized controlled trials [RCTs]). Corticosteroids are probably ineffective in treating cosmetically disabling or severe disease (SOR: A, meta-analysis and large RCT).
Improvement seen with corticosteroids in mild to moderate palsy
A 2010 Cochrane review of 8 RCTs (7 double-blind) compared corticosteroids with placebo in 1569 patients with Bell’s palsy, 24 months to 84 years of age.1 The definition of mild and moderate severity of symptoms differed across studies, as did corticosteroid doses. Only 6 trials required initiation of therapy within 3 days.
More patients in the corticosteroid group had completely recovered facial motor function at 6 months than patients taking placebo (77% vs 65%; 7 trials, 1507 patients; relative risk [RR]=0.71; 95% confidence interval [CI], 0.61-0.81; number needed to treat=10). Improvement in cosmetically disabling or severe disease wasn’t significant (5 trials, 668 patients; RR=0.97; 95% CI, 0.44-2.2).
Prednisolone with and without an antiviral reduces facial weakness
A 2012 prospective, randomized, double-blind, placebo-controlled, multicenter trial evaluated prednisolone (60 mg/day for 5 days, tapered for 5 days) in 829 adults, 18 to 75 years of age.2 Patients were randomized to one of 4 groups: placebo plus placebo, prednisolone plus placebo, valacyclovir plus placebo, and prednisolone plus valacyclovir. Facial function was assessed over 12 months using the Sunnybrook grading system (scored from 0 to 100; 0=complete paralysis, 100=normal function).
Compared to the groups not receiving any prednisolone, the 2 groups that received prednisolone, either with placebo or valacyclovir, had significantly less facial weakness at 12 months for both mild and moderate palsy (Sunnybrook scores <90: 184 patients; difference= −10.3%; 95% CI, −15.9 to −4.7; P<.001; Sunnybrook score <80: 134 patients; difference= −6.9%; 95% CI, −11.9 to −1.9; P=.01; Sunnybrook score <70: 98 patients; difference= −7.8%; 95% CI, −12.1 to −3.4; P<.001). Patients with severe disease (Sunnybrook score <50) didn’t show significant improvement (56 patients; difference= –2.9%; CI, −6.4 to 0.5; P=.10).
Guideline recommends corticosteroids for Bell’s palsy
The 2014 American Academy of Neurology evidence-based guideline reviewed all studies of the use of steroids in Bell’s palsy published after the original 2001 guideline.3 They found 2 high-quality RCTs, both of which are included in the 2010 Cochrane review. The 2014 guideline recommends corticosteroids for every patient who develops Bell’s palsy unless a medical contraindication exists (2 Class 1 studies [RCTs], Level A [must prescribe or offer]).
1. Salinas RA, Alvarez G, Daly F, et al. Corticosteroids for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst Rev. 2010;(3):CD001942.
2. Berg T, Bylund N, Marsk E, et al. The effect of prednisolone on sequelae in Bell’s palsy. Arch Otolaryngol Head Neck Surg. 2012;138:445-449.
3. Gronseth G, Paduga R, American Academy of Neurology. Evidence-based guideline update: steroids and antivirals for Bell’s palsy: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2012;79:2209-2213.
1. Salinas RA, Alvarez G, Daly F, et al. Corticosteroids for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst Rev. 2010;(3):CD001942.
2. Berg T, Bylund N, Marsk E, et al. The effect of prednisolone on sequelae in Bell’s palsy. Arch Otolaryngol Head Neck Surg. 2012;138:445-449.
3. Gronseth G, Paduga R, American Academy of Neurology. Evidence-based guideline update: steroids and antivirals for Bell’s palsy: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2012;79:2209-2213.
Evidence-based answers from the Family Physicians Inquiries Network
Stem cell therapy for MS: Steady progress but not ready for general use
NEW ORLEANS – Stem cell-mediated functional regeneration continues to attract interest in the treatment of multiple sclerosis (MS). The reality, however, is daunting.
“Several types of cell-based therapeutic strategies are under investigation, with different risks, benefits, and goals. Some of these strategies show promise but significant methodological questions need to be answered,” Dr. Andrew D. Goodman said at a meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
The present reality is that stem cell transplantation is not yet ready for general use to treat MS. Yet, the possible benefits of the approach demand further exploration, including clinical trials, according to Dr. Goodman, professor of neurology, chief of the neuroimmunology unit, and director of the multiple sclerosis center at the University of Rochester (N.Y.).
MS stem cell therapy hinges on the pluripotent nature of stem cells, particularly mesenchymal stem cells (MSCs) and hematopoietic stem cells. MS therapy would involve regeneration of nerve cell myelin in the brain and/or spinal cord and possibly suppression of inflammation.
Autologous HSC transplantation
Immunoablation followed by the autologous HSC transplantation (HSCT) has been explored in the ASTIMS phase II randomized trials (Neurology. Mar 10;84[10]:981-8 and HALT-MS, JAMA Neurol. 2015 Feb;72[2]:159-69), and in a Northwestern University case series (JAMA. 2015 Jan 20;313[3]:275-84).
ASTIMS compared high-dose chemotherapy followed by autologous HSCT with mitoxantrone (which is no longer used). Only 22% percent of patients had relapsing-remitting MS (RRMS; the one where HSCT generally works) and 78% had primary progressive MS (where HSCT generally does not work well or is not optimal). Yet, HSCT worked, with new T2 lesions reduced by 79%. No difference in disability progression was evident. Interim (3-year) results of HALT-MS were encouraging, with sustained remission of active RRMS and improved neurologic function. The Northwestern case series also documented improvements in neurologic disability and other clinical outcomes.
“The available data suggest that immunoablation and HSCT is highly effective in active RRMS. Patients most likely to benefit are young and still ambulatory with a relatively recent disease onset featuring highly active MS with MRI lesion activity and continued activity despite first- and second-line agents,” Dr. Goodman said.
While encouraging, the small patient numbers of the two trials and uncontrolled nature of the case series prevent conclusions concerning the therapeutic use of autologous HSCT in RRMS. Furthermore, risks of the approach include MS relapse, treatment-related adverse effects, adverse effects due to myelosuppression and immunoablation, and secondary autoimmune disorders that may arise at a later time.
Mesenchymal stem cell transplantation
MSCs offer the advantages of a variety of sources in adult tissue, established methods of culture, and either local or peripheral administration. Their finite capacity for proliferation is a drawback. Studies to date of MSC transplantation in MS have involved about 100 patients, so it is much too early to consider MSC use. Even if therapy is contemplated, whether it should be directed at quelling inflammation or to promote repair is undecided. As well, cell production and delivery issues need to be addressed, Dr. Goodman said.
Human oligodendrocyte progenitor cell transplantation
The implantation of CD 140a+ cell populations containing human oligodendrocyte progenitor cells (hOPCs) into the cerebral hemispheres of patients with non-relapsing secondary progressive MS as a means of stabilizing or improving neurological function is being planned. The NYSTEM project, with Dr. Goodman as a lead investigator, will first seek to identify the maximum tolerated dose of hOPCs.
The study is planned with the knowledge of safety issues that include cancer tumorigenesis. Yet exploration of the possible benefits will be evident only by testing in humans. “I don’t know of any way to find out except by trying,” Dr. Goodman said.
Dr. Goodman disclosed receiving research support and/or serving as a consultant to Avanir, Teva, Genzyme/Sanofi, Sun Pharma, Ono, Roche, AbbVie, Biogen, Novartis, Acorda, Purdue, and EMD Serono.
NEW ORLEANS – Stem cell-mediated functional regeneration continues to attract interest in the treatment of multiple sclerosis (MS). The reality, however, is daunting.
“Several types of cell-based therapeutic strategies are under investigation, with different risks, benefits, and goals. Some of these strategies show promise but significant methodological questions need to be answered,” Dr. Andrew D. Goodman said at a meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
The present reality is that stem cell transplantation is not yet ready for general use to treat MS. Yet, the possible benefits of the approach demand further exploration, including clinical trials, according to Dr. Goodman, professor of neurology, chief of the neuroimmunology unit, and director of the multiple sclerosis center at the University of Rochester (N.Y.).
MS stem cell therapy hinges on the pluripotent nature of stem cells, particularly mesenchymal stem cells (MSCs) and hematopoietic stem cells. MS therapy would involve regeneration of nerve cell myelin in the brain and/or spinal cord and possibly suppression of inflammation.
Autologous HSC transplantation
Immunoablation followed by the autologous HSC transplantation (HSCT) has been explored in the ASTIMS phase II randomized trials (Neurology. Mar 10;84[10]:981-8 and HALT-MS, JAMA Neurol. 2015 Feb;72[2]:159-69), and in a Northwestern University case series (JAMA. 2015 Jan 20;313[3]:275-84).
ASTIMS compared high-dose chemotherapy followed by autologous HSCT with mitoxantrone (which is no longer used). Only 22% percent of patients had relapsing-remitting MS (RRMS; the one where HSCT generally works) and 78% had primary progressive MS (where HSCT generally does not work well or is not optimal). Yet, HSCT worked, with new T2 lesions reduced by 79%. No difference in disability progression was evident. Interim (3-year) results of HALT-MS were encouraging, with sustained remission of active RRMS and improved neurologic function. The Northwestern case series also documented improvements in neurologic disability and other clinical outcomes.
“The available data suggest that immunoablation and HSCT is highly effective in active RRMS. Patients most likely to benefit are young and still ambulatory with a relatively recent disease onset featuring highly active MS with MRI lesion activity and continued activity despite first- and second-line agents,” Dr. Goodman said.
While encouraging, the small patient numbers of the two trials and uncontrolled nature of the case series prevent conclusions concerning the therapeutic use of autologous HSCT in RRMS. Furthermore, risks of the approach include MS relapse, treatment-related adverse effects, adverse effects due to myelosuppression and immunoablation, and secondary autoimmune disorders that may arise at a later time.
Mesenchymal stem cell transplantation
MSCs offer the advantages of a variety of sources in adult tissue, established methods of culture, and either local or peripheral administration. Their finite capacity for proliferation is a drawback. Studies to date of MSC transplantation in MS have involved about 100 patients, so it is much too early to consider MSC use. Even if therapy is contemplated, whether it should be directed at quelling inflammation or to promote repair is undecided. As well, cell production and delivery issues need to be addressed, Dr. Goodman said.
Human oligodendrocyte progenitor cell transplantation
The implantation of CD 140a+ cell populations containing human oligodendrocyte progenitor cells (hOPCs) into the cerebral hemispheres of patients with non-relapsing secondary progressive MS as a means of stabilizing or improving neurological function is being planned. The NYSTEM project, with Dr. Goodman as a lead investigator, will first seek to identify the maximum tolerated dose of hOPCs.
The study is planned with the knowledge of safety issues that include cancer tumorigenesis. Yet exploration of the possible benefits will be evident only by testing in humans. “I don’t know of any way to find out except by trying,” Dr. Goodman said.
Dr. Goodman disclosed receiving research support and/or serving as a consultant to Avanir, Teva, Genzyme/Sanofi, Sun Pharma, Ono, Roche, AbbVie, Biogen, Novartis, Acorda, Purdue, and EMD Serono.
NEW ORLEANS – Stem cell-mediated functional regeneration continues to attract interest in the treatment of multiple sclerosis (MS). The reality, however, is daunting.
“Several types of cell-based therapeutic strategies are under investigation, with different risks, benefits, and goals. Some of these strategies show promise but significant methodological questions need to be answered,” Dr. Andrew D. Goodman said at a meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
The present reality is that stem cell transplantation is not yet ready for general use to treat MS. Yet, the possible benefits of the approach demand further exploration, including clinical trials, according to Dr. Goodman, professor of neurology, chief of the neuroimmunology unit, and director of the multiple sclerosis center at the University of Rochester (N.Y.).
MS stem cell therapy hinges on the pluripotent nature of stem cells, particularly mesenchymal stem cells (MSCs) and hematopoietic stem cells. MS therapy would involve regeneration of nerve cell myelin in the brain and/or spinal cord and possibly suppression of inflammation.
Autologous HSC transplantation
Immunoablation followed by the autologous HSC transplantation (HSCT) has been explored in the ASTIMS phase II randomized trials (Neurology. Mar 10;84[10]:981-8 and HALT-MS, JAMA Neurol. 2015 Feb;72[2]:159-69), and in a Northwestern University case series (JAMA. 2015 Jan 20;313[3]:275-84).
ASTIMS compared high-dose chemotherapy followed by autologous HSCT with mitoxantrone (which is no longer used). Only 22% percent of patients had relapsing-remitting MS (RRMS; the one where HSCT generally works) and 78% had primary progressive MS (where HSCT generally does not work well or is not optimal). Yet, HSCT worked, with new T2 lesions reduced by 79%. No difference in disability progression was evident. Interim (3-year) results of HALT-MS were encouraging, with sustained remission of active RRMS and improved neurologic function. The Northwestern case series also documented improvements in neurologic disability and other clinical outcomes.
“The available data suggest that immunoablation and HSCT is highly effective in active RRMS. Patients most likely to benefit are young and still ambulatory with a relatively recent disease onset featuring highly active MS with MRI lesion activity and continued activity despite first- and second-line agents,” Dr. Goodman said.
While encouraging, the small patient numbers of the two trials and uncontrolled nature of the case series prevent conclusions concerning the therapeutic use of autologous HSCT in RRMS. Furthermore, risks of the approach include MS relapse, treatment-related adverse effects, adverse effects due to myelosuppression and immunoablation, and secondary autoimmune disorders that may arise at a later time.
Mesenchymal stem cell transplantation
MSCs offer the advantages of a variety of sources in adult tissue, established methods of culture, and either local or peripheral administration. Their finite capacity for proliferation is a drawback. Studies to date of MSC transplantation in MS have involved about 100 patients, so it is much too early to consider MSC use. Even if therapy is contemplated, whether it should be directed at quelling inflammation or to promote repair is undecided. As well, cell production and delivery issues need to be addressed, Dr. Goodman said.
Human oligodendrocyte progenitor cell transplantation
The implantation of CD 140a+ cell populations containing human oligodendrocyte progenitor cells (hOPCs) into the cerebral hemispheres of patients with non-relapsing secondary progressive MS as a means of stabilizing or improving neurological function is being planned. The NYSTEM project, with Dr. Goodman as a lead investigator, will first seek to identify the maximum tolerated dose of hOPCs.
The study is planned with the knowledge of safety issues that include cancer tumorigenesis. Yet exploration of the possible benefits will be evident only by testing in humans. “I don’t know of any way to find out except by trying,” Dr. Goodman said.
Dr. Goodman disclosed receiving research support and/or serving as a consultant to Avanir, Teva, Genzyme/Sanofi, Sun Pharma, Ono, Roche, AbbVie, Biogen, Novartis, Acorda, Purdue, and EMD Serono.
EXPERT ANALYSIS FROM ACTRIMS FORUM 2016
QI and Patient Safety: No Longer Just an Elective for Trainees
The demand for training in healthcare quality and patient safety, for both medical students and residents, has never been higher. The Quality and Safety Educators Academy (QSEA, sites.hospitalmedicine.org/qsea) responds to that demand by providing medical educators with the knowledge and tools to integrate quality improvement and safety concepts into their curricula.
Sponsored by the Society of Hospital Medicine (SHM) and the Alliance for Academic Internal Medicine (AAIM), QSEA 2016 is a two-and-a-half-day course designed as a faculty development program. This year, QSEA will be held at Tempe Mission Palms Hotel and Conference Center in Tempe, Ariz., from May 23 to 25.
Attendees will enjoy a hands-on, interactive learning environment with a 10-to-1 student-to-faculty ratio. Participants will develop a professional network and leave with a tool kit of educational resources and curricular tools for quality and safety education.
Think QSEA is for you? Make plans to attend now if you are:
- A program director or assistant program director interested in acquiring new curricular ideas to help meet the ACGME requirements, which require residency programs to integrate quality and safety in their curriculum
- A medical school leader or clerkship director developing quality and safety curricula for students
- A faculty member beginning a new role or expanding an existing role in quality and safety education
- A quality and safety leader who wishes to extend influence and effectiveness by learning strategies to teach and engage trainees
QSEA has sold out each of the past four years, so don’t delay. Register online at sites.hospitalmedicine.org/qsea/register.html or via phone at 800-843-3360. Questions? Email [email protected]. TH
Brett Radler is SHM’s communications coordinator.
The demand for training in healthcare quality and patient safety, for both medical students and residents, has never been higher. The Quality and Safety Educators Academy (QSEA, sites.hospitalmedicine.org/qsea) responds to that demand by providing medical educators with the knowledge and tools to integrate quality improvement and safety concepts into their curricula.
Sponsored by the Society of Hospital Medicine (SHM) and the Alliance for Academic Internal Medicine (AAIM), QSEA 2016 is a two-and-a-half-day course designed as a faculty development program. This year, QSEA will be held at Tempe Mission Palms Hotel and Conference Center in Tempe, Ariz., from May 23 to 25.
Attendees will enjoy a hands-on, interactive learning environment with a 10-to-1 student-to-faculty ratio. Participants will develop a professional network and leave with a tool kit of educational resources and curricular tools for quality and safety education.
Think QSEA is for you? Make plans to attend now if you are:
- A program director or assistant program director interested in acquiring new curricular ideas to help meet the ACGME requirements, which require residency programs to integrate quality and safety in their curriculum
- A medical school leader or clerkship director developing quality and safety curricula for students
- A faculty member beginning a new role or expanding an existing role in quality and safety education
- A quality and safety leader who wishes to extend influence and effectiveness by learning strategies to teach and engage trainees
QSEA has sold out each of the past four years, so don’t delay. Register online at sites.hospitalmedicine.org/qsea/register.html or via phone at 800-843-3360. Questions? Email [email protected]. TH
Brett Radler is SHM’s communications coordinator.
The demand for training in healthcare quality and patient safety, for both medical students and residents, has never been higher. The Quality and Safety Educators Academy (QSEA, sites.hospitalmedicine.org/qsea) responds to that demand by providing medical educators with the knowledge and tools to integrate quality improvement and safety concepts into their curricula.
Sponsored by the Society of Hospital Medicine (SHM) and the Alliance for Academic Internal Medicine (AAIM), QSEA 2016 is a two-and-a-half-day course designed as a faculty development program. This year, QSEA will be held at Tempe Mission Palms Hotel and Conference Center in Tempe, Ariz., from May 23 to 25.
Attendees will enjoy a hands-on, interactive learning environment with a 10-to-1 student-to-faculty ratio. Participants will develop a professional network and leave with a tool kit of educational resources and curricular tools for quality and safety education.
Think QSEA is for you? Make plans to attend now if you are:
- A program director or assistant program director interested in acquiring new curricular ideas to help meet the ACGME requirements, which require residency programs to integrate quality and safety in their curriculum
- A medical school leader or clerkship director developing quality and safety curricula for students
- A faculty member beginning a new role or expanding an existing role in quality and safety education
- A quality and safety leader who wishes to extend influence and effectiveness by learning strategies to teach and engage trainees
QSEA has sold out each of the past four years, so don’t delay. Register online at sites.hospitalmedicine.org/qsea/register.html or via phone at 800-843-3360. Questions? Email [email protected]. TH
Brett Radler is SHM’s communications coordinator.
U.S. flu activity continues steady climb
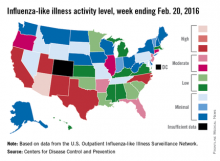
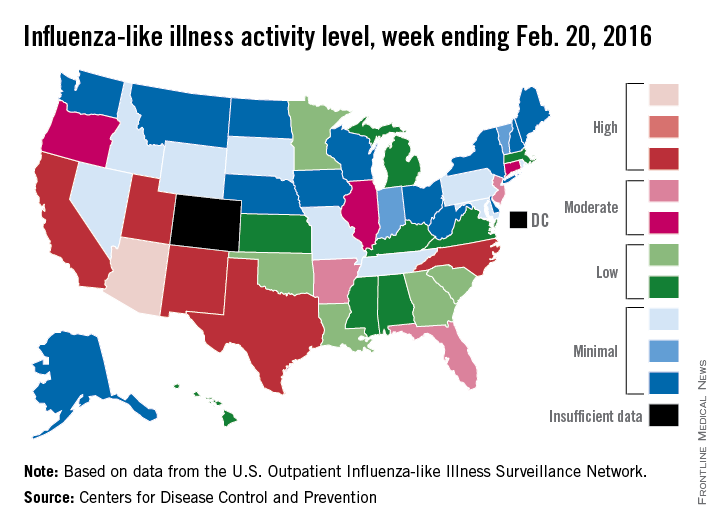
Influenza-like illness (ILI) activity reached a new national high for the 2015-2016 flu season during the week ending Feb. 20, according to the Centers for Disease Control and Prevention.
Nationwide, the proportion of outpatient visits for ILI was 3.2%, up from 3.1% the week before and well over the national baseline of 2.1%. Arizona and Puerto Rico were still at level 10 on the CDC’s 1-10 scale for ILI activity, and they were joined in the “high” range of activity by California, New Mexico, North Carolina, Texas, and Utah, which were all at level 8, the CDC reported Feb. 26.
States in the “moderate” range of activity for week 19 of the 2015-2016 flu season (week 7 of calendar year 2016) were Arkansas, Florida, and New Jersey at level 7 and Connecticut, Illinois, and Oregon at level 6. There were 13 states in the “low” range of activity – five at level 5 and eight states at level 4 – and 24 states in the “minimal” range, of which 13 were at level 1. Colorado and the District of Columbia had insufficient data to determine activity level, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
For the week, one flu-related pediatric death, associated with an influenza B virus, was reported to the CDC, bringing the total to 14 for the season. The only states reporting more that one death are California (two) and Florida (three), the CDC said. The average number of deaths for the three previous flu seasons is over 143.
During week 19, a total of 18,844 respiratory specimens were tested, 13.8% of which were positive: 76.1% for influenza A and 23.9% for influenza B. Since Oct. 1, 2015, 4.2% of specimens have tested positive for influenza, with a 70% to 30% split between influenza A and B, the CDC report showed.
Influenza-like illness (ILI) activity reached a new national high for the 2015-2016 flu season during the week ending Feb. 20, according to the Centers for Disease Control and Prevention.
Nationwide, the proportion of outpatient visits for ILI was 3.2%, up from 3.1% the week before and well over the national baseline of 2.1%. Arizona and Puerto Rico were still at level 10 on the CDC’s 1-10 scale for ILI activity, and they were joined in the “high” range of activity by California, New Mexico, North Carolina, Texas, and Utah, which were all at level 8, the CDC reported Feb. 26.
States in the “moderate” range of activity for week 19 of the 2015-2016 flu season (week 7 of calendar year 2016) were Arkansas, Florida, and New Jersey at level 7 and Connecticut, Illinois, and Oregon at level 6. There were 13 states in the “low” range of activity – five at level 5 and eight states at level 4 – and 24 states in the “minimal” range, of which 13 were at level 1. Colorado and the District of Columbia had insufficient data to determine activity level, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
For the week, one flu-related pediatric death, associated with an influenza B virus, was reported to the CDC, bringing the total to 14 for the season. The only states reporting more that one death are California (two) and Florida (three), the CDC said. The average number of deaths for the three previous flu seasons is over 143.
During week 19, a total of 18,844 respiratory specimens were tested, 13.8% of which were positive: 76.1% for influenza A and 23.9% for influenza B. Since Oct. 1, 2015, 4.2% of specimens have tested positive for influenza, with a 70% to 30% split between influenza A and B, the CDC report showed.
Influenza-like illness (ILI) activity reached a new national high for the 2015-2016 flu season during the week ending Feb. 20, according to the Centers for Disease Control and Prevention.
Nationwide, the proportion of outpatient visits for ILI was 3.2%, up from 3.1% the week before and well over the national baseline of 2.1%. Arizona and Puerto Rico were still at level 10 on the CDC’s 1-10 scale for ILI activity, and they were joined in the “high” range of activity by California, New Mexico, North Carolina, Texas, and Utah, which were all at level 8, the CDC reported Feb. 26.
States in the “moderate” range of activity for week 19 of the 2015-2016 flu season (week 7 of calendar year 2016) were Arkansas, Florida, and New Jersey at level 7 and Connecticut, Illinois, and Oregon at level 6. There were 13 states in the “low” range of activity – five at level 5 and eight states at level 4 – and 24 states in the “minimal” range, of which 13 were at level 1. Colorado and the District of Columbia had insufficient data to determine activity level, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
For the week, one flu-related pediatric death, associated with an influenza B virus, was reported to the CDC, bringing the total to 14 for the season. The only states reporting more that one death are California (two) and Florida (three), the CDC said. The average number of deaths for the three previous flu seasons is over 143.
During week 19, a total of 18,844 respiratory specimens were tested, 13.8% of which were positive: 76.1% for influenza A and 23.9% for influenza B. Since Oct. 1, 2015, 4.2% of specimens have tested positive for influenza, with a 70% to 30% split between influenza A and B, the CDC report showed.
FDA approves obinutuzumab for FL

The US Food and Drug Administration (FDA) has approved obinutuzumab (Gazyva) for certain patients with previously treated follicular lymphoma (FL).
Obinutuzumab is a glycoengineered, humanized, monoclonal antibody that selectively binds to the extracellular domain of the CD20 antigen on B cells.
The drug was previously approved by the FDA for use in combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia.
Now, obinutuzumab is approved for use in combination with bendamustine, followed by obinutuzumab alone, to treat patients with FL who did not respond to a rituximab-containing regimen or whose FL returned after such treatment.
The recommended dose and schedule for the regimen is:
- Obinutuzumab at 1000 mg by intravenous infusion on days 1, 8, and 15 of cycle 1; on day 1 of cycles 2-6 (28-day cycles); then every 2 months for 2 years.
- Bendamustine at 90 mg/m2 by intravenous infusion on days 1 and 2 of cycles 1-6.
Full prescribing information for obinutuzumab is available on the FDA website or at www.Gazyva.com.
Phase 3 study
The approval for obinutuzumab in FL is based on results from the phase 3 GADOLIN study. The trial included 413 patients with rituximab-refractory non-Hodgkin lymphoma, including 321 patients with FL, 46 with marginal zone lymphoma, and 28 with small lymphocytic lymphoma.
The patients were randomized to receive bendamustine alone (control arm) or a combination of bendamustine and obinutuzumab followed by obinutuzumab maintenance (every 2 months for 2 years or until progression).
The primary endpoint of the study was progression-free survival (PFS), as assessed by an independent review committee (IRC). The secondary endpoints were PFS as assessed by investigator review, best overall response, complete response (CR), partial response (PR), duration of response, overall survival, and safety profile.
Among patients with FL, the obinutuzumab regimen improved PFS compared to bendamustine alone, as assessed by IRC (hazard ratio [HR]=0.48, P<0.0001). The median PFS was not reached in patients receiving the obinutuzumab regimen but was 13.8 months in those receiving bendamustine alone.
Investigator-assessed PFS was consistent with IRC-assessed PFS. Investigators said the median PFS with the obinutuzumab regimen was more than double that with bendamustine alone—29.2 months vs 13.7 months (HR=0.48, P<0.0001).
Best overall response for patients receiving the obinutuzumab regimen was 78.7% (15.5% CR, 63.2% PR), compared to 74.7% for those receiving bendamustine alone (18.7% CR, 56% PR), as assessed by the IRC.
The median duration of response was not reached for patients receiving the obinutuzumab regimen and was 11.6 months for those receiving bendamustine alone.
The median overall survival has not yet been reached in either study arm.
The most common grade 3/4 adverse events observed in patients receiving the obinutuzumab regimen were neutropenia (33%), infusion reactions (11%), and thrombocytopenia (10%).
The most common adverse events of any grade were infusion reactions (69%), neutropenia (35%), nausea (54%), fatigue (39%), cough (26%), diarrhea (27%), constipation (19%), fever (18%), thrombocytopenia (15%), vomiting (22%), upper respiratory tract infection (13%), decreased appetite (18%), joint or muscle pain (12%), sinusitis (12%), anemia (12%), general weakness (11%), and urinary tract infection (10%).
About obinutuzumab
Obinutuzumab is being studied in a large clinical program, including the phase 3 GOYA and GALLIUM studies.
In GOYA, researchers are comparing obinutuzumab head-to-head with rituximab plus CHOP chemotherapy in first-line diffuse large B-cell lymphoma. In GALLIUM, researchers are comparing obinutuzumab plus chemotherapy head-to-head with rituximab plus chemotherapy in first-line indolent non-Hodgkin lymphoma.
Additional combination studies investigating obinutuzumab with other approved or investigational medicines, including cancer immunotherapies and small-molecule inhibitors, are planned or underway across a range of blood cancers.
Obinutuzumab was discovered by Roche Glycart AG, a wholly owned, independent research unit of Roche. In the US, obinutuzumab is part of a collaboration between Genentech and Biogen.
Genentech has a patient assistance program, Genentech Access Solutions, that can help qualifying patients access obinutuzumab and other Genentech medications.
The program is designed to help people navigate the access and reimbursement process and provide assistance to eligible patients in the US who are uninsured or cannot afford the out-of-pocket costs for their medicine. For more information, visit www.Genentech-Access.com. ![]()

The US Food and Drug Administration (FDA) has approved obinutuzumab (Gazyva) for certain patients with previously treated follicular lymphoma (FL).
Obinutuzumab is a glycoengineered, humanized, monoclonal antibody that selectively binds to the extracellular domain of the CD20 antigen on B cells.
The drug was previously approved by the FDA for use in combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia.
Now, obinutuzumab is approved for use in combination with bendamustine, followed by obinutuzumab alone, to treat patients with FL who did not respond to a rituximab-containing regimen or whose FL returned after such treatment.
The recommended dose and schedule for the regimen is:
- Obinutuzumab at 1000 mg by intravenous infusion on days 1, 8, and 15 of cycle 1; on day 1 of cycles 2-6 (28-day cycles); then every 2 months for 2 years.
- Bendamustine at 90 mg/m2 by intravenous infusion on days 1 and 2 of cycles 1-6.
Full prescribing information for obinutuzumab is available on the FDA website or at www.Gazyva.com.
Phase 3 study
The approval for obinutuzumab in FL is based on results from the phase 3 GADOLIN study. The trial included 413 patients with rituximab-refractory non-Hodgkin lymphoma, including 321 patients with FL, 46 with marginal zone lymphoma, and 28 with small lymphocytic lymphoma.
The patients were randomized to receive bendamustine alone (control arm) or a combination of bendamustine and obinutuzumab followed by obinutuzumab maintenance (every 2 months for 2 years or until progression).
The primary endpoint of the study was progression-free survival (PFS), as assessed by an independent review committee (IRC). The secondary endpoints were PFS as assessed by investigator review, best overall response, complete response (CR), partial response (PR), duration of response, overall survival, and safety profile.
Among patients with FL, the obinutuzumab regimen improved PFS compared to bendamustine alone, as assessed by IRC (hazard ratio [HR]=0.48, P<0.0001). The median PFS was not reached in patients receiving the obinutuzumab regimen but was 13.8 months in those receiving bendamustine alone.
Investigator-assessed PFS was consistent with IRC-assessed PFS. Investigators said the median PFS with the obinutuzumab regimen was more than double that with bendamustine alone—29.2 months vs 13.7 months (HR=0.48, P<0.0001).
Best overall response for patients receiving the obinutuzumab regimen was 78.7% (15.5% CR, 63.2% PR), compared to 74.7% for those receiving bendamustine alone (18.7% CR, 56% PR), as assessed by the IRC.
The median duration of response was not reached for patients receiving the obinutuzumab regimen and was 11.6 months for those receiving bendamustine alone.
The median overall survival has not yet been reached in either study arm.
The most common grade 3/4 adverse events observed in patients receiving the obinutuzumab regimen were neutropenia (33%), infusion reactions (11%), and thrombocytopenia (10%).
The most common adverse events of any grade were infusion reactions (69%), neutropenia (35%), nausea (54%), fatigue (39%), cough (26%), diarrhea (27%), constipation (19%), fever (18%), thrombocytopenia (15%), vomiting (22%), upper respiratory tract infection (13%), decreased appetite (18%), joint or muscle pain (12%), sinusitis (12%), anemia (12%), general weakness (11%), and urinary tract infection (10%).
About obinutuzumab
Obinutuzumab is being studied in a large clinical program, including the phase 3 GOYA and GALLIUM studies.
In GOYA, researchers are comparing obinutuzumab head-to-head with rituximab plus CHOP chemotherapy in first-line diffuse large B-cell lymphoma. In GALLIUM, researchers are comparing obinutuzumab plus chemotherapy head-to-head with rituximab plus chemotherapy in first-line indolent non-Hodgkin lymphoma.
Additional combination studies investigating obinutuzumab with other approved or investigational medicines, including cancer immunotherapies and small-molecule inhibitors, are planned or underway across a range of blood cancers.
Obinutuzumab was discovered by Roche Glycart AG, a wholly owned, independent research unit of Roche. In the US, obinutuzumab is part of a collaboration between Genentech and Biogen.
Genentech has a patient assistance program, Genentech Access Solutions, that can help qualifying patients access obinutuzumab and other Genentech medications.
The program is designed to help people navigate the access and reimbursement process and provide assistance to eligible patients in the US who are uninsured or cannot afford the out-of-pocket costs for their medicine. For more information, visit www.Genentech-Access.com. ![]()

The US Food and Drug Administration (FDA) has approved obinutuzumab (Gazyva) for certain patients with previously treated follicular lymphoma (FL).
Obinutuzumab is a glycoengineered, humanized, monoclonal antibody that selectively binds to the extracellular domain of the CD20 antigen on B cells.
The drug was previously approved by the FDA for use in combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia.
Now, obinutuzumab is approved for use in combination with bendamustine, followed by obinutuzumab alone, to treat patients with FL who did not respond to a rituximab-containing regimen or whose FL returned after such treatment.
The recommended dose and schedule for the regimen is:
- Obinutuzumab at 1000 mg by intravenous infusion on days 1, 8, and 15 of cycle 1; on day 1 of cycles 2-6 (28-day cycles); then every 2 months for 2 years.
- Bendamustine at 90 mg/m2 by intravenous infusion on days 1 and 2 of cycles 1-6.
Full prescribing information for obinutuzumab is available on the FDA website or at www.Gazyva.com.
Phase 3 study
The approval for obinutuzumab in FL is based on results from the phase 3 GADOLIN study. The trial included 413 patients with rituximab-refractory non-Hodgkin lymphoma, including 321 patients with FL, 46 with marginal zone lymphoma, and 28 with small lymphocytic lymphoma.
The patients were randomized to receive bendamustine alone (control arm) or a combination of bendamustine and obinutuzumab followed by obinutuzumab maintenance (every 2 months for 2 years or until progression).
The primary endpoint of the study was progression-free survival (PFS), as assessed by an independent review committee (IRC). The secondary endpoints were PFS as assessed by investigator review, best overall response, complete response (CR), partial response (PR), duration of response, overall survival, and safety profile.
Among patients with FL, the obinutuzumab regimen improved PFS compared to bendamustine alone, as assessed by IRC (hazard ratio [HR]=0.48, P<0.0001). The median PFS was not reached in patients receiving the obinutuzumab regimen but was 13.8 months in those receiving bendamustine alone.
Investigator-assessed PFS was consistent with IRC-assessed PFS. Investigators said the median PFS with the obinutuzumab regimen was more than double that with bendamustine alone—29.2 months vs 13.7 months (HR=0.48, P<0.0001).
Best overall response for patients receiving the obinutuzumab regimen was 78.7% (15.5% CR, 63.2% PR), compared to 74.7% for those receiving bendamustine alone (18.7% CR, 56% PR), as assessed by the IRC.
The median duration of response was not reached for patients receiving the obinutuzumab regimen and was 11.6 months for those receiving bendamustine alone.
The median overall survival has not yet been reached in either study arm.
The most common grade 3/4 adverse events observed in patients receiving the obinutuzumab regimen were neutropenia (33%), infusion reactions (11%), and thrombocytopenia (10%).
The most common adverse events of any grade were infusion reactions (69%), neutropenia (35%), nausea (54%), fatigue (39%), cough (26%), diarrhea (27%), constipation (19%), fever (18%), thrombocytopenia (15%), vomiting (22%), upper respiratory tract infection (13%), decreased appetite (18%), joint or muscle pain (12%), sinusitis (12%), anemia (12%), general weakness (11%), and urinary tract infection (10%).
About obinutuzumab
Obinutuzumab is being studied in a large clinical program, including the phase 3 GOYA and GALLIUM studies.
In GOYA, researchers are comparing obinutuzumab head-to-head with rituximab plus CHOP chemotherapy in first-line diffuse large B-cell lymphoma. In GALLIUM, researchers are comparing obinutuzumab plus chemotherapy head-to-head with rituximab plus chemotherapy in first-line indolent non-Hodgkin lymphoma.
Additional combination studies investigating obinutuzumab with other approved or investigational medicines, including cancer immunotherapies and small-molecule inhibitors, are planned or underway across a range of blood cancers.
Obinutuzumab was discovered by Roche Glycart AG, a wholly owned, independent research unit of Roche. In the US, obinutuzumab is part of a collaboration between Genentech and Biogen.
Genentech has a patient assistance program, Genentech Access Solutions, that can help qualifying patients access obinutuzumab and other Genentech medications.
The program is designed to help people navigate the access and reimbursement process and provide assistance to eligible patients in the US who are uninsured or cannot afford the out-of-pocket costs for their medicine. For more information, visit www.Genentech-Access.com. ![]()
Brain atrophy may be a clinically relevant measure in PPMS: Data from INFORMS
NEW ORLEANS – The recently published results of the INFORMS multicenter, double-blind, placebo-controlled parallel-group study (NCT00731692) that compared the efficacy of fingolimod in slowing disease progression in primary progressive multiple sclerosis (PPMS) with placebo proved disappointing. However, further scrutiny of the data has provided valuable insights, such as supportive evidence for brain atrophy as a clinically relevant measure in PPMS patients.
“The degree of clinical worsening was directly associated with patient’s extent of brain volume loss. Patients in the extreme category of disability progression had more brain volume loss than patients with only one progression or those who remained clinically stable,” wrote Dr. Jerry Wolinsky of the University of Texas Health Science Center at Houston and his colleagues in a poster presented Feb. 19 at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
In INFORMS, 970 patients with PPMS were randomly allocated (1:1) to receive oral fingolimod 0.5 mg or placebo for at least 36 months and for up to 5 years. The anti-inflammatory effects of fingolimod did not slow disease progression in PPMS.
While INFORMS did not pan out in terms of the primary endpoint, the long duration of the study, use of various progression measures, and rigorous patient selection offered the unique opportunity to assess the associations of magnetic resonance imaging of the brain over at least 3 years of the clinical progression occurring in patients with PPMS.
All patients had being clinically diagnosed with PPMS, with disease duration of 2-10 years and objective evidence of progression in disability in the prior 2 years. The composite endpoint of INFORMS based on the change in Expanded Disability Status Scale (EDSS), 25-Foot Timed Walk Test, or 9-Hole Peg Test was used to gauge 3-month confirmed disease progression (3CDP). The patients were classified according to EDSS-determined disease progression as extreme (more than one occurrence of 3CDP; n = 162), moderate (one occurrence of 3CDP; n = 309), and stable (no 3CDP; n = 499).
Mean age, % male, and baseline EDSS scores were similar across the three categories (whole population: 48.4 ± 8.4 years; 51.6%; and 4.7 ± 1.0, respectively). Mean number of gadolinium-positive lesions in the extreme and stable category was 0.39 ± 1.1 and 0.25 ± 1.0, respectively. Baseline T2 lesion volume was 10,160.8 ± 12,743.3 mm3 in extreme patients and 9,585.8 ± 12,421.6 mm3 in stable patients.
Patients in the extreme category displayed greater changes in the three endpoint measures than did the moderate and stable categories. At month 36, the mean change in brain volume, compared with baseline, was –1.76 ± 1.4 in the extreme group and –1.26 ± 0.9 in stable group. Corresponding values for mean number of gadolinium-positive lesions were 0.40 ± 1.4 and 0.10 ± 0.5. Corresponding numbers of new/newly enlarging T2 lesions from baseline to month 36 were 1.7 ± 4.6 and 0.9 ± 2.9.
Patients in the extreme category exceeded the recently proposed cut-off for pathologically increased brain volume loss by about 60%, compared with only about 14% for patients in the stable category.
The higher change in brain volume from baseline in patients who progressed to extreme disability “supports brain atrophy as a clinically relevant measure of neuroprotection in PPMS trials,” wrote Dr. Wolinsky and his colleagues.
The study was funded by Novartis Pharma AG. Dr. Wolinsky disclosed consulting fees from Genzyme/Sanofi, Hoffmann-La Roche/Genentech, Forward Pharma, Alkermes, AbbVie, Novartis Pharmaceuticals, Teva, and XenoPort and advisory board participation for Hoffman-La Roche/Genentech, Forward Pharma, EMD Serono, Actelion, Novartis Pharmaceuticals, Teva, and Xenoport. He performed contract research for Genzyme/Sanofi.
NEW ORLEANS – The recently published results of the INFORMS multicenter, double-blind, placebo-controlled parallel-group study (NCT00731692) that compared the efficacy of fingolimod in slowing disease progression in primary progressive multiple sclerosis (PPMS) with placebo proved disappointing. However, further scrutiny of the data has provided valuable insights, such as supportive evidence for brain atrophy as a clinically relevant measure in PPMS patients.
“The degree of clinical worsening was directly associated with patient’s extent of brain volume loss. Patients in the extreme category of disability progression had more brain volume loss than patients with only one progression or those who remained clinically stable,” wrote Dr. Jerry Wolinsky of the University of Texas Health Science Center at Houston and his colleagues in a poster presented Feb. 19 at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
In INFORMS, 970 patients with PPMS were randomly allocated (1:1) to receive oral fingolimod 0.5 mg or placebo for at least 36 months and for up to 5 years. The anti-inflammatory effects of fingolimod did not slow disease progression in PPMS.
While INFORMS did not pan out in terms of the primary endpoint, the long duration of the study, use of various progression measures, and rigorous patient selection offered the unique opportunity to assess the associations of magnetic resonance imaging of the brain over at least 3 years of the clinical progression occurring in patients with PPMS.
All patients had being clinically diagnosed with PPMS, with disease duration of 2-10 years and objective evidence of progression in disability in the prior 2 years. The composite endpoint of INFORMS based on the change in Expanded Disability Status Scale (EDSS), 25-Foot Timed Walk Test, or 9-Hole Peg Test was used to gauge 3-month confirmed disease progression (3CDP). The patients were classified according to EDSS-determined disease progression as extreme (more than one occurrence of 3CDP; n = 162), moderate (one occurrence of 3CDP; n = 309), and stable (no 3CDP; n = 499).
Mean age, % male, and baseline EDSS scores were similar across the three categories (whole population: 48.4 ± 8.4 years; 51.6%; and 4.7 ± 1.0, respectively). Mean number of gadolinium-positive lesions in the extreme and stable category was 0.39 ± 1.1 and 0.25 ± 1.0, respectively. Baseline T2 lesion volume was 10,160.8 ± 12,743.3 mm3 in extreme patients and 9,585.8 ± 12,421.6 mm3 in stable patients.
Patients in the extreme category displayed greater changes in the three endpoint measures than did the moderate and stable categories. At month 36, the mean change in brain volume, compared with baseline, was –1.76 ± 1.4 in the extreme group and –1.26 ± 0.9 in stable group. Corresponding values for mean number of gadolinium-positive lesions were 0.40 ± 1.4 and 0.10 ± 0.5. Corresponding numbers of new/newly enlarging T2 lesions from baseline to month 36 were 1.7 ± 4.6 and 0.9 ± 2.9.
Patients in the extreme category exceeded the recently proposed cut-off for pathologically increased brain volume loss by about 60%, compared with only about 14% for patients in the stable category.
The higher change in brain volume from baseline in patients who progressed to extreme disability “supports brain atrophy as a clinically relevant measure of neuroprotection in PPMS trials,” wrote Dr. Wolinsky and his colleagues.
The study was funded by Novartis Pharma AG. Dr. Wolinsky disclosed consulting fees from Genzyme/Sanofi, Hoffmann-La Roche/Genentech, Forward Pharma, Alkermes, AbbVie, Novartis Pharmaceuticals, Teva, and XenoPort and advisory board participation for Hoffman-La Roche/Genentech, Forward Pharma, EMD Serono, Actelion, Novartis Pharmaceuticals, Teva, and Xenoport. He performed contract research for Genzyme/Sanofi.
NEW ORLEANS – The recently published results of the INFORMS multicenter, double-blind, placebo-controlled parallel-group study (NCT00731692) that compared the efficacy of fingolimod in slowing disease progression in primary progressive multiple sclerosis (PPMS) with placebo proved disappointing. However, further scrutiny of the data has provided valuable insights, such as supportive evidence for brain atrophy as a clinically relevant measure in PPMS patients.
“The degree of clinical worsening was directly associated with patient’s extent of brain volume loss. Patients in the extreme category of disability progression had more brain volume loss than patients with only one progression or those who remained clinically stable,” wrote Dr. Jerry Wolinsky of the University of Texas Health Science Center at Houston and his colleagues in a poster presented Feb. 19 at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
In INFORMS, 970 patients with PPMS were randomly allocated (1:1) to receive oral fingolimod 0.5 mg or placebo for at least 36 months and for up to 5 years. The anti-inflammatory effects of fingolimod did not slow disease progression in PPMS.
While INFORMS did not pan out in terms of the primary endpoint, the long duration of the study, use of various progression measures, and rigorous patient selection offered the unique opportunity to assess the associations of magnetic resonance imaging of the brain over at least 3 years of the clinical progression occurring in patients with PPMS.
All patients had being clinically diagnosed with PPMS, with disease duration of 2-10 years and objective evidence of progression in disability in the prior 2 years. The composite endpoint of INFORMS based on the change in Expanded Disability Status Scale (EDSS), 25-Foot Timed Walk Test, or 9-Hole Peg Test was used to gauge 3-month confirmed disease progression (3CDP). The patients were classified according to EDSS-determined disease progression as extreme (more than one occurrence of 3CDP; n = 162), moderate (one occurrence of 3CDP; n = 309), and stable (no 3CDP; n = 499).
Mean age, % male, and baseline EDSS scores were similar across the three categories (whole population: 48.4 ± 8.4 years; 51.6%; and 4.7 ± 1.0, respectively). Mean number of gadolinium-positive lesions in the extreme and stable category was 0.39 ± 1.1 and 0.25 ± 1.0, respectively. Baseline T2 lesion volume was 10,160.8 ± 12,743.3 mm3 in extreme patients and 9,585.8 ± 12,421.6 mm3 in stable patients.
Patients in the extreme category displayed greater changes in the three endpoint measures than did the moderate and stable categories. At month 36, the mean change in brain volume, compared with baseline, was –1.76 ± 1.4 in the extreme group and –1.26 ± 0.9 in stable group. Corresponding values for mean number of gadolinium-positive lesions were 0.40 ± 1.4 and 0.10 ± 0.5. Corresponding numbers of new/newly enlarging T2 lesions from baseline to month 36 were 1.7 ± 4.6 and 0.9 ± 2.9.
Patients in the extreme category exceeded the recently proposed cut-off for pathologically increased brain volume loss by about 60%, compared with only about 14% for patients in the stable category.
The higher change in brain volume from baseline in patients who progressed to extreme disability “supports brain atrophy as a clinically relevant measure of neuroprotection in PPMS trials,” wrote Dr. Wolinsky and his colleagues.
The study was funded by Novartis Pharma AG. Dr. Wolinsky disclosed consulting fees from Genzyme/Sanofi, Hoffmann-La Roche/Genentech, Forward Pharma, Alkermes, AbbVie, Novartis Pharmaceuticals, Teva, and XenoPort and advisory board participation for Hoffman-La Roche/Genentech, Forward Pharma, EMD Serono, Actelion, Novartis Pharmaceuticals, Teva, and Xenoport. He performed contract research for Genzyme/Sanofi.
Key clinical point: Brain atrophy could be a clinically useful and relevant measure in primary progressing multiple sclerosis.
Major finding: Progression to extreme disability was associated with greater brain volume loss.
Data source: Data from the multinational, double-blind, placebo-controlled, parallel-group INFORMS trial.
Disclosures: The study was funded by Novartis Pharma AG. Dr. Wolinsky disclosed consulting fees from Genzyme/Sanofi, Hoffmann-La Roche/Genentech, Forward Pharma, Alkermes, AbbVie, Novartis Pharmaceuticals, Teva, and XenoPort, and advisory board participation for Hoffman-La Roche/Genentech, Forward Pharma, EMD Serono, Actelion, Novartis Pharmaceuticals, Teva, and Xenoport. He performed contract research for Genzyme/Sanofi.
Triage MS-related ED visits to reduce unnecessary treatment
NEW ORLEANS – The majority of multiple sclerosis–related emergency department visits in a recent chart review were related to pseudoflares or MS-related complications, rather than to true MS relapse.
The findings suggest that many diagnostic tests, treatments, and hospital admissions are unnecessary, Dr. Hesham Abboud of the Cleveland Clinic and his colleagues reported in a poster at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Of 97 MS-related visits among 75 patients, 33 were for new neurologic symptoms, 29 were for worsening of preexisting symptoms, and 36 were for MS-related complications. New relapse was diagnosed in only 27 visits (27.8%), and urinary tract infections were found in about one-third of patients presenting with either urinary or neurologic symptoms, the investigators said.
New MRIs were ordered in 37 patients (38.1%); 89 emergency department visits (91.7%) resulted in hospital admissions and 40.4% were related to neurology; and steroid treatment was used in 24 visits (24.7%), 7 of which were for worsening of preexisting symptoms, the investigators said.
Of the visits involving new neurologic symptoms, one-third were not from relapse; 59% of the MRIs done in those situations were positive for enhancing or new lesions. Of the visits with worsening preexisting symptoms, only 16.6% were associated with a new relapse, and 28.5% of MRIs done in those situations were positive, the investigators said.
Although many ED visits among MS patients are driven by neurologic complaints, true relapse is rarely present, and not all those with true relapse require hospital admission and steroid treatment, they noted, concluding that developing a care path and triaging system for MS patients in the ED could prevent unnecessary MRIs, steroid treatment, and hospital admissions.
The investigators reported having no disclosures.
NEW ORLEANS – The majority of multiple sclerosis–related emergency department visits in a recent chart review were related to pseudoflares or MS-related complications, rather than to true MS relapse.
The findings suggest that many diagnostic tests, treatments, and hospital admissions are unnecessary, Dr. Hesham Abboud of the Cleveland Clinic and his colleagues reported in a poster at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Of 97 MS-related visits among 75 patients, 33 were for new neurologic symptoms, 29 were for worsening of preexisting symptoms, and 36 were for MS-related complications. New relapse was diagnosed in only 27 visits (27.8%), and urinary tract infections were found in about one-third of patients presenting with either urinary or neurologic symptoms, the investigators said.
New MRIs were ordered in 37 patients (38.1%); 89 emergency department visits (91.7%) resulted in hospital admissions and 40.4% were related to neurology; and steroid treatment was used in 24 visits (24.7%), 7 of which were for worsening of preexisting symptoms, the investigators said.
Of the visits involving new neurologic symptoms, one-third were not from relapse; 59% of the MRIs done in those situations were positive for enhancing or new lesions. Of the visits with worsening preexisting symptoms, only 16.6% were associated with a new relapse, and 28.5% of MRIs done in those situations were positive, the investigators said.
Although many ED visits among MS patients are driven by neurologic complaints, true relapse is rarely present, and not all those with true relapse require hospital admission and steroid treatment, they noted, concluding that developing a care path and triaging system for MS patients in the ED could prevent unnecessary MRIs, steroid treatment, and hospital admissions.
The investigators reported having no disclosures.
NEW ORLEANS – The majority of multiple sclerosis–related emergency department visits in a recent chart review were related to pseudoflares or MS-related complications, rather than to true MS relapse.
The findings suggest that many diagnostic tests, treatments, and hospital admissions are unnecessary, Dr. Hesham Abboud of the Cleveland Clinic and his colleagues reported in a poster at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Of 97 MS-related visits among 75 patients, 33 were for new neurologic symptoms, 29 were for worsening of preexisting symptoms, and 36 were for MS-related complications. New relapse was diagnosed in only 27 visits (27.8%), and urinary tract infections were found in about one-third of patients presenting with either urinary or neurologic symptoms, the investigators said.
New MRIs were ordered in 37 patients (38.1%); 89 emergency department visits (91.7%) resulted in hospital admissions and 40.4% were related to neurology; and steroid treatment was used in 24 visits (24.7%), 7 of which were for worsening of preexisting symptoms, the investigators said.
Of the visits involving new neurologic symptoms, one-third were not from relapse; 59% of the MRIs done in those situations were positive for enhancing or new lesions. Of the visits with worsening preexisting symptoms, only 16.6% were associated with a new relapse, and 28.5% of MRIs done in those situations were positive, the investigators said.
Although many ED visits among MS patients are driven by neurologic complaints, true relapse is rarely present, and not all those with true relapse require hospital admission and steroid treatment, they noted, concluding that developing a care path and triaging system for MS patients in the ED could prevent unnecessary MRIs, steroid treatment, and hospital admissions.
The investigators reported having no disclosures.
AT ACTRIMS FORUM 2016
Key clinical point: The majority of multiple sclerosis–related emergency department visits in a recent chart review were related to pseudoflares or MS-related complications, rather than true MS relapse.
Major finding: New relapse was diagnosed in only 27 visits (27.8%).
Data source: A retrospective chart review of 75 patients with 97 MS-related ED visits.
Disclosures: The investigators reported having no disclosures.
For Men, Exercise-related Bone Loading During Adolescence Reaps Benefits Later in Life
Men who continuously participate in high-impact activities, such as jogging and tennis, during adolescence and young adulthood have greater hip and lumbar spine bone mineral density than those who do not take part in such activities, according to a study published in American Journal of Men’s Health.
In a cross-sectional study, researchers analyzed the physical histories of 203 healthy, physically active males ages 30 to 65. Participants’ sports and exercise histories varied in the type and level of activity and the length of time spent doing various physical activities.
Exercise-associated bone loading scores were calculated based on the biomechanical ground-reaction forces of the patients’ past and current physical activities. Current bone mineral density (BMD) was measured using dual-energy x-ray absorptiometry. In addition, participants were grouped based on current participation in a high-impact activity, resistance training, both, or neither.
Bone loading during adolescence and young adulthood were significant, positive predictors of BMD of the whole body, total hip, and lumbar spine, adjusting for lean body mass and/or age Individuals who currently participate in a high-impact activity had greater lumbar spine BMD than nonparticipants. Men who continuously participated in a high-impact activity had greater hip and lumbar spine BMD than those who did not.
Suggested Reading
Matthew A. Strope, Peggy Nigh, Melissa I. Carter, et al. Physical activity–associated bone loading during adolescence and young adulthood is positively associated with adult bone mineral density in men. Am J Mens Health. 2015 November. [Epub ahead of print].
Men who continuously participate in high-impact activities, such as jogging and tennis, during adolescence and young adulthood have greater hip and lumbar spine bone mineral density than those who do not take part in such activities, according to a study published in American Journal of Men’s Health.
In a cross-sectional study, researchers analyzed the physical histories of 203 healthy, physically active males ages 30 to 65. Participants’ sports and exercise histories varied in the type and level of activity and the length of time spent doing various physical activities.
Exercise-associated bone loading scores were calculated based on the biomechanical ground-reaction forces of the patients’ past and current physical activities. Current bone mineral density (BMD) was measured using dual-energy x-ray absorptiometry. In addition, participants were grouped based on current participation in a high-impact activity, resistance training, both, or neither.
Bone loading during adolescence and young adulthood were significant, positive predictors of BMD of the whole body, total hip, and lumbar spine, adjusting for lean body mass and/or age Individuals who currently participate in a high-impact activity had greater lumbar spine BMD than nonparticipants. Men who continuously participated in a high-impact activity had greater hip and lumbar spine BMD than those who did not.
Men who continuously participate in high-impact activities, such as jogging and tennis, during adolescence and young adulthood have greater hip and lumbar spine bone mineral density than those who do not take part in such activities, according to a study published in American Journal of Men’s Health.
In a cross-sectional study, researchers analyzed the physical histories of 203 healthy, physically active males ages 30 to 65. Participants’ sports and exercise histories varied in the type and level of activity and the length of time spent doing various physical activities.
Exercise-associated bone loading scores were calculated based on the biomechanical ground-reaction forces of the patients’ past and current physical activities. Current bone mineral density (BMD) was measured using dual-energy x-ray absorptiometry. In addition, participants were grouped based on current participation in a high-impact activity, resistance training, both, or neither.
Bone loading during adolescence and young adulthood were significant, positive predictors of BMD of the whole body, total hip, and lumbar spine, adjusting for lean body mass and/or age Individuals who currently participate in a high-impact activity had greater lumbar spine BMD than nonparticipants. Men who continuously participated in a high-impact activity had greater hip and lumbar spine BMD than those who did not.
Suggested Reading
Matthew A. Strope, Peggy Nigh, Melissa I. Carter, et al. Physical activity–associated bone loading during adolescence and young adulthood is positively associated with adult bone mineral density in men. Am J Mens Health. 2015 November. [Epub ahead of print].
Suggested Reading
Matthew A. Strope, Peggy Nigh, Melissa I. Carter, et al. Physical activity–associated bone loading during adolescence and young adulthood is positively associated with adult bone mineral density in men. Am J Mens Health. 2015 November. [Epub ahead of print].
New data points to slower course of labor
Only recently has evidence emerged that challenges our long-held understanding of “normal” and “abnormal” labor. We now know there is a much wider range of normal labor progress in women who go on to have good labor outcomes. We have a new labor curve to guide us – one that shows us, for example, that active labor occurs most commonly after 6 cm dilation rather than 4 cm as we’d previously thought.
By appreciating this new labor paradigm, we can potentially have a significant impact on the cesarean rate in the United States. While our use of the older labor curve is not the only reason for the rise in cesarean deliveries over the last 30 years, it very likely has played a role. A study published in 2011 of more than 32,000 live births at a major academic hospital demonstrated that one of the most common reasons for primary cesarean is abnormal labor or arrest (Obstet Gynecol. 2011 Jul;118[1]:29-38).
Another study by the Consortium on Safe Labor – an analysis of labor and delivery information from more than 228,000 women across the United States – showed that half of the cesarean deliveries performed for dystocia in women undergoing labor induction were performed before 6 cm of cervical dilation and relatively soon after the previous cervical examination (Am J Obstet Gynecol. 2010 Oct; 203[4]: 326.e1–326.e10).
Our new labor paradigm brings to the forefront a host of new issues and questions about how we can best manage labor to optimize outcomes. In a way, recent discoveries about labor progress have highlighted a dearth of evidence and made “old” issues in labor management seem new and urgent.
As we strive to learn more, however, we are challenged to change our practices and behavior at the bedside with the evidence we currently have. By appreciating both the new labor curve and our current understanding of how labor induction, obesity, and other patient characteristics and clinical conditions can affect labor progress, we can expect that many women will simply progress much more slowly than was historically expected.
As long as we have indications of the well-being of the baby and the well-being of the mother, a slower but progressive labor in the first stage should not prompt us to intervene. We should no longer apply the standards of active-phase progress – standards that have traditionally driven our diagnoses of labor dystocia – until the patient has achieved 6 cm of dilation.
The labor curve that had shaped our thinking about normal and abnormal labor progress until recently was developed by Dr. Emanuel Friedman. Based on findings from a prospective cohort study of 500 nulliparous women, Dr. Friedman plotted labor progress with centimeters of cervical dilation on the Y-axis and time on the X-axis, and divided labor into several stages and phases. In this curve, the rate of change of cervical dilation over time started increasing significantly at 4 cm; this period of increasing slope defined the active phase of labor.
Abnormal labor progress in the active phase was then defined, based on the 95th percentile, as cervical dilation of less than 1.2 cm per hour for nulliparous women and less than 1.5 cm per hour for multiparous women. Based on Dr. Friedman’s work, a woman was deemed to be in active-phase arrest when she had no cervical changes for 2 hours or more while having adequate uterine contractions and cervical dilation of at least 4 cm. These concepts came to govern labor management.
The paradigm shifted when the Consortium on Safe Labor reported in 2010 on a retrospective cohort study of more than 62,000 women at 19 U.S. hospitals. The women had a singleton term gestation, spontaneous labor, vertex presentation, vaginal delivery, and a normal perinatal outcome. In their analysis of labor and delivery information, Dr. Jun Zhang of the National Institutes of Health’s Eunice Kennedy Shriver National Institute of Child Health and Human Development and his colleagues accounted for the fact that the exact times of cervical change are unknown.
They used modern statistical methods and analytical tools that took into account the specific nature of cervical dilation data – that cervical measurements are interval-censored (we never know the exact time when a woman’s cervix changes) and that multiple exams of the cervix in the same patient are not independent (Obstet Gynecol. 2010 Dec;116[6]:1281-7).
The methodology used in the Consortium study accounted for both the interval-censored and repeated-measures nature of cervical dilation data. It thus addressed analytical flaws in the previous approach to labor data, which was purely descriptive of the exam findings and did not consider the nature of the data itself.
Under the new analysis and in the larger, contemporary population of patients, the period of increasing slope was found to occur most commonly after 6 cm, not 4 cm. The slowest 5% of nulliparous women had cervical dilation of 0.4 cm per hour (with the median at 1.9 cm per hour), compared with 1.2 cm per hour (with a median of 3.0 cm per hour) as in the Friedman data.
Dr. Zhang’s study showed us that labor may take more than 6 hours to progress from 4 to 5 cm dilation, and more than 3 hours to progress from 5 to 6 cm dilation – a rate of progress that is significantly slower than what Dr. Friedman had described. The new data showed us, moreover, that from 4 cm-6 cm dilation, nulliparous and multiparous women progressed similarly slowly. Beyond 6 cm, multiparous women dilated more rapidly, with a steeper acceleration phase than previously described.
A consensus statement published in 2014 by the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) on “Safe Prevention of the Primary Cesarean Delivery” encourages use of the Consortium data to revisit the definition of labor dystocia. While the data “do not directly address an optimal duration for the diagnosis of active-phase protraction or labor arrest, [they] do suggest that neither should be diagnosed before 6 cm dilation” (Obstet Gynecol. 2014 Mar;123[3]:693-711).
The ACOG-SMFM statement makes a series of recommendations for managing the first and second stages of labor, based not only on the Consortium data but on a broader literature review. It recommends that if mother and fetus appear well, cesarean delivery for active-phase arrest in the first stage of labor be reserved for women of at least 6 cm of dilation with ruptured membranes who fail to progress despite 4 hours of adequate uterine activity, or at least 6 hours of oxytocin administration with inadequate uterine activity and no cervical change.
Regarding the latent phase of labor, the statement says that most women with a prolonged latent phase ultimately will enter the active phase with expectant management. It advises that a prolonged latent phase (for example, greater than 20 hours in nulliparous women and greater than 14 hours in multiparous women) should not be an isolated indication for cesarean delivery.
The consensus statement also recognizes recent data showing that women who undergo labor induction have an even slower “normal” course of labor, particularly a longer latent phase, than women who labor spontaneously. A retrospective cohort study of more than 5,000 women, for instance, found that before 6 cm, women whose labor is induced can spend up to 10 hours to achieve each 1 cm of dilation (Obstet Gynecol. 2012 Jun;119[6]:1113-8).
As long as maternal and fetal status are reassuring, the statement says, cesarean deliveries for failed induction of labor in the latent phase can be avoided by allowing longer durations of the latent phase (up to 24 hours) and by requiring that oxytocin be administered for 12-18 hours after membrane rupture before deeming induction a failure.
Each of these described recommendations were graded in the ACOG-SMFM consensus document as “strong” recommendations with “moderate quality evidence.”
Examining our standards
Moving forward, we must further develop and define our thresholds for identifying who will most benefit from a cesarean delivery. We have many specific aspects of labor management to address as well, such as the optimal timing of artificial membrane rupture and the safety and efficacy of different oxytocin protocols. We may also want to revisit recommendations for serial cervical assessment, possibly adjusting the intervals given our understanding of the new labor curve.
Under the new labor paradigm, moreover, we must think not only about the clinical decisions we make at the bedside, but about the decisions we make early in the labor management process.
The timing of admission is one such decision. A statement published in 2012 on “Preventing the First Cesarean Delivery” by ACOG, SMFM, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development advises us to avoid admittance of women during the early latent phase of labor (Obstet Gynecol. 2012 Nov;120[5]:1181-93).
It may even be advisable that we consider admittance at higher cervical dilation. A study published this year shows that women admitted at less than 6 cm of dilation had an increased risk of cesarean delivery, compared with women admitted at higher cervical dilation (Am J Perinatol. 2016 Jan;33[2]:188-94). We have more to learn, but certainly, given what we know now about labor progress and the start of active labor, the timing of admission is an important factor to consider.
The second stage of labor, defined as the interval from complete cervical dilation through delivery of the fetus, presents many questions as well. There is a paucity of quality published data concerning what is normal, how long the stage should last, and how we should manage it. Historically, we have been taught to allow 2 hours of pushing for nulliparous women and 1 hour for multiparous women, when epidural anesthesia has not been administered, and to add an additional hour when epidural is used.
The 2014 ACOG-SMFM consensus statement recommends extending each of these limits by an hour, if maternal and fetal conditions permit, so that we allow at least 3 hours of pushing for nulliparous women and at least 2 hours for multiparous women before diagnosing arrest of labor in the second stage. Longer durations may be appropriate with the use of epidural anesthesia and on an individualized basis.
At this time, it is unclear whether there is any absolute maximum length of time beyond which all women in the second stage of labor should undergo cesarean delivery. We also still do not know the optimal technique for managing maternal pushing during the second stage. Should women with an epidural push right away or should they allow for a period of spontaneous descent? Many of the high-quality studies reported thus far that compare delayed and immediate pushing have limited applicability to current practice because they involved now-obsolete midpelvic forceps deliveries. A large multicenter randomized trial currently underway should provide us with some answers.
Dr. Cahill is an associate professor and chief of the division of maternal-fetal medicine in the department of obstetrics and gynecology at Washington University School of Medicine in St. Louis. She reported having no relevant financial disclosures.
Only recently has evidence emerged that challenges our long-held understanding of “normal” and “abnormal” labor. We now know there is a much wider range of normal labor progress in women who go on to have good labor outcomes. We have a new labor curve to guide us – one that shows us, for example, that active labor occurs most commonly after 6 cm dilation rather than 4 cm as we’d previously thought.
By appreciating this new labor paradigm, we can potentially have a significant impact on the cesarean rate in the United States. While our use of the older labor curve is not the only reason for the rise in cesarean deliveries over the last 30 years, it very likely has played a role. A study published in 2011 of more than 32,000 live births at a major academic hospital demonstrated that one of the most common reasons for primary cesarean is abnormal labor or arrest (Obstet Gynecol. 2011 Jul;118[1]:29-38).
Another study by the Consortium on Safe Labor – an analysis of labor and delivery information from more than 228,000 women across the United States – showed that half of the cesarean deliveries performed for dystocia in women undergoing labor induction were performed before 6 cm of cervical dilation and relatively soon after the previous cervical examination (Am J Obstet Gynecol. 2010 Oct; 203[4]: 326.e1–326.e10).
Our new labor paradigm brings to the forefront a host of new issues and questions about how we can best manage labor to optimize outcomes. In a way, recent discoveries about labor progress have highlighted a dearth of evidence and made “old” issues in labor management seem new and urgent.
As we strive to learn more, however, we are challenged to change our practices and behavior at the bedside with the evidence we currently have. By appreciating both the new labor curve and our current understanding of how labor induction, obesity, and other patient characteristics and clinical conditions can affect labor progress, we can expect that many women will simply progress much more slowly than was historically expected.
As long as we have indications of the well-being of the baby and the well-being of the mother, a slower but progressive labor in the first stage should not prompt us to intervene. We should no longer apply the standards of active-phase progress – standards that have traditionally driven our diagnoses of labor dystocia – until the patient has achieved 6 cm of dilation.
The labor curve that had shaped our thinking about normal and abnormal labor progress until recently was developed by Dr. Emanuel Friedman. Based on findings from a prospective cohort study of 500 nulliparous women, Dr. Friedman plotted labor progress with centimeters of cervical dilation on the Y-axis and time on the X-axis, and divided labor into several stages and phases. In this curve, the rate of change of cervical dilation over time started increasing significantly at 4 cm; this period of increasing slope defined the active phase of labor.
Abnormal labor progress in the active phase was then defined, based on the 95th percentile, as cervical dilation of less than 1.2 cm per hour for nulliparous women and less than 1.5 cm per hour for multiparous women. Based on Dr. Friedman’s work, a woman was deemed to be in active-phase arrest when she had no cervical changes for 2 hours or more while having adequate uterine contractions and cervical dilation of at least 4 cm. These concepts came to govern labor management.
The paradigm shifted when the Consortium on Safe Labor reported in 2010 on a retrospective cohort study of more than 62,000 women at 19 U.S. hospitals. The women had a singleton term gestation, spontaneous labor, vertex presentation, vaginal delivery, and a normal perinatal outcome. In their analysis of labor and delivery information, Dr. Jun Zhang of the National Institutes of Health’s Eunice Kennedy Shriver National Institute of Child Health and Human Development and his colleagues accounted for the fact that the exact times of cervical change are unknown.
They used modern statistical methods and analytical tools that took into account the specific nature of cervical dilation data – that cervical measurements are interval-censored (we never know the exact time when a woman’s cervix changes) and that multiple exams of the cervix in the same patient are not independent (Obstet Gynecol. 2010 Dec;116[6]:1281-7).
The methodology used in the Consortium study accounted for both the interval-censored and repeated-measures nature of cervical dilation data. It thus addressed analytical flaws in the previous approach to labor data, which was purely descriptive of the exam findings and did not consider the nature of the data itself.
Under the new analysis and in the larger, contemporary population of patients, the period of increasing slope was found to occur most commonly after 6 cm, not 4 cm. The slowest 5% of nulliparous women had cervical dilation of 0.4 cm per hour (with the median at 1.9 cm per hour), compared with 1.2 cm per hour (with a median of 3.0 cm per hour) as in the Friedman data.
Dr. Zhang’s study showed us that labor may take more than 6 hours to progress from 4 to 5 cm dilation, and more than 3 hours to progress from 5 to 6 cm dilation – a rate of progress that is significantly slower than what Dr. Friedman had described. The new data showed us, moreover, that from 4 cm-6 cm dilation, nulliparous and multiparous women progressed similarly slowly. Beyond 6 cm, multiparous women dilated more rapidly, with a steeper acceleration phase than previously described.
A consensus statement published in 2014 by the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) on “Safe Prevention of the Primary Cesarean Delivery” encourages use of the Consortium data to revisit the definition of labor dystocia. While the data “do not directly address an optimal duration for the diagnosis of active-phase protraction or labor arrest, [they] do suggest that neither should be diagnosed before 6 cm dilation” (Obstet Gynecol. 2014 Mar;123[3]:693-711).
The ACOG-SMFM statement makes a series of recommendations for managing the first and second stages of labor, based not only on the Consortium data but on a broader literature review. It recommends that if mother and fetus appear well, cesarean delivery for active-phase arrest in the first stage of labor be reserved for women of at least 6 cm of dilation with ruptured membranes who fail to progress despite 4 hours of adequate uterine activity, or at least 6 hours of oxytocin administration with inadequate uterine activity and no cervical change.
Regarding the latent phase of labor, the statement says that most women with a prolonged latent phase ultimately will enter the active phase with expectant management. It advises that a prolonged latent phase (for example, greater than 20 hours in nulliparous women and greater than 14 hours in multiparous women) should not be an isolated indication for cesarean delivery.
The consensus statement also recognizes recent data showing that women who undergo labor induction have an even slower “normal” course of labor, particularly a longer latent phase, than women who labor spontaneously. A retrospective cohort study of more than 5,000 women, for instance, found that before 6 cm, women whose labor is induced can spend up to 10 hours to achieve each 1 cm of dilation (Obstet Gynecol. 2012 Jun;119[6]:1113-8).
As long as maternal and fetal status are reassuring, the statement says, cesarean deliveries for failed induction of labor in the latent phase can be avoided by allowing longer durations of the latent phase (up to 24 hours) and by requiring that oxytocin be administered for 12-18 hours after membrane rupture before deeming induction a failure.
Each of these described recommendations were graded in the ACOG-SMFM consensus document as “strong” recommendations with “moderate quality evidence.”
Examining our standards
Moving forward, we must further develop and define our thresholds for identifying who will most benefit from a cesarean delivery. We have many specific aspects of labor management to address as well, such as the optimal timing of artificial membrane rupture and the safety and efficacy of different oxytocin protocols. We may also want to revisit recommendations for serial cervical assessment, possibly adjusting the intervals given our understanding of the new labor curve.
Under the new labor paradigm, moreover, we must think not only about the clinical decisions we make at the bedside, but about the decisions we make early in the labor management process.
The timing of admission is one such decision. A statement published in 2012 on “Preventing the First Cesarean Delivery” by ACOG, SMFM, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development advises us to avoid admittance of women during the early latent phase of labor (Obstet Gynecol. 2012 Nov;120[5]:1181-93).
It may even be advisable that we consider admittance at higher cervical dilation. A study published this year shows that women admitted at less than 6 cm of dilation had an increased risk of cesarean delivery, compared with women admitted at higher cervical dilation (Am J Perinatol. 2016 Jan;33[2]:188-94). We have more to learn, but certainly, given what we know now about labor progress and the start of active labor, the timing of admission is an important factor to consider.
The second stage of labor, defined as the interval from complete cervical dilation through delivery of the fetus, presents many questions as well. There is a paucity of quality published data concerning what is normal, how long the stage should last, and how we should manage it. Historically, we have been taught to allow 2 hours of pushing for nulliparous women and 1 hour for multiparous women, when epidural anesthesia has not been administered, and to add an additional hour when epidural is used.
The 2014 ACOG-SMFM consensus statement recommends extending each of these limits by an hour, if maternal and fetal conditions permit, so that we allow at least 3 hours of pushing for nulliparous women and at least 2 hours for multiparous women before diagnosing arrest of labor in the second stage. Longer durations may be appropriate with the use of epidural anesthesia and on an individualized basis.
At this time, it is unclear whether there is any absolute maximum length of time beyond which all women in the second stage of labor should undergo cesarean delivery. We also still do not know the optimal technique for managing maternal pushing during the second stage. Should women with an epidural push right away or should they allow for a period of spontaneous descent? Many of the high-quality studies reported thus far that compare delayed and immediate pushing have limited applicability to current practice because they involved now-obsolete midpelvic forceps deliveries. A large multicenter randomized trial currently underway should provide us with some answers.
Dr. Cahill is an associate professor and chief of the division of maternal-fetal medicine in the department of obstetrics and gynecology at Washington University School of Medicine in St. Louis. She reported having no relevant financial disclosures.
Only recently has evidence emerged that challenges our long-held understanding of “normal” and “abnormal” labor. We now know there is a much wider range of normal labor progress in women who go on to have good labor outcomes. We have a new labor curve to guide us – one that shows us, for example, that active labor occurs most commonly after 6 cm dilation rather than 4 cm as we’d previously thought.
By appreciating this new labor paradigm, we can potentially have a significant impact on the cesarean rate in the United States. While our use of the older labor curve is not the only reason for the rise in cesarean deliveries over the last 30 years, it very likely has played a role. A study published in 2011 of more than 32,000 live births at a major academic hospital demonstrated that one of the most common reasons for primary cesarean is abnormal labor or arrest (Obstet Gynecol. 2011 Jul;118[1]:29-38).
Another study by the Consortium on Safe Labor – an analysis of labor and delivery information from more than 228,000 women across the United States – showed that half of the cesarean deliveries performed for dystocia in women undergoing labor induction were performed before 6 cm of cervical dilation and relatively soon after the previous cervical examination (Am J Obstet Gynecol. 2010 Oct; 203[4]: 326.e1–326.e10).
Our new labor paradigm brings to the forefront a host of new issues and questions about how we can best manage labor to optimize outcomes. In a way, recent discoveries about labor progress have highlighted a dearth of evidence and made “old” issues in labor management seem new and urgent.
As we strive to learn more, however, we are challenged to change our practices and behavior at the bedside with the evidence we currently have. By appreciating both the new labor curve and our current understanding of how labor induction, obesity, and other patient characteristics and clinical conditions can affect labor progress, we can expect that many women will simply progress much more slowly than was historically expected.
As long as we have indications of the well-being of the baby and the well-being of the mother, a slower but progressive labor in the first stage should not prompt us to intervene. We should no longer apply the standards of active-phase progress – standards that have traditionally driven our diagnoses of labor dystocia – until the patient has achieved 6 cm of dilation.
The labor curve that had shaped our thinking about normal and abnormal labor progress until recently was developed by Dr. Emanuel Friedman. Based on findings from a prospective cohort study of 500 nulliparous women, Dr. Friedman plotted labor progress with centimeters of cervical dilation on the Y-axis and time on the X-axis, and divided labor into several stages and phases. In this curve, the rate of change of cervical dilation over time started increasing significantly at 4 cm; this period of increasing slope defined the active phase of labor.
Abnormal labor progress in the active phase was then defined, based on the 95th percentile, as cervical dilation of less than 1.2 cm per hour for nulliparous women and less than 1.5 cm per hour for multiparous women. Based on Dr. Friedman’s work, a woman was deemed to be in active-phase arrest when she had no cervical changes for 2 hours or more while having adequate uterine contractions and cervical dilation of at least 4 cm. These concepts came to govern labor management.
The paradigm shifted when the Consortium on Safe Labor reported in 2010 on a retrospective cohort study of more than 62,000 women at 19 U.S. hospitals. The women had a singleton term gestation, spontaneous labor, vertex presentation, vaginal delivery, and a normal perinatal outcome. In their analysis of labor and delivery information, Dr. Jun Zhang of the National Institutes of Health’s Eunice Kennedy Shriver National Institute of Child Health and Human Development and his colleagues accounted for the fact that the exact times of cervical change are unknown.
They used modern statistical methods and analytical tools that took into account the specific nature of cervical dilation data – that cervical measurements are interval-censored (we never know the exact time when a woman’s cervix changes) and that multiple exams of the cervix in the same patient are not independent (Obstet Gynecol. 2010 Dec;116[6]:1281-7).
The methodology used in the Consortium study accounted for both the interval-censored and repeated-measures nature of cervical dilation data. It thus addressed analytical flaws in the previous approach to labor data, which was purely descriptive of the exam findings and did not consider the nature of the data itself.
Under the new analysis and in the larger, contemporary population of patients, the period of increasing slope was found to occur most commonly after 6 cm, not 4 cm. The slowest 5% of nulliparous women had cervical dilation of 0.4 cm per hour (with the median at 1.9 cm per hour), compared with 1.2 cm per hour (with a median of 3.0 cm per hour) as in the Friedman data.
Dr. Zhang’s study showed us that labor may take more than 6 hours to progress from 4 to 5 cm dilation, and more than 3 hours to progress from 5 to 6 cm dilation – a rate of progress that is significantly slower than what Dr. Friedman had described. The new data showed us, moreover, that from 4 cm-6 cm dilation, nulliparous and multiparous women progressed similarly slowly. Beyond 6 cm, multiparous women dilated more rapidly, with a steeper acceleration phase than previously described.
A consensus statement published in 2014 by the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) on “Safe Prevention of the Primary Cesarean Delivery” encourages use of the Consortium data to revisit the definition of labor dystocia. While the data “do not directly address an optimal duration for the diagnosis of active-phase protraction or labor arrest, [they] do suggest that neither should be diagnosed before 6 cm dilation” (Obstet Gynecol. 2014 Mar;123[3]:693-711).
The ACOG-SMFM statement makes a series of recommendations for managing the first and second stages of labor, based not only on the Consortium data but on a broader literature review. It recommends that if mother and fetus appear well, cesarean delivery for active-phase arrest in the first stage of labor be reserved for women of at least 6 cm of dilation with ruptured membranes who fail to progress despite 4 hours of adequate uterine activity, or at least 6 hours of oxytocin administration with inadequate uterine activity and no cervical change.
Regarding the latent phase of labor, the statement says that most women with a prolonged latent phase ultimately will enter the active phase with expectant management. It advises that a prolonged latent phase (for example, greater than 20 hours in nulliparous women and greater than 14 hours in multiparous women) should not be an isolated indication for cesarean delivery.
The consensus statement also recognizes recent data showing that women who undergo labor induction have an even slower “normal” course of labor, particularly a longer latent phase, than women who labor spontaneously. A retrospective cohort study of more than 5,000 women, for instance, found that before 6 cm, women whose labor is induced can spend up to 10 hours to achieve each 1 cm of dilation (Obstet Gynecol. 2012 Jun;119[6]:1113-8).
As long as maternal and fetal status are reassuring, the statement says, cesarean deliveries for failed induction of labor in the latent phase can be avoided by allowing longer durations of the latent phase (up to 24 hours) and by requiring that oxytocin be administered for 12-18 hours after membrane rupture before deeming induction a failure.
Each of these described recommendations were graded in the ACOG-SMFM consensus document as “strong” recommendations with “moderate quality evidence.”
Examining our standards
Moving forward, we must further develop and define our thresholds for identifying who will most benefit from a cesarean delivery. We have many specific aspects of labor management to address as well, such as the optimal timing of artificial membrane rupture and the safety and efficacy of different oxytocin protocols. We may also want to revisit recommendations for serial cervical assessment, possibly adjusting the intervals given our understanding of the new labor curve.
Under the new labor paradigm, moreover, we must think not only about the clinical decisions we make at the bedside, but about the decisions we make early in the labor management process.
The timing of admission is one such decision. A statement published in 2012 on “Preventing the First Cesarean Delivery” by ACOG, SMFM, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development advises us to avoid admittance of women during the early latent phase of labor (Obstet Gynecol. 2012 Nov;120[5]:1181-93).
It may even be advisable that we consider admittance at higher cervical dilation. A study published this year shows that women admitted at less than 6 cm of dilation had an increased risk of cesarean delivery, compared with women admitted at higher cervical dilation (Am J Perinatol. 2016 Jan;33[2]:188-94). We have more to learn, but certainly, given what we know now about labor progress and the start of active labor, the timing of admission is an important factor to consider.
The second stage of labor, defined as the interval from complete cervical dilation through delivery of the fetus, presents many questions as well. There is a paucity of quality published data concerning what is normal, how long the stage should last, and how we should manage it. Historically, we have been taught to allow 2 hours of pushing for nulliparous women and 1 hour for multiparous women, when epidural anesthesia has not been administered, and to add an additional hour when epidural is used.
The 2014 ACOG-SMFM consensus statement recommends extending each of these limits by an hour, if maternal and fetal conditions permit, so that we allow at least 3 hours of pushing for nulliparous women and at least 2 hours for multiparous women before diagnosing arrest of labor in the second stage. Longer durations may be appropriate with the use of epidural anesthesia and on an individualized basis.
At this time, it is unclear whether there is any absolute maximum length of time beyond which all women in the second stage of labor should undergo cesarean delivery. We also still do not know the optimal technique for managing maternal pushing during the second stage. Should women with an epidural push right away or should they allow for a period of spontaneous descent? Many of the high-quality studies reported thus far that compare delayed and immediate pushing have limited applicability to current practice because they involved now-obsolete midpelvic forceps deliveries. A large multicenter randomized trial currently underway should provide us with some answers.
Dr. Cahill is an associate professor and chief of the division of maternal-fetal medicine in the department of obstetrics and gynecology at Washington University School of Medicine in St. Louis. She reported having no relevant financial disclosures.