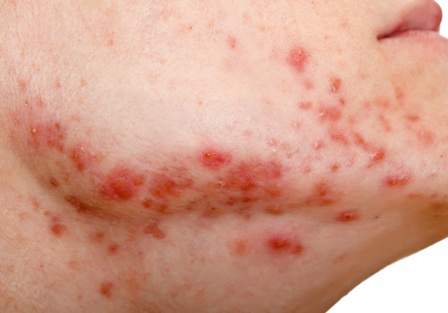
User login
Tool may provide new insight into pediatric cancers
and Xin Zhou, PhD
Photo by Peter Barta/St. Jude
Children’s Research Hospital
Researchers say they have developed a tool that may advance our understanding of the mutations that drive pediatric cancers.
The tool, called ProteinPaint, is a web application that allows the user to visualize genetic lesions and RNA expression in pediatric cancers.
ProteinPaint’s infographics let users see all mutations in individual genes and their corresponding proteins, including detailed information about mutation type, frequency in cancer subtype, and location in the protein domain.
That information provides clues about how a change might contribute to cancer’s start, progression, or relapse.
Jinghui Zhang, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and her colleagues described ProteinPaint in a letter to Nature Genetics.
ProteinPaint currently integrates information from 5 studies, but Dr Zhang and her colleagues said the data will be updated as new studies are published.
ProteinPaint now includes information on almost 27,500 mutations discovered in more than 1000 pediatric patients with 21 cancer subtypes. The application also includes RNA-sequencing data from 928 pediatric tumors belonging to 36 different subtypes.
Xin Zhou, PhD, also of St. Jude, developed ProteinPaint’s infographics to display the genomic information in an interactive format. A click of the mouse gives users additional details about the mutations listed, including the pediatric cancer subtype where the change has been validated and a link to the publication.
“ProteinPaint’s focus on pediatric cancer and presentation of mutations at the gene level complements existing cancer genome data portals,” Dr Zhang said. “For St. Jude, the application is the foundation for developing a global reference database for information about pediatric cancer.”
Dr Zhou added that the ProteinPaint software has the potential to help researchers studying other disorders, including sickle cell disease, that involve a mutation that affects protein function.
ProteinPaint is available at no cost to academic researchers who are free to use the tool to analyze their own data. The application also lets researchers compare information about pediatric and adult cancer genomes by providing a parallel view of data from COSMIC, the world’s largest database of somatic mutations, primarily from adult cancer.
ProteinPaint has already been used to study the role played by germline mutations in pediatric cancers. That research was published in NEJM in November.
More information about ProteinPaint is available on the St. Jude PeCan Data Portal. ![]()

and Xin Zhou, PhD
Photo by Peter Barta/St. Jude
Children’s Research Hospital
Researchers say they have developed a tool that may advance our understanding of the mutations that drive pediatric cancers.
The tool, called ProteinPaint, is a web application that allows the user to visualize genetic lesions and RNA expression in pediatric cancers.
ProteinPaint’s infographics let users see all mutations in individual genes and their corresponding proteins, including detailed information about mutation type, frequency in cancer subtype, and location in the protein domain.
That information provides clues about how a change might contribute to cancer’s start, progression, or relapse.
Jinghui Zhang, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and her colleagues described ProteinPaint in a letter to Nature Genetics.
ProteinPaint currently integrates information from 5 studies, but Dr Zhang and her colleagues said the data will be updated as new studies are published.
ProteinPaint now includes information on almost 27,500 mutations discovered in more than 1000 pediatric patients with 21 cancer subtypes. The application also includes RNA-sequencing data from 928 pediatric tumors belonging to 36 different subtypes.
Xin Zhou, PhD, also of St. Jude, developed ProteinPaint’s infographics to display the genomic information in an interactive format. A click of the mouse gives users additional details about the mutations listed, including the pediatric cancer subtype where the change has been validated and a link to the publication.
“ProteinPaint’s focus on pediatric cancer and presentation of mutations at the gene level complements existing cancer genome data portals,” Dr Zhang said. “For St. Jude, the application is the foundation for developing a global reference database for information about pediatric cancer.”
Dr Zhou added that the ProteinPaint software has the potential to help researchers studying other disorders, including sickle cell disease, that involve a mutation that affects protein function.
ProteinPaint is available at no cost to academic researchers who are free to use the tool to analyze their own data. The application also lets researchers compare information about pediatric and adult cancer genomes by providing a parallel view of data from COSMIC, the world’s largest database of somatic mutations, primarily from adult cancer.
ProteinPaint has already been used to study the role played by germline mutations in pediatric cancers. That research was published in NEJM in November.
More information about ProteinPaint is available on the St. Jude PeCan Data Portal. ![]()

and Xin Zhou, PhD
Photo by Peter Barta/St. Jude
Children’s Research Hospital
Researchers say they have developed a tool that may advance our understanding of the mutations that drive pediatric cancers.
The tool, called ProteinPaint, is a web application that allows the user to visualize genetic lesions and RNA expression in pediatric cancers.
ProteinPaint’s infographics let users see all mutations in individual genes and their corresponding proteins, including detailed information about mutation type, frequency in cancer subtype, and location in the protein domain.
That information provides clues about how a change might contribute to cancer’s start, progression, or relapse.
Jinghui Zhang, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and her colleagues described ProteinPaint in a letter to Nature Genetics.
ProteinPaint currently integrates information from 5 studies, but Dr Zhang and her colleagues said the data will be updated as new studies are published.
ProteinPaint now includes information on almost 27,500 mutations discovered in more than 1000 pediatric patients with 21 cancer subtypes. The application also includes RNA-sequencing data from 928 pediatric tumors belonging to 36 different subtypes.
Xin Zhou, PhD, also of St. Jude, developed ProteinPaint’s infographics to display the genomic information in an interactive format. A click of the mouse gives users additional details about the mutations listed, including the pediatric cancer subtype where the change has been validated and a link to the publication.
“ProteinPaint’s focus on pediatric cancer and presentation of mutations at the gene level complements existing cancer genome data portals,” Dr Zhang said. “For St. Jude, the application is the foundation for developing a global reference database for information about pediatric cancer.”
Dr Zhou added that the ProteinPaint software has the potential to help researchers studying other disorders, including sickle cell disease, that involve a mutation that affects protein function.
ProteinPaint is available at no cost to academic researchers who are free to use the tool to analyze their own data. The application also lets researchers compare information about pediatric and adult cancer genomes by providing a parallel view of data from COSMIC, the world’s largest database of somatic mutations, primarily from adult cancer.
ProteinPaint has already been used to study the role played by germline mutations in pediatric cancers. That research was published in NEJM in November.
More information about ProteinPaint is available on the St. Jude PeCan Data Portal. ![]()
Identifying druggable proteins

Photo by Darren Baker
A computer model that employs techniques used to analyze social networks could aid the development of new cancer treatments, according to researchers.
The model analyzes the unique behaviors of cancer-causing proteins, spotting what makes them different from normal proteins and mapping out molecular targets for drugs that could potentially be developed to treat cancers.
The researchers described this model in PLOS Computational Biology.
Bissan Al-Lazikani, PhD, of The Institute of Cancer Research in London, UK, and her colleagues compared proteins to members of an enormous social network, mapping the ways they interact. This allowed the team to predict which proteins might be most effectively targeted with drugs.
Cancer-causing proteins that have already been successfully targeted tended to have particular “social” characteristics that differed from non-cancer proteins. “Hub-like” proteins that were shown to “communicate” with lots of other proteins were more likely to cause cancers.
The researchers said this suggests that previously unexplored cancer proteins with similar characteristics could make good drug targets.
“Our study is the first to identify the rules of social behavior of cancer proteins and use it to predict new targets for potential cancer drugs,” Dr Al-Lazikani said.
“It shows that cancer drug targets behave very differently from normal proteins and often have a complex web of social interactions. Finding new targets is one of the most important steps in drug discovery, but it can be a lengthy, expensive process.”
“The map that we’ve made will help researchers design better new drugs, more quickly, saving time and money. It also sheds light on how resistance to treatments may occur and, in just a few years, could help doctors choose the best drug combinations to suit individual patients.”
All of the researchers’ target predictions are available on the canSAR website. The underlying data and tools are also available on the site. ![]()

Photo by Darren Baker
A computer model that employs techniques used to analyze social networks could aid the development of new cancer treatments, according to researchers.
The model analyzes the unique behaviors of cancer-causing proteins, spotting what makes them different from normal proteins and mapping out molecular targets for drugs that could potentially be developed to treat cancers.
The researchers described this model in PLOS Computational Biology.
Bissan Al-Lazikani, PhD, of The Institute of Cancer Research in London, UK, and her colleagues compared proteins to members of an enormous social network, mapping the ways they interact. This allowed the team to predict which proteins might be most effectively targeted with drugs.
Cancer-causing proteins that have already been successfully targeted tended to have particular “social” characteristics that differed from non-cancer proteins. “Hub-like” proteins that were shown to “communicate” with lots of other proteins were more likely to cause cancers.
The researchers said this suggests that previously unexplored cancer proteins with similar characteristics could make good drug targets.
“Our study is the first to identify the rules of social behavior of cancer proteins and use it to predict new targets for potential cancer drugs,” Dr Al-Lazikani said.
“It shows that cancer drug targets behave very differently from normal proteins and often have a complex web of social interactions. Finding new targets is one of the most important steps in drug discovery, but it can be a lengthy, expensive process.”
“The map that we’ve made will help researchers design better new drugs, more quickly, saving time and money. It also sheds light on how resistance to treatments may occur and, in just a few years, could help doctors choose the best drug combinations to suit individual patients.”
All of the researchers’ target predictions are available on the canSAR website. The underlying data and tools are also available on the site. ![]()

Photo by Darren Baker
A computer model that employs techniques used to analyze social networks could aid the development of new cancer treatments, according to researchers.
The model analyzes the unique behaviors of cancer-causing proteins, spotting what makes them different from normal proteins and mapping out molecular targets for drugs that could potentially be developed to treat cancers.
The researchers described this model in PLOS Computational Biology.
Bissan Al-Lazikani, PhD, of The Institute of Cancer Research in London, UK, and her colleagues compared proteins to members of an enormous social network, mapping the ways they interact. This allowed the team to predict which proteins might be most effectively targeted with drugs.
Cancer-causing proteins that have already been successfully targeted tended to have particular “social” characteristics that differed from non-cancer proteins. “Hub-like” proteins that were shown to “communicate” with lots of other proteins were more likely to cause cancers.
The researchers said this suggests that previously unexplored cancer proteins with similar characteristics could make good drug targets.
“Our study is the first to identify the rules of social behavior of cancer proteins and use it to predict new targets for potential cancer drugs,” Dr Al-Lazikani said.
“It shows that cancer drug targets behave very differently from normal proteins and often have a complex web of social interactions. Finding new targets is one of the most important steps in drug discovery, but it can be a lengthy, expensive process.”
“The map that we’ve made will help researchers design better new drugs, more quickly, saving time and money. It also sheds light on how resistance to treatments may occur and, in just a few years, could help doctors choose the best drug combinations to suit individual patients.”
All of the researchers’ target predictions are available on the canSAR website. The underlying data and tools are also available on the site. ![]()
The well-woman visit comes of age: What it offers, how we got here
When the Affordable Care Act (ACA) was passed in 2010, it represented an intended shift from reactive medicine, with its focus on acute and urgent needs, to a model focused on disease prevention.
OBG Management readers know about the important women’s health services ensured by the ACA, including well-woman care, as well as the key role played by the American Congress of Obstetricians and Gynecologists (ACOG) in winning this coverage. ACOG worked closely with the Institute of Medicine (IOM) to help define this set of services. And the ACA ensured that women have access to these services, often without copays and deductibles.
ACOG and the National Women’s Law Center (NWLC) work closely on many issues. At first independently and then together, the 2 organizations set out to explore some fundamental issues:
- How does a woman experience the new well-woman benefit when she visits her doctor?
- Does she receive a consistent care set?
- Do some patients have copays while patients in other clinics do not for the same services?
- What does well-woman care mean from one doctor to another, from an ObGyn to an internist to a family physician?
This article explores these issues.
2 initiatives focused on components of women’s health care
During her tenure as president of ACOG, Jeanne Conry, MD, PhD, decided to tackle clinical issues associated with well-woman care. She convened a Well-Woman Task Force, led by Haywood Brown, MD, and included the NWLC among other partner organizations (TABLE).
Table. Partipating organizations of the ACOG Well-Woman Task Force
• American Academy of Family Physicians
• American Academy of Pediatrics
• American Academy of Physician Assistants
• American College of Nurse–Midwives
• American College of Osteopathic Obstetricians and Gynecologists
• Association of Reproductive Health Professionals
• Association of Women’s Health, Obstetric, and Neonatal Nurses
• National Association of Nurse Practitioners in Women’s Health
• National Medical Association
• National Women’s Law Center
• Planned Parenthood Federation of America
• Society for Maternal-Fetal Medicine
• Society of Academic Specialists in General Obstetrics and Gynecology
• Society of Gynecologic Oncology
The NWLC and Brigham and Women’s Hospital also partnered with ACOG and others to help ensure a consistent patient experience. These 2 closely related initiatives were designed to work together to help patients and physicians understand and benefit from new coverage under the ACA.
1. How does a woman experience well-woman care?
Experts associated with these 2 initiatives recognized that well-woman care includes attention to the history, physical examination, counseling, and screening intended to maintain physical, mental, and social wellbeing and general health throughout a woman’s lifespan. Experts also recognized that the ACA guarantees coverage of at least one annual well-woman visit, although not all of the recommended components necessarily would be performed at the same visit or by the same provider.
For many women who have gained insurance coverage under the ACA, the well-woman visit represents their entry into the insured health care system. These women may have limited understanding of the services they should receive during this visit.
To address this issue, the NWLC invited ACOG to participate in its initiative with Brigham and Women’s Hospital to understand the well-woman visit from the patient’s point of view. This effort yielded patient education materials in English and Spanish that help women understand:
- that their health insurance now covers a well-woman visit
- what care is included in that visit
- that there is no deductible or copay for this visit
- how to prepare for this visit
- what questions to ask during the visit.
These materials help women understand that the purpose of the well-woman visit is to provide them with a chance to:
- “receive care and counseling that is appropriate, based on age, cognitive development, and life experience
- review their current health and risks to their health with their health care professional
- ask any questions they may have about their health or risk factors
- talk about what they can do to prevent future health problems
- build a trusting relationship with their health care provider, with an emphasis on confidentiality
- receive appropriate preventive screenings and immunizations and make sure they know which screenings and immunizations they should receive in the future
- review their reproductive plan and contraceptive choices.”1
The materials also advise patients that they may be asked about:
- current health concerns
- current medications, both prescription and over the counter
- family history on both the mother’s and father’s sides
- life management, including family relationships, work, and stress
- substance use habits, including alcohol and tobacco
- sexual activity
- eating habits and physical activity
- past reproductive health experience and any pregnancy complications
- any memory problems (older women)
- screening for depression, anxiety, substance use disorders, and interpersonal violence.
To view some of these materials, visit http://www.nwlc.org/sites/default/files/final_well-womanbrochure.pdf.
2. Does each woman receive consistent well-woman care?
ACOG’s Well-Woman Task Force was shaped by an awareness that many medical societies and government agencies provide recommendations and guidelines about the basic elements of women’s health. While these recommendations and guidelines all may be based on evidence and expert opinion, the recommendations vary. A goal of the task force was to work with providers across the women’s health spectrum to find consensus and provide guidance to women and clinicians with age-appropriate recommendations for a well-woman visit.
In the fall of 2015, the task force’s findings were published in an article entitled “Components of the well-woman visit” in the journal Obstetrics & Gynecology.2 Those findings outline a core set of well-woman care practices across a woman’s lifespan, from adolescence through the reproductive years and into maturity, and they are usable by any provider who cares for adolescent girls or women.
ACOG has summarized its well-woman recommendations, by age, on its website,3 at http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Well-Woman-Recommendations.
3. Do all women have a copay for the well-woman visit?
Because research has revealed that any type of copay or deductible for preventive care significantly lessens the likelihood that patients will seek out such care, the ACA sought to make basic preventive care available without cost sharing.4
The US Department of Health and Human Services notes that: “The Affordable Care Act requires most health plans to cover recommended preventive services without cost sharing. In 2011 and 2012, 71 million Americans with private health insurance gained access to preventive services with no cost sharing because of the law.”4
Grandfathered plans (those created or sold before March 23, 2010) are exempt from this requirement, as are Medicare, TRICARE, and traditional Medicaid plans.
4. What does well-woman care mean from one doctor to another?
Under the ACA, well-woman care can be provided by a “wide range of providers, including family physicians, internists, nurse–midwives, nurse practitioners, obstetrician-gynecologists, pediatricians, and physician assistants,” depending on the age of the patient, her particular needs and preferences, and access to health services.2
The ACOG Well-Woman Task Force “focused on delineating the well-woman visit throughout the lifespan, across all providers and health plans.”2
In determining the components of well-woman care, ACOG’s task force compiled existing guidelines from many sources, including the Department of Health and Human Services, the IOM, the US Preventive Services Task Force, and each member organization.
Members categorized guidelines as:
- single source (eg, abdominal examination)
- no agreement (breast cancer/mammography screening)
- limited agreement (pelvic examination)
- general agreement (hypertension, osteoporosis)
- sound agreement (screening for sexually transmitted infections)
The task force also agreed that final recommendations would rely on evidence-based guidelines, evidence-informed guidelines, and uniform expert agreement. Recommendations were considered “strong” if they relied primarily on evidence-based or evidence-informed guidelines and “qualified” if they relied primarily on expert consensus.
Guidelines were further separated into age bands:
- adolescents (13–18 years)
- reproductive-aged women (19–45 years)
- mature women (46–64 years)
- women older than 64 years.
The task force recommended that, during the well-woman visit, health care professionals educate patients about:
- healthy eating habits and maintenance of healthy weight
- exercise and physical activity
- seat belt use
- risk factors for certain types of cancer
- heart health
- breast health
- bone health
- safer sex practices and prevention of sexually transmitted infections
- healthy interpersonal relationships
- prevention and management of chronic disease
- resources for the patient (online, written, community, patient groups)
- medication use
- fall prevention.
Health care providers also should counsel patients regarding:
- recommended preventive screenings and immunizations
- any concerns about mood, such as prolonged periods of sadness, a failure to enjoy what they usually find pleasant, or anxiety or irritability that seems out of proportion to events
- what to expect in terms of effects on mood and anxiety at reproductive life transitions, including menarche, pregnancy, the postpartum period, and perimenopause
- body image issues
- what to expect in terms of the menstrual cycle during perimenopause and menopause
- reproductive health or fertility concerns
- reproductive life planning (contraception appropriate for life stage, reproductive plans, and risk factors, including risk factors for breast and ovarian cancer and cardiovascular disease)
- pregnancy planning, including attaining and maintaining a healthy weight and managing any chronic conditions before or during pregnancy
- what to expect during menopause, including signs and symptoms and options for addressing symptoms (midlife and older women)
- symptoms of cardiovascular disease
- urinary incontinence.
The task force acknowledged that not all of these recommendations can be carried out at a single well-woman visit or by a single provider.
See, again, ACOG’s specific well-woman recommendations, by age range, at http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Well-Woman-Recommendations.3
How to winnow a long list of recommendations to determine the most pressing issues for a specific patient
In an editorial accompanying the ACOG Well-Woman Task Force report, entitled “Re-envisioning the annual well-woman visit: the task forward,” George F. Sawaya, MD, of the University of California, San Francisco, devised a plan to determine the most pressing well-woman needs for a specific patient.1 He chose as an example a 41-year-old sexually active woman who does not smoke.
While Dr. Sawaya praised the Well-Woman Task Force recommendations for their “comprehensive scope,” he also noted that the sheer number of recommendations might be “overwhelming and difficult to navigate.”1 One tool for winnowing the recommendations comes from the Agency for Healthcare Research and Quality, which offers an Electronic Preventive Services Selector (http://epss.ahrq.gov/PDA/index.jsp), available both online and as a smartphone app. Once the clinician plugs in the patient’s age and a few risk factors, the tool generates a list of recommended preventive services. This list of services has been evaluated by the US Preventive Services Task Force, with each recommendation graded “A” through “D,” based on benefits versus harms.
Back to that 41-year-old sexually active woman: Using the Electronic Preventive Services Selector, a list of as many as 20 grade A and B recommendations would be generated. However, only 3 of them would be grade A (screening for cervical cancer, HIV, and high blood pressure). An additional 2 grade B recommendations might apply to an average-risk patient such as this: screening for alcohol misuse and depression. All 5 services fall within the Well-Woman Task Force’s recommendations. They also have “good face validity with clinicians as being important, so it seems reasonable that these be prioritized above the others, at least at the first visit,” Dr. Sawaya says.1
Clinicians can use a similar strategy for patients of various ages and risk factors.
Reference
1. Sawaya GF. Re-envisioning the annual well-woman visit: the task forward [editorial]. Obstet Gynecol. 2015;126(4):695–696.
The bottom line
By defining and implementing the foundational elements of women’s health, we can improve care for all women and ensure, as Dr. Conry emphasized during her tenure as ACOG president, “that every woman gets the care she needs, every time.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- National Women’s Law Center, Brigham and Women’s Hospital. Your Guide to Well-Woman Visits. http://www.nwlc.org/sites/default/files/final_well-womanbrochure.pdf. Accessed December 8, 2015.
- Conry JA, Brown H. Well-Woman Task Force: Components of the well-woman visit. Obstet Gynecol. 2015;126(4):697–701.
- American College of Obstetricians and Gynecologists. Well-Woman Recommendations. http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Well-Woman-Recommendations. Accessed December 4, 2015.
- US Department of Health and Human Services. Affordable Care Act Rules on Expanding Access to Preventive Services for Women. http://www.hhs.gov/healthcare/facts-and-features/fact-sheets/aca-rules-on-expanding-access-to-preventive-services-for-women/index.html. Updated June 28, 2013. Accessed December 4, 2015.
When the Affordable Care Act (ACA) was passed in 2010, it represented an intended shift from reactive medicine, with its focus on acute and urgent needs, to a model focused on disease prevention.
OBG Management readers know about the important women’s health services ensured by the ACA, including well-woman care, as well as the key role played by the American Congress of Obstetricians and Gynecologists (ACOG) in winning this coverage. ACOG worked closely with the Institute of Medicine (IOM) to help define this set of services. And the ACA ensured that women have access to these services, often without copays and deductibles.
ACOG and the National Women’s Law Center (NWLC) work closely on many issues. At first independently and then together, the 2 organizations set out to explore some fundamental issues:
- How does a woman experience the new well-woman benefit when she visits her doctor?
- Does she receive a consistent care set?
- Do some patients have copays while patients in other clinics do not for the same services?
- What does well-woman care mean from one doctor to another, from an ObGyn to an internist to a family physician?
This article explores these issues.
2 initiatives focused on components of women’s health care
During her tenure as president of ACOG, Jeanne Conry, MD, PhD, decided to tackle clinical issues associated with well-woman care. She convened a Well-Woman Task Force, led by Haywood Brown, MD, and included the NWLC among other partner organizations (TABLE).
Table. Partipating organizations of the ACOG Well-Woman Task Force
• American Academy of Family Physicians
• American Academy of Pediatrics
• American Academy of Physician Assistants
• American College of Nurse–Midwives
• American College of Osteopathic Obstetricians and Gynecologists
• Association of Reproductive Health Professionals
• Association of Women’s Health, Obstetric, and Neonatal Nurses
• National Association of Nurse Practitioners in Women’s Health
• National Medical Association
• National Women’s Law Center
• Planned Parenthood Federation of America
• Society for Maternal-Fetal Medicine
• Society of Academic Specialists in General Obstetrics and Gynecology
• Society of Gynecologic Oncology
The NWLC and Brigham and Women’s Hospital also partnered with ACOG and others to help ensure a consistent patient experience. These 2 closely related initiatives were designed to work together to help patients and physicians understand and benefit from new coverage under the ACA.
1. How does a woman experience well-woman care?
Experts associated with these 2 initiatives recognized that well-woman care includes attention to the history, physical examination, counseling, and screening intended to maintain physical, mental, and social wellbeing and general health throughout a woman’s lifespan. Experts also recognized that the ACA guarantees coverage of at least one annual well-woman visit, although not all of the recommended components necessarily would be performed at the same visit or by the same provider.
For many women who have gained insurance coverage under the ACA, the well-woman visit represents their entry into the insured health care system. These women may have limited understanding of the services they should receive during this visit.
To address this issue, the NWLC invited ACOG to participate in its initiative with Brigham and Women’s Hospital to understand the well-woman visit from the patient’s point of view. This effort yielded patient education materials in English and Spanish that help women understand:
- that their health insurance now covers a well-woman visit
- what care is included in that visit
- that there is no deductible or copay for this visit
- how to prepare for this visit
- what questions to ask during the visit.
These materials help women understand that the purpose of the well-woman visit is to provide them with a chance to:
- “receive care and counseling that is appropriate, based on age, cognitive development, and life experience
- review their current health and risks to their health with their health care professional
- ask any questions they may have about their health or risk factors
- talk about what they can do to prevent future health problems
- build a trusting relationship with their health care provider, with an emphasis on confidentiality
- receive appropriate preventive screenings and immunizations and make sure they know which screenings and immunizations they should receive in the future
- review their reproductive plan and contraceptive choices.”1
The materials also advise patients that they may be asked about:
- current health concerns
- current medications, both prescription and over the counter
- family history on both the mother’s and father’s sides
- life management, including family relationships, work, and stress
- substance use habits, including alcohol and tobacco
- sexual activity
- eating habits and physical activity
- past reproductive health experience and any pregnancy complications
- any memory problems (older women)
- screening for depression, anxiety, substance use disorders, and interpersonal violence.
To view some of these materials, visit http://www.nwlc.org/sites/default/files/final_well-womanbrochure.pdf.
2. Does each woman receive consistent well-woman care?
ACOG’s Well-Woman Task Force was shaped by an awareness that many medical societies and government agencies provide recommendations and guidelines about the basic elements of women’s health. While these recommendations and guidelines all may be based on evidence and expert opinion, the recommendations vary. A goal of the task force was to work with providers across the women’s health spectrum to find consensus and provide guidance to women and clinicians with age-appropriate recommendations for a well-woman visit.
In the fall of 2015, the task force’s findings were published in an article entitled “Components of the well-woman visit” in the journal Obstetrics & Gynecology.2 Those findings outline a core set of well-woman care practices across a woman’s lifespan, from adolescence through the reproductive years and into maturity, and they are usable by any provider who cares for adolescent girls or women.
ACOG has summarized its well-woman recommendations, by age, on its website,3 at http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Well-Woman-Recommendations.
3. Do all women have a copay for the well-woman visit?
Because research has revealed that any type of copay or deductible for preventive care significantly lessens the likelihood that patients will seek out such care, the ACA sought to make basic preventive care available without cost sharing.4
The US Department of Health and Human Services notes that: “The Affordable Care Act requires most health plans to cover recommended preventive services without cost sharing. In 2011 and 2012, 71 million Americans with private health insurance gained access to preventive services with no cost sharing because of the law.”4
Grandfathered plans (those created or sold before March 23, 2010) are exempt from this requirement, as are Medicare, TRICARE, and traditional Medicaid plans.
4. What does well-woman care mean from one doctor to another?
Under the ACA, well-woman care can be provided by a “wide range of providers, including family physicians, internists, nurse–midwives, nurse practitioners, obstetrician-gynecologists, pediatricians, and physician assistants,” depending on the age of the patient, her particular needs and preferences, and access to health services.2
The ACOG Well-Woman Task Force “focused on delineating the well-woman visit throughout the lifespan, across all providers and health plans.”2
In determining the components of well-woman care, ACOG’s task force compiled existing guidelines from many sources, including the Department of Health and Human Services, the IOM, the US Preventive Services Task Force, and each member organization.
Members categorized guidelines as:
- single source (eg, abdominal examination)
- no agreement (breast cancer/mammography screening)
- limited agreement (pelvic examination)
- general agreement (hypertension, osteoporosis)
- sound agreement (screening for sexually transmitted infections)
The task force also agreed that final recommendations would rely on evidence-based guidelines, evidence-informed guidelines, and uniform expert agreement. Recommendations were considered “strong” if they relied primarily on evidence-based or evidence-informed guidelines and “qualified” if they relied primarily on expert consensus.
Guidelines were further separated into age bands:
- adolescents (13–18 years)
- reproductive-aged women (19–45 years)
- mature women (46–64 years)
- women older than 64 years.
The task force recommended that, during the well-woman visit, health care professionals educate patients about:
- healthy eating habits and maintenance of healthy weight
- exercise and physical activity
- seat belt use
- risk factors for certain types of cancer
- heart health
- breast health
- bone health
- safer sex practices and prevention of sexually transmitted infections
- healthy interpersonal relationships
- prevention and management of chronic disease
- resources for the patient (online, written, community, patient groups)
- medication use
- fall prevention.
Health care providers also should counsel patients regarding:
- recommended preventive screenings and immunizations
- any concerns about mood, such as prolonged periods of sadness, a failure to enjoy what they usually find pleasant, or anxiety or irritability that seems out of proportion to events
- what to expect in terms of effects on mood and anxiety at reproductive life transitions, including menarche, pregnancy, the postpartum period, and perimenopause
- body image issues
- what to expect in terms of the menstrual cycle during perimenopause and menopause
- reproductive health or fertility concerns
- reproductive life planning (contraception appropriate for life stage, reproductive plans, and risk factors, including risk factors for breast and ovarian cancer and cardiovascular disease)
- pregnancy planning, including attaining and maintaining a healthy weight and managing any chronic conditions before or during pregnancy
- what to expect during menopause, including signs and symptoms and options for addressing symptoms (midlife and older women)
- symptoms of cardiovascular disease
- urinary incontinence.
The task force acknowledged that not all of these recommendations can be carried out at a single well-woman visit or by a single provider.
See, again, ACOG’s specific well-woman recommendations, by age range, at http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Well-Woman-Recommendations.3
How to winnow a long list of recommendations to determine the most pressing issues for a specific patient
In an editorial accompanying the ACOG Well-Woman Task Force report, entitled “Re-envisioning the annual well-woman visit: the task forward,” George F. Sawaya, MD, of the University of California, San Francisco, devised a plan to determine the most pressing well-woman needs for a specific patient.1 He chose as an example a 41-year-old sexually active woman who does not smoke.
While Dr. Sawaya praised the Well-Woman Task Force recommendations for their “comprehensive scope,” he also noted that the sheer number of recommendations might be “overwhelming and difficult to navigate.”1 One tool for winnowing the recommendations comes from the Agency for Healthcare Research and Quality, which offers an Electronic Preventive Services Selector (http://epss.ahrq.gov/PDA/index.jsp), available both online and as a smartphone app. Once the clinician plugs in the patient’s age and a few risk factors, the tool generates a list of recommended preventive services. This list of services has been evaluated by the US Preventive Services Task Force, with each recommendation graded “A” through “D,” based on benefits versus harms.
Back to that 41-year-old sexually active woman: Using the Electronic Preventive Services Selector, a list of as many as 20 grade A and B recommendations would be generated. However, only 3 of them would be grade A (screening for cervical cancer, HIV, and high blood pressure). An additional 2 grade B recommendations might apply to an average-risk patient such as this: screening for alcohol misuse and depression. All 5 services fall within the Well-Woman Task Force’s recommendations. They also have “good face validity with clinicians as being important, so it seems reasonable that these be prioritized above the others, at least at the first visit,” Dr. Sawaya says.1
Clinicians can use a similar strategy for patients of various ages and risk factors.
Reference
1. Sawaya GF. Re-envisioning the annual well-woman visit: the task forward [editorial]. Obstet Gynecol. 2015;126(4):695–696.
The bottom line
By defining and implementing the foundational elements of women’s health, we can improve care for all women and ensure, as Dr. Conry emphasized during her tenure as ACOG president, “that every woman gets the care she needs, every time.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
When the Affordable Care Act (ACA) was passed in 2010, it represented an intended shift from reactive medicine, with its focus on acute and urgent needs, to a model focused on disease prevention.
OBG Management readers know about the important women’s health services ensured by the ACA, including well-woman care, as well as the key role played by the American Congress of Obstetricians and Gynecologists (ACOG) in winning this coverage. ACOG worked closely with the Institute of Medicine (IOM) to help define this set of services. And the ACA ensured that women have access to these services, often without copays and deductibles.
ACOG and the National Women’s Law Center (NWLC) work closely on many issues. At first independently and then together, the 2 organizations set out to explore some fundamental issues:
- How does a woman experience the new well-woman benefit when she visits her doctor?
- Does she receive a consistent care set?
- Do some patients have copays while patients in other clinics do not for the same services?
- What does well-woman care mean from one doctor to another, from an ObGyn to an internist to a family physician?
This article explores these issues.
2 initiatives focused on components of women’s health care
During her tenure as president of ACOG, Jeanne Conry, MD, PhD, decided to tackle clinical issues associated with well-woman care. She convened a Well-Woman Task Force, led by Haywood Brown, MD, and included the NWLC among other partner organizations (TABLE).
Table. Partipating organizations of the ACOG Well-Woman Task Force
• American Academy of Family Physicians
• American Academy of Pediatrics
• American Academy of Physician Assistants
• American College of Nurse–Midwives
• American College of Osteopathic Obstetricians and Gynecologists
• Association of Reproductive Health Professionals
• Association of Women’s Health, Obstetric, and Neonatal Nurses
• National Association of Nurse Practitioners in Women’s Health
• National Medical Association
• National Women’s Law Center
• Planned Parenthood Federation of America
• Society for Maternal-Fetal Medicine
• Society of Academic Specialists in General Obstetrics and Gynecology
• Society of Gynecologic Oncology
The NWLC and Brigham and Women’s Hospital also partnered with ACOG and others to help ensure a consistent patient experience. These 2 closely related initiatives were designed to work together to help patients and physicians understand and benefit from new coverage under the ACA.
1. How does a woman experience well-woman care?
Experts associated with these 2 initiatives recognized that well-woman care includes attention to the history, physical examination, counseling, and screening intended to maintain physical, mental, and social wellbeing and general health throughout a woman’s lifespan. Experts also recognized that the ACA guarantees coverage of at least one annual well-woman visit, although not all of the recommended components necessarily would be performed at the same visit or by the same provider.
For many women who have gained insurance coverage under the ACA, the well-woman visit represents their entry into the insured health care system. These women may have limited understanding of the services they should receive during this visit.
To address this issue, the NWLC invited ACOG to participate in its initiative with Brigham and Women’s Hospital to understand the well-woman visit from the patient’s point of view. This effort yielded patient education materials in English and Spanish that help women understand:
- that their health insurance now covers a well-woman visit
- what care is included in that visit
- that there is no deductible or copay for this visit
- how to prepare for this visit
- what questions to ask during the visit.
These materials help women understand that the purpose of the well-woman visit is to provide them with a chance to:
- “receive care and counseling that is appropriate, based on age, cognitive development, and life experience
- review their current health and risks to their health with their health care professional
- ask any questions they may have about their health or risk factors
- talk about what they can do to prevent future health problems
- build a trusting relationship with their health care provider, with an emphasis on confidentiality
- receive appropriate preventive screenings and immunizations and make sure they know which screenings and immunizations they should receive in the future
- review their reproductive plan and contraceptive choices.”1
The materials also advise patients that they may be asked about:
- current health concerns
- current medications, both prescription and over the counter
- family history on both the mother’s and father’s sides
- life management, including family relationships, work, and stress
- substance use habits, including alcohol and tobacco
- sexual activity
- eating habits and physical activity
- past reproductive health experience and any pregnancy complications
- any memory problems (older women)
- screening for depression, anxiety, substance use disorders, and interpersonal violence.
To view some of these materials, visit http://www.nwlc.org/sites/default/files/final_well-womanbrochure.pdf.
2. Does each woman receive consistent well-woman care?
ACOG’s Well-Woman Task Force was shaped by an awareness that many medical societies and government agencies provide recommendations and guidelines about the basic elements of women’s health. While these recommendations and guidelines all may be based on evidence and expert opinion, the recommendations vary. A goal of the task force was to work with providers across the women’s health spectrum to find consensus and provide guidance to women and clinicians with age-appropriate recommendations for a well-woman visit.
In the fall of 2015, the task force’s findings were published in an article entitled “Components of the well-woman visit” in the journal Obstetrics & Gynecology.2 Those findings outline a core set of well-woman care practices across a woman’s lifespan, from adolescence through the reproductive years and into maturity, and they are usable by any provider who cares for adolescent girls or women.
ACOG has summarized its well-woman recommendations, by age, on its website,3 at http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Well-Woman-Recommendations.
3. Do all women have a copay for the well-woman visit?
Because research has revealed that any type of copay or deductible for preventive care significantly lessens the likelihood that patients will seek out such care, the ACA sought to make basic preventive care available without cost sharing.4
The US Department of Health and Human Services notes that: “The Affordable Care Act requires most health plans to cover recommended preventive services without cost sharing. In 2011 and 2012, 71 million Americans with private health insurance gained access to preventive services with no cost sharing because of the law.”4
Grandfathered plans (those created or sold before March 23, 2010) are exempt from this requirement, as are Medicare, TRICARE, and traditional Medicaid plans.
4. What does well-woman care mean from one doctor to another?
Under the ACA, well-woman care can be provided by a “wide range of providers, including family physicians, internists, nurse–midwives, nurse practitioners, obstetrician-gynecologists, pediatricians, and physician assistants,” depending on the age of the patient, her particular needs and preferences, and access to health services.2
The ACOG Well-Woman Task Force “focused on delineating the well-woman visit throughout the lifespan, across all providers and health plans.”2
In determining the components of well-woman care, ACOG’s task force compiled existing guidelines from many sources, including the Department of Health and Human Services, the IOM, the US Preventive Services Task Force, and each member organization.
Members categorized guidelines as:
- single source (eg, abdominal examination)
- no agreement (breast cancer/mammography screening)
- limited agreement (pelvic examination)
- general agreement (hypertension, osteoporosis)
- sound agreement (screening for sexually transmitted infections)
The task force also agreed that final recommendations would rely on evidence-based guidelines, evidence-informed guidelines, and uniform expert agreement. Recommendations were considered “strong” if they relied primarily on evidence-based or evidence-informed guidelines and “qualified” if they relied primarily on expert consensus.
Guidelines were further separated into age bands:
- adolescents (13–18 years)
- reproductive-aged women (19–45 years)
- mature women (46–64 years)
- women older than 64 years.
The task force recommended that, during the well-woman visit, health care professionals educate patients about:
- healthy eating habits and maintenance of healthy weight
- exercise and physical activity
- seat belt use
- risk factors for certain types of cancer
- heart health
- breast health
- bone health
- safer sex practices and prevention of sexually transmitted infections
- healthy interpersonal relationships
- prevention and management of chronic disease
- resources for the patient (online, written, community, patient groups)
- medication use
- fall prevention.
Health care providers also should counsel patients regarding:
- recommended preventive screenings and immunizations
- any concerns about mood, such as prolonged periods of sadness, a failure to enjoy what they usually find pleasant, or anxiety or irritability that seems out of proportion to events
- what to expect in terms of effects on mood and anxiety at reproductive life transitions, including menarche, pregnancy, the postpartum period, and perimenopause
- body image issues
- what to expect in terms of the menstrual cycle during perimenopause and menopause
- reproductive health or fertility concerns
- reproductive life planning (contraception appropriate for life stage, reproductive plans, and risk factors, including risk factors for breast and ovarian cancer and cardiovascular disease)
- pregnancy planning, including attaining and maintaining a healthy weight and managing any chronic conditions before or during pregnancy
- what to expect during menopause, including signs and symptoms and options for addressing symptoms (midlife and older women)
- symptoms of cardiovascular disease
- urinary incontinence.
The task force acknowledged that not all of these recommendations can be carried out at a single well-woman visit or by a single provider.
See, again, ACOG’s specific well-woman recommendations, by age range, at http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Well-Woman-Recommendations.3
How to winnow a long list of recommendations to determine the most pressing issues for a specific patient
In an editorial accompanying the ACOG Well-Woman Task Force report, entitled “Re-envisioning the annual well-woman visit: the task forward,” George F. Sawaya, MD, of the University of California, San Francisco, devised a plan to determine the most pressing well-woman needs for a specific patient.1 He chose as an example a 41-year-old sexually active woman who does not smoke.
While Dr. Sawaya praised the Well-Woman Task Force recommendations for their “comprehensive scope,” he also noted that the sheer number of recommendations might be “overwhelming and difficult to navigate.”1 One tool for winnowing the recommendations comes from the Agency for Healthcare Research and Quality, which offers an Electronic Preventive Services Selector (http://epss.ahrq.gov/PDA/index.jsp), available both online and as a smartphone app. Once the clinician plugs in the patient’s age and a few risk factors, the tool generates a list of recommended preventive services. This list of services has been evaluated by the US Preventive Services Task Force, with each recommendation graded “A” through “D,” based on benefits versus harms.
Back to that 41-year-old sexually active woman: Using the Electronic Preventive Services Selector, a list of as many as 20 grade A and B recommendations would be generated. However, only 3 of them would be grade A (screening for cervical cancer, HIV, and high blood pressure). An additional 2 grade B recommendations might apply to an average-risk patient such as this: screening for alcohol misuse and depression. All 5 services fall within the Well-Woman Task Force’s recommendations. They also have “good face validity with clinicians as being important, so it seems reasonable that these be prioritized above the others, at least at the first visit,” Dr. Sawaya says.1
Clinicians can use a similar strategy for patients of various ages and risk factors.
Reference
1. Sawaya GF. Re-envisioning the annual well-woman visit: the task forward [editorial]. Obstet Gynecol. 2015;126(4):695–696.
The bottom line
By defining and implementing the foundational elements of women’s health, we can improve care for all women and ensure, as Dr. Conry emphasized during her tenure as ACOG president, “that every woman gets the care she needs, every time.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- National Women’s Law Center, Brigham and Women’s Hospital. Your Guide to Well-Woman Visits. http://www.nwlc.org/sites/default/files/final_well-womanbrochure.pdf. Accessed December 8, 2015.
- Conry JA, Brown H. Well-Woman Task Force: Components of the well-woman visit. Obstet Gynecol. 2015;126(4):697–701.
- American College of Obstetricians and Gynecologists. Well-Woman Recommendations. http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Well-Woman-Recommendations. Accessed December 4, 2015.
- US Department of Health and Human Services. Affordable Care Act Rules on Expanding Access to Preventive Services for Women. http://www.hhs.gov/healthcare/facts-and-features/fact-sheets/aca-rules-on-expanding-access-to-preventive-services-for-women/index.html. Updated June 28, 2013. Accessed December 4, 2015.
- National Women’s Law Center, Brigham and Women’s Hospital. Your Guide to Well-Woman Visits. http://www.nwlc.org/sites/default/files/final_well-womanbrochure.pdf. Accessed December 8, 2015.
- Conry JA, Brown H. Well-Woman Task Force: Components of the well-woman visit. Obstet Gynecol. 2015;126(4):697–701.
- American College of Obstetricians and Gynecologists. Well-Woman Recommendations. http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Well-Woman-Recommendations. Accessed December 4, 2015.
- US Department of Health and Human Services. Affordable Care Act Rules on Expanding Access to Preventive Services for Women. http://www.hhs.gov/healthcare/facts-and-features/fact-sheets/aca-rules-on-expanding-access-to-preventive-services-for-women/index.html. Updated June 28, 2013. Accessed December 4, 2015.
6 Supreme Court decisions that affected ObGyns in 2015
You might ask, why do I need to know what the Supreme Court does, it will not affect me! Well, we all know that is not exactly true. We chose these 6 cases because of their importance to ObGyns. In a number of them, the American Medical Association (AMA) or specific specialty board filed amicus curiae briefs, which suggested that the profession felt these were especially critical cases. (An amicus brief is a “friend of the Court” brief filed not by one of the affected parties, but by an organization or person with an interest or special expertise in the case.) You can find additional analysis of the 2015 term of the Supreme Court at the website of the National Register (http://www.nationalregister.org/pub/the-national-registerreport-pub/the-register-report-fall-2015/the-aca-survives-and-same-sex-marriage-thrives-the-2014-2015-supreme-court/).
1. Affordable Care Act upheld
King v Burwell was likely the Court’s most important case for physicians and their patients during the 2015 term.
At stake. The question was whether or not people who use the federal Affordable Care Act (ACA) Exchange could receive the same subsidy as those who use the state established Exchanges.1
Final ruling. The Court said “yes,” they could receive the same subsidy, ruling in favor of King.
Key points of the case
The ACA provides for state Exchanges (an electronic marketplace in which people can compare and purchase health insurance policies), but most states did not establish Exchanges. As a result, in many states the federal Exchange became the default. Because the ACA subsidies (which help many people afford mandated insurance coverage) are processed through exchanges, the question of whether those who used the federal Exchange received the subsidy was enormously important. Subsidies for millions of individuals depended on it.
The language of the ACA provides that the insurance subsidy is available only if the person(s) enroll in “an Exchange established by the State” (emphasis added).1 This case was about what “Exchange established by the State” means. A 6-justice majority held that the best interpretation of the statute was that it permitted subsidies through the federal Exchange. This was a difficult decision because, as the majority noted, the ACA is sloppily written. Nonetheless, the Court had to do its best to read the language in the context of the whole statute. Six justices held that the statute meant that the subsidies would cover people who signed up through federal as well as state Exchanges.
The dissenting justices essentially took the position that the language of the statute is clear. Among other things, the dissent said, it means that the words “established by the state” have no meaning at all in the statute, and it is unclear why Congress did not say “Exchange” instead of “Exchange established by the state.”
The results of the case were that the subsidies granted through the federal Exchange will continue. It will not expand the subsidies. Had the decision gone the other way, there would have been a real challenge to the future of the ACA. For that reason a number of medical and health care organizations filed amicus briefs with the Court in this case.
2. State licensing boards and antitrust
At stake. Antitrust laws prohibit combinations and conspiracies in restraint of trade. Competitors cannot come together to seek to set prices, divide the market, or prevent new competitors from entering the market. Since the 1940s, however, the Supreme Court has recognized a “state action” exception to the antitrust laws. The question before the Court this term was whether or not a state licensing board is included in the state-action immunity.2
The AMA and others filed an amicus brief in this case, noting threat to the public health if the Court disrupted state medical boards’ regulation of professional licensing and unauthorized practice.
Final ruling. The Court rejected this argument. It held that, where a state board is “controlled by active market participants” (as most state professional boards are), antitrust immunity is not automatic. For the immunity to protect boards, 2 conditions must exist, the Court held:
1. The state must have articulated a clear policy to allow the regulation that is an anticompetitive conduct (eg, licensing)
2. The state must have provided active supervision of the anticompetitive conduct. This requires that the state appoint someone or some group to approve policies of the board.
The first of these requirements often would be met by the statute setting up the board. The Court focused some attention on the second requirement. It concluded that “the adequacy of supervision otherwise will depend on all the circumstances of a case.”2
Key points of the case
In most states, this decision will require some kind of restructuring so that the professional boards are not the final decision makers but, in effect, only make recommendations to a “supervisor.” The Court gave short shrift to the AMA’s concerns that the decision might make it more difficult to attract really good professionals to the boards. The possibility of personal liability probably can be dealt with, but it deserves attention. The problems with litigation and antitrust claims, and reviews of decisions of a board (potentially by nonprofessionals) hardly can be a plus in attracting the right professionals to the boards.
This case will give rise to considerable litigation for many years. Medical boards should endeavor to get ahead of the issue by immediately studying ways in which the concerns of the Federal Trade Commissioncan be accommodated without significantly reducing the public protection that is part of well-administered professional licensing.
3. State reimbursement for Medicaid services
Medicaid is a federal-state program, and federal law requires, in part, that states must “assure that payments are … sufficient to enlist enough providers so that care and services are available under the plan at least to the extent that such care and services are available to the general population in the geographic area.”3 Providers claimed that the state had failed to establish such a payment system. A number of medical groups, including the AMA, filed amicus briefs in support of the providers.
At stake. State funding for various Medicaid services has been a problem for many health care professionals for some time. But the Medicaid law does not clearly give providers the right to file lawsuits claiming inadequate reimbursement,4 so the question in this case was whether or not there was an implied right to do so.
Final ruling. The Court, in a 5-4 decision, held that Medicaid providers do not have the authority to sue states in federal courts torequire that the states provide higher Medicaid rates for services.4 As a practical matter, this decision leaves with the states broad authority to set Medicaid reimbursement rates. It is possible, of course, that in the future Congress would change the law to grant such rights or more clearly set reimbursement rates.
4. False Claims Act cases
Unfortunately, False Claims Act cases occur in health care. False claims transpire when someone (or an organization) presents to the government a claim for payment that is not legitimate or is for inadequate or low quality services. False claims include everything from fraudulent billing for services never performed to a pharmaceutical company’s promotion of a drug for off-label use.
The federal False Claims Act incentivizes private whistleblowers (often disgruntled employees) to initiate false claims lawsuits. (The government may then choose to take over the false claims or allow the private whistleblower to pursue them.)
At stake. Because Medicare and Medicaid are federally financed programs, it is common for providers who participate in those programs to be subject to false claims.5 This term the Court heard an important False Claims Act case involving the statute of limitations and multiple claims based on the same activity. The AMA joined an amicus brief urging the Court to prohibit both kinds of expansion.
Final ruling. The Court agreed that the statute of limitations for these claims should not be extended, but it did determine that the “first-to-file” limitation in the statute “keeps new claims out of court only while related claims are still alive, not in perpetuity.”5 The result of this second holding is that the “firstto-file” rule does not preclude another false claims suit that is duplicative to be filed as soon as the prior suit is no longer pending.
It is not clear that the practical effect of the decision will be great, but it may in some cases open up clinicians to multiple, serial lawsuits over the same claims.
5. Pregnancy and employment discrimination
The federal Pregnancy Discrimination Act (PDA) prohibits discrimination “because of or on the basis of pregnancy, childbirth, or related medical conditions.” It also requires that employers treat “women affected by pregnancy . . . the same for all employment-related purposes . . . as other persons not so affected but similar in their ability or inability to work.”
At stake. United Parcel Service (UPS) declined to give a pregnant employee a “light duty” accommodation during pregnancy so that she could avoid having to lift heavy packages. But it had given other employees just that kind of accommodation when they were injured or lost certification to drive a delivery truck. The suit claimed violation of the PDA.6
Final ruling. The Court held that UPS violated the PDA by giving some employees this accommodation but refusing it to pregnant workers who requested it.6 Once an organization voluntarily grants a particular accommodation to, for example, a temporarily injured worker, it may be required to provide similar accommodation to pregnant workers. The case probably will increase employment protection for pregnant women.
6. Children “testifying” in abuse cases
All states require physicians and teachers, among others, to report suspected child abuse. An ongoing question has been whether those reporters may be called to testify about what the child said at the time of abuse discovery/suspicion. This case involved a teacher, but it could as easily have been a physician.7
Key points of the case. Teachers found suspicious injuries on 3-year-old L.P. The child gave conflicting statements about what happened, but claimed that Clark had hurt him. At Clark’s criminal trial, L.P. did not testify, but the state wanted to introduce L.P.’s statements to the teachers as evidence of Clark’s guilt.
At stake. The question was whether or not this introduction would violate the right of Clark to “confront his accuser” as guaranteed by the Constitution.
Final ruling. All 9 justices said it was proper to allow L.P.’s statements to be introduced at trial.7 Essentially this was permitted because the primary purpose of L.P.’s statements was not to gather testimony to be presented at trial.
The final opinion creates incentives for some prosecutors to make abuse victims unavailable in order to allow their testimony by hearsay. Aside from the legal technicalities, the likelihood of wrongful convictions under such a system must be considered for the long run.
Other major 2015 Supreme Court decisions
- The Constitution requires states to recognize same-sex marriages and also accept such marriages performed in other states.1 (The American Medical Association joined an amicus brief in this case.)
- The Court turned down the appeal of several inmates who had received lethal injection death sentences. They challenged the mix of drugs that Oklahoma planned to use to execute them.2
- In 2 cases justices went out of the way to raise questions about the constitutionality of the death penalty. This may suggest that the Court will take up this issue in the near future.2,3
- Federal housing discrimination laws were expanded to cases in which there is “disparate impact” discrimination.4
- The Court narrowed prosecution for “threatening” statements in social network/Internet communications by holding that negligence in the communication is not sufficient for conviction under federal law.5
- The Court held that the Environmental Protection Agency violated the Clean Air Act with its power plant emission regulations because it failed to do a proper cost-benefit analysis.6
References
1. Obergefell et al v Hodges, Director, Ohio Department of Health et al, No. 14–556 (2015). http://www.supremecourt.gov/opinions/14pdf/14-556_3204.pdf. Accessed December 14, 2015.
2. Glossip et al v Gross et al, No. 14–7955 (2015). http://www.supremecourt.gov/opinions/14pdf/14-7955_aplc.pdf. Accessed December 14, 2015.
3. Davis, Acting Worden v Ayala, No. 13–1428 (2015). http://www.supremecourt.gov/opinions/14pdf/13-1428_1a7d.pdf. Accessed December 14, 2015.
4. Texas Department of Housing and Community Affairs et al v Inclusive Communities Project, Inc et al, No. 13–1371 (2015). http://www.supremecourt.gov/opinions/14pdf/13-1371_8m58.pdf. Accessed December 14, 2015.
5. Elonis v United States, No. 13–983 (2015). http://www.supremecourt.gov/opinions/14pdf/13-983_7148.pdf. Accessed December 14, 2015.
6. Michigan et al v Environmental Protection Agency et al, No. 14–46 (2015). http://www.supremecourt.gov/opinions/14pdf/14-46_bqmc.pdf. Accessed December 14, 2015.
What’s coming in 2016
This term of the Supreme Court is a reminder of how important the decisions of the Court are to the work of physicians and ObGyns in particular. This trend is continuing—the Court already has, for the next term, accepted cases regarding abortion, contraception, and health care delivery. It will, therefore, be worthwhile to follow the developments at the nation’s highest court.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. King et al v Burwell, Secretary of Health and Human Services et al, No. 14–114 (2015). http://www.supremecourt.gov/opinions/14pdf/14-114_qol1.pdf. Accessed December 14, 2015.
2. North Carolina State Board of Dental Examiners v Federal Trade Commission, No. 13–534 (2015). http://www.supremecourt.gov/opinions/14pd/13-534_19m2.pdf. Accessed December 14, 2015.
3. Federal Policy Guidance. Medicaid.gov website. http://medicaid.gov/federal-policy-guidance/federal-policyguidance.html. Accessed December 14, 2015.
4. Armstrong et al v Exceptional Child Center, Inc et al, No. 14–15 (2015). http://www.supremecourt.gov/opinions/14pdf/14-15_d1oe.pdf. Accessed December 14, 2015.
5. Kellogg Brown & Root Services, Inc et al v United States ex rel. Carter, No 12–1497 (2015). http://www.supremecourt.gov/opinions/14pdf/12-1497_2d8f.pdf. Accessed December 14, 2015.
6. Young v United Parcel Service, Inc., No. 12–1226 (2015). http://www.supremecourt.gov/opinions/14pdf/12-1226_k5fl.pdf. Accessed December 14, 2015.
7. Ohio v Clark, No. 13–1352 (2015). http://www.supremecourt.gov/opinion/14pdf/13-1352_ed9l.pdf. Accessed December 14, 2015.
You might ask, why do I need to know what the Supreme Court does, it will not affect me! Well, we all know that is not exactly true. We chose these 6 cases because of their importance to ObGyns. In a number of them, the American Medical Association (AMA) or specific specialty board filed amicus curiae briefs, which suggested that the profession felt these were especially critical cases. (An amicus brief is a “friend of the Court” brief filed not by one of the affected parties, but by an organization or person with an interest or special expertise in the case.) You can find additional analysis of the 2015 term of the Supreme Court at the website of the National Register (http://www.nationalregister.org/pub/the-national-registerreport-pub/the-register-report-fall-2015/the-aca-survives-and-same-sex-marriage-thrives-the-2014-2015-supreme-court/).
1. Affordable Care Act upheld
King v Burwell was likely the Court’s most important case for physicians and their patients during the 2015 term.
At stake. The question was whether or not people who use the federal Affordable Care Act (ACA) Exchange could receive the same subsidy as those who use the state established Exchanges.1
Final ruling. The Court said “yes,” they could receive the same subsidy, ruling in favor of King.
Key points of the case
The ACA provides for state Exchanges (an electronic marketplace in which people can compare and purchase health insurance policies), but most states did not establish Exchanges. As a result, in many states the federal Exchange became the default. Because the ACA subsidies (which help many people afford mandated insurance coverage) are processed through exchanges, the question of whether those who used the federal Exchange received the subsidy was enormously important. Subsidies for millions of individuals depended on it.
The language of the ACA provides that the insurance subsidy is available only if the person(s) enroll in “an Exchange established by the State” (emphasis added).1 This case was about what “Exchange established by the State” means. A 6-justice majority held that the best interpretation of the statute was that it permitted subsidies through the federal Exchange. This was a difficult decision because, as the majority noted, the ACA is sloppily written. Nonetheless, the Court had to do its best to read the language in the context of the whole statute. Six justices held that the statute meant that the subsidies would cover people who signed up through federal as well as state Exchanges.
The dissenting justices essentially took the position that the language of the statute is clear. Among other things, the dissent said, it means that the words “established by the state” have no meaning at all in the statute, and it is unclear why Congress did not say “Exchange” instead of “Exchange established by the state.”
The results of the case were that the subsidies granted through the federal Exchange will continue. It will not expand the subsidies. Had the decision gone the other way, there would have been a real challenge to the future of the ACA. For that reason a number of medical and health care organizations filed amicus briefs with the Court in this case.
2. State licensing boards and antitrust
At stake. Antitrust laws prohibit combinations and conspiracies in restraint of trade. Competitors cannot come together to seek to set prices, divide the market, or prevent new competitors from entering the market. Since the 1940s, however, the Supreme Court has recognized a “state action” exception to the antitrust laws. The question before the Court this term was whether or not a state licensing board is included in the state-action immunity.2
The AMA and others filed an amicus brief in this case, noting threat to the public health if the Court disrupted state medical boards’ regulation of professional licensing and unauthorized practice.
Final ruling. The Court rejected this argument. It held that, where a state board is “controlled by active market participants” (as most state professional boards are), antitrust immunity is not automatic. For the immunity to protect boards, 2 conditions must exist, the Court held:
1. The state must have articulated a clear policy to allow the regulation that is an anticompetitive conduct (eg, licensing)
2. The state must have provided active supervision of the anticompetitive conduct. This requires that the state appoint someone or some group to approve policies of the board.
The first of these requirements often would be met by the statute setting up the board. The Court focused some attention on the second requirement. It concluded that “the adequacy of supervision otherwise will depend on all the circumstances of a case.”2
Key points of the case
In most states, this decision will require some kind of restructuring so that the professional boards are not the final decision makers but, in effect, only make recommendations to a “supervisor.” The Court gave short shrift to the AMA’s concerns that the decision might make it more difficult to attract really good professionals to the boards. The possibility of personal liability probably can be dealt with, but it deserves attention. The problems with litigation and antitrust claims, and reviews of decisions of a board (potentially by nonprofessionals) hardly can be a plus in attracting the right professionals to the boards.
This case will give rise to considerable litigation for many years. Medical boards should endeavor to get ahead of the issue by immediately studying ways in which the concerns of the Federal Trade Commissioncan be accommodated without significantly reducing the public protection that is part of well-administered professional licensing.
3. State reimbursement for Medicaid services
Medicaid is a federal-state program, and federal law requires, in part, that states must “assure that payments are … sufficient to enlist enough providers so that care and services are available under the plan at least to the extent that such care and services are available to the general population in the geographic area.”3 Providers claimed that the state had failed to establish such a payment system. A number of medical groups, including the AMA, filed amicus briefs in support of the providers.
At stake. State funding for various Medicaid services has been a problem for many health care professionals for some time. But the Medicaid law does not clearly give providers the right to file lawsuits claiming inadequate reimbursement,4 so the question in this case was whether or not there was an implied right to do so.
Final ruling. The Court, in a 5-4 decision, held that Medicaid providers do not have the authority to sue states in federal courts torequire that the states provide higher Medicaid rates for services.4 As a practical matter, this decision leaves with the states broad authority to set Medicaid reimbursement rates. It is possible, of course, that in the future Congress would change the law to grant such rights or more clearly set reimbursement rates.
4. False Claims Act cases
Unfortunately, False Claims Act cases occur in health care. False claims transpire when someone (or an organization) presents to the government a claim for payment that is not legitimate or is for inadequate or low quality services. False claims include everything from fraudulent billing for services never performed to a pharmaceutical company’s promotion of a drug for off-label use.
The federal False Claims Act incentivizes private whistleblowers (often disgruntled employees) to initiate false claims lawsuits. (The government may then choose to take over the false claims or allow the private whistleblower to pursue them.)
At stake. Because Medicare and Medicaid are federally financed programs, it is common for providers who participate in those programs to be subject to false claims.5 This term the Court heard an important False Claims Act case involving the statute of limitations and multiple claims based on the same activity. The AMA joined an amicus brief urging the Court to prohibit both kinds of expansion.
Final ruling. The Court agreed that the statute of limitations for these claims should not be extended, but it did determine that the “first-to-file” limitation in the statute “keeps new claims out of court only while related claims are still alive, not in perpetuity.”5 The result of this second holding is that the “firstto-file” rule does not preclude another false claims suit that is duplicative to be filed as soon as the prior suit is no longer pending.
It is not clear that the practical effect of the decision will be great, but it may in some cases open up clinicians to multiple, serial lawsuits over the same claims.
5. Pregnancy and employment discrimination
The federal Pregnancy Discrimination Act (PDA) prohibits discrimination “because of or on the basis of pregnancy, childbirth, or related medical conditions.” It also requires that employers treat “women affected by pregnancy . . . the same for all employment-related purposes . . . as other persons not so affected but similar in their ability or inability to work.”
At stake. United Parcel Service (UPS) declined to give a pregnant employee a “light duty” accommodation during pregnancy so that she could avoid having to lift heavy packages. But it had given other employees just that kind of accommodation when they were injured or lost certification to drive a delivery truck. The suit claimed violation of the PDA.6
Final ruling. The Court held that UPS violated the PDA by giving some employees this accommodation but refusing it to pregnant workers who requested it.6 Once an organization voluntarily grants a particular accommodation to, for example, a temporarily injured worker, it may be required to provide similar accommodation to pregnant workers. The case probably will increase employment protection for pregnant women.
6. Children “testifying” in abuse cases
All states require physicians and teachers, among others, to report suspected child abuse. An ongoing question has been whether those reporters may be called to testify about what the child said at the time of abuse discovery/suspicion. This case involved a teacher, but it could as easily have been a physician.7
Key points of the case. Teachers found suspicious injuries on 3-year-old L.P. The child gave conflicting statements about what happened, but claimed that Clark had hurt him. At Clark’s criminal trial, L.P. did not testify, but the state wanted to introduce L.P.’s statements to the teachers as evidence of Clark’s guilt.
At stake. The question was whether or not this introduction would violate the right of Clark to “confront his accuser” as guaranteed by the Constitution.
Final ruling. All 9 justices said it was proper to allow L.P.’s statements to be introduced at trial.7 Essentially this was permitted because the primary purpose of L.P.’s statements was not to gather testimony to be presented at trial.
The final opinion creates incentives for some prosecutors to make abuse victims unavailable in order to allow their testimony by hearsay. Aside from the legal technicalities, the likelihood of wrongful convictions under such a system must be considered for the long run.
Other major 2015 Supreme Court decisions
- The Constitution requires states to recognize same-sex marriages and also accept such marriages performed in other states.1 (The American Medical Association joined an amicus brief in this case.)
- The Court turned down the appeal of several inmates who had received lethal injection death sentences. They challenged the mix of drugs that Oklahoma planned to use to execute them.2
- In 2 cases justices went out of the way to raise questions about the constitutionality of the death penalty. This may suggest that the Court will take up this issue in the near future.2,3
- Federal housing discrimination laws were expanded to cases in which there is “disparate impact” discrimination.4
- The Court narrowed prosecution for “threatening” statements in social network/Internet communications by holding that negligence in the communication is not sufficient for conviction under federal law.5
- The Court held that the Environmental Protection Agency violated the Clean Air Act with its power plant emission regulations because it failed to do a proper cost-benefit analysis.6
References
1. Obergefell et al v Hodges, Director, Ohio Department of Health et al, No. 14–556 (2015). http://www.supremecourt.gov/opinions/14pdf/14-556_3204.pdf. Accessed December 14, 2015.
2. Glossip et al v Gross et al, No. 14–7955 (2015). http://www.supremecourt.gov/opinions/14pdf/14-7955_aplc.pdf. Accessed December 14, 2015.
3. Davis, Acting Worden v Ayala, No. 13–1428 (2015). http://www.supremecourt.gov/opinions/14pdf/13-1428_1a7d.pdf. Accessed December 14, 2015.
4. Texas Department of Housing and Community Affairs et al v Inclusive Communities Project, Inc et al, No. 13–1371 (2015). http://www.supremecourt.gov/opinions/14pdf/13-1371_8m58.pdf. Accessed December 14, 2015.
5. Elonis v United States, No. 13–983 (2015). http://www.supremecourt.gov/opinions/14pdf/13-983_7148.pdf. Accessed December 14, 2015.
6. Michigan et al v Environmental Protection Agency et al, No. 14–46 (2015). http://www.supremecourt.gov/opinions/14pdf/14-46_bqmc.pdf. Accessed December 14, 2015.
What’s coming in 2016
This term of the Supreme Court is a reminder of how important the decisions of the Court are to the work of physicians and ObGyns in particular. This trend is continuing—the Court already has, for the next term, accepted cases regarding abortion, contraception, and health care delivery. It will, therefore, be worthwhile to follow the developments at the nation’s highest court.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
You might ask, why do I need to know what the Supreme Court does, it will not affect me! Well, we all know that is not exactly true. We chose these 6 cases because of their importance to ObGyns. In a number of them, the American Medical Association (AMA) or specific specialty board filed amicus curiae briefs, which suggested that the profession felt these were especially critical cases. (An amicus brief is a “friend of the Court” brief filed not by one of the affected parties, but by an organization or person with an interest or special expertise in the case.) You can find additional analysis of the 2015 term of the Supreme Court at the website of the National Register (http://www.nationalregister.org/pub/the-national-registerreport-pub/the-register-report-fall-2015/the-aca-survives-and-same-sex-marriage-thrives-the-2014-2015-supreme-court/).
1. Affordable Care Act upheld
King v Burwell was likely the Court’s most important case for physicians and their patients during the 2015 term.
At stake. The question was whether or not people who use the federal Affordable Care Act (ACA) Exchange could receive the same subsidy as those who use the state established Exchanges.1
Final ruling. The Court said “yes,” they could receive the same subsidy, ruling in favor of King.
Key points of the case
The ACA provides for state Exchanges (an electronic marketplace in which people can compare and purchase health insurance policies), but most states did not establish Exchanges. As a result, in many states the federal Exchange became the default. Because the ACA subsidies (which help many people afford mandated insurance coverage) are processed through exchanges, the question of whether those who used the federal Exchange received the subsidy was enormously important. Subsidies for millions of individuals depended on it.
The language of the ACA provides that the insurance subsidy is available only if the person(s) enroll in “an Exchange established by the State” (emphasis added).1 This case was about what “Exchange established by the State” means. A 6-justice majority held that the best interpretation of the statute was that it permitted subsidies through the federal Exchange. This was a difficult decision because, as the majority noted, the ACA is sloppily written. Nonetheless, the Court had to do its best to read the language in the context of the whole statute. Six justices held that the statute meant that the subsidies would cover people who signed up through federal as well as state Exchanges.
The dissenting justices essentially took the position that the language of the statute is clear. Among other things, the dissent said, it means that the words “established by the state” have no meaning at all in the statute, and it is unclear why Congress did not say “Exchange” instead of “Exchange established by the state.”
The results of the case were that the subsidies granted through the federal Exchange will continue. It will not expand the subsidies. Had the decision gone the other way, there would have been a real challenge to the future of the ACA. For that reason a number of medical and health care organizations filed amicus briefs with the Court in this case.
2. State licensing boards and antitrust
At stake. Antitrust laws prohibit combinations and conspiracies in restraint of trade. Competitors cannot come together to seek to set prices, divide the market, or prevent new competitors from entering the market. Since the 1940s, however, the Supreme Court has recognized a “state action” exception to the antitrust laws. The question before the Court this term was whether or not a state licensing board is included in the state-action immunity.2
The AMA and others filed an amicus brief in this case, noting threat to the public health if the Court disrupted state medical boards’ regulation of professional licensing and unauthorized practice.
Final ruling. The Court rejected this argument. It held that, where a state board is “controlled by active market participants” (as most state professional boards are), antitrust immunity is not automatic. For the immunity to protect boards, 2 conditions must exist, the Court held:
1. The state must have articulated a clear policy to allow the regulation that is an anticompetitive conduct (eg, licensing)
2. The state must have provided active supervision of the anticompetitive conduct. This requires that the state appoint someone or some group to approve policies of the board.
The first of these requirements often would be met by the statute setting up the board. The Court focused some attention on the second requirement. It concluded that “the adequacy of supervision otherwise will depend on all the circumstances of a case.”2
Key points of the case
In most states, this decision will require some kind of restructuring so that the professional boards are not the final decision makers but, in effect, only make recommendations to a “supervisor.” The Court gave short shrift to the AMA’s concerns that the decision might make it more difficult to attract really good professionals to the boards. The possibility of personal liability probably can be dealt with, but it deserves attention. The problems with litigation and antitrust claims, and reviews of decisions of a board (potentially by nonprofessionals) hardly can be a plus in attracting the right professionals to the boards.
This case will give rise to considerable litigation for many years. Medical boards should endeavor to get ahead of the issue by immediately studying ways in which the concerns of the Federal Trade Commissioncan be accommodated without significantly reducing the public protection that is part of well-administered professional licensing.
3. State reimbursement for Medicaid services
Medicaid is a federal-state program, and federal law requires, in part, that states must “assure that payments are … sufficient to enlist enough providers so that care and services are available under the plan at least to the extent that such care and services are available to the general population in the geographic area.”3 Providers claimed that the state had failed to establish such a payment system. A number of medical groups, including the AMA, filed amicus briefs in support of the providers.
At stake. State funding for various Medicaid services has been a problem for many health care professionals for some time. But the Medicaid law does not clearly give providers the right to file lawsuits claiming inadequate reimbursement,4 so the question in this case was whether or not there was an implied right to do so.
Final ruling. The Court, in a 5-4 decision, held that Medicaid providers do not have the authority to sue states in federal courts torequire that the states provide higher Medicaid rates for services.4 As a practical matter, this decision leaves with the states broad authority to set Medicaid reimbursement rates. It is possible, of course, that in the future Congress would change the law to grant such rights or more clearly set reimbursement rates.
4. False Claims Act cases
Unfortunately, False Claims Act cases occur in health care. False claims transpire when someone (or an organization) presents to the government a claim for payment that is not legitimate or is for inadequate or low quality services. False claims include everything from fraudulent billing for services never performed to a pharmaceutical company’s promotion of a drug for off-label use.
The federal False Claims Act incentivizes private whistleblowers (often disgruntled employees) to initiate false claims lawsuits. (The government may then choose to take over the false claims or allow the private whistleblower to pursue them.)
At stake. Because Medicare and Medicaid are federally financed programs, it is common for providers who participate in those programs to be subject to false claims.5 This term the Court heard an important False Claims Act case involving the statute of limitations and multiple claims based on the same activity. The AMA joined an amicus brief urging the Court to prohibit both kinds of expansion.
Final ruling. The Court agreed that the statute of limitations for these claims should not be extended, but it did determine that the “first-to-file” limitation in the statute “keeps new claims out of court only while related claims are still alive, not in perpetuity.”5 The result of this second holding is that the “firstto-file” rule does not preclude another false claims suit that is duplicative to be filed as soon as the prior suit is no longer pending.
It is not clear that the practical effect of the decision will be great, but it may in some cases open up clinicians to multiple, serial lawsuits over the same claims.
5. Pregnancy and employment discrimination
The federal Pregnancy Discrimination Act (PDA) prohibits discrimination “because of or on the basis of pregnancy, childbirth, or related medical conditions.” It also requires that employers treat “women affected by pregnancy . . . the same for all employment-related purposes . . . as other persons not so affected but similar in their ability or inability to work.”
At stake. United Parcel Service (UPS) declined to give a pregnant employee a “light duty” accommodation during pregnancy so that she could avoid having to lift heavy packages. But it had given other employees just that kind of accommodation when they were injured or lost certification to drive a delivery truck. The suit claimed violation of the PDA.6
Final ruling. The Court held that UPS violated the PDA by giving some employees this accommodation but refusing it to pregnant workers who requested it.6 Once an organization voluntarily grants a particular accommodation to, for example, a temporarily injured worker, it may be required to provide similar accommodation to pregnant workers. The case probably will increase employment protection for pregnant women.
6. Children “testifying” in abuse cases
All states require physicians and teachers, among others, to report suspected child abuse. An ongoing question has been whether those reporters may be called to testify about what the child said at the time of abuse discovery/suspicion. This case involved a teacher, but it could as easily have been a physician.7
Key points of the case. Teachers found suspicious injuries on 3-year-old L.P. The child gave conflicting statements about what happened, but claimed that Clark had hurt him. At Clark’s criminal trial, L.P. did not testify, but the state wanted to introduce L.P.’s statements to the teachers as evidence of Clark’s guilt.
At stake. The question was whether or not this introduction would violate the right of Clark to “confront his accuser” as guaranteed by the Constitution.
Final ruling. All 9 justices said it was proper to allow L.P.’s statements to be introduced at trial.7 Essentially this was permitted because the primary purpose of L.P.’s statements was not to gather testimony to be presented at trial.
The final opinion creates incentives for some prosecutors to make abuse victims unavailable in order to allow their testimony by hearsay. Aside from the legal technicalities, the likelihood of wrongful convictions under such a system must be considered for the long run.
Other major 2015 Supreme Court decisions
- The Constitution requires states to recognize same-sex marriages and also accept such marriages performed in other states.1 (The American Medical Association joined an amicus brief in this case.)
- The Court turned down the appeal of several inmates who had received lethal injection death sentences. They challenged the mix of drugs that Oklahoma planned to use to execute them.2
- In 2 cases justices went out of the way to raise questions about the constitutionality of the death penalty. This may suggest that the Court will take up this issue in the near future.2,3
- Federal housing discrimination laws were expanded to cases in which there is “disparate impact” discrimination.4
- The Court narrowed prosecution for “threatening” statements in social network/Internet communications by holding that negligence in the communication is not sufficient for conviction under federal law.5
- The Court held that the Environmental Protection Agency violated the Clean Air Act with its power plant emission regulations because it failed to do a proper cost-benefit analysis.6
References
1. Obergefell et al v Hodges, Director, Ohio Department of Health et al, No. 14–556 (2015). http://www.supremecourt.gov/opinions/14pdf/14-556_3204.pdf. Accessed December 14, 2015.
2. Glossip et al v Gross et al, No. 14–7955 (2015). http://www.supremecourt.gov/opinions/14pdf/14-7955_aplc.pdf. Accessed December 14, 2015.
3. Davis, Acting Worden v Ayala, No. 13–1428 (2015). http://www.supremecourt.gov/opinions/14pdf/13-1428_1a7d.pdf. Accessed December 14, 2015.
4. Texas Department of Housing and Community Affairs et al v Inclusive Communities Project, Inc et al, No. 13–1371 (2015). http://www.supremecourt.gov/opinions/14pdf/13-1371_8m58.pdf. Accessed December 14, 2015.
5. Elonis v United States, No. 13–983 (2015). http://www.supremecourt.gov/opinions/14pdf/13-983_7148.pdf. Accessed December 14, 2015.
6. Michigan et al v Environmental Protection Agency et al, No. 14–46 (2015). http://www.supremecourt.gov/opinions/14pdf/14-46_bqmc.pdf. Accessed December 14, 2015.
What’s coming in 2016
This term of the Supreme Court is a reminder of how important the decisions of the Court are to the work of physicians and ObGyns in particular. This trend is continuing—the Court already has, for the next term, accepted cases regarding abortion, contraception, and health care delivery. It will, therefore, be worthwhile to follow the developments at the nation’s highest court.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. King et al v Burwell, Secretary of Health and Human Services et al, No. 14–114 (2015). http://www.supremecourt.gov/opinions/14pdf/14-114_qol1.pdf. Accessed December 14, 2015.
2. North Carolina State Board of Dental Examiners v Federal Trade Commission, No. 13–534 (2015). http://www.supremecourt.gov/opinions/14pd/13-534_19m2.pdf. Accessed December 14, 2015.
3. Federal Policy Guidance. Medicaid.gov website. http://medicaid.gov/federal-policy-guidance/federal-policyguidance.html. Accessed December 14, 2015.
4. Armstrong et al v Exceptional Child Center, Inc et al, No. 14–15 (2015). http://www.supremecourt.gov/opinions/14pdf/14-15_d1oe.pdf. Accessed December 14, 2015.
5. Kellogg Brown & Root Services, Inc et al v United States ex rel. Carter, No 12–1497 (2015). http://www.supremecourt.gov/opinions/14pdf/12-1497_2d8f.pdf. Accessed December 14, 2015.
6. Young v United Parcel Service, Inc., No. 12–1226 (2015). http://www.supremecourt.gov/opinions/14pdf/12-1226_k5fl.pdf. Accessed December 14, 2015.
7. Ohio v Clark, No. 13–1352 (2015). http://www.supremecourt.gov/opinion/14pdf/13-1352_ed9l.pdf. Accessed December 14, 2015.
1. King et al v Burwell, Secretary of Health and Human Services et al, No. 14–114 (2015). http://www.supremecourt.gov/opinions/14pdf/14-114_qol1.pdf. Accessed December 14, 2015.
2. North Carolina State Board of Dental Examiners v Federal Trade Commission, No. 13–534 (2015). http://www.supremecourt.gov/opinions/14pd/13-534_19m2.pdf. Accessed December 14, 2015.
3. Federal Policy Guidance. Medicaid.gov website. http://medicaid.gov/federal-policy-guidance/federal-policyguidance.html. Accessed December 14, 2015.
4. Armstrong et al v Exceptional Child Center, Inc et al, No. 14–15 (2015). http://www.supremecourt.gov/opinions/14pdf/14-15_d1oe.pdf. Accessed December 14, 2015.
5. Kellogg Brown & Root Services, Inc et al v United States ex rel. Carter, No 12–1497 (2015). http://www.supremecourt.gov/opinions/14pdf/12-1497_2d8f.pdf. Accessed December 14, 2015.
6. Young v United Parcel Service, Inc., No. 12–1226 (2015). http://www.supremecourt.gov/opinions/14pdf/12-1226_k5fl.pdf. Accessed December 14, 2015.
7. Ohio v Clark, No. 13–1352 (2015). http://www.supremecourt.gov/opinion/14pdf/13-1352_ed9l.pdf. Accessed December 14, 2015.
Should you adopt the practice of vaginal cleansing with povidone-iodine prior to cesarean delivery?
There are approximately 4,000,000 births annually in the United States, and about 32% of them occur by cesarean delivery. Compared with vaginal birth, cesarean delivery is associated with an increased risk of endometritis (defined as fever plus uterine or abdominal tenderness). Although surgical complications cannot be eliminated entirely, surgeons are deeply dedicated to the continuous improvement of surgical practice in order to reduce the risk of complications.
With cesarean delivery, many surgical practices have been adopted universally to reduce postoperative complications, including administration of intravenous (IV) antibiotics before skin incision to minimize postoperative infection and the use of postoperative mechanical or pharmacologic interventions to help prevent venous thromboembolism and pulmonary embolism. Preoperative vaginal cleansing with povidone-iodine may reduce the risk of postoperative endometritis, but the practice is not currently common in the United States.
Should you adopt a policy of preoperative vaginal cleansing prior to cesarean delivery? The data suggest perhaps you should.
Data-driven support for povidone-iodine precesareanThree large randomized trials published within the past 10 years concluded that preoperative vaginal cleansing with povidone-iodine reduced the risk of postcesarean endometritis in women who also received prophylactic IV antibiotics (TABLE).1−3 Vaginal cleansing did not reduce the rate of postpartum fever or wound infection in these studies.
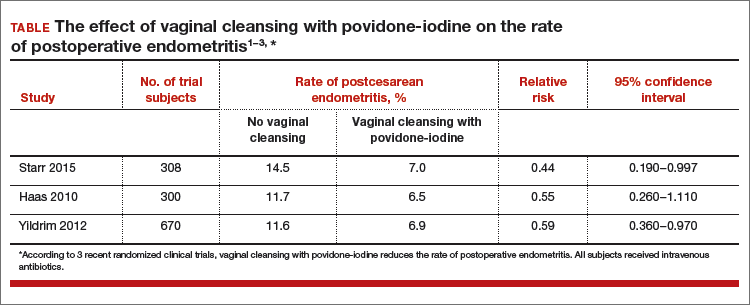
Clinical factors that increased the risk of postpartum endometritis independent of vaginal cleansing included:
- extended duration of cesarean surgery
- being in labor prior to cesarean delivery
- ruptured membranes
- advanced cervical examination
- maternal anemia
- use of intrapartum internal monitors
- prior history of genitourinary infection.
Authors of two recent, large nonrandomized studies also have reported that vaginal cleansing reduced the risk of postcesarean endometritis.4,5 By contrast, investigators from one large trial from 2001 did not observe a decrease in endometritis with vaginal cleansing.6
To test the impact of metronidazole vaginal gel on post‑cesarean endometritis, 224 women undergoing cesarean delivery for various indications were randomly assigned to placebo vaginal gel or metronidazole vaginal gel 5 g prior to surgery initiation.1 Most women also received intravenous antibiotics. The rates of endometritis were 17% and 7% in the placebo and metronidazole groups, respectively (relative risk, 0.42; 95% confidence interval, 0.19−0.92).
Vaginal antibiotic administration shows promise as an alternative to povidone-iodine cleansing in the prevention of postcesarean endometritis. Additional randomized clinical trials are necessary to fully evaluate the benefits and risks of this practice.
Reference
1. Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745−750.
Cochrane review of precesarean vaginal cleansingAuthors of a Cochrane review, in which they synthesized 7 studies involving 2,635 women, reported that vaginal cleansing with povidone-iodine immediately before cesarean delivery was associated with a reduced risk of postcesarean endometritis: 8.3% vs 4.3% in the control and vaginal cleansing groups, respectively, (risk ratio [RR], 0.45; 95% confidence interval [CI], 0.25−0.81).7
The positive effect of vaginal cleansing was particularly noteworthy in the subgroup of women with ruptured membranes (3 trials involving 272 women). The rates of endometritis in the control versus vaginal cleansing groups were 17.9% and 4.3%, respectively (RR, 0.24; 95% CI, 0.10−0.55).
Women who went into labor prior to cesarean delivery (523 women from 3 trials) also benefitted from vaginal cleansing, with endometritis rates of 13.0% and 7.4% in the control and vaginal cleansing groups, respectively (RR, 0.56; 95% CI, 0.34−0.95).
In this review, again, vaginal cleansing did not significantly reduce the rate of postoperative fever or wound infection.
The American College of Obstetricians and Gynecologists has noted that chlorohexidine gluconate solutions with high concentrations of alcohol are contraindicated for vaginal cleansing.1 However, although not approved for vaginal cleansing, solutions of chlorohexidine gluconate with low alcohol content (4% alcohol concentration) are safe and may be effective for off-label use as vaginal cleansings.
Reference
1. American College of Obstetricians and Gynecologists Women’s Health Care Physicians; Committee on Gynecologic Practice. Committee Opinion No. 571: solutions for surgical cleansing of the vagina. Obstet Gynecol. 2013;122(3):718−720.
Is vaginal cleansing prior to cesarean delivery best practice?In the United States, precesarean vaginal cleansing is not a common practice. To close the gap between current practice and what is potentially a best practice, two approaches to using vaginal cleansing could be instituted in delivery units.
Approach #1: A liberal clinical protocol. In this scenario, all women (who are not allergic to iodine or povidone-iodine) undergoing cesarean delivery should undergo vaginal cleansing. The World Health Organization conditionally recommends vaginal cleansing for all women undergoing a cesarean delivery.8
Approach #2: A focused clinical protocol. For this protocol, only women (again, who are not allergic to iodine or povidone-iodine) who have ruptured membranes or are in labor upon advanced cervical examination should receive vaginal cleansing.
The advantage of a liberal protocol is that vaginal preparation becomes embedded within the standard practice of cesarean delivery and, hence, is seldom overlooked. The upside of the focused protocol is that only those women most likely to benefit receive the intervention.
Tell me what you thinkWill you consider using vaginal cleansing in your practice? Please let me know your views on vaginal cleansing for cesarean delivery, as well as your clinical pearls on cesarean delivery surgery, at obgmanagement.com. In addition, weigh in on the Quick Poll posted to OBG Management’s homepage. Send your letter to the editor to [email protected].
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2015;105(5 pt 1):1024–1029.
- Haas DM, Pazouki F, Smith RR, et al. Vaginal cleansing before cesarean delivery to reduce postoperative infectious morbidity: a randomized, controlled trial. Am J Obstet Gynecol. 2010;202(3):310.e1–e6.
- Yildrim G, Gungorduk K, Asicioglu O, et al. Does vaginal preparation with povidone-iodine prior to cesarean delivery reduce the risk of endometritis?A randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25(11):2316–2321.
- Asghania M, Mirblouk F, Shakiba M, Faraji R. Preoperative vaginal preparation with povidone-iodine on post-cesarean infectious morbidity. J Obstet Gynaecol. 2011;31(5):400–403.
- Memon S, Qazi RA, Bibi S, Parveen N. Effect of preoperative vaginal cleansing with an antiseptic solution to reduce post caesarean infectious morbidity. J Pak Med Assoc. 2011;61(12):1179–1183.
- Reid VC, Hartmann KE, McMahon M, Fry EP. Vaginal preparation with povidone-iodine and postcesarean infectious morbidity: a randomized controlled trial. Obstet Gynecol. 2001;97(1):147–152.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2014;12:CD007892.
- Yildrim G, Gungorduk K, Asicioglu O, et al. Does vaginal preparation with povidone-iodine prior to
There are approximately 4,000,000 births annually in the United States, and about 32% of them occur by cesarean delivery. Compared with vaginal birth, cesarean delivery is associated with an increased risk of endometritis (defined as fever plus uterine or abdominal tenderness). Although surgical complications cannot be eliminated entirely, surgeons are deeply dedicated to the continuous improvement of surgical practice in order to reduce the risk of complications.
With cesarean delivery, many surgical practices have been adopted universally to reduce postoperative complications, including administration of intravenous (IV) antibiotics before skin incision to minimize postoperative infection and the use of postoperative mechanical or pharmacologic interventions to help prevent venous thromboembolism and pulmonary embolism. Preoperative vaginal cleansing with povidone-iodine may reduce the risk of postoperative endometritis, but the practice is not currently common in the United States.
Should you adopt a policy of preoperative vaginal cleansing prior to cesarean delivery? The data suggest perhaps you should.
Data-driven support for povidone-iodine precesareanThree large randomized trials published within the past 10 years concluded that preoperative vaginal cleansing with povidone-iodine reduced the risk of postcesarean endometritis in women who also received prophylactic IV antibiotics (TABLE).1−3 Vaginal cleansing did not reduce the rate of postpartum fever or wound infection in these studies.

Clinical factors that increased the risk of postpartum endometritis independent of vaginal cleansing included:
- extended duration of cesarean surgery
- being in labor prior to cesarean delivery
- ruptured membranes
- advanced cervical examination
- maternal anemia
- use of intrapartum internal monitors
- prior history of genitourinary infection.
Authors of two recent, large nonrandomized studies also have reported that vaginal cleansing reduced the risk of postcesarean endometritis.4,5 By contrast, investigators from one large trial from 2001 did not observe a decrease in endometritis with vaginal cleansing.6
To test the impact of metronidazole vaginal gel on post‑cesarean endometritis, 224 women undergoing cesarean delivery for various indications were randomly assigned to placebo vaginal gel or metronidazole vaginal gel 5 g prior to surgery initiation.1 Most women also received intravenous antibiotics. The rates of endometritis were 17% and 7% in the placebo and metronidazole groups, respectively (relative risk, 0.42; 95% confidence interval, 0.19−0.92).
Vaginal antibiotic administration shows promise as an alternative to povidone-iodine cleansing in the prevention of postcesarean endometritis. Additional randomized clinical trials are necessary to fully evaluate the benefits and risks of this practice.
Reference
1. Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745−750.
Cochrane review of precesarean vaginal cleansingAuthors of a Cochrane review, in which they synthesized 7 studies involving 2,635 women, reported that vaginal cleansing with povidone-iodine immediately before cesarean delivery was associated with a reduced risk of postcesarean endometritis: 8.3% vs 4.3% in the control and vaginal cleansing groups, respectively, (risk ratio [RR], 0.45; 95% confidence interval [CI], 0.25−0.81).7
The positive effect of vaginal cleansing was particularly noteworthy in the subgroup of women with ruptured membranes (3 trials involving 272 women). The rates of endometritis in the control versus vaginal cleansing groups were 17.9% and 4.3%, respectively (RR, 0.24; 95% CI, 0.10−0.55).
Women who went into labor prior to cesarean delivery (523 women from 3 trials) also benefitted from vaginal cleansing, with endometritis rates of 13.0% and 7.4% in the control and vaginal cleansing groups, respectively (RR, 0.56; 95% CI, 0.34−0.95).
In this review, again, vaginal cleansing did not significantly reduce the rate of postoperative fever or wound infection.
The American College of Obstetricians and Gynecologists has noted that chlorohexidine gluconate solutions with high concentrations of alcohol are contraindicated for vaginal cleansing.1 However, although not approved for vaginal cleansing, solutions of chlorohexidine gluconate with low alcohol content (4% alcohol concentration) are safe and may be effective for off-label use as vaginal cleansings.
Reference
1. American College of Obstetricians and Gynecologists Women’s Health Care Physicians; Committee on Gynecologic Practice. Committee Opinion No. 571: solutions for surgical cleansing of the vagina. Obstet Gynecol. 2013;122(3):718−720.
Is vaginal cleansing prior to cesarean delivery best practice?In the United States, precesarean vaginal cleansing is not a common practice. To close the gap between current practice and what is potentially a best practice, two approaches to using vaginal cleansing could be instituted in delivery units.
Approach #1: A liberal clinical protocol. In this scenario, all women (who are not allergic to iodine or povidone-iodine) undergoing cesarean delivery should undergo vaginal cleansing. The World Health Organization conditionally recommends vaginal cleansing for all women undergoing a cesarean delivery.8
Approach #2: A focused clinical protocol. For this protocol, only women (again, who are not allergic to iodine or povidone-iodine) who have ruptured membranes or are in labor upon advanced cervical examination should receive vaginal cleansing.
The advantage of a liberal protocol is that vaginal preparation becomes embedded within the standard practice of cesarean delivery and, hence, is seldom overlooked. The upside of the focused protocol is that only those women most likely to benefit receive the intervention.
Tell me what you thinkWill you consider using vaginal cleansing in your practice? Please let me know your views on vaginal cleansing for cesarean delivery, as well as your clinical pearls on cesarean delivery surgery, at obgmanagement.com. In addition, weigh in on the Quick Poll posted to OBG Management’s homepage. Send your letter to the editor to [email protected].
There are approximately 4,000,000 births annually in the United States, and about 32% of them occur by cesarean delivery. Compared with vaginal birth, cesarean delivery is associated with an increased risk of endometritis (defined as fever plus uterine or abdominal tenderness). Although surgical complications cannot be eliminated entirely, surgeons are deeply dedicated to the continuous improvement of surgical practice in order to reduce the risk of complications.
With cesarean delivery, many surgical practices have been adopted universally to reduce postoperative complications, including administration of intravenous (IV) antibiotics before skin incision to minimize postoperative infection and the use of postoperative mechanical or pharmacologic interventions to help prevent venous thromboembolism and pulmonary embolism. Preoperative vaginal cleansing with povidone-iodine may reduce the risk of postoperative endometritis, but the practice is not currently common in the United States.
Should you adopt a policy of preoperative vaginal cleansing prior to cesarean delivery? The data suggest perhaps you should.
Data-driven support for povidone-iodine precesareanThree large randomized trials published within the past 10 years concluded that preoperative vaginal cleansing with povidone-iodine reduced the risk of postcesarean endometritis in women who also received prophylactic IV antibiotics (TABLE).1−3 Vaginal cleansing did not reduce the rate of postpartum fever or wound infection in these studies.

Clinical factors that increased the risk of postpartum endometritis independent of vaginal cleansing included:
- extended duration of cesarean surgery
- being in labor prior to cesarean delivery
- ruptured membranes
- advanced cervical examination
- maternal anemia
- use of intrapartum internal monitors
- prior history of genitourinary infection.
Authors of two recent, large nonrandomized studies also have reported that vaginal cleansing reduced the risk of postcesarean endometritis.4,5 By contrast, investigators from one large trial from 2001 did not observe a decrease in endometritis with vaginal cleansing.6
To test the impact of metronidazole vaginal gel on post‑cesarean endometritis, 224 women undergoing cesarean delivery for various indications were randomly assigned to placebo vaginal gel or metronidazole vaginal gel 5 g prior to surgery initiation.1 Most women also received intravenous antibiotics. The rates of endometritis were 17% and 7% in the placebo and metronidazole groups, respectively (relative risk, 0.42; 95% confidence interval, 0.19−0.92).
Vaginal antibiotic administration shows promise as an alternative to povidone-iodine cleansing in the prevention of postcesarean endometritis. Additional randomized clinical trials are necessary to fully evaluate the benefits and risks of this practice.
Reference
1. Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745−750.
Cochrane review of precesarean vaginal cleansingAuthors of a Cochrane review, in which they synthesized 7 studies involving 2,635 women, reported that vaginal cleansing with povidone-iodine immediately before cesarean delivery was associated with a reduced risk of postcesarean endometritis: 8.3% vs 4.3% in the control and vaginal cleansing groups, respectively, (risk ratio [RR], 0.45; 95% confidence interval [CI], 0.25−0.81).7
The positive effect of vaginal cleansing was particularly noteworthy in the subgroup of women with ruptured membranes (3 trials involving 272 women). The rates of endometritis in the control versus vaginal cleansing groups were 17.9% and 4.3%, respectively (RR, 0.24; 95% CI, 0.10−0.55).
Women who went into labor prior to cesarean delivery (523 women from 3 trials) also benefitted from vaginal cleansing, with endometritis rates of 13.0% and 7.4% in the control and vaginal cleansing groups, respectively (RR, 0.56; 95% CI, 0.34−0.95).
In this review, again, vaginal cleansing did not significantly reduce the rate of postoperative fever or wound infection.
The American College of Obstetricians and Gynecologists has noted that chlorohexidine gluconate solutions with high concentrations of alcohol are contraindicated for vaginal cleansing.1 However, although not approved for vaginal cleansing, solutions of chlorohexidine gluconate with low alcohol content (4% alcohol concentration) are safe and may be effective for off-label use as vaginal cleansings.
Reference
1. American College of Obstetricians and Gynecologists Women’s Health Care Physicians; Committee on Gynecologic Practice. Committee Opinion No. 571: solutions for surgical cleansing of the vagina. Obstet Gynecol. 2013;122(3):718−720.
Is vaginal cleansing prior to cesarean delivery best practice?In the United States, precesarean vaginal cleansing is not a common practice. To close the gap between current practice and what is potentially a best practice, two approaches to using vaginal cleansing could be instituted in delivery units.
Approach #1: A liberal clinical protocol. In this scenario, all women (who are not allergic to iodine or povidone-iodine) undergoing cesarean delivery should undergo vaginal cleansing. The World Health Organization conditionally recommends vaginal cleansing for all women undergoing a cesarean delivery.8
Approach #2: A focused clinical protocol. For this protocol, only women (again, who are not allergic to iodine or povidone-iodine) who have ruptured membranes or are in labor upon advanced cervical examination should receive vaginal cleansing.
The advantage of a liberal protocol is that vaginal preparation becomes embedded within the standard practice of cesarean delivery and, hence, is seldom overlooked. The upside of the focused protocol is that only those women most likely to benefit receive the intervention.
Tell me what you thinkWill you consider using vaginal cleansing in your practice? Please let me know your views on vaginal cleansing for cesarean delivery, as well as your clinical pearls on cesarean delivery surgery, at obgmanagement.com. In addition, weigh in on the Quick Poll posted to OBG Management’s homepage. Send your letter to the editor to [email protected].
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2015;105(5 pt 1):1024–1029.
- Haas DM, Pazouki F, Smith RR, et al. Vaginal cleansing before cesarean delivery to reduce postoperative infectious morbidity: a randomized, controlled trial. Am J Obstet Gynecol. 2010;202(3):310.e1–e6.
- Yildrim G, Gungorduk K, Asicioglu O, et al. Does vaginal preparation with povidone-iodine prior to cesarean delivery reduce the risk of endometritis?A randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25(11):2316–2321.
- Asghania M, Mirblouk F, Shakiba M, Faraji R. Preoperative vaginal preparation with povidone-iodine on post-cesarean infectious morbidity. J Obstet Gynaecol. 2011;31(5):400–403.
- Memon S, Qazi RA, Bibi S, Parveen N. Effect of preoperative vaginal cleansing with an antiseptic solution to reduce post caesarean infectious morbidity. J Pak Med Assoc. 2011;61(12):1179–1183.
- Reid VC, Hartmann KE, McMahon M, Fry EP. Vaginal preparation with povidone-iodine and postcesarean infectious morbidity: a randomized controlled trial. Obstet Gynecol. 2001;97(1):147–152.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2014;12:CD007892.
- Yildrim G, Gungorduk K, Asicioglu O, et al. Does vaginal preparation with povidone-iodine prior to
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2015;105(5 pt 1):1024–1029.
- Haas DM, Pazouki F, Smith RR, et al. Vaginal cleansing before cesarean delivery to reduce postoperative infectious morbidity: a randomized, controlled trial. Am J Obstet Gynecol. 2010;202(3):310.e1–e6.
- Yildrim G, Gungorduk K, Asicioglu O, et al. Does vaginal preparation with povidone-iodine prior to cesarean delivery reduce the risk of endometritis?A randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25(11):2316–2321.
- Asghania M, Mirblouk F, Shakiba M, Faraji R. Preoperative vaginal preparation with povidone-iodine on post-cesarean infectious morbidity. J Obstet Gynaecol. 2011;31(5):400–403.
- Memon S, Qazi RA, Bibi S, Parveen N. Effect of preoperative vaginal cleansing with an antiseptic solution to reduce post caesarean infectious morbidity. J Pak Med Assoc. 2011;61(12):1179–1183.
- Reid VC, Hartmann KE, McMahon M, Fry EP. Vaginal preparation with povidone-iodine and postcesarean infectious morbidity: a randomized controlled trial. Obstet Gynecol. 2001;97(1):147–152.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2014;12:CD007892.
- Yildrim G, Gungorduk K, Asicioglu O, et al. Does vaginal preparation with povidone-iodine prior to
Can transabdominal ultrasound exclude short cervix?
Preterm birth (PTB) remains a major cause of perinatal morbidity and mortality, and so its prediction and prevention are 2 of the most important issues in obstetrics. Cervical length (CL) measured by ultrasound has been shown to be the best predictor; several interventions (vaginal progesterone and cerclage) have been shown to be effective at reducing PTB if a short CL is identified. In fact, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) recommend CL being measured every 2 weeks from 16 to 23 weeks in singletons with prior spontaneous PTB (sPTB), with cerclage placed for CL less than 25 mm. Moreover, both ACOG and SMFM recommend that “universal CL screening” (CL measured in singletons without a prior sPTB) be considered as a single measurement at about 18 to 23 weeks.
Details of the study
Rhoades and colleagues present data on CL screening done by transabdominal ultrasound (TAU), as an alternative to transvaginal ultrasound (TVU). This study confirms early data:
- TAU cannot visualize CL in several women (20.6%).
- To make sure a high sensitivity (92.9% in this study) is achieved to detect a TVU CL less than 30 mm, a high cutoff (in this case 35 mm) needs to be used with TAU. Nonetheless, 7% of women with a short TVU CL would not be detected, raising clinical and legal issues.
- A high percentage (in this case 32.4%; 103/318) of women screened by TAU would screen positive (TAU CL less than 35 mm) and therefore need to have a TVU anyway.
- Overall, more than 50% (in this study 53%–20.6% because TAU could not visualize CL, and 32.4% because TAU was less than 35 mm) of women having TAU CL screening would need to have TVU anyway! In the largest study comparing TAU to TVU CL screening (TABLE1–6), 66% of women screened by TAU would have to be screened also by TVU.5
There are several other reasons why TVU is considered the gold standard for CL screening, and instead TAU CL should be avoided as possible. All randomized controlled trials that showed benefit from interventions (vaginal progesterone, cerclage, pessary) aimed at decreasing PTB in women with short CL used TVU CL screening and never TAU CL screening. In addition, TAU CL is less accurate than TVU CL screening. On TAU, fetal parts can obscure the cervix, obesity makes it hard to visualize CL, the distance between probe and cervix is longer, manual pressure can mask CL shortening, and bladder filling can elongate CL.7 Cost-effectiveness studies show that TVU CL screening is more effective, and less costly, compared with TAU CL screening, even in singletons without a prior sPTB.8
Societies such as ACOG and SMFM all have recommended TVU CL for prediction and prevention of PTB, over TAU CL.9,10 Importantly, a TVU CL should be done by sonographers educated and trained formally, through such programs as those made available by SMFM.11
What this evidence means for practice
If CL assessment is done, TVU should be preferred, as it is the gold standard, and not TAU.
>>Vincenzo Berghella, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Saul LL, Kurtzman JT, Hagemann C, Ghamsary M, Wing DA. Is transabdominal sonography of the cervix after voiding a reliable method of cervical length assessment? J Ultrasound Med. 2008;27(9):1305−1311.
- Stone PR, Chan EH, McCowan LM, Taylor RS, Mitchell JM; SCOPE Consortium. Aust N Z J Obstet Gynaecol. 2010;50(6):523−527.
- To MS, Skentou C, Cicero S, Nicolaides KH. Cervical assessment at the routine 23-weeks’ scan: problems with transabdominal sonography. Ultrasound Obstet Gynecol 2000;15(4):292−296.
- Hernandez-Andrade E, Romero R, Ahn H, et al. Transabdominal evaluation of uterine cervical length during pregnancy fails to identify a substantial number of women with a short cervix. 2012;25(9):1682−1689.
- Friedman AM, Srinivas SK, Parry S, et al. Can transabdominal ultrasound be used as a screening test for short cervical length? Am J Obstet Gynecol. 2013;208(3):190.e1−e7.
- Rhoades JS, Park JM, Stout MJ, Macones GA, Cahill AG, Tuuli MG. Can transabdominal cervical length measurement exclude short cervix? 2015 Nov 2. [Epub ahead of print]
- Berghella V, Bega G, Tolosa JE, Berghella M. Ultrasound assessment of the cervix. Clin Obstet Gynecol. 2003; 46(4):947–623.
- Miller ES, Grobman WA. Cost-effectiveness of transabdominal ultrasound for cervical length screening for preterm birth prevention. Am J Obstet Gynecol. 2013;209(6): 546.e1–e6.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964–973.
- Society for Maternal-Fetal Medicine Publications Committee; Berghella V. Progesterone and preterm birth prevention: translating clinical trial data into clinical practice. Am J Obstet Gynecol. 2012;206(5):376–386.
- Cervical Length Education and Review (CLEAR) guidelines. https://clear.perinatalquality.org. Published 2015. Accessed December 15, 2015.
Preterm birth (PTB) remains a major cause of perinatal morbidity and mortality, and so its prediction and prevention are 2 of the most important issues in obstetrics. Cervical length (CL) measured by ultrasound has been shown to be the best predictor; several interventions (vaginal progesterone and cerclage) have been shown to be effective at reducing PTB if a short CL is identified. In fact, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) recommend CL being measured every 2 weeks from 16 to 23 weeks in singletons with prior spontaneous PTB (sPTB), with cerclage placed for CL less than 25 mm. Moreover, both ACOG and SMFM recommend that “universal CL screening” (CL measured in singletons without a prior sPTB) be considered as a single measurement at about 18 to 23 weeks.
Details of the study
Rhoades and colleagues present data on CL screening done by transabdominal ultrasound (TAU), as an alternative to transvaginal ultrasound (TVU). This study confirms early data:
- TAU cannot visualize CL in several women (20.6%).
- To make sure a high sensitivity (92.9% in this study) is achieved to detect a TVU CL less than 30 mm, a high cutoff (in this case 35 mm) needs to be used with TAU. Nonetheless, 7% of women with a short TVU CL would not be detected, raising clinical and legal issues.
- A high percentage (in this case 32.4%; 103/318) of women screened by TAU would screen positive (TAU CL less than 35 mm) and therefore need to have a TVU anyway.
- Overall, more than 50% (in this study 53%–20.6% because TAU could not visualize CL, and 32.4% because TAU was less than 35 mm) of women having TAU CL screening would need to have TVU anyway! In the largest study comparing TAU to TVU CL screening (TABLE1–6), 66% of women screened by TAU would have to be screened also by TVU.5
There are several other reasons why TVU is considered the gold standard for CL screening, and instead TAU CL should be avoided as possible. All randomized controlled trials that showed benefit from interventions (vaginal progesterone, cerclage, pessary) aimed at decreasing PTB in women with short CL used TVU CL screening and never TAU CL screening. In addition, TAU CL is less accurate than TVU CL screening. On TAU, fetal parts can obscure the cervix, obesity makes it hard to visualize CL, the distance between probe and cervix is longer, manual pressure can mask CL shortening, and bladder filling can elongate CL.7 Cost-effectiveness studies show that TVU CL screening is more effective, and less costly, compared with TAU CL screening, even in singletons without a prior sPTB.8
Societies such as ACOG and SMFM all have recommended TVU CL for prediction and prevention of PTB, over TAU CL.9,10 Importantly, a TVU CL should be done by sonographers educated and trained formally, through such programs as those made available by SMFM.11
What this evidence means for practice
If CL assessment is done, TVU should be preferred, as it is the gold standard, and not TAU.
>>Vincenzo Berghella, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Preterm birth (PTB) remains a major cause of perinatal morbidity and mortality, and so its prediction and prevention are 2 of the most important issues in obstetrics. Cervical length (CL) measured by ultrasound has been shown to be the best predictor; several interventions (vaginal progesterone and cerclage) have been shown to be effective at reducing PTB if a short CL is identified. In fact, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) recommend CL being measured every 2 weeks from 16 to 23 weeks in singletons with prior spontaneous PTB (sPTB), with cerclage placed for CL less than 25 mm. Moreover, both ACOG and SMFM recommend that “universal CL screening” (CL measured in singletons without a prior sPTB) be considered as a single measurement at about 18 to 23 weeks.
Details of the study
Rhoades and colleagues present data on CL screening done by transabdominal ultrasound (TAU), as an alternative to transvaginal ultrasound (TVU). This study confirms early data:
- TAU cannot visualize CL in several women (20.6%).
- To make sure a high sensitivity (92.9% in this study) is achieved to detect a TVU CL less than 30 mm, a high cutoff (in this case 35 mm) needs to be used with TAU. Nonetheless, 7% of women with a short TVU CL would not be detected, raising clinical and legal issues.
- A high percentage (in this case 32.4%; 103/318) of women screened by TAU would screen positive (TAU CL less than 35 mm) and therefore need to have a TVU anyway.
- Overall, more than 50% (in this study 53%–20.6% because TAU could not visualize CL, and 32.4% because TAU was less than 35 mm) of women having TAU CL screening would need to have TVU anyway! In the largest study comparing TAU to TVU CL screening (TABLE1–6), 66% of women screened by TAU would have to be screened also by TVU.5
There are several other reasons why TVU is considered the gold standard for CL screening, and instead TAU CL should be avoided as possible. All randomized controlled trials that showed benefit from interventions (vaginal progesterone, cerclage, pessary) aimed at decreasing PTB in women with short CL used TVU CL screening and never TAU CL screening. In addition, TAU CL is less accurate than TVU CL screening. On TAU, fetal parts can obscure the cervix, obesity makes it hard to visualize CL, the distance between probe and cervix is longer, manual pressure can mask CL shortening, and bladder filling can elongate CL.7 Cost-effectiveness studies show that TVU CL screening is more effective, and less costly, compared with TAU CL screening, even in singletons without a prior sPTB.8
Societies such as ACOG and SMFM all have recommended TVU CL for prediction and prevention of PTB, over TAU CL.9,10 Importantly, a TVU CL should be done by sonographers educated and trained formally, through such programs as those made available by SMFM.11
What this evidence means for practice
If CL assessment is done, TVU should be preferred, as it is the gold standard, and not TAU.
>>Vincenzo Berghella, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Saul LL, Kurtzman JT, Hagemann C, Ghamsary M, Wing DA. Is transabdominal sonography of the cervix after voiding a reliable method of cervical length assessment? J Ultrasound Med. 2008;27(9):1305−1311.
- Stone PR, Chan EH, McCowan LM, Taylor RS, Mitchell JM; SCOPE Consortium. Aust N Z J Obstet Gynaecol. 2010;50(6):523−527.
- To MS, Skentou C, Cicero S, Nicolaides KH. Cervical assessment at the routine 23-weeks’ scan: problems with transabdominal sonography. Ultrasound Obstet Gynecol 2000;15(4):292−296.
- Hernandez-Andrade E, Romero R, Ahn H, et al. Transabdominal evaluation of uterine cervical length during pregnancy fails to identify a substantial number of women with a short cervix. 2012;25(9):1682−1689.
- Friedman AM, Srinivas SK, Parry S, et al. Can transabdominal ultrasound be used as a screening test for short cervical length? Am J Obstet Gynecol. 2013;208(3):190.e1−e7.
- Rhoades JS, Park JM, Stout MJ, Macones GA, Cahill AG, Tuuli MG. Can transabdominal cervical length measurement exclude short cervix? 2015 Nov 2. [Epub ahead of print]
- Berghella V, Bega G, Tolosa JE, Berghella M. Ultrasound assessment of the cervix. Clin Obstet Gynecol. 2003; 46(4):947–623.
- Miller ES, Grobman WA. Cost-effectiveness of transabdominal ultrasound for cervical length screening for preterm birth prevention. Am J Obstet Gynecol. 2013;209(6): 546.e1–e6.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964–973.
- Society for Maternal-Fetal Medicine Publications Committee; Berghella V. Progesterone and preterm birth prevention: translating clinical trial data into clinical practice. Am J Obstet Gynecol. 2012;206(5):376–386.
- Cervical Length Education and Review (CLEAR) guidelines. https://clear.perinatalquality.org. Published 2015. Accessed December 15, 2015.
- Saul LL, Kurtzman JT, Hagemann C, Ghamsary M, Wing DA. Is transabdominal sonography of the cervix after voiding a reliable method of cervical length assessment? J Ultrasound Med. 2008;27(9):1305−1311.
- Stone PR, Chan EH, McCowan LM, Taylor RS, Mitchell JM; SCOPE Consortium. Aust N Z J Obstet Gynaecol. 2010;50(6):523−527.
- To MS, Skentou C, Cicero S, Nicolaides KH. Cervical assessment at the routine 23-weeks’ scan: problems with transabdominal sonography. Ultrasound Obstet Gynecol 2000;15(4):292−296.
- Hernandez-Andrade E, Romero R, Ahn H, et al. Transabdominal evaluation of uterine cervical length during pregnancy fails to identify a substantial number of women with a short cervix. 2012;25(9):1682−1689.
- Friedman AM, Srinivas SK, Parry S, et al. Can transabdominal ultrasound be used as a screening test for short cervical length? Am J Obstet Gynecol. 2013;208(3):190.e1−e7.
- Rhoades JS, Park JM, Stout MJ, Macones GA, Cahill AG, Tuuli MG. Can transabdominal cervical length measurement exclude short cervix? 2015 Nov 2. [Epub ahead of print]
- Berghella V, Bega G, Tolosa JE, Berghella M. Ultrasound assessment of the cervix. Clin Obstet Gynecol. 2003; 46(4):947–623.
- Miller ES, Grobman WA. Cost-effectiveness of transabdominal ultrasound for cervical length screening for preterm birth prevention. Am J Obstet Gynecol. 2013;209(6): 546.e1–e6.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964–973.
- Society for Maternal-Fetal Medicine Publications Committee; Berghella V. Progesterone and preterm birth prevention: translating clinical trial data into clinical practice. Am J Obstet Gynecol. 2012;206(5):376–386.
- Cervical Length Education and Review (CLEAR) guidelines. https://clear.perinatalquality.org. Published 2015. Accessed December 15, 2015.
Insulin resistance in 22% of men with acne
Young adult men with acne were more likely to have insulin resistance and to have higher fasting plasma glucose levels than were men of the same age who did not have acne, in a cross-sectional study of 20 to 32 year old men in India.
In a study published online in JAMA Dermatology, on Dec. 23 (doi: 10.1001/jamadermatol.2015.4499), Dr. Mohit Nagpal, of the Postgraduate Institute of Medical Education and Research, Chandigarh, India, and associates, wrote that “Insulin resistance may be a stage of prediabetes, and the patients may develop hyperinsulinemia or type 2 diabetes in the future. These patients should be followed up to determine whether they develop conditions associated with insulin resistance.”
The researchers compared 100 men with acne, aged 20 to 32 years, with 100 age-matched men who did not have acne and were being treated for non-acne dermatoses; all were being treated at the Institute’s dermatology outpatient department. Insulin resistance, as defined by a Homeostasis Model Assessment–Insulin Resistance (HOMA-IR) value greater than 2.5, was present in 22% of those with acne, vs. 11% of those without acne, a significant difference (P = .036). Metabolic syndrome, based on criteria of the modified National Cholesterol Education Program’s Adult Treatment Panel III (NCEP-ATP III), was more common among those with acne (17% vs. 9%), but the difference was not significant (P = .09).
The mean diastolic and systolic blood pressure values were also significantly higher among those with acne, compared with controls, as were mean fasting plasma glucose levels.
When evaluated by acne severity (mild, moderate, severe, or very severe), there were no significant differences in the prevalence of insulin resistance or metabolic syndrome between the four groups. However, the mean body mass index and the mean weights among those with very severe acne were significantly higher than among those with mild acne (P = .04).
The cross-sectional design of the study was a limitation, the authors noted, and future studies will “follow up patients with acne to assess the development of clinical conditions associated with insulin resistance,” such as acanthosis nigricans and metabolic syndrome.
In an accompanying editorial, Dr. Rachel V. Reynolds of Beth Israel Deaconess Medical Center, Boston, wrote that this study, and another study published in the same issue regarding insulin resistance and polycystic ovary syndrome, “highlight the important role that the dermatologist plays in identifying and characterizing patients with common skin disorders who may be at risk for metabolic and androgen-mediated disease.” The study, “to our knowledge, [is] the largest cohort to date examining the prevalence of insulin resistance and metabolic syndrome in postadolescent males with acne of varying severity,” she added (doi: 10.1001/jamadermatol.2015.4500).
The authors of the study had no disclosures.
Young adult men with acne were more likely to have insulin resistance and to have higher fasting plasma glucose levels than were men of the same age who did not have acne, in a cross-sectional study of 20 to 32 year old men in India.
In a study published online in JAMA Dermatology, on Dec. 23 (doi: 10.1001/jamadermatol.2015.4499), Dr. Mohit Nagpal, of the Postgraduate Institute of Medical Education and Research, Chandigarh, India, and associates, wrote that “Insulin resistance may be a stage of prediabetes, and the patients may develop hyperinsulinemia or type 2 diabetes in the future. These patients should be followed up to determine whether they develop conditions associated with insulin resistance.”
The researchers compared 100 men with acne, aged 20 to 32 years, with 100 age-matched men who did not have acne and were being treated for non-acne dermatoses; all were being treated at the Institute’s dermatology outpatient department. Insulin resistance, as defined by a Homeostasis Model Assessment–Insulin Resistance (HOMA-IR) value greater than 2.5, was present in 22% of those with acne, vs. 11% of those without acne, a significant difference (P = .036). Metabolic syndrome, based on criteria of the modified National Cholesterol Education Program’s Adult Treatment Panel III (NCEP-ATP III), was more common among those with acne (17% vs. 9%), but the difference was not significant (P = .09).
The mean diastolic and systolic blood pressure values were also significantly higher among those with acne, compared with controls, as were mean fasting plasma glucose levels.
When evaluated by acne severity (mild, moderate, severe, or very severe), there were no significant differences in the prevalence of insulin resistance or metabolic syndrome between the four groups. However, the mean body mass index and the mean weights among those with very severe acne were significantly higher than among those with mild acne (P = .04).
The cross-sectional design of the study was a limitation, the authors noted, and future studies will “follow up patients with acne to assess the development of clinical conditions associated with insulin resistance,” such as acanthosis nigricans and metabolic syndrome.
In an accompanying editorial, Dr. Rachel V. Reynolds of Beth Israel Deaconess Medical Center, Boston, wrote that this study, and another study published in the same issue regarding insulin resistance and polycystic ovary syndrome, “highlight the important role that the dermatologist plays in identifying and characterizing patients with common skin disorders who may be at risk for metabolic and androgen-mediated disease.” The study, “to our knowledge, [is] the largest cohort to date examining the prevalence of insulin resistance and metabolic syndrome in postadolescent males with acne of varying severity,” she added (doi: 10.1001/jamadermatol.2015.4500).
The authors of the study had no disclosures.
Young adult men with acne were more likely to have insulin resistance and to have higher fasting plasma glucose levels than were men of the same age who did not have acne, in a cross-sectional study of 20 to 32 year old men in India.
In a study published online in JAMA Dermatology, on Dec. 23 (doi: 10.1001/jamadermatol.2015.4499), Dr. Mohit Nagpal, of the Postgraduate Institute of Medical Education and Research, Chandigarh, India, and associates, wrote that “Insulin resistance may be a stage of prediabetes, and the patients may develop hyperinsulinemia or type 2 diabetes in the future. These patients should be followed up to determine whether they develop conditions associated with insulin resistance.”
The researchers compared 100 men with acne, aged 20 to 32 years, with 100 age-matched men who did not have acne and were being treated for non-acne dermatoses; all were being treated at the Institute’s dermatology outpatient department. Insulin resistance, as defined by a Homeostasis Model Assessment–Insulin Resistance (HOMA-IR) value greater than 2.5, was present in 22% of those with acne, vs. 11% of those without acne, a significant difference (P = .036). Metabolic syndrome, based on criteria of the modified National Cholesterol Education Program’s Adult Treatment Panel III (NCEP-ATP III), was more common among those with acne (17% vs. 9%), but the difference was not significant (P = .09).
The mean diastolic and systolic blood pressure values were also significantly higher among those with acne, compared with controls, as were mean fasting plasma glucose levels.
When evaluated by acne severity (mild, moderate, severe, or very severe), there were no significant differences in the prevalence of insulin resistance or metabolic syndrome between the four groups. However, the mean body mass index and the mean weights among those with very severe acne were significantly higher than among those with mild acne (P = .04).
The cross-sectional design of the study was a limitation, the authors noted, and future studies will “follow up patients with acne to assess the development of clinical conditions associated with insulin resistance,” such as acanthosis nigricans and metabolic syndrome.
In an accompanying editorial, Dr. Rachel V. Reynolds of Beth Israel Deaconess Medical Center, Boston, wrote that this study, and another study published in the same issue regarding insulin resistance and polycystic ovary syndrome, “highlight the important role that the dermatologist plays in identifying and characterizing patients with common skin disorders who may be at risk for metabolic and androgen-mediated disease.” The study, “to our knowledge, [is] the largest cohort to date examining the prevalence of insulin resistance and metabolic syndrome in postadolescent males with acne of varying severity,” she added (doi: 10.1001/jamadermatol.2015.4500).
The authors of the study had no disclosures.
FROM JAMA DERMATOLOGY
Key clinical point: Acne in young men may be a sign of insulin resistance.
Major finding: 22% of the young men with acne had insulin resistance, compared with 11% of the age-matched controls, a significant difference (P = .036).
Data source: The cross-sectional study compared the prevalence of insulin resistance and metabolic syndrome in 100 men aged 20-32 years with acne and 100 age-matched controls without acne.
Disclosures: The authors had no disclosures.
Study: One-third of patients with bipolar disorders abnormally metabolized glucose
One-third of patients with bipolar disorders abnormally metabolized glucose, in a study of outpatients from two university hospitals in Germany.
The study included 85 euthymic patients with bipolar disorders, who underwent an oral glucose tolerance test, laboratory screening, and clinical measurements.
Seven percent of the patients tested positive for diabetes mellitus, while 27% of the patients showed prediabetic abnormalities, including abnormalities in glucose metabolism. Patients in both of these groups had significantly lower quality of life and global functioning.
Additional study findings were that higher body mass index, leptin, triglycerides, and C-reactive protein levels significantly increased the likelihood of an individual having pre-diabetes abnormalities or diabetes.
Low sample size was a weakness of the study, according to Karolina Leopold and her colleagues.
Read the full study in the Journal of Affective Disorders (doi: 10.1016/j.jad.2015.09.041).
One-third of patients with bipolar disorders abnormally metabolized glucose, in a study of outpatients from two university hospitals in Germany.
The study included 85 euthymic patients with bipolar disorders, who underwent an oral glucose tolerance test, laboratory screening, and clinical measurements.
Seven percent of the patients tested positive for diabetes mellitus, while 27% of the patients showed prediabetic abnormalities, including abnormalities in glucose metabolism. Patients in both of these groups had significantly lower quality of life and global functioning.
Additional study findings were that higher body mass index, leptin, triglycerides, and C-reactive protein levels significantly increased the likelihood of an individual having pre-diabetes abnormalities or diabetes.
Low sample size was a weakness of the study, according to Karolina Leopold and her colleagues.
Read the full study in the Journal of Affective Disorders (doi: 10.1016/j.jad.2015.09.041).
One-third of patients with bipolar disorders abnormally metabolized glucose, in a study of outpatients from two university hospitals in Germany.
The study included 85 euthymic patients with bipolar disorders, who underwent an oral glucose tolerance test, laboratory screening, and clinical measurements.
Seven percent of the patients tested positive for diabetes mellitus, while 27% of the patients showed prediabetic abnormalities, including abnormalities in glucose metabolism. Patients in both of these groups had significantly lower quality of life and global functioning.
Additional study findings were that higher body mass index, leptin, triglycerides, and C-reactive protein levels significantly increased the likelihood of an individual having pre-diabetes abnormalities or diabetes.
Low sample size was a weakness of the study, according to Karolina Leopold and her colleagues.
Read the full study in the Journal of Affective Disorders (doi: 10.1016/j.jad.2015.09.041).
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Long spine fusions can give patients improved quality of life
SAN DIEGO – When necessary, long fusions that extend from the C-spine to the pelvis can result in health-related quality of life improvements, results from a multicenter study suggest.
“Patients with spinal deformities will sometimes require long fusion constructs that extend into the cervical spine,” lead study author Dr. Han-Jo Kim said at the annual meeting of the Cervical Spine Research Society. “The prevalence of these cases is increasing, especially as revision surgery for conditions such as proximal junctional kyphosis increase. They are also indicated for other diagnoses, such a progressive cervical deformity, cervical myelopathy as well as neuromuscular disorders.”
Prior investigations that have examined outcomes for these long constructs usually focus on patients who have had fusions from the upper thoracic spine to the pelvis, added Dr. Kim, an orthopedic spine surgeon at the Hospital for Special Surgery, New York. “To my knowledge, there are no studies in the literature that report on the subset of patients who have had fusions from the cervical spine to the pelvis,” he said. “The question is, even though these revisions may be necessary, does surgical intervention result in improved outcomes for these patients despite the extent of these long fusions?”
In an effort to determine the outcomes and rates of complications in patients who had fusions from the cervical spine to the pelvis, Dr. Kim and his associates conducted a retrospective review of patients who underwent fusions from the cervical spine to the pelvis at four institutions during 2003-2014. The researchers administered outcome scores utilizing the Scoliosis Research Society 22 (SRS-22r) questionnaire; the Oswestry Disability Index (ODI); and the Neck Disability Index (NDI); and collected demographic data including age, body mass index, and follow-up time; medical history including comorbidity data, operative details, radiographic and articular outcomes data; and postoperative complications.
Of 55 patients initially included in the study, complete data were available for 46 (84%). Their average age was 42 years, nearly one-third (30%) were classified as ASA III, 4.2% were smokers, and the average follow-up time was 2.7 years. “The majority of these cases were revision operations, and osteotomies were performed in close to 60% of these patients,” Dr. Kim said. “The average operating time was over 300 minutes, and there was an average of over 2 L of blood loss for these cases.”
The researchers observed improvements in the activity, pain, and mental health domains of the SRS, as well as an improvement in the SRS total score, which improved from an average of 3.0 preoperatively to 3.5 postoperatively (P less than .01). This was greater than the minimally clinically important difference for the SRS-22r. “At least one [minimally clinically important difference] was met in all of the SRS domains, as well as in the NDI,” Dr. Kim said. “There was no change in the ODI, as we would expect for this patient subset.”
Radiographic outcomes improved significantly, he continued, with an average 31-degree correction in maximum kyphosis and a 3.3-cm improvement in sagittal vertical axis. The overall rate of complications was 71%, with major complications comprising about 39% of these cases. Medical complications were high as well (a rate of 61%), as was the rate of surgical complications (43%). More than half of the patients (54%) required reoperation during the follow-up period, and the rate of pseudarthrosis was 29%.
“These results demonstrate improved outcomes following cervical to pelvic fusions, despite the magnitude of their operations and extent of fusion,” Dr. Kim concluded. “In addition, despite the high rate of complications and reoperations, we noted a significant improvement in radiographic and clinical outcomes.”
Dr. Kim disclosed that he is a consultant for Zimmer Biomet and K2M.
SAN DIEGO – When necessary, long fusions that extend from the C-spine to the pelvis can result in health-related quality of life improvements, results from a multicenter study suggest.
“Patients with spinal deformities will sometimes require long fusion constructs that extend into the cervical spine,” lead study author Dr. Han-Jo Kim said at the annual meeting of the Cervical Spine Research Society. “The prevalence of these cases is increasing, especially as revision surgery for conditions such as proximal junctional kyphosis increase. They are also indicated for other diagnoses, such a progressive cervical deformity, cervical myelopathy as well as neuromuscular disorders.”
Prior investigations that have examined outcomes for these long constructs usually focus on patients who have had fusions from the upper thoracic spine to the pelvis, added Dr. Kim, an orthopedic spine surgeon at the Hospital for Special Surgery, New York. “To my knowledge, there are no studies in the literature that report on the subset of patients who have had fusions from the cervical spine to the pelvis,” he said. “The question is, even though these revisions may be necessary, does surgical intervention result in improved outcomes for these patients despite the extent of these long fusions?”
In an effort to determine the outcomes and rates of complications in patients who had fusions from the cervical spine to the pelvis, Dr. Kim and his associates conducted a retrospective review of patients who underwent fusions from the cervical spine to the pelvis at four institutions during 2003-2014. The researchers administered outcome scores utilizing the Scoliosis Research Society 22 (SRS-22r) questionnaire; the Oswestry Disability Index (ODI); and the Neck Disability Index (NDI); and collected demographic data including age, body mass index, and follow-up time; medical history including comorbidity data, operative details, radiographic and articular outcomes data; and postoperative complications.
Of 55 patients initially included in the study, complete data were available for 46 (84%). Their average age was 42 years, nearly one-third (30%) were classified as ASA III, 4.2% were smokers, and the average follow-up time was 2.7 years. “The majority of these cases were revision operations, and osteotomies were performed in close to 60% of these patients,” Dr. Kim said. “The average operating time was over 300 minutes, and there was an average of over 2 L of blood loss for these cases.”
The researchers observed improvements in the activity, pain, and mental health domains of the SRS, as well as an improvement in the SRS total score, which improved from an average of 3.0 preoperatively to 3.5 postoperatively (P less than .01). This was greater than the minimally clinically important difference for the SRS-22r. “At least one [minimally clinically important difference] was met in all of the SRS domains, as well as in the NDI,” Dr. Kim said. “There was no change in the ODI, as we would expect for this patient subset.”
Radiographic outcomes improved significantly, he continued, with an average 31-degree correction in maximum kyphosis and a 3.3-cm improvement in sagittal vertical axis. The overall rate of complications was 71%, with major complications comprising about 39% of these cases. Medical complications were high as well (a rate of 61%), as was the rate of surgical complications (43%). More than half of the patients (54%) required reoperation during the follow-up period, and the rate of pseudarthrosis was 29%.
“These results demonstrate improved outcomes following cervical to pelvic fusions, despite the magnitude of their operations and extent of fusion,” Dr. Kim concluded. “In addition, despite the high rate of complications and reoperations, we noted a significant improvement in radiographic and clinical outcomes.”
Dr. Kim disclosed that he is a consultant for Zimmer Biomet and K2M.
SAN DIEGO – When necessary, long fusions that extend from the C-spine to the pelvis can result in health-related quality of life improvements, results from a multicenter study suggest.
“Patients with spinal deformities will sometimes require long fusion constructs that extend into the cervical spine,” lead study author Dr. Han-Jo Kim said at the annual meeting of the Cervical Spine Research Society. “The prevalence of these cases is increasing, especially as revision surgery for conditions such as proximal junctional kyphosis increase. They are also indicated for other diagnoses, such a progressive cervical deformity, cervical myelopathy as well as neuromuscular disorders.”
Prior investigations that have examined outcomes for these long constructs usually focus on patients who have had fusions from the upper thoracic spine to the pelvis, added Dr. Kim, an orthopedic spine surgeon at the Hospital for Special Surgery, New York. “To my knowledge, there are no studies in the literature that report on the subset of patients who have had fusions from the cervical spine to the pelvis,” he said. “The question is, even though these revisions may be necessary, does surgical intervention result in improved outcomes for these patients despite the extent of these long fusions?”
In an effort to determine the outcomes and rates of complications in patients who had fusions from the cervical spine to the pelvis, Dr. Kim and his associates conducted a retrospective review of patients who underwent fusions from the cervical spine to the pelvis at four institutions during 2003-2014. The researchers administered outcome scores utilizing the Scoliosis Research Society 22 (SRS-22r) questionnaire; the Oswestry Disability Index (ODI); and the Neck Disability Index (NDI); and collected demographic data including age, body mass index, and follow-up time; medical history including comorbidity data, operative details, radiographic and articular outcomes data; and postoperative complications.
Of 55 patients initially included in the study, complete data were available for 46 (84%). Their average age was 42 years, nearly one-third (30%) were classified as ASA III, 4.2% were smokers, and the average follow-up time was 2.7 years. “The majority of these cases were revision operations, and osteotomies were performed in close to 60% of these patients,” Dr. Kim said. “The average operating time was over 300 minutes, and there was an average of over 2 L of blood loss for these cases.”
The researchers observed improvements in the activity, pain, and mental health domains of the SRS, as well as an improvement in the SRS total score, which improved from an average of 3.0 preoperatively to 3.5 postoperatively (P less than .01). This was greater than the minimally clinically important difference for the SRS-22r. “At least one [minimally clinically important difference] was met in all of the SRS domains, as well as in the NDI,” Dr. Kim said. “There was no change in the ODI, as we would expect for this patient subset.”
Radiographic outcomes improved significantly, he continued, with an average 31-degree correction in maximum kyphosis and a 3.3-cm improvement in sagittal vertical axis. The overall rate of complications was 71%, with major complications comprising about 39% of these cases. Medical complications were high as well (a rate of 61%), as was the rate of surgical complications (43%). More than half of the patients (54%) required reoperation during the follow-up period, and the rate of pseudarthrosis was 29%.
“These results demonstrate improved outcomes following cervical to pelvic fusions, despite the magnitude of their operations and extent of fusion,” Dr. Kim concluded. “In addition, despite the high rate of complications and reoperations, we noted a significant improvement in radiographic and clinical outcomes.”
Dr. Kim disclosed that he is a consultant for Zimmer Biomet and K2M.
AT CSRS 2015
Key clinical point: Following cervical to pelvic fusions, patients can achieve improved clinical and quality of life outcomes.
Major finding: The Scoliosis Research Society total score improved from an average of 3.0 preoperatively to 3.5 postoperatively (P less than .01).
Data source: A retrospective review of 55 patients who underwent fusions from the cervical spine to the pelvis at four institutions during 2003-2014.
Disclosures: Dr. Kim disclosed that he is a consultant for Zimmer Biomet and K2M.
Resident Guide to Advocacy in Dermatology
It is never too early (or too late!) to get involved in dermatology advocacy. Residency is an ideal time to start learning about advocating on behalf of the specialty of dermatology as well as on behalf of our patients. Many opportunities are available for residents to gain experience and become advocates on national and grassroots levels. As residents, participating in these efforts can help set a solid foundation for future involvement in advocacy, regardless of our ultimate career goals.
American Medical Association
The mission of the American Medical Association (AMA) is “to promote the art and science of medicine and the betterment of public health.”1 Joining the AMA costs $45 for 1 year of resident membership (with a discounted rate for multiyear memberships). As a member, you are given the opportunity to cast a ballot for the national medical specialty society that best represents you in the House of Delegates, the AMA’s principle policy-making body.2 The more votes a particular society receives, the more delegates from that society are added to the House of Delegates, meaning more representation for that specialty organization. It is advised that members choose the society that best represents them: for dermatologists, this most likely would be the American Academy of Dermatology (AAD), among other dermatology organizations that are candidates (ie, the American College of Mohs Surgery, the American Society for Dermatologic Surgery, and the Society for Investigative Dermatology). This representation is key for a specialty like dermatology, which has a relatively smaller number of physicians compared to other larger specialties and therefore has less representation in the House of Delegates.
Additionally, AMA membership grants you access to the entire Journal of the American Medical Association network including a subscription to the specialty journal of your choice.
Patient Advocacy
Patient advocacy groups generally have 3 main goals: education (for patients, patient support networks, and the layperson), research, and lobbying for issues that are in the interest of patients and treatment of dermatologic conditions (eg, funding support, regulation of medical devices, etc).3 In dermatology, the number of patient advocacy groups is growing to represent a myriad of dermatologic conditions, from common conditions like psoriasis to rare genodermatoses (Table). As dermatologists in training, it is key for residents to be involved in patient advocacy and to be aware of the resources that exist for patients to access educational information and support for their respective conditions. These educational materials can help provide more comprehensive care for patients and give patients more autonomy in choosing a physician or hospital to manage their care, help patients become more knowledgeable about available treatment options, and arm patients with more information to address questions that may arise from laypeople regarding their condition.
In terms of patient education, the resources available to patients include informational websites, access to educational materials like pamphlets and multimedia (eg, videos), and special events; for example, the National Psoriasis Foundation hosts walks for patients and their friends and family to raise money for the organization as well as to promote psoriasis awareness and give patients an opportunity to build a support network. Patient advocacy groups also help raise funding for research and have shown to be influential in research initiatives that are granted funding.3 Often, these groups also play a political role and take part in lobbying efforts by patients and support groups by working with politicians to raise awareness or request financial support for particular skin diseases.
The Society for Investigative Dermatology sponsors an application for mobile devices that can assist residents in referring patients to support and advocacy groups (http://www.skinadvocateapp.com).
Grassroots Advocacy
Grassroots advocacy in dermatology means that an individual or group of individuals (in this case, a resident or group of residents) is motivated to take action by contacting legislators and other government officials about gaps in funding and regulation for particular dermatology issues. These efforts often are noticed and taken into consideration by politicians because it is in their best interest to listen to their constituents rather than risk losing support.
The American Academy of Dermatology Association, the advocacy entity of the AAD, hosts the Dermatology Advocacy Network (www.aad-dan.com/default.aspx), which is dedicated to helping dermatologists become advocates. The DAN website helps residents easily identify and contact their local, state, and national legislators to discuss issues or concerns related to the dermatology specialty and medicine as a whole. For example, tanning bed regulation currently is a priority among dermatologists, and the DAN website provides customizable form letters that can be sent electronically to legislators for review.
Furthermore, the AAD offers helpful resources and suggestions for dermatologists and dermatology residents who want to get involved with grassroots advocacy efforts. The website (www.aad.org/advocacy) details current AAD advocacy priorities as well as specific topics such as Medicare physician payment, skin cancer and indoor tanning, drug pricing and availability, state policy, and network adequacy, as these are high-priority issues identified by the AAD that would benefit from action by its members.
Final Thoughts
Many opportunities exist for dermatology residents to get involved in advocacy, from opportunities on the national level with the AMA to patient advocacy and grassroots efforts. It is important for dermatology residents to get involved in advocacy efforts during their training so they may continue to be involved in these efforts as their careers develop. Advocacy helps keep the dermatology specialty relevant and maintain its voice in the national medical arena. It also enhances the dermatology resident’s ability to provide comprehensive quality care for patients by addressing some of their educational and supportive needs that perhaps cannot be addressed in a clinic visit alone. Advocacy also gives residents the opportunity to network and meet colleagues and other individuals with similar goals and interests, which may be beneficial for their future careers. Thus, early involvement in advocacy may be a productive and interesting part of dermatology residency for trainees to be further involved in the specialty.
- AMA mission & guiding principles. American Medical Association Web site. http://www.ama-assn.org/ama/pub/about-ama.page?. Accessed December 22, 2015.
- Specialty Society Representation Ballot. American Medical Association Web site. http://www.ama-assn.org/ama/pub/about-ama/our-people/the-federation-medicine/specialty-society-ballot.page. Accessed December 22, 2015.
- Nijsten T, Bergstresser PR. Patient advocacy groups: let’s stick together. J Invest Dermatol. 2010;130:1757-1759.
It is never too early (or too late!) to get involved in dermatology advocacy. Residency is an ideal time to start learning about advocating on behalf of the specialty of dermatology as well as on behalf of our patients. Many opportunities are available for residents to gain experience and become advocates on national and grassroots levels. As residents, participating in these efforts can help set a solid foundation for future involvement in advocacy, regardless of our ultimate career goals.
American Medical Association
The mission of the American Medical Association (AMA) is “to promote the art and science of medicine and the betterment of public health.”1 Joining the AMA costs $45 for 1 year of resident membership (with a discounted rate for multiyear memberships). As a member, you are given the opportunity to cast a ballot for the national medical specialty society that best represents you in the House of Delegates, the AMA’s principle policy-making body.2 The more votes a particular society receives, the more delegates from that society are added to the House of Delegates, meaning more representation for that specialty organization. It is advised that members choose the society that best represents them: for dermatologists, this most likely would be the American Academy of Dermatology (AAD), among other dermatology organizations that are candidates (ie, the American College of Mohs Surgery, the American Society for Dermatologic Surgery, and the Society for Investigative Dermatology). This representation is key for a specialty like dermatology, which has a relatively smaller number of physicians compared to other larger specialties and therefore has less representation in the House of Delegates.
Additionally, AMA membership grants you access to the entire Journal of the American Medical Association network including a subscription to the specialty journal of your choice.
Patient Advocacy
Patient advocacy groups generally have 3 main goals: education (for patients, patient support networks, and the layperson), research, and lobbying for issues that are in the interest of patients and treatment of dermatologic conditions (eg, funding support, regulation of medical devices, etc).3 In dermatology, the number of patient advocacy groups is growing to represent a myriad of dermatologic conditions, from common conditions like psoriasis to rare genodermatoses (Table). As dermatologists in training, it is key for residents to be involved in patient advocacy and to be aware of the resources that exist for patients to access educational information and support for their respective conditions. These educational materials can help provide more comprehensive care for patients and give patients more autonomy in choosing a physician or hospital to manage their care, help patients become more knowledgeable about available treatment options, and arm patients with more information to address questions that may arise from laypeople regarding their condition.

In terms of patient education, the resources available to patients include informational websites, access to educational materials like pamphlets and multimedia (eg, videos), and special events; for example, the National Psoriasis Foundation hosts walks for patients and their friends and family to raise money for the organization as well as to promote psoriasis awareness and give patients an opportunity to build a support network. Patient advocacy groups also help raise funding for research and have shown to be influential in research initiatives that are granted funding.3 Often, these groups also play a political role and take part in lobbying efforts by patients and support groups by working with politicians to raise awareness or request financial support for particular skin diseases.
The Society for Investigative Dermatology sponsors an application for mobile devices that can assist residents in referring patients to support and advocacy groups (http://www.skinadvocateapp.com).
Grassroots Advocacy
Grassroots advocacy in dermatology means that an individual or group of individuals (in this case, a resident or group of residents) is motivated to take action by contacting legislators and other government officials about gaps in funding and regulation for particular dermatology issues. These efforts often are noticed and taken into consideration by politicians because it is in their best interest to listen to their constituents rather than risk losing support.
The American Academy of Dermatology Association, the advocacy entity of the AAD, hosts the Dermatology Advocacy Network (www.aad-dan.com/default.aspx), which is dedicated to helping dermatologists become advocates. The DAN website helps residents easily identify and contact their local, state, and national legislators to discuss issues or concerns related to the dermatology specialty and medicine as a whole. For example, tanning bed regulation currently is a priority among dermatologists, and the DAN website provides customizable form letters that can be sent electronically to legislators for review.
Furthermore, the AAD offers helpful resources and suggestions for dermatologists and dermatology residents who want to get involved with grassroots advocacy efforts. The website (www.aad.org/advocacy) details current AAD advocacy priorities as well as specific topics such as Medicare physician payment, skin cancer and indoor tanning, drug pricing and availability, state policy, and network adequacy, as these are high-priority issues identified by the AAD that would benefit from action by its members.
Final Thoughts
Many opportunities exist for dermatology residents to get involved in advocacy, from opportunities on the national level with the AMA to patient advocacy and grassroots efforts. It is important for dermatology residents to get involved in advocacy efforts during their training so they may continue to be involved in these efforts as their careers develop. Advocacy helps keep the dermatology specialty relevant and maintain its voice in the national medical arena. It also enhances the dermatology resident’s ability to provide comprehensive quality care for patients by addressing some of their educational and supportive needs that perhaps cannot be addressed in a clinic visit alone. Advocacy also gives residents the opportunity to network and meet colleagues and other individuals with similar goals and interests, which may be beneficial for their future careers. Thus, early involvement in advocacy may be a productive and interesting part of dermatology residency for trainees to be further involved in the specialty.
It is never too early (or too late!) to get involved in dermatology advocacy. Residency is an ideal time to start learning about advocating on behalf of the specialty of dermatology as well as on behalf of our patients. Many opportunities are available for residents to gain experience and become advocates on national and grassroots levels. As residents, participating in these efforts can help set a solid foundation for future involvement in advocacy, regardless of our ultimate career goals.
American Medical Association
The mission of the American Medical Association (AMA) is “to promote the art and science of medicine and the betterment of public health.”1 Joining the AMA costs $45 for 1 year of resident membership (with a discounted rate for multiyear memberships). As a member, you are given the opportunity to cast a ballot for the national medical specialty society that best represents you in the House of Delegates, the AMA’s principle policy-making body.2 The more votes a particular society receives, the more delegates from that society are added to the House of Delegates, meaning more representation for that specialty organization. It is advised that members choose the society that best represents them: for dermatologists, this most likely would be the American Academy of Dermatology (AAD), among other dermatology organizations that are candidates (ie, the American College of Mohs Surgery, the American Society for Dermatologic Surgery, and the Society for Investigative Dermatology). This representation is key for a specialty like dermatology, which has a relatively smaller number of physicians compared to other larger specialties and therefore has less representation in the House of Delegates.
Additionally, AMA membership grants you access to the entire Journal of the American Medical Association network including a subscription to the specialty journal of your choice.
Patient Advocacy
Patient advocacy groups generally have 3 main goals: education (for patients, patient support networks, and the layperson), research, and lobbying for issues that are in the interest of patients and treatment of dermatologic conditions (eg, funding support, regulation of medical devices, etc).3 In dermatology, the number of patient advocacy groups is growing to represent a myriad of dermatologic conditions, from common conditions like psoriasis to rare genodermatoses (Table). As dermatologists in training, it is key for residents to be involved in patient advocacy and to be aware of the resources that exist for patients to access educational information and support for their respective conditions. These educational materials can help provide more comprehensive care for patients and give patients more autonomy in choosing a physician or hospital to manage their care, help patients become more knowledgeable about available treatment options, and arm patients with more information to address questions that may arise from laypeople regarding their condition.

In terms of patient education, the resources available to patients include informational websites, access to educational materials like pamphlets and multimedia (eg, videos), and special events; for example, the National Psoriasis Foundation hosts walks for patients and their friends and family to raise money for the organization as well as to promote psoriasis awareness and give patients an opportunity to build a support network. Patient advocacy groups also help raise funding for research and have shown to be influential in research initiatives that are granted funding.3 Often, these groups also play a political role and take part in lobbying efforts by patients and support groups by working with politicians to raise awareness or request financial support for particular skin diseases.
The Society for Investigative Dermatology sponsors an application for mobile devices that can assist residents in referring patients to support and advocacy groups (http://www.skinadvocateapp.com).
Grassroots Advocacy
Grassroots advocacy in dermatology means that an individual or group of individuals (in this case, a resident or group of residents) is motivated to take action by contacting legislators and other government officials about gaps in funding and regulation for particular dermatology issues. These efforts often are noticed and taken into consideration by politicians because it is in their best interest to listen to their constituents rather than risk losing support.
The American Academy of Dermatology Association, the advocacy entity of the AAD, hosts the Dermatology Advocacy Network (www.aad-dan.com/default.aspx), which is dedicated to helping dermatologists become advocates. The DAN website helps residents easily identify and contact their local, state, and national legislators to discuss issues or concerns related to the dermatology specialty and medicine as a whole. For example, tanning bed regulation currently is a priority among dermatologists, and the DAN website provides customizable form letters that can be sent electronically to legislators for review.
Furthermore, the AAD offers helpful resources and suggestions for dermatologists and dermatology residents who want to get involved with grassroots advocacy efforts. The website (www.aad.org/advocacy) details current AAD advocacy priorities as well as specific topics such as Medicare physician payment, skin cancer and indoor tanning, drug pricing and availability, state policy, and network adequacy, as these are high-priority issues identified by the AAD that would benefit from action by its members.
Final Thoughts
Many opportunities exist for dermatology residents to get involved in advocacy, from opportunities on the national level with the AMA to patient advocacy and grassroots efforts. It is important for dermatology residents to get involved in advocacy efforts during their training so they may continue to be involved in these efforts as their careers develop. Advocacy helps keep the dermatology specialty relevant and maintain its voice in the national medical arena. It also enhances the dermatology resident’s ability to provide comprehensive quality care for patients by addressing some of their educational and supportive needs that perhaps cannot be addressed in a clinic visit alone. Advocacy also gives residents the opportunity to network and meet colleagues and other individuals with similar goals and interests, which may be beneficial for their future careers. Thus, early involvement in advocacy may be a productive and interesting part of dermatology residency for trainees to be further involved in the specialty.
- AMA mission & guiding principles. American Medical Association Web site. http://www.ama-assn.org/ama/pub/about-ama.page?. Accessed December 22, 2015.
- Specialty Society Representation Ballot. American Medical Association Web site. http://www.ama-assn.org/ama/pub/about-ama/our-people/the-federation-medicine/specialty-society-ballot.page. Accessed December 22, 2015.
- Nijsten T, Bergstresser PR. Patient advocacy groups: let’s stick together. J Invest Dermatol. 2010;130:1757-1759.
- AMA mission & guiding principles. American Medical Association Web site. http://www.ama-assn.org/ama/pub/about-ama.page?. Accessed December 22, 2015.
- Specialty Society Representation Ballot. American Medical Association Web site. http://www.ama-assn.org/ama/pub/about-ama/our-people/the-federation-medicine/specialty-society-ballot.page. Accessed December 22, 2015.
- Nijsten T, Bergstresser PR. Patient advocacy groups: let’s stick together. J Invest Dermatol. 2010;130:1757-1759.