User login
Histologic Correlation of Dermoscopy Findings in a Sebaceous Nevus
To the Editor:
Sebaceous nevus (SN) is a relatively common hamartoma that presents most often as a single congenital hairless plaque on the scalp. After puberty, histologic features characteristically include papillomatous hyperplasia of the epidermis, a large number of mature or nearly mature sebaceous glands, and a lack of terminally differentiated hair follicles; however, histologic findings can be misleading during childhood when sebaceous glands are still underdeveloped. Bright yellow dots, which are thought to indicate the presence of sebaceous glands, may be seen on dermoscopy and can be useful in differentiating SN from aplasia cutis congenita in newborns.
We report a case of an SN in an 18-year-old woman and discuss how the histology findings correlated with features seen on dermoscopy.
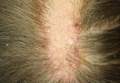
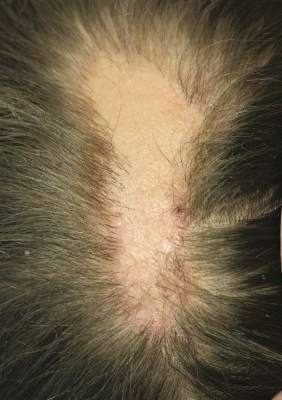
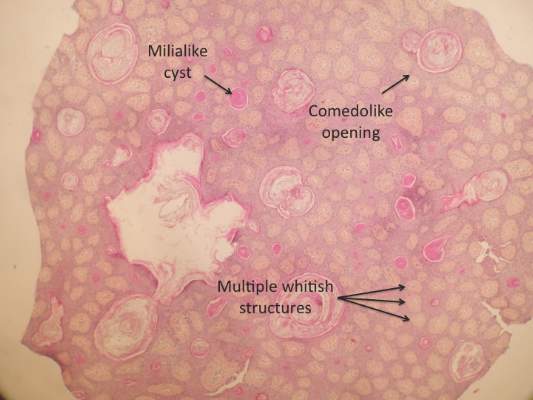
An 18-year-old woman presented to our dermatology clinic with an asymptomatic, hairless plaque on the right parietal scalp that had been present since birth. The patient noted that the plaque had recently become larger in size. On physical examination, an 8×3-cm plaque with a smooth, flesh-colored surface was noted with central comedolike structures and an erythematous, verrucous periphery (Figure 1).
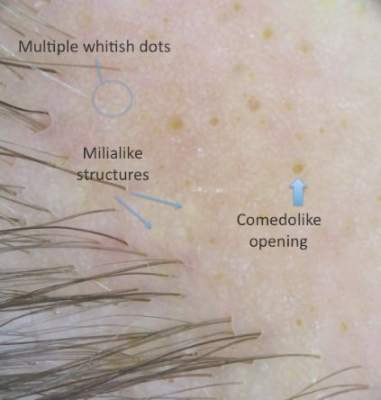
Dermoscopy (handheld dermoscope using polarized light) revealed 3 distinct types of round structures within the lesion: (1) comedolike openings (similar to those seen in seborrheic keratosis) that appeared as brownish-yellow, sharply circumscribed structures; (2) milialike cysts (also found in acanthotic seborrheic keratosis), which appeared as bright yellow structures; and (3) multiple whitish structures that were irregular in shape and size and covered the surface of the lesion where there were no other dermoscopic findings (Figure 2). The affected skin was pale to red in color and the verrucous aspect of the surface was better visualized at the edge of the lesion.
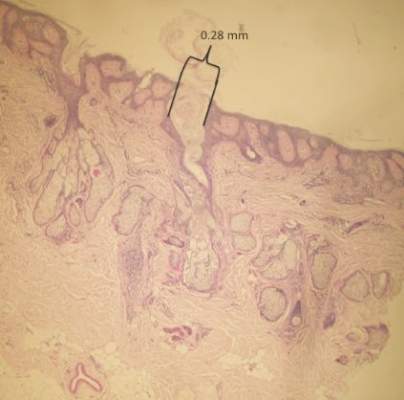
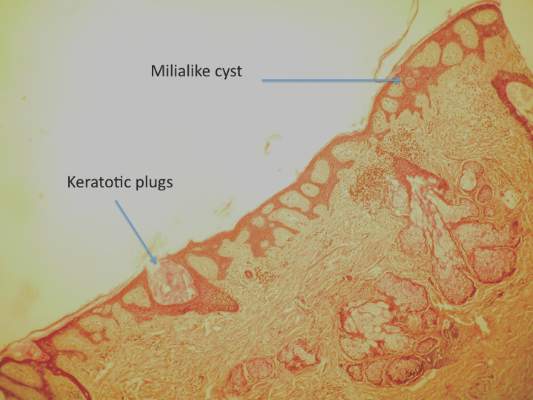
Two 4-mm punch biopsies were performed following dermoscopy: one for horizontal sectioning and one for vertical sectioning. Histologic analysis showed an acanthotic epidermis with an anastomosing network of elongated rete ridges in the superficial dermis. Numerous hyperplasic sebaceous glands were found in the mid dermis, with some also located above this level. Immature hair follicles were present and sebaceous gland ducts communicated directly with the epidermis through dilated hyperkeratinized pathways. Eccrine glands were normal, but no apocrine glands were present. A lymphocytic infiltrate was noted around the sebaceous glands and immature hair follicles and also around dilated capillaries in the superficial dermis. Moderate spongiosis and lymphocytic exocytosis were noted in the glandular epithelium and in the basal layer of the hair follicles and the epidermis. Superficial slides of horizontal sections of the biopsy specimen showed a correlation between the histology findings and dermoscopy images: multiple normal-appearing papilla surrounded by a network of anastomosing rete ridges correlated with multiple whitish structures, keratotic cysts with compact keratin corresponded to bright yellow dots, and larger conglomerates of loose lamelar keratin correlated with comedolike openings. Due to the presence of eczematous changes (eg, epithelial spongiosis, inflammatory cells) observed on histology, a diagnosis of an irritated sebaceous nevus was made, which explained the recent enlargement of the congenital lesion.
Sebaceous nevus is a benign, epidermal appendageal tumor with differentiation towards sebaceous glands that is composed of mature or nearly mature skin structures. Histologically, it is classified as a hamartoma.1 It commonly arises on the scalp as a yellowish or flesh-colored, hairless plaque of variable size. At birth, its surface is smooth and the differential diagnoses include aplasia cutis congenita, congenital triangular alopecia, and alopecia areata.2 As the patient ages, hormones stimulate the proliferation of sebaceous glands and the epidermis, and the lesion gradually acquires a verrucous, waxy surface.3 Benign appendageal tumors often develop inside SN. Basal cell epitheliomas are rarely found.4 Surgical excision is recommended for aesthetic purposes or to prevent the development of tumors.
Histology also varies with the patient’s age and can be misleading in childhood because the sebaceous glands are underdeveloped.5,6 After adrenarche, histology becomes more diagnostic, showing a dermis almost completely filled with sebaceous glands with varying degrees of maturity.2 The presence of incompletely differentiated follicles without hair shafts can be found in newborns and children and may be helpful for the correct histological diagnosis before puberty.1,5 The epidermis presents no abnormalities at birth but develops acanthosis and papillomatosis as the patient ages. Ectopic dilated apocrine glands sometimes can be found deeper in the dermis in the late stage of the lesion.5
In a report by Neri et al,7 multiple bright yellow dots were noted on dermoscopy in 2 children with SN. The investigators concluded that this characteristic feature, which was thought to represent the sebaceous glands, can be useful in differentiating SN from aplasia cutis congenita in early infancy, but no histologic analyses were performed.7 In our patient, we identified 3 different dermoscopic features that correlated with histologic findings. Comedolike openings correlated with the accumulated keratin (ie, keratotic plugs) inside dilated sebaceous gland ducts directly connected to the epidermis. The brownish-yellow color of these openings observed on dermoscopy may be due to the oxidation of kerat-inous material, such as those in seborrheic keratosis lesions (Figure 3). We also noted bright yellow dots similar to those reported by Neri et al7; however, histologic analysis in our patient showed these dots more closely correlated with keratotic cysts similar to milialike structures seen in acanthotic seborrheic keratosis. The material remained lightly colored because no oxidation process had occurred (Figure 4). The third structure found on dermoscopy in our patient was multiple whitish structures that were irregular in shape and size. According to our comparison of superficial horizontal histology slides with dermoscopy images, we hypothesized this finding was the result of epidermal papillomatosis over a dermis filled with enlarged sebaceous glands (Figure 5). This finding was likely absent in the cases previously reported by Neri et al7 because epidermal and glandular changes occur later in the evolution of SN and the patients in these cases were younger than 4 months old.
Our correlation of dermoscopic features with histology findings in an 18-year-old woman with an irritated SN highlights the need for more studies needed in order to establish the prevalence of certain dermoscopic findings in this setting, particularly considering the important morphological changes that occur in these lesions as patients age as well as the histological variation among different hamartomas. Over the last decade, dermoscopy has proven to be a useful tool in the diagnosis of various hair and scalp diseases.8 Histologic correlation of dermoscopy findings is essential for more precise understanding of this new imaging technique and should be conducted whenever possible.
- Lever WF, Schaumburg-Lever G. Tumors of the epidermal appendages. In: Lever WF, Schaumburg-Lever G, eds. Histopathology of the Skin. 5th ed. Philadelphia, PA: Lippincott Co; 1975:498-502.
- Civatte J. Tumeurs du cuir chevelu. In: Bouhanna P, Reygagne P, eds. Pathologie du Cheveu et du Cuir Cheveulu. Paris, France: Masson Co; 1999:208-209.
- Gruβendorf-Conen E-I. Adnexal cysts and tumors of the scalp. In: Orfanos CE, Happle R, eds. Hair and Hair Diseases. 1st ed. Berlin Germany: Springer-Verlag Berlin Heidelberg Co; 1990:710-711.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceous: a study of 596 cases. J Am Acad Dermatol. 2000;42(2 pt 1):263-268.
- Camacho F. Tumeurs du cuir chevelu. In: Camacho F, Montagna W, eds. Trichologie: Maladie du Follicule Pilosébacé. Madrid, Spain: Grupo Aula Medica; 1997:515-516.
- Wechsler J. Hamartome sebace. In: Wechsler J, Fraitag S, Moulonguet I, eds. Pathologie Cutanee Tumorale. Montpelier, France: Sauramps Medical Co; 2009:100-102.
- Neri I, Savoia F, Giacomini F, et al. Usefulness of dermatoscopy for the early diagnosis of sebaceous naevus and differentiation from aplasia cutis congenita [published online ahead of print May 5, 2009]. Clin Exp Dermatol. 2009;34:e50-e52.
- Miteva M, Tosti A. Hair and scalp dermatoscopy. J Am Acad Dermatol. 2012;67:1040-1048.
To the Editor:
Sebaceous nevus (SN) is a relatively common hamartoma that presents most often as a single congenital hairless plaque on the scalp. After puberty, histologic features characteristically include papillomatous hyperplasia of the epidermis, a large number of mature or nearly mature sebaceous glands, and a lack of terminally differentiated hair follicles; however, histologic findings can be misleading during childhood when sebaceous glands are still underdeveloped. Bright yellow dots, which are thought to indicate the presence of sebaceous glands, may be seen on dermoscopy and can be useful in differentiating SN from aplasia cutis congenita in newborns.
We report a case of an SN in an 18-year-old woman and discuss how the histology findings correlated with features seen on dermoscopy.
An 18-year-old woman presented to our dermatology clinic with an asymptomatic, hairless plaque on the right parietal scalp that had been present since birth. The patient noted that the plaque had recently become larger in size. On physical examination, an 8×3-cm plaque with a smooth, flesh-colored surface was noted with central comedolike structures and an erythematous, verrucous periphery (Figure 1).

Dermoscopy (handheld dermoscope using polarized light) revealed 3 distinct types of round structures within the lesion: (1) comedolike openings (similar to those seen in seborrheic keratosis) that appeared as brownish-yellow, sharply circumscribed structures; (2) milialike cysts (also found in acanthotic seborrheic keratosis), which appeared as bright yellow structures; and (3) multiple whitish structures that were irregular in shape and size and covered the surface of the lesion where there were no other dermoscopic findings (Figure 2). The affected skin was pale to red in color and the verrucous aspect of the surface was better visualized at the edge of the lesion.

Two 4-mm punch biopsies were performed following dermoscopy: one for horizontal sectioning and one for vertical sectioning. Histologic analysis showed an acanthotic epidermis with an anastomosing network of elongated rete ridges in the superficial dermis. Numerous hyperplasic sebaceous glands were found in the mid dermis, with some also located above this level. Immature hair follicles were present and sebaceous gland ducts communicated directly with the epidermis through dilated hyperkeratinized pathways. Eccrine glands were normal, but no apocrine glands were present. A lymphocytic infiltrate was noted around the sebaceous glands and immature hair follicles and also around dilated capillaries in the superficial dermis. Moderate spongiosis and lymphocytic exocytosis were noted in the glandular epithelium and in the basal layer of the hair follicles and the epidermis. Superficial slides of horizontal sections of the biopsy specimen showed a correlation between the histology findings and dermoscopy images: multiple normal-appearing papilla surrounded by a network of anastomosing rete ridges correlated with multiple whitish structures, keratotic cysts with compact keratin corresponded to bright yellow dots, and larger conglomerates of loose lamelar keratin correlated with comedolike openings. Due to the presence of eczematous changes (eg, epithelial spongiosis, inflammatory cells) observed on histology, a diagnosis of an irritated sebaceous nevus was made, which explained the recent enlargement of the congenital lesion.
Sebaceous nevus is a benign, epidermal appendageal tumor with differentiation towards sebaceous glands that is composed of mature or nearly mature skin structures. Histologically, it is classified as a hamartoma.1 It commonly arises on the scalp as a yellowish or flesh-colored, hairless plaque of variable size. At birth, its surface is smooth and the differential diagnoses include aplasia cutis congenita, congenital triangular alopecia, and alopecia areata.2 As the patient ages, hormones stimulate the proliferation of sebaceous glands and the epidermis, and the lesion gradually acquires a verrucous, waxy surface.3 Benign appendageal tumors often develop inside SN. Basal cell epitheliomas are rarely found.4 Surgical excision is recommended for aesthetic purposes or to prevent the development of tumors.
Histology also varies with the patient’s age and can be misleading in childhood because the sebaceous glands are underdeveloped.5,6 After adrenarche, histology becomes more diagnostic, showing a dermis almost completely filled with sebaceous glands with varying degrees of maturity.2 The presence of incompletely differentiated follicles without hair shafts can be found in newborns and children and may be helpful for the correct histological diagnosis before puberty.1,5 The epidermis presents no abnormalities at birth but develops acanthosis and papillomatosis as the patient ages. Ectopic dilated apocrine glands sometimes can be found deeper in the dermis in the late stage of the lesion.5
In a report by Neri et al,7 multiple bright yellow dots were noted on dermoscopy in 2 children with SN. The investigators concluded that this characteristic feature, which was thought to represent the sebaceous glands, can be useful in differentiating SN from aplasia cutis congenita in early infancy, but no histologic analyses were performed.7 In our patient, we identified 3 different dermoscopic features that correlated with histologic findings. Comedolike openings correlated with the accumulated keratin (ie, keratotic plugs) inside dilated sebaceous gland ducts directly connected to the epidermis. The brownish-yellow color of these openings observed on dermoscopy may be due to the oxidation of kerat-inous material, such as those in seborrheic keratosis lesions (Figure 3). We also noted bright yellow dots similar to those reported by Neri et al7; however, histologic analysis in our patient showed these dots more closely correlated with keratotic cysts similar to milialike structures seen in acanthotic seborrheic keratosis. The material remained lightly colored because no oxidation process had occurred (Figure 4). The third structure found on dermoscopy in our patient was multiple whitish structures that were irregular in shape and size. According to our comparison of superficial horizontal histology slides with dermoscopy images, we hypothesized this finding was the result of epidermal papillomatosis over a dermis filled with enlarged sebaceous glands (Figure 5). This finding was likely absent in the cases previously reported by Neri et al7 because epidermal and glandular changes occur later in the evolution of SN and the patients in these cases were younger than 4 months old.



Our correlation of dermoscopic features with histology findings in an 18-year-old woman with an irritated SN highlights the need for more studies needed in order to establish the prevalence of certain dermoscopic findings in this setting, particularly considering the important morphological changes that occur in these lesions as patients age as well as the histological variation among different hamartomas. Over the last decade, dermoscopy has proven to be a useful tool in the diagnosis of various hair and scalp diseases.8 Histologic correlation of dermoscopy findings is essential for more precise understanding of this new imaging technique and should be conducted whenever possible.
To the Editor:
Sebaceous nevus (SN) is a relatively common hamartoma that presents most often as a single congenital hairless plaque on the scalp. After puberty, histologic features characteristically include papillomatous hyperplasia of the epidermis, a large number of mature or nearly mature sebaceous glands, and a lack of terminally differentiated hair follicles; however, histologic findings can be misleading during childhood when sebaceous glands are still underdeveloped. Bright yellow dots, which are thought to indicate the presence of sebaceous glands, may be seen on dermoscopy and can be useful in differentiating SN from aplasia cutis congenita in newborns.
We report a case of an SN in an 18-year-old woman and discuss how the histology findings correlated with features seen on dermoscopy.
An 18-year-old woman presented to our dermatology clinic with an asymptomatic, hairless plaque on the right parietal scalp that had been present since birth. The patient noted that the plaque had recently become larger in size. On physical examination, an 8×3-cm plaque with a smooth, flesh-colored surface was noted with central comedolike structures and an erythematous, verrucous periphery (Figure 1).

Dermoscopy (handheld dermoscope using polarized light) revealed 3 distinct types of round structures within the lesion: (1) comedolike openings (similar to those seen in seborrheic keratosis) that appeared as brownish-yellow, sharply circumscribed structures; (2) milialike cysts (also found in acanthotic seborrheic keratosis), which appeared as bright yellow structures; and (3) multiple whitish structures that were irregular in shape and size and covered the surface of the lesion where there were no other dermoscopic findings (Figure 2). The affected skin was pale to red in color and the verrucous aspect of the surface was better visualized at the edge of the lesion.

Two 4-mm punch biopsies were performed following dermoscopy: one for horizontal sectioning and one for vertical sectioning. Histologic analysis showed an acanthotic epidermis with an anastomosing network of elongated rete ridges in the superficial dermis. Numerous hyperplasic sebaceous glands were found in the mid dermis, with some also located above this level. Immature hair follicles were present and sebaceous gland ducts communicated directly with the epidermis through dilated hyperkeratinized pathways. Eccrine glands were normal, but no apocrine glands were present. A lymphocytic infiltrate was noted around the sebaceous glands and immature hair follicles and also around dilated capillaries in the superficial dermis. Moderate spongiosis and lymphocytic exocytosis were noted in the glandular epithelium and in the basal layer of the hair follicles and the epidermis. Superficial slides of horizontal sections of the biopsy specimen showed a correlation between the histology findings and dermoscopy images: multiple normal-appearing papilla surrounded by a network of anastomosing rete ridges correlated with multiple whitish structures, keratotic cysts with compact keratin corresponded to bright yellow dots, and larger conglomerates of loose lamelar keratin correlated with comedolike openings. Due to the presence of eczematous changes (eg, epithelial spongiosis, inflammatory cells) observed on histology, a diagnosis of an irritated sebaceous nevus was made, which explained the recent enlargement of the congenital lesion.
Sebaceous nevus is a benign, epidermal appendageal tumor with differentiation towards sebaceous glands that is composed of mature or nearly mature skin structures. Histologically, it is classified as a hamartoma.1 It commonly arises on the scalp as a yellowish or flesh-colored, hairless plaque of variable size. At birth, its surface is smooth and the differential diagnoses include aplasia cutis congenita, congenital triangular alopecia, and alopecia areata.2 As the patient ages, hormones stimulate the proliferation of sebaceous glands and the epidermis, and the lesion gradually acquires a verrucous, waxy surface.3 Benign appendageal tumors often develop inside SN. Basal cell epitheliomas are rarely found.4 Surgical excision is recommended for aesthetic purposes or to prevent the development of tumors.
Histology also varies with the patient’s age and can be misleading in childhood because the sebaceous glands are underdeveloped.5,6 After adrenarche, histology becomes more diagnostic, showing a dermis almost completely filled with sebaceous glands with varying degrees of maturity.2 The presence of incompletely differentiated follicles without hair shafts can be found in newborns and children and may be helpful for the correct histological diagnosis before puberty.1,5 The epidermis presents no abnormalities at birth but develops acanthosis and papillomatosis as the patient ages. Ectopic dilated apocrine glands sometimes can be found deeper in the dermis in the late stage of the lesion.5
In a report by Neri et al,7 multiple bright yellow dots were noted on dermoscopy in 2 children with SN. The investigators concluded that this characteristic feature, which was thought to represent the sebaceous glands, can be useful in differentiating SN from aplasia cutis congenita in early infancy, but no histologic analyses were performed.7 In our patient, we identified 3 different dermoscopic features that correlated with histologic findings. Comedolike openings correlated with the accumulated keratin (ie, keratotic plugs) inside dilated sebaceous gland ducts directly connected to the epidermis. The brownish-yellow color of these openings observed on dermoscopy may be due to the oxidation of kerat-inous material, such as those in seborrheic keratosis lesions (Figure 3). We also noted bright yellow dots similar to those reported by Neri et al7; however, histologic analysis in our patient showed these dots more closely correlated with keratotic cysts similar to milialike structures seen in acanthotic seborrheic keratosis. The material remained lightly colored because no oxidation process had occurred (Figure 4). The third structure found on dermoscopy in our patient was multiple whitish structures that were irregular in shape and size. According to our comparison of superficial horizontal histology slides with dermoscopy images, we hypothesized this finding was the result of epidermal papillomatosis over a dermis filled with enlarged sebaceous glands (Figure 5). This finding was likely absent in the cases previously reported by Neri et al7 because epidermal and glandular changes occur later in the evolution of SN and the patients in these cases were younger than 4 months old.



Our correlation of dermoscopic features with histology findings in an 18-year-old woman with an irritated SN highlights the need for more studies needed in order to establish the prevalence of certain dermoscopic findings in this setting, particularly considering the important morphological changes that occur in these lesions as patients age as well as the histological variation among different hamartomas. Over the last decade, dermoscopy has proven to be a useful tool in the diagnosis of various hair and scalp diseases.8 Histologic correlation of dermoscopy findings is essential for more precise understanding of this new imaging technique and should be conducted whenever possible.
- Lever WF, Schaumburg-Lever G. Tumors of the epidermal appendages. In: Lever WF, Schaumburg-Lever G, eds. Histopathology of the Skin. 5th ed. Philadelphia, PA: Lippincott Co; 1975:498-502.
- Civatte J. Tumeurs du cuir chevelu. In: Bouhanna P, Reygagne P, eds. Pathologie du Cheveu et du Cuir Cheveulu. Paris, France: Masson Co; 1999:208-209.
- Gruβendorf-Conen E-I. Adnexal cysts and tumors of the scalp. In: Orfanos CE, Happle R, eds. Hair and Hair Diseases. 1st ed. Berlin Germany: Springer-Verlag Berlin Heidelberg Co; 1990:710-711.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceous: a study of 596 cases. J Am Acad Dermatol. 2000;42(2 pt 1):263-268.
- Camacho F. Tumeurs du cuir chevelu. In: Camacho F, Montagna W, eds. Trichologie: Maladie du Follicule Pilosébacé. Madrid, Spain: Grupo Aula Medica; 1997:515-516.
- Wechsler J. Hamartome sebace. In: Wechsler J, Fraitag S, Moulonguet I, eds. Pathologie Cutanee Tumorale. Montpelier, France: Sauramps Medical Co; 2009:100-102.
- Neri I, Savoia F, Giacomini F, et al. Usefulness of dermatoscopy for the early diagnosis of sebaceous naevus and differentiation from aplasia cutis congenita [published online ahead of print May 5, 2009]. Clin Exp Dermatol. 2009;34:e50-e52.
- Miteva M, Tosti A. Hair and scalp dermatoscopy. J Am Acad Dermatol. 2012;67:1040-1048.
- Lever WF, Schaumburg-Lever G. Tumors of the epidermal appendages. In: Lever WF, Schaumburg-Lever G, eds. Histopathology of the Skin. 5th ed. Philadelphia, PA: Lippincott Co; 1975:498-502.
- Civatte J. Tumeurs du cuir chevelu. In: Bouhanna P, Reygagne P, eds. Pathologie du Cheveu et du Cuir Cheveulu. Paris, France: Masson Co; 1999:208-209.
- Gruβendorf-Conen E-I. Adnexal cysts and tumors of the scalp. In: Orfanos CE, Happle R, eds. Hair and Hair Diseases. 1st ed. Berlin Germany: Springer-Verlag Berlin Heidelberg Co; 1990:710-711.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceous: a study of 596 cases. J Am Acad Dermatol. 2000;42(2 pt 1):263-268.
- Camacho F. Tumeurs du cuir chevelu. In: Camacho F, Montagna W, eds. Trichologie: Maladie du Follicule Pilosébacé. Madrid, Spain: Grupo Aula Medica; 1997:515-516.
- Wechsler J. Hamartome sebace. In: Wechsler J, Fraitag S, Moulonguet I, eds. Pathologie Cutanee Tumorale. Montpelier, France: Sauramps Medical Co; 2009:100-102.
- Neri I, Savoia F, Giacomini F, et al. Usefulness of dermatoscopy for the early diagnosis of sebaceous naevus and differentiation from aplasia cutis congenita [published online ahead of print May 5, 2009]. Clin Exp Dermatol. 2009;34:e50-e52.
- Miteva M, Tosti A. Hair and scalp dermatoscopy. J Am Acad Dermatol. 2012;67:1040-1048.
Cardiovascular risk assessment required with use of TKIs for CML
Treatment for chronic myeloid leukemia (CML) entails effective but mostly noncurative long-term use of tyrosine kinase inhibitors (TKIs) that require proactive, rational approaches to minimizing cardiovascular toxicities, according to a recent review.
Survival rates of patients with newly diagnosed CML are about 90%, and in those with a complete cytogenetic response, survival is comparable to that of age-matched controls. Although second-generation TKIs have increased efficacy, survival rates are similar to those of imatinib, possibly due in part to mortality from non-CML causes.
TKIs used in CML therapy target BCR-ABL1, but their potencies vary against other kinases, including receptors for vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF). The relationship between off-target activities and adverse events (AEs) remains unclear, and AE management is largely empirical, said Dr. Javid Moslehi of Vanderbilt University Medical Center, Nashville, Tenn., and Dr. Michael Deininger, professor at the University of Utah Huntsman Cancer Institute, Salt Lake City.
“Reports of cardiovascular AEs with nilotinib, pulmonary arterial hypertension (PAH) on dasatinib, and frequent cardiovascular AEs with ponatinib have caused a reassessment of the situation,” they noted.
“Given the high population frequency of cardiovascular disease and the increased frequency of vascular events with nilotinib and ponatinib, cardiovascular risk assessment and, if necessary, treatment need to be integrated into the management of patients with CML on TKIs,” they wrote (J Clin Onc. 2015 Dec 10. doi: 10.1200/JCO.2015.62.4718).
Retrospective studies have indicated that imatinib may have favorable metabolic and vascular effects, but prospective controlled trials are lacking. Defining the cardiovascular baseline risk of the specific CML population under study will be crucial in future studies.
Dasatinib was approved for front-line CML treatment based on superior cytogenic response rates, compared with imatinib, but in 2011 the Food and Drug Administration warned against cardiopulmonary risks and recommended that patients be evaluated for signs and symptoms of cardiopulmonary disease before and during dasatinib treatment. Results of DASISION (Dasatinib Versus Imatinib Study in Treatment-Naive CML Patients) showed that, at 36 months of follow-up, PAH was reported in 3% of patients on dasatinib and 0% on imatinib.
Nilotinib has shown superior efficacy to imatinib and was FDA approved for first-line therapy, with recommendations for arrhythmia monitoring and avoidance of QT interval–prolonging medications. There have been no subsequent reports of ventricular arrhythmias with nilotinib, but 36% of patients on nilotinib experienced hyperglycemia in the ENESTnd (Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients) study, compared with 20% on imatinib. Nilotinib also has been associated with hyperlipidemia and increased body mass. Recent results point to vascular toxicity with nilotinib. At the 6-year follow-up of the ENESTnd study, 10% of patients on nilotinib 300 mg twice per day and 16% on nilotinib 400 mg twice per day had cardiovascular events, compared with 2.5% of patients taking imatinib 400 mg once per day. The dose-dependent increased risk implicates a drug-dependent process.
Ponatinib is the only clinical TKI active against the BCR-ABL1T315I mutation. It is a potent inhibitor of numerous other kinases as well, including VEGF receptors. In the PACE (Ponatinib Ph-positive Acute Lymphoblastic Leukemia and CML Evaluation) study, 26% of patients on ponatinib developed hypertension, and traditional atherosclerosis risk factors (age, hypertension, and diabetes) predisposed patients to serious vascular AEs. Cardiovascular toxicity was shown to be dose dependent, and older patients with history of diabetes or ischemic events are the least tolerant of high dose intensity. A subset of patients will benefit from ponatinib, particularly those with BCR-ABL1T315I, but leukemia-related and cardiovascular risks must both be assessed.
Dr. Moslehi reported financial ties with Novartis, ARIAD, Takeda/Millennium, Bristol-Myers Squibb, and Acceleron Pharma. Dr. Deininger reported ties to Novartis, Bristol-Myers Squibb, Incyte, ARIAD, Pfizer, and Cellgene.
Treatment for chronic myeloid leukemia (CML) entails effective but mostly noncurative long-term use of tyrosine kinase inhibitors (TKIs) that require proactive, rational approaches to minimizing cardiovascular toxicities, according to a recent review.
Survival rates of patients with newly diagnosed CML are about 90%, and in those with a complete cytogenetic response, survival is comparable to that of age-matched controls. Although second-generation TKIs have increased efficacy, survival rates are similar to those of imatinib, possibly due in part to mortality from non-CML causes.
TKIs used in CML therapy target BCR-ABL1, but their potencies vary against other kinases, including receptors for vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF). The relationship between off-target activities and adverse events (AEs) remains unclear, and AE management is largely empirical, said Dr. Javid Moslehi of Vanderbilt University Medical Center, Nashville, Tenn., and Dr. Michael Deininger, professor at the University of Utah Huntsman Cancer Institute, Salt Lake City.
“Reports of cardiovascular AEs with nilotinib, pulmonary arterial hypertension (PAH) on dasatinib, and frequent cardiovascular AEs with ponatinib have caused a reassessment of the situation,” they noted.
“Given the high population frequency of cardiovascular disease and the increased frequency of vascular events with nilotinib and ponatinib, cardiovascular risk assessment and, if necessary, treatment need to be integrated into the management of patients with CML on TKIs,” they wrote (J Clin Onc. 2015 Dec 10. doi: 10.1200/JCO.2015.62.4718).
Retrospective studies have indicated that imatinib may have favorable metabolic and vascular effects, but prospective controlled trials are lacking. Defining the cardiovascular baseline risk of the specific CML population under study will be crucial in future studies.
Dasatinib was approved for front-line CML treatment based on superior cytogenic response rates, compared with imatinib, but in 2011 the Food and Drug Administration warned against cardiopulmonary risks and recommended that patients be evaluated for signs and symptoms of cardiopulmonary disease before and during dasatinib treatment. Results of DASISION (Dasatinib Versus Imatinib Study in Treatment-Naive CML Patients) showed that, at 36 months of follow-up, PAH was reported in 3% of patients on dasatinib and 0% on imatinib.
Nilotinib has shown superior efficacy to imatinib and was FDA approved for first-line therapy, with recommendations for arrhythmia monitoring and avoidance of QT interval–prolonging medications. There have been no subsequent reports of ventricular arrhythmias with nilotinib, but 36% of patients on nilotinib experienced hyperglycemia in the ENESTnd (Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients) study, compared with 20% on imatinib. Nilotinib also has been associated with hyperlipidemia and increased body mass. Recent results point to vascular toxicity with nilotinib. At the 6-year follow-up of the ENESTnd study, 10% of patients on nilotinib 300 mg twice per day and 16% on nilotinib 400 mg twice per day had cardiovascular events, compared with 2.5% of patients taking imatinib 400 mg once per day. The dose-dependent increased risk implicates a drug-dependent process.
Ponatinib is the only clinical TKI active against the BCR-ABL1T315I mutation. It is a potent inhibitor of numerous other kinases as well, including VEGF receptors. In the PACE (Ponatinib Ph-positive Acute Lymphoblastic Leukemia and CML Evaluation) study, 26% of patients on ponatinib developed hypertension, and traditional atherosclerosis risk factors (age, hypertension, and diabetes) predisposed patients to serious vascular AEs. Cardiovascular toxicity was shown to be dose dependent, and older patients with history of diabetes or ischemic events are the least tolerant of high dose intensity. A subset of patients will benefit from ponatinib, particularly those with BCR-ABL1T315I, but leukemia-related and cardiovascular risks must both be assessed.
Dr. Moslehi reported financial ties with Novartis, ARIAD, Takeda/Millennium, Bristol-Myers Squibb, and Acceleron Pharma. Dr. Deininger reported ties to Novartis, Bristol-Myers Squibb, Incyte, ARIAD, Pfizer, and Cellgene.
Treatment for chronic myeloid leukemia (CML) entails effective but mostly noncurative long-term use of tyrosine kinase inhibitors (TKIs) that require proactive, rational approaches to minimizing cardiovascular toxicities, according to a recent review.
Survival rates of patients with newly diagnosed CML are about 90%, and in those with a complete cytogenetic response, survival is comparable to that of age-matched controls. Although second-generation TKIs have increased efficacy, survival rates are similar to those of imatinib, possibly due in part to mortality from non-CML causes.
TKIs used in CML therapy target BCR-ABL1, but their potencies vary against other kinases, including receptors for vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF). The relationship between off-target activities and adverse events (AEs) remains unclear, and AE management is largely empirical, said Dr. Javid Moslehi of Vanderbilt University Medical Center, Nashville, Tenn., and Dr. Michael Deininger, professor at the University of Utah Huntsman Cancer Institute, Salt Lake City.
“Reports of cardiovascular AEs with nilotinib, pulmonary arterial hypertension (PAH) on dasatinib, and frequent cardiovascular AEs with ponatinib have caused a reassessment of the situation,” they noted.
“Given the high population frequency of cardiovascular disease and the increased frequency of vascular events with nilotinib and ponatinib, cardiovascular risk assessment and, if necessary, treatment need to be integrated into the management of patients with CML on TKIs,” they wrote (J Clin Onc. 2015 Dec 10. doi: 10.1200/JCO.2015.62.4718).
Retrospective studies have indicated that imatinib may have favorable metabolic and vascular effects, but prospective controlled trials are lacking. Defining the cardiovascular baseline risk of the specific CML population under study will be crucial in future studies.
Dasatinib was approved for front-line CML treatment based on superior cytogenic response rates, compared with imatinib, but in 2011 the Food and Drug Administration warned against cardiopulmonary risks and recommended that patients be evaluated for signs and symptoms of cardiopulmonary disease before and during dasatinib treatment. Results of DASISION (Dasatinib Versus Imatinib Study in Treatment-Naive CML Patients) showed that, at 36 months of follow-up, PAH was reported in 3% of patients on dasatinib and 0% on imatinib.
Nilotinib has shown superior efficacy to imatinib and was FDA approved for first-line therapy, with recommendations for arrhythmia monitoring and avoidance of QT interval–prolonging medications. There have been no subsequent reports of ventricular arrhythmias with nilotinib, but 36% of patients on nilotinib experienced hyperglycemia in the ENESTnd (Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients) study, compared with 20% on imatinib. Nilotinib also has been associated with hyperlipidemia and increased body mass. Recent results point to vascular toxicity with nilotinib. At the 6-year follow-up of the ENESTnd study, 10% of patients on nilotinib 300 mg twice per day and 16% on nilotinib 400 mg twice per day had cardiovascular events, compared with 2.5% of patients taking imatinib 400 mg once per day. The dose-dependent increased risk implicates a drug-dependent process.
Ponatinib is the only clinical TKI active against the BCR-ABL1T315I mutation. It is a potent inhibitor of numerous other kinases as well, including VEGF receptors. In the PACE (Ponatinib Ph-positive Acute Lymphoblastic Leukemia and CML Evaluation) study, 26% of patients on ponatinib developed hypertension, and traditional atherosclerosis risk factors (age, hypertension, and diabetes) predisposed patients to serious vascular AEs. Cardiovascular toxicity was shown to be dose dependent, and older patients with history of diabetes or ischemic events are the least tolerant of high dose intensity. A subset of patients will benefit from ponatinib, particularly those with BCR-ABL1T315I, but leukemia-related and cardiovascular risks must both be assessed.
Dr. Moslehi reported financial ties with Novartis, ARIAD, Takeda/Millennium, Bristol-Myers Squibb, and Acceleron Pharma. Dr. Deininger reported ties to Novartis, Bristol-Myers Squibb, Incyte, ARIAD, Pfizer, and Cellgene.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Most patients with chronic myeloid leukemia require long-term tyrosine kinase inhibitor (TKI) therapy, and cardiovascular effects are critical factors in treatment decisions.
Major finding: Second- and third-generation TKIs have been associated with more cardiovascular risk than first-generation imatinib.
Data source: Review of current literature on cardiovascular toxicity of BCR-ABL1 TKIs for treatment of chronic myeloid leukemia.
Disclosures: Dr. Moslehi reported financial ties with Novartis, ARIAD, Takeda/Millennium, Bristol-Myers Squibb, and Acceleron Pharma. Dr. Deininger reported ties to Novartis, Bristol-Myers Squibb, Incyte, ARIAD, Pfizer, and Cellgene.
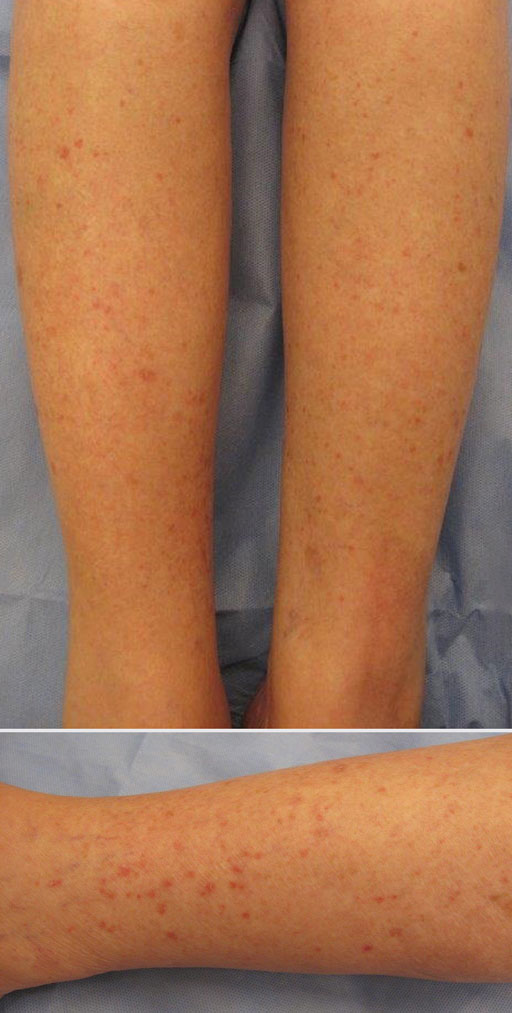
Erythematous Scaly Papules on the Shins and Calves
The Diagnosis: Hyperkeratosis Lenticularis Perstans
A shave biopsy of a lesion on the right leg was performed. Histopathology revealed a discrete papule with overlying compact hyperkeratosis. There was parakeratosis with an absent granular layer and a lichenoid lymphocytic infiltrate within the papillary dermis (Figure). Given the clinical context, these changes were consistent with a diagnosis of hyperkeratosis lenticularis perstans (HLP), also known as Flegel disease.
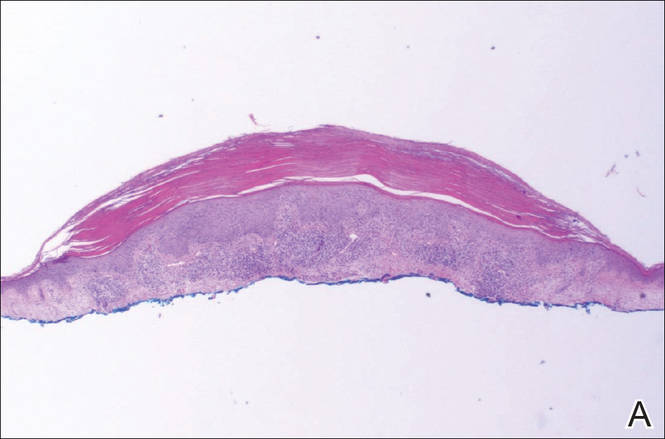
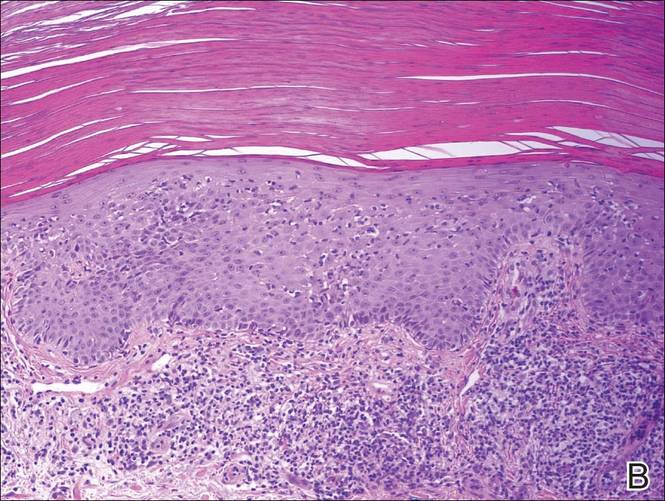
The patient was started on tretinoin cream 0.1% nightly for 3 months and triamcinolone ointment 0.1% as needed for pruritus but showed no clinical response. Given the benign nature of the condition and because the lesions were asymptomatic, additional treatment options were not pursued.
Originally described by Flegel1 in 1958, HLP is a rare skin disorder commonly seen in white individuals with onset in the fourth or fifth decades of life.1,2 While most cases are sporadic,3-6 HLP also has been associated with autosomal dominant inheritance.7-10
Patients with HLP typically present with multiple 1- to 5-mm reddish-brown, hyperkeratotic, scaly papules that reveal a moist, erythematous base with pinpoint bleeding upon removal of the scale. Lesions usually are distributed symmetrically and most commonly present on the extensor surfaces of the lower legs and dorsal feet.1,2,7 Lesions also may appear on the extensor surfaces of the arms, pinna, periocular region, antecubital and popliteal fossae, and oral mucosa and also may present as pits on the palms and soles.2,4,7,8 Furthermore, unilateral and localized variants of HLP have been described.11,12 Hyperkeratosis lenticularis perstans usually is asymptomatic but can present with mild pruritus or burning.3,5,13
The etiology and pathogenesis of HLP are unknown. Exposure to UV light has been implicated as an inciting factor14; however, reports of spontaneous resolution in the summer13 and upon treatment with psoralen plus UVA therapy15 make the role of UV light unclear. Furthermore, investigators disagree as to whether the primary pathogenic event in HLP is an inflammatory process or one of abnormal keratinization.1,3,7,10 Fernandez-Flores and Manjon16 suggested HLP is an inflammatory process with periods of exacerbations and remissions after finding mounds of parakeratosis with neutrophils arranged in different strata in the stratum corneum.
Histologically, compact hyperkeratosis usually is noted, often with associated parakeratosis, epidermal atrophy with thinning or absence of the granular layer, and a bandlike lymphohistiocytic infiltrate in the papillary dermis.1-3 Histopathologic differences between recent-onset versus longstanding lesions have been found, with old lesions lacking an inflammatory infiltrate.3 Furthermore, new lesions often show abnormalities in quantity and/or morphology of membrane-coating granules, also known as Odland bodies, in keratinocytes on electron microscopy,3,10,17 while old lesions do not.3 Odland bodies are involved in normal desquamation, leading some to speculate on their role in HLP.10 Currently, it is unclear whether abnormalities in these organelles cause the retention hyperkeratosis seen in HLP or if such abnormalities are a secondary phenomenon.3,17
There are questionable associations between HLP and diabetes mellitus type 2, hyperthyroidism, basal and squamous cell carcinomas of the skin, and gastrointestinal malignancy.4,9,18 Our patient had a history of basal cell carcinoma on the face, diet-controlled diabetes mellitus, and hypothyroidism. Given the high prevalence of these diseases in the general population, however, it is difficult to ascertain whether a true association with HLP exists.
While HLP can slowly progress to involve additional body sites, it is overall a benign condition that does not require treatment. Therapeutic options are based on case reports, with no single treatment showing a consistent response. From review of the literature, therapies that have been most effective include dermabrasion, excision,19 topical 5-fluorouracil,2,17,20 and oral retinoids.8 Hyperkeratosis lenticularis perstans generally is resistant to topical steroids, retinoids, and vitamin D3 analogs, although success with betamethasone dipropionate,5 isotretinoin
gel 0.05%,11 and calcipotriol have been reported.6 A case of HLP with clinical response to psoralen plus UVA therapy also has been described.15
- Flegel H. Hyperkeratosis lenticularis perstans. Hautarzt. 1958;9:363-364.
- Pearson LH, Smith JG, Chalker DK. Hyperkeratosis lenticularis perstans (Flegel’s disease). J Am Acad Dermatol. 1987;16:190-195.
- Ando K, Hattori H, Yamauchi Y. Histopathological differences between early and old lesions of hyperkeratosis lenticularis perstans (Flegel’s disease). Am J Dermatopathol. 2006;28:122-126.
- Fernández-Crehuet P, Rodríguez-Rey E, Ríos-Martín JJ, et al. Hyperkeratosis lenticularis perstans, or Flegel disease, with palmoplantar involvement. Actas Dermosifiliogr. 2009;100:157-159.
- Sterneberg-Vos H, van Marion AM, Frank J, et al. Hyperkeratosis lenticularis perstans (Flegel’s disease)—successful treatment with topical corticosteroids. Int J Dermatol. 2008;47:38-41.
- Bayramgürler D, Apaydin R, Dökmeci S, et al. Flegel’s disease: treatment with topical calcipotriol. Clin Exp Dermatol. 2002;27:161-162.
- Price ML, Jones EW, MacDonald DM. A clinicopathological study of Flegel’s disease (hyperkeratosis lenticularis perstans). Br J Dermatol. 1987;116:681-691.
- Krishnan A, Kar S. Photoletter to the editor: hyperkeratosis lenticularis perstans (Flegel’s disease) with unusual clinical presentation. response to isotretinoin therapy. J Dermatol Case Rep. 2012;6:93-95.
- Beveridge GW, Langlands AO. Familial hyperkeratosis lenticularis perstans associated with tumours of the skin. Br J Dermatol. 1973;88:453-458.
- Frenk E, Tapernoux B. Hyperkeratosis lenticularis perstans (Flegel): a biological model for keratinization occurring in the absence of Odland bodies? Dermatologica. 1976;153:253-262.
- Miranda-Romero A, Sánchez Sambucety P, Bajo del Pozo C, et al. Unilateral hyperkeratosis lenticularis perstans (Flegel's disease). J Am Acad Dermatol. 1998;39:655-657.
- Gutiérrez MC, Hasson A, Arias MD, et al. Localized hyperkeratosis lenticularis perstans (Flegel's disease). Cutis. 1991;48:201-204.
- Fathy S, Azadeh B. Hyperkeratosis lenticularis perstans. Int J Dermatol. 1988;27:120-121.
- Rosdahl I, Rosen K. Hyperkeratosis lenticularis perstans: report on two cases. Acta Derm Venerol. 1985;65:562-564.
- Cooper SM, George S. Flegel's disease treated with psoralen ultraviolet A. Br J Dermatol. 2000;142:340-342.
- Fernandez-Flores A, Manjon JA. Morphological evidence of periodical exacerbation of hyperkeratosis lenticularis perstans. Acta Dermatovenerol Croat. 2009;17:16-19.
- Langer K, Zonzits E, Konrad K. Hyperkeratosis lenticularis perstans (Flegel's disease). ultrastructural study of lesional and perilesional skin and therapeutic trial of topical tretinoin versus 5-fluorouracil. J Am Acad Dermatol. 1992;27:812-816.
- Ishibashi A, Tsuboi R, Fujita K. Familial hyperkeratosis lenticularis perstans. associated with cancers of the digestive organs. J Dermatol. 1984;11:407-409.
- Cunha Filho RR, Almeida Jr HL. Hyperkeratosis lenticularis perstans. An Bras Dermatol. 2011;86(4 suppl 1):S76-S77.
- Blaheta HJ, Metzler G, Rassner G, et al. Hyperkeratosis lenticularis perstans (Flegel's disease)—lack of response to treatment with tacalcitol and calcipotriol. Dermatology. 2001;202:255-258.
The Diagnosis: Hyperkeratosis Lenticularis Perstans
A shave biopsy of a lesion on the right leg was performed. Histopathology revealed a discrete papule with overlying compact hyperkeratosis. There was parakeratosis with an absent granular layer and a lichenoid lymphocytic infiltrate within the papillary dermis (Figure). Given the clinical context, these changes were consistent with a diagnosis of hyperkeratosis lenticularis perstans (HLP), also known as Flegel disease.


The patient was started on tretinoin cream 0.1% nightly for 3 months and triamcinolone ointment 0.1% as needed for pruritus but showed no clinical response. Given the benign nature of the condition and because the lesions were asymptomatic, additional treatment options were not pursued.
Originally described by Flegel1 in 1958, HLP is a rare skin disorder commonly seen in white individuals with onset in the fourth or fifth decades of life.1,2 While most cases are sporadic,3-6 HLP also has been associated with autosomal dominant inheritance.7-10
Patients with HLP typically present with multiple 1- to 5-mm reddish-brown, hyperkeratotic, scaly papules that reveal a moist, erythematous base with pinpoint bleeding upon removal of the scale. Lesions usually are distributed symmetrically and most commonly present on the extensor surfaces of the lower legs and dorsal feet.1,2,7 Lesions also may appear on the extensor surfaces of the arms, pinna, periocular region, antecubital and popliteal fossae, and oral mucosa and also may present as pits on the palms and soles.2,4,7,8 Furthermore, unilateral and localized variants of HLP have been described.11,12 Hyperkeratosis lenticularis perstans usually is asymptomatic but can present with mild pruritus or burning.3,5,13
The etiology and pathogenesis of HLP are unknown. Exposure to UV light has been implicated as an inciting factor14; however, reports of spontaneous resolution in the summer13 and upon treatment with psoralen plus UVA therapy15 make the role of UV light unclear. Furthermore, investigators disagree as to whether the primary pathogenic event in HLP is an inflammatory process or one of abnormal keratinization.1,3,7,10 Fernandez-Flores and Manjon16 suggested HLP is an inflammatory process with periods of exacerbations and remissions after finding mounds of parakeratosis with neutrophils arranged in different strata in the stratum corneum.
Histologically, compact hyperkeratosis usually is noted, often with associated parakeratosis, epidermal atrophy with thinning or absence of the granular layer, and a bandlike lymphohistiocytic infiltrate in the papillary dermis.1-3 Histopathologic differences between recent-onset versus longstanding lesions have been found, with old lesions lacking an inflammatory infiltrate.3 Furthermore, new lesions often show abnormalities in quantity and/or morphology of membrane-coating granules, also known as Odland bodies, in keratinocytes on electron microscopy,3,10,17 while old lesions do not.3 Odland bodies are involved in normal desquamation, leading some to speculate on their role in HLP.10 Currently, it is unclear whether abnormalities in these organelles cause the retention hyperkeratosis seen in HLP or if such abnormalities are a secondary phenomenon.3,17
There are questionable associations between HLP and diabetes mellitus type 2, hyperthyroidism, basal and squamous cell carcinomas of the skin, and gastrointestinal malignancy.4,9,18 Our patient had a history of basal cell carcinoma on the face, diet-controlled diabetes mellitus, and hypothyroidism. Given the high prevalence of these diseases in the general population, however, it is difficult to ascertain whether a true association with HLP exists.
While HLP can slowly progress to involve additional body sites, it is overall a benign condition that does not require treatment. Therapeutic options are based on case reports, with no single treatment showing a consistent response. From review of the literature, therapies that have been most effective include dermabrasion, excision,19 topical 5-fluorouracil,2,17,20 and oral retinoids.8 Hyperkeratosis lenticularis perstans generally is resistant to topical steroids, retinoids, and vitamin D3 analogs, although success with betamethasone dipropionate,5 isotretinoin
gel 0.05%,11 and calcipotriol have been reported.6 A case of HLP with clinical response to psoralen plus UVA therapy also has been described.15
The Diagnosis: Hyperkeratosis Lenticularis Perstans
A shave biopsy of a lesion on the right leg was performed. Histopathology revealed a discrete papule with overlying compact hyperkeratosis. There was parakeratosis with an absent granular layer and a lichenoid lymphocytic infiltrate within the papillary dermis (Figure). Given the clinical context, these changes were consistent with a diagnosis of hyperkeratosis lenticularis perstans (HLP), also known as Flegel disease.


The patient was started on tretinoin cream 0.1% nightly for 3 months and triamcinolone ointment 0.1% as needed for pruritus but showed no clinical response. Given the benign nature of the condition and because the lesions were asymptomatic, additional treatment options were not pursued.
Originally described by Flegel1 in 1958, HLP is a rare skin disorder commonly seen in white individuals with onset in the fourth or fifth decades of life.1,2 While most cases are sporadic,3-6 HLP also has been associated with autosomal dominant inheritance.7-10
Patients with HLP typically present with multiple 1- to 5-mm reddish-brown, hyperkeratotic, scaly papules that reveal a moist, erythematous base with pinpoint bleeding upon removal of the scale. Lesions usually are distributed symmetrically and most commonly present on the extensor surfaces of the lower legs and dorsal feet.1,2,7 Lesions also may appear on the extensor surfaces of the arms, pinna, periocular region, antecubital and popliteal fossae, and oral mucosa and also may present as pits on the palms and soles.2,4,7,8 Furthermore, unilateral and localized variants of HLP have been described.11,12 Hyperkeratosis lenticularis perstans usually is asymptomatic but can present with mild pruritus or burning.3,5,13
The etiology and pathogenesis of HLP are unknown. Exposure to UV light has been implicated as an inciting factor14; however, reports of spontaneous resolution in the summer13 and upon treatment with psoralen plus UVA therapy15 make the role of UV light unclear. Furthermore, investigators disagree as to whether the primary pathogenic event in HLP is an inflammatory process or one of abnormal keratinization.1,3,7,10 Fernandez-Flores and Manjon16 suggested HLP is an inflammatory process with periods of exacerbations and remissions after finding mounds of parakeratosis with neutrophils arranged in different strata in the stratum corneum.
Histologically, compact hyperkeratosis usually is noted, often with associated parakeratosis, epidermal atrophy with thinning or absence of the granular layer, and a bandlike lymphohistiocytic infiltrate in the papillary dermis.1-3 Histopathologic differences between recent-onset versus longstanding lesions have been found, with old lesions lacking an inflammatory infiltrate.3 Furthermore, new lesions often show abnormalities in quantity and/or morphology of membrane-coating granules, also known as Odland bodies, in keratinocytes on electron microscopy,3,10,17 while old lesions do not.3 Odland bodies are involved in normal desquamation, leading some to speculate on their role in HLP.10 Currently, it is unclear whether abnormalities in these organelles cause the retention hyperkeratosis seen in HLP or if such abnormalities are a secondary phenomenon.3,17
There are questionable associations between HLP and diabetes mellitus type 2, hyperthyroidism, basal and squamous cell carcinomas of the skin, and gastrointestinal malignancy.4,9,18 Our patient had a history of basal cell carcinoma on the face, diet-controlled diabetes mellitus, and hypothyroidism. Given the high prevalence of these diseases in the general population, however, it is difficult to ascertain whether a true association with HLP exists.
While HLP can slowly progress to involve additional body sites, it is overall a benign condition that does not require treatment. Therapeutic options are based on case reports, with no single treatment showing a consistent response. From review of the literature, therapies that have been most effective include dermabrasion, excision,19 topical 5-fluorouracil,2,17,20 and oral retinoids.8 Hyperkeratosis lenticularis perstans generally is resistant to topical steroids, retinoids, and vitamin D3 analogs, although success with betamethasone dipropionate,5 isotretinoin
gel 0.05%,11 and calcipotriol have been reported.6 A case of HLP with clinical response to psoralen plus UVA therapy also has been described.15
- Flegel H. Hyperkeratosis lenticularis perstans. Hautarzt. 1958;9:363-364.
- Pearson LH, Smith JG, Chalker DK. Hyperkeratosis lenticularis perstans (Flegel’s disease). J Am Acad Dermatol. 1987;16:190-195.
- Ando K, Hattori H, Yamauchi Y. Histopathological differences between early and old lesions of hyperkeratosis lenticularis perstans (Flegel’s disease). Am J Dermatopathol. 2006;28:122-126.
- Fernández-Crehuet P, Rodríguez-Rey E, Ríos-Martín JJ, et al. Hyperkeratosis lenticularis perstans, or Flegel disease, with palmoplantar involvement. Actas Dermosifiliogr. 2009;100:157-159.
- Sterneberg-Vos H, van Marion AM, Frank J, et al. Hyperkeratosis lenticularis perstans (Flegel’s disease)—successful treatment with topical corticosteroids. Int J Dermatol. 2008;47:38-41.
- Bayramgürler D, Apaydin R, Dökmeci S, et al. Flegel’s disease: treatment with topical calcipotriol. Clin Exp Dermatol. 2002;27:161-162.
- Price ML, Jones EW, MacDonald DM. A clinicopathological study of Flegel’s disease (hyperkeratosis lenticularis perstans). Br J Dermatol. 1987;116:681-691.
- Krishnan A, Kar S. Photoletter to the editor: hyperkeratosis lenticularis perstans (Flegel’s disease) with unusual clinical presentation. response to isotretinoin therapy. J Dermatol Case Rep. 2012;6:93-95.
- Beveridge GW, Langlands AO. Familial hyperkeratosis lenticularis perstans associated with tumours of the skin. Br J Dermatol. 1973;88:453-458.
- Frenk E, Tapernoux B. Hyperkeratosis lenticularis perstans (Flegel): a biological model for keratinization occurring in the absence of Odland bodies? Dermatologica. 1976;153:253-262.
- Miranda-Romero A, Sánchez Sambucety P, Bajo del Pozo C, et al. Unilateral hyperkeratosis lenticularis perstans (Flegel's disease). J Am Acad Dermatol. 1998;39:655-657.
- Gutiérrez MC, Hasson A, Arias MD, et al. Localized hyperkeratosis lenticularis perstans (Flegel's disease). Cutis. 1991;48:201-204.
- Fathy S, Azadeh B. Hyperkeratosis lenticularis perstans. Int J Dermatol. 1988;27:120-121.
- Rosdahl I, Rosen K. Hyperkeratosis lenticularis perstans: report on two cases. Acta Derm Venerol. 1985;65:562-564.
- Cooper SM, George S. Flegel's disease treated with psoralen ultraviolet A. Br J Dermatol. 2000;142:340-342.
- Fernandez-Flores A, Manjon JA. Morphological evidence of periodical exacerbation of hyperkeratosis lenticularis perstans. Acta Dermatovenerol Croat. 2009;17:16-19.
- Langer K, Zonzits E, Konrad K. Hyperkeratosis lenticularis perstans (Flegel's disease). ultrastructural study of lesional and perilesional skin and therapeutic trial of topical tretinoin versus 5-fluorouracil. J Am Acad Dermatol. 1992;27:812-816.
- Ishibashi A, Tsuboi R, Fujita K. Familial hyperkeratosis lenticularis perstans. associated with cancers of the digestive organs. J Dermatol. 1984;11:407-409.
- Cunha Filho RR, Almeida Jr HL. Hyperkeratosis lenticularis perstans. An Bras Dermatol. 2011;86(4 suppl 1):S76-S77.
- Blaheta HJ, Metzler G, Rassner G, et al. Hyperkeratosis lenticularis perstans (Flegel's disease)—lack of response to treatment with tacalcitol and calcipotriol. Dermatology. 2001;202:255-258.
- Flegel H. Hyperkeratosis lenticularis perstans. Hautarzt. 1958;9:363-364.
- Pearson LH, Smith JG, Chalker DK. Hyperkeratosis lenticularis perstans (Flegel’s disease). J Am Acad Dermatol. 1987;16:190-195.
- Ando K, Hattori H, Yamauchi Y. Histopathological differences between early and old lesions of hyperkeratosis lenticularis perstans (Flegel’s disease). Am J Dermatopathol. 2006;28:122-126.
- Fernández-Crehuet P, Rodríguez-Rey E, Ríos-Martín JJ, et al. Hyperkeratosis lenticularis perstans, or Flegel disease, with palmoplantar involvement. Actas Dermosifiliogr. 2009;100:157-159.
- Sterneberg-Vos H, van Marion AM, Frank J, et al. Hyperkeratosis lenticularis perstans (Flegel’s disease)—successful treatment with topical corticosteroids. Int J Dermatol. 2008;47:38-41.
- Bayramgürler D, Apaydin R, Dökmeci S, et al. Flegel’s disease: treatment with topical calcipotriol. Clin Exp Dermatol. 2002;27:161-162.
- Price ML, Jones EW, MacDonald DM. A clinicopathological study of Flegel’s disease (hyperkeratosis lenticularis perstans). Br J Dermatol. 1987;116:681-691.
- Krishnan A, Kar S. Photoletter to the editor: hyperkeratosis lenticularis perstans (Flegel’s disease) with unusual clinical presentation. response to isotretinoin therapy. J Dermatol Case Rep. 2012;6:93-95.
- Beveridge GW, Langlands AO. Familial hyperkeratosis lenticularis perstans associated with tumours of the skin. Br J Dermatol. 1973;88:453-458.
- Frenk E, Tapernoux B. Hyperkeratosis lenticularis perstans (Flegel): a biological model for keratinization occurring in the absence of Odland bodies? Dermatologica. 1976;153:253-262.
- Miranda-Romero A, Sánchez Sambucety P, Bajo del Pozo C, et al. Unilateral hyperkeratosis lenticularis perstans (Flegel's disease). J Am Acad Dermatol. 1998;39:655-657.
- Gutiérrez MC, Hasson A, Arias MD, et al. Localized hyperkeratosis lenticularis perstans (Flegel's disease). Cutis. 1991;48:201-204.
- Fathy S, Azadeh B. Hyperkeratosis lenticularis perstans. Int J Dermatol. 1988;27:120-121.
- Rosdahl I, Rosen K. Hyperkeratosis lenticularis perstans: report on two cases. Acta Derm Venerol. 1985;65:562-564.
- Cooper SM, George S. Flegel's disease treated with psoralen ultraviolet A. Br J Dermatol. 2000;142:340-342.
- Fernandez-Flores A, Manjon JA. Morphological evidence of periodical exacerbation of hyperkeratosis lenticularis perstans. Acta Dermatovenerol Croat. 2009;17:16-19.
- Langer K, Zonzits E, Konrad K. Hyperkeratosis lenticularis perstans (Flegel's disease). ultrastructural study of lesional and perilesional skin and therapeutic trial of topical tretinoin versus 5-fluorouracil. J Am Acad Dermatol. 1992;27:812-816.
- Ishibashi A, Tsuboi R, Fujita K. Familial hyperkeratosis lenticularis perstans. associated with cancers of the digestive organs. J Dermatol. 1984;11:407-409.
- Cunha Filho RR, Almeida Jr HL. Hyperkeratosis lenticularis perstans. An Bras Dermatol. 2011;86(4 suppl 1):S76-S77.
- Blaheta HJ, Metzler G, Rassner G, et al. Hyperkeratosis lenticularis perstans (Flegel's disease)—lack of response to treatment with tacalcitol and calcipotriol. Dermatology. 2001;202:255-258.

Drug disappoints in phase 3 HSCT trial
The antiviral drug brincidofovir did not meet the primary endpoint of the phase 3 SUPPRESS trial, according to the drug’s developer, Chimerix.
Brincidofovir did not prevent clinically significant cytomegalovirus (CMV) infection through week 24 after hematopoietic stem cell transplant (HSCT).
However, the president and CEO of Chimerix said the company still believes there is a “viable path forward” for the drug.
Brincidofovir is an oral nucleotide analog that has shown in vitro antiviral activity against all 5 families of DNA viruses that affect humans, including herpes viruses and adenovirus.
The SUPPRESS trial, which was initiated in August 2013 and fully enrolled in June 2015, was informed by a successful phase 2 trial conducted in HSCT recipients.
The SUPPRESS trial enrolled and treated 452 adults who received allogeneic HSCTs from more than 40 transplant centers in the US, Canada, and Europe.
Subjects received twice-weekly brincidofovir or placebo (2:1 ratio) from the early post-transplant period through week 14 post-transplant, the period of highest risk for viral infections.
All patients in the trial were CMV-seropositive, placing them at high risk of CMV infection. The most common indications leading to HSCT were acute myelogenous leukemia (43% of patients), myelodysplasia (17%), non-Hodgkin lymphoma (10%), and acute lymphocytic leukemia (9%).
During the on-treatment period through week 14 after HSCT, fewer patients in the brincidofovir arm had a CMV infection, which was consistent with results from the phase 2 study of the drug.
However, during the 10 weeks off treatment from week 14 to week 24, there was an increase in CMV infections in the brincidofovir arm compared to the control arm. And there was a non-statistically significant increase in mortality in the brincidofovir arm compared to the control arm.
Preliminary analysis suggests the failure in preventing CMV infections and the increased mortality in the brincidofovir arm were driven by confirmed cases of graft-versus-host-disease (GVHD), which resulted in a significantly higher use of corticosteroids than in the control arm.
Both GVHD and the use of corticosteroids are risk factors for “late” CMV infection that occurs after the discontinuation of the antiviral in HSCT recipients.
The rate of study drug discontinuation for gastrointestinal events was less than 10% in this study, which is comparable to that observed in the phase 2 trial of brincidofovir in a similar HSCT population.
“While we are clearly disappointed in the top-line results from SUPPRESS, we remain committed to better understanding the full data set as we consider potential paths forward for brincidofovir,” said M. Michelle Berrey, MD, president and CEO of Chimerix.
“We will be evaluating the subgroups of patients within SUPPRESS, such as T-cell-depleted transplant recipients who have a lower risk of GVHD, to better understand these results and inform our next steps,” said W. Garrett Nichols, MD, Chimerix’s chief medical officer.
“We are reaching out to investigators and other experts to help us assess the complete data set to understand what may have caused the results of the SUPPRESS trial to differ substantially from those seen in the phase 2 study. Additionally, we are in communication with the US Food and Drug Administration and other regulatory bodies and will share any updates on the brincidofovir clinical program when we can.”
“With data currently in hand, we believe that brincidofovir may ultimately demonstrate a positive risk-benefit profile for the treatment of adenovirus and smallpox, as well as use in other populations in need of a novel compound for DNA viral infections.”
Chimerix plans to continue the programs testing brincidofovir in serious adenovirus infections and in smallpox. Pending the availability of complete data from SUPPRESS, including secondary endpoints in other dsDNA viral infections, Chimerix has elected to pause further enrollment in the phase 3 SUSTAIN and SURPASS trials in kidney transplant recipients.
A full analysis of the SUPPRESS trial results is ongoing. The data are scheduled to be presented at the BMT Tandem Meetings in Honolulu, Hawaii, in February. ![]()
The antiviral drug brincidofovir did not meet the primary endpoint of the phase 3 SUPPRESS trial, according to the drug’s developer, Chimerix.
Brincidofovir did not prevent clinically significant cytomegalovirus (CMV) infection through week 24 after hematopoietic stem cell transplant (HSCT).
However, the president and CEO of Chimerix said the company still believes there is a “viable path forward” for the drug.
Brincidofovir is an oral nucleotide analog that has shown in vitro antiviral activity against all 5 families of DNA viruses that affect humans, including herpes viruses and adenovirus.
The SUPPRESS trial, which was initiated in August 2013 and fully enrolled in June 2015, was informed by a successful phase 2 trial conducted in HSCT recipients.
The SUPPRESS trial enrolled and treated 452 adults who received allogeneic HSCTs from more than 40 transplant centers in the US, Canada, and Europe.
Subjects received twice-weekly brincidofovir or placebo (2:1 ratio) from the early post-transplant period through week 14 post-transplant, the period of highest risk for viral infections.
All patients in the trial were CMV-seropositive, placing them at high risk of CMV infection. The most common indications leading to HSCT were acute myelogenous leukemia (43% of patients), myelodysplasia (17%), non-Hodgkin lymphoma (10%), and acute lymphocytic leukemia (9%).
During the on-treatment period through week 14 after HSCT, fewer patients in the brincidofovir arm had a CMV infection, which was consistent with results from the phase 2 study of the drug.
However, during the 10 weeks off treatment from week 14 to week 24, there was an increase in CMV infections in the brincidofovir arm compared to the control arm. And there was a non-statistically significant increase in mortality in the brincidofovir arm compared to the control arm.
Preliminary analysis suggests the failure in preventing CMV infections and the increased mortality in the brincidofovir arm were driven by confirmed cases of graft-versus-host-disease (GVHD), which resulted in a significantly higher use of corticosteroids than in the control arm.
Both GVHD and the use of corticosteroids are risk factors for “late” CMV infection that occurs after the discontinuation of the antiviral in HSCT recipients.
The rate of study drug discontinuation for gastrointestinal events was less than 10% in this study, which is comparable to that observed in the phase 2 trial of brincidofovir in a similar HSCT population.
“While we are clearly disappointed in the top-line results from SUPPRESS, we remain committed to better understanding the full data set as we consider potential paths forward for brincidofovir,” said M. Michelle Berrey, MD, president and CEO of Chimerix.
“We will be evaluating the subgroups of patients within SUPPRESS, such as T-cell-depleted transplant recipients who have a lower risk of GVHD, to better understand these results and inform our next steps,” said W. Garrett Nichols, MD, Chimerix’s chief medical officer.
“We are reaching out to investigators and other experts to help us assess the complete data set to understand what may have caused the results of the SUPPRESS trial to differ substantially from those seen in the phase 2 study. Additionally, we are in communication with the US Food and Drug Administration and other regulatory bodies and will share any updates on the brincidofovir clinical program when we can.”
“With data currently in hand, we believe that brincidofovir may ultimately demonstrate a positive risk-benefit profile for the treatment of adenovirus and smallpox, as well as use in other populations in need of a novel compound for DNA viral infections.”
Chimerix plans to continue the programs testing brincidofovir in serious adenovirus infections and in smallpox. Pending the availability of complete data from SUPPRESS, including secondary endpoints in other dsDNA viral infections, Chimerix has elected to pause further enrollment in the phase 3 SUSTAIN and SURPASS trials in kidney transplant recipients.
A full analysis of the SUPPRESS trial results is ongoing. The data are scheduled to be presented at the BMT Tandem Meetings in Honolulu, Hawaii, in February. ![]()
The antiviral drug brincidofovir did not meet the primary endpoint of the phase 3 SUPPRESS trial, according to the drug’s developer, Chimerix.
Brincidofovir did not prevent clinically significant cytomegalovirus (CMV) infection through week 24 after hematopoietic stem cell transplant (HSCT).
However, the president and CEO of Chimerix said the company still believes there is a “viable path forward” for the drug.
Brincidofovir is an oral nucleotide analog that has shown in vitro antiviral activity against all 5 families of DNA viruses that affect humans, including herpes viruses and adenovirus.
The SUPPRESS trial, which was initiated in August 2013 and fully enrolled in June 2015, was informed by a successful phase 2 trial conducted in HSCT recipients.
The SUPPRESS trial enrolled and treated 452 adults who received allogeneic HSCTs from more than 40 transplant centers in the US, Canada, and Europe.
Subjects received twice-weekly brincidofovir or placebo (2:1 ratio) from the early post-transplant period through week 14 post-transplant, the period of highest risk for viral infections.
All patients in the trial were CMV-seropositive, placing them at high risk of CMV infection. The most common indications leading to HSCT were acute myelogenous leukemia (43% of patients), myelodysplasia (17%), non-Hodgkin lymphoma (10%), and acute lymphocytic leukemia (9%).
During the on-treatment period through week 14 after HSCT, fewer patients in the brincidofovir arm had a CMV infection, which was consistent with results from the phase 2 study of the drug.
However, during the 10 weeks off treatment from week 14 to week 24, there was an increase in CMV infections in the brincidofovir arm compared to the control arm. And there was a non-statistically significant increase in mortality in the brincidofovir arm compared to the control arm.
Preliminary analysis suggests the failure in preventing CMV infections and the increased mortality in the brincidofovir arm were driven by confirmed cases of graft-versus-host-disease (GVHD), which resulted in a significantly higher use of corticosteroids than in the control arm.
Both GVHD and the use of corticosteroids are risk factors for “late” CMV infection that occurs after the discontinuation of the antiviral in HSCT recipients.
The rate of study drug discontinuation for gastrointestinal events was less than 10% in this study, which is comparable to that observed in the phase 2 trial of brincidofovir in a similar HSCT population.
“While we are clearly disappointed in the top-line results from SUPPRESS, we remain committed to better understanding the full data set as we consider potential paths forward for brincidofovir,” said M. Michelle Berrey, MD, president and CEO of Chimerix.
“We will be evaluating the subgroups of patients within SUPPRESS, such as T-cell-depleted transplant recipients who have a lower risk of GVHD, to better understand these results and inform our next steps,” said W. Garrett Nichols, MD, Chimerix’s chief medical officer.
“We are reaching out to investigators and other experts to help us assess the complete data set to understand what may have caused the results of the SUPPRESS trial to differ substantially from those seen in the phase 2 study. Additionally, we are in communication with the US Food and Drug Administration and other regulatory bodies and will share any updates on the brincidofovir clinical program when we can.”
“With data currently in hand, we believe that brincidofovir may ultimately demonstrate a positive risk-benefit profile for the treatment of adenovirus and smallpox, as well as use in other populations in need of a novel compound for DNA viral infections.”
Chimerix plans to continue the programs testing brincidofovir in serious adenovirus infections and in smallpox. Pending the availability of complete data from SUPPRESS, including secondary endpoints in other dsDNA viral infections, Chimerix has elected to pause further enrollment in the phase 3 SUSTAIN and SURPASS trials in kidney transplant recipients.
A full analysis of the SUPPRESS trial results is ongoing. The data are scheduled to be presented at the BMT Tandem Meetings in Honolulu, Hawaii, in February. ![]()
Study reveals germline variants in AML, other cancers

A study published in Nature Communications has shed light on the hereditary elements of 12 cancer types.
Investigators looked for rare germline mutations in genes known to be associated with cancer and found the frequency of these mutations varied widely, from 4% in the acute myeloid leukemia (AML) cases studied to 19% in cases of ovarian cancer.
The team’s analysis also revealed an unexpected inherited component to stomach cancer and provided some clarity on the consequences of certain mutations in the BRCA1 and BRCA2 genes.
Li Ding, PhD, of Washington University School of Medicine in St Louis, Missouri, and her colleagues conducted this study, analyzing genetic information from more than 4000 cancer cases included in The Cancer Genome Atlas project.
“In general, we have known that ovarian and breast cancers have a significant inherited component, and others, such as acute myeloid leukemia and lung cancer, have a much smaller inherited genetic contribution,” Dr Ding said. “But this is the first time, on a large scale, that we’ve been able to pinpoint gene culprits or even the actual mutations responsible for cancer susceptibility.”
To help tease out cancer’s inherited components, Dr Ding and her colleagues looked for germline truncations in 114 genes known to be associated with cancer.
“We looked for germline mutations in the tumor, but it was not enough for the mutations simply to be present,” Dr Ding said. “They needed to be enriched in the tumor—present at higher frequency. If a mutation is present in the germline and amplified in the tumor, there is a high likelihood it is playing a role in the cancer.”
The investigators found germline truncations in all 12 cancer types analyzed, but the mutations occurred in varying frequencies depending on the cancer.
The percentage of tumors with truncations in the germline was 4% for AML and glioblastoma; 5% for kidney cancer; 7% for lung adenocarcinoma and endometrial cancer; 8% for head and neck cancer, glioma, lung squamous cell carcinoma, and prostate cancer; 9% for breast cancer; 11% for stomach cancer; and 19% for ovarian cancer.
“We also found a significant number of germline truncations in the BRCA1 and BRCA2 genes present in tumor types other than breast cancer, including stomach and prostate cancers, for example,” Dr Ding said. “This suggests we should pay attention to the potential involvement of these 2 genes in other cancer types.”
The investigators said they identified 13 cancer genes with significant enrichment of rare truncations. Some of these were associated with specific cancers—for example, RAD51C in AML, PALB2 in stomach cancer, and MSH6 in endometrial cancer.
And the team observed significant, tumor-specific loss of heterozygosity in 9 genes—ATM, BAP1, BRCA1/2, BRIP1, FANCM, PALB2, and RAD51C/D.
Dr Ding said more research is needed to confirm these results before they can be used to advise patients making healthcare decisions.
“Our strategy of investigating germline-tumor interactions provides a good way to prioritize important mutations that we should focus on,” she said. “For the information to eventually be used in the clinic, we will need to perform this type of analysis on even larger numbers of patients.” ![]()

A study published in Nature Communications has shed light on the hereditary elements of 12 cancer types.
Investigators looked for rare germline mutations in genes known to be associated with cancer and found the frequency of these mutations varied widely, from 4% in the acute myeloid leukemia (AML) cases studied to 19% in cases of ovarian cancer.
The team’s analysis also revealed an unexpected inherited component to stomach cancer and provided some clarity on the consequences of certain mutations in the BRCA1 and BRCA2 genes.
Li Ding, PhD, of Washington University School of Medicine in St Louis, Missouri, and her colleagues conducted this study, analyzing genetic information from more than 4000 cancer cases included in The Cancer Genome Atlas project.
“In general, we have known that ovarian and breast cancers have a significant inherited component, and others, such as acute myeloid leukemia and lung cancer, have a much smaller inherited genetic contribution,” Dr Ding said. “But this is the first time, on a large scale, that we’ve been able to pinpoint gene culprits or even the actual mutations responsible for cancer susceptibility.”
To help tease out cancer’s inherited components, Dr Ding and her colleagues looked for germline truncations in 114 genes known to be associated with cancer.
“We looked for germline mutations in the tumor, but it was not enough for the mutations simply to be present,” Dr Ding said. “They needed to be enriched in the tumor—present at higher frequency. If a mutation is present in the germline and amplified in the tumor, there is a high likelihood it is playing a role in the cancer.”
The investigators found germline truncations in all 12 cancer types analyzed, but the mutations occurred in varying frequencies depending on the cancer.
The percentage of tumors with truncations in the germline was 4% for AML and glioblastoma; 5% for kidney cancer; 7% for lung adenocarcinoma and endometrial cancer; 8% for head and neck cancer, glioma, lung squamous cell carcinoma, and prostate cancer; 9% for breast cancer; 11% for stomach cancer; and 19% for ovarian cancer.
“We also found a significant number of germline truncations in the BRCA1 and BRCA2 genes present in tumor types other than breast cancer, including stomach and prostate cancers, for example,” Dr Ding said. “This suggests we should pay attention to the potential involvement of these 2 genes in other cancer types.”
The investigators said they identified 13 cancer genes with significant enrichment of rare truncations. Some of these were associated with specific cancers—for example, RAD51C in AML, PALB2 in stomach cancer, and MSH6 in endometrial cancer.
And the team observed significant, tumor-specific loss of heterozygosity in 9 genes—ATM, BAP1, BRCA1/2, BRIP1, FANCM, PALB2, and RAD51C/D.
Dr Ding said more research is needed to confirm these results before they can be used to advise patients making healthcare decisions.
“Our strategy of investigating germline-tumor interactions provides a good way to prioritize important mutations that we should focus on,” she said. “For the information to eventually be used in the clinic, we will need to perform this type of analysis on even larger numbers of patients.” ![]()

A study published in Nature Communications has shed light on the hereditary elements of 12 cancer types.
Investigators looked for rare germline mutations in genes known to be associated with cancer and found the frequency of these mutations varied widely, from 4% in the acute myeloid leukemia (AML) cases studied to 19% in cases of ovarian cancer.
The team’s analysis also revealed an unexpected inherited component to stomach cancer and provided some clarity on the consequences of certain mutations in the BRCA1 and BRCA2 genes.
Li Ding, PhD, of Washington University School of Medicine in St Louis, Missouri, and her colleagues conducted this study, analyzing genetic information from more than 4000 cancer cases included in The Cancer Genome Atlas project.
“In general, we have known that ovarian and breast cancers have a significant inherited component, and others, such as acute myeloid leukemia and lung cancer, have a much smaller inherited genetic contribution,” Dr Ding said. “But this is the first time, on a large scale, that we’ve been able to pinpoint gene culprits or even the actual mutations responsible for cancer susceptibility.”
To help tease out cancer’s inherited components, Dr Ding and her colleagues looked for germline truncations in 114 genes known to be associated with cancer.
“We looked for germline mutations in the tumor, but it was not enough for the mutations simply to be present,” Dr Ding said. “They needed to be enriched in the tumor—present at higher frequency. If a mutation is present in the germline and amplified in the tumor, there is a high likelihood it is playing a role in the cancer.”
The investigators found germline truncations in all 12 cancer types analyzed, but the mutations occurred in varying frequencies depending on the cancer.
The percentage of tumors with truncations in the germline was 4% for AML and glioblastoma; 5% for kidney cancer; 7% for lung adenocarcinoma and endometrial cancer; 8% for head and neck cancer, glioma, lung squamous cell carcinoma, and prostate cancer; 9% for breast cancer; 11% for stomach cancer; and 19% for ovarian cancer.
“We also found a significant number of germline truncations in the BRCA1 and BRCA2 genes present in tumor types other than breast cancer, including stomach and prostate cancers, for example,” Dr Ding said. “This suggests we should pay attention to the potential involvement of these 2 genes in other cancer types.”
The investigators said they identified 13 cancer genes with significant enrichment of rare truncations. Some of these were associated with specific cancers—for example, RAD51C in AML, PALB2 in stomach cancer, and MSH6 in endometrial cancer.
And the team observed significant, tumor-specific loss of heterozygosity in 9 genes—ATM, BAP1, BRCA1/2, BRIP1, FANCM, PALB2, and RAD51C/D.
Dr Ding said more research is needed to confirm these results before they can be used to advise patients making healthcare decisions.
“Our strategy of investigating germline-tumor interactions provides a good way to prioritize important mutations that we should focus on,” she said. “For the information to eventually be used in the clinic, we will need to perform this type of analysis on even larger numbers of patients.” ![]()
Education may increase clinical trial participation

Photo courtesy of NCI
and Matthews Media Group
A new study suggests that educating cancer patients about clinical trials—with either a general or patient-specific program—increases the likelihood that patients will enroll in such trials.
After completing either type of educational program, 21% of the cancer patients studied chose to enroll in clinical trials.
Traditionally, less than 5% of cancer patients decide to participate in trials, according to the American Cancer Society.
“Unfortunately, although clinical trials are critical for advancing cancer treatment and ultimately serve as the basis for new standards of care, very few patients participate,” said Neal J. Meropol, MD, of Case Western Reserve University School of Medicine in Cleveland, Ohio.
“We want to close the patient knowledge gap and positively affect their attitudes toward clinical trials.”
Dr Meropol and his colleagues described their effort to do just that in the Journal of Clinical Oncology.
The researchers compared a tailored video education program on clinical trials, PRE-ACT (Preparatory Education about Clinical Trials), to educational information delivered as written text.
PRE-ACT is an intervention in which patients access a website to take an online survey. The survey gauges the individual patient’s knowledge and attitudes about clinical trials, and then, based on that patient’s answers, video clips are presented addressing his or her specific concerns.
For example, patients may worry they will receive a placebo rather than active treatment, so one video clip explains how placebos are used ethically in cancer studies, and the fact that very few studies will include a placebo without any active treatment. The videos also help patients clarify their preferences in terms of quality of life or length of life.
Results
Dr Meropol and his colleagues enrolled 1255 cancer patients in the study. Half of them participated in the PRE-ACT program, and the other half received written information about clinical trials that was not specifically chosen based on their survey responses.
Both interventions improved patients’ knowledge and attitudes regarding clinical trials when compared with baseline (all P<0.001).
Patients in both arms said they felt more prepared to consider enrolling in a clinical trial after completing their assigned educational program (P<0.001), but there was a trend favoring the PRE-ACT arm (P<0.09).
Patients in the PRE-ACT arm also showed a significantly greater increase in knowledge (P<0.001) and a significantly greater decrease in attitudinal barriers (P<0.001) than patients in the text-only arm. And PRE-ACT was associated with greater patient satisfaction.
Financial concerns
During the course of this research, Dr Meropol and his colleagues made a surprising discovery. Video clips meant to address concerns about the costs of clinical trial treatment actually caused a spike in worries about the out-of-pocket costs of clinical trials.
These financial concerns generated yet another paper that appeared in the same edition of the Journal of Clinical Oncology.
“What was a surprise is that giving people information about costs in general terms made them more anxious,” Dr Meropol said. “It was not surprising to us that these concerns actually affect distress, add to decisional conflict, and interfere with decision-making.”
“This finding highlighted for us that communication about costs is both necessary and challenging. It indicates that we need to be sensitive to patients’ cost concerns as they navigate decisions about cancer care.”
The researchers are now planning to develop tools to assist patients with financial navigation. The team is also developing a web-based educational program for oncology nurses to help them discuss clinical trial participation with patients. ![]()

Photo courtesy of NCI
and Matthews Media Group
A new study suggests that educating cancer patients about clinical trials—with either a general or patient-specific program—increases the likelihood that patients will enroll in such trials.
After completing either type of educational program, 21% of the cancer patients studied chose to enroll in clinical trials.
Traditionally, less than 5% of cancer patients decide to participate in trials, according to the American Cancer Society.
“Unfortunately, although clinical trials are critical for advancing cancer treatment and ultimately serve as the basis for new standards of care, very few patients participate,” said Neal J. Meropol, MD, of Case Western Reserve University School of Medicine in Cleveland, Ohio.
“We want to close the patient knowledge gap and positively affect their attitudes toward clinical trials.”
Dr Meropol and his colleagues described their effort to do just that in the Journal of Clinical Oncology.
The researchers compared a tailored video education program on clinical trials, PRE-ACT (Preparatory Education about Clinical Trials), to educational information delivered as written text.
PRE-ACT is an intervention in which patients access a website to take an online survey. The survey gauges the individual patient’s knowledge and attitudes about clinical trials, and then, based on that patient’s answers, video clips are presented addressing his or her specific concerns.
For example, patients may worry they will receive a placebo rather than active treatment, so one video clip explains how placebos are used ethically in cancer studies, and the fact that very few studies will include a placebo without any active treatment. The videos also help patients clarify their preferences in terms of quality of life or length of life.
Results
Dr Meropol and his colleagues enrolled 1255 cancer patients in the study. Half of them participated in the PRE-ACT program, and the other half received written information about clinical trials that was not specifically chosen based on their survey responses.
Both interventions improved patients’ knowledge and attitudes regarding clinical trials when compared with baseline (all P<0.001).
Patients in both arms said they felt more prepared to consider enrolling in a clinical trial after completing their assigned educational program (P<0.001), but there was a trend favoring the PRE-ACT arm (P<0.09).
Patients in the PRE-ACT arm also showed a significantly greater increase in knowledge (P<0.001) and a significantly greater decrease in attitudinal barriers (P<0.001) than patients in the text-only arm. And PRE-ACT was associated with greater patient satisfaction.
Financial concerns
During the course of this research, Dr Meropol and his colleagues made a surprising discovery. Video clips meant to address concerns about the costs of clinical trial treatment actually caused a spike in worries about the out-of-pocket costs of clinical trials.
These financial concerns generated yet another paper that appeared in the same edition of the Journal of Clinical Oncology.
“What was a surprise is that giving people information about costs in general terms made them more anxious,” Dr Meropol said. “It was not surprising to us that these concerns actually affect distress, add to decisional conflict, and interfere with decision-making.”
“This finding highlighted for us that communication about costs is both necessary and challenging. It indicates that we need to be sensitive to patients’ cost concerns as they navigate decisions about cancer care.”
The researchers are now planning to develop tools to assist patients with financial navigation. The team is also developing a web-based educational program for oncology nurses to help them discuss clinical trial participation with patients. ![]()

Photo courtesy of NCI
and Matthews Media Group
A new study suggests that educating cancer patients about clinical trials—with either a general or patient-specific program—increases the likelihood that patients will enroll in such trials.
After completing either type of educational program, 21% of the cancer patients studied chose to enroll in clinical trials.
Traditionally, less than 5% of cancer patients decide to participate in trials, according to the American Cancer Society.
“Unfortunately, although clinical trials are critical for advancing cancer treatment and ultimately serve as the basis for new standards of care, very few patients participate,” said Neal J. Meropol, MD, of Case Western Reserve University School of Medicine in Cleveland, Ohio.
“We want to close the patient knowledge gap and positively affect their attitudes toward clinical trials.”
Dr Meropol and his colleagues described their effort to do just that in the Journal of Clinical Oncology.
The researchers compared a tailored video education program on clinical trials, PRE-ACT (Preparatory Education about Clinical Trials), to educational information delivered as written text.
PRE-ACT is an intervention in which patients access a website to take an online survey. The survey gauges the individual patient’s knowledge and attitudes about clinical trials, and then, based on that patient’s answers, video clips are presented addressing his or her specific concerns.
For example, patients may worry they will receive a placebo rather than active treatment, so one video clip explains how placebos are used ethically in cancer studies, and the fact that very few studies will include a placebo without any active treatment. The videos also help patients clarify their preferences in terms of quality of life or length of life.
Results
Dr Meropol and his colleagues enrolled 1255 cancer patients in the study. Half of them participated in the PRE-ACT program, and the other half received written information about clinical trials that was not specifically chosen based on their survey responses.
Both interventions improved patients’ knowledge and attitudes regarding clinical trials when compared with baseline (all P<0.001).
Patients in both arms said they felt more prepared to consider enrolling in a clinical trial after completing their assigned educational program (P<0.001), but there was a trend favoring the PRE-ACT arm (P<0.09).
Patients in the PRE-ACT arm also showed a significantly greater increase in knowledge (P<0.001) and a significantly greater decrease in attitudinal barriers (P<0.001) than patients in the text-only arm. And PRE-ACT was associated with greater patient satisfaction.
Financial concerns
During the course of this research, Dr Meropol and his colleagues made a surprising discovery. Video clips meant to address concerns about the costs of clinical trial treatment actually caused a spike in worries about the out-of-pocket costs of clinical trials.
These financial concerns generated yet another paper that appeared in the same edition of the Journal of Clinical Oncology.
“What was a surprise is that giving people information about costs in general terms made them more anxious,” Dr Meropol said. “It was not surprising to us that these concerns actually affect distress, add to decisional conflict, and interfere with decision-making.”
“This finding highlighted for us that communication about costs is both necessary and challenging. It indicates that we need to be sensitive to patients’ cost concerns as they navigate decisions about cancer care.”
The researchers are now planning to develop tools to assist patients with financial navigation. The team is also developing a web-based educational program for oncology nurses to help them discuss clinical trial participation with patients. ![]()
Team explains how artemisinin kills malaria parasite

infecting a red blood cell
Photo courtesy of St. Jude
Children’s Research Hospital
Researchers say they have gained a better understanding of how the antimalarial drug artemisinin kills the Plasmodium falciparum parasite.
A chemical proteomics analysis revealed more than 120 protein targets of artemisinin and the mechanism that activates its killing effect.
Given the emergence of artemisinin resistance, the team believes their findings could aid the design of new treatments against drug-resistant parasites.
They reported the findings in Nature Communications.
Previously, only 2 targets of artemisinin had been identified, and their correlation with the parasite-killing effect of the drug had been questioned.
Lin Qingsong, PhD, of the National University of Singapore, and his colleagues identified 124 protein targets of artemisinin in P falciparum. Many of these newly identified protein targets are involved in essential biological processes in the parasite, thus explaining artemisinin’s potent killing effect.
The research suggests that, through its promiscuous targeting mechanism, artemisinin targets the blood-eating nature of the malaria parasite, binding to a broad spectrum of targets simultaneously and fatally disrupting the biochemistry of the parasite.
The study also showed that the main activator of artemisinin is heme, an iron-containing compound that is either biosynthesized by the parasite at its early developmental ring stage or derived from hemoglobin digestion in the later stages.
The drug activation level was found to be much lower in ring-stage parasites, given that artemisinin activation requires heme, which is of much lower abundance and is biosynthesized by the parasite.
In comparison, during the late stages of its life cycle, the parasite actively digests the hemoglobin in infected blood cells as its primary energy source. This releases large amounts of heme, and the drug is able to specifically respond to parasite-infected cells and effectively attack the disease-causing parasites.
“The current findings not only provide a more complete picture of how artemisinin and its derivatives work but also suggest new ways of using the drug,” Dr Lin said. “For instance, to improve drug activation at ring stage, we can explore enhancing the level of heme biosynthesis in the parasite.”
“By understanding that hemoglobin digestion releases huge amounts of heme, which brings about the effective killing mechanism in the later stages, we can also consider prolonging the treatment time to ensure that the drug can effectively kill the parasite through multiple cycles.”
In addition, the researchers are planning to develop novel artemisinin analogues with more specific targeting properties. ![]()

infecting a red blood cell
Photo courtesy of St. Jude
Children’s Research Hospital
Researchers say they have gained a better understanding of how the antimalarial drug artemisinin kills the Plasmodium falciparum parasite.
A chemical proteomics analysis revealed more than 120 protein targets of artemisinin and the mechanism that activates its killing effect.
Given the emergence of artemisinin resistance, the team believes their findings could aid the design of new treatments against drug-resistant parasites.
They reported the findings in Nature Communications.
Previously, only 2 targets of artemisinin had been identified, and their correlation with the parasite-killing effect of the drug had been questioned.
Lin Qingsong, PhD, of the National University of Singapore, and his colleagues identified 124 protein targets of artemisinin in P falciparum. Many of these newly identified protein targets are involved in essential biological processes in the parasite, thus explaining artemisinin’s potent killing effect.
The research suggests that, through its promiscuous targeting mechanism, artemisinin targets the blood-eating nature of the malaria parasite, binding to a broad spectrum of targets simultaneously and fatally disrupting the biochemistry of the parasite.
The study also showed that the main activator of artemisinin is heme, an iron-containing compound that is either biosynthesized by the parasite at its early developmental ring stage or derived from hemoglobin digestion in the later stages.
The drug activation level was found to be much lower in ring-stage parasites, given that artemisinin activation requires heme, which is of much lower abundance and is biosynthesized by the parasite.
In comparison, during the late stages of its life cycle, the parasite actively digests the hemoglobin in infected blood cells as its primary energy source. This releases large amounts of heme, and the drug is able to specifically respond to parasite-infected cells and effectively attack the disease-causing parasites.
“The current findings not only provide a more complete picture of how artemisinin and its derivatives work but also suggest new ways of using the drug,” Dr Lin said. “For instance, to improve drug activation at ring stage, we can explore enhancing the level of heme biosynthesis in the parasite.”
“By understanding that hemoglobin digestion releases huge amounts of heme, which brings about the effective killing mechanism in the later stages, we can also consider prolonging the treatment time to ensure that the drug can effectively kill the parasite through multiple cycles.”
In addition, the researchers are planning to develop novel artemisinin analogues with more specific targeting properties. ![]()

infecting a red blood cell
Photo courtesy of St. Jude
Children’s Research Hospital
Researchers say they have gained a better understanding of how the antimalarial drug artemisinin kills the Plasmodium falciparum parasite.
A chemical proteomics analysis revealed more than 120 protein targets of artemisinin and the mechanism that activates its killing effect.
Given the emergence of artemisinin resistance, the team believes their findings could aid the design of new treatments against drug-resistant parasites.
They reported the findings in Nature Communications.
Previously, only 2 targets of artemisinin had been identified, and their correlation with the parasite-killing effect of the drug had been questioned.
Lin Qingsong, PhD, of the National University of Singapore, and his colleagues identified 124 protein targets of artemisinin in P falciparum. Many of these newly identified protein targets are involved in essential biological processes in the parasite, thus explaining artemisinin’s potent killing effect.
The research suggests that, through its promiscuous targeting mechanism, artemisinin targets the blood-eating nature of the malaria parasite, binding to a broad spectrum of targets simultaneously and fatally disrupting the biochemistry of the parasite.
The study also showed that the main activator of artemisinin is heme, an iron-containing compound that is either biosynthesized by the parasite at its early developmental ring stage or derived from hemoglobin digestion in the later stages.
The drug activation level was found to be much lower in ring-stage parasites, given that artemisinin activation requires heme, which is of much lower abundance and is biosynthesized by the parasite.
In comparison, during the late stages of its life cycle, the parasite actively digests the hemoglobin in infected blood cells as its primary energy source. This releases large amounts of heme, and the drug is able to specifically respond to parasite-infected cells and effectively attack the disease-causing parasites.
“The current findings not only provide a more complete picture of how artemisinin and its derivatives work but also suggest new ways of using the drug,” Dr Lin said. “For instance, to improve drug activation at ring stage, we can explore enhancing the level of heme biosynthesis in the parasite.”
“By understanding that hemoglobin digestion releases huge amounts of heme, which brings about the effective killing mechanism in the later stages, we can also consider prolonging the treatment time to ensure that the drug can effectively kill the parasite through multiple cycles.”
In addition, the researchers are planning to develop novel artemisinin analogues with more specific targeting properties. ![]()
Length of Different‐Hospital Readmissions
Readmissions within a relatively short time after discharge are receiving considerable attention as an area of quality improvement,[1, 2] with increasing emphasis on the relatively large share of readmissions to different hospitals, accounting for 20% to 30% of all readmissions.[3, 4, 5, 6] Returning to a different hospital may affect patient and healthcare outcomes due to breaches in continuity. When information from the previous recent hospitalization is not transferred efficiently and accurately to the next admitting hospital, omissions and duplications can occur, resulting in delayed care and potentially worse outcomes (compared to same hospital readmissions [SHRs]), such as longer length of readmission stay (LORS) and increased costs.[7]
Electronic health records (EHRs) and health information exchange (HIE) systems are increasingly used for storage and retrieval of patient information from various sources, such as laboratories and previous physician visits and hospitalizations, enabling informational continuity by providing vital historical medical information for decision‐making. Whereas EHRs collect, store, and present information that is locally created within a specific clinic or hospital, HIEs connect EHR systems between multiple institutions, allowing providers to share clinical data and achieve interorganizational continuity. Such integrative systems are increasingly being implemented across healthcare systems worldwide.[8, 9, 10] Yet, technical difficulties, costs, competitive concerns, data privacy, and workflow implementation challenges have been described as hindering HIE participation.[11, 12, 13, 14] Moreover, major concerns exist regarding the poor usability of EHRs, their limited ability to support multidisciplinary care, and major difficulties in achieving interoperability with HIEs, which undermine efforts to deliver integrated patient‐centered care.[15] Nonetheless, previous research has demonstrated that HIEs can positively affect healthcare resource use and outcomes, including reduced rates of repeated diagnostic imaging in the emergency evaluation of back pain,[16] reduction in admissions via the emergency department (ED),[17] and reduced rates of readmissions within 7 days.[18] However, it is not known whether HIEs can contribute to positive outcomes when patients are readmitted to a different hospital than the hospital from which they were recently (within the previous 30 days) discharged, potentially bridging the transitional‐care information divide.
In Israel, an innovative HIE system, OFEK (literally horizon), was implemented in 2005 at the largest not‐for‐profit insurer and provider of services, Clalit Health Services (Clalit). Clalit operates as an integrated healthcare delivery system, serving more than 50% of the Israeli population, as part of the country's national health insurance system. OFEK links information on all Clalit enrollees from all hospitals, primary care, and specialty care clinics, laboratories, and diagnostic services into a single, virtual, patient file, enabling providers to obtain complete, real‐time information needed for healthcare decision making at the point of care. Like similar HIE systems, OFEK includes information on previous medical encounters and hospitalizations, previous diagnoses, chronically prescribed medications, previous lab and imaging tests, known allergies, and some demographic information.[19] At the time of this study, OFEK was available in all Clalit hospitals as well as in 2 non‐Clalit (government‐owned and operated) large tertiary‐care centers, resulting in 40% coverage of all hospitalizations through the OFEK HIE system. As part of a large organization‐wide readmission reduction program recently implemented by Clalit for all its members admitted to any hospital in Israel, aimed at early detection and intervention,[20] OFEK was viewed as an important mechanism to help maintain continuity and improve transitions.
To inform current knowledge on different‐hospital readmissions (DHRs) and HIEs, we examined whether having HIE systems can contribute to information continuity and prevent delays in care and the need for more expensive, lengthy readmission stays when patients are readmitted to a different hospital. More specifically, we tested whether there is a difference in the LORS between SHRs and DHRs, and whether DHRs the LORS differ by the availability of an HIE (whether index and readmitting hospital are or are not connected through HIE systems).
METHODS
Study Design and Setting
We conducted a retrospective cohort study based on data of hospitalized Clalit members. Clalit has a centralized data warehouse with a comprehensive EHR containing data on all patients' medical encounters, administrative data, and clinical data compiled from laboratories, imaging centers, and hospitals. At the time of the study, OFEK was operating in all 8 Clalit hospitals and in 2 large government‐owned and operated hospitals in the central and northern parts of the country. Information is linked in the Clalit system and OFEK‐affiliated hospitals through an individual identity number assigned by the Israeli Interior Ministry to every Israeli resident for general identification purposes.
Population
The study examined all internal medicine and intensive‐care unit (ICU) readmissions of adult Clalit members (aged 18 years and older) previously (within the prior 30 days) discharged from internal medicine departments during January 1, 2010 until December 31, 2010 (ie, index hospitalizations). Only readmissions of index hospitalizations with more than a 24‐hour stay were included. A total of 146,266 index hospitalizations met the inclusion criteria. Index admissions that resulted in a transfer to another hospital, a long‐term care facility, or rehabilitation center were not included (N = 11,831). The final study sample included 27,057 readmissions (20.1% of the 134,435 index admissions), which could have resulted in any type of discharge (to patient's home, a long‐term care or rehabilitation facility, or due to death). The study was approved by Clalit's institutional review board.
Outcome Variable
We defined the LORS as the number of days from admission to discharge during readmission.
Main Independent Variable
We assessed information continuity as a categorical variable in which 0 = no information continuity (DHRs with either no HIE at either hospital or an HIE in only 1 of the hospitals), 1 = information continuity through an HIE (DHRs with both hospitals having an HIE), and 2 = full information continuity (readmission to the same hospital).
Covariates
We examined the following known correlates of length of stay (LOS): age, gender, residency in a nursing home, socioeconomic status (SES) based on an indicator of social security entitlement received by low‐income members,[21] and the occurrence of common chronic conditions registered in Clalit's EHR registries[22]: congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), chronic renal failure (CRF), malignancy, diabetes, hypertension, ischemic heart disease, atrial fibrillation, asthma, and disability (indication of a functional limitation). To provide comorbidity adjustment we used the Charlson Comorbidity Index.[23] Additionally, we assessed LOS of the index hospitalization. We included an indicator for the size of the index hospital: small, fewer than 100 beds; medium, 100 to 200 beds; and large, more than 200 beds. Finally, to account for a well‐known correlate of length of hospital stay,[24] we included an indicator for an ICU stay during the readmission.
Statistical Analysis
We first examined the study populations' characteristics and calculated the average LORS for each SHR and DHR category. Due to the skewed distribution of LORS, we also calculated the median and interquartile range (IQR) of LORS and evaluated the difference between categories using the Kruskall‐Wallis test.[25] Sample‐size calculations showed that we would need a sample of >3000 admissions to have 80% power to detect a difference of 0.8 hospitalization days given the 1:3 ratio between the DHR groups. To examine the association between LORS and information continuity, we employed a univariate marginal Cox model.[26] Variables that were significantly (P < 0.05) associated with LORS in the univariate model were entered into a multivariate marginal Cox model, clustering by patient and using a robust sandwich covariance matrix estimate. Additionally, we performed a sensitivity analysis using hierarchichal modeling to account for potential variations due to hospital level factors. A low hazard ratio (<1) represented an association of the covariate with decreased likelihood of discharge, that is, longer LORS. All analyses were conducted with SPSS version 20 (IBM, Armonk, NY) and SAS version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
The study included a total of 27,057 readmissions, of which 23,927 (88.4%) were SHRs and 3130 (11.6%) were DHRs. Of all DHRs, in 792 (2.9%) of the cases, both hospitals had HIEs (partial information continuity), and in 2338 (8.6%), either 1 or both did not have an HIE system (thus meaning there was no information continuity). Characteristics of the study population are shown in Table 1. Most (75%) of the readmissions were of patients over the age of 65 years, though only 7% were nursing home residents. More than half the study's population consisted of patients with low SES. The most common chronic conditions were hypertension (77%), ischemic heart disease (52%), and diabetes (48%). Other chronic conditions were arrhythmia (38%), CHF (35%), disability (31%), COPD (28%), malignancy (28%), and asthma (16%). In more than 55% of the index hospitalizations, the LOS was 4 days or less, and most index admissions (64%) were in large hospitals. Table 1 also displays the study population by the type of readmission: SHR, DHR with HIE, and DHR without HIE. As compared to patients readmitted to the same hospital, patients with DHRs were younger (P < 0.001), less likely to be nursing home residents (P < 0.001), and had longer LOS during the index admission (P < 0.001). Additionally, patients with SHRs were more likely to have their index admission at a large hospital (P < 0.001), had a higher comorbidity score (P < 0.043), and were less likely be treated in the ICU during their readmission (P < 0.001) compared to their DHR counterparts. Patients with DHRs without an HIE were similar in most characteristics to those with an HIE, except for having an ICU stay during their readmission (6.4% compared with 9.2%, respectively).
| Characteristics | All Readmissions, n = 27,057 | SHR, n = 23,927 | DHR With HIE, n = 792 | DHR Without HIE, n = 2,338 | P Value |
|---|---|---|---|---|---|
| |||||
| All personal characteristics | |||||
| Age, n (%) | <0.001 | ||||
| 1844 years | 1,328 (4.9) | 1,095 (4.6) | 58 (7.3) | 175 (7.5) | |
| 4564 years | 5,370 (19.8) | 4,597 (19.2) | 197 (24.9) | 576 (24.6) | |
| 6584 years | 14,059 (52.0) | 12,500 (52.2) | 402 (50.8) | 1,157 (49.5) | |
| 85+ years | 6,300 (23.3) | 5,735 (24.0) | 135 (17.0) | 430 (18.4) | |
| Female sex, n (%) | 13,742 (50.8) | 12,040 (50.3) | 418 (52.8) | 1,284 (54.9) | <0.001 |
| Low socioeconomic status, n (%) | 15,473 (57.2) | 13,670 (57.1) | 453 (57.2) | 1,350 (57.7) | |
| Residency in a nursing home, n (%) | 1,857 (6.9) | 1,743 (7.3) | 27 (3.4) | 87 (3.7) | <0.001 |
| Common chronic conditions, n (%) | |||||
| Hypertension | 20,797 (76.9) | 18,484 (77.3) | 588 (74.2) | 1,725 (73.8) | <0.001 |
| Ischemic heart disease | 14,150 (52.3) | 12,577 (52.6) | 397 (50.1) | 1,176 (50.3) | 0.052 |
| Diabetes | 13,052 (48.2) | 11,589 (48.4) | 345 (43.6) | 1,118 (47.8) | 0.024 |
| Arrhythmia | 10,306 (38.1) | 9,197 (38.4) | 292 (36.9) | 817 (34.9) | 0.003 |
| Chronic renal failure | 9,486 (35.1) | 8,454 (35.3) | 262 (33.1) | 770 (32.9) | 0.034 |
| Congestive heart failure | 9,216 (34.1) | 8,232 (34.4) | 270 (34.1) | 714 (30.5) | 0.001 |
| Disability | 8,362 (30.9) | 7,600 (31.8) | 165 (20.8) | 597 (25.5) | <0.001 |
| Chronic obstructive pulmonary disease | 7,671 (28.4) | 6,888 (28.8) | 201 (25.4) | 582 (24.9) | <0.001 |
| Malignancy | 7,642 (28.2) | 6,763 (28.3) | 220 (27.8) | 659 (28.2) | 0.954 |
| Asthma | 4,491 (16.6) | 4,040 (16.9) | 109 (13.8) | 342 (14.6) | 0.002 |
| Charlson score, mean [SD] | 4.54 [3.15] | 4.58 [3.14] | 4.14 [3.08] | 4.25 [3.24] | 0.043 |
| Index hospitalization characteristics (LOS during index hospitalization), n (%) | <0.001 | ||||
| 24 days | 14,961 (55.3) | 13,310 (55.6) | 428 (54.0) | 1,223 (52.3) | |
| 57 days | 6,366 (23.5) | 5,654 (23.6) | 174 (22.0) | 538 (23.0) | |
| 8 days and more | 5,730 (21.2) | 4,963 (20.7) | 190 (24.0) | 577 (24.7) | |
| Hospital size in index hospitalization (no. of hospitals in each category), n (%) | <0.001 | ||||
| Small, <100 beds (8) | 1,498 (5.5) | 1,166 (4.9) | 23 (2.9) | 309 (13.2) | |
| Medium, 100200 beds (9) | 8,129 (30.0) | 7,113 (29.7) | 316 (39.9) | 700 (29.9) | |
| Large, >200 beds (10) | 17,430 (64.4) | 15,648 (65.4) | 453 (57.2) | 1,329 (56.8) | |
| Intensive care unit during readmission, n (%) | 869 (3.2) | 647 (2.7) | 73 (9.2) | 149 (6.4) | <0.001 |
The mean LORS in SHRs was shorter by 1 day than the mean LORS for DHRs: 6.3 (95% confidence interval [CI]: 6.2‐6.4) versus 7.3 (95% CI: 7.0‐7.6), respectively. Mean LORS in DHRs with or without HIE was 7.6 (95% CI: 7.0‐8.3) and 7.2 (95% CI: 6.8‐7.6), respectively. Although median LORS was similar (4 days), the IQR differed, resulting in significant differences between the SHR and DHR groups (Table 2).
| Information Continuity | No. of Readmissions | Mean LORS (95% CI) | Median (Q1Q3) | Kruskal‐Wallis P Value |
|---|---|---|---|---|
| ||||
| SHRs | 23,927 (88.4) | 6.3 (6.26.4) | 4 (27) | |
| DHRs | 3,130 (11.6) | 7.3 (7.07.6) | 4 (28) | |
| DHRs with HIE | 792 (2.9) | 7.6 (7.08.3) | 4 (29) | |
| DHRs without HIE | 2,338 (8.7) | 7.2 (6.87.6) | 4 (28) | |
| Total | 27,057 | 6.4 (6.36.5) | 4 (27) | <0.001 |
In the multivariate model, partial continuity (DHRs with an HIE) was associated with decreased likelihood of discharge on any given day compared with full continuity (SHR) (hazard ratio [HR] = 0.85, 95% CI: 0.79‐0.91). Similar results were obtained for no continuity (DHRs without an HIE) (HR = 0.90, 95% CI: 0.86‐0.94). The difference between DHRs with and without an HIE was not significant (overlapping confidence intervals). Other factors associated with a lower HR for discharge during each day of the readmission were older age, residency in a nursing home, CHF, CRF, disability, malignancy, and long LOS (8+ days) during the index hospitalization. Patients with asthma or ischemic heart disease had a higher HR for discharge during each readmission day (Table 3). We performed a sensitivity analysis using hierarchical modeling (patients nested within hospitals), which resulted in similar findings in terms of directionality and magnitude of the relationships and significance levels.
| Characteristics | Univariate Model | Multivariate Model | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| ||||
| Information continuity | ||||
| SHR | Reference | Reference | ||
| DHR with HIE | 0.87 (0.810.93) | <0.001 | 0.86 (0.800.93) | <0.001 |
| DHR without HIE | 0.91 (0.870.94) | <0.001 | 0.90 (0.870.94) | <0.001 |
| Age | ||||
| 844 years | 1.22 (1.181.26) | <0.001 | 1.14 (1.071.22) | <0.001 |
| 4564 years | 1.16 (1.141.18) | <0.001 | 1.11 (1.061.1) | <0.001 |
| 6584 years | 1.01 (0.991.02) | 0.53 | 0.99 (0.961.02) | 0.60 |
| 85+ years | Reference | Reference | ||
| Sex | ||||
| Male | 0.97 (0.950.99) | 0.008 | 0.98 (0.961.01) | 0.19 |
| Female | Reference | Reference | ||
| Socioeconomic status | ||||
| Low | 0.98 (0.970.99) | 0.11 | ||
| Other | Reference | |||
| Residency in a nursing home | ||||
| Nursing home | 0.90 (0.880.92) | <0.001 | 0.90 (0.860.95) | <0.001 |
| All others | Reference | Reference | ||
| Common chronic conditions (reference: without condition) | ||||
| Hypertension | 0.94 (0.930.96) | <0.001 | 1.01 (0.971.04) | 0.69 |
| Ischemic heart disease | 1.00 (0.991.01) | 0.93 | 1.06 (1.031.09) | <0.001 |
| Diabetes | 0.97 (0.950.98) | 0.004 | 0.99 (0.971.02) | 0.64 |
| Arrhythmia | 0.96 (0.950.97) | 0.002 | 1.01 (0.981.04) | 0.39 |
| Chronic renal failure | 0.92 (0.910.93) | <0.001 | 0.96 (0.930.99) | 0.01 |
| Congestive heart failure | 0.93 (0.920.94) | <0.001 | 0.96 (0.930.99) | 0.01 |
| Disability | 0.86 (0.850.87) | <0.001 | 0.90 (0.870.92) | <0.001 |
| Chronic obstructive pulmonary disease | 0.99 (0.981.01) | 0.66 | ||
| Malignancy | 0.97 (0.960.98) | 0.03 | 0.98 (0.961.01) | 0.28 |
| Asthma | 1.04 (1.021.06) | 0.03 | 1.04 (1.001.07) | 0.03 |
| Charlson score | 0.99 (0.980.99) | <0.001 | 0.99 (0.991.00) | 0.04 |
| LOS during index hospitalization | ||||
| Days 24 | 1.52 (1.491.54) | <0.001 | 1.49 (1.451.54) | <0.001 |
| Days 57 | 1.21 (1.191.23) | <0.001 | 1.20 (1.161.24) | <0.001 |
| 8 days and more | Reference | Reference | ||
| Hospital size in index hospitalization | ||||
| Small, <100 beds (8) | 0.94 (0.920.97) | 0.02 | 1.00 (0.951.05) | 0.93 |
| Medium, 100200 beds (9) | 1.00 (0.991.02) | 0.78 | 1.01 (0.991.04) | 0.38 |
| Large, >200 beds (10) | Reference | Reference | ||
| Intensive care unit in readmission | ||||
| Yes | 0.75 (0.700.80) | <0.001 | 0.74 (0.690.79) | <0.001 |
| No | Reference | Reference | ||
DISCUSSION
This study shows that readmission to a different hospital results in longer duration of the readmission stay compared with readmission to the same index hospital. Our results also show that having HIE systems in both the index and readmitting hospitals does not protect against these negative outcomes, as there was no difference in the length of the readmission stay based on the availability of HIE systems. Factors that were found to be associated with longer readmission stays are well known indicators of the complexity of the patient's medical condition, such as the presence of disability, comorbidity, and ICU treatment during the readmission.[24, 27]
The shorter LORS in SHRs may be due to the familiarity of physicians and other healthcare providers with the patient and his or her condition, especially as the policy in SHRs in Israel is to readmit to the same unit from which the patient was recently discharged. This same hospital familiarity is especially important as hospital care in Israel follows the hospitalist model, in which responsibility for patient care is transferred from the patient's primary care physician to the hospital's physician, resulting in increased need for integration through HIE systems, especially when patients are readmitted to a different hospital.[28, 29]
Our findings, congruent with previous research on DHRs and poor outcomes,[7] could also be explained by the inefficiency associated with transitions. For example, patients frequently leave the hospital with pending lab tests, often with abnormal results that would change the course of care.[30] Because these pending tests are often omitted from the hospital discharge summaries,[31] if patients are hospitalized in a different hospital, the same tests may be ordered again, or a course of treatment that does not acknowledge the test results could be taken. Such time‐consuming duplication can be prevented in SHRs, where the index‐hospital records may be already more complete.
Our null findings regarding the contribution of HIE systems may be explained by the low levels of HIE actual use. Although we did not directly assess use, previous research reports that actual use of HIE is limited.[12] An Israeli study on the effects of the use of the OFEK system on ED physicians' admission decisions found that the patient's medical history was viewed in only 31.2% of all 281,750 ED referrals.[19] In another Israeli‐based ED study, even lower usage levels were found, with the OFEK system having been accessed in only 16% of all 3,219,910 ED referrals.[32] Low levels of HIE use have also been reported in the United States. An additional study, which tested the implementation of HIE in hospitals and clinics, showed that in only 2.3% of encounters did providers access the HIE record.[33] Another study conducted in 12 ED sites and 2 ambulatory clinics reported rates of 6.8% HIE use.[34] Moreover, the null effect of integrated health information reported here is congruent with findings from a US study on implementation of an electronic discharge instructions form with embedded computerized medication reconciliation, which was not found to be associated with postdischarge outcomes.[35]
A wide range of factors may influence decisions on HIE use: patient‐level factors,[36] perceived medical complexity of the patient,[33, 34] and the number of prior hospitalizations.[33, 34, 36] Healthcare systemlevel factors may include: time constraints, which may be positively[32] or negatively[33] associated with HIE use, and organizational policies or incentives.[33] Use may also be associated with features of the HIE technology itself, such as difficulty to access, difficulty to use once accessed, and the quality of information it contains.[37] Additionally, there is some evidence of the link between tight functional integration and higher proportions of usage.[38] Although comprehensive studies on factors affecting the use of the OFEK system in Israeli internal medicine units are still needed, the lack of its integration within each hospital's EHR system may serve as a major explanatory factor for the low usage levels.
The findings from this study should be interpreted in light of its limitations. First, compared with previously reported DHR rates (20%30%),[3, 5] the rate observed in our population was relatively low (about 12%). Previous research was restricted to heart failure patients[3] or assessed DHR in surgical, as well as internal medicine, patients.[5] Our lower rates may have been affected by the type of population (hospitalized internal medicine patients) and/or by characteristics of the Clalit healthcare system, which serves as an integrated provider network as well as insurer. Generalization from 1 health care system to others should be made with caution. Nonetheless, our results may underestimate the potential effect in other healthcare systems with less structural integration. Additionally, as noted above, information on the actual use of an HIE in the course of medical decision making during readmission was absent. Future studies should examine the potential benefit of an HIE with measures that capture providers' use of HIEs. Also, the LORS may be influenced by other factors not investigated here, and further future studies should examine additional outcomes such as costs, patient well‐being, and satisfaction. Finally, causality could not be determined, and future research in this realm should aim to search for the pathways connecting readmission to a different hospital, with and without HIEs, to readmission LOS and additional outcomes.
To conclude, our findings show that patients readmitted to a different hospital are at risk for prolonged LORS, regardless of the availability of HIE systems. Implementing HIE systems is the focus of substantial efforts by policymakers and is considered a key part of the meaningful use of electronic health information. HIE features in the provisions of the Health Information Technology for Economic and Clinical Health Act[39] because it can furnish providers with complete, timely information at the point of care. Moreover, although there has been substantial growth in the number of healthcare organizations that have operational an HIE, its ability to lead to improved outcomes has yet to be realized.[8, 10] The Israeli experience reported here suggests that provisions are needed that will ensure actual use of HIEs, which might in turn minimize the difference between DHRs and SHRs.
Acknowledgements
The authors acknowledge Chandra Cohen‐Stavi, MPA, and Orly Tonkikh, MA, for their contribution to this study.
Disclosures
The study was supported in part by a grant from the Israel National Institute for Health Policy Research (NIHP) (10/127). The authors report no conflicts of interest.
- , , , , , . Assessing preventability in the quest to reduce hospital readmissions. J Hosp Med. 2014;9:598–603.
- , , , et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174:1095–1107.
- , , , et al. Is same‐hospital readmission rate a good surrogate for all‐hospital readmission rate? Med Care. 2010;48:477–481.
- , , , . Hospital readmission rates: the impacts of age, payer, and mental health diagnoses. J Ambul Care Manage. 2013;36(2):147–155.
- , , , . Limitations of using same‐hospital readmission metrics. Int J Qual Health Care. 2013;25(6):633–639.
- Hospital inpatient and outpatient services. In: Report to the Congress: promoting greater efficiency in Medicare. Washington, DC: Medicare Payment Advisory Commission., March 2012;45–66.
- , , , , . For‐profit hospital status and rehospitalizations at different hospitals: an analysis of Medicare data. Ann Intern Med. 2010;153:718–727.
- , , , . Health information technology: an updated systematic review with a focus on meaningful use. Ann Intern Med. 2014;160:48–54.
- , , , . The benefits of health information technology: a review of the recent literature shows predominantly positive results. Health Aff (Millwood). 2011;30(3):464–471.
- , , . Health information exchange among US hospitals. Am J Manag Care. 2011;17:761–768.
- , , . A survey of health information exchange organizations in the United States: implications for meaningful use. Ann Intern Med. 2011;54:666–671.
- , , , , . Physicians' potential use and preferences related to health information exchange. Int J Med Inform. 2011;80:171–180.
- , , , et al. Provider stakeholders' perceived benefit from a nascent health information exchange: a qualitative analysis. J Med Syst. 2012;36:601–613.
- . More than just a question of technology: factors related to hospitals' adoption and implementation of health information exchange. Int J Med Inform. 2010;79:797–806.
- , , . Leveraging health information technology to achieve the “triple aim” of healthcare reform. J Am Med Inform Assoc. 2015;22(4):849–856.
- , , , , , . Health information exchange reduces repeated diagnostic imaging for back pain. Ann Emerg Med. 2013;62:16–24.
- , , , , . Association between use of a health information exchange system and hospital admissions. Appl Clin Inform. 2014;5:219.
- , , . The impact of EHR and HIE on reducing avoidable admissions: controlling main differential diagnoses. BMC Med Inform Decis Mak. 2013;13:49.
- , , , et al. The impact of an integrated hospital‐community medical information system on quality and service utilization in hospital departments. Int J Med Inform. 2010;79(9):649–657.
- , , , , , Predicting 30‐day readmissions with preadmission electronic health record data. Med Care. 2015;53:283–289.
- , , , , . Assessing socioeconomic health care utilization inequity in Israel: impact of alternative approaches to morbidity adjustment. BMC Public Health. 2011;11(1):609.
- , . Prevalence of selected chronic diseases in Israel. Isr Med Assoc J. 2001;3:404–408.
- , , , . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383.
- , , , , . Systematic review of risk adjustment models of hospital length of stay (LOS). Med Care. 2015;53:355–365.
- , . Use of ranks in one‐criterion variance analysis. J Am Stat Assoc. 1952;47:583–621.
- , , . Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc. 1989;84:1065–1073.
- , , , , , . Disability impacts length of stay in general internal medicine patients. J Gen Intern Med. 2014;29:885–890.
- . The hospitalist movement—time to move on. N Engl J Med. 2007;357:2627–2629.
- , , . Association of hospitalist presence and hospital‐level outcome measures among Medicare patients. J Hosp Med. 2014;9:1–6.
- , , , et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med. 2005;143:121–128.
- , , , et al. Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers. J Gen Intern Med. 2009;24:1002–1006.
- , , . Using electronic medical record systems for admission decisions in emergency departments: examining the crowdedness effect. J Med Syst. 2012;36:3795–3803.
- , , , , , . Factors motivating and affecting health information exchange usage. J Am Med Inform Assoc. 2011;18(2):143–149.
- , , , et al. Health information exchange usage in emergency departments and clinics: the who, what, and why. J Am Med Inform Assoc. 2011;18:690–697.
- , , , , . Effect of standardized electronic discharge instructions on post‐discharge hospital utilization. J Gen Intern Med. 2011;26:718–723.
- , , . Health information exchange technology on the front lines of healthcare: workflow factors and patterns of use. J Am Med Inform Assoc. 2012;19:392–400.
- , . The DeLone and McLean model of information systems success: a ten‐year update. J Manag Inf Syst. 2003;19:9–30.
- , , , et al. Architectural strategies and issues with health information exchange. AMIA Annu Symp Proc. 2006:814–818.
- . Launching HITECH. N Engl J Med. 2010;362(5):382–385.
Readmissions within a relatively short time after discharge are receiving considerable attention as an area of quality improvement,[1, 2] with increasing emphasis on the relatively large share of readmissions to different hospitals, accounting for 20% to 30% of all readmissions.[3, 4, 5, 6] Returning to a different hospital may affect patient and healthcare outcomes due to breaches in continuity. When information from the previous recent hospitalization is not transferred efficiently and accurately to the next admitting hospital, omissions and duplications can occur, resulting in delayed care and potentially worse outcomes (compared to same hospital readmissions [SHRs]), such as longer length of readmission stay (LORS) and increased costs.[7]
Electronic health records (EHRs) and health information exchange (HIE) systems are increasingly used for storage and retrieval of patient information from various sources, such as laboratories and previous physician visits and hospitalizations, enabling informational continuity by providing vital historical medical information for decision‐making. Whereas EHRs collect, store, and present information that is locally created within a specific clinic or hospital, HIEs connect EHR systems between multiple institutions, allowing providers to share clinical data and achieve interorganizational continuity. Such integrative systems are increasingly being implemented across healthcare systems worldwide.[8, 9, 10] Yet, technical difficulties, costs, competitive concerns, data privacy, and workflow implementation challenges have been described as hindering HIE participation.[11, 12, 13, 14] Moreover, major concerns exist regarding the poor usability of EHRs, their limited ability to support multidisciplinary care, and major difficulties in achieving interoperability with HIEs, which undermine efforts to deliver integrated patient‐centered care.[15] Nonetheless, previous research has demonstrated that HIEs can positively affect healthcare resource use and outcomes, including reduced rates of repeated diagnostic imaging in the emergency evaluation of back pain,[16] reduction in admissions via the emergency department (ED),[17] and reduced rates of readmissions within 7 days.[18] However, it is not known whether HIEs can contribute to positive outcomes when patients are readmitted to a different hospital than the hospital from which they were recently (within the previous 30 days) discharged, potentially bridging the transitional‐care information divide.
In Israel, an innovative HIE system, OFEK (literally horizon), was implemented in 2005 at the largest not‐for‐profit insurer and provider of services, Clalit Health Services (Clalit). Clalit operates as an integrated healthcare delivery system, serving more than 50% of the Israeli population, as part of the country's national health insurance system. OFEK links information on all Clalit enrollees from all hospitals, primary care, and specialty care clinics, laboratories, and diagnostic services into a single, virtual, patient file, enabling providers to obtain complete, real‐time information needed for healthcare decision making at the point of care. Like similar HIE systems, OFEK includes information on previous medical encounters and hospitalizations, previous diagnoses, chronically prescribed medications, previous lab and imaging tests, known allergies, and some demographic information.[19] At the time of this study, OFEK was available in all Clalit hospitals as well as in 2 non‐Clalit (government‐owned and operated) large tertiary‐care centers, resulting in 40% coverage of all hospitalizations through the OFEK HIE system. As part of a large organization‐wide readmission reduction program recently implemented by Clalit for all its members admitted to any hospital in Israel, aimed at early detection and intervention,[20] OFEK was viewed as an important mechanism to help maintain continuity and improve transitions.
To inform current knowledge on different‐hospital readmissions (DHRs) and HIEs, we examined whether having HIE systems can contribute to information continuity and prevent delays in care and the need for more expensive, lengthy readmission stays when patients are readmitted to a different hospital. More specifically, we tested whether there is a difference in the LORS between SHRs and DHRs, and whether DHRs the LORS differ by the availability of an HIE (whether index and readmitting hospital are or are not connected through HIE systems).
METHODS
Study Design and Setting
We conducted a retrospective cohort study based on data of hospitalized Clalit members. Clalit has a centralized data warehouse with a comprehensive EHR containing data on all patients' medical encounters, administrative data, and clinical data compiled from laboratories, imaging centers, and hospitals. At the time of the study, OFEK was operating in all 8 Clalit hospitals and in 2 large government‐owned and operated hospitals in the central and northern parts of the country. Information is linked in the Clalit system and OFEK‐affiliated hospitals through an individual identity number assigned by the Israeli Interior Ministry to every Israeli resident for general identification purposes.
Population
The study examined all internal medicine and intensive‐care unit (ICU) readmissions of adult Clalit members (aged 18 years and older) previously (within the prior 30 days) discharged from internal medicine departments during January 1, 2010 until December 31, 2010 (ie, index hospitalizations). Only readmissions of index hospitalizations with more than a 24‐hour stay were included. A total of 146,266 index hospitalizations met the inclusion criteria. Index admissions that resulted in a transfer to another hospital, a long‐term care facility, or rehabilitation center were not included (N = 11,831). The final study sample included 27,057 readmissions (20.1% of the 134,435 index admissions), which could have resulted in any type of discharge (to patient's home, a long‐term care or rehabilitation facility, or due to death). The study was approved by Clalit's institutional review board.
Outcome Variable
We defined the LORS as the number of days from admission to discharge during readmission.
Main Independent Variable
We assessed information continuity as a categorical variable in which 0 = no information continuity (DHRs with either no HIE at either hospital or an HIE in only 1 of the hospitals), 1 = information continuity through an HIE (DHRs with both hospitals having an HIE), and 2 = full information continuity (readmission to the same hospital).
Covariates
We examined the following known correlates of length of stay (LOS): age, gender, residency in a nursing home, socioeconomic status (SES) based on an indicator of social security entitlement received by low‐income members,[21] and the occurrence of common chronic conditions registered in Clalit's EHR registries[22]: congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), chronic renal failure (CRF), malignancy, diabetes, hypertension, ischemic heart disease, atrial fibrillation, asthma, and disability (indication of a functional limitation). To provide comorbidity adjustment we used the Charlson Comorbidity Index.[23] Additionally, we assessed LOS of the index hospitalization. We included an indicator for the size of the index hospital: small, fewer than 100 beds; medium, 100 to 200 beds; and large, more than 200 beds. Finally, to account for a well‐known correlate of length of hospital stay,[24] we included an indicator for an ICU stay during the readmission.
Statistical Analysis
We first examined the study populations' characteristics and calculated the average LORS for each SHR and DHR category. Due to the skewed distribution of LORS, we also calculated the median and interquartile range (IQR) of LORS and evaluated the difference between categories using the Kruskall‐Wallis test.[25] Sample‐size calculations showed that we would need a sample of >3000 admissions to have 80% power to detect a difference of 0.8 hospitalization days given the 1:3 ratio between the DHR groups. To examine the association between LORS and information continuity, we employed a univariate marginal Cox model.[26] Variables that were significantly (P < 0.05) associated with LORS in the univariate model were entered into a multivariate marginal Cox model, clustering by patient and using a robust sandwich covariance matrix estimate. Additionally, we performed a sensitivity analysis using hierarchichal modeling to account for potential variations due to hospital level factors. A low hazard ratio (<1) represented an association of the covariate with decreased likelihood of discharge, that is, longer LORS. All analyses were conducted with SPSS version 20 (IBM, Armonk, NY) and SAS version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
The study included a total of 27,057 readmissions, of which 23,927 (88.4%) were SHRs and 3130 (11.6%) were DHRs. Of all DHRs, in 792 (2.9%) of the cases, both hospitals had HIEs (partial information continuity), and in 2338 (8.6%), either 1 or both did not have an HIE system (thus meaning there was no information continuity). Characteristics of the study population are shown in Table 1. Most (75%) of the readmissions were of patients over the age of 65 years, though only 7% were nursing home residents. More than half the study's population consisted of patients with low SES. The most common chronic conditions were hypertension (77%), ischemic heart disease (52%), and diabetes (48%). Other chronic conditions were arrhythmia (38%), CHF (35%), disability (31%), COPD (28%), malignancy (28%), and asthma (16%). In more than 55% of the index hospitalizations, the LOS was 4 days or less, and most index admissions (64%) were in large hospitals. Table 1 also displays the study population by the type of readmission: SHR, DHR with HIE, and DHR without HIE. As compared to patients readmitted to the same hospital, patients with DHRs were younger (P < 0.001), less likely to be nursing home residents (P < 0.001), and had longer LOS during the index admission (P < 0.001). Additionally, patients with SHRs were more likely to have their index admission at a large hospital (P < 0.001), had a higher comorbidity score (P < 0.043), and were less likely be treated in the ICU during their readmission (P < 0.001) compared to their DHR counterparts. Patients with DHRs without an HIE were similar in most characteristics to those with an HIE, except for having an ICU stay during their readmission (6.4% compared with 9.2%, respectively).
| Characteristics | All Readmissions, n = 27,057 | SHR, n = 23,927 | DHR With HIE, n = 792 | DHR Without HIE, n = 2,338 | P Value |
|---|---|---|---|---|---|
| |||||
| All personal characteristics | |||||
| Age, n (%) | <0.001 | ||||
| 1844 years | 1,328 (4.9) | 1,095 (4.6) | 58 (7.3) | 175 (7.5) | |
| 4564 years | 5,370 (19.8) | 4,597 (19.2) | 197 (24.9) | 576 (24.6) | |
| 6584 years | 14,059 (52.0) | 12,500 (52.2) | 402 (50.8) | 1,157 (49.5) | |
| 85+ years | 6,300 (23.3) | 5,735 (24.0) | 135 (17.0) | 430 (18.4) | |
| Female sex, n (%) | 13,742 (50.8) | 12,040 (50.3) | 418 (52.8) | 1,284 (54.9) | <0.001 |
| Low socioeconomic status, n (%) | 15,473 (57.2) | 13,670 (57.1) | 453 (57.2) | 1,350 (57.7) | |
| Residency in a nursing home, n (%) | 1,857 (6.9) | 1,743 (7.3) | 27 (3.4) | 87 (3.7) | <0.001 |
| Common chronic conditions, n (%) | |||||
| Hypertension | 20,797 (76.9) | 18,484 (77.3) | 588 (74.2) | 1,725 (73.8) | <0.001 |
| Ischemic heart disease | 14,150 (52.3) | 12,577 (52.6) | 397 (50.1) | 1,176 (50.3) | 0.052 |
| Diabetes | 13,052 (48.2) | 11,589 (48.4) | 345 (43.6) | 1,118 (47.8) | 0.024 |
| Arrhythmia | 10,306 (38.1) | 9,197 (38.4) | 292 (36.9) | 817 (34.9) | 0.003 |
| Chronic renal failure | 9,486 (35.1) | 8,454 (35.3) | 262 (33.1) | 770 (32.9) | 0.034 |
| Congestive heart failure | 9,216 (34.1) | 8,232 (34.4) | 270 (34.1) | 714 (30.5) | 0.001 |
| Disability | 8,362 (30.9) | 7,600 (31.8) | 165 (20.8) | 597 (25.5) | <0.001 |
| Chronic obstructive pulmonary disease | 7,671 (28.4) | 6,888 (28.8) | 201 (25.4) | 582 (24.9) | <0.001 |
| Malignancy | 7,642 (28.2) | 6,763 (28.3) | 220 (27.8) | 659 (28.2) | 0.954 |
| Asthma | 4,491 (16.6) | 4,040 (16.9) | 109 (13.8) | 342 (14.6) | 0.002 |
| Charlson score, mean [SD] | 4.54 [3.15] | 4.58 [3.14] | 4.14 [3.08] | 4.25 [3.24] | 0.043 |
| Index hospitalization characteristics (LOS during index hospitalization), n (%) | <0.001 | ||||
| 24 days | 14,961 (55.3) | 13,310 (55.6) | 428 (54.0) | 1,223 (52.3) | |
| 57 days | 6,366 (23.5) | 5,654 (23.6) | 174 (22.0) | 538 (23.0) | |
| 8 days and more | 5,730 (21.2) | 4,963 (20.7) | 190 (24.0) | 577 (24.7) | |
| Hospital size in index hospitalization (no. of hospitals in each category), n (%) | <0.001 | ||||
| Small, <100 beds (8) | 1,498 (5.5) | 1,166 (4.9) | 23 (2.9) | 309 (13.2) | |
| Medium, 100200 beds (9) | 8,129 (30.0) | 7,113 (29.7) | 316 (39.9) | 700 (29.9) | |
| Large, >200 beds (10) | 17,430 (64.4) | 15,648 (65.4) | 453 (57.2) | 1,329 (56.8) | |
| Intensive care unit during readmission, n (%) | 869 (3.2) | 647 (2.7) | 73 (9.2) | 149 (6.4) | <0.001 |
The mean LORS in SHRs was shorter by 1 day than the mean LORS for DHRs: 6.3 (95% confidence interval [CI]: 6.2‐6.4) versus 7.3 (95% CI: 7.0‐7.6), respectively. Mean LORS in DHRs with or without HIE was 7.6 (95% CI: 7.0‐8.3) and 7.2 (95% CI: 6.8‐7.6), respectively. Although median LORS was similar (4 days), the IQR differed, resulting in significant differences between the SHR and DHR groups (Table 2).
| Information Continuity | No. of Readmissions | Mean LORS (95% CI) | Median (Q1Q3) | Kruskal‐Wallis P Value |
|---|---|---|---|---|
| ||||
| SHRs | 23,927 (88.4) | 6.3 (6.26.4) | 4 (27) | |
| DHRs | 3,130 (11.6) | 7.3 (7.07.6) | 4 (28) | |
| DHRs with HIE | 792 (2.9) | 7.6 (7.08.3) | 4 (29) | |
| DHRs without HIE | 2,338 (8.7) | 7.2 (6.87.6) | 4 (28) | |
| Total | 27,057 | 6.4 (6.36.5) | 4 (27) | <0.001 |
In the multivariate model, partial continuity (DHRs with an HIE) was associated with decreased likelihood of discharge on any given day compared with full continuity (SHR) (hazard ratio [HR] = 0.85, 95% CI: 0.79‐0.91). Similar results were obtained for no continuity (DHRs without an HIE) (HR = 0.90, 95% CI: 0.86‐0.94). The difference between DHRs with and without an HIE was not significant (overlapping confidence intervals). Other factors associated with a lower HR for discharge during each day of the readmission were older age, residency in a nursing home, CHF, CRF, disability, malignancy, and long LOS (8+ days) during the index hospitalization. Patients with asthma or ischemic heart disease had a higher HR for discharge during each readmission day (Table 3). We performed a sensitivity analysis using hierarchical modeling (patients nested within hospitals), which resulted in similar findings in terms of directionality and magnitude of the relationships and significance levels.
| Characteristics | Univariate Model | Multivariate Model | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| ||||
| Information continuity | ||||
| SHR | Reference | Reference | ||
| DHR with HIE | 0.87 (0.810.93) | <0.001 | 0.86 (0.800.93) | <0.001 |
| DHR without HIE | 0.91 (0.870.94) | <0.001 | 0.90 (0.870.94) | <0.001 |
| Age | ||||
| 844 years | 1.22 (1.181.26) | <0.001 | 1.14 (1.071.22) | <0.001 |
| 4564 years | 1.16 (1.141.18) | <0.001 | 1.11 (1.061.1) | <0.001 |
| 6584 years | 1.01 (0.991.02) | 0.53 | 0.99 (0.961.02) | 0.60 |
| 85+ years | Reference | Reference | ||
| Sex | ||||
| Male | 0.97 (0.950.99) | 0.008 | 0.98 (0.961.01) | 0.19 |
| Female | Reference | Reference | ||
| Socioeconomic status | ||||
| Low | 0.98 (0.970.99) | 0.11 | ||
| Other | Reference | |||
| Residency in a nursing home | ||||
| Nursing home | 0.90 (0.880.92) | <0.001 | 0.90 (0.860.95) | <0.001 |
| All others | Reference | Reference | ||
| Common chronic conditions (reference: without condition) | ||||
| Hypertension | 0.94 (0.930.96) | <0.001 | 1.01 (0.971.04) | 0.69 |
| Ischemic heart disease | 1.00 (0.991.01) | 0.93 | 1.06 (1.031.09) | <0.001 |
| Diabetes | 0.97 (0.950.98) | 0.004 | 0.99 (0.971.02) | 0.64 |
| Arrhythmia | 0.96 (0.950.97) | 0.002 | 1.01 (0.981.04) | 0.39 |
| Chronic renal failure | 0.92 (0.910.93) | <0.001 | 0.96 (0.930.99) | 0.01 |
| Congestive heart failure | 0.93 (0.920.94) | <0.001 | 0.96 (0.930.99) | 0.01 |
| Disability | 0.86 (0.850.87) | <0.001 | 0.90 (0.870.92) | <0.001 |
| Chronic obstructive pulmonary disease | 0.99 (0.981.01) | 0.66 | ||
| Malignancy | 0.97 (0.960.98) | 0.03 | 0.98 (0.961.01) | 0.28 |
| Asthma | 1.04 (1.021.06) | 0.03 | 1.04 (1.001.07) | 0.03 |
| Charlson score | 0.99 (0.980.99) | <0.001 | 0.99 (0.991.00) | 0.04 |
| LOS during index hospitalization | ||||
| Days 24 | 1.52 (1.491.54) | <0.001 | 1.49 (1.451.54) | <0.001 |
| Days 57 | 1.21 (1.191.23) | <0.001 | 1.20 (1.161.24) | <0.001 |
| 8 days and more | Reference | Reference | ||
| Hospital size in index hospitalization | ||||
| Small, <100 beds (8) | 0.94 (0.920.97) | 0.02 | 1.00 (0.951.05) | 0.93 |
| Medium, 100200 beds (9) | 1.00 (0.991.02) | 0.78 | 1.01 (0.991.04) | 0.38 |
| Large, >200 beds (10) | Reference | Reference | ||
| Intensive care unit in readmission | ||||
| Yes | 0.75 (0.700.80) | <0.001 | 0.74 (0.690.79) | <0.001 |
| No | Reference | Reference | ||
DISCUSSION
This study shows that readmission to a different hospital results in longer duration of the readmission stay compared with readmission to the same index hospital. Our results also show that having HIE systems in both the index and readmitting hospitals does not protect against these negative outcomes, as there was no difference in the length of the readmission stay based on the availability of HIE systems. Factors that were found to be associated with longer readmission stays are well known indicators of the complexity of the patient's medical condition, such as the presence of disability, comorbidity, and ICU treatment during the readmission.[24, 27]
The shorter LORS in SHRs may be due to the familiarity of physicians and other healthcare providers with the patient and his or her condition, especially as the policy in SHRs in Israel is to readmit to the same unit from which the patient was recently discharged. This same hospital familiarity is especially important as hospital care in Israel follows the hospitalist model, in which responsibility for patient care is transferred from the patient's primary care physician to the hospital's physician, resulting in increased need for integration through HIE systems, especially when patients are readmitted to a different hospital.[28, 29]
Our findings, congruent with previous research on DHRs and poor outcomes,[7] could also be explained by the inefficiency associated with transitions. For example, patients frequently leave the hospital with pending lab tests, often with abnormal results that would change the course of care.[30] Because these pending tests are often omitted from the hospital discharge summaries,[31] if patients are hospitalized in a different hospital, the same tests may be ordered again, or a course of treatment that does not acknowledge the test results could be taken. Such time‐consuming duplication can be prevented in SHRs, where the index‐hospital records may be already more complete.
Our null findings regarding the contribution of HIE systems may be explained by the low levels of HIE actual use. Although we did not directly assess use, previous research reports that actual use of HIE is limited.[12] An Israeli study on the effects of the use of the OFEK system on ED physicians' admission decisions found that the patient's medical history was viewed in only 31.2% of all 281,750 ED referrals.[19] In another Israeli‐based ED study, even lower usage levels were found, with the OFEK system having been accessed in only 16% of all 3,219,910 ED referrals.[32] Low levels of HIE use have also been reported in the United States. An additional study, which tested the implementation of HIE in hospitals and clinics, showed that in only 2.3% of encounters did providers access the HIE record.[33] Another study conducted in 12 ED sites and 2 ambulatory clinics reported rates of 6.8% HIE use.[34] Moreover, the null effect of integrated health information reported here is congruent with findings from a US study on implementation of an electronic discharge instructions form with embedded computerized medication reconciliation, which was not found to be associated with postdischarge outcomes.[35]
A wide range of factors may influence decisions on HIE use: patient‐level factors,[36] perceived medical complexity of the patient,[33, 34] and the number of prior hospitalizations.[33, 34, 36] Healthcare systemlevel factors may include: time constraints, which may be positively[32] or negatively[33] associated with HIE use, and organizational policies or incentives.[33] Use may also be associated with features of the HIE technology itself, such as difficulty to access, difficulty to use once accessed, and the quality of information it contains.[37] Additionally, there is some evidence of the link between tight functional integration and higher proportions of usage.[38] Although comprehensive studies on factors affecting the use of the OFEK system in Israeli internal medicine units are still needed, the lack of its integration within each hospital's EHR system may serve as a major explanatory factor for the low usage levels.
The findings from this study should be interpreted in light of its limitations. First, compared with previously reported DHR rates (20%30%),[3, 5] the rate observed in our population was relatively low (about 12%). Previous research was restricted to heart failure patients[3] or assessed DHR in surgical, as well as internal medicine, patients.[5] Our lower rates may have been affected by the type of population (hospitalized internal medicine patients) and/or by characteristics of the Clalit healthcare system, which serves as an integrated provider network as well as insurer. Generalization from 1 health care system to others should be made with caution. Nonetheless, our results may underestimate the potential effect in other healthcare systems with less structural integration. Additionally, as noted above, information on the actual use of an HIE in the course of medical decision making during readmission was absent. Future studies should examine the potential benefit of an HIE with measures that capture providers' use of HIEs. Also, the LORS may be influenced by other factors not investigated here, and further future studies should examine additional outcomes such as costs, patient well‐being, and satisfaction. Finally, causality could not be determined, and future research in this realm should aim to search for the pathways connecting readmission to a different hospital, with and without HIEs, to readmission LOS and additional outcomes.
To conclude, our findings show that patients readmitted to a different hospital are at risk for prolonged LORS, regardless of the availability of HIE systems. Implementing HIE systems is the focus of substantial efforts by policymakers and is considered a key part of the meaningful use of electronic health information. HIE features in the provisions of the Health Information Technology for Economic and Clinical Health Act[39] because it can furnish providers with complete, timely information at the point of care. Moreover, although there has been substantial growth in the number of healthcare organizations that have operational an HIE, its ability to lead to improved outcomes has yet to be realized.[8, 10] The Israeli experience reported here suggests that provisions are needed that will ensure actual use of HIEs, which might in turn minimize the difference between DHRs and SHRs.
Acknowledgements
The authors acknowledge Chandra Cohen‐Stavi, MPA, and Orly Tonkikh, MA, for their contribution to this study.
Disclosures
The study was supported in part by a grant from the Israel National Institute for Health Policy Research (NIHP) (10/127). The authors report no conflicts of interest.
Readmissions within a relatively short time after discharge are receiving considerable attention as an area of quality improvement,[1, 2] with increasing emphasis on the relatively large share of readmissions to different hospitals, accounting for 20% to 30% of all readmissions.[3, 4, 5, 6] Returning to a different hospital may affect patient and healthcare outcomes due to breaches in continuity. When information from the previous recent hospitalization is not transferred efficiently and accurately to the next admitting hospital, omissions and duplications can occur, resulting in delayed care and potentially worse outcomes (compared to same hospital readmissions [SHRs]), such as longer length of readmission stay (LORS) and increased costs.[7]
Electronic health records (EHRs) and health information exchange (HIE) systems are increasingly used for storage and retrieval of patient information from various sources, such as laboratories and previous physician visits and hospitalizations, enabling informational continuity by providing vital historical medical information for decision‐making. Whereas EHRs collect, store, and present information that is locally created within a specific clinic or hospital, HIEs connect EHR systems between multiple institutions, allowing providers to share clinical data and achieve interorganizational continuity. Such integrative systems are increasingly being implemented across healthcare systems worldwide.[8, 9, 10] Yet, technical difficulties, costs, competitive concerns, data privacy, and workflow implementation challenges have been described as hindering HIE participation.[11, 12, 13, 14] Moreover, major concerns exist regarding the poor usability of EHRs, their limited ability to support multidisciplinary care, and major difficulties in achieving interoperability with HIEs, which undermine efforts to deliver integrated patient‐centered care.[15] Nonetheless, previous research has demonstrated that HIEs can positively affect healthcare resource use and outcomes, including reduced rates of repeated diagnostic imaging in the emergency evaluation of back pain,[16] reduction in admissions via the emergency department (ED),[17] and reduced rates of readmissions within 7 days.[18] However, it is not known whether HIEs can contribute to positive outcomes when patients are readmitted to a different hospital than the hospital from which they were recently (within the previous 30 days) discharged, potentially bridging the transitional‐care information divide.
In Israel, an innovative HIE system, OFEK (literally horizon), was implemented in 2005 at the largest not‐for‐profit insurer and provider of services, Clalit Health Services (Clalit). Clalit operates as an integrated healthcare delivery system, serving more than 50% of the Israeli population, as part of the country's national health insurance system. OFEK links information on all Clalit enrollees from all hospitals, primary care, and specialty care clinics, laboratories, and diagnostic services into a single, virtual, patient file, enabling providers to obtain complete, real‐time information needed for healthcare decision making at the point of care. Like similar HIE systems, OFEK includes information on previous medical encounters and hospitalizations, previous diagnoses, chronically prescribed medications, previous lab and imaging tests, known allergies, and some demographic information.[19] At the time of this study, OFEK was available in all Clalit hospitals as well as in 2 non‐Clalit (government‐owned and operated) large tertiary‐care centers, resulting in 40% coverage of all hospitalizations through the OFEK HIE system. As part of a large organization‐wide readmission reduction program recently implemented by Clalit for all its members admitted to any hospital in Israel, aimed at early detection and intervention,[20] OFEK was viewed as an important mechanism to help maintain continuity and improve transitions.
To inform current knowledge on different‐hospital readmissions (DHRs) and HIEs, we examined whether having HIE systems can contribute to information continuity and prevent delays in care and the need for more expensive, lengthy readmission stays when patients are readmitted to a different hospital. More specifically, we tested whether there is a difference in the LORS between SHRs and DHRs, and whether DHRs the LORS differ by the availability of an HIE (whether index and readmitting hospital are or are not connected through HIE systems).
METHODS
Study Design and Setting
We conducted a retrospective cohort study based on data of hospitalized Clalit members. Clalit has a centralized data warehouse with a comprehensive EHR containing data on all patients' medical encounters, administrative data, and clinical data compiled from laboratories, imaging centers, and hospitals. At the time of the study, OFEK was operating in all 8 Clalit hospitals and in 2 large government‐owned and operated hospitals in the central and northern parts of the country. Information is linked in the Clalit system and OFEK‐affiliated hospitals through an individual identity number assigned by the Israeli Interior Ministry to every Israeli resident for general identification purposes.
Population
The study examined all internal medicine and intensive‐care unit (ICU) readmissions of adult Clalit members (aged 18 years and older) previously (within the prior 30 days) discharged from internal medicine departments during January 1, 2010 until December 31, 2010 (ie, index hospitalizations). Only readmissions of index hospitalizations with more than a 24‐hour stay were included. A total of 146,266 index hospitalizations met the inclusion criteria. Index admissions that resulted in a transfer to another hospital, a long‐term care facility, or rehabilitation center were not included (N = 11,831). The final study sample included 27,057 readmissions (20.1% of the 134,435 index admissions), which could have resulted in any type of discharge (to patient's home, a long‐term care or rehabilitation facility, or due to death). The study was approved by Clalit's institutional review board.
Outcome Variable
We defined the LORS as the number of days from admission to discharge during readmission.
Main Independent Variable
We assessed information continuity as a categorical variable in which 0 = no information continuity (DHRs with either no HIE at either hospital or an HIE in only 1 of the hospitals), 1 = information continuity through an HIE (DHRs with both hospitals having an HIE), and 2 = full information continuity (readmission to the same hospital).
Covariates
We examined the following known correlates of length of stay (LOS): age, gender, residency in a nursing home, socioeconomic status (SES) based on an indicator of social security entitlement received by low‐income members,[21] and the occurrence of common chronic conditions registered in Clalit's EHR registries[22]: congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), chronic renal failure (CRF), malignancy, diabetes, hypertension, ischemic heart disease, atrial fibrillation, asthma, and disability (indication of a functional limitation). To provide comorbidity adjustment we used the Charlson Comorbidity Index.[23] Additionally, we assessed LOS of the index hospitalization. We included an indicator for the size of the index hospital: small, fewer than 100 beds; medium, 100 to 200 beds; and large, more than 200 beds. Finally, to account for a well‐known correlate of length of hospital stay,[24] we included an indicator for an ICU stay during the readmission.
Statistical Analysis
We first examined the study populations' characteristics and calculated the average LORS for each SHR and DHR category. Due to the skewed distribution of LORS, we also calculated the median and interquartile range (IQR) of LORS and evaluated the difference between categories using the Kruskall‐Wallis test.[25] Sample‐size calculations showed that we would need a sample of >3000 admissions to have 80% power to detect a difference of 0.8 hospitalization days given the 1:3 ratio between the DHR groups. To examine the association between LORS and information continuity, we employed a univariate marginal Cox model.[26] Variables that were significantly (P < 0.05) associated with LORS in the univariate model were entered into a multivariate marginal Cox model, clustering by patient and using a robust sandwich covariance matrix estimate. Additionally, we performed a sensitivity analysis using hierarchichal modeling to account for potential variations due to hospital level factors. A low hazard ratio (<1) represented an association of the covariate with decreased likelihood of discharge, that is, longer LORS. All analyses were conducted with SPSS version 20 (IBM, Armonk, NY) and SAS version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
The study included a total of 27,057 readmissions, of which 23,927 (88.4%) were SHRs and 3130 (11.6%) were DHRs. Of all DHRs, in 792 (2.9%) of the cases, both hospitals had HIEs (partial information continuity), and in 2338 (8.6%), either 1 or both did not have an HIE system (thus meaning there was no information continuity). Characteristics of the study population are shown in Table 1. Most (75%) of the readmissions were of patients over the age of 65 years, though only 7% were nursing home residents. More than half the study's population consisted of patients with low SES. The most common chronic conditions were hypertension (77%), ischemic heart disease (52%), and diabetes (48%). Other chronic conditions were arrhythmia (38%), CHF (35%), disability (31%), COPD (28%), malignancy (28%), and asthma (16%). In more than 55% of the index hospitalizations, the LOS was 4 days or less, and most index admissions (64%) were in large hospitals. Table 1 also displays the study population by the type of readmission: SHR, DHR with HIE, and DHR without HIE. As compared to patients readmitted to the same hospital, patients with DHRs were younger (P < 0.001), less likely to be nursing home residents (P < 0.001), and had longer LOS during the index admission (P < 0.001). Additionally, patients with SHRs were more likely to have their index admission at a large hospital (P < 0.001), had a higher comorbidity score (P < 0.043), and were less likely be treated in the ICU during their readmission (P < 0.001) compared to their DHR counterparts. Patients with DHRs without an HIE were similar in most characteristics to those with an HIE, except for having an ICU stay during their readmission (6.4% compared with 9.2%, respectively).
| Characteristics | All Readmissions, n = 27,057 | SHR, n = 23,927 | DHR With HIE, n = 792 | DHR Without HIE, n = 2,338 | P Value |
|---|---|---|---|---|---|
| |||||
| All personal characteristics | |||||
| Age, n (%) | <0.001 | ||||
| 1844 years | 1,328 (4.9) | 1,095 (4.6) | 58 (7.3) | 175 (7.5) | |
| 4564 years | 5,370 (19.8) | 4,597 (19.2) | 197 (24.9) | 576 (24.6) | |
| 6584 years | 14,059 (52.0) | 12,500 (52.2) | 402 (50.8) | 1,157 (49.5) | |
| 85+ years | 6,300 (23.3) | 5,735 (24.0) | 135 (17.0) | 430 (18.4) | |
| Female sex, n (%) | 13,742 (50.8) | 12,040 (50.3) | 418 (52.8) | 1,284 (54.9) | <0.001 |
| Low socioeconomic status, n (%) | 15,473 (57.2) | 13,670 (57.1) | 453 (57.2) | 1,350 (57.7) | |
| Residency in a nursing home, n (%) | 1,857 (6.9) | 1,743 (7.3) | 27 (3.4) | 87 (3.7) | <0.001 |
| Common chronic conditions, n (%) | |||||
| Hypertension | 20,797 (76.9) | 18,484 (77.3) | 588 (74.2) | 1,725 (73.8) | <0.001 |
| Ischemic heart disease | 14,150 (52.3) | 12,577 (52.6) | 397 (50.1) | 1,176 (50.3) | 0.052 |
| Diabetes | 13,052 (48.2) | 11,589 (48.4) | 345 (43.6) | 1,118 (47.8) | 0.024 |
| Arrhythmia | 10,306 (38.1) | 9,197 (38.4) | 292 (36.9) | 817 (34.9) | 0.003 |
| Chronic renal failure | 9,486 (35.1) | 8,454 (35.3) | 262 (33.1) | 770 (32.9) | 0.034 |
| Congestive heart failure | 9,216 (34.1) | 8,232 (34.4) | 270 (34.1) | 714 (30.5) | 0.001 |
| Disability | 8,362 (30.9) | 7,600 (31.8) | 165 (20.8) | 597 (25.5) | <0.001 |
| Chronic obstructive pulmonary disease | 7,671 (28.4) | 6,888 (28.8) | 201 (25.4) | 582 (24.9) | <0.001 |
| Malignancy | 7,642 (28.2) | 6,763 (28.3) | 220 (27.8) | 659 (28.2) | 0.954 |
| Asthma | 4,491 (16.6) | 4,040 (16.9) | 109 (13.8) | 342 (14.6) | 0.002 |
| Charlson score, mean [SD] | 4.54 [3.15] | 4.58 [3.14] | 4.14 [3.08] | 4.25 [3.24] | 0.043 |
| Index hospitalization characteristics (LOS during index hospitalization), n (%) | <0.001 | ||||
| 24 days | 14,961 (55.3) | 13,310 (55.6) | 428 (54.0) | 1,223 (52.3) | |
| 57 days | 6,366 (23.5) | 5,654 (23.6) | 174 (22.0) | 538 (23.0) | |
| 8 days and more | 5,730 (21.2) | 4,963 (20.7) | 190 (24.0) | 577 (24.7) | |
| Hospital size in index hospitalization (no. of hospitals in each category), n (%) | <0.001 | ||||
| Small, <100 beds (8) | 1,498 (5.5) | 1,166 (4.9) | 23 (2.9) | 309 (13.2) | |
| Medium, 100200 beds (9) | 8,129 (30.0) | 7,113 (29.7) | 316 (39.9) | 700 (29.9) | |
| Large, >200 beds (10) | 17,430 (64.4) | 15,648 (65.4) | 453 (57.2) | 1,329 (56.8) | |
| Intensive care unit during readmission, n (%) | 869 (3.2) | 647 (2.7) | 73 (9.2) | 149 (6.4) | <0.001 |
The mean LORS in SHRs was shorter by 1 day than the mean LORS for DHRs: 6.3 (95% confidence interval [CI]: 6.2‐6.4) versus 7.3 (95% CI: 7.0‐7.6), respectively. Mean LORS in DHRs with or without HIE was 7.6 (95% CI: 7.0‐8.3) and 7.2 (95% CI: 6.8‐7.6), respectively. Although median LORS was similar (4 days), the IQR differed, resulting in significant differences between the SHR and DHR groups (Table 2).
| Information Continuity | No. of Readmissions | Mean LORS (95% CI) | Median (Q1Q3) | Kruskal‐Wallis P Value |
|---|---|---|---|---|
| ||||
| SHRs | 23,927 (88.4) | 6.3 (6.26.4) | 4 (27) | |
| DHRs | 3,130 (11.6) | 7.3 (7.07.6) | 4 (28) | |
| DHRs with HIE | 792 (2.9) | 7.6 (7.08.3) | 4 (29) | |
| DHRs without HIE | 2,338 (8.7) | 7.2 (6.87.6) | 4 (28) | |
| Total | 27,057 | 6.4 (6.36.5) | 4 (27) | <0.001 |
In the multivariate model, partial continuity (DHRs with an HIE) was associated with decreased likelihood of discharge on any given day compared with full continuity (SHR) (hazard ratio [HR] = 0.85, 95% CI: 0.79‐0.91). Similar results were obtained for no continuity (DHRs without an HIE) (HR = 0.90, 95% CI: 0.86‐0.94). The difference between DHRs with and without an HIE was not significant (overlapping confidence intervals). Other factors associated with a lower HR for discharge during each day of the readmission were older age, residency in a nursing home, CHF, CRF, disability, malignancy, and long LOS (8+ days) during the index hospitalization. Patients with asthma or ischemic heart disease had a higher HR for discharge during each readmission day (Table 3). We performed a sensitivity analysis using hierarchical modeling (patients nested within hospitals), which resulted in similar findings in terms of directionality and magnitude of the relationships and significance levels.
| Characteristics | Univariate Model | Multivariate Model | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| ||||
| Information continuity | ||||
| SHR | Reference | Reference | ||
| DHR with HIE | 0.87 (0.810.93) | <0.001 | 0.86 (0.800.93) | <0.001 |
| DHR without HIE | 0.91 (0.870.94) | <0.001 | 0.90 (0.870.94) | <0.001 |
| Age | ||||
| 844 years | 1.22 (1.181.26) | <0.001 | 1.14 (1.071.22) | <0.001 |
| 4564 years | 1.16 (1.141.18) | <0.001 | 1.11 (1.061.1) | <0.001 |
| 6584 years | 1.01 (0.991.02) | 0.53 | 0.99 (0.961.02) | 0.60 |
| 85+ years | Reference | Reference | ||
| Sex | ||||
| Male | 0.97 (0.950.99) | 0.008 | 0.98 (0.961.01) | 0.19 |
| Female | Reference | Reference | ||
| Socioeconomic status | ||||
| Low | 0.98 (0.970.99) | 0.11 | ||
| Other | Reference | |||
| Residency in a nursing home | ||||
| Nursing home | 0.90 (0.880.92) | <0.001 | 0.90 (0.860.95) | <0.001 |
| All others | Reference | Reference | ||
| Common chronic conditions (reference: without condition) | ||||
| Hypertension | 0.94 (0.930.96) | <0.001 | 1.01 (0.971.04) | 0.69 |
| Ischemic heart disease | 1.00 (0.991.01) | 0.93 | 1.06 (1.031.09) | <0.001 |
| Diabetes | 0.97 (0.950.98) | 0.004 | 0.99 (0.971.02) | 0.64 |
| Arrhythmia | 0.96 (0.950.97) | 0.002 | 1.01 (0.981.04) | 0.39 |
| Chronic renal failure | 0.92 (0.910.93) | <0.001 | 0.96 (0.930.99) | 0.01 |
| Congestive heart failure | 0.93 (0.920.94) | <0.001 | 0.96 (0.930.99) | 0.01 |
| Disability | 0.86 (0.850.87) | <0.001 | 0.90 (0.870.92) | <0.001 |
| Chronic obstructive pulmonary disease | 0.99 (0.981.01) | 0.66 | ||
| Malignancy | 0.97 (0.960.98) | 0.03 | 0.98 (0.961.01) | 0.28 |
| Asthma | 1.04 (1.021.06) | 0.03 | 1.04 (1.001.07) | 0.03 |
| Charlson score | 0.99 (0.980.99) | <0.001 | 0.99 (0.991.00) | 0.04 |
| LOS during index hospitalization | ||||
| Days 24 | 1.52 (1.491.54) | <0.001 | 1.49 (1.451.54) | <0.001 |
| Days 57 | 1.21 (1.191.23) | <0.001 | 1.20 (1.161.24) | <0.001 |
| 8 days and more | Reference | Reference | ||
| Hospital size in index hospitalization | ||||
| Small, <100 beds (8) | 0.94 (0.920.97) | 0.02 | 1.00 (0.951.05) | 0.93 |
| Medium, 100200 beds (9) | 1.00 (0.991.02) | 0.78 | 1.01 (0.991.04) | 0.38 |
| Large, >200 beds (10) | Reference | Reference | ||
| Intensive care unit in readmission | ||||
| Yes | 0.75 (0.700.80) | <0.001 | 0.74 (0.690.79) | <0.001 |
| No | Reference | Reference | ||
DISCUSSION
This study shows that readmission to a different hospital results in longer duration of the readmission stay compared with readmission to the same index hospital. Our results also show that having HIE systems in both the index and readmitting hospitals does not protect against these negative outcomes, as there was no difference in the length of the readmission stay based on the availability of HIE systems. Factors that were found to be associated with longer readmission stays are well known indicators of the complexity of the patient's medical condition, such as the presence of disability, comorbidity, and ICU treatment during the readmission.[24, 27]
The shorter LORS in SHRs may be due to the familiarity of physicians and other healthcare providers with the patient and his or her condition, especially as the policy in SHRs in Israel is to readmit to the same unit from which the patient was recently discharged. This same hospital familiarity is especially important as hospital care in Israel follows the hospitalist model, in which responsibility for patient care is transferred from the patient's primary care physician to the hospital's physician, resulting in increased need for integration through HIE systems, especially when patients are readmitted to a different hospital.[28, 29]
Our findings, congruent with previous research on DHRs and poor outcomes,[7] could also be explained by the inefficiency associated with transitions. For example, patients frequently leave the hospital with pending lab tests, often with abnormal results that would change the course of care.[30] Because these pending tests are often omitted from the hospital discharge summaries,[31] if patients are hospitalized in a different hospital, the same tests may be ordered again, or a course of treatment that does not acknowledge the test results could be taken. Such time‐consuming duplication can be prevented in SHRs, where the index‐hospital records may be already more complete.
Our null findings regarding the contribution of HIE systems may be explained by the low levels of HIE actual use. Although we did not directly assess use, previous research reports that actual use of HIE is limited.[12] An Israeli study on the effects of the use of the OFEK system on ED physicians' admission decisions found that the patient's medical history was viewed in only 31.2% of all 281,750 ED referrals.[19] In another Israeli‐based ED study, even lower usage levels were found, with the OFEK system having been accessed in only 16% of all 3,219,910 ED referrals.[32] Low levels of HIE use have also been reported in the United States. An additional study, which tested the implementation of HIE in hospitals and clinics, showed that in only 2.3% of encounters did providers access the HIE record.[33] Another study conducted in 12 ED sites and 2 ambulatory clinics reported rates of 6.8% HIE use.[34] Moreover, the null effect of integrated health information reported here is congruent with findings from a US study on implementation of an electronic discharge instructions form with embedded computerized medication reconciliation, which was not found to be associated with postdischarge outcomes.[35]
A wide range of factors may influence decisions on HIE use: patient‐level factors,[36] perceived medical complexity of the patient,[33, 34] and the number of prior hospitalizations.[33, 34, 36] Healthcare systemlevel factors may include: time constraints, which may be positively[32] or negatively[33] associated with HIE use, and organizational policies or incentives.[33] Use may also be associated with features of the HIE technology itself, such as difficulty to access, difficulty to use once accessed, and the quality of information it contains.[37] Additionally, there is some evidence of the link between tight functional integration and higher proportions of usage.[38] Although comprehensive studies on factors affecting the use of the OFEK system in Israeli internal medicine units are still needed, the lack of its integration within each hospital's EHR system may serve as a major explanatory factor for the low usage levels.
The findings from this study should be interpreted in light of its limitations. First, compared with previously reported DHR rates (20%30%),[3, 5] the rate observed in our population was relatively low (about 12%). Previous research was restricted to heart failure patients[3] or assessed DHR in surgical, as well as internal medicine, patients.[5] Our lower rates may have been affected by the type of population (hospitalized internal medicine patients) and/or by characteristics of the Clalit healthcare system, which serves as an integrated provider network as well as insurer. Generalization from 1 health care system to others should be made with caution. Nonetheless, our results may underestimate the potential effect in other healthcare systems with less structural integration. Additionally, as noted above, information on the actual use of an HIE in the course of medical decision making during readmission was absent. Future studies should examine the potential benefit of an HIE with measures that capture providers' use of HIEs. Also, the LORS may be influenced by other factors not investigated here, and further future studies should examine additional outcomes such as costs, patient well‐being, and satisfaction. Finally, causality could not be determined, and future research in this realm should aim to search for the pathways connecting readmission to a different hospital, with and without HIEs, to readmission LOS and additional outcomes.
To conclude, our findings show that patients readmitted to a different hospital are at risk for prolonged LORS, regardless of the availability of HIE systems. Implementing HIE systems is the focus of substantial efforts by policymakers and is considered a key part of the meaningful use of electronic health information. HIE features in the provisions of the Health Information Technology for Economic and Clinical Health Act[39] because it can furnish providers with complete, timely information at the point of care. Moreover, although there has been substantial growth in the number of healthcare organizations that have operational an HIE, its ability to lead to improved outcomes has yet to be realized.[8, 10] The Israeli experience reported here suggests that provisions are needed that will ensure actual use of HIEs, which might in turn minimize the difference between DHRs and SHRs.
Acknowledgements
The authors acknowledge Chandra Cohen‐Stavi, MPA, and Orly Tonkikh, MA, for their contribution to this study.
Disclosures
The study was supported in part by a grant from the Israel National Institute for Health Policy Research (NIHP) (10/127). The authors report no conflicts of interest.
- , , , , , . Assessing preventability in the quest to reduce hospital readmissions. J Hosp Med. 2014;9:598–603.
- , , , et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174:1095–1107.
- , , , et al. Is same‐hospital readmission rate a good surrogate for all‐hospital readmission rate? Med Care. 2010;48:477–481.
- , , , . Hospital readmission rates: the impacts of age, payer, and mental health diagnoses. J Ambul Care Manage. 2013;36(2):147–155.
- , , , . Limitations of using same‐hospital readmission metrics. Int J Qual Health Care. 2013;25(6):633–639.
- Hospital inpatient and outpatient services. In: Report to the Congress: promoting greater efficiency in Medicare. Washington, DC: Medicare Payment Advisory Commission., March 2012;45–66.
- , , , , . For‐profit hospital status and rehospitalizations at different hospitals: an analysis of Medicare data. Ann Intern Med. 2010;153:718–727.
- , , , . Health information technology: an updated systematic review with a focus on meaningful use. Ann Intern Med. 2014;160:48–54.
- , , , . The benefits of health information technology: a review of the recent literature shows predominantly positive results. Health Aff (Millwood). 2011;30(3):464–471.
- , , . Health information exchange among US hospitals. Am J Manag Care. 2011;17:761–768.
- , , . A survey of health information exchange organizations in the United States: implications for meaningful use. Ann Intern Med. 2011;54:666–671.
- , , , , . Physicians' potential use and preferences related to health information exchange. Int J Med Inform. 2011;80:171–180.
- , , , et al. Provider stakeholders' perceived benefit from a nascent health information exchange: a qualitative analysis. J Med Syst. 2012;36:601–613.
- . More than just a question of technology: factors related to hospitals' adoption and implementation of health information exchange. Int J Med Inform. 2010;79:797–806.
- , , . Leveraging health information technology to achieve the “triple aim” of healthcare reform. J Am Med Inform Assoc. 2015;22(4):849–856.
- , , , , , . Health information exchange reduces repeated diagnostic imaging for back pain. Ann Emerg Med. 2013;62:16–24.
- , , , , . Association between use of a health information exchange system and hospital admissions. Appl Clin Inform. 2014;5:219.
- , , . The impact of EHR and HIE on reducing avoidable admissions: controlling main differential diagnoses. BMC Med Inform Decis Mak. 2013;13:49.
- , , , et al. The impact of an integrated hospital‐community medical information system on quality and service utilization in hospital departments. Int J Med Inform. 2010;79(9):649–657.
- , , , , , Predicting 30‐day readmissions with preadmission electronic health record data. Med Care. 2015;53:283–289.
- , , , , . Assessing socioeconomic health care utilization inequity in Israel: impact of alternative approaches to morbidity adjustment. BMC Public Health. 2011;11(1):609.
- , . Prevalence of selected chronic diseases in Israel. Isr Med Assoc J. 2001;3:404–408.
- , , , . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383.
- , , , , . Systematic review of risk adjustment models of hospital length of stay (LOS). Med Care. 2015;53:355–365.
- , . Use of ranks in one‐criterion variance analysis. J Am Stat Assoc. 1952;47:583–621.
- , , . Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc. 1989;84:1065–1073.
- , , , , , . Disability impacts length of stay in general internal medicine patients. J Gen Intern Med. 2014;29:885–890.
- . The hospitalist movement—time to move on. N Engl J Med. 2007;357:2627–2629.
- , , . Association of hospitalist presence and hospital‐level outcome measures among Medicare patients. J Hosp Med. 2014;9:1–6.
- , , , et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med. 2005;143:121–128.
- , , , et al. Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers. J Gen Intern Med. 2009;24:1002–1006.
- , , . Using electronic medical record systems for admission decisions in emergency departments: examining the crowdedness effect. J Med Syst. 2012;36:3795–3803.
- , , , , , . Factors motivating and affecting health information exchange usage. J Am Med Inform Assoc. 2011;18(2):143–149.
- , , , et al. Health information exchange usage in emergency departments and clinics: the who, what, and why. J Am Med Inform Assoc. 2011;18:690–697.
- , , , , . Effect of standardized electronic discharge instructions on post‐discharge hospital utilization. J Gen Intern Med. 2011;26:718–723.
- , , . Health information exchange technology on the front lines of healthcare: workflow factors and patterns of use. J Am Med Inform Assoc. 2012;19:392–400.
- , . The DeLone and McLean model of information systems success: a ten‐year update. J Manag Inf Syst. 2003;19:9–30.
- , , , et al. Architectural strategies and issues with health information exchange. AMIA Annu Symp Proc. 2006:814–818.
- . Launching HITECH. N Engl J Med. 2010;362(5):382–385.
- , , , , , . Assessing preventability in the quest to reduce hospital readmissions. J Hosp Med. 2014;9:598–603.
- , , , et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174:1095–1107.
- , , , et al. Is same‐hospital readmission rate a good surrogate for all‐hospital readmission rate? Med Care. 2010;48:477–481.
- , , , . Hospital readmission rates: the impacts of age, payer, and mental health diagnoses. J Ambul Care Manage. 2013;36(2):147–155.
- , , , . Limitations of using same‐hospital readmission metrics. Int J Qual Health Care. 2013;25(6):633–639.
- Hospital inpatient and outpatient services. In: Report to the Congress: promoting greater efficiency in Medicare. Washington, DC: Medicare Payment Advisory Commission., March 2012;45–66.
- , , , , . For‐profit hospital status and rehospitalizations at different hospitals: an analysis of Medicare data. Ann Intern Med. 2010;153:718–727.
- , , , . Health information technology: an updated systematic review with a focus on meaningful use. Ann Intern Med. 2014;160:48–54.
- , , , . The benefits of health information technology: a review of the recent literature shows predominantly positive results. Health Aff (Millwood). 2011;30(3):464–471.
- , , . Health information exchange among US hospitals. Am J Manag Care. 2011;17:761–768.
- , , . A survey of health information exchange organizations in the United States: implications for meaningful use. Ann Intern Med. 2011;54:666–671.
- , , , , . Physicians' potential use and preferences related to health information exchange. Int J Med Inform. 2011;80:171–180.
- , , , et al. Provider stakeholders' perceived benefit from a nascent health information exchange: a qualitative analysis. J Med Syst. 2012;36:601–613.
- . More than just a question of technology: factors related to hospitals' adoption and implementation of health information exchange. Int J Med Inform. 2010;79:797–806.
- , , . Leveraging health information technology to achieve the “triple aim” of healthcare reform. J Am Med Inform Assoc. 2015;22(4):849–856.
- , , , , , . Health information exchange reduces repeated diagnostic imaging for back pain. Ann Emerg Med. 2013;62:16–24.
- , , , , . Association between use of a health information exchange system and hospital admissions. Appl Clin Inform. 2014;5:219.
- , , . The impact of EHR and HIE on reducing avoidable admissions: controlling main differential diagnoses. BMC Med Inform Decis Mak. 2013;13:49.
- , , , et al. The impact of an integrated hospital‐community medical information system on quality and service utilization in hospital departments. Int J Med Inform. 2010;79(9):649–657.
- , , , , , Predicting 30‐day readmissions with preadmission electronic health record data. Med Care. 2015;53:283–289.
- , , , , . Assessing socioeconomic health care utilization inequity in Israel: impact of alternative approaches to morbidity adjustment. BMC Public Health. 2011;11(1):609.
- , . Prevalence of selected chronic diseases in Israel. Isr Med Assoc J. 2001;3:404–408.
- , , , . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383.
- , , , , . Systematic review of risk adjustment models of hospital length of stay (LOS). Med Care. 2015;53:355–365.
- , . Use of ranks in one‐criterion variance analysis. J Am Stat Assoc. 1952;47:583–621.
- , , . Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc. 1989;84:1065–1073.
- , , , , , . Disability impacts length of stay in general internal medicine patients. J Gen Intern Med. 2014;29:885–890.
- . The hospitalist movement—time to move on. N Engl J Med. 2007;357:2627–2629.
- , , . Association of hospitalist presence and hospital‐level outcome measures among Medicare patients. J Hosp Med. 2014;9:1–6.
- , , , et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med. 2005;143:121–128.
- , , , et al. Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers. J Gen Intern Med. 2009;24:1002–1006.
- , , . Using electronic medical record systems for admission decisions in emergency departments: examining the crowdedness effect. J Med Syst. 2012;36:3795–3803.
- , , , , , . Factors motivating and affecting health information exchange usage. J Am Med Inform Assoc. 2011;18(2):143–149.
- , , , et al. Health information exchange usage in emergency departments and clinics: the who, what, and why. J Am Med Inform Assoc. 2011;18:690–697.
- , , , , . Effect of standardized electronic discharge instructions on post‐discharge hospital utilization. J Gen Intern Med. 2011;26:718–723.
- , , . Health information exchange technology on the front lines of healthcare: workflow factors and patterns of use. J Am Med Inform Assoc. 2012;19:392–400.
- , . The DeLone and McLean model of information systems success: a ten‐year update. J Manag Inf Syst. 2003;19:9–30.
- , , , et al. Architectural strategies and issues with health information exchange. AMIA Annu Symp Proc. 2006:814–818.
- . Launching HITECH. N Engl J Med. 2010;362(5):382–385.
© 2015 Society of Hospital Medicine
Aneuploidy screening: Newer noninvasive test gains traction
Discuss cell-free DNA testing when offering fetal aneuploidy screening to pregnant women.1,2
Strength of recommendation
A: Based on multiple large, multi-center cohort studies.
Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799-808.1
Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372:1589-1597.2
Illustrative case
A 28-year-old gravida 2, para 1001 at 10 weeks gestation presents to your clinic for a routine first-trimester prenatal visit. Her first child has no known chromosomal abnormalities and she has no family history of aneuploidy. She asks you which tests are available to screen her fetus for chromosomal abnormalities.
Pregnant women have traditionally been offered some combination of serum biomarkers and nuchal translucency to assess the risk of fetal aneuploidy. Cell-free DNA testing (cfDNA) is a form of noninvasive prenatal testing that uses maternal serum samples to conduct massively parallel sequencing of cell-free fetal DNA fragments. It has been offered to pregnant women as a screening test to detect fetal chromosomal abnormalities since 2011 after multiple clinical studies found high sensitivities, specificities, and negative predictive values (NPVs) for detecting aneuploidy.3-6 However until 2015, practice guidelines from the American Congress of Obstetricians and Gynecologists (ACOG) recommended that standard aneuploidy screening or diagnostic testing be offered to all pregnant women and cfDNA be reserved for women with pregnancies at high risk for aneuploidy (strength of recommendation: B).7
CARE (Comparison of Aneuploidy Risk Evaluation) and NEXT (Noninvasive Examination of Trisomy) are 2 large studies that compared cfDNA and standard aneuploidy screening methods in pregnant women at low risk for fetal aneuploidy. Based on new data from these and other studies, ACOG and the Society for Maternal-Fetal Medicine (SMFM) released a new consensus statement in June 2015 that addressed the use of cfDNA in the general obstetric population. The 2 groups still recommend conventional first- and second-trimester screening by serum chemical biomarkers and nuchal translucency as the first-line approach for low-risk women who want to pursue aneuploidy screening; however, they also recommend that the risks and benefits of cfDNA should be discussed with all patients.8
STUDY SUMMARIES
CARE was a prospective, blinded, multicenter (21 US sites across 14 states) study that compared the aneuploidy detection rates of cfDNA to those of standard screening. Standard aneuploidy screening included assays of first- or second-trimester serum biomarkers with or without fetal nuchal translucency measurement.
This study enrolled 2042 pregnant patients ages 18 to 49 (mean: 29.6 years) with singleton pregnancies. The population was racially and ethnically diverse (65% white, 22% black, 11% Hispanic, 7% Asian). This study included women with diabetes mellitus, thyroid disorders, and other comorbidities. cfDNA testing was done on 1909 maternal blood samples for trisomy 21 and 1905 for trisomy 18.
cfDNA and standard aneuploidy screening results were compared to pregnancy outcomes. The presence of aneuploidy was determined by physician-documented newborn physical exam (97%) or karyotype analysis (3%). In both live and non-live births, the incidence of trisomy 21 was 5 of 1909 cases (0.3%) and the incidence of trisomy 18 was 2 of 1905 cases (0.1%).
The NPV of cfDNA in this study was 100% (95% confidence interval, 99.8%-100%) for both trisomy 21 and trisomy 18. The positive predictive value (PPV) was higher with cfDNA compared to standard screening (45.5% vs 4.2% for trisomy 21 and 40% vs 8.3% for trisomy 18). This means that approximately 1 in 25 women with a positive standard aneuploidy screen actually has aneuploidy. In contrast, nearly one in 2 women with a positive cfDNA result has aneuploidy.
Similarly, false positive rates with cfDNA were significantly lower than those with standard screening. For trisomy 21, the cfDNA false positive rate was 0.3% compared to 3.6% for standard screening (P<.001); for trisomy 18, the cfDNA false positive rate was 0.2% compared to 0.6% for standard screening (P=.03).
NEXT was a prospective, blinded cohort study that compared cfDNA testing with standard first-trimester screening (with measurements of nuchal translucency and serum biochemical analysis) in a routine prenatal population at 35 centers in 6 countries.
This study enrolled 18,955 women ages 18 to 48 (mean: 31 years) who underwent traditional first-trimester screening and cfDNA testing. Eligible patients included pregnant women with a singleton pregnancy with a gestational age between 10 and 14.3 weeks. Prenatal screening results were compared to newborn outcomes using a documented newborn physical examination and, if performed, results of genetic testing. For women who had a miscarriage or stillbirth or chose to terminate the pregnancy, outcomes were determined by diagnostic genetic testing.
The primary outcome was the area under the receiver-operating-characteristic (ROC) curve for trisomy 21. Area under the ROC curve is a measure of a diagnostic test’s accuracy that plots sensitivity against 1-specificity; <.700 is considered a poor test, whereas 1.00 is a perfect test. A secondary analysis evaluated cfDNA testing in low-risk women (ages <35 years).
The area under the ROC curve was 0.999 for cfDNA compared with 0.958 for standard screening (P=.001). For diagnosis of trisomy 21, cfDNA had a higher PPV than standard testing (80.9% vs 3.4%; P<.001) and a lower false positive rate (0.06% vs 5.4%; P<.001). These findings were consistent in the secondary analysis of low-risk women.
Both the CARE and NEXT trials also evaluated cfDNA testing vs standard screening for diagnosis of trisomy 13 and 18 and found higher PPVs and lower false positive rates for cfDNA compared with traditional screening.
WHAT'S NEW
Previously, cfDNA was recommended only for women with high-risk pregnancies. The new data demonstrate that cfDNA has substantially better PPVs and lower false positive rates than standard fetal aneuploidy screening for the general obstetrical population.
So while conventional screening tests remain the most appropriate methods for aneuploidy detection in the general obstetrical population, according to ACOG and SMFM, the 2 groups now recommend that all screening options—including cfDNA—be discussed with every woman. Any woman may choose cfDNA but should be counseled about the risks and benefits.8
CAVEATS
Both the CARE and NEXT studies had limitations. They compared cfDNA testing with first- or second-trimester screening and did not evaluate integrated screening methods (sequential first- and second-trimester biomarkers plus first-trimester nuchal translucency), which have a slightly higher sensitivity and specificity than first-trimester screening alone.
Multiple companies offer cfDNA, and the test is not subject to Food and Drug Administration approval. The CARE and NEXT studies used tests from companies that provided funding for these studies and employ several of the study authors.
Although cfDNA has increased specificity compared to standard screening, there have been case reports of false negative results. Further testing has shown that such false negative results could be caused by mosaicism in either the fetus and/or placenta, vanishing twins, or maternal malignancies.8-10
In the CARE and NEXT trials, cfDNA produced no results in 0.9% and 3% of women, respectively. Patients for whom cfDNA testing yields no results have higher rates of aneuploidy, and therefore require further diagnostic testing.
Because the prevalence of aneuploidy is lower in the general obstetric population than it is among women whose pregnancies are at high risk for aneuploidy, the PPV of cfDNA testing is also lower in the general obstetric population. This means that there are more false positive results for women at lower risk for aneuploidy. Therefore, it is imperative that women with positive cfDNA tests receive follow-up diagnostic testing such as chorionic villus sampling or amniocentesis before making a decision about termination.
All commercially available cfDNA tests have high sensitivity and specificity for trisomy 21, 18, and 13. Some offer testing for sex chromosome abnormalities and microdeletions. However, current cfDNA testing methods are unable to detect up to 17% of other clinically significant chromosomal abnormalities,11 and cfDNA cannot detect neural tube or ventral wall defects. Therefore, ACOG and SMFM recommend that women who choose cfDNA as their aneuploidy screening method should also be offered maternal serum alpha-fetoprotein or ultrasound evaluation.
CHALLENGES TO IMPLEMENTATION
cfDNA testing is validated only for singleton pregnancies. Physicians should obtain a baseline fetal ultrasound to confirm the number of fetuses, gestational age, and viability before ordering cfDNA to ensure it is the most appropriate screening test. This may add to the overall number of early pregnancy ultrasounds conducted.
Counseling patients about aneuploidy screening options is time-consuming, and requires discussion of the limitations of each screening method and caution that a negative cfDNA result does not guarantee an unaffected fetus, nor does a positive result guarantee an affected fetus. However, aneuploidy screening is well within the scope of care for family physicians who provide prenatal care, and referral to genetic specialists is not necessary or recommended.
Some patients may request cfDNA in order to facilitate earlier identification of fetal sex. In such cases, physicians should advise patients that cfDNA testing also assesses trisomy risk. Patients who do not wish to assess their risk for aneuploidy should not receive cfDNA testing.
Finally, while cfDNA is routinely recommended for women with pregnancies considered at high risk for aneuploidy, many insurance companies do not cover the cost of cfDNA for women with low-risk pregnancies, and the test may cost up to $1,700.12 The overall cost-effectiveness of cfDNA for aneuploidy screening in low-risk women is unknown.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799-808.
2. Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372:1589-1597.
3. Chiu RW, Akolekar R, Zheng YW, et al. Non-invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: large scale validity study. BMJ. 2011;342:c7401.
4. Ehrich M, Deciu C, Zwiefelhofer T, et al. Noninvasive detection of fetal trisomy 21 by sequencing of DNA in maternal blood: a study in a clinical setting. Am J Obstet Gynecol. 2011;204:205.e1-11.
5. Bianchi DW, Platt LD, Goldberg JD, et al; MatERNal BLood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119:890-901.
6. Norton ME, Brar H, Weiss J, et al. Non-invasive chromosomal evaluation (NICE) study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;207:137.e1-8.
7. American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 545: Noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol. 2012;120:1532-1534.
8. Committee Opinion No. 640: Cell-Free DNA Screening For Fetal Aneuploidy. Obstet Gynecol. 2015;126:e31-37.
9. Wang Y, Zhu J, Chen Y, et al. Two cases of placental T21 mosaicism: challenging the detection limits of non-invasive prenatal testing. Prenat Diagn. 2013;33:1207-1210.
10. Choi H, Lau TK, Jiang FM, et al. Fetal aneuploidy screening by maternal plasma DNA sequencing: ‘false positive’ due to confined placental mosaicism. Prenat Diagn. 2013;33:198-200.
11. Norton ME, Jelliffe-Pawlowski LL, Currier RJ. Chromosome abnormalities detected by current prenatal screening and noninvasive prenatal testing. Obstet Gynecol. 2014;124:979-986.
12. Agarwal A, Sayres LC, Cho MK, et al. Commercial landscape of noninvasive prenatal testing in the United States. Prenat Diagn. 2013;33:521-531.
Discuss cell-free DNA testing when offering fetal aneuploidy screening to pregnant women.1,2
Strength of recommendation
A: Based on multiple large, multi-center cohort studies.
Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799-808.1
Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372:1589-1597.2
Illustrative case
A 28-year-old gravida 2, para 1001 at 10 weeks gestation presents to your clinic for a routine first-trimester prenatal visit. Her first child has no known chromosomal abnormalities and she has no family history of aneuploidy. She asks you which tests are available to screen her fetus for chromosomal abnormalities.
Pregnant women have traditionally been offered some combination of serum biomarkers and nuchal translucency to assess the risk of fetal aneuploidy. Cell-free DNA testing (cfDNA) is a form of noninvasive prenatal testing that uses maternal serum samples to conduct massively parallel sequencing of cell-free fetal DNA fragments. It has been offered to pregnant women as a screening test to detect fetal chromosomal abnormalities since 2011 after multiple clinical studies found high sensitivities, specificities, and negative predictive values (NPVs) for detecting aneuploidy.3-6 However until 2015, practice guidelines from the American Congress of Obstetricians and Gynecologists (ACOG) recommended that standard aneuploidy screening or diagnostic testing be offered to all pregnant women and cfDNA be reserved for women with pregnancies at high risk for aneuploidy (strength of recommendation: B).7
CARE (Comparison of Aneuploidy Risk Evaluation) and NEXT (Noninvasive Examination of Trisomy) are 2 large studies that compared cfDNA and standard aneuploidy screening methods in pregnant women at low risk for fetal aneuploidy. Based on new data from these and other studies, ACOG and the Society for Maternal-Fetal Medicine (SMFM) released a new consensus statement in June 2015 that addressed the use of cfDNA in the general obstetric population. The 2 groups still recommend conventional first- and second-trimester screening by serum chemical biomarkers and nuchal translucency as the first-line approach for low-risk women who want to pursue aneuploidy screening; however, they also recommend that the risks and benefits of cfDNA should be discussed with all patients.8
STUDY SUMMARIES
CARE was a prospective, blinded, multicenter (21 US sites across 14 states) study that compared the aneuploidy detection rates of cfDNA to those of standard screening. Standard aneuploidy screening included assays of first- or second-trimester serum biomarkers with or without fetal nuchal translucency measurement.
This study enrolled 2042 pregnant patients ages 18 to 49 (mean: 29.6 years) with singleton pregnancies. The population was racially and ethnically diverse (65% white, 22% black, 11% Hispanic, 7% Asian). This study included women with diabetes mellitus, thyroid disorders, and other comorbidities. cfDNA testing was done on 1909 maternal blood samples for trisomy 21 and 1905 for trisomy 18.
cfDNA and standard aneuploidy screening results were compared to pregnancy outcomes. The presence of aneuploidy was determined by physician-documented newborn physical exam (97%) or karyotype analysis (3%). In both live and non-live births, the incidence of trisomy 21 was 5 of 1909 cases (0.3%) and the incidence of trisomy 18 was 2 of 1905 cases (0.1%).
The NPV of cfDNA in this study was 100% (95% confidence interval, 99.8%-100%) for both trisomy 21 and trisomy 18. The positive predictive value (PPV) was higher with cfDNA compared to standard screening (45.5% vs 4.2% for trisomy 21 and 40% vs 8.3% for trisomy 18). This means that approximately 1 in 25 women with a positive standard aneuploidy screen actually has aneuploidy. In contrast, nearly one in 2 women with a positive cfDNA result has aneuploidy.
Similarly, false positive rates with cfDNA were significantly lower than those with standard screening. For trisomy 21, the cfDNA false positive rate was 0.3% compared to 3.6% for standard screening (P<.001); for trisomy 18, the cfDNA false positive rate was 0.2% compared to 0.6% for standard screening (P=.03).
NEXT was a prospective, blinded cohort study that compared cfDNA testing with standard first-trimester screening (with measurements of nuchal translucency and serum biochemical analysis) in a routine prenatal population at 35 centers in 6 countries.
This study enrolled 18,955 women ages 18 to 48 (mean: 31 years) who underwent traditional first-trimester screening and cfDNA testing. Eligible patients included pregnant women with a singleton pregnancy with a gestational age between 10 and 14.3 weeks. Prenatal screening results were compared to newborn outcomes using a documented newborn physical examination and, if performed, results of genetic testing. For women who had a miscarriage or stillbirth or chose to terminate the pregnancy, outcomes were determined by diagnostic genetic testing.
The primary outcome was the area under the receiver-operating-characteristic (ROC) curve for trisomy 21. Area under the ROC curve is a measure of a diagnostic test’s accuracy that plots sensitivity against 1-specificity; <.700 is considered a poor test, whereas 1.00 is a perfect test. A secondary analysis evaluated cfDNA testing in low-risk women (ages <35 years).
The area under the ROC curve was 0.999 for cfDNA compared with 0.958 for standard screening (P=.001). For diagnosis of trisomy 21, cfDNA had a higher PPV than standard testing (80.9% vs 3.4%; P<.001) and a lower false positive rate (0.06% vs 5.4%; P<.001). These findings were consistent in the secondary analysis of low-risk women.
Both the CARE and NEXT trials also evaluated cfDNA testing vs standard screening for diagnosis of trisomy 13 and 18 and found higher PPVs and lower false positive rates for cfDNA compared with traditional screening.
WHAT'S NEW
Previously, cfDNA was recommended only for women with high-risk pregnancies. The new data demonstrate that cfDNA has substantially better PPVs and lower false positive rates than standard fetal aneuploidy screening for the general obstetrical population.
So while conventional screening tests remain the most appropriate methods for aneuploidy detection in the general obstetrical population, according to ACOG and SMFM, the 2 groups now recommend that all screening options—including cfDNA—be discussed with every woman. Any woman may choose cfDNA but should be counseled about the risks and benefits.8
CAVEATS
Both the CARE and NEXT studies had limitations. They compared cfDNA testing with first- or second-trimester screening and did not evaluate integrated screening methods (sequential first- and second-trimester biomarkers plus first-trimester nuchal translucency), which have a slightly higher sensitivity and specificity than first-trimester screening alone.
Multiple companies offer cfDNA, and the test is not subject to Food and Drug Administration approval. The CARE and NEXT studies used tests from companies that provided funding for these studies and employ several of the study authors.
Although cfDNA has increased specificity compared to standard screening, there have been case reports of false negative results. Further testing has shown that such false negative results could be caused by mosaicism in either the fetus and/or placenta, vanishing twins, or maternal malignancies.8-10
In the CARE and NEXT trials, cfDNA produced no results in 0.9% and 3% of women, respectively. Patients for whom cfDNA testing yields no results have higher rates of aneuploidy, and therefore require further diagnostic testing.
Because the prevalence of aneuploidy is lower in the general obstetric population than it is among women whose pregnancies are at high risk for aneuploidy, the PPV of cfDNA testing is also lower in the general obstetric population. This means that there are more false positive results for women at lower risk for aneuploidy. Therefore, it is imperative that women with positive cfDNA tests receive follow-up diagnostic testing such as chorionic villus sampling or amniocentesis before making a decision about termination.
All commercially available cfDNA tests have high sensitivity and specificity for trisomy 21, 18, and 13. Some offer testing for sex chromosome abnormalities and microdeletions. However, current cfDNA testing methods are unable to detect up to 17% of other clinically significant chromosomal abnormalities,11 and cfDNA cannot detect neural tube or ventral wall defects. Therefore, ACOG and SMFM recommend that women who choose cfDNA as their aneuploidy screening method should also be offered maternal serum alpha-fetoprotein or ultrasound evaluation.
CHALLENGES TO IMPLEMENTATION
cfDNA testing is validated only for singleton pregnancies. Physicians should obtain a baseline fetal ultrasound to confirm the number of fetuses, gestational age, and viability before ordering cfDNA to ensure it is the most appropriate screening test. This may add to the overall number of early pregnancy ultrasounds conducted.
Counseling patients about aneuploidy screening options is time-consuming, and requires discussion of the limitations of each screening method and caution that a negative cfDNA result does not guarantee an unaffected fetus, nor does a positive result guarantee an affected fetus. However, aneuploidy screening is well within the scope of care for family physicians who provide prenatal care, and referral to genetic specialists is not necessary or recommended.
Some patients may request cfDNA in order to facilitate earlier identification of fetal sex. In such cases, physicians should advise patients that cfDNA testing also assesses trisomy risk. Patients who do not wish to assess their risk for aneuploidy should not receive cfDNA testing.
Finally, while cfDNA is routinely recommended for women with pregnancies considered at high risk for aneuploidy, many insurance companies do not cover the cost of cfDNA for women with low-risk pregnancies, and the test may cost up to $1,700.12 The overall cost-effectiveness of cfDNA for aneuploidy screening in low-risk women is unknown.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Discuss cell-free DNA testing when offering fetal aneuploidy screening to pregnant women.1,2
Strength of recommendation
A: Based on multiple large, multi-center cohort studies.
Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799-808.1
Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372:1589-1597.2
Illustrative case
A 28-year-old gravida 2, para 1001 at 10 weeks gestation presents to your clinic for a routine first-trimester prenatal visit. Her first child has no known chromosomal abnormalities and she has no family history of aneuploidy. She asks you which tests are available to screen her fetus for chromosomal abnormalities.
Pregnant women have traditionally been offered some combination of serum biomarkers and nuchal translucency to assess the risk of fetal aneuploidy. Cell-free DNA testing (cfDNA) is a form of noninvasive prenatal testing that uses maternal serum samples to conduct massively parallel sequencing of cell-free fetal DNA fragments. It has been offered to pregnant women as a screening test to detect fetal chromosomal abnormalities since 2011 after multiple clinical studies found high sensitivities, specificities, and negative predictive values (NPVs) for detecting aneuploidy.3-6 However until 2015, practice guidelines from the American Congress of Obstetricians and Gynecologists (ACOG) recommended that standard aneuploidy screening or diagnostic testing be offered to all pregnant women and cfDNA be reserved for women with pregnancies at high risk for aneuploidy (strength of recommendation: B).7
CARE (Comparison of Aneuploidy Risk Evaluation) and NEXT (Noninvasive Examination of Trisomy) are 2 large studies that compared cfDNA and standard aneuploidy screening methods in pregnant women at low risk for fetal aneuploidy. Based on new data from these and other studies, ACOG and the Society for Maternal-Fetal Medicine (SMFM) released a new consensus statement in June 2015 that addressed the use of cfDNA in the general obstetric population. The 2 groups still recommend conventional first- and second-trimester screening by serum chemical biomarkers and nuchal translucency as the first-line approach for low-risk women who want to pursue aneuploidy screening; however, they also recommend that the risks and benefits of cfDNA should be discussed with all patients.8
STUDY SUMMARIES
CARE was a prospective, blinded, multicenter (21 US sites across 14 states) study that compared the aneuploidy detection rates of cfDNA to those of standard screening. Standard aneuploidy screening included assays of first- or second-trimester serum biomarkers with or without fetal nuchal translucency measurement.
This study enrolled 2042 pregnant patients ages 18 to 49 (mean: 29.6 years) with singleton pregnancies. The population was racially and ethnically diverse (65% white, 22% black, 11% Hispanic, 7% Asian). This study included women with diabetes mellitus, thyroid disorders, and other comorbidities. cfDNA testing was done on 1909 maternal blood samples for trisomy 21 and 1905 for trisomy 18.
cfDNA and standard aneuploidy screening results were compared to pregnancy outcomes. The presence of aneuploidy was determined by physician-documented newborn physical exam (97%) or karyotype analysis (3%). In both live and non-live births, the incidence of trisomy 21 was 5 of 1909 cases (0.3%) and the incidence of trisomy 18 was 2 of 1905 cases (0.1%).
The NPV of cfDNA in this study was 100% (95% confidence interval, 99.8%-100%) for both trisomy 21 and trisomy 18. The positive predictive value (PPV) was higher with cfDNA compared to standard screening (45.5% vs 4.2% for trisomy 21 and 40% vs 8.3% for trisomy 18). This means that approximately 1 in 25 women with a positive standard aneuploidy screen actually has aneuploidy. In contrast, nearly one in 2 women with a positive cfDNA result has aneuploidy.
Similarly, false positive rates with cfDNA were significantly lower than those with standard screening. For trisomy 21, the cfDNA false positive rate was 0.3% compared to 3.6% for standard screening (P<.001); for trisomy 18, the cfDNA false positive rate was 0.2% compared to 0.6% for standard screening (P=.03).
NEXT was a prospective, blinded cohort study that compared cfDNA testing with standard first-trimester screening (with measurements of nuchal translucency and serum biochemical analysis) in a routine prenatal population at 35 centers in 6 countries.
This study enrolled 18,955 women ages 18 to 48 (mean: 31 years) who underwent traditional first-trimester screening and cfDNA testing. Eligible patients included pregnant women with a singleton pregnancy with a gestational age between 10 and 14.3 weeks. Prenatal screening results were compared to newborn outcomes using a documented newborn physical examination and, if performed, results of genetic testing. For women who had a miscarriage or stillbirth or chose to terminate the pregnancy, outcomes were determined by diagnostic genetic testing.
The primary outcome was the area under the receiver-operating-characteristic (ROC) curve for trisomy 21. Area under the ROC curve is a measure of a diagnostic test’s accuracy that plots sensitivity against 1-specificity; <.700 is considered a poor test, whereas 1.00 is a perfect test. A secondary analysis evaluated cfDNA testing in low-risk women (ages <35 years).
The area under the ROC curve was 0.999 for cfDNA compared with 0.958 for standard screening (P=.001). For diagnosis of trisomy 21, cfDNA had a higher PPV than standard testing (80.9% vs 3.4%; P<.001) and a lower false positive rate (0.06% vs 5.4%; P<.001). These findings were consistent in the secondary analysis of low-risk women.
Both the CARE and NEXT trials also evaluated cfDNA testing vs standard screening for diagnosis of trisomy 13 and 18 and found higher PPVs and lower false positive rates for cfDNA compared with traditional screening.
WHAT'S NEW
Previously, cfDNA was recommended only for women with high-risk pregnancies. The new data demonstrate that cfDNA has substantially better PPVs and lower false positive rates than standard fetal aneuploidy screening for the general obstetrical population.
So while conventional screening tests remain the most appropriate methods for aneuploidy detection in the general obstetrical population, according to ACOG and SMFM, the 2 groups now recommend that all screening options—including cfDNA—be discussed with every woman. Any woman may choose cfDNA but should be counseled about the risks and benefits.8
CAVEATS
Both the CARE and NEXT studies had limitations. They compared cfDNA testing with first- or second-trimester screening and did not evaluate integrated screening methods (sequential first- and second-trimester biomarkers plus first-trimester nuchal translucency), which have a slightly higher sensitivity and specificity than first-trimester screening alone.
Multiple companies offer cfDNA, and the test is not subject to Food and Drug Administration approval. The CARE and NEXT studies used tests from companies that provided funding for these studies and employ several of the study authors.
Although cfDNA has increased specificity compared to standard screening, there have been case reports of false negative results. Further testing has shown that such false negative results could be caused by mosaicism in either the fetus and/or placenta, vanishing twins, or maternal malignancies.8-10
In the CARE and NEXT trials, cfDNA produced no results in 0.9% and 3% of women, respectively. Patients for whom cfDNA testing yields no results have higher rates of aneuploidy, and therefore require further diagnostic testing.
Because the prevalence of aneuploidy is lower in the general obstetric population than it is among women whose pregnancies are at high risk for aneuploidy, the PPV of cfDNA testing is also lower in the general obstetric population. This means that there are more false positive results for women at lower risk for aneuploidy. Therefore, it is imperative that women with positive cfDNA tests receive follow-up diagnostic testing such as chorionic villus sampling or amniocentesis before making a decision about termination.
All commercially available cfDNA tests have high sensitivity and specificity for trisomy 21, 18, and 13. Some offer testing for sex chromosome abnormalities and microdeletions. However, current cfDNA testing methods are unable to detect up to 17% of other clinically significant chromosomal abnormalities,11 and cfDNA cannot detect neural tube or ventral wall defects. Therefore, ACOG and SMFM recommend that women who choose cfDNA as their aneuploidy screening method should also be offered maternal serum alpha-fetoprotein or ultrasound evaluation.
CHALLENGES TO IMPLEMENTATION
cfDNA testing is validated only for singleton pregnancies. Physicians should obtain a baseline fetal ultrasound to confirm the number of fetuses, gestational age, and viability before ordering cfDNA to ensure it is the most appropriate screening test. This may add to the overall number of early pregnancy ultrasounds conducted.
Counseling patients about aneuploidy screening options is time-consuming, and requires discussion of the limitations of each screening method and caution that a negative cfDNA result does not guarantee an unaffected fetus, nor does a positive result guarantee an affected fetus. However, aneuploidy screening is well within the scope of care for family physicians who provide prenatal care, and referral to genetic specialists is not necessary or recommended.
Some patients may request cfDNA in order to facilitate earlier identification of fetal sex. In such cases, physicians should advise patients that cfDNA testing also assesses trisomy risk. Patients who do not wish to assess their risk for aneuploidy should not receive cfDNA testing.
Finally, while cfDNA is routinely recommended for women with pregnancies considered at high risk for aneuploidy, many insurance companies do not cover the cost of cfDNA for women with low-risk pregnancies, and the test may cost up to $1,700.12 The overall cost-effectiveness of cfDNA for aneuploidy screening in low-risk women is unknown.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799-808.
2. Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372:1589-1597.
3. Chiu RW, Akolekar R, Zheng YW, et al. Non-invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: large scale validity study. BMJ. 2011;342:c7401.
4. Ehrich M, Deciu C, Zwiefelhofer T, et al. Noninvasive detection of fetal trisomy 21 by sequencing of DNA in maternal blood: a study in a clinical setting. Am J Obstet Gynecol. 2011;204:205.e1-11.
5. Bianchi DW, Platt LD, Goldberg JD, et al; MatERNal BLood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119:890-901.
6. Norton ME, Brar H, Weiss J, et al. Non-invasive chromosomal evaluation (NICE) study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;207:137.e1-8.
7. American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 545: Noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol. 2012;120:1532-1534.
8. Committee Opinion No. 640: Cell-Free DNA Screening For Fetal Aneuploidy. Obstet Gynecol. 2015;126:e31-37.
9. Wang Y, Zhu J, Chen Y, et al. Two cases of placental T21 mosaicism: challenging the detection limits of non-invasive prenatal testing. Prenat Diagn. 2013;33:1207-1210.
10. Choi H, Lau TK, Jiang FM, et al. Fetal aneuploidy screening by maternal plasma DNA sequencing: ‘false positive’ due to confined placental mosaicism. Prenat Diagn. 2013;33:198-200.
11. Norton ME, Jelliffe-Pawlowski LL, Currier RJ. Chromosome abnormalities detected by current prenatal screening and noninvasive prenatal testing. Obstet Gynecol. 2014;124:979-986.
12. Agarwal A, Sayres LC, Cho MK, et al. Commercial landscape of noninvasive prenatal testing in the United States. Prenat Diagn. 2013;33:521-531.
1. Bianchi DW, Parker RL, Wentworth J, et al; CARE Study Group. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799-808.
2. Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372:1589-1597.
3. Chiu RW, Akolekar R, Zheng YW, et al. Non-invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: large scale validity study. BMJ. 2011;342:c7401.
4. Ehrich M, Deciu C, Zwiefelhofer T, et al. Noninvasive detection of fetal trisomy 21 by sequencing of DNA in maternal blood: a study in a clinical setting. Am J Obstet Gynecol. 2011;204:205.e1-11.
5. Bianchi DW, Platt LD, Goldberg JD, et al; MatERNal BLood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119:890-901.
6. Norton ME, Brar H, Weiss J, et al. Non-invasive chromosomal evaluation (NICE) study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;207:137.e1-8.
7. American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 545: Noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol. 2012;120:1532-1534.
8. Committee Opinion No. 640: Cell-Free DNA Screening For Fetal Aneuploidy. Obstet Gynecol. 2015;126:e31-37.
9. Wang Y, Zhu J, Chen Y, et al. Two cases of placental T21 mosaicism: challenging the detection limits of non-invasive prenatal testing. Prenat Diagn. 2013;33:1207-1210.
10. Choi H, Lau TK, Jiang FM, et al. Fetal aneuploidy screening by maternal plasma DNA sequencing: ‘false positive’ due to confined placental mosaicism. Prenat Diagn. 2013;33:198-200.
11. Norton ME, Jelliffe-Pawlowski LL, Currier RJ. Chromosome abnormalities detected by current prenatal screening and noninvasive prenatal testing. Obstet Gynecol. 2014;124:979-986.
12. Agarwal A, Sayres LC, Cho MK, et al. Commercial landscape of noninvasive prenatal testing in the United States. Prenat Diagn. 2013;33:521-531.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Holiday Trip Marred by Illness
ANSWER
This ECG is representative of marked sinus bradycardia with second-degree atrioventricular block (Mobitz I) with an occasional junctional escape, left-axis deviation, and poor R-wave progression in the precordial leads.
Marked sinus bradycardia is evidenced by P waves that are regular except where expected, prior to the third QRS complex on the rhythm strip (lead 1).
Second-degree Mobitz I (Wenckebach) block is indicated by a gradual prolonging of the PR interval until there is loss of conduction from the atria to the ventricle (following the third P wave). Careful inspection of the third QRS complex shows a slight difference in the normally conducted sinus beat, indicative of a junctional escape beat. Left-axis deviation entails an R-wave axis of –78°.
Finally, there is poor R-wave progression in the precordial leads, including the lateral leads.
Although Mobitz I block is not an indication for pacemaker placement, symptomatic bradycardia is. The patient underwent implantation of a dual-chamber permanent pacemaker, with complete resolution of symptoms.
ANSWER
This ECG is representative of marked sinus bradycardia with second-degree atrioventricular block (Mobitz I) with an occasional junctional escape, left-axis deviation, and poor R-wave progression in the precordial leads.
Marked sinus bradycardia is evidenced by P waves that are regular except where expected, prior to the third QRS complex on the rhythm strip (lead 1).
Second-degree Mobitz I (Wenckebach) block is indicated by a gradual prolonging of the PR interval until there is loss of conduction from the atria to the ventricle (following the third P wave). Careful inspection of the third QRS complex shows a slight difference in the normally conducted sinus beat, indicative of a junctional escape beat. Left-axis deviation entails an R-wave axis of –78°.
Finally, there is poor R-wave progression in the precordial leads, including the lateral leads.
Although Mobitz I block is not an indication for pacemaker placement, symptomatic bradycardia is. The patient underwent implantation of a dual-chamber permanent pacemaker, with complete resolution of symptoms.
ANSWER
This ECG is representative of marked sinus bradycardia with second-degree atrioventricular block (Mobitz I) with an occasional junctional escape, left-axis deviation, and poor R-wave progression in the precordial leads.
Marked sinus bradycardia is evidenced by P waves that are regular except where expected, prior to the third QRS complex on the rhythm strip (lead 1).
Second-degree Mobitz I (Wenckebach) block is indicated by a gradual prolonging of the PR interval until there is loss of conduction from the atria to the ventricle (following the third P wave). Careful inspection of the third QRS complex shows a slight difference in the normally conducted sinus beat, indicative of a junctional escape beat. Left-axis deviation entails an R-wave axis of –78°.
Finally, there is poor R-wave progression in the precordial leads, including the lateral leads.
Although Mobitz I block is not an indication for pacemaker placement, symptomatic bradycardia is. The patient underwent implantation of a dual-chamber permanent pacemaker, with complete resolution of symptoms.
While visiting family over the holidays, an 84-year-old man is (plaintively) informed by his wife that he appears unwell; she suspects he has the flu. The patient’s son, whom they are visiting, learns through conversation that his father has been feeling very tired and lethargic and becomes dizzy if he stands too quickly. The son, concerned for his father’s well-being, brings him to your clinic in the hope of obtaining a prescription for antibiotics. According to the patient, his symptoms, which have waxed and waned for several weeks, have become constant in the past week. He denies fever, cough, nausea, and vomiting, as well as chest pain, shortness of breath, palpitations, and lower extremity swelling. He reports that he has not recently changed his medication regimen and, aside from this current visit, has not traveled anywhere; he reiterates that his symptoms started prior to this trip. Medical history is remarkable for hypertension, peripheral atherosclerosis, osteoarthritis, gout, and pneumonia. Surgical history is remarkable for appendectomy, cholecystectomy, and removal of multiple lipomas from the patient’s upper extremities. Via the family history, you learn that the man’s father had a myocardial infarction and died of complications from a stroke at age 92 and his mother died of complications of diabetes at age 87. The patient is married, with three sons and one daughter, all of whom are in good health. He is a retired owner of a hardware store. He has never smoked or used recreational drugs and says he rarely drinks alcohol. The patient’s medication list includes metoprolol, atorvastatin, furosemide, and a daily baby aspirin. He is allergic to sulfa, which causes shortness of breath and wheezing. Review of systems reveals that he wears corrective lenses and hearing aids. He walks with a cane due to pain in both knees but is not dependent on it. He denies constitutional symptoms. A review of cardiovascular, respiratory, gastrointestinal, urologic, neurologic, and integumentary systems is noncontributory. Chest x-ray and laboratory testing, including complete blood count and chemistry panel, yield normal results. Vital signs include a blood pressure of 162/92 mm Hg; pulse, 40 beats/min; respiratory rate, 14 breaths/min; temperature, 99.2°F; and O2 saturation, 97% on room air. Pertinent physical findings include clear lung fields bilaterally, no evidence of jugular venous distention, and a heart rate of 40 beats/min that is regular and without evidence of a murmur or rub. There are well-healed scars on the abdomen and no evidence of organomegaly. Peripheral pulses are diminished but present bilaterally in both lower extremities. The neurologic exam is grossly intact, and the patient is alert, cooperative, and cognizant. Your concern about the patient’s heart rate prompts you to order an ECG. It reveals a ventricular rate of 38 beats/min; no measurable PR interval; QRS duration, 78 ms; QT/QTc interval, 434/345 ms; P axis, 25°; R axis, –78°; and T axis, 13°. What is your interpretation of this ECG?