User login
ASH: Donor CAR-T cells elicit responses in mixture of progressive B-cell cancers
ORLANDO – A single infusion of donor-derived chimeric antigen receptor (CAR)-modified T cells targeting CD19 achieved remission in 9 of 20 patients with B-cell malignancies that progressed after allogeneic stem cell transplant, a study shows.
The seven complete remissions and two partial remissions occurred without any chemotherapy and without causing acute graft-versus-host disease (GVHD).
The experimental anti-CD 19 CAR T-cells seem particularly effective against acute lymphoid leukemia (ALL) and chronic lymphocytic leukemia (CLL), but responses also occurred in lymphoma, Dr. James Kochenderfer of the Center for Cancer Research, National Cancer Institute, in Bethesda, Md., reported at the annual meeting of the American Society of Hematology.
B-cell malignancies that persist after transplantation are often treated with unmanipulated donor lymphocytes, but these infusions are often ineffective and associated with significant morbidity and mortality from GVHD.
To improve on this approach, 20 patients were infused with T cells obtained from the original stem cell donor and transduced with a CD19-directed CAR that was encoded by a gamma-retroviral vector and included a CD28 co-stimulatory domain. The highest dose reached in the phase I study was 107 total cell/kg. Production of the anti-CD19 CAR T cells took only eight days for each patient, Dr. Kochenderfer said at a press briefing.
The patients had received at least one standard donor-leukocyte infusion, had to have minimal or no GVHD, and could not be receiving systemic immunosuppressive drugs.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The highest response rates were in ALL, where four of five patients obtained complete remission (CR) with no detectable minimal residual disease by multi-color flow cytometry, Dr. Kochenderfer said. Two of these patients later relapsed, one is in ongoing CR at 18 months, and one went on to a second allogeneic transplant and continues in complete remission.
The longest ongoing CR at 36 months occurred in a patient treated for CLL. Another patient achieved a partial remission (PR) ongoing at 18 months, two patients progressed, and one has stable disease.
In five patients treated for Mantle cell lymphoma, there is one CR ongoing at 31 months, one PR, and three stable diseases.
Three of the five patients treated for diffuse large B-cell lymphoma experienced stable disease, one progressive disease, and one obtained a CR, but is no longer evaluable because she received other therapies for chronic GVHD. Dr. Kochenderfer went on describe an impressive response in this patient, who had large lymphoma masses at the back of her head and in her eye socket before infusion.
“Amazingly, the tumor masses completely disappeared within five days of CAR T-cell infusion,” he said.
Patients with high tumor burdens, however, experienced severe cytokine-release syndrome with fever, tachycardia, and hypertension that was treated with the interleukin-6 receptor antagonist tocilizumab (Actemra).
Only one case of mild aphasia occurred, which contrasts with other CAR T-cell therapies where neurotoxicity is common, Dr. Kochenderfer said.
One patient had continued worsening of pre-existing chronic GVHD after CAR T-cell therapy, and one patient developed very mild chronic eye GVHD more than a year after infusion.
The press corps was not fully convinced by the findings, however, asking Dr. Kochenderfer why they should be excited by the 40% remission rate when other CAR T-cell therapies have yielded remission rates as high as 90%.
Dr. Kochenderfer pointed out that four of the five ALL patients (80%) achieved a MRD-negative complete response, which compares favorably with other protocols. The remaining patients had far more advanced, treatment-resident disease of varying histologies than evaluated in other trials and, unlike most trials, all patients had received an allogeneic transplant. Further, the investigators used no chemotherapy whatsoever, whereas other CAR T-cells trials have used chemotherapy, sometimes in huge does, he said.
ORLANDO – A single infusion of donor-derived chimeric antigen receptor (CAR)-modified T cells targeting CD19 achieved remission in 9 of 20 patients with B-cell malignancies that progressed after allogeneic stem cell transplant, a study shows.
The seven complete remissions and two partial remissions occurred without any chemotherapy and without causing acute graft-versus-host disease (GVHD).
The experimental anti-CD 19 CAR T-cells seem particularly effective against acute lymphoid leukemia (ALL) and chronic lymphocytic leukemia (CLL), but responses also occurred in lymphoma, Dr. James Kochenderfer of the Center for Cancer Research, National Cancer Institute, in Bethesda, Md., reported at the annual meeting of the American Society of Hematology.
B-cell malignancies that persist after transplantation are often treated with unmanipulated donor lymphocytes, but these infusions are often ineffective and associated with significant morbidity and mortality from GVHD.
To improve on this approach, 20 patients were infused with T cells obtained from the original stem cell donor and transduced with a CD19-directed CAR that was encoded by a gamma-retroviral vector and included a CD28 co-stimulatory domain. The highest dose reached in the phase I study was 107 total cell/kg. Production of the anti-CD19 CAR T cells took only eight days for each patient, Dr. Kochenderfer said at a press briefing.
The patients had received at least one standard donor-leukocyte infusion, had to have minimal or no GVHD, and could not be receiving systemic immunosuppressive drugs.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The highest response rates were in ALL, where four of five patients obtained complete remission (CR) with no detectable minimal residual disease by multi-color flow cytometry, Dr. Kochenderfer said. Two of these patients later relapsed, one is in ongoing CR at 18 months, and one went on to a second allogeneic transplant and continues in complete remission.
The longest ongoing CR at 36 months occurred in a patient treated for CLL. Another patient achieved a partial remission (PR) ongoing at 18 months, two patients progressed, and one has stable disease.
In five patients treated for Mantle cell lymphoma, there is one CR ongoing at 31 months, one PR, and three stable diseases.
Three of the five patients treated for diffuse large B-cell lymphoma experienced stable disease, one progressive disease, and one obtained a CR, but is no longer evaluable because she received other therapies for chronic GVHD. Dr. Kochenderfer went on describe an impressive response in this patient, who had large lymphoma masses at the back of her head and in her eye socket before infusion.
“Amazingly, the tumor masses completely disappeared within five days of CAR T-cell infusion,” he said.
Patients with high tumor burdens, however, experienced severe cytokine-release syndrome with fever, tachycardia, and hypertension that was treated with the interleukin-6 receptor antagonist tocilizumab (Actemra).
Only one case of mild aphasia occurred, which contrasts with other CAR T-cell therapies where neurotoxicity is common, Dr. Kochenderfer said.
One patient had continued worsening of pre-existing chronic GVHD after CAR T-cell therapy, and one patient developed very mild chronic eye GVHD more than a year after infusion.
The press corps was not fully convinced by the findings, however, asking Dr. Kochenderfer why they should be excited by the 40% remission rate when other CAR T-cell therapies have yielded remission rates as high as 90%.
Dr. Kochenderfer pointed out that four of the five ALL patients (80%) achieved a MRD-negative complete response, which compares favorably with other protocols. The remaining patients had far more advanced, treatment-resident disease of varying histologies than evaluated in other trials and, unlike most trials, all patients had received an allogeneic transplant. Further, the investigators used no chemotherapy whatsoever, whereas other CAR T-cells trials have used chemotherapy, sometimes in huge does, he said.
ORLANDO – A single infusion of donor-derived chimeric antigen receptor (CAR)-modified T cells targeting CD19 achieved remission in 9 of 20 patients with B-cell malignancies that progressed after allogeneic stem cell transplant, a study shows.
The seven complete remissions and two partial remissions occurred without any chemotherapy and without causing acute graft-versus-host disease (GVHD).
The experimental anti-CD 19 CAR T-cells seem particularly effective against acute lymphoid leukemia (ALL) and chronic lymphocytic leukemia (CLL), but responses also occurred in lymphoma, Dr. James Kochenderfer of the Center for Cancer Research, National Cancer Institute, in Bethesda, Md., reported at the annual meeting of the American Society of Hematology.
B-cell malignancies that persist after transplantation are often treated with unmanipulated donor lymphocytes, but these infusions are often ineffective and associated with significant morbidity and mortality from GVHD.
To improve on this approach, 20 patients were infused with T cells obtained from the original stem cell donor and transduced with a CD19-directed CAR that was encoded by a gamma-retroviral vector and included a CD28 co-stimulatory domain. The highest dose reached in the phase I study was 107 total cell/kg. Production of the anti-CD19 CAR T cells took only eight days for each patient, Dr. Kochenderfer said at a press briefing.
The patients had received at least one standard donor-leukocyte infusion, had to have minimal or no GVHD, and could not be receiving systemic immunosuppressive drugs.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The highest response rates were in ALL, where four of five patients obtained complete remission (CR) with no detectable minimal residual disease by multi-color flow cytometry, Dr. Kochenderfer said. Two of these patients later relapsed, one is in ongoing CR at 18 months, and one went on to a second allogeneic transplant and continues in complete remission.
The longest ongoing CR at 36 months occurred in a patient treated for CLL. Another patient achieved a partial remission (PR) ongoing at 18 months, two patients progressed, and one has stable disease.
In five patients treated for Mantle cell lymphoma, there is one CR ongoing at 31 months, one PR, and three stable diseases.
Three of the five patients treated for diffuse large B-cell lymphoma experienced stable disease, one progressive disease, and one obtained a CR, but is no longer evaluable because she received other therapies for chronic GVHD. Dr. Kochenderfer went on describe an impressive response in this patient, who had large lymphoma masses at the back of her head and in her eye socket before infusion.
“Amazingly, the tumor masses completely disappeared within five days of CAR T-cell infusion,” he said.
Patients with high tumor burdens, however, experienced severe cytokine-release syndrome with fever, tachycardia, and hypertension that was treated with the interleukin-6 receptor antagonist tocilizumab (Actemra).
Only one case of mild aphasia occurred, which contrasts with other CAR T-cell therapies where neurotoxicity is common, Dr. Kochenderfer said.
One patient had continued worsening of pre-existing chronic GVHD after CAR T-cell therapy, and one patient developed very mild chronic eye GVHD more than a year after infusion.
The press corps was not fully convinced by the findings, however, asking Dr. Kochenderfer why they should be excited by the 40% remission rate when other CAR T-cell therapies have yielded remission rates as high as 90%.
Dr. Kochenderfer pointed out that four of the five ALL patients (80%) achieved a MRD-negative complete response, which compares favorably with other protocols. The remaining patients had far more advanced, treatment-resident disease of varying histologies than evaluated in other trials and, unlike most trials, all patients had received an allogeneic transplant. Further, the investigators used no chemotherapy whatsoever, whereas other CAR T-cells trials have used chemotherapy, sometimes in huge does, he said.
AT ASH 2015
Key clinical point: Allogeneic anti-CD19 CAR T-cell therapy showed promise in a treatment approach for B-cell malignancies persisting after allogeneic transplantation.
Major finding: Nine of 20 patients achieved remission with anti-CD19 CAR T-cell therapy.
Data source: Phase I study in 20 patients with CD19-positive B-cell malignancies progressing after allogeneic transplant.
Disclosures: Dr. Kochenderfer reported research funding from Bluebird bio, the study sponsor.
ASH: Gene therapy reduces transfusion needs in beta-thalassemia major
ORLANDO – Lentiviral gene therapy with LentiGlobin BB305 boosts beta-globin production in patients with beta-thalassemia, but frees only some from lifelong dependence on blood transfusions, updated results of the Northstar study show.
Five patients with non-Beta-0/Beta-0 genotypes were able to stop transfusions shortly after their infusion, and remain transfusion independent for up to 16.4 months.
In four patients with the more severe form of beta-thalassemia, the Beta-0/Beta-0 genotype, red blood cell transfusion volume was reduced by 33% to 100%, with one patient stopping transfusions entirely, Dr. Mark C. Walters of the University of California-San Francisco Benioff Children’s Hospital in Oakland reported at the annual meeting of the American Society of Hematology.
Preliminary findings reported over the last two years have raised hopes that the experimental lentiviral-based therapy could be a functional cure for beta-thalassemia major and severe sickle cell disease.
Patients with beta-thalassemia major, also called Cooley’s anemia, rely on frequent blood transfusions to correct the anemia, with less than a quarter undergoing curative treatment with an allogeneic hematopoietic transplant.
In the ongoing Northstar study, 13 patients with transfusion-dependent beta-thalassemia major have been infused as of Oct. 28, 2015 with autologous CD34-positive cells transduced ex-vivo with LentiGlobin BB305 (Bluebird bio, Cambridge, Mass.), a self-inactivating, second-generation lentiviral vector containing a functioning, engineered beta-globin gene (A-T87Q). Their median age was 21 years and 11 were women.Vector-derived hemoglobin AT87Q was detectable at 6 months in 8 of 9 evaluable patients with at least six months follow-up and in 100% at 9 months, Dr. Walters reported. The median HbAT87Q level was 4.9 g/dL at 6 months, 6.5 g/dL at 9 months, and 4.2 g/dL at 12 months.
The difference in transfusion independence between genotypes is explained by endogenous non-HbAT87Q production, he said during a press briefing. While lentiglobin production was the same in patients with Beta-0/Beta-0 and non-Beta-0/Beta-0 genotypes, the Beta-0/Beta-0 patients made much smaller amounts of native hemoglobin.
Three serious post-infusion events occurred: grade 2 thrombosis, grade 3 skin infection and grade 3 veno-occlusive liver disease.
Importantly, there was no evidence of clonal dominance or replication competent lentivirus with up to 19 months follow-up.
“This is a significant advancement in the treatment of thalassemia for several reasons,” Dr. Walters told reporters. “First, compared to a bone marrow transplant, which is the only curative therapy that’s been approved, this appears to be a safer treatment in that none of these patients had a life-threatening complication. Second, because the treatment uses a thalassemia patient’s own stem cells, this bypasses the need to find a healthy bone marrow donor and thus should be more broadly available to patients affected by this disease.”
Dr. George Daley of Harvard Medical School in Boston and moderator of the press briefing, agreed that the results are an welcome advancement after decades of disappointments in the field of gene therapy including the development of insertional mutigenesis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In addition to the Northstar study (Ab. 201), results will be presented at the meeting from a second study examining LentiGlobin BB305 gene therapy for severe sickle cell disease and beta-thalassemia major (Ab. 202) and from the recently expanded phase I HGB-206 study in severe sickle cell disease (Ab. 3233).
Sickle cell disease represents a much larger potential market for LentiGlobin BB305, with an estimated 90,000 to 100,000 Americans affected compared with about 15,000 patients in America and Europe living with beta-thalassemia major.
ORLANDO – Lentiviral gene therapy with LentiGlobin BB305 boosts beta-globin production in patients with beta-thalassemia, but frees only some from lifelong dependence on blood transfusions, updated results of the Northstar study show.
Five patients with non-Beta-0/Beta-0 genotypes were able to stop transfusions shortly after their infusion, and remain transfusion independent for up to 16.4 months.
In four patients with the more severe form of beta-thalassemia, the Beta-0/Beta-0 genotype, red blood cell transfusion volume was reduced by 33% to 100%, with one patient stopping transfusions entirely, Dr. Mark C. Walters of the University of California-San Francisco Benioff Children’s Hospital in Oakland reported at the annual meeting of the American Society of Hematology.
Preliminary findings reported over the last two years have raised hopes that the experimental lentiviral-based therapy could be a functional cure for beta-thalassemia major and severe sickle cell disease.
Patients with beta-thalassemia major, also called Cooley’s anemia, rely on frequent blood transfusions to correct the anemia, with less than a quarter undergoing curative treatment with an allogeneic hematopoietic transplant.
In the ongoing Northstar study, 13 patients with transfusion-dependent beta-thalassemia major have been infused as of Oct. 28, 2015 with autologous CD34-positive cells transduced ex-vivo with LentiGlobin BB305 (Bluebird bio, Cambridge, Mass.), a self-inactivating, second-generation lentiviral vector containing a functioning, engineered beta-globin gene (A-T87Q). Their median age was 21 years and 11 were women.Vector-derived hemoglobin AT87Q was detectable at 6 months in 8 of 9 evaluable patients with at least six months follow-up and in 100% at 9 months, Dr. Walters reported. The median HbAT87Q level was 4.9 g/dL at 6 months, 6.5 g/dL at 9 months, and 4.2 g/dL at 12 months.
The difference in transfusion independence between genotypes is explained by endogenous non-HbAT87Q production, he said during a press briefing. While lentiglobin production was the same in patients with Beta-0/Beta-0 and non-Beta-0/Beta-0 genotypes, the Beta-0/Beta-0 patients made much smaller amounts of native hemoglobin.
Three serious post-infusion events occurred: grade 2 thrombosis, grade 3 skin infection and grade 3 veno-occlusive liver disease.
Importantly, there was no evidence of clonal dominance or replication competent lentivirus with up to 19 months follow-up.
“This is a significant advancement in the treatment of thalassemia for several reasons,” Dr. Walters told reporters. “First, compared to a bone marrow transplant, which is the only curative therapy that’s been approved, this appears to be a safer treatment in that none of these patients had a life-threatening complication. Second, because the treatment uses a thalassemia patient’s own stem cells, this bypasses the need to find a healthy bone marrow donor and thus should be more broadly available to patients affected by this disease.”
Dr. George Daley of Harvard Medical School in Boston and moderator of the press briefing, agreed that the results are an welcome advancement after decades of disappointments in the field of gene therapy including the development of insertional mutigenesis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In addition to the Northstar study (Ab. 201), results will be presented at the meeting from a second study examining LentiGlobin BB305 gene therapy for severe sickle cell disease and beta-thalassemia major (Ab. 202) and from the recently expanded phase I HGB-206 study in severe sickle cell disease (Ab. 3233).
Sickle cell disease represents a much larger potential market for LentiGlobin BB305, with an estimated 90,000 to 100,000 Americans affected compared with about 15,000 patients in America and Europe living with beta-thalassemia major.
ORLANDO – Lentiviral gene therapy with LentiGlobin BB305 boosts beta-globin production in patients with beta-thalassemia, but frees only some from lifelong dependence on blood transfusions, updated results of the Northstar study show.
Five patients with non-Beta-0/Beta-0 genotypes were able to stop transfusions shortly after their infusion, and remain transfusion independent for up to 16.4 months.
In four patients with the more severe form of beta-thalassemia, the Beta-0/Beta-0 genotype, red blood cell transfusion volume was reduced by 33% to 100%, with one patient stopping transfusions entirely, Dr. Mark C. Walters of the University of California-San Francisco Benioff Children’s Hospital in Oakland reported at the annual meeting of the American Society of Hematology.
Preliminary findings reported over the last two years have raised hopes that the experimental lentiviral-based therapy could be a functional cure for beta-thalassemia major and severe sickle cell disease.
Patients with beta-thalassemia major, also called Cooley’s anemia, rely on frequent blood transfusions to correct the anemia, with less than a quarter undergoing curative treatment with an allogeneic hematopoietic transplant.
In the ongoing Northstar study, 13 patients with transfusion-dependent beta-thalassemia major have been infused as of Oct. 28, 2015 with autologous CD34-positive cells transduced ex-vivo with LentiGlobin BB305 (Bluebird bio, Cambridge, Mass.), a self-inactivating, second-generation lentiviral vector containing a functioning, engineered beta-globin gene (A-T87Q). Their median age was 21 years and 11 were women.Vector-derived hemoglobin AT87Q was detectable at 6 months in 8 of 9 evaluable patients with at least six months follow-up and in 100% at 9 months, Dr. Walters reported. The median HbAT87Q level was 4.9 g/dL at 6 months, 6.5 g/dL at 9 months, and 4.2 g/dL at 12 months.
The difference in transfusion independence between genotypes is explained by endogenous non-HbAT87Q production, he said during a press briefing. While lentiglobin production was the same in patients with Beta-0/Beta-0 and non-Beta-0/Beta-0 genotypes, the Beta-0/Beta-0 patients made much smaller amounts of native hemoglobin.
Three serious post-infusion events occurred: grade 2 thrombosis, grade 3 skin infection and grade 3 veno-occlusive liver disease.
Importantly, there was no evidence of clonal dominance or replication competent lentivirus with up to 19 months follow-up.
“This is a significant advancement in the treatment of thalassemia for several reasons,” Dr. Walters told reporters. “First, compared to a bone marrow transplant, which is the only curative therapy that’s been approved, this appears to be a safer treatment in that none of these patients had a life-threatening complication. Second, because the treatment uses a thalassemia patient’s own stem cells, this bypasses the need to find a healthy bone marrow donor and thus should be more broadly available to patients affected by this disease.”
Dr. George Daley of Harvard Medical School in Boston and moderator of the press briefing, agreed that the results are an welcome advancement after decades of disappointments in the field of gene therapy including the development of insertional mutigenesis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In addition to the Northstar study (Ab. 201), results will be presented at the meeting from a second study examining LentiGlobin BB305 gene therapy for severe sickle cell disease and beta-thalassemia major (Ab. 202) and from the recently expanded phase I HGB-206 study in severe sickle cell disease (Ab. 3233).
Sickle cell disease represents a much larger potential market for LentiGlobin BB305, with an estimated 90,000 to 100,000 Americans affected compared with about 15,000 patients in America and Europe living with beta-thalassemia major.
AT ASH 2015
Key clinical point: Lentiviral-based gene therapy with LentiGlobin BB305 restarts hemoglobin production and leads to transfusion independence in some patients with beta-thalassemia major.
Major finding: Five patients with the non-Beta-0/Beta-0 genotype were transfusion independent post-infusion.
Data source: Phase I/II study in 13 patients with transfusion-dependent beta-thalassemia major.
Disclosures: Dr. Walters reported financial relationships with ViaCord and AllCells Inc. Several co-authors have financial relationships including employment with Bluebird bio, the study sponsor. Dr. Daley disclosed consultancy with True North Therapeutics and serving as an advisory committee member for Raze Therapeutics, Ocata Therapeutics, MPM Capital, and Solasia.
ASH: Gene therapy eases effects of rare Wiskott-Aldrich syndrome
ORLANDO – Genetic modification of autologous stem cells provided sustained clinical benefit with good safety for children with the rare immunodeficiency disorder Wiskott-Aldrich syndrome, an international team of investigators report.
Six of eight children who received infusions of autologous stem cells that had been modified with a lentiviral vector to restore normal expression of the WAS gene had marked reductions in severe infections, fewer hospitalizations, improved hematologic parameters, and more robust immune responses than they had prior to transplant, reported Dr. Francesca Ferrua from the San Raffaele Telethon Institute for Gene Therapy in Milan, Italy.
“Importantly, with regards to safety we did not detect any serious adverse events related to gene therapy in follow up, and we did not observe any evidence of abnormal clonal proliferation after gene therapy,” she said at the American Society of Hematology annual meeting here.
The Wiskott-Aldrich syndrome is an x-linked syndrome caused by mutations in the WAS gene encoding for the WAS protein (WASP), which is involved in regulation of the cytoskeleton. The disorder, which primarily affects males, leads to immunodeficiency, microthrombocytopenia and leukocyte abnormalities. Patients develop severe eczema and other inflammatory disorders, and are at increased risk for autoimmune diseases and malignancies.
The syndrome is estimated to occur in 1-10 per 1 million males worldwide, according to The National Library of Medicine.
Allogeneic hematopoietic stem cell transplantion (HSCT) can be curative for patients with Wiskott-Aldrich syndrome, but the technique is associated with both acute transplant-related complications and long-term morbidities, particularly when there is not a perfect match between donor and recipient, Dr. Ferrua explained.Prior studies using a gamma-retroviral vector under the control of a strong viral promoter showed that gene therapy was feasible in these patients and could result in immunological improvement. The earlier attempts, however, were associated with a high risk of genotoxicity and insertional mutagenesis; seven of nine patients treated in one study developed leukemia.
In their current line of research, Dr. Ferrua and colleagues had previously reported on the use of autologous hematopoietic stem/progenitor cells modified ex vivo to correct the inherent defect in three patients with severe mutations in WAS who had no suitable stem-cell donors.
The researchers collected CD34-positive cells from each patient’s bone marrow and/or mobilized peripheral blood and transduced the cells in the laboratory with a lentivirus modified to promote normal expression of WAS. They then returned the cells to the patients after they underwent a reduced-intensity conditioning regimen using an anti-CD20 monoclonal antibody, busulfan, and fludarabine.
Long-term follow-up
At ASH 2015, Dr. Ferrua reported results on the first 8 patients treated as of October 2015. The patients were treated at a median age of 2.2 years; all are alive after a median of 3.3 years of follow up, with the longest follow up being 5.5 years
All had marked reductions in the annualized estimated rate of severe infections compared with the pre-transplant period.
Of the seven patients followed for more than 1 year, all were able to discontinue prophylaxis for infections, at a median of 13-15 months after gene therapy, and five were able to discontinue immunoglobulin supplementation.
Additionally, four of four patients had evidence of a normal immune response based on the development of specific antibodies after vaccination.
Four patients had resolution of their eczemas, and the other two with eczema had only mild cases.
At a median of 4 months after genetic therapy, none of the patients required platelet transfusions. Out to at least 1 year, there was no evidence of autoimmunity.
Among all patients, there were reductions in the frequency or severity of bleeding, no severe bleeding episodes, no hospitalizations for bleeding and a reduction in the number of hospitalizations for infections.
There were no serious adverse events related to the transplant.
The study was sponsored by IRCCS San Raffaele with support from the Fondazione Telethon and GlaxoSmithKline. Dr. Ferrua reported having no conflicts of interest.
ORLANDO – Genetic modification of autologous stem cells provided sustained clinical benefit with good safety for children with the rare immunodeficiency disorder Wiskott-Aldrich syndrome, an international team of investigators report.
Six of eight children who received infusions of autologous stem cells that had been modified with a lentiviral vector to restore normal expression of the WAS gene had marked reductions in severe infections, fewer hospitalizations, improved hematologic parameters, and more robust immune responses than they had prior to transplant, reported Dr. Francesca Ferrua from the San Raffaele Telethon Institute for Gene Therapy in Milan, Italy.
“Importantly, with regards to safety we did not detect any serious adverse events related to gene therapy in follow up, and we did not observe any evidence of abnormal clonal proliferation after gene therapy,” she said at the American Society of Hematology annual meeting here.
The Wiskott-Aldrich syndrome is an x-linked syndrome caused by mutations in the WAS gene encoding for the WAS protein (WASP), which is involved in regulation of the cytoskeleton. The disorder, which primarily affects males, leads to immunodeficiency, microthrombocytopenia and leukocyte abnormalities. Patients develop severe eczema and other inflammatory disorders, and are at increased risk for autoimmune diseases and malignancies.
The syndrome is estimated to occur in 1-10 per 1 million males worldwide, according to The National Library of Medicine.
Allogeneic hematopoietic stem cell transplantion (HSCT) can be curative for patients with Wiskott-Aldrich syndrome, but the technique is associated with both acute transplant-related complications and long-term morbidities, particularly when there is not a perfect match between donor and recipient, Dr. Ferrua explained.Prior studies using a gamma-retroviral vector under the control of a strong viral promoter showed that gene therapy was feasible in these patients and could result in immunological improvement. The earlier attempts, however, were associated with a high risk of genotoxicity and insertional mutagenesis; seven of nine patients treated in one study developed leukemia.
In their current line of research, Dr. Ferrua and colleagues had previously reported on the use of autologous hematopoietic stem/progenitor cells modified ex vivo to correct the inherent defect in three patients with severe mutations in WAS who had no suitable stem-cell donors.
The researchers collected CD34-positive cells from each patient’s bone marrow and/or mobilized peripheral blood and transduced the cells in the laboratory with a lentivirus modified to promote normal expression of WAS. They then returned the cells to the patients after they underwent a reduced-intensity conditioning regimen using an anti-CD20 monoclonal antibody, busulfan, and fludarabine.
Long-term follow-up
At ASH 2015, Dr. Ferrua reported results on the first 8 patients treated as of October 2015. The patients were treated at a median age of 2.2 years; all are alive after a median of 3.3 years of follow up, with the longest follow up being 5.5 years
All had marked reductions in the annualized estimated rate of severe infections compared with the pre-transplant period.
Of the seven patients followed for more than 1 year, all were able to discontinue prophylaxis for infections, at a median of 13-15 months after gene therapy, and five were able to discontinue immunoglobulin supplementation.
Additionally, four of four patients had evidence of a normal immune response based on the development of specific antibodies after vaccination.
Four patients had resolution of their eczemas, and the other two with eczema had only mild cases.
At a median of 4 months after genetic therapy, none of the patients required platelet transfusions. Out to at least 1 year, there was no evidence of autoimmunity.
Among all patients, there were reductions in the frequency or severity of bleeding, no severe bleeding episodes, no hospitalizations for bleeding and a reduction in the number of hospitalizations for infections.
There were no serious adverse events related to the transplant.
The study was sponsored by IRCCS San Raffaele with support from the Fondazione Telethon and GlaxoSmithKline. Dr. Ferrua reported having no conflicts of interest.
ORLANDO – Genetic modification of autologous stem cells provided sustained clinical benefit with good safety for children with the rare immunodeficiency disorder Wiskott-Aldrich syndrome, an international team of investigators report.
Six of eight children who received infusions of autologous stem cells that had been modified with a lentiviral vector to restore normal expression of the WAS gene had marked reductions in severe infections, fewer hospitalizations, improved hematologic parameters, and more robust immune responses than they had prior to transplant, reported Dr. Francesca Ferrua from the San Raffaele Telethon Institute for Gene Therapy in Milan, Italy.
“Importantly, with regards to safety we did not detect any serious adverse events related to gene therapy in follow up, and we did not observe any evidence of abnormal clonal proliferation after gene therapy,” she said at the American Society of Hematology annual meeting here.
The Wiskott-Aldrich syndrome is an x-linked syndrome caused by mutations in the WAS gene encoding for the WAS protein (WASP), which is involved in regulation of the cytoskeleton. The disorder, which primarily affects males, leads to immunodeficiency, microthrombocytopenia and leukocyte abnormalities. Patients develop severe eczema and other inflammatory disorders, and are at increased risk for autoimmune diseases and malignancies.
The syndrome is estimated to occur in 1-10 per 1 million males worldwide, according to The National Library of Medicine.
Allogeneic hematopoietic stem cell transplantion (HSCT) can be curative for patients with Wiskott-Aldrich syndrome, but the technique is associated with both acute transplant-related complications and long-term morbidities, particularly when there is not a perfect match between donor and recipient, Dr. Ferrua explained.Prior studies using a gamma-retroviral vector under the control of a strong viral promoter showed that gene therapy was feasible in these patients and could result in immunological improvement. The earlier attempts, however, were associated with a high risk of genotoxicity and insertional mutagenesis; seven of nine patients treated in one study developed leukemia.
In their current line of research, Dr. Ferrua and colleagues had previously reported on the use of autologous hematopoietic stem/progenitor cells modified ex vivo to correct the inherent defect in three patients with severe mutations in WAS who had no suitable stem-cell donors.
The researchers collected CD34-positive cells from each patient’s bone marrow and/or mobilized peripheral blood and transduced the cells in the laboratory with a lentivirus modified to promote normal expression of WAS. They then returned the cells to the patients after they underwent a reduced-intensity conditioning regimen using an anti-CD20 monoclonal antibody, busulfan, and fludarabine.
Long-term follow-up
At ASH 2015, Dr. Ferrua reported results on the first 8 patients treated as of October 2015. The patients were treated at a median age of 2.2 years; all are alive after a median of 3.3 years of follow up, with the longest follow up being 5.5 years
All had marked reductions in the annualized estimated rate of severe infections compared with the pre-transplant period.
Of the seven patients followed for more than 1 year, all were able to discontinue prophylaxis for infections, at a median of 13-15 months after gene therapy, and five were able to discontinue immunoglobulin supplementation.
Additionally, four of four patients had evidence of a normal immune response based on the development of specific antibodies after vaccination.
Four patients had resolution of their eczemas, and the other two with eczema had only mild cases.
At a median of 4 months after genetic therapy, none of the patients required platelet transfusions. Out to at least 1 year, there was no evidence of autoimmunity.
Among all patients, there were reductions in the frequency or severity of bleeding, no severe bleeding episodes, no hospitalizations for bleeding and a reduction in the number of hospitalizations for infections.
There were no serious adverse events related to the transplant.
The study was sponsored by IRCCS San Raffaele with support from the Fondazione Telethon and GlaxoSmithKline. Dr. Ferrua reported having no conflicts of interest.
AT ASH 2015
Key clinical point: Gene therapy might safely and effectively correct an inherited immunodeficiency syndrome.
Major finding: Six of eight children with Wiskott-Aldrich syndrome who received genetically modified autologous stem cells had marked clinical improvements.
Data source: International collaborative trial studying the safety and efficacy of WAS gene transfer into hematopoietic stem/progenitor cells.
Disclosures: The study was sponsored by IRCCS San Raffaele with support from the Fondazione Telethon and GlaxoSmithKline. Dr. Ferrua reported having no conflicts of interest.
Pardon the interruption
It’s 7:30 Monday evening and the good news is that you were able to leave the office a little after 7:00 and are now at home. The bad news is that you are on call tonight and you are scheduled to work a usual 10-hour day tomorrow.
Should you:
A) Tuck the kids in, warm up a bowl of chili, and lie down on the couch in the den and hope to get a few hours of prophylactic sleep?
B) Go to bed at your usual bedtime of 10:30?
C) Stay up until midnight when experience tells you that the likelihood of getting a call drops significantly ... but unfortunately never approaches zero?
D) Say “To hell with it” and stay up all night binge-watching a whole season of “Justified?”
E) Or, stay up all night surfing the Internet looking for job opportunities that don’t include night call?
Of course, there is no correct answer because stuff happens whenever it chooses to and no strategy will ever guarantee you an uninterrupted 8 hours of sleep when you are on call. However, I learned from a recent article in the Wall Street Journal (A Good Night’s Sleep Is Tied to Interruptions, Not Just Hours – Sumathi Reddy – Nov. 30, 2015) that there is some evidence that “C” clearly is the best choice.
A study from John Hopkins University, Baltimore, published in the journal Sleep found that subjects who were awakened multiple times during the night exhibited a greater decline in positive mood than did those subjects who were sleep deprived by being made to stay up past their usual bedtime (2015 Nov 1;38[11]:1735-42).
Another study from the University of Pittsburgh discovered that elderly subjects’ cognitive performance was impaired when their sleep was interrupted but not when they were allowed to sleep uninterrupted for a shorter period of time (J Gerontol B Psychol Sci Soc Sci. 2009 Mar;64B[2]:180-7).
And, investigators at the University of Tel Aviv have found that subjects who endured 8 hours of fragmented sleep demonstrated declines in their positive mood and ability to attend that were similar to subjects who were allowed only 4 hours of uninterrupted sleep (Sleep Med. 2011 Mar;12[3]:257-61).
Where were these sleep researchers 45 years ago, when I was experimenting with my own strategies for navigating a night on call with a minimum of emotional and cognitive damage? It took me several years to discover that it was fruitless to try grabbing an hour or two of prophylactic sleep early in the evening when the risk of being awakened by a call was still relatively high. The rare occasion when I slept without interruption was of little comfort on the other nights when I could feel every wakening erode my feeble attempts at projecting a pleasant bedside (my bed that is) manner.
It took another few years of trial and error to improve my skill at determining the optimal time to turn in on a given night. It was never perfect, but eventually, I developed an instinct – based on the level of disease in the community, the pulse of the office during the day, and the weather – that kept the interruptions to a minimum. Despite what you may have heard, I never found the phase of moon to be terribly helpful in predicting when I could more safely go to bed.
There is no avoiding the unpleasant truth that being on call puts you at risk for sleep deprivation. One way or another, you are going to be sleep deprived when you show up at the office the next day. But, your best chance of continuing to appear to be a sensitive and intelligent physician is staying up late until the likelihood you will be awakened by a call has reached its traditional nadir.
It’s 7:30 Monday evening and the good news is that you were able to leave the office a little after 7:00 and are now at home. The bad news is that you are on call tonight and you are scheduled to work a usual 10-hour day tomorrow.
Should you:
A) Tuck the kids in, warm up a bowl of chili, and lie down on the couch in the den and hope to get a few hours of prophylactic sleep?
B) Go to bed at your usual bedtime of 10:30?
C) Stay up until midnight when experience tells you that the likelihood of getting a call drops significantly ... but unfortunately never approaches zero?
D) Say “To hell with it” and stay up all night binge-watching a whole season of “Justified?”
E) Or, stay up all night surfing the Internet looking for job opportunities that don’t include night call?
Of course, there is no correct answer because stuff happens whenever it chooses to and no strategy will ever guarantee you an uninterrupted 8 hours of sleep when you are on call. However, I learned from a recent article in the Wall Street Journal (A Good Night’s Sleep Is Tied to Interruptions, Not Just Hours – Sumathi Reddy – Nov. 30, 2015) that there is some evidence that “C” clearly is the best choice.
A study from John Hopkins University, Baltimore, published in the journal Sleep found that subjects who were awakened multiple times during the night exhibited a greater decline in positive mood than did those subjects who were sleep deprived by being made to stay up past their usual bedtime (2015 Nov 1;38[11]:1735-42).
Another study from the University of Pittsburgh discovered that elderly subjects’ cognitive performance was impaired when their sleep was interrupted but not when they were allowed to sleep uninterrupted for a shorter period of time (J Gerontol B Psychol Sci Soc Sci. 2009 Mar;64B[2]:180-7).
And, investigators at the University of Tel Aviv have found that subjects who endured 8 hours of fragmented sleep demonstrated declines in their positive mood and ability to attend that were similar to subjects who were allowed only 4 hours of uninterrupted sleep (Sleep Med. 2011 Mar;12[3]:257-61).
Where were these sleep researchers 45 years ago, when I was experimenting with my own strategies for navigating a night on call with a minimum of emotional and cognitive damage? It took me several years to discover that it was fruitless to try grabbing an hour or two of prophylactic sleep early in the evening when the risk of being awakened by a call was still relatively high. The rare occasion when I slept without interruption was of little comfort on the other nights when I could feel every wakening erode my feeble attempts at projecting a pleasant bedside (my bed that is) manner.
It took another few years of trial and error to improve my skill at determining the optimal time to turn in on a given night. It was never perfect, but eventually, I developed an instinct – based on the level of disease in the community, the pulse of the office during the day, and the weather – that kept the interruptions to a minimum. Despite what you may have heard, I never found the phase of moon to be terribly helpful in predicting when I could more safely go to bed.
There is no avoiding the unpleasant truth that being on call puts you at risk for sleep deprivation. One way or another, you are going to be sleep deprived when you show up at the office the next day. But, your best chance of continuing to appear to be a sensitive and intelligent physician is staying up late until the likelihood you will be awakened by a call has reached its traditional nadir.
It’s 7:30 Monday evening and the good news is that you were able to leave the office a little after 7:00 and are now at home. The bad news is that you are on call tonight and you are scheduled to work a usual 10-hour day tomorrow.
Should you:
A) Tuck the kids in, warm up a bowl of chili, and lie down on the couch in the den and hope to get a few hours of prophylactic sleep?
B) Go to bed at your usual bedtime of 10:30?
C) Stay up until midnight when experience tells you that the likelihood of getting a call drops significantly ... but unfortunately never approaches zero?
D) Say “To hell with it” and stay up all night binge-watching a whole season of “Justified?”
E) Or, stay up all night surfing the Internet looking for job opportunities that don’t include night call?
Of course, there is no correct answer because stuff happens whenever it chooses to and no strategy will ever guarantee you an uninterrupted 8 hours of sleep when you are on call. However, I learned from a recent article in the Wall Street Journal (A Good Night’s Sleep Is Tied to Interruptions, Not Just Hours – Sumathi Reddy – Nov. 30, 2015) that there is some evidence that “C” clearly is the best choice.
A study from John Hopkins University, Baltimore, published in the journal Sleep found that subjects who were awakened multiple times during the night exhibited a greater decline in positive mood than did those subjects who were sleep deprived by being made to stay up past their usual bedtime (2015 Nov 1;38[11]:1735-42).
Another study from the University of Pittsburgh discovered that elderly subjects’ cognitive performance was impaired when their sleep was interrupted but not when they were allowed to sleep uninterrupted for a shorter period of time (J Gerontol B Psychol Sci Soc Sci. 2009 Mar;64B[2]:180-7).
And, investigators at the University of Tel Aviv have found that subjects who endured 8 hours of fragmented sleep demonstrated declines in their positive mood and ability to attend that were similar to subjects who were allowed only 4 hours of uninterrupted sleep (Sleep Med. 2011 Mar;12[3]:257-61).
Where were these sleep researchers 45 years ago, when I was experimenting with my own strategies for navigating a night on call with a minimum of emotional and cognitive damage? It took me several years to discover that it was fruitless to try grabbing an hour or two of prophylactic sleep early in the evening when the risk of being awakened by a call was still relatively high. The rare occasion when I slept without interruption was of little comfort on the other nights when I could feel every wakening erode my feeble attempts at projecting a pleasant bedside (my bed that is) manner.
It took another few years of trial and error to improve my skill at determining the optimal time to turn in on a given night. It was never perfect, but eventually, I developed an instinct – based on the level of disease in the community, the pulse of the office during the day, and the weather – that kept the interruptions to a minimum. Despite what you may have heard, I never found the phase of moon to be terribly helpful in predicting when I could more safely go to bed.
There is no avoiding the unpleasant truth that being on call puts you at risk for sleep deprivation. One way or another, you are going to be sleep deprived when you show up at the office the next day. But, your best chance of continuing to appear to be a sensitive and intelligent physician is staying up late until the likelihood you will be awakened by a call has reached its traditional nadir.
Combo could target LSCs, treat CML

Image by Difu Wu
Researchers say they have identified a mechanism governing malignant reprogramming of progenitors into self-renewing leukemia stem cells (LSCs).
And their discovery has revealed a potential new therapeutic approach for chronic myeloid leukemia (CML).
Experiments in mice showed that a targeted monoclonal antibody could impair LSCs’ ability to regenerate and made them easier to eradicate with a tyrosine kinase inhibitor.
Catriona Jamieson, MD, PhD, of the University of California, San Diego, and her colleagues described this research in PNAS.
The researchers found that downregulation of Muscleblind-like 3 (MBNL3) RNA binding proteins resulted in re-expression of a human embryonic stem cell-specific alternative splicing gene regulatory network—a mechanism that controls embryonic stem cell pluripotency and fate. One effect of this was reprogramming of progenitor cells into LSCs in blast crisis CML.
“This is the first description of cancer stem cell generation through decreased expression of a transcriptional repressor of an embryonic pattern of alternative splicing that enhances stem cell self-renewal and survival,” Dr Jamieson said.
“Rather than acquiring multiple DNA mutations, as was previously thought, cancer stem cells in chronic myeloid leukemia switch to embryonic RNA splicing, which enhances their capacity to self-renew or clone themselves.”
“If we can detect and turn off embryonic splicing, we may be able to prevent cancer stem cells from propagating themselves. Also, if we target embryonic versions of proteins that are re-expressed by cancer, like CD44 variant 3, with specific antibodies together with tyrosine kinase inhibitors, we may be able to circumvent cancer relapse—a leading cause of cancer-related mortality.”
The researchers tested this theory in mice. They found that treatment with a humanized pan-CD44 monoclonal antibody and a targeted tyrosine kinase antagonist disrupted the development of LSCs in their protected microenvironment.
This forced the cells to enter the bloodstream, where dasatinib could effectively target them. ![]()

Image by Difu Wu
Researchers say they have identified a mechanism governing malignant reprogramming of progenitors into self-renewing leukemia stem cells (LSCs).
And their discovery has revealed a potential new therapeutic approach for chronic myeloid leukemia (CML).
Experiments in mice showed that a targeted monoclonal antibody could impair LSCs’ ability to regenerate and made them easier to eradicate with a tyrosine kinase inhibitor.
Catriona Jamieson, MD, PhD, of the University of California, San Diego, and her colleagues described this research in PNAS.
The researchers found that downregulation of Muscleblind-like 3 (MBNL3) RNA binding proteins resulted in re-expression of a human embryonic stem cell-specific alternative splicing gene regulatory network—a mechanism that controls embryonic stem cell pluripotency and fate. One effect of this was reprogramming of progenitor cells into LSCs in blast crisis CML.
“This is the first description of cancer stem cell generation through decreased expression of a transcriptional repressor of an embryonic pattern of alternative splicing that enhances stem cell self-renewal and survival,” Dr Jamieson said.
“Rather than acquiring multiple DNA mutations, as was previously thought, cancer stem cells in chronic myeloid leukemia switch to embryonic RNA splicing, which enhances their capacity to self-renew or clone themselves.”
“If we can detect and turn off embryonic splicing, we may be able to prevent cancer stem cells from propagating themselves. Also, if we target embryonic versions of proteins that are re-expressed by cancer, like CD44 variant 3, with specific antibodies together with tyrosine kinase inhibitors, we may be able to circumvent cancer relapse—a leading cause of cancer-related mortality.”
The researchers tested this theory in mice. They found that treatment with a humanized pan-CD44 monoclonal antibody and a targeted tyrosine kinase antagonist disrupted the development of LSCs in their protected microenvironment.
This forced the cells to enter the bloodstream, where dasatinib could effectively target them. ![]()

Image by Difu Wu
Researchers say they have identified a mechanism governing malignant reprogramming of progenitors into self-renewing leukemia stem cells (LSCs).
And their discovery has revealed a potential new therapeutic approach for chronic myeloid leukemia (CML).
Experiments in mice showed that a targeted monoclonal antibody could impair LSCs’ ability to regenerate and made them easier to eradicate with a tyrosine kinase inhibitor.
Catriona Jamieson, MD, PhD, of the University of California, San Diego, and her colleagues described this research in PNAS.
The researchers found that downregulation of Muscleblind-like 3 (MBNL3) RNA binding proteins resulted in re-expression of a human embryonic stem cell-specific alternative splicing gene regulatory network—a mechanism that controls embryonic stem cell pluripotency and fate. One effect of this was reprogramming of progenitor cells into LSCs in blast crisis CML.
“This is the first description of cancer stem cell generation through decreased expression of a transcriptional repressor of an embryonic pattern of alternative splicing that enhances stem cell self-renewal and survival,” Dr Jamieson said.
“Rather than acquiring multiple DNA mutations, as was previously thought, cancer stem cells in chronic myeloid leukemia switch to embryonic RNA splicing, which enhances their capacity to self-renew or clone themselves.”
“If we can detect and turn off embryonic splicing, we may be able to prevent cancer stem cells from propagating themselves. Also, if we target embryonic versions of proteins that are re-expressed by cancer, like CD44 variant 3, with specific antibodies together with tyrosine kinase inhibitors, we may be able to circumvent cancer relapse—a leading cause of cancer-related mortality.”
The researchers tested this theory in mice. They found that treatment with a humanized pan-CD44 monoclonal antibody and a targeted tyrosine kinase antagonist disrupted the development of LSCs in their protected microenvironment.
This forced the cells to enter the bloodstream, where dasatinib could effectively target them. ![]()
Cord blood banking resource
Stem cells collected from donated umbilical cord blood have been approved by the Food and Drug Administration to treat more than 80 diseases, including leukemia and lymphoma, according to the Cord Blood Center.
Expectant parents can save stem cells from their child’s umbilical cord blood for use in medical procedures and transplants for their child and can choose to store or donate these stem cells to a patient who might need them, which is called cord blood banking. There are more than half a million donated cord blood units worldwide, with thousands more units added every year.
By learning more about cord blood and about the science behind stem cell transplants, parents can then decide if storing their child’s leftover umbilical cord cells is the right option for their family.
The FDA does caution consumers to make sure that any stem cell treatment they are considering has been approved by the agency or is being studied in clinical trials.
For more information, go to the Cord Blood Center website.
Stem cells collected from donated umbilical cord blood have been approved by the Food and Drug Administration to treat more than 80 diseases, including leukemia and lymphoma, according to the Cord Blood Center.
Expectant parents can save stem cells from their child’s umbilical cord blood for use in medical procedures and transplants for their child and can choose to store or donate these stem cells to a patient who might need them, which is called cord blood banking. There are more than half a million donated cord blood units worldwide, with thousands more units added every year.
By learning more about cord blood and about the science behind stem cell transplants, parents can then decide if storing their child’s leftover umbilical cord cells is the right option for their family.
The FDA does caution consumers to make sure that any stem cell treatment they are considering has been approved by the agency or is being studied in clinical trials.
For more information, go to the Cord Blood Center website.
Stem cells collected from donated umbilical cord blood have been approved by the Food and Drug Administration to treat more than 80 diseases, including leukemia and lymphoma, according to the Cord Blood Center.
Expectant parents can save stem cells from their child’s umbilical cord blood for use in medical procedures and transplants for their child and can choose to store or donate these stem cells to a patient who might need them, which is called cord blood banking. There are more than half a million donated cord blood units worldwide, with thousands more units added every year.
By learning more about cord blood and about the science behind stem cell transplants, parents can then decide if storing their child’s leftover umbilical cord cells is the right option for their family.
The FDA does caution consumers to make sure that any stem cell treatment they are considering has been approved by the agency or is being studied in clinical trials.
For more information, go to the Cord Blood Center website.
Bearing the wait
If you have ever waited anxiously for the results of a blood test or biopsy, you may be surprised to learn that some psychologists at the University of California, Riverside, believe that there can be a bright side to those dark days you spent worrying (“Two Definitions of Waiting Well.” Emotion 2015 Oct 12 [epub ahead of print]).
Surveying more than 200 recent law school graduates every 2 weeks during their 4-month wait for the results of the California bar exam, the researchers discovered that those who rode it out anxiously and pessimistically handled the bad news of failure “more productively.” And they welcomed the good news “more joyously” than did their peers who had “suffered little during the wait.”
While these psychologists’ findings may be of some help to aspiring lawyers or freshly minted physicians waiting to hear if they have passed their boards, I don’t think we should take them to heart when ordering lab work or imaging studies on our patients. After all, flunking the bar exam may be a life-altering event, but it isn’t a life-ending one such as learning that the biopsy you waited a week for has detected a cancer that has metastasized beyond the reaches of radiation and chemotherapy.
The bottom line is that waiting for potentially bad news is anxiety provoking regardless of whether it is for the results of a qualifying exam or a simple CBC. And, as physicians, it is our responsibility to do whatever we can to minimize that anxiety by following some simple commonsense rules of courtesy and decency.
First, we must understand that even low-risk preop screening lab work that we may view as innocuous may trigger significant anxiety in many patients. For example, a patient who knew someone whose leukemia was discovered as the result of a preop screening CBC may worry that a similar fate will be revealed by his blood test.
Second, we should ask ourselves every time we order some lab work or imaging study if it is really necessary. Are we just trying to cover our behinds and protect ourselves from a malpractice suit? Do we know what we are going to do with an equivocal borderline result? An unnecessary blood test isn’t just a waste of someone’s money and a symptom of sloppy medicine. It can be the cause of an anxiety-provoking wait for the patient.
Finally, if we are going to order a lab test, even if it is just for preop screening, it is our obligation to inform the patient of the result in a timely fashion. In my universe, that means the same day that the physician receives the result. In today’s world with its panoply of communication platforms, informing the patient can be as simple as leaving a message on a system previously approved by the patient. Obviously, bad or complicated news should be delivered directly by the physician with a phone call. Of course, informing the patient of even normal lab work results takes time, but it is the courteous and decent thing to do and signals to the patient that she has a physician who cares. If it seems like too much work, it may be that the physician is ordering too much lab work.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.”
If you have ever waited anxiously for the results of a blood test or biopsy, you may be surprised to learn that some psychologists at the University of California, Riverside, believe that there can be a bright side to those dark days you spent worrying (“Two Definitions of Waiting Well.” Emotion 2015 Oct 12 [epub ahead of print]).
Surveying more than 200 recent law school graduates every 2 weeks during their 4-month wait for the results of the California bar exam, the researchers discovered that those who rode it out anxiously and pessimistically handled the bad news of failure “more productively.” And they welcomed the good news “more joyously” than did their peers who had “suffered little during the wait.”
While these psychologists’ findings may be of some help to aspiring lawyers or freshly minted physicians waiting to hear if they have passed their boards, I don’t think we should take them to heart when ordering lab work or imaging studies on our patients. After all, flunking the bar exam may be a life-altering event, but it isn’t a life-ending one such as learning that the biopsy you waited a week for has detected a cancer that has metastasized beyond the reaches of radiation and chemotherapy.
The bottom line is that waiting for potentially bad news is anxiety provoking regardless of whether it is for the results of a qualifying exam or a simple CBC. And, as physicians, it is our responsibility to do whatever we can to minimize that anxiety by following some simple commonsense rules of courtesy and decency.
First, we must understand that even low-risk preop screening lab work that we may view as innocuous may trigger significant anxiety in many patients. For example, a patient who knew someone whose leukemia was discovered as the result of a preop screening CBC may worry that a similar fate will be revealed by his blood test.
Second, we should ask ourselves every time we order some lab work or imaging study if it is really necessary. Are we just trying to cover our behinds and protect ourselves from a malpractice suit? Do we know what we are going to do with an equivocal borderline result? An unnecessary blood test isn’t just a waste of someone’s money and a symptom of sloppy medicine. It can be the cause of an anxiety-provoking wait for the patient.
Finally, if we are going to order a lab test, even if it is just for preop screening, it is our obligation to inform the patient of the result in a timely fashion. In my universe, that means the same day that the physician receives the result. In today’s world with its panoply of communication platforms, informing the patient can be as simple as leaving a message on a system previously approved by the patient. Obviously, bad or complicated news should be delivered directly by the physician with a phone call. Of course, informing the patient of even normal lab work results takes time, but it is the courteous and decent thing to do and signals to the patient that she has a physician who cares. If it seems like too much work, it may be that the physician is ordering too much lab work.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.”
If you have ever waited anxiously for the results of a blood test or biopsy, you may be surprised to learn that some psychologists at the University of California, Riverside, believe that there can be a bright side to those dark days you spent worrying (“Two Definitions of Waiting Well.” Emotion 2015 Oct 12 [epub ahead of print]).
Surveying more than 200 recent law school graduates every 2 weeks during their 4-month wait for the results of the California bar exam, the researchers discovered that those who rode it out anxiously and pessimistically handled the bad news of failure “more productively.” And they welcomed the good news “more joyously” than did their peers who had “suffered little during the wait.”
While these psychologists’ findings may be of some help to aspiring lawyers or freshly minted physicians waiting to hear if they have passed their boards, I don’t think we should take them to heart when ordering lab work or imaging studies on our patients. After all, flunking the bar exam may be a life-altering event, but it isn’t a life-ending one such as learning that the biopsy you waited a week for has detected a cancer that has metastasized beyond the reaches of radiation and chemotherapy.
The bottom line is that waiting for potentially bad news is anxiety provoking regardless of whether it is for the results of a qualifying exam or a simple CBC. And, as physicians, it is our responsibility to do whatever we can to minimize that anxiety by following some simple commonsense rules of courtesy and decency.
First, we must understand that even low-risk preop screening lab work that we may view as innocuous may trigger significant anxiety in many patients. For example, a patient who knew someone whose leukemia was discovered as the result of a preop screening CBC may worry that a similar fate will be revealed by his blood test.
Second, we should ask ourselves every time we order some lab work or imaging study if it is really necessary. Are we just trying to cover our behinds and protect ourselves from a malpractice suit? Do we know what we are going to do with an equivocal borderline result? An unnecessary blood test isn’t just a waste of someone’s money and a symptom of sloppy medicine. It can be the cause of an anxiety-provoking wait for the patient.
Finally, if we are going to order a lab test, even if it is just for preop screening, it is our obligation to inform the patient of the result in a timely fashion. In my universe, that means the same day that the physician receives the result. In today’s world with its panoply of communication platforms, informing the patient can be as simple as leaving a message on a system previously approved by the patient. Obviously, bad or complicated news should be delivered directly by the physician with a phone call. Of course, informing the patient of even normal lab work results takes time, but it is the courteous and decent thing to do and signals to the patient that she has a physician who cares. If it seems like too much work, it may be that the physician is ordering too much lab work.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.”
AHA: One in three black Americans will experience PAD
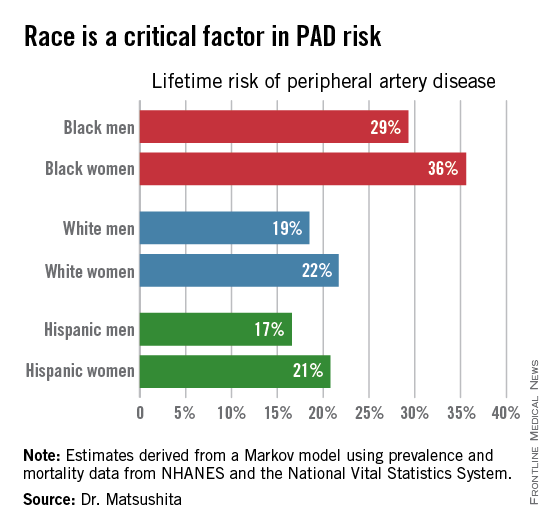
ORLANDO – One in three black Americans and one in five whites and Hispanics will develop lower extremity peripheral artery disease during their lifetime, according to the first-ever lifetime risk estimate calculated for this important manifestation of atherosclerotic vascular disease.
“Our results suggest that race is a critical factor in PAD [peripheral artery disease] risk. Current clinical guidelines recommend measuring ankle-brachial index according to age, traditional cardiovascular risk factors, and leg symptoms. Our results suggest race should also be taken into account,” Dr. Kunihiro Matsushita said at the American Heart Association scientific sessions.
This lifetime risk estimate for PAD was derived from national prevalence and mortality data from the National Health and Nutrition Examination Survey (NHANES) and the National Vital Statistics System. The analytic methods employed have previously been used to estimate lifetime risk of kidney disease and other major health issues with significant impact upon quality of life and longevity, noted Dr. Matsushita of Johns Hopkins University, Baltimore.
Over an 80-year time horizon, the projected risk of experiencing PAD was similar for men and women of the same race, but 1.5-fold higher for blacks, compared with whites or Hispanics (see chart).
An estimated 10% of black Americans will develop PAD by age 60. Among whites and Hispanics, a 10% prevalence is not reached until age 70. For individuals who don’t have PAD by age 65, their risk during the next 15 years is in the range of 28%-30% for black men and women, and 16%-18% in white or Hispanic men and women, according to Dr. Matsushita.
He declared having no financial conflicts related to this study. His work is supported by an AHA award.
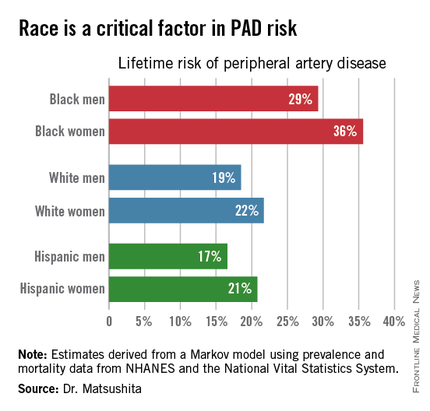
ORLANDO – One in three black Americans and one in five whites and Hispanics will develop lower extremity peripheral artery disease during their lifetime, according to the first-ever lifetime risk estimate calculated for this important manifestation of atherosclerotic vascular disease.
“Our results suggest that race is a critical factor in PAD [peripheral artery disease] risk. Current clinical guidelines recommend measuring ankle-brachial index according to age, traditional cardiovascular risk factors, and leg symptoms. Our results suggest race should also be taken into account,” Dr. Kunihiro Matsushita said at the American Heart Association scientific sessions.

This lifetime risk estimate for PAD was derived from national prevalence and mortality data from the National Health and Nutrition Examination Survey (NHANES) and the National Vital Statistics System. The analytic methods employed have previously been used to estimate lifetime risk of kidney disease and other major health issues with significant impact upon quality of life and longevity, noted Dr. Matsushita of Johns Hopkins University, Baltimore.
Over an 80-year time horizon, the projected risk of experiencing PAD was similar for men and women of the same race, but 1.5-fold higher for blacks, compared with whites or Hispanics (see chart).
An estimated 10% of black Americans will develop PAD by age 60. Among whites and Hispanics, a 10% prevalence is not reached until age 70. For individuals who don’t have PAD by age 65, their risk during the next 15 years is in the range of 28%-30% for black men and women, and 16%-18% in white or Hispanic men and women, according to Dr. Matsushita.
He declared having no financial conflicts related to this study. His work is supported by an AHA award.
ORLANDO – One in three black Americans and one in five whites and Hispanics will develop lower extremity peripheral artery disease during their lifetime, according to the first-ever lifetime risk estimate calculated for this important manifestation of atherosclerotic vascular disease.
“Our results suggest that race is a critical factor in PAD [peripheral artery disease] risk. Current clinical guidelines recommend measuring ankle-brachial index according to age, traditional cardiovascular risk factors, and leg symptoms. Our results suggest race should also be taken into account,” Dr. Kunihiro Matsushita said at the American Heart Association scientific sessions.

This lifetime risk estimate for PAD was derived from national prevalence and mortality data from the National Health and Nutrition Examination Survey (NHANES) and the National Vital Statistics System. The analytic methods employed have previously been used to estimate lifetime risk of kidney disease and other major health issues with significant impact upon quality of life and longevity, noted Dr. Matsushita of Johns Hopkins University, Baltimore.
Over an 80-year time horizon, the projected risk of experiencing PAD was similar for men and women of the same race, but 1.5-fold higher for blacks, compared with whites or Hispanics (see chart).
An estimated 10% of black Americans will develop PAD by age 60. Among whites and Hispanics, a 10% prevalence is not reached until age 70. For individuals who don’t have PAD by age 65, their risk during the next 15 years is in the range of 28%-30% for black men and women, and 16%-18% in white or Hispanic men and women, according to Dr. Matsushita.
He declared having no financial conflicts related to this study. His work is supported by an AHA award.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: The threshold for screening for peripheral artery disease should be lower in black patients.
Major finding: One in three black Americans will develop peripheral artery disease by age 80, as will one in five whites or Hispanics.
Data source: This lifetime risk estimate for peripheral artery disease was derived from a Markov model with data input on the prevalence of the disorder and its associated mortality obtained from NHANES and the National Vital Statistics System.
Disclosures: The presenter reported having no financial conflicts. His research is supported by an AHA award.
Bone can reactivate dormant MM cells, study suggests

to the tibia of a mouse
Image courtesy of the Garvan
Institute of Medical Research
Cancer cells that lie dormant in the bone can be “woken up” by changes in their surroundings, according to researchers.
The group used microscopy techniques to study multiple myeloma (MM) cells that lay “sleeping” in mouse bones.
The experiment revealed that dormant cells can be reactivated when bone tissue is broken down around them, suggesting new possibilities for
treating metastatic cancers in bone.
“Once a cancer spreads to bone, it becomes notoriously difficult to treat,” said study author Peter Croucher, PhD, of the Garvan Institute of Medical Research in Sydney, New South Wales, Australia.
“So it’s important to establish exactly what wakes those cells in bone. Is it some signal within the cells themselves, or is it a change in their environment?”
The researchers set out to discover which scenario is correct and reported their findings in Nature Communications.
Using a technique called intravital 2-photon microscopy, the team tracked the fate of dormant MM cells in the tibia of living mice.
They introduced MM cells into the mice and watched as a small number of the cells lodged in the tibia and “went to sleep.” These cells could be detected because they contained a fluorescent dye that was quickly lost from dividing cells.
“Because we were looking at a long bone like a tibia, we could watch the same sleeping cancer cells, in the same bone, in the same mouse, over a long period of time, and this is something that hasn’t been done before,” said Tri Giang Phan, PhD, of the Garvan Institute of Medical Research.
Dr Croucher said that studying the same set of cells over a period of months revealed vital clues about what caused them to reactivate.
“Because we’ve done it this way, we can show that there are a great many dormant cells, yet only some of them get woken up, and those that do wake, wake at different times,” he noted.
“We even saw some cells that woke then went back to sleep again. The fact that these myeloma cells behave so differently, despite coming from the same cancer cell line, gave us our first clue that it is a signal from outside the cells that is controlling when they wake.”
Explaining the phenomenon
The researchers’ next challenge was to determine the precise nature of the “wake-up call” from bone.
“[W]e’ve shown that bone’s dynamic process of building up and breaking down can send signals to cancer cells to stay sleeping or to wake,” said Michelle McDonald, PhD, of the Garvan Institute of Medical Research.
“We were able to show that myeloma cells are usually kept asleep by close association with a layer of osteoblast-like cells, called bone-lining cells, in the endosteum. The bone-lining cells are essentially inactive, so we can think of them as providing a quiet environment in which myeloma cells sleep undisturbed.”
“Crucially, we can wake those myeloma cells by activating osteoclasts, which break down bone tissue. We think the osteoclasts are physically changing the local environment of the cancer cells and waking them up in the process, as if they were literally throwing them out of bed.”
“We know that bone remodeling is going on in all of us. So a myeloma cell could be woken in an essentially random fashion, by having its local environment remodeled by osteoclasts. Essentially, a cancer cell could be woken by being in the wrong place at the wrong time.”
Implications for treatment
So what do these findings mean for treating secondary cancers in bone?
“Now we can see that the cancer cells are woken by changes in the surrounding bone, we can think in a whole new way about treating bone metastasis,” Dr Croucher said. “And there are 2 treatment approaches in particular that have promise.”
“The first is that we could inhibit the breakdown of bone by osteoclasts so as to keep cancer cells in long-term hibernation. In fact, there are already drugs that can do this, such as bisphosphonates, and there’s already evidence that these drugs do improve survival in breast cancer patients.”
“The other, more radical, option is to do the opposite—to wake the sleeping cells by activating osteoclasts and driving the breakdown of bone. Most cancer treatments target active, dividing cells. So waking the sleeping cells should make them susceptible to those therapies and, ultimately, could eradicate any residual disease.” ![]()

to the tibia of a mouse
Image courtesy of the Garvan
Institute of Medical Research
Cancer cells that lie dormant in the bone can be “woken up” by changes in their surroundings, according to researchers.
The group used microscopy techniques to study multiple myeloma (MM) cells that lay “sleeping” in mouse bones.
The experiment revealed that dormant cells can be reactivated when bone tissue is broken down around them, suggesting new possibilities for
treating metastatic cancers in bone.
“Once a cancer spreads to bone, it becomes notoriously difficult to treat,” said study author Peter Croucher, PhD, of the Garvan Institute of Medical Research in Sydney, New South Wales, Australia.
“So it’s important to establish exactly what wakes those cells in bone. Is it some signal within the cells themselves, or is it a change in their environment?”
The researchers set out to discover which scenario is correct and reported their findings in Nature Communications.
Using a technique called intravital 2-photon microscopy, the team tracked the fate of dormant MM cells in the tibia of living mice.
They introduced MM cells into the mice and watched as a small number of the cells lodged in the tibia and “went to sleep.” These cells could be detected because they contained a fluorescent dye that was quickly lost from dividing cells.
“Because we were looking at a long bone like a tibia, we could watch the same sleeping cancer cells, in the same bone, in the same mouse, over a long period of time, and this is something that hasn’t been done before,” said Tri Giang Phan, PhD, of the Garvan Institute of Medical Research.
Dr Croucher said that studying the same set of cells over a period of months revealed vital clues about what caused them to reactivate.
“Because we’ve done it this way, we can show that there are a great many dormant cells, yet only some of them get woken up, and those that do wake, wake at different times,” he noted.
“We even saw some cells that woke then went back to sleep again. The fact that these myeloma cells behave so differently, despite coming from the same cancer cell line, gave us our first clue that it is a signal from outside the cells that is controlling when they wake.”
Explaining the phenomenon
The researchers’ next challenge was to determine the precise nature of the “wake-up call” from bone.
“[W]e’ve shown that bone’s dynamic process of building up and breaking down can send signals to cancer cells to stay sleeping or to wake,” said Michelle McDonald, PhD, of the Garvan Institute of Medical Research.
“We were able to show that myeloma cells are usually kept asleep by close association with a layer of osteoblast-like cells, called bone-lining cells, in the endosteum. The bone-lining cells are essentially inactive, so we can think of them as providing a quiet environment in which myeloma cells sleep undisturbed.”
“Crucially, we can wake those myeloma cells by activating osteoclasts, which break down bone tissue. We think the osteoclasts are physically changing the local environment of the cancer cells and waking them up in the process, as if they were literally throwing them out of bed.”
“We know that bone remodeling is going on in all of us. So a myeloma cell could be woken in an essentially random fashion, by having its local environment remodeled by osteoclasts. Essentially, a cancer cell could be woken by being in the wrong place at the wrong time.”
Implications for treatment
So what do these findings mean for treating secondary cancers in bone?
“Now we can see that the cancer cells are woken by changes in the surrounding bone, we can think in a whole new way about treating bone metastasis,” Dr Croucher said. “And there are 2 treatment approaches in particular that have promise.”
“The first is that we could inhibit the breakdown of bone by osteoclasts so as to keep cancer cells in long-term hibernation. In fact, there are already drugs that can do this, such as bisphosphonates, and there’s already evidence that these drugs do improve survival in breast cancer patients.”
“The other, more radical, option is to do the opposite—to wake the sleeping cells by activating osteoclasts and driving the breakdown of bone. Most cancer treatments target active, dividing cells. So waking the sleeping cells should make them susceptible to those therapies and, ultimately, could eradicate any residual disease.” ![]()

to the tibia of a mouse
Image courtesy of the Garvan
Institute of Medical Research
Cancer cells that lie dormant in the bone can be “woken up” by changes in their surroundings, according to researchers.
The group used microscopy techniques to study multiple myeloma (MM) cells that lay “sleeping” in mouse bones.
The experiment revealed that dormant cells can be reactivated when bone tissue is broken down around them, suggesting new possibilities for
treating metastatic cancers in bone.
“Once a cancer spreads to bone, it becomes notoriously difficult to treat,” said study author Peter Croucher, PhD, of the Garvan Institute of Medical Research in Sydney, New South Wales, Australia.
“So it’s important to establish exactly what wakes those cells in bone. Is it some signal within the cells themselves, or is it a change in their environment?”
The researchers set out to discover which scenario is correct and reported their findings in Nature Communications.
Using a technique called intravital 2-photon microscopy, the team tracked the fate of dormant MM cells in the tibia of living mice.
They introduced MM cells into the mice and watched as a small number of the cells lodged in the tibia and “went to sleep.” These cells could be detected because they contained a fluorescent dye that was quickly lost from dividing cells.
“Because we were looking at a long bone like a tibia, we could watch the same sleeping cancer cells, in the same bone, in the same mouse, over a long period of time, and this is something that hasn’t been done before,” said Tri Giang Phan, PhD, of the Garvan Institute of Medical Research.
Dr Croucher said that studying the same set of cells over a period of months revealed vital clues about what caused them to reactivate.
“Because we’ve done it this way, we can show that there are a great many dormant cells, yet only some of them get woken up, and those that do wake, wake at different times,” he noted.
“We even saw some cells that woke then went back to sleep again. The fact that these myeloma cells behave so differently, despite coming from the same cancer cell line, gave us our first clue that it is a signal from outside the cells that is controlling when they wake.”
Explaining the phenomenon
The researchers’ next challenge was to determine the precise nature of the “wake-up call” from bone.
“[W]e’ve shown that bone’s dynamic process of building up and breaking down can send signals to cancer cells to stay sleeping or to wake,” said Michelle McDonald, PhD, of the Garvan Institute of Medical Research.
“We were able to show that myeloma cells are usually kept asleep by close association with a layer of osteoblast-like cells, called bone-lining cells, in the endosteum. The bone-lining cells are essentially inactive, so we can think of them as providing a quiet environment in which myeloma cells sleep undisturbed.”
“Crucially, we can wake those myeloma cells by activating osteoclasts, which break down bone tissue. We think the osteoclasts are physically changing the local environment of the cancer cells and waking them up in the process, as if they were literally throwing them out of bed.”
“We know that bone remodeling is going on in all of us. So a myeloma cell could be woken in an essentially random fashion, by having its local environment remodeled by osteoclasts. Essentially, a cancer cell could be woken by being in the wrong place at the wrong time.”
Implications for treatment
So what do these findings mean for treating secondary cancers in bone?
“Now we can see that the cancer cells are woken by changes in the surrounding bone, we can think in a whole new way about treating bone metastasis,” Dr Croucher said. “And there are 2 treatment approaches in particular that have promise.”
“The first is that we could inhibit the breakdown of bone by osteoclasts so as to keep cancer cells in long-term hibernation. In fact, there are already drugs that can do this, such as bisphosphonates, and there’s already evidence that these drugs do improve survival in breast cancer patients.”
“The other, more radical, option is to do the opposite—to wake the sleeping cells by activating osteoclasts and driving the breakdown of bone. Most cancer treatments target active, dividing cells. So waking the sleeping cells should make them susceptible to those therapies and, ultimately, could eradicate any residual disease.” ![]()
Antibody shows early promise for hemophilia A

Results observed in healthy subjects suggest ACE910, a factor VIIIa-mimetic bispecific antibody, may be safe and effective for patients with severe hemophilia A.
The data indicate that a weekly injection of ACE910 may prevent excessive bleeding, whereas existing hemophilia treatments require 2 to 3 injections per week.
Furthermore, ACE910 may be less likely to prompt factor VIII inhibitors, and the drug may be effective in patients who already have inhibitors.
Researchers reported the results of this phase 1 trial in Blood.
The team enrolled healthy male volunteers (ages 20 to 44), 40 of whom were Japanese and 24 of whom were Caucasian.
In part A of the study, the Japanese volunteers were randomized to receive 1 of 5 doses of ACE910 (ranging from 0.001 to 1 mg/kg) or placebo subcutaneously. There were 6 subjects per ACE910 dose group and 10 subjects who received placebo.
In part B, the Caucasian volunteers were randomized to receive 1 of 3 doses (ranging from 0.1 to 1 mg/kg) or placebo subcutaneously. There were 6 subjects in each group.
The volunteers were monitored based on their dose group, ranging from 4 weeks of observation for 0.001 mg/kg to 24 weeks for 1 mg/kg.
In all 48, subjects received ACE910. The researchers said that doses up to 1 mg/kg appeared to be safe. There were 15 adverse events (AEs) in 13 (27.1%) subjects receiving ACE910, compared to 6 AEs in 4 (25%) subjects receiving placebo.
There was 1 moderate AE (nasopharyngitis in 1 Caucasian subject receiving ACE910 at 0.1 mg/kg), but all other events were mild. There were no serious AEs or AEs that led to study withdrawal. The incidence of AEs did not differ according to dose or ethnicity.
The researchers did not observe any cases of hypercoagulability, hypersensitivity, serum cytokine concentration abnormality, or injection site reaction.
ACE910 absorbed into the plasma at a steady rate similar for both Japanese and Caucasian volunteers and remained in the blood with a half-life of 4 to 5 weeks, suggesting the drug’s therapeutic effects could be sustained with once-weekly subcutaneous dosing of ACE910.
Two subjects (1 Japanese and 1 Caucasian) were positive for anti-ACE910 antibodies. One subject was positive for antibodies before and after receiving ACE910, and the other was only positive after.
“These data are very encouraging for patients with severe hemophilia A, irrespective of the presence of factor VIII inhibitors, as ACE910 has the potential to offer the opportunity to live more normal lives without constantly planning around the next injection,” said study author Midori Shima, MD, PhD, of Nara Medical University in Kashihara, Japan.
“The first clinical investigation of this drug in hemophilia A patients with or without factor VIII inhibitors has already been implemented, and phase 3 studies are being planned to start in the near future.”
Interim results of a phase 1 study of ACE910 in hemophilia A patients (with and without inhibitors) were presented at ISTH 2015. And ACE910 was recently granted breakthrough designation from the US Food and Drug Administration. ![]()

Results observed in healthy subjects suggest ACE910, a factor VIIIa-mimetic bispecific antibody, may be safe and effective for patients with severe hemophilia A.
The data indicate that a weekly injection of ACE910 may prevent excessive bleeding, whereas existing hemophilia treatments require 2 to 3 injections per week.
Furthermore, ACE910 may be less likely to prompt factor VIII inhibitors, and the drug may be effective in patients who already have inhibitors.
Researchers reported the results of this phase 1 trial in Blood.
The team enrolled healthy male volunteers (ages 20 to 44), 40 of whom were Japanese and 24 of whom were Caucasian.
In part A of the study, the Japanese volunteers were randomized to receive 1 of 5 doses of ACE910 (ranging from 0.001 to 1 mg/kg) or placebo subcutaneously. There were 6 subjects per ACE910 dose group and 10 subjects who received placebo.
In part B, the Caucasian volunteers were randomized to receive 1 of 3 doses (ranging from 0.1 to 1 mg/kg) or placebo subcutaneously. There were 6 subjects in each group.
The volunteers were monitored based on their dose group, ranging from 4 weeks of observation for 0.001 mg/kg to 24 weeks for 1 mg/kg.
In all 48, subjects received ACE910. The researchers said that doses up to 1 mg/kg appeared to be safe. There were 15 adverse events (AEs) in 13 (27.1%) subjects receiving ACE910, compared to 6 AEs in 4 (25%) subjects receiving placebo.
There was 1 moderate AE (nasopharyngitis in 1 Caucasian subject receiving ACE910 at 0.1 mg/kg), but all other events were mild. There were no serious AEs or AEs that led to study withdrawal. The incidence of AEs did not differ according to dose or ethnicity.
The researchers did not observe any cases of hypercoagulability, hypersensitivity, serum cytokine concentration abnormality, or injection site reaction.
ACE910 absorbed into the plasma at a steady rate similar for both Japanese and Caucasian volunteers and remained in the blood with a half-life of 4 to 5 weeks, suggesting the drug’s therapeutic effects could be sustained with once-weekly subcutaneous dosing of ACE910.
Two subjects (1 Japanese and 1 Caucasian) were positive for anti-ACE910 antibodies. One subject was positive for antibodies before and after receiving ACE910, and the other was only positive after.
“These data are very encouraging for patients with severe hemophilia A, irrespective of the presence of factor VIII inhibitors, as ACE910 has the potential to offer the opportunity to live more normal lives without constantly planning around the next injection,” said study author Midori Shima, MD, PhD, of Nara Medical University in Kashihara, Japan.
“The first clinical investigation of this drug in hemophilia A patients with or without factor VIII inhibitors has already been implemented, and phase 3 studies are being planned to start in the near future.”
Interim results of a phase 1 study of ACE910 in hemophilia A patients (with and without inhibitors) were presented at ISTH 2015. And ACE910 was recently granted breakthrough designation from the US Food and Drug Administration. ![]()

Results observed in healthy subjects suggest ACE910, a factor VIIIa-mimetic bispecific antibody, may be safe and effective for patients with severe hemophilia A.
The data indicate that a weekly injection of ACE910 may prevent excessive bleeding, whereas existing hemophilia treatments require 2 to 3 injections per week.
Furthermore, ACE910 may be less likely to prompt factor VIII inhibitors, and the drug may be effective in patients who already have inhibitors.
Researchers reported the results of this phase 1 trial in Blood.
The team enrolled healthy male volunteers (ages 20 to 44), 40 of whom were Japanese and 24 of whom were Caucasian.
In part A of the study, the Japanese volunteers were randomized to receive 1 of 5 doses of ACE910 (ranging from 0.001 to 1 mg/kg) or placebo subcutaneously. There were 6 subjects per ACE910 dose group and 10 subjects who received placebo.
In part B, the Caucasian volunteers were randomized to receive 1 of 3 doses (ranging from 0.1 to 1 mg/kg) or placebo subcutaneously. There were 6 subjects in each group.
The volunteers were monitored based on their dose group, ranging from 4 weeks of observation for 0.001 mg/kg to 24 weeks for 1 mg/kg.
In all 48, subjects received ACE910. The researchers said that doses up to 1 mg/kg appeared to be safe. There were 15 adverse events (AEs) in 13 (27.1%) subjects receiving ACE910, compared to 6 AEs in 4 (25%) subjects receiving placebo.
There was 1 moderate AE (nasopharyngitis in 1 Caucasian subject receiving ACE910 at 0.1 mg/kg), but all other events were mild. There were no serious AEs or AEs that led to study withdrawal. The incidence of AEs did not differ according to dose or ethnicity.
The researchers did not observe any cases of hypercoagulability, hypersensitivity, serum cytokine concentration abnormality, or injection site reaction.
ACE910 absorbed into the plasma at a steady rate similar for both Japanese and Caucasian volunteers and remained in the blood with a half-life of 4 to 5 weeks, suggesting the drug’s therapeutic effects could be sustained with once-weekly subcutaneous dosing of ACE910.
Two subjects (1 Japanese and 1 Caucasian) were positive for anti-ACE910 antibodies. One subject was positive for antibodies before and after receiving ACE910, and the other was only positive after.
“These data are very encouraging for patients with severe hemophilia A, irrespective of the presence of factor VIII inhibitors, as ACE910 has the potential to offer the opportunity to live more normal lives without constantly planning around the next injection,” said study author Midori Shima, MD, PhD, of Nara Medical University in Kashihara, Japan.
“The first clinical investigation of this drug in hemophilia A patients with or without factor VIII inhibitors has already been implemented, and phase 3 studies are being planned to start in the near future.”
Interim results of a phase 1 study of ACE910 in hemophilia A patients (with and without inhibitors) were presented at ISTH 2015. And ACE910 was recently granted breakthrough designation from the US Food and Drug Administration. ![]()