User login
Surgical ablation endures at 5 years
The Cox-Maze IV procedure (CMPIV) has become the standard for surgical ablation for atrial fibrillation (AF), yet little information has been available on how late outcomes compare with catheter-based ablation. A recent analysis of 576 procedures found that after 5 years, most people who had the procedure remained free of atrial tachyarrhythmias and anticoagulation.
The study, by investigators from Washington University, Barnes-Jewish Hospital in St. Louis, was published in the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2015;150:1168-78). The researchers first presented the study in April at the American Association for Thoracic Surgery meeting in Seattle.
“The results of the CMPIV remain superior to those reported for catheter ablation and other forms of surgical AF ablation, especially for patients with persistent or long-standing AF,” wrote Dr. Matthew C. Henn and his colleagues.
They set out to evaluate late outcomes after CMPIV using current consensus definitions of treatment failure, noting that such outcomes had yet to be reported. They followed 576 patients with atrial fibrillation who had a CMPIV from 2002 to 2014 and compared long-term freedom from atrial fibrillation on and off antiarrhythmic drugs (AADs) across various subgroups. They included the left-sided CMPIV lesion in the analysis because, they said, it had success rates similar to those of biatrial CMPIV.
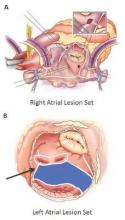
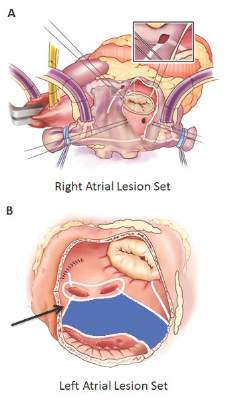
The Cox-Maze procedure was first introduced by Dr. James Cox in 1987 and updated from the original “cut-and-sew” technique in 2002 to combine bipolar radiofrequency and cryothermal ablation lines in place of most surgical incisions. This iteration was called the Cox-Maze IV procedure. In 2005, CMPIV was modified to include a superior connecting lesion, which formed a “box lesion” by completely isolating the entire posterior left atrium. The study included 512 people who underwent the “box lesion” set procedure.
“The modifications of the CMPIV have allowed it to be performed through a right minithoracotomy (RMT) approach, which has further reduced major morbidity, mortality, and hospital stay compared to those who underwent sternotomy while enjoying equivalent outcomes with regards to freedom from AF,” wrote Dr. Henn and his coauthors.
In the entire cohort, the overall freedom from atrial tachyarrhythmias (ATAs) and anticoagulation were 92% at 1 year, 88% at 2 years, 87% at 3 years, 81% at 4 years, and 73% at 5 years. Overall freedom from ATAs off antiarrhythmic drugs for the entire cohort ranged from 81% at 1 year to 61% at 5 years, and freedom from anticoagulation ranged from 65% at 1 year to 55% at 5 years.
“Freedoms from ATAs on or off AADs were significantly higher in those who underwent box lesion sets when compared to those who did not at 5 years,” noted Dr. Henn and his coauthors. Among the box lesion set group, 78% of those on AADs remained free of ATAs vs. 45% in the non–box lesion set group, and for those off AADs, 66% had no ATAs at 5 years while 33% of the non–box lesion set group did.
Of the overall study population, 41% had paroxysmal AF and 58% had nonparoxysmal AF. Among the latter group, 20% had persistent and 80% had long-standing persistent AF. The nonparoxysmal AF group had a longer duration of preoperative AF, larger left atria and more failed catheter ablations, Dr. Henn and coauthors reported. But, the study showed no differences in freedom from atrial fibrillation on or off AADs at 5 years between patients with paroxysmal AF or persistent/long-standing persistent AF, or between those who underwent stand-alone procedure and those who received a concomitant Cox-Maze procedure. Among those who had a concomitant procedure, 50% had a concomitant mitral valve procedure and 23% had coronary artery bypass grafting.
“The CMPIV results in our series were better than what has been achieved with catheter ablation,” the researchers wrote. They cited studies that showed arrhythmia-free survival after a single ablation procedure ranging from 17% to 29% and “equally poor results.” (Circ Arrhythm Electrophysiol. 2015;8:18-24; J Am Coll Cardiol. 2011;57:160-166; J Am Heart Assoc. 2013;2:e004549.)
“The CMPIV remains the most successful surgical treatment for AF, even in patients with non-paroxysmal AF and regardless of the complexity of the concomitant procedures,” Dr. Henn and his coauthors concluded.
Inconsistencies in this study of the Cox-Maze IV procedure include differing types of atrial fibrillation, heterogeneous concomitant operations, multiple lesion sets and energy sources and inconsistent postablation monitoring, all of which make direct comparisons of surgical ablation strategies or even catheter ablation difficult, Dr. Robert Hawkins and Dr. Gorav Ailawadi of the University of Virginia noted in their invited commentary (J Thorac Cardiovasc Surg. 2015;150:1179-80). “Moreover, without controls or selection criteria, it is difficult to account for selection bias,” they wrote.
Yet, this study has “some important findings” despite its shortcomings, namely the “respectable” rates of atrial tachyarrhythmias off antiarrhythmic drugs. These results are superior to other clinical trials, “in part due to the expertise at Washington University,” noted Dr. Hawkins and Dr. Ailawadi.
Adding patients who had the box lesion set approach also improved 5-year outcomes in the study substantially, and left atrium (LA) ablation alone has good results in patients with paroxysmal AF, left atria less than 5.0 cm, and no right atrial enlargement. “Yet, a direct comparison between biatrial and LA lesion sets cannot be made due to the above listed limitations,” they wrote.
The study makes a case for surgical ablation when the preoperative duration of AF is less than 5-10 years and left atrium size is not a problem, and the lesion-set requires further investigation, they said. “Finally, this study highlights the continued need for rigorous monitoring and comparisons of homogeneous patient populations to make stronger conclusions.”
Dr. Ailawadi disclosed relationships with Abbot Vascular, Mitralign, Edwards Lifesciences and St. Jude Medical. Dr. Hawkins had no relationships to disclose.
Inconsistencies in this study of the Cox-Maze IV procedure include differing types of atrial fibrillation, heterogeneous concomitant operations, multiple lesion sets and energy sources and inconsistent postablation monitoring, all of which make direct comparisons of surgical ablation strategies or even catheter ablation difficult, Dr. Robert Hawkins and Dr. Gorav Ailawadi of the University of Virginia noted in their invited commentary (J Thorac Cardiovasc Surg. 2015;150:1179-80). “Moreover, without controls or selection criteria, it is difficult to account for selection bias,” they wrote.
Yet, this study has “some important findings” despite its shortcomings, namely the “respectable” rates of atrial tachyarrhythmias off antiarrhythmic drugs. These results are superior to other clinical trials, “in part due to the expertise at Washington University,” noted Dr. Hawkins and Dr. Ailawadi.
Adding patients who had the box lesion set approach also improved 5-year outcomes in the study substantially, and left atrium (LA) ablation alone has good results in patients with paroxysmal AF, left atria less than 5.0 cm, and no right atrial enlargement. “Yet, a direct comparison between biatrial and LA lesion sets cannot be made due to the above listed limitations,” they wrote.
The study makes a case for surgical ablation when the preoperative duration of AF is less than 5-10 years and left atrium size is not a problem, and the lesion-set requires further investigation, they said. “Finally, this study highlights the continued need for rigorous monitoring and comparisons of homogeneous patient populations to make stronger conclusions.”
Dr. Ailawadi disclosed relationships with Abbot Vascular, Mitralign, Edwards Lifesciences and St. Jude Medical. Dr. Hawkins had no relationships to disclose.
Inconsistencies in this study of the Cox-Maze IV procedure include differing types of atrial fibrillation, heterogeneous concomitant operations, multiple lesion sets and energy sources and inconsistent postablation monitoring, all of which make direct comparisons of surgical ablation strategies or even catheter ablation difficult, Dr. Robert Hawkins and Dr. Gorav Ailawadi of the University of Virginia noted in their invited commentary (J Thorac Cardiovasc Surg. 2015;150:1179-80). “Moreover, without controls or selection criteria, it is difficult to account for selection bias,” they wrote.
Yet, this study has “some important findings” despite its shortcomings, namely the “respectable” rates of atrial tachyarrhythmias off antiarrhythmic drugs. These results are superior to other clinical trials, “in part due to the expertise at Washington University,” noted Dr. Hawkins and Dr. Ailawadi.
Adding patients who had the box lesion set approach also improved 5-year outcomes in the study substantially, and left atrium (LA) ablation alone has good results in patients with paroxysmal AF, left atria less than 5.0 cm, and no right atrial enlargement. “Yet, a direct comparison between biatrial and LA lesion sets cannot be made due to the above listed limitations,” they wrote.
The study makes a case for surgical ablation when the preoperative duration of AF is less than 5-10 years and left atrium size is not a problem, and the lesion-set requires further investigation, they said. “Finally, this study highlights the continued need for rigorous monitoring and comparisons of homogeneous patient populations to make stronger conclusions.”
Dr. Ailawadi disclosed relationships with Abbot Vascular, Mitralign, Edwards Lifesciences and St. Jude Medical. Dr. Hawkins had no relationships to disclose.
The Cox-Maze IV procedure (CMPIV) has become the standard for surgical ablation for atrial fibrillation (AF), yet little information has been available on how late outcomes compare with catheter-based ablation. A recent analysis of 576 procedures found that after 5 years, most people who had the procedure remained free of atrial tachyarrhythmias and anticoagulation.
The study, by investigators from Washington University, Barnes-Jewish Hospital in St. Louis, was published in the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2015;150:1168-78). The researchers first presented the study in April at the American Association for Thoracic Surgery meeting in Seattle.
“The results of the CMPIV remain superior to those reported for catheter ablation and other forms of surgical AF ablation, especially for patients with persistent or long-standing AF,” wrote Dr. Matthew C. Henn and his colleagues.
They set out to evaluate late outcomes after CMPIV using current consensus definitions of treatment failure, noting that such outcomes had yet to be reported. They followed 576 patients with atrial fibrillation who had a CMPIV from 2002 to 2014 and compared long-term freedom from atrial fibrillation on and off antiarrhythmic drugs (AADs) across various subgroups. They included the left-sided CMPIV lesion in the analysis because, they said, it had success rates similar to those of biatrial CMPIV.
The Cox-Maze procedure was first introduced by Dr. James Cox in 1987 and updated from the original “cut-and-sew” technique in 2002 to combine bipolar radiofrequency and cryothermal ablation lines in place of most surgical incisions. This iteration was called the Cox-Maze IV procedure. In 2005, CMPIV was modified to include a superior connecting lesion, which formed a “box lesion” by completely isolating the entire posterior left atrium. The study included 512 people who underwent the “box lesion” set procedure.
“The modifications of the CMPIV have allowed it to be performed through a right minithoracotomy (RMT) approach, which has further reduced major morbidity, mortality, and hospital stay compared to those who underwent sternotomy while enjoying equivalent outcomes with regards to freedom from AF,” wrote Dr. Henn and his coauthors.
In the entire cohort, the overall freedom from atrial tachyarrhythmias (ATAs) and anticoagulation were 92% at 1 year, 88% at 2 years, 87% at 3 years, 81% at 4 years, and 73% at 5 years. Overall freedom from ATAs off antiarrhythmic drugs for the entire cohort ranged from 81% at 1 year to 61% at 5 years, and freedom from anticoagulation ranged from 65% at 1 year to 55% at 5 years.
“Freedoms from ATAs on or off AADs were significantly higher in those who underwent box lesion sets when compared to those who did not at 5 years,” noted Dr. Henn and his coauthors. Among the box lesion set group, 78% of those on AADs remained free of ATAs vs. 45% in the non–box lesion set group, and for those off AADs, 66% had no ATAs at 5 years while 33% of the non–box lesion set group did.
Of the overall study population, 41% had paroxysmal AF and 58% had nonparoxysmal AF. Among the latter group, 20% had persistent and 80% had long-standing persistent AF. The nonparoxysmal AF group had a longer duration of preoperative AF, larger left atria and more failed catheter ablations, Dr. Henn and coauthors reported. But, the study showed no differences in freedom from atrial fibrillation on or off AADs at 5 years between patients with paroxysmal AF or persistent/long-standing persistent AF, or between those who underwent stand-alone procedure and those who received a concomitant Cox-Maze procedure. Among those who had a concomitant procedure, 50% had a concomitant mitral valve procedure and 23% had coronary artery bypass grafting.
“The CMPIV results in our series were better than what has been achieved with catheter ablation,” the researchers wrote. They cited studies that showed arrhythmia-free survival after a single ablation procedure ranging from 17% to 29% and “equally poor results.” (Circ Arrhythm Electrophysiol. 2015;8:18-24; J Am Coll Cardiol. 2011;57:160-166; J Am Heart Assoc. 2013;2:e004549.)
“The CMPIV remains the most successful surgical treatment for AF, even in patients with non-paroxysmal AF and regardless of the complexity of the concomitant procedures,” Dr. Henn and his coauthors concluded.
The Cox-Maze IV procedure (CMPIV) has become the standard for surgical ablation for atrial fibrillation (AF), yet little information has been available on how late outcomes compare with catheter-based ablation. A recent analysis of 576 procedures found that after 5 years, most people who had the procedure remained free of atrial tachyarrhythmias and anticoagulation.
The study, by investigators from Washington University, Barnes-Jewish Hospital in St. Louis, was published in the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2015;150:1168-78). The researchers first presented the study in April at the American Association for Thoracic Surgery meeting in Seattle.
“The results of the CMPIV remain superior to those reported for catheter ablation and other forms of surgical AF ablation, especially for patients with persistent or long-standing AF,” wrote Dr. Matthew C. Henn and his colleagues.
They set out to evaluate late outcomes after CMPIV using current consensus definitions of treatment failure, noting that such outcomes had yet to be reported. They followed 576 patients with atrial fibrillation who had a CMPIV from 2002 to 2014 and compared long-term freedom from atrial fibrillation on and off antiarrhythmic drugs (AADs) across various subgroups. They included the left-sided CMPIV lesion in the analysis because, they said, it had success rates similar to those of biatrial CMPIV.
The Cox-Maze procedure was first introduced by Dr. James Cox in 1987 and updated from the original “cut-and-sew” technique in 2002 to combine bipolar radiofrequency and cryothermal ablation lines in place of most surgical incisions. This iteration was called the Cox-Maze IV procedure. In 2005, CMPIV was modified to include a superior connecting lesion, which formed a “box lesion” by completely isolating the entire posterior left atrium. The study included 512 people who underwent the “box lesion” set procedure.
“The modifications of the CMPIV have allowed it to be performed through a right minithoracotomy (RMT) approach, which has further reduced major morbidity, mortality, and hospital stay compared to those who underwent sternotomy while enjoying equivalent outcomes with regards to freedom from AF,” wrote Dr. Henn and his coauthors.
In the entire cohort, the overall freedom from atrial tachyarrhythmias (ATAs) and anticoagulation were 92% at 1 year, 88% at 2 years, 87% at 3 years, 81% at 4 years, and 73% at 5 years. Overall freedom from ATAs off antiarrhythmic drugs for the entire cohort ranged from 81% at 1 year to 61% at 5 years, and freedom from anticoagulation ranged from 65% at 1 year to 55% at 5 years.
“Freedoms from ATAs on or off AADs were significantly higher in those who underwent box lesion sets when compared to those who did not at 5 years,” noted Dr. Henn and his coauthors. Among the box lesion set group, 78% of those on AADs remained free of ATAs vs. 45% in the non–box lesion set group, and for those off AADs, 66% had no ATAs at 5 years while 33% of the non–box lesion set group did.
Of the overall study population, 41% had paroxysmal AF and 58% had nonparoxysmal AF. Among the latter group, 20% had persistent and 80% had long-standing persistent AF. The nonparoxysmal AF group had a longer duration of preoperative AF, larger left atria and more failed catheter ablations, Dr. Henn and coauthors reported. But, the study showed no differences in freedom from atrial fibrillation on or off AADs at 5 years between patients with paroxysmal AF or persistent/long-standing persistent AF, or between those who underwent stand-alone procedure and those who received a concomitant Cox-Maze procedure. Among those who had a concomitant procedure, 50% had a concomitant mitral valve procedure and 23% had coronary artery bypass grafting.
“The CMPIV results in our series were better than what has been achieved with catheter ablation,” the researchers wrote. They cited studies that showed arrhythmia-free survival after a single ablation procedure ranging from 17% to 29% and “equally poor results.” (Circ Arrhythm Electrophysiol. 2015;8:18-24; J Am Coll Cardiol. 2011;57:160-166; J Am Heart Assoc. 2013;2:e004549.)
“The CMPIV remains the most successful surgical treatment for AF, even in patients with non-paroxysmal AF and regardless of the complexity of the concomitant procedures,” Dr. Henn and his coauthors concluded.
Key clinical point: Outcomes with the Cox-Maze IV procedure for surgical ablation are superior to catheter ablation and other forms of surgical ablation for atrial fibrillation for up to 5 years duration.
Major finding: Seventy-three percent of the study population was free from atrial tachyarrhythmias and 55% were free from anticoagulation at 5 years.
Data source: Prospective analysis of 576 consecutive patients with atrial fibrillation who had Cox-Maze IV procedure or a left-sized Cox-Maze IV procedure from 2002 to 2014 at a single institution
Disclosures: The National Institutes of Health provided grants for the study. Coauthor Dr. Ralph J. Damiano Jr. disclosed research grants and educational funding from AtriCure and Edwards LifeSciences. The other authors had no disclosures.
Standing with our patients
One-half of my practice is taking care of employees and dependents employed by the organization for which I work. Most of these patients sit … a lot … and present to me with musculoskeletal pain and weight concerns. My patients have a high degree of health literacy and are fully aware that 6 hours of sitting might have at least something to do with these problems.
We currently seem to be on the other side of the “walk station” mania. Sanity has been restored through a combination of concerns about medical liability for work-related treadmill injuries, expense, space issues, and reports that walkers were more forgetful and less focused. The last study resulted in some personal email for my own indulgences in walking while researching.
But let us not throw out an upright posture with the treadmill. Sitters have an increased risk for elevated blood sugars, cardiovascular disease, cancer, and death. Standers have been suggested to burn 50 more calories per hour. Some experts recommend that people should stand for at least 2 hours each day, and 4 hours is even better.
Dr. Graves and colleagues conducted a randomized controlled trial to evaluate the impact of a sit-stand workstation on sitting time, vascular, metabolic, and musculoskeletal outcomes and to investigate workstation acceptability and feasibility. Forty-seven participants without any bodily symptoms were randomized to either a sit-stand workstation or no intervention for 8 weeks. The sit-stand workstation was associated with decreased sit time (80 minutes per 8-hour work day), increased standing time (73 minutes per 8-hour work day), and a decrease in total cholesterol. No increase in musculoskeletal pain was observed with a suggestion of possible benefit in the neck and upper back (BMC Public Health. 2015;15:1145. doi 10.1186/s12889-015-2469-8).
Each of the devices cost about $550 to install for a single monitor ($20 more for a dual monitor). The intervention was only 8 weeks in duration and stronger effects in musculoskeletal and cardiovascular risk markers might be seen with longer durations of study. The qualitative work in this study suggested that several factors may influence use of a sit-stand desk such as social environment (for example, other colleagues not using it may decrease use), work tasks (for example, paperwork made difficult by limited elevated work surface), and design (for example, keyboard surface bounces too much). From personal experience, the sit-stand desk is ideal if the vast majority of work is on the computer. I’d also like to say I was standing when I wrote this. But I wasn’t. And I wasn’t walking either because I can’t remember where that desk is.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter @jonebbert.
One-half of my practice is taking care of employees and dependents employed by the organization for which I work. Most of these patients sit … a lot … and present to me with musculoskeletal pain and weight concerns. My patients have a high degree of health literacy and are fully aware that 6 hours of sitting might have at least something to do with these problems.
We currently seem to be on the other side of the “walk station” mania. Sanity has been restored through a combination of concerns about medical liability for work-related treadmill injuries, expense, space issues, and reports that walkers were more forgetful and less focused. The last study resulted in some personal email for my own indulgences in walking while researching.
But let us not throw out an upright posture with the treadmill. Sitters have an increased risk for elevated blood sugars, cardiovascular disease, cancer, and death. Standers have been suggested to burn 50 more calories per hour. Some experts recommend that people should stand for at least 2 hours each day, and 4 hours is even better.
Dr. Graves and colleagues conducted a randomized controlled trial to evaluate the impact of a sit-stand workstation on sitting time, vascular, metabolic, and musculoskeletal outcomes and to investigate workstation acceptability and feasibility. Forty-seven participants without any bodily symptoms were randomized to either a sit-stand workstation or no intervention for 8 weeks. The sit-stand workstation was associated with decreased sit time (80 minutes per 8-hour work day), increased standing time (73 minutes per 8-hour work day), and a decrease in total cholesterol. No increase in musculoskeletal pain was observed with a suggestion of possible benefit in the neck and upper back (BMC Public Health. 2015;15:1145. doi 10.1186/s12889-015-2469-8).
Each of the devices cost about $550 to install for a single monitor ($20 more for a dual monitor). The intervention was only 8 weeks in duration and stronger effects in musculoskeletal and cardiovascular risk markers might be seen with longer durations of study. The qualitative work in this study suggested that several factors may influence use of a sit-stand desk such as social environment (for example, other colleagues not using it may decrease use), work tasks (for example, paperwork made difficult by limited elevated work surface), and design (for example, keyboard surface bounces too much). From personal experience, the sit-stand desk is ideal if the vast majority of work is on the computer. I’d also like to say I was standing when I wrote this. But I wasn’t. And I wasn’t walking either because I can’t remember where that desk is.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter @jonebbert.
One-half of my practice is taking care of employees and dependents employed by the organization for which I work. Most of these patients sit … a lot … and present to me with musculoskeletal pain and weight concerns. My patients have a high degree of health literacy and are fully aware that 6 hours of sitting might have at least something to do with these problems.
We currently seem to be on the other side of the “walk station” mania. Sanity has been restored through a combination of concerns about medical liability for work-related treadmill injuries, expense, space issues, and reports that walkers were more forgetful and less focused. The last study resulted in some personal email for my own indulgences in walking while researching.
But let us not throw out an upright posture with the treadmill. Sitters have an increased risk for elevated blood sugars, cardiovascular disease, cancer, and death. Standers have been suggested to burn 50 more calories per hour. Some experts recommend that people should stand for at least 2 hours each day, and 4 hours is even better.
Dr. Graves and colleagues conducted a randomized controlled trial to evaluate the impact of a sit-stand workstation on sitting time, vascular, metabolic, and musculoskeletal outcomes and to investigate workstation acceptability and feasibility. Forty-seven participants without any bodily symptoms were randomized to either a sit-stand workstation or no intervention for 8 weeks. The sit-stand workstation was associated with decreased sit time (80 minutes per 8-hour work day), increased standing time (73 minutes per 8-hour work day), and a decrease in total cholesterol. No increase in musculoskeletal pain was observed with a suggestion of possible benefit in the neck and upper back (BMC Public Health. 2015;15:1145. doi 10.1186/s12889-015-2469-8).
Each of the devices cost about $550 to install for a single monitor ($20 more for a dual monitor). The intervention was only 8 weeks in duration and stronger effects in musculoskeletal and cardiovascular risk markers might be seen with longer durations of study. The qualitative work in this study suggested that several factors may influence use of a sit-stand desk such as social environment (for example, other colleagues not using it may decrease use), work tasks (for example, paperwork made difficult by limited elevated work surface), and design (for example, keyboard surface bounces too much). From personal experience, the sit-stand desk is ideal if the vast majority of work is on the computer. I’d also like to say I was standing when I wrote this. But I wasn’t. And I wasn’t walking either because I can’t remember where that desk is.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter @jonebbert.
Study: Hospitalists Can Drive Quality Improvement, Cut Costs
A quality improvement (QI) initiative can start with a single hospitalist, says Adam H. Corson, MD, a hospitalist at Seattle’s Swedish Medical Center.
In a study presented at SHM's annual meeting, Dr. Corson set out to determine whether critically evaluating how frequently common lab tests were ordered could help decrease hospital costs. Using a cohort of patients, Dr. Corson compared how often a complete blood count or a metabolic panel was ordered in a large hospitalist group. His QI intervention involved academic detailing, audit, and feedback, as well as transparent reporting of lab orders for 9,368 patients. At baseline, a mean of 2.06 common labs were ordered per patient day. The number of labs ordered post-intervention decreased by 10%.
“Within the hospitalist team itself, there was a 20% reduction,” Dr. Corson says. That percentage “got diluted down to 10% when you included all the other providers who care for a patient.” He found no adverse effects from this intervention on mortality, length-of-stay, or readmission rates. His report also cited a reduction in the volume of blood transfused per patient who received a transfusion and a $16.19 decrease in hospital costs—a total of $159,682—per admission annualized for the cohort.
Although better patient care was his main goal, a secondary goal was to demonstrate the potential value of hospitalists in today’s changing medical environment, particularly in terms of reimbursement. “In a fee-for-service world, hospitalists can’t participate as much as [physicians in] other areas of medicine,” he says. “But in a fee-for-outcome world, hospitalists can play a big role, and this is a demonstration of that.”
He also points out that this cost-effective intervention was basically done by him alone, “one hospitalist with access to electronic medical records and someone to pull some data out of there.”
Dr. Corson says he hopes his study will inspire other providers to look at this specific topic in their own practice and possibly expand it to other services they order each day. “The big headline these days is the United States spends more money on healthcare than everyone else does, but we don’t get better results,” he says. “Inherent in that is the idea that we do a lot of stuff that doesn’t need to be done or has no physical value. This is a small example of that.” TH
Visit our website for more information on other cost-cutting measures hospitalists can adopt.
A quality improvement (QI) initiative can start with a single hospitalist, says Adam H. Corson, MD, a hospitalist at Seattle’s Swedish Medical Center.
In a study presented at SHM's annual meeting, Dr. Corson set out to determine whether critically evaluating how frequently common lab tests were ordered could help decrease hospital costs. Using a cohort of patients, Dr. Corson compared how often a complete blood count or a metabolic panel was ordered in a large hospitalist group. His QI intervention involved academic detailing, audit, and feedback, as well as transparent reporting of lab orders for 9,368 patients. At baseline, a mean of 2.06 common labs were ordered per patient day. The number of labs ordered post-intervention decreased by 10%.
“Within the hospitalist team itself, there was a 20% reduction,” Dr. Corson says. That percentage “got diluted down to 10% when you included all the other providers who care for a patient.” He found no adverse effects from this intervention on mortality, length-of-stay, or readmission rates. His report also cited a reduction in the volume of blood transfused per patient who received a transfusion and a $16.19 decrease in hospital costs—a total of $159,682—per admission annualized for the cohort.
Although better patient care was his main goal, a secondary goal was to demonstrate the potential value of hospitalists in today’s changing medical environment, particularly in terms of reimbursement. “In a fee-for-service world, hospitalists can’t participate as much as [physicians in] other areas of medicine,” he says. “But in a fee-for-outcome world, hospitalists can play a big role, and this is a demonstration of that.”
He also points out that this cost-effective intervention was basically done by him alone, “one hospitalist with access to electronic medical records and someone to pull some data out of there.”
Dr. Corson says he hopes his study will inspire other providers to look at this specific topic in their own practice and possibly expand it to other services they order each day. “The big headline these days is the United States spends more money on healthcare than everyone else does, but we don’t get better results,” he says. “Inherent in that is the idea that we do a lot of stuff that doesn’t need to be done or has no physical value. This is a small example of that.” TH
Visit our website for more information on other cost-cutting measures hospitalists can adopt.
A quality improvement (QI) initiative can start with a single hospitalist, says Adam H. Corson, MD, a hospitalist at Seattle’s Swedish Medical Center.
In a study presented at SHM's annual meeting, Dr. Corson set out to determine whether critically evaluating how frequently common lab tests were ordered could help decrease hospital costs. Using a cohort of patients, Dr. Corson compared how often a complete blood count or a metabolic panel was ordered in a large hospitalist group. His QI intervention involved academic detailing, audit, and feedback, as well as transparent reporting of lab orders for 9,368 patients. At baseline, a mean of 2.06 common labs were ordered per patient day. The number of labs ordered post-intervention decreased by 10%.
“Within the hospitalist team itself, there was a 20% reduction,” Dr. Corson says. That percentage “got diluted down to 10% when you included all the other providers who care for a patient.” He found no adverse effects from this intervention on mortality, length-of-stay, or readmission rates. His report also cited a reduction in the volume of blood transfused per patient who received a transfusion and a $16.19 decrease in hospital costs—a total of $159,682—per admission annualized for the cohort.
Although better patient care was his main goal, a secondary goal was to demonstrate the potential value of hospitalists in today’s changing medical environment, particularly in terms of reimbursement. “In a fee-for-service world, hospitalists can’t participate as much as [physicians in] other areas of medicine,” he says. “But in a fee-for-outcome world, hospitalists can play a big role, and this is a demonstration of that.”
He also points out that this cost-effective intervention was basically done by him alone, “one hospitalist with access to electronic medical records and someone to pull some data out of there.”
Dr. Corson says he hopes his study will inspire other providers to look at this specific topic in their own practice and possibly expand it to other services they order each day. “The big headline these days is the United States spends more money on healthcare than everyone else does, but we don’t get better results,” he says. “Inherent in that is the idea that we do a lot of stuff that doesn’t need to be done or has no physical value. This is a small example of that.” TH
Visit our website for more information on other cost-cutting measures hospitalists can adopt.
New and Noteworthy Information—December 2015
The risk of epilepsy is increased in children with hospital-diagnosed pertussis infections, compared with the general population, but the absolute risk is low, according to a Danish study published in the November 3 issue of JAMA. Researchers used data from population-based medical registries covering all Danish hospitals to identify all patients with pertussis born between 1978 and 2011 and followed up through 2011. Investigators used the Civil Registration System to identify 10 individuals from the general population for each patient with pertussis, matched on sex and year of birth. They identified 4,700 patients with pertussis, of whom 90 developed epilepsy during the follow-up. The cumulative incidence of epilepsy at age 10 was 1.7% for patients with pertussis and 0.9% for the matched comparison cohort.
Chronic users of antiepileptic drugs have poorer standing balance, compared with nonusers, according to a longitudinal twin and sibling study published in the November issue of Epilepsia. Researchers studied 26 twin and sibling pairs. Siblings were of the same gender, but only one in each pair had exposure to antiepileptic drugs. Clinical and laboratory balance examinations were conducted twice, and at least one year elapsed between examinations. The mean within-pair differences in balance measures were calculated cross-sectionally at baseline and follow-up, and longitudinally. Researchers found no significant mean within-pair difference at baseline in age (mean, 44), weight, and height. Cross-sectional sway measures from posturography and clinical static balance tests showed poorer performance in antiepileptic drug users, compared with nonusers, on several test conditions at baseline and follow-up.
Treatment for symptomatic intracerebral hemorrhage (sICH) after thrombolysis for stroke does not significantly reduce the likelihood of in-hospital mortality or hematoma expansion, according to a retrospective study published online ahead of print October 26 in JAMA Neurology. Of 3,894 patients treated with IV rt-PA within 4.5 hours after onset of ischemic stroke symptoms, 128 had sICH. The median time from initiation of rt-PA to sICH diagnosis was 470 minutes, and the median time from diagnosis to treatment of sICH was 112 minutes. The in-hospital mortality rate was 52.3%, and 26.8% of participants had hematoma expansion. In multivariable models, code status change to comfort measures after sICH diagnosis was the sole factor associated with increased in-hospital mortality. Severe hypofibrinogenemia was associated with hematoma expansion and occurred in 36.3% of patients without hematoma expansion.
Gaucher disease or mutations in the β-glucocerebrosidase gene (GBA) may protect individuals from deficiency in visual color discrimination, according to a study published September 14 in Journal of Parkinson’s Disease. Investigators tested groups of patients on the Farnsworth-Munsell 100 hue test (FMHT) and calculated their mean Total Error Scores (TES). Patients were classified as having Parkinson’s disease only, Gaucher disease only, Parkinson’s disease and Gaucher disease, GBA mutations, GBA mutations and Parkinson’s disease, or as controls. Patients with Parkinson’s disease only had the highest mean TES, and patients with Gaucher disease only had the lowest mean TES. GBA carriers without Parkinson’s disease made more errors than patients with Gaucher disease only, which was approximately the same number of errors as healthy controls.
Brain scans of people in a coma may help predict who will regain consciousness, according to a study published online ahead of print November 11 in Neurology. Researchers compared 27 prospectively recruited comatose patients who had severe brain injury (14 with traumatic injury and 13 with anoxic injury) with 14 age-matched healthy participants. Standardized clinical assessment and functional MRI were performed at an average of four days after withdrawal of sedation. Patients who were comatose showed a significant disruption of functional connectivity of brain areas spontaneously synchronized with posterior cingulate cortex, regardless of etiology of injury. The functional connectivity strength between the posterior cingulate cortex and the medial prefrontal cortex was significantly different between comatose patients who subsequently recovered and those who subsequently scored an unfavorable outcome three months after brain injury.
Raloxifene does not have a significant cognitive effect for women with Alzheimer’s disease, according to a study published online ahead of print November 4 in Neurology. Investigators conducted a randomized, double-blind, placebo-controlled pilot study with a planned treatment period of 12 months. Women with mild to moderate late-onset Alzheimer’s disease were randomized to high-dose (ie, 120 mg) oral raloxifene or identical placebo provided once daily. Forty-two women randomized to raloxifene or placebo were included in intent-to-treat analyses, and 39 women contributed 12-month outcomes. Results on the Alzheimer’s Disease Assessment Scale, cognitive subscale showed no cognitive benefits in the raloxifene-treated group. Raloxifene and placebo groups did not differ significantly on secondary analyses of dementia rating, activities of daily living, behavior, or a global cognition composite score.
Nonpharmacologic sleep interventions may help optimize outcomes in patients with chronic pain, according to data published in the November issue of Sleep. Investigators analyzed 11 randomized controlled trials, involving 1,066 participants, that evaluated the effect of nonpharmacologic sleep treatments on self-reported sleep quality, pain, and well-being in patients with long-term pain. They extracted means and standard deviations of sleep quality, pain, fatigue, depression, anxiety, and physical and psychologic functioning for the treatment and control groups at baseline, post treatment, and final follow-up. Nonpharmacologic sleep treatments in patients with chronic pain were associated with a large improvement in sleep quality, a small reduction in pain, and moderate improvement in fatigue at post treatment. The effects on sleep quality and fatigue were maintained for as long as one year, when a moderate reduction in depression also was observed.
CSF biomarkers of angiogenesis are increased in Parkinson’s disease and associated with gait difficulties, blood–brain barrier dysfunction, white matter lesions, and cerebral microbleeds, according to a study published online ahead of print October 28 in Neurology. This cross-sectional analysis included 38 elderly controls and 100 patients with Parkinson’s disease. Patients with Parkinson’s disease without dementia displayed higher CSF levels of vascular endothelial growth factor, placental growth factor, and vascular endothelial growth factor 2, and lower levels of angiopoietin 2, compared with controls. Similar alterations in vascular endothelial growth factor, placental growth factor, and angiopoietin 2 levels were observed in patients with Parkinson’s disease with dementia. Abnormal angiogenesis may be important in Parkinson’s disease pathogenesis and contribute to dopa-resistant symptoms, said the researchers.
Despite comparable reductions in total sleep time, partial sleep loss from sleep continuity disruption is more detrimental to positive mood than partial sleep loss from delaying bedtime, even when controlling for concomitant increases in negative mood, according to a study published in the November issue of Sleep. Participants were randomized to receive three consecutive nights of sleep continuity disruption by forced nocturnal awakenings, or one of the following two control conditions: restricted sleep opportunity or uninterrupted sleep. Compared with controls with restricted sleep opportunity, participants who underwent forced awakenings had significantly less slow wave sleep after the first night of sleep deprivation, and significantly lower positive mood after the second night of sleep deprivation. The differential change in slow wave sleep statistically mediated the observed group differences in positive mood.
Among patients who underwent transcatheter atrial septal defect closure (ASD), the use of clopidogrel and aspirin, compared with aspirin plus placebo, resulted in a lower monthly frequency of migraine attacks over three months, according to a study published online ahead of print November 9 in JAMA. A total of 171 patients without migraine were randomized to receive dual antiplatelet therapy or single antiplatelet therapy (ie, aspirin and placebo) for three months following transcatheter ASD closure. The mean age of the participants was 49, and 62% were women. Among patients with migraines following the procedure, those who received clopidogrel had less-severe migraine attacks. No patients who received clopidogrel had moderately or severely disabling migraine attacks, and 37% of the placebo group had such attacks.
The Consortium of Multiple Sclerosis Centers (CMSC) has issued a statement asserting that prescribers must retain the right to decide on the best treatment and medication for each patient with MS. “The varied and individualized course of MS mandates full access to symptomatic management as well as disease-modifying therapies, which, in the best judgment of the prescriber, offer optimal treatment outcomes. Medications to treat symptoms are carefully decided on an individual basis and by best-practice regimens,” said the CMSC. “Lack of understanding of the disease course and the challenges of MS treatment result in poor decision making practices by the insurance plans and specialty pharmacies and subsequent denial of prescribed medications.... CMSC proposes a collaborative care model in which providers, patients, and insurers work together to address these concerns.”
APOE4 greatly increases the likelihood of microbleeds in some men, according to a study published online ahead of print October 16 in Neurobiology of Aging. These microbleeds contribute to memory loss. Investigators examined brain scans of 658 participants (ages 48 to 91) in the Alzheimer’s Disease Neuroimaging Initiative. Of those subjects, 402 had mild cognitive impairment (MCI), 90 had early-stage Alzheimer’s disease, and 166 were cognitively normal. Researchers also analyzed scans of 448 other subjects (ages 36 to 88). Of those people, 152 had MCI, 152 had Alzheimer’s disease, and 144 were cognitively normal. Male carriers of APOE4 with MCI or Alzheimer’s disease had twice as many microbleeds in their brains as women with similar diagnoses. Researchers should evaluate whether sex steroids can reduce the microbleeds, said the authors.
The Lewy Body Composite Risk Score (LBCRS) increases the diagnostic probability that Lewy body pathology is contributing to dementia and may improve clinical detection and enrollment for clinical trials, according to a study published September 1 in Alzheimer’s Dementia. The LBCRS was tested in a consecutive series of 256 patients and compared with the Clinical Dementia Rating and gold-standard measures of cognition, motor symptoms, function, and behavior. Mean LBCRS scores were significantly different between patients with dementia with Lewy bodies and those with Alzheimer’s disease. Mean LBCRS scores also were significantly different between patients with mild cognitive impairment (MCI) due to dementia with Lewy bodies and patients with MCI due to Alzheimer’s disease. The LBCRS also was able to discriminate between dementia with Lewy bodies and other causes of dementia.
—Kimberly Williams
The risk of epilepsy is increased in children with hospital-diagnosed pertussis infections, compared with the general population, but the absolute risk is low, according to a Danish study published in the November 3 issue of JAMA. Researchers used data from population-based medical registries covering all Danish hospitals to identify all patients with pertussis born between 1978 and 2011 and followed up through 2011. Investigators used the Civil Registration System to identify 10 individuals from the general population for each patient with pertussis, matched on sex and year of birth. They identified 4,700 patients with pertussis, of whom 90 developed epilepsy during the follow-up. The cumulative incidence of epilepsy at age 10 was 1.7% for patients with pertussis and 0.9% for the matched comparison cohort.
Chronic users of antiepileptic drugs have poorer standing balance, compared with nonusers, according to a longitudinal twin and sibling study published in the November issue of Epilepsia. Researchers studied 26 twin and sibling pairs. Siblings were of the same gender, but only one in each pair had exposure to antiepileptic drugs. Clinical and laboratory balance examinations were conducted twice, and at least one year elapsed between examinations. The mean within-pair differences in balance measures were calculated cross-sectionally at baseline and follow-up, and longitudinally. Researchers found no significant mean within-pair difference at baseline in age (mean, 44), weight, and height. Cross-sectional sway measures from posturography and clinical static balance tests showed poorer performance in antiepileptic drug users, compared with nonusers, on several test conditions at baseline and follow-up.
Treatment for symptomatic intracerebral hemorrhage (sICH) after thrombolysis for stroke does not significantly reduce the likelihood of in-hospital mortality or hematoma expansion, according to a retrospective study published online ahead of print October 26 in JAMA Neurology. Of 3,894 patients treated with IV rt-PA within 4.5 hours after onset of ischemic stroke symptoms, 128 had sICH. The median time from initiation of rt-PA to sICH diagnosis was 470 minutes, and the median time from diagnosis to treatment of sICH was 112 minutes. The in-hospital mortality rate was 52.3%, and 26.8% of participants had hematoma expansion. In multivariable models, code status change to comfort measures after sICH diagnosis was the sole factor associated with increased in-hospital mortality. Severe hypofibrinogenemia was associated with hematoma expansion and occurred in 36.3% of patients without hematoma expansion.
Gaucher disease or mutations in the β-glucocerebrosidase gene (GBA) may protect individuals from deficiency in visual color discrimination, according to a study published September 14 in Journal of Parkinson’s Disease. Investigators tested groups of patients on the Farnsworth-Munsell 100 hue test (FMHT) and calculated their mean Total Error Scores (TES). Patients were classified as having Parkinson’s disease only, Gaucher disease only, Parkinson’s disease and Gaucher disease, GBA mutations, GBA mutations and Parkinson’s disease, or as controls. Patients with Parkinson’s disease only had the highest mean TES, and patients with Gaucher disease only had the lowest mean TES. GBA carriers without Parkinson’s disease made more errors than patients with Gaucher disease only, which was approximately the same number of errors as healthy controls.
Brain scans of people in a coma may help predict who will regain consciousness, according to a study published online ahead of print November 11 in Neurology. Researchers compared 27 prospectively recruited comatose patients who had severe brain injury (14 with traumatic injury and 13 with anoxic injury) with 14 age-matched healthy participants. Standardized clinical assessment and functional MRI were performed at an average of four days after withdrawal of sedation. Patients who were comatose showed a significant disruption of functional connectivity of brain areas spontaneously synchronized with posterior cingulate cortex, regardless of etiology of injury. The functional connectivity strength between the posterior cingulate cortex and the medial prefrontal cortex was significantly different between comatose patients who subsequently recovered and those who subsequently scored an unfavorable outcome three months after brain injury.
Raloxifene does not have a significant cognitive effect for women with Alzheimer’s disease, according to a study published online ahead of print November 4 in Neurology. Investigators conducted a randomized, double-blind, placebo-controlled pilot study with a planned treatment period of 12 months. Women with mild to moderate late-onset Alzheimer’s disease were randomized to high-dose (ie, 120 mg) oral raloxifene or identical placebo provided once daily. Forty-two women randomized to raloxifene or placebo were included in intent-to-treat analyses, and 39 women contributed 12-month outcomes. Results on the Alzheimer’s Disease Assessment Scale, cognitive subscale showed no cognitive benefits in the raloxifene-treated group. Raloxifene and placebo groups did not differ significantly on secondary analyses of dementia rating, activities of daily living, behavior, or a global cognition composite score.
Nonpharmacologic sleep interventions may help optimize outcomes in patients with chronic pain, according to data published in the November issue of Sleep. Investigators analyzed 11 randomized controlled trials, involving 1,066 participants, that evaluated the effect of nonpharmacologic sleep treatments on self-reported sleep quality, pain, and well-being in patients with long-term pain. They extracted means and standard deviations of sleep quality, pain, fatigue, depression, anxiety, and physical and psychologic functioning for the treatment and control groups at baseline, post treatment, and final follow-up. Nonpharmacologic sleep treatments in patients with chronic pain were associated with a large improvement in sleep quality, a small reduction in pain, and moderate improvement in fatigue at post treatment. The effects on sleep quality and fatigue were maintained for as long as one year, when a moderate reduction in depression also was observed.
CSF biomarkers of angiogenesis are increased in Parkinson’s disease and associated with gait difficulties, blood–brain barrier dysfunction, white matter lesions, and cerebral microbleeds, according to a study published online ahead of print October 28 in Neurology. This cross-sectional analysis included 38 elderly controls and 100 patients with Parkinson’s disease. Patients with Parkinson’s disease without dementia displayed higher CSF levels of vascular endothelial growth factor, placental growth factor, and vascular endothelial growth factor 2, and lower levels of angiopoietin 2, compared with controls. Similar alterations in vascular endothelial growth factor, placental growth factor, and angiopoietin 2 levels were observed in patients with Parkinson’s disease with dementia. Abnormal angiogenesis may be important in Parkinson’s disease pathogenesis and contribute to dopa-resistant symptoms, said the researchers.
Despite comparable reductions in total sleep time, partial sleep loss from sleep continuity disruption is more detrimental to positive mood than partial sleep loss from delaying bedtime, even when controlling for concomitant increases in negative mood, according to a study published in the November issue of Sleep. Participants were randomized to receive three consecutive nights of sleep continuity disruption by forced nocturnal awakenings, or one of the following two control conditions: restricted sleep opportunity or uninterrupted sleep. Compared with controls with restricted sleep opportunity, participants who underwent forced awakenings had significantly less slow wave sleep after the first night of sleep deprivation, and significantly lower positive mood after the second night of sleep deprivation. The differential change in slow wave sleep statistically mediated the observed group differences in positive mood.
Among patients who underwent transcatheter atrial septal defect closure (ASD), the use of clopidogrel and aspirin, compared with aspirin plus placebo, resulted in a lower monthly frequency of migraine attacks over three months, according to a study published online ahead of print November 9 in JAMA. A total of 171 patients without migraine were randomized to receive dual antiplatelet therapy or single antiplatelet therapy (ie, aspirin and placebo) for three months following transcatheter ASD closure. The mean age of the participants was 49, and 62% were women. Among patients with migraines following the procedure, those who received clopidogrel had less-severe migraine attacks. No patients who received clopidogrel had moderately or severely disabling migraine attacks, and 37% of the placebo group had such attacks.
The Consortium of Multiple Sclerosis Centers (CMSC) has issued a statement asserting that prescribers must retain the right to decide on the best treatment and medication for each patient with MS. “The varied and individualized course of MS mandates full access to symptomatic management as well as disease-modifying therapies, which, in the best judgment of the prescriber, offer optimal treatment outcomes. Medications to treat symptoms are carefully decided on an individual basis and by best-practice regimens,” said the CMSC. “Lack of understanding of the disease course and the challenges of MS treatment result in poor decision making practices by the insurance plans and specialty pharmacies and subsequent denial of prescribed medications.... CMSC proposes a collaborative care model in which providers, patients, and insurers work together to address these concerns.”
APOE4 greatly increases the likelihood of microbleeds in some men, according to a study published online ahead of print October 16 in Neurobiology of Aging. These microbleeds contribute to memory loss. Investigators examined brain scans of 658 participants (ages 48 to 91) in the Alzheimer’s Disease Neuroimaging Initiative. Of those subjects, 402 had mild cognitive impairment (MCI), 90 had early-stage Alzheimer’s disease, and 166 were cognitively normal. Researchers also analyzed scans of 448 other subjects (ages 36 to 88). Of those people, 152 had MCI, 152 had Alzheimer’s disease, and 144 were cognitively normal. Male carriers of APOE4 with MCI or Alzheimer’s disease had twice as many microbleeds in their brains as women with similar diagnoses. Researchers should evaluate whether sex steroids can reduce the microbleeds, said the authors.
The Lewy Body Composite Risk Score (LBCRS) increases the diagnostic probability that Lewy body pathology is contributing to dementia and may improve clinical detection and enrollment for clinical trials, according to a study published September 1 in Alzheimer’s Dementia. The LBCRS was tested in a consecutive series of 256 patients and compared with the Clinical Dementia Rating and gold-standard measures of cognition, motor symptoms, function, and behavior. Mean LBCRS scores were significantly different between patients with dementia with Lewy bodies and those with Alzheimer’s disease. Mean LBCRS scores also were significantly different between patients with mild cognitive impairment (MCI) due to dementia with Lewy bodies and patients with MCI due to Alzheimer’s disease. The LBCRS also was able to discriminate between dementia with Lewy bodies and other causes of dementia.
—Kimberly Williams
The risk of epilepsy is increased in children with hospital-diagnosed pertussis infections, compared with the general population, but the absolute risk is low, according to a Danish study published in the November 3 issue of JAMA. Researchers used data from population-based medical registries covering all Danish hospitals to identify all patients with pertussis born between 1978 and 2011 and followed up through 2011. Investigators used the Civil Registration System to identify 10 individuals from the general population for each patient with pertussis, matched on sex and year of birth. They identified 4,700 patients with pertussis, of whom 90 developed epilepsy during the follow-up. The cumulative incidence of epilepsy at age 10 was 1.7% for patients with pertussis and 0.9% for the matched comparison cohort.
Chronic users of antiepileptic drugs have poorer standing balance, compared with nonusers, according to a longitudinal twin and sibling study published in the November issue of Epilepsia. Researchers studied 26 twin and sibling pairs. Siblings were of the same gender, but only one in each pair had exposure to antiepileptic drugs. Clinical and laboratory balance examinations were conducted twice, and at least one year elapsed between examinations. The mean within-pair differences in balance measures were calculated cross-sectionally at baseline and follow-up, and longitudinally. Researchers found no significant mean within-pair difference at baseline in age (mean, 44), weight, and height. Cross-sectional sway measures from posturography and clinical static balance tests showed poorer performance in antiepileptic drug users, compared with nonusers, on several test conditions at baseline and follow-up.
Treatment for symptomatic intracerebral hemorrhage (sICH) after thrombolysis for stroke does not significantly reduce the likelihood of in-hospital mortality or hematoma expansion, according to a retrospective study published online ahead of print October 26 in JAMA Neurology. Of 3,894 patients treated with IV rt-PA within 4.5 hours after onset of ischemic stroke symptoms, 128 had sICH. The median time from initiation of rt-PA to sICH diagnosis was 470 minutes, and the median time from diagnosis to treatment of sICH was 112 minutes. The in-hospital mortality rate was 52.3%, and 26.8% of participants had hematoma expansion. In multivariable models, code status change to comfort measures after sICH diagnosis was the sole factor associated with increased in-hospital mortality. Severe hypofibrinogenemia was associated with hematoma expansion and occurred in 36.3% of patients without hematoma expansion.
Gaucher disease or mutations in the β-glucocerebrosidase gene (GBA) may protect individuals from deficiency in visual color discrimination, according to a study published September 14 in Journal of Parkinson’s Disease. Investigators tested groups of patients on the Farnsworth-Munsell 100 hue test (FMHT) and calculated their mean Total Error Scores (TES). Patients were classified as having Parkinson’s disease only, Gaucher disease only, Parkinson’s disease and Gaucher disease, GBA mutations, GBA mutations and Parkinson’s disease, or as controls. Patients with Parkinson’s disease only had the highest mean TES, and patients with Gaucher disease only had the lowest mean TES. GBA carriers without Parkinson’s disease made more errors than patients with Gaucher disease only, which was approximately the same number of errors as healthy controls.
Brain scans of people in a coma may help predict who will regain consciousness, according to a study published online ahead of print November 11 in Neurology. Researchers compared 27 prospectively recruited comatose patients who had severe brain injury (14 with traumatic injury and 13 with anoxic injury) with 14 age-matched healthy participants. Standardized clinical assessment and functional MRI were performed at an average of four days after withdrawal of sedation. Patients who were comatose showed a significant disruption of functional connectivity of brain areas spontaneously synchronized with posterior cingulate cortex, regardless of etiology of injury. The functional connectivity strength between the posterior cingulate cortex and the medial prefrontal cortex was significantly different between comatose patients who subsequently recovered and those who subsequently scored an unfavorable outcome three months after brain injury.
Raloxifene does not have a significant cognitive effect for women with Alzheimer’s disease, according to a study published online ahead of print November 4 in Neurology. Investigators conducted a randomized, double-blind, placebo-controlled pilot study with a planned treatment period of 12 months. Women with mild to moderate late-onset Alzheimer’s disease were randomized to high-dose (ie, 120 mg) oral raloxifene or identical placebo provided once daily. Forty-two women randomized to raloxifene or placebo were included in intent-to-treat analyses, and 39 women contributed 12-month outcomes. Results on the Alzheimer’s Disease Assessment Scale, cognitive subscale showed no cognitive benefits in the raloxifene-treated group. Raloxifene and placebo groups did not differ significantly on secondary analyses of dementia rating, activities of daily living, behavior, or a global cognition composite score.
Nonpharmacologic sleep interventions may help optimize outcomes in patients with chronic pain, according to data published in the November issue of Sleep. Investigators analyzed 11 randomized controlled trials, involving 1,066 participants, that evaluated the effect of nonpharmacologic sleep treatments on self-reported sleep quality, pain, and well-being in patients with long-term pain. They extracted means and standard deviations of sleep quality, pain, fatigue, depression, anxiety, and physical and psychologic functioning for the treatment and control groups at baseline, post treatment, and final follow-up. Nonpharmacologic sleep treatments in patients with chronic pain were associated with a large improvement in sleep quality, a small reduction in pain, and moderate improvement in fatigue at post treatment. The effects on sleep quality and fatigue were maintained for as long as one year, when a moderate reduction in depression also was observed.
CSF biomarkers of angiogenesis are increased in Parkinson’s disease and associated with gait difficulties, blood–brain barrier dysfunction, white matter lesions, and cerebral microbleeds, according to a study published online ahead of print October 28 in Neurology. This cross-sectional analysis included 38 elderly controls and 100 patients with Parkinson’s disease. Patients with Parkinson’s disease without dementia displayed higher CSF levels of vascular endothelial growth factor, placental growth factor, and vascular endothelial growth factor 2, and lower levels of angiopoietin 2, compared with controls. Similar alterations in vascular endothelial growth factor, placental growth factor, and angiopoietin 2 levels were observed in patients with Parkinson’s disease with dementia. Abnormal angiogenesis may be important in Parkinson’s disease pathogenesis and contribute to dopa-resistant symptoms, said the researchers.
Despite comparable reductions in total sleep time, partial sleep loss from sleep continuity disruption is more detrimental to positive mood than partial sleep loss from delaying bedtime, even when controlling for concomitant increases in negative mood, according to a study published in the November issue of Sleep. Participants were randomized to receive three consecutive nights of sleep continuity disruption by forced nocturnal awakenings, or one of the following two control conditions: restricted sleep opportunity or uninterrupted sleep. Compared with controls with restricted sleep opportunity, participants who underwent forced awakenings had significantly less slow wave sleep after the first night of sleep deprivation, and significantly lower positive mood after the second night of sleep deprivation. The differential change in slow wave sleep statistically mediated the observed group differences in positive mood.
Among patients who underwent transcatheter atrial septal defect closure (ASD), the use of clopidogrel and aspirin, compared with aspirin plus placebo, resulted in a lower monthly frequency of migraine attacks over three months, according to a study published online ahead of print November 9 in JAMA. A total of 171 patients without migraine were randomized to receive dual antiplatelet therapy or single antiplatelet therapy (ie, aspirin and placebo) for three months following transcatheter ASD closure. The mean age of the participants was 49, and 62% were women. Among patients with migraines following the procedure, those who received clopidogrel had less-severe migraine attacks. No patients who received clopidogrel had moderately or severely disabling migraine attacks, and 37% of the placebo group had such attacks.
The Consortium of Multiple Sclerosis Centers (CMSC) has issued a statement asserting that prescribers must retain the right to decide on the best treatment and medication for each patient with MS. “The varied and individualized course of MS mandates full access to symptomatic management as well as disease-modifying therapies, which, in the best judgment of the prescriber, offer optimal treatment outcomes. Medications to treat symptoms are carefully decided on an individual basis and by best-practice regimens,” said the CMSC. “Lack of understanding of the disease course and the challenges of MS treatment result in poor decision making practices by the insurance plans and specialty pharmacies and subsequent denial of prescribed medications.... CMSC proposes a collaborative care model in which providers, patients, and insurers work together to address these concerns.”
APOE4 greatly increases the likelihood of microbleeds in some men, according to a study published online ahead of print October 16 in Neurobiology of Aging. These microbleeds contribute to memory loss. Investigators examined brain scans of 658 participants (ages 48 to 91) in the Alzheimer’s Disease Neuroimaging Initiative. Of those subjects, 402 had mild cognitive impairment (MCI), 90 had early-stage Alzheimer’s disease, and 166 were cognitively normal. Researchers also analyzed scans of 448 other subjects (ages 36 to 88). Of those people, 152 had MCI, 152 had Alzheimer’s disease, and 144 were cognitively normal. Male carriers of APOE4 with MCI or Alzheimer’s disease had twice as many microbleeds in their brains as women with similar diagnoses. Researchers should evaluate whether sex steroids can reduce the microbleeds, said the authors.
The Lewy Body Composite Risk Score (LBCRS) increases the diagnostic probability that Lewy body pathology is contributing to dementia and may improve clinical detection and enrollment for clinical trials, according to a study published September 1 in Alzheimer’s Dementia. The LBCRS was tested in a consecutive series of 256 patients and compared with the Clinical Dementia Rating and gold-standard measures of cognition, motor symptoms, function, and behavior. Mean LBCRS scores were significantly different between patients with dementia with Lewy bodies and those with Alzheimer’s disease. Mean LBCRS scores also were significantly different between patients with mild cognitive impairment (MCI) due to dementia with Lewy bodies and patients with MCI due to Alzheimer’s disease. The LBCRS also was able to discriminate between dementia with Lewy bodies and other causes of dementia.
—Kimberly Williams
Mid-Atlantic Hospital Medicine Symposium Delivers Practice Pearls for Hospitalists
What do Clostridium difficile, Staphylococcus aureus, and acute pulmonary embolism have in common? They were all topics of discussion at the 2015 Mid-Atlantic Hospital Medicine Symposium, held at the Icahn School of Medicine at Mount Sinai in New York City.
Vikas Saini, MD, FACC, president of the Lown Institute in Brookline, Mass., and associate physician at Brigham and Women’s Hospital in Boston, gave the keynote address on high-value care.
“We’re asking you to do hard things: to be kind to complete strangers, to feel their pain, to be compassionate when you’re on the run,” Dr. Saini said as he urged his fellow physicians to be more personable with patients. “Pull up a chair and sit down. It takes you 30 seconds, but for the patient, it feels like an eternity.” Dr. Saini, a guest lecturer at the symposium, also stressed the importance of being collaborative and encouraged clinicians to join their colleagues in implementing RightCare Rounds.
Following the keynote address, Louis DePalo, MD, an associate professor of pulmonary, critical care, and sleep at Icahn School of Medicine, gave a 30-minute presentation on the management of acute pulmonary embolism (PE).
Dr. DePalo said that in his early days of practicing medicine, PE patients were usually hospitalized without debate. Today, thanks to indices such as the Pulmonary Embolism Severity Index, there are algorithms to determine the severity of PE in patients, allowing doctors to determine whether patients should be discharged or hospitalized. Still, Dr. DePalo added, “Discussions about sending a patient home are complicated.”
If a patient has severe PE, Dr. DePalo advised doctors to analyze studies such as the Pulmonary Embolism Thrombolysis (PEITHO) trial and the Moderate Pulmonary Embolism Treated with Thrombolysis (MOPPETT) trial to determine when to use advanced treatments. “One study may not be sufficient for administering advanced therapies,” Dr. DePalo said. “One study doesn’t make us feel good, so get a lot of data.”
After Dr. DePalo’s presentation, physicians gave presentations on common healthcare-associated infections. Gopi Patel, MD, an assistant professor of infectious diseases at Icahn School of Medicine, discussed Clostridium difficile infection (CDI). “CDI is the most common [healthcare-associated infection] in the United States,” Dr. Patel said as she urged physicians to “be a role model” by practicing good hand hygiene.
Using the updated practice guidelines from the Infectious Diseases Society of America, Tim Sullivan, MD, an assistant professor of infectious diseases at Icahn School of Medicine, discussed skin and soft tissue infections, particularly methicillin-resistant Staphylococcus aureus (MRSA). The guidelines “make a very important distinction between purulent and nonpurulent infections,” Dr. Sullivan said. “The majority of nonpurulent infections are caused by strep, [and] treating for strep seems to be sufficient to cure the infection … Adding extra coverage for MRSA is either not helpful or may actually be harmful to patients.”
Purulent infections, which require drainage, “are mostly caused by Staph aureus, including MRSA,” Dr. Sullivan said. “You don’t always have to give antibiotics, but they are recommended when the patient is sick.”
Dr. Sullivan said although “it can be sort of confusing trying to choose the right antibiotics for your patient … almost everyone should just receive vancomycin.” The drug is well-studied, inexpensive at $2.80 per dose for a five-day treatment, and well-tolerated by patients, he added. Yet vancomycin should not be administered to everyone as some patients experience devastating adverse reactions, and vancomycin could potentially cause irreversible hearing loss, he said.
Dr. Sullivan mentioned three new antibiotic treatments for MRSA: telavancin, which the U.S. Food and Drug Administration approved in 2009, costs 75 times more than vancomycin; dalbavancin, which was approved last May, costs $5,300 for two doses; and oritavancin, which was approved last August, costs $3,400 for one dose.
The arguments for using these newer drugs “may be that you can discharge the patient home, assuming they’ll have an active antibiotic in their system for a week, and maybe that will be more cost-effective,” Dr. Sullivan said. “The role that these should play in the management of your inpatients is still not clear, but they’re very new and interesting developments in treatment.” TH
Visit our website for more information on antibiotic stewardship and hospitalists.
What do Clostridium difficile, Staphylococcus aureus, and acute pulmonary embolism have in common? They were all topics of discussion at the 2015 Mid-Atlantic Hospital Medicine Symposium, held at the Icahn School of Medicine at Mount Sinai in New York City.
Vikas Saini, MD, FACC, president of the Lown Institute in Brookline, Mass., and associate physician at Brigham and Women’s Hospital in Boston, gave the keynote address on high-value care.
“We’re asking you to do hard things: to be kind to complete strangers, to feel their pain, to be compassionate when you’re on the run,” Dr. Saini said as he urged his fellow physicians to be more personable with patients. “Pull up a chair and sit down. It takes you 30 seconds, but for the patient, it feels like an eternity.” Dr. Saini, a guest lecturer at the symposium, also stressed the importance of being collaborative and encouraged clinicians to join their colleagues in implementing RightCare Rounds.
Following the keynote address, Louis DePalo, MD, an associate professor of pulmonary, critical care, and sleep at Icahn School of Medicine, gave a 30-minute presentation on the management of acute pulmonary embolism (PE).
Dr. DePalo said that in his early days of practicing medicine, PE patients were usually hospitalized without debate. Today, thanks to indices such as the Pulmonary Embolism Severity Index, there are algorithms to determine the severity of PE in patients, allowing doctors to determine whether patients should be discharged or hospitalized. Still, Dr. DePalo added, “Discussions about sending a patient home are complicated.”
If a patient has severe PE, Dr. DePalo advised doctors to analyze studies such as the Pulmonary Embolism Thrombolysis (PEITHO) trial and the Moderate Pulmonary Embolism Treated with Thrombolysis (MOPPETT) trial to determine when to use advanced treatments. “One study may not be sufficient for administering advanced therapies,” Dr. DePalo said. “One study doesn’t make us feel good, so get a lot of data.”
After Dr. DePalo’s presentation, physicians gave presentations on common healthcare-associated infections. Gopi Patel, MD, an assistant professor of infectious diseases at Icahn School of Medicine, discussed Clostridium difficile infection (CDI). “CDI is the most common [healthcare-associated infection] in the United States,” Dr. Patel said as she urged physicians to “be a role model” by practicing good hand hygiene.
Using the updated practice guidelines from the Infectious Diseases Society of America, Tim Sullivan, MD, an assistant professor of infectious diseases at Icahn School of Medicine, discussed skin and soft tissue infections, particularly methicillin-resistant Staphylococcus aureus (MRSA). The guidelines “make a very important distinction between purulent and nonpurulent infections,” Dr. Sullivan said. “The majority of nonpurulent infections are caused by strep, [and] treating for strep seems to be sufficient to cure the infection … Adding extra coverage for MRSA is either not helpful or may actually be harmful to patients.”
Purulent infections, which require drainage, “are mostly caused by Staph aureus, including MRSA,” Dr. Sullivan said. “You don’t always have to give antibiotics, but they are recommended when the patient is sick.”
Dr. Sullivan said although “it can be sort of confusing trying to choose the right antibiotics for your patient … almost everyone should just receive vancomycin.” The drug is well-studied, inexpensive at $2.80 per dose for a five-day treatment, and well-tolerated by patients, he added. Yet vancomycin should not be administered to everyone as some patients experience devastating adverse reactions, and vancomycin could potentially cause irreversible hearing loss, he said.
Dr. Sullivan mentioned three new antibiotic treatments for MRSA: telavancin, which the U.S. Food and Drug Administration approved in 2009, costs 75 times more than vancomycin; dalbavancin, which was approved last May, costs $5,300 for two doses; and oritavancin, which was approved last August, costs $3,400 for one dose.
The arguments for using these newer drugs “may be that you can discharge the patient home, assuming they’ll have an active antibiotic in their system for a week, and maybe that will be more cost-effective,” Dr. Sullivan said. “The role that these should play in the management of your inpatients is still not clear, but they’re very new and interesting developments in treatment.” TH
Visit our website for more information on antibiotic stewardship and hospitalists.
What do Clostridium difficile, Staphylococcus aureus, and acute pulmonary embolism have in common? They were all topics of discussion at the 2015 Mid-Atlantic Hospital Medicine Symposium, held at the Icahn School of Medicine at Mount Sinai in New York City.
Vikas Saini, MD, FACC, president of the Lown Institute in Brookline, Mass., and associate physician at Brigham and Women’s Hospital in Boston, gave the keynote address on high-value care.
“We’re asking you to do hard things: to be kind to complete strangers, to feel their pain, to be compassionate when you’re on the run,” Dr. Saini said as he urged his fellow physicians to be more personable with patients. “Pull up a chair and sit down. It takes you 30 seconds, but for the patient, it feels like an eternity.” Dr. Saini, a guest lecturer at the symposium, also stressed the importance of being collaborative and encouraged clinicians to join their colleagues in implementing RightCare Rounds.
Following the keynote address, Louis DePalo, MD, an associate professor of pulmonary, critical care, and sleep at Icahn School of Medicine, gave a 30-minute presentation on the management of acute pulmonary embolism (PE).
Dr. DePalo said that in his early days of practicing medicine, PE patients were usually hospitalized without debate. Today, thanks to indices such as the Pulmonary Embolism Severity Index, there are algorithms to determine the severity of PE in patients, allowing doctors to determine whether patients should be discharged or hospitalized. Still, Dr. DePalo added, “Discussions about sending a patient home are complicated.”
If a patient has severe PE, Dr. DePalo advised doctors to analyze studies such as the Pulmonary Embolism Thrombolysis (PEITHO) trial and the Moderate Pulmonary Embolism Treated with Thrombolysis (MOPPETT) trial to determine when to use advanced treatments. “One study may not be sufficient for administering advanced therapies,” Dr. DePalo said. “One study doesn’t make us feel good, so get a lot of data.”
After Dr. DePalo’s presentation, physicians gave presentations on common healthcare-associated infections. Gopi Patel, MD, an assistant professor of infectious diseases at Icahn School of Medicine, discussed Clostridium difficile infection (CDI). “CDI is the most common [healthcare-associated infection] in the United States,” Dr. Patel said as she urged physicians to “be a role model” by practicing good hand hygiene.
Using the updated practice guidelines from the Infectious Diseases Society of America, Tim Sullivan, MD, an assistant professor of infectious diseases at Icahn School of Medicine, discussed skin and soft tissue infections, particularly methicillin-resistant Staphylococcus aureus (MRSA). The guidelines “make a very important distinction between purulent and nonpurulent infections,” Dr. Sullivan said. “The majority of nonpurulent infections are caused by strep, [and] treating for strep seems to be sufficient to cure the infection … Adding extra coverage for MRSA is either not helpful or may actually be harmful to patients.”
Purulent infections, which require drainage, “are mostly caused by Staph aureus, including MRSA,” Dr. Sullivan said. “You don’t always have to give antibiotics, but they are recommended when the patient is sick.”
Dr. Sullivan said although “it can be sort of confusing trying to choose the right antibiotics for your patient … almost everyone should just receive vancomycin.” The drug is well-studied, inexpensive at $2.80 per dose for a five-day treatment, and well-tolerated by patients, he added. Yet vancomycin should not be administered to everyone as some patients experience devastating adverse reactions, and vancomycin could potentially cause irreversible hearing loss, he said.
Dr. Sullivan mentioned three new antibiotic treatments for MRSA: telavancin, which the U.S. Food and Drug Administration approved in 2009, costs 75 times more than vancomycin; dalbavancin, which was approved last May, costs $5,300 for two doses; and oritavancin, which was approved last August, costs $3,400 for one dose.
The arguments for using these newer drugs “may be that you can discharge the patient home, assuming they’ll have an active antibiotic in their system for a week, and maybe that will be more cost-effective,” Dr. Sullivan said. “The role that these should play in the management of your inpatients is still not clear, but they’re very new and interesting developments in treatment.” TH
Visit our website for more information on antibiotic stewardship and hospitalists.
What’s the best way to predict the success of a trial of labor after a previous C-section?
While 8 scoring tools predict success rates for a trial of labor after previous cesarean section (TOLAC), it’s unclear which is the best because no trials have compared prediction tools against each other, and each tool has a unique set of variables.
A “close-to-delivery” scoring nomogram predicting the success rate of TOLAC correlates well (90% accuracy) with actual outcomes (strength of recommendation [SOR]: B, prospective and retrospective cohort studies) and has been externally validated with multiple additional cohorts.
All other point-prediction scoring tools are accurate within 10% when predicting the success rate of TOLAC (SOR: B, prospective and retrospective cohort studies).
EVIDENCE SUMMARY
Seven validated prospective scoring systems, and one unvalidated system, predict a successful TOLAC based on a variety of clinical factors (TABLE1-11). The systems use different outcome statistics, so their predictive accuracy can’t be directly compared.12
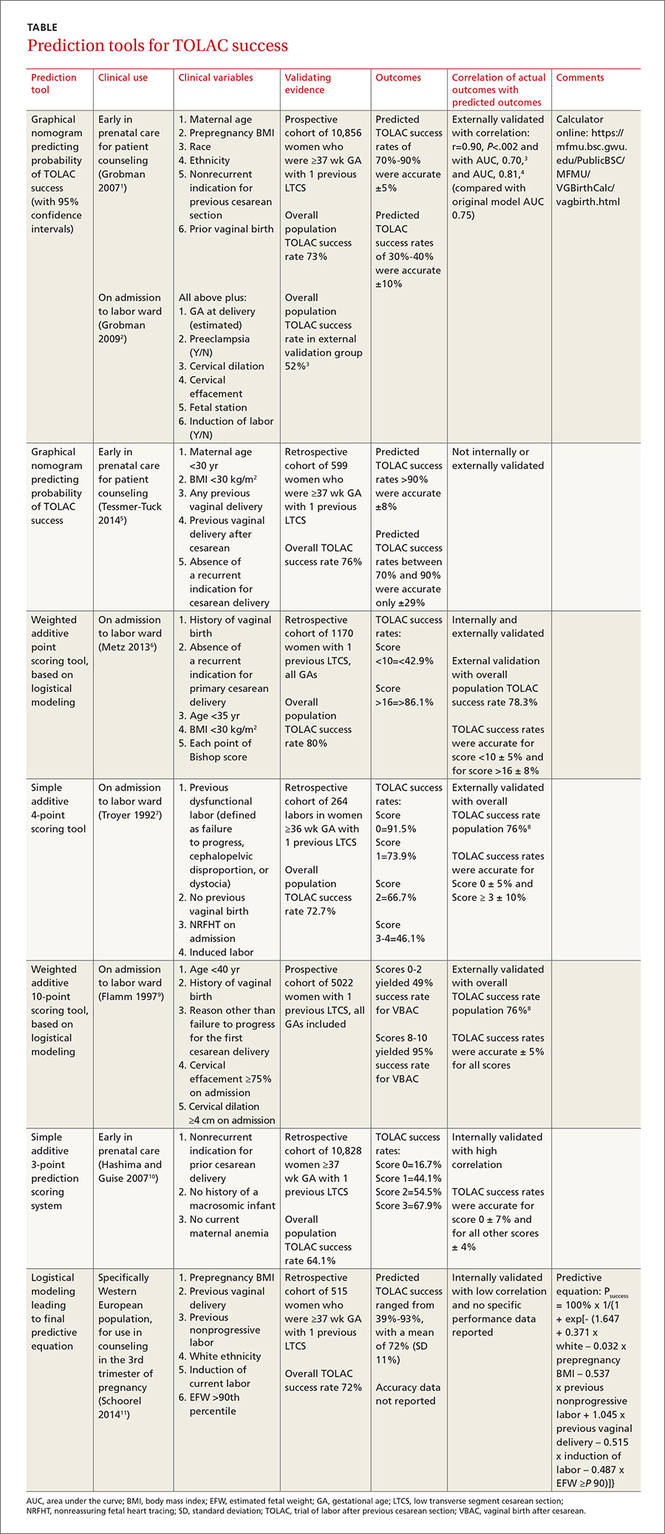
Grobman: Entry-to-care and close-to-delivery nomograms
Grobman et al created 2 prediction models, an “entry-to-care” model (used at the first prenatal visit), and a “close-to-delivery” model (used on admission to the labor ward).1,2 Both models display a graphic nomogram forecasting the probability of TOLAC success (with 95% confidence intervals [CIs]). The authors compared predicted TOLAC outcomes with actual TOLAC outcomes and found that the model predictions most successfully correlated with high-likelihood outcomes (70% to 90% chance of successful TOLAC, plus or minus approximately 5%). Both models were less accurate with low-likelihood outcomes (40% chance of successful TOLAC, plus or minus approximately 10%).
Many independent authors have validated the close-to-delivery model, comparing predicted with actual TOLAC success rates. In a retrospective cohort study of 490 women, Constantine et al found the correlation between the observed and predicted TOLAC rates to have an r of 0.90, P=.002, with an area under the curve (AUC) of 0.70.3 Yoki et al validated the model in a Japanese cohort of 729 women with an AUC of 0.81, consistent with the AUC of 0.75 reported in the development of the original model.4
Tessmer-Tuck: The close-to-delivery model without the race variable
Tessmer-Tuck et al developed a model similar to Grobman’s close-to-delivery model, but removed race/ethnicity as a variable and compared it to the accuracy of the Grobman nomogram.5 Variables considered in this model were maternal age <30 years (odds ratio [OR]=1.53; 95% CI, 1.00-2.36), body mass index (BMI) <30 kg/m2 (OR=1.82; 95% CI, 1.11-2.97), any previous vaginal delivery (OR=3.17; 95% CI, 1.50-6.80), previous vaginal delivery after cesarean (OR=2.24; 95% CI, 1.25-4.18), and absence of a recurrent indication for cesarean delivery (OR=1.81; 95% CI, 1.18-2.76).
The model provided a successful probability of vaginal birth after cesarean ranging from 38% to 98% with AUC of 0.723 (95% CI, 0.680-0.767). When compared with the Grobman model, the AUC for features in the Tessmer-Tuck model was 0.757 (95% CI, 0.713-0.801), similar to the AUC of 0.75 reported in the development of the original model. The predictive accuracy of TOLAC success between 70% and 90% was quite poor at only ±29%.
Metz: A 5-point scoring tool
Metz et al created a point scoring tool for use on admission to the labor ward, based on 5 variables weighted by degree of correlation with TOLAC success: a history of vaginal birth (OR=2.7; 95% CI, 1.8-4.1), absence of a recurrent indication for initial cesarean delivery (OR=2.0; 95% CI, 1.3-3.1), age <35 years (OR=2.0; 95% CI, 1.1-3.4), BMI <30 kg/m2 (OR=1.6; 95% CI, 1.1-2.4), and each point of Bishop score on admission (OR=1.3; 95% CI, 1.2-1.4).6
The authors internally validated this scoring tool with an AUC of 0.70 (95% CI, 0.67-0.74), then externally validated the tool with an independent cohort of 585 women and found an AUC of 0.80 (95% CI, 0.76-0.84). In the external validation cohort, TOLAC success rates were 37.4% (95% CI, 27.2-47.5) with a score <10 and 94.4% (95% CI, 90.9-97.8) with a score >16, performing within 8% of the prediction model.
Troyer: A simple 4-point tool
Troyer et al created a simple 4-point scoring tool for use on admission to the labor ward.7 The tool’s 4 variables—previous dysfunctional labor, no previous vaginal birth, nonreassuring fetal heart tracing (NRFHT) on admission, and induced labor—were found to reduce the success rate of a trial of labor (P<.05). Dinsmoor et al used this scoring tool in a group of 156 women with an overall TOLAC success rate of 76% (3% higher than Troyer’s group) and found that for labors with a favorable score (0), the tool performed within 5% and for labors with an unfavorable score (≥3), the tool performed within 10%.8
Flamm: 5 variables weighted by correlation with TOLAC success
Flamm et al also created a scoring tool for use on admission to the labor ward, based on 5 variables weighted according to degree of correlation with TOLAC success: age <40 years (OR=2.58; 95% CI, 1.55-4.3), history of a vaginal birth (OR=1.53-9.11 depending on where the vaginal birth fell in the woman’s reproductive history), reason other than failure to progress for the first cesarean delivery (OR=1.93; 95% CI, 1.58-2.35), cervical effacement ≥75% on admission (OR=2.72; 95% CI, 2.00-3.71), and cervical dilation ≥4 cm on admission (OR=2.16; 95% CI, 1.66-2.82).9 Dinsmoor validated this scoring tool as well in 156 women and found 100% TOLAC success for scores ≥7 (within 5% of the original tool) and 56% TOLAC success for scores ≤4 (compared with 49% for scores 0-2 in the original work).8
Hashima and Guise: A 3-point scoring tool
Hashima and Guise evaluated 16 variables and identified 7 associated with TOLAC outcome: indication for cesarean delivery (recurrent vs nonrecurrent), chorioamnionitis, macrosomicinfant, age, anemia, diabetes, and infant sex, from which they created a 3-point scoring tool using the variables most associated with TOLAC outcome. Each variable was assigned a score of 0 or 1, and the likelihood of TOLAC success was calculated.10
They found a relationship between score and TOLAC success. The original study population of 10,828 was randomly divided into a score development and validation group. TOLAC success percentages were most discordant between the tool development and internal validation groups for score 0 at 7%. Scores 1 to 3 were within 4% of each other.
Schoorel: A model designed for Western Europeann women
Finally, Schoorel et al developed and internally validated a prediction model for a Western European population, to be used during counseling in the third trimester of pregnancy.11 Six variables were identified and entered into the model calculations: prepregnancy BMI (entered as a continuous variable), (OR=0.96; 95% CI, 0.92-1.00); previous cesarean for nonprogressive labor (OR=0.50; 95% CI, 0.33-0.76); previous vaginal delivery (OR=3.81; 95% CI, 2.10-6.92); induction of labor (OR=0.52; 95% CI, 0.33-2.10); estimated fetal weight >90th percentile (OR=0.54; 95% CI, 0.14-2.02); and white ethnicity (OR=1.61; 95% CI, 0.97-2.66). The authors noted that the predicted probability of TOLAC success ranged from 39% to 93%, with a mean of 72% (standard deviation, 11%), and only noted the predicted probabilities were well calibrated from 65% upwards without additional data on specific performance.
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) lists strong predictors of a successful vaginal birth after cesarean as previous vaginal birth and spontaneous labor. Factors associated with decreased probability of success are recurrent indication for initial cesarean delivery (labor dystocia), increased maternal age, nonwhite ethnicity, gestational age greater than 40 weeks, maternal obesity, preeclampsia, short interpregnancy interval, and increased neonatal birth weight. ACOG does not offer any weighted or risk-based scoring tools for predicting success.13
Neither the American Academy of Family Physicians nor the American College of Nurse Midwives recommend specific scoring tools or success predictors.
1. Grobman WA, Lai Y, Landon MB, et al; National Institute of Child Health and Human Development (NICHD) Maternal- Fetal Medicine Units Network (MFMU). Development of a nomogram for prediction of vaginal birth after cesarean delivery. Obstet Gynecol. 2007;109:806-812.
2. Grobman WA, Lai Y, Landon MB, et al. Does information available at admission for delivery improve prediction of vaginal birth after cesarean? Am J Perinatol. 2009;26:693-701.
3. Costantine MM, Fox KA, Pacheco LD, et al. Does information available at delivery improve the accuracy of predicting vaginal birth after cesarean? Validation of the published models in an independent patient cohort. Am J Perinatol. 2011;28:293-298.
4. Yoki A, Ishikawa K, Miyazaki K, et al. Validation of the prediction model for success of vaginal birth after cesarean delivery in Japanese women. Int J Med Sci. 2012;9:488-491.
5. Tessmer-Tuck JA, El-Nashar SA, Racek AR, et al. Predicting vaginal birth after cesarean section: a cohort study. Gynecol Obstet Invest. 2014;77:121-126.
6. Metz TD, Stoddard GJ, Henry E, et al. Simple, validated vaginal birth after cesarean delivery prediction model for use at the time of admission. Obstet Gynecol. 2013;122:571-578.
7. Troyer LR, Parisi VM. Obstetric parameters affecting success in a trial of labor: designation of a scoring system. Am J Obstet Gynecol. 1992;167(4 pt 1):1099-1104.
8. Dinsmoor MJ, Brock EL. Predicting failed trial of labor after primary cesarean delivery. Obstet Gynecol. 2004;103:282-286.
9. Flamm BL, Geiger AM. Vaginal birth after cesarean delivery: an admission scoring system. Obstet Gynecol. 1997;90:907-910.
10. Hashima JN, Guise JM. Vaginal birth after cesarean: a prenatal scoring tool. Am J Obstet Gynecol. 2007;196:e22-e23.
11. Schoorel ENC, van Kuijk SMJ, Melman S, et al. Vaginal birth after a caesarean section: the development of a Western European population-based prediction model for deliveries at term. BJOG. 2014;121:194-201.
12. Guise JM, Eden K, Emeis C, et al. Vaginal birth after cesarean: New insights. Evidence Report/Technology Assessment No. 191. AHRQ Publication No. 10-E003. Rockville, MD: Agency for Healthcare Research and Quality; 2010.
13. American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 115: Vaginal birth after previous cesarean delivery. Obstet Gynecol. 2010;116(2 pt 1):450-463.
While 8 scoring tools predict success rates for a trial of labor after previous cesarean section (TOLAC), it’s unclear which is the best because no trials have compared prediction tools against each other, and each tool has a unique set of variables.
A “close-to-delivery” scoring nomogram predicting the success rate of TOLAC correlates well (90% accuracy) with actual outcomes (strength of recommendation [SOR]: B, prospective and retrospective cohort studies) and has been externally validated with multiple additional cohorts.
All other point-prediction scoring tools are accurate within 10% when predicting the success rate of TOLAC (SOR: B, prospective and retrospective cohort studies).
EVIDENCE SUMMARY
Seven validated prospective scoring systems, and one unvalidated system, predict a successful TOLAC based on a variety of clinical factors (TABLE1-11). The systems use different outcome statistics, so their predictive accuracy can’t be directly compared.12

Grobman: Entry-to-care and close-to-delivery nomograms
Grobman et al created 2 prediction models, an “entry-to-care” model (used at the first prenatal visit), and a “close-to-delivery” model (used on admission to the labor ward).1,2 Both models display a graphic nomogram forecasting the probability of TOLAC success (with 95% confidence intervals [CIs]). The authors compared predicted TOLAC outcomes with actual TOLAC outcomes and found that the model predictions most successfully correlated with high-likelihood outcomes (70% to 90% chance of successful TOLAC, plus or minus approximately 5%). Both models were less accurate with low-likelihood outcomes (40% chance of successful TOLAC, plus or minus approximately 10%).
Many independent authors have validated the close-to-delivery model, comparing predicted with actual TOLAC success rates. In a retrospective cohort study of 490 women, Constantine et al found the correlation between the observed and predicted TOLAC rates to have an r of 0.90, P=.002, with an area under the curve (AUC) of 0.70.3 Yoki et al validated the model in a Japanese cohort of 729 women with an AUC of 0.81, consistent with the AUC of 0.75 reported in the development of the original model.4
Tessmer-Tuck: The close-to-delivery model without the race variable
Tessmer-Tuck et al developed a model similar to Grobman’s close-to-delivery model, but removed race/ethnicity as a variable and compared it to the accuracy of the Grobman nomogram.5 Variables considered in this model were maternal age <30 years (odds ratio [OR]=1.53; 95% CI, 1.00-2.36), body mass index (BMI) <30 kg/m2 (OR=1.82; 95% CI, 1.11-2.97), any previous vaginal delivery (OR=3.17; 95% CI, 1.50-6.80), previous vaginal delivery after cesarean (OR=2.24; 95% CI, 1.25-4.18), and absence of a recurrent indication for cesarean delivery (OR=1.81; 95% CI, 1.18-2.76).
The model provided a successful probability of vaginal birth after cesarean ranging from 38% to 98% with AUC of 0.723 (95% CI, 0.680-0.767). When compared with the Grobman model, the AUC for features in the Tessmer-Tuck model was 0.757 (95% CI, 0.713-0.801), similar to the AUC of 0.75 reported in the development of the original model. The predictive accuracy of TOLAC success between 70% and 90% was quite poor at only ±29%.
Metz: A 5-point scoring tool
Metz et al created a point scoring tool for use on admission to the labor ward, based on 5 variables weighted by degree of correlation with TOLAC success: a history of vaginal birth (OR=2.7; 95% CI, 1.8-4.1), absence of a recurrent indication for initial cesarean delivery (OR=2.0; 95% CI, 1.3-3.1), age <35 years (OR=2.0; 95% CI, 1.1-3.4), BMI <30 kg/m2 (OR=1.6; 95% CI, 1.1-2.4), and each point of Bishop score on admission (OR=1.3; 95% CI, 1.2-1.4).6
The authors internally validated this scoring tool with an AUC of 0.70 (95% CI, 0.67-0.74), then externally validated the tool with an independent cohort of 585 women and found an AUC of 0.80 (95% CI, 0.76-0.84). In the external validation cohort, TOLAC success rates were 37.4% (95% CI, 27.2-47.5) with a score <10 and 94.4% (95% CI, 90.9-97.8) with a score >16, performing within 8% of the prediction model.
Troyer: A simple 4-point tool
Troyer et al created a simple 4-point scoring tool for use on admission to the labor ward.7 The tool’s 4 variables—previous dysfunctional labor, no previous vaginal birth, nonreassuring fetal heart tracing (NRFHT) on admission, and induced labor—were found to reduce the success rate of a trial of labor (P<.05). Dinsmoor et al used this scoring tool in a group of 156 women with an overall TOLAC success rate of 76% (3% higher than Troyer’s group) and found that for labors with a favorable score (0), the tool performed within 5% and for labors with an unfavorable score (≥3), the tool performed within 10%.8
Flamm: 5 variables weighted by correlation with TOLAC success
Flamm et al also created a scoring tool for use on admission to the labor ward, based on 5 variables weighted according to degree of correlation with TOLAC success: age <40 years (OR=2.58; 95% CI, 1.55-4.3), history of a vaginal birth (OR=1.53-9.11 depending on where the vaginal birth fell in the woman’s reproductive history), reason other than failure to progress for the first cesarean delivery (OR=1.93; 95% CI, 1.58-2.35), cervical effacement ≥75% on admission (OR=2.72; 95% CI, 2.00-3.71), and cervical dilation ≥4 cm on admission (OR=2.16; 95% CI, 1.66-2.82).9 Dinsmoor validated this scoring tool as well in 156 women and found 100% TOLAC success for scores ≥7 (within 5% of the original tool) and 56% TOLAC success for scores ≤4 (compared with 49% for scores 0-2 in the original work).8
Hashima and Guise: A 3-point scoring tool
Hashima and Guise evaluated 16 variables and identified 7 associated with TOLAC outcome: indication for cesarean delivery (recurrent vs nonrecurrent), chorioamnionitis, macrosomicinfant, age, anemia, diabetes, and infant sex, from which they created a 3-point scoring tool using the variables most associated with TOLAC outcome. Each variable was assigned a score of 0 or 1, and the likelihood of TOLAC success was calculated.10
They found a relationship between score and TOLAC success. The original study population of 10,828 was randomly divided into a score development and validation group. TOLAC success percentages were most discordant between the tool development and internal validation groups for score 0 at 7%. Scores 1 to 3 were within 4% of each other.
Schoorel: A model designed for Western Europeann women
Finally, Schoorel et al developed and internally validated a prediction model for a Western European population, to be used during counseling in the third trimester of pregnancy.11 Six variables were identified and entered into the model calculations: prepregnancy BMI (entered as a continuous variable), (OR=0.96; 95% CI, 0.92-1.00); previous cesarean for nonprogressive labor (OR=0.50; 95% CI, 0.33-0.76); previous vaginal delivery (OR=3.81; 95% CI, 2.10-6.92); induction of labor (OR=0.52; 95% CI, 0.33-2.10); estimated fetal weight >90th percentile (OR=0.54; 95% CI, 0.14-2.02); and white ethnicity (OR=1.61; 95% CI, 0.97-2.66). The authors noted that the predicted probability of TOLAC success ranged from 39% to 93%, with a mean of 72% (standard deviation, 11%), and only noted the predicted probabilities were well calibrated from 65% upwards without additional data on specific performance.
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) lists strong predictors of a successful vaginal birth after cesarean as previous vaginal birth and spontaneous labor. Factors associated with decreased probability of success are recurrent indication for initial cesarean delivery (labor dystocia), increased maternal age, nonwhite ethnicity, gestational age greater than 40 weeks, maternal obesity, preeclampsia, short interpregnancy interval, and increased neonatal birth weight. ACOG does not offer any weighted or risk-based scoring tools for predicting success.13
Neither the American Academy of Family Physicians nor the American College of Nurse Midwives recommend specific scoring tools or success predictors.
While 8 scoring tools predict success rates for a trial of labor after previous cesarean section (TOLAC), it’s unclear which is the best because no trials have compared prediction tools against each other, and each tool has a unique set of variables.
A “close-to-delivery” scoring nomogram predicting the success rate of TOLAC correlates well (90% accuracy) with actual outcomes (strength of recommendation [SOR]: B, prospective and retrospective cohort studies) and has been externally validated with multiple additional cohorts.
All other point-prediction scoring tools are accurate within 10% when predicting the success rate of TOLAC (SOR: B, prospective and retrospective cohort studies).
EVIDENCE SUMMARY
Seven validated prospective scoring systems, and one unvalidated system, predict a successful TOLAC based on a variety of clinical factors (TABLE1-11). The systems use different outcome statistics, so their predictive accuracy can’t be directly compared.12

Grobman: Entry-to-care and close-to-delivery nomograms
Grobman et al created 2 prediction models, an “entry-to-care” model (used at the first prenatal visit), and a “close-to-delivery” model (used on admission to the labor ward).1,2 Both models display a graphic nomogram forecasting the probability of TOLAC success (with 95% confidence intervals [CIs]). The authors compared predicted TOLAC outcomes with actual TOLAC outcomes and found that the model predictions most successfully correlated with high-likelihood outcomes (70% to 90% chance of successful TOLAC, plus or minus approximately 5%). Both models were less accurate with low-likelihood outcomes (40% chance of successful TOLAC, plus or minus approximately 10%).
Many independent authors have validated the close-to-delivery model, comparing predicted with actual TOLAC success rates. In a retrospective cohort study of 490 women, Constantine et al found the correlation between the observed and predicted TOLAC rates to have an r of 0.90, P=.002, with an area under the curve (AUC) of 0.70.3 Yoki et al validated the model in a Japanese cohort of 729 women with an AUC of 0.81, consistent with the AUC of 0.75 reported in the development of the original model.4
Tessmer-Tuck: The close-to-delivery model without the race variable
Tessmer-Tuck et al developed a model similar to Grobman’s close-to-delivery model, but removed race/ethnicity as a variable and compared it to the accuracy of the Grobman nomogram.5 Variables considered in this model were maternal age <30 years (odds ratio [OR]=1.53; 95% CI, 1.00-2.36), body mass index (BMI) <30 kg/m2 (OR=1.82; 95% CI, 1.11-2.97), any previous vaginal delivery (OR=3.17; 95% CI, 1.50-6.80), previous vaginal delivery after cesarean (OR=2.24; 95% CI, 1.25-4.18), and absence of a recurrent indication for cesarean delivery (OR=1.81; 95% CI, 1.18-2.76).
The model provided a successful probability of vaginal birth after cesarean ranging from 38% to 98% with AUC of 0.723 (95% CI, 0.680-0.767). When compared with the Grobman model, the AUC for features in the Tessmer-Tuck model was 0.757 (95% CI, 0.713-0.801), similar to the AUC of 0.75 reported in the development of the original model. The predictive accuracy of TOLAC success between 70% and 90% was quite poor at only ±29%.
Metz: A 5-point scoring tool
Metz et al created a point scoring tool for use on admission to the labor ward, based on 5 variables weighted by degree of correlation with TOLAC success: a history of vaginal birth (OR=2.7; 95% CI, 1.8-4.1), absence of a recurrent indication for initial cesarean delivery (OR=2.0; 95% CI, 1.3-3.1), age <35 years (OR=2.0; 95% CI, 1.1-3.4), BMI <30 kg/m2 (OR=1.6; 95% CI, 1.1-2.4), and each point of Bishop score on admission (OR=1.3; 95% CI, 1.2-1.4).6
The authors internally validated this scoring tool with an AUC of 0.70 (95% CI, 0.67-0.74), then externally validated the tool with an independent cohort of 585 women and found an AUC of 0.80 (95% CI, 0.76-0.84). In the external validation cohort, TOLAC success rates were 37.4% (95% CI, 27.2-47.5) with a score <10 and 94.4% (95% CI, 90.9-97.8) with a score >16, performing within 8% of the prediction model.
Troyer: A simple 4-point tool
Troyer et al created a simple 4-point scoring tool for use on admission to the labor ward.7 The tool’s 4 variables—previous dysfunctional labor, no previous vaginal birth, nonreassuring fetal heart tracing (NRFHT) on admission, and induced labor—were found to reduce the success rate of a trial of labor (P<.05). Dinsmoor et al used this scoring tool in a group of 156 women with an overall TOLAC success rate of 76% (3% higher than Troyer’s group) and found that for labors with a favorable score (0), the tool performed within 5% and for labors with an unfavorable score (≥3), the tool performed within 10%.8
Flamm: 5 variables weighted by correlation with TOLAC success
Flamm et al also created a scoring tool for use on admission to the labor ward, based on 5 variables weighted according to degree of correlation with TOLAC success: age <40 years (OR=2.58; 95% CI, 1.55-4.3), history of a vaginal birth (OR=1.53-9.11 depending on where the vaginal birth fell in the woman’s reproductive history), reason other than failure to progress for the first cesarean delivery (OR=1.93; 95% CI, 1.58-2.35), cervical effacement ≥75% on admission (OR=2.72; 95% CI, 2.00-3.71), and cervical dilation ≥4 cm on admission (OR=2.16; 95% CI, 1.66-2.82).9 Dinsmoor validated this scoring tool as well in 156 women and found 100% TOLAC success for scores ≥7 (within 5% of the original tool) and 56% TOLAC success for scores ≤4 (compared with 49% for scores 0-2 in the original work).8
Hashima and Guise: A 3-point scoring tool
Hashima and Guise evaluated 16 variables and identified 7 associated with TOLAC outcome: indication for cesarean delivery (recurrent vs nonrecurrent), chorioamnionitis, macrosomicinfant, age, anemia, diabetes, and infant sex, from which they created a 3-point scoring tool using the variables most associated with TOLAC outcome. Each variable was assigned a score of 0 or 1, and the likelihood of TOLAC success was calculated.10
They found a relationship between score and TOLAC success. The original study population of 10,828 was randomly divided into a score development and validation group. TOLAC success percentages were most discordant between the tool development and internal validation groups for score 0 at 7%. Scores 1 to 3 were within 4% of each other.
Schoorel: A model designed for Western Europeann women
Finally, Schoorel et al developed and internally validated a prediction model for a Western European population, to be used during counseling in the third trimester of pregnancy.11 Six variables were identified and entered into the model calculations: prepregnancy BMI (entered as a continuous variable), (OR=0.96; 95% CI, 0.92-1.00); previous cesarean for nonprogressive labor (OR=0.50; 95% CI, 0.33-0.76); previous vaginal delivery (OR=3.81; 95% CI, 2.10-6.92); induction of labor (OR=0.52; 95% CI, 0.33-2.10); estimated fetal weight >90th percentile (OR=0.54; 95% CI, 0.14-2.02); and white ethnicity (OR=1.61; 95% CI, 0.97-2.66). The authors noted that the predicted probability of TOLAC success ranged from 39% to 93%, with a mean of 72% (standard deviation, 11%), and only noted the predicted probabilities were well calibrated from 65% upwards without additional data on specific performance.
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) lists strong predictors of a successful vaginal birth after cesarean as previous vaginal birth and spontaneous labor. Factors associated with decreased probability of success are recurrent indication for initial cesarean delivery (labor dystocia), increased maternal age, nonwhite ethnicity, gestational age greater than 40 weeks, maternal obesity, preeclampsia, short interpregnancy interval, and increased neonatal birth weight. ACOG does not offer any weighted or risk-based scoring tools for predicting success.13
Neither the American Academy of Family Physicians nor the American College of Nurse Midwives recommend specific scoring tools or success predictors.
1. Grobman WA, Lai Y, Landon MB, et al; National Institute of Child Health and Human Development (NICHD) Maternal- Fetal Medicine Units Network (MFMU). Development of a nomogram for prediction of vaginal birth after cesarean delivery. Obstet Gynecol. 2007;109:806-812.
2. Grobman WA, Lai Y, Landon MB, et al. Does information available at admission for delivery improve prediction of vaginal birth after cesarean? Am J Perinatol. 2009;26:693-701.
3. Costantine MM, Fox KA, Pacheco LD, et al. Does information available at delivery improve the accuracy of predicting vaginal birth after cesarean? Validation of the published models in an independent patient cohort. Am J Perinatol. 2011;28:293-298.
4. Yoki A, Ishikawa K, Miyazaki K, et al. Validation of the prediction model for success of vaginal birth after cesarean delivery in Japanese women. Int J Med Sci. 2012;9:488-491.
5. Tessmer-Tuck JA, El-Nashar SA, Racek AR, et al. Predicting vaginal birth after cesarean section: a cohort study. Gynecol Obstet Invest. 2014;77:121-126.
6. Metz TD, Stoddard GJ, Henry E, et al. Simple, validated vaginal birth after cesarean delivery prediction model for use at the time of admission. Obstet Gynecol. 2013;122:571-578.
7. Troyer LR, Parisi VM. Obstetric parameters affecting success in a trial of labor: designation of a scoring system. Am J Obstet Gynecol. 1992;167(4 pt 1):1099-1104.
8. Dinsmoor MJ, Brock EL. Predicting failed trial of labor after primary cesarean delivery. Obstet Gynecol. 2004;103:282-286.
9. Flamm BL, Geiger AM. Vaginal birth after cesarean delivery: an admission scoring system. Obstet Gynecol. 1997;90:907-910.
10. Hashima JN, Guise JM. Vaginal birth after cesarean: a prenatal scoring tool. Am J Obstet Gynecol. 2007;196:e22-e23.
11. Schoorel ENC, van Kuijk SMJ, Melman S, et al. Vaginal birth after a caesarean section: the development of a Western European population-based prediction model for deliveries at term. BJOG. 2014;121:194-201.
12. Guise JM, Eden K, Emeis C, et al. Vaginal birth after cesarean: New insights. Evidence Report/Technology Assessment No. 191. AHRQ Publication No. 10-E003. Rockville, MD: Agency for Healthcare Research and Quality; 2010.
13. American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 115: Vaginal birth after previous cesarean delivery. Obstet Gynecol. 2010;116(2 pt 1):450-463.
1. Grobman WA, Lai Y, Landon MB, et al; National Institute of Child Health and Human Development (NICHD) Maternal- Fetal Medicine Units Network (MFMU). Development of a nomogram for prediction of vaginal birth after cesarean delivery. Obstet Gynecol. 2007;109:806-812.
2. Grobman WA, Lai Y, Landon MB, et al. Does information available at admission for delivery improve prediction of vaginal birth after cesarean? Am J Perinatol. 2009;26:693-701.
3. Costantine MM, Fox KA, Pacheco LD, et al. Does information available at delivery improve the accuracy of predicting vaginal birth after cesarean? Validation of the published models in an independent patient cohort. Am J Perinatol. 2011;28:293-298.
4. Yoki A, Ishikawa K, Miyazaki K, et al. Validation of the prediction model for success of vaginal birth after cesarean delivery in Japanese women. Int J Med Sci. 2012;9:488-491.
5. Tessmer-Tuck JA, El-Nashar SA, Racek AR, et al. Predicting vaginal birth after cesarean section: a cohort study. Gynecol Obstet Invest. 2014;77:121-126.
6. Metz TD, Stoddard GJ, Henry E, et al. Simple, validated vaginal birth after cesarean delivery prediction model for use at the time of admission. Obstet Gynecol. 2013;122:571-578.
7. Troyer LR, Parisi VM. Obstetric parameters affecting success in a trial of labor: designation of a scoring system. Am J Obstet Gynecol. 1992;167(4 pt 1):1099-1104.
8. Dinsmoor MJ, Brock EL. Predicting failed trial of labor after primary cesarean delivery. Obstet Gynecol. 2004;103:282-286.
9. Flamm BL, Geiger AM. Vaginal birth after cesarean delivery: an admission scoring system. Obstet Gynecol. 1997;90:907-910.
10. Hashima JN, Guise JM. Vaginal birth after cesarean: a prenatal scoring tool. Am J Obstet Gynecol. 2007;196:e22-e23.
11. Schoorel ENC, van Kuijk SMJ, Melman S, et al. Vaginal birth after a caesarean section: the development of a Western European population-based prediction model for deliveries at term. BJOG. 2014;121:194-201.
12. Guise JM, Eden K, Emeis C, et al. Vaginal birth after cesarean: New insights. Evidence Report/Technology Assessment No. 191. AHRQ Publication No. 10-E003. Rockville, MD: Agency for Healthcare Research and Quality; 2010.
13. American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 115: Vaginal birth after previous cesarean delivery. Obstet Gynecol. 2010;116(2 pt 1):450-463.
Evidence-based answers from the Family Physicians Inquiries Network
Prevention, recognition, and management of complications associated with sacrospinous colpopexy

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
This video is brought to you by ![]()
I will click those boxes, but first, I will care for my patient
I am a member of a large primary care group certified as a level 3 patient-centered medical home; we are in the midst of certifying for Meaningful Use Stage 2. Recently, my first patient of the day was a 65-year-old widowed man who used tobacco, had diabetes, hypertension, and elevated lipid levels, and hadn’t seen me in 2 years. He came in for a Medicare Advantage comprehensive physical examination.
To meet all Meaningful Use Stage 2 expectations during his physical exam, I had to:
• check the box to document discussion of body mass index (his was 26 kg/m2),
• check the box for functional status assessment,
• check the box to indicate that his blood pressure was under 140/90 mm Hg (the threshold for a previously diagnosed hypertensive patient),
• generate annual care guides for the “clinically important conditions” of hypertension with diabetes, tobacco use, and hyperlipidemia,
• review the quality information stoplight for lab tests to be ordered,
• remind the patient to complete his annual eye examination,
• identify hierarchical categorical coding to maximize the accurate morbidity determination of my patient and, therefore, funding for our medical group,
• click on the code for annual prostate examination screening,
• click on the code to bill for tobacco cessation counseling, and
• generate a visit summary.
Naturally, all of this was in addition to giving my patient my full, undivided attention, providing him with the opportunity to express his concerns, and then pursuing a careful examination of his health problems.
Documentation expectations, coding, billing, and the like degrade the clinician-patient relationship, and I’m not going to redirect my attention away from the patient’s concerns and toward these activities. I will continue to listen and respect what my patients have to say and engage with them, and not my keyboard. I will strive to identify and meet their health needs.
Click the boxes? Yes, I will click all the right boxes; my livelihood and my medical group’s future success depend on that. But how much congruence will there be between what I “click” and what I “do”? Well …
We are challenged by good intentions but crushingly poor execution—and it’s taking its toll.
H. Andrew Selinger, MD
Bristol, Conn
I am a member of a large primary care group certified as a level 3 patient-centered medical home; we are in the midst of certifying for Meaningful Use Stage 2. Recently, my first patient of the day was a 65-year-old widowed man who used tobacco, had diabetes, hypertension, and elevated lipid levels, and hadn’t seen me in 2 years. He came in for a Medicare Advantage comprehensive physical examination.
To meet all Meaningful Use Stage 2 expectations during his physical exam, I had to:
• check the box to document discussion of body mass index (his was 26 kg/m2),
• check the box for functional status assessment,
• check the box to indicate that his blood pressure was under 140/90 mm Hg (the threshold for a previously diagnosed hypertensive patient),
• generate annual care guides for the “clinically important conditions” of hypertension with diabetes, tobacco use, and hyperlipidemia,
• review the quality information stoplight for lab tests to be ordered,
• remind the patient to complete his annual eye examination,
• identify hierarchical categorical coding to maximize the accurate morbidity determination of my patient and, therefore, funding for our medical group,
• click on the code for annual prostate examination screening,
• click on the code to bill for tobacco cessation counseling, and
• generate a visit summary.
Naturally, all of this was in addition to giving my patient my full, undivided attention, providing him with the opportunity to express his concerns, and then pursuing a careful examination of his health problems.
Documentation expectations, coding, billing, and the like degrade the clinician-patient relationship, and I’m not going to redirect my attention away from the patient’s concerns and toward these activities. I will continue to listen and respect what my patients have to say and engage with them, and not my keyboard. I will strive to identify and meet their health needs.
Click the boxes? Yes, I will click all the right boxes; my livelihood and my medical group’s future success depend on that. But how much congruence will there be between what I “click” and what I “do”? Well …
We are challenged by good intentions but crushingly poor execution—and it’s taking its toll.
H. Andrew Selinger, MD
Bristol, Conn
I am a member of a large primary care group certified as a level 3 patient-centered medical home; we are in the midst of certifying for Meaningful Use Stage 2. Recently, my first patient of the day was a 65-year-old widowed man who used tobacco, had diabetes, hypertension, and elevated lipid levels, and hadn’t seen me in 2 years. He came in for a Medicare Advantage comprehensive physical examination.
To meet all Meaningful Use Stage 2 expectations during his physical exam, I had to:
• check the box to document discussion of body mass index (his was 26 kg/m2),
• check the box for functional status assessment,
• check the box to indicate that his blood pressure was under 140/90 mm Hg (the threshold for a previously diagnosed hypertensive patient),
• generate annual care guides for the “clinically important conditions” of hypertension with diabetes, tobacco use, and hyperlipidemia,
• review the quality information stoplight for lab tests to be ordered,
• remind the patient to complete his annual eye examination,
• identify hierarchical categorical coding to maximize the accurate morbidity determination of my patient and, therefore, funding for our medical group,
• click on the code for annual prostate examination screening,
• click on the code to bill for tobacco cessation counseling, and
• generate a visit summary.
Naturally, all of this was in addition to giving my patient my full, undivided attention, providing him with the opportunity to express his concerns, and then pursuing a careful examination of his health problems.
Documentation expectations, coding, billing, and the like degrade the clinician-patient relationship, and I’m not going to redirect my attention away from the patient’s concerns and toward these activities. I will continue to listen and respect what my patients have to say and engage with them, and not my keyboard. I will strive to identify and meet their health needs.
Click the boxes? Yes, I will click all the right boxes; my livelihood and my medical group’s future success depend on that. But how much congruence will there be between what I “click” and what I “do”? Well …
We are challenged by good intentions but crushingly poor execution—and it’s taking its toll.
H. Andrew Selinger, MD
Bristol, Conn
Sleep apnea treatments: Helping patients explore options
If you’re looking for a resource to provide to your patients with sleep apnea, you may want to consider this one from the National Heart, Lung, and Blood Institute. “How Is Sleep Apnea Treated?” discusses lifestyle changes, mouthpieces, breathing devices, and surgery, and explains which work options work best for which patients. The resource can be found at: http://www.nhlbi.nih.gov/health/health-topics/topics/sleepapnea/treatment.
If you’re looking for a resource to provide to your patients with sleep apnea, you may want to consider this one from the National Heart, Lung, and Blood Institute. “How Is Sleep Apnea Treated?” discusses lifestyle changes, mouthpieces, breathing devices, and surgery, and explains which work options work best for which patients. The resource can be found at: http://www.nhlbi.nih.gov/health/health-topics/topics/sleepapnea/treatment.
If you’re looking for a resource to provide to your patients with sleep apnea, you may want to consider this one from the National Heart, Lung, and Blood Institute. “How Is Sleep Apnea Treated?” discusses lifestyle changes, mouthpieces, breathing devices, and surgery, and explains which work options work best for which patients. The resource can be found at: http://www.nhlbi.nih.gov/health/health-topics/topics/sleepapnea/treatment.
Helping patients who are newly diagnosed with hepatitis C
Patients who are newly diagnosed with hepatitis C are likely to feel overwhelmed—but this detailed brochure from the American Liver Foundation provides answers to questions that patients are likely to have. The brochure, available at http://www.liverfoundation.org/downloads/alf_download_901.pdf, addresses issues such as available treatments and steps to take to prevent the transmission of hepatitis C to others.
Patients who are newly diagnosed with hepatitis C are likely to feel overwhelmed—but this detailed brochure from the American Liver Foundation provides answers to questions that patients are likely to have. The brochure, available at http://www.liverfoundation.org/downloads/alf_download_901.pdf, addresses issues such as available treatments and steps to take to prevent the transmission of hepatitis C to others.
Patients who are newly diagnosed with hepatitis C are likely to feel overwhelmed—but this detailed brochure from the American Liver Foundation provides answers to questions that patients are likely to have. The brochure, available at http://www.liverfoundation.org/downloads/alf_download_901.pdf, addresses issues such as available treatments and steps to take to prevent the transmission of hepatitis C to others.